Abstract
Background
Between 10% to 18% of people undergoing cholecystectomy for gallstones have common bile duct stones. Treatment of the bile duct stones can be conducted as open cholecystectomy plus open common bile duct exploration or laparoscopic cholecystectomy plus laparoscopic common bile duct exploration (LC + LCBDE) versus pre‐ or post‐cholecystectomy endoscopic retrograde cholangiopancreatography (ERCP) in two stages, usually combined with either sphincterotomy (commonest) or sphincteroplasty (papillary dilatation) for common bile duct clearance. The benefits and harms of the different approaches are not known.
Objectives
We aimed to systematically review the benefits and harms of different approaches to the management of common bile duct stones.
Search methods
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL, Issue 7 of 12, 2013) in The Cochrane Library, MEDLINE (1946 to August 2013), EMBASE (1974 to August 2013), and Science Citation Index Expanded (1900 to August 2013).
Selection criteria
We included all randomised clinical trials which compared the results from open surgery versus endoscopic clearance and laparoscopic surgery versus endoscopic clearance for common bile duct stones.
Data collection and analysis
Two review authors independently identified the trials for inclusion and independently extracted data. We calculated the odds ratio (OR) or mean difference (MD) with 95% confidence interval (CI) using both fixed‐effect and random‐effects models meta‐analyses, performed with Review Manager 5.
Main results
Sixteen randomised clinical trials with a total of 1758 randomised participants fulfilled the inclusion criteria of this review. Eight trials with 737 participants compared open surgical clearance with ERCP; five trials with 621 participants compared laparoscopic clearance with pre‐operative ERCP; and two trials with 166 participants compared laparoscopic clearance with postoperative ERCP. One trial with 234 participants compared LCBDE with intra‐operative ERCP. There were no trials of open or LCBDE versus ERCP in people without an intact gallbladder. All trials had a high risk of bias.
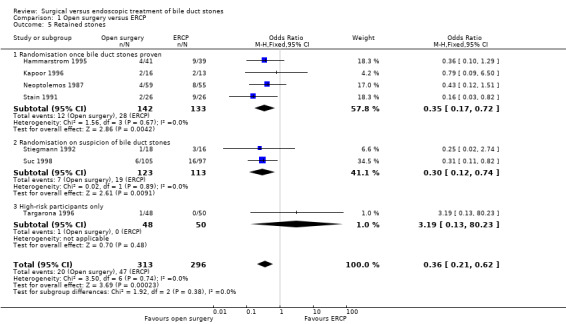
There was no significant difference in the mortality between open surgery versus ERCP clearance (eight trials; 733 participants; 5/371 (1%) versus 10/358 (3%) OR 0.51;95% CI 0.18 to 1.44). Neither was there a significant difference in the morbidity between open surgery versus ERCP clearance (eight trials; 733 participants; 76/371 (20%) versus 67/358 (19%) OR 1.12; 95% CI 0.77 to 1.62). Participants in the open surgery group had significantly fewer retained stones compared with the ERCP group (seven trials; 609 participants; 20/313 (6%) versus 47/296 (16%) OR 0.36; 95% CI 0.21 to 0.62), P = 0.0002.
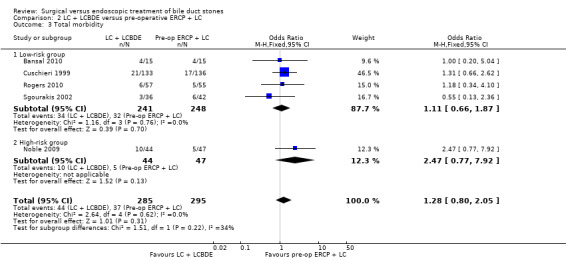
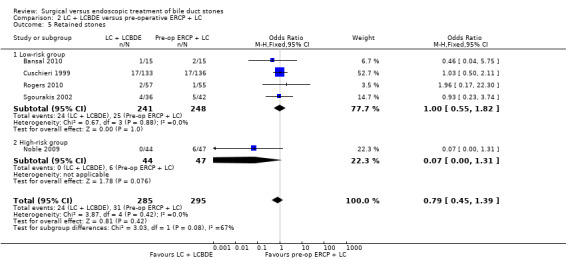
There was no significant difference in the mortality between LC + LCBDE versus pre‐operative ERCP +LC (five trials; 580 participants; 2/285 (0.7%) versus 3/295 (1%) OR 0.72; 95% CI 0.12 to 4.33). Neither was there was a significant difference in the morbidity between the two groups (five trials; 580 participants; 44/285 (15%) versus 37/295 (13%) OR 1.28; 95% CI 0.80 to 2.05). There was no significant difference between the two groups in the number of participants with retained stones (five trials; 580 participants; 24/285 (8%) versus 31/295 (11%) OR 0.79; 95% CI 0.45 to 1.39).
There was only one trial assessing LC + LCBDE versus LC+intra‐operative ERCP including 234 participants. There was no reported mortality in either of the groups. There was no significant difference in the morbidity, retained stones, procedure failure rates between the two intervention groups.
Two trials assessed LC + LCBDE versus LC+post‐operative ERCP. There was no reported mortality in either of the groups. There was no significant difference in the morbidity between laparoscopic surgery and postoperative ERCP groups (two trials; 166 participants; 13/81 (16%) versus 12/85 (14%) OR 1.16; 95% CI 0.50 to 2.72). There was a significant difference in the retained stones between laparoscopic surgery and postoperative ERCP groups (two trials; 166 participants; 7/81 (9%) versus 21/85 (25%) OR 0.28; 95% CI 0.11 to 0.72; P = 0.008.
In total, seven trials including 746 participants compared single staged LC + LCBDE versus two‐staged pre‐operative ERCP + LC or LC + post‐operative ERCP. There was no significant difference in the mortality between single and two‐stage management (seven trials; 746 participants; 2/366 versus 3/380 OR 0.72; 95% CI 0.12 to 4.33). There was no a significant difference in the morbidity (seven trials; 746 participants; 57/366 (16%) versus 49/380 (13%) OR 1.25; 95% CI 0.83 to 1.89). There were significantly fewer retained stones in the single‐stage group (31/366 participants; 8%) compared with the two‐stage group (52/380 participants; 14%), but the difference was not statistically significantOR 0.59; 95% CI 0.37 to 0.94).
There was no significant difference in the conversion rates of LCBDE to open surgery when compared with pre‐operative, intra‐operative, and postoperative ERCP groups. Meta‐analysis of the outcomes duration of hospital stay, quality of life, and cost of the procedures could not be performed due to lack of data.
Authors' conclusions
Open bile duct surgery seems superior to ERCP in achieving common bile duct stone clearance based on the evidence available from the early endoscopy era. There is no significant difference in the mortality and morbidity between laparoscopic bile duct clearance and the endoscopic options. There is no significant reduction in the number of retained stones and failure rates in the laparoscopy groups compared with the pre‐operative and intra‐operative ERCP groups. There is no significant difference in the mortality, morbidity, retained stones, and failure rates between the single‐stage laparoscopic bile duct clearance and two‐stage endoscopic management. More randomised clinical trials without risks of systematic and random errors are necessary to confirm these findings.
Keywords: Humans; Cholangiopancreatography, Endoscopic Retrograde; Cholangiopancreatography, Endoscopic Retrograde/mortality; Laparoscopy; Laparoscopy/mortality; Cholecystectomy, Laparoscopic; Cholecystectomy, Laparoscopic/mortality; Choledocholithiasis; Choledocholithiasis/diagnostic imaging; Choledocholithiasis/mortality; Choledocholithiasis/surgery; Common Bile Duct; Common Bile Duct/surgery; Randomized Controlled Trials as Topic; Sphincterotomy, Endoscopic; Sphincterotomy, Endoscopic/mortality
Plain language summary
Surgical versus endoscopic treatment of bile duct stones
Background Gallstones are a common problem in the general population and commonly cause problems with pain (biliary colic) and gallbladder infections (acute cholecystitis). Gallstones can sometimes migrate out of the gallbladder and become trapped in the tube between the gallbladder and the small bowel (common bile duct). Here, they obstruct the flow of bile from the liver and gallbladder into the small bowel and cause pain, jaundice (yellowish discolouration of the eyes, dark urine, and pale stools), and sometimes severe infections of the bile (cholangitis). Between 10% and 18% of people undergoing cholecystectomy for gallstones have common bile duct stones.
Treatment involves removal of the gallbladder as well as the gallstones from this tube. There are several methods to achieve this. Surgery is performed to remove the gallbladder. In the past, this was performed through a single large incision through the abdomen (open cholecystectomy). Newer keyhole techniques (laparoscopic surgery) are now the most common methods of removal of the gallbladder. Removal of the trapped gallstones in the common bile duct can be performed at the same time as the open or keyhole surgery. Alternatively, an endoscope (a narrow flexible tube equipped with a camera) is inserted through the mouth and into the small bowel to allow removal of the trapped gallstones from the common bile duct. This procedure can be performed before, during, and after the surgery to remove the gallbladder. This systematic review attempts to answer the question of the safest and most effective method to remove these trapped gallstones (in terms of open surgery or laparoscopic surgery compared with endoscopic removal), whether removal of the common bile duct stones should be performed during surgery to remove the gallbladder as a single‐stage treatment or as a separate treatment before or after surgery (two‐stage treatment).
Review questions We analysed results from randomised clinical trials in the literature to assess the benefits and harms of these procedures
Quality of evidence We identified a total of 16 trials including 1758 participants. All the trials were at high risk of bias (defects in study design which may result in overestimation of benefits or underestimation of harms). Overall the quality of the evidence is moderate because of the risk of systematic errors or bias (defects in study design) and random errors (insufficient number of participants were included in the trials) which can result in wrong conclusions.
Key results Our analysis suggests open surgery to remove the gallbladder and trapped gallstones appears to be as safe as endoscopy and may even be more successful than the endoscopic technique in clearing the duct stones. Keyhole (laparoscopic) surgery to remove the gallbladder and trapped gallstones appears to be as safe as and as effective as the endoscopic technique. More randomised clinical trials conducted with low risks of systematic errors (trials) and low risks of random errors (play of chances) are required to confirm or refute the present findings.
Summary of findings
Summary of findings for the main comparison. Open surgery compared to ERCP for bile duct stones.
| Open surgery compared to ERCP for bile duct stones | ||||||
| Patient or population: with common bile duct stones Settings: secondary or tertiary hospital Intervention: open surgery Comparison: ERCP + LC | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| ERCP + LC | Open surgery | |||||
| Mortality | Study population | 0.51 (0.18 to 1.44) | 733 (8 studies) | ⊕⊕⊕⊝ moderate1,2 | ||
| 3 per 100 | 1 per 100 (0 to 4) | |||||
| Moderate | ||||||
| 2 per 100 | 1 per 100 (0 to 3) | |||||
| Total morbidity | Study population | OR 1.12 (0.77 to 1.62) | 729 (8 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 19 per 100 | 21 per 100 (15 to 27) | |||||
| Moderate | ||||||
| 17 per 100 | 19 per 100 (14 to 25) | |||||
| Failure of procedure | Study population | OR 0.32 (0.21 to 0.48) | 943 (7 studies) | ⊕⊕⊕⊝ moderate1,2 | ||
| 200 per 1000 | 74 per 1000 (50 to 107) | |||||
| Moderate | ||||||
| 188 per 1000 | 69 per 1000 (46 to 100) | |||||
| Retained stones after primary intervention | Study population | OR 0.36 (0.23 to 0.57) | 943 (7 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 144 per 1000 | 57 per 1000 (37 to 87) | |||||
| Moderate | ||||||
| 165 per 1000 | 66 per 1000 (43 to 101) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 High‐risk surgical participants are included in one trial. 2Bornman 1992 is not a published trial and therefore could not be included in all the outcome analysis. 3 Randomisation of the studies was performed on confirmation of ductal stones and on suspicion of ductal stones in these studies.
Summary of findings 2. LC + LCBDE versus pre‐operative ERCP + LC for common bile duct stones.
| LC + LCBDE versuspre‐operative ERCP + LC for common bile duct stones | ||||||
| Patient or population: with common bile duct stones Settings: secondary or tertiary hospital Intervention: LC+ LCBDE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | LC+ LCBDE | |||||
| Mortality at 30 days | Study population | OR 0.72 (0.12 to 4.33) | 580 (5 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 10 per 1000 | 7 per 1000 (1 to 43) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Total morbidity | Study population | OR 1.28 (0.8 to 2.05) | 580 (5 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 125 per 1000 | 155 per 1000 (103 to 227) | |||||
| Moderate | ||||||
| 125 per 1000 | 155 per 1000 (103 to 227) | |||||
| Failure of procedure | Study population | OR 0.51 (0.16 to 1.59) | 580 (5 studies) | ⊕⊕⊕⊕ moderate1 | Random‐effects model | |
| 166 per 1000 | 92 per 1000 (31 to 241) | |||||
| Moderate | ||||||
| 169 per 1000 | 94 per 1000 (32 to 244) | |||||
| Retained stones after primary intervention | Study population | OR 0.79 (0.45 to 1.39) | 580 (5 studies) | ⊕⊕⊕⊕ moderate1 | ||
| 105 per 1000 | 85 per 1000 (50 to 140) | |||||
| Moderate | ||||||
| 125 per 1000 | 101 per 1000 (60 to 166) | |||||
| Conversion to open surgery | Study population | OR 1.46 (0.76 to 2.81) | 580 (5 studies) | ⊕⊕⊕⊕ moderate1 | ||
| 58 per 1000 | 82 per 1000 (44 to 147) | |||||
| Moderate | ||||||
| 59 per 1000 | 84 per 1000 (45 to 150) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Included low‐risk and high‐risk groups of surgical participants
Summary of findings 3. LC + LCBDE compared to LC + post‐operative ERCP for common bile duct stones.
| LC + LCBDE compared withLC + post‐operativeERCP for common bile duct stones | ||||||
| Patient or population: with common bile duct stones Settings: secondary or tertiary hospital Intervention: LC + LCBDE Comparison: LC + postoperative ERCP | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| LC + post‐operativeERCP | LC + LCBDE | |||||
| Total morbidity | Study population | OR 1.16 (0.5 to 2.72) | 166 (2 studies) | ⊕⊕⊕⊝ moderate1,2 | ||
| 141 per 1000 | 160 per 1000 (76 to 309) | |||||
| Moderate | ||||||
| 142 per 1000 | 161 per 1000 (76 to 310) | |||||
| Failure of procedure | Study population | OR 0.47 (0.21 to 1.06) | 166 (2 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 247 per 1000 | 134 per 1000 (64 to 258) | |||||
| Moderate | ||||||
| 247 per 1000 | 134 per 1000 (64 to 258) | |||||
| Retained stones after primary intervention | Study population | OR 0.28 (0.11 to 0.72) | 166 (2 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 247 per 1000 | 84 per 1000 (35 to 191) | |||||
| Moderate | ||||||
| 247 per 1000 | 84 per 1000 (35 to 191) | |||||
| Conversion to open surgery | Study population | OR 1.77 (0.23 to 13.81) | 166 (2 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 12 per 1000 | 21 per 1000 (3 to 141) | |||||
| Moderate | ||||||
| 11 per 1000 | 19 per 1000 (3 to 133) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Rhodes 1998 is considered to be at unclear risk of bias at randomisation. 2Nathanson 2005 randomised participants with ductal stones at laparoscopic cholecystectomy after failed transcystic clearance to laparoscopic choledochotomy or postoperative ERCP.
Background
Description of the condition
Gallstones occur in approximately 15% of the general population (Stinton 2012). In people who have cholecystectomy for gallbladder stones, approximately 10% to 18% also have common bile duct stones (Soltan 2000; Williams 2008). Common bile duct stones can be suspected pre‐operatively by symptoms or signs of jaundice, pancreatitis, or cholangitis, or by derangement in liver function tests, or on imaging showing duct dilation or actual ductal stones. Chronic obstruction can result in hepatic abscess, secondary biliary cirrhosis, and portal hypertension. In people without jaundice, with normal duct size on trans‐abdominal ultrasound, the prevalence of common bile duct stones at the time of cholecystectomy is less than 5% (Collins 2004; Williams 2008). The natural history of common bile duct stones is not known, though complications appear to be more frequent and severe than in those with asymptomatic gallstones (Ko 2002). Up to a third of people with stones identified at intra‐operative cholangiogram clear their ducts spontaneously after surgery (Collins 2004).
Description of the intervention
Open surgery Open surgical bile duct clearance is achieved by open surgical exploration of the common bile duct that could include flushing (with or without the aid of interventions like glucagon or buscopan), balloon extraction, mechanical lithotripsy or Dormia basket extraction or both (with or without the use of choledochoscopy), and either antegrade or retrograde sphincterotomy.
Laparoscopic surgery Laparoscopic surgery involves laparoscopic cholecystectomy combined with bile duct exploration (LCBDE) that is achieved either by transcystic or by choledochotomy techniques including flushing, balloon extraction, mechanical lithotripsy or Dormia basket extraction or both (with or without the use of choledochoscopy), with or without sphincterotomy.
Endoscopy Endoscopic retrograde cholangiopancreatography (ERCP) involves endoscopic intervention in the bile duct. A side‐viewing duodenoscope is used to identify the ampulla of Vater that is cannulated, and stone extraction is performed by endoscopic sphincterotomy or sphincteroplasty most commonly accompanied by either balloon or basket extraction of the common bile duct stones. Mechanical lithotripsy is used for larger stones.
Pre‐operative ERCP ERCP is performed prior to surgical intervention with the aim of clearing the common bile duct. Patients, later, underwent cholecystectomy (open or laparoscopic) as a separate procedure (irrespective of the duration between the ERCP and laparoscopic cholecystectomy).
Intra‐operative ERCP ERCP is performed at the time of surgical intervention to remove the gallbladder either by passing the guidewire through the cystic duct (rendezvous) or by the transampullary route.
Postoperative ERCP Patients underwent laparoscopic cholecystectomy as the initial procedure, and it was followed by ERCP if there were ductal stones identified on intra‐operative cholangiogram.
How the intervention might work
Common bile duct stones are often complicated by obstructive jaundice with or without superadded infection (cholangitis) or pancreatitis. Patients with asymptomatic bile duct stones are at a risk of developing these serious complications and require intervention (Tazuma 2006). Common bile duct exploration and removal of the ductal stones clear the ductal obstruction, and the patient can then proceed with laparoscopic cholecystectomy at the same operation, or as two different procedures.
Why it is important to do this review
The ideal treatment for common bile duct stones is still controversial. The options are that of surgical treatment alone (open or laparoscopic surgery) or a combination of endoscopy with surgical treatment (pre‐, intra‐ or post laparoscopic cholecystectomy ERCP) to clear the common bile duct stones.
In the era of open cholecystectomy, most common bile duct stones found at surgery were managed at the time, with only a minority managed by the alternative, namely, ERCP with or without endoscopic sphincterotomy (Fletcher 1994). Studies suggested that surgical common bile duct stone extraction was the recommended option for routine cases (Neoptolemos 1989). In the early days of laparoscopic biliary surgery, operative clearance of common bile duct stones along with laparoscopic cholecystectomy was not considered technically possible. Either open surgical clearance or, more commonly, ERCP/sphincterotomy became the techniques used to clear common bile duct stones.
Endoscopic intervention helps removal of stones from the duct so that surgical exploration of the bile duct can be avoided. When the duct is cleared by ERCP, the patient can then proceed to laparoscopic cholecystectomy. ERCP (either pre‐ or postoperatively) remains the preferred approach at most centres for managing patients with suspected common bile duct stones. However, ERCP is associated with complications such as pancreatitis, haemorrhage, cholangitis, duodenal perforation (5% to 11%) and mortality of up to 1% (Coelho‐Prabhu 2013). Failure rates of 5% to 10% are reported with ERCP. Also, when patients proceed to ERCP, a significant number of them may not have stones (Rhodes 1998; Nathanson 2005), yet patients risk these complications. The rate of negative ERCP (without stones), determined on the basis of absence of common bile duct stones, can vary from 15% to 25% (Collins 2004). A selective use of magnetic resonance cholangiopancreatography (MRCP) in patients with suspected choledocholithiasis is practised in the diagnosis of common bile duct stones, prior to definitive endoscopic or surgical intervention (Mercer 2007).
Laparoscopic exploration and clearance of common bile duct stones has become technically feasible, and several studies have shown that laparoscopic treatment of common bile duct stones is possible and is potentially as effective as ERCP (Lezoche 1996; Cuschieri 1999). Transcystic or transcholedochal exploration of the common bile duct could be performed at the time of laparoscopic cholecystectomy (Martin 1998; Decker 2003; Rojas‐Ortega 2003). Clayton 2006 demonstrated that ERCP and LCBDE have similar rates of stone clearance, morbidity, and mortality. Advantages of surgical common bile duct exploration are that the sphincter anatomy is not distorted and that the cholecystectomy is performed during the same procedure. However, surgical common bile duct exploration can be associated with the risk of bile leak (Nathanson 2005) and a possibility of long‐term complications of common bile duct stricture.
The current review is performed to compare the surgical and endoscopic options of management of common bile duct stones. This is an updated version of the Cochrane systematic review published by Martin 2006.
Objectives
To assess the benefits and harms of removing common bile duct stones using the following methods:
Open surgery versus ERCP.
Laparoscopic cholecystectomy + laparoscopic common bile duct exploration (LCBDE) versus pre‐operative ERCP + laparoscopic cholecystectomy.
Laparoscopic cholecystectomy + LCBDE versus intra‐operative ERCP + laparoscopic cholecystectomy.
Laparoscopic cholecystectomy + LCBDE versus laparoscopic cholecystectomy + postoperative ERCP.
Single‐stage management (LCBDE + laparoscopic cholecystectomy) versus two‐stage management (pre‐operative/postoperative ERCP + laparoscopic cholecystectomy). Earlier trials comparing the open surgical arm with endoscopic arm were not considered for this analysis and only the laparoscopic surgical studies were included. However, it does not include LCBDE versus intra‐operative ERCP as both the intervention arms were single‐stage procedures.
Open or laparoscopic common bile duct (CBD) exploration versus ERCP in participants with previous cholecystectomy.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised clinical trials that compared surgical (open or laparoscopic) treatment with ERCP for the management of common bile duct stones.
Quasi‐randomised clinical trials and observational studies were excluded. Trials were considered from journal articles, abstracts, and unpublished studies in any language, date of publication, and irrespective of blinding.
Types of participants
Adults (over 18 years) with suspected or proven common bile duct stones prior to open or laparoscopic cholecystectomy.
Types of interventions
Open surgery versus ERCP.
Laparoscopic cholecystectomy + laparoscopic common bile duct exploration (LCBDE) versus pre‐operative ERCP + laparoscopic cholecystectomy.
Laparoscopic cholecystectomy + LCBDE versus intra‐operative ERCP + laparoscopic cholecystectomy.
Laparoscopic cholecystectomy + LCBDE versus laparoscopic cholecystectomy + postoperative ERCP.
Single‐stage management (LCBDE + laparoscopic cholecystectomy) versus two‐stage management (pre‐operative/postoperative ERCP + laparoscopic cholecystectomy). Earlier trials comparing the open surgical arm with endoscopic arm were not considered for this analysis and only the laparoscopic surgical studies were included. However, it does not include LCBDE versus intra‐operative ERCP as both the intervention arms were single‐stage procedures.
Open or laparoscopic CBD exploration versus ERCP in participants with previous cholecystectomy.
Types of outcome measures
Primary and secondary outcomes are listed below.
Primary outcomes
Mortality at maximal follow‐up.
Morbidity: Complications from surgery and ERCP procedures, such as bile duct injuries, pancreatitis, cholangitis, post‐ERCP haemorrhage, postoperative complications requiring intervention and pulmonary/cardiac/renal complications.
Retained stones: Inability to clear the ductal stones with the planned technique (endoscopy or surgery) by the end of that procedure.
Secondary outcomes
Failure to complete the planned procedure: Inability to perform the planned procedure due to technical reasons such as failed cannulation or difficult Calot's dissection, or due to impacted stone.
Conversion to open surgery: Participants requiring conversion of laparoscopic surgery (LCBDE or LC) to open surgery (open common bile duct exploration (CBDE) or open cholecystectomy).
Quality of life.
Duration of procedure.
Duration of hospital stay.
Cost of the procedure.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2013), the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 7 of 12, 2013) in The Cochrane Library, MEDLINE (1946 to August 2013), EMBASE (1974 to August 2013), and Science Citation Index Expanded (1900 to August 2013). Search strategies are given in Appendix 1.
The search domains are:
1. Disease condition: common bile duct stone. 2. Intervention (and control): open common bile duct exploration, laparoscopic cholecystectomy, endoscopic sphincterotomy, or sphincteroplasty. 3. Study design: randomised controlled trial.
Searching other resources
We scanned the reference lists of the included trials for additional trials of interest.
Data collection and analysis
We collected data using a data collection form designed by the review author, BD. We entered data were entered into Review Manager 5 (RevMan 2012).
Selection of studies
Two review authors (BD and CJT) considered trials for inclusion. We included all randomised clinical trials which compared surgical (open or laparoscopic) versus ERCP treatment for common bile duct stones.
Data extraction and management
DJM and colleagues performed data extraction for the previously published version of the review. Two review authors (BD and CJT) reviewed and extracted data from the included trials according to the revised outcomes.
Extracted data (according to availability) included all relevant information to assess the described treatment outcomes and risk of bias. Additional data extracted included participant demographics, period of follow‐up, and inclusion and exclusion criteria. We planned to contact the authors of individual trials for any unclear or missing information. We resolved disagreements by discussion and revisiting the defined outcomes.
Assessment of risk of bias in included studies
We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module 2013 (Gluud 2013). According to empirical evidence (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Lundh 2012; Savovic 2012a; Savovic 2012b) the risk of bias of the trials was assessed based on the following domains: sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data, selective outcome reporting, for‐profit bias, and other bias. Risk of bias domains were classified as follows:
Allocation sequence generation ‐ Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if performed by an independent person not otherwise involved in the trial. ‐ Uncertain risk of bias: the method of sequence generation was not specified. ‐ High risk of bias: the sequence generation method was not random. Allocation concealment ‐ Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. The allocation sequence was unknown to the investigators (for example, if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes). ‐ Uncertain risk of bias: the method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during enrolment. ‐ High risk of bias: the allocation sequence was likely to be known to the investigators who assigned the participants. Blinding of participants, personnel, and outcome assessors ‐ Low risk of bias: blinding was performed adequately, or the assessment of outcomes was not likely to be influenced by lack of blinding. ‐ Uncertain risk of bias: there was insufficient information to assess whether blinding was likely to induce bias in the results. ‐ High risk of bias: no blinding or incomplete blinding, and the assessment of outcomes was likely to be influenced by lack of blinding.
Blinding of the participants and healthcare providers is not possible in a study comparing the endoscopic or surgical procedures. Also, it is not ethical to blind the surgeon when the patient might still require bile duct exploration.
Incomplete outcome data ‐ Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. Sufficient methods, such as multiple imputation, had been employed to handle missing data. ‐ Uncertain risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias in the results. ‐ High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting ‐ Low risk of bias: all outcomes were predefined and reported, or all clinically relevant and reasonably expected outcomes were reported. The trial was registered either on the www.clinicaltrials.gov web site or a similar register, or there was a published protocol. ‐ Uncertain risk of bias: it was unclear whether all predefined and clinically relevant and reasonably expected outcomes were reported. ‐ High risk of bias: one or more clinically relevant and reasonably expected outcomes were not reported, and data on these outcomes were likely to have been recorded.
For‐profit bias ‐ Low risk of bias: the trial appeared to be free of industry sponsorship or other kind of for‐profit support that might have manipulated the trial design, the conduct, or results of the trial. ‐ Uncertain risk of bias: the trial might or might not have been free of for‐profit bias as no information on clinical trial support or sponsorship was provided. ‐ High risk of bias: the trial was sponsored by the industry or had received other kind of for‐profit support. Other bias ‐ Low risk of bias: the trial appeared to be free of other components (for example, academic bias) that could put it at risk of bias. ‐ Uncertain risk of bias: the trial might or might not have been free of other components that could put it at risk of bias. ‐ High risk of bias: there were other factors in the trial that could put it at risk of bias (for example, authors have conducted trials on the same topic, etc).
Trials assessed as being at 'low risk of bias' in all of the specified domains were considered trials at 'low risk of bias'. Trials assessed as being at 'uncertain risk of bias' or at 'high risk of bias' in one or more of the specified domains were considered trials at 'high risk of bias'.
See Figure 1 and Figure 2 as well as Characteristics of included studies.
1.
