Abstract
Background
Hepatitis C virus (HCV) is a single‐stranded RNA (ribonucleic acid) virus that has the potential to cause inflammation of the liver. The traditional definition of acute HCV infection is the first six months following infection with the virus. Another commonly used definition of acute HCV infection is the absence of HCV antibody and subsequent seroconversion (presence of HCV antibody in a person who was previously negative for HCV antibody). Approximately 40% to 95% of people with acute HCV infection develop chronic HCV infection, that is, have persistent HCV RNA in their blood. In 2010, an estimated 160 million people worldwide (2% to 3% of the world's population) had chronic HCV infection. The optimal pharmacological treatment of acute HCV remains controversial. Chronic HCV infection can damage the liver.
Objectives
To assess the comparative benefits and harms of different pharmacological interventions in the treatment of acute HCV infection through a network meta‐analysis and to generate rankings of the available pharmacological treatments according to their safety and efficacy. However, it was not possible to assess whether the potential effect modifiers were similar across different comparisons. Therefore, we did not perform the network meta‐analysis and instead we assessed the comparative benefits and harms of different interventions versus each other or versus no intervention using standard Cochrane methodology.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, Science Citation Index Expanded, World Health Organization International Clinical Trials Registry Platform, and randomised controlled trials registers to April 2016 to identify randomised clinical trials on pharmacological interventions for acute HCV infection.
Selection criteria
We included only randomised clinical trials (irrespective of language, blinding, or publication status) in participants with acute HCV infection. We excluded trials which included previously liver transplanted participants and those with other coexisting viral diseases. We considered any of the various pharmacological interventions compared with placebo or each other.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We calculated the odds ratio (OR) and rate ratio with 95% confidence intervals (CI) using both fixed‐effect and random‐effects models based on the available‐participant analysis with Review Manager 5. We assessed risk of bias according to Cochrane, controlled risk of random errors with Trial Sequential Analysis, and assessed the quality of the evidence using GRADE.
Main results
We identified 10 randomised clinical trials with 488 randomised participants that met our inclusion criteria. All the trials were at high risk of bias in one or more domains. Overall, the evidence for all the outcomes was very low quality evidence. Nine trials (467 participants) provided information for one or more outcomes. Three trials (99 participants) compared interferon‐alpha versus no intervention. Three trials (90 participants) compared interferon‐beta versus no intervention. One trial (21 participants) compared pegylated interferon‐alpha versus no intervention, but it did not provide any data for analysis. One trial (41 participants) compared MTH‐68/B vaccine versus no intervention. Two trials (237 participants) compared pegylated interferon‐alpha versus pegylated interferon‐alpha plus ribavirin. None of the trials compared direct‐acting antivirals versus placebo or other interventions. The mean or median follow‐up period in the trials ranged from six to 36 months.
There was no short‐term mortality (less than one year) in any group in any trial except for one trial where one participant died in the pegylated interferon‐alpha plus ribavirin group (1/95: 1.1%). In the trials that reported follow‐up beyond one year, there were no further deaths. The number of serious adverse events was higher with pegylated interferon‐alpha plus ribavirin than with pegylated interferon‐alpha (rate ratio 2.74, 95% CI 1.40 to 5.33; participants = 237; trials = 2; I2 = 0%). The proportion of people with any adverse events was higher with interferon‐alpha and interferon‐beta compared with no intervention (OR 203.00, 95% CI 9.01 to 4574.81; participants = 33; trials = 1 and OR 27.88, 95% CI 1.48 to 526.12; participants = 40; trials = 1). None of the trials reported health‐related quality of life, liver transplantation, decompensated liver disease, cirrhosis, or hepatocellular carcinoma. The proportion of people with chronic HCV infection as indicated by the lack of sustained virological response was lower in the interferon‐alpha group versus no intervention (OR 0.27, 95% CI 0.09 to 0.76; participants = 99; trials = 3; I2 = 0%). The differences between the groups were imprecise or not estimable (because neither group had any events) for all the remaining comparisons.
Four of the 10 trials (40%) received financial or other assistance from pharmaceutical companies who would benefit from the findings of the research; the source of funding was not available in five trials (50%), and one trial (10%) was funded by a hospital.
Authors' conclusions
Very low quality evidence suggests that interferon‐alpha may decrease the incidence of chronic HCV infection as measured by sustained virological response. However, the clinical impact such as improvement in health‐related quality of life, reduction in cirrhosis, decompensated liver disease, and liver transplantation has not been reported. It is also not clear whether this finding is applicable in the current clinical setting dominated by the use of pegylated interferons and direct‐acting antivirals, although we found no evidence to support that pegylated interferons or ribavirin or both are effective in people with acute HCV infection. We could find no randomised trials comparing direct‐acting antivirals with placebo or other interventions for acute HCV infection. There is significant uncertainty in the benefits and harms of the interventions, and high‐quality randomised clinical trials are required.
Keywords: Humans; Acute Disease; Antiviral Agents; Antiviral Agents/adverse effects; Antiviral Agents/therapeutic use; Drug Therapy, Combination; Drug Therapy, Combination/adverse effects; Hepatitis C; Hepatitis C/drug therapy; Hepatitis C, Chronic; Hepatitis C, Chronic/drug therapy; Interferon‐alpha; Interferon‐alpha/adverse effects; Interferon‐alpha/therapeutic use; Network Meta‐Analysis; Polyethylene Glycols; Polyethylene Glycols/adverse effects; Polyethylene Glycols/therapeutic use; Quality of Life; Randomized Controlled Trials as Topic; Recombinant Proteins; Recombinant Proteins/adverse effects; Recombinant Proteins/therapeutic use; Ribavirin; Ribavirin/adverse effects; Ribavirin/therapeutic use; Viral Hepatitis Vaccines; Viral Hepatitis Vaccines/therapeutic use
Medical treatment of acute hepatitis C virus infection
Background
Hepatitis C virus (HCV) is a virus that affects the liver. It is usually transmitted by injectable drug abuse, transfusion of infected blood, unhygienic tattooing practices, coming into contact with blood infected with HCV, and unprotected sex. Acute HCV infection is the period that covers within six months of infection. While some people clear the virus after acute HCV infection, the virus remains in others. This is called chronic HCV infection and may cause major health problems such as excessive tiredness, and liver failure leading to vomiting blood, confusion, and death. Overall, an estimated 160 million people worldwide (2% to 3% of the world's population) have chronic HCV infection. A number of medical treatments have been used for acute HCV infection. The best way to treat acute HCV infection is not clear. We sought to resolve this issue by searching for existing studies on the topic. We included all randomised clinical trials (clinical studies where people are randomly put into one of two or more intervention groups) whose results were reported to April 2016. We included only trials in which participants had not undergone liver transplantation previously and those who did not have liver disease due to other viral infections. Apart from using standard Cochrane methods which allow comparison of only two interventions at a time (direct comparison), we planned to use advanced method which allows comparison of the many different interventions individually compared in the trials (network meta‐analysis). However, because of the nature of the information available, we could not determine whether the network meta‐analysis results were reliable. So, we used standard Cochrane methodology.
Study characteristics
We identified 10 randomised clinical trials which were eligible for our review. Nine randomised clinical trials (467 participants) provided information for one or more measures (outcomes). The main interventions compared included different forms of interferon (protein secreted in response to viral infection), namely, interferon‐alpha alone, interferon‐beta alone, pegylated interferon‐alpha alone, pegylated interferon‐alpha plus ribavirin (another antiviral drug), a vaccine called MTH‐68/B made from a different virus, versus no intervention. None of the trials compared direct‐acting antivirals (the latest option for treating HCV infection) versus placebo or other interventions. The average follow‐up period in the trials ranged from six months to three years.
Source of funding
Four of the 10 trials (40%) received financial or other assistance from pharmaceutical companies who would benefit from the findings of the research; the source of funding was not available in five trials (50%), and one trial (10%) was funded by a hospital.
Quality of evidence
All the trials were at high risk of bias, and the overall quality of the evidence was very low. This means that there is a possibility of making wrong conclusions overestimating benefits or underestimating harms of one intervention or the other because of the way that the trials were conducted.
Key results
No deaths occurred less than one year after treatment in any group in any trial except for one trial where one participant died in the pegylated interferon‐alpha plus ribavirin group (1/95: 1.1%). In the trials in which participants were followed up beyond one year, there were no further deaths. The number of serious complications was higher with pegylated interferon‐alpha plus ribavirin than with pegylated interferon‐alpha. The percentage of people with any complications was higher with interferon‐alpha and interferon‐beta than with no intervention. None of the trials reported health‐related quality of life, liver transplantation, liver failure, severe liver damage, or liver cancer. The percentage of people in whom the virus remained in the blood six months after the end of treatment was lower in the interferon‐alpha than in the no intervention groups. There was no evidence of differences between the groups for all the remaining comparisons. There is significant uncertainty about the size and direction of the results and high quality randomised clinical trials are required.
Summary of findings
Summary of findings for the main comparison.
Intervention versus no intervention or control intervention (control) for acute hepatitis C infection: primary outcomes
| Intervention versus no intervention or control intervention (control) for acute hepatitis C infection: primary outcomes | |||||
|
Patient or population: people with acute hepatitis C infection Intervention: multiple Control: multiple Settings: secondary or tertiary care | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Intervention | ||||
| Mortality: there was 1 mortality within 6 months (in the pegylated interferon‐alpha group (1/95 = 1.1%). There was no mortality in the remaining groups. There was no further mortality in the trials which reported mortality until maximal follow‐up. | |||||
| Serious adverse events: there were no serious adverse events in either group in the comparisons interferon‐beta versus control and MH‐68/B vaccine versus control. Trials in interferon‐alpha versus control did not report serious adverse events. | |||||
| Serious adverse events (proportion) ‐ pegylated interferon‐alpha plus ribavirin versus pegylated interferon‐alpha | 70 per 1000 | 115 per 1000 (50 to 242) | OR 1.72 (0.7 to 4.21) | 237 (2 RCTs) | ⊕⊝⊝⊝ Very low1,2,3 |
| Serious adverse events (number) ‐ pegylated interferon‐alpha plus ribavirin versus pegylated interferon‐alpha | 92 per 1000 | 251 per 1000 (128 to 488) | Rate ratio 2.74 (1.40 to 5.33) | 237 (2 RCTs) | ⊕⊝⊝⊝ Very low1,2 |
| Adverse events (proportion) ‐ interferon‐alpha versus no intervention | 10 per 1000 | 672 per 1000 (83 to 979) | OR 203 (9.01 to 4574.81) | 33 (1 RCT) | ⊕⊝⊝⊝ Very low1,2 |
| Adverse events (proportion) ‐ interferon‐beta versus no intervention | 10 per 1000 | 220 per 1000 (15 to 842) | OR 27.88 (1.48 to 526.12) | 40 (1 RCT) | ⊕⊝⊝⊝ Very low1,2 |
| Adverse events: there were no adverse events in the comparison MTH‐68/B vaccine versus control. The number of adverse events was not reported for the comparison interferon‐alpha versus control. The proportion of people with adverse events and number of adverse events was not reported for the comparison pegylated interferon‐alpha plus ribavirin versus pegylated interferon‐alpha. | |||||
| Adverse events (number) ‐ interferon‐beta versus no intervention | 10 per 1000 | 147 per 1000 (10 to 748) | OR 17 (0.98 to 294.53) | 40 (1 RCT) | ⊕⊝⊝⊝ Very low1,2,3 |
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| None of the trials reported health‐related quality of life, cirrhosis, decompensated liver disease, liver transplantation, or hepatocellular carcinoma. | |||||
| *The basis for the assumed risk is the mean control group proportion (or control group rate) unless there were no events in the control group when the control group proportion (or control group rate) was considered as 1%. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised clinical trial. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded 2 levels for high risk of bias. 2 Downgraded 1 level for small sample size (i.e. imprecision). 3 Downgraded 1 level for wide confidence intervals (i.e. imprecision).
Summary of findings 2.
Intervention versus no intervention or control intervention (control) for acute hepatitis C infection: secondary outcomes
| Intervention versus no intervention or control intervention (control) for acute hepatitis C infection: secondary outcomes | |||||
|
Patient or population: people with acute hepatitis C infection Intervention: multiple (see below) Control: multiple (see below) Settings: secondary or tertiary care | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Intervention | ||||
| Liver transplantation | None of the trials reported this outcome. | ||||
| Decompensated liver disease | None of the trials reported this outcome. | ||||
| Cirrhosis | None of the trials reported this outcome. | ||||
| Hepatocellular carcinoma | None of the trials reported this outcome. | ||||
| Chronic HCV infection†‐ interferon‐alpha versus no intervention | 848 per 1000 | 601 per 1000 (334 to 809) | OR 0.27 (0.09 to 0.76) | 99 (3 RCTs) | ⊕⊝⊝⊝ Very low1,2 |
| Chronic HCV infection†‐ interferon‐beta versus no intervention | 833 per 1000 | 259 per 1000 (0 to 861) | OR 0.07 (0 to 1.24) | 90 (3 RCTs) | ⊕⊝⊝⊝ Very low1,2,3,4 |
| Chronic HCV infection†‐ MTH‐68/B vaccine versus no intervention | 263 per 1000 | 91 per 1000 (18 to 371) | OR 0.28 (0.05 to 1.65) | 41 (1 RCT) | ⊕⊝⊝⊝ Very low1,2,3 |
| Chronic HCV infection†‐ pegylated interferon‐alpha plus ribavirin versus pegylated interferon‐alpha | 204 per 1000 | 181 per 1000 (95 to 315) | OR 0.86 (0.41 to 1.79) | 237 (2 RCTs) | ⊕⊝⊝⊝ Very low1,2,3 |
| *The basis for the assumed risk is the mean control group proportion. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). †Chronic HCV infection was measured by absence of sustained virological response (i.e. the presence of circulating virus at least 6 months after cessation of treatment). CI: confidence interval; HCV: hepatitis C virus; OR: odds ratio; RCT: randomised clinical trial. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded 2 levels for high risk of bias (i.e. within study risk of bias). 2 Downgraded 1 level for small sample size (i.e. imprecision). 3 Downgraded 1 level for wide confidence intervals (i.e. imprecision). 4 Downgraded 1 level (substantial heterogeneity in magnitude of effect) (i.e. heterogeneity).
Background
Description of the condition
Hepatitis C virus (HCV) is a single‐stranded RNA (ribonucleic acid) virus that has the potential to cause inflammation of the liver (NCBI 2014a). There is some variation in the RNA sequence of HCV and currently, seven genotypes (genotypes 1 to 7) are recognised (Smith 2014). The major mode of transmission of HCV is by parenteral routes, which includes parenteral drug abuse, transfusion of infected blood, unhygienic tattooing practices, and occupational exposure to the blood of HCV‐infected people (Beltrami 2000; Xia 2008; Kleinman 2009; Jafari 2010; Loomba 2011; Bunchorntavakul 2015). The other modes of transmission include sexual intercourse with infected people and perinatal transmission from mother to child (Syriopoulou 2005; Tohme 2010). Acute HCV infection is defined in different ways (Hajarizadeh 2012). The traditional definition of acute HCV infection is the first six months following exposure to viral infection (Grebely 2011). However, as most people are asymptomatic after acute HCV infection (Kamal 2008; Grebely 2011), it is difficult to identify the exact duration of infection (Hajarizadeh 2012). The absence of the HCV antibody and subsequent seroconversion (presence of HCV antibody in a person who was previously negative for HCV antibody) is the most common definition used in studies related to acute HCV infection (Hajarizadeh 2012). Other criteria that have been used to assess treatment effects include alanine transaminase elevation and HCV RNA detection (Hajarizadeh 2012). Presence of HCV RNA in the absence of HCV antibody in the serum and subsequent seroconversion accurately diagnoses very recent acute HCV infection, but it is uncommon in clinical practice (Hajarizadeh 2012). While HCV RNA can be detected in the circulation about one to two weeks after exposure to infection, seroconversion may be evident only two to six months after the exposure to infection (Kamal 2008).
