Abstract
Background
Water‐based exercises are used in rehabilitation and might help to reduce disability after stroke.
Objectives
To investigate the effect of water‐based exercises for reducing disability after stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched August 2010), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 4), MEDLINE (1966 to April 2010), EMBASE (1980 to April 2010), CINAHL (1982 to April 2010), AMED (1985 to April 2010), SPORTDiscus (1949 to April 2010), the Physiotherapy Evidence Database (PEDro, April 2010) and OT Seeker (1969 to April 2010). In an effort to identify further published, unpublished and ongoing trials we handsearched relevant journals and conference proceedings, searched trials and research registers, checked reference lists and contacted authors.
Selection criteria
We included studies using random assignment.
Data collection and analysis
Two review authors independently selected trials for inclusion, assessed trial quality and extracted the data. The primary outcome was activities of daily living.
Main results
We included four trials involving 94 participants in this review. There was a significant improvement in activity of daily living (mean difference (MD) 13.20 points on the 'Capacidad funcional' (functional capacity) subscale of the Brazilian‐Portuguese version of the SF‐36; 95% confidence interval (CI) 8.36 to 18.04; P < 0.00001) and on muscle strength (MD 1.01 Nm/kg; 95% CI 0.19 to 1.83; P = 0.02) but these results should be interpreted with caution because population numbers were small and the results are based on single studies. There was no significant improvement in ability to walk (MD 0.14 m/s; 95% CI ‐0.32 to 0.606; P = 0.55), postural balance (MD 3.05 points; 95% CI ‐3.41 to 9.52; P = 0.35) or fitness (MD 3.6 (VO2max; 95% CI ‐0.53 to 7.73; P = 0.09) after water‐based exercises treatment compared to control. Adverse effects were not reported.
Authors' conclusions
The evidence from randomised controlled trials so far does not confirm or refute that water‐based exercises after stroke might help to reduce disability after stroke. There is a lack of hard evidence for water‐based exercises after stroke. Better and larger studies are therefore required.
Keywords: Humans, Stroke Rehabilitation, Activities of Daily Living, Hydrotherapy, Hydrotherapy/methods, Muscle Strength, Postural Balance, Randomized Controlled Trials as Topic, Recovery of Function, Walking
Water‐based exercises for improving activities of daily living after stroke
Many people who have had a stroke have limited activities of daily living and reducing disability is one of the main goals of rehabilitation. Water‐based exercises are used in rehabilitation and might help to reduce disability after stroke. This review of four trials, which included 94 participants, found there is not enough evidence to decide if water‐based exercises may reduce disability after stroke. There is a lack of hard evidence for water‐based exercises after stroke. More research is therefore needed.
Background
Stroke presents a major global public health challenge. Together with ischaemic heart disease, stroke is the largest source of disease burden: e.g. in low‐ and middle‐income countries of Europe and Central Asia these health issues account for more than one‐quarter of the total disease burden (Lopez 2006) and stroke represents the leading cause of long‐term disability in Americans (Anderson 1995). Stroke is a major cause of chronic impaired function, and it is well known that activities of daily living after stroke are limited.
In an effort to address the problem, many people join multidisciplinary rehabilitation programmes soon after having a stroke. However, despite intensive rehabilitation efforts, only approximately 5% to 20% achieve complete functional recovery (Nakayama 1994). Thus, there still exists an urgent need for new inpatient and outpatient rehabilitation and training strategies that match the specific needs of people after stroke and their carers (Barker 2005).
There are many different rehabilitation approaches to improving disability after stroke. One example is water‐based exercises. Water‐based exercises, sometimes also called hydrotherapy, are defined according to the Hydrotherapy Association of Chartered Physiotherapists Guidance on Good Practice in Hydrotherapy as a therapy programme using the properties of water, designed by a suitably qualified physiotherapist, to improve function, ideally in a purpose‐built and suitably heated hydrotherapy pool (HACP 2006).
Apart from various specific techniques, simple exercises such as general body movements and walking in water might be beneficial because water's natural buoyancy allows many body movements by providing a type of body weight support. The natural resistance of water may encourage strengthening of weakened muscles.
Perhaps the interest in hydrotherapy is based on the treatments for rehabilitation after poliomyelitis developed some decades ago (Birk 1993). This experience may have influenced the way in which so much attention was given to hydrotherapy for those people with "weak muscles".
Water‐based exercises have recently been shown to improve fitness and strength in older people (Taunton 1996; Tsourlou 2006) and in people with rheumatoid arthritis (Danneskiold 1987). At the very least, water‐based exercises might have the potential to improve activities of daily living and might also improve impaired cardiovascular fitness in people who have had a stroke (Chu 2004). However, no systematic evaluation of the effectiveness of water‐based exercises for people after stroke currently exists. Therefore, the rationale for this review is to evaluate the effectiveness of water‐based exercises to improve activities of daily living, ability to walk, muscle strength, postural balance, and fitness after stroke.
Objectives
To conduct a systematic review of randomised controlled trials (RCTs) to evaluate the effect of water‐based exercises for improving activities of daily living, ability to walk, muscle strength, postural balance, and fitness after stroke.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs and randomised controlled cross‐over trials (we only analysed the first period as a parallel group trial). However, we did not include quasi‐controlled trials.
Types of participants
We included studies with participants of either gender over 18 years of age after stroke (using the World Health Organization (WHO) definition of stroke, or a clinical definition of stroke when the WHO definition was not specifically stated) (WHO 2006), regardless of the duration of illness or level of initial impairment. If we found RCTs with mixed populations (such as traumatic brain injury and stroke) we only included those RCTs with more than 50% of participants with stroke in our analysis.
Types of interventions
We defined water‐based exercises as any single or group intervention that involves participants being treated in water. To distinguish water‐based exercises from use of a bathtub or spa pool, we have used the term (water‐based) exercises to refer to an activity that is planned, structured and repetitive. We have included only those studies which considered treatments carried out by a trained health professional, such as a physiotherapist. We have excluded studies with treatments such as mud baths, day spas or the use of shallower water (e.g. Kneipp hydrotherapy: Schencking 2009). We aimed to compare water‐based exercises (of any kind and irrespective of whether they are targeted at improving activities of daily living) for improving activities of daily living, ability to walk, muscle strength, postural balance, and fitness after stroke with all other non‐water‐based interventions (including other rehabilitation strategies, or no treatment). We did not include studies if they compared one type of water‐based intervention with another water‐based intervention.
Types of outcome measures
Primary outcomes
We defined the primary outcome as activities of daily living (ADL). Possible scales which we considered include global measures of activities of daily living such as: Barthel ADL Index (BI) (Mahoney 1965; Wade 1988), Rivermead ADL Assessment (Whiting 1980), Rivermead Motor Ability Scale (Collen 1991), Modified Rankin Scale (Bonita 1988), Functional Independence Measure (FIM) (Hamilton 1994), Katz Index of Activities of Daily Living (Katz 1970), Rehabilitation Activities Profile (Van Bennekom 1995), and the Motor Assessment Scale (MAS) (Carr 1985). A brief description of most of these scales and generally accepted measures for ADL can be found in a statement by the American Heart Association (Miller 2010). Depending on the data provided by the studies and trialists, all the review authors discussed and reached consensus on which appropriate measures should be included in the analysis for the primary outcome.
