Abstract
Background
Chronic lung disease (CLD) remains a common complication among preterm infants. There is increasing evidence that inflammation plays an important role in the pathogenesis of CLD. Due to their strong anti‐inflammatory properties, corticosteroids are an attractive intervention strategy. However, there are growing concerns regarding short‐ and long‐term effects of systemic corticosteroids. Theoretically, administration of inhaled corticosteroids may allow for beneficial effects on the pulmonary system with a lower risk of undesirable systemic side effects.
Objectives
To determine the impact of inhaled corticosteroids administered to preterm infants with birth weight up to 1500 grams (VLBW) beginning in the first two weeks after birth for the prevention of CLD as reflected by the requirement for supplemental oxygen at 36 weeks' postmenstrual age (PMA).
Search methods
Randomised and quasi‐randomised trials were identified by searching the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 12) in the Cochrane Library (searched 5 January 2016), MEDLINE (1966 to 5 January 2016), Embase (1980 to 5 January 2016), CINAHL (1982 to 5 January 2016), reference lists of published trials and abstracts published in Pediatric Research or electronically on the Pediatric Academic Societies web‐site (1990 to May 2016).
Selection criteria
We included in this review randomised controlled trials of inhaled corticosteroid therapy initiated within the first two weeks of life in VLBW preterm infants.
Data collection and analysis
We evaluated data regarding clinical outcomes, including: CLD at 28 days or 36 weeks' PMA; mortality; combined outcome of death or CLD at 28 days of age and at 36 weeks' PMA; the need for systemic corticosteroids; failure to extubate within 14 days; and adverse effects of corticosteroids. All data were analysed using Review Manager (RevMan) 5. Meta‐analyses were performed using relative risk (RR) and risk difference (RD), along with their 95% confidence intervals (CI). If RD was significant, the number needed to treat for an additional beneficial outcome (NNTB) was calculated. We used the GRADE approach to assess the quality of evidence.
Main results
According to GRADE the quality of the studies was moderate. Three additional trials are included in this update. The present review includes data analyses based on 10 qualifying trials that enrolled 1644 neonates. There was no significant difference in the incidence of CLD at 36 weeks' PMA in the inhaled steroid versus the placebo group (5 trials, 429 neonates) among all randomised (typical RR 0.97, 95% CI 0.62 to 1.52; typical RD −0.00, 95% CI −0.07 to 0.06). There was no heterogeneity for this outcome (typical RR I² = 11%; typical RD I² = 0%). There was a significant reduction in the incidence of CLD at 36 weeks' PMA among survivors (6 trials, 1088 neonates) (typical RR 0.76, 95% CI 0.63 to 0.93; typical RD −0.07, 95% CI −0.13 to −0.02; NNTB 14, 95% CI 8 to 50). There was a significant reduction in the combined outcome of death or CLD at 36 weeks' PMA among all randomised neonates (6 trials, 1285 neonates) (typical RR 0.86, 95% CI 0.75 to 0.99; typical RD −0.06, 95% CI −0.11 to −0.00) (P = 0.04); NNTB 17, 95% CI 9 to infinity). There was no significant heterogeneity for any of these analyses (I² = 0%). A lower rate of reintubation was noted in the inhaled steroid group compared with the control group in one study. There were no statistically significant differences in short‐term complications between groups and no differences in adverse events at long‐term follow‐up reported. Long‐term follow‐up of infants enrolled in the study by Bassler 2015 is ongoing.
Authors' conclusions
Based on this updated review, there is increasing evidence from the trials reviewed that early administration of inhaled steroids to VLBW neonates is effective in reducing the incidence of death or CLD at 36 weeks' PMA among either all randomised infants or among survivors. Even though there is statistical significance, the clinical relevance is of question as the upper CI limit for the outcome of death or CLD at 36 weeks' PMA is infinity. The long‐term follow‐up results of the Bassler 2015 study may affect the conclusions of this review. Further studies are needed to identify the risk/benefit ratio of different delivery techniques and dosing schedules for the administration of these medications. Studies need to address both the short‐ and long‐term benefits and adverse effects of inhaled steroids with particular attention to neurodevelopmental outcome.
Plain language summary
Early administration of inhaled corticosteroids for preventing chronic lung disease in very low birth weight preterm neonates
Review question Do inhaled corticosteroids administered to preterm infants with birth weight of up to 1500 grams beginning in the first two weeks after birth prevent chronic lung disease as reflected by the requirement for supplemental oxygen at 36 weeks' postmenstrual age?
Background Preterm babies who require breathing support often develop chronic lung disease. It is thought that inflammation in the lungs may be part of the cause. Corticosteroid drugs when given orally or through a vein reduce this inflammation. However, the use of corticosteroids is associated with serious side effects including cerebral palsy (motor problem) and developmental delay. It is possible that inhaling steroids, so that the drug directly reaches the lung, may reduce the adverse effects. This review looked at trials that compared preterm babies who received steroids or placebo (inactive drug) by inhalation while they were receiving breathing support. There was no previous evidence that the early administration of inhaled steroids reduces chronic lung disease.
Study characteristics From literature searches updated to 5 January 2016, 10 randomised controlled trials that enrolled 1644 infants were included.
Study funding sources We are not aware of any financial support from the industry for the included studies.
Key results In this update of the review there was no significant reduction in the rate of chronic lung disease at 36 weeks' postmenstrual age. A significant reduction in the combined outcome of death or chronic lung disease at 36 weeks' postmenstrual age among all randomised neonates and among survivors was noted. Even though the results were significant, the upper confidence interval was infinity (i.e. we would have to treat every baby with inhaled steroid to prevent one baby dying or developing chronic lung disease at 36 weeks' postmenstrual age). This would not be acceptable in clinical practice. A lower rate of reintubation (the need for the insertion of a tube into the airway) was noted in the steroid group compared with the control group in one large study. There were no statistically significant differences in short‐ and long‐term complications between groups. The results of the long‐term follow‐up of one large study is awaited.
Quality of evidence In general the quality of the studies was good.
Summary of findings
Background
Description of the condition
Chronic lung disease (CLD) remains a common complication among survivors of neonatal intensive care. Despite the use of antenatal corticosteroids and postnatal surfactant treatment, the incidence of CLD has increased. This is partly explained by increased survival of extremely low birth weight infants (Shaw 1993), in that the incidence of CLD has an inverse relationship with birth weight and gestational age (Sinkin 1990).
O'Brodovich and Mellins have used the term "unresolved neonatal acute lung injury" to describe CLD (O'Brodovich 1985). According to their model, CLD results from disordered repair processes in a susceptible baby following acute lung injury induced by various pulmonary disorders and positive pressure ventilation. Preventive strategies can focus on any point in the cascade, including prevention or amelioration of the primary pulmonary disorders affecting preterm babies, reduction in ventilator‐induced lung injury or modification of the response to tissue injury (Sinkin 1987).
Description of the intervention
There is increasing evidence that inflammation plays an important role in the pathogenesis of CLD (Pierce 1995). In many infants, an inflammatory reaction is evident shortly after birth suggesting that the process may have been triggered in utero (Watterberg 1996). Therefore, interventions aimed at reducing or modulating the inflammatory process may reduce the incidence or severity of CLD.
How the intervention might work
Treatment with corticosteroids is an attractive intervention strategy to achieve this goal due to their strong anti‐inflammatory properties. Systematic reviews on the early use of postnatal systemic corticosteroids (≤ 7 days) and the late use of postnatal systemic corticosteroids (> 7 days) have demonstrated a reduction in CLD at 28 days and 36 weeks' postmenstrual age (PMA) (Bhuta 1998; Doyle 2014a; Doyle 2014b). Doyle 2014a and Doyle 2014b concluded that the benefits of early and postnatal use of corticosteroids may not outweigh actual or potential adverse effects. There are concerns regarding the short‐ and long‐term adverse effects of systemic corticosteroid therapy in this population (Ng 1993; Yeh 1998; Doyle 2014b). These include hyperglycaemia, hypertension, hypertrophic obstructive cardiomyopathy, gastrointestinal haemorrhage and perforation, growth failure and hypothalamic‐pituitary‐adrenal axis suppression. The potential effects on brain growth and neurodevelopment are most alarming: studies in animals have shown that steroids can permanently affect brain cell division, differentiation and myelination, as well as the ontogeny of cerebral cortical development (Weichsel 1977; Johnson 1979). As both dexamethasone and hydrocortisone administration within the first seven days of life is associated with an increased risk of cerebral palsy, early postnatal corticosteroid therapy is not recommended to prevent CLD. After seven days of life, dexamethasone has been shown to decrease the rate of CLD at 36 weeks’ postmenstrual age with less impact on neurodevelopmental outcome. No trials have examined whether the benefits of corticosteroids outweigh the adverse effects for infants at high risk of, or with, severe CLD (Jefferies 2012). Follow‐up of double‐blind randomised controlled trials of dexamethasone, commenced within 12 hours of birth, showed a two‐fold increase in neuromotor impairments in surviving dexamethasone‐treated infants as compared with controls at two years' corrected age (Yeh 1998; Doyle 1999). Two meta‐regression meta‐analyses have concluded that the effect of postnatal corticosteroids on the combined outcome of death or cerebral palsy varies with the level of risk for CLD (Doyle 2005; Doyle 2014c). Theoretically, administration of corticosteroids topically may allow for beneficial effects on the pulmonary system with a lower risk of undesirable systemic side effects.
Why it is important to do this review
A variety of Cochrane reviews address the use of systemic or inhaled corticosteroids in the prevention or treatment of bronchopulmonary dysplasia (BPD) or CLD.
These include reviews of the early use (≤ 7 days) of systemic postnatal corticosteroids to prevent chronic lung disease (Doyle 2014a), as well as the late use (> 7 days) of systemic postnatal corticosteroids for CLD (Doyle 2014b).
In addition, a variety of reviews address the use of inhaled corticosteroids in the prevention or treatment of CLD. Onland and colleagues have reviewed the late use (≥ 7 days) of inhaled corticosteroids to reduce BPD in preterm infants (Onland 2012). Shah and colleagues have compared the use of inhaled versus systemic corticosteroids for preventing CLD in ventilated very low birth weight (VLBW) preterm neonates (Shah 2003); and the use of inhaled versus systemic corticosteroids for the treatment of CLD in ventilated VLBW preterm infants (Shah 2007b).
The use of corticosteroids for other indications in neonates including intravenous dexamethasone to facilitate extubation (Davis 2001), corticosteroids for the treatment of hypotension (Ibrahim 2011), and corticosteroids for the treatment of meconium aspiration syndrome (Ward 2003) are reviewed.
This review aims to examine the impact of inhaled corticosteroid therapy when administered to VLBW preterm infants within the first two weeks of life for the prevention of CLD. This is an update of our review in 2007 (Shah 2007a), and in 2012 (Shah 2012).
Objectives
Primary objective: to determine the impact of inhaled corticosteroids administered to preterm infants with birth weight up to 1500 grams (VLBW) beginning in the first two weeks after birth for the prevention of CLD as reflected by the requirement for supplemental oxygen at 36 weeks' PMA.
Secondary objectives: assessment of the effect of inhaled corticosteroids on:
1. Other indicators of CLD including:
requirement for supplemental oxygen at 28 days of age;
death by, or CLD at, 28 days of age;
death by, or CLD at, 36 weeks' PMA;
duration of requirement for supplemental oxygen;
duration of assisted ventilation;
requirement for systemic corticosteroids;
change in pulmonary function tests.
2. The incidence of adverse events including:
mortality;
hyperglycaemia;
culture‐proven infection (positive blood or cerebrospinal fluid (CSF); or positive blood culture only) during hospital stay;
hypertension;
hypertrophic obstructive cardiomyopathy;
gastrointestinal haemorrhage or perforation;
growth (weight, length/height and head circumference);
cataracts;
hypertrophy of tongue;
nephrocalcinosis;
suppression of the hypothalamic‐pituitary‐adrenal axis.
3. Long‐term neurodevelopmental outcome: neurodevelopmental impairment is defined as presence of cerebral palsy and/or mental retardation (Bayley Scales of Infant Development (BSID) Mental Development Index (MDI) < 70 and/or legal blindness (< 20/200 visual acuity) and/or deafness (aided or < 60 dB on audiometric testing) assessed at 18 to 24 months.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised clinical trials of inhaled corticosteroid therapy in very low birth weight (VLBW) preterm infants enrolled in the first two weeks after birth (early administration). Studies which evaluated a combination of systemic and inhaled corticosteroids were excluded.
Types of participants
Preterm neonates with birth weight up to 1500 grams (VLBW) and postnatal age of less than 2 weeks.
Types of interventions
Inhaled corticosteroids versus placebo or no intervention.
Types of outcome measures
Primary outcomes
1. Chronic Lung Disease (CLD) at 36 weeks' postmenstrual age (PMA):
among all randomised;
among survivors.
Secondary outcomes
1. Among all randomised:
CLD at 28 days of age;
death by 28 days of age;
death by 36 weeks' PMA;
death by, or CLD at, 28 days of age;
death by, or CLD at, 36 weeks' PMA;
death during hospital stay (this outcome was added in the 2016 update of the review);
survival to hospital discharge without CLD (this outcome was added in the 2016 update of the review);
requirement for systemic steroids;
failure to extubate within 14 days;
adverse events: culture‐proven infection (blood or CSF), hyperglycaemia, hypertension, gastrointestinal bleeding, cataracts, intraventricular haemorrhage (IVH), periventricular leukomalacia (PVL), brain injury*, necrotizing enterocolitis (NEC), retinopathy of prematurity (ROP), pituitary‐adrenal suppression, and patent ductus arteriosus (PDA).
2. Among survivors:
CLD at 28 days of age.
3. Long‐term neurodevelopmental outcome: neurodevelopmental impairment is defined as presence of cerebral palsy and/or mental retardation (Bayley Scales of Infant Development (BSID), Mental Development Index (MDI) < 70) and/or legal blindness (< 20/200 visual acuity) and/or deafness (aided or < 60 dB on audiometric testing) assessed at 18 to 24 months.
* post hoc outcome
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register).
Electronic searches
For the 2016 update, we conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 12) in the Cochrane Library (searched 5 January 2016); MEDLINE via PubMed (1966 to 5 January 2016); Embase (1980 to 5 January 2016); and CINAHL (1982 to 5 January 2016) using the following search terms: (bronchopulmonary dysplasia OR lung diseases OR chronic lung disease) AND (anti‐inflammatory agents OR steroids OR dexamethasone OR inhalation OR aerosols OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate)), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). We did not apply language restrictions. We searched clinical trials' registries for ongoing or recently completed trials (ClinicalTrials.gov; the World Health Organization’s International Trials Registry and Platform www.whoint/ictrp/search/en/; and the ISRCTN Registry).
For the 2011 update, randomised controlled trials of inhaled corticosteroid therapy in preterm neonates were identified from MEDLINE via PubMed using MeSH headings: infant‐newborn; bronchopulmonary dysplasia; lung diseases; anti‐inflammatory agents; steroids; administration; inhalation; aerosols; budesonide; beclomethasone dipropionate; flunisolide; and fluticasone propionate. We searched Embase and CINAHL. See Appendix 2 for the complete search strategies.
Searching other resources
We searched the reference lists of published trials. One trial was identified through an additional search of MEDLINE in May 2016 for reasons unrelated to this review (Nakamura 2016).
Data collection and analysis
We used the methods of the Cochrane Neonatal Review Group for data collection and analysis.
Selection of studies
We included all randomised and quasi‐randomised controlled trials that fulfilled the selection criteria described in the previous section. We would have included cluster randomised trials if they had been identified. The review authors independently reviewed the results of the updated search and selected studies for inclusion. We resolved any disagreement by discussion.
Data extraction and management
We sought information regarding the method of randomisation, blinding and reporting of all outcomes of all the infants enrolled in the trial for each trial. We obtained data from the primary investigator for unpublished trials or when published data were incomplete. We assessed retrieved articles and three review authors (VS, MD, AO) abstracted data independently. The updates of the review in 2012 and 2016 were performed by two review authors (VS, AO).
For each study, final data were entered into Review Manager 5 by one review author (AO) and then checked for accuracy by a second reviewer author (VS). We resolved discrepancies through discussion.
