Abstract
Background
Endoscopic retrograde cholangiopancreatography (ERCP) and intraoperative cholangiography (IOC) are tests used in the diagnosis of common bile duct stones in people suspected of having common bile duct stones. There has been no systematic review of the diagnostic accuracy of ERCP and IOC.
Objectives
To determine and compare the accuracy of ERCP and IOC for the diagnosis of common bile duct stones.
Search methods
We searched MEDLINE, EMBASE, Science Citation Index Expanded, BIOSIS, and Clinicaltrials.gov to September 2012. To identify additional studies, we searched the references of included studies and systematic reviews identified from various databases (Database of Abstracts of Reviews of Effects (DARE)), Health Technology Assessment (HTA), Medion, and ARIF (Aggressive Research Intelligence Facility)). We did not restrict studies based on language or publication status, or whether data were collected prospectively or retrospectively.
Selection criteria
We included studies that provided the number of true positives, false positives, false negatives, and true negatives for ERCP or IOC. We only accepted studies that confirmed the presence of common bile duct stones by extraction of the stones (irrespective of whether this was done by surgical or endoscopic methods) for a positive test, and absence of common bile duct stones by surgical or endoscopic negative exploration of the common bile duct, or symptom‐free follow‐up for at least six months for a negative test as the reference standard in people suspected of having common bile duct stones. We included participants with or without prior diagnosis of cholelithiasis; with or without symptoms and complications of common bile duct stones; with or without prior treatment for common bile duct stones; and before or after cholecystectomy. At least two authors screened abstracts and selected studies for inclusion independently.
Data collection and analysis
Two authors independently collected data from each study. We used the bivariate model to summarise the sensitivity and specificity of the tests.
Main results
We identified five studies including 318 participants (180 participants with and 138 participants without common bile duct stones) that reported the diagnostic accuracy of ERCP and five studies including 654 participants (125 participants with and 529 participants without common bile duct stones) that reported the diagnostic accuracy of IOC. Most studies included people with symptoms (participants with jaundice or pancreatitis) suspected of having common bile duct stones based on blood tests, ultrasound, or both, prior to the performance of ERCP or IOC. Most studies included participants who had not previously undergone removal of the gallbladder (cholecystectomy). None of the included studies was of high methodological quality as evaluated by the QUADAS‐2 tool (quality assessment tool for diagnostic accuracy studies). The sensitivities of ERCP ranged between 0.67 and 0.94 and the specificities ranged between 0.92 and 1.00. For ERCP, the summary sensitivity was 0.83 (95% confidence interval (CI) 0.72 to 0.90) and specificity was 0.99 (95% CI 0.94 to 1.00). The sensitivities of IOC ranged between 0.75 and 1.00 and the specificities ranged between 0.96 and 1.00. For IOC, the summary sensitivity was 0.99 (95% CI 0.83 to 1.00) and specificity was 0.99 (95% CI 0.95 to 1.00). For ERCP, at the median pre‐test probability of common bile duct stones of 0.35 estimated from the included studies (i.e., 35% of people suspected of having common bile duct stones were confirmed to have gallstones by the reference standard), the post‐test probabilities associated with positive test results was 0.97 (95% CI 0.88 to 0.99) and negative test results was 0.09 (95% CI 0.05 to 0.14). For IOC, at the median pre‐test probability of common bile duct stones of 0.35, the post‐test probabilities associated with positive test results was 0.98 (95% CI 0.85 to 1.00) and negative test results was 0.01 (95% CI 0.00 to 0.10). There was weak evidence of a difference in sensitivity (P value = 0.05) with IOC showing higher sensitivity than ERCP. There was no evidence of a difference in specificity (P value = 0.7) with both tests having similar specificity.
Authors' conclusions
Although the sensitivity of IOC appeared to be better than that of ERCP, this finding may be unreliable because none of the studies compared both tests in the same study populations and most of the studies were methodologically flawed. It appears that both tests were fairly accurate in guiding further invasive treatment as most people diagnosed with common bile duct stones by these tests had common bile duct stones. Some people may have common bile duct stones in spite of having a negative ERCP or IOC result. Such people may have to be re‐tested if the clinical suspicion of common bile duct stones is very high because of their symptoms or persistently abnormal liver function tests. However, the results should be interpreted with caution given the limited quantity and quality of the evidence.
Keywords: Humans; Cholangiography; Cholangiopancreatography, Endoscopic Retrograde; Choledocholithiasis; Choledocholithiasis/diagnostic imaging; Intraoperative Period; Randomized Controlled Trials as Topic; Sensitivity and Specificity
Plain language summary
Endoscopic retrograde cholangiopancreatography versus intraoperative cholangiography for the diagnosis of common bile duct stones
Background
The liver has various functions. Production of bile is one of these functions. The common bile duct (CBD) is the tube through which bile flows from the gallbladder (where bile is temporarily stored) into the small bowel. Stones in the CBD (CBD stones) can obstruct the flow of bile from the liver into the small bowel. Usually such stones are formed in the gallbladder and migrate into the CBD. Obstruction of the flow of bile can lead to jaundice (yellowish discolouration of skin and white of the eyes, and dark urine), infection of the bile duct (cholangitis), and inflammation of the pancreas (pancreatitis), which can be life threatening. Various diagnostic tests can be performed to diagnose CBD stones. Depending upon the availability of resources, these stones are removed endoscopically (a tube inserted into the stomach and upper part of small bowel through mouth; usually the case), or may be removed as part of the laparoscopic operation (key hole surgery) or open operation performed to remove the gallbladder (cholecystectomy; it is important to remove the gallbladder since the stones continue to form in the gallbladder and can cause recurrent health problems). If the stones are removed endoscopically, presence of stones is confirmed by endoscopic retrograde cholangiopancreatography (ERCP) (injection of dye into the CBD using an endoscope) before endoscopic removal of CBD stones. Alternatively, intraoperative cholangiography (IOC) (injection of dye into the biliary tree during an operation to remove the CBD stones, usually combined with an operation to remove gallstones) can be performed to detect CBD stones prior to operative removal of the stones. We performed a thorough search for studies that reported the accuracy of ERCP or IOC for the diagnosis of CBD stones. The evidence is current to September 2012.
Study characteristics
We identified five studies including 318 participants that reported the diagnostic test accuracy of ERCP and five studies including 654 participants that reported the diagnostic test accuracy of IOC. Most studies included people with symptoms (participants with jaundice or pancreatitis) who were suspected of having CBD stones based on blood tests, ultrasound (use of sound waves higher than audible range to differentiate tissues based on how they reflect the sound waves), or both, prior to the having ERCP or IOC. Most studies included participants who had not previously undergone cholecystectomy.
Key results
Given an average sensitivity of 83% for ERCP, we would expect that on average 83 out of 100 people (this may vary between 72 and 90 out of 100 people) with CBD stones would be detected while the remaining 17 people would be missed and would not receive appropriate treatment. Based on an average specificity of 99% for ERCP, we would expect that on average 99 out of 100 people without CBD stones would be identified as not having CBD stones; 1 out of 100 (this could vary between 0 and 17 out of 100 people) would be false positive and would not receive appropriate treatment. For IOC, an average sensitivity of 99% means that on average 99 out of 100 people (this may vary between 83 and 100 out of 100 people) with CBD stones would be detected while only one person would be missed and would not receive appropriate treatment. In terms of specificity, an average of 99% for IOC means that 99 out of 100 people without CBD stones would be identified as not having CBD stones with only one false positive (this could vary between 0 and 5 out of 100 people) who would not receive appropriate treatment. It appears that both tests are fairly accurate in guiding further invasive treatment as most people diagnosed with CBD stones by these tests have CBD stones. However, some people may have CBD stones in spite of having a negative ERCP or IOC test result. Such people may have to be re‐tested if the clinical suspicion of CBD stones is very high because of their symptoms.
Quality of evidence
All the studies were of low methodological quality, which may question the validity of our findings.
Future research
Further studies of high methodological quality are necessary.
Summary of findings
Summary of findings'. 'Performance of endoscopic retrograde cholangiopancreatography and intraoperative cholangiography for diagnosis of common bile duct stones.
| Population | People suspected of having common bile duct stones based on symptoms, liver function tests, and ultrasound. | ||||
| Settings | Secondary and tertiary care setting in Brazil, China, France, the UK, and the USA. | ||||
| Index tests | ERCP and IOC. | ||||
| Reference standard | Endoscopic or surgical extraction of stones in people with a positive index test result or clinical follow‐up (minimum 6 months) in people with a negative index test result. | ||||
| Target condition | Common bile duct stones. | ||||
| Number of studies | 5 studies (180 cases, 318 participants) of ERCP and 5 studies (125 cases, 654 participants) of IOC. None of the studies evaluated both tests in the same participants. | ||||
| Methodological quality concerns | All the studies were of poor methodological quality; most studies were at high risk of bias or gave high concern about applicability across all domains of quality assessment, or both. | ||||
| Pre‐test probability1 | Test | Summary sensitivity (95% CI) | Summary specificity (95% CI) | Positive post‐test probability (95% CI)2 | Negative post‐test probability (95% CI)3 |
| 0.12 | ERCP | 0.83 (0.72 to 0.90) | 0.99 (0.94 to 1.00) | 0.90 (0.66 to 0.98) | 0.02 (0.01 to 0.04) |
| IOC | 0.99 (0.83 to 1.00) | 0.99 (0.95 to 1.00) | 0.94 (0.60 to 0.99) | 0.00 (0.00 to 0.03) | |
| 0.21 | ERCP | 0.83 (0.72 to 0.90) | 0.99 (0.94 to 1.00) | 0.94 (0.78 to 0.99) | 0.04 (0.03 to 0.07) |
| IOC | 0.99 (0.83 to 1.00) | 0.99 (0.95 to 1.00) | 0.97 (0.74 to 1.00) | 0.00 (0.00 to 0.05) | |
| 0.35 | ERCP | 0.83 (0.72 to 0.90) | 0.99 (0.94 to 1.00) | 0.97 (0.88 to 0.99) | 0.09 (0.05 to 0.14) |
| IOC | 0.99 (0.83 to 1.00) | 0.99 (0.95 to 1.00) | 0.98 (0.85 to 1.00) | 0.01 (0.00 to 0.10) | |
| 0.48 | ERCP | 0.83 (0.72 to 0.90) | 0.99 (0.94 to 1.00) | 0.98 (0.93 to 1.00) | 0.14 (0.09 to 0.22) |
| IOC | 0.99 (0.83 to 1.00) | 0.99 (0.95 to 1.00) | 0.99 (0.91 to 1.00) | 0.01 (0.00 to 0.16) | |
| 0.68 | ERCP | 0.83 (0.72 to 0.90) | 0.99 (0.94 to 1.00) | 0.99 (0.97 to 1.00) | 0.28 (0.18 to 0.39) |
| IOC | 0.99 (0.83 to 1.00) | 0.99 (0.95 to 1.00) | 1.00 (0.96 to 1.00) | 0.02 (0.00 to 0.30) | |
|
Comparison of the diagnostic accuracy of ERCP and IOC: For ERCP: at a pre‐test probability of 0.12, out of 100 people with positive ERCP test results, common bile duct stones would be present in 90 people; at a pre‐test probability of 0.35, common bile duct stones would be present in 97 people; and at a pre‐test probability of 0.68, common bile duct stones would be present in 99 people. For ERCP: at a pre‐test probability of 0.12, out of 100 people with negative ERCP test results, common bile duct stones would be present in 2 people; at a pre‐test probability of 0.35, common bile duct stones would be present in 9 people; and at a pre‐test probability of 0.68, common bile duct stones would be present in 28 people. For IOC: at a pre‐test probability of 0.12, out of 100 people with positive IOC test results, common bile duct stones would be present in 94 people; at a pre‐test probability of 0.35, common bile duct stones would be present in 98 peoplecommon bile duct stones and at a pre‐test probability of 0.68, common bile duct stones would be present in 100 people. For IOC: at a pre‐test probability of 0.12, out of 100 people with negative IOC test results, common bile duct stones would be present in 0 people; at a pre‐test probability of 0.35, common bile duct stones would be present in 1 person; and at a pre‐test probability of 0.68, common bile duct stones would be present in 2 people. CI: confidence interval; ERCP: endoscopic retrograde cholangiopancreatography; IOC: intraoperative cholangiography. | |||||
| Conclusions: Although the sensitivity of IOC appeared to be better than that of ERCP, the finding may be unreliable because none of the studies compared both tests in the same study population and most of the studies were methodologically flawed. It appeared that both tests were fairly accurate in guiding further invasive treatment as most people diagnosed with common bile duct stones by these tests have common bile duct stones. However, the results should be interpreted with caution, given the limited quantity and quality of the evidence. | |||||
1 We computed the pre‐test probability (proportion with common bile duct stones out of the total number of participants) for each included study. These numbers represented the minimum, lower quartile, median, upper quartile, and maximum values from the 10 studies. 2Post‐test probability of common bile duct stones in people with positive index test results. 3Post‐test probability of common bile duct stones in people with negative index test results.
Background
Biliary stones are conglomerates of precipitated bile salts that form in the gallbladder or common bile duct. The common bile duct carries bile from the liver to the duodenum (first part of the small intestine). The term 'gallstones' generally refers to the stones in the gallbladder, while 'common bile duct stones' refers to stones in the common bile duct. Common bile duct stones may form inside the common bile duct (primary common bile duct stones), or they may form in the gallbladder and migrate to the common bile duct (secondary common bile duct stones) (Williams 2008). A significant proportion of people presenting with common bile duct stones may be asymptomatic (Sarli 2000). In some people, the stones pass silently into the duodenum, and in other people, the stones cause clinical symptoms such as biliary colic, jaundice, cholangitis, or pancreatitis (Caddy 2006). The prevalence of gallstone disease in the general population is about 6% to 15% with a higher prevalence in females (Barbara 1987; Loria 1994). Only 2% to 4% of people with gallstones become symptomatic with biliary colic (pain), acute cholecystitis (inflammation), obstructive jaundice, or gallstone pancreatitis in one year (Attili 1995; Halldestam 2004), and removal of gallbladder is recommended in people with symptomatic gallstones (Gurusamy 2010). Among people who undergo laparoscopic cholecystectomy (removal of gallbladder) for symptomatic gallstones, 3% to 22% also have concomitant common bile duct stones (Arnold 1970; Lill 2010; Yousefpour Azary 2011).
Common bile duct stones present in multiple ways. Central and right‐sided upper abdominal pain is a common presentation (Anciaux 1986; Roston 1997). Jaundice, caused by an impacted stone in the common bile duct leading to obstruction of bile passage into the duodenum, is another presentation. It may subsequently resolve if the common bile duct stone passes spontaneously into the duodenum. This happens in 54% to 73% of people with common bile duct stones in whom cholecystectomy is performed for gallstones (Tranter 2003; Lefemine 2011). Another, more dangerous, complication of common bile duct stones is acute cholangitis. Cholangitis is clinically defined by Charcot's triad, which includes elevated body temperature, pain under the right ribcage, and jaundice (Raraty 1998; Salek 2009). Acute cholangitis is caused by an ascending bacterial infection of the common bile duct and the biliary tree along with biliary obstruction. This complication is present in 2% to 9% of people admitted for gallstone disease (Saik 1975; Tranter 2003), and a mortality of approximately 24% is recorded (Salek 2009). Common bile duct stones may also cause acute pancreatitis, accounting for 33% to 50% of all people with acute pancreatitis (Corfield 1985; Toh 2000). Acute pancreatitis is usually a self limiting disease and is usually sufficiently treated by conservative measures in its mild form (Neoptolemos 1988). However, a more severe pancreatitis may evolve in approximately 27% to 37% of people with common bile duct stone‐induced pancreatitis, with mortality around 6% to 9% (Mann 1994; Toh 2000).
Suspicion of common bile duct stones can be confirmed by laboratory liver function tests (Barkun 1994), or diagnostic tests such as abdominal ultrasound (Ripolles 2009). Further testing may include endoscopic ultrasound (EUS) (Aljebreen 2008), magnetic resonance cholangiopancreatography (MRCP) (Stiris 2000), endoscopic retrograde cholangiopancreatography (ERCP) (Geron 1999), and intraoperative cholangiography (IOC) (Fiore 1997).
IOC can only be done during an operation, as this test requires surgical cannulation of the common bile duct during cholecystectomy. EUS, MRCP, and ERCP may be used preoperatively or postoperatively.
Currently, recommended diagnostic tests for diagnosis of common bile duct stones are liver function tests, abdominal ultrasound, MRCP, EUS, ERCP, and IOC. There are other tests such as conventional computed tomogram (CT scan), CT cholangiogram, laparoscopic ultrasound, and ERCP‐guided intraductal ultrasound used for diagnosing common bile duct stones but these are of limited use (Maple 2010).
Usually, the first diagnostic tests that most people will undergo are liver function tests and abdominal ultrasound. Invasive diagnostic tests are usually reserved for people with suspected common bile duct stones based on non‐invasive diagnostic tests, or when therapeutic measures are necessary (Freitas 2006).
Target condition being diagnosed
Common bile duct stones. We did not differentiate the target condition with respect to common bile duct stone size, degree of common bile duct obstruction, and the presence or absence of symptoms.
Index test(s)
ERCP is a diagnostic test and therapeutic method that uses an endoscope with side‐viewing camera for visualisation of the opening of the common bile duct into the duodenum. A series of tubes with electrocautery knives for cutting, injection tubes for contrast material, or guidewire tubes for placing tools such as balloons or baskets can be inserted into the common bile duct (Prat 1996). When diagnosing common bile duct stones, radio‐opaque contrast material is injected into the common bile duct and a series of x‐rays are taken to visualise filling defects that indicate the presence of common bile duct stones. This method also has therapeutic possibilities because common bile duct stones can be extracted using baskets (such as a Dormia basket that is inserted through the endoscope) or crushed by mechanical lithotripsy and extracted by baskets or balloons (Prat 1996; Maple 2010). It is also combined with sphincterotomy (incision of the opening of the common bile duct into the duodenum) to make both the procedure and passage of possible stones easier. Endoscopic retrograde cholangiography is used predominantly as a therapeutic tool and it is usually preceded by other diagnostic tests such as EUS or MRCP (Maple 2010).
IOC is a diagnostic test used during cholecystectomy. A radio‐opaque material is injected into the common bile duct and series of x‐rays are taken to visualise possible filling defects in the common bile duct. As it is used intraoperatively; surgical steps to remove the identified stones can then be taken. As with ERCP, a positive test shows as a filling defect within the common bile duct (Amott 2005; Moon 2005).
