Abstract
Background
Levels of physical fitness are low after stroke. It is unknown whether improving physical fitness after stroke reduces disability.
Objectives
To determine whether fitness training after stroke reduces death, dependence, and disability and to assess the effects of training with regard to adverse events, risk factors, physical fitness, mobility, physical function, quality of life, mood, and cognitive function. Interventions to improve cognitive function have attracted increased attention after being identified as the highest rated research priority for life after stroke. Therefore we have added this class of outcomes to this updated review.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched February 2015), the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 1: searched February 2015), MEDLINE (1966 to February 2015), EMBASE (1980 to February 2015), CINAHL (1982 to February 2015), SPORTDiscus (1949 to February 2015), and five additional databases (February 2015). We also searched ongoing trials registers, handsearched relevant journals and conference proceedings, screened reference lists, and contacted experts in the field.
Selection criteria
Randomised trials comparing either cardiorespiratory training or resistance training, or both (mixed training), with usual care, no intervention, or a non‐exercise intervention in stroke survivors.
Data collection and analysis
Two review authors independently selected trials, assessed quality and risk of bias, and extracted data. We analysed data using random‐effects meta‐analyses. Diverse outcome measures limited the intended analyses.
Main results
We included 58 trials, involving 2797 participants, which comprised cardiorespiratory interventions (28 trials, 1408 participants), resistance interventions (13 trials, 432 participants), and mixed training interventions (17 trials, 957 participants). Thirteen deaths occurred before the end of the intervention and a further nine before the end of follow‐up. No dependence data were reported. Diverse outcome measures restricted pooling of data. Global indices of disability show moderate improvement after cardiorespiratory training (standardised mean difference (SMD) 0.52, 95% confidence interval (CI) 0.19 to 0.84; P value = 0.002) and by a small amount after mixed training (SMD 0.26, 95% CI 0.04 to 0.49; P value = 0.02); benefits at follow‐up (i.e. after training had stopped) were unclear. There were too few data to assess the effects of resistance training.
Cardiorespiratory training involving walking improved maximum walking speed (mean difference (MD) 6.71 metres per minute, 95% CI 2.73 to 10.69), preferred gait speed (MD 4.28 metres per minute, 95% CI 1.71 to 6.84), and walking capacity (MD 30.29 metres in six minutes, 95% CI 16.19 to 44.39) at the end of the intervention. Mixed training, involving walking, increased preferred walking speed (MD 4.54 metres per minute, 95% CI 0.95 to 8.14), and walking capacity (MD 41.60 metres per six minutes, 95% CI 25.25 to 57.95). Balance scores improved slightly after mixed training (SMD 0.27, 95% CI 0.07 to 0.47). Some mobility benefits also persisted at the end of follow‐up. The variability, quality of the included trials, and lack of data prevents conclusions about other outcomes and limits generalisability of the observed results.
Authors' conclusions
Cardiorespiratory training and, to a lesser extent, mixed training reduce disability during or after usual stroke care; this could be mediated by improved mobility and balance. There is sufficient evidence to incorporate cardiorespiratory and mixed training, involving walking, within post‐stroke rehabilitation programmes to improve the speed and tolerance of walking; some improvement in balance could also occur. There is insufficient evidence to support the use of resistance training. The effects of training on death and dependence after stroke are still unclear but these outcomes are rarely observed in physical fitness training trials. Cognitive function is under‐investigated despite being a key outcome of interest for patients. Further well‐designed randomised trials are needed to determine the optimal exercise prescription and identify long‐term benefits.
Keywords: Humans, Physical Fitness, Stroke Rehabilitation, Activities of Daily Living, Exercise Therapy, Exercise Therapy/methods, Randomized Controlled Trials as Topic, Resistance Training, Stroke, Stroke/mortality, Walking, Walking/physiology
Physical fitness training for stroke survivors
Review question
We reviewed the evidence that examines whether physical fitness training is beneficial for a range of health and function outcomes in people with stroke.
Background
Physical fitness is important to allow people to carry out everyday activities such as walking and climbing stairs. Physical fitness varies among everyone. For example, fitness in men tends to be a little higher than in women and everyone's fitness becomes reduced as we get older and if we become less physically active. Physical fitness is often particularly low in stroke survivors. It may limit their ability to perform everyday activities and also worsen any stroke‐related disability. For this reason fitness training has been proposed as a beneficial approach for people with stroke. However, taking part in fitness training could have a range of other benefits important to people with stroke such as improving cognitive function (thinking skills), improving mood, and quality of life, and it could reduce the chance of having another stroke.
Study characteristics
By February 2015 we identified 58 trials for inclusion in the review. The trials involved at total of 2797 participants at all stages of care including being in hospital or back living at home. Most of the people who took part were able to walk on their own. The trials tested different forms of fitness training; these included 1) cardiorespiratory or 'endurance' training, 2) resistance or 'strength' training, or 3) mixed training, which is a combination of cardiorespiratory plus resistance training.
Key results
We found that cardiorespiratory fitness training, particularly involving walking, can improve exercise ability and walking after stroke. Mixed training improves walking ability and improves balance. However, there was not enough information to draw reliable conclusions about the impact of fitness training on other areas such as quality of life, mood, or cognitive function. Cognitive function is under‐investigated despite being a key outcome of interest for stroke survivors. There was no evidence that any of the different types of fitness training caused injuries or other health problems; exercise appears to be a safe intervention. We need more studies to examine the benefits that are important to stroke survivors, in particular for those with more severe stroke who are unable to walk.
Quality of the evidence
Studies of fitness training can be difficult to carry out. This means most of the studies were small and of moderate quality. However, some consistent findings did emerge with different studies all tending to show the same effect.
Summary of findings
Summary of findings for the main comparison.
Cardiorespiratory training
| Cardiorespiratory training versus control in people with stroke | ||||||
| Patient or population: stroke patients Setting: inpatient; outpatient; community; home Intervention: cardiorespiratory training Comparison: control ‐ end of intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control ‐ end of intervention | Risk with cardiorespiratory training | |||||
| Case fatality | Death was a rare event. There were a total of only 4 deaths among 1437 participants | ‐ | 1437 (28 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | 2 deaths in the control group and 2 in the intervention group of a single study (Gordon 2013) | |
| Dead or dependent | ‐ | ‐ | (0 RCTs) | No studies reported the composite outcome of dead or dependent | ||
| Disability assessed with: combined disability scales | ‐ | The mean disability in the intervention group was 0.52 standard deviations more (0.19 more to 0.84 more) | ‐ | 462 (8 RCTs) | ⊕⊕⊕⊕ HIGH 2 | Although a benefit is suggested, a standardised mean difference of global scales of disability is difficult to interpret. The magnitude observed (> 0.5) can be generally categorised as 'medium' effect. This improvement may be reflecting improved mobility since mobility items are commonly included in these assessment tools. |
| Physical fitness ‐ peak VO2 (ml/kg/min) assessed with: VO2 (ml/kg/min) | ‐ | The mean physical fitness ‐ peak VO2 (ml/kg/min) in the intervention group was 2.86 higher (1.76 higher to 3.96 higher) | ‐ | 425 (9 RCTs) | ⊕⊕⊕⊕ HIGH | Higher values of oxygen uptake represent increased cardiorespiratory fitness. This can provide functional benefit and is also a marker associated with reduced risk of stroke. |
| Mobility assessed with: maximal gait speed (m/min) | ‐ | The mean mobility in the intervention group was 6.71 more (2.73 more to 10.69 more) | ‐ | 631 (14 RCTs) | ⊕⊕⊕⊕ HIGH | In trials with a follow‐up (n = 312; 5 RCTs) this benefit was retained MD 6.71 (2.40 to 11.02). Being able to walk faster, when required, could mean activities like crossing a road may be safer. |
| Mobility assessed with: preferred gait speed (m/min) | ‐ | The mean mobility in the intervention group was 4.28 more (1.71 more to 6.84 more) | ‐ | 505 (10 RCTs) | ⊕⊕⊕⊕ HIGH | This degree of improvement (+4.28 m/min) is just under half that suggested (+9.6 m/min) for stroke patients to experience a meaningful improvement in disability |
| Mobility assessed with: gait endurance ‐ 6‐minute walking test (metres) | ‐ | The mean mobility in the intervention group was 30.29 more (16.19 more to 44.39 more) | ‐ | 826 (15 RCTs) | ⊕⊕⊕⊕ HIGH | In trials with a follow‐up (n = 283; 5 RCTs) this benefit was retained MD 38.29 (7.19 to 69.39). This degree of improvement corresponds to minimal clinically important differences and reflects the ability to tolerate continuous activity, particularly walking. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; min: minute; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Most participants were high‐functioning patients; risk of death was low among this group.
2Some heterogeneity of effect arises from the high level of baseline disability in one study (Wang 2014). Overall, in 7/8 RCTs there was a positive beneficial effect.
Summary of findings 2.
Mixed training
| Mixed training versus control in people with stroke | ||||||
| Patient or population: stroke patients Setting: inpatient; outpatient; community; home Intervention: mixed training Comparison: control ‐ end of intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control ‐ end of intervention | Risk with mixed training | |||||
| Case fatality | Death was a rare event. There were a total of only 9 deaths among 979 participants. | ‐ | 957 (17 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | A total of 9 deaths restricted to 2/17 RCTs | |
| Dead or dependent | ‐ | ‐ | (0 RCTs) | No studies reported the composite outcome of dead or dependent | ||
| Disability assessed with: combined disability scales | ‐ | The mean disability in the intervention group was 0.26 standard deviations more (0.04 more to 0.49 more) | ‐ | 544 (7 RCTs) | ⊕⊕⊕⊕ HIGH 2 3 | A standardised mean difference of global scales of disability is difficult to interpret. The magnitude of increase observed (0.2 to 0.5) can be generally categorised as a 'small' effect: this is a negligible effect. Only 1/7 included trials had a design including balanced dose of exposure in the intervention and control; confounding could be exaggerating this effect. Any improvement may be reflecting improved mobility since mobility items are commonly included in these assessment tools. |
| Mobility assessed with: preferred gait speed (m/min) | ‐ | The mean mobility in the intervention group was 4.54 more (0.95 more to 8.14 more) | ‐ | 639 (9 RCTs) | ⊕⊕⊕⊕ HIGH 2 | No evidence of retention at follow‐up. This degree of improvement (+4.54 m/min) is just under half that suggested (+9.6 m/min) for stroke patients to experience a meaningful improvement in disability. |
| Mobility assessed with: gait endurance ‐ 6‐minute walking test (metres) | ‐ | The mean mobility in the intervention group was 41.6 more (25.25 more to 57.95 more) | ‐ | 561 (7 RCTs) | ⊕⊕⊕⊕ HIGH 2 | All trials in this analysis the intervention groups were confounded by additional training time, which could exaggerate the effect. In trials with a follow‐up (n = 365; 3 RCTs) benefit was retained. This degree of improvement exceeds the minimum clinically important differences and reflects the ability to tolerate continuous activity, particularly walking. |
| Physical function ‐ balance assessed with: Berg Balance scale Scale from: 0 to 56 | ‐ | The mean physical function ‐ balance in the intervention group was 1.97 more (0.36 more to 3.59 more) | ‐ | 260 (6 RCTs) | ⊕⊕⊕⊕ HIGH 2 3 | This demonstrates that a small benefit to functional walking balance would could theoretically augment fall reduction measures. Improvement was achieved even though balance training was not an explicit aim of the fitness training programmes. |
| Physical function ‐ balance assessed with: combined balance scales | ‐ | The mean physical function ‐ balance in the intervention group was 0.27 standard deviations more (0.07 more to 0.47 more) | ‐ | 596 (9 RCTs) | ⊕⊕⊕⊕ HIGH 2 | A standardised mean difference of a range of balance instruments is difficult to interpret. The magnitude of increase observed (0.2 to 0.5) can be generally categorised as a 'small' effect: this is a negligible effect. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; min: minute; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Most participants were high‐functioning patients; risk of death was low among this group.
2Trials were confounded for additional training time exposure; exclusion using sensitivity analyses reduced the effect.
3Poor reporting of risk of bias across included trials.
Background
Physical activity and exercise recommendations exist for a wide range of healthy, older, and patient populations (Nelson 2007; O'Donovan 2010), including those with specific health problems such as stroke (Billinger 2014). Although exercise and physical activity are promoted positively the evidence is still incomplete.
What is physical fitness training?
Exercise refers to a subset of physical activity that is planned, structured, repetitive, and deliberately performed to train (improve) one or more components of physical fitness (USDHHS 2008). Since the term 'exercise' is used more generically within stroke care we will refer to exercise as 'physical fitness training'.
What is physical fitness?
Physical fitness describes a set of physiological attributes that a person has or achieves, which confers the ability to perform physical activities without undue fatigue. Activities can range from day‐to‐day tasks to leisure activities (USDHHS 2008). The most important components of physical fitness are those responsible for muscular work, as follows.
Cardiorespiratory fitness is the ability to transport and use oxygen and is usually expressed as maximal oxygen uptake (VO2 max). Cardiorespiratory fitness confers 'endurance', that is the ability to perform physical activity for an extended period.
Muscle strength refers to the ability of a specific muscle or muscle group to exert force. Strength is associated with the ability to perform forceful movements such as pushing or lifting.
Muscle power refers to the rate at which muscular work can be performed during a single explosive contraction. Power is associated with the ability to carry out forceful movements, in particular those that are dynamic.
In addition, other components of fitness can influence the ability to perform physical activities, including flexibility (range of motion about a specific joint), balance (ability to maintain stability and posture), and body composition (for example relative amounts of fat and fat‐free mass).
Determinants of fitness
Physical fitness is lower in women compared with men and it deteriorates due to increasing age (1% to 4% in one year) (Young 2001), physical inactivity (12% to 14% in 10 days) (Kortebein 2008), and other secondary consequences of chronic disease such as inflammation (Degens 2006).
Functional importance of fitness
When the level of fitness is low (regardless of the reason) physical activities may either become limited by fatigue or impossible to perform (Young 2001). Levels of fitness below a threshold needed to perform instrumental activities of daily living (ADL) may mean loss of independence, for example cardiorespiratory fitness (Shephard 2009) and muscle strength (Hasegawa 2008).
Description of the condition
A common neurological consequence of stroke is unilateral loss or limitation of muscle function; the direct consequence can be limitation or loss of movement, mobility, and functional ability. In addition, a whole range of indirect complications occur after stroke (Indredavik 2008; Langhorne 2000). Low levels of physical activity are therefore common soon after stroke (Bernhardt 2004; Bernhardt 2007). In community‐dwelling stroke patients cardiorespiratory fitness ranges from 26% to 87% of the value expected in age and gender‐matched healthy people (Smith 2012). Muscle strength (Gerrits 2009; Horstman 2008) and muscle power (Saunders 2008) are also impaired with bilateral deficits, which suggest the influence of physical inactivity. The level of post‐stoke fitness may be low due to a range of factors directly and indirectly connected to stroke.
Pre‐stroke fitness levels may already be low since physical inactivity (Lee 2002) and low levels of fitness (Kurl 2003) are both risk factors for stroke. In addition, most stroke patients are elderly (more than 70 years of age) so levels of fitness will be low due to the effects of age (Malbut 2002) and the presence of comorbid diseases.
Direct neurological effects of stroke reduce the muscle mass available for activation (e.g. hemiparesis).
Post‐stroke physical inactivity (for whatever reason) will cause a longitudinal loss of fitness alongside the effects of comorbid diseases and increasing age. Limitation or loss of functional abilities after stroke (e.g. walking, stair climbing, chair rising) are associated with low cardiorespiratory fitness levels, muscle strength, and muscle power (Flansbjer 2006; Patterson 2007; Saunders 2008).
Therefore, inactivity, which commonly occurs after stroke, may result in low levels of physical fitness; this may exacerbate or cause some common post‐stroke physical limitations (Saunders 2013a). Restoration of motor function in order to improve functional ability is a key focus within stroke rehabilitation and a number of interventions have been investigated that involve physical activities and physical fitness training (Langhorne 2009).
Description of the intervention
Although the design of physical fitness training interventions varies across healthy people, older people, and patient groups, the structure and content remains guided by a common set of well‐established principles (ACSM 1998; ACSM 2011).
Type of training
Most physical fitness training programmes are classified as either: 1) cardiorespiratory training (to improve cardiorespiratory fitness), 2) resistance training (to improve muscular strength and muscle power), or 3) mixed training, which combines cardiorespiratory and resistance training. With regard to other aspects of fitness, all types of training programme have the potential to influence body composition (increase lean mass and reduce adiposity) and some may also incorporate elements that improve flexibility (stretching exercises) and balance.
Mode of training
The type of fitness training influences the mode(s) of exercise. For example, cardiorespiratory training commonly employs walking and cycling, whilst resistance training employs activities involving muscle contractions resisted by weights, body mass, or elastic devices.
Dose of training
The dose of training is controlled by influencing: 1) the amount of training (for example programme length (weeks, months), frequency (days/week), and duration (minutes) of sessions), and 2) the intensity of training (amount of work or effort).
It is the manipulation of type, mode, and dose that defines an exercise prescription; however, the effectiveness is also influenced by some other critically important principles of training (ACSM 1998; ACSM 2011), including progression of training, whether training is task‐related (specific), and the fact that training effects are reversible if training is reduced or stopped.
Physical fitness training is, therefore, very much a complex intervention with numerous component parts and this can give rise to variation in plausible benefits.
How the intervention might work
Regular physical activity is currently recommended where possible to people of all ages, including those with disabilities, in order to promote and maintain health (Haskell 2007; USDHHS 2008). The dose‐response relationship means additional benefits exist if physical fitness training is employed, in particular with regard to physical function. Physical fitness training interventions improve physical function in healthy elderly people (Chodzko‐Zajko 2009).
Post‐stroke physical activity and fitness levels are low, and these low levels are associated with common post‐stroke functional limitations. Increased fitness and physical function could benefit a range of other common post‐stroke problems, for example by reducing fatigue, reducing the incidence of falls and fractures, compensating for the increased energetic cost of a hemiparetic gait, reducing disability and improving independence, and improving quality of life and mood.
Physical therapies are known to promote structural brain remodelling (Gauthier 2008) and this can influence post‐stroke motor deficits. There is systematic review evidence that repetitive practice of some common day‐to‐day activities produces some modest improvements in mobility and ADL in stroke patients (French 2008). Therefore, participation in repetitive, task‐related fitness training may have functional benefits even if fitness is not improved.
Engagement with group training activities may have some psychosocial benefits in people with stroke (Carin‐Levy 2009; Mead 2005; Patterson 2009). Therefore, simply participating in physical fitness training may be beneficial, particularly where group activities are involved.
Cognitive function impairments are common after stroke and are predicted by low indices of physical fitness (Lee 2014). In older adults (> 65 years) with cognitive impairment exercise interventions have been shown to improve cognitive function (Heyn 2004). Therefore, there is some rationale that fitness training interventions could benefit cognition in people with stroke.
Physical fitness training is known to be beneficial for people with a number of conditions that are comorbid conditions or risk factors for stroke. Systematic review evidence shows that exercise interventions can reduce blood pressure (Cornelissen 2013), improve vascular risk factors in obesity (Shaw 2006) and type II diabetes (Thomas 2006), reduce mortality in people with coronary heart disease (CHD) (Heran 2011), and improve depressive symptoms in patients diagnosed with depression (Rimer 2012). Therefore, post‐stroke cardiorespiratory training, in particular, could reduce morbidity and mortality through secondary prevention of stroke and comorbid disease.
In summary, physical fitness training does not simply provide a mechanism to increase fitness, it has multiple mechanisms of action and has a spectrum of plausible benefits that are relevant to many people with stroke. However, there may also be risks, such as training‐induced soft tissue injuries, altered muscle tone, falls, and vascular events.
Why it is important to do this review
Physical fitness training for stroke survivors remains under‐investigated in two key areas.
Firstly, the range of possible benefits is not fully explored. The top 10 most important research priorities for 'life after stroke' have recently been defined by a partnership of patients, carers, and clinicians (Pollock 2012); exercise interventions may have a beneficial role in at least five of the top 10 research priorities (Saunders 2014a).
Secondly, although enough evidence is available to implement fitness training for stroke, the optimal exercise prescription has yet to be defined (Mead 2011).
There has been sustained interest in physical fitness interventions for stroke evidenced by the trials included in previous updates of this review: Saunders 2004a (12 trials), Saunders 2009 (24 trials), Brazzelli 2011 (32 trials), and Saunders 2013 (45 trials). The previous version of this Cochrane Review was the sixth most accessed Cochrane review (3276 full‐text accesses during 2014) about stroke as a whole (source: The Cochrane Library Impact Factor and Usage Report, 2014). Considering the degree of incomplete knowledge, the high level of interest, and the clinical relevance of this review for improving patient care, we believe it is essential to continue updating this review.
Objectives
To determine whether fitness training after stroke reduces death, dependence, and disability. The secondary aims were to determine the effects of training on adverse events, risk factors, physical fitness, mobility, physical function, health status and quality of life, mood, and cognitive function.
Interventions to improve cognitive function have attracted increased attention after being identified as the highest‐rated research priority for life after stroke (Pollock 2012). Therefore, we have added this class of outcomes to this updated review.
Methods
Criteria for considering studies for this review
Types of studies
All trials described as randomised controlled trials (RCTs), single‐blinded or open, which examined the effects of cardiorespiratory, resistance, or mixed training using any of the following six comparisons.
Cardiorespiratory training versus control: 1) at the end of intervention, 2) at the end of follow‐up.
Resistance training versus control: 3) at the end of intervention, 4) at the end of follow‐up.
Mixed training (cardiorespiratory plus resistance training) versus control: 5) at the end of intervention, 6) at the end of follow‐up.
In this review 'end of intervention' refers to the time point when a training programme finishes; 'end of follow‐up' refers to any time point occurring after the end of the intervention. Measures at the end of follow‐up allow us to examine whether training effects (if any) are retained after training is completed. For trials with multiple follow‐up phases we analysed data from the longest follow‐up period.
We included studies in which controls were exposed to either physical activity occurring during usual care or no training after usual care. By 'no training' we meant either no intervention or a non‐exercise intervention (for example cognitive tasks or sham training). Therefore, we deemed the following comparisons suitable for inclusion where 'usual care' refers to inpatient hospital care or other standard rehabilitation given to all stroke patients delivered as a normal part of stroke care in the region in which the trials were performed:
training plus usual care versus usual care (during usual care);
training versus no training (after usual care).
We included only full‐text reports of published and unpublished trials. We did not include conference proceedings alone (that is abstract and poster presentations) because usually they provide only limited data and do not allow full assessment of study quality. We did not exclude trials on the basis of their sample size. We included studies published in languages other than English only when a translation could be arranged. Where investigators published several reports based on data from a single study population, we selected the most recent or most complete report for data extraction and we listed the other reports as duplicate publications.
Types of participants
Adult stroke survivors who were considered suitable for fitness training by the trials' authors; we used the trialists' definition of stroke. Participants were considered eligible irrespective of the time since stroke onset.
Types of interventions
We assessed the following interventions.
Cardiorespiratory training
The aim of this type of training is to improve the cardiorespiratory component of physical fitness. It is typically performed for extended periods of time on devices or ergometers (for example treadmill, cycling, rowing) or by utilising modes of activity such as walking or climbing stairs.
Resistance training
This type of training is performed primarily to improve muscle strength and muscular endurance or muscle power output, or both. It is typically carried out by making repeated muscle contractions resisted by body weight, elastic devices, masses, free weights or specialised machine weights, and isokinetic devices.
Mixed training
This describes training interventions that comprise different activity components, some intended to improve cardiorespiratory fitness and others to improve strength, power or muscular endurance; for example, a training programme comprising both cycling and weight training.
We only included trials that aimed at training stroke survivors. We defined 'training' as a systematic, progressive increase in the intensity or resistance, frequency, or duration of the physical activity throughout a scheduled programme. We categorised the 'dose' of the cardiorespiratory or resistance training components of a training programme as falling within or below the American College of Sports Medicine (ACSM) criteria for developing and maintaining fitness (ACSM 1998). Although a more recent update of this is available (ACSM 2011), the recommendations are more difficult to apply as criteria; therefore for consistency with previous versions of this review we have retained the ACSM 1998 criteria in this update. This decision makes no difference to whether studies are included or not. We sought measures of adherence to training since this can modify the dose of training received by trial participants. For the purposes of this review, adherence included both: 1) attendance at training sessions, and 2) compliance with exercise instructions during training sessions.
We excluded trials that focused on different types of standard rehabilitation techniques but did not include a physical fitness component. We also excluded trials that combined fitness training with assistive technologies, such as robotic and electromechanical‐assisted gait training devices during body weight‐supported locomotor training, as well as trials investigating virtual reality approaches.
We excluded studies that compared upper and lower body training if an additional non‐exercise control group was not considered.
If any description of a training regimen was unclear, we contacted the authors for further information.
Types of outcome measures
We anticipated that existing trials in the literature would use different measures to assess outcomes relevant to this review; in particular they would use a variety of rating scales. For each outcome of interest we tried, therefore, to list the most common and relevant measures or tools. We only included rating scales that had been described in peer‐reviewed journals.
Primary outcomes
Case fatality: numbers of deaths from all causes.
Death or dependence: composite outcome where dependence is classified as having a Barthel Index score of less than 20 or modified Rankin Scale score of 3, 4, or 5 (Lindley 1994).
Disability: assessed by functional scales such as the Functional Independence Measure (Hamilton 1994); Barthel Index (Collin 1988); Rivermead Mobility Index (Collen 1991); Functional Ambulation Category (Holden 1984); Nottingham Extended Activities of Daily Living Scale (Wade 1992); Lawton Index of Activities of Daily Living (Lawton 1969); and the Stroke Impact Scale (Duncan 1999).
Since the review protocol was originally written, the use of the International Classification of Functioning, Disability and Handicap (ICF) is becoming more widespread (WHO 2001). In the ICF classification the term 'disability' is an umbrella term for impairments and activity limitations. In this review the primary outcome measure 'disability' refers to 'global indices of activity limitation'. Secondary outcome measures of mobility and physical function refer to 'specific activity limitations'.
Secondary outcomes
Adverse effects: recurrent non‐fatal cardiovascular or cerebrovascular events; altered muscle tone; training‐induced injury; incidence of falls; incidence of fractures.
Vascular risk factors: resting systolic and diastolic blood pressure; resting heart rate; total cholesterol; glucose tolerance; body mass index (BMI).
Physical fitness: exercise heart rate and maximum or peak oxygen uptake (peak VO2); muscle strength and power output.
Mobility: gait speed (maximum or preferred speed); gait capacity (e.g. six‐minute walking test (6‐MWT)).
Physical function: balance; stair climbing; weight bearing; 'timed up and go' test.
Health status and quality of life: any relevant scale such as the Short Form 36 Health Survey Questionnaire (http://www.sf‐36.org) and the Nottingham Health Profile (Hunt 1980).
Mood: any relevant scale such as the Hospital Anxiety and Depression Scale (HADS) (Zigmond 1983); the Beck Depression Index (Beck 1961).
Cognitive function: any sub‐scale of disability or health status outcomes that relate to cognitive function, or any specific cognition instrument, for example the Repeatable Battery for the Assessment of Neuropsychological Status (Randolph 1998); the Montreal Cognitive Assessment (MOCA) (Nasreddine 2005).
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for trials in all languages and arranged translation of relevant papers where necessary.
Electronic searches
We searched the Cochrane Stroke Group Trials Register, which was last searched by the Managing Editor in February 2015. In addition, we searched the following electronic bibliographic databases:
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2015, Issue 1: searched February 2015) (Appendix 1);
MEDLINE (1966 to February 2015) in Ovid (Appendix 2);
EMBASE (1980 to February 2015) in Ovid (Appendix 3);
CINAHL (1982 to February 2015) in EBSCO (Appendix 4);
SPORTDiscus (1949 to February 2015) in EBSCO (Appendix 5).
We developed the search strategies for the electronic databases with the help of the Cochrane Stroke Group Trials Search Co‐ordinator. The MEDLINE search strategy includes both MeSH controlled vocabulary (/) and free text terms (.tw.) for the relevant target condition (for example stroke, cerebrovascular diseases) and for specific interventions (for example fitness training, muscle strengthening, cycling, rowing, treadmill, circuit training). We limited the search to clinical trials and intervention studies carried out in humans. We did not apply any language restrictions. We adapted the MEDLINE search strategy, and accommodated differences in indexing and syntax, to search the other major electronic databases. We imported all citations identified by the electronic searches into a Reference Manager database and removed duplicate records.
We also searched the following electronic databases and websites using the terms 'stroke', 'exercise', and 'physical fitness' to identify additional relevant trials, ongoing trials, and thesis dissertations:
Science Citation Index Expanded (1981 to February 2015) (Web of Knowledge ‐ WOK);
Web of Science Proceedings (1982 to February 2015) (WOK);
Physiotherapy Evidence Database (PEDro) (last searched February 2015) (www.pedro.fhs.usyd.edu.au/);
REHABDATA (1956 to February 2015) (http://www.naric.com/);
ProQuest Dissertations & Theses Global February 2015 (http://www.proquest.com/products‐services/pqdtglobal.html);
Internet Stroke Centre's Stroke Trials Directory database (last searched February 2015) (www.strokecenter.org/trials/);
metaRegister of Controlled Trials (last searched February 2015) (www.controlled‐trials.com/mrct/);
ClinicalTrials.gov February 2015 (http://clinicaltrials.gov/);
World Health Organization (WHO) International Clinical Trials Registry Platform Search Portal February 2015 (http://apps.who.int/trialsearch/).
We performed citation tracking of all reports selected for inclusion using Google Scholar (http://scholar.google.co.uk/) (last searched February 2015).
Searching other resources
We scrutinised the proceedings of relevant stroke meetings (February 2015) listed on the Internet Stroke Centre's website (www.strokecenter.org/), including the European Stroke Conference (2000 to 2014), the International Stroke Conference (2000 to 2015), and the World Stroke Congress (2000 to 2014). We used proceedings to identify ongoing studies and full publications that may have been missed in other searches. We did not consider potentially relevant completed studies for inclusion if they were available only as conference proceedings; instead we retained them as 'studies awaiting classification'. We will consider these studies for inclusion in the next update of this review if a full publication has subsequently become available.
We handsearched relevant scientific journals that focus on exercise and physical fitness and are not currently included in the Cochrane handsearching programme:
Adapted Physical Activity Quarterly (1984 to February 2015);
British Journal of Sports Medicine (1974 to February 2015);
International Journal of Sports Medicine (1980 to February 2015);
Journal of Science and Medicine in Sport (1998 to February 2015);
Research Quarterly for Exercise and Sport (1985 to February 2015);
Sports Medicine (1984 to February 2015).
We examined the references lists of all relevant studies identified by the above methods and perused all relevant systematic reviews identified during the entire search process for further trials. We also checked all the references in both the studies awaiting classification and ongoing studies sections of the previous version of this review.
We contacted experts in the field and principal investigators of relevant studies to enquire about unpublished and ongoing trials.
Data collection and analysis
Selection of studies
One review author (DS or MS) read the titles and abstracts of all citations identified by the electronic searches and excluded obviously irrelevant reports. We retrieved the full text of the remaining papers and two review authors (DS and either SH, MK, MS) independently assessed these and selected trials which met the pre‐specified inclusion criteria. The two review authors resolved any disagreements by discussion and if necessary consulted with a third review author (GM or CG). One review author (DS) also screened the correspondence with experts and trial investigators for details of any additional published or unpublished trials.
Data extraction and management
Two review authors (DS and either SH, MK, MS) independently extracted data from the selected studies. We recorded the following characteristics for each individual study.
Publication details: authors, year of publication, publication status (published, unpublished, or ongoing), citation of other relevant trials.
Details of study conduct: study design, method of recruitment, inclusion and exclusion criteria, number of participants enrolled, number of participants excluded, number of participants assessed, losses to follow‐up, geographical location of the trial, setting in which the trial was conducted (e.g. hospital, community).
Characteristics of participants: total number, age, gender, stage of care, severity of stroke, time since stroke onset, co‐morbidity, walking ability.
Details of intervention: total number of intervention groups, type of training (i.e. cardiorespiratory, resistance, or mixed), training mode (e.g. treadmill walking, weight training), dose (i.e. intensity, frequency of delivery), timing (i.e. during or after usual care), length of training (i.e. duration and programme length), adherence to intervention (i.e. attendance, compliance).
Details of outcome measures: choice of outcomes (i.e. death, dependence, disability, physical fitness measures, gait assessment, physical function measures, health status and quality of life, mood, adverse events, risk factors), outcome data, reported outcomes, missing outcomes.
We classified all outcome data as being from time points at either: 1) the end of intervention, or 2) the end of follow‐up (that was defined as any period of time after the training intervention was completed). We resolved any disagreement by consensus or arbitration.
Assessment of risk of bias in included studies
Two review authors (DS and either SH, MK, MS) assessed the risk of bias for the following items, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We included one extra item 'confounded by increased training time' where we recorded trials that did not include a balanced exposure to an attention control as being at 'high risk' of exaggerating effects.
Random sequence generation
Allocation concealment
Blinding of participants *
Blinding of outcome assessment
Incomplete outcome data
Selective reporting
Other bias
Confounded by increased training time
* For trials of physical interventions like exercise it is not possible to blind participants or those delivering interventions. However, some trials may incorporate a degree of blinding if the control group participates in an attention control intervention that allows the investigators to disguise the exact purpose of the two interventions; the trial could be described simply as a 'comparison of two interventions'.
Data synthesis
We carried out statistical analysis using RevMan 5.3 (RevMan 2014). We calculated a summary statistic for each outcome measure to describe the observed treatment effect. All summary statistics reported in this review refer to effects at either: 1) the end of intervention, or 2) the end of follow‐up. We qualitatively assessed whether clinical heterogeneity was present among included studies and we combined studies in a meta‐analysis only when we judged them reasonably homogeneous in terms of participants, interventions, and outcomes. We presented relevant outcomes for the main comparisons of interest in 'Summary of findings' tables.
Continuous and dichotomous data
The data required for meta‐analyses of continuous data in RevMan 2014 were mean and standard deviation (SD). When collecting continuous data we took some precautions to check whether standard error (SE) was mistakenly reported as SD. We used SE or 95% confidence interval (CI) to compute SD when missing. The included studies presented results for continuous data either as mean and SD of final measurement values or as mean and SD of change from baseline for each intervention group, or both. We extracted final measurement values or change from baseline scores instead of final measurement values if required. In our analyses we combined final measurement values with any change from baselines scores using the mean difference (MD) method as we assumed that MDs based on changes from baseline scores addressed the same underlying treatment effects as MDs based on final measurements.
The data required for meta‐analyses of dichotomous data in RevMan 2014 were number of events in each intervention group and total number of participants in each intervention group.
In the case of missing outcome data, we attempted to analyse data according to the intention‐to‐treat (ITT) approach. When individual patient data were available we used the 'last observation carried forward' (LOCF) approach (that is the most recently reported outcome was assumed to hold for all subsequent outcome assessments).
Measures of effect
For continuous data we calculated mean differences with 95% CIs if the studies used the same instrument to measure the same outcome (for example disability). However, if studies used a variety of instruments (for example rating scales), we calculated the standardised mean difference (SMD) with 95% CI.
For dichotomous data we calculated odds ratios (OR) with 95% CIs.
We assessed statistical homogeneity between trial results by means of the Chi2 test for heterogeneity, which is included in the forest plots in RevMan 5. The Chi2 test has notoriously low power in meta‐analyses when studies have small sample size, or when the number of events is small, therefore we decided: 1) to set the significance level at 0.10 rather than at the conventional level of 0.05, and 2) to analyse data using a random‐effects model (a fixed‐effect model would have given the same quantitative conclusions but with narrower CIs).
To quantify inconsistency across studies we used the I2 statistic, which is included in the meta‐analysis graphs in RevMan 2014.
Where possible, we investigated publication bias by entering data from studies included in the relevant meta‐analyses in funnel plots (treatment effect versus trial size).
Subgroup analysis and investigation of heterogeneity
When sufficient data were available, we planned to investigate heterogeneity between included studies (both clinical and statistical) by means of subgroup analyses. We attempted to compare effect estimates in the following main subgroups:
type of training (cardiorespiratory versus resistance training versus mixed training);
time of training (during usual care versus after usual care).
The complexity of exercise interventions and low numbers of studies in the meta‐analyses mean that subgroup analyses are difficult to perform and difficult to interpret. We explored the following planned subgroups instead, where possible, using a sensitivity analysis approach:
training programmes that met the ACSM guidelines (ACSM 1998) versus those that did not;
type of control interventions (no intervention versus non‐exercise intervention versus other intervention);
duration of training (less than 12 weeks versus 12 weeks or more);
severity of stroke (mild symptoms versus severe symptoms).
Sensitivity analysis
When sufficient data were available we planned to explore the influence of some study characteristics by means of sensitivity analyses. We considered the effect of excluding studies in which the comparisons were confounded by increased training time and explored some of the factors originally intended for subgroup analyses.
Results
Description of studies
Results of the search
The previous version of this review included 45 trials (2188 participants) (Saunders 2013). In this updated version we repeated the previous electronic searches and other relevant searches (for example handsearching, screening of conference proceedings and relevant websites) in 2015. We screened a total of 8915 citations; this includes duplicates.
We identified new 31 new systematic reviews of exercise interventions and screened these for relevant trials (Ada 2013; Bonilha 2013; Cabanas‐Valdés 2013; Charalambous 2013; Cooke 2010; Cumming 2012; Dunn 2013; Eng 2014; Francica 2014; Garcia‐Soto 2013; Karttunen 2014; Lennon 2014; Mackay‐Lyons 2013; Marsden 2013; Mehrholz 2014; Mehta 2012; Mehta 2012a; Pang 2013; Pereira 2012; Pereira 2012a; Peurala 2014; Ploughman 2014; Pohl 2014; Polese 2013; Pollock 2014; Rodrigues‐Baroni 2014; Sorinola 2014; Stoller 2012; Veerbeek 2011).
The results of our searching activities are summarised in Figure 1. We identified and applied the inclusion criteria to a total of 83 potentially relevant new trials.
Figure 1.
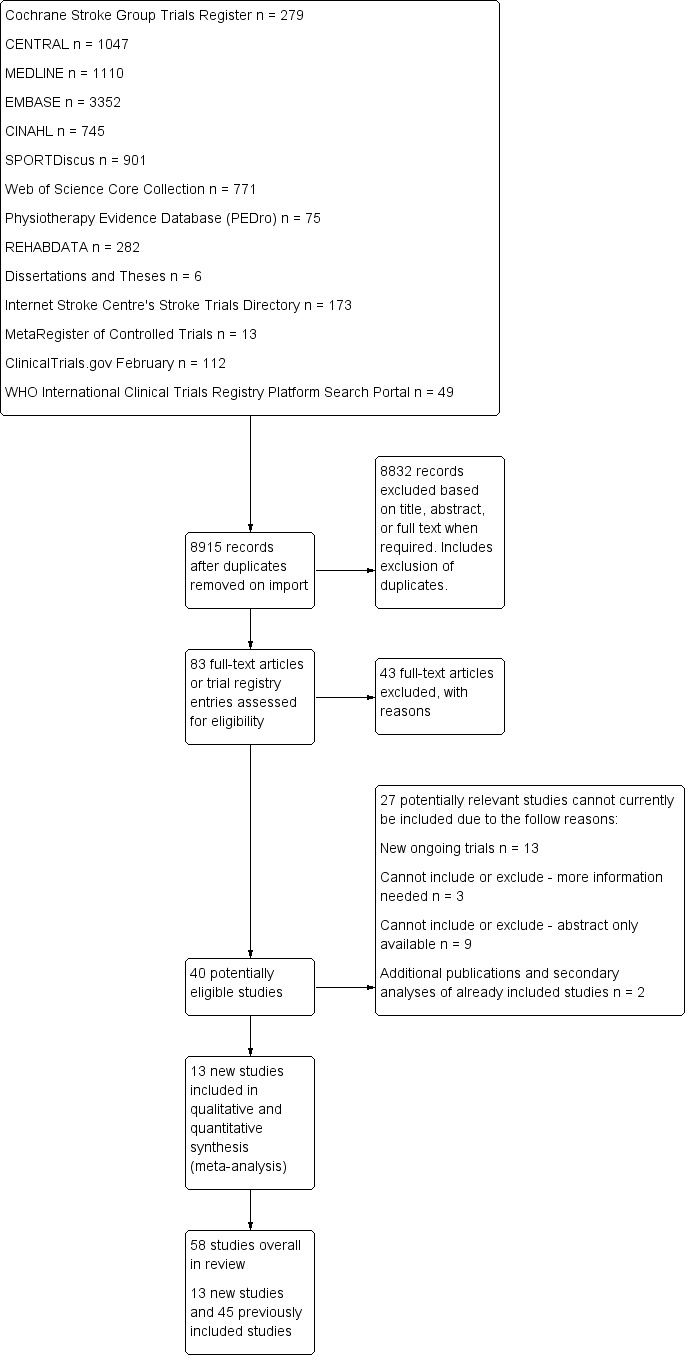
Study flow diagram for the current update of this review.
We included 13 additional completed trials (see Characteristics of included studies table).
We excluded 43 new trials (see Characteristics of excluded studies table).
We identified 13 new ongoing trials (see Characteristics of ongoing studies table).
We identified three trials for which we require more information to establish eligibility (Park 2014; Qu 2014; Rydwik 2006) (see Characteristics of studies awaiting classification table).
We identified nine trials that are awaiting classification because only the abstract is currently available (Buyukavci 2011; Kondo 2012; Kumaran 2014; Kwok 2012; Lee 2008; Malagoni 2013; Pagnussat 2014; Sen 2013; Vahlberg 2014) (see Characteristics of studies awaiting classification).
Two trials were additional publications and secondary analyses of studies already included (Ada 2013a; Galvin 2011).
Included studies
We included the 13 new studies in this update (Aidar 2014; Gordon 2013; Jin 2013; Kim 2014a; Lee 2013; Lee 2013a; Letombe 2010; MacKay‐Lyons 2013; Shin 2011; Son 2014; Verheyden 2009; Wang 2014; Yang 2014). This now brings the total number to 58 trials comprising 2797 participants).
Two trials are dissertations (Cuviello‐Palmer 1988; James 2002).
Participants
Characteristics
A total of 2797 stroke survivors (range 13 to 250 individuals) were randomised to physical fitness training or control interventions in the 58 included clinical trials. The mean age of the participants was approximately 62 years. The mean time since onset of symptoms ranged from 8.8 days in trials assessing participants before discharge from hospital (Richards 1993) to 7.7 years in trials assessing participants after hospital discharge (Teixeira 1999).
Two trials recruited non‐ambulatory stroke survivors (Richards 1993; Wang 2014), three trials recruited both ambulatory and non‐ambulatory participants (Bateman 2001; Cooke 2010a; Lennon 2008), four trials did not report this information (Donaldson 2009; Lee 2013; Verheyden 2009; Winstein 2004), and all the remaining trials recruited ambulatory stroke survivors.
Sample size
Of the 58 included trials:
13 trials had 20 participants or fewer (Bale 2008; Cuviello‐Palmer 1988; da Cunha 2002; Donaldson 2009; Duncan 1998; Glasser 1986; James 2002; Kim 2001; Letombe 2010; Moore 2010; Richards 1993; Smith 2008; Teixeira 1999).
four trials had over 100 participants (Ada 2013a 102 participants, Gordon 2013 128 participants, Jin 2013 128 participants, and van de Port 2012 250 participants).
41 remaining trials recruited between 21 and 100 participants.
Interventions
Cardiorespiratory training
Twenty‐eight trials with a total of 1408 randomised participants (range 15 to 128 individuals) examined cardiorespiratory training (Ada 2013a; Aidar 2007; Bateman 2001; Cuviello‐Palmer 1988; da Cunha 2002; Eich 2004; Glasser 1986; Globas 2012; Gordon 2013; Ivey 2010; Ivey 2011; Jin 2013; Kang 2012; Katz‐Leurer 2003; Kim 2014a; Kuys 2011; Lennon 2008; MacKay‐Lyons 2013; Moore 2010; Mudge 2009; Park 2011; Pohl 2002; Potempa 1995; Salbach 2004; Smith 2008; Takami 2010; Wang 2014; Yang 2014). Details of the nature and dose of the cardiorespiratory interventions are summarised in Table 10.
Table 1.
Outline of the studies that focused on cardiorespiratory training interventions
| Study ID | Mode of training | During or after usual care | Upper or lower body | Specific training | Intensity | Duration (minutes) |
Frequency (days) |
Programme length (weeks) | ACSM criteria met |
| Aidar 2007 | Water training | After | Both | Yes | Unknown | 45 to 60 | 2 | 12 | Unknown |
| Lennon 2008 | Cycle ergometer (cardiac rehabilitation programme) | After | Both | No | 50% to 60% maximum heart rate | 30 | 2 | 10 | Yes |
| Moore 2010 | Treadmill gait training with overhead harness | After | Lower body | Yes | 80% to 85% age‐predicted maximum heart rate | Unknown | 2 to 5 | 4 | Yes |
| Mudge 2009 | Circuit training | After | Lower body | Yes | Unknown | 30 | 3 | 4 | Unknown |
| Smith 2008 | Treadmill gait training | After | Lower body | Yes | Rate perceived exertion ≤ 13 | 20 | 3 | 4 | Yes |
| Glasser 1986 | Kinetron | During | Lower body | No | Unknown | 20 to 60 | 5 | 3 | Unknown |
| Cuviello‐Palmer 1988 | Kinetron | During | Lower body | No | Heart rate < resting + 20 beats/minute | 7 to 17 | 5 | 3 | No |
| da Cunha 2002 | Treadmill gait training with body weight support (BWS) | During | Lower body | Yes | Unknown | 20 | 5 | 2 to 3 | Unknown |
| Pohl 2002 | Treadmill gait training Group (1) STT (structured speed‐dependent treadmill training) Group (2) LTT (limited progressive treadmill training group) |
During | Lower body | Yes | Unknown | 30 | 3 | 4 | Unknown |
| Eich 2004 | Treadmill gait training | During | Lower body | Yes | 60% heart rate reserve | 30 | 5 | 6 | Yes |
| Bateman 2001 | Cycle ergometer | During | Lower body | No | 60% to 80% age‐related heart rate maximum | ≤ 30 | 3 | 12 | Yes |
| Katz‐Leurer 2003 | Cycle ergometer | After | Lower body | No | ≤ 60% heart rate reserve | 20 then 30 | 5 then 3 | 2 then 6 (total 8) | Yes |
| Potempa 1995 | Cycle ergometer | After | Lower body | No | 30% to 50% maximum effort | 30 | 3 | 10 | Yes |
| Salbach 2004 | Circuit training | After | Lower body | Yes | Unknown | 55 | 3 | 6 | Unknown |
| Ada 2013a | Treadmill + overground walking | After | Lower body | Yes | Unknown | 30min | 3 | Group 1 = 16 Group 2 = 8 |
Unknown |
| Globas 2012 | Treadmill | After | Lower body | Yes | 40% to 50% progressing to 60% to 80% heart rate reserve | 10 to 20 min increasing to 30 to 50 min | 3 | 12 | Yes |
| Ivey 2010 | Treadmill | After | Lower body | Yes | 40% to 50% progressing to 60% to 70% heart rate reserve | 10 to 20 min increasing to 40 min | 3 | 24 (6 months) |
Yes |
| Ivey 2011 | Treadmill | After | Lower body | Yes | 40% to 50% progressing to 60% to 70% heart rate reserve | 10 to 20 min increasing to 40 min | 3 | 24 (6 months) |
Yes |
| Kang 2012 | Treadmill | After | Lower body | Yes | Unknown | 30 | 3 | 4 | Unknown |
| Kuys 2011 | Treadmill | After | Lower body | Yes | 40% progressing to 60% heart rate reserve | 30 | 3 | 6 | Yes |
| Park 2011 | Overground community‐based walking | During | Lower | Yes | Unknown | 60 | 3 | 4 | Unknown |
| Takami 2010 | Treadmill gait training with body weight support (BWS) Group (1) Backward walking group Group (2) Forward walking group |
During | Lower body | Yes | Unknown | 10 | 6 | 3 | Unknown |
| Gordon 2013 | Overground community‐based walking | After | Lower body | Yes | Target heart rate was 60% to 85% of age‐predicted maximum heart rate (220‐age). | 15min progressing by +5 min per week | 3 | 12 | Yes |
| Yang 2014 | Cycle ergometer | During | Lower | Yes | Cycling training consisted of 15‐minute sessions each of forward and backward cycling including: 150‐second passive warm up; 10‐minute active pedaling at 50 to 70 rev/min at an intensity of stage 13 of the Borg scale; 150 seconds of passive cool down | 30 | 5 | 4 | Unclear |
| Wang 2014 | Wheelchair seated pedaling ergometry | During | Lower | Yes | Cycling training consisted of 30 minutes sessions including: 5‐minute warm up; 30‐minute active pedaling at an intensity based on an incremental graded exercise test (2.5W ramp every 3 minutes maintaining 50 rpm until exhaustion); followed by 5‐minute cool down. Target heart rate was calculated as ((peak heart rate in graded exercise test – resting heart rate) x 50% to 70%) + resting heart rate | 30 | 3 | 6 | Yes |
| MacKay‐Lyons 2013 | Body weight supported treadmill training | During | Both | Yes | Target heart rates corresponding to 60% to 75% of baseline VO2peak Initially treadmill speed 80% to 90% of self paced overground speed with 20% to 30% body weight supported for ambulatory independent participants and 70% to 80% of overground speed with 40% body weight supported for ambulatory dependent participants |
60 | 5/week for 6 weeks then 3/week for 6 weeks | 12 | Yes |
| Kim 2014a | Community walking programme | During | Lower | Yes | Unclear The walking environment was made more challenging with increased exposure to uneven ground, gradients, and stairs |
60 | 5 | 4 | Unclear |
| Jin 2013 | Cycle ergometry | During | Lower | No | Commencing at 40% to 50% heart rate reserve progressing 5% heart rate reserve every 2 weeks up to 70% heart rate reserve | 40 | 5 | 12 | Yes |
ACSM: American College of Sports Medicine min: minute(s)
Two of these trials assessed circuit training (Mudge 2009; Salbach 2004).
One trial assessed aquatic training (Aidar 2007).
Nine trials used some form of ergometry: six assessed cycle ergometry (Bateman 2001; Jin 2013; Katz‐Leurer 2003; Lennon 2008; Potempa 1995; Yang 2014), one assessed seated/recumbent cycle ergometry (Wang 2014), and two assessed a 'Kinetron' ergometer (Cuviello‐Palmer 1988; Glasser 1986).
Sixteen trials focused on walking using treadmills (da Cunha 2002; Eich 2004; Globas 2012; Ivey 2010; Ivey 2011; Kang 2012; Kuys 2011; MacKay‐Lyons 2013; Moore 2010; Pohl 2002; Smith 2008; Takami 2010), overground walking (Gordon 2013; Kim 2014a; Park 2011), or a combination of treadmill and overground walking (Ada 2013a).
The training programmes comprised regular weekly sessions of sufficient duration (usually greater than 20 minutes) but the exercise intensity was clearly described in only 17 of the 28 included trials. In 15 trials the cardiorespiratory training started after usual care, while in 13 trials it started during usual care. In four of these trials participants were recruited in the acute phase of stroke, less than one month post‐stroke (Cuviello‐Palmer 1988; da Cunha 2002; MacKay‐Lyons 2013; Takami 2010).
Three of the included cardiorespiratory training trials had more than one intervention group that met the eligibility criteria; these compare two different durations, intensities, and modes of training. Each of these studies therefore has two entries when included in any meta‐analyses, each sharing 50% of the number of participants in the single control group from each trial.
Ada 2013a: Group 1 ‐ duration four months training; Group 2 ‐ duration two months training.
Pohl 2002: Group 1 ‐ intensity high due to rapid progression; Group 2 ‐ intensity lower due to limited progression.
Takami 2010: Group 1 ‐ mode: backward walking on treadmill; Group 2 ‐ mode: forward walking on treadmill.
Resistance training
Thirteen trials with a total of 432 randomised participants (range 18 to 54 individuals) assessed the effects of resistance training (Aidar 2012; Aidar 2014; Bale 2008; Flansbjer 2008; Inaba 1973; Kim 2001; Lee 2013; Lee 2013a; Ouellette 2004; Sims 2009; Son 2014; Verheyden 2009; Winstein 2004). Details of the nature and dose of the resistance training intervention trials are summarised in Table 11).
Table 2.
Outline of the studies that focused on resistance training interventions
| Study ID | Mode of training | During/after usual care | Upper or lower body | Specific training | Intensity | Duration (minutes) | Frequency (days) | Programme length (weeks) | ACSM criteria |
| Bale 2008 | Resistance training; weights | During | Lower body | No | 10 to 15 repetitions to achieve moderate fatigue | 50 | 3 | 4 | Yes |
| Flansbjer 2008 | Dynamic and isokinetic resistance training (leg extension/curl rehab exercise machine) | After | Lower body | Yes | 6 to 10 repetitions equivalent to 80% of maximum load | 90 | Unknown | 10 | Unclear (criteria nearly met) |
| Sims 2009 | Resistance training; machine weights | After | Both | Yes | 3 x 8/10 repetitions at 80% one repetition maximum | Unknown | 2 | 10 | Unclear (criteria nearly met) |
| Inaba 1973 | Resistance training | During | Lower body | No | 50% and 100% maximum weight | Unknown | 'Daily' | 4 to 8 | Yes |
| Winstein 2004 | Resistance training; weights; Thera‐band and grip devices | During | Upper body | No | Unknown | 60 | 3 high 2 slow | 4 to 6 (target of 20 sessions) | Unknown |
| Kim 2001 | Resistance training; isokinetic dynamometer | After | Lower body | No | Maximal effort 3 x 10 repetitions | 30 | 3 | 6 | Yes |
| Ouellette 2004 | Resistance training; weights and pneumatic resistance machines | After | Lower body | No | 70% one repetition maximum: 3 x 8 to 10 repetitions | Not applicable | 3 | 12 | Unclear (criteria nearly met) |
| Aidar 2012 | Resistance training; machine weights | After | Both | No | OMNI Resistance Exercise Scale | 45 to 60 | 3 | 12 | Unclear |
| Aidar 2014 | Resistance training; machine weights | After | Both | No | OMNI Resistance Exercise Scale | 60 | 3 | 12 | Unclear |
| Verheyden 2009 | Functional strength | During | Upper (trunk) | Yes | Functional trunk flexion and extension strength in supine and sitting. Exercises gradually introduced and number of repetitions determined by physiotherapists on a patient's performance basis. No further details reported | 30 | 4 | 5 | Unclear |
| Lee 2013 | Closed chain and open chain progressive resistance training | After | Lower | No | 3 sets of 8 to 10 repetitions 70% of one repetition maximum |
Unclear (duration based on repetitions) | 5 | 6 | Yes |
| Lee 2013a | Closed chain and open chain progressive resistance training | After | Lower | No | 3 sets of 8 to 10 repetitions 70% of one repetition maximum |
Unclear (duration based on repetitions) | 5 | 6 | Yes |
| Son 2014 | Pneumatic leg press machine | Probably after | Lower | No | 3 sets of 8 to 10 repetitions 70% of one repetition maximum |
30 | 5 | 6 | Yes |
ACSM: American College of Sports Medicine
All employed muscle contractions resisted by weights, exercise machines, or elastic devices. One trial trained the upper limbs (Winstein 2004), one trained the trunk (Verheyden 2009), three trials trained both the upper and lower limbs (Aidar 2012; Aidar 2014; Sims 2009), and the remaining eight involved the lower limbs only. The training met or nearly met the ACSM 1998 criteria for strength training in five trials. Most programmes were short (less than 12 weeks) apart from Aidar 2012 and Ouellette 2004 (12 weeks). In nine trials resistance training started after usual care (Aidar 2012; Aidar 2014; Flansbjer 2008; Kim 2001; Lee 2013; Lee 2013a; Ouellette 2004; Sims 2009; Son 2014), whilst in four trials it started during usual care (Bale 2008; Inaba 1973; Verheyden 2009; Winstein 2004). In Winstein 2004 participants were recruited during the acute phase of stroke (less than one month post‐onset).
Three of the recent trials appear similar in terms of participants and interventions and have a shared authorship (Lee 2013; Lee 2013a; Son 2014). Although the sample sizes are different there is a possibility that these three publications share some of the same participants. We have not had a response to queries to establish this.
Mixed training
Seventeen trials with a total of 957 randomised participants (range 13 to 250 individuals) assessed the effects of mixed training (Cooke 2010a; Donaldson 2009; Duncan 1998; Duncan 2003; Galvin 2011; James 2002; Langhammer 2007; Letombe 2010; Mead 2007; Richards 1993; Richards 2004; Shin 2011; Teixeira 1999; Toledano‐Zarhi 2011; van de Port 2012; Yang 2006; Zedlitz 2012). Details of the nature and dose of the mixed training interventions are summarised in Table 12.
Table 3.
Outline of the studies that focused on mixed training interventions
| Study ID | Mode of training | During or after usual care | Upper or lower body | Specific training | Intensity | Duration (minutes) | Frequency (days) | Programme length (weeks) | ACSM criteria |
| Cooke 2010a | Resistance training plus treadmill training | During | Lower body | Yes | Unknown | 60 | 4 | 6 | Unknown |
| Donaldson 2009 | Paretic upper limb exercises and hand grip activities | During | Upper body | Yes | Unknown | 60 | 4 | 6 | Unknown |
| Langhammer 2007 | Walking, stationary bicycling, stair walking, treadmill, and resistance training | After | Both | Yes | 70% to 80% maximum pulse (cardiorespiratory component); 50% to 60% one repetition maximum (strength component) | 45 | 2/3 | Unclear. Minimum 20 hours every third month in the first year after stroke | Yes |
| Richards 1993 | Treadmill plus Kinetron plus tilt table | During | Lower body | Yes | Unknown | 104 | 5 | 5 | Unknown |
| Richards 2004 | Treadmill plus Kinetron plus limb load monitor | During | Lower body | Yes | Unknown | 60 | 5 | 8 | Unknown |
| Duncan 1998 | Walking or cycle ergometry; elastic‐resisted contractions | After | Both | Yes | Unknown | 90 | 3 | 12 | Cardio: no Strength: yes |
| Teixeira 1999 | Walking and stepping or cycle ergometry; resistance training body mass, weights, and elastic | After | Lower body | Yes | 50% to 70% maximum work rate (cardiorespiratory component) 50% to 80% one repetition maximum, 3 x 10 repetitions (strength component) | 60 to 90 | 3 | 10 | Cardio: yes Strength: yes |
| Duncan 2003 | Circuit training | After | Lower body | Yes | 50% to 60% heart rate reserve | 90 to 120 | 3 | 4 | Cardio: yes Strength: unclear |
| James 2002 | Circuit training | After | Both | Yes | Unknown | 90 | 3 | 12 to 14 (total of 36 sessions) | Cardio: no Strength: yes |
| Yang 2006 | Functional stepping and chair rising | After | Lower body | Yes | Unknown | 30 | 3 | 4 | No |
| Mead 2007 | Circuit including walking, stepping, cycle ergometry; resistance training body mass, weights, and elastic | After | Both | Yes | Rating of perceived exertion: 13 to 16 | 40 to 75 | 3 | 12 to 14 (total of 36 sessions) | Unknown |
| Galvin 2011 | Family mediated gait and strength training | During | Lower | Yes | Unknown | 35 | 7 | 8 | Unknown |
| Toledano‐Zarhi 2011 | Treadmill, hand bike, cycle ergometer plus group exercise for strength, balance and co‐ordination exercise | During | Both | Yes (treadmill) | Cardiorespiratory 50% to 70% of maximal heart rate | Cardiorespiratory 90 min Group 45 to 55 min |
Cardiorespiratory 2/wk Group 1/wk |
6 | Cardio: yes Strength: unknown |
| van de Port 2012 | Task‐oriented circuit training. 8 workstations targeting balance, stair walking, turning, transfers, and speed walking | After | Lower | Yes (task‐oriented) | Unknown | 90 | 2 | 12 | Unknown |
| Zedlitz 2012 | Treadmill walking, strength training, and home exercise assignments | After | Both | Yes (walking) | Cardiorespiratory and strength progressed from 40% to 70% | 120 | 2 | 12 | Cardio: yes Strength: unknown |
| Letombe 2010 | Cycle ergometry, treadmill walking, and isokinetic resistance training | During | Both including trunk | Yes (walking) | Cardiorespiratory training: 70% to 80% maximal cycling power Strength training; 6 x 10 repetitions at 50% to 60% maximum |
40 to 60 | 4 | 4 | Cardio; unclear Strength; yes |
| Shin 2011 | Functional strength training (bridging and stepping) plus treadmill and cycle ergometry | During | Lower | Yes (walking and stepping) | Cardiorespiratory progressive but < 40% heart rate reserve Strength training described only as 'medium intensity' of 5 to 15 repetitions |
60 | 5 | 4 | Cardio; no Strength; unclear |
ACSM: American College of Sports Medicine min: minute(s) wk: week
The modes of exercise are quite diverse since these comprise circuit training or various combinations of walking, treadmill training, and resistance training. All interventions contained one or more functionally relevant activity (such as walking). Intensity of exercise was reported sufficiently to classify the cardiorespiratory component of three trials (James 2002; Langhammer 2007; Teixeira 1999), and the strength component of six trials (Duncan 1998; Duncan 2003; Langhammer 2007; Letombe 2010; Teixeira 1999; possibly Toledano‐Zarhi 2011) as satisfying the ACSM 1998 criteria. Most programmes were short, with only five trials meeting or exceeding 12 weeks (Duncan 1998; James 2002; Mead 2007; van de Port 2012; Zedlitz 2012). Six trials occurred during usual care; four of these recruited participants in the acute phase of stroke, less than one month post‐onset (Galvin 2011; Letombe 2010; Richards 1993; Toledano‐Zarhi 2011), and two at a later stage of care (Richards 2004; Shin 2011).
Adherence to training interventions
Adherence to the interventions was defined in terms of: 1) attendance at the planned training sessions, and 2) compliance with the planned content of the training sessions.
Attendance
Rate of attendance (%) could be clearly determined in 26 of the 58 included trials (Ada 2013a; Aidar 2012; Bateman 2001; Duncan 1998; Duncan 2003; Eich 2004; Flansbjer 2008; Globas 2012; Kuys 2011; Langhammer 2007; MacKay‐Lyons 2013; Mead 2007; Mudge 2009; Park 2011; Ouellette 2004; Pohl 2002; Richards 1993; Richards 2004; Salbach 2004; Sims 2009; Toledano‐Zarhi 2011; van de Port 2012; Wang 2014; Winstein 2004; Yang 2006; Zedlitz 2012). The proportion of attended training sessions ranged from 65% up to 100%. Six trials measured attendance for the training and the control groups separately and showed similar rates between groups (Bateman 2001; Langhammer 2007; MacKay‐Lyons 2013; Mead 2007; Ouellette 2004; Salbach 2004). A few other trials described attempts to facilitate attendance and make up missed sessions, or reported that "attendance did not differ between intervention groups" but did not provide attendance rates (Bale 2008; Cooke 2010a; Teixeira 1999). One trial specifically excluded those participants who attended fewer than nine training sessions from the statistical analyses (thus preventing an intention‐to‐treat assessment of results) (da Cunha 2002).
Compliance
Compliance with the scheduled exercise programme during training sessions was described in few trials. For cardiorespiratory training interventions, Langhammer 2007 stated that the compliance with the individualised training levels was 'high'; other trials reported that participants 'tolerated' training (Globas 2012; MacKay‐Lyons 2013; Pohl 2002), or showed no discomfort (Jin 2013). Salbach 2004 maintained that most of the participants completed nine out of 10 circuit training exercises. For mixed training, Duncan 1998 reported 'good compliance' with home‐based training and Yang 2006 stated that mixed circuit training was "performed as planned". Mead 2007 reported 94% to 99% compliance with circuit training exercises 'tailored' to individual requirements. Information on compliance was not available for the remaining trials. Zedlitz 2012 described the compliance of participants with training as 'good'. Two trials reported good compliance of therapists in delivery of the content of the planned protocol (MacKay‐Lyons 2013; Zedlitz 2012).
Comparisons
Training interventions were compared with control interventions in different ways in the included studies. We identified seven different types of comparison, which has implications for establishing the effects of fitness training.
Balanced comparisons ‐ The nature of some of these comparisons allows intervention and control groups to be comparable in terms of exposure time (both groups are exposed to an intervention, the frequency and duration of which is similar between groups) and the 'attention' received by the therapists. Therefore, these comparisons allow one to separate the specific effects of fitness training from those of usual rehabilitation interventions.
Training plus a proportion of usual care versus usual care.
Training plus usual care versus non‐exercise intervention plus usual care.
Training versus non‐exercise intervention ‐ after usual care.
Training versus usual outpatient care.
Confounded comparisons ‐ Other comparisons make it impossible to have a comparable intervention and control group exposure time (for example the 'training versus no intervention' comparison). We will describe these comparisons in the review as 'confounded by additional training time'. With regard to interventions involving physical exercise, a greater exposure to the intervention has a known effect on rehabilitation outcomes ('augmented therapy time') (Kwakkel 2004). Therefore, although these comparisons allow comment on the overall effect of training programmes, they make it difficult to attribute any benefits to the content of the exercise prescription itself.
Training plus usual care versus usual care.
Training plus non‐exercise intervention versus non‐exercise intervention ‐ after usual care.
Training versus no intervention ‐ after usual care.
Outcome measures
Outcome measures were recorded at the end of the training period (end of intervention), or at any other defined point either within the trial duration or after completion of the training programme, or both (scheduled end of follow‐up).
A variety of outcome measures were used in the included studies; some trials shared the same outcome measures. This limited the opportunity to combine outcome measures in the meta‐analyses.
Some outcome measures involved continuous data (for example assessment scales) with skewed distributions. Due to time and resource constraints we did not attempt to transform these data. We therefore combined continuous skewed data and continuous normal‐distributed data.
Excluded studies
The most common reasons for exclusion were: a controlled trial in which the intervention did not meet the criteria for fitness training or did not include a suitable comparison, or a confounding of training with another active physical intervention.
Risk of bias in included studies
Details and justifications for 'Risk of bias' assessments in individual studies are shown in the Characteristics of included studies table. As this is a complex review we decided to apply the 'Risk of bias' assessments to 'all outcomes' for simplicity apart from incomplete outcome data, for which we assessed bias at 1) the end of the intervention, and 2) the end of follow‐up. We present the summary results in Figure 2 and Figure 3.
Figure 2.
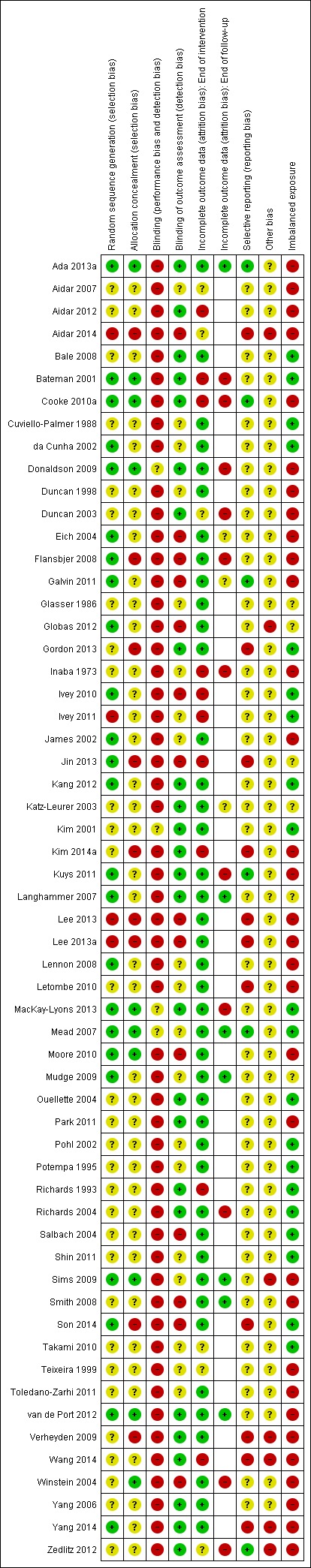
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study. In studies with no follow‐up measurement the risk of bias was not performed for the item labelled 'Incomplete outcome data (attrition bias): end of follow‐up'; this results in some blank spaces.
Figure 3.
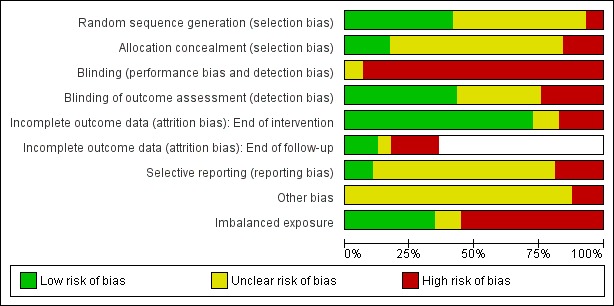
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. In studies with no follow‐up measurement the risk of bias was not performed for the item labelled 'Incomplete outcome data (attrition bias): end of follow‐up'; this results in some blank spaces.
Allocation
Randomisation
We assessed less than half (24/58, 41%) of the included studies as having a low risk of selection bias. All studies did identify that randomisation had occurred but many did not describe the actual mechanism of how this was achieved. Therefore, uncertainties remain among a number of trials. Most trials of fitness training are small; therefore, the use of techniques to balance participant numbers (e.g. block randomisation) and participant characteristics (e.g. stratification or minimisation based on age, gender, or outcomes of interest recorded at baseline) is quite common.
Allocation concealment
Mechanisms of allocation concealment were poorly reported; we considered nine of the included trials low risk of bias (15%). There are instances when centralised assignment mechanisms are used where allocation concealment is automatic (e.g. Mead 2007), in which case the risk of bias is rated as low. In other trials where allocation concealment mechanisms are needed envelopes were frequently used. Numbered, sealed, opaque envelopes (e.g. Cooke 2010a; Donaldson 2009) are appropriate. However, 14/19 (74%) of trials reporting the use of 'sealed envelopes' did not specify whether they were sequentially numbered or opaque therefore we were unable to exclude potential selection bias with certainty.
Blinding
Participant blinding
Participants cannot be blinded to physical interventions like fitness training; therefore, we judged no trials to be at low risk of bias. However, some trials utilised an attention control where the trialists attempted to blind participants to the 'true nature' of the comparison. In three trials, the participants were informed that they would receive one of two different, potentially beneficial interventions (Kim 2001; MacKay‐Lyons 2013; Mead 2007), without being given information on the types of interventions. Similarly, in another trial participants allocated to the experimental group were advised that they were to be offered extra therapy but were not told which type of therapy (Donaldson 2009). In these 4/58 (7%) instances we reported the judgement as 'unclear' risk of bias; the remaining 54/58 trials (93%) trials were all at 'high' risk of bias.
Investigator blinding
We considered the outcome assessment to be at low risk of detection bias in 25/58 (43%) of the included trials. Among trials that used blinded outcome assessment some instructed participants not to reveal group assignments (Bateman 2001; Duncan 2003; Flansbjer 2008; Mead 2007). However, some degree of unmasking can easily occur and was documented in some trials (e.g. Eich 2004; Mudge 2009; Salbach 2004). Outcome assessment was not blinded in six trials (Galvin 2011; Globas 2012; Ivey 2010; Moore 2010; Smith 2008; Winstein 2004).
Incomplete outcome data
Intention‐to‐treat (ITT) analysis
Twenty‐three trials reported the use of an ITT approach for their analyses. One of these trials did not analyse data for the participants who dropped out, therefore we imputed sometimes large numbers of missing values in data obtained from Bateman 2001; this did not influence any of the findings, therefore, only the imputed data are included in this review for simplicity.
Of the 35 trials that did not mention ITT, 21 did not have any missing data.
Incomplete outcome data
Incomplete outcome data arose from participant attrition meaning all outcomes were affected. At the end of intervention 48/58 (83%) included studies reported an attrition rate of 10% or less, 8/58 (14%) reported an attrition rate between 10% and 20% (Aidar 2012; Aidar 2014; da Cunha 2002; Kim 2014a; Langhammer 2007; Richards 1993; Wang 2014; Zedlitz 2012), and 2/58 (3%) trials exceeded an attrition rate of 20% (Ivey 2010 (25%) and Ivey 2011 (51%)).
At the end of follow‐up the attrition rate increased for 12 of the 21 trials (57%) that followed participants after completion of the intervention (Bateman 2001; Cooke 2010a; Donaldson 2009; Duncan 2003; Galvin 2011; Katz‐Leurer 2003; Kuys 2011; MacKay‐Lyons 2013; Mudge 2009; Richards 2004; Winstein 2004; Zedlitz 2012), and ranged from 14% to 40%. Overall, the proportion of withdrawals was similar for the intervention and control groups. The bias assessment could not be applied when no end of follow‐up measurement was included in trial designs. Therefore, some blank spaces occur in Figure 2.
Overall, we judged 10/58 (17%) trials as being at high risk of attrition bias at the end of intervention and 11/21 (52%) trials at the end of follow‐up.
Selective reporting
The majority of studies, particularly the older trials, do not have readily available protocols. In most cases, where these were available, there was no evidence of selective reporting of outcomes relevant to this review.
Other potential sources of bias
Most of the included trials recruited participants during hospital or community stroke care. In a few trials, however, participants' recruitment involved media advertisements (Ouellette 2004; Teixeira 1999), or databases of potential volunteers (Kim 2001; Lennon 2008; Mudge 2009; Sims 2009; Yang 2006). These methods of recruitment render these trials more prone to self selection bias and hamper the generalisability of their findings.
Confounded by additional training time (imbalanced exposure)
Trials in which the participants received an unequal amount of exposure to the intervention and comparison arms of the trial are judged to be at high risk of bias. Technically this could be described as a source of confounding rather than bias but it is appropriate to record it here. The design of more than half of the trials in this review means that in 32/58 trials (55%) the effects of fitness training could be exaggerated because the training intervention groups received greater time of exposure irrespective of the content of the training programme.
Effects of interventions
Effect of training on primary outcome measures
Case fatality
Overall there were few deaths: 13/2797 (0.46%) deaths before end of intervention and 9/1256 (0.72%) deaths between end of intervention and end of follow‐up time points.
Cardiorespiratory training (Comparisons 1 and 2)
End of intervention
Out of the 28 trials of cardiorespiratory training (1437 participants) only Gordon 2013 reported death (n = 2 in each trial arm) as a reason for participant losses (Analysis 1.1). Five of the trials in this analysis did report dropouts but could either not contact participants (Kuys 2011: n = 1) or did not fully describe reasons for dropouts (Aidar 2014; Bateman 2001; Ivey 2011; Jin 2013).
Analysis 1.1.
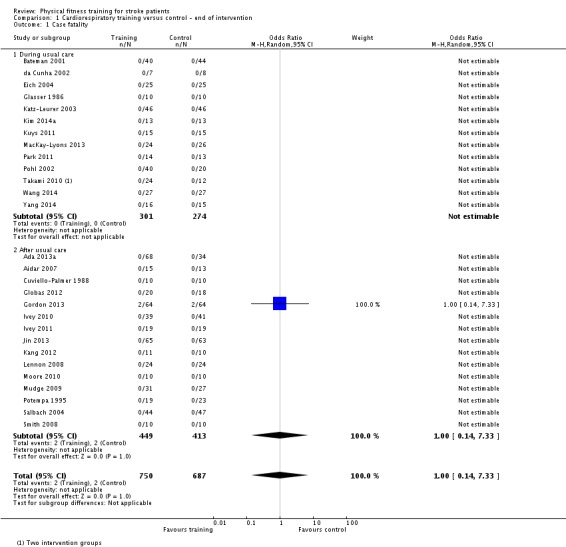
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 1 Case fatality.
End of follow‐up
One out of five trials (304 participants) reported that one participant died in the cardiorespiratory training group (1/46) compared with one participant in the control group (1/46) (Katz‐Leurer 2003) (Analysis 2.1).
Analysis 2.1.
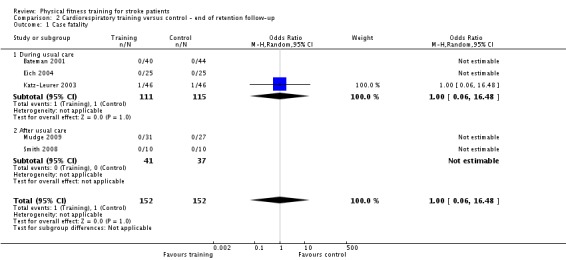
Comparison 2 Cardiorespiratory training versus control ‐ end of retention follow‐up, Outcome 1 Case fatality.
Resistance training (Comparisons 3 and 4)
End of intervention
None of the 13 trials (726 participants) reported deaths (Analysis 3.1), although two trials had undocumented attrition (Aidar 2014; Inaba 1973), including one with a large number of undocumented dropouts (Inaba 1973).
Analysis 3.1.
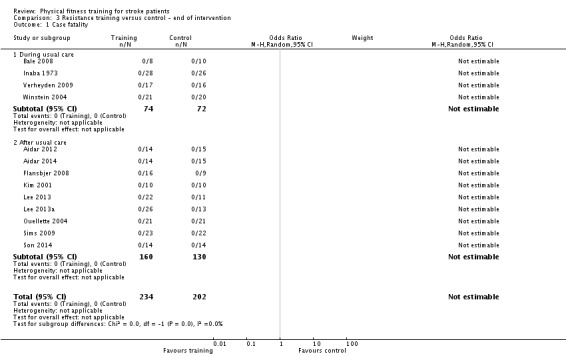
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 1 Case fatality.