We attempted to contact authors of the original reports to provide further details when information regarding any of the above was unclear.
Assessment of risk of bias in included studies
The review authors independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool (Higgins 2011) for the following domains:
selection bias;
performance bias;
detection bias;
attrition bias;
reporting bias;
or any other bias.
We resolved any disagreements by discussion or, in the event of deadlock, by a third assessor's adjudication. See Appendix 3 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We performed statistical analyses using Review Manager software (RevMan 2014). Dichotomous data were analysed using relative risk (RR), risk difference (RD) and the number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH). The 95% CI were reported on all estimates.
We analysed continuous data using mean difference (MD) or, if applicable, the standardized mean difference, to combine trials that measured the same outcome but used different methods.
Unit of analysis issues
We analysed the data as the proportion of neonates having one or more episodes for clinical outcomes such as episodes of sepsis.
Dealing with missing data
For included studies, levels of attrition were noted. The impact of including studies with high levels of missing data in the overall assessment of treatment effect was explored by using sensitivity analysis.
All outcomes analyses were on an intention‐to‐treat basis, i.e. we included all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We examined heterogeneity between trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I² statistic. If noted, we planned to explore the possible causes of statistical heterogeneity using pre‐specified subgroup analysis (for example, differences in study quality, participants, intervention regimens, or outcome assessments).
Based on the results of the I² statistic we used the following cut‐offs and labels for heterogeneity: less than 25% – no heterogeneity; 25% to 49% – low heterogeneity; 50% to 74% – moderate heterogeneity; and 75% or above – high heterogeneity.
Assessment of reporting biases
We planned to assess possible publication bias and other biases using symmetry/asymmetry of funnel plots if there had been 10 or more trials included in an analysis.
For included trials that were recently performed (and therefore prospectively registered), we explored possible selective reporting of study outcomes by comparing the primary and secondary outcomes in the reports with the primary and secondary outcomes proposed at trial registration, using the web sites ClinicalTrials.gov and Controlled‐Trials.com. If such discrepancies were found, we planned to contact the primary investigators to obtain missing outcome data on outcomes pre‐specified at trial registration.
Data synthesis
Meta‐analysis was done using Review Manager software (RevMan 2014), supplied by Cochrane. We used the Mantel‐Haenszel method for estimates of typical relative risk and risk difference. We analysed continuous measures using the inverse variance method.
We used the fixed‐effect model for all meta‐analyses.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: chronic lung disease at 36 weeks' PMA and death and CLD at 36 weeks' PMA amongst all randomised and amongst survivors.
Two authors independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro 2014 Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades:
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
Groups were analysed based on 'all randomised' and 'survivors only'.
Sensitivity analysis
We planned sensitivity analyses for situations where this might affect the interpretation of significant results (for example, where there is risk of bias associated with the quality of some of the included trials or missing outcome data). None was thought necessary in this review.
Results
Description of studies
Results of the search
For the results of our literature searches see Study flow diagram (Figure 1).
1.

Study flow diagram.
For this update of the review two additional trials were identified (Bassler 2015; Nakamura 2016). The study by Denjean 1998 was moved from excluded to included studies.
Fourteen trials assessing the impact of inhaled corticosteroids were identified, of which four trials were excluded (Kovacs 1998; Beresford 2002; Dugas 2005; Yeh 2016).
Ten trials qualified for inclusion in this review: Denjean 1998; Townsend 1998; Yong 1999; Merz 1999; Fok 1999; Cole 1999; Jonsson 2000; Jangaard 2002; Bassler 2015; and Nakamura 2016. Eight included studies have been published as complete articles while two studies were presented at scientific meetings and published in abstract form (Townsend 1998; Yong 1999). Complete data from the investigators were available for Yong 1999, while information published in abstract form is presented for Townsend 1998.
Although all studies attempted to include infants thought to be at risk of developing CLD, the inclusion criteria, the intervention (type of inhaled corticosteroid) and duration of therapy varied between studies. All studies except Jonsson 2000 used a metered dose inhaler (MDI) and an Aerochamber interposed between the endotracheal tube and the ventilatory circuit or a manual puffer (anaesthesia bag). In Cole 1999, the drug was administered to infants who were extubated using the same procedure through a nasopharyngeal tube. In Jonsson 2000, a dosimetric jet nebulizer was used to deliver the aerosol.
Details of each study are given in the table 'Characteristics of included studies'.
Included studies
See 'Characteristics of included studies'.
Ten trials qualified for inclusion in this review: Denjean 1998; Townsend 1998; Yong 1999; Merz 1999; Fok 1999; Cole 1999; Jonsson 2000; Jangaard 2002; Bassler 2015; and Nakamura 2016.
Bassler 2015: the study by Bassler and co‐workers enrolled 863 infants with postmenstrual age (PMA) 230/7 weeks to 276/7 weeks and chronological age of 12 hours or less, who required any form of positive pressure support. Exclusion criteria were: palliative care; dysmorphic feature or congenital abnormalities likely to affect life expectancy or neurologic development; strongly suspected cyanotic heart disease; and, to prevent a correlated data problem, the infant was from a multiple‐birth pregnancy (other than the second infant in birth order, who was considered eligible). The budesonide group (n = 441; 437 followed to first discharge home) received two puffs of budesonide (200 µg/puff) administered every 12 hours for the first 14 days of life and one puff administered every 12 hours from day 15 until the last dose of study drug had been administered. Study drugs were administered until infants no longer needed supplemental oxygen and positive pressure support or reached a PMA of 320/7 weeks, regardless of ventilator status. The control group (n = 422; 419 followed to first discharge home) received two puffs of placebo containing only hydrofluoroalkane propellant.
Primary outcome: a composite of death or bronchopulmonary dysplasia (BPD) at 36 weeks' PMA. BPD was defined as the requirement for positive pressure support, the requirement for supplemental oxygen at FiO₂ exceeding 0.30, or in infants receiving low amounts of oxygen, an inability to maintain an oxygen saturation value above 90% during a structured, short period of saturation monitoring coupled with gradual weaning from oxygen to ambient air (oxygen reduction test).
Secondary outcomes included: death by any cause at 36 weeks' PMA, BPD at 36 weeks' PMA (defined as per above); duration of positive pressure respiratory support or supplemental oxygen; ventriculomegaly with or without intraventricular haemorrhage (IVH) on ultrasound at or before 36 weeks' PMA; patent ductus arteriosus (PDA) requiring medical or surgical treatment; intestinal perforation or necrotizing enterocolitis (NEC); retinopathy of prematurity (ROP) (stage 2 or higher); culture‐proven infections; increase in body weight and head circumference from birth to day 28; length of hospital stay; need for reintubation after the last dose of drug had been administered; occurrence of oral candidiasis requiring treatment; hyperglycaemia requiring insulin treatment; and hypertension requiring treatment.
Neurodevelopmental disability testing is to be conducted at 18 to 22 months (hence is not reported in this current publication).
Cole 1999: the study by Cole and co‐workers enrolled infants of less than 33 weeks gestational age (GA) and with birth weight equal up to 1250 grams who required assisted ventilation at three to 14 days of life. The infants were randomly assigned to receive inhaled beclomethasone dipropionate (n = 123) or placebo (n = 130). The desired dose was calculated to deliver to the lung 40 µg/kg/day for the first week, 30 and 15 µg/kg/day for the second and third weeks respectively, and then 10 and 5 µg/kg/day during the fourth week. The primary outcome was the incidence of BPD in survivors defined as an abnormal chest x‐ray and need for supplemental oxygen at 28 days of life. Secondary outcomes included the incidence of BPD in survivors at 36 weeks' PMA (defined as an abnormal chest x‐ray and need for supplemental oxygen at that age), duration of respiratory support (oxygen, mechanical ventilation and continuous positive airway pressure), the need for systemic glucocorticoids, diuretic or bronchodilator therapy, death, length of hospitalisation and the incidence of complications possibly attributable to the use of inhaled steroids. Systemic glucocorticoid therapy was permitted at the discretion of the infant's physician. The baseline characteristics of the two groups were similar except for maternal race or ethnic group (P = 0.03). Two additional reports from the same trial (Cole 1999) have been published as separate articles. One of these reports evaluated the effect of inhaled beclomethasone therapy on adrenal response and the other report evaluated its effect on tracheal aspirate inflammatory mediators (IL‐8 and IL‐1ra).
Denjean 1998: the study by Denjean and co‐workers enrolled infants with respiratory distress syndrome and PMA less than 31 weeks. Infants were eligible for the study if they required ventilator support (intermittent mandatory ventilation (IMV) or nasal IMV/continuous positive airway pressure (CPAP)) on the 10th postnatal day. 178 infants were randomised, five were withdrawn leaving 173 infants in the trial who were assigned to four groups (placebo + placebo; placebo + salbutamol; placebo + beclomethasone; beclomethasone + salbutamol) of which two (placebo + placebo and placebo + beclomethasone) are included in our review. Beclomethasone (250 µg) was given four times a day (1000 µg daily). Treatment was started on the 10th or 11th postnatal day and was given for 28 days, with dose tapering over a period of eight days. Total number reported on: n = 43. Placebo (250 µg) was given four times a day (1000 µg daily). Treatment was started on the 10th or 11th postnatal day and was given for 28 days, with dose tapering over a period of 8 days. Total number reported on: n = 43. Beclomethasone and placebo were administered by metered‐dose inhalers.
The main outcome criterion was CLD. The diagnosis of CLD was made at 28 days of age on the basis of clinical (oxygen dependence) and radiographic criteria. CLD was categorized in three grades of severity: severe: ventilation with endotracheal tube (ET) for more than three months or oxygen supplementation for more than four months; moderate: ventilation with ET for more than one month or oxygen supplementation for more than two months; and mild: ventilation with ET for less than one month and oxygen supplementation for less than two months. The definition of CLD was unclear. Secondary outcomes included: survival without CLD, death (during hospital stay), ventilatory support (nasal IMV, CPA or IMV) (days), need for supplementary oxygen (days), need for systemic dexamethasone (IV), and sepsis (positive blood culture).
Fok 1999: the study by Fok and co‐workers enrolled 53 infants born at less than 32 weeks' GA, birth weight less than 1.5 kg, requiring mechanical ventilation with an arterial PO₂/alveolar PO₂ ratio of less than 0.25 at 6 to 10 hours after the second dose of surfactant was administered. Infants were excluded if they needed high‐frequency ventilation at the time of enrolment. Infants were randomised to receive inhaled fluticasone propionate or placebo. Two puffs of fluticasone propionate (250 micrograms/puff) or placebo were administered 12 hourly for two weeks. The first dose was administered within 24 hours of birth. The primary outcome was successful extubation by day seven or day 14 of life. Secondary outcomes included mortality, oxygen dependency at 28 days of postnatal age and at 36 weeks' PMA. The incidence of complications possibly attributable to the use of inhaled steroids was monitored. Twenty‐seven infants were enrolled in the fluticasone propionate group and 26 in the placebo group. The two groups were similar in their demographic and perinatal characteristics.
Jangaard 2002: the study by Jangaard and co‐workers enrolled 60 preterm infants weighing less than 1250 grams with respiratory distress syndrome (RDS) and requiring ventilatory support at 72 hours of age. Infants were randomly assigned to receive inhaled beclomethasone dipropionate (250 µg/puff) or placebo for four weeks. Medication dosage assumed a 10% deposition of the administered dose with the aim to provide a total dose of 0.2 mg/kg/day. When the infants were extubated, the study drug was administered using an infant‐sized Aerochamber (Boehringer Ingelheim, Canada) with an appropriately fitted mask. The primary outcome for this study was BPD defined as oxygen dependency at 28 days of life. The demographic characteristics of the two groups were similar. Jangaard 2002 has been previously published in abstract form. The results of the published report are presented in this review.
Jonsson 2000: The study by Jonsson and co‐workers randomised 30 VLBW infants with median (range) GA of 26 weeks (23 to 29 weeks) and median birth weight of 805 g (525 to 1227 g). Inclusion criteria were 1) mechanical ventilation on day six of life, or 2) if extubated, nasal continuous positive airway pressure with FiO₂ of 0.3 or higher. Infants with the following conditions were excluded: congenital malformations, congenital heart disease and grades III‐IV IVH. Infants on high frequency oscillatory ventilation (HFOV) were excluded as the inhalations could not be given through the electronic dosimetric jet nebulizer. Infants were randomised to receive 500 µg twice a day or placebo delivered using a dosimetric jet nebulizer with variable inspiratory time and breath sensitivity. Inhalations were started on day seven of life. The primary objective was to attain a 30% reduction in FiO₂ levels in the budesonide treatment group after 14 days of therapy. Secondary outcomes included: duration of supplemental oxygen, duration of mechanical ventilation, duration of nasal CPAP, oxygen requirements at 28 days of age and at 36 weeks' postmenstrual age. Adrenal cortisol response to stimulation was measured at baseline (prior to commencement of inhalation) and at the end of the study period. Information on adverse events — hyperglycaemia, hypertension, sepsis, PDA, IVH and gastrointestinal problems — were collected. Of the 30 infants enrolled, one parent declined to participate and two eligible infants could not be included due to ongoing HFOV on day seven of life. Thirteen infants were enrolled in the budesonide group, of which eight were ventilated, while 14 infants were enrolled in the placebo group, of which nine were ventilated at the commencement of therapy. Only one outcome for ventilated infants was reported (successful extubation during the study period ‒ 14 days).
Merz 1999: the study by Merz and co‐workers enrolled 24 infants with a birth weight of 750 to 1500 grams, GA of 25 to 32 weeks, ventilator dependency on day three of life with a rate of 15 breaths/minute or more and FiO₂ of 0.25 or more to maintain oxygen saturation 90% or more. Infants were randomly assigned to inhaled budesonide (200 µg/puff) or placebo. Two puffs were administered four times a day for a total of 10 days. The primary outcome was duration of artificial ventilation. Secondary outcomes included the duration of supplemental oxygen and the release of albumin and different inflammatory mediators in the tracheobronchial aspirate fluid. Adverse events such as frequency of acute infections, hypertension, hyperglycaemia, and adrenal suppression were evaluated. The demographic and perinatal data were similar in both groups on the day of randomisation.
Nakamura 2016: The study by Nakamura and co‐workers enrolled 211 infants with birth weight of less than 1000 grams, who needed endotracheal intubation and respiratory support due to respiratory failure. Infants were randomised to prophylactic inhaled steroids starting within 24 hours of birth and continuing until six weeks of age or extubation. Two doses of 50 µg fluticasone propionate (FP) were administered every 24 hours. The placebo group received two doses of placebo every 24 hours. The placebo contained only hydrofluoroalkane propellant. The primary outcome was death or oxygen dependency at discharge from NICU. Secondary outcomes included death, severe BPD and neurodevelopmental outcomes at 18 months' PMA and three years of age. Complications of preterm birth (grade 3 or 4 IVH; periventricular leukomalacia (PVL); NEC; sepsis and ROP) were reported in combination with deaths.
Townsend 1998: The study by Townsend and co‐workers enrolled 32 infants with GA less than 28 weeks and birth weight up to 1100 grams who were ventilator dependent with RDS. Infants were randomised to receive flunisolide or placebo in the dose of 500 µg three times a day delivered via spacer chamber. Treatment was begun at 48 to 96 hours of age and continued for 28 days or until extubation. Outcomes included were: need for systemic dexamethasone, duration of ventilation, duration of hospitalisation, duration of supplemental oxygen and the incidence of adverse events (hyperglycaemia, hypertension, weight gain).
Yong 1999: The study by Yong and co‐workers enrolled 40 infants born at less than 32 weeks' GA and requiring mechanical ventilation at birth within 18 hours after birth. Infants were randomly assigned to receive fluticasone propionate or placebo. One puff (250 µg/puff) was administered twice a day for two weeks. There was no difference in the baseline characteristics between groups. Study outcomes included frequency of BPD at 28 days of life and at 36 weeks' PMA, duration of respiratory support, need for systemic corticosteroids, mortality, duration of hospitalisation, successful extubation by seven and 14 days of age, pulmonary function tests (compliance and resistance), inflammatory markers in the tracheal aspirates and incidence of adverse events.
