Abstract
Background
Treadmill training, with or without body weight support using a harness, is used in rehabilitation and might help to improve walking after stroke. This is an update of a Cochrane review first published in 2005.
Objectives
To determine if treadmill training and body weight support, individually or in combination, improve walking ability, quality of life, activities of daily living, dependency or death, and institutionalisation or death, compared with other physiotherapy gait training interventions after stroke. The secondary objective was to determine the safety and acceptability of this method of gait training.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched June 2013), the Cochrane Central Register of Controlled Trials (CENTRAL) and the Database of Reviews of Effects (DARE) (The Cochrane Library 2013, Issue 7), MEDLINE (1966 to July 2013), EMBASE (1980 to July 2013), CINAHL (1982 to June 2013), AMED (1985 to July 2013) and SPORTDiscus (1949 to June 2013). We also handsearched relevant conference proceedings and ongoing trials and research registers, screened reference lists and contacted trialists to identify further trials.
Selection criteria
Randomised or quasi‐randomised controlled and cross‐over trials of treadmill training and body weight support, individually or in combination, for the treatment of walking after stroke.
Data collection and analysis
Two authors independently selected trials, extracted data and assessed methodological quality. The primary outcomes investigated were walking speed, endurance and dependency.
Main results
We included 44 trials with 2658 participants in this updated review. Overall, the use of treadmill training with body weight support did not increase the chances of walking independently compared with other physiotherapy interventions (risk difference (RD) ‐0.00, 95% confidence interval (CI) ‐0.02 to 0.02; P = 0.94; I² = 0%). Overall, the use of treadmill training with body weight support in walking rehabilitation for patients after stroke increased the walking velocity and walking endurance significantly. The pooled mean difference (MD) (random‐effects model) for walking velocity was 0.07 m/s (95% CI 0.01 to 0.12; P = 0.02; I² = 57%) and the pooled MD for walking endurance was 26.35 metres (95% CI 2.51 to 50.19; P = 0.03; I² = 60%). Overall, the use of treadmill training with body weight support in walking rehabilitation for patients after stroke did not increase the walking velocity and walking endurance at the end of scheduled follow‐up significantly. The pooled MD (random‐effects model) for walking velocity was 0.04 m/s (95% CI ‐0.06 to 0.14; P = 0.40; I² = 40%) and the pooled MD for walking endurance was 32.36 metres (95% CI ‐3.10 to 67.81; P = 0.07; I² = 63%). However, for ambulatory patients improvements in walking endurance lasted until the end of scheduled follow‐up (MD 58.88 metres, 95% CI 29.10 to 88.66; P = 0.0001; I² = 0%). Adverse events and drop outs did not occur more frequently in people receiving treadmill training and these were not judged to be clinically serious events.
Authors' conclusions
Overall, people after stroke who receive treadmill training with or without body weight support are not more likely to improve their ability to walk independently compared with people after stroke not receiving treadmill training, but walking speed and walking endurance may improve. Specifically, stroke patients who are able to walk (but not people who are not able to walk) appear to benefit most from this type of intervention. This review found that improvements in walking endurance in people able to walk may have persisting beneficial effects. Further research should specifically investigate the effects of different frequencies, durations or intensities (in terms of speed increments and inclination) of treadmill training, as well as the use of handrails, in ambulatory patients, but not in dependent walkers.
Keywords: Humans, Stroke Rehabilitation, Body Weight, Exercise Therapy, Exercise Therapy/instrumentation, Exercise Therapy/methods, Orthotic Devices, Randomized Controlled Trials as Topic, Walking, Weight‐Bearing
Treadmill training and body weight support for walking after stroke
Question: We wanted to assess whether treadmill training and body weight support, individually or in combination, could improve walking when compared with other gait training methods, placebo or no treatment.
Background: About 60% of people who have had a stroke have difficulties with walking, and improving walking is one of the main goals of rehabilitation. Treadmill training, with or without body weight support, uses specialist equipment to assist walking practice.
Study characteristics: We identified 44 relevant trials, involving 2658 participants, up to June 2013. Twenty‐two studies (1588 participants) compared treadmill training with body weight support to another physiotherapy intervention; 16 studies (823 participants) compared treadmill training without body weight support to other physiotherapy intervention, no intervention or sham; two studies (100 participants) compared treadmill training with body weight support to treadmill training without body weight support; and four studies (147 participants) did not state whether they used body weight support or not. The average age of the participants ranged from 50 to 75 years, and the studies were carried out in both inpatient and outpatient settings.
Key results and quality of the evidence: The results of this review were partly conclusive. People after stroke who receive treadmill training with or without body weight support are not more likely to improve their ability to walk independently. The quality of this evidence was low. However, treadmill training with or without body weight support may improve walking speed and walking capacity compared with people not receiving treadmill training. The quality of this evidence was moderate. More specifically, people after stroke who are able to walk at the start of therapy appear to benefit most from this type of intervention, but people who are not able to walk independently at therapy onset do not benefit. This review found that improvements in walking speed and endurance in people who can walk may have persisting beneficial effects. However, our review suggests that stroke patients who are not able to walk independently at the start of treatment may not benefit from treadmill training with or without body weight support. Adverse events and drop outs did not occur more frequently in people receiving treadmill training. Subgroup analysis showed that treadmill training in the first three months after stroke produces statistically and clinically relevant improvements in walking speeds and endurance. For people treated in the chronic phase (i.e. more than six months post‐stroke) the effects were lower. Treadmill training at higher frequencies may produce greater effects on walking speed and endurance; however, this was not significant.
In practice, treadmill training should be used when stroke patients can walk independently. Therapists should be aware that treadmill training may be used as an option but not as a stand‐alone treatment to improve walking speed and endurance in people who are able to walk independently. It appears that people who can walk after stroke, but not those who cannot, may profit from treadmill training (with and without body weight support) to improve their walking abilities. Further research should specifically investigate the effects of different frequencies, durations or intensities (in terms of speed increments and inclination) of treadmill training, as well as the use of handrails. Future trials should include people who can already walk, but not dependent walkers who are unable to walk unaided.
Summary of findings
Summary of findings for the main comparison.
Treadmill (with or without BWS) for walking after stroke
| Treadmill (with or without BWS) for walking after stroke | ||||||
| Patient or population: patients with walking after stroke Settings: Inpatient and outpatient Intervention: Treadmill (with or without BWS) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Treadmill (with or without BWS) | |||||
| Walking speed (m/sec) at end of treatment phase Measures of timed gait | The mean walking speed (m/sec) at end of treatment phase in the control groups was 0.59 m/sec | The mean walking speed (m/sec) at end of treatment phase in the intervention groups was 0.07 higher (0.03 to 0.11 higher) | 1891 (35 studies) | ⊕⊕⊝⊝ low1,2 | ||
| Walking speed (m/sec) at end of treatment phase ‐ dependent in walking at start of treatment Measures of timed gait | The mean walking speed (m/sec) at end of treatment phase ‐ dependent in walking at start of treatment in the control groups was 0.26 m/sec | The mean walking speed (m/sec) at end of treatment phase ‐ dependent in walking at start of treatment in the intervention groups was 0.01 lower (0.06 lower to 0.03 higher) | 752 (9 studies) | ⊕⊕⊝⊝ low1,3 | ||
| Walking speed (m/sec) at end of treatment phase ‐ independent in walking at start of treatment Measures of timed gait | The mean walking speed (m/sec) at end of treatment phase ‐ independent in walking at start of treatment in the control groups was 0.67 m/sec | The mean walking speed (m/sec) at end of treatment phase ‐ independent in walking at start of treatment in the intervention groups was 0.11 higher (0.06 to 0.16 higher) | 1139 (26 studies) | ⊕⊕⊕⊝ moderate1,2,4 | ||
| walking endurance (m) at the end of treatment Measures of timed gait | The mean walking endurance (m) at the end of treatment in the control groups was 203.7 m | The mean walking endurance (m) at the end of treatment in the intervention groups was 20.08 higher (6.14 to 34.03 higher) | 1388 (20 studies) | ⊕⊕⊝⊝ low1,2 | ||
| walking endurance (m) at the end of treatment ‐ dependent in walking at start of treatment Measures of timed gait | The mean walking endurance (m) at the end of treatment ‐ dependent in walking at start of treatment in the control groups was 115.3 m | The mean walking endurance (m) at the end of treatment ‐ dependent in walking at start of treatment in the intervention groups was 5.09 lower (23.41 lower to 13.22 higher) | 639 (5 studies) | ⊕⊕⊝⊝ low1,3 | ||
| walking endurance (m) at the end of treatment ‐ independent in walking at start of treatment Measures of timed gait | The mean walking endurance (m) at the end of treatment ‐ independent in walking at start of treatment in the control groups was 240.9 m | The mean walking endurance (m) at the end of treatment ‐ independent in walking at start of treatment in the intervention groups was 30.61 higher (14.02 to 47.2 higher) | 749 (15 studies) | ⊕⊕⊕⊝ moderate1,2,4 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded due to several ratings with "unclear" or even "high" risk of bias 2 Downgraded due to CIs embracing effect size of least clinically important benefit 3 Downgraded due to CIs embracing effect size of null hypothesis 4 Upgraded due to evidence of a dose response gradient
Background
Description of the condition
Stroke ranks as the sixth highest cause of burden of disease worldwide in terms of disability adjusted life years and is the single most important cause of severe disability in people living in their own homes (Murray 2012). An inability or an impaired ability to walk is a significant contributor to long‐term disability and burden of care after stroke. Approximately one‐third of people surviving acute stroke are unable to walk three months after admission to a general hospital (Langhorne 2009).
High‐quality evidence from systematic reviews indicates that organised (stroke unit) care decreases physical dependence after stroke compared with general medical care (SUTC 2013). This organised care is characterised by early mobilisation and multidisciplinary rehabilitation (including physiotherapy) co‐ordinated by regular team meetings (Langhorne 2002). The effectiveness of specific physiotherapy gait training strategies, however, is still not very clear. A review of studies comparing different physiotherapy treatments for patients with stroke concluded that "There is insufficient evidence to conclude that any one physiotherapy approach is more effective in promoting recovery of lower limb function or postural control following stroke than any other approach." (Pollock 2007).
Description of the intervention
Improving walking after stroke is one of the main goals of rehabilitation. There is increasing evidence that high‐intensity, repetitive, task‐specific training might result in better gait rehabilitation (French 2007; Langhorne 2009). One example of potentially intensive, repetitive, task‐specific gait training is treadmill training.
Walking on a treadmill, with or without body weight supported via a harness connected to an overhead support system, is a method of treating walking impairments post stroke that is becoming increasingly popular. Use of a treadmill permits a greater number of steps to be performed within a training session: that is, it increases the amount of task‐specific practice completed. For example, Hesse 2003 reported that people after stroke can perform up to 1000 steps in a 20‐minute treadmill training session, compared with only 50 to 100 steps during a 20‐minute session of conventional physiotherapy (neurophysiological approach). The speed of the treadmill, the amount of body weight support and the amount of assistance provided by the physiotherapist can all be adjusted in order to provide a sufficient training intensity. This intervention emerged from research involving spinalised cats (Barbeau 1987) and was first used in clinical settings in the 1980s (Finch 1985). Since then, treadmill training with partial body weight support has been increasingly promoted as a treatment to drive recovery after stroke (Charalambous 2013; Langhorne 2009).
Treadmill training with body weight support is costly in terms of equipment and human resources. The treadmill and body weight suspension system alone may cost up to USD 180,000 (Reyes 2000). In addition, the equipment is not portable, so stroke patients must attend a suitably equipped healthcare facility in order to access this treatment. Several published randomised controlled trials (RCTs) have evaluated treadmill training with or without body weight support (Charalambous 2013; Polese 2013).
Why it is important to do this review
Several non‐Cochrane systematic reviews evaluating treadmill training with and without body weight support have been published since this Cochrane review first appeared in The Cochrane Library 2003, Issue 3 (e.g. Manning 2003; Teasell 2003; van Peppen 2004) and in the last year (Charalambous 2013; Polese 2013). However, all of these reviews are now out of date or had some methodological weaknesses (for example they did not used a comprehensive search strategy for all relevant databases or were prone to language bias because non‐English studies were not included).
Updating this Cochrane review is required in order to justify the large equipment and human resource cost required to implement treadmill training as well as to confirm the safety and acceptance of this method of training. The first update of this review was published 2005 and included 15 trials with 622 participants. This is the second update of this Cochrane review. The search for trials was extended from March 2005 to July 2013. The aim of this review is to provide an update of the best available evidence about the above‐mentioned approach.
Objectives
To determine if treadmill training and body weight support, individually or in combination, improve walking ability, quality of life, activities of daily living, dependency or death, and institutionalisation or death, compared with other physiotherapy gait training interventions after stroke. The secondary objective was to determine the safety and acceptability of this method of gait training.
Methods
Criteria for considering studies for this review
Types of studies
We included truly randomised and quasi‐randomised controlled trials (including cross‐over trials) in the review. We considered procedures such as coin tossing and dice rolling as random. Quasi‐random allocation procedures included allocation by hospital record number or birth date, or alternation. We only included the first arm of the data from cross‐over trials. We assessed concealment, blinding and the number of withdrawals for all trials, but we did not use these data as inclusion or exclusion criteria.
Treadmill training and body weight support, individually or in combination, must have been implemented in one of the experimental conditions. We were looking for trials that made one of the following comparisons:
treadmill training with body weight support versus other physiotherapy, placebo or no intervention;
treadmill training without body weight support versus other physiotherapy, placebo or no intervention;
treadmill training with body weight support versus treadmill training without body weight support; and
body weight support (without treadmill training) versus other physiotherapy, placebo or no intervention.
Treadmill training and body weight support, individually or in combination, may have been implemented with physiotherapy co‐intervention(s). Where co‐intervention(s) were comparable for experimental and control groups, we grouped the trials according to the first four comparisons. In some cases, however, the co‐intervention(s) used were not the same for the treatment and control groups. For example, treadmill training with body weight support may be implemented as one component of a task‐oriented physiotherapy programme and compared with non task‐oriented physiotherapy (Richards 1993). Task‐oriented physiotherapy programmes involve task and context‐specific training of motor skills based on a movement science or motor relearning framework (Carr 1998). Non‐task‐oriented physiotherapy includes neurophysiological approaches to treatment, such as Bobath (Bobath 1990), Brunnstrom (Brunnstrom 1970), Rood (Goff 1969) and proprioceptive neuromuscular facilitation (Knott 1968). While these trials cannot differentiate the effects of treadmill training and body weight support from other co‐interventions, they do evaluate the intervention as part of a treatment package. We identified such trials and described them separately.
We included trials that evaluated any intensity and duration of treadmill training and body weight support that exceeded a single treatment session. Where necessary, we obtained details of the treatment and control interventions via correspondence with the trialists.
Types of participants
We included trials of adults who had suffered a stroke and exhibited an abnormal gait pattern. We used the World Health Organization's (WHO) definition of stroke: "rapidly developing clinical signs of focal (at times global) disturbance of cerebral function, lasting more than 24 hours or leading to death, with no apparent cause other than that of vascular origin." (Hatano 1976). We defined an abnormal gait pattern as walking with a slow speed, exhibiting kinematic deviations during gait (Moore 1993; Moseley 1993) or an inability to walk.
We envisaged that some trials may have included participants with other types of upper motor neurone lesions (e.g. traumatic brain injury, multiple sclerosis). However, we did not identify any mixed trials. If we identify trials using mixed types of participants in future updates of this review, we will attempt to obtain data for the stroke subgroup only via correspondence with the trialists.
Types of interventions
The primary question was whether treadmill training and body weight support, individually or in combination, could improve walking compared with other gait training methods, placebo or no treatment. We therefore included any trial that attempted to evaluate such a comparison. Treadmill training involves walking on a standard treadmill; assistance, feedback or guidance may be provided by a health professional (usually a physiotherapist). Some of the patient's body weight may be supported during this training using a harness attached to an overhead support system. Alternatively, this type of body weight support can be used without a treadmill.
Types of outcome measures
The primary analyses focused on the ability to walk both at the end of the treatment period (that is, immediate or short‐term effects) and at the end of the scheduled follow‐up (that is, long‐term effects). We examined the ability to walk using dichotomous and continuous variables.
The dichotomous variable was 'dependence on personal assistance', where we defined 'dependence' as the inability to walk indoors (with or without a gait aid) without personal assistance or supervision. If reported, we used data from functional scales (or parts of functional scales relating to walking) to define the level of dependence. Suitable scales (with criterion for 'dependence') are:
Motor Assessment Scale (Carr 1985), a score of two or less;
Functional Independence Measure (Hamilton 1994), a score of five or less for the walking item;
Barthel Index (Collin 1988), a score of three (independent, but may use any aid) or less for the ambulation item;
Rivermead Mobility Index (Collen 1991), an answer of 'no' to the 'walking inside, with an aid if necessary' item; and
Functional Ambulation Category (Holden 1984), a score of two or less.
We used walking dependence at the start of treatment to group trials in each comparison in the analyses.
The continuous variables were:
independent walking speed measured over a short distance (e.g. six to 10 metres); and
independent walking endurance measured over a long distance (e.g. Six‐Minute Walk Test) expressed as a total distance walked.
These tests could be performed with or without a gait aid, but must have been completed without personal assistance. Wade 1992 reported that independent walking speed over a short distance is a simple, reliable, valid and sensitive measure of walking performance. Walking over a long distance is a valid (Wade 1992) and reliable (Guyatt 1984) measure of walking endurance with established reference equations (Enright 1998). Where participants could not walk unless assisted, we allocated a speed and distance score of zero.
Secondary outcome measures included patient quality of life, ability to perform activities of daily living and the combined outcomes of death or dependency, and death or institutional care. Quality of life scales include the Frenchay Activities Index, Medical Outcomes Study Short Form Health Survey Questionnaire, Nottingham Health Profile, Quality of Life Index and Sickness Impact Profile (de Haan 1993).
Activities of daily living scales include the Barthel Index, Modified Rankin Scale and Nottingham Extended Activities of Daily Living Scale (Wade 1992); and the Index of Activities of Daily Living, Instrumental Activities of Daily Living Scale, Functional Activities Questionnaire and Blessed Functional Activities Scale (Pohjasvaara 1997).
We used the Stroke Unit Trialists' Collaboration definitions for death or dependency and death or institutional care (SUTC 2013). The criterion for dependency is scoring less than 18 on the Barthel Index or greater than two on the Modified Rankin Scale, while institutional care refers to care in a residential home, nursing home or hospital at the end of the scheduled follow‐up.
We determined the safety and acceptance of treadmill training. We used the prevalence of adverse events during the treatment period as a measure of safety. We categorised adverse events into injurious falls, other injury, major cardiovascular events and any other adverse outcomes. We examined the reason for participants withdrawing from the studies as a marker for acceptance. We analysed this withdrawal data qualitatively.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. For this update we extended the search for trials from March 2005 (when the first update of this review was published) to July 2013. We searched for trials in all languages and arranged translation of relevant trial reports published in languages other then English.
Electronic searches
We searched the Cochrane Stroke Group Trials Register (last searched June 2013) and the following electronic bibliographic databases:
The Cochrane Central Register of Controlled Trials (CENTRAL) and the Database of Reviews of Effects (DARE) (The Cochrane Library2013, Issue 7) (Appendix 1);
MEDLINE (1966 to July 2013) (Appendix 2);
EMBASE (1980 to July 2013) (Appendix 3);
CINAHL (1982 to June 2013) (Appendix 4);
AMED (1985 to July 2013) (Appendix 5); and
SPORTDiscus (1949 to June 2013) (Appendix 6).
We developed the search strategies with the help of the Cochrane Stroke Group Trials Search Co‐ordinator and adapted the MEDLINE search strategy for the other databases.
We identified and searched the following ongoing trials and research registers:
International Standard Randomised Controlled Trial Number Register at http://www.controlled‐trials.com/isrctn/ (searched September 2013);
Clinical trials.gov at www.clinicaltrials.gov (searched September 2013); and
Stroke Trials Register at www.strokecenter.org (searched September 2013).
Searching other resources
We also:
-
handsearched the following relevant conference proceedings:
World Congress of NeuroRehabilitation (2006, 2008, 2010 and 2012);
World Congress of Physical Medicine and Rehabilitation (2005, 2007, 2009, 2011 and 2013);
World Congress of Physical Therapy (2007 and 2011);
Deutsche Gesellschaft für Neurotraumatologie und Klinische Neurorehabilitation (2005 to 2013);
Deutsche Gesellschaft für Neurologie (2005 to 2013);
Deutsche Gesellschaft für Neurorehabilitation (2005 to 2013); and
Asian Oceania Conference of Physical and Rehabilitation (2008 to 2012);
screened reference lists of all relevant articles; and
contacted trialists, experts and researchers in our field of study.
Data collection and analysis
On 28 March 2013 we were contacted by the Cochrane Stroke Group; the authors of the 2005 version of the published Cochrane review of 'Treadmill training and body weight support for walking after stroke' intimated that they were no longer able to update this review. Our author team accepted the invitation to take over this review and update it.
We contacted the original review authors of the 2005 review and received data for all studies included in the 2005 version. We updated these original study data, including eligible studies from 2005 onwards.
Selection of studies
For this update, two review authors (BE and JM) read the titles and abstracts of the records identified from the electronic searches and eliminated obviously irrelevant studies. We retrieved the full texts of the remaining studies and two review authors (MP, BE) ranked the studies as relevant, possibly relevant or irrelevant according to our inclusion criteria (types of studies, participants, aims of interventions). Two review authors (JM, MP) then examined whether the relevant and possibly relevant publications fitted the population, intervention, comparison, outcome (PICO) strategy of our study question. We resolved disagreements by discussion with all authors. If we needed further information, we contacted trial authors.
We excluded studies that did not match our inclusion criteria regarding the type of study, participants or type of interventions and those that were not RCTs.
Data extraction and management
For this update, two review authors (BE, JM) independently extracted trial and outcome data from the selected trials. If one of the review authors was involved in an included trial, another review author extracted the trial and outcome data from that trial. In accordance with the 'Risk of bias' tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we used checklists to independently assess:
methods of random sequence generation;
methods of allocation concealment;
blinding of assessors;
blinding of patients;
adverse effects and drop outs;
important imbalances in prognostic factors at baseline;
participants (country, number of participants, age, gender, type of stroke, time from stroke onset to study entry, inclusion and exclusion criteria, cognition, pre‐existing neurological impairment(s), neurological history);
comparison (details of interventions in treatment and control groups, duration of treatment, details of co‐interventions in the groups);
outcomes and their time point of measurement.
All review authors checked the extracted data for agreement. If these authors could not reach consensus, a researcher not involved in data extraction arbitrated. If necessary, we contacted the researchers to request more information.
Assessment of risk of bias in included studies
For this update of the review two authors (BE and JM) independently assessed the risk of bias in the included trials in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We described the agreement between authors during the assessment of risk of bias and we resolved disagreement by reaching consensus through discussion. We contacted trialists for clarification and to request missing information.
Measures of treatment effect
For all outcomes representing continuous data, we entered means and standard deviations. We calculated a pooled estimate of the mean difference (MD) with 95% confidence interval (CI). If studies did not use the same outcome measure we calculated standardised mean differences (SMD) instead of MDs. For all binary outcomes we calculated risk differences (RD) with 95% CI. For all analyses we used The Cochrane Collaboration's Review Manager software, RevMan 5.2 (RevMan 2012) and used a random‐effects model for all analyses.
Dealing with missing data
We contacted the relevant principal investigators to retrieve missing data.
Assessment of heterogeneity
We used the I² statistic to assess heterogeneity. We used a random‐effects model, regardless of the level of heterogeneity. Thus, in the case of heterogeneity we did not violate the preconditions of a fixed‐effect model approach. We visually examined publication bias using funnel plots.
Subgroup analysis and investigation of heterogeneity
We did three subgroup analyses for time between the stroke and the start of training, the intensity of training and the duration of training. However, for the types of co‐interventions implemented in conjunction with treadmill training we were not able to conduct a subgroup analysis.
Sensitivity analysis
We performed a sensitivity analysis based on the methodological quality of trials (involving treadmill training) including true versus quasi‐randomisation, concealed versus unconcealed allocation and blinded versus non‐blinded outcome assessment.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification and Characteristics of ongoing studies.
Results of the search
Figure 1 shows the flow diagram for the selection of studies. The searches of the electronic databases and trials registers generated 8875 unique references for screening. After excluding non‐relevant citations we obtained the full texts of 246 papers; of these, we included 46 trials in the qualitative analysis and 44 trials in the quantitative analysis of the review.
Figure 1.
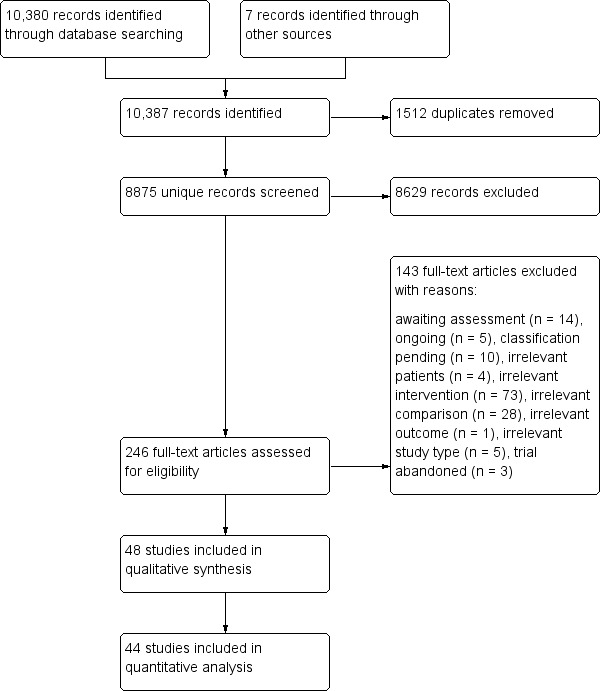
Flow diagram.
Included studies
We included 44 studies involving a total of 2658 participants in the quantitative analysis of this review (Ada 2003; Ada 2010; Ada 2013; Kim 2011; da Cunha Filho 2002; Deniz 2011; Du 2006; Duncan 2011; Eich 2004; Franceschini 2009; Gan 2012; Globas 2011; Hoyer 2012; Jaffe 2004; Kang 2012; Kosak 2000; Kuys 2011; Langhammer 2010; Laufer 2001; Liston 2000; Luft 2008; MacKay‐Lyons 2013; Macko 2005; Mehrberg 2001; Moore 2010; Nilsson 2001; Nilsson 2001a; Nilsson 2001b; Olawale 2009; Pohl 2002; Richards 1993; Richards 2004; Scheidtmann 1999; Smith 2008; Sullivan 2007; Suputtitada 2004; Takami 2010; Toledano‐Zarhi 2011; Visintin 1998; Visintin 1998a; Visintin 1998b; Weng 2004; Weng 2006; Werner 2002a; Yang 2010; Yen 2008; Zhang 2008; Zhu 2004; see the Characteristics of included studies). Two included studies have been split up into two sub‐studies each (Nilsson 2001; Visintin 1998).
22 studies (1588 participants) compared treadmill training with body weight support to other physiotherapy intervention.
16 studies (823 participants) compared treadmill training without body weight support to other physiotherapy intervention, no intervention or sham.
two studies (100 participants) compared treadmill training with body weight support to treadmill training without body weight support.
four studies (147 participants) did not state whether they used body weight support or not.
No studies compared body weight support without treadmill training to another physiotherapy intervention.
The data from two studies were sub‐divided for the analyses and the corresponding patients were not double counted. The Nilsson 2001 and Visintin 1998 studies recruited both dependent and independent walkers, so the data were sub‐divided into two comparisons for each trial. For the Nilsson 2001 trial, we separately analysed data from the 54 participants (26 experimental and 28 control) who were dependent walkers at the start of treatment (Nilsson 2001a) and data from the 19 participants (10 experimental and nine control) who were independent walkers at the start of treatment (Nilsson 2001b). For the Visintin 1998 trial, we performed separate analyses for data from the 59 participants (33 experimental and 26 control) (Visintin 1998a) and 20 participants (10 experimental and 10 control) (Visintin 1998b) who were dependent and independent walkers at the start of treatment, respectively. We obtained these walking dependency data through correspondence with the authors.
The characteristics of participants in the included studies are listed in Table 12. The characteristics of the experimental interventions are listed in Table 13. The outcomes used in the included studies are described in detail in the Characteristics of included studies. The reporting of adverse events and drop outs was incomplete for all trials and described in detail in Table 14 and Table 15. If these data were not explicitly reported, we attempted to obtain the missing information through correspondence with the trialists.
Table 1.
Participant characteristics
| Study ID | EXP age | CTL age | EXP gender | CTL gender | EXP time post stroke | CTL time post stroke | EXP paresis side | CTL paresis side |
| Ada 2003 | Mean 66 (SD 11) years (excluding 1 drop out) | Mean 66 (SD 11) years (excluding 1 drop out) | Male/female 9/4 | Male/female 10/4 | Mean 28 (SD 17) months | Mean 26 (SD 20) months | Left/right 5/8 | Left/right 8/6 |
| Ada 2010 | Mean 70 (SD 9) years | Mean 71 (SD 9) years | Male/female 38/26 | Male/female 33/29 | Mean 18 (SD 8) days | Mean 18 (SD 7) days | Left/right 34/30 | Left/right 36/26 |
| Ada 2013 | Mean 67 (SD 12) years | Mean 63 (SD 13) years | Male/female 52/16 | Male/female 19/15 | Mean 21 (SD 16) months | Mean 19 (SD 13) months | Left/right 32/34 | Left/right 13/21 |
| Kim 2011 | Mean 51 (SD 4) years | Mean 50 (SD 8) years | Male/female 11/9 | Male/female 14/10 | Mean 15 (SD 6) months | Mean 14 (SD 3) months | Left/right 8/12 | Left/right 8/16 |
| da Cunha Filho 2002 | Mean 57.8 (SD 5.5) years (excluding drop outs) | Mean 58.9 (SD 12.9) years (excluding drop outs) | Male/female 6/0 | Male/female 7/0 | Mean 15.7 (SD 7.7) days | Mean 19.0 (SD 12.7) days | Left/right/bilateral 1/4/1 | Left/right 4/3 |
| Deniz 2011 | Mean 61.5 (SD 4.7) years | Mean 61.5 (SD 12.5) years | Male/female 8/2 | Male/female 3/7 | Mean 71 (SD 40) days | Mean 81 (SD 47) months | Left/right 6/4 | Left/right 3/7 |
| Du 2006 | 56 (6) years | 58 (6) years | Male/female 35/32 | Male/female 30/31 | < 3 months | < 3 months | Left/right 31/36 | Left/right 29/32 |
| Duncan 2011 | Mean 62 (SD 12) years | Mean 63 (SD 13) years | Male/female 159/123 | Male/female 65/61 | Mean 64 (SD 9) days | Mean 63 (SD 8) days | Left/right 121/161 | Left/right 61/65 |
| Eich 2004 | Mean 62.4 (SD 4.8) years (all participants) | Mean 64.0 (SD 6.0) years (all participants) | Male/female 17/8 | Male/female 16/9 | Mean 6.1 (SD 2.2) weeks | Mean 6.3 (SD 2.5) weeks | Left/right 14/11 | Left/right 14/11 |
| Franceschini 2009 | Mean 66 (SD 12) years | Mean 71 (SD 12) years | Male/female 28/24 | Male/female 22/23 (only 45 described) |
Mean 17 (SD 10) days | Mean 14 (SD 7) days | Left/right 29/23 | Left/right 15/30 (only 45 described) |
| Gan 2012 | Not described | Not described | Not described | Not described | Not described | Not described | Not described | Not described |
| Globas 2011 | Mean 69 (SD 7) years | Mean 69 (SD 6) years | Male/female 14/4 (only 18 described) |
Male/female 15/3 (only 18 described) |
Mean 60 (SD 47) months | Mean 70 (SD 67) months | Left/right 4/14 (only 18 described) |
Left/right 9/9 (only 18 described) |
| Hoyer 2012 | Mean 52 (SD 13) years | Mean 52 (SD 6) years | Male/female 20/10 | Male/female 18/12 | Mean 99 (SD 39) days | Mean 96 (SD 42) days | Left/right 17/13 | Left/right 17/13 |
| Jaffe 2004 | Mean 58.2 (SD 11.2) years (excluding drop outs) | Mean 63.2 (SD 8.3) years (excluding drop outs) | Male/female 5/5 (excluding drop outs) | Male/female 7/3 (excluding drop outs) | Mean 3.9 (SD 2.3) years (excluding drop outs) | Mean 3.6 (SD 2.6) years (excluding drop outs) | Left/right 6/4 (excluding drop outs) | Left/right 4/6 (excluding drop outs) |
| Kang 2012 | Mean 56 (SD 7) years | Mean 56 (SD 8) years | Male/female 10/10 (excluding drop outs) |
Male/female 6/4 (excluding drop outs) |
Mean 14 (SD 4) months | Mean 15 (SD 7) months | Left/right 8/12 (excluding drop outs) |
Left/right 5/5 (excluding drop outs) |
| Kosak 2000 | Mean 74 (SEM 2) years (all participants) | Mean 70 (SEM 2) years | Male/female 13/9 | Male/female 18/16 | Mean 39 (SEM 3) days | Mean 40 (SEM 4) days | Left/right/bilateral 8/12/2 | Left/right/bilateral 12/16/6 |
| Kuys 2011 | Mean 63 (SD 14) years | Mean 72 (SD 17) years | Male/female 8/7 | Male/female 6/9 | Mean 52 (SD 32) days (excluding drop outs) |
Mean 49 (SD 30) days (excluding drop outs) |
Left/right 6/9 | Left/right 11/4 |
| Langhammer 2010 | Mean 74 (SD 13) years | Mean 75 (SD 10) years | Male/female 10/11 | Male/female 6/12 | Mean 419 (SD 1034) days | Mean 349 (SD 820) days | Left/right 15/6 | Left/right 13/5 |
| Laufer 2001 | Mean 66.6 (SD 7.2) years (excluding drop outs) | Mean 69.3 (SD 8.1) years (excluding drop outs) | Male/female 7/6 | Male/female 7/5 | Mean 32.6 (SD 21.2) days | Mean 35.8 (SD 17.3) days | Left/right 5/8 | Left/right 5/7 |
| Liston 2000 | Mean 79.1 (SD 6.8) years (all EXP and CTL participants) | Male/female 12/6 | Not reported | Not reported | Not reported | Not reported | ||
| Luft 2008 | Mean 64 (SD 10) years | Mean 63 (SD 9) years | Male/female 14/20 (excluding drop outs) |
Male/female 19/18 (excluding drop outs) |
Mean 55 months (excluding drop outs) |
Mean 63 months (excluding drop outs) |
Left/right 21/12 (excluding drop outs) |
Left/right 13/21 (excluding drop outs) |
| MacKay‐Lyons 2013 | Mean 62 (SD 15) years | Mean 59 (SD 13) years | Male/female 15/9 | Male/female 14/12 | Mean 23 (SD 6) days | Mean 23 (SD 4) days | Left/right 16/8 | Left/right 13/13 |
| Macko 2005 | Mean 63 (SD 10) years | Mean 64 (SD 8) years | Male/female 22/10 | Male/female 21/8 | Mean 35 (SD 29) months | Mean 39 (SD 59) months | Left/right 18/14 | Left/right 13/16 |
| Mehrberg 2001 | Not described | Not described | Not described | Not described | Not described | Not described | Not described | Not described |
| Moore 2010 | Mean 50 (SD 15) years (EXP and CTL participants) | Male/female 14/6 (EXP and CTL) | Mean 13 (SD 8) months (EXP and CTL) | Left/right 16/4 (EXP and CTL) | ||||
| Nilsson 2001 | Median 54 (range 24 to 67) years (all participants) | Median 56 (range 24 to 66) years | Male/female 20/16 | Male/female 20/17 | Median 22 (range 10 to 56) days | Median 17 (range 8 to 53) days | Left/right/bilateral 21/11/4 | Left/right/bilateral 18/14/5 |
| Olawale 2009 | Mean 56.8 (SD 6.4) years | Mean 57.0 (SD 7.1) years | Male/female 12/8 | Male/female 22/18 | Mean 10.2 (SD 6.9) months | Mean 10.5 (SD 6.3) months | Left/right 12/8 | Left/right 19/21 |
| Pohl 2002 | Mean 58.2 (SD 10.5) years for EXP 1 (excluding drop outs) Mean 57.1 (SD 13.9) years for EXP 2 (excluding drop outs) | Mean 61.6 (SD 10.6) years (excluding drop outs) | Male/female 16/4 for EXP 1 male/female 14/6 for EXP 2 | Male/female 13/7 | Mean 16.2 (SD 16.4) weeks for EXP 1 Mean 16.8 (SD 20.5) weeks for EXP 2 | Mean 16.1 (SD 18.5) weeks | Left/right 15/5 for EXP 1 Left/right 16/4 for EXP 2 | Left/right 16/4 |
| Richards 1993 | Mean 69.6 (SD 7.4) years (all participants) | Mean 67.3 (SD 11.2) years (CTL 1) | Male/female 5/5 | Male/female 2/6 | Mean 8.3 (SD 1.4) days | Mean 8.8 (SD 1.5) days | Left/right 8/2 | Left/right 2/6 |
| Richards 2004 | Mean 62.9 (SD 12) years | Mean 60.7 (SD 12) years | Male/female 22/10 | Male/female 21/10 | Mean 52.0 (SD 22) months | Mean 52.6 (SD 18) months | Left/right 15/17 | Left/right 20/11 |
| Scheidtmann 1999 | Mean 57.7 (SD 11.0) years (all participants) | Male/female 16/14 | Mean 52.2 (SD 29.6) days | Left/right 17/13 | ||||
| Smith 2008 | Mean 57.8 (SD 7.0) years | Mean 56.0 (SD 8.3) years | Male/female 8/2 | Male/female 4/6 | < 1 year: 8 > 1 < 2 years: 2 | < 1 year: 8 > 1< 2 years: 2 | Left/right 4/16 | |
| Sullivan 2007 | Mean 60.0 (SD 13.3) years | Mean 63.4 (SD 8.4) years | Male/female 34/26 | Male/female 11/9 | Mean 23.8 (SD 15.2) months | Mean 28.4 (SD 19.0) months | Left/right 28/32 | Left/right 10/10 |
| Suputtitada 2004 | Mean 61.1 (SD 10.2) years | Mean 64.9 (SD 10.7) years | Male/female 20/4 | Male/female 15/9 | Mean 27.3 (SD 26.6) months | Mean 21.6 (SD 27.7) months | Left/right 9/15 | Left/right 8/16 |
| Takami 2010 | Mean 68.6 (SD 8.9) years | Mean 66.9 (SD 10.6) years | Male/female 15/9 | Male/female 7/7 | Mean 14.0 (SD 8.1) days | Mean 13.7 (SD 8.9) days | Left/right 12/12 | Left/right 4/10 |
| Toledano‐Zarhi 2011 | Mean 65 (SD 10) years | Mean 65 (SD 12) years | Male/female 11/3 | Male/female 10/4 | Mean 11 (SD 5) days | Mean 11 (SD 4) days | Not described | Not described |
| Visintin 1998 | Mean 66.5 (SD 12.8) years (all participants) | Mean 66.7 (SD 10.1) years | Male/female 31/19 | Male/female 28/22 | Mean 68.1 (SD 26.5) days | Mean 78.4 (SD 30.0) days | Left/right 30/20 | Left/right 21/29 |
| Weng 2004 | 55.2 (15.4) years | 54.6 (15.2) years | Male/female 17/6 | Male/female 17/5 | Mean 36.1 (SD 11.3) days | Mean 35.6 (SD 14.5) days | Left/right 10/13 | Left/right 8/14 |
| Weng 2006 | 51 (12) years | 50 (14) years | Male/female 8/5 | Male/female 9/4 | Mean 62 (SD 24) days | Mean 63 (SD 34) days | Left/right 6/7 | Left/right 7/6 |
| Werner 2002a | Mean 59.7 (SD 10.2) years (all participants) | Mean 60.3 (SD 8.6) years (all participants) | Male/female 8/7 | Male/female 5/10 | Mean 7.4 (SD 2.0) weeks | Mean 6.9 (SD 2.1) weeks | Left/right 7/8 | Left/right 7/8 |
| Yang 2010 | Mean 57.2 (SD 9.3) years | Mean 55.0 (SD 10.1) years | Male/female 5/5 | Male/female 5/3 | Mean 1.2 (SD 1.1) years | Mean 1.6 (SD 1.5) years | Left/right 5/5 | Left/right 4/4 |
| Yen 2008 | Mean 57.3 (SD 16.4) years | Mean 56.1 (SD 12.7) years | Male/female 3/4 | Male/female 6/1 | Mean 2.0 (SD 0.6) months | Mean 2.0 (SD 2.4) months | Left/right 5/2 | Left/right 3/4 |
| Zhang 2008 | 63.3 (13.4) years | 62.8 (15.4) years | Male/female 12/7 | Male/female 13/7 | 68.7 (25.6) days | 66.3 (23.3) days | Left/right 7/12 | Left/right 8/12 |
| Zhu 2004 | 56.9 (12.9) years | 57.8 (12.16) years | Male/female 6/4 | Male/female 7/3 | Mean 4.1 (SD 4.8) months | Mean 3.1 (SD 4.2) months | Not stated by the authors | Not stated by the authors |
CTL: control EXP: experimental SD: standard deviation SEM: standard error of the mean
Table 2.