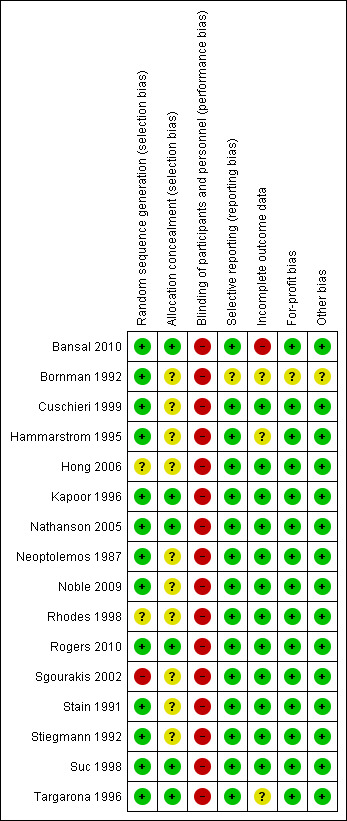
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
2.
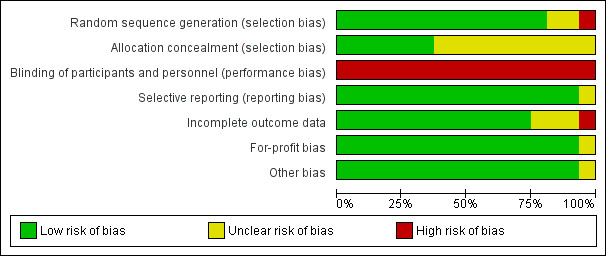
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Measures of treatment effect
For dichotomous variables, we calculated the odds ratio (OR) with a 95% confidence interval (CI). For data with zero events, the odds ratio cannot be calculated, and for analyses involving trials with such data we also calculated risk difference (RD) in addition to calculating the odds ratio.
For continuous data, authors generally present their results in medians with ranges due to suspicion of skewed data. However, for inclusion of such data in a meta‐analysis, data had to be presented in terms of the mean with its corresponding standard deviations (SD), or published in enough detail to allow accurate calculation of these factors, as needed or to calculate mean differences (MD) and 95% CIs (Hozo 2005).
Unit of analysis issues
The unit of analysis is the participant with confirmed or with suspected common bile duct stones. We performed subgroup analysis, where possible, for those only with suspected common bile duct stones.
Dealing with missing data
When details such as power calculations were not presented in the original publication, we listed it in the table Characteristics of included studies. We planned to contact the original investigators to request missing data.
The analyses were performed on an intention‐to‐treat basis (Newell 1992) whenever possible, in addition to per protocol analysis. We imputed the data for the total drop‐outs for the primary outcomes. For the number of drop‐outs post‐randomisation, we performed a 'good outcome' analysis (including all the drop‐outs in the total number of participants but not in the number of events), a 'poor outcome' analysis (including all the drop‐outs in the total number of participants and in the number of events), 'best‐case' for the experimental intervention (including the drop‐outs in the total number of intervention group eg, LCBDE but not to their events and including the drop‐outs in the total number of control group eg, ERCP in addition to the number of their events), 'worst‐case' for the control intervention (including all the drop‐outs in the total number of participants and for the events in the experimental intervention group, and including all the drop‐outs in the total number of controls but not for their events).
Assessment of heterogeneity
We explored heterogeneity by the Chi² test with significance set at a P value of 0.10. A low P value provides evidence of heterogeneity of intervention effects. I² is used to quantify inconsistency across the studies as an indicator of the presence of heterogeneity. Interpretaion of I² is as follows: 0% to 40% may not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity, and 75% to 100% may represent considerable heterogeneity. The importance of the observed value of I² depends on the magnitude and direction of effects as well as the strength of evidence for heterogeneity.
Assessment of reporting biases
We planned to construct funnel plots to explore reporting bias whenever there were at least 10 trials in a comparison (Egger 1997; Macaskill 2001).
Data synthesis
We calculated the odds ratio using both random‐effects and fixed‐effect models meta‐analyses. In the case of discrepancy in the results between the two models (e.g., one giving a significant intervention effect, the other no significant intervention effect), we reported both results; otherwise we reported only the fixed‐effect model in the cases where no significant statistical heterogeneity existed, and the random‐effects model meta‐analyses when statistical heterogeneity was present. We planned to perform meta‐analysis of continuous data using standardised mean difference where possible.
Trial sequential analysis
We used the trial sequential analysis to control for random errors due to sparse data and repetitive testing of the accumulating data for the primary outcomes (CTU 2011; Thorlund 2011). We added the trials according to the year of publication, and if more than one trial was published in a year, we added the trials in alphabetical order according to the last name of the first author. We planned to construct the trial sequential monitoring boundaries on the basis of the required diversity‐adjusted information size (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009, Wetterslev 2009; Thorlund 2010).
We applied trial sequential analysis (CTU 2011; Thorlund 2011) using a required sample size calculated from an alpha error of 0.05, a beta error of 0.20, a control group proportion obtained from the results of our meta‐analysis, and a risk ratio reduction of 20% for the primary outcomes (mortality, morbidity and retained stones after primary intervention) with two or more trials to determine whether more trials are necessary on this topic. If the trial sequential monitoring boundary and the required information size is reached or the futility zone is crossed, then more trials may not be necessary) (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009, Wetterslev 2009; Thorlund 2010).
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses, where appropriate: ‐ Trials with low‐risk surgical participants compared to the high‐risk surgical participants. ‐ Depending on when the randomisation was performed ‐ at the suspicion of CBD stones or confirmation of CBD stones. Randomisation at the suspicion of stones would include those who do not have the stones, resulting in a selection bias.
Sensitivity analysis
We performed a sensitivity analysis for reporting bias (drop‐outs) by imputing the outcomes for binary outcomes under different scenarios, namely 'good outcome' analysis, 'poor outcome' analysis, 'best‐case' analysis, and 'worst‐case' analysis (Gurusamy 2009; Gluud 2013) for the primary outcomes.
'Summary of findings' tables
We designed 'Summary of findings' tables using GRADEpro 3.6 (http://ims.cochrane.org/revman/other‐resources/gradepro) for the mortality, morbidity, retained stones, failure to clear the duct, and conversion of laparoscopic to open surgery.
Results
Description of studies
We identified a total of 4221 references through electronic searches of the Cochrane Hepato‐Biliary Group Controlled Trials Register (n = 317 hits), the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (n = 579), MEDLINE (n = 938), EMBASE (n = 1272), and Science Citation Index Expanded (n = 1115). We excluded 1758 duplicates and 2235 clearly irrelevant references through reading abstracts. Twenty‐four publications were scrutinised, of which, 16 trials fulfilled the inclusion criteria.
Participants The number of participants in each trial ranged from 30 to 300. The age of the participants in the included trials varied from 18 years to 80 years (Table 4). The proportion of women in the trials was about 50% (Table 5, Characteristics of included studies). Only five trials reported the duration of follow‐up (Neoptolemos 1987;Hammarstrom 1995;Targarona 1996; Sgourakis 2002;Noble 2009) (Table 6).
1. Participant age.
| Study ID | ERCP | Surgery |
| Bansal 2010 | Mean (range): 39.07 (23 to 64) | Mean (range): 47.1 (34 to72) |
| Bornman 1992 | Mean (SD): 54 (14) | Mean (SD): 55 (15) |
| Cuschieri 1999 | Range: 18 to 89 | Range: 19 to 88 |
| Hammarstrom 1995 | Median (range): 75 (56 to 85) | Median (range): 73.5 (56 to 85) |
| Hong 2006 | Not stated. | 15 to 82 years (mean, 48) |
| Kapoor 1996 | Mean (range): 42 (20 to 60) | Mean (range): 46 (24 to 75) |
| Nathanson 2005 | Median (range): 59.6 (18 to 92) | Median (range): 56.1 (17 to 91) |
| Neoptolemos 1987 | Median (range): 61 (20 to 83) | Median (range): 59 (20 to 82) |
| Noble 2009 | 74.3 (70.0 to 78.9) | 75.9 (70 to 80.8) |
| Rhodes 1998 | Mean (range): 68 (28 to 84) | Mean (range): 62 (24 to 83) |
| Rogers 2010 | Mean 44.6 | Mean 39.9 |
| Sgourakis 2002 | Range: 46 to 89 | Range: 43 to 88 |
| Stain 1991 | Mean (range): 48.4 (31 to 78) | Mean (range): 42.4 (20 to 86) |
| Stiegmann 1992 | Mean (SD): 46.3 (21.7) | Mean (SD): 38.1 (14.8) |
| Suc 1998 | Mean (SD): 66.8 (17.5) | Mean (SD): 66.7 (18.1) |
| Targarona 1996 | Mean (SD): 79 (9) | Mean (SD): 80 (7) |
2. Participant sex distribution.
| Study ID | ERCP | Surgery |
| Bansal 2010 | M:F 5:10 | M:F 4:11 |
| Bornman 1992 | M:F 17:45 | M:F 10:48 |
| Cuschieri 1999 | M:F 42:108 | M:F 60:90 |
| Hammarstrom 1995 | M:F 12:27 ) | M:F 16:25 |
| Hong 2006 | Not stated | M:F (ratio) 28:65 |
| Kapoor 1996 | Not stated | Not stated |
| Nathanson 2005 | M:F 17:28 | M:F 16:25 |
| Neoptolemos 1987 | M:F 29:26 | M:F 24:35 |
| Noble 2009 | M:F 22:25 | M:F 16:28 |
| Rhodes 1998 | M:F 14:26 | M:F 12:28 ) |
| Rogers 2010 | M:F 16:39 | M:F 17:40 |
| Sgourakis 2002 | M:F 17:25 | M:F 15:21 |
| Stain 1991 | M:F 6:20 | M:F 3:23 |
| Stiegmann 1992 | Not stated | Not stated |
| Suc 1998 | M:F 31:66 | M:F 33:72 |
| Targarona 1996 | M:F 15:35 | M:F 15:33 |
3. Follow‐up duration.
| Study ID | ERCP | Surgery |
| Bansal 2010 | not stated | not stated |
| Bornman 1992 | not stated | not stated |
| Cuschieri 1999 | not stated | not stated |
| Hammarstrom 1995 | median: 92 months | median: 82 months |
| Hong 2006 | not stated | not stated |
| Kapoor 1996 | not stated | not stated |
| Nathanson 2005 | not stated | not stated |
| Neoptolemos 1987 | minimum of 6 months | minimum of 6 months |
| Noble 2009 | at least 1 year | at least 1 year |
| Rhodes 1998 | not stated | not stated |
| Rogers 2010 | not stated | not stated |
| Sgourakis 2002 | median: 22.36 months | median: 22.36 months |
| Stain 1991 | not stated | not stated |
| Stiegmann 1992 | not stated | not stated |
| Suc 1998 | not stated | not stated |
| Targarona 1996 | mean (sd): 15 (11) months | mean (sd): 18 (10) months |
All trials detailed age distributions except Hong 2006. Three trials did not describe the sex distribution (Stiegmann 1992;Kapoor 1996; Hong 2006). Three trials specifically included participants in the older age group (more than 70 years) (Hammarstrom 1995; Targarona 1996; Noble 2009). One trial assessed high‐risk surgical candidates in a comparison of ERCP plus selective open cholecystectomy versus open cholecystectomy and exploration of the common duct (Targarona 1996). Targarona 1996 defined surgical high risk by at least one of the following: age over 70 years, Goldman cardiac index > 13, chronic pulmonary disease, Child‐Pugh B or C liver disease, severely impaired mobility, severe obesity (body mass index (BMI) > 30 kg/m2). Noble 2009 defined higher risk participants as being over 70 years age, over 60 with comorbidity, or those over 50 with a BMI greater than 40. We did not have to contact any of the authors about missing data for the included outcomes. None of the comparisons had more than 10 trials and we did not construct funnel plots.
Interventions In the open surgery comparison, four trials randomised participants at the time when common bile duct stones were diagnosed, which for the most part was during ERCP rather than on suspicion from blood tests or non‐invasive imaging or both (that is, ultrasound sonography and more recently magnetic resonance cholangiopancreatography (MRCP) (Neoptolemos 1987; Stain 1991; Hammarstrom 1995; Kapoor 1996)). This selection may give the ERCP group an advantage. In the laparoscopic surgery comparison, Rhodes 1998 randomised participants to laparoscopic exploration of the common duct versus postoperative ERCP following the identification of common bile duct stones at intra‐operative cholangiography. Participants in whom laparoscopic cholecystectomy or intra‐operative cholangiography were not technically feasible were excluded. Nathanson 2005 randomised participants only after failed transcystic clearance, ie, only more technically challenging participants, to either laparoscopic choledochotomy or postoperative ERCP; diagnosed during therapeutic manoeuvres at the operating table.
Three open‐surgery trials (Hammarstrom 1995; Targarona 1996; Suc 1998) proceeded to cholecystectomy on a selective basis in the ERCP arm after endoscopic clearance, while the other four proceeded routinely to cholecystectomy (Neoptolemos 1987; Stain 1991; Stiegmann 1992; Kapoor 1996).
Endoscopic stone extraction was either by basket (Stain 1991; Bornman 1992; Hammarstrom 1995; Kapoor 1996; Suc 1998; Sgourakis 2002), by balloon (Bornman 1992; Hammarstrom 1995; Sgourakis 2002), by mechanical lithotripsy (Hammarstrom 1995), by a combination (Nathanson 2005;Hong 2006;Noble 2009), or not described (Neoptolemos 1987; Stiegmann 1992; Targarona 1996; Rhodes 1998; Cuschieri 1999; Bansal 2010; Rogers 2010).
Reporting on the use of choledochoscopy for surgical stone extraction was variable. Routine use was reported by Nathanson 2005;Hong 2006;Noble 2009; Bansal 2010, while Sgourakis 2002 attempted its routine use. A further two trials reported its use in 6 of the 17 included participants (Kapoor 1996) and 25 of the 41 included participants (Hammarstrom 1995).
A distinction was not always made in the laparoscopic surgery trials between transcystic stone extraction and laparoscopic choledochotomy except for Nathanson 2005 (choledochotomy). The use of biliary drainage at the end of the surgical procedure with either T‐tubes (Stain 1991;Stiegmann 1992;Hammarstrom 1995;Rhodes 1998;Suc 1998;Sgourakis 2002; Nathanson 2005;Hong 2006;Noble 2009;Bansal 2010) or antegrade stents (Rhodes 1998; Nathanson 2005) was variably employed among the trials.
Results of the search
Please see the Study flow diagram (Figure 3). Details of the trials are shown in the table 'Characteristics of included studies'.
3.

Study flow diagram.
Included studies
There were 16 randomised clinical trials included in this systematic review, covering 1758 participants.
Eight randomised trials (737 participants) compared open surgery and CBD exploration versus ERCP (Neoptolemos 1987; Stain 1991; Bornman 1992; Stiegmann 1992; Hammarstrom 1995; Kapoor 1996; Targarona 1996; Suc 1998). These trials were performed mainly in the era of open cholecystectomy. Five randomised trials (621 participants) compared pre‐operative ERCP followed by laparoscopic cholecystectomy versus laparoscopic cholecystectomy and CBD exploration to clear the bile duct stones (Cuschieri 1999; Sgourakis 2002; Noble 2009; Rogers 2010; Bansal 2010). Of these, Noble 2009 included high anaesthetic risk participants only. One trial (234 participants) compared intra‐operative ERCP versus laparoscopic cholecystectomy and CBD exploration (Hong 2006). Two trials (166 participants) compared postoperative endoscopy versus laparoscopic cholecystectomy and CBD exploration (Rhodes 1998; Nathanson 2005).
Excluded studies
We excluded trials that compared the role of pre‐operative ERCP + LC versus postoperative ERCP + LC (Lella 2006;Morino 2006;Rabago 2006; El Geidie 2011) as these trials do not compare the surgical and endoscopic procedures as two different arms.
Risk of bias in included studies
Risk of bias in the included studies is assessed based on the following six domains and summarised in the tables of 'Characteristics of included studies'.
Allocation
Generation of the allocation sequence The majority reported the use of computer‐generated random number sequences or random number tables and are at low risk of selection bias (Neoptolemos 1987; Stain 1991; Bornman 1992; Stiegmann 1992; Hammarstrom 1995; Kapoor 1996; Targarona 1996; Suc 1998; Cuschieri 1999; Nathanson 2005;Hong 2006;Noble 2009;Bansal 2010). In two trials, the methodology merely described the process as being randomised, without further elaboration (unclear risk of bias) (Rhodes 1998; Rogers 2010). Sgourakis 2002 was considered to be at high risk of bias as the methods of randomisation were ambiguous.
Allocation concealment In six trials, allocation concealment was considered to be at low risk of bias with a phone‐in to a third party in two trials (Nathanson 2005, Suc 1998) and by sealed envelopes in four trials (Targarona 1996;Kapoor 1996; Bansal 2010;Rogers 2010) . In the remaining ten trials allocation concealment was not mentioned and the risk of bias was considered unclear (Neoptolemos 1987;Stain 1991;Bornman 1992;Stiegmann 1992;Hammarstrom 1995; Rhodes 1998; Cuschieri 1999;Sgourakis 2002;Hong 2006; Noble 2009).
Blinding
There was no blinding in any of the included trials. Blinding of the participant would have been beneficial, where possible, but none of the trials measured outcomes in this way. Also, all trials could have used blinded outcome assessors for the clinical outcomes.
Incomplete outcome data
Follow‐up and description of withdrawals and drop‐outs In all but four trials, withdrawals and drop‐outs were described (Stain 1991; Stiegmann 1992; Sgourakis 2002; Bansal 2010). We performed a sensitivity analysis of the primary outcomes to deal with the possible attrition bias.
Follow‐up duration Only three trials detailed precise data (Hammarstrom 1995; Targarona 1996; Sgourakis 2002) and a further trial described follow‐up 'for a minimum of six months' (Neoptolemos 1987). Most of the remaining trials described 30‐day mortality, so follow‐up was presumably of at least this duration, and certainly until discharge from hospital. Late complications, important for morbidity and procedural number analysis, occurring from 10 to 24 months after initial treatment, was variably reported (Bornman 1992; Nathanson 2005; Noble 2009).
Selective reporting
All the included trials were considered to be at low risk of bias except one (Bornman 1992), where the risk of bias was considered unclear as the data were from a published abstract.
Other potential sources of bias
All the included trials were considered to be at low risk of bias except one (Bornman 1992), where the risk of bias was considered unclear as the data were from a published abstract.
Effects of interventions
See: Table 1; Table 2; Table 3
Open surgical bile duct exploration versus ERCP
A total of 737 participants from eight trials were randomised to this comparison (Neoptolemos 1987; Stain 1991; Bornman 1992; Stiegmann 1992; Hammarstrom 1995; Kapoor 1996; Targarona 1996; Suc 1998). There were three post‐randomisation drop‐outs in Hammarstrom 1995, four in Kapoor 1996, and one in Neoptolemos 1987.
Mortality
Mortality was reported in eight trials (Neoptolemos 1987; Stain 1991; Bornman 1992; Stiegmann 1992; Hammarstrom 1995; Kapoor 1996; Targarona 1996; Suc 1998). There were 5 deaths/371 participants reported in the surgical group and 10 deaths/358 participants were reported in the ERCP group. There was no significant difference in the mortality between the two groups (Mantel‐Haenszel (M‐H) fixed‐effect odds ratio (OR) 0.51; 95% CI 0.18 to1.44), P = 0.20 (Analysis 1.1). There was no statistical heterogeneity (I² = 0%). Trial sequential analysis revealed that the proportion of information accrued was only 2.79% of the diversity‐adjusted required information size and so the trial sequential monitoring boundaries were not drawn (Figure 4). The cumulative Z‐curve does not cross the conventional statistical boundaries.
1.1. Analysis.
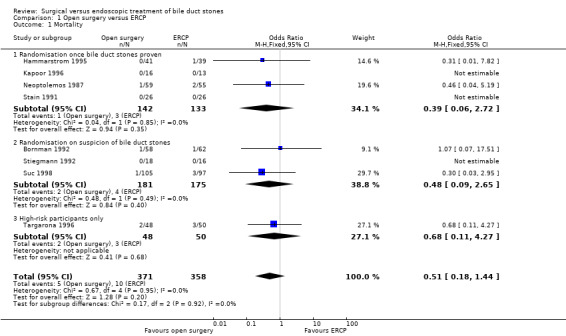
Comparison 1 Open surgery versus ERCP, Outcome 1 Mortality.
4.
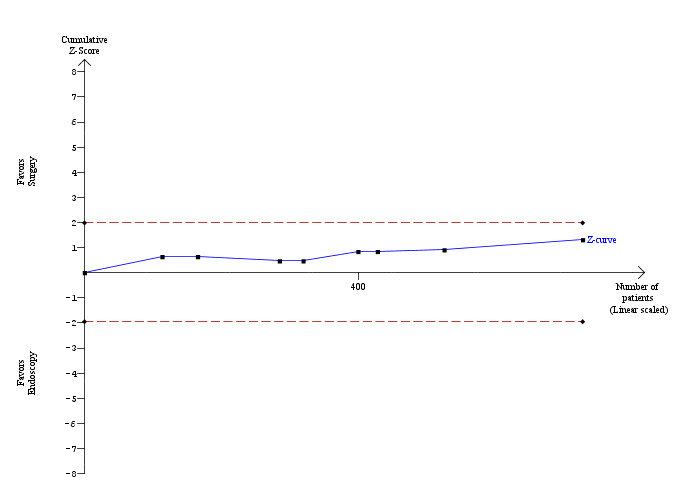
Trial sequential analysis of mortality (open surgery versus endoscopic retrograde cholangio pancreatography) The diversity‐adjusted required information size (DARIS) was calculated to 24,498 patients, based on the proportion of patients in the control group with the outcome of 2.79%, a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and a diversity of 0%. To account for zero event groups, a continuity correction of 0.01 was used in the calculation of the cumulative Z‐curve (blue line). After accruing a total of 729 participants in eight trials, only 2.98% of the DARIS has been reached. Accordingly, the trial sequential analysis does not show the required information size and the trial sequential monitoring boundaries. As shown, the conventional statistical boundaries (dotted red line) have also not been crossed by the cumulative Z‐curve.
Sensitivity analysis
'Good outcome' analysis: (OR 0.50; 95% CI 0.18 to 1.42), P = 0.19, I² = 0% (no significant difference) (Analysis 1.2.1). 'Poor outcome' analysis: (OR 1.00; 95% CI 0.43 to 2.32), P = 1.00, I² = 0% (no significant difference) (Analysis 1.2.2) 'Best‐case' for open surgery: (OR 0.46; 95% CI 0.17 to 1.25), P = 0.13, I² = 0% (no significant difference) (Analysis 1.2.3). 'Worst‐case' for open surgery: (OR 1.10; 95% CI 0.47 to 2.55), P = 0.83, I² = 0% (no significant difference) (Analysis 1.2.4).
1.2. Analysis.
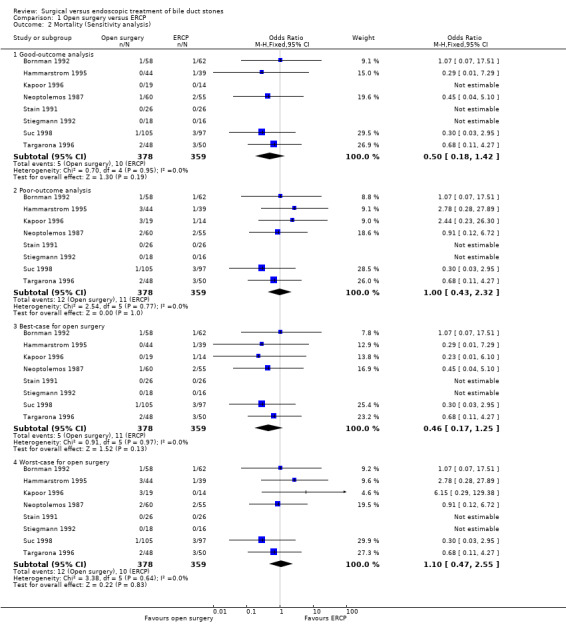
Comparison 1 Open surgery versus ERCP, Outcome 2 Mortality (Sensitivity analysis).
Total morbidity
Morbidity was reported in eight trials (Neoptolemos 1987;Stain 1991;Bornman 1992;Stiegmann 1992;Hammarstrom 1995;Kapoor 1996;Targarona 1996; Suc 1998). There was no significant difference in morbidity rates between open surgery versus endoscopy groups (M‐H fixed‐effect OR 1.12; 95% CI 0.77 to 1.62), P = 0.55, I² = 0% (Analysis 1.3). Trial sequential analysis revealed that only 23.18% of the diversity‐adjusted required information size has been reached, so the futility area was not drawn. The trial sequential analysis was consistent with absence of current evidence of any significant difference between open surgery and ERCP but significantly increased or decreased morbidity of open surgery compared with ERCP could not be ruled out (Figure 5).
1.3. Analysis.
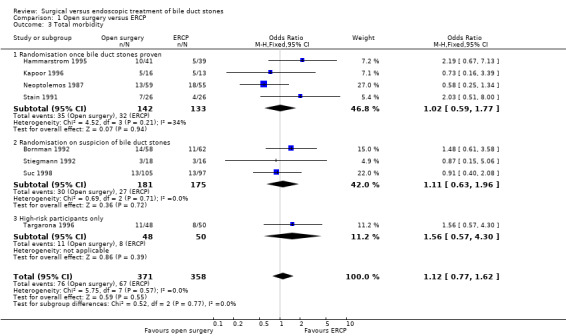
Comparison 1 Open surgery versus ERCP, Outcome 3 Total morbidity.
5.
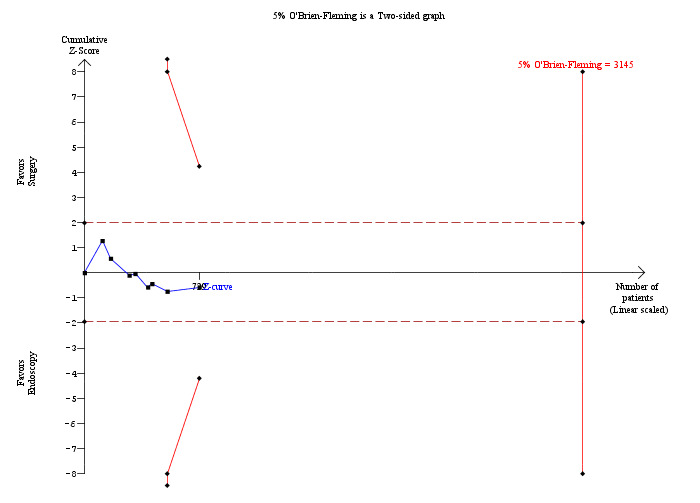
Trial sequential analysis of morbidity (open surgery versus endoscopic retrograde cholangio pancreatography (ERCP)) The diversity‐adjusted required information size (DARIS) was calculated to 3,145 patients, based on the proportion of patients in the control group with the outcome of 18.72%, a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and a diversity of 0%. After accruing a total of 729 participants in eight trials, only 23.18% of the DARIS has been reached. So, the futility area was not drawn. The cumulative Z‐curve (blue line) does not cross the trial sequential monitoring boundaries (red line) or the conventional boundaries (etched red line). This is consistent with absence of current evidence of any significant difference between open surgery and ERCP but significantly increased or decreased morbidity of open surgery compared to ERCP cannot be ruled out.
Sensitivity analysis
'Good outcome' analysis: (OR 1.09; 95% CI 0.76 to 1.58), P = 0.64, I² = 0% (no significant difference) (Analysis 1.4.1). 'Poor outcome' analysis: (OR 1.19; 95% CI 0.83 to 1.71), P = 0.35, I² = 0% (no significant difference) (Analysis 1.4.2). 'Best‐case' for open surgery: (OR 1.07; 95% CI 0.74 to 1.54), P = 0.71, I² = 0% (no significant difference) (Analysis 1.4.3). 'Worst‐case' for open surgery: (OR 1.22; 95% CI 0.84 to 1.75), P = 0.29, I² = 0% (no significant difference) (Analysis 1.4.4).
1.4. Analysis.
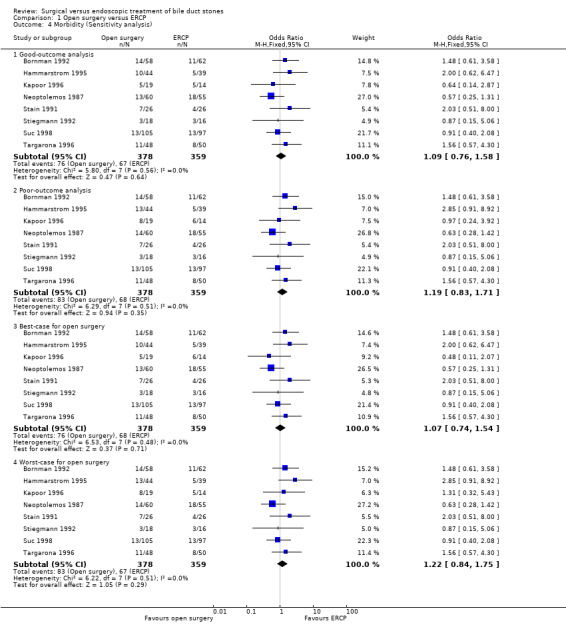
Comparison 1 Open surgery versus ERCP, Outcome 4 Morbidity (Sensitivity analysis).
