Abstract
Background
Vitamin E occurs naturally in the diet. It has several biological activities, including functioning as an antioxidant to scavenge toxic free radicals. Evidence that free radicals may contribute to the pathological processes behind cognitive impairment has led to interest in the use of vitamin E supplements to treat mild cognitive impairment (MCI) and Alzheimer's disease (AD). This is an update of a Cochrane Review first published in 2000, and previously updated in 2006 and 2012.
Objectives
To assess the efficacy of vitamin E in the treatment of MCI and dementia due to AD.
Search methods
We searched the Specialized Register of the Cochrane Dementia and Cognitive Improvement Group (ALOIS), the Cochrane Library, MEDLINE, Embase, PsycINFO, CINAHL, LILACS as well as many trials databases and grey literature sources on 22 April 2016 using the terms: "Vitamin E", vitamin‐E, alpha‐tocopherol.
Selection criteria
We included all double‐blind, randomised trials in which treatment with any dose of vitamin E was compared with placebo in people with AD or MCI.
Data collection and analysis
We used standard methodological procedures according to the Cochrane Handbook for Systematic Reviews of Interventions. We rated the quality of the evidence using the GRADE approach. Where appropriate we attempted to contact authors to obtain missing information.
Main results
Four trials met the inclusion criteria, but we could only extract outcome data in accordance with our protocol from two trials, one in an AD population (n = 304) and one in an MCI population (n = 516). Both trials had an overall low to unclear risk of bias. It was not possible to pool data across studies owing to a lack of comparable outcome measures.
In people with AD, we found no evidence of any clinically important effect of vitamin E on cognition, measured with change from baseline in the Alzheimer's Disease Assessment Scale ‐ Cognitive subscale (ADAS‐Cog) over six to 48 months (mean difference (MD) ‐1.81, 95% confidence interval (CI) ‐3.75 to 0.13, P = 0.07, 1 study, n = 272; moderate quality evidence). There was no evidence of a difference between vitamin E and placebo groups in the risk of experiencing at least one serious adverse event over six to 48 months (risk ratio (RR) 0.86, 95% CI 0.71 to 1.05, P = 0.13, 1 study, n = 304; moderate quality evidence), or in the risk of death (RR 0.84, 95% CI 0.52 to 1.34, P = 0.46, 1 study, n = 304; moderate quality evidence). People with AD receiving vitamin E showed less functional decline on the Alzheimer's Disease Cooperative Study/Activities of Daily Living Inventory than people receiving placebo at six to 48 months (mean difference (MD) 3.15, 95% CI 0.07 to 6.23, P = 0.04, 1 study, n = 280; moderate quality evidence). There was no evidence of any clinically important effect on neuropsychiatric symptoms measured with the Neuropsychiatric Inventory (MD ‐1.47, 95% CI ‐4.26 to 1.32, P = 0.30, 1 study, n = 280; moderate quality evidence).
We found no evidence that vitamin E affected the probability of progression from MCI to probable dementia due to AD over 36 months (RR 1.03, 95% CI 0.79 to 1.35, P = 0.81, 1 study, n = 516; moderate quality evidence). Five deaths occurred in each of the vitamin E and placebo groups over the 36 months (RR 1.01, 95% CI 0.30 to 3.44, P = 0.99, 1 study, n = 516; moderate quality evidence). We were unable to extract data in accordance with the review protocol for other outcomes. However, the study authors found no evidence that vitamin E differed from placebo in its effect on cognitive function, global severity or activities of daily living . There was also no evidence of a difference between groups in the more commonly reported adverse events.
Authors' conclusions
We found no evidence that the alpha‐tocopherol form of vitamin E given to people with MCI prevents progression to dementia, or that it improves cognitive function in people with MCI or dementia due to AD. However, there is moderate quality evidence from a single study that it may slow functional decline in AD. Vitamin E was not associated with an increased risk of serious adverse events or mortality in the trials in this review. These conclusions have changed since the previous update, however they are still based on small numbers of trials and participants and further research is quite likely to affect the results.
Keywords: Humans, Alzheimer Disease, Alzheimer Disease/drug therapy, Antioxidants, Antioxidants/therapeutic use, Cognitive Dysfunction, Cognitive Dysfunction/drug therapy, Disease Progression, Outcome Assessment (Health Care), Randomized Controlled Trials as Topic, Vitamin E, Vitamin E/therapeutic use
The use of vitamin E in the treatment of mild cognitive impairment and Alzheimer's disease (AD)
Background
Vitamin E is found in a variety of foods, including vegetable oils and fats, nuts and seeds. Some animal and non‐interventional studies have suggested it might have a role in the prevention or treatment of Alzheimer's disease (AD). However, evidence has linked vitamin E with potentially serious side effects and even an increased risk of death. In this review, we looked for evidence about the effect of vitamin E on people who had either dementia due to AD or milder problems with memory or thinking (mild cognitive impairment or MCI). People with MCI have an increased risk of developing dementia.
Included trials
We searched for clinical trials published up to April 2016 which had randomly allocated people with dementia due to AD or with MCI to treatment with vitamin E supplements or a placebo (pretend treatment). We found three trials that investigated the effects of vitamin E on people with AD, but we could extract data from only one of these trials (304 participants). We found only one trial with 516 participants which investigated the effects of vitamin E on people with MCI. The quality of these two trials was generally good.
Results
Vitamin E did not reduce the number of people with MCI who developed dementia over three years. We also found no evidence that vitamin E improved cognition (e.g. learning and memory) in people with MCI or with dementia due to AD. One trial found that people with dementia due to AD who took vitamin E could manage daily activities (e.g. bathing and dressing) better than people who took placebo. There was no evidence from these trials that vitamin E caused significant harm to participants, but these types of trials are not the best way to look for harmful effects unless the effects are very common. Because all the results came from single trials, it is likely that further research could lead to different conclusions.
Conclusion
From limited evidence, we found nothing to suggest that there are either benefits or harms from vitamin E supplements. As the quality of evidence was only moderate, further trials are needed to confirm the findings. It is possible that different types or doses of vitamin E might have different effects.
Summary of findings
Summary of findings for the main comparison.
Vitamin E (capsules 2000 IU/day in two divided doses) compared to placebo for people with Alzheimer's disease
| Vitamin E (capsules 2000 IU/day in 2 divided doses) compared to placebo for people with Alzheimer's disease | ||||||
|
Patient or population: people with Alzheimer's disease Settings: multicentre, US Intervention: vitamin E (capsules 2000 IU/day in 2 divided doses) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Vitamin E (capsules 2000 IU/day in 2 divided doses) | |||||
|
Cognitive function LS mean change from baseline using the ADAS‐Cog Scale from: 0 to 70 Follow‐up: 6 to 48 months |
The LS mean change from baseline in cognitive function in placebo group was 7.78 | The LS mean change from baseline in cognitive function in the intervention group was 1.81 lower (3.75 lower to 0.13 higher) | ‐ | 272 (1 study) | ⊕⊕⊕⊝ Moderate1 | Higher scores represent worse cognitive function. A 4‐point difference in ADAS‐cog has been considered the MCID. |
|
Adverse events Number of participants reporting ≥ 1 serious adverse event Follow‐up: 6 to 48 months |
625 per 1000 | 538 per 1000 (444 to 656) | RR 0.86 (0.71 to 1.05) | 304 (1 study) | ⊕⊕⊕⊝ Moderate1 | ‐ |
|
Deaths Number of deaths Follow‐up: 6 to 48 months |
204 per 1000 | 171 per 1000 (106 to 273) | RR 0.84 (0.52 to 1.34) | 304 (1 study) | ⊕⊕⊕⊝ Moderate1 | ‐ |
|
Activities of daily living LS mean change from baseline using the ADCS‐ADL Scale from: 0 to 78 Follow‐up: 6 to 48 months |
The LS mean change from baseline in activities of daily living in the placebo group was ‐16.96 | The LS mean change from baseline in activities of daily living in the intervention group was 3.15 higher (0.07 to 6.23 higher) | ‐ | 280 (1 study) | ⊕⊕⊕⊝ Moderate1 | Higher scores represent better activities of daily living. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADAS‐Cog: Alzheimer Disease Assessment Scale ‐ Cognitive Subscale; ADCS‐ADL: Alzheimer's Disease Cooperative Study/Activities of Daily Living Inventory;CI: confidence interval; LS: least square; MCID: minimum clinically important difference; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Quality downgraded one level due to imprecision. Evidence from a single study of modest size. This is supported by dichotomous data not reaching the optimal information size criterion (assuming α of 0.05, and β of 0.2) (Guyatt 2011).
Summary of findings 2.
