Abstract
Background
Major abdominal surgery can be associated with a number of serious complications that may impair patient recovery. In particular, postoperative pulmonary complications (PPCs), including respiratory complications such as atelectasis and pneumonia, are a major contributor to postoperative morbidity and may even contribute to increased mortality. Continuous positive airway pressure (CPAP) is a type of therapy that uses a high‐pressure gas source to deliver constant positive pressure to the airways throughout both inspiration and expiration. This approach is expected to prevent some pulmonary complications, thus reducing mortality.
Objectives
To determine whether any difference can be found in the rate of mortality and adverse events following major abdominal surgery in patients treated postoperatively with CPAP versus standard care, which may include traditional oxygen delivery systems, physiotherapy and incentive spirometry.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 9; Ovid MEDLINE (1966 to 15 September 2013); EMBASE (1988 to 15 September 2013); Web of Science (to September 2013) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (to September 2013).
Selection criteria
We included all randomized controlled trials (RCTs) in which CPAP was compared with standard care for prevention of postoperative mortality and adverse events following major abdominal surgery. We included all adults (adults as defined by individual studies) of both sexes. The intervention of CPAP was applied during the postoperative period. We excluded studies in which participants had received PEEP during surgery.
Data collection and analysis
Two review authors independently selected studies that met the selection criteria from all studies identified by the search strategy. Two review authors extracted the data and assessed risk of bias separately, using a data extraction form. Data entry into RevMan was performed by one review author and was checked by another for accuracy. We performed a limited meta‐analysis and constructed a summary of findings table.
Main results
We selected 10 studies for inclusion in the review from 5236 studies identified in the search. These 10 studies included a total of 709 participants. Risk of bias for the included studies was assessed as high in six studies and as unclear in four studies.
Two RCTs reported all‐cause mortality. Among 413 participants, there was no clear evidence of a difference in mortality between CPAP and control groups, and considerable heterogeneity between trials was noted (risk ratio (RR) 1.28, 95% confidence interval (CI) 0.35 to 4.66; I2 = 75%).
Six studies reported demonstrable atelectasis in the study population. A reduction in atelectasis was observed in the CPAP group, although heterogeneity between studies was substantial (RR 0.62, 95% CI 0.45 to 0.86; I2 = 61%). Pneumonia was reported in five studies, including 563 participants; CPAP reduced the rate of pneumonia, and no important heterogeneity was noted (RR 0.43, 95% CI 0.21 to 0.84; I2 = 0%). The number of participants identified as having serious hypoxia was reported in two studies, with no clear difference between CPAP and control groups, given imprecise results and substantial heterogeneity between trials (RR 0.48, 95% CI 0.22 to 1.02; I2 = 67%). A reduced rate of reintubation was reported in the CPAP group compared with the control group in two studies, and no important heterogeneity was identified (RR 0.14, 95% CI 0.03 to 0.58; I2 = 0%). Admission into the intensive care unit (ICU) for invasive ventilation and supportive care was reduced in the CPAP group, but this finding did not reach statistical significance (RR 0.45, 95% CI 0.18 to 1.14; I2 = 0).
Secondary outcomes such as length of hospital stay and adverse effects were only minimally reported.
A summary of findings table was constructed using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) principle. The quality of evidence was determined to be very low.
Authors' conclusions
Very low‐quality evidence from this review suggests that CPAP initiated during the postoperative period might reduce postoperative atelectasis, pneumonia and reintubation, but its effects on mortality, hypoxia or invasive ventilation are uncertain. Evidence is not sufficiently strong to confirm the benefits or harms of CPAP during the postoperative period in those undergoing major abdominal surgery. Most of the included studies did not report on adverse effects attributed to CPAP.
New, high‐quality research is much needed to evaluate the use of CPAP in preventing mortality and morbidity following major abdominal surgery. With increasing availability of CPAP to our surgical patients and its potential to improve outcomes (possibly in conjunction with intraoperative lung protective ventilation strategies), unanswered questions regarding its efficacy and safety need to be addressed. Any future study must report on the adverse effects of CPAP.
Plain language summary
Is continuous positive airway pressure (CPAP) during the postoperative period useful?
Review question
Does continuous positive airway pressure during the postoperative period help reduce death and major lung complications after major abdominal surgery?
Background
General anaesthesia can lead to reduced lung volumes and collapse of the alveoli as well as to reversible, patchy collapse of areas of lung (atelectasis) and subsequent low oxygenation. These problems are worse in those patients undergoing upper abdominal surgery, in those who have predisposing factors such as obesity and chronic lung disease and in smokers. Continuous positive airway pressure (CPAP) is a type of therapy that uses a high‐pressure gas source to deliver constant pressure to the airways throughout both inspiration and expiration in spontaneously breathing people; oxygen is added in appropriate amounts. CPAP uses a variety of masks, which are placed over the nose or mouth. The aim of this technique is to improve the oxygenation of patients while preventing common postoperative complications in vulnerable people, especially smokers and the obese.
This review was conducted to determine whether any difference can be found in death and major chest complications following major abdominal operations between patients treated with CPAP and those given standard care (oxygen by mask and physiotherapy).
Study characteristics
We searched the literature until 15 September 2013. We included all adults who underwent elective major abdominal surgery. We included only studies in which the intervention was started postoperatively.
We employed the standard methods of the Cochrane Anaesthesia Review Group for data collection and analysis. A total of 709 participants were included in the 10 selected trials. Considerable differences between studies were noted in the populations studied, duration of treatment and supportive care provided.
Key results
Two controlled trials (413 participants) reported deaths; no clear evidence showed a difference between CPAP and control groups. Six trials (249 participants) reported on atelectasis, which was reduced in the CPAP group. Pneumonia was reported in five trials (563 participants), and the rate of pneumonia was reduced in the CPAP group. The need for further respiratory support with artificial ventilation (reintubation) was reported in two studies, which favoured CPAP. No clear evidence revealed a difference between CPAP and control groups in rates of admission to intensive care units, nor were severely low oxygen levels reported.
Few studies reported on length of hospital stay and harm due to CPAP.
Quality of evidence
Substantial variability was seen in trial characteristics (heterogeneity), and risk of bias was high in six of the 10 studies. The included studies were small, and some were at least 20 years old; currently, computed tomography (CT) scans are used more often than chest x‐rays and clinical examination alone for diagnosis. The summary of findings (GRADE) suggests that the strength of evidence supporting the use of CPAP was ‘very low.’ This means that recommendations based on currently available evidence from randomized controlled trials investigating use of CPAP during the postoperative period are not definitive.
Summary of findings
for the main comparison.
| CPAP during postoperative period for participants having major abdominal surgery | ||||||
|
Patient or population: patients having major abdominal surgery Settings: major abdominal surgery Intervention: CPAP during postoperative period as intervention Comparison: usual postoperative care as control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | CPAP during postoperative period | |||||
|
Reported mortality As reported in the trials Follow‐up: up to 5‐7 days |
Study population |
RR 1.28 (0.35 to 4.66) |
413 (2 studies) | ⊕⊝⊝⊝ very lowa,b,c | Data contain only mortality figures reported in the studies | |
| 14 per 1000 | 18 per 1000 (5 to 65) | |||||
| Moderate | ||||||
| 14 per 1000 | 18 per 1000 (5 to 63) | |||||
|
Atelectasis As reported in the trials Follow‐up: up to 1‐10 days |
Study population |
RR 0.62 (0.45 to 0.86) |
249 (6 studies) | ⊕⊝⊝⊝ very lowd,e,f | Data include only the numbers identified in included studies | |
| 441 per 1000 | 240 per 1000 (148 to 371) | |||||
| Moderate | ||||||
| 402 per 1000 | 212 per 1000 (129 to 335) | |||||
|
Pneumonia As reported in the included trials Follow‐up: up to 5‐7 days |
Study population |
RR 0.43 (0.21 to 0.84) |
563 (5 studies) | ⊕⊝⊝⊝ very lowg,h,i | Data include only documented cases in included studies | |
| 81 per 1000 | 32 per 1000 (16 to 67) | |||||
| Moderate | ||||||
| 59 per 1000 | 23 per 1000 (11 to 49) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aRisk of bias for the 2 included studies (Bohner 2002; Squadrone 2005) assessed as unclear. bHeterogeneity is considerable in the methods of intervention and controls in the 2 studies (Bohner 2002; Squadrone 2005). cEven though the 2 studies (Bohner 2002; Squadrone 2005) included around 400 participants, the rate of (mortality) is very low. dFour of the 6 studies in this analysis (Carlsson 1981; Christensen 1991; Lindner 1987; Lotz 1984; Ricksten 1986: Stock 1985) are marked as high risk of bias, and Stock 1985, Carlsson 1981 and Christensen 1991 are marked as unclear risk of bias. eHeterogeneity between the 6 studies included in this analysis is considerable; intervention groups and control groups were somewhat different; duration of intervention and period of observations were also different in these studies. fNumbers of participants and event rates in included trials were low in the included trials. gThree of the selected studies (Bohner 2002; Christensen 1991; Squadrone 2005) were assessed as unclear risk of bias and two of the studies (Lindner 1987; Stock 1985) were assessed as high risk of bias. hIn the included trials, differences in control and intervention groups, methods of assessing outcomes (pneumonia) and time when outcomes were assessed were inconsistent, resulting in important clinical heterogeneity. iThe 5 included studies have varying numbers of participants and low rates of pneumonia. This leads to serious imprecision.
Background
Description of the condition
Major abdominal surgery, which is defined as abdominal surgery requiring laparotomy, can be associated with several serious complications that may impair patient recovery. In particular, postoperative pulmonary complications (PPCs) are a major contributor to postoperative morbidity (Warner 2000). Studies investigating the incidence of PPCs following abdominal surgery have suffered from the use of varying definitions of the term. Thus the documented incidence among patients varies between 9% and 40% (Arozullah 2000). Mortality following all types of inpatient surgery ranges from 0.4% to 1.5% (Haynes 2009). However, a large study conducted to evaluate mortality and morbidity in patients undergoing higher‐risk elective and emergency abdominal and vascular surgery reported mortality rates of 3.5% to 6.9% (Ghaferi 2009).
Atelectasis can be defined as reversible loss of aerated lung (Duggan 2007). Atelectasis may be the result of alveolar collapse from surfactant impairment, gas resorption or lung compression. Atelectasis is no longer considered a benign entity. It can result in reduced lung compliance, increased pulmonary vascular resistance and gas exchange abnormalities. Atelectasis is considered an important postoperative pulmonary complication that increases the risk of postoperative pneumonia and acute respiratory failure. Acute respiratory failure may result in endotracheal intubation, lengthened hospital stay and increased morbidity and mortality (Pelosi 2010). In a prospective cohort study of a broad range of surgical procedures, the 30‐day mortality rate was increased from 1% to 27% in the presence of postoperative respiratory failure (Arozullah 2000).
It has long been recognized that general anaesthesia can impair respiratory function, leading to hypoxaemia (Nunn 1962). Anaesthetic agents can impair central respiratory regulation as well as the function and co‐ordination of respiratory muscles (Warner 2000). The overall effect of this is reduced functional residual capacity, predisposing to atelectasis. It is now recognized that atelectasis occurs in dependent lung regions among most patients under general anaesthesia (Duggan 2005). These changes can persist for several days postoperatively (Lindberg 1992). Mechanisms of atelectasis formation include compression of lung tissue, absorption of alveolar air and impairment of surfactant function (Duggan 2005).
During the postoperative period, contributors to pulmonary dysfunction include residual anaesthetic effects, surgical trauma and pain (Warner 2000). Also important in the development of PPCs are patient risk factors such as age, smoking, pre‐existing respiratory disease, functional status and obesity (Arozullah 2000; Arunotai 2010).
Another potential cause of postoperative hypoxia is upper airway obstruction causing apnoea. It is recognized that obstructive sleep apnoea syndrome (OSAS) is a common and frequently undiagnosed condition. The incidence of OSAS among patients presenting for surgery is estimated to be between 1% and 9%, and most of these are undiagnosed cases (Kaw 2006). Symptoms of OSAS can be exacerbated during the postoperative period, predisposing to PPCs and adverse outcomes (Kaw 2006).
A previous Cochrane review found no evidence to support the use of incentive spirometry (a mechanical device that can increase lung volume by encouraging deep inspiration) for prevention of PPCs following upper abdominal surgery (Guimaraes 2009).
Description of the intervention
Continuous positive airway pressure (CPAP) is a form of non‐invasive respiratory support (NRS). It uses a high‐pressure gas source to deliver constant positive pressure to the airways throughout both inspiration and expiration (Weksler 1991). CPAP can deliver positive pressure to the airways in various ways and may involve one of a variety of masks (nasal, oral, oronasal, full face) or a helmet that covers the whole head (Pelosi 2010).
CPAP therapy in the immediate postoperative period requires staff who are well trained in its use. Traditionally, this treatment has been provided in a specialized environment (e.g. a high‐dependency unit), but it can be used on surgical wards in some hospitals. When CPAP is instituted, pressures of 7 to 10 cm H2O appear well tolerated with few adverse effects. CPAP is often instituted intermittently, for example, for 60 to 90 minutes at two‐ to three‐hourly intervals. Pressures greater than 20 cm H2O are generally avoided following abdominal surgery to reduce the presence of air in the digestive tract (Pelosi 2010).