Retained stones after primary intervention
Seven trials reported on this outcome (Neoptolemos 1987; Stain 1991; Stiegmann 1992; Hammarstrom 1995; Kapoor 1996; Targarona 1996; Suc 1998). These data could not be accurately analysed from Bornman 1992 where ERCP was repeated in up to five attempts to obtain CBD stones clearance. Fewer retained stones were encountered in the surgical group (M‐H fixed‐effect OR 0.36; 95% CI 0.21 to 0.62, P = 0.0002) (Analysis 1.5). Trial sequential analysis revealed that only 16.01% of the diversity‐adjusted required information size has been reached, so the futility area was not drawn. The trial sequential analysis suggested that although there is a statistically significant reduction in the proportion of people with retained stones in the open surgery group compared to the ERCP group, there is a high risk of random error and one cannot firmly conclude that open surgery has a significantly lower proportion of retained stones compared to the ERCP group (Figure 6).
1.5. Analysis.
Comparison 1 Open surgery versus ERCP, Outcome 5 Retained stones.
6.
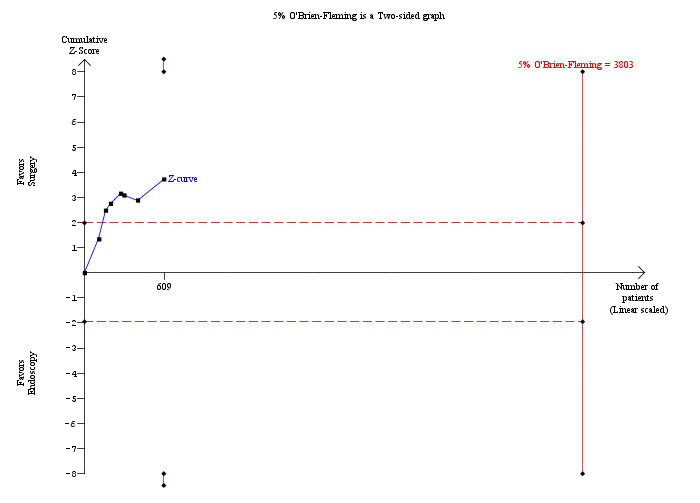
Trial sequential analysis of retained stones (open surgery versus endoscopic retrograde cholangio pancreatography (ERCP)) The diversity‐adjusted required information size (DARIS) was calculated to 3,803 patients, based on the proportion of patients in the control group with the outcome of 15.88%, a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and a diversity of 0%. After accruing a total of 609 participants in seven trials, only 16.01% of the DARIS has been reached. So, the futility area was not drawn. The cumulative Z‐curve (blue line) does not cross the trial sequential monitoring boundaries (red line) but crosses the conventional boundaries (etched red line). This suggests that although there is a statistically significant reduction in the proportion of people with retained stones in the open surgery group compared to the ERCP group, there is a high risk of random error and one cannot firmly conclude that open surgery has significantly lower retained stones proportion compared to the ERCP group.
Sensitivity analysis
'Good outcome' analysis: (OR 0.35; 95% CI 0.20 to 0.60), P = 0.0002, I² = 0% (favours surgery) (Analysis 1.6.1). 'Poor outcome' analysis: (OR 0.46; 95% CI 0.28 to 0.76), P = 0.002, I² = 0% (favours surgery) (Analysis 1.6.2). 'Best‐case' for open surgery : (OR 0.34; 95% CI 0.20 to 0.58), P < 0.0001, I² = 0% (favours surgery) (Analysis 1.6.3). 'Worst‐case' for open surgery : (OR 0.36; 95% CI 0.21 to 0.62), P = 0.0002, I² = 0% (favours surgery) (Analysis 1.6.4).
1.6. Analysis.
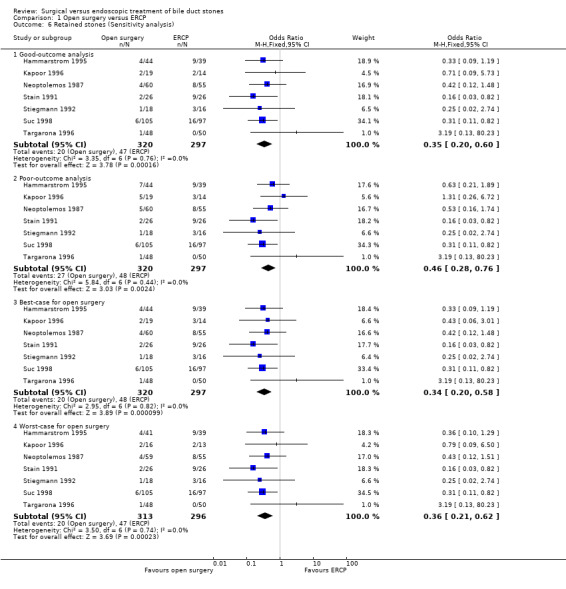
Comparison 1 Open surgery versus ERCP, Outcome 6 Retained stones (Sensitivity analysis).
Failure of procedure
Meta‐analysis of seven trials found a significantly lesser risk of failure to complete the procedure in the open surgery group compared with the ERCP group (M‐H fixed‐effect OR 0.31; 95% CI 0.19 to 0.51), P = 0.00001, I² = 0% (Analysis 1.7) (Neoptolemos 1987; Stain 1991; Stiegmann 1992; Hammarstrom 1995; Kapoor 1996; Targarona 1996; Suc 1998).
1.7. Analysis.
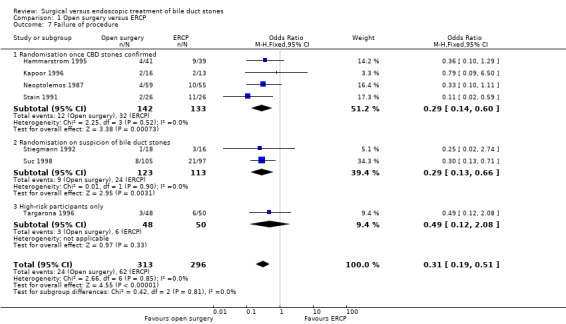
Comparison 1 Open surgery versus ERCP, Outcome 7 Failure of procedure.
A sensitivity analysis excluding the trials with randomisation at suspicion of stones (Stiegmann 1992; Targarona 1996; Suc 1998) but including only those trials that performed randomisation on confirmation of stones (Neoptolemos 1987; Stain 1991; Hammarstrom 1995; Kapoor 1996) was also in favour of the surgery group (M‐H fixed‐effect OR 0.29; 95% CI 0.14 to 0.60), P = 0.0007, I² = 0% (Analysis 1.7.1).
Quality of life
We found no data on quality of life.
Duration of procedure
There were two trials with data (Stain 1991; Stiegmann 1992). Because the data are non‐parametric, they cannot be subjected to meta‐analysis. In one of these trials (Stain 1991), there was a median operating time of 214 (range 115 to 420) minutes in the surgery versus 151 (range 80 to 310) minutes in the endoscopy group. It is, however, not apparent whether this refers to the combined time of endoscopy and surgery, or of surgery alone. In the other trial the data were reported as mean ± standard deviation (SD), with the assumption that these were normally distributed. The duration in the surgery group was 142 ± 72 minutes versus 114 ± 78 minutes in the endoscopy group, with no significance detected on parametric testing.
Hospital stay
All except one trial (Bornman 1992) had data concerning this outcome. However, since these data are also non‐parametric, they cannot be subjected to meta‐analysis. In five of the trials there were no statistical differences between the treatment groups as analysed by the individual trial authors. In one trial (Stain 1991) there was no indication whether or not a statistical analysis had been performed, with median (range) hospital stays of 5 (2 to 19) days for endoscopy and 6 (4 to 22) days for surgery. In the remaining trial (Neoptolemos 1987), there was a significant benefit favouring endoscopy with median (range) hospital stays of 16 (9 to 59) days for endoscopy and 21 (10 to 52) days for surgery (P = 0.0065). In the former trial (Stain 1991), the authors measured hospital stay from the day of first procedure, whereas in the latter it was measured from admission. Even allowing for this, there is clearly marked heterogeneity between the trials in this outcome variable (Analysis 1.8). Trial sequential analysis was not performed since the meta‐analysis was not performed.
1.8. Analysis.
Comparison 1 Open surgery versus ERCP, Outcome 8 Hospital stay.
| Hospital stay | ||
|---|---|---|
| Study |
Duration of hospital stay from the day of intervention (surgery group) |
Duration of hospital stay from the day of intervention (endoscopy group) |
| Randomisation once CBD stones were proven | ||
| Hammarstrom 1995 | Not reported | 13 |
| Kapoor 1996 | 11.3 (range 6 to 24) | 10.6 (range 6 to 18) |
| Neoptolemos 1987 | 11 (range 6 to 27) | 9 (range 4 to 57) |
| Stain 1991 | 7 ( range 4 to 22) | 5 (range 2 to 12) |
| Randomisation on suspicion of CBD stones | ||
| Stiegmann 1992 | 9.2 +/‐ 0.6 days (mean +/‐ SD) | 11.0 +/‐ 1.5 days (mean +/‐ SD) |
| Suc 1998 | 16 (range 6 to 60) | 12 (range 2 to 68) |
| High‐risk participants only | ||
| Targarona 1996 | Not reported. | Not reported. |
Costs
Only two trials reported costs. Stiegmann 1992 reported a significant difference favouring the endoscopy group (P < 0.007), whereas Kapoor 1996 reported a non significant difference between the surgical and endoscopy groups (mean of 4748 Rupees in the endoscopy group versus 4305 Rupees in the surgical group) (Analysis 1.9).
1.9. Analysis.
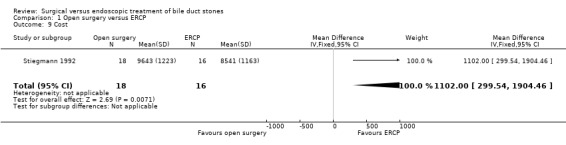
Comparison 1 Open surgery versus ERCP, Outcome 9 Cost.
Subgroup analysis
We performed sensitivity analyses on the following subgroups as required, based on our assessment of clinical variability.
‐ Randomisation once CBD stones proven (Neoptolemos 1987; Hammarstrom 1995; Kapoor 1996). ‐ Randomisation on suspicion of CBD stones (Bornman 1992; Stiegmann 1992; Suc 1998). ‐ High‐risk participants only (randomisation on suspicion of CBD stones) (Targarona 1996).
Timing of randomisation had no significant influence on the overall mortality (Analysis 1.1), morbidity (Analysis 1.3), retained stones (Analysis 1.5), and failure of procedure (Analysis 1.7) between the open and endoscopy groups.
Reporting bias
We did not generate a funnel plot because there were only eight trials for this comparison.
LC + LCBDE versus pre‐operative ERCP + LC
Five randomised trials with a total of 621 participants were found and included in the meta‐analysis (Cuschieri 1999; Sgourakis 2002; Noble 2009; Bansal 2010; Rogers 2010). One randomised trial compared the two interventions in a higher‐risk patient group and a relevant sub‐group analysis was performed (Noble 2009). There were 10 post‐randomisation drop‐outs in Rogers 2010 and 31 post‐randomisation drop‐outs in Cuschieri 1999.
Mortality
All the included trials reported mortality (Cuschieri 1999; Sgourakis 2002; Noble 2009; Bansal 2010; Rogers 2010); 2 deaths/241 participants in the LCBDE group and 3 deaths/248 participants in the pre‐operative ERCP group (Cuschieri 1999; Sgourakis 2002). No deaths were reported in the high‐risk group (Noble 2009). Meta‐analysis showed no significant difference between the two groups with (M‐H fixed‐effect OR 0.72; 95% CI 0.12 to 4.33), P = 0.72, I² = 0% (Analysis 2.1). Trial sequential analysis revealed that the proportion of information accrued was only 0.81% of the diversity‐adjusted required information size and so the trial sequential monitoring boundaries were not drawn (Figure 7). The cumulative Z‐curve does not cross the conventional statistical boundaries.
2.1. Analysis.
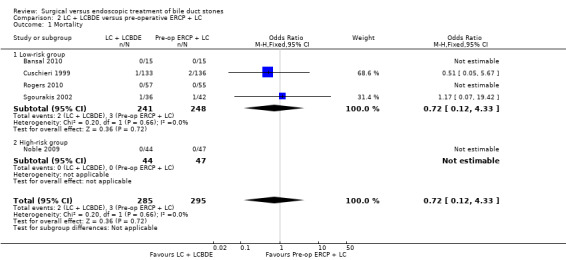
Comparison 2 LC + LCBDE versus pre‐operative ERCP + LC, Outcome 1 Mortality.
7.
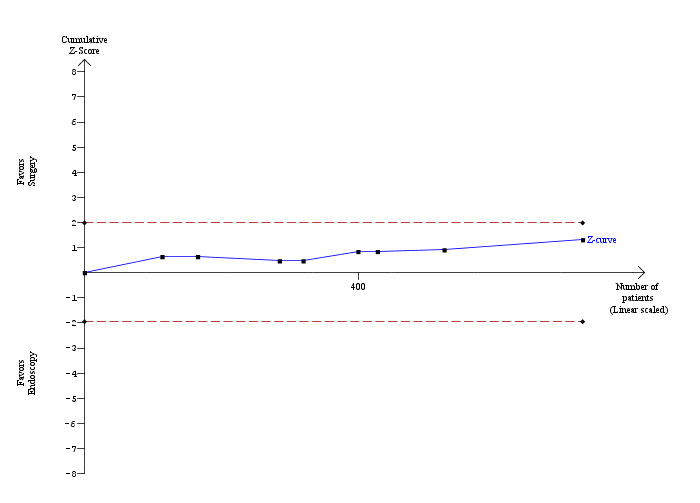
Trial sequential analysis of mortality (laparoscopic common bile duct exploration versus pre‐operative endoscopic retrograde cholangio pancreatography after laparoscopic cholecystectomy) The diversity‐adjusted required information size (DARIS) was calculated to 71,546 patients, based on the proportion of patients in the control group with the outcome of 1.02%, a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and a diversity of 0%. To account for zero event groups, a continuity correction of 0.01 was used in the calculation of the cumulative Z‐curve (blue line). After accruing a total of 580 participants in five trials, only 0.81% of the DARIS has been reached. Accordingly, the trial sequential analysis does not show the required information size and the trial sequential monitoring boundaries. As shown, the conventional statistical boundaries (etched red line) have also not been crossed by the cumulative Z‐curve.
Sensitivity analysis
'Good outcome' analysis: (OR 0.71; 95% CI 0.12 to 4.27), P = 0.71, I² = 0% (no significant difference) (Analysis 2.2.1). 'Poor outcome' analysis: (OR 1.01; 95% CI 0.55 to 1.85), P = 0.98, I² = 0% (no significant difference) (Analysis 2.2.2). 'Best‐case' for LCBDE: (OR 0.10; 95% CI 0.03 to 0.38), P = 0.0006, I² = 39% (favours LCBDE) (Analysis 2.2.3). 'Worst‐case' for LCBDE: (OR 7.46; 95% CI 2.39 to 23.27), P = 0.0005, I² = 0% (favours pre‐operative ERCP) (Analysis 2.2.4).
2.2. Analysis.
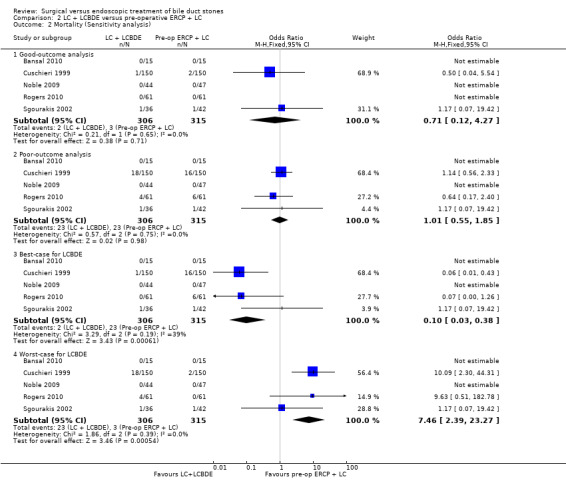
Comparison 2 LC + LCBDE versus pre‐operative ERCP + LC, Outcome 2 Mortality (Sensitivity analysis).
Total morbidity
All five randomised clinical trials (RCTs) reported morbidity (Cuschieri 1999; Sgourakis 2002; Noble 2009; Bansal 2010; Rogers 2010). Calculation of morbidity showed no significant difference favouring either group, M‐H fixed‐effect OR 1.28; 95% CI 0.80 to 2.05, P = 0.31, I² = 0% (Analysis 2.3). Trial sequential analysis revealed that only 11.62% of the diversity‐adjusted required information size had been reached, so the futility area was not drawn. The trial sequential analysis was consistent with absence of current evidence of any significant difference between LBCDE and ERCP but significantly increased or decreased morbidity of LBCDE compared with ERCP could not be ruled out (Figure 8).
2.3. Analysis.
Comparison 2 LC + LCBDE versus pre‐operative ERCP + LC, Outcome 3 Total morbidity.
8.
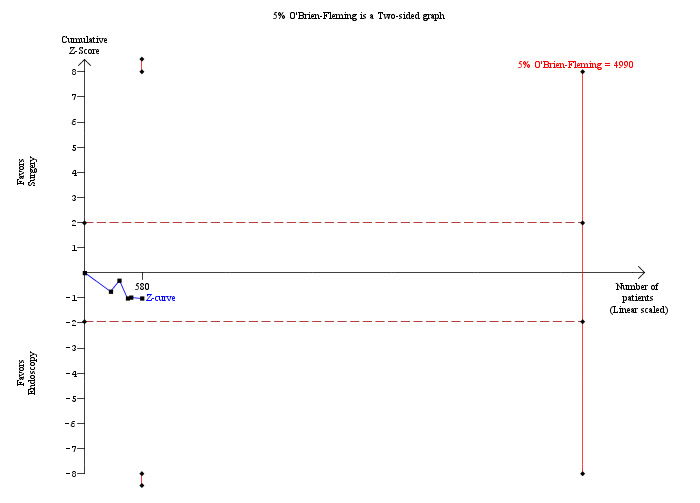
Trial sequential analysis of morbidity (laparoscopic common bile duct exploration (LCBDE) versus pre‐operative endoscopic retrograde cholangio pancreatography (ERCP) after laparoscopic cholecystectomy) The diversity‐adjusted required information size (DARIS) was calculated to 4,990 patients, based on the proportion of patients in the control group with the outcome of 12.54%, a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and a diversity of 0%. After accruing a total of 580 participants in five trials, only 11.62% of the DARIS has been reached. So, the futility area was not drawn. The cumulative Z‐curve (blue line) does not cross the trial sequential monitoring boundaries (red line) or the conventional boundaries (etched red line). This is consistent with absence of current evidence of any significant difference between LCBDE and ERCP but significantly increased or decreased morbidity of LCBDE compared to ERCP cannot be ruled out.
Sensitivity analysis
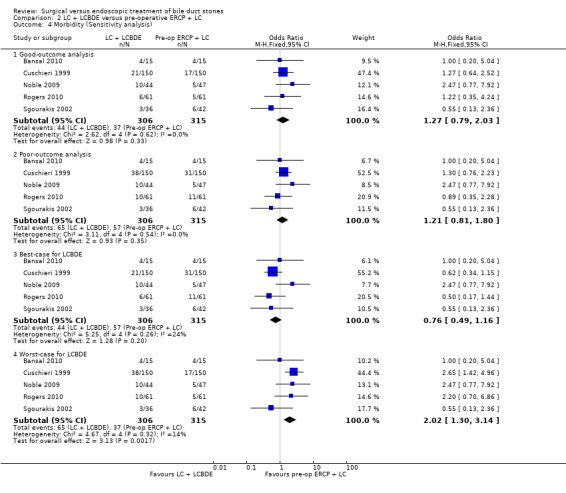
'Good outcome' analysis: (OR 1.27; 95% CI 0.79 to 2.03), P = 0.33, I² = 0% (no significant difference) (Analysis 2.4.1). 'Poor outcome' analysis: (OR 1.21; 95% CI 0.81 to 1.80), P = 0.35, I² = 0% (no significant difference) (Analysis 2.4.2). 'Best‐case' for LCBDE: (OR 0.76; 95% CI 0.49 to 1.16), P = 0.20, I² = 24% (no significant difference) (Analysis 2.4.3). 'Worst‐case' for LCBDE: (OR 2.02; 95% CI 1.30 to 3.14), P = 0.002, I² = 14% (favours pre‐operative ERCP) (Analysis 2.4.4).
2.4. Analysis.
Comparison 2 LC + LCBDE versus pre‐operative ERCP + LC, Outcome 4 Morbidity (Sensitivity analysis).
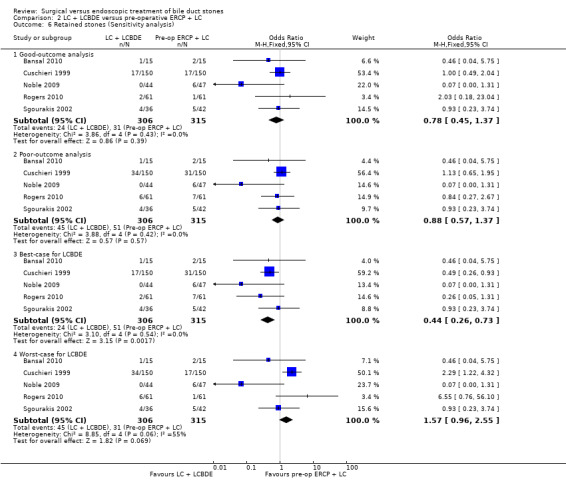
Retained stones after primary intervention
Based on the data from all five RCTs , the surgery group had retained stones after primary intervention in 24/285 participants versus 31/295 participants in the ERCP group (Cuschieri 1999; Sgourakis 2002; Noble 2009; Bansal 2010; Rogers 2010). Overall, there was no significant difference between the two groups with (M‐H fixed‐effect OR 0.79; 95% CI 0.45 to 1.39), P = 0.42. There was no substantial heterogeneity between studies (I² = 0%) (Analysis 2.5). Trial sequential analysis revealed that only 9.51% of the diversity‐adjusted required information size has been reached, so the futility area was not drawn. The trial sequential analysis was consistent with absence of current evidence of any significant difference between LBCDE and ERCP but significantly increased or decreased morbidity of LBCDE compared with ERCP could not be ruled out (Figure 9).
2.5. Analysis.
Comparison 2 LC + LCBDE versus pre‐operative ERCP + LC, Outcome 5 Retained stones.
9.
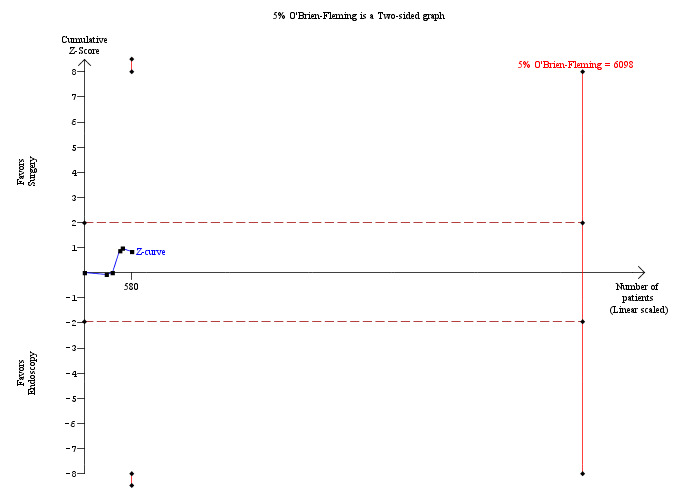
Trial sequential analysis of retained stones (laparoscopic common bile duct exploration versus pre‐operative endoscopic retrograde cholangio pancreatography after laparoscopic cholecystectomy) The diversity‐adjusted required information size (DARIS) was calculated to 6,098 patients, based on the proportion of patients in the control group with the outcome of 10.51%, a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and a diversity of 0%. To account for zero event groups, a continuity correction of 0.01 was used in the calculation of the cumulative Z‐curve (blue line). After accruing a total of 580 participants in five trials, only 9.51% of the DARIS has been reached. So, the futility area was not drawn. The cumulative Z‐curve does not cross the trial sequential monitoring boundaries (red line) or the conventional boundaries (etched red line). This is consistent with absence of current evidence of any significant difference between LCBDE and ERCP but significantly increased or decreased proportion of people with retained stones of LCBDE compared to ERCP cannot be ruled out.
Subgroup analysis
One randomised clinical trial with high‐risk surgical participants reported significantly higher duct clearance rates in the surgical group with no participants having retained stones (0/44) compared with the ERCP group (6/47), (P = 0.08) (Noble 2009) (Analysis 2.5).
Sensitivity analysis
'Good outcome' analysis: (OR 0.78; 95% CI 0.45 to 1.37), P = 0.39, I² = 0% (no significant difference) (Analysis 2.6.1). 'Poor outcome' analysis: (OR 0.88; 95% CI 0.57 to 1.37), P = 0.57, I² = 0% (no significant difference) (Analysis 2.6.2). 'Best‐case' for LCBDE: (OR 0.44; 95% CI 0.26 to 0.73), P = 0.002, I² = 0% (favours LCBDE) (Analysis 2.6.3). 'Worst‐case' for LCBDE: (OR 1.57; 95% CI 0.96 to 2.55), P = 0.07, I² = 55% (no significant difference) (Analysis 2.6.4).
2.6. Analysis.
Comparison 2 LC + LCBDE versus pre‐operative ERCP + LC, Outcome 6 Retained stones (Sensitivity analysis).
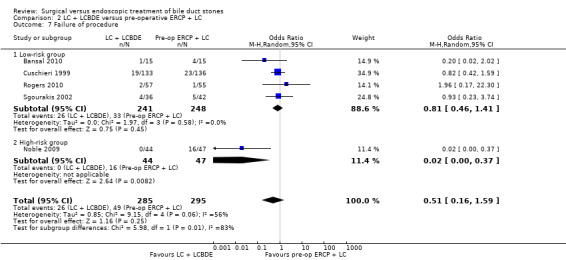
Failure of procedure
Reduced number of failures were encountered in the LCBDE (26/285) compared with the pre‐operative ERCP group (49/295), (M‐H random‐effects OR 0.51; 95% CI 0.16 to 1.59), P = 0.25, I² = 56% (Analysis 2.7). Using the fixed‐effect model this difference was significant, (M‐H OR 0.52; 0.31 to 0.85), P = 0.009.
2.7. Analysis.
Comparison 2 LC + LCBDE versus pre‐operative ERCP + LC, Outcome 7 Failure of procedure.
Subgroup analysis
Data were significantly influenced by a single study (Noble 2009). On excluding this study from the analysis, the heterogeneity was reduced to 0% and there was no significant difference between the two groups (P = 0.41) (Analysis 2.7).
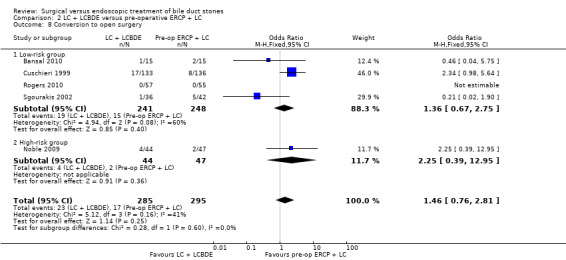
Conversion to open surgery
Based on the data from all five trials (Cuschieri 1999; Sgourakis 2002; Noble 2009; Bansal 2010;Rogers 2010), 23/285 in the LCBDE arm were converted to open surgery whereas 17/295 participants in the pre‐operative ERCP group underwent conversion of laparoscopic to open surgery with no statistically significant difference between the two groups on random‐effects analysis (M‐H OR 1.20; 95% CI 0.40 to 3.60), P = 0.75, I² = 41% (Analysis 2.8). On fixed‐effect analysis, there was no significant difference between the two groups (M‐H OR 1.46; 95% CI 0.76 to 2.81), P = 0.25, I² = 41%.
2.8. Analysis.
Comparison 2 LC + LCBDE versus pre‐operative ERCP + LC, Outcome 8 Conversion to open surgery.
