Abstract
Background
Empyema refers to pus in the pleural space, commonly due to adjacent pneumonia, chest wall injury, or a complication of thoracic surgery. A range of therapeutic options are available for its management, ranging from percutaneous aspiration and intercostal drainage to video‐assisted thoracoscopic surgery (VATS) or thoracotomy drainage. Intrapleural fibrinolytics may also be administered following intercostal drain insertion to facilitate pleural drainage. There is currently a lack of consensus regarding optimal treatment.
Objectives
To assess the effectiveness and safety of surgical versus non‐surgical treatments for complicated parapneumonic effusion or pleural empyema.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2016, Issue 9), MEDLINE (Ebscohost) (1946 to July week 3 2013, July 2015 to October 2016) and MEDLINE (Ovid) (1 May 2013 to July week 1 2015), Embase (2010 to October 2016), CINAHL (1981 to October 2016) and LILACS (1982 to October 2016) on 20 October 2016. We searched ClinicalTrials.gov and WHO International Clinical Trials Registry Platform for ongoing studies (December 2016).
Selection criteria
Randomised controlled trials that compared a surgical with a non‐surgical method of management for all age groups with pleural empyema.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data, and checked the data for accuracy. We contacted trial authors for additional information. We assessed the quality of the evidence using the GRADE approach.
Main results
We included eight randomised controlled trials with a total of 391 participants. Six trials focused on children and two on adults. Trials compared tube thoracostomy drainage (non‐surgical), with or without intrapleural fibrinolytics, to either VATS or thoracotomy (surgical) for the management of pleural empyema. Assessment of risk of bias for the included studies was generally unclear for selection and blinding but low for attrition and reporting bias. Data analyses compared thoracotomy versus tube thoracostomy and VATS versus tube thoracostomy. We pooled data for meta‐analysis where appropriate. We performed a subgroup analysis for children along with a sensitivity analysis for studies that used fibrinolysis in non‐surgical treatment arms.
The comparison of open thoracotomy versus thoracostomy drainage included only one study in children, which reported no deaths in either treatment arm. However, the trial showed a statistically significant reduction in mean hospital stay of 5.90 days for those treated with primary thoracotomy. It also showed a statistically significant reduction in procedural complications for those treated with thoracotomy compared to thoracostomy drainage. We downgraded the quality of the evidence for length of hospital stay and procedural complications outcomes to moderate due to the small sample size.
The comparison of VATS versus thoracostomy drainage included seven studies, which we pooled in a meta‐analysis. There was no statistically significant difference in mortality or procedural complications between groups. This was true for both adults and children with or without fibrinolysis. However, mortality data were limited: one study reported one death in each treatment arm, and seven studies reported no deaths. There was a statistically significant reduction in mean length of hospital stay for those treated with VATS. The subgroup analysis showed the same result in adults, but there was insufficient evidence to estimate an effect for children. We could not perform a separate analysis for fibrinolysis for this outcome because all included studies used fibrinolysis in the non‐surgical arms. We downgraded the quality of the evidence to low for mortality (due to wide confidence intervals and indirectness), and moderate for other outcomes in this comparison due to either high heterogeneity or wide confidence intervals.
Authors' conclusions
Our findings suggest there is no statistically significant difference in mortality between primary surgical and non‐surgical management of pleural empyema for all age groups. Video‐assisted thoracoscopic surgery may reduce length of hospital stay compared to thoracostomy drainage alone.
There was insufficient evidence to assess the impact of fibrinolytic therapy.
A number of common outcomes were reported in the included studies that were not directly examined in our primary and secondary outcomes. These included duration of chest tube drainage, duration of fever, analgesia requirement, and total cost of treatment. Future studies focusing on patient‐centred outcomes, such as patient functional scores, and other clinically relevant outcomes, such as radiographic improvement, treatment failure rates, and amount of fluid drainage, are needed to inform clinical decisions.
Plain language summary
Surgical versus non‐surgical treatment for pus in the space around the lungs
Review question
We investigated if there was a difference in outcomes for patients who develop pus in the space around the lungs (empyema) among those who had surgical treatment and people who had non‐surgical treatment. We looked for differences in proportions of children and adults who survived, time in hospital, and complications from treatments.
Background
Pus can form in the space around the lungs as a result of pneumonia, complication of chest wall trauma, or surgery. Solid divisions, called loculations, can form within the pus. The infection does not usually improve with antibiotic treatment alone.
There are several surgical and non‐surgical treatments. Non‐surgical treatments include draining pus using a needle inserted through the chest wall (thoracentesis) or by inserting a tube through the chest wall to drain infection (thoracostomy). If a chest tube is inserted, drugs can be injected into the space around the lungs to break down the divisions. This is called fibrinolysis. Non‐surgical treatments can cause harms, including air in the space around the lungs, damage to chest tissue, or lungs filling with fluid as they re‐expand. Surgical treatment involves either opening the chest cavity and clearing out the infection (thoracotomy) or clearing out infection through small cuts on the chest wall with the aid of a camera, known as video‐assisted thoracoscopic surgery (VATS). A chest tube drains any fluids after surgery. Risks from surgery include air in the space around the lungs, rib pain, and anaesthetic complications.
Search date
The evidence is current to October 2016.
Study characteristics
We included eight trials with a total of 391 participants. Six trials focused on children and two on adults. The trials compared chest tube drainage (non‐surgical), with or without fibrinolysis, to either VATS or thoracotomy (surgical).
Study funding sources
Two studies declared no financial conflicts of interest; the remaining six studies did not report funding source.
Key results
There was no difference in the proportion of patients of all ages who survived empyema in relation to surgical or non‐surgical treatment. However, this finding was based on limited data: one study reported one death with each treatment option, and seven studies reported no deaths. There was no difference in rates of complications between patients treated with surgical or non‐surgical options.
There was limited evidence to suggest that VATS reduced length of stay in hospital compared to non‐surgical treatments.
Quality of evidence
The quality of the evidence was moderate overall. The main limitations were few included studies for each analysis and inconsistencies among studies.
Summary of findings
Summary of findings for the main comparison. Open thoracotomy compared to thoracostomy drainage for pleural empyema.
| Open thoracotomy compared to thoracostomy drainage for pleural empyema | ||||||
| Patient or population: children with pleural empyema Intervention: open thoracotomy Comparison: thoracostomy drainage | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Thoracostomy drainage | Open thoracotomy | |||||
|
Mortality Follow‐up: up to 3 months after discharge |
Risk in study population | Not estimable | 30 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | No deaths occurred in either group. | |
| — | — | |||||
|
Length of hospital stay (days) Follow‐up: up to 3 months after discharge |
The mean length of hospital stay in the control group was 15.4 days. | The mean length of hospital stay in the intervention group was 5.9 days fewer (7.29 fewer to 4.51 fewer). | — | 30 (1 RCT) | ⊕⊕⊕⊝ MODERATE1 | |
|
Procedural complications Follow‐up: up to 3 months after discharge |
Risk in study population | OR 0.10 (0.02 to 0.63) | 30 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 600 per 1000 | 130 per 1000 (29 to 486) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level due to small sample size and only one study.
Summary of findings 2. VATS compared to thoracostomy drainage for pleural empyema.
| VATS compared to thoracostomy drainage for pleural empyema | ||||||
| Patient or population: children and adults with pleural empyema Intervention: VATS Comparison: thoracostomy drainage | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Thoracostomy drainage | VATS | |||||
| Mortality | Risk in study population | OR 0.80 (0.04 to 14.89) | 361 (7 RCTs) | ⊕⊕⊝⊝ LOW1 | Data only for adults | |
| 6 per 1000 | 5 per 1000 (0 to 78) | |||||
| Mortality: children | Risk in study population | Not estimable | 271 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | No deaths occurred in either group. | |
| Not pooled | Not pooled | |||||
|
Mortality: adults Follow‐up: not reported |
Risk in study population | OR 0.80 (0.04 to 14.89) | 90 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | No deaths occurred in Bilgin 2006. Data based on Wait 1997 | |
| 23 per 1000 | 18 per 1000 (1 to 257) | |||||
|
Length of hospital stay (days) Follow‐up: 1 year in Cobanoglu 2011 and 3 months in Marhuenda 2014 |
Control group | The mean length of hospital stay in the intervention group was 2.52 days fewer (4.26 fewer to 0.77 fewer). | — | 231 (5 RCTs) | ⊕⊕⊕⊝ MODERATE4 | Note: Follow‐up period not reported in Kurt 2006; Peter 2009; Wait 1997. |
|
Procedural complications Follow‐up: 6 months in Bilgin 2006 and not reported in Wait 1997 |
Risk in study population | OR 0.46 (0.08 to 2.75) | 271 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 5 | ||
| 23 per 1000 | 11 per 1000 (2 to 60) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial; VATS: video‐assisted thoracoscopic surgery | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded two levels due to wide confidence intervals (data from only one study) and indirectness (data only available for adults). 2Downgraded one level as data not available due to small sample size and no events occurring in the studies. 3Downgraded one level due to small sample size (imprecision) as data available from only one study. 4Downgraded one level due to heterogeneity. 5Downgraded one level due to wide confidence intervals.
Background
Description of the condition
Empyema refers to pus in the pleural space. The pathogenesis of empyema is not well understood, but it is hypothesised to be caused by transmigration of bacteria into the pleural space, most commonly from adjacent lung infection or pneumonia. Other potential causes include penetrating chest wall injuries and thoracic procedures (Ferguson 1996). Approximately 20% to 57% of people with pneumonia develop a parapneumonic effusion, of whom some can progress to pleural empyema (Sahn 2007). Progression of the condition begins with the exudative stage characterised by a shift of pulmonary interstitial fluid into the pleural space from an increased capillary permeability. The second is the fibropurulent stage, where the pleural space becomes infected and loculation (development of separate cavities) occurs. Lastly, organisation of the chronic inflammation results in proliferation of fibroblasts and a thickening of the pleural space, known as a pleural peel, which can be seen on imaging (Light 2006).
Clinically, the three stages can be referred to as uncomplicated parapneumonic effusion (UPPE), complicated parapneumonic effusion (CPPE), and pleural empyema, respectively. Uncomplicated parapneumonic effusion usually resolves with antibiotic therapy alone, but CPPE and empyema require additional interventions. The laboratory diagnosis of CPPE can be made with any one of the following pleural fluid features: positive bacterial studies, glucose level below 60 mg/dL, pH below 7.20, or lactate dehydrogenase (LDH) more than three times the upper normal limit for serum (Light 2006). Medical imaging examination findings of pleural loculations and septations on ultrasound, or pleural thickening and enhancement on computed tomography (CT), can also suggest the diagnosis; however, the definitive diagnosis is a positive culture (Sahn 2007).
This review examined the available evidence for the various treatments of CPPE and empyema. We compared outcomes from surgical and non‐surgical methods of treatment with the aim of determining what constitutes the optimal strategy for management.
Description of the intervention
Surgical interventions include video‐assisted thoracoscopic surgery (VATS) or open thoracotomy (Appendix 1). Non‐surgical management includes thoracentesis and insertion of a chest tube (thoracostomy), with or without the use of intrapleural fibrinolytics (Appendix 1). Descriptions of interventions follow.
Surgical management
Video‐assisted thoracoscopic surgery (VATS) (Appendix 1) enables visualisation of the pleural cavity for drainage of pus and disruption of septations. A temporary chest tube is left in place for postoperative drainage of any re‐accumulated effusions (Light 2006). Open thoracotomy involves surgical exploration of the pleural space and drainage of the empyema (Light 2006). Complications of both VATS and thoracotomy include postoperative pneumothorax, intercostal neuralgia, and associated anaesthetic risks (Yim 1996). Postoperative complications and longer recovery periods are associated with open thoracotomy (Jaffé 2003), hence VATS is the more commonly performed surgical procedure for empyema. Nonetheless, thoracotomy may be the initial choice in select cases, or alternatively, VATS may be converted to open thoracotomy if necessary (Lardinois 2005).