Vitamin E (capsules 2000 IU/day in two divided doses) compared to placebo for people with mild cognitive impairment
| Vitamin E (capsules 2000 IU/day in 2 divided doses) compared to placebo for people with mild cognitive impairment | ||||||
| Patient or population: people with mild cognitive impairment Settings: US and Canada Intervention: vitamin E (capsules 2000 IU/day in 2 divided doses) Comparison: placeb | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Vitamin E (capsules 2000 IU/day in 2 divided doses) | |||||
|
Progression to Alzheimer's disease Number of people progressing to AD Follow‐up: 36 months |
284 per 1000 | 293 per 1000 (224 to 383) | RR 1.03 (0.79 to 1.35) | 516 (1 study) | ⊕⊕⊕⊝ Moderate1 | ‐ |
|
Cognitive function Mean change from baseline of ADAS‐Cog Scale from: 0 to 70 Follow‐up: 36 months |
Not possible to extract data for analysis. | ‐ | Not possible to extract data for analysis. | Unable to evaluate quality of evidence. | Uncertainty about how missing data were handled. Study reports no significant difference between intervention and control groups. | |
|
Adverse events Number of participants reporting ≥ 1 serious adverse event. Follow‐up: 36 months |
Not possible to extract data for analysis. | ‐ | 516 (1 study) | Unable to evaluate quality of evidence. | Overall adverse event rates not reported. | |
|
Death Number of deaths over 36 months Follow‐up: 36 months |
19 per 1000 | 19 per 1000 (6 to 66) | RR 1.01 (0.3 to 3.44) | 516 (1 study) | ⊕⊕⊕⊝ Moderate1 | ‐ |
|
Activities of daily living Mean change from baseline using the ADCS Mild Cognitive Impairment Activities of Daily Living Scale from: 0 to 53 Follow‐up: 36 months |
Not possible to extract data for analysis. | ‐ | Not possible to extract data for analysis. | Unable to evaluate quality of evidence. | Uncertainty about how missing data were handled. Study reports no significant difference between intervention and control groups. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADAS‐Cog: Alzheimer Disease Assessment Scale ‐ Cognitive Subscale; ADCS‐ADL: Alzheimer's Disease Cooperative Study/Activities of Daily Living Inventory;CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Quality downgraded one level due to imprecision. Evidence from a single study of modest size. This was supported by dichotomous data not reaching the optimal information size criterion (assuming α of 0.05, and β of 0.2) (Guyatt 2011).
Background
Description of the condition
Dementia due to Alzheimer's disease (AD) is a progressive neurodegenerative condition in which people develop deficits in multiple cognitive domains, including memory, language and executive functioning, as well as a variety of emotional and behavioural symptoms. This leads to progressive functional impairment. AD causes huge emotional and financial burden to people, carers, and health and social care systems. Current treatments for AD have limited efficacy and cannot prevent progression of the condition. It is projected that 5.2% of the world's population will have dementia by 2050, equating to a worldwide prevalence of 131.5 million cases (Prince 2015).
Mild cognitive impairment (MCI) is a condition of cognitive deficits which are not accompanied by significant impairment in the performance of activities of daily living and hence do not meet the diagnostic criteria for dementia. People with MCI are at increased risk of developing dementia (Ganguli 2011), although this is not considered inevitable (Bruscoli 2004). In 2011, it was estimated that between 10% and 20% of people over the age of 65 years meet criteria for MCI (Petersen 2011).
Description of the intervention
Vitamin E is a generic term for a group of eight naturally occurring, fat‐soluble chemical derivatives of tocopherol and tocotrienol. It occurs naturally in a variety of food substances including vegetable oils and fats, and in nuts and seeds, such as almonds and sunflower seeds. Alpha‐tocopherol is the most commonly studied compound and it has been the only form of vitamin E used in clinical trials in AD. According to the Food and Nutrition Board (FNB) at the Institute of Medicine of the National Academies (US), the recommended daily allowance for men and women over 14 years of age is 22.4 IU (15 mg of alpha‐tocopherol) (National Academy of Sciences 2000).
Vitamin E has a number of biological activities which vary with the form of vitamin E (Boccardi 2016). One of its functions is to act as a scavenger of free radicals by working as an antioxidant (Uneri 2006). Oxygen free radicals contain oxygen atoms with unpaired electrons and are highly reactive, damaging proteins, DNA and cell membranes unless rapidly 'quenched' by antioxidants. They are produced as by‐products of the body's metabolism as well as by radiation. As vitamin E is lipophilic, it can protect cell membranes and plasma lipoproteins from peroxyl radicals, which react preferentially with vitamin E (Traber 2011). In particular, vitamin E is thought to inhibit the process of lipid peroxidation, which damages the polyunsaturated fatty acids essential to the integrity of cell membranes. Vitamin E's activity in protecting against the harmful effects of free radicals may be measured indirectly by assessing the state of the antioxidant‐oxidant system. Endogenous antioxidants such as glutathione provide an indication of the antioxidant status while the oxidised form of glutathione, glutathione disulphide (GSSG), and malondialdehyde (MDA) may provide an indication of the oxidative stress status and in particular lipid peroxidation.
High doses of vitamin E (over 3000 IU/day) are considered toxic, and have been implicated in a variety of symptoms including fatigue, gastrointestinal cramps and diarrhoea. Further, there is an increasing body of evidence that vitamin E supplementation can cause adverse effects, even at lower doses. For example, vitamin E increases bleeding tendency and can potentiate the effect of aspirin (Steiner 1995). One meta‐analysis found that vitamin E supplementation, while reducing risk of ischaemic stroke by 10%, increased the risk of haemorrhagic stroke by 22% (Schürks 2010). Evidence from the Selenium and Vitamin E Cancer Prevention Trial (SELECT) suggests that daily supplementation with vitamin E 400 IU in healthy men may significantly increase the risk of prostate cancer (Klein 2011), and there is evidence from the Heart Outcomes Prevention Evaluation (HOPE) trial that vitamin E supplementation increases the risk of heart failure and hospitalisation for heart failure in people over 55 years of age with diabetes mellitus and vascular disease (Lonn 2005). Miller 2005 carried out a meta‐analysis of 19 clinical trials testing vitamin E alone or in combination with other supplements. The median dose of vitamin E in these trials was 400 IU/day although the maximum dose was up to 2000 IU/day. The authors reported a statistically significant dose‐dependent relationship between vitamin E intake and all‐cause mortality. One Cochrane meta‐analysis also found that vitamin E given singly or combined with other antioxidants significantly increased mortality in 26 trials (risk ratio (RR) 1.04) (Bjelakovic 2010).
How the intervention might work
There are both theoretical arguments and empirical findings to suggest that free radical damage may be one of the mechanisms causing neuronal degeneration in a range of conditions including ageing, MCI and AD. This evidence was summarised by Grundman 2000. Many studies have found evidence of increased level of oxidative damage to neurons and mitochondrial DNA in AD and MCI (Butterfield 2002; Hensley 1995; Marcus 1998; Mecocci 1994; Mecocci 2004; Wang 2005; Wang 2006). Antioxidants, including vitamin E, improve cognitive functioning of aged rodents (Socci 1995), and protect against the effects of brain ischaemia (Hara 1990) and of some neurotoxins (Wortwein 1994). Transgenic mice overexpressing the amyloid precursor protein, implicated in AD, showed accelerated age‐associated brain degeneration (Hsiao 1995). Vitamin E can delay this deterioration (Behl 1992; Koppal 1998; Zhou 1996), and decrease oxidative DNA damage (Boothby 2005).
Central nervous tissue contains a high proportion of fatty material (lipid), and since vitamin E is fat‐soluble it can readily enter the brain (Vatassery 1988). In older‐adult population‐based studies, there have been reports of an association between low blood levels of vitamin E and impaired cognitive function (Cherubini 2005; Perrig 1997). Mean levels of vitamin E in the blood and cerebrospinal fluid of people with AD were reported to be lower than normal in several reports (Jeandel 1989; Jimenez‐Jimenez 1997; Polidori 2002; Tohgi 1994; Zaman 1992), though not in all (Ahlskog 1995). Lower levels of both forms of vitamin E ‐ tocotrienols and tocopherols ‐ have been observed in the plasma of people with AD and MCI (Mangialasche 2012). However, lower levels of vitamin E in AD may be a consequence of the disease rather than a cause of it, due in part to an overall decreased general dietary intake especially as the disease advances (Tabet 2001; Tabet 2002).
Results from clinical trials of vitamin E in non‐AD neurodegenerative disorders have not been promising. For example, neither people with Parkinson's disease (Parkinson's SG 1993; Pham 2005) nor Huntington's disease (Peyser 1995) have shown a significant overall benefit from vitamin E supplementation. In this review, we focused specifically on the role of vitamin E in the treatment of AD and MCI.
Why it is important to do this review
It is estimated that 46.8 million people worldwide have a diagnosis of dementia, and this is predicted to rise to 131.5 million cases by 2050 (Prince 2015), with AD being the most prevalent subtype. At present, there is no effective treatment for MCI or for dementia due to AD. Vitamin E continues to be used by some people as a non‐prescription supplement mainly because of its widely reported antioxidant properties and some previous positive findings. However, due to the potential health risks of taking vitamin E, it is important to determine whether vitamin E is an effective treatment of AD and MCI. This is an update of an earlier Cochrane Review, which was originally published in 2000 and was last updated in 2012. This review involved an updated search of the literature as well as changes to the conclusion, which better reflect emerging findings from vitamin E clinical trials in AD.
Objectives
To assess the efficacy of vitamin E in the treatment of MCI and dementia due to AD.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised, double‐blind trials in which vitamin E was compared with placebo in the treatment of people with MCI or dementia due to AD, or both.
Types of participants
Participants with AD were diagnosed with probable AD according to internationally accepted diagnostic criteria including NINCDS‐ADRDA (National Institute of Neurological and Communicative Disorders and Stroke‐Alzheimer's Disease and Related Disorders Association (McKhann 1984), Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) (APA 1994), or International Statistical Classification of Diseases and Related Health Problem, Tenth Revision (ICD‐10). In the absence of consensus criteria for MCI, we accepted MCI defined according to published criteria such as those by Petersen 2005, or any other criteria with face validity. If only a subset of the study population was eligible for inclusion, then we sought and included data relating only to the eligible participants.
Types of interventions
We included studies using any dosage of vitamin E or any of its constituent tocopherols or tocotrienols. A placebo comparator was required. Co‐administration of another drug with vitamin E was permitted if the same drug was also taken by the placebo group. There was no restriction on the dosage, mode of delivery or duration of the vitamin E intervention.
