Abstract
Background
Surgery remains an acceptable treatment modality for tubal infertility despite the rise in usage of in vitro fertilisation (IVF). Estimated livebirth rates after surgery range from 9% for women with severe tubal disease to 69% for those with mild disease; however, the effectiveness of surgery has not been rigorously evaluated in comparison with other treatments such as IVF and expectant management (no treatment). Livebirth rates have not been adequately assessed in relation to the severity of tubal damage. It is important to determine the effectiveness of surgery against other treatment options in women with tubal infertility because of concerns about adverse outcomes, intraoperative complications and costs associated with tubal surgery, as well as alternative treatments, mainly IVF.
Objectives
The aim of this review was to determine the effectiveness and safety of surgery compared with expectant management or IVF in improving the probability of livebirth in the context of tubal infertility (regardless of grade of severity).
Search methods
We searched the following databases in October 2016: the Cochrane Gynaecology and Fertility (CGF) Group trials register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL) and PsycINFO; as well as clinical trials registries, sources of unpublished literature and reference lists of included trials and related systematic reviews.
Selection criteria
We considered only randomised controlled trials to be eligible for inclusion, with livebirth rate per participant as the primary outcome of interest.
Data collection and analysis
We planned that two review authors would independently assess trial eligibility and risk of bias and would extract study data. The primary review outcome was cumulative livebirth rate. Pregnancy rate and adverse outcomes, including miscarriage rate, rate of ectopic pregnancy and rate of procedure‐related complications, were secondary outcomes. We planned to combine data to calculate pooled odds ratios (ORs) and 95% confidence intervals (CIs). We planned to assess statistical heterogeneity using the I2 statistic and to assess the overall quality of evidence for the main comparisons using GRADE methods.
Main results
We identified no suitable randomised controlled trials.
Authors' conclusions
The effectiveness of tubal surgery relative to expectant management and IVF in terms of livebirth rates for women with tubal infertility remains unknown. Large trials with adequate power are warranted to establish the effectiveness of surgery in these women. Future trials should not only report livebirth rates per patient but should compare adverse effects and costs of treatment over a longer time. Factors that have a major effect on these outcomes, such as fertility treatment, female partner's age, duration of infertility and previous pregnancy history, should be considered. Researchers should report livebirth rates in relation to severity of tubal damage and different techniques used for tubal repair, including microsurgery and laparoscopic methods.
Plain language summary
Surgery versus IVF or expectant management for women with tubal infertility
Review question
Cochrane review authors investigated the effectiveness of fallopian tube surgery compared with in vitro fertilisation (IVF) or expectant management in overcoming infertility caused by tubal disease.
Background
Tubal surgery to overcome infertility caused by tubal disease is becoming popular, in part because of risks and costs related to IVF, which offers another option for overcoming tubal infertility. Benefits obtained from tubal surgery would potentially be sustained over multiple cycles and many years, even resulting in multiple livebirths. However, tubal surgery is expensive, as it requires additional specialist training and experience among gynaecologists who perform the procedure, and it can involve adverse effects (including ectopic pregnancies) and operative risks. The effectiveness of tubal surgery in comparison with no treatment (expectant management) or IVF in women with tubal infertility is unknown.
Study characteristics
This review identified no suitable trials. Our literature searches are current to October 2016.
Key results
No randomised evidence is currently available. Research is needed to obtain information about adverse outcomes and costs.
Background
Description of the condition
Tubal disease of the fallopian tubes is responsible for 25% to 35% of cases of female infertility (Serafini 1989). Tubal disease can involve the proximal, distal or entire tube and varies in severity. Pelvic inflammatory disease is the most common cause of tubal disease, representing more than 50% of cases, and may affect the fallopian tube at multiple sites (Honore 1999). The Hull & Rutherford classification (2002) is a simple classification system that separates infertile women into three categories according to severity of tubal damage, namely, mild/grade I, moderate/grade II and severe/grade III (Akande 2004). This system is defined in the section on inclusion criteria. Diagnosis is confirmed by hysterosalpingography (HSG) or laparoscopy.
Description of the intervention
Treatment options include surgical tubal repair, expectant management (i.e. waiting/no specific intervention) and in vitro fertilisation (IVF). The effectiveness of these treatments has not been tested rigorously in the context of randomised controlled trials (RCTs).
Surgery
Despite operative risks (general anaesthetic, intraoperative and postoperative) and a high postoperative incidence of ectopic pregnancy, surgery for tubal infertility is considered an effective treatment option. Procedures such as salpingostomy (formation of an opening into a uterine tube for the purpose of drainage) or fimbrioplasty (breaking of scar tissue around the distal end of the tube) are widely performed for distal tubal obstruction. Surgery is considered a viable treatment option for women with mild tubal disease, and for severe disease, laparoscopic salpingectomy before IVF has a role in improving livebirth rates among women with hydrosalpinges (ASRM 2015; Johnson 2010; NICE 2004).
Tubal ectopic pregnancy ‐ pregnancy in the fallopian tubes ‐ is a potential adverse effect of tubal surgery. A large retrospective regional study from Denmark of 236 women who underwent tubal surgery or adhesiolysis (a procedure performed to remove scar tissue around the tube) reported an ectopic pregnancy rate of 16% (Mosgaard 1996). Higher ectopic pregnancy rates have been associated with increasing severity of tubal damage (Akande 2004). Compared with the 2% incidence of ectopic pregnancy reported in the general population, rates of ectopic pregnancy after surgical correction of tubal abnormalities are reported to be 1% to 10% in mild tubal disease, up to 40% in severe pathology and 2.1% to 11% when IVF is performed in patients with tubal infertility (Schippert 2012).
Expectant management
Pregnancies do occur without treatment in women with a diagnosis of tubal blockage (Collins 1983; Evers 1998; NICE 2004; Wiedemann 1996). It has been suggested that this could be the result of beneficial effects of diagnostic tests required to establish infertility and the therapeutic value of counselling provided during outpatient visits (Collins 1983). In addition, chance inclusion of normal couples (i.e. those at the boundaries of normal reference ranges of fertility) with infertile couples during clinical studies may be contributory. It is likely that "post hoc ergo propter hoc" (i.e. the temporal association between event A and event B immediately implies causation of event B by event A) does not apply for some types of infertility, as the widely held assumption that infertile women serve as their own controls and hence any pregnancy after treatment can be attributed to said treatment may not hold true.
IVF
Tubal infertility remains a major indication for IVF, which completely bypasses tubal blockage and offers an 18% to 29% livebirth rate per cycle (AIHW 2012; SART 2014). As IVF involves manual fertilisation outside the normal reproductive system, it is expensive, invasive and not available to all infertile patients. IVF is associated with several potential complications, including multiple births and foetal anomalies (ASRM 2015; El‐Chaar 2009). Ovarian hyperstimulation syndrome (OHSS) is a potentially life‐threatening adverse effect of ovulation induction. The intravascular depletion associated with OHSS can lead to dehydration, hypovolaemia (low volume of fluid in veins), electrolyte disturbances and thrombosis due to haemoconcentration. In IVF cycles, the rate of severe OHSS requiring hospitalisation is less than 0.01% (AIHW 2012; HFEA 2015). This figure increases with the number of oocytes retrieved at each cycle, reaching 4.0% when more than 20 oocytes have been retrieved (AIHW 2012; HFEA 2015). Older women have been shown to have poor success rates, and increased recognition of factors such as parity (number of children to whom a patient has given birth), duration of infertility, coexisting infertility factors and local IVF success rates can influence outcomes (AIHW 2012; SART 2014).
How the intervention might work
Surgery
The largest case series to date reported an impressive intrauterine pregnancy rate of 72.8% (2369/3254) for terminal salpingo‐neostomy and salpingo‐ovariolysis performed in patients with tubo‐peritoneal infertility (Ponomarev 2009). However, the time period over which the pregnancy rate was measured was not mentioned in the study publication, which was provided in the form of a conference abstract. A recent meta‐analysis combining 22 observational studies of women (N = 2810) undergoing salpingostomy for hydrosalpinges revealed a cumulative pregnancy rate of 20.0% (95% confidence interval (CI) 17.5% to 22.8%) at one year and 25.5% (95% CI 22.2% to 29.4%) at two years (Chu 2015). Although surgical techniques, participant characteristics and duration of follow‐up were heterogeneous, study authors cited these as reasons for generalisability.
The second largest case series to date (N = 1669), which stratified participants according to severity of tubal disease, reported favourable pregnancy outcomes of 55% to 80% for those with mild tubal disease, including prior tubal ligation (n = 1517), and poor pregnancy outcomes of 10% for participants with severe disease (e.g. concurrent proximal and distal lesions, extended dense adhesions, sclerohypertrophic tube, intra‐ampullary adhesions) (n = 152) at a minimum of two years of follow‐up (Tran 2010). However, the significance of these positive results is limited by the risk of bias inherent to retrospective case series.
Expectant management
A retrospective analysis of 109 women with proximal tubal occlusion reported a spontaneous pregnancy rate of 10% per patient and 1.6% per month (Wiedemann 1996). This study showed that when assisted reproductive technology – in particular, gamete intrafallopian transfer (GIFT) – was used as a subsequent treatment, the pregnancy rate was increased to 50%. A retrospective cohort study of 562 couples with tubal factor infertility who were on the waiting list for IVF found that the 12‐month cumulative spontaneous pregnancy rate was only 2.4% (95% CI 1.2% to 3.9%). More than 75% of these pregnancies occurred during the first three months on the waiting list (Evers 1998). Another study followed 1145 couples with infertility and noted that 61% (26/43) of conceptions among patients with infertility of tubal origin were treatment independent, defined as pregnancies that occur after no treatment, three months after medical management or 12 months after surgical management, respectively. Of note, a significant percentage of non‐treated infertile couples also conceived ‐ 35% (191/548). However, subgroup analysis revealed that pregnancies unrelated to treatment were less likely to occur in women with bilateral tubal occlusion (0%; 0/5) than in women with other less severe tubal lesions (68%; 26/38), further emphasising the need for comparative studies stratifying participants according to severity of disease (Collins 1983).
IVF
Analysis of cumulative data showed that women with tubal factor, both with and without other coexisting infertility factors, had a pregnancy rate in excess of 70% after four cycles of IVF and embryo transfer (Benadiva 1995). A meta‐analysis of 14 retrospective studies compared pregnancy rates in women with tubal infertility with and without hydrosalpinx (accumulation of watery fluid in the tube as a consequence of distal obstruction) and revealed the pregnancy rate to be 31.2% for the 4588 women without hydrosalpinx who underwent IVF (Camus 1999). Most of the studies included in this meta‐analysis did not specify the number of IVF cycles completed.
Why it is important to do this review
Considerable uncertainty remains about whether surgical treatment is superior to expectant management and IVF in women with tubal factor infertility. Surgery is still commonly performed, especially in areas where reimbursement for IVF is not available. This systematic review evaluated the effectiveness and safety of surgery in comparison with other available treatments for women with tubal infertility.
Objectives
The aim of this review was to determine the effectiveness and safety of surgery compared with expectant management or IVF in improving the probability of livebirth in the context of tubal infertility (regardless of grade of severity).
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished randomised controlled trials (RCTs) comparing the clinical effectiveness of tubal surgery versus expectant management or IVF. We included cross‐over trials if pre‐cross‐over data were available.
Types of participants
Inclusion criteria
Participants were required to meet all the criteria listed below.
Subfertile couples with infertility of at least one year’s duration.
