Abstract
Background
Fungating wounds arise from primary, secondary or recurrent malignant disease and are associated with advanced cancer. A small proportion of patients may achieve healing following surgical excision, but treatment is usually palliative. Fungating wound management usually aims to slow disease progression and optimise quality of life by alleviating physical symptoms, such as copious exudate, malodour, pain and the risk of haemorrhage, through selection of appropriate dressings and topical agents.
Objectives
To review the evidence of the effects of dressings and topical agents on quality of life, and symptoms that impact on quality of life, in people with fungating malignant wounds.
Search methods
For this third update we searched the Wounds Group Specialised Register in August 2013; the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library); Ovid MEDLINE; Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations); Ovid EMBASE and EBSCO CINAHL.
Selection criteria
Eligible studies comprised randomised controlled trials (RCTs) or, in their absence, controlled clinical trials (CCTs) with a concurrent control group.
Data collection and analysis
Data extraction and risk of bias assessment was undertaken by one review author and checked for accuracy by a second.
Main results
Four trials involving 164 people were included. One RCT in women with superficial breast lesions compared 6% miltefosine solution with placebo and found that miltefosine delayed tumour progression. The study reported that the time to treatment failure was significantly longer in the miltefosine group (median 56 days) than in the placebo group (median 21 days) (p value 0.007, log‐rank test). A second trial compared topical metronidazole with placebo but the results up to the point of cross‐over were not statistically significant. A third trial compared the effect of foam dressings containing silver to foam dressings without silver and found that more patients experienced decreased malodour in the foam with silver group than in the foam alone group (p value=0.049). The fourth trial compared the effect of manuka honey‐coated dressings with nanocrystalline silver‐coated dressings and found no statistically significant difference with regard to exudate, malodour and wound pain. All trials, however, had methodological limitations.
Authors' conclusions
There is weak evidence from one small trial that 6% miltefosine solution applied topically to people with superficial fungating breast lesions (smaller than 1cm) who have received either previous radiotherapy, surgery, hormonal therapy or chemotherapy for their breast cancer, may slow disease progression. There is also weak evidence to suggest that foam dressings containing silver may be effective in reducing malodour. There is insufficient evidence in this review to give a clear direction for practice with regard to improving quality of life or managing wound symptoms associated with fungating wounds. More research is needed.
Keywords: Female; Humans; Male; Anti‐Infective Agents, Local; Anti‐Infective Agents, Local/administration & dosage; Antineoplastic Agents; Antineoplastic Agents/administration & dosage; Biological Dressings; Disease Progression; Metronidazole; Metronidazole/administration & dosage; Odorants; Odorants/prevention & control; Ointments; Ointments/administration & dosage; Phosphorylcholine; Phosphorylcholine/administration & dosage; Phosphorylcholine/analogs & derivatives; Randomized Controlled Trials as Topic; Silver Compounds; Silver Compounds/administration & dosage; Skin Neoplasms; Skin Neoplasms/complications; Skin Neoplasms/drug therapy; Skin Ulcer; Skin Ulcer/drug therapy; Wounds and Injuries; Wounds and Injuries/drug therapy
Plain language summary
Topical agents and dressings for fungating wounds (ulcers caused by cancer)
Fungating wounds sometimes occur in people with advanced cancer. Care usually aims to slow down disease progression, and improve quality of life by relieving the physical symptoms caused by the wounds (leakage, bad smell, pain and the risk of haemorrhage) by means of appropriate dressings and other applied treatments. There is weak evidence to suggest that a 6% solution of miltefosine, applied as a fluid to small, superficial fungating wounds on the breast (in people with breast cancer who had previously had either radiotherapy, surgery, hormone therapy or chemotherapy) may slow down the progression of the disease (i.e. extend the time to disease progression). There is also weak evidence to suggest that foam dressings containing silver may be effective in reducing bad smell. There is very little evidence in this area of medicine, however, and what there is is insufficient to give clear directions to practice for improving quality of life or managing wound symptoms in people with fungating wounds. More research is needed in this area.
Background
Fungating wounds arise from primary, secondary or recurrent malignant disease (cancer). These lesions may also be referred to as ulcerating tumours, malignant wounds or neoplasmic lesions. Grocott 1995 defines a fungating wound as "the condition of ulceration and proliferation which arises when malignant tumour cells infiltrate and erode through the skin". The exact prevalence of fungating wounds is currently unknown, since these data are not collected by population‐based cancer registries. However, current estimates for the prevalence of fungating wounds in cancer patients are between 5 to 10% (Alexander 2009a ; Gibson 2013 ; Probst 2013 ; Selby 2009). It should be noted that some people with fungating wounds will never seek support from health services due to embarrassment. Life expectancy in people with fungating wounds is limited and it is estimated that more than half will die within six months of diagnosis of a fungating ulcer (Alexander 2009a). Fungating wounds can occur in a diverse range of locations on the body but in women, the most common fungating ulcers are breast tumours which have metastasised to the breast. In men, the most common occurrence are cutaneous metastases from primary lung cancers but head and neck are also common sites in both genders (Alexander 2009a).
Fungating wounds can present as either raised nodules similar in appearance to a cauliflower (proliferative) or as a crater‐like ulcer (destructive process) or a combination of both (Alexander 2009a). Fungating lesions are thought to interfere with tissue oxygenation, lymphatic drainage and haemostasis (maintenance of the healthy state of tissue). Reduced tissue perfusion, due to abnormalities in the vascularisation of solid tumours, leads to local cell anoxia (lack of oxygen), and sometimes cell death and tissue necrosis (Hirst 1992). Rapid tumour‐cell growth may also affect the pH of extracellular fluid; this interferes with the clotting mechanism that leads to coagulation, and may cause occlusion of blood vessels and subsequent necrosis (Grocott 1995). The lymphatic system may be impaired: interstitial fluid pressures (between parts of the tissue) can lead to vascular collapse causing infarction, hypoxia and necrosis (Bridel‐Nixon 1997). While anaerobic and aerobic bacteria thrive on necrotic tissue and produce malodour and profuse exudate, the fragility of tumour capillaries increases the risk of haemorrhage (Grocott 1995).
Although the challenge of managing a fungating wound depends on the site of the wound, certain symptoms are common to most fungating wounds. Malodour is the most frequently reported physical symptom but other symptoms such as copious exudate, pain and itching, risk of haemorrhage and reduced quality of life are common (Alexander 2009b ; Gibson 2013 ; Lo 2008 ; Probst 2013 ; Selby 2009). The management of fungating malignant wounds is complex and challenging, and the physical, and psychological distress for patients and their carers is well documented (Bale 1991; Fairburn 1994; Gibson 2013 ; Lund‐Nielson 2005 ; Probst 2013). Although a very small proportion of patients may achieve healing after surgical excision or radiotherapy (Alexander 2009a), treatment is usually palliative and aims to achieve the best quality of life for patients and their families. Palliative treatment may include radiotherapy, and systemic or topical chemotherapy to reduce tumour size, exudate and malodour. These palliative treatments may be evaluated by the following means: measuring progressive growth of any treated skin lesion; development of any new lesions within an adequately‐treated area; withdrawal of the patient because of poor tolerability of the study medication; non‐compliance; refusal; or death of the patient. Topical wound management aims to alleviate physical symptoms such as copious exudate, malodour, pain, and the risk of haemorrhage. Its interventions may be evaluated by validated objective tools designed to measure a specific wound symptom or quality of life (for example the Rotterdam Symptom Checklist (de Haes 1996)), withdrawal of the patient because of poor tolerability of the wound management product, non‐compliance or refusal.
Dressings and topical agents may be selected to extend the time to disease progression (e.g. topical cytotoxic chemotherapy), or to cope with exudate (e.g. foams and alginate dressings) and malodour (e.g. metronidazole gel, charcoal‐impregnated dressings, honey). Cutaneous pain management may be attempted with topical analgesia (e.g. topical diamorphine gel). The application of non‐adherent dressings to minimise the trauma of dressing removal may reduce cutaneous pain, and also reduce the risk of haemorrhage through minimising tissue trauma during dressing changes. Although debridement is the usual approach for managing necrotic tissue in other wounds, the friable vessels within fungating wounds significantly increase the risk of haemorrhage, and therefore debridement is used with great caution. Bleeding or haemorrhaging fungating wounds may be treated with coagulant topical agents (e.g. haemostatic sponges) to achieve haemostasis.
Several literature reviews have been published which consider the clinical issues relevant to the management of fungating wounds (Alexander 2009a ; Gibson 2013 ; Grocott 2002; Kelly 2002; Selby 2009) and these reviews identify the need for a systematic study of the problems encountered in the management of fungating wounds with wound dressings. The authors of this review have been unable to identify any previous systematic reviews of topical agents and dressings for the management of fungating wounds.
At present, good practice aims to extend the time to disease progression, or to achieve the best quality of life for patients by alleviating physical symptoms such as copious exudate, malodour, pain and the risk of haemorrhage. Symptom management is primarily through selection of an appropriate dressing and topical agent, although oral antibiotics are sometimes prescribed to reduce the bacterial count of necrotic tissue, and thus reduce malodour.
Objectives
To review the evidence for the effects of dressings and topical agents on quality of life and symptoms that impact on quality of life in people with fungating malignant wounds.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), or, in their absence, controlled clinical trials (CCTs) with a concurrent control group, that assessed the effects of a dressing or topical agent for the treatment of fungating wounds were eligible for inclusion in the review. Eligible trial reports could be published or unpublished, and written in any language. In RCTs with a cross‐over design, data were considered only up to the point of crossover. This was because fungating wounds are not stable, and outcomes following cross‐over are not easy to attribute to a particular treatment. In addition, there is a likelihood of carry‐over with certain interventions. Studies were included if they provided results on primary or secondary outcomes specified in this review.
