Abstract
Background
A number of conditions compromise the passage of food along the digestive tract. Nasogastric tube (NGT) feeding is a classic, time‐proven technique, although its prolonged use can lead to complications such as lesions to the nasal wing, chronic sinusitis, gastro‐oesophageal reflux, and aspiration pneumonia. Another method of infusion, percutaneous endoscopy gastrostomy (PEG), is generally used when there is a need for enteral nutrition for a longer time period. There is a high demand for PEG in patients with swallowing disorders, although there is no consistent evidence about its effectiveness and safety as compared to NGT.
Objectives
To evaluate the effectiveness and safety of PEG compared with NGT for adults with swallowing disturbances.
Search methods
We searched The Cochrane Library, MEDLINE, EMBASE, and LILACS from inception to January 2014, and contacted the main authors in the subject area. There was no language restriction in the search.
Selection criteria
We planned to include randomised controlled trials comparing PEG versus NGT for adults with swallowing disturbances or dysphagia and indications for nutritional support, with any underlying diseases. The primary outcome was intervention failure (e.g. feeding interruption, blocking or leakage of the tube, no adherence to treatment).
Data collection and analysis
We used standard methodological procedures expected by The Cochrane Collaboration. For dichotomous and continuous variables, we used risk ratio (RR) and mean difference (MD), respectively with the random‐effects statistical model and 95% confidence interval (CI). We assumed statistical heterogeneity when I² > 50%.
Main results
We included 11 randomised controlled studies with 735 participants which produced 16 meta‐analyses of outcome data. Meta‐analysis indicated that the primary outcome of intervention failure, occurred in lower proportion of participants with PEG compared to NGT (RR 0.18, 95% CI 0.05 to 0.59, eight studies, 408 participants, low quality evidence) and this difference was statistically significant. For this outcome, we also subgrouped the studies by endoscopic gastrostomy technique into pull, and push and not reported. We observed a significant difference favouring PEG in the pull subgroup (RR 0.07, 95% CI 0.01 to 0.35, three studies, 90 participants). Thepush subgroup contained only one clinical trial and the result favoured PEG (RR 0.05, 95% CI 0.00 to 0.74, one study, 33 participants) techniques. We found no statistically significant difference in cases where the technique was not reported (RR 0.43, 95% CI 0.13 to 1.44, four studies, 285 participants).
There was no statistically significant difference between the groups for meta‐analyses of the secondary outcomes of mortality (RR 0.86, 95% CI 0.58 to 1.28, 644 participants, nine studies, very low quality evidence), overall reports of any adverse event at any follow‐up time point (ITT analysis, RR 0.83, 95% CI 0.51 to 1.34), 597 participants, 6 studies, moderate quality evidence), specific adverse events including pneumonia (aspiration) (RR 0.70, 95% CI 0.46 to 1.06, 645 participants, seven studies, low quality evidence), or for the meta‐ analyses of the secondary outcome of nutritional status including weight change from baseline, and mid‐arm circumference at endpoint, although there was evidence in favour of PEG for meta‐analyses of mid‐arm circumference change from baseline (MD 1.16, 95% CI 1.01 to 1.31, 115 participants, two studies), and levels of serum albumin were higher in the PEG group (MD 6.03, 95% CI 2.31 to 9.74, 107 participants).
For meta‐analyses of the secondary outcomes of time on enteral nutrition, there was no statistically significant difference (MD 14.48, 95% CI ‐2.74 to 31.71; 119 participants, two studies). For meta‐analyses of quality of life measures (EuroQol) outcomes in two studies with 133 participants, for inconvenience (RR 0.03, 95% CI 0.00 to 0.29), discomfort (RR 0.03, 95% CI 0.00 to 0.29), altered body image (RR 0.01, 95% CI 0.00 to 0.18; P = 0.001) and social activities (RR 0.01, 95% CI 0.00 to 0.18) the intervention favoured PEG, that is, fewer participants found the intervention of PEG to be inconvenient, uncomfortable or interfered with social activities. However, there were no significant differences between the groups for pain, ease of learning to use, or the secondary outcome of length of hospital stay (two studies, 381 participants).
Authors' conclusions
PEG was associated with a lower probability of intervention failure, suggesting the endoscopic procedure may be more effective and safe compared with NGT. There is no significant difference in mortality rates between comparison groups, or in adverse events, including pneumonia related to aspiration. Future studies should include details of participant demographics including underlying disease, age and gender, and the gastrostomy technique.
Plain language summary
Nutritional support for adults with swallowing difficulties
Background
A number of conditions compromise the transport of food along the digestive tract. Patients with swallowing disturbances can develop low nutritional status, which affects their recovery from illness, surgery, and injury. Conditions associated with swallowing disorders include stroke, neurological diseases, dementia, cancers of the head and neck, amyotrophic lateral sclerosis, physical obstruction, and dysphagia from stroke. Nasogastric tube feeding is a time proven technique to provide nutritional support; the tube can be inserted by a nurse. Percutaneous endoscopy gastrostomy (PEG) involves a feeding tube inserted directly into the stomach through the abdomen and is particularly useful when enteral nutrition is needed for a length of time.
Review question
Prolonged use of a nasal tube can lead to adverse events such as damage to the nose and larynx, chronic sinusitis, gastro‐oesophageal reflux, and aspiration pneumonia (which can result from inhalation of stomach contents leading to lower respiratory tract infection and pneumonia).
Study characteristics
We obtained updated evidence for this review from 11 randomised controlled studies comparing a nasogastric tube with PEG in a total of 735 patients. Seven studies measured treatment failure i.e. feeding interruption, blocking or leakage of the feeding tube in 408 patients randomised to either a nasal gastric tube or PEG.
Key results
The studies showed a higher probability of treatment failure with a nasal gastric tube. The number of deaths was no different with the two methods; nor was the overall occurrence of adverse events. Participants with PEGs may have a better quality of life.
Quality of the evidence
Possible limitations of this review include the small number of participants in the majority of studies, explained by the high cost of PEG and requirements for endoscopy in its use, the operational challenges to accomplish a clinical trial in this area and the different length of follow‐up of the patients in the studies (from less than four weeks to six months). There were clinical differences between the trials, with the participants having different baseline diseases and different techniques used to insert the PEG. The findings of the present review of the literature should be interpreted with caution, given that there were methodological issues with most of the included studies which increase the risk of bias in the trial. This systematic review of the literature is valuable in analysing 11 studies, with a sample size of 735 patients. Nevertheless, further randomised clinical trials that adopt a rigorous method are warranted.
Summary of findings
Summary of findings for the main comparison. Percutaneous endoscopic gastrostomy compared with nasogastric tube feeding for adults with swallowing disturbances.
| Percutaneous endoscopic gastrostomy compared with nasogastric tube feeding for adults with swallowing disturbances | ||||||
| Patient or population: adult patients with swallowing disturbances Settings: in‐patient Intervention: percutaneous endoscopic gastrostomy Comparison: nasogastric tube feeding | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Nasogastric tube feeding | Percutaneous endoscopic gastrostomy | |||||
| Treatment failure Feeding interruption, blocking or leakage of the tube, non‐adherence Follow‐up: 0 to 6 months | Study population | RR 0.18 (0.05 to 0.59) | 408 (8 studies) | ⊕⊕⊝⊝ low1,3 | The subgroup of stroke/neurological diseases was associated with a lower risk of intervention failure compared with the subgroup composed of mixed diseases. Favours PEG |
|
| 391 per 1000 | 70 per 1000 (20 to 231) | |||||
| Low | ||||||
| 375 per 1000 | 30 per 1000 (7 to 124) | |||||
| High | ||||||
| 319 per 1000 | 102 per 1000 (26 to 421) | |||||
| Mortality irrespective of follow‐up time Follow‐up: 0 to 6 months | 366 per 1000 | 315 per 1000 (212 to 469) | RR 0.86 (0.58 to 1.28) | 644 (9 studies) | ⊕⊝⊝⊝ very low1,2,3 | Favours neither PEG nor NGT. |
| Pneumonia irrespective of follow‐up time Follow‐up: 0 to 6 months | 415 per 1000 | 291 per 1000 (24 to 45) | RR 0.7 (0.46 to 1.06) | 645 (7 studies) | ⊕⊕⊝⊝ low1,3 | Favours neither PEG nor NGT. |
| Adverse events irrespective of follow‐up time Follow‐up: 0‐17 months | 458 per 1000 | 380 per 1000 (234 to 614) | RR 0.83 (0.51 to 1.34) | 597 (6 studies) | ⊕⊕⊕⊝ moderate1,3 | Favours neither PEG nor NGT. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Design limitation (risk of bias), unclear sequence generation, allocation concealment and loss to follow‐up. 2 Relatively few participants and few events and/or wide confidence intervals 3 Widely differing estimates of the treatment effect (i.e. heterogeneity or variability in results) across studies
Background
A number of conditions compromise the passage of food along the digestive tract. Disturbances may be due to blockage, as seen in stenosis and cancer of the stomach or larynx, or due to swallowing difficulties such as in genetic diseases, stroke sequelae, cranial encephalic trauma, brain tumours, and amyotrophic lateral sclerosis (Heemskerk 2014; Löser 2005; Piecuch 2013; Schneider 2014). Several approaches are available to provide nutritional support (Nugent 2013). Nasogastric tube (NGT) feeding is a classic, time‐proven technique, although its prolonged use can lead to adverse events such as lesions to the nasal wing, chronic sinusitis, gastro‐oesophageal reflux, and aspiration pneumonia (Bastow 1986; Beavan 2010). Two meta‐analyses comparing tube placement into the stomach or duodenum revealed no significant difference between the methods in terms of length of hospital stay, mortality, or adverse events (Ho 2006; Marik 2003). In addition to adverse events, the need to change the tube due to blockage inherent to its narrow gauge coupled with its disagreeable appearance in social settings have led to the election of alternative techniques whenever possible (Zaherah 2012).
Gastrostomy has been used to gain access to the stomach for long‐term enteral feeding in patients with swallowing limitations who require nutritional support. The main criteria for indicating gastrostomy are (i) a reasonable prospect of patient survival and (ii) normal intestinal function (Friginal‐Ruiz 2011). This surgical procedure was first carried out successfully in humans in 1876, by Verneuil in France. Following various modifications, Stamm devised the technique most frequently used to this day (Ljungdahl 2006). In 1980, Gauderer et al described a new technique of feeding tube placement in gastrostomy using endoscopy, called percutaneous endoscopic gastrostomy (PEG). This involves a local anaesthetic and does not require laparotomy (Gauderer 1980). Since the introduction of PEG, a number of studies comparing methods of gastrostomy have been conducted, such as operative, push and pull PEG techniques (Köhler 2014; Stiegmann 1990; Tucker 2003).
Previous systematic reviews and meta‐analyses on enteral nutrition approaches have been performed, but not with the broad scope we propose. Langmore 2006 published a meta‐analysis that investigated enteral nutrition, specifically in amyotrophic lateral sclerosis, comparing the use of several types of feeding tubes in patients being fed orally. However, they did not find any controlled or randomised studies. Another meta‐analysis compared nutrition by endoscopic gastrostomy and NGT including only post‐stroke patients (Bath 1999). Thereafter, a number of controlled and randomised studies were published that compared the two methods of nutritional support in stroke patients and those admitted to intensive care units with a range of different pathologies, as well as individuals on mechanical ventilation (Dennis 2005; Douzinas 2006; Hamidon 2006; McClave 2005).
Assessment of these latest studies in patients with a range of pathologies, together with analysis of the optimal moment to commence nutritional support, warrant mapping by means of a systematic review so as to offer the best evidence available on which to base decisions.
Description of the condition
Malnutrition encompasses overnutrition and undernutrition, but undernutrition is a prevalent, and undesired condition affecting up to 40% of hospitalised patients (Barker 2011). This condition has important causal associations with morbidity and mortality, by affecting, for example, length of stay in hospital; recovery from illness, surgery and injury; cardiac function, weak muscles (including respiratory muscles), with consequent higher risk of thromboembolism, chest infection, and pressure sores (Geeganage 2012; Iwamoto 2014; Löser 2010; Pearce 2002; Valente da Silva 2012). Mortality rates tend to be higher in elderly and undernourished patients in comparison to other subgroups of hospitalised patients (Ordoñez 2013; Valente da Silva 2012). In this sense, swallowing disturbances are of special interest, because of its direct relationship with undernutrition (Poisson 2014).
The clinical diagnosis of swallowing disturbances can be given based on clinical signals such as delay in swallowing, pharyngeal sensibility, abnormality or absence of tongue movements; loosening of water from lips, pocketing of food in the cheek, under the tongue or on the hard palate, coughing or choking while eating or signs of penetration or aspiration (Falsetti 2009; Simons 2014). Although not usually used in daily practice, radiological tests like videofluoroscopic modified barium swallow and videofluoroscopic swallowing study can be used for diagnosis of dysphagia (Finestone 2003; Scheeren 2014; Stec 2008).
Patients with indications for enteral nutrition (nutrients intake by means of feeding tubes) include those with conditions associated with swallowing disorders, such as motor neuron disease and multiple sclerosis; physical obstruction to swallowing, such as oesophageal tumours; an inability to ingest food due to head injury or stroke; and those with anorexia due to an underlying disease such as chronic lung disease, irritable bowel disease, or cancer (Botella Romero 2012; de Aguilar‐Nascimento 2011; Fini 2014; Kolaček 2013; Manba 2014). Dysphagic patients and those with anorexia, malabsorption, or excessive catabolism also may need long‐term enteral feeding (Le 2010; Gentile 2012; Pearce 2002). Aspiration risk often is an indication for nutritional support using tubes (Corry 2008; Metheny 2010). Enteral nutrition can be provided in the form of drink supplements or, if a patient is unable to take adequate nutritional supplements orally, fed via an enteral tube into the stomach or small bowel (Granell Vidal 2014; Löser 2005).
Description of the intervention
In general, tube systems for artificial enteral nutrition can be positioned by nasal insertion, guided percutaneous application, or surgical techniques (Abdel‐Lah Mohamed 2006; Blumenstein 2014; Gopalan 2003; Schröder 2004). The superiority of percutaneously placed gastrostomies compared with the former surgical gastrostomy procedures (that is, Witzel, Stamm, Janeway techniques) has been clearly suggested (Löser 2005; Ljungdahl 2006). Lower complication rates, reduced hospital length of stay and costs have been reported (Grant 1988; Ljungdahl 2006). Most patients who require nutritional support need it for around one month or less, with the nasogastric sound probe being the main way of infusion (Blumenstein 2014; Pearce 2002). The probe used is made of thin polyurethane, size 14 with an internal diameter of 3.3 mm, and is inserted by a trained professional in order to prevent adverse events such as perforation and tracheobronchial location (Hamidon 2006; Löser 2005). Another method of infusion, percutaneous endoscopy gastrostomy (PEG), is generally used when there is a need for enteral nutrition for a longer time period (Löser 2005; Pearce 2002). This procedure can be done by either 'pull' or 'push' techniques, the former being simpler and more frequently used. Both techniques use a silicon probe (for example 24 Fr, internal diameter 5.5 mm). The puncture site is marked with gastroscopic monitoring of the anterior gastric wall in the region of the distal corpus, after adequate local anaesthesia and intravenous sedation (Hamidon 2006; Löser 2005). Prospective studies have shown that the early insertion of the probe via PEG improves the patient’s nutritional state (Hamidon 2006; Norton 1996). Patients treated for head and neck carcinoma have considered PEG to be more acceptable than a NGT, even though persistent dysphagia was associated with PEG (Mekhail 2001). A cohort study verified the acceptability of PEG, with significantly higher survival time and lower aspiration rates (Dwolatzky 2001) compared to NGT. On the other hand, a narrative review (Plonk 2005) reported increased risk of death in stroke patients with PEG compared with NGT and concluded that aspiration pneumonia rates were similar. Published guidelines on enteral nutrition recommend the performing of gastrostomy, preferably endoscopically (Löser 2005).
Radiologically placed gastrostomy (RIG) is another method of enteral nutrition, but operationally different from PEG. RIG is not an endoscopic procedure and utilises fluoroscopy, performed in an interventional radiologic suite (Barkmeier 1998; Chiò 2004).
How the intervention might work
The percutaneous gastronomy probe is of a larger calibre compared with an NGT and is placed in the abdomen. This leads to less interruption of nutrition caused by the probe being withdrawn as well as reduced reflux with consequent aspiration, thus being less embarrassing for the patient (Dwolatzky 2001; Pearce 2002). Patients and carers believe that nutrition via PEG helps in feeding and the ability to cope, being more convenient than NGT (Anis 2006). PEG‐related morbidity and mortality are 9.4% and 0.53%, respectively (Wollman 1995). There are, however, exclusive adverse events for endoscopy percutaneous gastrostomy, such as peritonitis, buried bumper syndrome, gastrocolocutaneous fistula, and wound infection (Potack 2008). Adverse events associated with NGT due to its nasogastric insertion and positioning are also cited, including sinusitis, laryngeal ulcerations, pneumothorax, and tracheoesophagic fistula; the latter due to incorrect positioning of the tube (Pearce 2002).