Secondary outcomes
We defined secondary outcomes as ability to walk, postural balance, muscle strength and fitness, with appropriate measures as reported in the studies. Other secondary outcomes include drop‐out from the study during the treatment phase, and adverse events (including death from all causes).
A possible scale to measure the ability to walk is the Functional Ambulation Categories (FAC) (Holden 1984). If FAC scores were not reported in the included studies we accepted alternative indicators of independent walking (Mehrholz 2006).
A possible scale to measure postural balance is the Berg Balance Scale (Berg 1992).
A possible scale to measure muscle strength is the Motricity Index (Demeurisse 1980) and a possible measure of cardiorespiratory (aerobic) fitness may include exercises heart rate and oxygen consumption (VO2) (Saunders 2004).
Depending on the aforementioned categories and the availability of variables used in the included trials, all the review authors discussed and reached consensus on which outcome measures should be included in the analysis.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module.
Electronic searches
We searched the Cochrane Stroke Group Trials Register, which was last searched by the Managing Editor on 31 August 2010, the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 4), MEDLINE (1966 to April 2010) (Appendix 1), EMBASE (1980 to April 2010), CINAHL (1982 to April 2010), AMED (1985 to April 2010), SPORTDiscus (1949 to April 2010), the Physiotherapy Evidence Database (PEDro, http://www.pedro.org.au/) (searched April 2010) and OT Seeker (http://www.otseeker.com/default.aspx) (1969 to April 2010).
Searching other resources
In an effort to identify further published, unpublished, and ongoing trials not available in the major databases:
we checked reference lists of all relevant articles;
we searched the following ongoing trials and research registers: Internet Stroke Center Stroke Trials Registry (http://www.strokecenter.org/trials/); ClinicalTrials.gov (http://clinicaltrials.gov/); and Current Controlled Trials (http://www.controlled‐trials.com/);
-
we identified and handsearched the following journals and conference proceedings:
3rd, 4th, 5th and 6th World Congress of NeuroRehabilitation (2002, 2006, 2008 and 2010);
1st, 2nd, 3rd, 4th and 5th World Congress of Physical Medicine and Rehabilitation (2001, 2003, 2005, 2007 and 2009);
World Congress of Physical Therapy (2003 and 2007);
Deutsche Gesellschaft für Neurotraumatologie und Klinische Neurorehabilitation (2001 to 2008);
Deutsche Gesellschaft für Neurologie (2000 to 2009);
Deutsche Gesellschaft für Neurorehabilitation (1999 to 2009);
Asian Oceania Conference of Physical and Rehabilitation (2008);
we contacted trialists, researchers and relevant special interest groups in our field of study.
We searched for trials in all languages and arranged translation of relevant trial reports published in languages other then English.
Data collection and analysis
Selection of studies
One review author (JM) independently read titles and abstracts of identified references and eliminated obviously irrelevant studies based on titles and, when available, abstracts. Two review authors (MP and JK) independently examined potentially relevant studies using predetermined criteria for including studies. We obtained the full‐text articles for the remaining studies. Based on our inclusion criteria (types of studies, participants, aims of interventions, outcome measures) two review authors (JM and MP) independently ranked these studies as relevant, irrelevant or possibly relevant. We excluded all trials ranked initially as irrelevant, but included all other trials at this stage. The authors resolved disagreements through discussion involving all review authors. If further information was needed to reach consensus on whether to select a study or not, we contacted trialists in an effort to obtain missing information.
Data extraction and management
Two review authors (JM and MP) independently extracted trial and outcome data from the selected trials. If one of the review authors was involved in an included trial, another review author extracted trial and outcome data from that trial. We established the characteristics of unpublished trials through correspondence with the trial co‐ordinator or principal investigator. We used checklists to independently record details of the:
methods of generating randomisation schedule;
method of concealment of allocation;
blinding of assessors;
use of an intention‐to‐treat analysis (we will include all participants initially randomised in the analyses as allocated to groups);
adverse events and drop‐outs for all reasons;
important imbalance in prognostic factors;
participants (country, number of participants, age, gender, type of stroke, time from stroke onset to entry into the study, inclusion and exclusion criteria);
comparison (details of the intervention in treatment and control groups; details of co‐intervention(s) in both groups; duration of treatment);
- outcomes and time points of measures (number of participants in each group and outcome, regardless of compliance) (see Characteristics of included studies, Table 2 and Table 3).
Table 1.
Demographics of included participantsStudy Number of participants (EXP/CON) Mean age (EXP/CON) Gender (F/M) Duration since stroke (EXP/CON) Side of stroke L/R (EXP/CON) Type of stroke ischaemic/haemorrhage (EXP/CON) Aidar 2007 31 (16/15) 51 (50/52) years 9/19 Not stated Not stated Not stated Chan 2010 25 (not stated) Not stated Not stated Not stated Not stated Not stated Chu 2004 13 (7/6) 63 (62/63) years 1/11 3/4 years 7/5 (4/3 / 3/2) 8/4 (3/4 / 5/0) Noh 2008 25 (13/12) 64 (62/66) years 14/11 2.8/1.6 years 12/13 (7/6 / 5/7) 13/12 (6/7 / 7/5) Table 2.
Methodological quality of included trials using the PEDro ScalePEDro score Aidar 2007 Chan 2010 Chu 2004 Noh 2008 Random allocation Yes Yes Yes Yes Concealed allocation Unclear Yes Unclear Unclear Baseline comparability Yes Unclear Yes Yes Blind participants No No No No Blind therapists No No No No Blind assessors No Yes Yes Yes Adequate follow up* (drop‐out rate) Yes (0%) Unclear Yes (8%) No (20%) Intention‐to‐treat analysis Unclear Unclear No No Between‐group comparisons Yes Yes Yes Yes Point estimates and variability Yes Yes Yes Yes Total PEDro score 5 (10) 5 (10) 6 (10) 5 (10)
The review authors checked all of the extracted data for agreement, with another review author (JK) arbitrating any items if consensus was not reached. If necessary, we contacted trialists to request more information, clarification or missing data.
Assessment of risk of bias in included studies
All review authors independently assessed the methodological quality of included trials using the PEDro Scale (Maher 2003). We present the results of quality ratings in Table 3. The items of the PEDro Scale are: specification of eligibility criteria; random allocation to groups; concealed allocation; groups similar at baseline; blinding of participants, therapists and assessors; outcome measurements obtained from more than 85% of participants; presence of an intention‐to‐treat (ITT) analysis; reporting of results of between‐group statistical comparisons; reporting of point measures and measures of variability (Herbert 1998). The maximum achievable PEDro sum score is 10 points. (It should be noted that the PEDro scale is an 11‐item scale, but the maximum sum score is 10 points because the first Item of the scale 'eligibility criteria were specified' is not used to calculate the sum score.)