Women younger than 40 years of age.
Women with minor/grade I, moderate/grade II or severe/grade III tubal damage confirmed before tubal surgery by hysterosalpingography (HSG) or laparoscopy.
Women who had undergone tubal surgery for minor/grade I, moderate/grade II or severe/grade III tubal damage after investigation.
According to the Hull & Rutherford 2002 classification of tubal damage (Akande 2004), minor/grade I tubal damage is defined as:
tubal fibrosis absent even if tube occluded (proximally);
tubal distension absent even if tube occluded (distally);
mucosal appearances favourable; and
flimsy adhesions (peritubal‐ovarian).
Moderate/grade II tubal damage is defined as:
unilateral severe tubal damage;
contralateral minor disease present or absent; and
'limited' dense adhesions of tubes and/or ovaries.
Severe/grade III tubal damage is defined as:
bilateral tubal damage;
extensive tubal fibrosis;
tubal distension greater than 1.5 cm;
abnormal mucosal appearance;
bipolar occlusion; and
'extensive' dense adhesions.
Exclusion criteria
Women 40 years of age or older.
Women with multiple or other causes of infertility such as ovulatory or sperm dysfunction.
Women who had undergone tubal sterilisation.
When trials included couples with infertility of various categories, we included only couples with tubal infertility. We excluded participants with other causes of infertility because their inclusion could have confounded outcomes. When we had doubt about the definitions of various grades of tubal infertility, we requested more information from study authors. If extraction of data is not possible for any reason, we will exclude that trial and will state the reason for exclusion. We will include in the review trials that cannot be included in the meta‐analysis owing to insufficient data.
Types of interventions
Included studies performed one or more comparisons of effectiveness of tubal surgery versus expectant management, or of tubal surgery versus IVF. We considered a variety of techniques for tubal surgery to be eligible, including microsurgery or macrosurgery, laparoscopy and minilaparotomy or laparotomy. No treatment for infertility was administered to couples undergoing expectant management. For women undergoing IVF, a standard IVF procedure was carried out according to standard protocols for controlled ovarian stimulation, oocyte retrieval under ultrasound guidance, insemination, embryo culture and transcervical replacement of embryos, most often between pro‐nucleate and eight‐cell stages. Embryo transfer up to the blastocyst stage and frozen replacement cycles were eligible for inclusion.
Types of outcome measures
Primary outcomes
Cumulative livebirth rate per couple, where cumulative refers to time‐specific or cycle‐specific rates over a given time or number of cycles, and livebirth is defined as the delivery of one or more living infants after 20 completed weeks of gestational age.
Secondary outcomes
Cumulative pregnancy rate per participant/couple (evidence of a gestational sac, confirmed on ultrasonography, defines clinical pregnancy).
Pregnancy rate per participant/couple (evidence of clinical pregnancy ‐ evidence of a gestational sac, confirmed on ultrasonography), including ectopic pregnancy, although multiple gestational sacs in one individual count as one clinical pregnancy).
Livebirth rate per cycle commenced.
Ectopic pregnancy rate per participant.
Multiple pregnancy rate per participant (demonstration of more than one sac with foetal pole on ultrasonographic scan defines multiple pregnancy).
Incidence of OHSS per participant.
Search methods for identification of studies
We searched for all published and unpublished RCTs comparing tubal surgery versus expectant management or IVF, without language restriction, and in consultation with the Gynaecology and Fertility Group (CGF) Information Specialist.
Electronic searches
We searched the following databases.
Gynaecology and Fertility Group (CGFG) Specialised Register of Controlled Trials Procite (searched 19 October 2016) (Appendix 1).
Central Register of Controlled Trials (CENTRAL) Ovid (searched 19 October 2016) (Appendix 2).
MEDLINE Ovid (1946 to 19 October 2016) (Appendix 3).
Embase Ovid (1974 to 19 October 2016) (Appendix 4).
PsycINFO Ovid (1806 to 19 October 2016) (Appendix 5).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) EBSCO (1982 to 19 October 2016) (Appendix 6).
Database of Abstracts of Reviews of Effects (DARE) in the Cochrane Library (for reference lists from relevant non‐Cochrane reviews) (searched 17 August 2015) (Appendix 7).
-
Trial registries for ongoing and registered trials.
http://www.clinicaltrials.gov (a service of the US National Institutes of Health) (searched 24 November 2016) (Appendix 8).
http://www.who.int/trialsearch/Default.aspx (World Health Organization International Trials Registry Platform search portal) (searched 24 November 2016) (Appendix 9).
Web of Science (searched 24 November 2016) (Appendix 10).
OpenGrey (unpublished literature from Europe) (searched 24 November 2016) (Appendix 11).
Latin American Caribbean Health Sciences Literature (LILACS, trials from the Portuguese and Spanish speaking world) (searched 24 November 2016) (Appendix 12).
PubMed and Google Scholar (for recent trials not yet indexed in MEDLINE) (searched 24 November 2016) (Appendix 13; Appendix 14).
ProQuest Dissertations & Theses Global (searched 17 August to 24 November 2016) (Appendix 15).
We combined the MEDLINE search with the Cochrane highly sensitive search strategy, which appears in the Cochrane Handbook of Systematic Reviews of Interventions (Version 5.0.2, Chapter 6, 6.4.11) (Higgins 2011), to identify randomised trials. We combined the Embase, PsycINFO and CINAHL searches with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN).
We designed a new search strategy that differed from the strategy used in the previous version of this review, necessitating database searches covering inception up to October 2015. All searches were current to 19 October 2016.
Searching other resources
We searched reference lists of articles retrieved by the search, along with conference abstracts not covered in the CGFG register, in liaison with the Information Specialist (Appendix 16). We communicated with trial authors and experts in the field regarding additional trials.
Data collection and analysis
Selection of studies
We planned that two review authors (SC and BM) would independently undertake selection of studies after an initial screen of titles and abstracts retrieved (by SC), employing the search strategy outlined above. We planned that study investigators would be contacted, as required, to clarify study eligibility. We resolved discrepancies by discussion. Review authors identified no RCTs via the search strategy. We documented the selection process on a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow chart (see Figure 1).
1.
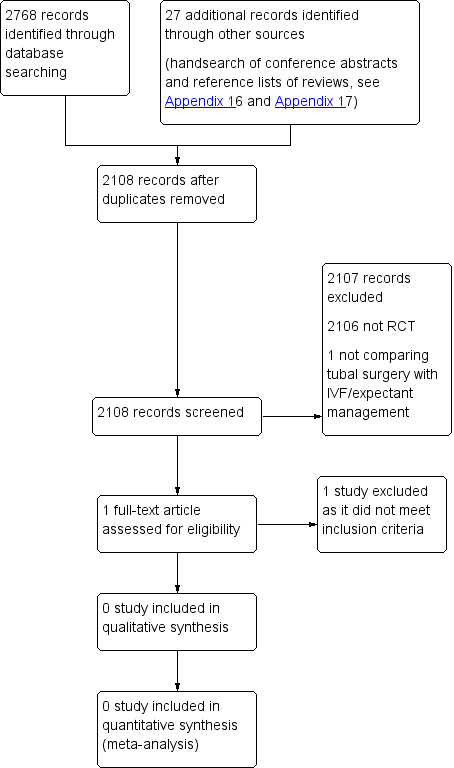
PRISMA study flow diagram.
Data extraction and management
We planned that two review authors (SC and BM) would independently extract data from eligible studies and would resolve disagreements by discussion or by consultation with the third review author. Data extracted would include study characteristics and outcome data (see data extraction form for details; Appendix 18). We would collate studies involving multiple publications in such a way that each study, with its unique study identifier and multiple references, rather than each report, would be considered a single unit of interest in the review.
We planned to extract the following data from studies selected for inclusion in the review.
Trial characteristics.
Characteristics of study participants.
Outcomes.
Analysis.
Assessment of risk of bias in included studies
We planned that two review authors (SC and BM) would independently assess included studies for risk of bias using the Cochrane risk of bias assessment tool (www.cochrane‐handbook.org) to assess selection (random sequence generation and allocation concealment), performance (blinding of participants and personnel), detection (blinding of outcome assessors), attrition (incomplete outcome data), reporting (selective reporting) and other bias. We planned to resolve disagreements by discussion with the third review author. We described all judgements fully and presented our conclusions in the risk of bias table.
We planned to search for within‐trial selective reporting when obvious outcomes were not reported or were not reported in insufficient detail to allow inclusion and to seek published protocols for comparison with the final published study.
Measures of treatment effect
For dichotomous data (e.g. livebirth rates), we planned to use numbers of events in the control and intervention groups of each study to calculate Peto odds ratios (ORs). We planned to present 95% confidence intervals for all outcomes. When data needed to calculate ORs were not available, we planned to utilise the most detailed numerical data available that might facilitate similar analyses of included studies (e.g. test statistics, P values). We planned to assess whether estimates calculated in the review for individual studies were compatible in each case with estimates reported in study publications.
Unit of analysis issues
We planned that the primary analysis would be randomised per woman, and we planned to include per pregnancy data for some outcomes (e.g. miscarriage). We would briefly summarise in an additional table data that did not allow valid analysis (e.g. "per cycle" data) and would not perform meta‐analysis. We would count multiple livebirths (e.g. twins, triplets) as one livebirth event and would include only first‐phase data obtained from cross‐over trials.
Dealing with missing data
We planned to analyse the data on an intention‐to‐treat basis as far as possible, and to attempt to obtain missing data from the original trialists. When these data could not be obtained, we planned to undertake imputation of individual values for the primary outcome only. We planned to assume that livebirths did not occur in participants without a reported outcome. For other outcomes, we planned to analyse only available data. We would perform sensitivity analysis of any imputation undertaken (see below).
Assessment of heterogeneity
We planned to consider whether clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We would have assessed statistical heterogeneity by using the I2 statistic (I2 greater than 50% would indicate substantial heterogeneity) (Higgins 2003; Higgins 2011).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, review authors planned to minimise their potential impact by ensuring a comprehensive search for eligible studies and by staying alert for duplication of data. If we included 10 or more studies in an analysis, we planned to use a funnel plot to explore the possibility of small study effects (the tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
If studies were sufficiently similar, we planned to combine the data using a fixed‐effect model for the following comparisons.
Tubal surgery versus expectant management.
Tubal surgery versus IVF.
Subgroup analysis and investigation of heterogeneity
We planned no subgroup analyses.
Sensitivity analysis
We planned to conduct sensitivity analyses for the primary outcome to determine whether conclusions were robust to arbitrary decisions made regarding eligibility and analysis. These analyses were to include consideration of whether review conclusions would have differed if:
eligibility were restricted to studies without high risk of bias;
a random‐effects model had been adopted;
alternative imputation strategies had been implemented; or
the summary effect measure was relative risk rather than odds ratio.
Overall quality of the body of evidence ‐ 'Summary of findings' table
We planned to prepare a 'Summary of findings' table using GRADEpro software (GRADEpro GDT 2014). This table would have evaluated the overall quality of the body of evidence for the main review outcomes (livebirth rate and pregnancy rate) according to GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias). We would have justified judgements about evidence quality (high, moderate or low) and would have documented and incorporated these into reporting of results for each outcome. Two review authors (SC and BM) would have made judgements independently as needed.
Results
Description of studies
We identified no eligible trials for inclusion.
Results of the search
We included no RCTs.