Non‐surgical management
Non‐surgical management of empyema includes thoracentesis and chest tube insertion (thoracostomy). Thoracentesis involves aspirating the pleural fluid through a percutaneously inserted catheter, which can be performed under ultrasound or CT guidance. Potential complications from this procedure include haemothorax, pneumothorax, catheter malposition, and bronchopleural fistula (Jones 2003). Chest tube insertion involves dissection of a small area of the chest wall muscle and the placement of a chest tube (Oddel 1994). Duration of treatment is usually no longer than 7 to 10 days or when drainage is minimal, as guided by evidence from clinical tests or radiographic images, or both, of empyema resolution (Oddel 1994). In patients not responding to treatment or who require a prolonged period of chest tube placement, surgical intervention may be considered. Complications associated with tube thoracostomy include chest tube malposition, tissue trauma, and re‐expansion pulmonary oedema (Miller 1987).
Intrapleural fibrinolysis initially used a combination of streptokinase and streptodornase and is an adjunct to chest tube drainage to facilitate fibrinolysis of loculations (Tillett 1951). Side effects due to impurities led to a decline in its use, but the availability of a more purified form and successful trial of urokinase have led to a re‐appraisal of this modality (Aye 1991; Temes 1996). More recently, a 2008 Cochrane review concluded that intrapleural fibrinolytic therapy conferred significant benefit in reducing the requirement for surgical interventions (Cameron 2008); however, when subgroup analysis was performed on high‐quality trials, the benefit was not significant.
How the intervention might work
A cornerstone of the management of patients with severe infection is source control. This refers to interventions which aim to control the foci of infections (Marshall 2009). There is a long history of the recognition that draining empyema is beneficial, dating back to the time of Hippocrates (Cameron 2008). However, during the progression of empyema there is a tendency for the formation of loculations (separate cavities) and adhesions which may limit the effectiveness of drainage (Light 1985). It is hypothesised that surgical intervention or the addition of intrapleural fibrinolytics to thoracostomy drainage may reduce the impact of loculations and adhesions on the drainage of empyema.
Why it is important to do this review
The British Thoracic Society pleural disease guidelines recommend that patients found to have a significant pleural collection associated with pneumonia should have a diagnostic pleural fluid aspirate performed (Davies 2010). Patients found to have CPPE or empyema would then have chest tube drainage along with appropriate antibiotic therapy. Patients who subsequently have persistent sepsis and pleural collection despite chest tube drainage and antibiotics should be referred for surgical management. However, some institutions proceed directly to surgical management if the initial pleural fluid aspirate is thick pus or there are extensive loculations present on imaging. There is currently no clear consensus as to which patients benefit from primary surgical intervention versus non‐surgical management.
An earlier Cochrane review concluded that there were insufficient large trials to suggest a preference for any particular intervention (Coote 2005). This review aimed to reconcile the issue by comparing the results of surgical and non‐surgical therapies for the treatment of CPPE and empyema, thereby providing clinicians with best evidence for management.
Objectives
To assess the effectiveness and safety of surgical versus non‐surgical treatments for CPPE or pleural empyema.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs comparing any surgical intervention to any non‐surgical intervention for the management of CPPE or pleural empyema were eligible for inclusion.
Types of participants
Participants of all ages and either gender with diagnosis of CPPE or empyema. Analysis of pleural aspirates or medical imaging examinations, such as ultrasound or CT, can be used to confirm the diagnosis of CPPE or empyema. The diagnosis of CPPE requires either positive pleural fluid biochemistry (pH < 7.2, glucose < 2.2 mM/L, LDH > 1000 IU/L), confirmation of loculation on imaging examination, or positive culture or gram stain from pleural aspirate. Pus aspirated from the pleural space is indicative of pleural empyema.
Exclusion criteria were any contraindications to either surgery, minimally invasive procedures, or to the fibrinolytic agent. We excluded tuberculous empyemas because of the different clinical presentation, associated severe complications, and poorer outcomes (Chapman 2004; Kundu 2010). We also excluded participants who were immunocompromised or had underlying malignancy (Kaifi 2012). We excluded participants with comorbid conditions who would have required hospitalisation beyond the course of the empyema.
Types of interventions
Surgical interventions
Video‐assisted thoracoscopy (VATS).
Open thoracotomy.
Comparator: procedural management
Thoracocentesis.
Chest tube insertion.
We included interventions with or without intrapleural fibrinolytics in the review.
Types of outcome measures
Primary outcomes
Mortality.
Secondary outcomes
Length of hospital stay (days).
Procedural complications.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, to Issue 9, 2016), part of the Cochrane Library,www.cochranelibrary.com (accessed 19 October 2016), which includes the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (Ebscohost) (1946 to October 2016), MEDLINE (Ovid) (from 1 May 2013 to July week 1 2015), Embase (2010 to October 2016), CINAHL (Cumulative Index to Nursing and Allied Health Literature) (1981 to October 2016), and LILACS (Latin American and Caribbean Health Sciences Literature) (1982 to October 2016).
We searched CENTRAL and MEDLINE using the search strategy in Appendix 2. The MEDLINE search was combined with the Cochrane highly sensitive search for identifying randomised trials in MEDLINE; sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted this search strategy to search Embase (Appendix 3), CINAHL (Appendix 4), and LILACS (Appendix 5). There were no language, publication year, or publication status restrictions on searching.
Searching other resources
We handsearched reference lists of identified publications for additional trials, either published or unpublished, and contacted trial authors where necessary. We searched ClinicalTrials.gov (clinicaltrials.gov/) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp/en/) for ongoing trials or trials which may have been published and were missed, on 21 December 2016.
Data collection and analysis
Selection of studies
Two review authors (TC, MR) independently assessed the studies obtained from the searches to determine eligibility. We assessed the full text of studies where abstracts were unavailable. Two review authors (TC, MR) independently assessed the retrieved trials for compliance with the inclusion and exclusion criteria. A third review author (MVD) was available to resolve any disagreements by acting as an arbiter.
Data extraction and management
We designed a standardised data extraction sheet. Two review authors (TC, MR) independently extracted data. A third review author (MVD) was available to act as an arbiter in case of disagreements. Two review authors (TC, MR) entered data into Review Manager 5 (RevMan 2014). One review author (MVD) was available to act as arbiter.
Assessment of risk of bias in included studies
Two review authors (TC, MR) independently assessed included studies for risk of bias using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the following domains for each study and deemed them to be low risk, high risk, or unclear risk.
Random sequence generation
Low risk: allocation was generated by a computer or random number table algorithm.
High risk: any non‐random sequence generation, e.g. dates, names, or identification numbers.
Unclear risk: sequence generation not mentioned.
Allocation concealment
Low risk: the process used a telephone or central allocation system or sealed, opaque envelopes to prevent participant recruiters, investigators, and participants from knowing the intervention allocation.
High risk: other methods of allocation concealment, e.g. open random allocation, unsealed or non‐opaque envelopes, date of birth.
Unclear risk: method of allocation concealment not mentioned.
Blinding of participants and outcome assessors
Low risk: blinding was performed adequately, or the outcome measurement is not likely to be influenced by lack of blinding.
High risk: no blinding or incomplete blinding, and the outcome or the outcome measurement is likely to be influenced by lack of blinding.
Unclear risk: there is insufficient information to assess whether the type of blinding used is likely to induce bias in the estimate of effect.
Incomplete outcome data
Low risk: there are no missing outcome data; the reasons for missing outcome data were unlikely to be related to a true outcome; or the missing outcome data were balanced in numbers or reasons across intervention groups.
High risk: there are missing outcome data that are likely to be related to the true outcome or there is an imbalance in numbers or reasons for missing data across intervention groups.
Unclear risk: there are incomplete outcome data that are not addressed.
Selective reporting
Low risk: the trial protocol is available and all of the trial's prespecified outcomes that are of interest in the review have been reported.
High risk: not all of the prespecified primary outcomes of the trial have been reported.
Unclear risk: there is insufficient information to assess whether the magnitude and direction of the observed effect is related to selective outcome reporting.
Other bias
For each study we described any concerns we had about other possible sources of bias. We assessed whether each study was free of other problems that could put it at risk of bias as low risk, high risk, or unclear risk.
Measures of treatment effect
When dealing with dichotomous outcome measures, we calculated a pooled estimate of the treatment effect for each outcome across trials using the odds ratio (OR) (the odds of an outcome among treatment‐allocated participants compared to the corresponding odds among controls) and the 95% confidence interval (CI). For continuous outcomes, we recorded mean postintervention values and standard deviations (SD) for each group. We then calculated a pooled estimate of treatment effect by calculating the mean difference (MD) and 95% CI.
Unit of analysis issues
The unit of analysis was each participant recruited into the trials. We planned to adjust for the cluster effect as described in the Cochrane Handbook for Systematic Reviews of Interventions if the level of analysis was not the same as the level of randomisation, such as in cluster‐RCTs (Higgins 2011). However, this was not required.
Dealing with missing data
We planned to contact the trial authors for missing data when possible. Otherwise, we planned to deal with missing data by performing an intention‐to‐treat (ITT) analysis that considers all missing data as unsuccessful outcomes. As there were no missing data in the included studies, this was not required.
Assessment of heterogeneity
We assessed heterogeneity based on face value (e.g. population, setting, risk of complications) and with the I² statistic (Higgins 2011). We then explored reasons for heterogeneity. A guide to interpretation of the I² statistic is described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011):
0% to 40% might not be important;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity; and
75% to 100% represents considerable heterogeneity.
The observed importance of the I² statistic depends on factors including the magnitude and direction of effects, and the strength of evidence for heterogeneity determined by the P value from the Chi² test or a CI for the I² statistic (Higgins 2011).
Assessment of reporting biases
We planned to assess reporting bias for each outcome by using funnel plots if there were 10 or more studies; however, only eight studies were included. Asymmetrical funnel plots can indicate outcome reporting bias or heterogeneity. If outcome reporting bias was suspected, we planned to contact study authors. Where the original protocol is not available, outcome reporting bias can be assessed by comparing the methods section of a published trial to the results section.
Data synthesis
We performed a meta‐analysis using Review Manager 5 (RevMan 2014). We planned to describe studies without pooling them if there was obvious face value heterogeneity, but this was not required. We planned to use the fixed‐effect model if there was no statistical heterogeneity. However, we used the random‐effects model because there was statistical heterogeneity (Higgins 2011).
GRADE and 'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes: mortality, length of hospital stay, and procedural complications. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used GRADEpro GDT software (GRADEpro GDT 2015). We justified all decisions to down‐ or upgrade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We performed a subgroup analysis for children and adults, with children defined as those under the age of 18 years, and adults as those aged 18 years or over, at the time of diagnosis. This is due to the lower mortality rate of empyema in children, compared to adult empyema, which is estimated at 20% (Balfour‐Lynn 2005; Ferguson 1996).
Sensitivity analysis
We performed a sensitivity analysis for trials involving the use of intrapleural fibrinolytic therapy, because studies have demonstrated an improved outcome with its inclusion in empyema therapy.
We planned to pool studies with a low risk of bias and gradually add studies with a high risk of bias to explore how that changed the overall estimate of effect. However, this was not performed because none of the included studies was assessed to have a high risk of bias. We explored which studies contributed to heterogeneity by gradually adding studies to the pooled analysis.
Results
Description of studies
Results of the search
The search strategy identified 2905 records. A preliminary screening excluded all but 35 records, which were then obtained in full text. Of the 35 studies, we excluded 23 because they compared either two surgical or two non‐surgical methods of pleural empyema management, or were not RCTs. (See Characteristics of excluded studies.)
We allocated four studies to Characteristics of studies awaiting classification (Ahmed 2016; Hasimoto 2015; NCT00234208; Zhang 2011). Zhang 2011 was a summary of a poster presentation with insufficient information to determine study characteristics. Hasimoto 2015 was available only as an abstract, and had insufficient information to assess for inclusion in the review; we contacted the authors for unpublished data. Ahmed 2016 was a RCT, however it was published immediately before publication of this review and will be assessed for inclusion in the update of this review. We identified NCT00234208 by searching ClinicalTrials.gov and WHO ICTRP; we have contacted the authors. We found no ongoing trials. (See Figure 1.)
1.