End of follow‐up
None of the three trials (138 participants) reported deaths (Analysis 4.1), although one had a large number of undocumented dropouts (Inaba 1973).
Analysis 4.1.
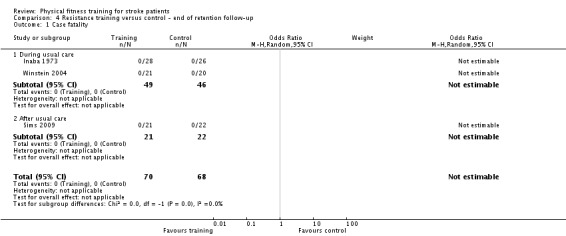
Comparison 4 Resistance training versus control ‐ end of retention follow‐up, Outcome 1 Case fatality.
Mixed training (Comparisons 5 and 6)
End of intervention
Two of the 17 trials (957 participants) reported nine deaths between the baseline and the end of intervention assessments of Langhammer 2007 (6/35 control, 1/32 training) and van de Port 2012 (2/124 control, 0/126 training). Among these two trials reporting deaths the odds of death from all causes whilst participating in mixed training showed a weak tendency favouring training (odds ratio (OR) 0.18, 95% confidence interval (CI) 0.03 to 1.03; P value = 0.05; Analysis 5.1). However, in the Langhammer 2007 trial, three of the six deaths in the control and the one death in the training group occurred before discharge and before the intervention began; after excluding these data, the odds of dying was OR 0.19, 95% CI 0.01 to 4.08 (P value = 0.29). The other 15 trials reported no deaths. However, two trials described undocumented losses: Richards 1993 (two control) and Richards 2004 (five training, seven control) mentioning only that some participants were not available.
Analysis 5.1.
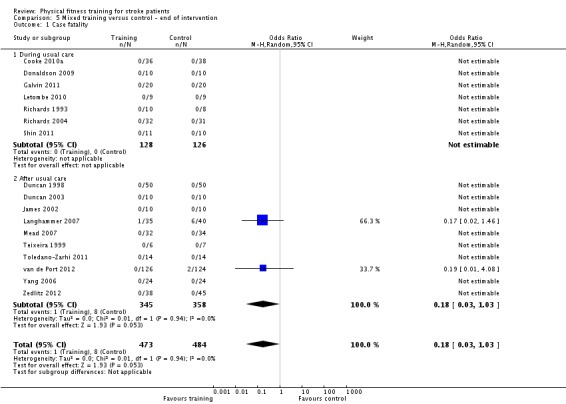
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 1 Case fatality.
End of follow‐up
Four of the 11 trials (762 participants) reported a total of nine deaths (Cooke 2010a; Duncan 2003; Galvin 2011; van de Port 2012). These data are cumulative and include seven new deaths occurring in the follow‐up period along with those deaths occurring before the end of intervention (van de Port 2012: n = 2). Among these four trials reporting deaths the odds of death from all causes at the end of the follow‐up period showed a tendency favouring the mixed training although this only approaches borderline significance (OR 0.27, 95% CI 0.06 to 1.11; P value = 0.07; Analysis 6.1). The other seven mixed trials reported that no losses to follow‐up were attributable to death apart from Richards 1993 (two control), Richards 2004 (five training, seven control), and Zedlitz 2012 (four control), which describe only that some participants were lost or not available for follow‐up.
Analysis 6.1.
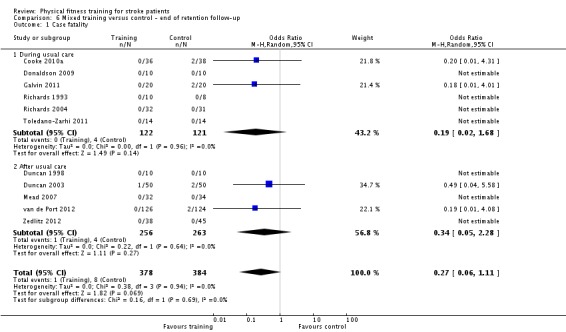
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 1 Case fatality.
Death or dependence
The composite outcome of death or dependence was not reported by any trial.
Disability
Cardiorespiratory training (Comparisons 1 and 2)
End of intervention
The Functional Independence Measure (FIM) was assessed by three trials: one during usual care (Bateman 2001) and two after usual care (Cuviello‐Palmer 1988; Katz‐Leurer 2003). Overall, there was no effect of training (standardised mean difference (SMD) 0.21, 95% CI ‐0.10 to 0.52; P value = 0.18; Analysis 1.2). However, the Bateman 2001 data are problematic because the procedures for obtaining FIM data at the end of intervention were not uniform and there was a high proportion of missing FIM data at the end of intervention (38%); exclusion of this trial does not change the result (SMD 0.17, 95% CI ‐0.29 to 0.63; P value = 0.46).
Analysis 1.2.
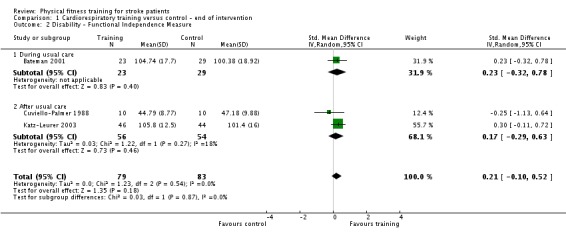
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 2 Disability ‐ Functional Independence Measure.
Barthel Index scores were assessed by three trials: two during usual care (Bateman 2001; Wang 2014) and one after usual care (Gordon 2013), and there was no overall effect with (MD 6.65, 95% CI ‐0.67 to 13.98; Analysis 1.3) or without the problematic data from Bateman 2001. The high heterogeneity within this analysis is likely to stem from the data from Wang 2014 since the participants were non‐ambulatory.
Analysis 1.3.
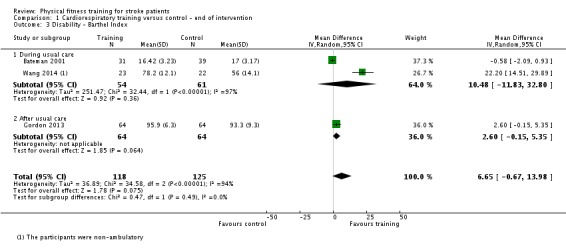
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 3 Disability ‐ Barthel Index.
Rivermead Mobility Index (RMI) scores were assessed by two trials during usual care (Bateman 2001; Takami 2010) and one trial after usual care (Globas 2012). There was a small overall improvement in scores (MD 1.56, 95% CI 0.20 to 2.92; P value = 0.02; Analysis 1.4). When the data from Bateman 2001 were excluded (risk of bias) the effect was strengthened (MD 2.18, 95% CI 0.99 to 3.37; P value = 0.0003).
Analysis 1.4.
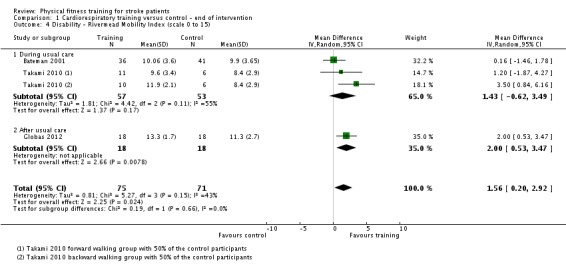
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 4 Disability ‐ Rivermead Mobility Index (scale 0 to 15).
Other individual studies reported scales relating to global assessments of disability. Neither the Physical Activity and Disability scale scores reported by Mudge 2009 (MD 16.90, 95% CI ‐15.15 to 48.95; P value = 0.3; Analysis 1.5) nor the Older Americans Resources and Services Questionnaire reported by Gordon 2013 (MD 0.60, 95% CI ‐0.37 to 1.57; P value = 0.23; Analysis 1.6) showed a significant effect in meta‐analysis.
Analysis 1.5.
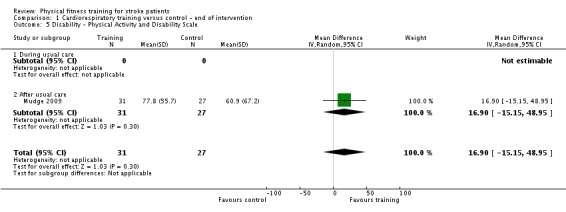
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 5 Disability ‐ Physical Activity and Disability Scale.
Analysis 1.6.
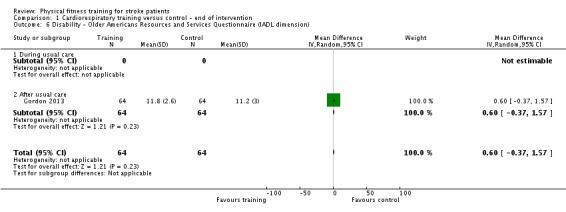
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 6 Disability ‐ Older Americans Resources and Services Questionnaire (IADL dimension).
When we combined all the disability scale data from these individual outcomes (using FIM data from Bateman 2001), there was a significant overall effect in favour of cardiorespiratory training (SMD 0.52, 95% CI 0.19 to 0.84; participants = 462, P value = 0.002; Analysis 1.7). Exclusion of the Bateman 2001 data made a trivial difference.
Analysis 1.7.
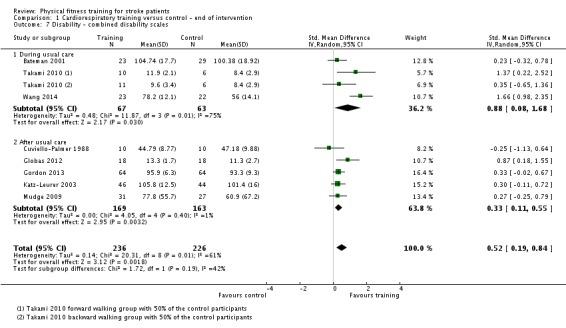
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 7 Disability ‐ combined disability scales.
End of follow‐up
RMI scores were assessed by Bateman 2001; there was no significant training effect at the end of follow‐up (Analysis 2.2).
Analysis 2.2.
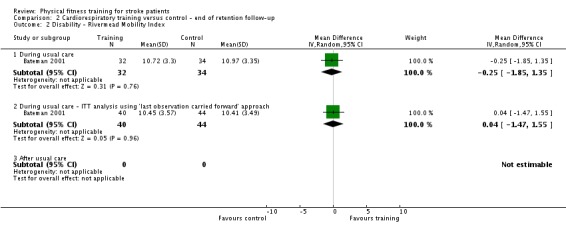
Comparison 2 Cardiorespiratory training versus control ‐ end of retention follow‐up, Outcome 2 Disability ‐ Rivermead Mobility Index.
Nottingham Extended ADL was assessed by Bateman 2001 at the end of follow‐up (Analysis 2.3). Although no significant training effect was evident there was a considerable proportion of missing data (21%) and therefore these results should be treated with caution.
Analysis 2.3.
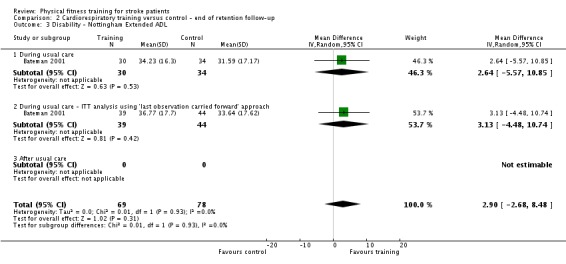
Comparison 2 Cardiorespiratory training versus control ‐ end of retention follow‐up, Outcome 3 Disability ‐ Nottingham Extended ADL.
Physical Activity and Disability scale scores were reported by Mudge 2009. There was no effect at the end of follow‐up (MD 19.90, 95% CI ‐17.58 to 57.38; P value = 0.3; Analysis 2.4).
Analysis 2.4.
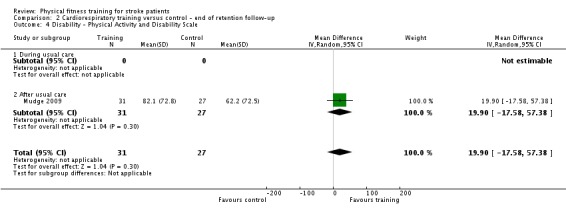
Comparison 2 Cardiorespiratory training versus control ‐ end of retention follow‐up, Outcome 4 Disability ‐ Physical Activity and Disability Scale.
The Frenchay Activities Index (FAI) was reported by Katz‐Leurer 2003. There was no effect at the end of follow‐up (MD 1.00, 95% CI ‐1.55 to 3.55; P value = 0.44; Analysis 2.5).
Analysis 2.5.
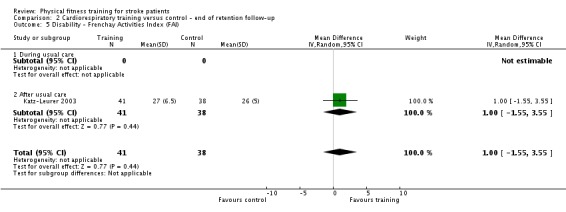
Comparison 2 Cardiorespiratory training versus control ‐ end of retention follow‐up, Outcome 5 Disability ‐ Frenchay Activities Index (FAI).
When we combined all the disability scale data from these individual outcomes (Nottingham Extended ADL data from Bateman 2001), there was no effect of cardiorespiratory training at the end of follow‐up (SMD 0.20, 95% CI ‐0.07 to 0.46; P value = 0.14; Analysis 2.6). When the analysis was repeated using RMI data from Bateman 2001 instead of Nottingham Extended ADL data there was still no effect.
Analysis 2.6.
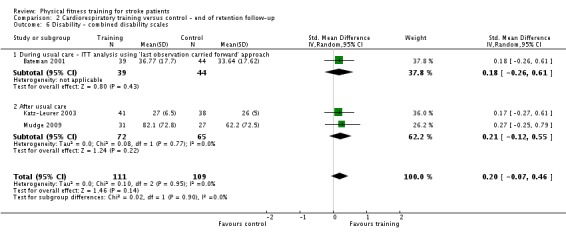
Comparison 2 Cardiorespiratory training versus control ‐ end of retention follow‐up, Outcome 6 Disability ‐ combined disability scales.
Resistance training (Comparisons 3 and 4)
Ouellette 2004 assessed participants' functional abilities and disability outcomes by means of the Late Life Function and Disability Instrument (LLFD). Ouellette 2004 reported the various subscales of this tool and noted that those who received resistance training felt less self perceived limitation. However, no significant effect sizes were apparent for either the disability frequency dimension (Analysis 3.2) or the disability limitation dimension (Analysis 3.3) subscales of this tool.
Analysis 3.2.
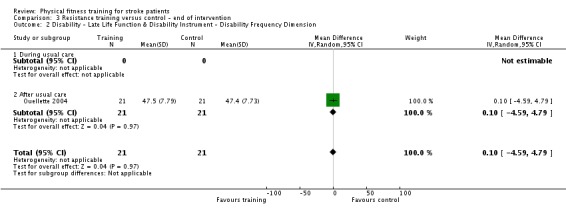
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 2 Disability ‐ Late Life Function & Disability Instrument ‐ Disability Frequency Dimension.
Analysis 3.3.
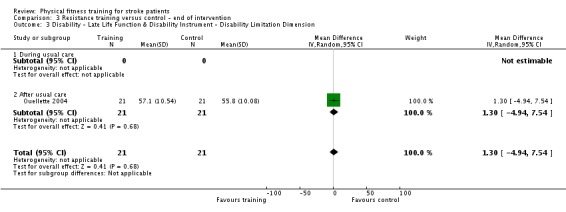
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 3 Disability ‐ Late Life Function & Disability Instrument ‐ Disability Limitation Dimension.
The remaining trials either did not measure disability outcomes or used sub‐scales or specific dimensions of existing functional scales (Inaba 1973; Winstein 2004), which we did not deem suitable for inclusion.
Mixed training (Comparisons 5 and 6)
End of intervention
Six trials assessed the effects of mixed training at the end of the treatment phase or at follow‐up using a variety of scales that measured disability outcomes: Lawton Instrumental Activities of Daily Living (IADL) scores reported by Duncan 1998 and Duncan 2003 at the end of intervention showed no significant effect (MD 0.83, 95% CI ‐0.51 to 2.17; P value = 0.22; Analysis 5.2).
Analysis 5.2.
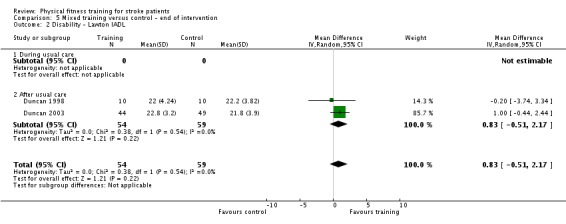
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 2 Disability ‐ Lawton IADL.
The Barthel Index was assessed by five trials during usual care (Galvin 2011; Letombe 2010) and after usual care (Duncan 1998; Duncan 2003; Langhammer 2007) at the end of intervention (MD 2.91, 95% CI ‐1.15 to 6.96; P value = 0.16; Analysis 5.3). Barthel Index scores reached ceiling level in five out of 20 participants at baseline and 10 out of 20 participants at follow‐up (Duncan 1998).
Analysis 5.3.
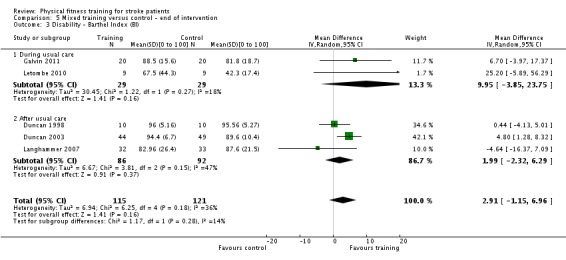
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 3 Disability ‐ Barthel Index (BI).
RMI was assessed by two trials after usual care (Mead 2007; van de Port 2012). These data showed a significant improvement at the end of intervention (MD 0.48, 95% CI 0.05 to 0.91; P value = 0.03; Analysis 5.4).
Analysis 5.4.
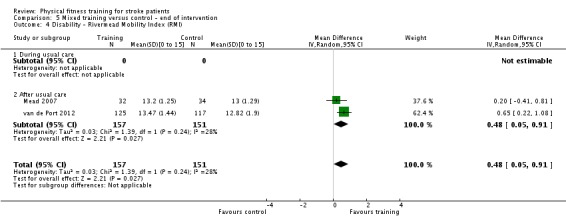
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 4 Disability ‐ Rivermead Mobility Index (RMI).
Nottingham Extended Activities of Daily Living (EADL) was reported by Mead 2007 and showed no significant effects at the end of intervention (MD ‐0.20, 95% CI ‐1.08 to 0.68; P value = 0.66; Analysis 5.5). In addition, van de Port 2012 separately reported four sub‐scales of the Nottingham EADL scale; only one was significantly affected in favour of the usual care rather than mixed training; all other sub‐scales were unaffected.
Analysis 5.5.
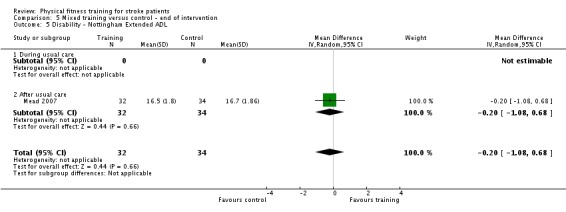
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 5 Disability ‐ Nottingham Extended ADL.
FIM was reported by Mead 2007 and showed no significant effects at the end of intervention (Analysis 5.6).
Analysis 5.6.
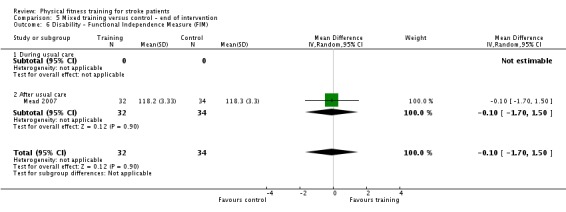
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 6 Disability ‐ Functional Independence Measure (FIM).
The Stroke Impact Scale was reported by one study (Duncan 2003), showing a marginal benefit (Analysis 5.7). In addition, van de Port 2012 separately reported 11 sub‐scales of the Stroke Impact Scale. One sub‐scale was significantly affected in favour of the usual care rather than mixed training; all other sub‐scales were unaffected.
Analysis 5.7.
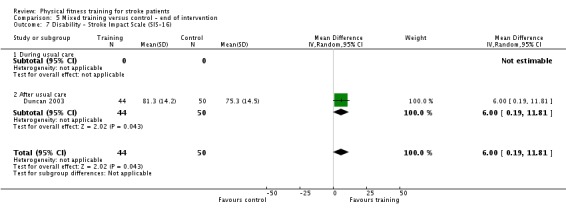
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 7 Disability ‐ Stroke Impact Scale (SIS‐16).
The Katz ADL scale was reported by Letombe 2010 and showed no significant effects at the end of intervention (Analysis 5.8).
Analysis 5.8.
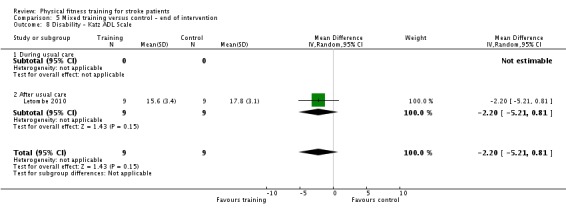
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 8 Disability ‐ Katz ADL Scale.
When we combined disability scale data from the end of intervention, including the Barthel Index (Duncan 1998; Duncan 2003; Galvin 2011; Langhammer 2007; Letombe 2010), FIM (Mead 2007), and RMI (van de Port 2012), there was a small significant effect of mixed training at the end of the intervention (SMD 0.26 0 to 100, 95% CI 0.04 to 0.49; P value = 0.02; Analysis 5.9). There were several potential combinations of data that could be included in this analysis as individual studies report more than one disability scale; therefore, we included Barthel Index and FIM data as these relate more to overall, 'global' disability. We observed moderate inconsistency among trials (Chi² = 7.62, df = 5 (P value = 0.18); I² = 34%) and this may relate to the different specific domains each tool addresses. Another possible explanation could be that five of the seven trials included in these analyses were confounded by increased training time (amount of contact with therapists in the experimental groups was greater than in the control groups) (Duncan 1998; Duncan 2003; Galvin 2011; Letombe 2010; van de Port 2012). The remaining two trials were those with the smallest effect (Langhammer 2007; Mead 2007).
Analysis 5.9.
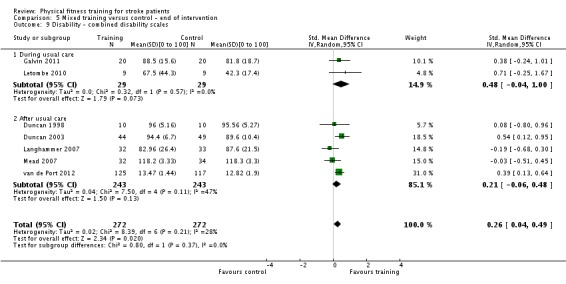
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 9 Disability ‐ combined disability scales.
End of follow‐up
The Barthel Index was assessed by two trials (Galvin 2011; Langhammer 2007); there was no significant effect at the end of follow‐up (MD 1.82, 95% CI ‐13.69 to 17.33; P value = 0.82; Analysis 6.2).
Analysis 6.2.
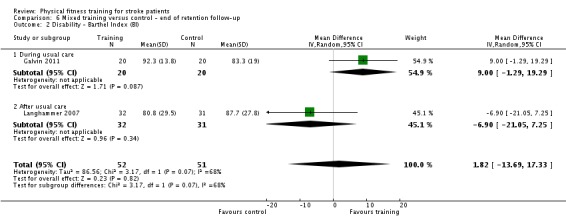
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 2 Disability ‐ Barthel Index (BI).
The FIM was reported by Mead 2007 and showed no significant effect at the end of follow‐up (MD 0.20, 95% CI ‐1.88 to 2.28; P value = 0.85; Analysis 6.3).
Analysis 6.3.
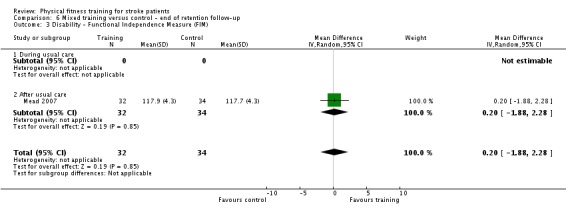
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 3 Disability ‐ Functional Independence Measure (FIM).
Nottingham EADL was reported by Mead 2007 and Galvin 2011 and showed no significant effects at the end of follow‐up (MD 3.10, 95% CI ‐5.20 to 11.40; P value = 0.46; Analysis 6.4).
Analysis 6.4.
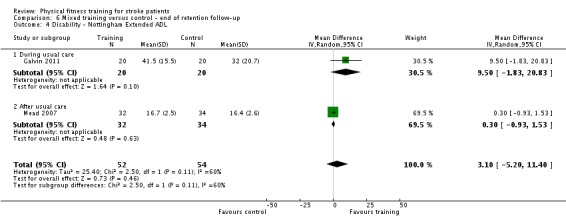
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 4 Disability ‐ Nottingham Extended ADL.
RMI was assessed by Mead 2007 and van de Port 2012; there was a significant benefit at the end of three to four months of follow‐up (MD 0.39, 95% CI 0.04 to 0.73; P value = 0.03; Analysis 6.5). The large trial of van de Port 2012 was confounded by increased training time in the intervention group; when we excluded these data from the analysis the advantage of mixed training disappeared.
Analysis 6.5.
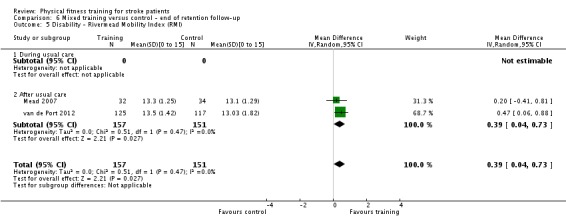
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 5 Disability ‐ Rivermead Mobility Index (RMI).
When we combined disability scale data from the end of follow‐up, including Barthel Index (Galvin 2011; Langhammer 2007), FIM (Mead 2007), and RMI (van de Port 2012), there was no effect (SMD 0.16, 95% CI ‐0.12 to 0.44; P value = 0.26; Analysis 6.6).
Analysis 6.6.
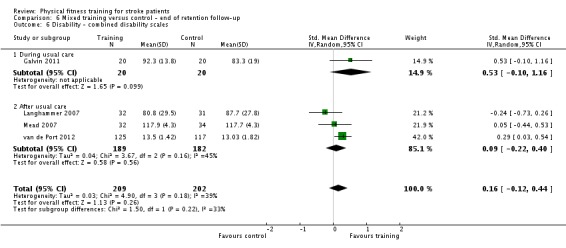
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 6 Disability ‐ combined disability scales.
It is worth noting that two trials included in these analyses were confounded by increased training time (Galvin 2011; van de Port 2012).
Comparison of cardiorespiratory training, resistance training, and mixed training (Comparison 7)
We performed a subgroup analysis to directly compare the effects of the different types of training (cardiorespiratory training versus mixed training versus resistance training) on pooled disability outcomes at the end of the intervention (Analysis 7.1). Cardiorespiratory and mixed training show similar beneficial effects with no statistically significant difference between these subgroups. The pooled effect of cardiorespiratory training and mixed training shows a significant overall effect (SMD 0.38, 95% CI 0.19 to 0.58; P value = 0.0001; participants = 1006; studies = 16).
Analysis 7.1.
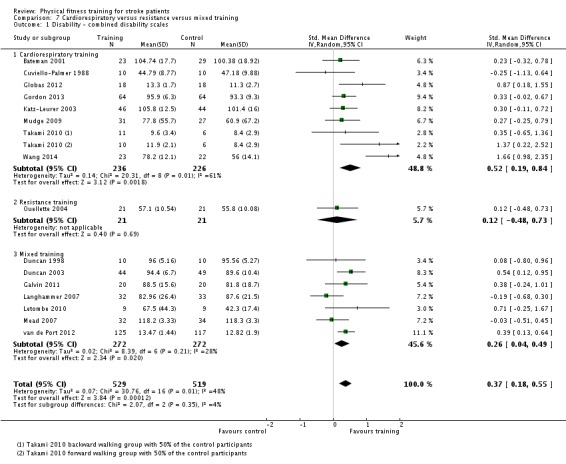
Comparison 7 Cardiorespiratory versus resistance versus mixed training, Outcome 1 Disability ‐ combined disability scales.
Effect of training on secondary outcomes
Adverse events
Mead 2007 reported 11 falls in eight of the 32 participants allocated mixed training and five falls in four of the 34 participants in the control group (P value = 0.21, non‐significant). None of these falls occurred within training sessions.
van de Port 2012 reported 29 falls in 126 participants allocated mixed training and 26 falls in those allocated usual care (P value = 0.93, non‐significant); one fall occurred during exercise training.
Adverse events were not typically sought (a priori) as an outcome measure but were instead reported in a more ad hoc fashion.
Ten of the included trials provided some comments on participant tolerance of the training programme and did not report any adverse events such as falls, fractures, or injuries arising during the intervention.
Considering all included trials, 10 participants (seven participants receiving the training intervention and three control participants) were reported to have suffered a cerebrovascular event between baseline and the end of the training intervention.
In the 17 trials that included a follow‐up assessment, 10 participants (four participants receiving the training intervention and six control participants) were reported to have suffered a stroke or cerebrovascular event between the end of intervention and the end of follow‐up.
Three participants (one participant receiving the training intervention and two control participants) were also reported to have suffered a cardiovascular event between baseline and the end of the training intervention.
Vascular risk factors
Few data regarding modification of risk factors for cardiovascular and cerebrovascular events were available in the included trials.
Blood pressure
Five trials of cardiorespiratory training, with a total of 318 participants, showed no significant training effects on systolic (MD ‐0.20, 95% CI ‐6.00 to 5.60; P value = 0.95; Analysis 1.8) or diastolic blood pressure (MD ‐0.15, 95% CI ‐2.28 to 1.98; P value = 0.89); P value = 0.81; Analysis 1.9) at the end of intervention (da Cunha 2002; Jin 2013; Katz‐Leurer 2003; Lennon 2008; Potempa 1995). One trial stated that there was an effect of cardiorespiratory training on blood pressure but did not provide data (Ivey 2011). Only one small trial of mixed training examined blood pressure (Toledano‐Zarhi 2011); it showed no effects (Analysis 5.10; Analysis 5.11). No resistance training trials reported blood pressure outcomes.
Analysis 1.8.
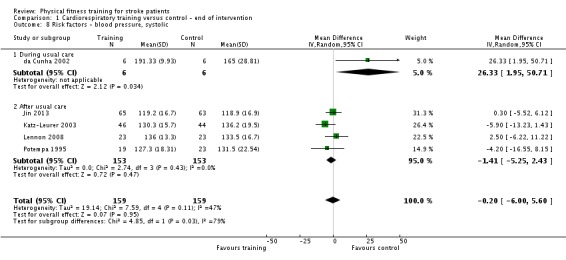
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 8 Risk factors ‐ blood pressure, systolic.
Analysis 1.9.
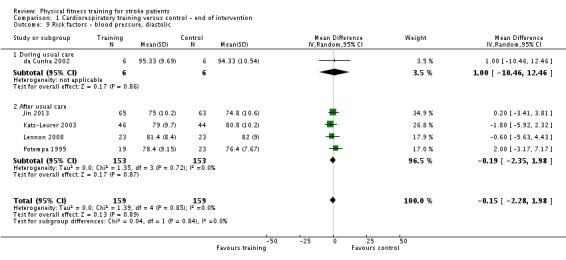
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 9 Risk factors ‐ blood pressure, diastolic.
Analysis 5.10.
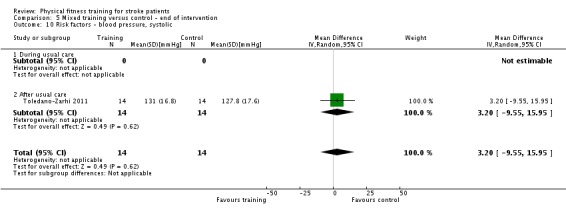
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 10 Risk factors ‐ blood pressure, systolic.
Analysis 5.11.
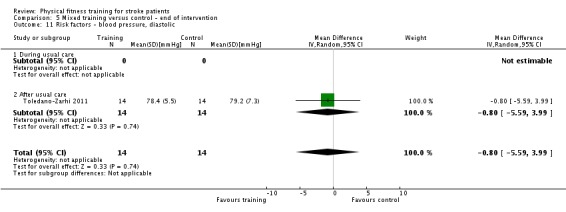
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 11 Risk factors ‐ blood pressure, diastolic.
Body mass index (BMI) data were reported by two studies at the end of cardiorespiratory training interventions (MD 1.19, 95% CI ‐0.38 to 2.76; Analysis 1.10) but there was no evidence of an effect. Waist girth measures and total cholesterol were reported by Lennon 2008 but were not affected by cardiorespiratory training.
Analysis 1.10.
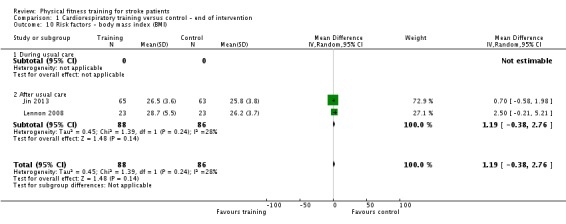
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 10 Risk factors ‐ body mass index (BMI).
In a small trial of 45 participants (Wang 2014), abnormal glucose tolerance and total triglycerides improved after cardiorespiratory training (abnormal glucose tolerance OR 0.08, 95% CI 0.02 to 0.33; P value = 0.0005; Analysis 1.11; total triglycerides MD ‐0.39 mmol/L, 95% CI ‐0.48 to ‐0.30; P value = 0.00001; Analysis 1.12).
Analysis 1.11.
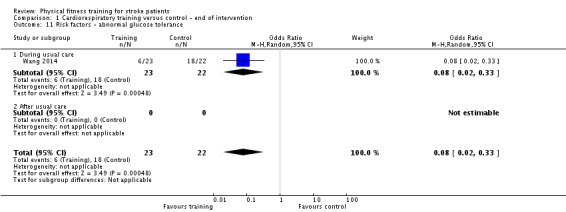
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 11 Risk factors ‐ abnormal glucose tolerance.
Analysis 1.12.
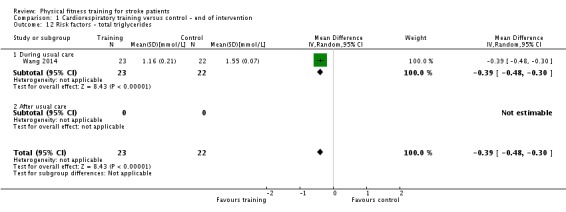
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 12 Risk factors ‐ total triglycerides.
Physical fitness
Cardiorespiratory training (Comparisons 1 and 2)
Cardiorespiratory fitness was assessed in nine trials (425 participants) using measures of peak VO2 (ml/kg/minute) at the end of the intervention. Most of the studies took place after usual care and there was a consistent pattern of improvement in measures of peak VO2 showing that cardiorespiratory fitness increased significantly in the training groups (MD 2.86 ml/kg/minute, 95% CI 1.76 to 3.96; P value = 0.00001; Analysis 1.13). Doses of training varied between four weeks and six months among the trials. Heterogeneity arose from a single study with a small effect (Lennon 2008); exclusion of these data resulted in no heterogeneity and a stronger effect (MD 3.48 ml/kg/minute, 95% CI 3.05 to 3.92; P value = 0.00001).
Analysis 1.13.
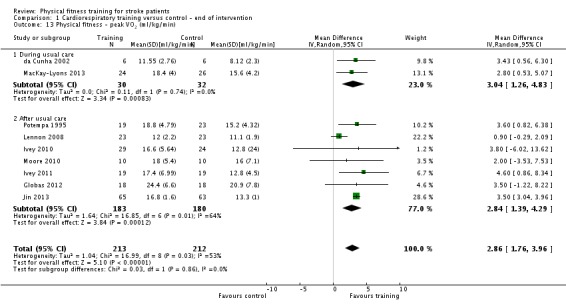
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 13 Physical fitness ‐ peak VO2 (ml/kg/min).
A single study suggests that training‐induced benefit to peak VO2 remained after a 12‐month follow‐up (MD 2.90 ml/kg/minute, 95% CI 0.56 to 5.24; P value = 0.02) (MacKay‐Lyons 2013). This study is small (n = 50) but at low risk of bias.
VO2 cost assessed during the 12‐minute walking test in Moore 2010 did not show any significant training effect at the end of intervention (Analysis 1.14).
Analysis 1.14.
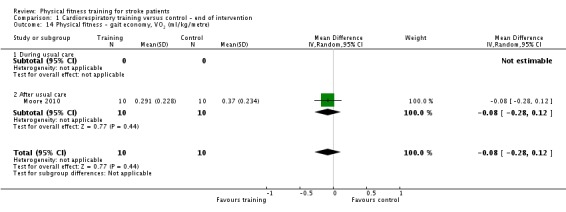
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 14 Physical fitness ‐ gait economy, VO2 (ml/kg/metre).
Similarly, in four trials that measured maximal cycling work rate at the end of intervention during (Bateman 2001; da Cunha 2002) and after (Katz‐Leurer 2003; Potempa 1995) usual care, cardiorespiratory fitness improved significantly in participants who received the training intervention (SMD 0.60, 95% CI 0.18 to 1.02; P value = 0.005; Analysis 1.15). The large number of dropouts in Bateman 2001 means these data are at risk of bias. When we excluded this study all statistical heterogeneity disappeared and the overall effect was strengthened (SMD 0.84, 95% CI 0.49 to 1.18; P value < 0.00001).
Analysis 1.15.
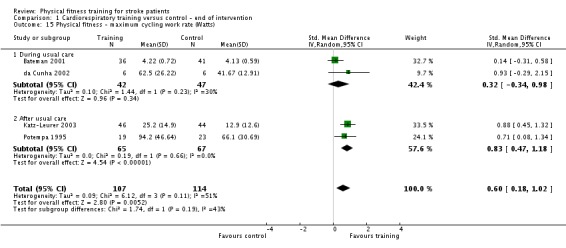
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 15 Physical fitness ‐ maximum cycling work rate (Watts).
Results from Bateman 2001 showed that the improvement measured by maximal cycling work rate was not maintained at follow‐up (MD 5.11, 95% CI ‐18.93 to 29.15; Analysis 2.7).
Analysis 2.7.
Comparison 2 Cardiorespiratory training versus control ‐ end of retention follow‐up, Outcome 7 Physical fitness ‐ maximum cycling work rate (Watts).
Resistance training (Comparisons 3 and 4)
Two trials with a total of 30 participants assessed the effects of resistance training on a composite measure of muscle strength at the end of intervention, during and after usual care (Kim 2001; Winstein 2004). Kim 2001 used a composite measure (that is the sum of the percentage change in six muscle groups) to assess the strength of the lower limbs, while Winstein 2004 used a composite measure (that is the sum of the torque of the extensors and flexors of the wrist, elbow, and shoulder) to assess the strength of the upper limbs. The pooled estimate of effect was only marginally in favour of the resistance training group (SMD 0.58, 95% CI 0.06 to 1.10; P value = 0.03; Analysis 3.4). However, Winstein 2004 was biased by the lack of blinding and the use of a dynamometer that was hand‐held by the investigator, and confounded by increased training time in the intervention group.
Analysis 3.4.
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 4 Physical fitness ‐ composite measure of muscle strength.
Two trials with a total of 42 participants assessed the effects of training on knee muscle strength measured with a dynamometer at the end of intervention during (Bale 2008) and after (Flansbjer 2008) usual care but did not detect any significant training effect on either knee extension (Analysis 3.5) or knee flexion (Analysis 3.6). Follow‐up data were available for only one of these two trials (Flansbjer 2008) and did not show any significant training effect over time (Analysis 4.2; Analysis 4.3).
Analysis 3.5.
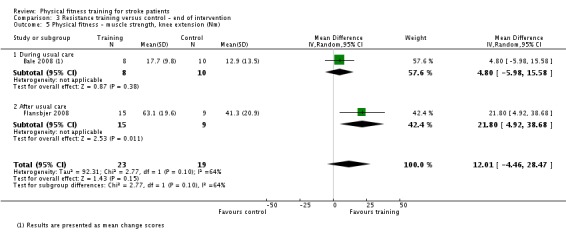
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 5 Physical fitness ‐ muscle strength, knee extension (Nm).
Analysis 3.6.
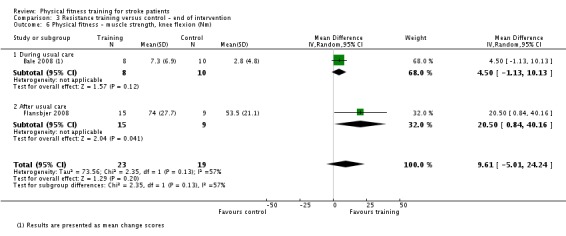
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 6 Physical fitness ‐ muscle strength, knee flexion (Nm).
Analysis 4.2.
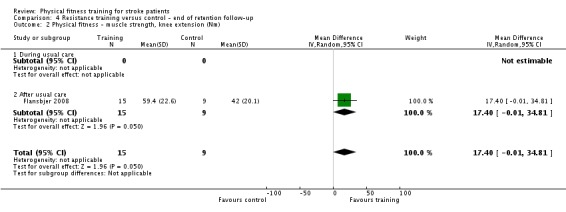
Comparison 4 Resistance training versus control ‐ end of retention follow‐up, Outcome 2 Physical fitness ‐ muscle strength, knee extension (Nm).
Analysis 4.3.
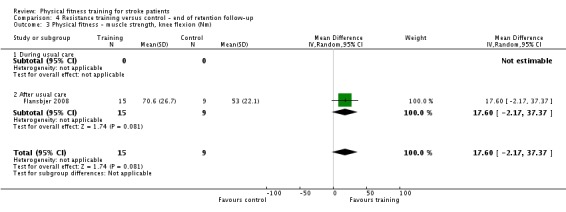
Comparison 4 Resistance training versus control ‐ end of retention follow‐up, Outcome 3 Physical fitness ‐ muscle strength, knee flexion (Nm).
Ouellette 2004 examined strength bilaterally in the lower limb extensors and unilaterally in the knee extensors and the ankle flexors (plantar and dorsi). All strength measures were reported to improve significantly after resistance training compared with the control group except for ankle dorsiflexion on the unaffected side. This study also suggested that peak power was improved during unilateral knee extensions but not during bilateral extension of the whole lower limb. However, as strength and power data were presented as graphs, we were not able to extrapolate them satisfactorily for further analyses.
Inaba 1973 reported that participants allocated to resistance training of the lower limbs achieved significantly greater gains in the 10‐repetition maximum exercise compared with controls (12.18 versus 8.58 kg, P value < 0.02) after one month of intervention. No significant differences were observed between groups after two months of training. No measures of variance were reported by this trial and therefore we were not able to include these data in our analyses.
Aidar 2012 and Aidar 2014 reported significant gains in maximal strength (1‐repetition maximum) in a range of upper and lower body muscle groups after resistance training compared with the control group.
Overall, meta‐analysis of muscle strength data is awkward because so many different muscles groups can be assessed using a range of different equipment and muscle contraction types.
Mixed training (Comparisons 5 and 6)
Effect sizes calculated for two individual trials show a small significant improvement in VO2 peak (Duncan 2003) and in gait economy (Mead 2007: net VO2 mL/kg per metre) at the end of intervention in participants who received mixed training (Analysis 5.12; Analysis 5.13). The benefit in gait economy, however, disappeared after a three‐month follow‐up (Analysis 6.7). Letombe 2010 also reported changes in VO2 peak (+ 30%) and peak power output (+ 20%) but incomplete reporting prevented incorporation in meta‐analysis.
Analysis 5.12.
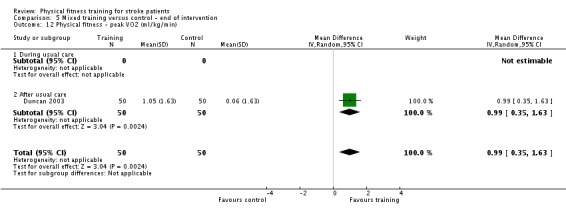
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 12 Physical fitness ‐ peak VO2 (ml/kg/min).
Analysis 5.13.
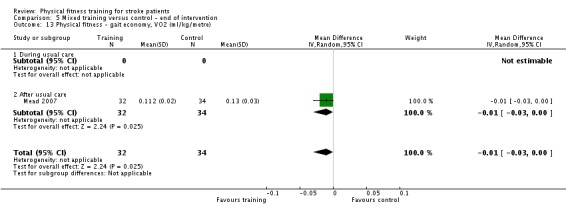
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 13 Physical fitness ‐ gait economy, VO2 (ml/kg/metre).
Analysis 6.7.
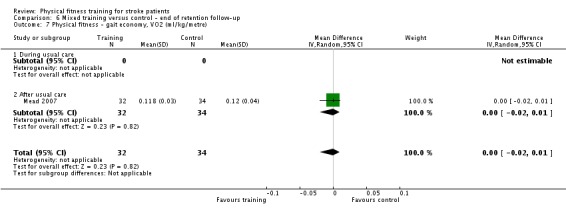
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 7 Physical fitness ‐ gait economy, VO2 (ml/kg/metre).
Toledano‐Zarhi 2011 reported no effect of mixed training on walking performance (time or METS) during a Modified Bruce treadmill protocol.
Two trials with a total of 148 participants (Duncan 2003; Yang 2006) did not show any significant improvement in ankle dorsiflexion strength after mixed training (Analysis 5.14) but there was considerable heterogeneity between their results (Chi2 17.67, df = 1) and both trials were confounded by increased training time. Yang 2006 also reported a range of lower limb strength improvements, but all measurements were potentially biased as they were obtained by means of a hand‐held dynamometer, which is not a reliable, objective method of measurement.
Analysis 5.14.
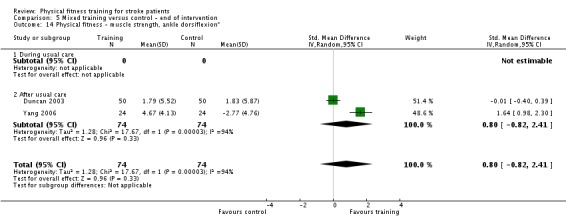
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 14 Physical fitness ‐ muscle strength, ankle dorsiflexion*.
The same two trials also assessed the effect of mixed training on knee extension strength. Data for knee extension strength were also available from the Cooke 2010a trial. The pooled SMD indicated a small effect size in favour of the mixed training group at the end of intervention (SMD 0.33, 95% CI 0.05 to 0.61; P value = 0.02; Analysis 5.15). Cooke 2010a showed that this training effect was not retained at the end of the scheduled follow‐up (Analysis 6.9). Cooke 2010a also assessed knee flexion strength but no significant training effect was observed either at the end of intervention or at follow‐up (Analysis 5.16; Analysis 6.8).
Analysis 5.15.
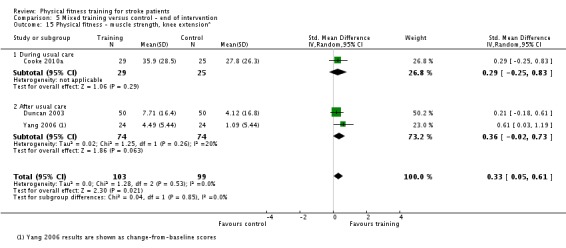
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 15 Physical fitness ‐ muscle strength, knee extension*.
Analysis 6.9.
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 9 Physical fitness ‐ muscle strength, knee extension.
Analysis 5.16.
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 16 Physical fitness ‐ muscle strength, knee flexion.
Analysis 6.8.
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 8 Physical fitness ‐ muscle strength, knee flexion.
Donaldson 2009 assessed the effect of mixed training on elbow extension, elbow flexion, and grip force at the end of intervention but did not detect any significant training effect (Analysis 5.17; Analysis 5.18; Analysis 5.19).
Analysis 5.17.
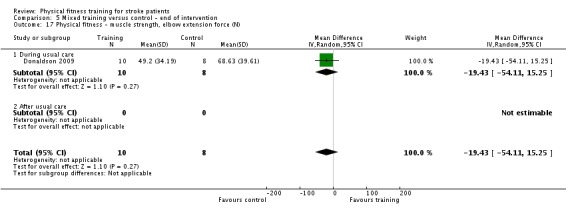
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 17 Physical fitness ‐ muscle strength, elbow extension force (N).
Analysis 5.18.
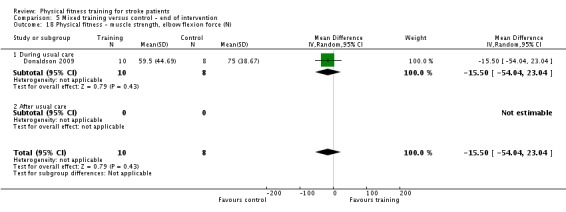
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 18 Physical fitness ‐ muscle strength, elbow flexion force (N).
Analysis 5.19.
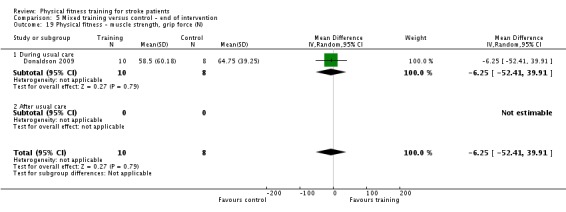
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 19 Physical fitness ‐ muscle strength, grip force (N).
Mead 2007 assessed the extensor power of the lower affected limb at the end of the training period and at follow‐up but found no differences between mixed training and a 'non‐exercise' control intervention (Analysis 5.21; Analysis 6.10).
Analysis 5.21.
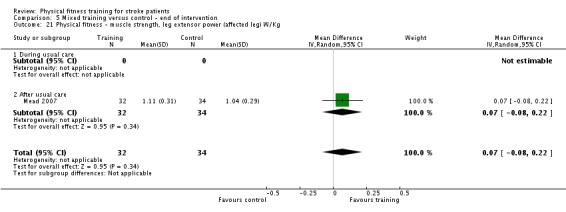
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 21 Physical fitness ‐ muscle strength, leg extensor power (affected leg) W/Kg.
Analysis 6.10.
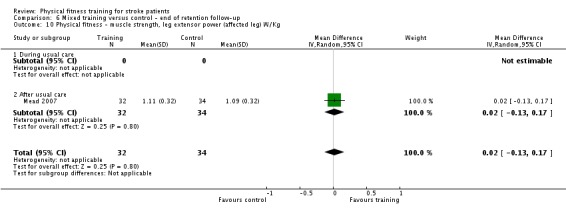
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 10 Physical fitness ‐ muscle strength, leg extensor power (affected leg) W/Kg.
The pooled results of two trials assessing grip strength of the paretic hand did not show any significant improvement after mixed training at the end of the intervention phase (SMD ‐0.05, 95% CI ‐0.36 to 0.26; P value = 0.75; Analysis 5.20) (Duncan 2003; Langhammer 2007). Langhammer 2007 also provided follow‐up data for grip strength, which failed to demonstrate any training effect over time (Analysis 6.11).
Analysis 5.20.
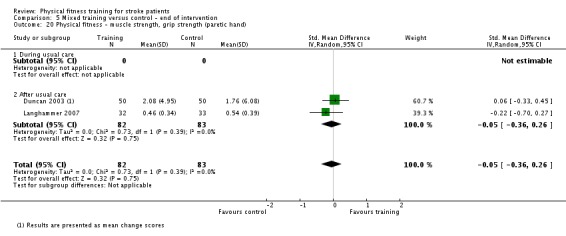
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 20 Physical fitness ‐ muscle strength, grip strength (paretic hand).
Analysis 6.11.
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 11 Physical fitness ‐ grip strength (paretic hand).
Mobility
Cardiorespiratory training (Comparisons 1 and 2)
Functional Ambulation Category
Two trials, which included three relevant comparisons and 73 participants, measured the effect of treadmill gait training using the Functional Ambulation Category (FAC) scale (da Cunha 2002; Pohl 2002). The pooled MD showed that the FAC score measured at the end of intervention was significantly better in stroke survivors who received cardiorespiratory training during usual care (MD 0.53, 95% CI 0.21 to 0.85; P value = 0.001; Analysis 1.16).
Analysis 1.16.
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 16 Mobility ‐ functional ambulation categories.
Maximum walking speed (MWS)
Fourteen trials with a total of 631 participants measured maximum walking speed (metres per minute) at the end of intervention. The mode of cardiorespiratory training in all these trials was walking‐specific apart from two trials that used cycle ergometry (Bateman 2001) and circuit type‐training (Mudge 2009), respectively. The pooled MD was significantly in favour of the training group (MD 6.71 m/minute, 95% CI 2.73 to 10.69; P value = 0.0009; Analysis 1.17). This analysis also shows a consistent effect across the studies as a whole and a similar magnitude of effect arising from training delivered during or after usual care. The Bateman 2001 data were not walking‐specific and were problematic due to high dropout rates; when we excluded the data heterogeneity was reduced and the confidence in the treatment effect strengthened. If we also excluded the longer trials (longer than 12 weeks; Ada 2013a; Globas 2012) there was little change.
Analysis 1.17.
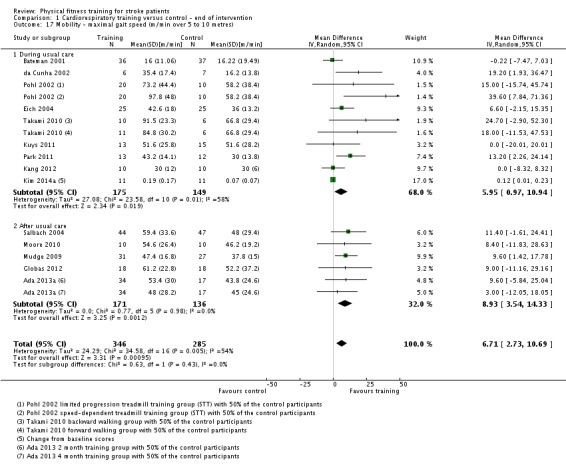
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 17 Mobility ‐ maximal gait speed (m/min over 5 to 10 metres).
A funnel plot of the 14 studies (including 17 relevant comparisons) that measured maximum walking speed showed a tendency toward asymmetry, suggesting potential publication bias during but not after usual care (Figure 4).
Figure 4.
Funnel plot of comparison: 1 Cardiorespiratory training versus control ‐ end of intervention, outcome: 1.17 Mobility ‐ maximal gait speed (m/min over 5 to 10 metres) [m/min].
Five trials (312 participants) also provided follow‐up data on maximum walking speed and a significant training effect was observed at the end of follow‐up (MD 6.71 m/minute, 95% CI 2.40 to 11.02; P value = 0.002; Analysis 2.9). Although the overall effect is consistent the two comparisons of Ada 2013a show the smallest effect. Ada 2013a used a 12‐month follow‐up whilst all the others used a three‐month follow‐up period. If we excluded the data heterogeneity was reduced and the confidence in the treatment effect strengthened.
Analysis 2.9.
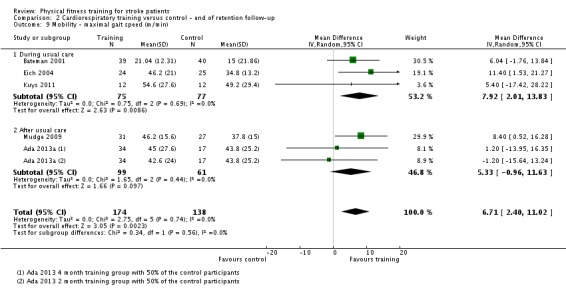
Comparison 2 Cardiorespiratory training versus control ‐ end of retention follow‐up, Outcome 9 Mobility ‐ maximal gait speed (m/min).
Preferred walking speed (PWS)
Ten trials measured the preferred gait speed (metres per minute) in a total of 505 stroke survivors at the end of the training period during and after usual care. The mode of cardiorespiratory training in all these trials was walking‐specific apart from two trials (Katz‐Leurer 2003; Yang 2014), which used cycle ergometry. The pooled MD indicated a significant training effect (MD 4.28 m/minute, 95% CI 1.71 to 6.84; P value = 0.001; Analysis 1.18). The majority of the interventions contributing to this effect took place after usual care. There is a consistent effect even though dose of training varies.
Analysis 1.18.
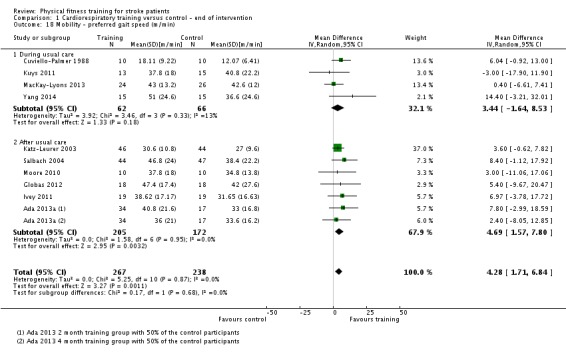
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 18 Mobility ‐ preferred gait speed (m/min).
Three trials provided follow‐up data three months (Kuys 2011) and 12 months (Ada 2013a; MacKay‐Lyons 2013) after the intervention. Pooling these data shows no evidence of retention (Analysis 2.10).
Analysis 2.10.
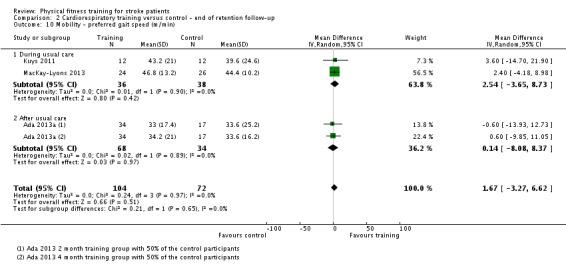
Comparison 2 Cardiorespiratory training versus control ‐ end of retention follow‐up, Outcome 10 Mobility ‐ preferred gait speed (m/min).
Six‐Minute Walking Test (6‐MWT)
Fifteen trials assessed walking endurance using the six‐minute walking test (total metres walked in six minutes: 6‐MWT) in a total of 826 stroke survivors. Cardiorespiratory training significantly increased the walking capacity at the end of intervention (MD 30.29, 95% CI 16.19 to 44.39; P value = 0.0001; Analysis 1.19). A consistent effect is demonstrated at all stages of care.
Analysis 1.19.
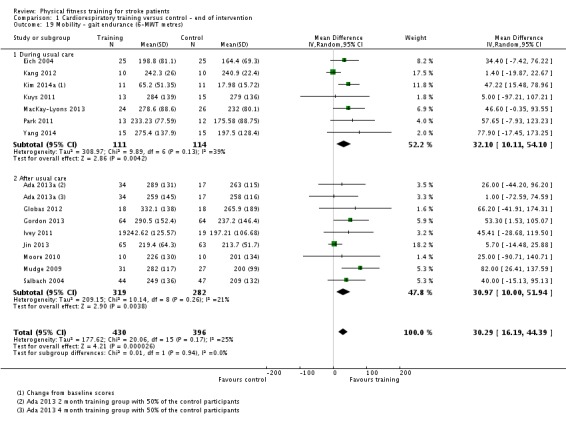
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 19 Mobility ‐ gait endurance (6‐MWT metres).
A funnel plot of the 15 studies (including 16 relevant comparisons) that measured 6‐MWT showed no evidence of asymmetry, suggesting no publication bias (Figure 5).
Figure 5.
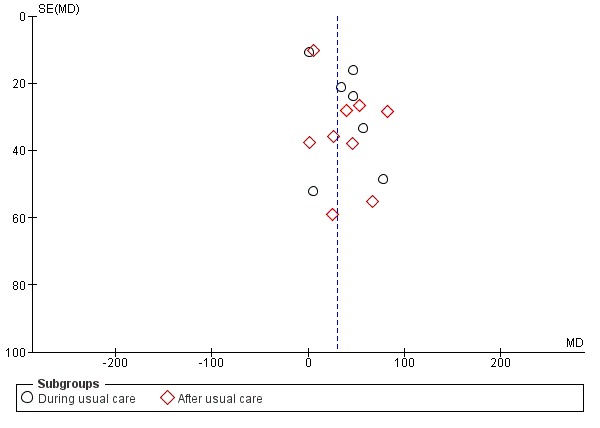
Funnel plot of comparison: 1 Cardiorespiratory training versus control ‐ end of intervention, outcome: 1.19 Mobility ‐ gait endurance (6‐MWT metres).
Five trials provided follow‐up data three months (Eich 2004; Kuys 2011; Mudge 2009) and 12 months (Ada 2013a; MacKay‐Lyons 2013) after the intervention. When pooled these data show some evidence of retention (MD 38.29 metres, 95% CI 7.19 to 69.39; P value = 0.02) (Analysis 2.11). Although overall heterogeneity is low, the effects are variable and not obviously associated to either the shorter or longer follow‐up periods.
Analysis 2.11.
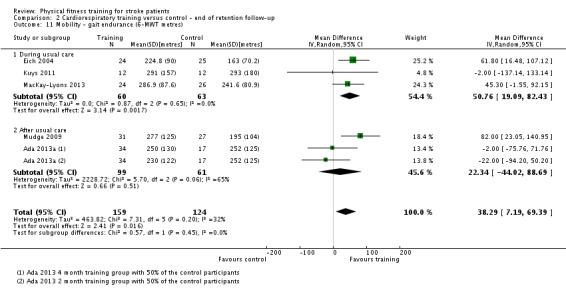
Comparison 2 Cardiorespiratory training versus control ‐ end of retention follow‐up, Outcome 11 Mobility ‐ gait endurance (6‐MWT metres).
Other mobility outcomes
Similar to the 6‐MWT data, three trials measured walking endurance (reported as metres per minute) in 154 stroke survivors at the end of intervention, during (da Cunha 2002; Eich 2004) and after (Salbach 2004) usual care. Walking capacity increased significantly in participants who received cardiorespiratory training (MD 8.87 metres/minute, 95% CI 1.35 to 16.40; P value = 0.02; Analysis 1.20).
Analysis 1.20.
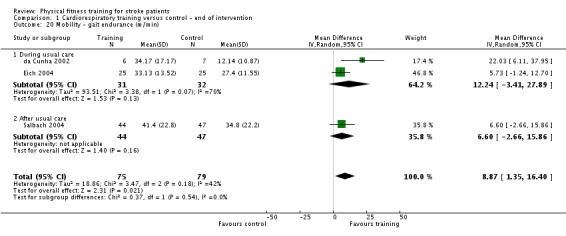
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 20 Mobility ‐ gait endurance (m/min).
Park 2011 and Kim 2014a reported time taken for the community walk test. There was a small difference between participants who received community ambulation training and controls at the end of intervention (MD ‐10.54 minutes, 95% CI ‐14.11 to ‐6.98; P value = 0.00001; Analysis 1.21).
Analysis 1.21.
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 21 Mobility ‐ community walk test (min).
Glasser 1986 measured the time taken by stroke participants to walk a six metre distance and did not find any significant difference between participants who received Kinetron walking training and controls (Analysis 1.22).
Analysis 1.22.
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 22 Mobility ‐ 6 metre walking time (sec).
Smith 2008 assessed the effect of cardiorespiratory training using the mobility domain of the Stroke Impact Scale (SIS). SIS scores were similar between intervention groups at the end of the intervention and at follow‐up (Analysis 1.23; Analysis 2.14).
Analysis 1.23.
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 23 Mobility ‐ Stroke Impact Scale (mobility domain).
Analysis 2.14.
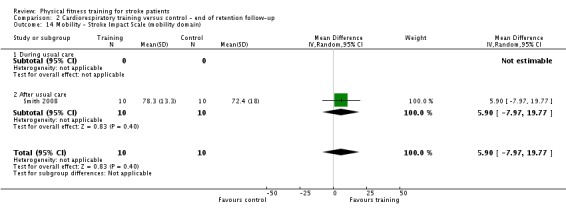
Comparison 2 Cardiorespiratory training versus control ‐ end of retention follow‐up, Outcome 14 Mobility ‐ Stroke Impact Scale (mobility domain).
It is worth noting that six trials, which assessed walking outcomes, were confounded by additional training time in the intervention groups (Ada 2013a; Katz‐Leurer 2003; Kuys 2011; Moore 2010; Park 2011; Smith 2008).
Resistance training (Comparisons 3 and 4)
Maximal walking speed (MWS)
Four trials with a total of 104 participants measured maximal walking speed (metres per minute) during (Bale 2008) and after (Flansbjer 2008; Kim 2001; Ouellette 2004) usual care. Overall, resistance training did not increase the walking velocity at the end of intervention (MD 1.92 m/minute, 95% CI ‐3.50 to 7.35; Analysis 3.7). There was, however, definite heterogeneity between trial results (Chi2 = 7.76, df = 3, P value = 0.05). The heterogeneity was mainly due to the results of one trial (Bale 2008), which involved specific walking‐related exercises and, in contrast to the results of the other three trials, showed a significant training effect during usual care (MD 8.40 m/minute, 95% CI 2.82 to 13.98). Follow‐up data were available from one trial only (Flansbjer 2008) and did not show any significant training effect (Analysis 4.4).
Analysis 3.7.
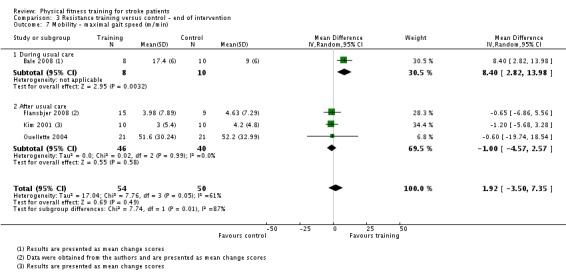
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 7 Mobility ‐ maximal gait speed (m/min).
Analysis 4.4.
Comparison 4 Resistance training versus control ‐ end of retention follow‐up, Outcome 4 Mobility ‐ maximal gait speed (m/min).
Preferred walking speed (PWS)
Three trials with a total of 80 participants also measured preferred gait speed (metres per minute) during (Bale 2008) and after (Kim 2001; Ouellette 2004) usual care, but failed to demonstrate any effect of resistance training on walking speed at the end of intervention (MD 2.34 m/min, 95% CI ‐6.77 to 11.45; Analysis 3.8). Heterogeneity between results (Chi2 = 9.18, df = 2, P value = 0.01) was again attributable to the results of the Bale 2008 trial.
Analysis 3.8.
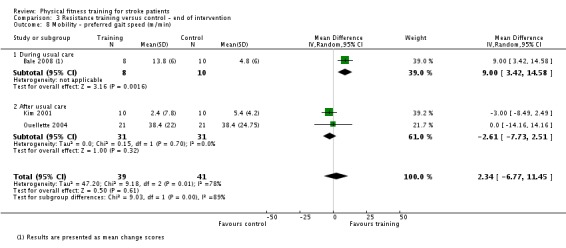
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 8 Mobility ‐ preferred gait speed (m/min).
Six‐Minute Walking Test (6‐MWT)
Two trials assessed the walking capacity (metres walked in six minutes) in a total of 66 stroke survivors (Flansbjer 2008; Ouellette 2004). Resistance training did not have any significant effect on walking capacity at the end of intervention (MD 3.78, 95% CI ‐68.56 to 76.11; level of heterogeneity Chi2 = 0.00, df = 1, P value = 0.99; Analysis 3.9). Flansbjer 2008 provided follow‐up data that confirmed the lack of training effect on walking capacity at the end of follow‐up (Analysis 4.5).
Analysis 3.9.
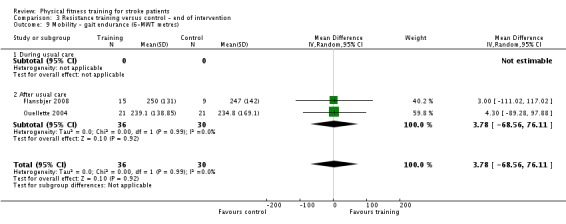
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 9 Mobility ‐ gait endurance (6‐MWT metres).
Analysis 4.5.
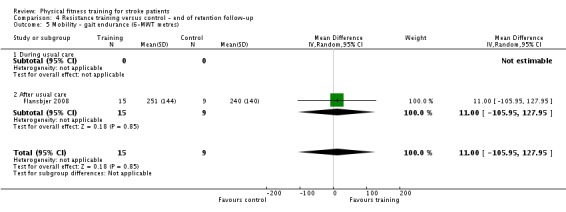
Comparison 4 Resistance training versus control ‐ end of retention follow‐up, Outcome 5 Mobility ‐ gait endurance (6‐MWT metres).
Mixed training (Comparisons 5 and 6)
Functional ambulation categories
One trial examined the effects of mixed training on Functional Ambulation Category scores (van de Port 2012); it showed no effect at the end of intervention (Analysis 5.22) and borderline beneficial effect after a follow‐up of three months (MD 0.11, 95% CI 0.00 to 0.22; P value = 0.05; Analysis 6.12).
Analysis 5.22.
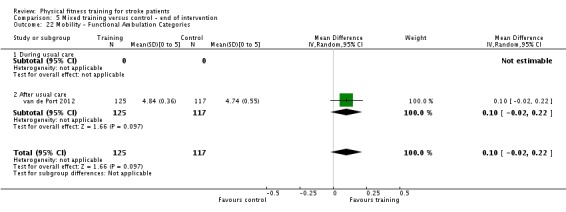
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 22 Mobility ‐ Functional Ambulation Categories.
Analysis 6.12.
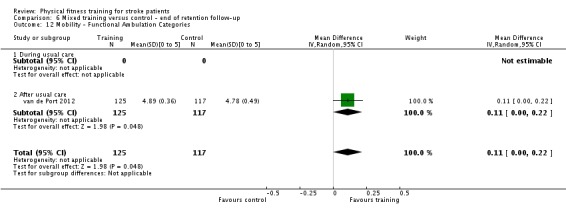
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 12 Mobility ‐ Functional Ambulation Categories.
Preferred walking speed (PWS)
Nine studies with a total of 639 participants measured the effects of mixed training on preferred walking speed (metres per minute). The walking speed increased at the end of intervention in stroke survivors who received mixed training (MD 4.54 m/minute, 95% CI 0.95 to 8.14; P value = 0.01; Analysis 5.23). The effect is influenced mostly by data from interventions delivered after usual care and there is significant heterogeneity within the after usual care subgroup (Chi² = 34.39, df = 5, P value < 0.00001). Only the interventions in three of the nine studies are not confounded by additional training time and show no effect (Mead 2007; Richards 1993; Richards 2004).
Analysis 5.23.
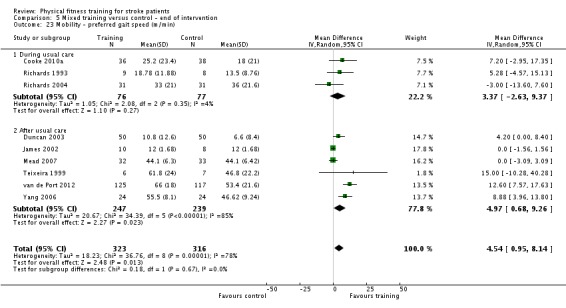
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 23 Mobility ‐ preferred gait speed (m/min).
Subgroup analysis of trials in which the experimental group was confounded by additional training time showed a significant difference in favour of mixed training (MD 6.32 metres/minute, 95% CI 1.08 to 11.55; P value = 0.02; Analysis 5.24), whilst those not confounded by additional training time did not (MD 0.49 metres/minute, 95% CI ‐2.96 to 3.94; P value = 0.78). The confounded data show significant heterogeneity (I2 = 85%; P value < 0.001) whilst the unconfounded data do not (I2 = 8%; P value = 0.34).
Analysis 5.24.
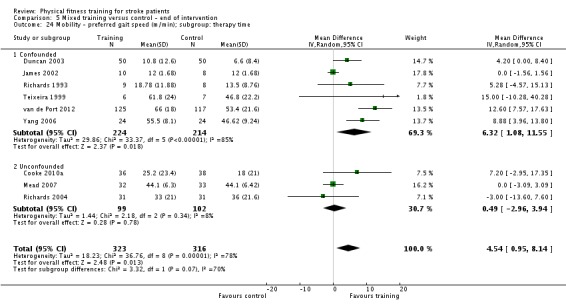
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 24 Mobility ‐ preferred gait speed (m/min); subgroup: therapy time.
A funnel plot that we generated using continuous measures for preferred walking speed at the end of intervention did not suggest the presence of publication bias as its shape did not show gross asymmetry (Figure 6).
Figure 6.
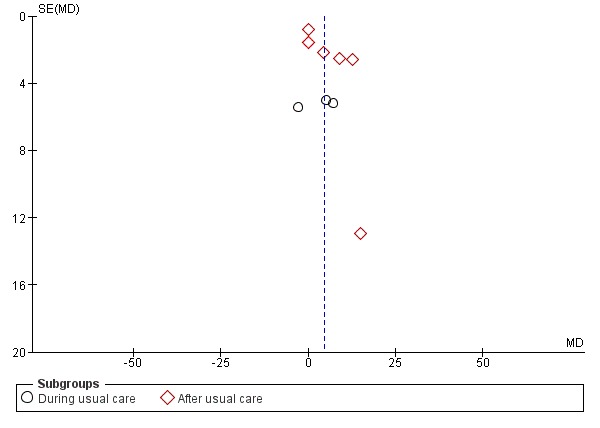
Funnel plot of comparison: 5 Mixed training versus control ‐ end of intervention, outcome: 5.23 Mobility ‐ preferred gait speed (m/min).
Four trials that provided follow‐up data for preferred gait speed did not show a significant training effect at the end of the scheduled follow‐up (Analysis 6.13).
Analysis 6.13.
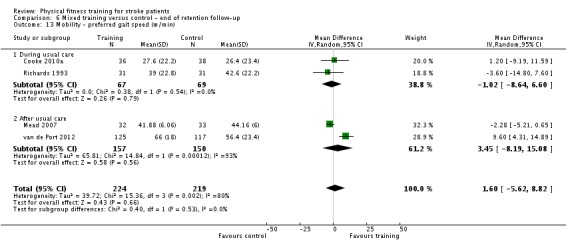
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 13 Mobility ‐ preferred gait speed (m/min).
One study showed some indication of dose‐response, where the improvement in preferred gait speed was positively associated with the amount of time spent on the gait training component (R2 = 0.63; Richards 1993).
Six‐Minute Walking Test (6‐MWT)
Seven trials measured the walking capacity (metres walked in six minutes) in a total of 561 participants. Walking capacity increased significantly in the mixed training group (MD 41.60 metres, 95% CI 25.25 to 57.95; P value < 0.00001; Analysis 5.25). Two trials included a follow‐up and showed that walking capacity remained significantly greater in the groups who had participated in training (MD 51.62 metres, 95% CI 25.20 to 78.03; P value = 0.0001; Analysis 6.14).
Analysis 5.25.
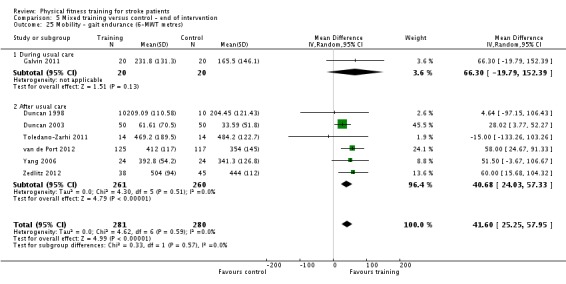
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 25 Mobility ‐ gait endurance (6‐MWT metres).
Analysis 6.14.
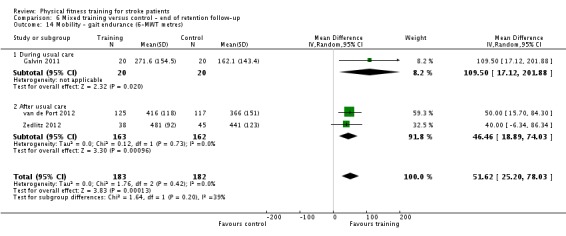
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 14 Mobility ‐ gait endurance (6‐MWT metres).
It is worth noting, however, that in all trials in this analysis the intervention groups were confounded by additional training time, which could exaggerate the effect.
Other mobility outcomes
Three trials measured community ambulation speed (the ability to walk at 0.8 metres per second or more) in a total of 232 participants during (Cooke 2010a) and after (Duncan 2003; Mead 2007) usual care. We did not observe any significant training effects either at the end of intervention (Analysis 5.26) or at follow‐up (Analysis 6.15).
Analysis 5.26.
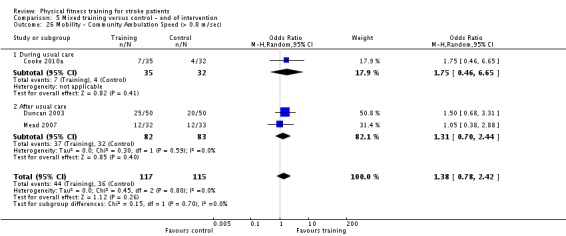
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 26 Mobility ‐ Community Ambulation Speed (> 0.8 m/sec).
Analysis 6.15.
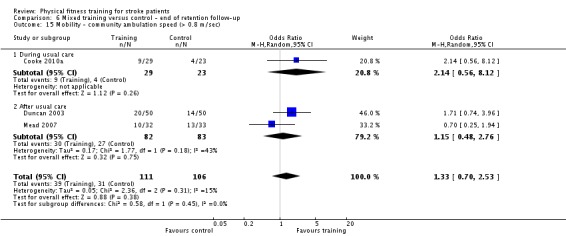
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 15 Mobility ‐ community ambulation speed (> 0.8 m/sec).
Comparison of cardiorespiratory, resistance training, and mixed training (Comparison 7)
We performed a subgroup analysis to compare the effects of the different types of training (cardiorespiratory training versus mixed training versus resistance training) on mobility outcomes at the end of intervention. Although we found no statistically significant subgroup differences there were still clear patterns within the data.