Excluded studies
See 'Characteristics of excluded studies'.
Four trials were excluded (Kovacs 1998; Beresford 2002; Dugas 2005; Yeh 2016). Beresford 2002 was excluded as infants had to be receiving supplemental oxygen at 36 weeks' PMA at the time of randomisation. Denjean 1998 was excluded in previous versions of this review as both ventilated and non‐ventilated infants were included and we were unable to obtain data for ventilated infants from the authors. In a deviation from our protocol for this update we included all infants on any form of assisted ventilatory support (nasal CPAP/IMV or endotracheal IMV). Dugas 2005 was excluded as infants were randomised between 28 and 60 days of age (late). Kovacs 1998 was excluded as investigators evaluated the impact of a combination of systemic and inhaled corticosteroid for prevention of CLD. Yeh 2016 compared the effect of intratracheal administration of surfactant/budesonide with that of surfactant alone on the incidence of death or BPD.
Risk of bias in included studies
For details please see Figure 2; Figure 3.
2.
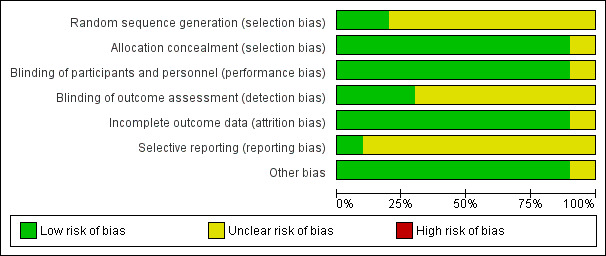
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The risk of bias regarding the random sequence generation was low in Bassler 2015 and Nakamura 2016 and the risk was unclear in the remaining eight studies.
The allocation concealment was of low risk of bias in all studies except for Denjean 1998 in which no information was provided.
Blinding
The risk of performance bias was low in nine studies (no information provided in Denjean 1998) and the risk of detection bias was low in three studies and unclear in seven studies.
Incomplete outcome data
Attrition bias was low in nine studies and unclear in one study (Cole 1999).
Exclusions after randomisation:
In Bassler 2015 four infants in the budesonide group and three infants in the placebo group had unknown outcomes because of withdrawal of consent or right to use the data.
In Cole 1999, three infants were withdrawn before the study drug was administered (two due to sepsis and one due to prior receipt of systemic glucocorticoid therapy). In the treatment group, one infant had been withdrawn prior to 28 days of life and eight more were withdrawn by 36 weeks' PMA. Similarly, in the placebo group, two infants had been withdrawn by 28 days of age and four more were withdrawn by 36 weeks' PMA. Reasons for these later withdrawals were not described. The outcome data for withdrawn infants were not provided. Thus, outcomes were reported at 28 days for 122 treated and 128 control infants, and at 36 weeks' PMA for 114 treated and 124 control infants.
In Denjean 1998 178 infants were initially randomised, but informed consent was either not obtained or withdrawn for five infants, leaving 173 infants in the trial. During the study the randomisation code was broken in three cases because of severe deterioration.
One infant in the placebo group was withdrawn from Merz 1999 due to severe sepsis.
Selective reporting
In only one study was the protocol available to us (Bassler 2015). In the other studies the study protocol was not available to us and we could not judge if there were any deviations from the protocol or not.
Other potential sources of bias
We could not ascertain if there was other bias in one study for which only the abstract was available to us (Townsend 1998). For the remaining studies we could not identify any other potential sources of bias.
Effects of interventions
Summary of findings for the main comparison. Early inhaled steroids (< 2 weeks) compared to placebo (among all randomised) for preventing chronic lung disease in very low birth weight preterm neonates.
| Early inhaled steroids (< 2 weeks) compared to placebo (among all randomised) for preventing chronic lung disease in very low birth weight preterm neonates | ||||||
| Patient or population: very low birth weight preterm neonates Settings: neonatal intensive care units Intervention: early inhaled steroids (< 2 weeks after birth) Comparison: placebo (among all randomised) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo (among all randomised) | Early inhaled steroids (< 2 weeks) | |||||
| CLD at 36 weeks' PMA oxygen dependency at 36 weeks' PMA | Study population | RR 0.97 (0.62 to 1.52) | 429 (5 studies) | ⊕⊕⊕⊝ moderate | ||
| 152 per 1000 | 148 per 1000 (94 to 231) | |||||
| Moderate | ||||||
| 115 per 1000 | 112 per 1000 (71 to 175) | |||||
| Death by, or CLD at, 36 weeks' PMA Death or oxygen dependency at 36 weeks' PMA | Study population | RR 0.86 (0.75 to 0.99) | 1285 (6 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 403 per 1000 | 346 per 1000 (302 to 398) | |||||
| Moderate | ||||||
| 350 per 1000 | 301 per 1000 (262 to 347) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Method of sequence generation was unclear in all included studies except for the study by Bassler 2015. In the studies by Fok 1999, Jangaard 2002, Merz 1999 and Yong 1999 blinding of outcome assessment was unclear. Except for the study by Bassler 2015, none of the included studies were registered and we were unable to identify whether there was selective reporting or not.
Summary of findings 2. Early inhaled steroid (< 2 weeks) compared to placebo (among survivors) for preventing chronic lung disease in very low birth weight preterm neonates.
| Early inhaled steroid (< 2 weeks) compared to placebo (among survivors) for preventing chronic lung disease in very low birth weight preterm neonates | ||||||
| Patient or population: Very low birth weight preterm neonates Settings: Neonatal intensive care units Intervention: Early inhaled steroid (< 2 weeks after birth) Comparison: placebo (among survivors) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo (among survivors) | Early inhaled steroid (< 2 weeks) | |||||
| CLD at 36 weeks' PMA | Study population | RR 0.76 (0.63 to 0.93) | 1088 (6 studies) | ⊕⊕⊕⊝ moderate | ||
| 314 per 1000 | 239 per 1000 (198 to 292) | |||||
| Moderate | ||||||
| 188 per 1000 | 143 per 1000 (118 to 175) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Nakamura 2016 reported all outcomes as a combination of death and a short‐ or long‐term clinical outcome. We wrote to the authors for clarifications regarding the outcomes on 20th May 2016 but as of 20th July 2016 we have not received a response. We report their findings separately in Analysis 1.23 to Analysis 1.32 as their data could not be incorporated in any of the meta‐analyses. This is in a deviation from our protocol.
1.23. Analysis.
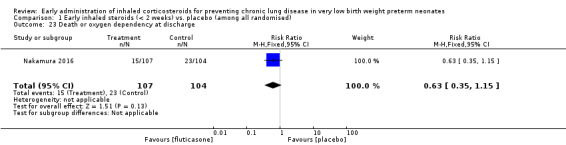
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 23 Death or oxygen dependency at discharge.
1.32. Analysis.
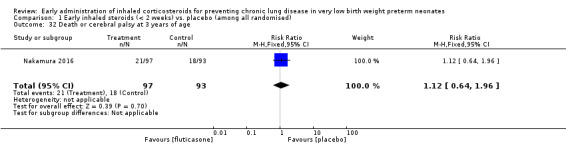
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 32 Death or cerebral palsy at 3 years of age.
Early inhaled steroids (< 2 weeks) vs. placebo among all randomised (Comparison 1)
If only one study is included in an analysis tests for heterogeneity are not applicable.
Primary outcome
CLD at 36 weeks' PMA (Outcome 1.1):
Five trials enrolling 429 neonates reported on the incidence of CLD at 36 weeks' PMA among all randomised (Cole 1999; Fok 1999; Merz 1999; Yong 1999; Jangaard 2002). There was no significant difference in the incidence of CLD at 36 weeks' PMA in the inhaled steroid group versus the placebo group (typical RR 0.97, 95% CI 0.62 to 1.52; typical RD −0.00, 95% CI −0.07 to 0.06). There was no heterogeneity for this outcome for RR (I² = 11%) nor for RD (I² = 0%). Analysis 1.1Figure 4.
1.1. Analysis.
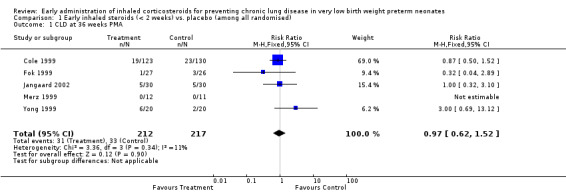
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 1 CLD at 36 weeks PMA.
4.

Forest plot of comparison: 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), outcome: 1.1 CLD at 36 weeks' PMA.
Secondary outcomes
CLD at 28 days of age (Outcome 1.2):
Five trials enrolling 429 neonates reported on the incidence of CLD at 28 days of age among all randomised (Cole 1999; Fok 1999; Merz 1999; Yong 1999; Jangaard 2002). There was no significant difference in the incidence of CLD at 28 days (typical RR 1.08, 95% CI 0.85 to 1.38; typical RD 0.03, 95% CI −0.08 to 0.14). There was no heterogeneity for this outcome for RR (I² = 4%) nor for RD (I² = 23%). Analysis 1.2.
1.2. Analysis.
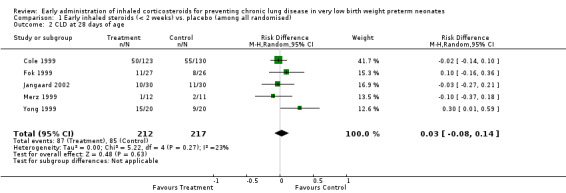
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 2 CLD at 28 days of age.
Death by 28 days of age (Outcome 1.3):
Five trials enrolling 429 neonates reported on the incidence of death by 28 days of age among all randomised (Cole 1999; Fok 1999; Merz 1999; Yong 1999; Jangaard 2002). There was no significant effect on death by 28 days of age (typical RR 0.66, 95% CI 0.39 to 1.14; typical RD −0.04, 95% CI −0.09 to 0.01). There was no heterogeneity for this outcome for RR (I² = 0%); however, there was moderate heterogeneity for RD (I² = 59%). Analysis 1.3.
1.3. Analysis.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 3 Death by 28 days of age.
Death by 36 weeks' PMA (Outcome 1.4):
Six trials enrolling 1285 neonates reported on the incidence of death by 36 weeks' PMA among all randomised (Cole 1999; Fok 1999; Merz 1999; Yong 1999; Jangaard 2002; Bassler 2015). No significant effect on mortality by 36 weeks' PMA was noted (typical RR 1.07, 95% CI 0.82 to 1.40; typical RD 0.01, 95% CI −0.03 to 0.05). There was moderate heterogeneity for this outcome for RR (I² = 51%); and there was low heterogeneity for RD (I² = 48%). Analysis 1.4.
1.4. Analysis.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 4 Death by 36 weeks PMA.
Death by, or CLD at, 28 days of age (Outcome 1.5):
Five trials enrolling 429 neonates reported on the incidence of death by or CLD at 28 days of age among all randomised (Cole 1999; Fok 1999; Merz 1999; Yong 1999; Jangaard 2002). There was no significant difference between the groups for the combined outcome of death by, or CLD at, 28 days of age (typical RR 0.96, 95% CI 0.80 to 1.14; typical RD −0.02, 95% CI −0.11 to 0.07). There was no statistically significant heterogeneity for this outcome (RR I² = 0%; RD I² = 0%). Analysis 1.5.
1.5. Analysis.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 5 Death by or CLD at 28 days of age.
Death by, or CLD at, 36 weeks' PMA (Outcome 1.6):
Six trials enrolling 1285 neonates reported on this outcome (Cole 1999; Fok 1999; Merz 1999; Yong 1999; Jangaard 2002; Bassler 2015). There was a significant difference noted for the combined outcome of death by, or CLD at, 36 weeks' PMA (typical RR 0.86, 95% CI 0.75 to 0.99; typical RD −0.06, 95% CI −0.11 to −0.00) (P = 0.04); NNTB 17 (95% CI 9 to infinity). There was no statistically significant heterogeneity for this outcome (RR I² = 0%; RD I² = 0%). Analysis 1.6; Figure 5.
1.6. Analysis.
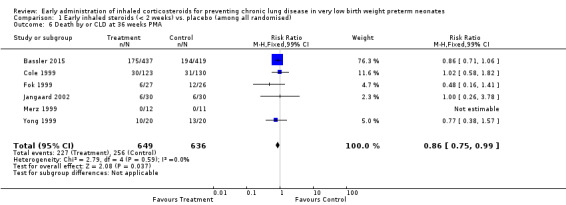
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 6 Death by or CLD at 36 weeks PMA.
5.

Forest plot of comparison: 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), outcome: 1.6 Death by or CLD at 36 weeks' PMA.
Survival to hospital discharge without CLD (Outcome 1.7):
One trial enrolling 86 infants reported on this outcome (Denjean 1998). There was no significant difference between the groups for survival to discharge without CLD (RR 1.50, 95% CI 0.83 to 2.72; RD 0.14, 95% CI −0.06 to 0.34). Analysis 1.7.
1.7. Analysis.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 7 Survival to hospital discharge without CLD.
Death during hospital stay (Outcome 1.8):
One trial enrolling 86 infants reported on this outcome (Denjean 1998). There was no significant difference between the groups for death during hospital stay (RR 1.75, 95% CI 0.55 to 5.55; RD 0.07, 95% CI −0.07 to 0.21). Analysis 1.8.
1.8. Analysis.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 8 Death during hospital stay.
Culture‐proven infection (Outcome 1.9):
Two trials reported on 896 neonates for the incidence of culture‐proven infection (positive blood or CSF culture) (Yong 1999; Bassler 2015). There was no statistically significant difference in the incidence of culture‐proven infection based on this definition (typical RR 1.17, 95% CI 0.97 to 1.41; typical RD 0.05, 95% CI −0.01 to 0.11). There was low heterogeneity for this outcome for RR (I² = 34 %) and for RD (I² = 47 %).
Four trials reported on 225 neonates for the incidence of culture‐proven infection (positive blood culture) (Denjean 1998; Fok 1999; Jonsson 2000; Jangaard 2002). There was no statistically significant difference in the incidence of culture‐proven infection based on this definition (typical RR 1.17, 95% CI 0.83 to 1.65; typical RD 0.05, 95% CI −0.06 to 0.16). There was no statistically significant heterogeneity for this outcome (RR I² = 0%; RD I² = 0%).
For the two outcomes (positive blood culture or CSF culture and positive blood culture) combined (N = 1121 neonates) there was no statistically significant difference for culture‐proven infection (typical RR 1.17, 95% CI 0.99 to 1.38; typical RD 0.05, 95% CI −0.00 to 0.11). There was no statistically significant heterogeneity for this outcome (RR I² = 0%; RD I² = 0%). Analysis 1.9.
1.9. Analysis.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 9 Culture proven infection during hospital stay.
Hyperglycaemia (Outcome 1.10):
Three trials enrolling 116 neonates reported on the incidence of hyperglycaemia (Fok 1999; Merz 1999; Yong 1999). There was no significant difference in the incidence of hyperglycaemia (typical RR 0.84, 95% CI 0.49 to 1.44; typical RD −0.05, 95% CI −0.19 to 0.10). There was no statistically significant heterogeneity for this outcome (RR I² = 0%; RD I² = 0%). Analysis 1.10.
1.10. Analysis.
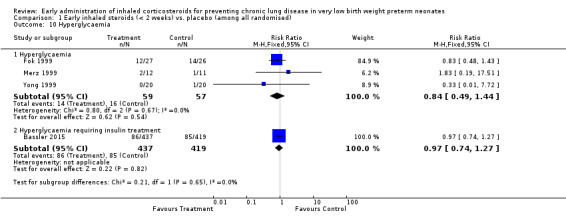
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 10 Hyperglycaemia.
Bassler 2015 found no significant difference in the occurrence of hyperglycaemia requiring insulin treatment between the two groups (n = 856) (RR 0.97, 95% CI 0.74 to 1.27; RD −0.01, 95% CI −0.06 to 0.05). Tests for heterogeneity were not applicable. Analysis 1.10.
