Abstract
Background
Pancreatic cancer causes severe pain in 50 to 70% of patients and is often difficult to treat. Celiac plexus block (CPB) is thought to be a safe and effective technique for reducing the severity of pain.
Objectives
To determine the efficacy and safety of celiac plexus neurolysis in reducing pancreatic cancer pain, and to identify adverse effects and differences in efficacy between the different techniques.
Search methods
We searched Cochrane CENTRAL, MEDLINE, GATEWAY and EMBASE from 1990 to December 2010.
Selection criteria
Randomised controlled trials (RCTs) of CPB by the percutaneous approach or endoscopic ultrasonography (EUS)‐guided neurolysis in adults with pancreatic cancer at any stage, with a minimum of four weeks follow‐up.
Data collection and analysis
We recorded details of study design, participants, disease, setting, outcome assessors, pain intensity (visual analogue scale (VAS)) and methods of calculation.
Main results
The search identified 102 potentially eligible studies. Judged from the information in the title and abstract six of these concerning the percutaneous block, involving 358 participants, fulfilled the inclusion criteria and were included in the review. All were RCTs in which the participants were followed for at least four weeks. We excluded studies published only as abstracts. We identified one RCT comparing EUS‐guided or computed tomography (CT) ‐guided CPB but its aim was to assess efficacy in controlling chronic abdominal pain associated with chronic pancreatitis rather than pancreatic cancer, so it was excluded.
For pain (VAS) at four weeks the mean difference was ‐0.42 in favour of CPB (95% confidence interval (CI) ‐0.70 to ‐ 0.13, P = 0.004, fixed‐effect model). At eight weeks the mean difference was ‐0.44 (95% CI ‐0.89 to ‐ 0.01, random‐effects model). At eight weeks there was significant heterogeneity (I2 = 89%).
Opioid consumption was significantly lower in the CPB group than the control group (P < 0.00001).
Authors' conclusions
Although statistical evidence is minimal for the superiority of pain relief over analgesic therapy, the fact that CPB causes fewer adverse effects than opioids is important for patients. Further studies and RCTs are recommended to demonstrate the potential efficacy of a less invasive technique under EUS guidance.
Plain language summary
Celiac plexus block (CPB) in patients with unresectable pancreatic cancer‐related pain
Abdominal pain is a major symptom in patients with inoperable pancreatic cancer and is often difficult to treat. Celiac plexus block (CPB) is a safe and effective method for reducing this pain. It involves the chemical destruction of the nerve fibres that convey pain from the abdomen to the brain. We searched for studies comparing CPB with standard analgesic therapy in patients with inoperable pancreatic cancer. We were interested in the primary outcome of pain, measured on a visual analogue scale (VAS). We also looked at the amount of opioid (morphine‐like drugs) patients took (opioid consumption) and adverse effects of the treatment. Six studies (358 participants) comparing CPB with standard therapy (painkillers) met our inclusion criteria. At four weeks pain scores were significantly lower in the CPB group. Opioid consumption was also significantly lower than in the control group. The main adverse effects were diarrhoea or constipation (this symptom was significantly more likely in the control group, where opioid consumption was higher). Endoscopic ultrasonography (EUS)‐guided CPB is becoming popular as a minimally invasive technique that has fewer risks, but we were not able to find any RCTs assessing this method (current medical literature on this subject is limited to studies without control groups). Although the data on EUS‐guided CPB and pain control are promising, we await rigorously designed RCTs that may validate these findings. We conclude that, although statistical evidence is minimal for the superiority of pain relief over analgesic therapy, the fact that CPB causes fewer adverse effects than opioids is important for patients.
Background
Pancreatic cancer is the fifth leading cause of cancer‐related mortality in the United States, with an estimated 33,370 deaths attributable to this disease in 2007. The annual mortality rate closely approximates the annual incidence rate, which reflects the generally short survival with pancreatic cancer ‐‐ mostly less than one year. Advancing age is the strongest risk factor for pancreatic cancer, with the vast majority of cases occurring after the age of 60 years. There is also an association with cigarette smoking but the roles of diet, alcohol, and coffee have not been substantiated and should not be considered proven risk factors (Maisonneuve 2010).
Pancreatic diseases such as cancer can cause clinically significant pain in the upper abdomen, which may radiate to the back. Pain management for pancreatic cancer patients is one of the most important aspects of their care, as it is one of the most weakening symptoms. The best approach involves adequate therapy with constant assessment.
The current management of pancreatic pain follows the WHO three‐step ladder for pain control, starting with non‐opioid analgesics such as nonsteroidal anti‐inflammatory drugs (NSAIDs) and progressing to increasing doses of opioid analgesics (WHO 2008). For pain that does not respond to drugs, or when oral or topical medication leads to unacceptable side effects such as nausea, constipation, somnolence, confusion, dependence, and addiction, an alcohol nerve block may be indicated. This provides pain relief by acting directly on the nerves (celiac plexus) that carry painful stimuli from the diseased pancreas to the brain.
The main aim of this review was to study the efficacy of CPB in the control of pancreatic cancer pain in comparison with standard analgesic therapy. We also did a meta‐analysis to help physicians choose the best treatment for patients with this pain.
Description of the condition
Pancreatic cancer causes severe pain in 50% to 70% of patients. This kind of pain is multi‐factorial (pancreatic duct obstruction and hypertension, neural invasion) and it is often difficult to treat (Staatas 2001). Different mechanisms perpetuate pancreatic pain: infiltration of nerve sheaths and neural ganglia, increased ductal and interstitial pressure, and gland inflammation. Pancreatic pain is generally transmitted through the celiac plexus, a neural structure located in the upper abdomen, near the emergence of the celiac trunk from the aorta.
Description of the intervention
Many different pharmacotherapeutic approaches have been used to control pancreatic pain caused by cancer, including NSAIDs and narcotic analgesics, but patients' responses are often unpredictable and variable. Opioids have adverse effects (constipation, dry mouth, nausea, vomiting and drowsiness) that may reduce quality of life (QoL) and NSAIDs may cause gastrointestinal disturbances and cardiovascular events. As a consequence, alternative approaches to pain management have been explored.
Celiac plexus neurolysis was first described by Kappis 1919 and is done at the level of the L1 vertebral body, with the patient in the prone position. There are a number of variations on the technique (Giménez 1993). It has been described in the literature since the 1950s but the first prospective study was published in 1990, and the first randomised study in 1992. Improvements in technique have made the treatment safer and more effective for patients. Celiac plexus neurolysis can be done surgically under fluoroscopic guidance, or under computed tomography (CT) guidance.
