Abstract
Background
Severe sepsis and septic shock are leading causes of death in the intensive care unit (ICU), despite advances in the treatment of patients with severe sepsis and septic shock, including early recognition, appropriate treatment with antibiotics and support of organs that may have been affected by the illness. High‐volume haemofiltration (HVHF) is a blood purification technique that may improve outcomes in severe sepsis or septic shock. The technique of HVHF has evolved from renal replacement therapies used in the ICU to treat critically ill patients with acute kidney injury (AKI). This review was first published in 2013 and was updated in 2016.
Objectives
To investigate whether HVHF improves outcomes in critically ill adults admitted to the intensive care unit with severe sepsis or septic shock. The primary outcome of this systematic review is patient mortality; secondary outcomes include duration of stay, severity of organ dysfunction and adverse events.
Search methods
For this updated version, we extended searches of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, Latin American Caribbean Health Sciences Literature (LILACS), Web of Science and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) to 31 December 2015. The original search was performed in 2011. We also searched trials registers.
Selection criteria
We included randomized controlled trials (RCTs) and quasi‐randomized trials comparing HVHF or high‐volume haemodiafiltration versus standard or usual dialysis therapy, as well as RCTs and quasi‐randomized trials comparing HVHF or high‐volume haemodiafiltration versus no similar dialysis therapy. These studies involved adults treated in critical care units.
Data collection and analysis
Three review authors independently extracted data and assessed trial quality. We sought additional information from trialists as required.
Main results
We included four randomized trials involving 200 participants. Owing to small numbers of studies and participants, it was not possible to combine data for all outcomes. Two trials reported 28‐day mortality, and one trial reported hospital mortality; in the third trial, the number of deaths stated did not match the quoted mortality rates. The pooled risk ratio (95% confidence interval) for 28‐day mortality associated with HVHF was 0.89 (0.60 to 1.32, two trials, 146 participants, low‐quality evidence). One study (137 participants, low‐quality evidence) reported length of stay in the ICU. Two trials (170 participants, low‐quality evidence) reported organ dysfunction, but we could not pool results owing to reporting differences. Three studies (189 participants, low‐quality evidence) reported on haemodynamic changes, but we could not pool results owing to reporting differences. Investigators reported no adverse events. Overall, the included studies had low risk of bias.
Authors' conclusions
Investigators reported no adverse effects of HVHF (low‐quality evidence). The results of this meta‐analysis show that very few studies have been conducted to investigate the use of HVHF in critically ill patients with severe sepsis or septic shock (four studies, 201 participants, low‐quality evidence). Researchers should consider additional randomized controlled trials that are large and multi‐centred and have clinically relevant outcome measures. The cost‐effectiveness of HVHF should also be studied..
Plain language summary
High‐volume haemofiltration for sepsis
Background
Severe sepsis and septic shock are among the most common causes of death in adults admitted to an intensive care unit (ICU). Sepsis often arises after infection, when the body responds by producing chemicals that cause massive inflammation throughout the body. This inflammation can cause organs such as kidneys, heart, circulation or lungs to fail. It is these organ failures, which result from inflammation, that lead to the high death rates associated with sepsis.
Theoretically, if it were possible to artificially neutralize or remove these chemicals from the bloodstream, patient outcomes (such as organ failure and death) might improve. High‐volume haemofiltration (HVHF) is one method that could be used. Standard haemofiltration is a treatment already used in the ICU to remove toxins that build up when a patient's kidneys have stopped working. This treatment involves removal of blood from the patient via a large catheter (a hollow, flexible tube placed into a large vein). After the blood has been removed, it is passed through a filter that removes toxins. The 'purified' blood is then returned to the patient via the catheter. HVHF, a more intense form of this treatment, aims to remove even more toxins (including some of the toxic chemicals produced during sepsis). However, HVHF presents potential disadvantages. This specialized technique requires specific equipment and extra training. Theoretically, it could have harmful effects on a patient's blood pressure or could remove beneficial chemicals (such as antibiotics). For this review, we assessed whether high‐volume haemofiltration improves outcomes such as risk of death among patients with severe sepsis.
Study characteristics
This review is current until December 2015. We included four trials involving 205 participants. All four studies assessed effects of HVHF compared with the current standard haemofiltration and included participants with severe sepsis or septic shock who had been admitted to an ICU. Three of the four studies were very small (fewer than 20 participants enrolled in each study). The maximum time that participants were followed up after inclusion in any of the studies was 28 days. Two studies received financial support from pharmaceutical companies, and one study received support from a health research organization.
Key results
Outcome data were limited ‐ two trials reported death rates at 28 days and one reported death rates in hospital; in the fourth study, the number of deaths stated did not match the quoted mortality rates. One study reported length of stay in the ICU, and one provided data on organ dysfunction. Investigators described no complications.
No clear evidence showed any benefit of HVHF in critically ill patients with severe sepsis or septic shock.
Quality of evidence
Evidence is insufficient to support the routine use of high‐volume haemofiltration in patients with severe sepsis or septic shock. Studies included in this review reported relatively small numbers of participants and measured different outcomes; therefore, we judged the quality of evidence with respect to the impact of HVHF as low. Larger trials, carried out at many centres, are required for full assessment of clinically relevant outcomes and for evaluation of cost versus benefit.
Summary of findings
Summary of findings for the main comparison. Should high‐volume haemofiltration vs standard or usual dialysis be used for sepsis?
| Should high‐volume haemofiltration vs standard or usual dialysis be used for sepsis? | |||||
| Patient or population: patients with sepsis Settings: ICUs in France, Belgium and the Netherlands Intervention: high‐volume haemofiltration Comparison: standard or usual dialysis | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) |
Quality of evidence (GRADE) |
|
| Assumed risk | Corresponding risk | ||||
| Standard or usual dialysis | High‐volume haemofiltration | ||||
| ICU mortality | Study population | RR 0.59 (0.19 to 1.59) | 19 (1 study) | Lowa | |
| 600 per 1000 | 354 per 1000 (114 to 954) | ||||
| 28‐Day mortality | Study population | RR 0.89 (0.60 to 1.32) | 156 (2 studies) | Lowb | |
| 500 per 1000 | 445 per 1000 (300 to 660) | ||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; RR: risk ratio. | |||||
aDowngraded two levels to low quality owing to study heterogeneity and imprecision.
bDowngraded two levels to low quality owing to study heterogeneity and imprecision.
Background
Description of the condition
Sepsis is the body’s response to infection. Sepsis is ‘severe’ when infection leads to organ dysfunction or failure. Septic shock is present when infection causes acute circulatory failure that leads to persistent hypotension despite adequate fluid resuscitation.
Severe sepsis and septic shock are leading causes of the multiple organ dysfunction syndrome, as well as death, in the intensive care unit (ICU). Associated mortality remains very high, ranging from 30% to 50% (Surviving Sepsis Campaign 2008) despite advances in the treatment of patients with severe sepsis and septic shock, including early recognition, source control, timely and appropriate administration of antimicrobial agents and goal‐directed haemodynamic, ventilatory and metabolic therapies (Alejandria 2010; Annane 2004; Gomes Silva 2010; Martí‐Carvajal 2011).
The number of patients with severe sepsis and septic shock continues to grow and is estimated to increase in the United States (USA) by a rate of 1.5% per year. To the current annual incidence of 3.0 cases per 1000 of the population, this would add an additional one million cases per year in the USA alone by 2020 (Angus 2001). This increase is thought to be due to rising numbers of elderly and high‐risk patients in the population and growing use of invasive procedures within hospital settings (Surviving Sepsis Campaign 2008).
Our understanding of the complex pathophysiology of sepsis is evolving. The sepsis syndrome is no longer seen just as a disorder of uncontrolled inflammation; it is regarded more as a syndrome reflecting loss of balance between pro‐inflammatory and anti‐inflammatory mediators (Hotchkiss 2003) resulting in organ damage and development of the multiple organ dysfunction syndrome with its associated high mortality. The sepsis syndrome can occur with or without acute kidney injury (AKI; formerly known as acute renal failure (ARF)).
It was once thought that immunological events in sepsis lead to elevated levels and activity of inflammatory mediators, and that the resulting cascade attempts to restore immunological homeostasis (Adrie 2000). Attempts were made to halt this 'inflammatory cascade' by blocking or antagonizing single inflammatory mediators. This did not lead to improvement in outcomes for patients with sepsis/septic shock (Abraham 2000). It is increasingly recognized that the systemic inflammatory reaction that characterizes sepsis involves very complex interactions between endothelial cells, platelets, leucocytes, the coagulation system and multiple inflammatory mediators (Joannidis 2009). It would appear that the initial concept of sepsis as a stepwise progression down an inflammatory cascade of mediator release is an oversimplification. In addition to the pro‐inflammatory systemic inflammatory response syndrome (SIRS) reaction (marked by overproduction of mediators such as interleukin‐1, interleukin‐6, interleukin‐8 and tumour necrosis factor‐α), sepsis comprises a ‘hyporesponsive’ component. This excessive anti‐inflammatory counterpart to sepsis is referred to as the ‘compensated anti‐inflammatory response syndrome’ (CARS) (Ronco 2003). The SIRS and CARS components may happen in sequence (as in ‘the sequential or serial sepsis theory’), with pro‐inflammatory and anti‐inflammatory mediators alternatively produced during high‐generation or low‐generation periods, leading to SIRS or CARS, or both. Alternatively, SIRS and CARS may occur simultaneously (as described in ‘the parallel sepsis theory’) (Ronco 2003).
Managing these excessive inflammatory and counter inflammatory responses while restoring balance to the immune system is an important therapeutic goal in the management of severe sepsis/septic shock.