Maximal walking speed increased significantly after cardiorespiratory training but not after resistance training (Analysis 7.2). No mixed training data were available for this outcome.
Preferred walking speed increased significantly after cardiorespiratory and mixed training but not after resistance (Analysis 7.3). Excluding trials that were confounded by additional training time, only cardiorespiratory training showed a significant training effect.
Gait endurance (6‐MWT) increased significantly after cardiorespiratory, and particularly mixed training, but not after resistance training (Analysis 7.4). All mixed training trials were confounded by additional training time.
Analysis 7.2.
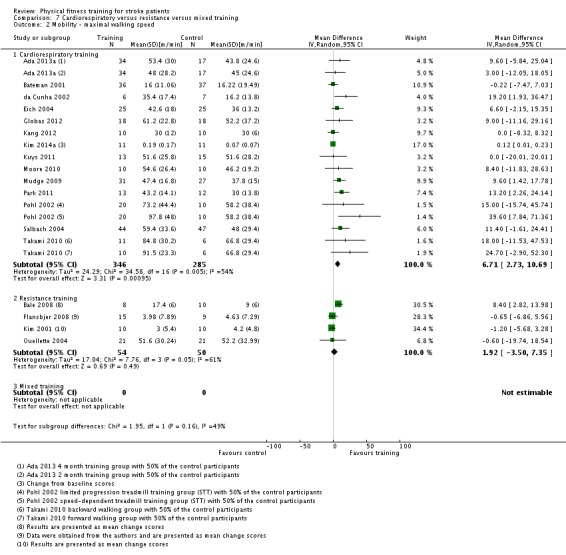
Comparison 7 Cardiorespiratory versus resistance versus mixed training, Outcome 2 Mobility ‐ maximal walking speed.
Analysis 7.3.
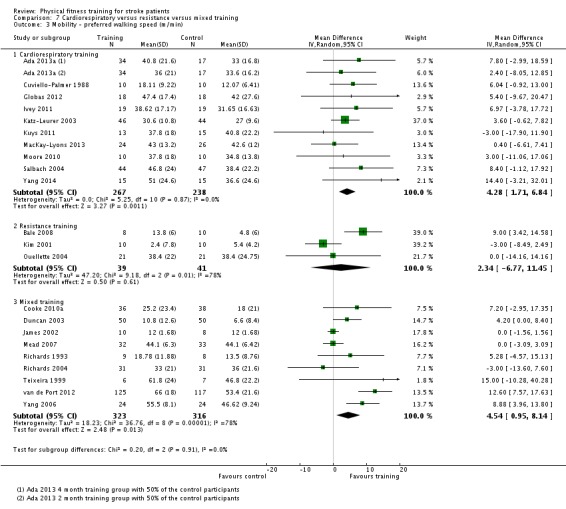
Comparison 7 Cardiorespiratory versus resistance versus mixed training, Outcome 3 Mobility ‐ preferred walking speed (m/min).
Analysis 7.4.
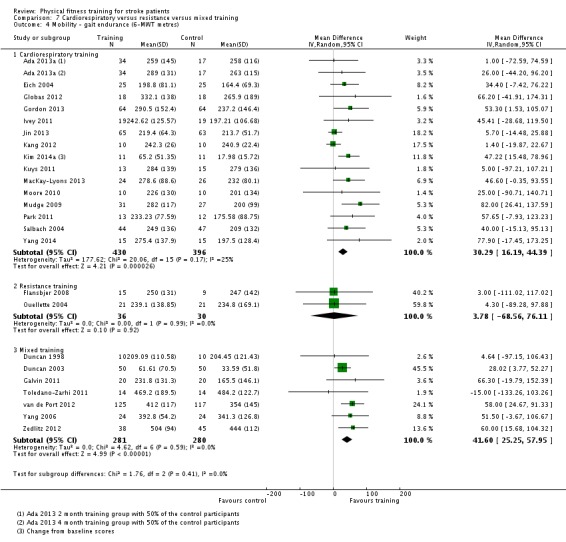
Comparison 7 Cardiorespiratory versus resistance versus mixed training, Outcome 4 Mobility ‐ gait endurance (6‐MWT metres).
Physical function
The included trials assessed participants' physical function using a variety of different measures including rating scales (for example Berg Balance Scale) and specific measures of functional performance (for example functional reach, timed up and go test, stair climbing).
Cardiorespiratory training (Comparisons 1 and 2)
Seven trials with a total of 435 participants assessed the effects of cardiorespiratory training on balance using the Berg Balance Scale. There was no significant improvement in the scores (MD 1.13, 95% CI ‐0.44 to 2.70; P value = 0.16; Analysis 1.28). All trials except Bateman 2001 and Jin 2013 involved walking. The Bateman 2001 data were also at risk of bias; if we excluded these trials the effect was strengthened but borderline (MD 2.57, 95% CI ‐0.03 to 5.17: P value = 0.05). The backwards walking group of Takami 2010 appeared to produce a larger (non‐significant) benefit compared with the forwards walking group from the same trial. Bateman 2001 and MacKay‐Lyons 2013 also assessed participants at the end of the follow‐up period but did not show any effect (Analysis 2.15).
Analysis 1.28.
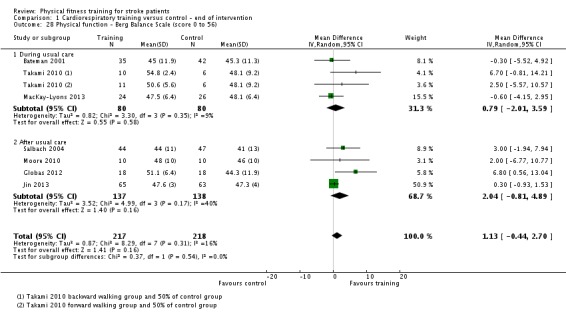
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 28 Physical function ‐ Berg Balance Scale (score 0 to 56).
Analysis 2.15.
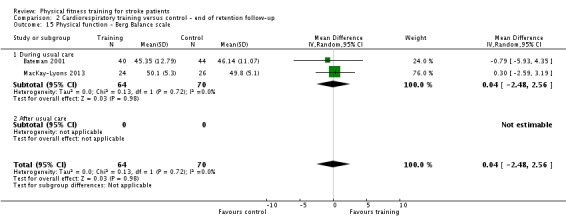
Comparison 2 Cardiorespiratory training versus control ‐ end of retention follow‐up, Outcome 15 Physical function ‐ Berg Balance scale.
Three trials that measured the performance of a total of 131 participants during the timed up and go test did not show any specific benefits of training at the end of the intervention after usual care (Analysis 1.26) (Kang 2012; Moore 2010; Salbach 2004).
Analysis 1.26.
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 26 Physical function ‐ Timed Up and Go (sec).
Resistance training (Comparisons 3 and 4)
One trial assessed the maximum weight‐bearing on the affected leg (% body weight) (Bale 2008). We observed a small training effect in the resistance training group compared with the usual rehabilitation group (MD 11.80, 95% CI 0.89 to 22.71; Analysis 3.10).
Analysis 3.10.
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 10 Physical function ‐ weight‐bearing (% body weight ‐ affected side).
Two trials did not find any significant differences between intervention groups in the time needed to ascend a 10‐stair flight at the end of the training period (Analysis 3.11) (Kim 2001; Ouellette 2004).
Analysis 3.11.
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 11 Physical function ‐ stair climbing, maximal (sec/step).
Two trials examined the effect of resistance training on the timed up and go test and showed a small improvement at the end of intervention (MD ‐6.45 sec, 95% CI ‐7.48 to ‐5.43; P value = 0.00001) (Analysis 3.12; Flansbjer 2008; Son 2014), but not after at follow‐up (Analysis 4.6; Flansbjer 2008 only).
Analysis 3.12.
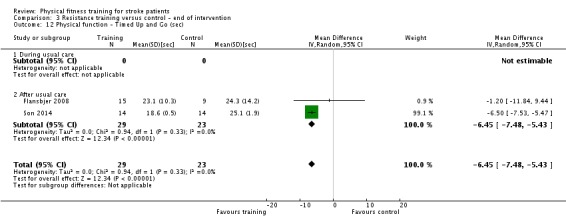
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 12 Physical function ‐ Timed Up and Go (sec).
Analysis 4.6.
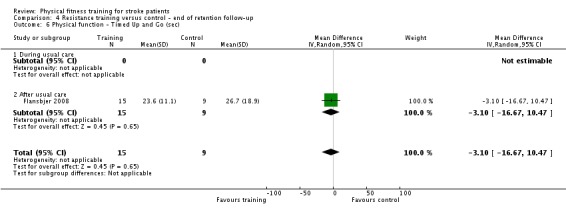
Comparison 4 Resistance training versus control ‐ end of retention follow‐up, Outcome 6 Physical function ‐ Timed Up and Go (sec).
Balance was assessed by two small studies (Lee 2013; Son 2014); resistance training was shown to have marginal, heterogenous effects on antero‐posterior sway (SMD ‐2.12 mm, 95% CI ‐4.07 to ‐0.16; P value = 0.03; Analysis 3.14) and mediolateral sway (SMD ‐2.51 mm, 95% CI ‐5.16 to 0.14; P value = 0.06; Analysis 3.15). Son 2014 also assessed effect on the Berg Balance scale showing significant benefit (MD 3.41, 95% CI 1.52 to 5.30; P value = 0.0004; Analysis 3.13).
Analysis 3.14.
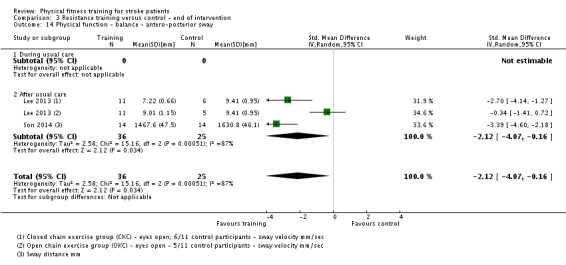
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 14 Physical function ‐ balance ‐ antero‐posterior sway.
Analysis 3.15.
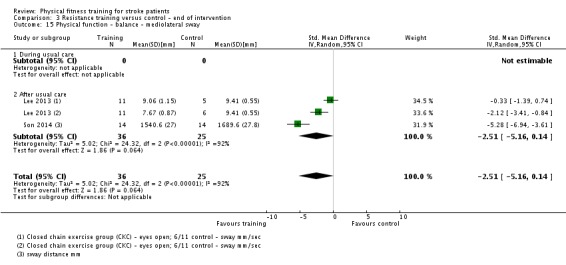
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 15 Physical function ‐ balance ‐ mediolateral sway.
Analysis 3.13.
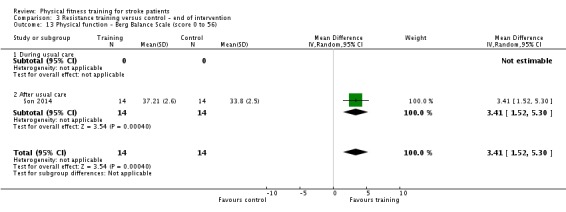
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 13 Physical function ‐ Berg Balance Scale (score 0 to 56).
One study examined the effect of resistance training on the Trunk Impairment Scale; there was no significant effect size (Verheyden 2009; Analysis 3.16).
Analysis 3.16.
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 16 Physical function ‐ Trunk Impairment Scale [scale 0 to 23].
Mixed training (Comparisons 5 and 6)
Balance outcomes
Six trials with a total of 260 participants assessed the participants' balance using the Berg Balance Scale. Scores show a tendency for beneficial improvements in balance, which are at the borderline of statistical significance (MD 1.97, 95% CI 0.36 to 3.59; P value = 0.02; Analysis 5.27). Follow‐up data from two trials did not show any significant training effect (Analysis 6.16).
Analysis 5.27.
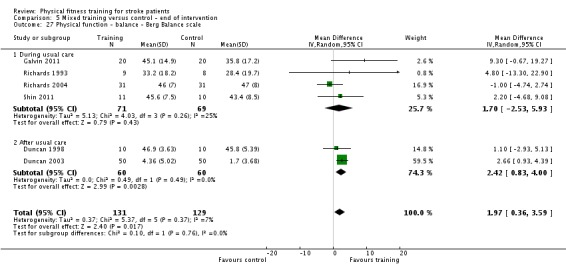
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 27 Physical function ‐ balance ‐ Berg Balance scale.
Analysis 6.16.
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 16 Physical function ‐ balance ‐ Berg Balance Scale.
Two trials with a total of 166 participants measured balance using the functional reach test but did not show any benefit of mixed training at the end of intervention (Duncan 2003; Mead 2007; Analysis 5.28). One trial also provided follow‐up data (Mead 2007), which did not show persistence of any training effect beyond the duration of intervention.
Analysis 5.28.
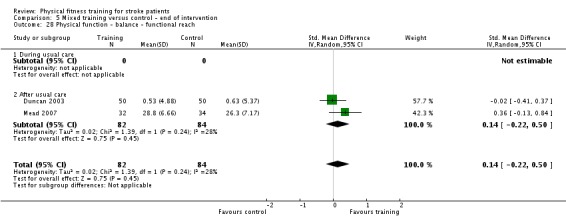
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 28 Physical function ‐ balance ‐ functional reach.
One trial measured balance using the Four Square Step Test and found no significant effect at the end of intervention (Toledano‐Zarhi 2011; Analysis 5.29); however these data were very different at baseline in a way that benefited the control group.
Analysis 5.29.
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 29 Physical function ‐ balance ‐ Four Square Step Test.
One trial measured balance using the timed balance test and showed a beneficial effect of training at the end of intervention (MD 0.32, 95% CI 0.06 to 0.58; P value = 0.02) (van de Port 2012; Analysis 5.30) and after a three‐month follow‐up (MD 0.46, 95% CI 0.09 to 0.83; P value = 0.02; Analysis 6.18).
Analysis 5.30.
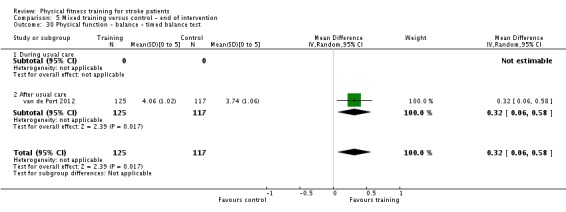
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 30 Physical function ‐ balance ‐ timed balance test.
Analysis 6.18.
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 18 Physical function ‐ balance ‐ timed balance test.
One trial measured postural sway (static balance) in a range of conditions and planes of movement (too many for a meaningful meta‐analysis); however, the study authors concluded there was no effect of mixed training (Shin 2011).
There were sufficient data among the different measures of balance used (nine trials, 596 participants) to be legitimately pooled. This showed an overall beneficial improvement in balance at the end of intervention (SMD 0.27, 95% CI 0.07 to 0.47; P value = 0.008; Analysis 5.31). If we excluded the problematic data from Toledano‐Zarhi 2011 the effect was strengthened and heterogeneity was reduced (SMD 0.33, 95% CI 0.16 to 0.49; P value = 0.0001). However, five of the nine included trials were confounded by additional training time; when we excluded these data, leaving only Mead 2007, Richards 1993, Richards 2004 and Shin 2011, there was no effect of training on balance.
Analysis 5.31.
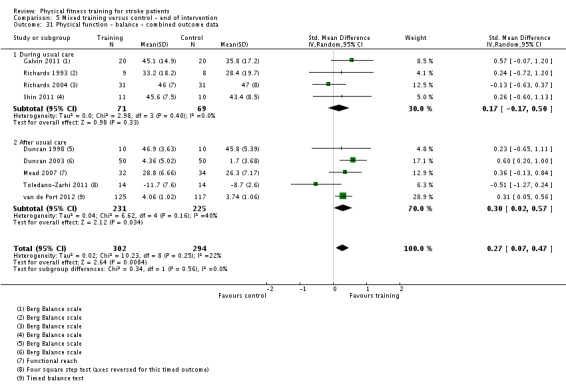
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 31 Physical function ‐ balance ‐ combined outcome data.
Other outcomes
Four trials measured the time to complete the timed up and go test in a total of 418 participants (Mead 2007; Richards 2004; van de Port 2012; Yang 2006). Participants in the training group were faster than those in the control group (MD ‐1.37 sec, 95% CI ‐2.26 to ‐0.47; P value = 0.003; Analysis 5.32) at the end of the mixed training phase. The Yang 2006 and van de Port 2012 data were, however, confounded by additional training time. After we removed these data from the analysis no significant training effect was evident (MD ‐1.13 seconds, 95% CI ‐2.91 to 0.65; Analysis 5.33). Follow‐up data in three trials did not show a significant retention of mixed training benefits (Mead 2007; Richards 2004; van de Port 2012; Analysis 6.19).
Analysis 5.32.
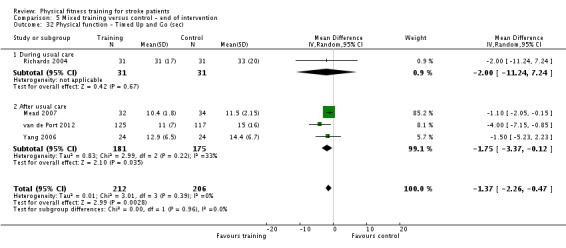
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 32 Physical function ‐ Timed Up and Go (sec).
Analysis 5.33.
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 33 Physical function ‐ Timed Up and Go (sec) ‐ sensitivity analysis ‐ unconfounded trials.
Analysis 6.19.
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 19 Physical function ‐ Timed Up and Go (sec).
One trial assessed upper extremity functional performance using the Action Research Arm test (Donaldson 2009). We observed no significant training effects (Analysis 5.34).
Analysis 5.34.
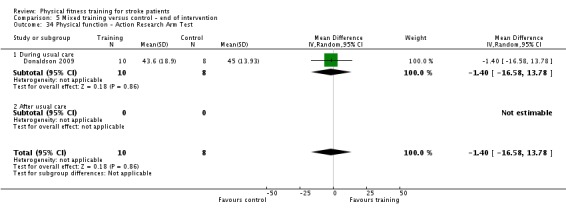
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 34 Physical function ‐ Action Research Arm Test.
Comparison of cardiorespiratory, resistance training, and mixed training (Comparison 7)
We performed a subgroup analysis to directly compare the effects of the different types of training (cardiorespiratory training versus mixed training versus resistance training) on the Berg Balance Scale at the end of the intervention (Analysis 7.5). There was an overall beneficial effect of mixed training on balance, and only a single study using resistance training. Overall, the effects of cardiorespiratory training were not significant but this may be influenced by individual study characteristics. There was no statistically significant difference between the subgroups.
Analysis 7.5.
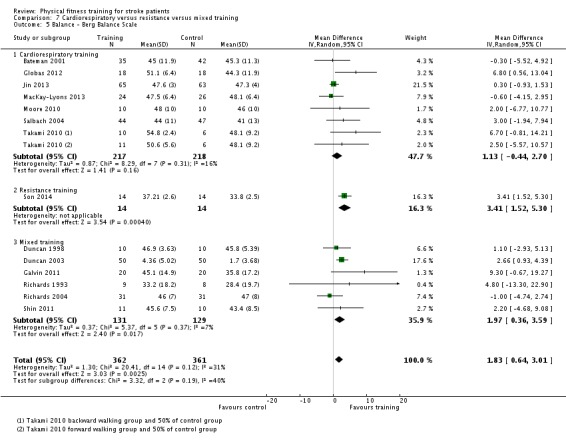
Comparison 7 Cardiorespiratory versus resistance versus mixed training, Outcome 5 Balance ‐ Berg Balance Scale.
Health status and quality of life
Cardiorespiratory training (Comparisons 1 and 2)
One trial assessed the effects of cardiorespiratory training on measures of quality of life in 28 participants (Aidar 2007). Both the SF‐36 physical functioning score (MD 10.60, 95% CI 6.51 to 14.69; P value = 0.00001; Analysis 1.29) and the SF‐36 emotional role (MD 11.00, 95% CI 6.15 to 15.85; P value = 0.0001; Analysis 1.30) scores were significantly better at the end of the training period in participants who underwent cardiorespiratory training.
Analysis 1.29.
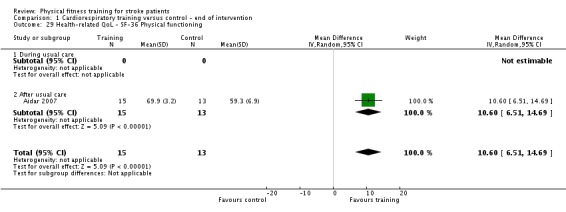
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 29 Health‐related QoL ‐ SF‐36 Physical functioning.
Analysis 1.30.
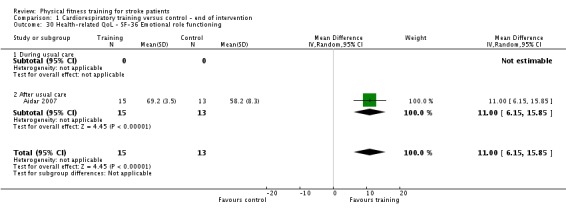
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 30 Health‐related QoL ‐ SF‐36 Emotional role functioning.
One trial examined effects on the SF‐36 and showed that cardiorespiratory training benefited the physical health component (MD 6.60, 95% CI 2.40 to 10.80; P value = 0.002; Analysis 1.31) but not the mental health component (Gordon 2013; Analysis 1.32).
Analysis 1.31.
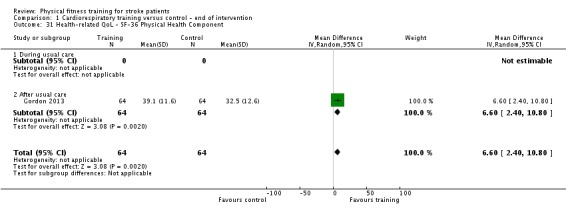
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 31 Health‐related QoL ‐ SF‐36 Physical Health Component.
Analysis 1.32.
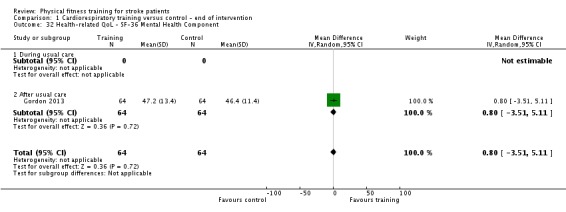
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 32 Health‐related QoL ‐ SF‐36 Mental Health Component.
One trial examined the effects of cardiorespiratory training on the SF‐12 and showed a significant improvement in the mental health domain (MD 9.30, 95% CI 4.31 to 14.29; P value = 0.0003; Analysis 1.33) but not the physical health domain (Globas 2012; Analysis 1.34).
Analysis 1.33.
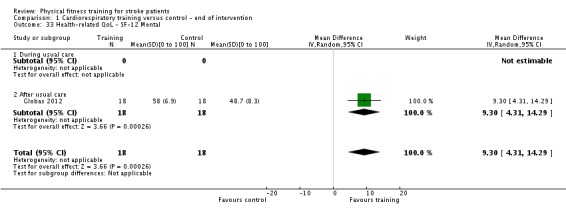
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 33 Health‐related QoL ‐ SF‐12 Mental.
Analysis 1.34.
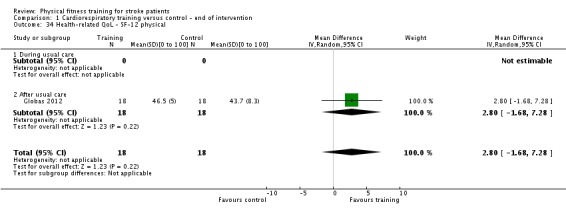
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 34 Health‐related QoL ‐ SF‐12 physical.
One trial examined the effects on EuroQoL scores and showed no effect at the end of the intervention (Analysis 1.35). There was also no effect after a 12‐month follow‐up although the effect approaches statistical significance (Ada 2013a; Analysis 2.16).
Analysis 1.35.
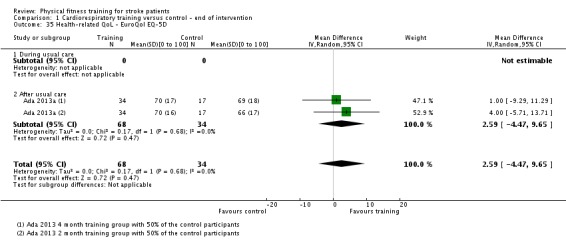
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 35 Health‐related QoL ‐ EuroQol EQ‐5D.
Analysis 2.16.
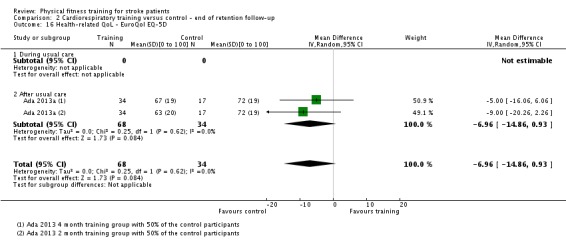
Comparison 2 Cardiorespiratory training versus control ‐ end of retention follow‐up, Outcome 16 Health‐related QoL ‐ EuroQol EQ‐5D.
Resistance training (Comparisons 3 and 4)
One small trial of 20 participants did not show any significant differences between the resistance training group and the control group in either the physical health or mental health component of the SF‐36 at the end of intervention (Kim 2001; Analysis 3.17; Analysis 3.18).
Analysis 3.17.
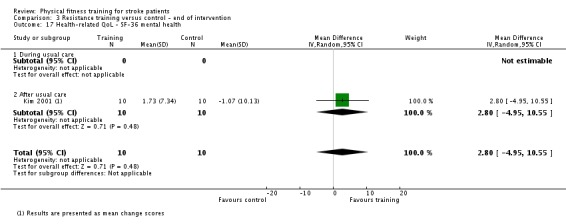
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 17 Health‐related QoL ‐ SF‐36 mental health.
Analysis 3.18.
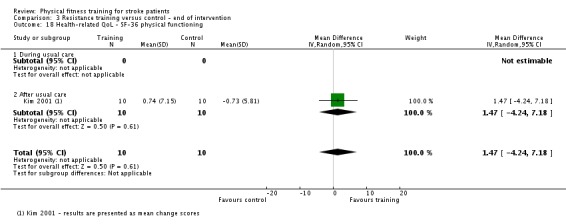
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 18 Health‐related QoL ‐ SF‐36 physical functioning.
Mixed training (Comparisons 5 and 6)
Several trials assessed the effects of mixed training on quality of life using different components of the SF‐36 survey questionnaire. In two trials with a total of 112 participants (Duncan 2003; James 2002), significantly better scores were obtained in the SF‐36 physical functioning component in the mixed training group at the end of intervention (SMD 0.48, 95% CI 0.10 to 0.85; 0.01; Analysis 5.35) but not in the social role functioning component (Analysis 5.36). Three trials with a total of 178 participants showed significantly better scores in the SF‐36 physical role functioning component for the mixed training group at the end of intervention (SMD 0.56, 95% CI 0.26 to 0.86; P value = 0.0003; Analysis 5.37) (Duncan 2003; James 2002; Mead 2007). This effect was retained at follow‐up (MD 11.61, 95% CI 2.38 to 20.84; P value = 0.01; Analysis 6.23).
Analysis 5.35.
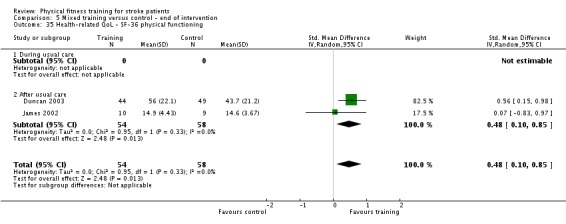
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 35 Health‐related QoL ‐ SF‐36 physical functioning.
Analysis 5.36.
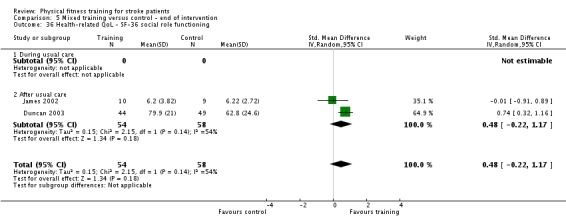
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 36 Health‐related QoL ‐ SF‐36 social role functioning.
Analysis 5.37.
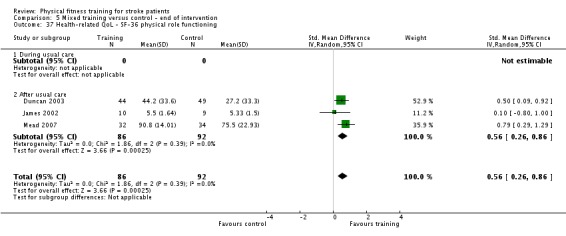
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 37 Health‐related QoL ‐ SF‐36 physical role functioning.
Analysis 6.23.
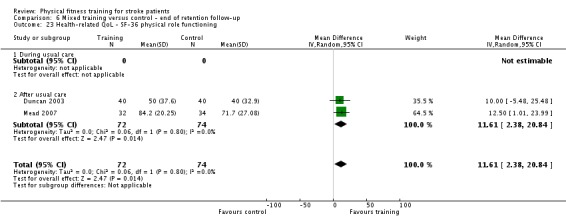
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 23 Health‐related QoL ‐ SF‐36 physical role functioning.
Duncan 2003 showed that participants receiving mixed training had significantly better results in the emotional role functioning component of the SF‐36 compared with controls at the end of the training period (MD 15.50, 95% CI 2.98 to 28.02; P value = 0.02 (Analysis 5.38) but not at follow‐up (Analysis 6.24).
Analysis 5.38.
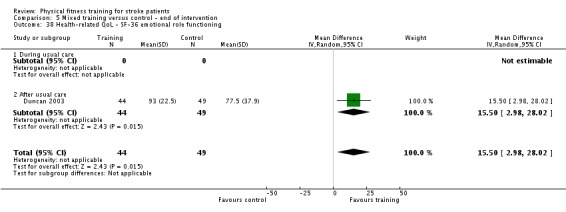
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 38 Health‐related QoL ‐ SF‐36 emotional role functioning.
Analysis 6.24.
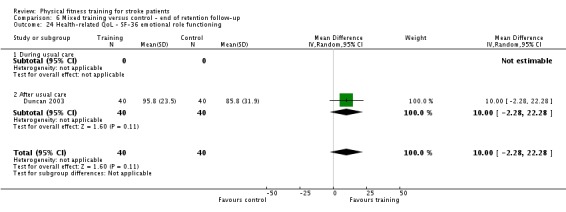
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 24 Health‐related QoL ‐ SF‐36 emotional role functioning.
Cooke 2010a measured the effects of mixed training on quality of life in 50 participants using two components of the EuroQol scale (health state and perceived health state). Scores were not significantly different between intervention groups at the end of the training phase (Analysis 5.40; Analysis 5.41) or at follow‐up (Analysis 6.20; Analysis 6.21).
Analysis 5.40.
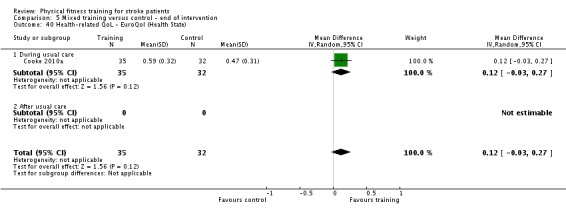
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 40 Health‐related QoL ‐ EuroQol (Health State).
Analysis 5.41.
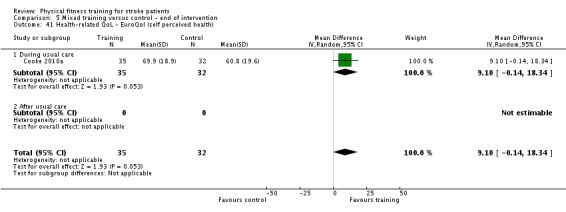
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 41 Health‐related QoL ‐ EuroQol (self perceived health).
Analysis 6.20.
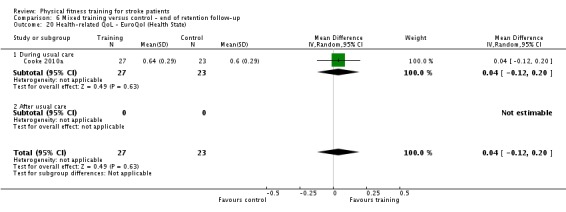
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 20 Health‐related QoL ‐ EuroQol (Health State).
Analysis 6.21.
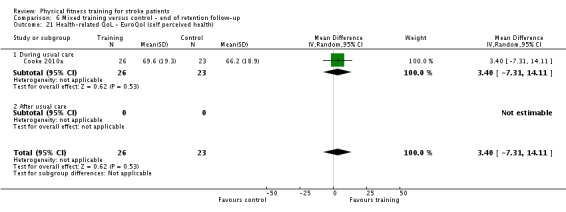
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 21 Health‐related QoL ‐ EuroQol (self perceived health).
Zedlitz 2012 assessed the effect of mixed training on the Stroke‐Adapted Sickness Impact profile and showed no effect at the end of intervention (Analysis 5.39) or end of six‐month follow‐up (Analysis 6.25).
Analysis 5.39.
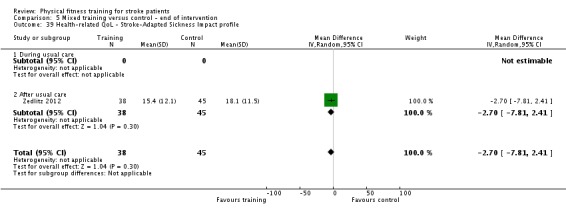
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 39 Health‐related QoL ‐ Stroke‐Adapted Sickness Impact profile.
Analysis 6.25.
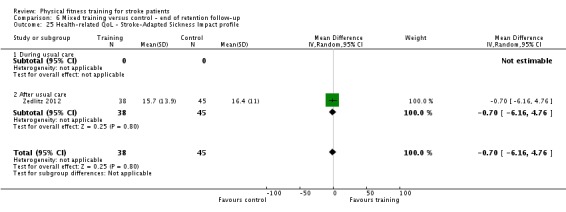
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 25 Health‐related QoL ‐ Stroke‐Adapted Sickness Impact profile.
It is worth noting that in the Duncan 2003, James 2002 and Zedlitz 2012 trials the intervention group was potentially confounded by additional training time.
Mood
Cardiorespiratory training (Comparisons 1 and 2)
Smith 2008 assessed the potential benefits of cardiorespiratory training on depression symptoms using the Beck Depression Index. We found no significant differences between intervention groups at the end of intervention (Analysis 1.37) and at follow‐up (Analysis 2.17).
Analysis 1.37.
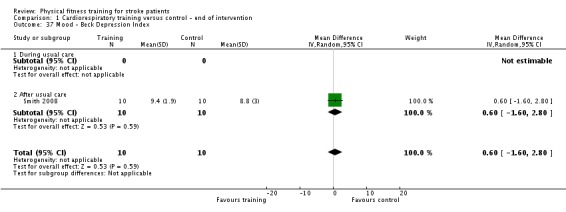
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 37 Mood ‐ Beck Depression Index.
Analysis 2.17.
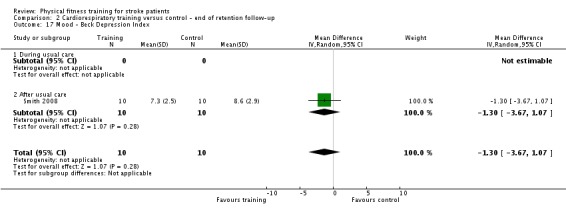
Comparison 2 Cardiorespiratory training versus control ‐ end of retention follow‐up, Outcome 17 Mood ‐ Beck Depression Index.
Bateman 2001 assessed participants using the anxiety and depression components of the Hospital Anxiety and Depression Scale (HADS). The anxiety score decreased immediately after cardiorespiratory training (MD ‐1.94, 95% CI ‐3.80 to ‐0.08; Analysis 1.38) but this small benefit was not retained at the follow‐up assessment (Analysis 2.18). In contrast, the depression score was not significantly different between groups at the end of the training phase (Analysis 1.36) but decreased significantly in the cardiorespiratory group at the end of the follow‐up period (MD ‐2.70, 95% CI ‐4.40 to ‐1.00; Analysis 2.19). This trial had, however, substantial missing values at the end of intervention (29%) and end of follow‐up (37%) and therefore these findings should be interpreted with caution. Another trial, which measured participants' mood using the HADS, reported that the depression score improved in the intervention group but not in the control group (Lennon 2008). We were, however, unable to include these trial data in our analyses as they were presented in a format not suitable for RevMan 2014.
Analysis 1.38.
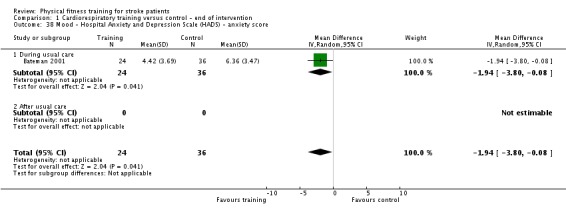
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 38 Mood ‐ Hospital Anxiety and Depression Scale (HADS) ‐ anxiety score.
Analysis 2.18.
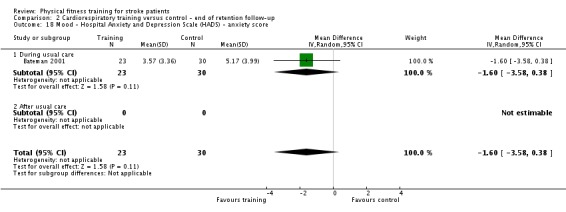
Comparison 2 Cardiorespiratory training versus control ‐ end of retention follow‐up, Outcome 18 Mood ‐ Hospital Anxiety and Depression Scale (HADS) ‐ anxiety score.
Analysis 1.36.
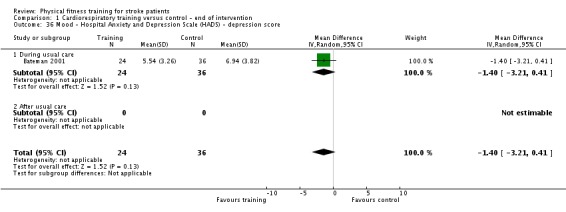
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 36 Mood ‐ Hospital Anxiety and Depression Scale (HADS) ‐ depression score.
Analysis 2.19.
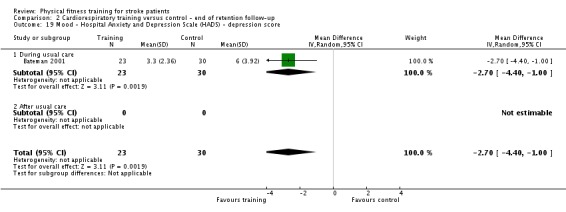
Comparison 2 Cardiorespiratory training versus control ‐ end of retention follow‐up, Outcome 19 Mood ‐ Hospital Anxiety and Depression Scale (HADS) ‐ depression score.
Combining data from different depression scales (Bateman 2001; Smith 2008) showed no effect at the end of intervention (Analysis 1.39), however a significant benefit was noted at the end of follow‐up (SMD ‐0.70, 95% CI ‐1.18 to ‐0.22; P value = 0.004; Analysis 2.20).
Analysis 1.39.
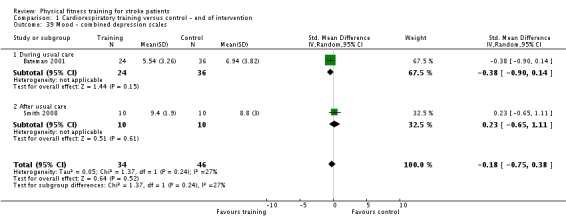
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 39 Mood ‐ combined depression scales.
Analysis 2.20.
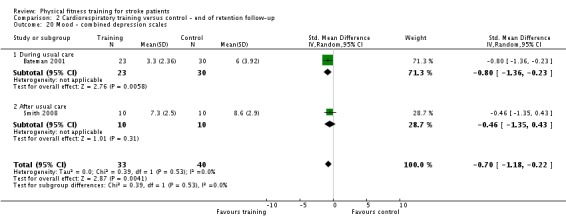
Comparison 2 Cardiorespiratory training versus control ‐ end of retention follow‐up, Outcome 20 Mood ‐ combined depression scales.
Resistance training (Comparisons 3 and 4)
Sims 2009 assessed 88 participants using the Centre for Epidemiological Studies for Depression scale (CES‐D). The mood in the resistance training group was significantly better at the end of intervention (MD ‐5.49, 95% CI ‐9.78 to ‐1.20; Analysis 3.19) and at follow‐up (MD ‐8.92, 95% CI ‐13.03 to ‐4.81; Analysis 4.7).
Analysis 3.19.
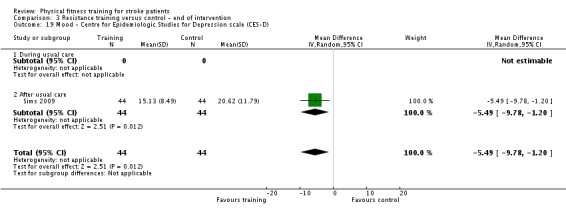
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 19 Mood ‐ Centre for Epidemiologic Studies for Depression scale (CES‐D).
Analysis 4.7.
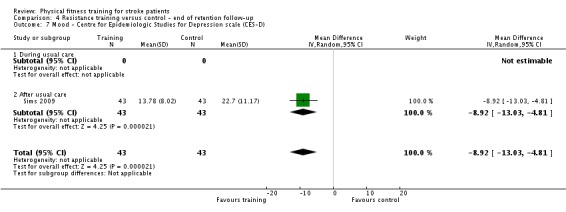
Comparison 4 Resistance training versus control ‐ end of retention follow‐up, Outcome 7 Mood ‐ Centre for Epidemiologic Studies for Depression scale (CES‐D).
Aidar 2012 used the Brazilian translation of the State‐Trait Anxiety Inventory and showed no effect on either trait anxiety (Analysis 3.20) or state anxiety (Analysis 3.21) at the end of intervention.
Analysis 3.20.
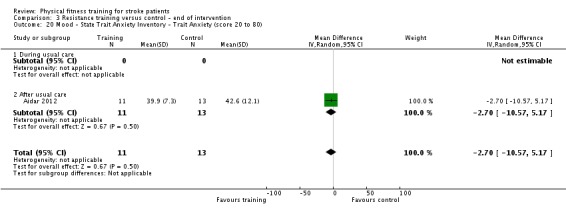
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 20 Mood ‐ State Trait Anxiety Inventory ‐ Trait Anxiety (score 20 to 80).
Analysis 3.21.
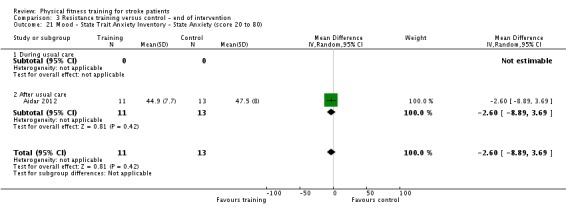
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 21 Mood ‐ State Trait Anxiety Inventory ‐ State Anxiety (score 20 to 80).
Aidar 2014 reported a significant benefit in measures of the Beck Depression Inventory at the end of intervention but this was not detectable in a meta‐analysis (Analysis 3.22).
Analysis 3.22.
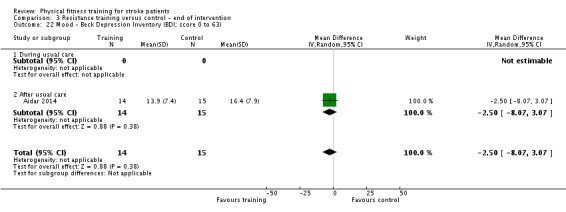
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 22 Mood ‐ Beck Depression Inventory (BDI; score 0 to 63).
Combining data from the different depression scales (Aidar 2014; Sims 2009) showed a significant benefit at the end of intervention (SMD ‐0.48, 95% CI ‐0.84 to ‐0.11; P value = 0.01; Analysis 3.23),
Analysis 3.23.
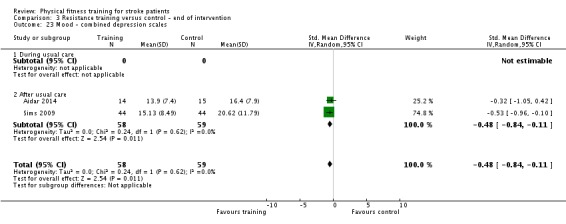
Comparison 3 Resistance training versus control ‐ end of intervention, Outcome 23 Mood ‐ combined depression scales.
Mixed training (Comparisons 5 and 6)
Three trials assessed 391 participants using the anxiety and depression components of the Hospital Anxiety and Depression Scale (HADS) (Mead 2007; van de Port 2012; Zedlitz 2012). No immediate training effects were observed on either HADS component at the end of the intervention (Analysis 5.42; Analysis 5.43). No retained training effects were observed on either HADS component at the end of follow‐up (Analysis 6.28; Analysis 6.29).
Analysis 5.42.
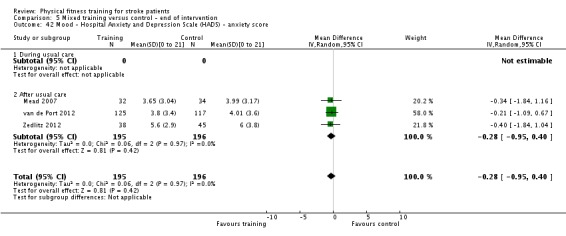
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 42 Mood ‐ Hospital Anxiety and Depression Scale (HADS) ‐ anxiety score.
Analysis 5.43.
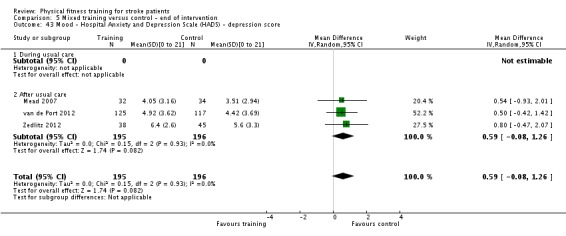
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 43 Mood ‐ Hospital Anxiety and Depression Scale (HADS) ‐ depression score.
Analysis 6.28.
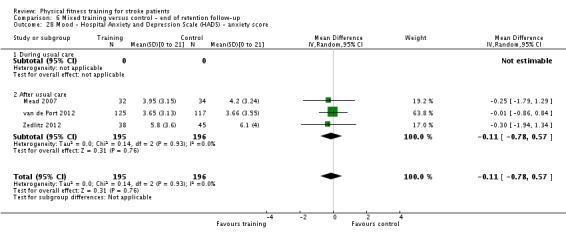
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 28 Mood ‐ Hospital Anxiety and Depression Scale (HADS) ‐ anxiety score.
Analysis 6.29.
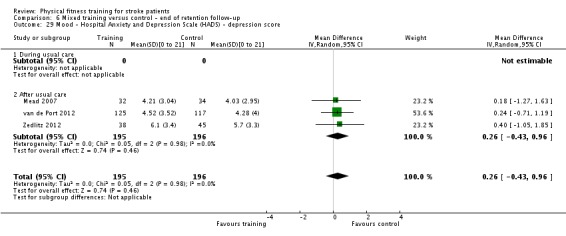
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 29 Mood ‐ Hospital Anxiety and Depression Scale (HADS) ‐ depression score.
Duncan 2003 and van de Port 2012 assessed mood in 335 participants using the emotion domain of the Stroke Impact Scale (SIS) and showed no significant effect at the end of intervention (Analysis 5.44) or after three‐month follow‐up (Analysis 6.26).
Analysis 5.44.
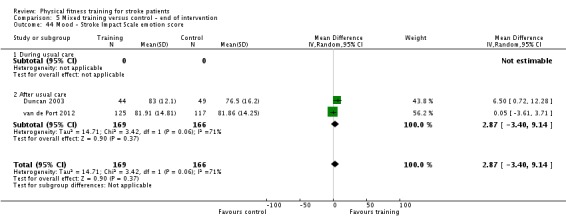
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 44 Mood ‐ Stroke Impact Scale emotion score.
Analysis 6.26.
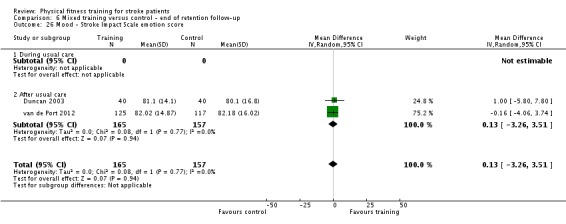
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 26 Mood ‐ Stroke Impact Scale emotion score.
Duncan 2003 showed improvements in Geriatric Depression Scale scores at the end of intervention (MD ‐1.90, 95% CI ‐3.10 to ‐0.70; P value = 0.002; Analysis 5.45) but not the end of follow‐up (Analysis 6.27).
Analysis 5.45.
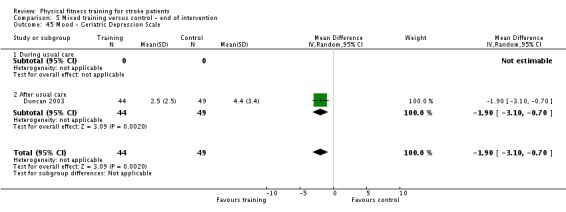
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 45 Mood ‐ Geriatric Depression Scale.
Analysis 6.27.
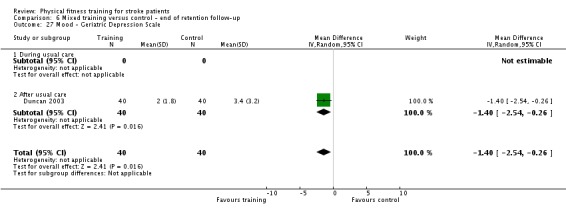
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 27 Mood ‐ Geriatric Depression Scale.
Combining data from the different depression scales showed no effect of training at the end of intervention (Analysis 5.46) or the end of follow‐up (Analysis 6.30).
Analysis 5.46.
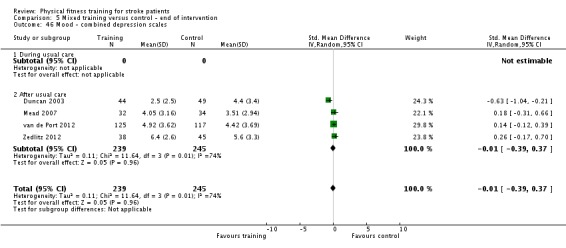
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 46 Mood ‐ combined depression scales.
Analysis 6.30.
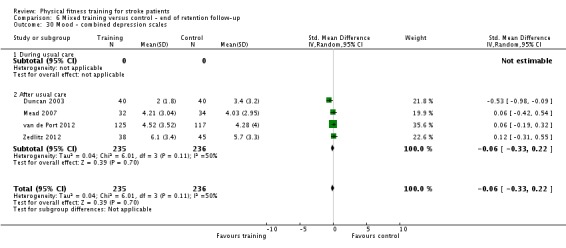
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 30 Mood ‐ combined depression scales.
Cognitive function
Three trials included cognitive function outcomes.
One trial of cardiorespiratory training showed no effect on FIM cognitive score (memory, problems solving questions) at the end of intervention (Analysis 1.40) (Bateman 2001). We did not consider end of follow‐up data due to the considerable proportions of missing data.
Analysis 1.40.
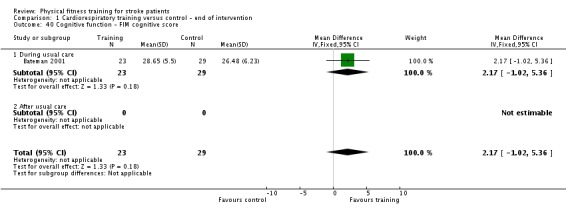
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 40 Cognitive function ‐ FIM cognitive score.
Two trials of mixed training, Duncan 2003 and Mead 2007, showed no effect on FIM cognitive score (memory, problems solving questions) at the end of intervention (Analysis 5.47) or end of follow‐up (Analysis 6.31). Duncan 2003 also used SIS domains of 'communication' and 'memory and thinking' to assess cognitive function. Although there were some trends in favour of exercise, the meta‐analysis results showed no significant effects at the end of intervention (Analysis 5.48; Analysis 5.49) or the end of the six‐month follow‐up (Analysis 6.32; Analysis 6.33).
Analysis 5.47.
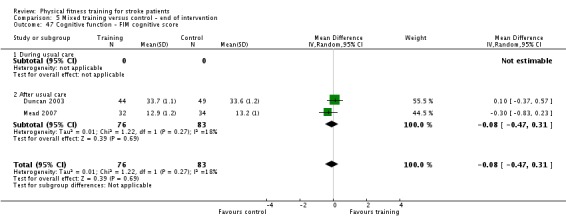
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 47 Cognitive function ‐ FIM cognitive score.
Analysis 6.31.
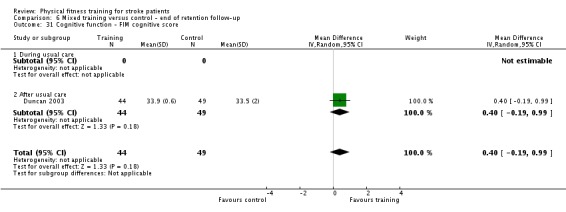
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 31 Cognitive function ‐ FIM cognitive score.
Analysis 5.48.
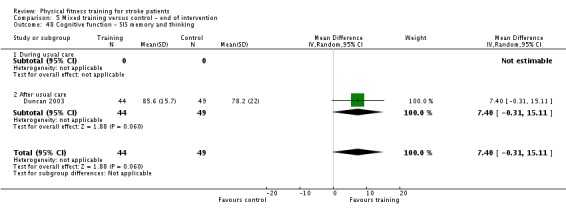
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 48 Cognitive function ‐ SIS memory and thinking.
Analysis 5.49.
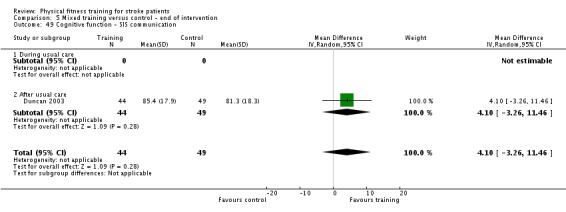
Comparison 5 Mixed training versus control ‐ end of intervention, Outcome 49 Cognitive function ‐ SIS communication.
Analysis 6.32.
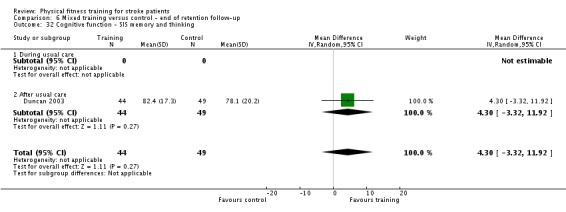
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 32 Cognitive function ‐ SIS memory and thinking.
Analysis 6.33.
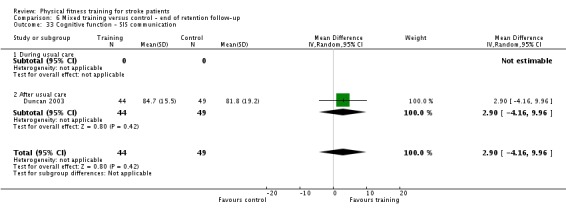
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 33 Cognitive function ‐ SIS communication.
'Summary of findings' tables
The key results are presented in 'Summary of findings' tables for cardiorespiratory training (Table 1) and for mixed training (Table 2). There were too few data to generate a meaningful 'Summary of findings' table for resistance training.
Discussion
The included trials encompassed a variety of outcome measures. This has been a typical drawback of stroke rehabilitation trials for some time (Greener 2002), and continues to be a problem when summarising and analysing data in a systematic review.
Effect of training on primary outcome measures
Case fatality
Death, from any cause, was not a common event among the participants of the trials included in this review. Only 13 out of the total 2797 participants died before the end of the intervention period and nine out of 1256 died before the end of follow‐up.
Where deaths did occur there may be a tendency toward these being more common among the control groups than among the intervention groups of mixed training trials. However, there are still too few data to draw any conclusions about the effect of fitness training on case fatality.
The observed numbers of deaths in this review may be low because the included participants were at lower risk of death compared with the wider stroke population. This may occur firstly because the inclusion criteria of the trials of exercise select participants with milder strokes (most were ambulatory) and reduced risk factors (such as blood pressure ceiling criteria). Secondly, there may be self selection by participants who are physically active with increased fitness. Higher physical activity is known to be associated with reduced risk of stroke (Lee 2003; Wendel‐Vos 2004), and higher VO2 peak is associated with reduced risk of stroke (Kurl 2003) and mortality (Lee 2002). In addition, the majority of the training programmes in this review were of short duration (12 weeks or less). A Cochrane Review of the effect of exercise‐based cardiac rehabilitation showed reduced mortality in people with coronary heart disease in the longer term (12 months follow‐up and more; Heran 2011); the training programmes tended to be much longer than those in this review. Since many stroke patients have coexisting heart disease, training might influence post‐stroke mortality provided it comprises cardiorespiratory training delivered over long periods of time. This requires investigation.
Although higher physical activity and higher cardiorespiratory fitness are linked to the primary prevention of stroke, there is a lack of data on the role of fitness training interventions in the secondary prevention of stroke. This gap in knowledge is currently a research priority and requires investigation (Pollock 2012).
Death or dependence
There were no data available to allow us to draw conclusions about the influence of training on the composite outcome of death or dependence after stroke. Death is infrequent and measures of dependency such as those based on simple questions, a Barthel Index score of less than 20, or a modified Rankin Scale score of 3, 4, or 5, are lacking (Lindley 1994). Both elements of this composite outcome are likely to be rare in stroke survivors who are eligible for physical fitness training.
Disability
We assessed a number of different global indices of disability. Data using the same scales were limited and this restricted the meta‐analyses, and a number of methodological issues weakened and biased the available data.
After cardiorespiratory training there was no improvement in Functional Independence Instrument scores (Analysis 1.2), Barthel Index scores (Analysis 1.3), or other individually reported outcomes. However, there was an improvement in Rivermead Mobility Index scores (Analysis 1.4). Pooling all available disability scale data from different scales showed a small beneficial effect (SMD; Analysis 1.7). This pattern of findings could occur because training influences the physical/mobility items of these various scales; such items dominate the scoring in tools like the Rivermead Mobility Index (eight out of 15 items) whereas they are less influential in more 'global' tools like the Functional Independence Measure (FIM) (two out of 18 items). Since walking is a common mode of cardiorespiratory exercise these findings could be precipitated by improvements in walking and mobility rather than more 'global' effects on disability.
In trials of mixed training various disability measurement instruments were used. Among these the only significant improvements were in Rivermead Mobility Index scores, both at the end of training (Analysis 5.4) and retained after a period of follow‐up (Analysis 6.5). Pooling all available data from different scales shows a hint of benefit at the end of intervention (Analysis 5.9). Like cardiorespiratory training these significant effects could be driven principally by changes in mobility. The study designs of several of the mixed training trials were confounded by additional training time; when these were excluded the benefits vanish. This means that participation in mixed training appeared effective but it is impossible to attribute any benefits to the actual content of the mixed training programmes.
The effects of cardiorespiratory training and mixed training at the end of intervention are similar in magnitude (Analysis 7.1). Overall, the findings show that interventions containing cardiorespiratory training, either alone or combined with resistance training, improve global measures of disability after stroke.
There are too few data to allow for any comment on the effect of resistance training.
Lack of benefits among many of the disability tools may arise from a lack of sensitivity due to the recruitment of people typically presenting with milder strokes. There was evidence of ceiling effects in the Barthel Index data from two trials (Bateman 2001; Duncan 1998). Similarly, the Functional Independence Instrument, which was assessed in some of the included studies, is known to be prone to ceiling effects, particularly in community‐living patients (Hall 1996). Thirdly, a lack of effect on disability measures despite functional benefits has been reported in trials of exercise for healthy elderly people (Keysor 2001).
It is worth pointing out that a lack of an immediate effect does not necessarily preclude longer‐term benefits. Increased fitness may provide some 'reserve capacity' to cope with the deterioration of function that will occur with increasing age and thus postpone crossing 'thresholds of independence' (Young 2001). Therefore, indicators of pre‐clinical disability (Fried 1996), coupled with long‐term follow‐up, may be a more useful approach for assessing outcomes in trials of fitness training after stroke.
Overall, the benefits after cardiorespiratory and mixed training detected using scale‐based measures of disability may be driven by improvements in mobility rather than being indicative of a change in more 'global' disability status. This would agree with the findings among the secondary outcomes (mobility).
Effect of training on secondary outcome measures
Adverse events
There was no evidence of any serious adverse events arising from training in people who participated in physical fitness training programmes. However, this finding cannot be generalisable to the wider stroke population as only a few trials specifically recorded or reported adverse events. There is a clear need to improve the reporting of adverse events in physical fitness training trials.
Vascular risk factors
Few trials reported vascular risk factors. There was no effect on blood pressure but there was an increase in peak VO2. As well as indicating poor cardiorespiratory fitness, low values of peak VO2 peak are associated with an increased risk of stroke (Kurl 2003) and stroke mortality (Lee 2002). Limited data meant that no conclusions could be drawn. Blood pressure is still rarely reported among trials of fitness training and yet it could be an important, plausible benefit.
Physical fitness
Cardiorespiratory fitness
Cardiorespiratory training, and to a smaller degree mixed training, significantly improved VO2 peak and exercise tolerance during continuous exercise. This improvement may be beneficial because a low VO2 peak is associated with functional limitation in elderly people (Young 2001). In people with stroke the functional benefits are, however, less clear (see for example the contradictory data by Patterson 2007 and Michael 2007).
Gait economy may improve in response to training that contains walking activity. A limited 'fitness reserve' caused by a low VO2 peak coupled with poor walking economy is a common post‐stroke problem (Macko 2001). Therefore, training to improve walking economy and increase the peak may be beneficial for walking performance and exercise tolerance after stroke. Only few, inconsistent data were available for the assessment of gait economy. Data from one individual trial suggested that mixed training may improve gait economy at the end of the training period even though this training effect appeared to disappear at follow‐up (Mead 2007). On the whole, the data were insufficient to draw reliable conclusions on the effect of training on gait economy as well as on the post‐training retention of cardiorespiratory fitness.
Musculoskeletal fitness
The few trials that assessed whether resistance training or mixed training improved muscle strength after stroke show inconsistent results. Most of the trials that showed positive training effects were either methodologically biased or confounded by additional training time.
One individual trial measured explosive lower limb extensor power but showed no immediate or retained effect of mixed training (Mead 2007). Non‐response could be due to a lack of explosive, fast movements during resistance training. In people with stroke, explosive power is associated with function and disability after stroke (Saunders 2008), and in elderly people explosive power output may be more important than strength for function and disability (Puthoff 2007). Interventions to improve explosive power after stroke remain under‐investigated; however, one ongoing trial does include training with fast movements (NCT01573585).
Mobility
All the meta‐analyses of walking performance outcomes are summarised in Table 13 and this shows a clear pattern of findings.
Table 4.
Pooled walking data for cardiorespiratory training, resistance training, and mixed training at the end of the training period and at follow‐up
| End of intervention | End of follow‐up | ||||||
| Intervention | Walking outcome |
Trials (number of participants) |
MD (95% CI) |
Significance level |
Trials (number of participants) |
MD (95% CI) |
Significance level |
|
Cardiorespiratory training |
Maximal gait speed | 14 (631) | 6.71 m/min (2.73 to 10.69) | P value < 0.0006 | 5 (312) | 6.71 m/min (2.40 to 11.02) |
P value = 0.002 |
| Preferred gait speed | 10 (505) | 4.28 m/min (1.71 to 6.84) | P value = 0.001 | 3 (176) | 1.67 m/min (‐3.27 to 6.62) | NS | |
| 6‐Minute Walking Test | 15 (826) | 30.29 metres (16.19 to 44.39) | P value < 0.0001 | 5 (283) | 38.29 metres (7.19 to 69.39) | P value = 0.02 | |
| Resistance training | Maximal gait speed | 4 (104) | 1.92 m/min (‐3.50 to 7.35) | NS | 1 (24) | ‐19.8 m/min (‐95.77 to 56.17) | NS |
| Preferred gait speed | 3 (80) | 2.34 m/min (‐6.77 to 11.45) | NS | ‐ | ‐ | ‐ | |
| 6‐Minute Walking Test | 2 (66) | 3.78 metres (‐68.56 to 76.11) | NS | 1 (24) | 11.0 m/min (‐105.95 to 127.95) | NS | |
|
Mixed training |
Maximal gait speed | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Preferred gait speed | 9 (639) | 4.54 m/min (0.95 to 8.14) | P value = 0.01 | 4 (443) | 1.60 m/min (‐5.62 to 8.82) |
NS | |
| 6‐Minute Walking Test | 7 (561) | 41.60 metres (25.25 to 57.95) | P value < 0.00001 | 3 (365) | 51.62 metres (25.20 to 78.03) | P value = 0.0001 | |
CI: confidence interval m: metre MD: mean difference min: minutes NS: non‐significant
Cardiorespiratory training increased preferred and maximal walking speed and walking capacity (6‐MWT) at the end of the training period (Analysis 1.17; Analysis 1.18; Analysis 1.19). Benefits were retained after follow‐up in both maximum walking speed (Analysis 2.9) and 6‐MWT (Analysis 2.11). Benefits to walking performance also emerge when walking in a community setting outside the research environment (Analysis 1.21). Gait improvements in stroke survivors after cardiorespiratory training may occur due to an increased fitness reserve (arising from an increased VO2 peak or improved gait economy, or both). Cardiorespiratory walking training is, however, also task‐related and repetitive in nature. These elements by themselves may facilitate motor learning and benefit gait performance even in the absence of an obvious improvement in physical fitness parameters. There is evidence that suggests cardiorespiratory training, as well as improving walking speed, may reduce the reliance of stroke survivors on other people to assist with ambulation (Functional Ambulation Categories score; Analysis 1.16).
Mixed training increased preferred walking speed and walking capacity at the end of the training period (Analysis 5.23; Analysis 5.25). Benefits were retained only in the 6‐MWT performance (Analysis 6.14). These findings were based, however, on trials that were heterogeneous and potentially confounded by additional training time. When we looked only at the results of the 'unconfounded' trials, we did not find any significant training effect (Analysis 5.24). Moreover, all trials except Yang 2006 included specific walking training. Therefore, benefits may be explained by the additional walking practice and treatment 'attention'.
Meta‐analyses revealed no significant effects of resistance training on walking outcomes. It is worth noting that most of the resistance training interventions did not incorporate walking as a mode of exercise. Improvements in muscle strength may not necessarily produce functional benefits (Kim 2001), which translate into a better walking performance. The relationships between 'fitness' and 'function' is indeed very complex and may arise from factors such as non‐linear associations (Buchner 1991) or the interaction of 'co‐impairments' such as lack of balance and low muscle strength (Rantanen 2001).
Therefore, on the whole, there is consistent evidence that measures of walking performance improve after both cardiorespiratory training and mixed training but not after resistance training. Although the improvements are clear one could still question whether they are clinically important. For example Fulk 2011 concluded that a clinically important increase in preferred walking speed after stroke would be 10.5 m/minute; this is greater than the upper 95% confidence interval (CI) margin of the effect sizes for preferred walking speed in this review.
Physical function
A variety of measures to assess functional limitations were used in the included trials. A number of balance outcomes were reported, which enabled data to be pooled.
Berg Balance scores did not improve after cardiorespiratory training but there were some hints of benefit among mixed and resistance training (Analysis 7.5). When balance data using other measurement tools were also combined (SMD; Analysis 5.31) a stronger beneficial effect was shown for mixed training. All of the mixed training interventions involved weight bearing and walking and some specifically included balance training; these components of the training could improve balance. However, this overall effect is difficult to attribute to the content of the mixed training because many of the studies were confounded by increased training time. A sensitivity analysis showed the benefit disappeared when confounded studies were excluded. Overall there is some uncertainty over whether balance can be improved.
Other systematic reviews have examined cardiorespiratory training effects (Pang 2013) and trunk training effects (Cabanas‐Valdés 2013; Sorinola 2014) on balance after stroke. Although Sorinola 2014 reported a significant effect of trunk exercise on standing balance (standardised mean difference (SMD) 0.72, 95% CI ‐0.01 to 1.45; P value = 0.05) the balance data as a whole are not convincing and these reviews have different eligibility criteria to the current review.
Health status and quality of life
Only a limited number of trials, with inconsistent results, included relevant quality of life measures. Therefore, few conclusions can be drawn on whether training can improve self perceived health status and quality of life after stroke.
Aidar 2007, a small trial, showed that both the physical functioning and the emotional role functioning of the SF‐36 survey were significantly better after cardiorespiratory training.
Two trials, confounded by additional training time, showed better results on the physical functioning but not the social role functioning of the SF‐36 survey after mixed training. Similarly, three trials demonstrated both immediate and long‐term benefits of mixed training on the 'physical role functioning' of the SF‐36 survey. The scoring of this domain is, however, problematic in people ‐ such as stroke survivors ‐ who are not engaged in employment (Johnson 1999). Furthermore, various elements of the SF‐36 survey are prone to ceiling effects (Hobart 2002).
A small individual trial did not show any significant effect on the physical functioning and mental health components of the SF‐36 health survey after resistance training.
A recent systematic review of exercise after stroke included quality of life outcomes and also concluded there was no consistent effect (Pang 2013).
Mood
Few trials of variable methodological quality were available to assess the effects of training on mood. Results were not consistent amongst trials and few conclusions can be drawn. Data could be pooled from different depression scales in all types of training with small benefits only after cardiorespiratory training follow‐up and at the end of resistance training interventions. There is no consistent pattern and the pool of studies are at risk of bias due to attrition and confounding due to imbalanced exposures.
A recent systematic review of exercise for depressive symptoms after stroke combined data from 13 studies, with a total of 1022 participants, and showed a small effect at the end of exercise (SMD ‐0.13, 95% CI ‐0.26 to 0.01; P value = 0.03) but not follow‐up (Eng 2014). These findings may differ to the current review since Eng 2014 pooled different exercise types, only 5/13 randomised controlled trials (RCTs) meet our eligibility criteria, and we made a methodological decision not to pool the data from Lennon 2008 in our meta‐analyses (as this would involve estimating mean and standard deviation from median and range).
In both reviews small benefits could be exaggerated due to confounded exposure. Also, any lack of effect could arise from the fact that the symptoms of depression are relatively mild; this could be due to confounding by antidepressant medications, which were not reported.
Cognitive function
There are only three included trials that have examined the effect of fitness training interventions on cognitive function; currently no conclusions can be drawn. There are two other systematic reviews that have examined the effects of exercise on cognitive function (Cumming 2012; Garcia‐Soto 2013).
Cumming 2012 showed that physical activity and exercise interventions produced significant improvements in cognitive function (SMD 0.20, 95% CI 0.04 to 0.36; P value = 0.015; nine trials; 716 participants) at the end of the intervention. Although 6/13 of the studies involved exercise interventions only 3/13 included studies meet our inclusion criteria (Bateman 2001; Duncan 2003 (cited as Studentski); Mead 2007).
Garcia‐Soto 2013 reviewed the effects of cardiorespiratory and resistance training interventions on cognitive function after stroke. None of the five included studies met our criteria for inclusion.
Inclusion of cognitive function outcomes is a protocol change in this review update. This change is justified because there is a rationale as to why cognitive function may be improved by fitness training (Heyn 2004), and interventions to improve cognitive function have recently emerged as the highest rated research priority with regard to life after stroke (Pollock 2012); there is a clear and important knowledge gap here.
Factors influencing primary and secondary outcome measures
Performing subgroup analyses is problematic when the number of trials is small; the consequences are reduced power and the influence of characteristics unrelated to the grouping factors.
Dose of training
All the training interventions occurred regularly and were progressive in nature. The interventions differed in the dose of training, quantified in terms of 1) overall volume of training time, and 2) the intensity of the exercise used.
The ACSM 1998 criteria were used to define an effective overall 'dose' of fitness training as defined by the parameters of intensity, duration, and frequency. Some study interventions may have provided a sufficient dose of training but failure to record or report intensity meant they could not be assigned to a category. Conversely, interventions meeting the criteria may have provided a low dose of training because they were of short duration (for example Kwakkel 2004).
Underestimation of benefits may arise if interventions are poorly attended or complied with. Full attendance was found in few included trials, where interventions occurred partly or completely during inpatient care, were home‐based, or were of very short duration (four weeks).
Overestimation of benefits may arise in trials where the intervention group is potentially confounded by increased training time compared with the control group. In these trials with no attention control additional benefits could arise from non‐specific effects of therapist input, psychosocial effects of contact with other participants and factors such as travel to and from a training location that could amount to a substantial dose of physical activity from which a real training effect could arise.
A further exaggeration of this simple 'dose' effect in confounded trials would also be expected for trials with a long duration or large volumes of training, or both. In most confounded trials the total volume of training was 20 hours or more, whilst only few unconfounded trials exceeded 20 hours of training. Published meta‐analyses have shown that augmented stroke rehabilitation may result in improvements in activities of daily living (Kwakkel 2004). This source of confounding may influence the outcome in trials of physical fitness training. For example, in a number of instances when we excluded confounded trials in sensitivity analyses, the effect sizes became smaller. The data from Richards 1993 supported these observations, showing that longer gait training was associated with improved mobility outcomes (this may also be indicative of a dose‐response effect).
Exercise programme intensity is one of the most important fitness training variables. Pohl 2002 demonstrated that higher intensity walking increased maximal walking speed compared with lower intensity walking. However, the training programme in Pohl 2002 was also the most rapidly progressing, so it is somewhat difficult to disentangle the effect derived from an increase in progression from the effect due to the intensity of the intervention.
The findings of this review indicate that stroke survivors may successfully complete a variety of short‐term training interventions. However, the optimal dose of training for people with stroke has yet to be established.
Type of training
None of the included trials directly compared cardiorespiratory, resistance, and mixed training. We were able to compare the effects of the different types of training on gait speed. Walking speed increased significantly after cardiorespiratory training and mixed training, but not after resistance training. Both cardiorespiratory interventions and mixed interventions comprised specific gait‐related training, which resulted in positive training effects.
Overall, the findings of this review show that benefits reflect the concept of the specificity of the training response. In particular, cardiorespiratory fitness (VO2 peak) improved after cardiorespiratory training; muscle strength improved after resistance training; walking performance improved after training interventions based on walking or walking‐like modes of exercise; walking did not improve after resistance training interventions, probably because functionally relevant movements are difficult to incorporate into resistance training interventions.
Timing of training
All our meta‐analyses were divided into 'during usual care' and 'after usual care' subgroups. However, this still does not have much value for a subgroup analysis since there are generally too few trials and too many other influential confounding factors. For instance, trial design tends to differ among these groups, interventions tend to be longer after usual care, etc.
Retention of benefits
Functional advantages observed at the end of rehabilitation interventions are known to be transient, disappearing at a later stage (Kwakkel 1999; Kwakkel 2002). This is probably due to continued improvements in the control group rather than deterioration in function (Langhorne 2002). Fitness improvements observed at the end of training interventions are also known to deteriorate.
Few trials included in this review assessed possible retention of benefits over time. Those that did were at increased risk of attrition bias. Most of the functional improvements observed at the end of the training period were not sustained at later assessments. We found, however, that cardiorespiratory and mixed training effects on some measures of walking performance were retained at the end of the follow‐up period. This retention effect could have arisen from an increase in habitual levels of physical activity (including walking) facilitated by participation in a training intervention. The extent to which short‐term fitness training influences longer‐term habitual physical activity after stroke is still unknown. Currently, there are no data examining either long‐term fitness training interventions or interventions to facilitate continued exercise after the training intervention is completed. Long‐term assessments should be incorporated into future trials of physical fitness training.
Effect of physical activity performed by control groups
Training effects arising from physical activity in the control group could partly explain the lack of effect observed in some of the included trials.
Effect of risk of bias
There are insufficient data to reliably examine the effects of risk of bias on estimates of effect. Overall, the methodological quality of most of the 58 included trials was limited. Only 4/58 trials enrolled more than 100 participants. Only 24/58 trials reported adequate methods of sequence generation and 25/58 trials had blinded outcome assessors (but some degree of unmasking occurred in three of these trials). The rate of attendance could only be determined in half of the included trials.
Summary of review findings
Most available data relate to ambulatory people in the chronic phase (more than one month) post‐stroke.
It is feasible for stroke survivors to participate in a variety of short‐term fitness training regimens presented in a range of settings, either during usual stroke care or after hospital discharge.
There were insufficient data to assess death and dependence outcomes reliably.
From the limited data reported in the included trials, there is an indication that participation in fitness training programmes is safe and does not result in serious adverse events.
There is some evidence that global indices of disability are reduced after training; this may be mediated largely by mobility improvements.
There is some evidence that cardiorespiratory training may improve cardiorespiratory fitness.
There is clear evidence that cardiorespiratory training improves measures of walking performance (e.g. walking speed and walking capacity) and reduces dependence on others for ambulation during usual care. Some training effects were retained at follow‐up.
There is some evidence that mixed training may improve measures of walking performance. Some training effects were retained at follow‐up.
There are insufficient data to assess reliably the effects of resistance training.
There is an indication that the training effect may be greater when fitness training is specific or 'task‐related'.
There is some evidence that balance improves after fitness training; the benefits are within mixed and resistance training.
There are few data relating to quality of life, mood, or cognitive function outcomes.
There are insufficient data to conduct meaningful subgroup analyses to explore the effects of the type, 'dose', and timing of training on outcome measures.
Limited methodological quality of included trials and relatively small sample sizes hamper the generalisability of findings.
The main findings are presented are presented in 'Summary of findings' tables for cardiorespiratory training (Table 1) and for mixed training (Table 2).