Clinical pathway
Figure 1 shows a diagnostic pathway. People that are at risk of having common bile duct stones or suspected of having common bile duct stones (such as people with gallbladder stones or people who show symptoms and signs of obstructive jaundice or pancreatitis) undergo liver function tests and abdominal ultrasound as the first step. An abdominal ultrasound is usually available by the time the person is at risk or suspected of having common bile duct stones. Usually both tests is used as triage tests before further testing is done in the second step, but these can be used as the definitive diagnostic test to carry out a therapeutic option directly (e.g., endoscopic or surgical common bile duct exploration) (Williams 2008; ASGE Standards of Practice Committee 2010). MRCP or EUS are tests in the second step of the diagnostic pathway and are used as optional triage tests prior to the tests used in the third step of the diagnostic pathway, but can also be used as definitive diagnostic tests to carry out a therapeutic option directly. MRCP and EUS are usually not combined, since a positive or negative result of one or the other test is usually accepted for making further clinical decisions without taking into consideration the results of liver function tests or transabdominal ultrasound because it is generally believed that MRCP and EUS have better diagnostic accuracy than liver function tests or transabdominal ultrasound. ERCP and IOC are used in the third step of the diagnostic pathway. Both these tests are done just before the therapeutic intervention. Therapeutic interventions, such as endoscopic or surgical stone extraction, can then be undertaken during the same session. ERCP is done before endoscopic sphincterotomy and removal of common bile duct stones using Dormia basket or balloon during the same endoscopic session (Prat 1996; Maple 2010), while IOC is done before surgical common bile duct exploration and removal of common bile duct stones using surgical instruments during the operation for cholecystectomy (Targarona 2004; Freitas 2006; Chen 2007; Williams 2008; ASGE Standards of Practice Committee 2010; Kelly 2010). Thus, ERCP and IOC can be considered as the final diagnostic tests prior to intervention. The choice of whether the person undergoes ERCP or IOC is very much dependent upon the surgical preference for management of common bile duct stones. There is currently no evidence to suggest that endoscopic management is better than surgical management and vice versa (Dasari 2013). Generally, ERCP followed immediately by endoscopic sphincterotomy performed preoperatively (before the person undergoes laparoscopic cholecystectomy) is the preferred method of management of common bile duct stones in people with an intact gallbladder (Ludwig 2001). IOC followed immediately by surgical exploration of common bile duct during the cholecystectomy operation is the less preferred operation (Ludwig 2001). Thus, ERCP and IOC can be considered as replacement tests for each other. However, it should be pointed out that a small proportion of surgeons also perform endoscopic sphincterotomy after the laparoscopic cholecystectomy (Ludwig 2001). In this small proportion of people who undergo endoscopic sphincterotomy after the laparoscopic cholecystectomy, IOC can be considered as an add‐on test similar to MRCP or EUS prior to ERCP in people with positive ultrasound or liver function tests, and the results of IOC is used to determine whether the person undergoes ERCP and endoscopic sphincterotomy irrespective of the results of the liver function tests or transabdominal ultrasound.
1.
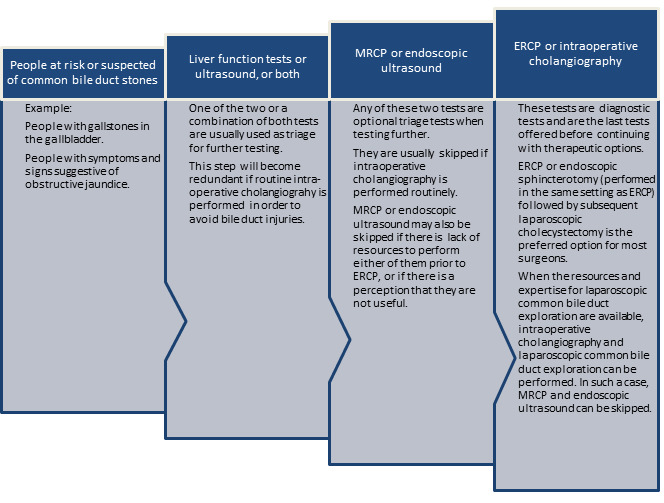
The diagnostic pathway for diagnosis of common bile duct stones. Note that ultrasound is generally performed in all people at risk or suspected of common bile duct stones.
ERCP: endoscopic retrograde cholangiopancreatography; MRCP: magnetic resonance cholangiopancreatography.
In people with gallstones and common bile duct stones anaesthetically suitable to undergo a major surgical procedure, the choice between ERCP and IOC depends upon surgical preference. However, if such people undergo a roux‐en‐Y gastric anastomosis, ERCP can be challenging (Lopes 2011), and IOC and surgical exploration may be the preferred option. In people who are not anaesthetically suitable to undergo major surgery, ERCP and endoscopic sphincterotomy is the only option available for the diagnosis and subsequent treatment of common bile duct stones.
Implications of negative tests
In general, people with negative test results in one step do not undergo further testing. For example, a person with no suggestion of common bile duct stones on liver function tests and ultrasound will not undergo further testing for common bile duct stones. Similarly, people with no suggestion of common bile duct stones on MRCP or EUS will not undergo further testing for common bile duct stones and people with no suggestion of common bile duct stones on ERCP or IOC will not undergo common bile duct clearance. People with a false‐negative test result can develop complications of common bile duct stones such as cholangitis and pancreatitis but the natural history of such people in terms of the frequency with which these complications develop, is unknown. However, it is generally recommended that common bile duct stones be removed when they are identified because of the serious complications that may develop (Williams 2008). Although this practice is not evidence‐based, this shows the perception among hepato‐pancreato biliary surgeons and gastroenterologists that it is important not to miss common bile duct stones.
Rationale
There are several other benign and malignant conditions that may cause obstructive jaundice where there are no identifiable common bile duct stones. Benign (non‐cancerous) causes of obstructive jaundice include primary sclerosing cholangitis (Penz‐Osterreicher 2011), primary biliary cirrhosis (Hirschfield 2011), chronic pancreatitis (Abdallah 2007), autoimmune pancreatitis (Lin 2008), inflammatory strictures of the common bile duct (Krishna 2008), and strictures of the common bile duct caused by prior instrumentation (Lillemoe 2000; Tang 2011). Malignant (cancerous) causes of obstructive jaundice include cholangiocarcinoma (Siddiqui 2011), cancer of the ampulla of Vater as well as other periampullary cancers (Hamade 2005; Choi 2011; Park 2011), and carcinoma of the pancreas (Singh 1990; Kalady 2004). It is important to differentiate between the causes of obstructive jaundice in order to initiate appropriate treatment. The correct diagnosis of common bile duct stones is an essential contribution to this differentiation.
Common bile duct stones are responsible for a range of complications and may lead to pancreatitis in about 33% to 50% of the people who have them (Corfield 1985; Toh 2000), and cause mortality in about 6% to 9% of these people (Mann 1994; Toh 2000). Acute cholangitis appears in 2% to 9% of people admitted for gallstone disease, with mortality around 24% (Salek 2009). Therefore, it is important to diagnose common bile duct stones in order to treat people and prevent such complications.
The preferred option for the treatment of common bile duct stones is currently endoscopic sphincterotomy with balloon trawling followed by laparoscopic cholecystectomy (Ludwig 2001; Spelsberg 2009). Other options include open cholecystectomy with open common bile duct exploration, laparoscopic cholecystectomy with laparoscopic common bile duct exploration, and laparoscopic cholecystectomy with endoscopic sphincterotomy (Hong 2006; Dasari 2013). Approximately half of people with jaundice, abnormal liver function tests, and common bile duct dilation on ultrasound do not actually have common bile duct stones (Hoyuela 1999), and, therefore, these people undergo invasive procedures unnecessarily. Accurate diagnosis of common bile duct stones may avoid unnecessary procedures and complications associated with these procedures. Invasive tests can result in complications, for example, ERCP with endoscopic sphincterotomy can have life‐threatening complications such as pancreatitis (Gurusamy 2011). Accurate diagnosis of common bile duct stones using non‐invasive tests can avoid these complications.
Currently, there are no Cochrane reviews of studies assessing the accuracy of different tests for diagnosing common bile duct stones. This review is one of three reviews evaluating the diagnostic accuracy of different tests used in the diagnosis of common bile duct stones and will help in the development of an evidence‐based algorithm for diagnosis of common bile duct stones.
Objectives
To determine and compare the accuracy of ERCP and IOC for the diagnosis of common bile duct stones.
Secondary objectives
The secondary objective of this review is to investigate variation in the diagnostic accuracy of ERCP and IOC according to the following potential sources of heterogeneity.
Studies at low risk of bias versus studies with unclear or high risk of bias (as assessed by the quality assessment tool for diagnostic accuracy studies (QUADAS‐2) tool (Table 2)).
Full‐text publications versus abstracts (this may indicate publication bias if there is an association between the results of the study and the study reaching full publication) (Eloubeidi 2001).
Prospective versus retrospective design.
Symptomatic versus asymptomatic common bile duct stones (the presence of symptoms may increase the pre‐test probability). People with symptoms were defined as people showing upper right quadrant abdominal pain, jaundice, acute cholangitis, or acute pancreatitis (Anciaux 1986; Roston 1997; Raraty 1998; Toh 2000; Tranter 2003).
Prevalence of common bile duct stones in each included study. The prevalence of common bile duct stones in the population analysed by each included study may vary and cause heterogeneity. Prevalence may also change with people with co‐morbidities that would predispose them to common bile duct stones, such as primary sclerosing cholangitis, Caroli's disease, hypercholesterolaemia, sickle cell anaemia, and sphincter of Oddi dysfunction.
Proportion of people with previous cholecystectomy. Cholecystectomy may cause dilation of the common bile duct (Benjaminov 2013), and subsequently change the accuracy of the index test particularly imaging modalities.
Proportion of people with common bile duct strictures (only for index tests that use contrast material, as strictures may prevent contrast material filling the common bile duct completely and, therefore, change the accuracy of the index test).
MRCP or EUS, if performed, prior to the index test.
1. The QUADAS 2 tool for assessing methodological quality of included studies.
| Domain 1: Participant sampling | Signalling question | Signalling question | Signalling question | Risk of bias | Concerns for applicability |
| Domain 1: Participant sampling | |||||
| Patient sampling | Was a consecutive or random sample of participants enrolled? | Was a case‐control design avoided? | Did the study avoid inappropriate exclusions? | Could the selection of participants have introduced bias? | Were there concerns that the included participants and setting did not match the review question? |
| Yes: all consecutive participants or random sample of participants with suspected common bile duct stones were enrolled. No: selected participants were enrolled. Unclear: this was not clear from the report. |
Yes: case‐control design was avoided. No: case‐control design was not avoided. Unclear: this was not clear from the report. |
Yes: the study avoided inappropriate exclusions (i.e., people who were difficult to diagnose). No: the study excluded participants inappropriately. Unclear: this was not clear from the report. |
Low risk: 'yes' for all signalling questions. High risk: 'no' or 'unclear' for at least 1 signalling question. |
Low concern: the selected participants represent the people in whom the tests would be used in clinical practice (see diagnostic pathway (Figure 1). High concern: there was high concern that participant selection was performed in a such a way that the included participants did not represent the people in whom the tests will be used in clinical practice. |
|
| Domain 2: Index test | |||||
| Index test(s) | Were the index test results interpreted without knowledge of the results of the reference standard? | If a threshold was used, was it pre‐specified? | ‐ | Could the conduct or interpretation of the index test have introduced bias? | Were there concerns that the index test, its conduct, or its interpretation differed from the review question? |
| Yes: index test results were interpreted without knowledge of the results of the reference standard. No: index test results were interpreted with knowledge of the results of the reference standard. Unclear: this was not clear from the report. |
Yes: if the criteria for a positive test result were pre‐specified. No: if the criteria for a positive test result were not pre‐specified. Unclear: this was not clear from the report. |
‐ | Low risk: 'yes' for all signalling questions. High risk: 'no' or 'unclear' for at least 1 of the 2 signalling questions. |
High concern: there was high concern that the conduct or interpretation of the index test differed from the way it is likely to be used in clinical practice. Low concern: there was low concern that the conduct or interpretation of the index test differed from the way it is likely to be used in clinical practice. |
|
| Domain 3: Reference standard | |||||
| Target condition and reference standard(s) | Was the reference standard likely to classify the target condition correctly? | Were the reference standard results interpreted without knowledge of the results of the index tests? | ‐ | Could the reference standard, its conduct, or its interpretation have introduced bias? | Were there concerns that the target condition as defined by the reference standard did not match the review question? |
| Yes: all participants underwent the acceptable reference standard. No: if all participants did not undergo an acceptable reference standard. Such studies were excluded from the review. Unclear: if the reference standard that the participants underwent was not stated. Such studies were excluded from the review. |
Yes: reference standard results were interpreted without knowledge of the results of the index test. No: reference standard results were interpreted with the knowledge of the results of the index test. Unclear: this was not clear from the report. |
‐ | Low risk: 'yes' for all signalling questions. High risk: 'no' or 'unclear' for at least 1 of the 2 signalling questions. |
Low concern: participants underwent endoscopic or surgical exploration for common bile duct stone. High concern: no participants underwent endoscopic or surgical exploration for common bile duct stone. |
|
| Domain 4: Flow and timing | |||||
| Flow and timing | Was there an appropriate interval between index test and reference standard? | Did all participants receive the same reference standard? | Were all participants included in the analysis? | Could the participant flow have introduced bias? | ‐ |
| Yes: the interval between index test and reference standard (including any repeat procedures) was ≤ 4 weeks (arbitrary choice). No: the interval between index test and reference standard was > 4 weeks. Unclear: this was not clear from the report. |
Yes: all participants underwent endoscopic or surgical exploration for common bile duct stone irrespective of the index test results. No: participants underwent endoscopic or surgical exploration if the index test results were positive and underwent clinical follow‐up for at least 6 months if the index test results were negative. Unclear: this was not clear from the report. Such studies were excluded. |
Yes: all participants meeting the selection criteria (selected participants) were included in the analysis, or data on all the selected participants were available so that a 2 x 2 table including all selected participants could be constructed. No: not all participants meeting the selection criteria were included in the analysis or the 2 x 2 table could not be constructed using data on all selected participants. Unclear: this was not clear from the report. |
Low risk: 'yes' for all signalling questions. High risk: 'no' or 'unclear' for at least 1 signalling question. |
‐ | |
Methods
Criteria for considering studies for this review
Types of studies
We included studies providing cross‐sectional information comparing one or more of the index tests against a reference standard in the appropriate patient population (see Participants). We included studies irrespective of language or publication status, or whether data were collected prospectively or retrospectively. We included comparative studies in which ERCP and IOC were performed in the same study population either by giving all participants both index tests or by randomly allocating participants to receive ERCP or IOC. We excluded diagnostic case‐control studies if there were at least four cross‐sectional or comparative studies.
Participants
People at risk of or suspected of having common bile duct stones, with or without prior diagnosis of cholelithiasis; with or without symptoms and complications of common bile duct stones, with or without prior treatment for common bile duct stones; and before or after cholecystectomy.
Index tests
ERCP and IOC.
Target conditions
Common bile duct stones.
Reference standards
We accepted the following reference standard.
For test positives, we accepted confirmation of a common bile duct stone by extraction of the stone (irrespective of whether this was done by surgical or endoscopic methods).
For test negatives, we acknowledged that there was no way of being sure that there were no common bile duct stones. However, we accepted negative results by surgical or endoscopic negative exploration of the common bile duct, or symptom‐free follow‐up for at least six months as the reference standard. Surgical or endoscopic exploration s adequate, but it is not commonly used in people with negative index tests because of its invasive nature. Therefore, we accepted follow‐up as a less adequate reference test. Negative exploration of common bile duct is likely to be a better reference standard than follow‐up for at least six months since most stones already present in the common bile duct are likely to be extracted in this fashion. Six months is an arbitrary choice, but we anticipated most common bile duct stones will become manifest during this period.
Search methods for identification of studies
Electronic searches
We searched MEDLINE via PubMed (January 1946 to September 2012), EMBASE via OvidSP (January 1947 to September 2012), Science Citation Index Expanded via Web of Knowledge (January 1898 to September 2012), BIOSIS via Web of Knowledge (January 1969 to September 2012), and Clinicaltrials.gov (September 2012). Appendix 1 shows the search strategies. We used a common search strategy for the three reviews of which this review is one. The other two reviews assess the diagnostic test accuracy of transabdominal ultrasound, liver function tests, EUS, and MRCP (Giljaca 2015; Gurusamy 2015). We also identified systematic reviews from the Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment (HTA), Medion, and ARIF (Aggressive Research Intelligence Facility) databases in order to search their reference lists (see Searching other resources).
Searching other resources
We searched the references of included studies and systematic reviews related to the topic to identify further studies. We also searched for additional articles related to included studies by performing the 'related search' function in MEDLINE (PubMed) and EMBASE (OvidSP) and a 'citing reference' search (search the articles that cited the included articles) (Sampson 2008) in Science Citation Index Expanded and EMBASE (OvidSP).
Data collection and analysis
Selection of studies
Two authors (VG and DH or GP) independently searched the references for identification of relevant studies. We obtained full texts for the references that at least one of the authors considered relevant. Two authors (VG and DH or GP) independently assessed the full‐text articles. One author (KG) arbitrated any differences in study selection. We selected studies that met the inclusion criteria for data extraction. We included abstracts if sufficient data to create a 2 x 2 table were provided.
Data extraction and management
Two authors (KG and VG) independently extracted the following data from each included study.
First author of report.
Year of publication of report.
Study design (prospective or retrospective; cross‐sectional studies or randomised clinical trials).
Inclusion and exclusion criteria for individual studies.
Total number of participants.
Number of males and females.
Mean age of the participants.
Tests carried out prior to index test.
Index test.
Reference standard.
Number of true positives, false positives, true negatives, and false negatives.
We sought further information on diagnostic test accuracy and assessment of methodological quality (see Assessment of methodological quality) from the authors of the studies, if necessary. We resolved any differences between the review authors by discussion until we reached a consensus. We extracted data excluding the indeterminates but recorded the number of indeterminates and the reference standard results of participants with indeterminate results.
Assessment of methodological quality
We adopted the quality assessment of diagnostic accuracy studies assessment tool (QUADAS‐2) for assessment of the methodological quality of included studies as described in Table 2 (Whiting 2006; Whiting 2011). We considered studies classified at low risk of bias and low concern regarding applicability to the review question as studies at low risk of bias. We resolved any differences in the methodological quality assessment by discussion between the review authors until a consensus was reached. We sought further information from study authors in order to assess the methodological quality of included studies accurately.
Statistical analysis and data synthesis
We plotted study estimates of sensitivity and specificity on forest plots and in receiver operating characteristic (ROC) space to explore between‐study variation in the performance of each test. Because our focus of inference was summary points, we used the bivariate model to summarise jointly the sensitivity and specificity of each test (Reitsma 2005; Chu 2006). This model accounts for between‐study variability in estimates of sensitivity and specificity through the inclusion of random effects for the logit sensitivity and logit specificity parameters of the bivariate model. Where sparse data precluded reliable estimation of the covariance matrix of the random effects, we simplified the model by assuming an exchangeable covariance structure (i.e., common variances for the random effects and a covariance) instead of the more complex unstructured covariance matrix that allows for separate variances for each random effect and a covariance.
We compared the diagnostic accuracy of ERCP and IOC by including covariate terms for test type in the bivariate model to estimate differences in the sensitivity and specificity of the two tests. We allowed the variances of the random effects and their covariance to depend also on test type thus allowing the variances to differ between tests. We assumed an exchangeable covariance structure for the variances of the random effects for each test. We used likelihood ratio tests to compare the fit of different models, and we compared the estimates of sensitivity and specificity between models to check the robustness of our assumptions about the variances of the random effects. If studies that evaluated ERCP and IOC in the same study population were available, we also performed a direct head‐to‐head comparison by limiting the test comparison to such studies. We performed meta‐analyses using the xtmelogit command in Stata version 13 (Stata‐Corp, College Station, Texas, USA). Confidence regions on summary ROC plots generated using Review Manager 5 are excessively conservative when there are few studies and they may appear inconsistent with the estimated confidence intervals (CI) (RevMan 2012). While estimation of the CIs relies on the standard errors, the confidence regions rely on the number of studies in addition to the standard errors and the covariance of the estimated mean logit sensitivity and specificity. Therefore, if fewer than 10 studies evaluated a test included in a meta‐analysis, we used 10 as the number of studies for generating the regions. This number is arbitrary but seems to provide a better approximation than using a small number of studies.