Description of the intervention
Continuous haemofiltration has been used for some time to treat critically ill patients with AKI who require renal replacement therapy. This blood purification technique involves removing water and solutes from the patient's blood by convection by applying positive hydrostatic pressure across a filter membrane. In contrast, haemodialysis utilizes the process of diffusion. The fluid removed during haemofiltration, which contains water and solutes, is known as 'ultrafiltrate'. The volume of ultrafiltrate removed during the process of continuous haemofiltration can vary depending on how the clinician prescribes treatment. The amount of ultrafiltrate removed by this treatment is described in millilitres (mL) of fluid per kilogram (kg) of the patient's body weight per hour (i.e. mL/kg/h). The 'standard' ultrafiltrate volume removed can be considered between 25 and 35 mL/kg/h. In 'high‐volume' haemofiltration, the ultrafiltration volume is greater than 35 mL/kg/h (Ronco 2000).
How the intervention might work
Pro‐inflammatory and anti‐inflammatory mediators involved in the sepsis syndrome can be found in the ultrafiltrate of patients who have received continuous haemofiltration (De Vriese 1999). It has been suggested that potential clinical benefit may result from the non‐specific reduction in peak concentrations of these mediators (Ronco 2001) that is achieved by increasing the volume of ultrafiltrate removed to levels above those used to treat patients with AKI.
Why it is important to do this review
The technical requirements of high‐volume haemofiltration (HVHF), including tightly controlled blood flow and ultrafiltration rates, good central venous vascular access, continuous anticoagulation and use of large amounts of sterile fluid, are both problematic and demanding for ICU staff. High‐volume haemofiltration may also be associated with increased, and unmeasured, removal of potentially beneficial substances such as medications or trace elements. The impact of these losses on patient outcomes is uncertain. In addition, considerable financial cost is associated with this procedure, and it is uncertain whether this relatively new technology offers clinically important benefit for patients with severe sepsis or septic shock (Reiter 2002).
Objectives
To investigate whether HVHF improves outcomes in critically ill adults admitted to the intensive care unit with severe sepsis or septic shock. The primary outcome of this systematic review is patient mortality; secondary outcomes include duration of stay, severity of organ dysfunction and adverse events.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and quasi‐randomized trials (trials in which the method of allocation is not truly random but could rely, for example, on allocation by date of birth) comparing HVHF or high‐volume haemodiafiltration versus standard or usual dialysis therapy; and RCTs and quasi‐randomized trials comparing HVHF or high‐volume haemodiafiltration versus no similar dialysis therapy.
We defined quasi‐randomized trials as those involving a method of allocation to study groups that was not truly random (e.g. by alternating allocation to treatment or control groups on the basis of the order of inclusion into the study).
We included only studies that clearly stated the dosage of high‐volume ultrafiltration used.
We excluded studies that did not report the primary outcome as detailed below.
We did not include conference proceedings and meeting abstracts in this review.
Types of participants
We included studies of adults aged 16 years and older with severe sepsis or septic shock in an ICU setting. We accepted study authors’ definitions of severe sepsis and septic shock. We included both patients with severe sepsis or septic shock and AKI and those with severe sepsis or septic shock but without AKI. We accepted study authors' definitions of AKI.
We excluded studies with participants younger than 16 years of age, those with participants without severe sepsis or septic shock and those not conducted in an ICU setting.
Types of interventions
High‐volume haemofiltration (or haemodiafiltration) defined as haemofiltration (or haemodiafiltration) in which an ultrafiltration rate greater than 35 mL/kg/h was achieved. High‐volume haemofiltration (or haemodiafiltration) was the experimental intervention.
Participants in the control intervention group were those who received ‘standard or usual’ dialysis therapy (continuous or intermittent haemofiltration, haemodiafiltration or haemodialysis) and those who received no similar dialysis therapy.
We accepted the study authors' definitions of pulse (if applicable) and continuous high‐volume haemofiltration (or haemodiafiltration).
Types of outcome measures
Primary outcomes
Mortality
1. ICU or hospital mortality, or both, with death reported at 7, 28 or 30 days
Secondary outcomes
Length of stay
1. Length of ICU stay
2. Length of hospital stay
Organ dysfunction
3. Duration of organ dysfunction
4. Number of organ dysfunctions
Adverse effects
5. Risk of haemodynamic changes during HVHF (systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), inotrope or vasopressor medication dose change)
6. Risk of central venous catheter‐related infection (attributable to the catheter used for HVHF)
7. Risk of mechanical complications related to catheter placement: malpositioned lines, haematoma, pneumothorax
8. Risk of bleeding related to anticoagulation
We planned to present pooled estimates of risk (with associated 95% confidence intervals) for all outcome measures in a 'Summary of findings' table. We planned to assess the quality of evidence provided by included studies for each outcome measure using the GRADE Working Group system.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 12); MEDLINE (Ovid SP, 1990 to December 2015); Embase (Ovid SP, 1990 to December 2015); Latin American Caribbean Health Sciences Literature (LILACS) via BIREME (1982 to December 2015); the Institute for Scientific Information (ISI) Web of Science (1990 to December 2015); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCO host (1982 to December 2015).
We developed a search strategy to define keywords for all searches. See Appendix 1 for the MEDLINE search, Appendix 2 for the Embase search, Appendix 3 for the CENTRAL search, Appendix 4 for the LILACS search, Appendix 5 for the CINAHL search and Appendix 6 for the Web of Science search.
In addition, we searched for relevant ongoing trials on specific websites (31 December 2015).
Searching other resources
When appropriate, we contacted the first authors of studies included in the review to obtain further information on unpublished studies or work in progress. We did not apply language or publication restrictions.
Data collection and analysis
Selection of studies
Two review authors (EMJB and KSR) independently assessed all titles and abstracts. We obtained full papers for studies that might have fulfilled the inclusion criteria. We (EMJB and KSR) independently assessed these studies to determine whether they fulfilled the inclusion criteria. When necessary, we planned to resolve disagreements by discussion and, if necessary, through the decision of a third review author (APM).
Data extraction and management
Two review authors (EMJB and CJH) independently extracted data using a modified version of the data extraction form of the Cochrane Anaesthesia, Critical and Emergency Care Group, as given in Appendix 7. We resolved disagreements by discussion and were not blinded with regard to the names of study authors, investigators or institutions, nor to study results. We listed excluded trials and reasons for exclusion. We piloted the data extraction form before use.
Assessment of risk of bias in included studies
Two review authors (EMJB, CJH) independently assessed risk of bias of included studies by addressing six specific domains (random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and other issues), as set forth in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion and planned to involve a fourth review author (APM) should this be necessary.
Each domain included specific entries in a ‘Risk of bias’ table. We assessed risk of bias as 'low', 'high' or 'unclear'. We contacted the first authors of included studies for further information on study design when we judged this to be necessary. We created plots of the risk of bias in RevMan 5.3.
Measures of treatment effect
We included and combined data using RevMan 5.3, when appropriate, by intervention, outcome and population.
For dichotomous data, we used risk ratios (RRs) with 95% confidence intervals (CIs).
For continuous data, we used, as appropriate, mean differences (MDs) with 95% CIs or standard mean differences (SMDs).
Unit of analysis issues
We had no unit of analysis issues.
Dealing with missing data
We contacted the original investigators to request missing data. We planned to make explicit assumptions about any methods used to cope with missing data, for example, that the data are assumed missing at random, or that missing values are assumed to have a particular value such as a poor outcome. This approach reflects the guidelines set forth in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of heterogeneity
We planned to test statistical heterogeneity by using the I2 statistic. We planned to pool clinically and statistically homogeneous studies using the fixed‐effect model. We planned to pool clinically homogeneous and statistically heterogeneous (I2 statistic > 50%) studies using the random‐effects model, if appropriate, as set forth in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We planned to assess clinical heterogeneity by judgement and to attempt to calculate pooled summary effects only in the absence of clinical heterogeneity.
Assessment of reporting biases
If sufficient studies were identified, we planned to construct funnel plots (trial effect vs standard error) to assess possible publication bias.
Data synthesis
We combined data, when possible, using random‐effects models and assessed heterogeneity using the I2 statistic, as described above. We reported the main outcomes of this review in Table 1. We incorporated the GRADE approach to interpret findings and used the GRADE profiler to import data from RevMan 5.3 to help to create Table 1. We included the following outcomes in Table 1.
ICU mortality.
28‐Day mortality.
Hospital mortality.
Subgroup analysis and investigation of heterogeneity
If adequate data were available, we planned to perform a priori subgroup analysis for the following categories.
Patients with severe sepsis or septic shock and AKI and those with severe sepsis or septic shock but without AKI.
Pulse HVHF and continuous HVHF.
Dosage of high‐volume ultrafiltration used.
Type of ICU: surgical (including cardiac and trauma), medical or mixed (including all patient categories).
Sensitivity analysis
If appropriate, we planned to perform a sensitivity analysis to look at review results with and without studies that we deemed to have high risk of bias.
Results
Description of studies
See Figure 1.
1.
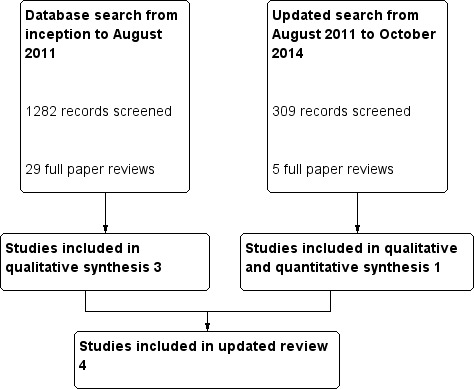
Study selection flow diagram.