Approximately 10% to 30% of people with acute HCV develop symptoms (Kamal 2008; Maheshwari 2008). The symptoms and signs related to acute HCV infection include jaundice, fatigue, muscle pain, influenza‐like illness, low‐grade fever, nausea, vomiting, and right upper abdominal pain (Kamal 2008; Maheshwari 2008). Acute HCV is rarely fulminant (Kamal 2008; Maheshwari 2008). Symptoms usually occur within six to eight weeks after exposure and last for about three to 12 weeks (Kamal 2008). Approximately 40% to 95% of people with acute HCV infection develop chronic HCV infection, that is, have persistent HCV RNA in their blood (Lehmann 2004; Wawrzynowicz‐Syczewska 2004; Kamal 2008; Maheshwari 2008; Beinhardt 2012; Bunchorntavakul 2015), depending upon the genotype of the HCV (Lehmann 2004). Various factors that can predict the development of chronic HCV infection have been proposed but none are sufficiently reliable to guide management (Maheshwari 2008; EASL 2014). Presence of jaundice, HCV genotypes 1 and 3, favourable interleukin‐28B genotype, female sex, being white by ethnic origin, having a low peak viral load, and having a rapid decline in viral load within the first four weeks of diagnosis are all associated with spontaneous clearance of the HCV infection (Maheshwari 2008; Grebely 2014). However, being of Afro‐Caribbean ethnic origin and having a coexistent HIV infection is associated with increased risk of chronic HCV infection (Maheshwari 2008).
Chronic HCV infection is a slowly progressive disease. Approximately 1% to 39% of people who develop chronic HCV infection develop liver cirrhosis (advanced liver fibrosis) after a period of seven to 30 years due to damage to the liver by HCV (Poynard 1997; Kenny‐Walsh 1999; Rodger 2000; Wiese 2005; Seeff 2009). Cirrhosis has two phases, an asymptomatic 'compensated cirrhosis' phase and a 'decompensated cirrhosis' phase, characterised by clinical symptoms such as upper gastrointestinal bleeding from varices, ascites, encephalopathy, jaundice, or renal failure (D'Amico 2006). Liver‐related complications such as cirrhosis, hepatocellular carcinoma, decompensated liver disease, and mortality usually occur 15 to 20 years after the initial infection in a proportion of people (Wiese 2005). Every year, 1% to 4% of people referred to the hospital with HCV‐related cirrhosis die (4%); develop liver failure manifested as ascites (3%); or develop jaundice (2%), gastrointestinal bleeding (1%), or hepatocellular carcinoma (4%) (Sangiovanni 2006). The median survival after compensated liver disease is more than 10 years while that of decompensated liver disease is less than two years (D'Amico 2006). The only definitive treatment for decompensated liver cirrhosis is liver transplantation. The median survival after liver transplantation for chronic HCV infection is between six and eight years (Uemura 2012; Singal 2013). There is also improvement in the quality of life of people with chronic liver disease after liver transplantation (Yang 2014). In 2010, an estimated 160 million people worldwide (2% to 3% of the world's population) had chronic HCV infection (Lavanchy 2011). There is a global variation in the prevalence of chronic HCV infection with highest prevalence in Africa and the Eastern Mediterranean region (Global Burden of Hepatitis C Working Group 2004). In 2010, on average, approximately USD 400 were spent on each person each year with chronic HCV infection without complications (El Khoury 2012). Once people develop complications, the healthcare costs increase exponentially. The annual healthcare costs associated with compensated HCV cirrhosis are USD 1080 per person; annual healthcare costs associated with decompensated HCV cirrhosis are USD 17,070 per person; and annual healthcare costs associated with liver transplantation associated with HCV cirrhosis are USD 146,960 per person (El Khoury 2012).
Description of the intervention
Various drugs such as interferon, direct‐acting antivirals (ribavirin; protease inhibitors such as telaprevir), or a combination of these have been used with the aim of eradicating acute HCV infection, thereby preventing progression to chronic HCV infection and subsequent complications related to chronic HCV infection (Maheshwari 2008; Grebely 2011; Fierer 2014). Interferons are proteins secreted by cells in response to a wide range of inducers that confer resistance against viruses and cancer cells (NCBI 2014b). The major types of interferon include interferon‐alpha, interferon‐beta, interferon‐omega, interferon‐lambda, and interferon‐gamma (Feld 2005; NCBI 2014b). Interferon‐alpha is the most common interferon used to treat acute HCV infection (Myers 2001; Maheshwari 2008; Grebely 2011), and interferon‐beta has also been evaluated (Myers 2001). Interferon is usually manufactured by recombinant technology where a sequence of human DNA is combined with the DNA of bacteria such as Escherichia coli so that large‐scale quantities of interferon are produced by Escherichia coli (Anonymous 1981). A variation of interferon‐alpha is pegylated interferon‐alpha where the structure of interferon is modified to make it long acting (Bailon 2001). Interferon‐alpha is usually administered by subcutaneous or intramuscular injections (Martindale 2011). Ribavirin is a guanosine analogue (Feld 2005). It is usually administered orally (Martindale 2011). Telaprevir, boceprevir, danoprevir, and ABT‐450 are called direct‐acting antiviral agents as they inhibit viral proteins (Lewis 2012; Welsch 2012; Poordad 2014). Of these, telaprevir and boceprevir are first‐generation direct‐acting antiviral agents as these were the first direct‐acting antiviral agents licensed for use in chronic HCV infections (Lewis 2012). The remaining antiviral agents are second‐generation direct‐acting antiviral agents (Lewis 2012). The direct‐acting antiviral agents are usually administered orally (Martindale 2011). It should be noted that these treatments have significant complications including mortality, severe infections, severe liver decompensation, refractory anaemia, neutropenia, thrombocytopenia, and neuropsychiatric disorders (homicidal and suicidal ideation) with different treatments having different safety profiles (Martindale 2011; Perry 2012; Furusyo 2013; Hezode 2013; Coilly 2014).
How the intervention might work
Interferon is one of the natural defence mechanisms of the body against viruses (Feld 2005; NCBI 2014b). Interferons induce interferon‐stimulated genes, which creates an antiviral state within the cells (Feld 2005). Ribavirin may act by inhibiting the HCV RNA polymerase (which plays a significant role in viral replication), inducing mutations in the virus and making them less infective, and by modifying the immune response (immunomodulation), which promotes clearance of virus from cells (Feld 2005). Direct‐acting antiviral agents inhibit viral proteins involved in the HCV life cycle and so inhibit replication of the virus (Welsch 2012).
Why it is important to do this review
There is significant controversy as to whether any intervention is beneficial in acute HCV infection. Currently, the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) have no recommendations for treatment directed at acute HCV infection (AASLD 2014; EASL 2014). However, some investigators recommend pegylated interferon for people with acute HCV infection, usually started three months after diagnosis after repeat serum testing to allow three months for spontaneous resolution (Maheshwari 2008; Grebely 2011). Thus, the treatment of acute HCV is controversial. In addition, several treatment combinations are available for the treatment of acute HCV infection, and the relative ranking of different treatments in terms of clinical effectiveness and harms is unknown.
Network meta‐analysis allows combination of the direct evidence and indirect evidence, and allows ranking of different treatments in terms of the different outcomes (Salanti 2011; Salanti 2012). There has been no network meta‐analysis on this topic although there has been one head‐to‐head comparison Cochrane systematic review comparing interferon versus placebo or no intervention in people with acute HCV infection (Myers 2001). The present systematic review and attempted network meta‐analysis intended to provide the best level of evidence for the role of different pharmacological interventions in the treatment of people with acute HCV infection.
Objectives
To assess the comparative benefits and harms of different pharmacological interventions in the treatment of acute HCV infection through a network meta‐analysis and to generate rankings of the available pharmacological treatments according to their safety and efficacy. However, it was not possible to assess whether the potential effect modifiers were similar across different comparisons. Therefore, we did not perform the network meta‐analysis, and instead, we assessed the comparative benefits and harms of different interventions versus each other or versus no intervention using standard Cochrane methodology.
When more trials become available with adequate description of potential effect modifiers, we will attempt to conduct network meta‐analysis to generate rankings of the available treatments according to their safety and efficacy. This is why we have retained the planned methodology for network meta‐analysis in our Appendix 1. Once data appear allowing for the conduct of network meta‐analysis, this Appendix 1 will be moved back into the Methods section.
Methods
Criteria for considering studies for this review
Types of studies
We considered only randomised clinical trials for the meta‐analysis irrespective of language, publication status, or date of publication. We excluded studies of other design because of the risk of bias in such studies. We are all aware that such exclusions make us focus much more on potential benefits and not fully assess the risks of serious adverse events as well as risks of adverse events.
Types of participants
We included randomised clinical trials with participants with acute HCV infection (absence of HCV antibody and subsequent seroconversion (presence of HCV antibody in a person who was previously negative for HCV antibody or less than six months of HCV infection)), irrespective of the method of diagnosis of seroconversion or HCV genotype. We excluded randomised clinical trials in which participants had undergone liver transplantation previously. We also excluded randomised clinical trials in which participants had other coexisting viral diseases such as HIV or hepatitis B virus coinfections.
Types of interventions
We included any of the following pharmacological interventions that are possible treatments for acute HCV infection, either alone or in combination and could be compared versus each other or versus placebo or no intervention.
The interventions that we considered a priori were:
interferon‐alpha;
pegylated interferon‐alpha;
interferon‐beta;
ribavirin;
first‐generation direct‐acting antiviral agents (boceprevir and telaprevir);
second‐generation direct‐acting antiviral agents (other direct‐acting antiviral agents).
The above list of interventions was not an exhaustive list. If we identified any other pharmacological interventions that we were not aware of (e.g. we included MTH‐68/B), we considered them eligible and included them in the review if they were used primarily for the treatment of acute HCV infection.
Types of outcome measures
We planned to assess the comparative benefits and harms of available pharmacological interventions aimed at treating people with acute HCV infection for the following outcomes.
Primary outcomes
Mortality at maximal follow‐up (time to death).
-
Mortality:
short‐term mortality (up to one year);
medium‐term mortality (one to five years).
-
Adverse events (within three months of cessation of treatment). We extracted adverse events and serious adverse events as reported in the studies. In general, we defined a non‐serious adverse event as any untoward medical occurrence not necessarily having a causal relationship with the treatment but resulting in a dose reduction or discontinuation of treatment (any time after commencement of treatment) (ICH‐GCP 1997), and defined a serious adverse event as any event that would increase mortality; was life threatening; required hospitalisation; resulted in persistent or significant disability; was a congenital anomaly/birth defect; or any important medical event that might jeopardise the person or require intervention to prevent it.
Proportion of participants with serious adverse events.
Number of serious adverse events.
Proportion of participants with any type of adverse event.
Number of any type of adverse event.
-
Health‐related quality of life as defined in the included trials using a validated scale such as EQ‐5D or 36‐item Short Form (SF‐36) (EuroQol 2014; Ware 2014):
short‐term (up to one year);
medium‐term (one to five years);
long‐term (beyond five years).
We planned to consider short‐term quality of life to be more important than medium‐term or long‐term quality of life, although medium‐term and long‐term quality of life are also important primary outcomes.
Secondary outcomes
-
Liver transplantation (maximal follow‐up):
proportion of participants with liver transplantation;
time to liver transplantation.
-
Decompensated liver disease (presence of one or more of bleeding varices, ascites, encephalopathy, jaundice) (maximal follow‐up):
proportion of participants with decompensated liver disease;
time to liver decompensation.
-
Cirrhosis (scarring of the liver caused by continuous, long‐term liver damage or stage IV fibrosis) (maximal follow‐up):
proportion of participants with cirrhosis;
time to cirrhosis.
Proportion of participants with hepatocellular carcinoma (maximal follow‐up).
Proportion of participants with chronic HCV infection (however defined by trial authors).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and Science Citation Index Expanded (which includes Conference Proceedings) (Royle 2003) from inception to 16 April 2016 for randomised clinical trials comparing two or more of the above interventions. We searched for all possible comparisons formed by the interventions of interest. To identify further ongoing or completed trials, we also searched the World Health Organization International Clinical Trials Registry Platform Search Portal (apps.who.int/trialsearch/), which searches various trial registers, including ISRCTN and ClinicalTrials.gov on 16 April 2016. Appendix 2 shows the search strategies we used.
Searching other resources
We searched the references of the identified trials and the existing Cochrane Reviews on acute HCV infection to identify additional trials for inclusion.
Data collection and analysis
Selection of studies
Two review authors (MK and EB) independently identified the trials for inclusion by screening the titles and abstracts. We sought full‐text articles for any references that at least one of the review authors identified for potential inclusion. We selected the trials for inclusion based on the full‐text articles. We planned to list the excluded full‐text references with reasons for their exclusion in the Characteristics of excluded studies table. We also planned to list any ongoing trials identified primarily through the search of the clinical trial registers for further follow‐up. We resolved discrepancies through discussion and by arbitration with KG, DT, and ET.
Data extraction and management
Two review authors (MK and EB) independently extracted the following data.
-
Outcome data (for each outcome and for each treatment arm whenever applicable):
number of participants randomised;
number of participants included for the analysis;
number of participants with events for binary outcomes, mean and standard deviation for continuous outcomes, number of events for count outcomes, and the number of participants with events and the mean follow‐up period for time‐to‐event outcomes;
definition of outcomes or scale used if appropriate.
-
Data on potential effect modifiers:
participant characteristics such as age, sex, comorbidities, proportion of participants with different HCV genotypes, and severity of acute HCV infection, however defined by trial authors;
details of the intervention and control (including dose, frequency, and duration);
risk of bias (assessment of risk of bias in included studies).
-
Other data:
year and language of publication;
country in which the participants were recruited;
year(s) in which the trial was conducted;
inclusion and exclusion criteria;
follow‐up time points of the outcome.
If available, we planned to obtain the data separately for different genotypes from the report. We sought unclear or missing information by attempting to contact the trial authors. If there was any doubt whether trials shared the same participants, completely or partially (by identifying common authors and centres), we planned to contact the trial authors to clarify whether the trial report was duplicated. We resolved any differences in opinion through discussion.
Assessment of risk of bias in included studies
We followed the guidance given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and in the Cochrane Hepato‐Biliary Module (Gluud 2015) to assess the risk of bias in included studies. Specifically, we assessed the risk of bias in included trials for the following domains, using the methods below (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Savović 2012a; Savović 2012b; Lundh 2017).
Allocation sequence generation
Low risk of bias: the study authors performed sequence generation using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if an independent person not otherwise involved in the study performed them.
Unclear risk of bias: the study authors did not specify the method of sequence generation.
High risk of bias: the sequence generation method was not random.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. The investigators were unaware of the allocation sequence (e.g. if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Unclear risk of bias: the study authors did not describe the method used to conceal the allocation so the intervention allocations may have been foreseen before, or during, enrolment.
High risk of bias: it is likely that the investigators who assigned the participants knew the allocation sequence.