NRS such as CPAP is now used increasingly to prevent postoperative respiratory complications such as atelectasis after major abdominal surgery. However, several potential contraindications to its use have been identified. These include inability to fit a mask, unco‐operative patients, medical instability and inability of patients to protect their airway (Nava 2009). Poor compliance with CPAP therapy is recognized in patients given long‐term treatment, 46% to 83% of whom are non‐adherent with treatment (Weaver 2008). Whether this will be an issue in the acute setting remains unclear. Potential reasons for non‐compliance include noise from the machine, discomfort caused by the mask, claustrophobia, skin trauma and nasal congestion.
How the intervention might work
CPAP may improve respiratory function postoperatively by increasing functional residual capacity, improving alveolar recruitment and reducing the work of breathing (Nava 2009; Pelosi 2010). Consequences of atelectasis such as pneumonia and acute respiratory failure may subsequently be prevented. Additionally, CPAP may help treat the symptoms of unrecognized OSAS, thus preventing hypoxia.
Cardiac function may improve through reduced left ventricular afterload (Sibbald 1985) and improved oxygenation.
Why it is important to do this review
Major abdominal surgery is frequently associated with postoperative complications. CPAP may help reduce the occurrence of postoperative complications while improving patient outcomes. Currently, no consensus has been reached on the role of prophylactic CPAP following major abdominal surgery.
Objectives
To determine whether any difference can be found in the rate of mortality and adverse events following major abdominal surgery in patients treated postoperatively with CPAP versus standard care, which may include traditional oxygen delivery systems, physiotherapy and incentive spirometry.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) in which CPAP was compared with standard care for prevention of postoperative mortality and morbidity following major abdominal surgery.
Types of participants
We included all adults (adults as defined by individual studies) of both sexes who underwent elective or emergency major abdominal surgery.
We did not exclude patients with co‐morbidities such as obesity, respiratory disease and a history of smoking.
We excluded patients who received bilevel positive airway pressure (BiPAP) and those treated with CPAP perioperatively, because the review was confined to postoperative use of CPAP.
Types of interventions
The intervention of CPAP was applied during the postoperative period. The control group was made up of those who received standard postoperative care, which may have included traditional oxygen delivery systems, physiotherapy and incentive spirometry.
Types of outcome measures
Primary outcomes
All‐cause mortality.
Major respiratory complications as defined in individual studies (significant atelectasis, pneumonia, significant hypoxia, tracheal reintubation, intensive care unit (ICU) admission).
We have accepted 'atelectasis' as defined by the authors of the individual studies and have explored obvious differences between varying definitions used in different studies, which were recorded as sources of clinical heterogeneity. However, if considerable heterogeneity existed between various studies, we did not proceed to meta‐analysis but presented the available data.
Secondary outcomes
Length of stay in hospital.
Cardiovascular complications (myocardial infarction, unstable angina, acute cardiac failure, arrhythmia).
Other postoperative complications (wound infection, anastomotic leak, renal failure).
Adverse effects of the intervention (pulmonary aspiration, upper airway or facial injury).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 9; Ovid MEDLINE (1966 to September 2013); EMBASE (1988 to September 2013); Web of Science (to September 2013); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (to September 2013). The search strategies are given in Appendix 1 (MEDLINE), Appendix 2 (EMBASE), Appendix 3 (CENTRAL), Appendix 4 (Web of Science) and Appendix 5 (CINAHL).
Searching other resources
We also searched reference lists and bibliographical data from all retrieved articles as well as reviews for any additional, relevant material. We endeavoured to contact the relevant authors and known experts in this area to ask for further information on published studies or for unpublished data. We tried to identify unpublished studies or ongoing studies from relevant clinical trial registries. We did not restrict our selection of studies on the basis of language or country of study.
We searched for ongoing trials through the following websites.
Data collection and analysis
Selection of studies
We evaluated all 5236 studies identified by the search methods for appropriateness of inclusion (see Figure 1). We first examined abstracts or summaries of publications. We obtained full publications for studies that required further assessment. Two review authors (CI, TC) evaluated these studies for appropriateness of inclusion without prior consideration of the results. Studies in languages other than English were selected, and translation by foreign language experts was required for some. Consensus on the final selection was reached; if necessary, another review author (MZ) helped to make the final selection.
1.

Study flow diagram.
Data extraction and management
Two review authors (MZ, SFM) independently extracted data using a suitable data extraction form (Appendix 6). Special focus was placed on study design, methods of analysis and relevant study results. Information regarding study methodological quality included method of randomization, concealment of allocation, blinding (masking) used, frequency and handling of withdrawals and completion of an intention‐to‐treat (ITT) analysis. We resolved disagreements through discussion and in consultation with another review author (CI). We attempted to contact the authors of all included trials to obtain additional details on study methodology and missing data as required.
All data were entered and double‐checked by MZ and SFM.
Assessment of risk of bias in included studies
The measures recommended by the Cochrane Anaesthesia Review Group (CARG) were used to assess risk of bias and included the following.
Adequate randomization and concealment of allocation (allocation bias)
Allocation bias: This was assessed as low risk of bias, high risk of bias or unclear risk of bias, as below.
Generation of the allocation sequence: This was considered adequate if a computer‐generated randomization sequence or a random number table was used.
Adequate concealment of allocation: Allocation was performed through a central office; an on‐site computer system with allocation was kept in a locked computer file or numbered and sealed opaque envelopes were used.
Inadequate concealment of allocation: alternation using date of birth, an open list of random numbers or day of the week.
Unclear concealment of allocation: Study did not report any concealment approach or stated that concealment was not used.
Blinding or masking (performance bias)
Information about blinding was sought in the trial reports. However, blinding may not be possible, as differences in the two techniques are very evident.
Completeness of follow‐up (attrition bias)
Information regarding loss of participants from a study after allocation was noted (e.g. withdrawal, dropout, protocol deviation).
Adequacy of follow‐up (detection bias)
This was determined from the following:
Outcomes clearly defined in the text.
Appropriate timing of outcome measures in the text.
Reporting bias (selective reporting)
Information was sought regarding the availability of a study protocol with prespecified outcomes reported in a prespecified way.
Based on the above criteria, the risk of bias was assessed by the review authors (CI, TC) as below; the two review authors resolved disagreements regarding the assessment by discussion and by reaching consensus, if necessary with the help of another review author (MZ).
Low risk of bias: All criteria were adequately met.
High risk of bias: One or more criteria were not met.
Unclear risk of bias: Insufficient information was available to permit assessment of "low" or "high" risk.
Risk of bias table
We generated a risk of bias (ROB) table as recommended by the Cochrane Anaesthesia Review Group (CARG) and as per recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The ROB is given in Figure 2 and Figure 3.
2.
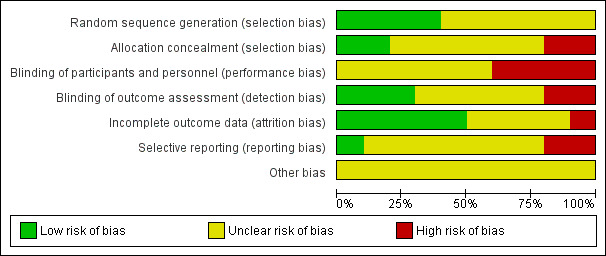
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
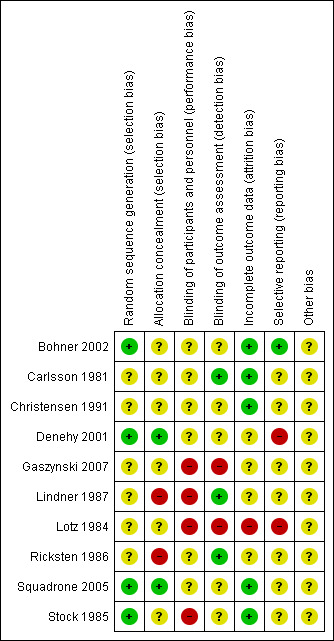
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
We summarized treatment effects using risk ratio (RR) for dichotomous outcomes. We had no continuous outcomes to incorporate in this review because of inconsistent and limited reporting of length of hospital stay, but we would have used mean difference (MD) for this purpose.
Unit of analysis issues
In the unlikely event that cluster‐randomized trials or cross‐over trials were identified, we planned to make sure that they were analysed correctly before they were included in meta‐analyses. It may have been possible to get corrected estimates from what was presented.
Dealing with missing data
We attempted to contact investigators using email as the means to inquire about missing data or to ask for further information on the methodological quality of the studies. Unfortunately, a number of the studies selected were many years old; hence this was mostly unsuccessful.
Assessment of heterogeneity
Clinical heterogeneity was judged by the review authors (CI, MZ, SFM, TC) and the results noted in the review. If significant clinical heterogeneity existed, pooling of data was to be avoided; data from individual studies would have been presented in a tabular format.
We tested for statistical heterogeneity using visual inspection of the forest plot and the I2 statistic (Higgins 2011). We used the following thresholds as a guide to interpretation of the I2 statistic.
0% to 40%: might not be important. 30% to 60%: may represent moderate heterogeneity. 50% to 90%: may represent substantial heterogeneity. 75% to 100%: show considerable heterogeneity.
Assessment of reporting biases
We would have looked for publication bias by using a funnel plot, plotting the size of the treatment effect for the outcome against trial precision (one/standard error), if at least 10 studies were identified in an individual meta‐analysis (Egger 1997). In this case, we would have used a formal statistical test for funnel plot asymmetry. If asymmetry existed, we would have explored and presented the reasons for this, such as publication bias or studies with poor methodology.
Data synthesis
We used Review Manager (RevMan 5.2) to perform quantitative analysis. A fixed‐effect model meta‐analysis was used for synthesis of all data.
Subgroup analysis and investigation of heterogeneity
If it were possible, we would have carried out subgroup analyses for the following.
Bariatric surgery.
Pre‐existing respiratory disease.
Elective versus emergency surgery.
Age.
Continuous versus intermittent CPAP.
Obstructive sleep apnoea syndrome.
If important heterogeneity was not explained by the subgroups above, we examined studies for other factors that may help to explain the heterogeneity.
Sensitivity analysis
Sensitivity analysis would have been performed for studies with low risk of bias versus the others, and for those with adequate allocation concealment versus the others.
Summary of findings table
We summarized the evidence in the Table 1, as recommended by CARG, using the programme GRADEpro (GRADEpro 2008). The GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach appraises the quality of a body of evidence on the basis of the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias.
We had planned to use the GRADEPro system to assess the quality of the body of evidence associated with specific outcomes, as below.
All‐cause mortality.
Major respiratory complications as defined in individual studies (significant atelectasis, pneumonia, significant hypoxia, tracheal reintubation, ICU admission).
Length of stay in hospital.
Cardiovascular complications (myocardial infarction, unstable angina, acute cardiac failure, arrhythmia).
Other postoperative complications (wound infection, anastomotic leak, renal failure).
Adverse effects of the intervention (pulmonary aspiration, upper airway or facial injury).
As we had limited data, we used the following outcomes to construct the SOF table (Table 1).
Reported mortality.
Atelectasis.
Pneumonia.
Results
Description of studies
Details of studies can be found in Characteristics of included studies, Characteristics of excluded studies and Characteristics of studies awaiting classification.
Results of the search
We searched the literature until September 2013. We searched MEDLINE, EMBASE, CENTRAL, CINAHL and Web of Science; the search details are given in Appendix 1; Appendix 2; Appendix 3; Appendix 4; and Appendix 5. The studies identified and subsequently selected are shown in Figure 1.
We searched reference lists and bibliographical data from all retrieved articles and reviews to look for additional, relevant material. We sought information from authors of unpublished studies and contacted recognized experts on this topic about any unpublished data. Unfortunately, many of the studies were old (over 20 years old); hence we were unable to reliably contact the study authors.
Our final selection yielded 10 studies for inclusion in the review (from 5229 identified articles); this occurred for multiple reasons, including use of broad search criteria and duplication in multiple databases.
Included studies
We selected 10 studies for the analysis (see Characteristics of included studies). However, these studies were found to have substantial clinical heterogeneity (Table 2) in the form of different operations, different nature and details of intervention, differences in control groups and differences in the duration of the study and in reporting. Reported outcomes were varied and infrequent (Table 3).
