Abstract
Background
Deep venous thrombosis (DVT) occurs in approximately one in 1000 adults every year, and has an annual mortality of 14.6%. In particular, iliofemoral DVT can lead to recurrent thrombosis and post‐thrombotic syndrome (PTS), a painful condition which can lead to chronic venous insufficiency, oedema, and ulceration. It causes significant disability, impaired quality of life, and economic burden. Early thrombus removal techniques have been advocated in patients with an iliofemoral DVT in order to improve vein patency, prevent valvular dysfunction, and reduce future complications, such as post‐thrombotic syndrome and venous ulceration. One such technique is pharmacomechanical thrombectomy, a combination of catheter‐based thrombectomy and catheter‐directed thrombolysis.
Objectives
To assess the effects of pharmacomechanical thrombectomy versus anticoagulation (alone or with compression stockings), mechanical thrombectomy, thrombolysis, or other endovascular techniques in the management of people with acute DVT of the iliofemoral vein.
Search methods
The Cochrane Vascular Information Specialist searched the Specialised Register (last searched December 2015) and the Cochrane Register of Studies (last searched December 2015). We searched clinical trials databases for details of ongoing or unpublished studies and the reference lists of relevant articles retrieved by electronic searches for additional citations.
Selection criteria
Randomised controlled trials in which patients with an iliofemoral deep vein thrombosis were allocated to receive pharmacomechanical thrombectomy versus anticoagulation, mechanical thrombectomy, thrombolysis (systemic or catheter directed thrombolysis), or other endovascular techniques for the treatment of iliofemoral DVT.
Data collection and analysis
At least two review authors independently assessed studies identified for potential inclusion.
Main results
We found no randomised controlled trials that met the eligibility criteria for this review. We identified one ongoing study.
Authors' conclusions
There were no randomised controlled trials that assessed the effects of pharmacomechanical thrombectomy versus anticoagulation (alone or with compression stockings), mechanical thrombectomy, thrombolysis, or other endovascular techniques in the management of people with acute DVT of the iliofemoral vein that met the eligibility criteria for this review. Further high quality randomised controlled trials are needed.
Plain language summary
Pharmacomechanical thrombectomy for iliofemoral deep vein thrombosis
Background
Deep vein thrombosis (DVT) is a condition in which a blood clot forms in the deep vein of the leg or pelvis. It affects approximately 1 in 1000 people. If it is not treated, the clot can travel in the blood, and block the arteries in the lungs. This life‐threatening condition is called a pulmonary embolism and occurs in approximately 3 to 4 in 10,000 people. Another side‐effect of DVT is post‐thrombotic syndrome (PTS), a condition in which the patient suffers pain, swelling, and changes in the skin of the leg, which can lead to an ulcer. This causes significant disability and diminished qualify of life, and is costly to the healthcare system.
One way to prevent another blood clot or PTS is to remove the clot. There are a number of ways to do this. A catheter can be inserted into the vein and the clot removed directly (mechanical thrombectomy), the clot can be broken down through the use of drugs infused into a vein in the foot or directly at the site of the clot using a catheter and X‐ray control (pharmacomechanical thrombolysis), or a combination of the two procedures. This review aimed to measure how safe and effective pharmacomechanical thrombectomy is, compared to other techniques.
Key results
There were no randomised controlled trials that met the inclusion criteria of this review (current until December 2015). We identified one ongoing study.
Quality of evidence
At present, there is a lack of randomised controlled trials that examine the comparative effectiveness and safety of pharmacomechanical thrombectomy in the management of patients with DVT.
Conclusion
Further research is required before conclusions can be made.
Background
Description of the condition
Deep vein thrombosis (DVT) is a condition in which a blood clot forms in the deep vein of the leg or pelvis. It is a common condition, resulting in significant morbidity and mortality. Deep vein thrombosis occurs in approximately one in 1000 adults annually (White 2003), and has an associated one year mortality of 14.6% (Ageno 2006). In particular, untreated proximal DVT or iliofemoral DVT carries an approximate 50% risk of developing into a symptomatic pulmonary embolism within three months, 10% of which carries a risk of mortality within one hour of symptom onset (Kearon 2003). A major cause of DVT‐related morbidity is post‐thrombotic syndrome (PTS), which is characterised by pain, chronic venous insufficiency, oedema, and associated skin changes, and can progress to venous ulceration, despite conventional treatment. Post‐thrombotic syndrome typically occurs within two years of a DVT, and is reported to occur in around 30% to 60% of patients, with 10% suffering from severe PTS (Prandoni 2009). It causes significant disability, impaired quality of life and economic burden (Access Economics Pty Limited 2008).
Deep vein thrombosis can be divided anatomically, into proximal DVT, affecting either the iliofemoral or femoral vein and distal DVT, affecting the popliteal vein or more distally (Jenkins 2011). Iliofemoral DVT occludes both draining venous systems of the lower limb, that of the femoral vein and the superficial femoral vein, so there is decreased room for collateralisation of venous drainage (Jenkins 2011). Consequently, iliofemoral DVT has an increased propensity for complications such as pulmonary embolism and PTS, compared to popliteal DVT.