Blinding of participants and personnel
Low risk of bias: any of the following: no blinding or incomplete blinding, but we judged that the outcome was not likely to be influenced by lack of blinding; or blinding of participants and key study personnel ensured, and it was unlikely that the blinding could have been broken.
Unclear risk of bias: any of the following: insufficient information to permit judgement of 'low risk' or 'high risk'; or the trial did not address this outcome.
High risk of bias: any of the following: no blinding or incomplete blinding, and the outcome was likely to be influenced by lack of blinding; or blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome was likely to be influenced by lack of blinding.
Blinding of outcome assessors
Low risk of bias: any of the following: no blinding of outcome assessment, but we judged that the outcome measurement was not likely to be influenced by lack of blinding; or blinding of outcome assessment ensured, and unlikely that the blinding could have been broken.
Unclear risk of bias: any of the following: insufficient information to permit judgement of 'low risk' or 'high risk'; or the trial did not address this outcome.
High risk of bias: any of the following: no blinding of outcome assessment, and the outcome measurement was likely to be influenced by lack of blinding; or blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement was likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. The study used sufficient methods, such as multiple imputation, to handle missing data.
Unclear risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: the trial reported the following predefined outcomes: long‐term outcomes related to the disease process (namely, mortality or decompensated liver disease, or requirement for transplantation along with treatment‐related adverse events). If the original trial protocol was available, the outcomes should have been those called for in that protocol. If the trial protocol was obtained from a trial registry (e.g. www.clinicaltrials.gov), the outcomes sought should have been those enumerated in the original protocol if the trial protocol was registered before or at the time that the trial was begun. If the trial protocol was registered after the trial was begun, those outcomes were not considered to be reliable.
Unclear risk of bias: not all predefined outcomes were reported fully, or it was unclear whether data on these outcomes were recorded or not.
High risk of bias: one or more predefined outcomes were not reported.
For‐profit bias
Low risk of bias: the trial appeared to be free of industry sponsorship or other type of for‐profit support that may manipulate the trial design, conductance, or results of the trial.
Unclear risk of bias: the trial may or may not have been free of for‐profit bias as no information on clinical trial support or sponsorship was provided.
High risk of bias: the trial was sponsored by industry or received other type of for‐profit support.
Other bias
Low risk of bias: the trial appeared to be free of other components (e.g. inappropriate control or dose or administration of control) that could put it at risk of bias.
Unclear risk of bias: the trial may or may not have been free of other components that could have put it at risk of bias.
High risk of bias: there were other factors in the trial that could have put it at risk of bias (e.g. inappropriate control or dose or administration of control).
We considered a trial at low risk of bias if we assessed the trial as at low risk of bias across all domains. Otherwise, we considered the trials at high risk of bias regarding one or more domains as at high risk of bias.
Measures of treatment effect
For dichotomous variables (e.g. short‐term and medium‐term mortality or liver transplantation, proportion of participants with adverse events, decompensated liver disease, cirrhosis, hepatocellular carcinoma, or sustained virological response), we calculated the odds ratio (OR) with 95% confidence intervals (CI). For continuous variables (e.g. quality of life reported on the same scale), we planned to calculate the mean difference with 95% CI. We planned to use standardised mean difference values with 95% CI for quality of life if included trials used different scales. For count outcomes (e.g. number of adverse events), we calculated the rate ratio with 95% CI. For time‐to‐event data (e.g. mortality at maximal follow‐up or requirement for liver transplantation, time to liver decompensation, and time to cirrhosis), we planned to use the hazard ratio (HR) with 95% CIs. We also calculated Trial Sequential Analysis‐adjusted CI to control random errors (Thorlund 2011).
Unit of analysis issues
The unit of analysis was people with acute HCV infection according to the intervention group to which they were randomly assigned.
Cluster randomised clinical trials
As expected, we found no cluster randomised clinical trials. If we had found them, we planned to include them provided that the effect estimate adjusted for cluster correlation was available.
Cross‐over randomised clinical trials
As expected, we found no cross‐over randomised clinical trials. If we had identified any, we planned to only include the outcomes after the period of first treatment because acute HCV infection may resolve before the cross‐over period.
Trials with multiple intervention groups
We collected data for all trial intervention groups that met the inclusion criteria.
Dealing with missing data
We performed an intention‐to‐treat analysis whenever possible (Newell 1992). Otherwise, we used the data that were available to us (e.g. a trial might have reported only per‐protocol analysis results). As such 'per‐protocol' analyses may be biased, we planned to conduct best‐worst case scenario analyses (good outcome in intervention group and bad outcome in control group) and worst‐best case scenario analyses (bad outcome in intervention group and good outcome in control group) as sensitivity analyses whenever possible but because of lack of the required information for such analyses, we did not perform them.
For continuous outcomes, we planned to impute the standard deviation from P values according to guidance given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If the data were likely to be normally distributed, we planned to use the median for meta‐analysis when the mean was not available. If it was not possible to calculate the standard deviation from the P value or the CIs, we planned to impute the standard deviation using the largest standard deviation in other trials for that outcome. This form of imputation may decrease the weight of the study for calculation of mean differences and may bias the effect estimate to no effect for calculation of standardised mean differences (Higgins 2011).
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by carefully examining the characteristics and design of included trials. We planned to assess the presence of clinical heterogeneity by comparing effect estimates in the different HCV genotypes and the different regimens (e.g. different agents, different doses, and different durations) of the pharmacological treatments. Different study designs and risk of bias may contribute to methodological heterogeneity. We used the I2 test and Chi2 test for heterogeneity, and overlapping of CIs to assess heterogeneity.
Assessment of reporting biases
We planned to use visual asymmetry on a funnel plot to explore reporting bias in the presence of at least 10 trials that could be included for a direct comparison (Egger 1997; Macaskill 2001). In the presence of heterogeneity that could be explained by subgroup analysis, we planned to produce a funnel plot for each subgroup in the presence of an adequate number of trials. We planned to use the linear regression approach described by Egger 1997 to determine funnel plot asymmetry.
We also considered selective reporting as evidence of reporting bias.
Data synthesis
We performed the meta‐analyses according to Cochrane recommendations (Higgins 2011), using the software package Review Manager 5 (RevMan 2014). We used a random‐effects model (DerSimonian 1986) and a fixed‐effect model (Demets 1987). In the case of a discrepancy between the two models, we have reported both results; otherwise, we reported only the results from the fixed‐effect model.
Calculation of required information size and Trial Sequential Analysis
For calculation of the required information size, see Appendix 3. We performed Trial Sequential Analysis to control the risks of random errors when there were at least two trials included in the meta‐analysis (Wetterslev 2008; Thorlund 2011; TSA 2011). We used an alpha error as per guidance of Jakobsen 2014, power of 90% (beta error of 10%), a relative risk reduction of 20%, a control group proportion observed in the trials, and the diversity observed in the meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We planned to assess the differences in the effect estimates between the following subgroups.
Trials with low risk of bias compared to trials with high risk of bias.
Different HCV genotypes.
Different regimens of pharmacological treatments. For example, boceprevir compared to telaprevir, different doses, and different durations.
However, we could not conduct these subgroup analyses.
We planned to use the Chi2 test for subgroup differences to identify subgroup differences.
Sensitivity analysis
If a trial reported only per‐protocol analysis results, we planned to re‐analyse the results using the best‐worst case scenario and worst‐best case scenario analyses as sensitivity analyses whenever possible. However, we did not perform these analyses because of insufficient information in the trials.
Presentation of results and GRADE assessments
We reported all outcomes in a 'Summary of findings' table format, downgrading the quality of evidence for risk of bias, inconsistency, indirectness, imprecision, and publication bias using GRADE (Guyatt 2011).
Results
Description of studies
Results of the search
We identified 2803 references through electronic searches of CENTRAL (n = 257), MEDLINE (n = 1681), Embase (n = 323), Science Citation Index Expanded (n = 514), World Health Organization International Clinical Trials Registry Platform (n = 8), and randomised controlled trials registers (n = 20). After the removal of 720 duplicates, we obtained 2083 references. We then excluded 2070 clearly irrelevant references through screening titles and reading abstracts. We retrieved 13 references for further assessment. No references were identified through scanning reference lists of the identified randomised trials. We included all 13 references (10 trials) that met the inclusion criteria (Omata 1991; Genesca 1993; Hwang 1994; Lampertico 1994; Omata 1994; Calleri 1998; Csatary 1998; Wang 2005; Deterding 2013; Santantonio 2014). The reference flow is summarised in the study flow diagram (Figure 1).
Figure 1.
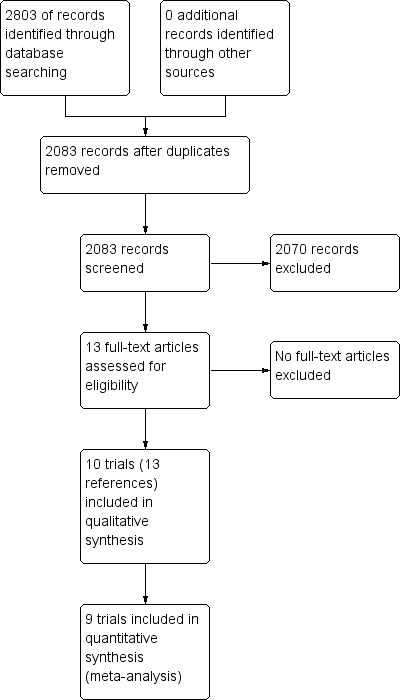
Study flow diagram.
Included studies
The 10 randomised clinical trials included 488 participants (Omata 1991; Genesca 1993; Hwang 1994; Lampertico 1994; Omata 1994; Calleri 1998; Csatary 1998; Wang 2005; Deterding 2013; Santantonio 2014). Nine trials (467 participants) provided information for one or more outcomes (Omata 1991; Genesca 1993; Hwang 1994; Lampertico 1994; Omata 1994; Calleri 1998; Csatary 1998; Deterding 2013; Santantonio 2014). One trial was a three‐armed trial which compared two different doses of pegylated interferon versus pegylated interferon plus ribavirin (Santantonio 2014). The remaining nine trials were two‐armed trials (Omata 1991; Genesca 1993; Hwang 1994; Lampertico 1994; Omata 1994; Calleri 1998; Csatary 1998; Wang 2005; Deterding 2013). Table 4 summarises the details of the intervention, control, the period of follow‐up, and the risk of bias in the trials arranged according to intervention and control. Three trials (99 participants) compared interferon‐alpha versus no intervention (Genesca 1993; Hwang 1994; Lampertico 1994). Three trials (90 participants) compared interferon‐beta versus no intervention (Omata 1991; Omata 1994; Calleri 1998). One trial (41 participants) compared MTH‐68/B vaccine versus no intervention (Csatary 1998). One trial (21 participants) compared pegylated interferon‐alpha versus no intervention, but it did not provide any data for the analysis (Wang 2005). Two trials (237 participants) compared pegylated interferon‐alpha versus pegylated interferon‐alpha plus ribavirin (Deterding 2013; Santantonio 2014). None of the trials compared direct‐acting antivirals versus other interventions. The mean or median follow‐up period in the trials ranged from six to 36 months.
Table 1.
Characteristics table
| Study name | Intervention | Control | Period of follow‐up (months) | Randomisation | Blinding of participants and healthcare professionals | Blinding of outcome assessors | Missing outcome bias | Selective outcome reporting bias | For‐profit bias |
| Genesca 1993 | Interferon‐alpha | No intervention | 12 | Unclear | High | Unclear | Low | High | Unclear |
| Hwang 1994 | Interferon‐alpha | No intervention | 12 | Unclear | High | Unclear | High | Low | High |
| Lampertico 1994 | Interferon‐alpha | No intervention | 18 | Unclear | High | Unclear | High | High | High |
| Omata 1991 | Interferon‐beta | No intervention | 36 | Low | High | Unclear | High | High | Unclear |
| Omata 1994 | Interferon‐beta | No intervention | 36 | Unclear | High | Unclear | Low | High | Unclear |
| Calleri 1998 | Interferon‐beta | No intervention | 22.5 | Unclear | High | High | Low | Low | Low |
| Csatary 1998 | MTH‐68/B vaccine | No intervention | 12 | Unclear | High | Unclear | Low | Low | Unclear |
| Wang 2005 | Pegylated interferon‐alpha | No intervention | 6 | Unclear | Unclear | Unclear | High | High | Unclear |
| Deterding 2013 | Pegylated interferon‐alpha | Pegylated interferon‐alpha plus ribavirin | 6 | Unclear | High | Low | High | Low | High |
| Santantonio 2014 | Pegylated interferon‐alpha | Pegylated interferon‐alpha plus ribavirin | 12 | Unclear | High | Unclear | Low | High | High |
The mean or median age in the trials that reported this information ranged from 29 to 54 years. The proportion of females in the trials that reported this information ranged from 15% to 56%. Three trials reported the proportion of participants with HCV genotype 1, which were 61.9% (Wang 2005); 68.2% (Deterding 2013); and 61.6% (Santantonio 2014). Two trials reported the proportion of participants with genotype 3, which were 16.8% (Deterding 2013); and 37,2% (Santantonio 2014). None of the trials provided information separately for the different genotypes.
Source of funding: four trials received financial or other assistance from pharmaceutical companies who would benefit from the findings of the research (Hwang 1994; Lampertico 1994; Deterding 2013; Santantonio 2014); one trial was funded by a hospital (Calleri 1998); the source of funding was not available in the remaining trials (Omata 1991; Genesca 1993; Omata 1994; Csatary 1998; Wang 2005).
Risk of bias in included studies
The risk of bias in the included trials is summarised in Figure 2 and Figure 3. The trials were at high risk of bias in one or more domains, so all trials were assessed at overall high risk of bias.
Figure 2.
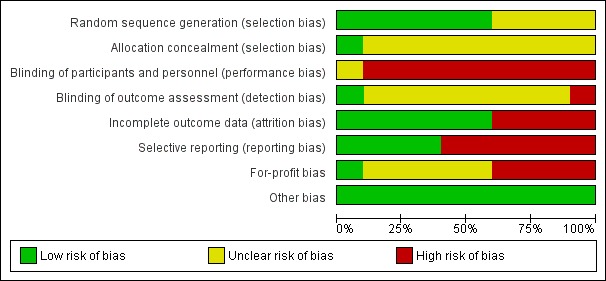
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
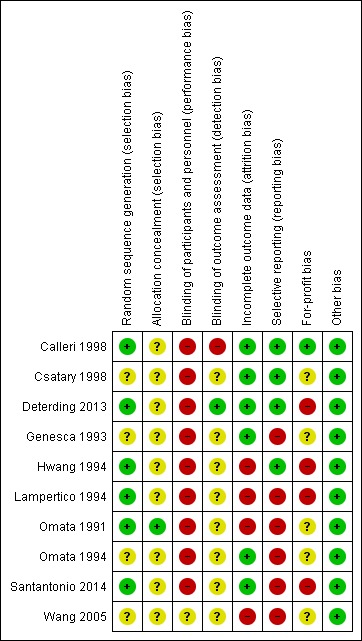
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Six trials were at low risk of random sequence generation bias (Omata 1991; Hwang 1994; Lampertico 1994; Calleri 1998; Deterding 2013; Santantonio 2014). One trial was at low risk of allocation concealment bias (Omata 1991).
Blinding
None of the trials reported blinding of participants and healthcare providers. One trial was at low risk of bias due to blinding of outcome assessors (Deterding 2013).
Incomplete outcome data
Six trials were at low risk of attrition bias (Genesca 1993; Omata 1994; Calleri 1998; Csatary 1998; Deterding 2013; Santantonio 2014).