Percutaneous celiac nerve block
The targets for celiac axis destruction are the splanchnic nerves and/or celiac ganglia. The splanchnic nerves cross the diaphragm, enter the abdominal cavity, and form the celiac plexus. The celiac ganglia are located around the celiac artery anterior to the aorta, in varying positions, from T12 to L2. They can be reached percutaneously by different routes, with one needle through the anterior approach (under computed tomography (CT) or ultrasound guidance) or with one or two needles through the posterior approach.
The classic retrocrural technique was first described by Kappis 1919. The landmarks include the spinous processes of the T12 and L1 vertebra, the lower border of the 12th rib, the dorsal midline and the lateral borders of the paraspinal muscles.
The standard technique places the patient in the prone position; skin and soft tissues of the mid‐back are anaesthetised with lidocaine and the needle is inserted and advanced along an antero‐lateral path to the superior portion of the first lumbar vertebral body on each side (Moore 1981). The needle insertion is followed utilising CT scan or fluoroscopy images. Once the needle is in the right position, alcohol is injected to destroy the nerves.
Intraoperative CPB
During abdominal surgical procedures for pancreatic cancer, chemical splanchnicectomy can be achieved by injecting the neurolytic solutions directly into the junction area of the splanchnic nerves with the celiac ganglia in the retroperitoneal area. This has sometimes been done as a laparoscopic procedure.
Celiac nerve block accomplished by endoscopic ultrasonography
With the advent of endoscopic ultrasonography (EUS) new therapeutic applications of endoscopy have been developed and a needle can now be guided safely into the celiac plexus (Puli 2009; Wiersema 1996). EUS‐guided celiac plexus neurolysis offers several advantages over other techniques (Michaels 2007). The celiac plexus is destroyed by alcohol injected under the guidance of real‐time endosonography. First, using a linear array echo‐endoscope, the region of the celiac ganglia is located from the lesser curve of the stomach, following the emergence of the celiac trunk from the aorta. The anterior approach avoids the retro‐crural space and minimises the risk of neurological complications such as paraesthesia or paralysis; these problems may arise during the posterior approach, because of spasm or thrombosis of the anterior spinal artery. In addition, EUS‐guided celiac plexus neurolysis is done with Doppler control and it therefore seems a safer alternative to percutaneous CPB in patients with cancer (Tran 2006).
This systematic review set out to establish the benefit of CPB for pancreatic cancer pain.
Objectives
To determine the efficacy and safety of celiac plexus neurolysis in reducing pancreatic cancer pain, and to identify adverse effects and differences in efficacy between the various techniques employed. Additionally, to compare the minimally invasive techniques for CPB (EUS‐, CT‐, fluoroscopy‐guided) with conventional medical therapy.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) with a minimum duration of four weeks follow‐up. We excluded studies reported only as abstracts or not in English.
Types of participants
Adults of either sex, aged 18 years or over, suffering from abdominal or back pain due to pancreatic cancer at any stage, confirmed by CT or ultrasound, EUS and clinical criteria. Patients with benign lesions and severe alterations of coagulation were excluded.
Types of interventions
We considered percutaneous CPB, the surgical approach, and EUS‐guided neurolysis. The control group included patients treated with NSAIDs and morphine.
Types of outcome measures
Reduction in pain intensity using a visual analogue scale (VAS) or other pain relief scales (during the procedure the patients are usually sedated, so no discomfort will be reported).
Consumptoin of analgesics.
Overall patient satisfaction after the procedure.
Adverse effects reported may include the following:
hypotension;
diarrhoea;
haematoma;
procedure‐related pain;
neurologic complications;
severe bleeding;
infection; and
mortality.
Search methods for identification of studies
Electronic searches
We conducted a systematic search of the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, issue 12), CENTRAL, MEDLINE, GATEWAY and EMBASE from January 1990 to December 2010. The search strategy used the following terms: ‘celiac plexus’ AND ‘nerve block’ AND ‘pancreatic cancer’ AND ‘pain’. Some of the search strategies used are set out in Appendix 1.
Searching other resources
We identified additional studies from the reference lists of the studies retrieved. We also reviewed the following: Proceedings of the European Congress of Endoscopic Ultrasonography (EURO‐EUS), Congresso Nazionale delle Malattie Digestive, Congresso Nazionale di Anestesiologia, Italian Journal of Gastroenterology and Endoscopy, Educational Synopses in Anesthesiology (ESIA, the online Journal of Anesthesiology), Proceedings of the Italian Society of Anesthesiology, and Analgesia and Intensive Care (SIAARTI) to identify further clinical trials.
Data collection and analysis
Selection of studies
The studies were selected after an electronic search (see above). We selected only trials which examined CPB for pain secondary to pancreatic malignancy. Patients with pain due to chronic inflammation (pancreatitis) were excluded. All the studies were randomized and controlled.
Data extraction and management
We recorded details of study design, participants, disease, setting, outcome assessors, pain intensity (established using a visual analogue scale ‐ VAS) and calculation methods.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of all included studies in this review using a domain based evaluation. The review authors made critical assessments for each of the following different domains: sequence generation (randomization), allocation concealment, and blinding. Review author judgment for each domain was entered into a 'Risk of Bias' table, with answers 'Yes' indicating low risk of bias, 'No' indicating high risk of bias, and 'Unclear' indicating either lack of information or uncertainty over the potential for bias (Higgins 2008).
Data synthesis
We divided the studies according to each treatment procedure: trans‐cutaneous or EUS‐guided block. For VAS scores and consumption of analgesic drugs, we estimated the effect size of celiac block by computing the standardised mean difference (SMD) between the treated and control groups. We calculated the mean difference (MD) and the 95% confidence interval (CI), weighting each study for the inverse of the variance.
We ran a test for heterogeneity to investigate whether differences between studies were greater than would be expected by chance. We calculated the I2 statistic in order to quantify the percentage total variation across studies due to heterogeneity. When heterogeneity was found we tried to clarify the reason for the differences in effects between trials and, depending on the results, we focused either on the average effect or on the range of effects. We used the random‐effects model when the test for heterogeneity was statistically significant, as the true treatment effect was assumed to vary from one study to the next.
For effects reported as binary data, such as adverse effects (yes/no), we calculated a common relative risk (RR) with 95% CI.
Results
Description of studies
The trials are summarized below, under 'Included studies'.
Results of the search
Our search identified 102 potentially eligible studies based on the information in the title and the abstract. Of these, six studies (358 participants) concerning the percutaneous block fulfilled the inclusion criteria and were included in the review. All were randomised controlled trials (RCTs) in which the participants were followed for at least four weeks. We excluded studies reported only as abstracts.
Included studies
Six studies (Kawamata 1996; Lillemoe 1993; Mercadante 1993; Polati 1998; Wong 2004; Zhang 2008), published between 1993 and 2008, were included in the review (their main details are set out in the 'Characteristics of included studies' table). Two were from Italy, two from the USA, one from Japan and one from China. The table also shows the design, the randomisation method, the number of participants, the experimental arm and the controls.