Excluded studies
We found one related single‐centre RCT that was performed at a university tertiary care centre in Iran from March 2002 to September 2004 (2.5‐year period). This study included 13 participants with unilateral hydrosalpinx and recurrent abortion, detected on ultrasonography and hysterosalpingography, and thus did not meet our inclusion criteria, which required that couples would be subfertile.
Ongoing studies
We found no currently ongoing studies undertaken to compare the effectiveness of tubal surgery versus expectant management or IVF.
Risk of bias in included studies
We identified no RCTs for inclusion in the review, so we could perform no assessment of methodological quality.
Effects of interventions
We found no RCTs that compared surgery versus expectant management or IVF in women with tubal infertility; therefore, we cannot report study data.
Discussion
Summary of main results
This review shows that evidence on this topic has not been provided by randomised controlled trials (RCTs).
Overall completeness and applicability of evidence
No evidence was available. Despite potential risks of surgery, such as possibly increased risk of ectopic pregnancy, and despite widespread availability of in vitro fertilisation (IVF), surgical treatment remains a popular option. This is reflected by recent epidemiological data on fertility services indicating that although the ratio of IVF services to tubal surgery favours IVF, actual prevalence of tubal surgeries performed has remained static. In the United States, 3.2% of women 25 to 44 years of age with fertility problems have ever used reproductive surgery, and 3.1% have used IVF (Chandra 2014).
Potential biases in the review process
As this systematic review applied a newly designed search strategy that encompassed an extensive number of databases from conception, it is not likely that we missed relevant studies. However, we could not adequately assess publication bias by using a funnel plot owing to the scarcity of RCTs on this topic. We may have automatically excluded good quality observational studies that may adequately answer the study question owing to the nature of the protocol of this systematic review. Consideration must be given to incorporating such studies in future reviews because well‐powered RCTs continue to be few.
Agreements and disagreements with other studies or reviews
As a result of the limited nature of available data, it has repeatedly been difficult to draw reliable conclusions on the effectiveness of surgery for tubal infertility. Until data from large RCTs with adequate power become available, clinical practice must be guided on the basis of available observational studies, many of which are confounded by bias due to the traditional method of using each couple as its own internal control, hence assuming that any fertility outcome could be totally attributed to the intervention performed. Moreover, very few observational studies have incorporated direct concurrent comparison of two or more cohorts undergoing different interventions, respectively.
A previous version of this review (Pandian 2008) evaluated this topic, and a related review examined use of pelvic surgery for subfertility (Ahmad 2006). Neither these reviews nor any of the numerous non‐Cochrane systematic reviews on this topic have produced solid answers over the past three decades. Reasons for the lack of well‐designed RCTs in this area are manifold. The validity of the classification systems used to assess severity of tubal damage is questionable. Extent of tubal disease and the presence of pelvic pathology are important factors in the prognosis for success after surgical repair. The pregnancy outcome has been found to be uniformly poor after surgical treatment in patients with severe tubal disease (less than 15% pregnancy rate) (Akande 2004; Wu 1988). Selection of appropriate patients is an important determinant of outcomes after surgery and is not possible in the absence of a reliable classification system. The group of patients thought to be eligible for participation in such an RCT may, therefore, comprise a misrepresentation of the typical patient population required. Recruitment for such trials is impaired by the provision of insufficient patient information; an accepted, reliable method that can provide precise prognostic information for women with tubal damage is needed. The advent of IVF has diminished the role of tubal surgery, and tubal infertility remains one of the major indications for IVF. Although it is expensive and invasive, IVF is the preferred choice for older women with severe tubal damage. With the reported livebirth rate per IVF cycle in most centres as high as 30% (SART 2014), and in light of uncertainties surrounding the outcomes of tubal surgery, a preference for IVF may contribute to poor recruitment for surgical RCTs. Furthermore, women with tubal damage find the spontaneous pregnancy rate unacceptably low (12‐month cumulative pregnancy rate (PR) of 2.4%); consequently, expectant management is an unattractive option for them (Evers 1998).
Funding constraints in some clinical situations mean that many women and clinicians continue to favour surgery. An additional advantage of surgery over IVF is the theoretically permanent restoration of the ability to naturally conceive for every ovulation cycle over a sustained period. This is compared with the high chance afforded by IVF over the few cycles performed and associated complications of multiple births, foetal anomalies and ovarian hyperstimulation syndrome (OHSS) (AIHW 2012; ASRM 2015; El‐Chaar 2009; HFEA 2015). Tubal surgery may be the only treatment option for couples who object to IVF for religious, moral or emotional reasons. Finally, when effective, tubal surgery leads to sustainable improvement in fertility prospects, whereas IVF (apart from frozen embryos) provides only one chance.
Specific problems have been noted with RCTs that involve surgical procedures. It is difficult to standardise the surgical procedures being tested, as procedures evolve continuously and complications decrease as surgeons gain experience. The success rate of a specific procedure depends on the experience and skill of the surgeon. It is important that all participating surgeons undergo appropriate training before the start of an RCT to reach a certain minimal level of standardisation, but this is not always possible. Blinding of participants and surgeons in surgical trials is a potential source of bias, particularly as it is not always possible to do this when one of the interventions being tested is surgical. Financial support for surgical clinical trials is limited, and this is an ongoing problem.
Despite the problems described above, serious consideration should be given to conducting RCTs to determine the effectiveness of surgery in comparison with expectant management and IVF for tubal infertility. Inclusion of women with mild and moderate tubal disease and exclusion of women with severe tubal disease may provide a reasonable way forward.
Authors' conclusions
Implications for practice.
The effectiveness of tubal surgery relative to expectant management and IVF in terms of livebirth rates for women with tubal infertility remains unknown.
Implications for research.
Randomised studies are needed to evaluate clinical outcomes and cost‐effectiveness of tubal surgery compared with no treatment and IVF. Large RCTs with sufficient power are warranted. These trials should include a prolonged period of follow‐up (possibly lasting several years). Treatment protocols, methods of sperm preparation (for IVF), numbers of embryos transferred (for IVF) and inclusion and exclusion criteria should be clearly stated. Participant characteristics should be clear (age, duration of infertility, infertility investigations and previous therapy). In addition, participants should be stratified according to severity of tubal lesions.
With regard to surgical interventions, researchers should describe the techniques used and the experience of the surgeon(s). Future trials should use adequate methods of randomisation and should clearly state numbers and reasons for drop‐out and withdrawal. Allocation concealment should be adequate, and intention‐to‐treat analysis performed. Investigators should perform a power calculation and should provide a clear description of the improvement in treatment outcome that is considered clinically significant. Use of parallel rather than cross‐over study design is also favourable for continued study of these events because the latter may exaggerate treatment effectiveness. Outcome measures should include pregnancy rate (PR) and livebirth rate (LBR) per participant/couple. Although rates per cycle are commonly reported, they constitute a 'unit of analysis' error and do not generate valid estimates or confidence intervals. Estimation of cumulative LBRs is also important. So results can be expressed as cumulative LBR after 'n' cycles, researchers should provide results after each cycle separately. They should evaluate cumulative LBRs by means of Cox proportional hazard analysis, which is a form of survival analysis (Cohlen 2002). It is important that they include all cycles in the denominator. If cycles with poor outcomes are excluded, effectiveness can be exaggerated. In trials that include tubal surgery, researchers should state the number of ectopic pregnancies. Trialists should report pregnancy outcomes in relation to different grades of tubal damage and must describe significant adverse outcomes such as ectopic pregnancy (this information is crucial).
What's new
| Date | Event | Description |
|---|---|---|
| 16 January 2017 | New search has been performed | The background and methods sections have been updated to current Cochrane standards. |
| 16 January 2017 | New citation required but conclusions have not changed | New searches did not identify any studies eligible for inclusion. |
History
Protocol first published: Issue 1, 2007 Review first published: Issue 3, 2008
| Date | Event | Description |
|---|---|---|
| 14 April 2008 | Amended | Converted to new review format |
| 3 March 2008 | New search has been performed | Contact details updated |
| 15 November 2006 | New citation required and conclusions have changed | Substantive amendments made |
Acknowledgements
Thanks to the Cochrane Gynaecology and Fertility Group for support provided.
The authors of the 2016 update acknowledge the contributions of Professor Bhattacharya, Dr Pandian and Dr Harrild to previous versions of this review.