Dose of experimental interventions
| Study ID | EXP ‐ treadmill | EXP ‐ support | EXP ‐ duration | EXP ‐ frequency | EXP ‐ N weeks | CTL ‐ interventions | CTL ‐ duration | CTL ‐ frequency | CTL ‐ N weeks |
| Ada 2003 | Gradually increased on an individual basis starting from 0.7 m/s at the start of the first session and finishing at 1.1 m/s at the end of the last session, on average | BWS ‐ no Hand support ‐ yes, use of hand rails if required Assistance from therapist ‐ only if required, 2 participants needed slight help with stepping through for the first 2 weeks |
30 minutes (24, 21, 18 and 15 minutes in treadmill training in the first, second, third and fourth training weeks, respectively) | 3 times per week | 4 weeks | Sham (task‐orientated home programme with an intensity insufficient to produce an effect, plus telephone follow‐up once each week) | 30 minutes | 3 times per week (plus encouraged to walk every day) | 4 weeks |
| Ada 2010 | Initial speed of the treadmill was set so that the therapist had time to assist the leg to swing through while maintaining a reasonable step length. If a participant was too disabled to walk on a moving treadmill with the assistance of a therapist, then the participant walked on the spot. Once they attained a speed of 0.4 m/s without body weight support, they commenced 10 minutes of overground walking | BWS ‐ yes Hand support ‐ no Assistance from therapist ‐ yes if required |
30 minutes | 5 times per week | Until they achieved independent walking or were discharged The experimental group participated in a total of 1336 sessions | Assisted overground walking. Aids such as knee splints, ankle–foot orthoses, parallel bars, forearm support frames and walking sticks could be used as part of the intervention. If a participant was too disabled to walk with the help of a therapist, then the participant practiced shifting weight and stepping forwards and backwards. Once participants could walk with assistance, they were instructed to increase their speed and assistance from both the therapist and aids was reduced | 30 minutes | 5 times per week | Until they achieved independent walking or were discharged. The experimental group participated in a total of 1490 sessions |
| Ada 2013 | Treadmill was run at a comfortable speed and participants were instructed to "walk as slowly as possible" and/or a metronome was used to decrease cadence thereby encouraging larger steps. When necessary, marching‐type steps were included to encourage hip and knee flexion during swing phase to improve toe clearance. When a normal step length was observed, the therapist increased the speed of the treadmill until step length was compromised. Workload was then progressed by increasing the incline of the treadmill. Overground walking was used each session and comprised 20% of intervention time in week 1 and was progressively increased each week so that it comprised 50% of the 30 minutes intervention time in week 8 of training. In week 9, the 4‐month training group returned to 20% overground walking, which was again increased to 50% by week 16 |
BWS ‐ no Hand support ‐ no Assistance from therapist ‐ no | 30 minutes | 3 times per week | Group 1: 16 weeks Group 2: eight weeks |
Control group received no intervention. | ‐ | ‐ | ‐ |
| Kim 2011 | Gradually increased starting from 0.3 m/s to 0.7 m/s | BWS ‐ no Hand support ‐ no Assistance from therapist ‐ no |
30 minutes | 5 times per week | 6 weeks | Control group received muscle strengthening (seated leg press, knee extension, leg abductor) | 30 minutes | 5 times per week | 6 weeks |
| da Cunha Filho 2002 | Gradually increased in increments of 0.01 m/s, starting at 0.01 m/s | BWS ‐ yes, starting at 30% body weight and progressively decreased to 0% Hand support ‐ not reported Assistance from therapist ‐ not reported |
20 minutes | 5 times per week | 2 to 3 weeks | Task‐orientated gait training | 20 minutes | 5 times per week | 2 to 3 weeks |
| Deniz 2011 | 10‐minute sessions, if necessary separated by 5‐minute resting period, training at comfortable walking speed every 3 to 5 minutes was increased by increments of 0.01 m/s | BWS ‐ yes Hand support – not reported Assistance from therapist – not reported |
60 minutes | 5 times per week | 4 weeks | Range of motion, stretching, strengthening, balance, co‐ordination exercises and conventional ambulation training treatment programme with parallel bars |
60 minutes | 5 times per week | 4 weeks |
| Du 2006 | Gradually increased starting from 0.1 m/s to 0.5 m/s; interval method, resting period gradually reduced | BWS ‐ yes, initial BWS 30% to 40% weight, gradually reduction Hand support ‐ not reported Assistance from therapist ‐ not reported |
40 minutes | 2 times per day | 4 weeks | Brunnstrom, Bobath, Rood therapy approaches as well as proprioceptive neuromuscular facilitation techniques and motor relearning programme, transfer training, trunk stabilisation | 40 minutes | Unclear | 4 weeks |
| Duncan 2011 | At 0.89 m/s, followed by a progressive programme of walking over ground for 15 minutes. The treadmill speeds ranged from 0 to 1.6 km per hour, increasing by increments of 0.16 km per hour | BWS ‐ yes Hand support ‐ not reported Assistance from therapist ‐ yes |
90 minute sessions | 3 times per week | 12 to 16 weeks (30 and 36 exercise sessions within this period) | Home exercise as an active control, not as a high‐intensity, task‐specific walking programme. Progression through the programme was managed by a physical therapist in the home, with the goals of enhancing flexibility, range of motion in joints, strength of arms and legs, co‐ordination, and static and dynamic balance. Participants in this programme were encouraged to walk daily | 90‐minute sessions | 3 times per week | 12 to 16 weeks (30 and 36 exercise sessions within this period) |
| Eich 2004 | Speed and inclination increased on an individual basis to achieve a training heart rate Mean speed increased from 0.35 m/s (SD 0.11) in week 1 to 0.64 m/s (SD 0.15) in week 6. In week 1 only 1/25 participants had an inclination of 4 degrees, this increased to 25/25 participants in week 6 with a mean inclination of 6.2 degrees | BWS ‐ yes, the harness was always secured and body weight was minimally supported (0 to 15%) according to participant need Hand support ‐ not reported Assistance from therapist ‐ yes, to set the paretic leg, weight shift and hip extension if required |
30 minutes | 5 times per week | 6 weeks | Non‐task‐orientated (neurophysiological) | 30 minutes | 5 times per week | 6 weeks |
| Franceschini 2009 | Speed starting from 0.1 m/s and aiming at 1.2 m/s according to the patient’s compliance and progress. Conventional treatment was performed for 40 minutes, not immediately after treadmill training | BWS ‐ yes, limited to 40% of body weight, gradually reduced Hand support ‐ not reported Assistance from therapist ‐ 2 trained physical therapists for each patient to control the paretic lower extremity and pelvis, when pelvic and paretic lower extremity control was considered adequate, training was administered by 1 physical therapist only |
20 minutes + 40 minutes | 2 times per day | 20 sessions within 5 weeks | 20 sessions of overground gait training of 60 minutes each | 60 minutes | 5 times per week | 20 sessions within 5 weeks |
| Gan 2012 | Body weight support treadmill (BWS‐T) training; treadmill speed was initially started at 0.5 mph | BWS ‐ yes, up to 40% of their body weight supported at the beginning of the training, gradually reduced Hand support ‐ unclear Assistance from therapist ‐ unclear |
Not described | Not described | 8 weeks | Body weight support overground (BWS‐O) ambulation training | Not described | Not described | 8 weeks |
| Globas 2011 | Beginning with 10 to 20 minutes) at 60% to 80% of the maximum heart rate reserve (HRR) (starting with 40% to 50% HRR). Duration was increased as tolerated by 1 to 5 minutes per week Treadmill speed was progressed by 0.1 to 0.3 km/hour every 1 to 2 weeks Training was a group intervention (3 participants trained in parallel) |
BWS ‐ no Hand support ‐ allowed Assistance from therapist ‐ unclear Treadmill inclination at 0° |
30 to 50 minutes | 3 times per week | 3 months (39 sessions) | Passive, muscle tone–regulating exercises for the upper and lower extremities with elements of balance training conducted on an outpatient basis in physiotherapy practices or rehabilitation centres. No aerobic fitness training was performed | 60 minutes | 3 times per week | 3 months (13 weeks) |
| Hoyer 2012 | Treadmill therapy with BWS and on days without TTBWS conventional gait training was conducted | BWS ‐ yes Hand support ‐ not reported Assistance from therapist ‐ not reported |
30 minutes | Daily for the first 4 weeks (20 sessions), and then 1 to 2 times a week (10 sessions) for the remaining 6 weeks | 30 sessions for a period of a minimum of 10 weeks | Intensive gait training (30 minutes) and functional training (30 minutes) daily for a minimum of 10 weeks | 30 minutes | daily | For a minimum of 10 weeks |
| Jaffe 2004 | Comfortable walking speed (speed not reported), speed was not progressed | BWS ‐ no, harness used to prevent falls only Hand support ‐ yes, use of hand rails if required Assistance from therapist ‐ no |
60 minutes | 3 times per week | 2 weeks | Task‐orientated (overground obstacle training) | 60 minutes | 3 times per week | 2 weeks |
| Kang 2012 | Group 1: treadmill training with optic flow (optic flow was applied and treadmill speed was increased by 0.1 km/hour each time once the patient could walk stably for more than 20 seconds) Group 2: treadmill training without optic flow (treadmill speed was increased by 0.1 km/hour each time once the participants could walk stably for more than 20 seconds) |
BWS ‐ no Hand support ‐ allowed but discouraged Assistance from therapist ‐ no |
30 minutes (2 times for 15 minutes with a rest between) | 3 times per week | 4 weeks | General stretching added range of motion exercises in the less and more affected sides of the trunk, arms and legs for the same time. Exercise therapy was performed using the traditional motor development theory and neurodevelopmental treatment based on motor learning theory | 30 minutes | 3 times per week | 4 weeks |
| Kosak 2000 | Gradually increased from 0.22 to 0.89 m/s, as tolerated | BWS ‐ yes, starting at 30% body weight and progressively decreased to 0% or eliminated Hand support ‐ yes, use of hand rails if required Assistance from therapist ‐ yes, assisted with swing phase, foot placement and weight shift if required |
45 minutes | 5 times per week | 2 to 3 weeks | Non‐task‐orientated (orthopaedic) | 45 minutes | 5 times per week | 2 to 3 weeks |
| Kuys 2011 | Walked on the treadmill at an intensity of 40% to 60% heart rate reserve or a Borg Rating of Perceived Exertion of 11 to 14. Participants commenced at an intensity level of 40% heart rate reserve for 30 minutes, progressing each week aiming for a 5% to 10% increase until 60% heart rate reserve was reached. For participants unable to reach 40% heart rate reserve on commencement of treadmill walking, treadmill speeds were set as fast as tolerated and progressed as quickly as possible. Also received task‐oriented physiotherapy, approximately 1 hour per day |
BWS ‐ no Hand support ‐ yes, were encouraged to hold the handrail Assistance from therapist ‐ yes, a physiotherapist provided assistance as required to ensure foot clearance during swing phase |
30 minutes | 3 times per week | 6 weeks | Received usual physiotherapy intervention only | Unclear (probably the same as the EXP group) |
Unclear (probably the same as the EXP group) | Unclear (probably the same as the EXP group) |
| Langhammer 2010 | Walking speed was started on the lowest level and was increased within the first minutes to the working level. The working load was increased in co‐operation with the participants to a level they felt comfortable with and they felt no insecurity in balance or discomfort otherwise | BWS ‐ no Hand support ‐ yes Assistance from therapist ‐ no, and no inclination |
30 minutes | (Up to) 5 times per week | Mean of 16 days of inpatient stay (mean 10 walking sessions) |
Outdoor walking at a comfortable speed and with the use of ordinary assistive devices when necessary | 30 minutes | (Up to) 5 times per week | Mean of 17 days of inpatient stay (mean 11 walking sessions) |
| Laufer 2001 | Comfortable walking speed, speed used and progression not reported | BWS ‐ no Hand support ‐ yes, use of hand rails if required Assistance from therapist ‐ yes, assisted with swing phase and trunk alignment |
8 to 20 minutes | 5 times per week | 3 weeks | Task‐orientated | 8 to 20 minutes | 5 times per week | 3 weeks |
| Liston 2000 | Speed used and progression not reported | BWS ‐ no Hand support ‐ not reported Assistance from therapist ‐ not reported |
60 minutes | 3 times per week | 4 weeks | Task‐orientated | 60 minutes | 3 times per week | 4 weeks |
| Luft 2008 | Aerobic intensity of 60% of heart rate reserve. Duration and intensity started low (10 to 20 minutes, 40% to 50% heart rate reserve) and increased approximately 5 minutes and 5% heart rate reserve every 2 weeks as tolerated. Treadmill velocity and incline were increased by 0.05 m/s and 1% increments, respectively | BWS ‐ no Hand support ‐ not reported Assistance from therapist ‐ not reported |
40 minutes | 3 times per week | 6 months | 13 supervised traditional stretching movements on a raised mat table with a therapist’s assistance. Each movement was performed actively if possible or passively with a therapist’s assistance. Movements included quadriceps, calf, hip and hamstring stretch, low back rotation and stretch, chest stretch, bridging, shoulder shrug, abduction, and flexion, heel slides and short arc of quadriceps | 40 minutes | 3 times per week | 6 month |
| MacKay‐Lyons 2013 | 5 to 10 minutes of active/passive stretching exercises 10 to 15 minutes of upper extremity training (active exercises and strengthening) 10 to 15 minutes of lower extremity training (active exercises and strengthening) 25 to 30 minutes of BWSTT including warm‐up and cool‐down BWSTT initiated in 5 to 10‐minute bouts at the heart rate achieved at 40% to 50% of baseline VO2 peak. The goal was to achieve a target exercise duration (at least 20 minutes, exclusive of warm‐up and cool‐down) and intensity (heart rates corresponding to 60% to 75% of baseline VO2 peak 27) by the fourth or fifth week. Initially, ambulatory‐independent participants walked at a treadmill speed of 80% to 90% of their self paced overground speed Ambulatory‐dependent participants walked at a treadmill speed of 70% to 80% of their overground speed Treadmill speed and grade were gradually increased and percentage of manual and body weight support decreased, as tolerated |
BWS ‐ yes 20% to 30% or 40% if necessary of their body weight Hand support ‐ handrail support was discouraged Assistance from therapist ‐ therapist emphasised trunk and limb alignment, loading of the stance limb, hip extension at terminal stance, and advancement of the swing limb |
40 minutes | 5 times per week (after 6 weeks 3 times per week) |
6 weeks (plus 6 weeks; total of 48 sessions) |
5 to 10 minutes of active/passive stretching exercises 10 to 15 minutes of upper extremity training (active exercises and strengthening) 10 to 15 minutes of lower extremity training (active exercises and strengthening) 25 to 30 minutes of overground gait training |
40 minutes | 5 times per week (after 6 weeks 3 times per week) |
6 weeks (plus 6 weeks; total of 48 sessions) |
| Macko 2005 | Increased from a mean of 0.48 (SE 0.30) m/s at baseline to 0.75 (SE 0.30) m/s at treatment end on an individual basis to achieve a target aerobic intensity of 60% to 70% heart rate reserve (treadmill slope increased from 0% at baseline to 2.2% (SE 2.2) at treatment end) | BWS ‐ no Hand support ‐ yes, use of handrails if required Assistance from therapist ‐ not reported |
40 minutes (including 5 minutes warm‐up and 5 minutes cool‐down) increased duration at target intensity from a mean of 12 (SE 6) minutes at baseline to 31 (SE 10) minutes at treatment end | 3 times per week | 6 months | Task‐orientated | 40 minutes | 3 times per week | 6 months |
| Mehrberg 2001 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Moore 2010 | Intensive locomotor training with walking velocity increased in 0.5 km/h increments until participants’ heart rate reached 80% to 85% of age‐predicted maximum or until the participants' Rating of Perceived Exertion increased to 17 on the Borg scale, and was reduced in 10% increments as tolerated | BWS ‐ up to 40% partial body weight support using a counterweight system attached to the safety harness was provided for those participants who walked 0.2 m/s overground Hand support ‐ handrail use for balance only Assistance from therapist ‐ therapists did not provide manual assistance |
Unclear | 2 to 5 times per week | 4 weeks | Did not receive locomotor training or any other interventions | Unclear | 2 to 5 times per week | 4 weeks |
| Nilsson 2001 | Gradually increased from 0.0 to 2.0 m/s on an individual basis | BWS ‐ yes, starting at 100% body weight and decreased to 0% Hand support ‐ yes, use of a cross bar if required Assistance from therapist ‐ yes, assisted with swing phase, hip and knee extension during stance phase, and weight shift if required |
30 minutes | 5 times per week | 9 to 10 weeks | Task‐orientated | 30 minutes | 5 times per week | 9 to 10 weeks |
| Olawale 2009 | Participants walked on a treadmill at a "pre‐determined natural safe walking speed" | BWS – not reported Hand support – not reported Assistance from therapist – not reported |
60 minutes of therapy, including 25 minutes treadmill training | 3 times per week | 12 weeks | Conventional physiotherapy, CTL 2 received overground gait training included in the hourly therapy sessions, whereas CTL 1 received conventional physiotherapy only (active and passive range of motion exercises, strength and balance training) | 60 minutes | 3 times per week | 12 weeks |
| Pohl 2002 | Speed‐dependent treadmill training (EXP 1) ‐ aggressive increase in speed starting from the highest speed the participant could walk at without stumbling and increasing at 10% increments of this speed several times within a session. The average treadmill speed increased from 0.68 m/s (SD 0.34) at the start of training to 2.05 m/s (SD 0.71) at the end of training; limited progressive treadmill training (EXP 2) ‐ gradually increased in increments of 5% of the initial maximum walking speed each week. The average treadmill speed increased from 0.66 m/s (SD 0.39) at the start of training to 0.79 m/s (SD 0.47) at the end of training | Speed‐dependent treadmill training BWS ‐ yes, no more than 10% body weight for the first 3 training sessions only (participants always wore an unweighted harness) Hand support ‐ not reported Assistance from therapist ‐ no Limited progressive treadmill training BWS ‐ yes, no more than 10% body weight for the first 3 training sessions only Hand support ‐ not reported Assistance from therapist ‐ yes, assisted with the walking cycle |
30 minutes | 3 times per week | 4 weeks | Non‐task‐orientated (neurophysiological) | 45 minutes | 3 times per week | 4 weeks |
| Richards 1993 | Speed used and progression not reported | BWS ‐ no Hand support ‐ not reported Assistance from therapist ‐ not reported |
105 minutes (about 35 minutes in treadmill training) | 5 times per week | 5 weeks | Non‐task‐orientated (neurophysiological) | 105 minutes | 5 times per week | 5 weeks |
| Richards 2004 | Specialised locomotor training including tilt table, reciprocal stepping on a Kinetron device | BWS ‐ no Hand support – not described Assistance from therapist – not described |
60 minutes | 5 times per week | 8 weeks | Conventional physiotherapy (traditional neurodevelopmental approach, task‐oriented motor learning, overground gait training, stepping exercises) | 60 minutes | 5 times per week | 8 weeks |
| Scheidtmann 1999 | Gradually increased from 0.0 to 1.3 m/s | BWS ‐ yes, amount of body weight support and progression not reported Hand support ‐ yes, use of hand rails if required Assistance from therapist ‐ yes, assisted with swing phase, foot placement, hip and knee extension during stance phase, and weight shift if required |
30 minutes | 5 times per week | 3 weeks | Non‐task‐orientated (neurophysiological) | 30 minutes | 5 times per week | 3 weeks |
| Smith 2008 | Patients walked for 5 minutes with a "slightly hard" rate of perceived exertion (RPE), then the speed was increased by increments of 0.2 m/hour every 10 minutes of walking with a "slightly hard" RPE | BWS – not clearly stated Hand support – not reported Assistance from therapist ‐ only if required, 2 participants needed slight help with stepping through for the first 2 weeks |
20 minutes | 12 times per month | 4 weeks | Sham (weekly phone calls, recording of a daily life log) | Not reported | 1 telephone call per week | 4 weeks |
| Sullivan 2007 | Initially 4 x 5‐minute training bouts at individualised speeds, initially within the range of 0.7 to 1.1 m/s, followed by 15 m overground walking and either (1) sham or (2) progressive resistive leg cycling or (3) individualised progressive resistive strength training | BWS – yes, initially between 30% and 40% of the participant's weight and being decreased as participants improved Hand support – not described Assistance from therapist – up to 3 therapists assisting in placing of both feet and the pelvis if necessary |
60 minutes | 4 times per week | 6 weeks | Sham (upper extremity cycle ergometry with minimal physical exertion) | 60 minutes | 4 times per week | 6 weeks |
| Suputtitada 2004 | Speed was initiated from 0.044 m/s for 10 minutes, followed by a rest for 5 minutes and then increased by increments of 0.044 m/s 10 minutes |
BWS – yes, 30% during the first week, 20% during the second week, I 0% during the third week and no BWS during the fourth week Hand support ‐ unclear Assistance from therapist – initially 2 therapists assisted in placing the foot and the pelvis |
25 minutes | 7 times per week | 4 weeks | Walking at a self adopted speed on a 15 m walkway for 10 minutes, rested 5 minutes, and walked again 10 minutes | 25 minutes | 7 times per week | 4 weeks |
| Takami 2010 | For 3 minutes twice (with 4 minute rest); week 1: 0.8 km/hour, week 2: 1.0 km/hour, week 3: 1.3 km/hour | BWS – yes 30% Hand support ‐ yes, use of hand rails if required Assistance from therapist – not described |
30 minutes control intervention followed by 10 minutes treadmill training either in forward or backward direction | 3 times per week | 4 weeks | Conventional training (stretching, strengthening), including overground walking < 200 m and ADL training | 80 minutes | 5.5 times per week | 4 weeks |
| Toledano‐Zarhi 2011 | Intervention consisted of treadmill training, training on a hand bike machine, and a stationary bicycle | BWS – not stated Hand support – not stated Assistance from therapist – not stated |
90 minutes exercise training, including 35 to 55 minutes treadmill training | 2 times per week | 6 weeks | Home exercise booklet with included instructions for flexibility and muscle strength exercises, patients were encouraged to stick to their normal community routine | NA | NA | 6 weeks |
| Visintin 1998 | Gradually increased in increments of 0.04 m/s, from 0.23 to 0.42 m/s, on average, on an individual basis | BWS ‐ yes, starting at 40% body weight and progressively decreased to 0% Hand support ‐ yes, use of hand rails if required Assistance from therapist ‐ yes, assisted with stepping and limb control during stance and swing phases, and weight shift if required |
20 minutes | 4 times per week | 6 weeks | Task‐orientated (treadmill only) ‐ gradually increased speed from 0.19 to 0.34 m/s, on average, on an individual basis | 20 minutes | 4 times per week | 6 weeks |
| Weng 2004 | Initial speed was half of the measured maximal walking speed prior to training session for 5 minutes as a warm‐up, then intervals of higher speed for 10 s were delivered, returning back to warm‐up speed for 2 minutes; in the next phase the speed would be increased or decreased by 10%, respectively | BWS ‐ no Hand support ‐ unclear Assistance from therapist ‐ yes, assisted with foot placing and pelvis rotation |
20 minutes | 5 times per week | 4 weeks | Neuromuscular facilitation techniques | 20 minutes | 5 times per week | 4 weeks |
| Weng 2006 | Patients walked backwards on a treadmill with increasing speed | BWS ‐ no Hand support ‐ unclear Assistance from therapist ‐ yes; assisted with foot placing and pelvis rotation |
30 minutes of control intervention and 30 minutes of treadmill training | 5 times per week | 3 weeks | Neuromuscular facilitation techniques including lower limb movements and overground gait exercises | 60 minutes | 5 times per week | 3 weeks |
| Werner 2002a | Increased from a mean of 0.32 (SD 0.05) m/s at baseline on an individual basis | BWS ‐ yes, starting at a mean of 8.93% (SD 1.84) body weight and progressively decreased Hand support ‐ yes, use of handrails if required Assistance from therapist ‐ yes, assisted with foot placement, swing phase, and hip and trunk extension during stance phase if required |
15 to 20 minutes | 5 times per week | 2 weeks | Task‐orientated | 15 to 20 minutes | 5 times per week | 2 weeks |
| Yang 2010 | Additional to the CTL intervention: Initial BWS of 40% was decreased to the maximum extent, if knee flexion of the paretic limb did not exceed 15°; speed was selected according to the patient’s ability | BWS ‐ yes Hand support ‐ no, patients were encouraged to refrain from handrails Assistance from therapist – yes, 1 or 2 therapists assisted |
30 minutes + 20 minutes control intervention | 3 times per week | 4 weeks | Stretching, muscle strengthening, balance, and overground walking training | 50 minutes | 3 times per week | 4 weeks |
| Yen 2008 | Additional to the CTL intervention: Initial BWS of 40% was decreased to the maximum extent, if knee flexion of the paretic limb did not exceed 15°; speed was selected according to the patient’s ability | BWS ‐ yes Hand support ‐ no, patients were encouraged to refrain from handrails Assistance from therapist – yes, 1 or 2 therapists assisted |
30 minutes + 20 minutes of control intervention | 3 times per week | 4 weeks | Stretching, muscle strengthening, balance and overground walking training | 50 minutes | 2 to 3 times per week | 4 weeks |
| Zhang 2008 | Increased from 0.2 km/hour and 40% weight‐bearing relief according to the patients capabilities | BWS ‐ yes Hand support ‐ unclear Assistance from therapist ‐ yes, assisted with foot placing, knee extension and pelvis rotation |
30 minutes | 5 times per week | 8 weeks | Not described | Not stated | Not stated | 8 weeks |
| Zhu 2004 | Walking speed and BWS were individualised to the patients' capabilities (with a mean walking speed of 0.13 m/s at baseline and 0.17 m/s at the end of the intervention phase) | BWS ‐ yes Hand support ‐ unclear Assistance from therapist: unclear |
Individualised | 5 times a week | 4 weeks | Individualised conventional motor rehabilitation aiming at improving strength and endurance | Not stated | 5 times a week | 4 weeks |
BWS: body weight support BWSTT: body weight support treadmill training CTL: control EXP: experimental NA: not applicable SE: standard error SD: standard deviation
Table 3.
Adverse events during the treatment phase
| Study ID | Injurious falls | Other injuries | Cardiovascular event | Other adverse event |
| Ada 2003 | EXP = 1 (hip fracture caused by a fall at home after the first week of training) CTL = 0 | EXP = 1 (missed post‐treatment measurement session due to low back pain) CTL = 0 | EXP = 0 CTL = 0 | EXP = 1 (fall during overground component of training but no injuries sustained) CTL = 0 |
| Ada 2010 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 47 reports CTL = 27 reports All reports included musculoskeletal problems (back, hip, knee, calf, foot pain and gout), headaches, dizziness or chest pain. There were 6 reports of falling, 1 of which resulted in a fracture and none of which occurred during the delivery of intervention. 2 participants in the experimental group experienced anxiety attributable to being on a treadmill that was severe enough for them to withdraw from the study |
| Ada 2013 | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported |
| Kim 2011 | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported |
| da Cunha Filho 2002 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 |
| Deniz 2011 | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported |
| Du 2006 | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported |
| Duncan 2011 | EXP = 0 CTL = 0 | EXP = 16 (fracture) CTL = not reported | EXP = 1 (myocardial infarction) CTL = 1 (myocardial infarction) | EXP = 139 + 143 (ALL reported events) CTL = 126 (ALL reported events) |
| Eich 2004 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 |
| Franceschini 2009 | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported |
| Gan 2012 | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported |
| Globas 2011 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 1 recurrent stroke, 1 transportation problem CTL = 0 |
| Hoyer 2012 | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported |
| Jaffe 2004 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 |
| Kang 2012 | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported |
| Kosak 2000 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 1 (acute myocardial infarction 2 days after last treatment session) CTL = 1 (stroke progression) | EXP = 0 CTL = 0 |
| Kuys 2011 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 |
| Langhammer 2010 | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported |
| Laufer 2001 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 |
| Liston 2000 | EXP = 0 CTL = not reported | EXP = 1 (knee pain after first 4 treadmill sessions) CTL = not reported | EXP = 0 CTL = not reported | EXP = 1 (hospitalised after first training session and subsequently died, reason for hospitalisation not reported) CTL = not reported |
| Luft 2008 | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported |
| MacKay‐Lyons 2013 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 |
| Macko 2005 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 11 (5 falls during treadmill training but no injuries sustained; 6 minor medical complications) CTL = 0 |
| Mehrberg 2001 | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported |
| Moore 2010 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 |
| Nilsson 2001 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 |
| Olawale 2009 | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported |
| Pohl 2002 | EXP 1 = 0 EXP 2 = 0 CTL = 0 | EXP 1 = 0 EXP 2 = 0 CTL = 0 | EXP 1 = 0 EXP 2 = 0 CTL = 0 | EXP 1 = 0 EXP 2 = 1 (vertigo, but did not have to terminate training) CTL = 0 |
| Richards 1993 | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported |
| Richards 2004 | EXP = not reported CTL = not reported | EXP = 1 (hip fracture) CTL = not reported | EXP = 1 ‐ (cardiac problems) CTL = not reported | EXP = not reported CTL = not reported |
| Scheidtmann 1999 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 |
| Smith 2008 | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported |
| Sullivan 2007 | EXP = 7 CTL = 2 | |||
| Suputtitada 2004 | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported |
| Takami 2010 | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported |
| Toledano‐Zarhi 2011 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 |
| Visintin 1998 | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported |
| Weng 2004 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 |
| Weng 2006 | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported |
| Werner 2002a | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 | EXP = 0 CTL = 0 |
| Yang 2010 | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported |
| Yen 2008 | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported |
| Zhang 2008 | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported |
| Zhu 2004 | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported | EXP = not reported CTL = not reported |
CTL: control EXP: experimental
Table 4.
Drop outs
| Study ID | EXP ‐ treatment phase | EXP ‐ follow‐up | CTL ‐ treatment phase | CTL ‐ follow‐up |
| Ada 2003 | 1 ‐ hip fracture caused by a fall at home after the first week of training 2 ‐ not measured at post‐test for medical reasons, 1 due to low back pain (these participants completed the follow‐up assessment) | No drop outs | 1 ‐ moved out of area | 1 ‐ moved out of area |
| Ada 2010 | 2 ‐ died 2 ‐ withdrew |
No follow‐up period | 2 ‐ died | No follow‐up period |
| Ada 2013 | 1 ‐ withdrew | No drop outs | 3 ‐ withdrew | No drop outs |
| Kim 2011 | Drop outs not stated | Drop outs not stated | Drop outs not stated | Drop outs not stated |
| da Cunha Filho 2002 | 1 ‐ completed fewer than 9 treadmill and body weight support sessions | No follow‐up period | 1 ‐ pulmonary complications (not related to the protocol) | No follow‐up period |
| Deniz 2011 | Drop outs not stated | Drop outs not stated | Drop outs not stated | Drop outs not stated |
| Du 2006 | No drop outs | No follow‐up period | No drop outs | No follow‐up period |
| Duncan 2011 | 35 (12 withdrew, 7 died, 13 moved, 3 other) | Unclear | 11 (2 withdrew, 6 died, 3 moved) | |
| Eich 2004 | No drop outs | 1 ‐ refusal | No drop outs | No drop outs |
| Franceschini 2009 | 10 ‐ drop outs | No follow‐up period | 10 ‐ drop outs | No follow‐up period |
| Gan 2012 | No drop outs | No follow‐up period | No drop outs | No follow‐up period |
| Globas 2011 | 1 ‐ recurrent stroke 1 ‐ transportation problem |
2 drop outs (but unclear which group) | No drop outs | 2 drop outs (but unclear which group) |
| Hoyer 2012 | No drop outs | No follow‐up period | No drop outs | No follow‐up period |
| Jaffe 2004 | 1 ‐ endurance level too low to continue treatment | No drop outs | 2 ‐ medical conditions unrelated to the study (1 participant with arthritis and 1 participant with a heart condition) | No drop outs |
| Kang 2012 | 1 ‐ drop out ‐ another treatment 1 ‐ lack of participation |
No drop outs | No drop outs | No drop outs |
| Kosak 2000 | 1 ‐ chose to discontinue treatment (did not want to walk on the treadmill) 1 ‐ acute myocardial infarction requiring readmission to acute care | No follow‐up period | 1 ‐ Stroke progression requiring readmission to acute care | No follow‐up period |
| Kuys 2011 | 1 ‐ withdrew 1 ‐ fall |
1 ‐ moved 1 ‐ medical condition |
No drop outs | No drop outs |
| Langhammer 2010 | 3 ‐ drop outs (unclear reasons) | No follow‐up period | 2 ‐ drop outs (unclear reasons) | No follow‐up period |
| Laufer 2001 | 2 ‐ discharged prior to completion of data collection | No follow‐up period | 1 ‐ discharged prior to completion of data collection 1 ‐ readmitted to an acute hospital (not related to the protocol) | No follow‐up period |
| Liston 2000 | 1 ‐ hospitalised after first treatment and subsequently died (reason for hospitalisation not reported) 1 ‐ chose to discontinue treatment due to knee pain 1 ‐ chose to discontinue treatment (felt unsafe and frightened on the treadmill) | No follow‐up period | No drop outs | No follow‐up period |
| Luft 2008 | 12 ‐ unrelated medical condition 2 ‐ recurrent stroke 6 ‐ non‐compliance |
No follow‐up period | 11 ‐ unrelated medical condition 11 ‐ non‐compliance |
No follow‐up period |
| MacKay‐Lyons 2013 | 1 ‐ seizure activity 1 ‐ moved |
1 ‐ refused | 2 ‐ medical reasons 1 ‐ disinterest |
1 ‐ refused 1 ‐ lost to follow‐up |
| Macko 2005 | 3 ‐ medical conditions (1 participant had sinus surgery, 1 participant had pre‐existing shoulder pain, 1 participant had a gastrointestinal bleed and recurrent stroke) 1 ‐ fall at home 3 ‐ chose to discontinue treatment (1 participant had transportation problems, 1 participant had poor adherence and 1 participant decided to train at home) | No follow‐up period | 4 ‐ medical conditions (1 participant had a hernia repair, 1 participant had elective cardiac surgery, 1 participant had a radiculopathy and 1 participant had a foot infection and poor control of hypertension) 2 ‐ fracture caused by a fall at home 3 ‐ chose to discontinue treatment (1 participant moved out of area, 1 participant returned to work and 1 participant was disinterested in stretching) | No follow‐up period |
| Mehrberg 2001 | Missing information | Missing information | Missing information | Missing information |
| Moore 2010 | ||||
| Nilsson 2001 | 2 ‐ chose to discontinue treatment (did not want to walk on the treadmill) 2 ‐ medical reasons | 2 ‐ medical reasons 1 ‐ death 1 ‐ moved out of area | 1 ‐ chose to discontinue treatment (wanted to walk on the treadmill) 1 ‐ medical reasons 1 ‐ death | 1 ‐ moved out of area 1 ‐ did not want to attend the follow‐up tests |
| Olawale 2009 | 2 ‐ did not attend all training sessions | No follow‐up period | 5 ‐ Did not attend all training sessions | No follow‐up period |
| Pohl 2002 | 2 ‐ medical conditions (1 participant with bladder infection and fever, and 1 participant with viral infection and fever) from EXP 1 2 ‐ medical conditions (1 participant with bladder infection and fever, and 1 participant with pneumonia) from EXP 2 | No follow‐up period | 5 ‐ medical conditions (3 participants with pneumonia and 2 with viral infection and fever) | No follow‐up period |
| Richards 1993 | 1 ‐ reason not reported | No follow‐up data reported | 2 ‐ reason not reported | No follow‐up data reported |
| Richards 2004 | 1 ‐ medical conditions (hip fracture) 1 ‐ medical conditions (cardiac problems) |
5 ‐ being unavailable | 1 ‐ reason not stated | 7 ‐ being unavailable |
| Scheidtmann 1999 | No drop outs | No follow‐up period | No drop outs | No follow‐up period |
| Smith 2008 | Drop outs not stated | Drop outs not stated | Drop outs not stated | Drop outs not stated |
| Sullivan 2007 | 6 ‐ withdrawn by administration 1 ‐ refused to participate |
4 ‐ refused to participate | 2 ‐ withdrawn by administration | 1 ‐ withdrawn by administration 3 ‐ refused to participate |
| Suputtitada 2004 | Drop outs not stated | No follow‐up period | Drop outs not stated | No follow‐up period |
| Takami 2010 | 3 ‐ for family reasons | No follow‐up period | Drop outs not stated | No follow‐up period |
| Toledano‐Zarhi 2011 | 1 ‐ chose to discontinue treatment | No follow‐up period | No drop outs | No follow‐up period |
| Visintin 1998 | 2 ‐ chose to discontinue treatment 2 ‐ medical reasons 2 ‐ discharged to chronic care prior to completion of data collection (no longer eligible) 1 ‐ discharged home prior to completion of data collection and was unwilling or unable to complete the training | 14 ‐ medical event, repeated stroke, lack of willingness to participate or moved away from area | 4 ‐ chose to discontinue treatment 5 ‐ medical reasons 3 ‐ discharged to chronic care prior to completion of data collection (no longer eligible) 2 ‐ discharged home prior to completion of data collection and were unwilling or unable to complete the training | 13 ‐ medical event, repeated stroke, lack of willingness to participate or moved away from area |
| Weng 2004 | 2 ‐ reasons unknown due to issues of translation | No follow‐up period | 3 ‐ reasons unknown due to issues of translation | No follow‐up period |
| Weng 2006 | Drop outs not stated | No follow‐up period | Drop outs not stated | No follow‐up period |
| Werner 2002a | No drop outs | No follow‐up period | No drop outs | No follow‐up period |
| Yang 2010 | No drop outs | No follow‐up period | No drop outs | No follow‐up period |
| Yen 2008 | No drop outs | No follow‐up period | No drop outs | No follow‐up period |
| Zhang 2008 | Drop outs not stated | No follow‐up period | Drop outs not stated | No follow‐up period |
| Zhu 2004 | No drop outs | No follow‐up period | No drop outs | No follow‐up period |
CTL: control EXP: experimental
Excluded studies
We excluded 55 studies for various reasons (see Characteristics of excluded studies).
Seventeen studies are still awaiting classification, mainly due to being conference abstracts with sparse outcome data reported and we were unable to contact the authors (see the Characteristics of studies awaiting classification).
Thirteen studies are ongoing (see the Characteristics of ongoing studies).
We excluded all these studies from the main analysis.
Risk of bias in included studies
Two authors independently assessed the methodological quality of the included trials using the 'Risk of bias' tool (using the categories random sequence generation, allocation concealment and blinding of outcome assessors; Figure 2).
Figure 2.
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
We wrote to all trialists requesting clarification of some design features or the provision of missing information in order to complete the quality ratings (correspondence was via email or letter, with a reminder being send after three weeks and then every three months if we did not get a response). If no data were provided or no contact achieved we used published data only for all analysis.
Three trials used a cross‐over design with random allocation to the order of treatments (Liston 2000; Scheidtmann 1999; Werner 2002a). All other studies used a parallel‐group design with true randomisation or quasi‐randomisation (Laufer 2001) to groups.
Random sequence generation (selection bias)
Twenty‐five of the 44 included studies described appropriately the method of random sequence generation (see Figure 2).
Allocation concealment (selection bias)
Twenty of the 44 included studies described appropriately the method of concealing allocation of participants to groups (see Figure 2).
Blinding (performance bias and detection bias)
Twenty‐one of the 44 included studies described the outcome assessors as being blinded to group allocation (see Figure 2).
We explored publication bias visually by inspecting funnel plots for all comparisons (plots only shown for analyses 1.1 and 1.2 (Figure 3, Figure 4)). Our inspection did not indicate clear evidence for publication bias or our inspection was not suggestive of systematic heterogeneity. The only systematic heterogeneity in the funnel plots was found between categories of people after stroke who were dependent or independent walkers at study onset (as we described in detail above).
Figure 3.
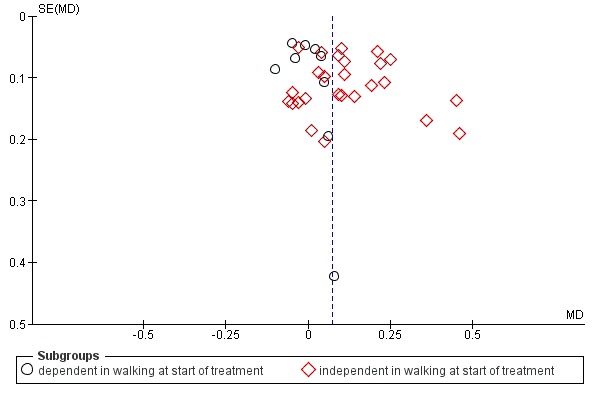
Funnel plot of comparison: 1 Treadmill (with or without body weight support) versus other intervention, outcome: 1.1 Walking speed (m/s) at end of treatment phase.
Figure 4.
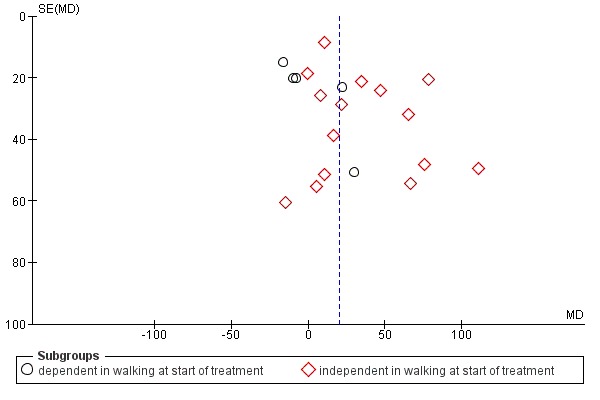
Funnel plot of comparison: 1 Treadmill (with or without body weight support) versus other intervention, outcome: 1.2 Walking endurance (m) at end of treatment.
Effects of interventions
See: Table 1
Comparison 1: Treadmill (with or without body weight support) versus another intervention
Outcome 1.1: Walking speed (m/s) at the end of the treatment phase
Thirty‐five studies with a total of 1891 participants provided data for walking velocity (metres per second, m/s) at study end (Analysis 1.1).
Analysis 1.1.
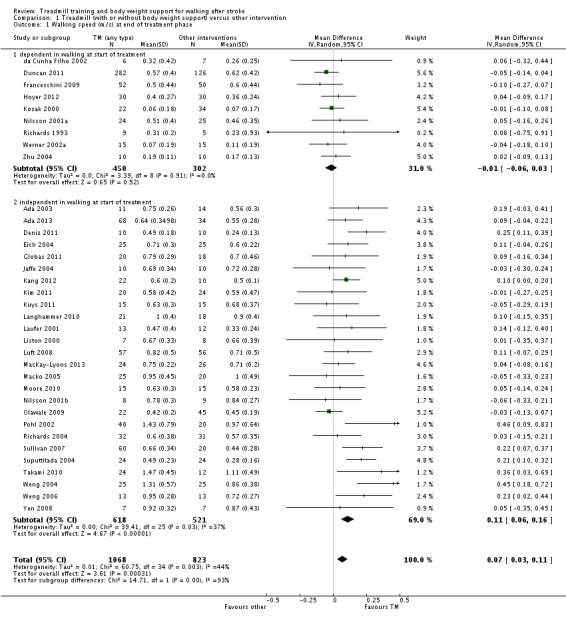
Comparison 1 Treadmill (with or without body weight support) versus other intervention, Outcome 1 Walking speed (m/s) at end of treatment phase.