We created a table of pre‐test probabilities (using the observed median and range of prevalence from the included studies) against post‐test probabilities. We calculated the post‐test probabilities using these pre‐test probabilities and the summary positive and negative likelihood ratios. We calculated the summary likelihood ratios and their CIs using the Stata _diparm command and functions of the parameter estimates from the bivariate model that we fitted to estimate the summary sensitivities and specificities.
Investigations of heterogeneity
We visually inspected forest plots of sensitivity and specificity, and summary ROC plots to identify heterogeneity. We investigated the sources of heterogeneity stated in the Secondary objectives. Where possible, given the number of included studies, we formally explored heterogeneity by adding each potential source of heterogeneity listed above as a covariate in the bivariate model (meta‐regression with one covariate at a time).
Sensitivity analyses
Exclusion of participants with uninterpretable results can result in overestimation of diagnostic test accuracy (Schuetz 2012). In practice, uninterpretable test results would be generally considered as test negatives. Therefore, we performed sensitivity analyses by including uninterpretable test results as test negatives if sufficient data were available.
Assessment of reporting bias
As described in the section on Investigations of heterogeneity, we planned to investigate whether the summary sensitivity and specificity of the tests differed between studies that were published as full texts and those that were available only as abstracts.
Results
Results of the search
We identified 22,789 references through electronic searches of MEDLINE (8292 references), EMBASE (10,029 references), Science Citation Index Expanded and BIOSIS (4276 references), and DARE and HTA in The Cochrane Library (192 references). We identified no additional studies by searching the other sources. We excluded 5866 duplicates and 16,773 clearly irrelevant references through reading abstracts. We retrieved 150 references for further assessment. We excluded 140 references for the reasons listed in the Characteristics of excluded studies table. Ten studies fulfilled the inclusion criteria and provided data for the review. We were able to obtain additional information from the authors of two studies (Prat 1996; Montariol 1998). Figure 2 shows the flow of studies through the selection process.
2.
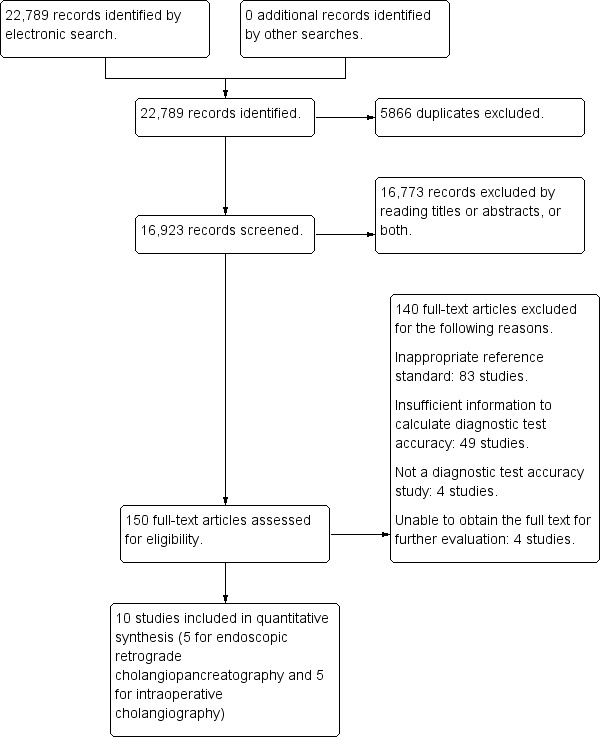
Flow of studies through the screening process.
Characteristics of included studies
We included 10 studies (Characteristics of included studies table). None of the studies compared ERCP and IOC in the same study population. Five studies including 318 participants (180 participants with and 138 participants without common bile duct stones) reported the diagnostic test accuracy of ERCP (Prat 1996; Norton 1997; Fazel 2002; Katz 2004; Ney 2005), and five studies including 654 participants (125 participants with and 529 participants without common bile duct stones) reported the diagnostic test accuracy of IOC (Fenton 1989; Montariol 1998; Silverstein 1998; Wu 2005; Li 2009). None of the 10 studies was diagnostic case‐control studies. The median pre‐test probability of common bile duct stones in the 10 studies was 0.35 (range 0.12 to 0.68).
Except for one study (Fazel 2002), the studies were full‐text publications. Five studies were prospective (Prat 1996; Montariol 1998; Silverstein 1998; Katz 2004; Li 2009), one was retrospective (Fenton 1989), and It was not clear whether the remaining studies were prospective or retrospective (Norton 1997; Fazel 2002; Ney 2005; Wu 2005). Based on the inclusion and exclusion criteria stated in nine studies, it appears participants were screened by ultrasound, liver function tests, or both prior to the performance of either ERCP or IOC but this information on prior testing was unclear in one study (Fenton 1989). Seven studies included people with suspicion of common bile duct stones based on the presence of clinical symptoms of obstructive jaundice or pancreatitis, or ultrasound or liver function tests suggestive of common bile duct stones (Prat 1996; Norton 1997; Silverstein 1998; Fazel 2002; Katz 2004; Wu 2005; Li 2009). One study excluded people with obstructive jaundice or pancreatitis with ultrasound or liver function tests suggestive of common bile stones (Montariol 1998). One study excluded people with obstructive jaundice or pancreatitis with unequivocal evidence of common bile duct stones on ultrasound or computed tomography scan or MRCP (Ney 2005). One study did not state whether participants were selected on the basis of symptoms or abnormal transabdominal ultrasound or liver function tests (Fenton 1989). It did not appear that EUS or magnetic resonance pancreatography was used prior to the index tests in any of the studies other than one study where some participants received magnetic resonance cholangiopancreatography (Ney 2005). Eight studies included only participants who had not undergone previous cholecystectomy (Fenton 1989; Norton 1997; Montariol 1998; Silverstein 1998; Katz 2004; Ney 2005; Wu 2005; Li 2009). For the remaining two studies, about 34% of participants had previously undergone cholecystectomy in one study (Prat 1996), and It was not clear whether the participants had undergone cholecystectomy in the other study (Fazel 2002). The proportion of people with common bile duct strictures was not stated in any of the studies.
Methodological quality of included studies
Figure 3 and Figure 4 summarise the methodological quality of the included studies. All the studies were of poor methodological quality.
3.
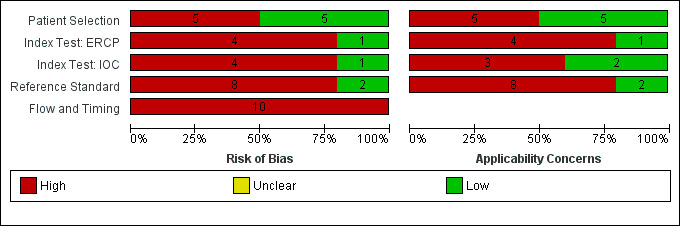
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies. Each bar shows the number of studies in each category. The index test domain was evaluated separately for each test. Of the 10 included studies, 5 studies evaluated endoscopic retrograde cholangio pancreatography (ERCP) and 5 studies evaluated intraoperative cholangiogram (IOC).
4.
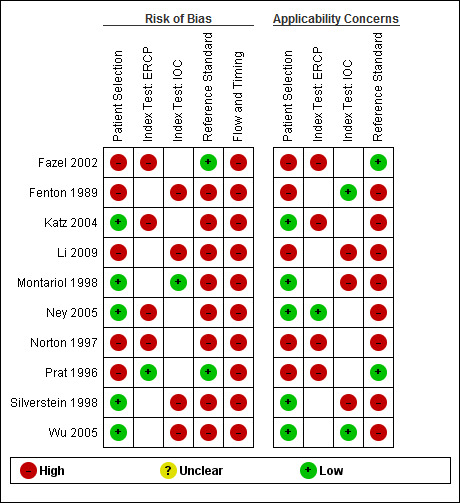
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study. In the index test domain, the empty white cell indicates that the study did not evaluate the test.
Patient selection
Five studies were at low risk of bias in the 'patient selection' domain (Montariol 1998; Silverstein 1998; Katz 2004; Ney 2005; Wu 2005). The same five studies were of low concern about applicability in this domain. The remaining five studies were at high risk of bias and with high concern about applicability because they either did not mention whether a consecutive or random sample of participants were included in the study (Fenton 1989; Norton 1997; Fazel 2002), or they excluded participants inappropriately (Prat 1996), or both (Li 2009).
Index test
Only one study of ERCP (Prat 1996) and one study of IOC (Montariol 1998) were at low risk of bias in the 'index study' domain. The remaining studies were at high risk of bias because it was not clear whether the index test results were interpreted without knowledge of the reference standard results. For two studies of IOC (Fenton 1989; Wu 2005) and one study of ERCP (Ney 2005), there was low concern about applicability. The remaining studies were of high concern regarding applicability because the studies did not mention the criteria for a positive test result (Prat 1996; Norton 1997; Montariol 1998; Silverstein 1998; Fazel 2002; Katz 2004; Li 2009).
Reference standard
Only two studies were at low risk of bias in the 'reference standard' domain (Prat 1996; Fazel 2002). The remaining studies were at high risk of bias in the 'reference standard' domain because it was either not clear whether the reference standards were interpreted without knowledge of the index test results (Fenton 1989; Norton 1997; Silverstein 1998; Katz 2004; Ney 2005; Wu 2005; Li 2009), or it was clear that the reference standards were interpreted with the knowledge of the index test results (Montariol 1998). We assess two studies as low concern about applicability (Prat 1996; Fazel 2002), while the remaining studies were of high concern because endoscopic or surgical clearance of common bile duct was achieved in people with a positive test result and clinical follow‐up was performed in people with negative test results (Fenton 1989; Norton 1997; Montariol 1998; Silverstein 1998; Katz 2004; Ney 2005; Wu 2005; Li 2009).
Flow and timing
None of the studies was at low risk of bias in the 'flow and timing' domain. Four studies did not report the time interval between the index test and reference standard (Fenton 1989; Fazel 2002; Katz 2004; Li 2009). In eight studies, the same reference standard was not used because endoscopic or surgical clearance of common bile duct was achieved in people with a positive test result and clinical follow‐up was performed in people with a negative test result (Fenton 1989; Norton 1997; Montariol 1998; Silverstein 1998; Katz 2004; Ney 2005; Wu 2005; Li 2009). It was not clear whether all the participants were included in the analysis in three studies (Norton 1997; Fazel 2002; Li 2009), while some participants were excluded from the analysis in four studies (Fenton 1989; Prat 1996; Montariol 1998; Wu 2005).
Findings
Table 1 summarises the results. The pre‐test probability (proportion with common bile duct stones out of the total number of participants) was computed for each included study. Based on the 10 studies, the minimum value was 0.12, the lower quartile was 0.19, the median was 0.29, the upper quartile was 0.40, and the maximum was 0.59.
Endoscopic retrograde cholangiopancreatography
Five studies including 318 participants reported the diagnostic accuracy of ERCP (Prat 1996; Norton 1997; Fazel 2002; Katz 2004; Ney 2005). The sensitivities ranged between 0.67 and 0.94, and the specificities ranged between 0.92 and 1.00 (Figure 5). The summary sensitivity was 0.83 (95% CI 0.72 to 0.90) and the summary specificity was 0.99 (95% CI 0.94 to 1.00). The summary positive likelihood ratio was 64 (95% CI 14 to 292) and summary negative ratio was 0.18 (95% CI 0.11 to 0.29). At the median pre‐test probability of 0.35, the post‐test probability associated with positive test result was 0.97 (95% CI 0.88 to 0.99) and negative test result was 0.09 (95% CI 0.05 to 0.14). At the minimum pre‐test probability of 0.12, the post‐test probability associated with positive test results was 0.90 (95% CI 0.66 to 0.98) and negative test results was 0.02 (95% CI 0.01 to 0.04). At the maximum pre‐test probability of 0.68, the post‐test probability associated with positive test results was 0.99 (95% CI 0.97 to 1.00) and negative test results was 0.28 (95% CI 0.18 to 0.39).
5.
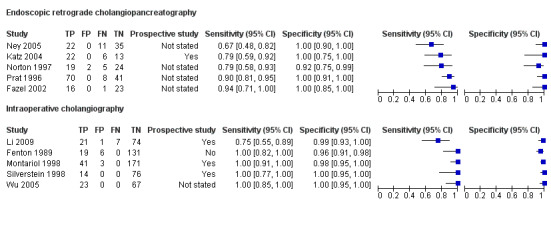
Forest plot of endoscopic retrograde cholangiopancreatography (ERCP) and intraoperative cholangiography (IOC) for diagnosis of common bile duct stones. Studies are ordered by sensitivity and study identifier. CI: confidence interval; FP: false positive; FN: false negative; TN: true negative; TP: true positive.
Intraoperative cholangiography
Five studies including 654 participants reported the diagnostic accuracy of IOC (Fenton 1989; Montariol 1998; Silverstein 1998; Wu 2005; Li 2009). The sensitivities ranged between 0.75 and 1.00, and the specificities ranged between 0.96 and 1.00 (Figure 5). The summary sensitivity was 0.99 (95% CI 0.83 to 1.00) and the summary specificity was 0.99 (95% CI 0.95 to 1.00). The summary positive likelihood ratio was 121 (95% CI 11 to 1370) and summary negative ratio was 0.01 (95% CI 0.00 to 0.20). At the median pre‐test probability of 0.35, the post‐test probability associated with positive test results was 0.98 (95% CI 0.85 to 1.00) and negative test results was 0.01 (95% CI 0.00 to 0.10). At the minimum pre‐test probability of 0.12, the post‐test probability associated with positive test results was 0.94 (95% CI 0.60 to 0.99) and negative test results was 0.00 (95% CI 0.00 to 0.03). At the maximum pre‐test probability of 0.68, the post‐test probability associated with positive test results was 1.00 (95% CI 0.96 to 1.00) and negative test results was 0.02 (95% CI 0.00 to 0.30).
Endoscopic retrograde cholangiopancreatography versus intraoperative cholangiography
We performed an indirect test comparison by including all studies in which either ERCP or IOC was performed. We had planned to include the information obtained only from a subgroup of the studies in order to ensure that the participants were similar in the studies included in the indirect comparison. However, all the studies included similar participants (i.e., most studies included participants identified at high risk of common bile duct stones based on the results of transabdominal ultrasound and liver function tests who did not undergo prior testing by MRCP or EUS and not undergone previous cholecystectomy) and so we included all the studies for the indirect comparison. Figure 6 shows the summary ROC plot comparing the accuracy of EUS and IOC. There was a statistically significant difference in sensitivity (P value = 0.05) with IOC showing higher sensitivity (0.99, 95% CI 0.83 to 1.00) compared to ERCP (0.83, 95% CI 0.72 to 0.90). In contrast, there was no evidence of a difference in specificity (P value = 0.7) with IOC having similar specificity (0.99, 95% CI 0.95 to 1.00) to that of ERCP (0.99, 95% CI 0.94 to 1.00).
6.
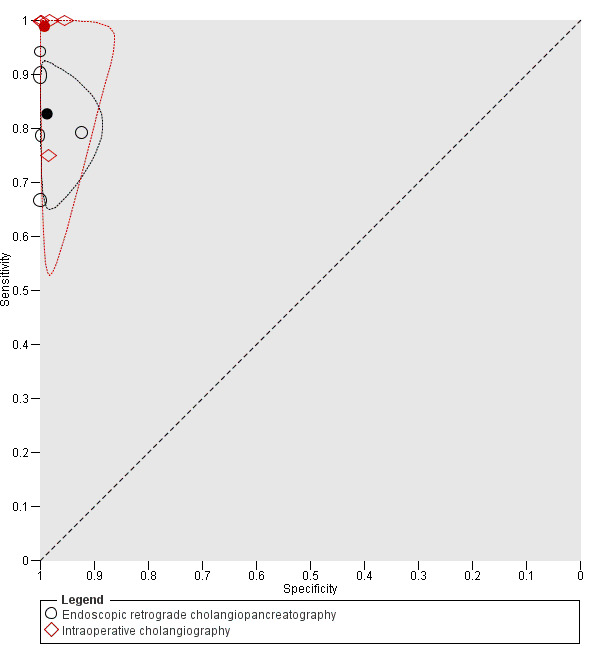
Summary receiver operating characteristic (ROC) plot comparing endoscopic retrograde cholangiopancreatography (ERCP) and intraoperative cholangiography (IOC) for diagnosis of common bile duct stones. The solid circles represent the summary estimates of sensitivity and specificity for each test, and are shown with 95% confidence regions (dotted lines around each summary point).
Investigations of heterogeneity
We carried out none of the planned investigations of heterogeneity using meta‐regression because few studies of each index test were included in the review.
Sensitivity analyses
Because there were only two participants with indeterminate test results in two studies (one participant in Prat 1996 and one participant in Wu 2005) and data were sparse, we did not perform sensitivity analyses.
Discussion
Summary of main results
Table 1 summarises the results. There was weak evidence to suggest that IOC had superior sensitivity (0.99, 95% CI 0.83 to 1.00) compared to ERCP (0.83, 95% CI 0.72 to 0.90). The specificities of the two tests were very similar; the specificities of IOC was 0.99 (95% CI 0.95 to 1.00) and of ERCP was 0.99 (95% CI 0.94 to 1.00). At the median pre‐test probability of common bile duct stones of 0.35 from the included studies, the post‐test probabilities associated with positive test results was 0.97 (95% CI 0.88 to 0.99) and negative test results was 0.09 (95% CI 0.05 to 0.14) for ERCP and positive test results was 0.98 (95% CI 0.85 to 1.00) and negative test results was 0.01 (95% CI 0.00 to 0.10) for IOC. The forest plots showed that specificity was consistent across the studies for both ERCP and IOC. With the exception of Li 2009, all studies of IOC reported 100% sensitivity but there was more variation in sensitivity for studies of ERCP. We were unable to explore heterogeneity because we included few studies evaluating ERCP or IOC in the review.
Strengths and weaknesses of the review
A major strength of this review was that we used recommended methods and searched the literature thoroughly, including full‐text publications and abstracts without any language restrictions. The determinants and extent of publication bias and selective reporting are not well known for diagnostic test accuracy studies. Inclusion of abstracts and non‐English articles may decrease the impact of publication bias to a certain extent even if publication bias existed in this field. The use of diagnostic test accuracy filters may lead to the elimination of some studies (Doust 2005), and so we did not use any diagnostic test accuracy filters. Two authors independently identified and extracted data from the studies potentially decreasing the errors related to single data extraction (Buscemi 2006).
There were some limitations. First, we were unable to explore heterogeneity formally because few studies were included in the review. We observed heterogeneity mainly in the estimates of the sensitivity of ERCP. Nevertheless, similar participants were included in the studies (i.e., people with abnormal liver function tests, transabdominal ultrasound, or both about to undergo cholecystectomy). The differences in sensitivity may be due to different intrinsic (or implicit) thresholds; however, one would expect a corresponding decrease in specificity in such a situation, which is not the case here. Overall, the heterogeneity in sensitivity remains unexplained.