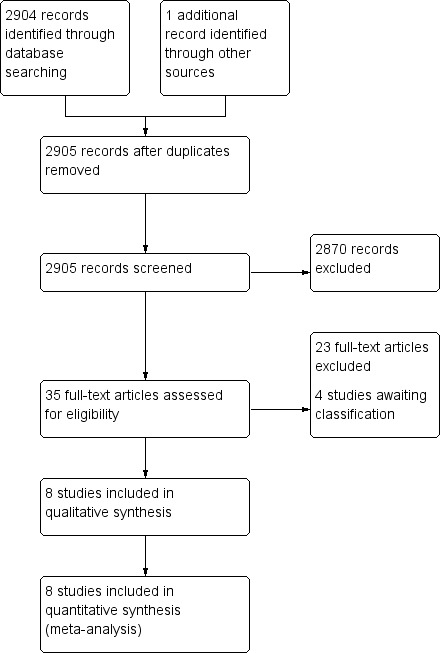
Study flow diagram.
Included studies
We included eight RCTs in our review. See Characteristics of included studies.
The largest multicentre trial included 103 children (aged under 15 years) recruited from six study centres (Marhuenda 2014). Other trials in children are Cobanoglu 2011, Karaman 2004, Kurt 2006, Peter 2009, and Sonnappa 2006. Two trials included participants under 18 years of age (Kurt 2006; Peter 2009); one trial included participants under 16 years of age (Sonnappa 2006); and two trials stated that they were paediatric studies but did not provide a maximum age (Cobanoglu 2011; Karaman 2004). Two studies included participants over the age of 18 years (Bilgin 2006; Wait 1997).
All eight studies listed specific methods of empyema diagnosis in their inclusion criteria. Seven studies used medical imaging as part of their diagnostic criteria (Bilgin 2006; Karaman 2004; Kurt 2006; Marhuenda 2014; Peter 2009; Sonnappa 2006; Wait 1997). This included chest X‐ray, CT, and ultrasound findings such as effusion size and presence of septations or loculations. Five trials used pleural fluid findings as part of their diagnostic criteria (Bilgin 2006; Cobanoglu 2011; Karaman 2004; Peter 2009; Wait 1997). These findings included biochemistry, gram stain, and culture. Four studies used clinical findings such as persistent fever, tachypnoea, and oxygen requirement to support a diagnosis of empyema (Kurt 2006; Marhuenda 2014; Sonnappa 2006; Wait 1997).
The modes of treatment that were compared varied slightly among the trials. Seven included studies compared VATS against conventional tube thoracostomy (Bilgin 2006; Cobanoglu 2011; Kurt 2006; Marhuenda 2014; Peter 2009; Sonnappa 2006; Wait 1997). Karaman 2004 compared open thoracotomy with tube thoracostomy.
Six trials used intrapleural fibrinolytics as an adjunct (Cobanoglu 2011; Kurt 2006; Marhuenda 2014; Peter 2009; Sonnappa 2006; Wait 1997). In these studies, fibrinolytics were used routinely in the thoracostomy group except for Kurt 2006, in which fibrinolytics were used for cases that had failed to resolve in either treatment arm. The four fibrinolytic agents used were streptokinase, urokinase, reteplase, and tissue plasminogen activator. Each of these studies applied different protocols for the frequency and duration of fibrinolytic therapy. Detailed notes regarding the fibrinolytic agent and treatment protocol used in each study are presented in Characteristics of included studies.
There were a number of commonly reported outcomes among the included studies. All studies included a measure of hospital stay. Seven studies reported total length of hospital stay (Bilgin 2006; Cobanoglu 2011; Karaman 2004; Kurt 2006; Marhuenda 2014; Sonnappa 2006; Wait 1997), of which two studies also reported postintervention length of hospital stay (Marhuenda 2014; Sonnappa 2006). One trial reported only postintervention length of hospital stay (Peter 2009). Other common outcomes included duration of chest tube drainage, duration of fever, analgesia duration, total cost of treatment, and occurrence of complications. One study reported mortality as a specific outcome (Wait 1997). However, the remaining seven studies reported no losses to follow‐up throughout study periods.
Excluded studies
We excluded 23 studies. Of these, 20 were RCTs, but compared either two surgical or two non‐surgical methods of empyema management. One study was not randomised (Nandeesh 2013); one study was a commentary on a RCT (Mathew 2015); and one study evaluated the effect of surgery as a rescue therapy rather than primary therapy (Bagheri 2013).
For further details, see Characteristics of excluded studies.
Risk of bias in included studies
Overall, risk of bias was unclear for selection and blinding but low for attrition and reporting bias. No studies were judged to be at high risk of bias for any of the domains assessed. Please see Figure 2 for 'Risk of bias' percentages and Figure 3 for a summary of the risk of bias.
2.
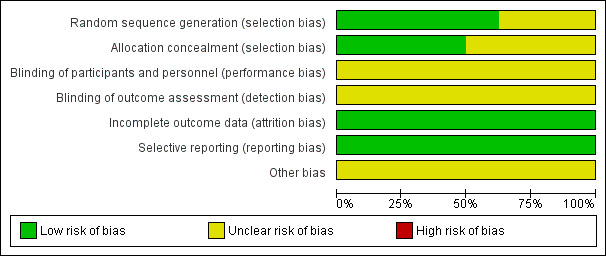
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Five studies adequately described the generation of the allocation sequence (Kurt 2006; Marhuenda 2014; Peter 2009; Sonnappa 2006; Wait 1997). Three studies did not describe the method of randomisation (Bilgin 2006; Cobanoglu 2011; Karaman 2004).
The method used to conceal allocation was adequately described in four studies (Bilgin 2006; Marhuenda 2014; Peter 2009; Sonnappa 2006), and not described in four studies (Cobanoglu 2011; Karaman 2004; Kurt 2006; Wait 1997).
Additionally, Cobanoglu 2011 did not adequately describe the protocol for exclusion; it is unclear if participants with contraindications to fibrinolysis were excluded before or after randomisation was performed (see Characteristics of included studies).
Three studies adequately described both methods of allocation sequence generation and allocation concealment (Marhuenda 2014; Peter 2009; Sonnappa 2006); the risk of selection bias for the remaining studies was unclear (Bilgin 2006; Cobanoglu 2011; Karaman 2004; Kurt 2006; Wait 1997).
Blinding
Blinding was not possible for either participants or assessors in any of the included studies due to the nature of the trials. As such, the risk for performance and detection bias was unclear. However, participants can potentially be blinded for fibrinolytic therapy.
Incomplete outcome data
In all eight studies, all participants were accounted for in the results published for the outcomes included in the meta‐analysis. In two studies, participants were lost to follow‐up at three and six months' chest X‐ray (Marhuenda 2014; Sonnappa 2006). However, this did not affect any of the main outcomes assessed. We assessed the risk of attrition bias as low for all studies.
Selective reporting
No protocols were published for seven of the eight studies. However, in those seven studies, all outcomes mentioned in the methods sections were included in their results. A brief protocol was published on ClinicalTrials.gov for Marhuenda 2014. All outcomes mentioned in the protocol were included in the results. Hence, we considered the risk of reporting bias to be low in all studies.
Other potential sources of bias
Two studies declared there were no financial conflicts of interest (Marhuenda 2014; Sonnappa 2006). The funding source was unclear for the remaining studies.
Effects of interventions
We performed two separate comparisons. One comparison examined open thoracotomy versus thoracostomy drainage for the treatment of pleural empyema and included one study (Karaman 2004). The other comparison examined VATS versus thoracostomy drainage for the treatment of pleural empyema and included seven studies (Bilgin 2006; Cobanoglu 2011; Kurt 2006; Marhuenda 2014; Peter 2009; Sonnappa 2006; Wait 1997). We performed subgroup analyses for children (aged younger than 18 years) and adults for the comparison of VATS versus thoracostomy.
We performed a sensitivity analysis to examine the effect of excluding studies that did not use intrapleural fibrinolytics in the management of participants in the non‐surgical treatment arm. We removed one study from all analyses before conducting the sensitivity analysis because it used fibrinolytics in both treatment arms for people who did not initially respond to treatment (Kurt 2006). Studies that did not use fibrinolytics were then removed from each analysis and the results compared. We did not perform a sensitivity analysis for the comparison of open thoracotomy versus thoracostomy because only one study was included. We also could not perform a sensitivity analysis for the comparison of VATS versus thoracostomy drainage length of hospital stay outcome because all of the included studies used fibrinolysis in the non‐surgical treatment arms. We did not perform the sensitivity analysis in reverse (exclusion of studies that used fibrinolytics) in the comparison of VATS versus thoracostomy drainage because this would have left only one study in the comparison.
We performed sensitivity analyses for analyses that showed considerable heterogeneity. Studies were gradually added to the pool to determine if any particular studies contributed significantly to the heterogeneity.
We did not perform a sensitivity analysis for risk of bias because none of the included studies were deemed to have high risk of bias.
Please see Table 1 and Table 2.
1. Comparison 1: Open thoracotomy versus thoracostomy drainage
Please see Data and analyses and Table 1 for further details.
Primary outcome
1.1. Mortality
Karaman 2004 reported no deaths in either treatment arm, and accordingly the OR for treatment effect on this outcome was not estimable (Analysis 1.1). We downgraded the quality of the evidence for this outcome by one level to moderate due to small sample size (imprecision).
1.1. Analysis.

Comparison 1 Open thoracotomy versus thoracostomy drainage, Outcome 1 Mortality.
Secondary outcomes
1.2. Length of hospital stay (days)
Karaman 2004 reported on length of hospital stay in 30 participants. Study results showed a statistically significant reduction in mean hospital stay of 5.90 days in those treated with open thoracotomy compared to thoracostomy drainage arm participants (95% CI ‐7.29 to ‐4.51) (Analysis 1.2). We downgraded the quality of the evidence for this outcome to moderate due to small sample size (one study) (imprecision).
1.2. Analysis.

Comparison 1 Open thoracotomy versus thoracostomy drainage, Outcome 2 Length of hospital stay (days).
1.3. Procedural complications
Karaman 2004 reported on procedural complications such as bronchopleural fistulas, unsuitable tube position, and subcutaneous emphysema. Study results showed a statistically significant reduction in procedural complications in the open thoracotomy group compared to those treated with tube thoracostomy (OR 0.10, 95% CI 0.02 to 0.63; Analysis 1.3). No participants were reported to have experienced more than one complication. We downgraded the quality of the evidence for this outcome to moderate due to small sample size (one study) (imprecision).
1.3. Analysis.

Comparison 1 Open thoracotomy versus thoracostomy drainage, Outcome 3 Procedural complications.
2. Comparison 2: VATS versus thoracostomy drainage
Please see Data and analyses for further details.
Primary outcome
2.1. Mortality
We included seven studies in this comparison (Bilgin 2006; Cobanoglu 2011; Kurt 2006; Marhuenda 2014; Peter 2009; Sonnappa 2006; Wait 1997). Six studies reported no deaths in either treatment arm. Wait 1997 reported one death in each treatment arm (Wait 1997). We found no statistically significant difference in mortality for those treated with VATS compared with those treated with thoracostomy drainage (OR 0.80, 95% CI 0.04 to 14.89; Analysis 2.1). We downgraded the quality of the evidence for this outcome to low due to wide confidence intervals (imprecision) and indirectness (data only available for adults). We excluded two studies from the fibrinolytic sensitivity analysis (Bilgin 2006; Kurt 2006). This had no effect on the outcome (OR 0.80, 95% CI 0.04 to 14.89).
2.1. Analysis.

Comparison 2 VATS versus thoracostomy drainage, Outcome 1 Mortality.
Five studies in the mortality comparison investigated children aged less than 18 years (Cobanoglu 2011; Kurt 2006; Marhuenda 2014; Peter 2009; Sonnappa 2006). No deaths were reported in either treatment arm, and accordingly the OR of mortality in children was not estimable.
Two studies in the mortality comparison evaluated adults aged over 18 years (Bilgin 2006; Wait 1997). Bilgin 2006 reported no deaths in either treatment arm; Wait 1997 reported one death in each treatment arm. We found no statistically significant difference in mortality for those treated with VATS compared to those treated with thoracostomy drainage (OR 0.80, 95% CI 0.04 to 14.89). We downgraded the quality of the evidence for this outcome to moderate due to wide confidence intervals (imprecision). We excluded Bilgin 2006 from the fibrinolytic sensitivity analysis, but this had no effect on this analysis.
