Abstract
Background
Eplerenone is an aldosterone receptor blocker that is chemically derived from spironolactone. In Canada, it is indicated for use as adjunctive therapy to reduce mortality for heart failure patients with New York Heart Association (NYHA) class II systolic chronic heart failure and left ventricular systolic dysfunction. It is also used as adjunctive therapy for patients with heart failure following myocardial infarction. Additionally, it is indicated for the treatment of mild and moderate essential hypertension for patients who cannot be treated adequately with other agents. It is important to determine the clinical impact of all antihypertensive medications, including aldosterone antagonists, to support their continued use in essential hypertension. No previous systematic reviews have evaluated the effect of eplerenone on cardiovascular morbidity, mortality, and magnitude of blood pressure lowering in patients with hypertension.
Objectives
To assess the effects of eplerenone monotherapy versus placebo for primary hypertension in adults. Outcomes of interest were all‐cause mortality, cardiovascular events (fatal or non‐fatal myocardial infarction), cerebrovascular events (fatal or non fatal strokes), adverse events or withdrawals due to adverse events, and systolic and diastolic blood pressure.
Search methods
We searched the Cochrane Hypertension Specialised Register, CENTRAL, MEDLINE, Embase, and two trials registers up to 3 March 2016. We handsearched references from retrieved studies to identify any studies missed in the initial search. We also searched for unpublished data by contacting the corresponding authors of the included studies and pharmaceutical companies involved in conducting studies on eplerenone monotherapy in primary hypertension. The search had no language restrictions.
Selection criteria
We selected randomized placebo‐controlled trials studying adult patients with primary hypertension. We excluded studies in people with secondary or gestational hypertension and studies where participants were receiving multiple antihypertensives.
Data collection and analysis
Three review authors independently reviewed the search results for studies meeting our criteria. Three review authors independently extracted data and assessed trial quality using a standardized data extraction form. A fourth independent review author resolved discrepancies or disagreements. We performed data extraction and synthesis using a standardized format on Covidence. We conducted data analysis using Review Manager 5.
Main results
A total of 1437 adult patients participated in the five randomized parallel group studies, with treatment durations ranging from 8 to 16 weeks. The daily doses of eplerenone ranged from 25 mg to 400 mg daily. Meta‐analysis of these studies showed a reduction in systolic blood pressure of 9.21 mmHg (95% CI −11.08 to −7.34; I2 = 58%) and a reduction of diastolic pressure of 4.18 mmHg (95% CI −5.03 to −3.33; I2 = 0%) (moderate quality evidence).
There may be a dose response effect for eplerenone in the reduction in systolic blood pressure at doses of 400 mg/day. However, this finding is uncertain, as it is based on a single included study with low quality evidence. Overall there does not appear to be a clinically important dose response in lowering systolic or diastolic blood pressure at eplerenone doses of 50 mg to 400 mg daily. There did not appear to be any differences in the number of patients who withdrew due to adverse events or the number of patients with at least one adverse event in the eplerenone group compared to placebo. However, only three of the five included studies reported adverse events. Most of the included studies were of moderate quality, as we judged multiple domains as being at unclear risk in the 'Risk of bias' assessment.
Authors' conclusions
Eplerenone 50 to 200 mg/day lowers blood pressure in people with primary hypertension by 9.21 mmHg systolic and 4.18 mmHg diastolic compared to placebo, with no difference of effect between doses of 50 mg/day to 200 mg/day. A dose of 25 mg/day did not produce a statistically significant reduction in systolic or diastolic blood pressure and there is insufficient evidence for doses above 200 mg/day. There is currently no available evidence to determine the effect of eplerenone on clinically meaningful outcomes such as mortality or morbidity in hypertensive patients. The evidence available on side effects is insufficient and of low quality, which makes it impossible to draw conclusions about potential harm associated with eplerenone treatment in hypertensive patients.
Plain language summary
Eplerenone for high blood pressure
Review question
The aim of this review was to determine the effectiveness of eplerenone for reducing blood pressure, its side effect profile, and its impact on clinically meaningful outcomes such as mortality and morbidity.
Background
Clinicians have used eplerenone to treat high blood pressure since 2002. It is important to determine the clinical impact of all antihypertensive medications used in patients to support their continued use in essential hypertension. We searched multiple databases and found five eligible studies in 1437 people who received either eplerenone or no medication in a random fashion.
Study characteristics
The doses of eplerenone used in these studies ranged from 25 mg to 400 mg daily. These studies followed patients for 8 to 16 weeks while on therapy. None of the studies reported on the clinically meaningful outcomes of eplerenone, such as whether eplerenone can reduce heart attacks, stroke, or death compared to placebo. Only three of the five studies reported on side effects.
Key results
There is currently no evidence that eplerenone has a beneficial effect on life expectancy or complications rleated to hypertension (e.g. heart attack, stroke). Evidence for risk of side effects with eplerenone is limited and of poor quality; it is difficult to tell the extent of possible harm with eplerenone versus placebo. This meta‐analysis shows that eplerenone 50 to 200 mg/day reduces systolic blood pressure by approximately 9 mmHg and diastolic blood pressure by 4 mmHg compared to taking no medication.
Quality of the evidence
We judged the five included trials to be of moderate quality, as authors did not extensively describe portions of their methodology.
Summary of findings
for the main comparison.
| Eplerenone compared with placebo for primary hypertension | |||
|
Patient or population: adults with primary hypertension Settings: primary care Intervention: eplerenone (25‐400 mg) Comparison: placebo | |||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) |
|
Effect on systolic blood pressure (mean difference in systolic blood pressure) |
(MD −9.21 mmHg, 95% CI −11.08 to −7.34) | 1437 (5) | ⊕⊕⊕⊝ Moderatea |
|
Effect on diastolic blood pressure (mean difference in diastolic blood pressure) |
(MD −4.18 mmHg, 95% CI −5.03 to −3.33) | 1437 (5) | ⊕⊕⊕⊝ Moderatea |
| Any adverse event | OR 1.07 (0.82 to 1.41) | 1105 (3) |
⊕⊕⊝⊝ Lowa,b |
| Adverse event leading to withdrawal | OR 1.10 (0.47 to 2.55) | 1105 (3) |
⊕⊕⊝⊝ Lowa,b |
| CI: confidence interval; OR: odds ratio. | |||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||
aDowngraded due to high risk of bias in many of the studies. bDowngraded as only 3 of the included 5 trials provided data.
Background
Description of the condition
Hypertension is associated with structural changes in the heart and blood vessels, which can lead to cardiovascular mortality and morbidity (e.g. cardiovascular disease, stroke, peripheral vascular disease, and renal disease). Hypertension is generally defined as having a systolic blood pressure (SBP) of 140 mmHg or more, a diastolic blood pressure (DBP) of 90 mmHg or more, or both (Arguedas 2009; CHEP 2015). For every 20 mmHg increase in SBP and 10 mmHg increase in DBP (through the range of 115/75 to 185/115 mmHg) in people aged 40 to 70 years, the risk of cardiovascular disease‐related morbidity doubles (JNC 7). Epidemiologic studies have shown increased blood pressure to be associated with increased incidence of stroke, ischemic heart disease, and other vascular mortality (Lewington 2002). While blood pressure is a surrogate goal of therapy for the prevention of hypertension‐associated target‐organ damage (DiPiro 2014), no research has convincingly shown that lowering blood pressure below the target value of 140/90 mmHg reduces cardiovascular morbidity and mortality (Arguedas 2009). At present, there is no definitive threshold to predict the blood pressure above which treatment provides more benefit than harm (Arguedas 2009). In very elderly individuals aged 75 years or older, there is no established association between SBP and all‐cause mortality (Banach 2014). Interestingly, there is some evidence that blood pressure values below 140/70 mmHg are associated with excess mortality in individuals aged 85 and over (Van Bemmel 2006). Current recommendations on target blood pressures are mostly based on expert opinion and cannot be generalized to all age groups (NCGC 2011). This variability underscores the importance of finding safe and effective antihypertensive agents that demonstrate a proven benefit for improving hard clinical outcomes (morbidity and mortality), rather than chasing arbitrary blood pressure values.
Description of the intervention
Eplerenone is an aldosterone receptor blocker that is chemically derived from spironolactone. In a recent Cochrane Review, spironolactone, considered fourth‐line therapy for hypertension in patients already treated with multiple medications, was shown to reduce systolic/diastolic blood pressure by approximately 20/7 mmHg compared to placebo. However, the review found no evidence on the effect of spironolactone on clinical outcomes in hypertensive patients (Batterink 2010). This review attempts to define the effect of eplerenone, another aldosterone antagonist, on hard clinical outcomes in hypertensive patients.
Compared to spironolactone, eplerenone exhibits less affinity for androgen, progesterone, and glucocorticoid receptors (Katzung 2012). As a result, many authors consider that it reduces the risk of gynaecomastia, menstrual irregularities, and sexual dysfunction. Eplerenone undergoes extensive hepatic metabolism, primarily through the enzyme cytochrome P450 3A4 (CYP3A4) to inactive metabolites, so clinicians should avoid co‐administration of potent CYP3A4 inhibitors (Muldowney 2009). Eplerenone itself neither induces nor inhibits CYP3A4 and does not alter the pharmacokinetics of co‐administered substrates or inducers of CYP3A4, highly protein‐bound drugs, or highly renally cleared drugs. As a result, dosage adjustments of other drugs are generally not necessary. Less than 5% of the administered dose is cleared unchanged in the urine and faeces. Current data suggest that adverse effects are generally rare and mild with eplerenone therapy. These include hyperkalemia, dizziness, elevation in serum creatinine, diarrhea, cough, fatigue, dyslipidemia, abdominal pain, and albuminuria (Muldowney 2009). Hyperkalemia is the most significant adverse effect of eplerenone and may result in fatal cardiac arrhythmias if serum potassium levels are inadequately monitored. Patients with declining renal function are at greater risk for hyperkalemia. Evidence also suggests that diabetic patients with proteinuria may be at increased risk of high serum potassium levels. No data is currently available for the use of eplerenone in patients with severe hepatic dysfunction, although those with mild to moderate impairment (Child‐Pugh class B) do not require dosage adjustments (eCPS 2014).
Eplerenone is contraindicated in people with known hypersensitivity to the drug or its excipients, clinically significant hyperkalemia or serum potassium of more than 5.0 mmol/L at initiation of treatment, severe hepatic dysfunction (Child‐Pugh class C), moderate to severe renal dysfunction (eGFR < 50 mL/min/1.73 m2), type 2 diabetes with microalbuminuria, and in people taking potassium‐sparing diuretics, potassium supplements, or potent CYP3A4 inhibitors (eCPS 2014).
In Canada, eplerenone is indicated for use as adjunctive therapy to reduce mortality for heart failure patients with New York Heart Association (NYHA) class II systolic chronic heart failure and left ventricular systolic dysfunction, and as adjunctive therapy for patients with heart failure following myocardial infarction. Additionally, it is indicated for treating mild and moderate essential hypertension in patients who cannot be treated adequately with other agents (eCPS 2014). The Canadian Hypertension Education Program (CHEP) recommends using aldosterone antagonists as adjuncts for patients with systolic dysfunction (ejection fraction < 40%) and recent cardiovascular hospitalization, acute myocardial infarction, elevated brain‐type natriuretic peptide (BNP) or N‐terminal pro b‐type BNP (NT‐proBNP) level, or NYHA class II to IV symptoms (CHEP 2015). The American Society of Hypertension recommends the addition of aldosterone antagonists when standard three‐drug regimens (angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker/calcium channel blocker/diuretic) are ineffective in treatment‐resistant patients (Weber 2014).
How the intervention might work
The renin‐angiotensin‐aldosterone system (RAAS) plays a key role in the pathophysiology of hypertension. Aldosterone increases blood pressure by inducing sodium reabsorption, vascular remodeling, and endothelial dysfunction, and possibly through other genomic and non‐genomic effects (eCPS 2014). The role of aldosterone itself has been established not only in hypertension secondary to hyperaldosteronism, but also in primary, or essential, hypertension. There is also some evidence that non‐hypertensive patients with high‐normal aldosterone levels are at increased risk for developing elevated blood pressure (Jansen 2009). Eplerenone is an aldosterone antagonist and competes with aldosterone for binding to the mineralocorticoid receptor. In the late distal and cortical collecting tubules of the kidney, this antagonism will lead to decreased activation of sodium channels and decreased numbers of sodium/potassium ATPase pumps. The net effect is diuresis: increased sodium and, as a consequence, water excretion (Katzung 2012).
Aldosterone antagonists may also have a role in treatment‐resistant hypertension. Non‐responders to typical antihypertensive therapies may benefit from a trial of an aldosterone antagonist (Weber 2014). Prolonged treatment with angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), which initially lower serum aldosterone levels, may result in a rebound increase in serum aldosterone to higher than pre‐treatment levels. This is referred to as 'aldosterone escape' or 'aldosterone breakthrough' and could be a key mechanism in resistance to ACE inhibitors and ARBs (Jansen 2009).
Aldosterone has also been implicated in blood pressure‐independent adverse events, including vascular inflammation and cardiac and perivascular fibrosis, which may promote end‐organ damage. Evidence suggests that patients with hyperaldosteronism may be at increased risk for stroke, non‐fatal myocardial infarction, atrial fibrillation, elevated left ventricular mass, and arterial wall stiffness versus matched controls with essential hypertension (Jansen 2009). Use of an aldosterone antagonist may thus reduce these pathologies in at‐risk patients.