Appendices
Appendix 1. Gynaecology and Fertility Group Specialised Register
(searched from inception to 19 October 2016) (PROCITE platform)
Keywords CONTAINS "Fallopian tube obstruction" or "tubal factor" or "tubal flushing" or "tubal infertility" or "tubal inflation" or "tubal occlusion" or "tubal occlusion ‐ proximal" or "tubal patency" or "tubal reconstruction" or "tubal subfertility" or "tube drainage" or "tuboplasty" or "Fallopian Tube Fixation" or "fallopian tubes" or "tubal anastomosis" or "tubal disorders" or "tubo‐ovarian abscess" or "salpingectomy" or "Salpingitis‐Physiopathology" or "salpingo‐oopherectomy" or "Salpingolysis" or "*Salpingostomy‐"or "salpingotomy" or "Hydrosalpinx" or "hydrosalpingies" or "hydrosalpinges" or "falloscopy" or "laparoscopic salpingectomy" or "laparoscopic salpingoovolysis" or "laparoscopic salpingotomy" or "laparoscopic tubal fulguration" or "microsurgery" or "microscopic" or "microdiathermy" or "microlaparoscopy" or "hydrotubation" (446 hits)
Appendix 2. CENTRAL CRSO search strategy
(searched from inception to 19 October 2016) (CRSO web platform)
#1 MESH DESCRIPTOR Fallopian Tube Diseases EXPLODE ALL TREES 137
#2 MESH DESCRIPTOR Pelvic Inflammatory Disease EXPLODE ALL TREES 422
#3 MESH DESCRIPTOR Salpingitis EXPLODE ALL TREES 42
#4 (tubal infertility):TI,AB,KY 52
#5 ( tubal factor):TI,AB,KY 55
#6 (disten* adj3 tub*):TI,AB,KY 5
#7 (tubal subfertility):TI,AB,KY 2
#8 (tub* adj3 occlusion*):TI,AB,KY 163
#9 (tube* adj3 damage*):TI,AB,KY 7
#10 (tubal adj3 damage*):TI,AB,KY 12
#11 (adhesion* adj3 tub*):TI,AB,KY 22
#12 fallopian:TI,AB,KY 534
#13 (peritubal adj3 adhesion*):TI,AB,KY 3
#14 (tub* adj3 block*):TI,AB,KY 78
#15 hydrosalpin*:TI,AB,KY 53
#16 (Tub* adj3 lesion*):TI,AB,KY 46
#17 (disease* adj3 tub*):TI,AB,KY 222
#18 (occlu* adj3 oviduct*):TI,AB,KY 2
#19 (adhesion* adj3 oviduct*):TI,AB,KY 2
#20 (Tub* adj3 obstruction*):TI,AB,KY 61
#21 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 1531
#22 MESH DESCRIPTOR Gynecologic Surgical Procedures EXPLODE ALL TREES 3630
#23 MESH DESCRIPTOR Salpingectomy EXPLODE ALL TREES 22
#24 MESH DESCRIPTOR salpingostomy EXPLODE ALL TREES 38
#25 MESH DESCRIPTOR Hand‐Assisted Laparoscopy EXPLODE ALL TREES 7
#26 MESH DESCRIPTOR Laparoscopy EXPLODE ALL TREES 4243
#27 Laparoscop*:TI,AB,KY 9365
#28 MESH DESCRIPTOR Laparotomy EXPLODE ALL TREES 622
#29 Laparotomy:TI,AB,KY 1812
#30 electrosurg*:TI,AB,KY 352
#31 MESH DESCRIPTOR Electrosurgery EXPLODE ALL TREES 204
#32 MESH DESCRIPTOR Microsurgery EXPLODE ALL TREES 518
#33 microsurg*:TI,AB,KY 732
#34 minilaparotom*:TI,AB,KY 106
#35 (tubo‐cornual anastomosis):TI,AB,KY 0
#36 fimbrioplasty:TI,AB,KY 6
#37 adhesiolysis:TI,AB,KY 85
#38 reconstruction:TI,AB,KY 3866
#39 (recanalizing or recanalising):TI,AB,KY 7
#40 (recanalisation or recanalization):TI,AB,KY 872
#41 (salpingostomy or salpingectomy):TI,AB,KY 142
#42 aspiration:TI,AB,KY 3801
#43 electrocoagulation:TI,AB,KY 716
#44 MESH DESCRIPTOR Sclerotherapy EXPLODE ALL TREES 432
#45 Sclerotherap*:TI,AB,KY 1153
#46 emboli?ation:TI,AB,KY 1111
#47 excision*:TI,AB,KY 3333
#48 #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 27783
#49 #21 AND #48 358
Appendix 3. MEDLINE search strategy
(searched form 1946 to 19 October 2016) (Ovid platform)
1 exp fallopian tube diseases/ or pelvic inflammatory disease/ or salpingitis/ (11952) 2 tubal infertility.tw. (707) 3 tubal subfertility.tw. (14) 4 tubal factor.tw. (719) 5 tubal fibrosis.tw. (6) 6 (disten$ adj3 tube).tw. (70) 7 (disten$ adj3 tubal).tw. (10) 8 tubal occlusion.tw. (912) 9 (occlusion adj3 tubes).tw. (70) 10 (occlusion adj3 tube).tw. (306) 11 ((tube adj3 damage) or (tubal adj3 damage)).tw. (426) 12 (tube adj3 damage).tw. (124) 13 (adhesion$ adj3 tubal).tw. (199) 14 (adhesion$ adj3 tube).tw. (216) 15 (adhesion$ adj3 tubes).tw. (66) 16 fallopian.tw. (9086) 17 (peritubal adj3 adhesion$).tw. (117) 18 (tube adj3 block$).tw. (530) 19 (tubal adj3 block$).tw. (160) 20 (tubes adj3 block$).tw. (206) 21 hydrosalpin$.tw. (842) 22 ((Tubal adj3 lesion$) or (Tube adj3 lesion$)).tw. (240) 23 ((disease$ adj3 tubal) or (disease$ adj3 tubes)).tw. (576) 24 (oviduct$ adj3 damage$).tw. (33) 25 (oviduct$ adj3 fibrosis).tw. (4) 26 (disten$ adj3 oviduct$).tw. (7) 27 (occlu$ adj3 oviduct$).tw. (48) 28 (adhesion$ adj3 oviduct$).tw. (24) 29 ((Tubal adj3 obstruction$) or (Tube adj3 obstruction$)).tw. (1058) 30 1 and 29 (266) 31 or/1‐28,30 (21711) 32 gynecologic surgical procedures/ or salpingectomy/ or salpingostomy/ (9590) 33 (surgery or surgical).tw. (1441006) 34 32 and 33 (6300) 35 laparoscopy/ or hand‐assisted laparoscopy/ (70173) 36 Laparoscop$.tw. (101252) 37 Laparotomy/ (17222) 38 Laparotomy.tw. (40722) 39 electrosurgery/ or microsurgery/ (28971) 40 microsurg$.tw. (22043) 41 minilaparotom$.tw. (994) 42 tubo‐cornual anastomosis.tw. (1) 43 fimbrioplasty.tw. (71) 44 adhesiolysis.tw. (1226) 45 reconstruction.tw. (160461) 46 (recanalizing or recanalising).tw. (173) 47 (recanalisation or recanalization).tw. (9700) 48 (salpingostomy or salpingectomy).tw. (1786) 49 aspiration.tw. (68032) 50 electrocoagulation.tw. (2763) 51 Sclerotherapy/ (4766) 52 Sclerotherap$.tw. (6126) 53 emboli?ation.tw. (39239) 54 or/32,34‐53 (469023) 55 31 and 54 (5028) 56 randomized controlled trial.pt. (432907) 57 controlled clinical trial.pt. (91818) 58 randomized.ab. (373391) 59 randomised.ab. (76600) 60 placebo.tw. (185046) 61 clinical trials as topic.sh. (180215) 62 randomly.ab. (265326) 63 trial.ti. (163366) 64 (crossover or cross‐over or cross over).tw. (71526) 65 or/56‐64 (1126803) 66 exp animals/ not humans.sh. (4325953) 67 65 not 66 (1039022) 68 55 and 67 (282)
Utilising the Cochrane Highly Sensitive Search Strategies for identifying randomised trials in MEDLINE (Higgins 2011)
Appendix 4. Embase search strategy
(searched from 1974 to 19 October 2016) (Ovid platform)
1 exp uterine tube disease/ or pelvic inflammatory disease/ or salpingitis/ (14863) 2 tubal infertility.tw. (828) 3 tubal subfertility.tw. (15) 4 tubal factor.tw. (875) 5 tubal fibrosis.tw. (6) 6 (disten$ adj3 tube).tw. (84) 7 (disten$ adj3 tubal).tw. (17) 8 tubal occlusion.tw. (949) 9 (occlusion adj3 tubes).tw. (64) 10 (occlusion adj3 tube).tw. (332) 11 ((tube adj3 damage) or (tubal adj3 damage)).tw. (476) 12 (tube adj3 damage).tw. (133) 13 (adhesion$ adj3 tubal).tw. (251) 14 (adhesion$ adj3 tube).tw. (222) 15 (adhesion$ adj3 tubes).tw. (62) 16 fallopian.tw. (9749) 17 (peritubal adj3 adhesion$).tw. (140) 18 (tube adj3 block$).tw. (617) 19 (tubal adj3 block$).tw. (221) 20 (tubes adj3 block$).tw. (242) 21 hydrosalpin$.tw. (1071) 22 ((Tubal adj3 lesion$) or (Tube adj3 lesion$)).tw. (309) 23 ((disease$ adj3 tubal) or (disease$ adj3 tubes)).tw. (649) 24 (oviduct$ adj3 damage$).tw. (26) 25 (oviduct$ adj3 fibrosis).tw. (3) 26 (disten$ adj3 oviduct$).tw. (5) 27 (occlu$ adj3 oviduct$).tw. (41) 28 (adhesion$ adj3 oviduct$).tw. (25) 29 ((Tubal adj3 obstruction$) or (Tube adj3 obstruction$)).tw. (1213) 30 1 and 29 (278) 31 or/1‐28,30 (25812) 32 gynecologic surgical procedures/ or salpingectomy/ or salpingostomy/ (14627) 33 (surgery or surgical).tw. (1658696) 34 32 and 33 (9079) 35 laparoscopy/ or hand‐assisted laparoscopy/ (56876) 36 Laparoscop$.tw. (134431) 37 Laparotomy/ (57049) 38 Laparotomy.tw. (46702) 39 electrosurgery/ or microsurgery/ (28040) 40 microsurg$.tw. (23216) 41 minilaparotom$.tw. (1149) 42 tubo‐cornual anastomosis.tw. (3) 43 fimbrioplasty.tw. (78) 44 adhesiolysis.tw. (1860) 45 reconstruction.tw. (163756) 46 (recanalizing or recanalising).tw. (235) 47 (recanalisation or recanalization).tw. (12911) 48 (salpingostomy or salpingectomy).tw. (2173) 49 aspiration.tw. (79956) 50 electrocoagulatioaintern.tw. (2749) 51 Sclerotherapy/ (8628) 52 Sclerotherap$.tw. (7813) 53 emboli?ation.tw. (47816) 54 or/32,34‐53 (552917) 55 31 and 54 (6793) 56 Clinical Trial/ (848860) 57 Randomized Controlled Trial/ (380154) 58 exp randomization/ (67586) 59 Single Blind Procedure/ (20772) 60 Double Blind Procedure/ (122634) 61 Crossover Procedure/ (43961) 62 Placebo/ (261129) 63 Randomi?ed controlled trial$.tw. (121529) 64 Rct.tw. (17856) 65 random allocation.tw. (1441) 66 randomly.tw. (296704) 67 randomly allocated.tw. (22974) 68 allocated randomly.tw. (2045) 69 (allocated adj2 random).tw. (734) 70 Single blind$.tw. (16181) 71 Double blind$.tw. (153400) 72 ((treble or triple) adj blind$).tw. (471) 73 placebo$.tw. (218534) 74 prospective study/ (302585) 75 or/56‐74 (1662347) 76 case study/ (33245) 77 case report.tw. (287952) 78 abstract report/ or letter/ (933800) 79 or/76‐78 (1248567) 80 75 not 79 (1622351) 81 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5341103) 82 80 not 81 (1508716) 83 55 and 82 (661)
Appendix 5. PsycINFO search strategy
(searched from 1806 to 19 October 2016) (Ovid platform)
1 exp Gynecological Disorders/ (1613) 2 tubal infertility.tw. (2) 3 tubal factor.tw. (4) 4 (disten$ adj3 tube).tw. (1) 5 tubal occlusion.tw. (5) 6 fallopian.tw. (46) 7 ((Tubal adj3 obstruction$) or (Tube adj3 obstruction$)).tw. (6) 8 2 or 3 or 4 or 5 or 6 or 7 (63) 9 1 and 8 (4) 10 8 or 9 (63) 11 exp Gynecology/ or exp Surgery/ (50237) 12 microsurg$.tw. (214) 13 Laparoscop$.tw. (393) 14 Laparotomy.tw. (121) 15 adhesiolysis.tw. (14) 16 reconstruction.tw. (8168) 17 (salpingostomy or salpingectomy).tw. (15) 18 aspiration.tw. (4129) 19 electrocoagulation.tw. (67) 20 emboli?ation.tw. (238) 21 (surgery or surgical).tw. (35051) 22 or/11‐21 (82130) 23 10 and 22 (20) 24 random*.ti,ab,hw,id. (159256) 25 trial*.ti,ab,hw,id. (148083) 26 controlled stud*.ti,ab,hw,id. (10491) 27 placebo*.ti,ab,hw,id. (35395) 28 ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).ti,ab,hw,id. (25155) 29 (cross over or crossover or factorial* or latin square).ti,ab,hw,id. (25065) 30 (assign* or allocat* or volunteer*).ti,ab,hw,id. (137432) 31 treatment effectiveness evaluation/ (20480) 32 mental health program evaluation/ (1970) 33 exp experimental design/ (52046) 34 "2000".md. (0) 35 or/24‐34 (434710) 36 23 and 35 (1)