Second, it was not possible to perform a direct comparison of the tests because none of the studies performed both tests within the same study population. It should be pointed out that indirect comparisons might give different results compared to the more reliable direct comparisons because differences in test accuracy may be confounded by differences in characteristics of the population and study methods (Takwoingi 2013). The preferred option for the treatment of gallbladder stones and common bile duct stones is currently endoscopic sphincterotomy followed by laparoscopic cholecystectomy (Ludwig 2001; Spelsberg 2009), which means that the same person is unlikely to receive both the tests (i.e., people with common bile duct stones on ERCP undergo endoscopic sphincterotomy during the same procedure and have their common bile duct stones removed before surgery). Randomised clinical trials comparing ERCP and IOC are possible but unlikely to be conducted since the choice of the test is usually based on the choice of treatment (i.e., endoscopic sphincterotomy versus operative exploration of the common bile duct). In this current era of laparoscopic surgery, endoscopic sphincterotomy and laparoscopic common bile duct exploration have similar results (Dasari 2013). The surgeon's preference determines the choice of the procedure and hence the test used prior to the procedure. Without evidence to support a significant difference in diagnostic test accuracy, the choice of treatment is likely to be based on surgeon's preference unless a value of information analysis can show that there is significant value in the question of which test to use for the diagnosis of common bile duct stones (Claxton 2006). Until then, the diagnostic accuracy of ERCP and IOC is likely to be compared indirectly despite the limitations of using this approach.
Third, none of the studies was of good methodological quality. The proportion of studies at high risk of bias and high concern regarding applicability was high in all the four domains. This makes the results unreliable. We considered endoscopic or surgical extraction of common bile duct stones in all participants as a better reference standard rather than a combination of extraction of common bile duct stones in participants with positive index test results and clinical follow‐up in those with negative index test. Endoscopic or surgical extraction was used in all participants in only two studies (Prat 1996; Fazel 2002). In the remaining eight studies, endoscopic or surgical clearance of common bile duct was achieved in people with a positive index test and clinical follow‐up was performed in people with a negative index test result (Fenton 1989; Norton 1997; Montariol 1998; Silverstein 1998; Katz 2004; Ney 2005; Wu 2005; Li 2009). This might result in overestimation of the diagnostic test accuracy although there is no evidence that this is the case. However, we acknowledge that even the best reference standard of endoscopic or surgical extraction of common bile duct stones can result in misclassification and hence alteration in diagnostic test accuracy if one or more stones reach the small bowel without the knowledge of the person who performed the common bile duct stone extraction. The use of different reference standards may also reflect the belief of the study authors about the probability of participants harbouring common bile duct stones. It is quite possible that in studies in which surgical or endoscopic clearance was performed in all participants (Prat 1996; Fazel 2002), included participants were at greater risk of having common bile duct stones because of their symptoms (i.e., they were more symptomatic) compared to the study in which participants with positive index test results underwent surgical or endoscopic extraction of stones and participants with negative index test results were followed clinically (Fenton 1989; Norton 1997; Montariol 1998; Silverstein 1998; Katz 2004; Ney 2005; Wu 2005; Li 2009). This was not evident from pre‐test probabilities of common bile duct stones in studies in which all participants underwent endoscopic or surgical extraction compared to those in which participants received different reference standards.
Another issue is that it is likely for the same person to perform the index test and the reference standard as part of the same clinical procedure since this is the usual clinical practice. This makes interpretation of index test blinded to the results of reference standard difficult and can result in overestimation of diagnostic test accuracy. This can be avoided if the index test results are documented prior to the performance of the reference standard and by documenting all subsequent alterations to the interpretation of the index test.
It has to be noted that most of the participants included in this study had not undergone EUS or MRCP. Using these tests prior to ERCP or IOC may improve the diagnostic test accuracy of ERCP and IOC.
Despite all these shortcomings, it should be pointed out that these studies provide the best available evidence on this topic.
Applicability of findings to the review question
Most of the participants included in this review were people who had not undergone previous cholecystectomy and were fit to undergo cholecystectomy with IOC or ERCP. Therefore, the findings of this review are applicable only to people suspected of common bile duct stones before they undergo cholecystectomy and are fit to undergo cholecystectomy with IOC or ERCP. Most participants either had symptoms of common bile duct obstruction (such as jaundice or pancreatitis) or features suggestive of common bile duct obstruction based on liver function tests and ultrasound. The findings of this review are applicable to only such people.
Previous research
This is the first systematic review on this topic.
Authors' conclusions
Implications for practice.
Some people may have common bile duct stones in spite of having a negative ERCP or IOC result. Such people may have to be re‐tested if the clinical suspicion of common bile duct stones is very high because of their symptoms or persistently abnormal liver function tests. However, it should be noted that the results of this review are based on few studies of poor methodological quality and so the results should be interpreted with caution.
Implications for research.
Studies of high methodological quality are necessary to assess the diagnostic accuracy of ERCP and IOC in the diagnosis of common bile duct stones. Considering that most people undergo triage tests such as ultrasound, liver function tests, and MRCP or EUS prior to ERCP or IOC, it is recommended that future studies either focus on such people or present the results separately for people with positive and negative triage test results so that it is possible to calculate the diagnostic test accuracy of ERCP and IOC separately for people with positive and negative triage test results. We acknowledge that differential verification cannot always be avoided if endoscopic sphincterotomy and extraction of stones is used as the reference standard because of the complications associated with this procedure (Gurusamy 2011). Surgical exploration of the common bile duct is a major surgical procedure and cannot be taken lightly. Based on these, people with positive test results are likely to undergo endoscopic sphincterotomy and extraction of stones or surgical exploration of the common bile duct while people with negative test results are likely to be followed up. Such people would benefit from being followed up for at least six months to ensure that they do not develop the symptoms of common bile duct stones. Future studies that avoid inappropriate exclusions would be useful to ensure that the true diagnostic accuracy of the tests for a given clinical context can be calculated. Long‐term follow‐up of people with negative tests will help in understanding the implications of false‐negative results and aid clinical decision making.
Notes
This review is based on a common protocolwhich needed to be split into three reviews (Giljaca 2013).
Acknowledgements
We thank the Cochrane Hepato‐Biliary Group staff, contact editors Mirella Fraquelli and Agostino Colli, and the UK Cochrane Diagnostic Test Accuracy Review Support Unit for their advice in the preparation of this review.
This project was funded by the National Institute for Health Research. Disclaimer of the Department of Health: "The views and opinions expressed in the review are those of the authors and do not necessarily reflect those of the National Institute for Health Research (NIHR), National Health Services (NHS), or the Department of Health".
Appendices
Appendix 1. Search strategies
| Database | Period of Search | Search Strategy |
| MEDLINE (PubMed) | 1946 to September 2012. | (((bile duct[tiab] or biliary[tiab] OR CBD[tiab]) AND (stone[tiab] OR stones[tiab] OR calculus[tiab] OR calculi[tiab])) OR choledocholithiasis[tiab] OR cholelithiasis[tiab] OR "Choledocholithiasis"[Mesh] OR "Common Bile Duct Calculi "[MESH] OR "Cholelithiasis "[MESH]) AND (CT[tiab] OR tomodensitometry[tiab] OR MRI[tiab] OR NMRI[tiab] OR zeugmatogra*[tiab] OR ((computed[tiab] OR computerised[tiab] OR computerized[tiab] OR magneti*[tiab] OR MR[tiab] OR NMR[tiab] OR proton[tiab]) AND (tomogra*[tiab] OR scan[tiab] OR scans[tiab] OR imaging[tiab] OR cholangiogra*[tiab])) OR "Tomography, X‐Ray Computed"[Mesh] OR "Magnetic Resonance Imaging"[Mesh] OR echogra*[tiab] OR ultrason*[tiab] OR ultrasound[tiab] OR EUS[tiab] OR "Ultrasonography"[Mesh] OR "Endosonography"[Mesh] OR cholangiogra*[tiab] OR cholangio?pancreatogra*[tiab] OR cholangiosco*[tiab] OR choledochosco*[tiab] OR ERCP[tiab] OR MRCP[tiab] OR "Cholangiography"[Mesh] OR "Cholangiopancreatography, Magnetic Resonance"[Mesh] OR liver function test[tiab] OR liver function tests[tiab] OR "Liver Function Tests"[Mesh]) |
| EMBASE (OvidSP) | 1947 to September 2012. | 1. (((bile duct or biliary or CBD) adj5 (stone or stones or calculus or calculi)) or choledocholithiasis or cholelithiasis).tw. 2. exp common bile duct stone/ or exp bile duct stone/ or exp cholelithiasis/ 3. 1 or 2 4. (CT or tomodensitometry or MRI or NMRI or zeugmatogra* or ((computed or computerised or computerized or magneti* or MR or NMR or proton) adj5 (tomogra* or scan or scans or imaging or cholangiogra*))).tw. 5. exp computer assisted tomography/ 6. exp nuclear magnetic resonance imaging/ 7. (echogra* or ultrason* or ultrasound or EUS).tw. 8. exp ultrasound/ 9. (cholangiogra* or cholangio?pancreatogra* or cholangiosco* or choledochosco* or ERCP or MRCP).tw. 10. exp cholangiography/ 11. (liver function test or liver function tests).tw. 12. exp liver function test/ 13. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14. 3 and 13 |
| Science Citation Index Expanded (ISI Web of Knowledge) | 1898 to September 2012. | #1 TS=(((bile duct or biliary OR CBD) AND (stone OR stones OR calculus OR calculi)) OR choledocholithiasis OR cholelithiasis) #2 TS=(CT OR tomodensitometry OR MRI OR NMRI OR zeugmatogra* OR ((computed OR computerised OR computerized OR magneti* OR MR OR NMR OR proton) AND (tomogra* OR scan OR scans OR imaging OR cholangiogra*))) #3 TS=(echogra* OR ultrason* OR ultrasound OR EUS) #4 TS=(cholangiogra* OR cholangio?pancreatogra* OR cholangiosco* OR choledochosco* OR ERCP OR MRCP) #5 TS=(liver function test OR liver function tests) #6 #5 OR #4 OR #3 OR #2 #7 #1 AND #6 |
| BIOSIS (ISI Web of Knowledge) | 1969 to September 2012. | #1 TS=(((bile duct or biliary OR CBD) AND (stone OR stones OR calculus OR calculi)) OR choledocholithiasis OR cholelithiasis) #2 TS=(CT OR tomodensitometry OR MRI OR NMRI OR zeugmatogra* OR ((computed OR computerised OR computerized OR magneti* OR MR OR NMR OR proton) AND (tomogra* OR scan OR scans OR imaging OR cholangiogra*))) #3 TS=(echogra* OR ultrason* OR ultrasound OR EUS) #4 TS=(cholangiogra* OR cholangio?pancreatogra* OR cholangiosco* OR choledochosco* OR ERCP OR MRCP) #5 TS=(liver function test OR liver function tests) #6 #5 OR #4 OR #3 OR #2 #7 #1 AND #6 |
| Clinicaltrials.gov | September 2012. | (bile duct) OR CBD OR choledocholithiasis OR cholelithiasis |
| Database of Abstracts of Reviews of Effects (DARE) and Health Technology Assessment (HTA) in The Cochrane Library (Wiley) |
September 2012. | #1 (((bile duct or biliary or CBD) NEAR/5 (stone OR stones OR calculus OR calculi)) OR choledocholithiasis OR cholelithiasis):ti,ab,kw #2 MeSH descriptor Choledocholithiasis explode all trees #3 (#1 OR #2) #4 (CT OR tomodensitometry OR MRI OR NMRI OR zeugmatogra* OR ((computed OR computerised OR computerized OR magneti* OR MR OR NMR OR proton) NEAR/5 (tomogra* OR scan OR scans OR imaging OR cholangiogra*))):ti,ab,kw #5 MeSH descriptor Tomography, X‐Ray Computed explode all trees #6 MeSH descriptor Magnetic Resonance Imaging explode all trees #7 (echogra* OR ultrason* OR ultrasound OR EUS):ti,ab,kw #8 MeSH descriptor Ultrasonography explode all trees #9 MeSH descriptor Endosonography explode all trees #10 (cholangiogra* OR cholangio?pancreatogra* OR cholangiosco* OR choledochosco* OR ERCP OR MRCP):ti,ab,kw #11 MeSH descriptor Cholangiography explode all trees #12 MeSH descriptor Cholangiopancreatography, Magnetic Resonance explode all trees #13 (liver function test OR liver function tests):ti,ab,kw #14 MeSH descriptor Liver Function Tests explode all trees #15 (#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14) #16 (#3 AND #15) |
| Medion (www.mediondatabase.nl/) | September 2012. | We will conduct four separate searches of the abstract using the terms: bile duct CBD choledocholithiasis cholelithiasis |
| ARIF (www.birmingham.ac.uk/research/activity/mds/projects/HaPS/PHEB/ARIF/databases/index.aspx) | September 2012. | (bile duct) OR CBD OR choledocholithiasis OR cholelithiasis |
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 Endoscopic retrograde cholangiopancreatography | 5 | 318 |
| 2 Intraoperative cholangiography | 5 | 654 |
1. Test.
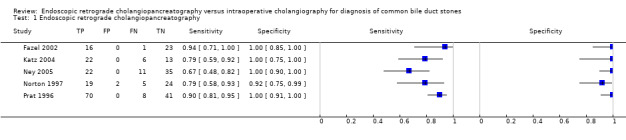
Endoscopic retrograde cholangiopancreatography.
2. Test.
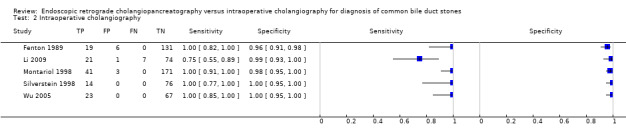
Intraoperative cholangiography.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fazel 2002.
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 40. Females: not stated. Age: not stated. Presentation: Inclusion criteria:
Setting: care setting: not stated, USA. |
||
| Index tests | Index test: endoscopic retrograde cholangiography. Further details: Technical specifications: not stated. Performed by: not stated. Criteria for positive diagnosis: not stated. | ||
| Target condition and reference standard(s) | Target condition: common bile duct stones. Reference standard: attempted endoscopic extraction of stones in all participants. Further details: Technical specifications: not applicable. Performed by: endoscopists and surgeons. Criteria for positive diagnosis: presence or absence of stones during endoscopic clearance. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated. Number of participants who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | Attempted to contact the authors in June 2013. Received no replies. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test ERCP | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| High | |||
Fenton 1989.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 156. Females: not stated. Age: not stated. Presentation: Inclusion criteria:
Setting: Department of Surgery, USA. |
||
| Index tests | Index test: intraoperative cholangiography. Further details: Technical specifications: not applicable. Performed by: surgeon. Criteria for positive diagnosis: filling defect. | ||
| Target condition and reference standard(s) | Target condition: common bile duct stones. Reference standard: extraction of stones in people with positive intraoperative cholangiography and clinical follow‐up of minimum 12 months in people with negative intraoperative cholangiography. Further details: Technical specifications: not applicable. Performed by: surgeons and clinicians. Criteria for positive diagnosis: extraction of stones in people with positive intraoperative cholangiography and clinical follow‐up of minimum 12 months in people with negative intraoperative cholangiography. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated. Number of participants who were excluded from the analysis: 15 (8.8%). | ||
| Comparative | |||
| Notes | Attempted to contact the authors in June 2013. Received no replies. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test IOC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Katz 2004.
| Study characteristics | |||
| Patient sampling | Type of study: prospective study. Consecutive or random sample: consecutive participants. | ||
| Patient characteristics and setting | Sample size: 41. Females: not stated. Age: not stated. Presentation: Inclusion criteria:
Setting: Department of Surgery, USA. |
||
| Index tests | Index test: endoscopic retrograde cholangiography. Further details: Technical specifications: not stated. Performed by: endoscopist. Criteria for positive diagnosis: not stated. | ||
| Target condition and reference standard(s) | Target condition: common bile duct stones. Reference standard: endoscopic extraction of bile duct stones in people with positive endoscopic retrograde cholangiography and follow‐up for at least 1 year for people with negative endoscopic retrograde cholangiography. Further details: Technical specifications: not applicable. Performed by: endoscopists and surgeons. Criteria for positive diagnosis: presence of stones during endoscopic clearance and clinical follow‐up with negative endoscopic retrograde cholangiography. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated. Number of participants who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | Attempted to contact the authors in June 2013. Received no replies. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test ERCP | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
Li 2009.
| Study characteristics | |||
| Patient sampling | Type of study: prospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 103. Females: 65 (63.1%). Age: 49 years. Presentation: Inclusion criteria: People undergoing cholecystectomy with ≥ 1 of the following features.
Setting: Department of Surgery, China. |
||
| Index tests | Index test: intraoperative cholangiography. Further details: Technical specifications: not applicable. Performed by: surgeon. Criteria for positive diagnosis: not stated. | ||
| Target condition and reference standard(s) | Target condition: common bile duct stones. Reference standard: extraction of stones in people with positive intraoperative cholangiography (surgical or endoscopic) and clinical follow‐up of minimum 12 months in people with negative intraoperative cholangiography. Further details: Technical specifications: not applicable. Performed by: surgeons and clinicians. Criteria for positive diagnosis: extraction of stones in people with positive intraoperative cholangiography (surgical or endoscopic) and clinical follow‐up of minimum 12 months in people with negative intraoperative cholangiography. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated. Number of participants who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | Attempted to contact the authors in June 2013. Received no replies. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test IOC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Unclear | ||
| High | |||
Montariol 1998.
| Study characteristics | |||
| Patient sampling | Type of study: prospective study. Consecutive or random sample: consecutive participants. | ||
| Patient characteristics and setting | Sample size: 240. Females: 171 (71.3%). Age: 57 years. Presentation: Inclusion criteria:
Exclusion criteria:
Setting: Surgery Departments, France. |
||
| Index tests | Index test: intraoperative cholangiography. Further details: Technical specifications: not applicable. Performed by: surgeon. Criteria for positive diagnosis: not stated. | ||
| Target condition and reference standard(s) | Target condition: common bile duct stones. Reference standard: surgical extraction of stones in people with positive intraoperative cholangiography and clinical follow‐up of minimum 12 months in people with negative intraoperative cholangiography. Further details: Technical specifications: not applicable. Performed by: surgeons and clinicians. Criteria for positive diagnosis: surgical extraction of stones in people with positive intraoperative cholangiography and clinical follow‐up of minimum 12 months in people with negative intraoperative cholangiography. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated. Number of participants who were excluded from the analysis: 25 (10.4%). | ||
| Comparative | |||
| Notes | Attempted to contact the authors in June 2013. Received replies in July 2013. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test IOC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Ney 2005.
| Study characteristics | |||
| Patient sampling | Type of study: prospective study. Consecutive or random sample: consecutive participants. | ||
| Patient characteristics and setting | Sample size: 68. Females: 49 (72.1%). Age: 57 years. Presentation: Inclusion criteria:
Exclusion criteria:
Setting: Department of Surgery, Brazil. |
||
| Index tests | Index test: endoscopic retrograde cholangiography. Further details: Technical specifications: standard videoduodenoscope. Performed by: experienced endoscopist. Criteria for positive diagnosis: filling defects in cholangiography. | ||
| Target condition and reference standard(s) | Target condition: common bile duct stones. Reference standard: endoscopic or surgical extraction of stones in people with positive endoscopic retrograde cholangiography and clinical follow‐up minimum 11 months in people with negative endoscopic retrograde cholangiography. Further details: Technical specifications: not applicable. Performed by: endoscopists, surgeons, and clinicians. Criteria for positive diagnosis: endoscopic or surgical extraction of stones in people with positive endoscopic retrograde cholangiography and clinical follow‐up minimum 11 months in people with negative endoscopic retrograde cholangiography. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated. Number of participants who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | Attempted to contact the authors in June 2013. Received no replies. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test ERCP | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
Norton 1997.