Overall, the use of treadmill training in walking rehabilitation for patients after stroke did increase the walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.07 m/s (95% CI 0.03 to 0.11; P = 0.0003; level of heterogeneity I2 = 44%) (Analysis 1.1).
In nine studies with a total of 752 participants who were dependent in walking at study onset the use of treadmill training in walking rehabilitation for patients after stroke did not increase the walking velocity significantly. The pooled mean difference (MD, random‐effects model) for walking velocity was ‐0.01 m/s (95% CI ‐0.06 to 0.03; P = 0.52; level of heterogeneity I2 = 0%) (Analysis 1.1).
In 26 studies with a total of 1139 participants who were independent in walking at study onset the use of treadmill training in walking rehabilitation for patients after stroke did increase the walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.11 m/s (95% CI 0.06 to 0.16; P < 0.0001; level of heterogeneity I2 = 37%) (Analysis 1.1).
We did find statistically significant differences in walking velocity between dependent and independent walkers (Chi2 = 14.71, df = 1, P = 0.0001).
Outcome 1.2: Walking endurance (m) at the end of treatment
Twenty trials with a total of 1388 participants provided data for walking endurance (walking capacity; metres (m) walked in six minutes) at study end (Analysis 1.2).
Analysis 1.2.
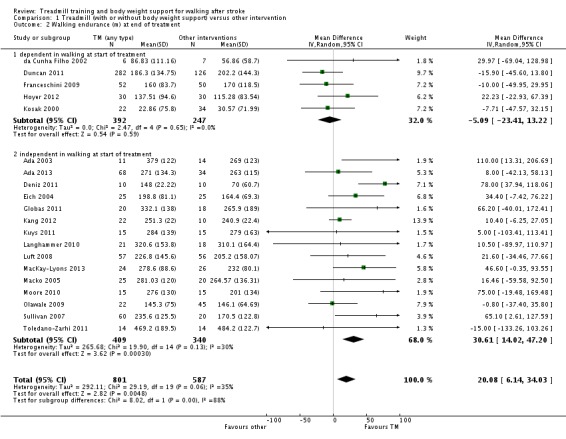
Comparison 1 Treadmill (with or without body weight support) versus other intervention, Outcome 2 Walking endurance (m) at end of treatment.
Overall, the use of treadmill training in walking rehabilitation for patients after stroke did increase the walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 20.08 m (95% CI 6.14 to 34.03; P = 0.005; level of heterogeneity I2 = 35%) (Analysis 1.2).
In five studies with a total of 639 participants who were dependent in walking at study onset the use of treadmill training in walking rehabilitation for patients after stroke did not increase the walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was ‐5.09 m (95% CI ‐23.41 to 13.22; P = 0.59; level of heterogeneity I2 = 0%) (Analysis 1.2).
In 15 studies with a total of 749 participants who were independent in walking at study onset the use of treadmill training in walking rehabilitation for patients after stroke did increase the walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 30.61 m (95% CI 14.02 to 47.20; P = 0.0003; level of heterogeneity I2 = 30%) (Analysis 1.2).
We did find statistically significant differences in walking endurance between dependent and independent walkers (Chi2 = 8.02, df = 1, P = 0.005).
Comparison 2: Treadmill training with body weight support compared to other physiotherapy interventions
Outcome 2.1: Dependence on personal assistance to walk at the end of the treatment phase
Nineteen studies with a total of 1210 participants measured dependence on personal assistance to walk at the end of the treatment phase (Analysis 2.1).
Analysis 2.1.
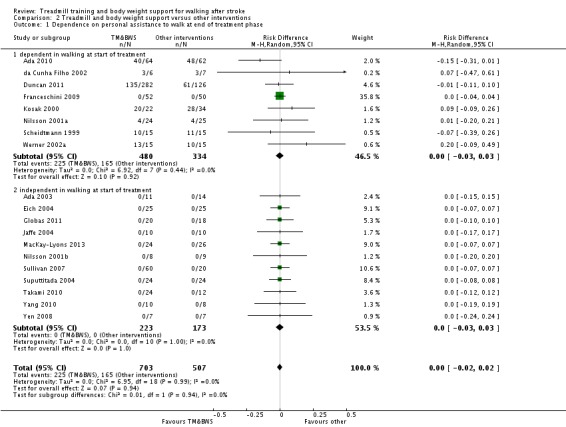
Comparison 2 Treadmill and body weight support versus other interventions, Outcome 1 Dependence on personal assistance to walk at end of treatment phase.
Overall, the use of treadmill training with body weight support in walking rehabilitation for patients after stroke did not increase the chance of walking independently compared with other physiotherapy interventions (RD 0.00, 95% CI ‐0.02 to 0.02; P = 0.92; level of heterogeneity I2 = 0%) (Analysis 2.1).
In eight studies with a total of 814 participants who were dependent in walking at study onset the use of treadmill training with body weight support in walking rehabilitation for patients after stroke did not increase the chance of walking independently compared with other physiotherapy interventions (RD ‐0.00, 95% CI ‐0.03 to 0.03; P = 0.92; level of heterogeneity I2 = 0%) (Analysis 2.1).
In 11 studies with a total of 396 participants who were independent in walking at study onset the use of treadmill training with body weight support in walking rehabilitation for patients after stroke did not increase the chance of walking independently compared with other physiotherapy interventions (RD ‐0.00, 95% CI ‐0.03 to 0.03; P = 1.00; level of heterogeneity I2 = 0%) (Analysis 2.1).
We did not find statistically significant differences between dependent and independent walkers (Chi2 = 0.01, df = 1, P = 0.94).
Outcome 2.2: Walking speed (m/s) at the end of the treatment phase
Nineteen studies with a total of 1163 participants provided data for walking velocity (metres per second, m/s) at study end (Analysis 2.2).
Analysis 2.2.
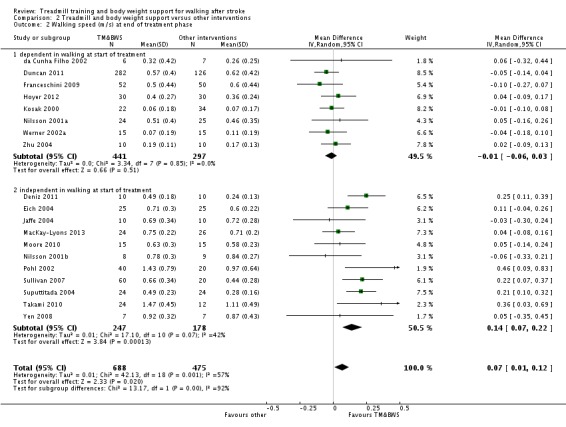
Comparison 2 Treadmill and body weight support versus other interventions, Outcome 2 Walking speed (m/s) at end of treatment phase.
Overall, the use of treadmill training with body weight support in walking rehabilitation for patients after stroke did increase the walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.07 m/s (95% CI 0.01 to 0.12; P = 0.02; level of heterogeneity I2 = 57%) (Analysis 2.2).
In eight studies with a total of 738 participants who were dependent in walking at study onset the use of treadmill training with body weight support in walking rehabilitation for patients after stroke did not increase the walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was ‐0.01 m/s (95% CI ‐0.06 to 0.03; P = 0.51; level of heterogeneity I2 = 0%) (Analysis 2.2).
In 11 studies with a total of 425 participants who were independent in walking at study onset the use of treadmill training with body weight support in walking rehabilitation for patients after stroke did increase the walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.14 m/s (95% CI 0.07 to 0.22; P < 0.001; level of heterogeneity I2 = 42%) (Analysis 2.2).
We did find statistically significant differences in walking velocity between dependent and independent walkers (Chi2 = 13.17, df = 1, P = 0.0003).
Outcome 2.3: Walking endurance (m) at the end of the treatment phase
Ten trials with a total of 869 participants provided data for walking endurance (walking capacity; metres (m) walked in six minutes) at study end (Analysis 2.3).
Analysis 2.3.
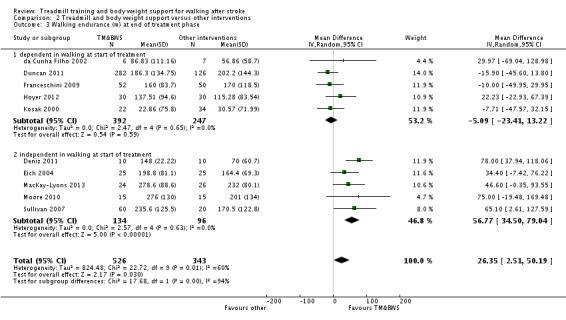
Comparison 2 Treadmill and body weight support versus other interventions, Outcome 3 Walking endurance (m) at end of treatment phase.
Overall, the use of treadmill training with body weight support in walking rehabilitation for patients after stroke did increase the walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 26.35 m (95% CI 2.51 to 50.19; P = 0.03; level of heterogeneity I2 = 60%) (Analysis 2.3).
In five studies with a total of 639 participants who were dependent in walking at study onset the use of treadmill training with body weight support in walking rehabilitation for patients after stroke did not increase the walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was ‐5.09 m (95% CI ‐23.41 to 13.22; P = 0.59; level of heterogeneity I2 = 0%) (Analysis 2.3).
In five studies with a total of 230 participants who were independent in walking at study onset the use of treadmill training with body weight support in walking rehabilitation for patients after stroke did increase the walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 56.77 m (95% CI 34.50 to 79.04; P < 0.00001; level of heterogeneity I2 = 0%) (Analysis 2.3).
We did find statistically significant differences in walking endurance between dependent and independent walkers (Chi2 = 17.68, df = 1, P < 0.0001).
Outcome 2.4: Dependence on personal assistance to walk at the end of scheduled follow‐up
Five studies with a total of 285 participants measured dependence on personal assistance to walk at the end of scheduled follow‐up (Analysis 2.4).
Analysis 2.4.
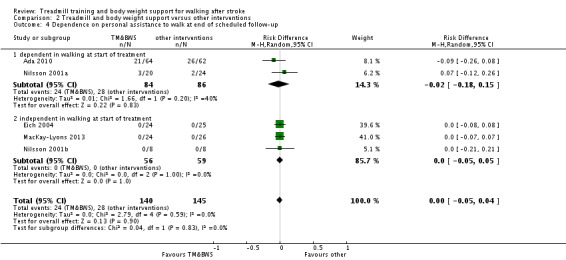
Comparison 2 Treadmill and body weight support versus other interventions, Outcome 4 Dependence on personal assistance to walk at end of scheduled follow‐up.
In two studies with a total of 170 participants who were dependent in walking at study onset the use of treadmill training with body weight support in walking rehabilitation for patients after stroke did not increase the chance of walking independently compared with other physiotherapy interventions (RD ‐0.02, 95% CI ‐0.18 to 0.15; P = 0.83; level of heterogeneity I2 = 40%) (Analysis 2.4).
In three studies with a total of 115 participants who were independent in walking at study onset the use of treadmill training with body weight support in walking rehabilitation for patients after stroke did not increase the chance of walking independently compared with other physiotherapy interventions (RD 0.00, 95% CI ‐0.05 to 0.05; P = 1.00; level of heterogeneity I2 = 0%) (Analysis 2.4).
Outcome 2.5: Walking speed (m/s) at the end of scheduled follow‐up
Seven trials with a total of 751 participants provided data for walking velocity (metres per second, m/s) at the end of scheduled follow‐up (Analysis 2.5).
Analysis 2.5.
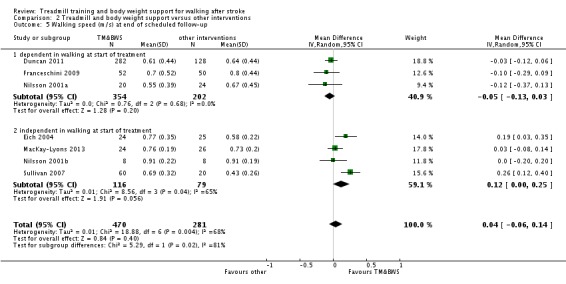
Comparison 2 Treadmill and body weight support versus other interventions, Outcome 5 Walking speed (m/s) at end of scheduled follow‐up.
Overall, the use of treadmill training with body weight support in walking rehabilitation for patients after stroke did not increase the walking velocity at the end of scheduled follow‐up significantly. The pooled MD (random‐effects model) for walking velocity was 0.04 m/s (95% CI ‐0.06 to 0.14; P = 0.40; level of heterogeneity I2 = 40%) (Analysis 2.5).
In three studies with a total of 556 participants who were dependent in walking at the end of scheduled follow‐up the use of treadmill training with body weight support in walking rehabilitation for patients after stroke did not increase the walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was ‐0.05 m/s (95% CI ‐0.13 to 0.03; P = 0.20; level of heterogeneity I2 = 0%) (Analysis 2.5).
In four studies with a total of 195 participants who were independent in walking at the end of scheduled follow‐up the use of treadmill training with body weight support in walking rehabilitation for patients after stroke did not increase the walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.12 m/s (95% CI ‐0.00 to 0.25; P = 0.06; level of heterogeneity I2 = 65%) (Analysis 2.5).
Outcome 2.6: Walking endurance (m) at the end of scheduled follow‐up
Five trials with a total of 689 participants provided data for walking endurance (walking capacity; metres (m) walked in six minutes) at the end of scheduled follow‐up (Analysis 2.6).
Analysis 2.6.
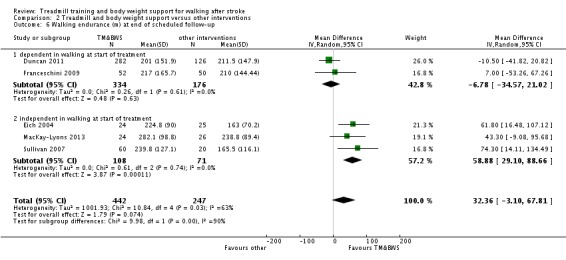
Comparison 2 Treadmill and body weight support versus other interventions, Outcome 6 Walking endurance (m) at end of scheduled follow‐up.
Overall, the use of treadmill training with body weight support in walking rehabilitation for patients after stroke did not increase the walking endurance at the end of scheduled follow‐up significantly. The pooled MD (random‐effects model) for walking endurance was 32.36 m (95% CI ‐3.10 to 67.81; P = 0.07; level of heterogeneity I2 = 63%) (Analysis 2.6).
In two studies with a total of 510 participants who were dependent in walking at study onset the use of treadmill training with body weight support in walking rehabilitation for patients after stroke did not increase the walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was ‐6.78 m (95% CI ‐34.57 to 21.02; P = 0.63; level of heterogeneity I2 = 0%) (Analysis 2.6).
In three studies with a total of 179 participants who were independent in walking at study onset the use of treadmill training with body weight support in walking rehabilitation for patients after stroke did increase the walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 58.88 m (95% CI 29.10 to 88.66; P = 0.0001; level of heterogeneity I2 = 0%) (Analysis 2.6).
Comparison 3: Treadmill training without body weight support compared to other physiotherapy intervention
Outcome 3.1: Walking speed (m/s) at the end of the treatment phase
Fifteen trials with a total of 714 participants who were ambulatory at study onset provided data for walking velocity (metres per second, m/s) at the end of the treatment phase (Analysis 3.1).
Analysis 3.1.
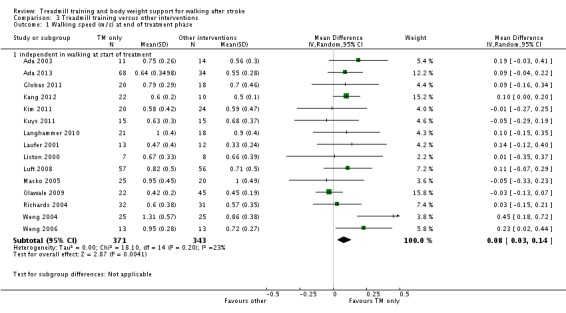
Comparison 3 Treadmill training versus other interventions, Outcome 1 Walking speed (m/s) at end of treatment phase.
Overall, the use of treadmill training without body weight support in gait rehabilitation for ambulatory patients after stroke did increase the walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.08 m/s (95% CI 0.03 to 0.14; P = 0.004; level of heterogeneity I2 = 23%) (Analysis 3.1).
Outcome 3.2: Walking endurance (m) at the end of the treatment phase
Ten trials with a total of 519 participants provided data for walking endurance (walking capacity; metres (m) walked in six minutes) at the end of the treatment phase (Analysis 3.2).
Analysis 3.2.
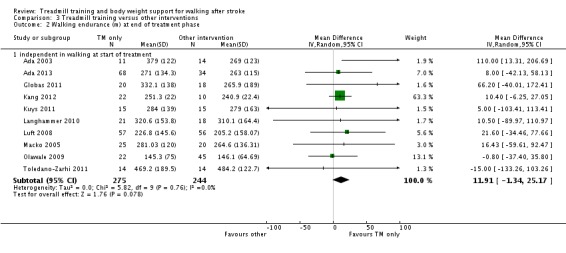
Comparison 3 Treadmill training versus other interventions, Outcome 2 Walking endurance (m) at end of treatment phase.
Overall, the use of treadmill training without body weight support in gait rehabilitation for patients after stroke did not increase the walking endurance significantly. The pooled MD (random‐effects model) for walking velocity was 11.91 m (95% CI ‐1.34 to 25.17; P = 0.08; level of heterogeneity I2 = 0%) (Analysis 3.2).
Comparison 4: Treadmill training with body weight support compared to treadmill training without body weight support
In this update of the review we did not find any additional studies for this comparison. Only one trial with 79 participants was included in this comparison (Visintin 1998a; Visintin 1998b) (more details may be found in Analysis 4.1).
Analysis 4.1.
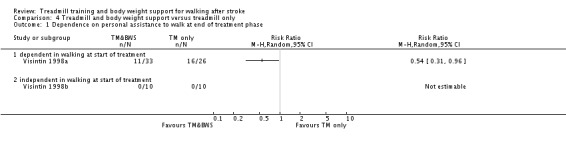
Comparison 4 Treadmill and body weight support versus treadmill only, Outcome 1 Dependence on personal assistance to walk at end of treatment phase.
Comparison 5: Adverse events for all included trials
Outcome 5.1: Adverse events during the treatment phase
Twenty‐four trials with a total of 1511 participants provided data for adverse events during the treatment phase (Analysis 5.1).
Analysis 5.1.
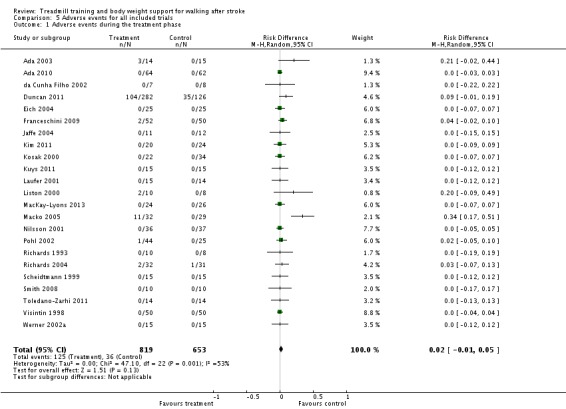
Comparison 5 Adverse events for all included trials, Outcome 1 Adverse events during the treatment phase.
Overall, the use of treadmill training with or without body weight support in gait rehabilitation for patients after stroke did not increase the risk of adverse events during the treatment phase (RD (random‐effects model) 0.02, 95% CI ‐0.01 to 0.05; P = 0.14; level of heterogeneity I2 = 51%). The adverse events during the treatment phase are described in detail for each trial in Table 14.
Comparison 6: Drop outs for all included trials
Outcome 6.1: Drop outs
Outcome 6.1.1: Drop outs by the end of the treatment phase
Forty‐four trials with a total of 2658 participants provided data for drop outs at study end (Analysis 6.1).
Analysis 6.1.
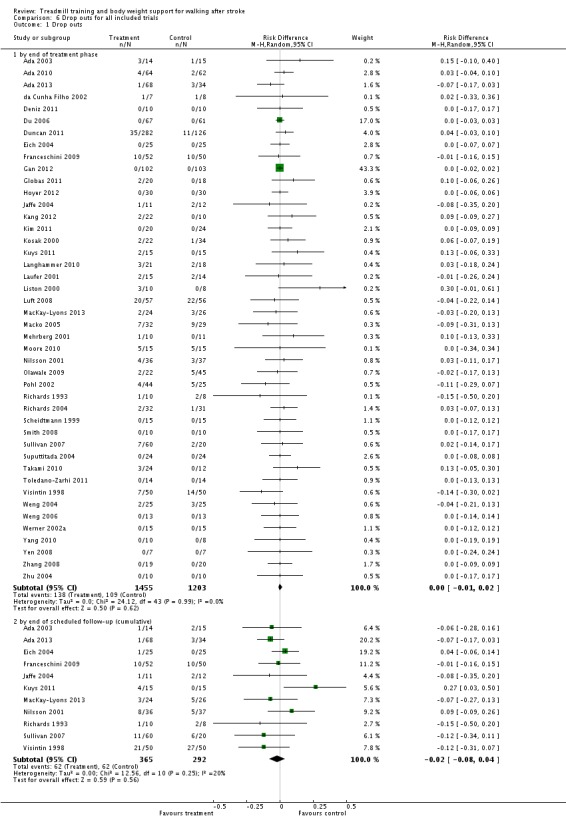
Comparison 6 Drop outs for all included trials, Outcome 1 Drop outs.
Overall, the use of treadmill training with or without body weight support in gait rehabilitation for patients after stroke did not increase the risk of patients dropping out by the end of the treatment phase (RD (random‐effects model) 0.00, 95% CI ‐0.01 to 0.02; P = 0.62; level of heterogeneity I2 = 0%). The reasons for drop outs and all adverse events during the treatment phase are described in detail for each trial in Table 14 and Table 15.
Outcome 6.1.2: Drop outs by the end of scheduled follow‐up (cumulative)
Eleven trials with a total of 657 participants provided data for drop outs by the end of scheduled follow‐up (cumulative) (Analysis 6.1).
Overall the use of treadmill training with or without body weight support in gait rehabilitation for patients after stroke did not increase the risk of patients dropping out by the end of scheduled follow‐up (cumulative) (RD (random‐effects model) ‐0.02, 95% CI ‐0.08 to 0.04; P = 0.56; level of heterogeneity I2 = 20%). The reasons for drop outs are described in detail for each trial in Table 14 and Table 15.
Comparison 7: Sensitivity analysis: by trial methodology
Outcome 7.1: Walking speed (m/s) at the end of the treatment phase (all trials involving treadmill training)
To examine the robustness of the results, we specified variables (adequate sequence generation process, adequate concealed allocation and blinded assessors for primary outcome) in a sensitivity analysis that we believed could influence the size of the effect observed for walking speed (m/s) at the end of the treatment phase (Analysis 7.1).
Analysis 7.1.
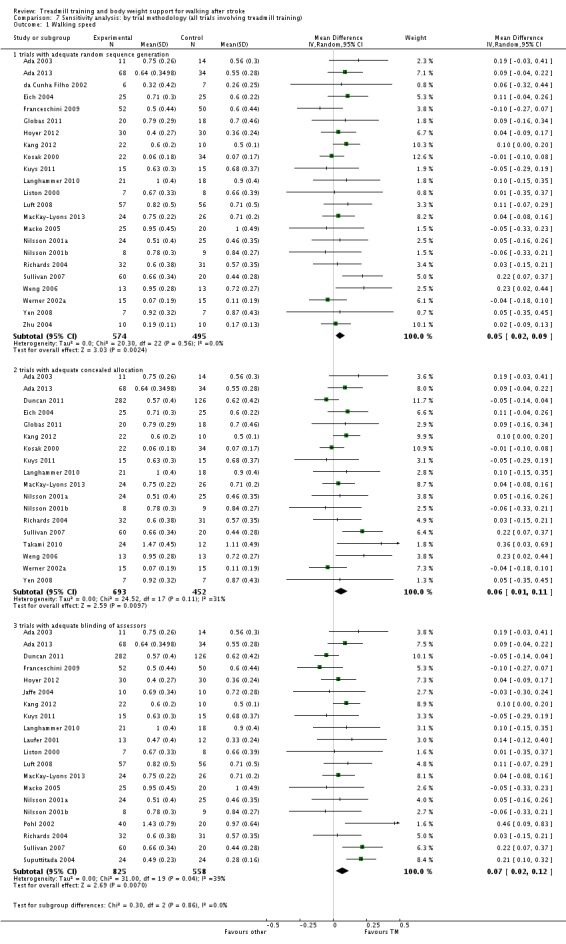
Comparison 7 Sensitivity analysis: by trial methodology (all trials involving treadmill training), Outcome 1 Walking speed.
Studies with adequate sequence generation process
We included 23 trials with a total of 1069 participants which had an adequate sequence generation process (Analysis 7.1). The use of treadmill training in walking rehabilitation for patients after stroke did increase the walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.05 m/s (95% CI 0.02 to 0.09; P = 0.002; level of heterogeneity I2 = 0%).
Studies with adequate concealed allocation
We included 18 trials with a total of 1145 participants which had adequate concealed allocation (Analysis 7.1). The use of treadmill training in walking rehabilitation for patients after stroke did increase the walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.06 m/s (95% CI 0.01 to 0.11; P = 0.010; level of heterogeneity I2 = 31%).
Studies with blinded assessors for the primary outcome
We included 20 trials with a total of 1383 participants which had blinded assessors for the primary outcome (Analysis 7.1). The use of treadmill training in walking rehabilitation for patients after stroke did increase the walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.07 m/s (95% CI 0.02 to 0.12; P = 0.007; level of heterogeneity I2 = 39%).
Comparison 8: Subgroup analysis: treadmill (with or without body weight support) versus other, by duration of illness (independent in walking only)
Outcome 8.1: Walking speed (m/s) at the end of the treatment phase
In our planned subgroup analysis comparing walking speed at the end of the intervention phase in patients in the acute and chronic phases of stroke we arranged all included studies in one of two subgroups (acute and chronic phase).
Acute phase: less than or equal to three months after stroke, independent in walking
Ten trials with a total of 318 participants investigated patients in the acute or subacute phase, defined as less than or equal to three months after stroke (Analysis 8.1). The use of treadmill training in walking rehabilitation for patients after stroke did increase the walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.15 m/s (95% CI 0.05 to 0.24; P = 0.002; level of heterogeneity I2 = 49%).
Analysis 8.1.
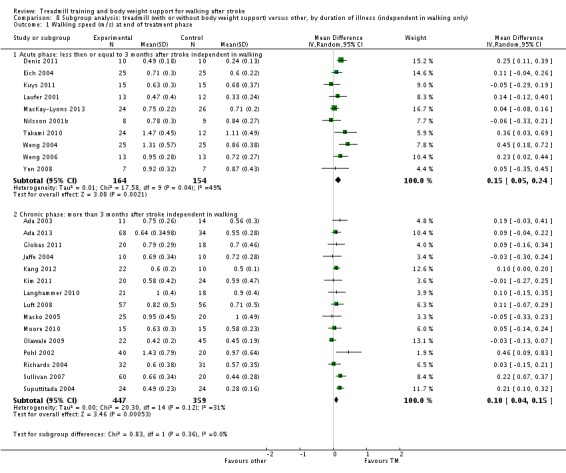
Comparison 8 Subgroup analysis: treadmill (with or without body weight support) versus other, by duration of illness (independent in walking only), Outcome 1 Walking speed (m/s) at end of treatment phase.
Chronic phase: more than three months after stroke, independent in walking
Fifteen trials with a total of 806 participants investigated patients in the chronic phase, defined as more than three months after stroke (Analysis 8.1). The use of treadmill training in walking rehabilitation for patients after stroke did increase the walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.10 m/s (95% CI 0.04 to 0.15; P = 0.0005; level of heterogeneity I2 = 31%).
We did not find statistically significant differences in walking velocity between participants treated in the acute/subacute phase compared with participants treated in the chronic phase after stroke (Chi2 = 0.83, df = 1, P = 0.36).
Outcome 8.2: Walking endurance (m) at the end of the treatment phase
Acute phase: less than or equal to three months after stroke, independent in walking
Five trials with a total of 178 participants investigated patients in the acute or subacute phase, defined as less than or equal to three months after stroke (Analysis 8.2). The use of treadmill training in walking rehabilitation for patients after stroke did increase the walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 48.6 m (95% CI 23.97 to 73.32; P = 0.0001; level of heterogeneity I2 = 6%).
Analysis 8.2.
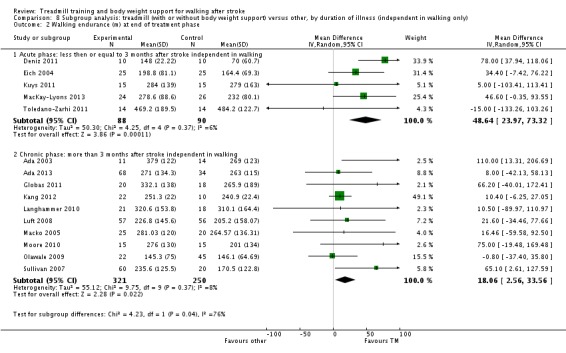
Comparison 8 Subgroup analysis: treadmill (with or without body weight support) versus other, by duration of illness (independent in walking only), Outcome 2 Walking endurance (m) at end of treatment phase.
Chronic phase: more than three months after stroke, independent in walking
Ten trials with a total of 571 participants investigated patients in the chronic phase, defined as more than three months after stroke (Analysis 8.2). The use of treadmill training in walking rehabilitation for patients after stroke did increase the walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 18.06 m (95% CI 2.56 to 33.56; P = 0.02; level of heterogeneity I2 = 8%).
We did find statistically significant differences in walking endurance between participants treated in the acute/subacute phase compared with participants treated in the chronic phase after stroke (Chi2 = 4.23, df = 1, P = 0.04).
Comparison 9: Subgroup analysis: treadmill (with or without body weight support) versus other interventions, by intensity (frequency) of training (independent in walking only)
In our planned subgroup analysis comparing walking speed at the end of the intervention phase at different intensities (frequencies) of training we arranged all included studies in one of three subgroups (treadmill training five times per week or more, three to four times per week, less than three times per week or unclear frequency).
Outcome 9.1: Walking speed (m/s) at the end of the treatment phase
Treadmill training five times per week or more
Thirteen trials with a total of 483 participants investigated patients with an intensity (frequency) of training of five times per week or more (Analysis 9.1). The use of treadmill training in walking rehabilitation for patients after stroke did increase the walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.13 m/s (95% CI 0.08 to 0.17; P < 0.0001; level of heterogeneity I2 = 38%).
Analysis 9.1.
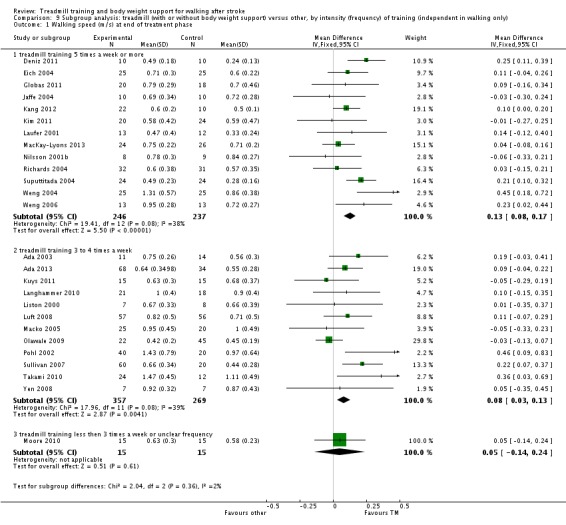
Comparison 9 Subgroup analysis: treadmill (with or without body weight support) versus other, by intensity (frequency) of training (independent in walking only), Outcome 1 Walking speed (m/s) at end of treatment phase.
Treadmill training three to four times per week
Twelve trials with a total of 626 participants investigated patients with an intensity (frequency) of training three to four times per week (Analysis 9.1). The use of treadmill training in walking rehabilitation for patients after stroke did increase the walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.08 m/s (95% CI 0.03 to 0.13; P = 0.004; level of heterogeneity I2 = 39%).
Treadmill training less than three times per week or unclear frequency
One trial with a total of 30 participants investigated patients with an intensity (frequency) of training less of than three times a week (Analysis 9.1). The use of treadmill training in walking rehabilitation for patients after stroke did not increase the walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.05 m/s (95% CI ‐0.14 to 0.24; P = 0.61; level of heterogeneity not applicable).
We did not find statistically significant differences in walking velocity between participants treated at different intensities of training (Chi2 = 2.04, df = 1, P = 0.36).
Outcome 9.2: walking endurance (m) at the end of the treatment phase
Treadmill training five times per week
Four trials with a total of 233 participants investigated patients with an intensity (frequency) of training of five times a week or more (Analysis 9.2). The use of treadmill training in walking rehabilitation for patients after stroke did increase the walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 48.54 m (95% CI 24.40 to 72.68; P < 0.0001; level of heterogeneity I2 = 12%).
Analysis 9.2.
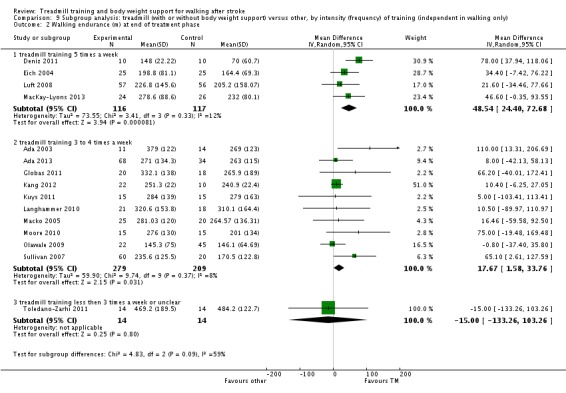
Comparison 9 Subgroup analysis: treadmill (with or without body weight support) versus other, by intensity (frequency) of training (independent in walking only), Outcome 2 Walking endurance (m) at end of treatment phase.
Treadmill training three to four times per week
Ten trials with a total of 488 participants investigated patients with an intensity (frequency) of training of three to four times per week (Analysis 9.2). The use of treadmill training in walking rehabilitation for patients after stroke did increase the walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 17.67 m (95% CI 1.58 to 33.76; P = 0.03; level of heterogeneity I2 = 8%).
Treadmill training less than three times per week or unclear
One trial with a total of 28 participants investigated patients with an intensity (frequency) of training of less than three times a week (Analysis 9.2). The use of treadmill training in walking rehabilitation for patients after stroke did not increase the walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was ‐15.00 m (95% CI ‐133.26 to 103.26; P =0.80; level of heterogeneity not applicable).
We did not find statistically significant differences in walking endurance between participants treated at different intensities of training (Chi2 = 4.83, df = 2, P = 0.09).
Comparison 10: Subgroup analysis: treadmill (with or without body weight support) versus other interventions, by duration of training period (independent in walking only)
In our planned subgroup analysis comparing walking speed at the end of the intervention phase after different durations of treatment we arranged all included studies into one of three subgroups (treadmill training duration of more than four weeks, equal to four weeks or less than four weeks).
Outcome 10.1 Walking speed (m/s) at the end of the treatment phase
Treadmill training duration of more than four weeks
Twelve trials with a total of 699 participants investigated patients with a duration of training of more than four weeks (Analysis 10.1). The use of treadmill training in walking rehabilitation for patients after stroke did increase the walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.05 m/s (95% CI 0.00 to 0.10; P = 0.03; level of heterogeneity I2 = 0%).
Analysis 10.1.
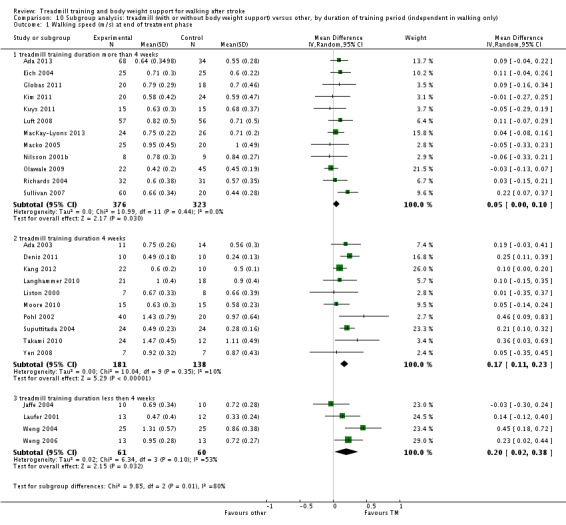
Comparison 10 Subgroup analysis: treadmill (with or without body weight support) versus other, by duration of training period (independent in walking only), Outcome 1 Walking speed (m/s) at end of treatment phase.
Treadmill training duration of four weeks
Ten trials with a total of 319 participants investigated patients with a duration of training of four weeks (Analysis 10.1). The use of treadmill training in walking rehabilitation for patients after stroke did increase the walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.17 m/s (95% CI 0.11 to 0.23; P < 0.0001; level of heterogeneity I2 = 10%).
Treadmill training duration of less than four weeks
Four trials with a total of 121 participants investigated patients with a duration of training of less than four weeks (Analysis 10.1). The use of treadmill training in walking rehabilitation for patients after stroke did increase the walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.20 m/s (95% CI 0.02 to 0.38; P = 0.03; level of heterogeneity I2 = 53%).
We did find statistically significant differences in walking velocity between participants treated with training for different durations (Chi2 = 9.85, df = 2, P = 0.007).
Outcome 10.2: Walking endurance (m) at the end of the treatment phase
In our planned subgroup analysis comparing walking endurance at the end of the intervention phase after different durations of treatment we arranged all included studies into one of three subgroups (treadmill training duration of more than four weeks, equal to four weeks or less than four weeks).
Treadmill training duration of more than four weeks
Ten trials with a total of 603 participants investigated patients with a duration of training of more than four weeks (Analysis 10.2). The use of treadmill training in walking rehabilitation for patients after stroke did increase the walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 23.72 m (95% CI 5.94 to 41.50; P = 0.009; level of heterogeneity I2 = 0%).
Analysis 10.2.
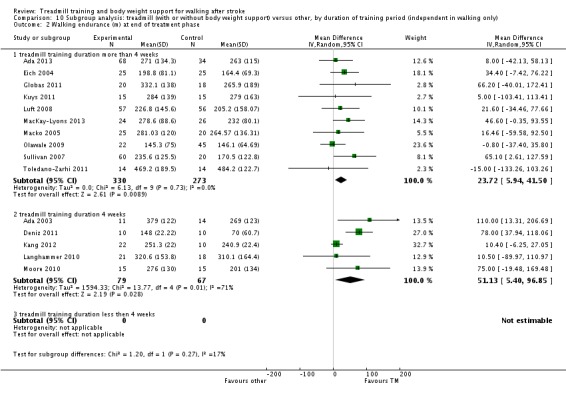
Comparison 10 Subgroup analysis: treadmill (with or without body weight support) versus other, by duration of training period (independent in walking only), Outcome 2 Walking endurance (m) at end of treatment phase.
Treadmill training duration of four weeks
Five trials with a total of 146 participants investigated patients with a duration of training of four weeks (Analysis 10.2). The use of treadmill training in walking rehabilitation for patients after stroke did increase the walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 51.13 m (95% CI 5.40 to 96.85; P = 0.03; level of heterogeneity I2 = 71%).
Treadmill training duration of less than four weeks
No trials investigated patients with a duration of training of less than four weeks.
We did not find statistically significant differences in walking endurance between participants treated with training for different durations (Chi2 = 1.20, df = 1, P = 0.27).
Other outcomes
We did not analyse the secondary outcomes of patient quality of life, ability to perform activities of daily living and the combined outcomes of death or dependency, and death or institutional care because these variables were not reported or due to insufficient data in many of the included studies.
We did not perform the planned subgroup analyses for the types of co‐interventions implemented in conjunction with treadmill training due to insufficient data.
Discussion
Summary of main results
The aim of this review was to evaluate the effect of treadmill training and body weight support, individually or in combination, for walking after stroke. We included 44 trials with 2658 participants in this update. Overall, the use of treadmill training with body weight support did not increase the chance of walking independently compared with people after stroke receiving other physiotherapy interventions but not treadmill training. The use of treadmill training with body weight support in walking rehabilitation for patients after stroke did increase the walking velocity and walking endurance significantly compared with other physiotherapy interventions.
Overall, treadmill training with or without body weight support produced statistically significant higher walking speed and endurance, 0.07 m/s and 20 m respectively, compared with people not receiving treadmill training. For people who could walk independently at the start of treatment, treadmill training with or without body weight support produced statistically significant higher walking speed and endurance, 0.11 m/s and 30 m respectively, compared with people not receiving treadmill training. These results raise the question: how clinically relevant are these statistically significant effects?
For people after stroke Flansbjer 2005 described the smallest possible change (the standard error of measurement (SEM) and the smallest real clinical differences (95% SRD)). The SEMs and the 95% SRDs for walking speed were 0.07 m/s and 0.15 to 0.25 m/s and the SEMs and the 95% SRDs for walking endurance were 18.6 m and 37 to 66 m. Our results might, according to Flansbjer 2005, be interpreted as follows: the overall effects of treadmill training with or without body weight support can be measured in practice but cannot be interpreted as a clinically relevant improvement.
We did not find any benefit for people after stroke who could not walk independently at the start of treatment. We did not find enough studies of the effects of treadmill training with or body weight support on activities and quality of life to draw any appropriate conclusions. We did not find enough studies of the effects of body weight support without treadmill training to draw any appropriate conclusions.
Adverse events and drop outs did not occur more frequently in people receiving treadmill training and these were not judged to be clinically serious events.
Our subgroup analysis showed that, for people after stroke who walk independently, treadmill training in the first three months after stroke produces walking speeds that are statistically and clinically relevant (Flansbjer 2005). For people treated in the chronic phase the effects on walking speed were lower (not clinically relevant). However, the subgroup differences did not differ significantly.
Our subgroup analysis showed that, for people after stroke who walk independently, treadmill training in the first three months after stroke produces a walking endurance which is statistically and clinically relevant (Flansbjer 2005). For people treated in the chronic phase the effects on walking endurance were lower (not clinically relevant). The subgroup differences did differ significantly, indicating that people treated in the first three months after stroke have higher gains in walking endurance compared with training in the chronic phase after stroke.