Standard treatment of DVT currently involves immediate intravenous anticoagulation with unfractionated heparin, or more recently, with the use of low molecular weight heparin (Merli 2008). Early ambulation and graduated compression stockings (30 mmHg to 40 mmHg) are also standard treatment and have been shown to be particularly effective in preventing the development of PTS (Musani 2010). A meta‐analysis indicated a 0.54 relative risk reduction in PTS with the use of graduated compression stockings post‐DVT (Musani 2010). A randomised controlled trial by Prandoni 2004 determined that the incidence of PTS in the control group versus the elastic stockings group was 40.0% versus 21.1% after six months, 46.7% versus 22.2% after one year, and 49.1% versus 24.5% after two years. However, the SOX trial, a randomised placebo‐controlled trial, showed that stockings offered no benefit in the prevention of PTS (Kahn 2014).
The goal of this current treatment strategy is to prevent thrombus propagation, embolisation, and recurrence of venous thromboembolism during both the early and later course of the disease. However, anticoagulation lacks fibrinolytic activity, so there is no attempt to remove or reduce existing thrombus load. In ultrasound based studies, complete resolution of DVT at one year was only around 50% (Kearon 2003). Furthermore, the one‐year incidence of PTS is around 25% following first‐time DVT, despite treatment with anticoagulation and elastic compression bandages (Pappy 2010).
Iliofemoral DVT in particular, carries a significant risk of both PTS and recurrent venous thromboembolism. Therefore, early thrombus removal techniques have been advocated in these select patient groups, in order to improve vein patency, preventing valvular dysfunction, and reduce future complications, such as PTS and venous ulceration.
Description of the intervention
Mechanical thrombectomy
Thrombectomy for DVT can be performed through either an open surgical approach or a percutaneous endovascular approach. Open thrombectomy is reserved for life‐ or limb‐threatening DVT, and is seldom used, as it is much more invasive than endovascular techniques, and is associated with a disproportionately high incidence of rethrombosis (Suwanabol 2013).
Percutaneous mechanical thrombectomy modalities are a relatively new intervention available for treating DVT (Jenkins 2011). A number of different percutaneous devices exist, however, they all reduce clot burden through either suction, rotation, rheolysis, ultrasound, or a combination there‐of.
Pharmacological thrombolysis
Thrombolysis has been used as a technique to remove clot burden, and can either be performed systemically, or locally in a procedure called catheter‐directed thrombolysis (Jenkins 2011). Systemic thrombolysis has been shown to enhance clot removal and prevent propagation of thrombosis. However, it carries an increased risk of major bleeding episodes, and therefore, is rarely used in the setting of an acute DVT. Catheter‐directed thrombolysis is a percutaneous technique involving the delivery of thrombolytics by an infusion catheter, directly to the venous thrombus, which allows clot lysis, whilst avoiding the major bleeding risks associated with systemic thrombolysis. Randomised controlled trials have demonstrated the feasibility and effectiveness of the technique in reducing PTS whilst minimising major bleeding risks to 1.7%, compared to 2% in the use of anticoagulation‐only groups (Enden 2009; Watson 2014).
Pharmacomechanical thrombectomy
Some medical devices combine the use of both percutaneous mechanical thrombectomy and thrombolysis, using the same endovascular techniques. This combination of catheter‐based thrombectomy and catheter‐directed thrombolysis is referred to as pharmacomechanical thrombectomy.
Pharmacomechanical thrombectomy, when compared to catheter‐directed thrombolysis alone, has been shown to result in similar levels of clot removal, but with significant reduction in use of hospital resources, catheterisation, infusion time, and total dose of thrombolytic, hence, potentially reducing adverse bleeding events as well (Jenkins 2011).
Percutaneous endovenous intervention refers to a variety of endovascular techniques used for thrombus removal. It includes any one or more endovascular techniques, such as thrombectomy, balloon venoplasty, stenting, and low‐dose thrombolysis, and has been shown to reduce DVT and PTS recurrence (TORPEDO 2012).
How the intervention might work
Evidence suggests that early removal of the thrombus with thrombolysis can reduce the incidence of post‐thrombotic syndrome, and improve venous haemodynamics (Elliot 1979; Plate 1990; Turpie 1990). In addition, mechanical thrombectomy facilitates a more rapid removal of the thrombus, improving venous patency, decreasing venous hypertension, and ultimately, preventing valvular dysfunction and the development of PTS (Suwanabol 2013).
Combining both mechanical thrombectomy with thrombolysis in pharmacomechanical thrombectomy can reduce the required dose of thrombolytic agent used compared to a thrombolytic technique alone, reducing the bleeding risks associated with either catheter‐directed thrombolysis or systemic thrombolysis, whilst conferring the immediate benefits of a mechanical reduction in clot load (Jenkins 2011).