Types of outcome measures
We sought data on the primary and secondary outcomes listed below. For continuous outcomes, we included only data derived from validated, published scales.
Primary outcomes
For MCI studies, development of, or time to development of, possible or probable AD.
Cognitive function.
Adverse events
Death.
Secondary outcomes
Global measure of dementia severity and deterioration.
Behavioural disturbance.
Mood.
Activities of daily living.
Carer burden.
Quality of life.
Permanent physical disability.
Institutionalisation.
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois) ‐ the Cochrane Dementia and Cognitive Improvement Group's Specialized Register on 22 April 2016. The search terms used were: "Vitamin E", vitamin‐E, alpha‐tocopherol.
ALOIS is maintained by the Trials Search Co‐ordinator of the Cochrane Dementia and Cognitive Improvement Group and contains studies in the areas of dementia prevention, dementia treatment and cognitive enhancement in healthy people. The studies are identified from:
monthly searches of a number of major healthcare databases: MEDLINE, Embase, CINAHL, PsycINFO and LILACS;
monthly searches of a number of trial registers: ISRCTN; UMIN (Japan's Trial Register); the World Health Organization (WHO) portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials and the Netherlands National Trials Register, plus others);
quarterly searches of the Cochrane Library’s Central Register of Controlled Trials (CENTRAL);
six‐monthly searches of a number of grey literature sources: ISI Web of Knowledge Conference Proceedings; Index to Theses and Australasian Digital Theses.
To view a list of all sources searched for ALOIS see About ALOIS on the ALOIS website.
Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL and conference proceedings can be viewed in the 'methods used in reviews' section within the editorial information about the Dementia and Cognitive Improvement Group.
Additional searches were performed in the sources listed above to cover the timeframe from the last searches performed for ALOIS to ensure that the search for the review was as up‐to‐date and as comprehensive as possible. The search strategies used can be seen in Appendix 1.
Searching other resources
We reviewed reference lists of included studies to identify any additional studies.
Data collection and analysis
Selection of studies
Two review authors (NF and DL) independently examined the title and abstract of the papers identified by the search and selected and retrieved them for their relevance to the review. We resolved any disagreements concerning inclusion by discussion among all review authors until a decision was made.
Data extraction and management
Two review authors (NF and DL for descriptive data, NF and NT for outcome data) independently extract data for all authors. We attempted to collect the following data:
report ‐ author, year and source of publication;
study ‐ study setting;
participants ‐ demographics, diagnostic criteria for AD or MCI, exclusion criteria, other concomitant medical conditions or medications that may affect cognition;
research design and features ‐ sampling mechanism, treatment assignment mechanism, blinding, dropout rates, length of follow‐up, pertinent design features (e.g. cross‐over design);
intervention ‐ type, duration, dose, timing, mode of delivery;
outcome measures;
results ‐ number of participants randomised, outcome data.
For each outcome measure, we sought data on every participant randomised We preferred intention‐to‐treat (ITT) data but if these were not available, we extracted data on participants who completed treatment. We did not extract data for any non‐randomised titration periods or any open‐label follow‐on phases.
For continuous data, we extracted the means, standard deviation (SD) and number of participants in each treatment group at each time point. We extracted change from baseline data if endpoint data were unavailable. For binary data, the data extracted was the number of participants with each outcome in each treatment group at each time point. For ordinal data, there were two possible approaches. If ordinal scale data appeared to be approximately normally distributed or if the analysis that the investigators performed suggested that parametric tests were appropriate, then ewe treated the outcome measures as continuous data. The second approach, which may not exclude the first, was to concatenate into two categories that best represent the contrasting states of interest, and to treat the variable as binary. For time‐to‐event data, we used a hazard ratio (HR).
Assessment of risk of bias in included studies
Two review authors (NF and DL) assessed risk of bias for each included study independently. This was assessed based on guidance from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the following criteria:
sequence generation;
allocation concealment;
blinding (participants);
blinding (investigators);
incomplete outcome reporting;
selective outcome reporting.
For each study, we gave a risk of bias rating ('Low risk', 'Unclear risk' or 'High risk') for each of the above criteria. Empirical research has shown that lack of adequate allocation concealment may be associated with bias. Trials with unclear concealment measures have been shown to yield more pronounced estimates of treatment effects than trials that have taken adequate measures to conceal allocation schedules, but less pronounced than inadequately concealed trials (Chalmers 1983; Schulz 1995). Thus, we included trials if they conformed to 'Low risk' or 'Unclear risk' allocation risk categories, while we excluded trials falling into the 'High risk' category.
Measures of treatment effect
For studies with continuous outcome measures, we calculated mean differences (MD) or standardised mean differences (SMD) with 95% confidence intervals (CI). For binary outcome measures, we calculated RRs. For time‐to‐event outcome measures, we calculated HRs.
Unit of analysis issues
The participant was the unit of analysis.
Dealing with missing data
An ITT analysis was carried out where possible. In cases where only completers' data were available, we explored the impact of the missing data on the findings, where possible. When appropriate, the review authors contacted relevant study authors to request missing information.
Assessment of heterogeneity
Owing to the clearly significant clinical and methodological heterogeneity between studies, we performed no statistical tests for heterogeneity. As such, data did not lend itself to performing a meta‐analysis. However, we carried out critical interpretive synthesis of data for individual studies.
Assessment of reporting biases
There were an insufficient number of studies identified to make an overall quantitative assessment. We assessed biases for individual studies.
Data synthesis
It was not possible to synthesise and compare data across studies as there were no comparable outcome measures. We carried out a critical interpretive synthesis of data for individual studies separately. We included 'Summary of findings' tables.
Subgroup analysis and investigation of heterogeneity
Although not specified in the protocol, we extracted and reported data for a subgroup analysis carried out in one included study. The subgroups were participants who did or did not show a decline in markers of oxidative stress in response to vitamin E treatment. This subgroup was considered important to include as a reduction of oxidative stress is thought to be the mechanism by which vitamin E may affect cognition.
Sensitivity analysis
Sensitivity analysis was planned if a sufficient number of comparable studies were identified.
Presentation of results ‐ 'Summary of findings' tables
We used the GRADE approach to assess the quality of the supporting evidence behind the treatment effects presented in this review (Guyatt 2011). For each comparison, we presented key outcomes in a 'Summary of findings' table including, for each outcome, a summary of the amount of data, the magnitude of the effect size and the quality of the evidence.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
Only four trials met the inclusion criteria: Dysken 2014; Lloret 2009;Petersen 2005; and Sano 1996. Three were trials of alpha‐tocopherol (vitamin E) for the treatment of dementia due to AD (Dysken 2014; Lloret 2009; Sano 1996) and one was a trial of alpha‐tocopherol to delay progression from amnestic MCI to dementia (Petersen 2005). See Figure 1 for the study flow diagram of included studies.
Figure 1.
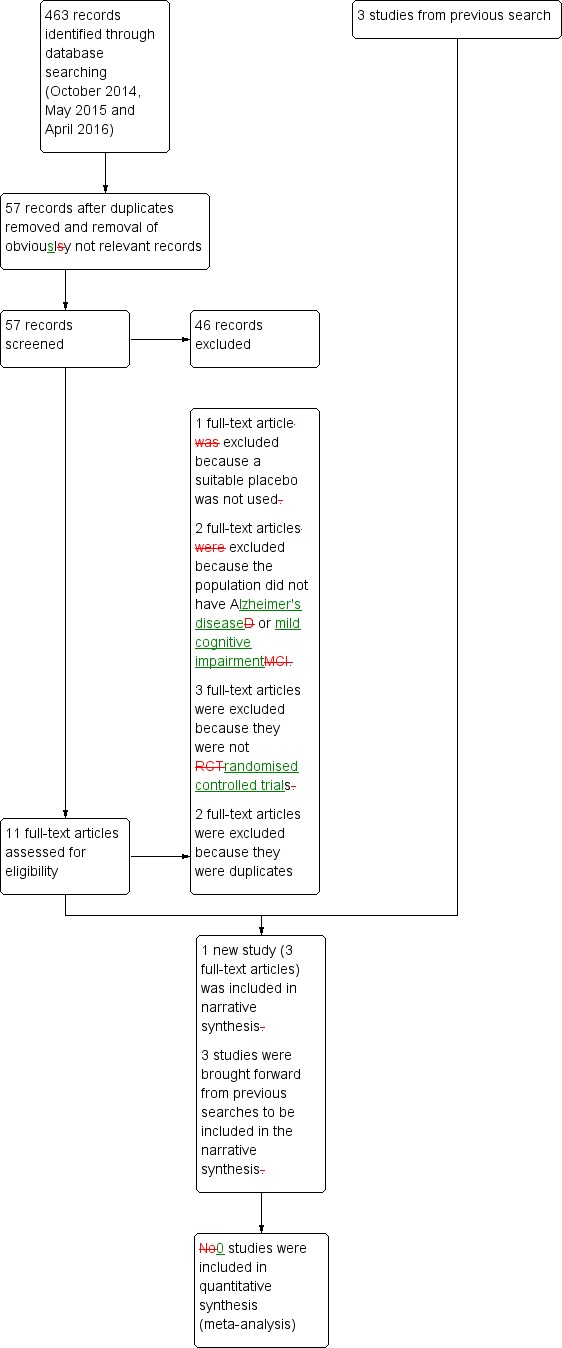
Study flow diagram for study for the search April 2016.