Our subgroup analysis showed that, for people after stroke who walk independently, treadmill training with higher intensities (frequency of training: five times versus three to four times versus less than three times per week) may produce greater effects on walking speed and endurance. However, this trend toward subgroup differences was not significant.
Our subgroup analysis showed that, for people after stroke who walk independently, treadmill training with shorter treatment periods may produce greater effects on walking speed and endurance. However, this trend toward subgroup differences was only significant for walking speed.
Possible recommendations based on our findings are that treadmill training should be used when people after stroke can walk independently and when improvement of walking speed and endurance is the aim of therapy. Therapists should apply higher intensities of treadmill training and may use relatively short periods of treatments, e.g. four weeks. The greatest effect of treadmill training is to be expected in the first three months after stroke.
Overall completeness and applicability of evidence
The results of this review seem to be quite generalisable to inpatient settings in industrialised countries. However, there are factors producing uncertainty for generalisations.
The investigated study population was quite heterogeneous (e.g. age, time post stroke, severity of stroke and especially walking ability).
The investigated experimental and control conditions were heterogeneous (e.g. type of training, frequency and duration of training; some studies had no active control group or compared with no intervention).
Hence, the results may be of limited applicability for all people after stroke.
Quality of the evidence
We found heterogeneity regarding trial design (parallel‐group or cross‐over design, two or more intervention groups), but it is not clear if this could have limited the quality of the evidence. Furthermore, in our sensitivity analysis examining the effects of methodological quality on the effectiveness of the intervention we found that the benefits (improving walking speed) were relatively robust when we removed trials with an inadequate sequence generation process, inadequate concealed allocation and no blinded assessors for the primary outcome (Analysis 7.1).
Although the methodological quality of the included trials seemed generally moderate (Figure 2), trials investigating treadmill training with or without body weight support are subject to potential methodological limitations. These limitations include inability to blind the therapist and participants, so‐called contamination (provision of the intervention to the control group) and co‐intervention (when the same therapist unintentionally provides additional care to either treatment or comparison group). All these potential methodological limitations introduce the possibility of performance bias. However, as discussed previously, this was not supported in our sensitivity analyses by methodological quality.
Potential biases in the review process
The methodological rigour of Cochrane reviews minimises bias in the process of conducting systematic reviews. We are confident that our detailed search strategy combined with detailed handsearching efforts identified all relevant trials. It is possible that we did not identify studies published in the grey literature, but it would be unlikely that this would have a significant impact on our results. Because the grey literature tends to include trials with relatively small numbers of participants and inconclusive results, inclusion of this literature may actually decrease the size of the effect detected in our review (McAuley 2000).
Another potential source for the introduction of bias could have been that two of the review authors (JM, MP) were involved in conducting and analysing one of the 44 included trials (Pohl 2002). However, the third review author (BE) extracted the outcome data from raw data and described the risk of bias of this trial. Excluding Pohl 2002 from the pooled analyses did not change the results significantly so we believe that this one trial has not biased our overall evidence.
Agreements and disagreements with other studies or reviews
There are several recent reviews about treadmill training with or without body weight support; for example, two reviews were published in 2013 (Charalambous 2013; Polese 2013).
The review of Polese 2013 included nine studies of treadmill training with 977 participants and concluded that treadmill training resulted in faster walking than no intervention or a non‐walking intervention immediately after the intervention period (MD 0.14 m/s, 95% CI 0.09 to 0.19). The review of Charalambous 2013 included 15 studies of treadmill training and concluded that treadmill‐based interventions post stroke may increase and retain walking speed, but a pooled analysis with forest plots was not provided. In comparison, we found more studies (44 studies included in this update) than in the reviews of Charalambous 2013 and Polese 2013 and we found smaller effects on walking speed, MD 0.07 m/s, 95% CI 0.03 to 0.11 (based on 35 included studies of treadmill training with 1891 participants). These differences could be due to the comprehensive search in our review update and to our inclusion of studies not published in English. This update is the most comprehensive review about the topic to date.
We have found in this update of the review significant effects for walking velocity and endurance but not for dependence, and that patients who can walk independently profit more from treadmill training than patients who cannot walk. Initially, this might be difficult to interpret. However, we believe that the overall results of this review are somewhat 'confounded' by the results of patients who cannot walk. We found evidence that this patient group may not profit from treadmill training. Treadmill training appears, therefore, to be an appropriate adjunct intervention that might improve certain important walking parameters such as speed and endurance for people who are already able walk alone. This might appear a little ironic to researchers because treadmill training with body weight support was designed to get non‐ambulatory walkers walking. Another Cochrane review found evidence that the chance of regaining independent walking ability for patients after stroke increases when electromechanical and robotic‐assisted gait training devices are used in combination with physiotherapy (Mehrholz 2013). Interestingly, whereas independent walking improved, neither walking velocity nor walking capacity improved. Perhaps one conclusion could be that different interventions are suitable for different patients. For example, for severely affected patients who cannot walk independently electromechanical and robotic‐assisted gait training devices in combination with physiotherapy are recommended (Mehrholz 2013). However, when patients after stroke recover and start walking, then treadmill training may improve important walking parameters such as speed and endurance, as our update showed. Therefore, the combination of approaches should be recommended.
Finally, it should be mentioned that treadmill training in and of itself is perhaps not the 'main issue'. We believe that treadmill training just offers a very easy approach for high‐intensity, repetitive, task‐specific walking training, which is recommended for gait rehabilitation (Langhorne 2009).
Authors' conclusions
The results of this review were conclusive in part. Overall, people after stroke who receive treadmill training with or without body weight support are not more likely to improve their ability to walk independently, but their speed of walking and their walking capacity may improve. More specifically, people after stroke who are able to walk independently (but not those who are unable to walk independently) seem to benefit from this type of intervention. This review found that improvements in walking speed and endurance in people who are able to walk independently have persisting beneficial effects. However, our review suggests that patients after stroke who are not able to walk independently at the start of treatment may not benefit from treadmill training with or without body weight support.
In practice, therapists should be aware that treadmill training may be used as an option but not as stand‐alone treatment to improve the walking speed and endurance of patients who are able to walk independently. It appears that patients who are able to walk independently, but not patients who are unable to walk independently, may profit from treadmill training with and without body weight support to improve their walking abilities.
Further research should specifically investigate the effects of different frequencies, durations or intensities (in terms of speed increments and inclination) of treadmill training, as well as the use of handrails. To answer these research questions future trials should include patients who are already ambulatory and exclude non‐ambulatory patients.
Acknowledgements
We thank the Cochrane Stroke Group and the original review author team of the 2005 published Cochrane review. We acknowledge the former review team for all requested data and their support for the present update. We therefore thank especially Anne Moseley, Angela Stark, Ian Cameron and Alex Pollock. We thank Brenda Thomas for help with developing the search strategy, Hazel Fraser for providing us with relevant information about trials and systematic reviews from the Cochrane Stroke Group Trials Register, and Gabi Voigt for conducting searches and for providing us with many helpful studies. We thank Louise Ada, Inacio da Cunha Filho, Catherine Dean, Stefan Hesse, David Jaffe, Marc Kosak, Yocheved Laufer, Richard Macko, Jane Mickelborough, Lena Nilsson, Klaus Scheidmann, Martha Visintin and Cordula Werner for providing additional or unpublished data from their trials; Jutta Jablonski, Pauline van Es and Rob de Bie for screening, quality rating and data extraction of German language trials; Yoetsu Ogata for screening the Japanese language trials; Ellen Wang and Chris Lin for screening the Chinese language trials; Dr Bing Gu for helping us translate Chinese trials; David McKenzie and Jarmila McKenzie for screening a Slovak language trial; Aurelien Descatoire for screening a French language trial; Stephanie Nelson for assisting with handsearching the conference proceedings and screening a French language trial; Paul Hansen for sharing his bibliography of treadmill training publications; and Michelle Starkey (Executive Officer, Stroke Recovery Association) for reviewing the plain language summary.
Appendices
Appendix 1. CENTRAL search strategy
#1. [mh ^"cerebrovascular disorders"] or [mh "basal ganglia cerebrovascular disease"] or [mh "brain ischemia"] or [mh "carotid artery diseases"] or [mh "intracranial arterial diseases"] or [mh "intracranial embolism and thrombosis"] or [mh "intracranial hemorrhages"] or [mh ^stroke] or [mh "brain infarction"] or [mh ^"stroke, lacunar"] or [mh ^"vasospasm, intracranial"] or [mh ^"vertebral artery dissection"] #2. stroke or poststroke or "post‐stroke" or cerebrovasc* or brain next vasc* or cerebral next vasc* or cva* or apoplex* or SAH #3. (brain* or cerebr* or cerebell* or intracran* or intracerebral) near/5 (isch*emi* or infarct* or thrombo* or emboli* or occlus*) #4. (brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) near/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*) #5. [mh ^hemiplegia] or [mh paresis] #6. hemipleg* or hemipar* or paresis or paretic #7. [mh ^"gait disorders, neurologic"] #8. #1 or #2 or #3 or #4 or #5 or #6 or #7 #9. [mh ^exercise] or [mh ^"exercise test"] or [mh ^"exercise therapy"] or [mh ^"motion therapy, continuous passive"] #10. [mh ^"body weight"] or [mh ^weight‐bearing] #11. treadmill* or tread next mill* or running next wheel* or running next machine* #12. (walking or walk or exercise) near/5 (machine* or device*) #13. (walking or gait or locomotor or ambulation) near/5 (train* or re‐train* or retrain*) #14. [mh ^walking] #15. machine* or device* or train* or re‐train* or retrain* #16. #14 and #15 #17. (weight or "body‐weight" or bodyweight) near/5 (support* or suspen* or relief) #18. (walk or walking or ambulat* or locomot* or gait or overhead) near/5 support* #19. harness* #20. #9 or #10 or #11 or #12 or #13 or #16 or #17 or #18 or #19 #21. [mh ^walking] or [mh ^gait] or [mh ^"mobility limitation"] or [mh ^locomotion] #22. walk* or gait* or ambulat* or mobil* or locomot* or stride #23. #21 or #22 #24. #8 and #20 and #23
Appendix 2. MEDLINE search strategy
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or stroke, lacunar/ or vasospasm, intracranial/ or vertebral artery dissection/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiplegia/ or exp paresis/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. exp gait disorders, neurologic/ 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. exercise/ or exercise test/ or exercise therapy/ or motion therapy, continuous passive/ 10. body weight/ or weight‐bearing/ 11. (treadmill$ or tread mill$ or running wheel$ or running machine$).tw. 12. ((walking or walk or exercise) adj5 (machine$ or device$)).tw. 13. ((walking or gait or locomotor or ambulation) adj5 (train$ or re‐train$ or retrain$)).tw. 14. exp walking/ and (machine$ or device$ or train$ or re‐train$ or retrain$).tw. 15. ((weight or body‐weight or bodyweight) adj5 (support$ or suspen$ or relief)).tw. 16. ((walk or walking or ambulat$ or locomot$ or gait or overhead) adj5 support$).tw. 17. harness$.tw. 18. 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 19. exp walking/ or gait/ or mobility limitation/ or locomotion/ 20. (walk$ or gait$ or ambulat$ or mobil$ or locomot$ or stride).tw. 21. 19 or 20 22. Randomized Controlled Trials as Topic/ 23. random allocation/ 24. Controlled Clinical Trials as Topic/ 25. control groups/ 26. clinical trials as topic/ 27. double‐blind method/ 28. single‐blind method/ 29. Placebos/ 30. placebo effect/ 31. cross‐over studies/ 32. Therapies, Investigational/ 33. Research Design/ 34. randomized controlled trial.pt. 35. controlled clinical trial.pt. 36. clinical trial.pt. 37. (random$ or RCT or RCTs).tw. 38. (controlled adj5 (trial$ or stud$)).tw. 39. (clinical$ adj5 trial$).tw. 40. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 41. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 42. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 43. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 44. (cross‐over or cross over or crossover).tw. 45. (placebo$ or sham).tw. 46. trial.ti. 47. (assign$ or allocat).tw. 48. or/22‐47 49. 8 and 18 and 21 and 48 50. exp animals/ not humans.sh. 51. 49 not 50
Appendix 3. EMBASE search strategy
1. cerebrovascular disease/ or exp basal ganglion hemorrhage/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or cerebral artery disease/ or exp cerebrovascular accident/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or vertebrobasilar insufficiency/ 2. stroke patient/ or stroke unit/ 3. exp neurologic gait disorder/ or hemiparesis/ or hemiplegia/ or paresis/ 4. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 5. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 6. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 7. (hemipleg$ or hemipar$ or paresis or paretic).tw. 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. treadmill/ or treadmill exercise/ or treadmill ergometry/ 10. walking harness/ or walking machine/ 11. exp exercise/ or exp kinesiotherapy/ or exercise test/ 12. body weight/ or weight bearing/ 13. (treadmill$ or tread mill$ or running wheel$ or running machine$).tw. 14. ((walking or walk or exercise) adj5 (machine$ or device$)).tw. 15. ((walking or gait or locomotor or ambulation) adj5 (train$ or re‐train$ or retrain$)).tw. 16. exp walking/ and (machine$ or device$ or train$ or re‐train$ or retrain$).tw. 17. ((weight or body‐weight or bodyweight) adj5 (support$ or suspen$ or relief)).tw. 18. ((walk or walking or ambulat$ or locomot$ or gait or overhead) adj5 support$).tw. 19. harness$.tw. 20. 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 21. walking/ or walking speed/ or gait/ or locomotion/ or walking difficulty/ 22. (walk$ or gait$ or ambulat$ or mobil$ or locomot$ or stride).tw. 23. 21 or 22 24. Randomized Controlled Trial/ 25. Randomization/ 26. Controlled Study/ 27. control group/ 28. clinical trial/ 29. Crossover Procedure/ 30. Double Blind Procedure/ 31. Single Blind Procedure/ or triple blind procedure/ 32. placebo/ 33. "types of study"/ 34. random$.tw. 35. (controlled adj5 (trial$ or stud$)).tw. 36. (clinical$ adj5 trial$).tw. 37. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 38. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 39. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 40. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 41. (cross‐over or cross over or crossover).tw. 42. placebo$.tw. 43. sham.tw. 44. (assign$ or allocat$).tw. 45. trial.ti. or (RCT or RCT).tw. 46. or/24‐45 47. 8 and 20 and 23 and 46 48. exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 49. human/ or normal human/ or human cell/ 50. 48 not 49 51. 47 not 50
Appendix 4. CINAHL search strategy
S48. S13 AND S24 AND S28 AND S47 S47. S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S40 OR S41 OR S44 OR S45 OR S46 S46. TI trial OR ( TI (RCT or RCTs) OR AB (RCT or RCTs) ) S45. TI ( counterbalance* or multiple baseline* or ABAB design ) or AB ( counterbalance* or multiple baseline* or ABAB design ) S44. S42 and S43 S43. TI trial* or AB trial* S42. TI ( clin* or intervention* or compar* or experiment* or preventive or therapeutic ) or AB ( clin* or intervention* or compar* or experiment* or preventive or therapeutic ) S41. TI ( crossover or cross‐over or placebo* or control* or factorial or sham ) or AB ( crossover or cross‐over or placebo* or control* or factorial or sham ) S40. S38 and S39 S39. TI ( blind* or mask*) or AB ( blind* or mask* ) S38. TI ( singl* or doubl* or tripl* or trebl* ) or AB ( singl* or doubl* or tripl* or trebl* ) S37. TI random* or AB random* S36. (MH "Community Trials") or (MH "Experimental Studies") or (MH "One‐Shot Case Study") or (MH "Pretest‐Posttest Design+") or (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") or (MH "Study Design") S35. (MH "Clinical Research") or (MH "Clinical Nursing Research") S34. (MH "Placebo Effect") or (MH "Placebos") or (MH "Meta Analysis") S33. (MH "Factorial Design") or (MH "Quasi‐Experimental Studies") or (MH "Nonrandomized Trials") S32. (MH "Control (Research)") or (MH "Control Group") S31. (MH "Crossover Design") or (MH "Clinical Trials+") or (MH "Comparative Studies") S30. (MH "Random Assignment") or (MH "Random Sample+") S29. PT randomized controlled trial or clinical trial S28. S25 OR S26 OR S27 S27. TI (walk* or gait* or ambulat* or mobil* or locomot* or stride) OR AB (walk* or gait* or ambulat* or mobil* or locomot* or stride) S26. (MH "Gait Analysis") OR (MH "Gait Training") S25. (MH "Locomotion") OR (MH "Walking") OR (MH "Gait") OR (MH "Step") S24. S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 S23. TI harness* OR AB harness* S22. ( TI (walk or walking or ambulat* or locomot* or gait or overhead) OR AB (walk or walking or ambulat* or locomot* or gait or overhead) ) AND ( TI support* OR AB support* ) S21. ( TI (weight or body‐weight or bodyweight) OR AB (weight or body‐weight or bodyweight) ) AND ( TI (support* or suspen* or relief) OR AB (support* or suspen* or relief) ) S20. ( (MH "Walking") OR (MH "Gait training") ) AND ( TI (machine* or device* or train* or re‐train* or retrain*) OR AB (machine* or device* or train* or re‐train* or retrain*) ) S19. ( TI (walking or gait or locomotor or ambulation) OR AB (walking or gait or locomotor or ambulation) ) AND ( TI (train* or re‐train* or retrain*) OR AB (train* or re‐train* or retrain*) ) S18. ( TI (walking or walk or exercise) OR AB (walking or walk or exercise) ) AND ( TI (machine* or device*) OR AB (machine* or device*) ) S17. TI ( treadmill* or tread mill* or running wheel* or running machine* ) OR AB ( treadmill* or tread mill* or running wheel* or running machine* ) S16. (MH "Weight‐Bearing") or (MH "Body Weight") S15. (MH "Exercise+") or (MH "Therapeutic Exercise+") or (MH "Exercise Test") S14. (MH "Treadmills") S13. S1 OR S2 OR S3 OR S6 OR S9 OR S10 OR S11 OR S12 S12. (MH "Gait Disorders, Neurologic+") S11. TI ( hemipleg* or hemipar* or paresis or paretic ) or AB ( hemipleg* or hemipar* or paresis or paretic ) S10. (MH "Hemiplegia") S9. S7 and S8 S8. TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) S7. TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) S6. S4 and S5 S5. TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) S4. TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) S3. TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) S2. (MH "Stroke Patients") OR (MH "Stroke Units") S1. (MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR (MH "Intracranial Embolism and Thrombosis") OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections")
Appendix 5. AMED search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ or stroke/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiplegia/ or gait disorders/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. 1 or 2 or 3 or 4 or 5 or 6 8. exercise/ or exercise testing/ or exercise therapy/ or continuous passive motion/ 9. body weight/ or weight bearing/ 10. (treadmill$ or tread mill$ or running wheel$ or running machine$).tw. 11. ((walking or walk or exercise) adj5 (machine$ or device$)).tw. 12. ((walking or gait or locomotor or ambulation) adj5 (train$ or re‐train$ or retrain$)).tw. 13. exp walking/ and (machine$ or device$ or train$ or re‐train$ or retrain$).tw. 14. ((weight or body‐weight or bodyweight) adj5 (support$ or suspen$ or relief)).tw. 15. ((walk or walking or ambulat$ or locomot$ or gait or overhead) adj5 support$).tw. 16. harness$.tw. 17. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 18. exp walking/ or gait/ or locomotion/ or mobility limitation/ or gait analysis/ 19. (walk$ or gait$ or ambulat$ or mobil$ or locomot$ or stride).tw. 20. 18 or 19 21. 7 and 17 and 20 22. (clinical trial or clinical trial phase iii or clinical trialb or clinical trials or controlled clinical trial or controlled trial or randomised controlled trial or randomized controlled trial).pt. 23. clinical trials/ or randomized controlled trials/ or double blind method/ or random allocation/ 24. (random$ or RCT or RCTs).tw. 25. (controlled adj5 (trial$ or stud$)).tw. 26. (clinical$ adj5 trial$).tw. 27. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 28. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 29. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 30. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 31. (cross‐over or cross over or crossover).tw. 32. (placebo$ or sham).tw. 33. trial.ti. 34. (assign$ or allocat).tw. 35. or/22‐34 36. 21 and 35
Appendix 6. SPORTDiscus search strategy
S30. S28 AND S29 S29. TI ( random* or RCT or trial* or placebo* or sham or double‐blind* or single‐blind or control or controls or assign* or allocat* ) OR AB ( random* or RCT or trial* or placebo* or sham or double‐blind* or single‐blind or control or controls or assign* or allocat* ) S28. S13 AND S24 AND S27 S27. S25 OR S26 S26. TI (walk* or gait* or ambulat* or mobil* or locomot* or stride) OR AB (walk* or gait* or ambulat* or mobil* or locomot* or stride) S25. (DE "WALKING" OR DE "GAIT in humans") AND (DE "LOCOMOTION" OR DE "HUMAN locomotion") S24. S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 S23. TI harness* OR AB harness* S22. ( TI (walk or walking or ambulat* or locomot* or gait or overhead) OR AB (walk or walking or ambulat* or locomot* or gait or overhead) ) AND ( TI support* OR AB support* ) S21. ( TI (weight or body‐weight or bodyweight) OR AB (weight or body‐weight or bodyweight) ) AND ( TI (support* or suspen* or relief) OR AB (support* or suspen* or relief) ) S20. (DE "WALKING" OR DE "FITNESS walking" OR DE "GAIT in humans") AND (TI (machine* or device* or train* or re‐train* or retrain*) OR AB (machine* or device* or train* or re‐train* or retrain*)) S19. ( TI (walking or gait or locomotor or ambulation) OR AB (walking or gait or locomotor or ambulation) ) AND ( TI (train* or re‐train* or retrain*) OR AB (train* or re‐train* or retrain*) ) S18. ( TI (walking or walk or exercise) OR AB (walking or walk or exercise) ) AND ( TI (machine* or device*) OR AB (machine* or device*) ) S17. TI ( treadmill* or tread mill* or running wheel* or running machine* ) OR AB ( treadmill* or tread mill* or running wheel* or running machine* ) S16. (DE "BODY weight") OR (DE "WEIGHT‐bearing (Orthopedics)") S15. DE "EXERCISE" OR DE "AEROBIC exercises" OR DE "EXERCISE for people with disabilities" OR DE "EXERCISE therapy" OR DE "KNEE exercises" OR DE "LEG exercises" OR DE "STRENGTH training" OR DE "EXERCISE therapy" OR DE "EXERCISE tests" OR DE "EXERCISE ‐‐ Equipment & supplies" S14. DE "TREADMILL exercise tests" OR DE "TREADMILL exercise" OR DE "TREADMILLS (Exercise equipment)" S13. S1 or S2 or S3 or S4 or S7 or S10 or S11 or S12 S12. DE "GAIT disorders" S11. TI ( hemipleg* or hemipar* or paresis or paretic ) or AB ( hemipleg* or hemipar* or paresis or paretic ) S10. S8 and S9 S9. TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) S8. TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) S7. S5 and S6 S6. TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) S5. TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) S4. TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) S3. DE "HEMIPLEGIA" OR DE "HEMIPLEGICS" S2. DE "CEREBROVASCULAR disease ‐‐ Patients" S1. DE "CEREBROVASCULAR disease" OR DE "BRAIN ‐‐ Hemorrhage" OR DE "CEREBRAL embolism & thrombosis"
Data and analyses
Comparison 1.
Treadmill (with or without body weight support) versus other intervention
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Walking speed (m/s) at end of treatment phase | 35 | 1891 | Mean Difference (IV, Random, 95% CI) | 0.07 [0.03, 0.11] |
| 1.1 dependent in walking at start of treatment | 9 | 752 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.06, 0.03] |
| 1.2 independent in walking at start of treatment | 26 | 1139 | Mean Difference (IV, Random, 95% CI) | 0.11 [0.06, 0.16] |
| 2 Walking endurance (m) at end of treatment | 20 | 1388 | Mean Difference (IV, Random, 95% CI) | 20.08 [6.14, 34.03] |
| 2.1 dependent in walking at start of treatment | 5 | 639 | Mean Difference (IV, Random, 95% CI) | ‐5.09 [‐23.41, 13.22] |
| 2.2 independent in walking at start of treatment | 15 | 749 | Mean Difference (IV, Random, 95% CI) | 30.61 [14.02, 47.20] |
Comparison 2.
Treadmill and body weight support versus other interventions
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dependence on personal assistance to walk at end of treatment phase | 19 | 1210 | Risk Difference (M‐H, Random, 95% CI) | ‐.00 [‐0.02, 0.02] |
| 1.1 dependent in walking at start of treatment | 8 | 814 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.03, 0.03] |
| 1.2 independent in walking at start of treatment | 11 | 396 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 2 Walking speed (m/s) at end of treatment phase | 19 | 1163 | Mean Difference (IV, Random, 95% CI) | 0.07 [0.01, 0.12] |
| 2.1 dependent in walking at start of treatment | 8 | 738 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.06, 0.03] |
| 2.2 independent in walking at start of treatment | 11 | 425 | Mean Difference (IV, Random, 95% CI) | 0.14 [0.07, 0.22] |
| 3 Walking endurance (m) at end of treatment phase | 10 | 869 | Mean Difference (IV, Random, 95% CI) | 26.35 [2.51, 50.19] |
| 3.1 dependent in walking at start of treatment | 5 | 639 | Mean Difference (IV, Random, 95% CI) | ‐5.09 [‐23.41, 13.22] |
| 3.2 independent in walking at start of treatment | 5 | 230 | Mean Difference (IV, Random, 95% CI) | 56.77 [34.50, 79.04] |
| 4 Dependence on personal assistance to walk at end of scheduled follow‐up | 5 | 285 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.05, 0.04] |
| 4.1 dependent in walking at start of treatment | 2 | 170 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.18, 0.15] |
| 4.2 independent in walking at start of treatment | 3 | 115 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 5 Walking speed (m/s) at end of scheduled follow‐up | 7 | 751 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.06, 0.14] |
| 5.1 dependent in walking at start of treatment | 3 | 556 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.13, 0.03] |
| 5.2 independent in walking at start of treatment | 4 | 195 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.00, 0.25] |
| 6 Walking endurance (m) at end of scheduled follow‐up | 5 | 689 | Mean Difference (IV, Random, 95% CI) | 32.36 [‐3.10, 67.81] |
| 6.1 dependent in walking at start of treatment | 2 | 510 | Mean Difference (IV, Random, 95% CI) | ‐6.78 [‐34.57, 21.02] |
| 6.2 independent in walking at start of treatment | 3 | 179 | Mean Difference (IV, Random, 95% CI) | 58.88 [29.10, 88.66] |
Comparison 3.
Treadmill training versus other interventions
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Walking speed (m/s) at end of treatment phase | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 independent in walking at start of treatment | 15 | 714 | Mean Difference (IV, Random, 95% CI) | 0.08 [0.03, 0.14] |
| 2 Walking endurance (m) at end of treatment phase | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 independent in walking at start of treatment | 10 | 519 | Mean Difference (IV, Random, 95% CI) | 11.91 [‐1.34, 25.17] |
Comparison 4.
Treadmill and body weight support versus treadmill only
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dependence on personal assistance to walk at end of treatment phase | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 dependent in walking at start of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 independent in walking at start of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Walking speed (m/s) at end of treatment phase | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 dependent in walking at start of treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 independent in walking at start of treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Walking endurance (m) at end of treatment phase | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 dependent in walking at start of treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 independent in walking at start of treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Dependence on personal assistance to walk at end of scheduled follow‐up | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 dependent in walking at start of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 independent in walking at start of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Walking speed (m/s) at end of scheduled follow‐up | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 dependent in walking at start of treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 independent in walking at start of treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Walking endurance (m) at end of scheduled follow‐up | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 dependent in walking at start of treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 independent in walking at start of treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Analysis 4.2.
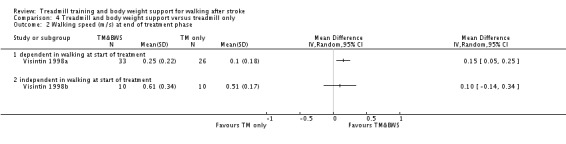
Comparison 4 Treadmill and body weight support versus treadmill only, Outcome 2 Walking speed (m/s) at end of treatment phase.
Analysis 4.3.
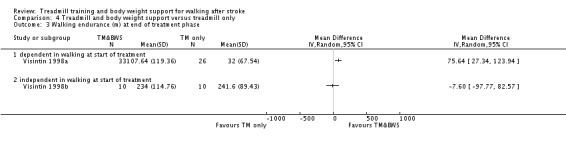
Comparison 4 Treadmill and body weight support versus treadmill only, Outcome 3 Walking endurance (m) at end of treatment phase.
Analysis 4.4.
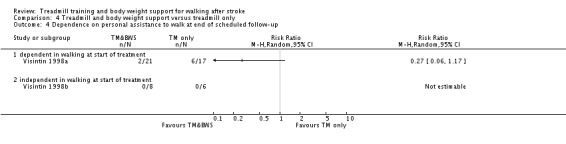
Comparison 4 Treadmill and body weight support versus treadmill only, Outcome 4 Dependence on personal assistance to walk at end of scheduled follow‐up.
Analysis 4.5.
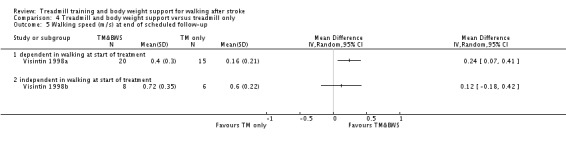
Comparison 4 Treadmill and body weight support versus treadmill only, Outcome 5 Walking speed (m/s) at end of scheduled follow‐up.
Analysis 4.6.
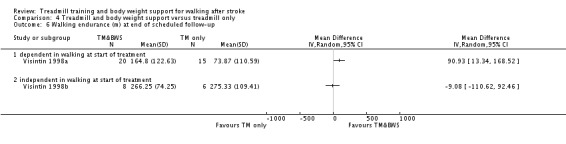
Comparison 4 Treadmill and body weight support versus treadmill only, Outcome 6 Walking endurance (m) at end of scheduled follow‐up.
Comparison 5.
Adverse events for all included trials
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events during the treatment phase | 23 | 1472 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.01, 0.05] |
Comparison 6.
Drop outs for all included trials
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Drop outs | 44 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 by end of treatment phase | 44 | 2658 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.02] |
| 1.2 by end of scheduled follow‐up (cumulative) | 11 | 657 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.08, 0.04] |
Comparison 7.
Sensitivity analysis: by trial methodology (all trials involving treadmill training)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Walking speed | 29 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 trials with adequate random sequence generation | 23 | 1069 | Mean Difference (IV, Random, 95% CI) | 0.05 [0.02, 0.09] |
| 1.2 trials with adequate concealed allocation | 18 | 1145 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.01, 0.11] |
| 1.3 trials with adequate blinding of assessors | 20 | 1383 | Mean Difference (IV, Random, 95% CI) | 0.07 [0.02, 0.12] |
Comparison 8.
Subgroup analysis: treadmill (with or without body weight support) versus other, by duration of illness (independent in walking only)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Walking speed (m/s) at end of treatment phase | 25 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Acute phase: less then or equal to 3 months after stroke independent in walking | 10 | 318 | Mean Difference (IV, Random, 95% CI) | 0.15 [0.05, 0.24] |
| 1.2 Chronic phase: more than 3 months after stroke independent in walking | 15 | 806 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.04, 0.15] |
| 2 Walking endurance (m) at end of treatment phase | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Acute phase: less then or equal to 3 months after stroke independent in walking | 5 | 178 | Mean Difference (IV, Random, 95% CI) | 48.64 [23.97, 73.32] |
| 2.2 Chronic phase: more than 3 months after stroke independent in walking | 10 | 571 | Mean Difference (IV, Random, 95% CI) | 18.06 [2.56, 33.56] |
Comparison 9.
Subgroup analysis: treadmill (with or without body weight support) versus other, by intensity (frequency) of training (independent in walking only)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Walking speed (m/s) at end of treatment phase | 26 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 treadmill training 5 times a week or more | 13 | 483 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.08, 0.17] |
| 1.2 treadmill training 3 to 4 times a week | 12 | 626 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [0.03, 0.13] |
| 1.3 treadmill training less then 3 times a week or unclear frequency | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.14, 0.24] |
| 2 Walking endurance (m) at end of treatment phase | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 treadmill training 5 times a week | 4 | 233 | Mean Difference (IV, Random, 95% CI) | 48.54 [24.40, 72.68] |
| 2.2 treadmill training 3 to 4 times a week | 10 | 488 | Mean Difference (IV, Random, 95% CI) | 17.67 [1.58, 33.76] |
| 2.3 treadmill training less then 3 times a week or unclear | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐15.0 [‐133.26, 103.26] |
Comparison 10.
Subgroup analysis: treadmill (with or without body weight support) versus other, by duration of training period (independent in walking only)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Walking speed (m/s) at end of treatment phase | 26 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 treadmill training duration more than 4 weeks | 12 | 699 | Mean Difference (IV, Random, 95% CI) | 0.05 [0.00, 0.10] |
| 1.2 treadmill training duration 4 weeks | 10 | 319 | Mean Difference (IV, Random, 95% CI) | 0.17 [0.11, 0.23] |
| 1.3 treadmill training duration less then 4 weeks | 4 | 121 | Mean Difference (IV, Random, 95% CI) | 0.20 [0.02, 0.38] |
| 2 Walking endurance (m) at end of treatment phase | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 treadmill training duration more than 4 weeks | 10 | 603 | Mean Difference (IV, Random, 95% CI) | 23.72 [5.94, 41.50] |
| 2.2 treadmill training duration 4 weeks | 5 | 146 | Mean Difference (IV, Random, 95% CI) | 51.13 [5.40, 96.85] |
| 2.3 treadmill training duration less then 4 weeks | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
What's new
Last assessed as up‐to‐date: 4 September 2013.
| Date | Event | Description |
|---|---|---|
| 30 August 2013 | New search has been performed | We have updated the searches to June 2013 and revised the text as appropriate. We have included 44 trials with 2658 participants in this update compared with 15 trials with 622 participants in the last version of this review from 2005. |
| 15 August 2013 | New citation required and conclusions have changed | The conclusions of the review have changed. The previous version of this review concluded that, overall, no statistically significant effect of treadmill training with or without body weight support could be detected. This updated version concludes that overall walking ability was not improved but a statistically significant effect of treadmill training with or without body weight support was detected for improving walking speed and walking endurance. The authorship of the review has changed. |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 18 August 2008 | Amended | Converted to new review format. |
| 14 April 2005 | New search has been performed | The search for trials was extended from March 2003 to March 2005. Four trials (Eich 2004; Jaffe 2004; Macko 2005; Werner 2002a) and one outcome measure (walking endurance) have been added to our original review. We have been able to obtain individual patient data for another trial (Visintin 1998). |
Differences between protocol and review
In the protocol it was stated that we would use the PEDro Scale to assess the methodological quality of the included trials. However, in Chapter 8 of the latest edition of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), it is suggested that scales that yield a summary score should be avoided. In accordance with this suggestion, we no longer used the PEDro Scale to assess the methodological quality of the included trials. Instead, we used the Cochrane 'Risk of bias' tool to analyse trial methodology as suggested by the Cochrane Handbook (Higgins 2011).
In the protocol it was planned to test the homogeneity between trial results using the Chi2 test and, if there was statistically significant heterogeneity (P < 0.10), to calculate the overall effects using a random‐effects model and perform a series of sensitivity analyses to investigate. In this update, we estimated all effects using a random‐effects model, regardless of the level of heterogeneity.
In the protocol it was planned to calculate relative risks and 95% confidence intervals for dichotomous variables. In this update, we used risk differences for dichotomous variables because many studies reported no events and it was therefore not possible to calculate relative risks.