Why it is important to do this review
In addition to anticoagulation, thrombectomy, catheter‐directed thrombolysis and pharmacomechanical thrombectomy are used as techniques to prevent thrombus propagation and thrombus removal, and to decrease the risk of both pulmonary embolism and PTS (Jenkins 2011). This review examined the evidence supporting the use of pharmacomechanical thrombectomy for iliofemoral DVT.
Objectives
To assess the effects of pharmacomechanical thrombectomy versus anticoagulation (alone or with compression stockings), mechanical thrombectomy, thrombolysis, or other endovascular techniques in the management of people with acute deep vein thrombosis of the iliofemoral vein.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs) that compared pharmacomechanical thrombectomy versus anticoagulation (alone or in combination with compression stockings), mechanical thrombectomy, thrombolysis (systemic or catheter‐directed thrombolysis), or other endovascular techniques for the treatment of iliofemoral deep vein thrombosis (DVT).
Types of participants
We included patients of all ages with DVT confirmed by objective testing, such as venography or duplex ultrasonography. We only considered DVTs at the iliofemoral level. We excluded patients with recurrent venous thromboembolism (VTE) or post‐thrombotic syndrome (PTS), and those with treatment commencing after 21 days. If we were unable to clarify the anatomical level of DVT after interrogation of the data in a study, we did not use the results in our data analysis.
Types of interventions
We included any pharmacomechanical thrombectomy, defined as a combination of locally delivered thrombolytic agent used in conjunction with mechanical thrombectomy. Patients who received any type of thrombolysis or thrombectomy intervention also received standard DVT management, which included anticoagulation and the use of compression stockings.
We considered the following comparisons:
pharmacomechanical thrombectomy versus anticoagulation alone;
pharmacomechanical thrombectomy versus anticoagulation and compression stocking use;
pharmacomechanical thrombectomy versus mechanical thrombectomy;
pharmacomechanical thrombectomy versus thrombolysis;
pharmacomechanical thrombectomy versus other endovascular techniques (including balloon venoplasty and stenting).
Types of outcome measures
For all outcomes, we had planned to collect data for an early (up to one month), intermediate (one month to two years), and a long‐term period (more than two years).
Primary outcomes
Post‐thrombotic syndrome: (including venous ulceration rates, as defined by the Villalta scale; Prandoni 1992)
Any improvement in venous patency (assessed by objective measures, such as venography, where pre‐ and post‐comparative data on the degree of restoration of the lumen were available)
-
Major bleeding (as defined by the International Society on Thrombosis and Haemostasis (ISTH); Schulman 2005):
Fatal bleeding
Symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial, or intramuscular with compartment syndrome
Bleeding causing a fall in haemoglobin level of 20 g/L (1.24 mmol/L) or more, or leading to transfusion of two or more units of whole blood or red cells
Any combination of points a to c
Secondary outcomes
Recurrent DVT
Pulmonary embolism
Mortality (all cause and pulmonary embolism‐related)
Stroke, in particular, haemorrhagic stroke (preferably documented by objective means such as a computerised tomography (CT) scan or autopsy
Venous function (assessed by duplex ultrasound or other objective means, such as foot volumetry or ambulatory venous pressure measurements)
Time in hospital
Quality of life
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials:
The Cochrane Vascular Specialised Register (December 2015);
The Cochrane Central Register of Controlled Trials (CENTRAL (2015, Issue 11)) via The Cochrane Register of Studies Online.
See Appendix 1 for details of the search strategy used to search CENTRAL.
The Cochrane Vascular Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE Ovid, Embase Ovid, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals, and conference proceedings that have been searched, and the search strategies used, are described in the Specialised Register section of the Cochrane Vascular module in the Cochrane Library (www.cochranelibrary.com).
The CIS also searched the following trial databases for details of ongoing and unpublished studies, using the terms thrombectomy and thrombosis:
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (apps.who.int/trialsearch/);
ClinicalTrials.gov (clinicaltrials.gov/);
ISRCTN Register (www.isrctn.com/)
Searching other resources
We searched citations within identified studies. We also contacted authors of relevant papers by email, to identify any unpublished RCTs.
Data collection and analysis
Selection of studies
Two review authors (LR and AB or OM) independently reviewed the results of all searches and identified any article that was eligible, given a reference to thrombectomy for DVT. The two review authors discussed each study to confirm eligibility for inclusion in the systematic review; those that did not fulfil the criteria as described in Criteria for considering studies for this review were excluded, and the reasons for exclusion were described in the review. Disagreements were resolved by consensus.
Data extraction and management
Two review authors (LR, AB) had intended to independently extract from each study, information about the study characteristics, participants, interventions, duration of follow‐up, and outcome parameters, using standardised forms. Where available, we had intended to extract data on the following items:
Study design.
Quality items.
Number of study patients.
Participants, including: age, sex, length of clot, diagnosis (clinical or ultrasound), presence of pulmonary embolism prior to treatment, presence of phlegmasia, and co‐morbidities.
Interventions, including: thrombectomy device used, adverse events (major and minor), dose and delivery of thrombolytics, length of stay in hospital, and veins treated.