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 50. Females: 34 (68.0%). Age: 63 years. Presentation: Inclusion criteria:
Setting: Surgery Department, UK. |
||
| Index tests | Index test: endoscopic retrograde cholangiography. Further details: Technical specifications: not stated. Performed by: not stated. Criteria for positive diagnosis: not stated. | ||
| Target condition and reference standard(s) | Target condition: common bile duct stones. Reference standard: endoscopic or surgical extraction of stones in people with positive endoscopic retrograde cholangiography and clinical follow‐up minimum 6 months in people with negative endoscopic retrograde cholangiography. Further details: Technical specifications: not applicable. Performed by: endoscopists, surgeons, and clinicians. Criteria for positive diagnosis: endoscopic or surgical extraction of stones in people with positive endoscopic retrograde cholangiography and clinical follow‐up minimum 6 months in people with negative endoscopic retrograde cholangiography. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated. Number of participants who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | Attempted to contact the authors in June 2013. Received no replies. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test ERCP | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Unclear | ||
| High | |||
Prat 1996.
| Study characteristics | |||
| Patient sampling | Type of study: prospective study. Consecutive or random sample: consecutive participants. | ||
| Patient characteristics and setting | Sample size: 121. Females: 69 (57.0%). Age: 70 years. Presentation: Inclusion criteria:
Exclusion criteria:
Setting: Gastroenterology Department, France. |
||
| Index tests | Index test: endoscopic retrograde cholangiography. Further details: Technical specifications: TJF 100 videoduodenoscope (Olympus). Performed by: expert endoscopist. Criteria for positive diagnosis: not stated. | ||
| Target condition and reference standard(s) | Target condition: common bile duct stones. Reference standard: attempted endoscopic extraction of stones in all participants. Further details: Technical specifications: not applicable. Performed by: endoscopists and surgeons. Criteria for positive diagnosis: presence or absence of stones during endoscopic clearance. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: 1 (0.8%) Number of participants who were excluded from the analysis: 1 (0.8%). | ||
| Comparative | |||
| Notes | Attempted to contact the authors in June 2013. Received replies. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test ERCP | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Silverstein 1998.
| Study characteristics | |||
| Patient sampling | Type of study: prospective study. Consecutive or random sample: consecutive participants. | ||
| Patient characteristics and setting | Sample size: 90. Females: not stated. Age: not stated. Presentation: Inclusion criteria:
Setting: Department of Surgery, USA. |
||
| Index tests | Index test: intraoperative cholangiography. Further details: Technical specifications: not applicable. Performed by: surgeon. Criteria for positive diagnosis: not stated. | ||
| Target condition and reference standard(s) | Target condition: common bile duct stones. Reference standard: surgical extraction of stones in people with positive intraoperative cholangiography and clinical follow‐up of minimum 2 years in people with negative intraoperative cholangiography. Further details: Technical specifications: not applicable. Performed by: surgeons and clinicians. Criteria for positive diagnosis: surgical extraction of stones in people with positive intraoperative cholangiography and clinical follow‐up of minimum 2 years in people with negative intraoperative cholangiography. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: not stated. Number of participants who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | Attempted to contact the authors in June 2013. Received no replies. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test IOC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
Wu 2005.
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study. Consecutive or random sample: consecutive participants. | ||
| Patient characteristics and setting | Sample size: 91. Females: not stated. Age: not stated. Presentation: Inclusion criteria:
Setting: Department of Surgery, Taiwan. |
||
| Index tests | Index test: intraoperative cholangiography. Further details: Technical specifications: not applicable. Performed by: surgeon. Criteria for positive diagnosis: filling defect. | ||
| Target condition and reference standard(s) | Target condition: common bile duct stones. Reference standard: surgical extraction of stones in people with positive intraoperative cholangiography and clinical follow‐up of minimum 2 years in people with negative intraoperative cholangiography. Further details: Technical specifications: not applicable. Performed by: surgeons and clinicians. Criteria for positive diagnosis: surgical extraction of stones in people with positive intraoperative cholangiography and clinical follow‐up of minimum 2 years in people with negative intraoperative cholangiography. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: 1 (1.1%) Number of participants who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | Attempted to contact the authors in June 2013. Received no replies. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test IOC | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdul Ghani 1989 | Insufficient information to calculate diagnostic test accuracy. |
| Al Quorain 1996 | Inappropriate reference standard. |
| Alhayaf 2008 | Insufficient information to calculate diagnostic test accuracy. |
| Almersjo 1966 | Inappropriate reference standard. |
| Ang 2007 | Inappropriate reference standard. |
| Ashton 1998 | Inappropriate reference standard. |
| Askew 1992 | Inappropriate reference standard. |
| Aubertin 1995 | Insufficient information to calculate diagnostic test accuracy. |
| Aubertin 1996 | Inappropriate reference standard. |
| Azary 2011 | Inappropriate reference standard. |
| Barkun 1993 | Inappropriate reference standard. |
| Barr 1999 | Inappropriate reference standard. |
| Barteau 1995 | Inappropriate reference standard. |
| Beliveau 1964 | Inappropriate reference standard. |
| Bennion 2002 | Inappropriate reference standard. |
| Berci 1994 | Insufficient information to calculate diagnostic test accuracy. |
| Berdah 2001 | Inappropriate reference standard. |
| Bergamaschi 1999 | Inappropriate reference standard. |
| Bodula 2011 | Inappropriate reference standard. |
| Bokobza 1988 | Not a diagnostic test accuracy study. |
| Bornman 1981 | Inappropriate reference standard. |
| Bostanci 2003 | Unable to obtain this article. |
| Buckley 1987 | Inappropriate reference standard. |
| Calero Ayala 1983 | Insufficient information to calculate diagnostic test accuracy. |
| Canto 1995 | Insufficient information to calculate diagnostic test accuracy. |
| Cariani 2006 | Inappropriate reference standard. |
| Carroll 1996 | Inappropriate reference standard. |
| Catheline 1997 | Inappropriate reference standard. |
| Catheline 2000 | Inappropriate reference standard. |
| Catheline 2002 | Inappropriate reference standard. |
| Chaib 1990 | Inappropriate reference standard. |
| Chattopadhyay 1992 | Insufficient information to calculate diagnostic test accuracy. |
| Chen 1993 | Inappropriate reference standard. |
| Chen 2012 | Not a diagnostic test accuracy study. |
| Chernev 2001 | Inappropriate reference standard. |
| Chodoff 1960 | Insufficient information to calculate diagnostic test accuracy. |
| Chowdhury 1999 | Insufficient information to calculate diagnostic test accuracy. |
| Coppola 2001 | Inappropriate reference standard. |
| Corff 1957 | Inappropriate reference standard. |
| Csendes 1998 | Inappropriate reference standard. |
| Cwik 2003 | Unable to obtain this article. |
| Dalton 2005 | Inappropriate reference standard. |
| de Dios Vega 1982 | Insufficient information to calculate diagnostic test accuracy. |
| Derodra 1986 | Not a diagnostic test accuracy study. |
| di Angelo 2010 | Insufficient information to calculate diagnostic test accuracy. |
| di Angelo 2011 | Insufficient information to calculate diagnostic test accuracy. |
| Diez 2000 | Inappropriate reference standard. |
| Duchmann 1999 | Insufficient information to calculate diagnostic test accuracy. |
| Elizondo 1989 | Insufficient information to calculate diagnostic test accuracy. |
| Endo 2007 | Inappropriate reference standard. |
| Endo 2011 | Inappropriate reference standard. |
| Eshghi 2008 | Insufficient information to calculate diagnostic test accuracy. |
| Familiari 2004 | Inappropriate reference standard. |
| Famos 1990 | Inappropriate reference standard. |
| Faris 1975 | Inappropriate reference standard. |
| Farrands 1982 | Insufficient information to calculate diagnostic test accuracy. |
| Fiore 1997 | Insufficient information to calculate diagnostic test accuracy. |
| Flowers 1992 | Inappropriate reference standard. |
| Friedrichs 1981 | Not a diagnostic test accuracy study. |
| Garcia‐Caballero 1994 | Inappropriate reference standard. |
| Geron 1999 | Inappropriate reference standard. |
| Goletti 1995 | Insufficient information to calculate diagnostic test accuracy. |
| Gregg 1979 | Inappropriate reference standard. |
| Griffin 2003 | Inappropriate reference standard. |
| Grundy 1972 | Inappropriate reference standard. |
| Hammarstrom 1996 | Inappropriate reference standard. |
| Hoyuela 1999 | Inappropriate reference standard. |
| Huddy 1989 | Inappropriate reference standard. |
| Huynh 1996 | Insufficient information to calculate diagnostic test accuracy. |
| Ijzermans 1989 | Inappropriate reference standard. |
| Jakimowicz 1987 | Inappropriate reference standard. |
| Joyce 1991 | Insufficient information to calculate diagnostic test accuracy. |
| Karakan 2009 | Inappropriate reference standard. |
| Kent 1994 | Insufficient information to calculate diagnostic test accuracy. |
| Kielar 2005 | Inappropriate reference standard. |
| Kitahama 1986 | Inappropriate reference standard. |
| Kruis 1997 | Inappropriate reference standard. |
| Kullman 1984 | Inappropriate reference standard. |
| Kushnirenko 1988 | Insufficient information to calculate diagnostic test accuracy. |
| Lakoma 1996 | Inappropriate reference standard. |
| Lane 1982 | Inappropriate reference standard. |
| Ledniczky 2006 | Inappropriate reference standard. |
| Lenriot 1993 | Insufficient information to calculate diagnostic test accuracy. |
| Lim 2003 | Inappropriate reference standard. |
| Lin 1997 | Inappropriate reference standard. |
| Linghu 2004 | Inappropriate reference standard. |
| Liu 2005 | Inappropriate reference standard. |
| Lomanto 1999 | We were unable to obtain this article. |
| Low 1992 | Inappropriate reference standard. |
| Machi 1999 | Inappropriate reference standard. |
| Macmahon 1993 | Inappropriate reference standard. |
| Madhavan 1995 | Insufficient information to calculate diagnostic test accuracy. |
| Mala 2000 | Insufficient information to calculate diagnostic test accuracy. |
| Matzen 1981 | Insufficient information to calculate diagnostic test accuracy. |
| McCormick 1974 | Inappropriate reference standard. |
| Meduri 1998 | Inappropriate reference standard. |
| Miao 2008 | Insufficient information to calculate diagnostic test accuracy. |
| Mlynek 1971 | Insufficient information to calculate diagnostic test accuracy. |
| Moon 2005 | Inappropriate reference standard. |
| Nataly 2002 | Insufficient information to calculate diagnostic test accuracy. |
| Neitlich 1997 | Inappropriate reference standard. |
| Neoptolemos 1986a | Inappropriate reference standard. |
| Neoptolemos 1986b | Inappropriate reference standard. |
| Neri 2000 | Inappropriate reference standard. |
| Nevah 2011 | Insufficient information to calculate diagnostic test accuracy. |
| Nickkholgh 2006 | Inappropriate reference standard. |
| Nies 1997 | Inappropriate reference standard. |
| Oconnor 1986 | Insufficient information to calculate diagnostic test accuracy. |
| Ohtani 1997 | Insufficient information to calculate diagnostic test accuracy. |
| Osnes 1978 | Insufficient information to calculate diagnostic test accuracy. |
| Pamos 2003 | Inappropriate reference standard. |
| Pasanen 1993 | Insufficient information to calculate diagnostic test accuracy. |
| Pasanen 1994 | Inappropriate reference standard. |
| Polat 2000 | Inappropriate reference standard. |
| Polkowski 2001 | Insufficient information to calculate diagnostic test accuracy. |
| Potashov 1987 | Inappropriate reference standard. |
| Puri 2012 | Insufficient information to calculate diagnostic test accuracy. |
| Rahman 2010 | Inappropriate reference standard. |
| Rijna 2000 | Insufficient information to calculate diagnostic test accuracy. |
| Rives 1981 | Insufficient information to calculate diagnostic test accuracy. |
| Robinson 1995 | Insufficient information to calculate diagnostic test accuracy. |
| Rogers 2010 | Insufficient information to calculate diagnostic test accuracy. |
| Roig 1995 | Insufficient information to calculate diagnostic test accuracy. |
| Romano 2002 | Insufficient information to calculate diagnostic test accuracy. |
| Sauerbruch 1979 | Inappropriate reference standard. |
| Schulenburg 1969 | Insufficient information to calculate diagnostic test accuracy. |
| Shafiq 2003 | Insufficient information to calculate diagnostic test accuracy. |
| Shah 2011 | Inappropriate reference standard. |
| Sheen‐Chen 1990 | We were unable to obtain this article. |
| Siddiqui 1994 | Insufficient information to calculate diagnostic test accuracy. |
| Sigel 1982 | Inappropriate reference standard. |
| Sikic 1996 | Insufficient information to calculate diagnostic test accuracy. |
| Singh 2000 | Inappropriate reference standard. |
| Skorka 1982 | Inappropriate reference standard. |
| Snow 2001 | Inappropriate reference standard. |
| Stiris 2000 | Insufficient information to calculate diagnostic test accuracy. |
| Stuart 1998 | Inappropriate reference standard. |
| Tobin 1984 | Insufficient information to calculate diagnostic test accuracy. |
| Ueno 1997 | Insufficient information to calculate diagnostic test accuracy. |
| Videhult 2009 | Inappropriate reference standard. |
Differences between protocol and review
We used the statistical package Stata instead of SAS to fit the bivariate models.
We performed one main analysis. In this analysis, we excluded indeterminates test results. We considered the planned sensitivity analyses inappropriate because there were only two participants with indeterminate test results and data were sparse.
Contributions of authors
KG extracted the data from identified studies and wrote the review. VG, GP, and DH evaluated studies for inclusion. VG extracted the data from included studies. YT analysed the data and contributed to the draft of the review. DS and BRD critically commented on the review.
Sources of support
Internal sources
University College London, UK.
External sources
-
University of Rijeka ‐ Medical Faculty, Croatia.
TransMedRi project, EU FP7 REGPOT‐2010‐5 programme (grant agreement No. 256686)
National Institute of Health Research, UK.
Declarations of interest
None.