Why it is important to do this review
According to Potack 2008, there is a high demand for PEG in patients with swallowing disorders, with 160,000 to 200,000 PEG procedures performed per year in the USA. This makes PEG the procedure of choice for nutritional support in adults. The same author commented that many such procedures are performed, although there is no consistent evidence about what is the more effective and safe method. Because NGT and PEG are the most commonly used methods for feeding access (Pearce 2002), a systematic review is worth performing to resolve such questions.
Objectives
To evaluate the effectiveness and safety of percutaneous endoscopic gastrostomy (PEG) as compared to a nasogastric tube (NGT) for adults with swallowing disturbances, by updating our previous Cochrane review (Other published versions of this review), assessing the included studies with the revised 'Risk of bias' assessments, and to assess the overall level of evidence using the GRADE approach.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials comparing percutaneous endoscopic gastrostomy (PEG) versus nasogastric tube (NGT) for nutrition in adults with swallowing disturbances.
Types of participants
Adult patients presenting with swallowing disturbances or dysphagia and indications for nutritional support, as identified by the authors of primary studies. Patients with any underlying diseases were also acceptable.
Types of interventions
The comparison arms of interest are as follows.
Intervention group: PEG performed by any method (e.g., pull and push methods, others).
Control group: NGT irrespective of technique (e.g., conventional and looping).
We did not include studies with radiologically inserted gastrostomy (PRG), nasojejunal tubes, and jejunal tube percutaneous endoscopy gastrostomy (JET‐PEG) in this review.
Types of outcome measures
Primary outcomes
Intervention failures as defined by any event leading to failure to introduce the tube, recurrent displacement and treatment interruption (feeding interruption, blocking or leakage of the tube, no adherence to treatment) (based on Norton 1996).
Secondary outcomes
Nutritional status, as measured by any validated instrument (such as upper‐arm skin fold thickness, mid‐arm circumference, body weight, serum albumin level, haemoglobin (Ramel 2008)).
Mortality.
Adverse events (e.g., aspiration, haemorrhage, pneumonia, wound infection, sinusitis, fistula).
Time on enteral nutrition.
Quality of life, as measured by any validated instrument (such as EUROQoL, SF‐36 (Dorman 1997)).
Length of hospital stay.
Costs and economic issues.
Search methods for identification of studies
Electronic searches
We performed a computerised literature search in, re‐running searches from the previous search date (August 2009). We carried out updated searches in September 2011 and in January 2014.
The Cochrane Central Register of Controlled Trials (CENTRAL, 2013, Issue 12) and other databases in The Cochrane Library (Appendix 1),
Ovid MEDLINE(R) Daily Update January 31, 2014, Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) 1946 to Present Appendix 2.
EMBASE via OVID (Embase 1980 to 2014 Week 05) Appendix 3.
LILACS via BIREME (from inception to January 2014) Appendix 4.
Search terms and their synonyms for clinical conditions of interest to us (swallowing disturbance or dysphagia) and interventions of interest (percutaneous endoscopic gastrostomy and nasogastric tube feeding) are given in the appendices. They were adapted for each of the databases. There was no language restriction in the search. Search filters to identify randomised controlled trials involving humans were used when appropriate.
Searching other resources
We compiled a reference list of relevant studies (irrespective of study design) to identify trials with the potential for inclusion. We contacted authors via email requesting the data from unpublished trials. We also tried to identify ongoing trials on the Current Controlled Trials Web site (www.currentcontrolledtrials.gov).
Data collection and analysis
Selection of studies
Two review authors (CG, RA) checked the titles and abstracts found by the search strategy and other sources researched. Whenever titles or abstracts seemed relevant to the review, we analysed them by reading the full article. If they were truly randomised controlled trials that met the previously stated criteria, we included them in the review. If there remained any doubt or disagreement, all of the authors assessed the study in question.
Data extraction and management
Two review authors (CG, DRW) extracted data based on CONSORT (Moher 2001). For the update in 2014, CB with CG and DRW extracted data from new included studies. We settled doubts by consensus of the authors.
Assessment of risk of bias in included studies
Two review authors (CG, RBA, with CB) independently assessed the methodological quality of included studies using the following items (Higgins 2011).
Random sequence generation (selection bias) . Biased allocation to interventions due to inadequate generation of a randomised sequence.
Allocation concealment (selection bias). Biased allocation to interventions due to inadequate concealment of allocations prior to assignment.
Blinding (performance bias and detection bias). Performance bias or detection bias due to knowledge of the allocated interventions after assignment.
Blinding of participants and personnel (performance bias). Performance bias due to knowledge of the allocated interventions by participants and personnel during the study.
Blinding of outcome assessment (detection bias). Detection bias due to knowledge of the allocated interventions by outcome assessors.
Incomplete outcome data (attrition bias). Attrition bias due to amount, nature or handling of incomplete outcome data.
Selective reporting (reporting bias). Reporting bias due to selective outcome reporting.
Other bias that is bias due to problems not covered elsewhere in the table.
For the above biases, we classified studies according to their risk of systematic error.
High risk: when the appropriate method to avoid systematic error was not met.
Unclear risk: when the appropriate method to avoid systematic error was not described or the information was not acquired by contacting the authors of primary studies.
Low risk: when the appropriate method to avoid systematic error was met.
We did not use performance bias as a criterion to analyse the risk of systematic error since this was not compatible with the characteristics of the intervention.
Measures of treatment effect
For dichotomous and continuous variables, we calculated risk ratio (RR), mean difference (MD), and 95% confidence intervals (CIs). When data from primary studies were not parametric (for example, effects were reported as medians, quartiles) or without sufficient statistical information (such as standard deviations, number of patients), we inserted them into Table 2 if authors did not provide the necessary information.
1. Continuous data unsuitable for inclusion in meta‐analyses.
| Outcome | PEG | NGT | P value | Mean difference (95% CI) | ||
| n | n | |||||
| mean albumin (at 3 months) (Yata 2001 abstract) | 3.6 | 42 | 3.2 | 40 | < 0.01 | |
| mean albumin (at 6 months) (Yata 2001 abstract) | 3.9 | 42 | 3.1 | 40 | < 0.01 | |
| mean haemoglobin (at 3 months) (Yata 2001 abstract) | 11.9 | 42 | 11.7 | 40 | no significant difference | |
| mean haemoglobin (at 6 months) (Yata 2001 abstract) | 12.4 | 42 | 11.1 | 40 | no significant difference | |
| median length of stay (days) (Dennis 2005) | 34.0 (IQR 17 to 66) | 162 | 37.0 (IQR 17 to 76) | 159 | not reported | |
| utility mean difference between comparison groups (endpoint) Derived from EuroQol between comparison groups (endpoint) favouring NGT group, no statistically significant difference (Dennis 2005) | 0.12 | 0.035 (‐0.024 to 0.093) | ||||
| median patient overall quality of life at first week (endpoint) (Corry 2008) | 4.0 (R 2.0 to 7.0) | 15 | 4.0 (R 2.0 to 7.0) | 18 | 0.89 | |
| anthropometric parameters (endpoint medians) (Hamidon 2006) | 8 | 10 | ||||
| median TSFT (mm) | 20.1 (R 9.6 to 34) | 12.7 (R 9.8 to 32) | 0.076 | |||
| median BSFT (mm) | 0.3 (R 4.8 to 13) | 7.4 (R 4.4 to 15) | 0.533 | |||
| median MAC (cm) | 31.4 (R 22 to 36) | 27.8 (R 21 to 37) | 0.182 | |||
| median serum albumin (g/L) | 39.5 (R 36 to 44) | 36.0 (R 31 to 45) | 0.045 | |||
| median change in gastro‐oesophageal reflux (%, endpoint) on day 7 (Douzinas 2006) | 2.7 (R 0 to 10.4) | 10.8 (R 6.3 to 36.6) | < 0.01 | |||
| anthropometric parameters (endpoint medians) (6 weeks)Corry 2008 | ||||||
| upper‐arm circumference (mm) at endpoint | 302.5 (R 270 to 370) | 15 | 300.0 (R 240 to 352) | 18 | 0.69 | Mean values stated in text (Page 506) to be 295 vs. 283 mm P = 0.25 |
| median TSFT (mm) | 13 (R 10 to 20) |
15 | 12 (R 10 to 23) |
18 | 0.65 | The NGT patients had significantly lower triceps skin fold thickness (9.5 vs 13.5 mm; P = 0.03) than the PEG patients at 6 weeks post‐treatment. |
BSTF: biceps skin fold thickness CI: confidence interval IQR: interquartile range MAC: mid‐arm circumference R: range TSFT: triceps skin fold thickness
Unit of analysis issues
The unit of analysis was based on the individual patient (unit to be randomised for interventions to be compared). We planned to analyse events happening to a person more than once (for example pneumonia, bronchoaspiration) by using risk ratio, which compares the rate of events in the two groups (PEG and NGT) by dividing one by the other. We planned to analyse cross‐over study designs separately from the parallel‐group randomised controlled trials.
Dealing with missing data
For continuous and dichotomous data, we carried out available case analysis. In this update, for mean values of outcome data with missing standard deviations, we calculated this from the difference between means (Cochrane Handbook for Systematic Reviews of Interventions 7.7.3.3. Higgins 2011). We investigated the effects of making these assumptions by performing sensitivity analyses where appropriate.
Assessment of heterogeneity
We assessed statistical heterogeneity using the I² statistic. We assumed a statistically significant heterogeneity between the estimated effects of included studies with an I² > 50%.
Assessment of reporting biases
We had planned to assess publication bias by preparing a funnel plot, and will do so in future versions of this review if a sufficient number of studies is available. However, we are aware that asymmetry in the funnel plot can be associated with reasons other than that of publication bias (for example, by chance, real heterogeneity, or clinical particulars inherent to each one of the included studies such as patients at high risk for the outcome).
Data synthesis
Qualitative information
We synthesised qualitative information relative to methods, risk of bias, description of participants, and outcomes measures in the Characteristics of included studies table.
Quantitative information
For dichotomous variables, we calculated the risk ratio (RR). For continuous variables, we calculated the mean difference (MD) when studies reported their results through the same variables measured with the same instruments (same units of measure). When continuous data were measured with different instruments (different and non‐interchangeable units of measure), we planned to pool them using the standardised mean difference (SMD). We used 95% CIs for all statistical methods to pool data.
Irrespective of the nature of the data, we used a random‐effects statistical model as we were expecting substantial clinical and methodological heterogeneity, which could generate substantial statistical heterogeneity.
Subgroup analysis and investigation of heterogeneity
We planned to carry out subgroup analyses using different NGT and PEG methods (for example pull, push, nasal loop, conventional). We assumed that heterogeneity between studies in both the direction and magnitude of estimate effect had a suspected causal relationship (the subgroup characteristic and the estimate of effect), and we have considered these in the Discussion section.
Sensitivity analysis
We planned sensitivity analysis to examine the effects of intention‐to‐treat (ITT) analysis and available data analysis for dichotomous data. We planned to carry out ITT analysis by using imputation based on the analysis of the total number of randomised participants, irrespective of how the original study authors analysed the data. We assumed that all missing participants experienced the event. The other factors were study quality, trials reported only in abstracts, and testing for fixed‐effect and random‐effects statistical models.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies for more information.
Results of the search
For details of the process of studies selection, see Figure 1.
1.
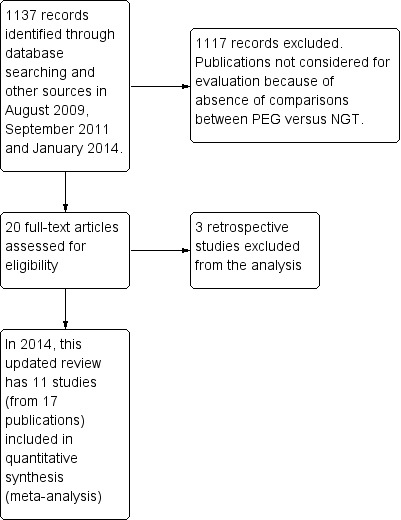
Study flow diagram.
The first literature search (August 2009 to September 2011) yielded 474 hits. From this, 18 papers were retrieved for full text review. Three papers were excluded due to inappropriate study design and intervention. In January 2014, an update search yielded 663 additional records and two additional studies were identified for inclusion in the review.
Included studies
The 11 randomised controlled studies selected were published in English. In many cases the data we required were not available in the published report of the study and we obtained further information from the study investigators (e.g. Bath 2009; Corry 2008b), which were used to estimate the effects of the interventions for clinically relevant outcomes (i.e., treatment failure, mortality, pneumonia, adverse events, and length of hospital stay). Yata 2001 was only available in abstract form, which hampered the gleaning of all the relevant data, and the corresponding author could not be contacted. Data from another study (Bath 1997) came from a systematic review by the same author, and doubts were resolved via email with the corresponding author. Elbadawy 2014 was an unpublished study and we obtained further information by correspondence with the study investigator.
Participants and study design
We sought to compare percutaneous endoscopic gastrostomy (PEG) (n = 373 participants) with nasogastric tube (NGT) (n = 362 participants) placement for enteral feeding in adults (n = 735 total randomised participants).
The sample in Baeten 1992 included patients with different diseases, including neoplasia of the ear, nose, and throat and neurologic and post‐operative diseases. The mean age of these patients was 72 years (range: 62 to 82 years). Park 1992 included only patients with dysphagia secondary to neurologic diseases in their sample. The mean age of these patients in the NGT group was 65 years, whereas the mean age of those in the PEG group was 56 years. Norton 1996 and Bath 1997 included in their sample patients with dysphagia after acute stroke with a mean age of 77 years. Yata 2001 studied patients with dysphagia in several diseases, such as dementia, Parkinson’s disease, and cerebrovascular disease. These patients had a mean age of 75.1 years (range: 50 to 96 years) in the PEG group and 76.5 years (range: 38 to 93 years) in the NGT group. Dennis 2005 included in their sample patients who presented with dysphagia after acute stroke. Their mean age was 76 years (SD = 10 years). Douzinas 2006 assessed patients with different diseases, some of whom presented with recurrent or persistent ventilator‐associated pneumonia. These patients had a median age of 53 years (range: 20 to 82 years) in the PEG group and 58 years (range: 25 to 85 years) in the NGT group. Hamidon 2006 investigated patients with dysphagia after acute stroke with a median age of 65 years (range: 48 to 79 years) in the PEG group and 72 years (range: 54 to 77 years) in the NGT group. Finally, Corry 2008 included in their sample patients with cancer of the head and neck with a median age of 60 years (range: 46 to 80 years). In Sadasivan 2012, participants had advanced stage two or three squamous cell carcinoma of the head and neck and were scheduled either for radical surgery with adjuvant radiotherapy (RT), chemo‐RT, or for concurrent chemo and radiation therapy were included in the study. The age of participants in the study was not reported and we were unable to obtain further data. Elbadawy 2014, included participants with close traumatic severe brain injury in a study to determine whether PEG or NGT resulted in lower rates of ventilator‐assisted pneumonia. The mean age of participants in the study was not reported and we were unable to obtain further data.
Interventions and comparisons
The interventions were PEG, inserted by any method, versus NGT. Further details can be found in the Characteristics of included studies tables.
In Elbadawy 2014, a three‐arm study, NGT plus intubation was compared with PEG plus intubation and PEG plus tracheostomy. For the purposes of this review, we combined the two PEG groups and compared these results with the NGT group.
Outcomes
Follow‐up times varied across the 11 studies analysed. Baeten 1992, Douzinas 2006, Park 1992, and Hamidon 2006 studied patients for no more than four weeks. On the contrary, the follow‐up times of Bath 1997, Dennis 2005, Norton 1996, Yata 2001, and Corry 2008 ranged from three to six months. Elbadawy 2014 and Sadasivan 2012 followed up participants at one week, six weeks and six months.
The included studies reported our review outcomes as follows:
Our primary outcome, intervention failure, was reported in eight studies (Baeten 1992; Bath 1997; Corry 2008; Hamidon 2006; Norton 1996; Park 1992; Sadasivan 2012; Yata 2001). Elbadawy 2014 reported the number of adverse events in each group; we requested further information, but the study investigators were not able to provide the number of patients with the primary review outcome of intervention failures (e.g., feeding interruption, blocking or leakage of the tube, no adherence to treatment). Participant non‐adherence to treatment was reported in Sadasivan 2012,
Mortality was reported in nine studies (Baeten 1992; Bath 1997; Corry 2008; Dennis 2005; Douzinas 2006; Elbadawy 2014; Hamidon 2006; Norton 1996; Park 1992).
Adverse effects were reported in seven studies (Baeten 1992; Corry 2008; Dennis 2005; Douzinas 2006; Elbadawy 2014; Norton 1996; Sadasivan 2012). Pneumonia, the result of aspirating food into the airway, was reported in seven studies (Baeten 1992; Corry 2008; Dennis 2005; Douzinas 2006; Elbadawy 2014; Norton 1996; Yata 2001). Reflux oesophagitis was reported in Yata 2001.
Two studies additionally reported measures related to the nutritional status of the participants: weight gain (Norton 1996; Sadasivan 2012), mid‐arm circumference (Norton 1996; Sadasivan 2012), serum albumin levels (Norton 1996), and haemoglobin levels (Sadasivan 2012).
