Abstract
Background
Urinary incontinence can affect 40‐60% of people admitted to hospital after a stroke, with 25% still having problems on hospital discharge and 15% remaining incontinent at one year.
Objectives
To determine the optimal methods for treatment of urinary incontinence after stroke in adults.
Search methods
We searched the Cochrane Incontinence and Stroke Groups specialised registers (searched 15 March 2007 and 5 March 2007 respectively), CINAHL (January 1982 to January 2007), national and international trial databases for unpublished data, and the reference lists of relevant articles.
Selection criteria
Randomised or quasi‐randomised controlled trials evaluating the effects of interventions designed to promote continence in people after stroke.
Data collection and analysis
Data extraction and quality assessment were undertaken by two reviewers working independently. Disagreements were resolved by a third reviewer.
Main results
Twelve trials with a total of 724 participants were included in the review. Participants were from a mixture of settings, age groups and phases of stroke recovery.
Behavioural interventions Three trials assessed behavioural interventions, such as timed voiding and pelvic floor muscle training. All had small sample sizes and confidence intervals were wide.
Specialised professional input interventions Two trials assessed variants of professional input interventions. Results tended to favour the intervention groups: in a small trial in early rehabilitation, fewer people had incontinence at discharge from hospital after structured assessment and management than in a control group (1/21 vs. 10/13; RR 0.06, 95% CI 0.01 to 0.43); in the second trial, assessment and management by Continence Nurse Advisors was associated with fewer participants having urinary symptoms (48/89 vs. 38/54; RR 0.77, 95% CI 0.59 to 0.99) and statistically significantly more being satisfied with care.
Complementary therapy interventions Three small trials all reported fewer participants with incontinence after acupuncture therapy (overall RR 0.44; 95% 0.23 to 0.86), but there were particular concerns about study quality.
Pharmacotherapy and hormonal interventions There were three small trials that included groups allocated meclofenoxate, oxybutinin or oestrogen. There were no apparent differences other than in the trial of meclofenoxate where fewer participants had urinary symptoms in the active group than in the control group (9/40 vs. 27/40; RR 0.33, 95% CI 0.18 to 0.62).
Authors' conclusions
Data from the available trials are insufficient to guide continence care of adults after stroke. However, there was suggestive evidence that professional input through structured assessment and management of care and specialist continence nursing may reduce urinary incontinence and related symptoms after stroke. Better quality evidence is required of the range of interventions that have been suggested for continence care after stroke.
Keywords: Adult, Female, Humans, Male, Acupuncture Therapy, Acupuncture Therapy/methods, Randomized Controlled Trials as Topic, Stroke, Stroke/complications, Stroke Rehabilitation, Urinary Incontinence, Urinary Incontinence/drug therapy, Urinary Incontinence/etiology, Urinary Incontinence/therapy
Treatment of urinary incontinence after stroke in adults
Urinary incontinence is a common consequence of stroke and has many causes. In early stroke rehabilitation, structured assessment and management of care shows promise in reducing the number of people with urinary incontinence. In the later phases of stroke recovery the use of specialist advisors may be helpful in reducing symptoms associated with urinary incontinence. Even late after stroke, interventions targeted at specific causes of incontinence may be helpful. Unfortunately, all the conclusions were limited by a lack of robust information.
Background
Urinary incontinence is defined as the complaint of any involuntary leakage of urine (Abrams 2002). 40‐60% of people admitted to hospital after a stroke can have problems with urinary incontinence, with 25% of stroke survivors still having problems on hospital discharge, and 15% remaining incontinent after one year (Barrett 2001). Not all the studies reviewed by Barrett 2001 excluded people with pre‐morbid incontinence, however, so figures presented may include old as well as new cases. Addressing problems with continence whilst the person is still in hospital may prevent long‐term problems for the patient and family.
The more severe the stroke, the greater is the likelihood of urinary incontinence (Burney 1996a). Other risk factors for urinary incontinence include older age, female sex, speech difficulties, motor weakness, visual field defects or cognitive impairment (Barrett 2001). Problems experienced may range from urinary retention to complete incontinence. The most likely pattern of incontinence is urinary frequency, urgency (a sudden compelling desire to pass urine which is difficult to defer) and urge incontinence (involuntary leakage) (Marinkovic 2001). This is generally the result of detrusor overactivity (Arunabh 1993), although this may depend on the site of the stroke lesion (Burney 1996b).
Damage to the frontal lobe, the area believed to be responsible for control of micturition, has been identified in several studies as associated with urinary dysfunction after stroke. However, evidence suggests that the size of the lesion, rather than its location, is more likely to predict urinary incontinence (Brittain 1999). It is unclear whether incontinence is a direct (i.e. site of brain lesion) or indirect (e.g. functional impairment preventing access) consequence of stroke. Other non‐neurological factors contributing to urinary incontinence, including pre‐morbid continence state, sphincter incompetence and polyuria (Barrett 2001), are regarded as prevalent in the population of patients with stroke (Brittain 1998b).
Because of their severity, the symptoms of urinary incontinence are reported to have more of an effect on the lives of stroke survivors, when compared with other groups of people with incontinence (Brittain 2000b). Urinary symptoms had more impact on sleep, daily activities, quality of life, physical discomfort, social life and relationships. Incontinence is not just a physical problem, but impacts on what people can do and how they feel. Depression is twice as common in stroke survivors who are incontinent (Brittain 1998a). Urinary incontinence is distressing for both those affected and their carers (Williams 1993).
Continuing incontinence is associated with poor outcome in both stroke survivor and carer (Nakayama 1997). Conversely, stroke outcome is better in those stroke survivors who remain continent or regain continence (Barer 1989). Improvement is common over time (Marinkovic 2001), which suggests that problems with continence may be transient in some stroke survivors, and/or amenable to intervention. Factors predicting early improvements in continence status are less impairment on admission, and the site of the stroke lesion (Ween 1996). Factors associated with poor recovery of continence include stroke type and being aged 75 or over (Patel 2001).
Incontinence is a strong predictor of stroke functional outcome (Meijer 2003). While there are problems with attributing better stroke outcome to improvements in continence, it is possible that recovery from incontinence may improve morale and self esteem and therefore speed overall stroke recovery (Barer 1989; Patel 2001). While differences in incontinence rates between centres will reflect difference in the case mix of individuals and the methods of reporting (Barrett 2001), they might also indicate variations in the processes of continence assessment and management.
It is known that early rehabilitation intervention in stroke results in better outcome overall (Cifu 1999). Some of the studies on which this conclusion is based have included incontinence as a measure of functional outcome. For example, a trial of a multidisciplinary rehabilitation intervention aiming to improve functional independence after stroke showed a positive impact on incontinence rates (Wikander 1998a). Other management techniques include biofeedback, pelvic floor muscle training, electrical stimulation, drug treatments, surgical interventions and mechanical devices.
Current guidelines for the management of urinary incontinence recommend an assessment to guide management (Thuroff 1999). This begins with physical assessment and history‐taking including identification of urological problems before the stroke occurred, such as bladder outlet obstruction in men or stress incontinence in women. The choice of method to promote continence will then depend on the individual's history and type of incontinence. Bladder training and/or anticholinergic drugs may be appropriate for urge incontinence, while problems with retention may require intermittent catheterisation. Alternatively, problems with memory loss or restriction of movement may benefit more from toileting assistance programmes such as prompted or timed voiding or habit retraining (Eustice 2000; Ostaszkiewicz 2004a; Ostaszkiewicz 2004b; Roe 2007 ).
A systematic review of methodologically robust studies ‐ randomised controlled trials ‐ is needed to identify interventions to promote continence that are effective in the stroke population. Trials that have evaluated different management strategies also need to be reviewed in relation to subgroups of stroke patients with specific patterns of incontinence, because the effects of management are likely to depend on the type of stroke that the person has suffered and the urinary problems they experienced.
Objectives
The objective of this review was to determine the optimal methods for the treatment of urinary continence after stroke in adults.
The following hypotheses were addressed: 1. intervention is more effective than no intervention; 2. intervention is more effective than placebo; 3. a specific intervention is more effective in comparison with another intervention; 4. combined interventions are more effective than single interventions.
Within each hypothesis, interventions were considered within four categories: 1. behavioural interventions 2. specialised professional input interventions 3. complementary therapy interventions 4. pharmacotherapy interventions.
Methods
Criteria for considering studies for this review
Types of studies
All randomised and quasi‐randomised trials evaluating the effects of interventions designed to promote continence in people who have had a stroke. Quasi‐random methods include: allocation by the person's date of birth, by the day of the week or month of the year, by a person's medical record number, or just allocating every alternate person.
Types of participants
Adults (i.e. 18 years of age and over) with a diagnosis of stroke, including people with incontinence who have had a stroke identified as a subgroup within a larger group for whom relevant data are reported.
Types of interventions
One arm of the study must include an intervention designed to promote urinary continence. Trials evaluating any of the following were included in the review:
pharmacotherapy e.g. anticholinergics, adrenergics, hormonal treatment;
physical therapy e.g. electrical stimulation, biofeedback;
physical aids e.g. catheters, pads, pessaries, other appliances;
behavioural interventions e.g. prompted or scheduled voiding, bladder training, habit retraining (i.e. identification of voiding pattern and development of an individualised toileting schedule), pelvic floor muscle training or other behavioural management programmes;
environmental or lifestyle interventions e.g. voiding position, diet and fluid management, alternative communication devices;
specialised professional input interventions e.g. provision of information or education, assessment schedules, generic multidisciplinary rehabilitation programmes, Continence Advisors, home‐support programmes, nurse practitioners;
complementary interventions e.g. homeopathy, acupuncture.
Trials relating solely to surgical or physical interventions for pre‐existing continence problems that are not associated with stroke (e.g. transurethral resection of the prostate) were excluded, unless it was a co‐intervention in a wider trial testing an included method of continence promotion. Trials relating to urological diagnosis, or to the management of incontinence or retention of urine in the acute phase of stroke were also excluded. The acute phase was defined as up to one month post stroke. Studies were excluded if solely in the acute phase because the aim of intervention is commonly to contain incontinence, monitor urine output and prevent adverse events, rather than promote continence.
Types of outcome measures
The primary outcome of interest was in/continence, measured by:
1. Participant symptoms
Number of participants regaining continence
Number of incontinent episodes over 24 hours (indicated by bladder charts, total and mean number of episodes)
Severity of incontinence e.g. index score
Perception of improvement or cure in continence (as reported by participant or caregiver)
2. Physical measures
Pad tests of quantified leakage
Volume of urine loss
Total and mean number of pads used
3. Secondary outcomes
a) Symptom scores or participant /carer report of other urinary symptoms including frequency, urgency, dysuria, polyuria, nocturia, discomfort, pain
b) Physical measures e.g. post‐void retention of urine, time to voiding onset, void volume, urodynamic measures
c) Health status or measures of psychological health Impact of incontinence e.g. Incontinence Impact Questionnaire (IIQ), Short Form 36 Health Survey Questionnaire (SF36), functional ability, knowledge, satisfaction, quality of life
d) Economic outcomes Impact of continence promotion interventions on cost or service use
e) Other outcomes Any other outcomes subsequently deemed appropriate to the review.
Search methods for identification of studies
The review used the search strategies developed for both the Cochrane Incontinence Group and the Cochrane Stroke Group. Relevant trials were initially identified from the Groups' specialised registers of controlled trials described under the groups' details in The Cochrane Library (For more details please see the ‘Specialized Register’ section of the Incontinence Group’s module in The Cochrane Library) (For details of the Stroke Group's Specialised Register please see the 'Specialized Register' section of their module). The dates of the most recent searches of the registers for this review update were conducted on 15 March 2007 (Stroke Register) and 5 March 2007 (Incontinence Register).