In the protocol it was planned to include patient quality of life, ability to perform activities of daily living, and the combined outcomes of death or dependency and death or institutional care. However, we did not find enough studies to perform such analyses.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ada 2003
| Methods | Parallel‐group design Concealed randomisation of participants by ranking the participants according to independent walking speed at baseline (from fastest to slowest) and then allocating each descending pair of participants by coin toss 14% drop outs at the end of treatment and 10% drop outs at the end of the follow‐up phase Outcome assessors were blinded to group allocation | |
| Participants | 14 participants in the EXP group and 15 participants in the CTL group Inclusion criteria: less than 5 years post stroke; first stroke; clinically diagnosed hemiparesis; aged 50 to 85 years; can walk 10 metres independently with a speed less than 1 m/s; discharged from rehabilitation Exclusion criteria: cardiovascular disease that would preclude participation in training (assessed by the participant's medical practitioner); severe cognitive deficits that would preclude participation in training | |
| Interventions | Treated as outpatients for 3 x 30‐minute sessions per week for 4 weeks Treadmill training (EXP): participants walk on a treadmill (no body weight support was provided using a harness) and complete some overground walking training (the proportion of overground training is gradually increased) Sham training (CTL): home‐based exercises based on written instructions with weekly telephone contact to review and update the exercises | |
| Outcomes | Assessed at baseline, after treatment phase and 3‐month follow‐up:
|
|
| Notes | Obtained unpublished data by interview and correspondence with the trialists. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated by coin toss to 1 of 2 groups |
| Allocation concealment (selection bias) | Low risk | By an investigator independent of recruitment and measurement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinded |
Ada 2010
| Methods | Parallel‐group design Concealed randomisation Outcome assessor was blinded to group allocation | |
| Participants | Country: Australia 64 participants in the EXP group and 62 participants in the CTL group Inclusion criteria: within 28 days of their first stroke, between 50 and 85 years of age, hemiparesis or hemiplegia clinically diagnosed, and nonambulatory (defined as scoring 0 or 1 on item 5 (walking) of the Motor Assessment Scale for Stroke) Exclusion criteria: clinically evident brain stem signs, severe cognitive and/or language deficits that precluded them from following instructions, unstable cardiac status or any premorbid conditions that precluded them from rehabilitation 126 stroke patients who were unable to walk were recruited and randomly allocated to an experimental or a control group within 4 weeks of stroke |
|
| Interventions | Both the EXP and the CTL groups underwent a maximum of 30 minutes per day of walking practice with assistance from 1 therapist for 5 days per week EXP group involved walking on a treadmill supported in a harness: initial body weight support was set so that the knee was within 15 degrees of extension in mid‐stance; initial speed of the treadmill was set so that the therapist had time to assist the leg to swing through while maintaining a reasonable step length CTL group involved assisted overground walking |
|
| Outcomes | The primary outcome was the proportion of participants achieving independent walking within 6 months Independent walking was defined as being able to walk 15 metres overground barefoot without any aids; participants were tested once per week until they achieved independent walking or were discharged from the rehabilitation unit and were tested again at 6 months |
|
| Notes | MOBILISE trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random permuted (computer‐generated) blocks |
| Allocation concealment (selection bias) | Low risk | A central office was used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor was blinded for primary outcome |
Ada 2013
| Methods | RCT Method of randomisation: computer‐generated Blinding of outcome assessors: stated as 'yes' by the investigator Adverse events: not stated Deaths: none Drop outs: 4 (0 in EXP group A, 1 in EXP group B, 3 in CTL group) ITT: yes | |
| Participants | Country: Australia 102 participants (34 in EXP group A, 34 in EXP group B, 34 in CTL group) Ambulatory at study onset Mean age: 63 years; 64 to 70 years (control and EXP groups respectively) Inclusion criteria: within 5 years of their first stroke, adults capable of providing consent (defined as having a MMSE score of > 23), had been discharged from formal rehabilitation, were community dwelling and walked slowly (defined as being able to walk 10 metres across flat ground in bare feet without any aids taking more than 9 seconds) Exclusion criteria: unstable cardiac status precluding them from participation in a treadmill training programme (i.e. permission not granted by their medical practitioner), or had severe cognitive and/or language deficits (aphasia) precluding them from participation in the training sessions (i.e. unable to follow 2‐step commands) | |
| Interventions | 3 arms: EXP group A undertook 30 minutes of treadmill and overground walking 3 times per week for 4 months EXP group B undertook treadmill training for 2 months CTL group had no intervention |
|
| Outcomes | Outcomes were recorded at baseline and after 2, 4, 6 and 12 months
|
|
| Notes | The AMBULATE trial We combined the results of both treadmill groups (EXP group A and EXP group B) as 1 group and compared with the results of the CTL group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, independent and concealed randomisation was used to assign each participant in this study |
| Allocation concealment (selection bias) | Low risk | Independent and concealed allocation was used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome measures were collected by therapists trained in the measurement procedures who were blind to group allocation |
da Cunha Filho 2002
| Methods | Parallel‐group design Participants randomised to groups using a random number table Allocation to groups was not concealed 13% drop outs at the end of the treatment phase Outcome assessors were not blinded to group allocation | |
| Participants | 7 participants in the EXP group and 8 participants in the CTL group Inclusion criteria: less than 6 weeks post stroke; hemiparetic stroke based on clinical examination or MRI, or both; significant gait deficit ‐ speed of no more than 36 m/min or FAC 0 to 2 (that is, needs assistance); sufficient cognition to participate in training (at least 21 on the MMSE); ability to stand and take at least 1 step with or without assistance; informed consent Exclusion criteria: any co‐morbidity or disability other than hemiparesis that would preclude gait training; recent myocardial infarction; any uncontrolled health condition for which exercise is contraindicated (e.g. diabetes); severe lower extremity joint disease or rheumatoid arthritis that would interfere with gait training; obesity (mass more than 110 kg) | |
| Interventions | Treated as inpatients for 5 x 20‐minute sessions per week for 2 to 3 weeks BWSTT (EXP): participants walked on a treadmill with up to 30% of their body weight supported using a harness Regular gait training (CTL): strengthening, functional and mobility activities | |
| Outcomes | Assessed at baseline and after treatment phase:
|
|
| Notes | The rating of drop outs and the allocation concealment classification were changed based on correspondence from the trialist | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | High risk | Inadequate (based on correspondence from the investigator) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded (based on correspondence from the investigator) |
Deniz 2011
| Methods | RCT Method of randomisation: not described Blinding of outcome assessors: yes Adverse events: not stated Deaths: none Drop outs: none ITT: yes | |
| Participants | Country: Turkey 20 participants (10 in EXP group, 10 in CTL group) Ambulatory at study onset: yes Mean age: 62 years (CTL and EXP groups respectively) Inclusion criteria: ischaemic or haemorrhagic stroke prior 6 weeks to study enrolment, confirmed by MRI, MMSE score > 21, supported or independent 1‐minute free‐standing, significant loss of ambulation (FAC < 3) Exclusion criteria: recurrent stroke interfering with the study, severe contractures of the lower extremity joints, severe cardiac conditions, uncontrolled diabetes mellitus, Parkinson's Disease, current thrombosis in the legs, aphasia, depression and body weight > 110 kg | |
| Interventions | 2 arms: CTL group used general physiotherapy, 5 times per week for 4 weeks (300 minutes a week) EXP group received BWSTT, 5 times per week for 4 weeks (300 minutes a week) | |
| Outcomes | Outcomes were recorded at baseline, at the end of the intervention phase and at 3‐month follow‐up FAC, Rivermead Motor Evaluation Gross (RMD1) and total gross function (RMD2), Berg Balance Scale, Barthel Index, walking capacity (6‐Minute Walk Test), walking speed (10 metre walk), cadence rate, ratios of right‐left step length, muscle activity (EMG) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
Du 2006
| Methods | RCT, parallel‐group design Method of randomisation: random number table Allocation concealment: unclear Blinding of outcome assessors: not stated by the authors Adverse events: not stated by the authors Deaths: not stated by the authors Drop outs: not stated by the authors ITT: unclear | |
| Participants | Country: China 128 participants (67 in EXP group, 61 in CTL group) Ambulatory at study onset: 26/61 participants (43%) of the EXP group and 22/67 participants (33%) of the CTL group Mean age: 58 to 56 years (CTL and EXP groups respectively) Inclusion criteria: ≤ 3 months after stroke, stable stroke, Brunnstrom stage > 2 Exclusion criteria: severe cognitive dysfunction, acute myocardial infarction, unstable angina pectoris, other severe medical conditions of the inner organs | |
| Interventions | 2 arms, treated as inpatients and outpatients: CTL group used conventional treatment techniques, 2 times per day for 4 weeks EXP group used BWSTT in addition to the same training as in the CTL group for the same time and frequency | |
| Outcomes | Outcomes were recorded at baseline and after the end of the intervention phase:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | High risk | To be confirmed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | To be confirmed |
Duncan 2011
| Methods | Parallel‐group design Participants were randomised to 3 groups using a stratified randomisation procedure Allocation to groups was concealed 11.5% drop outs at the end of the treatment phase Outcome assessors were not rigorously blinded to group allocation | |
| Participants | Country: USA 408 participants Inclusion criteria: age of 18 years or older, a stroke within 45 days before study entry and the ability to undergo randomisation within 2 months after the stroke, residual paresis in the leg affected by stroke, the ability to walk 3 metres with assistance from no more than 1 person and the ability to follow a 3‐step command, the treating physician’s approval of participation in the study, a self selected speed for walking 10 metres of less than 0.8 m per second, and residence in the community by the time of randomisation Exclusion criteria: dependency on assistance in activities of daily living before the stroke, contraindications to exercise, pre‐existing neurologic disorders and inability to travel to the treatment site |
|
| Interventions | 3 groups: Group 1 (EXP) received training on a treadmill with the use of BWS 2 months after the stroke had occurred (early locomotor training) Group 2 (EXP) received this training 6 months after the stroke had occurred (late locomotor training) Group 3 (CTL) participated in an exercise programme at home managed by a physical therapist 2 months after the stroke (home‐exercise programme) Each intervention included 36 sessions of 90 minutes each for 12 to 16 weeks |
|
| Outcomes | The primary outcome was the proportion of participants in each group who had an improvement in functional walking ability 1 year after the stroke Further outcomes were: walking speed; distance walked in 6 minutes; number of steps walked per day; Stroke Impact Scale; FMA legs; Berg Balance Scale; Specific Balance Confidence score |
|
| Notes | We combined the results of both EXP groups (Group 1 and Group 2) as 1 group and compared them with the results of the CTL group (Group 3) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors describe that participants were randomly assigned to 1 of 3 groups. Authors describe a stratified randomisation procedure in ratios of 140:120:120 stratified by severity. The method of randomisation generation is, however, not described |
| Allocation concealment (selection bias) | Low risk | The method of allocation concealment is described as: "The study coordinator registers the patient, enters the baseline data into the web based database system, and then obtains group assignment from the data management and analysis center." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Per diem therapists did the assessments |
Eich 2004
| Methods | Parallel‐group design Concealed randomisation of participants to groups by having a person independent of the study asking the participant to draw a sealed opaque envelope from a box (each envelope contained the group allocation and there were 25 EXP and 25 CTL envelopes) 0% drop outs at the end of treatment and 2% drop outs at the end of the follow‐up phase Outcome assessors were blinded to group allocation | |
| Participants | 25 participants in the EXP group, and 25 participants in the CTL group Inclusion criteria: first time supratentorial stroke; less than 6 weeks post stroke; aged 50 to 75 years; scores 50 to 80 on 100‐point Barthel Index; able to walk a minimum distance of 12 metres with either intermittent help or stand‐by assistance; cardiovascular stable; participation in a 12‐week comprehensive rehabilitation programme; no other neurologic or orthopaedic disease impairing walking; able to understand the purpose and content of the study; written consent | |
| Interventions | Treated as inpatients for 5 x 30‐minute sessions per week for 6 weeks TTBWS (EXP): participants walked on a treadmill with up to 15% of their body weight supported using a harness; the slope and speed of the treadmill were adjusted to achieve a training heart rate Regular gait training (CTL): tone‐inhibiting and gait preparatory manoeuvres and walking practice on the floor and stairs based on Bobath (non‐task‐oriented 'neurophysiological') | |
| Outcomes | Assessed at baseline, after treatment phase and 3 months later:
|
|
| Notes | Method of randomisation and the allocation concealment classification were changed based on correspondence from the trialist | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Using sealed envelopes chosen by an independent person |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The primary outcomes were not blinded, the secondary outcomes walking ability (Rivermead Motor Assessment scale) and walking quality were blinded |
Franceschini 2009
| Methods | RCT Method of randomisation: software generated Blinding of outcome assessors: stated as 'yes' by the trialists Adverse events: not stated Drop outs: 20 (10 in EXP group, 10 in CTL group) ITT: unclear | |
| Participants | Country: Italy 102 participants (52 in EXP group, 50 in CTL group) Not ambulatory at study onset Mean age: 66 to 71 years (CTL and EXP group respectively) Inclusion criteria: within 45 days of the onset of hemiparesis caused by right or left ischaemic or haemorrhagic stroke, able to control the sitting position on a rigid plane surface with the legs hanging freely and without the help of the arms for at least 30 seconds; able to control the trunk in the upright position even with the help of the upper extremities gripping a fixed support or other aid (cane, tripod); without lower limb spasticity (Ashworth scale 1), in stable cardiovascular condition with a low, although slightly greater, risk for vigorous exercise than apparently healthy persons (Class B according to the American College of Sports Medicine) Exclusion criteria: significant disability before stroke (modified Rankin Scale 2); significant pre stroke gait disability (Walking Handicap scale 2) and mild gait impairment at time of enrolment (ability to walk without aids for at least 3 metres or to walk for more than 6 metres with the aid of a cane or tripod); patients having done previous treadmill training and/or with a Class C or D exercise risk according to the American College of Sports Medicine criteria or Class III or IV in the New York Heart Association classification system; patients with orthopaedic or other disorders causing a gait limitation before stroke onset Participants who did not complete the treatment (EXP or CTL) within 5 weeks of study inclusion were excluded from the analysis |
|
| Interventions | EXP group received conventional rehabilitative treatment plus gait training with BWS on a treadmill CTL group received conventional treatment with overground gait training only All participants were treated in 60‐minute sessions every weekday for 4 weeks |
|
| Outcomes | Outcome measures were:
Assessments were done at baseline, after 20 sessions of treatment, 2 weeks after treatment and 6 months after stroke |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation scheme was generated by custom‐made software that used the Lehmer algorithm |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment is not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments were done by therapists and physicians not involved in the treatment of the patient |
Gan 2012
| Methods | RCT Method of randomisation: not stated Blinding of outcome assessors: unclear Adverse events: not stated Deaths: not stated Drop outs: unclear ITT: unclear | |
| Participants | Country: Philippines 205 participants (102 in EXP group, 103 in CTL group) Ambulatory status at study onset: unclear Mean age: unclear Inclusion criteria: unclear Exclusion criteria: unclear | |
| Interventions | Interventions: either to BWS supported overground gait training or BWS supported treadmill training group BWS was provide by using an overhead harness system with up to 40% of their BWS at the beginning of the training Treadmill speed in the BWS‐treadmill group was initially started at 0.5 mph Progression was accomplished by decreasing percentage of BWS or increasing treadmill speed based on gait pattern and endurance |
|
| Outcomes | Main outcome measures: study outcome measures included:
|
|
| Notes | Only published as abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of blinding not described |
Globas 2011
| Methods | RCT Method of randomisation: computer‐based Blinding of outcome assessors: not blinded Adverse events: 1 recurrent stroke (EXP group) Drop outs: 2 (2 in EXP group, 0 in CTL group) ITT: stated by the trialists |
|
| Participants | Country: Switzerland and Germany 38 participants (20 in EXP group, 18 in CTL group) Ambulatory at study onset Mean age: 69 years (both CTL and EXP groups) Inclusion criteria: hemiparetic gait as evaluated by a neurologist with at least 1 clinical sign for paresis, spasticity or circumduction of the affected leg while walking, and the ability to walk on the treadmill at ≥ 0.3 km/hour for 3 minutes with handrail support Exclusion criteria: unstable angina pectoris, heart failure (New York Health Association > II°), haemodynamically significant valvular dysfunction, peripheral arterial occlusive disease, dementia (MMSE < 20), aphasia (unable to follow 2 commands), major depression (CES‐D > 16) and other medical conditions precluding participation in aerobic exercise, as well as patients already performing aerobic exercise training for > 20 minutes per day and > 1 day per week |
|
| Interventions | 3 months (3 times per week) progressive graded, high‐intensity aerobic treadmill exercise (TAEX) or conventional care physiotherapy | |
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐based pseudo random number generator and the Moses–Oakford assignment algorithm were used to develop the randomisation schedule |
| Allocation concealment (selection bias) | Low risk | The procedure was performed by study independent staff at the Department of Biostatistics, University of Ulm, Germany |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcomes was done |
Hoyer 2012
| Methods | RCT Method of randomisation: computer‐based Blinding of outcome assessors: yes Adverse events: not described Drop outs: 0 ITT: not stated by the trialists, probably done because no drop outs were reported |
|
| Participants | Country: Norway 60 participants (30 in EXP group, 30 in CTL group) Not ambulatory at study onset Mean age: 52 years (both groups) Inclusion criteria: admission for a primary rehabilitation stay, mainly < 6 months after onset of stroke, use of wheelchair, dependent on assistance for walking with or without walking aids, medically stable, no neurological or orthopaedic contraindications for walking, and sufficient cognitive capacity to understand information and instructions Exclusion criteria: the patients' need of assistance should not be beyond 1 person for shorter transfer and for taking some steps over ground |
|
| Interventions | 2 arms: Traditional gait training or treadmill therapy In the traditional gait training group intensive gait training (30 minutes) and functional training (30 minutes) daily for minimum of 10 weeks was conducted In the treadmill therapy participants walked on a motorised, raised treadmill, secured by a harness combined with a suspension system releasing body weight; this group received 30 sessions of TTBWS, plus conventional gait training and other functional training for a period of minimum 10 weeks; TTBWS was conducted daily for the first 4 weeks (20 sessions), and then 1 to 2 times a week (10 sessions) for the remaining 6 weeks; on days without TTBWS, conventional gait training was conducted; each treadmill session lasted for 30 minutes, including necessary pauses, but excluding equipment preparation Time for daily training (5 days a week) was the same in the 2 intervention groups, 30 minutes for walking and 30 minutes for other functional training, including selective training of the trunk and extremities, balance and transfer, customised to individual deficits and needs Additional self training, individually or by the staff, was allowed |
|
| Outcomes | Outcomes were recorded at baseline and after 4 to 6 weeks and after 10 to 12 weeks Primary outcomes: walking ability (FAC and EU‐walking scale) Secondary outcomes: walking velocity and steps, walking endurance | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 60 numbers concealed in envelopes were prepared by an external statistician |
| Allocation concealment (selection bias) | Unclear risk | Not described, probably done because concealed envelopes were used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A pool of 8 experienced assessors blinded to group allocation were involved in testing |
Jaffe 2004
| Methods | Parallel‐group design Concealed randomisation of participants to groups by using an Excel spreadsheet with group allocation masked using black cells 15% drop outs at the end of the treatment phase and 15% drop outs at the end of the 2‐week follow‐up Blinding of outcome assessors to group allocation | |
| Participants | 11 participants in the EXP group and 12 participants in the CTL group Inclusion criteria: at least 6 months post stroke; hemiplegia secondary to documented lesion; able to walk independently or with stand‐by supervision (with or without a gait aid); asymmetric gait pattern and short step length; 'average' or 'minimal impairment' in all Cognistat test categories; informed consent Exclusion criteria: any medical condition that would prevent participation in a training programme; inability to follow instructions | |
| Interventions | Treated as outpatients for 6 x 1‐hour sessions per week for 2 weeks Virtual reality and treadmill training (EXP): participants practiced stepping over virtual objects while walking on a treadmill, with a harness to prevent falls (each session consisted of 12 trials of stepping over 10 obstacles) Overground training (CTL): participants practiced stepping over real objects while walking overground, with a gait belt for safety (each session consisted of 12 trials of stepping over 10 obstacles; task‐oriented) | |
| Outcomes | Assessed at baseline, after treatment phase and 2 weeks later:
|
|
| Notes | Rating of concealed allocation, assessor blinding and drop outs, and the allocation concealment classification were changed based on correspondence from the trialist | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Unclear concealed randomisation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessors to group allocation |
Kang 2012
| Methods | RCT Method of randomisation: sealed envelopes Blinding of outcome assessors: stated as 'yes' by the investigator Adverse events: not stated Drop outs: 2 (2 in EXP groups, 0 in CTL group) ITT: unclear | |
| Participants | Country: Republic of Korea 32 participants (11 in first EXP group, 11 in second EXP group and 10 in CTL group) Ambulatory at study onset Mean age: 56 years (CTL and EXP groups) Inclusion criteria: hemiparetic stroke patients 6 months after diagnosis; patients who could walk on their own for more than 15 minutes; patients without visual disabilities or hemianopia; (4) patients who had a mini‐mental state examination score of 21 or higher; Brunnstrum stage > 4 Exclusion criteria: cardiovascular problems; orthopaedic and other neurological diseases except stroke for influencing gait |
|
| Interventions | 3 arms
|
|
| Outcomes | Before and after treatment:
|
|
| Notes | We combined the results of both EXP groups (arms 1 and 2) as 1 group and compared with the results of the CTL group (arm 3) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Independent person who picked one of the sealed envelopes before the start of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Other physical therapists than the treating physical therapists used in this study for the blinding measurements |
Kim 2011
| Methods | RCT Method of randomisation: not described Blinding of outcome assessors: no Adverse events: not stated Deaths: none Drop outs: not described ITT: not described | |
| Participants | Country: Republic of Korea 20 participants in the EXP group and 24 participants in the CTL group Inclusion criteria: stroke, able to maintain standing independently for 30 seconds and to walk independently more than 30 metres and able to understand and follow instructions Exclusion criteria: orthopaedic surgery or impairment, Modified Ashworth scale of 2 or more |
|
| Interventions | 2 arms
Both groups received walking therapy for 30 minutes, 3 times a week for 6 weeks |
|
| Outcomes | Outcomes were recorded at baseline and after 6 weeks
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | High risk | Not described, probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described, probably not done |
Kosak 2000
| Methods | Parallel‐group design Participants randomised to groups using a random number table Concealed allocation to groups by a person independent of the study 5% drop outs at the end of the treatment phase Blinding of outcome assessors to group allocation | |
| Participants | 22 participants in the EXP group and 34 participants in the CTL group Inclusion criteria: no prior stroke; independent with ambulation prior to stroke; no active angina pectoris or orthostatic hypertension; free of other neurologic or orthopaedic disorders that might preclude walking; FIM walking subscore less than or equal to 3 (indicating at least moderate assistance is required for ambulation); hemiparesis with iliopsoas strength less than or equal to 3 out of 5 (indicating significant weakness ‐ full range of movement against gravity only); written informed consent | |
| Interventions | Treated as inpatients for 5 x 45‐minute sessions per week for an average of 12.5 (SD 4.7) total treatment sessions Treadmill training with body weight support (EXP): participants walked on a treadmill and were provided with manual guidance for weight shifting, leg advancement and foot placement Aggressive bracing assisted walking (CTL): participants walked with the assistance of knee‐ankle combination bracing and a hemi‐bar (non‐task‐oriented ‐ 'orthopaedic') | |
| Outcomes | Assessed at baseline and after treatment phase:
|
|
| Notes | Rating of concealed allocation and the allocation concealment classification were changed based on correspondence from the trialist | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Concealed allocation to groups by a person independent of the study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not described |
Kuys 2011
| Methods | RCT Method of randomisation: computer‐generated random number programme Blinding of outcome assessors: stated as 'yes' by the investigator Adverse events: none Drop outs: 2 (2 in EXP group, 0 in CTL group) ITT: described as ITT used | |
| Participants | Country: Australia 30 participants (15 in EXP group, 15 in CTL group) Ambulatory at study onset Mean age: 72 to 63 years (control and EXP group respectively) Inclusion criteria: diagnosis of first stroke confirmed by CT scan, were referred for physiotherapy rehabilitation and scored 2 or more on the walking item of the Motor Assessment Scale (i.e. were able to walk with stand‐by help), were medically stable, were able to understand simple instructions Exclusion criteria: normal walking speed was considered normal (> 1.2 m/s), any cardiovascular problems that limited their participation in rehabilitation or had other neurological or musculoskeletal conditions affecting their walking |
|
| Interventions | 2 arms:
|
|
| Outcomes | Details of treadmill walking (duration, heart rate reserve, treadmill speed and distance walked) were recorded for each session:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number programme |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed from the recruiter through the use of consecutively numbered envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Measures were taken by assessors blinded to group allocation |
Langhammer 2010
| Methods | RCT Method of randomisation: by sealed envelopes Blinding of outcome assessors: stated as 'yes' by the investigator Adverse events: not stated Deaths: no Drop outs: 5 (3 in EXP group, 2 in CTL group) ITT: unclear | |
| Participants | Country: Norway 39 participants (21 in EXP group, 18 in CTL group) Not ambulatory at study onset Mean age: 75 to 74 years (control and EXP group respectively) Inclusion criteria: stroke, neurological impairment and age above 50 years Exclusion criteria: barriers to taking part in a physical rehabilitation programme, insufficient language, an unstable cardiac status, neurosurgery and a premorbid history of orthopaedic problems or any problems that would prevent a patient from walking | |
| Interventions | 2 arm:
for 30 minutes 5 days a week during the inpatient stay until discharge from hospital (length of stay was 16 days in EXP group, and 17 days in CTL group) |
|
| Outcomes | Main measures: Six‐Minute Walk Test, a 10‐Metre Walk Test and pulse rates at rest and in activity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes |
| Allocation concealment (selection bias) | Low risk | By a person not involved; sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinded |
Laufer 2001
| Methods | Parallel‐group design Alternate assignment of participants to groups, therefore allocation to groups not concealed 14% drop outs at the end of the treatment phase Blinding of outcome assessors to group allocation | |
| Participants | 15 participants in the EXP group and 14 participants in the CTL group Inclusion criteria: first supratentorial stroke in anterior brain circulation as evidenced by CT scanning; no additional neurological or orthopaedic deficiencies impairing ambulation; no cardiac, respiratory or medical condition that could interfere with the protocol; no severe cognitive or communication impairment; onset of stroke no more than 90 days prior to recruitment; ability to walk on treadmill at a speed of at least 0.2 km/hour for 2 minutes without rest with minimal to moderate assistance; have begun ambulation training | |
| Interventions | Treated as inpatients for 5 sessions of up to 20 minutes per week for 3 weeks (15 treatment sessions) Treadmill training (EXP): participants walked on a treadmill at a comfortable speed with a therapist assisting leg movements, they were permitted use a handrail for external support if required; no body weight support using a harness was provided Overground walking (CTL): participants walked on a floor surface using gait aids, assistance and rest periods as needed | |
| Outcomes | Assessed at baseline and after treatment phase:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternately assigned to groups by order of admittance |
| Allocation concealment (selection bias) | High risk | Not described, inadequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessors to group allocation |
Liston 2000
| Methods | Cross‐over group design Participants randomised to groups by the toss of a coin Allocation concealment not reported 17% drop outs at the end of the first treatment phase Blinding of outcome assessors to group allocation | |
| Participants | 10 participants allocated to the EXP then CTL order, and 8 participants allocated to the CTL then EXP order Inclusion criteria: higher level gait disorder; CT scan with large vessel infarct, basal ganglia and white matter lacunes, or extensive leukoaraiosis; discharged from all rehabilitation services; informed consent Exclusion criteria: severe cognitive impairment; significant physical impairments from other causes | |
| Interventions | Treated as inpatients or outpatients for 3 x 1‐hour sessions per week for 4 weeks Treadmill training (EXP): participants walked on a treadmill for as long as they felt comfortable, rest breaks were allowed; no body weight support was provided using a harness Conventional physiotherapy (CTL): a schedule of 31 interventions in 3 treatment modules: gait ignition or failure, postural alignment and other | |
| Outcomes | Assessed at baseline, at cross‐over (4 weeks), after treatment phase (at 8 weeks) and 6 weeks after final treatment:
|
|
| Notes | The rating of drop outs was changed based on correspondence from the trialist Trial treated as a parallel‐group design for this review by using the first treatment phase data only (that is baseline and cross‐over data only) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By the toss of a coin |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessors to group allocation |
Luft 2008
| Methods | RCT Method of randomisation: computer‐based list Blinding of outcome assessors: stated as 'yes' by the investigator Adverse events: not stated Deaths: not stated Drop outs: 42 (20 in EXP group, 22 in CTL group) ITT: no | |
| Participants | Country: USA 113 participants (57 in EXP group, 56 in CTL group) Ambulatory at study onset Mean age: 64 to 63 years (CTL and EXP group respectively) Inclusion criteria: first clinical ischaemic stroke, older than 45 years of age with chronic hemiparetic gait 6 or more months after completion of conventional subacute rehabilitation Exclusion criteria: heart failure, unstable angina, peripheral arterial occlusive disease, dementia (MMSE ≤ 23 for those with 9th grade education or more and ≤ 17 for those with 8th grade education or less), significant aphasia (unable to follow 2‐point commands), untreated major depression (CES‐D 16) and other medical conditions precluding participation in aerobic exercise | |
| Interventions | 2 arms:
|
|
| Outcomes | Assessed at baseline, 3 and 6 months:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based list |
| Allocation concealment (selection bias) | High risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessors |
MacKay‐Lyons 2013
| Methods | RCT Method of randomisation: computer‐generated, blocked randomisation Blinding of outcome assessors: stated as 'yes' by the investigator Adverse events: not stated Deaths: not stated Drop outs: 5 (2 in EXP group, 3 in CTL group) ITT: all analyses were conducted on an ITT basis (that means carrying the last observation forward for those lost to follow‐up) |
|
| Participants | Country: Canada 50 participants (24 in EXP group, 26 in CTL group) Ambulatory at study onset Mean age: 59 to 62 years (control and EXP group respectively) Inclusion criteria: men and women older than 18 years, within 1 month of a first ischaemic stroke confirmed by neuroimaging, inpatients in the stroke rehabilitation unit and able to walk 5 metres with or without use of ambulatory aids, ankle orthoses or stand‐by assistance Exclusion criteria: contraindications to maximal exercise stress testing, musculoskeletal or cognitive limitations that could preclude participation in the programme, or involvement in other pharmacological or physical intervention studies |
|
| Interventions | 2 arms:
All individuals participated in 60‐minute physiotherapy sessions 5 times weekly as inpatients for 6 weeks and 3 times weekly as outpatients for another 6 weeks for a total of 48 sessions Substitute sessions for missed appointments were provided |
|
| Outcomes | Assessments were done at baseline, post‐training, at 6 and 12‐month follow‐up:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, blocked randomisation |
| Allocation concealment (selection bias) | Low risk | A person not involved in the study prepared and safeguarded individual, opaque sealed envelopes containing group and physiotherapist allocation, which were opened after completion of the baseline assessment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcome assessments were conducted by a blinded assessor located off‐site |
Macko 2005
| Methods | Parallel‐group design Participants randomised to groups using a computer‐generated randomisation scheme that was stratified by walking speed (less than 0.44 m/s and more than or equal to 0.44 m/s) and age (less than 65 years and more than or equal to 65 years) Concealed allocation to groups not reported 26% drop outs at the end of the treatment phase Blinding of outcome assessors to group allocation for gait and balance outcomes (i.e. outcomes 1, 2, 3 and 6) | |
| Participants | 32 participants in the EXP group and 29 participants in the CTL group Inclusion criteria: chronic ischaemic stroke (less than 6 months); residual mild to moderate hemiplegic gait deficits; completion of all conventional physiotherapy; aged 45 years or more; (5) independently ambulant with or without a gait aid or stand‐by help Exclusion criteria: heart failure, unstable angina, peripheral arterial occlusive disease; aphasia (inability to follow 2‐point commands); dementia; untreated major depression; other medical conditions precluding aerobic exercise | |
| Interventions | Treated as outpatients for 3 x 40‐minute sessions per week for 6 months Treadmill training (EXP): participants walked on a treadmill to achieve a target aerobic intensity of 60% to 70% heart rate reserve (progressive aerobic training); no body weight support was provided using a harness Conventional physiotherapy (CTL): participants completed a supervised stretching and low‐intensity walking programme (5 minutes walking on a treadmill at 30% to 40% heart rate reserve without body weight support; task‐oriented) | |
| Outcomes | Assessed at baseline and after treatment phase:
|
|
| Notes | Method of randomisation and rating of assessor blinding were changed based on correspondence from the trialist Obtained unpublished data by correspondence with the trialists |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation scheme |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessors to group allocation for gait and balance outcomes (i.e. outcomes 1, 2, 3 and 6) |
Mehrberg 2001
| Methods | RCT Method of randomisation: not stated Blinding of outcome assessors: not stated Adverse events: not stated Deaths: not stated Drop outs: not stated ITT: unclear | |
| Participants | Country: USA 21 participants (9 in EXP group, 11 in CTL group; according to the authors, 1 participant appears to be missing) Ambulatory status at study onset unclear Mean age: unclear Inclusion criteria: severe hemiparetic patients after stroke (defined as inability to raise and hold affected leg) Exclusion criteria: not stated |
|
| Interventions | 2 arms:
1 hour per day for 3 weeks |
|
| Outcomes | Tinetti Balance Scale Functional Ambulation Categories Scandinavian Stroke Scale |
|
| Notes | Only published as conference proceeding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
Moore 2010
| Methods | RCT with baseline period, followed by cross‐over design Method of randomisation: not stated Blinding of outcome assessors: stated as 'yes' by the investigator Adverse events: not stated Deaths: not stated Drop outs: 10 (unclear in which period/group) ITT: unclear | |
| Participants | Country: USA 30 participants (probably 15 in EXP group, 15 in CTL group) Ambulatory at study onset Mean age: 57 to 67 years (CTL and EXP group respectively) Inclusion criteria: ≤ 3 months after stroke, ability to stand or walk 5 metres Exclusion criteria: orthopaedic problems, contractures, NYHA III‐IV | |
| Interventions | 2 arms: A, A‐B, B‐A 20 out of 30 participants with chronic stroke completed a repeated baseline measure, randomised cross‐over trial in which walking performance was assessed during the last 4 weeks of clinical physical therapy before discharge secondary to reaching a plateau, followed by 4 weeks of intensive locomotor training and 4 weeks of no intervention |
|
| Outcomes | Outcome measures included clinical and physiological (metabolic) measures of walking overground and on a treadmill, and measures of daily stepping activity in the home and community, including during clinical physical therapy and subsequent locomotor therapy sessions | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
Nilsson 2001
| Methods | Parallel‐group design Participants randomised to groups using a random number computer program Concealed allocation to groups using sealed, opaque and consecutively numbered envelopes 10% drop outs at the end of the treatment phase, 18% drop outs at the 10‐month follow‐up Blinding of outcome assessors to group allocation | |
| Participants | 36 participants in the EXP group and 37 participants in the CTL group Inclusion criteria: first stroke with residual hemiparesis; aged less than 70 years; onset of stroke no more than 8 weeks prior to recruitment; take longer than 14 seconds to walk 10 metres; informed consent Exclusion criteria: patients with heart disease, psychiatric illness or incapable of co‐operating; patients with other severe disabilities (e.g. rheumatoid arthritis) that might hinder training; patients participating in other studies | |
| Interventions | Treated as inpatients for 5 x 30‐minute sessions per week for the duration of inpatient rehabilitation Treadmill training with body weight support (EXP): participants walked on a treadmill with up to 2 therapists assisting leg movements, they were permitted to use a handrail for external support if required Overground walking training (CTL): participants practiced walking on a floor surface based on a Motor Relearning Program guidelines | |
| Outcomes | Assessed at baseline, after treatment phase (when discharged from inpatient rehabilitation) and 10 months after stroke:
|
|
| Notes | Allocation concealment classification was changed based on correspondence from the trialist Data divided into 2 comparisons, see Nilsson 2001a and Nilsson 2001b |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number computer program |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque and consecutively numbered envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was done |
Nilsson 2001a
| Methods | See Nilsson 2001 | |
| Participants | See Nilsson 2001 | |
| Interventions | See Nilsson 2001 | |
| Outcomes | See Nilsson 2001 | |
| Notes | For Nilsson 2001a, data from the 54 participants who were dependent walkers at the start of treatment were used (26 EXP and 28 CTL); these walking dependency data were obtained through correspondence with the authors | |
Nilsson 2001b
| Methods | See Nilsson 2001 | |
| Participants | See Nilsson 2001 | |
| Interventions | See Nilsson 2001 | |
| Outcomes | See Nilsson 2001 | |
| Notes | For Nilsson 2001b, data from the 19 participants who were independent walkers at the start of treatment were used (10 EXP and 9 CTL); these walking dependency data were obtained through correspondence with the authors | |
Olawale 2009
| Methods | RCT Method of randomisation: not described Blinding of outcome assessors: unclear Adverse events: not reported Deaths: not reported Drop outs: 7 (2 in EXP group, 5 in CTL group) ITT: no | |
| Participants | Country: Nigeria 60 participants (20 in EXP group, 40 in CTL group) Ambulatory at study onset: yes Mean age: 57 years (CTL and EXP group respectively) Inclusion criteria: stroke > 3 months but < 24 months prior to enrolment, ability to walk 10 metres independently without the help of assistive devices, written informed consent Exclusion criteria: not reported | |
| Interventions | 3 arms:
|
|
| Outcomes | Outcomes were recorded at baseline, at 4, 8 and after 12 weeks (at the end of the intervention phase) Outcomes: walking speed (10‐Metre Walk Test), walking capacity (6‐Minute Walk Test) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
Pohl 2002
| Methods | Parallel‐group design Participants randomised to groups (block randomisation with participants stratified for walking speed) Concealed allocation to groups using sealed, opaque envelopes 13% drop outs at the end of the treatment phase Blinding of outcome assessors to group allocation | |
| Participants | 22 participants in the EXP 1 group, 22 participants in the EXP 2 group and 25 participants in the CTL group Inclusion criteria: hemiparesis caused by ischaemic stroke; impaired gait (takes 5 to 60 seconds to walk 10 metres); hemiparesis more than 4 weeks; no or slight spasticity (0 or 1 on the Ashworth scale); able to walk without assistance (FAC of 3 or more); informed consent Exclusion criteria: previous treadmill training; class C or D exercise risk (American College of Sports Medicine Guidelines); cognitive deficits (less than 26 out of 30 on Mini Mental State Examination); movement disorders, orthopaedic or other gait influencing disease | |
| Interventions | Treated as inpatients for 3 x 30‐minutes sessions (EXP 1 and EXP 2) or 45‐minute sessions (CTL) per week for 4 weeks Speed‐dependent treadmill training with body weight support (EXP 1): participants walked on a treadmill without therapist assistance, speed was progressed using an aggressive protocol Limited progressive treadmill training with body weight support (EXP 2): participants walked on a treadmill with therapists assisting the walking cycle, speed was progressed using conservative protocol Conventional gait therapy (CTL): traditional physiotherapy based on neurophysiological techniques | |
| Outcomes | Assessed at baseline and after treatment phase:
|
|
| Notes | The rating of concealed allocation and the allocation concealment classification were changed based on correspondence from the trialist In the update of 2005 the data from this study were divided into 2 comparisons: half of the control group data were used for each comparison. Based on the raw data we combined both experimental groups into 1 group. According to Chapter 16.5.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) we combined both treadmill groups, group LTT and group STT together to one treadmill group (to create a single pair‐wise comparison) and compared it with the control group We used raw data provided by the trialists |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded |
Richards 1993
| Methods | Parallel‐group design Participants randomised to groups using a stratified block randomisation scheme Concealed allocation to groups not reported 15% drop outs at the end of the treatment phase, number of drop outs not reported at 3 and 6‐month follow‐ups Blinding of outcome assessors to group allocation | |
| Participants | 10 participants in the EXP group, 8 participants in the CTL 1 group and 9 participants in the CTL 2 group Non‐ambulatory at study onset Inclusion criteria: resident within 50 km of Quebec; aged 40 to 80 years; less than 7 days after onset of first stroke; clinically identifiable middle cerebral artery syndrome of thromboembolic origin involving sub‐cortical structures confirmed by CT; under medical supervision of study neurologists; informed consent; middle‐band disability according to Garraway (i.e. excluded patients independent in ambulation as well as those who were unconscious) Exclusion criteria: other neurological problems; major medical problems that would incapacitate functional capacity (patients independent in ambulation were excluded) |
|
| Interventions | Treated as inpatients for 6 weeks for a mean of 1.74 (SD 0.15) (EXP), 1.79 (SD 0.10) (CTL 1) and 0.72 (SD 0.10) (CTL 2) hours per day Early intensive task‐oriented physiotherapy (EXP): treatment started as early as possible after stroke and included treadmill training (no body weight support was provided using a harness), tilt table exercises and resisted exercises using isokinetic equipment Early intensive traditional physiotherapy (CTL 1): treatment started as early as possible after stroke and included traditional physiotherapy based on neurophysiological techniques Delayed non‐intensive traditional physiotherapy (CTL 2): treatment started later after stroke and included less intense traditional physiotherapy based on neurophysiological techniques | |
| Outcomes | Assessed at baseline, after treatment phase and 3 and 6 months later:
|
|
| Notes | 3 and 6‐month follow‐up data not reported We chose to compare the EXP and CTL 1 groups only for this review because they had the same intensity and starting time of therapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Evaluators were blind to group allocation |
Richards 2004
| Methods | RCT Method of randomisation: stratified randomisation with random permuted blocks and random block size Blinding of outcome assessors: yes Adverse events: not reported Deaths: not reported Drop outs: 15 (7 in EXP group, 8 in CTL group) ITT: yes | |
| Participants | Country: Canada 63 participants (32 in EXP group, 31 in CTL group) Ambulatory at study onset Mean age: 61 to 63 years (CTL and EXP group respectively) Inclusion criteria: age between 30 and 89 years, with first or second episode of ischaemic stroke with residual deficit, Barthel Ambulation Subscore > 10, gait speed between 0.1 and 0.6 m/s Exclusion criteria: haemorrhagic stroke, ability to understand and follow verbal instructions, major medical problems (diabetes, cancer, aphasia, orthopaedic disorders) interfering with the intervention | |
| Interventions | 2 arms:
|
|
| Outcomes | Outcomes were recorded at baseline, at the end of the intervention phase and 3 months later Primary outcomes: gait speed by walking 5 metres, 10 metres or 30 metres at preferred speed Secondary outcomes: lower extremity function (FMA), Timed Up and Go, Functional Independence (Barthel Ambulation Subscore) | |
| Notes | Contamination addressed in the study design by issues of location and personnel | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation with random permuted blocks and random block size |
| Allocation concealment (selection bias) | Low risk | After randomisation, treating therapists were informed about assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded to group assignment |
Scheidtmann 1999
| Methods | Cross‐over group design Participants randomised to groups (method of randomisation and concealment not stated) 0% drop outs at the end of the first treatment phase Blinding of outcome assessors to group allocation not reported | |
| Participants | 15 participants allocated to the EXP then CTL order, and 15 participants allocated to the CTL then EXP order Inclusion criteria: hemiparesis; stroke (infarct or haemorrhage); at least 4 weeks post stroke; not able to walk; able to stand for 20 seconds Exclusion criteria: cardiovascular problems or infections with a decrease in general health | |
| Interventions | Treated as inpatients for 5 x 1‐hour sessions per week for 3 weeks Treadmill training with body weight support (EXP): participants walked on a treadmill with partial body weight support provided by a harness for 30 minutes plus completed 30 minutes of usual physiotherapy per day Usual physiotherapy (CTL): participants completed 2 x 30‐minute sessions of usual physiotherapy per day | |
| Outcomes | Assessed at baseline, at cross‐over (3 weeks) and after treatment phase (at 6 weeks):
|
|
| Notes | Trial treated as a parallel‐group design for this review by using the first treatment phase data only (that is baseline and cross‐over data only) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
Smith 2008
| Methods | RCT Method of randomisation: modified random assignment, matched‐pair CTL group design; stratified regarding (1) motor impairment (measured by FMA) and (2) side of hemiparesis Blinding of outcome assessors: no Adverse events: not reported Deaths: not reported Drop outs: not reported ITT: unclear | |
| Participants | Country: USA 20 participants (10 in EXP group, 10 in CTL group) Ambulatory at study onset: yes Mean age: 56 to 58 years (CTL and EXP group respectively) Inclusion criteria: informed consent, ischaemic stroke in the distribution of the middle cerebral artery < 3 months, but > 2 years prior to study enrolment, walking slower than prior to the stroke Exclusion criteria: cognitive impairment, inability to ambulate, concomitant pathology interfering with treadmill walking | |
| Interventions | 2 arms:
|
|
| Outcomes | Outcomes were recorded at baseline, at the end of the intervention phase and at 6‐week follow‐up Outcomes: depression (Beck Depression Inventory); Stroke Impact Scale (SIS) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessor was not blinded |
Sullivan 2007
| Methods | RCT, parallel‐group design Method of randomisation: stratified block randomisation (block size not stated) Blinding of outcome assessors: yes Adverse events: 21 cumulative adverse events in 18 patients until follow‐up Deaths: none Drop outs: 9 until follow‐up (6 in EXP group, 3 in CTL group) ITT: yes, last observation carried forward for primary outcomes | |
| Participants | Country: USA 80 participants (60 in EXP group, 20 in CTL group) Ambulatory at study onset: yes Mean age: 63 and 60 years (CTL and EXP group respectively) Inclusion criteria: aged 18 and above, ischaemic or haemorrhagic stroke confirmed by CT, MRI or clinical criteria, 4 to 60 months post stroke, ambulate at least 10 metres with assistive or orthotic device, FAC 2 or above, walking speed < 1 m/s, informed consent, approval of primary care physician Exclusion criteria: serious medical conditions interfering with the study protocol such as high blood pressure, high resting heart rate, lower limb orthopaedic conditions, recent botulinum toxin injections, recent baclofen delivery, MMSE score < 24, co‐interventions aiming at gait training or lower extremity strengthening, prior enrolment to similar studies, plans to move out of the area of study centres during the next year | |
| Interventions | 4 arms:
|
|
| Outcomes | Primary outcome was recorded at baseline, after 12 and 24 treatment sessions and at 6‐month follow‐up Secondary outcomes were recorded at baseline, at the end of the intervention phase and at 6‐month follow‐up Primary outcome: overground self selected walking speed Secondary outcomes: fast walking speed, 6‐Minute Walk Test, lower extremity FMA, Berg Balance Scale, 16‐item Stroke Impact Scale (SIS‐16), Medical Outcomes Study Short Form Health Survey (SF‐36), lower extremity isometric peak torque | |
| Notes | The 3 experimental groups (using body weight supported treadmill training) were collapsed together and compared with the CTL group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was generated at a central data management centre |
| Allocation concealment (selection bias) | Low risk | Allocation was performed by a central data management centre |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded |
Suputtitada 2004