Hypertension (Outcome 1.11):
Three trials enrolling 116 neonates reported on the incidence of hypertension (Fok 1999; Merz 1999; Yong 1999). There was no statistically significant difference in the incidence of hypertension between groups (typical RR 1.20, 95% CI 0.36 to 3.99; typical RD 0.01, 95% CI −0.09 to 0.12). Test for heterogeneity was not applicable for RR (two studies had no outcomes) and there was no heterogeneity for RD (I² = 0%). Analysis 1.11.
1.11. Analysis.
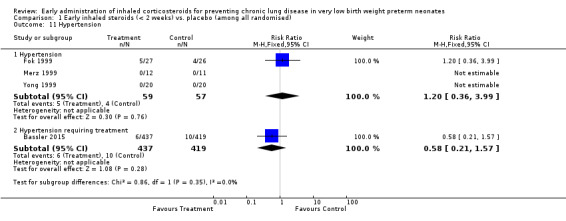
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 11 Hypertension.
Bassler 2015 reported no significant difference in the occurrence of hypertension requiring treatment between the two groups (n = 856) (RR 0.58, 95% CI 0.21 to 1.57; RD −0.01, 95% CI −0.03 to 0.01). Tests for heterogeneity were not applicable. Analysis 1.11.
Gastrointestinal bleeding (Outcome 1.12):
One trial enrolling 253 neonates reported on the incidence of gastrointestinal bleeding (Cole 1999). There was no significant difference for this outcome between groups (RR 0.35, 95% CI 0.04 to 3.34; RD −0.01, 95% CI −0.05 to 0.02). Tests for heterogeneity were not applicable. Analysis 1.12.
1.12. Analysis.
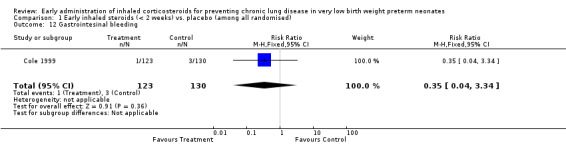
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 12 Gastrointesinal bleeding.
Bassler 2015 did not report on gastrointestinal bleeding, but reported on gastrointestinal disorders which occurred in 30 cases (6.86%) in the inhaled budesonide group and in 32 cases (7.64%) in the placebo group. The contribution of gastrointestinal bleeding to the total number is not reported.
Cataract (Outcome 1.13):
One trial enrolling 253 neonates reported on the incidence of cataracts (Cole 1999). There was no significant difference in the incidence of cataracts between groups (RR 0.35, 95% CI 0.01 to 8.56; RD −0.01, 95% CI −0.03 to 0.01). Tests for heterogeneity were not applicable. Analysis 1.13.
1.13. Analysis.
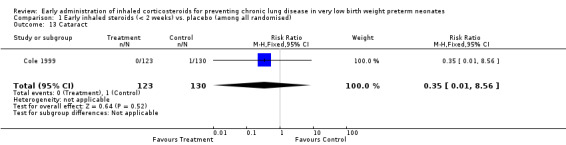
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 13 Cataract.
Intraventricular haemorrhage (IVH) (Outcome 1.14):
Two trials enrolling 306 neonates reported on the incidence of IVH (Fok 1999; Cole 1999). There was no significant difference in the incidence of IVH between groups (typical RR 1.04, 95% CI 0.77 to 1.41; typical RD 0.02, 95% CI −0.09 to 0.12). There was no statistically significant heterogeneity for this outcome (RR I² = 0%; RD I² = 0%). Analysis 1.14.
1.14. Analysis.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 14 Intraventricular haemorrhage.
Periventricular leukomalacia (PVL) (Outcome 1.15):
Two trials enrolling 306 neonates reported on the incidence of PVL (Fok 1999; Cole 1999). There was no significant difference in the incidence of PVL between groups (typical RR 1.43, 95% CI 0.59 to 3.46; typical RD 0.02, 95%CI −0.03 to 0.08). There was no statistically significant heterogeneity for this outcome (RR I² = 0%; RD I² = 0%). Analysis 1.15.
1.15. Analysis.
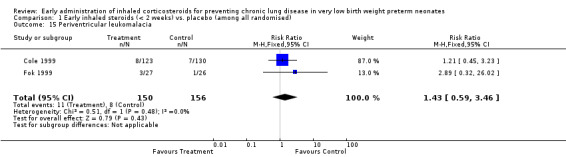
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 15 Periventricular leukomalacia.
Brain injury (Outcome 1.16):
One trial reporting on 838 infants reported on the incidence of brain injury diagnosed based on the worst cranial ultrasound finding performed at or before 36 weeks' PMA (Bassler 2015). There was no significant difference in the incidence of brain injury between the two groups (RR 1.25, 95% CI 0.94 to 1.65; RD 0.04, 95% CI −0.01 to 0.10). Tests for heterogeneity were not applicable. Analysis 1.16
1.16. Analysis.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 16 Brain injury.
Necrotizing enterocolitis (NEC) (Outcome 1.17):
Three trials enrolling 1162 neonates reported on the incidence of NEC (Cole 1999; Fok 1999; Bassler 2015). There was no significant difference in the incidence of NEC between groups (typical RR 0.92, 95% CI 0.68 to 1.24; typical RD −0.01, 95% CI −0.05 to 0.03). There was low heterogeneity for this outcome for RR (I² = 31%) and for RD (I² = 40%). Analysis 1.17.
1.17. Analysis.
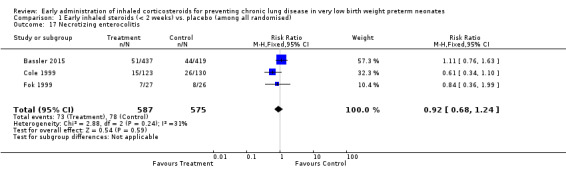
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 17 Necrotizing enterocolitis.
Retinopathy of prematurity (ROP) any stage (Outcome 1.18):
Two trials enrolling 306 neonates reported on the incidence of ROP (Fok 1999; Cole 1999). There was no significant difference in the incidence of ROP (typical RR 1.00, 95% CI 0.87 to 1.15; typical RD 0.00, 95% CI −0.09 to 0.09). There was no statistically significant heterogeneity for this outcome (RR I² = 0%; RD I² = 0%). Analysis 1.18
1.18. Analysis.
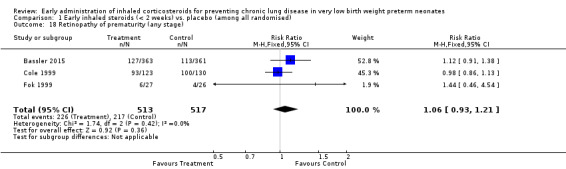
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 18 Retinopathy of prematurity (any stage).
Three trials enrolling 1030 neonates reported on the incidence of ROP (Cole 1999; Fok 1999; Bassler 2015). There was no significant difference in the incidence of ROP (typical RR 1.06, 95% CI 0.93 to 1.21; typical RD 0.03, 95% CI −0.03 to 0.08). There was no statistically significant heterogeneity for this outcome (RR I² = 0%; RD I² = 0%). Analysis 1.18.
Patent ductus arteriosus (Outcome 1.19):
One trial enrolling 53 neonates reported on the incidence of PDA (Fok 1999). There was no significant difference in the incidence of PDA between the two groups (RR 0.82, 95% CI 0.57 to 1.17; RD −0.14, 95% CI −0.38 to 0.10). Tests for heterogeneity were not applicable. Bassler 2015 reported on PDA treated with drugs (reported un stratified RR 0.88, 95% CI 0.76 to 1.01) and with surgical ligation (reported un stratified RR 0.55, 95% CI 0.36 to 0.84). There was overlap between these groups and we are awaiting clarification by the first author prior to inclusion in the analysis. Analysis 1.19.
1.19. Analysis.
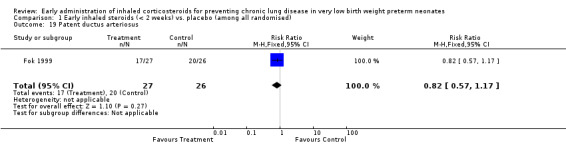
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 19 Patent ductus arteriosus.
Reintubation (Outcome 1.20):
One trial reporting on 856 infants reported a significantly lower risk of reintubation in the steroid group compared with the control group (RR 0.58, 95% CI 0.35 to 0.96; RD −0.04, 95% CI −0.07 to −0.00; NNTB 25, 95% CI 14 to ∞) (Bassler 2015). Tests for heterogeneity were not applicable. Analysis 1.20.
1.20. Analysis.
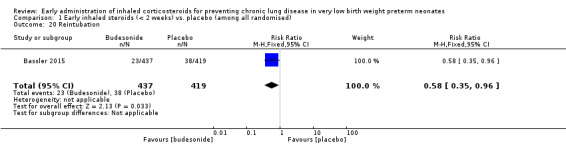
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 20 Reintubation.
Requirement for systemic steroids (Outcome 1.21):
Eight trials enrolling 1403 infants reported on the requirements for systemic steroids (Denjean 1998; Townsend 1998; Cole 1999; Fok 1999; Merz 1999; Yong 1999; Jangaard 2002; Bassler 2015). The need for systemic steroids was not statistically significantly different between groups (typical RR 0.89, 95% CI 0.77 to 1.02; typical RD −0.04, 95% CI −0.09 to 0.01). There was low heterogeneity for this outcome for RR (I² = 33%) and moderate heterogeneity for RD (I² = 63%). Analysis 1.21.
1.21. Analysis.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 21 Requirement for systemic steroids.
Failure to extubate within 14 days (Outcome 1.22):
Five trials enrolling 193 neonates reported on this outcome (Fok 1999; Merz 1999; Yong 1999; Jonsson 2000; Jangaard 2002). There was no significant difference noted for this outcome (typical RR 0.97, 95% CI 0.76 to 1.24; typical RD −0.02, 95% CI −0.15 to 0.12). There was moderate heterogeneity for this outcome for RR (I² = 61%) and high heterogeneity for RD (I² = 80%). Analysis 1.22.
1.22. Analysis.
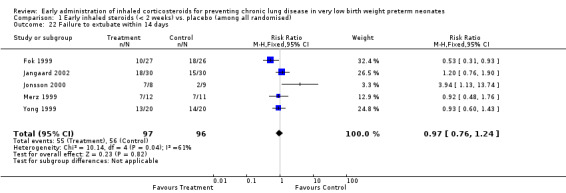
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 22 Failure to extubate within 14 days.
The following outcomes (Analysis 1.23 to Analysis 1.32) all pertain to the trial by Nakamura and co‐workers (Nakamura 2016), who for all outcomes reported a combination of deaths and complications of preterm birth. As there is only one trial included in these analyses tests for heterogeneity are not applicable. Some denominators exclude infants for which the outcome could not be ascertained.
Death or oxygen dependency at discharge (Outcome 1.23):
This outcome was reported for all 211 randomised infants (Nakamura 2016). There was no significant difference between the two groups (RR 0.63, 95% CI 0.35 to 1.15; RD −0.08, 95% CI −0.18 to 0.02). Analysis 1.23.
Death or severe BPD (Outcome 1.24):
This outcome was reported for all 211 randomised infants (Nakamura 2016). There was no significant difference between the two groups (RR 0.93, 95% CI 0.70 to 1.25; RD −0.03, 95% CI −0.17 to 0.10). Analysis 1.24.
1.24. Analysis.
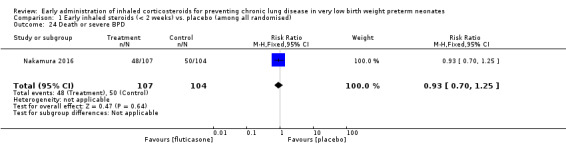
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 24 Death or severe BPD.
Death or grade 3 or 4 IVH (Outcome 1.25):
This outcome was reported for all 211 randomised infants (Nakamura 2016). There was no significant difference between the two groups (RR 1.05, 95% CI 0.65 to 1.68; RD 0.01, 95% CI −0.10 to 0.13). Analysis 1.25.
1.25. Analysis.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 25 Death or grade 3 or 4 IVH.
Death or PVL (Outcome 1.26):
This outcome was reported for all 211 randomised infants (Nakamura 2016). There was no significant difference between the two groups (RR 0.89, 95% CI 0.41 to 1.93; RD −0.01, 95% CI −0.10 to 0.07). Analysis 1.26.
1.26. Analysis.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 26 Death or PVL.
Death or NEC (Outcome 1.27):
This outcome was reported for all 211 randomised infants (Nakamura 2016). There was no significant difference between the two groups (RR 0.97, 95% CI 0.46 to 2.06; RD −0.00, 95% CI −0.09 to 0.08). Analysis 1.27.
1.27. Analysis.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 27 Death or NEC.
Death or sepsis (Outcome 1.28):
This outcome was reported for all 211 randomised infants (Nakamura 2016). There was no significant difference between the two groups (RR 0.79, 95% CI 0.44 to 1.40; RD −0.04, 95% CI −0.15 to 0.06). Analysis 1.28.
1.28. Analysis.
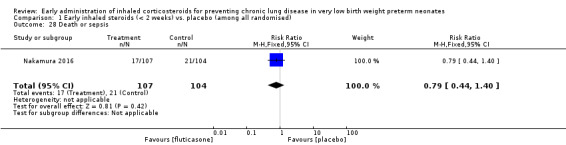
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 28 Death or sepsis.
Death or ROP (stage not stated) (Outcome 1.29):
This outcome was reported for all 211 randomised infants (Nakamura 2016). There was no significant difference between the two groups (RR 1.05, 95% CI 0.79 to 1.40; RD 0.02, 95% CI −0.11 to 0.16). Analysis 1.29.
1.29. Analysis.
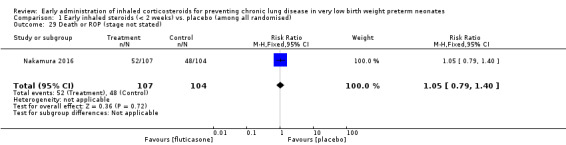
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 29 Death or ROP (stage not stated).
Death or neurodevelopmental impairment at 18 months PMA (Outcome 1.30):
This outcome was reported in 187 infants available at follow‐up (Nakamura 2016). There was no significant difference between the two groups (RR 1.09, 95% CI 0.70 to 1.70; RD 0.03, 95% CI −0.10 to 0.16). Analysis 1.30
1.30. Analysis.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 30 Death or neurodevelopmental impairment at 18 months PMA.
Death or neurodevelopmental impairment at 3 years of age (Outcome 1.31):
This outcome was reported in 179 infants available at follow‐up (Nakamura 2016). There was no significant difference between the two groups (RR 1.03, 95% CI 0.68 to 1.56; RD 0.01, 95% CI −0.13 to 0.15). Analysis 1.31.
1.31. Analysis.
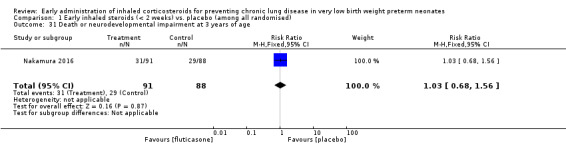
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 31 Death or neurodevelopmental impairment at 3 years of age.
Death or cerebral palsy at 3 years of age (Outcome 1.32):
This outcome was reported in 190 infants available at follow‐up (Nakamura 2016). There was no significant difference between the two groups (RR 1.12, 95% CI 0.64 to 1.96; RD 0.02, 95% CI −0.09 to 0.14). Analysis 1.32.
Early inhaled steroids (< 2 weeks) vs. placebo among survivors (Comparison 2)
Primary outcome
CLD at 36 weeks' PMA (Outcome 2.1):
Six trials enrolling 1088 neonates reported on this outcome among survivors (Cole 1999; Fok 1999; Merz 1999; Yong 1999; Jangaard 2002; Bassler 2015). There was a significant difference in the incidence of CLD at 36 weeks' PMA among survivors (typical RR 0.76, 95% CI 0.63 to 0.93; typical RD −0.07, 95% CI −0.13 to −0.02; NNTB 14, 95% CI 8 to 50). There was no statistically significant heterogeneity for this outcome (RR I² = 0%; RD I² = 0%). Analysis 2.1; Figure 6.