1. Details of study groups.
| Study ID | Surgical procedure | Duration of trial | Duration of follow‐up | Control group: details | Control group: number |
Intervention group: details |
Intervention group: number | Comments |
| Bohner 2002 | Midline laparotomy | 14.0 ± 4.3 hours | Longer than 7 days | O2 via mask | 105 | nCPAP at + 10 cm H2O | 99 | |
| Carlsson 1981 | Open cholecystectomy | 4 hours | 1 day | 30% O2 via bag | 11 | 30% O2 via bag, + 5 to 10 cm H2O | 13 | |
| Christensen 1991 | Upper abdominal surgery | 3 days postop | 3 days | Conventional physiotherapy | 17 | Conventional physio + CPAP using PEP mask | 17 | High‐risk patients; O2 only if hypoxia |
|
Denehy 2001 |
Upper abdominal surgery | 3 days postop | 5 days | Traditional physiotherapy | 13 | Traditional physiotherapy + nasal CPAP at + 10 cm H2O | 32 | Intervention groups × 2, CPAP for 15 and 30 minutes each |
| Gaszynski 2007 | Roux‐en‐Y gastric bypass | 8 hours | 1 day? | O2 via nasal cannula | 9 | CPAP Boussignac device, + 9.4 cm H2O | 10 | |
|
Lindner 1987 |
Upper abdominal surgery | 5 days | 5 days | Standard physiotherapy | 17 | Standard physio + CPAP for 5 days | 17 | |
|
Lotz 1984 |
Upper abdominal surgery | 2 hours in recovery ward | 10 days | O2 by face mask | 16 | CPAP during postoperative period | 16 | 2 further groups, both receiving PEEP during anaesthesia, excluded |
|
Ricksten 1986 |
Upper abdominal surgery | 3 days | 3 days | Deep breathing, hourly | 15 | Hourly, CPAP + 10 to 15 cm H2O | 28 | Combined CPAP and PEP groups combined |
| Squadrone 2005 | Abdominal surgery | 12 hours | 3‐7 days | Venturi mask (FiO2 0.5) | 104 | CPAP mask, + 7.5 cm H2O (FiO2 0.5) | 105 | |
|
Stock 1985 |
Upper abdominal surgery | 4‐72 hours | 3 days | Cough and deep breathing or incentive spirometry | 42 | CPAP mask | 23 | Combined 2 groups as control |
2. Reported outcomes.
| Study ID | All cause mortality | Major respiratory complications | Length of hospital stay, days | Cardiovascular complications | Other postop complications | Adverse effects of intervention | Comments |
| Bohner 2002 | 0/105; 5/99 | Pneumonia: 5/105; 2/99 Severe hypoxia: 17/105; 5/99 Intubation: 5/105; 1/99 |
11.8 ± 18.6; 9.5 ± 6.8 | Cardiac arrest: 2/105; 1/99 |
Delirium: 12/105; 6/99 Renal failure: 3/105; 3/99 |
Nose ulcers: 0/105; 4/99 |
ICU admission: 14/105; 6/99 |
|
Carlsson 1981 |
‐ | Atelectasis (24 hours): 10/11; 10/13 |
‐ | ‐ | ‐ | ‐ | ‐ |
| Christensen 1991 | ‐ | Atelectasis: 9/17; 11/17 Pneumonia: 5/17; 6/17 Intubation: 0/17; 1/17 |
10.4 ± 1.9/ 16.4 ± 3.2 |
‐ | 0/17; 0/17 | ‐ | Converted 95% CI into SD: Mean 10.4; 95% CI 4 to 26; 26 to 10.4 = 15.6 95% CI implies z = 1.96 Error = z (SE) 15.6= 1.96 (SE) 8.0 = SE SE = SD/SQRT (N) 8.0 = SD/SQRT (17) 1.9 = SD |
|
Denehy 2001 |
1 death (no group) |
Postop pulmonary complications: 4/18; 3/32 |
12.3 ± 4.8; 12.0 ± 4.5 |
‐ | ‐ | ‐ | ‐ |
| Gaszynski 2007 | 0/9; 0/11 | Intubation: 0 /9; 0/11 |
‐ | ‐ | ‐ | ‐ | ‐ |
| Lindner 1987 | ‐ | Atelectasis: 4/17; 0/17 Consolidation: 1/17; 1/17 Intubation: 0/17; 0/17 |
‐ | ‐ | ‐ | ‐ | ‐ |
|
Lotz 1984 |
‐ | Respiratory complications (?atelectasis): 6/16; 3/16 | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Ricksten 1986 |
‐ | Atelectasis: 6/15; 1/28 |
‐ | ‐ | ‐ | ‐ | ‐ |
| Squadrone 2005 | 3/104; 0/105 |
Pneumonia: 10/104; 2/105 |
17 ± 15; 15 ± 13 | ‐ | Infection: 11/104; 3/105 Sepsis: 9/104; 2/105 |
‐ | ‐ |
| Stock 1985 | ‐ | Atelectasis: 17/42; 5/23 |
‐ | ‐ | ‐ | ‐ | ‐ |
Excluded studies
We had to exclude many studies at the final selection process (see Characteristics of excluded studies). Reasons for this included interventions that were outside our selection criteria and lack of randomization. As we planned to select studies that used CPAP only during the postoperative period, we did not select studies in which positive end‐expiratory pressure (PEEP) was used intraoperatively, followed by CPAP in the postoperative period.
We identified two studies that are awaiting final reporting (Characteristics of ongoing studies), and we have been unable to get one publication translated to this point (Characteristics of studies awaiting classification). We hope to address this in our next update of this review.
Risk of bias in included studies
The most disappointing part of the review was that our assessment determined that most of the selected studies (six out of 10) were at high risk of bias, and in the remaining studies the risk of bias was unclear (Table 4). The main reason for this could be that most of the selected studies were old, and methodological quality and reporting in those studies were inadequate or insufficient. Many of the studies were at least 20 years old; therefore, we were unsuccessful in contacting most of the study authors to obtain further details (Denehy 2001; Squadrone 2005; Stock 1985; see Table 4).
3. Risk of bias data.
| Study ID | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) | Other bias | Overall risk of bias judgement |
| Bohner 2002 | Randomization using a random list | No description of allocation concealment | Anaesthesiologist; not sure of anyone else | Not described in the text | Dropouts described | No evidence for this | None reported |
Unclear |
| Carlsson 1981 | No details of randomization | Not described | Not described |
Radiologist was unaware of treatments | All participants accounted for | Not sure | Not sure | Unclear |
| Christensen 1991 | Randomly allocated into 3 groups | No evidence for it | No evidence for it | No evidence for it | Reasonable account | Not sure |
Not sure |
Unclear |
| Denehy 2001 | Randomly allocated into 3 groups with use of sealed envelopes | Sealed envelopes | Not described | Only radiologist possibly blinded (partial blinding only) | Not described | No detailed demographics of any complications | Not sure | High |
| Gaszynski 2007 | 'Randomly divided into two groups,' no further description | No description |
Not stated | Not stated | Not clear |
Not clear |
Not sure | High |
| Lindner 1987 | Randomized into 2 groups | No description for it | Not stated | Not stated | Probably OK | Not sure | Not sure | High |
|
Lotz 1984 |
Randomized into 4 groups | No description in text, but possible | No details | No details | Scarcity of outcomes of interest | No information | Not sure | High |
| Ricksten 1986 | Stratification and randomization (unclear method) | No evidence |
Not sure |
Blinded radiologist, not sure of others | Not clear | Not clear | Not sure | High |
| Squadrone 2005 | Centrally through dedicated website using computer‐generated block randomization schedule | Yes, concealed central randomization | Not described |
Not described |
Yes | Premature stopping of trial |
Not sure | Unclear |
|
Stock 1985 |
A computer random number generator was used to assign each participant | Most likely adequate |
Not described |
Not described |
Probably all participants accounted for |
Probable |
Probable |
High |
Allocation
Randomization was adequate in four trials (Bohner 2002; Denehy 2001; Squadrone 2005; Stock 1985) but was not reported in detail in the other studies.
Allocation concealment was described in only three studies (Denehy 2001; Squadrone 2005; Stock 1985), and these details are lacking in the other publications.
Blinding
Blinding to the radiologist was reported in three studies (Carlsson 1981; Denehy 2001; Ricksten 1986) but not in the other studies. No other evidence suggested blinding during the conduct of the trials.
Incomplete outcome data
Data reporting seems to be complete in four studies (Carlsson 1981; Christensen 1991; Lindner 1987; Stock 1985);information obtained from the other studies does not give evidence of any issues related to incomplete outcome reporting but does not confirm that the reporting is complete.
Selective reporting
Reporting bias could be identified in only one study (Stock 1985).
Other potential sources of bias
None of the studies revealed any other possible biases, but one study (Stock 1985) gave a clear indication of no further biases.
Effects of interventions
See: Table 1
As was previously mentioned, substantial heterogeneity exists between studies for some outcomes, and the methodological quality of the included studies was poor. We therefore were somewhat hesitant to perform meta‐analyses of the data; however, we did completedata analysis and prepared tables for the different outcomes of interest. We have provided a narrative description of the main outcomes, which are summarized in Table 3.
Primary outcomes
1. All‐cause mortality
Two included RCTs (Bohner 2002; Squadrone 2005) reported the main primary outcome of interest: all‐cause mortality. Seven postoperative deaths were reported among 413 participants: three of 209 (0.73%) participants in the control group, and four of 204 (0.97%) in the CPAP group. No clear evidence of a difference in mortality was found between CPAP and control groups, and considerable heterogeneity was noted between available trials (Analysis 1.1; RR 1.28, 95% confidence interval (CI) 0.35 to 4.66; I2 = 75%).
1.1. Analysis.
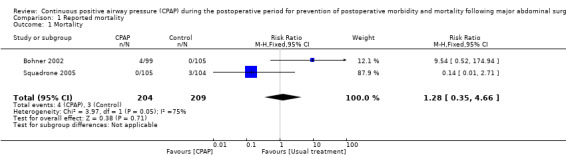
Comparison 1 Reported mortality, Outcome 1 Mortality.
2. Major respiratory complications as defined in individual studies (significant atelectasis, pneumonia, significant hypoxia, tracheal reintubation, ICU admission)
We have reported the data given in the selected publications and have used the criteria listed in those publications (Table 3).
Atelectasis: Six studies including 249 participants reported demonstrable atelectasis (Carlsson 1981; Christensen 1991; Lindner 1987; Lotz 1984; Ricksten 1986; Stock 1985). Atelectasis was a common finding, with 39 of 131 (29.8%) participants in the CPAP group and 52 of 118 (44.1%) in the control group diagnosed on days one to five postoperatively. A reduction in atelectasis in the CPAP group reached statistical significance, although clinical heterogeneity between studies was substantial (Analysis 2.1; RR 0.62, 95% CI 0.45 to 0.86; I2 = 61%).
2.1. Analysis.
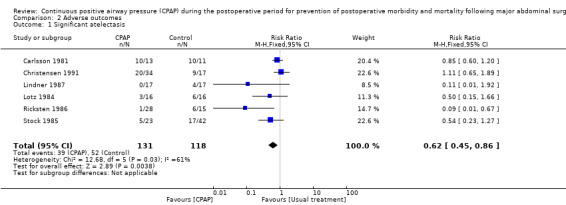
Comparison 2 Adverse outcomes, Outcome 1 Significant atelectasis.
Pneumonia was reported in five studies with 563 participants (Bohner 2002; Christensen 1991; Lindner 1987; Squadrone 2005; Stock 1985). This was seen in 12 participants in the CPAP group (4.3%) and 23 participants (8.1%) in the control group. Reduction of pneumonia in the CPAP group was statistically significant, with no important statistical heterogeneity observed between studies (Analysis 2.2; RR 0.43, 95% CI 0.21 to 0.84; I2 = 0%).
2.2. Analysis.
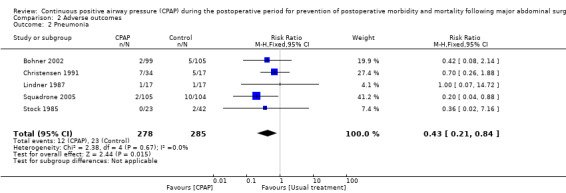
Comparison 2 Adverse outcomes, Outcome 2 Pneumonia.
Significant hypoxia: The number of participants identified as having severe hypoxia was reported in two studies with a total of 255 participants (Bohner 2002; Christensen 1991). The CPAP group reported 11 of 133 (8.3%) and the control group 19 of 122 (15.6%), with no statistically significant advantage for CPAP and substantial heterogeneity between trials (Analysis 2.3; RR 0.48, 95% CI 0.22 to 1.02; I2 = 67%).
2.3. Analysis.
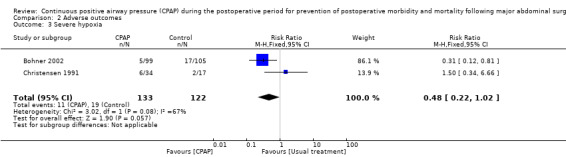
Comparison 2 Adverse outcomes, Outcome 3 Severe hypoxia.
Tracheal reintubation was reported in two studies with a total of 411 participants (Bohner 2002; Squadrone 2005). A statistically significant reduction was seen in postoperative reintubation of participants in the CPAP group versus the control group, and no important heterogeneity was noted between these studies (Analysis 2.4; RR 0.14, 95% CI 0.03 to 0.58; I2 = 0%).
2.4. Analysis.
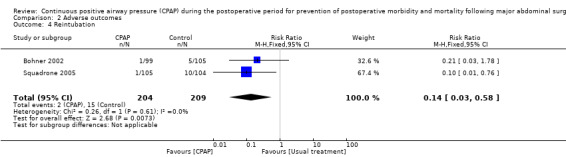
Comparison 2 Adverse outcomes, Outcome 4 Reintubation.