Types of participants
Studies involving people of any age, male and female, in any care setting, who had been clinically diagnosed with fungating wounds due to any type of carcinoma were included. As the method of diagnosis might have varied between studies, no standardised definition was applied, and the author's decision regarding diagnosis was accepted. Trials that included patients with other conditions too were included only if the outcomes for those with fungating wounds were reported separately.
Types of interventions
Studies evaluating topical agents and dressings, or dressing systems, applied to fungating wounds were included. Topical agents comprised all agents that may be applied topically, including drugs such as antimicrobial agents and topical cytotoxic agents. Dressings included all types of dressings that may be used as a primary contact layer (e.g. foams, alginates, hydrocolloids). Dressing systems consisted of combinations of topical agents, and primary and secondary dressings applied in a clearly‐described, systematic manner.
Types of outcome measures
Primary outcomes
Quality of life as measured by a recognised generic or disease‐specific tool (e.g. SF36)
Secondary outcomes
Fungating tumour containment or regression as measured by time to treatment failure, time to progression or an objective assessment of response using recognised generic or disease‐specific criteria. (This outcome is a post‐hoc addition following publication of the protocol, because at that time the authors were unaware of any topical agent that aimed to influence this outcome. The search strategy for this review identified the existence of topical agents relevant to this outcome, therefore, it was considered appropriate to include this additional outcome as one which is highly relevant to patients).
Malodour as measured by a recognised generic or disease‐specific tool (e.g. TELER system Grocott 2001b).
Cutaneous pain (acute and chronic) as measured by a recognised generic or disease‐specific pain‐assessment tool (e.g. McGill Pain Questionnaire Melzack 1987).
Exudate as measured by number and frequency of dressing changes, or use of other recognised means (e.g. visual analogue scales).
Haemorrhage as measured by number of episodes of fresh bleeding at dressing change.
Cost of dressings and topical agents.
Studies were eligible for inclusion even if they only reported secondary outcomes, since these outcomes are relevant and important to patients and can impact on quality of life.
Search methods for identification of studies
Electronic searches
The search methods section of the second update of this review can be found in Appendix 1
For this third update we searched the following electronic databases, and retrieved 53 citations:
Cochrane Wounds Group Specialised Register (searched 2 August 2013);
The Cochrane Central Register of Controlled Trials (CENTRAL) ‐ The Cochrane Library 2013 Issue 7;
Ovid MEDLINE (2010 to July Week 4 2013);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, August 01, 2013);
Ovid EMBASE (2010 to 2013 Week 30);
EBSCO CINAHL (2010 to 02 July 2013).
The following search strategy was used in The Cochrane Central Register of Controlled Trials (CENTRAL):
#1 MeSH descriptor: [Biological Dressings] explode all trees 63 #2 MeSH descriptor: [Occlusive Dressings] explode all trees 438 #3 MeSH descriptor: [Hydrogels] explode all trees 215 #4 MeSH descriptor: [Alginates] explode all trees 172 #5 MeSH descriptor: [Silicones] explode all trees 729 #6 MeSH descriptor: [Charcoal] explode all trees 211 #7 MeSH descriptor: [Honey] explode all trees 83 #8 MeSH descriptor: [Silver] explode all trees 155 #9 MeSH descriptor: [Silver Sulfadiazine] explode all trees 133 #10 MeSH descriptor: [Ointments] explode all trees 1608 #11 MeSH descriptor: [Metronidazole] explode all trees 1629 #12 MeSH descriptor: [Phosphorylcholine] explode all trees 134 #13 (dressing* or hydrocolloid* or alginate* or hydrogel* or "foam" or "bead" or "film" or "films" or tulle or gauze or non‐adherent or "non adherent" or silicon* or polymer* or charcoal or honey or silver or ointment* or "gel" or "gels" or cream* or lotion* or metronidazole or miltefosine):ti,ab,kw 26348 #14 ((odor or odour) near/3 absorb*):ti,ab,kw 3 #15 MeSH descriptor: [Anti‐Bacterial Agents] explode all trees 8499 #16 MeSH descriptor: [Administration, Topical] explode all trees 11946 #17 MeSH descriptor: [Administration, Cutaneous] explode all trees 2930 #18 #16 or #17 11946 #19 #15 and #18 461 #20 MeSH descriptor: [Analgesics, Opioid] explode all trees 4755 #21 #18 and #20 240 #22 MeSH descriptor: [Antineoplastic Agents] explode all trees 9259 #23 #18 and #22 264 #24 (topical near/3 (antibacterial* or antimicrobial* or antibiotic* or chemotherap*)):ti,ab,kw 486 #25 (topical near/3 (agent* or preparation* or therap* or treatment*)):ti,ab,kw 4830 #26 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #19 or #21 or #23 or #24 or #25 29978 #27 MeSH descriptor: [Skin Ulcer] explode all trees 1681 #28 MeSH descriptor: [Neoplasms] explode all trees 46935 #29 #27 and #28 17 #30 (fungat* near/3 (wound* or tumor* or tumour* or lesion*)):ti,ab,kw 9 #31 (ulcerating next (tumor* or tumour*)):ti,ab,kw 2 #32 malignant next wound*:ti,ab,kw 7 #33 ((cutaneous or skin) near/3 metastas*):ti,ab,kw 61 #34 #29 or #30 or #31 or #32 or #33 91 #35 #26 and #34 17
The search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 2, Appendix 3 and Appendix 4 respectively. The Ovid MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision); Ovid format (Lefebrve 2011). The EMBASE and CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (SIGN 2008). No date or language restrictions were applied.
Searching other resources
We contacted dressing manufacturers (3M Health Care Ltd, Activa Healthcare, Advancis Medical Ltd, Biosurgical Research Unit (Surgical Materials Testing Laboratory), Coloplast Ltd, ConvaTec Ltd, Paul Hartman Ltd, Johnson & Johnson Ltd, Medlogic Global Ltd, Molnlycke Health Care Ltd, Smith & Nephew Healthcare Ltd and Unomedical Ltd) to enquire about ongoing, and recently completed, relevant trials for the original review, this was not repeated for the updates. To date, no new citations have been identified through these contacts for this review. In addition, citations within obtained papers were scrutinised to identify additional studies. There was no restriction on language or date of publication.
Data collection and analysis
Selection of studies
One review author assessed titles and abstracts of the studies identified in terms of their relevance and design, according to the selection criteria. If they satisfied the inclusion criteria, full versions of articles were obtained. Those rejected by one review author were checked by the other author before being excluded. Disagreements about initial exclusions were resolved by discussion, and, if necessary, referred to the Cochrane Wounds Group Editor for adjudication. Two independent review authors assessed full papers against the review selection criteria. Any disagreements were resolved by discussion and, if necessary, referred to the Cochrane Wounds Group Editor for adjudication.
Data extraction and management
Details of the studies were extracted and summarised using a data extraction sheet. If data were missing from reports, we attempted to contact the study authors to obtain the missing information. Should eligible studies with multiple publications become available, data will be included only once. One review author extracted data and a second review author checked them for accuracy.
Types of data extracted included the following;
author;
title;
source of reference;
country of study;
study setting;
number and description of participants;
types of wound;
site of wound;
wound size (area and depth of invasion below skin surface);
previous palliative treatment (chemotherapy, radiotherapy, endocrine therapy);
intervention and comparison;
who delivered treatment;
study design;
outcomes measured (and methods of measuring outcomes);
duration of follow up;
withdrawals (and reasons);
evaluation of cost as a narrative summary.
Assessment of risk of bias in included studies
For this review update two review authors independently assessed each included study using the Cochrane Collaboration tool for assessing risk of bias (Higgins 2011). This tool addresses six specific domains, namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues (e.g. extreme baseline imbalance). We completed a risk of bias table for each eligible study. We discussed any disagreement amongst all review authors to achieve a consensus. We present assessment of risk of bias using a risk of bias summary Fig.1, which presents all of the judgements in a cross‐tabulation of study by entry. This display of internal validity indicates the weight the reader may give the results of each study.
Data synthesis
Each outcome was assessed separately. Because of the small number of heterogenous studies identified pooling was not appropriate and a narrative overview is presented.
Should studies be included in future updates with available data, that data will be entered into and analysed using Cochrane RevMan software. Results will be presented with 95% confidence intervals (CI). Estimates for dichotomous outcomes (e.g. number of wounds healed ‐yes or no) will be reported as risk ratio (RR). Continuous data (e.g. total area healed, or changes in volume of wound area) will be converted to the standardised mean difference (or a weighted mean difference, when plausible) and overall effect size (with 95% CI) will be calculated. Time to wound healing and time to return to work will be analysed as survival (time to event) data, using the appropriate analytical method (as per the Cochrane Reviewers' Handbook version 5). Methods of synthesising the studies will depend on their quality, design and heterogeneity. Both clinical and statistical heterogeneity will be explored. In the absence of clinical and statistical heterogeneity a fixed effect model will be applied to pool data. In the presence of statistical heterogeneity, as estimated by the I2 statistic (Higgins 2003), a random effects model will be applied for meta‐analysis.