Selective reporting
Four trials were at low risk of selecting outcome reporting bias (Hwang 1994; Calleri 1998; Csatary 1998; Deterding 2013).
Other potential sources of bias
One trial was at low risk of for‐profit bias (Calleri 1998). All the trials were at low risk of other bias.
Effects of interventions
Mortality
Mortality at maximal follow‐up
Eight trials (337 participants) reported mortality at maximal follow‐up (Omata 1991; Genesca 1993; Hwang 1994; Lampertico 1994; Omata 1994; Calleri 1998; Csatary 1998; Deterding 2013). The period of follow‐up ranged from six to 36 months. There were no deaths reported in any of these trials.
Short‐term mortality
Nine trials reported short‐term mortality (less than one year) (Omata 1991; Genesca 1993; Hwang 1994; Lampertico 1994; Omata 1994; Calleri 1998; Csatary 1998; Deterding 2013; Santantonio 2014). There was no short‐term mortality in any group in any trial except for one trial where one participant died (Santantonio 2014). The unadjusted rates of mortality (short‐term) were 0% in all intervention groups except pegylated interferon‐alpha plus ribavirin in which the mortality was 1.1% (1/95 participants).
Medium‐term mortality
Three trials (90 participants) reported medium‐term mortality (one to five years) (Omata 1991; Omata 1994; Calleri 1998). All three trials compared interferon‐beta versus no intervention. There was no mortality in either group after a follow‐up of two to three years.
Adverse events
Proportion of people with serious adverse events
Four trials (318 participants) reported the proportion of people with serious adverse events (Calleri 1998; Csatary 1998; Deterding 2013; Santantonio 2014). In two trials (one comparing interferon‐beta versus no intervention (Calleri 1998) and one comparing MTH‐68/B vaccine versus no intervention (Csatary 1998)), there were no serious adverse events in either group. In the remaining two trials, the proportion of people with serious adverse events in the pegylated interferon‐alpha plus ribavirin group was adjusted proportion: 11.5% and in pegylated interferon‐alpha group was 10/142 (7.0%). There was no evidence of difference between pegylated interferon‐alpha plus ribavirin versus pegylated interferon‐alpha (OR 1.72, 95% CI 0.70 to 4.21; participants = 237; trials = 2; I2 = 0%; Analysis 1.1).
Analysis 1.1.
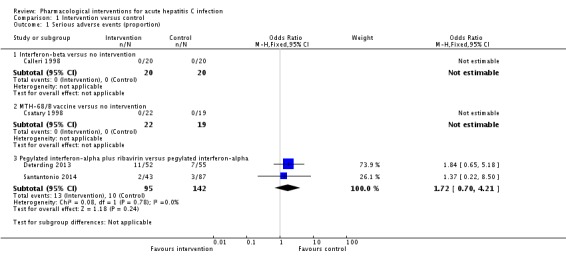
Comparison 1 Intervention versus control, Outcome 1 Serious adverse events (proportion).
Number of serious adverse events
Four trials (318 participants) reported the number of people with serious adverse events (Calleri 1998; Csatary 1998; Deterding 2013; Santantonio 2014). In two trials (one comparing interferon‐beta versus no intervention (Calleri 1998) and one comparing MTH‐68/B vaccine versus no intervention (Csatary 1998)), there were no serious adverse events in either group. In the remaining two trials, the rates for number of serious adverse events in the pegylated interferon‐alpha plus ribavirin group were 25.1 per 100 participants and in the pegylated interferon‐alpha group were 9.2 per 100 participants. The number of serious adverse events was significantly higher with pegylated interferon‐alpha plus ribavirin than with pegylated interferon‐alpha interventions (rate ratio 2.74, 95% CI 1.40 to 5.33; participants = 237; trials = 2; I2 = 0%; Analysis 1.2).
Analysis 1.2.
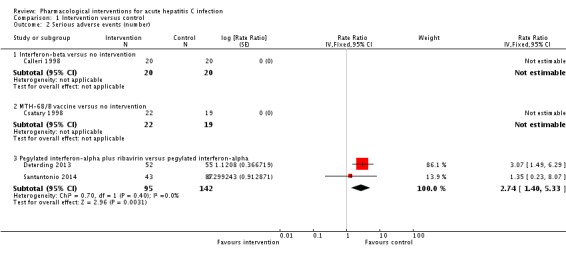
Comparison 1 Intervention versus control, Outcome 2 Serious adverse events (number).
Proportion of people with any type of adverse events
Three trials (114 participants) reported the proportion of people with any adverse events (Hwang 1994; Calleri 1998; Csatary 1998). There were no adverse events in people receiving MTH‐68/B vaccine versus no intervention. The proportions of people with adverse events in the interferon‐alpha group was 87.5% (14/16) and in the interferon‐beta group was 40% (8/20). Compared to the no intervention group the proportion of people with any adverse events was higher in the interferon‐alpha and the interferon‐beta group (interferon‐alpha: OR 203.00, 95% CI 9.01 to 4574.81; participants = 33; trials = 1; interferon‐beta: OR 27.88, 95% CI 1.48 to 526.12; participants = 40; trials = 1; Analysis 1.3). The difference in the proportion of people with adverse events was not estimable in the comparison between MTH‐68/B vaccine versus no intervention group as there were no adverse events in either group.
Analysis 1.3.
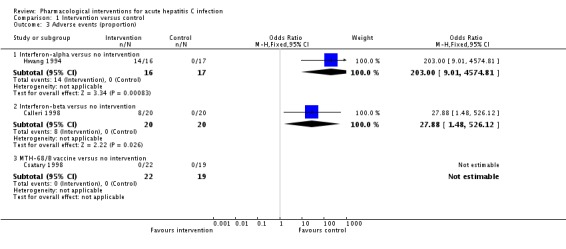
Comparison 1 Intervention versus control, Outcome 3 Adverse events (proportion).
Number of any type of adverse events
Two trials (81 participants) reported number of adverse events (Calleri 1998; Csatary 1998). There were no adverse events in the MTH‐68/B vaccine or no intervention groups. There were eight adverse events reported in 20 participants in the interferon‐beta group (adverse event rate: 14.7 per 100 participants). There was no statistically significant difference in the number of adverse events between the interferon‐beta group and the no intervention group (OR 17.00, 95% CI 0.98 to 294.53; participants = 40; trials = 1; Analysis 1.4).
Analysis 1.4.
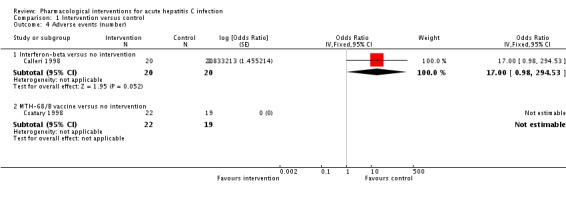
Comparison 1 Intervention versus control, Outcome 4 Adverse events (number).
Health‐related quality of life
None of the trials reported health‐related quality of life at any time point.
Liver transplantation
None of the trials reported liver transplantation.
Decompensated liver disease
None of the trials reported decompensated liver disease.
Cirrhosis
None of the trials reported cirrhosis.
Proportion of participants with hepatocellular carcinoma
None of the trials reported hepatocellular carcinoma.
Proportion of participants with chronic HCV infection
Nine trials (467 participants) reported chronic HCV infection as measured by absence of sustained virological response (i.e. the presence of circulating virus at least six months after cessation of treatment) (Omata 1991; Genesca 1993; Hwang 1994; Lampertico 1994; Omata 1994; Calleri 1998; Csatary 1998; Deterding 2013; Santantonio 2014) (Analysis 1.5). The proportion of people with chronic HCV infection was lower in the interferon‐alpha and interferon‐beta groups than in the no intervention group using the fixed‐effect model (interferon‐alpha: OR 0.27, 95% CI 0.09 to 0.76; participants = 99; trials = 3; I2 = 0%; interferon‐beta: OR 0.07, 95% CI 0.00 to 1.24; participants = 90; trials = 3; I2 = 81%). Using the random‐effects model, there was no change in the results from the interferon‐alpha versus the no intervention group (OR 0.27, 95% CI 0.09 to 0.76). However, there was no evidence of a difference between the interferon‐beta versus the no intervention groups on using the random‐effects model (OR 0.07, 95% CI 0.00 to 1.24). There was no evidence of difference in the proportion of participants with chronic HCV in the comparison between the MTH‐68/B vaccine versus the no intervention groups (OR 0.28, 95% CI 0.05 to 1.65; participants = 41; trials = 1) and that between the pegylated interferon‐alpha plus ribavirin versus the pegylated interferon‐alpha groups (OR 0.86, 95% CI 0.41 to 1.79; participants = 237; trials = 2; I2 = 0%).
Analysis 1.5.
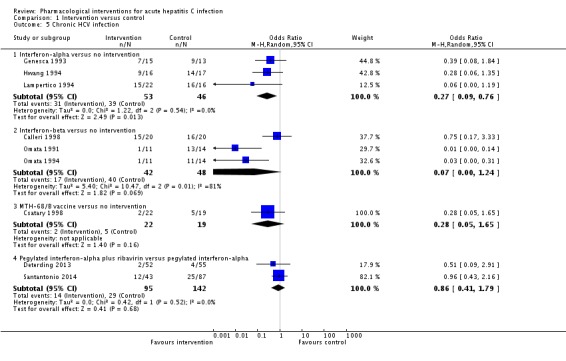
Comparison 1 Intervention versus control, Outcome 5 Chronic HCV infection.
Subgroup analysis
We did not perform any of the subgroup analyses as none of the trials were at low risk of bias, the trials did not report the data for different genotypes separately, and because there were few trials for performing a meaningful subgroup analysis based on dosage.
Reporting bias
We did not explore reporting bias using funnel plots because of the few trials included in the review.
Trial Sequential Analysis
Four comparisons had more than one trial and were eligible for Trial Sequential Analysis. The Z‐curves did not cross the trial sequential monitoring boundaries for any of the comparisons except for chronic HCV in pegylated interferon‐alpha plus ribavirin versus pegylated interferon‐alpha, where it has reached the futility zone (Figure 4; Figure 5; Figure 6; Figure 7); when a relative risk reduction of 10% was used, the Z‐curve did not cross any of the trial sequential monitoring boundaries for chronic HCV in pegylated interferon‐alpha plus ribavirin versus pegylated interferon‐alpha as well (Figure 7). The Trial Sequential Analysis‐adjusted CIs were as follows.
Figure 4.
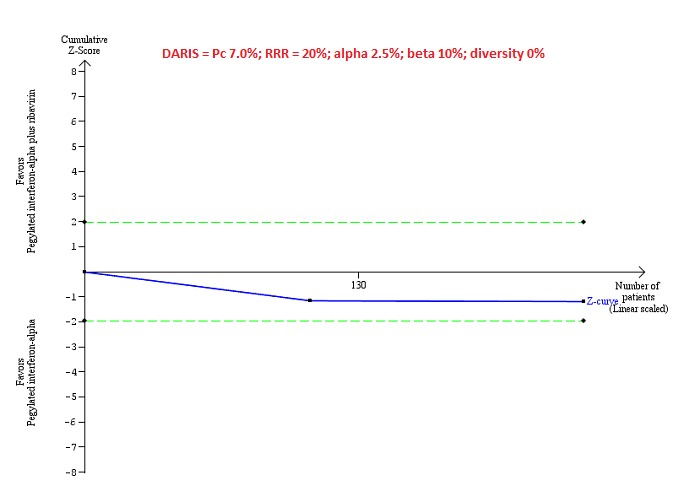
Trial Sequential Analysis of serious adverse events (proportion) for pegylated interferon‐alpha plus ribavirin versus pegylated interferon‐alpha performed using an alpha error of 2.5%, power of 90% (beta error of 10%), relative risk reduction (RRR) of 20%, control group proportion observed in trials (Pc = 7%), and observed heterogeneity in the trials (0%) shows that the accrued sample size was only a small fraction of the diversity‐adjusted required information size (DARIS); so the trial sequential monitoring boundaries were not drawn. The Z‐curve (blue line) does not cross the conventional boundaries (dotted green line). There was a high risk of random errors.
Figure 5.
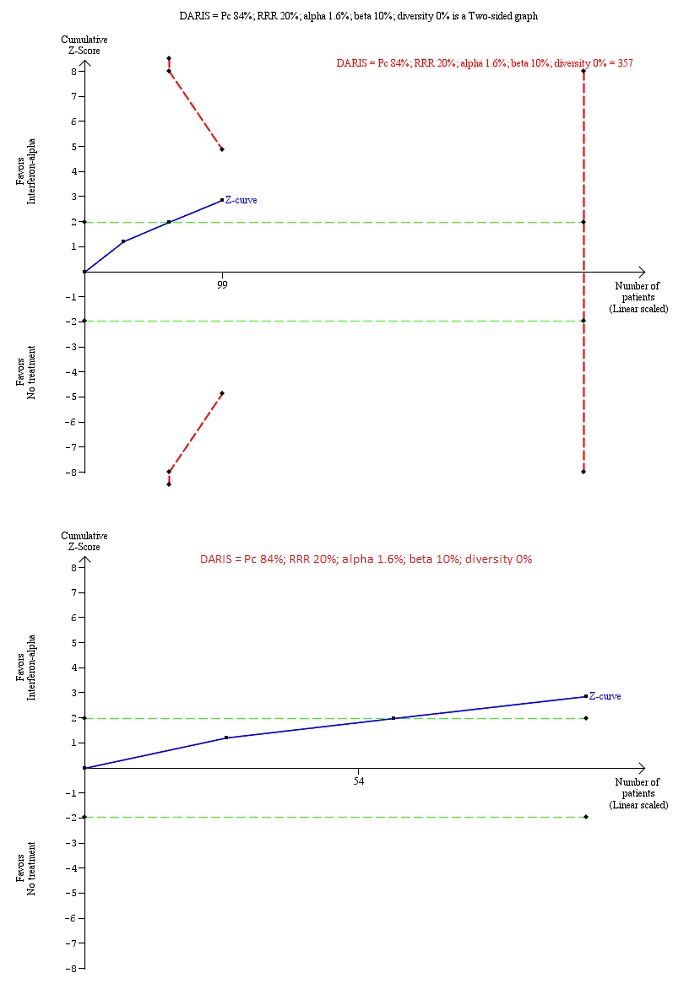
Trial Sequential Analysis of chronic hepatitis C virus for interferon‐alpha versus no intervention was performed using an alpha error of 1.6%, power of 90% (beta error of 10%), relative risk reduction (RRR) of 20%, control group proportion observed in trials (Pc = 84%; upper figure) and Pc = 20% (lower figure), and observed diversity in the trials (0%). The upper figure with Pc = 84% shows that the accrued sample size was only a small fraction of the diversity‐adjusted required information size (DARIS). The Z‐curve (blue line) crosses the conventional boundaries (dotted green line), but it does not cross any of the trial sequential monitoring boundaries (dotted red lines). The lower figure with Pc = 20% shows that the accrued sample size was so small that trial sequential monitoring boundaries were not drawn. There is a high risk of random errors.
Figure 6.