To search the Stroke Group register the following search terms were used: "incontinence (urine/faecal) or (nursing and bladder care) or urinary retention or urinary tract infection".
The terms used to search the Incontinence Group trials register are given below: (({Topic.urine.incon.Stroke.} OR {TOPIC.URINE.INCON.UNKNOWN.STROKE.} OR {TOPIC.URINE.INCON.NEUROGENIC.} (in keywords field)) OR ({stroke\*} OR {cerebrovascular\*} (in title1 field)) OR ({TOPIC.URINE.NEUROGENIC.} OR {TOPIC.URINE.NEUROGENIC.stroke.} (in keywords field))) AND ({DESIGN.CCT*} OR {DESIGN.RCT*} (in keywords field)) (All searches were in Reference Manager 9.5 N, ISI ResearchSoft).
The following extra specific searches were performed for this review. For more details, including the search terms used please see Appendix 1.
We did not impose any language or other restrictions on any of these searches.
Electronic searches
Due to the comprehensive nature of the searches already performed by the Cochrane Stroke Group, no additional searches were performed other than on CINAHL, combining stroke terms with terms for urinary incontinence without a research methods filter. The search was combined with the CINAHL search from the Cochrane Stroke Group. This was done because of the potentially poor indexing of nursing research. The CINAHL search covered the years January 1982 to January 2007 (on Dialog Datastar). More details, including search terms are given in Appendix 1.
Recent unpublished trial data were also searched for on national and international databases i.e. NHS National Research Register, NHS Research Findings Register, US Community of Science NIH Grants, MetaRegister of Controlled Clinical Trials and CRISP, by adapting terms drawn from the existing search strategies of the Incontinence and Stroke Review Groups.
Searching other resources
The reference lists of all relevant reviews and trial reports were searched to identify further relevant studies. Major investigators were contacted to ask for any other possible relevant trials, published or unpublished. In addition, contact was made with the authors of other relevant Cochrane reviews to ascertain whether defined subgroups of stroke survivors were identified in trials testing methods of promoting continence in a general population.
The review was publicised on the following websites: Joanna Briggs Institute, Royal College of Nursing Research Society, Royal College of Nursing Continence Interest Group, Association of Continence Advisors, Sigma Theta Tau.
Data collection and analysis
Trials were considered for inclusion independently by two reviewers (LT, BF). Studies were excluded from the review if they were not randomised or quasi‐randomised, or if on more detailed examination, they did not meet the review inclusion criteria. These studies are listed in the 'Characteristics of Excluded Studies Table'.
Data extraction forms, based on the protocol, were piloted and checked for the coding of intervention, outcome and quality assessment. Data extraction and review of the methodological quality of the eligible studies was independently conducted by two reviewers for each study (SC, BF, ML, LT) using the quality assessment tool described by the Cochrane Incontinence Review Group. Extracted data and quality assessment were cross checked and any disagreements discussed and if necessary resolved by a third reviewer (LT).
Attempts were made to obtain missing data, as well as data collected but not reported, by contacting trialists.
Outcomes are reported as unfavourable events. Planned subgroup analyses for the effect of urological diagnosis (i.e. detrusor overactivity versus other) and time from stroke onset (1 to 6 months, greater than 7 months) were not possible as the data were not available in the original studies. Included trial data were processed as described in the Cochrane Authors' Handbook (Deeks 2006), and analysed using the statistical analysis package RevMan Analyses. Effect estimates for continuous outcomes were summarised using weighted mean difference (WMD) and dichotomous outcomes were summarised using relative risk (RR). Where deemed appropriate, effects were summarised across studies using fixed or random effects meta‐analysis techniques appropriate to the form of the data. Random effects meta‐analysis (DerSimonian and Laird method) was used if the studies showed heterogeneity (defined by the studies' effects having an I‐squared statistic of greater than 50%), otherwise a fixed effect analysis (Mantel‐Haenszel for dichotomous and inverse variance for continuous data) was used. For continuous outcome data, if change from baseline data were available, these were used; otherwise the raw outcome data were used.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Included/excluded studies
The search identified 1234 papers and 126 studies were retrieved. The search initially identified ten potential trials evaluating the effectiveness of methods to promote continence in adults after stroke (Brittain 2000a; Chu 1997; Cook 1998; Gelber 1997a; Gelber 1997b; Gross 1990; Judge 1969; Lewis 1990; Tekeoglu 1998; Wikander 1998b).
One trial was published in Chinese, one in Korean, and the rest in English. Information from the Chinese trial was translated and extracted by nursing lecturers from the Shanghai Military Medical University. On further examination, three trials (Cook 1998; Gross 1990; Tekeoglu 1998) were excluded for reasons listed in the 'Characteristics of Excluded Studies Table'.
The review update identified a further five studies (Liu 2006; Tibaek 2005; Zhang 2002; Zhou 1999; Zhu 2003). With the exception of Tibaek 2005, all were published in Chinese and were translated by a native speaking Chinese nursing student undertaking postgraduate study in England.
Twelve trials were therefore included in the review update: seven were full reports (Brittain 2000a; Chu 1997; Judge 1969; Liu 2006; Tibaek 2005; Wikander 1998b; Zhu 2003), although one Chinese trial (Chu 1997) was only briefly described in a one page report; the remaining five trials were reported only in conference abstracts (Gelber 1997a; Gelber 1997b; Lewis 1990; Zhang 2002; Zhou 1999). Two of the trials (Gelber 1997a; Gelber 1997b) were in parts of the same report.
Description of interventions
The trials tested the following interventions: Behavioural interventions
timed voiding versus void on request (Gelber 1997a);
oxybutynin versus timed voiding (Gelber 1997b);
pelvic floor muscle training versus usual care (Tibaek 2005);
sensory‐motor biofeedback device (Uristop) combined with timed voiding against timed voiding alone (Lewis 1990).
Specialised professional input interventions:
care from a Continence Nurse Advisor (CNP) versus usual care provided by the general practitioner (GP) (Brittain 2000a);
a special intervention programme based on assessment using the Functional Independence Measure (FIM) versus usual rehabilitation care (Wikander 1998b).
Complementary therapy interventions:
scalp acupuncture versus no scalp acupuncture (Chu 1997);
eye and scalp acupuncture versus no acupuncture (Zhou 1999);
acupuncture versus usual care (Zhang 2002);
ginger‐salt‐partitioned moxibustion (involving filling the navel with salt, adding a piece of ginger and a taper and setting the taper alight) plus routine acupuncture versus routine acupuncture (Liu 2006).
Pharmacotherapy interventions:
oestrogen versus placebo (Judge 1969);
meclofenoxate (designed to improve glucose utilisation of brain cells) plus salvia miltirrhiza versus salvia miltirrhiza (Zhu 2003);
oxybutynin versus timed voiding (Gelber 1997b);
Physical therapy interventions:
sensory‐motor biofeedback device (Uristop) combined with timed voiding against timed voiding alone (Lewis 1990).
Intervention versus no intervention / usual care
Nine trials tested an intervention versus no intervention or usual care (Brittain 2000a; Chu 1997; Gelber 1997a; Liu 2006; Tibaek 2005; Wikander 1998b; Zhang 2002; Zhou 1999; Zhu 2003).
Behavioural interventions One trial (Gelber 1997a) tested a behavioural intervention comprising timed voiding versus void on request for participants with normal urodynamic studies. Normal urodynamic studies was not defined and no further details are given of the intervention. One trial (Tibaek 2005) tested an intensive pelvic floor muscle training programme, comprising individual and group exercises and feedback to participants, compared against normal rehabilitation with no specific treatment of urinary incontinence. Specialised professional input interventions Two trials (Brittain 2000a; Wikander 1998b) tested specialised professional input interventions. Brittain 2000a compared care given by a Continence Nurse Advisor against usual care provided by a general practitioner and existing specialised services for the management of continence. Wikander 1998b compared the impact of a special intervention programme based on assessment using the Functional Independence Measure against usual rehabilitation care based on the Bobath method.
Complementary therapy interventions Four trials tested complementary interventions. Chu 1997 tested scalp acupuncture plus usual care, compared against usual care with no scalp acupuncture; Zhang 2002 tested acupuncture against general treatment and Zhou 1999 tested eye and electriferous scalp acupuncture plus medication therapy against medication therapy only. Liu 2006 tested ginger‐salt‐partitioned moxibustion plus routine acupuncture against routine acupuncture.
Pharmacotherapy interventions One trial (Zhu 2003) tested meclofenoxate plus salvia miltirrhiza against salvia miltirrhiza alone.
Intervention versus placebo
One cross‐over trial (Judge 1969) tested a pharmacotherapy intervention (oestrogen) against placebo.
Specific intervention versus another intervention
One trial (Gelber 1997b) tested a specific intervention against another intervention, comparing the anticholinergic oxybutynin against timed voiding in participants with bladder hyperreflexia.
Combined intervention versus single intervention
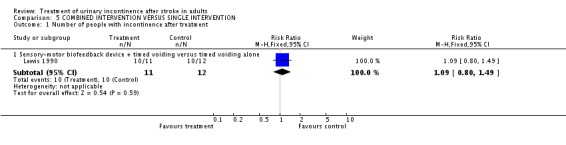
One trial (Lewis 1990) tested a combined intervention against a single intervention. The study tested a sensory‐motor biofeedback device combined with timed voiding, against timed voiding alone.
Participants
A total of 724 participants were enrolled in the trials. The numbers in individual trials ranged from 13 to 242. Two trials (Judge 1969; Tibaek 2005) enrolled only female participants. Three trials (Gelber 1997a; Gelber 1997b; Lewis 1990) gave no details of age or gender. The remaining trials (Brittain 2000a; Chu 1997; Liu 2006; Wikander 1998b; Zhang 2002; Zhou 1999; Zhu 2003) ranged from 40% to 61% males. The mean age of the study participants overall cannot be reported, but the highest mean age (82) was in the trial by Judge (Judge 1969), with the widest age range of 40‐96 in the Brittain trial (Brittain 2000a).
Diagnosis
Three trials (Liu 2006; Tibaek 2005; Wikander 1998b) only included participants who were continent prior to the stroke. Nine trials (Brittain 2000a; Chu 1997; Gelber 1997a; Gelber 1997b; Judge 1969; Lewis 1990; Zhang 2002; Zhou 1999; Zhu 2003) did not specify whether urinary incontinence was subsequent to the occurrence of stroke, although Lewis 1990 described participants as having "post‐stroke urinary urge incontinence".
Diagnostic criteria for incontinence were given in only two trials (Brittain 2000a; Tibaek 2005). Four trials specified a urological diagnosis, given as normal urodynamic studies (Gelber 1997a), bladder hyperreflexia (Gelber 1997b), urge incontinence (Lewis 1990) and urge, stress and mixed stress/urge incontinence (Tibaek 2005). Judge 1969 reported data for two groups of participants defined as mildly or severely incontinent. Liu 2006 reported data for three groups of participants, classified according to the Barthel continence item: completely incontinent, partially incontinent and independent. Chu 1997 included participants who had "urinary frequency or urinary incontinence" but did not define urinary frequency further. Two trials (Brittain 2000a; Wikander 1998b) did not include a urological diagnosis for participants, or group them by type of incontinence.