Issues for research
Control groups
In terms of trial design, there should be a concerted effort to balance total contact time across all arms in order to avoid confounded results. Preferably, the control intervention should be a non‐exercise intervention to avoid training effects. In reality this may be difficult to achieve since even performing activities of daily living may be sufficient to cause training effects in elderly people (Young 2001). However, a comparison of two different doses of training would be a robust way of clarifying whether the content of the training itself is beneficial.
Interventions
Currently there are few well‐controlled trials examining interventions to improve muscle force production. Trials of resistance training often focus on pre‐specified movements that bear little resemblance to those relevant to everyday life and, even though muscle strength may improve, no functional benefits arise. The nature of the association between physical fitness and functional benefits is complex, and this suggests that training interventions should also address other co‐impairments such as balance.
Outcome measures
To measure disability and dependence in stroke is problematic. A variety of disability and assessment scales are usually reported in trials of physical rehabilitation and fitness training. These scales do not always assess the same functional domain and therefore pose the problem of the validity and reliability of combining their results in a meta‐analysis. Furthermore, some of these scales are not validated in stroke survivors and, therefore, may lack specificity. Rating scales are also prone to a 'ceiling effect' and to skewed distributions. It would be useful if only well‐known, validated scales are used in future trials for the assessment of participants' functional performance and if trial investigators would clearly address the problems related to the use of these assessment scales.
Stroke survivors who are eligible for fitness training have typically mild levels of disability. Mild impairments may be difficult to assess and many of the existing disability scales may fail to detect them. However, functional decline over time that is simply due to increasing age and inactivity could mean that mild disability may progress quickly to more serious levels. Therefore, it would be useful to assess long‐term outcomes in mild stroke survivors using pre‐clinical disability measures (for example Fried 1996).
A priority setting exercise undertaken in 2012 identified the top 10 research priorities for life after stroke (Pollock 2012). Among the priorities identified by stroke patients and carers are a substantial proportion of areas for which fitness training could be beneficial: 1) cognitive function; 2) upper limb function; 3) mobility, balance and gait; and 4) the role of exercise in physical function, quality of life, and secondary stroke prevention (Saunders 2014a). Apart from mobility there is little evidence relating to the other areas important to patients. In particular cognitive function lacks investigation despite being the ranked the most important research priority.
Long‐term studies
Both improvements in physical fitness after training and improvements in physical function after rehabilitation are transient. Since physical fitness may be linked to functional status, the long‐term retention of benefit should be routinely examined in trials of fitness training. Fitness and function parameters are known to deteriorate with physical inactivity and to decrease with increasing age. Therefore, it is plausible that short‐term effects of training only emerge as being beneficial after a period of functional decline.
There is a need to examine strategies aimed at promoting physical activity and maintaining physical fitness in the long term after stroke.
In conclusion, there is a clear need for larger well‐designed trials of physical fitness training. Future trials should include participants with a greater spectrum of stroke severity that includes non‐ambulatory patients, have adequate control interventions, and use relevant outcome measures.
Authors' conclusions
Cardiorespiratory training and mixed training during or after usual stroke care is effective in increasing walking speed and walking capacity in stroke survivors. It is likely that improvements in fitness, mobility, and physical function outcomes are associated with 'task‐related' training. Guidance and services for exercise after stroke are developing worldwide, including:
UK (Exercise and Fitness Training after Stroke Instructor course; www.laterlifetraining.co.uk/courses/exercise‐for‐stroke‐instructor/);
Australia (http://heartmoves.heartfoundation.org.au);
Canada (Canadian Best Practice Recommendations for Stroke Care; Dawson 2013);
USA (APA Physical Activity and Exercise Recommendations for Stroke Survivors; Billinger 2014).
These initiatives are based on existing evidence about the benefits of exercise after stroke and the needs of stroke survivors to have ongoing access to rehabilitation after discharge from hospital. The findings of this review will inform the content of such services.
Larger, well‐designed clinical trials are needed to assess the effects of physical fitness training after stroke and to determine the optimal regimen for improving fitness.
Future trials should:
comply with the current CONSORT guidelines for reporting of randomised clinical trials (CONSORT 2010);
report exercise and control interventions more clearly; intervention reporting guidelines do exist (TIDiER; Hoffmann 2014), and new exercise‐specific guidance is emerging (CERT Consensus Exercise Reporting Template; Slade 2014);
include a broader population of stroke survivors (including non‐ambulatory stroke survivors) to allow stratification by gender, level of impairment, and functional ability;
assess the effects of physical fitness training in people with specific post‐stroke problems, for example people with depression or post‐stroke fatigue;
be of longer duration (12 weeks or longer);
have a long‐term follow‐up.
The training intervention and the control intervention should be comparable in terms of duration to prevent overestimation of training effects. The content of an attention control intervention should be chosen carefully to prevent underestimation of treatment effects caused by confounded physical activity in the control group.
Implications for future updates
The literature on physical fitness training interventions is constantly growing. Complex reviews such as this do attract suggestions to 'split' findings in some way. However, for ease of updating and to allow direct comparison of a range of different fitness interventions the current architecture should remain. It may be desirable to revise some of the inclusion criteria to allow more potentially relevant comparisons to be assessed especially where these are not covered by existing Cochrane Reviews.
Acknowledgements
We thank the Cochrane Stroke Group editorial team for their assistance in preparing the protocol. We are grateful to Brenda Thomas for her assistance in developing the search strategies. We would also thank all investigators who provided further information about existing trials.
We would also like to thank the consumer reviewers Julie Gildie and Sandra Paget for their perspectives on this work; their input relating to the clarity and accessibility of this information is invaluable.
We would be grateful if people who are aware of trials potentially relevant for this review could contact David Saunders.
Appendices
Appendix 1. CENTRAL search strategy (The Cochrane Library)
#1 [mh ^"cerebrovascular disorders"] or [mh "basal ganglia cerebrovascular disease"] or [mh "brain ischemia"] or [mh "carotid artery diseases"] or [mh "intracranial arterial diseases"] or [mh "intracranial arteriovenous malformations"] or [mh "intracranial embolism and thrombosis"] or [mh "intracranial hemorrhages"] or [mh ^stroke] or [mh "brain infarction"] or [mh ^"stroke, lacunar"] or [mh ^"vasospasm, intracranial"] or [mh ^"vertebral artery dissection"] or [mh ^"brain injuries"] or [mh ^"brain injury, chronic"]
#2 (stroke or poststroke or post‐stroke or cerebrovasc* or brain next vasc* or cerebral next vasc* or cva* or apoplex* or SAH):ti,ab
#3 ((brain* or cerebr* or cerebell* or intracran* or intracerebral) near/5 (isch*emi* or infarct* or thrombo* or emboli* or occlus*)):ti,ab
#4 ((brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) near/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)):ti,ab
#5 [mh ^hemiplegia] or [mh paresis]
#6 (hempar* or hemipleg* or brain next injur*):ti,ab
#7 [mh ^"Gait Disorders, Neurologic"]
#8 #1 or #2 or #3 or #4 or #5 or #6 or #7
#9 [mh ^exercise] or [mh ^"circuit‐based exercise"] or [mh ^"cool‐down exercise"] or [mh ^"physical conditioning, human"] or [mh ^"plyometric exercise"] or [mh running] or [mh ^swimming] or [mh ^walking] or [mh ^"warm‐up exercise"]
#10 [mh ^"exercise test"]
#11 [mh ^"physical exertion"]
#12 [mh ^"exercise therapy"]
#13 [mh ^"physical fitness"]
#14 [mh ^"muscle stretching exercises"] or [mh ^"resistance training"]
#15 [mh ^"isometric contraction"]
#16 [mh ^"isotonic contraction"]
#17 [mh sports]
#18 [mh "physical endurance"]
#19 [mh ^locomotion]
#20 [mh ^"early ambulation"]
#21 [mh ^"sports equipment"]
#22 [mh ^"tai ji"] or [mh ^yoga] or [mh ^"dance therapy"]
#23 [mh ^"exercise movement techniques"]
#24 [mh ^"fitness centers"]
#25 [mh ^"leisure activities"]
#26 [mh ^recreation]
#27 (physical near/3 (exercise* or exertion or endurance or therap* or conditioning or activit* or fitness)):ti,ab
#28 (exercise near/3 (train* or intervention* or protocol* or program* or therap* or activit* or regim*)):ti,ab
#29 (fitness near/3 (train* or intervention* or protocol* or program* or therap* or activit* or regim* or centre* or center*)):ti,ab
#30 ((training or conditioning) near/3 (intervention* or protocol* or program* or activit* or regim*)):ti,ab
#31 (sport* or recreation* or leisure or cycling or bicycl* or rowing or treadmill* or running or "circuit training" or swim* or walk* or dance* or dancing or "tai ji" or "tai chi" or yoga):ti,ab
#32 ((endurance or aerobic or cardio*) near/3 (fitness or train* or intervention* or protocol* or program* or therap* or activit* or regim*)):ti,ab
#33 ("muscle strengthening" or progressive next resist*):ti,ab
#34 ((weight or strength* or resistance) next (train* or lift* or exercise*)):ti,ab
#35 ((isometric or isotonic or eccentric or concentric) next (action* or contraction* or exercise*)):ti,ab
#36 #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35
#37 #8 and #36
Appendix 2. MEDLINE (Ovid) search strategy
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp intracranial arteriovenous malformations/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or stroke, lacunar/ or vasospasm, intracranial/ or vertebral artery dissection/ or brain injuries/ or brain injury, chronic/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. hemiplegia/ or exp paresis/
6. (hempar$ or hemipleg$ or brain injur$).tw.
7. Gait Disorders, Neurologic/
8. or/1‐7
9. exercise/ or circuit‐based exercise/ or cool‐down exercise/ or physical conditioning, human/ or plyometric exercise/ or exp running/ or swimming/ or walking/ or warm‐up exercise/
10. exercise test/
11. physical exertion/
12. exercise therapy/
13. physical fitness/
14. muscle stretching exercises/ or resistance training/
15. isometric contraction/
16. isotonic contraction/
17. exp sports/
18. exp physical endurance/
19. locomotion/
20. early ambulation/
21. sports equipment/
22. tai ji/ or yoga/ or dance therapy/
23. exercise movement techniques/
24. fitness centers/
25. leisure activities/
26. recreation/
27. (physical adj3 (exercise$ or exertion or endurance or therap$ or conditioning or activit$ or fitness)).tw.
28. (exercise adj3 (train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$)).tw.
29. (fitness adj3 (train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$ or centre$ or center$)).tw.
30. ((training or conditioning) adj3 (intervention$ or protocol$ or program$ or activit$ or regim$)).tw.
31. (sport$ or recreation$ or leisure or cycling or bicycl$ or rowing or treadmill$ or running or circuit training or swim$ or walk$ or dance$ or dancing or tai ji or tai chi or yoga).tw.
32. ((endurance or aerobic or cardio$) adj3 (fitness or train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$)).tw.
33. (muscle strengthening or progressive resist$).tw.
34. ((weight or strength$ or resistance) adj (train$ or lift$ or exercise$)).tw.
35. ((isometric or isotonic or eccentric or concentric) adj (action$ or contraction$ or exercise$)).tw.
36. or/9‐35
37. Randomized Controlled Trials as Topic/
38. random allocation/
39. Controlled Clinical Trials as Topic/
40. control groups/
41. clinical trials as topic/
42. double‐blind method/
43. single‐blind method/
44. Placebos/
45. placebo effect/
46. cross‐over studies/
47. randomized controlled trial.pt.
48. controlled clinical trial.pt.
49. clinical trial.pt.
50. (random$ or RCT or RCTs).tw.
51. (controlled adj5 (trial$ or stud$)).tw.
52. (clinical$ adj5 trial$).tw.
53. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
54. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
55. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
56. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
57. (cross‐over or cross over or crossover).tw.
58. (placebo$ or sham).tw.
59. trial.ti.
60. (assign$ or allocat$).tw.
61. controls.tw.
62. or/37‐61
63. 8 and 36 and 62
64. exp animals/ not humans.sh.
65. 63 not 64
Appendix 3. EMBASE (Ovid) search strategy
1. cerebrovascular disease/ or exp basal ganglion hemorrhage/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or cerebral artery disease/ or exp cerebrovascular accident/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/
2. stroke patient/ or stroke unit/
3. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
5. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
6. brain injury/ or acquired brain injury/
7. hemiparesis/ or hemiplegia/ or paresis/ or neurologic gait disorder/ or hemiplegic gait/
8. (hempar$ or hemipleg$ or brain injur$).tw.
9. or/1‐8
10. exercise/ or aerobic exercise/ or aquatic exercise/ or arm exercise/ or athletic performance/ or dynamic exercise/ or exercise intensity/ or isokinetic exercise/ or exp muscle exercise/ or pilates/ or static exercise/ or resistance training/ or plyometrics/
11. exercise test/
12. kinesiotherapy/ or isometric exercise/ or movement therapy/ or muscle training/ or neuromuscular facilitation/ or stretching exercise/ or tai chi/ or yoga/
13. muscle strength/
14. muscle contraction/ or muscle isometric contraction/ or muscle isotonic contraction/
15. mobilization/
16. locomotion/ or swimming/ or walking/ or dancing/
17. physical activity/ or jumping/ or lifting effort/ or stretching/ or weight lifting/
18. fitness/ or exp training/ or endurance/ or endurance training/
19. exp sport/ or recreation/ or leisure/
20. (physical adj3 (exercise$ or exertion or endurance or therap$ or conditioning or activit$ or fitness)).tw.
21. (exercise adj3 (train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$)).tw.
22. (fitness adj3 (train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$ or centre$ or center$)).tw.
23. ((training or conditioning) adj3 (intervention$ or protocol$ or program$ or activit$ or regim$)).tw.
24. (sport$ or recreation$ or leisure or cycling or bicycl$ or rowing or treadmill$ or running or circuit training or swim$ or walk$ or dance$ or dancing or tai ji or tai chi or yoga).tw.
25. ((endurance or aerobic or cardio$) adj3 (fitness or train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$)).tw.
26. (muscle strengthening or progressive resist$).tw.
27. ((weight or strength$ or resistance) adj (train$ or lift$ or exercise$)).tw.
28. ((isometric or isotonic or eccentric or concentric) adj (action$ or contraction$ or exercise$)).tw.
29. or/10‐28
30. Randomized Controlled Trial/ or "randomized controlled trial (topic)"/
31. Randomization/
32. Controlled clinical trial/ or "controlled clinical trial (topic)"/
33. control group/ or controlled study/
34. clinical trial/ or "clinical trial (topic)"/
35. Crossover Procedure/
36. Double Blind Procedure/
37. Single Blind Procedure/ or triple blind procedure/
38. placebo/ or placebo effect/
39. (random$ or RCT or RCTs).tw.
40. (controlled adj5 (trial$ or stud$)).tw.
41. (clinical$ adj5 trial$).tw.
42. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
43. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
44. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
45. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
46. (cross‐over or cross over or crossover).tw.
47. (placebo$ or sham).tw.
48. trial.ti.
49. (assign$ or allocat$).tw.
50. controls.tw.
51. or/30‐50
52. 9 and 29 and 51
53. (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/)
54. 52 not 53
Appendix 4. CINAHL (EBSCO) search strategy
S1. (MH "Cerebrovascular Disorders+") or (MH "stroke patients") or (MH "stroke units")
S2. TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH )
S3. TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral )
S4. TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* )
S5. S3 and S4
S6. TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid )
S7. TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )
S8. S6 and S7
S9. (MH "Hemiplegia")
S10. TI ( hemipleg* or hemipar* or paresis or paretic ) or AB ( hemipleg* or hemipar* or paresis or paretic )
S11. (MH "Gait Disorders, Neurologic+")
S12. S1 or S2 or S5 or S8 or S9 or S10 or S11
S13. (MH "Exercise+")
S14. (MH "Exercise Test+") or (MH "Exercise Test, Cardiopulmonary") or (MH "Exercise Test, Muscular+")
S15. (MH "Exertion+")
S16. (MH "Therapeutic Exercise+")
S17. (MH "Physical Fitness+")
S18. (MH "Physical Endurance+") or (MH "Endurance Sports") or (MH "Endurance (Iowa NOC)")
S19. (MH "Stretching")
S20. (MH "Muscle Strengthening+") or (MH "Athletic Training+") or (MH "Athletic Training Programs")
S21. (MH "Isometric Contraction") or (MH "Isotonic Contraction")
S22. (MH "Sports+")
S23. (MH "Locomotion+")
S24. (MH "Ambulation Therapy (Saba CCC)") or (MH "Early Ambulation") or (MH "Exercise Therapy: Ambulation (Iowa NIC)") or (MH "Ambulation: Walking (Iowa NOC)") or (MH "Walking+")
S25. (MH "Sports Equipment and Supplies+")
S26. (MH "Yoga")
S27. (MH "Dancing+") or (MH "Aerobic Dancing") or (MH "Dance Therapy")
S28. (MH "Tai Chi")
S29. (MH "Fitness Centers")
S30. (MH "Leisure Activities+")
S31. (MH "Recreation+") or (MH "Recreational Therapists") or (MH "Recreational Therapy") or (MH "Recreation Therapy (Iowa NIC)")
S32. (MH "Treadmills")
S33. TI physical or AB physical
S34. TI ( exercise* or exertion or endurance or therap* or conditioning or activit* or fitness ) or AB ( exercise* or exertion or endurance or therap* or conditioning or activit* or fitness )
S35. S33 and S34
S36. TI exercise or AB exercise
S37. TI (train* or intervention* or protocol* or program* or therap* or activit* or regim*) or AB (train* or intervention* or protocol* or program* or therap* or activit* or regim*)
S38. S36 and S37
S39. TI fitness or AB fitness
S40. TI (train* or intervention* or protocol* or program* or therap* or activit* or regim* or centre* or center*) or AB (train* or intervention* or protocol* or program* or therap* or activit* or regim* or centre* or center*)
S41. S39 and S40
S42. TI (training or conditioning) or AB (training or conditioning)
S43. TI (intervention* or protocol* or program* or activit* or regim*) or AB (intervention* or protocol* or program* or activit* or regim*)
S44. S42 and S43
S45. TI (sport* or recreation* or leisure or cycling or bicycl* or rowing or treadmill* or running or circuit training or swim* or walk* or dance* or dancing or tai ji or tai chi or yoga) or AB (sport* or recreation* or leisure or cycling or bicycl* or rowing or treadmill* or running or circuit training or swim* or walk* or dance* or dancing or tai ji or tai chi or yoga)
S46. TI (endurance or aerobic or cardio*) or AB (endurance or aerobic or cardio*)
S47. TI (fitness or train* or intervention* or protocol* or program* or therap* or activit* or regim*) or AB (fitness or train* or intervention* or protocol* or program* or therap* or activit* or regim*)
S48. S46 and S47
S49. TI (muscle strengthening or progressive resist*) or AB (muscle strengthening or progressive resist*)
S50. TI (weight or strength* or resistance) or AB (weight or strength* or resistance)
S51. TI (train* or lift* or exercise*) or AB (train* or lift* or exercise*)
S52. S50 and S51
S53. TI (isometric or isotonic or eccentric or concentric) or AB (isometric or isotonic or eccentric or concentric)
S54. TI (action* or contraction* or exercise*) or AB (action* or contraction* or exercise*)
S55. S53 and S54
S56. S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S35 or S38 or S41 or S44 or S45 or S48 or S49 or S52 or S55
S57. S12 and S56
S58. (MH "Randomized Controlled Trials") or (MH "Random Assignment") or (MH "Random Sample+")
S59. (MH "Clinical Trials") or (MH "Intervention Trials") or (MH "Therapeutic Trials")
S60. (MH "Double‐Blind Studies") or (MH "Single‐Blind Studies") or (MH "Triple‐Blind Studies")
S61. (MH "Control (Research)") or (MH "Control Group") or (MH "Placebos") or (MH "Placebo Effect")
S62. (MH "Crossover Design") OR (MH "Quasi‐Experimental Studies")
S63. PT (clinical trial or randomized controlled trial)
S64. TI (random* or RCT or RCTs) or AB (random* or RCT or RCTs)
S65. TI (controlled N5 (trial* or stud*)) or AB (controlled N5 (trial* or stud*))
S66. TI (clinical* N5 trial*) or AB (clinical* N5 trial*)
S67. TI ((control or treatment or experiment* or intervention) N5 (group* or subject* or patient*)) or AB ((control or treatment or experiment* or intervention) N5 (group* or subject* or patient*))
S68. TI ((control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*)) or AB ((control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*))
S69. TI ((singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*)) or AB ((singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*))
S70. TI (cross‐over or cross over or crossover) or AB (cross‐over or cross over or crossover)
S71. TI (placebo* or sham) or AB (placebo* or sham)
S72. TI trial
S73. TI (assign* or allocat*) or AB (assign* or allocat*)
S74. TI controls or AB controls
S75. TI (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*) or AB (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*)
S76. S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 OR S65 OR S66 OR S67 OR S68 OR S69 OR S70 OR S71 OR S72 OR S73 OR S74 OR S75
S77. S57 AND S76
Appendix 5. SPORTDiscus (EBSCO) search strategy
S16. (S7 and S15) S15. S8 or S9 or S10 or S11 or S12 or S13 or S14 S14. SU ( random* or trial or crossover or cross‐over or placebo* or control* or factorial or sham or counterbalance* or multiple baseline* or ABAB design or meta analysis* or metaanlaysis or meta‐anlaysis or systematic review* ) or KW ( random* or trial or crossover or cross‐over or placebo* or control* or factorial or sham or counterbalance* or multiple baseline* or ABAB design or meta analysis* or metaanlaysis or meta‐anlaysis or systematic review* ) S13. TI ( meta analysis* or metaanlaysis or meta‐anlaysis or systematic review* ) or AB ( meta analysis* or metaanlaysis or meta‐anlaysis or systematic review* ) S12. TI ( counterbalance* or multiple baseline* or ABAB design ) or AB ( counterbalance* or multiple baseline* or ABAB design ) S11. ( TI ( clin* or intervention* or compar* or experiment* or preventive or therapeutic ) or AB ( clin* or intervention* or compar* or experiment* or preventive or therapeutic ) ) and ( TI trial* or AB trial* ) S10. TI ( crossover or cross‐over or placebo* or control* or factorial or sham ) or AB ( crossover or cross‐over or placebo* or control* or factorial or sham ) S9. ( TI ( singl* or doubl* or tripl* or trebl* ) or AB ( singl* or doubl* or tripl* or trebl* ) ) and ( TI ( blind* or mask*) or AB ( blind* or mask* ) ) S8. TI random* or AB random* S7. S1 or S2 or S3 or S4 or S5 or S6 S6. TI ( hemipleg* or hemipar* or paresis or paretic ) or AB ( hemipleg* or hemipar* or paresis or paretic ) S5. DE "HEMIPLEGIA" S4. ( TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) ) and ( TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) ) S3. ( TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) ) and ( TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) ) S2. TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) S1. DE "CEREBROVASCULAR disease" or DE "BRAIN Hemorrhage" or DE "CEREBRAL embolism & thrombosis"
Data and analyses
Comparison 1.
Cardiorespiratory training versus control ‐ end of intervention
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Case fatality | 28 | 1437 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.14, 7.33] |
| 1.1 During usual care | 13 | 575 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 After usual care | 15 | 862 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.14, 7.33] |
| 2 Disability ‐ Functional Independence Measure | 3 | 162 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.10, 0.52] |
| 2.1 During usual care | 1 | 52 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.32, 0.78] |
| 2.2 After usual care | 2 | 110 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.29, 0.63] |
| 3 Disability ‐ Barthel Index | 3 | 243 | Mean Difference (IV, Random, 95% CI) | 6.65 [‐0.67, 13.98] |
| 3.1 During usual care | 2 | 115 | Mean Difference (IV, Random, 95% CI) | 10.48 [‐11.83, 32.80] |
| 3.2 After usual care | 1 | 128 | Mean Difference (IV, Random, 95% CI) | 2.60 [‐0.15, 5.35] |
| 4 Disability ‐ Rivermead Mobility Index (scale 0 to 15) | 3 | 146 | Mean Difference (IV, Random, 95% CI) | 1.56 [0.20, 2.92] |
| 4.1 During usual care | 2 | 110 | Mean Difference (IV, Random, 95% CI) | 1.43 [‐0.62, 3.49] |
| 4.2 After usual care | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 2.0 [0.53, 3.47] |
| 5 Disability ‐ Physical Activity and Disability Scale | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 16.9 [‐15.15, 48.95] |
| 5.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 After usual care | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 16.9 [‐15.15, 48.95] |
| 6 Disability ‐ Older Americans Resources and Services Questionnaire (IADL dimension) | 1 | 128 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.37, 1.57] |
| 6.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 After usual care | 1 | 128 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.37, 1.57] |
| 7 Disability ‐ combined disability scales | 8 | 462 | Std. Mean Difference (IV, Random, 95% CI) | 0.52 [0.19, 0.84] |
| 7.1 During usual care | 3 | 130 | Std. Mean Difference (IV, Random, 95% CI) | 0.88 [0.08, 1.68] |
| 7.2 After usual care | 5 | 332 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [0.11, 0.55] |
| 8 Risk factors ‐ blood pressure, systolic | 5 | 318 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐4.00, 5.60] |
| 8.1 During usual care | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 26.33 [1.95, 50.71] |
| 8.2 After usual care | 4 | 306 | Mean Difference (IV, Random, 95% CI) | ‐1.41 [‐5.25, 2.43] |
| 9 Risk factors ‐ blood pressure, diastolic | 5 | 318 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐2.28, 1.98] |
| 9.1 During usual care | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐10.46, 12.46] |
| 9.2 After usual care | 4 | 306 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐2.35, 1.98] |
| 10 Risk factors ‐ body mass index (BMI) | 2 | 174 | Mean Difference (IV, Random, 95% CI) | 1.19 [‐0.38, 2.76] |
| 10.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 After usual care | 2 | 174 | Mean Difference (IV, Random, 95% CI) | 1.19 [‐0.38, 2.76] |
| 11 Risk factors ‐ abnormal glucose tolerance | 1 | 45 | Odds Ratio (M‐H, Random, 95% CI) | 0.08 [0.02, 0.33] |
| 11.1 During usual care | 1 | 45 | Odds Ratio (M‐H, Random, 95% CI) | 0.08 [0.02, 0.33] |
| 11.2 After usual care | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Risk factors ‐ total triglycerides | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.48, ‐0.30] |
| 12.1 During usual care | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.48, ‐0.30] |
| 12.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Physical fitness ‐ peak VO2 (ml/kg/min) | 9 | 425 | Mean Difference (IV, Random, 95% CI) | 2.86 [1.76, 3.96] |
| 13.1 During usual care | 2 | 62 | Mean Difference (IV, Random, 95% CI) | 3.04 [1.26, 4.83] |
| 13.2 After usual care | 7 | 363 | Mean Difference (IV, Random, 95% CI) | 2.84 [1.39, 4.29] |
| 14 Physical fitness ‐ gait economy, VO2 (ml/kg/metre) | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.28, 0.12] |
| 14.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 After usual care | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.28, 0.12] |
| 15 Physical fitness ‐ maximum cycling work rate (Watts) | 4 | 221 | Std. Mean Difference (IV, Random, 95% CI) | 0.60 [0.18, 1.02] |
| 15.1 During usual care | 2 | 89 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.34, 0.98] |
| 15.2 After usual care | 2 | 132 | Std. Mean Difference (IV, Random, 95% CI) | 0.83 [0.47, 1.18] |
| 16 Mobility ‐ functional ambulation categories | 2 | 73 | Mean Difference (IV, Random, 95% CI) | 0.53 [0.21, 0.85] |
| 16.1 During usual care | 2 | 73 | Mean Difference (IV, Random, 95% CI) | 0.53 [0.21, 0.85] |
| 16.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Mobility ‐ maximal gait speed (m/min over 5 to 10 metres) | 14 | 631 | Mean Difference (IV, Random, 95% CI) | 6.71 [2.73, 10.69] |
| 17.1 During usual care | 9 | 324 | Mean Difference (IV, Random, 95% CI) | 5.95 [0.97, 10.94] |
| 17.2 After usual care | 5 | 307 | Mean Difference (IV, Random, 95% CI) | 8.93 [3.54, 14.33] |
| 18 Mobility ‐ preferred gait speed (m/min) | 10 | 505 | Mean Difference (IV, Random, 95% CI) | 4.28 [1.71, 6.84] |
| 18.1 During usual care | 4 | 128 | Mean Difference (IV, Random, 95% CI) | 3.44 [‐1.64, 8.53] |
| 18.2 After usual care | 6 | 377 | Mean Difference (IV, Random, 95% CI) | 4.69 [1.57, 7.80] |
| 19 Mobility ‐ gait endurance (6‐MWT metres) | 15 | 826 | Mean Difference (IV, Random, 95% CI) | 30.29 [16.19, 44.39] |
| 19.1 During usual care | 7 | 225 | Mean Difference (IV, Random, 95% CI) | 32.10 [10.11, 54.10] |
| 19.2 After usual care | 8 | 601 | Mean Difference (IV, Random, 95% CI) | 30.97 [10.00, 51.94] |
| 20 Mobility ‐ gait endurance (m/min) | 3 | 154 | Mean Difference (IV, Random, 95% CI) | 8.87 [1.35, 16.40] |
| 20.1 During usual care | 2 | 63 | Mean Difference (IV, Random, 95% CI) | 12.24 [‐3.41, 27.89] |
| 20.2 After usual care | 1 | 91 | Mean Difference (IV, Random, 95% CI) | 6.60 [‐2.66, 15.86] |
| 21 Mobility ‐ community walk test (min) | 2 | 47 | Mean Difference (IV, Random, 95% CI) | ‐10.54 [‐14.11, ‐6.98] |
| 21.1 During usual care | 2 | 47 | Mean Difference (IV, Random, 95% CI) | ‐10.54 [‐14.11, ‐6.98] |
| 21.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Mobility ‐ 6 metre walking time (sec) | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐3.32 [‐8.52, 1.88] |
| 22.1 During usual care | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐3.32 [‐8.52, 1.88] |
| 22.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Mobility ‐ Stroke Impact Scale (mobility domain) | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐3.20 [‐17.14, 10.74] |
| 23.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 After usual care | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐3.20 [‐17.14, 10.74] |
| 24 Mobility ‐ walking ability questionnaire (score 0 to 76) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 1.04 [‐6.71, 8.79] |
| 24.1 During usual care | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 1.04 [‐6.71, 8.79] |
| 24.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Mobility ‐ Activities‐Specific Balance Confidence scale (scores 0 to 100) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 10.66 [‐4.66, 25.98] |
| 25.1 During usual care | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 10.66 [‐4.66, 25.98] |
| 25.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Physical function ‐ Timed Up and Go (sec) | 3 | 131 | Mean Difference (IV, Random, 95% CI) | ‐2.52 [‐6.18, 1.15] |
| 26.1 During usual care | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐2.10 [‐6.27, 2.07] |
| 26.2 After usual care | 2 | 111 | Mean Difference (IV, Random, 95% CI) | ‐3.94 [‐11.65, 3.77] |
| 27 Physical function ‐ Functional Reach | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 2.20 [0.09, 4.31] |
| 27.1 During usual care | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 2.20 [0.09, 4.31] |
| 27.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Physical function ‐ Berg Balance Scale (score 0 to 56) | 7 | 435 | Mean Difference (IV, Random, 95% CI) | 1.13 [‐0.44, 2.70] |
| 28.1 During usual care | 3 | 160 | Mean Difference (IV, Random, 95% CI) | 0.79 [‐2.01, 3.59] |
| 28.2 After usual care | 4 | 275 | Mean Difference (IV, Random, 95% CI) | 2.04 [‐0.81, 4.89] |
| 29 Health‐related QoL ‐ SF‐36 Physical functioning | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 10.60 [6.51, 14.69] |
| 29.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 After usual care | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 10.60 [6.51, 14.69] |
| 30 Health‐related QoL ‐ SF‐36 Emotional role functioning | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 11.0 [6.15, 15.85] |
| 30.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.2 After usual care | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 11.0 [6.15, 15.85] |
| 31 Health‐related QoL ‐ SF‐36 Physical Health Component | 1 | 128 | Mean Difference (IV, Random, 95% CI) | 6.60 [2.40, 10.80] |
| 31.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 After usual care | 1 | 128 | Mean Difference (IV, Random, 95% CI) | 6.60 [2.40, 10.80] |
| 32 Health‐related QoL ‐ SF‐36 Mental Health Component | 1 | 128 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐3.51, 5.11] |
| 32.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 After usual care | 1 | 128 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐3.51, 5.11] |
| 33 Health‐related QoL ‐ SF‐12 Mental | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 9.30 [4.31, 14.29] |
| 33.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 After usual care | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 9.30 [4.31, 14.29] |
| 34 Health‐related QoL ‐ SF‐12 physical | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 2.80 [‐1.68, 7.28] |
| 34.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.2 After usual care | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 2.80 [‐1.68, 7.28] |
| 35 Health‐related QoL ‐ EuroQol EQ‐5D | 1 | 102 | Mean Difference (IV, Random, 95% CI) | 2.59 [‐4.47, 9.65] |
| 35.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 After usual care | 1 | 102 | Mean Difference (IV, Random, 95% CI) | 2.59 [‐4.47, 9.65] |
| 36 Mood ‐ Hospital Anxiety and Depression Scale (HADS) ‐ depression score | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐3.21, 0.41] |
| 36.1 During usual care | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐3.21, 0.41] |
| 36.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Mood ‐ Beck Depression Index | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐1.60, 2.80] |
| 37.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.2 After usual care | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐1.60, 2.80] |
| 38 Mood ‐ Hospital Anxiety and Depression Scale (HADS) ‐ anxiety score | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐1.94 [‐3.80, ‐0.08] |
| 38.1 During usual care | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐1.94 [‐3.80, ‐0.08] |
| 38.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39 Mood ‐ combined depression scales | 2 | 80 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.75, 0.38] |
| 39.1 During usual care | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.90, 0.14] |
| 39.2 After usual care | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.65, 1.11] |
| 40 Cognitive function ‐ FIM cognitive score | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 2.17 [‐1.02, 5.36] |
| 40.1 During usual care | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 2.17 [‐1.02, 5.36] |
| 40.2 After usual care | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Analysis 1.24.
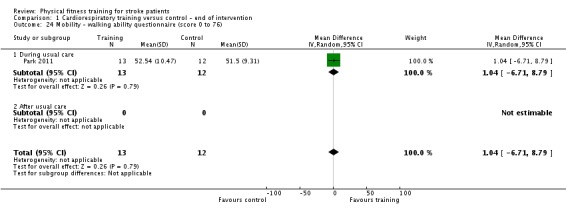
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 24 Mobility ‐ walking ability questionnaire (score 0 to 76).
Analysis 1.25.
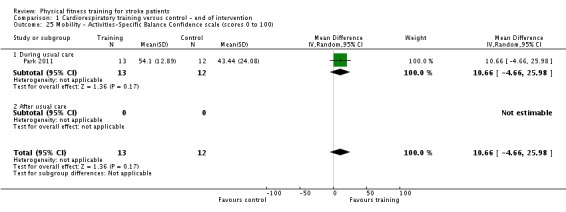
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 25 Mobility ‐ Activities‐Specific Balance Confidence scale (scores 0 to 100).
Analysis 1.27.
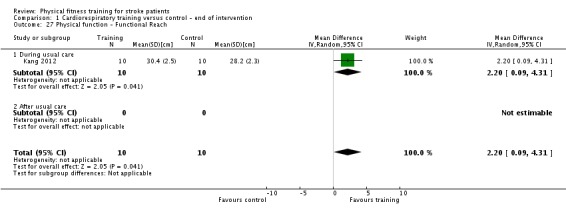
Comparison 1 Cardiorespiratory training versus control ‐ end of intervention, Outcome 27 Physical function ‐ Functional Reach.
Comparison 2.
Cardiorespiratory training versus control ‐ end of retention follow‐up
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Case fatality | 5 | 304 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 16.48] |
| 1.1 During usual care | 3 | 226 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 16.48] |
| 1.2 After usual care | 2 | 78 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Disability ‐ Rivermead Mobility Index | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 During usual care | 1 | 66 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐1.85, 1.35] |
| 2.2 During usual care ‐ ITT analysis using 'last observation carried forward' approach | 1 | 84 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐1.47, 1.55] |
| 2.3 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Disability ‐ Nottingham Extended ADL | 1 | 147 | Mean Difference (IV, Random, 95% CI) | 2.90 [‐2.68, 8.48] |
| 3.1 During usual care | 1 | 64 | Mean Difference (IV, Random, 95% CI) | 2.64 [‐5.57, 10.85] |
| 3.2 During usual care ‐ ITT analysis using 'last observation carried forward' approach | 1 | 83 | Mean Difference (IV, Random, 95% CI) | 3.13 [‐4.48, 10.74] |
| 3.3 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Disability ‐ Physical Activity and Disability Scale | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 19.90 [‐17.58, 57.38] |
| 4.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 After usual care | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 19.90 [‐17.58, 57.38] |
| 5 Disability ‐ Frenchay Activities Index (FAI) | 1 | 79 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐1.55, 3.55] |
| 5.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 After usual care | 1 | 79 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐1.55, 3.55] |
| 6 Disability ‐ combined disability scales | 3 | 220 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.07, 0.46] |
| 6.1 During usual care ‐ ITT analysis using 'last observation carried forward' approach | 1 | 83 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.26, 0.61] |
| 6.2 After usual care | 2 | 137 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.12, 0.55] |
| 7 Physical fitness ‐ maximum cycling work rate (Watts) | 1 | 84 | Mean Difference (IV, Random, 95% CI) | 5.11 [‐18.93, 29.15] |
| 7.1 During usual care | 1 | 84 | Mean Difference (IV, Random, 95% CI) | 5.11 [‐18.93, 29.15] |
| 7.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Physical fitness ‐ peak VO2 (ml/kg/min) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 2.90 [0.56, 5.24] |
| 8.1 During usual care | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 2.90 [0.56, 5.24] |
| 8.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Mobility ‐ maximal gait speed (m/min) | 5 | 312 | Mean Difference (IV, Random, 95% CI) | 6.71 [2.40, 11.02] |
| 9.1 During usual care | 3 | 152 | Mean Difference (IV, Random, 95% CI) | 7.92 [2.01, 13.83] |
| 9.2 After usual care | 2 | 160 | Mean Difference (IV, Random, 95% CI) | 5.33 [‐0.96, 11.63] |
| 10 Mobility ‐ preferred gait speed (m/min) | 3 | 176 | Mean Difference (IV, Random, 95% CI) | 1.67 [‐3.27, 6.62] |
| 10.1 During usual care | 2 | 74 | Mean Difference (IV, Random, 95% CI) | 2.54 [‐3.65, 8.73] |
| 10.2 After usual care | 1 | 102 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐8.08, 8.37] |
| 11 Mobility ‐ gait endurance (6‐MWT metres) | 5 | 283 | Mean Difference (IV, Random, 95% CI) | 38.29 [7.19, 69.39] |
| 11.1 During usual care | 3 | 123 | Mean Difference (IV, Random, 95% CI) | 50.76 [19.09, 82.43] |
| 11.2 After usual care | 2 | 160 | Mean Difference (IV, Random, 95% CI) | 22.34 [‐44.02, 88.69] |
| 12 Mobility ‐ peak activity index (steps/min) | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 12.20 [1.38, 23.02] |
| 12.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 After usual care | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 12.20 [1.38, 23.02] |
| 13 Mobility ‐ max step rate in 1 min | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 12.10 [0.93, 23.27] |
| 13.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 After usual care | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 12.10 [0.93, 23.27] |
| 14 Mobility ‐ Stroke Impact Scale (mobility domain) | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 5.90 [‐7.97, 19.77] |
| 14.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 After usual care | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 5.90 [‐7.97, 19.77] |
| 15 Physical function ‐ Berg Balance scale | 2 | 134 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐2.48, 2.56] |
| 15.1 During usual care | 2 | 134 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐2.48, 2.56] |
| 15.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Health‐related QoL ‐ EuroQol EQ‐5D | 1 | 102 | Mean Difference (IV, Random, 95% CI) | ‐6.96 [‐14.86, 0.93] |
| 16.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 After usual care | 1 | 102 | Mean Difference (IV, Random, 95% CI) | ‐6.96 [‐14.86, 0.93] |
| 17 Mood ‐ Beck Depression Index | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐3.67, 1.07] |
| 17.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 After usual care | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐3.67, 1.07] |
| 18 Mood ‐ Hospital Anxiety and Depression Scale (HADS) ‐ anxiety score | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐1.6 [‐3.58, 0.38] |
| 18.1 During usual care | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐1.6 [‐3.58, 0.38] |
| 18.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Mood ‐ Hospital Anxiety and Depression Scale (HADS) ‐ depression score | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐2.7 [‐4.40, 1.00] |
| 19.1 During usual care | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐2.7 [‐4.40, 1.00] |
| 19.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Mood ‐ combined depression scales | 2 | 73 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.18, ‐0.22] |
| 20.1 During usual care | 1 | 53 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.36, ‐0.23] |
| 20.2 After usual care | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐1.35, 0.43] |
Analysis 2.8.
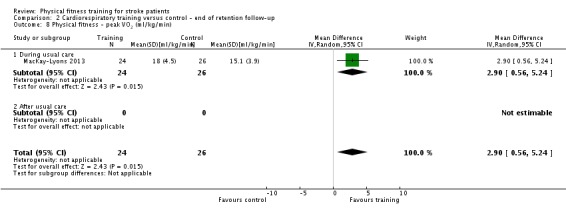
Comparison 2 Cardiorespiratory training versus control ‐ end of retention follow‐up, Outcome 8 Physical fitness ‐ peak VO2 (ml/kg/min).
Analysis 2.12.
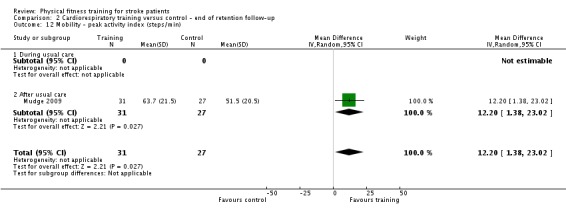
Comparison 2 Cardiorespiratory training versus control ‐ end of retention follow‐up, Outcome 12 Mobility ‐ peak activity index (steps/min).
Analysis 2.13.
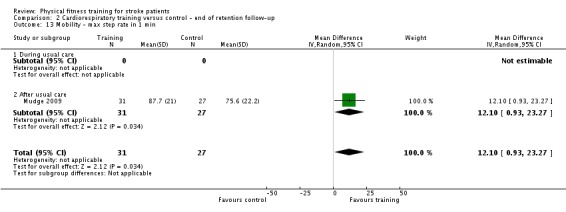
Comparison 2 Cardiorespiratory training versus control ‐ end of retention follow‐up, Outcome 13 Mobility ‐ max step rate in 1 min.
Comparison 3.
Resistance training versus control ‐ end of intervention
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Case fatality | 13 | 436 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 During usual care | 4 | 146 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 After usual care | 9 | 290 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Disability ‐ Late Life Function & Disability Instrument ‐ Disability Frequency Dimension | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐4.59, 4.79] |
| 2.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 After usual care | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐4.59, 4.79] |
| 3 Disability ‐ Late Life Function & Disability Instrument ‐ Disability Limitation Dimension | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐4.94, 7.54] |
| 3.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 After usual care | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐4.94, 7.54] |
| 4 Physical fitness ‐ composite measure of muscle strength | 2 | 60 | Std. Mean Difference (IV, Random, 95% CI) | 0.58 [0.06, 1.10] |
| 4.1 During usual care | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 During and after usual care | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.16, 1.10] |
| 4.3 After usual care | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | 0.84 [‐0.09, 1.76] |
| 5 Physical fitness ‐ muscle strength, knee extension (Nm) | 2 | 42 | Mean Difference (IV, Random, 95% CI) | 12.01 [‐4.46, 28.47] |
| 5.1 During usual care | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 4.80 [‐5.98, 15.58] |
| 5.2 After usual care | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 21.80 [4.92, 38.68] |
| 6 Physical fitness ‐ muscle strength, knee flexion (Nm) | 2 | 42 | Mean Difference (IV, Random, 95% CI) | 9.61 [‐5.01, 24.24] |
| 6.1 During usual care | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 4.5 [‐1.13, 10.13] |
| 6.2 After usual care | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 20.5 [0.84, 40.16] |
| 7 Mobility ‐ maximal gait speed (m/min) | 4 | 104 | Mean Difference (IV, Random, 95% CI) | 1.92 [‐3.50, 7.35] |
| 7.1 During usual care | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 8.40 [2.82, 13.98] |
| 7.2 After usual care | 3 | 86 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐4.57, 2.57] |
| 8 Mobility ‐ preferred gait speed (m/min) | 3 | 80 | Mean Difference (IV, Random, 95% CI) | 2.34 [‐6.77, 11.45] |
| 8.1 During usual care | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 9.0 [3.42, 14.58] |
| 8.2 After usual care | 2 | 62 | Mean Difference (IV, Random, 95% CI) | ‐2.61 [‐7.73, 2.51] |
| 9 Mobility ‐ gait endurance (6‐MWT metres) | 2 | 66 | Mean Difference (IV, Random, 95% CI) | 3.78 [‐68.56, 76.11] |
| 9.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 After usual care | 2 | 66 | Mean Difference (IV, Random, 95% CI) | 3.78 [‐68.56, 76.11] |
| 10 Physical function ‐ weight‐bearing (% body weight ‐ affected side) | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 11.80 [0.89, 22.71] |
| 10.1 During usual care | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 11.80 [0.89, 22.71] |
| 10.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Physical function ‐ stair climbing, maximal (sec/step) | 2 | 61 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.86, 0.77] |
| 11.1 During usual care | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 After usual care | 2 | 61 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.86, 0.77] |
| 12 Physical function ‐ Timed Up and Go (sec) | 2 | 52 | Mean Difference (IV, Random, 95% CI) | ‐6.45 [‐7.48, ‐5.43] |
| 12.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 After usual care | 2 | 52 | Mean Difference (IV, Random, 95% CI) | ‐6.45 [‐7.48, ‐5.43] |
| 13 Physical function ‐ Berg Balance Scale (score 0 to 56) | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 3.41 [1.52, 5.30] |
| 13.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 After usual care | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 3.41 [1.52, 5.30] |
| 14 Physical function ‐ balance ‐ antero‐posterior sway | 2 | 61 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.12 [‐4.07, ‐0.16] |
| 14.1 During usual care | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 After usual care | 2 | 61 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.12 [‐4.07, ‐0.16] |
| 15 Physical function ‐ balance ‐ mediolateral sway | 2 | 61 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.51 [‐5.16, 0.14] |
| 15.1 During usual care | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 After usual care | 2 | 61 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.51 [‐5.16, 0.14] |
| 16 Physical function ‐ Trunk Impairment Scale [scale 0 to 23] | 1 | 33 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐1.52, 2.52] |
| 16.1 During usual care | 1 | 33 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐1.52, 2.52] |
| 16.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Health‐related QoL ‐ SF‐36 mental health | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 2.8 [‐4.95, 10.55] |
| 17.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 After usual care | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 2.8 [‐4.95, 10.55] |
| 18 Health‐related QoL ‐ SF‐36 physical functioning | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 1.47 [‐4.24, 7.18] |
| 18.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 After usual care | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 1.47 [‐4.24, 7.18] |
| 19 Mood ‐ Centre for Epidemiologic Studies for Depression scale (CES‐D) | 1 | 88 | Mean Difference (IV, Random, 95% CI) | ‐5.49 [‐9.78, ‐1.20] |
| 19.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 After usual care | 1 | 88 | Mean Difference (IV, Random, 95% CI) | ‐5.49 [‐9.78, ‐1.20] |
| 20 Mood ‐ State Trait Anxiety Inventory ‐ Trait Anxiety (score 20 to 80) | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐2.70 [‐10.57, 5.17] |
| 20.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 After usual care | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐2.70 [‐10.57, 5.17] |
| 21 Mood ‐ State Trait Anxiety Inventory ‐ State Anxiety (score 20 to 80) | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐2.60 [‐8.89, 3.69] |
| 21.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 After usual care | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐2.60 [‐8.89, 3.69] |
| 22 Mood ‐ Beck Depression Inventory (BDI; score 0 to 63) | 1 | 29 | Mean Difference (IV, Random, 95% CI) | ‐2.50 [‐8.07, 3.07] |
| 22.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 After usual care | 1 | 29 | Mean Difference (IV, Random, 95% CI) | ‐2.50 [‐8.07, 3.07] |
| 23 Mood ‐ combined depression scales | 2 | 117 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.84, ‐0.11] |
| 23.1 During usual care | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 After usual care | 2 | 117 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.84, ‐0.11] |
Comparison 4.
Resistance training versus control ‐ end of retention follow‐up
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Case fatality | 3 | 138 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 During usual care | 2 | 95 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 After usual care | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Physical fitness ‐ muscle strength, knee extension (Nm) | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 17.4 [‐0.01, 34.81] |
| 2.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 After usual care | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 17.4 [‐0.01, 34.81] |
| 3 Physical fitness ‐ muscle strength, knee flexion (Nm) | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 17.60 [‐2.17, 37.37] |
| 3.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 After usual care | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 17.60 [‐2.17, 37.37] |
| 4 Mobility ‐ maximal gait speed (m/min) | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐19.80 [‐95.77, 56.17] |
| 4.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 After usual care | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐19.80 [‐95.77, 56.17] |
| 5 Mobility ‐ gait endurance (6‐MWT metres) | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 11.0 [‐105.95, 127.95] |
| 5.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 After usual care | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 11.0 [‐105.95, 127.95] |
| 6 Physical function ‐ Timed Up and Go (sec) | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐16.67, 10.47] |
| 6.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 After usual care | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐16.67, 10.47] |
| 7 Mood ‐ Centre for Epidemiologic Studies for Depression scale (CES‐D) | 1 | 86 | Mean Difference (IV, Random, 95% CI) | ‐8.92 [‐13.03, ‐4.81] |
| 7.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 After usual care | 1 | 86 | Mean Difference (IV, Random, 95% CI) | ‐8.92 [‐13.03, ‐4.81] |
Comparison 5.
Mixed training versus control ‐ end of intervention
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Case fatality | 17 | 957 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.03, 1.03] |
| 1.1 During usual care | 7 | 254 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 After usual care | 10 | 703 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.03, 1.03] |
| 2 Disability ‐ Lawton IADL | 2 | 113 | Mean Difference (IV, Random, 95% CI) | 0.83 [‐0.51, 2.17] |
| 2.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 After usual care | 2 | 113 | Mean Difference (IV, Random, 95% CI) | 0.83 [‐0.51, 2.17] |
| 3 Disability ‐ Barthel Index (BI) | 5 | 236 | Mean Difference (IV, Random, 95% CI) | 2.91 [‐1.15, 6.96] |
| 3.1 During usual care | 2 | 58 | Mean Difference (IV, Random, 95% CI) | 9.95 [‐3.85, 23.75] |
| 3.2 After usual care | 3 | 178 | Mean Difference (IV, Random, 95% CI) | 1.99 [‐2.32, 6.29] |
| 4 Disability ‐ Rivermead Mobility Index (RMI) | 2 | 308 | Mean Difference (IV, Random, 95% CI) | 0.48 [0.05, 0.91] |
| 4.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 After usual care | 2 | 308 | Mean Difference (IV, Random, 95% CI) | 0.48 [0.05, 0.91] |
| 5 Disability ‐ Nottingham Extended ADL | 1 | 66 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.08, 0.68] |
| 5.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 After usual care | 1 | 66 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.08, 0.68] |
| 6 Disability ‐ Functional Independence Measure (FIM) | 1 | 66 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.70, 1.50] |
| 6.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 After usual care | 1 | 66 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.70, 1.50] |
| 7 Disability ‐ Stroke Impact Scale (SIS‐16) | 1 | 94 | Mean Difference (IV, Random, 95% CI) | 6.0 [0.19, 11.81] |
| 7.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 After usual care | 1 | 94 | Mean Difference (IV, Random, 95% CI) | 6.0 [0.19, 11.81] |
| 8 Disability ‐ Katz ADL Scale | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐5.21, 0.81] |
| 8.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 After usual care | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐5.21, 0.81] |
| 9 Disability ‐ combined disability scales | 7 | 544 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [0.04, 0.49] |
| 9.1 During usual care | 2 | 58 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.04, 1.00] |
| 9.2 After usual care | 5 | 486 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.06, 0.48] |
| 10 Risk factors ‐ blood pressure, systolic | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 3.20 [‐9.55, 15.95] |
| 10.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 After usual care | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 3.20 [‐9.55, 15.95] |
| 11 Risk factors ‐ blood pressure, diastolic | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐5.59, 3.99] |
| 11.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 After usual care | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐5.59, 3.99] |
| 12 Physical fitness ‐ peak VO2 (ml/kg/min) | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.99 [0.35, 1.63] |
| 12.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 After usual care | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.99 [0.35, 1.63] |
| 13 Physical fitness ‐ gait economy, VO2 (ml/kg/metre) | 1 | 66 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, ‐0.00] |
| 13.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 After usual care | 1 | 66 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, ‐0.00] |
| 14 Physical fitness ‐ muscle strength, ankle dorsiflexion* | 2 | 148 | Std. Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.82, 2.41] |
| 14.1 During usual care | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 After usual care | 2 | 148 | Std. Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.82, 2.41] |
| 15 Physical fitness ‐ muscle strength, knee extension* | 3 | 202 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [0.05, 0.61] |
| 15.1 During usual care | 1 | 54 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.25, 0.83] |
| 15.2 After usual care | 2 | 148 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.02, 0.73] |
| 16 Physical fitness ‐ muscle strength, knee flexion | 1 | 54 | Mean Difference (IV, Random, 95% CI) | 6.40 [‐3.76, 16.56] |
| 16.1 During usual care | 1 | 54 | Mean Difference (IV, Random, 95% CI) | 6.40 [‐3.76, 16.56] |
| 16.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Physical fitness ‐ muscle strength, elbow extension force (N) | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐19.43 [‐54.11, 15.25] |
| 17.1 During usual care | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐19.43 [‐54.11, 15.25] |
| 17.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Physical fitness ‐ muscle strength, elbow flexion force (N) | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐15.50 [‐54.04, 23.04] |
| 18.1 During usual care | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐15.50 [‐54.04, 23.04] |
| 18.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Physical fitness ‐ muscle strength, grip force (N) | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐6.25 [‐52.41, 39.91] |
| 19.1 During usual care | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐6.25 [‐52.41, 39.91] |
| 19.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Physical fitness ‐ muscle strength, grip strength (paretic hand) | 2 | 165 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.36, 0.26] |
| 20.1 During usual care | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 After usual care | 2 | 165 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.36, 0.26] |
| 21 Physical fitness ‐ muscle strength, leg extensor power (affected leg) W/Kg | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.08, 0.22] |
| 21.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 After usual care | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.08, 0.22] |
| 22 Mobility ‐ Functional Ambulation Categories | 1 | 242 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.02, 0.22] |
| 22.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 After usual care | 1 | 242 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.02, 0.22] |
| 23 Mobility ‐ preferred gait speed (m/min) | 9 | 639 | Mean Difference (IV, Random, 95% CI) | 4.54 [0.95, 8.14] |
| 23.1 During usual care | 3 | 153 | Mean Difference (IV, Random, 95% CI) | 3.37 [‐2.63, 9.37] |
| 23.2 After usual care | 6 | 486 | Mean Difference (IV, Random, 95% CI) | 4.97 [0.68, 9.26] |
| 24 Mobility ‐ preferred gait speed (m/min); subgroup: therapy time | 9 | 639 | Mean Difference (IV, Random, 95% CI) | 4.54 [0.95, 8.14] |
| 24.1 Confounded | 6 | 438 | Mean Difference (IV, Random, 95% CI) | 6.32 [1.08, 11.55] |
| 24.2 Unconfounded | 3 | 201 | Mean Difference (IV, Random, 95% CI) | 0.49 [‐2.96, 3.94] |
| 25 Mobility ‐ gait endurance (6‐MWT metres) | 7 | 561 | Mean Difference (IV, Random, 95% CI) | 41.60 [25.25, 57.95] |
| 25.1 During usual care | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 66.30 [‐19.79, 152.39] |
| 25.2 After usual care | 6 | 521 | Mean Difference (IV, Random, 95% CI) | 40.68 [24.03, 57.33] |
| 26 Mobility ‐ Community Ambulation Speed (> 0.8 m/sec) | 3 | 232 | Odds Ratio (M‐H, Random, 95% CI) | 1.38 [0.78, 2.42] |
| 26.1 During usual care | 1 | 67 | Odds Ratio (M‐H, Random, 95% CI) | 1.75 [0.46, 6.65] |
| 26.2 After usual care | 2 | 165 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.70, 2.44] |
| 27 Physical function ‐ balance ‐ Berg Balance scale | 6 | 260 | Mean Difference (IV, Random, 95% CI) | 1.97 [0.36, 3.59] |
| 27.1 During usual care | 4 | 140 | Mean Difference (IV, Random, 95% CI) | 1.70 [‐2.53, 5.93] |
| 27.2 After usual care | 2 | 120 | Mean Difference (IV, Random, 95% CI) | 2.42 [0.83, 4.00] |
| 28 Physical function ‐ balance ‐ functional reach | 2 | 166 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.22, 0.50] |
| 28.1 During usual care | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.2 After usual care | 2 | 166 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.22, 0.50] |
| 29 Physical function ‐ balance ‐ Four Square Step Test | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 3.00 [‐1.21, 7.21] |
| 29.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 After usual care | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 3.00 [‐1.21, 7.21] |
| 30 Physical function ‐ balance ‐ timed balance test | 1 | 242 | Mean Difference (IV, Random, 95% CI) | 0.32 [0.06, 0.58] |
| 30.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.2 After usual care | 1 | 242 | Mean Difference (IV, Random, 95% CI) | 0.32 [0.06, 0.58] |
| 31 Physical function ‐ balance ‐ combined outcome data | 9 | 596 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.07, 0.47] |
| 31.1 During usual care | 4 | 140 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.17, 0.50] |
| 31.2 After usual care | 5 | 456 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [0.02, 0.57] |
| 32 Physical function ‐ Timed Up and Go (sec) | 4 | 418 | Mean Difference (IV, Random, 95% CI) | ‐1.37 [‐2.26, ‐0.47] |
| 32.1 During usual care | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐11.24, 7.24] |
| 32.2 After usual care | 3 | 356 | Mean Difference (IV, Random, 95% CI) | ‐1.75 [‐3.37, ‐0.12] |
| 33 Physical function ‐ Timed Up and Go (sec) ‐ sensitivity analysis ‐ unconfounded trials | 2 | 128 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐2.91, 0.65] |
| 33.1 During usual care | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐11.24, 7.24] |
| 33.2 After usual care | 1 | 66 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐2.91, 0.71] |
| 34 Physical function ‐ Action Research Arm Test | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐16.58, 13.78] |
| 34.1 During usual care | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐16.58, 13.78] |
| 34.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Health‐related QoL ‐ SF‐36 physical functioning | 2 | 112 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [0.10, 0.85] |
| 35.1 During usual care | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 After usual care | 2 | 112 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [0.10, 0.85] |
| 36 Health‐related QoL ‐ SF‐36 social role functioning | 2 | 112 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.22, 1.17] |
| 36.1 During usual care | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.2 After usual care | 2 | 112 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.22, 1.17] |
| 37 Health‐related QoL ‐ SF‐36 physical role functioning | 3 | 178 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.26, 0.86] |
| 37.1 During usual care | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.2 After usual care | 3 | 178 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.26, 0.86] |
| 38 Health‐related QoL ‐ SF‐36 emotional role functioning | 1 | 93 | Mean Difference (IV, Random, 95% CI) | 15.5 [2.98, 28.02] |
| 38.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38.2 After usual care | 1 | 93 | Mean Difference (IV, Random, 95% CI) | 15.5 [2.98, 28.02] |
| 39 Health‐related QoL ‐ Stroke‐Adapted Sickness Impact profile | 1 | 83 | Mean Difference (IV, Random, 95% CI) | ‐2.70 [‐7.81, 2.41] |
| 39.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.2 After usual care | 1 | 83 | Mean Difference (IV, Random, 95% CI) | ‐2.70 [‐7.81, 2.41] |
| 40 Health‐related QoL ‐ EuroQol (Health State) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.03, 0.27] |
| 40.1 During usual care | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.03, 0.27] |
| 40.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41 Health‐related QoL ‐ EuroQol (self perceived health) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 9.10 [‐0.14, 18.34] |
| 41.1 During usual care | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 9.10 [‐0.14, 18.34] |
| 41.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 42 Mood ‐ Hospital Anxiety and Depression Scale (HADS) ‐ anxiety score | 3 | 391 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.95, 0.40] |
| 42.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 42.2 After usual care | 3 | 391 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.95, 0.40] |
| 43 Mood ‐ Hospital Anxiety and Depression Scale (HADS) ‐ depression score | 3 | 391 | Mean Difference (IV, Random, 95% CI) | 0.59 [‐0.08, 1.26] |
| 43.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 43.2 After usual care | 3 | 391 | Mean Difference (IV, Random, 95% CI) | 0.59 [‐0.08, 1.26] |
| 44 Mood ‐ Stroke Impact Scale emotion score | 2 | 335 | Mean Difference (IV, Random, 95% CI) | 2.87 [‐3.40, 9.14] |
| 44.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 44.2 After usual care | 2 | 335 | Mean Difference (IV, Random, 95% CI) | 2.87 [‐3.40, 9.14] |
| 45 Mood ‐ Geriatric Depression Scale | 1 | 93 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐3.10, ‐0.70] |
| 45.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 45.2 After usual care | 1 | 93 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐3.10, ‐0.70] |
| 46 Mood ‐ combined depression scales | 4 | 484 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.39, 0.37] |
| 46.1 During usual care | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 46.2 After usual care | 4 | 484 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.39, 0.37] |
| 47 Cognitive function ‐ FIM cognitive score | 2 | 159 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.47, 0.31] |
| 47.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 47.2 After usual care | 2 | 159 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.47, 0.31] |
| 48 Cognitive function ‐ SIS memory and thinking | 1 | 93 | Mean Difference (IV, Random, 95% CI) | 7.40 [‐0.31, 15.11] |
| 48.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 48.2 After usual care | 1 | 93 | Mean Difference (IV, Random, 95% CI) | 7.40 [‐0.31, 15.11] |
| 49 Cognitive function ‐ SIS communication | 1 | 93 | Mean Difference (IV, Random, 95% CI) | 4.10 [‐3.26, 11.46] |
| 49.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 49.2 After usual care | 1 | 93 | Mean Difference (IV, Random, 95% CI) | 4.10 [‐3.26, 11.46] |
Comparison 6.
Mixed training versus control ‐ end of retention follow‐up
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Case fatality | 11 | 762 | Odds Ratio (M‐H, Random, 95% CI) | 0.27 [0.06, 1.11] |
| 1.1 During usual care | 6 | 243 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.02, 1.68] |
| 1.2 After usual care | 5 | 519 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.05, 2.28] |
| 2 Disability ‐ Barthel Index (BI) | 2 | 103 | Mean Difference (IV, Random, 95% CI) | 1.82 [‐13.69, 17.33] |
| 2.1 During usual care | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 9.0 [‐1.29, 19.29] |
| 2.2 After usual care | 1 | 63 | Mean Difference (IV, Random, 95% CI) | ‐6.90 [‐21.05, 7.25] |
| 3 Disability ‐ Functional Independence Measure (FIM) | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.88, 2.28] |
| 3.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 After usual care | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.88, 2.28] |
| 4 Disability ‐ Nottingham Extended ADL | 2 | 106 | Mean Difference (IV, Random, 95% CI) | 3.10 [‐5.20, 11.40] |
| 4.1 During usual care | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 9.5 [‐1.83, 20.83] |
| 4.2 After usual care | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.93, 1.53] |
| 5 Disability ‐ Rivermead Mobility Index (RMI) | 2 | 308 | Mean Difference (IV, Random, 95% CI) | 0.39 [0.04, 0.73] |
| 5.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 After usual care | 2 | 308 | Mean Difference (IV, Random, 95% CI) | 0.39 [0.04, 0.73] |
| 6 Disability ‐ combined disability scales | 4 | 411 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.12, 0.44] |
| 6.1 During usual care | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [‐0.10, 1.16] |
| 6.2 After usual care | 3 | 371 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.22, 0.40] |
| 7 Physical fitness ‐ gait economy, VO2 (ml/kg/metre) | 1 | 66 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 7.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 After usual care | 1 | 66 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 8 Physical fitness ‐ muscle strength, knee flexion | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 4.20 [‐9.36, 17.76] |
| 8.1 During usual care | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 4.20 [‐9.36, 17.76] |
| 8.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Physical fitness ‐ muscle strength, knee extension | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 4.20 [‐12.71, 21.11] |
| 9.1 During usual care | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 4.20 [‐12.71, 21.11] |
| 9.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Physical fitness ‐ muscle strength, leg extensor power (affected leg) W/Kg | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.13, 0.17] |
| 10.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 After usual care | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.13, 0.17] |
| 11 Physical fitness ‐ grip strength (paretic hand) | 1 | 63 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.26, 0.18] |
| 11.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 After usual care | 1 | 63 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.26, 0.18] |
| 12 Mobility ‐ Functional Ambulation Categories | 1 | 242 | Mean Difference (IV, Random, 95% CI) | 0.11 [0.00, 0.22] |
| 12.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 After usual care | 1 | 242 | Mean Difference (IV, Random, 95% CI) | 0.11 [0.00, 0.22] |
| 13 Mobility ‐ preferred gait speed (m/min) | 4 | 443 | Mean Difference (IV, Random, 95% CI) | 1.60 [‐5.62, 8.82] |
| 13.1 During usual care | 2 | 136 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐8.64, 6.60] |
| 13.2 After usual care | 2 | 307 | Mean Difference (IV, Random, 95% CI) | 3.45 [‐8.19, 15.08] |
| 14 Mobility ‐ gait endurance (6‐MWT metres) | 3 | 365 | Mean Difference (IV, Random, 95% CI) | 51.62 [25.20, 78.03] |
| 14.1 During usual care | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 109.50 [17.12, 201.88] |
| 14.2 After usual care | 2 | 325 | Mean Difference (IV, Random, 95% CI) | 46.46 [18.89, 74.03] |
| 15 Mobility ‐ community ambulation speed (> 0.8 m/sec) | 3 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.70, 2.53] |
| 15.1 During usual care | 1 | 52 | Odds Ratio (M‐H, Random, 95% CI) | 2.14 [0.56, 8.12] |
| 15.2 After usual care | 2 | 165 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.48, 2.76] |
| 16 Physical function ‐ balance ‐ Berg Balance Scale | 2 | 102 | Mean Difference (IV, Random, 95% CI) | 2.22 [‐7.79, 12.22] |
| 16.1 During usual care | 2 | 102 | Mean Difference (IV, Random, 95% CI) | 2.22 [‐7.79, 12.22] |
| 16.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Physical function ‐ balance ‐ functional reach | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 2.5 [‐0.97, 5.97] |
| 17.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 After usual care | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 2.5 [‐0.97, 5.97] |
| 18 Physical function ‐ balance ‐ timed balance test | 1 | 242 | Mean Difference (IV, Random, 95% CI) | 0.46 [0.09, 0.83] |
| 18.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 After usual care | 1 | 242 | Mean Difference (IV, Random, 95% CI) | 0.46 [0.09, 0.83] |
| 19 Physical function ‐ Timed Up and Go (sec) | 3 | 370 | Mean Difference (IV, Random, 95% CI) | ‐1.37 [‐3.86, 1.12] |
| 19.1 During usual care | 1 | 62 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐6.97, 6.97] |
| 19.2 After usual care | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐1.65 [‐4.84, 1.53] |
| 20 Health‐related QoL ‐ EuroQol (Health State) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.12, 0.20] |
| 20.1 During usual care | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.12, 0.20] |
| 20.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Health‐related QoL ‐ EuroQol (self perceived health) | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 3.40 [‐7.31, 14.11] |
| 21.1 During usual care | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 3.40 [‐7.31, 14.11] |
| 21.2 After usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Health‐related QoL ‐ SF‐36 physical functioning | 2 | 146 | Mean Difference (IV, Random, 95% CI) | 2.46 [‐7.20, 12.11] |
| 22.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 After usual care | 2 | 146 | Mean Difference (IV, Random, 95% CI) | 2.46 [‐7.20, 12.11] |
| 23 Health‐related QoL ‐ SF‐36 physical role functioning | 2 | 146 | Mean Difference (IV, Random, 95% CI) | 11.61 [2.38, 20.84] |
| 23.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 After usual care | 2 | 146 | Mean Difference (IV, Random, 95% CI) | 11.61 [2.38, 20.84] |
| 24 Health‐related QoL ‐ SF‐36 emotional role functioning | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 10.0 [‐2.28, 22.28] |
| 24.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 After usual care | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 10.0 [‐2.28, 22.28] |
| 25 Health‐related QoL ‐ Stroke‐Adapted Sickness Impact profile | 1 | 83 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐6.16, 4.76] |
| 25.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 After usual care | 1 | 83 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐6.16, 4.76] |
| 26 Mood ‐ Stroke Impact Scale emotion score | 2 | 322 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐3.26, 3.51] |
| 26.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 After usual care | 2 | 322 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐3.26, 3.51] |
| 27 Mood ‐ Geriatric Depression Scale | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐1.4 [‐2.54, ‐0.26] |
| 27.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.2 After usual care | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐1.4 [‐2.54, ‐0.26] |
| 28 Mood ‐ Hospital Anxiety and Depression Scale (HADS) ‐ anxiety score | 3 | 391 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.78, 0.57] |
| 28.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.2 After usual care | 3 | 391 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.78, 0.57] |
| 29 Mood ‐ Hospital Anxiety and Depression Scale (HADS) ‐ depression score | 3 | 391 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.43, 0.96] |
| 29.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 After usual care | 3 | 391 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.43, 0.96] |
| 30 Mood ‐ combined depression scales | 4 | 471 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.33, 0.22] |
| 30.1 During usual care | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.2 After usual care | 4 | 471 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.33, 0.22] |
| 31 Cognitive function ‐ FIM cognitive score | 1 | 93 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.19, 0.99] |
| 31.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 After usual care | 1 | 93 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.19, 0.99] |
| 32 Cognitive function ‐ SIS memory and thinking | 1 | 93 | Mean Difference (IV, Random, 95% CI) | 4.30 [‐3.32, 11.92] |
| 32.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 After usual care | 1 | 93 | Mean Difference (IV, Random, 95% CI) | 4.30 [‐3.32, 11.92] |
| 33 Cognitive function ‐ SIS communication | 1 | 93 | Mean Difference (IV, Random, 95% CI) | 2.90 [‐4.16, 9.96] |
| 33.1 During usual care | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 After usual care | 1 | 93 | Mean Difference (IV, Random, 95% CI) | 2.90 [‐4.16, 9.96] |
Analysis 6.17.
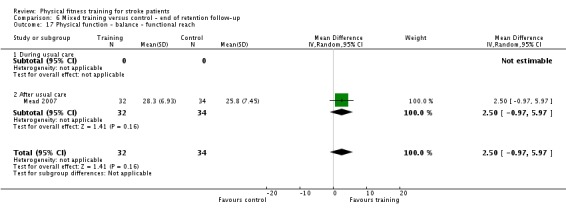
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 17 Physical function ‐ balance ‐ functional reach.
Analysis 6.22.
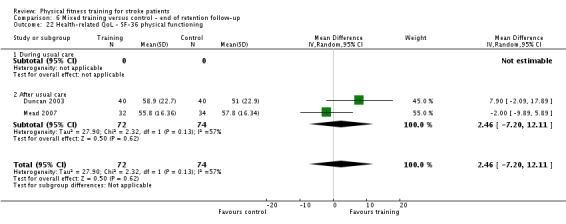
Comparison 6 Mixed training versus control ‐ end of retention follow‐up, Outcome 22 Health‐related QoL ‐ SF‐36 physical functioning.
Comparison 7.
Cardiorespiratory versus resistance versus mixed training
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disability ‐ combined disability scales | 16 | 1048 | Std. Mean Difference (IV, Random, 95% CI) | 0.37 [0.18, 0.55] |
| 1.1 Cardiorespiratory training | 8 | 462 | Std. Mean Difference (IV, Random, 95% CI) | 0.52 [0.19, 0.84] |
| 1.2 Resistance training | 1 | 42 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.48, 0.73] |
| 1.3 Mixed training | 7 | 544 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [0.04, 0.49] |
| 2 Mobility ‐ maximal walking speed | 18 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Cardiorespiratory training | 14 | 631 | Mean Difference (IV, Random, 95% CI) | 6.71 [2.73, 10.69] |
| 2.2 Resistance training | 4 | 104 | Mean Difference (IV, Random, 95% CI) | 1.92 [‐3.50, 7.35] |
| 2.3 Mixed training | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Mobility ‐ preferred walking speed (m/min) | 22 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Cardiorespiratory training | 10 | 505 | Mean Difference (IV, Random, 95% CI) | 4.28 [1.71, 6.84] |
| 3.2 Resistance training | 3 | 80 | Mean Difference (IV, Random, 95% CI) | 2.34 [‐6.77, 11.45] |
| 3.3 Mixed training | 9 | 639 | Mean Difference (IV, Random, 95% CI) | 4.54 [0.95, 8.14] |
| 4 Mobility ‐ gait endurance (6‐MWT metres) | 24 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Cardiorespiratory training | 15 | 826 | Mean Difference (IV, Random, 95% CI) | 30.29 [16.19, 44.39] |
| 4.2 Resistance training | 2 | 66 | Mean Difference (IV, Random, 95% CI) | 3.78 [‐68.56, 76.11] |
| 4.3 Mixed training | 7 | 561 | Mean Difference (IV, Random, 95% CI) | 41.60 [25.25, 57.95] |
| 5 Balance ‐ Berg Balance Scale | 14 | 723 | Mean Difference (IV, Random, 95% CI) | 1.83 [0.64, 3.01] |
| 5.1 Cardiorespiratory training | 7 | 435 | Mean Difference (IV, Random, 95% CI) | 1.13 [‐0.44, 2.70] |
| 5.2 Resistance training | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 3.41 [1.52, 5.30] |
| 5.3 Mixed training | 6 | 260 | Mean Difference (IV, Random, 95% CI) | 1.97 [0.36, 3.59] |
What's new
Last assessed as up‐to‐date: 1 February 2015.
| Date | Event | Description |
|---|---|---|
| 12 November 2015 | New citation required and conclusions have changed | New trials have changed where significant benefits emerge. Improvements in global indices of disability are apparent now for mixed training as well as cardiorespiratory training. Improvements in balance are now only apparent among trials of mixed training. We have added a new patient‐important outcome (cognitive function) but there is a lack of evidence and this highlights an important knowledge gap. |
| 29 October 2015 | New search has been performed | We have updated all main electronic search strategies to February 2015. We have included 13 additional randomised controlled trials, bringing the total number of included trials to 58, involving 2797 participants. We have added a cognitive function outcome to the review because this has been identified as a research priority. Secondly an application to carry out a Cochrane review of exercise interventions for cognition after stroke was judged to overlap and to be more efficiently combined with this review of fitness training interventions after stroke. We checked all previously included trials for cognitive outcomes as well as those in the updated searches. We have added two new co‐authors. |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 5 July 2013 | New citation required and conclusions have changed | Additional co‐author. We have revised the main text and conclusions of the review according to the findings of the new included trials. |
| 28 January 2013 | New search has been performed | We have updated all main electronic search strategies to January 2013. We have included 13 additional randomised clinical trials, bringing the total number of included trials to 45, involving 2188 participants. We have incorporated 'Risk of bias' tables. |
| 22 November 2010 | New citation required and conclusions have changed | New first author. We have revised the main text and conclusions of the review according to the findings of the new included trials. |
| 22 November 2010 | New search has been performed | We have updated all main electronic search strategies to March 2010. We have included 11 additional randomised clinical trials and 7 ongoing trials. We have better clarified our inclusion criteria and objectives. |
| 2 March 2009 | New search has been performed | We updated the search of the Cochrane Stroke Group Trials Register in March 2009. |
| 3 November 2008 | New citation required and conclusions have changed | There is sufficient evidence to incorporate cardiorespiratory training, using walking as a mode of exercise, into the rehabilitation of patients with stroke in order to improve speed, tolerance, and independence during walking, but further trials are needed to determine the optimal exercise prescription after stroke and to establish whether any long‐term benefits exist. |
| 3 November 2008 | New search has been performed | We updated the searches to March 2007. There are now 24 trials, involving 1147 participants, included in the review; 12 more trials than in the previous version. The text of the review has been revised throughout. |
| 23 October 2008 | Amended | Converted to new review format. |
Differences between protocol and review
Subgroup analyses were on the whole not possible as there were too few trials within the meta‐analyses and too many other influential factors.