ICU admission for invasive ventilation and supportive care was reported in one study (Bohner 2002), which included a total of 204 participants. Reported rates of intubation were reasonably high, with six of 99 (6.1%) in the CPAP group and 14 of 105 (13.3%) in the control group admitted to the ICU (RR 0.45, 95% CI 0.18 to 1.14; I2 = 0%). This study reported a total of 20 postoperative ICU admissions, the reasons for which are not clearly documented.
Secondary outcomes
1. Length of hospital stay: Four studies (Bohner 2002; Christensen 1991; Denehy 2001; Squadrone 2005) including 497 participants reported on length of hospital stay (see Table 3). We were reluctant to formally analyse these data because of the multiplicity of reasons presented for discharge from the hospital.
2. Cardiovascular complications (myocardial infarction, unstable angina, acute cardiac failure, arrhythmia): Only one study reported cardiac complications of any kind. Bohner 2002 described cardiac arrest in two of 105 participants in the control group and in one of 99 participants in the CPAP group. None of our predetermined outcomes (myocardial infarction, unstable angina, acute cardiac failure, arrhythmia) were reported.
3. Other postoperative complications (wound infection, anastomotic leak, renal failure): Three studies (Bohner 2002; Christensen 1991; Squadrone 2005) reported instances of renal failure, anastomotic leak and/or infection during the postoperative period (Table 3).
4. Adverse effects of the intervention (pulmonary aspiration, upper airway or facial injury): One RCT (Bohner 2002) reported the incidence of nasal ulcers, which were noted in four of 99 participants receiving nasal CPAP and in no participants in the control group (0/105) (Table 3).
Discussion
Summary of main results
We conducted a limited meta‐analysis of available outcome data from the 10 selected studies (see the table of data and analysis and Table 1).
No clear evidence of a difference in postoperative mortality was found between CPAP and control groups in the two studies that reported this outcome (Bohner 2002; Squadrone 2005), and clinical heterogeneity between these studies was substantial. The rate of mortality following major abdominal surgery in these RCTs was consistent with previously documented rates (Haynes 2009). No deaths were reported in either group in one study (Gaszynski 2007), possibly because both of these studies involved younger participants undergoing bariatric surgery. All three deaths in the control group were reported by Squadrone 2005 among participants undergoing elective major abdominal surgery;no details were given as to the cause of death. All four postoperative deaths in the CPAP group were reported by Bohner 2002 among participants undergoing vascular surgery by midline laparotomy. Two participants died of surgical complications: one from cardiac failure and another from septic shock of unknown source. It may be that the small number of included studies was not sufficiently powered to determine a difference in mortality—a relatively uncommon outcome—between CPAP and control groups.
We identified major respiratory complications as they were defined and reported in the individual studies.
Atelectasis was common on days two to five postoperatively, and this outcome was reduced in the CPAP groups compared with the control groups (Analysis 2.1). However, the methodological quality of these earlier trials was poor, and heterogeneity between trials was substantial. The presence of atelectasis has been shown to impair lung compliance, increase pulmonary vascular resistance, impair oxygenation and predispose to lung injury (Duggan 2005); it has been cited as an important factor in the development of postoperative respiratory complications and as a clinical entity in itself, requiring targeted postoperative treatment to prevent hypoxia (Pelosi 2010; Tusman 2012); therefore, this finding is encouraging. However, all of the included studies were small and at least 20 years old, and CT scanning has evolved in recent times as a more accurate diagnostic tool than chest x‐ray or clinical evaluation (Brismar 1985; Lindberg 1992). Therefore, our finding must be interpreted with caution.
It is thought that atelectasis is a precursor to the development of pneumonia, and although this seems likely clinically, no direct causal association between the presence of atelectasis and the development of pneumonia itself has been confirmed to date (Tusman 2012). Fewer participants developed postoperative pneumonia in the CPAP groups compared with the control groups. Evidence of this in the meta‐analysis is significant (Analysis 2.2). Similarly, some evidence of a reduction in severe hypoxia was seen in the CPAP groups compared with the control groups, but the two included studies reporting severe hypoxia during the postoperative period were assessed as having an unclear risk of bias (Table 4), and substantial heterogeneity between them was noted.
A reduction in postoperative admission to the ICU in the CPAP groups versus the control groups was noted in one study, although this finding did not reach statistical significance. In the Bohner 2002 study, a total of 20 postoperative ICU readmissions were reported. The causes were not specifically reported, although the study authors commented that their cohort of participants were elderly with significant co‐morbidities, and that admission was due primarily to cardiac and pulmonary complications. The possibility of a reduction in ICU admissions among patients receiving postoperative CPAP is encouraging; however, our analysis cannot confirm this as fact.
We found a statistically significant reduction in postoperative reintubation in the CPAP groups versus the control groups. Two studies (Bohner 2002; Squadrone 2005) including 411 participants evaluated this outcome. Documentation as to whether the reintubations were due to respiratory complications was incomplete, but nonetheless, the use of CPAP may have prevented this significant clinical event in some participants. Although no important heterogeneity was observed between these studies, this result again must be interpreted with caution, as the risk of bias in these trials is unclear.
Reporting of cardiovascular complications was minimal, with one study reporting cardiac arrest in three participants undergoing elective vascular surgery via a midline laparotomy (Bohner 2002), and analysis of the data was not possible.
Length of hospital stay was reported in some studies, but we were unable to analyse these data. Available data have been reported in Table 3. The modern practice of 'fast track surgery' will make it impossible to combine data from previous years with those of recent years (Olsen 2011).
Bohner 2002 was the sole study to describe any adverse effects of the use of CPAP, but data were insufficient for analysis. Of note, pulmonary aspiration, a recognized and serious complication of CPAP use that is associated with considerable morbidity and mortality, was not reported in any of the included RCTs.
Overall completeness and applicability of evidence
Only 10 eligible studies were included in this review; this number was insufficient to allow firm conclusions to be drawn regarding our primary outcome measures. We identified major respiratory complications as they were defined and reported in the individual studies; however, this lead to clinical heterogeneity between the included studies. Despite this fact, we were able to analyse data for all primary outcome measures, although only a few eligible trials were available. A paucity of secondary outcomes measured was noted; some were not reported at all in any of the selected trials.
We were unable to perform subgroup analysis because of the inadequate number of studies identified. Therefore, we were unable to evaluate whether observed differences in the CPAP groups versus the control groups were more representative of certain patient groups, such as those with underlying respiratory disease or undergoing emergency surgery. We did not complete a sensitivity analysis to examine outcomes in any studies at low risk of bias, as all studies were at high or unclear risk. We were unable to contact some study authors to obtain further information, as many of the studies were at least 20 years old, and contact details were scarce.
Quality of the evidence
Unfortunately, the overall quality of evidence available for this review was disappointing, and several trials were more than 20 years old with poor methodological quality. Four trials had an unclear risk of bias, and the remaining trials were classified as high risk. A lot of information required to assess the risk of bias was unclear or was not stated. Most commonly, allocation concealment and blinding of participants and personnel were not adequately addressed. All trials were RCTs, but in only three trials was the method of allocation clearly described (Denehy 2001; Squadrone 2005; Stock 1985). It is impossible to blind all personnel to the use of CPAP against standard care, as the difference is very evident, but consistent blinding of some participants and observers such as radiologists was not achieved.
We constructed the summary of findings (SOF) table in accordance with the GRADE principle (see Table 1). Even though the meta‐analysis suggests an advantage of CPAP over control measures, the SOF tables show that the quality of evidence is 'very low' for the reported outcomes of mortality, atelectasis and pneumonia. Reasons for this include the methodological quality of selected studies (high risk of bias of the included studies) and inconsistency and imprecision of reporting (caused most often by clinical heterogeneity among selected studies).
Potential biases in the review process
The review protocol was thorough and included a comprehensive search strategy using multiple sources, independent screening of trials for inclusion and independent data extraction. Risk of bias of individual studies was assessed using measures recommended by the Cochrane Anaesthesia Review Group (CARG). We analysed pooled data for the primary outcome measures, despite the presence of substantial heterogeneity between studies for some outcomes, which may introduce bias into this review. As such, we have been cautious in interpreting these results.
The GRADE method of construction of SOF tables may have been influenced by interpretation of the review authors. Only three of the six predefined outcome measures were included for evaluation in the SOF tables because of insufficient data; we acknowledge that this could be a source of bias in the review. We plan to expand the table in the next review update to include the remaining outcomes.
Agreements and disagreements with other studies or reviews
Only one previous systematic review has explored this topic; it was published in 2008 (Ferreyra 2008). Differences between the 2008 review and the current review include the exclusion of emergency and vascular surgery and differing definitions of pulmonary complications. Ferreyra 2008 concluded that CPAP significantly reduces the risk of postoperative pulmonary complications, namely, atelectasis and pneumonia. The current review also found a significant reduction in atelectasis and pneumonia. We used the GRADE method to construct SOF tables in this review; these pointed to the strength of evidence as 'very low.' The previous review (Ferreyra 2008) suggested that evidence supports the use of CPAP in patients undergoing abdominal surgery. We are unable to make such a recommendation because the quality of evidence as indicated from the SOF table was 'very low.' Also note that our review is confined to studies in which CPAP was initiated after major abdominal surgery and does not include studies in which CPAP is initiated during the intraoperative period and is continued into the postoperative phase.
Authors' conclusions
Implications for practice.
Very low‐quality evidence from this systematic review suggests that CPAP initiated during the postoperative period after major abdominal surgery might reduce postoperative atelectasis, pneumonia and reintubation, but its effects on mortality, hypoxia and invasive ventilation are uncertain. Evidence is not sufficiently strong to permit conclusions on the benefits or harms of CPAP during the postoperative period in reducing mortality and morbidity following major abdominal surgery. Summary of findings data obtained from the GRADE analysis reveal that the strength of evidence supporting the use of CPAP during the postoperative period is very low. None of the studies assessed provided reasons to be cautious about using CPAP during the postoperative period, mainly because most of these studies did not report on adverse effects attributed to CPAP.
Implications for research.
New, high‐quality research is much needed to definitively evaluate the use of CPAP in preventing mortality and morbidity following major abdominal surgery. A targeted approach investigating the use of CPAP in patients at higher risk for postoperative respiratory complications would be of value. A focus on well‐defined, pertinent outcomes, including adverse events, of CPAP use should be employed. With increasing availability of CPAP for our surgical patients and its potential to improve outcomes (possibly in conjunction with intraoperative lung protective ventilation strategies), unanswered questions regarding its efficacy and safety need to be addressed.
Future studies must report on all adverse effects of CPAP. These studies should standardize the equipment used for CPAP, CPAP pressures applied and duration of treatment provided. Reporting standards should be more uniform and should include such items as the number of participants with adverse effects and the duration of reporting of outcomes of interest.
History
Protocol first published: Issue 1, 2011 Review first published: Issue 8, 2014
| Date | Event | Description |
|---|---|---|
| 18 January 2012 | Amended | Contact details updated. |
Acknowledgements
We acknowledge help received from Karen Hovhannisyan (CARG Trial Search Co‐ordinator) in development of the search strategy.
We would like to thank Andrew Smith (content editor); Nathan Pace (statistical editor); and Rodrigo Cavallazzi, Shekhar T Venkataraman and William McIlvaine (peer reviewers) for help and editorial advice provided during preparation of the protocol for this systematic review. We would like to thank Dr Jochen Maierl, Dr Martin Dvoracek and Suzanne Mosser for translations of Russian and German publications. We would like to thank Andrew Smith (content editor); Nathan Pace (statistical editor); Rodrigo Cavallazzi and Andrea Cortegiani (peer reviewers); and Janet Wale (consumer editor) for help and editorial advice provided during preparation of this review. We acknowledge Dolores Matthews for the excellent copyediting of this manuscript.