Included studies
Treatment of dementia due to Alzheimer's disease
The primary purpose of the first treatment study was to determine whether alpha‐tocopherol (vitamin E), selegiline (a monoamine oxidase inhibitor) or a combination of the two agents would slow the clinical deterioration associated with AD (Sano 1996). There were four groups: placebo, vitamin E (2000 IU total daily dose divided into two doses), selegiline and selegiline plus vitamin E. The study recruited 341 participants with a diagnosis of probable AD dementia of moderate severity (Clinical Dementia Rating (CDR) 2 (Berg 1988)) from 23 centres in the US. We considered only the placebo (n = 84) and vitamin E (n = 85) groups in this review. Assessments were conducted one month after enrolment and at three‐monthly intervals for two years. The primary outcome was the survival time to any one of four end points; death, institutionalisation, change in severity of dementia to a CDR of three or loss of two basic activities of daily living. After the primary end point was reached, every effort was made to continue further assessment of the secondary outcomes if possible. The secondary outcomes were the Cognitive section of the Alzheimer's Disease Assessment Scale (ADAS‐Cog) (Rosen 1984), the Mini‐Mental State Examination (MMSE) (Folstein 1975), the Blessed Dementia Scale (BDS) (Blessed 1968), the Dependence Scale (DS) (Stern 1994), the Behavior Rating Scale for Dementia (BRSD) (Tariot 1995), and adverse events. The primary analysis compared survival to end point for each of the three treatment groups in comparison with the placebo group, using Kaplan‐Meier estimation and log‐rank tests with no correction for other factors or variables, and the Cox proportional hazards model including covariates. A small number of participants were lost to follow‐up without having reached a primary end point (placebo 6/84, vitamin E 8/85).
The purpose of the second treatment study was to explore the effects of vitamin E on AD progression and markers of oxidative stress (Lloret 2009). Vitamin E 800 IU or placebo was given daily for six months. There were 57 participants diagnosed with probable AD. All AD participants were taking a cholinesterase inhibitor and were not taking any other antioxidant medication. Assessments were conducted on the first day of enrolment and after six months. Oxidative stress was assessed in two ways. The first method used blood concentrations of the oxidised glutathione molecule, GSSG, with greater levels indicating greater oxidative stress. GSSG was also assessed as a ratio with the antioxidant molecule, glutathione. The second method measured the blood marker MDA as a marker of lipid peroxidation, which is a strong indicator of oxidative damage. AD progression was measured by the Clock Drawing Test (Sunderland 1989), the BDS and MMSE. The primary analysis was to compare performance on the cognitive tasks in relation to the effects of vitamin E on oxidative stress. The effects of vitamin E on oxidative stress markers were investigated using a Mann‐Whitney test to compare GSSG and MDA values at the beginning and the end of the study in both the placebo and active treatment groups. Vitamin E‐treated participants were divided into responders and non‐responders according to whether vitamin E was effective or not in reducing markers of oxidative stress. The responder, non‐responder and placebo group means were compared using a Kruskal‐Wallis test. Comparison between groups was also undertaken following removal of participants with cerebrovascular disease. To determine the effect of modifying oxidative stress on cognitive performance, the relationship between MMSE change and GSSG change in participants treated with vitamin E was assessed using Spearman's coefficient of correlation. We contacted two authors of the Lloret 2009 study for additional information, but following an initial response from one author, we received no additional responses.
The purpose of the third treatment study was to determine if alpha tocopherol (vitamin E), memantine, or both slowed the progression of mild to moderate AD (Dysken 2014). In a double‐blind, placebo‐controlled randomised controlled trial (RCT), 613 people with mild to moderate AD were randomly assigned to one of four groups: alpha tocopherol (2000 IU total daily divided into two doses), memantine 20 mg/day, alpha tocopherol plus memantine or placebo. We considered only the placebo (n = 152) and vitamin E (n = 152) groups in this review. All participants were recruited in the US and had a diagnosis of possible or probable AD of mild to moderate severity, as defined by a MMSE score between 12 and 26. Duration of treatment ranged from six months to four years and participants were assessed every six months. The primary outcome was the Alzheimer's Disease Cooperative Study/Activities of Daily Living (ADCS‐ADL) scale (Galasko 2006). Secondary outcomes were the ADAS‐Cog, Neuropsychiatric Inventory (NPI) (Cummings 1994), the Caregiver Activity Survey (CAS) (Davis 1997), the DS and adverse events. Participants and their carers were asked specifically about participant falls, syncope and congestive heart failure as these were linked to high‐dose alpha tocopherol in previous trials (Lonn 2005; Sano 1996). Serious adverse events were coded on the basis of Medical Dictionary for Regulatory Activities. A longitudinal repeated‐measure mixed‐effects model adjusted for medical centre as a random effect and for baseline ADCS‐ADL.
Prevention of progression of cognitive impairment
The primary purpose of the single MCI study was to determine whether treatment with vitamin E 2000 IU/day or donepezil in people with the amnestic form of MCI decreased the conversion rate into dementia due to AD (Petersen 2005). There were three treatment groups: vitamin E, donepezil and placebo. All participants were also taking a multivitamin. This review included the vitamin E and the placebo groups only. Out of 769 participants enrolled from 69 sites in the US and Canada, 516 received either placebo or vitamin E. The secondary outcomes were scores on various measures: the MMSE (Folstein 1975), ADAS‐Cog (Rosen 1984), CDR (Sum of Boxes), the Alzheimer's Disease Assessment Scale Mild Cognitive Impairment Activities of Daily Living (ADCS‐MCI‐ADL) scale, the Global Deterioration Scale, a neuropsychological battery and adverse events. For further details about the study design refer to the Characteristics of included studies table.
The relevant primary analysis in the paper used the Cox proportional hazards model to determine whether there was a significant reduction in time to progression to AD among participants treated with vitamin E compared with participants given placebo. Participants were included on an ITT basis. Several baseline variables were included in the analysis as covariates. The secondary outcomes were examined using analysis of covariance for the change in scores without correction for multiple comparisons. Missing values were imputed using a projection method considered appropriate for assessing responses among people with neurodegenerative diseases (Aisen 2003).
Excluded studies
Following the latest search (April 2016), we initially excluded 46 articles based on the screening of the abstract and title. Of the 11 remaining articles, we excluded one because it did not use a suitable control intervention for our question (Naeini 2014), two because the study population was ineligible (Kryscio 2013a; MacPherson 2012), and three because they were not RCTs (Corbett 2014; Evans 2014; Hermann 2014). The five remaining articles all described one study which was eligible for inclusion (Dysken 2014), however, we subsequently excluded two because they were identified as duplicates. Details of the excluded studies are provided in Characteristics of excluded studies table.
Risk of bias in included studies
See Figure 2 for the summary of the risk of bias, and Figure 3 for the 'Risk of bias' graph. See Characteristics of included studies table for further details of included studies.
Figure 2.
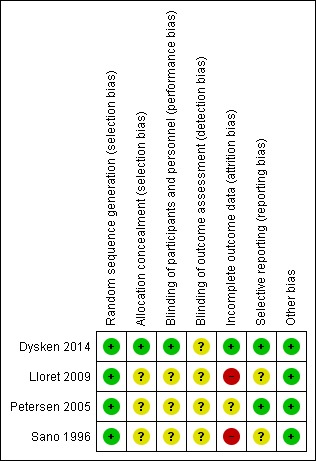
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Figure 3.
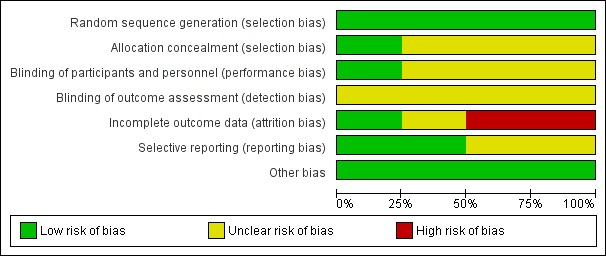
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
We judged that all four studies had a low risk of bias in terms of random sequence generation.
Only Dysken 2014 sufficiently described their method of allocation concealment. In the other three studies, the means of ensuring allocation concealment was unclear. However, we considered it sufficiently likely that allocation was concealed to include the studies.
Blinding
All studies report that they were double‐blinded, though no study described how this was maintained throughout the studies or whether blinding was successful. However, Dysken 2014 did use placebo capsules with identical appearance to the vitamin E capsules, so we considered this to have a low risk of bias. We judged the remaining studies to have an unclear risk of performance bias.
We considered all studies at unclear risk of detection bias because of a lack of information on blinding of outcome assessors.
Incomplete outcome data
Missing data was balanced across groups in Dysken 2014, thus we judged risk of bias to be low. Petersen 2005 reported using an ITT analysis with an appropriate imputation method to deal with missing data and stated that demographic and neuropsychological outcomes did not differ significantly between missing and non‐missing participants. However, it was difficult to tell how the authors had handled data from participants who had developed dementia and switched to open‐label donepezil. Only 42% of participants randomised to vitamin E and 46% randomised to placebo completed the study on their allocated treatment. We judged the study to have an unclear risk of attrition bias. Sano 1996 reported the attrition rate but did not give reasons. In addition, Sano 1996 only reported attrition up to the point a participant reached one of their primary end points, therefore we judged it to be at high risk of bias for our outcomes of interest. Lloret 2009 reported a very high attrition rate and did not give reasons; we judged it to be at high risk of bias.