Why it is important to do this review
Hypertension can be difficult to manage. Patients may be intolerant to antihypertensive medications, and many require multiple classes of medications to control their blood pressure. More importantly, lowering blood pressure below 140/90 mmHg is not a validated surrogate outcome for clinically significant endpoints such as mortality and morbidity. It is important to determine the clinical impact of all antihypertensive medications, including aldosterone antagonists, to support their continued use in essential hypertension. Previous systematic reviews have not evaluated the effect of eplerenone on cardiovascular morbidity, mortality, and magnitude of blood pressure lowering in people with hypertension (Wright 2009).
Objectives
To assess the effects of eplerenone monotherapy versus placebo for primary hypertension in adults. Outcomes of interest were all‐cause mortality, cardiovascular events (fatal or non‐fatal myocardial infarction), cerebrovascular events (fatal or non fatal strokes), adverse events or withdrawals due to adverse events, and systolic and diastolic blood pressure.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials that compare oral eplerenone monotherapy to placebo.
Types of participants
Trials including participants of both sexes older than 18 years of age with primary hypertension defined by SBP greater than 140 mmHg, DBP greater than 90 mmHg, or both, with no known secondary cause for the high blood pressure. We included trials involving participants with and without co‐morbidities.
Types of interventions
The intervention of interest was oral eplerenone monotherapy (at any dose). The comparative intervention was placebo.
Types of outcome measures
Primary outcomes
All‐cause mortality
Cardiovascular mortality
Non‐cardiovascular mortality
Number of patients experiencing at least one serious adverse event
Fatal and non‐fatal, disabling stroke
Fatal and non‐fatal myocardial infarction
Secondary outcomes
Number of patients with at least one adverse event
Number of patients who withdrew due to adverse events
-
Change blood pressure
Change in systolic blood pressure
Change in diastolic blood pressure
Search methods for identification of studies
Electronic searches
We searched the following databases for primary studies.
Cochrane Hypertension Specialised Register (searched 3 March 2016).
Cochrane Register of Controlled Trials (CENTRAL; 2016, Issue 3) via the Cochrane Register of Studies Online (searched 3 March 2016).
MEDLINE Ovid (1946 to 3 March 2016).
Embase Ovid (1974 to 3 March 2016).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 3 May 2016).
The Cochrane Hypertension Specialised Register includes controlled trials from searches of CENTRAL, MEDLINE, Embase, CAB Abstracts, CINAHL, Food Science and Technology Abstracts (FSTA), Global Health, LILACS,ProQuest Dissertations & Theses, PsycINFO, the Web of Science, and the WHO International Clinical Trials Registry Platform (ICTRP).
We searched electronic databases using a strategy combining the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision) with selected medical subject headings (MeSH) and free text words. We used no language restrictions and translated the MEDLINE search strategy into the other databases using the appropriate controlled vocabulary as applicable. We present full strategies in Appendix 1.
Searching other resources
We also tried to collect information from the following additional resources.
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch).
Reference lists of all papers and relevant reviews identified.
Authors of relevant papers, regarding any further published or unpublished work.
Authors of trials reporting incomplete information, to obtain the missing information.
ISI Web of Science for papers that cite studies included in the review.
European Medicines Agency Database.
United States Food and Drug Administration (FDA) Database.
Manufacturer of eplerenone.
Database of Abstracts of Reviews of Effectiveness (DARE), for related reviews.
Data collection and analysis
Selection of studies
Three review authors (SM, TT, MW) independently assessed all identified studies to determine whether they met the predefined inclusion criteria. We reviewed all references identified in the search that mentioned the use of eplerenone and included them if they met the criteria. We documented reasons for excluding studies and resolved any differences regarding inclusion through discussion with a fourth independent review author.
Data extraction and management
Once we identified all studies, three independent review authors examined those that fulfilled the inclusion criteria in detail. We used a web‐based systematic review program, Covidence, to create a standardized data extraction form.
Assessment of risk of bias in included studies
We assessed the following parameters.
Method of random sequence generation.
Method of allocation concealment.
Blinding to treatment allocation (including healthcare provider, assessor, and patient).
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Funding of clinical trial.
We resolved any differences in interpretation of the data through consensus. If additional information was required, we contacted the original authors of the study and then reassessed the study once the missing information was available, where possible. Three review authors independently collected study characteristics and the outcome measures of interest using a pre‐formulated data extraction sheet on Covidence. We collected all data, regardless of compliance or completion of follow‐up, in order to allow for intention‐to‐treat analysis.
Measures of treatment effect
For evaluation of the primary outcomes (all‐cause mortality, cardiovascular events, and cerebrovascular events), we planned to record the total number of patients with at least one event within each trial, calculating proportions for these dichotomous outcomes and presenting comparisons between groups as relative risk ratios (RRs) with corresponding 95% confidence intervals (CIs).
We planned to combine data for blood pressure reduction using a weighted mean difference method. This combines a weight based on the number of individuals in the trial and the within‐study variance. If the trial did not report the within‐study variance for decrease in blood pressure, we imputed the standard deviation (SD) from the average SD from the other trials.
We first performed all analyses using a fixed‐effect model.
Unit of analysis issues
We used data from all patients individually randomized to each intervention in the analyses, taking care to identify situations in which studies had censored/excluded data and to differentiate data presented as the total number of events or the total number of patients with a first event. We contacted authors for clarification if necessary. We intended to calculate proportions for the first relevant event that occurred for each patient randomized to a particular intervention.
Dealing with missing data
In general if there were missing data, we contacted the authors of the study for clarification and documented this process. If we could not obtain the SD of the change in blood pressure, we imputed the value (using SD of the change data from other similar trials).
Assessment of heterogeneity
We assessed heterogeneity across the studies using the I2 statistic (defining important heterogeneity at a threshold of 30% to 60%) and the Chi2 statistic (with statistical significance set at P < 0.10). Where we did not detect heterogeneity, we used a fixed‐effect model. Otherwise, we used a random‐effects model to determine if the effects of eplerenone changed depending on the analysis model. More importantly, we planned to explore clinical and methodological sources of heterogeneity, considering characteristics like: baseline risk factors for the outcomes of interest, duration of studies, age, race, and sex distribution of participants across the studies. Based on the exploration of sources of heterogeneity, we made decisions about the appropriateness of meta‐analyzing data.
Assessment of reporting biases
In the event that we assumed that missing data represented a poor outcome, or where we imputed data, we planned to carry out sensitivity analyses to see if results were sensitive to the assumptions being made. However, since we did not find any clinical outcome data, this type of imputation was not necessary.
Data synthesis
We used Cochrane Review Manager 5 (RevMan 5) software , for all data analyses. We based quantitative analyses of outcomes on intention‐to‐treat results. We used RRs and the fixed‐effect or random‐effects model (depending on heterogeneity results) to combine outcomes across trials. We calculated absolute risk reduction (ARR) as risk difference × 100, and the numbers needed to treat for an additional beneficial outcome (NNTB) as 1/risk difference for all dichotomous outcomes. We pooled data for blood pressure reduction using a weighted mean difference method with standard deviations.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following four subgroup analyses.
Trials of less than 6 months' duration, of 6 to 12 months' duration, and of more than 12 months' duration.
Effect of ethnic group on blood pressure and adverse events.
Effect of age on blood pressure and adverse events.
Effect of dose on blood pressure and adverse events.
Sensitivity analysis
We planned to carry out sensitivity analyses in order to test for the robustness of the results. We intended to analyze the following categories separately.
Trials without proper randomization compared to those with proper randomization.
Trials performed without proper allocation concealment versus those with proper allocation concealment.
Unblinded versus blinded trials.
Inclusion versus exclusion of data from trials where we imputed blood pressure standard deviations.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The search strategy identified 307 citations in CENTRAL, MEDLINE, and Embase. Following a review of their titles and abstracts, we excluded 229 citations that obviously did not meet our inclusion criteria and selected 78 studies for further review. We reviewed the full text of these 78 studies and excluded 73 that did not meet our inclusion criteria.
Of the six citations that met our inclusion criteria, one proved to be a duplicate publication, leaving five unique trials that met our inclusion criteria. See Figure 1.
1.

Study flow diagram.
Included studies
Refer to Characteristics of included studies for further details regarding the studies.
All five included studies were parallel‐group, randomized controlled trials. Together, they involved a total of 1437 participants, who received treatment for 8 to 16 weeks. The daily doses of eplerenone ranged from 25 mg to 400 mg. Participants' average age was 54 years, and there were slightly more men than women (64%). The mean baseline blood pressure (BP) in the eplerenone group was 153/101 mmHg, compared to 152/100 mmHg in the placebo group.
Excluded studies
See: Characteristics of excluded studies.
We excluded 73 trials for the following reasons.
Not a randomized controlled trial (k = 32).
Did not study patients with essential hypertension (k = 16).
Treatment arm was not eplerenone monotherapy (k = 9).
Compared eplerenone with other antihypertensive agents (k = 7).
Did not study any of our primary or secondary outcomes of interest (k = 5).
Terminated study on clinicaltrials.gov; efforts to contact the responsible party were fruitless in obtaining usable information for the meta‐analysis (k = 1, NCT01373086).
Completed study on clinicaltrials.gov but with no published results; efforts to contact the responsible party were fruitless in obtaining usable information for the meta‐analysis (k = 1, NCT02345044).
Unpublished trial not providing enough information to be used in the analysis (k = 1, Trial 015 1999); found through the FDA medical reviews on the approval of eplerenone.
Risk of bias in included studies
We assessed the risk of bias on the basis of five major criteria: allocation concealment, blinding, completeness of outcome data addressed, presence of selective reporting, and other potential sources of bias (see Figure 2). Three review authors (SM, TT, MW) independently performed the 'Risk of bias' assessment on Covidence, resolving any differences in interpretation of the data through consensus with a fourth review author (AT). If additional information was required, we contacted the original author of the study and reassessed the study in light of the availability of missing information. We summarize our contact with the original authors of the five included studies in Figure 3.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Summary of data extraction and contact with corresponding authors
Allocation
Calhoun 2011 reported performing allocation by generating randomization sequences using an interactive voice‐response system provider. A validated system automated the random assignment of participant numbers to randomization numbers, linking them to the different treatment groups and the medication numbers on treatment packs. Study drugs were identical in packaging, labeling, schedule of administration, appearance, taste, and odor in order to conceal the nature of the treatment.
Flack 2003 and Saruta 2004 did not describe methods of allocation concealment; the trial reports noted that the order in which participants received eplerenone and placebo was randomized; however, authors did not describe the method for randomization or respond to our attempts to contact them.
Weinberger 2002 allocated all participants at the same time as randomization after the run‐in period by using a randomized computer‐generated schedule for sequence generation.
White 2003 stated that the study coordinator performed allocation concealment via an interactive voice response system. Through email contact, the primary author of White 2003 clarified details on the randomization of the participants, which investigators accomplished using standard multicenter block randomization techniques.
Blinding
Flack 2003, Saruta 2004, and Weinberger 2002 described blinding methods poorly, stating that they conducted double‐blind trials but without describing how blinding was maintained beyond use of a placebo pill. Thus, the information available for assessing the appropriateness of blinding in these studies was inadequate.
Only two of the five studies adequately described blinding of participants and outcome assessors (Calhoun 2011; White 2003). Calhoun 2011 stated that participants, investigators, outcome assessors, and data analysts remained blinded to treatment assignments. Moreover, study drugs were identical in packaging, labeling, schedule of administration, appearance, taste, and odor in order to conceal the nature of the treatment.
Additional correspondence with White 2003 served to clarify details on the blinding of the study participants and outcome assessors. White 2003 stated both study participants and site staff were blinded to treatment assignment. The study medication was in bottles of unidentified investigational product and was sent from a central location, so the outcome assessors had no way of knowing the randomization medication or code.
Incomplete outcome data
Only one of the five included studies adequately reported how they dealt with incomplete outcome data (Saruta 2004). In this case, Saruta 2004 excluded one participant in the eplerenone 50 mg group from the efficacy analysis due to withdrawal of consent. The results in a group of 49 participants are unlikely to be affected by missing data from 1 participant.
However, there was a high risk of bias in Flack 2003. Four participants in the placebo and 8 participants in the eplerenone group had no post baseline assessment and were not included in the efficacy analysis. In addition, 41% of participants withdrew from the placebo group, compared to 26% in the eplerenone group, and authors did not describe details of imputing missing values using the last observation carried forward method. The high rates of withdrawal in the placebo group may cause smaller mean changes in blood pressure and thus exaggerate the effects seen in the eplerenone group.
The remaining three included studies were at unclear risk of bias for incomplete outcome data reporting. In White 2003, there was insufficient reporting of incomplete outcome data. Other than treatment failure, the authors did not specify the other reasons for withdrawals in each treatment group and how they accounted for missing data in the primary outcomes. There was not enough information to assess the risk of bias due to incomplete outcome data.
In Weinberger 2002, eight participants were not included in the efficacy analyses. In addition, 39 participants did not complete the study. The authors of Weinberger 2002 did not specify how withdrawals were distributed across groups or how authors dealt with missing data.
In Calhoun 2011, the percentage of withdrawals from treatment groups ranged from 6.8% to 13.0%. The authors did not thoroughly document reasons for withdrawal but noted that they conducted all efficacy analyses with the full analysis set and imputed missing measurements by carrying forward the last available observation. However, there was not enough available information to assess the risk of bias from the missing data.
Selective reporting
In most of the included studies, there did not seem be selective reporting of outcomes (Calhoun 2011; Flack 2003; Saruta 2004).
Calhoun 2011 reported all outcomes specified in their protocol registered on ClinicalTrials.gov. Flack 2003 reported all primary study outcomes but failed to report some secondary endpoints and serious adverse events. Weinberger 2002 and White 2003 reported all outcomes specified in the Methods section. However, there was no protocol available to assess the a priori design.