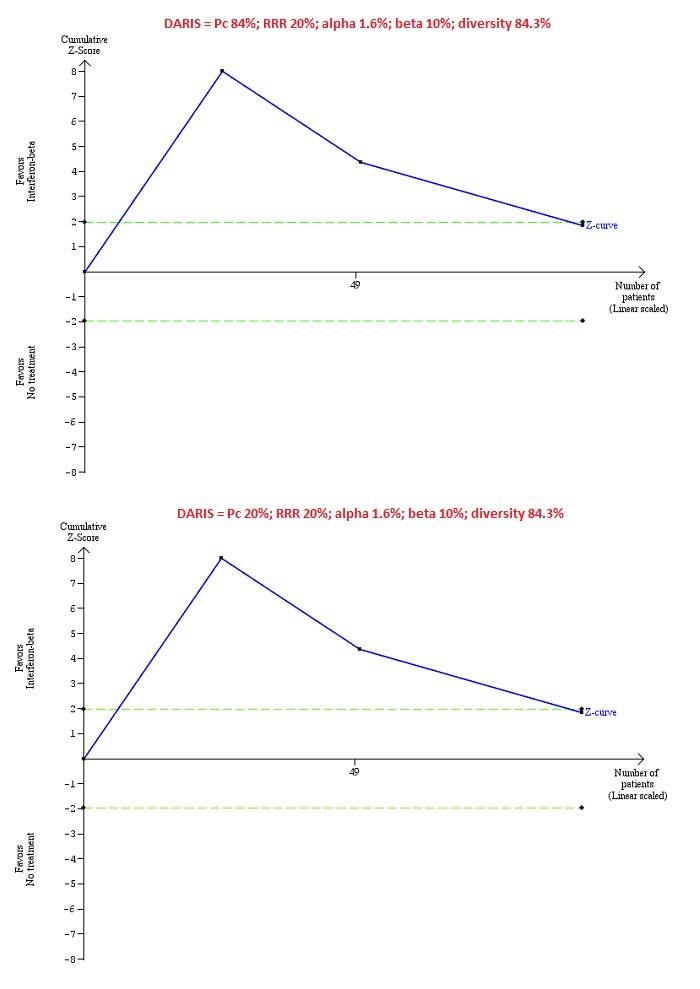
Trial Sequential Analysis of chronic hepatitis C virus for interferon‐beta versus no intervention performed using an alpha error of 1.6%, power of 90% (beta error of 10%), relative risk reduction (RRR) of 20%, control group proportion observed in the trials (Pc = 84%; upper figure) and a Pc of 20% (lower figure), and observed heterogeneity in the trials (84%) shows that the accrued sample size was only a small fraction of the diversity‐adjusted required information size (DARIS); so the trial sequential monitoring boundaries were not drawn. The Z‐curve (blue line) crosses the conventional boundaries (dotted green line). There is a high risk of random errors.
Figure 7.
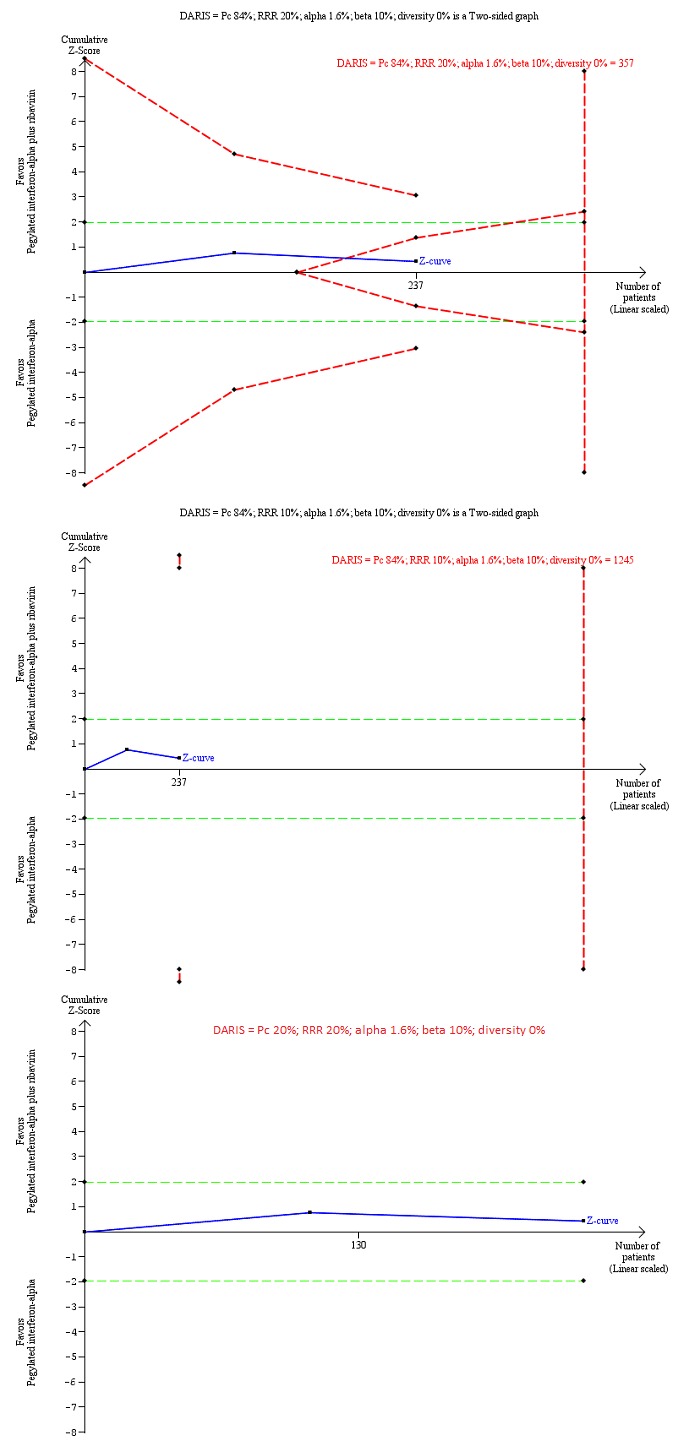
Trial Sequential Analysis of chronic hepatitis C virus infection for pegylated interferon‐alpha plus ribavirin versus pegylated interferon‐alpha performed using an alpha error of 1.6%, power of 90% (beta error of 10%), relative risk reduction (RRR) of 20% (top figure and bottom figure) and 10% (middle figure), control group proportion observed in the trials (Pc = 84%; top figure and middle figure) and Pc = 20% (bottom figure), and observed heterogeneity in the trials (0%) shows that the Z‐curve (blue line) has reached the zone of futility for a RRR of 20% (top figure). However, when a RRR of 10% or when a Pc = 20% was used, the accrued sample size was only a small fraction of the diversity adjusted required information size (DARIS); the Z‐curve (blue line) does not cross the conventional boundaries (dotted green line) or trial sequential monitoring boundaries (dotted red line) (middle figure). For a Pc = 20%, the accrued sample size was so small that the trial sequential monitoring boundaries were not drawn. There is a high risk of random errors.
Serious adverse events: pegylated interferon‐alpha plus ribavirin versus pegylated interferon‐alpha: could not be calculated because of too little information.
Chronic HCV: alpha interferon versus no intervention: 0.23 (95% CI 0.02 to 2.19).
Chronic HCV: alpha interferon versus no intervention: could not be calculated because of too little information.
Chronic HCV: pegylated interferon‐alpha plus ribavirin versus pegylated interferon‐alpha: 0.85 (95% CI 0.31 to 2.34) and relative risk reduction of 10%: 0.85% (95% CI 0.04 to 17.00).
In addition, as a post hoc sensitivity analysis, we used a control group proportion of 20% because the control group proportion observed in the trials was higher than the expected proportion of progression of acute HCV to chronic HCV. This revealed that the accrued sample was so small that none of the trial sequential monitoring boundaries were drawn.
Quality of evidence
None of the trials was at low risk of bias in all the domains. As a result, the quality of evidence was downgraded two levels for risk of bias in the trials in all the comparisons. In addition, the quality of evidence was downgraded one level for imprecision because of the small sample size for all the comparisons. The quality of evidence was downgraded by one more level for imprecision because of wide CIs for all comparisons and one more level for inconsistency because of substantial heterogeneity in magnitude of effect for some comparisons. Overall, the quality of evidence was very low for all comparisons (Table 1; Table 2).
Discussion
Summary of main results
A total of 488 participants in 10 trials were included in this review. A total of 467 participants in nine trials contributed to one or more outcomes. A total of six interventions were evaluated in the nine trials that contributed with analysis data to the review. The interventions included interferon‐alpha, interferon‐beta, MTH‐68/B vaccine, pegylated interferon‐alpha, pegylated interferon‐alpha plus ribavirin, all versus no intervention in the control groups (except pegylated interferon‐alpha plus ribavirin, which was compared with pegylated interferon‐alpha). None of the trials compared direct‐acting antivirals versus placebo or other interventions.
Overall, the mortality at six months to one year following treatment in people with acute HCV infection was very low. Only one of the participants died during this period. There was no evidence of differences in proportion of people with serious adverse events or number of serious adverse events in any of the comparisons. However, it should be noted that 7.0% of the people receiving pegylated interferon‐alpha and 11.5% of the people receiving pegylated interferon‐alpha plus ribavirin developed serious adverse events. The proportion of people with adverse events was higher in the interferon‐alpha and interferon‐beta groups with 87.5% (14/16) and 40% (8/20) of people developing one or more adverse events.
The proportion of people with acute HCV who developed chronic HCV as measured by lack of sustained virological response was approximately 84% in the no intervention group. This appears very high in relation to the conventional wisdom that only about 20% of people develop chronic HCV. This may be due to the definition used for chronic HCV, that is, presence of circulating virus after six months, rather than the presence of active hepatitis, which involves some measure of inflammation such as abnormal transaminases or biopsy which demonstrates inflammation. Only a proportion of people with circulating HCV virus develop chronic inflammation. This may be the reason for the difference in the chronic HCV proportions observed in this review and the conventional wisdom. The proportion of people who developed chronic HCV infection as measured by lack of sustained virological response was less in the interferon‐alpha versus the no intervention groups. However, the Trial Sequential Analysis showed that the Z‐curve did not cross the trial sequential monitoring boundaries indicating that further trials are required to confirm this finding. Long‐term follow‐up and assessment of clinical outcomes such as cirrhosis, decompensated cirrhosis, and requirement for liver transplantation are necessary to confirm that this translates into clinical benefit. However, none of the trials reported these outcomes. So, we are unable to conclude that interferon‐alpha is clinically beneficial.
None of the trials reported health‐related quality of life, and so, we were unable to determine the impact of these drugs on health‐related quality of life.
Overall completeness and applicability of evidence
We have included all the major treatments used for treating acute HCV infection in this review. We found no trials comparing pegylated interferon or direct acting antivirals versus placebo or any other intervention. While we found trials comparing different second‐generation antivirals with each other, we did not find any trials comparing second‐generation antivirals with first‐generation antivirals or any form of interferons, that is, none of the trials comparing different second‐generation antivirals were eligible for this review. One possible reason is the difficulty in distinguishing between acute HCV infection and chronic HCV infection. As most people are asymptomatic after acute HCV infection (Hajarizadeh 2012), the only definitive way of diagnosis of acute HCV infection is to have a baseline sample with absent HCV antibody and HCV RNA followed by presence of HCV antibody or HCV RNA, but it is uncommon in clinical practice (Hajarizadeh 2012). So, the clinicians might consider that the person has chronic HCV infection and treat them accordingly. Another possible reason is that clinicians may feel that the effectiveness of a treatment may not be dependent on whether someone has infection for less than six months (acute HCV infection) or more than six months (chronic HCV infection). So, the clinicians may use the same treatment as for chronic HCV infection. Both these reasons (difficulty in establishing a diagnosis of acute HCV infection and the belief that the treatment effect in people with less than six months of duration of HCV infection is probably the same as that in more than six months of HCV infection) contribute to the very few trials conducted in this field. So, it is not clear whether the findings of this review are applicable in the current clinical setting. If they are applicable, they are applicable only in people who have not undergone liver transplantation and those who do not have other coexisting viral diseases as we excluded such trials.
Quality of the evidence
The overall quality of evidence was very low for all the outcomes. All the trials were at high risk of bias for at least one of the domains, mainly because blinding of participants and healthcare providers was not performed in any of the trials and due to risk of industry bias (Lundh 2017). There were risks of bias in other domains also as shown in Figure 1 and Figure 2. The sample size was small for all the comparisons. There were also wide CIs (the CIs overlapped 20% increase or decrease and no effect) for many of the comparisons. In general, there was no evidence of heterogeneity in most comparisons with the exception of the chronic HCV infection in the comparison interferon‐beta versus no intervention.
Potential biases in the review process
We followed the guidance of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) with two review authors independently selecting trials and extracting data. We performed a thorough search of literature. However, the search period included the premandatory trial registration era and it is possible that some trials on treatments that were not effective or were harmful were not reported at all.
We only included randomised clinical trials which are known to focus mostly on benefits and do not collect and report harms in a detailed manner. According to our choice of studies (i.e. only randomised clinical trials), we might have missed a large number of studies that addressed reporting of harms. Accordingly, this review is biased towards benefits ignoring harms.
We did not search for interventions and trials registered at regulatory authorities (e.g. US Food and Drug Administration, European Medicines Agency, etc.). This may have overlooked trials and as such trials usually are unpublished, the lack of inclusion of such trials may make our comparisons look more advantageous than they really are. However, this is of academic interest only because there is no evidence of benefit of any treatment in people with acute HCV infection, that is, there is no reason to suggest that any of the treatments should be used in routine clinical practice regardless of the adverse event profile of the treatment.
We planned to perform a network meta‐analysis. However, it was not possible to assess whether the potential effect modifiers were similar across different comparisons. Performing a network meta‐analysis in this scenario can be misleading. Therefore, we did not perform the network meta‐analysis, and assessed the comparative benefits and harms of different interventions using standard Cochrane methodology.
Agreements and disagreements with other studies or reviews
We agree with the Cochrane systematic review on interferon therapy of acute HCV that interferons may improve the sustained virological response, but the clinical effects of the interferons are not known (Myers 2001).
Authors' conclusions
Very low quality evidence suggests that interferon‐alpha may decrease the incidence of chronic hepatitis C virus (HCV) infection as measured by lack of sustained virological response. However, the clinical impact (such as improvement in health‐related quality of life, reduction in cirrhosis, decompensated liver disease, and liver transplantation) has not been reported. It is also not clear whether this finding is applicable in the current clinical setting dominated by the use of pegylated interferons and direct‐acting antivirals, although we did not find any evidence to support that pegylated interferons or ribavirin or both, are effective in people with acute HCV infection. We could find no randomised clinical trials comparing direct‐acting antivirals versus placebo or other interventions for acute HCV infection. There is significant uncertainty in the benefits and harms of the interventions, and high‐quality randomised clinical trials are required.
The trial design that is most likely to be accepted by hepatologists will probably involve direct‐acting antivirals and pegylated interferon. Researchers should use clinical outcomes for research on this topic. The ideal trial should include placebo as one of the intervention arms because there has been no evidence from randomised clinical trials that any of the interventions are effective in improving clinical outcomes. However, as many hepatologists and researchers believe that sustained virological response equates to cure of disease (although, this belief has been challenged (Gluud 2014; Koretz 2015)), it may be difficult to recruit into such a trial. The trials should be designed and reported using guidance from the SPIRIT statement (Standard Protocol Items: Recommendations for Interventional Trials; Chan 2013) and the CONSORT statement (Schulz 2010).
Acknowledgements
We thank the Cochrane Comparing of Multiple Interventions Methods Group and the Cochrane Hepato‐Biliary Group for their support and advice. We thank the copy editors for their support.
Cochrane Review Group funding acknowledgement: the Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark.