Secondary outcomes
2.2. Length of hospital stay
We initially included seven studies in this comparison (Bilgin 2006; Cobanoglu 2011; Kurt 2006; Marhuenda 2014; Peter 2009; Sonnappa 2006; Wait 1997). Unfortunately, two studies were excluded because their data were not reported in means and standard deviations and could not be combined with the other studies (Bilgin 2006; Sonnappa 2006). We were unable to contact the trial authors for additional data. When the five remaining studies were combined in the meta‐analysis, a statistically significant reduction in mean length of hospital stay was found for those treated with VATS when compared to those treated with thoracostomy drainage (MD ‐2.52 days, 95% CI ‐4.26 days to ‐0.77 days; Analysis 2.2). However, considerable heterogeneity was present (I² = 81%). We performed a heterogeneity sensitivity analysis, which revealed that no one trial contributed significantly more than the others to the level of heterogeneity. We downgraded the quality of the evidence for this outcome to moderate due to this heterogeneity. We could not perform a fibrinolysis sensitivity analysis for this outcome because all included studies used fibrinolysis in the non‐surgical treatment arm.
2.2. Analysis.
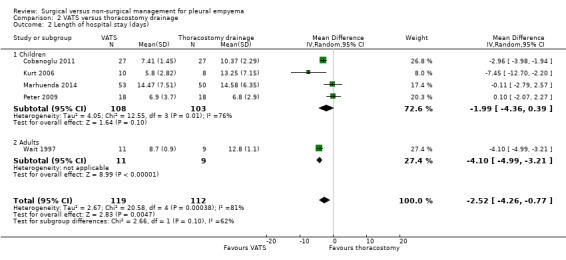
Comparison 2 VATS versus thoracostomy drainage, Outcome 2 Length of hospital stay (days).
We performed a subgroup analysis comparing results in adults and children (Analysis 2.2). The test for subgroup differences showed no difference between subgroups (P = 0.10).
Five studies initially included in the length of hospital stay comparison evaluated children aged up to 18 years (Cobanoglu 2011; Kurt 2006; Marhuenda 2014; Peter 2009; Sonnappa 2006). We excluded Sonnappa 2006 because data were not reported as means and standard deviations. The remaining four studies did not show a statistically significant difference in mean length of hospital stay for those treated with VATS when compared to those treated with thoracostomy drainage (MD ‐1.99 days, 95% CI ‐4.36 days to 0.39 days; Analysis 2.2.1). Considerable heterogeneity was also present (I² = 76%). We performed a heterogeneity sensitivity analysis, which revealed that no one trial contributed significantly more than the others to the level of heterogeneity. We downgraded the quality of the evidence for this outcome to moderate due to this heterogeneity. We could not perform a fibrinolysis sensitivity analysis for this outcome because all included studies used fibrinolysis in the non‐surgical treatment arm.
Two studies initially included in the length of hospital stay comparison investigated adults aged over 18 years (Bilgin 2006; Wait 1997). We excluded Bilgin 2006 because data were not reported as means and standard deviations. Wait 1997 showed a statistically significant reduction in mean length of hospital stay for those treated with VATS compared to those treated with thoracostomy drainage (MD ‐4.10 days, 95% CI ‐4.99 days to ‐3.21 days; Analysis 2.2). We downgraded the quality of the evidence to moderate due to small sample size (one study). We could not perform a fibrinolysis sensitivity analysis for this outcome because all included studies used fibrinolysis in the non‐surgical treatment arm.
2.3. Procedural complications
Five studies reported on procedural complications and were included in this comparison (Bilgin 2006; Kurt 2006; Marhuenda 2014; Sonnappa 2006; Wait 1997). The reported complications included bronchospasm, bronchopleural fistulas, chest tube insertion through diaphragm, and chest tubes requiring re‐insertion after falling out. No participants were reported to have experienced more than one complication. We found no statistically significant difference in procedural complications for those treated with VATS when compared to those treated with thoracostomy drainage (OR 0.46, 95% CI 0.08 to 2.75; Analysis 2.3). The level of heterogeneity in this comparison was low (I² = 0%). We downgraded the quality of the evidence for this outcome to moderate due to wide confidence intervals (imprecision). The fibrinolytic sensitivity analysis excluded two studies (Bilgin 2006; Kurt 2006). This did not produce any statistically significant results (OR 0.54, 95% CI 0.06 to 4.59) (I² = 0%).
2.3. Analysis.

Comparison 2 VATS versus thoracostomy drainage, Outcome 3 Procedural complications.
We performed a subgroup analysis comparing results in adults and children (Analysis 2.3). The test for subgroup differences showed no difference between subgroups (P = 0.52).
Three studies in the procedural complications comparison evaluated children aged up to 18 years (Kurt 2006; Marhuenda 2014; Sonnappa 2006). When these three studies were combined, no statistically significant difference in procedural complications was found for those treated with VATS compared to those treated with thoracostomy drainage (OR 0.94, 95% CI 0.06 to 15.48; Analysis 2.3.1). We downgraded the quality of the evidence for this outcome to moderate due to wide confidence intervals (imprecision). Sensitivity analysis excluded one study, which had no effect on the results (Kurt 2006).
Two studies in the procedural complications comparison evaluated adults aged over 18 years (Bilgin 2006; Wait 1997). These studies showed no statistically significant difference in procedural complications for those treated with VATS compared to those treated with thoracostomy drainage (OR 0.28, 95% CI 0.03 to 2.88; Analysis 2.3.2). The level of heterogeneity in this comparison was low (I² = 0%). We downgraded the quality of the evidence for this outcome to moderate due to wide confidence intervals (imprecision). The fibrinolytic analysis excluded one study, which produced no statistically significant results (OR 0.25, 95% CI 0.01 to 6.82) (Bilgin 2006).
Discussion
Summary of main results
Mortality
The included studies showed no statistically significant difference in mortality between primary surgical and non‐surgical management of pleural empyema in all age groups. However, mortality data were limited because only one study reported deaths in both treatment arms; the remaining studies reported no deaths.
Length of hospital stay
The data we reviewed showed a statistically significant reduction in length of hospital stay of 2.52 days for those treated with VATS compared to those treated with (non‐surgical) chest tube drainage when we included all age groups. This effect was larger for adults aged over 18 years (4.10 days), but no statistically significant difference in length of hospital stay was found in children. The quality of the data was limited by considerable heterogeneity.
The one included study that compared open thoracotomy to chest tube drainage in children showed a statistically significant reduction in length of hospital stay of 5.90 days for those on the surgical arm. However, data were limited by the small sample size.
Procedural complications
We found no statistically significant difference in procedural complications for participants of all ages treated with VATS compared to those treated with (non‐surgical) chest tube drainage.
The one included study that compared open thoracotomy to chest tube drainage in children showed a statistically significant reduction in procedural complications Karaman 2004. However, data were limited by the small sample size.
Overall completeness and applicability of evidence
We found few RCTs that investigated this topic. Seven studies compared VATS with thoracostomy drainage, of which two investigated adults and the remaining five investigated children. Only one included study compared open thoracotomy with thoracostomy and included children. All included studies had small sample sizes.
Data from two studies could not be included in the length of stay analysis for the comparison of VATS with thoracostomy drainage because means and standard deviations were not reported (Bilgin 2006; Sonnappa 2006). We were unable to obtain additional data from the trial authors.
The included studies used inclusion criteria that confirmed the diagnosis of CPPE/empyema in participants. Some studies based the diagnosis on pleural fluid biochemistry and microscopy, while others relied on imaging findings or a combination of both. As such, we consider the results of this review to be applicable to patients with pleural empyema diagnosed by the criteria listed in Types of participants.
A number of common outcomes were reported in the included studies that were not listed in the outcomes of the protocol for this review. Five trials reported cost of treatment (Cobanoglu 2011; Kurt 2006; Peter 2009; Sonnappa 2006; Wait 1997). Three of these found total cost of treatment to be lower for thoracostomy, with or without fibrinolytics, when compared to VATS (Cobanoglu 2011; Peter 2009; Sonnappa 2006). Two studies showed the cost of thoracostomy to be greater than for VATS (Kurt 2006; Wait 1997). However, these data were not statistically significant (Table 3). Five trials reported duration of chest tube drainage (Cobanoglu 2011; Karaman 2004; Kurt 2006; Marhuenda 2014; Wait 1997). All five studies found the duration to be shorter with surgical management when compared to thoracostomy with or without fibrinolytics; all of these findings were statistically significant (Table 4). Six studies reported on postintervention duration of fever (Cobanoglu 2011; Karaman 2004; Kurt 2006; Marhuenda 2014; Peter 2009; Sonnappa 2006). All showed fever duration to be shorter with surgical management. However, the difference in fever duration was not statistically significant in all trials (Table 5).
1. Total cost of treatment.
| Trial | Total treatment cost (USD): thoracostomy arm | Total treatment cost (USD): VATS arm | P value |
| Cobanoglu 2011 | Mean 386.672 ± 72.06 | Mean 957.487 ± 137.238 | < 0.001 |
| Kurt 2006 | Median (IQR) 21,947 (17,895 to 37,458) | Median (IQR) 19,714 (17,325 to 23,000) | 0.315 |
| Sonnappa 2006 | Mean 9127 | Mean 11,379 | < 0.001 |
| Peter 2009 | Mean 7600 ± 5400 | Mean 11,700 ± 2900 | 0.02 |
| Wait 1997 | Mean 24,052 ± 3466 | Mean 16,642 ± 2841 | 0.11 |
IQR: interquartile range
2. Duration of chest tube drainage.
| Trial | Duration of chest tube drainage: thoracostomy arm (days) | Duration of chest tube drainage: surgical arm (days) | P value |
| Cobanoglu 2011 | Mean 9.48 ± 2.50 | Mean 6.56 ± 1.55 | < 0.001 |
| Karaman 2004 | Mean 13.8 ± 2.3 | Mean 7.5 ± 1.1 | < 0.05 |
| Kurt 2006 | Mean 9.63 ± 5.45 | Mean 2.80 ± 0.63 | < 0.001 |
| Marhuenda 2014 | Median (IQR) 5 (4 to 6) | Median (IQR) 4 (3 to 5) | < 0.001 |
| Wait 1997 | Mean 9.8 ± 1.3 | Mean 5.8 ± 1.1 | 0.03 |
IQR: interquartile range
3. Postintervention fever duration.
| Trial | Postintervention fever duration: thoracostomy arm (days) | Postintervention fever duration: surgical arm (days) | P value |
| Cobanoglu 2011 | Mean 3.9 ± 2.1 | Mean 3.4 ± 2.4 | 0.782 |
| Kurt 2006 | Mean 6.25 ± 4.10 | Mean 3.60 ± 2.95 | 0.146 |
| Marhuenda 2014 | Median (IQR) 6 (3 to 7) | Median (IQR) 4 (2 to 7) | 0.62 |
| Sonnappa 2006 | Median (range) 2.5 (0 to 25) | Median (range) 2.5 (0 to 10) | 0.635 |
| Peter 2009 | Mean 3.8 ± 2.9 | Mean 3.1 ± 2.7 | 0.46 |
IQR: interquartile range
Quality of the evidence
The methodological quality of the included studies was generally good based on the 'Risk of bias' assessment. We assessed no domains as at high risk of bias. Five of the eight included studies reported low‐risk methods of randomisation (Kurt 2006; Marhuenda 2014; Peter 2009; Sonnappa 2006; Wait 1997), and three studies were assessed as at unclear risk of bias because methods were not reported. Four studies demonstrated low‐risk methods of allocation concealment, and four studies did not report methods and were assessed as at unclear risk of bias (Bilgin 2006; Marhuenda 2014; Peter 2009; Sonnappa 2006). All studies had low risk of attrition bias and reporting bias. However, the risk of performance/detection bias was unclear in all studies because blinding methods were not reported.