Appendices
Appendix 1. Ovid MEDLINE search strategy
1. exp Positive‐Pressure Respiration/ or exp Continuous Positive Airway Pressure/ or Respiration, Artificial/ 2. (positive adj5 (airway or pressure)).mp. or (sustained adj3 inflation).ti,ab. or CPAP.mp. 3. 1 or 2 4. (((surger* or surgic* or perat*) adj3 (abdom?n* or hepar* or hepat* or gastro* or pancrea* or biliar* or chole* or stomach* or intestin* or bowel* or colon*)) or post?operat*).ti,ab. 5. exp Postoperative Care/ or exp Postoperative Complications/ or exp Postoperative Period/ or Pulmonary Atelectasis/ or exp General Surgery/ or exp Surgical Procedures, Operative/ or Surgical Procedures, Elective/ or exp Colorectal Surgery/ or exp Bariatric Surgery/ or exp Digestive System Surgical Procedures/ or exp Biliary Tract Surgical Procedures/ 6. 5 or 4 7. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 8. 3 and 7 and 6
Appendix 2. EMBASE search strategy
1 positive end expiratory pressure/ or ((sustained adj3 inflat*) or CPAP or (spontaneous adj3 breathing) or (Continuous adj3 Airway Pressure)).ti,ab. 2 postoperative care/ or postoperative period/ or postoperative complication/ or atelectasis/ or general surgery/ or surgical technique/ or elective surgery/ or colorectal surgery/ or bariatric surgery/ or abdominal surgery/ or biliary tract surgery/ or (((surger* or surgic* or perat*) adj3 (abdom?n* or hepar* or hepat* or gastro* or pancrea* or biliar* or chole* or stomach* or intestin* or bowel* or colon*)) or post?operat* or prevent* or treatment*).ti,ab. or (elective adj3 emergenc*).ti,ab. 3 (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab.) not (animals not (humans and animals)).sh. 4 1 and 2 and 3
Appendix 3. CENTRAL search strategy
#1MeSH descriptor Continuous Positive Airway Pressure explode all trees #2(((sustained adj3 inflat*) or CPAP or (spontaneous adj3 breathing))):ti,ab #3(#1 OR #2) #4MeSH descriptor Postoperative Care explode all trees #5MeSH descriptor Postoperative Complications explode all trees #6MeSH descriptor Postoperative Period explode all trees #7MeSH descriptor Pulmonary Atelectasis explode all trees #8MeSH descriptor General Surgery, this term only #9MeSH descriptor Surgical Procedures, Operative, this term only #10MeSH descriptor Surgical Procedures, Elective explode all trees #11MeSH descriptor Colorectal Surgery explode all trees #12MeSH descriptor Bariatric Surgery explode all trees #13MeSH descriptor Digestive System Surgical Procedures explode all trees #14MeSH descriptor Biliary Tract Surgical Procedures explode all trees #15(((surger* or surgic* or perat*) adj3 (abdom?n* or hepar* or hepat* or gastro* or pancrea* or biliar* or chole* or stomach* or intestin* or bowel* or colon*)) or post?operat* or prevent* or treatment*):ti,ab #16(elective and emergenc*):ti,ab #17(#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16) #18(#3 AND #17)
Appendix 4. Web of Science search strategy
#1 TS=(Continuous SAME (Airway Pressure)) or TS=(sustained SAME inflat*) or TS=(spontaneous SAME breathing) or TS=CPAP
#2 TS=((surger* or surgic* or perat*) SAME (abdom?n* or hepar* or hepat* or gastro* or pancrea* or biliar* or chole* or stomach* or intestin* or bowel* or colon*)) or TS=(post?operat* or prevent* or treatment*) or TS=(elective and emergenc*)
#3 TS=(random* or (clinical SAME trial*) or placebo* or multicenter* or prospectiv* or ((single or double or triple) SAME (mask* or blind*)))
#4 #3 AND #2 AND #1
Appendix 5. CINAHL search strategy
S1 MH Continuous Positive Airway Pressure
S2 (sustained adj3 inflat*) or CPAP or (spontaneous adj3 breathing)
S3 S1 or S2
S4 MH Postoperative Care or MH Postoperative Complications or MH Postoperative Period or Pulmonary Atelectasis or MH General Surgery or MH Surgical Procedures, Operative or Surgical Procedures, Elective or MH Colorectal Surgery or MH Bariatric Surgery or MH Digestive System Surgical Procedures or MH Biliary Tract Surgical Procedures
S5 AB ( surger* or surgic* or perat* ) and ( abdomen* or hepar* or hepat* or gastro* or pancrea* or biliar* or chole* or stomach* or intestin* or bowel* or colon* )
S6 TX elective and emergenc*
S7 AB post?operat* or prevent* or treatment*
S8 S4 or S5 or S6 or S7
S9 (MH "Random Assignment")
S10 (MH "Clinical Trials+")
S11 (MM "Double‐Blind Studies") or (MM "Single‐Blind Studies") or (MM "Triple‐Blind Studies")
S12 (MH "Placebos")
S13 (MM "Multicenter Studies")
S14 (MH "Prospective Studies+")
S15 S9 or S10 or S11 or S12 or S13 or S14
S16 S3 and S8 and S15
Appendix 6. Data extraction form
| Author who extracted data? |
CI/TC/MZ | Date: | |
| Article |
MEDLINE ID |
Language | |
| Authors |
Year of publication | Volume/No. | Pages |
| Include? |
Yes/No | ||
| Reasons for exclusion | |||
| Need further details | |||
| Study type |
RCT | CCT | |
| Intervention group |
No. | Duration | Sex |
| Nature of CPAP |
Duration of CPAP | Weight/BMI | |
| Control group |
No. | Duration | Sex |
| Nature of control |
Duration of control | Weight/BMI | |
| Randomization |
Intervention | Control | Bias level |
| Concealment of allocation |
Intervention | Control | Bias level |
| Blinding |
Intervention | Control | Bias level |
| Dropouts |
Intervention | Control | Bias level |
| Outcome details |
Intervention | Control | Bias level |
| Reporting |
Bias level | ||
| Overall risk of bias |
Bias level | ||
| Mortality |
Intervention | Control | |
| Major respiratory complications: (significant atelectasis, pneumonia, significant hypoxia, tracheal reintubation, ICU admission) |
Intervention |
Control | |
| Length of hospital stay |
Intervention | Control | |
| Cardiovascular complications: (myocardial infarction, unstable angina, acute cardiac failure, arrhythmia) |
Intervention | Control | |
| Other postoperative complications: (wound infection, anastomotic leak, renal failure) |
Intervention | Control | |
| Adverse effects of the intervention: (pulmonary aspiration, upper airway or facial injury) |
Intervention | Control |
Data and analyses
Comparison 1. Reported mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 2 | 413 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.35, 4.66] |
Comparison 2. Adverse outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Significant atelectasis | 6 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.45, 0.86] |
| 2 Pneumonia | 5 | 563 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.21, 0.84] |
| 3 Severe hypoxia | 2 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.22, 1.02] |
| 4 Reintubation | 2 | 413 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.03, 0.58] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bohner 2002.
| Methods | Study was done in the hospitals of Heinrich‐Heine University, Germany | |
| Participants | Adults undergoing midline laparotomy for elective vascular surgery; abdominal aortic aneurysm surgery; and surgery of visceral, renal and iliac arteries or thrombectomy of IVC Excluded were emergency surgery, thoracoabdominal surgery or retroperitoneal approach Participants were randomly assigned to 2 groups with use of a random list: Control group: 105 participants; age, years: 64.5 ± 11.3; male/female: 82:23; BMI: 25.0 ± 3.3 Intervention group: 99 participants; age, years: 64.1 ± 12.3; male/female: 84:15; BMI: 25.4 ± 3.5 All participants received similar fluid regimen, analgesic routine and medications All participants were extubated soon after surgery and were admitted to intermediate care unit or intensive care unit |
|
| Interventions | Control group: Oxygen was administered at ambient pressure via a non‐occlusive face mask, including mouth and nose or nose cannulas to keep oxygen saturation > 95%; FiO2 was adjusted to achieve this Intervention group: received prophylactic nCPAP. nCPAP mask was placed on admission to the unit, using a high gas flow source and a standard PEEP valve set at 10 cm H2O. FiO2 was adjusted to keep SpO2 > 95% and to keep nCPAP mask on for at least 12 hours |
|
| Outcomes | Duration of intervention: 14.0 ± 4.3 hours Duration of follow‐up: longer than 7 days All‐cause mortality: control group, 0/105: intervention group, 4/99 Major respiratory complications as defined in individual studies Significant atelectasis: none reported Pneumonia: control group, 5/105: intervention group, 2/99 Respiratory failure: none reported Severe hypoxia: control group, 17/105: intervention group, 5/99 Severe delirium: control group, 12/105: intervention group, 6/99 Need for tracheal intubation and invasive ventilation: control group, 5/105: intervention group, 1/99 Readmission to ICU/IMC: control group, 14/105: intervention group, 6/99 Length of stay in hospital: control group, 11.81 ± 18.61 days: intervention group, 9.45 ± 6.79 days Cardiovascular complications (myocardial infarction, unstable angina, acute cardiac failure, arrhythmia): Cardiac arrest: control group, 2/105: intervention group, 1/99 Other postoperative complications (wound infection, anastomotic leak, renal failure): Renal failure: control group, 3/105: intervention group, 3/99 Adverse effects of the intervention (pulmonary aspiration, upper airway or facial injury): Nose ulcers: control group, 0/105: intervention group, 4/99 |
|
| Notes | BMI = body mass index FiO2 = Inspired oxygen fraction IVC = inferior vena cava nCPAP = nasal CPAP PEEP = positive end‐expiratory pressure ICU = intensive care unit IMC = intermediate care unit No response to email |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization using a random list |
| Allocation concealment (selection bias) | Unclear risk | Randomization using a random list, but no description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Anaesthesiologist at the operation site did not receive any information about the results of randomization; no evidence of anyone else being blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described in the text |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts described because 9/99 participants did not want to continue with nCPAP after 5.75 ± 4.80 hours |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting in text |
| Other bias | Unclear risk | None reported |
Carlsson 1981.
| Methods | Study done at University Hospital, Lund, Sweden | |
| Participants | Healthy patients undergoing open elective cholecystectomy using right subcostal incision (no intercostal blocks) Control group: 11 participants; age, years: 68.09 ± 9.72; male/female: 2:9 Intervention group: 13 participants; age, years: 62.08 ± 9.52; male/female: 8:5 20/24 participants in total were > 20% overweight according to Broca Index |
|
| Interventions | Male/female, 10:14; age, years: 50‐78 Control group: 11 participants; 30% prewarmed and humidified oxygen without a rubber bag, but with no PEEP Intervention group: 13 participants; 30% prewarmed and humidified oxygen via a rubber bag and PEEP of 5 to 10 cm H2O Treatment and control for 4 hours during the immediate postoperative period in the ward |
|
| Outcomes | Duration of intervention: 4 hours Duration of follow‐up: 24 hours (1 day) All‐cause mortality: none reported Major respiratory complications as defined in individual studies: Significant atelectasis (seen on 24‐hour x‐ray): control group, 10/11; intervention group, 10/13 Pneumonia: none reported (changes on x‐ray "such as atelectasis and pneumonia" were noted but not distinguished further; hence the data are included under atelectasis) Respiratory failure: none reported Severe hypoxia: not reported Need for tracheal intubation and invasive ventilation: none reported Admission to ICU/IMC: none reported Length of stay in hospital: not reported Cardiovascular complications (myocardial infarction, unstable angina, acute cardiac failure, arrhythmia): none reported Other postoperative complications (wound infection, anastomotic leak, renal failure): none reported Adverse effects of the intervention (pulmonary aspiration, upper airway or facial injury): none reported |
|
| Notes | PEEP = positive end‐expiratory pressure ICU = intesive care unit IMC = intermediate care unit Old study, no chance of getting additional information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | On the postoperative ward, participants were randomly assigned to 2 groups; no details |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Radiologist was unaware of treatments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Unclear risk | Not sure |
| Other bias | Unclear risk | Not sure |
Christensen 1991.
| Methods | Study done in Department of Anesthesiology and Respiratory Medicine, University Hospital of Aarhus, Denmark | |
| Participants | High‐risk adult patients scheduled for upper abdominal surgery (elective biliary and ventricular surgery). Participants were divided into 3 groups: control group; PEP group (PEP group used a mask similar to a CPAP mask, employs an expiratory resistance during breathing); and RMT group (RMT group is similar to PEP group, but the mask provides both inspiratory and expiratory resistance). We combined PEP and RMT groups as the intervention (CPAP) group for inclusion in the review Control group: 17 participants; male/female, 5:12; age, years: 62 (51‐83); weight, kg: 68.5 (53‐94) Intervention group: PEP group: 17 participants; male/female: 8/9; age, years: 63.7 (range 50‐80); weight, kg: 68.7 (range 39‐88) RMT group: 17 participants; male/female: 3/14; age, years: 64.2 (53‐79); weight, kg: 63.5 (range 43‐90) |
|
| Interventions | Control group: conventional physiotherapy, given to all participants in all groups; started during preoperative period; continued into postoperative period for 3 days; consisted of breathing exercises and forced expiration techniques; twice a day for 3 days and every hour during waking hours by participants Intervention group: conventional physiotherapy as well as CPAP using a PEEP mask; 5 to 15 cm H2O expiratory pressure, given preoperatively for practice; continued during postoperative period, twice daily for 3 days. RMT group had PEP mask (5‐7 cm H2O) + inspiratory resistance chosen according to participants' ability to tolerate the mask We combined CPAP and RMT groups for the purpose of this review and interventions Oxygen was given only if hypoxia was present |
|
| Outcomes | Duration of intervention: 3 days Duration of follow‐up: 3 days All‐cause mortality: none reported Major respiratory complications as defined in individual studies: Atelectasis: control group, 9/17; intervention groups: PEP group, 11/17; RMT, 9/17 Pneumonia: control group, 5/17; intervention groups; PEP group, 6/17; RMT, 1/17 Respiratory failure: none reported Severe hypoxia: control group, 2/17; intervention groups: PEP, 4/17; RMT, 2/17 Need for tracheal intubation and invasive ventilation: control group, 0/17; intervention groups: PEP, 1/17; RMT, 1/17 Admission to ICU/IMC: none reported Length of stay in hospital, days: control group: 10.4 (95% CI 4 to 26) (SD = 1.9); intervention groups: PEP, 16.4 (range 5‐42); RMT, 11.5 (range 5‐42) Cardiovascular complications (myocardial infarction, unstable angina, acute cardiac failure, arrhythmia): none reported Other postoperative complications (wound infection, anastomotic leak, renal failure): Pulmonary embolism: control group: 0/17; intervention groups: PEP, 0/17; RMT, 1/17 Wound infection: control group: 0/17, intervention groups: PEP, 4/17; RMT, 3/17 Adverse effects of the intervention (pulmonary aspiration, upper airway or facial injury): none reported |
|
| Notes | CI = confidence interval CPAP = continuous positive airway pressure ICU = intensive care unit IMC = intermediate care unit PEP = positive expiratory pressure, variable, mask RMT = PEP mask with inspiratory resistance, mask SD = standard deviation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomly allocated' into 3 groups; no other details |
| Allocation concealment (selection bias) | Unclear risk | No evidence for it |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No evidence for it |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No evidence for it |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasonable account given |
| Selective reporting (reporting bias) | Unclear risk | Not sure |
| Other bias | Unclear risk | Not sure |
Denehy 2001.