Outcome measures, including: PTS, venous patency and valve competency, major bleeding, recurrent DVT, pulmonary embolism, mortality, stroke, venous function, time in hospital, and quality of life.
Length of follow‐up.
Assessment of risk of bias in included studies
Two review authors (LR, AB) had intended to independently assess the design and execution of each study according to the following criteria: random sequence generation, allocation concealment of treatment, blinding of participants, personnel, and outcomes, incomplete outcome data, selective outcome reporting, and other sources of bias in accordance with Cochrane's tool for assessing risk of bias (Higgins 2011). We had intended to judge the studies as either low risk of bias, high risk of bias or, unclear (due to either lack of information or uncertainty over the potential for bias). We had planned to resolve disagreements by consensus between the two review authors, and involve a third review author (OM) if necessary.
Measures of treatment effect
We had intended to assess dichotomous data using risk ratio (RRs) with 95% confidence intervals (CIs). We had planned to analyse continuous outcomes using mean difference (MDs) with 95% CIs where the scales were the same, and where scales were different but outcome measured was the same, we had planned to use the standardised mean difference (SMD) with 95% CIs.
Unit of analysis issues
We did not include cross‐over trials. The individual patient was the unit of the analysis.
Dealing with missing data
Where information was missing, we had planned to contact the authors of the relevant study. If unsuccessful, we had intended to exclude the data from the meta‐analysis, but report it in the review. We had planned to include outcome measures only if it had been the intention of the study authors to perform the necessary assessments in all randomised patients. If less than 50% of the patients in a study had an acceptable follow‐up for a particular outcome measure, due to the associated high risk of attrition bias, we had planned to not report the results of this outcome measure.
Assessment of heterogeneity
If the included studies were comparable with regard to age, sex, treatment, and outcome definitions, we had planned to perform a pooled analysis. We had intended to assess heterogeneity with the use of forest plots and by a formal statistical test for heterogeneity, i.e. the I² statistic. Substantial heterogeneity was defined as I² greater than 50% (Higgins 2011). We had planned to explore possible causes of heterogeneity, and take appropriate measures.
Assessment of reporting biases
If more than ten studies had been included in a meta‐analysis, we had planned to construct a funnel plot to graphically ascertain the existence of publication bias (Higgins 2011).
Data synthesis
We had planned to enter data into the Cochrane software, Review Manager 2014, and analyse them according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We had intended to use a fixed‐effect model if we found no substantial heterogeneity, and a random‐effects model if we found heterogeneity (I² greater than 50%).
Subgroup analysis and investigation of heterogeneity
We had planned to perform subgroup analysis based on systemic thrombolysis and catheter‐directed thrombolysis, to determine the effect it may have had on outcome and possible heterogeneity between studies. We also had intended to examine heterogeneity for these by visual inspection of forest plots and the I² statistic.
Sensitivity analysis
We had planned to perform a sensitivity analysis by excluding studies that were at high risk of bias.
Summary of findings
We had planned to present the main findings of the review results concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the primary outcomes (Types of outcome measures) in a 'Summary of findings' table, according to the GRADE principles as described by Higgins 2011 and Atkins 2004. Since we had planned to assess different comparisons of interventions, we had planned to develop a 'Summary of findings' table for each comparison using the GRADEpro (GRADEproGDT) software to assist in the preparation of the 'Summary of findings' table.
Results
Description of studies
Results of the search
See Figure 1
1.
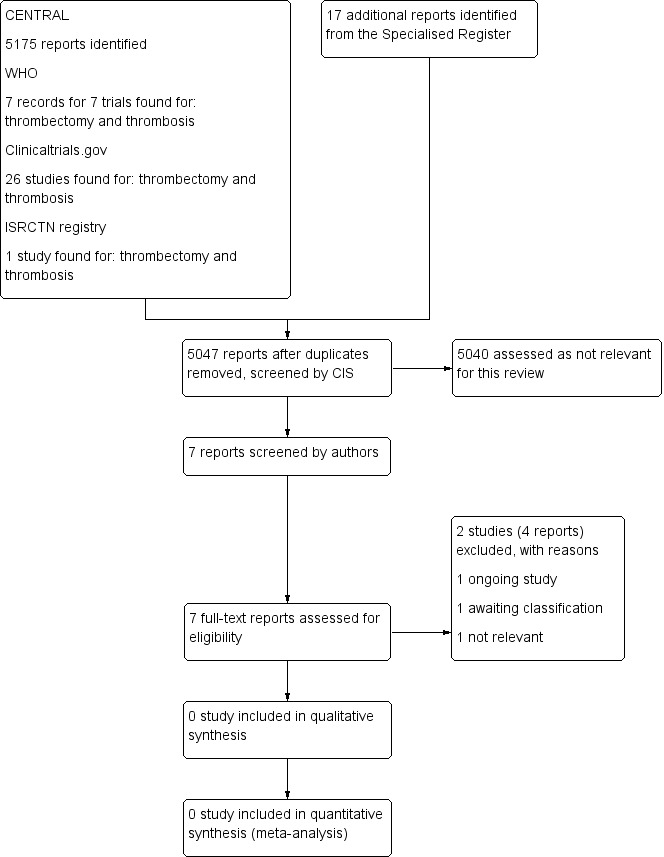
Study flow diagram.