The trial by Tibaek 2005 stated that participants must be diagnosed according to the World Health Organisation definition of ischaemic stroke verified by CT scan. Three trials (Gelber 1997a; Gelber 1997b; Wikander 1998b) reported that strokes were unilateral, and one trial (Chu 1997) reported the stroke type as multi‐focal infarction. Liu 2006 and Zhou 1999 included participants with cerebral infarction and haemorrhage. Two trials (Zhang 2002; Zhu 2003) included only participants with infarction, and the site of infarction is specified for all participants in the trial by Zhang 2002. Judge 1969 included participants with cerebrovascular accident or "multiple little strokes". Brittain 2000a included participants who self‐reported that they had had a stroke, subarachnoid haemorrhage or transient ischaemic attack on a screening questionnaire. One trial (Wikander 1998b) included side of stroke lesion in baseline comparison between groups.
It was not possible in all but one of the trials (Tibaek 2005) to determine whether only participants with a first stroke were included. Participants in two trials (Wikander 1998b; Zhu 2003) were in the acute phase of stroke (i.e. 0 to 1 month) on enrolment to the study, but in one study (Wikander 1998b) the study period extended into early rehabilitation, i.e. up to three months. Participants in the trial by Liu 2006 were 70.74 ± 35.26 days post‐stroke, while Zhou 1999 presented findings for participants who were less than or more than three months post‐stroke. Participants in three trials (Brittain 2000a; Judge 1969; Tibaek 2005) were also less likely to be in the early rehabilitation phase, as they were either occupying long‐stay geriatric hospital beds or living at home. It is difficult to identify the phase of stroke recovery for participants in the other trials (Chu 1997; Gelber 1997a; Gelber 1997b; Lewis 1990; Zhang 2002).
Five trials (Brittain 2000a; Judge 1969; Liu 2006; Tibaek 2005; Zhu 2003) reported exclusion criteria. The remaining seven trials did not detail inclusion or exclusion criteria (Chu 1997; Gelber 1997a; Gelber 1997b; Lewis 1990; Wikander 1998b; Zhou 1999; Zhang 2002). Three trials (Liu 2006; Tibaek 2005; Wikander 1998b) reported detailed baseline comparisons.
Setting
Care was provided in a hospital setting in five of the trials: on a rehabilitation ward in a Department of Geriatrics in Sweden (Wikander 1998b); in two long stay geriatric wards in two hospitals in Scotland (Judge 1969) and in hospitals in China (Liu 2006; Zhu 2003) and Denmark (Tibaek 2005) (although participants were outpatients at the time of this study). Brittain 2000a included participants living in the community in England, excluding those living in residential care. The six remaining trials do not specify the setting of care (Chu 1997; Gelber 1997a; Gelber 1997b; Lewis 1990; Zhang 2002; Zhou 1999).
Description of outcomes
Outcomes addressed by the trials were diverse. Six trials expressed the primary outcome as the number of people with urinary incontinence (Brittain 2000a; Chu 1997; Lewis 1990; Wikander 1998b; Zhang 2002; Zhou 1999), with six trials reporting number of incontinent episodes (Gelber 1997a; Gelber 1997b; Judge 1969; Lewis 1990; Liu 2006; Tibaek 2005). One trial (Brittain 2000a) also reported a measure of the severity of incontinence. The trials also measured primary outcomes at different time points.
In terms of secondary outcomes, four trials measured urinary symptoms (Brittain 2000a; Liu 2006; Tibaek 2005; Zhu 2003). One trial (Tibaek 2005) included a physical measure of incontinence in the form of pad tests of quantified leakage. Four trials measured functional and psychological outcomes (Brittain 2000a; Tibaek 2005; Wikander 1998b; Zhu 2003). One trial included an economic measure (Wikander 1998b).
All outcomes reported in the original trials are included in the review, except global functional index scores reported by Wikander 1998b.
Risk of bias in included studies
Methodological quality was assessed using the quality assessment tool described by the Cochrane Incontinence Review Group.
Allocation
Three of the trials (Brittain 2000a; Judge 1969; Tibaek 2005) were classed as A (adequate concealment of allocation). Eight of the trials (Chu 1997; Lewis 1990; Liu 2006; Gelber 1997a; Gelber 1997b; Wikander 1998b; Zhang 2002; Zhou 1999) were classed as B (unclear). The trial by Zhu (Zhu 2003) was a quasi‐experimental study with subjects allocated in sequence according to the time of admission; allocation was therefore not concealed. The trial by Brittain (Brittain 2000a) had a 2:1 randomisation.
Blinding
In the trials of complementary therapies (Chu 1997; Liu 2006; Zhang 2002; Zhou 1999) and meclofenoxate (Zhu 2003), blinding of staff, trial participants and outcome assessment was unclear. In the trials of professional input interventions (Brittain 2000a; Wikander 1998b) and pelvic floor muscle training (Tibaek 2005), health care providers and trial participants could not be blind to treatment status. Brittain 2000a and Tibaek 2005 used independent outcome assessors. In the trial by Wikander (Wikander 1998b), outcomes were assessed by ward staff. In the cross‐over trial of oestrogen versus placebo (Judge 1969), blinding of staff and trial participants is likely to have been effective, but it was unclear who undertook outcome assessment.
Follow‐up and exclusions
Loss to follow up was unclear in the trial of scalp acupuncture (Chu 1997). The trial of rehabilitation governed by a functional independence measure (Wikander 1998b) reported no loss to follow up. It is unclear why there were fewer patients in the control group (n=13) than the intervention group (n=21). There was also no reported loss to follow up in the trials of acupuncture versus usual care (Zhang 2002), eye and scalp acupuncture versus no acupuncture (Zhou 1999) and meclofenoxate plus salvia miltirrhiza versus salvia mitirrhiza (Zhu 2003). The cross‐over trial of oestrogen versus placebo (Judge 1969) and the trials of pelvic floor muscle training versus usual care (Tibaek 2005) and ginger‐salt‐partitioned moxibustion plus routine acupuncture versus routine acupuncture (Liu 2006) state numbers and reasons for withdrawals. In the trial of a Continence Nurse Advisor versus general practitioner care (Brittain 2000a), the numbers of withdrawals at three and six months are stated and reasons given, but are not reported separately for intervention and control group. There was missing data at both three and six months, because some participants were not yet due for their assessment when results were reported. The proportions for which data are reported are 82% at three months, and 63% at six months. Comparisons on age and gender for those excluded from analyses showed no significant differences. Power calculations and intention‐to‐treat analyses were not reported in any of the trials, although four trials (Wikander 1998b; Zhang 2002; Zhou 1999; Zhu 2003) reported complete data and are therefore likely to have applied intention to treat.
Effects of interventions
1. INTERVENTION VERSUS NO INTERVENTION/USUAL CARE (Comparison 01)
Nine trials (Brittain 2000a; Chu 1997; Gelber 1997a; Liu 2006; Tibaek 2005; Wikander 1998b; Zhang 2002; Zhou 1999; Zhu 2003) with a total of 670 participants compared an intervention to promote urinary continence against no intervention or usual care. The interventions included:
Behavioural interventions:
timed voiding versus void on request (Gelber 1997a);
pelvic floor muscle training versus usual care (Tibaek 2005).
Specialised professional input interventions:
a special intervention programme based on assessment using the Functional Independence Measure (FIM) versus usual rehabilitation care (Wikander 1998b);
care from a Continence Nurse Advisor (CNP) versus usual care provided by the general practitioner (GP) (Brittain 2000a).
Complementary therapy interventions:
scalp acupuncture versus no scalp acupuncture (Chu 1997);
eye and scalp acupuncture versus no acupuncture (Zhou 1999);
acupuncture versus usual care (Zhang 2002);
ginger‐salt‐partitioned moxibustion plus routine acupuncture versus routine acupuncture (Liu 2006);
Pharmacotherapy interventions:
meclofenoxate plus salvia miltirrhiza versus salvia miltirrhiza (Zhu 200
Incontinence
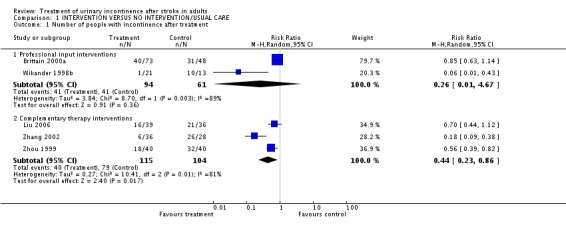
Five trials (Brittain 2000a; Liu 2006; Wikander 1998b; Zhang 2002; Zhou 1999) recruiting 485 participants measured number of people with incontinence after treatment; random effects models were used for the meta‐analysis because of heterogeneity. All five trials were of specialised professional input interventions or of complimentary therapy interventions.
Specialist professional input interventions In both the two trials of structured assessment and management, the rate of incontinence was lower in the intervention group than in the control group (Comparison 01.01.01). There was, however, marked heterogeneity between the trials: Wikander (Wikander 1998b), 1/21 vs. 10/13 (RR 0.06; 95% CI 0.01 to 0.43); Brittain (Brittain 2000a), 40/73 vs. 31/48 (RR 0.85; 95% CI 0.63 to 1.14). Although the overall RR (0.26) was consistent with a large treatment effect, applying a random effects model (because of the heterogeneity) led to a wide confidence interval which was not statistically significant (0.01 to 4.67; p = 0.36).
Complementary therapy interventions In the three trials of complementary therapy interventions for which data were available (Liu 2006; Zhang 2002; Zhou 1999), the rate of incontinence was reported to be lower in the intervention group and this difference was statistically significant for two of the studies. This comparison also showed heterogeneity (I2 = 80.8%). Applying a random effects model, the overall RR was 0.44 (95% CI 0.23 to 0.86; p = 0.02).
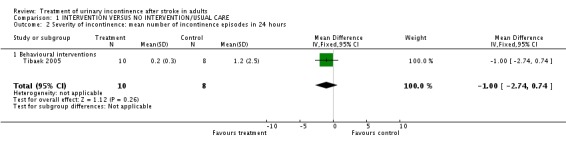
Severity of incontinence
Behavioural interventions One trial (Tibaek 2005) measured the mean number of incontinence episodes in 24 hours. The impact of pelvic floor muscle training indicated no significant difference (WMD ‐1.00, 95% CI ‐2.74, 0.74, Comparison 01.02.01).
In the trial by Gelber (Gelber 1997a), timed voiding was compared against void on request (which was interpreted as usual care). The data reported were too few even for tentative conclusions, and no further data could be obtained from the investigators. The study was reported as ongoing, but for the purposes of this review was considered closed.
Urinary symptoms
Behavioural interventions
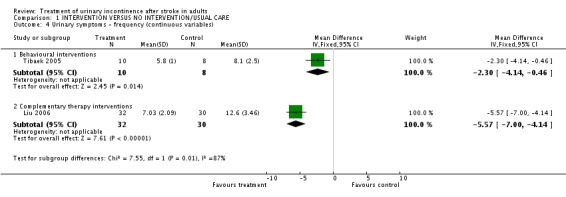
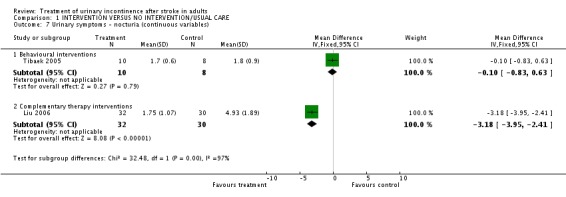
The impact of pelvic floor muscle training on mean daytime voiding frequency (Tibaek 2005) indicated a significant difference (WMD ‐2.30, 95% CI ‐4.14, ‐0.46, Comparisons 01.04.01). The impact on mean nighttime voiding frequency indicated no significant difference (WMD ‐0.10, 95% CI ‐0.83, 0.63, Comparison 01.07.01).