New
References
References to studies included in this review
Fazel 2002 {published data only}
- Fazel A, Catalano MF, Quadri A, Geenen JE. A comparison of the diagnostic accuracy of EUS and ERCP in identifying common bile duct stones. Gastrointestinal Endoscopy 2002;55(5):AB246. [Google Scholar]
Fenton 1989 {published data only}
- Fenton AH, Guyton DP, Evans DM. The utility of intraoperative cholangiography with acute cholecystitis. American Surgeon 1989;55(6):392‐5. [PubMed] [Google Scholar]
Katz 2004 {published data only}
- Katz D, Nikfarjam M, Sfakiotaki A, Christophi C. Selective endoscopic cholangiography for the detection of common bile duct stones in patients with cholelithiasis. Endoscopy 2004;36(12):1045‐9. [DOI] [PubMed] [Google Scholar]
Li 2009 {published data only}
- Li JW, Feng B, Wu L, Wang ML, Lu AG, Zang L, et al. Intraoperative cholangiography in combination with laparoscopic ultrasonography for the detection of occult choledocholithiasis. Medical Science Monitor 2009;15(9):MT126‐30. [PubMed] [Google Scholar]
Montariol 1998 {published data only}
- Montariol T, Charlier A, Rey C, Bataille N, Hay JM, Lacaine F, et al. Diagnosis of asymptomatic common bile duct stones: preoperative endoscopic ultrasonography versus intraoperative cholangiography ‐ a multicenter, prospective controlled study. Surgery 1998;124(1):6‐13. [PubMed] [Google Scholar]
Ney 2005 {published data only}
- Ney MVS, Maluf‐Filho F, Sakai P, Zilberstein B, Gama‐Rodrigues J, Rosa H. Endoscopic ultrasound versus endoscopic retrograde cholangiography for the diagnosis of choledocholithiasis: the influence of the size of the stone and diameter of the common bile duct. Arquivos de Gastroenterologia 2005;42(4):239‐43. [DOI] [PubMed] [Google Scholar]
Norton 1997 {published data only}
- Norton SA, Alderson D. Prospective comparison of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in the detection of bile duct stones. British Journal of Surgery 1997;84(10):1366‐9. [PubMed] [Google Scholar]
Prat 1996 {published data only}
- Prat F, Amouyal G, Amouyal P, Pelletier G, Fritsch J, Choury AD, et al. Prospective controlled study of endoscopic ultrasonography and endoscopic retrograde cholangiography in patients with suspected common‐bile duct lithiasis. Lancet 1996;347(8994):75‐9. [DOI] [PubMed] [Google Scholar]
Silverstein 1998 {published data only}
- Silverstein JC, Wavak E, Millikan KW. A prospective experience with selective cholangiography. American Surgeon 1998;64(7):654‐9. [PubMed] [Google Scholar]
Wu 2005 {published data only}
- Wu SC, Chen FC, Lo CJ. Selective intraoperative cholangiography and single‐stage management of common bile duct stone in laparoscopic cholecystectomy. World Journal of Surgery 2005;29(11):1402‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdul Ghani 1989 {published data only}
- Abdul Ghani AK. Selective per‐operative cholangiography and scoring method for selection. Bangladesh Medical Research Council Bulletin 1989;15(2):81‐9. [PubMed] [Google Scholar]
Alhayaf 2008 {published data only}
- Alhayaf N, Lalor E, Bain V, McKaigney J, Sandha GS. The clinical impact and cost implication of endoscopic ultrasound on use of endoscopic retrograde cholangiopancreatography in a Canadian university hospital. Canadian Journal of Gastroenterology 2008;22(2):138‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Almersjo 1966 {published data only}
- Almersjo O, Hultborn KA, Jensen C. Diagnosis of choledocholithiasis. Relations between historical, laboratory, operative, and peroperative roentgen data. Acta Chirurgica Scandinavica 1966;131(1):112‐26. [PubMed] [Google Scholar]
Al Quorain 1996 {published data only}
- Al Quorain A, Ibrahim EM, Al Sultan AI. Diagnostic and therapeutic value of ERCP and prediction of outcome: a retrospective analysis. Saudi Journal of Gastroenterology 1996;2(3):138‐41. [PubMed] [Google Scholar]
Ang 2007 {published data only}
- Ang TL, Teo EK, Fock KM. Endosonography‐ vs. endoscopic retrograde cholangiopancreatography‐based strategies in the evaluation of suspected common bile duct stones in patients with normal transabdominal imaging. Alimentary Pharmacology and Therapeutics 2007;26(8):1163‐70. [DOI] [PubMed] [Google Scholar]
Ashton 1998 {published data only}
- Ashton CE, McNabb WR, Wilkinson ML, Lewis RR. Endoscopic retrograde cholangiopancreatography in elderly patients. Age and Ageing 1998;27(6):683‐8. [DOI] [PubMed] [Google Scholar]
Askew 1992 {published data only}
- Askew A, Battersby C. Operative cholangiography ‐ a perspective. Australian and New Zealand Journal of Surgery 1992;62(7):530‐2. [DOI] [PubMed] [Google Scholar]
Aubertin 1995 {published data only}
- Aubertin JM, Prat F, Fritsch J, Choury AD, Etienne JP. Diagnostic ERCP in the era of laparoscopic surgery. Hepato‐Gastroenterology 1995;42(5):607‐11. [PubMed] [Google Scholar]
Aubertin 1996 {published data only}
- Aubertin JM, Levoir D, Becheur H, Bouillot JL, Bloch F, Petite JP. Prospective comparison of preoperative endosonography and intraoperative cholangiography during laparoscopic cholecystectomy in patients with common bile duct stone risk. Gastroenterology 1996;110(4 Supplement S):A446. [Google Scholar]
Azary 2011 {published data only}
- Azary SY, Kalbasi H, Setayesh A, Mousavi M, Hashemi A, Khodadoostan M, et al. Predictive value and main determinants of abnormal features of intraoperative cholangiography during cholecystectomy. Hepatobiliary and Pancreatic Diseases International 2011;10(3):308‐12. [DOI] [PubMed] [Google Scholar]
Barkun 1993 {published data only}
- Barkun JS, Fried GM, Barkun AN, Sigman HH, Hinchey EJ, Garzon J, et al. Cholecystectomy without operative cholangiography: implications for common bile duct injury and retained common bile duct stones. Annals of Surgery 1993;218(3):371‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barr 1999 {published data only}
- Barr LL, Frame BC, Coulanjon A. Proposed criteria for preoperative endoscopic retrograde cholangiography in candidates for laparoscopic cholecystectomy. Surgical Endoscopy‐Ultrasound and Interventional Techniques 1999;13(8):778‐81. [DOI] [PubMed] [Google Scholar]
Barteau 1995 {published data only}
- Barteau JA, Castro D, Arregui ME, Tetik C. A comparison of intraoperative ultrasound versus cholangiography in the evaluation of the common bile‐duct during laparoscopic cholecystectomy. Surgical Endoscopy‐Ultrasound and Interventional Techniques 1995;9(5):490‐6. [DOI] [PubMed] [Google Scholar]
Beliveau 1964 {published data only}
- Beliveau D, Bessette G, Laurendeau F, Lamoureux M. Clinical evaluation of perioperative cholangiography [Italian]. Union Medicale du Canada 1964;93(6):399‐407. [PubMed] [Google Scholar]
Bennion 2002 {published data only}
- Bennion RS, Wyatt LE, Thompson JE Jr. Effect of intraoperative cholangiography during cholecystectomy on outcome after gallstone pancreatitis. Journal of Gastrointestinal Surgery 2002;6(4):575‐81. [DOI] [PubMed] [Google Scholar]
Berci 1994 {published data only}
- Berci G, Morgenstern L. Laparoscopic management of common bile duct stones. A multi‐institutional SAGES study. Society of American Gastrointestinal Endoscopic Surgeons. Surgical Endoscopy 1994;8(10):1168‐74. [DOI] [PubMed] [Google Scholar]
Berdah 2001 {published data only}
- Berdah SV, Orsoni P, Bege T, Barthet M, Grimaud JC, Picaud R. Follow‐up of selective endoscopic ultrasonography and/or endoscopic retrograde cholangiography prior to laparoscopic cholecystectomy: a prospective study of 300 patients. Endoscopy 2001;33(3):216‐20. [DOI] [PubMed] [Google Scholar]
Bergamaschi 1999 {published data only}
- Bergamaschi R, Tuech JJ, Braconier L, Marvik R, Boyet J, Arnaud JP. Selective endoscopic retrograde cholangiography prior to laparoscopic cholecystectomy for gallstones. American Journal of Surgery 1999;178(1):46‐9. [DOI] [PubMed] [Google Scholar]
Bodula 2011 {published data only}
- Bodula A, Pazurek M, Wozniak B, Biernacki R, Antosik‐Biernacka A, Winter K, et al. Comparison of magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography in the diagnosis of pancreatobiliary diseases. Przeglad Gastroenterologiczny 2011;6(3):187‐94. [Google Scholar]
Bokobza 1988 {published data only}
- Bokobza B, Leblanc I, Michot F, Bouvier P, Teniere P. Peroperative investigations during surgery for biliary lithiasis. Endoscopy and ultrasonography. Revue Francaise de Gastro‐Enterologie 1988;24(236):729‐31. [Google Scholar]
Bornman 1981 {published data only}
- Bornman P, Marks IN, Kottler R, Denyer M, Hatfield A, Girdwood A. Value of endoscopic retrograde cholangiography in non‐jaundiced patients with suspected biliary calculi. South African Medical Journal 1981;60(7):289. [Google Scholar]
Bostanci 2003 {published data only}
- Bostanci EB, Yol S, Ozogul YB, Kayaalp C, Atalay F, Akoglu M. Comparison of magnetic resonance cholangiopancreatography with endoscopic retrograde cholangiopancreatography in patients with suspected common bile duct stone. Turkish Journal of Surgery 2003;19(3):157‐62. [Google Scholar]
Buckley 1987 {published data only}
- Buckley AR, Scudamore CH, Becker CD, Cooperberg PL. Intraoperative imaging of the biliary tree. Sonography vs. operative cholangiography. Journal of Ultrasound in Medicine 1987;6(10):589‐95. [DOI] [PubMed] [Google Scholar]
Calero Ayala 1983 {published data only}
- Calero Ayala B, Mino Fugarolas G, Dios Vega JF, Jimenez Sanchez JR. Ultrasound and ERCP in the diagnosis of common bile duct stones. Gastroenterologia y Hepatologia 1983;6(7):341‐4. [Google Scholar]
Canto 1995 {published data only}
- Canto M, Chak A, Sivak MV, Blades E, Stellato T. Endoscopic ultrasonography (EUS) versus cholangiography for diagnosing extrahepatic biliary stones ‐ a prospective, blinded study in precholecystectomy and postcholecystectomy patients. Gastrointestinal Endoscopy 1995;41(4):391. [Google Scholar]
Cariani 2006 {published data only}
- Cariani G, Marco M, Roda E, Solmi L. Efficacy and safety of ERCP in patients 90 years of age and older. Gastrointestinal Endoscopy 2006;64(3):471‐2. [DOI] [PubMed] [Google Scholar]
Carroll 1996 {published data only}
- Carroll BJ, Phillips EH, Rosenthal R, Gleischman S, Bray JF. One hundred consecutive laparoscopic cholangiograms ‐ Results and conclusions. Surgical Endoscopy‐Ultrasound and Interventional Techniques 1996;10(3):319‐23. [DOI] [PubMed] [Google Scholar]
Catheline 1997 {published data only}
- Catheline JM, Rizk N, Barrat C, Buenos P, Champault G. Peroperative investigation of the common bile duct during laparoscopic cholecystectomy. Laparoscopic ultrasonography versus cholangiography. A prospective study of 150 cases. Annales de Chirurgie 1997;51(1):46‐53. [PubMed] [Google Scholar]
Catheline 2000 {published data only}
- Catheline JM, Capelluto E, Turner R, Rizk N, Barrat C, Cazacu F, et al. Comparison of laparoscopic ultrasound and cholangiography during laparoscopic cholecystectomies. Results of a prospective study. Gastroenterologie Clinique et Biologique 2000;24(6‐7):619‐25. [PubMed] [Google Scholar]
Catheline 2002 {published data only}
- Catheline JM, Turner R, Paries J. Laparoscopic ultrasonography is a complement to cholangiography for the detection of choledocholithiasis at laparoscopic cholecystectomy. British Journal of Surgery 2002;89(10):1235‐9. [DOI] [PubMed] [Google Scholar]
Chaib 1990 {published data only}
- Chaib E, Freire AN, Cantanhede AV, Santana LL, Onofrio PL, Mello JB. Is it necessary to perform routine intraoperative cholangiography during cholecystectomy?. Arquivos de Gastroenterologia 1990;27(1):10‐3. [PubMed] [Google Scholar]
Chattopadhyay 1992 {published data only}
- Chattopadhyay TK, Gupta S, Kumar A, Kapoor VK. Peroperative cholangiogram ‐ routine or selective results of a prospective study. Tropical Gastroenterology 1992;13(2):75‐7. [PubMed] [Google Scholar]
Chen 1993 {published data only}
- Chen YK, McCarter TL, Santoro MJ, Hanson BL, Collen MJ. Utility of endoscopic retrograde cholangiopancreatography in the evaluation of idiopathic abdominal‐pain. American Journal of Gastroenterology 1993;88(9):1355‐8. [PubMed] [Google Scholar]
Chen 2012 {published data only}
- Chen CC. The efficacy of endoscopic ultrasound for the diagnosis of common bile duct stones as compared to CT, MRCP, and ERCP. Journal of the Chinese Medical Association 2012;75(7):301‐2. [DOI] [PubMed] [Google Scholar]
Chernev 2001 {published data only}
- Chernev N. Endoscopic retrograde cholangiopancreatography evaluation in diagnosing diseases of biliary tract and pancreas. Rentgenologiya i Radiologiya 2001;40(2):106‐9. [Google Scholar]
Chodoff 1960 {published data only}
- Chodoff RJ. Choledocholithiasis: failure of routine operative cholangiography to improve results. Journal of the Albert Einstein Medical Center, Philadelphia 1960;8:215‐7. [PubMed] [Google Scholar]
Chowdhury 1999 {published data only}
- Chowdhury A, Bourke MJ, Valiozis I, Williams SJ, Peduto A, Markson G, et al. Magnetic resonance cholangiography (MRC) versus endoscopic retrograde cholangio‐pancreaticography (ERCP) in the diagnosis of bile duct stones & strictures. Gastrointestinal Endoscopy 1999;49(4):AB154. [Google Scholar]
Coppola 2001 {published data only}
- Coppola R, Riccioni ME, Ciletti S, Cosentino L, Ripetti V, Magistrelli P, et al. Selective use of endoscopic retrograde cholangiopancreatography to facilitate laparoscopic cholecystectomy without cholangiography ‐ A review of 1139 consecutive cases. Surgical Endoscopy‐Ultrasound and Interventional Techniques 2001;15(10):1213‐6. [DOI] [PubMed] [Google Scholar]
Corff 1957 {published data only}
- Corff M. Operative cholangiography in surgery of the biliary tract. American Journal of Surgery 1957;94(1):9‐18. [DOI] [PubMed] [Google Scholar]
Csendes 1998 {published data only}
- Csendes A, Burdiles P, Diaz JC, Maluenda F, Korn O, Vallejo E, et al. Prevalence of common bile duct stones according to the increasing number of risk factors present: a prospective study employing routinely intraoperative cholangiography in 477 cases. Hepato‐Gastroenterology 1998;45(23):1415‐21. [PubMed] [Google Scholar]
Cwik 2003 {published data only}
- Cwik G, Wallner G, Ciechanski A, Zinkiewicz K, Zgodzinski W, Polkowski W. Endoscopic sphincterotomy in 100 patients scheduled for laparoscopic cholecystectomy: ultrasound evaluation. Hepato‐Gastroenterology 2003;50(53):1225‐8. [PubMed] [Google Scholar]
Dalton 2005 {published data only}
- Dalton SJ, Balupuri S, Guest J. Routine magnetic resonance cholangiopancreatography and intra‐operative cholangiogram in the evaluation of common bile duct stones. Annals of the Royal College of Surgeons of England 2005;87(6):469‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Dios Vega 1982 {published data only}
- Dios Vega JF, Mino Fugarolas G, Rodriguez Vives A, Guerrero Gimenez P, Gomez Camacho F, Robles Olid J. Ultrasonics and endoscopic retrograde cholangiopancreatography. Correlation in biliopancreatic pathology. Revista Espanola de Las Enfermedades del Aparato Digestivo 1982;62(1):45‐9. [PubMed] [Google Scholar]
Derodra 1986 {published data only}
- Derodra J. Predictive ability of choledocholithiasis indicators. Annals of Surgery 1986;203(3):335. [PMC free article] [PubMed] [Google Scholar]
di Angelo 2010 {published data only}
- Angelo IT, Prochazka V, Holinka M, Konecny M, Zapletalova J. Sensitivity, specificity and predictive value of choledocholithiasis by EUS and ERCP. Gastrointestinal Endoscopy 2010;71(5):AB293. [Google Scholar]
di Angelo 2011 {published data only}
- Angelo IT, Prochazka V, Holinka M, Zapletalova J. Endosonography versus endoscopic retrograde cholangiopancreatography in diagnosing extrahepatic biliary obstruction. Biomedical Papers 2011;155(4):339‐46. [DOI] [PubMed] [Google Scholar]
Diez 2000 {published data only}
- Diez J, Ferraina P, Oria M, Napolitano A, Caracoche M, Merello J. Results of routine intraoperative cholangiography in laparoscopic cholecystectomy. HPB 2000;2(3):317‐9. [Google Scholar]
Duchmann 1999 {published data only}
- Duchmann JC, Benkirane A, Herve E, Barbare JC, Latrive JP, Messerschmitt C. Endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography performed during the same anesthesia session. Gastroenterologie Clinique et Biologique 1999;23(10):1028‐32. [PubMed] [Google Scholar]
Elizondo 1989 {published data only}
- Elizondo J, Gallo S, Valdovinos MA, Paez R. Retrospective evaluation of 500 endoscopic cholangiopancreatographies performed at the Instituto Nacional de la Nutricion "Salvador Zubiran". Revista de Gastroenterologia de Mexico 1989;54(1):19‐26. [PubMed] [Google Scholar]
Endo 2007 {published data only}
- Endo T, Ito K, Fujita N, Noda Y, Kobayashi G, Horaguchi J, et al. The clinical significance of intraductal ultrasonography (IDUS) for patients with suspected common bile duct stones. Gastrointestinal Endoscopy 2007;65(5 Suppl S):AB217. [Google Scholar]
Endo 2011 {published data only}
- Endo T, Ito K, Fujita N, Noda Y, Kobayashi G, Obana T, et al. Intraductal ultrasonography in the diagnosis of bile duct stones: when and whom?. Digestive Endoscopy 2011;23(2):173‐5. [DOI] [PubMed] [Google Scholar]
Eshghi 2008 {published data only}
- Eshghi F. Routine magnetic resonance cholangiography compared to intra‐operative cholangiography in patients with suspected common bile duct stones. Journal of Medical Sciences 2008;8(1):98‐101. [PubMed] [Google Scholar]
Familiari 2004 {published data only}
- Familiari LT. MRCP vs ERCP: a comparative study in diagnosis of common bile duct stones. Gastrointestinal Endoscopy 2004;59(5 Suppl S):AB198. [Google Scholar]
Famos 1990 {published data only}
- Famos M, Stadler P, Schneekloth G. Selective intraoperative cholangiography. Helvetica Chirurgica Acta 1990;56(6):897‐901. [PubMed] [Google Scholar]
Faris 1975 {published data only}
- Faris I, Thomason JPS, Grundy DJ, Quesne LP. Operative cholangiography: a reappraisal based on a review of 400 cholangiograms. British Journal of Surgery 1975;62(12):966‐72. [DOI] [PubMed] [Google Scholar]
Farrands 1982 {published data only}
- Farrands PA, Britton DC. Peroperative cholangiography. Journal of the Royal College of Surgeons of Edinburgh 1982;27(3):150‐3. [PubMed] [Google Scholar]
Fiore 1997 {published data only}
- Fiore NF, Ledniczky G, Wiebke EA, Broadie TA, Pruitt AL, Goulet R, et al. An analysis of perioperative cholangiography in one thousand laparoscopic cholecystectomies. Surgery 1997;122(4):817‐21. [DOI] [PubMed] [Google Scholar]
Flowers 1992 {published data only}
- Flowers JL, Zucker KA, Graham SM, Scovill WA, Imbembo AL, Bailey RW. Laparoscopic cholangiography: results and indications. Annals of Surgery 1992;215(3):209‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Friedrichs 1981 {published data only}
- Friedrichs O. Diagnosis and therapy of the bile‐duct stone ‐ ERCP, fine needle PTC and endoscopic sphincterotomy in combination. Medizinische Welt 1981;32(32‐3):1204. [PubMed] [Google Scholar]