We checked all methodological quality assessments for agreement between review authors. We resolved any disagreements by discussion involving all the review authors. We contacted study authors for clarification and to request missing information. We planned to use the methodological features ‐ concealment of allocation, ITT analysis, and blinding of assessors ‐ to test the robustness of the main results in a post‐hoc sensitivity analysis, but there were not enough studies for such an analysis.
Measures of treatment effect
For all outcomes representing continuous data (e.g. the primary outcome ‐ activities of daily living) we entered means and standard deviations. We calculated a pooled estimate of the mean differences (MD) with 95% confidence intervals (CI). If studies did not use the same outcome (for example one study used the BI and another used the FIM to measure activities of daily living), we used the standardised mean difference (SMD) instead of MD.
For all binary outcomes (such as the secondary outcome 'drop‐out from all causes') we calculated risk ratio (RR) with 95% CI. As it is possible that some trials (or groups within a trial) have no adverse events or no drop‐outs, we calculated risk differences (RD) instead of RRs in these specific situations, again with 95% CI.
We quantified inconsistency across studies by using the I2 statistic. We considered an I2 value greater than 50% as substantial heterogeneity. In the presence of heterogeneity we used a random‐effects model instead of a fixed‐effect model approach. For all statistical comparisons we used the current version of the Cochrane Review Manager software, RevMan 5 (RevMan 2008).
Subgroup analysis and investigation of heterogeneity
In Table 2 we have described the variability in participants, interventions and outcomes studied (clinical diversity).
Because there are only four small studies we did not conduct any subgroup analysis (Higgins 2008).
Sensitivity analysis
To test the robustness of the results, we planned to undertake a post‐hoc sensitivity analysis for methodological quality (Mehrholz 2010). However, because there are only four small studies, we did not conduct a sensitivity analysis.
Results
Description of studies
Results of the search
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
We identified 43 potential studies through the electronic searching and searching of other resources. One review author excluded 34 of these as they were not relevant, leaving 10 studies which two review authors considered independently.
Included studies
We included four trials involving a total of 94 participants in the review (Aidar 2007; Chan 2010; Chu 2004; Noh 2008) (see the Characteristics of included studies). All included studies investigated the effect of water‐based exercises after stroke.
Excluded studies
We excluded three studies (Marroni 1988; Revnic 2004; Wang 2004) (see the Characteristics of excluded studies); one study is an ongoing trial (Gunn 2007) and two studies are still awaiting classification (Lee 2006; Xu 2008).
Risk of bias in included studies
For full details of methodology and risk of bias assessments, see Characteristics of included studies, Figure 1 and Figure 2.
Figure 1.
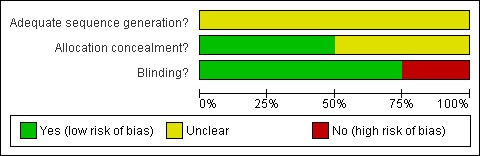
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 2.
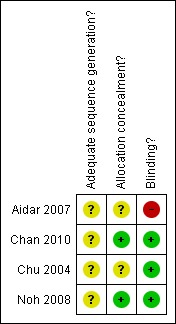
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We judged some methodological aspects of the included studies to be of uncertain methodological quality and therefore at risk of bias (see also Methodological quality of included trials using the PEDro Scale ‐ Table 3).
All four studies adequately reported the method of randomisation (Aidar 2007; Chan 2010; Chu 2004; Noh 2008). One study reported adequate allocation concealment, but the method is unclear (Noh 2008). Two studies reported blinding of outcome assessors (Chu 2004; Noh 2008). No studies reported the use of ITT analysis.
Effects of interventions
Primary outcome
In all following comparisons we used the data taken at the end of the trial, not the change between baseline and the end of the trial. We were interested to see if the use of water‐based exercises for people after stroke improved predefined outcomes more than the control intervention (differences between intervention and control at the end of the trial).
Comparison 1.1: Water‐based exercises versus no water‐based exercises (activities of daily living at the end of intervention phase)
Only one trial with a total of 31 participants (Aidar 2007) measured activities of daily living at study end. As a measure of activities of daily living, the authors used the 'Capacidad funcional' (functional capacity) subscale of the Brazilian‐Portuguese version of the SF‐36 (Ciconelli 1999). The use of water‐based exercises for people after stroke did improve activities of daily living significantly. The pooled mean difference (fixed‐effect model) for activities of daily living was 13.20 points (95% CI 8.36 to 18.04; P < 0.00001; level of heterogeneity I2 not applicable).
Secondary outcomes
Comparison 1.2: Water‐based exercises versus no water‐based exercises (ability to walk at the end of intervention phase)
Only one trial with a total of 13 participants (Chu 2004) measured walking speed (metres/second, m/s) at study end. The use of water‐based exercises for people after stroke did not improve walking speed significantly. The pooled mean difference (fixed‐effect model) for walking speed was 0.14 m/s (95% CI ‐0.32 to 0.60; P = 0.55; level of heterogeneity I2 not applicable).
Comparison 1.3: Water‐based exercises versus no water‐based exercises (postural control at the end of intervention phase)
Only two trials with a total of 38 participants (Chu 2004; Noh 2008) measured postural control (points of the Berg Balance Scale) at study end. The use of water‐based exercises for people after stroke did not improve postural control significantly. The pooled mean difference (random‐effects model) for postural control (was 3.05 points (95% CI ‐3.41 to 9.52; P = 0.35; level of heterogeneity I2 = 81% ).
Comparison 1.4: Water‐based exercises versus no water‐based exercises (muscle strength at the end of intervention phase)
Only one trial with a total of 13 participants (Chu 2004) measured muscle strength (Nm/kg) at study end. The use of water‐based exercises for people after stroke did improve muscle strength significantly. The pooled mean difference (fixed‐effect model) for muscle strength was 1.01 Nm/kg (95% CI 0.19 to 1.83; P = 0.02; level of heterogeneity I2 not applicable).
Comparison 1.5: Water‐based exercises versus no water‐based exercises (aerobic fitness at the end of intervention phase)
Only one trial with a total of 13 participants (Chu 2004) measured aerobic fitness ( VO2max ml/kg/min) at study end. The use of water‐based exercises for people after stroke did not improve aerobic fitness significantly. The pooled mean difference (fixed‐effect model) for aerobic fitness was 3.60 VO2max ml/kg/min (95% CI ‐0.53 to 7.73; P = 0.09; level of heterogeneity I2 not applicable).