2.1. Analysis.
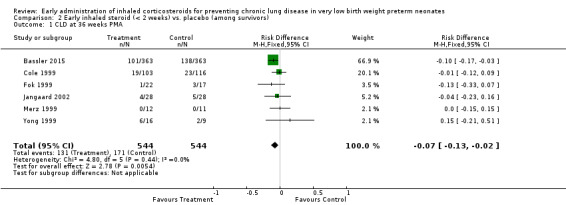
Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 1 CLD at 36 weeks PMA.
6.
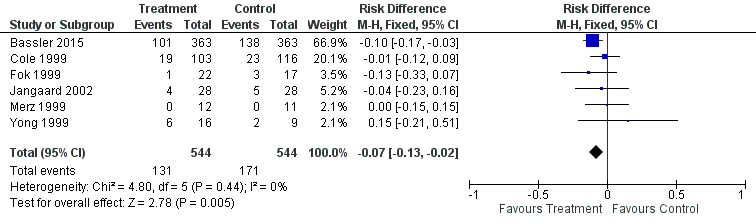
Forest plot of comparison: 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), outcome: 2.1 CLD at 36 weeks' PMA.
Secondary outcomes
CLD at 28 days of age (Outcome 2.2):
Five trials enrolling 380 neonates reported on this outcome (Cole 1999; Fok 1999; Merz 1999; Yong 1999; Jangaard 2002). There was no significant difference in the incidence of CLD at 28 days of age among survivors (typical RR 0.97, 95% CI 0.78 to 1.21; typical RD −0.01, 95% CI −0.11 to 0.08). There was no statistically significant heterogeneity for this outcome (RR I² = 0%; RD I² = 0%). Analysis 2.2.
2.2. Analysis.
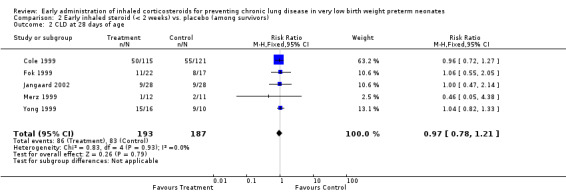
Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 2 CLD at 28 days of age.
Cerebral palsy (Outcome 2.3):
One trial enrolling 56 neonates reported on this outcome (Jangaard 2002). There was no significant difference in the incidence of cerebral palsy among survivors (RR 1.33, 95% CI 0.33 to 5.42; RD 0.04, 95% CI −0.14 to 0.21). Tests for heterogeneity were not applicable. Analysis 2.3.
2.3. Analysis.
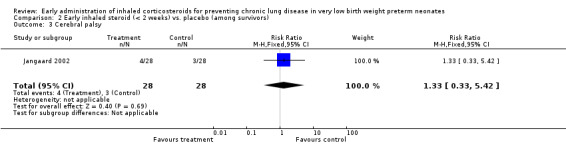
Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 3 Cerebral palsy.
Mean developmental index on BSID‐II < 2 SD of the mean (Outcome 2.4):
One trial enrolling 56 neonates reported on this outcome (Jangaard 2002). There was no significant difference in the incidence of mean developmental index on BSID‐II less than 2 SD of the mean among survivors (RR 1.25, 95% CI 0.37 to 4.17; RD 0.04, 95% CI −0.16 to 0.23). Tests for heterogeneity were not applicable. Analysis 2.4.
2.4. Analysis.
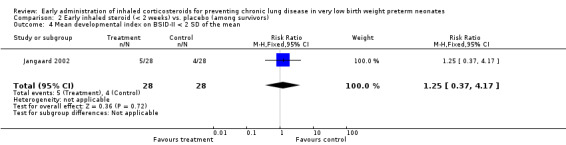
Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 4 Mean developmental index on BSID‐II < 2 SD of the mean.
Respiratory readmission (Outcome 2.5):
One trial enrolling 56 neonates reported on this outcome (Jangaard 2002). There was no statistically significant difference in the incidence of respiratory readmission (RR 1.00, 95% CI 0.44 to 2.29; RD 0.00, 95% CI −0.24 to 0.24). Tests for heterogeneity were not applicable. Analysis 2.5.
2.5. Analysis.
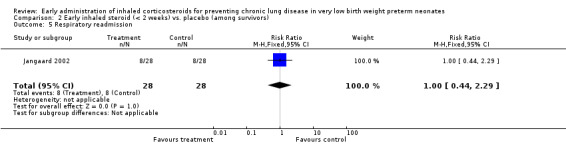
Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 5 Respiratory readmission.
Additional outcomes
The functioning of the hypothalamic‐pituitary‐adrenal axis in a subset of infants enrolled in the randomised controlled trial by Cole and co‐workers is reported in a separate publication (Cole 1999). Inhaled beclomethasone therapy was associated with a small decrease in the basal cortisol levels. There was no evidence of adrenal suppression as reflected by the response to cosyntropin stimulation. Ng 1993 reported on pituitary‐adrenal suppression in preterm, VLBW infants after inhaled fluticasone propionate treatment. This study evaluated a subset of infants enrolled in the trial of Fok 1999 using the human CRH stimulation test. Basal and post‐stimulation plasma ACTH and serum cortisol concentrations were significantly suppressed in the group receiving inhaled fluticasone when compared to the control group.
No relevant data for the following outcomes were available for analysis: duration of requirement for supplemental oxygen, duration of assisted ventilation, measurements of pulmonary function, growth, hypertrophy of the tongue, hypertrophic obstructive cardiomyopathy and nephrocalcinosis.
Discussion
Summary of main results
This review update identified one additional published trial that previously was listed as an ongoing trial in Europe (Bassler 2015); and one new trial from Japan (Nakamura 2016). Both trials were of high GRADE quality, but Nakamura 2016 reported all outcomes as a combination of death and a complication of preterm birth. We therefore could not include the results in meta‐analyses. Denjean 1998 was moved from excluded to included trials. The study lacks information on most items in the 'Risk of bias' assessment. The review now includes information from 10 trials which have enrolled 1644 infants.
There is now evidence that inhaled steroids are effective in reducing the incidence of death or CLD at 36 weeks' PMA among all randomised infants. There was a significant difference noted for the combined outcome of death by, or CLD at, 36 weeks' PMA (typical RR 0.86, 95% CI 0.75 to 0.99; typical RD −0.06, 95% CI −0.11 to −0.00 (P = 0.04); NNTB 17, 95% CI 9 to infinity). There was no statistically significant heterogeneity for this outcome (RR I² = 0%; RD I² = 0%). In addition there is evidence that inhaled steroids reduce the risk of need for reintubation. Even though there is evidence, the concern is whether this benefit is of any clinical relevance. The NNTB to prevent one infant from developing death or CLD at 36 weeks' PMA is 17 with the upper 95% CI of infinity and therefore not clinically important. Based on this result the use of inhaled steroids in this population cannot be recommended. The long‐term follow‐up results of Bassler 2015 may affect the conclusion of this review.
Among survivors, inhaled steroids are effective in reducing the incidence of CLD at 36 weeks' PMA (typical RR 0.76, 95% CI 0.63 to 0.93; typical RD −0.07, 95% CI −0.13 to −0.02 (P = 0.005); NNTB 14, 95% CI 8 to 15). There was no statistically significant heterogeneity for this outcome (RR I² = 0%; RD I² = 0%). In one trial enrolling 56 neonates, no significant difference in the incidence of cerebral palsy was noted.
A major concern with studies of inhalation therapy in neonates is the uncertainty regarding drug delivery and deposition in the peripheral airways. Numerous factors affect drug delivery and deposition including the number of particles in the respirable range, the delivery technique (use of MDI in combination with a spacer or face mask and nebuliser (ultrasonic or jet)) and the presence or absence of an endotracheal tube. Previous workers have shown that the amount of aerosol delivery varies from 0.4% to 14% based on the technique used (Arnon 1992; Grigg 1992; O'Callaghan 1992). Delivery technique, type of inhaled steroid and dosage used varied among the studies included in this review, making the interpretation of aggregate data difficult.
In conclusion, there is now some evidence from this systematic review that inhaled steroids reduce the incidence of death or CLD at 36 weeks' PMA amongst all randomised but the evidence is not of clinical relevance.
Studies are needed to examine the effect of different delivery techniques and dosing schedules for the administration of these medications. It is possible that, with improved and/or more consistent delivery of inhaled steroids to the target organ, an important therapeutic effect will be demonstrated. Long‐term outcomes are awaited from the trial by Bassler 2015.
Overall completeness and applicability of evidence
By 2016 a total of 1644 infants have been enrolled in trials testing the effectiveness of early administration of inhaled steroids. There is now moderate GRADE evidence that early administration of inhaled steroids reduces the incidence of death or CLD at 36 weeks' PMA among all randomised infants and among survivors only, but the significance is not clinically relevant. In addition there is evidence that inhaled steroids reduces the risk of need for reintubation.
Quality of the evidence
According to GRADE the quality of evidence was moderate for the outcomes of chronic lung disease at 36 weeks' PMA and death and CLD at 36 weeks' PMA amongst all randomised and amongst survivors. There was serious risk of bias, with method of sequence generation being unclear in all included studies except for Bassler 2015. In Denjean 1998, Fok 1999, Jangaard 2002, Merz 1999 and Yong 1999 blinding of outcome assessment was unclear. Except for the study by Bassler 2015, none of the included studies were registered and we were unable to identify whether there was selective reporting or not. There was no evidence to support inconsistency, imprecision or indirectness among the included studies.
Potential biases in the review process
We are not aware of any potential bias in our review process.
Agreements and disagreements with other studies or reviews
Of the included studies in this review, only the study by Bassler 2015 showed a reduction in CLD at 36 weeks' PMA amongst survivors. No difference was noted for the outcome of CLD at 36 weeks' PMA amongst all randomised infants. The inclusion of the trial by Bassler 2015 for the combined outcome of death and CLD at 36 weeks' PMA amongst all randomised was significant; however the CI is close to 1 and of questionable clinical relevance. In a systematic review published by Beam and colleagues the authors concluded that inhaled steroids were ineffective in preventing bronchopulmonary dysplasia (Beam 2014). This review was completed before the publication by Bassler 2015. In an editorial regarding the Bassler 2015 study Schmidt concluded that "Uncertainty prevails with respect to the use of early inhaled glucocorticosteroids to prevent bronchopulmonary dysplasia in extremely preterm infants" (Schmidt 2015). In contrast in an editorial to the excluded study by Yeh 2016, Bancalari and Jobe concluded: "If the striking pulmonary benefits with absent detrimental effects described by Yeh and colleagues are replicated in large randomised controlled trials done in different clinical setting, this could very well become a 'magic bullet' in the prevention of BPD" (Bancalari 2016). We excluded the study by Yeh 2016, as the intervention was a combination of intratracheal steroids and surfactant.
Authors' conclusions
Implications for practice.
Based on this updated review, there is now some evidence that early treatment of preterm infants with inhaled steroids reduces the incidence of death by, or CLD at, 36 weeks' PMA but the effect may not be of clinical relevance.
Implications for research.
Studies are needed to identify the risk/benefit ratio of different delivery techniques and dosing schedules for the administration of these medications. Studies need to address both short‐ and long‐term benefits and adverse effects of inhaled steroids, with particular attention to neurodevelopmental outcome.
What's new
| Date | Event | Description |
|---|---|---|
| 27 January 2020 | Amended | Arne Ohlsson deceased. |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 1, 2000
| Date | Event | Description |
|---|---|---|
| 25 July 2016 | New search has been performed | The previously listed ongoing trial (Bassler 2010a) has now been published — Bassler 2015. The trial by Bassler 2015 included non‐ventilated infants and we changed our inclusion criteria to include such trials. The previously excluded trial by Denjean 1998 is now eligible for inclusion. Denjean 1998 included infants on conventional intermittent mandatory ventilation with an endotracheal tube (ET IMV) or nasal ventilation or continuous positive airway pressure (nasal IMV/CPAP). One additional trial — Nakamura 2016 — was identified when a literature search was being conducted for a reason unrelated to this review. This search was conducted after the searches in January 2016. |
| 1 June 2016 | New citation required and conclusions have changed | With evidence from 3 new included trials, the conclusions of the review have changed. |
| 1 March 2012 | New search has been performed | This review updates the existing review "Early administration of inhaled corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates" published in the Cochrane Database of Systematic Reviews (Shah 2007a). Search updated in July 2011. |
| 3 June 2008 | Amended | Converted to new review format. |
| 3 August 2007 | New citation required but conclusions have not changed | Substantive amendment |
| 3 June 2007 | New search has been performed | This updates the review "Early administration of inhaled corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates" published in The Cochrane Library, Issue 1, 2000 (Shah 2000). The updated search conducted in August 2007 identified three additional trials. Two of these trials were excluded. The results of the seven included trials found no evidence that the early use of inhaled steroids prevents the development of chronic lung disease. |
Acknowledgements
We thank the following for providing information regarding their studies: Dr D Bassler, Department of Neonatology, University Hospital Zurich, University of Zurich, Switzerland; Dr C Cole, Department of Pediatrics, Floating Hospital for Children, New England Medical Center, Boston, USA; Dr T F Fok, Department of Paediatrics, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong, People's Republic of China; Dr U Merz, Children's Hospital, Neonatal Intensive Care Unit, Aachen University of Technology, Aachen, Germany; and Dr WSC Yong, Jessop Hospital for Women, Sheffield, UK.
Appendices
Appendix 1. Standard search methodology ‐ 2016 update
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh])) Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial) CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) Cochrane Library: (infant or newborn or neonate or neonatal or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. 2011 Search Strategy
PubMed:
((bronchopulmonary dysplasia OR lung diseases OR chronic lung disease) AND (anti‐inflammatory agents OR steroids OR dexamethasone OR inhalation OR aerosols OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate)) AND ((infant, newborn[MeSH] OR newborn OR neon* OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh])) AND (("2007"[PDat] : "3000"[PDat]))
CINAHL:
( (bronchopulmonary dysplasia OR lung diseases OR chronic lung disease) AND (anti‐inflammatory agents OR steroids OR dexamethasone OR inhalation OR aerosols OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate) ) and ( ( infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW) AND ( randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) ) 2007 ‐ Present
Cochrane Central Register of Controlled Trials
(bronchopulmonary dysplasia OR lung diseases OR chronic lung disease) AND (anti‐inflammatory agents OR steroids OR dexamethasone OR inhalation OR aerosols OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate) and (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW), from 2007 to 2011
Embase:
1 ((bronchopulmonary dysplasia or lung diseases or chronic lung disease) and (anti‐inflammatory agents or steroids or dexamethasone or inhalation or aerosols or budesonide or beclomethasone dipropionate or flunisolide or fluticasone propionate)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (1849)
2 (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (603948)
3 (human not animal).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (11849457)
4 (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (1256505)
5 1 and 2 and 3 and 4 (336)
6 limit 5 to yr="2007 ‐Current" (76)
ClinicalTrials.gov:
(infant OR newborn) AND (bronchopulmonary dysplasia OR lung disease) AND (anti‐inflammatory agents OR steroids OR dexamethasone OR inhalation OR aerosols OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate)
Controlled‐trials.com:
(infant OR newborn) AND (bronchopulmonary dysplasia OR lung disease) AND (anti‐inflammatory agents OR steroids OR dexamethasone OR inhalation OR aerosols OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate)
Appendix 3. Risk of bias tool
The following issues were evaluated and entered into the 'Risk of bias' table: 1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated? For each included study, we categorized the method used to generate the allocation sequence as: a. low risk (any truly random process e.g. random number table; computer random number generator); b. high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); c. unclear risk. 2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed? For each included study, we categorized the method used to conceal the allocation sequence as: a. low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); b. high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); c. unclear risk. 3. Blinding (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment? For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorized the methods as: a. low risk, high risk or unclear risk for participants; b. low risk, high risk or unclear risk for personnel; c. low risk, high risk or unclear risk for outcome assessors. 4. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed? For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods as: a. low risk (< 20% missing data); b. high risk (≥ 20% missing data); c. unclear risk. 5. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting? For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as: a. low risk (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported); b. high risk (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); c. unclear risk. 6.Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias? For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as: a. low risk; b. high risk; c. unclear risk.