Disclaimer: the views and opinions expressed in this review are those of the review authors and do not necessarily reflect those of the Danish State or The Copenhagen Trial Unit.
Peer reviewers of review: Goran Poropat, Croatia; Karl Heinz Weiss, Germany. Contact editor: Janus Christian Jakobsen, Denmark. Sign‐off editor: Christian Gluud, Denmark.
Appendices
Appendix 1. Methods for network meta‐analysis if we find this is possible in the future
Measures of treatment effect
Relative treatment effects
For dichotomous variables (e.g. proportion of participants with serious adverse events or any adverse events), we will calculate the odds ratio with 95% credible interval (or Bayesian confidence interval) (Severini 1993). For continuous variables (e.g. quality of life reported on the same scale), we will calculate the mean difference with 95% credible interval. We will use standardised mean difference values with 95% credible interval for quality of life if included trials use different scales. For count outcomes (e.g. number of adverse events and serious adverse events), we will calculate the rate ratio with 95% credible interval. For time‐to‐event data (e.g. mortality at maximal follow‐up), we will calculate hazard ratio with 95% credible interval.
Relative ranking
We will estimate the ranking probabilities for all treatments of being at each possible rank for each intervention. Then, we will obtain the surface under the cumulative ranking curve (SUCRA) (cumulative probability) and rankogram (Salanti 2011; Chaimani 2013).
Unit of analysis issues
We will collect data for all trial treatment groups that meet the inclusion criteria. The codes for analysis, that we will use, accounts for the correlation between the effect sizes from trials with more than two groups.
Assessment of heterogeneity
We will assess clinical and methodological heterogeneity by carefully examining the characteristics and design of included trials. We will assess the presence of clinical heterogeneity by comparing effect estimates under different categories of potential effect modifiers. Different study designs and risk of bias may contribute to methodological heterogeneity.
We will assess statistical heterogeneity by comparing the results of the fixed‐effect model meta‐analysis and the random‐effects model meta‐analysis, between‐study standard deviation (tau2 and comparing this with values reported in the study of the distribution of between‐study heterogeneity (Turner 2012)), and by calculating the I2 statistic (using Stata/SE 14.2). If we identify substantial heterogeneity, clinical, methodological, or statistical, we will explore and address heterogeneity in a subgroup analysis (see 'Subgroup analysis and investigation of heterogeneity for network meta‐analysis' section).
Assessment of transitivity across treatment comparisons
We will evaluate the plausibility of transitivity assumption (the assumption that the participants included in the different studies with different immunosuppressive regimens can be considered to be a part of a multi‐arm randomised clinical trial and could potentially have been randomised to any of the treatments) (Salanti 2012). In other words, any participant that meets the inclusion criteria is, in principle, equally likely to be randomised to any of the above eligible interventions. If there is any concern that the clinical safety and effectiveness are dependent upon the effect modifiers, we will continue to do traditional Cochrane pair‐wise comparisons and we will not perform a network meta‐analysis on all participant subgroups.
Assessment of reporting biases
For the network meta‐analysis, we will judge the reporting bias by the completeness of the search (i.e. searching various databases and including conference abstracts), as we do not currently find any meaningful order to perform a comparison‐adjusted funnel plot as suggested by Chaimani 2012. However, if we find any meaningful order, for example, the control group used depended upon the year of conduct of the trial, we will use comparison‐adjusted funnel plot as suggested by Chaimani 2012.
Data synthesis
Methods for indirect and mixed comparisons
We will conduct network meta‐analyses to compare multiple interventions simultaneously for each of the primary and secondary outcomes. Network meta‐analysis combines direct evidence within trials and indirect evidence across trials (Mills 2012). We will obtain a network plot to ensure that the trials were connected by treatments using Stata/SE 14.2 (Chaimani 2013). We will exclude any trials that were not connected to the network. We will conduct a Bayesian network meta‐analysis using the Markov chain Monte Carlo method in OpenBUGS 3.2.3 as per the guidance from the National Institute for Health and Care Excellence (NICE) Decision Support Unit (DSU) documents (Dias 2014a). We will model the treatment contrast (i.e. log odds ratio for binary outcomes, mean difference or standardised mean difference for continuous outcomes, log rate ratio for count outcomes, and log hazard ratio for time‐to‐event outcomes) for any two interventions ('functional parameters') as a function of comparisons between each individual intervention and an arbitrarily selected reference group ('basic parameters') (Lu 2006) using appropriate likelihood functions and links. We will use binomial likelihood and logit link for binary outcomes, Poisson likelihood and log link for count outcomes, binomial likelihood and complementary log‐log link for time‐to‐event outcomes, and normal likelihood and identity link for continuous outcomes. We will perform a fixed‐effect model and random‐effects model for the network meta‐analysis. We will report both models for comparison with the reference group in a forest plot. For pair‐wise comparison, we will report the fixed‐effect model if the two models reported similar results; otherwise, we will report the more conservative model.
We will use a hierarchical Bayesian model using three different initial values using codes provided by NICE DSU (Dias 2014a). We will use a normal distribution with large variance (10,000) for treatment effect priors (vague or flat priors). For the random‐effects model, we will use a prior distributed uniformly (limits: 0 to 5) for between‐trial standard deviation but assumed similar between‐trial standard deviation across treatment comparisons (Dias 2014a). We will use a 'burn‐in' of 5000 simulations, check for convergence visually, and run the models for another 10,000 simulations to obtain effect estimates. If we did not obtain convergence, we will increase the number of simulations for 'burn‐in'. If we do not obtain convergence still, we will use alternate initial values and priors using methods suggested by van Valkenhoef 2012. We will also estimate the probability that each intervention ranks at one of the possible positions using the NICE DSU codes (Dias 2014a).
Assessment of inconsistency
We will assess inconsistency (statistical evidence of the violation of transitivity assumption) by fitting both an inconsistency model and a consistency model. We will use the inconsistency models used in the NICE DSU manual, as we plan to use a common between‐study deviation for the comparisons (Dias 2014b). In addition, we will use the design‐by‐treatment full interaction model (Higgins 2012) and IF (inconsistency factor) plots (Chaimani 2013) to assess inconsistency. In the presence of inconsistency, we will assess whether the inconsistency is because of clinical or methodological heterogeneity by performing separate analyses for each of the different subgroups mentioned in the 'Subgroup analysis and investigation of heterogeneity for network meta‐analysis' section below.
If there is evidence of inconsistency, we will identify areas in the network where substantial inconsistency might be present in terms of clinical and methodological diversities between trials and, when appropriate, limit network meta‐analysis to a more compatible subset of trials.
Direct comparison
We will perform the direct comparisons using the same codes and the same technical details.
Sample size calculations
To control for the risk of random errors, we will interpret the information with caution when the accrued sample size in the network meta‐analysis (i.e. across all treatment comparisons) was less than the required sample size (required information size). For calculation of the required information size, see Appendix 3.
Subgroup analysis and investigation of heterogeneity for network meta‐analysis
We will assess the differences in the effect estimates between the subgroups listed in 'Subgroup analysis and investigation of heterogeneity' using meta‐regression with the help of the OpenBUGS code (Dias 2012a) if we include a sufficient number of trials. We will use the potential modifiers as study level covariates for meta‐regression. We will calculate a single common interaction term (Dias 2012a). If the 95% credible intervals of the interaction term do not overlap zero, we will consider this as evidence of difference in subgroups.
Presentation of results
We will present the effect estimates with 95% CrI for each pair‐wise comparisons calculated from the direct comparisons and network meta‐analysis. We will also present the cumulative probability of the treatment ranks (i.e. the probability that the treatment is within the top two, the probability that the treatment is within the top three, etc.) in graphs (surface under the cumulative ranking curve or SUCRA) (Salanti 2011). We will also plot the probability that each treatment is best, second best, third best etc. for each of the different outcomes (rankograms), which are generally considered more informative (Salanti 2011; Dias 2012b).
We will present the 'Summary of findings' tables for mortality. In the 'Table 1', we will follow the approach suggested by Puhan and colleagues (Puhan 2014). First, we will calculate the direct and indirect effect estimates and 95% credible intervals using the node‐splitting approach (Dias 2010), i.e. calculate the direct estimate for each comparison by including only trials in which there was direct comparison of treatments and the indirect estimate for each comparison by excluding the trials in which there was direct comparison of treatments. Then we will rate the quality of direct and indirect effect estimates using GRADE which takes into account the risk of bias, inconsistency, directness of evidence, imprecision, and publication bias (Guyatt 2011). Then, we will present the estimates of the network meta‐analysis and rate the quality of network meta‐analysis effect estimates as the best quality of evidence between the direct and indirect estimates (Puhan 2014). In addition, in the same table, we will present illustrations and information on the number of trials and participants as per the standard 'Summary of Findings' Table.
Appendix 2. Search strategies
| Database | Time span | Search strategy |
| The Central Register of Controlled Trials (CENTRAL) (Wiley) | Issue 4,2016. | #1 MeSH descriptor: [Hepatitis C] explode all trees #2 "hepatitis C" or HCV #3 #1 or #2 #4 acute #5 #3 and #4 |
| MEDLINE (OvidSP) | January 1947 to April 2016. | 1. exp hepatitis C/ 2. ("hepatitis C" or HCV).ti,ab. 3. 1 or 2 4. acute.ti,ab. 5. 3 and 4 6. randomized controlled trial.pt. 7. controlled clinical trial.pt. 8. randomized.ab. 9. placebo.ab. 10. drug therapy.fs. 11. randomly.ab. 12. trial.ab. 13. groups.ab. 14. 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 15. exp animals/ not humans.sh. 16. 14 not 15 17. 5 and 16 |
| Embase (OvidSP) | January 1974 to April 2016. | 1. exp hepatitis C/ 2. ("hepatitis C" or HCV).ti,ab. 3. 1 or 2 4. acute.ti,ab. 5. 3 and 4 6. exp crossover‐procedure/ or exp double‐blind procedure/ or exp randomized controlled trial/ or single‐blind procedure/ 7. (((((random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or double*) adj blind*) or single*) adj blind*) or assign* or allocat* or volunteer*).af. 8. 6 or 7 9. 5 and 8 |
| Science Citation Index Expanded (Web of Knowledge) | January 1945 to April 2016. | #1 TS=("hepatitis C" or HCV) #2 TS=(acute) #3 TS=(random* OR rct* OR crossover OR masked OR blind* OR placebo* OR meta‐analysis OR systematic review* OR meta‐analys*) #4 #1 AND #2 AND #3 |
| World Health Organization International Clinical Trials Registry Platform Search Portal (apps.who.int/trialsearch/Default.aspx) | April 2016. | Condition: "acute hepatitis C" |
| ClinicalTrials.gov | April 2016. | Interventional Studies | "acute hepatitis C" | Phase 2, 3, 4 |
Appendix 3. Sample size calculation
The five‐year mortality in people with chronic HCV infection is about 20% (Seeff 2001). The main aim of treatment for acute HCV infection is to prevent its progress to chronic HCV infection and its complications. Approximately 20% of people with acute HCV infection progress to chronic HCV infection (expert opinion). Based on this, we estimate the five‐year mortality in people with acute HCV infection to be 4% (20% proportion of acute HCV infection to chronic HCV infection × 20% five‐year mortality rate in people with chronic HCV infection). The required information size based on a control group proportion of 4%, a relative risk reduction of 20% in the intervention group, type I error of 5%, and type II error of 20% is 17,026 participants. Network analyses are more prone to the risk of random errors than direct comparisons (Del Re 2013). Accordingly, a greater sample size is required in indirect comparisons than direct comparisons (Thorlund 2012). The power and precision in indirect comparisons depend upon various factors, such as the number of participants included under each comparison and the heterogeneity between the trials (Thorlund 2012). If there is no heterogeneity across the trials, the sample size in indirect comparisons would be equivalent to the sample size in direct comparisons. The effective indirect sample size can be calculated using the number of participants included in each direct comparison (Thorlund 2012). For example, a sample size of 2500 participants in the direct comparison A versus C (nAC) and a sample size of 7500 participants in the direct comparison B versus C (nBC) results in an effective indirect sample size of 1876 participants. However, in the presence of heterogeneity within the comparisons, the sample size required is higher. In the above scenario, for an I2 statistic for each of the comparisons A versus C (IAC2) and B versus C (IBC2) of 25%, the effective indirect sample size is 1407 participants. For an I2 statistic for each of the comparisons A versus C and B versus C of 50%, the effective indirect sample size is 938 participants (Thorlund 2012). If there were only three groups and the sample size in the trials is more than the required information size, we will calculate the effective indirect sample size using the following generic formula (Thorlund 2012):
((nAC × (1 ‐ IAC2)) × (nBC × (1 ‐ IBC2))/((nAC × (1 ‐ IAC2)) + (nBC × (1 ‐ IBC2)).
There is currently no method to calculate the effective indirect sample size for a network analysis involving more than three intervention groups.
Data and analyses
Comparison 1.
Intervention versus control
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serious adverse events (proportion) | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Interferon‐beta versus no intervention | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 MTH‐68/B vaccine versus no intervention | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Pegylated interferon‐alpha plus ribavirin versus pegylated interferon‐alpha | 2 | 237 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.70, 4.21] |
| 2 Serious adverse events (number) | 4 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 Interferon‐beta versus no intervention | 1 | 40 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 MTH‐68/B vaccine versus no intervention | 1 | 41 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Pegylated interferon‐alpha plus ribavirin versus pegylated interferon‐alpha | 2 | 237 | Rate Ratio (Fixed, 95% CI) | 2.74 [1.40, 5.33] |
| 3 Adverse events (proportion) | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Interferon‐alpha versus no intervention | 1 | 33 | Odds Ratio (M‐H, Fixed, 95% CI) | 203.0 [9.01, 4574.81] |
| 3.2 Interferon‐beta versus no intervention | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 27.88 [1.48, 526.12] |
| 3.3 MTH‐68/B vaccine versus no intervention | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Adverse events (number) | 2 | Odds Ratio (Fixed, 95% CI) | Subtotals only | |
| 4.1 Interferon‐beta versus no intervention | 1 | 40 | Odds Ratio (Fixed, 95% CI) | 17.00 [0.98, 294.53] |
| 4.2 MTH‐68/B vaccine versus no intervention | 1 | 41 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Chronic HCV infection | 9 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Interferon‐alpha versus no intervention | 3 | 99 | Odds Ratio (M‐H, Random, 95% CI) | 0.27 [0.09, 0.76] |
| 5.2 Interferon‐beta versus no intervention | 3 | 90 | Odds Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.24] |
| 5.3 MTH‐68/B vaccine versus no intervention | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.05, 1.65] |
| 5.4 Pegylated interferon‐alpha plus ribavirin versus pegylated interferon‐alpha | 2 | 237 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.41, 1.79] |
What's new
| Date | Event | Description |
|---|---|---|
| 12 April 2017 | Amended | The Cochrane Central Editorial Unit requested removal of the 'attempted network meta‐analysis' phrase from the end of the review title, as this further description of the review might create confusion in the reader. Although we followed the planned methodology for network meta‐analysis, it was not possible to assess whether the potential effect modifiers were similar across different comparisons. Therefore, we did not perform the network meta‐analysis and instead assessed the comparative benefits and harms of different interventions versus each other or versus no intervention using standard Cochrane methodology. |
Differences between protocol and review
It was not possible to assess whether the potential effect modifiers were similar across different comparisons. Therefore, we did not perform the network meta‐analysis, and we assessed the comparative benefits and harms of different interventions using standard Cochrane methodology. The methodology that we plan to use if we conduct a network meta‐analysis in the future is available in Appendix 1.