| Methods | RCT, parallel‐group design Method of randomisation: block randomisation (block size of 4) Blinding of outcome assessors: yes Adverse events: not reported Deaths: not reported Drop outs: not reported ITT: unclear | |
| Participants | Country: Thailand 48 participants (24 in EXP group, 24 in CTL group) Ambulatory at study onset: yes Mean age: 65 to 61 years (CTL and EXP group respectively) Inclusion criteria: stroke > 6 months prior to enrolment, able to sit at the edge of the bed independently, independent ambulation with or without gait aids, being able to communicate with therapists, informed consent Exclusion criteria: cardiac risk factors, hyperkinetic movement disorders, using orthoses or prostheses, training less than 2 consecutive weeks | |
| Interventions | 2 arms:
|
|
| Outcomes | Outcomes were recorded at baseline and the end of the intervention phase Measures of timed gait (10‐Metre Walk Test); balance ability (Berg Balance Scale) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded |
Takami 2010
| Methods | RCT Method of randomisation: drawing envelopes containing a lot Blinding of outcome assessors: not described Adverse events: not reported Deaths: not reported Drop outs: 3 (1 in EXP group 1, 2 in EXP group 2, none in the CTL group) ITT: unclear |
|
| Participants | Country: Japan 36 participants (12 in EXP group 1, 12 in EXP group 2, 12 in CTL group) Ambulatory at study onset: yes Mean age: 67/71/66 years (CTL and EXP groups 1 and 2 respectively) Inclusion criteria: receive physical therapy, being able to walk 10 metres unassisted, less than 5 weeks post stroke, FIM‐L score < 5, perfect score on the Berg Balance Scale (BBS) or the Rivermead Mobility Index (RMI) Exclusion criteria: time to complete 10‐Metre Walk Test < 4 sec, factors interfering with the study like parkinsonism, dementia, severe communication disorders and orthopaedic conditions | |
| Interventions | 3 arms:
|
|
| Outcomes | Primary outcomes were recorded at baseline and once weekly during the 3‐week intervention phase Primary outcomes: balance ability (BBS), RMI, 10‐metre maximum walking speed, walk ratios during 10 metres of forward walking and 5 metres of backward walking Secondary outcomes: Motricity Index, Functional Independence Measure Locomotor (FIM‐L), modified Borg scale | |
| Notes | Both EXP groups (using body weight supported treadmill training) were collapsed together and compared with the CTL group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Quote: "[subjects] were randomly allocated [...] using an envelope method." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clearly described by the authors, however (quote:) "...a physical therapist measured the required time and number of steps [of measures of timed gait]." |
Toledano‐Zarhi 2011
| Methods | RCT, parallel‐group design Method of randomisation: not described Blinding of outcome assessors: not described Adverse events: none Deaths: none Drop outs: 1 in EXP group ITT: yes | |
| Participants | Country: Israel 28 participants (14 in EXP group, 14 in CTL group) Ambulatory at study onset: yes Mean age: 65 years Inclusion criteria: ischaemic stroke within 1 to 3 weeks after the event, modified Rankin scale < 2 Exclusion criteria: systolic blood pressure > 200 mm Hg, diastolic blood pressure > 110 mm Hg, unstable heart conditions, dementia, age > 80 years | |
| Interventions | 2 arms:
|
|
| Outcomes | Outcomes were recorded at baseline and at the end of the intervention phase:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
Visintin 1998
| Methods | Parallel‐group design Participants randomised to groups using a stratified block randomisation scheme Allocation was concealed using sealed and numbered envelopes 21% drop outs at the end of the treatment phase, 48% drop outs at the 3‐month follow‐up Blinding of outcome assessors to group allocation | |
| Participants | 50 participants in the EXP group and 50 participants in the CTL group Inclusion criteria: admitted to the Jewish Rehabilitation Hospital for physical rehabilitation after stroke; abnormal gait; no severe cardiac problems; no comorbid conditions contraindicating treadmill training; not cerebellar, bilateral or brain stem stroke; able to understand simple commands; anticipated length of stay of at least 4 weeks; onset of stroke no more than 6 months prior to recruitment; able to ambulate pre‐stroke; first admission during study period; treadmill training time slot available; informed consent | |
| Interventions | Treated as inpatients for 4 x 20‐minute session per week for 6 weeks Treadmill training with body weight support (EXP): participants walked on a treadmill with partial body weight support using a harness and the assistance of 1 to 2 therapists Treadmill training only (CTL): participants walked on a treadmill with the assistance of 1 to 2 therapists; no body weight support was provided using a harness | |
| Outcomes | Assessed at baseline, after treatment phase and 3 months later:
|
|
| Notes | The rating of concealed allocation and the allocation concealment classification were changed based on correspondence from the trialist Data divided into 2 comparisons, see Visintin 1998a and Visintin 1998b | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing lots out of a box |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed using sealed and numbered envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blind to group allocation |
Visintin 1998a
| Methods | See Visintin 1998 | |
| Participants | See Visintin 1998 | |
| Interventions | See Visintin 1998 | |
| Outcomes | See Visintin 1998 | |
| Notes | For Visintin 1998a, data from the 59 participants who were dependent walkers at the start of treatment and who did not drop out before the end of the treatment phase were used (33 EXP and 26 CTL); these walking dependency data were obtained through correspondence with the authors | |
Visintin 1998b
| Methods | See Visintin 1998 | |
| Participants | See Visintin 1998 | |
| Interventions | See Visintin 1998 | |
| Outcomes | See Visintin 1998 | |
| Notes | For Visintin 1998b, data from the 20 participants who were independent walkers at the start of treatment and who did not drop out before the end of the treatment phase were used (10 EXP and 10 CTL); these walking dependency data were obtained through correspondence with the authors | |
Weng 2004
| Methods | RCT, parallel‐group design Method of randomisation: stratified randomisation, generation of random sequence not stated Allocation concealment: not described Blinding of outcome assessors: unclear Adverse events: none Deaths: none Drop outs: 5 (2 in EXP group, 3 in CTL group) ITT: no |
|
| Participants | Country: China 50 participants (25 in EXP group, 25 in CTL group) Ambulatory at study onset: yes (FAC ≥ 3) Mean age: 55 years (CTL and EXP group) Inclusion criteria: comply with the Fourth National Stroke diagnostic criteria; stable disease, blood pressure and heart rate control in the normal range, lower extremity Brunnstrom stage ≥ 2, lower extremity limb paralysis without severe clonus and joint stiffness (Ashworth scale ≤ 2),patients being able to walk more than 10 metres independently or under supervision and without the help of assistive devices, walking speed ≥ 0.17 m/s Exclusion criteria: history of myocardial infarction, severe ventricular arrhythmias, chronic heart failure; lower extremity total joint replacement or severe arthritis, recurrent stroke, other severe conditions |
|
| Interventions | 2 arms, treated as inpatients:
|
|
| Outcomes | Outcomes were assessed at baseline and at the end of the intervention phase:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
Weng 2006
| Methods | RCT, parallel‐group design Method of randomisation: random number table Allocation concealment: sealed envelopes Blinding of outcome assessors: unclear Adverse events: not stated by the authors Deaths: not stated by the authors Drop outs: unclear ITT: unclear |
|
| Participants | Country: China 26 participants (13 in EXP group, 13 in CTL group) Ambulatory at study onset: able to walk 10 metres without aids Mean age: 50 to 51 years (CTL and EXP group respectively) Inclusion criteria: comply with the Fourth National Stroke diagnostic criteria; stable disease, blood pressure and heart rate control in the normal range, lower extremity Brunnstrom stage ≥ 2, lower extremity limb paralysis without severe clonus and joint stiffness (Ashworth scale ≤ 2), patients being able to walk more than 10 m independently and without the help of assistive devices Exclusion criteria: history of myocardial infarction, severe ventricular arrhythmias, chronic heart failure, lower extremity total joint replacement or severe arthritis, recurrent stroke, other severe conditions |
|
| Interventions | 2 arms, treated as inpatients:
|
|
| Outcomes | Outcomes were assessed at baseline and at 3 weeks follow‐up:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
Werner 2002a
| Methods | Cross‐over group design Participants randomised to groups (group allocation in envelopes that were drawn by an independent person) 0% drop outs at the end of the first treatment phase Blinding of outcome assessors to group allocation | |
| Participants | 15 participants allocated to the EXP then CTL order, and 15 participants allocated to the CTL then EXP order Inclusion criteria: first stroke; supratentorial lesion; 4 to 12 weeks post stroke; aged less than 75 years; not able to walk (FAC of 2 or less); able to sit unsupported on the edge of a bed; able to stand for at least 10 seconds with help; written informed consent Exclusion criteria: hip and knee extension deficit of more than 20 degrees; passive dorsiflexion of the affected ankle to less than a neutral position; severe impairment of cognition or communication; evidence of cardiac ischaemia, arrhythmia, decompression or heart failure; feeling of 'overexertion' or heart rate exceeding the age‐predicted maximum (i.e. 190 beats/minute minus age) during training; resting systolic blood pressure exceeding 200 mmHg at rest or dropping by more than 10 mmHg with increasing workload | |
| Interventions | Treated as inpatients for 5 x 15 to 20‐minute sessions per week for 2 weeks
|
|
| Outcomes | This was an A‐B‐A (or B‐A‐B) design, so participants were assessed at baseline, at first cross‐over (2 weeks), at second cross‐over (4 weeks) and after treatment phase (6 weeks):
|
|
| Notes | The number of drop outs was changed based on correspondence with the trialists Trial treated as a parallel‐group design for this review by using the first treatment phase data only (that is baseline and first cross‐over data only) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Lots with sealed opaque envelopes that were drawn by an independent person |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes that were drawn by an independent person |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors were blinded to group assignment |
Yang 2010
| Methods | RCT in parallel‐group design Method of randomisation: drawing lots out of an envelope Blinding of outcome assessors: yes Adverse events: not reported Deaths: none Drop outs: none ITT: yes | |
| Participants | Country: Taiwan 18 participants (10 in EXP group, 8 in CTL group) Mean age: 55 to 57 years (CTL and EXP group respectively) Inclusion criteria: diagnosis with unilateral hemiparesis due to stroke with < 6 months or > 12 months post stroke, being able to follow simple verbal commands Exclusion criteria: unstable medical conditions, history of other diseases interfering with the study, history of seizure, severe cardiovascular conditions/pacemaker | |
| Interventions | 4 arms:
|
|
| Outcomes | Outcomes were recorded at baseline and at the end of the intervention phase Primary outcomes: motor threshold and cortical map size Secondary outcomes: lower limb function (FMA) | |
| Notes | We combined the experimental groups and compared them with the combined controlled groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Drawing lots out of an envelope |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded |
Yen 2008
| Methods | RCT Method of randomisation: not described Adverse events: not stated Deaths: none Drop outs: none ITT: yes | |
| Participants | Country: Taiwan 14 participants (7 in EXP group, 7 in CTL group) Ambulatory at study onset: able to walk 10 metres Mean age: 56 to 57 years (CTL and EXP group respectively) Inclusion criteria: unilateral stroke with unilateral hemiparesis, ≥ 6 months post stroke, ability to walk at least 10 metres independently with or without assistance, no severe, cognitive impairment, stable medical condition Exclusion criteria: history of seizure, any orthopaedic or neurological conditions interfering with the study, cardiac problems/pacemaker, metallic implants in the head, walk with normal gait pattern, inability to walk pre‐stroke | |
| Interventions | 2 arms:
|
|
| Outcomes | Outcomes were recorded at baseline and at the end of the intervention phase
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent person selected one of the sealed envelopes containing a lot |
| Allocation concealment (selection bias) | Low risk | Independent person selected one of the sealed envelopes containing a lot |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessor was not blinded |
Zhang 2008
| Methods | RCT, parallel‐group design Method of randomisation: no details described by the authors Allocation concealment: no details described by the authors Blinding of outcome assessors: no blinding Adverse events: not stated by the authors Deaths: not stated by the authors Drop outs: not clearly stated by the authors ITT: unclear |
|
| Participants | Country: China 39 participants (19 in EXP group, 20 in CTL group) Ambulatory at study onset: not stated by the authors Mean age: 63 years (CTL and EXP group respectively) Inclusion criteria: ischaemic or haemorrhagic stroke confirmed by CT or MRI; aged 52 to 70 years; stable vital signs, conscious, being able to adhere to instructions; lower limb dysfunction Brunnstrom stage 2; blood pressure > 140/90 mm Hg, no myocardial infarction or angina pectoris Exclusion criteria: not stated by the authors |
|
| Interventions | 2 arms, treated as inpatients:
|
|
| Outcomes | Outcomes were assessed at baseline and at the end of the intervention phase:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not described |
Zhu 2004
| Methods | RCT, parallel‐group design Method of randomisation: random number table Allocation concealment: unclear Blinding of outcome assessors: no Adverse events: not reported by the authors Drop outs: none, all participants completed the study ITT: yes |
|
| Participants | Country: China 20 participants (10 in EXP group, 10 in CTL group) Ambulatory at study onset: not stated by the authors Mean age: 58 to 57 years (CTL and EXP group respectively) Inclusion criteria: aged 30 to 80 years; ischaemic or haemorrhagic stroke; confirmed by CT or MRI; not able to walk (FAC of 2 or less); being able to stand up without help; MMSE ≥ 21 points Exclusion criteria: other conditions than stroke affecting ambulation, such as history of spinal cord injury or amputation; myocardial infarction; severe heart failure; poor kidney function; uncontrolled diabetes mellitus; activated rheumatic diseases; MMSE < 21 points; body weight ≥ 110 kg |
|
| Interventions | 2 arms, treated as inpatients:
|
|
| Outcomes | Assessed at baseline and at the end of the intervention phase:
The following outcomes were measured by footprint analysis:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessor was not blinded |
ADL: activities of daily living BBS: Berg Balance Scale BWS: body weight support BWSTT: body weight supported treadmill training CT: computed tomography CTL: control EMG: electromyographic activity EXP: experimental FAC: Functional Ambulation Category FIM: Functional Independence Measure FMA: Fugl‐Meyer Assessment ITT: intention‐to‐treat km/hr: kilometres per hour LTT: limited progressive treadmill training m/min: metre per minute m/s: metre per second MMSE: Mini Mental State Examination MRI: magnetic resonance imaging NYHA: New York Heart Association RCT: randomised controlled trial RMAS: Rivermead Motor Assessment Scale RMI: Rivermead Mobility Index SD: standard deviation STT: speed‐dependent treadmill training TBC: to be confirmed TTBWS: treadmill training with body weight support
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aschbacher 2006 | Irrelevant intervention: electromechanical device training |
| Bayat 2005 | Described only a single‐session application of treadmill training |
| Bleckert 2006 | Both groups received treadmill training and differed only in the speed of the treadmill |
| Blennerhassett 2004 | Irrelevant intervention: circuit class training |
| Borsje 2003 | Correspondence with the author revealed that the trial was abandoned |
| Brissot 2006 | Investigated electromechanically assisted gait training |
| Caldwell 2000 | Correspondence with the author revealed that the trial was abandoned after the recruitment of only 5 participants (each allocated to 1 of 3 treatment groups) |
| Daly 2004 | Both groups received treadmill training; the parameter that was experimentally manipulated was electrical stimulation |
| Daly 2011 | Both groups received treadmill training and differed only by means of functional electrical stimulation |
| Dean 2000 | Irrelevant intervention: circuit class training |
| DEGAS 2007 | Irrelevant intervention: electromechanical device training |
| Dias 2007 | Irrelevant intervention: electromechanical device training |
| English 2007 | Irrelevant intervention: circuit class training |
| Fisher 2008 | Irrelevant intervention: electromechanical device training |
| Forrester 2004 | Evaluated a single treatment session, not a full course of treatment |
| Freivogel 2009 | Mixed population of patients with traumatic brain injury, spinal cord injury and stroke; only 2 out of 16 included patient had a stroke |
| Globokar 2005 | Irrelevant intervention: electromechanical device training |
| Hidler 2009 | Irrelevant intervention: electromechanical device training |
| Hornby 2008 | Irrelevant intervention: robotic device training |
| Husemann 2007 | Irrelevant intervention: electromechanical device training |
| Jang 2005 | Irrelevant intervention: electromechanical device training |
| Jeong 2008 | Irrelevant intervention: electromechanical device training |
| Khanna 2003 | Correspondence with the author revealed that the trial was abandoned before the commencement of recruitment |
| Kim 2001 | Irrelevant intervention: electromechanical device training |
| Kim 2008 | Irrelevant intervention: electromechanical device training |
| Kovrazhkina 2009 | Irrelevant intervention: electromechanical device training |
| Kwakkel 1999 | Correspondence with the author revealed that less than 20% of participants in the EXP group participated in treadmill training (i.e. only 6 out of 31 participants) |
| Langhammer 2000 | Correspondence with the author revealed that treadmill training (with or without body weight support) was not used in either group |
| Langhammer 2007 | Less than 20% of participants in the EXP group received treadmill training |
| Lau 2010 | Both groups received treadmill training which differed only by speed |
| Lindquist 2011 | Quasi‐experimental study, without randomisation |
| Macko 2006 | Both groups received treadmill training which differed only by duration and speed |
| Mayr 2007 | EXP group used an electromechanical device on a treadmill |
| Mayr 2008 | Irrelevant intervention: electromechanical device training |
| McCain 2008 | Not a RCT |
| Nielsen 2007 | Irrelevant intervention: electromechanical device training |
| Pang 2010 | Not a RCT |
| Park 2012 | Both groups received treadmill training and differed only in the setting (underwater treadmill versus overground treadmill) |
| Peurala 2005 | Did not use treadmill training |
| Peurala 2009 | Irrelevant intervention: electromechanical device training |
| Ploughman 2008 | Evaluation of a single treatment session |
| Rimmer 2000 | Correspondence with the author revealed that only one‐third of participants in the EXP group participated in treadmill training |
| Salbach 2004 | Irrelevant intervention: circuit class training |
| Saltuari 2004 | Irrelevant intervention: electromechanical device training |
| Schwartz 2009 | Irrelevant intervention: electromechanical device training |
| Shafshak 2012 | All groups received treadmill training with partial body weight support: the parameter that was experimentally manipulated was upper limb swinging |
| Sullivan 2002 | All groups received treadmill training with partial body weight support; the parameter that was experimentally manipulated was treadmill speed |
| Tong 2006 | Irrelevant intervention: electromechanical device training |
| Trueblood 2001 | A non‐random process was used to allocate participants to groups in Part II and Part III Participants chose which treatment they would receive |
| Tsai 2004 | All groups received treadmill training (without partial body weight support); the parameters that were experimentally manipulated were walking direction and treadmill slope |
| Tsang 2012 | Irrelevant outcome: echocardiography |
| Werner 2002b | Both groups received treadmill training with body weight support; the parameter that was experimentally manipulated was 'conventional' physiotherapy gait training |
| Westlake 2009 | Used robot‐assisted training (Lokomat) |
| Yagura 2006 | Both groups received treadmill training with body weight support; the parameter that was experimentally manipulated was therapeutic facilitation |
| Yang 2008 | Both groups received treadmill training and differed only by the EXP group receiving virtual reality as well |
EXP: experimental RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Al‐Jarrah 2011
| Methods | Method: not clearly stated, probably RCT Method of randomisation: not described Blinding of outcome assessors: not described Adverse events: not stated Deaths: not stated Drop outs: not stated ITT: unclear |
| Participants | Country: Jordan 30 people with chronic stroke: 21 in the EXP group and 10 (sic) in the CTL group Ambulatory at study onset: not described Inclusion criteria: not stated Exclusion criteria: not stated |
| Interventions | 2 arms:
|
| Outcomes | Outcomes were recorded at baseline and after 4 weeks of therapy:
|
| Notes | Characteristics derived from conference abstract |
Baer 2009
| Methods | Method: multicentre RCT Method of randomisation: stratified randomisation based on side of lesion and initial FAC score Blinding of outcome assessors: not described Adverse events: not stated Deaths: not stated Drop outs: 8 during intervention phase ITT: unclear |
| Participants | Country: UK 77 people with subacute stroke within 3 months of stroke onset Ambulatory at study onset: not described Inclusion criteria: stroke as defined by WHO; age over 18; medically stable; 1 minute standing balance (with or without support), ability to understand and follow verbal instructions |
| Interventions | 2 arms:
|
| Outcomes | Outcomes were recorded at baseline and after 8 weeks of therapy: Measures of timed gait (10‐Metre Walk Test)
|
| Notes | Characteristics derived from conference abstract |
Bartloff 2009
| Methods | Still unclear |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes |
DePaul 2011
| Methods | Method: RCT, parallel‐group design Method of randomisation: permuted block randomisation with stratification by baseline walking speed Allocation concealment: central randomisation service Blinding of outcome assessors: yes Adverse events: will be reported Deaths: yes Drop outs: will be reported ITT: yes |
| Participants | Country: Canada Estimated enrolment: 70 people with chronic stroke, 35 in the EXP group and 35 in the CTL group Ambulatory at study onset: yes Inclusion criteria: living in the community at time of entry into study; age > 40 years; within 12 months of onset of a physician‐diagnosed ischaemic or haemorrhagic stroke in any brain location (with or without diagnostic imaging); ability to walk 10 metres without assistance with self selected gait speed < 1.0 m/s (or typically use a walking aid); ability to follow a 2‐step verbal command; independent with community ambulation prior to most recent stroke; received physician approval to participate in the study Exclusion criteria: severe cognitive impairment (i.e. MMSE < 24/30 or score less than predicted according to age and education level); severe visual impairment; lower extremity amputation; presence of serious conditions that would limit safe participation in walking exercise |
| Interventions | 2 arms:
|
| Outcomes | Outcomes were recorded at baseline, at the end of 5‐week intervention phase and at 2‐month follow‐up: Primary outcomes: post‐intervention comfortable gait speed Secondary outcomes:
|
| Notes | Study was completed in June 2011 |
Hornby 2012
| Methods | Method: RCT, cross‐over assignment Method of randomisation: not described Blinding of outcome assessors: yes Adverse events: not described Deaths: not described Drop outs: not described ITT: not described |
| Participants | Country: USA 30 people with chronic stroke Ambulatory at study onset: not clearly described, probably yes Inclusion criteria: unilateral supratentorial stroke; MMSE > 22; > 6 months stroke duration; < 0.9 m/s gait speed overground. Exclusion criteria: lower extremity contracture; osteoporosis; cardiovascular/metabolic/respiratory instability; previous central/peripheral nerve injury; concurrent medications interacting with selective serotonin reuptake inhibitors (SSRIs) |
| Interventions | 2 arms:
|
| Outcomes | Outcomes will be assessed at baseline and at the end of the intervention phase at 4 weeks: Primary outcomes: peak treadmill speed Secondary outcomes: overground walking speed Other outcomes: gait kinematics, EMG activity |
| Notes |
Ivey 2010
| Methods | Method: RCT, parallel‐group design Method of randomisation: by a computer‐generated randomisation scheme that was stratified by walking speed (less than 0.44 m/s and more than or equal to 0.44 m/s) and age (less than 65 years and more than or equal to 65 years) Drop outs: 27 drop outs at the end of the treatment phase Blinding of outcome assessors: no ITT: no |
| Participants | Country: USA 29 participants in the EXP group and 24 participants in the CTL group Inclusion criteria: chronic ischaemic stroke (more than 6 months); residual mild to moderate hemiplegic gait deficits; completion of all conventional physiotherapy; independently ambulant with or without a gait aid or stand‐by help Exclusion criteria: vascular surgery; vascular disorders in the lower extremities; symptomatic peripheral arterial occlusive disease |
| Interventions | Treated as outpatients for 3 x 40‐minute sessions per week for 6 months Treadmill training (EXP): participants walked on a treadmill to achieve a target aerobic intensity of 60% to 70% heart rate reserve (progressive aerobic training); no body weight support was provided using a harness Conventional physiotherapy (CTL): participants completed an exercise programme consisting of 13 targeted active and passive supervised stretching movements of the upper and lower body |
| Outcomes | Assessed at baseline and after treatment phase:
|
| Notes |
Ivey 2011
| Methods | Method: RCT, parallel‐group design Method of randomisation: by a computer‐generated randomisation scheme that was stratified by walking speed (less than 0.44 m/s and more than or equal to 0.44 m/s) and age (less than 65 years and more than or equal to 65 years) Drop outs: 27 drop outs at the end of the treatment phase. Blinding of outcome assessors: no ITT: no |
| Participants | Country: USA 19 participants in the EXP group and 19 participants in the CTL group Inclusion criteria: chronic ischaemic stroke (more than 6 months); residual mild to moderate hemiplegic gait deficits; completion of all conventional physiotherapy; independently ambulant with or without a gait aid or stand‐by help Exclusion criteria: patients who had insufficient time for outcome measurement by Doppler sonography |
| Interventions | Treated as outpatients for 3 x 40‐minute sessions per week for 6 months Treadmill training (EXP): participants walked on a treadmill to achieve a target aerobic intensity of 60% to 70% heart rate reserve (progressive aerobic training); no body weight support was provided using a harness Conventional physiotherapy (CTL): participants completed an exercise programme consisting of 13 targeted active and passive supervised stretching movements of the upper and lower body |
| Outcomes | Assessed at baseline and after treatment phase:
|
| Notes |
Michael 2011
| Methods | Method: not described Method of randomisation: not described Drop outs: not explicitly stated Blinding of outcome assessors: unclear ITT: unclear |
| Participants | Country: USA 10 participants in the EXP group and 13 participants in the CTL group Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Treated for 3 x 60‐minute sessions per week for 6 months Treadmill training (EXP): participants received treadmill training in combination with adaptive physical activity Conventional physiotherapy (CTL): participants received adaptive physical activity |
| Outcomes | Assessed at baseline and after treatment phase:
|
| Notes | Characteristics derived from conference abstract |
Mokrusch 2004
| Methods | Method: not described Method of randomisation: not described Drop outs: not stated Blinding of outcome assessors: unclear ITT: unclear |
| Participants | Country: Germany 7 participants Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Treated for 4 weeks Treadmill training (EXP): participants received treadmill training in combination with functional electrical stimulation Conventional physiotherapy (CTL): based on the Bobath/neurodevelopmental approach |
| Outcomes | Assessed at baseline and after treatment phase:
|
| Notes | Characteristics derived from conference abstract |
Muller 2004
| Methods | Method: not described Method of randomisation: not described Drop outs: not stated Blinding of outcome assessors: unclear ITT: unclear |
| Participants | Country: Germany 50 participants in the EXP group, 44 participants in the CTL group Ambulatory at study onset: unclear Inclusion criteria: not clearly described, quote "stroke and spinal patients" Exclusion criteria: not described |
| Interventions | Treatment duration: unknown Treadmill training (EXP): participants received treadmill training for 45 minutes per session Electromechanical assisted gait training (CTL): using the Lokomat on a treadmill for 45 minutes per session |
| Outcomes | Assessed at baseline and after treatment phase:
|
| Notes | Characteristics derived from conference abstract |
Shintani 2005
| Methods | Unclear |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes |
Srivastava 2008
| Methods | RCT Method of randomisation: not described Blinding of outcome assessors: not described Adverse events: not reported Deaths: not reported Drop outs: not reported ITT: unclear |
| Participants | Country: India 45 patients Ambulatory at study onset: yes Inclusion criteria: first supratentorial stroke at least 3 months before enrolment, ability to walk (FAC 2 to 4) Exclusion criteria: not described |
| Interventions | 3 arms:
|
| Outcomes | Outcomes were recorded at baseline, at the end of the intervention phase and at 3‐month follow‐up:
|
| Notes | Abstract only |
Stephenson 2004
| Methods | Unclear |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes |
Thompson 2006
| Methods | RCT Method of randomisation: not described Blinding of outcome assessors: not described Adverse events: not stated Deaths: not stated Drop outs: not stated ITT: not stated |
| Participants | Country: USA 22 participants Ambulatory at study onset: not stated Mean age: 58 years Inclusion criteria: not stated Exclusion criteria: not stated |
| Interventions | 3 arms:
|
| Outcomes | Outcomes were recorded at baseline, post intervention and after 1‐month and 6‐month follow‐up:
|
| Notes | Abstract only |
Venkadesan 2009
| Methods | Method: not described Method of randomisation: not described Drop outs: not stated Blinding of outcome assessors: unclear ITT: unclear |
| Participants | Country: India 10 participants in the EXP group, 10 participants in the CTL group Ambulatory at study onset: yes Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Treatment duration: unknown Treadmill training (EXP): participants received treadmill training and conventional gait training Conventional gait training (CTL): participants received conventional gait training alone |
| Outcomes | Time points of assessments unknown:
|
| Notes | Characteristics derived from abstract |
Xu 2008
| Methods | Method: not described Method of randomisation: not described Drop outs: not stated Blinding of outcome assessors: unclear ITT: unclear |
| Participants | Country: China 36 participants in the EXP group, 40 participants in the CTL group Ambulatory at study onset: not described Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Treatment duration: unknown Pneu‐weight walking training (EXP): participants received Pneu‐weight walking training Underwater gait training (CTL): participants received underwater gait training |
| Outcomes | Time points of assessments unknown:
|
| Notes | Characteristics derived from conference abstract |
Yang 2007
| Methods | Method: RCT, parallel‐group design Method of randomisation: not described Blinding of outcome assessors: not described Adverse events: not stated Deaths: not stated Drop outs: not stated ITT: unclear |
| Participants | Country: Taiwan 13 participants in the EXP group and 13 in the CTL group Ambulatory at study onset: not described Inclusion criteria: hemiparetic gait disturbances and coronary artery disease Exclusion criteria: not stated |
| Interventions | 2 arms:
|
| Outcomes | Outcomes were recorded at baseline and after 4 weeks of therapy:
|
| Notes | Characteristics derived from conference abstract |
ADL: activities of daily living BWSTT: body weight supported treadmill training CTL: control EMG: electromyographic activity EXP: experimental FAC: Functional Ambulation Categories FIM: Functional Independence Measure ITT: intention‐to‐treat MMSE: Mini Mental State Examination RCT: randomised controlled trial WHO: World Health Organization
Characteristics of ongoing studies [ordered by study ID]
Combs 2012
| Trial name or title | Body weight supported treadmill training versus overground walking training in persons with chronic stroke |
| Methods | Method: RCT Method of randomisation: not described Blinding of outcome assessors: yes ITT: unclear |
| Participants | Country: USA 20 people with chronic stroke Ambulatory at study onset: yes Inclusion criteria: independent ambulation, walking speed ≤ 0.8 m/s Exclusion criteria: not stated |
| Interventions | 2 arms:
|
| Outcomes | Outcomes will be assessed at baseline, at the end of the intervention phase and at 3‐month follow‐up: Primary outcomes: gait speed (10‐Metre Walk Test) Secondary outcomes:
|
| Starting date | August 2010 |
| Contact information | Stephanie A Combs, PT, PhD, NCS University of Indianapolis, Krannert School of Physical Therapy, Indianapolis, IN, USA |
| Notes |
Dawes 2013
| Trial name or title | Improving community walking after a stroke, a new approach |
| Methods | Method: pilot RCT Method of randomisation: not described Blinding of outcome assessors: not described ITT: unclear |
| Participants | Country: UK 50 people with chronic stroke Ambulatory at study onset: yes Inclusion criteria: more than 6 months after first ischaemic stroke; reduced gait capacity (6‐Minute Walk Test); being able to perform a simple reciprocal bilateral foot tapping task and to walk safely on a treadmill; informed consent Exclusion criteria: high risk of psychosis; severe aphasia; history of previous stroke; other known contraindication to safe participation; contraindication to MRI |
| Interventions | 2 arms:
|
| Outcomes | Outcomes will be assessed at 0, 10 and 20 weeks:
|
| Starting date | February 2013 |
| Contact information | Prof Helen Dawes Oxford Brookes University, Movement Science Group, School of Life Email: hdawes@brookes.ac.uk |
| Notes |
Forrester 2011
| Trial name or title | Ankle robotics training after stroke: effects on gait and balance |
| Methods | RCT with 3 arms |
| Participants | Inclusion criteria: ischaemic or haemorrhagic stroke > 6 months prior in men or women aged 18 to 80 years, clear indications of hemiparetic gait by clinical observation, completed all conventional physical therapy, ability to walk on a treadmill with handrail support Exclusion criteria: cardiac history of (1) unstable angina, (2) recent (< 3 months) myocardial infarction, congestive heart failure (NYHA category II), (3) haemodynamically significant valvular dysfunction; major clinical depression: CES‐D score > 16 and judgment of clinical depression; medical history: (1) recent hospitalisation (< 3 months) for severe medical disease, (2) symptomatic peripheral arterial occlusive disease, (3) orthopaedic or chronic pain conditions that significantly alter gait function, (4) pulmonary or renal failure, (5) active cancer; history of non‐stroke neuromuscular disorder restricting gait; aphasia or cognitive functioning that confounds participation, defined as unable to follow 2‐step commands; the MMSE will be administered with a cut‐off of < 23 (< 17 if education level at or below 8th grade) or judgement of the medical officer; hypertension that is a contraindication for a bout of treadmill training (greater than 160/100 on 2 assessments); self report of pregnancy |
| Interventions | EXP Arm 1: seated robot training group: participants at least 6 months post stroke will use the ankle robot in a seated visuo‐motor training paradigm; they will train on the robot 3 times per week for 6 weeks (18 sessions) by playing video games with the paretic ankle; they will be evaluated on outcomes at baseline, post 6 weeks training and again after a 6‐week retention period with no training EXP Arm 2: treadmill training with ankle robot group: participants at least 6 months post stroke will wear the ankle robot during treadmill locomotor training; they will walk on a treadmill with the ankle robot adjusted to promote paretic ankle engagement during 3 x weekly training sessions over 6 weeks (18 sessions); they will be evaluated on outcomes at baseline, post‐6 weeks training and again after a 6‐week retention period with no training Active comparator: Arm 3: treadmill‐only group: this group will consist of participants at least 6 months post stroke who engage in treadmill training 3 x weekly for 6 weeks without robotic support; they will be volunteers from another treadmill training study and will be evaluated on outcomes at baseline and post 6 weeks training; they will not receive retention testing at 12 weeks because they will be continuing with regular treadmill training beyond the 6‐week period |
| Outcomes | Primary outcomes: self selected floor walking velocity, velocity and associated spatio‐temporal gait parameters from self selected; most comfortable and fastest floor walking over 10 metres Secondary outcomes: gait kinetics, anterior‐posterior and medio‐lateral ground reaction forces during walking to assess propulsive impulses from paretic and non‐paretic sides, Berg Balance Scale, Dynamic Gait Index, Anticipatory Postural Adjustments |
| Starting date | July 2011 |
| Contact information | Contact: Larry Forrester, PhD Email: Larry.Forrester@va.gov |
| Notes | Estimated primary completion date: March 2013 (final data collection date for primary outcome measure) |
Hollands 2012
| Trial name or title | Visual cues for gait training post stroke |
| Methods | Method: RCT, parallel assignment Method of randomisation: not described Blinding of outcome assessors: yes ITT: unclear |
| Participants | Country: Australia Target sample size: 60 people with stroke Ambulatory at study onset: yes Inclusion criteria: diagnosis of stroke; being able to walk 10 metres with or without assistance; residual paresis in the lower limb (Fugl‐Meyer lower limb score less than 34), informed written consent Exclusion criteria: gait speed more than 0.8 m/s; patients with a premorbid (retrospective) modified Rankin Scale score of greater than 3; gait deficits attributable to non‐stroke pathology; visual impairments preventing use of visual cue training (as assessed by Apple Cancellation test), concurrent progressive neurologic disorder, acute coronary syndrome, severe heart failure, confirmed or suspected lower‐limb fracture preventing mobilisation, those requiring palliative care, inability to follow a 3‐step command (as assessed by Modified MMSE) |
| Interventions | 3 arms:
|
| Outcomes | Outcomes will be assessed at baseline, at the end of the intervention phase and at 3‐month follow‐up: Primary outcome: participant enrolment, recruitment and retention Secondary outcomes:
|
| Starting date | May 2012 |
| Contact information | Trudy A Pelton, MRes Email: t.a.pelton@bham.ac.uk Kristen Hollands, PhD Email: k.hollands@salford.ac.uk |
| Notes |
Hornby 2013
| Trial name or title | Very Intensive Early Walking in Stroke (VIEWS) |
| Methods | Method: RCT, parallel assignment Method of randomisation: not described Blinding of outcome assessors: yes ITT: unclear |
| Participants | Country: USA 56 people with chronic stroke Ambulatory at study onset: yes Inclusion criteria: subacute (< 6 months) stroke; 18 to 75 years old; history of unilateral, supratentorial, ischaemic or haemorrhagic stroke; being able to walk 10 metres without physical assistance; gait speed less than or equal to 0.8 m/s; medical clearance Exclusion criteria: significant cardiorespiratory or metabolic disease that may limit exercise participation; weight limit > 113 kg; history of previous orthopaedic or neurological conditions which may impair walking; MMSE < 23 Exclusion for transcranial magnetic stimulation (TMS): pacemaker, metal implants in the head region, history of epilepsy or seizures, skull fractures or skull deficits, concussion within the last 6 months, unexplained recurring headaches, medications that lower seizure threshold, pregnancy Exclusion for the MRI: aneurysm clip or coil, metal or wire implants, heart valve prosthesis |
| Interventions | 2 arms:
|
| Outcomes | Primary outcomes will be assessed at baseline, at the end of the intervention phase at 8 weeks and at 3‐month follow‐up:
Secondary outcomes will be assessed at baseline, at the end of the intervention phase at 8 weeks and at 2‐month follow‐up:
|
| Starting date | October 2008 |
| Contact information | Carey Holleran, MPT, NCS Email: cholleran@ric.org Abigal Leddy, PT, DPT Email: aleddy@ric.org |
| Notes |
Kilbreath 2006
| Trial name or title | PBWST (partial body weight supported treadmill training) and muscle power training after sub‐acute stroke |
| Methods | Method: RCT, factorial assignment. Method of randomisation: not reported Blinding of outcome assessors: not clearly stated, probably yes ITT: unclear |
| Participants | Estimated enrolment: 102 participants aged 45 to 80 years Inclusion criteria: first stroke resulting in hemiplegia; MMSE score > 15; distance walked in 6‐Minute Walk Test less than the lower limit of 'normal' according to reference equations for healthy adults (adjusted for gender, age, BMI); score on walking subscale of the Motor Assessment Scale of ≥ 2 Exclusion criteria: unstable cardiac disease; known un‐repaired aortic or cerebral aneurysm; haemorrhagic stroke, symptomatic hernias, symptom limiting peripheral vascular disease; end‐stage congestive cardiac failure; any of the exclusion criteria contraindicating moderate exercise as outlined by American College of Sports Medicine guidelines for cardiac disease rehabilitation or for frail and elderly adults; significant musculotendinous or bony restrictions of either limb; any serious chronic disease independently causing significant disability or profound atrophy of the affected limb will comprise further exclusion criteria |
| Interventions | 2 arms:
|
| Outcomes | Assessed at baseline, at the end of the intervention phase and at 6‐month follow‐up: Primary outcome measure: gait capacity (6‐Minute Walk Test) Secondary outcome measures:
|
| Starting date | March 2004 |
| Contact information | School of Physiotherapy, University of Sydney, Sydney, New South Wales, Australia, 2141 Contact: Sharon L Kilbreath, PhD Email: s.kilbreath@fhs.usyd.edu.au |
| Notes |
Lennihan 2003
| Trial name or title | Treadmill with partial body weight support versus conventional gait training after stroke |
| Methods | Unclear |
| Participants | 42 participants will be recruited for the EXP group and 41 participants for the CTL group Inclusion criteria: within 30 days of first stroke; hemiparesis; dependent on supervision or physical assistance from at least 1 person to walk; not ataxic |
| Interventions | Treated as inpatients for 12 x 30‐minute per day sessions over 3 weeks Treadmill training (EXP): participants will walk on a treadmill with partial body weight support using a harness Conventional physiotherapy (CTL): participants will participate in conventional physiotherapy (standing, walking, sit‐to‐stand, and standing and walking with activity) |
| Outcomes | Assessed 90 days after stroke:
|
| Starting date | Unknown |
| Contact information | Unknown |
| Notes | Characteristics derived from conference abstract |
Macko 2013
| Trial name or title | Exercise for sub‐acute stroke patients in Jamaica |
| Methods | Method: RCT, parallel assignment Method of randomisation: stratified based on glucose tolerance (normal versus abnormal) and gait deficit severity Blinding of outcome assessors: no ITT: unclear |
| Participants | Country: Jamaica 150 people with chronic stroke Ambulatory at study onset: unclear Inclusion criteria: ischaemic stroke within 2 months; BMI of 18 to 40 kg/m²; being able to walk 3 minutes with handrails, assistive device or stand‐by aid Exclusion criteria: actively exercising for > 30 minutes per day for 5 days per week; increased alcohol consumption; active abuse of other illegal and illicit drugs; history of severe cardiac conditions; history of (1) peripheral arterial disease with vascular claudication making exercise challenging, (2) orthopaedic or chronic pain condition(s) restricting exercise, (3) pulmonary or renal failure, (4) active cancer, (5) untreated poorly controlled hypertension measured on at least 2 occasions (greater than 160/100), (6) HIV‐AIDS or other known inflammatory responses, (7) sickle cell anaemia, (8) medications: heparin, warfarin, Lovenox or oral steroids, (9) currently pregnant, (10) history of type 1 diabetes or insulin dependent type 2 diabetes, (11) poorly controlled type 2 diabetes (HbA1C > 10), (12) dementia (MMSE score < 23 or < 17 if education level at or below 8th grade) and clinical confirmation by clinical evaluation, (13) severe receptive or global aphasia that confounds testing and/or training, operationally defined as unable to follow 2 point commands, (14) hemiparetic gait from a prior stroke preceding the index stroke defining eligibility (more than one stroke), (15) neurologic disorder restricting exercise such as Parkinson's or myopathy, (16) untreated major depression (CES‐D > 16 or clinical confirmation), (17) muscular disorder (s) restricting exercise; muscle biopsy exclusion criteria: (1) anticoagulation therapy with heparin, warfarin or Lovenox (antiplatelet therapy is permitted), (2) bleeding disorder |
| Interventions | 2 arms:
|
| Outcomes | Outcomes will be assessed at baseline and at the end of the intervention phase at 6 months: Primary outcomes:
Secondary outcomes:
|
| Starting date | July 2011 |
| Contact information | Richard F Macko, MD Email: rmacko@grecc.umaryland.edu |
| Notes |
McDonnell 2009
| Trial name or title | Aerobic exercise to improve cardiovascular and neurological health outcomes in the chronic stroke population |
| Methods | Method: RCT, parallel‐group design Method of randomisation: secure web‐based computer generation, stratified according to age (< 65 versus > 65) and mobility (the 6‐Minute Walk Test, < 160 metres versus > 160 metres) Blinding of outcome assessors: yes ITT: unclear |
| Participants | Country: Australia Target sample size: 150 participants Ambulatory at study onset: not described Inclusion criteria: aged between 45 and 80 years, diagnosis of first or recurrent stroke, haemorrhage or infarct at least 6 months prior to study entry Exclusion criteria: unable to participate in an exercise programme due to medical conditions such as heart failure, unstable angina, dementia and receptive aphasia, patients on beta‐blockers, patients already participating in a supervised aerobic exercise programme, patients who have epilepsy, metallic implants in the skull or cardiac pacemakers will be excluded from the transcranial magnetic stimulation |
| Interventions | 2 arms:
|
| Outcomes | Outcomes were recorded at baseline, at the end of the 12‐week intervention period and at 6 months follow‐up: Primary outcome: peak oxygen uptake (VO2 peak) Secondary outcomes:
|
| Starting date | August 2009 |
| Contact information | Dr Michelle McDonnell School of Nursing and Midwifery GPO Box 2471 Adelaide SA 5001, Australia Email: michelle.mcdonnell@unisa.edu.au |
| Notes |
Sale 2012
| Trial name or title | Robot walking rehabilitation in stroke patients |
| Methods | RCT with 3 arms |
| Participants | Inclusion criteria: between the ages of 18 and 95 years, able to walk 25 feet unassisted or with assistance, first acute event of cerebrovascular stroke, unilateral paresis, ability to understand and follow simple instructions, ability to walk without assistance before stroke, endurance sufficient to stand at least 20 minutes unassisted per participant report Exclusion criteria: unable to understand instructions required by the study (Informed Consent Test of Comprehension), medical or neurological comorbidities that might contribute to significant gait dysfunction, uncontrolled hypertension > 190/110 mm Hg, significant symptoms of orthostasis when standing up, circulatory problems, history of vascular claudication or significant (+ 3) pitting oedema, lower extremity injuries or joint problems (hip or leg) that limit range of motion or function or cause pain with movement, bilateral impairment, severe sensory deficits in the paretic upper limb, cognitive impairment or behavioural dysfunction that would influence the ability to comprehend or participate in the study, women who are pregnant or lactating or both |
| Interventions | EXP group: robot G‐EO: each participant will be asked to perform 15 sessions (3 to 5 days a week for 4 up to 5 weeks) consisting of a treatment cycle using the GE‐O system device, according to individually tailored exercise scheduling CTL group: treadmill training: each participant will be asked to perform 15 sessions (3 to 5 days a week for 4 up to 5 weeks) consisting of a treatment cycle using the treadmill system device, according to individually tailored exercise scheduling CTL group: ground treatment: Ground Control Group (cCG): each participant will be asked to perform 15 sessions (3 to 5 days a week for 4 up to 5 weeks) of traditional lower limb physiotherapy |
| Outcomes | Outcomes will be assessed at baseline and at 6 months follow‐up: Primary outcomes: 6‐Minute Walk Test Secondary outcomes:
|
| Starting date | September 2012 |
| Contact information | Contact: Patrizio Sale, MD Email: patrizio.sale@gmail.com Contact: Marco Franceschini, MD Email: marco.franceschini@sanraffaele.it |
| Notes | Estimated enrolment: 90 Estimated study completion date: September 2015 Estimated primary completion date: August 2014 (final data collection date for primary outcome measure) |
Smania 2013
| Trial name or title | High intensity interval training in chronic stroke patients |
| Methods | Method: RCT Method of randomisation: not described Blinding of outcome assessors: yes ITT: unclear |
| Participants | Country: Italy Target sample size: 100 people with stroke Ambulatory at study onset: not described Inclusion criteria: diagnosis of ischaemic or haemorrhagic stroke, confirmed by MRI or CT at least 6 months before the onset of the study; ability to walk in the treadmill at > 0.3 km/hour for 3 minutes handrail support; be able to give informed consent and be motivated to participate in 3‐month intensive physical fitness training Exclusion criteria: MMSE < 20; unstable angina pectoris; unstable cardiac conditions; complex ventricular arrhythmia; resting systolic blood pressure > 200 mm/Hg, resting diastolic blood pressure > 100 mm/Hg; aphasia (unable to follow 2 commands); other medical conditions precluding participation in aerobic exercise |
| Interventions | 3 arms:
|
| Outcomes | Primary outcome will be assessed at baseline, at the end of the intervention phase at 12 weeks: 6‐Minute Walk Test Secondary outcomes:
|
| Starting date | March 2013 |
| Contact information | Nicola Smania Email: nicola.smania@univr.it |
| Notes |
Stookey 2013
| Trial name or title | Task‐oriented training for stroke: impact on function mobility |
| Methods | Method: RCT, parallel assignment Method of randomisation: not described Blinding of outcome assessors: no ITT: no |
| Participants | Country: USA 60 people with stroke Ambulatory at study onset: yes Inclusion criteria: stroke > 6 months prior with residual hemiparetic gait in women or men aged 40 to 85 years, completion of all regular post stroke physical therapy, adequate language and neurocognitive function to participate in testing and training and to give adequate informed consent, able to rise from a chair unaided and able to walk 10 metres without human assistance Exclusion criteria: regular structured aerobic exercise (> 2 x week), raised alcohol consumption by self report, clinical history of severe heart conditions, peripheral arterial obstructive disease with claudication, major orthopaedic, chronic pain or non‐stroke neuromuscular disorders restricting exercise, pulmonary or renal failure, poorly controlled hypertension (> 190/110), measured on at least 2 separate occasions, recent hospitalisation for severe disease or surgery, severe or global receptive aphasia which confounds reliable testing and training, untreated major depression as documented by a CES‐D score of > 16 and confirmed by clinical interview, pregnancy |
| Interventions | 2 arms:
|
| Outcomes | Outcomes will be assessed at baseline and at 3 months: Primary outcomes: economy of gait Secondary outcomes:
|
| Starting date | July 2011 |
| Contact information | Alyssa D Stookey, PhD MS Email: alyssa.stookey@va.gov |
| Notes |
Zielke 2003
| Trial name or title | Partial body weight supported treadmill training in early acute stroke rehabilitation |
| Methods | Unclear |
| Participants | 5 participants will be recruited for the EXP group and 5 participants for the CTL group Inclusion criteria: admitted to inpatient stroke unit between 2 and 30 days following stroke; single infarct stroke confirmed by MRI or CT scan; aged 50 to 75 years; no orthopaedic or additional neurologic conditions that impair ambulation (independent walker, with or without a gait aid, before the stroke); no history of previous stroke (based on medical chart review); no cardiac, respiratory or other medical condition that might interfere with the treatment protocol; able to follow instructions (no significant cognitive or communication deficits); scores at least 1 out of 5 on manual muscle testing of the hip flexors |
| Interventions | Treated for 3 sessions per week for 2 weeks Treadmill training (EXP): participants will walk on a treadmill with partial body weight support using a harness Overground walking training (CTL): participants will complete overground walking training |
| Outcomes | Assessed at baseline, and after the treatment phase (2 weeks):
|
| Starting date | February 2002 |
| Contact information | Donna Zielke, PT MPT Email: dzielke@marionjoy.org |
| Notes |
BMI: body mass index BWS: body weight support BWSTT: body weight supported treadmill training CES‐D: Center for Epidemiologic Studies Depression Scale CT: computed tomography CTL: control EXP: experimental FAC: Functional Ambulation Categories FIM: Functional Independence Measure ITT: intention‐to‐treat MMSE: Mini Mental State Examination MRI: magnetic resonance imaging RCT: randomised controlled trial
Contributions of authors
On 28 March 2013 we were contacted by the Cochrane Stroke Group and our author team (BE, MP, JM) took over this review and updated it from 2005. We contacted the former review team from 2005 and received all requested data. We used the data collection provided by the former review team and, based on this information, we updated the review by including all eligible studies from 2005 onwards.