If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CLD at 36 weeks PMA | 5 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.62, 1.52] |
| 2 CLD at 28 days of age | 5 | 429 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.08, 0.14] |
| 3 Death by 28 days of age | 5 | 429 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.09, 0.01] |
| 4 Death by 36 weeks PMA | 6 | 1285 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 5 Death by or CLD at 28 days of age | 5 | 429 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.11, 0.07] |
| 6 Death by or CLD at 36 weeks PMA | 6 | 1285 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.86 [0.75, 0.99] |
| 7 Survival to hospital discharge without CLD | 1 | 86 | Risk Difference (M‐H, Fixed, 95% CI) | 0.14 [‐0.06, 0.34] |
| 8 Death during hospital stay | 1 | 86 | Risk Difference (M‐H, Fixed, 95% CI) | 0.07 [‐0.07, 0.21] |
| 9 Culture proven infection during hospital stay | 6 | 1121 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.00, 0.11] |
| 9.1 Positive blood or CSF culture | 2 | 896 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.01, 0.11] |
| 9.2 Positive blood culture | 4 | 225 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.06, 0.16] |
| 10 Hyperglycaemia | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Hyperglycaemia | 3 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.49, 1.44] |
| 10.2 Hyperglycaemia requiring insulin treatment | 1 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.74, 1.27] |
| 11 Hypertension | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Hypertension | 3 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.36, 3.99] |
| 11.2 Hypertension requiring treatment | 1 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.21, 1.57] |
| 12 Gastrointesinal bleeding | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.34] |
| 13 Cataract | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.56] |
| 14 Intraventricular haemorrhage | 2 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.77, 1.41] |
| 15 Periventricular leukomalacia | 2 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.59, 3.46] |
| 16 Brain injury | 1 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.94, 1.65] |
| 17 Necrotizing enterocolitis | 3 | 1162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.68, 1.24] |
| 18 Retinopathy of prematurity (any stage) | 3 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.93, 1.21] |
| 19 Patent ductus arteriosus | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.57, 1.17] |
| 20 Reintubation | 1 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.35, 0.96] |
| 21 Requirement for systemic steroids | 8 | 1403 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.02] |
| 22 Failure to extubate within 14 days | 5 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.76, 1.24] |
| 23 Death or oxygen dependency at discharge | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.35, 1.15] |
| 24 Death or severe BPD | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.70, 1.25] |
| 25 Death or grade 3 or 4 IVH | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.65, 1.68] |
| 26 Death or PVL | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.41, 1.93] |
| 27 Death or NEC | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.46, 2.06] |
| 28 Death or sepsis | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.44, 1.40] |
| 29 Death or ROP (stage not stated) | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.79, 1.40] |
| 30 Death or neurodevelopmental impairment at 18 months PMA | 1 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.70, 1.70] |
| 31 Death or neurodevelopmental impairment at 3 years of age | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.68, 1.56] |
| 32 Death or cerebral palsy at 3 years of age | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.64, 1.96] |
Comparison 2. Early inhaled steroid (< 2 weeks) vs. placebo (among survivors).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CLD at 36 weeks PMA | 6 | 1088 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.07 [‐0.13, ‐0.02] |
| 2 CLD at 28 days of age | 5 | 380 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.78, 1.21] |
| 3 Cerebral palsy | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.33, 5.42] |
| 4 Mean developmental index on BSID‐II < 2 SD of the mean | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.37, 4.17] |
| 5 Respiratory readmission | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.44, 2.29] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bassler 2015.
| Methods | Multi‐national randomised placebo‐controlled clinical trial. Study setting: 40 centres in 9 European countries. Study period: 1 April 1 2010 to 3 August 2013. Blinding of randomisation: yes Blinding of intervention: yes Complete follow‐up: yes Blinding of outcome measurement: yes |
|
| Participants | Infants with PMA 230/7 weeks to 276/7 weeks and chronological age of 12 hours or less, who required any form of positive pressure support. Exclusion criteria: palliative care; dysmorphic feature or congenital abnormalities likely to affect life expectancy or neurologic development; strongly suspected cyanotic heart disease; were from a multiple‐birth pregnancy (other than the second infant in birth order). Demographic data: values are presented as mean (SD) or percentage or median (IQR) Budesonide group: n = 437; followed to first discharge home Birth weight (g): 798 (193) Gestational age (weeks): 26.1 (1.3) Sex (% male): 50.8 Age at randomisation (hours): 6.7 (4.0 to 10.3) Placebo group: n = 419; followed to first discharge home Birth weight (g): 803 (189) Gestational age (weeks): 26.1 (1.2) Sex (% male): 50.8 Age at randomisation (hours): 6.6 (3.8 to 10.6) |
|
| Interventions | The Budesonide group (n = 441 were assigned to budesonide; 437 were followed to first discharge home) received two puffs of budesonide (200µg/puff) administered every 12 hours for the first 14 days of life and one puff administered every 12 hours from day 15 until the last dose of study drug had been administered. Study drugs were administered until infants no longer needed supplemental oxygen and positive pressure support or reached a PMA of 320/7 weeks, regardless of ventilator status. The control group (n = 422; 419 were followed to first discharge home) received placebo containing hydrofluoroalkane propellant |
|
| Outcomes | Primary outcome: a composite of death or BPD at 36 weeks' PMA. BPD defined as the requirement for positive pressure support, the requirement for supplemental oxygen at FiO₂ > 0.30, or, if infants receiving low amounts of oxygen, an inability to maintain an oxygen saturation value above 90% during a structured, short period of saturation monitoring coupled with gradual weaning from oxygen to ambient air (oxygen reduction test). Secondary outcomes: death by any cause at 36 weeks' PMA, BPD at 36 weeks' PMA (defined as per above), duration of positive pressure respiratory support or supplemental oxygen, ventriculomegaly with or without IVH on ultrasound at or before 36 weeks' PMA, PDA requiring medical or surgical treatment, intestinal perforation or NEC (we included this outcome under NEC), ROP (≥ stage 2), culture‐proven infections, increase in body weight and head circumference from birth to day 28, length of hospital stay, need for reintubation after the last dose of drug had been administered, occurrence of oral candidiasis requiring treatment, hyperglycaemia requiring insulin treatment, hypertension requiring treatment. Neurodevelopmental disability testing to be conducted at 18 to 22 months (results not reported in this publication). |
|
| Notes | ClinicalTrials.gov: NCT01035190 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer generated randomisation scheme. |
| Allocation concealment (selection bias) | Low risk | The manufacturer of the study drug received the sequence of study drug assignments from a statistician at the coordinating centre and prepared drug packages, each of which contained 8 sequentially numbered metered dose inhalers that were identical in appearance. Packages of coded inhalers containing the study drugs were delivered to each participating centre to ensure concealment of randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | See comments above. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No one involved in patient care or in the assessment and analysis of outcomes was aware of the individual study group assignments before completion of the analysis. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 in the budesonide group and 3 in the control group had unknown outcome because of withdrawal of consent or right to use data. |
| Selective reporting (reporting bias) | Low risk | The study was registered as ClinicalTrials.gov number NCT01035190. There does not seem to be any major deviations from the protocol. |
| Other bias | Low risk | Appears free of other bias. |
Cole 1999.
| Methods | Multicentre randomised, double‐blind, placebo‐controlled trial. Infants were stratified for randomisation according to: study site, sex, birth weight (≤ 900 g or > 900 g), and severity of pulmonary disease (oxygenation index ≤ 5). Blinding of randomisation: yes Blinding of intervention: yes Complete follow‐up: no Blinding of outcome measurement: yes |
|
| Participants | Preterm infants < 33 weeks gestational age and birth weight ≤ 1250 g who required assisted ventilation between 3 and 14 days of life were eligible. 256 infants were enrolled in the study, 3 excluded due to sepsis (n = 2) and one infant had received systemic glucocorticoid therapy prior to enrolment. Demographic data: values presented as mean (SD) Beclomethasone dipropionate group: n = 123 7 died and 1 withdrawn prior to 28 days of age 4 died and 8 withdrawn between 28 days and 36 weeks' PMA Birth weight (g): 800 (193) Gestational age (weeks): 26 (2) Sex (%) male: 51 Age at enrolment (days): 5.7 (3.4) Oxygenation index at entry: 3.7 (3.1) Placebo group: n = 130 7 died and 2 withdrawn prior to 28 days 1 died and 4 withdrawn between 28 days and 36 weeks' PMA Birth weight (g): 802 (189) Gestational age (weeks): 26 (2) Sex (%) male: 53 Age at enrolment (days): 5.4 (2.9) Oxygenation index at entry: 4.1 (3.8) Exclusion criteria: Preterm infants with evidence of sepsis (clinical diagnosis or positive blood, cerebrospinal fluid, or urine culture), glucose intolerance (blood glucose > 6.7 mmol/L), hypertension (systolic blood pressure > 100 mmHg), NEC (according to physical and radiographic findings), abnormal renal function (serum creatinine > 186 mmol/L and a urine output of < 0.3 ml/kg/day), abnormal liver function (serum alanine aminotransferase concentration of > 108 U/L and serum aspartate aminotransferase concentration of > 150 U/L), major congenital anomalies, or prior systemic glucocorticoid therapy. Study centres: 3 in the US Study period: October 1993 to April 1997 |
|
| Interventions | Beclomethasone dipropionate (n = 123) Placebo (n = 130) Beclomethasone dipropionate (Beclovent, Allen and Hansbury, Glaxo Wellcome) and placebo metered‐dose inhalers (MDI) providing 42 µg/actuation were obtained from the drug manufacturer. Mode of delivery: from the MDI with a valve‐holding chamber (Aerochamber, Monaghan Medical) interposed between the neonatal anaesthesia bag and infant's endotracheal tube. The delivery procedure was standardized with respect to ventilation technique and actuation procedure for the MDI. For infants not requiring mechanical ventilation the study drug was administered by the same procedure through endotracheal tube in the pharynx. Dose: based on the desired dose of study drug to be delivered (µg/kg/day) times the infant's weight (in kg) divided by the dose exiting the endotracheal tube (in µg/actuation) equalled the total number of actuations per day. The mean dose of beclomethasone exiting the endotracheal tube was 1.7 µg/actuation (4%/actuation dose), as measured in prior studies in vitro. The desired dose was calculated to deliver 40 µg/kg/day for the first week, 30 and 15 µg/kg/day for second and third week respectively, and 10 and then 5 µg/kg/day in the fourth week. Duration of treatment: 4 weeks. Systemic glucocorticoid therapy permitted at the discretion of infant's physician (if the infant had an increasing oxygen requirement that was greater than the baseline for at least 5 days and had received the study drug for a minimum of 7 days). Treatment with the study drug was discontinued but intention‐to‐treat analysis was performed. | |
| Outcomes | Primary outcome:
Frequency of BPD at 28 days of life Secondary outcomes: Frequency of BPD at 36 weeks' PMA Duration of respiratory support Need for systemic glucocorticoid, diuretic, or bronchodilator therapy Frequency of air leak Death Length of hospitalisation The incidence of adverse events: Hypertension, hyperglycaemia, NEC, gastrointestinal haemorrhage, intracranial haemorrhage, periventricular leukomalacia, ROP, cataracts, suppression of pituitary‐adrenal axis, and growth. Chest x‐rays obtained at 28 days of age and 36 weeks' PMA were reviewed by a single radiologist unaware of the infants' study‐group assignment. |
|
| Notes | Randomisation schedule was provided by the data coordinating centre to the pharmacy at each study centre. An interim analysis was performed after 125 infants had reached 28 days of age. According to the Lan‐DeMets data monitoring rule, no significant difference was noted (P = 0.20) and therefore the study was continued. Reason for withdrawal of infants not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Multicentre randomised, double‐blind, placebo‐controlled trial. Infants were stratified for randomisation according to: study site, sex, birth weight (≤ 900 grams or > 900 grams), and severity of pulmonary disease (oxygenation index ≥ 5 or < 5). Method of sequence allocation unknown. |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete follow‐up: no |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered so we cannot tell if there was selective reporting or not. |
| Other bias | Low risk | Appears free of other bias. |
Denjean 1998.
| Methods | Multi‐centre randomised placebo‐controlled clinical trial. Study setting: 6 neonatal intensive care units in France. Study period: April 1993 to April 1995 Blinding of randomisation: no information provided Blinding of intervention: no information provided Complete follow‐up: yes Blinding of outcome measurement: no information provided |
|
| Participants | Infants with respiratory distress syndrome and PMA < 31 weeks were eligible for the study if they required ventilator support on the 10th postnatal day. 178 infants were randomised, 5 were withdrawn leaving 173 infants in the trial who were assigned to 4 groups of which 2 are included in our review. Beclomethasone: n = 43 Birth weight (g): 1082 (260) Gestational age (weeks): 27.8 (1.6) Placebo group: n = 43 Birth weight (g): 1060 (248) Gestational age (weeks): 27.6 (1.5) Exclusion criteria: infants with major malformations, sepsis, current bronchopulmonary infection, or treatment with corticosteroids or bronchodilators were not included. |
|
| Interventions | Beclomethasone (n = 43): 250 µg was given 4 times a day (1000 µg daily) starting on the 10th or 11th postnatal day and given for 28 days, with dose tapering over a period of 8 days. Placebo (n = 43): 250 µg was given 4 times a day (1000 µg daily) starting on the 10th or 11th postnatal day and given for 28 days, with dose tapering over a period of 8 days. |
|
| Outcomes | The main outcome criterion was CLD. The diagnosis of CLD was made at 28 days of age on the basis of clinical (oxygen dependence) and radiographic criteria. CLD was categorized in 3 grades of severity: severe: ventilation with ET > 3 months or oxygen supplementation > 4 months; moderate: ventilation with ET > 1 month or oxygen supplementation > 2 months; and mild: ventilation with ET < month and oxygen supplementation < 2 months Survival without CLD Death (during hospital stay) Ventilatory support (nasal IMV, CPAP or IMV) (days) Need for supplementary oxygen (days) Need for systemic dexamethasone (IV) Sepsis (positive blood culture) (no mention of meningitis) |
|
| Notes | The definition of CLD is unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The trial is described as a prospective, randomised, double‐blind trial, but no supporting evidence is provided by the authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial is described as a prospective, randomised, double‐blind trial, but no supporting evidence is provided by the authors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Initially 178 infants were randomised, but informed consent was either not obtained or withdrawn for five infants leaving 173 infants in the trial. Data reported for those 173 infants who were randomised into 4 groups. Data reported for 43 infants in each of the beclomethasone and placebo groups, which are included in this review. |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us so we cannot judge if there were any deviations from the protocol. |
| Other bias | Low risk | Appears free of other bias. |
Fok 1999.
| Methods | Randomised controlled trial. Blinding of randomisation: yes Blinding of intervention: yes Complete follow‐up: yes Blinding of outcome measurement: can't tell |
|
| Participants | Preterm infants < 32 weeks gestational age, birth weight < 1.5 kg and requiring mechanical ventilation were eligible if 6 to 10 hours after the second dose of surfactant the arterial PO₂:alveolar PO₂ was < 0.25. Demographic data: values are presented as mean (SD) Fluticasone propionate group: n = 27 Birth weight (g): 993 (369) Gestational age (weeks): 27.9 (2.6) arterial PO₂: alveolar PO₂ ratio at enrolment: 0.19 (0.05) Placebo group: n = 26 Birth weight (g): 981 (362) Gestational age (weeks): 27.1 (2.6) arterial PO₂: alveolar PO₂ ratio at enrolment: 0.19 (0.05) Exclusion criteria: Infants who were dying and those with significant congenital anomalies were excluded. Study centre: Hong Kong, China Study period: not stated |
|
| Interventions | Fluticasone propionate group (n = 27) Placebo group (n = 26) Mode of delivery: aerosol delivery was carried out using an MV15s Aerochamber inserted between the Y‐connector of the ventilator circuit and endotracheal tube. Infants extubated before day 14 received aerosol through a neonatal Aerochamber (Trudell, Canada), which was modified by removing its one way non‐rebreathing valve. This modification has been shown to increase the amount of aerosol delivery. The face mask of Aerochamber was replaced with a Laerdal Resuscitation mask (Laerdal, Stavanger, Norway) as it has a smaller dead space and a tighter fit to the face. Dose: two puffs of Fluticasone propionate (Flixotide; Glaxo, UK; 250 µg/ puff) or placebo by metered dose inhaler 12 hourly. Duration of treatment: 2 weeks. The first dose was given within 24 hours of birth. | |
| Outcomes | Primary outcomes:
Successful extubation by days 7 and 14 of age Secondary outcomes: Mortality Oxygen dependency at 28 days of postnatal age and 36 weeks' PMA Adverse events: hyperglycaemia, hypertension, sepsis confirmed by blood culture, pulmonary air leak (interstitial emphysema, pneumothorax, or pneumomediastinum), periventricular haemorrhage and leukomalacia, ROP, PDA, NEC and bacterial colonization of the airway Hyperglycemia was defined as a blood glucose reading > 7 mmol/L. Hypertension was defined as 2 consecutive readings of systolic or diastolic blood pressure ˃ 80 mmHg and 45 mmHg respectively. Symptomatic PDA was treated with intravenous indomethacin after confirmation by echocardiogram and refractory duct was closed by surgical ligation. Cranial ultrasound scans were performed at 6, 14 and 28 days of age, and when periventricular haemorrhage was suspected clinically. Ophthalmology screening for ROP was started at 4 weeks of age. NEC was diagnosed by the presence of pneumatosis intestinalis or intestinal perforation on abdominal radiograph, of for those requiring surgical intervention, on laparotomy. Tracheal aspirates for bacterial and fungal cultures were obtained immediately before the first dose of aerosol, and at 3, 5, 7 and 14 days of age. Static respiratory system compliance (Crs) and resistance (Rrs) were measured immediately before the start of aerosol treatment, and repeated on days 3, 7, and 14 in infants who remained intubated and ventilated. Both Crs and Rrs were measured using a SensorMedics Pulmonary Cart (SensorMedics Inc., Yorba Linda, CA, USA) using the passive flow‐volume technique. The measurement were carried out using a pneumotachograph (Hans Rudolph Inc., USA) with a small dead space (1.8 ml) connected to the endotracheal tube. |
|
| Notes | Infants were randomised using computer‐generated random numbers into treatment and control groups and allocation to the groups was performed using opaque, sealed envelopes.