It is worth noting that none of the included studies reported clinically meaningful outcomes (i.e. any of our primary outcomes) related to hypertension. Thus, there may be a high risk of selective reporting based on the omission of outcomes that should inform clinical practice and be collected in clinical trials involving human participants (e.g. all‐cause mortality, serious adverse events).
Other potential sources of bias
Four of the five included studies received industry funding. Pharmacia Corporation, which Pfizer purchased in 2002, financed Weinberger 2002, and Pfizer Inc funded Saruta 2004. Novartis funded Calhoun 2011, which academic authors and Novartis Parma designed together. Novartis was also responsible for collecting data from investigational sites to create the clinical database for the data analysis. As per the additional information provided by the corresponding author, Pharmacia Corporation sponsored White 2003.
Flack 2003 did not report the source of funding.
Effects of interventions
See: Table 1
See: Table 1.
Primary outcomes
Unfortunately, none of the included studies reported results for the following clinical outcomes: all‐cause mortality, cardiovascular mortality, non‐cardiovascular mortality, serious adverse events, fatal and non‐fatal myocardial infarction, or fatal and non‐fatal stroke.
Secondary outcomes
Number of participants with at least one adverse event
There was no difference in the incidence of participants experiencing any adverse event in three trials(Flack 2003; Weinberger 2002; White 2003; Analysis 1.1; Figure 4). However, we could not stratify these results based on dose, and the potential harms associated with increased daily doses of eplerenone are uncertain. We did not specifically assess rates of hyperkalemia in this review but will do so in future updates.
1.1. Analysis.

Comparison 1 Eplerenone monotherapy vs placebo, Outcome 1 Any adverse event.
4.

Forest plot of comparison: 29 Eplerenone Monotherapy vs Placebo, outcome: Any Adverse Event.
Number of participants who withdrew due to adverse events
There was no difference in the incidence of adverse events leading to discontinuation in the eplerenone group compared to placebo (Flack 2003; Weinberger 2002; White 2003; Analysis 1.2).
1.2. Analysis.

Comparison 1 Eplerenone monotherapy vs placebo, Outcome 2 Adverse event leading to withdrawal.
Change in blood pressure
Four studies reported mean systolic and diastolic blood pressures from participants who were seated (Calhoun 2011; Saruta 2004; Weinberger 2002; White 2003). Flack 2003 measured participants blood pressure while standing/supine, which could have raised/lowered the reading with respect to the other studies. Ideally, all studies would have reported blood pressures measured in the same position.
White 2003 studied four different daily doses of eplerenone (25 mg, 50 mg, 100 mg, and 200 mg). We used the mean change from baseline in seated blood pressure in our analysis, and we extracted data from Figure 1 in the published study report. The authors did not specify whether the error bars presented in Figure 1 in the published study report were standard deviations or standard errors. Using graphing methods, we estimated the variances in Figure 1 based on the pictorial error bars. Comparing these variances with the other studies, we assumed they were standard errors, as the numbers were similar. We felt this to be an appropriate estimate, as authors reported changes from baseline in daytime and nighttime blood pressures in Table 2 in the published study report as standard errors with similar numbers. When inputting our data for analysis, we distributed the sample size of the placebo group equally among the four different treatment groups.
Weinberger 2002 studied three different daily doses of eplerenone (50 mg, 100 mg, and 400 mg). The authors reported the change from baseline in systolic and diastolic blood pressure in seated position in Figure 1 in the published study report. Using the same assumptions and methods described in White 2003, we estimated the variance numbers from the error bars in Figure 1 in the published study report and assumed them to be standard errors in our calculations. Again, we distributed the sample size of the placebo group equally among the three different treatment groups when inputting our data for analysis.
Saruta 2004 studied three different daily doses of eplerenone (50 mg, 100 mg, and 200 mg). The mean change in cuff seated systolic and diastolic blood pressure from baseline was extracted from Figure 1 in the published study report. We estimated the variance as we did in White 2003 and Weinberger 2002. We felt this to be an appropriate estimate, as adjusted mean change in pulse pressure, presented in Table 2 in the published study report, showed variance with similar numbers and were reported as standard errors. Similarly to White 2003 and Weinberger 2002, we distributed the sample size of the placebo group equally among the three different treatment groups when inputting our data for analysis.
Flack 2003 reported mean changes in systolic and diastolic blood pressure but did not specify the position or method of blood pressure monitoring. Flack 2003 studied eplerenone 50 mg/day against placebo. We used the data presented in Table 3 in the published study report of the mean changes in SBP and DBP for placebo and eplerenone for all participants. Calhoun 2011 reported mean changes in sitting systolic and diastolic blood pressures, comparing the daily dose of 100 mg against placebo. Using the data presented in Figure 3 in the published study report, we used graphing methods in the bar graph to estimate standard errors. We did not need to distribute the sample size of the placebo group in Flack 2003 and Calhoun 2011 because only one eplerenone treatment group was used in their analyses.
We performed meta‐analysis of the blood pressure lowering effects of eplerenone versus placebo for the five included studies (Calhoun 2011; Flack 2003; Saruta 2004; Weinberger 2002; White 2003). There were a total of 1437 participants receiving eplerenone or placebo in parallel group randomized controlled trials. The duration that participants were in these trials ranged from 8 to 16 weeks.
Change in systolic blood pressure
The analysis of the mean difference in SBP showed that eplerenone reduced SBP by 9.21 mmHg (95% CI −11.08 to −7.34; P < 0.001; Analysis 1.3; Figure 5). There appears to be moderate heterogeneity in the results (I2 = 58%).
1.3. Analysis.
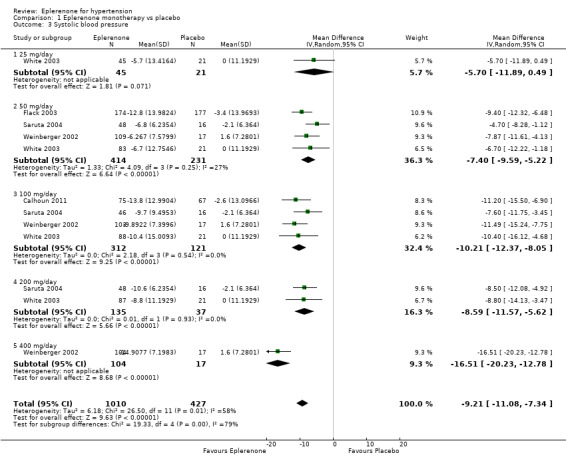
Comparison 1 Eplerenone monotherapy vs placebo, Outcome 3 Systolic blood pressure.
5.
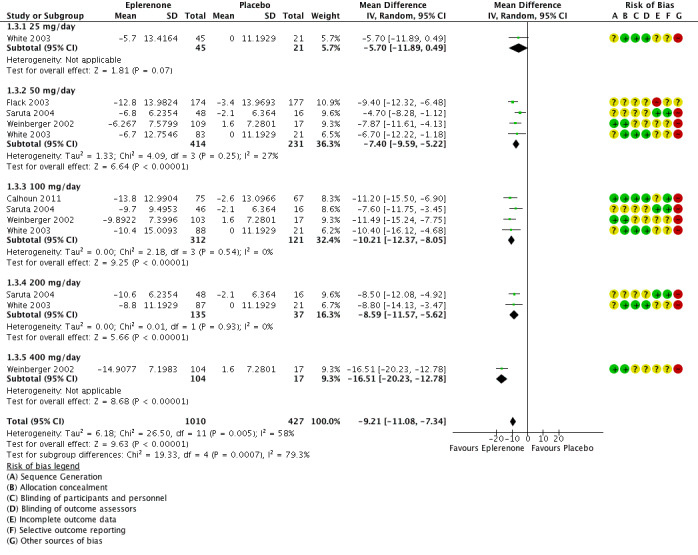
Forest plot of comparison: 1 Eplerenone monotherapy vs placebo, outcome: 1.3 Systolic blood pressure.
Change in diastolic blood pressure
The analysis of the mean difference in DBP found that eplerenone reduced DBP by 4.18 mmHg (95% CI −5.03 to −3.33; P < 0.001; Analysis 1.4; Figure 6). There was no statistical heterogeneity in the results (I2 = 0%) when using a fixed‐effect model. We summarize the results in the Table 1.
1.4. Analysis.
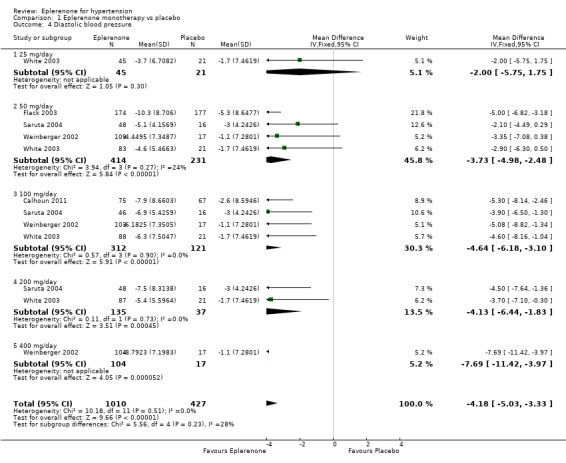
Comparison 1 Eplerenone monotherapy vs placebo, Outcome 4 Diastolic blood pressure.
6.
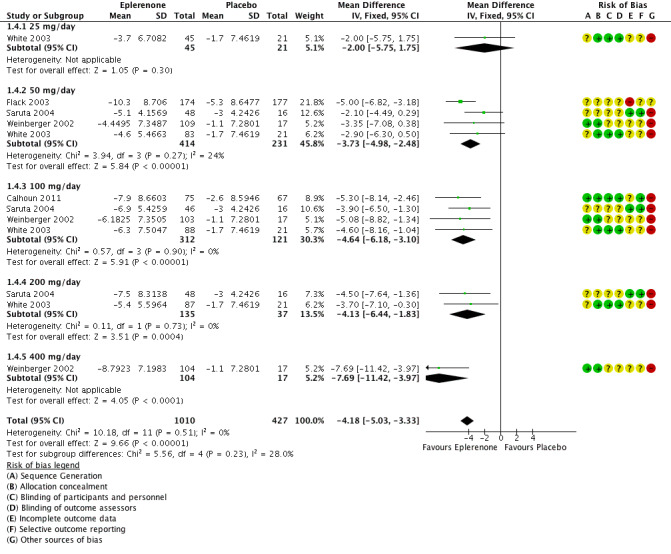
Forest plot of comparison: 1 Eplerenone monotherapy vs placebo, outcome: 1.4 Diastolic blood pressure.
Subgroup analyses
Because all five included studies were of short duration, there was insufficient evidence to perform a subgroup analysis of trials of less than 6 months' duration, 6 to 12 months' duration, and more than 12 months' duration. There was also no information provided on the blood pressure lowering effects of participants in different age groups, so a subgroup analysis was not possible. Only one study analyzed the effect of ethnicity on blood pressure (Flack 2003), so we did not perform a subgroup analysis on its influence as an effect modifier.
However, due to the variation in doses used in the included studies, we could stratify blood pressure data by dose in the meta‐analysis in order to examine the possibility of a dose response. For mean changes of both systolic and diastolic blood pressure, eplerenone 25 mg/day did not have a statistically significantly effect.
For changes in systolic blood pressure, the confidence intervals overlapped at eplerenone doses of 50 mg/day to 200 mg/day. However, eplerenone 400 mg/day decreased mean systolic blood pressure by 16.5 mmHg (95% CI −20.23 to −12.78; Analysis 1.3.5), which does not overlap with any other doses studied. This may lead us to believe eplerenone does exhibit a dose response at doses of 400 mg/day or higher. However, these data were only available from Weinberger 2002 as none of the other authors explored doses higher than 200 mg/day. Since only one study at high risk of bias investigated this dose, we cannot be confident of this effect until other studies replicate it.
The confidence intervals for change in DBP all overlapped at eplerenone doses of 50 mg/day to 400 mg/day. Thus, there is no apparent dose response. In other words, doses of 400 mg/day will not lower diastolic blood pressure more than doses of 50 mg/day (Analysis 2.2).
2.2. Analysis.
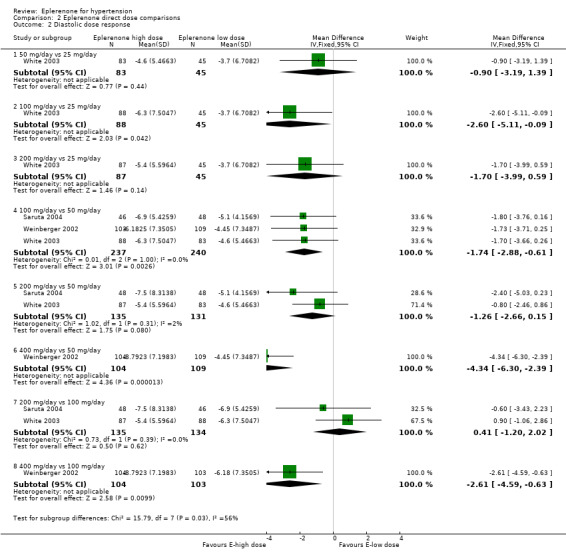
Comparison 2 Eplerenone direct dose comparisons, Outcome 2 Diastolic dose response.