Results
Description of studies
For this third update 53 citations were assessed from the new searches. Of these, six studies were retrieved in full text for further assessment and after examination, two studies met the inclusion criteria for the review and four studies were excluded (Ingle 2006; Lo 2012; Lund‐Nielsen 2011; Udwadia 2011 ). Following communication with the trial author it was confirmed that one paper which had been identified in the previous update as an ongoing trial (Lund‐Nielsen 2008) had subsequently been reported as one of the included trials in this update (Lund‐Nielsen 2011a).
Included studies
Four studies have been included in this review (Bower 1992; Leonard 2001; Kalemikerakis 2012; Lund‐Nielsen 2011a). All evaluated different topical preparations. In all trials all the patients were diagnosed as having a fungating wound although only two of the trials reported the criteria by which the diagnoses of cancer had been made (Leonard 2001; Lund‐Nielsen 2011a).
Leonard 2001 study was a multi‐centre, multi‐national (British, Dutch and French), double‐blind placebo‐controlled trial that evaluated a topical form of cytotoxic chemotherapy, miltefosine solution, for containment or regression of fungating tumour (primary outcome), and effect on quality of life (secondary outcome) in 52 patients with superficial fungating breast lesions. The participants in this trial were women 18 years and older (with confirmed breast cancer and superficial or flat skin lesions (where the estimated depth of invasion below skin level was less than, or equal to 1cm). All participants had received at least one prior systemic endocrine or chemotherapy treatment. The two groups of patients were comparable in terms of patient characteristics, except for mean age (68 years in miltefosine group compared with 60 years in placebo group; P < 0.05), and pre‐treatment characteristics (trend for less prior treatment in miltefosine group).
The Bower 1992 study was a double‐blind, randomised, placebo‐controlled trial that evaluated topical application of metronidazole gel for its effect on wound odour (primary outcome) in 11 people living at home with fungating wounds. The participants in this trial were predominantly female (10 female, one male), with a mean age of 68 years (range 51 to 85 years), open fungating primary or metastatic tumours (nine breast, one ovarian, one lung) producing an offensive odour graded at least 6/10 on a visual analogue scale (where 0 represents no odour) by both the patient and medical staff.
The Kalemikerakis 2012 study was a RCT, with 16 female participants (mean age female 73.94 +/‐ 8.58 years) and 10 male participants (mean age male 76.2 +/‐5.85 years). 11 patients had fungating breast wounds, 7 patients had head and neck fungating wounds, and 8 patients had fungating wounds in other parts of the body. All the fungating wounds were malodorous. The trial compared foam dressings containing silver with a foam dressings without silver. Outcome of odour was measured as "No change", or "Reduction of odour", or "Increased odour".
The Lund‐Nielsen 2011a study refers to bandages, yet communication with the study author confirms that the term 'dressing' would be appropriate and this term is used throughout this review. This study was a RCT with 75 participants with advanced stage cancer and malignant wounds. Only 69 completed the intervention. Of these, 61 were female, 8 were male and their combined age ranged from 47.4 to 89.6 years. 55 patients had malignant breast wounds, 8 in the head and neck, and 6 in other parts of the body. The trial compared manuka honey‐coated dressings with silver‐coated dressings. Four outcomes were assessed; malodour measured by a verbal rating scale (Haughton 1995) and by a visual analogue scale, wound pain measured by a visual analogue scale, change of wound size measured using digital photos and "Quantify Image" software (KLONK 2014), and exudate measured by dressing changes.
Excluded studies
Eighteen studies were excluded (Ashford 1984; Bale 2004; Clive 1999; Efendiev 1991; Gostishchev 1983; Gostishchev 1985; Gostishchev 1993; Grocott 2001a; Healy 1969; Moller 2000; Russell 2001; Stuwe 1983; Taranenko 1984; Upright 1994). Three studies were excluded because they were not RCTs or CCTs (Clive 1999; Grocott 2001a; Moller 2000). One trial was excluded because it dealt with cancers of the skin rather than fungating wounds (Healy 1969). Five trials were excluded because they dealt with wounds that were not fungating wounds (Bale 2004; Gostishchev 1983; Gostishchev 1985; Stuwe 1983; Taranenko 1984). A further three trials were excluded because it was unclear whether fungating wounds were included, and the authors could not be contacted to clarify this matter (Efendiev 1991; Gostishchev 1993; Russell 2001). One trial with a cross‐over design was excluded because the authors could not be contacted to obtain data up to the point of cross‐over (Upright 1994). One trial was excluded because the intervention was delivered systemically (Ashford 1984). One trial was excluded because it dealt with wounds that were not fungating (Ingle 2006). Two studies were excluded because they were not RCTs or CCTs (Lo 2012 ; Udwadia 2011). One trial was excluded because it dealt with qualitative bacteriology rather than the outcomes specified in this review (Lund‐Nielsen 2011).
Risk of bias in included studies
Leonard 2001
The Leonard 2001 trial report stated that the sample size calculation was informed by a meta‐analysis of previous studies of 6% miltefosine, and determined by described statistical methods. This calculation allowed for a planned interim statistical analysis after the recruitment of 50% of the original sample, with a view to early discontinuation if a statistically significant difference between the two groups became evident. This occurred, and so recruitment ceased after the recruitment of 52 participants.
The methods of randomisation and allocation concealment were not described, but the trial was reported to be "a double‐blind, placebo‐controlled, multicenter phase III trial". Blinding of outcome assessment was reported. The groups were comparable at baseline except for age and pre‐treatment. The miltefosine group had a mean age of 68 years, compared with 60 years in the placebo group, and this difference was statistically significant (P value < 0.05). The miltefosine group also had a trend for less prior treatment. An intention‐to‐treat analysis was performed for the outcome of containment or regression of fungating tumours, and follow up for this outcome was complete.
With regard to assessment of quality of life (QoL), the study design contained a cross‐over point at four weeks. Although QoL questionnaires were administered at week four, the study only reported QoL data at eight weeks. The authors were approached with a request to supply us with the QoL data from four weeks, but replied that these data are irretrievable. Therefore, there are no valid data available from this trial concerning QoL.
Bower 1992
The Bower 1992 trial report did not describe the method of randomisation and so it was unclear whether sequence generation had been truly random (i.e. by means of random number tables, computer random number generation), however the trial was reported to be a "double‐blind randomised placebo controlled trial". Furthermore, the method of allocation concealment was not described, nor was the baseline comparability of the intervention groups. Although the trial was described as a double‐blind trial, it was unclear whether blinded outcome assessment was used (i.e. assessment by an assessor who is unaware of the intervention group to which participants have been allocated). An intention‐to‐treat analysis was not undertaken. (Some patients who were randomised were not included in the analysis because they did not receive the study intervention, or they withdrew from the study or were not included because of protocol violation). Two withdrawals from the trial were reported along with the reasons for withdrawal. Follow up was complete, in that there were complete data for all participants at the defined study end‐point.
Kalemikerakis 2012
The Kalemikerakis 2012 trial report stated that the patients were randomly assigned to the study groups but the methods of randomisation and allocation concealment were not detailed. Blinding of outcome assessment was not reported. The groups were reported to be similar at baseline in terms of age and ulcer surface area (P value < 0.05). An intention‐to‐treat analysis was not performed.
Lund‐Nielsen 2011a
The Lund‐Nielsen 2011a trial report stated that the method of randomisation was computer‐based and stratified for gender, cancer diagnosis (+/‐ breast cancer), and treatment (+/‐ anti‐neoplastic treatment). The method of allocation concealment was not described. The baseline comparability of the intervention groups was reported but did not achieve statistical significance for homogeneity. Blinding was not reported. An intention‐to‐treat analysis was not performed.
See Figure 1 for additional information.
1.
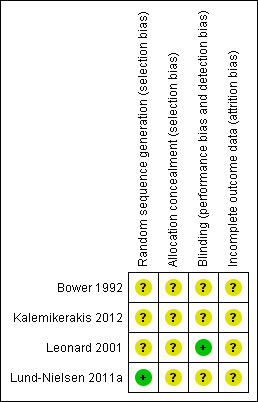
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Effects of interventions
The results are reported with reference to the original outcome measures identified within the review.
The four included studies evaluate different topical preparations. Leonard 2001 evaluated the effect of topical cytotoxic chemotherapy, miltefosine solution, on quality of life (QoL), and containment or regression of fungating tumour. Miltefosine (Miltex; Asta Medica, Frankfurt, Germany) is a therapeutic cytostatic agent that has been demonstrated to have an effect in a range of tumours. It induces major gastrointestinal toxicity when administered systemically, but can be delivered topically when combined with glycerol ethers, which enable absorption through the skin. Delivery of the drug via this route significantly reduces the severity of side effects.
In this trial, 52 patients were randomised to receive either 6% miltefosine solution or placebo solution applied at the dose of two drops/10 cm2 of skin area including the affected skin and a margin of 3cm around each lesion, once daily during the first week and twice daily thereafter until treatment failure. Miltefosine solution (6%) is a clear, colourless and odourless, slightly viscous liquid, provided in 10 ml glass vials with a dropper that allows the delivery of the solution in drops of approximately 0.025 ml (38 to 40 drops/ml). The treatment was continued until progression occurred, unless the patient developed intolerable local skin reactions. If a complete response occurred, patients were asked to apply the treatment for at least a further four weeks. A complete response was defined as the complete disappearance of all lesions for at least 4 weeks without the appearance of any new lesions in the treated area. Patients with progression of the treated lesions within the first four weeks of treatment were withdrawn from the study and could be treated with the open label 6% miltefosine solution according to the investigator's decision. Patients with progression of the treated lesions after more than four weeks of treatment were not allowed to receive open‐label miltefosine solution. Therefore, data from this trial were considered only for the first four weeks of treatment, i.e. to the point of cross‐over.