Quality of life
We found no data on quality of life apart from Rogers et al (Rogers 2010) who observed no significant difference.
Duration of procedure
Two randomised clinical trials reported the duration of procedure (Sgourakis 2002; Rogers 2010). One trial reported a median procedure time in the surgery group of 90 (70 to 310) minutes versus 105 (60 to 255) minutes in the ERCP group (Sgourakis 2002). The other trial reported mean procedure time of 174 minutes (SD ± 67) in the surgery group compared with 183 (SD ± 39) minutes in the ERCP group (P = 0.44) (Rogers 2010).
Hospital stay
Cuschieri 1999 and Rogers 2010 reported a significant difference in favour of the surgery‐only arm with P < 0.05 and P < 0.001 respectively. Sgourakis 2002, Noble 2009, and Bansal 2010 reported median total postoperative hospital stay but did not find a significant difference between the two groups (Analysis 2.9). Trial sequential analysis was not performed since no meta‐analysis was performed.
2.9. Analysis.
Comparison 2 LC + LCBDE versus pre‐operative ERCP + LC, Outcome 9 Duration of hospital stay.
| Duration of hospital stay | |||
|---|---|---|---|
| Study | Pre‐op ERCP + LC | LC + LCBDE | P ‐ value |
| Bansal 2010 | 4 (range 2 to 11) days | 4.2 (range 3 to 9) days | |
| Cuschieri 1999 | 9 (IQR, 6 to 14) days | 6 (IQR, 4 to 12) days | <0.05 |
| Noble 2009 | 3 (IQR, 2 to 7) days | 5 (IQR, 2 to 7) days | 0.825 |
| Rogers 2010 | 98hrs | 55hrs | <0.001 |
| Sgourakis 2002 | 9 days | 7.4 days | |
Costs
Only one randomised clinical trial compared the costs of the two different interventions (Rogers 2010). There was no significant difference in total charges between the two intervention groups.
Reporting bias
We did not generate a funnel plot because only five trials were included in this comparison.
LC + LCBDE versus LC + intra‐operative ERCP
There was only one randomised clinical trial included in this comparison with a total of 234 participants (Hong 2006). There were no drop‐outs after randomisation. Trial sequential analysis was not performed because of the presence of only one trial.
Mortality
No deaths were reported in either of the intervention arms in this trial.
Total morbidity
There was no significant difference in the total morbidity. There were 6/141 complications in LCBDE group compared with 8/93 complications in the intra‐operative ERCP group (M‐H fixed‐effect OR 0.47, 95% CI 0.16 to 1.41), P = 0.18 (Analysis 3.1).
3.1. Analysis.
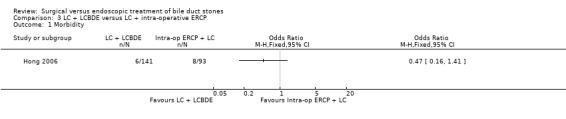
Comparison 3 LC + LCBDE versus LC + intra‐operative ERCP, Outcome 1 Morbidity.
Retained stones
6/141 participants in the LCBDE group and 6/93 participants in the intra‐op ERCP group had retained stones. This difference was not statistically significant (P = 0.46) (Analysis 3.2).
3.2. Analysis.
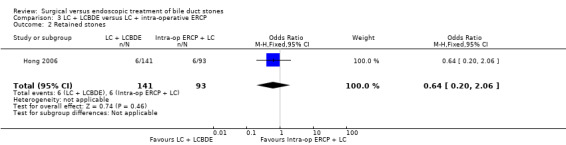
Comparison 3 LC + LCBDE versus LC + intra‐operative ERCP, Outcome 2 Retained stones.
Failure of procedure
Data on the failure to perform the planned procedure did not show a significant difference between to modalities of treatment (M‐H fixed‐effect OR 0.88; 95% CI 0.19 to 4.01), P = 0.10 (Analysis 3.3).
3.3. Analysis.
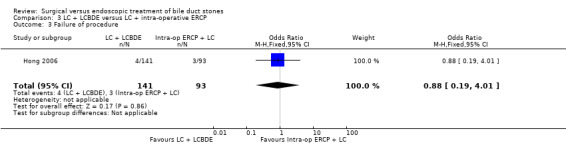
Comparison 3 LC + LCBDE versus LC + intra‐operative ERCP, Outcome 3 Failure of procedure.
Conversion to open surgery
There was no significant difference in the number of participants that required conversion to open procedure between the two groups (15/141 in the LCBDE versus 8/93 in the intra‐op ERCP group; P = 0.61) (Analysis 3.4).
3.4. Analysis.
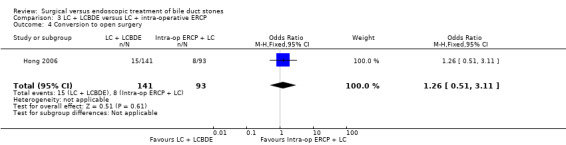
Comparison 3 LC + LCBDE versus LC + intra‐operative ERCP, Outcome 4 Conversion to open surgery.
Quality of life
We found no data on quality of life.
Duration of procedure
The randomised clinical trial included reported no significant difference in surgical times between the two intervention arms. The mean procedural time in the surgical group was 133.83 (SD ± 58.24) minutes versus the mean intra‐operative ERCP procedural time of 140.32 (SD ± 56.55) minutes (Analysis 3.5).
3.5. Analysis.
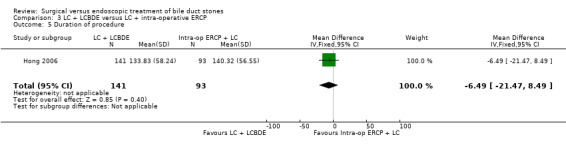
Comparison 3 LC + LCBDE versus LC + intra‐operative ERCP, Outcome 5 Duration of procedure.
Hospital stay
There was no significant difference in the length of postoperative hospital stay between the two groups. The intra‐operative ERCP group reported a mean postoperative hospital stay of 4.25 (SD ± 3.46) days compared to the surgical group with a mean postoperative hospital stay of 4.66 (SD ± 3.07) days (Analysis 3.6).
3.6. Analysis.
Comparison 3 LC + LCBDE versus LC + intra‐operative ERCP, Outcome 6 Duration of hospital stay.
| Duration of hospital stay | ||
|---|---|---|
| Study | Intra‐op ERCP + LC | LC + LCBDE |
| Hong 2006 | 4.25 +/‐ 3.46 | 4.66 +/‐ 3.07 |
Costs
There was no significant difference found in the reported hospital charges between the two intervention arms in this randomised clinical trial (Analysis 3.7).
3.7. Analysis.
Comparison 3 LC + LCBDE versus LC + intra‐operative ERCP, Outcome 7 Cost.
| Cost | ||
|---|---|---|
| Study | Intra‐op ERCP + LC | LC + LCBDE |
| Hong 2006 | 17279.96 + / ‐ 4097.43 | 13559.20 +/‐ 3452.10 |
LC + LCBDE versus LC + postoperative ERCP
There were two trials included in this comparison (Rhodes 1998; Nathanson 2005), randomising a total of 166 participants. In the first trial (Rhodes 1998), 80 participants were randomised after intra‐operative cholangiogram to laparoscopic bile duct exploration (transcystic or choledochotomy) or postoperative ERCP. In the other trial (Nathanson 2005), 86 participants were randomised after failed transcystic clearance to laparoscopic choledochotomy or postoperative ERCP. There were no post‐randomisation drop‐outs.
Mortality
There were no deaths reported in either of these two trials. Trial sequential analysis could not be performed using the control group proportion because of the absence of mortality in the control group. We therefore used a control group proportion of [1.02%] which was the mortality observed in laparoscopic cholecystectomy with pre‐operative ERCP. Trial sequential analysis revealed that the proportion of information accrued was only 0.24% of the diversity‐adjusted required information size and so the trial sequential monitoring boundaries were not drawn (Figure 10). The cumulative Z curve does not cross the conventional statistical boundaries.
10.
Trial sequential analysis of mortality (laparoscopic common bile duct exploration versus post‐operative endoscopic retrograde cholangio pancreatography after laparoscopic cholecystectomy) The diversity‐adjusted required information size (DARIS) was calculated to 71,546 patients, based on the proportion of patients in the control group with the outcome of 1.02%, a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and a diversity of 0%. To account for zero event groups, a continuity correction of 0.01 was used in the calculation of the cumulative Z‐curve (blue line). After accruing a total of 166 participants in two trials, only 0.24% of the DARIS has been reached. Accordingly, the trial sequential analysis does not show the required information size and the trial sequential monitoring boundaries. As shown, the conventional statistical boundaries (etched red line) have also not been crossed by the cumulative Z‐curve.
Total morbidity
Both the included trials reported morbidity (Rhodes 1998; Nathanson 2005). There was no significant difference between the two arms ((M‐H fixed‐effect OR 1.16; 95% CI 0.50 to 2.72), P = 0.73. There was no significant heterogeneity (I² = 0%) (Analysis 4.1). Trial sequential analysis revealed that the proportion of information accrued was only 3.79% of the diversity‐adjusted required information size and so the trial sequential monitoring boundaries were not drawn (Figure 11). The cumulative Z curve does not cross the conventional statistical boundaries.
4.1. Analysis.
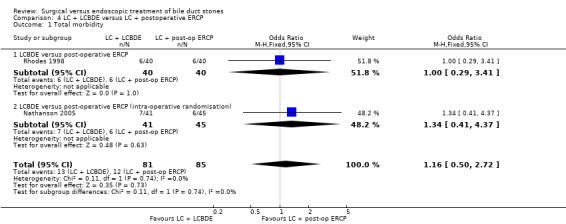
Comparison 4 LC + LCBDE versus LC + postoperative ERCP, Outcome 1 Total morbidity.
11.
Trial sequential analysis of morbidity (laparoscopic common bile duct exploration versus post‐operative endoscopic retrograde cholangio pancreatography after laparoscopic cholecystectomy) The diversity‐adjusted required information size (DARIS) was calculated to 4,381 patients, based on the proportion of patients in the control group with the outcome of 14.12%, a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and a diversity of 0%. After accruing a total of 166 participants in two trials, only 3.79% of the DARIS has been reached. Accordingly, the trial sequential analysis does not show the required information size and the trial sequential monitoring boundaries. As shown, the conventional statistical boundaries (etched red line) have also not been crossed by the cumulative Z‐curve (blue line).
Retained stones after primary intervention
Both the included trials reported data about retained stones (Rhodes 1998; Nathanson 2005). There was a significant difference (on fixed‐effect analysis) in the number of participants with retained stones between the two arms: 7/81 in the laparoscopy arm and 21/85 participants in the endoscopy arm had retained stones (M‐H fixed‐effect OR 0.28; 95% CI 0.11 to 0.72; P = 0.008). However, on random‐effects analysis, this difference is not significant (M‐H OR 0.25; 95% CI 0.04 to 1.65), P = 0.15, I² = 62% (Analysis 4.2). The trial sequential analysis revealed that only 2.17% of the diversity‐adjusted required information size has been reached. So, trial sequential monitoring boundaries were not drawn. The trial sequential analysis suggested that although there may be a statistically significant reduction in the proportion of people with retained stones in the LCBDE group compared with the ERCP group, there is a high risk of random error and one cannot firmly conclude that the LCBDE group had a significantly lower proportion of retained stones than the ERCP group (Figure 12).
4.2. Analysis.
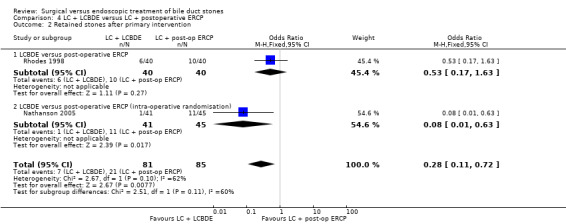
Comparison 4 LC + LCBDE versus LC + postoperative ERCP, Outcome 2 Retained stones after primary intervention.
12.
Trial sequential analysis of retained stones (laparoscopic common bile duct exploration (LCBDE) versus post‐operative endoscopic retrograde cholangio pancreatography (ERCP) after laparoscopic cholecystectomy) The diversity‐adjusted required information size (DARIS) was calculated to 7,661 patients, based on the proportion of patients in the control group with the outcome of 24.71%, a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and a diversity of 0%. After accruing a total of 166 participants in two trials, only 2.17% of the DARIS has been reached. Accordingly, the trial sequential analysis does not show the required information size and the trial sequential monitoring boundaries. The cumulative Z‐curve (blue line) crosses the conventional boundaries (etched red line). This suggests that although there is statistically significant reduction in the proportion of people with retained stones in the LCBDE group compared to the post‐operative ERCP group, there is a high risk of random error and one cannot firmly conclude that LCBDE has significantly lower retained stones proportion compared to the post‐operative ERCP group. The random‐effects model also did not reveal significant difference between the groups.
Failure of procedure
Both trials reported the number of failed procedures (Rhodes 1998; Nathanson 2005). In the laparoscopic choledochotomy trial there was a significantly lower risk of failure of procedure in the surgical groups compared with the endoscopic group(1/41 versus 11/45) (M‐H OR 0.08; 95% CI 0.01 to 0.63, P = 0.02) (Nathanson 2005). Meta‐analysis demonstrated marked heterogeneity (I² = 80%) with no significant difference between the two groups on fixed‐effect analysis (OR 0.47; 95% CI 0.21 to 1.06) as well as on random‐effect analysis (OR 0.33; 95% CI 0.02 to 4.31) (Analysis 4.3).
4.3. Analysis.
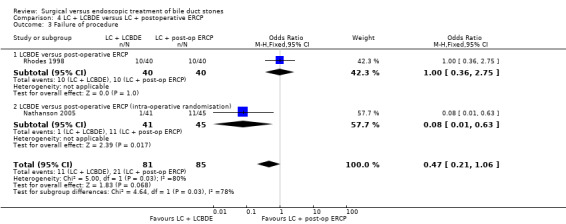
Comparison 4 LC + LCBDE versus LC + postoperative ERCP, Outcome 3 Failure of procedure.
Conversion to open surgery
Both included trials reported the number of conversions to open surgery (Rhodes 1998; Nathanson 2005). There was no significant difference in the proportion of participants who underwent conversion to open surgery between the two groups (M‐H fixed‐effect OR 1.77; 95% CI 0.23 to 13.81), P = 0.58 (Analysis 4.4).
4.4. Analysis.
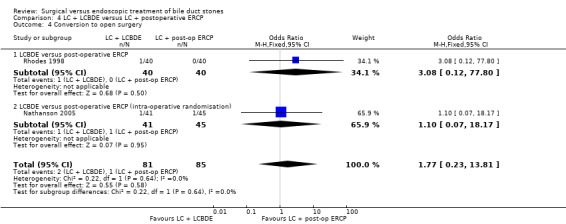
Comparison 4 LC + LCBDE versus LC + postoperative ERCP, Outcome 4 Conversion to open surgery.
Quality of life
We found no data on quality of life.
Duration of procedure
Both trials reported no significant difference between laparoscopy and endoscopy arms (Rhodes 1998; Nathanson 2005). Meta‐analysis could not be performed due to the parametric data. Rhodes 1998 reported median duration of procedure to be 90 (25 to 310) minutes for the surgical versus 105 (60 to 255) minutes for endoscopy groups (P = 0.1). Nathanson 2005 reported 158.8 minutes for the surgical and 147.9 for the endoscopy group(P = 0.49) (Analysis 4.5).
4.5. Analysis.
Comparison 4 LC + LCBDE versus LC + postoperative ERCP, Outcome 5 Duration of procedure.
| Duration of procedure | |||
|---|---|---|---|
| Study | Post‐op ERCP + LC | LC + LCBDE | P |
| Nathanson 2005 | 147.9 min (both procedures together) | 158.8 min (both procedures together) | 0.49 |
| Rhodes 1998 | 105 (60‐255) min (both procedures together) | 90 (25‐310) min (both procedures together) | 0.1 |
Hospital stay
Both trials reported a shorter stay in the surgical arm (Rhodes 1998; Nathanson 2005). Median hospital stay was shorter in the LCBDE group compared with the ERCP group (1 day versus 3.5 days; P = 0.0001) (Rhodes 1998). Nathanson 2005 reported a small difference, 6.4 days versus 7.7 days, with no P value reference (Analysis 4.6). Trial sequential analysis was not performed since a meta‐analysis was not performed.
4.6. Analysis.
Comparison 4 LC + LCBDE versus LC + postoperative ERCP, Outcome 6 Duration of hospital stay.
| Duration of hospital stay | |||
|---|---|---|---|
| Study | LC + post‐op ERCP | LC + LCBDE | P |
| Nathanson 2005 | Mean: 7.7 days | Mean: 6.4 days | 0.57 |
| Rhodes 1998 | 3.5 days (1‐11 days) | 1 day (1‐26 days) | 0.0001 |
Costs
Costs were not reported.
Reporting bias
We did not generate a funnel plot because only two trials were included in this comparison.
Single‐stage versus two‐stage management of CBD stones
We have analysed the data from the studies comparing the single‐stage procedures (LC+LCBDE) with two‐stage procedures (pre‐operative ERCP + LC or LC + postoperative ERCP). Seven studies were included in this meta‐analysis (Rhodes 1998; Cuschieri 1999; Sgourakis 2002; Nathanson 2005; Noble 2009; Bansal 2010; Rogers 2010). Earlier trials comparing the open surgical arm with the endoscopic arm were not considered for this analysis and only the laparoscopic surgical studies were included. Furthermore, it does not include LCBDE versus intra‐operative ERCP as both the intervention arms were single‐stage procedures (Hong 2006).
Mortality
All seven trials reported mortality rates (Rhodes 1998; Cuschieri 1999; Sgourakis 2002; Nathanson 2005; Noble 2009; Bansal 2010; Rogers 2010). Only two studies encountered mortality following the interventions and there was no significant difference in the proportion of participants who died (M‐H fixed‐effect OR 0.72; 95% CI 0.12 to 4.33; P = 0.72) (Cuschieri 1999; Sgourakis 2002) (Analysis 5.1). Trial sequential analysis revealed that the proportion of information accrued was only 0.86% of the diversity‐adjusted required information size and so the trial sequential monitoring boundaries were not drawn (Figure 13). The cumulative Z curve does not cross the conventional statistical boundaries.
5.1. Analysis.
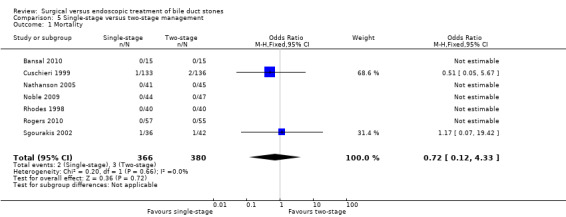
Comparison 5 Single‐stage versus two‐stage management, Outcome 1 Mortality.
13.
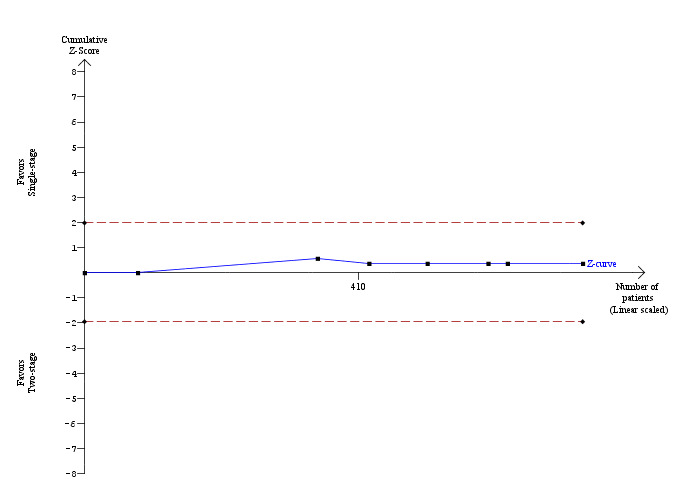
Trial sequential analysis of mortality (single‐stage versus two‐stage procedures) The diversity‐adjusted required information size (DARIS) was calculated to 86,456 patients, based on the proportion of patients in the control group with the outcome of 0.79%, a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and a diversity of 0%. To account for zero event groups, a continuity correction of 0.01 was used in the calculation of the cumulative Z‐curve (blue line). After accruing a total of 746 participants in seven trials, only 0.86% of the DARIS has been reached. Accordingly, the trial sequential analysis does not show the required information size and the trial sequential monitoring boundaries. As shown, the conventional boundaries have also not been crossed by the cumulative Z‐curve.
Sensitivity analysis
'Good outcome' analysis: (OR 0.71; 95% CI 0.12 to 4.27), P = 0.71, I² = 0% (no significant difference) (Analysis 5.2.1). 'Poor outcome' analysis: (OR 1.01; 95% CI 0.55 to 1.85), P = 0.98, I² = 0% (no significant difference) (Analysis 5.2.2). 'Best‐case' for single‐stage: (OR 0.10; 95% CI 0.03 to 0.38), P = 0.0006, I² = 39% (favours single‐stage) (Analysis 5.2.3) 'Worst‐case' for single‐stage : (OR 7.46; 95% CI 2.39 to 23.27), P = 0.0005, I² = 0% (favours two‐stage).(Analysis 5.2.4)
5.2. Analysis.
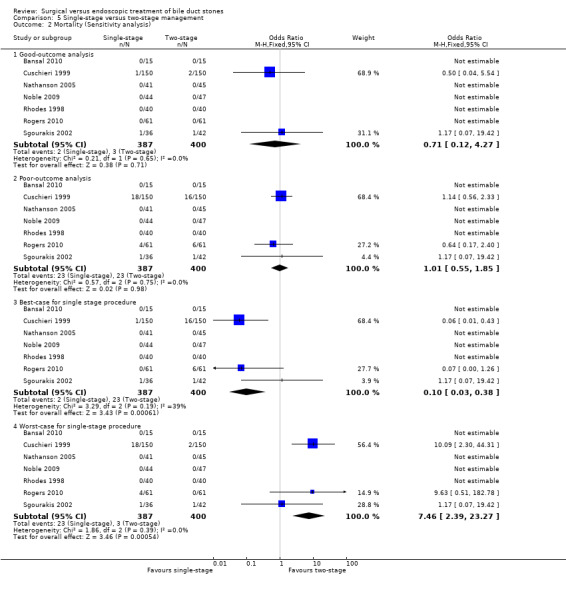
Comparison 5 Single‐stage versus two‐stage management, Outcome 2 Mortality (Sensitivity analysis).
Morbidity
All seven trials reported morbidity (Cuschieri 1999; Sgourakis 2002; Nathanson 2005; Noble 2009; Rhodes 1998; Bansal 2010; Rogers 2010). There was no significant difference in the morbidity that was encountered between the two groups: 57/366 participants in the single‐stage procedure and 49/380 participants in the two‐stage procedure (M‐H fixed‐effect OR 1.25; 95% CI 0.83 to 1.89; P = 0.29) (Analysis 5.3). Trial sequential analysis revealed that only 15.42% of the diversity‐adjusted required information size has been reached so the futility area was not drawn. The trial sequential analysis was consistent with absence of current evidence of any significant difference between single‐stage and two‐stage procedures but significantly increased or decreased morbidity of single‐stage compared to two‐stage could not be ruled out (Figure 14).
5.3. Analysis.
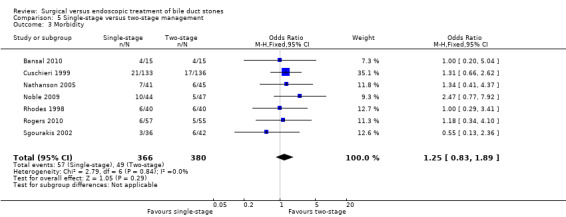
Comparison 5 Single‐stage versus two‐stage management, Outcome 3 Morbidity.
14.
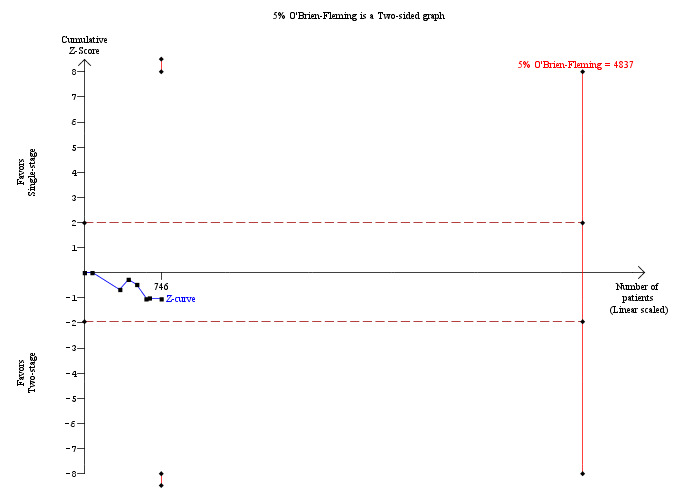
Trial sequential analysis of morbidity (single‐stage versus two‐stage procedures) The diversity‐adjusted required information size (DARIS) was calculated to 4,837 patients, based on the proportion of patients in the control group with the outcome of 12.89%, a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and a diversity of 0%. After accruing a total of 746 participants in seven trials, only 15.42% of the DARIS has been reached. So, the futility area was not drawn. The cumulative Z‐curve (blue line) does not cross the trial sequential monitoring boundaries (red line) or the conventional boundaries (etched red line). This is consistent with absence of current evidence of any significant difference between single‐stage and two‐stage procedures but significantly increased or decreased morbidity of single‐stage compared to two‐stage procedures cannot be ruled out.
Sensitivity analysis
'Good outcome' analysis: (OR 1.24; 95% CI 0.82 to 1.87), P = 0.31, I² = 0% (no significant difference) (Analysis 5.4.1). 'Poor outcome' analysis: (OR 1.20; 95% CI 0.84 to 1.72), P = 0.32, I² = 0% (no significant difference) (Analysis 5.4.2). 'Best‐case' for single‐stage: (OR 0.82; 95% CI 0.56 to 1.21), P = 0.32, I² = 3% (no significant difference) (Analysis 5.4.3). 'Worst‐case' for single‐stage : (OR 1.80; 95% CI 1.22 to 2.66), P = 0.003, I² = 1% (favours two‐stage) (Analysis 5.4.4).
5.4. Analysis.
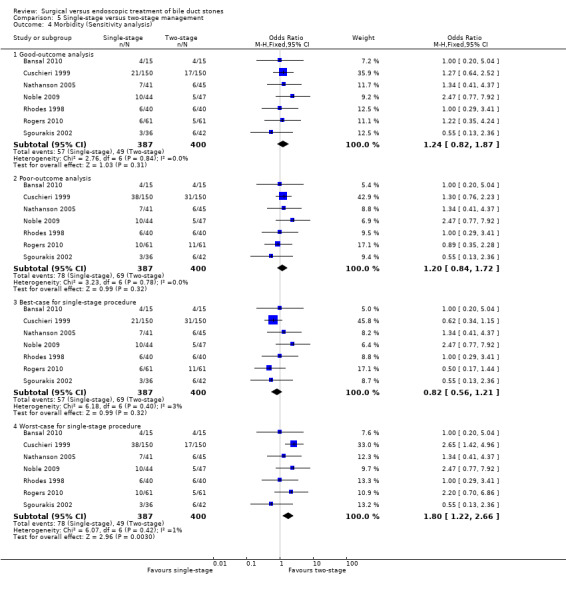
Comparison 5 Single‐stage versus two‐stage management, Outcome 4 Morbidity (Sensitivity analysis).
Retained stones
All seven trials reported the incidence of retained stones (Rhodes 1998; Cuschieri 1999; Sgourakis 2002; Nathanson 2005; Noble 2009; Bansal 2010; Rogers 2010). There was a significantly lower proportion of participants with retained stones in the single‐stage group (31/366 participants) compared with the two‐stage group (52/380 participants). This difference was not significant in random‐effects model (OR 0.58; 95% CI 0.28 to 1.22, P = 0.15, I² = 36%) (Analysis 5.5) but was significant in a fixed‐effect model (OR 0.59; 95% CI 0.37 to 0.94, P = 0.03). Trial sequential analysis revealed that only 8.29% of the diversity‐adjusted required information size has been reached. So, the futility area was not drawn. The trial sequential analysis suggested that although there is a statistically significant reduction in the proportion of people with retained stones in the single‐stage group compared with the two‐stage group, there is a high risk of random error and one cannot firmly conclude that the single‐stage group has a significantly lower proportion of retained stones compared with the two‐stage group (Figure 15).