Appendix 6. CINAHL search strategy
(searched from 1982 to 19 October 2016) (EBSCO platform)
| # | Query | Results |
| S57 | S44 AND S56 | 200 |
| S56 | S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 | 1,081,306 |
| S55 | TX allocat* random* | 5,281 |
| S54 | (MH "Quantitative Studies") | 14,919 |
| S53 | (MH "Placebos") | 9,827 |
| S52 | TX placebo* | 39,650 |
| S51 | TX random* allocat* | 5,281 |
| S50 | (MH "Random Assignment") | 41,699 |
| S49 | TX randomi* control* trial* | 110,746 |
| S48 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 857,082 |
| S47 | TX clinic* n1 trial* | 190,012 |
| S46 | PT Clinical trial | 79,719 |
| S45 | (MH "Clinical Trials+") | 203,397 |
| S44 | S22 AND S43 | 1,006 |
| S43 | S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 | 539,009 |
| S42 | TX salpingo neostom* | 0 |
| S41 | TX recanalisation or TX recanalization | 1,140 |
| S40 | TX salpingostomy or salpingectomy | 231 |
| S39 | TX recanalizing or TX recanalising | 17 |
| S38 | TX lysis N2 adhesion* | 67 |
| S37 | TX reconstruction | 19,742 |
| S36 | TX adhesiolysis | 129 |
| S35 | TX fimbrioplasty | 5 |
| S34 | TX tubo‐cornual anastomosis | 0 |
| S33 | TX excision | 9,388 |
| S32 | TX minilaparotom* | 65 |
| S31 | (MM "Microsurgery+") | 1,269 |
| S30 | TX Laparotomy | 4,259 |
| S29 | (MM "Laparotomy") | 839 |
| S28 | TX microsurg* | 2,853 |
| S27 | TX Laparoscop* | 20,801 |
| S26 | (MH "Surgery, Laparoscopic+") | 4,692 |
| S25 | TX surgical | 160,422 |
| S24 | TX surgery | 467,855 |
| S23 | (MM "Surgery, Gynecologic+") | 6,174 |
| S22 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 | 2,773 |
| S21 | TX disease* N3 tubal | 27 |
| S20 | TX disease* N3 tubes | 322 |
| S19 | TX Tube N3 lesion* | 8 |
| S18 | TX tubes N3 lesion* | 8 |
| S17 | TX Tubal N3 lesion* | 6 |
| S16 | TX hydrosalpin* | 61 |
| S15 | TX tubal N3 block* | 13 |
| S14 | TX tube* N3 block* | 115 |
| S13 | TX pelvic inflammatory | 1,041 |
| S12 | TX peritubal N3 adhesion* | 1 |
| S11 | TX fallopian | 1,155 |
| S10 | TX adhesion* N3 tub* | 30 |
| S9 | TX tubal occlusion | 71 |
| S8 | TX disten* N3 tube* | 10 |
| S7 | TX tubal fibrosis | 2 |
| S6 | TX tub* N3 damage | 271 |
| S5 | (MM "Pelvic Inflammatory Disease") | 416 |
| S4 | TX tubal factor | 113 |
| S3 | TX tubal subfertility | 10 |
| S2 | TX tubal infertility | 112 |
| S1 | (MM "Fallopian Tube Diseases+") | 268 |
Appendix 7. DARE search strategy
Cochrane Library (searched 24 November 2016)
All fields: "Fallopian tube obstruction" or "tubal factor" or "tubal flushing" or "tubal infertility" or "tubal inflation" or "tubal occlusion" or "tubal occlusion ‐ proximal" or "tubal patency" or "tubal reconstruction" or "tubal subfertility" or "tube drainage" or "tuboplasty" or "Fallopian Tube Fixation" or "fallopian tubes" or "tubal anastomosis" or "tubal disorders" or "tubo‐ovarian abcess" or "salpingectomy" or "Salpingitis‐Physiopathology" or "salpingo‐oopherectomy" or "Salpingolysis" or "*Salpingostomy" or "salpingotomy" or "Hydrosalpinx" or "hydrosalpingies" or "hydrosalpinges" or "falloscopy" or "laparoscopic salpingectomy" or "laparoscopic salpingoovolysis" or "laparoscopic salpingotomy" or "laparoscopic tubal fulguration" or "microsurgery" or "microscopic" or "microdiathermy" or "microlaparoscopy" or "hydrotubation"
(142 hits)
Appendix 8. http://www.clinicaltrials.gov search strategy
| PATIENT | INTERVENTION | COMPARATOR | OUTCOME |
| (Infertile OR infertility OR subfertile OR subfertility) AND (Tubal OR tube OR tubes OR oviduct OR oviducts) |
Surgery OR surgical OR surgically |
(Infertile OR infertility OR subfertile OR subfertility) AND (Tubal OR tube OR tubes OR oviduct OR oviducts) AND (Surgery OR surgical OR surgically)
(searched 24 November 2016)
45 hits
Appendix 9. World Health Organization International Trials Registry Portal search strategy
(searched 24 November 2016)
| PATIENT | INTERVENTION | COMPARATOR | OUTCOME |
| (Subfertility OR subfertile OR infertility OR infertile) AND (“Fallopian tube” OR “Fallopian tubes” OR oviduct OR oviducts OR tubal) NOT male |
Subfertility AND tubal NOT male (0 trials)
Infertility AND tubal NOT male (11 trials)
Infertile AND tubal NOT male (11 duplicates)
Subfertile AND tubal NOT male (0 trials)
Subfertility AND oviduct* NOT male (0 trials)
Infertility AND oviduct* NOT male (1 trials)
Infertile AND oviduct* NOT male (1 duplicate)
Subfertile AND oviduct* NOT male (0 trials)
Subfertility AND Fallopian tube* NOT male (0 trials)
Infertility AND Fallopian tube* NOT male (2 trials)
Infertile AND Fallopian tube* NOT male (2 duplicate)
Subfertile AND Fallopian tube* NOT male (0)
28 hits (13 hits minus duplicates)
Appendix 10. Web of Science search strategy
Version 5.18, limited to Web of Science Core Collection database
Basic Search option
(searched 24 November 2016)
| PATIENT | INTERVENTION | COMPARATOR | OUTCOME |
| search tag:TOPIC “fallopian tube disease*” or “pelvic inflammatory disease” or “salpingitis” OR “tubal infertil*” OR “tubal subfertil*” OR tubal factor OR “tubal fibrosis” OR (disten* NEAR/3 tube) OR (disten* NEAR/3 tubal) OR “tubal occlusion” OR (occlusion NEAR/3 tubes) OR (occlusion NEAR/3 tube) OR ((tube NEAR/3 damage) or (tubal NEAR/3 damage)) OR (tube NEAR/3 damage) OR (adhesion* NEAR/3 tubal) OR (adhesion* NEAR/3 tube) OR (adhesion* NEAR/3 tubes) OR fallopian OR (peritubal NEAR/3 adhesion*) OR (tube NEAR/3 block*) OR (tubal NEAR/3 block*) OR (tubes NEAR/3 block*) OR hydrosalpin* OR ((Tubal NEAR/3 lesion*) or (Tube NEAR/3 lesion*)) OR ((disease* NEAR/3 tubal) or (disease* NEAR/3 tubes)) OR (oviduct* NEAR/3 damage*) OR (oviduct* NEAR/3 fibrosis) OR (disten* NEAR/3 oviduct*) OR (occlu* NEAR/3 oviduct*) OR (adhesion* NEAR/3 oviduct*) OR (((Tubal NEAR/3 obstruction*) or (Tube NEAR/3 obstruction*)) AND (“fallopian tube disease*” or “pelvic inflammatory disease” or salpingitis)) |
search tag: TOPIC ((“gynecologic surgical procedures” or salpingectomy or salpingostomy) AND (surgery or surgical)) OR (“gynecologic surgical procedures” or salpingectomy or salpingostomy) or “hand‐assisted laparoscopy“ OR Laparoscop* OR Laparotomy OR electrosurgery or microsurg* OR minilaparotom* OR tubo‐cornual anastomosis OR fimbrioplasty OR adhesiolysis OR reconstruction OR (recanalizing or recanalising) OR (recanalisation or recanalization) OR (salpingostomy or salpingectomy) OR aspiration OR electrocoagulation OR Sclerotherapy OR Sclerotherap* OR emboli?ation |
search tag: TOPIC ((randomi* NEAR/1 “controlled trial*”) OR “controlled clinical trial” OR “random allocation*” OR “double‐blind” OR “single‐blind” OR (clin* NEAR/25 trial*) OR ((singl* or doubl* or tripl* or trebl*) NEAR/25 (blind* or mask*)) OR placebo* OR “Research design”) NOT (animal* not human*) |
The RCT filter was adapted from the Medline RCT filter provided by Cochrane (Higgins 2005)
(193 hits)
Appendix 11. OpenGrey search strategy
(searched 24 November 2016)
| PATIENT | INTERVENTION | COMPARATOR | OUTCOME |
| “fallopian tube disease*” or “pelvic inflammatory disease” or “salpingitis” OR “tubal infertil*” OR “tubal subfertil*” OR tubal factor OR “tubal fibrosis” OR (disten* NEAR/3 tube) OR (disten* NEAR/3 tubal) OR “tubal occlusion” OR (occlusion NEAR/3 tubes) OR (occlusion NEAR/3 tube) OR ((tube NEAR/3 damage) or (tubal NEAR/3 damage)) OR (tube NEAR/3 damage) OR (adhesion* NEAR/3 tubal) OR (adhesion* NEAR/3 tube) OR (adhesion* NEAR/3 tubes) OR fallopian OR (peritubal NEAR/3 adhesion*) OR (tube NEAR/3 block*) OR (tubal NEAR/3 block*) OR (tubes NEAR/3 block*) OR hydrosalpin* OR ((Tubal NEAR/3 lesion*) or (Tube NEAR/3 lesion*)) OR ((disease* NEAR/3 tubal) or (disease* NEAR/3 tubes)) OR (oviduct* NEAR/3 damage*) OR (oviduct* NEAR/3 fibrosis) OR (disten* NEAR/3 oviduct*) OR (occlu* NEAR/3 oviduct*) OR (adhesion* NEAR/3 oviduct*) OR (((Tubal NEAR/3 obstruction*) or (Tube NEAR/3 obstruction*)) AND (“fallopian tube disease*” or “pelvic inflammatory disease” or salpingitis)) |
((“gynecologic surgical procedures” or salpingectomy or salpingostomy) AND (surgery or surgical)) OR (“gynecologic surgical procedures” or salpingectomy or salpingostomy) or “hand‐assisted laparoscopy“ OR Laparoscop* OR Laparotomy OR electrosurgery or microsurg* OR minilaparotom* OR tubo‐cornual anastomosis OR fimbrioplasty OR adhesiolysis OR reconstruction OR (recanalizing or recanalising) OR (recanalisation or recanalization) OR (salpingostomy or salpingectomy) OR aspiration OR electrocoagulation OR Sclerotherapy OR Sclerotherap* OR embolization OR embolization | ((randomi* NEAR/1 “controlled trial*”) OR “controlled clinical trial” OR “random allocation*” OR “double‐blind” OR “single‐blind” OR (clin* NEAR/25 trial*) OR ((singl* or doubl* or tripl* or trebl*) NEAR/25 (blind* or mask*)) OR placebo* OR “Research design”) NOT (animal* not human*) |
The RCT filter was adapted from the Medline RCT filter provided by Cochrane (Higgins 2005)