Interestingly, when we exclude the results from Weinberger 2002's analysis with 400 mg/day, there is no heterogeneity (I2 = 5%) with our results in changes in systolic blood pressure. In addition, when we exclude the 100 mg daily dose of Weinberger 2002, the I2 decreases to 0%. There may be some unknown factor that is contributing to the heterogeneity of the results we found with changes in systolic blood pressure that is inherent in Weinberger 2002 study. We could not identify any apparent reason for heterogeneity based on our analysis of risk of bias, characteristics of included participants, or specifics of interventions tested. When we changed the analysis from a fixed‐effect model to a random‐effects model to test the robustness of the finding, the point estimate and confidence interval for SBP reduction did not change substantially (−9.21 mmHg, 95% CI −11.08 to −7.34; Analysis 1.3).
We conducted additional dose response comparisons based on dose comparisons within trials (Analysis 2.1; Analysis 2.2). Readers should be cautious of drawing definitive conclusions from these comparisons, as at best, three trials made the same dose‐to‐dose comparisons and at worst, one trial compared a particular dose‐to‐dose comparison. With these qualifications in mind, we can make some general observations.
2.1. Analysis.
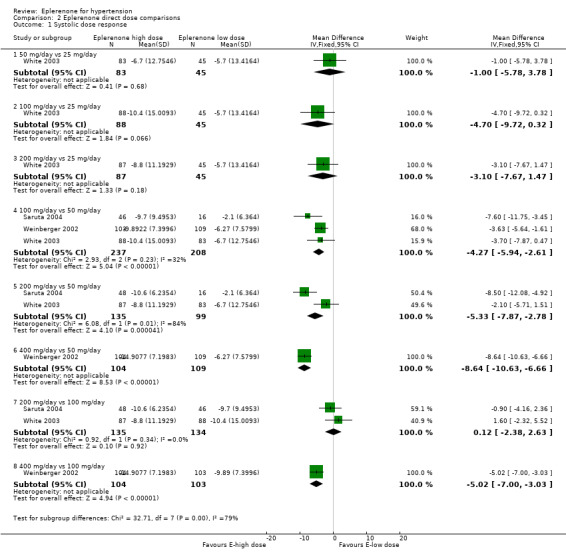
Comparison 2 Eplerenone direct dose comparisons, Outcome 1 Systolic dose response.
SBP direct dose comparisons
See Analysis 2.1.
It appears that 100 mg/day of eplerenone reduces SBP more than 50 mg/day eplerenone by 4.27 mmHg (95% CI −5.94 to −2.61), based on three trials with low statistical heterogeneity. These differences in SBP are within the variability of BP measurements and are unlikely to be clinically important (Musini 2009).
It is possible that 400 mg/day of eplerenone reduces SBP more than 50 mg/day or 100 mg/day of eplerenone based on one experiment at unclear risk of bias. These differences in SBP are within the variability of BP measurements and are unlikely to be clinically important (Musini 2009).
The difference in SBP reduction between 200 mg/day and 50 mg/day of eplerenone is unclear. One experiment demonstrated a significant difference while another experiment demonstrated no difference in SBP reductions with 200 mg/day versus 50 mg/day (high statistical heterogeneity).
Two experiments showed no statistical difference in SBP reduction between 200 mg/day and 100 mg/day of eplerenone (no statistical heterogeneity).
DBP direct dose comparisons
See Analysis 2.2.
It appears that 100 mg/day of eplerenone reduces DBP by 1.74 mmHg more than 50 mg/day eplerenone (Analysis 2.1), based on three trials with low statistical heterogeneity. These differences in SBP are within the variability of BP measurements and are unlikely to be clinically important (Musini 2009).
It is possible that 400 mg/day of eplerenone reduces DBP more than 50 mg/day or 100 mg/day of eplerenone based on one experiment with unclear risk of bias. These differences in SBP are within the variability of BP measurements and are unlikely to be clinically important (Musini 2009).
The difference in DBP reduction between 200 mg/day and 50 mg/day of eplerenone is unclear. One experiment demonstrated a numerical difference (not statistically significant) while another experiment demonstrated no difference in lowering SBP with 200 mg/day versus 50 mg/day (high statistical heterogeneity).
Two experiments showed no statistical difference in SBP reduction between 200 mg/day and 100 mg/day of eplerenone (no statistical heterogeneity).
One experiment showed no difference when comparing 50 mg/day, 100 mg/day, or 200 mg/day versus 25 mg/day.
Sensitivity analyses
In our sensitivity analysis, only including studies with proper randomization, proper allocation concealment, and proper blinding yielded similar results with respect to mean changes in systolic and diastolic blood pressure, although the number of trials was small, so there may be important differences in treatment effects based on risk of bias. Therefore, we do not present any of these results, as they did not contribute to a difference in our conclusions. We did not impute any of the blood pressure standard deviations, so a sensitivity analysis for this domain was not necessary.
Discussion
Summary of main results
There is insufficient evidence to draw any conclusions on the effects of eplerenone versus placebo for mortality, morbidity, or serious adverse events, as none of the included studies reported on any clinically meaningful outcomes.
Of the three studies that had adequate adverse event reporting, there were no differences in the incidence of participants experiencing any adverse event or adverse events leading to withdrawal in the eplerenone group compared to placebo (Figure 4). However, we could not analyze these results based on dose, and the potential harms related to increased daily doses of eplerenone are unknown.
Meta‐analysis of the five parallel‐group randomized controlled trials found a reduction in systolic blood pressure of 9.21 mmHg (95% CI −11.08 to −7.34; P < 0.001) and a reduction in diastolic blood pressure of 4.18 mmHg (95% CI −5.03 to −3.33; P < 0.001) (Figure 5; Figure 6). These results were statistically significant. There was no evidence of heterogeneity between the studies in changes in diastolic blood pressure. We found moderate heterogeneity between the studies in changes in systolic blood pressure. There may be a dose‐response effect with eplerenone at a dose of 400 mg/day; however, this finding is based on a single study (Weinberger 2002). The confidence intervals around the mean end‐of‐study blood pressure for doses ranging from 50 mg/day to 200 mg/day all overlapped. Thus, it appears that doses of more than 50 mg/day do not produce further reductions in systolic or diastolic blood pressure.
Overall completeness and applicability of evidence
While we attempted to contact authors of all five included studies, we only obtained further study information for White 2003. Contact with authors of White 2003 did not yield further information on clinical outcomes of mortality, morbidity, or serious adverse events. However, we were able to obtain further detail on blinding, allocation concealment, and randomization to better assess the risk of bias in the study. There was an underreporting of adverse effects in two of the studies (Calhoun 2011; Saruta 2004), which did not report the number of participants who withdrew due to adverse events or the number of participants with at least one adverse event.
It is interesting to note that the baseline mean BP was 153/101 mmHg in the intervention group and 152/100 mmHg in the placebo group across the included studies. This level of BP corresponds to the categorization of mild hypertension. A previous review (Diao 2014) suggested that there is no clear evidence that treatment of mild hypertension leads to reductions in the risk of morbidity and mortality.
Quality of the evidence
The effect sizes for reducing blood pressure that we calculated could be overestimates based on the unclear blinding used in three of the five included studies. Inappropriate blinding of outcome assessors, study participants, or both could exaggerate the effects of eplerenone on blood pressure by over‐reporting expected or desirable results. Only three included trials adequately reported on the number of participants who withdrew due to adverse events and the number of participants with at least one adverse event. Lastly, the reporting of adverse events was not stratified by dose. Therefore, we could not assess the harm due to eplerenone or based on its dose. This would be of particular interest to determine whether doses of eplerenone of 400 mg/day contributed to more significant harm.
Potential biases in the review process
Three independent review authors assessed the studies, and a fourth validated their judgement when any differences in interpretation arose. We undertook this process to screen 307 titles and abstracts as well as 78 full‐text articles, and to extract data from the five included studies for qualitative and quantitative analysis. None of the authors in this review have any conflicts of interest to declare.
Agreements and disagreements with other studies or reviews
The authors of this review are aware of one published review assessing the use of monotherapy eplerenone for treating essential hypertension (Pelliccia 2014). That review found statistically significant decreases in systolic blood pressure of 8.1 mmHg (95% CI −8.2 to −8.0) and in diastolic blood pressure of 4.1 mmHg (95% CI −4.1 to −4.0 mmHg). These results are very similar; however, Pelliccia 2014 included Krum 2002, which compared eplerenone plus ACE inhibitor or an angiotensin receptor blocker combination versus placebo plus ACE inhibitor or an angiotensin receptor blocker combination; this was not eplerenone monotherapy, so we excluded it from our review.
In a meta‐analysis of spironolactone versus placebo for hypertension, Batterink 2010 found that spironolactone lowered SBP by 20.09 mmHg and DBP by 6.75 mmHg. In a meta‐analysis of ACE inhibitors versus placebo, maximal blood pressure lowering for the ACE inhibitor class of drugs was −7.68 mmHg (95% CI ‐8.45, ‐6.91) for SBP and −4.59 mmHg (95% CI −4.99, −4.19) for DBP (Heran 2009). Another review found that beta‐blockers reduced systolic and diastolic blood pressure by approximately 11 mmHg and 6 mmHg, respectively, compared to placebo (Wiysonge 2017). Indirect evidence suggests that eplerenone lowers blood pressure to a lesser extent than spironolactone but to a similar extent as ACE inhibitors and beta‐blockers.
Authors' conclusions
Implications for practice.
Eplerenone 50 to 200 mg/day lowers blood pressure in people with primary hypertension by 9.21 mmHg systolic and 4.18 mmHg diastolic compared to placebo, with no difference of effect between doses of 50 mg/day to 200 mg/day. A dose of 25 mg/day did not produce a statistically significant reduction in systolic or diastolic blood pressure and there is insufficient evidence for doses above 200 mg/day. There is currently no available evidence to determine the effect of eplerenone on clinically meaningful outcomes such as all‐cause mortality, cardiovascular mortality, non‐cardiovascular mortality, serious adverse events, fatal and non‐fatal stroke, or fatal and non‐fatal myocardial infarction in hypertensive patients. The evidence available on side effects is insufficient and of low quality, which makes it impossible to draw conclusions about potential harm associated with eplerenone treatment in hypertensive patients.
Implications for research.
Although practitioners have used eplerenone for hypertension since 2002, studies have not yet shown a reduction in adverse cardiovascular events. Because the use of eplerenone in hypertension is generally limited to people already receiving other antihypertensive medications, further studies of eplerenone 50 mg/day to 100 mg/day as first‐line, second‐line, or third‐line therapy are necessary. Additionally, studies with longer follow‐up are necessary to allow for analysis of the effect of eplerenone on clinically meaningful outcomes such as mortality and morbidity.
History
Protocol first published: Issue 2, 2011 Review first published: Issue 2, 2017
| Date | Event | Description |
|---|---|---|
| 9 February 2016 | Amended | Updated protocol with most recent guidelines and references. New authors added. |
Acknowledgements
We would like to acknowledge the assistance provided by the Cochrane Hypertension Group.