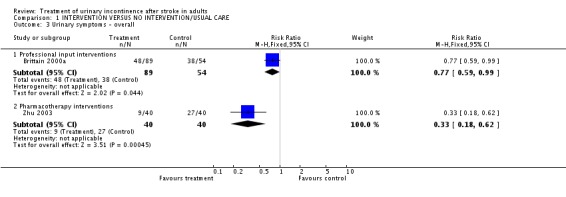
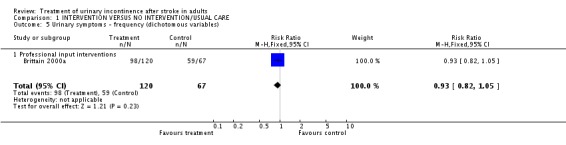
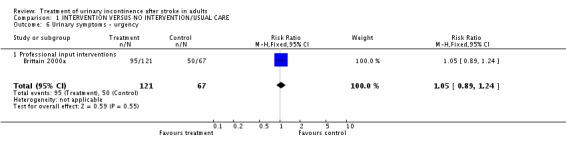
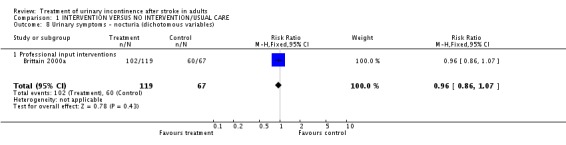
Specialised professional input interventions The impact of the Continence Nurse Advisor intervention on the number of people not cured of urinary symptoms at three months (Brittain 2000a) indicated a borderline significant difference (48/89 treatment versus 38/54 control, RR 0.77, 95% CI 0.59, 0.99, Comparison 01.03.01). No significant difference was found in the number of people with urinary frequency at three months (98/120 treatment versus 59/67 control, RR 0.93, 95% CI 0.82, 1.05, Comparison 01.05.01), the number of people with urinary urgency at three months.(95/121 treatment versus 50/67 control, RR 1.05, 95% CI 0.89, 1.24, Comparison 01.06.01) or the number of people with nocturia at three months (102/119 treatment versus 60/67 control, RR 0.96, 95% CI 0.86, 1.07, Comparison 01.08.01).
There were no data suitable for analysis in RevMan Analyses in relation to changes in the mean number of symptoms (see 'Characteristics of Included Studies Table'). However, the trial report suggested that the evidence favoured a Continence Nurse Advisor compared with usual care in terms of reducing the total number of symptoms experienced (p<0.01) at three months, with weak evidence in favour of the Continence Nurse Advisor in reducing the total number of symptoms at six months (p=0.06) (Brittain 2000a). There were no data suitable for analysis in RevMan Analyses in relation to changes in day and night‐time leakage scores (see 'Characteristics of Included Studies Table'). However, the trial report suggested that the evidence favoured a Continence Nurse Advisor compared with usual care in terms of day‐time severity of leakage at three months (p=0.038).
Complementary therapy interventions
The impact of ginger‐salt‐partitioned moxibustion on mean daytime voiding frequency (Liu 2006) indicated a significant difference (WMD ‐5.57, 95% CI ‐7.00, ‐4.14, Comparison 01.04.02). There was also a significant difference on mean nighttime voiding frequency (WMD ‐3.18, 95% CI ‐3.95, ‐2.41, Comparison 01.07.02).
There were no data suitable for analysis in RevMan Analyses in the trial of scalp acupuncture versus no scalp acupuncture (Chu 1997). After two weeks and two courses of treatment, the investigators reported a reduction in urinary frequency or incontinence of 90.3% in the intervention group, with two people not regaining "normal urine", 12 people partly regaining "normal urine" and 16 people regaining "normal urine". A significant difference between the experimental and control groups was reported as "p (0.05˜0.001)". No results are reported for the control group. No further data could be obtained for this study.
Pharmacotherapy interventions
The impact of meclofenoxate indicated a significant difference in the number of people whose incontinence did not improve (Zhu 2003) (9/40 treatment versus 27/40 control, RR 0.33, 95% CI 0.18, 0.62, Comparison 01.03.02).
Urological measures
Behavioural interventions
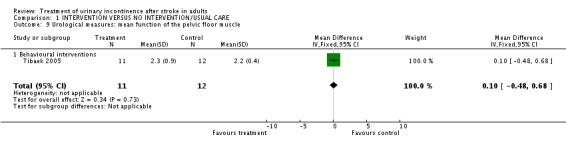
One trial (Tibaek 2005) measured mean function of the pelvic floor muscle. The impact of pelvic floor muscle training indicated no significant difference (WMD 0.10, 95% CI ‐0.48, 0.68, Comparison 01.09.01).
Health status and quality of life
Behavioural interventions
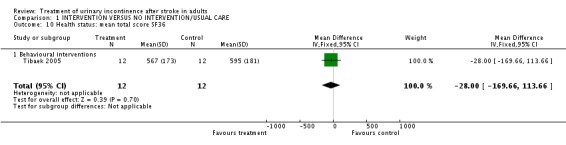
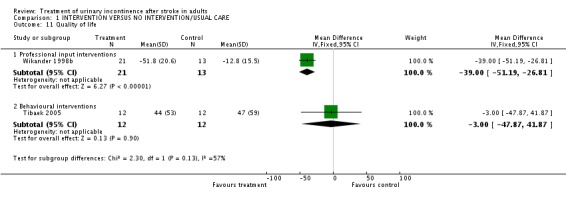
Tibaek 2005 measured health status in terms of the mean total score on the SF36. The impact of pelvic floor muscle training indicated no significant difference (WMD ‐28.00, 95% CI ‐169.66, 113.66, Comparison 01.10.01). Quality of life was measured using the mean total score on the IIQ; no significant difference was found (WMD ‐3.00, 95% CI ‐47.87, 41.87, Comparison 01.11.02).
Specialised professional input interventions Wikander 1998b measured quality of life in terms of mean change in psychological well‐being measured by the Psychological General Well‐Being Index. The impact of a functional assessment programme indicated a significant difference (WMD ‐39.00, 95% CI ‐51.19, ‐26.81, Comparison 01.11.01).
Function
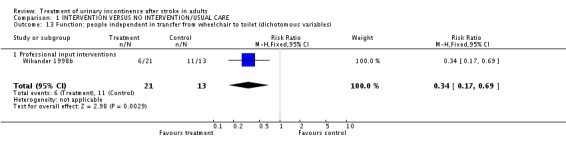
Specialised professional input interventions Wikander 1998b measured function in terms of the number of people independent in transfer from wheelchair to toilet. The impact of a functional assessment programme indicated a significant difference (6/21 treatment versus 11/13 control, RR 0.34, 95% CI 0.17 to 0.69, Comparison 01.13.01).
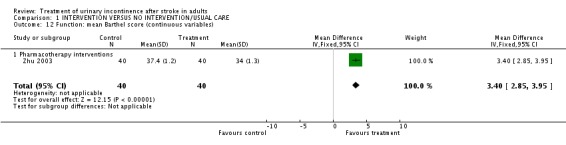
Pharmacotherapy interventions One trial measured function in terms of mean Barthel score (Zhu 2003). The impact of meclofenoxate indicated a significant difference (WMD 3.40, 95% CI 2.85 to 3.95, Comparison 01.12.01).
Patient satisfaction
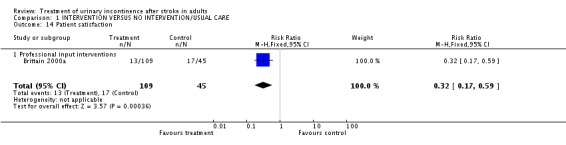
Specialised professional input interventions One trial (Brittain 2000a) measured satisfaction in terms of people not satisfied with the service at three months. The impact of the Continence Nurse Advisor indicated a significant difference (13/109 versus 17/45 control, RR 0.32, 95% CI 0.17, 0.59, Comparison 01.14.01).
Cost/service use
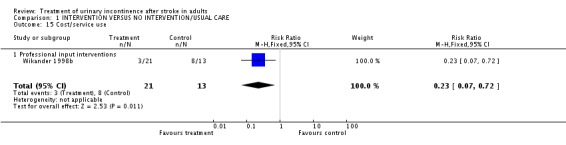
Specialised professional input interventions One trial (Wikander 1998b) measured service use in terms of people discharged to a setting other than their home. The impact of a functional assessment programme indicated a significant difference (3/21 treatment versus 8/13 control, RR 0.23, 95% CI 0.07 to 0.72, Comparison 01.15.01).
Six month follow‐up
Behavioural interventions
Tibaek 2005 measured outcomes at six months and found a pelvic floor muscle training programme indicated no significant difference in terms of mean SF36 score (WMD ‐46.00, 95% CI ‐165.05, 73.05) and mean IIQ score (WMD ‐4.00, 95% CI ‐56.17, 48.17). Specialised professional input interventions Brittain 2000a measured outcomes at six months and found some evidence of an impact of the Continence Nurse Advisor in terms of people with urinary symptoms at 6 months (48/89 treatment versus 38/54 control, RR 0.77, 95% CI 0.59 to 0.99), but little difference in terms of the number of people with incontinence after treatment (75/91 treatment versus 47/55 control, RR 0.96, 95% CI 0.83, 1.11), urinary frequency (73/89 treatment versus 47/54 control, RR 0.94, 95% CI 0.82, 1.09), urinary urgency (65/91 treatment versus 40/54 control, RR 0.96, 95% CI 0.79, 1.18) and nocturia (77/89 treatment versus 46/53 control, RR 1.00, 95% CI 0.87, 1.14). There were no data suitable for analysis in RevMan Analyses in relation to changes in the total number of symptoms; however, the trial report suggested that the evidence favoured a Continence Nurse Advisor compared with usual care in terms of reducing the total number of symptoms at six months (p=0.06).
2. INTERVENTION VERSUS PLACEBO (Comparison 02)
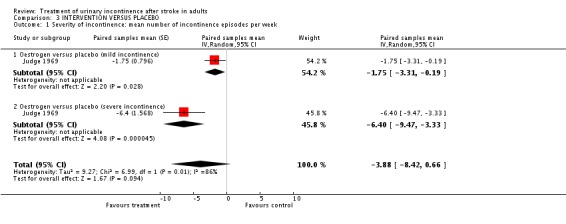
The only trial assessing this comparison was in the pharmacotherapy interventions category. One small cross‐over trial (Judge 1969) compared an intervention designed to promote urinary continence (oestrogen) against placebo in a long term care setting in 13 women with a history of stroke. Results were reported separately for people with mild or severe incontinence and in view of the significant heterogeneity between the two groups; a random effects model was used.
There was evidence in favour of oestrogen in both groups, with a stronger result in severe incontinence:
mild incontinence: paired samples mean difference in number of incontinent episodes per week ‐1.75; 95% CI ‐3.31 to ‐0.19, Comparison 02.01.01 (Judge 1969);
severe incontinence: paired samples mean difference ‐6.4; 95% CI ‐9.47 to ‐3.33. Comparison 02.01.02 (Judge 1969).
Combined results favoured oestrogen but were not statistically significant (paired samples mean ‐3.88 95% CI ‐8.42 to 0.66) (Judge 1969).