Comparison 1.6: Water‐based exercises versus no water‐based exercises (drop‐out from study during the intervention period)
All four included trials with a total of 94 participants (Aidar 2007; Chan 2010; Chu 2004; Noh 2008) provided information about the rate at which participants dropped out from all causes during the trial period. The drop‐out rates were between 0% (Aidar 2007; Chan 2010) and 20% (Noh 2008). The use of water‐based exercises for people after stroke did not significantly increase the risk of drop‐out (RD (fixed) ‐0.01, 95% CI ‐0.12 to 0.11, P = 0.92; level of heterogeneity I2 = 0%). The reasons for drop‐outs, if stated by the trialists, are described in detail for each trial in the Characteristics of included studies table.
Comparison 1.7: Water‐based exercises versus no water‐based exercises (adverse events)
None of the four included studies with a total of 94 participants (Aidar 2007; Chan 2010; Chu 2004; Noh 2008) provided information about any adverse events during the trial period. It is not clear if none occurred or if adverse events were not reported. Therefore, we did not do a pooled analysis for comparison 1.7.
Subgroup and sensitivity analyses
We initially planned in our protocol for this review to undertake subgroup and sensitivity analyses. However, due to the small number of included studies we did not perform either a subgroup analysis or a sensitivity analysis.
Discussion
Summary of main results
The aim of this review was to evaluate the effect of water‐based exercises for people after stroke. Only four trials with a total of 94 participants were eligible and were included in this review. There were significant differences in favour of water‐based exercises for people after stroke for activities of daily living and muscle strength, but these results are based only on a single study. However, adverse events and drop‐outs did not appear to be more frequent in those participants who received water‐based exercises. This indicates that the use of different types of water‐based exercises appears to be acceptable to most participants in the trials included in this review. However, the number of participants is too small to conclude anything about the safety of the intervention.
Potential biases in the review
A risk of publication bias is present in all systematic reviews. However, we searched extensively for relevant literature in databases and handsearched conference abstracts. Additionally, we contacted authors, trialists and experts in the field for other unpublished and ongoing trials.
The included studies had a small number of participants and reported a broad range of outcome measures, of which many were unique to individual studies. No study included adequate follow‐up after the end of the study. It is, therefore, not possible to draw any conclusions regarding how long any benefits last. All these factors limit the completeness of the evidence relevant to this review.
One could argue that the generalisability of our results in terms of representation of the stroke population is limited. Our included study population had a mean age between 50 and 67 years. Compared with data from a stroke registry, the mean age of people after stroke in our review was low. The highest annual incidence rates of first‐ever‐in‐a‐lifetime stroke are to be expected between 65 to 74 years of age (Kolominsky‐Rabas 1998).
There is one ongoing study (Gunn 2007). It is currently not clear how this study will address clinical uncertainties about the value of water‐based exercises. However, with a target sample size of 45 participants this ongoing trial seems to have the potential to give a more precise estimation of the effects of water‐based exercises after stroke.
Methodological issues
There was heterogeneity between the trials in terms of trial design (duration of study, selection criteria for people after stroke), characteristics of the therapy interventions (exercises used), participant characteristics (duration of illness, age). There were also methodological differences in the mechanism of randomisation and allocation concealment methods used, blinding of primary outcomes and the presence or use of ITT analysis. There were not enough studies to do a sensitivity analysis by methodological quality as planned and stated above.
Limitations of the review
One limitation of this review is that we only identified four relevant small randomised trials and most had methodological limitations, including lack of concealed allocation or lack of blinded assessors. The four studies were clinically diverse as different experimental intensities were used, and the setting, severity of stroke and the age of the participants differed between the trials (Table 2).
Another limitation is that interventions given to the control group in some studies are not comparable. In the control group in two studies some form of exercises was applied (arm exercises Chu 2004; general conditioning exercises Noh 2008), whereas for the other two studies (Aidar 2007; Chan 2010) the exercises given in the control group are not clearly described.
One could argue that our review is limited by the inclusion of RCTs only. However, the use of a rigorous study design is very important to minimise bias. Therefore, the Cochrane Handbook for Systematic Reviews of Interventions states that only RCTs should be considered to reduce bias and when addressing questions regarding therapeutic effectiveness (Higgins 2008).
Another limitation of this review is that we did not receive all the information we requested from trialists.
We are confident that our detailed search strategy, combined with handsearching, identified all relevant trials. However, it is still possible that we did not identify some of the 'grey' literature, but it would be unlikely that this would have a significant impact on our results. This review provides a template for the inclusion of future trials and could be used, in addition to guidelines, to guide further research.
There is a clear need for well‐designed large studies to evaluate the effects of water‐based exercises for people after stroke and such research should be given a higher priority. It is important that future trials should use relevant variables such as activities of daily living and walking ability. Future trials should also include detailed information about each participant's experience of water prior to their stroke (e.g. ability to swim, fear of water or limited experience of water buoyancy).
Authors' conclusions
There is insufficient evidence to conclude that water‐based exercises for people after stroke are effective for reducing disability. However, there is also insufficient evidence to conclude that water‐based exercises are ineffective or even harmful. Current clinical practice should be improved through the design of better and larger studies.
The insufficient evidence is mainly due to the small number of trials and the small number of included participants. Therefore, we recommend that there should be better and larger RCTs to evaluate the effectiveness of water‐based exercises for people after stroke.
Acknowledgements
We thank Brenda Thomas for her help with developing the search strategy and for giving us helpful support, Hazel Fraser for giving us very helpful support and for proof reading the review, Gabi Voigt for providing us with helpful studies, and the ZVK‐Stiftung Forschungsförderung.
Appendices
Appendix 1. MEDLINE search strategy
We used the following search strategy for MEDLINE (Ovid) and adapted it for the other databases. 1. exp cerebrovascular disorders/ or brain injuries/ or brain injury, chronic/ 2. (stroke$ or cva or poststroke or post‐stroke).tw. 3. (cerebrovasc$ or cerebral vascular).tw. 4. (cerebral or cerebellar or brain$ or vertebrobasilar).tw. 5. (infarct$ or isch?emi$ or thrombo$ or emboli$ or apoplexy).tw. 6. 4 and 5 7. (cerebral or brain or subarachnoid).tw. 8. (haemorrhage or hemorrhage or haematoma or hematoma or bleed$).tw. 9. 7 and 8 10. exp hemiplegia/ or exp paresis/ 11. (hempar$ or hemipleg$ or brain injur$).tw. 12. Gait Disorders, Neurologic/ 13. 1 or 2 or 3 or 6 or 9 or 10 or 11 or 12 14. exp cerebrovascular disorders/th, rh or brain injuries/th, rh or brain injury, chronic/th, rh 15. exp hemiplegia/th, rh or exp paresis/th, rh 16. Gait Disorders, Neurologic/th, rh 17. 16 or 15 or 14 18. water/ or exp fresh water/ or exp seawater/ or hydrotherapy/ or swimming pools/ or swimming/ or balneology/ 19. (water or water‐based or seawater or aqua or aquatic$ or hydrokinetic$ or hydro‐kinetic$ or pool or pool‐based or swimming pool).tw. 20. 18 or 19 21. 17 and 20 22. exp exercise/ or movement/ or exp locomotion/ or physical exertion/ or exp exercise therapy/ or physical endurance/ or physical fitness/ or sports/ or exercise movement techniques/ or fitness centers/ or physical therapy modalities/ or rehabilitation/ or gymnastics/ 23. 22 and 20 24. ((water or water‐based or seawater or aqua or aquatic$ or hydrokinetic$ or hydro‐kinetic$ or pool or pool‐based or swimming pool) adj10 (exercise$ or fitness or physiotherap$ or activit$ or aerobic or training or therap$ or rehabilitation or treadmill or walking or gymnastic$ or calisthenic$)).tw. 25. (treading water or swimming or swim or aquarobics or aquatone or Ai Chi or Halliwick or hydrotherap$ or whirlpool bath$).tw. 26. 25 or 24 or 23 27. 26 and 13 28. 27 or 21 29. limit 28 to humans
Data and analyses
Comparison 1.
