Abstract
Background
The use of ultrasound guidance for regional anaesthesia has become popular over the past two decades. However, it is not recognized by all experts as an essential tool. The cost of an ultrasound machine is substantially higher than the cost of other tools such as a nerve stimulator.
Objectives
To determine whether ultrasound guidance offers any clinical advantage when neuraxial and peripheral nerve blocks are performed in children in terms of increasing the success rate or decreasing the rate of complications.
Search methods
We searched the following databases to March 2015: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (OvidSP), EMBASE (OvidSP) and Scopus (from inception to 27 January 2015).
Selection criteria
We included all parallel randomized controlled trials (RCTs) that evaluated the effects of ultrasound guidance used when a regional blockade technique was performed in children, and that included any of our selected outcomes.
Data collection and analysis
We assessed selected studies for risk of bias by using the assessment tool of The Cochrane Collaboration. Two review authors independently extracted data. We graded the level of evidence for each outcome according to the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) Working Group scale.
Main results
We included 20 studies (1241 participants) for which the source of funding was a government organization (two studies), a charitable organization (one study), an institutional department (four studies) or an unspecified source (11 studies); two studies declared that they received help from the industry (equipment loan). In 14 studies (939 participants), ultrasound guidance increased the success rate by decreasing the occurrence of a failed block: risk difference (RD) ‐0.11 (95% confidence interval (CI) ‐0.17 to ‐0.05); I2 = 64%; number needed for additional beneficial outcome for a peripheral nerve block (NNTB) 6 (95% CI 5 to 8). Blocks were performed under general anaesthesia (usual clinical practice in this population); therefore, haemodynamic changes to the surgical stimulus (rather than classic sensory/motor blockade evaluation) were used to define success. For peripheral nerve blocks, the younger the child, the greater was the benefit. In eight studies (414 participants), pain scores at one hour in the post‐anaesthesia care unit were reduced when ultrasound guidance was used; however, the clinical relevance of the difference was unclear (equivalent to ‐0.2 on a scale from 0 to 10). In eight studies (358 participants), block duration was longer when ultrasound guidance was used: standardized mean difference (SMD) 1.21 (95% CI 0.76 to 1.65; I2 = 73%; equivalent to 62 minutes). Here again, younger children benefited most from ultrasound guidance. Time to perform the procedure was reduced when ultrasound guidance was used for pre‐scanning before a neuraxial block (SMD ‐1.97, 95% CI ‐2.41 to ‐1.54; I2 = 0%; equivalent to 2.4 minutes; two studies with 122 participants) or as an out‐of‐plane technique (SMD ‐0.68, 95% CI ‐0.96 to ‐0.40; I2 = 0%; equivalent to 94 seconds; two studies with 204 participants). In two studies (122 participants), ultrasound guidance reduced the number of needle passes required to perform the block (SMD ‐0.90, 95% CI ‐1.27 to ‐0.52; I2 = 0%; equivalent to 0.6 needle pass per participant). For two studies (204 participants), we could not demonstrate a difference in the incidence of bloody puncture when ultrasound guidance was used for neuraxial blockade, but we found that the number of participants was well below the optimal information size (RD ‐0.07, 95% CI ‐0.19 to 0.04). No major complications were reported for any of the 1241 participants. We rated the quality of evidence as high for success, pain scores at one hour, block duration, time to perform the block and number of needle passes. We rated the quality of evidence as low for bloody punctures.
Authors' conclusions
Ultrasound guidance seems advantageous, particularly in young children, for whom it improves the success rate and increases the block duration. Additional data are required before conclusions can be drawn on the effect of ultrasound guidance in reducing the rate of bloody puncture.
Keywords: Child; Humans; Ultrasonography, Interventional; Nerve Block; Nerve Block/methods; Perioperative Care; Perioperative Care/methods; Peripheral Nervous System; Randomized Controlled Trials as Topic
Ultrasound guidance for injecting local anaesthetics in children to block pain transmission
Background
A local anaesthetic can be injected into the spine or around the nerves to block pain transmission to avoid putting the patient to sleep for surgery or to treat postoperative pain. This is called ‘regional blockade’. Finding an effective alternative to general anaesthetics or traditional painkillers is particularly important for children because they might be more likely to suffer adverse effects from general anaesthesia or opioid painkillers, and because pain in early life might do long‐term harm. Regional blockade can be performed by inserting a needle into the skin at a place that is determined by palpation of bones or a pulsatile vessel. An electric needle producing a muscle contraction can also be used to find a suitable location. Over the past three decades, clinicians have started to use ultrasound to locate the nerves, but these machines are expensive and require additional clinician expertise. A Cochrane review has already found that ultrasound guidance does not increase the rate of success of regional blockade but does reduce harmful effects in adults. We wanted to know whether effects in children are the same.
Search date
Evidence is current to March 2015.
Study characteristics
We included 20 randomized controlled trials in which ultrasound was compared with another method of nerve localization for regional blockade in children.
Study funding sources
Sources of funding included a government organization (two studies), a charitable organization (one study) and an institutional department (four studies). Two studies declared that they received help from the industry (equipment loan). The source of funding was unclear for 11 studies.
Key results
Ultrasound guidance decreased the occurrence of a failed block (actual rate without ultrasound 25%). If six blocks were performed, one fewer participant would have a failed block if ultrasound guidance was used. The identified studies used children from different age groups. If we compare results by age, we find that the younger the child, the greater was the reduction in failed blocks. Pain scores at one hour after surgery were reduced when ultrasound guidance was used, but the reduction in pain was small. When ultrasound guidance was used, the time that lapsed before the child needed additional painkillers after surgery was increased by approximately 62 minutes from the usual mean time ranging from 11 minutes to seven hours. Here again, the younger the child, the longer was the difference in delay to the appearance of pain. Time to perform the block was reduced when ultrasound guidance was used for pre‐scanning before a block in the spine was performed (equivalent to 2.4 minutes less from a mean time of 3.2 minutes in the control group). Ultrasound guidance reduced the number of needle passes required to perform the block: mean 0.6 needle pass per participant (from a mean of 1.6 in the control group). Data are needed to show whether ultrasound guidance also reduces the number of unwanted needle entrances into a blood vessel (actual rate without ultrasound 14%). No major complications were reported in any of the 1241 participants.
Quality of evidence
The quality of the evidence was rated as high for decreased occurrence of a failed block, improved pain scores at one hour, increased block duration, reduced time needed to perform regional blockade when ultrasound guidance was used as pre‐scanning before a block in the spine and a decreased number of needle passes. The level of evidence was rated as low for the number of unwanted needles entered into a blood vessel.
Summary of findings
Summary of findings for the main comparison.
Ultrasound guidance compared with no ultrasound guidance for children
| Ultrasound guidance compared with no ultrasound guidance for children | ||||||
| Patient or population: children Settings: Data were collected in Austria (1 study), Belgium (1 study), Canada (1 study), China (3 studies), Egypt (1 study), India (2 studies), Japan (1 study), Ireland (1 study), South Africa (4 studies), Turkey (2 studies) and United States of America (3 studies) Intervention: ultrasound guidance Comparison: no ultrasound guidance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No ultrasound guidance | Ultrasound guidance | |||||
| Success (analysed as decreased failure rate) | Study population | Risk difference 0 (0 to ‐0.07) | 507 (8 studiesa) | ⊕⊕⊕⊕ highb,c,d,e,f,g,h,i | Only studies on peripheral nerve blocks were retained for this outcome | |
| 254 per 1000 | 0 per 1000 (0 to ‐18) | |||||
| Low | ||||||
| 25 per 1000 | 0 per 1000 (0 to ‐2) | |||||
| High | ||||||
| 350 per 1000 | 0 per 1000 (0 to ‐25) | |||||
| Pain scores at 1 hour after surgery | Mean pain scores at 1 hour after surgery in the intervention groups was 0.29 standard deviations lower (0.54 to 0.04 lower) | 308 (7 studies) | ⊕⊕⊕⊕ highb,d,f,h,j,k,l,m | One study (Lorenzo 2014) was excluded for this outcome to reduce the amount of heterogeneity (see Effects of interventions). The reduction is equivalent to 0.2 on the Children’s and Infant’s Postoperative Pain Scale (CHIPPS scale: 0 = no pain, 10 = maximal pain) |
||
| Block duration Time to request of first analgesic Follow‐up: 0 to 1 day | Mean block duration in intervention groups was 1.21 standard deviations higher (0.76 to 1.65 higher) | 358 (8 studies) | ⊕⊕⊕⊕ highb,c,d,f,h,k,n,o | Mean prolongation of the block was equivalent to 62 minutes | ||
| Time to perform the procedure Ultrasound guidance used as pre‐scanning before neuraxial block | Mean time to perform the procedure in intervention groups was 1.97 standard deviations lower (2.41 to 1.54 lower) | 122 (2 studies) | ⊕⊕⊕⊕ highd,h,k,m,p,q,r,s | This is equivalent to 2.4 minutes when ultrasound guidance was used as pre‐scanning before neuraxial block | ||
| Number of needle passes | Mean number of needle passes in intervention groups was 0.90 standard deviations lower (1.27 to 0.52 lower) | 122 (2 studies) | ⊕⊕⊕⊕ highd,h,k,m,p,q,r,t | Mean difference is equivalent to 0.6 needle pass per participant | ||
| Bloody puncture | Study population | Risk difference ‐0.07 (‐0.19 to 0.04) | 204 (2 studiesu) | ⊕⊕⊝⊝ lowb,d,e,h,l,m,r,v | For this outcome, we retained only studies in which ultrasound guidance was used for neuraxial block | |
| 135 per 1000 | ‐9 per 1000 (‐26 to 5) | |||||
| Low | ||||||
| 20 per 1000 | ‐1 per 1000 (‐4 to 1) | |||||
| High | ||||||
| 200 per 1000 | ‐14 per 1000 (‐38 to 8) | |||||
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aFor this outcome, we retained only studies performed on peripheral nerve blocks bAllocation concealment and blinding of outcome assessors were judged adequate for 50% or more of the studies cAn explanation was found for the heterogeneity dDirect comparisons performed on the population of interest; the outcome is not a surrogate marker eThe number of participants included is lower than the optimal information size for a large trial fNo evidence of publication bias, or correcting for the possibility of one, would not modify the conclusion gRisk ratio smaller than 0.5 hNo confounding factor justifying upgrading the evidence identified iFor peripheral nerve blocks, an inverse correlation between effect size and age was noted jFor this outcome, we excluded 1 study. I2 = 31% after exclusion of 1 study kOptimal information size achieved lNo evidence of a large effect mNo evidence of a dose‐response effect nSMD 1.21 oThe effect was inversely proportional to age; younger participants benefitted most from ultrasound guidance pWe retained only 2 studies for this outcome: Allocation concealment was unclear and blinding of the outcome assessor was not feasible for this outcome qI2statistic smaller than 25% rPublication bias assessment not available because of the low number of studies retained sSMD ‐1.97 tSMD ‐0.90 uFor this outcome, we retained only studies on neuraxial blocks vI2 statistic greater than 50%
Background
In 2005, in the United States alone, approximately 647,000 children were discharged from a short stay hospital after they had undergone a surgical procedure (DeFrances 2007). Anaesthesiologists are involved in these procedures at various steps of the process, amongst which anaesthesia for the procedure itself and treatment of postoperative pain are of the utmost importance. Although a vast majority of surgeries in children are performed with the child under general anaesthesia, concerns have been raised about the safety of inhalational agents for the developing brain of a child (Chiao 2014). As such, regional anaesthesia has been identified as a possible favourable replacement for general anaesthesia for specific surgeries (Nemergut 2014). Furthermore, in 2000, the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) suggested that pain should be considered as the fifth vital sign, and that under treatment of pain should constitute abrogation of a fundamental human right (White 2007). After this statement was issued, an increase in the use of opioids for treatment of patients with acute postoperative pain was observed, as was an increase in opioid side effects (White 2007). Postoperative pain relief is of particular importance in children. Pain experienced early in life may induce organic brain changes that can make children susceptible to an exaggerated brain response when pain is experienced later in life (Hohmeister 2010). These brain changes are frequently referred to as neuroplasticity. Young children often are unable to understand what is happening to them, and this may increase their distress, leading to an increase in their inability to convey what exactly is making them uncomfortable. In response to this situation, care providers may under treat or over treat children experiencing postoperative pain. In one study performed in children, adverse events requiring an intervention (therefore judged as clinically relevant) occurred in 22% and 24% of patients with patient‐controlled analgesia (PCA) administered by trained relatives or nurses and by the patients themselves, respectively (Voepel‐Lewis 2008). Opioid‐based regimens may therefore provide suboptimal treatment for postoperative pain in children.
Description of the condition
Regional blockade interrupts pain transmission to the brain and may be used during the surgery itself as a replacement for general anaesthesia (regional anaesthesia), or for treatment of postoperative pain (regional analgesia). In adults, regional analgesic techniques decrease postoperative opioid consumption (Guay 2006), making them a potentially interesting alternative or adjunct to opioid‐based regimens for treatment of children with postoperative pain. Regional blockade techniques can be classified as central neuraxial blocks (spinal, epidural, combined spinal and epidural or caudal) or as peripheral nerve blocks. The use of regional blockade in children is considered reasonably safe today (Long 2014; Polaner 2012). For a total of 14,917 regional blocks performed on 13,725 study participants from 1 April 2007 through 31 March 2010, no deaths or complications with sequelae lasting longer than three months were reported (95% confidence interval (CI) 0 to 2 per 10,000 blocks) (Polaner 2012). For neuraxial blocks, 183 adverse events were reported, at an incidence of 3% (95% CI 26 to 35 per 1000) (Polaner 2012). The most common adverse event (104, or 2% of the total and 57% of all events) was inability to place the block, or block failure (Polaner 2012). Ninety‐three per cent of neuraxial blocks were placed without imaging guidance. Inability to place the block or a failed block was also the most common adverse event for upper extremity blocks and was reported in six of 1000 lower limb blocks (Polaner 2012).
Description of the intervention
Ultrasound refers to an oscillating sound pressure wave at a frequency above the upper limit audible to the human ear (approximately 20 kHz). In nature, bats use ultrasounds as a guide for night flights. Ultrasounds emitted by the animal are reflected when they hit an obstacle. The same principle has been applied to develop devices in which ultrasound is used to create two‐dimensional (2‐D) or even three‐dimensional (3‐D) pictures (Feinglass 2007). Ultrasound has been used for regional blockade for almost three decades. The pioneers to whom use of ultrasound for regional blockade could be attributed include P. La Grange (La Grange 1978), R.L. Ting (Ting 1989), T.‐J. Wu (Wu 1993) and S. Kapral (Kapral 1994). The probe emitting and receiving ultrasounds is placed over the area of the body in which the local anaesthetic will be injected. After appropriate visualization of the target, the needle may be advanced in‐plane (parallel to the beam), allowing visualization of the entire needle during its trajectory, or out‐of‐plane (perpendicular to the beam). The local anaesthetic is then injected under visualization. For neuraxial blocks, ultrasound guidance can be used in real time to observe advancement of the needle within the epidural space or within the intrathecal canal (Niazi 2014), but most often it is used as a pre‐puncture guide to identify the exact vertebral level needed, to find an appropriate intervertebral space sufficient to allow passage of the needle, to determine the depth to which the needle should be advanced for placement of its tip at the chosen location and to visualize the spread of the local anaesthetic. For peripheral nerve blocks, ultrasound guidance allows visualization of target nerves, advancement of the needle (in‐plane technique) and spread of the local anaesthetic.
How the intervention might work
In children, regional anaesthetic techniques are usually performed with the child under deep sedation or under general anaesthesia. Fortunately, this does not seem to increase the risk of complications associated with regional anaesthesia (Taenzer 2014). However, as the child cannot express any paraesthesia‐related discomfort (with potential needle placement inside a neural structure), visualization may be even more important in this age group. Regional blockade may be performed with the use of landmarks, a nerve stimulator or ultrasound guidance. Ultrasound guidance allows adequate visualization of nerves and other structures relevant to the performance of both neuraxial and peripheral nerve blocks, particularly in children, in whom relevant structures are relatively superficial. Failed block is the most common problem in paediatric regional anaesthesia when neuraxial blocks are performed without the use of an imaging technique (Polaner 2012), and inadvertent vascular puncture occurs in 2% (95% CI 12 to 21 per 1000) of children undergoing neuraxial block. Ultrasound may decrease inadvertent vascular puncture (Walker 2009). Thus ultrasound guidance for regional anaesthesia in children may improve the success rate while decreasing the rate of complications.
Why it is important to do this review
The use of ultrasound guidance for regional anaesthesia has become popular over the past two decades. However, ultrasound is not recognized by all experts as an essential tool. Indeed, many authorities believe that no actual evidence suggests that ultrasound guidance would decrease the occurrence of important complications such as neurological damage (Neal 2008). A Cochrane review determined that ultrasound guidance appeared to reduce the incidence of vascular puncture or haematoma formation in adults but yielded a similar success rate (Walker 2009). The cost of an ultrasound machine varies, but most machines used in the clinical practice of regional anaesthesia cost approximately USD 40,000 or more (Liu 2010). Thus, equipment required to use ultrasound guidance is substantially more expensive than other tools, such as those used for nerve stimulation, which can be acquired for approximately USD 1000 or less (Liu 2010). In 2010, a comprehensive review of the paediatric literature concluded that additional outcome‐based, prospective, randomized controlled trials (RCTs) were needed to prove the benefits of ultrasound guidance over conventional methods in children (Tsui 2010).
Objectives
To determine whether ultrasound guidance offers any clinical advantage when neuraxial and peripheral nerve blocks are performed in children in terms of increasing the success rate or decreasing the rate of complications.
Methods
Criteria for considering studies for this review
Types of studies
We included all parallel RCTs that evaluated the effect of ultrasound guidance when a regional blockade technique was performed in children and that included any of our selected outcomes.
We excluded observational studies, quasi‐randomized trials, cross‐over trials and cluster‐randomized trials.
We excluded no studies on the basis of language of publication or publication status.
Types of participants
We included studies performed in children (≤ 18 years of age) undergoing any type of surgical procedure (open or laparoscopic) for which a neuraxial (spinal, epidural, caudal or combined spinal and epidural) or peripheral nerve block (any peripheral nerve block including fascial (fascia iliaca, transversus abdominis plane, rectus sheath blocks) or perivascular blocks), for surgical anaesthesia (alone or in combination with general anaesthesia) or for postoperative analgesia, was performed with ultrasound guidance.
We excluded studies in which regional blockade was used to treat chronic pain.