Appendices
Appendix 1. Search strategies
Database: Ovid MEDLINE(R) 1946 to Present with Daily Update
Search Date: 3 March 2016
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 eplerenon$.mp. (951)
2 (epoxymexrenone or inspra).mp. (32)
3 1 or 2 (955)
4 hypertension/ (206970)
5 hypertens$.tw. (322830)
6 exp blood pressure/ (260668)
7 (blood pressure or bloodpressure).tw. (223000)
8 or/4‐7 (616055)
9 randomized controlled trial.pt. (407468)
10 controlled clinical trial.pt. (90116)
11 randomized.tw. (326346)
12 placebo.tw. (159611)
13 drug therapy/ (28738)
14 randomly.tw. (216170)
15 trial.tw. (372508)
16 groups.tw. (1387094)
17 or/9‐16 (2077578)
18 animals/ not (humans/ and animals/) (4161070)
19 17 not 18 (1710532)
20 3 and 8 and 19 (92)
21 remove duplicates from 20 (92)
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Cochrane Central Register of Controlled Trials <2016, Issue 3> via Cochrane Register of Studies Online
Search Date: 3 March 2016
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
#1 eplerenon* 152
#2 (epoxymexrenone or inspra) 2
#3 #1 OR #2 152
#4 MESH DESCRIPTOR Hypertension 13753
#5 hypertens*:TI,AB 31403
#6 MESH DESCRIPTOR blood pressure EXPLODE ALL TREES 24184
#7 (blood pressure or bloodpressure or bp) 55509
#8 #4 OR #5 OR #6 OR #7 70392
#9 #3 AND #8 73
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Embase <1974 to 2016 March 02>
Search Date: 3 March 2016
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 eplerenon$.mp. (3697)
2 (epoxymexrenone or inspra).mp. (183)
3 or/1‐2 (3699)
4 exp hypertension/ (563188)
5 hypertens$.tw. (495963)
6 exp blood pressure/ (454413)
7 (blood pressure or bloodpressure).mp. (496246)
8 or/4‐7 (1067177)
9 randomized controlled trial/ (396252)
10 crossover procedure/ (46218)
11 double‐blind procedure/ (128975)
12 random$.tw. (1059968)
13 (crossover$ or cross‐over$).tw. (80579)
14 placebo$.tw. (233767)
15 (doubl$ adj blind$).tw. (165925)
16 allocat$.tw. (101519)
17 comparison.ti. (354702)
18 trial.ti. (199337)
19 or/9‐18 (1697693)
20 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5789426)
21 19 not 20 (1469922)
22 3 and 8 and 21 (270)
23 remove duplicates from 22 (266)
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Cochrane Hypertension Specialised Register
Search Date: 3 March 2016
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
#1 (eplerenon* or epoxymexrenone or inspra)
#2 RCT:DE
#3 (Review OR Meta‐Analysis):MISC2
#4 #2 OR #3 #5 #1 AND #4 (61)
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: ClinicalTrials.gov
Search Date: 3 March 2016
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Study type: Interventional
Studies Conditions: hypertension
Interventions: eplerenone
Search terms: randomized (18)
Data and analyses
Comparison 1. Eplerenone monotherapy vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any adverse event | 3 | 1121 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.82, 1.41] |
| 2 Adverse event leading to withdrawal | 3 | 1132 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.47, 2.55] |
| 3 Systolic blood pressure | 5 | 1437 | Mean Difference (IV, Random, 95% CI) | ‐9.21 [‐11.08, ‐7.34] |
| 3.1 25 mg/day | 1 | 66 | Mean Difference (IV, Random, 95% CI) | ‐5.7 [‐11.89, 0.49] |
| 3.2 50 mg/day | 4 | 645 | Mean Difference (IV, Random, 95% CI) | ‐7.40 [‐9.59, ‐5.22] |
| 3.3 100 mg/day | 4 | 433 | Mean Difference (IV, Random, 95% CI) | ‐10.21 [‐12.37, ‐8.05] |
| 3.4 200 mg/day | 2 | 172 | Mean Difference (IV, Random, 95% CI) | ‐8.59 [‐11.57, ‐5.62] |
| 3.5 400 mg/day | 1 | 121 | Mean Difference (IV, Random, 95% CI) | ‐16.51 [‐20.23, ‐12.78] |
| 4 Diastolic blood pressure | 5 | 1437 | Mean Difference (IV, Fixed, 95% CI) | ‐4.18 [‐5.03, ‐3.33] |
| 4.1 25 mg/day | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐5.75, 1.75] |
| 4.2 50 mg/day | 4 | 645 | Mean Difference (IV, Fixed, 95% CI) | ‐3.73 [‐4.98, ‐2.48] |
| 4.3 100 mg/day | 4 | 433 | Mean Difference (IV, Fixed, 95% CI) | ‐4.64 [‐6.18, ‐3.10] |
| 4.4 200 mg/day | 2 | 172 | Mean Difference (IV, Fixed, 95% CI) | ‐4.13 [‐6.44, ‐1.83] |
| 4.5 400 mg/day | 1 | 121 | Mean Difference (IV, Fixed, 95% CI) | ‐7.69 [‐11.42, ‐3.97] |
Comparison 2. Eplerenone direct dose comparisons.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Systolic dose response | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 50 mg/day vs 25 mg/day | 1 | 128 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐5.78, 3.78] |
| 1.2 100 mg/day vs 25 mg/day | 1 | 133 | Mean Difference (IV, Fixed, 95% CI) | ‐4.7 [‐9.72, 0.32] |
| 1.3 200 mg/day vs 25 mg/day | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐7.67, 1.47] |
| 1.4 100 mg/day vs 50 mg/day | 3 | 445 | Mean Difference (IV, Fixed, 95% CI) | ‐4.27 [‐5.94, ‐2.61] |
| 1.5 200 mg/day vs 50 mg/day | 2 | 234 | Mean Difference (IV, Fixed, 95% CI) | ‐5.33 [‐7.87, ‐2.78] |
| 1.6 400 mg/day vs 50 mg/day | 1 | 213 | Mean Difference (IV, Fixed, 95% CI) | ‐8.64 [‐10.63, ‐6.66] |
| 1.7 200 mg/day vs 100 mg/day | 2 | 269 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐2.38, 2.63] |
| 1.8 400 mg/day vs 100 mg/day | 1 | 207 | Mean Difference (IV, Fixed, 95% CI) | ‐5.02 [‐7.00, ‐3.03] |
| 2 Diastolic dose response | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 50 mg/day vs 25 mg/day | 1 | 128 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐3.19, 1.39] |
| 2.2 100 mg/day vs 25 mg/day | 1 | 133 | Mean Difference (IV, Fixed, 95% CI) | ‐2.60 [‐5.11, ‐0.09] |
| 2.3 200 mg/day vs 25 mg/day | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐3.99, 0.59] |
| 2.4 100 mg/day vs 50 mg/day | 3 | 477 | Mean Difference (IV, Fixed, 95% CI) | ‐1.74 [‐2.88, ‐0.61] |
| 2.5 200 mg/day vs 50 mg/day | 2 | 266 | Mean Difference (IV, Fixed, 95% CI) | ‐1.26 [‐2.66, 0.15] |
| 2.6 400 mg/day vs 50 mg/day | 1 | 213 | Mean Difference (IV, Fixed, 95% CI) | ‐4.34 [‐6.30, ‐2.39] |
| 2.7 200 mg/day vs 100 mg/day | 2 | 269 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [‐1.20, 2.02] |
| 2.8 400 mg/day vs 100 mg/day | 1 | 207 | Mean Difference (IV, Fixed, 95% CI) | ‐2.61 [‐4.59, ‐0.63] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Calhoun 2011.
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Eplerenone 50 mg twice daily
Placebo
Inclusion criteria:
Exclusion criteria:
Pretreatment: eplerenone group had a higher mean duration of hypertension |
|
| Interventions |
Intervention characteristics Placebo Eplerenone 50 mg twice daily |
|
| Outcomes |
Blood pressure (seated)
Mean sitting clinic SBP
Mean sitting clinic DBP
Change from baseline in 24h mean DBP
Change from baseline in 24h mean SBP
|
|
| Identification |
Sponsorship source: Novartis Pharma AG (Switzerland) Country: Argentina, Australia, France, Germany, the Netherlands, Romania, Spain, Sweden, and the USA Setting: Clinics/physician's offices in Argentina, Australia, France, Germany, the Netherlands, Romania, Spain, Sweden, and the USA Comments: patients recruited between 11 September 2008 and 6 April 2009; treatment continued until 2 July 2009 Author's name: David A Calhoun Institution: Vascular Biology and Hypertension Program, University of Alabama at Birmingham Email: dcalhoun@uab.edu Address: 1530 3rd Ave SBirmingham, AL 35294 |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Quote: "Randomization sequences were generated by the interactive voice‐response system provider." Quote: "A validated system automated the random assignment of patient numbers to randomization numbers, which were linked to the different treatment groups and the medication numbers on treatment packs." Quote: "interactive voice‐response system was used to randomly assign patients to 1 of 6 treatment groups" |
| Allocation concealment | Low risk | Quote: "A validated system automated the random assignment of patient numbers to randomization numbers, which were linked to the different treatment groups and the medication numbers on treatment packs." Quote: "The identity of the treatments was concealed by the use of study drugs that were identical in packaging, labelling, schedule of administration, appearance, taste, and odor." |
| Blinding of participants and personnel All outcomes | Low risk | Quote: "Patients, investigators, people performing the assessments, and data analysts remained blinded to treatment assignments from the time of randomization until database lock. The identity of the treatments was concealed by the use of study drugs that were identical in packaging, labelling, schedule of administration, appear‐ ance, taste, and odor." Quote: "Both patients and investigators were blinded to the treatment assigned at randomization." |
| Blinding of outcome assessors All outcomes | Low risk | Quote: "Patients, investigators, people performing the assessments, and data analysts remained blinded to treatment assignments from the time of randomization until database lock." |
| Incomplete outcome data All outcomes | Unclear risk | Quote: "Of the randomised patients, 522 had post baseline BP measurements, and 474 completed the 8‐week double‐blind period . . .The proportion of patients who discontinued during the double‐blind treatment period was higher in the placebo group (13.0%, 10 of 77) compared with the 5 active treatment groups (values ranged from 6.8% [6 of 88] in the 0.5‐mg once‐daily LCI699 group to 10.7% [9/84] in the 50‐mg twice‐daily eplerenone group), primarily because of a lack of efficacy as judged by the investigator." Judgement comment: percentage of withdrawals from groups ranges from 6.8% to 13.0%. Not enough information to assess the risk of bias from missing data. |
| Selective outcome reporting | Low risk | Quote: "The primary end point for this trial was mean sitting DBP. Secondary end points included mean sitting systolic BP and 24‐hour ambulatory DBP and SBP." Judgement comment: all outcomes specified in registered clinicaltrials.gov protocol were reported. |
| Other sources of bias | High risk | Quote: "This support was funded by Novartis." Quote: "This study was designed collaboratively by the academic authors and the sponsor, Novartis Pharma AG (Switzerland). The sponsor was responsible for collecting data from investigational sites to create the clinical database and for the data analysis." Quote: "None of the authors received compensation for the evaluation, writing, or editing of this article. Dr Calhoun has received consulting fees, honoraria, and research funding from Novartis. Dr White has received consulting fees for safety committee work with aliskiren, and research funding from Novartis has been granted to his university; in addition, Dr White was reimbursed by Novartis for travel/accommodation to present this study at the American College of Cardiology in Atlanta in 2010. Dr Krum has received a research grant from Novartis. Dr Me ´ nard has received consulting fees from Novartis, Roche, and Actelion and is a member of the scientific council of Actelion. Drs Guo, Bermann, Lefkowitz, and Trapani are employees of Novartis." |
Flack 2003.
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Eplerenone 50 mg daily
Placebo
Inclusion criteria:
Exclusion criteria:
Pretreatment: missing baseline data for serum aldosterone in N = 61 (placebo); missing baseline UA/CR for N = 63 (placebo) and N = 50 (eplerenone); missing baseline RAAS profile in N = 60 (placebo) and N = 45 (eplerenone). They report no significant differences between the three groups, but higher % female in eplerenone group (64.3% vs 53.6%) |
|
| Interventions |
Intervention characteristics Eplerenone 50 mg daily Placebo |
|
| Outcomes |
Mean change in SBP
Mean change in DBP
Any adverse event
Adverse event leading to discontinuation
|
|
| Identification |
Sponsorship source: Pharmacia Corporation, Skokie Illinois Country: South Africa and USA Setting: 8 centers in South Africa, 41 centers in USA Comments: 4 January 2000 to 19 January 2001 Author's name: John M Flack Institution: Department of Internal Medicine, Wayne State University, Detroit, Michigan Email: JFlack@intmed.wayne.edu Address: 4201 St. Antoine, Suite 2EDetroit, Michigan 48201 |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Judgement comment: method of sequence generation not described |
| Allocation concealment | Unclear risk | Judgement comment: method of allocation concealment not described |
| Blinding of participants and personnel All outcomes | Unclear risk | Quote: "double‐blind" Judgement comment: not described how blinding was achieved or which participants were blinded |
| Blinding of outcome assessors All outcomes | Unclear risk | Judgement comment: blinding of outcome assessors not described |
| Incomplete outcome data All outcomes | High risk | Quote: "A total of 551 patients were randomized to study treatment, and 352 completed 16 weeks of treatment. Of these, 16 patients (4 placebo, 8 eplerenone, and 4 losartan) had no post‐baseline assessment; therefore, 535 patients were included in the cohort for efficacy analysis (Table 1)." Judgement comment: 16 patients had no post baseline assessment and were not included in the efficacy analysis. Blood pressure data from these 16 patients was not known. |
| Selective outcome reporting | Unclear risk | Quote: "The primary study end point was the mean change in DBP from baseline to the final visit. Secondary end points were the mean change from baseline to the final visit for SBP and DBP within and between racial groups; improvement in urinary protein excretion as measured by changes in the urinary albumin/creatinine ratio (UA/CR); and the effect of eplerenone in selected subpopulations, including women, obese patients, patients with SBP 160 mm Hg, elderly patients, and patients with microalbuminuria. Exploratory analyses assessed the rate of response to therapy and the relationships between BP changes and baseline active renin or aldosterone levels." Judgement comment: all primary study outcomes specified appear to have been reported. Secondary end points of improvement in urinary protein excretion as measured by changes in the urinary albumin/creatinine ratio and the effect of eplerenone in obese patients, elderly patients, and patients with microalbuminuria were not reported. |
| Other sources of bias | Unclear risk | Judgement comment: funding source unknown |
Saruta 2004.
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Eplerenone 50 mg daily
Placebo
Eplerenone 100 mg daily
Eplerenone 200 mg daily
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention characteristics Eplerenone 50 mg daily Placebo Eplerenone 100 mg daily Eplerenone 200 mg daily |
|
| Outcomes |
Blood pressure (seated)
Pulse pressure (change from baseline)
Mean change in SBP
Mean change in DBP
Mean change in 24 h ambulatory SBP
Mean change in 24 h ambulatory DBP
|
|
| Identification |
Sponsorship source: Grant from Pfizer Inc Country: Japan Setting: 22 centers in Japan Comments: Author's name: Takao Saruta Institution: Department of Internal Medicine, Keio University School of Medicine Email: takao_saruta@ybb.ne.jp or saruta@sc.itc.keio.ac.jp Address: 35 Shinanomachi, Shinjuku‐ku Tokyo 160‐8582, Japan |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Judgement comment: No information provided. An email was sent to both takao_saruta@ybb.ne.jp and saruta@sc.itc.keio.ac.jp on 14 November 2015 for clarification. |
| Allocation concealment | Unclear risk | Judgement comment: not reported |
| Blinding of participants and personnel All outcomes | Unclear risk | Judgement comment: the study did not specify how blinding of participants or personnel was done. |
| Blinding of outcome assessors All outcomes | Unclear risk | Judgement comment: study did not address this. An email has been sent to both takao_saruta@ybb.ne.jp and saruta@sc.itc.keio.ac.jp on 14 November2015 for clarification. |
| Incomplete outcome data All outcomes | Low risk | Quote: "one patient in the eplerenone 50‐mg group had no post dose BP measurement due to withdrawal of informed consent and was therefore excluded from the efficacy analysis." Judgement comment: only 1 patient dropped out. No other missing data for adjusted mean change in systolic and diastolic blood pressure at week 8. Unlikely to affect results in the group of 49. |
| Selective outcome reporting | Low risk | Judgement comment: the study protocol is not available but published report included all of the prespecified outcomes |
| Other sources of bias | High risk | Quote: "Disclosure: This research was supported by a grant from Pfizer Inc." |
Weinberger 2002.