Water‐based exercises versus no water‐based exercises
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activities of daily living | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 13.20 [8.36, 18.04] |
| 2 Ability to walk | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.32, 0.60] |
| 3 Postural control | 2 | 38 | Mean Difference (IV, Random, 95% CI) | 3.05 [‐3.41, 9.52] |
| 4 Muscle strength | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | 1.01 [0.19, 1.83] |
| 5 Fitness | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | 3.60 [‐0.53, 7.73] |
| 6 Drop out from study during the treatment phase | 4 | 94 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.12, 0.11] |
Analysis 1.1.
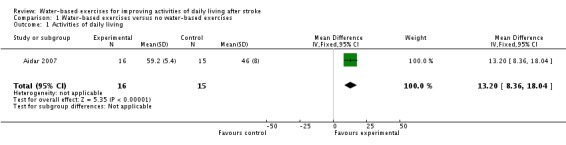
Comparison 1 Water‐based exercises versus no water‐based exercises, Outcome 1 Activities of daily living.
Analysis 1.2.
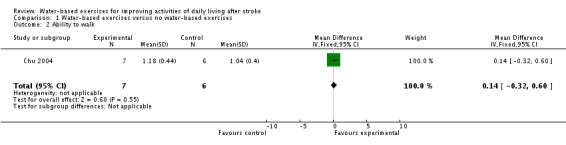
Comparison 1 Water‐based exercises versus no water‐based exercises, Outcome 2 Ability to walk.
Analysis 1.3.
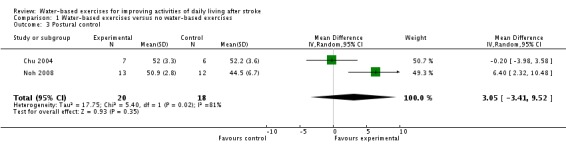
Comparison 1 Water‐based exercises versus no water‐based exercises, Outcome 3 Postural control.
Analysis 1.4.
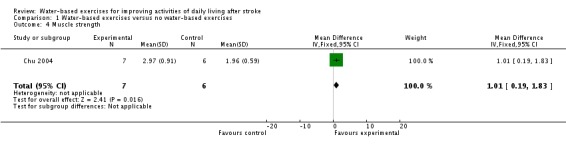
Comparison 1 Water‐based exercises versus no water‐based exercises, Outcome 4 Muscle strength.
Analysis 1.5.
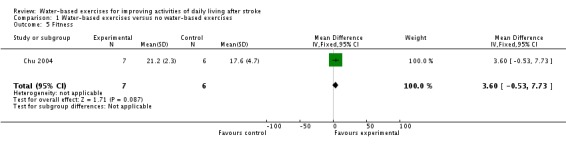
Comparison 1 Water‐based exercises versus no water‐based exercises, Outcome 5 Fitness.
Analysis 1.6.
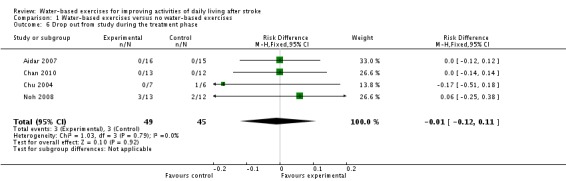
Comparison 1 Water‐based exercises versus no water‐based exercises, Outcome 6 Drop out from study during the treatment phase.
Differences between protocol and review
We planned to carry out subgroup and sensitivity analyses (Mehrholz 2010). However, as we only identified four studies, we did not conduct these analyses.
For the primary outcome 'activities of daily living', we used the 'Capacidad funcional' (functional capacity) subscale of the Brazilian‐Portuguese version of the SF‐36 because no other scales for measuring activities of daily living were used.
For the secondary outcome 'gait ability', we used the continuous measure 'walking speed' because no other scales for measuring activities were used.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aidar 2007
| Methods | Randomised controlled trial (random assignment stated) Method of randomisation: not stated Allocation concealment: not stated ITT analysis: unclear Blinding of outcome assessors: not stated Adverse events: not stated Deaths: not stated Drop‐outs: none reported Ethical approval: yes Informed consent to participation: yes | |
| Participants | Country: Spain 31 people after stroke (16 in treatment group, 15 in control group) Mean age: 50 and 52 years (treatment and control group respectively) Inclusion criteria: stroke, hemiplegia or hemiparesis, clinical stable Exclusion criteria: not reported | |
| Interventions | 2 arms: (1) experimental group used aquatic physical exercises12 weeks (45 to 60 minutes per day) (2) control group used no aquatic physical exercises | |
| Outcomes | Outcomes were recorded at baseline and after 12 weeks Primary outcomes: quality of life (SF‐36) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method not stated |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | High risk | Not stated |
Chan 2010
| Methods | Randomised controlled trial (random assignment stated) Method of randomisation: not stated Allocation concealment: yes ITT analysis: not stated Blinding of outcome assessors: yes, as stated by the authors Adverse events: not stated Deaths: not stated Drop‐outs: none reported Ethical approval: yes Informed consent to participation: yes | |
| Participants | Country: Canada 25 people after stroke (unknown how many in treatment and control group; for statistical analysis we assumed 13 in the treatment group and 12 in the control group) Unclear if ambulatory at study onset Mean age: unclear Inclusion criteria: not described Exclusion criteria: not described | |
| Interventions | 2 arms: (1) experimental group used hydrotherapy + land therapy group (unclear for how long and how much per day and how often) (2) control group used land therapy only group (unclear for how long and how much per day and how often) | |
| Outcomes | Unclear when outcomes were recorded Outcome assessments used were Berg Balance Score, Community Mobility and Balance Score, Time Up and Go, 2 Minute Walk Test and Biodex Balance Scores (Postural Stability Index and Limit of Stability Index) with the use of a Biodex Balance System SD | |
| Notes | Data collection is still ongoing. However, first results were presented at the 1st Canadian Stroke Conference 2010 The trialists initially proposed to include 44 consecutive out‐patients who meet the inclusion criteria into the study 22 participants are included in each group Data collection started in May 2007 and currently there is completed data collection for 25 participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not exactly stated |
| Allocation concealment? | Low risk | Described as "The randomization is known to the researcher only and when participants are included in the study, the treating therapists are informed of the group the participants are placed." |
| Blinding? All outcomes | Low risk | Described as "The assessor is blinded to the type of treatment. The participants and treating therapists are not blinded." |
Chu 2004
| Methods | Randomised controlled trial (random assignment stated) Method of randomisation: stratified randomisation Allocation concealment: not stated ITT analysis: no Blinding of outcome assessors: yes, as stated by the authors Adverse events: not stated Deaths: not stated Drop‐outs: 1 drop‐out in the control group Ethical approval: yes Informed consent to participation: yes | |
| Participants | Country: Canada 13 people after stroke (7 in the treatment and 6 in the control group) Mean age: 62 to 63 years (treatment and control group respectively) Inclusion criteria: at least 1 year post‐stroke from a single cerebrovascular accident; independent in walking (with or without assistive device); medically stable (see exclusion criteria); no previous myocardial infarction; and no significant musculoskeletal problems from conditions other than stroke Exclusion criteria: uncontrolled hypertension, arrhythmia, or unstable cardiovascular status | |