Garcia‐Caballero 1994 {published data only}
- Garcia‐Caballero M, Martin‐Palanca A, Vara‐Thorbeck C. Common bile duct stones after laparoscopic cholecystectomy and its treatment. The role of ultrasound and intravenous and intraoperative cholangiography. Surgical Endoscopy 1994;8(10):1182‐5. [DOI] [PubMed] [Google Scholar]
Geron 1999 {published data only}
- Geron N, Reshef R, Shiller M, Kniaz D, Eitan A. The role of endoscopic retrograde cholangiopancreatography in the laparoscopic era. Surgical Endoscopy 1999;13(5):452‐6. [DOI] [PubMed] [Google Scholar]
Goletti 1995 {published data only}
- Goletti O, Buccianti P, Ferrari M, Celona G, Chiarugi M, Cavina E. Laparoscopic sonography: a real alternative to cholangiography during laparoscopic cholecystectomy?. Hepato‐Gastroenterology 1995;42(5):612‐8. [PubMed] [Google Scholar]
Gregg 1979 {published data only}
- Gregg JA, McDonald DG. Endoscopic retrograde cholangiopancreatography and gray‐scale abdominal ultrasound in the diagnosis of jaundice. American Journal of Surgery 1979;137(5):611‐5. [DOI] [PubMed] [Google Scholar]
Griffin 2003 {published data only}
- Griffin N, Wastle ML, Dunn WK, Ryder SD, Beckingham IJ. Magnetic resonance cholangiopancreatography versus endoscopic retrograde cholangiopancreatography in the diagnosis of choledocholithiasis. European Journal of Gastroenterology and Hepatology 2003;15(7):809‐13. [DOI] [PubMed] [Google Scholar]
Grundy 1972 {published data only}
- Grundy DJ, King PA, Lloyd G. Comparative evaluation of preoperative and operative radiology in biliary‐tract disease. British Journal of Surgery 1972;59(3):205‐8. [DOI] [PubMed] [Google Scholar]
Hammarstrom 1996 {published data only}
- Hammarstrom LE, Holmin T, Stridbeck H, Ihse I. Routine preoperative infusion cholangiography at elective cholecystectomy: a prospective study in 694 patients. British Journal of Surgery 1996;83(6):750‐4. [DOI] [PubMed] [Google Scholar]
Hoyuela 1999 {published data only}
- Hoyuela C, Cugat E, Bretcha P, Collera P, Espinos J, Marco C. Must ERCP be routinely performed choledocholithiasis is suspected?. Digestive Surgery 1999;16(5):411‐4. [DOI] [PubMed] [Google Scholar]
Huddy 1989 {published data only}
- Huddy SP, Southam JA. Is intravenous cholangiography an alternative to the routine per‐operative cholangiogram?. Postgraduate Medical Journal 1989;65(770):896‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huynh 1996 {published data only}
- Huynh CH, Stadt J, Deviere J, Mehdi A, Nakadi I, Cremer M, et al. Preoperative endoscopic retrograde cholangiopancreatography: therapeutic impact in a general population of patients needing a cholecystectomy. Hepato‐Gastroenterology 1996;43(12):1484‐91. [PubMed] [Google Scholar]
Ijzermans 1989 {published data only}
- Ijzermans JN, Waard P, Merkelbach JW. Cholangiography during cholecystectomy: a plea for selective use. Netherlands Journal of Surgery 1989;41(4):79‐81. [PubMed] [Google Scholar]
Jakimowicz 1987 {published data only}
- Jakimowicz JJ, Rutten H, Jurgens PJ, Carol EJ. Comparison of operative ultrasonography and radiography in screening of the common bile‐duct for calculi. World Journal of Surgery 1987;11(5):628‐34. [DOI] [PubMed] [Google Scholar]
Joyce 1991 {published data only}
- Joyce WP, Keane R, Burke GJ, Daly M, Drumm J, Egan TJ, et al. Identification of bile duct stones in patients undergoing laparoscopic cholecystectomy. British Journal of Surgery 1991;78(10):1174‐6. [DOI] [PubMed] [Google Scholar]
Karakan 2009 {published data only}
- Karakan T, Cindoruk M, Alagozlu H, Ergun M, Dumlu S, Unal S. EUS versus endoscopic retrograde cholangiography for patients with intermediate probability of bile duct stones: a prospective randomized trial. Gastrointestinal Endoscopy 2009;69(2):244‐52. [DOI] [PubMed] [Google Scholar]
Kent 1994 {published data only}
- Kent AL, Cox MR, Wilson TG, Padbury RTA, Toouli J. Endoscopic retrograde cholangiopancreatography following laparoscopic cholecystectomy. Australian and New Zealand Journal of Surgery 1994;64(6):407‐12. [DOI] [PubMed] [Google Scholar]
Kielar 2005 {published data only}
- Kielar A, Toa H, Sekar A, Mimeault R, Jaffey J. Comparison of CT duodeno‐cholangiopancreatography to ERCP for assessing biliary obstruction. Journal of Computer Assisted Tomography 2005;29(5):596‐601. [DOI] [PubMed] [Google Scholar]
Kitahama 1986 {published data only}
- Kitahama A, Kerstein MD, Overby JL. Routine intraoperative cholangiogram. Surgery Gynecology and Obstetrics 1986;162(4):317‐22. [PubMed] [Google Scholar]
Kruis 1997 {published data only}
- Kruis W, Roehrig H, Hardt M, Pohl C, Schlosser D. A prospective evaluation of the diagnostic work‐up before laparoscopic cholecystectomy. Endoscopy 1997;29(7):602‐8. [DOI] [PubMed] [Google Scholar]
Kullman 1984 {published data only}
- Kullman E, Borch K, Tarpila E, Liedberg G. Endoscopic retrograde cholangiopancreatography (ERCP) in patients with jaundice and suspected biliary obstruction. Acta Chirurgica Scandinavica 1984;150(8):657‐63. [PubMed] [Google Scholar]
Kushnirenko 1988 {published data only}
- Kushnirenko O, Golubev VV, Ruchkin VI, Egorov A. Retrograde pancreaticocholangiography in the diagnosis of residual choledocholithiasis. Khirurgiia 1988;9:64‐6. [PubMed] [Google Scholar]
Lakoma 1996 {published data only}
- Lakoma S, Malczak J, Dziekan R, Kryskow A. Choledocholithiasis ‐ the evaluation of diagnostic effectiveness of USG and ERCP in own material [Polish]. Acta Endoscopica Polona 1996;6(4):165‐7. [Google Scholar]
Lane 1982 {published data only}
- Lane RJ, Coupland GA. Ultrasonic indications to explore the common bile duct. Surgery 1982;91(3):268‐74. [PubMed] [Google Scholar]
Ledniczky 2006 {published data only}
- Ledniczky G, Fiore N, Bognar G, Ondrejka P, Grosfeld JL. Evaluation of perioperative cholangiography in one thousand laparoscopic cholecystectomies. Chirurgia 2006;101(3):267‐72. [PubMed] [Google Scholar]
Lenriot 1993 {published data only}
- Lenriot JP, Neel JC, Hay JM, Jaeck D, Millat B, Fagniez PL. Retrograde cholangiopancreatography and endoscopic sphincterotomy for biliary lithiasis. Prospective evaluation in surgical circle. Gastroenterologie Clinique et Biologique 1993;17(4):244‐50. [PubMed] [Google Scholar]
Lim 2003 {published data only}
- Lim KP, Khan AL, Kirk HJ, Deans H, Koruth M. A prospective comparison of endoscopic retrograde cholangiography with magnetic resonance cholangiography in choledocholithiasis. British Journal of Surgery 2003;90(Suppl 1):94. [Google Scholar]
Lin 1997 {published data only}
- Lin G, Halevy A, Girtler O, GoldDeutch R, Zisman A, Scapa E. The role of endoscopic retrograde cholangiopancreatography in management of patients recovering from acute biliary pancreatitis in the laparoscopic era. Surgical Endoscopy‐Ultrasound and Interventional Techniques 1997;11(4):371‐5. [DOI] [PubMed] [Google Scholar]
Linghu 2004 {published data only}
- Linghu EQ, Cheng LF, Wang XD, Wang ZQ, Yang YS, Li W, et al. Intraductal ultrasonography and endoscopic retrograde cholangiography in diagnosis of extrahepatic bile duct stones: a comparative study. Hepatobiliary and Pancreatic Diseases International 2004;3(1):129‐32. [PubMed] [Google Scholar]
Liu 2005 {published data only}
- Liu CL, Fan ST, Lo CM, Tso WK, Wong Y, Poon RTP, et al. Comparison of early endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in the management of acute biliary pancreatitis: a prospective randomized study. Clinical Gastroenterology and Hepatology 2005;3(12):1238‐44. [DOI] [PubMed] [Google Scholar]
Lomanto 1999 {published data only}
- Lomanto D, Carlei F, Paganini AM, Nardovino M, Simone P, Angelis L, et al. Validation of predictive factors for choledocholithiasis. Gastroenterology 1999;116(4):A1330‐1. [Google Scholar]
Low 1992 {published data only}
- Low SC, Ng FC, Yap YL, Chng HC. Operative cholangiography. Singapore Medical Journal 1992;33(3):252‐4. [PubMed] [Google Scholar]
Machi 1999 {published data only}
- Machi J, Tateishi T, Oishi AJ, Furumoto NL, Oishi RH, Uchida S, et al. Laparoscopic ultrasonography versus operative cholangiography during laparoscopic cholecystectomy: review of the literature and a comparison with open intraoperative ultrasonography. Journal of the American College of Surgeons 1999;188(4):360‐7. [DOI] [PubMed] [Google Scholar]
Macmahon 1993 {published data only}
- Macmahon M, Walsh TN, Brennan P, Osborne H, Courtney MG. Endoscopic retrograde cholangiopancreatography in the elderly ‐ a single unit audit. Gerontology 1993;39(1):28‐32. [DOI] [PubMed] [Google Scholar]
Madhavan 1995 {published data only}
- Madhavan KK, Macintyre IMC, Wilson RG, Saunders JH, Nixon SJ, Hamer‐Hodges DW. Role of intraoperative cholangiography in laparoscopic cholecystectomy. British Journal of Surgery 1995;82(2):249‐52. [DOI] [PubMed] [Google Scholar]
Mala 2000 {published data only}
- Mala T, Lunde OC, Nesbakken A, Aadland E, Stiris M. Endoscopic retrograde cholangiopancreatography ‐ a 4‐year retrospective study. Tidsskrift for Den Norske Laegeforening 2000;120(5):560‐2. [PubMed] [Google Scholar]
Matzen 1981 {published data only}
- Matzen P, Haubek A, Holst‐Christensen J. Accuracy of direct cholangiography by endoscopic or transhepatic route in jaundice: a prospective study. Gastroenterology 1981;81(2):237‐41. [PubMed] [Google Scholar]
McCormick 1974 {published data only}
- McCormick JS, Bremner DN, Thomson JW, McNair TJ, Philp T. The operative cholangiogram: its interpretation, accuracy and value in association with cholecystectomy. Annals of Surgery 1974;180(6):902‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meduri 1998 {published data only}
- Meduri B, Aubert A, Chiche R, Fritsch J. Laparoscopic cholecystectomy and common bile duct stones: value of preoperative endoscopic ultrasonography and endoscopic retrograde cholangiography. Gastroenterologie Clinique et Biologique 1998;22(10):759‐65. [PubMed] [Google Scholar]
Miao 2008 {published data only}
- Miao L, Fan ZN, Ji GZ, Wen W, Wang X, Xiong GY, et al. Comparative study of ultrasonography, magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography in common duct stones. Chinese Journal of Surgery 2008;46(19):1465‐7. [PubMed] [Google Scholar]
Mlynek 1971 {published data only}
- Mlynek HJ, Schenker U. Diagnostic value of routine cholangiography during surgical treatment of the biliary ducts. Zentralblatt fur Chirurgie 1971;96(45):1537‐49. [PubMed] [Google Scholar]
Moon 2005 {published data only}
- Moon JH, Cho YD, Cha SW, Cheon YK, Ahn HC, Kim YS, et al. The detection of bile duct stones in suspected biliary pancreatitis: comparison of MRCP, ERCP, and intraductal US. American Journal of Gastroenterology 2005;100(5):1051‐7. [DOI] [PubMed] [Google Scholar]
Nataly 2002 {published data only}
- Nataly Y, Merrie AE, Stewart ID. Selective use of preoperative endoscopic retrograde cholangiopancreatography in the era of laparoscopic cholecystectomy. Australian and New Zealand Journal of Surgery 2002;72(3):186‐9. [DOI] [PubMed] [Google Scholar]
Neitlich 1997 {published data only}
- Neitlich JD, Topazian M, Smith RC, Gupta A, Burrell MI, Rosenfield A. Detection of choledocholithiasis: comparison of unenhanced helical CT and endoscopic retrograde cholangiopancreatography. Radiology 1997;203(3):753‐7. [DOI] [PubMed] [Google Scholar]
Neoptolemos 1986a {published data only}
- Neoptolemos JP, London N, Bailey I. The role of clinical and biochemical criteria and endoscopic retrograde cholangiopancreatography in the urgent diagnosis of common bile duct stones in acute pancreatitis. Surgery 1986;100(4):732‐42. [PubMed] [Google Scholar]
Neoptolemos 1986b {published data only}
- Neoptolemos JP, London N, Bailey I, Shaw D, Carrlocke DL, Fossard DP. Detection of common bile‐duct stones during acute gallstone pancreatitis ‐ the role of biochemical criteria and ERCP. British Journal of Surgery 1986;73(12):1030‐1. [Google Scholar]
Neri 2000 {published data only}
- Neri V, Ambrosi A, Lauro G, Melino R, Valentino TP. Role of ERCP, MRCP and laparoscopic exploration of common biliary duct. E.A.E.S: Proceedings of the 8th International Congress of the European Association for Endoscopic Surgery. 2000:167‐71.
Nevah 2011 {published data only}
- Nevah MI, Thosani N, Tanikella R, Ko TC, Wolf DS, Fallon MB, et al. ERCP for suspected choledocholithiasis: evaluating the ASGE guidelines. Gastrointestinal Endoscopy 2011;73(4 Suppl):AB116. [Google Scholar]
Nickkholgh 2006 {published data only}
- Nickkholgh A, Soltaniyekta S, Kalbasi H. Routine versus selective intraoperative cholangiography during laparoscopic cholecystectomy ‐ a survey of 2,130 patients undergoing laparoscopic cholecystectomy. Surgical Endoscopy 2006;20(6):868‐74. [DOI] [PubMed] [Google Scholar]
Nies 1997 {published data only}
- Nies C, Bauknecht F, Groth C, Clerici T, Bartsch D, Lange J, et al. Intraoperative cholangiography as a routine method? A prospective, controlled, randomized study. Der Chirurg 1997;68(9):892‐7. [DOI] [PubMed] [Google Scholar]
Oconnor 1986 {published data only}
- Oconnor HJ, Hamilton I, Ellis WR, Watters J, Lintott DJ, Axon ATR. Ultrasound detection of choledocholithiasis ‐ prospective comparison with ERCP in the postcholecystectomy patient. Gastrointestinal Radiology 1986;11(2):161‐4. [DOI] [PubMed] [Google Scholar]
Ohtani 1997 {published data only}
- Ohtani T, Kawai C, Shirai Y, Kawakami K, Yoshida K, Hatakeyama K. Intraoperative ultrasonography versus cholangiography during laparoscopic cholecystectomy: a prospective comparative study. Journal of the American College of Surgeons 1997;185(3):274‐82. [DOI] [PubMed] [Google Scholar]
Osnes 1978 {published data only}
- Osnes M, Gronseth K, Larsen S, Lotveit T, Lowe P, Nordshus T. Comparison of endoscopic retrograde and intravenous cholangiography in diagnosis of biliary calculi. Lancet 1978;2(8083):230. [DOI] [PubMed] [Google Scholar]
Pamos 2003 {published data only}
- Pamos S, Benages A, Medina E, Martinez Sanjuan V. Prospective evaluation of magnetic resonance cholangiopancreatography in patients with biliary disease: comparative study with conventional ultrasonography and endoscopic retrograde cholangiopancreatography diagnostic algorithm. Digestive and Liver Disease 2003;35(3):186‐92. [DOI] [PubMed] [Google Scholar]
Pasanen 1993 {published data only}
- Pasanen PA, Partanen KP, Pikkarainen PH, Alhava EM, Janatuinen EK, Pirinen AE. A comparison of ultrasound, computed tomography and endoscopic retrograde cholangiopancreatography in the differential diagnosis of benign and malignant jaundice and cholestasis. European Journal of Surgery (Acta Chirurgica) 1993;159(1):23‐9. [PubMed] [Google Scholar]
Pasanen 1994 {published data only}
- Pasanen PA, Partanen K, Pikkarainen P, Alhava E, Pirinen A, Janatuinen E. A prospective study on the value of ultrasound, computed tomography and endoscopic retrograde cholangiopancreatography in the diagnosis of unjaundiced cholestasis. In Vivo 1994;8(2):227‐30. [PubMed] [Google Scholar]
Polat 2000 {published data only}
- Polat FR, Abci I, Coskun I, Uranues S. The importance of intraoperative cholangiography during laparoscopic cholecystectomy. Journal of the Society of Laparoendoscopic Surgeons 2000;4(2):103‐7. [PMC free article] [PubMed] [Google Scholar]
Polkowski 2001 {published data only}
- Polkowski M, Regula J, Tilszer A, Rupinski M, Wronska E, Butruk E. Endosonography instead of endoscopic retrograde cholangiography in patients with low‐to‐moderate probability of bile duct stones ‐ a randomised, prospective comparison of two management strategies. Gastroenterologia Polska 2001;8(3):269‐76. [Google Scholar]
Potashov 1987 {published data only}
- Potashov LV, Sidorov AI, Nurmakov A, Shchetinin VN. Use of endoscopic retrograde pancreatocholangiography in diagnosing and treating choledocholithiasis. Klinichna Khirurhiia 1987;9:62‐3. [PubMed] [Google Scholar]
Puri 2012 {published data only}
- Puri R, Sud R, Thandassery RB. EUS versus ERC in patients with moderate risk of common bile duct stones. Gastrointestinal Endoscopy 2012;75(4 Suppl):AB197‐8. [Google Scholar]
Rahman 2010 {published data only}
- Rahman R, Ju J, Shamma's J, Goebel S, Sundaram U. Correlation between MRCP and ERCP findings at a tertiary care hospital. West Virginia Medical Journal 2010;106(5):14‐9. [PubMed] [Google Scholar]
Rijna 2000 {published data only}
- Rijna H, Kemps WG, Eijsbouts Q, Meuwissen SG, Cuesta MA. Preoperative ERCP approach to common bile duct stones: results of a selective policy. Digestive Surgery 2000;17(3):229‐33. [DOI] [PubMed] [Google Scholar]
Rives 1981 {published data only}
- Rives J, Flament JB, Palot JP, Delattre JF. Modern peroperative radiology in biliary surgery. Results obtained in a continuous series of 341 cases. Journal de Chirurgie 1981;118(1):19‐24. [PubMed] [Google Scholar]
Robinson 1995 {published data only}
- Robinson BL, Donohue JH, Gunes S, Thompson GB, Grant CS, Sarr MG, et al. Selective operative cholangiography. Appropriate management for laparoscopic cholecystectomy. Archives of Surgery 1995;130(6):625‐30. [DOI] [PubMed] [Google Scholar]
Rogers 2010 {published data only}
- Rogers SJ, Cello JP, Horn JK, Siperstein AE, Schecter WP, Campbell AR, et al. Prospective randomized trial of LC+LCBDE vs ERCP/S+LC for common bile duct stone disease. Archives of Surgery 2010;145(1):28‐33. [DOI] [PubMed] [Google Scholar]
Roig 1995 {published data only}
- Roig MVP, Espinosa RG, Rodero DR. Risk of common bile‐duct stones ‐ selective criteria for preoperative ERCP or intraoperative cholangiography in laparoscopic cholecystectomy. British Journal of Surgery 1995;82(Suppl 1):15. [Google Scholar]
Romano 2002 {published data only}
- Romano F, Franciosi CM, Caprotti R, Fina S, Lomazzi A, Colombo G, et al. Preoperative selective endoscopic retrograde cholangiopancreatography and laparoscopic cholecystectomy without cholangiography. Surgical Laparoscopy, Endoscopy and Percutaneous Techniques 2002;12(6):408‐11. [DOI] [PubMed] [Google Scholar]
Sauerbruch 1979 {published data only}
- Sauerbruch T, Wotzka R, Rehkamp D, Rummel T. Upper‐abdominal ultrasonography and ERCP?. Deutsche Medizinische Wochenschrift 1979;104(5):165‐6, 169‐71. [DOI] [PubMed] [Google Scholar]
Schulenburg 1969 {published data only}
- Schulenburg CAR. Operative cholangiography: 1,000 cases. Surgery 1969;65(5):723‐39. [PubMed] [Google Scholar]
Shafiq 2003 {published data only}
- Shafiq K, Thomas E, Brett B, Jamieson C. Evaluating MRC against ERCP in the assessment of bile duct stones. Gut 2003;52(Suppl 1):A25. [Google Scholar]
Shah 2011 {published data only}
- Shah JN, Shah C. A five years review intra‐operative cholangiogram. Journal of Nepal Health Research Council 2011;9(1):52‐5. [PubMed] [Google Scholar]
Sheen‐Chen 1990 {published data only}
- Sheen‐Chen SM, Chou FF. Value of five predictive parameters for choledocholithiasis. Journal of Surgical Association Republic of China 1990;23(2):128‐31. [Google Scholar]
Siddiqui 1994 {published data only}