The six studies comprised of 358 participants (CPB 176; control 182). There were 196 males and 162 females and their mean age was 61 years. The inclusion criteria were similar in all studies (severe pain in patients with histologically proven unresectable pancreatic cancer).
Mercadante 1993 randomised 20 participants with severe pain due to pancreatic cancer. Ten were treated with the CPB, the other ten with analgesics (NSAID). Lillemoe 1993 carried out a RCT in 137 participants. Kawamata 1996 studied 21 participants with pancreatic cancer, treating ten of them with CPB. Polati 1998 enrolled 24 participants: 12 received the celiac block and 12 pharmacological therapy. Wong 2004 did a double‐blind RCT randomising 100 participants with unresectable pancreatic cancer to receive neurolytic CPB or systemic analgesic therapy. The participants were followed for one year. Zhang 2008 enrolled 56 participants.
Excluded studies
We excluded uncontrolled and not randomised trials, and any dealing with pain not related to pancreatic cancer (e.g. chronic pancreatitis). We identified one RCT comparing EUS‐guided and CT‐guided CPB (Gress 1999), but its aim was to assess efficacy in controlling chronic abdominal pain associated with chronic pancreatitis rather than pancreatic cancer. We also excluded a recent study that compared the effectiveness of standard EUS‐CPB and EUS‐guided broad plexus neurolysis (EUS‐BPN) that extends over the superior mesenteric artery using a 25‐gauge needle, since this study did not have a control population (Sakamoto 2010).
Risk of bias in included studies
The inclusion criteria were similar in all studies including abdominal pain due to unresectable pancreatic cancer. Only one clearly specified how the allocation sequence had been generated and how allocation had been concealed (Figure 1 and Figure 2).
1.
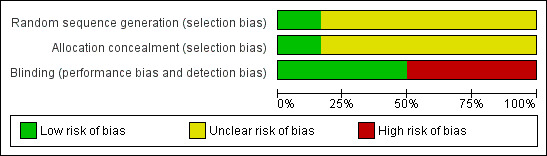
Risk of bias graph: it shows the lack of information in the studies: in most of the studies the sequence generation was not explained. The 50% of studies were blinded (see also Figure 2).
2.
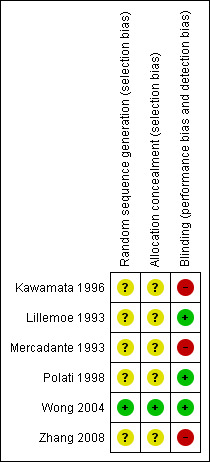
Risk of bias summary. Only one clearly specified how the allocation sequence had been generated and how allocation had been concealed. Three studies were double‐blinded and the others were not.
Four studies used the classical posterior bilateral approach under Rx‐guidance (Mercadante 1993; Kawamata 1996; Polati 1998; Wong 2004); one used the same approach but under CT‐guidance (Zhang 2008), and one used an intraoperative approach (Lillemoe 1993). All used ethanol as the neurolytic agent, but at different concentrations: three used 100%, one 80%, one 30% and one 75%. The total mL injected also varied. The analgesic therapy was similar in all the studies, as it has been demonstrated that the first choice drugs are NSAIDs and morphine.
Allocation
Allocation was properly concealed from the participants only in one study in which the authors called a central telephone number to randomise the patients (in blocks of four), stratified according to the TNM staging system (Wong 2004). In the other studies it was either not clear or not stated how allocation had been concealed and it was not possible to obtain this information from the authors.
Blinding
Three studies were double‐blinded and the others were not. In the studies defined as 'blinded' participants could receive opioids managed by a clinician blinded to the treatment assignment (Mercadante 1993; Polati 1998; Wong 2004). In the study by Zhang 2008 CPB was done by one operator who was not involved in the patients' follow‐up.
Incomplete outcome data
The primary outcome was pain based on the 10‐point VAS. VAS scores at four weeks were available in four studies, and at eight weeks in five. Daily opioid use was clearly reported in all the studies except the one by Lillemoe 1993. All described the complications and side effects of the treatments.
Selective reporting
Data on participant selection were available from the tables or text of the studies. The two groups of participants (CPB or opioids) were similar in all the studies as regards age, sex, and tumour site and stage. The anatomical approach to the celiac plexus was explained well in all the studies; all but one used the posterior approach.
Other potential sources of bias
All the potential sources of bias are described under the previous points.
Effects of interventions
The 'Characteristics of included studies' table summarises the trial design, randomisation method, and number of participants in the experimental and control arms.
In Mercadante 1993 the authors concluded that the CPB reduced the opioid consumption needed to control pancreatic cancer pain, with an effect that was evident for four weeks and persisted partially until death. Even in cases where analgesics gave a lower VAS score, they had more unpleasant side effects than CPB.
Lillemoe 1993 compared chemical splanchnicectomy with 50% alcohol to a placebo injection of saline. The pain score was significantly lower in the group undergoing chemical splanchnicectomy at two, four and six months follow‐up (P <0.05).
Kawamata 1996 found significantly lower VAS scores in the first four weeks after the CPB procedure than in participants given analgesics.
In Polati 1998, immediately after the block CPB participants reported significant pain relief compared with those given pharmacological therapy (P <0.05), but long‐term results did not differ in the two groups. Mean analgesic consumption was lower in the CPB group. Complications related to celiac block were transient diarrhoea and hypotension (P not significant between groups). Drug‐related adverse effects were nausea or vomiting (or both), one gastric ulcer and one gluteal abscess.
In Wong 2004 in the first week after the treatment pain intensity and quality of life (QOL) improved, with greater pain relief in CPB patients (53% reduction from baseline, P = 0.05).
Zhang 2008 found that participants who received the CT‐guided CPB had significantly lower VAS scores than those given pharmacological therapy; the scores were respectively 1.3 ± 0.8 and 4.1 ± 0.9 on day one, 1.7 ± 1.1 and 3.1 ± 1.1 on day seven, and 2.0 ± 1.1 and 2.9 ± 0.6 on day 14. QOL did not differ in the two groups.