| Interventions | 2 arms: (1) experimental group used an 8‐week water‐based exercise program that focused on leg exercises to improve cardiovascular fitness (3 days a week for 1 hour a session). The exercise intervention consisted of 10 minutes of land‐based stretching, 5 minutes of light aerobic warm‐up in the water (marching on the spot, single and double‐legged hopping holding onto the pool edge), 30 minutes of moderate to high aerobic activities (shallow water walking, running, side stepping) at the target heart rate prescribed for that week, 5 minutes of a light cool down (marching on the spot), and 10 minutes of gentle stretching in the water. Target heart rates were set at 50% to 70%, 75%, and 80% heart rate reserve 5 beats/minute (as determined by an initial maximal exercise test) for weeks 1 to 2, 3 through 5, and 6 through 8, respectively. (2) control group used no water‐based exercises but did arm exercise. Participants exercised for the same amount of time as the experimental group. The main objective was to improve upper‐extremity function. Each session began with a 5‐minute warm‐up of active upper‐extremity movements. A 6‐station circuit then focused on gross upper‐limb movement (e.g. reaching), fine motor movement (e.g. adjusting small screws and bolts), and muscle strengthening of the shoulder, elbow, wrist, and fingers (e.g. exercises using hand putty, Theraband, weights). Participants were seated at each station, but had to stand and walk a few steps to the next station. The program ended with a 5‐minute cool‐down from upper‐extremity exercises. | |
| Outcomes | Outcomes were recorded at baseline and after 8 weeks Primary outcomes: VO2max Secondary outcomes: maximal workload (watts), self‐selected gait speed (m/s) over 8 metres, Berg Balance Scale and muscle strength (N/kg) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of sequence generation not stated; stratified randomisation stated |
| Allocation concealment? | Unclear risk | Stated as follows: "One researcher undertook all randomization procedures, and the assessments were done by testers who had no knowledge of the participants’ groupings (researcher blinding)." |
| Blinding? All outcomes | Low risk | See above |
Noh 2008
| Methods | Randomised controlled trial (random assignment stated) Method of randomisation: blocked randomisation Allocation concealment: yes ITT analysis: no Blinding of outcome assessors: yes, as stated by the authors Adverse events: not stated Deaths: not stated Drop‐outs: 2 drop‐outs in the control group (1 due to hip fracture after a fall and 1 incomplete participation) and 3 drop‐outs in the treatment group (2 due to personal reasons and time constraints and 1 due to poor general condition) Ethical approval: yes Informed consent to participation: yes | |
| Participants | Country: Korea 25 people after stroke (14 in the treatment and 16 in the control group) Ambulatory at study onset Mean age: 57 to 67 years (control and treatment group respectively) Inclusion criteria: stroke at least 6 months before enrolment, hemiparesis secondary to a first stroke, able to walk independently (with or without an assistive device), medically stable, no previous myocardial infarction, and no significant musculoskeletal problems as a result of conditions other than stroke Exclusion criteria: uncontrolled hypertension, arrhythmia and unstable cardiovascular status | |
| Interventions | 2 arms: (1) experimental group used aquatic therapy group participated in a programme consisting of Ai Chi and Halliwick methods, which focused on balance and weight‐bearing exercises, 3 times a week for 8 weeks (1 hour session) (2) control group used performed gym exercises (such as general conditioning exercises, including a warm‐up, lower extremity strengthening (e.g. bicycle, leg extensor), upper‐extremity strengthening (e.g. upper body ergometer) and gait training (e.g. marching with 0.5 kg ankle weights for 10 minutes) for the same amount of time as the aquatic therapy group spent in the pool | |
| Outcomes | Outcomes were recorded at baseline and after 8 weeks Primary outcomes: Berg Balance Scale score and weight‐bearing ability (measured by vertical ground reaction force during four standing tasks. rising from a chair and weight‐shifting forward, backward and laterally) Secondary outcomes: muscle strength and gait | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Author statement: "The subjects were then randomly assigned, so that the numbers of subjects in each stratum were approximately equal between the aquatic and conventional therapy groups." |
| Allocation concealment? | Low risk | Author statement: "One researcher performed the randomization and kept the tables of patient allocation and random numbers; the other researcher evaluated the subjects and did not have access to these tables." |
| Blinding? All outcomes | Low risk | Author statement: "All assessments were graded by one clinician who was blinded to group assignment." |
ITT: intention‐to‐treat
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Marroni 1988 | Evaluated hyperbaric oxygen therapy |
| Revnic 2004 | Not a randomised controlled trial |
| Wang 2004 | Evaluated the effect of seawater exercise on cerebral and cardiac haemodynamics |
Characteristics of studies awaiting assessment [ordered by study ID]
Lee 2006
| Methods | Randomised controlled trial: unclear; random assignment not stated Method of randomisation: unclear Allocation concealment: unclear ITT analysis: unclear Blinding of outcome assessors: unclear Adverse events: unclear Deaths: unclear Drop‐outs: unclear |
| Participants | Country: Korea 24 people after ischaemic stroke (14 people after stroke in experimental group and 10 in control group) Mean age: unclear Inclusion criteria: unclear Exclusion criteria: unclear |
| Interventions | 2 arms: (1) Hydrotherapy (aquatic treadmill walking, range of motion exercises, stretching and balance exercises) plus NDT (2) NDT alone |
| Outcomes | Unclear when outcomes were recorded Primary outcomes and secondary outcomes: Functional Independence Measure, Modified Barthel Index and MAS (unclear for what this abbreviation stands) |
| Notes |
Xu 2008
| Methods | Randomised controlled trial: unclear method of random assignment Method of randomisation: unclear Allocation concealment: unclear ITT analysis: unclear Blinding of outcome assessors: unclear Adverse events: unclear Deaths: unclear Drop‐outs: unclear |
| Participants | Country: China 76 people (40 in experimental group and 36 patients in control group) diagnosis not clearly stated Mean age: unclear Inclusion criteria: unclear Exclusion criteria: unclear |
| Interventions | 2 arms: (1) walking in water (2) pneu‐weight walking |
| Outcomes | Walking ability |
| Notes |
ITT: intention‐to‐treat NDT: neurodevelopmental therapy
Characteristics of ongoing studies [ordered by study ID]
Gunn 2007