3. SPECIFIC INTERVENTION VERSUS ANOTHER INTERVENTION (Comparison 03)
The only trial assessing this comparison compared a behavioural intervention (timed voiding) with a pharmacotherapy intervention (oxybutinin)(Gelber 1997b). The data were too few for useful analysis, and no further data could be obtained from the investigators. The study was reported as ongoing, but for the purposes of this review was considered closed.
4. COMBINED INTERVENTION VERSUS SINGLE INTERVENTION (Comparison 04)
The only trial assessing this comparision compared a behavioural intervention with a physical therapy intervention; no studies assessed combinations involving any of the other categories of intervention. Lewis 1990 compared a combined intervention (sensory motor biofeedback plus timed voiding) designed to promote urinary continence against a single intervention (timed voiding). The number of incontinence episodes were fewer in the control group (WMD 2.20; 95% CI 0.12 to 4.28, Comparison 04.02.01) (Lewis 1990).
Discussion
Summary of main results
The review aimed to consider all interventions designed to treat urinary incontinence after stroke in adults, rather than testing a specific hypothesis. Experimental studies were found testing a wide range of interventions.
Behavioural interventions
The small trial testing pelvic floor muscle training against general rehabilitation (Tibaek 2005) found evidence in favour of the treatment group in terms of mean voiding frequency over 24 hours and mean daytime voiding frequency; however findings were only significant when outcomes were measured over three rather than two days. No significant differences were found in the primary outcome (24 hour pad test) or secondary outcomes including vaginal palpation of the pelvic floor muscle, the SF36 or the IIQ.
Specialised professional input interventions
One of the two trials testing specialised professional input (Wikander 1998b) suggests that structured assessment and management is promising in helping people to regain continence in the early post‐stroke period: this type of intervention merits further research. Some features of this trial have to be considered in interpreting the large effects suggested. Because the intervention was delivered on a separate ward, equivalence of treatment other than the intervention could not be assessed, particularly in relation to staff mix and numbers. The sample size was small, it is not clear why the numbers in the two trial groups differed, and outcome measurement was not blinded. Furthermore, if there is an effect, it is not possible to separate out the influences of structured assessment which could include training of the staff, multi‐disciplinary coordination or the focus provided to staff by regular feedback of outcome, from the involvement of the patient in the assessment and management of their care (Wikander 1998b).
The other trial of specialised professional input (Brittain 2000a) found that outcomes from Continence Nurse Advisor and usual care groups were similar at all data points, with the exception of the number of people cured of all urinary symptoms at six months and satisfaction with the service at three months, where findings favoured the treatment group (although the confidence intervals were wide). The intervention used by the Continence Nurse Advisor was well defined, with a structured assessment and intervention protocol. This was particularly appropriate where reasons for continuing problems with continence had not been investigated. The generalisability of these results has to take into consideration the wide definition of urinary incontinence used, which included people suffering from a range of other urinary symptoms such as frequency or urgency. While the trial overall was relatively large and well designed, the proportion of participants for whom results were available at six months was quite low (63%) and some of the data could not be analysed.
If there are differences between the two professional input intervention trials, these could be related both to the intensity of the interventions, and to the type of trial participant. Participants in the trial by Wikander 1998b were between 11‐19 days post‐stroke when the hospital‐based intervention began, whereas the trial by Brittain 2000a was conducted in a community setting. This is likely to have included people with a much less recent stroke. Participants in the trial by Wikander 1998b were also known to have developed continence problems only subsequent to their stroke, while this is not known for the other trial (Brittain 2000a).
Complementary therapy interventions
Three trials of complementary therapies found significant differences favouring the treatment group: acupuncture was more effective than usual care in terms of people with incontinence after treatment (Zhang 2002), eye and scalp acupuncture was more effective than no acupuncture in terms of people with incontinence after treatment (Zhou 1999) and ginger‐salt‐partitioned moxibustion plus routine acupuncture was more effective than routine acupuncture in terms of mean daytime voiding frequency and mean nighttime voiding frequency (Liu 2006). However, these trials reported minimal methodological detail and it is likely they were of poor quality. It is also not clear whether, and how, these interventions are transferable to the health services of other countries.
Pharmacotherapy interventions
In the small cross‐over trial testing an intervention (oral oestrogen) against placebo (Judge 1969), there were fewer incontinence episodes per week during oestrogen treatment. However, it is only known that the participants had a history of stroke. The high mean age of the participants in the trial, the presence of confusion and lack of mobility, and the setting in which the trial took place (two geriatric hospitals) suggest that continence problems may have been secondary to other conditions as well as stroke. For this reason it is problematic to generalise these results to women after stroke. Furthermore, the dose prescribed is not that currently recommended, hormone replacement therapy is anyway now widely considered to be contraindicated in patients who have had, or are at risk of, stroke, and there is evidence that oestrogens increase incontinence in postmenopausal women (Hendrix 2005).
It is notable that the most promising results for regaining continence were from the Wikander 1998b trial, where the time since stroke onset of the participants was short. Early intervention could have greatest impact on the numbers regaining continence. However, 25% of people discharged from hospital after stroke still have continence problems. There is a lack of evidence on whether the many other forms of intervention that have been shown to have some value in the general population would also be relevant to people after stroke. In particular, there are no usable results from trials testing the use of bladder relaxants such as anticholinergics, or programmes of timed voiding or bladder training.
Overall completeness and applicability of the evidence
The major point of interest in the included studies is the mix of categories of intervention, including physical, behavioural and complementary therapies, drugs, and professional input interventions such as using trained personnel and specific methods of managing care. However, within categories it was generally not possible to combine studies due to use of different outcome measures.
Quality of the evidence
The trials included were generally small (n=724 participants in total; median =49 participants); only three trials (Brittain 2000a; Judge 1969; Tibaek 2005) had adequate allocation concealment and many were limited by poor reporting. For most, it was impossible to judge the extent to which they might have been prone to bias. The small sample sizes meant that confidence intervals were wide and did not rule out clinically important differences when there was no statistically significant difference. Even the largest trial (Brittain 2000a) is not fully reported, and it has not been possible to obtain full data from the investigators. The review is also limited by the lack of full data for four other trials (Chu 1997; Gelber 1997a; Gelber 1997b; Lewis 1990).
Potential biases in the review process
The original protocol for this review specified inclusion criteria for participants as having had a stroke in the previous 12 months, but this definition proved unworkable, because most of the trials did not specify or report time since stroke. There was also a lack of clarity about whether urinary incontinence was subsequent to stroke. Only three trials (Liu 2006; Tibaek 2005; Wikander 1998b) specified that urinary continence problems were subsequent to stroke, and detailed the time since stroke onset. For this reason, the extent to which the results for the remaining trials can be generalised to people with continence problems solely as the result of a stroke is unclear.
Authors' conclusions
There is very little evidence from stroke‐specific studies to guide practice. The lack of trials testing the same category of intervention means that recommendations for practice are based on the results of a few, usually small, trials. The Wikander 1998b and Brittain 2000a trials provide some evidence to suggest that specialised professional input using systematic methods to assess and manage continence problems may improve some outcomes. The limited evidence suggests that the greatest impact on urinary incontinence may be in the acute phase of rehabilitation after stroke. However, the Brittain (Brittain 2000a) trial suggests that specialist input and individualised care management may improve the number of symptoms of urinary incontinence even in the longer term.
Evidence suggesting that beneficial outcomes may be achieved by structuring the management of care for people with urinary continence problems following a stroke points to the need for larger trials. Given the variety of problems that can hinder the maintenance of continence after stroke, the use of individualised assessment and goal setting to tailor interventions to the neurological and functional problems of the individual would seem to be especially worthy of consideration.
Methods of managing continuing urinary incontinence such as intermittent catheterisation or the use of catheter valves are also needed.
Three trials of complementary therapies, namely acupuncture (Zhou 1999; Zhang 2002) and ginger‐salt‐partitioned moxibustion (Liu 2006), suggest these interventions may be worth investigating further with more rigorous study design.
There is a need for more well‐designed studies. Further research should use standardised definitions and classification systems to record details of the type and severity of stroke, and the type and severity of urinary incontinence. Pre‐stroke continence status, time since stroke and stroke recurrence should also be recorded, with clear inclusion criteria for continence status. Exclusion criteria should be given for comorbidities and clinical indicators of underlying urogenital or systemic conditions such as infection. Specific details of structured assessment and intervention protocols need to be given, with standardisation of treatment, measures of between groups contamination or differences, and tailoring of intervention to the early or later phases of rehabilitation. Outcome measures of urinary incontinence and of urinary symptoms should be standardised, with attention to their validity and reliability and the blinding of outcome assessment. The measurement of changes in health related quality of life would be valuable. The time periods for review should be standardised for the acute, early and later phases of rehabilitation. Lastly, sample size calculations and secure randomisation at either the cluster or individual patient level should be used appropriately.
Acknowledgements
We wish to thank members of the Editorial team in the Incontinence Review Group, in particular Sheila Wallace and Cathryn Glazener, and Hazel Fraser and Brenda Thomas of the Stroke Review Group. Our thanks also to the Faculty of Health of the University of Central Lancashire for supporting this review, to trial authors for kindly responding to our requests for information and to the members of the Stroke Incontinence Interest Group for their valuable comments. Thank you to Gui Li of the Shanghai Medical Military University and Luyan Fang of the University of Central Lancashire for kindly translating some of our included studies.
Appendices
Appendix 1. Search methods and terms used for the extra specific searching for this review
Due to the comprehensive nature of the searches already performed by the Stroke Collaborative Review Group, no additional searches were performed other than on CINAHL, combining stroke terms with terms for urinary incontinence without a research methods filter. This was done because of the potentially poor indexing of nursing research. The following strategy was used to search CINAHL (January 1982 to January 2007) on Dialog Datastar:
1. Urination‐disorders#.de 2. Urinary‐tract‐infections#.de 3. Bladder‐neurogenic.de 4. Bowel‐and‐bladder‐management#.de 5. Urologic‐nursing.de 6. Urologic‐care.de 7. Catheters‐urinary#.de 8. Catheter‐care‐urinary#.de 9. Urinary‐catheterization#.de 10. Urinary‐bladder‐irrigation.de 11. Catheter‐irrigation‐urinary.de 12. Diagnosis‐urologic#.de 13. Urinary‐tract‐physiology#.de 14. Incontinence‐aids#.de 15. Urinary‐incontinence‐and frequency‐comfort‐questionnaire.de 16. Tube‐care‐urinary‐IOWA‐NIC.de 17. Urinary‐catheterisation‐IOWA‐NIC.de 18. Urinary‐elimination‐management‐IOWA‐NIC.de 19. Urinary‐incontinence‐care‐IOWA‐NIC.de 20. Urinary‐retention‐care‐IOWA‐NIC.de 21. Urinary‐continence‐IOWA‐NOC.de 22. Urinary‐elimination‐IOWA‐NOC.de 23. Altered‐urinary‐elimination‐NANDA#.de 24. Urinary‐elimination‐component‐SABA‐HHCC#.de 25. Genito‐urinary‐function‐OMAHA.de 26. Urin$ or bladder or urethra$).ti,ab. 27. Catheterization.w..de 28. Catheters.w..de 29. Catheter‐care.de 30. Catheter‐occlusion.de 31. Catheter‐removal.de 32. Tube‐removal.de 33. Catheter‐placement‐determination.de 34. Catheter‐related‐complications#.de 35. 26 and (27 or 28 or 29 or 30 or 31 or 32 or 33 or 34) 36. Void$ near (difficult$ or impair$ or problem$ or disorder$ or impair$ or control$) 37. 36.ti or 36.ab 38. Urin$ with (incontinen$ or continen$) 39. 38.ti or 38.ab 40. Bladder with (incontin$ or continen$) 41. 40.ti or 40.ab 42. Detrusor with (instability or stability or stable or unstable) 43. 42.ti or 42.ab 44. Bladder with (instability or stability or stable or unstable) 45. 44.ti or 44.ab 46. (stress or urge or pad$) with (continen$ or incontinen$) 47. 46.ti or 46.ab 48. Urodynamic$.ti or urodynamic$.ab 49. 37 or 39 or 41 or 43 or 45 or 47 or 48 50. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 51. 35 or 49 or 50
Key: # = explode, .de = index word, $ = wild card.