Pain control (visual analogue scale (VAS))
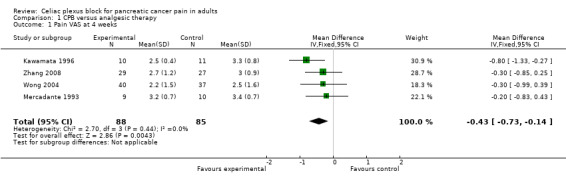
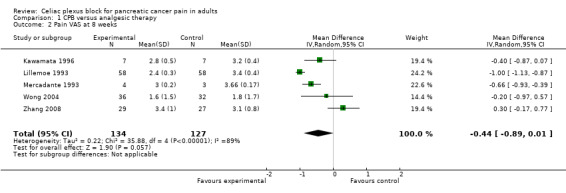
The studies calculated VAS scores at different times after the treatment so we compared the scores for the two groups (CPB and control) at four and eight weeks after treatment. All VAS scores were normalised to a 0‐10 scale, with 0 = no pain and 10 = worst pain imaginable. The VAS score could not be calculated at four weeks in two studies (Lillemoe 1993; Polati 1998) and at eight weeks in one (Polati 1998). At four weeks we calculated the mean difference in the VAS scores, using the fixed‐effect model, which gave ‐0.43 in favour of CPB with a 95% confidence interval (CI) between ‐0.73 and ‐ 0.14 (P = 0.004). (see Figure 3). At eight weeks the mean difference was ‐0.44 (95% CI ‐0.89 to ‐ 0.01, random‐effects model). At eight weeks there was significant heterogeneity (I2 = 89%) (see Figure 4).
3.
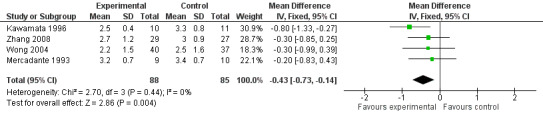
Forest plot of comparison: 1 'CPB versus analgesic therapy (VAS)' follow up at 4 weeks.
4.
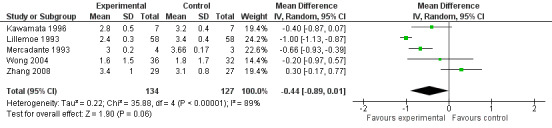
Forest plot of comparison: 1 CPB versus analgesic therapy, outcome: 1.2 Pain VAS at 8 weeks.
Opioid use
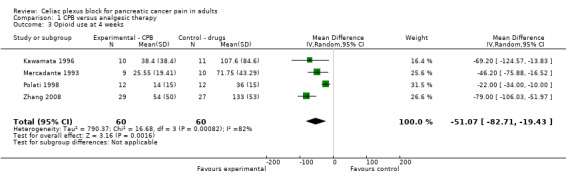
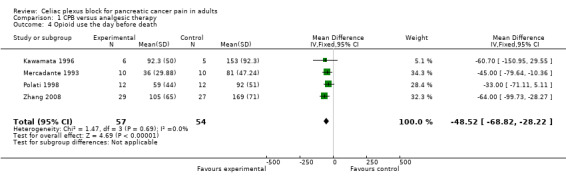
Opioid consumption was lower in the CPB group. At four weeks the mean difference, in mg of opioids, was ‐51.07 (95% CI ‐82.1 to ‐19.43, P =0.002) (see Figure 5). The last consumption figures before death showed a mean difference of ‐48.52 (95% CI ‐68.82 to ‐28.22) (see Figure 6).
5.
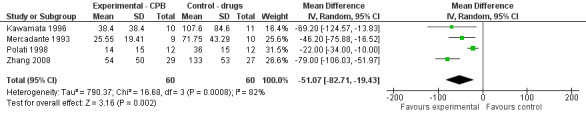
Forest plot of comparison: 1 CPB versus analgesic therapy, outcome: 1.3 Opioid use at 4 weeks.
6.
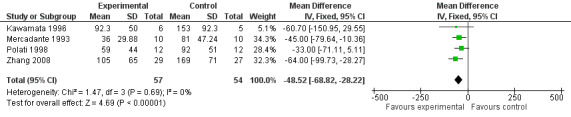
Forest plot of comparison: 1 CPB versus analgesic therapy, outcome: 1.4 Opioid use the day before death.
Opioid use was expressed in two ways: the mean dose of p.o. morphine per day calculated from the total consumption each week (Kawamata 1996, Polati 1998) or as a single dose the last day of the week (Mercadante 1993, Zhang 2008).
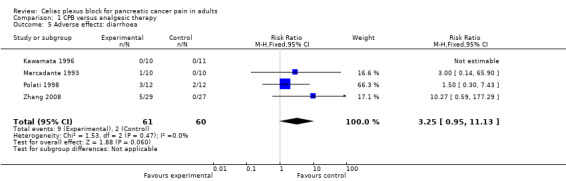
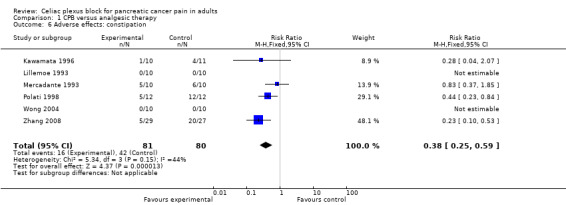
Adverse effects
The main adverse effects were diarrhoea and constipation (this latter was significantly more likely in the control group, whose opioid consumption was higher).
There was a significant reduction in constipation in patients treated with celiac block compared to those treated with standard analgesic care (RR 0.67, 95% CI 0.49–0.91, P = 0.01). In Mercadante’s study one patient treated with celiac block presented prolonged diarrhoea resistant to loperamide, but responsive to octreotide until death (Mercadante 1993).
In Polati’s study complications related to NCPB were transient diarrhoea and hypotension (P not significant between groups) (Polati 1998).
Discussion
In this review we have analyzed RCTs which compared participants with pain related to unresectable pancreatic cancer, treated with CPB, with participants given standard analgesic therapy (NSAIDs and morphine). Although EUS‐guided CPB is becoming popular as a minimally invasive technique that can easily reach the celiac plexus with fewer risks, we found no RCTs on this question. The literature is currently limited to observational uncontrolled series. One meta‐analysis of EUS‐guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain has been published (Puli 2009), but none of the trials included there met our selection criteria. The review authors selected eight papers, four of which were published as abstracts only (excluded from this review), and none of the remaining four was a RCT with a control arm (most published studies on the role of EUS‐guided CPB establish pain relief by comparing the VAS scores before and after treatment, without a control population).
The only RCT that compared EUS‐guided with CT‐guided CPB (Gress 1999) referred to chronic pancreatitis‐related pain. It is hoped that future trials will compare this new technique with standard therapy, and with Rx‐ or CT‐guided CPB.
Pancreatic cancer‐related pain is one of the main symptoms at presentation of a disease which is often unresectable at diagnosis. Palliative care and analgesics are therefore important in these patients. Most studies focus on the effects of the treatment on VAS pain scores and on the reduction of morphine intake. In this meta‐analysis we identified six RCTs, published between 1993 and 2008, which compared the percutaneous posterior bilateral block (five studies) or the intraoperative block (one study) with standard analgesic therapy. The mean difference for the VAS pain score at four weeks was significant (P = 0.004) for the experimental group (CPB). This improvement in pain control coincided with a reduction in opioid consumption; the mean difference in the use of analgesic therapy in the two groups was significantly in favour of the CPB group (P <0.00001). This effect persisted until the death of the patient, with significantly lower opioid requirements in the CPB group (P <0.00001). Although morphine was never completely stopped, its reduction translated into fewer side effects such as constipation, which was significantly more disturbing in the control group (P <0.0001).