Selective reporting
All studies reported outcomes stated in their protocol although often in insufficient detail for our purposes. Lloret 2009 tended to report significance values alone, without means and SDs. Similarly, Sano 1996 did not report SDs for the changes in score of their secondary outcomes. Thus, we judged the Lloret 2009 and Sano 1996 studies to have an unclear risk of bias in this domain. We judged the remaining studies to have a low risk of reporting bias.
Other potential sources of bias
We found no other sources of bias.
Effects of interventions
Vitamin E for dementia due to Alzheimer's disease
Three studies assessed the efficacy of vitamin E in people with dementia due to AD (Dysken 2014; Lloret 2009; Sano 1996). For a summary of findings related to the treatment of AD, see Table 1.
Sano 1996 had four treatment groups of which the groups receiving placebo only and vitamin E only were relevant to this review. Six of 84 participants in the placebo group and 8 of 85 participants in the vitamin E group were lost to follow‐up without reaching one of the predefined primary end points of death, institutionalisation, change in severity of dementia to a CDR score of three, or loss of two basic activities of daily living, but there was no information on the time points at which these participants were lost or on how many participants were lost to follow‐up after a primary end point.
Lloret 2009 divided participants treated with vitamin E into two groups depending on changes in oxidative stress markers with treatment. These groups had not been defined in advance of data collection. Participants who showed a decrease in GSSG values of more than 10 nmol/mL of blood after six months of vitamin E treatment were termed 'responders' and participants who did not were termed 'non‐responders'. They reported 'completers' results only (33/57 randomised participants).
Dysken 2014 had four treatment groups, but we evaluated only data for the vitamin E and placebo groups for this review (Dysken 2014). There was a wide range of durations of follow‐up within the trial (six to 48 months), with participants completing at least one follow‐up being included in the analysis. The final analysis included 140/152 (92.1%) participants in the vitamin E group and 140/152 (92.1%) participants in the placebo group. The authors calculated a least squares (LS) mean change from baseline in each treatment group and reported this with its standard error (SE). Dysken 2014 also reported results as a mean annual rate of change. For each relevant continuous outcome variable, we extracted the LS mean change from baseline, and derived SDs from reported SEs. We then calculated the MD (with 95% CI) between vitamin E and placebo groups.
Primary outcomes
Cognitive function
We were unable to extract the data we had specified for cognitive function for Sano 1996. Although the ADAS‐Cog and MMSE were secondary outcomes in the trial, only mean change scores to the time point where a participant was last assessed were reported and participants had been assessed over varying and unidentified time spans. SDs were not reported. The authors reported that changes from baseline score on the MMSE and the ADAS‐Cog did not differ significantly among the four groups.
Lloret 2009 measured cognition using the MMSE and the Clock Drawing Test, though we were unable to extract the data. The authors did not report means and SDs of their cognitive outcomes. There were no data on cognitive measures in the vitamin E‐treated group as a whole.
Dysken 2014 measured cognition using the ADAS‐Cog and MMSE. At six to 48 months, participants taking vitamin E had a smaller increase from baseline on the ADAS‐Cog (an increase in score representing worsening cognitive function) than participants taking placebo, but the result was uncertain and unlikely to be of clinical importance (completers: MD ‐1.81, 95% CI ‐3.75 to 0.13, P = 0.07, 1 study, n = 272; moderate quality evidence, downgraded due to imprecision because data were from a single small study). There was no evidence of a difference between groups in cognitive function measured with the MMSE (where a decrease represents worsening function) (completers: MD 0.19, 95% CI ‐0.72 to 1.10, P = 0.68, 1 study, n = 273; moderate quality evidence, downgraded due to imprecision).
Adverse events
Sano 1996 did not fully report adverse events. A total of 49 categories of adverse events were defined but the only results initially reported were for three categories (dental events, falls and syncopal episodes), in which there were significant differences between one or more of the treatment groups and the placebo group. However, once the authors adjusted for multiple comparisons, there were no significant differences between groups.
Lloret 2009 did not report adverse events. We requested additional information but this was not made available.
Dysken 2014 found no evidence of a difference between vitamin E and placebo groups in the number of participants reporting any adverse event or the number reporting at least one severe adverse event. The numbers of participants reporting adverse events during the study were 91/152 (59.9%) in the vitamin E group and 89/152 (58.6%) in the placebo group (RR 1.02, 95% CI 0.85 to 1.23, P = 0.82, 1 study, n = 304; moderate quality evidence, downgraded due to imprecision). The number of participants reporting at least one severe adverse event were 82/152 (54.0%) in the vitamin E group and 95/152 (62.5%) in the placebo group over the 48‐month trial (RR 0.86, 95% CI 0.71 to 1.05, P = 0.13, 1 study, n = 304; moderate quality evidence, downgraded due to imprecision).
Death
In Sano 1996, participants remained in the study until they reached one of the four predefined end points, one of which was death. In both vitamin E and placebo groups, 12% of participants died. The authors also reported that treatment had no effect on the cause of death.
Lloret 2009 did not report deaths. We requested additional information but this was not made available.
Dysken 2014 found no evidence of a difference between vitamin E and placebo groups in the number of deaths. There were 26/152 (17.1%) deaths in the vitamin E group compared to 31/152 (20.4%) deaths in the placebo group over the 48‐month trial (RR 0.84, 95% CI 0.52 to 1.34, P = 0.46, 1 study, n = 304; moderate quality evidence, downgraded due to imprecision).
Secondary outcomes
Global measure of dementia severity and deterioration
None of the studies measured dementia severity and deterioration (Dysken 2014; Lloret 2009; Sano 1996).
Behavioural disturbance
In Sano 1996, we were unable to extract the data for behavioural disturbances despite these being measured as secondary outcomes in the trial. Only the mean change scores to the time point where a participant was last assessed were reported and participants had been assessed over varying and unidentified time spans. SDs were not reported.
Lloret 2009 did not measure behavioural disturbance.
Dysken 2014 found that neuropsychiatric symptoms increased slightly more in the placebo group than in the vitamin E group over six to 48 months using the NPI but the result was uncertain and unlikely to be of clinical importance (completers: MD ‐1.47, 95% CI ‐4.26 to 1.32, P = 0.30, 1 study, n = 280; moderate quality evidence, downgraded due to imprecision).
Mood
None of the studies measured mood (Dysken 2014; Lloret 2009; Sano 1996).
Activities of daily living
In Sano 1996, we were unable to extract the data we had specified for activities of daily living, despite these being measured as secondary outcomes in the trial. Only the mean change scores to the time point where a participant was last assessed were reported and participants had been assessed over varying and unidentified time spans. SDs were not reported. The authors reported that there was significantly less decline in activities of daily living, as measured by the BDS and DS, in the vitamin E compared to the placebo group, but no significant difference between groups on the BRSD.
Lloret 2009 measured activities of daily living using the BDS. The authors did not report means and SDs, and therefore we were unable to extract the data.
Dysken 2014 measured activities of daily living using the ADCS‐ADL. Participants receiving vitamin E showed less functional decline on the ADCS‐ADL than participants receiving placebo at six to 48 months (completers: MD 3.15, 95% CI 0.07 to 6.23, P = 0.04, 1 study, n = 280; moderate quality evidence, downgraded due to imprecision).
Carer burden
None of the studies measured carer burden (Dysken 2014; Lloret 2009; Sano 1996).
Quality of life
None of the studies measured quality of life (Dysken 2014; Lloret 2009; Sano 1996).
Permanent physical disability
None of the studies measured permanent physical disability (Dysken 2014; Lloret 2009; Sano 1996).
Institutionalisation
Sano 1996 reported that participants receiving vitamin E treatment significantly delayed institutionalisation compared to participants receiving placebo (RR 0.42, no CI reported; P = 0.003).
Neither Dysken 2014 nor Lloret 2009 measured institutionalisation.
Other study outcomes
Sano 1996 reported the number in each outcome group who reached one of their primary end points (death, institutionalisation, change in severity of dementia to a CDR score of three or loss of two basic activities of daily living) within two years; this was 45/85 (53%) of participants in the vitamin E group and 58/84 (69%) in the placebo group. Because the trial was limited in time, an analysis comparing a count of events without taking into account their timing is less precise than the survival analysis reported by Sano 1996. Using Kaplan‐Meier estimation and log‐rank testing in their primary analysis, Sano 1996 found no significant difference in survival time to one of the four end points between the vitamin E and placebo groups (RR 0.70, no CI reported; P = 0.08). However, the study groups differed in baseline MMSE scores and these were correlated with clinical course. The vitamin E group began with a mean MMSE of 11.3 (SD 5.7) and the placebo group with a mean MMSE of 13.3 (SD 4.9). When the analysis was repeated using the Cox proportional hazards model and controlling for baseline MMSE, there emerged a significant difference favouring vitamin E (RR 0.47, no CI reported; P = 0.001).
The primary outcomes reported by Lloret 2009 were the percentage change in performance from baseline to six months on the BDS, Clock Drawing Test and MMSE, compared across responders (n = 9), non‐responders (n = 10) and placebo‐treated groups (n = 14), where participants who showed a decrease in GSSG values of more than 10 nmol/mL of blood after six months of vitamin E treatment were termed 'responders' and participants who did not were termed 'non‐responders'. We found no evidence that these groups had been defined before data collection. The authors reported no significant difference between groups on the Clock Drawing Test or BDS in an analysis of 'completers' only. The MMSE score increased in the responder group but decreased in the placebo group and more so in the non‐responders. There was a significant difference between the two vitamin E‐treated groups on MMSE change score (P < 0.05). The responders did not differ significantly from the placebo‐treated group. The decline in MMSE in the non‐responders was significantly greater than in the placebo group (P < 0.05). Lloret 2009 also reported a negative correlation between change in MMSE score and change in blood levels of GSSG from baseline to six months; that is, there was a greater decline in cognitive performance in people with AD whose blood GSSG levels stayed higher (reflecting higher oxidative stress).