- Siddiqui MN, Hamid S, Khan H, Ahmed M. Per‐operative endoscopic retrograde cholangio‐pancreatography for common bile duct stones. Gastrointestinal Endoscopy 1994;40(3):348‐50. [DOI] [PubMed] [Google Scholar]
Sigel 1982 {published data only}
- Sigel B, Coelho JC, Nyhus LM, Donahue PE, Velasco JM, Spigos DG. Comparison of cholangiography and ultrasonography in the operative screening of the common bile duct. World Journal of Surgery 1982;6(4):440‐4. [DOI] [PubMed] [Google Scholar]
Sikic 1996 {published data only}
- Sikic N, Pavan G, Baskot A, Tutek Z, Polovic A. Routine versus selective intraoperative cholangiography during open cholecystectomy?. Radiology and Oncology 1996;30(3):171‐6. [Google Scholar]
Singh 2000 {published data only}
- Singh G, Gupta PC, Sridar G, Katariya RN. Role of selective intra‐operative cholangiography during cholecystectomy. Australian and New Zealand Journal of Surgery 2000;70(2):106‐9. [DOI] [PubMed] [Google Scholar]
Skorka 1982 {published data only}
- Skorka B, Preuss HJ, Baldauf K. Informative value and validity of pre‐, per‐ and postoperative radiological bile‐duct diagnostics. Zeitschrift fur Arztliche Fortbildung 1982;76(1‐2):24‐9. [PubMed] [Google Scholar]
Snow 2001 {published data only}
- Snow LL, Weinstein LS, Hannon JK, Lane DR. Evaluation of operative cholangiography in 2043 patients undergoing laparoscopic cholecystectomy: a case for the selective operative cholangiogram. Surgical Endoscopy 2001;15(1):14‐20. [DOI] [PubMed] [Google Scholar]
Stiris 2000 {published data only}
- Stiris MG, Tennoe B, Aadland E, Lunde OC. MR cholangiopancreaticography and endoscopic retrograde cholangiopancreaticography in patients with suspected common bile duct stones. Acta Radiologica 2000;41(3):269‐72. [DOI] [PubMed] [Google Scholar]
Stuart 1998 {published data only}
- Stuart SA, Simpson TI, Alvord LA, Williams MD. Routine intraoperative laparoscopic cholangiography. American Journal of Surgery 1998;176(6):632‐7. [DOI] [PubMed] [Google Scholar]
Tobin 1984 {published data only}
- Tobin MV, Gilmore IT, Lamb GHR. Choledocholithiasis ‐ discrepancies between ultrasound and retrograde cholangiogram findings. Gut 1984;25(5):A560. [Google Scholar]
Ueno 1997 {published data only}
- Ueno N, Nishizono T, Tamada K, Ichiyama M, Wada S, Tomiyama T, et al. Diagnosing extrahepatic bile duct stones using intraductal ultrasonography: a case series. Endoscopy 1997;29(5):356‐60. [DOI] [PubMed] [Google Scholar]
Videhult 2009 {published data only}
- Videhult P, Sandblom G, Rasmussen IC. How reliable is intraoperative cholangiography as a method for detecting common bile duct stones? A prospective population‐based study on 1171 patients. Surgical Endoscopy 2009;23(2):304‐12. [DOI] [PubMed] [Google Scholar]
Additional references
Abdallah 2007
- Abdallah AA, Krige JEJ, Bornman PC. Biliary tract obstruction in chronic pancreatitis. HPB 2007;9(6):421‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aljebreen 2008
- Aljebreen A, Azzam N, Eloubeidi MA. Prospective study of endoscopic ultrasound performance in suspected choledocholithiasis. Journal of Gastroenterology and Hepatology 2008;23(5):241‐5. [DOI] [PubMed] [Google Scholar]
Amott 2005
- Amott D, Webb A, Tulloh B. Prospective comparison of routine and selective operative cholangiography. Australian and New Zealand Journal of Surgery 2005;75(6):378‐82. [DOI] [PubMed] [Google Scholar]
Anciaux 1986
- Anciaux ML, Pelletier G, Attali P, Meduri B, Liguory C, Etienne JP. Prospective study of clinical and biochemical features of symptomatic choledocholithiasis. Digestive Diseases and Sciences 1986;31(5):449‐53. [DOI] [PubMed] [Google Scholar]
Arnold 1970
- Arnold DJ, Zollinger RW, Bartlett RM, Telesz W, King RG, Strait J, et al. 28621 cholecystectomies in Ohio. Results of a survey in Ohio hospitals by the gallbladder survey committee, Ohio chapter, American College of Surgeons. American Journal of Surgery 1970;119(6):714‐7. [DOI] [PubMed] [Google Scholar]
ASGE Standards of Practice Committee 2010
- Maple JT, Ben‐Menachem T, Anderson MA, Appalaneni V, Banerjee S, ASGE Standards of Practice Committee 2010. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointestinal Endoscopy 2010;71(1):1‐9. [DOI] [PubMed] [Google Scholar]
Attili 1995
- Attili AF, Santis A, Capri R, Repice AM, Maselli S. The natural history of gallstones: the GREPCO experience. The GREPCO group. Hepatology 1995;21(3):655‐60. [DOI] [PubMed] [Google Scholar]
Barbara 1987
- Barbara L, Sama C, Morselli Labate AM, Taroni F, Rusticali AG, Festi D, et al. A population based study on the prevalence of gallstone disease: the Sirmione study. Hepatology 1987;7(5):913‐7. [DOI] [PubMed] [Google Scholar]
Barkun 1994
- Barkun AN, Barkun JS, Fried GM, Ghitulescu G, Steinmetz O, Pham C, et al. Useful predictors of bile duct stones in patients undergoing laparoscopic cholecystectomy. Annals of Surgery 1994;220(1):32‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benjaminov 2013
- Benjaminov F, Leichtman G, Naftali T, Half EE, Konikoff FM. Effects of age and cholecystectomy on common bile duct diameter as measured by endoscopic ultrasonography. Surgical Endoscopy 2013;27(1):303‐7. [DOI] [PubMed] [Google Scholar]
Buscemi 2006
- Buscemi N, Hartling L, Vandermeer B, Tjosvold L, Klassen TP. Single data extraction generated more errors than double data extraction in systematic reviews. Journal of Clinical Epidemiology 2006;59(7):697‐703. [DOI] [PubMed] [Google Scholar]
Caddy 2006
- Caddy GR, Tham DC. Gallstone disease: symptoms, diagnosis and endoscopic management of common bile duct stones. Best Practice & Research. Clinical Gastroenterology 2006;20(6):1085‐101. [DOI] [PubMed] [Google Scholar]
Chen 2007
- Chen YK, Pleskow DK. SpyGlass single‐operator peroral cholangiopacreatoscopy system for the diagnosis and therapy of bile duct disorders: a clinical feasibility study (with video). Gastrointestinal Endoscopy 2007;65(6):832‐41. [DOI] [PubMed] [Google Scholar]
Choi 2011
- Choi SB, Kim Wb, Song TJ, Suh SO, Kim YC, Choi SY. Surgical outcomes and prognostic factors for ampulla of Vater cancer. Scandinavian Journal of Surgery 2011;100(2):92‐8. [DOI] [PubMed] [Google Scholar]
Chu 2006
- Chu H, Cole SR. Bivariate meta‐analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. Journal of Clinical Epidemiology 2006;59(12):1331‐2. [DOI] [PubMed] [Google Scholar]
Claxton 2006
- Claxton KP, Sculpher MJ. Using value of information analysis to prioritise health research: some lessons from recent UK experience. Pharmaco Economics 2006;24(11):1055‐68. [DOI] [PubMed] [Google Scholar]
Corfield 1985
- Corfield AP, Cooper MJ, Williamson RCN. Acute pancreatitis: a lethal disease of increasing incidence. Gut 1985;26(7):724‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dasari 2013
- Dasari BV, Tan CJ, Gurusamy KS, Martin DJ, Kirk G, McKie L, et al. Surgical versus endoscopic treatment of bile duct stones. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD003327.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doust 2005
- Doust JA, Pietrzak E, Sanders S, Glasziou PP. Identifying studies for systematic reviews of diagnostic tests was difficult due to the poor sensitivity and precision of methodologic filters and the lack of information in the abstract. Journal of Clinical Epidemiology 2005;58(5):444‐9. [DOI] [PubMed] [Google Scholar]
Eloubeidi 2001
- Eloubeidi MA, Wade SB, Provenzale D. Factors associated with acceptance and full publication of GI endoscopic research originally published in abstract form. Gastrointestinal Endoscopy 2001;53(3):275‐82. [DOI] [PubMed] [Google Scholar]
Freitas 2006
- Freitas ML, Bell RL, Duffy AJ. Choledocholithiasis: evolving standard for diagnosis and management. World Journal of Gastroenterology 2006;12(20):3162‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giljaca 2015
- Giljaca V, Gurusamy KS, Higgie D, Poropat G, Stimac D, Davidson BR. Endoscopic ultrasound versus magnetic resonance cholangiopancreatography for common bile duct stones. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD011549] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurusamy 2010
- Gurusamy KS, Davidson BR. Surgical treatment of gallstones. Gastroenterology Clinics of North America 2010;39(2):229‐44, viii. [DOI] [PubMed] [Google Scholar]
Gurusamy 2011
- Gurusamy K, Sahay SJ, Burroughs AK, Davidson BR. Systematic review and meta‐analysis of intraoperative versus preoperative endoscopic sphincterotomy in patients with gallbladder and suspected common bile duct stones. British Journal of Surgery 2011;98(7):908‐16. [DOI] [PubMed] [Google Scholar]
Gurusamy 2015
- Gurusamy KS, Giljaca V, Higgie D, Poropat G, Stimac D, Davidson BR. Ultrasound versus liver function tests for diagnosis of common bile duct stones. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD011548] [DOI] [PMC free article] [PubMed] [Google Scholar]
Halldestam 2004
- Halldestam I, Enell EL, Kullman E, Borch K. Development of symptoms and complications in individuals with asymptomatic gallstones. British Journal of Surgery 2004;91(6):734‐8. [PUBMED: 15164444] [DOI] [PubMed] [Google Scholar]
Hamade 2005
- Hamade AM, Al‐Bahrani AZ, Owera AMA, Hamoodi AA, Abid GH, Bani Hani OI, et al. Therapeutic, prophylactic, and preresection applications of laparoscopic gastric and biliary bypass for patients with periampullary malignancy. Surgical Endoscopy 2005;19(10):1333‐40. [DOI] [PubMed] [Google Scholar]
Hirschfield 2011
- Hirschfield GM, Gershwin ME. Primary biliary cirrhosis: one disease with many faces. Israel Medical Association Journal 2011;13(1):55‐9. [PubMed] [Google Scholar]
Hong 2006
- Hong DF, Xin Y, Chen DW. Comparison of laparoscopic cholecystectomy combined with intraoperative endoscopic sphincterotomy and laparoscopic exploration of the common bile duct for cholecystocholedocholithiasis. Surgical Endoscopy and Other Interventional Techniques 2006;20(3):424‐7. [DOI] [PubMed] [Google Scholar]
Kalady 2004
- Kalady MF, Peterson B, Baillie J, Onaitis MW, Abdul‐Wahab OI, Howden JK, et al. Pancreatic duct strictures: identifying risk of malignancy. Annals of Surgical Oncology 2004;11(6):581‐8. [DOI] [PubMed] [Google Scholar]
Kelly 2010
- Kelly MD. Results of laparoscopic bile duct exploration via choledochotomy. ANZ Journal of Surgery 2010;80(10):694‐8. [DOI] [PubMed] [Google Scholar]
Krishna 2008
- Krishna RP, Kumar A, Singh RK, Sikora S, Saxena R, Kapoor VK. Xanthogranulomatous inflammatory strictures of extrahepatic biliary tract: presentation and surgical management. Journal of Gastrointestinal Surgery 2008;12(5):836‐41. [DOI] [PubMed] [Google Scholar]
Lefemine 2011
- Lefemine V, Morgan RJ. Spontaneous passage of common bile duct stones in jaundiced patients. Hepatobiliary and Pancreatic Diseases International 2011;10(2):209‐13. [DOI] [PubMed] [Google Scholar]
Lill 2010
- Lill S, Rantala A, Pekkala E, Sarparanta H, Huhtinen H, Rautava P, et al. Elective laparoscopic cholecystectomy without routine intraoperative cholangiography: a retrospective analysis of 1101 consecutive cases. Scandinavian Journal of Surgery 2010;99(4):197‐200. [DOI] [PubMed] [Google Scholar]
Lillemoe 2000
- Lillemoe KD, Melton GB, Cameron JL, Pitt HA, Campbell KA, Talamini MA, et al. Postoperative bile duct strictures: management and outcome in the 1990s. Annals of Surgery 2000;232(3):430‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2008
- Lin LF, Huang PT, Ho KS, Tung JN. Autoimmune chronic pancreatitis. Journal of the Chinese Medical Association 2008;71(1):14‐22. [DOI] [PubMed] [Google Scholar]
Lopes 2011
- Lopes TL, Baron TH. Endoscopic retrograde cholangiopancreatography in patients with Roux‐en‐Y anatomy. Journal of Hepato‐Biliary‐Pancreatic Sciences 2011;18(3):332‐8. [DOI] [PubMed] [Google Scholar]
Loria 1994
- Loria P, Dilengite MA, Bozzzoli M, Carubbi F, Messora R, Sassatelli R, et al. Prevalence rates of gallstone disease in Italy. The Chianciano population study. European Journal of Epidemiology 1994;10(2):143‐50. [DOI] [PubMed] [Google Scholar]
Ludwig 2001
- Ludwig K, Kockerling F, Hohenberger W, Lorenz D. Surgical therapy for cholecysto‐/choledocholithiasis. Results of a Germany‐wide questionnaire sent to 859 clinics including 123,090 cholecystectomies [Die chirurgische Therapie der cholecysto‐/choledocholithiasis. Ergenbisse einer deutschlandweiten Umfrage an 859 Kliniken mit 123.090 cholecystektomien]. Der Chirurg 2001;72(10):1170‐8. [DOI] [PubMed] [Google Scholar]
Mann 1994
- Mann DV, Hershman MJ, Hittinger R, Glazer G. Multicentre audit of death from acute pancreatitis. British Journal of Surgery 1994;81(6):890‐3. [DOI] [PubMed] [Google Scholar]
Maple 2010
- Maple JT, Ben‐Menachem T, Anderson MA, Appalaneni V, Banerjee S, ASGE Standards of Practice Commitee 2010. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointestinal Endoscopy 2010;71(1):1‐9. [DOI] [PubMed] [Google Scholar]
Neoptolemos 1988
- Neoptolemos JP, London NJ, James D, Carr‐Locke DL, Bailey IA, Fossard DP. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet 1988;2(8618):979‐83. [DOI] [PubMed] [Google Scholar]
Park 2011
- Park SY, Park CH, Cho SB, Lee WS, Kim JC, Cho CK, et al. What is appropriate procedure for preoperative biliary drainage in patients with obstructive jaundice awaiting pancreaticoduodenectomy. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2011;21(5):344‐8. [DOI] [PubMed] [Google Scholar]
Penz‐Osterreicher 2011
- Penz‐Osterreicher M, Osterreicher CH, Trauner M. Fibrosis in autoimmune and cholestatic liver disease. Best Practices & Research. Clinical Gastroenterology 2011;25(2‐4):245‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raraty 1998
- Raraty MG, Finch M, Neoptolemos JP. Acute cholangitis and pancreatitis secondary to common duct stones: management update. World Journal of Surgery 1998;22(11):1155‐61. [DOI] [PubMed] [Google Scholar]
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AWS, Scholten RJPM, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982‐90. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Ripolles 2009
- Ripolles T, Ramirez‐Fuentes C, Martinez‐Perez MJ, Delgado F, Blanc E, Lopez A. Tissue harmonic sonography in the diagnosis of common bile duct stones: a comparison with endoscopic retrograde cholangiography. Journal of Clinical Ultrasound 2009;37(9):501‐6. [DOI] [PubMed] [Google Scholar]
Roston 1997
- Roston AD, Jacobson IM. Evaluation of the pattern of liver tests and yield of cholangiography in symptomatic choledocholithiasis: a prospective study. Gastrointestinal Endoscopy 1997;45(5):394‐9. [DOI] [PubMed] [Google Scholar]
Saik 1975
- Saik RP, Greenburg AG, Farris JM, Peksin GW. Spectrum of cholangitis. American Journal of Surgery 1975;130(2):143‐50. [DOI] [PubMed] [Google Scholar]
Salek 2009
- Salek J, Livote E, Sideridis K, Bank S. Analysis of risk factors predictive of early mortality and urgent ERCP in acute cholangitis. Journal of Clinical Gastroenterology 2009;43(2):171‐5. [DOI] [PubMed] [Google Scholar]
Sampson 2008
- Sampson M, Shojania KG, McGowan J, Daniel R, Rader T, Iansavichene AE, et al. Surveillance search techniques identified the need to update systematic reviews. Journal of Clinical Epidemiology 2008;61(8):755‐62. [DOI] [PubMed] [Google Scholar]
Sarli 2000
- Sarli L, Costi R, Gobbi S, Sansebastiano G, Roncoroni L. Asymptomatic bile duct stones: selection criteria for intravenous cholangiography and/or endoscopic retrograde cholangiography prior to laparoscopic cholecystectomy. European Journal of Gastroenterology & Hepatology 2000;12(11):1175‐80. [PubMed] [Google Scholar]
Schuetz 2012
- Schuetz GM, Schlattmann P, Dewey M. Use of 3x2 tables with an intention to diagnose approach to assess clinical performance of diagnostic tests: meta‐analytical evaluation of coronary CT angiography studies. BMJ (Clinical Research Ed.) 2012;345:e6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siddiqui 2011
- Siddiqui AA, Sreenarasimhaiah J, Lara LF, Harford W, Lee C, Eloubeidi MA. Endoscopic ultrasound‐guided transduodenal placement of a fully covered metal stent for palliative biliary drainage in patients with malignant biliary obstruction. Surgical Endoscopy 2011;25(2):549‐55. [DOI] [PubMed] [Google Scholar]
Singh 1990
- Singh SM, Londmire WP, Reber HA. Surgical palliation for pancreatic cancer: the UCLA experience. Annals of Surgery 1990;212(2):132‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spelsberg 2009
- Spelsberg FW, Nusser F, Huttl TK, Obeidat FW, Lang RA, Jauch KW, et al. Management of cholecysto‐ and choledocholithiasis ‐ survey and analysis of 16 615 cholecystectomies and common bile duct explorations in Bavaria. Zentralblatt fur Chirurgie 2009;134(2):120‐6. [DOI] [PubMed] [Google Scholar]
Takwoingi 2013
- Takwoingi Y, Leeflang MM, Deeks JJ. Empirical evidence of the importance of comparative studies of diagnostic test accuracy. Annals of Internal Medicine 2013;158(7):544‐54. [DOI] [PubMed] [Google Scholar]
Tang 2011
- Tang S, Singh S, Singh S. Sphincterotome stricturoplasty for long ampullary stenoses and benign biliary strictures (with video). Surgical Endoscopy 2011;25(4):1313‐8. [DOI] [PubMed] [Google Scholar]
Targarona 2004
- Targarona EM, Bendahan GE. Management of common bile duct stones: controversies and future perspectives. HPB 2004;6(3):140‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toh 2000
- Toh SKC, Phillips S, Johnson CD. A prospective audit against national standards of the presentation and management of acute pancreatitis in the south of England. Gut 2000;46(2):239‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tranter 2003
- Tranter SE, Thompson MH. Spontaneous passage of bile duct stones: frequency of occurrence and relation to clinical presentation. Annals of the Royal College of Surgeons of England 2003;85(3):174‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2006
- Whiting PF, Weswood ME, Rutjes AWS, Reitsma JB, Bossuyt PNM, Kleijnen J. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Medical Research Methodology 2006;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, The QUADAS‐2 group. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529‐36. [DOI] [PubMed] [Google Scholar]
Williams 2008
- Williams EJ, Green J, Beckingham I, Parks R, Martin D, Lombard M. Guidelines on the management of common bile duct stones (CBDS). Gut 2008;57(7):1004‐21. [DOI] [PubMed] [Google Scholar]
Yousefpour Azary 2011
- Yousefpour Azary S, Kalbasi H, Setayesh A, Mousavi M, Hashemi A, Khodadoostan M, et al. Predictive value and main determinants of abnormal features of intraoperative cholangiography during cholecystectomy. Hepatobiliary and Pancreatic Diseases International 2011;10(3):308‐12. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Giljaca 2013
- Giljaca V, Gurusamy KS, Vaughan J, Stimac D, Davidson BR. Tests for diagnosis of common bile duct stones. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD010339] [DOI] [PMC free article] [PubMed] [Google Scholar]