Results of the search
For our published review (Borthwick 2013), the combined search of MEDLINE, Embase, CINAHL and CENTRAL yielded 1282 potentially relevant studies. After reviewing titles and abstracts, we excluded 1253 studies. We retrieved the full‐text versions of 29 studies, and we excluded 26 of these study reports. The major reason for exclusion was that identified studies were not randomized, or that participants were not appropriate. Examples of inappropriate participants included patients with pancreatitis, patients with AKI who were not all septic and patients with AKI plus non‐renal organ failure who were not necessarily septic. One study was published as an abstract (Zainudin 2006) and as a paper with a different first author (Ghani 2006). We included the full paper.
The updated search from August 2011 to December 2015 revealed 309 additional potentially relevant studies. We retrieved the full‐text versions of five studies. We excluded four of these studies as they were systematic reviews, provided inappropriate interventions or enrolled inappropriate participants. Finally, we included in the qualitative synthesis four studies (Boussekey 2008; Cole 2001; Ghani 2006; Joannes‐Boyau 2013) published in five reports with a total of 200 participants. We reported in the Characteristics of included studies table the characteristics of populations and interventions described in the included trials.
We contacted the first author of three studies (Boussekey 2008; Cole 2001; Ghani 2006) to ask for further information on the randomization process and the outcome measures described. All three study authors gave expanded details of the randomization process, and two study authors provided further information on and clarification of outcome measures. Joannes‐Boyau 2013 provided sufficient information in the published material on the randomization process used in this study.
Included studies
Only four studies with 200 participants met the entry criteria for this updated systematic review (Boussekey 2008; Cole 2001; Ghani 2006; Joannes‐Boyau 2013).
Boussekey 2008 was a single‐centre randomized controlled trial that investigated vasopressor doses and other clinical outcomes, such as mortality, in 19 participants over a 28‐day period following study inclusion. The mean age of participants was 68 years, and 78% were male. Investigators compared HVHF (65 mL/kg/h) versus control (35 mL/kg/h).
Cole 2001 was a single‐centre randomized cross‐over trial that investigated haemodynamic changes and plasma concentrations of inflammatory mediators in 11 participants over a 48‐hour period following study inclusion. The mean age of participants was 63.1 years, and 73% were male. Investigators compared HVHF (6000 mL/h for 8 hours) versus control (1000 mL/h for 8 hours).
Ghani 2006 was a single‐centre parallel randomized controlled trial that investigated haemodynamic status, inflammatory mediator concentrations and organ dysfunction scores in 33 participants. The mean age of participants was 58 years, and 53% were male. Investigators compared HVHF (6000 mL/h for 6 hours) versus control (2000 mL/h for 6 hours).
Joannes‐Boyau 2013 was a multi‐centre randomized controlled trial that investigated 28‐day mortality and other clinical outcomes in 137 participants. The mean age of participants was 68 years, and 68% were male. Investigators compared HVHF (70 mL/kg/h for 96 hours) versus control (35 mL/kg/h for 96 hours).
For full details, see Characteristics of included studies.
Excluded studies
We excluded 32 studies from the analysis. For details of excluded studies, see Characteristics of excluded studies. Nine were not RCTs (Bellomo 2009; Bouman 2007; Castro 2008; Clark 2014; Honore 2003; Honore 2004; Honore 2007; Honore 2009; Oudemans‐van Straaten1999).
In 10 trials, participants were not appropriate in that they did not all clearly have severe sepsis or septic shock (Abe 2010; Bouman 2002; Chu 2013; Combes 2015; Guo 2014; Palevsky 2008; Ronco 2000; Storck 1991; Yu 2008; Xie 2009).
In 11 cases, the intervention, including high‐absorption haemofiltration, plasma filtration and high cut‐off haemofiltration, was not appropriate (Haase 2007; Han 2011; Hoffman 1996, Mao 2009; Morgera 2006; Payen 2009; Peng 2010; Quenot 2015; Wang 2009; Zhang 2004; Zhang 2012).
In one case, the outcome measurement ‐ assessment of acid‐base balance ‐ was not appropriate (Cole 2003). One paper was an abstract for a paper published in full and already included (Zainudin 2006).
Studies awaiting assessment
There are no studies awaiting classification.
Ongoing studies
We found no ongoing studies on HVHF.
Risk of bias in included studies
All included studies were randomized controlled trials; one had a cross‐over design (Cole 2001), and none were quasi‐randomized clinical trials. Figure 2 shows the 'Risk of bias' summary, and Figure 3 the 'Risk of bias' graph.
2.
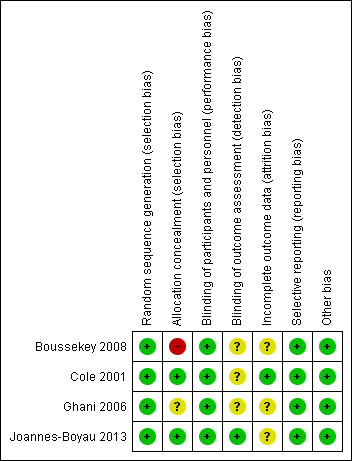
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.
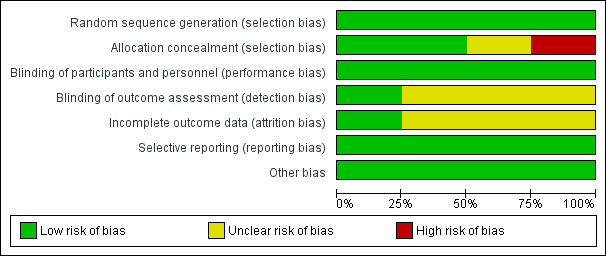
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All four included studies had low risk of selection bias in the random sequence generation domain. Two studies had low risk of bias in the allocation concealment domain. One study used sealed opaque envelopes (Cole 2001), and another used computer‐generated randomized allocation (Joannes‐Boyau 2013). One study had unclear risk of bias (insufficient information available as to the process of randomization) (Ghani 2006), and another had high risk of bias (the randomized group for the last participant in each block was known in advance) (Boussekey 2008).
Blinding
All four included studies had low risk of performance bias. For outcomes assessed in this review, it was unclear to whom the blinding referred, leading to unclear risk of detection bias in all four included studies.
Incomplete outcome data
One study had low risk of attrition bias (Cole 2001), and in the other three studies, this was unclear.
Selective reporting
All four included studies had low risk of reporting bias.
Other potential sources of bias
We found the included studies to be at low risk for other potential sources of bias.
One study author provided a statement that he had no conflicts of interest (Boussekey 2008). Two studies reported that investigators received pharmaceutical company funding (Cole 2001; Ghani 2006). Cole 2001 stated that this group received pharmaceutical funding for cytokine and complement measurements only. Ghani 2006 stated that research was supported by a pharmaceutical company grant, but review authors could not determine the extent of the company's involvement in the study by reviewing the manuscript. Joannes‐Boyau 2013 stated that one study author received support from a health research organization.
Effects of interventions
See: Table 1
Primary outcomes
Mortality
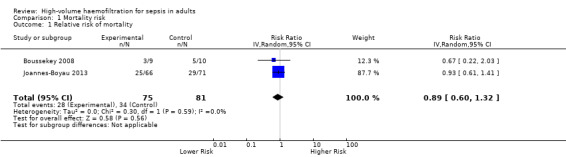
Boussekey 2008 described ICU mortality of 33.3% (three out of nine participants) in the treatment intervention group and 60% (six out of 10 participants) in the control intervention group (risk ratio (RR) 0.59, 95% CI 0.19 to 1.59). Investigators reported 28‐day mortality of 33.3% (three out of nine participants) in the treatment intervention group and 50% (five out of 10 participants) in the control intervention group (RR 0.67, 95% CI 0.22 to 2.03). Joannes‐Boyau 2013 described 28‐day mortality of 37.9% (25 out of 66 participants) in the treatment intervention group and 40.8% (29 out of 71 participants) in the control intervention group. We pooled results from Boussekey 2008 and Joannes‐Boyau 2013 using random‐effects modelling and have presented these results in Figure 4 and in Table 1. The pooled estimate of risk ratio (RR) was 0.89 (95% CI 0.60 to 1.32, two studies, 156 participants). We classified the strength of this evidence as low, using the GRADE rating scheme (grade reduced owing to imprecision).
4.
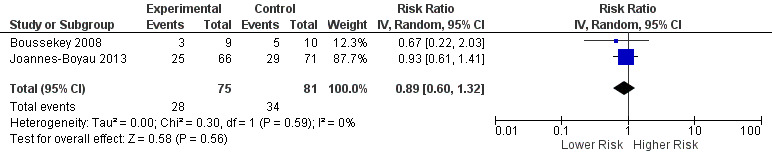
Forest plot of comparison: 1 Mortality risk, outcome: 1.1 Relative risk of mortality.
One study (Cole 2001) reported only hospital mortality rates, stated as 54.5% (six out of 11 participants), and as this was a cross‐over study, between‐group comparisons were not possible.
The fourth study (Ghani 2006) reported a mortality rate of 76% (25 out of 33 participants). It was not clear if this referred to ICU, hospital or other (e.g. 28‐day mortality). In addition, closer examination of the data published in this paper revealed that the number of deaths stated did not match the quoted mortality rates, nor was it reflected in the survival function curve. We have not been able to receive clarification from the study author on this; therefore, we have not included it in our analysis of mortality.
Secondary outcomes
Length of stay
One study (Joannes‐Boyau 2013) investigated length of ICU stay; researchers reported no difference in the median number of ICU‐free days (75 days in the intervention group and 74 in the control group).