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Eplerenone 50 mg daily
Placebo
Eplerenone 100 mg daily
Eplerenone 400 mg daily
Eplerenone 25 mg twice daily
Eplerenone 50 mg twice daily
Eplerenone 200 mg twice daily
Inclusion criteria:
Exclusion criteria:
Pretreatment: study notes no differences in baseline characteristics between groups. Groups look well‐balanced |
|
| Interventions |
Intervention characteristics Eplerenone 50 mg daily Placebo Eplerenone 100 mg daily Eplerenone 400 mg daily Eplerenone 25 mg twice daily Eplerenone 50 mg twice daily Eplerenone 200 mg twice daily |
|
| Outcomes |
Change from baseline in SBP (seated)
Change from baseline in DBP (seated)
Change from baseline in 24 h ambulatory BP monitoring (SBP)
Change from baseline in 24 h ambulatory BP monitoring (DBP)
Number of patients with any adverse events (occurring in at least 5% of patients)
Number of patients discontinued medication due to AE
|
|
| Identification |
Sponsorship source: Pharmacia Corporation, Skokie IL Country: 48 US sites Author's name: Myron H Weinberger Institution: Indiana University School of Medicine, Hypertension Research Center Email: mweinbe@iupui.edu Address: 541 Clinical Drive #423Indianapolis, IN46202‐5111 |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Quote: "Qualified patients in the double‐blind treatment period were randomised by a computer‐generated schedule to 50, 100, or 400 mg of eplerenone administered once daily or in divided doses; 50 mg of spironolactone twice daily; or placebo." |
| Allocation concealment | Low risk | Judgement comment: allocation of all patients simultaneously after run‐in via computer randomization. |
| Blinding of participants and personnel All outcomes | Unclear risk | Quote: "8‐week double‐blind treatment period." Judgement comment: unclear, because exactly who was blinded is not known. No mention of matching placebo used to mimic twice daily dosing of eplerenone groups |
| Blinding of outcome assessors All outcomes | Unclear risk | Judgement comment: no reference to blinding of assessors made. No mention of double dummy design. |
| Incomplete outcome data All outcomes | Unclear risk | Quote: "Overall, 417 patients were randomised to receive at least one dose of study medication and were subsequently included in the safety analyses. Of these, 409 had at least one evaluation after their baseline evaluation and were included in the efficacy analyses. Five patients were excluded because of protocol noncompliance and three were lost to follow‐up. An additional 39 patients did not complete the study because of treatment failure (7), loss to follow‐up (2), protocol noncompliance (16), pre‐existing protocol violation (3), or adverse events (11)." Judgement comment: not clear how 11.3% patient withdrawals were distributed across groups or how missing data was dealt with. |
| Selective outcome reporting | Unclear risk | Judgement comment: no protocol available. |
| Other sources of bias | High risk | Quote: "This study was supported by Pharmacia Corporation, Skokie, IL." |
White 2003.
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Eplerenone 50 mg daily
Placebo
Eplerenone 25 mg daily
Eplerenone 100 mg daily
Eplerenone 200 mg daily
Inclusion criteria: adult men and women were included if they had untreated hypertension, their seated clinic systolic BPs were < 180 mmHg, the clinic diastolic BP was 95‐110 mmHg, and the 24‐hour mean diastolic BP was ≥ 85 mm Hg. Exclusion criteria: participant were excluded from the trial if they had recent myocardial infarction or unstable angina, congestive heart failure, clinically significant liver or renal disease, known secondary hypertension, or uncontrolled diabetes mellitus (glycohaemoglobin > 10%). Men and women whose serum creatinine was > 1.5 mmol/L or > 1.3 mmol/L, respectively, or whose serum potassium was > 5.0 mmol/L at baseline were also excluded from the trial. Pretreatment: no significant group differences. |
|
| Interventions |
Intervention characteristics Eplerenone 50 mg daily Placebo Eplerenone 25 mg daily Eplerenone 100 mg daily Eplerenone 200 mg daily |
|
| Outcomes |
Change in SBP (seated)
Change in DBP (seated)
Change in 24 h ambulatory SBP
Change in 24 h ambulatory DBP
Change from baseline in daytime SBP
Change from baseline in daytime DBP
Change from baseline in nighttime SBP
Change from baseline in nighttime DBP
|
|
| Identification |
Sponsorship source: Country: USA, Brazil Setting: Investigation sites, offices, clinics Author's name: William B White Institution: Section of Hypertension and Clinical Pharmacology, University of Connecticut School of Medicine, Farmington, Connecticut, USA Email: wwhite@nso1.uchc.edu Address: University of Connecticut School of Medicine, 263 Farmington Avenue, Farmington, Connecticut 06030‐3940, USA |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Judgement comment: email response was unclear as to how sequence was generated as it was done by the sponsor Pharmacia, which no longer exists. |
| Allocation concealment | Low risk | Quote: "The study was a multicenter, double‐ blind, randomised, placebo‐controlled, parallel arm trial." Judgement comment: allocation concealment was done via "bottles of unidentified investigational product", which was distributed from a central coordination site by the sponsor via an interactive voice response system to the study coordinator as per email response from Dr White. |
| Blinding of participants and personnel All outcomes | Low risk | Judgement comment: as per email response, "both study patients and site staff were blinded to treatment assignment ‐ the study medication was in bottles of unidentified investigational product. The study coordinator used an IVRS process to obtain which bottles to use." |
| Blinding of outcome assessors All outcomes | Low risk | Judgement comment: as per email response, "the medication bottles are sent from a central location and are blinded. The outcome assessors had absolutely no way of knowing the randomization medication/code." |
| Incomplete outcome data All outcomes | Unclear risk | Quote: "There were 400 patients randomised into the 5 treatment arms by 47 investigative sites in the United States and Brazil with similar demographics and baseline clinic and ambulatory BP values . . . The percentage of patients withdrawn from the study was 22% in the placebo group, 27% in the eplerenone 25‐mg group, 21% in the eplerenone 50‐mg group, 11% in the eplerenone 100‐mg group, and 14% in the eplerenone 200‐mg group. The main reason for withdrawal after randomization was due to treatment failure: 12%, 9%, 7%, 1%, and 6% in the placebo, and eplerenone 25‐, 50‐, 100‐, and 200‐mg groups, respectively. Other reasons included lost to follow‐up, protocol violations, noncompliance, adverse events, or patient withdrawal of consent." Judgement comment: unclear reasons for withdrawals in each group and how missing data was accounted for in the blood pressure outcomes. |
| Selective outcome reporting | Unclear risk | Judgement comment: per email response by Dr White, he is unable to "find a study protocol at this late date and there is no way for [him] to retrieve that since no one associated with the sponsor works for any manufacturer of eplerenone." |
| Other sources of bias | High risk | Judgement comment: research was sponsored by Pharmacia Corporation as per Dr White's email response. |
ADBP: ambulatory diastolic blood pressure; AE: adverse event; ASBP: ambulatory systolic blood pressure; BMI: body mass index; CYP3A4: cytochrome P450 3A4;DBP: diastolic blood pressure; ECG: electrocardiogram; eGFR: estimated glomerular filtration rate; GI: gastrointestinal; NSAID: nonsteroidal anti‐inflammatory drug; NYHA: New York Hear Association; SBP: systolic blood pressure; SD: standard deviation; UA/CR: urine albumin‐to‐creatinine ratio.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amar 2013 | Wrong intervention |
| Ando 2010 | Wrong outcomes |
| Ando 2014a | Wrong intervention |
| Ando 2014b | Wrong intervention |
| Basile 2009 | Wrong study design |
| Blanchard 2015 | Wrong patient population |
| Burger 2005 | Wrong study design |
| Burgess 2003 | Wrong study design |
| Calhoun 2008 | Wrong comparator |
| CCOHTA 2003 | Wrong study design |
| Chaturvedi 2014 | Wrong patient population |
| Christou 2011 | Wrong outcomes |
| Christou 2012 | Wrong outcomes |
| Cleland 2007 | Wrong study design |
| Collier 2013 | Wrong patient population |
| Conti 2003 | Wrong study design |
| Dahal 2015 | Studies involved resistant hypertension patients on multiple antihypertensives |
| Davis 2003 | Wrong study design |
| Derer 2010 | Wrong study design |
| Deswal 2010 | Wrong patient population |
| Deswal 2011 | Wrong patient population |
| Dieterich 2005 | Wrong study design |
| Dobrucki 2003 | Wrong study design |
| Epstein 1998 | Wrong study design |
| Epstein 2002 | Wrong comparator |
| Eschalier 2013 | Wrong patient population |
| Flammer 2013 | Wrong patient population |
| Funder 2005 | Wrong study design |
| Funder 2010 | Wrong study design |
| Hameedi 2000 | Wrong study design |
| Hollenberg 2003 | Wrong comparator |
| Hollenberg 2004 | Wrong study design |
| Hwang 2011 | Wrong outcomes |
| Hwang 2013 | Wrong patient population |
| Jansen 2008 | Wrong intervention |
| Joffe 2007 | Wrong comparator |
| Karagiannis 2009 | Wrong study design |
| Karns 2013 | Wrong patient population |
| Krum 2002 | Wrong intervention |
| Levy 2004 | Wrong outcomes |
| Li 2010 | Wrong patient population |
| Magill 2003 | Wrong study design |
| Magni 2005 | Wrong study design |
| Mantero 2000 | Wrong study design |
| Montalescot 2014 | Wrong patient population |
| NCT00147589 | Wrong patient population |
| NCT00147615 | Wrong patient population |
| NCT00649311 | Wrong comparator |
| NCT00758524 | Wrong study design |
| NCT00817635 | Wrong intervention |
| NCT00825188 | Wrong comparator |
| NCT00980031 | Wrong patient population |
| NCT01275352 | Wrong intervention |
| NCT01373086 | No usable information obtained from responsible parties of terminated study |
| NCT02345044 | Study protocol |
| Pelliccia 2014 | Wrong study design |
| Pelliccia 2015 | Review did not identify any new studies compared to our search |
| Pitt 2004 | Wrong comparator |
| Romero 2011 | Wrong study design |
| Roush 2016 | Review did not identify any new studies compared to our search |
| Schmidt 2009 | Wrong intervention |
| Stier 2003 | Wrong study design |
| Struthers 2008 | Wrong study design |
| Stults 2006 | Wrong study design |
| Tomaschitz 2012 | Wrong patient population |
| Toto 2010 | Wrong study design |
| Trial 015 1999 | No usable information obtained from responsible parties of unpublished study |
| Van Zwieten 2001 | Wrong study design |
| Weber 2002 | Wrong study design |
| Wenger 2008 | Wrong study design |
| White 2010 | Duplicate study |
| Yoo 2011 | Wrong patient population |
Contributions of authors
All eight authors were responsible for searching for trials, data extraction, data analyses, interpretation of data, and writing/editing of the final report.
Sources of support
Internal sources
Fraser Health Authority, Canada.
-
Cochrane Hypertension Group, Canada.
Assistance in the electronic search
External sources
No sources of support supplied
Declarations of interest
Sarah C Masson: none known.
Aaron M Tejani: none known.
Sarah N Stabler: none known.
Matthew P Tsang: none known.
Anthony Tung: none known.
Angus TV Kinkade: none known.
Tina SC Tam: none known.
May HY Wu: none known.