The length of hospital stay was reported in two studies (Dennis 2005; Elbadawy 2014); and the time of entry nutrition in days was reported in Baeten 1992 and Park 1992.
Other outcome measures included quality‐of‐life measures using the EORTC QLQ‐H&N35 scale in Corry 2008 and Sadasivan 2012. Scores of patient satisfaction and inconvenience of maintaining PEG or NGT by nursing staff were reported in Baeten 1992; it is unclear if these were validated scales. Participant functional ability (modified Rankin scale (MRS)), an indicator of quality of life, was reported in Dennis 2005.
The mean survival time in months was reported in Yata 2001.
Excluded studies
The three excluded studies did not meet the aforementioned inclusion criteria. McClave 2005 conducted a randomised controlled trial without interventions of interest for this review; Mekhail 2001 and Schulz 2009 performed retrospective studies. McClave 2008 was excluded following contact with the corresponding author to clarify the randomisation process employed.
Risk of bias in included studies
2.
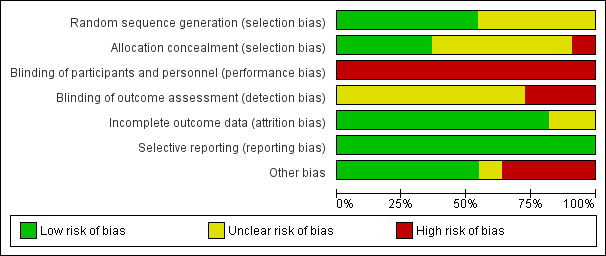
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
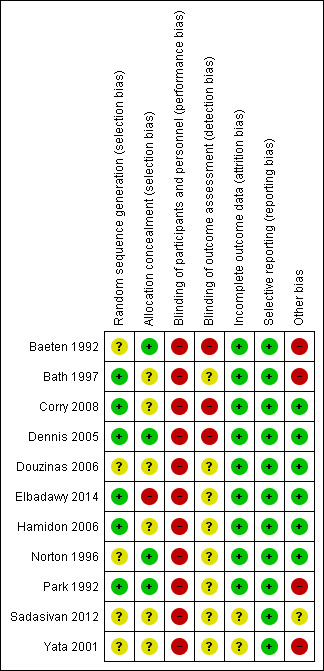
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The methods employed for allocation by Bath 1997; Corry 2008; Dennis 2005; Elbadawy 2014; Hamidon 2006; Park 1992 were suitable for this procedure; therefore, they were deemed low risk for systemic errors of a methodological nature. The remaining studies in this review (i.e., Baeten 1992; Douzinas 2006; Norton 1996; Sadasivan 2012; Yata 2001) were considered to be unclear for risk of bias because the methods used for allocation were not reported.
The methods used for allocation by Dennis 2005; Baeten 1992; Park 1992; and Norton 1996 were sufficiently sound to ensure concealment of the allocation process. Consequently, they were deemed low risk for systematic errors of a methodological nature. On the contrary, the studies by Bath 1997; Corry 2008; Douzinas 2006; Hamidon 2006; Sadasivan 2012; Yata 2001 were considered to be unclear for risk of bias. Although the authors described random allocation, they did not report the methods used for allocation concealment. No attempt was made to conceal allocation in Elbadawy 2014.
Overall, no unusually large differences were noted in the demographic characteristics of patients from each group on study entry, except in Sadasivan 2012, where there were more participants in the PEG group who had radical surgery and adjuvant radio or chemotherapy, and more participants in the NGT group had concurrent chemo or radio therapy. Participants in the NGT group weighed more at the start of the trial.
Blinding
The characteristics of the interventions compared in this systematic review prevented the patients and physicians from being blinded to the interventions. Eight studies made no mention of blinding data assessors (Bath 1997; Douzinas 2006; Elbadawy 2014; Hamidon 2006; Norton 1996; Park 1992; Sadasivan 2012; Yata 2001). Three studies were considered as of high risk of detection bias, because their authors explicitly described either the absence of (Baeten 1992; Corry 2008), or flawed method of blinding data assessors (Dennis 2005).
Incomplete outcome data
Nine studies clearly reported both missing data and the flow of the patients during the study. As a result, they were considered low risk for systematic errors in follow‐up losses. However, Yata 2001 and Sadasivan 2012 did not report losses or patient flow in their work; therefore, the study was considered to be unclear for risk of bias for this domain.
In Park 1992, 18 of the 19 patients in the NGT group presented intervention failure. The researchers did not follow these patients for the full 28 days. In contrast, all 19 patients from the PEG group completed the recommended follow‐up period. Despite the significant number of failures in the NGT group, this clinical trial was considered low risk for systematic error for dichotomous variables because the authors clearly described the flow of patients from randomisation through to the study endpoint.
Selective reporting
All of the studies were associated with a low risk of bias, given that relevant outcomes were reported in all cases.
Other potential sources of bias
The following studies were rated as having a high risk of bias: Baeten 1992 (follow‐up not previously established), Bath 1997 and Yata 2001 (unpublished studies), Park 1992 (dropout rate of 95% (19/20) in the NGT group due to treatment failure and death).
Effects of interventions
See: Table 1
Comparison 1: percutaneous endoscopic gastrostomy versus nasogastric tube
Primary outcomes
Intervention failure
The outcome of intervention failure (e.g., feeding interruption, blocking or leakage of the tube, no adherence to treatment) was reported in eight studies comprising 408 participants (Baeten 1992; Bath 1997; Corry 2008; Hamidon 2006; Norton 1996; Park 1992; Sadasivan 2012; Yata 2001). We were unable to obtain data on overall intervention failure rates in each group from Elbadawy 2014.
Failure occurred in 9.22% (19 out of 206 participants) in the PEG group and 39.11% (79 out of 202 participants) in the NGT group. A meta‐analysis of these eight studies using the random‐effects model favoured the PEG group, that is, fewer participants in the PEG group experienced an intervention failure (risk ratio (RR) 0.18, 95% confidence interval (CI) 0.05 to 0.59, P = 0.005; Analysis 1.1) (Mantel‐Haenszel’s statistical method). We found significant statistical heterogeneity in this analysis; I2 = 73%.
1.1. Analysis.
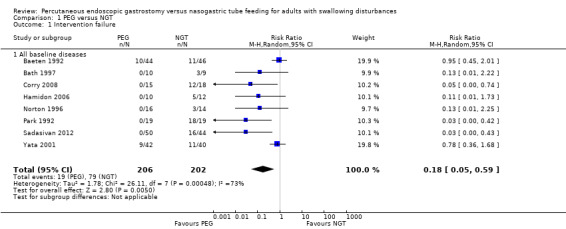
Comparison 1 PEG versus NGT, Outcome 1 Intervention failure.
Non‐adherence to treatment
Non‐adherence to treatment at six weeks was reported in only one study, Sadasivan 2012 and was not statistically significantly different in an analysis of 94 participants (RR 0.07, 95% CI 0.00 to 1.17). Intention‐to‐treat (ITT) analyses of non‐adherence at six weeks (RR 0.02, 95% CI 0.00 to 0.36) and at six months (RR 0.01, 95% CI 0.00 to 0.16) however, were statistically significantly different and favoured the PEG group Analysis 1.2.
1.2. Analysis.
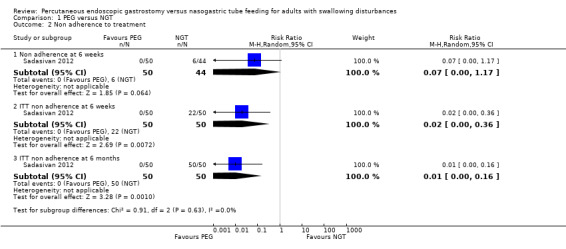
Comparison 1 PEG versus NGT, Outcome 2 Non adherence to treatment.
Subgroup analyses
We further subgrouped the studies by endoscopic gastrostomy technique into pull (n = 90), push (n = 33), and not reported (n = 285) in Analysis 1.3. We observed a significant difference favouring PEG in the pull subgroup (RR 0.07, 95% CI 0.01 to 0.35, three studies, P = 0.001). Thepush subgroup contained only one clinical trial and the result favoured PEG (RR 0.05, 95% CI 0.00 to 0.74, P = 0.03) techniques. We found no statistically significant difference in cases where technique was not reported (RR 0.43, 95% CI 0.13 to 1.44). Statistically significant heterogeneity was found in the unreported technique subgroup (I² statistic = 73%), and the statistical significance of this result was unchanged in ITT analyses (RR 0.37, 95% CI 0.09 to 1.45) Analysis 1.5.1.
1.3. Analysis.
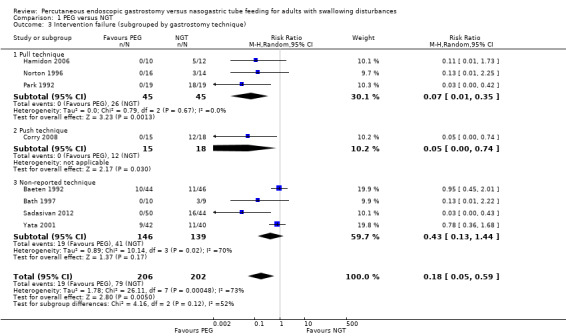
Comparison 1 PEG versus NGT, Outcome 3 Intervention failure (subgrouped by gastrostomy technique).
1.5. Analysis.
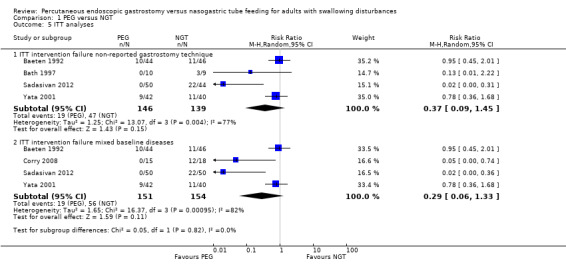
Comparison 1 PEG versus NGT, Outcome 5 ITT analyses.
We made a post‐hoc decision to investigate the possible reasons for this heterogeneity in Analysis 1.4 using subgroup analysis. Therefore we subgrouped the studies by participant condition (Analysis 1.4). For participants with cerebrovascular events or neurological baseline diseases (n = 109), the result favoured the PEG group (RR 0.08, 95% CI 0.02 to 0.33, P = 0.0005). There was no statistical heterogeneity in this analysis. For participants with mixed baseline diseases (n = 299), the intervention favoured neither PEG nor NGT(RR 0.32, 95% CI 0.08 to 1.32), and statistical heterogeneity was high (I2 = 79%), The statistical non‐significance of this result, was unchanged in ITT analyses (RR 0.29, 95% CI 0.06 to 1.33; Analysis 1.5.2).
1.4. Analysis.
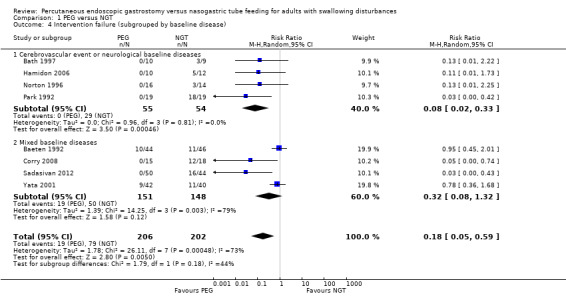
Comparison 1 PEG versus NGT, Outcome 4 Intervention failure (subgrouped by baseline disease).
Secondary outcomes
Mortality
The outcome of mortality was examined in nine studies (Baeten 1992; Bath 1997; Corry 2008; Dennis 2005; Douzinas 2006; Elbadawy 2014; Hamidon 2006; Norton 1996; Park 1992) (644 participants) and was assessed independently of study follow‐up time. The results showed 35.76% (118 out of 330 participants) in the PEG group and 36.62% (115 out of 314 participants) in the NGT group (RR 0.86, 95% CI 0.58 to 1.28) (Mantel‐Haenszels statistical method). The result of the meta‐analysis for mortality revealed no statistically significant difference between comparison groups. Finally, we observed statistical heterogeneity between included studies: I² statistic = 47%. Because of the radiologically placed gastrostomy technique used in a small number of participants in Dennis 2005, we carried out a sensitivity analysis to test the differences in the estimate effects by including and excluding this study. The sensitivity analysis shows that the inclusion of the FOOD study (Dennis 2005) did not change the statistical significance of the result for mortality (RR 0.81 (95% CI 0.47 to 1.41, P = 0.84; Analysis 1.6) without Dennis 2005 (analysis not shown).
1.6. Analysis.
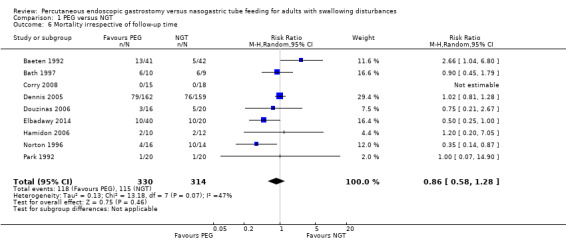
Comparison 1 PEG versus NGT, Outcome 6 Mortality irrespective of follow‐up time.
One study (n = 82) reported the mean survival time in months (Yata 2001) (MD 4.3, 95% CI 3.28 to 5.32; Analysis 1.7). The result favoured the PEG group, that is participants in the PEG group survived longer, for a mean of 11.4 months compared with 7.1 months in the NGT group.
1.7. Analysis.
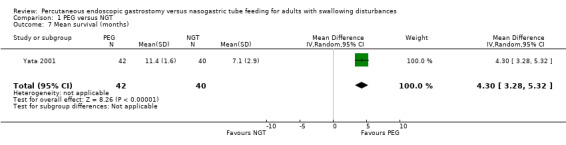
Comparison 1 PEG versus NGT, Outcome 7 Mean survival (months).
Complications and adverse effects
Complications and adverse effects (e.g., aspiration, haemorrhage, wound infection, sinusitis, fistula) were examined in six studies (Baeten 1992; Corry 2008; Dennis 2005; Douzinas 2006; Norton 1996; Sadasivan 2012) (597 participants) and was assessed independently of study follow‐up time or severity of adverse effect. Although some of adverse events were characteristic of only one intervention, we analysed them together for the purposes of this review. The results showed 35.67% (107 out of 300 participants) in the PEG group and 45.79% (136 out of 297 participants) in the NGT group had adverse effects (RR 0.83, 95% CI 0.51 to 1.34; Analysis 1.8) (Mantel‐Haenszel's statistical method). The result of the meta‐analysis for adverse effects revealed no statistically significant difference between the groups. We observed high statistical heterogeneity in the comparison: I² statistic = 87%. An ITT analysis of these data did not change the statistical significance of the result (RR 0.81, 95% CI 0.48 to 1.35; Analysis 1.9)
1.8. Analysis.
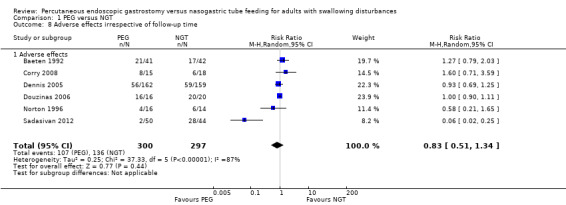
Comparison 1 PEG versus NGT, Outcome 8 Adverse effects irrespective of follow‐up time.
1.9. Analysis.
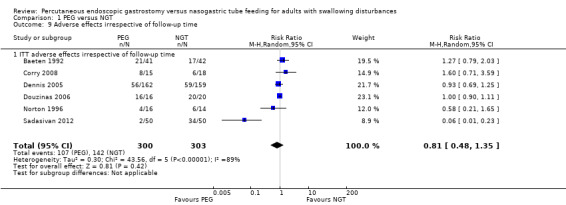
Comparison 1 PEG versus NGT, Outcome 9 Adverse effects irrespective of follow‐up time.
In Elbadawy 2014, which was a study of critically ill participants who had experienced head injury, adverse events associated with PEG tracheostomy and nasogastric tube were reported. Adverse events were reported as number of events, rather than number of participants experiencing adverse events (that is, participants may have experienced more than one type of adverse event). In this study, the adverse events in the PEG group were infection in the gastrostomy tube in 19 participants, leakage around the gastrostomy tube in 21 participants, dislodgement of the gastrostomy tube in 19 and obstruction of the PEG tube in two participants. Fistulas, perforations and 'buried pumper' syndrome (where the PEG tube migrates) were not seen. In the NGT group, paranasal sinusitis from the nasogastric tube was found in 12 participants (60%) (Table 3).