We included cognition as a class of outcome.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ada 2013a
| Methods | Design: randomised trial of cardiorespiratory training versus no intervention – after usual care Randomised: computer‐generated randomisation stratified on walking disability by independent researcher Allocation concealment: not applicable Blinding: assessors blind to group allocation ITT: yes Measurements: end of interventions (2 and 4 months) and 6 and 12 months follow‐up Withdrawals: 2 months treadmill training group: 1 participant withdrew; control group: 3 participants withdrew ‐ reasons unclear |
|
| Participants | Randomised: 102 participants Intervention: treadmill training 2 months group: 34 participants; 28 males and 6 females; mean age 64 years (SD 12); 20 months post‐stroke (SD 15). Treadmill training 4 months group: 34 participants; 24 males and 10 females; mean age 70 years (SD 11); 22 months post‐stroke (SD 16) Control: 34 participants; 19 males and 15 females; mean age 63 years (SD 13); 19 months post‐stroke (SD 13) Inclusion criteria: within first 5 years post‐stroke; MMSE score of > 23; discharged from rehabilitation; community dwelling; 10 metre unaided walking speed > 9 seconds Exclusion criteria: unstable cardiac status; severe cognitive and/or asphasia |
|
| Interventions | Invention group: both 2 months and 4 months treadmill training group received 30 minutes treadmill walking 3 times/week for 8 or 16 weeks respectively Progressive in nature. Both groups also received overground walking training (20% of intervention during week 1, increasing to 50% at week 8; for those in 4‐month group, overground walking reduced to 20% of intervention increasing again to 50% at week 16) Control group: no intervention Setting: rehabilitation centre |
|
| Outcomes | Included outcomes: 6‐MWT; EuroQol Health Status; Adelaide Activities Profile; walking and falls self efficacy | |
| Notes | There were 2 intervention groups. The extracted data correspond to:
A subgroup analysis was performed (Dean 2014) to examine the effects of the intervention on slower (≤ 4 m/sec) and faster (> 4 m/sec) walkers at baseline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation stratified on walking disability by independent researcher |
| Allocation concealment (selection bias) | Low risk | Allocation concealment ensured because all available participants allocated in groups of 15 to blocks of 3 after baseline measures recorded |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinded |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT analysis performed Few (2/102) losses; 2‐month treadmill training group: 1 participant withdrew; control group: 3 participants withdrew Reasons and timing unclear |
| Incomplete outcome data (attrition bias) End of follow‐up | Low risk | ITT analysis performed Few losses (2/102); 2‐month treadmill training group: 1 participant withdrew; control group: 3 participants withdrew Reasons and timing unclear |
| Selective reporting (reporting bias) | Low risk | Reported outcomes correspond to trial registry ACTRN12607000227493 |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Intervention group has uncontrolled exposure |
Aidar 2007
| Methods | Design: randomised trial of cardiorespiratory training (aquatic physical exercises) versus no intervention ‐ after usual care Randomisation: stated 'random' but no further details provided Allocation concealment: not reported Blinding: not reported ITT: no Measurements: at the end of intervention (12 weeks) Withdrawals: 1 participant in the intervention group refused the training ‐ at the beginning of the programme; 2 participants in the control group were not assessed at the end of the intervention |
|
| Participants | Randomised: 31 participants, assessed 28 (15 participants in the intervention group and 13 in the control group) Intervention: 15 participants; 10 males and 5 females; mean age 50.3 years (SD 9.1) Control: 13 participants; 9 males and 4 females; mean age 52.5 years (SD 7.7) Inclusion criteria: ischaemic cerebrovascular accident; hemiplegia or hemiparesis Exclusion criteria: cognitive impairment; significant co‐morbidities |
|
| Interventions | Intervention group: aquatic physical sessions (e.g. walking activity and physical exercises in the water; swimming) 45 to 60 minutes each session; 2 times/week for 12 weeks Control group: no intervention ‐ delayed started of the same programme Setting: community setting |
|
| Outcomes | Included outcome: SF‐36 | |
| Notes | Content of the intervention not very detailed. Unclear whether the trial met the ACSM criteria for fitness training | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'random' but no further details provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) End of intervention | Unclear risk | 1/16 lost from intervention and 2/15 from control group. No ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure |
Aidar 2012
| Methods | Design: randomised trial of strength training versus usual care Randomised mechanism: lottery allocation into groups Allocation concealment: not reported Blinding: assessor blinded to group allocation Measurements: end of intervention (12 weeks) Withdrawals: 3 participants from intervention group during second week of intervention and 2 participants from control group were not assessed at the end of the intervention |
|
| Participants | Randomised: 24 participants Intervention: 11 participants; 6 males and 5 females; mean age 51.7 years (SD 8.0) Control: 13 participants; 9 males and 4 females; mean age 52.5 years (SD 7.7) Inclusion criteria: ischaemic stroke at least 1 year prior to testing; hemiplegia or hemiparesis Exclusion criteria: aphasia |
|
| Interventions | Intervention group: strength training sessions (3 sets of 8 to 10 repetitions, leg press, front pulley and bench press) 45 to 60 minutes each session; 3 times/week for 12 weeks Control group: no intervention Setting: indoor basketball court |
|
| Outcomes | Included outcomes: State‐Trait Anxiety Inventory; muscle strength | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Lottery" allocation into groups; still unclear exactly what was done |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment: not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessors |
| Incomplete outcome data (attrition bias) End of intervention | High risk | 5/29 dropouts (17%) with no ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | Self selection bias may occur as advertisements were used |
| Imbalanced exposure | High risk | Intervention group has uncontrolled exposure |
Aidar 2014
| Methods | Design: randomised trial of resistance training versus no intervention after usual care Randomisation: unclear "subjects divided at random" Allocation concealment: information not included Blinding: information not included ITT: not completed Measurements: end of intervention (12 weeks) Withdrawals: 5 dropouts ‐ 3 from experimental group and 2 from control group |
|
| Participants | Randomised: total 29 participants; 14 participants were randomised to intervention, 15 to control Intervention: 14 participants; gender numbers unknown; mean age: males 52.8 years (SD 9.0); female 52.6 years (SD 7.6); days after stroke unknown (> 1 year) Control: 15 participants; gender numbers unknown; mean age: males 52.3 years (SD 9.0); female 50.8 years (SD 10.6); days after stroke unknown (> 1 year) Inclusion criteria: below "per‐capita" income below minimum wage, medical authorisation "clinically healthy people", stroke more than 1 year ago, clinically stable, presence of hemiplegia or hemiparesis Exclusion criteria: no recurrent strokes, asymptomatic with a non‐disabling deficit or with severe disabilities |
|
| Interventions | Intervention: resistance training; 12‐week intervention, 3 times/week, each session lasting 60 minutes ‐ conducted in the morning. Minimum of 48 hours rest between sessions. Warm up including 10 to 15 minutes walking followed by upper and lower body strengthening exercises: bar guided squat, machine bench press, horizontal leg press, military press machine, abdominal crunch, front lat pull downs and bar guided lunges. 3 sets of 8 to 10 reps with 2‐minute rest between sets Control: no intervention Setting: unclear; community‐based project |
|
| Outcomes | Included outcomes: depression (BDI), muscle strength (1RM kg for various lifts; squat, bench press, leg press, military press, lat pull downs, lunges) Other outcomes: level of deficit in the dominant and non‐dominant hand, disability (Rankin scale; not recorded at end of intervention); perceived exertion (OMNI scale) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not reported |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) End of intervention | Unclear risk | 5/29 (17%) total losses: 3 participants were lost from the intervention group (no reasons given in paper) 2 in the control group (no reasons given in paper) All 3 criteria for ITT analysis have not been met; methods suggest a per‐protocol analysis |
| Selective reporting (reporting bias) | High risk | Trial register or protocol not available |
| Other bias | High risk | External validity of this study: below minimal wage people with stroke; lack of sample size calculation |
| Imbalanced exposure | High risk | Intervention group has uncontrolled exposure |
Bale 2008
| Methods | Design: randomised trial of resistance training plus % usual care versus usual care ‐ during usual care Sample size calculation reported Randomisation: drawing lots ‐ not clearly described Allocation concealment: unclear Blinding: outcome assessors blinded ITT: planned but no withdrawals Measurements: at the end of intervention (4 weeks) Withdrawals: none |
|
| Participants | Randomised: 18 participants Intervention: 8 participants; 3 males and 5 females; mean age 68.0 years (SD 13); time since stroke 49.4 (SD 22.1) days Control: 10 participants; 4 males and 6 females; mean age 64.9 years (SD 8.8); time since stroke 32.0 (SD 18.5) days Inclusion criteria: first onset of stroke with reduced muscle strength in the affected leg; ability to understand verbal information; ability to sit without support Exclusion criteria: significant sensory or cognitive sequels; arrhythmia; uncontrolled angina pectoris or hypertension; co‐morbidities that could mask the sequels from the stroke; lack of motor control of the affected leg |
|
| Interventions | Intervention group: resistance training 50 minutes a day 3 days per week for 4 weeks. 8 individually tailored exercises for the affected lower limb involving weight bearing, stepping, sit‐to‐stand, heel/toe raising, and bridging. Tailored progression included using weights, reducing speed, adding more sets, etc. Other functional activities sometimes included too (walking, stair climbing, sit‐to‐stand). One set of 10 to 15 repetitions to moderate fatigue. Control group: usual care (Bobath) 50 minutes a day 3 days per week for 4 weeks, plus usual care (other) 50 minutes/day, 2 days per week for 4 weeks. Total training: 50 minutes a day 5 days per week for 4 weeks Setting: 2 rehabilitation units |
|
| Outcomes | Included outcomes: isometric muscle strength; preferred walking speed; maximal walking speed Other outcomes: maximum weight bearing; 2 items of the MAS; Patient Global Impression of Change tool |
|
| Notes | Very small sample size Poor external validity |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Drawing lots ‐ not clearly described |
| Allocation concealment (selection bias) | Unclear risk | Poorly reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Attention control exposure |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT planned but no withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Low risk | Balanced exposure |
Bateman 2001
| Methods | Design: multicentre randomised trial of cardiorespiratory training plus usual care versus non‐exercise intervention plus usual care ‐ during usual care Randomisation: mechanism ‐ computer; method ‐ blocks size of 10 participants Allocation concealment: numbered, sealed envelopes Blinding: investigator blinded; participants encouraged to maintain blinding; efficacy unknown ITT: yes, but participants were excluded after recruitment and baseline assessments due to discharge Measurements: end of intervention (12 weeks) and at follow‐up Withdrawals: intervention group (12 participants: 4 before and 8 after the 12‐week assessment); control group (12 participants: 2 before and 10 after the 12‐week assessment) Reasons unclear but included early discharge |
|
| Participants | Randomised: 84 participants Intervention: 40 participants; males 20, females 20; age 47.0 years (SD 13.1); 144 days (SD 84) post‐stroke Control: 44 participants; males 29, females 14; age 50.3 years (SD 10.1); 184 days (SD 127) day post‐stroke Inclusion criteria: single stroke; could comply with planned interventions; could sit on a cycle ergometer Exclusion criteria: likely to be inpatient for < 3 months; impairments severe enough to limit training compliance and participation; cardiac disease; co‐morbidities contraindicated for exercise | |
| Interventions | Intervention: cardiorespiratory training; cycle ergometry at 60% to 80% of age‐related heart rate maximum for up to 30 minutes per day 3 days per week for 12 weeks Control: relaxation ‐ programme individualised: included breathing exercises, progressive muscle relaxation, autogenic exercises, visualisation techniques Setting: multicentre, 4 rehabilitation units | |
| Outcomes | Included outcomes: FIM; BI (0 to 20 scale); NEADL; RMI; HADS; BBS; gait maximum speed; maximum cycling workload (data transformed to Log base e); BMI Other outcomes: fatigue questionnaire | |
| Notes | Mixed brain injury data provided by authors; stroke‐only data retained and re‐analysed. High rate of missing data made statistical analyses difficult | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based block (n = 10) randomisation |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigator blinded; participants encouraged to maintain blinding; efficacy unknown |
| Incomplete outcome data (attrition bias) End of intervention | High risk | ITT employed 6/84 (7%) lost: intervention group 4; control group 2 Reasons for losses not clear but included exclusion after recruitment and baseline assessments due to discharge Large amounts of missing outcome data |
| Incomplete outcome data (attrition bias) End of follow‐up | High risk | ITT employed 24/85 (29%) total losses; intervention group 8; control group 10 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Low risk | Balanced exposure |
Cooke 2010a
| Methods | Design: phase I multicentre trial; 4 centres; mixed training plus usual care versus usual care ‐ during usual care ‐ i.e. functional strength training (FST) plus conventional physiotherapy (CPT) versus conventional physiotherapy alone and versus conventional physiotherapy plus conventional physiotherapy (CPT + CPT) Randomisation: computer‐generated random allocation in blocks of 9 per trial centre (stratified allocation by baseline scores for visual spatial neglect) Allocation concealment: sequentially numbered, sealed, opaque envelopes Blinding: assessor blinded to group allocation ITT: attempt to measure participants at outcome and follow‐up even if they withdraw but analyses were not performed according to ITT principle Measurements: at the end of intervention (6 weeks) and 12 weeks later (follow‐up) Withdrawals: at outcome 7/74 (9%) participants were lost at outcome in the control CPT group (3 unwell, 3 withdrew, 1 moved abroad). At follow‐up, a further 21 participants had withdrawn (total 28/74 26%). 14 participants were lost in the CPT group (5 unwell, 4 withdrew, 1 moved abroad, 2 housebound, 2 died) and 7 in the CPT + FST group (5 unwell, 2 withdrew) |
|
| Participants | Randomised: total 109 participants. 38 participants were randomised to CPT, 35 to CPT + CPT, and 36 to FST + CPT (only the results from the CPT and the CPT + FST groups were included in this review) Number randomised in comparisons used in this review this review = 74 Intervention: FST + CPT = 36 participants; 22 males (61%) and 14 females (39%); mean age: 71.17 (SD 10.6); 33.86 (SD 16.50) days after stroke Control: CPT = 38 participants; 21 males (55%) and 17 females (45%); mean age: 66.37 (SD 13.7); 36.76 (SD 22.41) days after stroke Inclusion criteria: inpatients between 1 and 13 weeks after anterior circulation stroke (ischaemic and haemorrhagic); independently mobile; some voluntary contraction in the lower affected limb; no orthopaedic surgery or trauma affecting the lower limb in the last 8 weeks; no previous history of neurological diseases; able to follow a 1‐stage command Exclusion criteria: not reported |
|
| Interventions | Intervention: FST/mixed training plus CPT. FST consisted of increasing the amount of body weight the patients needed to move; increasing movements resistance; reducing amount of body weight support during treadmill training. Frequency of intervention: 1 hour for 4 days/week for 6 weeks Control: CPT included soft issue mobilisation, facilitation of muscle activity, facilitation of co‐ordinated multi‐joint movement; tactile and proprioceptive input, resistive exercise, and functional retraining. Frequency of intervention: 1 hour for 4 days/week for 6 weeks Setting: hospital |
|
| Outcomes | Included outcomes: walking speed; health‐related quality of life measures (e.g. EuroQol) Other outcomes: gait parameters; paretic knee torque force analysis; modified RMI |
|
| Notes | Trial authors stated 'strength training' but intervention was actually mixed training | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random allocation in blocks of 9 per trial centre (stratified allocation by baseline scores for visual spatial neglect) |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed, opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comparison used means no attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinded to group allocation |
| Incomplete outcome data (attrition bias) End of intervention | High risk | Attempt to measure participants at outcome and follow‐up even if they withdraw but analyses were not performed according to ITT principle. Imbalanced losses at the end of intervention 7/74 (9%) participants were lost from the control CPT group (3 unwell, 3 withdrew, 1 moved abroad) |
| Incomplete outcome data (attrition bias) End of follow‐up | High risk | Attempt to measure participants at outcome and follow‐up even if they withdraw but analyses were not performed according to ITT principle. Imbalanced large losses at the end of follow‐up 28/74 (38%) total losses: 14 participants were lost from the CPT group (5 unwell, 4 withdrew, 1 moved abroad, 2 housebound, 2 died) and 7 in the intervention group CPT + FST group (5 unwell, 2 withdrew) |
| Selective reporting (reporting bias) | Low risk | Reported outcome correspond with those in trial register NCT00322192 |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure (CPT + CPT group although balanced does not meet inclusion criteria) |
Cuviello‐Palmer 1988
| Methods | Design: randomised trial of cardiorespiratory training plus % usual care versus usual care ‐ after usual care Randomisation: unknown Allocation concealment: unknown Blinding: unknown ITT: no Measurements: end of intervention (3 weeks) Withdrawals: none |
|
| Participants | Randomised: 20 participants Intervention: 10 participants; 6 males and 4 females; age 69.5 years (SD 14.1); 20.7 days post‐stroke (SD 13.2) Control: 10 participants; 7 males and 3 females; age 71.8 years (SD 12.0); 12.0 days post‐stroke (SD 16.8) Inclusion criteria: unknown Exclusion criteria: unknown | |
| Interventions | Intervention: cardiorespiratory training: isokinetic ergometer allowing resisted reciprocal leg movements (Kinetron II); commencing at 2 x 7 minutes/day for 5 days/week and 1 x 7 minutes/day for 1 day/week (total 6 days/week) for 3 weeks progressing to 10 minutes per session in week 2 and 12 minutes in week 3 Exercise intensity maintained at a heart rate of < 20 beats/minute above resting Control: usual care: 2 x 45 minutes/day for 5 days/week and 1 x 45 minutes/day for 1 day/week (total 6 days/week) for 3 weeks Gait training, mat exercises, and transfer training achieved via strengthening exercises, post neuromuscular facilitation (PNF), functional electrical stimulation (FES), Brunnstrom, Rood, and neurodevelopment techniques Setting: rehabilitation centre | |
| Outcomes | Included outcomes: FIM (old version); preferred gait speed (7 seconds) Other outcomes: stance symmetry; contact time (seconds); stride cadence steps/minute and other biomechanical gait parameters | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Some degree of attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | No withdrawals, no planned ITT |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Low risk | Exposure balanced |
da Cunha 2002
| Methods | Design: randomised trial of cardiorespiratory training plus % usual care versus usual care ‐ during usual care Randomisation mechanism: random number table Allocation concealment: unknown Blinding: unknown ITT: no Measurements: end of intervention (2/3 weeks ‐ until discharge) Withdrawals: none |
|
| Participants | Randomised: 15 participants Intervention: 7 participants; 6 males and 1 females; age 57.8 years (SD 5.5); 15.7 days post‐stroke (SD 7.7) Control: 8 participants; 7 males and 1 female; age 58.9 years (SD 12.9); 19.0 days post‐stroke (SD 12.7) Inclusion criteria: recent stroke (onset < 6 weeks); significant gait deficit (< 36 metres/minute; FAC score of 0, 1 or 2); sufficient cognition to participate in training (Mini Mental State Examination ‐ MMSE ≥ 21); able to stand and take 1 or more steps without assistance Exclusion criteria: co‐morbidity or disability other than hemiparesis; recent myocardial infarct; any uncontrolled health condition; joint disease or rheumatoid arthritis; obesity (> 110 kg); cognitive impairment (MMSE < 21) | |
| Interventions | Intervention: cardiorespiratory training: treadmill walking with body weight support 20 minutes/day 6 days/week for 2 to 3 weeks (until discharge); intensity unknown but rapid progression imposed by increasing speed and reducing body weight support; the 20‐minute training replaced the 20‐minute gait training component of the control Control: usual care 3 hours per day for 6 days per week for 2 to 3 weeks until discharge; included kinesitherapy (1 hour per day), occupational therapy (1 hour per day), and physical therapy (1 hour per day): the physical therapist included 20 minutes of gait training comprising stepping, standing, turning, etc, but not continuous walking Setting: rehabilitation centre | |
| Outcomes | Included outcomes: cycle performance work rate (Watts); VO2 peak; blood pressure; FAC; FIM (lower limb); gait speed maximal (5 metres); gait endurance (5 minutes); gait economy Other outcomes: stance symmetry; contact time (seconds); stride cadence steps/minute and other biomechanical gait parameters | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by using random numbers to pre‐assign participants based on recruitment order |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Some degree of attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | No withdrawals, no planned ITT |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Low risk | Balanced exposure |
Donaldson 2009
| Methods | Design: phase II randomised multicentre trial; 3 centres; mixed training plus usual care versus usual care ‐ during usual care ‐ i.e. functional strength training (FST) plus conventional physiotherapy (CPT) versus CPT alone and versus CPT plus CPT Randomisation: computer‐generated random allocation. Allocation was stratified by baseline Action Research Arm Test score in blocks of 3 within each stratum Allocation concealment: sequentially numbered, sealed, opaque envelopes held by an independent investigator Blinding: assessor blinded to group allocation ITT: yes Measurements: at the end of intervention (6 weeks) and 12 weeks after (follow‐up) Withdrawals: 2 participants were lost at outcome in the CPT group (new stroke = 1; bail = 1). A further 11 participants were lost at follow‐up. 5 participants in the CPT group (3 unwell, 1 moved abroad, 1 bail) and 2 in the CPT + FST group (1 unwell, 1 moved abroad) |
|
| Participants | Randomised: total 30 participants. 10 participants were randomised to CPT, 10 to CPT + CPT, and 10 to CPT + FST (only the results from the CPT and the CPT + FST groups were included in this review, total 20) Intervention: CPT + FST = 10 participants, 3 males and 7 females; mean age: 72.6 Control: CPT = 10 participants, 5 males and 5 females; mean age: 72.6 Inclusion criteria: inpatients; infarction of the anterior cerebral circulation between 1 weeks and 3 months after stroke; some voluntary contraction in the upper affected limb; no obvious unilateral visuospatial neglect; ability, prior to the stroke, to use the paretic upper limb to lift a cup and drink; ability to follow a 1‐stage command Exclusion criteria: not reported |
|
| Interventions | Intervention: CPT + FST. FST = repetition and goal directed functional activity of the upper limb; hand positioning; hand grip activities; hand manipulation involving objects; improving power of shoulder/elbow muscles to enable appropriate hand position. Frequency of intervention: 1 hour for 4 days/week for 6 weeks Control: CPT included soft issue mobilisation, facilitation of muscle activity/movement, positioning; joint alignment; tactile and proprioceptive input. Frequency of intervention: 1 hour for 4 days/week for 6 weeks Setting: hospital setting |
|
| Outcomes | Included outcomes: upper limb strength (hand grip force, pinch grip force; isometric elbow flexion and extension force); upper limb function (ARAT) Other outcomes: dexterity (i.e. 9‐HPT) |
|
| Notes | Not clear how this relates to NCT00322192 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random allocation Allocation was stratified by baseline ARAT score in blocks of 3 within each stratum |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed, opaque envelopes held by an independent investigator |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No attention control in the comparison, however: Quote: "The majority of subjects (68%) who completed outcome measures were unsure as to which group they had been allocated (CPT 75%, CPT + CPT 60%, CPT + FST 70%; Table 3). Only 4 of the 28 subjects (14%) correctly identified the treatment they received. Even in the CPT group who had been told that they would receive no extra therapy, only 1 person correctly identified their grouping." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinded to group allocation; efficacy unknown |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT analysis planned 2/20 (10%) lost at the end of intervention: control CPT group (new stroke = 1; bail = 1) |
| Incomplete outcome data (attrition bias) End of follow‐up | High risk | ITT analysis planned 9/20 (45%) total losses at the end of follow‐up: additional 5 participants in the control CPT group (3 unwell, 1 moved abroad, 1 bail) and 2 in the intervention CPT + FST group (1 unwell, 1 moved abroad) |
| Selective reporting (reporting bias) | Unclear risk | Unclear how the trial relates to NCT00322192; outcomes do not correspond |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure in comparison used CPT versus CPT + FST |
Duncan 1998
| Methods | Design: randomised trial of mixed training versus usual care ‐ after usual care (outpatient) Randomisation mechanism: unknown; method: blocks of 10 Allocation concealment: third party involvement Blinding: unclear ITT: yes Measurements: end of intervention (12 weeks) Withdrawals: none |
|
| Participants | Randomised: 20 participants Intervention: 10 participants; number of males and females unknown; age 67.3 years (SD 9.6); 66 days post‐stroke Control: 10 participants; number of males and females unknown; age 67.8 years (SD 7.2); 56 days post‐stroke Inclusion criteria: 30 to 90 days post‐stroke; minimal/moderately impaired sensorimotor function; available to attend all training sessions; ambulatory with or without supervision or walking aids; living at home within 50 miles Exclusion criteria: medical condition that compromised outcome assessment or prevented fitness training; MMSE score < 18 or receptive aphasia | |
| Interventions | Intervention: mixed training, performed approximately 90 minutes/day 3 days/week for 12 weeks (8 weeks supervised 1:1 with therapist and 4 weeks alone), functional exercises comprising assistive/resistive exercise, balance exercises, upper limb functional activities, walking or cycling; apart from some resisted exercise the training intensity was not quantified Control: usual outpatient care, physical and occupational therapy as advised by the patient's physician, averaging 44 minutes per day, 3.25 days per week for 12 weeks, therapeutic interventions were during home or outpatient visits and comprised balance training (60%), strength training (40%), bimanual activities (50%), and facilitative exercise (30%); cardiorespiratory training was not provided (0%) Setting: home‐based, therapist‐supervised for first 8 weeks | |
| Outcomes | Included outcomes: BI; Lawton Activities of Daily Living; gait endurance (6‐MWT); BBS; gait preferred speed (data lack variance measures) Other outcomes: SF‐36 (non‐standard pooling of data), Jebsen Hand Test; Fugl Meyer (upper and lower extremity) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation used (blocks of 10), method unknown |
| Allocation concealment (selection bias) | Unclear risk | Third party involvement |
| Blinding (performance bias and detection bias) All outcomes | High risk | Degree of attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | Planned ITT; no losses |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure |
Duncan 2003
| Methods | Design: randomised trial of mixed training versus usual care ‐ after usual care (outpatient) Randomisation mechanism: unknown; method: blocks of 6 Allocation concealment: sealed envelopes Blinding: investigator; participants asked to maintain blinding ITT: yes Measurements: end of intervention (12/14 weeks) and 6‐month follow‐up Withdrawals: intervention (10 participants: 6 before (1 renal insufficiency, 1 subclavian steal syndrome, 1 chose withdrawal, 3 recurrent stroke), 4 after the 3‐months follow‐up (1 died, 1 hospital, 2 recurrent stroke); control (11 participants: 2 before (1 withdrew, 1 non‐return), 9 after 3‐months follow‐up (2 died, 2 hospital, 5 withdrew) |
|
| Participants | Randomised: 100 participants Intervention: 50 participants; 23 males and 27 females; age 68.5 years (SD 9.0); 77.5 days post‐stroke (SD 28.7) Control: 50 participants; males and 27 females 23; age 70.2 years (SD 11.4); 73.5 days post‐stroke (SD 27.1) Inclusion criteria: 30 to 150 days post‐stroke; independent ambulation for 25 feet; Fugl‐Meyer scores 27 to 90; Orpington Prognostic Scale 2.0 to 5.2); Folstein Mini‐Mental State score 16 Exclusion criteria: serious cardiac condition; oxygen dependence; severe weight bearing pain; serious organ system disease; life expectancy < 1 year | |
| Interventions | Intervention: mixed training, performed approximately 90 minutes per day 3 days per week for 12 to 14 weeks (36 sessions); training included range of motion and flexibility, strength training, balance, functional upper extremity practice, endurance training via interval training on cycle ergometer. All elements progressive but intensity not quantified Control: usual outpatient care including physiotherapy and occupational therapy for participants who needed. All controls received 30‐minute visit every 2 weeks including provision of health promotion information Setting: home‐based, therapist‐supervised for first 8 weeks | |
| Outcomes | Included outcomes: cognitive and motor subscales of the FIM; SF‐36 subscales; ankle dorsiflexion and knee extension isometric strength (Nm); isometric grip strength (N); BBS; functional reach; VO2 peak; gait speed preferred (10 metre); 6‐MWT; community ambulation (> 0.8 metres/second) Other outcomes: Stroke Impact Scale; cycle duration; Fugl Meyer scores | |
| Notes | Some outcomes reported as change from baseline scores, others reported as means at the end of 6‐month follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation used (blocks of 6), method unknown |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Degree of attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigator blinded; participants asked to maintain blinding |
| Incomplete outcome data (attrition bias) End of intervention | Unclear risk | ITT used 8/100 (8%) losses before outcome assessment intervention 6 (1 renal insufficiency, 1 subclavian steal syndrome, 1 chose withdrawal, 3 recurrent stroke) Control 2 (1 withdrew, 1 non‐return) |
| Incomplete outcome data (attrition bias) End of follow‐up | High risk | ITT used 21/100 (21%) total losses at the end of follow‐up intervention 4 (1 died, 1 hospital, 2 recurrent stroke) Control 9 (2 died, 2 hospital, 5 withdrew) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure |
Eich 2004
| Methods | Design: randomised trial of cardiorespiratory training plus usual care versus usual care ‐ during usual care Randomisation mechanism: picking envelopes; method: restricted Allocation concealment: sealed envelopes Blinding: investigator; efficacy was compromised ITT: yes Measurements: end of intervention (6 weeks) and 3‐month follow‐up Withdrawals: intervention 1 participant (refusal) after the 6‐week training |
|
| Participants | Randomised: 50 participants Intervention: 25 participants; 17 males and 8 females; age 62.4 years (SD 4.8); 43 days post‐stroke (SD 15) Control: 25 participants; 16 males and 9 females; age 64 years (SD 9); 44 days post‐stroke (SD 18) Inclusion criteria: aged 50 to 75 years; first stroke; time since stroke < 6 weeks; walk 12 metres with/without assistance; Barthel score 50 to 80; participating in 12‐week comprehensive rehabilitation programme; stable cardiovascular responses; no non‐stroke walking impairments; able to understand purpose and content of study | |
| Interventions | Intervention: cardiorespiratory training, performed 30 minutes per day 5 days per week for 6 weeks; progressive treadmill training with either no or minimal support of body weight; intensity was 60% of heart rate reserve Control: both groups received usual care comprising individual physiotherapy based on Bobath concept plus occupational and speech therapy, and neuropsychology as required Setting: rehabilitation unit ‐ inpatient care | |
| Outcomes | Included outcomes: gait speed maximal (10 metres); gait endurance (6‐MWT) Other outcomes: RMA (non‐normal data); walking quality scale (non‐normal data) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Restricted randomisation; independent person picking one of (initially) 50 sealed envelopes |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes; opaque and numbered unknown |
| Blinding (performance bias and detection bias) All outcomes | High risk | No suitable attention control |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "one could not fully exclude the possibility that the outcome observers were not totally blind" |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT planned Only 1/50 (2%) lost: intervention 1 participant (refusal) after the 6‐week training |
| Incomplete outcome data (attrition bias) End of follow‐up | Unclear risk | ITT planned Only 1/50 (2%) lost overall |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure |
Flansbjer 2008
| Methods | Design: randomised trial of resistance training versus no training ‐ after usual care Randomisation: stratified unequal randomisation (2:1) Allocation concealment: non‐sealed envelopes Blinding: physiotherapists who assessed isokinetic strength and gait performance outcomes were blinded to group assignment but the physiotherapist who assessed dynamic strength and muscle tone outcomes was not blinded; patients were not blinded but were told not to disclose group assignment ITT: yes Measurements: at the end of intervention (10 weeks), 5‐month follow‐up, and a 4‐year follow‐up Withdrawals: 1 participant dropped out from the intervention group due to an accident unrelated to strength training. 2 participants were unable to perform follow‐up assessments due to new illness, 4 participants did not wish to continue at follow‐up stage (but were reported in general good health) |
|
| Participants | Randomised: total 25 participants Intervention: 15 participants (16 randomised), 9 males and 6 females; mean age 61 (SD 5) years; time since stroke 18.9 (SD 7.9) months Control: 9 participants, 5 males and 4 females; mean age 60 (SD 5) years; time since stroke 20.0 (SD 11.6) months Inclusion criteria: age 40 to 70 years; 6 months post‐stroke; able to perform isolated extension and flexion movements of the knee; at least 15% reduction in muscle strength in the paretic limb (mean isokinetic peak torque at 60º/sec); walk unsupervised for 200 metres with or without walking aid; no medication, physical, cognitive, or mental dysfunction that could impact upon knee muscle strength, gait performance, or perceived participation; able to understand verbal and written information Exclusion criteria: not reported |
|
| Interventions | Intervention group: 10 weeks of dynamic and isokinetic knee muscle strength training. Each training session started with a warm up of 5 minutes of stationary cycling, 5 repetitions without resistance and 5 repetitions at 25% of maximum load. The participants then performed 6 to 8 repetitions at about 80% of their maximum load with a 2‐minute rest between each set. The participants performed as many repetitions as possible. The load was adjusted every 2 weeks to remain at 80% of their maximum load. Each training session lasted about 90 minutes but the actual progressive strength training time was less than 6 minutes Control group: participants were encouraged to continue daily activities and training but not to engage in any progressive strength training Setting: community dwelling; training in hospital |
|
| Outcomes | Included outcomes: dynamic and isokinetic muscle strength; 3 metre TUG; maximum walking speed; 6‐MWT; SIS ‐ Swedish version; muscle tone assessed with the MAS Other outcomes: none |
|
| Notes | Maximum walking speed data obtained from authors. The physiotherapist that supervised the resistance training was the same that assessed dynamic strength and muscle tone outcomes 4‐year follow‐up data available in secondary publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by gender unequal randomisation (2:1) |
| Allocation concealment (selection bias) | High risk | Non‐sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attention control |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Physiotherapists who assessed isokinetic strength and gait performance outcomes were blinded to group assignment but the physiotherapist who assessed dynamic strength and muscle tone outcomes was not blinded; patient were not blinded but were told not to disclose group assignment Therapists not blinded at 4‐year follow‐up |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT analysis 1/25 (4%) losses; 1 participant dropped out from the intervention group due to an accident unrelated to strength training |
| Incomplete outcome data (attrition bias) End of follow‐up | High risk | 1/25 (4%) total losses at the end of 5‐month follow‐up, ITT analysis used 7/25 (28%) total losses at the end of 4‐year follow‐up and no ITT analysis used. 2 participants were unable to perform follow‐up assessments due to new illness, 4 participants did not wish to continue at follow‐up stage (but were reported in general good health) |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure |
Galvin 2011
| Methods | Design: randomised trial of mixed family‐mediated exercise (FAME) plus usual care versus usual care ‐ during (and after usual care) Randomised mechanism: independent person using computer‐generated random numbers Allocation concealment: sealed envelopes Blinding: assessor not blinded to group allocation ITT: all randomised participants analysed using LOCF Measurements: end of intervention (8 weeks) and at follow‐up (3 months) Withdrawals: 2 participants in the intervention group before outcome assessment (MI and stroke). In the control group 1 withdrew before outcome assessment (1 unwell), 2 died before follow‐up assessment |
|
| Participants | Randomised: 37 participants Intervention: 19 participants; 7 males and 13 females; mean age 69.95 years (SD 11.7) Control: 18 participants; 13 males and 7 females; mean age 63.15 years (SD 13.3) Inclusion criteria: 2 weeks after stroke onset; diagnosed as first unilateral stroke; older than 18 years of age; participating in a physiotherapy programme; medically stable family member willing to participant in the programme Exclusion criteria: impairment of cognition, younger than 18 years |
|
| Interventions | Invention group: individualised FAME programmes daily for 35 minutes for 8 weeks aiming to improve stability, gait velocity, and lower limb strength plus usual care (routine physiotherapy) Control group: usual care (routine physiotherapy) Setting: rehabilitation unit |
|
| Outcomes | Included outcome: lower limb Fugl‐Meyer Assessment; MAS; BBS; 6‐MWT | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent person using computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelope; whether opaque and numbered unknown |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attention control |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessor not blinded to group allocation |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT used; all randomised participants analysed using LOCF 3/37 (8%) lost from intervention group 2 (MI and stroke); control group 1 (1 unwell), 2 died before follow‐up assessment |
| Incomplete outcome data (attrition bias) End of follow‐up | Unclear risk | ITT used; all randomised participants analysed using LOCF 5/37 (14%) total losses; control group 2 (died) |
| Selective reporting (reporting bias) | Low risk | Reported outcomes correspond to protocol NCT00666744 |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure |
Glasser 1986
| Methods | Design: randomised trial of cardiorespiratory training plus % usual care versus usual care ‐ during usual care Randomisation: unknown Allocation concealment: unknown Blinding: unknown ITT: no withdrawals Measurements: end of intervention (10 weeks) Withdrawals: none |
|
| Participants | Randomised: 20 participants Intervention: 10 participants; 4 males and 6 females Control: 10 participants; 6 males and 4 females All participants age 40 to 75 years and were 3 to 6 months post‐stroke; all participants exhibited hemiparesis with upper and lower extremity motor dysfunction; some showed sensory deficits and mild expressive or receptive aphasia Inclusion criteria: unknown Exclusion criteria: unknown | |
| Interventions | Intervention: cardiorespiratory training: isokinetic ergometer (Kinetron) training twice a day 5 days per week for 10 weeks; the intensity was maintained at 50 to 100 psi and duration of each session progressed from 10 to 30 minutes over the first 5 weeks Control: therapeutic exercise and gait training 1 hour per session 2 sessions per day, 5 days per week for 5 weeks Setting: physical therapy department | |
| Outcomes | Included outcomes: gait speed maximal (6 metres) Other outcomes: FAPS | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Some attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Unclear risk | Some attention control; may be a balanced exposure |
Globas 2012
| Methods | Design: randomised, cross‐over, controlled trial of high‐intensity cardiorespiratory training plus usual care versus usual care ‐ after usual care Randomised mechanism: computer‐based pseudo‐random number generator and Moses‐Oakford assignment algorithm to perform stratified block allocation scheme (3 blocks, allocation 1:1) Allocation concealment: not reported Blinding: not blinded to participants; unknown if blinded to assessors Measurements: end of intervention (3 months); follow‐up data (12 months) not used Withdrawals: 2 participants in the intervention group, 1 due to recurrent stroke, 1 due to transport problems. Other dropouts were reported but these occurred after the cross‐over part of the trial began and are therefore uncontrolled |
|
| Participants | Randomised: 36 participants completed endpoint investigation, 32 participants completed 12‐month follow‐up Intervention: 18 participants; 14 males and 4 females; mean age 68.6 years (SD 6.7) Control: 18 participants; 15 males and 3 females; mean age 68.7 years (SD 6.1) Inclusion criteria: greater than 6 months post‐stroke, confirmed diagnosis of ischaemic stroke via CT and/or MRI scans; hemiparetic gait as evaluated by a neurologist; at least 1 clinical sign for paresis, spasticity, or circumduction during gait; ability to treadmill walk at greater than 0.3 km/hr for 3 minutes Exclusion criteria: unstable angina pectoris; heart failure; haemodynamically significant valvular dysfunction; peripheral arterial occlusive disease; dementia; aphasia; major depression; already performing aerobic exercise training (> 20 minutes/day, > 1 day/week) |
|
| Interventions | Invention group: 39 sessions of 30 to 50 minutes of treadmill training 3 times/week for 3 months. Training intensity was 60% to 80% maximum heart rate. Treadmill training was progressed as tolerated by 1 to 5 minutes/week and by 0.1 to 0.3 km/hr every 1 to 2 weeks. Treadmill inclination was 0° Control group: usual care physiotherapy included passive, muscle tone‐regulating exercises for upper and lower limbs with element of balance training. Performed for 1 hour for 1 to 3 times/week. Control group also completed cross‐over period of treadmill training which was similar in protocol except for 2° inclination Setting: outpatients rehabilitation clinic |
|
| Outcomes | Included outcome: peak exercise capacity (VO2 peak); 6‐MWT; 10 Metre Timed Walks; 5‐Chair Rise Test; BBS; RMI; SF‐12 | |
| Notes | Cross‐over part of the trial not included Advertisements used for recruitment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based, stratified, block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attention control |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment not blinded |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT (LOCF) used 2/36 (6%) dropouts from intervention group (1 recurrent stroke,1 transportation problems) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | May be self selection bias due to use of newspaper adverts |
| Imbalanced exposure | Unclear risk | Some attention control but time appears not to be balanced |
Gordon 2013
| Methods | Design: randomised controlled trial of aerobic (walking) training versus massage Randomisation: block randomised Allocation concealment: unclear Blinding: blinded assessor ITT: completed Measurements: 6 weeks and 12 weeks (end of intervention) Withdrawals: 7 participants from the intervention and 5 participants from the control dropped out. | |
| Participants | Randomised: total 128 participants; 64 participants were randomised to intervention, 64 to control Intervention: 64 participants; 29 males (45.3%) and 35 females (54.7%); mean age: 63.4 (SD 9.4); 384 (SD 108) days after stroke Control: 64 participants; 29 males (45.3%) and 35 females (54.7%); mean age: 64.9 (SD 11.1); 354 (SD 108) days after stroke Inclusion criteria: ≥ 40 years of age, community‐dwelling, 6 to 24 months post‐stroke, able to walk with or without an assistive device Exclusion criteria: not currently in rehabilitation or regular exercise programme, not having any disorder that would compromise exercise training such as unstable cardiovascular diseases, not having any cognitive deficits |
|
| Interventions | Intervention: cardiorespiratory walking training. Participants were supervised by trained instructors to walk briskly along a prescribed course for 15 minutes, 3 times per week, for 12 weeks, initially progressing by 5 minutes over week up to 30 minutes in their home or community. Target heart rate was 60% to 85% of age‐predicted maximum heart rate (220‐age). Training progression was also carried out by increasing speed Control: light massage to the affected limbs for 25 minutes, 3 times per week, for 12 weeks, at home Setting: community/home |
|
| Outcomes | Included outcomes: Physical and Mental Component Summary scores of the Medical Outcomes Survey, 36‐item Short Form Health Survey (SF‐36), Barthel Index, Instrumental activities of daily living dimension of the Older Americans Resources and Services Questionnaire; 6 Minute Walk Test Other outcomes: resting heart rate, lower limb Motricity Index. | |
| Notes | Author provided details of dropouts and withdrawals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "block randomised" but with no further information on how this was achieved |
| Allocation concealment (selection bias) | High risk | Not reported in paper |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of participants or deliverers of intervention or control programmes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinded to group allocation |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | Missing data have been imputed using appropriate methods 12/128 (9.4%) total losses:
|
| Selective reporting (reporting bias) | High risk | Trial register or protocol not available. "Trial was not registered as enrollment commenced before 2005" |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Low risk | Balanced exposure: intervention and control groups were exposed to the same frequency and duration of treatment |
Inaba 1973
| Methods | Design: randomised trial of resistance training plus usual care versus usual care ‐ during usual care Randomisation: unknown Allocation concealment: unknown Blinding: outcome assessor ‐ unclear ITT: no Measurements: end of intervention (4 to 8 weeks) and 2‐month follow‐up Withdrawals: unclear: 101/177 patients lost to follow‐up across the control and both intervention groups; 54 patients completed the control versus strength training comparison; estimated dropouts approximately 60 1 reason given for dropouts was discharge before end of the study |
|
| Participants | Randomised: 54 participants Intervention: 28 participants; 11 males and 17 females; age 55.6 years; < 3 months post‐stroke Control: 26 participants; 15 males and 11 females; age 56.9 years; < 3 months post‐stroke All participants had hemiparesis Inclusion criteria: hemiparesis arising from cerebrovascular accident secondary to thrombosis; embolus or haemorrhage; able to follow verbal or demonstrated directions; extend the involved lower limb against a load of 1.1 kg; independent ambulation Exclusion criteria: aetiology of aneurysm or trauma | |
| Interventions | Intervention: progressive resistive exercise once per day for 4 to 8 weeks; extension of the affected lower limb from 90º to full‐knee extension whilst in the supine position on an Elgin table (machine weights), 5 repetitions at 50% maximum weight, and 10 at maximum Control: usual care: conventional functional training, including stretching, 4 to 8 weeks until discharge Setting: rehabilitation centre | |
| Outcomes | Included outcomes: leg strength (10 repetition maximum) lacked variance measures number of participants able to perform 10 activities of daily living | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) End of intervention | High risk | Large numbers of undocumented losses and no ITT analysis Unclear: 101/177 patients lost to follow‐up across the control and both intervention groups; 54 patients completed the control versus strength training comparison; estimated dropouts approximately 60 1 reason given for dropouts was discharge before end of the study |
| Incomplete outcome data (attrition bias) End of follow‐up | High risk | Large numbers of undocumented losses and no ITT analysis Unclear: 101/177 patients lost to follow‐up across the control and both intervention groups; 54 patients completed the control versus strength training comparison; estimated dropouts approximately 60 1 reason given for dropouts was discharge before end of the study |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure |
Ivey 2010
| Methods | Design: randomised trial of cardiorespiratory training versus usual care ‐ after usual care Randomised: blocked allocation schema and computer‐based pseudo‐random number generator Allocation concealment: not reported Blinding: assessors not blinded ITT: no Measurements: end of intervention (6 months) Withdrawals: intervention group 10 participants and control group 17 participants lost to follow‐up, 7 in both groups due to medical reasons unrelated to study procedures; 3 and 10 respectively due to general compliance issues |
|
| Participants | Randomised: 53 participants Intervention: 29 participants; 18 males and 11 females; mean age 62 years (SD 8) Control: 24 participants; 11 males and 13 women; mean age 60 years (SD 8) Inclusion criteria: chronic hemiparetic stroke (> 6 months); completed all conventional usual care Exclusion criteria: history of vascular surgery; vascular disorders of the lower limb; symptomatic peripheral arterial occlusive disease |
|
| Interventions | Invention group: treadmill training for 40 minutes 3 times/week for 6 months at a target intensity of 60% to 70% heart rate reserve, initially started with discontinuous training which progressed to continuous Control group: usual care: 13 targeted active and passive supervised stretching movements of the upper and lower body for 30 to 40 minutes 3 times/week for 6 months Setting: rehabilitation unit |
|
| Outcomes | Included outcome: peak aerobic capacity during treadmill protocol Other outcomes: resting and reactive hyperaemic calf blood flow in both paretic and non‐paretic legs |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked allocation schema and computer‐based pseudo‐random number generator |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants described as not blinded, although there was matched exposure to staff |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors not blinded |
| Incomplete outcome data (attrition bias) End of intervention | High risk | ITT not reported 27/53 (51%) losses; intervention group 10 and control group 17 due to medical reasons unrelated to study procedures; 3 and 10 respectively due to general compliance issues |
| Selective reporting (reporting bias) | Unclear risk | Relationship to trial register entries unclear |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Low risk | Matched exposure |
Ivey 2011
| Methods | Design: randomised trial of cardiorespiratory training versus usual care ‐ after usual care Randomised: mechanism unknown Allocation concealment: unknown Blinding: unknown ITT: no Measurements: end of intervention (6 months) Withdrawals: 13 participants withdrew at the end of intervention, reasons unknown |
|
| Participants | Randomised: 38 participants completed study; 51 may have been randomised Intervention: 19 participants; mean age 61 years (SD 8) Control: 19 participants; mean age 62 years (SD 10) Inclusion criteria: chronic hemiparetic stroke with mild to moderate hemiparetic gait; completed all conventional usual care; still present with residual hemiparetic gait deficits more than 6 months post‐stroke Exclusion criteria: inability for insonation of the middle cerebral artery bilaterally |
|
| Interventions | Invention group: treadmill training for 40 minutes 3 times/week for 6 months at a target intensity of 60% to 70% heart rate reserve, initially started with discontinuous training which progressed to continuous Control group: usual care: 13 targeted active and passive supervised stretching movements of the upper and lower body for 30 to 40 minutes 3 times/week for 6 months Setting: rehabilitation unit |
|
| Outcomes | Included outcomes: 6‐MWT, peak aerobic capacity during treadmill protocol Other outcomes: middle cerebral artery blood flow velocity bilaterally during normocapnia and hypercapnia (6% CO2) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Mechanism not described, number randomised not clear |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Attention control was included |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) End of intervention | High risk | ITT analysis not reported There may have been losses after randomisation; up to 13/51 (25%) |
| Selective reporting (reporting bias) | Unclear risk | Relationship to trial register entries unclear |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Low risk | Matched exposure |
James 2002
| Methods | Design: randomised trial of mixed training versus no intervention ‐ after usual care Randomisation mechanism: computer; method: blocks of 4 Allocation concealment: sealed envelopes Blinding: investigator ITT: yes Measurements: end of intervention (4 weeks) Withdrawals: control group 2 dropped out (neurological problems) |
|
| Participants | Randomised: 20 participants Intervention: 10 participants; 4 males and 6 females; age 76.1 years (SD 12.33); 1826 days post‐stroke Control: 10 participants; 2 males and 8 females; age 80.8 years (SD 9.0); 1845 days post‐stroke Inclusion criteria: stroke with hemiplegia; ability to give informed consent Exclusion criteria: no complicating medical history (cardiac, pulmonary, or neurological); no severe deficits in communication, memory or understanding; no painful orthopaedic conditions which could limit participation | |
| Interventions | Intervention: mixed training, performed 90 to 120 minutes per day 3 days per week for 4 weeks Warm up followed by half squats; chair squats; small knee bends; standing on affected leg; single‐leg half squat on affected leg; standing on unaffected leg and bending affected hip and knee; stair stepping; stepping on spot; walking indoors and outdoors; stepping forwards, backwards and sideways; opening and closing doors; walking and placing/lifting objects; placing objects on shelves. Finished with a cool down; progression achieved increasing pulse rate from 50% (first 2 weeks) to 60% (last 2 weeks) of heart rate reserve, increasing total distance walked, and increasing step height and repetition number Control: no intervention Setting: patients' homes | |
| Outcomes | Included outcomes: gait speed preferred (5 metres with mixed surfaces and a dead turn at 2.5 metres) Other outcomes: functional walking ability questionnaire; upright motor control test; SF‐36 ‐ older version | |
| Notes | Unpublished thesis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation (groups of 4) using computer software |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes; opaque and numbered unknown |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Investigator blinded |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT analysis used 2/20 (10%) losses; 2/10 in control group (neurological problems) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure |
Jin 2013
| Methods | Design: randomised controlled trial of progressive aerobic cycling training versus control Randomisation: stratified randomisation; stratification based on age, gender and deficit severity Allocation concealment: information not included Blinding: "single blind" but does not outline in the paper who was blinded ITT: not completed Measurements: 12 weeks (end of intervention) Withdrawals: before randomisation | |
| Participants | Randomised: total 128 participants. 65 participants were randomised to intervention, 63 to control Intervention: 65 participants; 46 males (71%) and 19 females (29%); mean age: 57.6 (SD 6.6); 561 (SD 156) days after stroke Control: 63 participants; 45 males (71%) and 18 females (29%); mean age: 56.3 (SD 6.5); 537 (SD 144) days after stroke Inclusion criteria: 42 to 68 years, Chinese Han population, first ischaemic stroke (< 6 months), independent mobility with or without an assistive device Exclusion criteria: haemorrhagic stroke, brainstem lesions and/or bilateral signs, diabetes mellitus or other concomitant nervous system disorders, cardiac of pulmonary disease possibly affecting the autonomic nervous system, any clinically relevant arrhythmia, heart failure, renal failure, unstable angina, uncontrolled hypertension, peripheral arterial occlusive disease, aphasia, dementia, untreated major depression, and other medical conditions that precluded participation in exercise training and conventional treatment |
|
| Interventions | Intervention: cardiorespiratory training: cycling training for 40 minutes per day, 5 times per week, target intensity of 50% to 70% for 12 weeks. The training was started at a low intensity (40% to 50% HR reserve) for 10 to 20 minutes and increased by approximately 5 minutes every 2 weeks as tolerated. Aerobic intensity was similarly progressed by 5% HR reserve every 2 weeks. Participants pedalled for 6 to 10 minutes in each task condition, and 2 to 3 minutes of rest were provided between each task Control: matched duration of conventional therapy including supervised stretching movements lasting 35 minutes and 5‐minute low‐intensity over‐ground walking training at 20% to 30% HR reserve, 5 times per week Setting: rehabilitation centre |
|
| Outcomes | Included outcomes: peak VO2 (L/min), peak VO2, mL/kg/min, resting systolic BP, resting diastolic BP, BMI, 6 Minute Walk Distance, Berg Balance Scale, spasticity (modified Ashworth Scale), paretic knee strength, non‐paretic knee strength Other outcomes: resting HR, peak HR, peak SBP, peak DBP |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After baseline testing, the subjects were stratified according to age, gender and deficit severity, then, randomly assigned to either an aerobic cycling training group or a control group by drawing lots." |
| Allocation concealment (selection bias) | High risk | Not reported in paper |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not clear who was blinded; exposure was balanced |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported in paper |
| Incomplete outcome data (attrition bias) End of intervention | High risk | No information on dropouts or incomplete outcome data after randomisation and at end of intervention. ITT not carried out |
| Selective reporting (reporting bias) | High risk | Trial register or protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Unclear risk | Balanced exposure: matched duration of treatment exposure between intervention and control groups |
Kang 2012
| Methods | Design: randomised trial of cardiorespiratory training plus usual care versus non‐exercise intervention plus usual care ‐ after usual care Randomised: picking sealed envelopes Allocation concealment: sealed envelopes Blinding: assessor blinded to group allocation ITT: not reported Measurements: end of intervention (4 weeks) Withdrawals: intervention group 1 participant due to lack of lack of participation |
|
| Participants | Randomised: 21 participants Intervention: 11 participants; 6 males and 4 females, mean age 56.3 (SD 7.6); 13.5 days post‐stroke (SD 4.0) Control: 10 participants; 6 males and 4 females, mean age 56.1 (SD 7.8); 15.1 days post‐stroke (SD 7.4) Inclusion criteria: hemiparetic stroke 6 months after diagnosis; ability to walk for 15 minutes; without visual disabilities; MMSE score of 21 or higher; Brunnstrom stage greater than 4 Exclusion criteria: cardiovascular problems, orthopaedic, and other neurological diseases except stroke for influencing gait |
|
| Interventions | Invention group: treadmill training for 30 minutes/day 3 times/week for 4 weeks, progressed by 0.1 km/h each time stable walking for 20 seconds was achieved Control group: non‐exercise intervention of general stretching added range of motion exercises plus usual care Setting: rehabilitation centre |
|
| Outcomes | Included outcomes: TUG; Functional Reach Test; 10 metre Maximal Walk Test; 6‐MWT | |
| Notes | 1 arm of this 3‐group RCT was not used (treadmill with optic flow intervention) 10 metre Maximal Walk Test data converted from m/sec into m/min |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent person picking sealed envelopes |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes; whether opaque or numbered unknown |
| Blinding (performance bias and detection bias) All outcomes | High risk | Attention control was included |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessors |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT not reported 1/21 (5%) losses; intervention group 1 participant (lack of participation) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Low risk | Balanced exposure |
Katz‐Leurer 2003
| Methods | Design: randomised trial of cardiorespiratory training plus usual care versus usual care ‐ during usual care Randomisation mechanism: unknown; method: blocks based on side of lesion Allocation concealment: not reported Blinding: investigator; efficacy unknown ITT: unknown Measurements: end of intervention and 6‐month post‐stroke follow‐up Withdrawals: intervention: no losses at the end of intervention, 5 losses at 6‐month follow‐up (4 not located, 1 died); control: 2 discontinued intervention (1 acute myocardial infarction, 1 deep vein thrombosis), 6 losses to follow‐up (3 not located, 1 died, 2 recurrent stroke) |
|
| Participants | Randomised: 92 participants Intervention: 46 participants; 26 males and 20 females; age 62 years (SD 11); time since stroke unknown Control: 46 participants; 23 males and 23 females; age 65 years (SD 11); time since stroke unknown Inclusion criteria: age > 50 years; > 6 months after first ever stroke; walk 40 metres with +/‐ rest, +/‐ assistive device; ≥ stage 3 of Chedoke‐McMaster Stroke Assessment: tolerate 45 minutes of exercise with rest intervals; non‐participation in other therapy programmes Exclusion criteria: comprehensive aphasia; not medically stable; musculoskeletal problems not associated with stroke | |
| Interventions | Intervention: cardiorespiratory training: cycle ergometer; 8‐week programme: (1) 20 minutes per day 5 days per week for 2 weeks of intermittent (10 x 1 minute) exercise progressing to 20 minutes continuous exercise by end of week 2; (2) 30 minutes per day 3 days per week for 6 weeks not exceeding 60% heart rate reserve; ACSM criteria for cardiorespiratory training met Control: usual physiotherapy, occupational therapy, speech therapy, and group activity/exercise Setting: rehabilitation centre | |
| Outcomes | Included outcomes: FIM; blood pressure; maximum cycle workload (Watts); comfortable walking speed (10 metre) gait endurance; distance until fatigue; FAI; stair climbing Other outcomes: SSS | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation based on side of lesion; mechanism not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigator blinded; efficacy unknown |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT not reported 2/96 (2%) lost at the end of intervention Intervention: no losses, control: 2 discontinued (1 acute myocardial infarction, 1 deep vein thrombosis) |
| Incomplete outcome data (attrition bias) End of follow‐up | Unclear risk | ITT not reported 13/96 (14%) total losses at the end of 6‐month follow‐up Intervention: 5 (4 not located, 1 died); control 6 (3 not located, 1 died, 2 recurrent stroke) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Unclear risk | Unclear |
Kim 2001
| Methods | Design: randomised trial of resistance training versus non‐exercise intervention ‐ after usual care Randomisation mechanism: unknown; method: stratified based on gender, age (50 to 59 or 60+ years), and time since onset of stroke (6 months to 2 years/2+ years) Allocation concealment: unknown Blinding: investigator; participants blinded to purpose of interventions ITT: unknown Measurements: end of intervention (6 weeks) Withdrawals: none |
|
| Participants | Randomised: 20 participants Intervention: 10 participants; 7 males and 3 females; age 60.4 years (SD 9.5); 4.9 years post‐stroke (SD 3.3) Control: 10 participants; 7 males and 3 females; age 61.9 years (SD 7.5); 3.2 years post‐stroke (SD 1.2) All participants had hemiparesis Inclusion criteria: age > 50 years; > 6 months after first ever stroke; walk 40 metres with +/‐ rest, +/‐ assistive device; stage 3 of Chedoke‐McMaster Stroke Assessment; tolerate 45 minutes of exercise with rest intervals; non‐participation in other therapy programmes Exclusion criteria: comprehensive aphasia; not medically stable; musculoskeletal problems not associated with stroke | |
| Interventions | Intervention: isokinetic dynamometer (Kin‐Com); 45 minutes per day 3 days per week for 6 weeks; after a warm up this comprised 30 minutes of 3 x 10 resisted repetitions of maximal effort concentric hip flexion/extension, knee flexion/extension and ankle dorsiflexion/plantarflexion of the affected lower limb; progression in the resistance was achieved by increasing the preload on the Kin‐Com device; ACSM criteria for resistance training met Control: exactly the same as intervention except the resisted contractions replaced with passive range of motion movements Setting: rehabilitation centre | |
| Outcomes | Included outcomes: gait preferred speed (metres/minute over 8 metres); gait maximum speed (metres/minute); stair climbing speed (stairs/second); composite strength score for the affected (trained) lower limb Other outcomes: stair walking performance (4 x 18 cm steps) self selected and maximal; physical functioning and mental health components of the SF‐36; composite strength score for the affected (trained) lower limb | |
| Notes | Data reported as change scores | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mechanism unknown; method stratified based on gender, age (50 to 59 or 60+ years), and time since onset of stroke (6 months to 2 years/2+ years) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Attention control used; participants blinded to purpose of interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigator blinded; efficacy unknown |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT not reported No losses |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Low risk | Balanced exposure |
Kim 2014a
| Methods | Design: randomised trial of community walking training programme plus usual care versus usual care (conventional PT and OT) during usual care Randomisation: sealed envelopes prepared in advance Allocation concealment: sealed envelopes Blinding: outcome assessment ITT: no Measurements: end of intervention (4 weeks) Withdrawals: 4 dropouts ‐ 2 from intervention group and 2 from control group |
|
| Participants | Randomised: total 26 participants; 13 to intervention and 13 to control Intervention: 11 participants; 6 male, 5 female; mean age: 50.18 years (SD 10.29); 109 days post‐stroke (SD 108) Control: 11 participants; 7 male, 4 female; mean age 50.73 years (SD 7.24); 273 days post‐stroke (SD 108) Inclusion criteria: hemiparesis from single stroke occurring at least 6 months previously; sufficient cognition to comprehend study purpose; gait speed < 0.8 m/s; ability to walk 10 m independently without device; no musculoskeletal condition that could potentially affect ability to walk safely; no hemispatial neglect Exclusion criteria: participation in other studies or rehab programmes; severe heart disease or other uncontrolled hypertension or pain |
|
| Interventions | Intervention: cardiorespiratory training, walking programme, 30 minutes per day 5 times/week for 4 weeks Week 1 – 200 m route, walking near hospital Week 2 – 300 m route, outside hospital/uneven ground Week 3 – 400 m route, uneven ground, gradual slope, unpaved road, obstacles Week 4 – walking around shopping centre Control: conventional PT and OT Setting: community‐based intervention delivered during inpatient care |
|
| Outcomes | Included outcomes: walking function measured using 10 m walk test, 6 minute walk assessment and "Community walking assessment" (300 m route, different terrains and gradients) Other outcomes: social participation measured with SIS |
|
| Notes | Change from baseline scores reported and used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sealed envelopes marked on the inside with an "O or X". Unclear how this was administered. |
| Allocation concealment (selection bias) | High risk | Unclear whether sealed envelopes were numbered, opaque, or opened sequentially. Also the envelopes were "… marked on the inside with an O or X". Therefore concealment may be threatened. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "… the assessor was blinded" |
| Incomplete outcome data (attrition bias) End of intervention | High risk | ITT analysis not used 4/26 (15%) losses; 2 participants lost from each group Due to health, personal reasons or discharge; distribution between groups unknown |
| Selective reporting (reporting bias) | High risk | No trial protocol available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure |
Kuys 2011
| Methods | Design: randomised, single‐blind trial of cardiorespiratory plus usual care versus usual care ‐ during usual care Randomised: independent researcher generated random sequence in blocks of 4 using computer‐generated random number sequence Allocation concealment: consecutively numbered envelopes Blinding: outcome assessors ITT: yes Measurements: end of intervention (6 weeks) and 3‐month follow‐up Withdrawals: intervention group (2 participants before end of intervention (1 withdrew, 1 due to fall); 2 participants before follow‐up (1 moved, 1 medical condition); control group (3 participants before follow‐up (1 unable to be contacted, 1 medical condition, 1 moved) |
|
| Participants | Randomised: 30 participants Intervention: 15 participants; 7 males and 8 females; mean age 63 years (SD 14); 52 days post‐stroke (SD 32) Control: 15 participants; 7 males and 8 females; mean age 72 years (SD 17); 49 days post‐stroke (SD 30) Inclusion criteria: first stroke diagnosed via CT; referred for physiotherapy rehabilitation; scored 2 or more MAS; medically stable; MMSE score of at least 24 Exclusion criteria: normal gait speed (> 1.2 m/s); cardiovascular problems |
|
| Interventions | Invention group: treadmill walking for 30 minutes 3 times/week for 6 weeks at 40% to 60% heart rate reserve (initially starting at 40% heart rate reserve, progressing by 5% to 10% increase each week until 60% reached) Control group: usual physiotherapy care Setting: 2 rehabilitation units |
|
| Outcomes | Included outcomes: 10 metre Walk Test; comfortable walking speed; 6‐MWT Other outcomes: walking kinematic data |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent researcher generated random sequence in blocks of 4 using computer‐generated random number sequence |
| Allocation concealment (selection bias) | Unclear risk | Consecutively numbered envelopes; not reported whether these were sealed and opaque |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessors |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT analysis used 2/30 (7%) losses Intervention group 2 (1 withdrew, 1 due to fall) |
| Incomplete outcome data (attrition bias) End of follow‐up | High risk | ITT analysis used 7/30 (23%) total losses Intervention group 2 (1 moved, 1 medical condition); control group 3 (1 unable to be contacted, 1 medical condition, 1 moved) |
| Selective reporting (reporting bias) | Low risk | All included outcomes were described in trial registry ACTRN12607000412437. Planned oxygen uptake measures not reported |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure |
Langhammer 2007
| Methods | Design: randomised trial of mixed training versus usual care ‐ after usual care ‐ i.e. intensive exercise (with emphasis on endurance, strength, and balance) versus regular exercise (no specific treatment was recommended) at discharge. Sample size calculation reported Randomisation: stratified randomisation according to gender and hemisphere lesion (minimisation). Method of randomisation: dice (uneven numbers versus even numbers). Randomisation was performed by an investigator not involved with the patients or the treatment Allocation concealment: unclear. Protocol was sealed for 1.5 years from the start of the study Blinding procedure: outcome assessor blinded ITT: planned but not performed Measurements: 3, 6, and 12 months Withdrawals: 3 participants in the intensive group at discharge (1 dead and 2 withdrawals) and 5 (3 dead and 2 withdrawals) in the regular exercise group at discharge. 1 dead and 1 withdrawal at 3 months and 2 dead at 6 months in the regular exercise control group |
|
| Participants | Randomised: 75 participants Intervention: 35 participants, gender not reported; mean age 76 years (SD 12.7) Control: 40 participants, gender not reported; mean age 72 years (SD 13.6) Inclusion criteria: first‐time stroke, confirmed by CT and voluntary participation Exclusion criteria: more than 1 stroke event, subarachnoid bleeding, tumour, other serious illness, brainstem, or cerebellar stroke |
|
| Interventions | Intervention: intensive individualised training programme supervised by physiotherapists. Endurance = walking indoors and outdoors, stationary bicycling, stair walking, treadmill, etc, at 70% to 80% maximal pulse. Strength = push‐ups, sit‐ups, weight lifting, pulley, etc, at 50% to 60% calculated from 1 repetition maximum. Participants were also encouraged to maintain high activity level apart from that in the training sessions. Frequency: 2/3 times per week (daily in rehabilitation ward); minimum 20 hours every third month, in the first year after stroke Control: rehabilitation and follow‐up treatments according to participants' needs but not on regular basis. No specific treatment was recommended. Participants were, however, encouraged to maintain high activity level Setting: general hospital, patients homes, and community service centres |
|
| Outcomes | Included outcomes: MAS; BI; grip strength measured with a Martin Vigorimeter; occurrences of falls and pain Other outcomes: none |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of dice (uneven numbers versus even numbers). In addition, randomisation was stratified according to gender and hemisphere lesion (minimisation). Randomisation was performed by an investigator not involved with the patients or the treatment |
| Allocation concealment (selection bias) | Unclear risk | Unclear; protocol was sealed for 1.5 years from the start of the study |
| Blinding (performance bias and detection bias) All outcomes | High risk | Some unstructured attention control "The amount of training was equal in the two groups". However, the control intervention was not given on a regular basis |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Experienced investigator, blinded to group allocation |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT analysis 8/75 (11%) losses at the end of intervention; 3 participants in the intensive exercise group at discharge (1 dead and 2 withdrawals) and 5 (3 dead and 2 withdrawals) in the control group at discharge |
| Incomplete outcome data (attrition bias) End of follow‐up | Low risk | ITT analysis 12/75 (16%) losses at the end of follow‐up; 1 dead and 1 withdrawal at 3 months and 2 dead at 6 months in the control group |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Unclear risk | Imbalanced exposure |
Lee 2013
| Methods | Design: randomised trial of close kinetic chain resistance exercise versus open kinetic chain resistance exercise versus no intervention Randomisation: unclear Allocation concealment: information not included Blinding: information not included ITT: no ITT but no losses Measurements: end of intervention (6 weeks) Withdrawals: none |
|
| Participants | Randomised: total 33 participants. 11 to close kinetic chain exercise (CKC), 11 to open kinetic chain exercise (OKC) and 11 to control Intervention 1 (CKC): 11 participants; 7 male, 4 female; mean age: 59.3 (SD 8.87); months after stroke 19.9 (SD 7.59) Intervention 2 (OKC) 11 participants; 7 male, 4 female; mean age 58.8 (SD 6.81); months post‐stroke 20.3 (SD 8.13) Control: 11 participants 6 male 5 female; mean age 60.10 (SD 7.01); months after stroke 19.70 (SD 9.42) Inclusion criteria: age 30 to 65 years; stroke occurring at least 6 months before start of study; sufficient cognition to comprehend study purpose; one‐sided hemiparesis of lower extremity Exclusion criteria: severe cognitive, communicative, perceptual or sensory problems preventing understanding of study purpose; other neurologic or psychiatric problems causing difficulties in following programme; unstable cardiovascular/ventilatory problems |
|
| Interventions | Resistance training 5 times/week for 6 weeks Warm up – 4 reps at 25% of 1‐RM followed by 3 sets (8 to 10 reps) at 70% of 1‐RM adjusted weekly Intervention 1 (CKC): – seated, paretic foot on pedal of a leg press machine with pneumatic resistance, extend leg and slowly flex Intervention 2 (OKC) – sat in chair, back facing leg press exercise machine, knee maintained at 90 degrees of flexion with free distal extremity. Extend and slowly flex knee Control: no intervention (maintained routine activity) Setting: unclear |
|
| Outcomes | Included outcomes: balance (postural sway) Other outcomes: muscle activation (limb muscle EMG recordings) |
|
| Notes | Very similar to Lee 2013a | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not reported in paper |
| Allocation concealment (selection bias) | High risk | Not reported in paper |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not reported in paper |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported in paper |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | No losses |
| Selective reporting (reporting bias) | High risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure between control group and both intervention groups |
Lee 2013a
| Methods | Design: randomised trial of close kinetic chain resistance exercise versus open kinetic chain resistance exercise versus no intervention Randomisation: unclear Allocation concealment: information not included Blinding: information not included ITT: no ITT but no losses Measurements: end of intervention (6 weeks) Withdrawals: none |
|
| Participants | Randomised: total 39 participants. 13 to close kinetic chain exercise (CKC), 13 to open kinetic chain exercise (OKC) and 13 to control Intervention 1 (CKC): 13 participants; 8 male, 5 female; mean age: 49.3 (SD 8.87); months after stroke 14.9 (SD 9.59) Intervention 2 (OKC): 13 participants; 8 male, 5 female; mean age 50.8 (SD 6.81); months post‐stroke 15.7 (SD 8.13) Control: 13 participants; 9 male, 4 female; mean age 49.10 (SD 7.01); months after stroke 15.10 (SD 8.73) Inclusion criteria: hemiparesis secondary to ingle onset unilateral stroke; ability to ambulate independently over 10 m (with/without device); absence of significant lower extremity joint pain and major sensory deficits; absence of significant lower limb contractures; no significant cardiovascular or respiratory symptoms contradictive to walking Exclusion criteria: none stated |
|
| Interventions | Resistance training 5 times/week for 6 weeks Warm up – 4 reps at 25% of 1‐RM followed by 3 sets (8 to 10 reps) at 70% of 1‐RM adjusted weekly Intervention 1 (CKC): seated, paretic foot on pedal of a leg press machine with pneumatic resistance, extend leg and slowly flex Intervention 2 (OKC): sat in chair, back facing leg press exercise machine, knee maintained at 90 degrees of flexion with free distal extremity. Extend and slowly flex knee Control: no intervention (maintained routine activity) Setting: unclear |
|
| Outcomes | Included outcomes: none Other outcomes: barefoot plantar pressure distributions |
|
| Notes | Very similar to Lee 2013 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not reported in paper |
| Allocation concealment (selection bias) | High risk | Not reported in paper |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not reported in paper |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported in paper |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | No losses |
| Selective reporting (reporting bias) | High risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure between control group and both intervention groups |
Lennon 2008
| Methods | Design: pilot randomised study of cardiorespiratory training versus usual care ‐ after usual care. Sample size calculation reported Randomisation: stratified randomisation (by age and sex) into 4 blocks of 6 using a sequence generator by an independent party Allocation concealment: opaque envelopes Blinding: single‐blinded; unclear who was blinded ITT: no but only 1 participant dropped out in the control group Measurements: end of intervention (10 weeks) Withdrawals: 1 participant (refusal) in the control group |
|
| Participants | Randomised: total 48 participants. Participants were recruited from the Stroke Rehabilitation Database (Dublin). Volunteers contacted the research team for initial screening Intervention: 24 participants; 14 males (58%) and 10 females (42%); mean age 59.0 years (SD 10.3); mean number of weeks from stroke 237.3 (SD 110.7) Control: 24 participants; 14 males (58%) and 10 females (42%); mean age 60.5 years (SD 10.0); mean number of weeks from stroke 245.3 (SD 169.8) Inclusion criteria: > 1 year post ischaemic stroke and over 18 years of age; participants were recruited irrespective of their ability to ambulate independently Exclusion criteria: O2 dependence, angina, unstable cardiac conditions, uncontrolled diabetes mellitus, major medical conditions, claudication, cognitive impairment, or beta blocker medication |
|
| Interventions | Intervention: the Cardiac Rehabilitation Programme consisted of cycle ergometry training using either the upper or lower limbs. Exercise load was set at 50% to 60% of the participants' maximal heart rate. Resistance and speed were adjusted daily to ensure progression. Frequency: participants trained twice weekly for 30 minutes each time, for 10 weeks. Measurements performed at week 1 and re‐assessment at week 10. All sessions were supervised by a physiotherapist Control: conventional physiotherapy and occupational therapy; no therapy contained an aerobic exercise component; measurements at week 1 and re‐assessment at week 10. No further details provided Setting: outpatient rehabilitation |
|
| Outcomes | Included outcomes: VO2; BMI; maximum cycle workload; resting systolic blood pressure; resting diastolic blood pressure; total cholesterol; FAI; HADS Other outcomes: resting heart rate; cardiac risk score; rate of perceived exertion | |