The above search was combined with the CINAHL search from the Cochrane Stroke Group.
Recent unpublished trial data were also searched for on national and international databases i.e. NHS National Research Register, NHS Research Findings Register, US Community of Science NIH Grants, MetaRegister of Controlled Clinical Trials and CRISP, by adapting terms drawn from the existing search strategies of the Incontinence and Stroke Review Groups.
The reference lists of all relevant reviews and trial reports were searched to identify further relevant studies. Major investigators were contacted to ask for any other possible relevant trials, published or unpublished. In addition, contact was made with the authors of other relevant Cochrane reviews to ascertain whether defined subgroups of stroke survivors were identified in trials testing methods of promoting continence in a general population.
The review was publicised on the following websites: Joanna Briggs Institute, Royal College of Nursing Research Society, Royal College of Nursing Continence Interest Group, Association of Continence Advisors, Sigma Theta Tau.
We did not impose any language or other restrictions on any of these searches.
Data and analyses
Comparison 1.
INTERVENTION VERSUS NO INTERVENTION/USUAL CARE
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of people with incontinence after treatment | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Professional input interventions | 2 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.01, 4.67] |
| 1.2 Complementary therapy interventions | 3 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.23, 0.86] |
| 2 Severity of incontinence: mean number of incontinence episodes in 24 hours | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐2.74, 0.74] |
| 2.1 Behavioural interventions | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐2.74, 0.74] |
| 3 Urinary symptoms ‐ overall | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Professional input interventions | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.59, 0.99] |
| 3.2 Pharmacotherapy interventions | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.18, 0.62] |
| 4 Urinary symptoms ‐ frequency (continuous variables) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Behavioural interventions | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐2.3 [‐4.14, ‐0.46] |
| 4.2 Complementary therapy interventions | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐5.57 [‐7.00, ‐4.14] |
| 5 Urinary symptoms ‐ frequency (dichotomous variables) | 1 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.82, 1.05] |
| 5.1 Professional input interventions | 1 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.82, 1.05] |
| 6 Urinary symptoms ‐ urgency | 1 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.89, 1.24] |
| 6.1 Professional input interventions | 1 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.89, 1.24] |
| 7 Urinary symptoms ‐ nocturia (continuous variables) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Behavioural interventions | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.83, 0.63] |
| 7.2 Complementary therapy interventions | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐3.18 [‐3.95, ‐2.41] |
| 8 Urinary symptoms ‐ nocturia (dichotomous variables) | 1 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.86, 1.07] |
| 8.1 Professional input interventions | 1 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.86, 1.07] |
| 9 Urological measures: mean function of the pelvic floor muscle | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.48, 0.68] |
| 9.1 Behavioural interventions | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.48, 0.68] |
| 10 Health status: mean total score SF36 | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐28.0 [‐169.66, 113.66] |
| 10.1 Behavioural interventions | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐28.0 [‐169.66, 113.66] |
| 11 Quality of life | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 Professional input interventions | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐39.0 [‐51.19, ‐26.81] |
| 11.2 Behavioural interventions | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐47.87, 41.87] |
| 12 Function: mean Barthel score (continuous variables) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 3.40 [2.85, 3.95] |
| 12.1 Pharmacotherapy interventions | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 3.40 [2.85, 3.95] |
| 13 Function: people independent in transfer from wheelchair to toilet (dichotomous variables) | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.17, 0.69] |
| 13.1 Professional input interventions | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.17, 0.69] |
| 14 Patient satisfaction | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.17, 0.59] |
| 14.1 Professional input interventions | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.17, 0.59] |
| 15 Cost/service use | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.07, 0.72] |
| 15.1 Professional input interventions | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.07, 0.72] |
Analysis 1.1.
Comparison 1 INTERVENTION VERSUS NO INTERVENTION/USUAL CARE, Outcome 1 Number of people with incontinence after treatment.
Analysis 1.2.
Comparison 1 INTERVENTION VERSUS NO INTERVENTION/USUAL CARE, Outcome 2 Severity of incontinence: mean number of incontinence episodes in 24 hours.
Analysis 1.3.
Comparison 1 INTERVENTION VERSUS NO INTERVENTION/USUAL CARE, Outcome 3 Urinary symptoms ‐ overall.
Analysis 1.4.
Comparison 1 INTERVENTION VERSUS NO INTERVENTION/USUAL CARE, Outcome 4 Urinary symptoms ‐ frequency (continuous variables).
Analysis 1.5.
Comparison 1 INTERVENTION VERSUS NO INTERVENTION/USUAL CARE, Outcome 5 Urinary symptoms ‐ frequency (dichotomous variables).
Analysis 1.6.
Comparison 1 INTERVENTION VERSUS NO INTERVENTION/USUAL CARE, Outcome 6 Urinary symptoms ‐ urgency.
Analysis 1.7.
Comparison 1 INTERVENTION VERSUS NO INTERVENTION/USUAL CARE, Outcome 7 Urinary symptoms ‐ nocturia (continuous variables).
Analysis 1.8.
Comparison 1 INTERVENTION VERSUS NO INTERVENTION/USUAL CARE, Outcome 8 Urinary symptoms ‐ nocturia (dichotomous variables).
Analysis 1.9.
Comparison 1 INTERVENTION VERSUS NO INTERVENTION/USUAL CARE, Outcome 9 Urological measures: mean function of the pelvic floor muscle.
Analysis 1.10.
Comparison 1 INTERVENTION VERSUS NO INTERVENTION/USUAL CARE, Outcome 10 Health status: mean total score SF36.
Analysis 1.11.
Comparison 1 INTERVENTION VERSUS NO INTERVENTION/USUAL CARE, Outcome 11 Quality of life.
Analysis 1.12.
Comparison 1 INTERVENTION VERSUS NO INTERVENTION/USUAL CARE, Outcome 12 Function: mean Barthel score (continuous variables).
Analysis 1.13.
Comparison 1 INTERVENTION VERSUS NO INTERVENTION/USUAL CARE, Outcome 13 Function: people independent in transfer from wheelchair to toilet (dichotomous variables).
Analysis 1.14.
Comparison 1 INTERVENTION VERSUS NO INTERVENTION/USUAL CARE, Outcome 14 Patient satisfaction.
Analysis 1.15.
Comparison 1 INTERVENTION VERSUS NO INTERVENTION/USUAL CARE, Outcome 15 Cost/service use.
Comparison 3.
INTERVENTION VERSUS PLACEBO
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severity of incontinence: mean number of incontinence episodes per week | 1 | Paired samples mean (Random, 95% CI) | ‐3.88 [‐8.42, 0.66] | |
| 1.1 Oestrogen versus placebo (mild incontinence) | 1 | Paired samples mean (Random, 95% CI) | ‐1.75 [‐3.31, ‐0.19] | |
| 1.2 Oestrogen versus placebo (severe incontinence) | 1 | Paired samples mean (Random, 95% CI) | ‐6.4 [‐9.47, ‐3.33] |
Analysis 3.1.
Comparison 3 INTERVENTION VERSUS PLACEBO, Outcome 1 Severity of incontinence: mean number of incontinence episodes per week.
Comparison 5.
COMBINED INTERVENTION VERSUS SINGLE INTERVENTION
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of people with incontinence after treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Sensory‐motor biofeedback device + timed voiding versus timed voiding alone | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.80, 1.49] |
| 2 Severity of incontinence: mean number of incontinence episodes | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Sensory‐motor biofeedback device + timed voiding vs timed voiding alone | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 2.2 [0.12, 4.28] |
Analysis 5.1.
Comparison 5 COMBINED INTERVENTION VERSUS SINGLE INTERVENTION, Outcome 1 Number of people with incontinence after treatment.
Analysis 5.2.
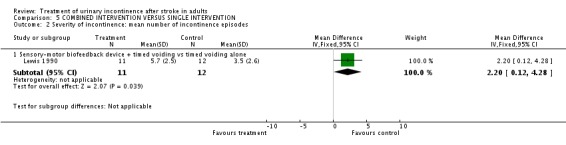
Comparison 5 COMBINED INTERVENTION VERSUS SINGLE INTERVENTION, Outcome 2 Severity of incontinence: mean number of incontinence episodes.
What's new
Last assessed as up‐to‐date: 13 November 2007.