Included studies
We found no randomised controlled trials that met the eligibility criteria for this review.
Excluded studies
See Characteristics of excluded studies
We excluded two studies. One study examined percutaneous aspiration thrombectomy versus medical treatment only (Cakir 2014), and the second study was not a randomised controlled trial (Srinivas 2014).
Ongoing studies and studies awaiting classification
We identified one eligible ongoing study (NCT02414802). This study is currently enrolling participants.
We identified one study that appeared to meet the inclusion criteria (TORPEDO 2012). However, on examination of the TORPEDO 2012 trial, it was clear that not all patients had an iliofemoral DVT, and not all were treated with pharmacomechanical thrombectomy. We contacted the authors of this trial to obtain the data on iliofemoral DVT patients stratified by pharmacomechanical thrombectomy, but to date, we have not had a response. If obtained, we hope to include this data in future updates of this review.
Risk of bias in included studies
As we identified no eligible completed studies, it was not possible to assess risk of bias.
Effects of interventions
We found no randomised controlled trials that met the eligibility criteria for this review.
Discussion
Summary of main results
We found no randomised controlled trials investigating the effects of pharmacomechanical thrombectomy versus anticoagulation (alone or with compression stockings), mechanical thrombectomy, thrombolysis, or other endovascular techniques in the management of people with acute deep venous thrombosis (DVT) of the iliofemoral vein that met the eligibility criteria for this review.
Overall completeness and applicability of evidence
We found no randomised controlled trials that met the eligibility criteria for this review.
At present, there is a lack of randomised controlled trials on the effectiveness and safety of pharmacomechanical thrombectomy in the management of people with iliofemoral DVT.
Quality of the evidence
We found no randomised controlled trials that met the eligibility criteria for this review; therefore, we were unable to assess the quality of the evidence.
Potential biases in the review process
None of the review authors have any commercial or other conflict of interest. The Cochrane Vascular Information Specialist performed a comprehensive search of the literature, and review authors selected studies in accordance with recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion.
Agreements and disagreements with other studies or reviews
To date, no other systematic review has examined pharmacomechanical thrombectomy for the management of people with acute DVT of the iliofemoral vein.
One study compared the effectiveness of catheter‐directed thrombolysis in conjunction with assisted mechanical thrombolysis versus routine anticoagulation (unfractionated or low molecular weight heparin) in patients with a lower limb DVT (Srinivas 2014). The study was not included in this review as it was not randomised. Results showed that after six months, iliofemoral patency was found in 20 out of 25 patients (80%) in the intervention group versus seven out of 26 patients (23%) treated with anticoagulation alone (P < 0.01). Post‐thrombotic syndrome was diagnosed in 5 patients (20%) in the intervention group versus 19 (77%) in the anticoagulation alone group (P < 0.01). Pulmonary embolism occurred in 4 patients (15%) in the intervention group versus 6 (21%) of the patients who received anticoagulation. Death due to pulmonary embolism occurred in two patients in each treatment group. In the intervention group, 4 patients (15%) also developed anaemia, and required a blood transfusion. There was no difference in prolonged hospital stay between the catheter‐directed thrombolysis in conjunction with assisted mechanical thrombolysis group (mean 5 days, standard deviation (SD) 1.3 days) and the routine anticoagulation arm (mean 4.8 days, SD 1.4 days).
A recent review discussed the three main types of pharmacomechanical thrombectomy devices (rotating motorised systems, rheolytic instruments and ultrasound enhanced devices; Blackwood 2016). The devices were compared for success rate, clinical patency at follow‐up, and complications, based on 3077 participants in seven studies. The studies varied in size from 40 to 2204 participants. Patency rates varied from 65% to 98%, major bleeding complications ranged from 0% to 11%, and the rate of post‐thrombotic syndrome ranged from 3% to 48%. However, the quality of the studies was extremely varied. Only two randomised controlled trials were included, only one of which appeared to meet the inclusion criteria for this review (TORPEDO 2012). However, on examination of the TORPEDO 2012 trial, it was clear that not all patients had an iliofemoral DVT, and not all were treated with pharmacomechanical thrombectomy. We contacted the authors of this trial to obtain the data on iliofemoral DVT patients stratified by pharmacomechanical thrombectomy but, to date, we have not had a response. If obtained, we hope to include this data in future updates of this review.
Authors' conclusions
Implications for practice.
There is insufficient evidence to draw any conclusions about effectiveness and safety of pharmacomechanical thrombectomy versus anticoagulation (alone or with compression stockings), mechanical thrombectomy, thrombolysis, or other endovascular techniques in the management of people with acute deep venous thrombosis of the iliofemoral vein, because no randomised trials were eligible for inclusion in this review.
Implications for research.