We performed Trial Sequential Analysis in addition to conventional methods of assessing the risk of random errors using P values.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised clinical trial. | |
| Participants | Country: Italy. Number randomised: 40. Post‐randomisation dropouts: 0 (0%). Revised sample size: 40. Mean age: 29 years. Females: 6 (15%). Genotype 1: not stated. Genotype 3: not stated. Other genotypes: not stated. Mean follow‐up period in months (for all groups): 22.5. Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: interferon‐beta (n = 20). Further details: interferon‐beta 3 MU IM once daily for 5 days then 3 times per day for 3 more weeks. Group 2: no intervention (n = 20). Duration of treatment: 1 month. |
|
| Outcomes | Sustained virological response. Biochemical response. Severity and frequency of adverse events. |
|
| Notes | 6 participants were not randomised for logistical issues and they were allocated to the immediate treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomized, computer generated according to the author's reply." |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: "different treatments" (author replies). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: "different treatments" (author replies). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Low risk | Quote: "Italian NHS". Comment: according to the author's reply. |
| Other bias | Low risk | Comment: no other risk of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Hungary, USA. Number randomised: 41. Post‐randomisation dropouts: 0 (0%). Revised sample size: 41. Mean age: not stated. Females: not stated. Genotype 1: not stated. Genotype 3: not stated. Other genotypes: not stated. Mean follow‐up period in months (for all groups): 12. Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: MTH‐68/B (n = 22). Further details: MTH‐68/B (live attenuated infectious bursal disease virus (IBDV)) 4000 U/day for 1 week, then 3 times per week for 2 weeks, then once monthly for 6 months. Group 2: no intervention (n = 19). Duration of treatment: 6 months |
|
| Outcomes | Sustained virological response. Frequency of adverse events. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated to two groups." Comment: further details not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no use of placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Germany. Number randomised: 107. Post‐randomisation dropouts: 0 (0%). Revised sample size: 107. Mean age: 39 years. Females: 46 (43%). Genotype 1: 73 (68.2%). Genotype 3: 18 (16.8%). Other genotypes: 10 (9.3%). Mean follow‐up period in months (for all groups): 6. Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: immediate pegylated interferon‐alpha‐2b (n = 55). Further details: immediate pegylated interferon‐alfa‐2b 1.5 μg/kg. Group 2: delayed pegylated interferon‐alfa‐2b (n = 52). Further details: delayed pegylated interferon‐alfa‐2b 1.5 μg/kg + ribavirin > 10.6 mg/kg. Duration of treatment: 6 months. |
|
| Outcomes | Sustained virological response. Biochemical response. Severity and frequency of adverse events. Analysis of responses to the respective treatment approaches according to severity of symptoms. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised via a web‐based randomisation service provided by the Hep‐Net Study House." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "We used stratified block randomisation with block sizes of eight, independent across strata." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This investigator‐initiated study was designed as an open‐label, phase 3, multicentre study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcome assessors blind according to the author reply. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | High risk | Quote: "The study was supported by the German Network of Competence on Viral Hepatitis (Hep‐Net, funded by the Federal Ministry of Education and Research). The study was also supported by a research grant from Essex Pharma, Schering‐Plough, and MSD. MSD provided study drugs and financial support." |
| Other bias | Low risk | Comment: no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Spain. Number randomised: 28. Post‐randomisation dropouts: 0 (0%). Revised sample size: 28. Mean age: not stated. Females: not stated. Genotype 1: not stated. Genotype 3: not stated. Other genotypes: not stated. Mean follow‐up period in months (for all groups): 12 months. Inclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: interferon‐alpha‐2b (n = 15). Further details: interferon‐alpha‐2b 3 MU 3 times per week. Group 2: no treatment (n = 13). Duration of treatment: 3 months. |
|
| Outcomes | Sustained virological response. Biochemical response. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A randomised, controlled trial was undertaken." Comment: further details not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Twenty eight patients with acute HCV (15 treated and 13 controls) were included in the trial." Comment: placebo not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: some important outcomes which would generally be assessed were not reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: China. Number randomised: 33. Post‐randomisation dropouts: 0 (0%). Revised sample size: 33. Mean age: 54 years. Females: 9 (27.3%). Genotype 1: not stated. Genotype 3: not stated. Other genotypes: not stated. Mean follow‐up period in months (for all groups): 12. Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: interferon‐alpha‐2b (n = 16). Further details: interferon‐alpha‐2b 3 MU 3 times per week. Group 2: no treatment (n = 17). Duration of treatment: 3 months. |
|
| Outcomes | Sustained virological response. Frequency of adverse events. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "These patients were randomly allocated to either the IFN‐treated group or the control group by a random number table." |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Seventeen patients in the control group received no specific treatment." Comment: placebo not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Only one patient in the control group was unwilling to continue and withdrew from the study after 6 months of follow up." Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: all important outcomes were reported. |
| For‐profit bias | High risk | Quote: "This study was supported by grants from the National Science Council (NSC82‐0419‐B075‐092) and National Health Research Institutes (DOH‐83‐HR‐208), Republic of China. Drug supplied by pharm. company." |
| Other bias | Low risk | Comment: no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Italy. Number randomised: 41. Post‐randomisation dropouts: 3 (7.3%). Revised sample size: 38. Mean age: 47 years. Females: 19 (50%). Genotype 1: not stated. Genotype 3: not stated. Other genotypes: not stated. Mean follow‐up period in months (for all groups): 18. Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: interferon‐alpha‐2b (n = 22). Further details: interferon‐alpha‐2b 3 MU IM 3 times per week. Group 2: no treatment (n = 16). Duration of treatment: 3 months. |
|
| Outcomes | Sustained virological response. Biochemical response. Mortality at the end of follow‐up (18 months). |
|
| Notes | Reasons for post‐randomisation dropouts: of the 48 participants enrolled in the study, 1 refused therapy and 2 untreated people were lost to follow‐up during month 1. 7 participants (16% of total) were thought to have been infected with a non‐A, non‐B, non‐C agent. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomisation was computerised according to the author's reply. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: multicentre, prospective, open, randomised study comparing interferon treatment and no treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: some important outcomes which would generally be assessed were not reported. |
| For‐profit bias | High risk | Quote: "We thank Dr. Paola Mazzanti and Dr. Cristina Pintus (Schering‐Plough) for their assistance." |
| Other bias | Low risk | Comment: there was no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Japan. Number randomised: 27. Post‐randomisation dropouts: 2 (7.4%). Revised sample size: 25. Mean age: 40 years. Females: 14 (56%). Genotype 1: not stated. Genotype 3: not stated. Other genotypes: not stated. Mean follow‐up period in months (for all groups): 36. Inclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: interferon‐beta (n = 11). Further details: interferon‐beta, 3 MU IV for 5 consecutive days in the first week, and then 3 times per week for the next 3 weeks. Group 2: no treatment (n = 14). Duration of treatment: 1 month. |
|
| Outcomes | Sustained virological response. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "25 patients with acute non‐A, non‐B hepatitis drew lots for allocation to treatment with interferon." |
| Allocation concealment (selection bias) | Low risk | Quote: "25 patients with acute non‐A, non‐B hepatitis drew lots for allocation to treatment with interferon." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: placebo not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "2 patients in the untreated group were lost to follow‐up." Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: some important outcomes which would generally be assessed were not reported. |
| For‐profit bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: there was no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Japan. Number randomised: 25. Post‐randomisation dropouts: 0 (0%). Revised sample size: 25. Mean age: 39 years. Females: 14 (56%). Genotype 1: not stated. Genotype 3: not stated. Other genotypes: not stated. Mean follow‐up period in months (for all groups): 36. Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: interferon‐beta (n = 11). Further details: interferon‐beta 3 MU IV for 5 days, then 3 times per week for 3 weeks. Group 2: no treatment (n = 14). Duration of treatment: 1 month. |
|
| Outcomes | Sustained virological response. Fluctuation of ALT concentrations. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "25 patients randomly assigned to." Comment: further details not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no use of placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: some important outcomes which would generally be assessed were not reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: there was no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Italy. Number randomised: 130. Post‐randomisation dropouts: 0 (0%). Revised sample size: 130. Mean age: 34 years. Females: 41 (31.5%). Genotype 1: 53 (40.8%). Genotype 3: 32 (24.6%). Other genotypes: 45 (34.6%). Mean follow‐up period in months (for all groups): 12. Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 3 groups. Group 1: pegylated interferon‐alpha‐2b (n = 44). Further details: pegylated interferon‐alpha‐2b 1.5 µg/kg/week (24 weeks). Group 2: pegylated interferon‐alpha‐2b (n = 43). Further details: pegylated interferon‐alpha‐2b 1.5 µg/kg/week (12 weeks). Group 3: pegylated interferon‐alpha‐2b plus ribavirin (n = 43). Further details: pegylated interferon‐alpha‐2b 1.5 µg/kg/week + ribavirin 10.6 mg/kg/day orally (12 weeks). Duration of treatment: 12 to 24 weeks (see above). |
|
| Outcomes | Sustained virological response. Virological responses after 2 weeks of treatment (very rapid virological response), after 4 weeks of treatment (rapid virological response), at the end of treatment (end‐of‐treatment virological response), and at 12 months' post‐treatment follow‐up (long‐term virological response). ALT level normalisation at the end of treatment and at 6 and 12 months' post‐treatment follow‐up. Safety (adverse events). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization list was generated centrally by an independent biostatistician using the Proc Plan of the SAS system (version 9.2; SAS Institute Inc., Cary, NC) and consisted of a computer‐generated treatment allocation list in blocks of 9 patients each." |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: different treatments, no placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "ITT analysis, Patients who discontinued the study for any reason before the 6‐month follow‐up visit were considered as nonresponders." Comment: low for sustained virological response, high for 1‐year mortality because there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: adverse events not clearly reported. |
| For‐profit bias | High risk | Quote: "The study sponsor for drug supply and financial support was Schering‐Plough (now Merck) SpA, Milan, Italy." |
| Other bias | Low risk | Comment: there was no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Number randomised: 21. Post‐randomisation dropouts: 0 (0%). Revised sample size: 21. Mean age: not stated. Females: not stated. Genotype 1: 13 (61.9%). Genotype 3: not stated. Other genotypes: not stated. Mean follow‐up period in months (for all groups): 6. Inclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 groups. Group 1: pegylated interferon‐alpha (n = 9). Further details: pegylated interferon‐alpha (no further details of treatment regimen). Group 2: no treatment (n = 12). Duration of treatment: 6 months. |
|
| Outcomes | Sustained virological response was reported but was not reported in sufficient details to include for analysis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: information not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: information not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: some important outcomes which would generally be assessed were not reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: there was no other bias. |
ALT: alanine transaminase; DNA: deoxy ribonucleic acid; HAV: hepatitis A virus; HBV: hepatitis B virus; HCV: hepatitis C virus; HCV‐RNA: hepatitis C virus ribonucleic acid; IM: intramuscular; ITT: intention‐to‐treat analysis; IU: international units; IV: intravenous; MU: million units.
Contributions of authors
MK and EB extracted data and provided them in a format that could be analysed. DT, ET, and BD critically commented on the review. KG wrote the review. All review authors agreed on this version before publication.
Sources of support
Internal sources
University College London, UK.
External sources
-
National Institute for Health Research, UK.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure, Cochrane Programme Grant, or Cochrane Incentive funding to the Cochrane Hepato‐Biliary and Upper Gastrointestinal and Pancreatic Diseases Groups. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, the National Health Service, or the Department of Health. The funding covered salary, equipment, and other resources to complete this review.
Declarations of interest
This report is independent research funded by the National Institute for Health Research (NIHR Cochrane Programme Grants, 13/89/03 ‐ evidence‐based diagnosis and management of upper digestive, hepato‐biliary, and pancreatic disorders). The views expressed in this publication are those of the review authors and not necessarily those of the National Health Service (NHS), the NIHR, or the Department of Health.
MK: no financial disclosures. EB: no financial disclosures. DT: Astellas funded Douglas Thorburn for his attendance at the International Liver Transplantation Society meeting in 2014. Douglas Thorburn has also received GBP 25,000 from Boston Scientific to fund a clinical research fellow in 2013. There are no other financial disclosures to report. BD: no financial disclosures. ET: no financial disclosures. KG: no financial disclosures.
Notes
Considerable overlap is evident in the 'Methods' sections of this review and those of several other reviews written by the same group of authors.