Lillemoe 1993 stratified the participants into two subgroups, with and without preoperative pain, and reported that in patients without preoperative pain, CPB significantly reduced pain scores and delayed or prevented the subsequent onset of pain (P <0.05).
In our meta‐analysis there were no major complications or deaths related to the experimental procedure. In particular, no vascular damage with intra‐abdominal bleeding, paraparesis or infection were reported. Constipation was the main adverse effect of opioid therapy (RR 0.38; 95% CI 0.25 to 0.59). Based on limited data, therefore, the procedure appears to be safe.
The technique used to perform CPB (anterior or posterior approach; amount and concentration of ethanol) may affect the results and the duration of the analgesic effect. All but one study used the posterior approach. It is reasonable to assume that an anterior approach might induce fewer side effects and could give better results. The best way to perform this kind of CPB is to use EUS guidance, since the echo‐endoscope, placed in the stomach just below the cardia, is extremely close to where the celiac trunk emerges from the aorta. For this reason, RCTs comparing the efficacy of EUS and the standard posterior percutaneous approach, and with standard analgesic therapy, are needed.
The only RCT we found on EUS‐guided CPB assessed pain relief in patients with chronic pancreatitis and found that the procedure was safe, cost‐effective, and provided longer‐lasting relief than CT‐guided CPB (posterior approach) (Gress 1999). In a prospective study on EUS‐guided CPB for pancreatic cancer pain, Gunaratnam 2001 reported a reduction in pain scores two weeks after the procedure (P = 0.0001) and found that chemotherapy with and without radiation also reduced pain after EUS‐guided CPB.
The timing of CPB and its use together with adjuvant therapy is another interesting issue that could be usefully investigated in the future. We can assume that CPB done at the time of diagnosis, before uncontrolled pain appears, should be more effective and require lower opioid consumption, but this has still to be demonstrated.
In conclusion, CPB appears to be safe and effective for the reduction of pain in patients with pancreatic cancer, with a significant (though limited) advantage over standard analgesic therapy. Further studies are needed to assess the potential role of EUS‐guided CPB and demonstrate its utility in clinical practice.
Authors' conclusions
Implications for practice.
Pain control in patients with unresectable pancreatic cancer is a major challenge. Although the current management of pancreatic pain, according to the WHO three‐step ladder for pain control, includes non‐opioid analgesics such as NSAIDs and progression to increasing doses of opioid analgesics (WHO 2008), in many cases the pain does not respond to drugs, or leads to unacceptable side effects. In such cases an alcohol nerve block may be indicated. It provides pain relief by acting directly on the nerves (celiac plexus) that carry painful stimuli from the diseased pancreas to the brain and it has been demonstrated to reduce pain scores and opioid consumption. Since the first percutaneous CPB was performed by Kappis, modifying percutaneous approaches have been developed with multiple technical variations. We argue that the presence of many options indicates some controversy and a lack of any relevant progress. Anyway, although statistical evidence is minimal for the superiority of pain relief over analgesic therapy, the fact that CPB causes fewer adverse effects than opioids is important for patients.
EUS‐CPN is theoretically safer than posterior percutaneous CPN as it provides detailed Doppler imaging of the blood vessels surrounding the stomach, but the absence of comparative data prohibits any assessment of the relative safety and efficacy. Therefore RCTs are needed to demonstrate the efficacy of EUS‐guided CPB and the fewer complications in comparison to the standard analgesic treatment.
Implications for research.
Future studies are required to demonstrate the real efficacy of the new, less invasive, EUS‐guided CPB. At present the trials available in the literature describe the procedure and its efficacy in reducing pain scores, but do not compare this technique with standardised percutaneous CPB and with opioid therapy.
Feedback
Dr Pease and Dr Nagels
Summary
Date of Submission: 04‐Apr‐2012
Submitters:
Werner Nagels, Heilig‐Hart Hospital Roeselare‐Menen, Wilgenstraat 2, 8800 Roeselare, Belgium
Nikki Pease: Palliative Medicine Education Department, Cardiff University, Velindre Hospital, Cardiff CF14 2TL United Kingdom
Feedback: In March 2011 the full text Cochrane review 'Coeliac plexus block for pancreatic cancer pain in adults' was published in Issue 3 of The Cochrane Library. The systematic review was conducted by Arcidiacono and his colleagues. The results of 6 papers were used for analysis (Kawamata, Ishitani et al. 1996) (Mercadante 1993) (Wong, Schroeder et al. 2004) (Zhang, Zhang et al. 2008) (Polati, Finco et al. 1998) (Lillemoe, Cameron et al. 1993). Their conclusion was: 'Although statistical evidence is minimal for the superiority of pain relief over analgesic therapy, the fact that coeliac plexus block causes fewer adverse effects than opioids is important for patients'. However, major remarks need to be made. Although the Cochrane review stated that the adverse events are constipation and diarrhoea, there was no data presented that supported this finding. Errors were made in the meta‐analyses in Arcidiacono's Cochrane Review. In all evaluations the patient numbers at the start of the included studies were used and not the real numbers at week 4 and 8. In some studies the exact number of patients were not available, but they could be estimated in a well‐founded way based on the patient numbers from the other studies. Even when the correct numbers at the different time points were presented in the included studies, they were not used. Not doing this leads to wrong levels of significance, heterogeneity and potentially incorrect conclusions. It is unclear why a FEM and not a REM was used for the meta‐analysis of the opioid usage at week 4, because an important heterogeneity (I2 = 76 %) was found. For the opioid usage at 4 weeks wrong values for the study of Polati were used (Polati, Finco et al. 1998). The dosage at ?one‐quarter of survival time? presented in Polati?s study was used as the dosage at 4 weeks. For the opioid dosages at week 4 and the day before death the medians were used as means for Wong's study (Wong, Schroeder et al. 2004). It is unclear how the standard deviations for the meta‐analysis were produced, because Wong only presented ranges in his publication. For the opioid usage at the day before death it is uncertain what dosages were used for Kawamata's study, because this time point was not used in that paper (Kawamata, Ishitani et al. 1996). For the study of Wong (Wong, Schroeder et al. 2004) and Zhang (Zhang, Zhang et al. 2008) the values of week 24 and 90 days were taken respectively. These studies did not present opioid dosages at the day before death.
Reply
1.Although the Cochrane review stated that the adverse events are constipation and diarrhoea, there was no data presented that supported this finding.
There was a significant reduction in constipation in patients treated with celiac block compared to those treated with standard analgesic care (RR 0.67, 95% CI 0.49–0.91, P = 0.01). In Mercadante’s study one patient treated with celiac block presented prolonged diarrhoea resistant to loperamide, but responsive to octreotide until death.