Types of interventions
We included studies in which ultrasound guidance was used to perform the technique in real time (in‐plane or out‐of‐plane), as pre‐scanning before the procedure or to evaluate the spread of the local anaesthetic so the position of the needle could be adjusted or the block complemented. For control groups, any other technique used to perform the block including landmarks, loss of resistance (air or fluid), click, paraesthesia, nerve stimulator, transarterial or infiltration was accepted.
We discarded no studies on the basis of the specific technique used as the comparator.
Types of outcome measures
We evaluated differences between treatment and control groups based on the following outcomes.
Primary outcomes
Success rate (study author's definition).
Pain scores in the post‐anaesthesia care unit (PACU).
Block duration (study author's definition).
Secondary outcomes
Time to perform the procedure.
Number of needle passes.
Minor complications (bloody puncture).
Major complications: local anaesthetic toxicity (signs of systemic toxicity including seizure or cardiac arrest), infection, neurological injury (transient or lasting longer than one month).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (Appendix 1; 2015, Issue 3), MEDLINE (OvidSP) (from 1946 to March 2015; Appendix 2), EMBASE (OvidSP) (from 1982 to March 2015; Appendix 3) and Scopus (from inception to 27 January 2015) (Appendix 4).
Searching other resources
We looked at http://www.clinicaltrials.gov (January 2015), http://isrctn.org (January 2015), http://www.umin.ac.jp/ctr/index.htm (January 2015),
http://www.anzctr.org.au (January 2015), http://www.trialregister.nl/ (January 2015) and https://udract.ema.europa.eu/ (January 2015) to identify trials in progress.
We screened the reference lists of all studies retained (during data extraction) and of the recent meta‐analysis or reviews related to the topic (March 2015). We screened conference proceedings of anaesthesiology societies for 2012, 2013 and 2014, published in three major anaesthesiology journals: British Journal of Anaesthesiology (January 2015),European Journal of Anaesthesiology (January 2015) and Regional Anesthesia and Pain Medicine (January 2015). We also looked for abstracts on the Website of the American Society of Anesthesiologists for the same years (2012 through 2014) (January 2015).
Data collection and analysis
Selection of studies
We (JG and SK) screened the list of all titles and abstracts identified by the search above. We retrieved and independently read potential articles for inclusion to determine their eligibility. We resolved discrepancies by discussion without the need for help from the third review author (SS). We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), We listed all reasons for exclusion in the Characteristics of excluded studies table.
Data extraction and management
We selected studies, extracted data (assessment of risk of bias in included studies; types of outcome measures; assessment of heterogeneity) and entered the data onto our data extraction sheet. We entered first the site where the study was performed and the date of data collection (to facilitate exclusion of duplicate publications), then whether the study was included in the review or the reason for rejection. After we reached agreement, one review author (JG) entered the data and the moderators for heterogeneity exploration into Comprehensive meta‐analysis (https://www.meta‐analysis.com/). Also, after agreement was reached, the same review author (JG) entered the risk of bias evaluation into RevMan. We resolved disagreements by discussion and contacted study authors to request additional information when required. We then transferred data for analysis to RevMan in the format required to include the maximal number of studies (events and total number of participants for each group; mean, standard deviation and number of participants included in each group; or generic inverse variance if necessary). When possible, we entered the data into an intention‐to‐treat (ITT) analysis.
Assessment of risk of bias in included studies
We assessed the quality of the included studies by using the tool of The Cochrane Collaboration found in RevMan 5.3 (Higgins 2011). We resolved disagreements by discussion. We considered a trial as having low risk of bias if we assessed all of the following criteria as adequate, and as having high risk of bias if one or more of the criteria were assessed as inadequate. We assessed the risk of bias based on the basis of information presented in the reports, with no assumptions made.
Generation of the allocation sequence of interventions: We considered randomization adequate if it was generated by a computer or by a random number table algorithm. We judged other processes, such as tossing of a coin, adequate if the whole sequence was generated before the start of the trial. We considered the trial as quasi‐randomized if a non‐random system, such as dates, names or identification numbers, was used.
Concealment of allocation: We considered concealment adequate if the process that was used prevented patient recruiters, investigators and participants from knowing the intervention allocation of the next participant to be enrolled in the study. We considered concealment inadequate if the allocation method allowed patient recruiters, investigators or participants to know the treatment allocation of the next participant to be enrolled in the study.
Blinding of participants and personnel: We considered blinding adequate if both the participant and personnel taking care of the participant were blinded to the intervention. We considered blinding inadequate if participants or personnel were not blinded to the intervention.
Blinding of outcome assessment: We considered blinding adequate if the outcome assessor was blinded to the intervention. We considered blinding inadequate if the outcome assessor was not blinded to the intervention.
Incomplete outcome data (attrition bias): We considered the trial adequate if all dropouts or withdrawals were accounted for, the number of dropouts was small (< 20%) and similar for both interventions and reasons for dropping out of participants sounded reasonable. We considered the trial inadequate if reasons for dropping out of the patient were not stated or did not sound reasonable, the number was high (≥ 20%) or the number differed greatly between groups.
Selective reporting (reporting bias): We considered a trial as having low risk of bias if all measurements stated in the Methods section were included in the Results, and as having high risk if only a portion of the results mentioned in the Methods section were given in the Results section. We considered per‐protocol results (not intention‐to‐treat (ITT)) as selective reporting.
Any other risk of bias: We considered any other reason that may have influenced study results. We considered an apparent conflict of interest as representing a risk of bias.
Measures of treatment effect
We reported results as risk differences (RD) and 95% confidence intervals (95% CIs) for dichotomous data (success rate, minor and major complications) and as mean differences (MDs) and 95% CIs for continuous data (pain scores, block duration, time to perform the procedure) as much as was feasible. If some of the continuous data were given on different scales (pain scores), or if results were provided with P values (number of attempts or needle passes), we presented the results as standardized mean differences (SMDs) and 95% CIs. For SMDs, we considered 0.2 a small effect, 0.5 a medium effect and 0.8 a large effect (Pace 2011). When an effect was noted, a number needed to treat for an additional beneficial outcome (NNTB) or a number needed to treat for an additional harmful outcome (NNTH) was calculated from the odds ratio. We gave results for dichotomous data as risk ratios (RRs or RDs) as the odds ratio (OR) is not easily understood by clinicians (Deeks 2002; McColl 1998). We used the OR for calculation of the NNTB and NNTH (http://www.nntonline.net/visualrx/), as this value is less likely to be affected by the side (benefit or harm) on which data are entered (Cates 2002; Deeks 2002). When no effect was noted, we calculated the optimal information size to make sure that enough participants were included in the retained studies to justify a conclusion on the absence of effect (Pogue 1998) (http://www.stat.ubc.ca/˜rollin/stats/ssize/b2.html). We considered a difference of 15% (increase or decrease) as the minimal clinically relevant difference.
Unit of analysis issues
We included only parallel‐group trials. If a study contained more than two groups, we fused two groups (by using the appropriate formula for adding standard deviations when required) if we thought that they were equivalent according to the criteria of our protocol (taking our factors for heterogeneity exploration into account), or we separated them and split the control group in half if we thought that they were different.
Dealing with missing data
We contacted study authors to ask for apparent missing data. We did not consider medians as equivalent to means. Instead, we used the P value and the number of participants included in each group to calculate the effect size. We did not use imputed results. We entered data as ITT data as much as was feasible. If we could not do this, we assigned the study as having high risk of bias for selective reporting and entered the data on a per‐protocol basis.
Assessment of heterogeneity
We considered clinical heterogeneity before pooling results and examined statistical heterogeneity before carrying out any meta‐analysis. We quantified statistical heterogeneity by using the I2 statistic and entered data in the way (benefit or harm) that yielded the least heterogeneity. We quantified the amount of heterogeneity as low (< 25%), moderate (50%) or high (75%), depending on the value obtained for the I2 statistic (Higgins 2003).
Assessment of reporting biases
We examined publication bias by using a funnel plot followed by Duval and Tweedie’s trim and fill technique for each outcome. Publication bias is the risk of bias introduced by the possibility that medical journals publish studies favouring one treatment more often than studies favouring another. When no publication bias and no small‐study effect are noted, if a graph is constructed with standard error or precision (1/standard error) on the y‐axis and the logarithm of the OR on the x‐axis, studies should be equally distributed on both sides of a vertical line passing through the effect size found (log odds ratio). The entire graph should have the shape of a reversed funnel. Duval and Tweedie’s trim and fill analysis corrects the asymmetry by removing extremely small studies from the positive side (re‐computing the effect size at each iteration until the funnel plot is symmetrical around the new effect size). The algorithm then adds the original studies back into the analysis and imputes a mirror image for each. The latter step does not modify the ’new effect size’ but corrects the variance, which was falsely reduced by the first step. Duval and Tweedie’s trim and fill analysis yields an estimate of what would be the effect size (OR, RR, etc) if no publication bias was present.
Data synthesis
We analysed the data by using RevMan 5.3 and Comprehensive Meta‐Analysis Version 2.2.044 (www.Meta‐Analysis.com) with fixed‐effect models for comparisons with a low level of heterogeneity as assessed by the I2 statistic (I2 < 25%) and random‐effects models for comparisons containing a moderate or high amount of heterogeneity (I2 ≥ 25%) (Higgins 2003). Fixed‐effect and random‐effects models provide the same results in the absence of statistical heterogeneity (I2 = 0%). When statistical heterogeneity is noted, random‐effects models usually widen the CI, thus decreasing the chance of finding an effect when no effect is present. However, they may increase the weight of smaller studies. We presented the characteristics of included and excluded studies in the Characteristics of included studies and Characteristics of excluded studies tables. We presented the risk of bias assessment in a risk of bias graph. We presented results for each comparison as forests plots when appropriate. For comparisons with fewer than two available studies, and for those that included a moderate or high level of heterogeneity after heterogeneity exploration, we provided the results in narrative format.
Subgroup analysis and investigation of heterogeneity
We explored any amount of heterogeneity, but we focused more specifically on comparisons with more than a small amount of heterogeneity (I2 ≥ 25%) (Higgins 2003), and we explored the heterogeneity by applying Egger’s regression intercept (to assess the possibility of a small‐study effect; Rucker 2011) and by performing visual inspection of the forest plots with studies placed in order according to a specific moderator, subgroupings (categorical moderators) or meta‐regressions (continuous moderators). We considered the following factors when exploring heterogeneity: type of block (neuraxial vs peripheral nerve block), type of comparator (nerve stimulator vs other), age and type of guidance (pre‐scanning vs real‐time (in‐plane or out‐of‐plane)) and combined methods (ultrasound plus nerve stimulator compared with other modalities vs ultrasound alone compared with other modalities).
Sensitivity analysis
A sensitivity analysis (based mainly on the risk of bias assessment (allocation concealment and blinding of the assessor) or on an outlier) could also be performed for results with heterogeneity.
Summary of findings
We used the principles of the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system (Guyatt 2008) to assess the quality of the body of evidence associated with the following specific outcomes in our review.
Success rate.
Pain scores in PACU.
Block duration.
Time to perform the procedure.
Number of needle passes.
Minor complications.
We constructed a 'Summary of findings' (SoF) table using GRADE software (http://tech.cochrane.org/revman/gradepro).
The GRADE approach appraises the quality of a body of evidence on the basis of the extent to which one can be confident that an estimate of effect or association reflects the item assessed. The quality of a body of evidence reflects within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates, amplitude of the effect size and risk of publication bias.
For risk of bias, we judged the quality of evidence as adequate when most information was derived from studies at low risk of bias; we downgraded the quality by one level when most information was provided by studies at high or unclear risk of bias and we downgraded the quality by two levels when the proportion of information from studies at high risk of bias was sufficient to affect interpretation of results. For inconsistency, we downgraded the quality of evidence by one level when the I2 statistic was 50% or higher without satisfactory explanation, and by two levels when the I2 statistic was 75% or higher with no explanation. We did not downgrade the quality of evidence for indirectness, as all outcomes were based on direct comparisons, were performed on the population of interest and were not surrogate markers (Guyatt 2011a). For imprecision (Guyatt 2011b), we downgraded the quality of evidence by one level when the confidence interval around the effect size was large or overlapped an absence of effect and failed to exclude an important benefit or harm; when the number of participants was lower than the optimal information size (unless the sample size was ≥ 2000 participants or the number of events included was ≥ 400); and we downgraded the quality by two levels when the confidence interval was very wide and included both appreciable benefit and harm. For publication bias, we downgraded the quality of evidence by one level when correcting for the possibility of publication as assessed by Duval and Tweedie’s fill and trim analysis changed the conclusion. We upgraded the quality of evidence by one level when the effect size was large (risk ratio < 0.5 or > 2.0), and by two levels when the effect size was very large (RR < 0.2 or > 5.0) (Guyatt 2011c). For SMD, we used 0.8 as the cutoff point for a large effect (Pace 2011). We also upgraded the quality by one level when evidence of a dose‐related response was found. We upgraded the quality by one level when possible effect of confounding factors would reduce a demonstrated effect or would suggest a spurious effect when results show no effect. When the quality of the body of evidence is high, further research is very unlikely to change our confidence in the estimate of effect. When the quality is moderate, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. When the quality is low, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. When the quality is very low, any estimate of effect is very uncertain (Guyatt 2008).
Results
Description of studies
Results of the search
Upon completing the electronic search, we screened 640 titles/abstracts. Among these abstracts or from the reference list of potentially relevant studies, we found 29 trials that met our criteria for inclusion. Seven are ongoing trials (Characteristics of ongoing studies), one studied a different intervention and one was withdrawn by the study authors because of a problem with data collection (Characteristics of excluded studies). Therefore, we retained 20 studies (Figure 1) for this review.
Figure 1.
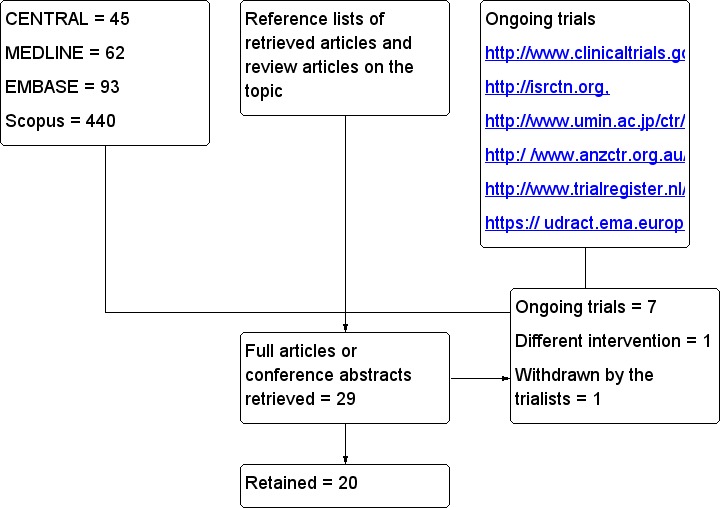
Flow diagram. Search results.
Included studies
The 20 studies included 1241 participants: in 624, the block was performed with ultrasound, and in 617, it was performed without ultrasound. The mean age of participants ranged from 0.9 to nine years.
The following surgeries were performed: circumcision (Faraoni 2010; O'Sullivan 2011), umbilical hernia repair (Dingeman 2013; Flack 2014; Gurnaney 2011), inguinal hernia repair (Kendigelen 2014; Nan 2012; Sahin 2013; Wang 2013; Weintraud 2009), inguinal hernia/orchidopexy (Willschke 2005), low urological/perineal surgery (Liu 2012), open pyeloplasty (Lorenzo 2014), major abdominal or thoracic surgery (Willschke 2006), the Nuss procedure for pectus excavatum (Tachibana 2012) and upper (Elnour 2009; Marhofer 2004; Ponde 2009) or lower limb surgery (Oberndorfer 2007; Ponde 2013).
The following blocks were performed: brachial plexus block (Elnour 2009; Marhofer 2004; Ponde 2009), sciatic and femoral nerve blocks (Oberndorfer 2007; Ponde 2013), ilioinguinal/iliohypogastric nerve blocks (Nan 2012; Weintraud 2009; Willschke 2005), penile nerve block (Faraoni 2010; O'Sullivan 2011), rectus sheath block (Dingeman 2013; Flack 2014; Gurnaney 2011), transversus abdominis plane blocks (Kendigelen 2014; Lorenzo 2014; Sahin 2013), thoracic epidural (Tachibana 2012), thoracic or lumbar epidural (Willschke 2006) and caudal blocks (Liu 2012; Wang 2013).
Ultrasound guidance was used in real time with an in‐plane (Elnour 2009; Faraoni 2010; Flack 2014; Gurnaney 2011; Lorenzo 2014; O'Sullivan 2011; Ponde 2009; Ponde 2013; Sahin 2013), out‐of‐plane (Marhofer 2004; Nan 2012; Oberndorfer 2007; Wang 2013; Weintraud 2009; Willschke 2005; Willschke 2006) or unspecified technique (Dingeman 2013; Kendigelen 2014), or as pre‐scanning (Liu 2012; Tachibana 2012). Ultrasound guidance was compared with infiltration (Dingeman 2013; Flack 2014; Gurnaney 2011; Kendigelen 2014; Lorenzo 2014), with landmarks (Faraoni 2010; Liu 2012; Nan 2012; O'Sullivan 2011; Sahin 2013; Tachibana 2012; Wang 2013; Weintraud 2009; Willschke 2005; Willschke 2006) or with a nerve stimulator (Elnour 2009; Marhofer 2004; Oberndorfer 2007; Ponde 2009; Ponde 2013).
The source of funding was a government organization (Flack 2014; Nan 2012), a charitable organization (Dingeman 2013) or an institutional department (Faraoni 2010; O'Sullivan 2011; Ponde 2013; Wang 2013), or the source was unspecified (Elnour 2009; Gurnaney 2011; Kendigelen 2014; Liu 2012; Lorenzo 2014; Marhofer 2004; Oberndorfer 2007; Ponde 2009; Sahin 2013; Tachibana 2012; Weintraud 2009). Two studies declared that they received help from the industry (equipment loan; Willschke 2005; Willschke 2006).
Excluded studies
We excluded one study (Triffterer 2012), which evaluated the cranial spread of caudally administered local anaesthetics in infants and children by means of real‐time ultrasonography. Ultrasound was used for all participants; therefore the study included no comparator, as was required by our inclusion criteria (see Characteristics of excluded studies).
Ongoing studies
We found seven ongoing trials (ACTRN12608000488303; ACTRN12613000595718; AT032012; NCT01136668; NCT01698268; NCT02321787; NCT02341144) that could fit our criteria for inclusion (Characteristics of ongoing studies).