Organ dysfunction
Investigators in two studies (Ghani 2006; Joannes‐Boyau 2013) stated that they measured the sequential organ failure assessment score (SOFA score). Ghani 2006 measured SOFA scores at days zero, one and seven, and at ICU and hospital discharge. The SOFA scores were similar in treatment and control intervention groups at baseline and fell in both groups by day seven, with a statistically significant fall in the control intervention group (P = 0.048 in the treatment group and P = 0.006 in the control intervention group). Joannes‐Boyau 2013 measured SOFA scores at days four and 28 and reported no difference between median SOFA scores in the intervention and control groups at either time point. Joannes‐Boyau 2013 also reported the simplified acute physiology score II (SAPS II) at days four and 28 and revealed no significant difference at either time point. We downgraded the evidence to low quality owing to imprecision.
Adverse events
Haemodynamic change
HVHF did not appear to cause haemodynamic instability in these studies. In Ghani 2006, study authors stated that they observed no significant drop in systolic blood pressure (SBP), diastolic blood pressure (DBP) or mean arterial pressure (MAP) after treatment. Boussekey 2008 showed that HVHF was associated with a decrease in norepinephrine dose greater than 75% in 24 hours in eight out of nine participants in the treatment intervention group compared with four out of 10 participants in the control intervention group (RR 2.22, 95% CI 1.01 to 4.51). Similarly, Cole 2001 noted that the dose of norepinephrine required for maintenance of target MAP during treatment decreased more during HVHF than during continuous veno‐venous haemofiltration (CVVH). This was a median dose reduction of 10.5 µg/min (interquartile range (IQR) 11.0) versus 1.0 µg/min (IQR 6.0), for a proportional decrease of 68% (IQR 28%) versus 7% (IQR 59%). Owing to differing reporting methods, we were unable to produce pooled estimates of effect. We downgraded this evidence to low quality owing to imprecision.
No researchers specifically commented on risk of central venous catheter‐related infection, risk of mechanical complications related to catheter placement (malpositioned lines, haematoma, pneumothorax) or risk of bleeding related to anticoagulation. Ghani 2006 stated, "there were no reported cases of excessive bleeding and adverse side‐effects". Boussekey 2008 reported, "no adverse event associated with HVHF, like severe hypophosphataemia or hypokalaemia, was recorded" and Cole 2001 said, "no adverse events were noted". Joannes‐Boyau 2013 described three serious adverse events but stated that investigators judged none of these to have been directly related to the study intervention.
Discussion
Severe sepsis and septic shock are associated with poor patient outcomes. High‐volume haemofiltration (HVHF) has been theorised to confer potential benefit in this setting. This systematic review shows that, despite the potential benefits of HVHF, very few studies have been conducted to investigate its use in patients with severe sepsis or septic shock. The studies included in this analysis included only small numbers of participants and used clinically diverse case definitions, treatment strategies and outcome measures.
The clinical heterogeneity of the included studies resulted in difficulty in pooling estimates for major outcomes. The evidence presented in Table 1 and Figure 4 incorporates data from only two studies (Boussekey 2008; Joannes‐Boyau 2013) and therefore should be interpreted with caution. Joannes‐Boyau 2013 stated that the trial was prematurely terminated because of slow recruitment and, therefore, did not enrol the anticipated number of participants required to achieve the desired 85% power. These limited data suggest that HVHF does not appear to confer improved mortality risk over standard therapy. Limited evidence also shows a trend towards reduced vasopressor requirements in patients treated with HVHF. Boussekey 2008 and Cole 2001 commented on this finding, but differing methods of reporting precluded the possibility of a pooled estimate. The review authors believe that this updated systematic review has highlighted the continued paucity of evidence in this field and the need for additional large‐scale clinical trials to determine the effects of HVHF.
Summary of main results
We found very weak evidence to support the use of high‐volume haemofiltration in critically ill patients with severe sepsis/septic shock to improve outcomes. We found no evidence to suggest that the treatment intervention was harmful.
Overall completeness and applicability of evidence
The evidence reported here is weak as a result of the limited number of trials identified with few participants. Therefore, one must interpret the results of this review with caution.
Quality of the evidence
Flaws in reporting of outcome results further reduced the data available for analysis. Clinical heterogeneity in terms of time points for assessing mortality rates meant that it was difficult to pool estimates for all primary outcomes. The small number of studies identified and, in particular, the single‐centre nature of three of the included studies (Boussekey 2008; Cole 2001; Ghani 2006) meant that review authors had to downgrade the quality of evidence produced in this review to low (reduced by two levels for imprecision).
Potential biases in the review process
The only potential bias in the review process of which review authors are aware is that we were unable to receive a response from the authors of Ghani 2006; this could have biased some of the results pertaining to mortality. However, given that Ghani 2006 was a small single‐centre study, it seems unlikely that the results of this review would have been significantly altered by its inclusion in the quantitative synthesis for mortality.
Agreements and disagreements with other studies or reviews
We are not aware of any similar studies or reviews meeting our inclusion and exclusion criteria.
Authors' conclusions
Implications for practice.
The evidence presented in this review, although of low quality, suggests that HVHF does not provide significant benefit in terms of reduction in mortality among critically ill patients with severe sepsis or septic shock.
Implications for research.
As noted, the evidence presented in this review is of low quality owing to the relatively small numbers of participants studied, the single‐centre nature of many studies and the heterogeneity of reporting of key outcomes. Future studies should be multi‐centre in nature and sufficiently powered to address key outcomes such as mortality. These studies should adopt standard definitions for the critical outcomes measured. For example, researchers should reach consensus as to the time point for assessing mortality rates, such as by limiting studies to measuring 28‐day mortality from time of randomization. This would also apply to secondary outcomes for which studies should adopt standard protocols, for example, for administration of vasopressor agents, which would allow data to be pooled across studies. The financial implications of providing HVHF, including those related to equipment and staffing costs, should be assessed in a cost‐benefit analysis. The review authors are not aware of any new trials, either registered or ongoing, involving the use of HVHF for sepsis.
What's new
| Date | Event | Description |
|---|---|---|
| 14 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 4, 2009 Review first published: Issue 1, 2013
| Date | Event | Description |
|---|---|---|
| 31 December 2015 | New citation required but conclusions have not changed | Overall, the conclusions of this updated review remain the same, despite the addition of one extra RCT. |
| 31 December 2015 | New search has been performed | This is an update of a previous Cochrane systematic review that included three RCTs (Borthwick 2013). The authors of the initial version updated this review. We used the initial search criteria again but extended the search date to 31 December 2015. Inclusion and exclusion criteria remained unchanged from the original version. We found only one extra study (Joannes‐Boyau 2013) to include in the quantitative synthesis. We constructed a forest plot by using mortality data. |
| 3 January 2013 | Amended | We updated contact details. |
Acknowledgements
This published review was funded by a Cochrane Fellowship Award from the Research and Development Office, Northern Ireland, and the Health Research Board, Ireland (Borthwick 2013).
We would like to thank Nicola Petrucci (Content Editor), Angela C Webster and Marlies Ostermann (Peer Reviewers) and Anne Lyddiatt (Consumer Representative) for help and editorial advice provided during preparation of this systematic review (Borthwick 2013).
We also would like to thank Nicola Petrucci (Content Editor), Angela C Webster (Peer Reviewer), Marlies Ostermann (Peer Reviewer), Rinaldo Bellomo (Peer Reviewer), Suzanne Cunliffe and Anne Lyddiatt (Consumer Representatives) and Nikhil Pawa (Cochrane Consumer Network Representative) for help and editorial advice provided during preparation of the protocol (Borthwick 2009) for this systematic review.
Appendices
Appendix 1. MEDLINE (Ovid SP) search strategy
#1 (Volume adj10 (ultrafiltrat* or h?emofiltrat*)).mp.
#2 (HVHF or HVHF).ti,ab.
#3 exp Hemodiafiltration/
#4 exp Hemofiltration/ or exp Ultrafiltration/
#5 (Extracorporeal adj10 ultrafiltration).mp.
#6 (h?emofiltrat* or h?emodiafiltrat* or ultrafiltrat*).mp.
#7 (purification adj5 therap*).mp.
#8 (Blood adj5 purificat*).mp.
#9 (purificat* adj3 therap*).mp.
#10 or/1‐9
#11 exp Sepsis/ or exp Shock, Septic/
#12 Systemic Inflammatory Response Syndrome/
#13 Multiple Organ Failure/
#14 (multi?organ adj5 failure).mp.
#15 SIRS.mp.
#16 (sepsis* or septic* or SIRS).mp.
#17 or/11‐16
#18 #10 and #17
#19 randomised controlled trial.pt.
#20 controlled clinical trial.pt.
#21 randomized.ab.
#22 placebo.ab.
#23 drug therapy.fs.
#24 randomly.ab.
#25 trial.ab.
#26 groups.ab.
#27 or/19‐26
#28 humans.sh.
#29 #27 and #28
#30 #18 and #29
Appendix 2. Embase (Ovid SP) search strategy
#1 (Volume adj10 (ultrafiltrat* or h?emofiltrat*)).mp.
#2 (HVHF or HVHF).ti,ab.
#3 exp HEMODIAFILTRATION/
#4 exp HEMOFILTRATION/
#5 exp ULTRAFILTRATION/
#6 (Extracorporeal adj10 ultrafiltration).mp.
#7 (h?emofiltrat* or h?emodiafiltrat* or ultrafiltrat*).mp.
#8 (purification adj5 therap*).mp.
#9 (purificat* adj5 therap*).mp.
#10 or/1‐9
#11 exp Sepsis/ or exp Shock, Septic/ or exp Septicemia/
#12 (sepsis* or septic*).mp.
#13 Multiple Organ Failure/
#14 (multi?organ adj6 failure).mp.