This review highlights the gap in evidence for the use of pharmacomechanical thrombectomy for acute deep venous thrombosis (DVT) of the iliofemoral vein. Future randomised controlled trials are required. Future studies should also aim to incorporate the Lower Extremity Thrombosis (LET) score (Strijkers 2015), a relatively new classification system designed to identify patients at high risk for developing post‐thrombotic syndrome in the acute DVT phase, using thrombus location and extent.
Acknowledgements
We would like to thank the Cochrane Vascular Information Specialist for assistance with search strategies.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MESH DESCRIPTOR Thrombosis | 1231 |
| #2 | MESH DESCRIPTOR Thromboembolism | 892 |
| #3 | MESH DESCRIPTOR Venous Thromboembolism | 233 |
| #4 | MESH DESCRIPTOR Venous Thrombosis EXPLODE ALL TREES | 1996 |
| #5 | (thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol*):TI,AB,KY | 16760 |
| #6 | MESH DESCRIPTOR Pulmonary Embolism EXPLODE ALL TREES | 729 |
| #7 | (PE or DVT or VTE):TI,AB,KY | 4422 |
| #8 | ((vein* or ven*) near thromb*):TI,AB,KY | 6043 |
| #9 | (blood near3 clot*):TI,AB,KY | 2424 |
| #10 | (pulmonary near3 clot*):TI,AB,KY | 5 |
| #11 | (lung near3 clot*):TI,AB,KY | 4 |
| #12 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 | 21706 |
| #13 | MESH DESCRIPTOR Thrombectomy EXPLODE ALL TREES | 143 |
| #14 | (percutaneous near2 endovascular):TI,AB,KY | 13 |
| #15 | PEVI:TI,AB,KY | 4 |
| #16 | thrombecto*:TI,AB,KY | 428 |
| #17 | excis* | 3652 |
| #18 | suction:TI,AB,KY | 1970 |
| #19 | aspiration or thromboaspirat* | 3693 |
| #20 | angiojet:TI,AB,KY | 10 |
| #21 | jet*:TI,AB,KY | 756 |
| #22 | pharmaco*:TI,AB,KY | 122292 |
| #23 | mechanical*:TI,AB,KY | 11751 |
| #24 | (Trerotola OR Rotarex or Aspirex):TI,AB,KY | 3 |
| #25 | (amplatz OR angiovac):TI,AB,KY | 26 |
| #26 | rotat*:TI,AB,KY | 4215 |
| #27 | rheoly*:TI,AB,KY | 26 |
| #28 | ultrasound:TI,AB,KY | 11469 |
| #29 | Endowave:TI,AB,KY | 0 |
| #30 | (power near2 pulse):TI,AB,KY | 20 |
| #31 | #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 | 154857 |
| #32 | #12 AND #31 | 5175 |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cakir 2014 | Study examined percutaneous aspiration thrombectomy versus medical treatment only |
| Srinivas 2014 | Study was not a randomised trial (personal communication with the author) |
Characteristics of studies awaiting assessment [ordered by study ID]
TORPEDO 2012.
| Methods | Study design: randomised controlled trial |
| Participants | Setting: hospital Country: United States of America Inclusion criteria: adult patients with symptomatic DVT, involving popliteal vein or more proximal venous segments, diagnosed by venous duplex sonography or multislice CT venography. The affected veins were divided into five segments, based on the anatomical involvement of DVT: inferior vena cava (IVC), right and left iliac veins, and right and left femoropopliteal veins Exclusion criteria: patients were excluded if they had serious bleeding in the previous 4 weeks, contra‐indication to unfractionated or low‐molecular weight heparin, or severe thrombocytopaenia (platelet count of < 30,000/mm³) |
| Interventions | Intervention 1: PEVI + anticoagulation. For acute DVT with otherwise preserved venous anatomy, thrombectomy was performed using any of the following: Angiojet DVX catheter (Medrad/Possis, Warrendale, PA), Trellis device (Bacchus Vascular, Santa Clara, CA), or manual aspiration with an 8‐F guide catheter. No preference was given to any thrombectomy device, and its use was based on operator discretion and device availability. For severe 'venosclerotic' disease with distorted anatomy, a venous conduit was created using balloon venoplasty and stents. If residual thrombus was more than 30% of the luminal area, an infusion catheter was placed and low‐dose thrombolytic therapy with tissue plasminogen activator (tPA) at 1 mg/hr delivered for 20 to 24 hr. In this scenario, the patient was brought back to the angiography suite for re‐evaluation after tPA administration Intervention 2: anticoagulation alone. Initial anticoagulation therapy consisted of subcutaneous enoxaparin at 1 mg/kg twice daily, administered subcutaneously. For those with renal insufficiency or concomitant massive PE, unfractionated heparin (UFH) was started at 80 IU/kg intravenously as loading dose, followed by 18 IU/hr, with subsequent adjustments to keep the activated partial thromboplastin time 1.5 to 2 times the baseline level. Warfarin was initiated on admission |
| Outcomes | Primary: post‐thrombotic syndrome and recurrence of VTE at 6 months Secondary: bleeding, duration of hospitalisation, reduction of leg oedema, reduction of skin induration, and patient's subjective perception of improvement |
| Notes | Authors were contacted for data on participants with iliofemoral DVT and for data stratified by pharmacomechanical thrombectomy. To date, no response has been received |
CT: computed tomography DVT: deep vein thrombosis PE: pulmonary embolism PEVI: percutaneous endovenous intervention VTE: venous thromboembolism
Characteristics of ongoing studies [ordered by study ID]
NCT02414802.