| Notes | The trial authors maintained that their pilot study was too small for detecting functional benefits (a minimum of 120 participants in each group would have been required to show expected change in all primary outcomes); possible Hawthorn effect due to the fact that the control group did not receive the comparable non‐exercise related attention to the intervention group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation (by age and sex) into 4 blocks of 6 using a sequence generator by an independent party |
| Allocation concealment (selection bias) | Unclear risk | Opaque envelopes; sealed and numbered unknown |
| Blinding (performance bias and detection bias) All outcomes | High risk | Control group did not receive the comparable non‐exercise related attention to the intervention group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who was blinded |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | No ITT analysis 1/48 (2%) participant dropped out 1 (refusal) in the control group |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure |
Letombe 2010
| Methods | Design: randomised trial of mixed training plus usual care versus usual care Randomisation: information not included Allocation concealment: information not included Blinding: information not included ITT: not completed Measurements: before and end of intervention (4 weeks) Withdrawals: none reported | |
| Participants | Randomised: total 18 participants: 9 participants were randomised to intervention, 9 to control Intervention: 9 participants; 5 males, 4 females; mean age: (combined males and females) 59.1 years (SD 9.4); mean height: unknown; mean weight: unknown; type of stroke: ischaemic 5, haemorrhagic 4; paretic side: right 4, left 5; time since stroke onset: 20 days Control: 9 participants; 6 males, 3 females: mean age: (combined males and females) 60.6 years (SD 8.2); mean height: unknown; mean weight: unknown; type of stroke: ischaemic 5, haemorrhagic 4; paretic side: right 4, left 5; time since stroke onset: 20 days Inclusion criteria: right or left hemiplegia following ischaemic or haemorrhagic hemispheric stroke; a full set of aetiological data (CT and/or MRI scans, Holter ECG, Doppler, cardiac ultrasound); a stable clinical state; well‐balanced treatment (particular in terms of antihypertensives and anticoagulants) Exclusion criteria: existence of disorders associated with hemiplegic motor damage, such as cognitive and memory disorders; hemisensory neglect; the existence of an intercurrent affection or unstable brain lesions |
|
| Interventions | Intervention: participants in the training group (n = 9) received conservative physical therapy for 3 hours per day, 5 days per week, for a period of 4 weeks. Conservative physical therapy consisted of gait exercises, stance exercises, the treatment of orthopaedic disorders, balance work (with a view to subsequently withdrawing gait aids), use of support stockings and braces and maintenance of the freedom of movement of the proximal‐distal limb joints. In addition general exercise training was implemented, with cardiorespiratory exercise (monitored by a heart rate monitor), muscle strengthening, gait exercises, and work focused on executive functions, lasting for between 40 and 60 minutes per day, 4 times a week. Aerobic exercise was included in the form of steady exercise on a semi‐recumbent cycle ergometer (with both feet pedalling) was performed at between 70% and 80% of maximum power Control: Participants in the control group (n = 9) received conservative physical therapy for 3 hours per day, 5 days per week, for a period of 4 weeks. Conservative physical therapy consisted of gait exercises, stance exercises, the treatment of orthopaedic disorders, balance work (with a view to subsequently withdrawing gait aids), use of support stockings and braces and maintenance of the freedom of movement of the proximal‐distal limb joints Setting: hospital setting |
|
| Outcomes | Included outcomes: triangular maximal aerobic power test using a cycle ergometer | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described only as "randomized into two groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not reported; not attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT not reported No participant losses |
| Selective reporting (reporting bias) | High risk | Trial register or protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure |
MacKay‐Lyons 2013
| Methods | Design: randomised trial of body weight supported treadmill training versus control (usual care) Randomisation: computer‐generated blocked randomisation. Allocation concealment: yes Blinding: yes ITT: yes Measurements: end of intervention (12 weeks), 6 months, 12 months Withdrawals: 5 withdrawals by end of intervention ‐ 3 from intervention and 2 from control; 3 more withdrawals by 6‐month follow‐up ‐ 1 from intervention, 2 from control; 5 more withdrawals by 12 month follow‐up – 3 from intervention, 2 from control |
|
| Participants | Randomised: total 50 participants: 45 of these completed the exercise programme 24 randomised to intervention (22 completed intervention) 26 randomised to control (23 completed) Intervention: 24 participants; 15 male, 9 female; mean age: 61.5 (SD 15.4); days after stroke 23.3 (SD 5.7) Control: 26 participants; 14 male, 12 female; mean age 59.0 (SD 12.7); days after stroke 23.1 (SD 4.4) Inclusion criteria: over 18 years, within 1 month of first ischaemic stroke confirmed by neuroimaging, inpatients in stroke rehabilitation centre, able to walk 5 m with or without ambulatory aids, ankle orthoses, or stand by assistance Exclusion criteria: contraindications to maximal exercise stress testing; musculoskeletal or cognitive limitations that could preclude participation in the programme, involvement in other pharmacological or physical intervention studies |
|
| Interventions | Intervention: 60 minutes, 5 days/week for 6 weeks then 3 days/week for 6 weeks 5 to 10 minutes active/passive stretching; 10 to 15 minutes upper extremity training (active exercises and stretching); 10 to 15 minutes lower extremity training (active exercises and stretching) 25 to 30 minutes treadmill gait training (initially treadmill speed 80% to 90% of self paced overground speed with 20% to 30% body weight supported for ambulatory independent participants and 70% to 80% of overground speed with 40% body weight supported for ambulatory dependent participants) Control: 60 minutes, 5 days/week for 6 weeks then 3 days/week for 6 weeks 5 to 10 minutes active/passive stretching; 10 to 15 minutes upper extremity training (active exercises and stretching); 10 to 15 minutes lower extremity training (active exercises and stretching) 5 to 10 minutes of pre‐gait activities in standing followed by 20 to 25 minutes overground walking at comfortable self selected speeds Setting: stroke rehabilitation unit |
|
| Outcomes | Included outcomes: cardiovascular fitness (VO2 peak); 6‐MWT; comfortable walking speed; Berg Balance Scale Other outcomes: Chedoke‐McMaster stages of Recovery Leg and Foot; participant satisfaction with programme |
|
| Notes | 12‐month follow‐up data used in meta‐analyses of end‐of‐retention time point | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, permuted block randomisation stratified by ambulatory status |
| Allocation concealment (selection bias) | Low risk | "A person not involved in the study prepared and safeguarded individual, opaque sealed envelopes containing group and physiotherapist allocation, which were opened after completion of the baseline assessment" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | There was a similar dose of exposure across both groups. Participants were informed they would be allocated to 1 of 2 'intervention' groups. The groups were kept separate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment. Participants instructed not to discuss their intervention with outcome assessor. A test of blinding was also performed and analysed statistically to demonstrate no significant unblinding |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT analysis 5/50 (10%) total losses) 2 lost from BWSTT group (1 moved, 1 for medical reasons) 3 lost from UC group (2 for medical reasons, 1 dropped out as disinterested) "All analyses were conducted on an intention‐to treat basis, carrying the last observation forward for those lost to follow‐up" |
| Incomplete outcome data (attrition bias) End of follow‐up | High risk | ITT analysis (last observation carried forward) 11/50 (22%) total losses 6 months follow‐up: 3 did not complete: 1 from intervention (1 refused); 2 from control (1 refused; 1 lost to follow‐up) 12 month follow‐up: 5 did not complete; 3 from intervention (2 lost to follow up, 1 refused); 2 from control (1 for medical reasons, 1 lost to follow‐up) |
| Selective reporting (reporting bias) | Unclear risk | Not clear whether a protocol exists |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Low risk | Balanced exposure |
Mead 2007
| Methods | Design: explanatory randomised trial of mixed training versus non‐exercise intervention ‐ after usual care Randomisation mechanism: Internet application; minimisation dichotomised on sex; FIM score (120); age (70 years) Allocation concealment: sequence generation and allocation occurred simultaneously Blinding: investigator; participants encouraged to maintain blinding ITT: yes Measurements: end of intervention (12 to 14 weeks) and 4‐month follow‐up Withdrawals: intervention 0; control 4: 1 withdrew before intervention; 3 after end of intervention follow‐up (1 stroke‐related illness, 1 fall, 1 recurrent stroke) |
|
| Participants | Randomised: 66 participants Intervention: 32 participants; 18 males and 14 females; age 72.0 years (SD 10.4); median 171 (IQR 55 to 287) days post‐stroke Control: 34 participants; 18 males and 16 females; age 71.7 years (SD 9.6); median 147.5 (IQR 78.8 to 235.5) days post‐stroke Inclusion criteria: independently ambulatory; living within central or south Edinburgh Exclusion criteria: dysphasia or confusion severe enough to prevent informed consent or impair safety in exercise classes; medical contraindications to exercise training | |
| Interventions | Intervention: mixed training: group circuit training performed 40 to 75 minutes per day 3 days per week for 12 to 14 weeks (36 sessions); after a warm up the training comprised 2 components: (1) a cardiorespiratory circuit (cycle ergometry, raising and lowering an exercise ball, shuttle walking, standing chest press, and stair climbing and descending); (2) resistance training circuit (upper back exercise and triceps extension using Thera‐Band, lifting a weighted pole, a sit‐to‐stand exercise); progression in duration, repetition number, speed, mass of objects and resistance of Thera‐Band whilst maintaining a rate of perceived exertion (6 to 20 scale) of 13 to 60 Control: non‐exercise intervention; seated relaxation involving deep breathing and progressive muscular relaxation; no muscle contractions were involved Setting: rehabilitation hospital | |
| Outcomes | Included outcomes: FIM; NEADL; RMI; functional reach; TUG; sit‐to‐stand time; SF‐36 ‐ version 2; HADS; gait preferred speed; gait economy (VO2 ml/kg/m); lower limb extensor explosive power (W/kg) Other outcomes: EMS (ceiling effect); FAC (ceiling effect) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Internet software based minimisation dichotomised on sex; FIM score (120); age (70 years) |
| Allocation concealment (selection bias) | Low risk | Not applicable; sequence generation and allocation occurred simultaneously |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Suitable attention control Quote: "Patients were blinded to the underlying hypothesis by reiterating the possible benefits of both interventions" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded Quote: "Outcome assessors were blinded by asking patients not to discuss their allocated intervention" |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT analysis 1/66 (2%) lost at the end of intervention; intervention 0; control 1 |
| Incomplete outcome data (attrition bias) End of follow‐up | Low risk | ITT analysis 4/66 (6%) total losses at the end of follow‐up; intervention 0; control group (1 stroke‐related illness, 1 fall, 1 recurrent stroke) |
| Selective reporting (reporting bias) | Low risk | Reported outcome correspond to proposal; Chief Scientist Office of the Scottish Executive (CZB/4/46) |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Low risk | Balanced exposure |
Moore 2010
| Methods | Design: randomised, cross‐over trial of cardiorespiratory training versus no intervention ‐ after usual care ‐ (i.e. intensive locomotor training ‐ including treadmill training ‐ versus delayed cardiovascular training) Randomisation: stratified randomisation according to severity of gait impairment Allocation concealment: sealed envelopes Blinding: investigators were not blinded ITT: not reported Measurements: end of intervention (4 weeks) Withdrawals: none reported |
|
| Participants | Randomised: 20 participants; mean age 50 years (SD 15); males 14, females 6; duration of post‐stroke symptoms 13 months (SD 8); moderate/severe gait limitations 13/7 Intervention: the number of participants randomised to the immediate locomotor training group was not clearly reported Control: the number of participants randomised to the delayed locomotor training group was not clearly reported Inclusion criteria: patients with hemiparesis of > 6 months duration who were attending physical therapy after unilateral supratentorial stroke; all patients were required to walk > 10 metres overground without physical assistance and medical clearance Exclusion criteria: lower extremity contractures; significant osteoporosis; cardiovascular instability; previous history of peripheral or central nervous system injury, cognitive or communication impairment; inability to adhere to study requirements |
|
| Interventions | Intervention: the immediate locomotor training group received 4 weeks of intensive locomotor training after discharge from clinical physical therapy, which consisted of high‐intensity stepping practice on a motorised treadmill while wearing an overhead harness attached to a safety system. Frequency: 2 to 5 days per week for 4 weeks. Intensity: highest tolerable speed with velocity increased in 0.5 kph increments until participants reached 80% to 85% of predicted maximum heart rate or until the participants Rating of Perceived Exertion increased to 17 on the Borg scale. Partial weighted support was reduced in 10% increments as tolerated by participants who needed partial weighted support. Measurements were performed: 4 weeks before termination of usual physical therapy; soon after termination of usual physical therapy; after completion of the 4‐week locomotor training; and again after a delay of 4 weeks after termination of locomotor training Control: delayed locomotor training group. The delayed group was also assessed 4 weeks before and after termination of usual physical therapy, but did not receive locomotor training or any other interventions for 4 weeks after termination of usual physical therapy. After this 4‐week delay the participants received locomotor training as described above Setting: rehabilitation centre |
|
| Outcomes | Included outcomes: preferred gait speed; fastest gait speed; 12‐MWT; O2 cost; peak treadmill speed; VO2 peak, TUG; BBS | |
| Notes | Only data at the end of the first cross‐over period were used for analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation according to severity of gait impairment |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attention control |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Investigators were not blinded |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT not reported No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure |
Mudge 2009
| Methods | Design: randomised trial of cardiorespiratory training versus non‐exercise intervention training ‐ after usual care (circuit‐based rehabilitation versus social and educational sessions); power calculation reported Randomisation: computer‐generated random numbers by an individual not associated with the trial Allocation concealment: not reported Blinding: assessor blinded (unmasking of the independent assessor occurred in 3 cases who inadvertently stated or implied their group allocation) ITT: yes Measurements: end of intervention (4 weeks) and 3‐month follow‐up Withdrawals: 1 participant in the intervention group (disinterest) and 2 participants in the control group (too busy) withdrew at the end of intervention. 3 further participants withdrew from the intervention group (health problems = 2; another stroke = 1) and 2 from the control group (health problems = 1; another stroke = 1) before the end of follow‐up |
|
| Participants | Randomised: 58 participants; median age 71.5 years (range 39.0 to 89.0 years); median 3.9 years after stroke (range 0.5 to 18.7 years); participants were recruited through the Stroke Foundation of New Zealand, stroke clubs, and the local hospital stroke service. Potential candidates were invited to contact the investigators if they wished to participate. All participants walked independently and 26 (45%) used an assistive device. 55 participants completed the trial Intervention: 31 participants were randomised to circuit training; 19 males and 12 females; median age 76.0 (range 39.0 to 89.0); median onset of stroke 3.33 years (range 0.6 to 13.3) Control: 27 participants were randomised to social and educational sessions; 13 males and 14 females; median age 71.0 (range 44.0 to 86.0); median onset of stroke 5.8 years (range 0.5 to 18.7) Inclusion criteria: participants with 1 or more strokes more than 6 months earlier, had been discharged from rehabilitation and were able to walk independently (with an aid if necessary). Some residual gait difficulty was required, as defined by a score of less than 2 on at least 1 of the walking items of the physical functioning scale of the SF‐36 Exclusion criteria: participants were excluded if they had progressive neurological diseases or significant health problems, more than 2 falls in the previous 6 months, unstable cardiac conditions, uncontrolled hypertension, or congestive heart failure |
|
| Interventions | Intervention: participants in the intervention group attended 12 group circuit sessions 3 times per week for 4 weeks. Groups were led by 1 of the principal investigators assisted by 2 physiotherapist students. There were 15 stations in the circuit that were graded to each participant's ability and progressed as tolerated. Each station contained either a task‐oriented gait or standing balance activity (e.g. step‐ups, balance beam, marching in place) or strengthening of a lower extremity muscle with the purpose to improve gait (e.g. lunges, Swiss ball squats, side leg lifts). Total exercise time was 30 minutes including stretching. Measurements performed post‐intervention and at 3‐month follow‐up Control: participants in the control group attended 8 sessions ‐ 4 social and 4 educational sessions (e.g. provide participants with relevant and useful information for everyday activities; provide intellectual stimulation and enjoyment sessions; play a game; cafe outing). Each session lasted 90 minutes. The control group was led by an occupational therapist. Measurements performed post‐intervention and at 3‐month follow‐up Setting: rehabilitation clinic |
|
| Outcomes | Included outcomes: mean number of steps a day measured by the StepWatch Activity Monitor; walking speed and walking endurance Other outcomes: self reported confidence during activity of daily living and self reported mobility assessed by the ABCS, the RMI, and the PADS |
|
| Notes | Randomisation was revealed to each participant by the principal investigator after the second baseline assessment. The trial was limited by the small number of participants. Participants volunteered to participate and were likely to be highly motivated. The sample appeared in fact to be higher functioning in terms of gait speed. A gait endurance component was not included in the training circuit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers by an individual not associated with the trial |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Attention control incorporated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinded; unmasking of the independent assessor occurred in 3 cases who inadvertently stated or implied their group allocation |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT reported 3/58 (5%) lost at the end of intervention: 1 participant in the intervention group (disinterest) and 2 participants in the control group (too busy) withdrew at the end of intervention |
| Incomplete outcome data (attrition bias) End of follow‐up | Low risk | ITT reported 8/58 (14%) lost overall at the end of follow‐up: 3 further participants withdrew from the intervention group (health problems = 2; another stroke = 1) and 2 from the control group (health problems = 1; another stroke = 1) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Unclear risk | Attention control used but there is not an equivalent exposure |
Ouellette 2004
| Methods | Design: randomised trial of resistance training versus non‐exercise intervention ‐ after usual care Randomisation: unknown Allocation concealment: unknown Blinding: investigator ITT: yes Measurements: end of intervention (12 weeks) Withdrawals: intervention: 1 withdrew (cardiac problem) and 1 was lost at follow‐up (hernia); control: 2 withdrew during intervention, 1 was lost at follow‐up (abnormal ECG) |
|
| Participants | Randomised: 42 participants Intervention: 21 participants; number of males and females unknown; age 65.8 years (SD 11.5); 968 days post‐stroke (SD 460) Control: 21 participants; number of males and females unknown; age 66.1 years (SD 9.62); 779 days post‐stroke (SD 558) Inclusion criteria: age ≥ 50 years; 6 months to 6 years after single unilateral mild/moderate stroke with residual lower extremity hemiparesis; community dwelling; independently ambulatory +/‐ walking aids; report of ?2 limitations on the physical function subscale of the SF‐36; ability to travel to the exercise laboratory; willing to be randomised | |
| Interventions | Intervention: progressive resistance training of both lower limbs performed 3 days/week for 12 weeks comprising 3 sets of 8 to 10 repetitions at 70% of 1 repetition maximum (1‐RM); exercises were (1) seated bilateral leg press, and (2) unilateral knee extension, both using pneumatic resistance, and unilateral ankle; dorsiflexion; plantarflexion, both using weights; progression achieved via weekly assessment of 1‐RM; warm up for each exercise was 4 repetitions of 25% 1‐RM Control: non‐exercise: bilateral range of motion and upper body flexibility exercises 3 days/week for 12 weeks Setting: exercise laboratory | |
| Outcomes | Included outcomes: muscle strength (bilateral lower limb extension force); muscle strength (unilateral knee extension, ankle dorsiflexion and ankle plantarflexion); gait endurance (6‐MWT), preferred speed (10 metres) and maximal speed (10 metres); chair rise time (5 repetitions); stair climb time (10 steps); late life function and disability instrument scale; SF‐36 physical function subscale Other outcomes: muscle power ‐ bilateral lower limb extension and unilateral knee extension; geriatric depression scale (data not reported); sickness impact profile; Ewarts self efficacy scale | |
| Notes | Variance reported as standard error and converted to standard deviation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Attention control incorporated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigator blinded; efficacy unknown |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT 5/42 (12%) lost at the end of intervention: Intervention: 1 withdrew (cardiac problem) and 1 was lost at follow‐up (hernia); control: 2 withdrew during intervention, 1 was lost at follow‐up (abnormal ECG) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Low risk | Balanced exposure |
Park 2011
| Methods | Design: randomised, single‐blind trial of cardiorespiratory training plus usual care versus usual care ‐ during usual care Randomisation mechanism: participants blindly pick 1 of 2 cards Allocation concealment: envelopes used Blinding: outcome assessor blind to group allocation ITT: not reported Measurements: end of intervention (4 weeks) Withdrawals: 2 participants (1 from both intervention and control groups) not regularly participating |
|
| Participants | Randomised: 27 participants Intervention: 14 participants; 7 males and 6 females; mean age 59.4 years (SD 8.5) Control: 13 participants; 5 males and 7 females; mean age 56.9 years (SD 7.8) Inclusion criteria: 6 months to 5 years post first stroke; walking speed < 0.7 m/s Exclusion criteria: auditory or visual deficits; no orthopaedic or cardiovascular conditions; cognitive impairment (> 25 MMSE score) |
|
| Interventions | Invention group: 4‐phased walking training programme (progressing 150 metres to 200 metres to 300 metres to 500 metres) 1 hour 3 times/week for 4 weeks Control group: usual physiotherapy care 1 hour daily based on Bobath concept Setting: community based |
|
| Outcomes | Included outcomes: 10 metre Walk Test; 6‐MWT; Community Walk test Other outcomes: walking ability questionnaire; activities‐specific balance confidence scale |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants drew 1 of 2 cards from an envelope |
| Allocation concealment (selection bias) | Unclear risk | Envelopes used; nature of concealment unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Person assessing outcome and analysing data blinded |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT analysis not reported 2/27 (7%) lost at the end of intervention: 1 from intervention and 1 from control groups (not regularly participating) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure |
Pohl 2002
| Methods | Design: randomised trial of cardiorespiratory training plus % usual care versus usual care ‐ during usual care Randomisation mechanism: unknown; method: equal block based on gait speed Allocation concealment: unknown Blinding: investigator; efficacy unknown ITT: no Measurements: end of intervention (4 weeks) Withdrawals: none |
|
| Participants | Randomised: 60 participants. 20 participants were randomised to the speed‐dependent treadmill training group (STT); 20 participants to the limited progressive treadmill training group (LTT) and 20 participants to a conventional gait training group (CGT) Intervention: STT group = 20 participants; 14 males, 6 females; age 57.1 years (SD 13.9); 16.8 (20.5) weeks post‐stroke. LTT group = 20 participants; 16 males, 4 females; age 58.2 years (SD 10.5); 16.2 (16.4) weeks post‐stroke Control: 20 participants; 13 males, 7 females; age 61.6 years (SD 10.6); 16.10 (SD 18.5) weeks post‐stroke Inclusion criteria: left or right hemiparesis for > 4 weeks; impaired gait; no or slight abnormal muscle tone (Ashworth Score 0 and 1); walk without assistance (FAC = 3); 10 metre walk time > 5 seconds and < 60 seconds; class B exercise risk (ACSM 1998); absence of known heart disease; no evidence of heart failure, ischaemia or angina at rest or exercise; appropriate rise in systolic blood pressure and absence of ventricular tachycardia during exercise Exclusion criteria: previous treadmill training; class C or D exercise risk (ACSM 1998); cognitive deficits (MMSE < 26 of 30); movement disorders; orthopaedic or gait‐influencing diseases | |
| Interventions | Intervention: Group 1: STT (structured speed‐dependent treadmill training); 30 minutes per day 3 days per week for 4 weeks; minimal body weight support (10%) for first 3 sessions; speed was increased progressively to the highest speed at which the patient could walk safely. The maximum‐achieved speed was held for 10 seconds followed by a recovery period. Each time the patient successfully completed 10 seconds of walking at the set speed, the speed was increased during the next phase by 10%. Treadmill was run at 0% incline Group 2: LTT (limited progressive treadmill training group); 30 minutes per day 3 days per week for 4 weeks; minimal body weight support for first 3 sessions; speed was increased by no more than 5% of the maximum initial speed each week (20% over 4 weeks); treadmill was run at 0% incline Both intervention groups also received conventional physiotherapy 45 minutes/day 2 days/week for 4 weeks (included some gait training); total 12 hours of treatment Control: conventional gait training that comprised post neuromuscular facilitation and Bobath techniques; 30 minutes/day 3 days/week for 4 weeks. The control group also received conventional physiotherapy 45 minutes per day 2 days per week for 4 weeks (included some gait training); total 15 hours of treatment Setting: rehabilitation centre |
|
| Outcomes | Included outcomes: gait maximum speed; FAC Other outcomes: stride cadence (steps/minute); stride length (metres) | |
| Notes | The control group (20 participants) was divided between the 2 relevant comparisons to avoid exaggeration of overall participant numbers in the analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mechanism unknown; randomised to equal blocks based on gait speed |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Attention control used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Investigator; efficacy unknown |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT no reported No losses |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Low risk | Imbalanced exposure favouring training (control 15 hours > intervention 12 hours) |
Potempa 1995
| Methods | Design: randomised trial of cardiorespiratory training versus non‐exercise intervention ‐ after usual care Randomisation: unknown Allocation concealment: unknown Blinding: unknown ITT: no Measurements: end of intervention (10 weeks) Withdrawals: none |
|
| Participants | Randomised: 42 participants Intervention: 19 participants; 8 males and 11 females Control: 23 participants; 15 males and 8 females All participants aged 43 to 70 years and were 216 days post‐stroke (SD 43) All participants had upper and lower limb hemiparesis Inclusion criteria: medically stable; at least 6 months post‐stroke; completed formal rehabilitation Exclusion criteria: patients with brain stem lesions; any clinical evidence that would preclude maximal exercise testing |
|
| Interventions | Intervention: cardiorespiratory training: cycle ergometer training for 30 minutes per day 3 days per week for 10 weeks; intensity 30% to 50% of maximal effort increasing to maximum sustainable over first 4 weeks Control: non‐exercise intervention: passive range of motion exercises for 30 minutes per day 3 days per week for 10 weeks Setting: unknown | |
| Outcomes | Included outcomes: blood pressure; maximum cycling work rate (Watts) Other outcomes: heart rate at rest and during maximal exercise; respiratory exchange rate and other respiratory variables; exercise duration; Fugl Meyer score | |
| Notes | Variance reported as standard error and converted to standard deviation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT not reported No losses |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Low risk | Balanced exposure |
Richards 1993
| Methods | Design: randomised trial of mixed training plus usual care versus usual care ‐ during usual care Randomisation mechanism: unknown; method: stratified on BI scores Allocation concealment: unknown Blinding: investigator; efficacy unknown ITT: no Measurements: end of intervention (5 weeks) Withdrawals: control group 3 (1 refusal, 2 unknown) |
|
| Participants | Randomised: 18 participants Intervention: 10 participants; 5 males and 5 females; age 69.6 years (SD 7.4 years); 8.3 days post‐stroke (SD 1.4) Control: 8 participants; 2 males and 6 females; age 67.3 years (SD 11.2); 8.8 days post‐stroke (SD 1.5) Inclusion criteria: within 50 km of treatment centre; males and females aged 40 to 80 years; 0 to 7 days after first stroke; middle cerebral artery syndrome identified by CT; under care of neurologist involved in study; willing to sign informed consent Exclusion criteria: other major medical conditions that would interfere with functional capacity or interfere with rehabilitation; patients who were independently ambulatory 1 week after stroke; patients who were unconscious at onset | |
| Interventions | Intervention: mixed training: task‐oriented gait training programme which used a tilt table, resisted exercises using a Kinetron, and treadmill walking, 104 minutes/day 5 days per week for 5 weeks; progression achieved via velocity and resistance (Kinetron) increments Control: traditional neurophysical techniques 109 minutes/day 5 days per week for 5 weeks Setting: hospital | |
| Outcomes | Included outcomes: Barthel Ambulation scores; BBS; gait velocity Other outcomes: Fugl‐Meyer balance; Fugl‐Meyer upper and lower extremity scores |
|
| Notes | A second control group of early conventional therapy was not used for comparison since it differed from the institution usual care; it commenced earlier than usual during hospital care and had substantially longer contact time | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified randomisation based on BI scores |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Suitable attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigator blinded; efficacy unknown |
| Incomplete outcome data (attrition bias) End of intervention | High risk | No ITT analysis 3/18 (17%) total losses at the end of intervention: intervention 0; control group 3 (1 refusal, 2 unknown) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Low risk | Balanced exposure |
Richards 2004
| Methods | Design: randomised trial of mixed training plus % usual care versus usual care ‐ during usual care Randomisation mechanism: unknown; method: variable blocks stratified on time since stroke, disability, and age Allocation concealment: unknown Blinding: investigator; efficacy unknown ITT: yes Measurements: end of intervention (8 weeks) and 3‐month follow‐up Withdrawals: intervention: 9 (2 discontinued intervention: 1 hip fracture, 1 cardiac problem), 5 unavailable for follow‐up; control: 8 (1 withdrew from intervention, 7 unavailable for follow‐up) |
|
| Participants | Randomised: 63 participants Intervention: 32 participants; 22 males and 10 females; age 62.9 years (SD 12); 52 days post‐stroke (SD 22) Control: 31 participants; 21 males and 10 females; age 60.7 years (SD 12); 52.8 days post‐stroke (SD 18) Inclusion criteria: first or second stroke; men or women aged 30 to 89 years; impaired walking; follow verbal instructions; Barthel ambulation score ?10; gait speed of 10 to 60 cm/second Exclusion criteria: cerebral and subarachnoid haemorrhage; major medical problems (cancer, heart conditions, diabetes); receptive or expressive aphasia; lower extremity musculoskeletal disorders affecting gait | |
| Interventions | Intervention: mixed training: task‐oriented gait training programme which used a limb‐load monitor, resisted exercises using a Kinetron, and treadmill walking, intervention occurred during physiotherapy sessions of 60 minutes per day 5 days per week for 8 weeks, progression achieved via velocity and resistance (Kinetron) increments Control: physiotherapy sessions of 60 minutes per day 5 days per week for 8 weeks not including the task‐oriented gait training content above Setting: 2 rehabilitation units | |
| Outcomes | Included outcomes: preferred walking speed; TUG; BI (ambulation subscore); BBS Other outcomes: kinematic gait analysis weakened by missing data in 50% participants; Fugl‐Meyer leg and arm scores | |
| Notes | A second control group of conventional therapy was not used for comparison since (1) it was much shorter in duration, and (2) started later than the training intervention Outcome data imputed from graphs in publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear; randomisation based on variable blocks stratified on time since stroke, disability, and age |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Suitable attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigator blinded |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT analysis 5/63 (8%) losses at the end of intervention; intervention (2 discontinued intervention: 1 hip fracture, 1 cardiac problem); control (1 withdrew from intervention) |
| Incomplete outcome data (attrition bias) End of follow‐up | High risk | ITT analysis 17/63 (27%) total losses at the end of follow‐up; intervention (5 not available); control (7 not available) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Low risk | Balanced exposure |
Salbach 2004
| Methods | Design: randomised trial of cardiorespiratory training versus non‐exercise intervention ‐ after usual care Randomisation mechanism: computer; method: stratified on gait speed Allocation concealment: unknown Blinding: investigator blinded (unblinded during assessment of intervention group 18/42 and control group 16/43) ITT: yes Measurements: end of intervention (6 weeks) Withdrawals: intervention: 3 discontinued (refused to travel, wanted both interventions, groin pain) with 2 of these lost to follow‐up; control: 4 discontinued (MI, prostate cancer, fall + fracture, wanted other intervention) with 3 of these lost to follow‐up |
|
| Participants | Randomised: 91 participants Intervention: 44 participants; 26 males and 18 females; age 71 years (SD 12); 239 days post‐stroke (SD 83) Control: 47 participants; 30 males and 17 females; age 73 years (SD 8); 217 days post‐stroke (SD 73) Inclusion criteria: first or recurrent stroke; gait deficit from recent stroke; mental competency; independently ambulatory for 10 metres +/‐ aids or supervision; ability to comprehend instructions; resident in community; discharged from rehabilitation; recent stroke 1 year or less Exclusion criteria: neurological deficit caused by metastatic disease; gait function (6‐MWT) equivalent to healthy norms; discharged to permanent care; comorbidity preventing participation in either intervention | |
| Interventions | Intervention: cardiorespiratory training: task‐oriented circuit training, performed 55 minutes per day 3 days per week for 6 weeks, comprising a warm up followed by 10 walking‐related tasks (step ups, balance beam, kicking ball, stand up and walk, obstacle course, treadmill, walk and carry, speed walk, backward walking, stairs); progression of speed, load and degree of assistance Control: functional practice, whilst seated, of writing, keyboard use, and manipulating cards; some practice encouraged at home. 3 days per week for 6 weeks Setting: 2 rehabilitation centres or hospitals | |
| Outcomes | Included outcomes: gait endurance 6‐MWT; gait comfortable speed; gait maximal speed; TUG; BBS Other outcomes: activity‐specific balance confidence scale | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Computer‐based randomisation stratified on gait speed |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Suitable attention control |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Investigator blinded Unblinded occurred during assessment of intervention group (18/42) and control group (16/43) |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT analysis 7/91 (8%) losses at the end of intervention assessment Intervention: 3 discontinued (refused to travel, wanted both interventions, groin pain) with 2 of these lost to follow‐up; control: 4 discontinued (MI, prostate cancer, fall + fracture, wanted other intervention) with 3 of these lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Low risk | Balanced exposure |
Shin 2011
| Methods | Design: randomised trial of mixed training versus usual care Randomisation: information not included Allocation concealment: information not included Blinding: information not included ITT: not completed Measurements: before and end of intervention (4 weeks) Withdrawals: none reported | |
| Participants | Randomised: total 21 participants. 11 participants were randomised to intervention, 10 to control Intervention: 11 participants; 5 males, 6 females; mean age: (combined males and females) 58.1 years (SD 4.6); mean height: 160.6 cm (SD 6.6); mean weight: 65.2 kg (SD 8.3); type of stroke: unknown; paretic side: right 8, left 3; time since stroke onset: between 6 months and 5 years Control: 10 participants; 3 male, 7 females; mean age: (combined males and females) 57.3 years (SD 4.4); mean height: 164.5 cm (SD 7.1); mean weight: 65.0 kg (SD 7.5); type of stroke: unknown; paretic side: right 5, left 5; time since stroke onset: between 6 months and 5 years Inclusion criteria: 6 months to 5 years post‐stroke with lower limb hemiplegia Exclusion criteria: unable to ride a bicycle or perform functional exercise due to arthritis, low‐back pain or degenerative joint disease; receiving treatment for other symptoms; unable to follow instructions due to low perceptive abilities, cognitive or communication disorder |
|
| Interventions | Intervention: participants in the training group (n = 11) received a total of 60 minutes per day combined exercise training, consisting of 30 minutes of functional strength training and 30 minutes of aerobic exercise 5 days per week for 4 weeks. Functional strength training included bridging, stepping and stair exercises. Aerobic exercise was completed using a cycle ergometer and treadmill for 15 minutes each at less than 40% HR reserve based on age matched maximum HR Control: participants in the control group (n = 10) received conservative physical therapy for 60 minutes per day, 5 days per week, for 4 weeks. Conservative physical therapy consisted of balance, postural control, and gait exercises Setting: community setting |
|
| Outcomes | Included outcomes: static and dynamic balance (force platform measurements), Berg Balance Scale (eyes open and eyes closed) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described only as "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not reported but there was an attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT not reported No participant losses |
| Selective reporting (reporting bias) | Unclear risk | Trial register or protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Low risk | Balanced exposure |
Sims 2009
| Methods | Design: pilot randomised study of resistance training versus no intervention (i.e. a waiting list comparison group) ‐ after usual care. Sample size calculation reported Randomisation: computer‐generated block randomisation by an independent investigator ‐ blocks of 6 stratified by gender Allocation concealment: unclear Blinding: unclear ITT: yes Measurements: at the end of the training programme (10 weeks) and at 6‐month follow‐up Withdrawals: 1 participant did not complete the 10‐week assessment; 5 participants (3 intervention, 2 control) did not complete the physical assessment at 10 weeks due to health reasons unrelated to the programme or time commitments. 43 participants completed the 6‐month survey assessment |
|
| Participants | Randomised: 45 participants; 27 males and 18 females; mean age 67.13 years (SD 15.23), average time since stroke 13.2 months (SD 4.95) Intervention: 23 participants were allocated to the progressive resistance training group. 21 participants completed the 10‐week programme (2 people became medically ineligible) Control: 22 participants were allocated to the waiting list control group Inclusion criteria: stroke survivors with depressive symptoms Exclusion criteria: under 18 years; stroke < 6 months ago; inability to walk a distance of at least 20 metres independently with or without a gait assistive device; Prime‐MD Patients Health Questionnaire (PHQ‐9) score < 5; depression with psychotic features; alcohol or drug‐related depression, schizophrenia; bipolar disorder; other psychiatric diagnoses; suicidal ideation; dementia; terminally ill; uncontrolled hypertension; unstable angina; and unstable insulin dependent diabetes |
|
| Interventions | Intervention: participants in the intervention group attended a community gymnasium twice/week for 10 weeks and trained under the supervision of an accredited fitness trainer. The training programme entailed moderate strengthening exercises (3 sets of 8/10 repetitions at a resistance of 80% of 1‐RM) using machine weights for the major upper and lower limb muscle groups. Resistance was increased when participants were able to complete 3 sets of 10 repetitions of an exercise Control: the waiting list controls received usual care and were asked not to do any resistance‐type exercise (content of the 'usual care' intervention not specified) Setting: community‐based setting |
|
| Outcomes | Included outcomes: CES‐D; AQoL SF‐12 Other outcomes: SIS; SWLS; LOT‐R; Self Esteem Scale; RLOC |
|
| Notes | Sample size calculation performed but sample obtained was smaller than that of the calculation (45 participants instead of 60). Small sample size. At baseline the intervention group had significantly lower depression scores than the comparison group. Impact of social interaction was not assessed The participants in the control group received more attention than simply usual care as they received a 10‐week strength assessment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Following the baseline assessments participants were randomly allocated to the intervention or comparison group by a centrally located independent person using a computer generated block randomisation list, with blocks of six, stratified by gender." |
| Allocation concealment (selection bias) | Low risk | Not applicable as participants allocated in blocks after recruitment and baseline assessment |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attention control (waiting list comparison) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT analysis 1 participant did not complete the 10‐week assessment; 5 participants (3 intervention, 2 control) did not complete the physical assessment at 10 weeks due to health reasons unrelated to the programme or time commitments |
| Incomplete outcome data (attrition bias) End of follow‐up | Low risk | ITT analysis 43/45 participants completed the 6‐month survey assessment |
| Selective reporting (reporting bias) | Unclear risk | Included outcomes correspond with protocol ACTRN12605000613606 |
| Other bias | High risk | At baseline the intervention group had significantly lower depression scores than the comparison group |
| Imbalanced exposure | High risk | Imbalanced exposure |
Smith 2008
| Methods | Design: randomised trial of cardiorespiratory training versus non‐exercise intervention ‐ after usual care (i.e. treadmill gait training versus weekly telephone calls ‐ the main purpose of the trial was to explore the potential additional benefits of treadmill training) Randomisation: random matched‐pair assignment. The investigator assigned a number to suitable participants and placed them in 1 of the intervention groups by 'the roll of a dice' (odd control, even treatment), or systematically allocated a participant to match a randomly assigned participant in the alternate group (minimisation?) Allocation concealment: unclear Blinding: clinical assessor not blinded ITT: not reported, but no withdrawals Measurements: at the end of the intervention (4 weeks) and then 6 weeks later Withdrawals: none |
|
| Participants | Randomised: 20 participants; age range 42 to 72 years Intervention: 10 participants, 8 males and 2 females; mean age 57.8 years (SD 7.0); time from stroke: 8 participants < 1 year and 2 participants ≥ 1 year < 2 years Control: 10 participants, 4 males and 6 females; mean age 56 years (SD 8.3); time from stroke: 8 participants < 1 year and 2 participants ≥ 1 year < 2 years Inclusion criteria: stroke in the middle cerebral artery territory more than 3 months but less than 2 years prior to enrolling in the trial; walking slower than pre‐stroke Exclusion criteria: cognitive impairment; unable to ambulate; concomitant pathology that prevented walking on a treadmill |
|
| Interventions | Intervention: participants in the intervention group received 12 sessions of treadmill training (20 minutes each session) over 4 weeks plus weekly calls from the investigator enquiring about the quality of their week and encouraging them to keep a quality of life log. They wore a standard gait belt on the treadmill and had a practice session prior to the start of the trial. The starting speed on the treadmill was the speed at which the participant could walk during the practice session for 5 minutes with a rate of perceived exertion (RPE) ≤ 13. The speed was increased by 0.2 mph each time the participant walked for 10 consecutive minutes with a RPE ≤ 13 Control: participants in the control group received weekly calls from the investigator enquiring about the quality of their week and encouraging them to keep a quality of life log only Setting: community‐based setting |
|
| Outcomes | Included outcomes: depression (Beck Depression Index), mobility Other outcomes: social participation (Stroke Impact Scale 3.0 subscales) |
|
| Notes | Very small sample size. Fitness outcomes not considered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random matched‐pair assignment. The investigator assigned a number to suitable participants and placed them in 1 of the intervention groups by 'the roll of a dice' (odd control, even treatment), or systematically allocated a participant to match a randomly assigned participant in the alternate group (minimisation?) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | No suitable attention control |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Clinical assessor not blinded |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT analysis not reported No withdrawals |
| Incomplete outcome data (attrition bias) End of follow‐up | Low risk | ITT analysis not reported No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure |
Son 2014
| Methods | Design: randomised trial of resistance training plus usual care versus sham training (no resistance) plus usual care (most likely after discharge from usual hospital care approximately 18 months post‐stroke) Randomisation: software used to randomise participants Allocation concealment: information not included Blinding: information not included ITT: not completed but no losses Measurements: before and end of intervention (6 weeks) Withdrawals: none reported | |
| Participants | 28 participants randomised: 14 to intervention, 15 to control Intervention: 14 participants; 8 males, 6 females; mean age: (combined males and females) 57.4 years (SD not given); type of stroke: ischaemic 7, haemorrhagic 7; paretic side: right 6, left 8; time since stroke onset: 17.9 months Control: 14 participants; 7 male, 7 females: mean age: (combined males and females) 56.6 years (SD not given); type of stroke: ischaemic 8, haemorrhagic 6; paretic side: right 7, left 7; time since stroke onset: 18.7 months Inclusion criteria: a Brunnstrom stage higher or equal to stage 3, ability to independently walk 10 m with or without supervision or an aid or orthosis, and a minimum score of 20 on the Korean Mini‐Mental State Examination (K‐MMSE) Exclusion criteria: joint contracture, pain, or fracture of the musculoskeletal system, and hemianopia |
|
| Interventions | Intervention: participants in the training group (n = 14) received lower limb resistance training for 30 minutes, 5 times per week for 6 weeks. Warm up included 1 set of 4 reps of resisted knee extension at 25% 1 RM, followed by 3 sets of 8 to 10 reps of resisted knee extension at 70% 1 RM. Resistance load was progressed weekly via reassessment of 1 RM Control: participants in the control group (n = 14) received sham lower limb training with no resistance for 30 minutes, 5 times per week for 6 weeks. Warm up included 1 set of 4 reps of knee extension at no resistance, followed by 3 sets of 8 to 10 reps of knee extension at no resistance All participants received conservative physical therapy for 30 minutes per day, 5 days per week, for a period of 6 weeks Conservative physical therapy consisted of joint mobilisation, muscle strengthening, and balance training Setting: unclear; community‐based project |
|
| Outcomes | Included outcomes: balance outcomes including antero‐posterior (A‐P), medio‐lateral (M‐L) sway distances, and the Berg balance scale (BBS); timed up and go (TUG) times | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Software‐generated randomisation |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not reported although there was a suitable attention control |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | All 3 criteria for ITT analysis have not been met ‐ methods suggest a per‐protocol analysis; however there were no losses |
| Selective reporting (reporting bias) | High risk | Trial register or protocol not available |
| Other bias | Unclear risk | No sample size calculation |
| Imbalanced exposure | Low risk | Balanced exposure (sham training) |
Takami 2010
| Methods | Design: randomised trial of cardiorespiratory training plus % usual care versus usual care ‐ during usual care Randomised: envelope method Allocation concealment: unknown Blinding: unknown ITT: not reported Measurements: end of intervention (3 weeks) Withdrawals: 2 participants from backward walking group and 1 participant from forward walking group due to family reasons |
|
| Participants | Randomised: 36 participants Intervention 1: 12 participants in backward walking group; 6 males and 6 females; mean age 66.1 years (SD 6.3); 13.2 days post‐stroke (SD 8.4) Intervention 2: 12 participants in forward walking group; 9 males and 3 females; mean age 71.1 years (SD 10.6); 14.7 days post‐stroke (SD 8.1) Control: 12 participants; 5 males and 7 females; mean age 66.9 years (SD 10.6); 13.7 days post‐stroke (SD 8.9) Inclusion criteria: ability to walk 10 metres using aids; post‐stroke period of less than 5 weeks; FIM‐Locomotion score of 5 or lower; perfect BBS and RMI scores Exclusion criteria: unknown |
|
| Interventions | Invention groups: body weight supported treadmill walking for 30 minutes then 10 minutes of either: backward or forward walking 6 times/week for 3 weeks Treadmill speed was progressed each week (0.8, 1.0, and 1.3 km/h) Control group: conventional training overground walking (150 to 200 m) for 40 minutes 6 times/week for 3 weeks Setting: rehabilitation unit and community settings |
|
| Outcomes | Included outcomes: BBS; RMI; 10 metre maximum walking speed; walking ratios during 10 metre forward walking and 5 metre backward walking; Motricity Index; FIM‐Locomotion | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described only as "envelope method" |
| Allocation concealment (selection bias) | Unclear risk | Nature of envelopes not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Attention control is incorporated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) End of intervention | Unclear risk | ITT not reported 3/36 (8%) losses at the end of intervention; 2 participants from backward walking training group and 1 participant from forward walking training group due to family reasons |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | Low risk | Balanced exposure |
Teixeira 1999
| Methods | Design: randomised trial of mixed training versus no intervention ‐ after usual care First iteration only of a lag control design; participants randomly allocated to immediate or delayed ‐ participants allocated delayed intervention initially received no intervention Randomisation mechanism: unknown; method: unclear ("balanced blocks") Allocation concealment: unknown Blinding: unknown ITT: no Measurements: end of intervention (10 weeks) Withdrawals: none |
|
| Participants | Randomised: 13 participants Intervention: 6 participants; 1 male and 5 females; age 65.9 years (SD 10.2); 9.15 years post‐stroke (SD 12.7) Control: 7 participants; 1 male and 6 females; age 69.4 years (SD 8.85); 6.4 years post‐stroke (SD 6.2) All participants had unilateral stroke resulting in residual weakness or abnormal muscle tone or both Inclusion criteria: at least 9 months post‐stroke; independently ambulatory with or without walking aids; no comprehensive aphasia Exclusion criteria: non‐stroke related disability | |
| Interventions | Intervention: mixed training: cardiorespiratory and lower extremity strength training 60 to 90 minutes per day 3 days per week for 10 weeks; cardiorespiratory training: graded walking plus stepping or cycling progressing from 10 to 20 minutes per day and from 50% to 70% of maximal cycling work rate over first 5 weeks; strength training: 7 exercises involving use of body weight and progressive resistive exercise using different masses and elastic bands (Thera‐Band), each performed as 3 x 10 repetitions and progressing from 50% to 80% of 1 repetition maximum; warm up and warm down 10 to 20 minutes per day Control: no intervention Setting: unclear | |
| Outcomes | Included outcomes: gait preferred speed (22 metre); Adjusted Activity Score; NHP Other outcomes: insufficient data to compare lower limb muscle strength (peak torque Nm); muscle tone assessment; and stair climbing | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear. Quote: "randomly assigned to one of the two groups (treatment and control) with equal probability and balanced into similar blocks" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) End of intervention | Unclear risk | ITT not reported No losses |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure |
Toledano‐Zarhi 2011
| Methods | Design: mixed training plus non‐exercise intervention versus non‐exercise intervention after usual care Randomisation mechanism: mechanism not reported Allocation concealment: unknown Blinding: unknown ITT: yes (LOCF) Measurements: end of intervention (6 weeks) Withdrawals: 1 from intervention group (discontinued intervention) |
|
| Participants | Randomised: 28 participants Intervention: 14 participants; 11 male and 3 females; age 65 years (SD 10); 1 to 3 weeks post‐stroke Control: 14 participants; 10 male and 4 females; age 65 years (SD 12); 1 to 3 weeks post‐stroke All participants had very minor ischaemic stroke Exclusion criteria: systolic BP > 200 mmHg; diastolic BP > 110 mmHg; unstable angina; arrhythmia; congestive heart failure; ST depression ≧ 2 mm on resting ECG; arterioventricular block with no pacemaker; severe peripheral vascular disease; severe lung disease; orthopaedic or neurological disability; dementia or major depression |
|
| Interventions | Intervention: mixed training; 2 days per week for total of 3 hours/week for 6 weeks. Twice per week 35 to 55 minutes of treadmill, hand bike, and cycle ergometer at 50% to 70% heart rate maximum. Once per week 45 to 55 minutes of group strength, flexibility, and co‐ordination Control: home‐based booklet with guidance on strength and flexibility and encouragement to continue with usual community routine Setting: hospital | |
| Outcomes | Included outcomes: 6‐MWT; Four Square Step Test; stair ascending and descending; treadmill performance (Bruce protocol); blood pressure | |
| Notes | Described as 'aerobic' training but this is mixed training | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | No suitable attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT 1/28 (4%) lost overall; from intervention group (discontinued intervention) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure |
van de Port 2012
| Methods | Design: multicentre randomised trial of mixed training versus usual outpatient care ‐ after usual care Randomised: online minimisation procedure Allocation concealment: unknown Blinding: assessors blinded to group allocation ITT: yes Measurements: end of intervention (12 weeks) and follow‐up (24 weeks) Withdrawals: intervention group (4 participants did not start intervention, 1 participant withdrew without reason); control (1 participant at the end of intervention missing assessment, 2 participants died from cancer, 2 participants had recurrent stroke, 2 participants withdrew without reason) |
|
| Participants | Randomised: 250 participants Intervention: 124 participants; 82 males and 42 females; mean age 56 years (SD 10); time post‐stroke 80.9 days (SD 13.0) Control: 126 participants; 80 males and 46 females; mean age 58 years (SD 10); time post‐stroke 77.8 days (SD 15.0) Inclusion criteria: verified stroke (according to WHO definition); able to walk a minimum of 10 metres unassisted; discharged home from rehabilitation centre; requirement to continue physiotherapy during outpatients care Exclusion criteria: cognitive deficits (MMSE < 24 score); unable to communicate; lived more than 30 km from rehabilitation centre |
|
| Interventions | Invention group: circuit training programme for 90 minutes twice/week for 12 weeks. Training included 8 stations intended to improve walking competency. Each station exercise was performed for 3 minutes with 3 minutes recovery Control group: usual outpatient physiotherapy care, no restriction or detail given regarding time or duration of these sessions Setting: rehabilitation outpatient centre |
|
| Outcomes | Included outcomes: mobility domain of SIS; RMI; falls efficacy scale; NEADL; HADS; fatigue severity scale; Motricity index; 6‐MWT; 5 metre comfortable walking speed test; timed balance test; TUG; modified stair test | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants stratified by rehabilitation centre using an online minimisation procedure |
| Allocation concealment (selection bias) | Low risk | Risk removed due to online dynamic allocation mechanism: i.e. there is no allocation list to conceal |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment. The efficacy of blinding was confirmed though statistical analysis of guesses of allocation |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT analysis used 8/250 (3%) losses. Slight imbalance in losses in the control group 7/124 and training group 1/126 Intervention group (4 participants did not start intervention, 1 participant withdrew without reason); control group (1 participant at the end of intervention missing assessment, 2 participants died from cancer, 2 participants had recurrent stroke, 2 participants withdrew without reason) |
| Incomplete outcome data (attrition bias) End of follow‐up | Low risk | ITT analysis used 8/250 (3%) overall losses |
| Selective reporting (reporting bias) | Unclear risk | Some planned secondary outcomes in the trial register (Dutch Trial Register NTR1534) were not reported or not followed up beyond baseline (chair rise, Motricity index). Other unplanned outcomes appear in report including functional ambulation categories (included in review) and the Letter Cancellation Task (but this is not included in this review) |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure Quote: "The circuit training group received 4461 treatment sessions compared with 4378 for the usual care group. The average treatment time per session was 72 (SD 39) minutes for the intervention group compared with 34 (SD 10) minutes for the control group (P < 0.05)." |
Verheyden 2009
| Methods | Design: randomised trial of functional strength training plus usual care versus usual care Randomisation: simple randomisation Allocation concealment: information not included Blinding: assessor blinded ITT: not completed Measurements: before and end of intervention (5 weeks) Withdrawals: none, however 2 participants received fewer hours of intervention therapy due to early discharge. In the control group, 3 participants were discharged early and therefore received fewer hours of usual care. All participants were evaluated before discharge and included in analysis | |
| Participants | Randomised: total 33 participants. 17 participants were randomised to intervention, 16 to control Intervention: 17 participants; 11 males, 6 females; mean age: (combined males and females) 55 years (SD 11); mean height: unknown; mean weight: unknown; type of stroke: ischaemic 15, haemorrhagic 2; paretic side: right 9, left 8; time since stroke onset: 53 (SD 24) days Control: 16 participants; 9 male, 7 females; mean age: (combined males and females) 62 years (SD 14); mean height: unknown; mean weight: unknown; type of stroke: ischaemic 13, haemorrhagic 3; paretic side: right 7, left 9; time since stroke onset: 49 (SD 28) days Inclusion criteria: stroke‐related hemiparesis. Full recovery from an earlier stroke. Exclusion criteria: an age of 80 years or greater; unable to understand instructions; other disorders which could affect motor performance or an ability to obtain maximum trunk performance |
|
| Interventions | Intervention: participants in the training group (n = 17) received resistance training for the trunk for 30 minutes, 4 times per week for 5 weeks. Seated exercises included selective movements of the upper and lower part of the trunk in supine and in sitting. In addition conventional multidisciplinary stroke rehabilitation, such as neuro‐developmental treatment and motor learning strategies, was provided. No other details were reported. Control: participants in the control group (n = 16) received conventional multidisciplinary stroke rehabilitation, such as neuro‐developmental treatment and motor learning strategies. No other details were reported Setting: rehabilitation centre |
|
| Outcomes | Included outcomes: Trunk Impairment Scale Other outcomes: Tinetti Scale (only reported at baseline) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method described only as simple randomisation by personnel not involved in the study |
| Allocation concealment (selection bias) | High risk | Not reported in paper |
| Blinding (performance bias and detection bias) All outcomes | High risk | "...patients nor the physiotherapists who delivered the interventions were blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinded |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | A total of 2 participants from the intervention group completed 3 and 4 hours fewer of additional therapy due to early discharge. The participants were evaluated before discharge and included in the analysis. A total of 3 participants from the control group were discharged early after 21, 23, and 25 days respectively and therefore received fewer hours of conventional physiotherapy (number of fewer hours not reported) Although ITT was not referred to specifically there were "no dropouts during the course" of the study, and "..all participants were evaluated before discharge" |
| Selective reporting (reporting bias) | High risk | Trial register or protocol not available |
| Other bias | High risk | Lack of sample size calculation reported |
| Imbalanced exposure | High risk | Imbalanced exposure |
Wang 2014
| Methods | Design: randomised trial of cardiovascular training plus usual care versus usual care ‐ during usual care Randomisation: sealed card selection by participants Allocation concealment: information not included Blinding: investigators were not blinded, outcome assessors and therapists were blinded ITT: not completed Measurements: before and end of intervention (6 weeks) Withdrawals: in the intervention group, unclear if up to 9 participants withdrew due to lower leg discomfort during intervention, transfer to another hospital or withdrawal from study |
|
| Participants | 54 participants randomised: 27 were randomised to intervention, 27 to control Intervention: 23 participants; 19 males, 8 females; mean age: (combined males and females) 54 years (SD 7.2); mean height: unknown; mean weight: 71.1 kg (SD 10.2); type of stroke: ischaemic 15, haemorrhagic 12; paretic side: right 15, left 12; time since stroke onset: 1 to 6 months Control: 27 participants; 17 males, 10 females: mean age: (combined males and females) 52 years (SD 12.1); mean height: unknown; mean weight: 75.2 kg (SD8.1); type of stroke: ischaemic 16, haemorrhagic 11; paretic side: right 18, left 9; time since stroke onset: 1 to 6 months Inclusion criteria: time since stroke onset of 1 to 6 months; age > 45 years; severely impaired with the affected leg marked 3 or less on the 7‐point Chedoke–McMaster Stroke Assessment scale; unable to walk even with walk aids; the unaffected leg can move against normal resistance; fasting glucose < 7 mmol/L; no physician diagnosed diabetes; never using medications that may significantly alter heart rate and blood glucose level; and able to understand the purpose and content of the study Exclusion criteria: signs and symptoms of subarachnoid haemorrhage, transient ischaemic attack, severe cerebral oedema, O2 dependence, angina, unstable cardiac conditions, peripheral arterial occlusive disease, abnormal high fever, severe pneumonia, high blood pressure over 200/110 mmHg, dementia, aphasia operationally defined as incapacity to follow 2‐point commands, untreated major depression, and other medical conditions that precluded participation in exercise training |
|
| Interventions | Intervention: participants in the training group (n = 23) received conventional stroke rehabilitation 5 days per week for 6 weeks, (3 x 40‐minute physical therapy sessions; 2 x 15‐minute occupational therapy sessions; 1 x 30‐minute acupuncture or traditional Chinese manipulation session; and 1 x 30‐minute physical agents therapy session). 1 x 40‐minute physical training session was replaced by low‐intensity aerobic cardiovascular training 3 days a week for 6 weeks using a cycle ergometer. Cycling training consisted of 30 minutes sessions including: 5 minute warm up; 30 minute active pedalling at an intensity based on an incremental graded exercise test (2.5 W ramp every 3 minutes maintaining 50 rpm until exhaustion); followed by 5‐minute cool down. Target heart rate was calculated as ((peak heart rate in graded exercise test – resting heart rate) x 50% to 70%) + resting heart rate Control: participants in the training group (n = 27) received conventional stroke rehabilitation 5 days per week for 6 weeks, (3 x 40‐minute physical therapy sessions; 2 x 15‐minute occupational therapy sessions; 1 x 30‐minute acupuncture or traditional Chinese manipulation session; and 1 x 30‐minute physical agents therapy session) Setting: rehabilitation centre |
|
| Outcomes | Included outcomes: Barthel Index; exercise test time; glucose tolerance variables (fasting glucose; fasting insulin; 2‐hour plasma glucose; homeostasis model assessment–insulin resistance index), and serum lipid profiles (total triglycerides, HDL cholesterol, LDL cholesterol) Other outcomes: Fugl–Meyer motor scores; peak and resting heart rate |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 1 of 2 cards selected from a sealed envelope by participants |
| Allocation concealment (selection bias) | Unclear risk | Not reported in paper |
| Blinding (performance bias and detection bias) All outcomes | High risk | Therapists blinded but there was imbalanced intervention exposure |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome measures administered by a blinded rater |
| Incomplete outcome data (attrition bias) End of intervention | High risk | 9/54 (17%) overall losses A total of up to 9 participants withdrew due discomfort, hospital transfer, and general withdrawal from study. Unclear how these withdrawals were distributed between intervention and control groups. No details given of whether pre‐intervention assessment data were included in analysis All 3 criteria for ITT analysis have not been met ‐ methods suggest a per‐protocol analysis |
| Selective reporting (reporting bias) | High risk | Trial register or protocol not available |
| Other bias | High risk | Lack of power analysis based on sample size calculations |
| Imbalanced exposure | High risk | Intervention group was exposed to an additional exposure volume on top of usual care physiotherapy |
Winstein 2004
| Methods | Design: randomised trial of resistance training plus usual care versus usual care ‐ during and after usual care Randomisation mechanism: unknown; method: stratified on Orpington Prognostic Scale (1.6 to 1.4 and 4.2 to 6.8) Allocation concealment: sealed envelopes Blinding: principal investigator but not outcome assessor ITT: no Measurements: end of intervention (4 to 6 weeks) and 9‐month post‐stroke follow‐up Withdrawals: before end of intervention: 1 (treatment group, medical complications), 1 (control group, lost interest); before end of follow‐up: 9 (treatment group 4, control group 5 ‐ moved away or lost contact) |
|
| Participants | Randomised: 42 participants Intervention: 21 participants; 12 males and 8 females; time since stroke 17.3 days (SD 10.6) Control: 20 participants; 2 males and 8 females; time since stroke 15.4 days (SD 5.5) Age: 29 to 76 years, most 35 to 75 years Inclusion criteria: first stroke; 2 to 35 days post‐stroke; FIM score Exclusion criteria: peripheral nerve or orthopaedic condition limiting arm movement; function limited by cardiac disease; subarachnoid haemorrhage without infarction; progressive hydrocephalus; history of brain injury; severe aphasia, neglect, agitation or depression which could limit participation | |
| Interventions | Intervention: upper limb movements resisted by gravity, free weights, Thera‐Band and grip devices for fingers, 60 minutes/day 5 days per week for 4 to 6 weeks, high‐intensity for 3 days per week and low‐intensity higher velocity for 2 days/week, training target 20 hours total Control: standard care delivered by occupational therapy, included muscle facilitation exercises using neuro‐developmental approach, electrical stimulation, stretching, ADL and caregiver training; activities included use of upper limbs Setting: inpatient rehabilitation hospital and outpatient clinic | |
| Outcomes | Included outcomes: FIM (mobility and self care scores); FTHUE; composite measure of strength (sum of torque from extension and flexion of the wrist elbow and shoulder); grip and pinch force Other outcomes: Fugl‐Meyer scores |
|
| Notes | Change from baseline scores reported and analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mechanism unknown; stratification based on Orpington Prognostic Scale (1.6 to 1.4 and 4.2 to 6.8) |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | No suitable attention control Quote: "This treatment regimen was separate (i.e. it was added to the standard dose of occupational and physical therapy)." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessor not blinded |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT not reported 2/42 (5%) losses at the end of intervention: 1 treatment group (medical complications), 1 control group (lost interest) |
| Incomplete outcome data (attrition bias) End of follow‐up | High risk | ITT not reported 11/42 (26%) losses at the end of follow‐up: 4 intervention group; 5 control group (moved away or lost contact) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure |
Yang 2006
| Methods | Design: randomised trial of mixed training versus no intervention ‐ after usual care Randomisation mechanism: picking envelopes Allocation concealment: sealed envelopes Blinding: investigator ITT: unknown Measurements: end of intervention (4 weeks) Withdrawals: none |
|
| Participants | Randomised: 48 participants Intervention: 24 participants; 16 males and 8 females; age 56.8 years (SD 10.2); time since stroke > 1 year Control: 24 participants; 18 males and 8 females; age 60 years (SD 10.4); time since stroke > 1 year Inclusion criteria: first stroke < 1 year ago; not receiving rehabilitation; ambulatory, independent with no aids; medically stable to participate; able to understand instructions and follow commands Exclusion criteria: medical condition preventing participation; uncontrolled health condition for which exercise was contraindicated | |
| Interventions | Intervention: mixed training performed as a circuit 30 minutes per day 3 days per week for 4 weeks; circuit comprised 6 x 5‐minute lower extremity workstations (standing and reaching, sit‐to‐stand from chair, stepping forwards and backwards onto blocks, stepping sideways onto blocks, forward step‐up onto blocks), participants encouraged to work hard, progression achieved by increasing number of repetitions in each 5‐minute block, and increasing step and chair height, and the complexity of task; extended periods (5‐minute) warrant acknowledgement of a cardiorespiratory component despite the author's title (progressive resistance strength training) Control: no intervention | |
| Outcomes | Included outcomes: gait endurance (6‐MWT ‐ outcome assessor not blinded); gait speed preferred (10 metres); 3 metre TUG; step test; isometric strength of knee and hip ankle extension and flexion; and ankle dorsi‐flexion and plantar‐flexion (using handheld dynamometer) Other outcomes: gait cadence and stride length | |
| Notes | Trial authors stated 'strength training' but intervention was actually mixed training. Data reported as absolute and change scores | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...independent person who picked one of the sealed envelopes 30 min before the start of the intervention." |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes; opaque and numbered not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigator blinded |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | ITT not reported No losses |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear |
| Imbalanced exposure | High risk | Imbalanced exposure |
Yang 2014
| Methods | Design: randomised crossover trial of cardiorespiratory training plus usual (outpatient) care versus usual (outpatient) care Randomisation: computer‐generated randomisation Allocation concealment: sealed envelopes Blinding: outcome assessor ITT: not completed Measurements: before and end (4 weeks) of intervention Withdrawals: in the intervention group, 1 participant withdrew due to a fall at home |
|
| Participants | 30 participants randomised: 15 participants were randomised to intervention, 15 to control Intervention: 15 participants; 9 males, 6 females; mean age: (combined males and females) 53.9 years (SD 10.5); mean height: unknown; mean weight: unknown; type of stroke: ischaemic 9, haemorrhagic 11; paretic side: right 11, left 4; time since stroke onset: 11.1 months (SD 8.1) Control: 15 participants; 13 male, 2 females: mean age: (combined males and females) 54.5 years (SD 8.0); mean height: unknown mean weight: unknown; type of stroke: ischaemic 8, haemorrhagic 7; paretic side: right 8, left 7; time since stroke onset: 11.1 months (SD 9.7) Inclusion criteria: first ever stroke; stroke onset greater than 3 months; unilateral hemiplegia; between 18 and 70 years of age; ability to walk 10 m with or without assistance; zero score of 3 levels of the National Institute of Health Stroke Scale Exclusion criteria: patients with aphasia who were unable to follow instructions; blindness or visual impairment; musculoskeletal disorders; cardiac disorders and peripheral neuropathy |
|
| Interventions | Intervention: participants in the training group (n = 15) received conventional stroke rehabilitation (1 hour physical therapy; 1 hour occupational therapy). In addition extra cardiovascular training was given for 30 minutes, 5 times per week for 4 weeks. Cycling training consisted of 15 minutes sessions each of forward and backward cycling including: 150‐second passive warm up; 10‐minute active pedalling at 50 to 70 rpm at an intensity of stage 13 of the Borg scale; 150 seconds of passive cool down Control: participants in the control group (n = 15) received conventional stroke rehabilitation (1 hour physical therapy; 1 hour occupational therapy) Setting: rehabilitation centre ‐ usual outpatient care |
|
| Outcomes | Included outcomes: 6 minute walk test (6MWT); comfortable walking speed using 10 metre walk test (10MWT) Other outcomes: lower limb subscale of Fugl‐Meyer assessment (LE‐FMA); modified Ashworth scale | |
| Notes | The first iteration of this cross‐over study is equivalent to a RCT The data in this paper, which can be analysed as a 2 group RCT, correspond to 'Group A' (intervention n = 15 per protocol) and 'Group B' (control n = 15) at the end of the first iteration ('T2') Authors indicate the 10MWT was at a comfortable speed not a maximal speed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... computer‐generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Some evidence of concealment but the description is inadequate; only reported as "... held in sealed envelopes by an independent individual" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded rater used |
| Incomplete outcome data (attrition bias) End of intervention | Low risk | 1/30 (3%) total dropouts A total of 1 participant withdrew due to a fall at home; no details given of whether pre‐intervention assessment data were included in analysis All 3 criteria for ITT analysis have not been met ‐ methods suggest a per‐protocol analysis, however only 1 participant affected |
| Selective reporting (reporting bias) | High risk | Trial register or protocol not available |
| Other bias | High risk | Lack of sample size calculation reported |
| Imbalanced exposure | High risk | Imbalanced exposure |
Zedlitz 2012
| Methods | Design: multicentre randomised trial of mixed training plus non‐exercise intervention versus non‐exercise intervention ‐ after usual care Randomised: block randomisation; implemented individually but also as a cluster when numbers were low Allocation concealment: sealed envelopes Blinding: assessor blind to group allocation ITT: yes Measurements: end of intervention (12 weeks) and end of 6‐month follow‐up Withdrawals: 1 participant withdrew consent before allocation into group; intervention group (5 participants, 3 withdrew consent before end of intervention, 1 participant withdrew due to poor health before end of intervention; 1 participant withdrew due to recurrent stroke before follow‐up); control group (6 participants, 3 withdrew consent, 1 got new job; 1 family emergency, 1 participant recurrent stroke all before end of intervention; 4 participants lost to follow‐up) |
|
| Participants | Randomised: 84 participants Intervention: 38 participants (1 withdrew consent); 22 males and 23 females; mean age 54.8 years (SD 9.1); 4.4 years post‐stroke (SD 4.2) Control: 45 participants; 21 males and 17 females; mean age 55.6 years (SD 8.8); 3.3 years post‐stroke (SD 3.9) Inclusion criteria: sustained stroke > 4 months; reported severe fatigue; between ages 18 to 70 years; able to walk independently Exclusion criteria: severe cognitive deficits; severe comorbidity (cardiac disease, pulmonary disease); depression |
|
| Interventions | Invention group: treadmill walking and strength training ranging from 40% to 70% maximum heart rate for 2 hours twice/week for 12 weeks Control group: non‐exercise control intervention (cognitive therapy) Setting: 8 rehabilitation centres |
|
| Outcomes | Included outcomes: Checklist Individual Strength‐subscale Fatigue; HADS; SIS; 6‐MWT | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation implemented (individually) in groups of 8 in each centre by picking 1 of 8 sealed envelopes. If only 4 patients were available in 1 centre then they were allocated as a group (cluster) |
| Allocation concealment (selection bias) | Unclear risk | Full nature and use of envelopes is unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attention control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessors used |
| Incomplete outcome data (attrition bias) End of intervention | Unclear risk | ITT analyses used 11/84 (13%) losses: intervention group (5 participants, 3 withdrew consent before end of intervention, 1 participant withdrew due to poor health before end of intervention); control group (6 participants, 3 withdrew consent, 1 got new job; 1 family emergency, 1 participant recurrent stroke all before end of intervention) |
| Incomplete outcome data (attrition bias) End of follow‐up | High risk | ITT analyses used 16/84 (19%) total losses: intervention group (1 participant withdrew due to recurrent stroke before follow‐up); control group (4 participants lost to follow‐up) |
| Selective reporting (reporting bias) | Low risk | Included outcomes correspond to trial registry NTR2704. Some proposed cognitive outcomes not present in publication (not relevant to this review) |
| Other bias | High risk | Self report questionnaires used Monitoring period before randomisation to identify those with potentially poor compliance Risk of self selection bias as newspaper adverts used for recruitment |
| Imbalanced exposure | High risk | Imbalanced exposure |
6‐MWT: 6‐Metre Walking Test 9‐HPT: 9‐Hole Peg Test 12‐MWT: 12‐minute walk test ABCS: Activities‐Specific Balance Confidence Scale ACSM: American College of Sports Medicine ADL: activities of daily living AQoL: Assessment of Quality of Life Instrument ARAT: Action Research Arm Test BBS: Berg Balance scale BDI: Beck Depression Inventory BI: Barthel Index BMI: body mass index BP: blood pressure BWSTT: body weight supported treadmill training CES‐D: Centre for Epidemiological Studies Depression scale CT: computerised tomography DBP: diastolic blood pressure ECG: electrocardiogram EMS: Elderly Mobility Scale FAC: Functional Ambulation Classification FAI: Frenchay Activity Index FAME: family‐mediated exercise FAPS: Functional Ambulation Profile Score FIM: Functional Independence Measure FTHUE: Functional Test of the Hemiparetic Upper Extremity HADS: Hospital Anxiety and Depression Scale HDL: high‐density lipoprotein HR: heart rate ITT: intention‐to‐treat LDL: low‐density lipoprotein LOCF: last observation carried forward LOT‐R: Life Orientation Test ‐ Revised MAS: Motor Assessment Scale MI: myocardial infarction MMSE: Mini Mental State Examination MRI: magnetic resonance imaging NEADL: Nottingham Extended Activities of Daily Living NHP: Nottingham Health Profile OMNI: the term OMNI is a contraction of the word 'omnibus' OT: occpational therapy PADS: Peripheral Arterial Diseases Walking Impairment questionnaire PT: physiotherapy RCT: randomised controlled trial RLOC: Recovery Locus of Control Scale RM: repetition maximum RMA: Rivermead Motor Assessment RMI: Rivermead Mobility Index SBP: systolic blood pressure SD: standard deviation SF‐12: Short Form‐12 Health Survey Questionnaire SF‐36: Short Form 36 Health Survey SIS: Stroke Impact Scale SSS: Scandinavian Stroke Scale SWLS: Satisfaction with Life Scale TUG: Timed Up and Go test UC: usual care WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ada 2003 | Control intervention was described as training and included prescribed walking, which confounds this walking study |
| Ada 2010 | Not a valid comparison (treadmill gait training with body weight support versus overground gait training) |
| Adie 2014 | Intervention not progressive intervention; not physical fitness training |
| Aidar 2013 | Intervention not progressive |
| Akbari 2006 | Not a valid control group |
| Au‐Yeung 2009 | Intervention not physical fitness training (short‐form Tai Chi). Not a valid control |
| Baek 2014 | Wrong control group or active control group |
| Barreca 2007 | Not progressive physical fitness training |