Awaiting classification
No studies are awaiting classification.
Risk of bias in included studies
The risk of bias of the retained studies can be found in Figure 2 and Figure 3. Kendigelen 2014 was available as an abstract only. When information available in the report was insufficient, we rated the item as having high risk for blinding and as having unclear risk for all other items.
Figure 2.
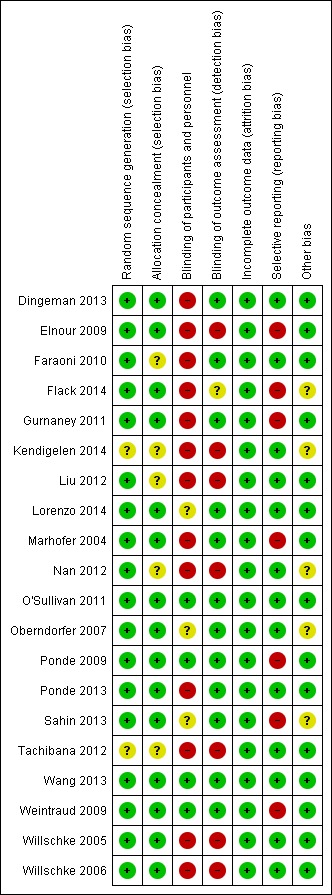
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Figure 3.
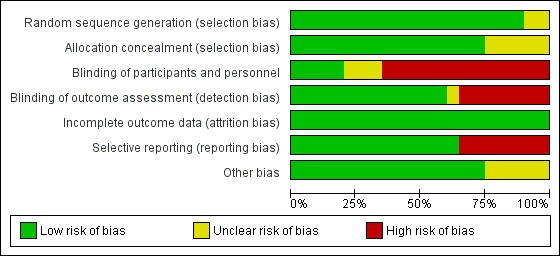
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
We judged the sequence of randomization as appropriate for all studies except Kendigelen 2014 (abstract only) and Tachibana 2012 (for which no details were provided). Allocation concealment was judged as adequate for almost 75% of the included studies (Dingeman 2013; Elnour 2009; Flack 2014; Gurnaney 2011; Lorenzo 2014; Marhofer 2004; O'Sullivan 2011; Oberndorfer 2007; Ponde 2009; Ponde 2013; Sahin 2013; Wang 2013; Weintraud 2009; Willschke 2005; Willschke 2006). We rated this item as having unclear risk for the remaining six studies, one of which was available only as an abstract (Kendigelen 2014).
Blinding
We judged blinding of personnel taking care as adequate for less than 25% of the included studies (O'Sullivan 2011; Ponde 2009; Wang 2013; Weintraud 2009). We judged blinding of outcome assessors as adequate for over 50% of the studies (Dingeman 2013; Faraoni 2010; Gurnaney 2011; Lorenzo 2014; Marhofer 2004; O'Sullivan 2011; Oberndorfer 2007; Ponde 2009; Ponde 2013; Sahin 2013; Wang 2013; Weintraud 2009).
Incomplete outcome data
We judged all studies as adequate for this item.
Selective reporting
Data were not reported in intention‐to‐treat for more than 50% of the studies (Elnour 2009; Flack 2014; Gurnaney 2011; Marhofer 2004; Ponde 2009; Sahin 2013; Weintraud 2009). For three studies (Flack 2014; Marhofer 2004; Ponde 2009), pain scores were measured but were not provided.
Other potential sources of bias
We judged more than 75% of the studies (Dingeman 2013; Elnour 2009; Faraoni 2010; Gurnaney 2011; Liu 2012; Lorenzo 2014; Marhofer 2004; O'Sullivan 2011; Ponde 2009; Ponde 2013; Tachibana 2012; Wang 2013; Weintraud 2009; Willschke 2005; Willschke 2006) as exempt from other risks of bias.
Effects of interventions
See: Table 1
Primary outcomes
Success rate (study authors’ definition)
This outcome was available for 14 studies (Elnour 2009; Faraoni 2010; Liu 2012; Marhofer 2004; Nan 2012; O'Sullivan 2011; Oberndorfer 2007; Ponde 2009; Ponde 2013; Tachibana 2012; Wang 2013; Weintraud 2009; Willschke 2005; Willschke 2006) (939 participants). We included in Table 3 the definition used by study authors for failed blocks. Data are presented as 'decreased failure rate'. Ultrasound guidance decreased the failure rate (RD ‐0.11, 95% CI ‐0.17 to ‐0.05), with I2 statistic of 64% (Analysis 1.1). Egger's regression intercept showed the possibility of a small‐study effect (P value = 0.02; two‐sided test). Duval and Tweedie's trim and fill analysis showed no evidence of publication bias. This effect differed from one subgroup to another (Analysis 1.1; I2 = 67%). For peripheral nerve blocks, the effect was inversely proportional to the age of the participant; younger children benefited most from ultrasound guidance (P value = 0.003; Figure 4). If a failure rate of 25% is assumed (incidence in the study population; Table 1), the NNTB for peripheral nerve blocks would be six (95% CI 5 to 8). The optimal information size for a large trial for a 25% decrease in failure rate would be 1724 (862 per group) (alpha 0.05; beta 0.2; one‐sided test).
Table 1.
Definitions used by study authors for successful blockade
| Study | Type of block | Timing of blockade | Definition |
| Elnour 2009 | Axillary brachial plexus block | Under general anaesthesia before surgical incision in both groups | The procedure was considered a failure when:
|
| Faraoni 2010 | Penile nerve block | Under general anaesthesia before surgical incision in both groups | Ineffective block was defined as an increase in heart rate and mean arterial pressure > 20% above baseline values |
| Liu 2012 | Caudal block | Under general anaesthesia 10 to 15 minutes before surgical incision in both groups | Unsuccessful caudal puncture after 4 attempts (n = 2 in the control group) or signs of pain, such as body movement, tachycardia and tachypnoea during surgery |
| Marhofer 2004 | Infraclavicular brachial plexus block | Propofol sedation, 30 minutes before surgical incision in both groups | Procedure was considered a failure if ≥ 2 of the 4 nerves (ulnar, radial, median and musculocutaneous) could not be blocked effectively |
| Nan 2012 | Ilioinguinal and iliohypogastric nerve blocks | Under general anaesthesia before surgical incision in both groups | Inadequate analgesia was defined as an increase of heart rate > 10% of baseline level, which needed to elevate the sevoflurane concentration to 3% to 4% during surgery |
| O'Sullivan 2011 | Penile nerve block | Under general anaesthesia ≥ 10 minutes before surgical incision in both groups | Procedure was considered a failure if a rise in heart rate or respiratory rate > 25% from baseline occurred in response to surgical stimulus |
| Oberndorfer 2007 | Sciatic and femoral nerve blocks | Under general anaesthesia ≥ 20 minutes before surgical incision in both groups | Procedure was considered a failure if a rise in heart rate > 15% of baseline value occurred at skin incision or during surgery |
| Ponde 2009 | Infraclavicular brachial plexus block | Under general anaesthesia before surgical incision in both groups | Procedure was considered a failure if a pain response to surgical stimulus occurred, defined as an increase in heart rate and arterial blood pressure > 20% of basal rate or non‐specific body movement in response to surgical stimulus and withdrawal of blocked limb in response to incision |
| Ponde 2013 | Sciatic and femoral nerve blocks | Under general anaesthesia ≥ 20 minutes before surgical incision in both groups | Procedure was considered a failure when:
|
| Tachibana 2012 | Thoracic epidural anaesthesia | Under general anaesthesia before surgical incision in both groups | Procedure was considered a failure if a participant complained of severe postoperative pain despite sufficient epidural administration of local anaesthetics |
| Wang 2013 | Caudal block | Under general anaesthesia ≥ 15 minutes before surgical incision in both groups | Procedure was considered a failure if a participant had motor or haemodynamic response, as indicated by an increase in mean arterial pressure or heart rate > 15% compared with baseline values obtained just before skin incision and subsequent to surgical procedure |
| Weintraud 2009 | Ilioinguinal and iliohypogastric nerve blocks | Under general anaesthesia ≥ 15 minutes before surgical incision in both groups | Procedure was considered a failure if participant had an increase in heart rate or mean arterial blood pressure > 10% compared with baseline during operation |
| Willschke 2005 | Ilioinguinal and iliohypogastric nerve blocks | Under general anaesthesia ≥ 15 minutes before surgical incision in both groups | Procedure was considered a failure if participant had an increase in heart rate or mean arterial pressure > 10% after skin incision or during surgery |
| Willschke 2006 | Thoracic or lumbar epidural anaesthesia | Under general anaesthesia ≥ 15 minutes before surgical incision in both groups | An increase in heart rate or blood pressure > 20% from baseline was considered to reflect inadequate analgesia and was managed by bolus administration of levobupivacaine 0.25% 0.3 millilitres per kilogram of body weight through the epidural catheter. If this was unsuccessful, the epidural block was considered to have failed |
Analysis 1.1.
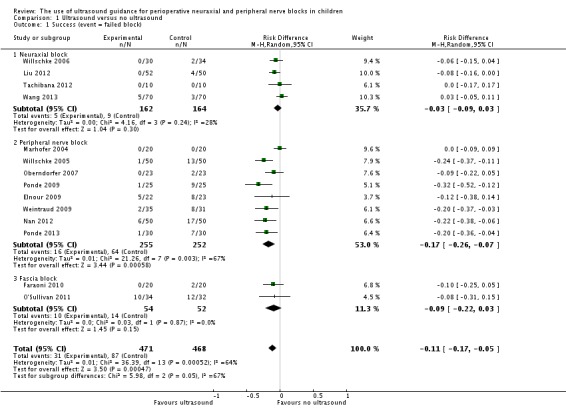
Comparison 1 Ultrasound versus no ultrasound, Outcome 1 Success (event = failed block).
Figure 4.
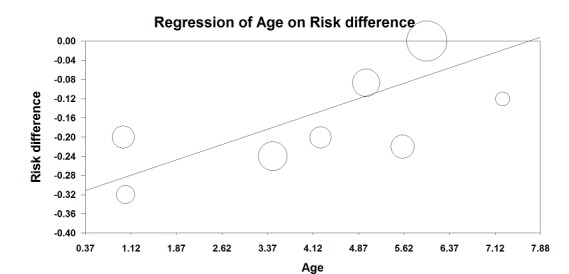
Occurence of a failed peripheral nerve block for ultrasound guidance versus no ultrasound (log risk ratio of failure vs age in years). The superiority of ultrasound guidance was greater in younger children; P value = 0.003.
We retained only studies on peripheral nerve block for quality of evidence for this outcome. We did not downgrade the evidence for risk of bias because 50% or more of the included studies were judged as adequate for allocation concealment and blinding of outcome assessors. We did not downgrade the quality for inconsistency because we could explain the heterogeneity (Analysis 1.1; Figure 4). We used only direct comparisons and downgraded the level by one for imprecision due to a low number of participants (below the optimal information size) (Guyatt 2011). We found no evidence of publication bias. We upgraded the evidence for a large effect size and for a dose‐response effect for success rate when ultrasound guidance was used for peripheral nerve blocks (Figure 4; the younger the child, the greater was the effect size). We found no reason to upgrade the level of evidence for confounding factors. We rated the level of evidence for this item as high.
Pain scores in the post‐anaesthesia care unit (PACU)
Pain scores at one hour in the PACU were available for eight studies (Dingeman 2013; Faraoni 2010; Gurnaney 2011; Lorenzo 2014; Oberndorfer 2007; Ponde 2013; Willschke 2005; Willschke 2006) (414 participants). We did not find differences for pain scores at one hour in the PACU (SMD ‐0.20, 95% CI ‐0.52 to 0.13; I2 = 62%) when all studies were included (Analysis 1.2). Upon exclusion of one study (Lorenzo 2014), in which a transversus abdominis plane block was used for open pyeloplasty, ultrasound guidance would decrease pain scores in the PACU at one hour (SMD ‐0.29, 95% CI ‐0.54 to ‐0.04; I2 = 31%). Egger's regression intercept showed no evidence of a small‐study effect, and Duval and Tweedie's trim and fill analysis showed no evidence of publication bias. When the study at lowest risk of bias among the studies for which pain scores were available as means and standard deviations (Ponde 2013; standard deviation in the control group 0.9) is considered, the mean reduction in pain at one hour in the PACU found in our meta‐analysis would be equivalent to 0.2 on the Children’s and Infants’ Postoperative Pain Scale (CHIPPS scale; 0 = no pain, 10 = maximal pain; Buttner 2000). On the basis of Gurnaney 2011 (mean value 4.35; standard deviation 3.1 for the control group), 238 (119 per group) would be required for a large trial, eliminating a difference of 1 on a score from 0 to 10 (alpha 0.05; beta 0.2; one‐sided test).
Analysis 1.2.
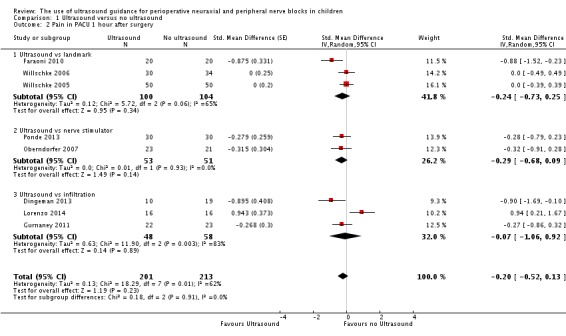
Comparison 1 Ultrasound versus no ultrasound, Outcome 2 Pain in PACU 1 hour after surgery.
For pain scores in the PACU at one hour, we did not downgrade the evidence for risk of bias because 50% or more of the included studies were judged as adequate for allocation concealment and blinding of outcome assessors. We did not downgrade for inconsistency because the amount of heterogeneity was low (I2 = 31%) after exclusion of one study in which the type of block used might have not been ideal for the type of surgery performed (Lorenzo 2014). We used direct comparisons only. The optimal information size was achieved; therefore, we did not downgrade for imprecision. No evidence of publication bias, large effect size or dose response was found, and we did not identify other significant confounding factors to justify upgrading. We rated the level of evidence for this outcome as high.
Block duration (study authors’ definition)
This outcome was available for eight studies (358 participants) (Faraoni 2010; Flack 2014; Gurnaney 2011; Lorenzo 2014; Marhofer 2004; Oberndorfer 2007; Ponde 2013; Sahin 2013). Ultrasound guidance increased block duration (SMD 1.21, 95% CI 0.76 to 1.65; I2 = 73% (Analysis 1.3). Egger's regression intercept showed no statistically significant evidence of a small‐study effect. Duval and Tweedie's trim and fill analysis showed that one study might be missing to the right of mean for an adjusted point of estimate (1.31, 95% CI 0.87 to 1.75; random‐effects model). This effect was inversely proportional to age (P value = 0.04); younger children benefited most from ultrasound guidance (Figure 5). The mean prolongation of the block found in our meta‐analysis is equivalent to 62 minutes. On the basis of Ponde 2013 (mean time and standard deviation before request of the first analgesic in the control group 457 ± 51 minutes), eight participants (four per group) would be required in a simple trial to eliminate a 25% difference (alpha 0.05; beta 0.2; two‐sided test).
Analysis 1.3.
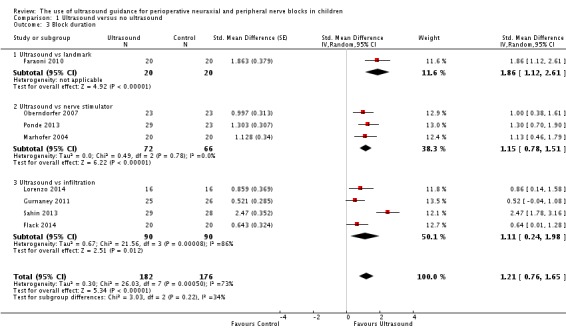
Comparison 1 Ultrasound versus no ultrasound, Outcome 3 Block duration.
Figure 5.
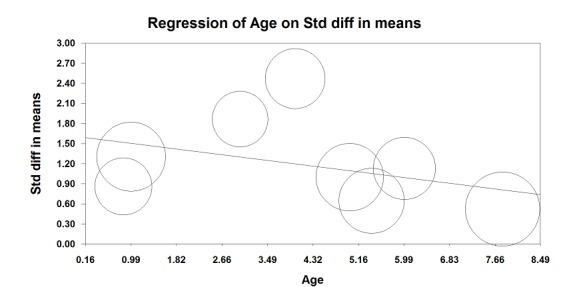
Meta‐regression: time to request for the first analgesic versus age. Younger children are those in whom the effect of ultrasound guidance is larger (P value = 0.04).
For block duration, we did not downgrade the evidence for risk of bias because 50% or more of the included studies were judged as adequate for allocation concealment and blinding of outcome assessors. We did not downgrade the evidence for inconsistency because we could explain the heterogeneity (Analysis 1.3; Figure 5). We included only direct comparisons. The optimal information size was achieved; therefore, we did not downgrade for imprecision. We did not downgrade for publication bias because correcting for this possibility would not change the conclusion. We upgraded the evidence by one because we found a large effect size (SMD 1.21 or > 0.8), and we upgraded the evidence because we found a dose‐response gradient (Figure 5; the younger the child, the larger was the effect size). We did not identify other significant confounding factors to justify upgrading. We rated the level of evidence for this item as high.
Secondary outcomes
Time to perform the procedure
This outcome was available for six studies (Elnour 2009; Liu 2012; O'Sullivan 2011; Tachibana 2012; Wang 2013; Willschke 2006) (362 participants). We found no differences between treatment groups when all studies were included in the analysis (SMD ‐0.77, 95% CI ‐1.57 to 0.02; I2 = 93%), and Egger's regression intercept showed no significant evidence of a small‐study effect. Duval and Tweedie's trim and fill analysis showed the possibility of publication bias for an adjusted point of estimate of SMD ‐1.10 (95% CI ‐2.04 to ‐0.17). Ultrasound guidance decreased the time required to perform the block only when used as an out‐of‐plane technique (SMD ‐0.68, 95% CI ‐0.96 to ‐0.40; I2 = 0%) or as pre‐scanning before a neuraxial block (SMD ‐1.97, 95% CI ‐2.41 to ‐1.54; I2 = 0%) (Analysis 1.4). A significant difference was noted among the three subgroups (I2 = 93%). This difference between ultrasound guidance versus no ultrasound guidance was equivalent to 94 seconds for an out‐of‐plane technique and 2.4 minutes when ultrasound guidance was used as pre‐scanning before a neuraxial block was performed. Based on Liu 2012 (mean time and standard deviation of the control group 3.2 ± 1.2 minutes), 46 participants (23 per group) would have been required, to eliminate a one‐minute difference (alpha 0.05; beta 0.2; two‐sided test).