(“fallopian tube disease*” or “pelvic inflammatory disease” or “salpingitis” OR “tubal infertil*” OR “tubal subfertil*” OR tubal factor OR “tubal fibrosis” OR (disten* NEAR/3 tube) OR (disten* NEAR/3 tubal) OR “tubal occlusion” OR (occlusion NEAR/3 tubes) OR (occlusion NEAR/3 tube) OR ((tube NEAR/3 damage) or (tubal NEAR/3 damage)) OR (tube NEAR/3 damage) OR (adhesion* NEAR/3 tubal) OR (adhesion* NEAR/3 tube) OR (adhesion* NEAR/3 tubes) OR fallopian OR (peritubal NEAR/3 adhesion*) OR (tube NEAR/3 block*) OR (tubal NEAR/3 block*) OR (tubes NEAR/3 block*) OR hydrosalpin* OR ((Tubal NEAR/3 lesion*) or (Tube NEAR/3 lesion*)) OR ((disease* NEAR/3 tubal) or (disease* NEAR/3 tubes)) OR (oviduct* NEAR/3 damage*) OR (oviduct* NEAR/3 fibrosis) OR (disten* NEAR/3 oviduct*) OR (occlu* NEAR/3 oviduct*) OR (adhesion* NEAR/3 oviduct*) OR (((Tubal NEAR/3 obstruction*) or (Tube NEAR/3 obstruction*)) AND (“fallopian tube disease*” or “pelvic inflammatory disease” or salpingitis))) AND (((“gynecologic surgical procedures” or salpingectomy or salpingostomy) AND (surgery or surgical)) OR (“gynecologic surgical procedures” or salpingectomy or salpingostomy) or “hand‐assisted laparoscopy“ OR Laparoscop* OR Laparotomy OR electrosurgery or microsurg* OR minilaparotom* OR tubo‐cornual anastomosis OR fimbrioplasty OR adhesiolysis OR reconstruction OR (recanalizing or recanalising) OR (recanalisation or recanalization) OR (salpingostomy or salpingectomy) OR aspiration OR electrocoagulation OR Sclerotherapy OR Sclerotherap* OR embolization OR embolization ) AND (((randomi* NEAR/1 “controlled trial*”) OR “controlled clinical trial” OR “random allocation*” OR “double‐blind” OR “single‐blind” OR (clin* NEAR/25 trial*) OR ((singl* or doubl* or tripl* or trebl*) NEAR/25 (blind* or mask*)) OR placebo* OR “Research design”) NOT (animal* not human*))
0 hits
Appendix 12. LILACS search strategy
(searched 24 November 2016)
Limited to the LILACs database using the "Controlled Clinical Trial" tag as provided by the search portal
| PATIENT | INTERVENTION | COMPARATOR | OUTCOME |
| (TW:Fallopian Tube Disease*) or (TW:tubal infertility) OR (TW:tubal subfertility) OR (TW:tubal factor*) or (TW:Pelvic Inflammatory Disease) OR (TW:tubal factor infertil*) OR (TW:tubal factor subfertil*) OR (TW:tubal damage) OR (TW:tubal fibrosis) OR (TW:tube damage*) OR (TW:tube fibrosis) OR (TW:oviduct* damage*) OR (TW:oviduct* fibrosis) OR (TW:tubal distension*) OR (TW:tube distension*) OR (TW:distended tube) OR (TW:distended tubes) OR (TW:distended oviduct*) OR (TW:oviduct distension*) OR (TW:tubal occlusion) OR (TW:occluded tube) OR (TW:occluded tubes) OR (TW:tube occlu*) OR (TW:occluded oviduct*) OR (TW:oviduct occlu*) OR (TW:tubal adhesion*) OR (TW:tube adhesion*) OR (TW:oviduct adhesion*) |
(TW:Laparoscop*) or (TW:Microsurg*) or (TW:tubal surgery) OR (TW:surgery oviduct*) OR (TW:surgical* oviduct*) OR (TW:infertility surgery) OR (TW:surgery infertil*) OR (TW:surgery subfertil*) OR (TW:surgical* infertil*) OR (TW:surgical* subfertil*) |
((TW:Fallopian Tube Disease*) or (TW:tubal infertility) OR (TW:tubal subfertility) OR (TW:tubal factor*) or (TW:Pelvic Inflammatory Disease) OR (TW:tubal factor infertil*) OR (TW:tubal factor subfertil*) OR (TW:tubal damage) OR (TW:tubal fibrosis) OR (TW:tube damage*) OR (TW:tube fibrosis) OR (TW:oviduct* damage*) OR (TW:oviduct* fibrosis) OR (TW:tubal distension*) OR (TW:tube distension*) OR (TW:distended tube) OR (TW:distended tubes) OR (TW:distended oviduct*) OR (TW:oviduct distension*) OR (TW:tubal occlusion) OR (TW:occluded tube) OR (TW:occluded tubes) OR (TW:tube occlu*) OR (TW:occluded oviduct*) OR (TW:oviduct occlu*) OR (TW:tubal adhesion*) OR (TW:tube adhesion*) OR (TW:oviduct adhesion*)) AND ((TW:Laparoscop*) or (TW:Microsurg*) or (TW:tubal surgery) OR (TW:surgery oviduct*) OR (TW:surgical* oviduct*) OR (TW:infertility surgery) OR (TW:surgery infertil*) OR (TW:surgery subfertil*) OR (TW:surgical* infertil*) OR (TW:surgical* subfertil*))
5 hits
Appendix 13. PubMed search strategy
(From 2012 to 24 November 2016) (limit to last 5 years)
| PATIENT | INTERVENTION | COMPARATOR | OUTCOME |
| Fallopian Tube Disease*[tw] or tubal infertility[all] OR tubal subfertility[all] OR tubal factor*[all] or Pelvic Inflammatory Disease[tw] OR tubal factor infertil*[all] OR tubal factor subfertil*[all] OR tubal damage[all] OR tubal fibrosis[all] OR tube damage*[all] OR tube fibrosis[all] OR oviduct* damage*[all] OR oviduct* fibrosis[all] OR tubal distension*[all] OR tube distension*[all] OR distended tube[all] OR distended tubes[all] OR distended oviduct*[all] OR oviduct distension* [all] OR tubal occlusion[all] OR occluded tube[all] OR occluded tubes[all] OR tube occlu* [all] OR occluded oviduct*[all] OR oviduct occlu* [all] OR tubal adhesion*[all] OR tube adhesion*[all] OR oviduct adhesion*[all] OR hydrosalpin* [all] OR fallopian [all] OR oviducts)Fallopian Tube Disease*[tw] or tubal infertility[all] OR tubal subfertility[all] OR tubal factor*[all] or Pelvic Inflammatory Disease[tw] OR tubal factor infertil*[all] OR tubal factor subfertil*[all] OR tubal damage[all] OR tubal fibrosis[all] OR tube damage*[all] OR tube fibrosis[all] OR oviduct* damage*[all] OR oviduct* fibrosis[all] OR tubal distension*[all] OR tube distension*[all] OR distended tube[all] OR distended tubes[all] OR distended oviduct*[all] OR oviduct distension* [all] OR tubal occlusion[all] OR occluded tube[all] OR occluded tubes[all] OR tube occlu* [all] OR occluded oviduct*[all] OR oviduct occlu* [all] OR tubal adhesion*[all] OR tube adhesion*[all] OR oviduct adhesion*[all] OR hydrosalpin* [all] OR fallopian [all] |
Laparoscop*[tw] or Microsurg*[tw] or laparotomy*[tw] or aspiration [tw] or tubal surgery[all] OR surgery oviduct*[all] OR surgical* oviduct*[all] OR infertility surgery[all] OR surgery infertil*[all] OR surgery subfertil*[all] OR surgical* infertil*[all] OR surgical* subfertil*[all] OR adhesiolysis [all] OR salpingostomy [all] OR salpingectomy [all] OR embolisation[all] OR embolization[all] OR reconstruction[all] OR surgical OR surgically |
(randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tw] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT (animals[mh] NOT humans[mh]) |
(Fallopian Tube Disease*[tw] or tubal infertility[all] OR tubal subfertility[all] OR tubal factor*[all] or Pelvic Inflammatory Disease[tw] OR tubal factor infertil*[all] OR tubal factor subfertil*[all] OR tubal damage[all] OR tubal fibrosis[all] OR tube damage*[all] OR tube fibrosis[all] OR oviduct* damage*[all] OR oviduct* fibrosis[all] OR tubal distension*[all] OR tube distension*[all] OR distended tube[all] OR distended tubes[all] OR distended oviduct*[all] OR oviduct distension* [all] OR tubal occlusion[all] OR occluded tube[all] OR occluded tubes[all] OR tube occlu* [all] OR occluded oviduct*[all] OR oviduct occlu* [all] OR tubal adhesion*[all] OR tube adhesion*[all] OR oviduct adhesion*[all] OR hydrosalpin* [all] OR fallopian [all]) AND (Laparoscop*[tw] or Microsurg*[tw] or laparotomy*[tw] or aspiration [tw] or tubal surgery[all] OR surgery oviduct*[all] OR surgical* oviduct*[all] OR infertility surgery[all] OR surgery infertil*[all] OR surgery subfertil*[all] OR surgical* infertil*[all] OR surgical* subfertil*[all] OR adhesiolysis [all] OR salpingostomy [all] OR salpingectomy [all] OR embolisation[all] OR embolization[all] OR reconstruction[all]) AND ((randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tw] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT (animals[mh] NOT humans[mh]))
This search utilised the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision) (Higgins 2011)
(76 hits)
Appendix 14. Google Scholar search strategy
The Google Scholar search was run via the Publish or Perish program. (Harzing 2007)
(searched 24 November 2016)
| PATIENT | INTERVENTION | COMPARATOR | OUTCOME |
| (tubal OR fallopian OR oviduct) AND (infertility OR infertile OR subfertile OR subfertility) NOT male NOT men NOT animal |
surgery | Random | pregnancy |
Year of publication between: 2016 and 2016
1. Search field (all of the words): tubal, infertility, surgery, random, pregnancy AND Search Field (none of the words): male, men, animal (107 hits)
2. Search field (all of the words): tubal, infertile, surgery, random, pregnancy AND Search Field (none of the words): male, men, animal (87 hits)
3. Search field (all of the words): tubal, subfertile, surgery, random, pregnancy AND Search Field (none of the words): male, men, animal (11 hits)
4. Search field (all of the words): tubal, subfertility, surgery, random, pregnancy AND Search Field (none of the words):male, men, animal (106 hits)
5. Search field (all of the words): fallopian, infertility, surgery, random, pregnancy AND, Search field (none of the words): male, men, animal (77 hits)
6. Search field (all of the words): fallopian, infertile, surgery, random, pregnancy AND Search field (none of the words): male, men, animal (61 hits)
7. Search field (all of the words): fallopian, subfertile, surgery, random, pregnancy AND Search field (none of the words): male, men, animal (9 hits)
8. Search field (all of the words): fallopian, subfertility, surgery, random, pregnancy AND Search field (none of the words): male, men, animal (79 hits)
9. Search field (all of the words): oviduct, infertility, surgery, random, pregnancy AND Search field (none of the words): male, men, animal (5 hits)
10. Search field (all of the words): oviduct, infertile, surgery, random, pregnancy AND Search field (none of the words): male, men, animal (3 hits)