We downgraded the quality of evidence for mortality, length of hospital stay, and procedural complications in the open thoracotomy versus thoracostomy comparison to moderate due to the small sample size (one study provided data).
We deemed the quality of evidence as low for mortality in the VATS versus thoracostomy comparison due to wide confidence intervals (imprecision) and indirectness (data for adults available from only one study).
We deemed the quality of evidence as moderate for mortality (in children and adults) in the VATS versus thoracostomy comparison due to wide confidence intervals (imprecision) (data for adults available from only one study).
We deemed the quality of evidence as moderate for procedural complications (in children and adults) in the VATS versus thoracostomy comparison due to wide confidence intervals (imprecision).
For children and the total population, we downgraded the quality of evidence for length of hospital stay in the VATS versus thoracostomy comparison to moderate due to considerable heterogeneity. For adults in the same comparison, we downgraded the quality of evidence to moderate due to the small sample size.
Potential biases in the review process
While there is a risk that some studies may have been missed in the search process, we took a number of precautions to minimise this risk. We searched multiple databases with a comprehensive search strategy. We searched clinical trials registers and contacted the authors of relevant trials. We performed an updated search prior to publishing to check for trials published during the review process.
There is a potential for bias in the selection of included studies and data extraction from included studies. To minimise this risk, two review authors independently selected articles from the search results. The review authors compared findings, and a third review author was available as an arbiter for any disagreements. Two review authors then independently extracted data and compared their findings.
For the VATS versus thoracostomy length of hospital stay outcome, one study reported postintervention length of hospital stay (Peter 2009), and four studies reported total hospital stay. We pooled these studies in the meta‐analysis. It is unclear if this had a significant influence on conclusions.
Agreements and disagreements with other studies or reviews
Krenke 2010 conducted a systematic review of RCTs that compared intrapleural fibrinolysis to a range of interventions, one of which was VATS. For their review of fibrinolysis versus VATS, Krenke 2010 found two studies that we also included in our review (Peter 2009; Sonnappa 2006). Krenke 2010 found no significant difference in length of postintervention hospital stay between fibrinolysis and VATS. As both included studies in Krenke 2010 were in children, results from both reviews agree.
Authors' conclusions
Implications for practice.
Our findings suggest that there is no significant difference in mortality between primary surgical and non‐surgical management of pleural empyema for all age groups. However, this finding was based on limited data. There was no statistically significant difference in procedural complications between (surgical) video‐assisted thoracoscopic surgery and (non‐surgical) thoracostomy in all age groups. Video‐assisted thoracoscopic surgery may reduce length of hospital stay compared to thoracostomy drainage alone. In children, open thoracotomy may reduce the length of hospital stay compared to thoracostomy alone. However, conclusions about length of hospital stay are based on limited evidence.
We found insufficient evidence to enable comment on the impact of fibrinolytic therapy. All but two included studies used fibrinolysis in the non‐surgical treatment arms, which limited the utility of the sensitivity analysis. However, the effect of the addition of fibrinolysis compared to thoracostomy drainage alone has been explored elsewhere in the literature (Cameron 2008).
In the absence of further high‐quality data it is likely that institutions will continue with the currently accepted management outlined in the British Thoracic Society pleural disease guidelines (Davies 2010). The evidence presented in this systematic review appears to support both surgical and non‐surgical treatment options and is certainly not sufficient to suggest any significant change from current practice.
Implications for research.
The findings of this review show no difference in mortality between the treatment options assessed. Future studies should place emphasis on patient‐centred outcomes such as disability and functional scores at admission, discharge, and longer‐term follow‐up. Other outcomes to be considered for inclusion in future studies are radiographic improvement, treatment failure rates, amount of fluid drainage, duration of chest tube drainage, duration of fever, analgesia duration, and total cost of treatment. In general, more high‐quality randomised controlled trials with larger numbers of participants are required to enable more rigorous conclusions to be drawn on preferred treatment options.
A number of common outcomes were reported in the included studies that were not directly examined in our primary and secondary outcomes. These include duration of chest tube drainage, duration of fever, analgesia duration, and total cost of treatment. Examination of these and other factors, such as radiographic improvement, treatment failure rates, amount of fluid drainage, and patient functional scores, might be required to inform clinical decisions.
Acknowledgements
We would like to acknowledge the previous authors of this review, Nicky Coote and Elspeth Kay. We also thank Sarah Thorning, Information Specialist for the Cochrane Acute Respiratory Infections Group, for designing the search strategy and conducting the searches for this review, and Liz Dooley, Managing Editor of the Cochrane Acute Respiratory Infections Group, for her guidance throughout the publication process. We thank Lars Eriksson, librarian at the University of Queensland, for conducting the search updates. We thank Dr Charlie C‐T Hsu for his advice on interpretation of the review findings. We thank the following people for commenting on the draft of the protocol: Amy Zelmer, Mario Kopljar, Craig Mellis, Nelcy Rodrigues, and Meenu Singh. Finally, we thank the following people for commenting on the draft of this review: Janet Yarrow, Devi Prasad Mohapatra, Craig Mellis, Hiren Mehta, Robert Ware, and Meenu Singh.
Appendices
Appendix 1. Glossary of terms
Decortication: surgical removal of fibrous tissue in the pleural space.
Dyspnoea: symptom of breathlessness.
Fibropurulent: also known as fibrinopurulent. It pertains to pus or suppurative exudate that contains a relatively large amount of fibrin.
Intrapleural fibrinolytics: therapy involving administration of fibrinolytics (streptokinase or urokinase) through a chest tube drain to facilitate breakdown of any fibrous loculations and improve the drainage of the pleural collections.
Lactate dehydrogenase (LDH): an enzyme that functions in anaerobic glucose metabolism and glucose synthesis.
Mediastinum: a group of structures in the middle of the thorax, including the heart and its surrounding structures.
Open thoracotomy: surgical procedure to open up the chest cavity (typically a posterolateral incision in an intercostal space), which is widened by rib spreaders (retractors) to gain direct visualisation and access to the pleural space.
Thoracentesis: bedside procedure involving a fine needle (usually 12 to 14 G cannula) connected to a syringe that is inserted into the pleural space to drain any fluid for therapeutic or diagnostic purposes.
Thoracostomy: bedside procedure involving a small incision between two ribs and the insertion of a flexible plastic tube into the pleural space to drain pleural collections.
Video‐assisted thoracoscopic surgery (VATS): surgical procedure involving the insertion of a small video camera along with other surgical instruments through three to four small incision ports into the patient's chest, enabling the chest cavity to be visualised for the performance of surgical procedures.
Appendix 2. MEDLINE search strategy
MEDLINE (Ovid)
1 empyema/ or empyema, pleural/ (5754) 2 Pleural Effusion/ (13675) 3 empyema*.tw. (7147) 4 (pleur* adj3 (effus* or exud* or purulent* or suppurat* or pus)).tw. (18190) 5 pyothora*.tw. (539) 6 ((parapneumon* or para‐pneumon* or para pneumon*) adj3 effus*).tw. (530) 7 or/1‐6 (32019)
We combined the MEDLINE search strategy with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE (Lefebvre 2011).
Appendix 3. Embase (Elsevier) search strategy
#9 #7 AND #8 1572 #8 798343 #8.8 #8.3 NOT #8.7 798343 #8.7 #8.4 NOT #8.6 #8.6 #8.4 AND #8.5 #8.5 'human'/de #8.4 'animal'/de OR 'nonhuman'/de OR 'animal experiment'/de #8.3 #8.1 OR #8.2 #8.2 random*:ab,ti OR placebo*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR allocat*:ab,ti OR trial:ti OR (doubl* NEXT/1 blind*):ab,ti #8.1 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp #7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 37679 #6 ((parapneumon* OR 'para‐pneumonia' OR 'para pneumonia') NEAR/3 effus*):ab,ti 650 #5 pyothora*:ab,ti 398 #4 (pleur* NEAR/3 (effus* OR exud* OR purulent* OR suppurat* OR pus)):ab,ti 18502 #3 empyema*:ab,ti 6015 #2 'pleura effusion'/de 26464 #1 'empyema'/de OR 'pleura empyema'/de 6876
Appendix 4. CINAHL (Ebsco) search strategy
S18 S7 AND S17 143 S17 S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 194,657 S16 (MH "Quantitative Studies") 8,886 S15 TI placebo* OR AB placebo* 20,650 S14 (MH "Placebos") 6,726 S13 (MH "Random Assignment") 29,577 S12 TI random* OR AB random* 103,219 S11 TI ( (singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*) ) OR AB ( (singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*) ) 14,983 S10 TI clinic* trial* OR AB clinic* trial* 31,567 S9 PT 50,578 S8 (MH "Clinical Trials+") 116,013 S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6 1,693 S6 TI ( (parapneumon* or para‐pneumon* or para pneumon*) N3 effus* ) OR AB ( (parapneumon* or para‐pneumon* or para pneumon*) N3 effus* ) 54 S5 TI pyothora* OR AB pyothora* 5 S4 TI ( pleur* N3 (effus* or exud* or purulent* or suppurat* or pus) ) OR AB ( pleur* N3 (effus* or exud* or purulent* or suppurat* or pus) ) 974 S3 TI empyema* OR AB empyema* 311 S2 (MH "Pleural Effusion") 943 S1 (MH "Empyema") 267
Appendix 5. LILACS (BIREME) search strategy
mh:empyema OR empiema OR mh:"Empyema, Pleural" OR pyothorax OR piotórax OR mh:"Pleural Effusion" OR "Derrame Pleural" OR "pleural effusion" OR "empyema thoracis" OR "para‐pneumonic effusion" OR "para‐pneumonic effusions" AND db:("LILACS") AND type_of_study:("clinical_trials")
Data and analyses
Comparison 1. Open thoracotomy versus thoracostomy drainage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Length of hospital stay (days) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐5.9 [‐7.29, ‐4.51] |
| 3 Procedural complications | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 0.10 [0.02, 0.63] |
Comparison 2. VATS versus thoracostomy drainage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 7 | 361 | Odds Ratio (M‐H, Random, 95% CI) | 0.8 [0.04, 14.89] |
| 1.1 Children | 5 | 271 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 2 | 90 | Odds Ratio (M‐H, Random, 95% CI) | 0.8 [0.04, 14.89] |
| 2 Length of hospital stay (days) | 5 | 231 | Mean Difference (IV, Random, 95% CI) | ‐2.52 [‐4.26, ‐0.77] |
| 2.1 Children | 4 | 211 | Mean Difference (IV, Random, 95% CI) | ‐1.99 [‐4.36, 0.39] |
| 2.2 Adults | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐4.10 [‐4.99, ‐3.21] |
| 3 Procedural complications | 5 | 271 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.08, 2.75] |
| 3.1 Children | 3 | 181 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.06, 15.48] |
| 3.2 Adults | 2 | 90 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.03, 2.88] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bilgin 2006.
| Methods | Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Country: Turkey Number of study centres: 1 |
|
| Participants | N recruited = 70 N randomised = 70 N reported outcomes = 70 Mean age = 47.35 years Gender (m/f) = 59/11 N tube thoracostomy = 35 N VATS = 35 Inclusion criteria: Diagnosis of empyema by:
Required values for pleural fluid measurements not given. Exclusion criteria: not reported |
|
| Interventions | Treatment before study: not reported Titration period and treatment:
|
|
| Outcomes | Time of outcome measurements: not reported Primary outcome(s):
Secondary outcome(s): Outcomes not divided into primary and secondary. Other outcome(s): not reported |
|
| Notes | Language of publication: English Publication status: peer‐reviewed journal Type of funding: not reported Conflicts of interest: none reported Stated aim for study: "We studied whether insertion of a chest tube by initially using video‐assisted thoracoscopy (VAT) results in shortening of hospitalisation time and reduces the necessity of open decortication." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Low risk | Closed‐envelope method |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol published. However, all outcomes in methods were included in results. |
| Other bias | Unclear risk | Funding source not reported. |
Cobanoglu 2011.
| Methods | Parellel randomised controlled trial Randomisation ratio: 1:1 Superiority design Country: Turkey Number of study centres: 1 |
|
| Participants | N recruited = 54 N randomised = 54 (Stage II = 23, Stage III = 31) N reported outcomes = 54 Mean age = 8.02 years Gender (m/f) = 32/22 N fibrinolytics = 27 N VATS = 27 Inclusion criteria: (Children's study, however maximum age not stated.)