Dysken 2014 reported that for functional decline, the mean treatment effect of 3.15 units on the ADCS‐ADL, favouring participants receiving vitamin E, translates into a clinically meaningful delay in progression of 6.2 months (95% CI 5.4 to 7.4), based on the rate of change in the control group.
Vitamin E for mild cognitive impairment
One study assessed vitamin E in people with MCI (Petersen 2005). For a summary of findings for the treatment of MCI, see Table 2.
Primary outcomes
Development of, or time to development of, possible or probable Alzheimer's disease
Petersen 2005 found no evidence of an effect of vitamin E on the numbers of participants with MCI who progressed to possible or probable AD. As their primary outcome, Petersen 2005 reported that 33/257 participants in the vitamin E group and 38/259 participants in the placebo group progressed to possible or probable AD in the first 12 months (RR 1.02, 95% CI 0.96 to 1.10, P = 0.05, 1 study, n = 516). By 36 months, 76/257 participants in the vitamin E group and 73/259 in the placebo group had progressed to AD (RR 1.03, 95% CI 0.79 to 1.35, P = 0.81, 1 study, n = 516). We considered this moderate quality evidence, downgraded due to imprecision.
Cognitive function
Petersen 2005 reported no significant difference between those receiving vitamin E and placebo on the change from baseline of the MMSE, ADAS‐Cog and modified ADAS‐Cog. However, it was difficult to tell how missing data had been handled and no sample size was reported, so we were unable to conduct an analysis.
Adverse events
Petersen 2005 did not fully report adverse events and therefore we were unable to extract the data we required. The authors reported the rates of 10 types of adverse events that occurred in at least 5% of participants in the vitamin E and at least two times in the placebo group during the double‐blind phase of the study. Diarrhoea and cataract extraction were the most frequently reported adverse events in the vitamin E group. Neither of these adverse events differed significantly in frequency between the vitamin E and placebo groups (P > 0.05). Overall, 66/257 participants discontinued in the vitamin E group and 72/259 participants discontinued treatment in the placebo group. Reasons were not given by group, but in the study as a whole, most discontinuations resulted from withdrawal of consent or adverse events.
Death
Petersen 2005 reported that five deaths occurred in each of the vitamin E and placebo groups during the 36‐month double‐blind phase (RR 1.01, 95% CI 0.30 to 3.44, P = 0.99, 1 study, n = 516; moderate quality evidence, downgraded due to imprecision). All deaths were deemed to be unrelated to treatment.
Secondary outcomes
Global measure of dementia severity and deterioration
Petersen 2005 reported no significant difference between those receiving vitamin E and placebo on change scores of the CDR Sum of Boxes or the Global Deterioration Scale. However, it was difficult to tell how missing data had been handled and no sample size was reported, so we were unable to conduct an analysis.
Behavioural disturbance
Petersen 2005 did not measure behavioural disturbance.
Mood
Petersen 2005 did not measure mood.
Activities of daily living
Petersen 2005 reported no significant difference between those receiving vitamin E and placebo on change scores of the ADCS MCI‐ADL. However, it was difficult to tell how missing data had been handled and no sample size was reported, so we were unable to conduct an analysis.
Carer burden
Petersen 2005 did not measure carer burden.
Quality of life
Petersen 2005 did not measure quality of life.
Permanent physical disability
Petersen 2005 did not measure permanent physical disability.
Institutionalisation
Petersen 2005 did not measure institutionalisation.
Other study outcomes
Petersen 2005 also created standardised z‐scores of individual cognitive domains (memory, executive, language, visuospatial and overall cognition) and reported the change from baseline for each. There were few significant differences in cognitive function change scores between the vitamin E and placebo groups. Those that were seen (in the executive, language and overall cognitive scores) were confined to the first 18 months of the study. On average, executive function improved in both groups at six months, but the improvement was significantly greater in the vitamin E group compared to the placebo group (P < 0.05). Change in language scores significantly favoured vitamin E at six months (P < 0.05), 12 months (P < 0.05) and 18 months (P < 0.05). Overall cognitive changes scores significantly favoured vitamin E at six months (P < 0.01), with an improvement in cognition in the vitamin E group and a decline in cognition in the placebo group. There were no differences between groups in the second 18 months of the study (18 to 36 months).
Discussion
Summary of main results
Four studies met the inclusion criteria and were selected for this review. To our knowledge there are currently no relevant ongoing trials. Three studies assessed the efficacy of vitamin E in people with AD (Dysken 2014; Lloret 2009; Sano 1996), while one study assessed vitamin E in people with MCI (Petersen 2005). A significant limitation of this review is that synthesis of data from the AD studies was not possible owing to different outcome measures, heterogeneity in designs and the inability to access relevant data sets from authors' reports. The Lloret 2009 study used a dose of vitamin E of 800 IU/day in the intervention group while the studies by Dysken 2014, Sano 1996, and Petersen 2005 used a dose of vitamin E of 2000 IU/day. Participants in the Sano 1996 study withdrew from the study once they reached a series of clinical end points. We could use data only from the Dysken 2014 and Petersen 2005 studies.
Vitamin E for dementia due to Alzheimer's disease
We found no evidence from the one study from which we could extract data that vitamin E was efficacious in improving cognitive outcomes in dementia due to AD (Dysken 2014). We could not extract data from Sano 1996 or Lloret 2009.
For our other primary outcomes, adverse events and deaths, there was no evidence that participants with AD receiving vitamin E were more at risk than participants receiving placebo (Dysken 2014; Sano 1996). From this review, we were unable to comment on the safety or tolerability of higher doses of alpha‐tocopherol or of other tocopherol and tocotrienol forms. In what appeared to be a post hoc analysis, Lloret 2009 reported that the subgroup of participants with AD who responded to vitamin E with a reduction in measures of oxidative stress had similar cognitive outcomes to participants taking placebo, while participants showing no reduction in oxidative stress measures in response to vitamin E had significantly poorer cognitive outcomes. This was very low quality evidence due to the post hoc definition of response, the small sample size and other significant methodological limitations, but the apparent accelerated decline in cognitive abilities in those participants in whom vitamin E did not reduce oxidative stress measures suggests a need for caution in future trials.
Among our secondary outcomes, we could extract data from Dysken 2014 on behavioural disturbance, which did not differ between groups, and on performance of activities of daily living, which declined less over a mean follow‐up of 2.27 years in the vitamin E than in the placebo group. We were unable to extract any data on our secondary outcomes of global dementia severity, mood, carer burden, quality of life, physical disability or institutionalisation.
Vitamin E for mild cognitive impairment
Petersen 2005 was the only study identified that investigated the effects of vitamin E on the progression from MCI to AD. We found no evidence that vitamin E treatment affected the risk of progression to AD over 36 months compared to placebo. The evidence presented by Petersen 2005 also suggests that vitamin E was not efficacious in improving cognitive outcomes in MCI.
There was no evidence that vitamin E treatment increased the risk of death, and while we were unable to extract data for adverse events, the data presented by the authors indicated that the risk of adverse events also did not differ between treatment groups. As with the AD sample, we were unable to comment on the safety or tolerability of higher doses of alpha‐tocopherol or of other tocopherol and tocotrienol forms in an MCI population.
Among our secondary outcomes, we were unable to analyse data on activities of daily living and global severity because sample sizes were not clearly reported. However, the data presented by the authors showed no evidence that either outcome benefited from vitamin E treatment compared to placebo. The study did not measure mood, carer burden, quality of life, physical disability or institutionalisation, so we could not draw any conclusions about the effect of vitamin E on these outcomes in an MCI population.
Overall completeness and applicability of evidence
The review was based on a small number of RCTs that were heterogeneous in design thus preventing a meta‐analysis. Notably, no two studies included in this review had the same primary outcome, while only a single study investigated vitamin E treatment in a MCI population (Petersen 2005).
Quality of the evidence
Using the GRADE approach, we rated the overall quality of evidence for all outcomes to be moderate. We considered the studies from which we extracted data to be at low to unclear risk of bias. However, we downgraded all quality judgements one level due to imprecision because all data came from single modest‐sized studies and we therefore considered it likely that further research could have an impact on the effect estimates.
Potential biases in the review process
To minimise the possibility of bias, we tried to locate all RCTs by a broad and comprehensive search strategy in a number of databases. We sought additional information from the authors of one study but were unable to obtain it (Lloret 2009). The review was limited by our inability to obtain or extract the data we needed from two of the included studies.
Agreements and disagreements with other studies or reviews
Habitual intake of dietary antioxidants has not consistently been found to improve cognitive performance or reduce the risk of dementia (Crichton 2013). While vitamin E assessed in epidemiological studies in relation to cognition has consisted of the various naturally occurring forms (Engelhart 2002; Morris 2002; Morris 2005), the relationship between vitamin E and cognition is unclear. In the present systematic review, there was no evidence that vitamin E improved either cognition or risk of developing AD. Morris 2005 suggested that the combined intake of tocopherols is likely to be more important than alpha‐tocopherol alone. This is further supported by Mangialasche 2010 who studied the relationship between cognitive impairment and the eight individual forms of vitamin E and concluded that any potential neuroprotective effect for vitamin E may result from interaction of the various forms.