| Date | Event | Description |
|---|---|---|
| 13 October 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 14 November 2007 | New citation required and conclusions have changed | Substantive amendment. Five new studies added |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brittain 2000a
| Methods | RCT Setting: at home | |
| Participants | 232 male (61%) and female (39%) adult stroke survivors with urinary incontinence. Inclusion: Stroke diagnosed by self‐report (questionnaire) as stroke, subarachnoid haemorrhage or transient ischaemic attack. Self‐reported clinically significant urinary symptoms, including leakage of urine, stress incontinence, frequency, nocturia or urgency. Exclusion: Pregnancy, physical causes of urinary tract dysfunction (prolapse, urethral stricture, prostatic obstruction, pelvic mass or malignancy in past 5 years), active treatment for incontinence in past 6 months, neurogenic retention, raised post‐residual volumes, glycosuria or haematuria Age 40 to 96 years, mean 70 | |
| Interventions | A: 152 people allocated to Continence Nurse Practitioner (CNP) assessment and treatment. CNP received 3 months training, and used formatted assessment and guidelines for treatment, which could include habit retraining, pelvic floor awareness, dietary advice, the provision of continence garments, and GP referral for treatment of atrophic vaginitis, candidiasis or constipation. The intervention comprised six contacts and five treatment visits at weeks 1, 2, 4, 6 and 8. Week 1: History + physical exam, Week 2: Diagnostic visit + development of management plan, Weeks 4 and 6: review, adapt + reinforce management plan, Week 8: re‐assessment. B: 80 people allocated to usual care provided by general practitioner and referral to existing services for the management of continence. | |
| Outcomes | Incontinence after treatment at 3 months: A: 40/73, B: 31/48 Incontinence after treatment at 6 months: A: 75/91, B: 47/55 Mean day‐time leakage severity at 3 months: A: ‐0.27, B: +0.05 Mean day‐time leakage severity at 6 months: A: ‐0.69, B: ‐0.52 Mean night‐time leakage severity at 3 months: A: ‐0.19, B: ‐0.02 Mean night‐time leakage severity at 6 months: A: ‐0.40, B: +0.02 Urinary frequency at 3 months: A: 98/120, B: 59/67 Urinary frequency at 6 months: A: 73/89, B: 47/54 Urinary urgency at 3 months: A: 95/121, B: 50/67 Urinary urgency at 6 months: A: 65/91, B: 40/54 Nocturia at 3 months: A: 102/119, B: 60/67 Nocturia at 6 months: A: 77/89, B: 46/53 Not cured of all urinary symptoms at 3 months: A: 75.3%, B: 82.1% Not cured of all urinary symptoms at 6 months: A: 48/89, B: 38/54 Mean number of urinary symptoms at 3 months: A: ‐0.58, B: ‐0.41 Mean number of urinary symptoms at 6 months: A: ‐0.47, B: ‐0.20 Not satisfied with service: A: 13/109, B: 17/45 |
|
| Notes | Sub sample of the MRC Incontinence Study 2:1 randomisation. No baseline differences in age and gender between the groups Proportions for which data reported: 82% at 3 months, 63% at 6 months. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Yes | A ‐ Adequate |
Chu 1997
| Methods | RCT | |
| Participants | 32 (53%) male and 28 (47%) female adult ischaemic stroke survivors with urinary incontinence/frequency. Subjects described as having multi‐focal cerebral infarction. Age: male 50‐71, female 45‐61. | |
| Interventions | A: 30 people allocated to scalp acupuncture using the "bai hui" channel. Needle in situ for 1‐2 days in hot weather, and 3‐7 days in cold weather. Needle manipulated every 4 hours. Treatment period: 1‐2 weeks. B: 30 people allocated to usual care. No details given. | |
| Outcomes | A: people totally regaining "normal urine" = 16/30, partly regaining "normal urine" = 12/30 , not regaining "normal urine" = 2/30. B: no results given. | |
| Notes | Both groups received acupuncture in the channels "guan yuan" and "san yinjiao" combined with nursing care, which was interpreted as usual care. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Gelber 1997a
| Methods | RCT | |
| Participants | 37 adult unilateral stroke survivors with urinary incontinence and normal urodynamic studies. Age and gender mix not given | |
| Interventions | A: 8 people allocated to timed voiding B: 10 people allocated to void on request No further details given | |
| Outcomes | Number of incontinence episodes per day. No numerical results reported. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Gelber 1997b
| Methods | RCT | |
| Participants | 37 adult unilateral stroke survivors with urinary incontinence and bladder hyperreflexia. Age and gender mix not given | |
| Interventions | A: 9 people allocated to Oxybutinin B: 10 people allocated to timed voiding No further details given | |
| Outcomes | Number of incontinence episodes per day. No numerical results reported | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Judge 1969
| Methods | Cross‐over trial Setting: Long stay geriatric hospitals | |
| Participants | 13 females with a diagnosis of cerebrovascular accident or "multiple little strokes" , 7 with mild incontinence (average 12.3 episodes/week), and 6 with severe incontinence (average 41.1 episodes/week). Inclusion: None stated Exclusion: Faecal impaction, urinary infection Age: mean 82, range 66‐92 | |
| Interventions | Oestrogen as Quinestradol 0.25mg 4 times a day for one month or placebo with a wash‐out period of one month. | |
| Outcomes | Number of incontinent episodes per week: Group 1 (mildly incontinent) placebo week 10.14 (SD 4.76), active preparation week 8.43 (SD 2.42) Group 2 (severely incontinent) placebo week 38.80 (SD 5.07), active preparation week 32.4 (SD 2.42) | |
| Notes | Participants in group 1 (defined as 'mildly incontinent') and group 2 (defined as 'severely incontinent') were from two different hospitals. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Yes | A ‐ Adequate |
Lewis 1990
| Methods | RCT | |
| Participants | 23 adults with post‐stroke urinary urge incontinence. 5 (22%) haemorrhagic stroke, 18 (78%) with ischaemic stroke. Age and gender mix not given | |
| Interventions | A: 11 people allocated to sensory‐motor biofeedback device (Uristop) + timed voiding B: 12 people allocated to timed voiding Treatment time of 2 weeks. | |
| Outcomes | Incontinence after treatment: A: 10/11, B: 10/12 Number of incontinence episodes: A: 5.7 (SD 2.5), B: 3.5 (SD 2.6) |
|
| Notes | Continence defined as zero incontinence episodes in the last two days of the study | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Liu 2006
| Methods | RCT Setting: inpatient | |
| Participants | 35 male (47%) and 40 female (53%) adult stroke survivors with urinary incontinence. Inclusion: cerebral infarction or haemorrhage, time between stroke onset and admission to hospital less than 6 months, continent of urine pre‐stroke, presence of urinary incontinence, urgent or frequent micturition when condition stabilised after stroke, micturition problems affected quality of life, conscious and able to communicate, normal recognition, aged between 40 and 75 years. Exclusion: co‐morbidities involving the heart, kidney or other important organs, pre‐stroke chronic urinary retention and urinary incontinence, unable to communicate, long‐standing chronic urinary tract infection. Age: mean 64 years |
|
| Interventions | A (39): Ginger‐salt‐partitioned moxibustion at Sheque (CV 8) and routine acupuncture (e.g. Tsusanli, Yinlingquan). B (36): Routine acupuncture. | |
| Outcomes | Mean number of urination times each day: A: 7.03, B: 12.60 Mean number of times requesting toileting at night: A: 1.75, B: 4.93 Number of participants with incontinence at 72 hours: A: 16/39; B: 21/36 |
|
| Notes | No baseline differences in age, gender, type of stroke, stroke course, frequency symptoms and grade of urinary incontinence between the groups. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Tibaek 2005
| Methods | RCT Setting: outpatient | |
| Participants | 26 women adult stroke survivors with mixed stress/urge urinary incontinence. Inclusion: women diagnosed with first ever ischaemic stroke according to WHO definition and verified by CAT scan; stroke symptoms in at least 1 month; normal cognitive function (mini‐mental state examination score <24); UI according to ICS definition; independent walking ability indoors >100m with/without aids; independence in toilet visits; age between 40 and 85 years. Exclusion: urinary tract infection; symptom of vaginal prolapse; chronic respiratory diseases; psychiatric diseases; other neurological diseases; unable to speak Danish. Age: median 60 years |
|
| Interventions | A (14): Intervention group: "systematic, controlled, intensive pelvic floor muscle (PFM) training programme". Programme consisted of 1) introduction to theory 2) home exercises, including strength PFM exercise by performing close to maximum contraction (6 sec contraction/6 sec rest) Subjects instructed to repeat exercise programme gradually 6‐10 times in supine, standing and sitting positions, 1‐2 times daily. 3) group treatment once per week, including isolating PFM contraction (6 sec contraction/6 sec rest) and strength exercises (3 sec contraction/3 sec rest, and 6 sec contraction/6 sec rest). All techniques were repeated 4‐8 times while supine, sitting and standing, and also prior to daily activities such as rising, sitting and walking. Vaginal palpation was performed to ensure correct contraction, to evaluate contraction strength and to give feedback to subjects. B (12) Comparison group received normal, general rehabilitation with no specific treatment of urinary incontinence. |
|
| Outcomes | Two day mean voiding frequency over 24 hours: A: 7.6, B: 9.2 Two day mean daytime voiding frequency: A: 5.9, B: 7.3 Two day mean nighttime voiding frequency: A: 1.7, B: 1.9 Two day mean number of incontinence episodes/24 hours: A: 0.2, B: 1.1 Two day mean number of used pads/24 hours: A: 1.1, B: 2.0 Three day mean voiding frequency over 24 hours: A: 7.6, B: 10.0 Three day mean daytime voiding frequency: A: 5.8, B: 8.1 Three day mean nighttime voiding frequency: A: 1.7, B: 1.8 Three day mean number of incontinence episodes/24 hours: A: 0.2, B: 1.2 Three day mean number of used pads/24 hours: A: 1.1, B: 1.8 Mean function of pelvic floor muscle: A: 2.3, B: 2.2 Mean strength of pelvic floor muscle: A: 2.6, B: 2.8 Mean static endurance of pelvic floor muscle: A: 23.4, B: 21.3 Mean dynamic endurance of pelvic floor muscle: A:15.7, B: 9.8 Short Form Health Status Questionnaire (SF36) post‐intervention: A: 567, B: 595 Short Form Health Status Questionnaire (SF36) at 6 months: A: 550, B: 596 Incontinence Impact Questionnaire (IIQ) post‐intervention: A: 44, B:47 Incontinence Impact Questionnaire (IIQ) at 6 months: A: 43, B: 47 |
|
| Notes | No baseline differences in age, number of births or mobility between the groups Proportions for which data reported: 92% at post‐test and 6 months |
|
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Yes | A ‐ Adequate |
Wikander 1998b
| Methods | RCT Setting: hospital rehabilitation ward | |
| Participants | 15 (43%) male and 19 (57%) female adults admitted to hospital within 1 month of acute hemispheric stroke Inclusion: New urinary incontinence since stroke Exclusion: none specified Age: mean 74 (SD 6.87) | |
| Interventions | A: 21 people allocated to intervention programme based on assessment using the Functional Independence Measure (FIM). Ward staff educated in use of FIM. Participants assessed on admission and at weekly goal setting meetings. B: 13 people allocated to usual rehabilitation care based on Bobath method | |
| Outcomes | Incontinence after treatment: A: 1/21, B: 3/13 Functional ability (median values of Katz ADL index scale A‐G (B = dependent in 1 activity) A: F/B, B: F/F Change in psychological well‐being: A: 51.8, B: 12.8 Not discharged to home setting: A: 3/21, B: 8/13 Not independent in transfer from bed to wheelchair: A: 6/21, B: 9/13 Not independent in transfer from wheelchair to toilet: A: 6/21, B: 11/13 Not independent in management of wheelchair: A: 0/21, B: 8/13 |
|
| Notes | Rehabilitation period 75‐91 days No baseline differences in age, gender, side of stroke lesion, time from stroke onset to admission, duration of hospital stay, dysphasia, functional ability, independence in activities of daily living, mobility or psychological well‐being between groups. Participants were randomised to two different wards. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Zhang 2002
| Methods | RCT Setting: unclear | |
| Participants | 38 male (59%) and 26 female (41%) adult stroke survivors with urinary incontinence. Inclusion and exclusion: unclear Age: range 42‐62 years |
|
| Interventions | A (36): acupuncture at the following points: Tsusanli, Yinlingquan, Sanyinjiao B (28): general treatment using mannite and other medicines | |
| Outcomes | Numbers not regaining continence: A: 6/36, B: 26/28 | |
| Notes | No description of baseline comparisons | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Zhou 1999
| Methods | RCT Setting: unclear | |
| Participants | 32 male (40%) and 48 female (60%) adult stroke survivors with urinary incontinence. Inclusion and exclusion: unclear |
|
| Interventions | A (40): eye acupuncture and electriferous scalp acupuncture once per day, six days per week for 4 weeks Medication therapy, e.g. Hua Duo Zai shi pill, Xi bi lin, Nao fu kang, vitamins C and E B (40): Medication therapy, e.g. Hua Duo Zai shi pill, Xi bi lin, Nao fu kang, vitamins C and E | |
| Outcomes | Numbers not regaining continence: A: 18/40, B: 32/40 | |
| Notes | No description of baseline comparisons | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | Unclear | B ‐ Unclear |
Zhu 2003
| Methods | Quasi‐experiment Setting: inpatient | |
| Participants | 45 male (56%) and 35 female (44%) adult stroke survivors with urinary incontinence. Inclusion: Acute cerebral infarction confirmed by CT scan, urinary incontinence Exclusion: Unconscious, mental abnormality Age: mean 63 years |
|
| Interventions | A (40): Meclofenoxate 0.3g three times per day for 1 month, in addition to salvia miltirrhiza injection 16 ml, IV, 4 times per day for 2 weeks. B (40%): salvia miltirrhiza injection 16 ml, IV, 4 times per day for 2 weeks. | |
| Outcomes | Number whose urinary symptoms did not improve: A: 9/40, B: 27/40 Mean cognitive function: A: 34.5, B: 30.42 Mean activities of daily living: A: 37.4, B: 34.0 |
|
| Notes | Groups described as 'comparable' | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Allocation concealment? | No | C ‐ Inadequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cook 1998 | Does not include a measure of continence |
| Gross 1990 | Does not include a measure of continence |
| Tekeoglu 1998 | Does not directly test a method of promoting continence |
Contributions of authors
The review was conceived and designed by Lois Thomas. Searching was completed by Beverley French. Trials were considered for inclusion independently by two reviewers (Lois Thomas, Beverley French). Data extraction and review of the methodological quality of the eligible studies was then independently conducted by two reviewers for each study (Chris Sutton, Michael Leathley, Stephen Cross, Beverley French and Lois Thomas). Extracted data and quality assessment were crosschecked and any disagreements discussed and if necessary resolved by Lois Thomas. Lois Thomas and Beverley French wrote the text of the review. James Barrett and Caroline Watkins acted as external reviewers, and contributed to constructing the implications for practice and research.