For this 2013 update, BE and JM conducted the literature selection, data extraction and analyses, and were responsible for the major content of the review. BE, JM and MP interpreted the data from the individual trials and the statistically pooled results, and contributed to the manuscript. All authors edited the manuscript.
Sources of support
Internal sources
Rehabilitation Studies Unit, Northern Clinical School, Faculty of Medicine, The University of Sydney, Australia.
School of Physiotherapy, The University of Sydney, Australia.
Department of Public Health, Medizinische Fakultät 'Carl Gustav Carus', TU Dresden, Germany.
Wissenschaftliches Institut, Private Europäische Medizinische Akademie der Klinik Bavaria in Kreischa GmbH, An der Wolfsschlucht 1‐201731 Kreischa, Germany.
External sources
No sources of support supplied
Declarations of interest
Marcus Pohl and Jan Mehrholz were authors of one included trial (Pohl 2002). They did not participate in quality assessment and data extraction for this study.
No other potential conflicts of interest are known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
- Ada L, Dean C, Crompton S, Hall J, Bampton J. The efficacy of treadmill training in improving walking in individuals after stroke in the community: a placebo‐controlled, randomised trial. Proceedings of the VIIth International Physiotherapy Congress. Sydney: Australian Physiotherapy Association, 2002:61. [Google Scholar]; Ada L, Dean C, Hall J, Bampton J, Crompton S. A treadmill and overground walking program improves walking in individuals residing in the community after stroke: a placebo‐controlled, randomised trial (abstract). Proceedings of the 14th International Congress of The World Confederation for Physical Therapy. Spain, Barcelona. 2003:RR‐PL‐1170. [Google Scholar]; Ada L, Dean C, Morris M. The efficacy of treadmill training in establishing walking after stroke (NCT00167531) (protocol). ClinicalTrials.gov.2002. ; Ada L, Dean CM, Hall JM, Bampton J, Crompton S. A treadmill and overground walking program improves walking in individuals residing in the community after stroke: a placebo‐controlled randomised trial (abstract). Internal Medicine Journal2004; Vol. 34, issue 1‐2:A7. [DOI] [PubMed]; Ada L, Dean CM, Hall JM, Bampton J, Crompton S. A treadmill and overground walking program improves walking in persons residing in the community after stroke: a placebo‐controlled, randomized trial. Archives of Physical Medicine and Rehabilitation2003; Vol. 84, issue 10:1486‐91. [DOI] [PubMed]
- Ada L, Dean C, Morris M. Establishing walking using treadmill training in non‐ambulatory patients during inpatient stroke rehabilitation: the MOBILISE trial. Australian Journal of Physiotherapy 2009;4 Suppl:2. [Google Scholar]; Ada L, Dean C, Morris M, Simpson J, Katrak P. Establishing walking using treadmill walking with body weight support in subacute non‐ambulatory stroke: the MOBILISE Trial I. International Journal of Stroke 2010;5:24‐5. [Google Scholar]; Ada L, Dean CM, Morris ME. Supported treadmill training to establish walking in non‐ambulatory patients early after stroke (protocol). BMC Neurology 2007;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ada L, Dean CM, Morris ME, Simpson JM, Katrak P. Randomized trial of treadmill walking with body weight support to establish walking in subacute stroke: the MOBILISE trial. Stroke 2010;41(6):1237‐42. [DOI] [PubMed] [Google Scholar]; Dean C, Ada L, Bampton J, Morris M, Katrak P, Potts S. Improving walking speed and capacity using treadmill walking with body weight support in subacute non‐ambulatory stroke: the Mobilise trial II. International Journal of Stroke 2010;5:12‐3. [Google Scholar]; Dean C, Ada L, Morris M. Improving walking using treadmill training in non‐ambulatory patients during inpatient stroke rehabilitation: the MOBILISE trial. Australian Journal of Physiotherapy 2009;4 Suppl:8. [Google Scholar]; Dean CM, Ada L, Bampton J, Morris ME, Katrak PH, Potts S. Treadmill walking with body weight support in subacute non‐ambulatory stroke improves walking capacity more than overground walking: a randomised trial. Journal of Physiotherapy 2010;56(2):97‐103. [DOI] [PubMed] [Google Scholar]
- Ada L. AMBULATE: 2 months versus 4 months of walking training to improve community ambulation after stroke (ACTRN12607000227493) (protocol). Australian New Zealand Clinical Trials Registry (ANZCTR) http://www.anzctr.org.au2007. ; Ada L, Dean C, Lindley R. Randomized trial of treadmill training to improve walking in community‐dwelling people after stroke: the AMBULATE trial. International Journal of Stroke 2013;8(6):436‐44. [DOI] [PubMed] [Google Scholar]; Ada L, Dean CM, Lindley R, Lloyd G. Improving community ambulation after stroke: the AMBULATE trial (protocol). BMC Neurology 2009;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ada L, Dean CM, Lindley R, Vargas J. Improving walking after stroke: The ambulate trial. Neurorehabilitation and Neural Repair 2012;26(6):674‐5. [Google Scholar]; Lindley RI, Dean C, Ada L. Can treadmill training improve walking in the chronic phase of stroke? The AMBULATE randomised controlled trial. Cerebrovascular Diseases2012; Vol. 33, issue Suppl 2:61.
- Lim PAC, Henson H, Cunha I, Qureshy H, Monga TN, Protas EJ. Body weight‐supported gait training in stroke patients (abstract). American Journal of Physical Medicine 2000;79(2):203. [Google Scholar]; Protas E. Stroke rehabilitation outcomes with supported treadmill ambulation training. ClinicalTrials.gov2003. ; Cunha Filho IT. Acute stroke rehabilitation outcomes with supported treadmill ambulation training. Texas Woman's University, PhD thesis2001. [PubMed]; Cunha Filho IT, Lim PA, Qureshy H, Henson H, Monga T, Protas EJ. Gait outcomes after acute stroke rehabilitation with supported treadmill ambulation training: a randomized controlled pilot study. Archives of Physical Medicine and Rehabilitation 2002;83(9):1258‐65. [DOI] [PubMed] [Google Scholar]; Cunha Filho IT, Lim PAC, Qureshy H, Henson H, Monga T, Protas EJ. A comparison of regular rehabilitation and regular rehabilitation with supported treadmill ambulation training for acute stroke patients. Journal of Rehabilitation Research and Development 2001;38(2):245‐55. [PubMed] [Google Scholar]
- Deniz L, Armagan O, Ozgen M, Oner S. Effectiveness of gait training with partial body‐weight support in subacute stroke patients. [Turkish]. Turk Serebrovaskuler Hastaliklar Dergisi 2011;17(1):13‐9. [Google Scholar]
- Du JB, Song WQ, Wang MB. The application of partial body weight support treadmill training in hemiplegia rehabilitation after stroke [Chinese ‐ simplified characters]. Chinese Journal of Cerebrovascular Diseases 2006;3(8):361‐4. [Google Scholar]
- Duncan P, Sullivan K, Behrman A, Azen S, Wu S, Dobkin B, et al. Locomotor Experience Applied Post‐Stroke (LEAPS): a randomized controlled trial (abstract PO01‐167). International Journal of Stroke 2008;3(Suppl 1):132. [Google Scholar]; Duncan PW. Locomotor Experience Applied Post‐Stroke (LEAPS) (abstract). Stroke2007; Vol. 38, issue 10:e120. ; Duncan PW. Locomotor experience applied post stroke (LEAPS) trial (NCT00243919) (protocol). ClinicalTrials.gov2006. [DOI] [PMC free article] [PubMed]; Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, et al. Body‐weight‐supported treadmill rehabilitation after stroke. New England Journal of Medicine 2011;364(21):2026‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]; Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, et al. Locomotor Experience Applied Post‐Stroke (LEAPS): a randomized controlled trial (abstract ‐ CT P20). Proceedings of the International Stroke Conference 2008 February 20‐22. USA, New Orleans: American Stroke Association, 2008. [Google Scholar]; Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, et al. Protocol for the Locomotor Experience Applied Post‐stroke (LEAPS) trial: a randomized controlled trial. BMC Neurology2007; Vol. 7:39. [DOI] [PMC free article] [PubMed]; Nadeau SE, Wu SS, Dobkin BH, Azen SP, Rose DK, Tilson JK, et al. Effects of task‐specific and impairment‐based training compared with usual care on functional walking ability after inpatient stroke rehabilitation: Leaps trial. Neurorehabilitation and Neural Repair 2013;27(4):370‐80. [DOI] [PubMed] [Google Scholar]; Rose DK, Behrman AL, Cen Y, Sullivan KJ, Martin D, Schofield RS, et al. Response to exercise tolerance testing in subacute stroke across severity levels (abstract). Stroke 2008;39(2):618‐9. [Google Scholar]; Tilson JK, Sullivan KJ, Cen SY, Rose DK, Koradia CH, Azen SP, et al. Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference. Physical Therapy 2010;90(2):196‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eich HJ, Hesse S, Mach H. Aerobes ausdauertraining gehfähiger hemiparetischer patienten, ergebnisse einer prospektiven randomisierten studie (Aerobic endurance training of hemiparetic patients who are able to walk, results of a prospective randomised study). Deutsche Zeitschrift fur Sportmedizin 2003;54(7‐8):S98. [Google Scholar]; Eich HJ, Mach H, Werner C, Hesse S. Aerobic treadmill plus Bobath walking training improves walking in subacute stroke: a randomised controlled trial. Clinical Rehabilitation 2004;18:640‐51. [DOI] [PubMed] [Google Scholar]; Eich HJ, Parchmann H, Hesse S, Mach H, Werner C. Aerobic treadmill training plus physiotherapy improves walking ability in subacute stroke patients. A randomized controlled study. Neurologie und Rehabilitation 2004;10(4):187‐216. [Google Scholar]; Hesse S, Eich HJ, Mach H, Parchmann H, Werner C. Aerobic treadmill training plus physiotherapy improves walking speed and capacity in subacute, moderately affected patients after stroke [Aerobes laufbandtraining plus physiotherapie verbessert das gehen von mäßig schwer betroffenen patienten nach schlaganfall]. Neurologie und Rehabilitation 2005;11(1):7‐12. [Google Scholar]; Hesse S, Eich HJ, Mach H, Werner C. Aerobic treadmill training of ambulatory hemiparetic patients: a randomised study. Proceedings of the 3rd World Congress in Neurological Rehabilitation; 2002; Venice, Italy: World Federation for NeuroRehabilitation. 2002. [Google Scholar]; Hesse S, Eich HJ, Mach H, Werner C. Aerobic treadmill training of ambulatory hemiparetic patients: a randomized study. Neurorehabilitation & Neural Repair 2001;15(4):311. [Google Scholar]; Mach H, Werner C, Eich HJ, Hesse S. Aerobic treadmill training in hemiparetic patients: a randomized study (abstract). Neurologie und Rehabilitation 2003;9(6):S6. [Google Scholar]
- Franceschini M, Carda S, Agosti M, Antenucci R, Malgrati D, Cisari C. Walking after stroke: what does treadmill training with body weight support add to overground gait training in patients early after stroke?: A single‐blind, randomized, controlled trial. Stroke 2009;40(9):3079‐85. [DOI] [PubMed] [Google Scholar]; Saccavini M, Zaccaria B, Franceschini M, Maestrini E, Agosti M, Mammi P, et al. Treadmill walking with bodyweight support in stroke patients during acute phase: a randomized controlled trial. Cerebrovascular Diseases 2009;27(Suppl 6):216‐7. [Google Scholar]
- Gan SW, Azarcon AC, Cadiao JJ, Gabua AM, Javier RS, Orayle EM, et al. A randomized controlled trial on the efficacy of body weight support overground ambulation versus body weight support treadmill training among post‐stroke patients of a tertiary hospital. PM & R: the Journal of Injury, Function, and Rehabilitation 2012;1:S182. [Google Scholar]
- Globas C, Becker C, Cerny J, Lam JM, Lindemann U, Forrester LW, et al. Chronic stroke survivors benefit from high‐intensity aerobic treadmill exercise: a randomized controlled trial. Neurorehabilitation and Neural Repair 2011;26(1):85‐95. [DOI] [PubMed] [Google Scholar]; Globas C, Becker C, Cerny J, Lam JM, Lindemann U, Forrester LW, et al. Elderly chronic stroke survivors benefit from aerobic treadmill exercise: a randomized, controlled trial. Stroke 2011;42(3):e323. [DOI] [PubMed] [Google Scholar]; Lam JM, Globas C, Cerny J, Hertler B, Uludag K, Forrester LW, et al. Predictors of response to treadmill exercise in stroke survivors. Neurorehabilitation and Neural Repair 2010;24(6):567‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]; Luft A. Structural neuroplasticity associated with aerobic treadmill training in geriatric chronic stroke survivors. ClinicalTrials.gov.2008.
- Hoyer E, Jahnsen R, Stanghelle JK, Strand LI. Body weight supported treadmill training versus traditional training in patients dependent on walking assistance after stroke: a randomized controlled trial. Disability Rehabilitation 2012;34(3):210‐9. [DOI] [PubMed] [Google Scholar]
- Brown DA, Jaffe DL, Buckley EL. Use of virtual objects to improve gait velocity in individuals with post‐stroke hemiplegia. Neurology Report 2002;26:105. [Google Scholar]; Jaffe DL. Results using stepping‐over response training to improve walking in individuals with post‐stroke hemiplegia. Proceedings of the 3rd National Rehabilitation Research and Development Conference. Arlington; USA, 2002. 2002. [Google Scholar]; Jaffe DL. Results using stepping‐over response training to improve walking in individuals with poststroke hemiplegia. Proceedings of the 3rd National Rehabilitation Research and Development Conference. Arlington, USA, 2002. [DOI] [PubMed] [Google Scholar]; Jaffe DL. Using virtual reality to improve walking following stroke. Proceedings of the Center on Disabilities Technology and Persons with Disabilities Conference. Northridge, California USA, 2002. [Google Scholar]; Jaffe DL, Brown DA. Improving stepping‐over responses in the elderly using simulated obstacles. http://www.stanford.edu/˜dljaffe/SOR/jaffe.pdf2002. ; Jaffe DL, Brown DA, Pierson‐Carey CD, Buckley EL, Lew HL. Stepping over obstacles to improve walking in individuals with poststroke hemiplegia. Journal of Rehabilitation Research and Development 2004;41(3A):283‐92. [DOI] [PubMed] [Google Scholar]
- Kang H‐K, Kim Y, Chung Y, Hwang S. Effects of treadmill training with optic flow on balance and gait in individuals following stroke: randomized controlled trials. Clinical Rehabilitation 2012;26(3):246‐55. [DOI] [PubMed] [Google Scholar]
- Kim C, Gong W, Kim S. The effects of lower extremity muscle strengthening exercise and treadmill walking exercise on the gait and balance of stroke patients. Journal of Physical Therapy Science 2011;23(3):405‐8. [Google Scholar]
- Kosak M, Reding M. Early aggressive mobilization is as effective as treadmill training for ambulation recovery in patients with stroke. Journal of Stroke and Cerebrovascular Diseases 1998;7(5):372. [Google Scholar]; Kosak MC, Brennan JA, Slomovicz LG, Tachkov A, Reding MJ. Body weight supported treadmill training versus traditional physical therapy. Stroke 1997;28(1):268. [Google Scholar]; Kosak MC, Reding MJ. Comparison of partial body weight‐supported treadmill gait training versus aggressive bracing assisted walking post stroke. Neurorehabilitation and Neural Repair 2000;14(1):13‐9. [DOI] [PubMed] [Google Scholar]
- Kuys S. Treadmill walking to improve walking and fitness following stroke: a single blinded pilot randomised controlled trial. Australian New Zealand Clinical Trials Registry (ANZCTR) http://www.anzctr.org.au2007. ; Kuys S, Brauer S, Ada L. Treadmill training to improve walking following stroke: a randomised controlled trial. International Journal of Stroke 2008;3(Suppl):347. [Google Scholar]; Kuys SS, Brauer SG, Ada L. High‐intensity treadmill walking during inpatient rehabilitation: feasibility of a randomised trial. Australian Journal of Physiotherapy2009, issue 4 Suppl:12‐3. ; Kuys SS, Brauer SG, Ada L. Higher‐intensity treadmill walking during rehabilitation after stroke in feasible and not detrimental to walking pattern or quality: a pilot randomized trial. Clinical Rehabilitation 2011;25(4):316‐26. [DOI] [PubMed] [Google Scholar]
- Langhammer B, Johan, Stanghelle K. Outdoors or indoors walking, what is more beneficial? A comparison of exercise methods in a randomized trial. Brain Injury 2010;24(3):172. [Google Scholar]; Langhammer B, Stanghelle JK. Exercise on a treadmill or walking outdoors? A randomized controlled trial comparing effectiveness of two walking exercise programmes late after stroke. Clinical Rehabilitation 2010;24(1):46‐54. [DOI] [PubMed] [Google Scholar]; Langhammer B, Stanghelle JK. Improving gait after stroke‐treadmill or walking; quantity or quality. Journal of Cyber Therapy and Rehabilitation 2009;2(3):191‐8. [Google Scholar]
- Laufer Y, Dickstein R, Chefez Y, Marcovitz E. The effect of treadmill training on the ambulation of stroke survivors in the early stages of rehabilitation: a randomized study. Journal of Rehabilitation Research and Development 2001;38(1):69‐78. [PubMed] [Google Scholar]
- Liston R, Mickelborough J, Harris B, Hann AW, Tallis RC. Conventional physiotherapy and treadmill re‐training for higher‐level gait disorders in cerebrovascular disease. Age & Ageing 2000;29(4):311‐8. [DOI] [PubMed] [Google Scholar]; Mickelborough J, Liston R, Harris B, Wynn Hann A, Tallis RC. An evaluation of conventional physiotherapy and treadmill re‐training of higher‐level gait disorders in patients with cerebral multi‐infarct states. Age & Ageing 1999;28 Suppl 2:54. [Google Scholar]
- Lam JM, Globas C, Cerny J, Hertler B, Uludag K, Forrester LW, et al. Predictors of response to treadmill exercise in stroke survivors. Neurorehabilitation and Neural Repair 2010;24(6):567‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]; Luft AR, Macko RF, Forrester LW, Villagra F, Ivey F, Sorkin JD, et al. Treadmill exercise activates subcortical neural networks and improves walking after stroke: a randomized controlled trial. Stroke 2008;39(12):3341‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay‐Lyons M, McDonald A, Matheson J, Howlett J. Physiological changes following a 12‐week program of body‐weight supported treadmill training early post‐stroke: a randomized clinical trial. International Journal of Stroke 2008;3(Suppl):348. [Google Scholar]; Mackay‐Lyons M, McDonald A, Matheson J, Eskes G, Klus MA. Dual effects of body‐weight supported treadmill training on cardiovascular fitness and walking ability early after stroke: a randomized controlled trial. Neurorehabilitation and Neural Repair 2013;27(7):644‐53. [DOI] [PubMed] [Google Scholar]
- Clark B, Harris‐Love M, Forrester L, Macko R, Smith GV. Effects of treadmill training on dynamic balance measures in chronic hemiparesis. Proceedings of the 3rd World Congress in Neurological Rehabilitation; Venice, Italy. World Federation for NeuroRehabilitation, 2002. [Google Scholar]; Clark B, Harris‐Love M, Forrester L, Macko R, Smith GW. Effects of treadmill training on dynamic balance measures in chronic hemiparesis. Neurorehabilitation and Neural Repair 2001;15(4):315. [Google Scholar]; Ivey FM, Ryan AS, Hafer‐Macko CE, Goldberg AP, Macko RF. Treadmill aerobic training improves glucose tolerance and indices of insulin sensitivity in disabled stroke survivors: a preliminary report. Stroke 2007;38(10):2752‐8. [DOI] [PubMed] [Google Scholar]; Limpar P, Macko RF, Sorkin JD, Katzel LI, Hanley DF. Safety of treadmill aerobic exercise in chronic hemiparetic stroke patients. Stroke 2004;35(1):286. [Google Scholar]; Macko RF. Effects of exercise on patients with hemiparetic stroke. ClinicalTrials.gov/show/NCT000184212001. ; Macko RF, Ivey FM, Forrester LW, Hanley D, Sorkin JD, Katzel LI, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: a randomized, controlled trial. Stroke 2005;36(10):2206‐11. [DOI] [PubMed] [Google Scholar]
- Mehrberg RD, Flick C, Dervay J, Carmody J, Carrington C, Jermer M. Clinical evaluation of a new over ground partial body weight support assistive device in hemiparetic stroke patients. Archives of Physical Medicine & Rehabilitation 2001;82:1293. [Google Scholar]
- Moore JL, Hornby G, Killian C. Intensive training facilitates locomotor improvements beyond a "plateau" in motor recovery post‐stroke. Journal of Neurologic Physical Therapy2009, issue 4. ; Moore JL, Roth EJ, Killian C, Hornby TG. Locomotor training improves daily stepping activity and gait efficiency in individuals poststroke who have reached a "plateau" in recovery. Stroke 2010;41(1):129‐35. [DOI] [PubMed] [Google Scholar]
- Hansen PD, Grimby G, Carlsson J, Nilsson L. Body‐weight‐support gait training. Clinical Rehabilitation 2002;16(3):343‐5. [DOI] [PubMed] [Google Scholar]; Nilsson L, Carlsson J, Danielsson A, Fugl‐Meyer A, Hellstrom K, Kristensen L, et al. Walking training of patients with hemiparesis at an early stage after stroke. Proceedings of the 14th International Congress of The World Confederation for Physical Therapy. Spain, Barcelona. 2003:RR‐PL‐1729. [Google Scholar]; Nilsson L, Carlsson J, Danielsson A, Fugl‐Myer A, Hellstrom K, Kristensen L, et al. Walking training of patients with hemiparesis at an early stage after stroke: a comparison of walking training on a treadmill with body weight support and walking training on the ground. Clinical Rehabilitation 2001;15(5):515‐27. [DOI] [PubMed] [Google Scholar]
- Nilsson L, Carlsson J, Danielsson A, Fugl‐Myer A, Hellstrom K, Kristensen L, et al. Walking training of patients with hemiparesis at an early stage after stroke: a comparison of walking training on a treadmill with body weight support and walking training on the ground. Clinical Rehabilitation 2001;15(5):515‐27. [DOI] [PubMed] [Google Scholar]
- Nilsson L, Carlsson J, Danielsson A, Fugl‐Myer A, Hellstrom K, Kristensen L, et al. Walking training of patients with hemiparesis at an early stage after stroke: a comparison of walking training on a treadmill with body weight support and walking training on the ground. Clinical Rehabilitation 2001;15(5):515‐27. [DOI] [PubMed] [Google Scholar]
- Olawale O, Appiah‐Kubi K, Jones‐Okai D. Exercise training improves walking function in an African group of stroke survivors: a randomized controlled trial. Physiotherapy 2007;93(Suppl 1):s562‐3. [DOI] [PubMed] [Google Scholar]; Olawale OA, Jaja SI, Anigbogu CN, Appiah‐Kubi KO, Jones‐Okai D. Effects of two exercise training techniques on walking function in adult patients with stroke. Nigerian Quarterly Journal of Hospital Medicine 2009;19(2):88‐94. [PubMed] [Google Scholar]; Olawale OA, Jaja SI, Anigbogu CN, Appiah‐Kubi KO, Jones‐Okai D. Exercise training improves walking function in an African group of stroke survivors: a randomized controlled trial. Clinical Rehabilitation 2011;5:442‐50. [DOI] [PubMed] [Google Scholar]
- Mehrholz J, Ritschel C, Ruckriem S, Pohl M. Speed‐dependent treadmill training in hemiparetic stroke patients. A randomized controlled trial. Proceedings of the 14th International Congress of The World Confederation for Physical Therapy. Spain, Barcelona. 2003:RR‐PL‐0168. [Google Scholar]; Pohl M, Mehrholz J, Ritschel C, Ruckriem S. Speed‐dependent treadmill training in ambulatory hemiparetic stroke patients: a randomized controlled trial. Neurorehabilitation and Neural Repair 2001;15:311. [DOI] [PubMed] [Google Scholar]; Pohl M, Mehrholz J, Ritschel C, Ruckriem S. Speed‐dependent treadmill training in ambulatory hemiparetic stroke patients: a randomized controlled trial. Stroke 2002;33:553‐8. [DOI] [PubMed] [Google Scholar]; Pohl M, Mehrholz J, Ritschel C, Ruckriem S. Speed‐dependent treadmill training in ambulatory stroke patients: a randomized controlled trial. Proceedings of the 3rd World Congress in Neurological Rehabilitation. Venice, Italy, 2002:T3. [DOI] [PubMed] [Google Scholar]
- Malouin F, Richards CL, Wood‐Dauphinee S, Williams JI. Effects of an intense task‐oriented gait‐training program in acute stroke patients: a pilot study. In: Woollacott M, Horak F editor(s). Posture and Gait: Control Mechanisms. Portland ORE: University of Oregon Books, 1992:407‐10. [Google Scholar]; Malouin F, Richards CL, Wood‐Dauphinee S, Williams JY. Effects of early and intensive gait training in stroke patients: a pilot study. Physical Therapy 1991;71(6):S58. [Google Scholar]; Malouin F, Richards Cl, Wood‐Dauphinee S, Williams JI. A randomized controlled trial comparing early and intensive task‐specific therapy to conventional therapy in acute‐stroke patients. Canadian Journal of Rehabilitation 1993;7(1):27‐8. [Google Scholar]; Malouin F, Richards Cl, Wood‐Dauphinee S, Williams JI. Early standing and intensive locomotor training after stroke (abstract). Proceedings of the International Congress on Stroke Rehabilitation. Berlin: German Society for Neurological Rehabilitation, 1993:41. [Google Scholar]; Richards CL, Malouin F. Evaluation and therapy of disturbed motor control in spastic paresis: therapeutic considerations for locomotor disorders. Neurology Report 1997;21:85‐90. [Google Scholar]; Richards CL, Malouin F, Wood‐Dauphinee S, Williams JI, Bouchard JP, Brunet D. Task‐specific physical therapy for optimization of gait recovery in acute stroke patients. Archives of Physical Medicine & Rehabilitation 1993;74(6):612‐20. [DOI] [PubMed] [Google Scholar]
- Richards CL, Malouin F, Bravo G, Dumas F, Wood‐Dauphinee S. The role of technology in task‐oriented locomotor training in acute stroke: a randomized controlled trial (abstract). Proceedings of the 14th International Congress of The World Confederation for Physical Therapy. Spain, Barcelona. 2003:RR‐PL‐1592. [Google Scholar]; Richards CL, Malouin F, Bravo G, Dumas F, Wood‐Dauphinee S. The role of technology in task‐oriented training in persons with subacute stroke: a randomized controlled trial. Neurorehabilitation & Neural Repair 2004;18(4):199‐211. [DOI] [PubMed] [Google Scholar]
- Scheidtmann K, Brunner H, Muller F, Weinandy‐Trapp M, Wulf D, Koenig E. Treadmill training in early poststroke patients ‐ do timing and walking ability matter? [Sequenzeffekte in der laufbandtherapie]. Neurological Rehabilitation 1999;5(4):198‐202. [Google Scholar]
- Smith PS. The Effect of Treadmill Training on Functional Limitation and Disability Measures in Persons in the Chronic Stage of Recovery from Stroke (Thesis). Texas Woman's University, 2006. [Google Scholar]; Smith PS, Thompson M. Treadmill training post stroke: are there any secondary benefits? A pilot study. Clinical Rehabilitation 2008;22(10‐11):997‐1002. [DOI] [PubMed] [Google Scholar]
- Carey JR. Locomotor and strength training in adults who were ambulatory after stroke: invited commentary. Physical Therapy 2007;87(12):1603‐5. [DOI] [PubMed] [Google Scholar]; Klassen T, Mulroy SJ, Sullivan KJ. Gait parameters associated with responsiveness to a task‐specific and/or strength training program post‐stroke. Journal of Neurologic Physical Therapy 2005;29(4):198. [Google Scholar]; Mulroy SJ, Klassen T, Gronley JK, Eberly VJ, Brown DA, Sullivan KJ. Gait parameters associated with responsiveness to treadmill training with body‐weight support after stroke: an exploratory study. Physical Therapy 2010;90(2):209‐23. [DOI] [PubMed] [Google Scholar]; Sullivan KJ, Brown DA, Klassen T, Mulroy S, Ge T, Azen SP, et al. Effects of task‐specific locomotor and strength training in adults who were ambulatory after stroke: results of the STEPS randomized clinical trial. Physical Therapy 2007;87(12):1580‐602. [DOI] [PubMed] [Google Scholar]; Sullivan KJ, Brown DA, Mulroy S, Winstein CJ. Author response: Locomotor and Strength Training in Adults Who Were Ambulatory After Stroke. Physical Therapy 2007;87(12):1605‐7. [DOI] [PubMed] [Google Scholar]
- Suputtitada A, Yooktanan P, Rarerng‐Ying T. Effect of partial body weight support treadmill training in chronic stroke patients. Chotmaihet Thangphaet (Journal of the Medical Association of Thailand) 2004;87 Suppl 2:S107‐11. [PubMed] [Google Scholar]
- Takami A, Wakayama S. Effects of partial body weight support while training acute stroke patients to walk backwards on a treadmill ‐ a controlled clinical trial using randomized allocation. Journal of Physical Therapy Science 2010;22(2):177‐87. [Google Scholar]
- Toledano A, Katz‐Leurer M, Carmeli E, Kamerman T, Merzeliak O, Adler Y, et al. A pilot randomized clinical trial of an early supervised aerobic exercise training program after minor ischemic strokes. Stroke 2009;40(4):e252. [Google Scholar]; Toledano‐Zarhi A, Tanne D, Carmeli E, Katz‐Leurer M. Feasibility, safety and efficacy of an early aerobic rehabilitation program for patients after minor ischemic stroke: a pilot randomized controlled trial. NeuroRehabilitation 2011;28(2):85‐90. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Visintin M. Optimal outcomes obtained with body‐weight support combined with treadmill training in stroke subjects. Archives of Physical Medicine & Rehabilitation 2003;84(10):1458‐65. [DOI] [PubMed] [Google Scholar]; Selzer ME, Zorowitz RD. Frontiers in neurorehabilitation: translating basic research into clinical advances. Journal of Neurologic Rehabilitation 1998;12:149‐51. [Google Scholar]; Visintin M, Barbeau H, Korner‐Bitensky N, Mayo NE. A new approach to retrain gait in stroke patients through body weight support and treadmill stimulation. Stroke 1998;29(6):1122‐8. [DOI] [PubMed] [Google Scholar]; Visintin M, Korner‐Bitensky N, Barbeau H, Mayo N. A new approach to retraining gait following stroke through body weight support and treadmill simulation. Proceedings of the 12th International Congress of the World Confederation of Physical Therapy. Washington DC: American Physical Therapy Association, 1995:812. [Google Scholar]
- Visintin M, Barbeau H, Korner‐Bitensky N, Mayo NE. A new approach to retrain gait in stroke patients through body weight support and treadmill stimulation. Stroke 1998;29(6):1122‐8. [DOI] [PubMed] [Google Scholar]
- Visintin M, Barbeau H, Korner‐Bitensky N, Mayo NE. A new approach to retrain gait in stroke patients through body weight support and treadmill stimulation. Stroke 1998;29(6):1122‐8. [DOI] [PubMed] [Google Scholar]
- Weng CS, Bi S, Tian Z, Yu ZZ, Xu J, Bi SQ, et al. Application of structured speed‐dependent treadmill training in hemiplegic patients after stroke [Chinese]. Zhongguo Linchuang Kangfu 2004;8(34):7617‐9. [Google Scholar]
- Weng CS, Wang J, Pan XY, Yu ZZ, Wang G, Gao LP, et al. Effectiveness of backward walking treadmill training in lower extremity function after stroke (Chinese ‐ simplified characters). Zhonghua Yi Xue Za Zhi (National Medical Journal of China) 2006;86(37):2635‐8. [PubMed] [Google Scholar]
- Hesse S, Werner C, Bardeleben A, Frankenberg S. Treadmill therapy with partial body weight support and an automated gait trainer for restoration of gait after stroke: a randomized study. Neurorehabilitation & Neural Repair 2001;15:310‐1. [DOI] [PubMed] [Google Scholar]; Hesse S, Werner C, Bardeleben A, Frankenberg S. Treadmill therapy with partial body weight support and an automated gait trainer for restoration of gait after stroke: a randomized study. Proceedings of the 3rd World Congress in Neurological Rehabilitation. Venice, Italy, 2002:T1. [Google Scholar]; Hesse S, Werner C, Frankenberg S, Bardeleben A. Electromechanical gait trainer for restoration of gait after stroke. Proceedings of the 1st World Congress of the International Society of Physical Rehabilitation Medicine (ISPRM). 2001 July 7‐13. 2001:489‐94. [Google Scholar]; Werner C, Frankenberg S, Treig T, Konrad M, Hesse S. Treadmill training with partial body weight support and an electromechanical gait trainer for restoration of gait in subacute stroke patients: a randomized crossover study. Stroke 2002;33:2895‐901. [DOI] [PubMed] [Google Scholar]
- Yang YR, Chen IH, Liao KK, Huang CC, Wang RY. Cortical reorganization induced by body weight‐supported treadmill training in patients with hemiparesis of different stroke durations. Archives of Physical Medicine and Rehabilitation 2010;91(4):513‐8. [DOI] [PubMed] [Google Scholar]
- Yen CL, Wang RY, Liao KK, Huang CC, Yang YR. Gait training induced change in corticomotor excitability in patients with chronic stroke. Neurorehabilitation & Neural Repair 2008;22(1):22‐30. [DOI] [PubMed] [Google Scholar]
- Zhang J‐P, Wang J‐H, Cao P‐W, Chen M. The effect of body weight supported treadmill training on drop foot of hemiplegia. Sichuan Medical Journal 2008;10:1331‐4. [Google Scholar]; Zhang J‐P, Wang J‐H, Chen W, Liu D‐Y. The effect of body‐weight supported treadmill training on drop foot of hemiplegia. Journal of Rehabilitation Medicine 2008;46(Suppl):104. [Google Scholar]
- Zhu HX, Dou ZL, Li K, Lan Y, Hu XQ. A preliminary investigation on the correlation of partial body weight support training with hemiplegic gait and ambulation function after brain injury [Chinese]. Zhongguo Linchuang Kangfu 2004;8(25):5205‐7. [Google Scholar]
References to studies excluded from this review
- Aschbacher B. Comparing gait training in patients after stroke with task oriented physiotherapy or robot‐assisted treadmill training, a feasibility study. Unpublished presentation2006.
- Bayat R, Barbeau H, Lamontagne A. Speed and temporal‐distance adaptations during treadmill and overground walking following stroke. Neurorehabilitation & Neural Repair 2005;2:115‐24. [DOI] [PubMed] [Google Scholar]
- Bleckert MG, Felder H, Grunebert C. Treadmill therapy in the acute rehabilitation stage in hemiparetic patients [German] [Laufbandtherapie in der akuten rehabilitationsphase bei patienten mit hemiparese. Pilotstudie zum vergleich lansamer und schneller ganggeschwindigkeiten]. Physioscience 2006;2(2):67‐72. [Google Scholar]
- Blennerhassett J, Dite W. A randomised controlled trial evaluating additional task‐related practice during stroke rehabilitation. Australian Journal of Physiotherapy 2003;49(4 Suppl):s6‐7. [DOI] [PubMed] [Google Scholar]; Blennerhassett J, Dite W. Additional task‐related practice improves mobility and upper limb function early after stroke: a randomised controlled trial. Australian Journal of Physiotherapy 2004;50(4):219‐24. [DOI] [PubMed] [Google Scholar]
- Borsje S, Hochstenbach JBH, Postema K, Mulder TH. Clinical value of motor imagery and bodyweight supported treadmill training for recovery of gait performance of stroke patients in the early phase. Proceedings of the European Stroke Conference; 2003 May 21‐24. Valencia, Spain, 2003. [Google Scholar]
- Brissot R, Laviolle B. Efficacy of a mechanical gait repetitive training technique in hemiparetic stroke patients. ClinicalTrials.gov2006.