| Trial name or title | Study on the application of a novel aspiration thrombectomy device combined with catheter‐directed thrombolysis for the treatment of acute iliofemoral deep venous thrombosis |
| Methods | Study design: randomised, parallel assignment study |
| Participants | Setting: hospital Country: China Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention 1: combined thrombectomy device. A manual spiral thrombus broken suction device will be used for thrombectomy before catheter‐directed thrombolysis. Ten million U of urokinase once every 4 to 6 hours will be used during catheter‐directed thrombolysis therapy. Anticoagulation therapy will be administered via subcutaneous injection of low molecular weight heparin calcium (LMWH‐Ca 5000 U/12 h) at discharge Intervention 2: participants will undergo catheter‐directed thrombolysis alone. A total of 100,000 units urokinase will be pulse‐spray injected through the catheter once every 4 to 6 hours. Anticoagulation therapy will be administered via subcutaneous injection of LMWH‐Ca 5000 U/12 h at discharge |
| Outcomes | Primary: patency of lower extremity deep venous system Secondary: technical success rate, thrombus removal rate, complications, blood loss, improvement of clinical symptoms and signs and incidence of post‐thrombotic syndrome |
| Starting date | December 2014 |
| Contact information | Qingqiao Zhang, Xuzhou Medical College |
| Notes |
LMWH‐Ca: low molecular weight heparin calcium
Contributions of authors
LR: drafted the protocol, selected studies for inclusion and wrote the review OM: contributed to the protocol, selected studies for inclusion, and provided clinical input to the review AB: drafted the protocol, selected studies for inclusion
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Insitute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Programme Grant funding (13/89/23) to Cochrane Vascular. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
LR: none known OM: none known AD: none known
New
References
References to studies excluded from this review
Cakir 2014 {published data only}
- Cakir V, Gulcu A, Akay E, Capar AE, Gencpinar T, Kucuk B, et al. Use of percutaneous aspiration thrombectomy vs. anticoagulation therapy to treat acute iliofemoral venous thrombosis: 1‐year follow‐up results of a randomised, clinical trial. Cardiovascular and Interventional Radiology 2014;37(4):969‐76. [DOI] [PubMed] [Google Scholar]
- Cakir V, Gülcü A, Kucuk B, Capar AE, Gencpinar T, Karabay O, et al. Early results of percutaneous aspiration thrombectomy vs. anticoagulation in acute iliofemoral venous trombosis: a randomised clinical trial. Cardiovascular and Interventional Radiology 2011;34(Suppl 3):524. [DOI] [PubMed] [Google Scholar]
Srinivas 2014 {published and unpublished data}
- Patra S. Catheter directed thrombolysis along with mechanical thromboaspiration versus anticoagulation alone in the management of lower limb deep venous thrombosis ‐ a comparative study. Journal of the American College of Cardiology 2014;64(16 Suppl 1):C203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas BC, Patra S, Nagesh CM, Reddy B, Manjunath CN. Catheter‐directed thrombolysis along with mechanical thromboaspiration versus anticoagulation alone in the management of lower limb deep venous thrombosis ‐ a comparative study. International Journal of Angiology 2014;23(4):247‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
TORPEDO 2012 {published data only}
- Sharifi M, Bay C, Mehdipour M, Sharifi J, for the TORPEDO Investigators. Thrombus Obliteration by Rapid Percutaneous Endovenous Intervention in Deep Venous Occlusion (TORPEDO) trial: midterm results. Journal of Endovascular Therapy 2012;19(2):273‐80. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT02414802 {published data only}
- NCT02414802. Study of a novel thrombectomy device to treat acute iliofemoral deep venous thrombosis. Available from clinicaltrials.gov/ct2/show/NCT02414802 (accessed 17 December 2015).
Additional references
Access Economics Pty Limited 2008
- Access Economics Pty Limited. The burden of venous thromboembolism in Australia. Report by Access Economics Pty Limited for The Australia and New Zealand Working Party on the Management and Prevention of Venous Thromboembolism. 1 May 2008. Available from www.deloitteaccesseconomics.com.au/uploads/File/The%20burden%20of%20VTE%20in%20Australia.pdf (accessed 28 January 2015).