Sources of support
Internal sources
Faculty of Health, University of Central Lancashire, UK.
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
- Brittain KR, Potter JF. The treatment of urinary incontinence in stroke survivors (MS9). Report for NHS R&D Programme on Cardiovascular Disease and Stroke Project, Division of Medicine for the Elderly, Dept of Medicine, University of Leicester, in collaboration with the MRC Incontinence Study2000. [DOI] [PubMed]
- Chu M, Feng J. Discussion on treating frequent urine due to multiple cerebral embolism with scalp acupuncture (Translation from Chinese). Information on Traditional Chinese Medicine 1997;5:42. [Google Scholar]
- Gelber DA, Swords L. Treatment of post‐stroke urinary incontinence (Abstract). Journal of Neurologic Rehabilitation 1997;11(2):131. [Google Scholar]
- Gelber DA, Swords L. Treatment of post‐stroke urinary incontinence (Abstract). Journal of Neurologic Rehabilitation 1997;11:131. [Google Scholar]
- Judge TG. The use of quinestradol in elderly incontinence women, a preliminary report. Gerontologica Clinica 1969;11:159‐64. [DOI] [PubMed] [Google Scholar]
- Lewis AM, Travis ML, Gordon AL, Weaver HB, Reding MJ. Sensory‐motor biofeedback for the treatment of urinary urge incontinence following stroke (Abstract). Clinical Research 1990;38(1):A10. [Google Scholar]
- Liu H, Wang L. Randomized controlled study on ginger‐salt‐partitioned moxibustion at Shenque (CV 8) on urination disorders poststroke. Chinese Acupuncture & Moxibustion 2006;26(9):621‐624. [PubMed] [Google Scholar]
- Tibaek S, Gard G, Jensen R. Is there a long‐lasting effect of pelvic floor muscle training in women with urinary incontinence after ischemic stroke?. International Urogynecology Journal 2007;18:281‐287. [DOI] [PubMed] [Google Scholar]; Tibaek S, Gard G, Jensen R. Pelvic floor muscle training is effective in women with urinary incontinence after stroke: a randomised, controlled and blinded study. Neurology and Urodynamics 2005;24:348‐357. [DOI] [PubMed] [Google Scholar]; Tibaek S, Jensen R, Lindskov G. Can quality of life be improved by pelvic floor muscle training in women with urinary incontinence after ischemic stroke? A randomised, controlled and blinded study. International Urogynecology Journal 2004;15:117‐123. [DOI] [PubMed] [Google Scholar]
- Wikander B, Ekelund P, Milsom I. An evaluation of multidisciplinary intervention governed by functional independence measure (FIMSM) in incontinent stroke patients. Scandinavian Journal of Rehabilitation Medicine 1998;30(1):15‐21. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Ma F, Ma Y. Observation on the effects of acupuncture in the treatment of urinary retention due to cerebral infarction in 36 patients. Heilongjiang Medicine and Pharmacy. 2002; Vol. 25, issue 3:71. [Google Scholar]
- Zhou G, Wu D. 40 examples of using eye acupuncture and electriferous scalp acupuncture to treat urinary incontinence after cerebrovascular accident. Journal of Clinical Acupuncture. 1999; Vol. 15, issue 9:33‐34. [Google Scholar]
- Zhu Y, Zhu X, Zhu D, Jin Z. Meclofenoxate in treating urinary incontinence after acute cerebral infarction. Chinese Journal of New Drugs and Clinical Remedies 2003;9:520‐522. [Google Scholar]
References to studies excluded from this review
- Cook D, Huboky E, Hasskarl J, Hochrien S, Reding M. Effect of voiding position on urinary retention post stroke. Journal of Stroke and Cerebrovascular Diseases 1998;7:382. [Google Scholar]
- Gross J C. Bladder dysfunction after a stroke: it's not always inevitable. Journal of Gerontological Nursing 1990;16:20‐5,41‐2. [DOI] [PubMed] [Google Scholar]
- Tekeoglu Y, Adak B, Goksoy T. Effect of transcutaneous electrical nerve stimulation (TENS) on Barthel Activities of Daily Living (ADL) Index score following stroke. Clinical Rehabilitation 1998;12:277‐80. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Engberg S, Sereika SM, McDowell J, Weber E, Brodak I. Effectiveness of prompted voiding in treating urinary incontinence in cognitively impaired homebound older adults. Journal of Wound, Ostomy and Continence Nursing 2002;29(5):252‐265. [DOI] [PubMed] [Google Scholar]
- McDowell BJ, Engberg S, Sereika S, Donovan N, Jubeck ME, Weber E, Engberg R. Effectiveness of behavioral therapy to treat incontinence in homebound older adults. Journal of the American Geriatrics Society 1999;47:309‐318. [DOI] [PubMed] [Google Scholar]
Additional references
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the standardisation subcommittee of the International Continence Society. Neurourology and Urodynamics 2002;21:167‐68. [DOI] [PubMed] [Google Scholar]
- Arunabh MB, Badlani GH. Urologic problems in cerebrovascular accidents. Probl Urol 1993;7:41. [Google Scholar]
- Barer DH. Continence after stroke: useful predictor or goal of therapy?. Age & Ageing 1989;18(3):183‐91. [DOI] [PubMed] [Google Scholar]
- Barrett JA. Bladder and bowel problems after a stroke. Reviews in Clinical Gerontology 2001;12:253‐67. [Google Scholar]
- Brittain KR. Urinary symptoms and depression in stroke survivors. Age & Ageing 1998;27(Suppl 1):116‐7. [Google Scholar]
- Brittain KR, Peet SM, Castleden MD. Stroke and incontinence. Stroke 1998;29:524‐8. [DOI] [PubMed] [Google Scholar]
- Brittain KR, Peet SM, Potter JF, Castleden CM. Prevalence and management of urinary incontinence in stroke survivors. Age & Ageing 1999;28(6):509‐11. [DOI] [PubMed] [Google Scholar]
- Brittain KR, Perry SI, Peet SM, Shaw C, Dallosso H, Assassa RP, et al. Prevalence and impact of urinary symptoms among community dwelling stroke survivors. Stroke 2000;31:886‐91. [DOI] [PubMed] [Google Scholar]
- Burney TL, Senapati M, Desai S, Choudary ST, Badlani GH. Effects of cerebrovascular accident on micturition. Urologic Clinics of North America 1996;23:483‐90. [DOI] [PubMed] [Google Scholar]
- Burney TL, Senepati M, Desai S, Choudhary MS, Badlani GH. Acute cerebrovascular accident and lower urinary tract dysfunction: a prospective correlation of the site of brain injury with urodynamic findings. Journal of Urology 1996;156:1748. [DOI] [PubMed] [Google Scholar]
- Cifu DX, Stewart DG. Factors affecting functional outcome after stroke: a critical review of rehabilitation interventions. Archives of Physical Medicine and Rehabilitation 1999;80(Suppl):S35‐9. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Higgins, JPT, Altman DG, editors. Analysing and presenting results. In: Higgins JPT, Green S editor(s). Cochrane Reviewers' Handbook 4.2.6 [updated September 2006]; Section 8. http://www.cochrane.org.resources/handbook/hbook.htm (accessed 1 August 2007). Chichester, UK: John Wiley & Sons, Ltd., 2006. [Google Scholar]
- Eustice S, Roe B, Paterson J. Prompted voiding for the management of urinary incontinence in adults. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002113] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix SL, Cochrane BB, Nygaard IE, Handa VL, Barnabei VM, Iglesia C, et al. Effects of estrogen with and without progestin on urinary incontinence. JAMA 2005;293(8):935‐48. [DOI] [PubMed] [Google Scholar]
- Marinkovic S, Badlani G. Voiding and sexual dysfunction after cerebrovascular accidents. Journal of Urology 2001;165(2):359‐70. [DOI] [PubMed] [Google Scholar]
- Meijer R, Ihnenfeldt DS, Groot IJM, Limbeek J, Vermeulen M, Haan RJ. Prognostic factors for ambulation and activities of daily living in the subacute phase after stroke. A systematic review of the literature. Clinical Rehabilitation 2003;17(2):119‐29. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Jorgensen HS, Pedersen PM, Raaschou HO, Olsen TS. Prevalence and risk factors of incontinence after stroke. The Copenhagen Stroke Study. Stroke 1997;28(1):58‐62. [DOI] [PubMed] [Google Scholar]
- Ostaszkiewicz J, Johnston L, Roe B. Habit retraining for the management of urinary incontinence in adults. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD002801.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostaszkiewicz J, Johnston L, Roe B. Timed voiding for the management of urinary incontinence in adults. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD002802.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Coshall C, Lawrence E, Rudd AG, Wolfe CD. Recovery from poststroke urinary incontinence: associated factors and impact on outcome. Journal of the American Geriatrics Society 2001;49(9):1229‐33. [DOI] [PubMed] [Google Scholar]
- Roe B, Ostaszkiewicz J, Milne J, Wallace S. Systematic review of bladder training and voiding programmes in adults: a synopsis of findings from data analysis and outcomes using metastudy techniques. Journal of Advanced Nursing 2007;57(1):15‐31. [DOI] [PubMed] [Google Scholar]
- Thuroff JW, Abrams P, Artibani W, Haab F, Khoury S, Madersbacher H. Clinical Guidelines For The Management Of Incontinence. In: Abrams P, Khoury S, Wein A editor(s). Incontinence: 1st International Consultation on Incontinence, Monaco. Plymouth, UK: Health Publication Ltd, 1999:933‐43. [Google Scholar]
- Ween JE, Alexander MP, D'Esposito M, Roberts M. Incontinence after stroke in a rehabilitation setting: outcome associations and predictive factors. Neurology 1996;47(3):659‐63. [DOI] [PubMed] [Google Scholar]
- Wikander B, Ekelund P, Milsom I. An evaluation of multidisciplinary intervention governed by functional independence measure (FIMSM) in incontinent stroke patients. Scandinavian Journal of Rehabilitation Medicine 1998;30(1):15‐21. [DOI] [PubMed] [Google Scholar]
- Williams A. Caregivers of persons with stroke: their physical and emotional well‐being. Quality of Life Research 1993;2:213‐20. [DOI] [PubMed] [Google Scholar]