All infants were given two doses (5 ml/kg/dose) of intratracheal synthetic surfactant (Exosurf) 12 hours apart. The first dose was given within 1 hour of birth. Extubation was considered when the FiO₂ and ventilator rate decreased to < 0.4 and < 10 breaths/minute, respectively. The decision to extubate was made by the attending neonatologists who were blinded to the study protocol and the nature of the aerosol given to the infants. All infants were given a loading dose of intravenous aminophylline (6 mg/kg) prior to extubation followed by a maintenance dose of 2.5 mg/kg every 12 hourly. Extubation was considered successful if the infant was able to breathe spontaneously without the endotracheal tube or assisted ventilation for at least 48 hours without a significant increase in respiratory effort and deterioration in blood gas values. In both groups infants with significant respiratory problems after 14 days of age were given open label systemic dexamethasone as decided by the attending neonatologists. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised controlled trial Method of sequence generation unknown |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome measurement: can't tell |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered so we cannot tell if there was selective reporting or not. |
| Other bias | Low risk | Appears free of other bias. |
Jangaard 2002.
| Methods | Randomised controlled trial Blinding of randomisation: yes Blinding of intervention: yes Complete follow‐up: yes Blinding of outcome measurement: can't tell |
|
| Participants | Preterm infants < 1250 grams diagnosed with RDS and requiring ventilatory support at 72 hours Demographic data: values presented as mean (SD) Beclomethasone group: n = 30 Birth weight (g): 882 (204) Gestational age (weeks): 27.2 (2) Sex (%) male: 43 Age at enrolment (hours): 72 Placebo group: n = 30 Birth weight (g): 917 (178) Gestational age (weeks): 27.9 (2) Gender (%) male: 43 Age at enrolment (hours): 72 Exclusion criteria: Infants with congenital anomalies Non‐survival to 72 hours Study centre: Halifax, Canada Study period: October 1996 to October 1998 |
|
| Interventions | Beclomethasone dipropionate (n = 30) Placebo (n = 30) Beclomethasone dipropionate (250 µg/puff) Mode of delivery: in‐line in respiratory limb of ventilator circuit with Medilife spacer via Aerochamber with mask. Dose: medication dosage assumed a deposition of 10% of the dose given and aimed for a total dose of 0.2 mg/kg/day. Based on birth weight: 500 to 749 g 1 puff q6h 750 to 999 g 2 puffs q8h 1000 to 1249 g 2 puffs q6h Duration of treatment: 28 days | |
| Outcomes | Primary outcome:
BPD ‒ defined by 28 day oxygen dependency Secondary outcomes: Need for systemic corticosteroid therapy, incidence of sepsis, PVL, ROP, hypertension. |
|
| Notes | Randomisation was performed for 3 weight strata in blocks of 4 using sealed envelopes. Data obtained from the author regarding the mode of delivery, demographic characteristics of the study groups, and adverse events. Data published in the abstract differ from those obtained from the investigator. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised controlled trial Method of sequence generation unknown |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome measurement: can't tell |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered so we cannot tell if there was selective reporting or not. |
| Other bias | Low risk | Appears free of other bias. |
Jonsson 2000.
| Methods | Randomised double‐blind placebo‐controlled trial Blinding of randomisation: yes Blinding of intervention: yes Complete follow‐up: yes Blinding of outcome measurement: can't tell |
|
| Participants | Very low birth weight infants who were mechanically ventilated on day 6 of life or if extubated, nasal continuous positive airway pressure with FiO₂ of ≥ to 0.3 were included. Exclusion criteria: Congenital malformations, congenital heart disease and IVH (grades III‐IV) Demographic data: values are presented as median (range) or number (%) Budesonide group: n = 15 Gestational age (weeks): 25 (23 to 27) Birth weight (g): 766 (525 to 1122) Sex (M/F): 7/8 Prenatal steroids: 12 (80%) Surfactant treatment: 14 (93%) Placebo group: n = 15 Gestational age (weeks): 26 (24 to 29) Birth weight (g): 813 (630 to 1227) Sex (M/F): 5/10 Prenatal steroids: 10 (67%) Surfactant treatment: 15 (100%) |
|
| Interventions | Budesonide (Pulmicort) (Astra Draco, Lund, Sweden) or placebo aerosol was used. The drug was delivered using an electronic dosimetric jet nebulizer (Spira Electro 4, Respiratory Centre, Hameenlinna, Finland) Dose: 500 µg twice a day | |
| Outcomes | Primary outcome: reduction in the FiO₂ levels after 14 days of treatment Secondary outcomes include: duration of supplemental oxygen, duration of mechanical ventilation, duration of nasal CPAP, oxygen requirements at 28 days of age and at 36 weeks' PMA, adrenal cortisol response to stimulation at baseline (prior to commencement of inhalation) and at the end of the study period. Information on adverse events: hyperglycaemia, hypertension, sepsis, PDA, IVH and gastrointestinal problems were collected. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised controlled trial Method of sequence generation unknown |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome measurement: can't tell |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered so we cannot tell if there was selective reporting or not. |
| Other bias | Low risk | Appears free of other bias |
Merz 1999.
| Methods | Randomised double‐blind placebo controlled trial Blinding of randomisation: yes Blinding of intervention: yes Complete follow‐up: yes Blinding of outcome measurement: can't tell |
|
| Participants | Preterm infants with birth weight of 750 to 1500 grams, gestational age of 25 to 32 weeks, ventilator dependency on day 3 of life with a ventilator rate ≥ 15 breaths/min and FiO₂ of > 0.25 to maintain an oxygen saturation of > 90%. 24 infants were enrolled in the study, one infant in the placebo group withdrawn due to severe sepsis 1 day after starting inhalation therapy. Demographic data: values are presented as median (range) Budesonide group: n = 12 Birth weight (g): 1108 (820 to 1420) Gestational age (weeks): 28 (27 to 32) Sex (% male): 42 Age at enrolment (hours): 72 Placebo group: n = 11 Birth weight (g): 1120 (880 to 1480) Gestational age (weeks): 29 (27 to 31) Sex (% male): 45 Age at enrolment (hours): 72 Exclusion criteria: Infants with multiple or severe congenital anomalies such as complex congenital heart disease, suspected chromosomal abnormalities, evidence or even suspected sepsis or pneumonia, IVH grade III or IV at the time of randomisation and infants intubated with an endotracheal tube size < 2.5 mm. Study centre: Aachen, Germany Study period: November 1995 to August 1996 |
|
| Interventions | Budesonide (Astra Draco, Lund, Sweden) or placebo aerosol were used. Two puffs of budesonide (200 µg/puff) or placebo was administered 4 times a day for a total of 10 days or until the infants were extubated. The aerosol was delivered using metered dose inhaler and an Aerochamber (Aerochamber MV15, Trudell Medical, Ontario, Canada). The spacer was directly connected to the endotracheal tube and the distal end of the spacer was connected to a manual puffer. | |
| Outcomes | Primary outcome:
duration of artificial ventilation Secondary outcomes: duration of supplemental oxygen Release of albumin and different inflammatory mediators in the tracheobronchial aspirate fluid Adverse events: frequency of acute infections, hypertension, hyperglycaemia and adrenal suppression was evaluated. CLD was defined as requirement of supplemental oxygen at 28 days of life and at 36 weeks' PMA. Hyperglycaemia was defined as blood glucose > 8.3 mmol/L. Hypertension was defined as systolic and diastolic blood pressure > 2 SD from mean values. Acute infection was suspected if clinical deterioration was observed accompanied by a rise in C‐reactive protein or by ratio of immature to mature granulocytes above 0.2 in complete blood cell count. A corticotropin‐releasing hormone stimulation test (CRH test) was performed on day 14 after completion of the inhalation treatment. |
|
| Notes | Infants were ventilated with Stephan respirator HF 300 (Fa. Stephan, Gackenbach, Germany) in the IPPV or IMV mode. To facilitate weaning from the ventilator infants were treated with fluid restriction (120 ml/kg/day). No nebulized bronchodilators or diuretics or supplemental vitamin A or E were used. Infants with respiratory distress syndrome were treated with natural surfactant (Alveofact) up to a maximum of 3 doses. Extubation was performed if the ventilator rate was down to 8 breaths/minute and 2 periods of tracheal continuous positive airway pressure lasting 20 minutes were tolerated. Theophylline or caffeine citrate was used to treat apnoea of prematurity. Infants who could not be weaned from ventilator on day 14 were treated with systemic glucocorticoids after day 14. Dexamethasone was administered intravenously and divided into two doses: starting dose of 0.5 mg/kg/day for the first 3 days followed by 0.3 mg/kg/day of day 4‐6. From day 7 the dose was reduced to 0.1 mg/kg/day and this was administered on alternate day from day 10 to day 16. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised controlled trial Method of sequence generation unknown |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome measurement: can't tell |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered so we cannot tell if there was selective reporting or not. |
| Other bias | Low risk | Appears free of other bias. |
Nakamura 2016.
| Methods | Randomised double‐blind placebo controlled trial Blinding of randomisation: yes Blinding of intervention: yes Complete follow‐up: yes Blinding of outcome measurement: yes for all outcomes except for neuromotor examination |
|
| Participants | Infants (n = 211) with birth weight < 1000 grams who needed endotracheal intubation and respiratory support due to respiratory failure. Demographic data: values are presented as mean (SD) or mean (range) or percentage Fluticasone propionate group: n = 107 Birthweight (g): 784 (135) Gestational age (weeks): 26.1 (25.1 to 27.3) Sex (% male): 58.9 Placebo group: n = 104 Birth weight (g): 784 (127) Gestational age (weeks): 26.2 (25.1 to 27.3) Sex (% male): 48.1 |
|
| Interventions | Prophylactic inhaled steroids starting within 24 h of birth and continuing until 6 weeks of age or extubation. Two doses of 50 µg fluticasone propionate (FP) were administered every 24 h (n = 107). The placebo contained only hydrofluoroalkane propellant (n = 104). | |
| Outcomes | The primary outcome measure used to indicate the morbidity of severe BPD was death or oxygen dependence at discharge. The secondary outcomes were death, severe BPD and neurodevelopmental outcomes at 18 months' PMA and 3 years of age | |
| Notes | Because of financial constraints the study was stopped early. The authors reported all outcomes as a combination of death and a clinical complication of preterm birth. We wrote to the first author on 20 May 2016, for clarifications regarding outcomes, but as of 20th July 2016 we have not received a response. We chose to report the data as per the authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent Internet‐based patient registration and randomisation robot. Central randomisation. |
| Allocation concealment (selection bias) | Low risk | Fluticasone propionate (FP) and placebo metered‐dose inhalers providing 50 g per actuation were obtained from the drug manufacturer. Fluticasone propionate and placebo were delivered from the metered‐dose inhaler with a valve space chamber interposed between the neonatal anaesthesia bag and the endotracheal tube. Double‐blind placebo‐controlled trial. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Staff was blinded to FP and placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcomes were assessed blinded to the groups except for the neuromotor exams. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants enrolled are accounted for. |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us so we cannot ascertain if there were any deviations from the protocol. Because of financial constraints the study was stopped early. |
| Other bias | Low risk | Appears free of other bias. |
Townsend 1998.
| Methods | Randomised double‐blind placebo‐controlled trial Blinding of randomisation: yes Blinding of intervention: yes Complete follow‐up: yes Blinding of outcome measurement: can't tell |
|
| Participants | Preterm infants < 28 weeks and ≤ 1100 grams at birth who were ventilator dependent due to RDS were enrolled at 48 to 96 hours of age. Demographic values: Flunisolide group: n = 15 Gestational age (weeks): 25.8 Birth weight (g): 728 g Age at enrolment: 3.1 days Placebo group: n = 17 Gestational age (weeks): 25.5 Birth weight (g): 695 g Age at enrolment: 3.4 days |
|
| Interventions | Flunisolide or placebo 500 µg 3 times a day via spacer chamber connected to the ventilator | |
| Outcomes | Outcome assessed: Need for systemic steroids Days on ventilator, in hospital and oxygen supplementation Information on adverse events were collected: hypertension, hyperglycaemia, infection, weight gain and complications of prematurity | |
| Notes | The authors do not state whether the demographic data are presented as means or medians | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised controlled trial Method of sequence generation unknown |
| Allocation concealment (selection bias) | Low risk | Randomised double‐blind placebo controlled trial Blinding of randomisation: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome measurement: can't tell |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered so we cannot tell if there was selective reporting or not. |
| Other bias | Unclear risk | Study published in abstract form and not enough information was provided to judge if there were other bias or not. |
Yong 1999.
| Methods | Randomised double‐blind placebo controlled trial.
Infants were stratified for gestational age: 24 to 26 weeks and 27 to 32 weeks. Blinding of randomisation: yes Blinding of intervention: yes Complete follow‐up: yes Blinding of outcome measurement: can't tell |
|
| Participants | Preterm infants < 32 weeks and requiring mechanical ventilation from birth were recruited within 18 hours of birth. 40 infants enrolled in the study. Demographic data: values are presented as mean (SD) Fluticasone propionate group: n = 20 Birth weight (g): 1011 (223) Gestational age (weeks): 27.4 (1.7) Sex (%) male: 65 Placebo group: n = 20 Birth weight (g): 932 (401) Gestational age (weeks): 27.7 (1.7) Gender (%) male: 60 Exclusion criteria: Preterm infants with major congenital anomalies, congenital pneumonia, pneumothorax and pulmonary hypoplasia Study centre: Jessop Hospital for Women, Sheffield, UK. |
|
| Interventions | Fluticasone propionate or placebo (expient without active ingredient) Mode of delivery: MDI and Aerochamber if ventilated, Babyhaler if extubated Dose: one puff of fluticasone propionate (250 µg/puff) twice daily Duration of therapy: 14 days |
|
| Outcomes | Data were collected on survival, duration of mechanical ventilation and oxygen supplementation and other measures of morbidity (BP, glucose and IVH). Weight gain and skeletal growth was assessed by knemometry. | |
| Notes | Additional data from the investigators were available for this trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised double‐blind placebo controlled trial.