| Baskett 1999 | Intervention not physical fitness training: it is described as exercise and activities but no evidence of progressive cardiorespiratory or strength elements, or both |
| Batchelor 2009 | Intervention not physical fitness training (falls prevention programme) |
| Batchelor 2012 | Exercise group also participate in non‐exercise falls prevention including education and injury risk minimisation strategies |
| Benvenuti 2014 | Wrong study design ‐ not a RCT |
| Bernhardt 2015 | Intervention not physical fitness training |
| Blennerhassett 2004 | Control group perform upper limb training intervention ‐ this could theoretically influence lower limb outcome measures |
| Boss 2014 | Wrong control group or active control group |
| Bourbonnais 2002 | Comparison of upper and lower body exercise |
| Boysen 2009 | Intervention does not meet the criteria for physical fitness training (self regulated exercise programme) |
| Brown 2002 | Comparison of 2 exercise regimens |
| Butefisch 1995 | Non‐random, alternate allocation on admission method |
| Carr 2003 | No relevant comparisons: comparison of cardiorespiratory training and mixed training |
| Chanruengvanich 2006 | Intervention does not meet the criteria for physical fitness training (self regulated exercise programme). Control not specified |
| Choi 2010 | Groups not randomly allocated |
| Chu 2004 | Control group perform upper limb training intervention ‐ this could theoretically influence lower limb outcome measures |
| Chumbler 2010 | Intervention had no definite intention of improving fitness |
| Chumbler 2012 | Intervention had no definite intention of improving fitness |
| Chung 2013 | Intervention not physical fitness training |
| Corsilles‐Sy 2013 | Intervention not physical fitness training |
| Corti 2012 | Control group not classified as usual care |
| da Silva 2015 | Intervention not progressive |
| Davis 2003 | No relevant comparisons: comparison of cardiorespiratory training and strength training |
| Davis 2006 | Control group included physical activity: comprised 30 minutes 'sham' aerobic training (which was motorised and passive) and 30 minutes of 'sham' resistance training; resistance training was not passive as it involved movement of legs against gravity and it included some stretching |
| Dean 1997 | Intervention not physical fitness training: although an element of progression is present the intervention is more 'practice' than training as defined in this review |
| Dean 2000 | Not a valid comparison (upper body versus lower body) |
| Dean 2012 | Control not usual care, therefore comparison of 2 interventions containing exercise |
| Deniz 2011 | Full English text unavailable |
| Desrosiers 2005 | Not a valid comparison: control contained additional dose of 'usual arm therapy'. Intervention not physical fitness training: repetition and practice |
| Di Lauro 2003 | Not a valid comparison. It is 'training' versus usual care; the intervention is also not physical fitness training |
| Dias 2007 | Not a valid control (not usual care) |
| Dickstein 1986 | Intervention not physical fitness training: although post neuromuscular facilitation and Bobath approaches may contain resistive exercises. Patient allocation not randomised: based on hospital administration procedures |
| Dickstein 1997 | Intervention not physical fitness training: muscle contractions not resisted and not progressive. Patient allocation not randomised: patients were sequentially assigned |
| Dobkin 2010 | Not a valid comparison. Both groups received physiotherapy plus 10 metre walk. The experimental group received feedback about walking speed |
| Dong 2012 | Non‐exercise co‐intervention |
| Dromerick 2005 | Intervention not physical fitness training: constraint‐induced movement therapy |
| Drummond 1996 | Interventions not physical fitness training: 2 interventions: (1) leisure therapy, and (2) conventional occupational therapy |
| Duncan 2011 | Control group not usual care |
| El‐Senousey 2012 | Not an exercise intervention |
| El‐Tamawy 2014 | Intervention not progressive |
| Faulkner 2012 | Exercise co‐intervention |
| Faulkner 2013 | Wrong participant population |
| Feys 1998 | Intervention not physical fitness training: the physical activity (rocking movements) showed no progression of intensity |
| Fletcher 1994 | Mixed population (35% of sample were not stroke) |
| Foley 2004 | Mixed population. Only 15 of 338 participants (4%) had stroke |
| Franceschini 2009 | Not a valid comparison (treadmill gait training versus overground gait training) |
| Friedman 2014 | Intervention not physical fitness training |
| Gelber 1995 | Intervention not physical fitness training: comparison of traditional functional retraining and neurodevelopmental techniques. No relevant comparisons |
| Gilbertson 1998 | Intervention not physical fitness training: home‐based occupational therapy |
| Gregson 2006 | Intervention was not fitness training, it was repetitive practice with no progression of exercise load except for some participants initially unable to complete the target number of repetitions (10) |
| Harrington 2010 | Not a valid comparison (exercise and education programme versus standard care) |
| Harris 2009 | Intervention does not meet the criteria for physical fitness training (upper limb supplementary programme) |
| Hart 2004 | Control intervention not a valid comparison: not usual care, not non‐exercise and balance exercises confound |
| Helbostad 2004 | Only 16 of 77 participants with stroke. Not a valid comparison, both groups receiving home training |
| Hidler 2007 | Not a valid comparison: comparison of 2 types of training |
| Higgins 2006 | Intervention not fitness training: experimental group dexterity practice. Control group not valid: included physical activity (walking) |
| Holmgren 2010 | Control group not usual care Intervention not physical fitness training Intervention has co‐intervention components |
| Howe 2005 | Intervention not physical fitness training |
| Hu 2003 | Intervention (Bobath) not physical fitness training |
| Hu 2006 | Intervention not physical fitness training |
| Ishida 2001 | Regular rehabilitation was suspended in some participants during a period of usual care. Not an exercise intervention |
| Jeong 2007 | Intervention not physical fitness training (rhythmic music and specialised rehabilitation movements) |
| Jongbloed 1989 | No relevant control group: comparison of 2 occupational therapy interventions. Interventions not physical fitness training |
| Jongbloed 1991 | Intervention not physical fitness training: occupational therapy related to leisure activities |
| Kamps 2005 | Not a relevant control group: participants recruited after usual care yet were exposed to physiotherapy and 'ergotherapeutic' interventions |
| Kim 2012 | Not usual care |
| Kirk 2014 | Intervention has co‐intervention components |
| Klassen 2005 | Not a valid control group: low‐intensity upper body exercise |
| Kono 2013 | Intervention has co‐intervention components |
| Kumaran 2013 | Intervention not physical fitness training |
| Kwakkel 1999 | Intervention not physical fitness training: investigation of rehabilitation of functional tasks. The principal author clarified that there was no progression of training intensity, the content of training was variable, and the treadmill training volume comprised only approximately 10% of participants |
| Kwon 2013 | Intervention not physical fitness training |
| Langhammer 2008 | Previously excluded |
| Langhammer 2009 | Not a valid comparison (physiotherapy versus self initiated exercise) |
| Langhammer 2010 | Not a valid comparison (treadmill gait training versus walking outdoors) |
| Langhammer 2014 | Previously excluded |
| Langhammer 2014a | Previously excluded |
| Laufer 2001 | Intervention not physical fitness training: comparison of treadmill ambulation and overground walking. No relevant comparisons |
| LEAPS | No relevant comparisons |
| Lee 2010 | Not a valid control |
| Lee 2013b | Intervention not progressive |
| Lemoncello 2011 | Intervention not physical fitness training (swallowing exercises) |
| Lennon 2009 | Not a valid comparison (aerobic exercises plus lifestyle counselling and risk reduction programme versus risk reduction programme) |
| Leveille 1998 | Contained few people with stroke: intervention (8%), control (9%). Not a valid intervention ‐ other healthy living interventions included. Not a valid control ‐ provided access to training facilities of intervention group |
| Lin 2004 | Intervention not physical fitness training |
| Lincoln 1999 | Interventions not physical fitness training: comprised additional physiotherapy |
| Lincoln 2003 | Comparison of 2 physiotherapy approaches |
| Lindsley 1994 | This was published as an abstract only, the numerical data were not included and could not be recovered from the authors This intervention may have been training although the abstract contained no mention of progression |
| Liston 2000 | Intervention not physical fitness training |
| Liu‐Ambrose 2015 | Intervention has co‐intervention components |
| Logan 2003 | Intervention not physical fitness training: comprised leisure activities, although sport was included |
| Logigian 1983 | No relevant comparisons: comparison of traditional and facilitation techniques. Intervention not physical fitness training: although training elements may have been included it would be difficult to separate the effect of training from therapy |
| Lord 2008 | Not a valid comparison (functional gait activities in community environments versus physiotherapy including treadmill gait training) |
| Luft 2004 | Intervention not physical fitness training. Control group contained physical activity not linked to usual care |
| Luft 2008 | Not a valid comparison (treadmill gait training versus stretching exercises) |
| MacKay‐Lyons 2010 | Co‐intervention (multi‐component lifestyle intervention) |
| Macko 2005 | Control group is not non‐exercise or conventional treatment |
| Maeshima 2003 | Not a relevant comparison: 2 exercise groups, with and without family members present |
| Marigold 2005 | Not a relevant comparison: comparison of agility and stretching/weight shifting; neither is physical fitness training |
| Marzolini 2012 | Not a RCT; no control group |
| Mayr 2007 | Not a valid comparison (Lokomat automatised gait training versus Bobath exercises) |
| McClellan 2004 | Control group not non‐exercise |
| Mehrholz 2008 | Not a valid comparison (automated locomotor gait training with physiotherapist assistance versus physical therapy) |
| Michaelsen 2006 | Control group is not non‐exercise |
| Miklitsch 2013 | Intervention not physical fitness training |
| Miller 2000 | Intervention not physical fitness training |
| Moreland 2003 | Control group not non‐exercise |
| Muhl 2014 | Intervention not physical fitness training |
| Nadeau 2013 | Previously excluded |
| Nelles 2001 | Not a valid comparison. Intervention not physical fitness training. Included non‐stroke healthy controls |
| Nilsson 2001 | Comparison not relevant: comparison of treadmill training with a physiotherapy approach to gait training (motor relearning programme) during usual care |
| Noh 2008 | Not a valid comparison. Active control. Experimental group received aquatic therapy ‐ Ai Chi ‐ whilst control group performed gym exercises |
| Olney 2006 | Not a valid comparison: trial of supervised versus unsupervised exercise |
| Outermans 2010 | Not a valid comparison (high‐intensity training programme versus low‐intensity circuit rehabilitation programme) |
| Pan 2004 | Not a valid comparison: trial of training versus unsupervised training |
| Pang 2006 | Control group not non‐exercise |
| Pang 2006a | Wrong control group or active control group |
| Pang 2008 | Not a valid comparison (leg exercise programme versus arm exercise programme) |
| Pang 2010 | Not a RCT |
| Park 2012 | Wrong control group or active control group |
| Parker 2001 | Intervention not physical fitness training: leisure therapy and occupational therapy |
| Parry 1999 | Intervention not physical fitness training: physiotherapy using Bobath and movement science approaches |
| Partridge 2000 | Intervention not physical fitness training: comparison of amount of physiotherapy |
| Patterson 2010 | Not a RCT |
| Peng 2002 | Intervention not physical fitness training |
| Peurala 2005 | Not a valid comparison (control group physical activity) |
| Peurala 2009 | Not a valid comparison (electromechanical gait training with physio assistance versus conventional physiotherapy) |
| Pitsch 2006 | Intervention not physical fitness training |
| Platz 2001 | Intervention not physical fitness training: arm ability training comprised simple functional and manipulative tasks |
| Platz 2005 | 2 interventions, neither were physical fitness training |
| Pohl 2007 | Not a valid comparison (electromechanical gait training with body support) |
| Pomeroy 2001 | Intervention not physical fitness training: weighted garments may offer increased resistance to muscle contraction but physical activity was neither controlled nor accurately monitored (patient's log book) |
| Puckree 2014 | Intervention not physical fitness training |
| Quaney 2009 | Not a valid comparison (bicycle training versus strength training) |
| Ribeiro 2013 | Wrong control group or active control group |
| Rimmer 2000 | Patient allocation not randomised: influenced by geographical location. The intervention was physical fitness training and comprised elements of cardiorespiratory, strength, and flexibility training |
| Rimmer 2009 | Not a valid comparison (moderate short duration exercise programme versus long‐intensity longer duration exercise programme versus rehabilitation programme including walking training and strength exercises). No valid control |
| Rose 2011 | Not a RCT |
| Saeys 2012 | Not usual care co‐intervention |
| Schmid 2012 | Exercise group involved a co‐intervention (yoga plus 20 minutes breathing exercises) |
| Severinsen 2014 | Wrong control group or active control group |
| Shatil 2005 | Intervention not physical fitness training. Control involved some strengthening |
| Sherrington 2008 | Mixed population (results are not provided separately for stroke participants) |
| Shimada 2003 | Only 25% of cohort were people with stroke (only 1 with stroke in control group) |
| Shimizu 2002 | Non‐random allocation (order of admission). Only 11 of 16 participants were people with stroke |
| Shimodozono 2013 | Intervention not physical fitness training |
| Shimodozono 2014 | Intervention not physical fitness training |
| Sivenius 2007 | Comparison not relevant: comparison of 2 therapies |
| Smith 1981 | Intervention not physical fitness training: intensive and conventional physiotherapy and occupational therapy |
| Sullivan 2002 | Comparison not relevant: participants allocated 3 different treadmill training speeds |
| Sullivan 2007 | Not a valid comparison (treadmill gait training with body weight support versus leg cycling versus upper‐extremity ergometry) |
| Sunderland 1994 | Intervention not physical fitness training: comparison of orthodox and enhanced physiotherapy |
| Suputtitada 2004 | Control is active walking |
| Takao 2015 | Intervention not physical fitness training |
| Takatori 2012 | Not a RCT and co‐intervention (strength training + whole body vibration) |
| Tamura 2011 | Quasi‐experimental |
| Tang 2009 | Wrong study design ‐ not a RCT |
| Tang 2014 | Wrong control group or active control group |
| Taylor‐Piliae 2012 | Intervention not physical fitness training (Tai Chi) |
| Thielman 2004 | Not a relevant comparison: resistance training versus task‐related training |
| Thielman 2005 | Not a relevant comparison: resistance training versus task‐related training |
| Thielman 2013 | Previously excluded |
| Tripp 2014 | Intervention not physical fitness training |
| Van der Lee 1999 | Intervention not physical fitness training. Comparison not relevant: comparison between forced use of affected arm and use of both arms |
| van Wijk 2012 | Intervention not physical fitness training |
| Walker 1999 | Intervention not physical fitness training: occupational therapy |
| Wang 2015 | Intervention has co‐intervention components |
| Werner 1996 | Intervention not physical fitness training: physical and occupational therapy |
| Werner 2002 | Not a valid comparison: comparison of 2 forms of training |
| Widén Holmqvist 1998 | Intervention not physical fitness training: home‐based physical and occupational therapy |
| Wing 2006 | Control group exposed to exercise (upper body) |
| Winstein 2013 | Intervention has co‐intervention components |
| Wolfe 2000 | Intervention not physical fitness training: community‐based physical and occupational therapy |
| Wu 2011 | Intervention not physical fitness training |
| Xiao 2002 | Not a valid comparison |
| Yang 2005 | Not a valid comparison: control intervention included strengthening, function, mobility, and gait training after completion of usual care |
| Yang 2007 | Intervention not physical training (ball exercise programme versus rehabilitation training) |
| Yen 2008 | Not a valid control (not usual care) |
| Yokokawa 1999 | Ongoing rehabilitation classes were randomised, not individuals; this is biased |
| Zheng 2014 | Wrong participant population |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Arya 2012
| Methods | Randomised, controlled, double‐blinded trial |
| Participants | Intervention n = 51; control n = 52; mean 12.15 weeks post‐stroke |
| Interventions | Meaningful task‐specific training 4 to 5 days/week for 4 weeks |
| Outcomes | Fugl‐Meyer Assessment, ARAT, Graded WMFT, MAL |
| Notes | Only abstract available |
Askim 2010
| Methods | Randomised, controlled, single‐blinded trial |
| Participants | Intervention n = 30; control n = 32; within 14 days post‐stroke |
| Interventions | Intensive motor training programme every week for 4 weeks |
| Outcomes | BBS, BI, MAS, Step Test, 5‐MWT, SIS |
| Notes | Cannot include as further detail needed from authors about intervention |
Buyukavci 2011
| Methods | Randomised, single‐blinded, controlled trial |
| Participants | Intervention n = 31; control n = 32 |
| Interventions | Trunk training |
| Outcomes | Motor recovery (Brunnstrom staging), RMI, BBS, trunk balance (Trunk impairment Scale, TIS), FIM, SIS |
| Notes | Only abstract available |
Byun 2011
| Methods | Non‐randomised cross‐over design |
| Participants | Intervention n = 15; control n = 15 |
| Interventions | Sliding rehabilitation machine for 2 weeks followed by conventional training |
| Outcomes | FAC, BBS, 6‐MWT, TUG, Korean Modified BI, MAS, MMT |
| Notes | Cannot include as further detail needed from authors about intervention |
Dean 2010
| Methods | Randomised, single‐blinded, controlled trial |
| Participants | 126 participants; unclear intervention or control group numbers |
| Interventions | Treadmill walking with supported body weight for 30 minutes, unclear frequency per week and length of intervention |
| Outcomes | Walking capacity, walking quality, walking perception, community participation and falls |
| Notes | Only abstract available |
Hoyer 2012
| Methods | Randomised, single‐blinded, controlled trial |
| Participants | — |
| Interventions | Treadmill walking for 30 minutes daily, 5 times/week for 10 weeks |
| Outcomes | FAC, FIM, 10‐MWT, 6‐MWT |
| Notes | Cannot include as further detail needed from authors about intervention |
Kondo 2012
| Methods | Randomised, single‐blinded, controlled trial |
| Participants | n = 22; intervention n = 11; control n = 11; > 6 months after stroke |
| Interventions | Dynamic‐intensive exercise (stepping forward and backward onto a block, squatting, a 30‐metre walk as fast as possible and jumping exercise) |
| Outcomes | Maximal isometric strength, TUG, maximal and comfortable 10‐MWT, 6‐MWT |
| Notes | Only abstract available |
Kumaran 2014
| Methods | Randomised controlled trial |
| Participants | n = 33; intervention n = 19; control n = 14; community dwelling |
| Interventions | Task‐based exercise programme |
| Outcomes | SIS |
| Notes | Only abstract available |
Kwok 2012
| Methods | Single‐blind clinical controlled trial |
| Participants | n = 142; intervention n = 74(?); control n = 68; community dwelling |
| Interventions | Active Lifestyle Therapeutic Exercise Program (ALTEP) |
| Outcomes | Energy expenditure, 6‐MWT, comfortable walking speed, SF‐36, blood pressure, fasting blood glucose, lipid profile |
| Notes | Only abstract available |
Lee 2008
| Methods | RCT |
| Participants | n = 142; intervention n = 17; control n = 17 |
| Interventions | Aquatic exercise |
| Outcomes | 10‐MWT, 12 minute walking test, balance |
| Notes | Only abstract available |
Malagoni 2013
| Methods | RCT; 3 groups |
| Participants | n = 16 |
| Interventions | Group 1: supervised exercise (stretching, balance exercises, supervised walking, and stair climbing) Group 2: home‐based walking training |
| Outcomes | 6‐MWT, TUG, Stair Test (ST), repeated (x 5) stand‐to‐sit test |
| Notes | Only abstract available |
Mayo 2011
| Methods | Randomised trial |
| Participants | 242 participants |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes | Only abstract available |
Moore 2012
| Methods | RCT |
| Participants | Intervention n = 20; control n = 20 |
| Interventions | Unclear type of intervention; exercise for 1 hour 3 times/week for 19 weeks |
| Outcomes | Maximal cardiopulmonary exercise testing, 6‐MWT, 10‐MWT, TUG, BBS |
| Notes | Only abstract available |
Olawale 2011
| Methods | RCT |
| Participants | Intervention n = 20 (treadmill walking); n = 20 (overground walking); control n = 20 |
| Interventions | Either treadmill walking or overground walking for 12 weeks |
| Outcomes | 10‐MWT, 6‐MWT |
| Notes | Cannot include as further detail needed from authors about intervention |
Pagnussat 2014
| Methods | RCT, single‐blind |
| Participants | n = unknown |
| Interventions | Task‐oriented strength training with personalized load resistance |
| Outcomes | The Upper Extremity Performance Test (TEMPA), shoulder flexor and handgrip strength, shoulder active range of motion (ROM), motor impairment (Fugl‐Meyer scale) and muscle tone |
| Notes | Only abstract available |
Park 2014
| Methods | RCT |
| Participants | Intervention n = 11; control n = 11 |
| Interventions | Underwater treadmill gait training |
| Outcomes | Static and dynamic balance |
| Notes | Cannot include as further detail needed from authors about intervention |
Podubecka 2011
| Methods | RCT |
| Participants | Unclear |
| Interventions | Cyclic movement training for 4 weeks |
| Outcomes | Power, balance, cardiorespiratory fitness, and quality of life |
| Notes | Only abstract available; non‐English full‐text available |
Qi 2011
| Methods | RCT |
| Participants | Intervention n = 13; control n = 12 |
| Interventions | Graded elastic strengthening training 3 times/week for 12 weeks |
| Outcomes | Fugl‐Meyer Assessment, 6‐MWT, BBS, muscle strength testing |
| Notes | Only abstract available |
Qu 2014
| Methods | Controlled study; whether it was randomised is unclear |
| Participants | Intervention n = 20; control n = 20 |
| Interventions | Biodex assisted walking training |
| Outcomes | BBS, TUG, maximum walking speed and stride length, Fugl‐Meyer Assessment Score (lower‐extremity), modified Barthel Index (MBI),and Functional Ambulation Categories (FAC) |
| Notes | Only abstract available; full text exists but not accessible |
Rydwik 2006
| Methods | RCT |
| Participants | Intervention n = 9; control n = 9 |
| Interventions | Ankle exercise; passive and active dorsal extension and plantar flexion |
| Outcomes | Range of motion, muscle strength, FIM, Modified Ashworth Scale, 10‐MWT, 6‐MWT, Romberg balance test, Instrumental Activity Measure (ADL), and health‐related quality of life (SF‐36) |
| Notes | Cannot include as further detail needed from authors about intervention |
Sen 2013
| Methods | RCT |
| Participants | n = 50 |
| Interventions | Isokinetic strength training |
| Outcomes | Muscle strength, FIM, Stroke Specific Quality of Life Scale, 10‐MWT, 6‐MWT, Stair‐Climbing Test, TUG, BBS, RMI |
| Notes | Only abstract available |
Shaughnessy 2012
| Methods | RCT; parallel assignment; open‐label |
| Participants | n = 90 stroke patients aged 40 to 85 years |
| Interventions | Intervention: home‐based exercise prescriptions with weekly motivational telephone calls |
| Outcomes | AAP |
| Notes | Only abstract available |
Shaughnessy 2012a
| Methods | RCT |
| Participants | Intervention n = 57; control n = 56 |
| Interventions | Treadmill intervention for 40 minutes 3 times/week for 6 months |
| Outcomes | Short Self‐efficacy and Outcome Expectations for Exercise, Yale Physical Activity Survey, SIS |
| Notes | Cannot include as further detail needed from authors about intervention |
Srivastava 2011
| Methods | RCT |
| Participants | Unclear |
| Interventions | Intervention: treadmill with body weight support; treadmill without body weight support each for 20 minutes/day, 5 days/week for 4 weeks |
| Outcomes | Walking distance, speed and endurance, no further details given |
| Notes | Only abstract available |
Tung 2010
| Methods | RCT |
| Participants | Intervention n = 16; control n = 16 |
| Interventions | Sit‐to‐stand training for 15 minutes, 3 times/week for 4 weeks |
| Outcomes | BBS, extensor muscle strength of lower extremity |
| Notes | Cannot include as further detail needed from authors about control intervention |
Vahlberg 2014
| Methods | RCT |
| Participants | Intervention n = 34; control n = 33; community dwelling |
| Interventions | Progressive resistance and balance training |
| Outcomes | Mobility (Short Physical Performance Battery), BBS, Physical Activity Scale for the Elderly, 6‐MWT, comfortable walking speed |
| Notes | Only abstract available |
Van Puymbroeck 2014
| Methods | RCT |
| Participants | Intervention n = 37; control n = 10 |
| Interventions | Yoga intervention for 1 hour, twice/week for 8 weeks |
| Outcomes | ICF Measure of Participation and Activity, Stroke Survivor Quality of Life |
| Notes | Only abstract available |
Yang 2010
| Methods | Randomised, controlled, single‐blind trial |
| Participants | Intervention n = 10; control n = 8 |
| Interventions | Body weight supported treadmill training for 30 minutes, 3 times/week for 4 weeks |
| Outcomes | Motor threshold of abductor hallucis muscle, Fugl‐Meyer Assessment |
| Notes | Cannot include as further detail needed from authors about usual care |
5‐MWT: 5‐Metre Walk Test 6‐MWT: 6‐Minute Walk Test 10‐MWT: 10‐Metre Walking Test AAP: Ambulatory Activity Profile ARAT: Action Research Arm Test BBS: Berg Balance Scale BI: Barthel Index CES‐D: Center for Epidemiologic Studies Depression Scale COPD: chronic obstructive pulmonary disease FAC: Functional Ambulation Classification FIM: Functional Independence Measure MAL: Motor Activity Log MAS: Motor Assessment Scale MMSE: Mini Mental State Examination MMT: Manual Muscle Test RCT: randomised controlled trial RMI: Rivermead Mobility Index SIS: Stroke Impact Scale TUG: Timed Up‐and Go Test WMFT: Wolf Motor Function Test
Characteristics of ongoing studies [ordered by study ID]
ACTRN12608000457347
| Trial name or title | The efficacy of a novel, non‐robotic intervention to train reaching post stroke |
| Methods | RCT |
| Participants | 75 participants |
| Interventions | Arm training |
| Outcomes | Primary: MAS item 6 Secondary: distance reached, force during reaching, MAS items 7 and 8, SIS |
| Starting date | Start: February 2010 |
| Contact information | Sandra Brauer Email: s.brauer@uq.edu.au |
| Notes | — |
ACTRN12610000096055
| Trial name or title | CIRCIT Trial |
| Methods | RCT |
| Participants | 282 participants |
| Interventions | Group circuit class therapy 5 days/week |
| Outcomes | 6‐MWT |
| Starting date | Unclear |
| Contact information | Susan Hillier Email: susan.hillier@unisa.edu.au |
| Notes | ACTRN 12610000096055 |
ACTRN12613000822785
| Trial name or title | The effect of moderate intensity cardiovascular fitness training compared to standard care in people with a diagnosis of stroke: a randomised controlled trial |
| Methods | RCT |
| Participants | 20 participants, aged 18 or older Stroke 6 weeks to 12 months previously |
| Interventions | Moderate intensity cardiorespiratory training |
| Outcomes | Primary: VO2 peak Secondary: functional independence, walking speed and endurance, mood, QoL |
| Starting date | Start: July 2013 End: unclear |
| Contact information | Johanna Reynolds Email: hanna.reynolds@wh.org.au |
| Notes | ACTRN12613000822785 |
Hariohm 2013
| Trial name or title | Efficacy of deep knee flexion exercises |
| Methods | RCT |
| Participants | 40 community‐dwelling participants |
| Interventions | Knee flexion exercise |
| Outcomes | Primary: activity goal attainment, QoL (social participation domain) Secondary: lower limb muscle strength, fear of falling, functional ambulation status |
| Starting date | Unknown |
| Contact information | K Hariohm, MSAJ Collage of PT, Chennai, India |
| Notes | No known trial registry entry |
ISRCTN 45392701
| Trial name or title | Study protocol for a RCT |
| Methods | RCT |
| Participants | 24 participants aged 18+ years 3 to 30 days post‐stroke |
| Interventions | Cardiorespiratory training (lower limb cycling) versus usual care |
| Outcomes | Motricity Index, electromyography |
| Starting date | Start: November 2009 End: March 2011 |
| Contact information | Nicola J Hancock Email: n.hancock@uea.ac.uk |
| Notes | ISRCTN 45392701 |
ISRCTN19090862
| Trial name or title | Clinical efficacy of functional strength training for upper limb motor recovery early after stroke: neural correlates and prognostic indicators (FAST INdICATE) |
| Methods | RCT |
| Participants | 288 participants 14 to 60 days post‐stroke |
| Interventions | Resistance training versus conventional physiotherapy |
| Outcomes | Primary outcome measure: ARAT Secondary outcome measure: WMFT, hand grip force, pinch grip force |
| Starting date | Start: September 2012 End: May 2015 |
| Contact information | Andrew Walker, University of East Anglia, Faculty of Medicine, Queens Building, Norwich Research Park, Norwich, NR4 7TJ, UK Email: andrew.walker@uea.ac.uk |
| Notes | ISRCTN19090862 |
ISRCTN56716589
| Trial name or title | Home‐based reach‐to‐grasp training after stroke |
| Methods | RCT |
| Participants | 60 participants living at home with upper limb deficit |
| Interventions | Task‐specific arm training |
| Outcomes | Primary: ARAT Secondary: Motor Activity Log, SIS, WMFT |
| Starting date | Start: November 2011 End: December 2012 |
| Contact information | Dr Ailie Turton Email: ailie.turton@uwe.ac.uk |
| Notes | ISRCTN56716589 |
| Trial name or title | Cardiac rehabilitation for TIA patients (CR‐TIA) |
| Methods | RCT, parallel assignment; single‐blind (outcomes assessor) |
| Participants | 200 participants Inclusion criteria: age > 20 years; documented TIA or mild non‐disabling stroke within the previous 3 months; at least 1 of the following vascular risk factors: hypertension, ischaemic heart disease, diabetes mellitus, dyslipidaemia, or cigarette smoking Exclusion criteria: inability to speak or understand English or provide informed consent; severe aphasia that renders communication difficult or impossible; mRS ≥ 3; MMSE ≤ 20; evidence of intracranial haemorrhage confirmed by CT scan or MRI study; anticipated or recent (< 30 days) carotid endarterectomy, angioplasty and/or stenting; resides > 1 hour travel time from London or Ottawa; prior participation in a CCR programme; inability to perform expected exercise training of CCR programme; evidence of cardioembolic source for TIA/stroke such as atrial fibrillation, valvular disease, septal defect, or left ventricular wall motion abnormality; participation in another clinical trial that could interfere with the intervention or outcomes of the current study |
| Interventions | Intervention: comprehensive CCR programme plus usual care (include home‐based exercise 2 days/week for 6 months) Control: usual care alone |
| Outcomes | Primary outcome measures: functional capacity, lipid profile, depression symptoms, cognition Secondary outcome measures: cerebrovascular and cardiovascular events; physiological, anthropometric, and behavioural vascular risk factors; neurocognitive measure; QoL Time frame: 6 months |
| Starting date | Start: September 2007 End: June 2014 |
| Contact information | Neville G Suskin, MBChB, MSc, University of Western Ontario and London Health Sciences Centre, London, Ontario, Canada, N6A 5A5 Email: neville.suskin@lhsc.on.ca |
| Notes | NCT00536562 |
| Trial name or title | Strength training for skeletal muscle adaptation after stroke |
| Methods | RCT; parallel assignment; open‐label |
| Participants | 38 participants Inclusion criteria: men and women aged 40 to 85 years, ?6 months post‐stroke Completion of rehabilitation |
| Interventions | Intervention: lower extremity strength training (leg extension, press and curl), 45 to 60 minutes per day, 3 days per week for 3 months Control: active and passive upper and lower body stretching and range of motion, 45 to 60 minutes per day, 3 days per week for 3 months |
| Outcomes | VO2 peak, bilateral single limb strength testing (leg extension and leg press), bilateral single limb muscle endurance (static and dynamic), mobility (timed 10‐MWT and 6‐MWT), BBS |
| Starting date | Start: April 2009 End: May 2016 |
| Contact information | Fred Ivey, VA Maryland Health Care System, Baltimore, USA |
| Notes | NCT00827827 |
| Trial name or title | Inflammation and exercise in stroke |
| Methods | RCT |
| Participants | 150 participants, aged 40 to 75 years ≥ 6 months post‐stroke |
| Interventions | Cardiorespiratory training versus non‐exercise intervention |
| Outcomes | Primary outcome measures: tumour necrosis factor alpha, whole body insulin sensitivity, VO2 peak, muscle insulin signalling Secondary outcome measures: circulating glucose, body composition, muscle triglyceride, number of macrophages |
| Starting date | Start: May 2009 End: April 2014 |
| Contact information | Jessica Hammers Email: jhammers@grecc.umaryland.edu |
| Notes | NCT00891514 |
| Trial name or title | The effect of an aerobic exercise programme in stroke patients |
| Methods | RCT, parallel assignment, double‐blind |
| Participants | 50 participants Inclusion criteria: 3 to 6 weeks after first stroke; ability to follow simple verbal instructions and cycle for ? 1 minute at 20 Watt (at 50 revolution/minute) |
| Interventions | Intervention: regular rehabilitation plus cardiorespiratory training; 30 minutes per day, 3 days per week for 12 weeks. Cycle ergometry. After 12 weeks the experimental group is randomised to receive either feedback on how to continue training or no feedback Control: regular rehabilitation plus passive mobilisation |
| Outcomes | VO2 peak, strength, walking, activities of daily living, post‐stroke fatigue, depression, lifestyle, cardiovascular risk factors |
| Starting date | Start: February 2010 End: December 2010 |
| Contact information | Vanroy Christel, University College Antwerp |
| Notes | NCT01070459 |
| Trial name or title | Fit For Function: a community wellness program for persons with stroke (FFF) |
| Methods | RCT |
| Participants | 61 participants |
| Interventions | Community‐based exercise programme at the YMCA |
| Outcomes | Primary: 6MWT, hand grip strength, Rapid Assessment of Physical Activity (RAPA) Secondary: Patient Activation Measure (PAM) |
| Starting date | Study: October 2010 End: October 2012 |
| Contact information | Dr Julie Richardson, McMaster University |
| Notes | NCT01194102 |
| Trial name or title | Exercise for sub‐acute stroke patients in Jamaica (JAMMS) |
| Methods | RCT |
| Participants | 150 participants with ischaemic stroke within 8 weeks |
| Interventions | Task‐oriented mixed training versus usual care |
| Outcomes | Primary outcome measures: thigh and abdominal muscle and fat, whole body protein and skeletal muscle, muscle myosin heavy chain isoform (MHC) proportions, leg strength, fitness VO2 peak, glucose tolerance Secondary outcome measures: muscle tumour necrosis factor, mobility and balance |
| Starting date | Study: July 2011 End: April 2016 |
| Contact information | Contact: Richard Macko, MD Email: rmacko@grecc.umaryland.edu |
| Notes | NCT01392391 |
| Trial name or title | Life After Stroke ‐ the LAST study |
| Methods | RCT |
| Participants | 390 participants; 10 to 16 weeks post‐stroke |
| Interventions | Coaching on physical activity; 45 to 60 minutes, once/week for 18 months |
| Outcomes | MAS, BI, mRS, BBS, TUG, SIS, HADS, MMSE |
| Starting date | Start: November 2011 End: December 2015 |
| Contact information | Torunn Askim Email: torunn.askim@ntnu.no |
| Notes | NCT01467206 |
| Trial name or title | Training dual‐task walking after stroke |
| Methods | RCT |
| Participants | 44 participants |
| Interventions | Dual‐task gait training |
| Outcomes | Primary: dual‐task cost on gait speed Secondary: executive function, spontaneous physical activity assessed with an activity monitor, kinematics of gait during obstacle crossing, SIS |
| Starting date | Start: September 2011 End: June 2016 |
| Contact information | Prudence Plummer Tel: 919‐843‐8658, Email: pplummer@med.unc.edu |
| Notes | NCT01568957 |
| Trial name or title | Fast muscle Activation and Stepping Training (FAST) post‐stroke |
| Methods | RCT |
| Participants | 60 participants with first stroke < 6 months previously |
| Interventions | Rapid movement training versus usual care |
| Outcomes | Primary outcome measures: Community Balance and Mobility Scale Secondary outcome measures: gait assessment self selected speed and changes in electromyography, physiological balance assessment by internal and external, activities‐specific Balance Confidence Scale |
| Starting date | Start: July 2012 End: June 2017 |
| Contact information | Principal Investigator: S Jayne Garland, PT, PhD University of British Columbia |
| Notes | NCT01573585 |
| Trial name or title | Use of repetitive facilitative exercise program in established stroke |
| Methods | RCT |
| Participants | 40 people with stroke > 6 months duration |
| Interventions | Repetitive exercise versus usual care |
| Outcomes | Primary outcome measures: Fugl‐Meyer Arm Assessment Secondary outcome measures: Motor Activity Log, 9‐Hole Peg Test, Box and Block Test, grasp, active range of motion |
| Starting date | Start: April 2012 End: April 2016 |
| Contact information | Billie A Schultz, MD Email: schultz.billie@mayo.edu |
| Notes | NCT01574599 |
| Trial name or title | Combined effects of aerobic exercise and cognitive training on cognition after stroke |
| Methods | RCT |
| Participants | 20 participants with stroke > 6 months ago |
| Interventions | Aerobic BWSTT exercise versus non‐exercise comparison |
| Outcomes | Primary outcome measures: Flanker Test, Raven's Matrices Test, Sternberg Digit Memory Task Secondary outcome measures: peak oxygen consumption, Fatigue Severity Scale, Cognitive Failures Questionnaire, Montreal Cognitive Assessment, expression of BDNF and IGF‐1 in peripheral blood samples |
| Starting date | Start: September 2012 End: June 2015 |
| Contact information | Marilyn MacKay‐Lyons, PhD Email: m.mackay‐lyons@dal.ca |
| Notes | NCT01674790 |
| Trial name or title | Effects of a community‐based group rehabilitation program for dynamic balance and mobility post stroke |
| Methods | RCT |
| Participants | 24 participants, 3 to 12 months post‐stroke Age 50 to 70 years English speaking with adequate cognitive function |
| Interventions | Mixed training: stretching, strengthening, treadmill, and cycling |
| Outcomes | Primary: gait speed, SIS Secondary: BBS, timed up and go test |
| Starting date | Start: April 2013 End: September 2015 |
| Contact information | Sepideh Pooyania, MD Email: spooyania@rhc.mb.ca |
| Notes | NCT01818271 |
| Trial name or title | A randomised, non‐inferiority clinical trial of CVA telerehabilitation treatments ‐ TelePhysioTaiChi |
| Methods | RCT |
| Participants | 240 participants aged 45 years or older |
| Interventions | Tai Chi‐based exercise programme |
| Outcomes | Primary: change from baseline in balance, change from baseline in mobility Secondary: change from baseline of: cost of services, psychological attitudes related to balance and mobility, QoL, satisfaction with care received, strength of lower limbs, walking endurance, walking speed |
| Starting date | Start: June 2013 End: October 2017 |
| Contact information | Michel Tousignant, PhD Email: michel.tousignant@usherbrooke.ca |
| Notes | NCT01848080 |
| Trial name or title | Physical fitness training in subacute stroke (PHYS‐STROKE) |
| Methods | Multicentre RCT |
| Participants | 215 participants |
| Interventions | Cardiorespiratory training versus relaxation |
| Outcomes | Primary outcome measures: gait speed, BI Secondary outcome measures: motor function, mobility, cognitive function, disability, QoL, sleep, mood, physical fitness VO2 max and gait energy, resting systolic and diastolic blood pressure, laboratory parameters, hair cortisol concentration, assessment of safety, resting heart rate, body mass index, waist to hip ratio, markers of inflammation, markers of peripheral immunity, blood lipid profile |
| Starting date | Start: October 2013 End: March 2017 |
| Contact information | Regina Schlieder Email: regina.schlieder@charite.de |
| Notes | NCT01953549 |
| Trial name or title | Ballistic strength training in stroke: a pilot study |
| Methods | RCT |
| Participants | 30 participants aged > 18 years |
| Interventions | Ballistic resistance training |
| Outcomes | Feasibility, 10‐MWT, QoL, FAC, timed up and go test |
| Starting date | Start: February 2014 End: April 2016 |
| Contact information | Genevieve C Tole, BPhys (Hons) Email: g.tole@alfred.org.au |
| Notes | NCT01958736 |
| Trial name or title | Aerobic exercise in early subacute stroke |
| Methods | RCT |
| Participants | 56 participants who understand Swedish language |
| Interventions | Cardiorespiratory training |
| Outcomes | Balance and walking capacity |
| Starting date | Start: January 2011 End: September 2013 |
| Contact information | Klas Sandberg, MSc, RPT, Ostergotland County Council |
| Notes | NCT02107768 |
| Trial name or title | Early intervention with a low‐intensity leg cycling exercise program for individuals after stroke |
| Methods | RCT |
| Participants | 120 participants aged 18 years to 80 years |
| Interventions | 2 intervention arms
|
| Outcomes | Primary: change values of symptom‐limit exercise capacity Secondary: change values of sympathetic nerve tests |
| Starting date | Start: March 2014 End: March 2017 |
| Contact information | Miao‐Ju Hsu, PhD Email: mjhsu@kmu.edu.tw |
| Notes | NCT02437006 |
10‐MWT: 10‐Metre Walk Test 6‐MWT: 6‐Minute Walk Test ARAT: Action Research Arm Test BBS: Berg Balance Scale BI: Barthel Index BWSTT: body weight supported treadmill training CCR: Circulatory, Cardiac and Respiratory Research Program FAC: Functional Ambulation Classification HADS: Hospital Anxiety and Depression Scale MAS: Motor Assessment Scale MMSE: Mini Mental State Examination mRS: modified Rankin Scale QoL: quality of life RCT: randomised controlled trial SIS: Stroke Impact Scale TIA: transient ischaemic attack TUG: Timed Up and Go Test WMFT: Wolf Motor Function Test
Contributions of authors
Original review
DH Saunders, CA Greig, GE Mead, and A Young contributed to writing the review protocol. DH Saunders developed and ran searches, selected studies, extracted and interpreted data, performed the analyses, and co‐wrote the review. CA Greig and GE Mead selected studies, extracted and interpreted data, performed the analyses, and co‐wrote the review. A Young provided comments on interim drafts of the review.
For this update
DH Saunders developed and ran searches, selected studies, extracted and interpreted data, performed the analyses, and wrote the review. MF Sanderson, S Hayes, and M Kilrane selected studies, extracted and interpreted data, and contributed to writing the review. M Brazzelli advised on the methodology and analyses and provided comments on a draft version of the review. GE Mead and CA Greig helped select studies and provided comments on a draft version of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
Cochrane Review Incentive Scheme 2012
Declarations of interest
DH Saunders and CA Greig were co‐authors of one included study (Mead 2007). GE Mead has received research funding for exercise after stroke. She has received honoraria from Later Life Training to develop an educational course of exercise after stroke for exercise professionals. She has also received honoraria and expenses to present work on exercise after stroke at conferences. She has led a trial of exercise after stroke that is included in the review (Mead 2007). S Hayes has no declarations of interest. M Brazzelli has no declarations of interest.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
- Ada L, Dean CM, Lindley R. Randomized trial of treadmill training to improve walking in community‐dwelling people after stroke: the AMBULATE trial. International Journal of Stroke 2013;8(6):436‐44. [DOI] [PubMed] [Google Scholar]; Dean CM, Ada L, Lindley RI. Treadmill training provides greater benefit to the subgroup of community‐dwelling people after stroke who walk faster than 0.4m/s: a randomised trial. Journal of Physiotherapy (Australian Physiotherapy Association) 2014;60(2):97‐101. [DOI] [PubMed] [Google Scholar]
- Aidar FJ, Silva AJ, Reis VM, Carniero A, Carniero‐Cotta S. A study on the quality of life in ischaemic vascular accidents and its relation to physical activity [Estudio de la calidad de vida en el accidente vascular isquémico y su relación con la acividad física]. Revista de Neurología 2007;45:518‐22. [PubMed] [Google Scholar]
- Aidar FJ, Oliveira RJ, Silva AJ, Matos Dihogo G, Mazini Filho ML, Hickner RC, et al. The influence of resistance exercise training on the levels of anxiety in ischemic stroke. Stroke Research and Treatment 2012;2012:298‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aidar FJ, Matos DG, Oliveira RJ, Carneiro AL, Cabral BG de AT, Dantas PMS, et al. Relationship between depression and strength training in survivors of the ischemic stroke. Journal of Human Kinetics 2014;43:7‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale M, Strand LI. Does functional strength training of the leg in subacute stroke improve physical performance? A pilot randomized controlled trial. Clinical Rehabilitation 2008;22(10‐11):911‐21. [DOI] [PubMed] [Google Scholar]
- Bateman A, Culpan FJ, Pickering AD, Powell JH, Scott OM, Greenwood RJ. The effect of aerobic training on rehabilitation outcomes after recent severe brain injury: a randomized controlled evaluation. Archives of Physical Medicine and Rehabilitation 2001;82(2):174‐82. [DOI] [PubMed] [Google Scholar]
- Cooke E, Pomeroy VM, Miller S, Tallis RC. The effects of functional strength training on lower limb strength and function after stroke. Cerebrovascular Diseases 2006;21 Suppl(4):104. [Google Scholar]; Cooke E, Tallis R, Miller S, Pomeroy V. The effects of functional strength training on lower limb strength and function after stroke. Proceedings of the UK Stroke Forum Conference. 2006. [Google Scholar]; Cooke E, Tallis R, Pomeroy V. The effects of different intensity of exercise‐based physiotherapy on motor recovery in the lower limb after stroke: a meta‐analysis. Proceedings of the UK Stroke Forum Conference 4‐6 December 2007. The Stroke Association, 2007. [Google Scholar]; Cooke EV, Tallis RC, Clark A, Pomeroy VM. Efficacy of functional strength training on restoration of lower‐limb motor function early after stroke: phase I randomized controlled trial. Neurorehabilitation and Neural Repair 2010;24(1):88‐96. [DOI] [PubMed] [Google Scholar]; Cooke EV, Tallis RC, Miller S, Pomeroy VM. The effects of functional strength training on lower limb strength and function after stroke. Cerebrovascular Diseases 2008;25 Suppl 2:45‐6. [Google Scholar]; Cooke EV, Tallis RC, Miller S, Pomeroy VM. The effects of type and intensity of physiotherapy on lower limb strength and function after stroke. Cerebrovascular Diseases 2007;23 Suppl 2:129. [Google Scholar]
- Cuviello‐Palmer ED. Effect of the Kinetron II on Gait and Functional Outcome in Hemiplegic Subjects. Texas, USA: Texas Women's University, 1988. [Google Scholar]
- Lim PAC, Henson H, Cunha I, Qureshy H, Monga TN, Protas EJ. Body weight‐supported gait training in stroke patients. American Journal of Physical Medicine and Rehabilitation 2000;79(2):203. [Google Scholar]; Cunha IT, Lim PA, Qureshy H, Henson H, Monga T, Protas EJ. A comparison of regular rehabilitation with supported treadmill ambulation training for acute stroke patients. Journal of Rehabilitation Research and Development 2001;38(2):245‐55. [PubMed] [Google Scholar]; Cunha IT, Lim PA, Qureshy H, Henson H, Monga T, Protas EJ. Gait outcomes after acute stroke rehabilitation with supported treadmill training: a randomized controlled pilot study. Archives of Physical Medicine and Rehabilitation 2002;83(9):1258‐65. [DOI] [PubMed] [Google Scholar]
- Donaldson C, Tallis R, Miller S, Sunderland A, Lemon R, Pomeroy V. Effects of conventional physical therapy and functional strength training on upper limb motor recovery after stroke: a randomized phase II study. Neurorehabilitation and Neural Repair 2009;23(4):389‐97. [DOI] [PubMed] [Google Scholar]; Donaldson C, Tallis R, Miller S, Sunderland A, Lemon R, Pomeroy V. The effects of current physical therapy and functional strength training on upper limb function and neuromuscular weakness after stroke: a pilot study (Abst. poster 156). Proceedings of the UK Stroke Forum Conference. The Stroke Association, 4‐6 December 2007. [Google Scholar]; Donaldson C, Tallis R, Pomeroy V. The effects of current physical therapy and functional strength training on upper limb function and neuromuscular weakness after stroke: a pilot study. Cerebrovascular Diseases 2007;23 Suppl 2:130. [Google Scholar]
- Duncan P, Richards L, Wallace D, Stoker‐Yates J, Pohl P, Luchies C, et al. A randomized, controlled pilot study of a home‐based exercise program for individuals with mild and moderate stroke. Stroke 1998;29(10):2055‐60. [DOI] [PubMed] [Google Scholar]
- Duncan P, Studenski S, Richards L, Gollub S, Lai SM, Reker D, et al. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke 2003;34(9):2173‐80. [DOI] [PubMed] [Google Scholar]; Lai SM, Studenski S, Richards L, Perera S, Reker D, Rigler S, et al. Therapeutic exercise and depressive symptoms after stroke. Journal of the American Geriatric Society 2006;54:240‐7. [DOI] [PubMed] [Google Scholar]; Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. Journal of the American Geriatrics Society 2006;54:743‐9. [DOI] [PubMed] [Google Scholar]; Schmid A, Duncan PW, Studenski S, Lai SM, Richards L, Perera S, et al. Improvements in speed‐based gait classifications are meaningful. Stroke 2007;38:2096‐100. [DOI] [PubMed] [Google Scholar]; Studenski S, Duncan PW, Perera S, Reker D, Lai SM, Richards L. Daily functioning and quality of life in a randomized controlled trial of therapeutic exercise for subacute stroke survivors. Stroke 2005;36(8):1764‐70. [DOI] [PubMed] [Google Scholar]
- Eich HJ, Hesse S, Mach H. Aerobic endurance training of hemiparetic patients who are able to walk. Results of a prospective randomised study. Deutsche Zeitschrift für Sportmedizin 2003;54(7/8):S98. [Google Scholar]; Eich HJ, Mach H, Werner C, Hesse S. Aerobic treadmill plus Bobath walking training improves walking in subacute stroke: a randomized controlled trial. Clinical Rehabilitation 2004;18(6):640‐51. [DOI] [PubMed] [Google Scholar]; Eich HJ, Parchmann H, Hesse S, Mach H, Werner C. Aerobic treadmill training plus physiotherapy improves walking ability in subacute stroke patients. A randomized controlled study. Neurologie und Rehabilitation 2004;10(4):187‐216. [Google Scholar]; Fitch S, Elkins M, Moseley A. Was CAP summary faithful?. Australian Journal of Physiotherapy 2005;51:265‐6. [DOI] [PubMed] [Google Scholar]; Hesse S, Eich HJ, Mach H, Parchmann H, Werner C. Aerobic treadmill training plus physiotherapy improves walking speed and capacity in subacute, moderately affected patients after stroke. Neurologie und Rehabilitation 2005;11(1):7‐12. [Google Scholar]; Moseley A. Treadmill training more effective than Bobath training in improving walking following stroke. Australian Journal of Physiotherapy 2005;51(3):192. [DOI] [PubMed] [Google Scholar]
- Flansbjer UB, Lexell J, Brogardh C. Long‐term benefits of progressive resistance training in chronic stroke: a 4‐year follow‐up. Journal of Rehabilitation Medicine 2012;44(3):218‐21. [DOI] [PubMed] [Google Scholar]; Flansbjer UB, Miller M, Downham D, Lexell J. Progressive resistance training after stroke: effects on muscle strength, muscle tone, gait performance and perceived participation. Journal of Rehabilitation Medicine 2008;40(1):42‐8. [DOI] [PubMed] [Google Scholar]; Flansbjer UB, Miller M, Lexell J. Progressive resistance training after stroke: effects on muscle function, gait performance and perceived participation. Stroke Rehabilitation 2006. Evidence for Stroke Rehabilitation ‐ Bridging into the Future. Goteborg, 2006. [DOI] [PubMed] [Google Scholar]
- Galvin R, Cusack T, O'Grady E, Murphy TB, Stokes E. Family‐mediated exercise intervention (FAME): evaluation of a novel form of exercise delivery after stroke. Stroke 2011;42(3):681‐6. [DOI] [PubMed] [Google Scholar]; Galvin R, Stokes E, Cusack T. Family‐mediated exercises (FAME): an exploration of participant's involvement in a novel form of exercise delivery after stroke. Topics in Stroke Rehabilitation 2014;21(1):63‐74. [DOI] [PubMed] [Google Scholar]
- Glasser L. Effects of isokinetic training on the rate of movement during ambulation in hemiparetic patients. Physical Therapy 1986;66(5):673‐6. [DOI] [PubMed] [Google Scholar]
- Globas C, Becker C, Cerny J, Lam JM, Lindemann U, Forrester LW, et al. Chronic stroke survivors benefit from high‐intensity aerobic treadmill exercise: a randomized control trial. Neurorehabilitation and Neural Repair 2012;26(1):85‐95. [DOI] [PubMed] [Google Scholar]; MacKay‐Lyons M. Aerobic treadmill training effectively enhances cardiovascular fitness and gait function for older persons with chronic stroke. Journal of Physiotherapy 2012;58(4):271. [DOI] [PubMed] [Google Scholar]
- Gordon CD, Wilks R, McCaw‐Binns A. Effect of aerobic exercise (walking) training on functional status and health‐related quality of life in chronic stroke survivors: a randomized controlled trial. Stroke 2013;44(4):1179‐81. [DOI] [PubMed] [Google Scholar]
- Inaba M, Edberg E, Montgomery J, Gillis MK. Effectiveness of functional training, active exercise, and resistive exercise for patients with hemiplegia. Physical Therapy 1973;53(1):28‐35. [DOI] [PubMed] [Google Scholar]
- Ivey FM, Hafer‐Macko CE, Ryan AS, Macko RF. Impaired leg vasodilatory function after stroke: adaptations with treadmill exercise training. Stroke 2010;41(12):2913‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey FM, Ryan AS, Hafer‐Macko CE, Macko RF. Improved cerebral vasomotor reactivity after exercise training in hemiparetic stroke survivors. Stroke 2011;42(7):1994‐2000. [DOI] [PubMed] [Google Scholar]
- James JEP. Closed kinetic chain training to enhance muscle power, control and retrain dynamic balance under task specific conditions improves functional walking ability in chronic stroke survivors. Dublin, Ireland: National University of Ireland, 2002. [Google Scholar]
- Jin H, Jiang Y, Wei Q, Chen L, Ma G. Effects of aerobic cycling training on cardiovascular fitness and heart rate recovery in patients with chronic stroke. NeuroRehabilitation 2013;32(2):327‐35. [DOI] [PubMed] [Google Scholar]
- Kang HK, Kim Y, Chung Y, Hwang S. Effects of treadmill training with optic flow on balance and gait in individuals following stroke: randomized controlled trials. Clinical Rehabilitation 2012;26(3):246‐55. [DOI] [PubMed] [Google Scholar]
- Katz‐Leurer M, Carmeli E, Shochina M. The effect of early aerobic training on independence six months post stroke. Clinical Rehabilitation 2003;17(7):735. [DOI] [PubMed] [Google Scholar]; Katz‐Leurer M, Seren I, Keren O, Dvir Z. The influence of early cycling training on balance in stroke patients at the subacute stage. Results of a preliminary trial. Clinical Rehabilitation 2006;20:398‐405. [DOI] [PubMed] [Google Scholar]; Katz‐Leurer M, Shochina M. The influence of autonomic impairment on aerobic exercise outcome in stroke patients. Neurorehabilitation 2007;22(4):267‐72. [PubMed] [Google Scholar]; Katz‐Leurer M, Shochina M, Carmeli E, Friedlander Y. The influence of early aerobic training on the functional capacity in patients with cerebrovascular accident at the subacute stage. Archives of Physical Medicine and Rehabilitation 2003;84:1609‐14. [DOI] [PubMed] [Google Scholar]
- Kim CM, Eng JJ, MacIntyre DL, Dawson AS. Effects of isokinetic strength training on walking in persons with stroke: a double‐blind controlled pilot study. Journal of Stroke and Cerebrovascular Diseases 2001;10(6):265‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Cho K, Lee W. Community walking training program improves walking function and social participation in chronic stroke patients. Tohoku Journal of Experimental Medicine 2014;234(4):281‐6. [DOI] [PubMed] [Google Scholar]
- Kuys SS, Brauer SG, Ada L. Higher‐intensity treadmill walking during rehabilitation after stroke in feasible and not detrimental to walking pattern or quality: a pilot randomized trial. Clinical Rehabilitation 2011;25(4):316‐26. [DOI] [PubMed] [Google Scholar]
- Langhammer B, Lindmark B, Stanghelle JK. Stroke patients and long‐term training: is it worthwhile? A randomized comparison of two different training strategies after rehabilitation. Clinical Rehabilitation 2007;21(6):495‐510. [DOI] [PubMed] [Google Scholar]; Langhammer B, Lindmark B, Stanghelle KJ. Activity and exercise in a long perspective. A solution for chronic stroke patients?. Stroke Rehab 2006. Evidence for Stroke Rehabilitation ‐ Bridging into the Future. Goteborg, 2006. [Google Scholar]
- Lee NK, Kwon JW, Son SM, Kang KW, Kim K, Hyun‐Nam S. The effects of closed and open kinetic chain exercises on lower limb muscle activity and balance in stroke survivors. NeuroRehabilitation 2013;33(1):177‐83. [DOI] [PubMed] [Google Scholar]
- Lee NK, Kwon JW, Son SM, Nam SH, Choi YW, Kim CS. Changes of plantar pressure distributions following open and closed kinetic chain exercise in patients with stroke. NeuroRehabilitation 2013;32(2):385‐90. [DOI] [PubMed] [Google Scholar]
- Lennon O, Carey A, Gaffney N, Stephenson J, Blake C. A pilot randomized controlled trial to evaluate the benefit of the cardiac rehabilitation paradigm for the non‐acute ischaemic stroke population. Clinical Rehabilitation 2008;22(2):125‐33. [DOI] [PubMed] [Google Scholar]
- Letombe A, Cornille C, Delahaye H, Khaled A, Morice O, Tomaszewski A, et al. Early post‐stroke physical conditioning in hemiplegic patients: a preliminary study. Annals of Physical and Rehabilitation Medicine 2010;53(10):632‐42. [DOI] [PubMed] [Google Scholar]
- MacKay‐Lyons M, McDonald A, Matheson J, Eskes G, Klus MA. Dual effects of body‐weight supported treadmill training on cardiovascular fitness and walking ability early after stroke: a randomized controlled trial. Neurorehabilitation & Neural Repair 2013;27(7):644‐53. [DOI] [PubMed] [Google Scholar]
- Mead G. Exercise or relaxation after stroke?. BMJ 2005;330(7503):1337. [Google Scholar]; Mead GE, Greig CA, Cunningham I, Lewis SJ, Dinan S, Saunders DH, et al. Stroke: a randomised trial of exercise or relaxation. Journal of the American Geriatrics Society 2007;55(6):892‐9. [DOI] [PubMed] [Google Scholar]; Mead GE, Greig CA, Cunningham I, Lewis SJ, Dinan S, Saunders DH, et al. Stroke: a randomised trial of exercise or relaxation. Proceedings of the UK Stroke Forum Conference 4‐6 December 2007. Harrogate, UK: The Stroke Association, 2007. [DOI] [PubMed] [Google Scholar]
- Moore JL, Roth EJ, Killian C, Hornby TG. Locomotor training improves daily stepping activity and gait efficiency in individuals poststroke who have reached a "plateau" in recovery. Stroke 2010;41(1):129‐35. [DOI] [PubMed] [Google Scholar]
- Mudge S. The impact of a group exercise programme on usual walking performance in adults who are at least 6 months post stroke: a single blinded randomised controlled trial. Australian New Zealand Clinical Trials Registry (ANZCTR) http://www.anzctr.org.au/. 2007. ; Mudge S, Barber PA, Stott NS. Circuit‐based rehabilitation improves gait endurance but not usual walking activity in chronic stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2009;90(12):1989‐96. [DOI] [PubMed] [Google Scholar]
- Ouellette MM, LeBrasseur NK, Bean JF, Phillips E, Stein J, Frontera WR, et al. High‐intensity resistance training improves muscle strength, self‐reported function, and disability in long‐term stroke survivors. Stroke 2004;35(6):1404‐9. [DOI] [PubMed] [Google Scholar]
- Park HJ, Oh DW, Kim SY, Choi JD. Effectiveness of community‐based ambulation training for walking function of post‐stroke hemiparesis: a randomized controlled pilot trial. Clinical Rehabilitation 2011;25(5):451‐9. [DOI] [PubMed] [Google Scholar]
- Pohl M, Mehrholz J, Ritschel C, Ruckriem S. Speed‐dependent treadmill training in ambulatory hemiparetic stroke patients: a randomized controlled trial. Stroke 2002;33(2):553‐8. [DOI] [PubMed] [Google Scholar]
- Potempa K, Lopez M, Braun LT, Szidon JP, Fogg L, Tincknell T. Physiological outcomes of aerobic exercise training in hemiparetic stroke patients. Stroke 1995;26(1):101‐5. [DOI] [PubMed] [Google Scholar]
- Malouin F, Richards CL, Wood‐Dauphinee S, Williams JI. A randomized controlled trial comparing early and intensive task‐specific therapy to conventional therapy in acute stroke patients. Canadian Journal of Rehabilitation 1993;7(1):27‐8. [Google Scholar]; Richards CL, Malouin F, Wood‐Dauphinee S, Williams JI, Bouchard JP, Brunet D. Task‐specific physical therapy for optimization of gait recovery in acute stroke patients. Archives of Physical Medicine and Rehabilitation 1993;74(6):612‐20. [DOI] [PubMed] [Google Scholar]
- Richards CL, Malouin F, Bravo G, Dumas F, Wood‐Dauphinee S. The role of technology in task‐oriented training in persons with subacute stroke: a randomized controlled trial. Neurorehabilitation and Neural Repair 2004;18(4):199‐211. [DOI] [PubMed] [Google Scholar]
- Salbach NM, Mayo NE, Robichaud‐Ekstrand S, Hanley JA, Richards CL, Wood‐Dauphinee S. The effect of a task‐oriented walking intervention on improving balance self‐efficacy poststroke: a randomized, controlled trial. Journal of the American Geriatrics Society 2005;53(4):576‐82. [DOI] [PubMed] [Google Scholar]; Salbach NM, Mayo NE, Wood‐Dauphinee S, Hanley JA, Richards CL, Côté R. A task‐orientated intervention enhances walking distance and speed in the first year post stroke: a randomized controlled trial. Clinical Rehabilitation 2004;18(5):509‐19. [DOI] [PubMed] [Google Scholar]
- Shin WS, Lee SW, Lee YW, Choi SB, Song CH. Effects of combined exercise training on balance of hemiplegic stroke patients. Journal of Physical Therapy Science 2011;23(4):639‐43. [Google Scholar]
- Sims J. Regenerate: a strength training program to enhance the physical and mental health of chronic post stroke patients with depression. Australian New Zealand Clinical Trials Registry (ANZCTR) http://www.anzctr.org.au/. 2005. [DOI] [PubMed]; Sims J, Galea M, Taylor N, Dodd K, Jespersen S, Joubert L, et al. Regenerate: assessing the feasibility of a strength‐training program to enhance the physical and mental health of chronic post stroke patients with depression. International Journal of Geriatric Psychiatry 2009;24(1):76‐83. [DOI] [PubMed] [Google Scholar]
- Smith PS, Thompson M. Treadmill training post stroke: are there any secondary benefits? A pilot study. Clinical Rehabilitation 2008;22(10‐11):997‐1002. [DOI] [PubMed] [Google Scholar]
- Son SM, Park MK, Lee NK. Influence of resistance exercise training to strengthen muscles across multiple joints of the lower limbs on dynamic balance functions of stroke patients. Journal of Physical Therapy Science 2014;26(8):1267‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami A, Wakayama S. Effects of partial body weight support while training acute stroke patients to walk backwards on a treadmill: a controlled clinical trial using randomized allocation. Journal of Physical Therapy Science 2010;22(2):177‐87. [Google Scholar]
- Teixeira L, Nadeau S, Olney S, McBride I, Culham E, Zee B. The impact of a muscle strengthening and physical conditioning program on gait and stair climbing performance in chronic stroke subjects. Gait and Posture 1998;7(2):144‐5. [Google Scholar]; Teixeira‐Salmela LF, Nadeau S, McBride I, Olney SJ. Effects of muscle strengthening and physical conditioning training on temporal, kinematic and kinetic variables during gait in chronic stroke survivors. Journal of Rehabilitation Medicine 2001;33(2):53‐60. [DOI] [PubMed] [Google Scholar]; Teixeira‐Salmela LF, Olney SJ, Nadeau S, Brouwer B. Muscle strengthening and physical conditioning to reduce impairment and disability in chronic stroke survivors. Archives of Physical Medicine and Rehabilitation 1999;80(10):1211‐8. [DOI] [PubMed] [Google Scholar]
- Toledano‐Zarhi A, Tanne D, Carmeli E, Katz‐Leurer M. Feasibility, safety and efficacy of an early aerobic rehabilitation program for patients after minor ischemic stroke: a pilot randomized controlled trial. Neurorehabilitation 2011;28(2):85‐90. [DOI] [PubMed] [Google Scholar]
- Dean C. Group task‐specific circuit training for patients discharged home after stroke may be as effective as individualised physiotherapy in improving mobility. Journal of Physiotherapy 2012;58(4):269. [DOI] [PubMed] [Google Scholar]; Port IGL, Wevers LEG, Lindeman E, Kwakkel G. Effects of circuit training as alternative to usual physiotherapy after stroke: randomised controlled trial. BMJ 2012;344:e2672. [DOI] [PMC free article] [PubMed] [Google Scholar]; Port IGL, Wevers L, Roelse H, Kats L, Lindeman E, Kwakkel G. Cost‐effectiveness of a structured progressive task‐oriented circuit class training programme to enhance walking competency after stroke: the protocol of the FIT‐Stroke trial. BMC Neurology 2009;9:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheyden G, Vereeck L, Truijen S, Troch M, Lafosse C, Saeys W, et al. Additional exercises improve trunk performance after stroke: a pilot randomized controlled trial. Neurorehabilitation and Neural Repair 2009;23(3):281‐6. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang L, Fan H, Lu X, Wang T. Effect of low‐intensity ergometer aerobic training on glucose tolerance in severely impaired nondiabetic stroke patients. Journal of Stroke and Cerebrovascular Diseases 2014;23(3):e187‐93. [DOI] [PubMed] [Google Scholar]
- Rose DK, Winstein CJ, Tan SM, Azen SP, Chui HC. Comparison of upper extremity intervention strategies at six and nine months post‐stroke. Neurology Report 2001;25(4):130. [Google Scholar]; Rose DK, Winstein CJ, Yang AN, Weiss WB, Tan SM, Azen SP, et al. Relationship between upper extremity function and impairment in individuals with unilateral stroke. Neurology Report 1999;23(5):186. [Google Scholar]; Winstein CJ, Rose DK, Chui HC, Yang AN, Weiss WB, Tan SM, et al. Recovery and rehabilitation of arm use after stroke. Journal of Stroke and Cerebrovascular Diseases 2001;10(4):197. [Google Scholar]; Winstein CJ, Rose DK, Tan SM, Lewthwaite R, Chui HC, Azen SP. A randomized controlled comparison of upper‐extremity rehabilitation strategies in acute stroke: a pilot study of immediate and long‐term outcomes. Archives of Physical Medicine and Rehabilitation 2004;85(4):620‐8. [DOI] [PubMed] [Google Scholar]
- Yang YR, Wang RY, Lin KH, Chu MY, Chan RC. Task‐oriented progressive resistance strength training improves muscle strength and functional performance in individuals with stroke. Clinical Rehabilitation 2006;20:860‐70. [DOI] [PubMed] [Google Scholar]
- Yang HC, Lee CL, Lin R, Hsu MJ, Chen CH, Lin JH, et al. Effect of biofeedback cycling training on functional recovery and walking ability of lower extremity in patients with stroke. Kaohsiung Journal of Medical Sciences 2014;30(1):35‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zedlitz AMEE, Rietveld TCM, Geurts AC, Fasotti L. Cognitive and graded activity training can alleviate persistent fatigue after stroke: a randomized, controlled trial. Stroke 2012;43(4):1046‐51. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Ada L, Dean CM, Hall JM, Bampton J, Crompton S. A treadmill and overground walking program improves walking in individuals residing in the community after stroke: a placebo‐controlled randomised trial. Internal Medicine Journal 2004;34(1‐2):A7. [DOI] [PubMed] [Google Scholar]; Ada L, Dean CM, Hall JM, Bampton J, Crompton S. A treadmill and overground walking program improves walking in persons residing in the community after stroke: a placebo‐controlled, randomized trial. Archives of Physical Medicine and Rehabilitation 2003;84(10):1486‐91. [DOI] [PubMed] [Google Scholar]
- Ada L, Dean CM, Morris ME, Simpson JM, Katrak P. Randomized trial of treadmill walking with body weight support to establish walking in subacute stroke. The MOBILISE Trial. Stroke 2010;41:1237‐42. [DOI] [PubMed] [Google Scholar]
- Adie K, Schofield C, Berrow M, Pritchard C, Freeman J, Humfryes J, et al. Does the use of Nintendo Wii Sports improve arm function and is it acceptable to patients after stroke? Publication of the protocol of the Trial of Wii in Stroke ‐ TWIST. International Journal of General Medicine 2014;7:475‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aidar F, Garrido N, Silva A, Reis V, Marinho D, Oliveira RJ. Effects of aquatic exercise on depression and anxiety in ischemic stroke subjects. Health 2013;5(2):222‐8. [Google Scholar]
- Akbari A, Karimi H. The effect of strengthening exercises on exaggerated muscle tonicity in chronic hemiparesis following stroke. Journal of Medical Sciences 2006;6(3):382‐8. [Google Scholar]
- Au‐Yeung SSY, Hui‐Chan CWY, Tang JCS. Short‐form Tai Chi improves standing balance of people with chronic stroke. Neurorehabilitation and Neural Repair 2009;23(5):515‐22. [DOI] [PubMed] [Google Scholar]
- Baek IH, Lee T, Song M, Goo BO. Effect of circuit class training for eight weeks on changes in ratios of F‐Trp/BCAAs and depression in people with poststroke depression. Journal of Physical Therapy Science 2014;26(2):243‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreca SR, Masters L, Sigouin CS. Sit‐to‐stand training improves standing performance and quality of life in residents living in long‐term care homes following a stroke: a cluster randomized controlled trial. Stroke 2007;38(2):474. [Google Scholar]
- Baskett JJ, Broad JB, Reekie G, Hocking C, Green G. Shared responsibility for ongoing rehabilitation: a new approach to home‐based therapy after stroke. Clinical Rehabilitation 1999;13(1):23‐33. [DOI] [PubMed] [Google Scholar]
- Batchelor FA, Hill KD, Mackintosh SF, Said CM, Whitehead CH. The FLASSH study: protocol for a randomised controlled trial evaluating falls prevention after stroke and two sub‐studies. BMC Neurology 2009;9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor FA, Hill KD, MacKintosh SF, Said CM, Whitehead CH. Effects of a multifactorial falls prevention program for people with stroke returning home after rehabilitation: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2012;93(9):1648‐55. [DOI] [PubMed] [Google Scholar]
- Benvenuti F, Stuart M, Cappena V, Gabella S, Corsi S, Taviani A, et al. Community‐based exercise for upper limb paresis: a controlled trial with telerehabilitation. Neurorehabilitation & Neural Repair 2014;28(7):611‐20. [DOI] [PubMed] [Google Scholar]
- Bernhardt J, Churilov L, Dewey H, Lindley RI, Moodie M, Collier J, et al. Statistical analysis plan (SAP) for A Very Early Rehabilitation Trial (AVERT): an international trial to determine the efficacy and safety of commencing out of bed standing and walking training (very early mobilization) within 24 h of stroke onset vs. usual stroke unit care. International Journal of Stroke 2015;10(1):23‐4. [DOI] [PubMed] [Google Scholar]
- Blennerhassett J, Dite W. Additional task‐related practice improves mobility and upper limb function early after stroke: a randomised controlled trial. Australian Journal of Physiotherapy 2004;50(4):219‐24. [DOI] [PubMed] [Google Scholar]
- Boss HM, Schaik SM, Deijle IA, Melker EC, Berg BTJ, Scherder EJA, et al. Safety and feasibility of post‐stroke care and exercise after minor ischemic stroke or transient ischemic attack: MotiveS & MoveIT. NeuroRehabilitation 2014;34(3):401‐7. [DOI] [PubMed] [Google Scholar]
- Bourbonnais D, Bilodeau S, Lepage Y, Beaudoin N, Gravel D, Forget R. Effect of force‐feedback treatments in patients with chronic motor deficits after a stroke. American Journal of Physical Medicine and Rehabilitation 2002;81(12):890‐7. [DOI] [PubMed] [Google Scholar]
- Boysen G, Krarup LH, Zeng X, Oskedra A, Korv J, Andersen G, et al. ExStroke Pilot Trial of the effect of repeated instructions to improve physical activity after ischaemic stroke: a multinational randomised controlled clinical trial. BMJ 2009;339:b2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Burgar CG. Graded weight‐bearing exercise for improved ambulation after stroke. http://guide.stanford.edu/projects/2kprojects/stroke06.html2002.
- Butefisch C, Hummelsheim H, Denzler P, Mauritz KH. Repetitive training of isolated movements improves the outcome of motor rehabilitation of the centrally paretic hand. Journal of the Neurological Sciences 1995;130(1):59‐68. [DOI] [PubMed] [Google Scholar]
- Carr M, Jones J. Physiological effects of exercise on stroke survivors. Topics in Stroke Rehabilitation 2003;9(4):57‐64. [DOI] [PubMed] [Google Scholar]
- Chanruengvanich W, Kasemkitwattana S, Charoenyooth C, Towanabut S, Pongurgsorn C. RCT: self‐regulated exercise program in transient ischemic attack and minor stroke patients. Thai Journal of Nursing Research 2006;10(3):165‐79. [Google Scholar]
- Choi HJ, Kang HJ, Kang SJ, Kim MW, Kang DH, Im JH, et al. The effect of wheelchair exercise on physical function and health status by duration of stroke patients. Medicine and Science in Sports and Exercise 2010;42(5):735. [Google Scholar]; Choi HJ, Kim YS, Park DS, Kang HJ. Effects of wheelchair‐based rehabilitation on the physical functions and health perception of stroke patients. Personal and Ubiquitous Computing 2013;17(7):1365‐72. [DOI: 10.1007/s00779-012-0571-9] [DOI] [Google Scholar]
- Chu KS, Eng JJ, Dawson AS, Harris JE, Ozkaplan A, Gylfadottir S. Water‐based exercise for cardiovascular fitness in people with chronic stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2004;85(6):870‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumbler NR, Rose DK, Griffiths P, Quigley P, Gee‐Hernandez N, Carlson KA, et al. Study protocol: home‐based telehealth stroke care: a randomized trial for veterans. Trials 2010;11(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumbler NR, Quigley P, Li X, Morey M, Rose D, Sanford J, et al. Effects of telerehabilitation on physical function and disability for stroke patients: a randomized, controlled trial. Stroke 2012;43(8):2168‐74. [DOI] [PubMed] [Google Scholar]
- Chung EJ, Kim JH, Lee BH. The effects of core stabilization exercise on dynamic balance and gait function in stroke patients. Journal of Physical Therapy Science 2013;25(7):803‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsilles‐Sy C. Effects of Bilateral Task‐Oriented Training on Arm Function After Stroke [Dissertation]. Seattle: University of Washington, 2013. [Google Scholar]
- Corti M, McGuirk TE, Wu SS, Patten C. Differential effects of power training versus functional task practice on compensation and restoration of arm function after stroke. Neurorehabilitation and Neural Repair 2012;26(7):842‐54. [DOI] [PubMed] [Google Scholar]
- Silva PB, Antunes FN, Graef P, Cechetti F, Pagnussat Ade S. Strength training associated with task‐oriented training to enhance upper‐limb motor function in elderly patients with mild impairment after stroke. American Journal of Physical Medicine & Rehabilitation 2015;94(1):11‐9. [DOI] [PubMed] [Google Scholar]
- Davis GMF, Kelly JO, Kilbreath SL, Zeman BD. Home‐based exercise training improves walking endurance in chronic stroke patients: a pilot study. Medicine and Science in Sports and Exercise 2003;35(5):S232. [Google Scholar]
- Davis GM, Lee M‐J, Kilbreath SL, Fiatarone Singh SM, Zeman B. Effect of aerobic or resistance training on cardiorespiratory fitness after chronic stroke. Neurorehabilitation and Neural Repair 2006;20:188. [Google Scholar]; Davis GM, Lee M‐J, Kilbreath SL, Fiatarone Singh SM, Zeman B. Specificity of training improves aerobic fitness in chronic stroke patients: a randomised controlled trial. Medicine and Science in Sports and Exercise 2004;36(5):S250. [Google Scholar]; Lee M‐J, Kilbreath SL, Davis GM, Fiatarone Singh SM, Zeman B. Progressive resistance training improves leg muscle strength and stair climbing power in persons following stroke. Medicine and Science in Sports and Exercise 2004;36(5):S250. [Google Scholar]; Lee MJ, Kilbreath SL, Fiatarone SM, Zeman B, Lord SR. Comparison of effect of aerobic cycle training and progressive resistance training on walking ability after stroke: a randomized sham exercise‐controlled study. Journal of the American Geriatrics Society 2008;56:976‐85. [DOI] [PubMed] [Google Scholar]
- Dean CM, Shepherd RB. Task‐related training improves performance of seated reaching tasks after stroke. A randomized controlled trial. Stroke 1997;28(4):722‐8. [DOI] [PubMed] [Google Scholar]
- Dean CM, Richards CL, Malouin F. Task‐related circuit training improves performance of locomotor tasks in chronic stroke: a randomized, controlled pilot trial. Archives of Physical Medicine and Rehabilitation 2000;81(4):409‐17. [DOI] [PubMed] [Google Scholar]; Richards CL, Malouin F, Dean C. Maximizing locomotor recovery after stroke. Archives of Physiology and Biochemistry 2000;108(1‐2):1. 11077567 [Google Scholar]
- Dean CM, Rissel C, Sherrington C, Sharkey M, Cumming R, Lord SR, et al. Weight‐bearing exercise improves mobility in stroke survivors and may prevent falls in faster walkers. Neurorehabilitation and Neural Repair 2012;26(6):730‐1. [DOI] [PubMed] [Google Scholar]; Dean CM, Rissel C, Sherrington C, Sharkey M, Cumming RG, Lord SR, et al. Exercise to enhance mobility and prevent falls after stroke: the community stroke club randomized trial. Neurorehabilitation and Neural Repair 2012;26(9):1046‐57. [DOI] [PubMed] [Google Scholar]
- Deniz L, Armagan O, Ozgen M, Oner S. Effectiveness of gait training with partial body‐weight support in subacute stroke patients. Turk Serebrovaskuler Hastaliklar Dergisi 2011;17(1):13‐9. [Google Scholar]
- Desrosiers J, Bourbonnais D, Corriveau H, Gosselin S, Bravo G. Effectiveness of unilateral and symmetrical bilateral task training for arm during the subacute phase after stroke: a randomized controlled trial. Clinical Rehabilitation 2005;19(6):581‐93. [DOI] [PubMed] [Google Scholar]
- Dias D, Lains J, Pereira A, Nunes R, Caldas J, Amaral C. Can we improve gait skills in chronic hemiplegics? A randomised control trial with gait trainer. Europa Medicophysica 2007;43:499‐504. [PubMed] [Google Scholar]; Dias D, Lains J, Pereira A, Nunes R, Caldas J, Amaral C. Partial body weight support in chronic hemiplegics: a randomized control trial. Personal communication2007. [PubMed]
- Dickstein R, Hocherman S, Pillar T, Shaham R. Stroke rehabilitation. Three exercise therapy approaches. Physical Therapy 1986;66(8):1233‐8. [DOI] [PubMed] [Google Scholar]
- Dickstein R, Heffes Y, Laufer Y, Abulaffio N, Shabtai EL. Repetitive practice of a single joint movement for enhancing elbow function in hemiparetic patients. Perceptual and Motor Skills 1997;85(3 Pt 1):771‐85. [DOI] [PubMed] [Google Scholar]
- Lauro A, Pellegrino L, Savastano G, Ferraro C, Fusco M, Balzarano F, et al. A randomized trial on the efficacy of intensive rehabilitation in the acute phase of ischemic stroke. Journal of Neurology 2003;250:1206‐8. [DOI] [PubMed] [Google Scholar]
- Dobkin BH, Plummer‐D'Amato P, Elashoff R, Lee J. International randomized clinical trial, stroke inpatient rehabilitation with reinforcement of walking speed (SIRROWS), improves outcomes. Neurorehabilitation and Neural Repair 2010;24(3):235‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong KS, Oh SK, Jee HK, Dong YL. The effect of trunk stabilization exercise on the thickness of the deep abdominal muscles and balance in patients with chronic stroke. Journal of Physical Therapy Science 2012;24(2):181‐5. [Google Scholar]
- Dromerick AW, Lang CE, Birkenmeier R, Hahn MG, Sahrmann SA, Edwards DF. Relationships between upper‐limb functional limitation and self‐reported disability 3 months after stroke. Journal of Rehabilitation Research and Development 2006;43(3):401‐8. [DOI] [PubMed] [Google Scholar]; Edwards DF, Lang CE, Birkenmeier R, Powers WJ, Dromerick AW. VECTORS (Very Early Constraint Therapy for Recovery from Stroke) Phase II RCT: results of secondary analyses. Stroke 2008;39(2):563. [Google Scholar]
- Drummond A, Walker M. Generalisation of the effects of leisure rehabilitation for stroke patients. British Journal of Occupational Therapy 1996;59(7):330‐4. [Google Scholar]; Drummond A, Walker MF. A randomized controlled trial of leisure rehabilitation after stroke. Clinical Rehabilitation 1995;9(4):283‐90. [Google Scholar]
- Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, et al. Body‐weight‐supported treadmill rehabilitation after stroke. New England Journal of Medicine 2011;364(21):2026‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Senousey MYA, Aboelsafa AA, El‐Heneedy YA, Khalil MK, Kamel LI, Abd‐El‐Aziz AL. Effect of comprehensive training on ischemic stroke patients. Egyptian Journal of Neurology, Psychiatry and Neurosurgery 2012;49(3):259‐69. [Google Scholar]
- El‐Tamawy MS, Abd‐Allah F, Ahmed SM, Darwish MH, Khalifa HA. Aerobic exercises enhance cognitive functions and brain derived neurotrophic factor in ischemic stroke patients. NeuroRehabilitation 2014;34(1):209‐13. [DOI] [PubMed] [Google Scholar]
- Faulkner J, Lambrick D, Woolley B, Stoner L, Wong L‐K, McGonigal G. Health‐enhancing physical activity programme (HEPAP) for transient ischaemic attack and non‐disabling stroke: recruitment and compliance. New Zealand Medical Journal 2012;125(1364):86‐76. [PubMed] [Google Scholar]; Faulkner J, Lambrick D, Woolley B, Stoner L, Wong LK, McGonigal G. Effects of early exercise engagement on vascular risk in patients with transient ischemic attack and nondisabling stroke. Journal of Stroke and Cerebrovascular Diseases 2013;22(8):E388‐96. [DOI] [PubMed] [Google Scholar]; Faulkner J, Woolley B, Wong L‐K, McGonigal G. A Health Enhancing Physical Activity Programme (HEPAP) on transient ischaemic attack (TIA) and non‐disabling stroke: feasibility of participant recruitment. International Journal of Stroke 2011;6:8‐9. [PubMed] [Google Scholar]
- Faulkner J, McGonigal G, Woolley B, Stoner L, Wong L, Lambrick D. The effect of a short‐term exercise programme on haemodynamic adaptability: a randomised controlled trial with newly diagnosed transient ischaemic attack patients. Journal of Human Hypertension 2013;27(12):736‐43. [DOI] [PubMed] [Google Scholar]
- Feys HM, Weerdt WJ, Selz BE, Cox Steck GA, Spichiger R, Vereeck LE, et al. Effect of a therapeutic intervention for the hemiplegic upper limb in the acute phase after stroke: a single‐blind, randomized, controlled multicenter trial. Stroke 1998;29(4):785‐92. [DOI] [PubMed] [Google Scholar]
- Fletcher BJ, Dunbar SB, Felner JM, Jensen BE, Almon L, Cotsonis G, et al. Exercise testing and training in physically disabled men with clinical evidence of coronary artery disease. American Journal of Cardiology 1994;73(2):170‐4. [DOI] [PubMed] [Google Scholar]
- Foley SM, O'Sullivan PS, Means KM. Postexercise outcome: does exercise affect functional obstacle course performance in stroke patients?. American Journal of Physical Medicine and Rehabilitation 2004;83:249. [Google Scholar]
- Franceschini M, Carda S, Agosti M, Antenucci R, Malgrati D, Cisari C. Walking after stroke: what does treadmill training with body weight support add to overground gait training in patients early after stroke?: a single‐blind, randomized, controlled trial. Stroke 2009;40(9):3079‐85. [DOI] [PubMed] [Google Scholar]
- Friedman N, Chan V, Reinkensmeyer AN, Beroukhim A, Zambrano GJ, Bachman M, et al. Retraining and assessing hand movement after stroke using the MusicGlove: comparison with conventional hand therapy and isometric grip training. Journal of Neuroengineering and Rehabilitation 2014;11:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelber DA, Josefczyk PB, Herrman D, Good DC, Verhulst SJ. Comparison of two therapy approaches in the rehabilitation of the pure motor hemiparetic stroke patient. Journal of Neurologic Rehabilitation 1995;9(4):191‐6. [Google Scholar]
- Gilbertson L, Langhorne P, Walker A, Allen A. A randomised controlled trial of home‐based occupational therapy for stroke patients: results at 7 weeks. Cerebrovascular Diseases 1998;8 Suppl 4:84. [Google Scholar]
- Gregson J. Exercise training early after stroke: a feasibility study. Personal communication2006.
- Harrington R, Taylor G, Hollinghurst S, Reed M, Kay H, Wood VA. A community‐based exercise and education scheme for stroke survivors: a randomized controlled trial and economic evaluation. Clinical Rehabilitation 2010;24(1):3‐15. [DOI] [PubMed] [Google Scholar]
- Harris JE, Eng JJ, Miller WC, Dawson AS. A self‐administered graded repetitive arm supplementary program (GRASP) improves arm function during inpatient stroke rehabilitation: a multi‐site randomized controlled trial. Stroke 2009;40(6):2123‐8. [DOI] [PubMed] [Google Scholar]
- Hart J, Kanner H, Gilboa‐Mayo R, Haroeh‐Peer O, Rozenthul‐Sorokin N, Eldar R. Tai Chi Chuan practice in community‐dwelling persons after stroke. International Journal of Rehabilitation Research 2004;27(4):303‐4. [DOI] [PubMed] [Google Scholar]
- Helbostad JL, Sletvold O, Moe‐Nilssen R. Effects of home exercises and group training on functional abilities in home‐dwelling older persons with mobility and balance problems. A randomized study. Aging‐Clinical and Experimental Research 2004;16(2):113‐21. [DOI] [PubMed] [Google Scholar]; Helbostad JL, Sletvold O, Moe‐Nilssen R. Home training with and without additional group training in physically frail old people living at home: effect on health‐related quality of life and ambulation. Clinical Rehabilitation 2004;18(5):498‐508. [DOI] [PubMed] [Google Scholar]
- Hidler JM. Walking therapy in hemiparetic stroke patients using robotic‐assisted treadmill training. ClinicalTrials.gov2007.