It has been previously reported that vitamin E, especially in the large doses used in the included studies, may be associated with potentially significant adverse effects and even an increased rate of all‐cause mortality (Miller 2005). High doses of alpha‐tocopherol also have the potential to induce enzymes involved in drug metabolism with an associated risk of serious interactions with some concomitant medication (Brigelius‐Flohé 2007). However, this review found no evidence that vitamin E treatment significantly affected the number of serious adverse events or deaths, although the relatively short study lengths and small sample sizes mean that no strong conclusions about the safety of vitamin E can be drawn from these data.
Authors' conclusions
This review found little evidence for the efficacy of vitamin E (alpha‐tocopherol) in the treatment of Alzheimer's disease (AD). We found no evidence that it affects cognition, although there was moderate quality evidence from one study that it may slow functional decline, changing our conclusions from the previous update. However, based upon the quality of evidence, it is likely that further trials could have an impact on this finding. We consider it important to inform patients, carers and professionals about the lack of evidence for efficacy and the potential hazards of using vitamin E in AD. It should be noted that the conclusions are based only on the alpha‐tocopherol formulation of vitamin E, and hence no comment can be made on the efficacy of other preparations.
Vitamin E is a powerful antioxidant and plays an important role in protecting cells against the harmful effects of free radicals. There is sufficient evidence from laboratory and animal studies to consider it of potential interest as a treatment for people with AD. Overall, however, there continues to be a paucity of randomised controlled trials investigating the efficacy of vitamin E in AD. The findings from Dysken 2014 are interesting and indicate that vitamin E may slow functional decline in AD. Further research is now warranted to assess this further. Additional research is also needed to explore the effects of vitamin E treatment on other clinically relevant outcomes, such as quality of life, which was not assessed in any of the trials identified in this review. This review is a good example of the need to have more uniform outcomes across trials, with a focus on identifying outcomes that are meaningful to patients and carers. Lloret 2009 was a small study, but interesting as it showed a harmful effect on cognition in those participants whose oxidative stress markers did not respond to vitamin E intake. However, the size and high number of dropouts in this study limits the conclusions that can be drawn. Other studies included in this review did not measure antioxidant or nutritional levels prior to randomisation. The assessment of oxidative stress markers in future trials should be considered in order to understand further the oxidant‐antioxidant balance in relation to vitamin E effects in AD. In addition, the exclusive use of the alpha‐tocopherol form of vitamin E in clinical trials has been criticised. Future studies should investigate other tocopherol and tocotrienol forms. We will consider investigating subgroups by vitamin E formulation and oxidative stress response in future updates of this review.
Acknowledgements
The review authors are grateful to Sue Marcus, Managing Editor, Cochrane Dementia and Cognitive Improvement Group, for her support throughout the review.
The review authors would also like to thank Dr Jenny McCleery, Assistant Co‐ordinating Editor, Cochrane Dementia and Cognitive Improvement Group, for her comments and contributions in the amendment process of the review (June 2012 and April 2016).
Appendices
Appendix 1. Source, search strategy and hits retrieved: June 2012 search
| Source | Search strategy | Hits received |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) | Advanced search: [Study design: RCT OR CCT] AND [Health condition: Alzheimer OR MCI] AND [Intervention: "vitamin E"] (all dates) | Jun 2012: 39 (all dates) |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950 to present (OvidSP) | 1. *Vitamin E/ 2. "vitamin E".ti,ab. 3. "alpha‐tocopherol".ti,ab. 4. or/1‐3 5. Alzheimer*.ti,ab. 6. Alzheimer Disease/ 7. AD.ti,ab. 8. "cognit* impair*".ti,ab. 9. MCI.ti,ab. 10. (AACI or memory or CIND or ARCD or ACMI).ti,ab. 11. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 12. (nMCI or aMCI or mMCI).ti,ab. 13. (CDR adj2 "0.5").ab. 14. or/5‐13 15. 4 and 14 16. randomized controlled trial.pt. 17. controlled clinical trial.pt. 18. randomi?ed.ab. 19. placebo.ab. 20. drug therapy.fs. 21. randomly.ab. 22. trial.ab. 23. groups.ab. 24. or/16‐23 25. (animals not (humans and animals)).sh. 26. 24 not 25 27. 15 and 26 |
Jun 2012: 16 |
| 3. EMBASE 1980 to 2011 week 27 (OvidSP) |
1. *Vitamin E/ 2. "vitamin E".ti,ab. 3. "alpha‐tocopherol".ti,ab. 4. or/1‐3 5. Alzheimer*.ti,ab. 6. Alzheimer Disease/ 7. AD.ti,ab. 8. "cognit* impair*".ti,ab. 9. MCI.ti,ab. 10. (AACI or memory or CIND or ARCD or ACMI).ti,ab. 11. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 12. (nMCI or aMCI or mMCI).ti,ab. 13. (CDR adj2 "0.5").ab. 14. or/5‐13 15. 4 and 14 16. randomi?ed.ab. 17. placebo.ab. 19. trial.ab. 20. groups.ab. 21. randomized controlled trial/ 22. controlled clinical trial/ 23. ("double‐blind*" or "single‐blind*").ti,ab. 24. or/16‐23 25. 15 to 24 |
Jun 2012: 28 |
| 4. PsycINFO 1806 to July week 2 2011 (OvidSP) |
1. "vitamin E".ti,ab. 2. "alpha‐tocopherol".ti,ab. 3. Alzheimer*.ti,ab. 4. Alzheimer Disease/ 5. AD.ti,ab. 6. "cognit* impair*".ti,ab. 7. MCI.ti,ab. 8. (AACI or memory or CIND or ARCD or ACMI).ti,ab. 9. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 10. (nMCI or aMCI or mMCI).ti,ab. 11. (CDR adj2 "0.5").ab. 12. randomi?ed.ab. 13. placebo.ab. 14. randomly.ab. 15. trial.ab. 16. groups.ab. 17. ("double‐blind*" or "single‐blind*").ti,ab. 18. Clinical Trials/ 19. 1 or 2 20. or/3‐11 21. or/12‐18 22. 19 and 20 and 21 |
Jun 2012: 6 |
| 5. CINAHL (EBSCOhost) | S1 (MM "Vitamin E") S2 TX "vitamin E" S3 TX "alpha‐tocopherol" S4 S1 or S2 or S3 S5 (MH "Alzheimer's Disease") S6 TX AD OR alzheimer* S7 "mild cognitive impairment" S8 TX "cognit* impair*" S9 TX AACI OR memory OR CIND OR ARCD OR ACMI S10 TX MCI S11 TX nMCI OR aMCI OR mMCI S12 S5 or S6 or S7 or S8 or S9 or S10 or S11 S13 S4 and S12 S14 TX random* S15 TX placebo* S16 TX trial S17 TX groups S18 TX RCT OR CCT S19 (MH "Randomized Controlled Trials") S20 S14 or S15 or S16 or S17 or S18 or S19 S21 S13 and S20 S22 EM 2009 S23 EM 2010 S24 EM 2011 S25 S22 or S23 or S24 S26 S21 and S25 |
Jun 2012: 6 |
| 6. ISI Web of Knowledge – all databases [includes: Web of Science (1945 to present); BIOSIS Previews (1926 to present); MEDLINE (1950 to present); Journal Citation Reports] | #1 Topic=(alzheimer* OR AD OR MCI OR memory OR cognitive OR "cognit* impair*") AND Topic=("vitamin e" OR "alpha‐tocopherol") AND Year Published=(2009‐2011) Timespan=All Years #2 Topic=(random* OR placebo* OR "double‐blind*" OR "single‐blind*") Timespan=All Years #3 #2 AND #1 Timespan=All Years |
Jun 2012: 174 |
| 7. LILACS (BIREME) | vitamin‐e OR alpha‐tocopherol | Jun 2012: 0 |
| 8. CENTRAL (the Cochrane Library) (Issue 4 of 4, Oct 2010) | #1 “vitamin e” #2 “alpha‐tocopherol” #3 (#1 OR #2) #4 MeSH descriptor Vitamin E, this term only #5 (#3 OR #4) #6 alzheimer* OR AD OR “cognit* impair*” OR MCI #7 (#5 AND #6), from 2011 to 2012 |