New
References
References to studies included in this review
Calhoun 2011 {published data only}
- Calhoun DA, White WB, Krum H, Guo W, Bermann G, Trapani A, et al. Effects of a novel aldosterone synthase inhibitor for treatment of primary hypertension: results of a randomized, double‐blind, placebo‐ and active‐controlled phase 2 trial. Circulation 2011;124(18):1945‐55. [DOI: 10.1161/CIRCULATIONAHA.111.029892] [DOI] [PubMed] [Google Scholar]
Flack 2003 {published data only}
- Flack JM, Oparil S, Pratt JH, Roniker B, Garthwaite S, Kleiman JH, et al. Efficacy and tolerability of eplerenone and losartan in hypertensive black and white patients. Journal of the American College of Cardiology 2003;41(7):1148‐55. [DOI: 10.1016/S0735-1097(03)00054-8] [DOI] [PubMed] [Google Scholar]
Saruta 2004 {published data only}
- Saruta T, Kageyama S, Ogihara T, Hiwada K, Ogawa M, Tawara K, et al. Efficacy and safety of the selective aldosterone blocker eplerenone in Japanese patients with hypertension: a randomized, double‐blind, placebo‐controlled, dose‐ranging study. Journal of Clinical Hypertension (Greenwich) 2004;6(4):175‐83; quiz 184‐5. [DOI: 10.1111/j.1524-6175.2004.03146.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinberger 2002 {published data only}
- Weinberger MH, Roniker B, Krause SL, Weiss RJ. Eplerenone, a selective aldosterone blocker, in mild‐to‐moderate hypertension. American Journal of Hypertension 2002;15(8):709‐16. [PUBMED: 12160194] [DOI] [PubMed] [Google Scholar]
White 2003 {published data only}
- White WB, Carr AA, Krause S, Jordan R, Roniker B, Oigman W. Assessment of the novel selective aldosterone blocker eplerenone using ambulatory and clinical blood pressure in patients with systemic hypertension. American Journal of Cardiology 2003;92(1):38‐42. [DOI: 10.1016/S0002-9149(03)00461-2] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amar 2013 {published data only}
- Amar L, Azizi M, Menard J, Peyrard S, Plouin PF. Sequential comparison of aldosterone synthase inhibition and mineralocorticoid blockade in patients with primary aldosteronism. Journal of Hypertension 2013;31(3):624‐9; discussion 629. [DOI: 10.1097/HJH.0b013e32835d6d49] [DOI] [PubMed] [Google Scholar]
Ando 2010 {published data only}
- Ando K, Ohtsu H, Arakawa Y, Kubota K, Yamaguchi T, Nagase M, EVALUATE investigators. Rationale and design of the Eplerenone combination Versus conventional Agents to Lower blood pressure on Urinary Antialbuminuric Treatment Effect (EVALUATE) trial: a double‐blinded randomized placebo‐controlled trial to evaluate the antialbuminuric effects of an aldosterone blocker in hypertensive patients with albuminuria. Hypertension Research 2010;33(6):616‐21. [DOI: 10.1038/hr.2010.46] [DOI] [PubMed] [Google Scholar]
Ando 2014a {published data only}
- Ando K, Ohtsu H, Uchida S, Kaname S, Arakawa Y, Fujita T, EVALUATE Study Group. Anti‐albuminuric effect of the aldosterone blocker eplerenone in non‐diabetic hypertensive patients with albuminuria: a double‐blind, randomised, placebo‐controlled trial. Lancet Diabetes & Endocrinology 2014;2(12):944‐53. [DOI: 10.1016/s2213-8587(14)70194-9] [DOI] [PubMed] [Google Scholar]
Ando 2014b {published data only}
- Ando K, Ohtsu H, Uchida S, Kaname S, Arakawa Y, Fujita T, EVALUATE Study Group. Correction to Antialbuminuric effect of the aldosterone blocker eplerenone in non‐diabetic hypertensive patients with albuminuria: a double‐blind, randomised, placebo controlled trial [Lancet Diabetes Endocrinol, 2, (2014) 944‐953]. Lancet Diabetes & Endocrinology 2015;3(4):e3. [DOI: 10.1016/S2213-8587(15)00055-8] [DOI] [PubMed] [Google Scholar]
Basile 2009 {published data only}
- Basile J. New therapeutic options in patients prone to hypertension: a focus on direct Renin inhibition and aldosterone blockade.. The American Journal of the Medical Sciences 2009;337(6):438‐44. [DOI: 10.1097/MAJ.0b013e31819b3a80] [DOI] [PubMed] [Google Scholar]
Blanchard 2015 {published data only}
- Blanchard A, Vargas‐Poussou R, Vallet M, Caumont‐Prim A, Allard J, Desport E, et al. Indomethacin, amiloride, or eplerenone for treating hypokalemia in Gitelman syndrome. Journal of the American Society of Nephrology: JASN 2015;26:468‐75. [DOI: 10.1681/ASN.2014030293] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burger 2005 {published data only}
- Burger PC, Brunner‐La Rocca H. Pharmacotherapy of congestive heart failure in elderly patients. Journal of Cardiovascular Pharmacology and Therapeutics 2005;10(2):85‐94. [DOI: 10.1177/107424840501000202] [DOI] [PubMed] [Google Scholar]
Burgess 2003 {published data only}
- Burgess ED, Lacourciere Y, Ruilope‐Urioste LM, Oparil S, Kleiman JH, Krause S, et al. Long‐term safety and efficacy of the selective aldosterone blocker eplerenone in patients with essential hypertension. Clinical Therapy 2003;25(9):2388‐404. [DOI] [PubMed] [Google Scholar]
Calhoun 2008 {published data only}
- Calhoun DA, White WB. Effectiveness of the selective aldosterone blocker, eplerenone, in patients with resistant hypertension. Journal of the American Society of Hypertension 2008;2(6):462‐8. [DOI: 10.1016/j.jash.2008.05.005] [DOI] [PubMed] [Google Scholar]
CCOHTA 2003 {published data only}
- The Canadian Coordinating Office for Health Technology Assessment (CCOHTA). Eplerenone for the treatment of hypertension. Emerging Drug List 2003; Vol. 37:1‐3.
Chaturvedi 2014 {published data only}
- Chaturvedi S, Lipszyc DH, Licht C, Craig JC, Parekh R. Pharmacological interventions for hypertension in children. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD008117.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Christou 2011 {published data only}
- Christou DD, Hwang MH, Yoo JK, Luttrell MJ, Cernosek MM, Meade TH, et al. Effect of acute mineralocorticoid receptor blockade on flow‐mediated dilation in middle aged and older adults with metabolic syndrome. Journal of Diabetes 2011;3:239‐40. [DOI: 10.1111/j.1753-0407.2011.00122.x] [DOI] [Google Scholar]
Christou 2012 {published data only}
- Christou DD, Yoo JK, Hwang MH, Luttrell M, Kim HK, Meade TH, et al. Mineralocorticoid receptor blockade does not result in arterial destiffening in healthy older adults. Hypertension 2012;60(Suppl 1):A13. [Google Scholar]
Cleland 2007 {published data only}
- Cleland JGF, Coletta AP, Clark AL. Clinical trials update from the American College of Cardiology 2007: ALPHA, EVEREST, FUSION II, VALIDD, PARR‐2, REMODEL, SPICE, COURAGE, COACH, REMADHE, pro‐BNP for the evaluation of dyspnoea and THIS‐diet. European Journal of Heart Failure 2007;9(6‐7):740‐5. [DOI: 10.1016/j.ejheart.2007.04.004] [DOI] [PubMed] [Google Scholar]
Collier 2013 {published data only}
- Collier TJ, Pocock SJ, McMurray JJV, Zannad F, Krum H, Veldhuisen DJ, et al. The impact of eplerenone at different levels of risk in patients with systolic heart failure and mild symptoms: insight from a novel risk score for prognosis derived from the EMPHASIS‐HF trial. European Heart Journal 2013;34(36):2823‐9. [DOI: 10.1093/eurheartj/eht247] [DOI] [PubMed] [Google Scholar]
Conti 2003 {published data only}
- Conti CR. Aldosterone antagonism and hypertension. Clinical Cardiology 2003;26(5):209‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dahal 2015 {published data only}
- Dahal K, Kunwar S, Rijal J, Alqatahni F, Panta R, Ishak N, et al. The effects of aldosterone antagonists in patients with resistant hypertension: a meta‐analysis of randomized and nonrandomized studies. American Journal of Hypertension 2015;28(11):1376‐85. [DOI: 10.1093/ajh/hpv031] [DOI] [PubMed] [Google Scholar]
Davis 2003 {published data only}
- Davis KL, Nappi JM. The cardiovascular effects of eplerenone, a selective aldosterone‐receptor antagonist. Clinical Therapeutics 2003;25(11):2647‐68. [DOI] [PubMed] [Google Scholar]
Derer 2010 {published data only}
- Derer W, Dechend R, Muller DN. Mineralocorticoid receptor antagonists: inhibition of the renin angiotensin system [Welchen Stellenwert haben sie in der antihypertensien Therapie?]. MMW Fortschritte der Medizin 2010;152(6):48‐9. [DOI] [PubMed] [Google Scholar]
Deswal 2010 {published data only}
- Deswal A, Richardson P, Bozkurt B, Mann DL. Randomized trial of aldosterone AntagonisM in diastolic heart failure (RAAM‐DHF). Journal of Cardiac Failure 2010;1:S7. [DOI: 10.1016/j.cardfail.2010.06.026] [DOI] [PubMed] [Google Scholar]
Deswal 2011 {published data only}
- Deswal A, Richardson P, Bozkurt B, Mann DL. Results of the randomized aldosterone antagonism in heart failure with preserved ejection fraction trial (RAAM‐PEF). Journal of Cardiac Failure 2011;17(8):634‐42. [DOI: 10.1016/j.cardfail.2011.04.007] [DOI] [PubMed] [Google Scholar]
Dieterich 2005 {published data only}
- Dieterich HA, Wendt C, Saborowski F. Cardioprotection by aldosterone receptor antagonism in heart failure. Part I. The role of aldosterone in heart failure. Fiziol Cheloveka 2005;31(6):97‐105. [PubMed] [Google Scholar]
Dobrucki 2003 {published data only}
- Dobrucki T, Januszewicz A, Sitkiewicz D, Januszewicz W. A New Face of Aldosterone [Aldosteron ‐ Hormon o nowym obliczu]. Nadcisnienie Tetnicze 2003;7(4):271‐9. [Google Scholar]
Epstein 1998 {published data only}
- Epstein M, Alexander JC, Roniker B. Eplerenone, a new selective aldosterone receptor antagonist (SARA): efficacy in patients with mild to moderate hypertension [Abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):322A‐3A. [Google Scholar]
Epstein 2002 {published data only}
- Epstein M, Buckalew V, Martinez F. Antiproteinuric efficacy of eplerenone, enalapril, and eplerenone/enalapril combination therapy in diabetic hypertensives with microalbuminuria [Abstract no: OR54]. American Journal of Hypertension 2002;15(4 Suppl 1):24A. [Google Scholar]
Eschalier 2013 {published data only}
- Eschalier R, McMurray JJ, Swedberg K, Veldhuisen DJ, Krum H, Pocock SJ, et al. Safety and efficacy of eplerenone in patients at high risk for hyperkalemia and/or worsening renal function: analyses of the EMPHASIS‐HF study subgroups (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure). Journal of the American College of Cardiology 2013;62:1585‐93. [DOI: 10.1016/j.jacc.2013.04.086] [DOI] [PubMed] [Google Scholar]
Flammer 2013 {published data only}
- Flammer A, Sudano I, Enseleit F, Luscher TF, Noll G Ruschitzka F. Mineralocorticoid receptor antagonism in patients with coronary artery disease and preserved ejection fraction‐a randomized, double‐blind trial. European Journal of Heart Failure 2013;12:S222‐S223. [DOI: 10.1093/eurjhf/hst009] [DOI] [Google Scholar]
Funder 2005 {published data only}
- Funder JW. ACE inhibitors and mineralocorticoid receptor blockade in patients with congestive heart failure. Current Diabetes Reports 2005;5(1):36‐40. [DOI] [PubMed] [Google Scholar]
Funder 2010 {published data only}
- Funder, J. W. Eplerenone in chronic renal disease: the EVALUATE trial. Hypertens Res 2010;33(6):539‐40. [DOI: 10.1038/hr.2010.71] [DOI] [PubMed] [Google Scholar]
Hameedi 2000 {published data only}
- Hameedi A, Chadow HL. The promise of selective aldosterone receptor antagonists for the treatment of hypertension and congestive heart failure. Current Hypertension Reports 2000;2(4):378‐83. [DOI] [PubMed] [Google Scholar]
Hollenberg 2003 {published data only}
- Hollenberg NK, Williams GH Anderson R, Akhras KS, Bittman RM, Krause SL. Symptoms and the distress they cause: comparison of an aldosterone antagonist and a calcium channel blocking agent in patients with systolic hypertension. Archives of Internal Medicine 2003;163(13):1543‐8. [DOI] [PubMed] [Google Scholar]
Hollenberg 2004 {published data only}
- Hollenberg NK. Aldosterone in the development and progression of renal injury. Kidney International 2004;66(1):1‐9. [DOI: 10.1111/j.1523-1755.2004.00701.x] [DOI] [PubMed] [Google Scholar]
Hwang 2011 {published data only}
- Hwang MH, Yoo JK, Luttrell MJ, Cernosek MM, Meade TH, English MW, et al. Mineralocorticoid receptor blockade does not improve vascular endothelial function in older adults with metabolic syndrome [Abstract]. FASEB Journal 2011;25:Abstract no: 821.45. [Google Scholar]
Hwang 2013 {published data only}
- Hwang MH, Yoo JK, Luttrell M, Kim HK, Meade TH, et al. Role of mineralocorticoid receptors in arterial stiffness in human aging. Experimental Gerontology 2013;48(8):701‐4. [DOI: 10.1016/j.exger.2013.05.058] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jansen 2008 {published data only}
- Jansen PM, Boomsma F, Meiracker AH, Dutch ARRAT investigators. Aldosterone‐to‐renin ratio as a screening test for primary aldosteronism‐‐the Dutch ARRAT Study. Netherlands Journal of Medicine 2008;66(5):220‐8. [PubMed] [Google Scholar]
Joffe 2007 {published data only}
- Joffe HV, Kwong RY, Gerhard‐Herman MD, Rice C, Feldman K, Adler GK. Beneficial effects of eplerenone versus hydrochlorothiazide on coronary circulatory function in patients with diabetes mellitus. The Journal of Clinical Endocrinology and Metabolism 2007;92(7):2552‐8. [DOI: 10.1210/jc.2007-0393] [DOI] [PubMed] [Google Scholar]
Karagiannis 2009 {published data only}
- Karagiannis A, Athyros VG. A comparison of the aldosterone‐blocking agents eplerenone and spironolactone. Clinical Cardiology 2009;32(4):230. [DOI: 10.1002/clc.20442] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karns 2013 {published data only}