Exclusion criteria:
|
|
| Interventions | Treatment before study: Pre‐hospital: 76% nil, remainder had irregular antibiotics After diagnosis: All received empiric treatment of ampicillin sulbactam plus cefotaxime, and an 18 to 24 Fr chest tube was inserted in all cases and connected to a closed drainage system with negative suction. Titration period and treatment: After diagnosis, participants received either STK or VATS VATS: After procedure, chest tube was left in place. 6 participants required conversion to mini‐thoracotomy. STK: Normal saline with 250,000 U/100 mL STK was administered into the pleural cavity through the chest tube in 70‐ to 120‐mL volume once a day, and the tube was held by a clamp for 4 to 6 hours. The period of fibrinolytic treatment was determined as 4.45 days (3 to 5 days). 8 participants required further VATS. Common: Chest tube removed when daily drainage less than 50 mL and radiological healing and full expansion detected. |
|
| Outcomes | Primary outcome(s): Success was based on whether further intervention was required. Lung was fully expanded and the procedure was considered successful: STK: 19 of 27 cases (70.37%) versus VATS: 21 cases of 27 (77.77%) (P = 0.533) Secondary outcome(s):
|
|
| Notes | Language of publication: English Publication status: peer‐reviewed journal Stated aim for study: "The aim of this study was to prospectively compare thoracoscopic debridement and fibrinolytic treatment in cases with stage II and III empyema and to present a perspective for treatment options." Funding: not disclosed Conflicts of interest: not disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not possible given the nature of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol published. However, all outcomes in methods were included in results. |
| Other bias | Unclear risk | The following statement might indicate potential selection bias, however, based on data in the paper, it is unclear if any participants were excluded from the fibrinolytics group after randomisation: "Patients with hemorrhagic diathesis, stroke or manifest haemorrhage having occurred in the previous six months and those who had used fibrinolytic agents for any reason in the previous two years were excluded from the fibrinolytic treatment group." Funding source not reported. |
Karaman 2004.
| Methods | Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Number of study centres: 1 |
|
| Participants | N recruited = 30 N randomised = 30 N reported outcomes = 30 Mean age = 3.9 years (study in children) Gender m/f = 15/15 N tube thoracostomy = 15 N open thoracotomy = 15 Inclusion criteria: Patients with pleural empyema confirmed by:
Required values for pleural fluid measurements not given. Exclusion criteria:
|
|
| Interventions | Treatment before study: 80% of participants had been treated with antibiotics for periods ranging from 2 to 20 days prior to admission (mean 4.7 days). 4 of these participants were treated in another centre for pneumonia and were given 2nd‐ or 3rd‐generation cephalosporins and aminoglycosides. The other participants were treated with beta‐lactams. Titration period and treatment:
|
|
| Outcomes | Time of outcome measurements: not reported Primary outcome(s):
Secondary outcome(s): Outcomes not divided into primary and secondary. Other outcome(s): In both groups, duration of chest tube drainage was found to be longer in participants whose pleural fluid pH was < 7.2. |
|
| Notes | Language of publication: English Publication status: peer‐reviewed journal Type of funding: not reported Conflicts of interest: none reported Stated aim for study: "The aim of this study is to compare the effectiveness of closed‐tube thoracostomy and open thoracotomy procedures in the management of empyema in children." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not possible given the nature of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol published. However, all outcomes in methods were included in results. |
| Other bias | Unclear risk | Funding source not reported. |
Kurt 2006.
| Methods | Parallel randomised controlled clinical trial Randomisation ratio: VATS:thoracostomy = 10:8 Country: USA Number of study centres: 1 |
|
| Participants | N recruited = 18 N randomised = 18 N reported outcomes = 18 Mean age = 64.5 months (study in children) Gender m/f = 10/8 N tube thoracostomy = 8 N VATS = 10 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Treatment before study: All participants had received antibiotic therapy that was appropriate for the suspected micro‐organisms prior to enrolment. Titration period and treatment:
|
|
| Outcomes | Time of outcome measurements: not reported Primary outcome(s):
Secondary outcome(s):
Other outcome(s):
|
|
| Notes | Language of publication: English Publication status: peer‐reviewed journal Type of funding: not reported Conflicts of interest: none reported Stated aim for study: "This trial of paediatric patients with community‐acquired pneumonia and associated parapneumonic processes compared primary video‐assisted thoracoscopic surgery with conventional thoracostomy drainage." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a random number method in groups of 10 generated by a Spectrum Health research nurse" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol published. However, all outcomes in methods were included in results. |
| Other bias | Unclear risk | Funding source not reported. |
Marhuenda 2014.
| Methods | Parellel randomised controlled trial Randomisation ratio: 1:1 Number of study centres: 6 |
|
| Participants | N recruited = 149 N randomised = 105 N reported outcomes = 103 Mean age = VATS 4.14 years; urokinase 4.63 years Gender m/f = 61/42 N Urokinase = 50 N VATS = 53 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Treatment before study: none reported Titration period and treatment: After diagnosis, participants were randomly allocated to either the VATS debridement group or the urokinase instillation group. VATS: The procedure was carried out by or under direct supervision of a senior surgeon. 1 or 2 chest tubes were left in place after the procedure. Urokinase: 12Fr to 14Fr chest tubes were used with insertion site selected using sonography. The pleural fluid was first drained, then urokinase was instilled into the pleural cavity through the tube. 10 mL of a 1000 IU/mL solution of urokinase in children aged 1 year and 40 mL in older children was administered every 12 hours for 3 days. After instillation, the chest tube was clamped for 4 hours. It was then unclamped and connected to a suction system at –20 cm H₂O for 8 hours. Common:
|
|
| Outcomes | Primary outcome(s):
Secondary outcome(s):
Other outcome(s): At 3 months: n = VATS (36 (67.9%)) versus urokinase (42 (84%)) Of those, normal X‐ray or only small changes: VATS (66.7%) versus urokinase (59.5%); P = 0.4 |
|
| Notes | Language of publication: English Publication status: peer‐reviewed journal Stated aim for study: "The aim of this study was to compare the efficacy of drainage plus urokinase versus video‐assisted thoracoscopic surgery in the treatment of PPE in childhood." Funding: Institute of Health Carlos III, under the Spanish Ministry of Science and Innovation Conflicts of interest: 2 of the authors (Moreno‐Galdo, Perez‐Yarza) are advisory board members of AbbVie Inc, and have received funding from several pharmaceutical companies for travel to conferences and fees for speaking. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence, stratified according to centre and with varying block sizes to ensure balance in both groups |
| Allocation concealment (selection bias) | Low risk | "The attending physician accessed a Web platform and obtained the treatment assigned to each new patient," after consent had been signed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not possible given the nature of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of participants to 3‐month follow‐up, but this would not have affected any of the primary or secondary outcomes. |
| Selective reporting (reporting bias) | Low risk | All outcomes published in protocol on ClinicalTrials.gov were included in the results. |
| Other bias | Unclear risk | 2 authors (Moreno‐Galdo, Perez‐Yarza) are advisory board members of AbbVie Inc, and have received funding from several pharmaceutical companies for travel to conferences and fees for speaking. |
Peter 2009.
| Methods | Parellel randomised controlled trial Randomisation ratio: 1:1 Superiority design Number of study centres: 1 Country: USA |
|
| Participants | N recruited = 36 N randomised = 36 N reported outcomes = 36 Mean age = 5 years Gender = not reported N tPA = 18 N VATS = 18 Inclusion criteria: Only patients under 18 years of age were included. Diagnosis of empyema:
Exclusion criteria:
|
|
| Interventions | Titration period and treatment: VATS: Performed by 1 of 5 staff surgeons. Chest drain left in place post procedure. tPA: 12Fr chest tube inserted and drained with suction. Fibrinolysis was performed by mixing 4 mg of tPA into 40 mL of sterile normal saline. Tube clamped for 1 hr before continuing suction. This was done once on tube insertion and 2 additional doses each at 24 hrs. Common: Clindamycin (10 mg/kg/dose) every 6 hours and ceftriaxone (25 mg/kg per dose) every 12 hours. If haemodynamic instability existed (hypotension, need for vasoactive medications, or persistent tachycardia), then vancomycin (15 mg/kg per dose) every 6 hours was added. Antibiotic therapy was tailored toward positive culture results and the individual patient’s course. Tubes removed when no air leakage and drainage output was < 1 mL/kg per day calculated for the previous 12 hours. |
|
| Outcomes | At diagnosis, there were no differences between groups in age, weight, degree of oxygen support, white blood cell count, or days of symptoms. No difference in LOS after intervention, days of oxygen requirement, days until afebrile, or analgesic requirements VATS higher cost. 3 in tPA group required VATS. 1 in VATS group required ventilator, 1 required ventilator and temporary dialysis Primary outcome(s):
|
|
| Notes | Language of publication: English Publication status: peer‐reviewed journal Stated aim for study: "we conducted a prospective, randomised trial comparing VATS to fibrinolytic therapy in children with empyema." Funding: not disclosed Conflicts of interest: not disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated using an individual unit of randomization in an unstratified sequence in blocks of 4" |
| Allocation concealment (selection bias) | Low risk | "The randomization sequence was accessed to identify the next allotment after the consent form was signed" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol. However, all outcomes in methods were included in results. |
| Other bias | Unclear risk | Funding source not reported. |
Sonnappa 2006.
| Methods | Parallel randomised controlled trial Randomisation ratio: 1:1 Superiority design Number of study centres: 1 Country: UK |
|
| Participants | N recruited = 60 N randomised = 60 N reported outcomes = 60 Median age = VATS 3.57 years; urokinase 3.07 years Gender m/f = 33/27 N Urokinase = 30 N VATS = 30 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Treatment before study: none reported Titration period and treatment: VATS: After the procedure, 1 or 2 chest drains were placed in the portholes on negative suction pressure. 4 children required conversion to mini‐thoracotomy. Urokinase: Pleural fluid was allowed to drain out first, after which intrapleural urokinase was instilled every 12 hours for 3 days, in a dose of 10,000 U in 10 mL normal saline in children under 1 yr of age and 40,000 U in 40 mL normal saline in children above 1 yr of age. 5 children required conversion to VATS. Common: Chest drains were removed when there was minimal drainage (40 to 60 mL/24 hours), and children were discharged home if they remained afebrile for 24 hours after drain removal and at the attending paediatrician's discretion. |
|
| Outcomes | Ultrasound staging at entry: Stage I: VATS (6) versus Uro (10) Stage II: VATS (13) versus Uro (8) Stage III: VATS (11) versus Uro (12) Primary outcome(s):
Secondary outcome(s):
At 6 months: 16 lost to follow‐up Chest X‐ray on 24 VATS and 20 Uro "Abnormal chest x‐ray" VATS 21 versus Uro 18 Stage I: VATS had a reduced hospital stay of borderline statistical significance: VATS (5 (3 to 5) days) versus Uro (6 (4 to 25) days) (P = 0.056). However, there was an outlier of 25 days in the urokinase group. No difference in Stage II or III |
|
| Notes | Language of publication: English Publication status: peer‐reviewed journal Stated aim for study: "Our study is the first randomised prospective trial to compare chest drain with intrapleural urokinase against primary VATS for the treatment of empyema in children." Funding: not disclosed Conflicts of interest:"None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization scheme was generated by using the internet web site http://www.randomization.com" |
| Allocation concealment (selection bias) | Low risk | "The trial coordinator was contacted by telephone to reveal treatment allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not possible given the nature of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of patients to 6‐month chest radiograph would not influence any other outcome measure. |
| Selective reporting (reporting bias) | Low risk | No protocol published. However, all outcomes in methods were included in results. |
| Other bias | Unclear risk | Funding source not reported. |
Wait 1997.