Infants were stratified for gestational age: 24 to 26 weeks and 27 to 32 weeks. Method of sequence generation unknown. |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome measurement: can't tell |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered so we cannot tell if there was selective reporting or not. |
| Other bias | Low risk | The principal investigator provided additional data. |
BPD = bronchopulmonary dysplasia
CLD = chronic lung disease
CPAP = continuous positive airway pressure
ET = endotracheal tube
FiO2 = fraction of inspired oxygen
g = grams
IMV = intermittent mandatory ventilation
IV = intravenous
IVH = intraventricular haemorrhage
IQR = inter‐quartile range
µg = micrograms
n = number
NEC = necrotizing enterocolitis
PDA = patent ductus arteriosus
PMA = postmenstrual age
PO2 = partial pressure of oxygen
ROP = retinopathy of prematurity
SD = standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beresford 2002 | Excluded as infants who required supplemental oxygen at 36 weeks' PMA were included. |
| Dugas 2005 | Excluded as infants were randomised between 28 and 60 days of age. |
| Kovacs 1998 | Excluded because a combination of systemic (dexamethasone) and inhaled corticosteroid (budesonide) was used. |
| Yeh 2016 | The study compared the effect of intratracheal administration of surfactant/budesonide with that of surfactant alone on the incidence of death or BPD. This study design did not meet our review objectives of "To determine the impact of inhaled corticosteroids administered to ventilated preterm infants with birth weight of ≤ 1500 grams beginning in the first two weeks of life for the prevention of CLD as reflected by the requirement for supplemental oxygen at 36 weeks' PMA". |
Differences between protocol and review
In a deviation from our protocol for this update we included all infants on any form of assisted ventilatory support (nasal CPAP/IMV or endotracheal IMV). Nakamura 2016 reported all outcomes as a combination of death and a complication of preterm birth. We wrote to the authors for clarifications regarding the outcomes on 20 May 2016 but as of 20 July 2016 we have not received a response. We report their findings separately in Analysis 1.23 to Analysis 1.32 as their data could not be incorporated in any of the meta‐analyses. This is a deviation from our protocol.
Contributions of authors
Vibhuti Shah (VS): performance of literature search, abstraction and analysis of data, writing of the original and updated reviews.
Arne Ohlsson (AO): performance of literature search, abstraction and analysis of data and editing of the original and updated reviews.
Henry L Halliday (HLH): editing of this update of the review.
Michael Dunn (MD): editing of this update of the review.
The searches for the 2011 update were completed by VS and AO. The searches in 2016 were conducted by Yolanda Brosseau at Cochrane Neonatal in Burlington, Vermont. This update was reviewed and approved by VS, AO, MD and HH.
Sources of support
Internal sources
Mount Sinai Hospital, Toronto, Canada.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201600005C.
Declarations of interest
Vibhuti Shah: no conflict of interest to declare
Arne Ohlsson: no conflict of interest to declare
Henry Halliday is the co‐author of one included trial (Bassler 2015). He acts as a consultant for Chiesi Farmaceutici, Parma, Italy and is also joint Editor‐in‐Chief of the journal Neonatology.
Michael Dunn: no conflict of interest to declare
Deceased
Edited (no change to conclusions)
References
References to studies included in this review
Bassler 2015 {published data only}
- Bassler D, Halliday HL, Plavka R, Hallman M, Shinwell ES, Jarreau PH, et al. The Neonatal European Study of Inhaled Steroids (NEUROSIS): An eu‐funded international randomised controlled trial in preterm infants. Neonatology 2010;97(1):52‐5. [DOI] [PubMed] [Google Scholar]
- Bassler D, Plavka R, Shinwell ES, Hallman M, Jarreau PH, Carnielli V, et al. Early inhaled budesonide for the prevention of bronchopulmonary dysplasia. New England Journal of Medicine 2015;373(16):1497‐506. [DOI: 10.1056/NEJMoa1501917] [DOI] [PubMed] [Google Scholar]
Cole 1999 {published data only}
- Cole CH, Colton T, Shah BL, Abbasi S, MacKinnon BL, Demissie S, et al. Early inhaled glucocorticoid therapy to prevent bronchopulmonary dysplasia. New England Journal of Medicine 1999;340(13):1005‐10. [DOI] [PubMed] [Google Scholar]
- Cole CH, Shah B, Abbasi S, Demissie S, MacKinnon B, Colton T, et al. Adrenal function in premature infants during inhaled beclomethasone therapy. Journal of Pediatrics 1999;135(1):65‐70. [DOI] [PubMed] [Google Scholar]
- Gupta GK, Cole CH, Abbasi S, Demissie S, Njinimbam C, Nielsen HC, et al. Effects of early inhaled beclomethasone therapy on tracheal aspirate inflammatory mediators IL‐8 and IL‐1ra in ventilated preterm infants at risk for bronchopulmonary dysplasia. Pediatric Pulmonology 2000;30(4):275‐81. [DOI] [PubMed] [Google Scholar]
Denjean 1998 {published data only}
- Denjean A, Paris‐Llado J, Zupan V, Debillon T, Kieffer F, Magny JF, et al. Inhaled salbutamol and beclomethasone for preventing broncho‐pulmonary dysplasia: a randomised double‐blind study. European Journal of Pediatrics 1998;157(11):926‐31. [DOI] [PubMed] [Google Scholar]
Fok 1999 {published data only}
- Fok TF, Lam K, Dolovich M, Ng PC, Wong W, Cheung KL, et al. Randomised controlled study of early use of inhaled corticosteroid in preterm infants with respiratory distress syndrome. Archives of Disease in Childhood. Fetal and Neonatal Edition 1999;80(3):F203‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng PC, Fok TF, Wong GWK, Lam CWK, Lee CH, Wong MY, et al. Pituitary‐adrenal suppression in preterm, very low birth weight infants after inhaled fluticasone propionate treatment. Journal of Clinical Endocrinology and Metabolism 1998;83(7):2390‐3. [DOI] [PubMed] [Google Scholar]
Jangaard 2002 {published data only}
- Jangaard KA, Frent GA, Vincer MJ. Prophylactic inhaled beclomethasone dipropionate in infants < 1250 gms for the prevention of BPD. Pediatric Research 1998;43:177A. [Google Scholar]
- Jangaard KA, Stinson DA, Allen AC, Vincer MJ. Early prophylactic inhaled beclomethasone in infants < 1250 gms for the prevention of chronic lung disease. Paediatrics & Child Health 2002;7(1):13‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jonsson 2000 {published data only}
- Jonsson B, Eriksson M, Soder O, Broberger U, Lagercrantz H. Budesonide delivered by dosimetric jet nebulization to preterm very low birthweight infants at high risk for development of chronic lung disease. Acta Paediatrica 2000;89(12):1449‐55. [DOI] [PubMed] [Google Scholar]
Merz 1999 {published data only}
- Merz U, Kusenbach G, Hausler M, Peschgens T, Hornchen H. Inhaled budesonide in ventilator dependent preterm infants: A randomized, double‐blind pilot study. Biology of the Neonate 1999;75:46‐53. [DOI] [PubMed] [Google Scholar]
Nakamura 2016 {published data only}
Townsend 1998 {unpublished data only}
- Townsend SF, Hale KA, Thilo EH. Early treatment with inhaled steroids does not improve outcome in extremely premature infants with respiratory distress. Pediatric Research 1998;43:300A. [Google Scholar]
Yong 1999 {published and unpublished data}
- Yong WSC, Carney S, Pearse RG, Gibson AT. The effect of inhaled fluticasone propionate (FP) on premature babies at risk for developing chronic lung disease of prematurity. Archives of Disease in Childhood 1999;80:G64. [Google Scholar]
References to studies excluded from this review
Beresford 2002 {published data only}
- Beresford MW, Primhak R, Subhedar NV, Shaw NJ. Randomised double blind placebo controlled trial of inhaled fluticasone propionate in infants with chronic lung disease. Archives of Diseases in Childhood. Fetal and Neonatal Edition 2002;87:62‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dugas 2005 {published data only}
- Dugas MA, Nguyen D, Frenette L, Lachance C, St‐Onge O, Fougeres A, et al. Fluticasone inhalation in moderate cases of bronchopulmonary dysplasia. Pediatrics 2005;115:e566‐72. [DOI] [PubMed] [Google Scholar]
Kovacs 1998 {published data only}
- Kovacs L, Davis GM, Faucher D, Papageorgiou A. Efficacy of sequential early systemic and inhaled corticosteroid therapy in the prevention of chronic lung disease of prematurity. Acta Paediatrica 1998;87:792‐8. [DOI] [PubMed] [Google Scholar]
Yeh 2016 {published data only}
- Yeh TF, Chen CM, Wu SY, Husan Z, Li TC, Hsieh WS, et al. Intratracheal administration of budesonide/surfactant to prevent bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine 2016;193(1):86‐95. [DOI] [PubMed] [Google Scholar]
Additional references
Arnon 1992
- Arnon S, Grigg J, Nikander K, Silverman M. Delivery of micronised budesonide suspension by metered dose inhaler and jet nebuliser into a neonatal ventilator circuit. Pediatric Pulmonology 1992;13(3):172‐5. [DOI] [PubMed] [Google Scholar]
Bancalari 2016
- Bancalari E, Jain D, Jobe AH. Prevention of bronchopulmonary dysplasia: Are intratracheal steroids with surfactant a magic bullet?. American Journal of Respiratory and Critical Care Medicine 2016;193(1):12‐3. [DOI] [PubMed] [Google Scholar]
Beam 2014
- Beam KS, Aliaga S, Ahlfeld SK, Cohen‐Wolkowiez M, Smith PB, Laughon MM. A systematic review of randomized controlled trials for the prevention of bronchopulmonary dysplasia in infants. Journal of Perinatology 2014;34(9):705‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhuta 1998
- Bhuta T, Ohlssson A. Systematic review and meta‐analysis of early postnatal dexamethasone for prevention of chronic lung disease. Archives of Disease in Childhood. Fetal and Neonatal Edition 1998;79(1):F26‐F33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 2001
- Davis PG, Henderson‐Smart DJ. Intravenous dexamethasone for extubation of newborn infants. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD000308] [DOI] [PubMed] [Google Scholar]
Doyle 1999
- Doyle LW, Davis PG. Postnatal corticosteroids in preterm infants ‐ effects on mortality and cerebral palsy. Pediatric Research 1999;45:194A. [Google Scholar]
Doyle 2005
- Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. Impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk of chronic lung disease. Pediatrics 2005;115(3):655‐61. [DOI] [PubMed] [Google Scholar]
Doyle 2014a
- Doyle LW, Halliday HL, Ehrenkranz RA. Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD001146.pub3] [DOI] [PubMed] [Google Scholar]
Doyle 2014b
- Doyle LW, Ehrenkranz RA, Halliday HL. Late (> 7 days) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD001145.pub3] [DOI] [PubMed] [Google Scholar]
Doyle 2014c
- Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. An update on the impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk of bronchopulmonary dysplasia. The Journal of Pediatrics 2014;165(6):1258‐60. [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- McMaster University, www.gradepro.org. The Cochrane Collaboration. GRADEpro Version [20150131]. 2014.. Hamilton, Ontario, Canada: McMaster University, www.gradepro.org. The Cochrane Collaboration, 2014.
Grigg 1992
- Grigg J, Arnon S, Jones T, Clarke A, Silverman M. Delivery of therapeutic aerosols to intubated babies. Archives of Disease in Childhood 1992;67(1 Spec No):25‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ibrahim 2011
- Ibrahim H, Sinha IP, Subhedar NV. Corticosteroids for treating hypotension in preterm infants. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD003662.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jefferies 2012
- Jefferies AL, Lacaze‐Masmonteil T, Newhook LA, Peliowski A, Sorokan ST, Stanwick R, et al. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Paediatrics and Child Health 2012;17(10):573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 1979
- Johnson WCJ, Mitzner W, London WT, Palmer AE, Scott R. Betamethasone and the rhesus fetus: multisystemic effects. American Journal of Obstetrics and Gynecology 1979;133(6):677‐84. [DOI] [PubMed] [Google Scholar]
Ng 1993
- Ng PC. The effectiveness and side effects of dexamethasone in preterm infants with bronchopulmonary dysplasia. Archives of Disease in Childhood 1993;68(3 Spec No):330‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Brodovich 1985
- O'Brodovich HM, Mellins RB. Bronchopulmonary dysplasia. Unresolved neonatal acute lung injury. American Review of Respiratory Disease 1985;132(3):694‐709. [DOI] [PubMed] [Google Scholar]
O'Callaghan 1992
- O'Callaghan C, Hardy J, Stammers J, Stephenson T, Hull D. Evaluation of techniques for delivery of steroids to lungs of neonates using a rabbit model. Archives of Disease in Childhood 1992;67(1 Spec No):20‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Onland 2012
- Onland W, Offringa M, Kaam A. Late (≥ 7 days) inhalation corticosteroids to reduce bronchopulmonary dysplasia in preterm infants. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD002311.pub2] [DOI] [PubMed] [Google Scholar]
Pierce 1995
- Pierce MR, Bancalari E. The role of inflammation in the pathogenesis of bronchopulmonary dysplasia. Pediatric Pulmonology 1995;19(6):371‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schmidt 2015
- Schmidt B. No end to uncertainty about inhaled glucocorticosteroids in preterm infants. New England Journal of Medicine 2015;373(16):1566‐7. [DOI] [PubMed] [Google Scholar]
Schünemann 2013 [Computer program]
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GWG. GRADE handbook for grading quality of evidence and strength of recommendations. www.guidelinedevelopment.org/handbook, 2013.
Shah 2003
- Shah SS, Ohlsson A, Halliday HL, Shah VS. Inhaled versus systemic corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD002058] [DOI] [PubMed] [Google Scholar]
Shah 2007a
- Shah VS, Ohlsson A, Halliday HL, Dunn M. Early administration of inhaled corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD001969.pub2] [DOI] [PubMed] [Google Scholar]
Shah 2007b
- Shah SS, Ohlsson A, Halliday HL, Shah VS. Inhaled versus systemic corticosteroids for the treatment of chronic lung disease in ventilated very low birth weight preterm infants. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD002057.pub2] [DOI] [PubMed] [Google Scholar]
Shaw 1993
- Shaw NJ, Gill AB, Weindling AM, Cooke RW. The changing incidence of chronic lung disease. Health Trends 1993;25(2):50‐3. [PubMed] [Google Scholar]
Sinkin 1987
- Sinkin RA, Phelps DL. New strategies for the prevention of bronchopulmonary dysplasia. Clinics in Perinatology 1987;14(3):599‐620. [PubMed] [Google Scholar]
Sinkin 1990
- Sinkin RA, Cox C, Phelps DL. Predicting risk for bronchopulmonary dysplasia: selection criteria for clinical trials. Pediatrics 1990;86(5):728‐36. [PubMed] [Google Scholar]
Ward 2003
- Ward MC, Sinn JKH. Steroid therapy for meconium aspiration syndrome in newborn infants. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003485] [DOI] [PMC free article] [PubMed] [Google Scholar]
Watterberg 1996
- Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 1996;97(2):210‐5. [PubMed] [Google Scholar]
Weichsel 1977
- Weichsel ME. The therapeutic use of glucocorticoid hormones in the perinatal period: Potential neurologic hazards. Annals of Neurology 1977;2(5):364‐6. [DOI] [PubMed] [Google Scholar]
Yeh 1998
- Yeh TF, Lin YJ, Huang CC, Chen YJ, Lin CH, Lin HC, et al. Early dexamethasone therapy in preterm infants: a follow‐up study. Pediatrics 1998;101(5):E7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Shah 2000
- Shah V, Ohlsson A, Halliday HL, Dunn MS. Early administration of inhaled corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD001969] [DOI] [PubMed] [Google Scholar]
Shah 2007
- Shah VS, Ohlsson A, Halliday HL, Dunn M. Early administration of inhaled corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD001969.pub2] [DOI] [PubMed] [Google Scholar]
Shah 2012
- Shah VS, Ohlsson A, Halliday HL, Dunn M. Early administration of inhaled corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD001969.pub3] [DOI] [PubMed] [Google Scholar]