In Polati’s study complications related to NCPB were transient diarrhoea and hypotension (P not significant between groups).
We add the sentences above in the revised text.
2.Errors were made in the meta‐analyses in Arcidiacono's Cochrane Review. In all evaluations the patient numbers at the start of the included studies were used and not the real numbers at week 4 and 8. In some studies the exact number of patients were not available, but they could be estimated in a well‐founded way based on the patient numbers from the other studies. Even when the correct numbers at the different time points were presented in the included studies, they were not used. Not doing this leads to wrong levels of significance, heterogeneity and potentially incorrect conclusions.
The number of patients was corrected by using the number reported in the studies at the specified time and the analysis was updated
3.It is unclear why a FEM and not a REM was used for the meta‐analysis of the opioid usage at week 4, because an important heterogeneity (I2 = 76 %) was found. The REM was applied as appropriate according to the results of the Heterogeneity test.
4.For the opioid usage at 4 weeks wrong values for the study of Polati were used (Polati, Finco et al. 1998). The dosage at 'one‐quarter of survival time' presented in Polati's study was used as the dosage at 4 weeks.
Being the survival time after treatment = 102 days, the one quarter survival time corresponds to 3 weeks + 4.5 days, so, approximately 4 weeks. For this reason that dosage was used.
5.For the opioid dosages at week 4 and the day before death the medians were used as means for Wong's study (Wong, Schroeder et al. 2004). It is unclear how the standard deviations for the meta‐analysis were produced, because Wong only presented ranges in his publication.
Since we did not received any reply from Wong we delete the data concerning the opioid usage from the meta‐analysis
6.For the opioid usage at the day before death it is uncertain what dosages were used for Kawamata's study, because this time point was not used in that paper (Kawamata, Ishitani et al. 1996).
Since this time point was not clear from the study, the last dosage presented in figure 1.B was interpreted as constant until death (since it was approximately constant also during the last 3 weeks of observation ‐150 mg/day‐). 7.For the study of Wong (Wong, Schroeder et al. 2004) and Zhang (Zhang, Zhang et al. 2008) the values of week 24 and 90 days were taken respectively. These studies did not present opioid dosages at the day before death.
As far as Wong data is concerned, they were deleted from the meta‐analysis. Concerning Zhang data, in the absence of clear values of opioid dosage the day before death, the values at week 24 were interpreted as opioid dosages at the day before death since, for a patient who suffers of pancreatic cancer, 24 weeks (6 months) are the mean survival time.
Contributors
Giliola Calori, Silvia Carrara.
Karen Pettersen
Summary
Date of submission: 11 February 2013
Karen Pettersen, Editor on Cochrane Clinical Answers, a new Cochrane Derivative product available at: http://cochraneclinicalanswers.com/.
We are writing a Cochrane Clinical Answer based on: Arcidiacono PG, Calori G, Carrara S, McNicol ED, Testoni PA. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database of Systematic Reviews 2011 , Issue 3 . Art. No.: CD007519. DOI: 10.1002/14651858.CD007519.pub2.
A query has arisen that I cannot seem to find an answer to within the review. The review authors refer to a mean difference in opioid use at 4 weeks of ‐34.33 mg, 95% CI ‐44.42 mg to ‐24.24 mg. Is this a difference in average daily dose over 4 weeks or a single dose on the day at 4 weeks? Or maybe it’s morphine‐equivalent dose?
Reply
Date of response: 21 May 2013
The authors added the following statement to clarify the section under 'Effects of interventions: Opioid use':
Opioid use was expressed in two ways: the mean dose of p.o. morphine per day calculated from the total consumption each week (Kawamata 1996, Polati 1998) or as a single dose the last day of the week (Mercadante 1993, Zhang 2008).
Contributors
Giliola Calori, Silvia Carrara.
What's new
| Date | Event | Description |
|---|---|---|
| 21 June 2019 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 1, 2009 Review first published: Issue 3, 2011
| Date | Event | Description |
|---|---|---|
| 19 January 2017 | Review declared as stable | See Published notes. |
| 23 May 2013 | Feedback has been incorporated | The authors have incorporated feedback, see Feedback 1; Feedback 2. |
Notes
2017
We performed a full search in May 2016 and found two new studies dealing with CPN under EUS‐guidance, but they did not change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
We acknowledge the work of Paolo G Arcidiacono in preparing the potential update from 2014 to 2016. We also acknowledge the work of additional authors Sabrina Testoni, Emanuele Dabizzi, and Maria Chiara Petrone, of Pancreato‐Biliary Endoscopy and Endosonography Division, San Raffaele Scientific Institute, Vita Salute University, Milan, Italy, in preparing the potential update from 2014 to 2016.
2019
We performed a full updated search in January 2019 and found no new studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be reassessed for updating in two years. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
We thank Jessica Thomas for her support and patience while the protocol and review were being written and Judith Baggott for her support with the language.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL)
#1 CELIAC PLEXUS #2 "celiac plexus" or "coeliac plexus" #3 neurolysis or "nerve block*" #4 ((#1 or #2) AND #3) #5 (("celiac plexus" near block*) or ("coeliac plexus" near block*)) #6 NCPB in ti or ab #7 "neurolytic sympathetic plexus" near block* #8 #4 or #5 or #6 or #7
MEDLINE via OVID
CELIAC PLEXUS
"celiac plexus" or "coeliac plexus"
neurolysis or "nerve block$"
((1 or 2) AND 3)
(("celiac plexus" adj5 block$) or ("coeliac plexus" adj5 block$))
NCPB in ti or ab
"neurolytic sympathetic plexus" adj5 block$
OR/4‐7
"Splanchnic neurolysis" was not used for the search strategy. We manually screened the titles and abstracts of the retrieved citations to identify potentially relevant studies. We excluded studies published in abstract form only.
Data and analyses
Comparison 1. CPB versus analgesic therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain VAS at 4 weeks | 4 | 173 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.73, ‐0.14] |
| 2 Pain VAS at 8 weeks | 5 | 261 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.89, 0.01] |
| 3 Opioid use at 4 weeks | 4 | 120 | Mean Difference (IV, Random, 95% CI) | ‐51.07 [‐82.71, ‐19.43] |
| 4 Opioid use the day before death | 4 | 111 | Mean Difference (IV, Fixed, 95% CI) | ‐48.52 [‐68.82, ‐28.22] |
| 5 Adverse effects: diarrhoea | 4 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.25 [0.95, 11.13] |
| 6 Adverse effects: constipation | 6 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.25, 0.59] |
1.1. Analysis.
Comparison 1 CPB versus analgesic therapy, Outcome 1 Pain VAS at 4 weeks.