| Methods | RCT done at Austin and Repatriation Medical Centre, Melbourne, Australia | |
| Participants | Adult patients undergoing upper abdominal surgery. Inclusion criteria: incision above the umbilicus; FEV1 greater than 50% predicted | |
| Interventions | All participants received preoperative education on effects of surgery on lung function and were instructed on deep breathing exercises, including sustained maximal inspiration Post surgery, all participants received physiotherapy twice daily for 3 days, for a minimum of 10 minutes each session. This traditional physiotherapy consisted of deep breathing exercises, forced expiration technique and supported cough. Early ambulation was encouraged, and physiotherapy was done in the sitting position on bed or chair Control group: received the above traditional physiotherapy Intervention groups (CPAP, 15 minutes; CPAP, 30 minutes): nasal mask for CPAP, PEEP set at 10 cm H2O. 30% O2 was used for CPAP. This was given 4 times each day, following traditional physiotherapy Control group: 18 participants; male/female: 15:3; age, years: 73.3 ± 5.8; no data on BMI Intervention group (2 groups combined): 17 and 15 participants; male/female: 12:5 and 12:3; age, years: 72.5 ± 6.5 and 70.5 ± 6.3; no data on BMI |
|
| Outcomes | Duration of intervention: 3 postoperative days Duration of follow‐up: at least 5 days No differences in pain scores between groups All‐cause mortality: 1 participant died after 32 days, but no details of surgical complications are given Major respiratory complications as defined in individual studies: Postoperative pulmonary complications: control, 4/18; intervention, 2/17 and 1/15 Significant atelectasis: not reported Pneumonia: not reported ("chest radiograph changes" reported but not significant (88% control group, 58.5% intervention group)) Respiratory failure: not reported Severe hypoxia: not reported Severe delirium: not reported Need for tracheal intubation and invasive ventilation: not reported Readmission to ICU/IMC: intervention group: not reported Length of stay in hospital, days: control group, 12.3 ± 4.8; intervention group, 11.5 ± 4.1 and 12.5 ± 4.8 Cardiovascular complications (myocardial infarction, unstable angina, acute cardiac failure, arrhythmia): none reported Cardiac arrest: not reported Other postoperative complications (wound infection, anastomotic leak, renal failure): not reported Adverse effects of the intervention (pulmonary aspiration, upper airway or facial injury): not reported |
|
| Notes | BMI = body mass index CPAP = continuous positive airway pressure FEV1 = forced expiratory volume in 1 second ICU = intensive care unit IMC = intermediate care unit PEEP = positive end‐expiratory pressure RCT = randomized controlled trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated into 3 groups with use of sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Randomly allocated into 3 groups with use of sealed envelopes, but no details of allocation concealment provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not discussed, but physiotherapists blinded to FRC (functional residual capacity) values, so only partially blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Only radiologist possibly blinded (partial blinding only) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described, but only those who completed 5 days of lung volume measurements (40/50) were included in FRC and VC (vital capacity) analyses |
| Selective reporting (reporting bias) | High risk | Poor reporting of participant demographics (no weight) and no details on complications |
| Other bias | Unclear risk | Not sure |
Gaszynski 2007.
| Methods | Study done at Medical University of Lodz, Poland | |
| Participants | Patients undergoing open Roux‐en‐Y gastric bypass Male/female, 8:11; BMI, kg/m2: 42.43 ± 3.3; age, years: 35.84 ± 9.05 |
|
| Interventions | After surgery, participants were monitored in PACU (postanaesthesia care unit) for 8 hours Control group: 9 participants; oxygen via nasal cannula, 4 L/min Intervention group: 10 participants; CPAP Boussignac device, + 9.4 cm H2O Continuous SpO2 monitoring and frequent blood gases |
|
| Outcomes | Duration of intervention: 8 hours Duration of follow‐up: most likely less than 1 day No differences in pain scores between groups All‐cause mortality: none Major respiratory complications as defined in individual studies: Postoperative pulmonary complications: none Significant atelectasis: not reported Pneumonia: not reported Respiratory failure: not reported Severe hypoxia: not reported Severe delirium: not reported Need for tracheal intubation and invasive ventilation: no Admission to ICU/IMC: intervention group: not reported Length of stay in hospital: not reported Cardiovascular complications (myocardial infarction, unstable angina, acute cardiac failure, arrhythmia): none reported Other postoperative complications (wound infection, anastomotic leak, renal failure): none, not sure for how long Adverse effects of intervention (pulmonary aspiration, upper airway or facial injury): not reported |
|
| Notes | BMI = body mass index CPAP = continuous positive airway pressure ICU = intensive care unit IMC = intermediate care unit PACU = postanaesthesia care unit SpO2 = oxygen saturation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomly divided into two groups,' no further description |
| Allocation concealment (selection bias) | Unclear risk | No description in text |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear |
| Selective reporting (reporting bias) | Unclear risk | Not clear |
| Other bias | Unclear risk | Not sure |
Lindner 1987.
| Methods | Study done at Ulm University Clinic, Ulm, Germany | |
| Participants | Participants undergoing elective major upper abdominal surgery; all were moderately healthy patients Control group: 17 participants; male/female: 6:11; age, years: 65 (range 52‐77); weight, kg: 65 (range 47‐85) Intervention group: 17 participants; male/female: 12:5; age, years: 66 (range 50‐77); weight, kg: 66 (50‐95) |
|
| Interventions | Control group: standard physiotherapy (deep breathing and coughing) at 3‐hourly intervals during daytime for 48 hours Intervention group: standard physiotherapy as well as continuous CPAP at FiO2 of 0.35, 12 cm H2O, 3 hours per day for 5 days |
|
| Outcomes | Duration of intervention: 5 days Duration of follow‐up: 5 days All participants received same analgesic routine All‐cause mortality: none reported Major respiratory complications as defined in the study by radiograph (3rd day): Atelectasis: control group, 4/17; intervention group, 0/17 Consolidation: control group, 1/17; intervention group, 1/17 Need for tracheal intubation and invasive ventilation: none in either group Admission to ICU/IMC: intervention group: not reported Length of stay in hospital: not reported Cardiovascular complications (myocardial infarction, unstable angina, acute cardiac failure, arrhythmia): none reported Other postoperative complications (wound infection, anastomotic leak, renal failure): none reported Adverse effects of the intervention (pulmonary aspiration, upper airway or facial injury): not reported |
|
| Notes | CPAP = continuous positive airway pressure FiO2 = inspired oxygen fraction ICU = intensive care unit IMC = intermediate care unit |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomized' into 2 groups |
| Allocation concealment (selection bias) | High risk | No description for it (randomly assigned into 2 groups) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Pulmonary function measurements were performed by a researcher who was not aware of which group the patients were in" Radiologist interpreted x‐ray films "without knowledge of whether the patient was receiving CPAP or not" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Probably OK |
| Selective reporting (reporting bias) | Unclear risk | Not sure |
| Other bias | Unclear risk | Not sure |
Lotz 1984.
| Methods | Study done at Universitat Ulm/Donau, Germany | |
| Participants | Patients undergoing upper abdominal surgery. Participants were randomly allocated into 4 groups. ZEEP (zero end‐expiratory pressure) or PEEP (positive end‐expiratory pressure) was given during surgery, followed by oxygen via mask or CPAP, thus making up 4 groups. For this review, we have chosen only 2 groups: control group, received ZEEP (no PEEP) and no CPAP after surgery. Intervention group received ZEEP during surgery and CPAP after surgery Control group: ZEEP during anaesthesia, followed by oxygen via mask during the postoperative period: 16 participants; male/female, 9:6; age, years: 58 ± 8; BMI: weight not given (only Broca Index given) Intervention group: ZEEP during anaesthesia, followed by CPAP during postoperative period: 16 participants; male/female: 8:8; age, years: 58 ± 10; BMI: weight not given (only Broca Index given) |
|
| Interventions | Control group: Participants received oxygen via nasal cannula in the recovery ward Intervention group: Participants received CPAP (+ 5 cm H2O) for 2 hours in the recovery ward |
|
| Outcomes | Duration of intervention: 2 hours in the recovery ward Duration of follow‐up: up to 10 days (days 2, 5 and 10) All‐cause mortality: not indicated Major respiratory complications as defined in the study: not differentiated between atelectasis, pneumonia and consolidation, but we have included it as atelectasis on day 2 Atelectasis (day 2): control group, 6/16; intervention group, 3/16 Consolidation/Pneumonia: not sure Need for tracheal intubation and invasive ventilation: control, 0/16; intervention, 0/16 Admission to ICU/IMC: no data Length of stay in hospital: no data Cardiovascular complications (myocardial infarction, unstable angina, acute cardiac failure, arrhythmia): Other postoperative complications (wound infection, anastomotic leak, renal failure): no data Adverse effects of intervention (pulmonary aspiration, upper airway or facial injury): no data |
|
| Notes | CPAP = continuous positive airway pressure ICU = intensive care unit IMC = intermediate care unit PEEP = positive end‐expiratory pressure ZEEP = zero end‐expiratory pressure |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Yes, randomly assigned to 4 groups |
| Allocation concealment (selection bias) | Unclear risk | No details given, but possible |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No outcome data of interest reported |
| Selective reporting (reporting bias) | High risk | No information in text |
| Other bias | Unclear risk | Not sure |
Ricksten 1986.
| Methods | Study done at Sahlgren's Hospital, Gothenburg, Sweden | |
| Participants | Patients having elective upper abdominal surgery Groups were stratified by preoperative lung function, age, sex, body weight and smoking habit |
|
| Interventions | General anaesthesia and epidural morphine (for at least 2 days) were given to all participants Participants were randomly assigned to 3 groups: Control group: deep breathing exercises every waking hour (30 breaths) CPAP group: hourly 30 breaths with CPAP mask (+ 10‐15 cm H2O) given every waking hour PEP (positive expiratory pressure) group: hourly 30 breaths with a mask, which generated + 10 to 15 cm H2O during expiration, given every waking hour We combined CPAP and PEP groups for the purpose of this review All treatments continued for 3 postoperative days if participants tolerated it Control group: 15 participants; male/female, 6:9; age, years: 51.7 ± 4.7; weight, kg: 90.4 ± 7.4 CPAP: 13 participants; male/female, 7:6; age, years: 52.5 ± 3.5; weight, kg: 93.4 ± 6.3 PEP group: 15 participants; male/female, 8:7; age, years 56.9 ± 3.8; weight, kg: 89.0 ± 7.0 |
|
| Outcomes | Duration of intervention: 3 days Duration of follow‐up: 3 days All‐cause mortality: none reported Major respiratory complications as defined in individual studies: Postoperative pulmonary complications: Significant atelectasis/consolidation (radiology confirmation): 1st postop day: control, 5/15; CPAP group: 2/13; PEP group, 1/15 Significant atelectasis/consolidation (radiology confirmation): 3rd postop day: control, 6/15; CPAP group: 1/13; PEP group, 0/15 Pneumonia: not reported Respiratory failure: not reported Severe hypoxia: lower A‐a difference reported, but no numbers given Need for tracheal intubation and invasive ventilation: none reported Admission to ICU: intervention group: not reported Length of stay in hospital: not reported Cardiovascular complications (myocardial infarction, unstable angina, acute cardiac failure, arrhythmia): none reported Other postoperative complications (wound infection, anastomotic leak, renal failure): none reported Adverse effects of the intervention (pulmonary aspiration, upper airway or facial injury): not reported |
|
| Notes | CPAP = continuous positive airway pressure ICU = intensive care unit PEP = positive expiratory pressure |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | After stratification, randomly assigned into 3 groups, not sure how |
| Allocation concealment (selection bias) | High risk | No evidence |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not sure |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Radiologist blinded, not sure whether anyone else was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear |
| Selective reporting (reporting bias) | Unclear risk | Not clear |
| Other bias | Unclear risk | Not sure |
Squadrone 2005.