Bower 1992 evaluated the effect of topical application of metronidazole gel on wound odour. In this study, 11 patients with open fungating primary or metastatic tumours were randomised to receive either 0.8% metronidazole gel 1 g/cm2 lesion or placebo gel applied daily for six days. Doses varied between 3.75 g and 15 g per depending on the size of the lesion but were constant for each patient. None of the patients received anti‐tumour therapy or antibiotics within the preceding four weeks, or for the duration of the trial. The initial trial was followed by an open assessment period of five days when all patients received metronidazole gel. Therefore, this trial was considered up to the point when the open assessment period commenced.
Kalemikerakis 2012 evaluated the effect of foam dressings with silver versus foam dressings without silver in the care of malodorous malignant fungating wounds. The foam dressing with silver was an adhesive or a non‐adhesive foam dressing containing 1mg/cm2 of silver and measuring either 10cm x 10cm or 15cm x 15 cm depending on the ulcer size and peri‐wound skin. The foam dressing without silver was an adhesive or a non‐adhesive foam dressing measuring either 10cm x 10cm or 15cm x 15 cm depending on the ulcer size and peri‐wound skin. The make of dressings used was not reported. 26 patients with malodorous malignant fungating wounds were randomised to receive either foam dressings with silver or foam dressings without silver. Wounds were cleansed using normal saline and a 10% povidone‐iodine solution. Wound dressings were changed 2‐3 times per week and patients were monitored for four weeks.
Lund‐Nielsen 2011a evaluated the effect of honey‐coated dressings compared with silver‐coated dressings on treatment of malignant fungating wounds. 75 patients with advanced stage cancer and malignant fungating wounds were randomly allocated to one of two groups. The first group received manuka honey‐coated dressings (Algivon/Activon Tulle UMF 12+, AdvaNordic Medical Group A/S, Soroe, Denmark) and absorbent dressing (Sorbion/Drymax, Mediq Danmark A/S, Broendby, Denmark) as well as foam dressings (Allevyn Adhesive, Smith & Nephew A/S, Hoersholm, Denmark). The second group received nanocrystalline silver‐coated dressings (Acticoat/Acticoat Absorbent, Smith & NephewA/S) and foam dressings (Allevyn Adhesive, Smith&NephewA/S). Wounds were cleansed using tap water and liquid medicinal soap (ph 4.5). Each home visit took on average 1.5 hours per patient and wounds were redressed every 2 to3 days over a 28 day intervention period.
Primary outcome
Quality of life as measured by a recognised generic or disease specific tool
Leonard 2001 evaluated quality of life (QoL), but since no data were reported, or available, from the cross‐over point at four weeks, no data assessing QoL can be presented for this trial.
Bower 1992 ; Kalemikerakis 2012; Lund‐Nielsen 2011a did not report on quality of life.
Secondary outcomes
Fungating tumour containment or regression as measured by time to treatment failure, time to progression or an objective assessment of response using recognised generic or disease‐specific criteria.
Leonard 2001evaluated the effect of miltefosine on containment or regression of fungating tumours. Skin lesions were assessed at baseline by written description, measured and photographed, and this procedure was repeated weekly for two weeks, fortnightly and then at four‐week intervals.
The time from the start of the treatment to treatment failure was referred to as 'time to treatment failure'. Treatment failure was defined as:
progressive growth of any treated skin lesion according to the WHO definition (WHO 1979);
development of any new lesions within an adequately treated area;
withdrawal of the patient because of poor tolerability of the study medication;
withdrawal because of noncompliance, refusal or death of the patient.
The time from the start of treatment to progression (i.e. local progression within the treated area) was referred to as time to progression.
The WHO criteria were used to provide objective evaluation of the response of individual lesions and the overall response of each patient (WHO 1979). The complete disappearance of all lesions for at least four weeks without the appearance of any new lesions in the treated area was defined as a complete response. A partial response was defined as a decrease by at least 50% of the sum of the products of two perpendicular diameters of lesions without the appearance of any new lesions within the treated area, or the increase of any treated lesion by more than 25% of the sum of the products of two perpendicular diameters. A decrease in size of less than 50%, or an increase of less than 25%, was defined as no change. An increase in size of 25% of any measurable lesions within the treated area (with reference to the smallest size of the lesion recorded during the study period in case of transient regression) was defined as progressive disease. A remission had to be confirmed by a second measurement after four weeks.
The study found that the time to treatment failure was significantly longer in the miltefosine group (median 56 days, range 8 to 324 days) than in the placebo group (median 21 days, range 8 to 197 days) (P value 0.007, log‐rank test). Since no data were reported at four weeks for the outcomes of time to progression and the objective response rate, these could not be included in this review.
Lund‐Nielsen 2011a measured change in wound size. The median decrease in wound size in Group A (honey‐coated dressings) was 15 cm2 compared with 8 cm2 in Group B (silver‐coated dressings). This difference was not statistically significant (p = 0.563). There was no significant reduction in wound size for all patients (p = 0.388) in spite of the fact that 62% of the patients experienced some decrease in wound size.
Malodour as measured by a recognised generic or disease‐specific tool
Bower 1992 evaluated malodour using a visual analogue scale rated 0 to 10 (where 0 = no odour). The odour was graded daily by the patient and by one investigator. In the placebo group (five participants), the mean patient and medical staff odour assessment remained above 6/10 (the minimum severity required for inclusion in the study). In the metronidazole group (four participants) the mean patient odour assessment fell from 7.8 on day 0, to 5.0 on day 6 (P value > 0.1), and the mean medical staff odour assessment fell from 6.5 on day 0, to 4.3 on day 6 (P value > 0.1). There was no statistically significant difference between the two groups.
Kalemikerakis 2012 evaluated odour using three measures, 'increased', 'decreased' and 'remained the same'. The odour was graded weekly for four weeks by health professionals. In the final assessment (week 4), in the group who received foam with silver, a decrease of malodour was reported in 10 patients (76.9%) while in 3 patients (23.1%) malodour stayed the same. Odour did not increase in any patients. In the group who received the foam dressings without silver,decrease of malodour was reported in 4 patients (30.8%) while in 9 (69.2%) malodour stayed the same. Odour did not increase in any patients. The difference in odour reduction between the two groups was statistically significant (p=0.049).
Lund‐Nielsen 2011a evaluated malodour using a verbal rating scale (Haughton 1995), and a visual analogue scale. No statistically significant difference was found between the patients treated with honey‐coated dressings and those treated with silver‐coated dressings.
Cutaneous pain (acute and chronic) as measured by a recognised generic or disease specific pain assessment tool
Leonard 2001 measured pain as an aspect of cutaneous adverse events using a recognised tool designed for this specific purpose, rather than a recognised generic or disease‐specific pain assessment tool. Since the incidence of pain can be measured as a simple yes/no (present/absent), it can be argued that this is a valid measure of the incidence of pain. In this study, one patient in the miltefosine group reported pain. There was no statistically significant difference between the two groups.
Lund‐Nielsen 2011a evaluated wound pain using a visual analogue scale. No statistically significant difference was found between the patients treated with honey‐coated dressings and those treated with silver‐coated dressings .
Exudate as measured by number and frequency of dressing changes
Lund‐Nielsen 2011a evaluated levels of exudate using a visual analogue scale. There was no statistically significant difference in levels of exudate between the patients treated with honey‐coated dressings and those treated with silver‐coated dressings.
Haemorrhage as measured by number of episodes of fresh bleeding at dressing change
None of the four studies evaluated haemorrhage.
Cost of dressings and topical agents
None of the four studies evaluated costs.
Discussion
The results of this review highlight the lack of research in this area. There is relatively little evidence to direct practice for improving quality of life through the use of dressings and topical agents for people with fungating malignant wounds. While the results of one included trial suggest that topical miltefosine solution slows disease progression (Leonard 2001), the poor reporting of the methods of randomisation and allocation concealment, allied with the lack of comparability between the intervention groups at baseline for mean age and pre‐treatment characteristics, mean that we cannot be confident that this is a valid result. In addition, this trial evaluated only small and superficial fungating wounds of the breast in patients in reasonable general health, which limits the generalisability of the findings.
Evidence that might guide clinical practice in managing the symptoms associated with fungating wounds was restricted to three studies. One small study suggested that metronidazole gel might be useful in reducing malodour (Bower 1992). A second study suggested that foam dressings containing silver may also be effective in reducing malodour (Kalemikerakis 2012). The third study found no evidence of difference between honey‐coated dressings and silver‐coated dressings. Issues such as the poor reporting of exclusion criteria, randomisation methods, allocation concealment, baseline comparability, blinded outcome assessment, the lack of an intention‐to‐treat analysis and use of small sample sizes means there is uncertainty about the validity of these results. A replication of these studies using larger sample sizes, improved methodology and better reporting to investigate the effects of these interventions for improving quality of life, and reducing malodour and exudate levels, might be useful. No evidence was found concerning management of any other symptoms of fungating wounds.