11. Search field (all of the words): oviduct, subfertile, surgery, random, pregnancy AND Search field (none of the words): male, men, animal (0 hits)
12. Search field (all of the words):oviduct, subfertility, surgery, random, pregnancy AND Search field (none of the words): male, men, animal (5 hits)
Total: 550 hits (146 hits excluding duplicates)
Appendix 15. ProQuest Dissertations & Theses Global search strategy
Searched 24th November 2016
| PATIENT | INTERVENTION | COMPARATOR | OUTCOME |
| “fallopian tube disease*” or “pelvic inflammatory disease” or salpingitis OR “tubal infertil*” OR” tubal subfertil*” OR “tubal factor” OR “tubal fibrosis” OR (disten* NEAR/3 tube) OR (disten* NEAR/3 tubal) OR “tubal occlusion” OR (occlusion NEAR/3 tubes) OR (occlusion NEAR/3 tube) OR ((tube NEAR/3 damage) or (tubal NEAR/3 damage)) OR (tube NEAR/3 damage) OR (adhesion* NEAR/3 tubal) OR (adhesion* NEAR/3 tube) OR (adhesion* NEAR/3 tubes) OR fallopian OR (peritubal NEAR/3 adhesion*) OR (tube NEAR/3 block*) OR (tubal NEAR/3 block*) OR (tubes NEAR/3 block*) OR hydrosalpin* OR ((Tubal NEAR/3 lesion*) or (Tube NEAR/3 lesion*)) OR ((disease* NEAR/3 tubal) or (disease* NEAR/3 tubes)) OR (oviduct* NEAR/3 damage*) OR (oviduct* NEAR/3 fibrosis) OR (disten* NEAR/3 oviduct*) OR (occlu* NEAR/3 oviduct*) OR (adhesion* NEAR/3 oviduct*) OR (((Tubal NEAR/3 obstruction*) or (Tube NEAR/3 obstruction*)) AND (exp fallopian tube diseases or pelvic inflammatory disease or salpingitis)) NOT male NOT male |
((“gynecologic surgical procedure*” or salpingectomy or salpingostomy) AND (surgery or surgical)) OR (“gynecologic surgical procedure*” or salpingectomy or salpingostomy) OR “hand‐assisted laparoscopy” OR Laparoscop* OR Laparotomy OR electrosurgery or microsurg* OR minilaparotom* OR tubo‐cornual anastomosis OR fimbrioplasty OR adhesiolysis OR reconstruction OR (recanalizing or recanalising) OR (recanalisation or recanalization) OR (salpingostomy or salpingectomy) OR aspiration OR electrocoagulation OR Sclerotherap* OR emboli?ation | ((randomi* NEAR/1 “controlled trial*”) OR “controlled clinical trial” OR “random allocation*” OR “double‐blind” OR “single‐blind” OR (clin* NEAR25 trial*) OR ((singl* or doubl* or tripl* or trebl*) NEAR/25 (blind* or mask*)) OR placebo* OR “Research design”) NOT (animal* not human*) |
Pregnan* OR birth* |
(“fallopian tube disease*” or “pelvic inflammatory disease” or salpingitis OR “tubal infertil*” OR” tubal subfertil*” OR “tubal factor” OR “tubal fibrosis” OR (disten* NEAR/3 tube) OR (disten* NEAR/3 tubal) OR “tubal occlusion” OR (occlusion NEAR/3 tubes) OR (occlusion NEAR/3 tube) OR ((tube NEAR/3 damage) or (tubal NEAR/3 damage)) OR (tube NEAR/3 damage) OR (adhesion* NEAR/3 tubal) OR (adhesion* NEAR/3 tube) OR (adhesion* NEAR/3 tubes) OR fallopian OR (peritubal NEAR/3 adhesion*) OR (tube NEAR/3 block*) OR (tubal NEAR/3 block*) OR (tubes NEAR/3 block*) OR hydrosalpin* OR ((Tubal NEAR/3 lesion*) or (Tube NEAR/3 lesion*)) OR ((disease* NEAR/3 tubal) or (disease* NEAR/3 tubes)) OR (oviduct* NEAR/3 damage*) OR (oviduct* NEAR/3 fibrosis) OR (disten* NEAR/3 oviduct*) OR (occlu* NEAR/3 oviduct*) OR (adhesion* NEAR/3 oviduct*) OR (((Tubal NEAR/3 obstruction*) or (Tube NEAR/3 obstruction*)) AND (exp fallopian tube diseases or pelvic inflammatory disease or salpingitis)) NOT male) AND (((“gynecologic surgical procedure*” or salpingectomy or salpingostomy) AND (surgery or surgical)) OR (“gynecologic surgical procedure*” or salpingectomy or salpingostomy) OR “hand‐assisted laparoscopy” OR Laparoscop* OR Laparotomy OR electrosurgery or microsurg* OR minilaparotom* OR tubo‐cornual anastomosis OR fimbrioplasty OR adhesiolysis OR reconstruction OR (recanalizing or recanalising) OR (recanalisation or recanalization) OR (salpingostomy or salpingectomy) OR aspiration OR electrocoagulation OR Sclerotherap* OR emboli?ation ) AND (Pregnan* OR birth*) AND (((controli* NEAR/1 “controlled trial*”) OR “controlled clinical trial” OR “control allocation*” OR “double‐blind” OR “single‐blind” OR (clin* NEAR25 trial*) OR ((singl* or doubl* or tripl* or trebl*) NEAR/25 (blind* or mask*)) OR placebo* OR “Research design”) NOT (animal* not human*))
The RCT filter was adapted from the Medline RCT filter provided by Cochrane (Higgins 2005)
132 hits
Appendix 16. ESHRE and ASRM search strategy
Handsearching of the ESHRE 2007, ESHRE 2015 and ASRM 2008 conference abstracts as these are not covered by the search of the Gynaecology and Fertility Group specialised register.
-
ESHRE 2007 (2)
Gordts S, Campo R, Puttemans P, Valkenburg M, Brosens I, Gordts S. Microsurgical reversal of tubal sterilisation: to be preferred? Hum Reprod. 2007;22(Suppl 1):i227.
Hotineanu AL, Moshin VN, Hotineanu AV, Croitor ME. The effect of the proximal tubal “clamping” prior to the IVF in patients with distal tubal occlusion. Hum Reprod. 2007;22(Suppl 1):i126.
-
ASRM 2008 (5)
Fukuda A, Hamada A, Sawabe M, Sonoda M, Nakaoka Y, Morimoto Y. Pregnancy rate of bilateral tubal occlusion patients by IVF improves after recovery of tubal patency by falloposcopic tuboplasty. Fertility and sterility. 2008;90(Supplement):S155.
Poncelet C, Ducarme G, Yazbeck C, Uzan M, Madelenat P, Carbonnel M. Efficacy and safety of transient ovariopexy in severe endometriotic patients. A ten year experience. . Fertility and sterility. 2008;90(Supplement):S167‐8.
Sawabe M, Fukuda A, Hamada A, Sonoda M, Nakaoka Y, Morimoto Y. Experience of 1000 falloposcopic tuboplasty (FT) cases: FT is a novel, patient friendly and effective regimen for tubal factor infertility before ART. Fertility and sterility. 2008;90(Supplement):S40.
Jindal UN, Verma YB, Sodhi S, Verma S. Comparative evaluation of laparoscopy and endometrial polymerase chain reaction for the diagnosis of female genital tuberculosis in infertile women in India. Fertility and sterility. 2008;90(Supplement):S152.
Hirano Y, Shibahara H, Shimada K, Suzuki T, Takamizawa S, Suzuki M. Clinical role of transvaginal hydrolaparoscopy for the diagnosis of early stage endometriosis. Fertility and sterility. 2008;90(Supplement):S441.
-
ESHRE 2015 (3)
Chu J, Harb HM, Gallos ID, Dhillon RK, Al‐Rshoud FM, Robinson L, et al. Salpingostomy in the treatment of hydrosalpinx: a systematic review and meta‐analysis. Hum Reprod. 2015;30(Supp 1):i448.
Wang XR, Bao HC, Hao CF. Core‐pulling Salpingectomy: A Novel Surgical for Hydrosalpinx before IVF‐ET. Hum Reprod. 2015;30(Supp 1):i33‐4.
Lind T, Olofsson JI, Holte J, Hadziosmanovic N, Berglund L, Gudmundsson J, et al. Reduced clinical pregnancy rates by ART in women with a history of unilateral oophorectomy. Results of a large multi‐center cohort study. Hum Reprod. 2015;30(Supp 1):i33.
Total = 10 abstracts
Appendix 17. Reference lists of included trials and related reviews
1. Boer‐Meisel, ME, te Velde, ER, Habbema, JD & Kardaun, JW 1986, 'Predicting the pregnancy outcome in patients treated for hydrosalpinx: a prospective study', Fertil Steril, vol. 45, no. 1, Jan, pp. 23‐29.
2. Vasquez, G, Boeckx, W & Brosens, I 1995, 'Prospective study of tubal mucosal lesions and fertility in hydrosalpinges', Hum Reprod, vol. 10, no. 5, May, pp. 1075‐1078.
3. Marcoux, S, Maheux, R & Berube, S 1997, 'Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group on Endometriosis', N Engl J Med, vol. 337, no. 4, Jul 24, pp. 217‐222.
4. Hughes, EG, Fedorkow, DM & Collins, JA 1993, 'A quantitative overview of controlled trials in endometriosis‐associated infertility', Fertil Steril, vol. 59, no. 5, May, pp. 963‐970.
5. Parazzini, F 1999, 'Ablation of lesions or no treatment in minimal‐mild endometriosis in infertile women: a randomized trial. Gruppo Italiano per lo Studio dell'Endometriosi', Hum Reprod, vol. 14, no. 5, May, pp. 1332‐1334.
6. Bontis JN, Dinas KD. Management of hydrosalpinx: reconstructive surgery or IVF? Ann NY Acad Sci 2000;900:260 –71.
7. Murray DL, Sagoskin AW, Widra EA, Levy MJ. The adverse effect of hydrosalpinges on in vitro fertilization pregnancy rates and the benefit of surgical correction. Fertil Steril 1998;69:41–5.
8. Sagoskin AW, Lessey BA, Mottla GL, Richter KS, Chetkowski RJ, Chang AS, et al. Salpingectomy or proximal tubal occlusion of unilateral hydrosalpinx increases the potential for spontaneous pregnancy. Hum Reprod 2003;18:2634 –7.
9. Nackley AC, Muasher SJ. The significance of hydrosalpinx in in vitro fertilization. Fertil Steril 1998;69:373–84.
10. Strandell A, Lindhard A. Why does hydrosalpinx reduce fertility? The importance of hydrosalpinx fluid. Hum Reprod 2002;17:1141–5.
11. Eytan O, Azem F, Gull I, Wolman I, Elad D, Jaffa AJ. The mechanism of hydrosalpinx in embryo implantation. Hum Reprod 2001;16:2662–7.
12. Bildirici I, Bukulmez O, Ensari A, Yarali H, Gurgan T. A prospective evaluation of the effect of salpingectomy on endometrial receptivity in cases of women with communicating hydrosalpinges. Hum Reprod 2001;16:2422– 6.
13. Zeyneloglu HB. Hydrosalpinx and assisted reproduction: options andrationale for treatment. Curr Opin Obstet Gynecol 2001;13:281–6.
14. Dechaud H. Hydrosalpinx and ART: hydrosalpinges suitable for salpingectomy before IVF. Hum Reprod 2000;15:2464–5
15. Choe J, Check JH. Salpingectomy for unilateral hydrosalpinx may improve in vivo fecundity. Gynecol Obstet Invest 1999;48:285–7.
16. Barmat LI, Rauch E, Spandorfer S, Kowalik A, Sills ES, Schattman G, et al. The effect of hydrosalpinges on IVF‐ET outcome. J Assist Reprod Genet 1999;16:350–4.