#15 or/11‐14
#16 Randomized Controlled Trial/
#17 RANDOMIZATION/
#18 Controlled Study/
#19 Multicenter Study/
#20 Phase 3 Clinical Trial/
#21 Phase 4 Clinical Trial/
#22 Double Blind Procedure/
#23 Single Blind Procedure/
#24 (RANDOM* or CROSS?OVER* or FACTORIAL* or PLACEBO* or VOLUNTEER*).ti,ab.
#25 ((SINGL* or DOUBL* or TREBL* or TRIPL*) adj6 (BLIND* or MASK*)).ti,ab.
#26 or/16‐25
#27 "human*".ec,hw,fs.
#28 #27 and #26
#29 #28 and #10 and #15
Appendix 3. CENTRAL (the Cochrane Library) search strategy
#1 (HVHF or HVHF):ti,ab
#2 MeSH descriptor Hemodiafiltration explode all trees
#3 MeSH descriptor Hemofiltration explode all trees
#4 MeSH descriptor Ultrafiltration explode all trees
#5 h?emofiltrat* or h?emodiafiltrat* or ultrafiltrat*
#6 purification NEAR therap*
#7 Blood near purificat*
#8 (blood near therap*):ti,ab
#9 (#1 OR #2 OR #3 OR #5 OR #6 OR #7 OR #8)
#10 MeSH descriptor Sepsis explode all trees
#11 MeSH descriptor Shock, Septic explode all trees
#12 MeSH descriptor Systemic Inflammatory Response Syndrome explode all trees
#13 MeSH descriptor Multiple Organ Failure explode all trees
#14 sepsis* or septic*
#15 (#10 OR #11 OR #12 OR #13 OR #14)
#16 (#9 AND #15)
Appendix 4. LILACS (BIREME) search strategy
HEMODIAFILTRATION/" or "HEMOFILTRATION" or "ULTRAFILTRATION/" or "haemofiltrat$" or "haemodiafiltrat$" or "ultrafiltrat*" or "purificat$ therap$" or "Blood purificat$" or "HVHF" or "HVHF" or "terapia de la purificación" or "terapia da purificação" or "Purificação do sangue" or "Purificación de la sangre" [Words] and "SEPSIS" or "SEPSIS SYNDROME/" or "SEPTIC SHOCK/" or "SYSTEMIC INFLAMMATORY RESPONSE SYNDROME/" or "MULTIPLE ORGAN FAILURE/" or "SIRS" or "septic$" or "sepsis" or "Falta múltiple del órgano" or "Falha múltipla do órgão"
Appendix 5. CINAHL (EBSCO host) search strategy
S1 purification therap*
S2 Blood purificat*
S3 MW Hemodiafiltration
S4 MW Hemofiltration
S5 MW Ultrafiltration
S6 S5 or S4 or S3 or S2 or S1
S7 MW Sepsis
S8 MW Shock, Septic
S9 MW Systemic Inflammatory Response Syndrome
S10 TX Multiple Organ Failure
S11 TX multiorgan failure
S12 TX sepsis* or septic* or SIRS
S13 S12 or S11 or S10 or S9 or S8 or S7
S14 S13 and S6
Appendix 6. ISI Web of Science search strategy
#1 TS=(purification SAME therap*) or TS=(Blood SAME purificat*) or TS=(purificat* SAME therap*) or TS=(h?emofiltrat* or h?emodiafiltrat* or ultrafiltrat*) or TS=(HVHF or HVHF) or TS=(Volume SAME (ultrafiltrat* or h?emofiltrat*))
#2 TS=(sepsis* or septic* or SIRS) or TS=(Systemic Inflammatory Response Syndrome) or TS=(Multiple Organ Failure) or TS=(multi?organ SAME failure)
#3 #2 AND #1
#4 TS=(random*) or TS=(clinical trial*) or TS=(controlled trial*) or TS=placebo
#5 #4 AND #3
Appendix 7. Data extraction form
Study selection, quality assessment & data extraction form
Name of author extracting data: ____________________________
Date form completed: ____________________________
Study ID
| Title |
|
Study ID for RevMan (Family name of first author and year of publication + letter if more than one per year, e.g. Smith 2001b) |
| Are there other articles on the same study? (Yes, No, Unclear. If Yes, write Study IDs) |
Study eligibility
| (please circle) | Source (page no. in report) | |
|
Type of study Can the study be described as randomized or quasi‐randomized, i.e. method of allocation is known but study is not considered strictly randomized? |
Yes, Unclear, No | |
|
Participants 1. Were participants adults (≥ 16 years) in ICUs? 2. Did they have severe sepsis or septic shock (study authors' definitions)? |
Yes, Unclear, No Yes, Unclear, No |
|
|
Interventions 1. HVHF0r HVHDF (> 35 mL/kg/h) vs some form of RRT OR 2. HVHF or HVHDF(> 35 mL/kg/h) vs no similar dialysis therapy |
Yes, Unclear, No Yes, Unclear, No |
|
|
Outcomes: Did the study report any one of: 1. Mortality rates, ICU or hospital with deaths reported at 7, 28 or 30 days |
Yes, Unclear, No |
|
|
Conclusion: If study to be ‘included’ or ‘excluded & listed in excluded table’, record below the information to be inserted into tables. If included, continue to page 2. Included Excluded and should be listed in the excluded table Excluded and should NOT be listed in the excluded table More information needed before inclusion decision (specify): Record for tables: |
||
Source of key information
|
Electronic database (Which one?) |
|
Unpublished source (Where?) |
|
Personal communication (From whom?) |
Risk of bias table
| Domain | Description | Review authors’ judgement |
|
Randon sequence generation (selection bias) Outcome: |
Was the allocation (selection bias) sequence adequately generated? Low risk High risk Unclear risk |
|
|
Allocation concealment (selection bias) Outcome: |
Was allocation adequately concealed? Low risk High risk Unclear risk |
|
|
Blinding of participants, personnel and outcome assessors (performance bias and detection bias) Outcome: |
Was knowledge of the allocated intervention adequately prevented during the study? Low risk High risk Unclear risk |
|
|
Incomplete outcome data (attrition bias) Outcome: |
Were incomplete outcome data adequately addressed? Low risk High risk Unclear risk |
|
|
Selective outcome reporting (reporting bias) Outcome: |
Are reports of the study free of suggestion of selective outcome reporting? Low risk High risk Unclear risk |
|
|
Other potential sources of bias Outcome: |
Was the study apparently free of other problems that could put it at high risk of bias? Low risk High risk Unclear risk |
Setting
| Country | |
| Setting | Single ICU > 1 ICU (specify no.) |
|
Type of ICU (& no.) |
Medical Surgical Mixed medical and surgical unit Other (specify) |
Participants
|
No of participants who were randomized |
Intervention group n = Control group n = |
|
No of participants who were analysed |
Intervention group n = Control group n = |
|
Age (mean/SD) |
Intervention group Control group |
|
Sex of participants (M/F numbers or %) |
Intervention group Control group |
| Inclusion criteria | |
| Exclusion criteria |
Intervention delivery
|
HVHF Dose (in mL/kg/h) Modality Duration of therapy delivery (hours) Pulse or continuous |
Haemofiltration Haemodiafiltration |
|
‘Standard or usual’ practice If RRT: Dose (mL/kg/h) Modality Duration/Frequency |
No RRT Intermittent CRRT HF HDF HD |
Outcomes relevant to the review reported in the paper
Mortality
|
Yes/No Yes/No |
Length of stay
|
Yes/No Yes/No |
Organ dysfunction
|
Yes/No Yes/No |
Adverse events:
|
Yes/No Yes/No Yes/No Yes/No |
Outcomes: continuous data
| Outcomes | Unit of measurement | Intervention group | Control group | 95% CI or any further details if outcome described only in text | ||||
| n |
Mean (SD) |
Median (IQR) | n | Mean (SD) | Median (IQR) | P value | ICU length of stay |
|
| Hospital length of stay | ||||||||
| Type + Duration of organ dysfunction | ||||||||
| Type + Number of organ dysfunctions | ||||||||
| Haemodynamic change when undergoing HVHF: SBP DBP MAP Inotrope/Vasopressor dose change |
||||||||
Outcomes: dichotomous data
| Outcomes |
Intervention group (n = ) |
Control group (n = ) |
P value | Any further information |
| ICU mortality | ||||
| Hospital mortality | ||||
| Mechanical complications related to catheter placement: Malpositioned lines Haematoma Pneumothorax |
||||
| Catheter‐related infection (due to HVHF catheter) | ||||
| Bleeding related to anticoagulation |
Please specify the number of participants in each group experiencing specified outcomes.