Overall, we found that the evidence base for guiding selection of dressing(s) and topical agent(s) for management of fungating wounds is negligible. We did not identify any previous systematic review of this subject, but found several literature reviews that included similar patient outcomes (malodour, cutaneous pain, tumour progression and exudate management) (Grocott 2002; Alexander 2009b; Gibson 2013) as well as several qualitative research studies into the experience of having a malignant fungating wound (Lo 2008 ; Lund‐Nielson 2005 ; Probst 2013). This research confirms the relevance of the outcomes of this review to patients with fungating malignant wounds and it is encouraging to see the emergence of more research in this field. However, there has been little advance in terms of robust research evidence to answer questions of clinical effectiveness in the management of fungating wounds.
The outcomes to be measured in RCTs need careful consideration. Traditionally, trials in wound care have focused on wound healing, which is an inappropriate outcome for patients with fungating wounds. Since treatment for fungating wounds is mainly palliative, the primary aim of future research should be to improve quality of life. In clinical terms, there is a close link between clinically effective alleviation of distressing symptoms and a patient's quality of life. In research terms, however, quality of life has a very specific meaning and is measured by specific tools. Evaluating quality of life as a primary outcome, or designing a study to be sufficiently powered to evaluate it adequately as a secondary outcome, is of particular significance for this patient group.
Other outcomes that are highly relevant for patients with fungating wounds are the palliation of distressing symptoms, such as copious exudate, malodour and pain and these are outcomes that can be measured objectively using an achievable sample size, and thus should be evaluated within an RCT.The outcomes of research into care for patients with fungating wounds need to be more specific and achievable, with a focus on obtaining objective measurements of symptom control in the clinical situation. Subjective approaches to measurement, such as seeking the opinion of the patient or clinician, or based on in vitro, laboratory‐based studies, rather than measuring how products function on real people are fundamentally flawed. Systematic, objective approaches that enable the use of a common language to evaluate symptom management, dressing use, dressing performance and patient experiences are being developed (Grocott 2001a), and should be more widely utilised.
The emergence of two new RCTs which can be included in this review is encouraging but it must be acknowledged that it is difficult to carry out RCTs for fungating wounds. RCTs may be possible for some outcomes, but there may be other outcomes relevant to management of fungating wounds which are very difficult to measure. For these outcomes, less robust study designs, such as multiple case study designs (Grocott 2001b), may be the highest level of evidence available. There may also be difficulties in conducting RCTs particularly because of the ethical challenges of recruiting patients who are approaching the end‐stage of their lives. Although all ethical trials should be well designed and reported, it is particularly important that the design of trials for this group of patients should be sufficiently elegant to be capable of providing relevant and reliable information, while minimising the burden on the patients recruited into them. For example, RCTs that measure pain or malodour are unlikely to need long follow‐up periods or large sample sizes, since any clinically‐meaningful changes are likely to have a large effect size, and be achieved relatively swiftly. We would argue that to deprive this group of patients of robust clinical information derived from high quality RCTs is ethically questionable, since this information is needed to direct the selection of clinically‐effective interventions that will benefit patients.
Authors' conclusions
Implications for practice.
There is weak evidence to suggest that patients with superficial fungating breast lesions (smaller than 1cm in depth) who have received either previous radiotherapy, surgery, hormonal therapy or chemotherapy for their breast cancer may extend the time to disease progression by receiving topical 6% miltefosine solution (Leonard 2001). There is also weak evidence to suggest that foam dressings containing silver may be effective in reducing malodour (Kalemikerakis 2012). There is insufficient evidence in this review to give a clear direction for practice with regard to improving quality of life or managing other wound symptoms associated with fungating wounds.
Implications for research.
The four studies summarised in this review were small and had methodological problems; they would benefit from being replicated in larger, well‐designed studies. The four studies addressed only some of the outcomes relevant to patients with fungating wounds. Since treatment for fungating wounds is mainly palliative, the primary aim of future research should be to concentrate on improving quality of life through improvements in symptom management.
Future research and research reports should aim to include the following:
evaluation of outcomes relevant to patients with fungating wounds as identified in case series, cohort data and qualitative studies of patients with fungating wounds;
clearly defined and reported inclusion and exclusion criteria for participants;
sample size with sufficient power to detect clinically‐important treatment effects;
clear reporting of a priori power calculations, use of true randomisation with allocation concealment (e.g. remote randomisation);
clear reporting of the methods of randomisation and measures to help ensure comparability of treatment groups at baseline (e.g. stratification by lesion size);
detailed reporting of baseline characteristics (including underlying factors which may affect outcomes);
blinded outcome assessment;
use of objective outcome measurement (e.g. use of recognised generic or disease‐specific tools);
use of intention‐to‐treat analysis;
detailed reporting of numbers and characteristics of dropouts by treatment group;
detailed reporting of co‐interventions such as oral antibiotics or anti tumour therapies.
What's new
| Date | Event | Description |
|---|---|---|
| 2 August 2013 | New citation required but conclusions have not changed | Conclusions remain unchanged |
| 2 August 2013 | New search has been performed | Third update, new searches. Two new studies included (Kalemikerakis 2012; Lund‐Nielsen 2011a). Four additional studies excluded (Ingle 2006; Lo 2012; Lund‐Nielsen 2008; Lund‐Nielsen 2011). |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 2, 2007
| Date | Event | Description |
|---|---|---|
| 26 February 2011 | New search has been performed | New searches, no new studies included, two studies added to the Table of Excluded studies (Lo 2010; Lo 2010a). The review's conclusions remain unchanged. |
| 9 November 2010 | Amended | Contact details updated. |
| 16 March 2010 | Amended | Contact details updated. |
| 13 May 2009 | Amended | Contact details updated. |
| 21 January 2009 | New search has been performed | First update of review no new studies identified, conclusions remain unchanged. |
| 5 August 2008 | Amended | Converted to new review format. |
| 26 November 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors would like to thank the Cochrane Wounds Group referees (Belinda Archer, Jacq Dinnes, Kate Flemming, Stephanie Kondos and Catriona McDaid), and Editors (Michelle Briggs, Gill Cranny, Nicky Cullum and Andrea Nelson) for their comments for improving the review. The authors would like to acknowledge the comments of the copy editor for the updated review, Elizabeth Royle. Ruth Smith participated in the original review but has not participated for the updates of this review, the current authors would like to acknowledge her contribution.
Appendices
Appendix 1. Search strategy ‐ Second update 2011
Electronic searches
For this second update we searched the following electronic databases:
Cochrane Wounds Group Specialised Register (searched 28 October 2010);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010 Issue 4);
Ovid MEDLINE (2005 to October Week 3 2010);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, October 27, 2010);
Ovid EMBASE (2008 to 2010 Week 42);
EBSCO CINAHL (1982 to 22 October 2010)
The following search strategy was used in The Cochrane Central Register of Controlled Trials (CENTRAL):
#1 MeSH descriptor Biological Dressings explode all trees #2 MeSH descriptor Occlusive Dressings explode all trees #3 MeSH descriptor Hydrogels explode all trees #4 MeSH descriptor Alginates explode all trees #5 MeSH descriptor Silicones explode all trees #6 MeSH descriptor Charcoal explode all trees #7 MeSH descriptor Honey explode all trees #8 MeSH descriptor Silver explode all trees #9 MeSH descriptor Silver Sulfadiazine explode all trees #10 MeSH descriptor Ointments explode all trees #11 MeSH descriptor Metronidazole explode all trees #12 MeSH descriptor Phosphorylcholine explode all trees #13 (dressing* or hydrocolloid* or alginate* or hydrogel* or "foam" or "bead" or "film" or "films" or tulle or gauze or non‐adherent or "non adherent" or silicon* or polymer* or charcoal or honey or silver or ointment* or “gel” or “gels” or cream* or lotion* or metronidazole or miltefosine):ti,ab,kw #14 ((odor or odour) NEAR/3 absorb*):ti,ab,kw #15 MeSH descriptor Anti‐Bacterial Agents explode all trees #16 MeSH descriptor Administration, Topical explode all trees #17 MeSH descriptor Administration, Cutaneous explode all trees #18 (#16 OR #17) #19 (#15 AND #18) #20 MeSH descriptor Analgesics, Opioid explode all trees #21 (#18 AND #20) #22 MeSH descriptor Antineoplastic Agents explode all trees #23 (#18 AND #22) #24 (topical NEAR/3 (antibacterial* or antimicrobial* or antibiotic* or chemotherap*)):ti,ab,kw #25 (topical NEAR/3 (agent* or preparation* or therap* or treatment*)):ti,ab,kw #26 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #19 OR #21 OR #23 OR #24 OR #25) #27 MeSH descriptor Skin Ulcer explode all trees #28 MeSH descriptor Neoplasms explode all trees #29 (#27 AND #28) #30 (fungat* NEAR/3 (wound* or tumor* or tumour* or lesion*)):ti,ab,kw #31 (ulcerating NEXT (tumor* or tumour*)):ti,ab,kw #32 malignant NEXT wound*:ti,ab,kw #33 ((cutaneous or skin) NEAR/3 metastas*):ti,ab,kw #34 (#29 OR #30 OR #31 OR #32 OR #33) #35 (#26 AND #34)
The search strategies for Ovid MEDLINE, Ovid EMBASE and Ovid CINAHL can be found in Appendix 2, Appendix 3 and Appendix 4respectively. The Ovid MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision); Ovid format (Lefebrve 2009). The EMBASE and CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (SIGN 2008). No date or language restrictions were applied.