| Trial name or title | The effectiveness of a higher intensity water‐based exercise program: a randomized controlled trial following stroke (pilot) ANZCTR registration title: The effectiveness of higher intensity water‐ and gym‐based exercise programs on gait speed: a randomised controlled trial following stroke (pilot) |
| Methods | Randomised controlled trial The study will compare 6 weeks higher intensity water‐based exercise with gym‐based exercise of similar intensity for people within 3 years of their first stroke |
| Participants | Country: Australia Inclusion criteria: 6 months to 3 years since first stroke (ischaemic stroke or primary intracerebral haemorrhage), community dwelling, independent ambulation with or without gait aids, Mini Mental State Exam score for at least 18, ability to accept instruction and give consent, a minimum age of 18 years Exclusion criteria: subsequent stroke, major medical complications following stroke, unstable cardiac conditions, urinary or faecal incontinence, open wounds, tinea, unstable epilepsy or seizures, other comorbid conditions that might contraindicate participation in gym‐ or water‐based exercise, inability to carry out the exercise program, current participation in an exercise program |
| Interventions | Intervention group: a water‐based intervention consisting of 3 pool sessions per week for a total of 6 weeks. Each session will be 40 minutes duration and consist of a standardised resistance program, the intensity of which will be varied according to each individual's ability, with progressive increase in load over the 6‐week period. Control group 1: gym‐based intervention consisting of 3 gym sessions per week for a total of 6 weeks. Each session will be 40 minutes duration and consist of a standardised resistance program, the intensity of which will be varied according to each individual's ability, with progressive increase in load over the 6‐week period. Control group 2: will consist of attendance at a chronic disease self‐management course once a week for 6 weeks. Each session will be 2.5 hours duration and will consist of instruction on how to manage symptoms, effectively communicate with your doctor, lessen frustration, fight fatigue, make daily tasks easier, and get more out of life. |
| Outcomes | Outcome measures: baseline, 6 weeks and 3 months post intervention Primary outcome: Six minute walk test Secondary outcomes: Modified Berg Balance Scale, Bioelectrical Impedance, Functional independence using the Modified Barthel Index, Goal Attainment Scale, Quality of Life using EuroQoL, Motor Assessment Scale, The Medical Outcomes Study (MOS) Sleep Scale, Fatigue Assessment Scale The people assessing the outcomes and the people analysing the results/data are masked/blinded |
| Starting date | Anticipated or actual date of first participant enrolment: 1 October 2007 |
| Contact information | Dr Simon Gunn Repatriation General Hospital Daws Road Daw Park SA 5041 Australia Tel: 08 8275 1103 Fax: 08 8275 1130 Email: simon.gunn@rgh.sa.gov.au |
| Notes | ACTR Number: ACTRN12607000506493 Target sample size: 45 http://www.anzctr.org.au/trial_view.aspx?ID=82304 |
Contributions of authors
Jan Mehrholz (JM) contributed to the conception and design of the protocol and approved the final manuscript. He searched electronic databases and conference proceedings, screened titles and abstracts of references identified by the search, selected and assessed trials, extracted trial and outcome data, guided the analysis and the interpretation of the data, and contributed to and approved the final manuscript of the review.
Joachim Kugler (JK) screened titles and abstracts of references identified by the search, located, selected and assessed trials, extracted trial and outcome data, assessed the methodological quality of selected trials, and contributed to and approved the final manuscript of the review.
Marcus Pohl (MP) contributed to the conception and design of the review, drafted the review, and assessed the methodological quality of selected trials. He contacted, together with JM, trialists about unpublished data and also entered the data, carried out statistical analysis, helped with the interpretation of the data, and approved the final manuscript of the review.
Sources of support
Internal sources
Private Europäische Medizinische Akademie der Klinik Bavaria Kreischa, Germany.
SRH Fachhochschule für Gesundheit Gera, Germany.
Technische Universität Dresden, Germany.
External sources
ZVK‐Stiftung Forschungsförderung, Germany.
Declarations of interest
None known.
New
References
References to studies included in this review
- Aidar FJ, Silva AJ, Reis VM, Carneiro A, Carneiro‐Cotta S. A study on the quality of life in ischaemic vascular accidents and its relation to physical activity. Revista de Neurologia 2007;45(9):518‐22. [PubMed] [Google Scholar]
- Chan K, Suter L, Heth D, Pauley T, Boulias C, Ismail F. A study examining the effect of hydrotherapy on balance in post stroke out‐patients. Stroke 2010;41:e492. [Google Scholar]
- Chu KS, Eng JJ, Dawson AS, Harris JEm Ozkaplan A, Gylfadottir S. Water‐based exercise for cardiovascular fitness in people with chronic stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2004;85:870‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh DK, Lim JY, Shin HI, Paik NJ. The effect of aquatic therapy on postural balance and muscle strength in stroke survivors ‐ a randomized controlled pilot trial. Clinical Rehabilitation 2008;22:966‐76. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Marronie A. Hyperbaric oxygen and in‐water rehabilitation in complete stroke. Journal of Hyperbaric Medicine 1988;3(1):15‐27. [Google Scholar]
- Revnic CRS, Teleki NG, Revnic FG. Rehabilitation therapy in hemiplegic patients post‐stroke. European Journal of Neurology 2004;11 Suppl 2:150 (Abst P1439). [Google Scholar]
- Wang Y‐H, Dai R, Dong M‐T, Lin L. Effects of seawater physical exercise on cerebral and cardiac hemodynamics in patients with cerebral infarction. Chinese Journal of Clinical Rehabilitation 2004;8(16):3006‐7. [Google Scholar]
References to studies awaiting assessment
- Lee SG, Lee SY, Im HL, Kim JH. Effect of hydrotherapy on the functional status in ischemic stroke patients. Neurorehabilitation and Neural Repair 2006;20(1):171 (Abst P2‐074). [Google Scholar]
- Xu W, Zhang L, Fan J. The comparison of gait rehabilitation in patients with hemiplegia by walking in water and the pneu‐weight walking therapies. Journal of Rehabilitation Medicine 2008;73 Suppl 46:Abst PP001‐055. [Google Scholar]
References to ongoing studies
- Gunn S. The effectiveness of higher intensity water‐ and gym‐based exercise programs on gait speed: a randomised controlled trial following stroke (pilot). Australian New Zealand Clinical Trials Registry (ANZCTR) http://www.anzctr.org.au/2007.