17. Camus E, Poncelet C, Goffinet F, Wainer B, Merlet F, Nisand I, et al. Pregnancy rates after in‐vitro fertilization in cases of tubal infertility with and without hydrosalpinx: a meta‐analysis of published comparative studies. Hum Reprod 1999;14:1243–9.
17 articles identified
Appendix 18. Data extraction
The following information was extracted from the studies selected for the review:
Trial characteristics 1. Method of randomisation a. Third‐party randomisation: e.g. computer, telephone randomisation. b. True randomisation by trialist: e.g. sequentially numbered, sealed, opaque envelopes, register, on‐site computer system. c. Method not stated. 2. Study design a. Cross‐over or parallel design. b. Duration of follow up. c. Type of follow up. 3. Size of the studies a. Number of women recruited. b. Number of women randomised. c. Number of women excluded. d. Number of women analysed. e. Number of women lost to follow up. f. Details of drop‐outs given. g. Duration of follow up. 4. Study setting a. Single or multi‐ centred. b. Location. c. Timing. 5. Analysis a. Sample size with power calculation. b. Whether or not analysed by intention‐to‐treat: b1. done; b2. not done, but possible; b3. not possible; b4. uncertain. 6. The extent to which the Consolidated Standards of Reporting Trials criteria (CONSORT) are met. Characteristics of the study participants a. Subfertile couples with at least one year’s duration of infertility. b. Females under forty years of age. c. Minor/grade I, moderate/grade II, or severe/grade III tubal damage confirmed prior to tubal surgery by means of HSG or laparoscopy. d. Women who have had tubal surgery for minor/grade I, moderate/grade II, or severe/grade III tubal damage carried out following investigation. 1. Baseline characteristics a. Age of the female partner. b. Primary or secondary infertility. c. Duration of subfertility. d. Previous fertility treatment. 2. Interventions used a. Tubal surgery. b. Expectant management. c. IVF. Outcomes 1. Primary
Cumulative livebirth rate per couple.
2. Secondary
a. Cumulative pregnancy rate per patient/couple.
b. Pregnancy rate per patient/ couple.
c. Livebirth rate per treatment cycle commenced.
d. Ectopic pregnancy rate per patient.
e. Multiple pregnancy rate per patient.
f. Incidence of OHSS per patient.
All assessments of trial quality and data extraction will be independently performed by three review authors (SJ, VA, BM) using forms designed according to Cochrane guidelines. Any discrepancies will be resolved by a senior review author (BM). Additional information on trial methodology or actual original trial data will be sought from the corresponding authors of trials which appear to meet the eligibility criteria but are unclear in aspects of methodology, or where the data are in a form unsuitable for meta‐analysis. Analysis
Should suitable trials become available in future, statistical analysis will be performed in accordance with the guidelines developed by the Gynaecology and Fertility group. Heterogeneity between the results of different studies will be examined by inspecting the scatter in the data points and the overlap in their confidence intervals and, more formally, using I2 tests. The possible contribution of differences in trial design to any heterogeneity identified in this manner, will be investigated. Where possible, the outcomes will be pooled statistically. For cross‐over trials, only the data from the first phase (i.e. before cross‐over) will be used. For dichotomous data (e.g. pregnancy rate), results for each study will be expressed as an odds ratio with 95% confidence interval and combined for meta‐analysis where appropriate, with RevMan software using the Peto‐modified Mantel‐Haenzel method. If possible, a sub‐group analysis will be performed to assess the clinical effectiveness of tubal surgery in women with grades I, II and III tubal damage separately. Sensitivity analysis will be undertaken to examine the stability of the results in relation to a number of factors including study quality and the source of the data.
Time line
The review is expected to be updated within two years of publication on the Cochrane Library or earlier should a seminal piece of research become available. New searches for RCTs will be performed every two years thereafter, and the review updated accordingly.
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Zolghadri 2006 | Did not satisfy inclusion criteria. Participants were not subfertile. |
Differences between protocol and review
For the 2016 update, we ensured that the definition of clinical pregnancy was consistent across review outcomes.
Contributions of authors
Su Jen Chua: literature search, data extraction, trial selection, quality assessment, data entry and analysis, writing of the first draft of the review.
Valentine Akande: development of the protocol, commenting on the draft of the review.
Ben Mol: trial selection, quality assessment, revising of the final draft of the review.
Sources of support
Internal sources
Robinson Research Institute, Adelaide, Australia.
External sources
None, Other.
Declarations of interest
BM has received payment for consultancy from biopharmaceutical company ObsEva Geneva. SC and VA have no interests to declare.
New search for studies and content updated (no change to conclusions)
References
References to studies excluded from this review
Zolghadri 2006 {published data only (unpublished sought but not used)}
- Zolghadri J, Momtahan M, Alborzi S, Mohammadinejad A, Khosravi D. Pregnancy outcome in patients with early recurrent abortion following laparoscopic tubal corneal interruption of a fallopian tube with hydrosalpinx. Fertility and Sterility 2006;86(1):149‐51. [DOI] [PubMed] [Google Scholar]
Additional references
Ahmad 2006
- Ahmad G, Watson A, Vandekerckhove P, Lilford R. Techniques for pelvic surgery in subfertility. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD000221.pub3] [DOI] [PubMed] [Google Scholar]
AIHW 2012
- Macaldowie A, Wang YA, Chambers GM, Sullivan EA. Assisted Reproductive Technology in Australia and New Zealand 2010. Canberra: AIHW, 2012. [Google Scholar]
Akande 2004
- Akande VA, Cahill DJ, Wardle PG, Rutherford AJ, Jenkins JM. The predictive value of the 'Hull & Rutherford' classification for tubal damage. British Journal of Obstetrics & Gynaecology 2004;111:123‐41. [DOI] [PubMed] [Google Scholar]
ASRM 2015
- Practice Committee of the American Society for Reproductive Medicine. Role of tubal surgery in the era of assisted reproductive technology: a committee opinion. Fertility and Sterility 2015;103:e37‐43. [DOI] [PubMed] [Google Scholar]
Benadiva 1995
- Benadiva CA, Kligman I, Davis O, Rozenwaks Z. In vitro fertilisation versus tubal surgery: is pelvic reconstructive surgery obsolete?. Fertility and Sterility 1995;64:1051‐61. [DOI] [PubMed] [Google Scholar]
Camus 1999
- Camus E, Poncelet C, Goffinet F, Wainer B, Merlet F, Nisand I, et al. Pregnancy rates after in‐vitro fertilization in cases of tubal infertility with and without hydrosalpinx: a meta‐analysis of published comparative studies. Human Reproduction 1999;14(5):1243‐49. [DOI] [PubMed] [Google Scholar]
Chandra 2014
- Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: data from the National Survey of Family Growth, 1982‐2010. National Health Statistics Report 2014;73:1‐21. [PubMed] [Google Scholar]
Chu 2015
- Chu J, Harb HM, Gallos ID, Dhillon R, Al‐Rshoud FM, Robinson L, et al. Salpingostomy in the treatment of hydrosalpinx: a systematic review and meta‐analysis. Human Reproduction 2015;30:1882‐95. [DOI] [PubMed] [Google Scholar]
Cohlen 2002
- Cohlen BJ, Hughes E, Velde ER. Intra‐uterine insemination for unexplained subfertility (Protocol for a Cochrane Review). Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD003854] [DOI] [PubMed] [Google Scholar]
Collins 1983
- Collins JA, Wrixon W, Janes LB, Wilson EH. Treatment‐independent pregnancy among infertile couples. New England Journal of Medicine 1983;309:1201‐6. [DOI] [PubMed] [Google Scholar]
El‐Chaar 2009
- El‐Chaar D, Yang Q, Gao J, Bottomley J, Leader A, Wen SW, et al. Risk of birth defects increased in pregnancies conceived by assisted human reproduction. Fertility and Sterility 2009;92:1557‐61. [DOI] [PubMed] [Google Scholar]
Evers 1998
- Evers JLH, Haas HW, Land JA, Dumoulin JCM, Dunselman GAJ. Treatment‐independent pregnancy rate in patients with severe reproductive disorders. Human Reproduction 1998;13(5):1206‐9. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 27 January 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Harzing 2007
- Harzing, A‐W. Publish or perish. Research in international management. http://www.harzing.com/pop.htm. 2007 (accessed 24 November 2016).
HFEA 2015
- Human Fertilisation, Embryology Authority. Adverse incidents in fertility clinics: lessons to learn. www.HFEA.gov.uk. (accessed 11th July 2016) September 2015.
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2005
- Higgins J, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 4.2.5. May 2005. Chichester, UK: John Wiley & Sons, Ltd, 2005. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. March 2011. London, UK: The Cochrane Collaboration, 2011. [Google Scholar]
Honore 1999
- Honore GM, Holden AE, Schenken RS. Pathophysiology and management of proximal tubal blockage. Fertility and Sterility 1999;71:785‐95. [DOI] [PubMed] [Google Scholar]
Johnson 2010
- Johnson N, Voorst S, Sowter MC, Strandell A, Mol BW. Surgical treatment for tubal disease in women due to undergo in vitro fertilisation. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD002125.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mosgaard 1996
- Mosgaard B, Hertz J, Steenstrup BR, Soorensen SS, Lindhard A, Anderson AN. Surgical management of tubal infertility: a regional study. Acta Obstetricia et Gynecologica Scandinavica 1996;75(5):469‐74. [DOI] [PubMed] [Google Scholar]
NICE 2004
- National Collaborating Centre for Women's and Children's Health. Fertility: Assessment and Treatment for People With Fertility Problems. Clinical Guideline. London: RCOG Press, 2004. [PubMed]
Ponomarev 2009
- Ponomarev VV, Zhuyko AA, Artyushkov VV, Bashirov EV, Vengerenko ME. Our experience in laparoscopic treatment of tubo‐peritoneal infertility. Gynecological Surgery 2009;6:S149‐50. [Google Scholar]
SART 2014
- Centers for Disease Control and Prevention/American Society for Reproductive Medicine/Society for Assisted Reproductive Technology. 2012 Assisted Reproductive Technology National Summary Report. Atlanta, GA: US Department of Health and Human Services, 2014. [Google Scholar]
Schippert 2012
- Schippert C, Soergel P, Staboulidou I, Bassler C, Gagalick S, Hillemanns P, et al. The risk of ectopic pregnancy following tubal reconstructive microsurgery and assisted reproductive technology procedures. Archives of Gynecology and Obstetrics 2012;285:863‐71. [DOI] [PubMed] [Google Scholar]
Serafini 1989
- Serafini P, Batzofin J. Diagnosis of female infertility. Journal of Reproductive Medicine 1989;34:29‐40. [PubMed] [Google Scholar]
Tran 2010
- Tran DK. Can open tubal microsurgery still be helpful in tubal infertility treatment?. Gynecological Surgery 2010;7:385‐400. [Google Scholar]
Wiedemann 1996
- Wiedemann R, Sterzik K, Gombisch V, Stuckensen J, Montag M. Beyond recanalizing proximal tubal occlusion: the argument for further diagnosis and classification. Human Reproduction 1996;11(5):986‐91. [DOI] [PubMed] [Google Scholar]
Wu 1988
- Wu CH, Gocial B. A pelvic scoring system for infertility surgery. International Journal of Fertility 1988;33:341‐6. [PubMed] [Google Scholar]
References to other published versions of this review
Pandian 2008
- Pandian Z, Akande VA, Harrild K, Bhattacharya S. Surgery for tubal infertility. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD006415.pub2] [DOI] [PubMed] [Google Scholar]