5.5. Analysis.
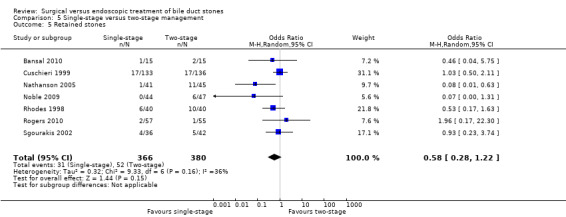
Comparison 5 Single‐stage versus two‐stage management, Outcome 5 Retained stones.
15.
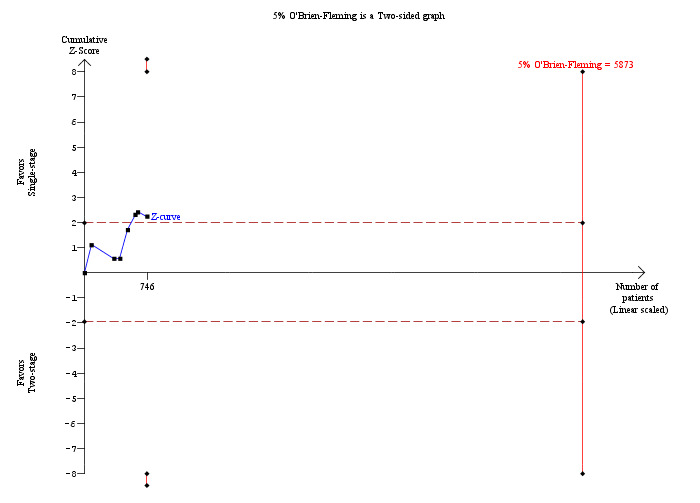
Trial sequential analysis of retained stones (single‐stage versus two‐stage procedures) The diversity‐adjusted required information size (DARIS) was calculated to 9,003 patients, based on the proportion of patients in the control group with the outcome of 13.68%, a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and a diversity of 49.85%. To account for zero event groups, a continuity correction of 0.01 was used in the calculation of the cumulative Z‐curve (blue line). After accruing a total of 746 participants in seven trials, only 8.29% of the DARIS has been reached. So, the futility area was not drawn. The cumulative Z‐curve does not cross the trial sequential monitoring boundaries (red line) but crosses the conventional boundaries (etched red line). This suggests that although there is statistically significant reduction in the proportion of people with retained stones in the single‐stage group compared to the two‐stage group, there is a high risk of random error and one cannot firmly conclude that single‐stage group has significantly lower retained stones proportion compared to the two‐stage group.
Sensitivity analysis
'Good outcome' analysis: (OR 0.58; 95% CI 0.37 to 0.93), P = 0.02, I² = 35% (favours single‐stage) (Analysis 5.6.1). 'Poor outcome' analysis: (OR 0.70; 95% CI 0.47 to 1.03), P = 0.07, I² = 40% (no significant difference) (Analysis 5.6.2). 'Best‐case' for single‐stage: (OR 0.39; 95% CI 0.25 to 0.62), P < 0.0001, I² = 1% (favours single‐stage) (Analysis 5.6.3). 'Worst‐case' for single‐stage: (OR 1.03; 95% CI 0.69 to 1.56), P = 0.88, I² = 70% (no significant difference) (Analysis 5.6.4).
5.6. Analysis.
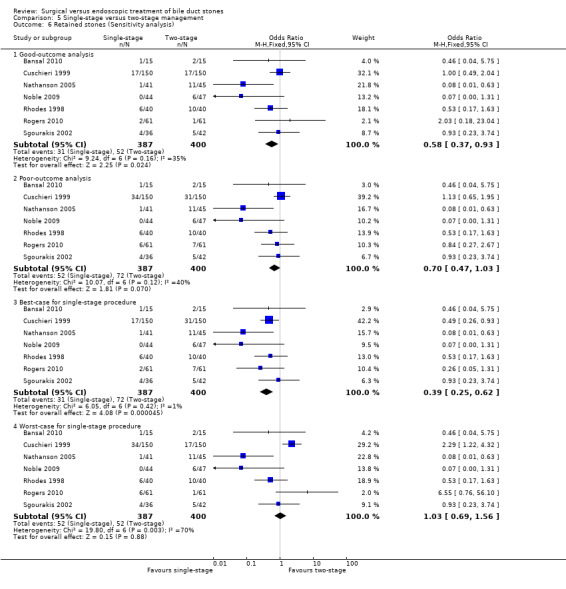
Comparison 5 Single‐stage versus two‐stage management, Outcome 6 Retained stones (Sensitivity analysis).
Failure to complete the procedure
All seven trials reported the incidence of failed procedures (Rhodes 1998; Cuschieri 1999; Sgourakis 2002; Nathanson 2005; Noble 2009; Bansal 2010; Rogers 2010). The planned procedure was completed successfully in more participants in the single‐stage procedure (37 failures in 366 participants) compared to the two‐stage procedure (70 failures in 380 participants). This difference is statistically significant and favours the single‐stage procedure with a fixed‐effect model (M‐H OR 0.50; 95% CI 0.33 to 0.77; P = 0.002, I² = 58%) but the difference was not significant with a random‐effects model (OR 0.49; 95% CI 0.20 to 1.18; P = 0.11) (Analysis 5.7).
5.7. Analysis.
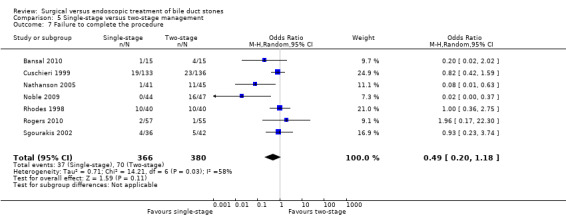
Comparison 5 Single‐stage versus two‐stage management, Outcome 7 Failure to complete the procedure.
Conversion to open surgery
All seven trials reported the rates of conversion to open surgery (Rhodes 1998; Cuschieri 1999; Sgourakis 2002; Nathanson 2005; Noble 2009; Bansal 2010; Rogers 2010). There was no significant difference between the two groups (OR 1.49; 95% CI 0.80 to 2.77; P = 0.21) (Analysis 5.8).
5.8. Analysis.
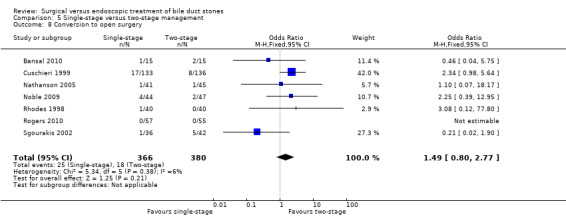
Comparison 5 Single‐stage versus two‐stage management, Outcome 8 Conversion to open surgery.
Reporting bias
We did not generate a funnel plot because only seven trials were included in this comparison.
Open or laparoscopic CBDE versus ERCP in patients with previous cholecystectomy
There were no trials applicable for comparison of these interventions in participants with previous cholecystectomy.
Summary of Findings tables
The summary of findings are reported (Table 1, Table 2, Table 3).
Discussion
Summary of main results
Overall, based on 16 randomised clinical trials, we still seem to lack sufficient evidence to recommend or refute one approach compared with another, regarding treatment of bile duct stones. This is due to the facts that all included trials have a high risk of bias and random errors.
Open surgery versus endoscopic retrograde cholangiopancreatography (ERCP)
Open surgery resulted in a significantly reduced number of retained stones, and lower rates of failure of planned treatment. There was no significant difference in the mortality and morbidity between the two groups. However, it is important to remember that these comparative trials are from the early days of endoscopy (1987 to 1998) and might have been influenced by the early experience of the endoscopist as well as the limited technological support.
Duration of surgery and the duration of hospital stay were difficult to assess from the trials included. Evaluation of these two outcomes requires inclusion of the duration of each procedure (endoscopic clearance and surgical removal of gallbladder). There were insufficient data to comment on the effect of the size and number of stones on the outcomes, costs involved, postoperative quality of life and postoperative analgesic requirements.
The studies are, however, a little dated and interpretation in the context of modern practice must be guarded. It is entirely possible that the results might have been influenced by the early experience of endoscopists in performing ERCP. It is unlikely that there will be any future trials comparing open surgery with ERCP, and the data from this review represent the best evidence comparing these two interventions.
One relevant scenario for the surgeon may be in the planning or performance of an open cholecystectomy (performed in preference to, or converted from, a laparoscopic cholecystectomy for whatever technical reason, eg, multiple previous laparotomies/adhesions) in a person with unexpected or CBD stones that are considered surgically removable. Open surgery and CBDE is one of the treatment options for these specific groups of patients, where an open cholecystectomy is considered as the follow‐up gallbladder surgery and not a laparoscopic surgery.
Laparoscopic cholecystectomy (LC) + LCBDE versus ERCP + LC or versus ERCP and LC or versus LC + ERCP
Several randomised clinical trials comparing the laparoscopy and endoscopic options were published since this review was first published (Hong 2006; Noble 2009; Bansal 2010; Rogers 2010). Pre‐operative ERCP to deal with the CBD stones followed by laparoscopic cholecystectomy is a popular option as the surgeon is assured a clear duct with no distal obstruction, reducing the risk of postoperative bile leak and the need for further postoperative procedures. However, studies performing intra‐operative cholangiogram demonstrated the presence of silent ductal stones in 5% to 10% that have eventually passed without significant clinical symptoms, questioning the need for ERCP in these people. Some authors therefore advocated postoperative ERCP for those people who had ductal stones at intra‐operative cholangiogram. Besides, about 2% to 15% of people who underwent pre‐operative ERCP and sphincterotomy had residual ductal stones at intra‐operative cholangiogram. This provides an argument to deal with the ductal stones by pre‐operative, intra‐operative or postoperative ERCP. Laparoscopic common bile duct exploration (LCBDE) offers the advantage of dealing with bile duct stones and gallbladder together, by a minimally invasive surgical procedure, during a single episode of hospitalisation as well as anaesthesia, and without the need for ERCP and endoscopic sphincterotomy.
There was no significant difference in the mortality and overall morbidity rates between LCBDE and the pre‐, intra‐ and postoperative ERCP groups. It was not possible to assess the procedure‐specific morbidity such as post‐ERCP (+ endoscopic sphincterotomy) pancreatitis, cholangitis and bleeding; post‐LCBDE bile leak and intra‐abdominal abscesses, due to lack of a standard reporting pattern for these complications (for example, there was an overlap between post‐interventional hyperamylasaemia/pancreatitis; no grading of severity of abscess or bile leak that required no or conservative or surgical treatment).
There was no significant difference in the retained stones between the LCBDE and the pre‐operative and intra‐operative ERCP groups. However, fewer retained stones were encountered in the postoperative ERCP group. These results could have been influenced by the fact that Nathanson 2005 and colleagues opted to randomise the participants at failed transcystic clearance to ERCP clearance or choledochotomy, adding to the heterogeneity of the included trials.
There was no significant difference in the conversion rates of LCBDE to open surgery when compared with pre‐operative, intra‐operative, and postoperative ERCP groups.
Single‐stage (LC + LCBDE) versus two‐stage management (pre‐operative ERCP + LC or LC + postoperative ERCP)
We compared the single‐stage laparoscopic surgical procedures with two ‐stage endoscopic procedures. However, we did not include the open surgical trials in the single‐stage group for the obvious reasons that the open and laparoscopic surgeries were two different techniques and their results could not be pooled. Also, open trials were from the early endoscopy era. There was no significant difference in the proportion of participants with morbidity, mortality or conversion to open surgery between the two groups. There were less number of patients with retained stones and failed procedures in the single‐stage group but this difference was not significant on the random‐effect meta‐analysis.
It was not possible to assess some important outcomes in this review. Rogers 2010 is the only trial that compared the quality of life (SF‐36) and the Karnofsky performance score between the endoscopy and surgical groups, finding no significant difference between the arms. None of the other trials reported patient satisfaction or quality of life. Postoperative pain scores were reported only by Bansal 2010 using visual analogue scales and there was no significant difference between the LC + LCBDE versus the pre‐operative ERCP + LC groups. With participants in both the arms subjected to laparoscopic or open cholecystectomy, the pain scores might simply be a surrogate outcome, but it would be interesting to know the influence of an additional procedure, ERCP, on patient satisfaction scores in future trials.
Data about the duration of hospital stay were not included in the meta‐analysis as the data were either not described (Bornman 1992; Targarona 1996), presented as parametric data (Stiegmann 1992; Nathanson 2005), as a mixture of parametric and non‐parametric data (Cuschieri 1999; Kapoor 1996), or were presented in non‐parametric data format, that is, median and range (Neoptolemos 1987; Stain 1991; Hammarstrom 1995; Rhodes 1998; Suc 1998; Sgourakis 2002). Four trials measured treatment cost (Stiegmann 1992; Kapoor 1996; Hong 2006; Rogers 2010) but it was not possible to tease out the procedure‐specific costs. Outcomes such as postoperative pain scores, length of hospital stay, cost of the procedure, quality of life and patient satisfaction need to be assessed and reported in a more standard fashion and must be included in future randomised clinical trials.
The number of stones and the size of stones were not included in the updated review. Successful extraction of the duct stones could be influenced by the technical expertise and preference of the endoscopists (or laparoscopic surgeon performing LCBDE) and the gadgets available in their unit. Moreover, none of the trials have reported their outcomes based on the stone size and number criteria. The influence of the technique of laparoscopic bile duct clearance ‐ transcystic or transcholedochal approach, impact of the biliary drainage procedure (placement of a T‐tube or an antegrade biliary stent) on the outcomes of the LCBDE patients was not planned to be assessed in this review.
Overall in this review the primary and the secondary outcomes that were evaluated on an intention‐to‐treat basis have shown no significant difference between the surgical and endoscopic modalities of the management of bile duct stones ‐ but, as stated, data were too sparse.
Overall completeness and applicability of evidence
This review is applicable only to people fit to undergo endoscopic or surgical (open or laparoscopic) intervention in the management of common bile duct stones.
Quality of the evidence
This review includes a total of 1758 participants from 16 randomised trials with the primary outcomes addressed in all the papers (except for the data on retained stones in Bornman 1992). The risk of bias in the included trials was high and the quality of the evidence was moderate. One of the major sources of heterogeneity among the trials was the lack of uniform criteria among the trials to confirm the presence of ductal stones at the time of randomisation. Participants who were suspected of having CBD stones, confirmed to have CBD stones by pre‐operative imaging and those with randomisation at intra‐operative cholangiogram were all included in the review. The expertise of the endoscopists and the laparoscopic surgeons in the initial trials might have affected their outcomes. These issues should be addressed in future trials.
We included the Sgourakis 2002 trial as a randomised clinical trial with high risk of bias as one of the five trials assessing LC + LCBDE versus preoperative ERCP + LC. This decision was taken in spite of the fact that we have suspicion that it may not be a randomised clinical trial as we cannot exclude irregularities. On the other hand, it did not contribute very much to our analyses and most findings were neutral. Accordingly, excluding this trial would not lead to noticeable changes in our findings.
Potential biases in the review process
We have followed the Cochrane Collaboration methodology for performing the review. Most of the included studies were assessed to be at high risk of bias in one of the six domains (blinding), and at unclear risk of bias in another domain (allocation concealment) (Figure 1; Figure 2). The high risk of bias was mainly attributable to the lack of blinding in all the trials as well as unclear allocation concealment. Blinding of the assessor might be feasible (in future trials), if the postoperative outcomes were to be evaluated by an independent assessor and not by the surgeon or endoscopist.
Agreements and disagreements with other studies or reviews
The current updated review is in agreement with the conclusions of the previously published version (Martin 2006) that open surgery seems superior to ERCP in the management of bile duct stones. There is no significant difference in the safety and efficacy of laparoscopic bile duct clearance and the endoscopic options. There is no significant reduction in the number of retained stones and failure rates in the laparoscopy arm compared to that of the pre‐operative or intra‐operative endoscopy arms, although this was not the case for the postoperative ERCP group.
Authors' conclusions
Implications for practice.
Open bile duct surgery seems superior to open cholecystectomy plus endoscopic retrograde cholangiopancreatography (ERCP) in its ability to achieve bile duct stone clearance. It is important to remember that these comparative trials are from the initial days of endoscopy (1987 to 1998) and the success rates of endoscopic procedures might have improved over the past decade. However, the evidence would suggest that when a surgeon is required to perform an open cholecystectomy in a person with common bile duct (CBD) stones, then surgical duct clearance is a worthy option.
There seems to be no significant differences in the safety and efficacy of laparoscopic bile duct exploration versus the endoscopic options. There was no significant difference in the proportion of participants with retained stones between the laparoscopic and the endoscopic arms (with the exception of the postoperative ERCP group). Similarly, there was no significant difference in the measured outcomes between the single‐stage laparoscopic bile duct clearance and the two‐stage endoscopic management.
Implications for research.
While it appears that LCBDE is safe and effective, larger good quality randomised clinical trials have a role to validate the findings of this review and also to explore the benefit of assessing evolving practices in the surgical community that may occur not only with increasing experience with laparoscopic techniques, but also with improvements in both endoscopic and laparoscopic technology.
In terms of future trials and trial designs, the following suggestions can be made to investigators:
Based on the available evidence, there is a definite place for open CBD exploration in people who are not suitable for laparoscopic surgery. New multicentre randomised clinical trials (to recruit enough participants) comparing the open CBDE and endoscopy might be able to update the current evidence.
The trial design should reflect realistic clinical situations. Randomisation should occur on suspicion of CBD stones and not at ERCP, since this latter situation exposes the surgery‐only group to the complications of ERCP without the potential benefits.
Further trials comparing the LCBDE and endoscopic procedures are needed to address and evaluate the outcomes that were discussed in the review, in addition to the other clinically relevant outcomes such as: procedure‐specific complications, additional procedures required to deal with the complications, hospital stay, total treatment cost and health economics, and, importantly, quality of life and patient satisfaction.
Follow‐up was generally poorly reported in the trials included in this review. It is particularly relevant for the accurate analysis of morbidity and long‐term outcomes. Long‐term outcomes might be helpful in making the clinical decision about the management of young patients with CBD stones.
With the current advances in laparoscopic surgical technology and expertise, options of laparoscopic cholecystectomy (LC) + intra‐operative ultrasound scan with or without LCBDE, transcystic or transcholedochal exploration of the CBD, and the role of biliary stents need evaluated in future trials.
Future trial ought to be planned according to the SPIRIT Statement (SPIRIT 2013).
A plea must be made to remind all researchers to present data in appropriate formats, to employ appropriate statistical tests, and report the appropriate features of trial methodology. This is best summarised in the CONSORT statement (Begg 1996) and its associated online electronic checklist that serves not only as a guide for trial publication but for pre‐trial preparation. We would also like to emphasise the publication of results in a manner allowing for interpretation of individual patient outcomes, thus allowing the inclusion of a wide range of data in meta‐analysis. Appropriate reporting of methodology will allow for the rapid assessment of trial quality. Journal editors are increasingly demanding that trial reporting adheres to the formats described in the CONSORT Statement (www.consort‐statement.org).
What's new
| Date | Event | Description |
|---|---|---|
| 15 July 2013 | New citation required and conclusions have changed | Conclusions from previous review: In the laparoscopic era, data are close to excluding a significant difference between laparoscopic and ERCP clearance of common bile duct stones. Conclusions from current review: Open bile duct surgery seems superior to ERCP in achieving common bile duct stone clearance based on the evidence available from the early endoscopy era. There is no significant difference in the mortality and morbidity between laparoscopic bile duct clearance and the endoscopic options. There is no reduction of the number of retained stones and failure rates in the laparoscopy group compared with that of the endoscopy group. There is no significant difference in the mortality, morbidity, and duct clearance rates between the single‐stage procedure (LCBDE) and two‐stage procedures. |
| 14 July 2013 | Amended | Updated objectives:
|
| 8 July 2013 | New search has been performed | Review is an updated version of Martin 2006. There are 16 trials included in the update. The objectives of the review, study groups, and primary and secondary outcomes were redefined. |
| 17 December 2012 | New search has been performed | The following outcomes were included in the protocol but are not included in the review:
|
| 14 July 2012 | Amended | Updated outcomes: Primary outcomes
Secondary outcomes
|
Acknowledgements
David Martin and James Toouli, Australia for performing previous work that was the foundation of the current study.
Dimitrinka Nikolova (Review Group Managing Editor) and the Cochrane Hepato‐Biliary Group at the Copenhagen Trial Unit, for the invaluable advice, support, and extremely prompt responses. Sarah Klingenberg, the Trials Search Co‐ordinator, for designing the electronic data search. Keith Gardiner for his encouragement, support and help in initiating the review.
Peer‐reviewers: Emmanouil Giorgakis, UK; Amir Taefi, USA. Contact Editors: Frederik Keus, The Netherlands; Christian Gluud, Denmark.
Appendices
Appendix 1. Search strategies
| Database | Time of search | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | August 2013. | (bile duct stone* or gall‐stone* or gall stone* or cholelithiasis or choledolithiasis or common bile duct calculi) AND (cholecystectom* or endoscopic retrograde cholangiopancreatograph* or ERCP or endoscopic sphincterotom* or sphincteroplast*) |
| Cochrane Central Register of Controlled Trials in The Cochrane Library | Issue 7 of 12, 2013. | #1 MeSH descriptor: [Gallstones] explode all trees #2 MeSH descriptor: [Cholelithiasis] explode all trees #3 bile duct stone* or gall*stone* or chole*lithiasis or common bile duct calculi #4 #1 or #2 or #3 #5 MeSH descriptor: [Cholecystectomy, Laparoscopic] explode all trees #6 MeSH descriptor: [Cholangiopancreatography, Endoscopic Retrograde] explode all trees #7 MeSH descriptor: [Sphincterotomy, Endoscopic] explode all trees #8 cholecystectom* or endoscopic retrograde cholangiopancreatograph* or ERCP or endoscopic sphincterotom* or sphincteroplast* #9 #5 or #6 or #7 or #8 #10 #4 and #9 |
| MEDLINE (Ovid SP) | 1946 to August 2013. | 1. exp Gallstones/ 2. exp Cholelithiasis/ 3. (bile duct stone* or gall*stone* or chole*lithiasis or common bile duct calculi).mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier] 4. 1 or 2 or 3 5. exp Cholecystectomy, Laparoscopic/ 6. exp Cholangiopancreatography, Endoscopic Retrograde/ 7. exp Sphincterotomy, Endoscopic/ 8. (cholecystectom* or endoscopic retrograde cholangiopancreatograph* or ERCP or endoscopic sphincterotom* or sphincteroplast*).mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier] 9. 5 or 6 or 7 or 8 10. 4 and 9 11. (random* or blind* or placebo* or meta‐analysis).mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier] 12. 10 and 11 |
| EMBASE (Ovid SP) | 1974 to August 2013. | 1. exp cholelithiasis/ 2. (bile duct stone* or gall*stone* or chole*lithiasis or common bile duct calculi).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 3. 1 or 2 4. exp cholecystectomy/ 5. exp endoscopic retrograde cholangiopancreatography/ 6. exp endoscopic sphincterotomy/ 7. (cholecystectom* or endoscopic retrograde cholangiopancreatograph* or ERCP or endoscopic sphincterotom* or sphincteroplast*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 8. 4 or 5 or 6 or 7 9. 3 and 8 10. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 11. 9 and 10 |
| Science Citation Index Expanded | 1900 to August 2013. | #5 #4 AND #3 #4 TS=(random* or blind* or placebo* or meta‐analys*) #3 #2 AND #1 #2 TS=( cholecystectom* or endoscopic retrograde cholangiopancreatograph* or ERCP or endoscopic sphincterotom* or sphincteroplast*) #1 TS=(bile duct stone* or gall*stone* or chole*lithiasis or common bile duct calculi) |
Data and analyses
Comparison 1. Open surgery versus ERCP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 8 | 729 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.18, 1.44] |
| 1.1 Randomisation once bile duct stones proven | 4 | 275 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.06, 2.72] |
| 1.2 Randomisation on suspicion of bile duct stones | 3 | 356 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.09, 2.65] |
| 1.3 High‐risk participants only | 1 | 98 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.11, 4.27] |
| 2 Mortality (Sensitivity analysis) | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Good‐outcome analysis | 8 | 737 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.18, 1.42] |
| 2.2 Poor‐outcome analysis | 8 | 737 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.43, 2.32] |
| 2.3 Best‐case for open surgery | 8 | 737 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.17, 1.25] |
| 2.4 Worst‐case for open surgery | 8 | 737 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.47, 2.55] |
| 3 Total morbidity | 8 | 729 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.77, 1.62] |
| 3.1 Randomisation once bile duct stones proven | 4 | 275 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.59, 1.77] |
| 3.2 Randomisation on suspicion of bile duct stones | 3 | 356 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.63, 1.96] |
| 3.3 High‐risk participants only | 1 | 98 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.57, 4.30] |
| 4 Morbidity (Sensitivity analysis) | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Good‐outcome analysis | 8 | 737 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.76, 1.58] |
| 4.2 Poor‐outcome analysis | 8 | 737 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.83, 1.71] |
| 4.3 Best‐case for open surgery | 8 | 737 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.74, 1.54] |
| 4.4 Worst‐case for open surgery | 8 | 737 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.84, 1.75] |
| 5 Retained stones | 7 | 609 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.21, 0.62] |
| 5.1 Randomisation once bile duct stones proven | 4 | 275 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.17, 0.72] |
| 5.2 Randomisation on suspicion of bile duct stones | 2 | 236 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.12, 0.74] |
| 5.3 High‐risk participants only | 1 | 98 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.19 [0.13, 80.23] |
| 6 Retained stones (Sensitivity analysis) | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Good‐outcome analysis | 7 | 617 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.20, 0.60] |
| 6.2 Poor‐outcome analysis | 7 | 617 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.28, 0.76] |
| 6.3 Best‐case for open surgery | 7 | 617 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.20, 0.58] |
| 6.4 Worst‐case for open surgery | 7 | 609 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.21, 0.62] |
| 7 Failure of procedure | 7 | 609 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.19, 0.51] |
| 7.1 Randomisation once CBD stones confirmed | 4 | 275 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.14, 0.60] |
| 7.2 Randomisation on suspicion of bile duct stones | 2 | 236 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.13, 0.66] |
| 7.3 High‐risk participants only | 1 | 98 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.12, 2.08] |
| 8 Hospital stay | Other data | No numeric data | ||
| 8.1 Randomisation once CBD stones were proven | Other data | No numeric data | ||
| 8.2 Randomisation on suspicion of CBD stones | Other data | No numeric data | ||
| 8.3 High‐risk participants only | Other data | No numeric data | ||
| 9 Cost | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 1102.0 [299.54, 1904.46] |
Comparison 2. LC + LCBDE versus pre‐operative ERCP + LC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 5 | 580 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.12, 4.33] |
| 1.1 Low‐risk group | 4 | 489 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.12, 4.33] |
| 1.2 High‐risk group | 1 | 91 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Mortality (Sensitivity analysis) | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Good‐outcome analysis | 5 | 621 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.12, 4.27] |
| 2.2 Poor‐outcome analysis | 5 | 621 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.55, 1.85] |
| 2.3 Best‐case for LCBDE | 5 | 621 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.38] |
| 2.4 Worst‐case for LCBDE | 5 | 621 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.46 [2.39, 23.27] |
| 3 Total morbidity | 5 | 580 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.80, 2.05] |
| 3.1 Low‐risk group | 4 | 489 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.66, 1.87] |
| 3.2 High‐risk group | 1 | 91 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.47 [0.77, 7.92] |
| 4 Morbidity (Sensitivity analysis) | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Good‐outcome analysis | 5 | 621 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.79, 2.03] |
| 4.2 Poor‐outcome analysis | 5 | 621 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.81, 1.80] |
| 4.3 Best‐case for LCBDE | 5 | 621 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.49, 1.16] |
| 4.4 Worst‐case for LCBDE | 5 | 621 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.30, 3.14] |
| 5 Retained stones | 5 | 580 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.45, 1.39] |
| 5.1 Low‐risk group | 4 | 489 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.55, 1.82] |
| 5.2 High‐risk group | 1 | 91 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.31] |
| 6 Retained stones (Sensitivity analysis) | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Good‐outcome analysis | 5 | 621 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.45, 1.37] |
| 6.2 Poor‐outcome analysis | 5 | 621 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.57, 1.37] |
| 6.3 Best‐case for LCBDE | 5 | 621 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.26, 0.73] |
| 6.4 Worst‐case for LCBDE | 5 | 621 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.96, 2.55] |
| 7 Failure of procedure | 5 | 580 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.16, 1.59] |
| 7.1 Low‐risk group | 4 | 489 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.46, 1.41] |
| 7.2 High‐risk group | 1 | 91 | Odds Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.37] |
| 8 Conversion to open surgery | 5 | 580 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.76, 2.81] |
| 8.1 Low‐risk group | 4 | 489 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.67, 2.75] |
| 8.2 High‐risk group | 1 | 91 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.39, 12.95] |
| 9 Duration of hospital stay | Other data | No numeric data |
Comparison 3. LC + LCBDE versus LC + intra‐operative ERCP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Morbidity | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Retained stones | 1 | 234 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.20, 2.06] |
| 3 Failure of procedure | 1 | 234 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.19, 4.01] |
| 4 Conversion to open surgery | 1 | 234 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.51, 3.11] |
| 5 Duration of procedure | 1 | 234 | Mean Difference (IV, Fixed, 95% CI) | ‐6.49 [‐21.47, 8.49] |
| 6 Duration of hospital stay | Other data | No numeric data | ||
| 7 Cost | Other data | No numeric data |
Comparison 4. LC + LCBDE versus LC + postoperative ERCP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total morbidity | 2 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.50, 2.72] |
| 1.1 LCBDE versus post‐operative ERCP | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.29, 3.41] |
| 1.2 LCBDE versus post‐operative ERCP (intra‐operative randomisation) | 1 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.41, 4.37] |
| 2 Retained stones after primary intervention | 2 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.11, 0.72] |
| 2.1 LCBDE versus post‐operative ERCP | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.17, 1.63] |
| 2.2 LCBDE versus post‐operative ERCP (intra‐operative randomisation) | 1 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.01, 0.63] |
| 3 Failure of procedure | 2 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.21, 1.06] |
| 3.1 LCBDE versus post‐operative ERCP | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.36, 2.75] |
| 3.2 LCBDE versus post‐operative ERCP (intra‐operative randomisation) | 1 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.01, 0.63] |
| 4 Conversion to open surgery | 2 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.23, 13.81] |
| 4.1 LCBDE versus post‐operative ERCP | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.12, 77.80] |
| 4.2 LCBDE versus post‐operative ERCP (intra‐operative randomisation) | 1 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.07, 18.17] |
| 5 Duration of procedure | Other data | No numeric data | ||
| 6 Duration of hospital stay | Other data | No numeric data |
Comparison 5. Single‐stage versus two‐stage management.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 7 | 746 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.12, 4.33] |
| 2 Mortality (Sensitivity analysis) | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Good‐outcome analysis | 7 | 787 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.12, 4.27] |
| 2.2 Poor‐outcome analysis | 7 | 787 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.55, 1.85] |
| 2.3 Best‐case for single stage procedure | 7 | 787 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.38] |
| 2.4 Worst‐case for single‐stage procedure | 7 | 787 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.46 [2.39, 23.27] |
| 3 Morbidity | 7 | 746 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.83, 1.89] |
| 4 Morbidity (Sensitivity analysis) | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Good‐outcome analysis | 7 | 787 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.82, 1.87] |
| 4.2 Poor‐outcome analysis | 7 | 787 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.84, 1.72] |
| 4.3 Best‐case for single‐stage procedure | 7 | 787 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.56, 1.21] |
| 4.4 Worst‐case for single‐stage procedure | 7 | 787 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.22, 2.66] |
| 5 Retained stones | 7 | 746 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.28, 1.22] |
| 6 Retained stones (Sensitivity analysis) | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Good‐outcome analysis | 7 | 787 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.37, 0.93] |
| 6.2 Poor‐outcome analysis | 7 | 787 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.47, 1.03] |
| 6.3 Best‐case for single‐stage procedure | 7 | 787 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.25, 0.62] |
| 6.4 Worst‐case for single‐stage procedure | 7 | 787 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.69, 1.56] |
| 7 Failure to complete the procedure | 7 | 746 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.20, 1.18] |
| 8 Conversion to open surgery | 7 | 746 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.80, 2.77] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bansal 2010.