Searching other resources
We contacted dressing manufacturers (3M Health Care Ltd, Activa Healthcare, Advancis Medical Ltd, Biosurgical Research Unit (Surgical Materials Testing Laboratory), Coloplast Ltd, ConvaTec Ltd, Paul Hartman Ltd, Johnson & Johnson Ltd, Medlogic Global Ltd, Molnlycke Health Care Ltd, Smith & Nephew Healthcare Ltd and Unomedical Ltd) to enquire about ongoing, and recently completed, relevant trials for the original review, this was not repeated for the updates. To date, no new citations have been identified through these contacts for this review. In addition, citations within obtained papers were scrutinised to identify additional studies. There was no restriction on language or date of publication.
Appendix 2. Ovid MEDLINE search strategy
1 exp Biological Dressings/ 2 exp Occlusive Dressings/ 3 exp Hydrogels/ 4 exp Alginates/ 5 exp Silicones/ 6 exp Charcoal/ 7 exp Honey/ 8 exp Silver/ 9 exp Silver Sulfadiazine/ 10 exp Ointments/ 11 exp Metronidazole/ 12 exp Phosphorylcholine/ 13 (dressing$ or hydrocolloid$ or alginate$ or hydrogel$ or foam or bead or film$1or tulle or gauze or non‐adherent or non adherent or silicon$ or polymer$ or charcoal or honey or silver or ointment$ or gel$1 or cream$ or lotion$ or metronidazole or miltefosine).ti,ab. (531271) 14 ((odor or odour) adj3 absorb$).ti,ab. 15 exp Anti‐Bacterial Agents/ 16 exp Administration, Topical/ 17 exp Administration, Cutaneous/ 18 or/16‐17 19 15 and 18 20 exp Analgesics, Opioid/ 21 18 and 20 22 exp Antineoplastic Agents/ 23 18 and 22 24 (topical adj3 (antibacterial$ or antimicrobial$ or antibiotic$ or chemotherap$)).ti,ab. 25 (topical adj3 (agent* or preparation* or therap* or treatment*)).ti,ab. 26 or/1‐14,19,21,23‐25 27 exp Skin Ulcer/ 28 exp Neoplasms/ 29 and/27‐28 30 (fungat$ adj3 (wound$ or tumor$ or tumour$ or lesion$)).mp. 31 (ulcerating adj (tumor$ or tumour$)).mp. 32 malignant wound$.mp. 33 ((cutaneous or skin) adj3 metastas$).mp. 34 or/29‐33 35 26 and 34
Appendix 3. Ovid EMBASE search strategy
1 exp Wound Dressing/ 2 exp Hydrogel Dressing/ 3 exp Hydrogels/ 4 exp Alginic Acid/ 5 exp Silicone/ 6 exp Charcoal/ 7 exp Honey/ 8 exp Silver/ 9 exp Sulfadiazine Silver/ 10 exp Ointment/ 11 exp Metronidazole/ 12 exp Phosphorylcholine/ 13 (dressing$ or hydrocolloid$ or alginate$ or hydrogel$ or foam or bead or film$1or tulle or gauze or non‐adherent or non adherent or silicon$ or polymer$ or charcoal or honey or silver or ointment$ or gel$1 or cream$ or lotion$ or metronidazole or miltefosine).ti,ab. (440090) 14 ((odor or odour) adj3 absorb$).ti,ab. 15 exp Antiinfective Agent/ 16 exp Topical Drug Administration/ 17 15 and 16 18 exp Narcotic Analgesic Agent/ 19 16 and 18 20 exp Cytotoxic Agent/ 21 16 and 20 22 (topical adj3 (antibacterial$ or antimicrobial$ or antibiotic$ or chemotherap$)).ti,ab. 23 (topical adj3 (agent* or preparation* or therap* or treatment*)).ti,ab. 24 or/1‐14,17,19,21‐23 25 exp Skin Ulcer/ 26 exp Neoplasm/ 27 25 and 26 28 exp Skin Metastasis/ 29 (fungat$ adj3 (wound$ or tumor$ or tumour$ or lesion$)).mp. 30 (ulcerating adj (tumor$ or tumour$)).mp. 31 malignant wound$.mp. 32 ((cutaneous or skin) adj3 metastas$).mp. 33 or/27‐32 34 24 and 33
Appendix 4. EBSCO CINAHL search strategy
S31 S30 and S24 S30 S29 or S28 or S27 or S26 or S25 S29 skin N3 metasta* or cutaneous N3 metasta* S28 malignant wound* S27 ulcerating tumor* or ulcerating tumour* S26 fungat* N3 wound* or fungat* N3 tumour* or fungat* N3 tumor* or fungat* N3 lesion* S25 (MH "Fungating Wounds") S24 S23 or S22 or S21 or S19 or S17 or S14 or S13 or S12 or S11 or S10 or S9 or S8 or S7 or S6 or S5 or S4 or S3 or S2 or S1 S23 topical N3 agent* or topical N3 preparation* or topical N3 therap* and topical N3 treatment* S22 topical N3 antibacterial* or topical N3 antimicrobial* or topical N3 antibiotic* or topical N3 chemotherap* S21 S20 and S16 S20 (MH "Antineoplastic Agents+") S19 S18 and S16 S18 (MH "Analgesics, Opioid+") S17 S16 and S15 S16 (MH "Administration, Topical+") S15 (MH "Antiinfective Agents+") S14 TI odor N3 absorb* or AB odor N3 absorb* S13 TI odour N3 absorb* or AB odour N3 absorb* S12 AB dressing* or hydrocolloid* or alginate* or hydrogel* or foam or bead or film* or tulle or gauze or non‐adherent or non adherent or silicon* or polymer* or charcoal or honey or silver or ointment* or gel or gels or cream* or lotion* or metronidazole or phosphorylcholine or miltefosine S11 TI dressing* or hydrocolloid* or alginate* or hydrogel* or foam or bead or film* or tulle or gauze or non‐adherent or non adherent or silicon* or polymer* or charcoal or honey or silver or ointment* or gel or gels or cream* or lotion* or metronidazole or phosphorylcholine or miltefosine S10 (MH "Metronidazole") S9 (MH "Ointments") S8 (MH "Silver") or (MH "Silver Sulfadiazine") S7 (MH "Honey") S6 (MH "Charcoal") S5 (MH "Silicones") S4 (MH "Alginates") S3 (MH "Hydrogel Dressings") S2 (MH "Occlusive Dressings") S1 (MH "Biological Dressings")
Appendix 5. Search strategy ‐ Second update 2011
For this second update we searched the following electronic databases:
Cochrane Wounds Group Specialised Register (searched 28 October 2010);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010 Issue 4);
Ovid MEDLINE (2005 to October Week 3 2010);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, October 27, 2010);
Ovid EMBASE (2008 to 2010 Week 42);
EBSCO CINAHL (1982 to 22 October 2010)
The following search strategy was used in The Cochrane Central Register of Controlled Trials (CENTRAL):
#1 MeSH descriptor Biological Dressings explode all trees #2 MeSH descriptor Occlusive Dressings explode all trees #3 MeSH descriptor Hydrogels explode all trees #4 MeSH descriptor Alginates explode all trees #5 MeSH descriptor Silicones explode all trees #6 MeSH descriptor Charcoal explode all trees #7 MeSH descriptor Honey explode all trees #8 MeSH descriptor Silver explode all trees #9 MeSH descriptor Silver Sulfadiazine explode all trees #10 MeSH descriptor Ointments explode all trees #11 MeSH descriptor Metronidazole explode all trees #12 MeSH descriptor Phosphorylcholine explode all trees #13 (dressing* or hydrocolloid* or alginate* or hydrogel* or "foam" or "bead" or "film" or "films" or tulle or gauze or non‐adherent or "non adherent" or silicon* or polymer* or charcoal or honey or silver or ointment* or “gel” or “gels” or cream* or lotion* or metronidazole or miltefosine):ti,ab,kw #14 ((odor or odour) NEAR/3 absorb*):ti,ab,kw #15 MeSH descriptor Anti‐Bacterial Agents explode all trees #16 MeSH descriptor Administration, Topical explode all trees #17 MeSH descriptor Administration, Cutaneous explode all trees #18 (#16 OR #17) #19 (#15 AND #18) #20 MeSH descriptor Analgesics, Opioid explode all trees #21 (#18 AND #20) #22 MeSH descriptor Antineoplastic Agents explode all trees #23 (#18 AND #22) #24 (topical NEAR/3 (antibacterial* or antimicrobial* or antibiotic* or chemotherap*)):ti,ab,kw #25 (topical NEAR/3 (agent* or preparation* or therap* or treatment*)):ti,ab,kw #26 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #19 OR #21 OR #23 OR #24 OR #25) #27 MeSH descriptor Skin Ulcer explode all trees #28 MeSH descriptor Neoplasms explode all trees #29 (#27 AND #28) #30 (fungat* NEAR/3 (wound* or tumor* or tumour* or lesion*)):ti,ab,kw #31 (ulcerating NEXT (tumor* or tumour*)):ti,ab,kw #32 malignant NEXT wound*:ti,ab,kw #33 ((cutaneous or skin) NEAR/3 metastas*):ti,ab,kw #34 (#29 OR #30 OR #31 OR #32 OR #33) #35 (#26 AND #34)
The search strategies for Ovid MEDLINE, Ovid EMBASE and Ovid CINAHL can be found in Appendix 2, Appendix 3 and Appendix 4 respectively. The Ovid MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision); Ovid format (Lefebrve 2011). The EMBASE and CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (SIGN 2008). No date or language restrictions were applied.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bower 1992.