Edited (no change to conclusions)
References
References to studies included in this review
- Calleri G, Colombatto P, Gozzelino M, Chieppa F, Romano P, Delmastro B, et al. Natural beta interferon in acute type‐C hepatitis patients: a randomized controlled trial. Italian Journal of Gastroenterology and Hepatology 1998;30(2):181‐4. [PubMed] [Google Scholar]
- Csatary LK, Telegdy L, Gergely P, Bodey B, Bakacs T. Preliminary report of a controlled trial of MTH‐68/B virus vaccine treatment in acute B and C hepatitis: a phase II study. Anticancer Research 1998;18(2B):1279‐82. [PubMed] [Google Scholar]
- Deterding K, Gruner N, Buggisch P, Wiegand J, Gale PR, Spengler U, et al. Delayed versus immediate treatment for patients with acute hepatitis C: a randomised controlled non‐inferiority trial. Lancet Infectious Diseases 2013;13(6):497‐506. [DOI] [PubMed] [Google Scholar]
- Genesca J. Interferon alfa in acute posttransfusion hepatitis C: a randomized, controlled trial. Gastroenterology 1992;103(5):1702‐3. [PubMed] [Google Scholar]; Genesca J, Esteban JI, Quer J, Viladomiu LL, Gonzalez A, Esteban R, et al. Hepatitis C virus markers in patients with acute post‐transfusion hepatitis treated with interferon alfa‐2b. Gut 1993;34(2 Suppl):S62‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Lee SD, Chan CY, Lu RH, Lo KJ. A randomized controlled trial of recombinant interferon alpha‐2b in the treatment of Chinese patients with acute post‐transfusion hepatitis C. Journal of Hepatology 1994;21(5):831‐6. [DOI] [PubMed] [Google Scholar]; Hwang SJ, Lee SD, Lee YH, Wu JC, Chan CY, Huang YS, et al. A randomized controlled clinical trial of recombinant interferon alpha‐2b in the treatment of acute post‐transfusion hepatitis C: a preliminary report. Journal of Gastroenterology and Hepatology 1993;8:S92‐8. [Google Scholar]
- Lampertico P, Rumi M, Romeo R, Craxi A, Soffredini R, Biassoni D, et al. A multicenter randomized controlled trial of recombinant interferon‐alpha 2b in patients with acute transfusion‐associated hepatitis C. Hepatology 1994;19(1):19‐22. [PubMed] [Google Scholar]; Lampertico P, Rumi MG, De FF, Romeo R, Soffredini R, Biassoni D, et al. Treatment of acute post‐transfusional non‐a, non‐b hepatitis with recombinant interferon alpha‐2b. A multicenter randomized controlled trial. Argomenti Gastroenterologia Clinica 1994;7(3):111‐5. [Google Scholar]
- Omata M, Yokosuka O, Takano S, Kato N, Hosoda K, Imazeki F, et al. Resolution of acute hepatitis C after therapy with natural beta interferon. Lancet 1991;338(8772):914‐5. [DOI] [PubMed] [Google Scholar]
- Omata M, Takano S. A randomized, controlled trial of interferon‐beta treatment for acute hepatitis C. Proceedings of the international symposium on viral hepatitis and liver Disease. 1st Edition. Tokyo: Springer‐Verlag, 1994:601‐3. [Google Scholar]
- Santantonio T, Fasano M, Sagnelli E, Tundo P, Babudieri S, Fabris P, et al. Acute hepatitis C: a 24‐week course of pegylated interferon alpha‐2b versus a 12‐week course of pegylated interferon alpha‐2b alone or with ribavirin. Hepatology 2014;59(6):2101‐9. [DOI] [PubMed] [Google Scholar]
- Wang C, Cook L, Krows M, Ng K, Hough E, Thiede H. Randomized trial of pegylated interferon for the treatment of acute HCV in Seattle injection drug users. Hepatology 2005;42(4 Suppl 1):673a. [Google Scholar]
Additional references
- American Association for the Study of Liver Diseases, Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C, 2014. www.hcvguidelines.org/fullreport (accessed 12 October 2014). [DOI] [PMC free article] [PubMed]
- Anonymous. Interferon production by genetic engineering. British Medical Journal (Clinical Research Ed.) 1981;282(6265):674‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailon P, Palleroni A, Schaffer CA, Spence CL, Fung WJ, Porter JE, et al. Rational design of a potent, long‐lasting form of interferon: a 40 kDa branched polyethylene glycol‐conjugated interferon alpha‐2a for the treatment of hepatitis C. Bioconjugate Chemistry 2001;12(2):195‐202. [DOI] [PubMed] [Google Scholar]
- Beinhardt S, Aberle JH, Strasser M, Dulic‐Lakovic E, Maieron A, Kreil A, et al. Serum level of IP‐10 increases predictive value of IL28B polymorphisms for spontaneous clearance of acute HCV infection. Gastroenterology 2012;142(1):78‐85. [DOI] [PubMed] [Google Scholar]
- Beltrami EM, Williams IT, Shapiro CN, Chamberland ME. Risk and management of blood‐borne infections in health care workers. Clinical Microbiology Reviews 2000;13(3):385‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunchorntavakul C, Jones LM, Kikuchi M, Valiga ME, Kaplan DE, Nunes FA, et al. Distinct features in natural history and outcomes of acute hepatitis C. Journal of Clinical Gastroenterology 2015;40(4):e31‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaimani A, Salanti G. Using network meta‐analysis to evaluate the existence of small‐study effects in a network of interventions. Research Synthesis Methods 2012;3(2):161‐76. [DOI] [PubMed] [Google Scholar]
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta‐analysis in STATA. PLoS One 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza‐Jeric K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158(3):200‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coilly A, Roche B, Dumortier J, Leroy V, Botta‐Fridlund D, Radenne S, et al. Safety and efficacy of protease inhibitors to treat hepatitis C after liver transplantation, a multicenter experience. Journal of Hepatology 2014;60(1):78‐86. [DOI] [PubMed] [Google Scholar]
- D'Amico G, Garcia‐Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. Journal of Hepatology 2006;44(1):217‐31. [DOI] [PubMed] [Google Scholar]
- Re AC, Spielmans GI, Flückiger C, Wampold BE. Efficacy of new generation antidepressants: differences seem illusory. PLoS One 2013;8(6):e63509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Statistics in Medicine 2010;29(7‐8):932‐44. [DOI] [PubMed] [Google Scholar]
- Dias S, Sutton AJ, Welton NJ, Ades AE. NICE DSU Technical Support Document 3: heterogeneity: subgroups, meta‐regression, bias and bias‐adjustment, September 2011 (last updated April 2012). www.nicedsu.org.uk/TSD3%20Heterogeneity.final%20report.08.05.12.pdf (accessed 27 March 2014).
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 1: introduction to evidence synthesis for decision making, April 2011 (last updated April 2012). www.nicedsu.org.uk/TSD1%20Introduction.final.08.05.12.pdf (accessed 27 March 2014).
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: a generalised linear modelling framework for pairwise and network meta‐analysis of randomised controlled trials, August 2011 (last updated April 2014). www.nicedsu.org.uk/TSD2%20General%20meta%20analysis%20corrected%2015April2014.pdf (accessed 8 October 2014). [PubMed]
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU Technical Support Document 4: inconsistency in networks of evidence based on randomised controlled trials, May 2011 (last updated April 2014). www.nicedsu.org.uk/TSD4%20Inconsistency.final.15April2014.pdf (accessed 8 October 2014). [PubMed]
- European Association for Study of Liver. EASL clinical practice guidelines: management of hepatitis C virus infection. Journal of Hepatology 2014;60(2):392‐420. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury AC, Wallace C, Klimack WK, Razavi H. Economic burden of hepatitis C‐associated diseases: Europe, Asia Pacific, and the Americas. Journal of Medical Economics 2012;15(5):887‐96. [DOI] [PubMed] [Google Scholar]
- EuroQol. About EQ‐5D, 2014. www.euroqol.org/about‐eq‐5d.html (accessed 8 October 2014).
- Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature 2005;436(7053):967‐72. [DOI] [PubMed] [Google Scholar]
- Fierer DS, Dieterich DT, Mullen MP, Branch AD, Uriel AJ, Carriero DC, et al. Telaprevir in the treatment of acute hepatitis C virus infection in HIV‐infected men. Clinical Infectious Diseases 2014;58(6):873‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusyo N, Ogawa E, Nakamuta M, Kajiwara E, Nomura H, Dohmen K, et al. Telaprevir can be successfully and safely used to treat older patients with genotype 1b chronic hepatitis C. Journal of Hepatology 2013;59(2):205‐12. [DOI] [PubMed] [Google Scholar]
- Global Burden of Hepatitis C Working Group. Global burden of disease (GBD) for hepatitis C. Journal of Clinical Pharmacology 2004;44(1):20‐9. [DOI] [PubMed] [Google Scholar]
- Gluud C, Koretz R, Gurusamy K. Hepatitis C: a new direction, but an old story?. Lancet (London, England)2014; Vol. 383, issue 9935:2122‐3. [PUBMED: 24953469] [DOI] [PubMed]
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2015, Issue 1. Art. No.: LIVER. [PubMed]
- Grebely J, Matthews GV, Dore GJ. Treatment of acute HCV infection. Nature Reviews. Gastroenterology & Hepatology 2011;8(5):265‐74. [DOI] [PubMed] [Google Scholar]
- Grebely J, Page K, Sacks‐Davis R, Loeff MS, Rice TM, Bruneau J, et al. The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology 2014;59(1):109‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
- Hajarizadeh B, Grebely J, Dore GJ. Case definitions for acute hepatitis C virus infection: a systematic review. Journal of Hepatology 2012;57(6):1349‐60. [DOI] [PubMed] [Google Scholar]
- Hezode C, Fontaine H, Dorival C, Larrey D, Zoulim F, Canva V, et al. Triple therapy in treatment‐experienced patients with HCV‐cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20‐CUPIC) ‐ NCT01514890. Journal of Hepatology 2013;59(3):434‐41. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta‐analysis: concepts and models for multi‐arm studies. Research Synthesis Methods 2012;3(2):98‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, Pennsylvania: Barnett International/PAREXEL, 1997. [Google Scholar]
- Jafari S, Copes R, Baharlou S, Etminan M, Buxton J. Tattooing and the risk of transmission of hepatitis C: a systematic review and meta‐analysis. International Journal of Infectious Diseases 2010;14(11):e928‐40. [DOI] [PubMed] [Google Scholar]
- Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta‐analytic methods. BMC Medical Research Methodology 2014;14(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal SM. Acute hepatitis C: a systematic review. American Journal of Gastroenterology 2008;103(5):1283‐97. [DOI] [PubMed] [Google Scholar]
- Kenny‐Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti‐D immune globulin. Irish Hepatology Research Group. New England Journal of Medicine 1999;340(16):1228‐33. [DOI] [PubMed] [Google Scholar]
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
- Kleinman SH, Lelie N, Busch MP. Infectivity of human immunodeficiency virus‐1, hepatitis C virus, and hepatitis B virus and risk of transmission by transfusion. Transfusion 2009;49(11):2454‐89. [DOI] [PubMed] [Google Scholar]
- Koretz RL, Lin KW, Ioannidis JP, Lenzer J. Is widespread screening for hepatitis C justified?. BMJ (Clinical Research Ed.) 2015;350:g7809. [DOI] [PubMed] [Google Scholar]
- Lavanchy D. Evolving epidemiology of hepatitis C virus. Clinical Microbiology and Infection 2011;17(2):107‐15. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Meyer MF, Monazahian M, Tillmann HL, Manns MP, Wedemeyer H. High rate of spontaneous clearance of acute hepatitis C virus genotype 3 infection. Journal of Medical Virology 2004;73(3):387‐91. [DOI] [PubMed] [Google Scholar]
- Lewis H, Cunningham M, Foster G. Second generation direct antivirals and the way to interferon‐free regimens in chronic HCV. Best Practice & Research Clinical Gastroenterology 2012;26(4):471‐85. [DOI] [PubMed] [Google Scholar]
- Loomba R, Rivera MM, McBurney R, Park Y, Haynes‐Williams V, Rehermann B, et al. The natural history of acute hepatitis C: clinical presentation, laboratory findings and treatment outcomes. Alimentary Pharmacology & Therapeutics 2011;33(5):559‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. Journal of the American Statistical Association 2006;101(474):447‐59. [Google Scholar]
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Ray S, Thuluvath PJ. Acute hepatitis C. Lancet 2008;372(9635):321‐32. [DOI] [PubMed] [Google Scholar]
- Sweetman S, editor. Martindale: the complete drug reference (online version), 37th edition, 2011. www.pharmpress.com/product/MC_MART/martindale‐the‐complete‐drug‐reference (accessed 23 September 2014).
- Mills EJ, Ioannidis JP, Thorlund K, Schünemann HJ, Puhan MA, Guyatt GH. How to use an article reporting a multiple treatment comparison meta‐analysis. JAMA 2012;308(12):1246‐53. [DOI] [PubMed] [Google Scholar]
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
- Myers RP, Regimbeau C, Thevenot T, Leroy V, Mathurin P, Opolon P, et al. Interferon for acute hepatitis C. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD000369] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCBI. Hepatitis C, 2014. www.ncbi.nlm.nih.gov/mesh/68006526 (accessed 11 October 2014).
- NCBI. Interferons, 2014. www.ncbi.nlm.nih.gov/mesh/68007372 (accessed 11 October 2014).
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
- Members of OpenBUGS Project Management Group. OpenBUGS. Version 3.2.3. Members of OpenBUGS Project Management Group, 2014.
- Perry CM. Telaprevir: a review of its use in the management of genotype 1 chronic hepatitis C. Drugs 2012;72(5):619‐41. [DOI] [PubMed] [Google Scholar]
- Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, et al. ABT‐450/r‐ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. New England Journal of Medicine 2014;370(21):1973‐82. [DOI] [PubMed] [Google Scholar]
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997;349(9055):825‐32. [DOI] [PubMed] [Google Scholar]
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello‐Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis. BMJ (Clinical Research Ed.) 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Rodger AJ, Roberts S, Lanigan A, Bowden S, Brown T, Crofts N. Assessment of long‐term outcomes of community‐acquired hepatitis C infection in a cohort with sera stored from 1971 to 1975. Hepatology 2000;32(3):582‐7. [DOI] [PubMed] [Google Scholar]
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163‐71. [DOI] [PubMed] [Google Scholar]
- Salanti G. Indirect and mixed‐treatment comparison, network, or multiple‐treatments meta‐analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80‐97. [DOI] [PubMed] [Google Scholar]
- Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, et al. The natural history of compensated cirrhosis due to hepatitis C virus: a 17‐year cohort study of 214 patients. Hepatology 2006;43(6):1303‐10. [DOI] [PubMed] [Google Scholar]
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeff LB, Hollinger FB, Alter HJ, Wright EC, Cain CM, Buskell ZJ, et al. Long‐term mortality and morbidity of transfusion‐associated non‐A, non‐B, and type C hepatitis: a National Heart, Lung, and Blood Institute collaborative study. Hepatology 2001;33(2):455‐63. [DOI] [PubMed] [Google Scholar]
- Seeff LB. The history of the "natural history" of hepatitis C (1968‐2009). Liver International 2009;29(Suppl 1):89‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severini TA. Bayesian interval estimates which are also confidence intervals. Journal of the Royal Statistical Society. Series B (Methodological) 1993;55(2):533‐40. [Google Scholar]
- Singal AK, Hmoud BS, Guturu P, Kuo YF. Outcome after liver transplantation for cirrhosis due to alcohol and hepatitis C: comparison to alcoholic cirrhosis and hepatitis C cirrhosis. Journal of Clinical Gastroenterology 2013;47(8):727‐33. [DOI] [PubMed] [Google Scholar]
- Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology 2014;59(1):318‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp LP. Stata/SE 14.2 for Windows[64‐bit x86‐64]. Version 14. College Station: StataCorp LP, 2017.
- Syriopoulou V, Nikolopoulou G, Daikos GL, Theodoridou M, Pavlopoulou I, Nicolaidou P, et al. Mother to child transmission of hepatitis C virus: rate of infection and risk factors. Scandinavian Journal of Infectious Diseases 2005;37(5):350‐3. [DOI] [PubMed] [Google Scholar]
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 30 November 2016).
- Thorlund K, Mills EJ. Sample size and power considerations in network meta‐analysis. Systematic Reviews 2012;1:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohme RA, Holmberg SD. Is sexual contact a major mode of hepatitis C virus transmission?. Hepatology 2010;52(4):1497‐505. [DOI] [PubMed] [Google Scholar]
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. Version 0.9 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta‐analysis, using empirical data from the Cochrane Database of Systematic Reviews. International Journal of Epidemiology 2012;41(3):818‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Ramprasad V, Hollenbeak CS, Bezinover D, Kadry Z. Liver transplantation for hepatitis C from donation after cardiac death donors: an analysis of OPTN/UNOS data. American Journal of Transplantation 2012;12(4):984‐91. [DOI] [PubMed] [Google Scholar]
- Valkenhoef G, Lu G, Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta‐analysis. Research Synthesis Methods 2012;3(4):285‐99. [DOI] [PubMed] [Google Scholar]
- Ware JE. SF‐36® health survey update, 2014. www.sf‐36.org/tools/sf36.shtml (accessed on 8 October 2014).
- Wawrzynowicz‐Syczewska M, Kubicka J, Lewandowski Z, Boron‐Kaczmarska A, Radkowski M. Natural history of acute symptomatic hepatitis type C. Infection 2004;32(3):138‐43. [DOI] [PubMed] [Google Scholar]
- Welsch C, Jesudian A, Zeuzem S, Jacobson I. New direct‐acting antiviral agents for the treatment of hepatitis C virus infection and perspectives. Gut 2012;61(Suppl 1):i36‐46. [DOI] [PubMed] [Google Scholar]
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
- Wiese M, Grungreiff K, Guthoff W, Lafrenz M, Oesen U, Porst H. Outcome in a hepatitis C (genotype 1b) single source outbreak in Germany ‐ a 25‐year multicenter study. Journal of Hepatology 2005;43(4):590‐8. [DOI] [PubMed] [Google Scholar]
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Luo J, Bai J, Yu R. Epidemiology of hepatitis C virus infection among injection drug users in China: systematic review and meta‐analysis. Public Health 2008;122(10):990‐1003. [DOI] [PubMed] [Google Scholar]
- Yang LS, Shan LL, Saxena A, Morris DL. Liver transplantation: a systematic review of long‐term quality of life. Liver International 2014;34(9):1298‐313. [DOI] [PubMed] [Google Scholar]