Analysis 1.4.
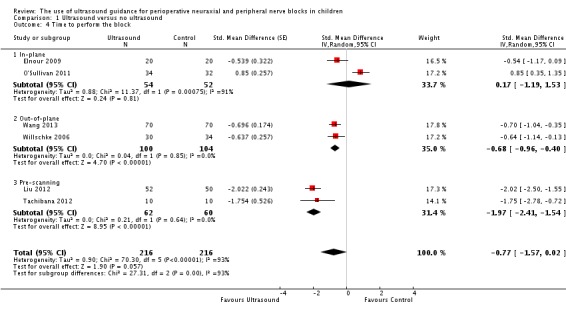
Comparison 1 Ultrasound versus no ultrasound, Outcome 4 Time to perform the block.
For time to perform the procedure, we rated the quality for pre‐scanning before a neuraxial block. We downgraded the level of evidence for risk of bias because for the two studies retained, allocation concealment was unclear and blinding of the outcome assessor was not feasible for this outcome. We found no significant inconsistency (I2 < 25%). We included only direct comparisons and did not downgrade the level of evidence for imprecision because the optimal information size was achieved. Publication bias could not be assessed. We upgraded the quality of evidence because the effect size was large (SMD ‐1.97 or > 0.8). We did not identify significant confounding factors to justify upgrading or dose response. We rated the level of evidence as high.
Number of needle passes
This outcome was available for only two studies (Liu 2012; Tachibana 2012) (122 participants). Ultrasound guidance reduced the number of needle passes (SMD ‐0.90, 95% CI ‐1.27 to ‐0.52; I2 = 0%) (Analysis 1.5). This difference was equivalent to a mean of 0.6 fewer needle passes per participant. On the basis of Liu 2012 (mean 1.6 and standard deviation 0.6), a trial would have to include 12 participants (six per group) to eliminate a difference of one needle pass (alpha 0.05; beta 0.2; two‐sided test).
Analysis 1.5.
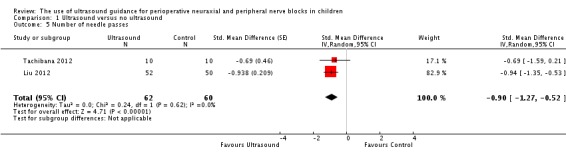
Comparison 1 Ultrasound versus no ultrasound, Outcome 5 Number of needle passes.
For number of needle passes, we downgraded the level of evidence for risk of bias because for the two studies retained, allocation concealment was unclear and blinding of the outcome assessor was not feasible for this outcome. We found no significant inconsistency (I2 < 25%) and included only direct comparisons. We did not downgrade the level of evidence for imprecision because the optimal information size was achieved, and we upgraded the evidence because the effect size was large (SMD ‐0.90 or > 0.8). We did not identify significant confounding factors to justify upgrading or dose response. We rated the level of evidence as high.
Minor complications (bloody puncture)
This outcome was available for six studies (Marhofer 2004; Nan 2012; Oberndorfer 2007; Wang 2013; Willschke 2005; Willschke 2006) (490 participants). Ultrasound did not decrease the incidence of bloody punctures (RD ‐0.02, 95% CI ‐0.06 to 0.02; I2 = 53%; Analysis 1.6). Eger's regression intercept showed no evidence of a small‐study effect, and Duval and Tweedie's trim and fill analysis showed the possibility of publication bias for an adjusted point of estimate (RD ‐0.04, 95%CI ‐0.04 to 0.01) (random‐effects model). Only two trials studied the effect of ultrasound for neuraxial blocks (Wang 2013; Willschke 2006) and showed a moderate amount of heterogeneity (RD ‐0.07, 95% CI ‐0.19 to 0.04; I2 = 68%). Given a basal rate of 13.5% for bloody puncture during neuraxial block in children, the optimal information size for a large trial for a 25% decrease would be 2226 (1113 per group).
Analysis 1.6.
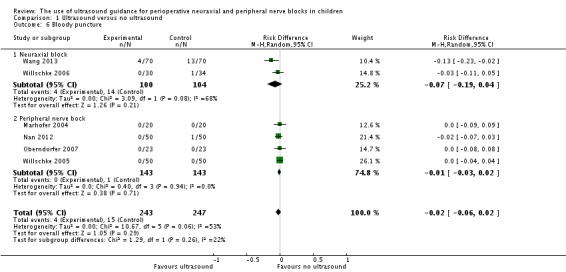
Comparison 1 Ultrasound versus no ultrasound, Outcome 6 Bloody puncture.
For bloody punctures, we did not downgrade the evidence for risk of bias because 50% or more of the included studies were judged as adequate for allocation concealment and blinding of outcome assessors. We downgraded the quality by one for inconsistency (I2 > 50%). We used only direct comparisons and downgraded the level by one for imprecision due to a low number of participants (below the optimal information size). We did not downgrade the level for publication bias because applying a correction would not modify the conclusion. We found no evidence of a large effect size or dose response gradient, and we did not identify any significant confounding factors to justify upgrading. We rated the level of evidence as low.
Major complications
No major complications were reported in any of the included studies (see Table 4).
Table 2.
Complications
| Peripheral nerve block | |||
| Study | Type of block | Minor complications | Major complications |
| Elnour 2009 | Axillary brachial plexus block | No intravascular injection Local bruising 2/17 in the ultrasound group vs 3/15 in the nerve stimulator group Local axillary pain 3/17 in the ultrasound group and 8/15 in the nerve stimulator group Transient post‐block paraesthesia 2/17 in the ultrasound group and 4/15 in the nerve stimulator group (which resolved within 5 days as reported by parents and participants in the follow‐up surgical clinic 1 week later) |
Major complications (e.g. unintentional intravascular injection, persistent neurological deficit) did not occur in either group |
| Marhofer 2004 | Infraclavicular brachial plexus block | No clinical signs of inadvertent puncture of major vessels | No clinical signs of pneumothorax, infection or haematoma |
| Nan 2012 | Ilioinguinal or iliohypogastric nerve block | 1 case in group no ultrasound had needle puncturing into blood vessels | No other adverse event was observed in the 2 groups |
| Oberndorfer 2007 | Sciatic and femoral nerve blocks | No clinical signs of inadvertent puncture of major vessels | No clinical signs of nerve damage, infection or haematoma |
| Ponde 2009 | Infraclavicular brachial plexus block | No complications were related to the regional anaesthetic technique | No complications were related to the regional anaesthetic technique |
| Ponde 2013 | Sciatic and femoral nerve blocks | Not reported | Not reported |
| Weintraud 2009 | Ilioinguinal‐iliohypogastric nerve block | Not reported | The ultrasound‐guided technique resulted in higher Cmax (SD) and AUC values (Cmax: 1.78 (0.62) vs 1.23 (0.70) mcg/mL, P value < 0.01; AUC: 42.4 (15.9) vs 27.2 (18.1) mcg 30 min/mL, P value < 0.001). No signs of clinical toxicity |
| Willschke 2005 | ilioinguinal block | All anaesthetic procedures were uneventful; no clinical evidence of complications such as small bowel or major vessel puncture | All anaesthetic procedures were uneventful |
| Fascia block | |||
| Study | Type of block | Minor complications | Major complications |
| Dingeman 2013 | Rectus sheath block | No adverse events requiring immediate medical attention associated with the surgical procedure or the postoperative course were reported in either group | No adverse events requiring immediate medical attention associated with the surgical procedure or the postoperative course were reported in either group |
| Faraoni 2010 | Penile nerve block | Not reported | Not reported |
| Flack 2014 | Rectus sheath block | Not reported | Peak plasma bupivacaine concentration was higher following ultrasound rectus sheath block: median: 631.9 ng/mL (IQR: 553.9 to 784.1 vs 389.7 ng/mL, IQR: 250.5 to 502.7; P value = 0.002). Time to peak concentration was longer in the USGRSB group (median 45 minutes, IQR: 30 to 60 vs 20 minutes, IQR: 20 to 45; P value = 0.006). No measured plasma bupivacaine concentration exceeded 1 mcg/mL. No adverse events and no clinical evidence of toxicity were noted |
| Gurnaney 2011 | Rectus sheath block | Not reported | Not reported |
| Kendigelen 2014 | Transersus abdominis plane block | No adverse effects related to the transversus abdominis plane block were identified | No adverse effects related to the transversus abdominis plane block were identified |
| Lorenzo 2014 | Transersus abdominis plane block | Not reported | No local anaesthetic‐specific adverse events were noted |
| O'Sullivan 2011 | Penile nerve block | No complications of either technique were reported | No complications of either technique were reported |
| Sahin 2013 | Transersus abdominis plane block | Not reported | Not reported |
| Neuraxial block | |||
| Study | Type of block | Minor complications | Major complications |
| Liu 2012 | Caudal | Not reported | Not reported |
| Tachibana 2012 | Thoracic epidural | Not reported | No participants experienced severe side effects |
| Wang 2013 | Caudal | Bloody puncture had an incidence of 18.6% in group landmarks and 5.7% in group ultrasound (P value < 0.05) | No dural puncture nor systemic reaction to LA was reported in both groups |
| Willschke 2006 | Thoracic (n = 59) or lumbar (n = 5) epidural | Blood was aspirated in 1 child in the control group | No dural puncture occurred in either group |
AUC: area under the curve for blood concentrations of local anaesthetics
Cmax: maximal blood concentration of local anaesthetic
IQR: interquartile range
LA: local anaesthetic
N: number
mcg: microgram
SD: standard deviation
USGRSB: ultrasound‐guided rectus sheath block
Discussion
In their large prospective study, Polaner et al (Polaner 2012) found that failed block and inadvertent vascular puncture were common problems encountered in paediatric regional anaesthesia. Our meta‐analysis showed that ultrasound guidance increases the success rate (or decreases the failure rate) (Table 1). However, results revealed some heterogeneity when all studies were included. The increased success rate was most evident for peripheral nerve block, for which the amplitude of the effect size (difference between ultrasound guidance and no ultrasound guidance) was inversely proportional to the age of the participant; younger children benefited most from ultrasound guidance (Figure 4). Ultrasound guidance also significantly prolonged block duration (longer time to first request of an analgesic); here again the effect was more evident in studies performed in younger participants (Figure 5). Thus, the younger the child, the more likely he/she is to benefit from ultrasound guidance. Findings of our review did not allow us to determine the exact age at which ultrasound will no longer be useful.
Pain scores at one hour after surgery were reduced when ultrasound guidance was used; however, this difference probably was not clinically relevant (equivalent to ‐0.2 on a scale from 0 to 10). For this outcome, we excluded one study from the analysis (Lorenzo 2014). The amount of heterogeneity was moderate (62%) when all studies were included and low (31%) when the study from Lorenzo et al was excluded. Lorenzo et al compared transversus abdominis plane blocks (0.4 mL/kg bupivacaine 0.25% with epinephrine) versus wound infiltration for open pyeloplasty (both before surgical incision). All surgeries followed a similar muscle‐splitting access by which the tip of the ipsilateral 12th rib was used as a landmark for incisions systematically smaller than 2 to 2.5 cm. Involved dermatomes were approximately T7 to T10 (Lorenzo 2014). The exact location of the injection for the transversus abdominis plane block (subcostal/iliac or mid‐axillary/posterior area) was not pre‐specified. The study was stopped prematurely at the interim analysis after enrolment of one‐third of the planned total recruitment on the basis that the transversus abdominis plane block was ineffective for this type of surgery. This block has been received with mixed degrees of enthusiasm in the literature because of its high variability in numbers and in the distribution of dermatoma blocked depending on the exact site of injection and the dose or volume used (Borglum 2012; Lee 2010). Therefore, the distribution of the sensory block may not have covered the surgical incision in the study by Lorenzo et al. For this reason, we chose to exclude this study from the analysis. In our opinion, the failure was specific to this type of block for this specific type of surgery and should not be considered failure of ultrasound guidance.
Time to perform the block was reduced by ultrasound guidance when used as an out‐of‐plane technique or as pre‐scanning before neuraxial block. The mean difference was small ‐ equivalent to 2.4 minutes when used as pre‐scanning. Ultrasound guidance accordingly reduced the number of needle passes required to perform the block (0.6 per procedure).
We could not demonstrate a difference in minor complications (bloody punctures), but our findings might show a trend towards a reduction in complications for the subgroup of studies in which ultrasound guidance was used for neuraxial block. Additional data are required before firm conclusions can be drawn.
None of the included studies reported major complications (Table 4). As a result of the extremely low incidence of major complications associated with paediatric regional anaesthesia, the incidence of these very rare events is best evaluated by large prospective studies.
Summary of main results
Ultrasound guidance increases the success rate of regional anaesthesia and decreases pain at one hour after surgery, time to perform the block and the number of needle passes. It also increases block duration (Table 1). For the success rate of peripheral nerve block and block duration, the effect was inversely correlated with age, meaning that the younger the child, the more likely it is that he/she will benefit from ultrasound guidance (Figure 4; Figure 5). The reduction in pain scores at one hour after surgery was small and may not have been clinically relevant. No major complications were reported in any of the included studies.
Overall completeness and applicability of evidence
Our review included both peripheral and neuraxial blocks in children from birth to 18 years of age undergoing a wide variety of surgeries. For this reason, we had to subgroup the studies according to pre‐defined criteria in our heterogeneity exploration. However, we are confident that the evidence obtained is sufficient to allow us to draw valid conclusions as reflected by the high level of evidence for five of our outcomes (success, pain at one hour, block duration, time to perform the procedure and number of needle passes; Table 1). Major complications could not be evaluated in our review because of their extremely rare incidence. More data would be useful regarding the possibility of decreased bloody puncture when ultrasound is used for neuraxial block.
Quality of the evidence
Details of the reasons justifying upgrading or downgrading the quality of evidence are given in the results section under effects of intervention (Effects of interventions). We rated the quality of evidence as high for success, pain at one hour, block duration, time to perform the procedure and number of needle passes. We rated the quality as low for minor complications (Table 1).
Potential biases in the review process
As the result of our extensive search, we are confident that we included the available literature on this topic. Studies included were all relatively recent (from 2004 to 2014) and therefore probably reflect quite well actual medical practice and technology. Although doses and volumes of injected solution varied, they seemed appropriate for the surgical indications for which they were used in most studies.
Agreements and disagreements with other studies or reviews
Unlike the Cochrane review on ultrasound guidance for peripheral nerve block in adults, we could not demonstrate a reduction in the rate of inadvertent vascular puncture (Walker 2009). However, we believe that additional paediatric data are required before firm conclusions can be drawn. Our finding that increased success and longer duration of block was inversely correlated with the age of participants represents interesting new information. We also found that ultrasound reduces the time to perform the block, but in our review, this proved true only when ultrasound was used with an out‐of‐plane technique or as pre‐scanning before neuraxial block.
Authors' conclusions
Evidence of high quality suggests that ultrasound guidance increases the success rate and increases block duration for regional blockade in children. Although we do not have enough information to state a specific age limit, evidence indicates that improved success rate and increased block duration were more pronounced in studies including younger children. Ultrasound guidance also seems to lead to slight improvement in pain scores at one hour after surgery and decreases time to perform the block in some situations (pre‐scanning before neuraxial block and out‐of‐plane techniques). The amplitude of the effect for these two outcomes was very small and therefore may not be clinically relevant. Ultrasound guidance also decreases the number of needle passes, but as a vast majority of blocks in children are performed with the child under deep sedation or general anaesthesia, the clinical relevance of this finding is arguable. No major complications were reported in any of the included studies. The incidence of lasting severe neurological complications is fortunately very low; therefore, it is unlikely that an optimal sample size could ever be achieved for this outcome with RCTs. No local anaesthetic toxicity was reported in the included studies, but here again, the number of participants included in our review is probably insufficient to eliminate a difference in the incidence of local anaesthetic toxicity when ultrasound guidance is used for regional blockade in children. Altogether, whether or not these differences justify the extra cost of ultrasound guidance may need to be evaluated.
Additional studies are required to support or refute the use of regional anaesthesia for postoperative pain in children (Suresh 2014). For peripheral nerve blocks, we found only studies on single‐shot blocks. If ultrasound guidance also improves the success rate for continuous peripheral nerve blocks in children, differences in pain and opioid‐related adverse effects after surgery may become more clinically relevant. Studies conducted to evaluate the cost/benefit ratio of ultrasound guidance versus nerve stimulator, while taking into account savings of time spent in the operating room (blocks are often performed with the child asleep in the operating room), could prove helpful. Finally, additional data are required before it can be determined whether ultrasound guidance can reduce the incidence of bloody puncture during neuraxial blockade in children.
Acknowledgements
We would like to thank Dr Jason Hayes, David Faraoni, Harshad Gurnaney, Peter Marhofer, Stephan Kettner, Michael O'Sullivan, Vrushali Ponde and Gildasio S. De Oliveira Jr., who provided additional information on their studies or took the time to inform us that their original data were no longer available.
We are also in debt to Jiang Jia for translation of Liu 2012 and Nan 2012.
Finally, we would like to thank Rodrigo Cavallazzi (Content Editor); Jing Xie (Statistical Editor); Kevin Walker and Vaughan L Thomas (Peer Reviewers) and Sheila Page (Consumer Referee) for help and editorial advice provided during preparation of this systematic review.
Appendices
Appendix 1. CENTRAL (The Cochrane Library) search strategy
#1 ultrasound near guidanc* #2 MeSH descriptor: [Nerve Block] explode all trees #3 MeSH descriptor: [Anesthesia, Local] explode all trees #4 MeSH descriptor: [Anesthesia, Spinal] explode all trees #5 ((central or spinal or epidural or caudal or neuraxial or peripheral) near nerve block*) or (nerv near block*) or (regional near (an?est* or techniq*)) #6 #1 and (#2 or #3 or #4 or #5) and (child* or neanat* or preschool* or adolescent*)
Appendix 2. MEDLINE (OvidSP) search strategy
1. (ultrasound adj5 guidanc*).mp.
2. exp Nerve Block/ or exp Anesthesia, Local/ or exp Anesthesia, Spinal/ or ((central or spinal or epidural or caudal or neuraxial or peripheral) adj5 nerve block*).mp. or (nerv adj3 block*).ti,ab. or (regional adj5 (an?est* or techniq*)).mp. and (child* or neanat* or preschool* or adolescent*).af.
3. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab. or (meta?analysis or review or systematic review).mp.) not (animals not (humans and animals)).sh.
4. 1 and 2 and 3
Appendix 3. EMBASE (OvidSP) search strategy
1. ultrasound/ or (ultrasound adj5 guidanc*).mp. 2. (exp nerve block/ or exp local anesthesia/ or exp spinal anesthesia/ or ((central or spinal or epidural or caudal or neuraxial or peripheral) adj5 nerve block*).mp. or (nerv adj3 block*).ti,ab. or (regional adj5 (an?est* or techniq*)).mp.) and (child* or neanat* or preschool* or adolescent*).af. 3. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 4. 1 and 2 and 3
Appendix 4. Scopus
Ultrasound AND (epidural OR caudal OR block)
Data and analyses
Comparison 1.
Ultrasound versus no ultrasound
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success (event = failed block) | 14 | 939 | Risk Difference (M‐H, Random, 95% CI) | ‐0.11 [‐0.17, ‐0.05] |
| 1.1 Neuraxial block | 4 | 326 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.09, 0.03] |
| 1.2 Peripheral nerve block | 8 | 507 | Risk Difference (M‐H, Random, 95% CI) | ‐0.17 [‐0.26, ‐0.07] |
| 1.3 Fascia block | 2 | 106 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.22, 0.03] |
| 2 Pain in PACU 1 hour after surgery | 8 | 414 | Std. Mean Difference (Random, 95% CI) | ‐0.20 [‐0.52, 0.13] |
| 2.1 Ultrasound vs landmark | 3 | 204 | Std. Mean Difference (Random, 95% CI) | ‐0.24 [‐0.73, 0.25] |
| 2.2 Ultrasound vs nerve stimulator | 2 | 104 | Std. Mean Difference (Random, 95% CI) | ‐0.29 [‐0.68, 0.09] |
| 2.3 Ultrasound vs infiltration | 3 | 106 | Std. Mean Difference (Random, 95% CI) | ‐0.07 [‐1.06, 0.92] |
| 3 Block duration | 8 | 358 | Std. Mean Difference (Random, 95% CI) | 1.21 [0.76, 1.65] |
| 3.1 Ultrasound vs landmark | 1 | 40 | Std. Mean Difference (Random, 95% CI) | 1.86 [1.12, 2.61] |
| 3.2 Ultrasound vs nerve stimulator | 3 | 138 | Std. Mean Difference (Random, 95% CI) | 1.15 [0.78, 1.51] |
| 3.3 Ultrasound vs infiltration | 4 | 180 | Std. Mean Difference (Random, 95% CI) | 1.11 [0.24, 1.98] |
| 4 Time to perform the block | 6 | 432 | Std. Mean Difference (Random, 95% CI) | ‐0.77 [‐1.57, 0.02] |
| 4.1 In‐plane | 2 | 106 | Std. Mean Difference (Random, 95% CI) | 0.17 [‐1.19, 1.53] |
| 4.2 Out‐of‐plane | 2 | 204 | Std. Mean Difference (Random, 95% CI) | ‐0.68 [‐0.96, ‐0.40] |
| 4.3 Pre‐scanning | 2 | 122 | Std. Mean Difference (Random, 95% CI) | ‐1.97 [‐2.41, ‐1.54] |
| 5 Number of needle passes | 2 | 122 | Std. Mean Difference (Random, 95% CI) | ‐0.90 [‐1.27, ‐0.52] |
| 6 Bloody puncture | 6 | 490 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 6.1 Neuraxial block | 2 | 204 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.19, 0.04] |
| 6.2 Peripheral nerve bock | 4 | 286 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.02] |
What's new
Last assessed as up‐to‐date: 10 March 2015.
| Date | Event | Description |
|---|---|---|
| 3 January 2017 | Amended | Co‐published in Anesthesia and Analgesia (Guay 2016) |
History
Protocol first published: Issue 12, 2014 Review first published: Issue 2, 2016
| Date | Event | Description |
|---|---|---|
| 1 March 2016 | Amended | Peer's name corrected |
Differences between protocol and review
We made no changes to our published protocol (Guay 2014).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomized controlled trial Approved by the ethics committee; informed consents obtained NCT01015053 Setting: tertiary‐referral urban children's hospital, United States of America Funding: charity: a pilot grant from Harvard Catalyst/The Harvard Clinical and Translational Science Center, Boston Children's Hospital Surgical Foundation and the Department of Anesthesiology, Perioperative and Pain Medicine Date of collection: November 2009 through May 2011 |
|
| Participants | 52 participants from 3 to 12 years of age undergoing elective umbilical hernia repair Exclusion criteria consisted of the following: ASA physical status ≥ 3, history of a complex regional pain syndrome, history of long‐term analgesic use, use of any analgesic (e.g. an opioid medication, acetaminophen, a non‐steroidal anti‐inflammatory agent) within 24 hours before surgery, history of renal insufficiency or a bleeding disorder, concurrent additional surgery at another anatomical site, being a ward of the state, a non‐English or non‐Spanish‐speaking patient or primary caregiver, inability to document postoperative pain level using FACES scores, inability of the primary caregiver to comply with home instructions |
|
| Interventions |
Treatment group: bilateral rectus sheath block with real‐time ultrasonographic guidance with 0.5 mL/kg per side of ropivacaine 0.25%, cephalad to the umbilicus (n = 27) Control group: infiltration by the surgeon (subcutaneous or intradermal) with 0.4 mL/kg of ropivacaine 0.5% (n = 25) All participants received general anaesthesia, and the block was performed at the end of surgery. Participants received no acetaminophen nor midazolam before surgery. Fentanyl citrate (1 mcg/kg) was administered before incision for intraoperative analgesia. All study participants received IV ketorolac tromethamine, 0.5 mg/kg, at the end of surgery |
|
| Outcomes |
|
|
| Notes | No adverse events requiring immediate medical attention associated with the surgical procedure or the postoperative course were reported in either group. Study authors were contacted on February 8 and 27, 2015: no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization scheme was created using a uniform (0 or 1) random number generator in commercially available statistical software incorporating an age‐stratified permuted block method of size 4 to achieve optimal balance (patient age strata, 3‐7 and 8‐12 years)" |
| Allocation concealment (selection bias) | Low risk | "Randomization proceeded after written informed consent was obtained in the preoperative area and was assigned by a research team member blinded to the group allocation. Sealed envelopes for each age stratum containing the random allocations were opaque and tamperproof" |
| Blinding of participants and personnel | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The patient and family, recovery room nurses, and study coordinator who collected the data from families were blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. Data were obtainable for 19/27 and 10/25 participants at 10 minutes |
| Selective reporting (reporting bias) | Low risk | Intention‐to‐treat |
| Other bias | Low risk | Groups well balanced |
| Methods | Randomized controlled trial Approved by the ethics committee; written parental informed consents obtained Setting: University Hospital, Egypt Funding: unspecified Date of collection: November 2007 through August 2008 |
|
| Participants | 50 ASA 1 or 2 children from 5 to 10 years scheduled for forearm and hand surgery Patients were excluded from this study in cases of parent or patient refusal, known or suspected sensitivity to amide local anaesthetics (bupivacaine), evidence of soft tissue infection near the proposed injection site, history of coagulopathy, uncooperative or mentally unstable child, axillary lymphadenopathy and significant neurological disorder of the upper extremity |
|
| Interventions |
Treatment group : axillary brachial plexus with ultrasound, 13‐6 MHz linear probe, real‐time in‐plane technique, circumferential spread around each target nerve (n = 25) Control group: axillary brachial plexus with nerve stimulator 0.5 mA, 0.3 ms distal response for median, radial and ulnar nerves. A triceps response could also be accepted for the radial nerve (n = 25) Total dose 0.5 mL/kg of 0.5% bupivacaine. General anaesthesia with a laryngeal mask airway, propofol and nitrous oxide for all participants |
|
| Outcomes |
|
|
| Notes | Pain scores: Postoperatively, the quality of analgesia was assessed by 2 pain scoring systems: the visual analogue scale score and the objective behavioural pain score. Visual analogue score consists of a single horizontal line 5 cm long, ranging from 1 = no pain to 5 = maximum pain, and the child marks the line at any point to indicate pain intensity. Objective behavioural pain score is a multi‐dimensional pain assessment based on 5 criteria: arterial blood pressure, crying, movement, agitation and verbal evaluation (localization of pain). Each criterion is given a score of 0 to 2, with 2 = worst, making the total worst possible score 10 and a total score < 5 regarded as an indication of adequate analgesia. The quality of analgesia was assessed immediately postoperatively, then at 2‐hour intervals for the next 12 hours Block duration: Paracetamol was given for a visual analogue scale score ≥ 3 or an objective pain scale score ≥ 5. Duration was defined as the interval between brachial plexus puncture and the first dose of paracetamol. In both study groups, no participants required rescue analgesia in the first 6 hours |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated by a computer‐generated table" |
| Allocation concealment (selection bias) | Low risk | "The randomization sequence was concealed in sealed envelopes" |
| Blinding of participants and personnel | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Five participants were excluded because of cancelled surgery (n = 2), change in anaesthetic plan (n = 2) or incomplete patient information (n = 1). Among the remaining 45 participants, 5 had a performance time longer than 20 minutes, leaving 40 complete participant data sets available for analysis. Two of these participants belonged to the ultrasound group, and 3 belonged to the nerve stimulator group. We included them as failed blocks for this meta‐analysis |
| Selective reporting (reporting bias) | High risk | Not intention‐to‐treat |
| Other bias | Low risk | No significant differences in demographic data between both study groups |
| Methods | Randomized controlled trial Approved by the ethics committee; parental informed consents obtained Setting: Belgium Funding: departmental resources Date of collection: not reported |
|
| Participants | 40 boys, 1 to 14 years old, who were scheduled for circumcision Exclusion criteria included allergy to amino‐amide local anaesthetics and a general contraindication for peripheral nerve block |
|
| Interventions |
Treatment group: real time (in‐plane) ultrasound guidance was used to guide bilateral injections into the subpubic space, deep to Scarpa’s fascia, with ropivacaine 0.75% 0.1 mL/kg per side plus 0.05 mL/kg at the base of the penis (n = 20) Control group: landmarks, same volumes and locations (n = 20) All participants were placed under standard anaesthesia with sevoflurane. The skin incision was performed 10 minutes after the ropivacaine injection. Paracetamol at 15 mg/kg IV was the first choice of rescue agent when the OPS score was > 3 |
|
| Outcomes |
|
|
| Notes | Additional information received from study authors, 30 January 2015 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization was performed using a computerized randomization table" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel | High risk | Blocks performed by the investigator |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The nurse in charge of the patient was blinded to the regional anaesthesia technique used. On arrival and every 30 min, the modified Objective Pain Scale (OPS) without the arterial pressure measures was recorded by the nurse trained to use this scale" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No cross‐over |
| Other bias | Low risk | Groups well balanced |
| Methods | Randomized controlled trial Approved by the ethics committee; written informed parental consents obtained NCT‐00836134 Setting: university hospital, United States of America Funding: government: supported in part by National Insitutes of Health Grant ULI RR025014‐01 of the University of Washington Institute of Translational Health Sciences Date of collection: not reported |
|
| Participants | 40 children undergoing umbilical hernia repair Exclusion criteria included younger than 1 or older than 17 years, bupivacaine or morphine allergy, local infection, coagulopathy, emergency surgery, additional surgical procedures and patient or parent refusal |
|
| Interventions |
Treatment group: real‐time (in‐plane) ultrasound‐guided rectus sheath block with 0.2 mL/kg 0.25% bupivacaine (1 mg/kg) to each side at least 10 minutes before incision (n = 20) Control group: fentanyl 2 mcg/kg before incision and wound infiltration of 0.4 mL/kg 0.25% bupivacaine (1 mg/kg) at the end of surgery (n = 20) All blocks performed under general anaesthesia with sevoflurane. Rescue analgesia for both groups consisted of fentanyl 1 mcg/kg, administered if intraoperative heart rate or blood pressure exceeded 20% of baseline. No other intraoperative analgesics or antiemetics were administered |
|
| Outcomes |
|
|
| Notes | Pain scores were assessed by a blinded observer, who used an age‐appropriate scale (FLACC age 1 to 3 years or FACES age 4 to 17 years). Study authors contacted on 28 January 2015. Replied on 29 January 2015 that they would send the data but never did so, despite multiple reminders | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomization was stratified based on age (1–3 and 4–17 years), and sequences of sealed envelopes were provided by biostatistical services" |
| Allocation concealment (selection bias) | Low risk | "Computer‐generated randomization was stratified based on age (1–3 and 4–17 years), and sequences of sealed envelopes were provided by biostatistical services" |
| Blinding of participants and personnel | High risk | Although observers measuring pain scores were blinded, recovery room nurses administering rescue analgesia were not. This potentially introduced bias and influenced rescue analgesia administration |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Pain scores were assessed by a blinded observer; morphine use may not have been blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | High risk | No cross‐over. Pain scores measured but not provided |
| Other bias | Unclear risk | Rectus sheath blocks performed 10 minutes or longer before surgical incision and infiltration at the end of the procedure |
| Methods | Randomized controlled trial Approved by the ethics committee; parental informed consents obtained NCT00578136 Setting: university hospital, United States of America Funding: unspecified Date of collection: not reported |
|
| Participants | 54 ASA physical status 1 or 2 participants between 5 and 18 years of age who were scheduled to undergo an umbilical hernia repair. 52 completed the study Patients with developmental delay that parents believed would interfere with postoperative pain score assessment and those with allergy to bupivacaine were excluded from the study |
|
| Interventions |
Treatment group: real‐time (in‐plane) rectus sheath block, 1 cm cephalad to the umbilicus with 0.25% bupivacaine, volume according to a table (n = 26) Control group: infiltration of 0.25% bupivacaine at the end of surgery. Volume according to a table (n = 26) Total volume: < 12 kg = 0.5 mL/kg; ≥ 12 to 30 = 12 mL; ≥ 30 to 40 = 16 mL; ≥ 40 = 20 mL Acetaminophen 15 mg/kg (maximum 650 mg) orally before surgery; general anaesthesia including fentanyl 1 mcg/kg |
|
| Outcomes |
|
|
| Notes | Additional information provided by study authors 30 January 2015 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "assigned using computer‐generated random numbers" |
| Allocation concealment (selection bias) | Low risk | "After obtaining written informed consent, the study patients were assigned using computer‐generated random numbers" |
| Blinding of participants and personnel | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The PACU team was blinded to the method of administering local anaesthetic. A blinded member of the research team made the initial assessment of postoperative pain using the revised Bieri FACES pain scale" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 lost in each group |
| Selective reporting (reporting bias) | High risk | Not intention‐to‐treat. One child excluded after randomization for developmental delay |
| Other bias | Low risk | Groups well balanced |
| Methods | Randomized controlled trial Setting: university hospital, Turkey Funding: unspecified Date of collection: not reported |
|
| Participants | 60 patients, between 6 and 12 years of age (ASA 1 to 2) undergoing unilateral inguinal surgery | |
| Interventions |
Treatment group: ultrasound transversus abdominis plane block with bupivacaine 0.25% (2 mg/kg; maximal volume 20 mL) (n = 30) Control group: infiltration by the surgeon with bupivacaine 0.5% (2 mg/kg) (n = 30) Blocks performed at the end of surgery |
|
| Outcomes |
|
|
| Notes | No adverse effects related to transversus abdominis plane block were identified Conference abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No cross‐over |
| Other bias | Unclear risk | Abstract only |
| Methods | Randomized controlled trial Approved by the ethics committee; written parental informed consent obtained Setting: China Funding: unspecified Date of collection: not reported |
|
| Participants | 102 ASA 1 or 2 paediatric patients from 1 month to 8 years of age and scheduled for urological or perineal surgery Children with pulmonary, neurological or coagulation dysfunction; history of multiple surgeries; spinal or sacral malformation or skin abnormalities were excluded |
|
| Interventions |
Treatment group: caudal anaesthesia with ultrasound for pre‐scanning; positive reaction in caudal space was monitored simultaneously by ultrasound (n = 52) Control group: caudal anaesthesia with landmarks; positive reaction in caudal space was monitored simultaneously by classic swoosh test (n = 50) All blocks performed under general anaesthesia with sevoflurane and loss of resistance to air. Mixture of 0.5% or 1.0% lidocaine and 0.125% to 0.250% levobupivacaine 0.5 to 1.0 mL /kg was injected. Sevoflurane was closed and was replaced with midazolam or a propofol infusion thereafter. The surgical incision was performed 10 to 15 minutes later. Children with block failures were operated while under general anaesthesia |
|
| Outcomes |
|
|
| Notes | Translated in English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly divided random number table" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced |
| Methods | Randomized controlled trial Approved by the ethics committee; parental consents obtained NCT01243593 Setting: university hospital, Canada Funding: unspecified Date of collection: 2.5‐year period; exact dates not reported |
|
| Participants | 32 children birth to 6 years old and ASA 1 to 3 undergoing open unilateral pyeloplasty Children who underwent additional unrelated surgical procedures and patients with a solitary kidney, history of pyeloplasty, history of or concern for malignant hyperthermia, previous adverse reaction to bupivacaine and history of chronic pain requiring opioid analgesics were excluded from the study |
|
| Interventions |
Treatment group : real‐time, in‐plane, ultrasound‐guided transversus abdominis plane block with 0.4 mL/kg bupivacaine 0.25% with 1:200,000 epinephrine before incision. Blocks were performed by a group of experienced anaesthesiologists specifically trained in the procedure, who had independently performed more than 25 successful blocks (n = 16) Control group : wound infiltration with 0.4 mL/kg bupivacaine 0.25% with 1:200,000 epinephrine before incision (n = 16) Anaesthesia was induced with sevoflurane in a nitrous oxide and oxygen mixture, followed by 2 mcg/kg fentanyl and 2 mg/kg propofol after IV access was secured. Administration of 0.6 mg/kg rocuronium was performed to facilitate endotracheal intubation. Participants received 40 mg/kg acetaminophen rectally immediately after induction or acetaminophen 15 mg/kg orally beforehand in the pre‐anaesthesia playroom. Anaesthesia was maintained by sevoflurane given in an oxygen/air mixture to an age‐appropriate minimum alveolar concentration of 1.1 to 1.5. All participants received 1 mcg/kg fentanyl intravenously at the start of wound closure |
|
| Outcomes |
|
|
| Notes | Additional information obtained from study authors (30 January 2015) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was carried out using a schedule derived from a table of random numbers with a simple 1:1" |
| Allocation concealment (selection bias) | Low risk | "Recruited patients were assigned in a concealed fashion (sequentially numbered, sealed envelopes) to 1 of 2 groups" |