Other information that you believe is relevant to the results:
| Indicate if any data were obtained from the primary author; or if results were estimated from graphs, etc, or were calculated by you using a formula (this should be stated and the formula given). In general, if results not reported in the paper(s) are obtained, this should be made clear here to be cited in the review. Freehand space for writing actions such as contact with study authors and changes. |
Data and analyses
Comparison 1. Mortality risk.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relative risk of mortality | 2 | 156 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.60, 1.32] |
1.1. Analysis.
Comparison 1 Mortality risk, Outcome 1 Relative risk of mortality.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Boussekey 2008.
| Methods | Country: France Setting: single‐centre ICU Time frame: 18 months (Aug 2005 to Jan 2007) Parallel RCT Intention‐to‐treat analysis: yes Follow‐up period: 28 days Lost to follow‐up:‐ 0/19 |
|
| Participants | No. randomized: 20 No. analysed: 19 (1 participant in HVHF group secondarily excluded, as diagnosis was mesenteric ischaemia rather than septic shock) Treatment intervention N = 9 Mean age: 68 years Male, %: 77.77 Mean SAPS II score: 66 Mean APACHE II score: 32 Control intervention N = 10 Mean age: 72.5 years Male, %: 80 Mean SAPS II score: 67 Mean APACHE II score: 33.50 Inclusion criteria Septic shock and ARF requiring RRT Exclusion criteria Obstructive or prerenal renal failure Severe chronic kidney disease (creatinine clearance < 30 mL/min) Included in another study Severe immunosuppression (study authors’ definitions) Moribund Decision that therapy was limited Septic shock or renal failure > 5 days after ICU admission Absence of written consent Secondarily excluded if a participant died within first day after randomization or was found to have a disease other than septic shock |
|
| Interventions |
Treatment intervention HVHF: 65 mL/kg/h ultrafiltrate flow for maximum of 4 days, or until norepinephrine was discontinued for at least 4 hours with persistent MAP > 65 mmHg Blood flow: 200‐300 mL/min Bicarbonate replacement fluid: replaced one‐third pre‐filter and two‐thirds post‐filter Bicarbonate buffered fluid Filter: 1.4 m2 polyethersulfone Control intervention 35 mL/kg/h ultrafiltrate flow for maximum of 4 days, or until norepinephrine was discontinued for at least 4 hours with persistent MAP > 65 mmHg Blood flow: 180‐250 mL/min Bicarbonate replacement fluid: replaced one‐third pre‐filter and two‐thirds post‐filter Filter: 1.4 m2 polyethersulfone |
|
| Outcomes | Vasopressor use with stable MAP (> 65 mmHg) within 24 hours of haemofiltration Duration mechanical ventilation and RRT ICU length of stay Mortality in ICU and day 28 |
|
| Notes | Funding source: Tourcoing Hospital No conflicts of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate: block randomization with sequentially number sealed envelopes |
| Allocation concealment (selection bias) | High risk | The group for the last participant in each block was known in advance. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind personnel to allocated treatment, although considered to be at low risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated as for whom or how outcome assessment was made |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No incomplete data were reported. |
| Selective reporting (reporting bias) | Low risk | Protocol available and predefined outcomes reported |
| Other bias | Low risk | Appears to be free of other sources of bias |
Cole 2001.
| Methods | Country: Australia Setting: tertiary‐centre ICU Time frame: unclear Cross‐over RCT Intention‐to‐treat analysis: yes Follow‐up period: 2 days Lost to follow‐up: none |
|
| Participants | No. of participants randomized: 11 No. of participants analysed: 11 Mean age: 63.1 Male, no. (%): 8/11 (73) Inclusion criteria Septic shock (criteria of bone) Established ARF secondary to septic shock (with oliguria or anuria) Established need for RRT Recognized source of sepsis that had been treated with antibiotics and surgical drainage of source if necessary Exclusion criteria ESRD AIDS Life expectancy < 6 months Withdrawal of therapy possible |
|
| Interventions |
Treatment intervention HVHF 8‐Hour session Filter: 1.6 m2 AN 69 Blood flow: 300 mL/min; ultrafiltrate flow: 100 mL/min Lactate buffered replacement fluid: one‐third delivered pre‐filter, two‐thirds post‐filter Control intervention CVVH 8‐Hour session Filter: 1.2 m2 AN 69 polyacrylonitrile Blood flow: 200 mL/min; ultrafiltrate flow: 1 L/h Lactate buffered replacement fluid delivered pre‐filter MAP to be maintained at > 70 mmHg throughout |
|
| Outcomes | Mean arterial pressure and norepinephrine dose | |
| Notes | Funding source: Austin & Repatriation Medical Centre Anaesthesia and Intensive Care Trust Fund and Hospal Pty Ltd No conflicts of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation by computer programme |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind personnel to allocated treatment, although considered to be at low risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated as for whom or how outcome assessment was made |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Protocol available and predefined outcomes reported |
| Other bias | Low risk | Appears to be free of other sources of bias |
Ghani 2006.
| Methods | Country: Malaysia Setting: single centre Time frame: not stated Parallel RCT Intention‐to‐treat analysis: yes Follow‐up period: not stated Lost to follow‐up: 0/33 |
|
| Participants | No. of participants randomized: 33 No. of participants analysed: 33 3 participants excluded on account of inadequate blood sample collection Treatment intervention N = 15 Mean age: 58 years Male, %: 53.33 Pre‐existing CKD: 40% Diabetes: 46.66% Baseline MAP: 99.3 mmHg Control intervention N = 18 Mean age: 57.50 years Male, %: 61.11 Pre‐existing CKD: 50.00% Diabetes: 50.00% Baseline MAP: 87.6 mmHg Inclusion criteria Patients who fulfilled criteria for sepsis (study author’s definition) with 1 additional major end‐organ dysfunction or septic shock as defined by the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Exclusion criteria ESRD (end‐stage renal disease) Underlying malignancy AIDS Life expectancy < 6 months |
|
| Interventions |
Treatment intervention 6 hours of HVHF Ultrafiltration rate: 6 L/h (equivalent to 100 mL/kg/h, whichever was higher) Blood flow: 250–350 mL/min Bicarbonate substitution fluid 1.4 m2 polyethersulfone dialysis membrane Control intervention 6 hours of CVVH Ultrafiltration rate: 2 L/h (equivalent to almost 35 mL/kg/h) Blood flow: 200‐250 mL/min 1.4 m2 polyethersulfone dialysis membrane |
|
| Outcomes | Blood pressure (systolic, diastolic and mean arterial pressure) SOFA scores |
|
| Notes | Funding source: Edwards Life Sciences No conflicts of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization carried out |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind personnel to allocated treatment, although considered to be at low risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information available to comment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information available to comment |
| Selective reporting (reporting bias) | Low risk | Protocol available and predefined outcomes reported |
| Other bias | Low risk | Appears to be free of other sources of bias |
Joannes‐Boyau 2013.
| Methods | Country: France, Belgium and Netherlands Setting: multi‐centre ICUs Time frame: 53 months (October 2005‐March 2010) Parallel RCT Intention‐to‐treat analysis: yes Follow‐up period: 90 days Lost to follow‐up: none |
|
| Participants | No. randomized: 140 (3 excluded, 1 by steering committee for eligibility reason, 2 for consent reasons) No. analysed: 137 Treatment intervention N = 66 Mean age: 68 years Male, %: 68 SAPS II: 68 Mean APACHE II score: not described Control intervention N = 71 Mean age: 70 years Male, %: 54 Mean SAPS II score: 64 Mean APACHE II score: not described Inclusion criteria Septic shock and AKI (scoring ‘INJURY’ or greater in RIFLE criteria) Exclusion criteria Age ≥ 80 years Estimated life expectancy ≤ 3 months Metastatic cancer Decompensated cirrhosis Acute necrotizing pancreatitis Prior diagnosis of ESRD Confirmed pregnancy Severe coagulopathy Lack of commitment to full medical support |
|
| Interventions |
Treatment intervention HVHF 70 mL/kg/h ultrafiltrate flow for 96 hours Blood flow: average blood flow rates 200‐320 mL/min Bicarbonate replacement fluid: replaced one‐third pre‐filter and two‐thirds post‐filter Filter: 1.9 m2 polyethersulfone Control intervention HVHF 35 mL/kg/h ultrafiltrate flow for 96 hours Blood flow: average blood flow rates 200‐320 mL/min Bicarbonate replacement fluid: replaced one‐third pre‐filter and two‐thirds post‐filter Filter: 1.9 m2 polyethersulfone |
|
| Outcomes |
Primary endpoint 28‐Day mortality Secondary endpoint Change in haemodynamic profile Change in SOFA and SAPS II scores Duration of mechanical ventilation Duration of RRT and recovery of renal function Duration of stay in ICU and hospital 60‐Day and 90‐day mortality Adverse events attributable to haemofiltration |
|
| Notes | Funding source: French Health Ministry One study author declared an award received from a health research organization |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate: block randomization |
| Allocation concealment (selection bias) | Low risk | Adequate: allocation process centralized and allocation group concealed until implementation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind personnel to allocated treatment, although considered to be at low risk |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trained personnel using standardized report form |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No incomplete data were reported. |
| Selective reporting (reporting bias) | Low risk | Protocol available and predefined outcomes reported |
| Other bias | Low risk | Appears to be free of other sources of bias |
AKI: acute kidney injury.
AN: acrylonitrile.
APACHE II: Acute Physiology and Chronic Health Evaluation II. ARF: acute renal failure. CKD: chronic kidney disease. CVVH: continuous veno‐venous haemofiltration. ESRD: end‐stage renal disease.
HVHF: high‐volume haemofiltration.
INJURY: part of RIFLE (see below) criteria for assessment of severity of acute kidney injury. ICU: intensive care unit.
MAP: mean arterial pressure.
RCT: randomized controlled trial.