- Caldwell C, Medley A. Effects of bicycling, treadmill, and variable surfaces on gait in people following a CVA. Neurology Report 2000;24(5):203. [Google Scholar]
- Daly J, Fryer J, Rochleau N. FNS and weight support treadmill training for gait component restoration. ClinicalTrials.gov2001. ; Daly JJ, Roenigk K, Holcomb J, Rogers JM, Butler K, Gansen J, et al. A randomized controlled trial of functional neuromuscular stimulation in chronic stroke subjects. Stroke 2006;37(1):172‐8. [DOI] [PubMed] [Google Scholar]; Daly JJ, Roenigk KL, Butler KM, Gansen JL, Fredrickson E, Marsolais EB, et al. Response of sagittal plane gait kinematics to weight‐supported treadmill training and functional neuromuscular stimulation following stroke. Journal of Rehabilitation Research & Development 2004;41(6):807‐20. [DOI] [PubMed] [Google Scholar]; Daly JJ, Rogers J, Strasshofer B, Debogorski AA, Roenigk K, Ruff RL. Treadmill training, weight support, and FNS for stroke gait training. Platform and poster presentations for CSM 2003. Neurology Report 2002;26(4):202‐3. [Google Scholar]; Daly JJ, Ruff RL. Feasibility of combining multi‐channel functional neuromuscular stimulation with weight‐supported treadmill training. Journal of the Neurological Sciences 2004;225(1‐2):105‐15. [DOI] [PubMed] [Google Scholar]; Daly JJ, Sng K, Roenigk K, Fredrickson E, Dohring M. Intra‐limb coordination deficit in stroke survivors and response to treatment. Gait & Posture 2007;25(3):412‐8. [DOI] [PubMed] [Google Scholar]; Hansen K, Lucarelli J, Torres AM, Roenigk KL, Daly JJ. Muscle activation latency gains for gait, in response to combined treadmill training, weight support, and FNS following stroke. National Science Conference, American Physical Therapy Association; February 2003; Tampa, Florida, USA. 2003. [Google Scholar]; Pundik S, Holcomb J, Daly JJ. Improvements in life‐role participation after intensive gait training of chronic stroke survivors. Stroke 2009;40(4):e123. [Google Scholar]
- Daly J, Zimbelman J, Roenigk K, McCabe J, Rogers J, Butler K, et al. Recovery of coordinated gait: randomized controlled stroke trial of functional electrical stimulation (FES) versus No FES, with weight‐supported treadmill and over‐ground training. Neurorehabilitation & Neural Repair 2011;25(7):588‐96. [DOI] [PubMed] [Google Scholar]
- Dean CM, Richards CL, Malouin F. Task‐related circuit training improves performance of locomotor tasks in chronic stroke: a randomized, controlled pilot study. Archives of Physical Medicine & Rehabilitation 2000;81(4):409‐17. [DOI] [PubMed] [Google Scholar]; Richards CL. Task‐oriented gait training for patients with cerebral palsy and stroke. Proceedings of the 2nd World Congress in Neurological Rehabilitation. Toronto, 1999:218‐27. [Google Scholar]; Richards CL, Malouin F, Dean C. Maximizing locomotor recovery after stroke. Archives of Physiology & Biochemistry 2000;108(1‐2):1. 11077567 [Google Scholar]
- Mehrholz J, Werner C, Hesse S, Pohl M. Immediate and long‐term functional impact of repetitive locomotor training as an adjunct to conventional physiotherapy for non‐ambulatory patients after stroke. Disability and Rehabilitation 2008;30(11):830‐6. [DOI] [PubMed] [Google Scholar]; Pohl M, Mehrholz J, Rutte K, Dressler C, Gold S, Werner C, et al. Results of aerobic exercise training in patients after stroke. Gait trainer vs conventional therapy. A randomized controlled longitudinal study. First results. Neurologie und Rehabilitation 2003;9(6):S6‐7. [Google Scholar]; Pohl M, Mehrholz J, Werner C, Hesse S. Comparison of aerobic exercise training in patients after stroke ‐ gait trainer versus conventional physiotherapy. A randomized controlled longitudinal study [Vergleich der aeroben ubungsintensitat bei patienten nach schlaganfall ‐ gangtrainer versis konventionelle physiotherapie. Eine randomisierte and kontrollierte longitudinalstudie]. Neurologie und Rehabilitation 2004;10(4):187‐216. [Google Scholar]; Pohl M, Werner C, Holzgraefe M, Kroczek G, Mehrholz J, Wingendorf I, et al. Repetitive locomotor training and physiotherapy improve walking and basic activities of daily living after stroke: a single‐blind, randomized multicentre trial (DEutsche GAngtrainerStudie, DEGAS). Clinical Rehabilitation 2007;21(1):17‐27. [DOI] [PubMed] [Google Scholar]; Werner C, Pohl M, Holzgraefe M, Kroczek G, Mehrholz J, Wingendorf I, et al. 'DEGAS' ‐ German gait training study to evaluate the gait trainer (GT1) combined with physiotherapy compared with physiotherapy alone in acute stroke patients. Neurologie und Rehabilitation2004; Vol. 10, issue 4:187‐216. ; Werner C, Pohl M, Holzgraefe M, Kroczek G, Mehrholz J, Wingendorf I, et al. Design of a multicentre study. Neurologie und Rehabilitation 2003;9(6):S6. [DOI] [PubMed] [Google Scholar]; Werner C, Pohl M, Holzgraefe M, Kroczek G, Mehrholz J, Wingendorf I, et al. Locomotor training in subacute stroke patients: Results of a multicenter study (DEGAS). Neurologie und Rehabilitation 2006;12(5):262‐9. [Google Scholar]
- Dias D, Lains J, Pereira A, Nunes R, Caldas J, Amaral C, et al. Can we improve gait skills in chronic hemiplegics? A randomised control trial with gait trainer. Europa Medicophysica 2007;43(4):499‐504. [PubMed] [Google Scholar]; Dias D, Lains J, Pereira A, Nunes R, Caldas J, Amaral C, et al. Partial body weight support in chronic hemiplegics: a randomized control trial. Proceedings of the 6th Mediterranean Congress of Physical and Rehabilitation Medicine; 2006 18‐21 October; Vilamoura, Portugal. 2006. [Google Scholar]
- English CK, Hillier SL, Stiller KR, Warden‐Flood A. Circuit class therapy versus individual physiotherapy sessions during inpatient stroke rehabilitation: a controlled trial. Archives of Physical Medicine and Rehabilitation 2007;88(8):955‐63. [0003‐9993: (Print)] [DOI] [PubMed] [Google Scholar]
- Fisher S. Use of Autoambulator for mobility improvement in patients with central nervous system (CNS) injury or disease. Neurorehabilitation & Neural Repair 2008;22(5):556. [Google Scholar]
- Forrester LW, Villagra F, Macko RF, Hanley DF. Treadmill vs. stretching: short‐term CNS adaptations to single bouts of submaximal exercise in chronic stroke patients. Stroke 2004;35(6):e312. [Google Scholar]
- Freivogel S, Schmalohr D, Mehrholz J. Improved walking ability and reduced therapeutic stress with an electromechanical gait device. Journal of Rehabilitation Medicine 2009;41(9):734‐9. [DOI] [PubMed] [Google Scholar]
- Globokar D. Gait trainer in neurorehabilitation of patients after stroke. Proceedings of 3rd World Congress of the International Society of Physical and Rehabilitation Medicine ‐ ISPRM; 2005 April 10‐14; Sao Paulo, Brazil. 2005. [Google Scholar]
- Hidler J, Hornby G. Gait restoration in hemiparetic stroke patients using goal‐directed robotic‐assisted treadmill training. http://www.ric.org/research/clinical‐trials/detail/?id=1692007. ; Hidler J, Nichols D, Pelliccio M, Brady K, Campbell DD, Kahn JH, et al. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabilitation & Neural Repair 2009;23(1):5‐13. [DOI] [PubMed] [Google Scholar]; Hidler JM. Walking therapy in hemiparetic stroke patients using robotic‐assisted treadmill training. ClinicalTrials.gov2007.
- Anonymous. Enhanced gait‐related improvements after therapist‐versus robotic‐assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke2008; Vol. 39, issue 8:e143. [DOI] [PubMed]; Hornby TG, Campbell DD, Kahn JH, Demott T, Moore JL, Roth HR. Enhanced gait‐related improvements after therapist ‐‐ versus robotic‐assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke 2008;39(6):1786‐92. [DOI] [PubMed] [Google Scholar]; Kahn J, Campbell D, Demott T, Moore J, Roth H, Hornby G. Alterations in locomotor performance in individuals with hemiplegia post‐stroke following robotic‐ or therapist‐assisted locomotor training. Journal of Neurologic Physical Therapy 2006;30(4):212. [Google Scholar]; Lewek M, Hayes T, Moore J, Roth H, Hornby TG. Alterations in joint kinesmatics following locomotor training in individuals with chronic stroke. Platforms, thematic posters, and posters for CSM 2007. Journal of Neurologic Physical Therapy 2006;30(4):196. [Google Scholar]; Lewek MD, Cruz TH, Moore JL, Roth HR, Dhaher YY, Hornby TG. Allowing intralimb kinematic variability during locomotor training poststroke improves kinematic consistency: a subgroup analysis from a randomized clinical trial. Physical Therapy 2009;89(8):829‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husemann B, Muller F, Krewer C, Heller S, Koenig E. Effects of locomotion training with assistance of a robot‐driven gait orthosis in hemiparetic patients after stroke: a randomized controlled pilot study. Stroke 2007;38(2):349‐54. [DOI] [PubMed] [Google Scholar]; Husemann B, Muller F, Krewer C, Lab A, Gille C, Heller S, et al. Effects of locomotion training with assistance of a driven gait orthosis in hemiparetic patients after stroke. Neurologie und Rehabilitation 2004;10(4):187‐217. [Google Scholar]
- Jang SJ, Park SW, Kim ES, Wee HM, Kim YH. Electromechanical gait trainer for restoring gait in hemiparetic stroke patients. Proceedings of 3rd World Congress of the International Society of Physical and Rehabilitation Medicine ‐ ISPRM; 2005 April 10‐14; Sao Paulo, Brazil. 2005. [Google Scholar]
- Jeong K, Ha H, Shin H, Ohn S, Sung D, Lee P, et al. Effects of robot‐assisted gait therapy on locomotor recovery in stroke patients. Journal of Rehabilitation Medicine 2008;40(Suppl 46):148. [Google Scholar]
- Khanna PB. A randomised control study of the immediate and long term benefits of conventional stroke rehabilitation with task related group therapy in chronic stroke patients. http://www.controlled‐trials.com/ (electronic database, accessed 2003).
- Kim BO, Lee JJ, Cho KH, Kim SH. Gait training robot (gaitTrainer) in rehabilitation (abstract). Proceedings of the 1st International Congress of International Society of Physical and Rehabilitation Medicine (ISPRM); 2001 July 7‐13; Amsterdam, Netherlands. International Society of Physical and Rehabilitation Medicine (ISPRM), 2001. [Google Scholar]
- Kim M, Kim Y, Lee P, Kim G, You J, Huh J. Effect of robot‐assisted gait therapy on cardio‐pulmonary fitness in subacute stroke patients. Neurorehabilitation & Neural Repair 2008;22(5):594. [DOI] [PubMed] [Google Scholar]
- Kovrazhkina E, Rumianzeva N, Starizin A, Ivanova G. Rehabilitation of walking in patients with an acute stroke with assistance of a robotic device gait trainer (abstract 13). Cerebrovascular Diseases 2009;27(Suppl 6):210. [Google Scholar]; Skvortsova V, Ivanova G, Kovrazhkina E, Rumyantseva N, Staritsin A, Sogomonyan E. The efficacy of gait rehabilitation after stroke training with assistance of a robotic device gait trainer: a pilot study (abstract PO02‐352). International Journal of Stroke 2008;3(Suppl):355. [Google Scholar]; Skvortsova VI, Ivanova GE, Kovrazhkina EA, Rumiantseva NA, Staritsyn AN, Suvorov A, et al. The use of a robot‐assisted Gait Trainer GT1 in patients in the acute period of cerebral stroke: a pilot study [Russian]. Zh Nevrol Psikhiatr Im S S Korsakova 2008;Suppl 23:28‐34. [PubMed] [Google Scholar]; Skvortsova VI, Ivanova GE, Rumiantseva NA, Staritsyn AN, Kovrazhkina EA, Suvorov A. Modern approach to gait restoration in patients in the acute period of cerebral stroke [Russian]. Zh Nevrol Psikhiatr Im S S Korsakova 2010;110(4):25‐30. [PubMed] [Google Scholar]
- Kwakkel G, Kollen BJ, Wagenaar RC. Long term effects of intensity of upper and lower limb training after stroke: a randomised trial. Journal of Neurology, Neurosurgery, and Psychiatry 2002;72(4):473‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kwakkel G, Wagenaar RC. Effect of duration of upper‐ and lower‐extremity rehabilitation sessions and walking speed on recovery of interlimb co‐ordination in hemiplegic gait. Physical Therapy 2002;82:432‐48. [PubMed] [Google Scholar]; Kwakkel G, Wagenaar RC, Twisk JWR, Lankhorst GJ, Koetsier JC. Intensity of leg and arm training after primary middle‐cerebral‐ artery stroke: a randomised trial. Lancet 1999;354:191‐6. [DOI] [PubMed] [Google Scholar]
- Langhammer B, Stanghelle JK. Bobath or motor relearning programme? A comparison of two different approaches of physiotherapy in stroke rehabilitation: a randomized controlled study. Clinical Rehabilitation 2000;14:361‐9. [DOI] [PubMed] [Google Scholar]
- Langhammer B, Lindmark B, Stanghelle JK. Living with stroke: exercising for life. 7th World Congress on Aging and Physical Activity. Journal of Aging & Physical Activity 2008;16:S80. [Google Scholar]; Langhammer B, Lindmark B, Stanghelle JK. Stroke patients and long‐term training: is it worthwhile? A randomized comparison of two different training strategies after rehabilitation. Clinical Rehabilitation 2007;21(6):495‐510. [DOI] [PubMed] [Google Scholar]; Langhammer B, Lindmark B, Tanghelle JKS. Motor function, activity and participation one year post stroke: A l follow‐up of a randomised controlled trial in persons with stroke. Brain Injury 2010;24(3):171‐2. [Google Scholar]; Langhammer B, Stanghelle JK, Lindmark B. An evaluation of two different exercise regimes during the first year following stroke: a randomised controlled trial. Physiotherapy Theory and Practice 2009;25(2):55‐68. [DOI] [PubMed] [Google Scholar]; Langhammer B, Stanghelle JK, Lindmark B. Exercise and health‐related quality of life during the first year following acute stroke. A randomized controlled trial. Brain Injury 2008;22(2):135‐45. [DOI] [PubMed] [Google Scholar]
- Lau KW, Mak MK. Speed‐dependent treadmill training is effective to improve gait and balance performance in patients with sub‐acute stroke. Journal of Rehabilitation Medicine 2011;43(8):709‐13. [DOI] [PubMed] [Google Scholar]; Lau WK, Mak MKY. The effects of speed‐dependent treadmill training on gait and balance performance in patients with sub‐acute stroke. Hong Kong Physiotherapy Journal 2010;28(1):27. [Google Scholar]
- Lindquist A, Ribeiro T, Silva E, Galvao E. Influence of treadmill training with body weight support and proprioceptive neuromuscular facilitation on hemiparetic gait. Archives of Physical Medicine and Rehabilitation 2011;92(10):1718. [PubMed] [Google Scholar]
- Macko RF. Exercise training for hemiparetic stroke. CRISP (Computer Retrieval of Information on Scientific Projects) Database http://crisp.cit.nih.gov/2002. ; Macko RF. Treadmill exercise prescriptions to improve fitness versus ambulatory function after stroke. ClinicalTrials.gov/show/NCT004304562006.
- Mayr A, Kofler M, Quirbach E, Matzak H, Frohlich K, Saltuari L. Prospective, blinded, randomized crossover study of gait rehabilitation in stroke patients using the Lokomat gait orthosis. Neurorehabilitation & Neural Repair 2007;21(4):307‐14. [DOI] [PubMed] [Google Scholar]
- Mayr A, Saltuari L, Quirbach E. Impact of Lokomat training on gait rehabilitation: a prospective randomized controlled trial in stroke patients. Neurorehabilitation & Neural Repair 2008;22(5):596. [DOI] [PubMed] [Google Scholar]
- McCain KJ, Pollo FE, Baum BS, Coleman SC, Baker S, Smith PS. Locomotor treadmill training with partial body‐weight support before overground gait in adults with acute stroke: a pilot study. Archives of Physical Medicine and Rehabilitation 2008;89(4):684‐91. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Kock‐Jensen C, Brincks J. Gait training for persons with stroke (GTS). ClinicalTrials.gov.2007.
- Pang MYC, Lau RWK. The effects of treadmill exercise training on hip bone density and tibial bone geometry in stroke survivors: a pilot study. Neurorehabilitation & Neural Repair 2010;24(4):368‐76. [DOI] [PubMed] [Google Scholar]
- Park SE, Kim SH, Lee SB, An HJ, Choi WS, Moon OG, et al. Comparison of underwater and overground treadmill walking to improve gait pattern and muscle strength after stroke. Journal of Physical Therapy Science 2012;24(11):1087‐90. [Google Scholar]
- Peurala SH, Pitkanen K, Sivenius J, Tarkka I. Body‐weight supported gait exercise compared with floor walking in chronic stroke patients. Archives of Physical Medicine & Rehabilitation 2004;85(9):E7. [DOI] [PubMed] [Google Scholar]; Peurala SH, Tarkka IM, Pitkanen K, Sivenius J. The effectiveness of body weight‐supported gait training and floor walking in patients with chronic stroke. Archives of Physical Medicine & Rehabilitation 2005;86(8):1557‐64. [DOI] [PubMed] [Google Scholar]; Pitkanen K, Tarkka I, Sivenius J. Walking training with partial body weight support versus conventional walking training of chronic stroke patients: preliminary findings. Neurorehabilitation & Neural Repair 2001;15(4):312. [Google Scholar]; Pitkanen K, Tarkka IM, Sivenius J. Walking training with partial body weight support versus conventional walking training of chronic stroke patients: preliminary findings. Proceedings of the 3rd World Congress in Neurological Rehabilitation; Venice, Italy. 2002:T7. [Google Scholar]
- Peurala S, Airaksinen O, Jakala P, Tarkka I, Sivenius J. Intensive walking and exercise therapy during early acute stage of stroke. 18th International Conference of the International Society for Posture and Gait Research; Vermont, USA. 2007:103‐4. [Google Scholar]; Peurala SH, Airaksinen O, Huuskonen P, Jakala P, Juhakoski M, Sandell K, et al. Effects of intensive therapy using gait trainer or floor walking exercises early after stroke. Journal of Rehabilitation Medicine 2009;41(3):166‐73. [DOI] [PubMed] [Google Scholar]; Peurala SH, Airaksinen O, Jakala P, Tarkka IM, Sivenius J. Effects of intensive gait‐oriented physiotherapy during early acute phase of stroke. Journal of Rehabilitation Research and Development 2007;44(5):637‐48. [DOI] [PubMed] [Google Scholar]; Peurala SH, Pitkanen K, Sivenius J, Tarkka IM. Body‐weight supported gait trainer exercises with or without functional electrical stimulation improves gait in patients with chronic stroke. Neurorehabilitation & Neural Repair 2006;20(1):98. [Google Scholar]; Sivenius J, Peurala SH. Gait trainer vs traditional physiotherapy in acute stroke. ClinicalTrials.gov2007.
- Ploughman M, McCarthy J, Bosse M, Sullivan HJ, Corbett D. Does treadmill exercise improve performance of cognitive or upper‐extremity tasks in people with chronic stroke? A randomized cross‐over trial. Archives of Physical Medicine and Rehabilitation 2008;89(11):2041‐7. [DOI] [PubMed] [Google Scholar]
- Rimmer JH, Riley B, Creviston T, Nicola T. Exercise training in a predominantly African‐American group of stroke survivors. Medicine & Science in Sports & Exercise 2000;32:1990‐6. [DOI] [PubMed] [Google Scholar]
- Salbach NM, Mayo NE, Robichaud‐Ekstrand S, Hanley JA, Richards CL, Wood‐Dauphinee S. The effect of a task‐oriented walking intervention on improving balance self‐efficacy poststroke: a randomized, controlled trial. Journal of the American Geriatrics Society 2005;53(4):576‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Salbach NM, Mayo NE, Wood‐Dauphinee S, Hanley JA, Richards CL, Cote R. A task‐orientated intervention enhances walking distance and speed in the first year post stroke: a randomized controlled trial. Clinical Rehabilitation 2004;18(5):509‐19. [DOI] [PubMed] [Google Scholar]
- Saltuari L. Efficiency of Lokomat training in stroke patients. Neurologie und Rehabilitation 2004;10(4):169‐78. [Google Scholar]
- Schwartz I, Katz‐Leurer M, Fisher I, Sajin A, Shochina M, Meiner Z. The effectiveness of early locomotor therapy in patients with first CVA. Proceedings of the Collaborative Evaluation of Rehabilitation in Stroke Across Europe (CERISE) Congress; 2006 February 10‐11. 2006. [Google Scholar]; Schwartz I, Sajin A, Fisher I, Neeb M, Shochina M, Katz‐Leurer M, et al. The effectiveness of locomotor therapy using robotic‐assisted gait training in subacute stroke patients: a randomized controlled trial. PM and R 2009;1(6):516‐23. [DOI] [PubMed] [Google Scholar]
- Shafshak TS. Central neuroplasticity and upper limbs functional outcome following repetitive lower limb locomotor training in stroke patients. PM and R 2012;10:S298. [Google Scholar]; Shahine EM, Shafshak TS. Central neuroplasticity and upper limbs functional outcome following repetitive lower limb locomotor training in chronic stroke patients. European Journal of Neurology 2012;19:570. 21999175 [Google Scholar]
- Sullivan KJ, Knowlton BJ, Dobkin BH. Step training with body weight support: effect of treadmill speed on practice paradigms on poststroke locomotor recovery. Archives of Physical Medicine & Rehabilitation 2002;83:683‐91. [DOI] [PubMed] [Google Scholar]; Sullivan KJ, Knowlton BJ, Dobkin BH. The effect of varying treadmill speed to enhance overground walking in patients with chronic stroke. Stroke 2000;31(1):292. [Google Scholar]; Sullivan KJ, Knowlton BJ, Dobkin H. Stroke severity and treadmill training as predictors of locomotor recovery in chronic stroke. Neurology Report 2000;24(5):173‐4. [Google Scholar]
- Li LSW. Motor training after stroke. Journal of Neurology, Neurosurgery, and Psychiatry 2005;76(4):605‐6. [Google Scholar]; Li LSW, Tong RKY, Ng MFW, So EFM. Gait training by mechanical gait trainer and functional electrical stimulation for subacute stroke patients: a randomised controlled study. Proceedings of 3rd World Congress of the International Society of Physical and Rehabilitation Medicine ‐ ISPRM; 2005 10‐14 April; Sao Paulo, Brazil. 2005. [Google Scholar]; Li LSW, Tong RYU, Ng MFW, So EFM. Effectiveness of gait trainer in stroke rehabilitation. Journal of the Neurological Sciences 2005;238 Suppl 1:S81. [Google Scholar]; Ng MF, Tong RK, Li LS. A pilot study of randomized clinical controlled trial of gait training in subacute stroke patients with partial body‐weight support electromechanical gait trainer and functional electrical stimulation: six‐month follow‐up. Stroke 2008;39(1):154‐60. [DOI] [PubMed] [Google Scholar]; Ng MFW, Tong KY, So EFM, Li LSW. The therapeutic effect of electromechanical gait trainer and functional electrical stimulation for patients with acute stroke. Neurorehabilitation & Neural Repair 2006;20(1):97. [Google Scholar]; Tong R, Ng M, Li L. The effect of electromechanical gait trainer combined with functional electrical stimulation for subacute stroke rehabilitation. International Journal of Stroke 2008;3(Suppl):357. [Google Scholar]; Tong RK, Ng MF, Li LS. Effectiveness of gait training using an electromechanical gait trainer, with and without functional electric stimulation, in subacute stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2006;87(10):1298‐304. [DOI] [PubMed] [Google Scholar]
- Trueblood PR. Partial body weight treadmill training in persons with chronic stroke. Neurorehabilitation 2001;16:141‐53. [PubMed] [Google Scholar]
- Tsai YC, Yang S, Chern JS. Effect of backward‐walk training in proving the balance and weight shifting skill of stroke patients. Stroke 2004;35(6):e319. [Google Scholar]
- Tsang MYC, Eng JJ, Tang A, Jue J, Gin KG, Nair P, et al. Impact of aerobic exercise training on cardiac function in stroke patients: a prospective randomized controlled study. Journal of the American Society of Echocardiography 2012;25(6):B11. [Google Scholar]
- Bardeleben A, Schaffrin A, Werner C, Hesse S. Treadmill therapy with and without physiotherapy after stroke: a randomized trial (abstract) [Laufbandtherapie mit und ohne physiotherapie nach schlaganfall: eine randomisierte studie]. Proceedings of the Deutsche Gesellschaft fur Neurologische Rehabilitation Annual Conference; 2000 November 23‐25. 2000. [Google Scholar]; Hesse S, Lucke D, Bardeleben A. [Chronic nonambulatory hemiparetic subjects: effects of a treadmill training alone and in combination with regular physiotherapy]. Proceedings of the 2nd World Congress in Neurological Rehabilitation. Toronto, Canada, 1999. [Google Scholar]; Hesse S, Lucke D, Bardeleben A. Chronic nonambulatory hemiplegic subjects: effects of a treadmill training alone and in combination with regular physiotherapy (abstract). Neurorehabilitation & Neural Repair 1999;13(1):54. [Google Scholar]; Werner C, Bardeleben A, Mauritz KH, Kirker S, Hesse S. Treadmill training with partial body weight support and physiotherapy in stroke patients: a preliminary comparison. European Journal of Neurology 2002;9:639‐44. [DOI] [PubMed] [Google Scholar]
- Patten C. Internally versus externally guided body weight supported treadmill training [BWSTT] for locomotor recovery post‐stroke. Stroke Trials Directory, Internet Stroke Center: www.strokecenter.org/trials/2005. ; Westlake KP, Patten C. Pilot study of Lokomat versus manual‐assisted treadmill training for locomotor recovery post‐stroke. Journal of NeuroEngineering and Rehabilitation 2009;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagura H, Hatakenaka M, Miyai I. Does therapeutic facilitation add to locomotor outcome of body weight‐supported treadmill training in nonambulatory patients with stroke? A randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2006;87(4):529‐35. [DOI] [PubMed] [Google Scholar]
- Yang S, Hwang W‐H, Tsai Y‐C, Liu F‐K, Hsieh L‐F, Chern J‐S. Improving balance skills in patients who had stroke through virtual reality treadmill training. American Journal of Physical Medicine & Rehabilitation 2011;90(12):969‐78. [DOI] [PubMed] [Google Scholar]; Yang YR, Tsai MP, Chuang TY, Sung WH, Wang RY. Virtual reality‐based training improves community ambulation in individuals with stroke: a randomized controlled trial. Gait & Posture 2008;28(2):201‐6. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Al‐Jarrah MD, Obaidat S. The impact of combined balance and treadmill exercise training on functional outcomes after chronic stroke. European Journal of Neurology 2011;18:440. [Google Scholar]
- Baer G. Treadmill training and sub‐acute stroke: a phase II feasibility study (abstract). Proceedings of the 3rd UK Stroke Forum Conference; Harrogate, UK. 2008:21. [Google Scholar]; Baer G, Dennis M, Pitman J, Salisbury L, Smith M. Does treadmill training improve walking after stroke ‐ the long term follow‐up from a phase II randomised controlled trial. International Journal of Stroke 2009;4(Suppl 2):8. [Google Scholar]; Salisbury L, Baer G, Dennis M, Pitman J, Smith M. Does treadmill training affect activities of daily living or quality of life after stroke? Results of a phase II randomised controlled trial. International Journal of Stroke 2009;4 Suppl 2:38. 19236496 [Google Scholar]; Smith M, Baer G, Dennis M, Pitman J, Salisbury L. How feasible is the delivery of treadmill training early after stroke within the NHS: findings of a phase II randomised controlled trial. International Journal of Stroke 2009;4 Suppl 2:38. 19236496 [Google Scholar]; Statt TC. Treadmill training in sub‐acute stroke: report of an ongoing phase II feasibility study of a complex intervention. Proceedings of the UK Stroke Forum Conference 2007; Harrogate, UK. 2007. [Google Scholar]
- Bartloff J, Bitting J, Lueke A, Sbertoli C, Sofen L, Walsh J, et al. After‐effects of slow isokinetic walk speed training on self‐selected gait velocity in persons with chronic post‐stroke hemiparesis. Journal of Neurologic Physical Therapy2009, issue 4.
- DePaul VG, Wishart LR. A comparison of two intensive walking training interventions in community dwelling individuals with history of stroke. ClinicalTrials.gov2006. ; DePaul VG, Wishart LR, Richardson J, Lee TD, Thabane L. Varied overground walking‐task practice versus body‐weight‐supported treadmill training in ambulatory adults within one year of stroke: a randomized controlled trial protocol. BMC Neurology2011; Vol. 11:129. [DOI] [PMC free article] [PubMed]
- Enhanced motor recovery using serotonergic agents in stroke. ClinicalTrials.gov/show/NCT01751854 (accessed 2 September 2013).
- Ivey FM, Hafer‐Macko CE, Ryan AS, Macko RF. Impaired leg vasodilatory function after stroke: adaptations with treadmill exercise training. Stroke 2010;41(12):2913‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey FM, Ryan AS, Hafer‐Macko CE, Macko RF. Improved cerebral vasomotor reactivity after exercise training in hemiparetic stroke survivors. Stroke 2011;42(7):1994‐2000. [DOI] [PubMed] [Google Scholar]
- Testing adaptive physical activity in stroke. ClinicalTrials.gov/show/NCT01042990 (accessed 2 September 2013). ; Michael KM. Combined adaptive physical activity and treadmill training in stroke. Stroke 2011;42(3):e355. [Google Scholar]
- Mokrusch T, Busch K. Treadmill training with functional electric stimulation in stroke patients. Benefits compared with Bobath physiotherapy. Neurologie und Rehabilitation 2004;10(4):187‐216. [Google Scholar]
- Muller F, Heller S, Krewer C, Husemann B, Koenig E. Effective gait training on the treadmill and the Lokomat: comparison of achievable training time and speed. Neurologie und Rehabilitation 2004;10(4):187‐216. [Google Scholar]
- Shintani M, Nagai S, Shimoda S, Wada Y, Sonoda S. High‐speed treadmill exercise for stroke hemiplegics. Proceedings of 3rd World Congress of the International Society of Physical and Rehabilitation Medicine ‐ ISPRM; Sao Paulo, Brazil. 2005. [Google Scholar]
- Srivastava A, Gupta A, Murali T, Taly AB. Body‐weight‐supported treadmill training in retraining gait among chronic stroke survivors: Randomized controlled study. PM and R 2011;1:S344‐5. [DOI] [PubMed] [Google Scholar]; Srivastava A, Gupta A, Taly A, Kumar S, Murali T. Role of body weight supported treadmill training in retraining gait after stroke: randomized controlled study. International Journal of Stroke 2008;3 Suppl:356. [Google Scholar]
- Stephenson J, Maitland M, Beckstead J. Body weight support treadmill training compared with PNF training in persons with chronic stroke. Journal of Neurologic Physical Therapy 2004;28(4):186. [Google Scholar]
- Thompson M, Medley A. Post stroke locomotor training: does type of practice make a difference?. Journal of Neurologic Physical Therapy 2006;30(4):209‐10. [Google Scholar]
- Venkadesan R, Kumar MKN. A comparative study of conventional gait training versus conventional and treadmill gait training in subacute stroke patients. Indian Journal of Physiotherapy & Occupational Therapy 2009;3(4):58‐62. [Google Scholar]
- Xu W, Zhang L‐Y, Fan J‐T. The comparison of gait rehabilitation in patients with hemiplegia by walking in water and the pneu‐weight walking therapies. Journal of Rehabilitation Medicine 2008;46 Suppl:73. [Google Scholar]
- Yang A, Su C, Lin K. Effects of long‐term exercise intervention on aerobic capacity and functional ability in stroke patients with prior coronary artery disease. Physiotherapy 2007;93 Suppl 1:s260. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- Body weight supported treadmill training vs. overground walking training in persons with chronic stroke. ClinicalTrials.gov/show/NCT01180738 (accessed 2 September 2013). ; Combs SA, Tucker L, Harmeyer A, Ertel T, Colburn D, Parameswaran AK. Body weight‐supported treadmill training vs. over‐ground walking training for persons with chronic stroke: a randomized controlled trial. Archives of Physical Medicine & Rehabilitation 2012;93(10):e36. [Google Scholar]
- Improving community walking after a stroke, a new approach. www.controlled‐trials.com/ISRCTN50586966 (accessed 2 September 2013).
- Ankle robotics training after stroke. ClinicalTrials.gov/show/NCT01337960 (accessed 2 September 2013).
- Visual cues for gait training post‐stroke. ClinicalTrials.gov/show/NCT01600391 (accessed 2 September 2013).
- Very intensive early walking in stroke. ClinicalTrials.gov/show/NCT01789853 (accessed 2 September 2013).
- Kilbreath SL. PBWST (Partial body‐weight supported treadmill training) and muscle power training after sub‐acute stroke. ClinicalTrials.gov/ct2/show/NCT001080302006.
- Lennihan L, Wootten ME, Wainwright M, Tenteromano L, McMahon D, Cotier J. Treadmill with partial body‐weight support versus conventional gait training after stroke. Archives of Physical Medicine & Rehabilitation 2003;84(9):A5. [Google Scholar]
- Exercise for sub‐acute stroke patients in Jamaica. ClinicalTrials.gov/show/NCT01392391 (accessed 2 September 2013).
- McDonnell M. Aerobic exercise to improve cardiovascular and neurological health outcomes in the chronic stroke population. Australian New Zealand Clinical Trials Registry (ANZCTR)2009.
- Robot walking rehabilitation in stroke patients. ClinicalTrials.gov/show/NCT01678547 (accessed 2 September 2013).
- High intensity interval training in chronic stroke patients. ClinicalTrials.gov/show/NCT01777113 (accessed 2 September 2013).
- Task‐oriented training for stroke: impact on function mobility. ClinicalTrials.gov/show/NCT01322607 (accessed 2 September 2013).
- Zielke DR. The effect of partial body weight supported treadmill training on gait rehabilitation in early acute stroke patients: preliminary data. Journal of Neurologic Physical Therapy 2003;27(4):177. [Google Scholar]
Additional references
- Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Research 1987;412:84‐95. [DOI] [PubMed] [Google Scholar]
- Bobath B. Adult Hemiplegia: Evaluation and Treatment. 2nd Edition. London: Butterworth‐Heinemann, 1990. [Google Scholar]
- Brunnstrom S. Movement Therapy in Hemiplegia. New York: Harper and Row, 1970. [Google Scholar]
- Carr JH, Shepherd RB, Nordholm L, Lynne D. Investigation of a new motor assessment scale for stroke patients. Physical Therapy 1985;65:175‐80. [DOI] [PubMed] [Google Scholar]
- Carr JH, Shepherd RB. Neurological Rehabilitation: Optimizing Motor Performance. Oxford: Butterworth‐Heinemann, 1998. [Google Scholar]
- Charalambous CC, Bonilha HS, Kautz SA, Gregory CM, Bowden MG. Rehabilitating walking speed poststroke with treadmill‐based interventions: a systematic review of randomized controlled trials. Neurorehabilitation and Neural Repair 2013 Jun 13 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Collen FM, Wade DT, Robb GF, Bradshaw CM. The Rivermead Mobility Index: a further development of the Rivermead Motor Assessment. International Disability Studies 1991;13:50‐4. [DOI] [PubMed] [Google Scholar]
- Collin C, Wade DR, Davies S, Horne V. The Barthel ADL Index: a reliability study. International Disability Studies 1988;10:61‐3. [DOI] [PubMed] [Google Scholar]
- Haan R, Aaronson N, Limburg M, Hewer RL, Crevel H. Measuring quality of life in stroke. Stroke 1993;24:320‐7. [DOI] [PubMed] [Google Scholar]
- Enright PL, Sherrill DL. Reference equations for the six‐minute walk in healthy adults. American Journal of Respiratory and Critical Care Medicine 1998;158:1384‐7. [DOI] [PubMed] [Google Scholar]
- Finch L, Barbeau H. Hemiplegic gait: new treatment strategies. Physiotherapy Canada 1985;38:36‐41. [Google Scholar]
- Flansbjer UB, Holmbäck AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. Journal of Rehabilitation Medicine 2005;37(2):75‐82. [DOI] [PubMed] [Google Scholar]
- French B, Thomas LH, Leathley MJ, Sutton CJ, McAdam J, Forster A, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006073.pub2] [DOI] [PubMed] [Google Scholar]
- Goff B. Appropriate afferent stimulation. Physiotherapy 1969;55:9‐17. [PubMed] [Google Scholar]
- Guyatt GH, Pugsley SO, Sullivan MJ, Thompson PJ, Berman L, Jones NL, et al. Effect of encouragement on walking test performance. Thorax 1984;39:818‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton BB, Laughlin JA, Fiedler RC, Granger CV. Interrater reliability of the 7‐level functional independence measure (FIM). Scandinavian Journal of Rehabilitation Medicine 1994;26:115‐9. [PubMed] [Google Scholar]
- Hatano S. Experience from a multi‐centre stroke register: a preliminary report. Bulletin of the World Health Organization 1976;54:541‐53. [PMC free article] [PubMed] [Google Scholar]
- Hesse S, Werner C. Poststroke motor dysfunction and spasticity: novel pharmacological and physical treatment strategies. CNS Drugs 2003;17(15):1093‐107. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Holden MK, Gill KM, Magliozzi MR, Nathan J, Peihl‐Baker L. Clinical gait assessment in the neurologically impaired: reliability and meaningfulness. Physical Therapy 1984;64(1):35‐40. [DOI] [PubMed] [Google Scholar]
- Knott M, Voss DE. Proprioceptive Neuromuscular Facilitation. New York: Harper and Row, 1968. [Google Scholar]
- Langhorne P, Pollock A. What are the components of effective stroke unit care?. Age & Ageing 2002;31:365‐71. [DOI] [PubMed] [Google Scholar]
- Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurology 2009;8(8):741‐54. [DOI] [PubMed] [Google Scholar]
- Manning CD, Pomeroy VM. Effectiveness of treadmill retraining on gait of hemiparetic stroke patients. Physiotherapy 2003;89:337‐49. [Google Scholar]
- McAuley L, Pham B, Tugwell P, Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta‐analyses?. Lancet 2000;356:1228‐31. [DOI] [PubMed] [Google Scholar]
- Mehrholz J, Elsner B, Werner C, Kugler J, Pohl M. Electromechanical‐assisted training for walking after stroke. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD006185.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S, Schurr K, Moseley A, Wales A, Herbert RD. Observation and analysis of hemiplegic gait. II: swing phase. Australian Journal of Physiotherapy 1993;39:271‐7. [DOI] [PubMed] [Google Scholar]
- Moseley A, Wales A, Herbert RD, Schurr K, Moore S. Observation and analysis of hemiplegic gait. I: stance phase. Australian Journal of Physiotherapy 1993;39:251‐6. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability‐adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2197‐223. [DOI] [PubMed] [Google Scholar]
- Pohjasvaara T, Erkinjuntti T, Vataja R, Kaste M. Comparison of stroke features and disability in daily life in patients with ischemic stroke aged 55 to 70 and 71 to 85 years. Stroke 1997;28(4):729‐35. [DOI] [PubMed] [Google Scholar]
- Polese JC, Ada L, Dean CM, Nascimento LR, Teixeira‐Salmela LF. Treadmill training is effective for ambulatory adults with stroke: a systematic review. Journal of Physiotherapy 2013;59(2):73‐80. [DOI] [PubMed] [Google Scholar]
- Pollock A, Baer G, Pomeroy VM, Langhorne P. Physiotherapy treatment approaches for the recovery of postural control and lower limb function following stroke. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD001920] [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
- Reyes L (Hospital Supplies of Australia). Personal communication2000.
- Stroke Unit Trialists' Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD000197.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasell RW, Bhogal SK, Foley NC, Speechley MR. Gait retraining post stroke. Topics in Stroke Rehabilitation 2003;10:34‐65. [DOI] [PubMed] [Google Scholar]
- Peppen RP, Kwakkel G, Wood‐Dauphinee S, Hendriks HJ, Wees PJ, Dekker J. The impact of physical therapy on functional outcomes after stroke: what's the evidence?. Clinical Rehabilitation 2004;18(8):833‐62. [DOI] [PubMed] [Google Scholar]
- Wade DT. Measurement in Neurological Rehabilitation. Oxford: Oxford University Press, 1992. [PubMed] [Google Scholar]
References to other published versions of this review
- Moseley A, Stark A, Cameron I, Pollock A. Treadmill training and body weight support for walking after stroke: a systematic review. Proceedings of the 7th International Physiotherapy Congress 25‐28 May. Sydney, Australia: Australian Physiotherapy Association, 2002. [Google Scholar]
- Moseley AM, Stark A, Cameron ID, Pollock A. Treadmill training and body weight support for walking after stroke. Stroke 2003;34(12):3006. [DOI] [PubMed] [Google Scholar]
- Moseley AM, Stark A, Cameron ID, Pollock A. Treadmill training and body weight support for walking after stroke. Physiotherapy 2003;89(9):515. [DOI] [PubMed] [Google Scholar]
- Moseley AM, Stark A, Cameron ID, Pollock A. Treadmill training and body weight support for walking after stroke: a Cochrane systematic review. Proceedings of the Stroke Society of Australasia 2003 Annual Scientific Meeting 17‐19 September. Sydney, Australia: Stroke Society of Australasia, 2003. [Google Scholar]
- Moseley AM, Stark A, Cameron ID, Pollock A. Treadmill training and body weight support for walking after stroke: a Cochrane systematic review. Proceedings of the Combined Australian Capital Territory Branch and New South Wales Branch of the Australian Physiotherapy Association Mini‐Conference 3 May. Sydney, Australia: Australian Physiotherapy Association, 2003. [Google Scholar]