| Blinding of participants and personnel | Unclear risk | "blinded (to assessor, post‐anaesthesia care unit health care provider(s), statistician, patient and family)" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "A blinded assessor regularly captured pain scores in the recovery room using the FLACC (Face, Legs, Activity, Cry, Consolability) scale." "All patients had an identical bandage to maintain blinding of the assessors" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | "At the first interim analysis (a third of recruitment attained), there was a highly clinically and statistically significant difference in the primary outcome measurements as well as secondary outcome measurements. Therefore, the study was stopped early due to lack of benefit" |
| Other bias | Low risk | "The groups were balanced for patient demographics, operating time and hospital stay In both groups, the local anaesthetic was administered before the surgical incision" |
| Methods | Randomized controlled trial Approved by the ethics committee; written parental informed consents obtained Setting: university hospital, Austria Funding: unspecified Date of collection: not reported |
|
| Participants | 40 ASA physical status 1 or 2, 1 to 10 years of age; children scheduled for arm and forearm surgery for traumatic conditions Exclusion criteria included coagulopathy; cardiac, hepatic, renal or neurological disease; malformations of the upper limb and surgical contraindications to regional anaesthesia |
|
| Interventions |
Treatment group: infraclavicular lateral brachial plexus blocks with 0.5% ropivacaine 0.5 mL/kg guided by ultrasound visualization (out‐of‐plane), injection around the brachial plexus (n = 20) Control group: infraclavicular brachial plexus blocks with 0.5% ropivacaine 0.5 mL/kg guided by nerve stimulation (coracoid process, 0.3 mA and 0.3 ms) (n = 20) If necessary, propofol was given to produce sedation during brachial plexus anaesthesia. In the ultrasound group, children were given a median (range) dose of midazolam of 1.3 (0.7 to 2.1) mg/kg, and 11 ⁄ 20 children received a median (range) dose of propofol of 10.4 (0 to 14) mg. In the nerve stimulator group, children were given midazolam 1.3 (0.8 to 2.1) mg/kg, and 13 ⁄ 20 children were given propofol 11.3 (0 to 14) mg. Surgical procedures were started 30 minutes after induction of brachial plexus anaesthesia |
|
| Outcomes |
|
|
| Notes | All anaesthetic procedures were uneventful, with no clinical signs of pneumothorax, inadvertent puncture of major vessels, infection or haematoma Study authors were contacted 11 February 2015. Original data have been destroyed (> 10 years) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised to one of two study groups by sealed envelope" |
| Allocation concealment (selection bias) | Low risk | "randomised to one of two study groups by sealed envelope" |
| Blinding of participants and personnel | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Sensory and motor blockades were assessed by an anaesthetist who was not involved in the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | High risk | No failed block. Pain scores measured but not provided. Study authors contacted, and original data destroyed |
| Other bias | Low risk | Groups well balanced |
| Methods | Randomized controlled trial Approved by the ethics committee; written parental informed consents obtained Setting: university hospital, China Funding: government: Research Foundation of Zhejiang Provincial Health Department (2009A145) Date of collection: July through September 2010 |
|
| Participants | 100 children with ASA status 1, between 4 and 8 years old, scheduled for surgery for unilateral inguinal repair or high hydrocoele ligation or descent and fixation of testis for cryptorchidism Children with cardiovascular, respiratory or kidney disease; a history of allergic to local anaesthetic; coagulation abnormalities or neuromuscular diseases were excluded |
|
| Interventions |
Treatment group: ilioinguinal or iliohypogastric block under ultrasonic guidance (linear 5 to 10 MHz probe; real‐time; out‐of‐plane) with a mixture of 0.8% lidocaine and 0.25% levobupivacaine at 0.2 mlL/kg (n = 50) Control group: ilioinguinal or iliohypogastric block performed according to the traditional method (Schulte‐Steinbery's method) of anatomical localization with the same local anaesthetic at 0.3 mL//kg(n = 50) All blocks performed with participants under general anaesthesia with sevoflurane 2% in nitrous oxide and oxygen 50% |
|
| Outcomes |
|
|
| Notes | Translated in English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "a random number remainder grouping method" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Unclear risk | Lower dose of local anaesthetic in the ultrasound group |
| Methods | Randomized controlled trial Approved by the ethics committee; written informed parental/guardian consent obtained Setting: Ireland Funding: departmental resources Date of collection: not reported |
|
| Participants | 66 boys of ASA physical status 1 or 2 scheduled for day case circumcision Exclusion criteria included allergy to local anaesthetic or an additional surgical procedure, other than circumcision, under the same general anaesthetic |
|
| Interventions |
Treatment group: penile block under ultrasound guidance. ‘Hockey‐stick’ probe (6 to 13 MHz, 25 mm), real‐time (in‐plane; information from study authors) guidance. Two puncture techniques with 0.5% bupivacaine 1 to 2 mL up to 3 years and 1 additional mL per each additional 3 years up to a maximum of 5 to 6 mL (n = 34) Control group: penile block with landmarks with the same doses (n = 32) All blocks performed under general anaesthesia maintained with sevoflurane in oxygen/air gas flow. In both groups, an additional bleb of local anaesthetic was deposited subcutaneously at the ventral penoscrotal junction to block the perineal nerve, which provides sensation to the ventral portion of the glans penis. All blocks were performed or were supervised by an experienced consultant (attending) anaesthetist. Routine analgesia with rectal paracetamol (30 to 40 mg/kg) and diclofenac (1 to 2 mg/kg) was administered after induction of anaesthesia. An end‐tidal sevoflurane value of 2.5% to 3.0% was established at the time of incision. The incision was performed at least 10 minutes after the block was completed |
|
| Outcomes |
|
|
| Notes | No complications of either technique were reported Pain in PACU. For older children able to communicate their level of pain, a visual analogue scale was used; the FLACC (face, legs, activity, cry and consolability) pain scale was used for those unable to communicate their level of pain. Study authors were contacted for results at 1 hour in the postoperative care unit; data not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized by drawing from a sealed envelope" |
| Allocation concealment (selection bias) | Low risk | "Patients were randomized by drawing from a sealed envelope" |
| Blinding of participants and personnel | Low risk | Attending anaesthetist and nurse in PACU blinded to the treatment group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Attending anaesthetist and nurse in PACU blinded to the treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were excluded from the study |
| Selective reporting (reporting bias) | Low risk | No cross‐over |
| Other bias | Low risk | Demographic data in both groups were similar |
| Methods | Randomized controlled trial Approved by the ethics committee; written informed consent obtained from the parents Setting: South Africa Funding: unspecified Date of collection: not reported |
|
| Participants | 46 children up to 8 years of age scheduled for surgery on 1 lower extremity Exclusion criteria included history of seizures or neurological, neuromuscular, psychiatric or blood clotting disorders |
|
| Interventions |
Treatment group: sciatic and femoral nerve block under ultrasound guidance using a multiple injection technique until the nerves were surrounded by levobupivacaine (n = 23). Portable ultrasound unit (SonoSiteTM, Bothell, WA, USA) with a 5 to 10 MHz linear hockey stick probe, real‐time (out‐of plane) technique Control group: Sciatic and femoral nerve blocks under nerve stimulator guidance using a pre‐defined dose of 0.3 mL/kg of levobupivacaine injected when a current of 0.3 mA over 0.3 ms at 2 Hz elicited plantar flexion (sciatic) or cephalic movement of the patella (femoral) (n = 23) Anaesthesia was maintained with 1 MAC halothane in nitrous oxide and oxygen. All blocks were performed under general anaesthesia by the same anaesthetist, who was experienced in both nerve stimulator and ultrasound‐guided regional anaesthesia in children. Sciatic nerve blocks were performed in all children in the subgluteal or popliteal area, as indicated by the surgical procedure. Femoral nerve blocks were performed when indicated by the planned operation. In both groups, skin incision was performed at least 20 minutes after the block |
|
| Outcomes |
|
|
| Notes | On the first postoperative day, all puncture sites were checked for haematoma or signs of infection, and sensorial examination of the blocked nerves was performed "no clinical signs of nerve damage, inadvertent puncture of major vessels, infection, or haematoma" Additional information provided by study authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Before randomization, participants were stratified into 2 groups on the basis of the planned surgical procedure and the need for sciatic nerve block or sciatic nerve block plus femoral nerve block. Children were then randomized to receive nerve blocks under nerve stimulator guidance (nerve stimulator group) or ultrasound guidance (ultrasound group). Randomization was based on computer‐generated codes that were kept in sequentially numbered opaque envelopes until just before use |
| Allocation concealment (selection bias) | Low risk | Randomization was based on computer‐generated codes that were kept in sequentially numbered opaque envelopes until just before use |
| Blinding of participants and personnel | Unclear risk | OPS scores were evaluated by an anaesthetist who was blinded to the randomization |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | OPS scores were evaluated by an anaesthetist who was blinded to the randomization |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | No cross‐over mentioned: "Ultrasound visualization of the sciatic and the femoral nerve was possible in all cases." Pain scores provided by study authors |
| Other bias | Unclear risk | Fixed dose of local anaesthetic with nerve stimulation and total dose of local anaesthetic administered were higher in this group. The volume of local anaesthetic in sciatic and femoral nerve blocks was reduced with ultrasound compared with nerve stimulator guidance (0.2 (SD 0.06) vs 0.3 mL/kg (P value < 0.001) and 0.15 (SD 0.04) vs 0.3 mL/kg (P value < 0.001), respectively). This could have favoured nerve stimulator guidance for time to first request of an analgesic |
| Methods | Randomized controlled trial Approved by the ethics committee; written informed parental consents obtained Setting: India Funding: unspecified Date of collection: not reported |
|
| Participants | 50 ASA physical status 1 and 2 children, between 1 and 2 years of age, scheduled for radial club hand repair (centralization of ulna) Exclusion criteria included cardiac, renal or neurological diseases and coagulopathies |
|
| Interventions |
Treatment group: real‐time (in‐plane) ultrasound‐guided infraclavicular brachial plexus block with a 38‐mm linear 5 to 10 mHz probe and with 0.5 mL/kg of 0.5% of bupivacaine as a 1‐injection technique (n = 25) Control group: lateral infraclavicular brachial plexus block guided by nerve stimulator with 0.5 mL/kg of 0.5% bupivacaine injected at 0.5 mA over 250 ms. if after 3 redirections, a wrist response could not be obtained, an elbow response was accepted (n = 25) All blocks were performed with participants under general anaesthesia, and anaesthesia was maintained with 50% nitrous oxide in oxygen 2 mg/kg/h propofol infusion. The first study author performed all blocks. The surgical procedure commenced 20 minutes after administration of the respective blocks. An anaesthesiologist who was blinded to the block administration technique finished the case |
|
| Outcomes |
|
|
| Notes | "There were no complications related to the regional anaesthetic technique"
Study authors contacted: data no longer available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized by sealed envelope to one of two study groups based on regional anaesthetic technique" |
| Allocation concealment (selection bias) | Low risk | "randomized by sealed envelope to one of two study groups based on regional anaesthetic technique" |
| Blinding of participants and personnel | Low risk | "An anaesthesiologist who was blinded to the block administration technique finished the case" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "An anaesthesiologist who was blinded to the block administration technique finished the case" "The Children’s Hospital Eastern Ontario Pain Scale pain score was recorded at 1, 4, 6, 8, and 10 postoperative hours by an anaesthesiologist not involved in the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Pain scores and time to first request of an analgesic measured but not provided |
| Other bias | Low risk | Groups were comparable for age, weight and duration of surgery |
| Methods | Randomized controlled trial Approved by the ethics committee; written informed parental/guardian consents obtained Setting: India Funding: departmental resources Date of collection: 2009 |
|
| Participants | 60 children 6 months to 5 years of age diagnosed with distal arthrogryposis multiplex congenita posted for foot surgery (vertical talus correction) Children were excluded from the study if they had coagulopathies or cardiac and renal disorders |
|
| Interventions |
Treatment group: femoral and sciatic block under real‐time (in‐plane) ultrasound guidance with a 5 to 10 mHz probe and 0.5 mL/kg of 0.25% bupivacaine for the sciatic nerve and 0.7 mL/kg of lidocaine 1% for the femoral nerve. The procedure was abandoned in the absence of visualization of the sciatic nerve (n = 30) Control group: femoral and sciatic block with nerve stimulator. Procedure abandoned if sciatic nerve stimulation was not obtained after 3 attempts (each pass was accounted as an attempt). Injection of 0.5 mL/kg of bupivacaine 0.25% with plantar or dorsiflexion (ankle movement for the sciatic block) and 0.7 mL/kg of lidocaine 1% with a quadriceps contraction (femoral block) obtained at 0.5 mA. A fascia iliaca block (loss of resistance technique) was administered to patients in whom a femoral block could not be performed (n = 30) All blocks performed under general anaesthesia with nitrous oxide and propofol 2 to 3 mg/kg/h IV. Fpr participants in whom sciatic block could not be performed, femoral block was not attempted. All blocks were performed by the same anaesthesiologist, who had extensive experience in nerve stimulator and ultrasound use. All blocks were allowed to set up for 20 minutes before surgical incision |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed by sealed envelopes prepared by the statistical staff" |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed by sealed envelopes prepared by the statistical staff. The anaesthesiologist performing the block was given randomly generated group allocations within sealed opaque envelopes |
| Blinding of participants and personnel | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Postoperatively, CHIPPS pain score was recorded at 1, 4, 6, 8 and 10 hours by the second anaesthesiologist on duty, who was blinded to the block technique |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 60 patients were recruited, and all completed the study |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced |
| Methods | Randomized controlled trial Approved by the ethics committee; informed parental written consents obtained ACTRN12611000585921 (7/06/2011) from Australian New Zealand Clinical Trials Registry Setting: university hospital, Turkey Funding: none declared Date of collection: December 2010 through May 2011 |
|
| Participants | 57 ASA 1 or 2 children between 2 and 8 years of age undergoing unilateral inguinal hernia repair Exclusion criteria included psychiatric illness, kidney failure and known hypersensitivity to relevant drugs |
|
| Interventions |
Treatment group: transversus abdominis plane block with ultrasound (real‐time; in‐plane) and 0.5 mL/kg of 0.25% levobupivacaine. The block was performed after anaesthesia induction with a 10 to 18 mHz probe placed between the iliac crest and the subcostal area at the mid‐axillary line and the needle directed posteriorly. The surgical procedure began 5 to 10 minutes after local anaesthetic administration (n = 29) Control group: wound infiltration with 0.2 mL/kg of 0.25% levobupivacaine between external aponeurosis and the skin was performed by surgeons during wound closure (n = 28) In both groups, rectal paracetamol 40 mg/kg was administered as a loading dose after induction of anaesthesia |
|
| Outcomes |
|
|
| Notes | Pain in PACU: Pain scores were assessed by using the modified Children’s Hospital of Eastern Ontario Pain Scale. Data were not included in the report. We contacted the study authors twice (26 January 2015 and 14 February 2015) but received no reply from them | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised by sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | "Patients were randomised by sealed envelopes" |
| Blinding of participants and personnel | Unclear risk | Blinding or masking began in the recovery room |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding or masking began in the recovery room. The recovery room nurse assigned to each participant was blinded to study groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants lost to follow‐up: 1 in ultrasound group and 2 in infiltration group |
| Selective reporting (reporting bias) | High risk | Not intention‐to‐treat |
| Other bias | Unclear risk | Higher volume of local anaesthetic in the ultrasound group. Blocks performed after anaesthesia induction for the ultrasound group and during wound closure for the infiltration group |
| Methods | Randomized controlled trial Approved by the ethics committee; written informed consent obtained from patients and their parents Setting: university hospital, Japan Funding: unspecified Date of collection: not reported |
|
| Participants | 20 ASA physical status classification I children from 5 to 7 years of age and scheduled to undergo the minimally invasive Nuss procedure for pectus excavatum. Patients known or suspected to have neurological disease, local infection, coagulation abnormality, seizures, allergy to a local anaesthetic or anatomical malformation of centroaxial structures were excluded from the study |
|
| Interventions |
Treatment group: ultrasound was used to determine the level, the wider space and the puncture point (shortest depth from the skin (n = 10) Control group: landmarks (n = 10) Thoracic epidural (T5‐T6 or T6‐T7) placed under general anaesthesia with nitrous oxide and sevoflurane. All epidural punctures were performed by a senior attending anaesthesiologist using an 18‐gauge Touhy needle, and the epidural space was identified by the loss of resistance technique with 2 mL physiological saline. Finally, a catheter was introduced 4 cm into the epidural space through the needle |
|
| Outcomes |
|
|
| Notes | No severe complications Study authors contacted twice (14 and 27 February 2015): no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", no details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No failed block |
| Other bias | Low risk | Groups were similar with respect to all demographic variables |
| Methods | Randomized controlled trial Approved by the ethics committee; informed parental consents obtained Setting: China Funding: departmental resources Date of collection: over a 5‐month period |
|
| Participants | 140 ASA 1 to 2 children, up to 72 months of age, weighing up to 25 kg, undergoing elective inguinal hernia repair Patients were excluded from this study if they had contraindications to caudal block or lack of parental consent |
|
| Interventions |
Treatment group: ultrasound guidance. Pre‐scanning with a 5 to 10 MHz linear 38 probe, real‐time (out of‐plane), confirmed with upward displacement of the sacrococcygeal ligament upon injection (n = 70) Control group: landmarks (n = 70) All caudal blocks were performed with the participant under general anaesthesia with sevoflurane, and anaesthesia was maintained with propofol 5 mg/kg/h thereafter. The same LA dosing regimen of 1 mL/kg of 0.25% ropivacaine (AstraZeneca) with epinephrine 1:200,000 was applied in both groups. 4 cm 21G "regional needle". All block procedures were performed by 2 anaesthetists who were experienced in caudal block and ultrasound technique. After the cranial dermatomal level was tested by the pinprick method (no movement response to cutaneous pinprick) 15 minutes after LA injection, skin incision was performed |
|
| Outcomes |
|
|