RIFLE: "Risk, Injury, Failure, Loss, End‐stage" criteria for assessment of severity of acute kidney injury. RRT: renal replacement therapy. SAPS: Simplified Acute Physiology Score. SOFA: Sequential Organ Failure Assessment score.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abe 2010 | Type of participant not appropriate (not all septic) |
| Bellomo 2009 | Type of participant not appropriate (not all septic) |
| Bouman 2002 | Type of participant not appropriate (not all septic) |
| Bouman 2007 | Review paper, not a trial report |
| Castro 2008 | Study design not appropriate (an algorithm for use in emergency room) |
| Chu 2013 | Type of participant not appropriate (not all septic) |
| Clark 2014 | Review paper, not a trial report |
| Cole 2003 | Outcome measures not appropriate (outcomes focused on acid base measurements) |
| Combes 2015 | Type of participant not appropriate (not all septic) |
| Guo 2014 | Type of participant not appropriate (not all septic) |
| Haase 2007 | Type of intervention not appropriate (high adsorption filters under investigation, not HVHF) |
| Han 2011 | Outcome measures not appropriate (outcomes focused on endothelial cell function) |
| Hoffman 1996 | Type of intervention not appropriate (not HVHF) |
| Honore 2003 | Review paper, not a trial report |
| Honore 2004 | Review paper, not a trial report |
| Honore 2007 | Review paper, not a trial report |
| Honore 2009 | Review paper, not a trial report |
| Mao 2009 | Type of intervention not appropriate (not HVHF, plasma adsorption under investigation) |
| Morgera 2006 | Type of intervention not appropriate (not HVHF, high cut‐off filters under investigation) |
| Oudemans‐van Straaten1999 | Type of participant and study design not appropriate (not all septic, not randomized) |
| Palevsky 2008 | Type of participant not appropriate (not all septic) |
| Payen 2009 | Type of intervention not appropriate (not HVHF) |
| Peng 2010 | Outcome measures not appropriate (outcomes focused on cytokine levels) |
| Quenot 2015 | Type of intervention not appropriate (extra high volume haemofiltration vs HVHF) |
| Ronco 2000 | Type of participant not appropriate (not all septic) |
| Storck 1991 | Type of participant and intervention not appropriate (not all septic, not HVHF) |
| Wang 2009 | Outcome measures not appropriate (outcomes focused on lactic acid and cytokine levels) |
| Xie 2009 | Type of participant not appropriate (not all septic) |
| Yu 2008 | Type of participant not appropriate (not septic, all pancreatitis) |
| Zainudin 2006 | Abstract only, published paper included (different first study author) |
| Zhang 2004 | Outcome measures not appropriate (outcomes focused on cytokine levels) |
| Zhang 2012 | Type of intervention not appropriate (extra high volume haemofiltration vs HVHF) |
HVHF: high‐volume haemofiltration.
Differences between protocol and review
We made the following changes to the published protocol (Borthwick 2009).
It was not necessary for protocol authors C Cardwell and P Glover to contribute to the review. We added Dr C Hill as an additional author of this review after the protocol had been finalized.
Contributions of authors
Emma MJ Borthwick (EMJB), Christopher J Hill (CJH), Kannaiyan S Rabindranath (KSR), Alexander P Maxwell (APM), Danny F McAuley (DFM), Bronagh Blackwood (BB).
Joint first authors: EMJB and CJH.
Conceiving the review: EMJB.
Co‐ordinating the review: EMJB, BB.
Undertaking manual searches: EMJB.
Screening search results: EMJB, KSR, APM.
Organizing retrieval of papers: EMJB.
Screening retrieved papers against inclusion criteria: EMJB, KSR.
Appraising quality of papers: EMJB, KSR.
Abstracting data from papers: EMJB, KSR, BB.
Writing to authors of papers for additional information: EMJB.
Providing additional data about papers: EMJB.
Obtaining and screening data on unpublished studies: EMJB.
Managing data for the review: EMJB.
Entering data into Review Manager (RevMan 5.3): EMJB, KSR, BB.
Analysing RevMan statistical data: EMJB, KSR.
Performing double entry of data: data entered by person one: EMJB; data entered by person two: KSR.
Interpreting data: EMJB, KSR, APM, DFM.
Writing the review: EMJB, CJH, BB, KSR, APM, DFM.
Securing funding for the review: EMJB.
Performing previous work that was the foundation of the present study:
Serving as guarantor for the review (one review author): EMJB.
Taking responsibility for reading and checking the review before submission: EMJB, BB.
Sources of support
Internal sources
-
Northern Ireland Kidney Research Fund, UK.
EMJB and CJH received funding from the NIKRF
External sources
-
Cochrane Fellowship, Health and Social Care, Research and Development Division, Public Health Agency, Northern Ireland, UK.
EMJB received funding and educational support from the Cochrane Fellowship programme
Declarations of interest
Christopher J Hill: The Northern Ireland Kidney Research Fund (NIKRF), a charitable organization, provided salary support for the duration of my postgraduate research. The NIKRF has had no influence (direct or indirect) on the performance or content of this research study.
Emma MJ Borthwick: Development of the Protocol (Borthwick 2009) was supported by a Cochrane Fellowship Award from the Northern Ireland Research and Development Office, and Health Research Board, Ireland.
Kannaiyan S Rabindranath: none known.
Alexander P Maxwell: none known.
Danny F McAuley: none known.
Bronagh Blackwood: none known.
Joint first author
Joint first author
Edited (no change to conclusions)
References
References to studies included in this review
Boussekey 2008 {published data only}
- Boussekey N, Chiche A, Faure K, Devos P, Guery B, d’Escrivan T, et al. A pilot randomized study comparing high and low volume hemofiltration on vasopressor use in septic shock. Intensive Care Medicine 2008;34:1646‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cole 2001 {published data only}
- Cole L, Bellomo R, Journois D, Davenport P, Baldwin I, Tipping P. High‐volume haemofiltration in human septic shock. Intensive Care Medicine 2001;27(6):978‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ghani 2006 {published data only}
- Ghani RA, Zainudin S, Ctkong N, Rahman AF, Wafa SR, Mohamad M, et al. Serum IL‐6 and IL‐1‐ra with sequential organ failure assessment scores in septic patients receiving high‐volume haemofiltration and continuous venovenous haemofiltration. Nephrology 2006;11(5):386‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Joannes‐Boyau 2013 {published data only}
- Joannes‐Boyau O, Honore PM, Perez P, Bagshaw SM, Grand H, Canivet JL, et al. High‐volume versus standard‐volume haemofiltration for septic shock patients with acute kidney injury (IVOIRE study). Intensive Care Medicine 2013;39(9):1535‐46. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abe 2010 {published data only}
- Abe M, Okada K, Suzuki M, Nagura C, Ishihara Y, Fujii Y, et al. Comparison of sustained hemodiafiltration with continuous venovenous hemodiafiltration for the treatment of critically ill patients with acute kidney injury. Artificial Organs 2010;34(4):331‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bellomo 2009 {published data only}
- Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, et al. on behalf of the RENAL Replacement Study Investigators. Intensity of continuous renal‐replacement therapy in critically ill patients. New England Journal of Medicine 2009;361(17):1627‐38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bouman 2002 {published data only}
- Bouman CSC, Oudemans‐van Straaten HM, Tijssen JGP, Zandstra DF, Kesecioglu J. Effects of early high‐volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: a prospective, randomized trial. Critical Care Medicine 2002;30(10):2205‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bouman 2007 {published data only}
- Bouman CSC, Straaten HMO, Schultz MJ, Vroom MB. Hemofiltration in sepsis and systemic inflammatory response syndrome: the role of dosing and timing. Journal of Critical Care 2007;22(1):1‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Castro 2008 {published data only}
- Castro R, Regueira T, Aguirre ML, Llanos OP, Bruhn A, Bugedo G, et al. An evidence‐based resuscitation algorithm applied from the emergency room to the ICU improves survival of severe septic shock. Minerva Anestesiologica 2008;74(6):223‐31. [MEDLINE: ] [PubMed] [Google Scholar]
Chu 2013 {published data only}
- Chu LP, Zhou JJ, Yu YF, Huang Y, Dong WX. Clinical effects of pulse high‐volume hemofiltration on severe acute pancreatitis complicated with multiple organ dysfunction syndrome. Therapeutic Apheresis and Dialysis 2013;17:78‐83. [DOI] [PubMed] [Google Scholar]
Clark 2014 {published data only}
- Clark E, Molnar AO, Joannes‐Bayou O, Honore PM, Sikora L, Bagshaw SM. High‐volume hemofiltration for septic acute kidney injury: a systematic review and meta‐analysis. Critical Care 2014;18(1):R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cole 2003 {published data only}
- Cole L, Bellomo R, Baldwin I, Hayhoe M, Ronco C. The impact of lactate‐buffered high‐volume hemofiltration on acid‐base balance. Intensive Care Medicine. 2003;29(7):1113‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Combes 2015 {published data only}
- Combes A, Bréchot N, Amour J, Cozic N, Lebreton G, Guidon C, et al. Early high‐volume hemofiltration versus standard care for post‐cardiac surgery shock. The HEROICS study. American Journal of Respiratory and Critical Care Medicine 2015;192(10):1179‐90. [DOI] [PubMed] [Google Scholar]
Guo 2014 {published data only}
- Guo J, Huang W, Yang XN, Jin T, Altaf K, Javed MA, et al. Short‐term continuous high‐volume hemofiltration on clinical outcomes of severe acute pancreatitis. Pancreas 2014;43(2):250‐4. [DOI] [PubMed] [Google Scholar]
Haase 2007 {published data only}
- Haase M, Silvester W, Uchino S, Goldsmith D, Davenport P, Tipping P, et al. A pilot study of high‐adsorption hemofiltration in human septic shock. International Journal of Artificial Organs 2007;30(2):108‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Han 2011 {published data only}
- Han SS, Sun T, Li Z, Jia LZ, Shang QM, Wang XZ. Effect of continuous blood purification on endothelial cell function in patients with severe sepsis. Chinese Critical Care Medicine 2011;23(2):81‐4. [PubMed] [Google Scholar]