| Methods | Randomised trial. LC + CBDE vs pre‐operative ERCP +LC. Drop‐outs or protocol violations reported. Sample size calculation: no. |
|
| Participants | All India Institute of Medical Sciences, India. July 2007 to April 2008. Mean age: Group I: 47.1 yrs vs Group II 39.07 yrs. Pre‐operative confirmation of CBD stones: EUS or MRCP. CBD more than 10 mm size. |
|
| Interventions | Gr I: LC + LCBDE (15 pts). Gr II: pre‐operative ERCP + LC 4 ‐ 6 weeks later (15 pts). |
|
| Outcomes | CBD clearance, bleeding, bile leak, pancreatitis, average hospital stay, pain scores (VAS). | |
| Notes | Choledochoscopy performed. T‐tube placed after lap CBD exploration. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequences. |
| Allocation concealment (selection bias) | Low risk | Concealed envelopes with block randomisation design. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible. |
| Selective reporting (reporting bias) | Low risk | All outcomes were clearly defined and reported. |
| Incomplete outcome data | High risk | Missing pts were not accounted. |
| For‐profit bias | Low risk | None. |
| Other bias | Low risk | None. |
Bornman 1992.
| Methods | Randomised trial of pre‐operative ERCP versus open cholecystectomy & cholangiogram with or without CBD exploration. Drop‐outs or protocol violations reported. Time period: not stated. Sample size calculation: no. |
|
| Participants | Cape Town. South Africa. 110 pts randomised. Pts enrolled between October 1989 and January 1992. 172 screened and 62 excluded ‐ reasons stated. Inclusion criteria: surgically fit pts with gallbladder stones and suspected CBD stones on US or biochemistry. |
|
| Interventions | Group 1: 62 pts.
Pre‐operative ERCP/ES and open surgery including subtotal cholecystectomy with or without cholangiogram and bile duct surgery where necessary. Group 2: 58 pts. Open cholecystectomy and cholangiogram with or without CBD exploration. Bile duct surgery in Group 2 also included: choledocho‐duodenostomies in 5 and transduodenal sphincteroplasty in 2. |
|
| Outcomes | Successful clearance, bile leak, postoperative death, morbidity, duration of procedure, post‐procedural hospital stay. | |
| Notes | First 90 pts. Published as abstract. Updated data received on request from author. Manuscript in provisional form with some specifics in data tables and text unclear. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Incomplete outcome data | Unclear risk | Unclear. |
| For‐profit bias | Unclear risk | Unclear. |
| Other bias | Unclear risk | Unclear. |
Cuschieri 1999.
| Methods | Multicentre randomised trial comparing pre‐operative ERC/ES and stone extraction followed by LC versus LC +/‐ laparoscopic stone extraction. Adequate report of protocol violations and drop‐outs. Sample size calculations: yes. |
|
| Participants | International trial based in Dundee. UK. Study commenced 1994, completed August 1997. 300 pts randomised. Inclusion criteria: ASA I or II. Ductal stones proven or suspected on clinical (jaundice, recent pancreatitis), biochemical (raised LFTs), or US findings. Essential investigations: LFTs, US. Optional investigations: IVC, CT. |
|
| Interventions | Group 1: 136/150 received correct treatment: Pre‐operative ERCP +/‐ ES and stone extraction when found. Subsequent laparoscopic cholecystectomy. IOC left to discretion of surgeon. Group 2: 133/150 received correct treatment: Laparoscopic cholecystectomy. IOC in all cases. Laparoscopic stone extraction attempted when stones found. |
|
| Outcomes | Mortality, morbidity, hospital stay. | |
| Notes | 10% protocol violations.
Pts in Group 1 could have had more than one pre‐operative attempt at ERCP. In Group 2 conversions to open surgery treated as successful clearance. Transcystic CBDE was performed for small non‐occluding stones and transcholedochal CBDE was performed for large or occluding stones. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was by a random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Trial was described as randomised, but the method used to conceal the allocation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible. |
| Selective reporting (reporting bias) | Low risk | All outcomes were clearly defined and reported. |
| Incomplete outcome data | Low risk | No missing data. |
| For‐profit bias | Low risk | Appears free of for‐profit support. |
| Other bias | Low risk | None. |
Hammarstrom 1995.
| Methods | Randomised clinical trial comparing ERCP/ES and stone removal versus open surgery alone for pts found to have CBDS proven on ERCP, intravenous cholangiogram, or USS, with an intact gallbladder.
Drop‐outs: 3 (all in surgery arm); 2 refused operation, 1 missing set of notes. Follow‐up: Median 92 (63 to 113) months Group 1, 82 (60 to 113) months Group 2. Sample size calculations: no. |
|
| Participants | Trial from Sweden. Commenced Sept 1984, completed Jan 1989, but with 5‐year follow‐up data. 83 pts randomised. Inclusion criteria: CBDS found either on ERC, USS or IVC, intact gallbladder, age < 85 yrs (arbitrary), informed consent. Exclusion criteria: Previous B2 anastomosis, malignancy, perforated cholecystitis, unfit for surgery. |
|
| Interventions | Group 1 (ERCP/ES):
Proceeded to ES and stone extraction by a variety of means (basket, balloon, mechanical lithotriptor). Subsequent surgery only if ongoing biliary symptoms. Group 2 (Surgery): Open cholecystectomy and ECBD on next available list. T‐tube always used. Choledochoscopy optional. |
|
| Outcomes | Successful stone clearance, additional endoscopic procedures, median hospital stay, complications ‐ bile leak, gastric retention, duodenal injury after surgery, biliary colic (no surgery), pancreatitis (no surgery), re‐operation for bleeding, bile duct injuries, late complications: incisional hernia, retained stone. | |
| Notes | Surgery arm all had ERCP to diagnose CBD stones. Unclear if surgery for late symptoms was included in complication assessment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible. |
| Selective reporting (reporting bias) | Low risk | All outcomes were clearly defined and reported. |
| Incomplete outcome data | Unclear risk | No missing data. |
| For‐profit bias | Low risk | Appears free of for‐profit support. |
| Other bias | Low risk | None. |
Hong 2006.
| Methods | Randomised clinical trial. LC + LCBDE vs LC + intra‐operative ERCP. Sample size estimation: No. Follow‐up: not mentioned. |
|
| Participants | Medical College of Zhejiang University, People's Republic of China
January 2002 to December 2003. LC+CBDE: 141 pts. LC + intra‐operative ERCP: 93 pts. Confirmation: USS/MRCP/IOC. Inclusion criteria: History, examination, USS, MRCP or cholangiogram. USS was positive in 174 pts, MRCP was positive in 3 and 57 had positive intra‐ operative cholangiogram. Exclusion criteria: none mentioned. |
|
| Interventions | Primary closure of CBD using 3'0 Vicryl in 45 cases and T‐tube placement in 96 cases. A second cholangiogram was performed to ensure an unobstructed CBD stone. |
|
| Outcomes | Success rates, surgical time, postoperative hospital stay, hospital charges, complications. | |
| Notes | ERCP ‐ small stones of 5 ‐ 8mm size were cleared by saline irrigation, larger stones by basket/balloon catheter. Sphincterotomy and lithotriptor were used only for stones larger than 15mm. Transcystic extraction for smaller stones and transcholedochal extraction for larger stones were performed. T‐tube was used in 96 cases and primary closure of CBD was performed in 46 pts. Cholangioscope was used. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised according to their identification numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible. |
| Selective reporting (reporting bias) | Low risk | All outcomes were clearly defined and reported. |
| Incomplete outcome data | Low risk | No missing data |
| For‐profit bias | Low risk | Appears free of for‐profit support. |
| Other bias | Low risk | None. |
Kapoor 1996.
| Methods | Randomised clinical trial of pts with CBD stones found at ERCP randomised to either ERCP/ES and extraction followed by open cholecystectomy (ES + S group), or open cholecystectomy and CBDE (Surgery group).
Single centre.
Drop‐outs: ES + S group ‐ 2 failures to complete treatment, 1 carcinoma of gallbladder; SA group 1 carcinoma of gallbladder. Sample size calculations: no. Exclusions: unfit (1), cholangitis (9), unable to perform ERCP (3), large stone (12), no stone (2). |
|
| Participants | Lucknow, India. Commenced July 1991 and completed October 1993. 33 pts randomised. Inclusion criteria: Pts proven to have CBD stones at ERCP, i.e, ERCP achieved. Fit for surgery. Exclusion criteria: Pregnancy, cholangitis/septicaemia, CBD cannulation failed at ERCP, stone larger than 15 mm. Essential investigations: USS, serum biochemistry. 420 pts seen with gallstones, 60 suspected of having BDS (bilirubin > 34.2 umol/l, ALP > 235 IU/l, CBD diameter > 10 mm or BDS on USS), all underwent ERCP. |
|
| Interventions | ES + S group:
CBD cleared at time of ERCP by basket or spontaneous passage.
Subsequent surgery scheduled within 6 weeks. SA group: Following ERCP, surgery undertaken on next available elective list. Choledochoscopy optional. |
|
| Outcomes | Mortality, morbidity, clearance rates, hospital stay. | |
| Notes | "good risk" pt not defined. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random assignments. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible. |
| Selective reporting (reporting bias) | Low risk | None. |
| Incomplete outcome data | Low risk | No missing data. |
| For‐profit bias | Low risk | Appears free of for‐‐profit support. |
| Other bias | Low risk | None. |
Nathanson 2005.
| Methods | Randomised clinical trial.
Randomisation of pts with CBS at laparoscopic cholecystectomy after failed transcystic clearance to laparoscopic choledochotomy or postoperative ERCP. Follow‐up: no drop‐outs reported. Time period not stated. Trial closed prior to reaching original sample size calculations due to slow accrual. |
|
| Participants | Brisbane. Australia. Commenced June 1998 and completed October 2003. 86 pts randomised. (286 pts had successful laparoscopic transcystic stone clearance from a total of 372 pts). Exclusion criteria: ERCP prior to referral for LC. CBD diameter less than 7 mm at LC or if bilioenteric drainage required at same time. |
|
| Interventions | 41 pts randomised to laparoscopic choledochotomy with or without biliary drainage with T‐tube or stent. 45 pts randomised to postoperative ERCP during same admission. |
|
| Outcomes | Mortality, morbidity, bile leak, stone clearance rates, re‐operation rate, hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised. |
| Allocation concealment (selection bias) | Low risk | Phone call to the trial centre available 24 hours a day. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible. |
| Selective reporting (reporting bias) | Low risk | None. |
| Incomplete outcome data | Low risk | No significant missing data. |
| For‐profit bias | Low risk | Appears free of for‐profit support. |
| Other bias | Low risk | None. |
Neoptolemos 1987.
| Methods | Randomised trial of pts found at ERCP, USS, or PTC to have CBDS, with intact gallbladder and fit for surgery; randomised to either ES and endoscopic extraction followed by OC or OC + CBDE. Single centre. Follow‐up: Drop‐outs: 1 pt in Group 2 who had an MI after ERCP and deemed unfit for surgery; 5 pts in Group 1 refused surgery after endoscopic CBDS extraction ‐ these latter were included in results on intention‐to‐treat analyses. Sample size calculations: yes. Based on reduction in morbidity from 40% in the surgical group (Group 2) to 20% in the endoscopic group (Group 1), requiring 79 pts in each group (a = 0.05, b = 0.2). |
|
| Participants | Leicester Royal Infirmary, UK Commenced April 1981 and completed December 1985. 120 pts entered based on the finding of CBD stones at ERCP (113), USS (6), or PTC (1). 5 early withdrawals pre‐treatment, not available for analysis. Inclusion criteria: CBDS found on ERCP, USS or PTC, intact gallbladder, fit for surgery, consent. Exclusion criteria: Pregnant. NB: Cholangitis and jaundice not exclusions. |
|
| Interventions | Group 1:
ES and clearance performed at same time as diagnostic ERCP (if performed), or else on next available list.
OC performed on next available operating list. Group 2: OC performed on next available operating list. Both ES and OC covered with prophylactic antibiotic cefazolin 1 g IV/IM unless cholangitic, in which case penicillin/gentamycin/metronidazole given. |
|
| Outcomes | Mortality, morbidity, endoscopic clearance rates, retained stones, median total hospital stay. | |
| Notes | Study treated as pilot, with termination before calculated optimal numbers recruited, based on there being no likelihood of reaching significance. Note authors claim that endoscopic clearance was 91%, but should be 87%, since 2 pts developed interval BDS. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible. |
| Selective reporting (reporting bias) | Low risk | None. |
| Incomplete outcome data | Low risk | No missing data. |
| For‐profit bias | Low risk | Appears free of for‐profit support. |
| Other bias | Low risk | None |
Noble 2009.
| Methods | ES followed by LC (Grp A) vs LCBDE during LC (Grp B). Randomised clinical trial. Sample size calculation: yes. Median length of follow‐up: 1.88 (IQR, 1.38 to 3.15) yrs. |
|
| Participants | Single centre. Southmead Hospital, Bristol, UK Higher‐risk pts: defined as being > 70 yrs age, > 60 with comorbidity, or > 50 with a BMI greater than 40. Pts with proven CBD stones on imaging or those with strong evidence of CBD stones (15 pts). Strong evidence of those with CBD stones was defined as those with a dilated CBD on transabdominal USS (5 mm in a 50‐yr old and 5+1 mm per decade) in addition to abnormal lLFTs. 2000 to 2006 Exclusion criteria: Pts with previous sphincterotomy, previous Bilroth II gastrectomy, pts unfit for general anaesthesia. |
|
| Interventions | Total of 91 pts. Group A ‐ 47 pts and Group B ‐ 44 pts. |
|
| Outcomes | Morbidity, bile duct clearance, conversion to open surgery, median postoperative stay. | |
| Notes | If stones were confirmed on cholangiogram, sphincterotomy was performed and stones retrieved using balloon, basket, mechanical lithotripsy. During post‐ERCP LC, lap USS or cholangiography was performed. If stones were present, proceeded to LCBDE. LCBDE group: Transcystic/transcholedochal approach was decided by intra‐operative USS or cholangiogram. Choledochoscope was used. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was by an independent computer‐generated random number system. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible. |
| Selective reporting (reporting bias) | Low risk | None. |
| Incomplete outcome data | Low risk | No missing data. |
| For‐profit bias | Low risk | Appears free of for‐profit support. |
| Other bias | Low risk | None. |
Rhodes 1998.
| Methods | Randomised clinical trial comparing LC and lap stone extraction versus LC and postoperative ERCP/ES and endoscopic stone extraction. No protocol violations. Pre‐study power analysis apparently conducted, but details not listed. |
|
| Participants | Norwich. UK Study commenced August 1995 and completed August 1997. 80 pts found to have stones and randomised. Inclusion criteria: LC for treatment of symptomatic gallstones with CBDS demonstrated on cholangiogram. Exclusion criteria: Pre‐operative ERCP/ES. No informed consent to proceed to randomisation. Exclusions listed: 8 pre‐operative ERCP/ES, 1 emergency OC and ECD 347 had no stones on IOC. Essential investigations: Pre‐operative USS, IOC. |
|
| Interventions | Laparoscopic group:
Trans‐cystic stone extraction if CBD < 9 mm diameter.
If failed or CBD ≥ 9 mm, stone extraction performed via choledochotomy, followed by stent or T‐tube insertion.
Postoperative ERCP required for stent removal.
Post‐operative ERCP or open conversion and duct exploration if laparoscopic extraction failed. Endoscopic group: ERCP and ES pre‐operatively. Repeated procedures until ducts clear. Followed by laparoscopic cholecystectomy. |
|
| Outcomes | Successful laparoscopic clearance, converted to open surgery, median (range) duration of all procedures, median (range) hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible. |
| Selective reporting (reporting bias) | Low risk | None. |
| Incomplete outcome data | Low risk | No missing data. |
| For‐profit bias | Low risk | Appears free of for‐profit support. |
| Other bias | Low risk | None. |
Rogers 2010.
| Methods | LC+LCBDE vs ERCP/S+LC. Randomised trial. Follow‐up: 10 protocol violators were excluded. Duration: 24 months. Pre‐study power analysis conducted, but not detailed. |
|
| Participants | University of California, San Francisco. 1997 to 2003. Inclusion criteria: Age > 18 yrs, ability to consent, classic biliary pain, USS‐cholecystolithiasis, platelet count/prothrombin time ‐ normal, ASA grade 1 or 2, 'Likely' choledocholithiasis suggested by one of the following: CBD ≥ 6 mm by USS or CT scan, intrahepatic duct dilation as determined by USS or CT scan, serum bilirubin ≥ 2 mg/dl, ALP and/or lipase levels ≥ 1.5 times upper limit of normal within 48 hrs of intended procedure. Exclusion criteria: History of bleeding disorders, uraemia, USS/CT evidence of cirrhosis, intrahepatic gallbladder, liver mass or abscess or periampullary neoplasm, clinical or sonographic evidence of suppurative or necrotising cholecystitis, gallbladder empyema or perforation, IDDM, multiple prior laparotomies/morbid obesity/portal vein thrombosis, pregnancy. |
|
| Interventions | 122 pts. LC+LCBDE: 61 pts = 57 pts ERCP/S+LC: 61 pts = 55 pts 10 exclusions. |
|
| Outcomes | CBD stones cleared, complications, procedure time. | |
| Notes | Sphincterotomy was performed after confirming the presence of stones. Transcystic exploration was performed for LCBDE. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised according to serially numbered, sealed, opaque envelopes. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible. |
| Selective reporting (reporting bias) | Low risk | None. |
| Incomplete outcome data | Low risk | No missing data. |
| For‐profit bias | Low risk | Appears free of for‐profit support. |
| Other bias | Low risk | None. |
Sgourakis 2002.
| Methods | Randomised clinical trial comparing laparoscopic surgery vs pre‐operative ERCP and surgery. Single centre. Study commenced April 1997, completed August 2000. Follow‐up: drop‐outs not reported. Median postoperative 22.36 months (7 to 36). Sample size calculations: no. |
|
| Participants | Athens, Greece. 78 pts randomised (36 Group A, 42 Group B) with high risk for CDS on USS and/or biochemical criteria. ASA I or II pts. 8 pts excluded because of 'poor performance' status. Further 6 refused consent. |
|
| Interventions | Laparoscopic group ‐ transcystic and choledochotomy approaches described in detail. In ERCP group ‐ surgery performed usually within 2 days. | |
| Outcomes | Primary clearance success, morbidity, mortality, median hospital stay. | |
| Notes | 8/36 of surgical arm (Group A) and 10/42 endoscopy arm (Group B) had no CBDS found at the time of the procedure. No explanation given for choice of transcystic or direct CBD approaches in Group A. 1 pt in each group with CBDS in situ at end of trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods of randomisation were ambiguous. The guidelines of a randomised trial with the probability of samples method and stratified sampling were applied. A preliminary retrospective study was conducted. Authors also mentioned: "Patients were assigned in two groups (LCBDE vs ERCP). All patients had an informed consent for their randomisation. We had to take into account and the preference of the surgeon responsible for their treatment." The author is not contactable at the provided email address. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible. |
| Selective reporting (reporting bias) | Low risk | Unclear. |
| Incomplete outcome data | Low risk | Unclear. |
| For‐profit bias | Low risk | Unclear. |
| Other bias | Low risk | Unclear. |
Stain 1991.
| Methods | Randomised clinical trial of pts found to have CBDS on ERCP and fit to undergo surgery, randomised to either ERCP/ES + surgery or surgery "alone" . Single centre. All pts suspected of having CBDS (bilirubin > 2 mg/dl, raised amylase, USS evidence of CBDS) underwent ERCP. Follow‐up: exclusions not listed. Drop‐outs not listed. Sample size calculations: no. No power analysis stated. |
|
| Participants | Los Angeles. USA
52 pts completed the study. Commencement and completion dates not stated. Inclusion criteria: Intact gallbladder, gallstones on USS, CBDS proven on ERC. Exclusion criteria: None stated. Essential investigations: USS, serum biochemistry, ERC, IOC, T‐tube cholangiogram in all having CBDE. |
|
| Interventions | Group 1:
ERCP followed by ES and stone extraction by basket or spontaneous passage. Subsequent OC +/‐ CBDE in all cases. Surgery scheduled electively.
CBDE performed on basis of ERCP findings and IOC. Group 2: ERCP followed by OC scheduled electively. CBDE performed as necessary. |
|
| Outcomes | Mortality, morbidity, stone clearance rate, retained stones after surgery, operation time, hospital stay. | |
| Notes | Pts in both groups had ERC, therefore surgery arm had complications of ERC. Low ERCP/ES stone clearance rate (65%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible. |
| Selective reporting (reporting bias) | Low risk | None. |
| Incomplete outcome data | Low risk | No missing data. |
| For‐profit bias | Low risk | Appears free of for‐profit support. |
| Other bias | Low risk | None. |
Stiegmann 1992.
| Methods | Randomised clinical trial.
Open cholecystectomy, IOC +/‐ bile duct exploration vs pre‐operative ERCP/ES followed by OC. Single centre. Commenced June 1986, completed March 1990. Exclusions not listed. No protocol violations listed. Sample size calculations: no. |
|
| Participants | Denver. USA. 34 pts randomised. Inclusion criteria: Pts for elective cholecystectomy, with suspected CBD stones. Suspicion of CBDS based on having at least one of: serum bilirubin > 2 mg/dl (twice upper normal), serum ALP > 235 U/l (twice upper normal), serum amylase > 240 U/l (twice upper normal), ultrasound measured CD diameter > 8 mm, or USS visualisation of CBDS. Exclusion criteria: Asc cholangitis, op for acute cholecystitis, liver disease, bleeding disorders, previous gastric surgery precluding ERCP, previous biliary tract surgery. Exclusions listed: 1 pt with cholangiocarcinoma found at ERCP and one with cirrhosis found at surgery excluded from further analysis. |
|
| Interventions | Endoscopic/Operative group:
ERCP/ES followed by OC plus IOC (usually the following day). Operative only group: OC + IOC +/‐ CBDE Essential investigations: Serum bilirubin, ALP, amylase, USS. IOC in all pts. Choledochoscopy in some of the surgical group. ERCP/ES in the endoscopic group. T‐tube cholangiography in the surgical group at 10 days postoperatively. Costing retrieved from hospital finance office. |
|
| Outcomes | Mortality, morbidity, stone clearance rates, hospital stay, procedure time, cost. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised. |
| Allocation concealment (selection bias) | Unclear risk | Not clear. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible. |
| Selective reporting (reporting bias) | Low risk | None. |
| Incomplete outcome data | Low risk | No missing data. |
| For‐profit bias | Low risk | Appears free of for‐profit support. |
| Other bias | Low risk | None. |
Suc 1998.
| Methods | Randomised clinical trial.