| Methods | Double‐blind, placebo controlled trial. | |
| Participants | 10 female, 1 male, mean age 68 years old with open fungating primary or metastatic tumours (9 breast, 1 ovarian, 1 lung) that produced an offensive odour graded at least 6/10 on a visual analogue scale (where 0 = no odour). | |
| Interventions | 0.8% metronidazole gel 1 g/cm2 lesion applied daily. | |
| Outcomes | Reduction of odour. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The report states that this was a 'randomised placebo controlled trial' but the method of generating the randomisation sequence was not described. |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) outcome assessment | Unclear risk | The report states that this was a double‐blind... trial' but who was blinded was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The report states that two patients withdrew from the study, one from each treatment group. It was not stated whether an ITT analysis was conducted. |
Kalemikerakis 2012.
| Methods | Randomised controlled trial | |
| Participants | 16 female, 10 male, mean age female 73.94 +/‐ 8.58 years; mean age male 76.2 +/‐5.85 years. 11 patients have malodorous fungating breast wounds, 7 in head and neck, and 8 in other parts of the body. |
|
| Interventions | Foam dressing containing silver compared to foam dressing without silver. | |
| Outcomes | Odour measured as 'no change', or 'Reduction of odour', or 'Increased odour' | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The report states that this is a 'randomised controlled trial' but the method of generating the randomisation sequence was not described. |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) outcome assessment | Unclear risk | The report states that this was a double‐blind... trial' but who was blinded was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The report states that 26 patients were randomised and report on 26 patients. |
Leonard 2001.
| Methods | Double‐blind, placebo controlled, multi‐centred trial. | |
| Participants | 52 female patients, > 18 years old. Inclusion criteria: confirmed breast cancer with superficial, or flat, skin lesions where the estimated depth of invasion below skin level was ≤ 1cm. All participants had received at least 1 prior systemic endocrine or chemotherapy treatment. Participants required adequate bone marrow status, and renal and hepatic function, to ensure that those at risk of requiring systemic therapeutic interventions ‐ due to concomitant or incipient visceral disease ‐ were excluded. Exclusion criteria: skin lesions manageable with surgery, radiotherapy, systemic endocrine therapy or chemotherapy; progressive systemic metastases, or other malignancies within the last five years (except treated and cured cervical carcinoma in situ, non melanoma skin cancer or cutaneous lymphoepithelioma); recent major surgery; concomitant treatment with other investigational drugs; uncontrolled clinically‐significant non‐cancer medical illness; and those who were lactating, pregnant, or had childbearing potential and were not taking adequate contraceptive precautions. | |
| Interventions | 6% miltefosine solution 2 drops/10 cm2 of skin area including the affected skin and a margin of 3cm around each lesion, once daily during first week and twice daily thereafter. | |
| Outcomes | Quality of life. Tumour containment/remission. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The report states that this is a 'placebo controlled ...trial' but the method of generating the randomisation sequence was not described. |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) outcome assessment | Low risk | Participants, clinicians and outcome assessors were blinded. ("The placebo was plyethene glycol solution, having a similar appearance and viscosity" and "All responses were assessed by an independent reviewer before the randomization codes were unblinded.") |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The report states that an ITT analysis was conducted. There were 9 withdrawals. |
Lund‐Nielsen 2011a.
| Methods | Randomised controlled trial | |
| Participants | 75 participants with advanced stage cancer and malignant wounds were recruited but only 69 completed the intervention. 61 were female, 8 were male. Combined age range 47.4 to 89.6 years. 55 patients have malignant breast wounds, 8 in head and neck, and 6 in other parts of the body. | |
| Interventions | Manuka honey‐coated bandages and silver‐coated bandages. | |
| Outcomes | Malodour. Wound pain. Change of wound size. | |
| Notes | The honey‐coated and silver‐coated bandages cited by the authors would appear to be dressings rather than bandages. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The report states that the randomisation process was "computer‐based and was stratified for gender, cancer diagnosis, (+/‐ breast cancer) and treatment (+/‐ antineoplastic treatment)". |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) outcome assessment | Unclear risk | not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The report states that 75 participants were randomised but only 69 completed the intervention. An ITT analysis was not conducted. |
Abbreviations
> = more than ≤ = less than or equal to y = year(s)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ashford 1984 | Intervention was systemic, not topical. |
| Bale 2004 | No participants had fungating wounds. |
| Clive 1999 | Not a RCT, CCT or cross‐over trial. |
| Efendiev 1991 | Translated abstract did not make clear whether any of these patients had fungating wounds. The abstract stated, however, that the intervention "intensifies reparative processes" which suggests that healing takes place, which would not occur with fungating wounds. |
| Gostishchev 1983 | Participants included patients with post‐injectional and paraossal phlegmons, postoperative suppurations and trophic ulcers, but did not list fungating wounds. |
| Gostishchev 1985 | No participants had fungating wounds. |
| Gostishchev 1993 | The translated abstract did not state whether any participants had fungating wounds. The abstract referred to granulation and epithelialisation of the wounds following treatment, which would be unlikely to occur with fungating wounds. |
| Grocott 2001a | Multiple case study design, i.e. not an RCT, CCT or cross‐over trial. |
| Healy 1969 | Participants had keratoses or early stage skin cancer, not fungating wounds. |
| Ingle 2006 | Participants had acute wounds, not fungating wounds. |
| Lo 2010 | Conference abstract only, insufficient information to make a decision on inclusion, study authors contacted but no reply received. |
| Lo 2010a | Conference abstract only, insufficient information to make a decision on inclusion, study authors contacted but no reply received. |
| Lo 2012 | Cross‐sectional survey, i.e. not an RCT, CCT or cross‐over trial. |
| Lund‐Nielsen 2008 | Communication with the author has confirmed that the results of this report have been subsequently published as three different papers. All these papers have been considered for this review but only one (Lund‐Nielsen 2011a) meets the inclusion criteria for this review. |
| Lund‐Nielsen 2011 | Outcomes not relevant to outcomes stated for this review. |
| Moller 2000 | An in vitro study i.e. not an RCT, CCT or cross‐over trial. |
| Russell 2001 | Participants had established necrotic/sloughy wounds. There was no mention of participants with fungating wounds, and, since the intervention (Vacutex) would be unsuitable for use with fungating wounds, due to the increased risk of haemorrhage, it is unlikely that there were any. |
| Stuwe 1983 | Participants had skin cancers, not fungating wounds. |
| Taranenko 1984 | No participants had fungating wounds. |
| Udwadia 2011 | Case series, i.e. not an RCT, CCT or cross‐over trial. |
| Upright 1994 | Cross‐over trial: we wrote to author requesting data up to the point of cross‐over but received no response. |
Differences between protocol and review
The outcome measure of fungating tumour containment or regression was a post‐hoc inclusion, this outcome was judged to be relevant and of interest to both patients and clinicians. The outcome measure of exudate was amended to include visual analogue scales.
Contributions of authors
Una Adderley and Ruth Smith jointly developed the protocol and addressed all peer referee comments. Ruth Smith commented on the first review and checked the data extraction. Una Adderley undertook the review and all subsequent updates. Ian Holt undertook this latest review update with Una Adderley.
Contributions of editorial base
Sally Bell‐Syer co‐ordinated the editorial process, advised on methodology, interpretation and content; edited and copy‐edited the review, and also the updated review. Ruth Foxlee designed the search strategy, ran the searches and edited the search methods section for the update.
Sources of support
Internal sources
Department of Health Sciences, University of York, York, UK.
External sources
The National Institue for health Research (NIHR) is the sole funder of the Cochrane Wounds Review Group, UK.