| Methods | Patients undergoing elective abdominal surgery from centres of the Piedmont Intensive Care Units Network, Italy | |
| Participants | Adult patients undergoing elective abdominal surgery, requiring laparotomy and visceral exposure for longer than 90 minutes | |
| Interventions | Randomization was done after surgery and 1 hour of monitoring (and PaO2/FiO2 < 300). Inclusion and exclusion criteria clearly detailed Control group: 104; male/female: 64:40; age, years: 65 ± 10; BMI, kg/m2: 26.3 ± 4.5 Intervention group: 105; male/female: 71:34; age, years: 66 ± 9; BMI, kg/m2: 26.5 ± 4.7 Control group: Venturi mask with FiO2 of 0.5 for 6 hours, followed by further assessment of PaO2/FiO2, followed by further treatment of Ventimask with FiO2 of 0.5 if PaO2/FiO2 ratio < 300 Intervention group: CPAP mask with FiO2 of 0.5 and CPAP of + 7.5 cm H2O for 6 hours, followed by further assessment of PaO2/FiO2, followed by further use of CPAP mask with FiO2 of 0.5 and CPAP of + 7.5 cm H2O if PaO2/FiO2 ratio < 300 |
|
| Outcomes | Duration of intervention: minimum of 6 hours Duration of follow‐up: longer than 3 days (up to 7 days) All‐cause mortality: control group, 3/104; intervention group, 0/105 Major respiratory complications as defined in individual studies: Pneumonia: control group, 10/104; intervention group, 2/105 Need for tracheal intubation and invasive ventilation: control group, 10/104; intervention group, 1/105 ICU length of stay: control group, 2.6; intervention group, 1.4 Length of stay in hospital: control group: 17 ± 15 days; intervention group, 15 ± 13 days Other postoperative complications (wound infection, anastomotic leak, renal failure): Infection: control group, 11/104; intervention group, 3/105 Sepsis: control group, 9/104; intervention group, 2/105 Anastomotic leakage: control group, 6/104; intervention group, 1/105 |
|
| Notes | BMI = body mass index CPAP = continuous positive airway pressure FiO2 = inspired oxygen fraction ICU = intensive care unit PaO2 = arterial oxygen pressure |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed randomization was conducted centrally through dedicated website using computer‐generated block randomization schedule |
| Allocation concealment (selection bias) | Low risk | Concealed randomization was conducted centrally through dedicated website using computer‐generated block randomization schedule |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported |
| Selective reporting (reporting bias) | Unclear risk | Not sure; premature stopping of trial by data monitoring committee |
| Other bias | Unclear risk | Not sure |
Stock 1985.
| Methods | Study done at Mercy Hospital, Northwest University, Chicago, USA | |
| Participants | Patients having elective upper abdominal surgery were selected. | |
| Interventions | A computer random number generator was used to assign each participant to 1 of 3 treatment groups for postoperative respiratory therapy Three groups were studied CBD group was given coughing and deep breathing exercises, starting 4 hours after extubation. This was given for 15 minutes, every 2 hours during waking hours. Duration of treatment was 4 to 72 hours IS group received incentive spirometry for 15 minutes, every 2 waking hours, for 4 to 72 hours (starting 4 hours after extubation) We combined CBD and IS groups as controls for the purpose of this review CPAP group received continuous positive airway pressure using a soft self‐sealing mask, + 7.5 cm H2O, for 15 minutes, every 2 waking hours, for 4 to 72 hours (starting 4 hours after extubation) Control groups: CDB group: number of participants, 20; male/female: 9:11; weight/ BMI, not given; age, years: 48 ± 4 (SEM); duration: 4 to 72 hours IS group: number of participants, 22; male/female: 8:14; weight/BMI: not given; age, years: 54 ± 4 (SEM); duration: 4 to 72 hours Intervention group: 23 participants; male/female: 8:15; weight/ BMI: not given; age, years: 49 ± 5 (SEM); duration: 4 to 72 hours |
|
| Outcomes | Duration of intervention: 4 to 72 hours Duration of follow‐up for 3 days All‐cause mortality: not given Major respiratory complications as defined in individual studies: X‐ray confirmed atelectasis at 24 hours: control group: CDB, 6/20; IS, 11/22 = 17/42; intervention group, 9/23 X‐ray confirmed atelectasis at 72 hours: control group: CDB, 8/20; IS, 9/22 = 17/42; intervention group, 5/23 Pneumonia: CDB, 1/20, IS, 1/22 (control, 2/42); intervention group, 0/23 No other information offered in the paper |
|
| Notes | BMI = body mass index CDB = coughing and deep breathing CPAP = continuous positive airway pressure IS = incentive spirometry SEM = standard error of the mean |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer random number generator was used to assign each participant to 1 of the different groups |
| Allocation concealment (selection bias) | Unclear risk | A computer random number generator was used to assign each participant to 1 of the different groups; most likely adequate, but no description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Radiologists blinded for x‐ray interpretation, but not sure of others |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Probably all participants accounted for |
| Selective reporting (reporting bias) | Unclear risk | Probable |
| Other bias | Unclear risk | Probable |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anderes 1979 | Participants in one group were intubated for 3 hours, whereas participants in the other group were extubated soon after surgery. Unequal treatment of groups |
| Celli 1984 | Intervention is not CPAP, but positive‐pressure breathing |
| Conti 2007 | Study is a case‐controlled study, not an RCT |
| Drummond 2002 | Even though this is an RCT (stratified), study was done mainly to observe effects of CPAP on sleep pattern and hypoxaemia. Study lasted only 1 day; no relevant data available from this publication for the purpose of the review |
| Ebeo 2002 | This RCT deals with BiPAP ventilation, which is different from CPAP; hence excluded from this review |
| Huerta 2002 | This is not an RCT |
| Jaber 2005 | This study of participants who already have established respiratory failure is not an RCT; it also falls into the exclusion criteria for the review |
| Joris 1997 | This RCT uses BiPAP (bilevel positive airway pressure) during the postoperative period. This is an exclusion criterion |
| Kindgen‐Milles 2005 | This RCT is dealing with thoraco‐abdominal aortic surgery and does not conform with inclusion criteria |
| Neligan 2009 | A randomized trial, but the intervention group receives the same treatment as the control group, except that onset of treatment is delayed by 30 minutes. Therefore, this study was excluded |
| Olsen 2002 | This RCT is dealing with thoraco‐abdominal operations, an exclusion criterion for the review |
| Rieg 2012 | This is not an RCT; participants were allocated into 2 groups on 2 different timelines |
| Roeseler 1982 | Study population is a mixture of randomized and non‐randomized cases, allocated into 2 groups. Therefore, excluded from review |
| Vartanov 2007 | Intervention group received BiPAP ventilation during the postoperative period, even though the study was an RCT |
| Wong 2011 | Used PEEP during surgery; follow‐up with CPAP in the recovery ward, but only for 1 hour |
BiPAP = bilevel positive airway pressure
CPAP = continuous positive airway pressure
PEEP = positive end expiratory pressure
RCT = randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Damgaard 1982.
| Methods | Waiting for a translation |
| Participants | Waiting for a translation |
| Interventions | Waiting for a translation |
| Outcomes | Waiting for a translation |
| Notes | Waiting for a translation |
Characteristics of ongoing studies [ordered by study ID]
McKay 2012.
| Trial name or title | Prophylactic nCPAP Following Bowel Surgery (Bio‐REB File 11‐27) |
| Methods | Randomized controlled trial |
| Participants | Adults undergoing bowel surgery |
| Interventions | Oxygen via nCPAP vs via masks in the postoperative period |
| Outcomes | Some relevant to this review |
| Starting date | 2012 |
| Contact information | Dr William McKay, University of Saskatchewan, Canada |
| Notes | Study being repeated |
Wong 2010.
| Trial name or title | Oxygenation and Pulmonary Function in Morbidly Obese Patients Undergoing Bariatric Surgery |
| Methods | Boussignac(TM) CPAP compared with Venturi mask |
| Participants | Adult patients undergoing bariatric surgery |
| Interventions | Boussignac(TM) CPAP |
| Outcomes | PaO2/FiO2 ratio for 24 hours |
| Starting date | 2010 |
| Contact information | Emailed lead author, Dr David Wong, University Health Network, Toronto, Canada |
| Notes | Study should have been finished by 2012, but not yet published |
CPAP = continuous positive airway pressure
FiO2 = inspired oxygen fraction
nCPAP = nasal CPAP
PaO2 = arterial oxygen pressure
Differences between protocol and review
A new author joined the review team: Suneeth F Mathew.
We planned, but were unable, to complete many of the tasks planned in the protocol (Ireland 2011), mostly because of insufficient data.
We excluded those who received bilevel positive airway pressure (BiPAP) during the postoperative period.
We searched www.controlled‐trials.com and http://clinicaltrials.gov/ct2/ instead of www.who.int/ictrp/en/, as per the protocol.
We planned, but were unable, to perform subgroup analysis or sensitivity analysis because of inadequate studies/data.
Results section in the protocol included differences under "major respiratory complications" listed as "significant atelectasis, pneumonia, respiratory failure, need for tracheal intubation and invasive ventilation." In this review, we recorded "significant atelectasis, pneumonia, significant hypoxia, tracheal reintubation, ICU admission." This was necessitated by the reporting pattern noted in the selected studies.
Contributions of authors
Conceiving of the review: Claire Ireland (CI).
Designing the review: CI.
Co‐ordinating the review: Tim Chapman (TC).
Undertaking manual searches: CI, TC.
Screening search results: CI, TC.
Organizing retrieval of papers: Mathew Zacharias (MZ).
Screening retrieved papers against inclusion criteria: CI, TC.
Appraising quality of papers: CI, TC, MZ.
Abstracting data from papers: CI, TC, Suneeth Fiona Mathew (SFM).
Writing to authors of papers for additional information: MZ.
Providing additional data about papers: CI, TC, MZ.
Obtaining and screening data on unpublished studies: CI, TC.
Managing data for the review: CI, TC, MZ, G Peter Herbison (PH).
Entering data into Review Manager (RevMan 5.2): CI, TC.
Handling RevMan statistical data: PH.
Performing double entry of data: data entered by person one: MZ; data entered by person two:SFM.
Interpreting data: CI, TC, MZ, SFM.
Making statistical inferences: PH.
Writing the review: CI, TC.
Providing guidance on the review: MZ.
Securing funding for the review: MZ.
Performing previous work that served as the foundation of the present study: CI, MZ, TC.
Serving as guarantor for the review (one review author): MZ.
Taking responsibility for reading and checking the review before submission: MZ.
Sources of support
Internal sources
-
Southern District Health Board and Dunedin School of Medicine, University of Otago, New Zealand.
Library access, sourcing of article reprints, photocopying
External sources
None received, Other.
Declarations of interest
Claire J Ireland: none declared.
Timothy M Chapman: none declared.
Suneeth F Mathew: none declared.
G Peter Herbison: none declared.
Mathew Zacharias: none declared.