2. Additional data of adverse events.
| Adverse events from Elbadawy 2014 | Group I (NGT + intubation) | Group II (PEG + intubation) | Group III (PEG + tracheostomy) | P1 | P2 | P3 | |||
| No. | % | No. | % | No. | % | ||||
| Infection of tracheostomy wound | 0 | 0.0 | 0 | 0.0 | 16 | 80.00 | ‐ | ‐ | ‐ |
| Bleeding from tracheostomy | 0 | 0.0 | 0 | 0.0 | 0 | 0.00 | ‐ | ‐ | ‐ |
| Pneumothorax | 0 | 0.0 | 0 | 0.0 | 3 | 15.00 | ‐ | ‐ | ‐ |
| Tracheo‐oesophageal fistula | 0 | 0.0 | 0 | 0.0 | 5 | 25.00 | ‐ | ‐ | ‐ |
| Infection of gastrostomy wound | 0 | 0.0 | 10 | 50.00 | 9 | 45.00 | ‐ | ‐ | 0.635 |
| Leakage around gastrostomy tube | 0 | 0.0 | 11 | 55.00 | 10 | 50 | ‐ | ‐ | 0.732 |
| Dislodgement of gastrostomy tube | 0 | 0.0 | 10 | 50.00 | 9 | 45.00 | ‐ | ‐ | 0.751 |
| GIT Fistula | 0 | 0.0 | 0 | 0.00 | 0 | 0.00 | ‐ | ‐ | ‐ |
| GIT Perforation | 0 | 0.0 | 0 | 0.00 | 0 | 0.00 | ‐ | ‐ | ‐ |
| Buried Pumper syndrome | 0 | 0.0 | 0 | 0.00 | 0 | 0.00 | ‐ | ‐ | ‐ |
| Obstruction | 0 | 0.0 | 1 | 5.00 | 1 | 0.00 | ‐ | ‐ | 0.742 |
| Paransal sinusitis | 12 | 60.0 | 0 | 0.0 | 0 | 0.0 | ‐ | ‐ | ‐ |
P1 is the comparison between group I and group II
P2 is the comparison between group I and group III
P3 is comparison between group II and group III
Aspriration (pneumonia)
The outcome of pneumonia (as a result of aspiration) was examined in seven studies (Baeten 1992; Corry 2008; Dennis 2005; Douzinas 2006; Elbadawy 2014; Norton 1996; Yata 2001) (645 participants) and was assessed independently of study follow‐up time. The results showed 31.93% (106 out of 332 participants) pneumonia in the PEG group and 41.54% (130 out of 313 participants) in the NGT group (RR 0.70, 95% CI 0.46 to 1.06; Analysis 1.10). However, the result of the meta‐analysis for the pneumonia outcome did not favour PEG. We observed high levels of statistical heterogeneity between studies: I² statistic = 81%.
1.10. Analysis.
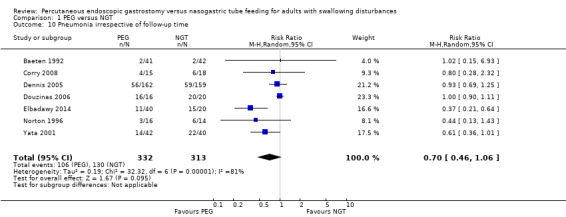
Comparison 1 PEG versus NGT, Outcome 10 Pneumonia irrespective of follow‐up time.
Reflux oesophagitis
Douzinas 2006 reported median change in gastro‐oesophageal reflux at endpoint (day seven) as percentage of the time when the oesophageal pH was less than 4 in a given 24‐hour period of time. The percentage was statistically significant, that is, less severe reflux was seen in the PEG group.
Yata 2001 reported reflux oesophagitis. In this single study analysis of 82 patients in total, there was a statistically significant result that favoured the PEG group (RR 0.45, 95% CI 0.22 to 0.92; Analysis 1.11).
1.11. Analysis.
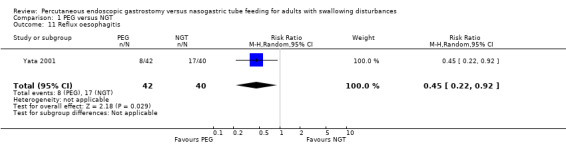
Comparison 1 PEG versus NGT, Outcome 11 Reflux oesophagitis.
Nutritional status
We analysed data for nutritional status, as measured by any validated instrument (e.g. as upper‐arm skin fold thickness, mid‐arm circumference, body weight, serum albumin level, haemoglobin)
Weight
In a single study analysis of weight (kg) at the study endpoint (Norton 1996) (mean difference (MD) 3.20, 95% CI ‐5.95 to 12.35; Analysis 1.12) The outcome favoured neither NGT or PEG. Three studies contributed to an analysis of weight change from baseline (n = 148, Corry 2008; Norton 1996; Sadasivan 2012) (MD 3.11, 95% CI ‐0.52 to 6.75; Analysis 1.13), that is, the outcome favoured neither NGT or PEG. In this analysis statistical heterogeneity was high I2 = 93%.
1.12. Analysis.
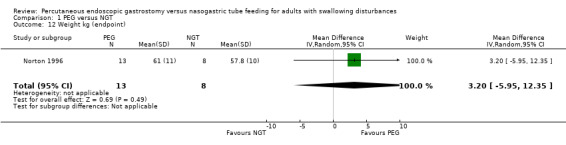
Comparison 1 PEG versus NGT, Outcome 12 Weight kg (endpoint).
1.13. Analysis.
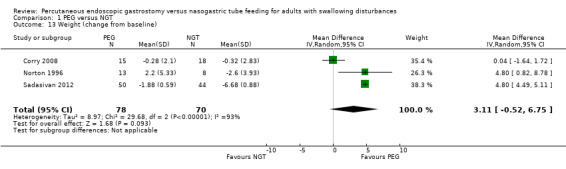
Comparison 1 PEG versus NGT, Outcome 13 Weight (change from baseline).
Mid‐arm circumference
Norton 1996 reported mid‐arm circumference in centimetres at the end point of the study and the change from baseline. The published report of Corry 2008 provided upper‐arm circumference data for the NGT and PEG group as the median 300 mm (range 240 to 352) verus PEG 302.5, P= 0.69 (range 270 to 370) (mean 283 mm versus 295 mm respectively, P=0.25, not statistically significant, no standard deviations (SDs) reported Table 2). We calculated the missing SD values for the data from Corry 2008 and the result for a meta‐analysis of both studies (n = 54) for arm circumference favoured neither intervention or control (MD 1.58, 95% CI ‐0.11 to 3.27; Analysis 1.14). No statistical heterogeneity was observed in this analysis I2 = 0%. This overall result was unchanged in a sensitivity analysis (MD 2.50, 95% CI ‐0.64 to 5.64; Analysis 1.14.2)
1.14. Analysis.
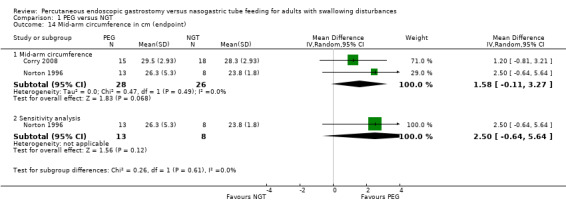
Comparison 1 PEG versus NGT, Outcome 14 Mid‐arm circumference in cm (endpoint).
The change in mid‐arm circumference from baseline was measured in Norton 1996 and Sadasivan 2012. In this analysis of 115 participants, the results were statistically significant in favour of PEG (MD 1.16, 95% CI 1.01 to 1.31; Analysis 1.15).
1.15. Analysis.
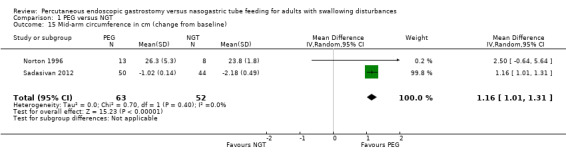
Comparison 1 PEG versus NGT, Outcome 15 Mid‐arm circumference in cm (change from baseline).
The included studies also reported anthropometric outcome data as median values which we could not include in our meta‐analyses (Table 2). Median triceps skin fold thickness was reported in Corry 2008 and Hamidon 2006 and these were not significantly different in either study, however in Corry 2008, the study reports states that the NGT patients had significantly lower triceps skin fold thickness (mean 9.5 versus 13.5 mm; P = 0.03 than the PEG patients at six weeks post‐treatment). Median biceps skin fold (mm) and median arm circumference was reported in Hamidon 2006 (Table 2) and the differences between groups were not statistically significantly different in either case.
Serum albumin
Mean serum albumin levels (g/dL) were reported in Yata 2001 and Norton 1996.
Yata 2001 was a short conference report and did not include SD values but reported that the serum albumin levels at three and six months were significantly different in the study report of Yata 2001 favouring PEG (P = <0.01) (Table 2). We calculated SD for this study using the difference between means and in an analysis of albumin levels of two studies of 107 participants, the result was statistically significant favouring the PEG group (MD 6.03, 95% CI 2.31 to 9.74; P = 0.001). Statistical heterogeneity was high I2 = 75%. In a sensitivity analysis excluding Yata 2001, the result remained statistically significant, that is, using data only from Norton 1996, an analysis of albumin levels at endpoint in 25 participants indicated a statistically significant result in favour of PEG (MD 7.80, 95% CI 5.52 to 10.08; Analysis 1.16).
1.16. Analysis.
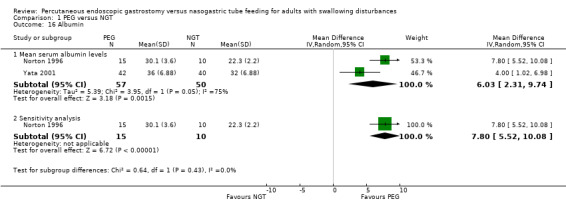
Comparison 1 PEG versus NGT, Outcome 16 Albumin.
Sadasivan 2012 reported change in albumin levels from baseline and again this result was statistically significant in an analysis of 94 participants favouring PEG (MD 0.12, 95% CI 0.11 to 0.14; Analysis 1.17).
1.17. Analysis.
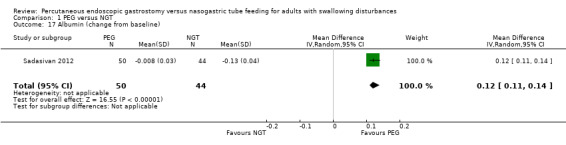
Comparison 1 PEG versus NGT, Outcome 17 Albumin (change from baseline).
The median serum albumin endpoint values were lower in the NGT group in Hamidon 2006 (P = 0.054) (Table 2).
Hamidon 2006 also reported nutritional status outcome data as median values which we could not include in our meta‐analyses (Table 2). Median serum albumin (g/L) was 39.5 (R 36 to 44) in the PEG groups versus 36.0 (R 31 to 45) in the NGT group. The P value was 0.045, which was statistically significantly different .
Haemoglobin
Haemoglobin levels were reported as a change from baseline in Sadasivan 2012, In this single study analysis of 94 participants, the results favoured PEG and was statistically significant (MD 0.59, 95% CI 0.49 to 0.69; Analysis 1.18).
1.18. Analysis.
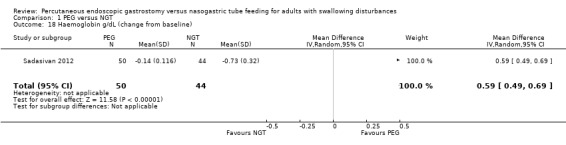
Comparison 1 PEG versus NGT, Outcome 18 Haemoglobin g/dL (change from baseline).
Yata 2001 reported that mean haemoglobin levels (g/L) were 11.7 in the NG group and in the PEG group were 11.9 at three months, and 11.1 versus 12.4 at six months (Table 2).
Time of enteral nutrition
Two studies (n = 119) reported the duration of enteral feeding in days (Baeten 1992; Park 1992) (MD 14.48, 95% CI ‐2.74 to 31.71; Analysis 1.21), this favoured neither NGT nor PGT and there were high levels of statistical heterogeneity present in this analysis (I² = 94%). These results should be interpreted cautiously as the assumption of normality for these outcomes may not be met.
1.21. Analysis.
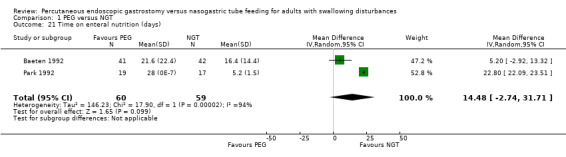
Comparison 1 PEG versus NGT, Outcome 21 Time on enteral nutrition (days).
Length of hospital stay
Two studies (n= 381) reported the length of hospital stay in days (Dennis 2005; Elbadawy 2014) (MD ‐12.67, 95% CI ‐40.18 to 14.84; Analysis 1.24), this favoured neither NGT nor PGT. There were high levels of statistical heterogeneity present in this analysis (I² = 93%). These results should be interpreted cautiously as the assumption of normality for these outcomes may not be met.
1.24. Analysis.
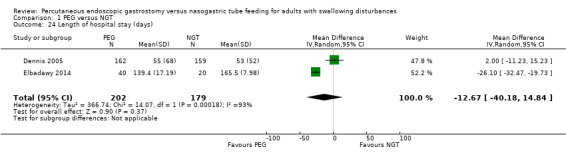
Comparison 1 PEG versus NGT, Outcome 24 Length of hospital stay (days).
Quality of life
Patient satisfaction
Patient satisfaction was reported in Baeten 1992 (a five‐point graded scale graded from 1 = very satisfied to 5 = very dissatisfied). In an analysis of 43 participants, the result favoured neither PEG nor NGT (MD ‐0.56, 95% CI ‐1.32 to 0.20) (Analysis 1.19). The inconvenience score (that is, inconvenience of maintaining the intervention to nursing staff in a scale with five categories) was also a statistically non‐significantly different in an analysis of 68 patients in Baeten 1992 (MD ‐0.58, 95% CI ‐1.18 to 0.02; Analysis 1.20).
1.19. Analysis.
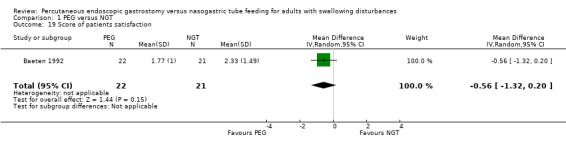
Comparison 1 PEG versus NGT, Outcome 19 Score of patients satisfaction.
1.20. Analysis.
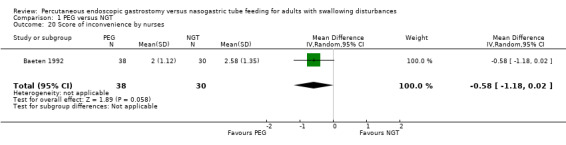
Comparison 1 PEG versus NGT, Outcome 20 Score of inconvenience by nurses.
Quality‐of‐life was measured in two studies (Corry 2008; Sadasivan 2012) and included in a meta‐analysis (Analysis 1.22), Using the EORTC QLQ‐H & N 35 Scale, and the number of participants who scored three or four (in this scale a high score is a worse outcome), the outcomes of pain, in an analysis of 133 participants, (RR 0.33, 95% CI 0.00 to 471.74) and ease of learning to use (RR 0.18, 95% CI 0.00 to 149.53), there was no statistically significant difference between the PEG and the NGT group. In analyses of 133 participants each for the outcomes of inconvenience (RR 0.03, 95% CI 0.00 to 0.29; P=0.002) and discomfort (RR 0.03, 95% CI 0.00 to 0.29; P = 0.002), altered body image (RR 0.01, 95% CI 0.00 to 0.18; P = 0.001), and social activities (RR 0.01, 95% CI 0.00 to 0.18; n= 100, P = 0.001), the intervention favoured PEG, that is, fewer participants found the intervention of PEG to be inconvenient, uncomfortable or interfered with family life or social activities, and this was a statistically significantly different between the groups. There was statistical heterogeneity present in the analysis of pain (I² = 95%) and ease of learning to use (I² = 94%), and low levels of statistical heterogeneity in the analyses of inconvenience and discomfort (I² = 21%).
1.22. Analysis.
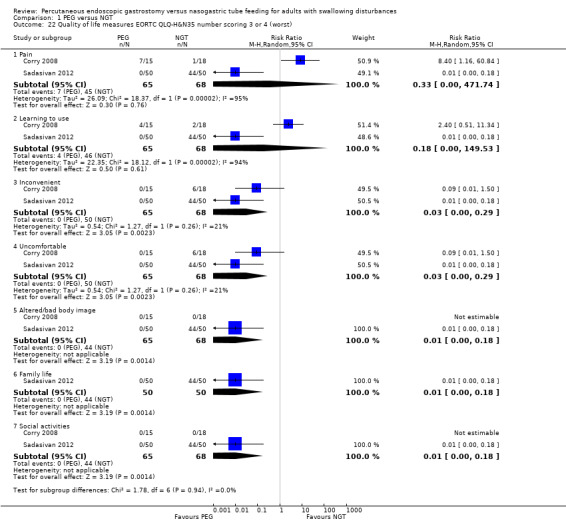
Comparison 1 PEG versus NGT, Outcome 22 Quality of life measures EORTC QLQ‐H&N35 number scoring 3 or 4 (worst).
The outcome of family life could not be entered into a meta‐analysis as Corry 2008 did not report this subscale. Using data from Sadasivan 2012 only, this outcome favoured the PEG group and this was a statistically significantly different (RR 0.01, 95% CI 0.00 to 0.18; n=100, P = 0.001).
Dennis 2005 reported the mean difference between comparison groups at endpoint derived from the EuroQol (reported as 0.035 95% CI ‐0.024 to 0.093). We could not include these data in our meta‐analyses, but the report of the study states that the results were not statistically significantly different.