Declarations of interest
None.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bower 1992 {published data only}
- Bower M, Stein R, Evans TR, Hedley A, Pert P, Coombes RC. A double‐blind study of the efficacy of metronidazole gel in the treatment of malodorous fungating tumours. European Journal of Cancer 1992;28A(4‐5):888‐9. [DOI] [PubMed] [Google Scholar]
Kalemikerakis 2012 {published data only}
- Kalemikerakis J. Comparison of foam dressings with silver versus foam dressings without silver in the care of malodorous malignant fungating wounds. Journal of B.U.On 2012;17(3):560‐4. [PubMed] [Google Scholar]
Leonard 2001 {published data only}
- Leonard R, Hardy J, Tienhoven G, Houston S, Simmonds P, David M, et al. Randomized, double‐blind, placebo‐controlled, multicenter trial of 6% miltefosine solution, a topical chemotherapy in cutaneous metastases from breast cancer. Journal of Clinical Oncology 2001;19(21):4150‐9. [DOI] [PubMed] [Google Scholar]
Lund‐Nielsen 2011a {published data only}
- Lund‐Nielsen B, Adamsen L, Kolmos HJ, Rorth M, Tolver A, Gottrup F. The effect of honey‐coated bandages compared with silver‐coated bandages on treatment of malignant wounds ‐ a randomized study. Wound Repair and Regeneration 2011;19(6):664‐70. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ashford 1984 {published data only}
- Ashford R, Plant G, Maher J, Teare L. Double blind trial of metronidazole in malodorous ulcerating tumours. Lancet 1984;June 2:1232‐3. [DOI] [PubMed] [Google Scholar]
Bale 2004 {published and unpublished data}
- Bale S, Tebbie N, Price P. A topical metronidazole gel used to treat malodorous wounds. British Journal of Nursing 2004;13(11):4S‐11S. [DOI] [PubMed] [Google Scholar]
Clive 1999 {published data only}
- Clive S, Gardiner J, Leonard RC. Miltefosine as a topical treatment for cutaneous metastases in breast carcinoma. Cancer Chemotherapy Pharmacology 1999;44(Suppl):29S‐30S. [DOI] [PubMed] [Google Scholar]
Efendiev 1991 {published data only}
- Efendiev AI, Tolstykh PI, Dadashev AI, Marshava AM. Topical prolonged enzyme therapy of purulent wounds. Khirurgiia (Mosk) 1991;Jul(7):48‐50. [PubMed] [Google Scholar]
Gostishchev 1983 {published data only}
- Gostishchev VK, Vasilkova ZF, Khanin AG, Vavilova GS, Lebedskoj AG. Debrisan in the treatment of purulent wounds. Vestnik Chirurgii Imemi Grekova 1983;131(9):56‐9. [PubMed] [Google Scholar]
Gostishchev 1985 {published data only}
- Gostishchev VK, Tolstykh PI, Khanin AG, Vasilkova ZF, Yusupuv KA, Vlasov, LT, et al. The use of Trypsin immobilised on textile cellulose matrix in the treatment of purulent wounds of soft tissues. Vestnik Chirurgii Imemi Grekova 1985;134(6):68‐71. [PubMed] [Google Scholar]
Gostishchev 1993 {published data only}
- Gotstishchev VK, Mulyaev LF, Nikolaev AV, Khanin AG, Kassin VY. Regenkur, a polymer sorbent, in the treatment of purulent wounds. Khirurgiya 1993;69(11):3‐6. [PubMed] [Google Scholar]
Grocott 2001a {published data only}
- Grocott P, Cowley S. The palliative management of fungating malignant wounds ‐ generalising from multiple‐case study data using a system of reasoning. International Journal of Nursing Studies 2001;38(5):533‐45. [DOI] [PubMed] [Google Scholar]
Healy 1969 {published data only}
- Healy JB. The use of topical 5‐fluorouracil. Journal of the Irish Medical Association 1969;62(380):41‐6. [PubMed] [Google Scholar]
Ingle 2006 {published data only}
- Ingle R, Levin J, Polinder K. Wound healing with honey ‐ a randomised controlled trial. South African Medical Journal 2006;96(9):831‐5. [PubMed] [Google Scholar]
Lo 2010 {published data only}
- Lo SF. A cost‐effectiveness analysis of a silver dressing for malignant fungating wound of cancer patients. EWMA Journal 2010;10:P116. [Google Scholar]
Lo 2010a {published data only}
- Lo SF, Huang CU. The effect wound bed status of a ionic silver dressings in cancer patients with malignant fungating wound. EWMA Journal. 2010; Vol. 10:P28.
Lo 2012 {published data only}
- Lo S‐F, Hayter M, Hu W‐Y, Tai C‐Y, Hsu M‐Y, Li Y‐F. Symptom burden and quality of life in patients with malignant fungating wounds. Journal of Advanced Nursing 2012;68(6):1312‐21. [DOI] [PubMed] [Google Scholar]
Lund‐Nielsen 2008 {published data only}
- Lund‐Nielsen B. Malignant wounds. A randomised clinical trial investigating a complimentary multidimensional intervention. EWMA Conference. 2008; Vol. 8, issue 2 (Supp):49, Abstract no. 60.
Lund‐Nielsen 2011 {published data only}
- Lund‐Nielsen B, Adamsen L, Gottrup F, Rorth M, Tolver A, Kolmos HJ. Qualitative bacteriology in malignant wounds ‐ A prospective randomized clinical study to compare the effect of honey and silver dressings. Ostomy/Wound Management 2011;57(7):28‐36. [PubMed] [Google Scholar]
Moller 2000 {published data only}
- Moller F, Jakobsen V, Jensen K. Performance of Contreet antibacterial hydrocolloid dressing compared to other antibacterial dressings. Tenth Annual Meeting of the European Tissue Repair Society; 2000, 24‐27 May; Brussels, Belgium. Brussels, 2000:A427.
Russell 2001 {published data only}
- Russell L, Deeth M, Jones HM, Reynolds T. Vacutex capillary action dressing: a multicentre, randomized trial. British Journal of Nursing 2001;10(11):66S‐70S. [DOI] [PubMed] [Google Scholar]
Stuwe 1983 {published data only}
- Stuwe VU. Enzymatische wundreinigung. Fortschritte des Medizin 1983;101(41):1883‐8. [PubMed] [Google Scholar]
Taranenko 1984 {published data only}
- Taranenko LD, Bondarev VI, Nefedov GP, Shlopov VG, Verkhuletsky IE, Kuznetsov AS. Experience in the use of SKN‐1K sorbent in the treatment of purulent wounds. Klinichna Khirurhiia 1984;Jan(1):44‐7. [PubMed] [Google Scholar]
Udwadia 2011 {published data only}
- Udwadia TE. Ghee and honey dressing for infected wounds. Indian Journal of Surgery 2011;73(4):278‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Upright 1994 {published data only}
- Upright CA, Salton C, Roberts F, Murphy J. Evaluation of Mesalt dressings and continuous wet saline dressings in ulcerating metastatic skin lesions. Cancer Nursing 1994;17(2):149‐55. [PubMed] [Google Scholar]
Additional references
Alexander 2009a
- Alexander S. Malignant fungating wounds: epidemiology, aetiology, presentation and assessment. Journal of Wound Care 2009;18(7):273‐80. [DOI] [PubMed] [Google Scholar]
Alexander 2009b
- Alexander S. Malignant fungating wounds: key symptoms and psychosocial. Journal of Wound Care 2009;18(8):325‐9. [DOI] [PubMed] [Google Scholar]
Bale 1991
- Bale S, Harding K. Fungating breast wounds. Journal of District Nursing 1991;9(12):4‐5. [Google Scholar]
Bridel‐Nixon 1997
- Bridel‐Nixon J. Other chronic wounds. In: Morison M, Moffatt C, Bridel‐Nixon J, Bale S editor(s). Nursing Management of Chronic Wounds. 1st Edition. London: Mosby, 1997:221‐45. [Google Scholar]
de Haes 1996
- Haes JCJM de, Olschewski M, Fayers P, Visser MRM, Cull A, Hopwood P, Sanderman, R. Measuring the quality of life of cancer patients with the Rotterdam Symptom Checklist: a manual. Groningen, the Netherlands: Noordelijk Centrum voor Gezondheidsvraagstukken / Northern Centre for Healthcare Research. University of Groningen, 1996. [Google Scholar]
Fairburn 1994
- Fairburn K. A challenge that requires further research. Management of fungating breast lesions. Professional Nurse 1994;9:272‐7. [PubMed] [Google Scholar]
Gibson 2013
- Gibson S, Green J. Review of patients' experiences with fungating wounds and associated quality of life. Journal of Wound Care 2013;22(5):265‐75. [DOI] [PubMed] [Google Scholar]
Grocott 1995
- Grocott P. The palliative management of fungating malignant wounds. Journal of Wound Care 1995;4(5):240‐2. [DOI] [PubMed] [Google Scholar]
Grocott 2001b
- Grocott P. Developing a tool for researching fungating wounds. World Wide Wounds 2001; Vol. http://www.worldwidewounds.com/2001/july/Grocott/Fungating‐Wounds.html.
Grocott 2002
- Grocott P. A review of advances in fungating wound management since EWMA 1991. EWMA Journal 2002;2(1):21‐4. [Google Scholar]
Haughton 1995
- Haughton W, Young T. Common problems in wound care: malodorous wounds. British Journal of Nursing 1995;4(16):959‐63. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group (Editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Available from www.cochrane‐handbook.org.
Hirst 1992
- Hirst DG. Tumour vascular physiology. Annual report of the Cancer Research Campaign Grey Laboratory 1992.
Kelly 2002
- Kelly N. Malodorous fungating wounds: a review of current literature. Professional Nurse 2002;17(5):323‐6. [Google Scholar]
KLONK 2014
- KLONK. KLONK Image Measurement. http://www.imagemeasurement.com/ (accessed February 15 2014).
Lefebrve 2011
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lo 2008
- Lo S, Hu W, Hayter M, Chang SC, Hsu MY, Wu LY. Experiences of living with a malignant fungating wound: a qualitative study.. Journal of Clinical Nursing 2008;17(20):2699‐708. [DOI] [PubMed] [Google Scholar]
Lund‐Nielson 2005
- Lund‐Nielsen B, Muller K, Adamsen L. Malignant wounds in women with breast cancer: feminine and sexual perspectives. Journal of Clinical Nursing 2005;14:56‐64. [DOI] [PubMed] [Google Scholar]
Melzack 1987
- Melzack R. The short‐form McGill Pain Questionnaire. Pain 1987;30(2):191‐7. [DOI] [PubMed] [Google Scholar]
Probst 2013
- Probst S, Arber A, Faithfull S. Coping with an exulcerated breast carcinoma: an interpretative phenomenological study. Journal of Wound Care 2013;22(7):352‐60. [DOI] [PubMed] [Google Scholar]
Selby 2009
- Selby T. Managing exudate in malignant fungating wounds and solving problems for patients. Nursing Times 2009;105(18):14‐17. [PubMed] [Google Scholar]
SIGN 2008
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. Available from http://www.sign.ac.uk/methodology/filters.html#random (accessed August 2008).
WHO 1979
- World Health Organisation. WHO Handbook for Reporting Results of Cancer Treatment. Geneva: World Health Organisation, 1979. [Google Scholar]