New
References
References to studies included in this review
Bohner 2002 {published data only}
- Bohner H, Kindgen‐Milles D, Grust A, Buhl R, Lillotte W‐C, et al. Prophylactic nasal continuous positive airway pressure after major vascular surgery: results of a prospective randomized trial. Archives of Surgery 2002;387:21‐6. [PUBMED: 11981680 ] [DOI] [PubMed] [Google Scholar]
Carlsson 1981 {published data only}
- Carlsson C, Sonden B, Thylen U. Can postoperative continuous positive airway pressure (CPAP) prevent pulmonary complications after abdominal surgery?. Intensive Care Medicine 1981;7:225‐9. [PUBMED: 7024383] [DOI] [PubMed] [Google Scholar]
Christensen 1991 {published data only}
- Christensen EF, Schultz P, Jensen OV, Egebo K, Engberg M, Gron I, et al. Postoperative pulmonary complications and lung function in high‐risk patients: a comparison of three physiotherapy regimens after upper abdominal surgery in general anesthesia. Acta Anaesthesiologica Scandinavica 1991;35:97‐104. [PUBMED: 2024569] [DOI] [PubMed] [Google Scholar]
Denehy 2001 {published data only}
- Denehy L, Carroll S, Ntoumenopoulos G, Jenkins S. A randomized controlled trial comparing periodic mask CPAP with physiotherapy after abdominal surgery. Physiotherapy Research International 2001;6(4):236‐50. [PUBMED: 11833245] [DOI] [PubMed] [Google Scholar]
Gaszynski 2007 {published data only}
- Gaszynski T, Tokarz A, Piotrowski D, Machala W. Boussignac CPAP in the postoperative period in morbidly obese patients. Obesity Surgery 2007;17:452‐6. [PUBMED: 17608255] [DOI] [PubMed] [Google Scholar]
Lindner 1987 {published data only}
- Lindner KH, Lotz P, Ahnefeld FW. Continous positive airway pressure effect on functional residual capacity, vital capacity and its subdivisions. Chest 1987;92(1):66‐70. [PUBMED: 3297521] [DOI] [PubMed] [Google Scholar]
Lotz 1984 {published data only}
- Lotz P, Heise U, Schaffer J, Wollinsky K‐H. The effect of Intraoperative PEEP‐ventilation and postoperative pulmonary function following upper abdominal surgery [(Article in German)]. Anaesthesist 1984;33:177‐88. [PUBMED: 6428260] [PubMed] [Google Scholar]
Ricksten 1986 {published data only}
- Ricksten SE, Bengtsson A, Soderberg C, Thorden M, Kvist H. Effects of periodic positive airway pressure by mask on postoperative pulmonary function. Chest 1986;89(6):774‐81. [PUBMED: 3519107] [DOI] [PubMed] [Google Scholar]
Squadrone 2005 {published data only}
- Squadrone V, Coha M, Cerutti E, Schellino MM, Biolino P, Occella P, et al. Continuous positive airway pressure for treatment of postoperative hypoxemia: a randomized controlled trial. JAMA 2005;293(5):589‐95. [PUBMED: 15687314] [DOI] [PubMed] [Google Scholar]
Stock 1985 {published data only}
- Stock MC, Downs JB, Gauer PK, Alster JM, Imrey PB. Prevention of postoperative pulmonary complications with CPAP, incentive spirometry, and conservative therapy. Chest 1985;87(2):151‐7. [PUBMED: 3881226] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anderes 1979 {published data only}
- Anderes C, Anderes U, Gasser D, Dittmann M, Turner J, Brennwald J, et al. Postoperative spontaneous breathing with CPAP to normalize late postoperative oxygenation. Intensive Care Medicine 1979;5(1):15‐21. [PUBMED: PMID: 374444] [DOI] [PubMed] [Google Scholar]
Celli 1984 {published data only}
- Celli BR, Rodriguez KS, Snider GL. A controlled trial of intermittent positive pressure breathing, incentive spirometry, and deep breathing exercises in preventing complications after abdominal surgery. The American review of respiratory disease 1984;130:12‐5. [PUBMED: 6377994] [DOI] [PubMed] [Google Scholar]
Conti 2007 {published data only}
- Conti G, Cavaliere F, Costa R, Craba A, Catarci S, Festa V, et al. Noninvasive positive pressure ventilation with different interfaces in patients with respiratory failure after abdominal surgery: a matched‐control study. Respiratory Care 2007;52(11):1463‐71. [PUBMED: 17971249] [PubMed] [Google Scholar]
Drummond 2002 {published data only}
- Drummond GB, Stedul K, Kingshott R, Rees K, Nimmo AF, Wraith P, et al. Automatic CPAP compared with conventional treatment for episodic hypoxemia and sleep disturbance after major abdominal surgery. Anesthesiology 2002;96:817‐26. [PUBMED: 11964587] [DOI] [PubMed] [Google Scholar]
Ebeo 2002 {published data only}
- Ebeo CT, Benotti PN, Byrd Jr RP, Elmaghraby Z, Lui J. The effect of bi‐level positive airway pressure on postoperative pulmonary function following gastric surgery for obesity. Respiratory Medicine 2002;96:672‐6. [PUBMED: 12243311] [DOI] [PubMed] [Google Scholar]
Huerta 2002 {published data only}
- Huerta S, DeShields S, Sbpiner R, Li Z, Liu C, Sawicki M, et al. Safety and efficacy of postoperative continuous positive airway pressure to prevent pulmonary complications after Roux‐en‐Y gastric bypass. Journal of Gastrointestinal Surgery 2002;6(3):354‐8. [PUBMED: 12022987] [DOI] [PubMed] [Google Scholar]
Jaber 2005 {published data only}
- Jaber S, Delay J‐M, Chanques G, Sebbane M, Jacquet E, Souche B, et al. Outcomes of patients with acute respiratory failure after abdominal surgery treated with noninvasive positive pressure ventilation. Chest 2005;128(4):2688‐95. [PUBMED: 16236943] [DOI] [PubMed] [Google Scholar]
Joris 1997 {published data only}
- Joris JL, Sottiaux TM, Chiche JD, Desaice CJ, Lamy ML. Effect of bi‐level positive airway pressure (BiPAP) nasal ventilation on the postoperative pulmonary restrictive syndrome in obese patients undergoing gastroplasty. Chest 1997;111(3):665‐70. [PUBMED: 9118706] [DOI] [PubMed] [Google Scholar]
Kindgen‐Milles 2005 {published data only}
- Kindgen‐Milles D, Muller E, Buhl R, Bohner H, Ritter D, Sandmann W, et al. Nasal‐continuous positive airway pressure reduces pulmonary morbidity and length of hospital stay following thoracoabdominal aortic surgery. Chest 2005;128(2):821‐8. [PUBMED: 16100174] [DOI] [PubMed] [Google Scholar]
Neligan 2009 {published data only}
- Neligan PJ, Malhothra G, Fraser M, Williams N, Greenblatt EP, Cereda M, et al. Continuous positive airway pressure via the Boussignac system immediately after extubation improves lung function in morbidly obese patients with obstructive sleep apnea undergoing laparoscopic bariatric surgery. Anesthesiology 2009;110(4):878‐84. [PUBMED: 19293693] [DOI] [PubMed] [Google Scholar]
Olsen 2002 {published data only}
- Olsen MF, Wennberg E, Johnsson E, Josefson K, Lonroth H, Lundell L. Randomized clinical study of the prevention of pulmonary complications after thoracoabdominal resection by two different breathing techniques. British Journal of Surgery 2002;89(10):1228‐34. [PUBMED: 12296888] [DOI] [PubMed] [Google Scholar]
Rieg 2012 {published data only}
- Rieg AD, Stoppe C, Rossaint R, Coburn M, Hein M, Schalte G. EzPAP® therapy of postoperative hypoxemia in the recovery room: experiences with the new compact system of end‐expiratory positive airway pressure. Der Anaesthesist 2012;61(10):867‐74. [PUBMED: 23011043] [DOI] [PubMed] [Google Scholar]
Roeseler 1982 {published data only}
- Roeseler J, Themouroux J, Soete M, Reynaert M, Francis CH. Effect of three types of respiratory assistance (continuous positive pressure, intermittent positive pressure, classical respiratory physiotherapy) on PaO2, PaCO2 in patients undergoing digestive tract surgery. Le Poumon et le Coeur 1982;38:135‐42. [PUBMED: 6813834] [PubMed] [Google Scholar]
Vartanov 2007 {published data only}
- Vartanov IV, Khrapov KN, Polushin IUS. Use of noninvasive ventilation in patients at high postoperative risk for cardiopulmonary complications. Anesteziologiia i Reanimatologiia 2007;3:17‐9. [PUBMED: 17684982] [PubMed] [Google Scholar]
Wong 2011 {published data only}
- Wong DT, Adly E, Ip HY, Thapar S, Maxted GR, Chung FF. A comparison between the Boussignac™ continuous positive airway pressure mask and the venturi mask in terms of improvement in the PaO2/F(I)O2 ratio in morbidly obese patients undergoing bariatric surgery: a randomized controlled trial. Canadian Journal of Anesthesia 2011;58(6):532‐9. [PUBMED: 21465320] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Damgaard 1982 {published data only}
- Damgaard B, Andersen BN, Andersen A. Postoperative CPAP therapy following surgery in the kidney area. Ugeskrift for Laeger 1982;144(8):545‐7. [MEDLINE: ] [PubMed] [Google Scholar]
References to ongoing studies
McKay 2012 {published data only}
- Prophylactic nCPAP Following Bowel Surgery (Bio‐REB File 11‐27). Ongoing study 2012.
Wong 2010 {published data only}
- Oxygenation and Pulmonary Function in Morbidly Obese Patients Undergoing Bariatric Surgery. Ongoing study 2010.
Additional references
Arozullah 2000
- Arozullah AM, Daley J, Henderson WG, Khuri S. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. Annals of Surgery 2000;232(2):242‐53. [PUBMED: 10903604] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arunotai 2010
- Arunotai S, Sahatsa M, Jathuporn T, Piyasak V, Somsak A, Jaruwan L. Obesity, epidural analgesia, and subcostal incision are risk factors for postoperative desaturation. Canadian Journal of Anaesthesia 2010;57(5):415‐22. [PUBMED: 20349163] [DOI] [PubMed] [Google Scholar]
Brismar 1985
- Brismar B, Hedenstierna G, Lundquist H, Strandberg A, Svensson L, Tokics L. Pulmonary densities during anesthesia with muscular relaxation: a proposal of atelectasis. Anesthesiology 1985;62:422‐8. [DOI] [PubMed] [Google Scholar]
Duggan 2005
- Duggan M, Kavanagh BP. Pulmonary atelectasis: a pathogenic perioperative entity. Anesthesiology 2005;102(4):838‐54. [PUBMED: 15791115 ] [DOI] [PubMed] [Google Scholar]
Duggan 2007
- Duggan M, Kavanagh BP. Atelectasis in the perioperative patient. Current Opinion in Anesthesiology 2007;20(1):37‐42. [PUBMED: 17211165 ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Phillips AN. Meta‐analysis: principles and procedures. BMJ 1997;316(7121):1533‐7. [PUBMED: 9432252] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferreyra 2008
- Ferreyra GP, Baussano I, Squadrone V, Richiardi L, Marchiaro G, et al. Continous positive airway pressure for treatment of respiratory complications after abdominal surgery: a systematic review and meta‐analysis. Annals of Surgery 2008;247(4):617‐26. [PUBMED: 18362624] [DOI] [PubMed] [Google Scholar]
Ghaferi 2009
- Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. New England Journal of Medicine 2009;361(14):1368‐75. [DOI] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro.. Version 3.2 for Windows. Brozek J, Oxman A, Schünemann H, 2008.
Guimaraes 2009
- Guimarães MMF, Dib RP, Smith AF, Matos D. Incentive spirometry for prevention of postoperative pulmonary complications in upper abdominal surgery. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006058] [DOI] [PubMed] [Google Scholar]
Haynes 2009
- Haynes AB, Weiser TG, Berry WR, Lipsitz SR, Breizat AS, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. New England Journal of Medicine 2009;360(5):491‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions, Version 5.1.0. [updated March 2011]. The Cochrane Collaboration 2011, www.cochrane‐handbook.org.
Kaw 2006
- Kaw R, Michota F, Jaffer A, Ghamande S, Auckley D, Golish J. Unrecognized sleep apnea in the surgical patient: implications for the perioperative setting. Chest 2006;129:198‐205. [PUBMED: 16424433] [DOI] [PubMed] [Google Scholar]
Lindberg 1992
- Lindberg P, Gunnarsson L, Tokics L, Secher E, Lundquist H, et al. Atelectasis and lung function in the postoperative period. Acta Anaesthesiologica Scandinavica 1992;36(6):546‐53. [PUBMED: 1514340 ] [DOI] [PubMed] [Google Scholar]
Nava 2009
- Nava S, Hill S. Non‐invasive ventilation in acute respiratory failure. Lancet 2009;374(9685):250‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nunn 1962
- Nunn JF, Payne JP. Hypoxaemia after general anaesthesia. Lancet 1962;280(7257):631‐2. [PUBMED: 13939226] [DOI] [PubMed] [Google Scholar]
Olsen 2011
- Olsen MF, Wennberg E. Fask‐track concepts in major open abdominal and thoracoabdominal surgery: a review. World Journal of Surgery 2011;35(12):2586‐93. [DOI] [PubMed] [Google Scholar]
Pelosi 2010
- Pelosi P, Jaber S. Noninvasive respiratory support in the perioperative period. Current Opinion in Anesthesiology 2010;23(2):233‐8. [PUBMED: 20019602] [DOI] [PubMed] [Google Scholar]
RevMan 5.2 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Sibbald 1985
- Sibbald WJ. Myocardial function in the critically ill: factors influencing left and right ventricular performance in patients with sepsis and trauma. Surgical Clinics of North America 1985;65(4):867‐93. [PUBMED: 3901347] [PubMed] [Google Scholar]
Tusman 2012
- Tusman G, Bohm SH, Warner DO, Sprung J. Atelectasis and perioperative pulmonary complications in high‐risk patients. Current Opinion in Anaesthesiology 2012;25(1):1‐10. [DOI] [PubMed] [Google Scholar]
Warner 2000
- Warner DO. Preventing postoperative pulmonary complications: the role of the anesthesiologist. Anestheiology 2000;92(5):1467‐72. [PUBMED: 10781293] [DOI] [PubMed] [Google Scholar]
Weaver 2008
- Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. The Proceedings of the American Thoracic Society 2008;5(2):173‐8. [PUBMED: 18250209] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weksler 1991
- Weksler N, Ovadia L. Nasal continuous positive airway pressure: an alternative method for respiratory assistance. Journal of Clinical Anesthesia 1991;6:442‐6. [PUBMED: 1760165] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ireland 2011
- Ireland CJ, Chapman TM, Herbison GP, Zacharias M. Continuous positive airway pressure (CPAP) in the postoperative period for the prevention of postoperative morbidity and mortality following major abdominal surgery. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD008930] [DOI] [PMC free article] [PubMed] [Google Scholar]