| Methods | Parallel randomised controlled clinical trial Randomisation ratio: VATS:thoracostomy = 11:9 Number of study centres: 1 |
|
| Participants | N recruited = 20 N randomised = 20 N reported outcomes = 20 Mean age = 42.45 years Gender m/f = 15/5 N tube thoracostomy + streptokinase = 9 N VATS = 11 Inclusion criteria:
Exclusion criteria: not reported |
|
| Interventions | Treatment before study: not reported Titration period and treatment:
|
|
| Outcomes | Time of outcome measurements: not reported Primary outcome(s):
Secondary outcome(s): Outcomes not divided into primary and secondary. Other outcome(s): not reported |
|
| Notes | Language of publication: English Publication status: peer‐reviewed journal Type of funding: not reported Conflicts of interest: none reported Stated aim for study: "To determine the optimal treatment of empyema thoracis (within the fibrinopurulent phase of illness) comparing pleural drainage and fibrinolytic therapy versus video‐assisted thoracoscopic surgery (VATS), with regard to efficacy and duration of hospitalisation." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. However, outcomes were objectively measured. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol published. However, all outcomes in methods were included in results. |
| Other bias | Unclear risk | Funding source not reported. |
cm H₂O: centimetre of water (measurement of pressure) CT: computed tomography ICU: intensive care unit IQR: interquartile range IU: international unit Fr: French (catheter‐sizing scale) LDH: lactate dehydrogenase LOS: length of stay N: number STK: streptokinase tPA: tissue plasminogen activator Uro: urokinase VATS: video‐assisted thoracoscopic surgery WCC: white cell count
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Angelillo 1996 | Compared 2 surgical treatments |
| Bagheri 2013 | Patients that failed to recover after 2 weeks of thoracostomy were randomised to receive either VATS or ongoing medical management. |
| Bouros 1997 | Compared 2 non‐surgical treatments |
| Bouros 1999 | Compared 2 non‐surgical treatments |
| Chin 1997 | Compared 2 non‐surgical treatments |
| Cho 2000 | Compared 2 non‐surgical treatments |
| Davies 1997 | Compared 2 non‐surgical treatments |
| Diacon 2004 | Compared 2 non‐surgical treatments |
| Hewidy 2014 | Compared 2 non‐surgical treatments |
| Lin 2011 | Compared 2 non‐surgical treatments |
| Maskell 2005 | Compared 2 non‐surgical treatments |
| Mathew 2015 | Paper was a commentary of a RCT. |
| Minchev 2004 | Compared 2 surgical treatments |
| Misthos 2005 | Compared 2 non‐surgical treatments |
| Nandeesh 2013 | Trial was not randomised. |
| Rahman 2009 | Compared 2 non‐surgical treatments |
| Rahman 2011 | Compared 2 non‐surgical treatments |
| Sahn 1998 | Compared 2 non‐surgical treatments |
| Shah 2006 | Compared 2 non‐surgical treatments |
| Singh 2004 | Compared 2 non‐surgical treatments |
| Talib 2003 | Compared 2 non‐surgical treatments |
| Thommi 2012 | Compared 2 non‐surgical treatments |
| Thomson 2002 | Compared 2 non‐surgical treatments |
RCT: randomised controlled trial VATS: video‐assisted thoracoscopic surgery
Characteristics of studies awaiting assessment [ordered by study ID]
Ahmed 2016.
| Methods | RCT Randomisation ratio: open thoracotomy:fibrinolytics = 43:35 Number of study centres: 1 |
| Participants | N recruited = 78 N randomised =78 N reported outcomes = 78 Mean age = thoracotomy 55.53 years; fibrinolytics 56.42 years Gender m/f = 67/11 N fibrinolysis = 35 N thoracotomy = 43 Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Treatment before study: none reported Titration period and treatment: After diagnosis, participants were randomly allocated to receive either open thoracotomy or chest tube with fibrinolysis. Open thoracotomy: Postero‐lateral thoracotomy with complete decortication of parietal and visceral pleura. 2 large‐bore chest tubes (32Fr) were left in place following the procedure. Fibrinolysis: 14Fr chest tube inserted using Seldinger technique. 250,000 units of streptokinase in 100 mL normal saline was injected and left in place for 4 hours prior to draining. This was repeated every 24 hours for a maximum of 14 days. If drainage continued after 14 days, open drainage was performed. Common: For both procedures, chest tubes were removed when the drainage volume < 50 mL per day. |
| Outcomes | Duration of treatment, mean (SD) days: thoracotomy 13.95 (1.02) versus fibrinolysis 12.91 (1.01); P = 0.66 Treatment success, number of participants (%): thoracotomy 42 (97.7) versus fibrinolysis 30 (85.7); P = 0.04 Duration of hospitalisation, mean (SD) days: thoracotomy 12.09 (2.18) versus fibrinolysis 17.6 (1.95); P < 0.0001 Survival, number of participants (%): thoracotomy 42 (97.7) versus fibrinolysis 33 (94.3); P = 0.43 |
| Notes | Language of publication: English Publication status: peer‐reviewed journal Stated aim for study: "To confirm that either Fibrinolytic therapy or open decortication which of the two is an effective First line treatment of pleural empyema." Funding: no funding Conflicts of interest: no conflicts of interest |
Hasimoto 2015.
| Methods | RCT Randomisation ratio: VATS:thoracostomy = 26:28 Number of study centres: not stated |
| Participants | N recruited = 54 N randomised =54 N reported outcomes = 54 Mean age = not stated Gender m/f = 31/23 N thoracostomy = 28 N VATS = 26 Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Treatment before study: none reported Titration period and treatment: After diagnosis, participants were randomly allocated to receive either VATS or thoracostomy. VATS: No further details provided. Thoracostomy: No further details provided. |
| Outcomes | Duration of chest tube drainage (days): VATS 4.46 ± 1.79; thoracostomy 10.36 ± 5.16; P < 0.001 |
| Notes | Language of publication: English Publication status: peer‐reviewed journal Stated aim for study: "To analyse cases of parapneumonic pleural empyema in children undergoing chest tube drainage alone or early video‐thoracoscopy in our hospital in order to determine the factors associated with a favourable treatment outcome." Funding: not stated Conflicts of interest: no significant relationships |
NCT00234208.
| Methods | Allocation: randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants | Ages eligible for study: 18 years and older Inclusion criteria:
Exclusion criteria:
Conducted RCT in 100 patients with complicated parapneumonic effusions with septa or empyema with frank pus |
| Interventions | Participants will be randomised to receive either simple chest tube drainage or early medical thoracoscopy. The latter will be performed in local anaesthesia and analgosedation according to the standards set by the European Study on Medical Video‐Assisted Thoracoscopy (ESMEVAT) group. Fibrinolysis will be used routinely. A nested study on the intrapleural pharmacokinetics of linezolid as antibiotic agent will be performed in 20 participants. Follow‐up will be structured on day 1, day 7, before discharge, and after 3 months including chest radiographs and clinical and laboratory evaluations. |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Notes | Responsible party: University Hospital Basel, Switzerland |
Zhang 2011.
| Methods | Patients with empyema who failed to recover with antibiotics and closed‐tube drainage were divided into 2 groups. Group A underwent VATS. Group B were treated with continued drainage and the addition of fibrinolytic therapy. No further details are available. |
| Participants | 68 patients Group A = 32 patients Group B = 36 patients Patient demographics not available. |
| Interventions | Group A underwent VATS debridement and decortication. Group B were treated with continued thoracostomy drainage and the addition of fibrinolytic therapy. No further details are available. |
| Outcomes | Length of hospital stay Chest tube drainage time Fever duration Conversion to thoracotomy Complications |
| Notes | Study start date: October 2005 Completed: January 2007 |
RCT: randomised controlled trial SD: standard deviation VATS: video‐assisted thoracoscopic surgery
Differences between protocol and review
We replaced the planned subgroup analysis for trials involving the use of intrapleural fibrinolytic therapy with a sensitivity analysis exploring the same topic.
We rewrote the review objective from “To assess the validity of surgical versus procedural interventions for CPPE or pleural empyema” to “To assess the effectiveness and safety of surgical versus non‐surgical treatments for complicated parapneumonic effusion or pleural empyema”. This was done to capture both the effectiveness and safety of the interventions investigated.
Contributions of authors
Tze Yang Chin and Mark Redden conducted the searches, extracted data, and drafted the review under the guidance of Mieke van Driel. All review authors read and approved the final draft.
Sources of support
Internal sources
No funding support received, Other.
External sources
No funding support received, Other.
Declarations of interest
Mark Redden: None known. Tze Yang Chin: None known. Mieke van Driel: None known.
New
References
References to studies included in this review
Bilgin 2006 {published data only}
- Bilgin M, Akcali Y, Oguzkaya F. Benefits of early aggressive management of empyema thoracis. ANZ Journal of Surgery 2006;76(3):120‐2. [DOI] [PubMed] [Google Scholar]
Cobanoglu 2011 {published data only}
- Cobanoglu U, Sayir F, Bilici S, Melek M. Comparison of the methods of fibrinolysis by tube thoracostomy and thoracoscopic decortication in children with stage II and III empyema: a prospective randomized study. Pediatric Reports 2011;3(4):e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karaman 2004 {published data only}
- Karaman I, Erdogan D, Karaman A, Cakmak O. Comparison of closed‐tube thoracostomy and open thoracotomy procedures in the management of thoracic empyema in childhood. European Journal of Pediatric Surgery 2004;14(4):250‐4. [DOI] [PubMed] [Google Scholar]
Kurt 2006 {published data only}
- Kurt BA, Winterhalter KM, Connors RH, Betz BW, Winters JW. Therapy of parapneumonic effusions in children: video‐assisted thoracoscopic surgery versus conventional thoracostomy drainage. Pediatrics 2006;118(3):e547‐53. [DOI] [PubMed] [Google Scholar]
Marhuenda 2014 {published data only}
- Marhuenda C, Barceló C, Fuentes I, Guillén G, Cano I, López M, et al. Urokinase vs VATS for treatment of empyema: A randomised multicenter clinical trial. Pediatrics 2014;134(5):e1300. [DOI] [PubMed] [Google Scholar]
Peter 2009 {published data only}
- Peter SD, Tsao K, Spilde TL, Keckler SJ, Harrison C, Jackson MA, et al. Thoracoscopic decortication vs tube thoracostomy with fibrinolysis for empyema in children: a prospective, randomized trial. Journal of Pediatric Surgery 2009;44(1):106‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sonnappa 2006 {published data only}
- Sonnappa S, Cohen G, Owens CM, Doorn C, Cairns J, Stanojevic S, et al. Comparison of urokinase and video‐assisted thoracoscopic surgery for treatment of childhood empyema. American Journal of Respiratory & Critical Care Medicine 2006;174(2):221‐7. [DOI] [PubMed] [Google Scholar]
Wait 1997 {published data only}
- Wait MA, Sharma S, Hohn J, Dal Nogare A. A randomised trial of empyema therapy. Chest 1997;111(6):1548‐51. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Angelillo 1996 {published data only}
- Angelillo Mackinlay TA, Lyons GA, Chimondeguy DJ, Piedras MA, Angaramo G, Emery J. VATS debridement versus thoracotomy in the treatment of loculated postpneumonia empyema. Annals of Thoracic Surgery 1996;61(6):1626‐30. [DOI] [PubMed] [Google Scholar]
Bagheri 2013 {published data only}
- Bagheri R, Tavassoli A, Haghi SZ, Attaran D, Sadrizadeh A, Asnaashari A, et al. The role of thoracoscopic debridement in the treatment of parapneumonic empyema. Asian Cardiovascular & Thoracic Annals Aug 2013;21(4):443‐6. [DOI] [PubMed] [Google Scholar]