Functional ability
A decline in functional ability while under treatment may be related to overall quality of life. Functional ability is the ability to perform basic activities of daily life without support, an important aspect of overall independence and quality of life. Just one study reported functional ability by using a modified Rankin Scale (MRS) (Dennis 2005). There was no statistically significant difference between comparison groups (Analysis 1.23) for the following ranges of Modified Rankin Scales (MRS): MRS 0 to 3 (RR 0.59, 95% CI 0.34 to 1.01, P = 0.06) and MRS 4 to 5 (RR 1.20, 95% CI 0.90 to 1.61, P = 0.21) and for the outcome composed by MRS scales from 4 to 5 or death as showed by the RR of 1.10, 95% CI 1.00 to 1.20, P = 0.05).
1.23. Analysis.
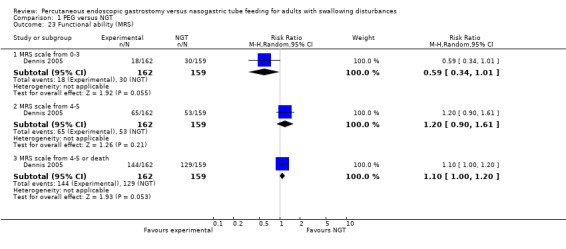
Comparison 1 PEG versus NGT, Outcome 23 Functional ability (MRS).
Costs and economic issues
Only one study provided information about costs and we did not include these data in any analyses. Corry 2008 stated that the "cost of each feeding tube is $26 for a NGT and $110 for a PEG tube" and "The insertion costs are significantly different as the NGT are inserted by nursing staff in outpatients and the PEG tubes are inserted by surgeons in theatre. The cost for insertion of a NGT is $50 (includes nursing time and cost of chest X‐ray), whereas the cost of insertion of a PEG tube is $626".
Discussion
Summary of main results
This systematic review of 11 included studies comprising 735 randomised participants in total (373 receiving percutaneous endoscopic gastrostomy (PEG) and 362 nasogastric tube (NGT)), produced 16 meta‐analyses in total, for the primary outcome of intervention failure (subgrouped by gastrostomy technique and by baseline disease) and for the secondary outcomes of mortality, adverse effects in total and also pneumonia as a result of aspiration, nutritional status including weight change from baseline, mid‐arm circumference at endpoint and change from baseline, time of enteral nutrition in days, length of stay in days, and quality of life measured by the EuroQol scale.
In our meta‐analyses, overall, the estimated effects for the primary outcome of intervention failure showed a statistically significant lower risk in the PEG group compared with the NGT group, and this was confirmed in subgroup analyses of intervention failure for both the 'push' and 'pull' gastrostomy techniques (subgroup analysis of those studies which did not report the gastrostomy technique showed no statistically significant difference between PEG or NGT). However, we cannot infer from the effect sizes that one technique (push or pull) is superior to the other as we did not carry out comparisons (indirect analysis) of the different techniques using data from separate studies.
We carried out additional intention‐to‐treat (ITT) analyses for the outcome of intervention failure specifically for the four studies with participants with mixed baseline diseases, and for intervention failure in the four studies where the gastrostomy technique was not reported, and we found no statistically significant differences between the PEG and NGT groups.
No direct causal relationship with the procedures was established for the secondary outcome of mortality i.e. there was no statistically significant difference between PEG or NGT for this outcomes. Only Dennis 2005 and Baeten 1992 reported a relationship between procedure‐related mortality and global mortality, ranging from 0% to 10%. These low rates support the notion that the use of these methods may have no significant influence on risk of death.
Meta‐anaysis of adverse effects irrespective of follow‐up time showed no statistically significant differences between the groups, and an ITT analysis of five studies for this outcome showed no statistically significant differences between the PEG and NGT groups. Fewer participants in the PEG group experienced pneumonia, an adverse event precipitated by aspiration of stomach contents or oro‐pharyngeal secretions into the airway, but this difference was not statistically significant.
The meta‐analyses of the secondary outcome of nutritional status i.e. weight change from baseline showed no statistically significant difference between the groups; endpoint mid‐arm circumference was not statistically significantly different between the groups, although the outcome of mid‐arm circumference in centimetres (change from baseline) was statistically significant in favour of PEG.
The meta‐analysis of quality‐of‐life measures (a secondary outcome) was statistically significant favouring PEG (that is, more patients in the NGT group reported worse outcomes) for the outcomes of inconvenience, discomfort, altered or bad body image, social activities and in a single study analysis, interference with family life.
We also present analyses of data from single studies for the primary outcome of intervention failure that is non‐adherence to treatment, and the secondary outcomes of adverse effects (specifically reflux oesophagitis), nutritional status including weight at endpoint, serum albumin levels and change from baseline, changes in haemoglobin levels g/dL from baseline, and measures of quality of life including scores of patient satisfaction and of inconvenience in maintaining the PEG or NGT by nurses, participant functional ability, and impact on family life measured by the EORTCQLQ‐H&N35 (in one study).The single study analyses of the primary outcome non‐adherence to treatment was statistically significant in favour of the PEG group at the six‐week and six‐month follow‐up point in Sadasivan 2012 and notably all the dropouts from treatment were from the NGT group in that study (at six months there were no patients in the NGT group due to resumption of oral feeds (n = 10) or conversion to a PEG tube (n = 34).
For the secondary outcome of adverse effects, fewer patients in the Yata 2001 study reported reflux oesophagitis in the PEG group and this was statistically significant favouring PEG. For the secondary outcome of nutritional status, the mean participant body weight in kilograms at the endpoint, showed no statistically significant difference favouring PEG or NGT. Serum albumin levels at endpoint were statistically significant in Norton 1996, favouring the PEG group and also the serum albumin change from baseline were statistically significant favouring PEG in Sadasivan 2012. Haemoglobin levels expressed as a change from baseline also were higher in the PEG group and this was a statistically significant in the only study that reported this outcome (Sadasivan 2012).
Outcomes relating to quality of life, including the scores of patient satisfaction and inconvenience in maintaining the intervention by nurses as reported in Baeten 1992, were not statistically significant in favour of either PEG or NGT. Functional ability reported in Dennis 2005 favoured neither PEG nor NGT.
Analyses of time on enteral nutrition and length of hospital stay favoured neither PEG nor NGT. However, these analyses of time are very unlikely to follow a normal distribution, so the analyses of mean differences are not necessarily accurate.These results should be interpreted cautiously as the assumption of normality for these outcomes may not be met.
These conclusions were not changed by the 2014 update of the review.
Overall completeness and applicability of evidence
Based on the findings of this review, outcomes in participants who received nutritional support via a PEG may be more favourable that in those who have a NGT, especially for the outcome of intervention failure, based on an examination of 408 participants who had heterogeneous clinical and demographic characteristics.
Participants receiving PEG may be more likely to adhere to treatment at six weeks and six months. However, we found no evidence of a difference in mortality or adverse events (aspiration pneumonia) between the comparison groups. This non significant result does not imply no difference and we suggest that the review may not have had sufficient power to look at these less common events. Participants receiving PEG may experience less reflux oesophagitis (an adverse event). There is limited evidence, derived from single study results and small meta‐analyses that PEG results in better outcomes in terms participants' nutritional status (mid‐arm circumference, haemoglobin levels and serum albumin), and report better quality of life.
We found clinical heterogeneity between the studies and noted statistical heterogeneity in some of our analyses. For example, for our analyses of intervention failure, our primary outcome, we observed high levels of statistical heterogeneity resulting from the inclusion of the Baeten 1992 and Yata 2001 trials. One explanation for this may be the clinical heterogeneity between the trials, with the participants having different baseline diseases. We made a post‐hoc decision to investigate the possible reasons for heterogeneity in the intervention failure meta‐analysis as we assumed that the source of this statistical heterogeneity would be related to clinical heterogeneity. We hypothesised that baseline disease may have contributed to clinical heterogeneity and we categorised the studies by baseline disease, i.e. cerebrovascular event or neurological disorder versus mixed baseline disease (i.e. participants who may have had severe co‐morbidities including cancer) and found that for the outcome of intervention failures in participants with cerebrovascular or neurological disease only, the results favoured PEG (i.e. fewer participants in the PEG group experienced any of the adverse events evaluated in the studies), but there was no difference between the groups for the mixed baseline disease subgroups and these studies included Baeten 1992 and Yata 2001. However, our hypothesis and the results of this analysis only point to one possible cause of heterogeneity, and this should be adequately tested in future studies. One further source of clinical heterogeneity in the remaining analyses could be because of the different techniques used to insert the PEG.
Many of the studies reported continuous outcome data in a format that could not be incorporated in to our meta‐analyses for example, median values. This limited the number of analyses that we could perform and we reported these data narratively in the review. Information reported in this way should be regarded as providing additional information only and the analyses we performed including meta‐analysis, forest plots, tests for statistical heterogeneity provide more precise estimates of effects.
Quality of the evidence
The findings of the present review of the literature should be interpreted with caution, given that almost half of the authors failed to report the method used to sequence and conceal the allocation (Figure 2; Figure 3). This is one of the main causes of error in randomised systematic studies. In addition, other potential risks of bias stemmed from the absence of prior planning of follow‐up time, as well as the unpublished or high rates of losses during follow‐up. However, almost all of the authors attempted to prevent attrition by making the flow of patients clear and through selective reporting bias by selecting clinically relevant outcomes. There are also challenges relating to the study design in terms of the numbers available for randomisation, following up such seriously ill patients and the high cost of the procedures in question. These factors may explain why the majority of studies involve small samples. It should be noted that all of the studies were judged at high risk of performance bias because it is not possible to blind participants and personnel in studies of this nature. In all cases of uncertainly we attempted to obtain further information or disaggregated data from the trial investigator, but where this was not available it was because the investigator no longer had access to historical trial data, or was unable to provide additional information. This systematic review of the literature is valuable in analysing 11 studies, thereby increasing the sample size to 735 participants. Nevertheless, further randomised clinical trials that adopt a rigorous method are warranted.
We rated the overall quality of the evidence as moderate or low for the key outcomes of treatment failure, mortality, pneumonia and adverse events (Table 1), resulting in lower confidence in the estimate of effect for those outcomes and further research is likely to have an important impact in our confidence in the estimate of effect and may even change the estimate. Where we downgraded the evidence, it was because there was risk of bias in the trial, out of eight estimates of potential bias (random sequence generation; allocation concealment; incomplete outcome data; selective reporting; blinding of participants and personnel; blinding of outcome assessment, and other bias) only six studies obtained scores of four or more. The included studies involved relatively few participants and wide confidence intervals (imprecision), although it is accepted that large scale studies of this type would be very difficult to perform. The results of many meta‐analyses had high levels of statistical heterogeneity (inconsistency).
Potential biases in the review process
In view of the sensitive search strategy involving electronic correspondence with the eminent authors in this area of research, we believe that it is highly unlikely that other studies meeting the inclusion criteria of this systematic review were overlooked, however this remains a possibility and could be regarded as a limitation of this review.
While we included adverse effects reported in the studies included in this the review, we may not have detected reports of all of serious and/or rare adverse events associated with PEG or NGT, and in common with many systematic review and meta‐analyses, this is a could be limitation of this review.
As outlined, all efforts were made to ensure that relevant qualitative or quantitative data were included in this review.
Agreements and disagreements with other studies or reviews
In one of the major controlled randomised trials performed to date (Dennis 2005), the authors suggested that NGT should be the method of choice in the first two to three weeks of enteral feeding, probably in light of the increased absolute risk of death associated with the use of PEG (RR 1.02, P = 0.86) and the absolute risk of the outcome composed by MRS scale (modified Rankin scale) from four to five or death (RR 1.10, P = 0.05). However, combining the results of 11 different studies with ≅ 400 patients, it seems that the PEG option is associated with a lower risk of intervention failure. Given the importance of this finding, selecting PEG might reduce the difference in cost between the two procedures. The findings of all of the other studies included in this analysis seem to support the use of PEG. Guidelines suggest that PEG is a highly effective and safe procedure when modern equipment is used, established standards are followed (Löser 2005). However, a careful patient selection and professional proficiency are fundamental for better outcomes (Blumenstein 2014; Skitt 2011). In a narrative review, Plonk 2005 suggested that the use of PEG should only be considered in amyotrophic lateral sclerosis, intestinal blockage by malignant tumour with incoercible vomiting, persistent dysphagia after acute stroke, and early head and neck cancer. However, the results of a systematic review that included studies with different designs suggests that PEG and NGT have the same effectiveness and safety for patients with head and neck cancer, even considering relevant outcomes, such as mortality and nutritional status (Wang 2014). Although no study included in our systematic review made available information about the use of nasal looping technique, there is some evidence that such NGT technique has potential to be preferable over PEG (Anderson 2004).
Authors' conclusions
Implications for practice.
Based on the findings of this meta‐analysis, the results favoured PEG rather than NGT for intervention failure, but not for mortality and pneumonia rates, and other adverse events. There may be some advantage in terms of nutritional status in using PEG over NGT, and patients may report better quality of life when using a PEG tube.
In routine practice, however, the costs and benefits of both procedures should be taken into account. Some health service providers, particularly under the public health system, face difficulties acquiring endoscopic gastrostomy apparatus due to their high cost. Possible reasons for the current state of the research in this area include the high cost of the procedures in question. Corry 2008 provided an example of this stating that the "cost of each feeding tube is $26 for a NGT and $110 for a PEG tube" However, it is noteworthy that because nasogastric tubes are easier to introduce (more often by the nursing team) and less weight is placed on the cost of constantly changing them as stated in Corry 2008 "The insertion costs are significantly different as the NGT are inserted by nursing staff in outpatients and the PEG tubes are inserted by surgeons in theatre. The cost for insertion of a NGT is $50 (includes nursing time and cost of chest X‐ray), whereas the cost of insertion of a PEG tube is $626". Therefore endoscopic gastrostomies may be less frequently indicated (Corry 2008).
It is important to note that in clinical practice, an endoscopic examination performed prior to PEG insertion is indicated in all cases, as the patient might present with lesions of the gastrointestinal tract, which prevents the passage of the endoscopy device and even tubes. In such patients, gastric tumours might also be present, which precludes gastrostomy. Partial gastric resections can also influence patients to elect to use alternative methods of enteral feeding.
Implications for research.
Our systematic review of the current evidence, carried out in 2014, indicated that information is available on important outcomes such as intervention failure, mortality, pneumonia and adverse events. The included studies were carried out with participants with varying baseline diseases including neurological baseline diseases and those with malignancies. Future studies should provide adequate baseline information such as baseline disease, gender and age of the participants. The gastrostomy technique was described only in some of the included studies, and future researchers should ideally specify the technique used and the experience of the professionals involved to allow for the analysis of more specific subgroups. Data on the nutritional status of the patients would prove valuable, as would a cost/benefit analysis of the number of feeding tubes used. Quality‐of‐life measures provide useful information about patient important outcomes and may help explain differences in adherence to treatment.
The high cost of the procedures in question combined with the difficulties associated with the randomisation and long‐term follow‐up of patients and explain why the majority of studies examine a small number of participants. Nevertheless, we believe that further randomised clinical trials should be conducted with rigorous observation of internal validity. They should also include previously planned and executed follow‐up periods.
What's new
| Date | Event | Description |
|---|---|---|
| 11 January 2017 | Amended | Data in Table 1 from Yata 2001 corrected. |
History
Protocol first published: Issue 4, 2009 Review first published: Issue 11, 2010
| Date | Event | Description |
|---|---|---|
| 20 January 2015 | New citation required but conclusions have not changed | Updated with two new studies. Conclusions not changed. |
| 20 January 2015 | New search has been performed | New review author (CB), updated with news studies and revised text to comply with current standards for systematic review reporting. |
| 15 December 2011 | New citation required but conclusions have not changed | No new studies identified and conclusions unchanged. |
| 15 December 2011 | New search has been performed | Literature searches rerun. No new studies identified and conclusions unchanged. |
| 14 June 2011 | Amended | Information about number of studies were amended in the Summary of Findings table and risk of bias terminology updated with no change to overall assessments. |
Acknowledgements
We would like to thank the methodological support of the Brazilian Cochrane Centre and the CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) ‐ Brazilian Ministry of Education for the scholarship.
We thank the Cochrane UGPD group for their support in the preparation of this updated review.
Appendices
Appendix 1. CENTRAL search strategy
esophag*
oesophag*
1 or 2
disease*
Neoplasms/
cancer*
Adenocarcinoma/
or/4‐7
3 and 8
Pathologic Constriction
stenosis
stenoses
dysmotilit*
stricture
or/10‐14
3 and 15
(Esophageal Motility Disorders) or (Esophageal Diverticulum) or (Esophageal Diverticulosis) or (Esophageal Stenosis) or (Esophageal Achalasia)
Deglutition Disorders/
dysphagia
swallowing disorder*
swallowing disturbance*
Esophageal Diseases/
or/16‐22
Enteral Nutrition/
Gastrointestinal Intubation/
tube feeding
gastroenteral tube
nasoenteral tube
nasojejunal feeding tube
nasojejunal tube
enteral feeding
gastric feeding tube*
Feeding Apparatus/ or Nutritional Support/ or Enteric Feeding/ or Tube Feeding/
force feeding*
Nasogastric Tube/
post‐pyloric feeding
postpyloric feeding
Enteric Feeding/
trans‐pyloric feeding
nasoduodenal tube
Gastrointestinal Endoscopy/ or Digestive System Endoscopy/
endoscop*
Endoscopic Surgical Procedure*
Gastrostom*
Gastrostomy/
percutaneous endoscopic gastrostomy
or/24‐46
(9 or 23) and 47
Appendix 2. MEDLINE search strategy
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
clinical trials as topic.sh.
randomly.ab.
trial.ti.
or/1‐7
(animals not (humans and animals)).sh.