Open cholecystectomy +/‐ ECD versus ERCP/ES. Cholecystectomy not necessarily performed in ERCP group. Multicentre. Exclusions ‐ none listed. Protocol violations ‐ 9, all withdrawn. Other withdrawals ‐ 9, due to incorrect diagnosis (malignant biliary obstruction). Sample size calculations: yes. Power analysis based on primary outcome variable of additional procedures required, with 95 pts per group required with a power of 90% at the 0.05 level. |
|
| Participants | France. Commenced September 1989 and completed September 1994. 220 consecutive adult pts randomised. Inclusion criteria: Adult (> 18 yrs). One of: jaundice, mild AP, mild cholangitis, biliary colic + raised ALP, CDS or dilated CD on USS. Exclusion criteria: Cholecystitis (thick gallbladder wall on USS). No stones on USS and CBD < 1 cm. Pts unable to have ERCP (previous total or B2 gastrectomy, or choledochoenterostomy). |
|
| Interventions | All cholecystectomies by surgeons, all ERCPs by gastroenterologists. Surgical group: OC (if not performed previously ‐ 3 pts). CBDE +/‐ choledochoscopy. Duct closure either primary, +/‐ T‐tube, or choledochoenterostomy. Endoscopic group: ERCP and cholangiogram. Basket extraction of stones OC subsequently only if cholecystitis or cholangitis developed. |
|
| Outcomes | Retained stones, additional procedures, mortality, morbidity, total duration of hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. |
| Allocation concealment (selection bias) | Low risk | Telephone call to co‐ordinating centre. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible. |
| Selective reporting (reporting bias) | Low risk | None. |
| Incomplete outcome data | Low risk | No missing data. |
| For‐profit bias | Low risk | Appears free of for‐profit support. |
| Other bias | Low risk | None. |
Targarona 1996.
| Methods | Randomised clinical trial of high‐risk surgical pts suspected of having CBDS to either OC +/‐ CBDE alone or ERCP/ES and stone extraction alone. Single centre. 9 excluded pre‐randomisation due to no consent (5), acute cholecystitis (1), severe cholangitis requiring urgent surgery (2), severe pancreatitis requiring urgent ERCP/ES (1). Sample size calculations: yes. Power analysis based on reduced mortality of 14% with endoscopic treatment, requiring 48 pts per group (a = 0.05, b = 0.1). If BDS diagnosed at time of ERCP, randomisation occurred during this procedure prior to ES. |
|
| Participants | Barcelona, Spain. Commenced September 1991 and completed September 1994. 109 pts eligible: 9 exclusions pre‐randomisation, 100 pts randomised, 2 early withdrawals post‐randomisation. 98 pts with analysable data. Inclusion criteria: Pts with any combination of: biliary colic and jaundice, pancreatitis, and cholangitis, suspected of having BDS based on having: cholestasis on LFTs, dilated BD > 8 mm on USS, CBDS on USS or ERCP, + deemed 'high surgical risk' on the basis of at least 1 of: age > 70 yrs, Goldman cardiac risk index > 13, COPD with PPO‐MSV < 10 l/min, Child‐Pugh class B or C, severely impaired mobility (neurological or locomotor), BMI > 30, informed consent, intact gallbladder. Exclusion criteria: previous ES, previous cholecystectomy. |
|
| Interventions | If the allocated therapy could not be performed within 30 days post‐randomisation, it was classed as a primary failure of that therapy. Group 1: (surgery) OC performed post‐randomisation. IOC performed in all and CBDE as required. Group 2: (endoscopy) ERCP performed post‐randomisation. ES performed regardless of presence of stones on cholangiogram. |
|
| Outcomes | Primary duct clearance rate, total morbidity, mortality, total hospital stay, recurrent biliary symptoms, readmissions due to recurrent symptoms. | |
| Notes | ES performed in Group 2 even if no stones present ‐ this does not reflect normal practice and may increase risk in this group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Closed envelopes with group distribution. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible. |
| Selective reporting (reporting bias) | Low risk | None. |
| Incomplete outcome data | Unclear risk | Incomplete outcome data (hospital stay). |
| For‐profit bias | Low risk | Appears free of for‐profit support. |
| Other bias | Low risk | None. |
AP = acute pancreatitis AC = acute cholangitis ALP = alkaline phosphatase BMI = body mass index CBDE = exploration of common duct CBD = common bile duct CBDS = common bile duct stones CT = computed tomography ERCP = endoscopic retrograde cholangiopancreatogram ES = endoscopic sphincterotomy EUS = endoscopic ultrasonography GRP = group IDDM = insulin‐dependent diabetes mellitusIQR ‐ interquartile range LC = laparoscopic cholecystectomy LFT = liver function test MRCP = magnetic resonance cholangiopancreatography OC = open cholecystectomy MI = myocardial infarction PTC = percutaneous transhepatic cholangiography pts = participants US = ultrasound USS = ultrasound scan (trans‐abdominal unless otherwise stated) VAS = visual analogue scale yrs = years
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Airan 1992 | Retrospective and prospective case series of LC +/‐ laparoscopic exploration of common bile duct. |
| Ammori 2000 | Prospective study comparing pre‐operative ERCP/ES + LC compared to LC + laparoscopic exploration of common bile duct . Not randomised. |
| Andreasen 1998 | Retrospective series of peri‐operative ERCP + LC. |
| Berci 1994 | Case series of laparoscopic exploration of common duct. |
| Bergamaschi 1999 | Case series of pre‐operative ERCP/ES + LC. |
| Boeckl 1988 | Study comparing pre‐operative ERCP/ES + OC versus OC +/‐ (open) exploration of common duct (with a view to stone extraction). |
| Boerma 2002 | A randomised trial comparing wait‐and‐see or laparoscopic cholecystectomy after endoscopic sphincterotomy for bile‐duct stones. |
| Bonatsos 1996 | Case series of pre‐operative ERCP/ES + LC. |
| Budzynski 1997 | Case series of pre‐operative ERCP/ES + LC. |
| Cemachovic 2000 | Retrospective case series of LC + intra‐operative ERCP/ES. |
| Chan 1996 | Prospective case series of pre‐operative ERCP/ES + LC. |
| Chang 2000 | Randomised clinical trial comparing pre‐operative versus postoperative ERCP and LC in mild to moderate gallstone pancreatitis. 60 randomised pts with gallstone pancreatitis and suspicion of common duct calculi on US and biochemistry. ERCP required in only 24% of postoperative group based on IOC. Treatment failure 10% in both groups, but higher costs and longer hospital stay in pre‐operative group. Excluded as the pre ‐ and postoperative ERP were the comparison groups. |
| Cisek 1994 | Case series of pre‐operative ERCP/ES + LC. |
| Conigliaro 1995 | Non‐randomised study for CBD stone treatment into 4 groups: (1) ES and LC, (2) Pre‐op ERCP and LC, (3) Open surgery, (4) Laparoscopic bile duct exploration. |
| Coppola 1996 | Case series of pre‐operative ERCP/ES + LC. |
| Daradkeh 2000 | Prospective case series of pre‐operative ERCP/ES + LC. |
| Davis 1997 | Case series of peri‐operative ERCP/ES + LC. |
| Decker 2003 | Cohort of 100 laparoscopic choledochotomies with primary closure of the bile duct. |
| Dias 2002 | Survey of surgeons in NSW Australia regarding management of CBD stones. |
| Drouard 1995 | Case series of laparoscopic exploration of common duct (with a view to stone extraction). |
| Drouard 1997 | Case series of laparoscopic exploration of common duct (with a view to stone extraction). |
| Ebner 2004 | Cohort of 200 pts undergoing laparoscopic management of bile duct stones (115 transcystic, 85 choledochotomy). 91% clearance. 7% 'complication rate'. 0.5% mortality. |
| El Geidie 2011 | Compares the timing of ERCP in relation to LC. |
| Fanning 1997 | Retrospective study comparing pre‐operative ERCP/ES + LC to LC +/‐ laparoscopic exploration of common duct (with a view to stone extraction). |
| Frazee 1993 | Case series of pre‐operative ERCP/ES + LC. |
| Galloway 1994 | Prospective non‐randomised study of combined laparoscopic and endoscopic treatment of gallstones and bile duct stones: |
| Giurgiu 1999 | Prospective case series of laparoscopic exploration of common duct (with a view to stone extraction). |
| Gonzalez 1989 | Prospective study comparing pre‐operative ERCP/ES + open cholecystectomy versus open cholecystectomy + (open) exploration of common duct (with a view to stone extraction). |
| Hamy 2003 | Case series of pre‐operative ERCP prior to laparoscopic cholecystectomy. |
| Heili 1999 | Retrospective study comparing pre‐operative ERCP/ES + LC to LC +/‐ laparoscopic exploration of common duct (with a view to stone extraction). |
| Heinerman 1989 | Non‐randomised comparison between surgical and endoscopic common bile duct stone extraction. |
| Hoyuela 1999 | Case series of pre‐operative ERCP/ES + LC. |
| Hui 2002 | A randomised study of ERCP vs no ERCP in acute acalculous cholangitis. |
| Huynh 1996 | Case series of pre‐operative ERCP/ES + LC. |
| Kapoor 1994 | Non‐randomised comparison between open cholecystectomy +/‐ open exploration of common duct (with a view to stone extraction) and ERCP/ES +/‐ open cholecystectomy. |
| Khuroo 1989 | Randomisation to biliary drainage (ie, not stone removal) and surgery. |
| Kullman 1996 | Prospective case series of pre‐operative and postoperative ERCP/ES + LC. Not a RCT. |
| Lai 1992 | Randomisation to biliary drainage (ie, not stone removal) and surgery. |
| Lella 2006 | Compares the timing of ERCP in relation to LC. |
| Lezoche 1996 | Prospective case series of LC +/‐ laparoscopic exploration of common duct (with a view to stone extraction). Not a RCT. |
| Lezoche 2000 | Case series of laparoscopic exploration of common duct (with a view to stone extraction). Not a RCT. |
| Liberman 1996 | Retrospective study comparing LC +/‐ postoperative ERCP/ES to LC +/‐ laparoscopic exploration of common duct (with a view to stone extraction). |
| Liu 1996 | Prospective case series of pre‐operative ERCP/ES + LC. Not a RCT. |
| Magnanini 1994 | Case series of pre‐operative ERCP/ES + LC. |
| Martin 1998 | Case series of 300 LC + laparoscopic exploration of common duct (with a view to stone extraction). |
| Martin 2002 | Cohort series of 56 pts undergoing attempted laparoscopic transcystic bile duct stenting in the management of common bile duct stones. |
| Masci 1999 | Case series of pre‐operative ERCP/ES + LC. |
| Materia 1996 | Case series of pre‐operative ERCP/ES + LC. |
| Meyer 1999 | Study comparing peri‐operative ERCP/ES + LC to LC +/‐ laparoscopic exploration of common duct (with a view to stone extraction) to open cholecystectomy +/‐ open exploration of common duct (with a view to stone extraction). |
| Meyer 2002 | Cohort of common bile duct stone management in a single operation combining laparoscopic cholecystectomy and pre‐operative endoscopic sphincterotomy. |
| Michel 2000 | Retrospective case series of laparoscopic exploration of common duct (with a view to stone extraction). |
| Mijal 1997 | Study comparing pre‐operative ERCP/ES + LC to open cholecystectomy + open exploration of common duct (with a view to stone extraction). Not a RCT. |
| Millat 1995 | Prospective case series of laparoscopic exploration of common duct (with a view to stone extraction). Not a RCT. |
| Millat 1996 | Prospective case series of laparoscopic exploration of common duct (with a view to stone extraction). Not a RCT. |
| Millat 1997 | Case series of laparoscopic exploration of common duct (with a view to stone extraction). |
| Miller 1988 | Retrospective study comparing ERCP/ES to open cholecystectomy +/‐ open exploration of common duct (with a view to stone extraction). |
| Mo 2002 | Study of pre‐operative endoscopic sphincterotomy in the treatment of pts with cholecystocholedocholithiasis. |
| Moreaux 1995 | Prospective case series of open cholecystectomy +/‐ open exploration of common duct (with a view to stone extraction). Not a RCT. |
| Morino 2006 | Compares the timing of ERCP in relation to LC. |
| Neoptolemos 89 | Retrospective and prospective study comparing open cholecystectomy +/‐ open exploration of common duct (with a view to stone extraction) to ERCP/ES +/‐ open cholecystectomy. |
| Neuhaus 1992 | Prospective case series of pre‐operative ERCP + LC. Not a RCT. |
| Niu 1995 | Case series of laparoscopic exploration of common duct (with a view to stone extraction). |
| Paganini 1998 | Prospective case series of LC +/‐ laparoscopic exploration of common duct (with a view to stone extraction). Not a RCT. |
| Palacios‐Macedo 1995 | Prospective case series of pre‐operative ERCP/ES + LC. Not a RCT. |
| Pedersen 1998 | Retrospective case series of pre‐operative ERCP/ES. |
| Pereira‐Lima 2001 | Cohort series of ERCP CDS clearence in the era of laparoscopic cholecystectomy: prospective analysis of 386 pts. |
| Perniceni 2001 | Case series of laparoscopic exploration of common duct (with a view to stone extraction). |
| Phillips 1995 | Retrospective case series of LC +/‐ laparoscopic exploration of common duct (with a view to stone extraction). |
| Quershi 1993 | Case series of pre‐ and postoperative ERCP/ES. |
| Rabago 2006 | Compares the timing of ERCP in relation to LC. |
| Rhodes 1995 | Retrospective case series of laparoscopic exploration of common duct (with a view to stone extraction). |
| Rieger 1994 | Case series of pre‐operative ERCP/ES + LC. |
| Rieger 1995 | Prospective case series of pre‐operative ERCP/ES + LC. Not a RCT. |
| Rijna 2000 | Case series of pre‐operative ERCP/ES + LC. |
| Robertson 1996 | Case series of ERCP/ES followed by LC. |
| Robinson 1995 | Case series of LC +/‐ laparoscopic exploration of common duct (with a view to stone extraction). |
| Roush 1995 | Retrospective case series of laparoscopic exploration of common duct (with a view to stone extraction). |
| Santucci 1996 | Prospective case series of pre‐operative ERCP/ES + LC. Not a RCT. |
| Sarli 1999 | Prospective case series of pre‐operative ERCP/ES + LC. Not a RCT. |
| Schwab 1992 | Prospective study comparing OC+/‐ ECD to ERCP/ES. Not a RCT. |
| Seo 2000 | Prospective case series of ERCP/ES. Not an RCT. |
| Stoker 1995 | Prospective case series of LC +/‐ laparoscopic exploration of common duct (with a view to stone extraction). Not a RCT. |
| Sugiyama 1999 | Prospective case series of laparoscopic exploration of common duct (with a view to stone extraction). |
| Sungler 1993 | Prospective case series of pre‐operative ERCP/ES + LC. |
| Sungler 1997 | Prospective case series of pre‐operative ERCP/ES + LC. |
| Tham 1998 | Case series of pre‐operative ERCP/ES + LC. |
| Trias 1997 | Prospective case series of pre‐operative ERCP/ES + LC. |
| Trondsen 1995 | Retrospective case series of pre‐operative ERCP/ES + open cholecystectomy. |
| Turcu 1997 | Prospective study comparing pre‐operative ERCP/ES + open cholecystectomy to open cholecystectomy + open exploration of common duct (with a view to stone extraction). Not a RCT. |
| Waage 2003 | Cohort series of 175 attempted laparoscopic bile duct exploration (110 transcystic, 52 lap choledochotomy and 13 open conversion). Morbidity 6.9%. Mortality 0%. Median follow‐up 36 months with 1 recurrence of CBS and no strictures. |
| Welbourn 1995 | Retrospective case series of pre‐operative ERCP/ES + LC. |
| Wenner 2005 | Cohort series of 23 pts undergoing laparoscopic bile duct exploration using a Multichannel Instrument Guide. 95% stone clearance rate. |
| Widdison 1994 | Prospective case series of pre‐operative ERCP/ES + LC. Not a RCT. |
| Wilson 1993 | Case series of peri‐operative ERCP/ES + LC. |
| Worthley 1989 | Prospective study comparing pre‐operative ERCP/ES + open cholecystectomy to open cholecystectomy + open exploration of common duct (with a view to stone extraction). Not a RCT. |
| Zargar 2002 | Case series of ERCP in the treatment of common bile duct stones. |
ERCP/ES = Endoscopic retrograde cholangio pancreatography with endoscopic sphincterotomy (usually with a view to endoscopic stone extraction) LC = Laparoscopic cholecystectomy Pt = participant RCT = Randomised clinical trial
Differences between protocol and review
The following outcomes were redefined in the updated review:
Retained stones: Inability to clear the ductal stones with planned technique (endoscopy or surgery) by the end of that procedure.
Failure to complete the planned procedure: Inability to perform the planned procedure and clear the duct by the end of the intervention (endoscopic or surgery). This can be due to technical reasons such as failed cannulation or difficult Calot's dissection, or due to impacted stone.There is a subtle but significant difference between these two comparisons. Retained stones indicate failure to clear the duct due to technical difficulty in getting the stone out, while failure to complete the planned procedure involves difficulty in getting to the stone. Inability to get the stone removed from the duct either due to less expertise or lack of gadgets (inability to get the guidewire into the CBD at ERCP or dissect the Calots is different from inability to remove the stone ‐ in the former, one has not explored the CBD).
Conversion to open surgery: Participants requiring conversion of laparoscopic surgery to open surgery.
The following outcomes were not included in the review:
Numbers and size of stones cleared by the procedures is not included in the update. This is because the trials have assessed the outcomes (failure rates, complication rates) that could have been influenced by the size of the stones. However, none of the trials have reported the outcomes based on the stone configuration. We therefore have not included the stone configuration in the review.
Number of procedures per participant was not analysed in the update of the review. The number of procedures appears to have been influenced by the number of additional procedures required for duct clearance. We have therefore included rates of retained stones and additional procedures required for duct clearance.
Contributions of authors
Conceiving the review: DJM (Martin J David). Designing the review: BD (Bobby VM Dasari). Co‐ordinating the review: BD. Data collection for the review: BD; CJT (Tan, Chuan Jin). Designing search strategies: BD. Screening search results: BD; CJT. Organising retrieval of papers: BD; CJT. Screening retrieved papers against inclusion criteria: BD; CJT. Appraising quality of papers: BD. Extracting data from papers: BD; CJT. Data management for the review: BD; CJT. Entering data into Review Manager 5: BD. Analysis of data: BD; KG (Kurinchi Selvan Gurusamy). Interpretation of data: BD; CJT; KG. Providing a methodological perspective: BD; KG. Providing a clinical perspective: BD; DM; LMcK (Lloyd McKie); GK (Gareth Kirk); MT (Mark A Taylor); TD (Tom Diamond). Writing the review: BD; GK; LMcK; MT; TD; DM.
Sources of support
Internal sources
No sources of support supplied
External sources
Copenhagen Hospital Corporation Research Grant on Getting Research into Practice (GRIP), Denmark.
-
National Institute of Health Research, UK.
NIHR provided financial support to update this review
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Bansal 2010 {published data only}
- Bansal VK, Misra MC, Garg P, Prabhu M. A prospective randomized trial comparing two‐stage versus single‐stage management of patients with gallstone disease and common bile duct stones. Surgical Endoscopy 2010;24:1986‐9. [DOI] [PubMed] [Google Scholar]
Bornman 1992 {unpublished data only}
- Bornman PC, Funnell IC, Wyk MEC, Krige JEJ, Graham S. Does ERCP before planned cholecystectomy benefit patients with suspected bile duct stones? A randomised trial [abstract]. South African Medical Journal 1991;81:41. [Google Scholar]
Cuschieri 1999 {published data only}
- Cuschieri A, Lezoche E, Mornino M, Croce E, Lacy A, Tooulo J, et al. E.A.E.S. multicenter prospective randomized trial comparing two‐stage vs single‐stage management of patients with gallstone disease and ductal calculi. Surgical Endoscopy 1999;13(10):952‐7. [DOI] [PubMed] [Google Scholar]
Hammarstrom 1995 {published data only}
- Hammarstrom LE, Holmin T, Stridbeck H, Ihse I. Long‐term follow‐up of a prospective randomized study of endoscopic versus surgical treatment of bile duct calculi in patients with gallbladder in situ. British Journal of Surgery 1995;82(11):1516‐21. [DOI] [PubMed] [Google Scholar]
Hong 2006 {published data only}
- Hong DF, Xin Y, Chen DW. Comparision of laparoscopic cholecystectomy combined with intraoperative endoscopic sphincterotomy and laparoscopic exploration of the common bile duct for cholecystocholedocholithiasis. Surgical Endoscopy 2006;20:424‐7. [DOI] [PubMed] [Google Scholar]
- Xin Y, Hong D, Cai X, Mou Y, Li L, Wang G, et al. Comparision of laparoscopic cholecystectomy combined with intraoperative endoscopic sphincterotomy and combined with laparoscopic common bile duct exploration in treatment of cholithiasis and calculus of common bile duct [Chinese]. National Medical Journal of China 2007;87(38):2703‐5. [PubMed] [Google Scholar]
Kapoor 1996 {published data only}
- Kapoor R, Kaushik SP, Saraswat VA, Choudhuri G, Sikora SS, Saxena R, et al. Prospective randomized trial comparing endoscopic sphincterotomy followed by surgery with surgery alone in good risk patients with choledocholithiasis. HPB Surgery 1996;9(3):145‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nathanson 2005 {published data only}
- Nathanson LK, O'Rourke NA, Martin IJ, Fielding GA, Cowen AE, Roberts RK, et al. Postoperative ERCP versus laparoscopic choledochotomy for clearance of selected bile duct calculi: a randomized trial. Annals of Surgery 2005;242(2):188‐92. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neoptolemos 1987 {published data only}
- Cotton PB. Endoscopic sphincterotomy before cholecystectomy?. HPB Surgery 1989;1(3):244‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neoptolemos JP, Carr‐Locke DL, Fossard DP. Prospective randomised study of preoperative endoscopic sphincterotomy versus surgery alone for common bile duct stones. BMJ (Clinical Research Ed) 1987;294(6570):470‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Noble 2009 {published data only}
- Noble H, Tranter S, Chesworth T, Norton S, Thompson MD. A randomized, clinical trial to compare endoscopic sphincterotomy and subsequent laparoscopic cholecystectomy with primary laparoscopic bile duct exploration during cholecystectomy in higher risk patients with choledocholithiasis. Journal of Laparoendoscopic and Advanced Surgical Techniques 2009;19(6):713‐20. [DOI] [PubMed] [Google Scholar]
Rhodes 1998 {published data only}
- Rhodes M, Sussman L, Cohen L, Lewis MP. Randomised trial of laparoscopic exploration of common bile duct versus postoperative endoscopic retrograde cholangiography for common bile duct stones. Lancet 1998;351(9097):159‐61. [DOI] [PubMed] [Google Scholar]
Rogers 2010 {published data only}
- Rogers S, Cello JP, Horn JK, Siperstein A, Campbell A, Mackersie R, et al. Randomized controlled clinical trial of laparascopic cholecystectomy plus laparoscopic common bile duct exploration (LC+LCBDE) vs ERCP sphincterotomy plus laparascopic cholecystectomy (ERCP/S+LC) for common bile duct stone disease [abstract]. Journal of Gastroenterology and Hepatology 1999;14(Suppl):S110. [Google Scholar]
- Rogers SJ, Cello JP, Horn JK, Siperstein AE, Schecter WP, Campbell AR, et al. Prospective randomised trial of LC+LCBDE vs ERCP/S+LC for common bile duct stone disease. Archives of Surgery 2010;145(1):28‐33. [DOI] [PubMed] [Google Scholar]
Sgourakis 2002 {published data only}
- Sgourakis G, Karaliotas K. Laparoscopic common bile duct exploration and cholecystectomy versus endoscopic stone extraction and laparoscopic cholecystectomy for choledocholithiasis. A prospective randomized study. Minerva Chirurgica 2002;57(4):467‐74. [PubMed] [Google Scholar]
Stain 1991 {published data only}
- Stain SC, Cohen H, Tsuishoysha M, Donovan AJ. Choledocholithiasis. Endoscopic sphincterotomy or common bile duct exploration. Annals of Surgery 1991;213(6):627‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stiegmann 1992 {published data only}
- Stiegmann GV, Goff JS, Mansour A, Pearlman N, Reveille RM, Norton L. Precholecystectomy endoscopic cholangiography and stone removal is not superior to cholecystectomy, cholangiography, and common duct exploration. American Journal of Surgery 1992;163(2):227‐30. [DOI] [PubMed] [Google Scholar]
Suc 1998 {published data only}
- Suc B, Escat J, Cherqui D, Fourtanier G, Hay JM, Fingerhut A, et al. Surgery vs endoscopy as primary treatment in symptomatic patients with suspected common bile duct stones: a multicenter randomized trial. French Associations for Surgical Research. Archives of Surgery 1998;133(7):702‐8. [DOI] [PubMed] [Google Scholar]
Targarona 1996 {published data only}
- Targarona EM, Perez Ayuso RM, Bordas JM, Ros E, Pros I, Martinez J, et al. Randomised trial of endoscopic sphincterotomy with gall bladder left in situ versus open surgery for common bile duct calculi in high risk patients. Lancet 1996;347:926‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Airan 1992 {published data only}
- Airan M, Appel M, Berci G, Coburg AJ, Cohen M, Cuschieri A, et al. Retrospective and prospective multi‐institutional laparoscopic cholecystectomy study organized by the Society of American Gastrointestinal Endoscopic Surgeons. Surgical Endoscopy 1992;6(4):169‐78. [DOI] [PubMed] [Google Scholar]
Ammori 2000 {published data only}
- Ammori BJ, Birbas K, Davides D, Vezakis A, Larvin M, McMahon MJ. Routine vs "on demand" postoperative ERCP for small bile duct calculi detected at intraoperative cholangiography. Clinical evaluation and cost analysis. Surgical Endoscopy 2000;14(12):1123‐6. [DOI] [PubMed] [Google Scholar]
Andreasen 1998 {published data only}
- Andreasen DA, Larsen JF. ERCP and laparoscopic cholecystectomy [ERCP og laparoskopisk kolecystektomi]. Ugeskrift for Laeger 1998;160(32):4626‐9. [PubMed] [Google Scholar]
Berci 1994 {published data only}
- Berci G, Morgenstern L. Laparoscopic management of common bile duct stones. A multi‐institutional SAGES study. Society of American Gastrointestinal Endoscopic Surgeons. Surgical Endoscopy 1994;8(10):1168‐75. [DOI] [PubMed] [Google Scholar]
Bergamaschi 1999 {published data only}
- Bergamaschi R, Tuech JJ, Braconier L, Walsoe HK, Marvik R, Boyet J, et al. Selective endoscopic retrograde cholangiography prior to laparoscopic cholecystectomy for gallstones. American Journal of Surgery 1999;178(1):46‐9. [DOI] [PubMed] [Google Scholar]
Boeckl 1988 {published data only}
- Boeckl O, Heinerman M, Pimpl W. Influence of preoperative selective endoscopy on the results and treatment concept of bile duct surgery [Einfluss der praoperativen selektiven Endoskopie auf Resultate und Therapiekonzept der Gallengangschirurgie]. Deutsche Medizinische Wochenschrift 1988;113(50):1950‐5. [DOI] [PubMed] [Google Scholar]
Boerma 2002 {published data only}
- Boerma D, Rauws EA, Keulemans YC, Janssen IM, Bolwerk CJ, Timmer R, et al. Wait‐and‐see policy or laparoscopic cholecystectomy after endoscopic sphincterotomy for bile‐duct stones: a randomised trial. Lancet 2002;360:761‐5. [DOI] [PubMed] [Google Scholar]
Bonatsos 1996 {published data only}
- Bonatsos G, Leandros E, Polydorou A, Romanos A, Dourakis N, Birbas C, et al. ERCP in association with laparoscopic cholecystectomy. A strategy to minimize the number of unnecessary ERCPs. Surgical Endoscopy 1996;10(1):37‐40. [DOI] [PubMed] [Google Scholar]
Budzynski 1997 {published data only}
- Budzynski A, Bobrzynski A, Rembiasz K, Biesiada Z, Dutkiewicz W. The problem of common bile duct calculi in the era of laparoscopic cholecystectomy [Problem kamicy przewodowej w dobie cholecystektomii laparoskopowej]. Wiadomosci Lekarskie 1997;50(Suppl 1 Pt 1):235‐8. [PubMed] [Google Scholar]
Cemachovic 2000 {published data only}
- Cemachovic I, Letard JC, Begin GF, Rousseau D, Nivet JM. Intraoperative endoscopic sphincterotomy is a reasonable option for complete single‐stage minimally invasive biliary stones treatment: short‐term experience with 57 patients. Endoscopy 2000;32(12):956‐62. [DOI] [PubMed] [Google Scholar]
Chan 1996 {published data only}
- Chan AC, Chung SC, Wyman A, Kwong KH, Ng EK, Lau JY, et al. Selective use of preoperative endoscopic retrograde cholangiopancreatography in laparoscopic cholecystectomy. Gastrointestinal Endoscopy 1996;43(3):212‐5. [DOI] [PubMed] [Google Scholar]