Bouros 1997 {published data only}
- Bouros D, Schiza S, Patsourakis G, Chalkiadakis G, Panagou P, Siafakas NM. Intrapleural streptokinase versus urokinase in the treatment of complicated parapneumonic effusions: a prospective, double‐blind study. American Journal of Respiratory & Critical Care Medicine 1997;155(1):291‐5. [DOI] [PubMed] [Google Scholar]
Bouros 1999 {published data only}
- Bouros D, Schiza S, Tzanakis N, Chalkiadakis G, Drositis J, Siafakas N. Intrapleural urokinase versus normal saline in the treatment of complicated parapneumonic effusions and empyema. A randomized, double‐blind study. American Journal of Respiratory & Critical Care Medicine 1999;159(1):37‐42. [DOI] [PubMed] [Google Scholar]
Chin 1997 {published data only}
- Chin NK, Lim TK. Controlled trial of intrapleural streptokinase in the treatment of pleural empyema and complicated parapneumonic effusions. Chest 1997;111(2):275‐9. [DOI] [PubMed] [Google Scholar]
Cho 2000 {published data only}
- Cho CH, Kwak SM, Park CS, Bae IY, Moon TH, Cho JH, et al. The effects of urokinase instillation therapy via percutaneous transthoracic catheter drainage in loculated pleural effusion: a randomised prospective study. European Respiratory Journal 2000;16(31):579. [Google Scholar]
Davies 1997 {published data only}
- Davies RJ, Traill ZC, Gleeson FV. Randomised controlled trial of intrapleural streptokinase in community acquired pleural infection. Thorax 1997;52(5):416‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diacon 2004 {published data only}
- Diacon AH, Theron J, Schuumans MM, Wal BW, Bolliger CT. Intrapleural streptokinase for empyema and complicated parapneumonic effusions. American Journal of Respiratory & Critical Care Medicine 2004;170(1):49‐53. [DOI] [PubMed] [Google Scholar]
Hewidy 2014 {published data only}
- Hewidy A, Elshafey M. Medical thoracoscopy versus intrapleural fibrinolytic therapy in complicated parapneumonic effusion and empyema. Egyptian Journal of Chest Diseases and Tuberculosis 2014;63(4):896‐9. [Google Scholar]
Lin 2011 {published data only}
- Lin JC, Zhang CR, Xu WM, Li M, Cui WL. Effectiveness and safety of intrapleural tissue plasminogen activator in the prevention of pleural thickening and loculated effusions by infective pleurisy. International Journal of Infectious Diseases 2011;15(Suppl):104‐5. [Google Scholar]
Maskell 2005 {published data only}
- Maskell NA, Davies CW, Nunn AJ, Hedley EL, Gleeson FV, Miller R, et al. UK controlled trial of intrapleural streptokinase for pleural infection. New England Journal of Medicine 2005;352(9):865‐74. [DOI] [PubMed] [Google Scholar]
Mathew 2015 {published data only}
- Mathew JL, Lodha R, Sharma S. VATS or urokinase for treatment of empyema?. Indian Pediatrics 2015;52(1):57‐60. [DOI] [PubMed] [Google Scholar]
Minchev 2004 {published data only}
- Minchev TS, Dzhambazov V, Petrov D, Stanoev V, Aleksov S, Petkov R. Video‐assisted thoracoscopic surgery (VATS) for treatment of pleural empyema [Video‐asistirana torakoskopska khirurgiia (VATS) za lechenie na plevralniia empiem]. Khirurgiia 2004;60(2):15‐7. [PubMed] [Google Scholar]
Misthos 2005 {published data only}
- Misthos P, Sepsas E, Konstantinou M, Athanassiadi K, Skottis L, Lioulias A. Early use of intrapleural fibrinolytics in the management of postpneumonic empyema. A prospective study. European Journal of Cardio‐Thoracic Surgery 2005;28(4):599‐603. [DOI] [PubMed] [Google Scholar]
Nandeesh 2013 {published data only}
- Nandeesh M, Sharathchandra BJ, Thrishuli PB. ICD versus VATS as primary treatment in fibrinopurulent stage of empyema thoracis. Journal of Clinical and Diagnostic Research 2013;7(12):2855‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahman 2009 {published data only}
- Rahman NM, Maskell N, Davies CW, West A, Teoh R, Arnold A, et al. Primary result of the Second Multicentre Intrapleural Sepsis (MIST2) trial; randomised trial of intrapleural TPA and DNase in pleural infection. Thorax 2009;64:A1. [Google Scholar]
Rahman 2011 {published data only}
- Rahman NM, Maskell NA, West A, Teoh R, Arnold A, Mackinlay C, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. New England Journal of Medicine 2011;365(6):518‐26. [DOI] [PubMed] [Google Scholar]
Sahn 1998 {published data only}
- Sahn SA. Use of fibrinolytic agents in the management of complicated parapneumonic effusions and empyemas. Thorax 1998;53(Suppl):65‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2006 {published data only}
- Shah NN, Bachh AA, Bhargava R, Ahmad Z, Panday DK, Shameem M. Role of intrapleural streptokinase in management of multiloculated thoracic empyemas. JK Practitioner 2006;13(2):91‐4. [Google Scholar]
Singh 2004 {published data only}
- Singh M, Mathew JL, Chandra S, Katariya S, Kumar L. Randomized controlled trial of intrapleural streptokinase in empyema thoracis in children. Acta Paediatrica 2004;93(11):1443‐5. [DOI] [PubMed] [Google Scholar]
Talib 2003 {published data only}
- Talib SH, Verma GR, Arshad M, Tayade BO, Rafeeque A. Utility of intrapleural streptokinase in management of chronic empyemas. Journal of the Association of Physicians of India 2003;51:464‐8. [PubMed] [Google Scholar]
Thommi 2012 {published data only}
- Thommi G, Shehan JC, Robison KL, Christensen M, Backmeyer LA, McLeay MT. A double blind randomized cross over trial comparing rate of decortication and efficacy of intrapleural instillation of alteplase vs placebo in patients with empyemas and complicated parapneumonic effusions. Respiratory Medicine 2012;106(5):716‐23. [DOI] [PubMed] [Google Scholar]
Thomson 2002 {published data only}
- Thomson AH, Hull J, Kumar MR, Wallis C, Balfour Lynn IM. Randomised trial of intrapleural urokinase in the treatment of childhood empyema. Thorax 2002;57(4):343‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Ahmed 2016 {published data only}
- Ahmed S, Azam H, Basheer I. Is open decortication superior to fibrinolytic therapy as a first line treatment in the management of pleural empyema?. Pakistan Journal of Medical Sciences 2016;32(2):329‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hasimoto 2015 {published data only (unpublished sought but not used)}
- Hasimoto EN, Hasimoto FN, Cataneo DC, Cataneo AJM. Pleural empyema treatment in children: Simple pleural drainage or videothoracoscopy? A controlled clinical trial. Interactive Cardiovascular and Thoracic Surgery 2015;21(Suppl 1):59‐510. [Google Scholar]
NCT00234208 {published and unpublished data}
- NCT00234208. Early medical thoracoscopy versus simple chest tube drainage in complicated parapneumonic effusion and pleural empyema. clinicaltrials.gov/ct2/show/NCT00234208?term=empyema&rank=1 (first received 5 October 2005).
Zhang 2011 {published data only}
- Zhang J, Liu J, Li Y, Huang SH, Chen HG, Wang CP, et al. Video‐assisted thoracic surgery (VATS) is superior to chest tube drainage in fibropurulent empyema. International Journal of Infectious Diseases 2011;15(Suppl 1):51‐2. [Google Scholar]
Additional references
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aye 1991
- Aye RW, Froese DP, Hill LD. Use of purified streptokinase in empyema and hemothorax. American Journal of Surgery 1991;161(5):560‐2. [DOI] [PubMed] [Google Scholar]
Balfour‐Lynn 2005
- Balfour‐Lynn IM, Abrahamson E, Cohen G, Hartley J, King S, Parikh D, et al. British Thoracic Society: Guidelines for the management of pleural infection in children. Thorax 2005;60(Suppl 1):1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cameron 2008
- Cameron R, Davies HR. Intra‐pleural fibrinolytic therapy versus conservative management in the treatment of adult parapneumonic effusions and empyema. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD002312.pub3] [DOI] [PubMed] [Google Scholar]
Chapman 2004
- Chapman SJ, Davies RJ. The management of pleural space infections. Respirology 2004;9(1):4‐11. [DOI] [PubMed] [Google Scholar]
Davies 2010
- Davies HE, Davies RJO, Davies CWH. Management of pleural infection in adults: British Thoracic Society pleural disease guideline 2010. Thorax 2010;65:45‐53. [DOI] [PubMed] [Google Scholar]
Ferguson 1996
- Ferguson AD, Prescott RJ, Selkon JB, Watson D, Swinburn CR. The clinical course and management of thoracic empyema. Quarterly Journal of Medicine 1996;89:285‐9. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADEpro GDT. GRADEpro Guideline Development Tool [www.guidelinedevelopment.org]. Hamilton: McMaster University (developed by Evidence Prime, Inc.), 2015.
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Jaffé 2003
- Jaffé A, Cohen G. Thoracic empyema. Archives of Disease in Childhood 2003;88:839‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2003
- Jones PW, Moyers JP, Rogers JT, Rodriguez RM, Lee YCG, Light RW. Ultrasound‐guided thoracentesis. Is it a safer method?. Chest 2003;123(2):418‐23. [DOI] [PubMed] [Google Scholar]
Kaifi 2012
- Kaifi JT, Toth JW, Gusani NJ, Kimchi ET, Staveley‐O'Carroll KF, Belani CP, et al. Multidisciplinary management of malignant pleural effusion. Journal of Surgical Oncology 2012;105(7):731‐8. [DOI] [PubMed] [Google Scholar]
Krenke 2010
- Krenke K, Peradzynska J, Lange J, Ruszczynski M, Kulus M, Szajewska H. Local treatment of empyema in children: a systematic review of randomized controlled trials. Acta Paediatrica 2010;99(10):1449‐53. [DOI] [PubMed] [Google Scholar]
Kundu 2010
- Kundu S, Mitra S, Mukherjee S, Das S. Adult thoracic empyema: a comparative analysis of tuberculous and nontuberculous etiology in 75 patients. Lung India 2010;27(4):196‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lardinois 2005
- Lardinois D, Gock M, Pezzetta E, Buchli C, Rousson V, Furrer M, et al. Delayed referral and gram‐negative organisms increase the conversion thoracotomy rate in patients undergoing video‐assisted thoracoscopic surgery for empyema. Annals of Thoracic Surgery 2005;79(6):1851‐6. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Light 1985
- Light RW. Parapneumonic effusions and empyema.. Clinical Chest Medicine 1985;6(1):55‐62. [PubMed] [Google Scholar]
Light 2006
- Light RW. Parapneumonic effusions and empyema. Proceedings of the American Thoracic Society 2006;3:75‐80. [DOI] [PubMed] [Google Scholar]
Marshall 2009
- Marshal JC, Naqbi AA. Principles of Source Control in the Management of Sepsis. Critical Care Clinics 2009;35(4):753‐68. [DOI] [PubMed] [Google Scholar]
Miller 1987
- Miller KS, Sahn SA. Chest tubes. Indications, technique, management and complications. Chest 1987;91:258‐64. [DOI] [PubMed] [Google Scholar]
Oddel 1994
- Oddel J. Management of empyema thoracis. Journal of the Royal Society of Medicine 1994;87:466‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sahn 2007
- Sahn SA. Diagnosis and management of parapneumonic effusions and empyema. Clinical Infectious Diseases 2007;45(11):1480‐6. [DOI] [PubMed] [Google Scholar]
Temes 1996
- Temes RT, Follis F, Kessler RM, Pett SB Jr, Wernly JA. Intrapleural fibrinolytics in management of empyema thoracis. Chest 1996;110(1):102‐6. [DOI] [PubMed] [Google Scholar]
Tillett 1951
- Tillett WS, Sherry S, Read CT. The use of streptokinase‐streptodornase in the treatment of chronic empyema; with an interpretive discussion of enzymatic actions in the field of intrathoracic diseases. Journal of Thoracic Surgery 1951;21(4):325‐41. [PubMed] [Google Scholar]
Yim 1996
- Yim A, Liu H. Complications and failures of video‐assisted thoracic surgery: experience from two centers in Asia. Annals of Thoracic Surgery 1996;61:538‐41. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Chin 2013
- Chin TY, Redden MD, Hsu CCT, Driel ML. Surgical versus non‐surgical management for pleural empyema. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD010651] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coote 2005
- Coote N, Kay E. Surgical versus non‐surgical management of pleural empyema. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD001956.pub3] [DOI] [PubMed] [Google Scholar]