Ageno 2006
- Ageno W, Squizzato A, Garcia D, Imberti D. Epidemiology and risk factors of venous thromboembolism. Seminars in Thrombosis and Hemostasis 2006;32(7):651‐8. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blackwood 2016
- Blackwood S, Dietzek AM. Pharmacomechanical thrombectomy: 2015 update. Expert Review of Cardiovascular Therapy 2016;17:1‐13. [DOI: 10.1586/14779072.2016.1140038] [DOI] [PubMed] [Google Scholar]
Elliot 1979
- Elliot MS, Immelman EJ, Jeffery P, Benatar SR, Funston MR, Smith JA, et al. A comparative randomized trial of heparin versus streptokinase in the treatment of acute proximal venous thrombosis: an interim report of a prospective trial. British Journal of Surgery 1979;66(12):838‐43. [DOI] [PubMed] [Google Scholar]
Enden 2009
- Enden T, Klow NE, Sandvik L, Slagsvold CE, Ghanima W, Hafsahl G, et al. Catheter‐directed thrombolysis vs. anticoagulant therapy alone in deep vein thrombosis: results of an open randomized, controlled trial reporting on short‐term patency. Journal of Thrombosis and Haemostasis 2009;7(8):1268‐75. [DOI] [PubMed] [Google Scholar]
GRADEproGDT [Computer program]
- GRADE Working Group, McMaster University. GRADEproGDT. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jenkins 2011
- Jenkins JS. Endovascular therapies to treat iliofemoral deep venous thrombosis. Progress in Cardiovascular Disease 2011;54(1):70‐6. [DOI] [PubMed] [Google Scholar]
Kahn 2014
- Kahn SR, Shapiro S, Wells PS, Rodger MA, Kovacs MJ, Anderson DR, et al. SOX trial investigators. Compression stockings to prevent post‐thrombotic syndrome: a randomised placebo‐controlled trial. Lancet 2014;383(9920):880‐8. [DOI] [PubMed] [Google Scholar]
Kearon 2003
- Kearon C. Natural history of venous thromboembolism. Circulation 2003;107(23 Suppl 1):I22‐30. [DOI] [PubMed] [Google Scholar]
Merli 2008
- Merli GJ. Treatment of venous thromboembolism. American Journal of Medicine 2008;121(11A):S8‐3. [Google Scholar]
Musani 2010
- Musani MH, Matta F, Yaekoub AY, Liang J, Hull RD, Stein PD. Venous compression for prevention of postthrombotic syndrome: a meta‐analysis. American Journal of Medicine 2010;123(8):735‐40. [DOI] [PubMed] [Google Scholar]
Pappy 2010
- Pappy R, Hanna EB, Abu‐Fadel MS, Hennebry TA. Isolated pharmacomechanical thrombectomy for the management of chronic DVT. Journal of Interventional Cardiology 2011;24(1):104‐99. [DOI] [PubMed] [Google Scholar]
Plate 1990
- Plate G, Akesson H, Einarsson E, Ohlin P, Eklof B. Long‐term results of venous thrombectomy combined with a temporary arterio‐venous fistula. European Journal of Vascular Surgery 1990;4(5):483‐9. [DOI] [PubMed] [Google Scholar]
Prandoni 1992
- Prandoni P, Villalta S, Polistena P, Bernardi E, Cogo A, Girolami A. Symptomatic deep‐vein thrombosis and the post‐thrombotic syndrome. Haematologica 1995;80(2):42‐8. [PubMed] [Google Scholar]
Prandoni 2004
- Prandoni P, Lensing AW, Prins MH, Frulla M, Marchiori A, Bernardi E, et al. Below‐knee elastic compression stockings to prevent the post‐thrombotic syndrome: a randomized, controlled trial. Annals of Internal Medicine 2004;141(4):249‐56. [DOI] [PubMed] [Google Scholar]
Prandoni 2009
- Prandoni P, Kahn SR. Post‐thrombotic syndrome: prevalence, prognostication and need for progress. British Journal of Haematology 2009;145(3):286‐95. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schulman 2005
- Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. Journal of Thrombosis and Haemostasis 2005;3(4):692‐4. [DOI] [PubMed] [Google Scholar]
Strijkers 2015
- Strijkers RH, Arnoldussen CW, Wittens CH. Validation of the LET classification. Phlebology 2015;30(1 Suppl):14‐9. [DOI] [PubMed] [Google Scholar]
Suwanabol 2013
- Suwanabol PA, Hoch JR. Venous thromboembolic disease. Surgical Clinics of North America 2013;93(4):983‐95. [DOI] [PubMed] [Google Scholar]
Turpie 1990
- Turpie AG, Levine MN, Hirsh J, Ginsberg JS, Cruickshank M, Jay R, et al. Tissue plasminogen activator (rt‐PA) vs heparin in deep vein thrombosis. Results of a randomized trial. Chest 1990;97(4 Suppl):172S‐5S. [PubMed] [Google Scholar]
Watson 2014
- Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD002783.pub3] [DOI] [PubMed] [Google Scholar]
White 2003
- White RH. The epidemiology of venous thromboembolism. Circulation 2003;107(23):14‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Robertson 2015
- Robertson L, Burdess A, McBride O. Pharmacomechanical thrombectomy for iliofemoral deep vein thrombosis. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD011536] [DOI] [PMC free article] [PubMed] [Google Scholar]