1.2. Analysis.
Comparison 1 CPB versus analgesic therapy, Outcome 2 Pain VAS at 8 weeks.
1.3. Analysis.
Comparison 1 CPB versus analgesic therapy, Outcome 3 Opioid use at 4 weeks.
1.4. Analysis.
Comparison 1 CPB versus analgesic therapy, Outcome 4 Opioid use the day before death.
1.5. Analysis.
Comparison 1 CPB versus analgesic therapy, Outcome 5 Adverse effects: diarrhoea.
1.6. Analysis.
Comparison 1 CPB versus analgesic therapy, Outcome 6 Adverse effects: constipation.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kawamata 1996.
| Methods | Randomised control trial (RCT) UB (unblinded) | |
| Participants | 21; M/F = 9/12; age: 62.3 Inclusion criteria: severe pain; palliative care unit Exclusion criteria: not stated. No significant differences between the 2 groups with regards to sex, age, weight and survival time |
|
| Interventions | CPB (X‐ray posterior bilateral 15 to 20 ml 80% alcohol) versus analgesic therapy (NSAID, morphine) | |
| Outcomes | Pain relief (VAS); quality of Life (QoL) | |
| Notes | Morphine consumption and the VAS score were recorded at regular weekly intervals and the performance status and the QoL score were measured every 2 weeks thereafter. In the results, although the VAS scores were lower in the CPB group than in the control group, they were maintained at about 3 to 4 cm in both groups. Thus, the pain management in both groups appeared to be sufficient. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The generation of the allocation sequence is not specified in the text |
| Allocation concealment (selection bias) | Unclear risk | Not stated in the text |
| Blinding (performance bias and detection bias) All outcomes | High risk | The authors were not blinded to the allocated interventions |
Lillemoe 1993.
| Methods | RCT DB | |
| Participants | 137; M/F = 76/54; age: 59 Inclusion criteria: unresectable, histologically proven pancreatic cancer Exclusion criteria: periampullary tumour; benign inflammation No significant differences between the 2 groups with regards to sex, age, weight and survival time |
|
| Interventions | All patients underwent surgical exploration with biopsy of the tumour and palliative biliary or gastrointestinal bypass. Chemical splanchnicectomy was performed by the operating surgeon by the injection of 20 ml of either 50% alcohol or saline solution each side of the aorta at the level of the celiac axis using a 20 or 22 G spinal needle. | |
| Outcomes | VAS | |
| Notes | To further determine the effect of CPB patients were stratified into those with and without preoperative pain. An unexpected finding of this study was a highly significant improvement in actuarial survival observed in patients with preoperative pain who received alcohol chemical splanchnicectomy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The generation of the allocation sequence is not specified in the text |
| Allocation concealment (selection bias) | Unclear risk | Not stated in the text |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The attending surgeon, assistants and patients were unaware of the content of the solution, which had been prepared by an operating room nurse |
Mercadante 1993.
| Methods | RCT UB | |
| Participants | 20; M/F = 11/9; age 62.3 Inclusion criteria: severe pain; palliative care unit Exclusion criteria: not stated No significant differences between the 2 groups with regards to sex, age, weight and survival time |
|
| Interventions | X‐ray posterior bilateral 25 ml 75% alcohol versus NSAID and morphine | |
| Outcomes | VAS | |
| Notes | This study demonstrated that CPB reduces opioid consumption for controlling pancreatic cancer pain: this effect is evident for 4 weeks and persists partially until the day before death in advanced patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The generation of the allocation sequence is not specified in the text |
| Allocation concealment (selection bias) | Unclear risk | Not stated in the text |
| Blinding (performance bias and detection bias) All outcomes | High risk | The authors were not blinded to the allocated interventions |
Polati 1998.
| Methods | RCT | |
| Participants | 24; M/F = 17/7; age: 58.5 Inclusion criteria: unresectable, histologically proven cancer. Outpatient pain centre Exclusion criteria: not stated No significant differences between the 2 groups with regards to sex, age, weight and survival time |
|
| Interventions | Fluoroscopic posterior bilateral 7 ml 100% alcohol versus NSAID and morphine | |
| Outcomes | VAS | |
| Notes | Follow up was conducted until death with daily visits during the hospital admission and then regular assessment by telephone or by office visits at least twice a week by physicians blinded to the treatment group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The generation of the allocation sequence is not specified in the text |
| Allocation concealment (selection bias) | Unclear risk | Not stated in the text |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The treating physicians were blinded to the randomisation status |
Wong 2004.
| Methods | RCT B | |
| Participants | 100; M/F = 53/47; age: 63 Inclusion criteria: histologically proven or radiologically consistent unresectable cancer; Mayo Pain Clinic; palliative surgery allowed; VAS > 3 or opioids required and VAS < 6 Exclusion criteria: epidural or intrathecal analgesia |
|
| Interventions | Fluoroscopic posterior bilateral 10 ml 100% alcohol versus NSAID and morphine | |
| Outcomes | VAS | |
| Notes | All patients could receive additional opioids managed by a clinician blinded to the treatment assignment The major finding of this study is that CPB significantly improves pain relief in patients with pancreatic cancer compared with optimised systemic analgesic therapy alone, but does not affect quality of life or survival. The analgesic effect of CPB over drugs alone was sustained over the longer term until death. The authors conclude that both CPB and optimised systemic analgesic therapy (SAT) can provide effective analgesia, though CPB can provide significantly better analgesia. However, CPB had no effect on opioid consumption, QoL or survival. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation sequence was adequately generated |
| Allocation concealment (selection bias) | Low risk | Central telephone number; blocks of 4 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The treating physicians were blinded to the randomisation status |
Zhang 2008.
| Methods | RCT UB | |
| Participants | 56; M/F = 35/31; age: 58 Inclusion criteria: unresectable, histologically proven cancer Exclusion criteria: previous neurolytic celiac plexus block; psychiatric disease that could have affected the study assessments No significant differences between the 2 groups with regards to sex, age, weight and survival time, however, within 90 days 10 patients died of cancer |
|
| Interventions | CT‐guided posterior bilateral block with 20 ml 100% alcohol | |
| Outcomes | VAS; QoL | |
| Notes | The CPB was performed by one operator who was not involved in the patients' follow up treatment. The authors suggest early intervention before the tumour spreads over the celiac plexus, based on the pain mechanism: if the plexus is affected by a growing tumour, the alcohol cannot spread enough to block the nerve conduction. This explains why some patients may not respond to the CPB. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The generation of the allocation sequence is not specified in the text |
| Allocation concealment (selection bias) | Unclear risk | Not stated in the text |
| Blinding (performance bias and detection bias) All outcomes | High risk | The authors were not blinded to the allocated interventions |
B: blinded CPB: celiac plexus block DB: double‐blinded QOL: quality of life NSAID: non‐steroidal anti‐inflammatory drug RCT: randomised controlled trial UB: unblinded VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gress 1999 | This study was excluded because it concerns the treatment of pain related to chronic pancreatitis, not pain related to pancreatic cancer |
| Gunaratnam 2001 | Excluded as not a RCT |
| Levy 2008 | Excluded as not a RCT |
| Sakamoto 2010 | No control |
| Tran 2006 | Excluded as not a RCT |
| Wiersema 1996 | Excluded as not a RCT |
RCT: randomised controlled trial
Contributions of authors
CG: ran the statistical analysis. APG: provided an overview of the study. CS: searched for papers, ran the MEDLINE search, wrote the review and will be responsible for conducting the update of this review. TPA: provided an overview of the study EM: support from the Cochrane Pain, Palliative and Supportive Care Review Group.