Chang 2000 {published data only}
- Chang L, Lo S, Stabile BE, Lewis RJ, Toosie K, Virgilio C. Preoperative versus postoperative endoscopic retrograde cholangiopancreatography in mild to moderate gallstone pancreatitis: a prospective randomized trial. Annals of Surgery 2000;231(1):82‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cisek 1994 {published data only}
- Cisek PL, Greaney GC. The role of endoscopic retrograde cholangiopancreatography with laparoscopic cholecystectomy in the management of choledocholithiasis. American Surgeon 1994;60(10):772‐6. [PubMed] [Google Scholar]
Conigliaro 1995 {published data only}
- Conigliaro R, Ricci E, Sassatelli R, Pacchione D, Bertoni G, Mortilla MG, et al. Common bile duct stones and cholecystectomy: a prospective multicentric study comparing different treatments. Giornale Italiano di Endoscopia Digestiva 1995;18(4):237‐45. [Google Scholar]
Coppola 1996 {published data only}
- Coppola R, D'Ugo D, Ciletti S, Riccioni ME, Cosentino L, Magistrelli P, et al. ERCP in the era of laparoscopic biliary surgery. Experience with 407 patients. Surgical Endoscopy 1996;10(4):403‐6. [DOI] [PubMed] [Google Scholar]
Daradkeh 2000 {published data only}
- Daradkeh S, Shennak M, Abu‐Khalaf M. Selective use of perioperative ERCP in patients undergoing laparoscopic cholecystectomy. Hepato‐Gastroenterology 2000;47(35):1213‐5. [PubMed] [Google Scholar]
Davis 1997 {published data only}
- Davis WZ, Cotton PB, Arias R, Williams D, Onken JE. ERCP and sphincterotomy in the context of laparoscopic cholecystectomy: academic and community practice patterns and results. American Journal of Gastroenterology 1997;92(4):597‐601. [PubMed] [Google Scholar]
Decker 2003 {published data only}
- Decker G, Borie F, Millat B, Berthou JC, Deleuze A, Drouard F, et al. One hundred laparoscopic choledochotomies with primary closure of the common bile duct. Surgical Endoscopy 2003;17(1):12‐8. [DOI] [PubMed] [Google Scholar]
Dias 2002 {published data only}
- Dias MM, Martin CJ, Cox MR. Pattern of management of common bile duct stones in the laparoscopic era: a NSW survey. Australian and New Zealand Journal of Surgery 2002;72(3):181‐5. [DOI] [PubMed] [Google Scholar]
Drouard 1995 {published data only}
- Drouard F, Passone‐Szerzyna N, Berthou JC. Laparoscopic treatment of common bile duct calculi [Traitement laparoscopique de la lithiase de la voie biliaire principale]. Annales de Chirurgie 1995;49(7):596‐601. [PubMed] [Google Scholar]
Drouard 1997 {published data only}
- Drouard F, Passone‐Szerzyna N, Berthou JC. Laparoscopic treatment of common bile duct stones. Hepato‐Gastroenterology 1997;44(13):16‐21. [PubMed] [Google Scholar]
Ebner 2004 {published data only}
- Ebner S, Rechner J, Beller S, Erhart K, Riegler FM, Szinicz G. Laparoscopic management of common bile duct stones. Surgical Endoscopy and Other Interventional Techniques 2004;18(5):762‐5. [DOI] [PubMed] [Google Scholar]
El Geidie 2011 {published data only}
- Geidie AA, Ebidy GK, Naeem YM. Preoperative versus intraoperative endoscopic sphincterotomy for management of common bile duct stones. Surgical Endoscopy 2011;25:1230‐7. [DOI] [PubMed] [Google Scholar]
Fanning 1997 {published data only}
- Fanning NF, Horgan PG, Keane FBV. Evolving management of common bile duct stones in the laparoscopic era. Journal of the Royal College of Surgeons of Edinburgh 1997;42:389‐94. [PubMed] [Google Scholar]
Frazee 1993 {published data only}
- Frazee RC, Roberts J, Symmonds R, Hendricks JC, Snyder S, Smith R, et al. Combined laparoscopic and endoscopic management of cholelithiasis and choledocholithiasis. American Journal of Surgery 1993;166(6):702‐6. [DOI] [PubMed] [Google Scholar]
Galloway 1994 {published data only}
- Galloway SW, Blazeby J, Tulloh B, Poskitt KR. Combined laparoscopic and endoscopic treatment of gallstones and bile duct stones: a prospective study. British Journal of Surgery 1994;81(11):1699‐70. [DOI] [PubMed] [Google Scholar]
Giurgiu 1999 {published data only}
- Giurgiu DI, Margulies DR, Carroll BJ, Gabbay J, Iida A, Takagi S, et al. Laparoscopic common bile duct exploration: long‐term outcome. Archives of Surgery 1999;134(8):839‐44. [DOI] [PubMed] [Google Scholar]
Gonzalez 1989 {published data only}
- Gonzalez JC, Gomis J, Zoghbi S, Capozzolo N, Aldana K, Nino M, et al. Preoperative endoscopic sphincterotomy [Esfinterotomia endoscopica preoperatoria]. Genetic Engineering News 1989;43(4):247‐50. [PubMed] [Google Scholar]
Hamy 2003 {published data only}
- Hamy A, Hennekinne S, Pessaux P, Lada P, Randriamananjo S, Lermite E, et al. Endoscopic sphincterotomy prior to laparoscopic cholecystectomy for the treatment of cholelithiasis. Surgical Endoscopy 2003;17:872‐5. [DOI] [PubMed] [Google Scholar]
Heili 1999 {published data only}
- Heili M, Wintz NK, Fowler DL. Choledocholithiasis: endoscopic versus laparoscopic management. American Surgeon 1999;2:135‐8. [PubMed] [Google Scholar]
Heinerman 1989 {published data only}
- Heinerman PM, Boeckl O, Pimpl W. Selective ERCP and preoperative stone removal in bile duct surgery. Annals of Surgery 1989;209(3):267‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoyuela 1999 {published data only}
- Hoyuela C, Cugat E, Bretcha P, Collera P, Espinos J, Marco C. Must ERCP be routinely performed if choledocholithiasis is suspected?. Digestive Surgery 1999;16(5):411‐4. [DOI] [PubMed] [Google Scholar]
Hui 2002 {published data only}
- Hui CK, Lai KC, Wong WM, Yuen MF, Lam SK, Lai CL. A randomised controlled trial of endoscopic sphincterotomy in acute cholangitis without common bile duct stones. Gut 2002;51:245‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huynh 1996 {published data only}
- Huynh CH, Stadt J, Deviere J, Mehdi A, Nakadi I, Cremer M, et al. Preoperative endoscopic retrograde cholangiopancreatography: therapeutic impact in a general population of patients needing a cholecystectomy. Hepato‐Gastroenterology 1996;43(12):1484‐91. [PubMed] [Google Scholar]
Kapoor 1994 {published data only}
- Kapoor R, Pradeep R, Sikora SS, Saxena R, Kapoor VK, Kaushik SP. Appraisal of surgical and endoscopic management of choledocholithiasis. Australian and New Zealand Journal of Surgery 1994;64(9):599‐603. [DOI] [PubMed] [Google Scholar]
Khuroo 1989 {published data only}
- Khuroo MS, Mahajan R, Zargar SA, Javid G, Banday M. Endoscopic vs surgical drainage of biliary tract in acute pyogenic cholangitis: a controlled study. Indian Journal of Gastroenterology 1989;8(2):119. [PubMed] [Google Scholar]
Kullman 1996 {published data only}
- Kullman E, Borch K, Lindstrom E, Svanvik J, Anderberg B. Management of bile duct stones in the era of laparoscopic cholecystectomy: appraisal of routine operative cholangiography and endoscopic treatment. European Journal of Surgery 1996;162(11):873‐80. [PubMed] [Google Scholar]
Lai 1992 {published data only}
- Lai EC, Mok FP, Tan ES, Lo CM, Fan ST, You KT, et al. Endoscopic biliary drainage for severe acute cholangitis. New England Journal of Medicine 1992;326(24):1582‐6. [DOI] [PubMed] [Google Scholar]
Lella 2006 {published data only}
- Lella F, Bagnolo F, Rebuffat C, Scalambra M, Bonassi U, Colombo E. Use of the laparoscopic‐endoscopic approach, the so‐called "rendezvous" technique, in cholecystocholedocholithiasis. Surgical Endoscopy 2006;20:419‐23. [DOI] [PubMed] [Google Scholar]
Lezoche 1996 {published data only}
- Lezoche E, Paganini AM, Carlei F, Feliciotti F, Lomanto D, Guerrieri M. Laparoscopic treatment of gallbladder and common bile duct stones: a prospective study. World Journal of Surgery 1996;20(5):535‐41. [DOI] [PubMed] [Google Scholar]
Lezoche 2000 {published data only}
- Lezoche E, Paganini AM. Technical considerations and laparoscopic bile duct exploration: transcystic and choledochotomy. Seminars in Laparoscopic Surgery 2000;7(4):262‐78. [PubMed] [Google Scholar]
Liberman 1996 {published data only}
- Liberman MA, Phillips EH, Carroll BJ, Fallas MJ, Rosenthal R, Hiatt J. Cost‐effective management of complicated choledocholithiasis: laparoscopic transcystic duct exploration or endoscopic sphincterotomy. Journal of the American College of Surgeons 1996;182:488‐94. [PubMed] [Google Scholar]
Liu 1996 {published data only}
- Liu CL, Lai EC, Lo CM, Chu KM, Fan ST, Wong J. Combined laparoscopic and endoscopic approach in patients with cholelithiasis and choledocholithiasis. Surgery 1996;119(5):534‐7. [DOI] [PubMed] [Google Scholar]
Magnanini 1994 {published data only}
- Magnanini F, Peralta C, Pardo R, Curras A, Zalar A, Olmos M. Endoscopic retrograde cholangiopancreatography: before and after laparoscopic cholecystectomy. Acta Gastroenterologica Latinoamericana 1994;24(4):213‐7. [PubMed] [Google Scholar]
Martin 1998 {published data only}
- Martin IJ, Bailey IS, Rhodes M, O'Rourke N, Nathanson L, Fielding G. Towards T‐tube free laparoscopic bile duct exploration: a methodologic evolution during 300 consecutive procedures. Annals of Surgery 1998;228(1):29‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2002 {published data only}
- Martin CJ, Cox MR, Vaccaro L. Laparoscopic transcystic bile duct stenting in the management of common bile duct stones. Australian and New Zealand Journal of Surgery 2002;72(4):258‐64. [DOI] [PubMed] [Google Scholar]
Masci 1999 {published data only}
- Masci E, Fanti L, Mariani A, Guerini S, Zuliani W, Baccari P, et al. Selection criteria for pre‐operative endoscopic retrograde cholangiography and endoscopic‐laparoscopic treatment of biliary stones. European Journal of Gastroenterology & Hepatology 1999;11(7):781‐4. [DOI] [PubMed] [Google Scholar]
Materia 1996 {published data only}
- Materia A, Pizzuto G, Silecchia G, Fiocca F, Fantini A, Spaziani E, et al. Sequential endoscopic‐laparoscopic treatment of cholecystocholedocholithiasis. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 1996;6(4):273‐7. [PubMed] [Google Scholar]
Meyer 1999 {published data only}
- Meyer C, Le JV, Rohr S, Thiry LC, Bourtoul C, Duclos B, et al. Management of common bile duct stones by laparoscopic cholecystectomy and endoscopic sphincterotomy: pre‐, per‐ or postoperative sphincterotomy?. Digestive Surgery 1999;16(1):26‐31. [DOI] [PubMed] [Google Scholar]
Meyer 2002 {published data only}
- Meyer C, Le JV, Rohr S, Duclos B, Reimund JM, Baumann R. Management of common bile duct stones in a single operation combining laparoscopic cholecystectomy and peroperative endoscopic sphincterotomy. Journal of Hepato‐Biliary‐Pancreatic Surgery 2002;9(2):196‐200. [DOI] [PubMed] [Google Scholar]
Michel 2000 {published data only}
- Michel J, Navarro F, Montpeyroux F, Burgel JS, Moine MC, Daures JP, et al. Treatment of common bile duct stones with laparoscopy. Retrospective multicenter study with 612 patients [Traitement de la lithiase de la voie biliaire principale sous laparoscopie. Etude retrospective multicentrique chez 612 malades]. Gastroenterologie Clinique et Biologique 2000;24(4):404‐8. [PubMed] [Google Scholar]
Mijal 1997 {published data only}
- Mijal M, Ciechanski A, Chmurzynski M, Zinkiewicz K, Cwik G, Misiuna P. Comparison of combined (endoscopic sphincterotomy + laparoscopic cholecystectomy) and classical treatment of obstructive jaundice in the course of cholelithiasis [Porownanie wynikow skojarzonego (ES + ChL) i klasycznego leczenia zoltaczki mechanicznej w przebiegu kamicy zolciowej]. Wiadomosci Lekarskie 1997;50(Suppl 1 Pt 1):242‐5. [PubMed] [Google Scholar]
Millat 1995 {published data only}
- Millat B, Fingerhut A, Deleuze A, Briandet H, Marrel E, Seguin C, et al. Prospective evaluation in 121 consecutive unselected patients undergoing laparoscopic treatment of choledocholithiasis. British Journal of Surgery 1995;82(9):1266‐9. [DOI] [PubMed] [Google Scholar]
Millat 1996 {published data only}
- Millat B, Deleuze A, Atger J, Briandet H, Fingerhut A, Marrel E, et al. Treatment of common bile duct lithiasis under laparoscopy. A prospective multicenter study in 189 patients [Traitement de la lithiase de la voie biliaire principale sous laparoscopie]. Gastroenterologie Clinique et Biologique 1996;20(4):339‐45. [PubMed] [Google Scholar]
Millat 1997 {published data only}
- Millat B, Atger J, Deleuze A, Briandet H, Fingerhut A, Guillon F, et al. Laparoscopic treatment for choledocholithiasis: a prospective evaluation in 247 consecutive unselected patients. Hepato‐Gastroenterology 1997;44(13):28‐34. [PubMed] [Google Scholar]
Miller 1988 {published data only}
- Miller BM, Kozarek RA, Ryan JA, Ball TJ, Traverso LW. Surgical versus endoscopic management of common bile duct stones. Annals of Surgery 1988;207(2):135‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mo 2002 {published data only}
- Mo LR, Chang KK, Wang CH, Yau MP, Yang TM. Preoperative endoscopic sphincterotomy in the treatment of patients with cholecystocholedocholithiasis. Journal of Hepato‐Biliary‐Pancreatic Surgery 2002;9(2):191‐5. [DOI] [PubMed] [Google Scholar]
Moreaux 1995 {published data only}
- Moreaux J. Traditional surgical management of common bile duct stones: a prospective study during a 20‐year experience. American Journal of Surgery 1995;169(2):220‐6. [DOI] [PubMed] [Google Scholar]
Morino 2006 {published data only}
- Morino M, Baracchi F, Miglietta C, Furlan N, Ragona R, Garbarini A. Preoperative endoscopic sphincterotomy versus laparo endoscopic rendezvous in patients with gallbladder and bile duct stones. Annals of Surgery 2006;244(6):889‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neoptolemos 89 {published data only}
- Neoptolemos JP, Shaw DE, Carr‐Locke DL. A multivariate analysis of preoperative risk factors in patients with common bile duct stones. Implications for treatment. Annals of Surgery 1989;209(2):157‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neuhaus 1992 {published data only}
- Neuhaus H, Ungeheuer A, Feussner H, Classen M, Siewert JR. Laparoscopic cholecystectomy: ERCP as standard preoperative diagnostic technique [Laparoskopische Cholezystektomie: ERCP als preoperative Standarddiagnostik?]. Deutsche Medizinische Wochenschrift 1992;117(49):1863‐7. [DOI] [PubMed] [Google Scholar]
Niu 1995 {published data only}
- Niu J, Shou NH, Forbes JF, Sun XY, Hu SY, Liu FJ. Laparoscopic exploration of intra‐ and extrahepatic bile ducts and T‐tube drainage. Australian and New Zealand Journal of Surgery 1995;65(3):189‐93. [DOI] [PubMed] [Google Scholar]
Paganini 1998 {published data only}
- Paganini AM, Lezoche E. Follow‐up of 161 unselected consecutive patients treated laparoscopically for common bile duct stones. Surgical Endoscopy 1998;12(1):23‐9. [DOI] [PubMed] [Google Scholar]
Palacios‐Macedo 1995 {published data only}
- Palacios‐Macedo A, Herrera MF, Moran MA, Gonzalez Garcia M, Chan Nunez C, Hernandez‐Ortiz J. Endoscopy and laparoscopy in the treatment of lithiasic cholecystitis associated with benign bile duct obstruction [Endoscopia y laparoscopia en el tratamiento de la colecistitis litiasica asociada a obstruccion benigna de via biliar]. Revista de Investigacion Clinica 1995;47(2):103‐7. [PubMed] [Google Scholar]
Pedersen 1998 {published data only}
- Pedersen FM, Brandt CJ, Schaffalitzky de Muckadell OB. Choledocholithiasis. Endoscopic treatment of 416 consecutive patients [Choledocholitiasis. Endoskopisk behandling af 416 konsekutive patienter]. Ugeskrift for Laeger 1998;160(45):6526‐9. [PubMed] [Google Scholar]
Pereira‐Lima 2001 {published data only}
- Pereira‐Lima JC, Rynkowski CB, Rhoden EL. Endoscopic treatment of choledocholithiasis in the era of laparoscopic cholecystectomy: prospective analysis of 386 patients. Hepato‐Gastroenterology 2001;48(41):1271‐4. [PubMed] [Google Scholar]
Perniceni 2001 {published data only}
- Perniceni T, Alves A, Levard H, Boudet MJ, Denet C, Gayet B. Can common bile duct lithiasis be removed laparoscopically without external biliary drainage? [Le traitement laparoscopique de la lithiase de la voie biliaire principale sans drainage biliaire externe est‐il possible?]. Gastroenterologie Clinique et Biologique 2001;25(2):149‐53. [PubMed] [Google Scholar]
Phillips 1995 {published data only}
- Phillips EH, Liberman M, Carroll BJ, Fallas MJ, Rosenthal R, Hiatt J. Bile duct stones in the laparoscopic era: is pre‐operative sphincterotomy necessary?. Archives of Surgery 1995;130:880‐6. [DOI] [PubMed] [Google Scholar]
Quershi 1993 {published data only}
- Quershi A, Browne A, Leahy AL, Courtney G, Osborne H, Broe PJ, et al. ERCP in the management of patients having laparoscopic cholecystectomy: re‐appraising current indications. Irish Journal of Medical Science 1993;162(12):510‐2. [DOI] [PubMed] [Google Scholar]
Rabago 2006 {published data only}
- Rabago LR, Vincente C, Soler F, Delgado M, Moral I, Guerra I, et al. Two‐stage treatment with preoperative endoscopic retrograde cholangiopancreatography (ERCP) compared with single‐stage treatment with intraoperative ERCP for patients with symptomatic cholelithiasis with possible choledocholithiasis. Endoscopy 2006;38(8):779‐86. [DOI] [PubMed] [Google Scholar]
Rhodes 1995 {published data only}
- Rhodes M, Nathanson L, O'Rourke N, Fielding G. Laparoscopic exploration of the common bile duct: lessons learned from 129 consecutive cases. British Journal of Surgery 1995;82:666‐8. [DOI] [PubMed] [Google Scholar]
Rieger 1994 {published data only}
- Rieger R, Sulzbacher H, Woisetschlager R, Schrenk P, Wayand W. Selective use of ERCP in patients undergoing laparoscopic cholecystectomy. World Journal of Surgery 1994;18(6):900‐4; discussion 904‐5. [DOI] [PubMed] [Google Scholar]
Rieger 1995 {published data only}
- Rieger R, Wayand W. Yield of prospective, noninvasive evaluation of the common bile duct combined with selective ERCP/sphincterotomy in 1390 consecutive laparoscopic cholecystectomy patients. Gastrointestinal Endoscopy 1995;42(1):6‐12. [DOI] [PubMed] [Google Scholar]
Rijna 2000 {published data only}
- Rijna H, Kemps WG, Eijsbouts Q, Meuwissen SG, Cuesta MA. Preoperative ERCP approach to common bile duct stones: results of a selective policy. Digestive Surgery 2000;17(3):229‐33. [DOI] [PubMed] [Google Scholar]
Robertson 1996 {published data only}
- Robertson GS, Jagger C, Johnson PR, Rathbone BJ, Wicks AC, Lloyd DM, et al. Selection criteria for preoperative endoscopic retrograde cholangiopancreatography in the laparoscopic era. Archives of Surgery 1996;131(1):89‐94. [DOI] [PubMed] [Google Scholar]
Robinson 1995 {published data only}
- Robinson G, Hollinshead J, Falk G, Moulton J. Technique and results of laparoscopic choledochotomy for the management of bile duct calculi. Australian and New Zealand Journal of Surgery 1995;65(5):347‐9. [DOI] [PubMed] [Google Scholar]
Roush 1995 {published data only}
- Roush TS, Traverso LW. Management and long‐term follow‐up of patients with positive cholangiograms during laparoscopic cholecystectomy. American Journal of Surgery 1995;169(5):484‐7. [DOI] [PubMed] [Google Scholar]
Santucci 1996 {published data only}
- Santucci L, Natalini G, Sarpi L, Fiorucci S, Solinas A, Morelli A. Selective endoscopic retrograde cholangiography and preoperative bile duct stone removal in patients scheduled for laparoscopic cholecystectomy: a prospective study. American Journal of Gastroenterology 1996;91(7):1326‐30. [PubMed] [Google Scholar]
Sarli 1999 {published data only}
- Sarli L, Pietra N, Franze A, Colla G, Costi R, Gobbi S, et al. Routine intravenous cholangiography, selective ERCP, and endoscopic treatment of bile duct stones before laparoscopic cholecystectomy. Gastrointestinal Endoscopy 1999;50(2):200‐8. [DOI] [PubMed] [Google Scholar]
Schwab 1992 {published data only}
- Schwab G, Pointner R, Wetscher G, Glaser K, Foltin E, Bodner E. Treatment of calculi of the common bile duct. Surgery, Gynecology and Obstetrics 1992;175(2):115‐20. [PubMed] [Google Scholar]
Seo 2000 {published data only}
- Seo DW. Prospective analysis of endoscopic papillary balloon dilatation and endoscopic sphincterotomy for removal of common bile duct stones. Gastrointestinal Endoscopy 2000;52(1):140‐2. [PubMed] [Google Scholar]
Stoker 1995 {published data only}
- Stoker ME. Common bile duct exploration in the era of laparoscopic surgery. Archives of Surgery 1995;130(3):265‐9. [DOI] [PubMed] [Google Scholar]
Sugiyama 1999 {published data only}
- Sugiyama M, Izumisato Y, Hatano N, Mori T, Atomi Y. Management of unsuspected common bile duct stones found during laparoscopic cholecystectomy by means of transcystic catheter placement and papillary dilation. Gastrointestinal Endoscopy 1999;50(6):837‐40. [DOI] [PubMed] [Google Scholar]
Sungler 1993 {published data only}
- Sungler P, Heinerman PM, Mayer F, Boeckl O. Laparoscopic cholecystectomy in cholecysto‐choledocholithiasis. "Therapeutic splitting" or conventional surgical procedure?. Chirurg 1993;64(12):1012‐5; discussion 1016‐7. [PubMed] [Google Scholar]
Sungler 1997 {published data only}
- Sungler P, Holzinger J, Heinerman PM, Waclawiczek HW, Boeckl O. Preoperative therapeutic splitting [Preoperatives therapeutisches splitting]. Zentralblatt fur Chirurgie 1997;122(12):1083‐7. [PubMed] [Google Scholar]
Tham 1998 {published data only}
- Tham TC, Lichtenstein DR, Vandervoort J, Wong RC, Brooks D, Dam J, et al. Role of endoscopic retrograde cholangiopancreatography for suspected choledocholithiasis in patients undergoing laparoscopic cholecystectomy. Gastrointestinal Endoscopy 1998;47(1):50‐6. [DOI] [PubMed] [Google Scholar]
Trias 1997 {published data only}
- Trias M, Targarona EM, Ros E, Bordas JM, Perez Ayuso RM, Balague C, et al. Prospective evaluation of a minimally invasive approach for treatment of bile‐duct calculi in the high‐risk patient. Surgical Endoscopy 1997;11(6):632‐5. [DOI] [PubMed] [Google Scholar]
Trondsen 1995 {published data only}
- Trondsen E, Edwin B, Reiertsen O, Fagertun H, Rosseland AR. Selection criteria for endoscopic retrograde cholangiopancreaticography (ERCP) in patients with gallstone disease. World Journal of Surgery 1995;19(6):852‐7. [DOI] [PubMed] [Google Scholar]
Turcu 1997 {published data only}
- Turcu F. A minimally invasive approach in choledocholithiasis [Abordul miniinvaziv al litiazei de cale biliara principala]. Chirurgia (Bucuresti) 1997;92(3):145‐53. [PubMed] [Google Scholar]
Waage 2003 {published data only}
- Waage A, Stromberg C, Leijonmarck CE, Arvidsson D. Long‐term results from laparoscopic common bile duct exploration. Surgical Endoscopy and Other Interventional Techniques 2003;17(8):1181‐5. [DOI] [PubMed] [Google Scholar]
Welbourn 1995 {published data only}
- Welbourn CR, Mehta D, Armstrong CP, Gear MW, Eyre‐Brook IA. Selective preoperative endoscopic retrograde cholangiography with sphincterotomy avoids bile duct exploration during laparoscopic cholecystectomy. Gut 1995;37(4):576‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wenner 2005 {published data only}
- Wenner DE, Whitwam P, Rosser J, Hashmi S, Wenner DE. A stone extraction facilitation device to achieve an improved technique for performing LCBDE. Surgical Endoscopy and Other Interventional Techniques 2005;19(1):120‐5. [DOI] [PubMed] [Google Scholar]
Widdison 1994 {published data only}
- Widdison AL, Longstaff AJ, Armstrong CP. Combined laparoscopic and endoscopic treatment of gallstones and bile duct stones: a prospective study. British Journal of Surgery 1994;81(4):595‐7. [DOI] [PubMed] [Google Scholar]
Wilson 1993 {published data only}
- Wilson TG, Jeans PL, Anthony A, Cox MR, Toouli J. Laparoscopic cholecystectomy and management of choledocholithiasis. Australian and New Zealand Journal of Surgery 1993;63(6):443‐50. [DOI] [PubMed] [Google Scholar]
Worthley 1989 {published data only}
- Worthley CS, Watts JM, Toouli J. Common duct exploration or endoscopic sphincterotomy for choledocholithiasis?. Australian and New Zealand Journal of Surgery 1989;59(3):209‐15. [DOI] [PubMed] [Google Scholar]
Zargar 2002 {published data only}
- Zagar SA, Javid G, Khan, et al. Endoscopic sphincterotomy in the management of the commmon bile duct stones: results in 170 patients. JK Practitioner 2002;9:20‐3. [Google Scholar]
Additional references
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276:637‐9. [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive‐‐Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
Clayton 2006
- Clayton ES, Connor S, Alexakis N, Leandros E. Meta‐analysis of endoscopy and surgery versus surgery alone for common bile duct stones with the gallbladder in situ. British Journal of Surgery 2006;93(10):1185‐91. [DOI] [PubMed] [Google Scholar]
Coelho‐Prabhu 2013
- Coelho‐Prabhu N, Shah ND, Houten H, Kamath PS, Baron TH. Endoscopic retrograde cholangiopancreatography: utilisation and outcomes in a 10‐year population‐based cohort. BMJ Open 2013;3(5):e002689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 2004
- Collins C, Maguire D, Ireland A Fitzgerald E, O'Sullivan GC. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: a natural history of choledocholithiasis revisited. Annals of Surgery 2004;239:28‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
CTU 2011
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. http://ctu.dk/tsa/ 2011 (accessed 5 July 2013).
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test.. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fletcher 1994
- Fletcher DR. Changes in the practice of biliary surgery and ERCP during the introduction of laparoscopic cholecystectomy to Australia: their possible significance. Australian and New Zealand Journal of Surgery 1994;64(2):75‐80. [DOI] [PubMed] [Google Scholar]
Gluud 2013
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2013, Issue 7. Art. No.: LIVER.
Gurusamy 2009
- Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. British Journal of Surgery 2009;96(4):342‐9. [DOI] [PubMed] [Google Scholar]
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Ko 2002
- Ko CW, Lee SP. Epidemiology and natural history of common bile duct stones and prediction of disease. Review. Gastrointestinal Endoscopy 2002;56(6 Suppl):S165‐S169. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparision of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
Mercer 2007
- Mercer S, Singh S, Paterson I. Selective MRCP in the management of suspected common bile duct stones. HPB (Oxford) 2007;9:125‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Neoptolemos 1989
- Neoptolemos JP, Rowley S. Advantages of nonsurgical treatment of bile duct stones. Hepato‐Gastroenterology 1989;36(5):313‐6. [PubMed] [Google Scholar]
Newell 1992
- Newell DJ. Intention‐tot‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rojas‐Ortega 2003
- Rojas‐Ortega S, Arizpe‐Bravo D, Marín López ER, Cesin‐Sánchez R, Roman GR, Gómez C. Transcystic common bile duct exploration in the management of patients with choledocholithiasis. Journal of Gastrointestinal Surgery 2003;7(4):492‐6. [DOI] [PubMed] [Google Scholar]
Savovic 2012a
- Savovic J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Savovic 2012b
- Savovic J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Soltan 2000
- Soltan, Kow, Toouli. A simple scoring system for predicting bile duct stones in patients with cholelithiasis. Journal of Gastrointestinal Surgery 2001;5(4):434‐7. [DOI] [PubMed] [Google Scholar]
SPIRIT 2013
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža‐Jerić K, et al. SPIRIT 2013 Statement: defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158:200‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stinton 2012
- Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut and Liver 2012;6:172‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tazuma 2006
- Tazuma S. Epidemiology, pathogenesis, and classification of biliary stones (common bile duct and intrahepatic). Best Practice and Research Clinical Gastroenterology 2006;20:1075‐83. [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual forTrial Sequential Analysis (TSA). http://ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 5 July 2013).
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2008
- Williams EJ, Green J, Beckingham I, Parks R, Martin D, Lombard M. Guidelines on the management of common bile duct stones (CBDS). Gut 2008;57:1004‐21. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Martin 2006
- Martin DJ, Vernon D, Toouli J. Surgical versus endoscopic treatment of bile duct stones. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003327.pub2] [DOI] [PubMed] [Google Scholar]