- Karns AD, Bral JM, Hartman D, Peppard T, Schumacher C. Study of aldosterone synthase inhibition as an add‐on therapy in resistant hypertension. Journal of Clinical Hypertension (Greenwich) 2013;15(3):186‐92. [DOI: 10.1111/jch.12051] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krum 2002 {published data only}
- Krum H, Nolly H, Workman D, He W, Roniker B, Krause S, et al. Efficacy of eplerenone added to renin‐angiotensin blockade in hypertensive patients. Hypertension 2002;40(2):117‐23. [DOI] [PubMed] [Google Scholar]
Levy 2004 {published data only}
- Levy DG, Rocha R, Funder JW. Distinguishing the antihypertensive and electrolyte effects of eplerenone. Journal of Clinical and Endocrinology Metabolism 2004;89(6):2736‐40. [DOI] [PubMed] [Google Scholar]
Li 2010 {published data only}
- Li, JS, Flynn JT, Portman R, Davis I, Ogawa M, Shi H, Pressler ML. The efficacy and safety of the novel aldosterone antagonist eplerenone in children with hypertension: a randomized, double‐blind, dose‐response study. Journal of Pediatrics 2010;157(2):282‐7. [DOI: 10.1016/j.jpeds.2010.02.042] [DOI] [PubMed] [Google Scholar]
Magill 2003 {published data only}
- Magill MK, Gunning K, Saffel‐Shrier S, Gay C. New developments in the management of hypertension. American Family Physician 2003;68(5):853‐8. [PubMed] [Google Scholar]
Magni 2005 {published data only}
- Magni P, Motta M. Aldosterone receptor antagonists: biology and novel therapeutic applications. Current Hypertension Reports 2005;7(3):206‐11. [DOI] [PubMed] [Google Scholar]
Mantero 2000 {published data only}
- Mantero F, Lucarelli G. Aldosterone antagonists in hypertension and heart failure. Annales d'endocrinologie 2000;61(1):52‐60. [PubMed] [Google Scholar]
Montalescot 2014 {published data only}
- Montalescot G, Pitt B, Lopez De Sa E, Hamm CW, Flather M, Verheugt F, et al. Early eplerenone treatment in patients with acute ST‐elevation myocardial infarction without heart failure: the Randomized Double‐Blind Reminder Study. European Heart Journal 2014;35:2295‐302. [DOI: 10.1093/eurheartj/ehu164] [DOI] [PubMed] [Google Scholar]
NCT00147589 {published data only}
- Pfizer. Peds I (Pediatric Eplerenone Development Study I): a randomized, double‐blind, placebo withdrawal, parallel group, dose‐response study to evaluate the efficacy and safety of eplerenone In the treatment of hypertension in children. ClinicalTrials.gov 2008. [NCT00147589]
NCT00147615 {published data only}
- Pfizer. Peds II (Pediatric Eplerenone Development Study II)‐‐An open label, long‐term study to evaluate the safety of eplerenone in the treatment of hypertension in children. ClinicalTrials.gov 2004. [NCT00147615]
NCT00649311 {published data only}
- Pfizer. A study to evaluate the efficacy of eplerenone compared with losartan for the treatment of patients with mild to moderate hypertension. ClinicalTrials.gov 2009. [NCT00649311]
NCT00758524 {published data only}
- Novartis. A multi‐center, randomized, double‐blind, placebo and active controlled, parallel group, dose finding study to evaluate the efficacy and safety of LCI699, a new experimental antihypertensive drug, in patients with essential hypertension. ClinicalTrials.gov 2012. [NCT00758524]
NCT00817635 {published data only}
- Novartis. A study to evaluate the effects of LCI699 on safety and efficacy in subjects with resistant hypertension receiving combination therapy with three or more antihypertensive drugs, including a diuretic. ClinicalTrials.gov 2012. [NCT00817635]
NCT00825188 {published data only}
- Wofford M, University of Mississippi Medical Center. A study of the effects of eplerenone and amlodipine on blood pressure and basal metabolic rate in obese hypertensives. ClinicalTrials.gov 2014. [NCT00825188]
NCT00980031 {published data only}
- Holmberg MJ, Creighton University. Aldosterone blockade to prevent myocardial remodeling in patients with controlled essential hypertension. ClinicalTrials.gov 2014. [NCT00980031]
NCT01275352 {published data only}
- Washington University School of Medicine. A randomized, double blind pilot study evaluating CLCNKA (Ka Renal Chloride Channel[ClC‐Ka]) polymorphism effects on hypertrophy regression in caucasian hypertensive patients treated with eplerenone. ClinicalTrials.gov 2015. [NCT01275352]
NCT01373086 {published data only}
- Novartis. LFF269 compared to placebo after treatment in subjects with essential hypertension. ClinicalTrials.gov 2012. [NCT01373086]
NCT02345044 {published data only}
- Daiichi Sankyo Inc. A study to evaluate efficacy and safety of CS‐3150 in Japanese hypertensive subjects. ClinicalTrials.gov 2016. [NCT02345044]
Pelliccia 2014 {published data only}
- Pelliccia F, Patti G, Rosano G, Greco C, Gaudio C. Efficacy and safety of eplerenone in the management of mild to moderate arterial hypertension: systematic review and meta‐analysis. International Journal of Cardiology 2014;177:219‐28. [DOI: 10.1016/j.ijcard.2014.09.091] [DOI] [PubMed] [Google Scholar]
Pelliccia 2015 {published data only}
- Pelliccia F, Rosano G, Patti G, Volterrani M, Greco C, Gaudio C. Efficacy and safety of mineralocorticoid receptors in mild to moderate arterial hypertension. International Journal of Cardiology 2015;200:8‐11. [DOI: 10.1016/j.ijcard.2014.10.150] [DOI] [PubMed] [Google Scholar]
Pitt 2004 {published data only}
- Pitt B, Reichek N, Willenbrock R, et al. Trial suggests that combining eplerenone and enalapril reduces left ventricular hypertrophy in hypertension more effectively than either treatment alone. Evidence‐based Cardiovascular Medicine 2004;8(1):16‐7; discussion 18‐9, 20‐1. [DOI: 10.1016/j.ebcm.2003.12.012] [DOI] [PubMed] [Google Scholar]
Romero 2011 {published data only}
- Romero J, Makani H, Kahan J, Wever‐Pinzon O, Colaco C, Messerli FH. Hyperkalemia with aldosterone antagonist monotherapy in essential hypertension. A meta‐analysis. Journal of Clinical Hypertension 2011;1:A65‐A66. [DOI: 10.1111/j.1751-7176.2011.00459.x] [DOI] [Google Scholar]
Roush 2016 {published data only}
- Roush GC, Ernst ME, Kostis JB, Yeasmin S, Sica DA. Dose doubling, relative potency, and dose equivalence of potassium‐sparing diuretics affecting blood pressure and serum potassium: systematic review and meta‐analyses. Journal of Hypertension 2016;34(1):11‐9. [DOI: 10.1097/HJH.0000000000000762] [DOI] [PubMed] [Google Scholar]
Schmidt 2009 {published data only}
- Schmidt BM, Raff U, Schwab J, Bar I, Schmieder RE. MR blockade improves cardiac and vascular target organ damage independent of blood pressure in resistant hypertension [Abstract]. Hypertension 2009;54(4):e37. [DOI: 10.1161/01.HYP.0000359702.48610.72] [DOI] [Google Scholar]
Stier 2003 {published data only}
- Stier CT Jr. Eplerenone: a selective aldosterone blocker. Cardiovascular Drug Review 2003;21(3):169‐84. [DOI] [PubMed] [Google Scholar]
Struthers 2008 {published data only}
- Struthers A, Krum H, Williams GH. A comparison of the aldosterone‐blocking agents eplerenone and spironolactone. Clinical Cardiology 2008;31(4):153‐8. [DOI: 10.1002/clc.20324] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stults 2006 {published data only}
- Stults B, Jones RE. Management of hypertension in diabetes. Diabetes Spectrum 2006;19(1):25‐31. [DOI: 10.2337/diaspect.19.1.25] [DOI] [Google Scholar]
Tomaschitz 2012 {published data only}
- Tomaschitz A, Fahrleitner‐Pammer A, Pieske B, Verheyen N, Amrein K, Ritz E, et al. Effect of eplerenone on parathyroid hormone levels in patients with primary hyperparathyroidism: a randomized, double‐blind, placebo‐controlled trial. BMC Endocrine Disorders 2012;12:19. [DOI: 10.1186/1472-6823-12-19] [DOI] [PMC free article] [PubMed] [Google Scholar]
Toto 2010 {published data only}
- Toto RD. Aldosterone blockade in chronic kidney disease: can it improve outcomes?. Current Opinion in Nephrology and Hypertension 2010;19(5):444‐9. [DOI: 10.1097/MNH.0b013e32833ce6d5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trial 015 1999 {published and unpublished data}
- U.S. Food, Drug Administration. Clinical review: detailed study reviews section. Drug approvals and databases.
Van Zwieten 2001 {published data only}
- Zwieten PA. Drug treatment of isolated systolic hypertension. Nephrology Dialysis Transplantation 2001;16(6):1095‐7. [DOI] [PubMed] [Google Scholar]
Weber 2002 {published data only}
- Weber MA. Clinical implications of aldosterone blockade. American Heart Journal 2002;144(5 Suppl):S12‐S18. [DOI] [PubMed] [Google Scholar]
Wenger 2008 {published data only}
- Wenger NK. Drugs for cardiovascular disease prevention in women: Implications of the AHA guidelines ‐ 2007 Update. Drugs 2008;68(3):339‐58. [DOI: 10.2165/00003495-200868030-00006] [DOI] [PubMed] [Google Scholar]
White 2010 {published data only}
- White WB, Calhoun DA, Krum H, Guo W, Trapani AJ, Lefkowitz H, et al. Blockade of aldosterone production as a novel approach to the management of high blood pressure: efficacy and tolerability of the aldosterone synthase inhibitor LCI699 in patients with stage 1‐2 hypertension [Abstract]. Journal of the American College of Cardiology 2010;55(10 Suppl 1):A61.E582. [DOI: 10.1016/S0735-1097%2810%2960583-9] [DOI] [Google Scholar]
Yoo 2011 {published data only}
- Yoo JK, Hwang MH, Luttrell MJ, Cernosek MM, Meade TH, English MW, et al. Mineralocorticoid receptor blockade does not affect arterial compliance in middle‐aged and older adults with and without metabolic syndrome. FASEB Journal 2011;25(1 Suppl):lb480. [Google Scholar]
Additional references
Arguedas 2009
- Arguedas JA, Perez MI, Wright JM. Treatment blood pressure targets for hypertension. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD004349.pub2] [DOI] [PubMed] [Google Scholar]
Banach 2014
- Banach M, Bromfield S, Howard G, Howard VJ, Zanchetti A, Aronow WS, et al. Association of systolic blood pressure levels with cardiovascular events and all‐cause mortality among older adults taking antihypertensive medication. International Journal of Cardiology 2014;176(1):219‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Batterink 2010
- Batterink J, Stabler SN, Tejani AM, Fowkes CT. Spironolactone for hypertension. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD008169.pub2] [DOI] [PubMed] [Google Scholar]
CHEP 2015
- Canadian Hypertension Education Program (CHEP). The 2015 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Canadian Journal of Cardiology 2015;31:549‐68. [DOI] [PubMed] [Google Scholar]
Diao 2014
- Diao D, Wright JM, Cundiff DK, Gueyffier F. Pharmacotherapy for mild hypertension. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD006742.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
DiPiro 2014
- DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacotherapy: A Pathophysiologic Approach. 9th Edition. New York: McGraw‐Hill Education, 2014. [Google Scholar]
eCPS 2014
- Inspra. Compendium of pharmaceuticals and specialties, online version (e‐CPS). www.e‐therapeutics.ca/cps.select.preliminaryFilter.action?simplePreliminaryFilter=eplerenone#m701861n00006 (accessed 10 June 2014).
Heran 2009
- Heran BS, Wong MMY, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003823.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jansen 2009
- Jansen PM, Danser AHJ, Imholz BP, Meiracker AH. Aldosterone‐receptor antagonism in hypertension. Journal of Hypertension 2009;27:680‐91. [DOI] [PubMed] [Google Scholar]
JNC 7
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289(19):2560‐72. [DOI: 10.1001/jama.289.19.2560] [DOI] [PubMed] [Google Scholar]
Katzung 2012
- Katzung BG, Masters SB, Trevor AJ. Basic and Clinical Pharmacology. 12th Edition. The McGraw‐Hill Companies, 2012. [Google Scholar]
Lewington 2002
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration. Age‐specific relevance of usual blood pressure to vascular mortality: A meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360(9349):1903‐13 [Erratum in: Lancet. 2003 Mar 22;361(9362):1060]. [DOI: 10.1016/S0140-6736(02)11911-8] [DOI] [PubMed] [Google Scholar]
Muldowney 2009
- Muldowney JA 3rd, Schoenhard JA, Benge CD. The clinical pharmacology of eplerenone. Expert Opinion on Drug Metabolism & Toxicology 2009;5(4):425‐32. [DOI: 10.1517/17425250902837973] [DOI] [PubMed] [Google Scholar]
Musini 2009
- Musini V, Wright JM. Factors affecting blood pressure variability: lessons learned from two systematic reviews of randomized controlled trials. PLOS ONE 2009;4(5):e5673. [DOI: 10.1371/journal.pone.0005673] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCGC 2011
- National Clinical Guideline Centre (UK). Hypertension: The Clinical Management of Primary Hypertension in Adults. London: Royal College of Physicians, 2011. [Google Scholar]
Van Bemmel 2006
- Bemmel T, Gussekloo J, Westendorp RG, Blauw GJ. In a population‐based prospective study, no association between high blood pressure and mortality after age 85 years. Journal of Hypertension 2006;24(2):287‐92. [PUBMED: 16508574] [DOI] [PubMed] [Google Scholar]
Weber 2014
- Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, et al. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. Journal of Hypertension 2014;32(1):3‐15. [PUBMED: 24270181] [DOI] [PubMed] [Google Scholar]
Wiysonge 2017
- Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Mbewu A, Opie LH. Beta‐blockers for hypertension. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD002003.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 2009
- Wright JM, Musini VM. First‐line drugs for hypertension. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001841.pub2] [DOI] [PubMed] [Google Scholar]