| Notes | "The dilation of the hiatus was seen in all patients including those deemed failure block in group ultrasound" Time to perform the procedure: duration of performance of the block (defined as the time interval from initial skin puncture to the end of LA injection). Study authors contacted on 15 and 27 February 2015: no reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was based on computer‐generated codes with SPSS 13.0 (SPSS Inc., Chicago, IL, USA)" |
| Allocation concealment (selection bias) | Low risk | "Maintained in sequentially numbered opaque envelopes" |
| Blinding of participants and personnel | Low risk | "A separate anaesthetist who then entered the operating room after the block was assigned to subsequent anaesthetic assessment and management" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Thus, the observer of anaesthetic effect was blinded with regards to the block technique" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study successfully |
| Selective reporting (reporting bias) | Low risk | Intention‐to‐treat |
| Other bias | Low risk | "There were no differences between two groups in terms of patients’ characteristics" |
| Methods | Randomized controlled trial Approved by the ethics committee; written parental informed consents obtained Setting: university hospital, South Africa Funding: unspecified Date of collection: not reported |
|
| Participants | 70 children (8 to 84 months of age) ASA 1 to 2, scheduled for inguinal hernia repair Exclusion criteria included prior surgical procedures in the groin area, general contraindications for the blocks, known allergy to amino‐amide local anaesthetics, inability of the parents to understand the study protocol and lack of parental informed consent |
|
| Interventions |
Treatment group: ilioinguinal‐iliohypogastric nerve blockade with real‐time (out of‐plane) ultrasound guidance using a 5 to 10 mHz linear hockey stick probe (n = 37 included; 35 analysed) Control group: ilioinguinal‐iliohypogastric nerve blockade with landmarks (single‐pop) (n = 33 included; 31 analysed) All blocks performed under general anaesthesia with halothane 1.0 MAC. Blocks were performed by experienced anaesthesiologists with 0.25 mL/kg of ropivacaine 0.5% (1.25 mg/kg) as the total dose. Skin incision was performed 15 minutes after the block |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The computer‐generated randomization protocol was prepared outside the study centre and delivered in opaque envelopes that were sealed and sequentially numbered" |
| Allocation concealment (selection bias) | Low risk | "The computer‐generated randomization protocol was prepared outside the study centre and delivered in opaque envelopes that were sealed and sequentially numbered" |
| Blinding of participants and personnel | Low risk | "The anaesthesiologist who performed general anaesthesia was blinded to the method of performance of the treatment group" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The anaesthesiologist who performed general anaesthesia was blinded to the method of performance of the treatment group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants excluded ‐ 2 in each study group |
| Selective reporting (reporting bias) | High risk | Not intention‐to‐treat |
| Other bias | Low risk | Groups well balanced |
| Methods | Randomized controlled trial Approved by the ethics committee; written parental informed consents obtained Setting: university hospital, South Africa Funding: equipment loan from the industry Date of collection: not reported |
|
| Participants | 100 children up to 8 years of age who were scheduled for inguinal hernia repair, orchidopexy or hydrocoele repair were included in this study Those with a history of seizures or neurological, neuromuscular, psychiatric or blood clotting disorders were excluded |
|
| Interventions |
Teatment group: real‐time (out of‐plane) ultrasound‐guided ilioinguinal block with levobupivacaine 0.25% in amount sufficient to surround the nerves (n = 50) Control group: ilioinguinal block using the traditional fascial click method. The needle was inserted vertically through the ‘tented’ skin, 1 to 2 cm medial and 1 to 2 cm inferior to the anterior superior iliac spine. After the first ‘fascial click’ was detected, and following a negative aspiration, levobupivacaine 0.25% (0.3 mL/kg) was injected. The spread of local anaesthetic was examined with ultrasound after injection but with no further intervention consequential to this information (n = 50) All blocks performed under general anaesthesia with halothane 1 MAC. In both groups, skin incision was performed 15 minutes after the block. All surgical procedures were performed by the same surgeon, and all blocks were performed by the same 2 anaesthetists, both experienced in ultrasonographic‐guided regional anaesthetic techniques in children |
|
| Outcomes |
|
|
| Notes | Additional information received from study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed outside the study centre and was delivered in sealed opaque envelopes, which were numbered sequentially |
| Allocation concealment (selection bias) | Low risk | Randomization was performed outside the study centre and was delivered in sealed opaque envelopes, which were numbered sequentially |
| Blinding of participants and personnel | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No cross‐over |
| Other bias | Low risk | The volume of local anaesthetic administered was less in the ultrasound group but should not influence the rate of success. The amount of local anaesthetic given in the ultrasound group was significantly less than that given in the fascial click group (0.19 (0.05) vs 0.3 mL/kg; P value < 0.0001) |
| Methods | Randomized controlled trial Approved by the ethics committee; parental consents obtained Setting: university hospital, South Africa Funding: equipment loan from the industry Date of collection: not reported |
|
| Participants | 64 children who were undergoing major abdominal or thoracic surgery, ranging from neonates to children 6 years of age Children with neurological disorders, seizures, local infection or coagulopathies were excluded |
|
| Interventions |
Treatment group: Epidural catheter placement was guided by direct ultrasound visualization (real‐time, out‐of‐plane for needle placement). Images obtained by an assistant with 5 to 10 mHz hockey‐stick probe to obtain a paramedian longitudinal view. Midline needle insertion. Confirmation of the position of the tip of the catheter and spread of local anaesthetics through the catheter. Levobupivacaine 0.25% for confirmation of needle placement and through the catheter (0.2 mg/kg for the latter) (n = 30) Control group: epidural catheter with standard loss of resistance technique with air or saline. Levobupivacaine 0.2% 0.2 mg/kg through the needle and 0.2 mg/kg through the catheter (n = 34) All epidural catheters were placed with participants under general anaesthesia with halothane 1.0 MAC. The initial bolus dose was topped up to a total of levobupivacaine 0.25% 0.5 to 0.7 mL/kg at lumbar level, or 0.4 to 0.5 mL/kg at the thoracic level. A continuous infusion of levobupivacaine 0.125% (0.2 mL/kg at lumbar sites and 0.1 mL/kg at thoracic sites) was started during the operation. All puncture sites were checked daily for inflammation or other adverse reactions. Epidural catheters were removed 2 to 3 days after surgery. The surgical incision was made ≥ 15 minutes after placement of the epidural block |
|
| Outcomes |
|
|
| Notes | Additional information received from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization protocol was prepared outside the study centre and delivered in opaque envelopes that were sealed and sequentially numbered" |
| Allocation concealment (selection bias) | Low risk | "The randomization protocol was prepared outside the study centre and delivered in opaque envelopes that were sealed and sequentially numbered" |
| Blinding of participants and personnel | High risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No cross‐over |
| Other bias | Low risk | "The patient characteristic data and epidural puncture level were similar in both groups" |
ASA: American Society of Anesthesiologists.
CHIPPS: Children's and Infants' Postoperative Pain Scale.
FACES: score on the FACES pain rating scale.
FLACC: Face, Legs, Activity, Cry and Consolability Pain Scale.
Hz: hertz.
IV: intravenous.
LA: local anaesthetic.
MAC: minimal alveolar concentration.
mcg/kg: microgram per kilogram of body weight.
mL: millilitres.
ms: millisecond.
mA: milliampere.
N = numbers.
OPS: objective pain scale.
PACU: post‐anaesthesia care unit.
SD: standard deviation.
USA: United States of America.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Sohn 2010 | This study was withdrawn by the trialists because of problems involving data collection |
| Triffterer 2012 | Different intervention. The aim of the study was to measure the cranial spread of caudally administered local anaesthetics in infants and children by means of real‐time ultrasonography, with a special focus on comparing the effects of using 2 different speeds of injection |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Comparison of Ultrasound Guided Placement of Local Anaesthetic in the Abdominal Muscle and Local Anaesthetic Under the Skin for Pain Relief during and Following Surgical Repair of Belly Button Hernias in Children |
| Methods | RCT |
| Participants | Children |
| Interventions |
Treatment group: Bilateral ultrasound‐guided rectus sheath blocks with a pre‐determined volume of 1% lignocaine and 1% ropivacaine will be injected following a negative aspiration test. A total of 0.3 mL/kg (to a maximum of 5 mL) of combined 1% lignocaine and 1% ropivacaine will be infiltrated bilaterally 5 minutes before skin incision Control group: Once general anaesthesia has been established, the surgeon will perform all subcutaneous periumbilical local anaesthetic infiltrations. Following aseptic preparation of the site, a total of 0.3 mL/kg (to a maximum of 5 mL) of combined 1% lignocaine and 1% ropivacaine will be infiltrated 5 minutes before skin incision |
| Outcomes | Primary outcome: number of intraoperative fentanyl doses, number of morphine doses in post‐anaesthesia care unit, time to first dose of paracetamol and ibuprofen |
| Starting date | 18/02/2008 |
| Contact information | Michael Fredrickson, Newmarket, Auckland |
| Notes | Retrospectively registered |
| Trial name or title | Ultrasound Guided Transversus Abdominis Plane Block Versus Local Wound Infiltration for Post‐operative Analgesia in Children Undergoing Appendectomy: A Randomized Controlled Trial |
| Methods | RCT |
| Participants | 40 children 4 to 14 years of age, ASA 1 to 2 undergoing laparoscopic appendectomy |
| Interventions |
Treatment group: ultrasound‐guided transversus abdominis plane block at the end of surgery using 0.4 mL/kg of bupivacaine 0.25% with 1:200,000 epinephrine. Total dose of bupivacaine will not exceed 2 mg/kg, and total volume will not be more than 20 mL Control group: local anaesthetic infiltration using 0.4 mL/kg of bupivacaine 0.25% by the surgeon at the end of surgery for a laparoscopic appendectomy |
| Outcomes | Primary outcome: requirement for morphine post surgery (24 hours) Secondary outcome: assessment of pain scores post surgery using a visual analogue scale (24 hours) |
| Starting date | 27/05/2013 |
| Contact information | Ahmad Ramzy, King Faisal Specialist Hospital and Research Centre, Saudi Arabia |
| Notes |
| Trial name or title | Dorsal Penile Nerve Block for Circumcision: A Comparison of Ultrasound‐Guided Versus Landmark Technique |
| Methods | RCT |
| Participants | Boys undergoing circumcision |
| Interventions |
Treatment group: ultrasound penile block Control group: penile block with landmarks |
| Outcomes | Postoperative pain and recovery after circumcision |
| Starting date | 2012‐06‐05 |
| Contact information | UZLeuven |
| Notes | EudraCT Number: 2012‐001217‐16 |
| Trial name or title | Transversus Abdominis Plane Block in Children Undergoing Ostomy Surgery |
| Methods | RCT |
| Participants | Children undergoing revision or closure of ostomy ASA physical status 1 to 3 and age ≥ 3 months up to 18 years |
| Interventions |
Treatment group: A high‐frequency (5 to 10 mHz) ultrasound probe (SonoSite Micromaxx, Licence No. 12407) will be placed on the flank at the midpoint between the iliac crest and the lower costal margin. The 3 muscle layers of external oblique, internal oblique and transversus abdominis will be visualized. A 22G short‐bevel block needle will be advanced in an anterior‐to‐posterior direction, in‐plane with the probe, until the tip is visualized in the transversus abdominis plane. After negative aspiration, 0.4 mL/kg of bupivacaine 0.25% with 1:200,000 epinephrine will be injected. The total dose of bupivacaine will not exceed 2 mg/kg, and the total volume will not be greater than 20 mL Control group: circumferential subcutaneous infiltration of the ostomy wound with bupivacaine 0.25% with 1:200,000 epinephrine 0.4 mL/kg by the surgeon after skin closure |
| Outcomes | Primary outcome measures: morphine requirement (within the first 48 hours after surgery). The primary endpoint will be the proportion of children in each group requiring 2 or more (≥ 2) boluses of morphine in the PACU Secondary outcome measures: dose of opioids administered (within first 48 hours after surgery), pain scores (within first 48 hours after surgery assessed using the Faces‐Legs‐Activity‐Cry‐Consolibility Scale for children ≤ 7 years old and the Numerical Rating Scale for children > 7 years old) and signs of adverse events related to the block (such as persistent pain or redness, swelling and/or discharge from the puncture site) or to opioids |
| Starting date | April 2010 |
| Contact information | Carolyne Pehora Hospital for Sick Children, Totonto, Ontario, Canada |
| Notes |
| Trial name or title | Study of Transverse Abdominis Plane Block in Children Undergoing Hydrocelectomy and/or Hernia Repair Surgery |
| Methods | RCT |
| Participants | Children 2 to 8 years of age undergoing elective inguinal hernia repair, inguinal hernia repair with peritoneoscopy and/or hydrocoelectomy, ASA physical status 1 and 2 (patients that have no systemic illness or mild systemic disease that is well controlled, e.g. mild asthma) |
| Interventions |
Treatment group: 25 participants will receive a transversus abdominis plane block with 0.5 mL/kg of 0.25% ropivacaine. Blocks will be performed under ultrasound guidance via SonoSite device by an in‐plane technique Control group: 25 participants will receive a transversus abdominis plane block with 0.5 mL/kg of 0.25% ropivacaine by the surgeon |
| Outcomes | Primary outcome measures: pain scores (assessed with Face, Legs, Activity, Cry and Consolability Pain Assessment Scale in the post‐anaesthesia care unit until hospital discharge (between 1 and 2 hours), pain medication (at 24 hours post hospital discharge) Secondary outcome measures: parental satisfaction with pain control (as measured by a 10‐point Likert scale) |
| Starting date | February 2012 |
| Contact information | Kaveh Aslani, MD, Principal Investigator, William Beaumont Hospitals |
| Notes |
| Trial name or title | Ultrasonography for Confirmation of Caudal Injection |
| Methods | RCT |
| Participants | Children younger than 8 years and weighing ≤ 20 kg who are undergoing lower abdominal, lower extremity orthopaedic or urological procedures |
| Interventions |
Treatment group: ultrasound caudal block Control group: landmark caudal block |
| Outcomes | Primary outcome measures: rate of block success (within 4 hours as estimated by ultrasound spread, heart rate, need for additional medications and pain scores) Secondary outcome measures: opioid administration (within 4 hours) |
| Starting date | December 2014 |
| Contact information | Justin B. Long, MD, and John Hajduk; Ann & Robert H. Lurie Children's Hospital of Chicago |
| Notes |
| Trial name or title | Percutaneous Rectus Sheath Block Versus Intra‐operative Rectus Sheath Block for Pediatric Umbilical Hernia Repair |
| Methods | RCT |
| Participants | Patients 3 to 18 years old with a diagnosis of umbilical hernia |
| Interventions |
Treatment group: After induction of anaesthesia, the attending anaesthesiologist will use a portable ultrasound probe to locate the rectus sheath. A 22‐gauge needle will be used to inject a pre‐determined volume of 0.2% ropivacaine (1 mL/kg, max dose 10 mL, divided into equal doses bilaterally). The analgesic will be injected percutaneously under ultrasound guidance between the rectus abdominis muscle and the posterior rectus sheath at the lateral border bilaterally Control group: After completion of umbilical hernia repair, after fascial closure, but before skin closure, a pre‐determined volume of 0.2% ropivacaine (1 mL/kg, max dose 10 mL, divided into equal doses bilaterally) will be administered under direct visualization into the rectus sheath bilaterally by the attending surgeon |
| Outcomes | Primary outcome measures: postoperative pain rating (within 5 days, as assessed by the Wong‐Baker FACES Pain Rating Scale) Secondary outcome measures: operative time, use of postoperative intravenous/oral opioid and non‐opioid (within 5 days), time to first rescue analgesic (within 1 day), number of participants with side effects such as nausea, vomiting, allergic reactions (within 5 days), number of participants with complications (such as infection, bleeding, intravascular injection, bowel puncture within 30 days) |
| Starting date | 9 December 2014 |
| Contact information | Nicole Chandler, Assistant Professor of Surgery, All Children's Hospital Johns Hopkins Medicine |
| Notes |
ASA: American Society of Anesthesiologists.
FACES: Score on the FACES pain rating scale.
PACU: post‐anaesthetic care unit.
RCT: randomized controlled trial.
Contributions of authors
Joanne Guay (JG), Santhanam Suresh (SS), Sandra Kopp (SK).
Conceiving of the review: JG and SS.
Co‐ordinating the review: JG.
Screening search results: JG and SK.
Organizing retrieval of papers: JG.
Screening retrieved papers against inclusion criteria: JG and SK.
Appraising the quality of papers: JG and SK.
Abstracting data from papers: JG and SK.
Writing to authors of papers to ask for additional information: JG.
Obtaining and screening data from unpublished studies: JG.
Managing data for the review: JG.
Entering data into Review Manager (RevMan 5.3): JG.
Analysing RevMan statistical data: JG.
Performing other statistical analysis not using RevMan: JG.
Interpreting data: JG, SS and SK.
Making statistical inferences: JG.
Writing the review: JG, SS and SK.
Securing funding for the review: departmental resources only.
Performing previous work that was the foundation of the present study: JG and SS.
Serving as guarantor for the review (one review author): JG.
Taking responsibility for reading and checking the review before submission: JG, SS and SK.
Sources of support
Internal sources
-
University of Sherbrooke, Canada.
University of Sherbrooke granted access to electronic databases and to major medical journals.
-
University of Quebec in Abiti‐Temiscamingue, Canada.
University of Quebec in Abiti‐Temiscamingue provided access to electronic databases and medical journals.
-
Cochrane Anaesthesia Review Group, Denmark.
The review authors wish to thank Karen Hovhannisyan, who designed the search strategy.
External sources
No sources of support supplied
Declarations of interest
Joanne Guay: I have had no direct relationship with any pharmaceutical company or equipment manufacturer in the past five years. I have not acted as a witness expert in the past five years. I am not an author of any of the included or excluded studies. I do not hold stock other than mutual funds. I am the editor of a multi‐author textbook on anaesthesia (including notions on general and regional anaesthesia). I receive fees for a course on airway management presented at University of Quebec en Abitibi‐Temiscamingue.
Santhanam Suresh: I am co‐author of one excluded trial (Sohn 2010) and one ongoing trial (NCT02321787).
Sandra Kopp: none known.
Notes
The review authors thank Karen Hovhannisyan, who designed the search strategy, as well as the University of Sherbrooke and the University of Quebec in Abitibi‐Temiscamingue for granting access to electronic databases and to medical journals. We would also like to thank Rodrigo Cavallazzi (Content Editor); Vibeke E. Horstmann (Statistical Editor) and Vaughan L. Thomas and Kevin J. Walker (Peer Reviewers) for help and editorial advice provided during preparation of the protocol (Guay 2014) for this systematic review.
We thank Rodrigo Cavallazzi (Content Editor), Kevin J. Walker and Vaughan L. Thomas (Peer Reviewers), Jing Xie (Statistical Editor) and Sheila Page (Consumer Reviewer) for help provided in preparation of this review.