8 not 9
esophag$.mp. [mp=title, original title, abstract, name of substance word, subject heading word]
oesophag$.mp. [mp=title, original title, abstract, name of substance word, subject heading word]
11 or 12
disease$.ab,ti.
exp Neoplasms/
cancer$.mp.
exp Adenocarcinoma/
or/14‐17
13 and 18
exp Constriction, Pathologic/
stenosis.mp.
stenoses.mp.
dysmotilit$.mp.
stricture.mp.
or/20‐24
13 and 25
Esophageal Motility Disorders/ or Diverticulum, Esophageal/ or Diverticulosis, Esophageal/ or Esophageal Stenosis/ or Esophageal Achalasia/
exp Deglutition Disorders/
dysphagia.ab,ti.
swallowing disorder$.ab,ti.
swallowing disturbance$.ab,ti.
Esophageal Diseases/
or/26‐32
exp Enteral Nutrition/
exp Intubation, Gastrointestinal/
tube feeding.ab,ti.
gastroenteral tube.ab,ti.
nasoenteral tube.ab,ti.
nasojejunal feeding tube.ab,ti.
nasojejunal tube.ab,ti.
enteral feeding.ab,ti.
gastric feeding tube$.ab,ti.
exp Feeding Apparatus/ or exp Nutritional Support/ or exp Enteric Feeding/ or exp Tube Feeding/
force feeding$.ab,ti.
Nasogastric Tube/
post‐pyloric feeding.ab,ti.
postpyloric feeding.ab,ti.
Enteric Feeding/
trans‐pyloric feeding.ab,ti.
nasoduodenal tube.ab,ti.
exp Endoscopy, Gastrointestinal/ or exp Endoscopy, Digestive System/
endoscop$.ab,ti.
Endoscopic Surgical Procedure$.mp.
Gastrostom$.mp.
exp Gastrostomy/
percutaneous endoscopic gastrostomy.mp.
or/34‐56
(19 or 33) and 57
10 and 58
Appendix 3. EMBASE search strategy
(random$ or placebo$).ti,ab.
((single$ or double$ or triple$ or treble$) and (blind$ or mask$)).ti,ab.
controlled clinical trial$.ti,ab.
RETRACTED ARTICLE/
or/1‐4
(animal$ not human$).sh,hw.
5 not 6
esophag$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
oesophag$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
8 or 9
disease$.ab,ti.
exp Neoplasms/
cancer$.mp.
exp Adenocarcinoma/
or/11‐14
10 and 15
exp Constriction, Pathologic/
stenosis.mp.
stenoses.mp.
dysmotilit$.mp.
stricture.mp.
or/17‐21
10 and 22
Esophageal Motility Disorders/ or Diverticulum, Esophageal/ or Diverticulosis, Esophageal/ or Esophageal Stenosis/ or Esophageal Achalasia/
exp Deglutition Disorders/
dysphagia.ab,ti.
swallowing disorder$.ab,ti.
swallowing disturbance$.ab,ti.
Esophageal Diseases/
or/23‐29
exp Enteral Nutrition/
exp Intubation, Gastrointestinal/
tube feeding.ab,ti.
gastroenteral tube.ab,ti.
nasoenteral tube.ab,ti.
nasojejunal feeding tube.ab,ti.
nasojejunal tube.ab,ti.
enteral feeding.ab,ti.
gastric feeding tube$.ab,ti.
exp Feeding Apparatus/ or exp Nutritional Support/ or exp Enteric Feeding/ or exp Tube Feeding/
force feeding$.ab,ti.
Nasogastric Tube/
post‐pyloric feeding.ab,ti.
postpyloric feeding.ab,ti.
Enteric Feeding/
trans‐pyloric feeding.ab,ti.
nasoduodenal tube.ab,ti
exp Endoscopy, Gastrointestinal/ or exp Endoscopy, Digestive System/
endoscop$.ab,ti.
Endoscopic Surgical Procedure$.mp.
Gastrostom$.mp.
exp Gastrostomy/
percutaneous endoscopic gastrostomy.mp.
or/31‐53
(16 or 30) and 54
7 and 5
Appendix 4. LILACS search strategy
pt ensaio controlado aleatorio
pt ensaio clinico controlado
mh ensaios controlados aleatorios
mh distribuicao aleatoria
mh método duplo‐cego
mh método simples‐cego
pt estudo multicentrico
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7
tw ensaio
tw ensayo
tw trial
#9 OR #10 OR #11
tw azar
tw acaso
tw placebo
tw control$
tw aleat$
tw random$
#13 OR #14 OR #15 OR #16 OR #17 OR #18
tw duplo
tw cego
#20 AND #21
tw doble
tw ciego
#23 AND #24
tw double
tw blind
#26 AND #27
#19 OR #22 OR #25 OR #28
tw clinic$
#12 AND #29 AND #30
#8 OR #31
Data and analyses
Comparison 1. PEG versus NGT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Intervention failure | 8 | 408 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.05, 0.59] |
| 1.1 All baseline diseases | 8 | 408 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.05, 0.59] |
| 2 Non adherence to treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Non adherence at 6 weeks | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.17] |
| 2.2 ITT non adherence at 6 weeks | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.36] |
| 2.3 ITT non adherence at 6 months | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.01 [0.00, 0.16] |
| 3 Intervention failure (subgrouped by gastrostomy technique) | 8 | 408 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.05, 0.59] |
| 3.1 Pull technique | 3 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.01, 0.35] |
| 3.2 Push technique | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.00, 0.74] |
| 3.3 Non‐reported technique | 4 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.13, 1.44] |
| 4 Intervention failure (subgrouped by baseline disease) | 8 | 408 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.05, 0.59] |
| 4.1 Cerebrovascular event or neurological baseline diseases | 4 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.02, 0.33] |
| 4.2 Mixed baseline diseases | 4 | 299 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.08, 1.32] |
| 5 ITT analyses | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 ITT intervention failure non‐reported gastrostomy technique | 4 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.09, 1.45] |
| 5.2 ITT intervention failure mixed baseline diseases | 4 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.06, 1.33] |
| 6 Mortality irrespective of follow‐up time | 9 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.58, 1.28] |
| 7 Mean survival (months) | 1 | 82 | Mean Difference (IV, Random, 95% CI) | 4.30 [3.28, 5.32] |
| 8 Adverse effects irrespective of follow‐up time | 6 | 597 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.51, 1.34] |
| 8.1 Adverse effects | 6 | 597 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.51, 1.34] |
| 9 Adverse effects irrespective of follow‐up time | 6 | 603 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.48, 1.35] |
| 9.1 ITT adverse effects irrespective of follow‐up time | 6 | 603 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.48, 1.35] |
| 10 Pneumonia irrespective of follow‐up time | 7 | 645 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.46, 1.06] |
| 11 Reflux oesophagitis | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.22, 0.92] |
| 12 Weight kg (endpoint) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 3.20 [‐5.95, 12.35] |
| 13 Weight (change from baseline) | 3 | 148 | Mean Difference (IV, Random, 95% CI) | 3.11 [‐0.52, 6.75] |
| 14 Mid‐arm circumference in cm (endpoint) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 Mid‐arm circumference | 2 | 54 | Mean Difference (IV, Random, 95% CI) | 1.58 [‐0.11, 3.27] |
| 14.2 Sensitivity analysis | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 2.5 [‐0.64, 5.64] |
| 15 Mid‐arm circumference in cm (change from baseline) | 2 | 115 | Mean Difference (IV, Random, 95% CI) | 1.16 [1.01, 1.31] |
| 16 Albumin | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 Mean serum albumin levels | 2 | 107 | Mean Difference (IV, Random, 95% CI) | 6.03 [2.31, 9.74] |
| 16.2 Sensitivity analysis | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 7.80 [5.52, 10.08] |
| 17 Albumin (change from baseline) | 1 | 94 | Mean Difference (IV, Random, 95% CI) | 0.12 [0.11, 0.14] |
| 18 Haemoglobin g/dL (change from baseline) | 1 | 94 | Mean Difference (IV, Random, 95% CI) | 0.59 [0.49, 0.69] |
| 19 Score of patients satisfaction | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.32, 0.20] |
| 20 Score of inconvenience by nurses | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.18, 0.02] |
| 21 Time on enteral nutrition (days) | 2 | 119 | Mean Difference (IV, Random, 95% CI) | 14.48 [‐2.74, 31.71] |
| 22 Quality of life measures EORTC QLQ‐H&N35 number scoring 3 or 4 (worst) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 22.1 Pain | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.00, 471.74] |
| 22.2 Learning to use | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.00, 149.53] |
| 22.3 Inconvenient | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 0.29] |
| 22.4 Uncomfortable | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 0.29] |
| 22.5 Altered/bad body image | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.01 [0.00, 0.18] |
| 22.6 Family life | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.01 [0.00, 0.18] |
| 22.7 Social activities | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.01 [0.00, 0.18] |
| 23 Functional ability (MRS) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 23.1 MRS scale from 0‐3 | 1 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.34, 1.01] |
| 23.2 MRS scale from 4‐5 | 1 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.90, 1.61] |
| 23.3 MRS scale from 4‐5 or death | 1 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [1.00, 1.20] |
| 24 Length of hospital stay (days) | 2 | 381 | Mean Difference (IV, Random, 95% CI) | ‐12.67 [‐40.18, 14.84] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baeten 1992.
| Methods | Single‐centre parallel randomised controlled trial Setting: 1 hospital in the Netherlands Sample size: not reported |
|
| Participants | Ninety patients with neurologic problems, ear, nose and throat tumours and surgical problems. 56 male, 34 female; mean age 72 (62 to 82) Inclusion criteria: indication for enteral nutrition Exclusion criteria: contra‐indication for either method |
|
| Interventions | PEG (n = 44) ‐ Freka set (Fresenius) NGT (n = 46) ‐silicone tube 14 inch inserted by nurse |
|
| Outcomes |
|
|
| Notes | Follow‐up: mean nutrition time 17.9 ± 19.9 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors not blinded as explicitly referred by the authors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no withdrawals reported by the study investigators |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes analysed |
| Other bias | High risk | Follow‐up was not previously established |
Bath 1997.
| Methods | Single‐centre parallel randomised controlled trial Setting: 1 hospital in UK Sample size: not reported |
|
| Participants | Nineteen patients (8 male, 11 female); mean age: 77 years (11) Baseline disease: 13 Ischaemic stroke, six haemorrhagic stroke Inclusion criteria: stroke within two weeks of stroke onset Exclusion criteria: oro‐gastrointestinal disease concurrent severe illness, coagulopathy, pre‐morbid dependency, severe dementia, psychiatric illness |
|
| Interventions | PEG: details not available NGT: details not available |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Follow‐up: three months Risks of bias was judged from a systematic review previously published by the author (Bath 2009) and by email contact with the author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated by minimisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not explicitly stated to be blinded by the study investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were analysed |
| Other bias | High risk | Unpublished study |
Corry 2008.
| Methods | Parallel randomised controlled trial Setting: hospitals in Australia; enteral feeding on an outpatient basis Sample size: the study planned to recruit 150 patients over two years, allowing a difference of at least 1.4 kg in mean weight loss to be detected between the two feeding tubes with 80% power using a two‐sided test with significance level of 5% |
|
| Participants | 42 patients; 24 male, 9 female; median age 60 (46 to 80) Inclusion criteria: patients with squamous cell carcinoma of the head and neck planned for curative radiotherapy or chemoradiation who were anticipated to require enteral feeding Exclusion criteria: refusal to be randomised and refusal to receive any tube for nutrition |
|
| Interventions | PEG (n = 22); push technique by Tucker (Kimberley‐Clark MIC e Wilson‐Cook) NGT (n = 20); fine bore tube inserted by nurse and confirmed the correct placement by a chest X‐ray and aspiration of stomach contents All patients received enteral feeding at home |
|
| Outcomes |
All patients were assessed 6 months post‐treatment |
|
| Notes | Nine patients did not receive the intervention to which they were allocated Outcome four was not considered for analysis because the instrument of evaluation is not formally validated Outcome one was not suitable for analysis because it was not explicitly informed if they were reported as means or medians |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adaptive biased coin technique |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors not blinded as explicitly referred by the authors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow of patients was clearly reported |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were analysed |
| Other bias | Low risk | None suspected |
Dennis 2005.
| Methods | Multicentric parallel randomised controlled trial Setting: multicentric study involving many countries, mainly UK Sample size: 1000 patients based on 85% power to detected and absolute risk difference for death or poor outcome of 9%. Type one error: 0.05 |
|
| Participants | 321 patients: 144 male, 177 female; mean age 76 (10); dysphagic stroke patients Inclusion criteria: recent stroke (within 7 days before admission), first‐ever or recurrent, if the responsible clinician was uncertain of the best feeding (PEG or NGT) Exclusion criteria: patients with subarachnoid haemorrhage |
|
| Interventions | PEG (n = 162) NGT (n = 159) |
|
| Outcomes |
|
|
| Notes | Follow‐up: six months Outcomes 3, 10 and 13 were not suitable for analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, stratified by country, age, gender, and predicted probability of poor outcome (by minimisation) |
| Allocation concealment (selection bias) | Low risk | The randomisation systems were housed on a secure server with access permitted, via a password. Participating centres were issued with codes in order for them to access the randomisation services (three separate numerical codes). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | According to the authors, "the randomising clinician, the clinical team, and the patients were not unaware to treatment allocation— doing so would have been impossible". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | According to the authors, "the randomising clinician, the clinical team, and the patients were not unaware to treatment allocation— doing so would have been impossible". However, 6 month of follow‐up was carried out for the following variables: patients’ vital status, functionalability with themodified Rankin score (MRS), 19 place of residence, method of feeding, and quality of life with the EUROQoL. For these variables, the authors referred that "follow‐up was masked to treatment allocation (except where patients or carers inadvertently unmasked an interviewer at follow‐up; such occurrences were unusual but their frequency was not systematically recorded)". Because of these divergences the study was considered as of high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow of patients was clearly reported |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were analysed |
| Other bias | Low risk | None suspected |
Douzinas 2006.
| Methods | Single‐centre parallel randomised controlled trial Setting: 1 hospital (intensive care unit) in Greece Sample size: not reported; pilot study was made |
|
| Participants | 39 patients; 22 male, 14 female; median age: PEG 53 (20 to 82), NGT 58 (25 to 85). Inclusion criteria: 1. patients on mechanical ventilation with NGT in place for more than 10 days, suffering from persistent or recurrent ventilator‐associated pneumonia and reflux rate above 6%. Exclusion criteria: unstable haemodynamic state, administration of morphine, atropine, theophylline, barbiturates, and cisapride, and a past history of GER or hiatal hernia. |
|
| Interventions | PEG (n = 19): pull technique NGT (n = 20): fine bore 14 |
|
| Outcomes |
|
|
| Notes | Follow‐up: 20 days Three patients randomly allocated to receive PEG were excluded because of hiatal hernia (2) and intestinal bloating |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not explicitly stated by the study investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow of patients was clearly reported |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were analysed |
| Other bias | Low risk | None suspected |
Elbadawy 2014.
| Methods | Single‐centre parallel randomised controlled 3‐arm trial Setting: Department of Critical Care Medicine, Egypt Sample size; minimum sample size required was 20 patients for each group to achieve a power of 80 % and alpha of 0.05. |
|
| Participants | 60 participants, with closed traumatic severe brain injury in need for prolonged MV who continued to have a Glasgow coma score (GCS) of less than 8 after initial stabiliSation of their haemodynamic and oxygenation. Mean age not available. Gender (male/female ratio): NGT + intubation: 8/12 PEG + intubation: 9/11 PEG + tracheostomy: 11/9 Exclusion criteria: History of known respiratory disease, thoracic trauma, multiple traumatic injuries including abdominal or spinal trauma, massive or untreatable loculated ascites, previous abdominal surgery, uncorrected coagulopathy. |
|
| Interventions |
NGT + intubation (n = 20): nasogastric tube and endotracheal tube was inserted through which MV was applied. PEG + intubation (n = 20): PEG was done within 24 hours of endotracheal intubation using percutaneous pull gastrostomy kit using Bard Ponsky pull through technique PEG + tracheostomy (n = 20): percutaneous dilatational tracheostomy (PDT) and PEG were done within 24 hours of endotracheal intubation. In all study groups, bolus enteral nutrition was given which was initiated within 24 hours after intubation for patients in group (A) and 24 hours after performance of gastrostomy for group (B and C). All the patients were nursed in a semi recumbent position (30‐45o). Proton pump inhibitor was given intravenously for stress ulcer prophylaxis (pantoprazole 40mg once daily) for each patient in all the study groups |
|
| Outcomes | Primary
Secondary
Adverse events including: infection of tracheostomy wound, bleeding from tracheostomy, pneumothorax, tracheo‐oesophageal fistula, infection of gastrostomy wound, GIT Fistula, GIT Perforation, buried pumper syndrome (PEG tube erodes and migrates through the gastric wall), paranasal sinusitis. |
|
| Notes | No statistically or clinically significant differences between comparison groups at baseline for gender, mechanism of injury, characteristics based on computer tomography, APACHE II score, Glasgow coma score, or other vital sign sand biochemical parameters. We combined data for the PEG + intubation and PEG + tracheostomy groups into a single PEG group for comparison with NGT. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as randomised, consecutive computer randomisation (further information from study investigator) |
| Allocation concealment (selection bias) | High risk | Not concealed (further information from study investigator) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not explicitly stated by the study investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition reported |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were analysed, protocol not available for assessment |
| Other bias | Low risk | None |
Hamidon 2006.