Hoffman 1996 {published data only}
- Hoffmann JN, Hartl WH, Deppisch R, Faist E, Jochum M, Inthorn D. Effect of hemofiltration on hemodynamics and systemic concentrations of anaphylatoxins and cytokines in human sepsis. Intensive Care Medicine 1996;22:1360‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Honore 2003 {published data only}
- Honore PM, Zydney A, Matson JR. High volume and high permeability haemofiltration for sepsis: the evidence and the key issues. Care of the Critically Ill 2003;19(3):69. [Google Scholar]
Honore 2004 {published data only}
- Honore PM, Joannes‐Boyau O. High volume hemofiltration (HVHF) in sepsis: a comprehensive review of rationale, clinical applicability, potential Indications and recommendations for future research. International Journal of Artificial Organs 2004;27(12):1077‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Honore 2007 {published data only}
- Honore PM, Joannes‐Boyau O, Gressens B. Blood and plasma treatments: high‐volume hemofiltration ‐ a global view. Contributions to Nephrology 2007;156:371‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Honore 2009 {published data only}
- Honore PM, Joannes‐Boyau O, Boer W, Collin V. High‐volume hemofiltration in sepsis and SIRS: current concepts and future prospects. Blood Purification 2009;28(1):1‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mao 2009 {published data only}
- Mao HJ, Yu S, Yu XB, Zhang B, Zhang L, Xu XR. Effects of coupled plasma filtration adsorption on immune function of patients with multiple organ dysfunction syndrome. The International Journal of Artificial Organs 2009;32(1):31‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Morgera 2006 {published data only}
- Morgera S, Haase M, Kuss T, Vargas‐Hein O, Zuckermann‐Becker H, Melzer C, et al. Pilot study on the effects of high cutoff hemofiltration on the need for norepinephrine in septic patients with acute renal failure. Critical Care Medicine 2006;34(8):2099‐104. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oudemans‐van Straaten1999 {published data only}
- Oudemans‐van Straaten HM, Bosman RJ, Spoel JI, Zandstra DF. Outcome of critically ill patients treated with intermittent high‐volume haemofiltration: a prospective cohort analysis. Intensive Care Medicine 1999;25(8):814‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Palevsky 2008 {published data only}
- VA/NIH Acute Renal Failure Trial Network, Palevsky PM, Zhang JH, O'Connor TZ, Chertow GM, Crowley ST, Choudhury D, et al. Intensity of renal support in critically ill patients with acute kidney injury. New England Journal of Medicine 2008;359(1):7‐20. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Payen 2009 {published data only}
- Payen D, Mateo J, Cavaillon JM, Fraisse F, Floriot C, Vicaut E, Hemofiltration and Sepsis Group of the Collège National de Réanimation et de Médecine d'Urgence des Hôpitaux extra‐Universitaires. Impact of continuous venovenous hemofiltration on organ failure during the early phase of severe sepsis: a randomized controlled trial. Critical Care Medicine 2009;37(3):803‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Peng 2010 {published data only}
- Peng Z, Pai P, Han‐Min W, Jun Z, Hong‐Bao L, Rong L, et al. Evaluation of the effects of pulse high‐volume hemofiltration in patients with severe sepsis: a preliminary study. Internation Journal of Artificial Organs 2010;33(8):505‐11. [DOI] [PubMed] [Google Scholar]
Quenot 2015 {published data only}
- Quenot JP, Binquet C, Vinsonneau C, Barbar SD, Vinault S, Deckert V, et al. Very high volume hemofiltration with the Cascade system in septic shock patients. Intensive Care Medicine 2015;41(12):2111‐20. [DOI] [PubMed] [Google Scholar]
Ronco 2000 {published data only}
- Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, et al. Effects of different doses in continuous veno‐venous haemofiltrationon outcomes of acute renal failure: a prospective randomised trial. Lancet 2000;355:26‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Storck 1991 {published data only}
- Storck M, Hartl WH, Zimmerer E, Inthorn D. Comparison of pump‐driven and spontaneous continuous haemofiltration in postoperative acute renal failure. Lancet 1991;337:452‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang 2009 {published data only}
- Wang CT, Ren HS, Jiang JJ, Zhang JC, Meng M, Yu JB, et al. Study the effects of high‐volume hemofiltration and fluid resuscitation on removing blood lactic acid and pro‐inflammatory cytokines in patients with refractory septic shock. Chinese Critical Care Medicine 2009;21(7):421‐4. [PubMed] [Google Scholar]
Xie 2009 {published data only}
- Xie J, Yang J. Effect of continuous high‐volume hemofiltration on patients with acute respiratory distress syndrome and multiple organ dysfunction syndrome. Chinese Critical Care Medicine 2009;21(7):402‐4. [PubMed] [Google Scholar]
Yu 2008 {published data only}
- Yu C, Liu ZH, Chen ZH, Gong DH, Ji DX, Li LSH. Improvement of monocyte function and immune homeostasis by high volume continuous venovenous hemofiltration in patients with severe acute pancreatitis. International Journal of Artificial Organs 2008;31(10):882‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zainudin 2006 {published data only}
- Zainudin S, Ghani RA, Mohd M, Wafa SRW, Tong NKC. Stability of haemodynamic parameters during CRRT: a comparison between standard continuous venovenous haemofiltration and high‐volume haemofiltration in patients with acute renal failure and sepsis [abstract no: SP286]. Nephrology Dialysis Transplantation 2006;21[Suppl 4]:iv108. [Google Scholar]
Zhang 2004 {published data only}
- Zhang J, Tao LJ, Ning JP, Xu H, Ai YH, Zhao SP. Effects of high‐volume hemofiltration on serum levels of tumor necrosis factor and its receptors in patients with multiple organ dysfunction syndromes. Chinese Critical Care Medicine 2004;16(2):81‐4. [PubMed] [Google Scholar]
Zhang 2012 {published data only}
- Zhang P, Yang Y, Lv R, Zhang YT, Xie WQ, Chen JH. Effect of the intensity of continuous renal replacement therapy in patients with sepsis and acute kidney injury: a single centre randomised clinical trial. Nephrology Dialysis Transplantation 2012;27(3):967‐73. [DOI] [PubMed] [Google Scholar]
Additional references
Abraham 2000
- Abraham E, Matthay MA, Dinarello CA, Vincent JL, Cohen J, Opal SM, et al. Consensus conference definitions for sepsis, septic shock, acute lung injury, and acute respiratory distress syndrome: time for a reevaluation. Critical Care Medicine 2000;28(1):232‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Adrie 2000
- Adrie C, Pinsky MR. The inflammatory balance in human sepsis. Intensive Care Medicine 2000;26(4):364‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Alejandria 2010
- Alejandria MM, Lansang MAD, Dans LF, Mantaring JB III. Intravenous immunoglobulin for treating sepsis, severe sepsis and septic shock. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD001090] [DOI] [PMC free article] [PubMed] [Google Scholar]
Angus 2001
- Angus DC, Linde‐Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky M. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical Care Medicine 2001;29(7):1303‐10. [PUBMED: 11445675 ] [DOI] [PubMed] [Google Scholar]
Annane 2004
- Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for treating severe sepsis and septic shock. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD002243.pub2] [DOI] [PubMed] [Google Scholar]
De Vriese 1999
- Vriese AS, Colardyn FA, Philippe JJ, Vanholder RC, Sutter JH, Lamiere NH. Cytokine removal during continuous hemofiltration in septic patients. Journal of the American Society of Nephrology 1999;10:846‐53. [PUBMED: 10203370] [DOI] [PubMed] [Google Scholar]
Gomes Silva 2010
- Gomes Silva BN, Andriolo RB, Atallah ÁN, Salomão R. De‐escalation of antimicrobial treatment for adults with sepsis, severe sepsis or septic shock. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD007934.pub2] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hotchkiss 2003
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. New England Journal of Medicine 2003;348(2):138‐50. [PUBMED: 12519925] [DOI] [PubMed] [Google Scholar]
Joannidis 2009
- Joannidis M. Continuous renal replacement therapy in sepsis and multisystem organ failure. Seminars in Dialysis 2009;22(2):160‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Martí‐Carvajal 2011
- Martí‐Carvajal AJ, Solà I, Lathyris D, Cardona AF. Human recombinant activated protein C for severe sepsis. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.CD004388.pub4] [DOI] [PubMed] [Google Scholar]
Reiter 2002
- Reiter K, Bellomo R, Ronco C, Kellum JA. Pro/con clinical debate: is high‐volume hemofiltration beneficial in the treatment of septic shock?. Critical Care 2002;6(1):18‐21. [PUBMED: 11940261 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Review Manager (RevMan): The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ronco 2001
- Ronco C, Ricci Z, Bellomo R. Importance of increased ultrafiltration volume and impact on mortality: sepsis and cytokine story and the role of continuous veno‐venous haemofiltration. Current Opinions in Nephrology and Hypertension 2001;10:755‐61. [PUBMED: 11706302] [DOI] [PubMed] [Google Scholar]
Ronco 2003
- Ronco C, Inguaggiato P, D'Intini V, Cole L, Bellomo R, Poulin S, et al. The role of extracorporeal therapies in sepsis. International Journal of Nephrology 2003;16(Suppl 7):S34‐41. [MEDLINE: ] [PubMed] [Google Scholar]
Surviving Sepsis Campaign 2008
- The Surviving Sepsis Campaign. www.survivingsepsis.org (accessed 5 November 2008).
References to other published versions of this review
Borthwick 2009
- Borthwick EMJ, Blackwood B, Rabindranath KS, Glover P, Cardwell CR, McAuley DF, et al. High‐volume haemofiltration for sepsis. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD008075] [DOI] [Google Scholar]
Borthwick 2013
- Borthwick EMJ, Hill CJ, Rabindranath KS, Maxwell AP, McAuley DF, Blackwood B. High‐volume haemofiltration for sepsis. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD008075.pub2] [DOI] [PubMed] [Google Scholar]