Additional references
- Anderson CS, Linto J, Stewart‐Wynne EG. A population‐based assessment of the impact and burden of caregiving for long‐term stroke survivors. Stroke 1995;26:843–9. [DOI] [PubMed] [Google Scholar]
- Barker RN, Brauer SG. Upper limb recovery after stroke: the stroke survivors' perspective. Disability and Rehabilitation 2005;30(20):1213‐23. [DOI] [PubMed] [Google Scholar]
- Berg K, Wood‐Dauphinee S, Williams J, Maki B. Measuring balance in the elderly: validation of an instrument. Canadian Journal of Public Health1992; Vol. 83 Suppl 2:S7‐11. [PubMed]
- Birk TJ. Poliomyelitis and the post‐polio syndrome: exercise capacities and adaptation‐‐current research, future directions, and widespread applicability. Medicine and Science in Sports and Exercise 1993;4:466‐72. [PubMed] [Google Scholar]
- Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke1988; Vol. 19, issue 12:1497‐500. [DOI] [PubMed]
- Carr JH, Shepherd RB, Nordholm L, Lynne D. Investigation of a new motor assessment scale for stroke patients. Physical Therapy 1985;65(2):175‐80. [DOI] [PubMed] [Google Scholar]
- Ciconelli RM, Ferraz MB, Santos W, Meinão I, Quaresma MR. Brazilian‐Portuguese version of the SF‐36. A reliable and valid quality of life outcome measure [Tradução para a língua portuguesa e validação do questionário genérico deavaliação de qualidade de vida SF‐36 (Brasil SF‐36)]. Revista Brasileira de Reumatologia 1999;39:143‐50. [Google Scholar]
- Collen FM, Wade DT, Robb GF, Bradshaw CM. The Rivermead Mobility Index: a further development of the Rivermead Motor Assessment. International Disability Studies 1991;13(2):50‐4. [DOI] [PubMed] [Google Scholar]
- Danneskiold‐Samsøe B, Lyngberg K, Risum T, Telling M. The effect of water exercise therapy given to patients with rheumatoid arthritis. Scandinavian Journal of Rehabilitation Medicine 1987;19:31‐5. [PubMed] [Google Scholar]
- Demeurisse G, Demol O, Robaye E. Motor evaluation in vascular hemiplegia. European Neurology 1980;19(6):382‐9. [DOI] [PubMed] [Google Scholar]
- Hydrotherapy Association of Chartered Physiotherapists, HACP. Guidance on good practice in hydrotherapy. www.csp.org.uk accessed 14 July 2009.
- Hamilton BB, Laughlin JA, Fiedler RC, Granger CV. Interrater reliability of the 7‐level functional independence measure (FIM). Scandinavian Journal of Rehabilitation Medicine 1994;26(3):115‐9. [PubMed] [Google Scholar]
- Herbert R, Moseley A, Sherrington C. PEDro: a database of randomised controlled trials in physiotherapy. Health Information Management: Journal of the Health Information Management Association of Australia 1998;28(4):186‐8. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
- Holden MK, Gill KM, Magliozzi MR, Nathan J, Piehl‐Baker L. Clinical gait assessment in the neurologically impaired. Reliability and meaningfulness. Physical Therapy 1984;64(1):35‐40. [DOI] [PubMed] [Google Scholar]
- Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist1970; Vol. 10, issue 1:20‐30. [DOI] [PubMed]
- Kolominsky‐Rabas PL, Sarti C, Heuschmann PU, Graf C, Siemonsen S, Neundoerfer B, et al. A prospective community‐based study of stroke in Germany ‐ the Erlangen Stroke Project (ESPro). Stroke 1998;29(12):2501‐6. [DOI] [PubMed] [Google Scholar]
- Lopez A, Mathers C, Ezzati M, Jamison D, Murray C. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006;367:1747–57. [DOI] [PubMed] [Google Scholar]
- Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Physical Therapy 2003;83(8):713‐21. [PubMed] [Google Scholar]
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Maryland State Medical Journal1965; Vol. 14:61‐5. [PubMed]
- Mehrholz J, Werner C, Kugler J, Pohl M. Electromechanical‐assisted training for walking after stroke. Cochrane Database of Systematic Reviews 2006, Issue 4. [Art. No.: CD006185. DOI: 10.1002/14651858.CD006185] [DOI] [PubMed] [Google Scholar]
- Mehrholz J, Kugler J, Pohl M. Water‐based exercise for reducing disability after stroke. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD008186] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EL, Murray L, Richards L, Zorowitz RD, Bakas T, Clark P, the American Heart Association Council on Cardiovascular Nursing and the Stroke Council. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient. A scientific statement from the American Heart Association. Stroke 2010;2 September [Epub ahead of print]:online first. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Jørgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Archives of Physical Medicine and Rehabilitation 1994;75(4):394‐8. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version Version 5.0 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
- Saunders DH, Greig CA, Young A, Mead GE. Physical fitness training for stroke patients. Cochrane Database of Systematic Reviews 2004, Issue 1. [Art. No.: CD003316 DOI: 10.1002/14651858.CD003316] [DOI] [PubMed] [Google Scholar]
- Schencking M, Otto A, Deutsch T, Sandholzer H. A comparison of Kneipp hydrotherapy with conventional physiotherapy in the treatment of osteoarthritis of the hip or knee: protocol of a prospective randomised controlled clinical trial. BMC Musculoskeletal Disorders 2009;10:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taunton JE, Rhodes EC, Wolski LA, Donelli M, Warren J, Elliot J, et al. Effect of land‐based and water‐based fitness programs on the cardiovascular fitness, strength and flexibility of women aged 65‐75 years. Gerontology 1996;42(4):204‐10. [DOI] [PubMed] [Google Scholar]
- Tsourlou T, Benik A, Dipla K, Zafeiridis A, Kellis S. The effects of a twenty‐four‐week aquatic training program on muscular strength performance in healthy elderly women. Journal of Strength Condition Research 2006;20(4):811‐8. [DOI] [PubMed] [Google Scholar]
- Bennekom CA, Jelles F, Lankhorst GJ, Bouter LM. The Rehabilitation Activities Profile: a validation study of its use as a disability index with stroke patients. Archives of Physical Medicine and Rehabilitation1995; Vol. 76, issue 6:501‐7. [DOI] [PubMed]
- Wade DT, Collin C. The Barthel ADL Index: a standard measure of physical disability?. International Disability Studies 1988;10(2):64‐7. [DOI] [PubMed] [Google Scholar]
- Whiting S, Lincoln N. An ADL assessment for stroke patients. British Journal of Occupational Therapy1980; Vol. 43:44‐6.
- World Health Organization. Cerebrovascular accident, stroke. http://www.who.int/topics/cerebrovascular_accident/en/.