| Methods | Single‐centre parallel randomised controlled trial Setting: 1 hospital in Malaysia; patients were discharged in one or two days after the intervention Sample size: not reported |
|
| Participants | 23 patients; 11 male, 11 female; median age: PEG 65 (48 to 79), NGT 72 (54 to 77) Inclusion criteria: patients with acute Ischaemic stroke and persistent dysphagia for seven or more days Exclusion criteria: not related |
|
| Interventions | PEG (n = 10): pull technique, Wilson CooK silicone tube 24 FR, inserted by a doctor NGT (n = 12): Steril Cathline polyurethane tube, size 14 inserted by a nurse and checked by aspirating asteric contents |
|
| Outcomes |
|
|
| Notes | There was one dropout because it was impossible to contact the patient after four weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible for this type of intervention; although only surgeons were responsible for the PEG and nurses by the NGT |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information given by the patients by telephone, but blinding of outcome assessment was not explicitly stated by the study investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow of patients was clearly reported (1 dropout due to failure to turn‐up) |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were analysed |
| Other bias | Low risk | None suspected |
Norton 1996.
| Methods | Parallel randomised controlled trial Setting: 1 university hospital and one district general hospital in UK Sample size: not reported |
|
| Participants | 30 patients: 11 male, 19 female; mean age 77 Inclusion criteria: acute cerebrovascular accident with persisting dysphagia for eight or more days, in need for sedation and prolonged mechanical ventilation. Exclusion criteria: patients with a previous history of gastrointestinal disease which would preclude siting a gastrostomy tube or who were unfit for upper gastrointestinal endoscopy and IV sedation |
|
| Interventions | PEG (n = 16): pull technique, Wilson Cook tube 24 FR or 12 FR Fresenius NGT (n = 14): fine bore tube Flocare 500, inserted by a senior nurse |
|
| Outcomes |
|
|
| Notes | Follow‐up: six weeks for main outcomes For continuous data, results were not available for all patients |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not explicitly stated by the study investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow of patients was clearly reported |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were analysed |
| Other bias | Low risk | None suspected |
Park 1992.
| Methods | Parallel randomised controlled trial Setting: three teaching hospitals in Glasgow Sample size: 40 patients was selected to detect a two‐sided difference between the success of gastrostomy feeding at 90% and NGT feeding at 40% with a power of 0.9 and significance of 0.05 |
|
| Participants | 40 patients with neurological dysphagia, 22 male, 18 female; mean age: PEG 56, NGT 65 Inclusion criteria: longstanding (4 weeks or more) dysphagia due to neurological disease; stable medical condition with likely survival of at least one month; ability to communicate verbally or in writing; and presence of a normal gastrointestinal tract Exclusion criteria: dementia; mechanical lesions causing obstruction of the oesophagus or stomach; active intra‐abdominal inflammation including inflammatory bowel disease or pancreatitis; history of partial gastrectomy, reflux oesophagitis, or intestinal obstruction; and presence of ascites, notable hepatomegaly, severe obesity, coagulopathy, untreated aspiration pneumonia, and major systemic disease including malignancy and respiratory, liver, or renal failure |
|
| Interventions | PEG (n = 20) Bard 20Fr silicone tube, technique by Ponsky ‐ Gauderer NGT (n = 20) fine bore Abbott Flexitube, polyurethane, 850 mm length,1.5 mm internal diameter |
|
| Outcomes |
|
|
| Notes | Outcome six was not considered for analysis because only one patient completed the follow‐up Outcome seven was not considered clinically relevant by itself, unless it causes failure or affects nutritional status (anthropometric parameters) Follow‐up: 28 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers (Epistat Statistical Package) |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not explicitly stated by the study investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow of patients was clearly reported |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were analysed |
| Other bias | High risk | There was 95% (19/20) of dropouts in the NGT group due to failures in the treatment and death |
Sadasivan 2012.
| Methods | Single‐centre parallel randomised controlled trial. Sample size: a minimum of 40 cases in each group, with 80%‐ to –90% power and 95% confidence (80% on tube dislodgement and 90% on infection). So, 50 cases were included in each group. Setting: India, Department of ENT (Ear, Nose, Throat; Otorhinolaryngology). |
|
| Participants | 100 participants Gender: PEG: 34/16 (male/female ratio); NGT: 33/17 (male/female ratio) Age (mean): not reported Inclusion criteria: patients with advanced stage 2 or 3 squamous cell carcinoma of the head and neck and who were scheduled either for radical surgery with adjuvant radiotherapy (RT), chemo‐RT, or for concurrent chemo and radiation therapy were included in the study. Exclusion criteria: patients with early stage 1 or 2 head and neck cancer were excluded from the study |
|
| Interventions | PEG n = 50; NGT n = 50 The majority of NG tubes were inserted by nurses, all PEG tubes were inserted by gastroenterologists. |
|
| Outcomes | Follow‐up: 1 week; 6 weeks and 6 month Primary outcomes
Secondary outcomes
|
|
| Notes | Statistical differences at baseline: radical surgery and adjuvant radiotherapy or chemo and radiation therapy (PEG: 92%; NGT: 72%; P = 0.01); concurrent chemo‐ and radiation therapy (PEG: 8%; NGT: 28% P = 0.01); baseline weight: PEG: 56.5 versus NGT: 61 (P < 0.01) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported by the study investigators |
| Allocation concealment (selection bias) | Unclear risk | Not reported by the study investigators |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported by the study investigators |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study investigators did not perform ITT analysis |
| Selective reporting (reporting bias) | Low risk | None suspected: relevant variables were analysed. The protocol was not assessed. |
| Other bias | Unclear risk | None suspected |
Yata 2001.
| Methods | Single‐centre parallel randomised controlled trial. Sample size: not reported Setting: 1 hospital in Inagawa Town (Japan) |
|
| Participants | 82 patients: 22 male, 60 female; mean age: PEG 75.1 (50 to 96), NGT 76.5 (38 to 93) Inclusion criteria: dysphagic patients Exclusion criteria: not reported |
|
| Interventions | PEG n = 42 NGT n = 40 |
|
| Outcomes |
|
|
| Notes | Study available only as a meeting abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible for this type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not explicitly described by the study investigators |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow of patients was not clearly reported |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were analysed, Outcome 7. was reported only for NGT group Outcomes 8 and 9 were reported only for the PEG group |
| Other bias | High risk | Unpublished study |
GER: gastroesophogeal reflux ITT: intention‐to‐treat IV: intravenous NGT: nasogastric tube PEG: percutaneous endoscopic gastrostomy QoL: quality of life
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| McClave 2005 | Retrospective study |
| Mekhail 2001 | Randomised controlled trial with intervention out of interest for this review (patients randomised to stop the enteral nutrition according to different residual gastric volume) |
| Schulz 2009 | Retrospective study |
Differences between protocol and review
Previous criteria to evaluate the risk of bias are indicated below. The criteria were modified according to the new Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)
Selection bias
Was the allocation sequence adequately generated?
Was allocation adequately concealed?
Were there systematic differences between the baseline characteristics of the groups that were compared?
Attrition bias
Were there systematic differences between groups in withdrawals from a study?
Detection bias
Were there systematic differences between groups in how outcomes were determined?
We included data in the analyses of scores of patient satisfaction and inconvenience to nursing staff from Baeten 1992, theses are five‐point scales and it is unclear if these were validated scales.
In this update, for mean values of outcome data with missing standard deviations, we calculated this from the difference between means (Cochrane Handbook for Systematic Reviews of Interventions 7.7.3.3. Higgins 2011). We investigated the effects of making these assumptions by performing sensitivity analyses where appropriate.
Outcomes
We report outcomes as specified in the protocol and clarify the following: pneumonia in this instance occurs as a direct result of aspiration of food. Functional ability is included as an indicator of quality of life. Oesophageal reflux and reflux oesophagitis are adverse effects. We have included survival time as an additional outcome grouped with mortality.
Data synthesis
We planned to pool continuous data using SMD, but where the units of measurement were the same we used MD.
Subgroup analyses
We made a post‐hoc decision to investigate the possible reasons for heterogeneity in the intervention failure meta‐analysis as we assumed that the source of this statistical heterogeneity would be related to clinical heterogeneity. We categorised the studies by baseline disease, i.e. cerebrovascular event or neurological disorder versus mixed baseline disease (i.e. participants who may have had severe co‐morbidities including cancer).
Contributions of authors
Conceiving the review: CG, JW and DM Co‐ordinating the review: CG Screening search results: CG and SL Organising retrieval of papers: CG and DRW Screening retrieved papers against inclusion criteria: CG, SL, DM and JW with CB Apraising quality of papers: CG, SL, RBA and DRW with CB Extracting data from papers: CG, DRW, SL and RBA with CB Writing to authors of papers for additional information: CG with CB Providing additional data about papers: CG with CB Obtaining and screening data on unpublished studies: CG and DRW with CB Data management for the review: CG and SL Entering data into Review Manager (RevMan 5.0): CG and RBA, with CB Other statistical analysis not using RevMan: RBA Interpretation of data: CG,DM, SL,RBA and JW with CB Statistical inferences: CG, RBA and SL Writing the review: CG with CB
Person responsible for reading and checking review before submission: CG, DM, JW and SL
Sources of support
Internal sources
No sources of support supplied
External sources
CAPES ‐ Ministry of Education for the postgraduate scholarship, Brazil.
Declarations of interest
None known.
Dr Cathy Bennett is the proprietor of Systematic Research Ltd and received a consultancy fee from the Cochrane UGPD group to assist the authors with the update of their review in 2014.
Edited (no change to conclusions)
References
References to studies included in this review
Baeten 1992 {published data only}
- Baeten C, Hoefnagels J. Feeding via nasogastric tube or percutaneous endoscopic gastrostomy. A comparison. Scandinavian Journal of Gastroenterology 1992;194:95‐8. [PUBMED: 1298056] [DOI] [PubMed] [Google Scholar]
Bath 1997 {published data only}
- Bath PMW, Bath‐Hextall FJ, Smithard D. Interventions for dysphagia in acute stroke. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD000323] [DOI] [PubMed] [Google Scholar]
Corry 2008 {published data only}
- Corry J, Poon W, McPhee N, Milner AD, Cruickshank D, Porceddu SV, et al. Randomized study of percutaneous endoscopic gastrostomy versus nasogastric tubes for enteral feeding in head and neck cancer patients treated with (chemo)radiation. Journal of Medical Imaging and Radiation Oncology 2008;52(5):503‐10. [PUBMED: 19032398] [DOI] [PubMed] [Google Scholar]
Dennis 2005 {published data only}
- Dennis M, Lewis S, Cranswick G, Forbes J, FOOD Trial Collaboration. FOOD: a multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke. Health Technology Assessment 2006;10(2):iii‐iv, ix‐x, 1‐120. [DOI] [PubMed] [Google Scholar]
- Dennis MS, Lewis SC, Warlow C. Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD):a multicentre randomised controlled trial. Lancet 2005;365:764‐72. [PUBMED: 15733717] [DOI] [PubMed] [Google Scholar]
Douzinas 2006 {published data only}
- Douzinas EE, Tsapalos A, Dimitrakopoulos A, Diamanti‐Kandarakis E, Rapidis AD, Roussos C. Effect of percutaneous endoscopic gastrostomy on gastro‐esophageal reflux in mechanically‐ventilated patients. World Journal of Gastroenterology 2006;12(1):114‐8. [PUBMED: 16440428] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbadawy 2014 {unpublished data only}
- Elbadawy TH, Gamal MA, Fayed AM, Habib TN. Early gastrostomy and tracheostomy prevent ventilator associated pneumonia in traumatic brain injured patients. European Society of Intensive Care Medicine (ESICM) Congress. Springer Verlag. S123 http://www.esicm.org/flash‐conferences/lisbon‐2012/3143 accessed 4th April 2014 13‐17 October 2012.
- Fayed AM, Elbadawy TH, Gamal MA, Habib TN. Role of early gastrostomy and tracheostomy in prevention of VAP in traumatic brain injured patients. Unpublished manuscript received 8 April 2014.
Hamidon 2006 {published data only}
- Hamidon BB, Abdullah SA, Zawawi MF, Sukumar N, Aminuddin A, Raymond AA. A prospective comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding in patients with acute dysphagic stroke. Medical Journal of Malaysia 2006;61(1):59‐66. [PUBMED: 16708735] [PubMed] [Google Scholar]
Norton 1996 {published data only}
- Kearns PJ. A randomized prospective comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding after acute dysphagic stroke. Journal of Parenteral and Enteral Nutrition 1996;20(5):374‐5. [DOI: 10.1177/014860719602000513] [DOI] [Google Scholar]
- Norton B, Homer‐Ward M, Donnelly MT, Long RG, Holmes GK. A randomised prospective comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding after acute dysphagic stroke. BMJ 1996;312(7022):13‐6. [PUBMED: 8555849] [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 1992 {published data only}
- Park RHR, Allison MC, Lang J, Spence E, Morris AJ, Danesh BJ, et al. Randomised comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding in patients with persisting neurological dysphagia. BMJ 1992;304(6839):1406‐9. [PUBMED: 1628013] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sadasivan 2012 {published data only}
Yata 2001 {published data only}
- Yata M, Date K, Miyoshi H, Matsuo N, Nishida M, Harima T, et al. Comparison between nasogastric tube feeding and percutaneous endoscopic gastrostomy feeding: a long‐term randomized controlled study. Gastrointestinal Endoscopy. 2001; Vol. 53, issue 5:AB206.
References to studies excluded from this review
McClave 2005 {published data only}
- McClave SA, Lukan JK, Stefater JA, Lowen CC, Looney SW, Matheson PJ, et al. Poor validity of residual volumes as a marker for risk of aspiration in critically ill patients. Critical Care Medicine 2005;33(2):324‐30. [DOI] [PubMed] [Google Scholar]
Mekhail 2001 {published data only}
- Mekhail TM, Adelstein DJ, Rybicki LA, Larto MA, Saxton JP, Lavertu P. Enteral nutrition during the treatment of head and neck carcinoma: is a percutaneous endoscopic gastrostomy tube preferable to a nasogastric tube?. Cancer 2001;91(9):1785‐90. [PubMed] [Google Scholar]
Schulz 2009 {published data only}
- Schulz RJ, Nieczaj R, Moll A, Azzaro M, Egge K, Becker R. Dysphagia treatment in a clinical‐geriatric setting PEG and functional therapy of dysphagia [Behandlung der Dysphagie in einem klinisch‐geriatrischen Setting: funktionelle Dysphagietherapie und PEG‐Einsatz.]. Zeitschrift für Gerontologie und Geriatrie 2009;42(4):328‐35. [PUBMED: 19618229] [DOI] [PubMed] [Google Scholar]
Additional references
Abdel‐Lah Mohamed 2006
- Abdel‐Lah Mohamed A, Abdel‐Lah Fernández O, Sánchez Fernández J, Pina Arroyo J, Gómez Alonso A. Surgical access routes in enteral nutrition [A Vías de acceso quirúrgico en nutrición enteral]. Cirugía, Ginecología y Urología 2006;79(6):331‐41. [PUBMED: 16768996] [DOI] [PubMed] [Google Scholar]
Anderson 2004
- Anderson MR, O'Connor M, Mayer P, O'Mahony D, Woodward J, Kane K. The nasal loop provides an alternative to percutaneous endoscopic gastrostomy in high‐risk dysphagic stroke patients. Clinical Nutrition 2004;23(4):501‐6. [PUBMED: 15297085] [DOI] [PubMed] [Google Scholar]
Anis 2006
- Anis MK, Abid S, Jafri W, Abbas Z, Shah HA, Hamid S, et al. Acceptability and outcomes of the percutaneous endoscopic gastrostomy (PEG) tube placement‐‐patients' and care givers' perspectives. BMC Gastroenterology 2006;24(6):37. [PUBMED: 17125502] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barker 2011
- Barker LA, Gout BS, Crowe TC. Hospital malnutrition: prevalence, identification and impact on patients and the healthcare system. International Journal of Environmental Research and Public Health 2011;8(2):514‐27. [PUBMED: 21556200] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barkmeier 1998
- BarkmeierJM, Trerotola SO, Wiebke EA, Sherman S, Harris VJ, Snidow JJ, et al. Percutaneous radiologic, surgical endoscopic, and percutaneous endoscopic gastrostomy/gastrojejunostomy: comparative study and cost analysis. Cardiovascular and Interventional Radiology 1998;21:324‐8. [DOI] [PubMed] [Google Scholar]
Bastow 1986
- Bastow MD. Complications of enteral nutrition. Gut 1986;27 Suppl 1:51‐5. [PUBMED: 3098642] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bath 1999
- Bath PMW, Bath‐Hextall FJ, Smithard DG. Interventions for dysphagia in acute stroke. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD000323] [DOI] [PubMed] [Google Scholar]
Bath 2009
- Bath PMW. Personal correspondence (email to Cláudio Gomes Jr asking for the full text) 2009 (July‐16).