Edited (no change to conclusions)
References
References to studies included in this review
- Dingeman RS, Barus LM, Chung HK, Clendenin DJ, Lee CS, Tracy S, et al. Ultrasonography‐guided bilateral rectus sheath block vs local anesthetic infiltration after pediatric umbilical hernia repair: a prospective randomized clinical trial. JAMA Surgery 2013;148(8):707‐13. [; PUBMED: 23760519] [DOI] [PubMed] [Google Scholar]; 3317101
- Elnour HA, Hana MG, Rizk SN, Soaaida S. Ultrasound guided axillary brachial plexus block in paediatric surgical patients. Egyptian Journal of Anaesthesia 2009;25(3):281‐90. [CENTRAL: 00789695; ] [Google Scholar]; 3317103
- Faraoni D, Gilbeau A, Lingier P, Barvais L, Engelman E, Hennart D. Does ultrasound guidance improve the efficacy of dorsal penile nerve block in children?. Paediatric Anaesthesia 2010;20(10):931‐6. [; PUBMED: 20849498] [DOI] [PubMed] [Google Scholar]; 3317105
- Flack SH, Martin LD, Walker BJ, Bosenberg AT, Helmers LD, Goldin AB, et al. Ultrasound‐guided rectus sheath block or wound infiltration in children: a randomized blinded study of analgesia and bupivacaine absorption. Paediatric Anaesthesia 2014;24(9):968‐73. [; PUBMED: 24853314] [DOI] [PMC free article] [PubMed] [Google Scholar]; 3317107
- Gurnaney HG, Maxwell LG, Kraemer FW, Goebel T, Nance ML, Ganesh A. Prospective randomized observer‐blinded study comparing the analgesic efficacy of ultrasound‐guided rectus sheath block and local anaesthetic infiltration for umbilical hernia repair. British Journal of Anaesthesia 2011;107(5):790‐5. [; PUBMED: 21856778] [DOI] [PubMed] [Google Scholar]; 3317109
- Kendigelen P, Tututncu A, Erbabacan E, Koksal G, Ekici B. Preliminary experience with ultrasound guided transversus abdominis plane (TAP) block versus wound infiltration for unilateral inguinal surgery in paediatric patients. Regional Anesthesia and Pain Medicine. 2014; Vol. 39, issue 5 Suppl 1:E266. [] [Google Scholar]; 3317111
- Liu JZ, Wu XQ, Li R, Zhang YJ. [A comparison of ultrasonography versus traditional approach for caudal block in children]. Zhonghua Yi Xue Za Zhi 2012;92(13):882‐5. [; PUBMED: 22781527] [PubMed] [Google Scholar]; 3317113
- Lorenzo AJ, Lynch J, Matava C, El‐Beheiry H, Hayes J. Ultrasound guided transversus abdominis plane vs surgeon administered intraoperative regional field infiltration with bupivacaine for early postoperative pain control in children undergoing open pyeloplasty. The Journal of Urology 2014;192(1):207‐13. [; PUBMED: 24518763] [DOI] [PubMed] [Google Scholar]; 3317115
- Marhofer P, Sitzwohl C, Greher M, Kapral S. Ultrasound guidance for infraclavicular brachial plexus anaesthesia in children. Anaesthesia 2004;59(7):642‐6. [; PUBMED: 15200537] [DOI] [PubMed] [Google Scholar]; 3317117
- Nan Y, Zhou J, Ma Q, Li T, Lian QQ, Li J. [Application of ultrasound guidance for ilioinguinal or iliohypogastric nerve block in paediatric inguinal surgery]. Zhonghua Yi Xue Za Zhi 2012;92(13):873‐7. [; PUBMED: 22781525] [PubMed] [Google Scholar]; 3317119
- O'Sullivan MJ, Mislovic B, Alexander E. Dorsal penile nerve block for male paediatric circumcision ‐ randomized comparison of ultrasound‐guided vs anatomical landmark technique. Paediatric Anaesthesia 2011;21(12):1214‐8. [; PUBMED: 22023417] [DOI] [PubMed] [Google Scholar]; 3317121
- Oberndorfer U, Marhofer P, Bosenberg A, Willschke H, Felfernig M, Weintraud M, et al. Ultrasonographic guidance for sciatic and femoral nerve blocks in children. British Journal of Anaesthesia 2007;98(6):797‐801. [; PUBMED: 17449890] [DOI] [PubMed] [Google Scholar]; 3317123
- Ponde VC, Diwan S. Does ultrasound guidance improve the success rate of infraclavicular brachial plexus block when compared with nerve stimulation in children with radial club hands?. Anesthesia and Analgesia 2009;108(6):1967‐70. [; PUBMED: 19448233] [DOI] [PubMed] [Google Scholar]; 3317125
- Ponde V, Desai AP, Shah D. Comparison of success rate of ultrasound‐guided sciatic and femoral nerve block and neurostimulation in children with arthrogryposis multiplex congenita: a randomized clinical trial. Paediatric Anaesthesia 2013;23(1):74‐8. [; PUBMED: 23004225] [DOI] [PubMed] [Google Scholar]; 3317127
- Sahin L, Sahin M, Gul R, Saricicek V, Isikay N. Ultrasound‐guided transversus abdominis plane block in children: a randomised comparison with wound infiltration. European Journal of Anaesthesiology 2013;30(7):409‐14. [; PUBMED: 23338056] [DOI] [PubMed] [Google Scholar]; 3317129
- Tachibana N, Yamauchi M, Sugino S, Watanabe A, Yamakage M. Utility of longitudinal paramedian view of ultrasound imaging for middle thoracic epidural anaesthesia in children. Journal of Anaesthesia 2012;26(2):242‐5. [; PUBMED: 22081114] [DOI] [PubMed] [Google Scholar]; 3317131
- Wang LZ, Hu XX, Zhang YF, Chang XY. A randomized comparison of caudal block by sacral hiatus injection under ultrasound guidance with traditional sacral canal injection in children. Paediatric Anaesthesia 2013;23(5):395‐400. [; PUBMED: 23278906] [DOI] [PubMed] [Google Scholar]; 3317133
- Weintraud M, Lundblad M, Kettner SC, Willschke H, Kapral S, Lonnqvist PA, et al. Ultrasound versus landmark‐based technique for ilioinguinal‐iliohypogastric nerve blockade in children: the implications on plasma levels of ropivacaine. Anesthesia and Analgesia 2009;108(5):1488‐92. [; PUBMED: 19372326] [DOI] [PubMed] [Google Scholar]; 3317135
- Willschke H, Marhofer P, Bosenberg A, Johnston S, Wanzel O, Cox SG, et al. Ultrasonography for ilioinguinal/iliohypogastric nerve blocks in children. British Journal of Anaesthesia 2005;95(2):226‐30. [; PUBMED: 15923270] [DOI] [PubMed] [Google Scholar]; 3317137
- Willschke H, Marhofer P, Bosenberg A, Johnston S, Wanzel O, Sitzwohl C, et al. Epidural catheter placement in children: comparing a novel approach using ultrasound guidance and a standard loss‐of‐resistance technique. British Journal of Anaesthesia 2006;97(2):200‐7. [; PUBMED: 16720672] [DOI] [PubMed] [Google Scholar]; 3317139
References to studies excluded from this review
- Sohn L, Voronov P, Sawardekar A, Jagannathan N, Suresh S. Postoperative pain control in children undergoing laparoscopic appendectomy: comparison of ultrasound‐guided peripheral nerve blocks to local anaesthetic infiltration analgesia. Regional Anesthesia and Pain Medicine. 2010; Vol. 35, issue 5:461. [] [Google Scholar]; 3317141
- Triffterer L, Machata AM, Latzke D, Willschke H, Rebhandl W, Kimberger O, et al. Ultrasound assessment of cranial spread during caudal blockade in children: effect of the speed of injection of local anaesthetics. British Journal of Anaesthesia 2012;108(4):670‐4. [; PUBMED: 22315328] [DOI] [PubMed] [Google Scholar]; 3317143
References to ongoing studies
- ACTRN12608000488303. A comparison of ultrasound‐guided rectus sheath block and subcutaneous local anaesthetic infiltration for pain relief following paediatric umbilical hernia repair. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=82999 accessed January 2015. [] ; 3317145
- ACTRN12613000595718. Ultrasound guided transversus abdominis plane block versus local wound infiltration for post‐operative analgesia in children undergoing appendectomy: A Randomized Controlled Trial. http://www.anzctr.org.au/TrialSearch.aspx?searchTxt=ACTRN12613000595718 accessed January 2015. [] ; 3317147
- AT032012. Dorsal penile nerve block(DPNB) for circumcision: a comparison of ultrasound‐guided vs. landmark technique. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=AT032012 accessed January 2015. [] ; 3317149
- NCT01136668. Transversus abdominis plane block in children undergoing ostomy surgery. https://clinicaltrials.gov/ct2/show/NCT01136668 accessed January 2015. [] ; 3317151
- NCT01698268. Study of transverse abdominis plane (TAP) block in children undergoing hydrocelectomy and/or hernia repair surgery (TAP). https://clinicaltrials.gov/ct2/show/NCT01698268 accessed January 2015. [] ; 3317153
- NCT02321787. Ultrasonography for confirmation of caudal injection. https://clinicaltrials.gov/ct2/show/NCT02321787 accessed January 2015. [] ; 3317155
- NCT02341144. Percutaneous rectus sheath block versus intra‐operative rectus sheath block for pediatric umbilical hernia repair. https://clinicaltrials.gov/ct2/show/NCT02341144 accessed January 2015. [] ; 3317157
Additional references
- Borglum J, Jensen K, Christensen AF, Hoegberg LC, Johansen SS, Lonnqvist PA, et al. Distribution patterns, dermatomal anaesthesia, and ropivacaine serum concentrations after bilateral dual transversus abdominis plane block. Regional Anesthesia and Pain Medicine 2012;37(3):294‐301. [PUBMED: 22476239] [DOI] [PubMed] [Google Scholar]
- Buttner W, Finke W. Analysis of behavioural and physiological parameters for the assessment of postoperative analgesic demand in newborns, infants and young children: a comprehensive report on seven consecutive studies. Paediatric Anaesthesia 2000;10(3):303‐18. [PUBMED: 10792748] [DOI] [PubMed] [Google Scholar]
- Cates CJ. Simpson's paradox and calculation of number needed to treat from meta‐analysis. BMC Medical Research Methodology 2002;2:1. [PUBMED: 11860604] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao S, Zuo Z. A double‐edged sword: volatile anaesthetic effects on the neonatal brain. Brain Sciences 2014;4(2):273‐94. [PUBMED: 24961761] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks JJ. Issues in the selection of a summary statistic for meta‐analysis of clinical trials with binary outcomes. Statistics in Medicine 2002;21(11):1575‐600. [PUBMED: 12111921] [DOI] [PubMed] [Google Scholar]
- DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital and Health Statistics. Series 13, Data from the National Health Survey 2007;13(165):1‐209. [PUBMED: 18350768] [PubMed] [Google Scholar]
- Feinglass NG, Clendenen SR, Torp KD, Wang RD, Castello R, Greengrass RA. Real‐time three‐dimensional ultrasound for continuous popliteal blockade: a case report and image description. Anesthesia and Analgesia 2007;105(1):272‐4. [PUBMED: 17578987] [DOI] [PubMed] [Google Scholar]
- Guay J. The benefits of adding epidural analgesia to general anaesthesia: a metaanalysis. Journal of Anesthesia 2006;20(4):335‐40. [PUBMED: 17072704] [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ (Clinical Research Ed.) 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical Research Ed.) 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
- Hohmeister J, Kroll A, Wollgarten‐Hadamek I, Zohsel K, Demirakca S, Flor H, et al. Cerebral processing of pain in school‐aged children with neonatal nociceptive input: an exploratory MRI study. Pain 2010;150(2):257‐67. [PUBMED: 20471751] [DOI] [PubMed] [Google Scholar]
- Kapral S, Krafft P, Eibenberger K, Fitzgerald R, Gosch M, Weinstabl C. Ultrasound‐guided supraclavicular approach for regional anesthesia of the brachial plexus. Anesthesia and Analgesia 1994;78(3):507‐13. [PUBMED: 8109769] [DOI] [PubMed] [Google Scholar]
- Grange P, Foster PA, Pretorius LK. Application of the Doppler ultrasound bloodflow detector in supraclavicular brachial plexus block. British Journal of Anaesthesia 1978;50(9):965‐7. [PUBMED: 708565] [DOI] [PubMed] [Google Scholar]
- Lee TH, Barrington MJ, Tran TM, Wong D, Hebbard PD. Comparison of extent of sensory block following posterior and subcostal approaches to ultrasound‐guided transversus abdominis plane block. Anaesthesia and Intensive Care 2010;38(3):452‐60. [PUBMED: 20514952] [DOI] [PubMed] [Google Scholar]
- Liu SS, John RS. Modeling cost of ultrasound versus nerve stimulator guidance for nerve blocks with sensitivity analysis. Regional Anesthesia and Pain Medicine 2010;35(1):57‐63. [PUBMED: 20052815] [DOI] [PubMed] [Google Scholar]
- Long JB, Birmingham PK, Oliveira GS Jr, Schaldenbrand KM, Suresh S. Transversus abdominis plane block in children: a multicenter safety analysis of 1994 cases from the PRAN (Pediatric Regional Anesthesia Network) Database. Anesthesia and Analgesia 2014;119(2):395‐9. [PUBMED: 24918899] [DOI] [PubMed] [Google Scholar]
- McColl A, Smith H, White P, Field J. General practitioner's perceptions of the route to evidence based medicine: a questionnaire survey. BMJ (Clinical Research Ed.) 1998;316(7128):361‐5. [PUBMED: 9487174] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher 2009: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
- Neal JM, Bernards CM, Hadzic A, Hebl JR, Hogan QH, Horlocker TT, et al. ASRA Practice Advisory on Neurologic Complications in Regional Anesthesia and Pain Medicine. Regional Anesthesia and Pain Medicine 2008;33(5):404‐15. [PUBMED: 18774509] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemergut ME, Crow S, Flick RP. Cognitive outcomes after infant spinal anesthesia: the other side of the coin. Anesthesia and Analgesia2014; Vol. 119, issue 3:514‐5. [PUBMED: 25136997] [DOI] [PMC free article] [PubMed]
- Niazi AU, Chin KJ, Jin R, Chan VW. Real‐time ultrasound‐guided spinal anesthesia using the SonixGPS ultrasound guidance system: a feasibility study. Acta Anaesthesiologica Scandinavica 2014;58(7):875‐81. [PUBMED: 24943307] [DOI] [PubMed] [Google Scholar]
- Pace NL. Research methods for meta‐analyses. Best Practice & Research. Clinical Anaesthesiology 2011;25(4):523‐33. [PUBMED: 22099918] [DOI] [PubMed] [Google Scholar]
- Pogue J, Yusuf S. Overcoming the limitations of current meta‐analysis of randomised controlled trials. Lancet 1998;351(9095):47‐52. [PUBMED: 9433436] [DOI] [PubMed] [Google Scholar]
- Polaner DM, Taenzer AH, Walker BJ, Bosenberg A, Krane EJ, Suresh S, et al. Pediatric Regional Anesthesia Network (PRAN): a multi‐institutional study of the use and incidence of complications of pediatric regional anesthesia. Anesthesia and Analgesia 2012;115(6):1353‐64. [PUBMED: 22696610] [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Rucker G, Schwarzer G, Carpenter JR, Binder H, Schumacher M. Treatment‐effect estimates adjusted for small‐study effects via a limit meta‐analysis. Biostatistics (Oxford, England) 2011;12(1):122‐42. [PUBMED: 20656692] [DOI] [PubMed] [Google Scholar]
- Suresh S, Schaldenbrand K, Wallis B, Oliveira GS Jr. Regional anaesthesia to improve pain outcomes in paediatric surgical patients: a qualitative systematic review of randomized controlled trials. British Journal of Anaesthesia 2014;113(3):375‐90. [PUBMED: 24907283] [DOI] [PubMed] [Google Scholar]
- Taenzer AH, Walker BJ, Bosenberg AT, Martin L, Suresh S, Polaner DM, et al. Asleep versus awake: does it matter?: Pediatric regional block complications by patient state: a report from the Pediatric Regional Anesthesia Network. Regional Anesthesia and Pain Medicine 2014;39(4):279‐83. [PUBMED: 24918334] [DOI] [PubMed] [Google Scholar]
- Ting PL, Sivagnanaratnam V. Ultrasonographic study of the spread of local anaesthetic during axillary brachial plexus block. British Journal of Anaesthesia 1989;63(3):326‐9. [PUBMED: 2679832] [DOI] [PubMed] [Google Scholar]
- Tsui BC, Suresh S. Ultrasound imaging for regional anesthesia in infants, children, and adolescents: a review of current literature and its application in the practice of neuraxial blocks. Anesthesiology 2010;112(3):719‐28. [PUBMED: 20179511] [DOI] [PubMed] [Google Scholar]
- Voepel‐Lewis T, Marinkovic A, Kostrzewa A, Tait AR, Malviya S. The prevalence of and risk factors for adverse events in children receiving patient‐controlled analgesia by proxy or patient‐controlled analgesia after surgery. Anesthesia and Analgesia 2008;107(1):70‐5. [PUBMED: 18635469] [DOI] [PubMed] [Google Scholar]
- Walker KJ, McGrattan K, Aas‐Eng K, Smith AF. Ultrasound guidance for peripheral nerve blockade. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD006459.pub2] [DOI] [PubMed] [Google Scholar]
- White PF, Kehlet H. Improving pain management: are we jumping from the frying pan into the fire?. Anesthesia and Analgesia2007; Vol. 105, issue 1:10‐2. [PUBMED: 17578944] [DOI] [PubMed]
- Wu TJ, Lin SY, Liu CC, Chang HC, Lin CC. Ultrasound imaging aids infraclavicular brachial plexus block. Ma Zui Xue Za Zhi = Anaesthesiologica Sinica 1993;31(2):83‐6. [PUBMED: 7934690] [PubMed] [Google Scholar]
References to other published versions of this review
- Guay J, Suresh S, Kopp S. The use of ultrasound guidance for perioperative neuraxial and peripheral nerve blocks in children. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD011436] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guay J, Suresh S, Kopp S. The use of ultrasound guidance for perioperative neuraxial and peripheral nerve blocks in children: A Cochrane Review. Anesthesia and Analgesia 2016:[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