Declarations of interest
None known
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Kawamata 1996 {published data only}
- Kawamata M, Ishitani K, Ishikawa K, Sasaki H, Ota K, Omote K, et al. Comparison between celiac plexus block and morphine treatment on quality of life in patients with pancreatic cancer pain. Pain 1996;64(3):597‐602. [DOI] [PubMed] [Google Scholar]
Lillemoe 1993 {published data only}
- Lillemoe KD, Cameron JL, Kaufman HS, Yeo CJ, Pitt HA, Sauter PK, et al. Chemical splanchnicectomy in patients with unresectable pancreatic cancer. A prospective randomized trial. Annals of Surgery 1993;217(5):447‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mercadante 1993 {published data only}
- Mercadante S. Celiac plexus block versus analgesics in pancreatic cancer pain. Pain 1993;52(2):187‐92. [DOI] [PubMed] [Google Scholar]
Polati 1998 {published data only}
- Polati E, Finco G, Gottin L, Bassi C, Pederzoli P, Ischia S. Prospective randomized double‐blind trial of neurolytic coeliac plexus block in patients with pancreatic cancer. British Journal of Surgery 1998;85(2):199‐201. [DOI] [PubMed] [Google Scholar]
Wong 2004 {published data only}
- Wong GY, Schroeder DR, Carns PE, Wilson JL, Martin DP, Kinney MO, et al. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. JAMA 2004;291(9):1092‐9. [DOI] [PubMed] [Google Scholar]
Zhang 2008 {published data only}
- Zhang CL, Zhang TJ, Guo YN, Yang LQ, He MW, Shi JZ, et al. Effect of neurolytic celiac plexus block guided by computerized tomography on pancreatic cancer pain. Digestive Diseases Sciences 2008;53(3):856‐60. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Gress 1999 {published data only}
- Gress F, Schmitt C, Sherman S, Ikenberry S, Lehman G. A prospective randomized comparison of endoscopic ultrasound‐ and computed tomography‐guided celiac plexus block for managing chronic pancreatitis pain. American Journal of Gastroenterology 1999;94(4):872‐4. [DOI] [PubMed] [Google Scholar]
Gunaratnam 2001 {published data only}
- Gunaratnam NT, Sarma AV, Norton ID, Wiersema MJ. A prospective study of EUS‐guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointestinal Endoscopy 2001;54(3):316‐24. [DOI] [PubMed] [Google Scholar]
Levy 2008 {published data only}
- Levy MJ, Topazian MD, Wiersema MJ, Clain JE, Rajan E, Wang KK, et al. Initial evaluation of the efficacy and safety of endoscopic ultrasound‐guided direct ganglia neurolysis and block. American Journal of Gastroenterology 2008;103(1):98‐103. [DOI] [PubMed] [Google Scholar]
Sakamoto 2010 {published data only}
- Sakamoto H, Kitano M, Kamata K, Komaki T, ImaiH, Chikugo T, et al. EUS‐guided broad plexus neurolysis over the superior mesenteric artery using a 25‐gauge needle. American Journal of Gastroenterology 2010;105(12):2599‐606. [DOI] [PubMed] [Google Scholar]
Tran 2006 {published data only}
- Tran QN, Urayama S, Meyers FJ. Endoscopic ultrasound‐guided celiac plexus neurolysis for pancreatic cancer pain: a single institution experience and review of the literature. Journal of Supportive Oncology 2006;4(9):460‐2. [PubMed] [Google Scholar]
Wiersema 1996 {published data only}
- Wiersema MJ, Wiersema LM. Endosonography‐guided celiac plexus neurolysis. Gastrointestinal Endoscopy. 1996;44(6):656‐62. [DOI] [PubMed] [Google Scholar]
Additional references
Giménez 1993
- Giménez A, Martinez‐Noguera A, Donoso L, Català E, Serra R. Percutaneous neurolysis of the celiac plexus via the anterior approach with sonographic guidance. American Journal of Roentgenology 1993;161(5):1061‐3. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0. The Cochrane Collaboration 2008. Available from www.cochrane‐handbook.org, updated February 2008. [Google Scholar]
Kappis 1919
- Kappis M. Sensitivity (means "pain sensation" in this context) and local anesthesia in the surgical area of the abdominal cavity, with special focus on anesthesia of the splanchnic nerve [Sensibilitat und lokale Anasthesie im chirurgischen Gebiet der Bauchhohle mit besonderer Berucksichtigung der splanchnicus‐Aasthesie]. Beitrage zur klinischen chirurgie 1919;115:161‐75. [Google Scholar]
Maisonneuve 2010
- Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an update. Dig Dis. 2010;28(4‐5):645‐56. [DOI] [PubMed] [Google Scholar]
Michaels 2007
- Michaels AJ, Draganov PV. Endoscopic ultrasonography guided celiac plexus neurolysis and celiac plexus block in the management of pain due to pancreatic cancer and chronic pancreatitis. World Journal of Gastroenterology 2007;13(26):3575‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 1981
- Moore DC, Busch WH, Burnett LL. Celiac plexus block: a roentgenographic, anatomic study of technique and spread of solution in patients and corpses. Anesthesia and Analgesia 1981;60:369‐79. [PubMed] [Google Scholar]
Puli 2009
- Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS‐guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta‐analysis and systematic review. Digestive Diseases Science 2009;54(11):2330‐7. [DOI] [PubMed] [Google Scholar]
Staatas 2001
- Staatas PS, Hekmat H, Sauter P, Lillemoe K. The effects of alcohol celiac block, pain, and mood on longevity in patients with unresectable pancreatic pain: a double blind, randomised, placebo‐controlled study. Pain Medicine 2001;2(1):28‐34. [DOI] [PubMed] [Google Scholar]
WHO 2008
- Scoping Document for WHO Treatment Guideline on Pain Related to Cancer, HIV and other progressive life‐threatening illnesses in adults Adopted in WHO Steering Group on Pain Guidelines, 14 October 2008. WHO Steering Group on Pain Guidelines.