Beavan 2010
- Beavan J, Conroy SP, Harwood R, Gladman JR, Leonardi‐Bee J, Sach T, et al. Does looped nasogastric tube feeding improve nutritional delivery for patients with dysphagia after acute stroke? A randomised controlled trial. Age and Ageing 2010;39(10):624‐30. [PUBMED: 20667840] [DOI] [PubMed] [Google Scholar]
Blumenstein 2014
- Blumenstein I, Shastri YM, Stein J. Gastroenteric tube feeding: techniques, problems and solutions. World Journal of Gastroenterology 2014;20(26):8505‐24. [PUBMED: 25024606] [DOI] [PMC free article] [PubMed] [Google Scholar]
Botella Romero 2012
- Botella Romero F, Alfaro Martínez JJ, Luna López V, Galicia Martín I, Grupo de Trabajo sobre Calcio y Vitamina D en Nutrición Enteral. Enteral nutrition in neurological patients: is there enough vitamin D content in commonly used formulas? [Nutricion enteral en el paciente neurologico: inverted question markes suficiente el contenido en vitamina D en las formulas de uso habitual?]. Nutrición Hospitalaria 2012;27(2):341‐8. [PUBMED: 22732955] [DOI] [PubMed] [Google Scholar]
Chiò 2004
- Chiò A, Galletti R, Finocchiaro C, Righi D, Ruffino MA, Calvo A, et al. Percutaneous radiological gastrostomy: a safe and effective method of nutritional tube placement in advanced ALS. Journal of Neurology, Neurosurgery and Psychiatry 2004;75(4):645‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corry 2008b
- Corry J. Personal correspondence (e‐mail to Cláudio Gomes Jr asking for data about pneumonia and complications) 2008.
de Aguilar‐Nascimento 2011
- Aguilar‐Nascimento JE, Prado Silveira BR, Dock‐Nascimento DB. Early enteral nutrition with whey protein or casein in elderly patients with acute ischemic stroke: a double‐blind randomized trial. Nutrition 2011;27(4):440‐4. [PUBMED: 21167685] [DOI] [PubMed] [Google Scholar]
Dorman 1997
- Dorman PJ, Slattery J, Farrell B, Dennis MS, Sandercock PA. A randomised comparison of the EuroQol and Short Form‐36 after stroke. United Kingdom collaborators in the International Stroke Trial. Britsh Medical Journal 1997;315(7106):461. [PUBMED: 9284664] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dwolatzky 2001
- Dwolatzky T, Berezovski S, Friedmann R, Paz J, Clarfield AM, Stessman J, et al. A prospective comparison of the use of nasogastric and percutaneous endoscopic gastrostomy tubes for long‐term enteral feeding in older people. Clinical Nutrition 2001;20(6):535‐40. [PUBMED: 11884002 ] [DOI] [PubMed] [Google Scholar]
Falsetti 2009
- Falsetti P, Acciai C, Palilla R, Bosi M, Carpinteri F, Zingarelli A, et al. Oropharyngeal dysphagia after stroke: incidence, diagnosis, and clinical predictors in patients admitted to a neurorehabilitation unit. Journal of Stroke and Cerebrovascular Diseases 2009;18(5):329‐35. [PUBMED: 19717014] [DOI] [PubMed] [Google Scholar]
Finestone 2003
- Finestone HM, Greene‐Finestone LS. Rehabilitation medicine: 2. Diagnosis of dysphagia and its nutritional management for stroke patients. Canadian Medical Association Journal 2003;169(10):1041‐4. [PUBMED: 14609974] [PMC free article] [PubMed] [Google Scholar]
Fini 2014
- Fini N, Georgoulopoulou E, Vinceti M, Monelli M, Pinelli G, Vacondio P, et al. Noninvasive and invasive ventilation and enteral nutrition for ALS in Italy. Muscle & Nerve 2014;50(4):508‐16. [PUBMED: 24448736] [DOI] [PubMed] [Google Scholar]
Friginal‐Ruiz 2011
- Friginal‐Ruiz AB, González‐Castillo S, Lucendo AJ. Endoscopic percutaneous gastrostomy: an update on the indications, technique and nursing care [Gastrostomía endoscópica percutánea: una actualización sobre indicaciones, técnica y cuidados de enfermería]. Enfermería Clínica 2011;21(3):173‐8. [PUBMED: 21530347] [DOI] [PubMed] [Google Scholar]
Gauderer 1980
- Gauderer MWL, Ponsky JL, Izant RJ Jr. Gastrostomy without laparotomy:a percutaneous endoscopic technique. Journal of Pediatric Surgery 1980;15(6):872‐5. [PUBMED: 6780678] [DOI] [PubMed] [Google Scholar]
Geeganage 2012
- Geeganage C, Beavan J, Ellender S, Bath PM. Interventions for dysphagia and nutritional support in acute and subacute stroke. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD000323; PUBMED: 23076886] [DOI] [PubMed] [Google Scholar]
Gentile 2012
- Gentile MG. Enteral nutrition for feeding severely underfed patients with anorexia nervosa. Nutrients 2012;4(9):1293‐303. [PUBMED: 23112917] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gopalan 2003
- Gopalan S, Khanna S. Enteral nutrition delivery technique. Current Opinion in Clinical Nutrition and Metabolic Care 2003;6(3):313‐7. [PUBMED: 12690265] [DOI] [PubMed] [Google Scholar]
Granell Vidal 2014
- Granell Vidal L, Sánchez Juan C, Alfonso García A. Sensory evaluation of enteral nutritional supplements. Nutrición Hospitalaria 2014;30(1):104‐12. [PUBMED: 25137268] [DOI] [PubMed] [Google Scholar]
Grant 1988
- Grant JP. Comparison of percutaneous endoscopic gastrostomy with Stamm gastrostomy. Annals of Surgery 1988;207(5):598‐603. [PUBMED: 3377569] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heemskerk 2014
- Heemskerk AW, Verbist BM, Marinus J, Heijnen B, Sjögren EV, Roos RA. The Huntington's disease dysphagia scale. Movement Disorders 2014;29(10):1312‐6. [PUBMED: 24862624] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ho 2006
- Ho KM, Dobb GJ, Webb SA. A comparison of early gastric and post‐pyloric feeding in critically ill patients:a meta‐analysis. Intensive Care Medicine 2006;32(5):639‐49. [PUBMED: 16570149] [DOI] [PubMed] [Google Scholar]
Iwamoto 2014
- Iwamoto M, Higashibeppu N, Arioka Y, Nakaya Y. Swallowing rehabilitation with nutrition therapy improves clinical outcome in patients with dysphagia at an acute care hospital. The Journal of Medical Investigation 2014;61(3‐4):353‐60. [PUBMED: 25264054] [DOI] [PubMed] [Google Scholar]
Kolaček 2013
- Kolaček S. Enteral nutrition. World Review of Nutrition and Dietetics 2013;108:86‐90. [PUBMED: 24029791] [DOI] [PubMed] [Google Scholar]
Köhler 2014
- Köhler G, Kalcher V, Koch OO, Luketina RR, Emmanuel K, Spaun G. Comparison of 231 patients receiving either "pull‐through" or "push" percutaneous endoscopic gastrostomy. Surgical Endoscopy 2014;Epub ahead of print:3673‐9. [PUBMED: 24993173] [DOI] [PubMed] [Google Scholar]
Langmore 2006
- Langmore SE, Kasarskis EJ, Manca ML, Olney RK. Enteral tube feeding for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD004030.pub2] [DOI] [PubMed] [Google Scholar]
Le 2010
- Le HD, Fallon EM, Meijer VE, Malkan AD, Puder M, Gura KM. Innovative parenteral and enteral nutrition therapy for intestinal failure. Seminars in Pediatric Surgery 2010;19(1):27‐34. [PUBMED: 20123271] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ljungdahl 2006
- Ljungdahl M, Sundbom M. Complication rate lower after percutaneous endoscopic gastrostomy than after surgical gastrostomy: a prospective, randomized trial. Surgical Endoscopy 2006;20:1248‐51. [PUBMED: 16865614] [DOI] [PubMed] [Google Scholar]
Löser 2005
- Löser C, Aschl G, Hébuteme X, Mathus‐Vliegen EM, Muscaritoli M, Niv Y, et al. ESPEN guidelines on artificial enteral nutrition‐percutaneous endoscopic gastrostomy (PEG). Clinical Nutrition 2005;24(5):848‐61. [PUBMED: 16261664] [DOI] [PubMed] [Google Scholar]
Löser 2010
- Löser C. Malnutrition in hospital: the clinical and economic implications. Deutsches Ärzteblatt :International 2010;107(51‐52):911‐7. [PUBMED: 21249138] [DOI] [PMC free article] [PubMed] [Google Scholar]
Manba 2014
- Manba N, Koyama Y, Kosugi S, Ishikawa T, Ichikawa H, Minagawa M, et al. Is early enteral nutrition initiated within 24 hours better for the postoperative course in esophageal cancer surgery?. Journal of Clinical Medicine Research 2014;6(1):53‐8. [PUBMED: 24400032] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marik 2003
- Marik PE, Zaloga GP. Gastric versus post pyloric feeding: a systematic review. Critical Care 2003;7(3):R46‐51. [PUBMED: 12793890] [DOI] [PMC free article] [PubMed] [Google Scholar]
McClave 2008
- McClave 2008. Personal correspondence (email to Cláudio Gomes Jr concerning randomization) 2008.
Metheny 2010
- Metheny NA, Davis‐Jackson J, Stewart BJ. Effectiveness of an aspiration risk‐reduction protocol. Nursing Research 2010;59(1):18‐25. [PUBMED: 20010041] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG, for the CONSORT Group. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 2001;357(9263):1191‐4. [PUBMED: 11323066] [PubMed] [Google Scholar]
Nugent 2013
- Nugent B, Lewis S, O'Sullivan JM. Enteral feeding methods for nutritional management in patients with head and neck cancers being treated with radiotherapy and/or chemotherapy. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD007904.pub3; PUBMED: 23440820] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ordoñez 2013
- Ordoñez AM, Madalozzo Schieferdecker ME, Cestonaro T, Cardoso Neto J, Ligocki Campos AC. Nutritional status influences the length of stay and clinical outcomes in patients hospitalized in internal medicine wards. Nutrición Hospitalaria 2013;28(4):1313‐20. [PUBMED: 23889658] [DOI] [PubMed] [Google Scholar]
Pearce 2002
- Pearce CB, Duncan HD. Enteral feeding. Nasogastric, nasojejunal, percutaneous endoscopic gastrostomy, or jejunostomy: its indications and limitations. Postgraduate Medical Journal 2002;78(918):198‐204. [PUBMED: 11930022] [DOI] [PMC free article] [PubMed] [Google Scholar]
Piecuch 2013
- Piecuch J, Wiewiora M, Latos W. Surgical treatment of a rare case of granular cell tumour of the cervical oesophagus. Wideochirurgia i Inne Techniki Mało Inwazyjne 2013;8(2):166‐9. [PUBMED: 23837102] [DOI] [PMC free article] [PubMed] [Google Scholar]
Plonk 2005
- Plonk WM Jr. To PEG or not to PEG. Practical Gastroenterology 2005;29(7):16‐31. [https://www.healthsystem.virginia.edu/internet/digestive‐health/nutritionarticles/PlonkArticlejuly2005.pdf] [Google Scholar]
Poisson 2014
- Poisson P, Laffond T, Campos S, Dupuis V, Bourdel‐Marchasson I. Relationships between oral health, dysphagia and undernutrition in hospitalised elderly patients. Gerodontology 2014;Epub ahead of print:1‐8. [DOI: 10.1111/ger.12123; PUBMED: 24612262] [DOI] [PubMed] [Google Scholar]
Potack 2008
- Potack JZ, Chokhavatia S. Complications of and controversies associated with percutaneous endoscopic gastrostomy: report of a case and literature review. Medscape Journal of Medicine 2008;10(6):142. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Ramel 2008
- Ramel A, Jonsson PV, Bjornsson S, Thorsdottir I. Anemia, nutritional status, and inflammation in hospitalized elderly. Nutrition 2008;24(11‐12):1116‐22. [PUBMED: 18692363] [DOI] [PubMed] [Google Scholar]
Scheeren 2014
- Scheeren B, Maciel AC, Barros SG. Videofluoroscopic Swallowing Study: esophageal alterations in patients with dysphagia [Videofluoroscopia da deglutição: alterações esofágicas em pacientes com disfagia]. Arquivos de Gastroenterologia 2014;51(3):221‐5. [PUBMED: 25296083] [DOI] [PubMed] [Google Scholar]
Schneider 2014
- Schneider AS, Schettler A, Markowski A, Luettig B, Kaufmann B, Klamt S, et al. Complication and mortality rate after percutaneous endoscopic gastrostomy are low and indication‐dependent. *Conference presentation: 36th ESPEN Congress in Leipzig, Germany on August 31st – September 3rd, 2013. Scandinavian Journal of Gastroenterology 2014;49(7):891‐8. [PUBMED: 24896841] [DOI] [PubMed] [Google Scholar]
Schröder 2004
- Schröder O, Hoepffner N, Stein J. Enteral nutrition by endoscopic means; I. Techniques, indications, types of enteral feed. Zeitschrift für Gastroenterologie 2004;42(12):1385‐92. [PUBMED: 15592963] [DOI] [PubMed] [Google Scholar]
Simons 2014
- Simons JA, Fietzek UM, Waldmann A, Warnecke T, Schuster T, Ceballos‐Baumann AO. Development and validation of a new screening questionnaire for dysphagia in early stages of Parkinson's disease. Parkinsonism & Related Disorders 2014;20(9):992‐8. [PUBMED: 25012695] [DOI] [PubMed] [Google Scholar]
Skitt 2011
- Skitt LC, Hurley JJ, Turner JK, Green AJ, Pinch N, Dolwani S, et al. Helping the general physician to improve outcomes after PEG insertion: how we changed our practice. Clinical Medicine 2011;11(2):132‐7. [PUBMED: 21526693] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stec 2008
- Stec S, Tarnowski W, Binda A, Kulakowski P. Videofluoroscopic modified barium swallow study for premature ventricular complexes‐associated dysphagia. Circulation. Arrhythmia and Electrophysiology 2008;1(1):e1. [PUBMED: 19808385] [DOI] [PubMed] [Google Scholar]
Stiegmann 1990
- Stiegmann GV, Goff JS, Silas D, Pearlman N, Sun J, Norton L. Endoscopic versus operative gastrostomy: final results of a prospective randomized trial. Gastrointestinal Endoscopy 1990;36(1):1‐5. [PUBMED: 2107116] [DOI] [PubMed] [Google Scholar]
Tucker 2003
- Tucker AT, Gourin CG, Ghegan MD, Porubsky ES, Martindale RG, Terris DJ. 'Push' versus 'pull' percutaneous endoscopic gastrostomy tube placement in patients with advanced head and neck cancer. Laryngoscope 2003;113(11):1898‐902. [PUBMED: 14603043] [DOI] [PubMed] [Google Scholar]
Valente da Silva 2012
- Valente da Silva HG, Santos SO, Silva NO, Ribeiro FD, Josua LL, Moreira AS. Nutritional assessment associated with length of inpatients' hospital stay. Nutrición Hospitalaria 2012;27(2):542‐7. [PUBMED: 22732981] [DOI] [PubMed] [Google Scholar]
Wang 2014
- Wang J, Liu M, Liu C, Ye Y, Huang G. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for patients with head and neck cancer: a systematic review. Journal of Radiation Research 2014;55(3):559‐67. [PUBMED: 24453356] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wollman 1995
- Wollman B, D'Agostino HB, Walus‐Wigle JR, Easter DW, Beale A. Radiologic, endoscopic, and surgical gastrostomy: an institutional evaluation and meta‐analysis of the literature. Radiology 1995;197(3):699‐704. [PUBMED: 7480742] [DOI] [PubMed] [Google Scholar]
Zaherah 2012
- Zaherah Mohamed Shah F, Suraiya HS, Poi PJ, Tan KS, Lai PS, Ramakrishnan K, et al. Long‐term nasogastric tube feeding in elderly stroke patients‐‐an assessment of nutritional adequacy and attitudes to gastrostomy feeding in Asians. Journal of Nutrition, Health & Aging 2012;16(8):701‐6. [PUBMED: 23076512] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gomes 2010
- Gomes CAR Jr, Lustosa SA, Matos D, Andriolo RB, Waisberg DR, Waisberg J. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for adults with swallowing disturbances. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD008096.pub2] [DOI] [PubMed] [Google Scholar]
Gomes 2012
- Gomes CAR Jr, Lustosa SAS, Matos D, Andriolo RB, Waisberg DR, Waisberg J. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for adults with swallowing disturbances. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD008096.pub3] [DOI] [PubMed] [Google Scholar]