- Higgins J, Salbach NM, Wood‐Dauphinee S, Richards CL, Cote R, Mayo NE. The effect of a task‐oriented intervention on arm function in people with stroke: a randomized controlled trial. Clinical Rehabilitation 2006;20(4):296‐310. [DOI] [PubMed] [Google Scholar]
- Holmgren E, Gosman‐Hedstrom G, Lindstrom B, Wester P. What is the benefit of a high‐intensive exercise program on health‐related quality of life and depression after stroke? A randomized controlled trial. Advances in Physiotherapy 2010;12(3):125‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]; Holmgren ES, Wester P, Lindstrom B, Nordin E. The impact of an high intensive exercise program on gait after stroke: a randomized controlled trial. Cerebrovascular Diseases 2012;33:468‐9. [Google Scholar]
- Howe TE, Taylor I, Finn P, Jones H. Lateral weight transference exercises following acute stroke: a preliminary study of clinical effectiveness. Clinical Rehabilitation 2005;19(1):45‐53. [DOI] [PubMed] [Google Scholar]
- Hu Z, Hu Y, Lu Q. Impact of early rehabilitation therapy on post stroke depression. Chinese Journal of Clinical Rehabilitation 2003;7(5):849. [Google Scholar]
- Hu Y‐S. Effects study of standardized tertiary rehabilitation on promoting of the neurological functions in stroke patients with hemiplegia. Chinese Medical Journal 2006;86(37):2621‐6. [PubMed] [Google Scholar]
- Ishida A, Tanaka H, Toyokura M, Izumi S. Early rehabilitative intervention for stroke ‐ prospective study. 1st World Congress of the International Society of Physical Rehabilitation Medicine (ISPRM I) 7‐13 July 2001. The Netherlands, Amsterdam: International Society of Physical Rehabilitation Medicine (ISPRM I), 2001:500‐4. [Google Scholar]
- Jeong S, Kim MT. Effects of a theory‐driven music and movement program for stroke survivors in a community setting. Applied Nursing Research 2007;20(3):125‐31. [DOI] [PubMed] [Google Scholar]
- Jongbloed L, Stacey S, Brighton C. Stroke rehabilitation: sensorimotor integrative treatment versus functional treatment. American Journal of Occupational Therapy 1989;43(6):391‐7. [DOI] [PubMed] [Google Scholar]
- Jongbloed L, Morgan D. An investigation of involvement in leisure activities after a stroke. American Journal of Occupational Therapy 1991;45(5):420‐7. [DOI] [PubMed] [Google Scholar]
- Kamps A, Schule K. Cyclic movement training of the lower limb in stroke rehabilitation. Neurologie und Rehabilitation 2005;11(5):259‐69. [Google Scholar]
- Kim B, Lee S, Bae Y, Yu J, Kim T. The effect of a task‐oriented training on trunk control ability, balance and gait of stroke patients. Journal of Physical Therapy Science 2012;24(6):519‐22. [Google Scholar]
- Kirk H, Kersten P, Crawford P, Keens A, Ashburn A, Conway J. The cardiac model of rehabilitation for reducing cardiovascular risk factors post transient ischaemic attack and stroke: a randomized controlled trial. Clinical Rehabilitation 2014;28(4):339‐49. [DOI] [PubMed] [Google Scholar]
- Klassen T, Mulroy SJ, Sullivan KJ. Gait parameters associated with responsiveness to a task‐specific and/or strength training program post‐stroke. Journal of Neurologic Physical Therapy 2005;29(4):198. [Google Scholar]
- Kono Y, Yamada T, Yamaguchi J, Hagiwara Y, Iritani N, Ishida S, et al. Secondary prevention of new vascular events with lifestyle intervention in patients with noncardioembolic mild ischemic stroke: a single‐center randomized controlled trial. Cerebrovascular Diseases 2013;36(2):88‐97. [DOI] [PubMed] [Google Scholar]
- Kumaran PS, Vanan T. Effect of early mobilisation training on gross motor function and functional outcome in hemi paretic stroke patients. International Journal of Pharmacy and Technology 2013;5(3):5637‐50. [Google Scholar]
- Kwakkel G, Wagenaar RC, Twisk JW, Lankhorst GJ, Koetsier JC. Intensity of leg and arm training after primary middle‐cerebral‐artery stroke: a randomised trial. Lancet 1999;354:191‐6. [DOI] [PubMed] [Google Scholar]
- Kwon OS, Lee SW. Effect of continuing repeated passive and active exercises on knee's position senses in patients with hemiplegia. NeuroRehabilitation 2013;33(3):391‐7. [DOI] [PubMed] [Google Scholar]
- Langhammer B, Stanghelle JK, Lindmark B. Exercise and health‐related quality of life during the first year following acute stroke. A randomized controlled trial. Brain Injury 2008;22(2):135‐45. [DOI] [PubMed] [Google Scholar]
- Langhammer B, Stanghelle JK, Lindmark B. An evaluation of two different exercise regimes during the first year following stroke: a randomised controlled trial. Physiotherapy Theory and Practice 2009;25(2):55‐68. [DOI] [PubMed] [Google Scholar]
- Langhammer B, Stanghelle JK. Exercise on a treadmill or walking outdoors? A randomized controlled trial comparing effectiveness of two walking exercise programmes late after stroke. Clinical Rehabilitation 2010;24(1):46‐54. [DOI] [PubMed] [Google Scholar]
- Langhammer B, Lindmark B, Stanghelle J. Are effects of a 1‐year long‐term intervention period in persons with stroke sustained 3 years after? The longitudinal follow‐up of a randomized controlled trial. Brain Injury 2014;28(5‐6):556. [DOI] [PubMed] [Google Scholar]
- Langhammer B, Lindmark B, Stanghelle J. Physiotherapy and physical functioning post‐stroke: exercise habits and functioning 4 years later? Long‐term follow‐up after a 1‐year long‐term intervention period: a randomized controlled trial. Brain Injury 2014;28(11):1396‐405. [DOI] [PubMed] [Google Scholar]
- Laufer Y, Dickstein R, Chefez Y, Marcovitz E. The effect of treadmill training on the ambulation of stroke survivors in the early stages of rehabilitation: a randomized study. Journal of Rehabilitation Research and Development 2001;38(1):69‐78. [PubMed] [Google Scholar]
- Behrman A, Sullivan KJ, Wu SS, Nadeau SE, Dobkin BH, Azen SP, et al. Functional walking ability 2 to 6 months after stroke: effects of progressive task‐specific and impairment‐based exercise rehabilitation programs compared to usual care. Stroke 2011;42(11):E610. [Google Scholar]; Duncan PW. Locomotor Experience Applied Post‐Stroke (LEAPS). Stroke 2007;38:e120. [DOI] [PMC free article] [PubMed] [Google Scholar]; Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Dobkin BH, et al. Locomotor Experience Applied Post‐Stroke (LEAPS): a randomized controlled trial. International Journal of Stroke 2008;3 Suppl 1:132. [Google Scholar]; Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, et al. Locomotor Experience Applied Post‐Stroke (LEAPS): a randomized controlled trial. International Stroke Conference 2008. New Orleans, 2008. [Google Scholar]; Rose DK, Behrman AL, Cen CY, Sullivan KJ, Martin AD, Schofield RS, et al. Response to exercise tolerance testing in subacute stroke across severity levels. International Journal of Stroke 2008;3 Suppl 1:329. [Google Scholar]; Rose DK, Behrman AL, Cen Y, Sullivan KJ, Martin D, Schofield RS. Response to exercise tolerance testing in subacute stroke across severity levels. Stroke 2008;39(2):618‐9. [Google Scholar]; Tilson JK, Sullivan KJ, Cen SY, Rose DK, Behrman AL, Wu SS, et al. Determination of the minimal clinically important difference in the lower extremity Fugl‐Meyer Motor Score in the first 60 days post‐stroke. Stroke 2008;39(2):693. [Google Scholar]
- Lee M, Kilbreath SL, Singh MF, Zeman B, Lord SR, Raymond J, et al. Comparison of effect of aerobic cycle training and progressive resistance training on walking ability after stroke: a randomized sham exercise‐controlled study. Journal of the American Geriatrics Society 2008;56(6):976‐85. [DOI] [PubMed] [Google Scholar]; Lee MJ, Kilbreath SL, Singh MF, Zeman B, Davis GM. Effect of progressive resistance training on muscle performance after chronic stroke. Medicine and Science in Sports and Exercise 2010;42(1):23‐34. [DOI] [PubMed] [Google Scholar]
- Lee SB, Kang KY. The effects of isokinetic eccentric resistance exercise for the hip joint on functional gait of stroke patients. Journal of Physical Therapy Science 2013;25(9):1177‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoncello R, Sohlberg MM, Fickas S, Albin Ri, Harn BE. Phase I evaluation of the television assisted prompting system to increase completion of home exercises among stroke survivors. Disability and Rehabilitation 2011;6(5):440‐52. [DOI] [PubMed] [Google Scholar]
- Lennon O, Blake C. Cardiac rehabilitation adapted to transient ischaemic attack and stroke (CRAFTS): a randomised controlled trial. BMC Neurology 2009;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveille SG, Wagner EH, Davis C, Grothaus L, Wallace J, LoGerfo M, et al. Preventing disability and managing chronic illness in frail older adults: a randomized trial of a community‐based partnership with primary care. Journal of the American Geriatrics Society 1998;46(10):1191‐8. [DOI] [PubMed] [Google Scholar]
- Lin J‐H, Hsieh C‐L, Lo SK, Chai H‐M, Liao L‐R. Preliminary study of the effect of low‐intensity home‐based physical therapy in chronic stroke patients. Kaohsiung Journal of Medical Sciences 2004;20(1):18‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln NB, Parry RH, Vass CD. Randomized, controlled trial to evaluate increased intensity of physiotherapy treatment of arm function after stroke. Stroke 1999;30(3):573‐9. [DOI] [PubMed] [Google Scholar]
- Lincoln N. Comparison of Bobath‐based and movement science based treatment for stroke: a randomised controlled trial. Personal communication2003. [DOI] [PMC free article] [PubMed]
- Lindsley HG, Musser L, Stewart MR, Burton L, Forgione KO, Martin M, et al. The effects of Kinetron training on gait patterns of patients with strokes. Tar Heel Journal 1994;91:8. [Google Scholar]
- Liston R, Mickelborough J, Harris B, Hann A, Tallis R. Conventional physiotherapy and treadmill re‐training for higher‐level gait disorders in cerebrovascular disease. Age and Ageing 2000;29(4):311‐8. [DOI] [PubMed] [Google Scholar]
- Liu‐Ambrose T, Eng JJ. Exercise training and recreational activities to promote executive functions in chronic stroke: a proof‐of‐concept study. Journal of Stroke and Cerebrovascular Diseases 2015;24(1):130‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan PA, Gladman JR, Drummond AE, Radford KA, TOTAL Study Group. A study of interventions and related outcomes in a randomized controlled trial of occupational therapy and leisure therapy for community stroke patients. Clinical Rehabilitation 2003;17(3):249‐55. [DOI] [PubMed] [Google Scholar]
- Logigian MK, Samuels MA, Falconer J, Zagar R. Clinical exercise trial for stroke patients. Archives of Physical Medicine and Rehabilitation 1983;64(8):364‐7. [PubMed] [Google Scholar]
- Lord S, McPherson KM, McNaughton HK, Rochester L, Weatherall M. How feasible is the attainment of community ambulation after stroke? A pilot randomized controlled trial to evaluate community‐based physiotherapy in subacute stroke. Clinical Rehabilitation 2008;22(3):215‐25. [DOI] [PubMed] [Google Scholar]
- Luft AR, McCombe‐Waller S, Whitall J, Forrester LW, Macko R, Sorkin JD, et al. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA 2004;292(15):1853‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]; Whitall J. Upper extremity training for chronic stroke. CRISP Database2002.
- Luft AR, Macko RF, Forrester LW, Villagra F, Ivey F, Sorkin JD, et al. Treadmill exercise activates subcortical neural networks and improves walking after stroke: a randomized controlled trial. Stroke 2008;39(12):3341‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay‐Lyons M, Gubitz G, Giacomantonio N, Wightman H, Marsters D, Thompson K, et al. Program of rehabilitative exercise and education to avert vascular events after non‐disabling stroke or transient ischemic attack (PREVENT Trial): a multi‐centred, randomised controlled trial. BMC Neurology 2010;10(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macko RF. Effects of exercise on patients with hemiparetic stroke. ClinicalTrials.gov2001. ; Macko RF, Ivey FM, Forrester LW, Hanley D, Sorkin JD, Katzel LI, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: a randomized, controlled trial. Stroke 2005;36(10):2206‐11. [DOI] [PubMed] [Google Scholar]
- Maeshima S, Ueyoshi A, Osawa A, Ishida K, Kunimoto K, Shimamoto Y, et al. Mobility and muscle strength contralateral to hemiplegia from stroke: benefit from self‐training with family support. American Journal of Physical Medicine and Rehabilitation 2003;82(6):456‐62. [PubMed] [Google Scholar]
- Marigold DS, Eng JJ, Dawson AS, Inglis JT, Harris JE, Gylfadottir S. Exercise leads to faster postural reflexes, improved balance and mobility, and fewer falls in older persons with chronic stroke. Journal of the American Geriatrics Society 2005;53(3):416‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzolini S, Oh P, McIlroy W, Brooks D. The effects of an aerobic and resistance exercise training program on cognition following stroke. Neurorehabilitation and Neural Repair 2012;27(5):392‐402. [DOI] [PubMed] [Google Scholar]
- Mayr A, Kofler M, Quirbach E, Matzak H, Fröhlich K, Saltuari L. Prospective, blinded, randomized crossover study of gait rehabilitation in stroke patients using the Lokomat gait orthosis. Neurorehabilitation and Neural Repair 2007;21(4):307‐14. [DOI] [PubMed] [Google Scholar]
- McClellan R, Ada L. A six‐week, resource‐efficient mobility program after discharge from rehabilitation improves standing in people affected by stroke: placebo‐controlled, randomised trial. Australian Journal of Physiotherapy 2004;50(3):163‐7. [DOI] [PubMed] [Google Scholar]; McClellan R, Ada L. Efficacy of a resource‐efficient exercise program in reducing disability and handicap in stroke survivors: a randomised controlled clinical trial. Internal Medicine Journal 2004;34(1‐2):A11. [Google Scholar]
- Mehrholz J, Werner C, Hesse S, Pohl M. Immediate and long‐term functional impact of repetitive locomotor training as an adjunct to conventional physiotherapy for non‐ambulatory patients after stroke. Disability and Rehabilitation 2008;30(11):830‐6. [DOI] [PubMed] [Google Scholar]
- Michaelsen SM, Dannenbaum R, Levin MF. Task‐specific training with trunk restraint on arm recovery in stroke: randomized control trial. Stroke 2006;37(1):186‐92. [DOI] [PubMed] [Google Scholar]
- Miklitsch C, Krewer C, Freivogel S, Steube D. Effects of a predefined mini‐trampoline training programme on balance, mobility and activities of daily living after stroke: a randomized controlled pilot study. Clinical Rehabilitation 2013;27(10):939‐47. [DOI] [PubMed] [Google Scholar]
- Miller K, Galea M, Kilbreath S. Early task‐related upper limb training is effective following stroke. Stroke 2000;31(11):2816. [Google Scholar]
- Moreland JD, Goldsmith CH, Huijbregts MP, Anderson RE, Prentice DM, Brunton KB, et al. Progressive resistance strengthening exercises after stroke: a single‐blind randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2003;84(10):1433‐40. [DOI] [PubMed] [Google Scholar]
- Muhl L, Kulin J, Dagonnier M, Churilov L, Dewey H, Linden T, et al. Mobilization after thrombolysis (rtPA) within 24 hours of acute stroke: what factors influence inclusion of patients in A Very Early Rehabilitation Trial (AVERT)?. BMC Neurology 2014;14:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau SE, Wu SS, Dobkin BH, Azen SP, Rose DK, Tilson JK, et al. Effects of task‐specific and impairment‐based training compared with usual care on functional walking ability after inpatient stroke rehabilitation: LEAPS trial. Neurorehabilitation and Neural Repair 2013;27(4):370‐80. [DOI] [PubMed] [Google Scholar]
- Nelles G, Jentzen W, Jueptner M, Muller S, Diener HC. Arm training induced brain plasticity in stroke studied with serial positron emission tomography. NeuroImage 2001;13(6):1146‐54. [DOI] [PubMed] [Google Scholar]
- Nilsson L, Carlsson J, Danielsson A, Fugl‐Meyer A, Hellstrom K, Kristensen L, et al. Walking training of patients with hemiparesis at an early stage after stroke: a comparison of walking training on a treadmill with body weight support and walking training on the ground. Clinical Rehabilitation 2001;15(5):515‐27. [DOI] [PubMed] [Google Scholar]
- Noh DK, Lim JY, Shin HI, Paik NJ. The effect of aquatic therapy on postural balance and muscle strength in stroke survivors ‐ a randomized controlled pilot trial. Clinical Rehabilitation 2008;22(10‐11):966‐76. [DOI] [PubMed] [Google Scholar]
- Olney SJ, Nymark J, Brouwer B, Culham E, Day A, Heard J, et al. A randomized controlled trial of supervised versus unsupervised exercise programs for ambulatory stroke survivors. Stroke 2006;37(2):476‐81. [DOI] [PubMed] [Google Scholar]
- Outermans JC, Peppen RPS, Wittink H, Takken T, Kwakkel G. Effects of a high‐intensity task‐oriented training on gait performance early after stroke: a pilot study. Clinical Rehabilitation 2010;24(11):979‐87. [DOI] [PubMed] [Google Scholar]
- Pan C‐H, He J‐Q, Pu S‐X, Wan X‐L, Gao C. Effects of early rehabilitation therapy on the motor function of limbs and ability of daily living in patients with hemiplegia after stroke. Zhongguo Linchuang Kangfu 2004;8(13):2404‐5. [Google Scholar]
- Pang MY, Eng JJ, Dawson AS, McKay HA, Harris JE. A community‐based fitness and mobility exercise program for older adults with chronic stroke: a randomized, controlled trial. Journal of the American Geriatrics Society 2005;53(10):1667‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pang MY, Harris JE, Eng JJ. A community‐based upper‐extremity group exercise program improves motor function and performance of functional activities in chronic stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2006;87(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang MYC, Ashe MC, Eng JJ, Mckay HA, Dawson AS. A 19‐week exercise program for people with chronic stroke enhances bone geometry at the tibia: a peripheral quantitative computed tomography study. Osteoporosis International 2006;17(11):1615‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang MYC, Eng JJ. Determinants of improvement in walking capacity among individuals with chronic stroke following a multi‐dimensional exercise program. Journal of Rehabilitation Medicine 2008;40(4):284‐90. [DOI] [PubMed] [Google Scholar]
- Pang MYC, Lau RWK. The effects of treadmill exercise training on hip bone density and tibial bone geometry in stroke survivors: a pilot study. Neurorehabilitation and Neural Repair 2010;24(4):368‐76. [DOI] [PubMed] [Google Scholar]
- Park S, Kim S, Lee S, An H, Choi W, Moon O, et al. Comparison of underwater and overground treadmill walking to improve gait pattern and muscle strength after stroke. Journal of Physical Therapy Science 2012;24(11):1087‐90. [Google Scholar]
- Parker CJ, Gladman JR, Drummond AE, Dewey ME, Lincoln NB, Barer D, et al. A multicentre randomized controlled trial of leisure therapy and conventional occupational therapy after stroke. TOTAL Study Group. Trial of Occupational Therapy and Leisure. Clinical Rehabilitation 2001;15(1):42‐52. [DOI] [PubMed] [Google Scholar]
- Parry RH, Lincoln NB, Vass CD. Effect of severity of arm impairment on response to additional physiotherapy early after stroke. Clinical Rehabilitation 1999;13(3):187‐98. [DOI] [PubMed] [Google Scholar]
- Partridge C, Mackenzie M, Edwards S, Reid A, Jayawardena S, Guck N, et al. Is dosage of physiotherapy a critical factor in deciding patterns of recovery from stroke: a pragmatic randomized controlled trial. Physiotherapy Research International 2000;5(4):230‐40. [DOI] [PubMed] [Google Scholar]
- Patterson SA, Ross‐Edwards BM, Gill HL. Stroke maintenance exercise group: pilot study on daily functioning in long‐term stroke survivors. Australian Journal of Primary Health 2010;16(1):93‐7. [DOI] [PubMed] [Google Scholar]
- Peng H‐L. Influence of facilitation techniques on early active rehabilitation in upper extremity, hand and walk function of hemiplegia patients with stroke. Zhongguo Linchuang Kangfu 2002;6(9):1255‐6. [Google Scholar]
- Peurala SH, Pitkanen K, Sivenius J, Tarkka I. Body‐weight supported gait exercise compared with floor walking in chronic stroke patients. Archives of Physical Medicine and Rehabilitation 2004;85:E7. [DOI] [PubMed] [Google Scholar]; Peurala SH, Pitkanen K, Sivenius J, Tarkka IM. Body‐weight supported gait trainer exercises with or without functional electrical stimulation improves gait in patients with chronic stroke. Neurorehabilitation and Neural Repair 2006;20(1):98. [Google Scholar]; Peurala SH, Tarkka IM, Pitkanen K, Sivenius J. The effectiveness of body weight‐supported gait training and floor walking in patients with chronic stroke. Archives of Physical Medicine and Rehabilitation 2005;86(8):1557‐64. [DOI] [PubMed] [Google Scholar]
- Peurala SH, Airaksinen O, Huuskonen P, Jakala P, Juhakoski M, Sandell K, et al. Effects of intensive therapy using gait trainer or floor walking exercises early after stroke. Journal of Rehabilitation Medicine 2009;41(3):166‐73. [DOI] [PubMed] [Google Scholar]
- Pitsch E, Byl N, Abrams G. Training volume heightens recovery in patients stable post‐stroke. Archives of Physical Medicine and Rehabilitation 2006;87:E4. [Google Scholar]
- Platz T, Winter T, Muller M, Pinkowski C, Eickhof C, Mauritz K‐H. Arm ability training for stroke and traumatic brain injury patients with mild to moderately severe arm paresis. Neurologie und Rehabilitation 2000;6(5):245‐50. [DOI] [PubMed] [Google Scholar]; Platz T, Winter T, Muller N, Pinkowski C, Eickhof C, Mauritz KH. Arm ability training for stroke and traumatic brain injury patients with mild arm paresis: a single‐blind, randomized, controlled trial. Archives of Physical Medicine and Rehabilitation 2001;82(7):961‐8. [DOI] [PubMed] [Google Scholar]
- Platz T, Eickhof C, Kaick S, Engel U, Pinkowski C, Kalok S, et al. Impairment‐oriented training for arm paresis after stroke: a single blind, randomised, controlled multicentre trial. Neurologie und Rehabilitation 2004;10(4):169‐78. [Google Scholar]; Platz T, Eickhof C, Kaick S, Engel U, Pinkowski C, Kalok S, et al. Impairment‐oriented training or Bobath therapy for severe arm paresis after stroke: a single‐blind, multicentre randomized controlled trial. Clinical Rehabilitation 2005;19(7):714‐24. [DOI] [PubMed] [Google Scholar]; Platz T, Kaick S, Moller L, Freund S, Winter T, Kim IH. Impairment‐oriented training and adaptive motor cortex reorganisation after stroke: a fTMS study. Journal of Neurology 2005;252:1363‐71. [DOI] [PubMed] [Google Scholar]
- Pohl M, Werner C, Holzgraefe M, Kroczek G, Mehrholz J, Wingendorf I, et al. Repetitive locomotor training and physiotherapy improve walking and basic activities of daily living after stroke: a single‐blind, randomized multicentre trial (DEutsche GAngtrainerStudie, DEGAS). Clinical Rehabilitation 2007;21(1):17‐27. [DOI] [PubMed] [Google Scholar]
- Pomeroy VM, Evans B, Falconer M, Jones D, Hill E, Giakas G. An exploration of the effects of weighted garments on balance and gait of stroke patients with residual disability. Clinical Rehabilitation 2001;15(4):390‐7. [DOI] [PubMed] [Google Scholar]
- Puckree T, Naidoo P. Balance and stability‐focused exercise program improves stability and balance in patients after acute stroke in a resource‐poor setting. PM & R: the Journal of Injury, Function, and Rehabilitation 2014;6(12):1081‐7. [DOI] [PubMed] [Google Scholar]
- Quaney BM, Boyd LA, McDowd JM, Zahner LH, He J, Mayo MS, et al. Aerobic exercise improves cognition and motor function poststroke. Neurorehabilitation and Neural Repair 2009;23(9):879‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro T, Britto H, Oliveira D, Silva E, Galvao E, Lindquist A. Effects of treadmill training with partial body weight support and the proprioceptive neuromuscular facilitation method on hemiparetic gait: a randomized controlled study. European Journal of Physical and Rehabilitation Medicine 2013;49(4):451‐61. [PubMed] [Google Scholar]
- Rimmer JH, Riley B, Creviston T, Nicola T. Exercise training in a predominantly African‐American group of stroke survivors. Medicine and Science in Sports and Exercise 2000;32(12):1990‐6. [DOI] [PubMed] [Google Scholar]
- Rimmer JH, Rauworth AE, Wang EC, Nicola TL, Hill B. A preliminary study to examine the effects of aerobic and therapeutic (nonaerobic) exercise on cardiorespiratory fitness and coronary risk reduction in stroke survivors. Archives of Physical Medicine and Rehabilitation 2009;90(3):407‐12. [DOI] [PubMed] [Google Scholar]
- Rose D, Paris T, Crews E, Wu SS, Sun A, Behrman AL, et al. Feasibility and effectiveness of circuit training in acute stroke rehabilitation. Neurorehabilitation and Neural Repair 2011;25(2):140‐8. [DOI] [PubMed] [Google Scholar]
- Saeys W, Vereeck L, Truijen S, Lafosse C, Wuyts FP, Heyning P. Randomized controlled trial of truncal exercises early after stroke to improve balance and mobility. Neurorehabilitation and Neural Repair 2012;26(3):231‐8. [DOI] [PubMed] [Google Scholar]
- Schmid AA, Puymbroeck M, Altenburger PA, Schalk NL, Dierks TA, Miller Kristine K, et al. Poststroke balance improves with yoga: a pilot study. Stroke 2012;43(9):2402‐7. [DOI] [PubMed] [Google Scholar]
- Severinsen K, Jakobsen JK, Pedersen AR, Overgaard K, Andersen H. Effects of resistance training and aerobic training on ambulation in chronic stroke. American Journal of Physical Medicine and Rehabilitation 2014;93(1):29‐42. [DOI] [PubMed] [Google Scholar]
- Shatil S, Ivanova TD, Mochizuki G, Garland SJ. Effects of therapeutic golf rehabilitation on golf performance, balance, and quality of life in individuals following stroke: pilot study. Physiotherapy Canada 2005;57(2):101‐11. [Google Scholar]
- Sherrington C, Pamphlett PI, Jacka JA, Olivetti LM, Nugent JA, Hall JM, et al. Group exercise can improve participants' mobility in an outpatient rehabilitation setting: a randomized controlled trial. Clinical Rehabilitation 2008;22:493‐502. [DOI] [PubMed] [Google Scholar]
- Shimada H, Uchiyama Y, Kakurai S. Specific effects of balance and gait exercises on physical function among the frail elderly. Clinical Rehabilitation 2003;17(5):472. [DOI] [PubMed] [Google Scholar]
- Shimizu ME, Ishizaki F, Nakamura S. Results of a home exercise program for patients with osteoporosis resulting from neurological disorders. Hiroshima Journal of Medical Sciences 2002;51(1):15‐22. [PubMed] [Google Scholar]
- Harris JE. Repetitive facilitative exercise improves upper limb function in patients with subacute stroke. Journal of Physiotherapy 2013;59(3):208. [DOI] [PubMed] [Google Scholar]; Shimodozono M. Repetitive facilitative exercise: recent evidence and development for combination therapy. Clinical Neurology 2013;53(11):1267‐9. [DOI] [PubMed] [Google Scholar]; Shimodozono M, Noma T, Nomoto Y, Hisamatsu N, Kamada K, Miyata R, et al. Benefits of a repetitive facilitative exercise program for the upper paretic extremity after subacute stroke: a randomized controlled trial. Neurorehabilitation and Neural Repair 2013;27(4):296‐305. [DOI] [PubMed] [Google Scholar]
- Shimodozono M, Noma T, Matsumoto S, Miyata R, Etoh S, Kawahira K. Repetitive facilitative exercise under continuous electrical stimulation for severe arm impairment after sub‐acute stroke: a randomized controlled pilot study. Brain Injury 2014;28(2):203‐10. [DOI] [PubMed] [Google Scholar]
- Peurala SH, Airaksinen O, Jakala P, Tarkka IM, Sivenius J. Intensive walking and exercise therapy during early acute stage of stroke. ISPGR 2007. 18th International Conference of the International Society for Posture and Gait Research. Vermont, 2007. [Google Scholar]; Sivenius J, Peurala S. Gait trainer vs traditional physiotherapy in acute stroke. ClinicalTrials.gov2007.
- Smith DS, Goldenberg E, Ashburn A, Kinsella G, Sheikh K, Brennan PJ, et al. Remedial therapy after stroke: a randomised controlled trial. BMJ 1981;282(6263):517‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KJ, Knowlton BJ, Dobkin BH. Step training with body weight support: effect of treadmill speed and practice paradigms on poststroke locomotor recovery. Archives of Physical Medicine and Rehabilitation 2002;83(5):683‐91. [DOI] [PubMed] [Google Scholar]
- Sullivan KJ, Brown DA, Klassen T, Mulroy S, Ge T, Azen SP, et al. Effects of task‐specific locomotor and strength training in adults who were ambulatory after stroke: results of the STEPS randomized clinical trial. Physical Therapy 2007;87(12):1580‐607. [DOI] [PubMed] [Google Scholar]
- Sunderland A, Fletcher D, Bradley L, Tinson D, Hewer RL, Wade DT. Enhanced physical therapy for arm function after stroke: a one year follow up study. Journal of Neurology Neurosurgery and Psychiatry 1994;57(7):856‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]; Sunderland A, Tinson DJ, Bradley EL, Fletcher D, Langton HR, Wade DT. Enhanced physical therapy improves recovery of arm function after stroke. A randomised controlled trial. Journal of Neurology Neurosurgery and Psychiatry 1992;55(7):530‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suputtitada A, Yooktanan P, Rarerng‐Ying T. Effect of partial body weight support treadmill training in chronic stroke patients. Journal of the Medical Association of Thailand 2004;87 Suppl 2:107‐11. [PubMed] [Google Scholar]
- Takao T, Tanaka N, Iizuka N, Saitou H, Tamaoka A, Yanagi H. Improvement of gait ability with a short‐term intensive gait rehabilitation program using body weight support treadmill training in community dwelling chronic poststroke survivors. Journal of Physical Therapy Science 2015;27(1):159‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatori K, Matsumoto D, Okada Y, Nakamura J, Shomoto K. Effect of intensive rehabilitation on physical function and arterial function in community‐dwelling chronic stroke survivors. Topics in Stroke Rehabilitation 2012;19(5):377‐83. [DOI] [PubMed] [Google Scholar]
- Tamura A, Ichihara T, Minagawa T, Kuwamura Y, Kondo H, Takata S, et al. Exercise intervention soon after stroke onset to prevent muscle atrophy. British Journal of Neuroscience Nursing 2011;7(4):574‐9. [Google Scholar]
- Tang A, Sibley KM, Thomas SG, Bayley MT, Richardson D, McIlroy WE, et al. Effects of an aerobic exercise program on aerobic capacity, spatiotemporal gait parameters, and functional capacity in subacute stroke. Neurorehabilitation and Neural Repair 2009;23(4):398‐406. [DOI] [PubMed] [Google Scholar]
- Tang A, Eng JJ, Krassioukov AV, Madden KM, Mohammadi A, Tsang MYC, et al. Exercise‐induced changes in cardiovascular function after stroke: a randomized controlled trial. International Journal of Stroke 2014;9(7):883‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor‐Piliae R, Coull B. Community‐based Yang‐style Tai Chi is safe and feasible in chronic stroke: a pilot study. Clinical Rehabilitation 2012;26(2):121‐31. [DOI] [PubMed] [Google Scholar]
- Thielman GT, Dean CM, Gentile AM. Rehabilitation of reaching after stroke: task‐related training versus progressive resistive exercise. Archives of Physical Medicine and Rehabilitation 2004;85(10):1613‐8. [DOI] [PubMed] [Google Scholar]
- Thielman GT. Training with Trunk Restraint During Reaching for Individuals Post‐stroke [Dissertation]. Teachers College, Columbia University, 2005. [Google Scholar]
- Thielman G. Insights into upper limb kinematics and trunk control one year after task‐related training in chronic post‐stroke individuals. Journal of Hand Therapy 2013;26(2):156‐61. [DOI] [PubMed] [Google Scholar]
- Tripp F, Krakow K. Effects of an aquatic therapy approach (Halliwick‐Therapy) on functional mobility in subacute stroke patients: a randomized controlled trial. Clinical Rehabilitation 2014;28(5):432‐9. [DOI] [PubMed] [Google Scholar]
- Lee JH, Wagenaar RC, Lankhorst GJ, Vogelaar TW, Deville WL, Bouter LM. Forced use of the upper extremity in chronic stroke patients: results from a single‐blind randomized clinical trial. Stroke 1999;30(11):2369‐75. [DOI] [PubMed] [Google Scholar]
- Wijk R, Cumming T, Churilov L, Donnan G, Bernhardt J. An early mobilization protocol successfully delivers more and earlier therapy to acute stroke patients: further results from phase II of AVERT. Neurorehabilitation and Neural Repair 2012;26(1):20‐6. [DOI] [PubMed] [Google Scholar]
- Walker MF, Gladman J, Lincoln NB, Siemonsma P, Whiteley T. A randomized controlled trial of occupational therapy for stroke patients not admitted to hospital. Clinical Rehabilitation 1999;13:527. [DOI] [PubMed] [Google Scholar]; Walker MF, Gladman JR, Lincoln NB, Siemonsma P, Whiteley T. Occupational therapy for stroke patients not admitted to hospital: a randomised controlled trial. Lancet 1999;354(9175):278‐80. [DOI] [PubMed] [Google Scholar]; Walker MF, Gladman JRF, Lincoln NB. A randomised controlled trial of occupational therapy for stroke patients not admitted to hospital. Cerebrovascular Diseases 1999;9 Suppl 1:123. [DOI] [PubMed] [Google Scholar]; Walker MF, Gladman JRF, Lincoln NB. A randomised controlled trial of occupational therapy: results at one year. Cerebrovascular Disease 2000;10 Suppl 2:61. [Google Scholar]
- Wang T‐C, Tsai AC, Wang J‐Y, Lin Y‐T, Lin K‐L, Chen JJ, et al. Caregiver‐mediated intervention can improve physical functional recovery of patients with chronic stroke: a randomized controlled trial. Neurorehabilitation and Neural Repair 2015;29(1):3‐12. [DOI] [PubMed] [Google Scholar]
- Werner RA, Kessler S. Effectiveness of an intensive outpatient rehabilitation program for postacute stroke patients. American Journal of Physical Medicine and Rehabilitation 1996;75(2):114‐24, 140. [DOI] [PubMed] [Google Scholar]
- Werner C, Frankenberg S, Treig T, Konrad M, Hesse S. Treadmill training with partial body weight support and an electromechanical gait trainer for restoration of gait in subacute stroke patients: a randomized crossover study. Stroke 2002;33(12):2895‐901. [DOI] [PubMed] [Google Scholar]
- Widén Holmqvist L, Koch L, Kostulas V, Holm M, Widsell G, Tegler H, et al. A randomized controlled trial of rehabilitation at home after stroke in southwest Stockholm. Stroke 1998;29(3):591‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wing A, Johannsen L, Pelton T, Vliet P, Riddoch J, Sackley C, et al. Bilateral leg movement training in chronic hemiparesis: a pilot RCT. UK Stroke Forum Conference. Harrogate, 2006. [Google Scholar]
- Winstein CJ, Wolf SL, Dromerick AW, Lane CJ, Nelsen MA, Lewthwaite R, et al. Interdisciplinary Comprehensive Arm Rehabilitation Evaluation (ICARE): a randomized controlled trial protocol. BMC Neurology 2013;13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe CD, Tilling K, Rudd AG. The effectiveness of community‐based rehabilitation for stroke patients who remain at home: a pilot randomized trial. Clinical Rehabilitation 2000;14(6):563‐9. [DOI] [PubMed] [Google Scholar]
- Wu CY, Chuang LL, Lin KC, Chen HC, Tsay PK. Randomized trial of distributed constraint‐induced therapy versus bilateral arm training for the rehabilitation of upper‐limb motor control and function after stroke. Neurorehabilitation and Neural Repair 2011;25(2):130‐9. [DOI] [PubMed] [Google Scholar]
- Xiao X‐H, Xu J‐X, Tan J‐W. Effect of unilateral bridging activities on regional cerebral blood flow and the recovery of the affected limb function of cerebral infarction in the early stage post stroke. Zhongguo Linchuang Kangfu 2002;6(5):644‐5. [Google Scholar]
- Yang YR, Yen JG, Wang RY, Yen LL, Lieu FK. Gait outcomes after additional backward walking training in patients with stroke: a randomized controlled trial. Clinical Rehabilitation 2005;19(3):264‐73. [DOI] [PubMed] [Google Scholar]
- Yang YR, Wang RY, Chen YC, Kao MJ. Dual‐task exercise improves walking ability in chronic stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2007;88(10):1236‐40. [DOI] [PubMed] [Google Scholar]
- Yen CL, Wang RY, Liao KK, Huang CC, Yang YR. Gait training‐induced change in corticomotor excitability in patients with chronic stroke. Neurorehabilitation and Neural Repair 2008;22:22‐30. [DOI] [PubMed] [Google Scholar]
- Yokokawa Y, Minamisawa H, Sato H, Kai I, Nakajima T, Fukusima Y. Psychological effect of a physical activity program on community people with cerebral apoplexy (Abstract PO‐RR‐575‐27). 13th International Congress of the World Confederation for Physical Therapy May 23‐28 1999. Japan, Yokohama: World Confederation for Physical Therapy, 1999:559. [Google Scholar]
- Zheng G, Chen B, Fang Q, Yi H, Lin Q, Chen L, et al. Primary prevention for risk factors of ischemic stroke with Baduanjin exercise intervention in the community elder population: Study protocol for a randomized controlled trial. Trials 2014;15:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Arya K, Verma R, Garg RK, Sharma VP, Agarwal M, Aggarwal GG. Meaningful Task‐Specific Training (MTST) for stroke rehabilitation: a randomized controlled trial. Topics in Stroke Rehabilitation 2012;19(3):193‐211. [DOI] [PubMed] [Google Scholar]
- Askim T, Morkved S, Engen A, Roos K, Aas T, Indredavik B. Effects of a community‐based intensive motor training program combined with early supported discharge after treatment in a comprehensive stroke unit: a randomized, controlled trial. Stroke 2010;41(8):1697‐703. [DOI] [PubMed] [Google Scholar]
- Buyukavci R, Iahin F, Sat S, Dotu B, Kuran B. The effect of trunk balance training on motor recovery, trunk balance, ambulation and quality of life in subacute stroke patients: a randomized controlled trial. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2011;57:270. [Google Scholar]
- Byun SD, Jung TD, Kim CH, Lee YS. Effects of the sliding rehabilitation machine on balance and gait in chronic stroke patients ‐ a controlled clinical trial. Clinical Rehabilitation 2011;25(5):408‐15. [DOI] [PubMed] [Google Scholar]
- Dean CM, Ada L, Bampton J, Morris ME, Katrak PH, Potts S. Treadmill walking with body weight support in subacute non‐ambulatory stroke improves walking capacity more than overground walking: a randomised trial. Journal of Physiotherapy 2010;56(2):97‐103. [DOI] [PubMed] [Google Scholar]
- Hoyer E, Jahnsen R, Stanghelle JK, Strand LI. Body weight supported treadmill training versus traditional training in patients dependent on walking assistance after stroke: a randomized controlled trial. Disability and Rehabilitation 2012;34(3):210‐9. [DOI] [PubMed] [Google Scholar]
- Kondo R. Poster 64: Effects of dynamic‐intensive exercise for gait ability in chronic stroke patients: a randomized controlled trial. Archives of Physical Medicine & Rehabilitation 2012;93(10):e33. [Google Scholar]
- Kumaran DS, Rao BK, Rao SN. Effectiveness of task based exercise program using ICF on quality of life in stroke patients. Cerebrovascular Diseases 2014;38:108‐9. [Google Scholar]
- Kwok CM, Lui HT, Hui KF, Wong KY, Lam HY, Yick CY. Intensify secondary stroke prevention with quality of life wellbeing and functions restoration in the active lifestyle therapeutic exercise program. Cerebrovascular Diseases 2012;34:135. [Google Scholar]
- Lee YH, Kim JH, Yu YJ, Kang SJ. The effects of aquatic rehabilitation exercise on maximal walking velocity, gait endurance, and balance in male hemiplegia patients after stroke. Medicine and Science in Sports and Exercise 2008;40(5):S64. [Google Scholar]
- Malagoni AM, Cavazza S, Basaglia N, Grassi G, Ferraresi G, Felisatti M, et al. Conventional therapy versus a test in‐train out, walking program to improve physical functioning in chronic stroke patients. A randomized pilot study. Acta Clinica Croatica, Supplement 2013;52:66. [Google Scholar]
- Mayo NE, Bayley M, Eng J, Teasell R, Kay‐Lyons M, Richards CL, et al. Getting on with the rest of your life after stroke: a community‐based randomized trial. Stroke 2011;42(11):e615. [Google Scholar]
- Moore S, Hallsworth K, Jakovljevic D, Blamire A, He J, Ford G, et al. Effects of exercise therapy on metabolic risk factors, brain atrophy and cerebral blood flow following stroke: a randomised controlled trial. International Journal of Stroke 2014;9:32. 22928705 [Google Scholar]; Moore SA, Jakovljevic DG, Ford GA, Rochester L, Trenell MI. The effect of a community exercise intervention on physiological and physical function following stroke: a randomized, controlled trial. International Journal of Stroke 2012;7:76. [Google Scholar]
- Olawale OA, Jaja SI, Anigbogu CN, Appiah‐Kubi KO, Jones‐Okai D. Exercise training improves walking function in an African group of stroke survivors: a randomized controlled trial. Clinical Rehabilitation 2011;25(5):442‐50. [DOI] [PubMed] [Google Scholar]
- Pagnussat A, Silva P, Antunes F, Graef P, Cechetti F. Strength training associated with task‐oriented training to enhance upper limb motor function in people with chronic stroke. Brain Injury 2014;28(5‐6):684‐5. [Google Scholar]
- Park SW, Lee KJ, Shin DC, Shin SH, Lee MM, Song CH. The effect of underwater gait training on balance ability of stroke patients. Journal of Physical Therapy Science 2014;26(6):899‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podubecka J, Scheer S, Theilig S, Wiederer R, Oberhoffer R, Nowak DA. Cyclic movement training versus conventional physiotherapy for rehabilitation of hemiparetic gait after stroke: a pilot study. Fortschritte der Neurologie Psychiatrie 2011;79(7):411‐8. [DOI] [PubMed] [Google Scholar]
- Qi L, Yuling W, Zhou Z, Zhiqin X, Wen Feng L, Haojie Z, et al. Effect of graded elastic band exercise and balance training on cardiovascular capacity and functional mobility in subacute and chronic stroke. Heart 2011;97:A228. [Google Scholar]
- Qu Y‐P, Sun L, Zhu L, Song W‐Q. Effects of Biodex system assisted task‐specific walking training on lower limb motor function in patients with stroke. Chinese Journal of Cerebrovascular Diseases 2014;11(5):233‐7. [Google Scholar]
- Rydwik E, Eliasson S, Akner G. The effect of exercise of the affected foot in stroke patients ‐ a randomized controlled pilot trial. Clinical Rehabilitation 2006;20(8):645‐55. [DOI] [PubMed] [Google Scholar]
- Sen SB, Demir SO, Ekiz T, Ozgirgin N. Effects of the isokinetic strengthening training on functional parameters, gait and quality of life in patients with stroke: a randomized controlled trial. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2013;59:396. [Google Scholar]
- Shaughnessy M, Stookey A. Reshaping exercise habits and beliefs (REHAB): a randomized trial of home‐based exercise in sub‐acute stroke. Stroke2012; Vol. 43, issue ANS11.
- Shaughnessy M, Michael K, Resnick B. Impact of treadmill exercise on efficacy expectations, physical activity, and stroke recovery. Journal of Neuroscience Nursing 2012;44(1):27‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Gupta A, Murali T, Taly AB. Body‐weight‐supported treadmill training in retraining gait among chronic stroke survivors: randomized controlled study. PM&R 2011;3(10 Suppl 1):S344‐5. [DOI] [PubMed] [Google Scholar]
- Tung FL, Yang YR, Lee CC, Wang RY. Balance outcomes after additional sit‐to‐stand training in subjects with stroke: a randomized controlled trial. Clinical Rehabilitation 2010;24(6):533‐42. [DOI] [PubMed] [Google Scholar]
- Vahlberg B, Cederholm T, Lindmark B, Zetterberg L, Hellstrm K. Effects of progressive resistance and balance training 1‐3 years after stroke: a randomized controlled trial. FASEB Journal2014; Vol. 28, issue 1 Suppl 1.
- Puymbroeck M, Allsop J, Miller KK, Schmid AA. Improvements in ICF components in individuals with chronic stroke following a yoga‐based intervention. Archives of Physical Medicine and Rehabilitation 2014;95(10):e24. [Google Scholar]; Puymbroeck M, Schmid A, Miller K, Schalk N. Improved activity, participation, and quality of life for individuals with chronic stroke following an 8‐week yoga intervention. BMC Complementary and Alternative Medicine 2012;12 Suppl 1:O39. [DOI: 10.1186/1472-6882-12-S1-O39] [DOI] [Google Scholar]
- Yang YR, Chen IH, Liao KK, Huang CC, Wang RY. Cortical reorganization induced by body weight‐supported treadmill training in patients with hemiparesis of different stroke durations. Archives of Physical Medicine and Rehabilitation 2010;91(4):513‐8. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- Brauer S. The efficacy of a novel, non‐robotic intervention to train reaching post stroke. ANZCTR. [ ACTRN12608000457347 ] ; Brauer S, Hayward K, Carson R, Cresswell A, Barker R. The efficacy of SMART Arm training early after stroke for stroke survivors with severe upper limb disability: a protocol for a randomised controlled trial. BMC Neurology 2013;13:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English C, Bernhardt J, Crotty M, Esterman A, Segal L, Hillier S. Circuit class therapy or seven‐day week therapy for increasing rehabilitation intensity of therapy after stroke (CIRCIT): a randomized controlled trial. International Journal of Stroke 2015;10(4):594‐602. [DOI] [PubMed] [Google Scholar]; English C, Bernhardt J, Hillier S. Circuit class therapy and 7‐day‐week therapy increase physiotherapy time, but not patient activity: early results from the CIRCIT Trial. Stroke 2014;45(10):3002‐7. [DOI] [PubMed] [Google Scholar]; Hillier S. Circuit class therapy for rehabilitation after stroke. A pragmatic randomised controlled trial (CIRCIT). ANZCTR. [ACTRN12610000096055] ; Hillier S, English C, Bernhardt J, Crotty M, Esterman A, Segal L. Free communications 3: Multidisciplinary rehabilitation circuit class and 7‐day week therapy for increasing rehabilitation intensity of therapy after stroke (CIRCIT): Six month follow‐up and cost analysis of the CIRCIT RCT. International Journal of Stroke 2014;9:21. [Google Scholar]; Hillier S, English C, Crotty M, Segal L, Bernhardt J, Esterman A. Circuit class or seven‐day therapy for increasing intensity of rehabilitation after stroke: protocol of the CIRCIT trial. International Journal of Stroke 2011;6(6):560‐5. [DOI] [PubMed] [Google Scholar]
- Reynolds J. The effect of moderate‐intensity cardiovascular fitness training compared to standard care in people with a diagnosis of stroke: a pilot randomised controlled trial. ANZCTR. [ACTRN12613000822785]
- Hariohm K, Prakash V, Vasanthan R, Daran JKD, Samuel RJ. An RCT protocol on efficacy of deep knee flexion exercises on improving activities involving deep knee flexion and quality of life in persons with stroke. Cerebrovascular Diseases 2013;36:15. [Google Scholar]
- Pomeroy VM, Ward NS, Johansen‐Berg H, Vliet P, Burridge J, Hunter SM, et al. FAST INdiCATE Trial protocol. Clinical efficacy of functional strength training for upper limb motor recovery early after stroke: Neural correlates and prognostic indicators. International Journal of Stroke 2014;9(2):240‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]; Walker A. Clinical efficacy of functional strength training for upper limb motor recovery early after stroke: neural correlates and prognostic indicators. http://www.controlled‐trials.com/ (accessed January 2013). [DOI] [PMC free article] [PubMed]
- Hancock N, Shepstone L, Rowe P, Myint P, Pomeroy V. Clinical efficacy and prognostic indicators for lower limb pedalling exercise early after stroke: study protocol for a pilot randomised controlled trial. Trials 2011;12:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turton A. Home based reach to grasp training after stroke. ICTRN. [DOI 10.1186/ISRCTN56716589; ISRCTN56716589] ; Turton A, Cunningham P, Heron E, Wijck F, Sackley C, Rogers Cs, et al. Home‐based reach‐to‐grasp training for people after stroke: study protocol for a feasibility randomized controlled trial. Trials 2013;14:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suskin NG. Cardiac rehabilitation for TIA patients (CR‐TIA). ClincalTrials.gov (accessed 2007).
- Ivey FM. Strength training for skeletal muscle adaptation after stroke. ClinicalTrials.gov2009.
- Hammers J. Inflammation and exercise in stroke. ClinicalTrials.gov.
- Vanroy C. The effect of an aerobic exercise programme in stroke patients. ClinicalTrials.gov2010.
- Richardson J. Fit for Function: A Community Wellness Program for Persons With Stroke (FFF). ClinicalTrials.gov. [NCT01194102] ; Richardson J, Fleck R, Hladysh G, McBay C, MacKay E, Thorlakson R, et al. Fit for function: a community wellness program for persons with stroke. Stroke; a journal of cerebral circulation 2011;42(11):e596. [Google Scholar]
- Macko RF. Exercise for sub‐acute stroke patients in Jamaica (JAMMS). ClinicalTrials.gov.
- Askim T, Langhammer B, Ihle‐Hansen H, Magnussen J, Engstad T, Indredavik B. A long‐term follow‐up programme for maintenance of motor function after stroke: protocol of the life after stroke ‐ The LAST study. Stroke Research and Treatment 2012;2012:392101. [DOI: 10.1155/2012/392101] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer‐D'Amato. Training dual‐task walking after stroke. ClinicalTrials.gov. [NCT01568957] ; Plummer‐D'Amato P, Kyvelidou A, Sternad D, Najafi B, Villalobos R, Zurakowski D. Training dual‐task walking in community‐dwelling adults within 1 year of stroke: a protocol for a single‐blind randomized controlled trial. BMC Neurology 2012;12(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland SJ. Fast muscle Activation and Stepping Training (FAST) post‐stroke. ClinicalTrials.gov. [DOI] [PMC free article] [PubMed]
- Schultz B. Use of repetitive facilitative exercise program in established stroke. ClinicalTrials.gov.
- Freeman MJ. Combined effects of aerobic exercise and cognitive training on cognition after stroke. ClinicalTrials.gov.
- Pooyania S, Szturm T. Effects of a community‐based group rehabilitation program for dynamic balance and mobility post stroke. ClinicalTrials.gov.
- Tousignant M. A randomized, non‐inferiority clinical trial of CVA telerehabilitation treatments ‐ TelePhysioTaiChi. ClinicalTrials.gov. ; Tousignant M, Corriveau H, Kairy D, Berg K, Dubois MF, Gosselin S, et al. Tai Chi‐based exercise program provided via telerehabilitation compared to home visits in a post‐stroke population who have returned home without intensive rehabilitation: study protocol for a randomized, non‐inferiority clinical trial. Trials 2014;15(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floel A, Werner C, Grittner U, Hesse S, Jobges M, Knauss J, et al. Physical fitness training in Subacute Stroke (PHYS‐STROKE) ‐ study protocol for a randomised controlled trial. Trials 2014;15:45. [DOI] [PMC free article] [PubMed] [Google Scholar]; Flöel A. Physical Fitness Training in Subacute Stroke (PHYS‐Stroke). ClinicalTrials.gov. [NCT01953549] [DOI] [PMC free article] [PubMed]
- Tole GC, Holland A, Williams G, Clark R. Ballistic strength training in stroke: a pilot study. ClinicalTrials.gov.
- Sandberg K, Kleist M, Falk L, Enthoven P. Aerobic exercise in early subacute stroke. ClinicalTrials.gov. [DOI] [PubMed]
- Hsu M‐J. Early intervention with a low‐intensity leg cycling exercise program for individuals after stroke. ClinicalTrials.gov.
Additional references
- American College of Sports Medicine. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Medicine and Science in Sports and Exercise 1998;30(6):975‐91. [DOI] [PubMed] [Google Scholar]
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, LaMonte MJ, Lee IM, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Medicine & Science in Sports & Exercise 2011;43(7):1334‐59. [DOI] [PubMed] [Google Scholar]
- Ada L, Dean CM, Nascimento LR, Teixeira‐Salmela LF. Treadmill training is effective for ambulatory adults with stroke: a systematic review. Journal of Physiotherapy 2013;59(2):73‐80. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561‐71. [DOI] [PubMed] [Google Scholar]
- Bernhardt J, Dewey H, Thrift A, Donnan G. Inactive and alone: physical activity within the first 14 days of acute stroke unit care. Stroke 2004;35(4):1005‐9. [DOI] [PubMed] [Google Scholar]
- Bernhardt J, Chan J, Nicola I, Collier JM. Little therapy, little physical activity: rehabilitation within the first 14 days of organized stroke unit care. Journal of Rehabilitation Medicine 2007;39:43‐8. [DOI] [PubMed] [Google Scholar]
- Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American heart association/American stroke association. Stroke 2014;45(8):2532‐53. [DOI] [PubMed] [Google Scholar]
- Bonilha HS, Kautz SA, Gregory CM, Bowden MG. Rehabilitating walking speed poststroke with treadmill‐based interventions: a systematic review of randomized controlled trials. Neurorehabilitation and Neural Repair 2013;27(8):709‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner DM, Lateur BJ. The importance of skeletal muscle strength to physical function in older adults. Annals of Behavioral Medicine 1991;13(3):91‐8. [Google Scholar]
- Cabanas‐Valdés R, Cuchi G, Bagur‐Calafat C. Trunk training exercises approaches for improving trunk performance and functional sitting balance in patients with stroke: a systematic review. NeuroRehabilitation 2013;33(4):575‐92. [DOI] [PubMed] [Google Scholar]
- Carin‐Levy G, Kendall M, Young A, Mead G. The psychosocial effects of exercise and relaxation classes for persons surviving a stroke. Canadian Journal of Occupational Therapy 2009;76:73‐80. [DOI] [PubMed] [Google Scholar]
- Charalambous C, Bonilha H, Kautz S, Gregory C, Bowden M. Rehabilitating walking speed poststroke with treadmill‐based interventions: a systematic review of randomized controlled trials. Neurorehabilitation & Neural Repair 2013;27(8):709‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodzko‐Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, et al. Exercise and physical activity for older adults. Medicine and Science in Sports and Exercise 2009;41(7):1510‐30. [DOI] [PubMed] [Google Scholar]
- Collen FM, Wade DT, Robb GF, Bradshaw CM. The Rivermead Mobility Index: a further development of the Rivermead Motor Assessment. International Disability Studies 1991;13:50‐4. [DOI] [PubMed] [Google Scholar]
- Collin C, Wade DR, Davies S, Horne V. The Barthel ADL Index: a reliability study. International Disability Studies 1988;10:61‐3. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, Moher D for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke EV, Mares K, Clark A, Tallis RC, Pomeroy VM. The effects of increased dose of exercise‐based therapies to enhance motor recovery after stroke: a systematic review and meta‐analysis. BMC Medicine 2010;8(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen VA, Buys R, Smart NA. Endurance exercise beneficially affects ambulatory blood pressure: a systematic review and meta‐analysis. Journal of Hypertension 2013;31(4):639‐48. [DOI] [PubMed] [Google Scholar]
- Cumming TB, Tyedin K, Churilov L, Morris ME, Bernhardt J. The effect of physical activity on cognitive function after stroke: a systematic review. International Psychogeriatrics/IPA 2012;24(4):557‐67. [DOI] [PubMed] [Google Scholar]
- Dawson AS, Knox J, McClure A, Foley N, Teasell R, on behalf of the Stroke Rehabilitation Writing Group. Stroke rehabilitation. Canadian Best Practice Recommendations for Stroke Care. 4th Edition. Ottawa, Ontario Canada: Heart and Stroke Foundation and the Canadian Stroke Network, 2013. [http://strokebestpractices.ca/wp‐content/uploads/2013/07/SBP2013_Stroke‐Rehabilitation‐Update_July‐10_FINAL.pdf] [Google Scholar]
- Degens H, Alway SE. Control of muscle size during disuse, disease, and aging. International Journal of Sports Medicine 2006;27(2):94‐9. [DOI] [PubMed] [Google Scholar]
- Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The Stroke Impact Scale Version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke 1999;30:2131‐40. [DOI] [PubMed] [Google Scholar]
- Dunn A, Callister R, Levi CR, Spratt NJ. Characteristics of exercise training interventions to improve cardiorespiratory fitness after stroke: a systematic review with meta‐analysis. Neurorehabilitation and Neural Repair 2013;27(9):775‐88. [DOI] [PubMed] [Google Scholar]
- Eng JJ, Reime B. Exercise for depressive symptoms in stroke patients: a systematic review and meta‐analysis. Clinical Rehabilitation 2014;28(8):731‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flansbjer UB, Downham D, Lexell J. Knee muscle strength, gait performance, and perceived participation after stroke. Archives of Physical Medicine and Rehabilitation 2006;87(7):974‐80. [DOI] [PubMed] [Google Scholar]
- Francica JV, Bigongiari A, Mochizuki L, Miranda MLJ, Rodrigues B. Aerobic program in persons with stroke: a systematic review. Acta Medica Portuguesa 2014;27(1):108‐15. [DOI] [PubMed] [Google Scholar]
- French B, Leathley M, Sutton C, McAdam J, Thomas L. A systematic review of repetitive functional task practice with modelling of resource use, costs and effectiveness. Health Technology Assessment 2008;12(30):140. [DOI] [PubMed] [Google Scholar]
- Fried LP, Bandeen‐Roche K, Williamson JD, Prasada‐Rao P, Chee E, Tepper S, et al. Functional decline in older adults: expanding methods of ascertainment. Journals of Gerontology Series A ‐ Biological Sciences and Medical Sciences 1996;51(5):M206‐14. [DOI] [PubMed] [Google Scholar]
- Fulk GD, Ludwig M, Dunning K, Golden S, Boyne P, West T. Estimating clinically important change in gait speed in people with stroke undergoing outpatient rehabilitation. Journal of Neurologic Physical Therapy 2011;35:82‐9. [DOI] [PubMed] [Google Scholar]
- Garcia‐Soto E, Lourdes Lopez de Munain M, Santibanez M. Effects of combined aerobic and resistance training on cognition following stroke: a systematic review. Revista de Neurologia 2013;57(12):535‐41. [PubMed] [Google Scholar]
- Gauthier LV, Taub E, Perkins C, Ortmann M, Mark VW, Uswatte GI. Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke 2008;39(5):1520‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits KH, Beltman MJ, Koppe PA, Konijnenbelt H, Elich PD, Haan A, et al. Isometric muscle function of knee extensors and the relation with functional performance in patients with stroke. Archives of Physical Medicine and Rehabilitation 2009;90(3):480‐7. [DOI] [PubMed] [Google Scholar]
- Greener J, Langhorne P. Systematic reviews in rehabilitation for stroke: issues and approaches to addressing them. Clinical Rehabilitation 2002;16(1):69‐74. [DOI] [PubMed] [Google Scholar]
- Hall K, Mann NR, Wright JM, Kreutzer JS, Wood D. Functional measures after traumatic brain injury: ceiling effects of FIM, FIM+FAM, DRS, and CIQ. Journal of Head Trauma Rehabilitation 1996;11:27‐39. [Google Scholar]
- Hamilton BB, Laughlin JA, Fiedler RC, Granger CV. Interrater reliability of the 7‐level functional independence measure (FIM). Scandinavian Journal of Rehabilitation Medicine 1994;26:115‐9. [PubMed] [Google Scholar]
- Hasegawa R, Islam MM, Sung CL, Koizumi D, Rogers ME, Takeshima N. Threshold of lower body muscular strength necessary to perform ADL independently in community‐dwelling older adults. Clinical Rehabilitation 2008;22(10‐11):902‐10. [DOI] [PubMed] [Google Scholar]
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine and Science in Sports and Exercise 2007;39:1423‐34. [DOI] [PubMed] [Google Scholar]
- Heran BS, Chen JMH, Ebrahim S, Moxham T, Oldridge N, Rees K, et al. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD001800.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta‐analysis. Archives of Physical Medicine and Rehabilitation 2004;85(10):1694‐704. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Hobart JC, Williams LS, Moran K, Thompson AJ. Quality of life measurement after stroke: uses and abuses of the SF‐36. Stroke 2002;33(5):1348‐56. [DOI] [PubMed] [Google Scholar]
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:1687. [DOI] [PubMed] [Google Scholar]
- Holden MK, Gill KM, Magliozzi MR, Nathan J, Peihl‐Baker L. Clinical gait assessment in the neurologically impaired: reliability and meaningfulness. Physical Therapy 1994;64:35‐40. [DOI] [PubMed] [Google Scholar]
- Horstman AM, Beltman MJ, Gerrits KH, Koppe P, Janssen TW, Elich P, et al. Intrinsic muscle strength and voluntary activation of both lower limbs and functional performance after stroke. Clinical Physiology and Functional Imaging 2008;28(4):251‐61. [DOI] [PubMed] [Google Scholar]
- Hunt SM, McKenna SP, McEwen J, Backett EM, Williams J, Papp E. A quantitative approach to perceived health status: a validation study. Journal of Epidemiology and Community Health 1980;34(4):281‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indredavik B, Rohweder GI, Naalsund E, Lydersen S. Medical complications in a comprehensive stroke unit and an early supported discharge service. Stroke 2008;39(2):414‐20. [DOI] [PubMed] [Google Scholar]
- Johnson EM. Has SF‐36's role physical measure fallen short?. Journal of Public Health Medicine 1999;21(2):234. [DOI] [PubMed] [Google Scholar]
- Karttunen AH, Sjogren T, Paltamaa J, Heinonen A. Evidence for the effectiveness of walking training on walking and self‐care after stroke: a systematic review and meta‐analysis of randomized controlled trials. Journal of Rehabilitation Medicine 2014;46(5):387‐99. [DOI] [PubMed] [Google Scholar]
- Keysor JJ, Jette AM. Have we oversold the benefit of late‐life exercise?. Journals of Gerontology Series A ‐ Biological Sciences and Medical Sciences 2001;56(7):M412‐23. [DOI] [PubMed] [Google Scholar]
- Kortebein P, Symons TB, Ferrando A, Paddon‐Jones D, Ronsen O, Protas E, et al. Functional impact of 10 days of bed rest in healthy older adults. Journals of Gerontology Series A ‐ Biological Sciences and Medical Sciences 2008;63(10):1076‐81. [DOI] [PubMed] [Google Scholar]
- Kurl S, Laukkanen JA, Rauramaa R, Lakka TA, Sivenius J, Salonen JT. Cardiorespiratory fitness and the risk for stroke in men. Archives of Internal Medicine 2003;163(14):1682‐8. [DOI] [PubMed] [Google Scholar]
- Kwakkel G, Kollen BJ, Wagenaar RC. Long term effects of intensity of upper and lower limb training after stroke: a randomised trial. Journal of Neurology, Neurosurgery, and Psychiatry 2002;72(4):473‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakkel G, Peppen R, Wagenaar RC, Wood Dauphinee S, Richards C, Ashburn A, et al. Effects of augmented exercise therapy time after stroke: a meta‐analysis. Stroke 2004;35(11):2529‐39. [DOI] [PubMed] [Google Scholar]
- Langhorne P, Stott DJ, Robertson L, MacDonald J, Jones L, McAlpine C, et al. Medical complications after stroke: a multicenter study. Stroke 2000;31(6):1223‐9. [DOI] [PubMed] [Google Scholar]
- Langhorne P. Intensity of rehabilitation: some answers and more questions?. Journal of Neurology, Neurosurgery, and Psychiatry 2002;72(4):430‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurology 2009;8(8):741‐54. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self‐maintaining and instrumental activities of daily living. Gerontologist 1969;9(3):179‐86. [PubMed] [Google Scholar]
- Lee CD, Blair SN. Cardiorespiratory fitness and stroke mortality in men. Medicine and Science in Sports and Exercise 2002;34(4):592‐5. [DOI] [PubMed] [Google Scholar]
- Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk: a meta‐analysis. Stroke 2003;34(10):2475‐81. [DOI] [PubMed] [Google Scholar]
- Lee YH, Yoon ES, Park SH, Heffernan KS, Lee C, Jae SY. Associations of arterial stiffness and cognitive function with physical fitness in patients with chronic stroke. Journal of Rehabilitation Medicine 2014;46(5):413‐7. [DOI] [PubMed] [Google Scholar]
- Lennon O, Galvin R, Smith K, Doody C, Blake C. Lifestyle interventions for secondary disease prevention in stroke and transient ischaemic attack: a systematic review. European Journal of Preventive Cardiology 2014;21(8):1026‐39. [DOI] [PubMed] [Google Scholar]
- Lindley R, Waddell F, Livingstone M. Can simple questions assess outcome after stroke?. Cerebrovascular Diseases 1994;4:314‐24. [Google Scholar]
- Mackay‐Lyons M, Thornton M, Ruggles T, Che M. Non‐pharmacological interventions for preventing secondary vascular events after stroke or transient ischemic attack. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD008656.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macko RF, Smith GV, Dobrovolny CL, Sorkin JD, Goldberg AP, Silver KH. Treadmill training improves fitness reserve in chronic stroke patients. Archives of Physical Medicine and Rehabilitation 2001;82(7):879‐84. [DOI] [PubMed] [Google Scholar]
- Malbut KE, Dinan S, Young A. Aerobic training in the 'oldest old': the effect of 24 weeks of training. Age and Ageing 2002;31(4):255‐60. [DOI] [PubMed] [Google Scholar]
- Marsden DL, Dunn A, Callister R, Levi CR, Spratt NJ. Characteristics of exercise training interventions to improve cardiorespiratory fitness after stroke: a systematic review with meta‐analysis. Neurorehabilitation and Neural Repair 2013;27(9):775‐88. [DOI] [PubMed] [Google Scholar]
- Mead G. Exercise or relaxation after stroke?. BMJ 2005;330:1337. [Google Scholar]
- Mead G, Bernhardt J. Physical fitness training after stroke, time to implement what we know: more research is needed. International Journal of Stroke 2011;6:506‐8. [DOI] [PubMed] [Google Scholar]
- Mehrholz J, Pohl M, Elsner B. Treadmill training and body weight support for walking after stroke. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD002840.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S, Pereira S, Janzen S, Mays R, Viana R, Lobo L, et al. Cardiovascular conditioning for comfortable gait speed and total distance walked during the chronic stage of stroke: a meta‐analysis. Topics in Stroke Rehabilitation 2012;19(6):463‐70. [DOI] [PubMed] [Google Scholar]
- Mehta S, Pereira S, Viana R, Mays R, McIntyre A, Janzen S, et al. Resistance training for gait speed and total distance walked during the chronic stage of stroke: a meta‐analysis. Topics in Stroke Rehabilitation 2012;19(6):471‐8. [DOI] [PubMed] [Google Scholar]
- Michael K, Macko RF. Ambulatory activity intensity profiles, fitness, and fatigue in chronic stroke. Topics in Stroke Rehabilitation 2007;14(2):5‐12. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society 2005;53(4):695‐9. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Medicine and Science in Sports and Exercise 2007;39:1435‐45. [DOI] [PubMed] [Google Scholar]
- O'Donovan G, Blazevich AJ, Boreham C, Cooper AR, Crank H, Ekelund U, et al. The ABC of physical activity for health: a consensus statement from the British Association of Sport and Exercise Sciences. Journal of Sports Sciences 2010;28(6):573‐91. [DOI] [PubMed] [Google Scholar]
- Pang MYC, Charlesworth SA, Lau RWK, Chung RCK. Using aerobic exercise to improve health outcomes and quality of life in stroke: evidence‐based exercise prescription recommendations. Cerebrovascular Diseases 2013;35(1):7‐22. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Forrester LW, Rodgers MM, Ryan AS, Ivey FM, Sorkin JD, et al. Determinants of walking function after stroke: differences by deficit severity. Archives of Physical Medicine and Rehabilitation 2007;88(1):115‐9. [DOI] [PubMed] [Google Scholar]
- Patterson S, Ross‐Edwards B. Long‐term stroke survivors' needs and perceptions of an exercise maintenance model of care. International Journal of Therapy and Rehabilitation 2009;16(12):659‐69. [Google Scholar]
- Pereira S, Viana R, Mays R, McIntyre A, Janzen S, Teasell RW. Resistance training for gait speed and total distance walked during the chronic stage of stroke: a meta‐analysis. Topics in Stroke Rehabilitation 2012;19(6):471‐8. [DOI] [PubMed] [Google Scholar]
- Pereira S, Janzen S, Mays R, Viana R, Lobo L, Teasell RW. Cardiovascular conditioning for comfortable gait speed and total distance walked during the chronic stage of stroke: a meta‐analysis. Topics in Stroke Rehabilitation 2012;19(6):463‐70. [DOI] [PubMed] [Google Scholar]
- Peurala SH, Karttunen AH, Sjogren T, Paltamaa J, Heinonen A. Evidence for the effectiveness of walking training on walking and self‐care after stroke: a systematic review and meta‐analysis of randomized controlled trials. Journal of Rehabilitation Medicine 2014;46(5):387‐99. [DOI] [PubMed] [Google Scholar]
- Ploughman M, Austin MW, Glynn L, Corbett D. The effects of poststroke aerobic exercise on neuroplasticity: a systematic review of animal and clinical studies. Translational Stroke Research 2014;6(1):13‐28. [DOI] [PubMed] [Google Scholar]
- Pohl M, Elsner B. Treadmill training and body weight support for walking after stroke. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD002840.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polese JC, Ada L, Dean CM, Nascimento LR, Teixeira‐Salmela LF. Treadmill training is effective for ambulatory adults with stroke: a systematic review. Journal of Physiotherapy 2013;59(2):73‐80. [DOI] [PubMed] [Google Scholar]
- Pollock A, George B, Fenton M, Firkins L. Top ten research priorities relating to life after stroke. Lancet Neurology 2012;11:209. [DOI] [PubMed] [Google Scholar]
- Pollock A, Gray C, Culham E, Durward BR, Langhorne P. Interventions for improving sit‐to‐stand ability following stroke. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD007232.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthoff ML, Nielsen DH. Relationships among impairments in lower‐extremity strength and power, functional limitations, and disability in older adults. Physical Therapy 2007;87(10):1334‐47. [DOI] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. Journal of Clinical and Experimental Neuropsychology 1998;20(3):310‐9. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Guralnik JM, Ferrucci L, Penninx BWJH, Leveille S, Sipila S, et al. Coimpairments as predictors of severe walking disability in older women. Journal of the American Geriatrics Society 2001;49(1):21‐7. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Rimer J, Dwan K, Lawlor DA, Greig CA, McMurdo M, Morley W, et al. Exercise for depression. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD004366.pub5] [DOI] [PubMed] [Google Scholar]
- Rodrigues‐Baroni JM, Nascimento LR, Ada L, Teixeira‐Salmela LF. Walking training associated with virtual reality‐based training increases walking speed of individuals with chronic stroke: systematic review with meta‐analysis. Brazilian Journal of Physical Therapy 2014;18(6):502‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders DH, Greig CA, Young A, Mead GE. Association of activity limitations and lower‐limb explosive extensor power in ambulatory people with stroke. Archives of Physical Medicine and Rehabilitation 2008;89(4):677‐83. [DOI] [PubMed] [Google Scholar]
- Saunders DH, Greig CA. Physical fitness and function After stroke. In: Mead GE, Wijck F editor(s). Exercise and Fitness Training after Stroke ‐ A Handbook for Evidence‐based Practice. 1st Edition. London: Churchill Livingston Elsevier, 2013:77‐91. [Google Scholar]
- Saunders DH, Greig CA, Mead GE. Physical activity and exercise after stroke: review of multiple meaningful benefits. Stroke 2014;45(12):3742‐7. [DOI] [PubMed] [Google Scholar]
- Shaw KA, Gennat HC, O'Rourke P, Mar C. Exercise for overweight or obesity. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003817.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard RJ. Maximal oxygen intake and independence in old age. British Journal of Sports Medicine 2009;43(5):342‐6. [DOI] [PubMed] [Google Scholar]
- Slade SC, Dionne CE, Underwood M, Buchbinder R. Standardised method for reporting exercise programmes: protocol for a modified Delphi study. BMJ Open 2014;4(12):e00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Saunders DH, Mead G. Cardiorespiratory fitness after stroke: a systematic review. International Journal of Stroke 2012;7:499‐510. [DOI] [PubMed] [Google Scholar]
- Sorinola IO, Powis I, White CM. Does additional exercise improve trunk function recovery in stroke patients? A meta‐analysis. NeuroRehabilitation 2014;35(2):205‐13. [DOI] [PubMed] [Google Scholar]
- Stoller O, Bruin ED, Knols RH, Hunt KJ. Effects of cardiovascular exercise early after stroke: systematic review and meta‐analysis. BMC Neurology 2012;12(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD002968.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion. Physical Activity Guidelines Advisory Committee Report. Atlanta: Centers for Disease Control and Prevention, 2008. [Google Scholar]
- Veerbeek JM, Koolstra M, Ket JCF, Wegen EEH, Kwakkel G. Effects of augmented exercise therapy on outcome of gait and gait‐related activities in the first 6 months after stroke: a meta‐analysis. Stroke 2011;42(11):3311‐5. [DOI] [PubMed] [Google Scholar]
- Wade DT. Measurement in Neurological Rehabilitation. Oxford University Press, 1992. [PubMed] [Google Scholar]
- Wendel‐Vos GCW, Schuit AJ, Feskens EJM, Boshuizen HC, Verschuren WMM, Saris WHM, et al. Physical activity and stroke. A meta‐analysis of observational data. International Journal of Epidemiology 2004;33(4):787‐98. [DOI] [PubMed] [Google Scholar]
- International Classification of Functional Disability and Handicap. World Health Organization (WHO). Fifty‐Fourth World Health Assembly WHA54.21, 2001. http://www.who.int/classifications/icf/en/ (accessed November 2010).
- Young A. For a healthier old age. In: Young A, Harries M editor(s). Physical Activity for Patients: An Exercise Prescription. London: Royal College of Physicians, 2001. [Google Scholar]
- Zigmond AS, Snaith PR. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67:361‐70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Brazzelli M, Saunders DH, Greig CA, Mead GE. Physical fitness training for stroke patients: a systematic review of the evidence. International Journal of Stroke2011; Vol. 6:11. [DOI] [PubMed]
- Brazzelli M, Saunders DH, Greig CA, Mead GE. Physical fitness training for stroke patients. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD003316.pub4] [DOI] [PubMed] [Google Scholar]
- Brazzelli M, Saunders DH, Greig CA, Mead GE. Physical fitness training for patients with stroke updated review. Stroke 2012;43(4):E39‐40. [DOI] [PubMed] [Google Scholar]
- Saunders DH, Greig CA, Young A, Mead GE. Physical fitness training for acute stroke patients ‐ a systematic review. Cerebrovascular Diseases 2002;13 Suppl 3:63. [Google Scholar]
- Saunders DH, Greig C, Young A, Mead G. Physical fitness training for stroke patients. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003316.pub2] [DOI] [PubMed] [Google Scholar]
- Saunders DH, Greig C, Young A, Mead G. Physical fitness training for stroke patients. Stroke 2004;35:2235. [DOI] [PubMed] [Google Scholar]
- Saunders DH, Greig CA, Mead GE, Young A. Physical fitness training for stroke patients. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003316.pub3] [DOI] [PubMed] [Google Scholar]
- Saunders David H, Sanderson Mark, Brazzelli Miriam, Greig Carolyn A, Mead Gillian E. Physical fitness training for stroke patients. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD003316.pub5] [DOI] [PubMed] [Google Scholar]
- Saunders DH, Sanderson M, Brazzelli M, Greig CA, Mead GE. Physical fitness training for patients with stroke: an updated review. Stroke 2014;45:e45‐e55. [DOI] [PubMed] [Google Scholar]