Jun 2012: 9 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) | Interventional Studies | alzheimer OR alzheimer's OR alzheimers OR MCI OR cognitive OR cognition OR memory | vitamin E OR alpha‐tocopherol | received from 01/01/2011 to 07/15/2012 | Jun 2012: 2 |
| 10. ICTRP Search Portal (apps.who.int/trialsearch) (includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register) | Interventional Studies | alzheimer OR alzheimer's OR alzheimers OR MCI OR cognitive OR cognition OR memory | vitamin E OR alpha‐tocopherol | received from 01/07/2011 to 15/07/2012 | Jun 2012: 2 |
| TOTAL before de‐duplication | 282 | |
| TOTAL after de‐duplication and first assessment | 14 | |
Appendix 2. Sources searched, search strategies and hits retrieved: October 2014, May 2015, April 2016
| Source | Search strategy | Hits received |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) | Advanced search: [Study design: RCT OR CCT] AND [Health condition: Alzheimer OR MCI] AND [Intervention: "vitamin E"] (all dates) | Oct 2014: 11 May 2015: 0 Apr 2016: 0 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950 to present (OvidSP) | 1. *Vitamin E/ 2. "vitamin E".ti,ab. 3. "alpha‐tocopherol".ti,ab. 4. or/1‐3 5. Alzheimer*.ti,ab. 6. Alzheimer Disease/ 7. AD.ti,ab. 8. "cognit* impair*".ti,ab. 9. MCI.ti,ab. 10. (AACI or memory or CIND or ARCD or ACMI).ti,ab. 11. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 12. (nMCI or aMCI or mMCI).ti,ab. 13. (CDR adj2 "0.5").ab. 14. or/5‐13 15. 4 and 14 16. randomized controlled trial.pt. 17. controlled clinical trial.pt. 18. randomi?ed.ab. 19. placebo.ab. 20. drug therapy.fs. 21. randomly.ab. 22. trial.ab. 23. groups.ab. 24. or/16‐23 25. (animals not (humans and animals)).sh. 26. 24 not 25 27. 15 and 26 |
Oct 2014: 51 May 2015: 6 Apr 2016: 43 |
| 3. EMBASE 1980 to 2011 week 27 (OvidSP) |
1. *Vitamin E/ 2. "vitamin E".ti,ab. 3. "alpha‐tocopherol".ti,ab. 4. or/1‐3 5. Alzheimer*.ti,ab. 6. Alzheimer Disease/ 7. AD.ti,ab. 8. "cognit* impair*".ti,ab. 9. MCI.ti,ab. 10. (AACI or memory or CIND or ARCD or ACMI).ti,ab. 11. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 12. (nMCI or aMCI or mMCI).ti,ab. 13. (CDR adj2 "0.5").ab. 14. or/5‐13 15. 4 and 14 16. randomi?ed.ab. 17. placebo.ab. 19. trial.ab. 20. groups.ab. 21. randomized controlled trial/ 22. controlled clinical trial/ 23. ("double‐blind*" or "single‐blind*").ti,ab. 24. or/16‐23 25. 15 to 24 |
Oct 2014: 100 May 2015: 65 Apr 2016: 40 |
| 4. PsycINFO 1806 to July week 2 2011 (OvidSP) |
1. "vitamin E".ti,ab. 2. "alpha‐tocopherol".ti,ab. 3. Alzheimer*.ti,ab. 4. Alzheimer Disease/ 5. AD.ti,ab. 6. "cognit* impair*".ti,ab. 7. MCI.ti,ab. 8. (AACI or memory or CIND or ARCD or ACMI).ti,ab. 9. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 10. (nMCI or aMCI or mMCI).ti,ab. 11. (CDR adj2 "0.5").ab. 12. randomi?ed.ab. 13. placebo.ab. 14. randomly.ab. 15. trial.ab. 16. groups.ab. 17. ("double‐blind*" or "single‐blind*").ti,ab. 18. Clinical Trials/ 19. 1 or 2 20. or/3‐11 21. or/12‐18 22. 19 and 20 and 21 |
Oct 2014: 10 May 2015: 4 Apr 2016: 0 |
| 5. CINAHL (EBSCOhost) | S1 (MM "Vitamin E") S2 TX "vitamin E" S3 TX "alpha‐tocopherol" S4 S1 or S2 or S3 S5 (MH "Alzheimer's Disease") S6 TX AD OR alzheimer* S7 "mild cognitive impairment" S8 TX "cognit* impair*" S9 TX AACI OR memory OR CIND OR ARCD OR ACMI S10 TX MCI S11 TX nMCI OR aMCI OR mMCI S12 S5 or S6 or S7 or S8 or S9 or S10 or S11 S13 S4 and S12 S14 TX random* S15 TX placebo* S16 TX trial S17 TX groups S18 TX RCT OR CCT S19 (MH "Randomized Controlled Trials") S20 S14 or S15 or S16 or S17 or S18 or S19 S21 S13 and S20 S22 EM 2009 S23 EM 2010 S24 EM 2011 S25 S22 or S23 or S24 S26 S21 and S25 |
Oct 2014: 7 May 2015: 5 Apr 2016: 3 |
| 6. ISI Web of Knowledge – all databases [includes: Web of Science (1945 to present); BIOSIS Previews (1926 to present); MEDLINE (1950 to present); Journal Citation Reports] | #1 Topic=(alzheimer* OR AD OR MCI OR memory OR cognitive OR "cognit* impair*") AND Topic=("vitamin e" OR "alpha‐tocopherol") AND Year Published=(2009‐2011) Timespan=All Years #2 Topic=(random* OR placebo* OR "double‐blind*" OR "single‐blind*") Timespan=All Years #3 #2 AND #1 Timespan=All Years |
Oct 2014: 91 May 2015: 58 Apr 2016: 54 |
| 7. LILACS (BIREME) | vitamin‐e OR alpha‐tocopherol | Oct 2014: 1 May 2015: 0 Apr 2016: 0 |
| 8. CENTRAL (the Cochrane Library) (Issue 4 of 4, Oct 2010) | #1 “vitamin e” #2 “alpha‐tocopherol” #3 (#1 OR #2) #4 MeSH descriptor Vitamin E, this term only #5 (#3 OR #4) #6 alzheimer* OR AD OR “cognit* impair*” OR MCI #7 (#5 AND #6), from 2011 to 2012 |
Oct 2014: 33 May 2015: 15 Apr 2016: 0 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) | Interventional Studies | alzheimer OR alzheimer's OR alzheimers OR MCI OR cognitive OR cognition OR memory | vitamin E OR alpha‐tocopherol | received from 01/01/2011 to 07/15/2012 | Oct 2014: 1 May 2015: 2 Apr 2016: 1 |
| 10. ICTRP Search Portal (apps.who.int/trialsearch) (includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register) | Interventional Studies | alzheimer OR alzheimer's OR alzheimers OR MCI OR cognitive OR cognition OR memory | vitamin E OR alpha‐tocopherol | received from 01/07/2011 to 15/07/2012 | Oct 2014: 14 May 2015: 4 Apr 2016: 1 |
| TOTAL before de‐duplication | Oct 2014: 321 May 2015: 159 Apr 2016: 142 |
|
| TOTAL after de‐duplication and first assessment | Oct 2014: 41 May 2015: 8 Apr 2016: 8 |
|
Data and analyses
Comparison 1.
Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease
Analysis 1.1.
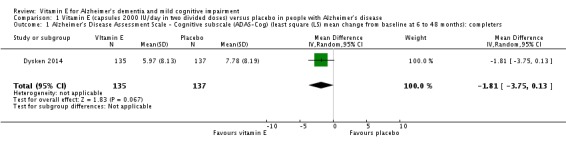
Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 1 Alzheimer's Disease Assessment Scale ‐ Cognitive subscale (ADAS‐Cog) (least square (LS) mean change from baseline at 6 to 48 months): completers.
Analysis 1.2.
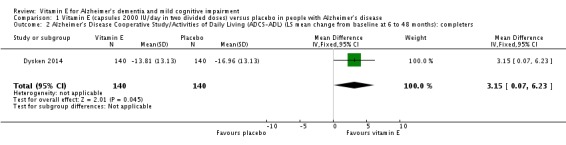
Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 2 Alzheimer's Disease Cooperative Study/Activities of Daily Living (ADCS‐ADL) (LS mean change from baseline at 6 to 48 months): completers.
Analysis 1.3.
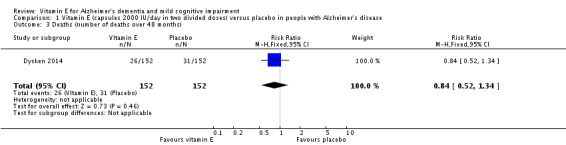
Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 3 Deaths (number of deaths over 48 months).
Analysis 1.4.
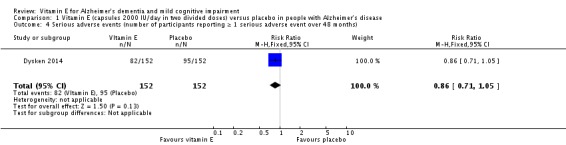
Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 4 Serious adverse events (number of participants reporting ≥ 1 serious adverse event over 48 months).
Analysis 1.5.
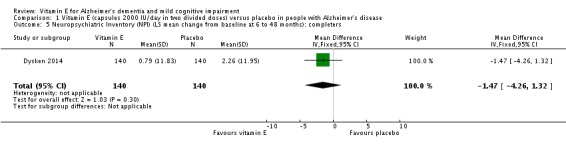
Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 5 Neuropsychiatric Inventory (NPI) (LS mean change from baseline at 6 to 48 months): completers.
Analysis 1.6.
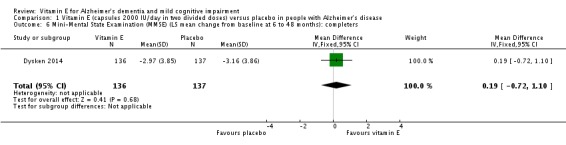
Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 6 Mini‐Mental State Examination (MMSE) (LS mean change from baseline at 6 to 48 months): completers.
Analysis 1.7.
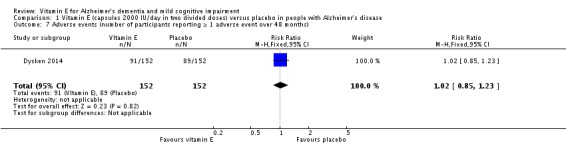
Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 7 Adverse events (number of participants reporting ≥ 1 adverse event over 48 months).
Comparison 2.
Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with mild cognitive impairment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Progression to Alzheimer's disease (AD) (number of people progressing to AD by 36 months) | 1 | 516 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.35] |
| 2 Deaths (number of deaths over 36 months) | 1 | 516 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.30, 3.44] |
Analysis 2.1.
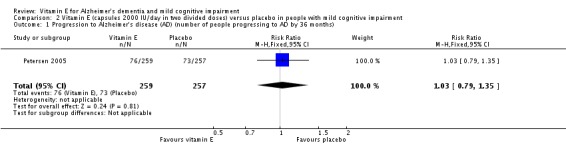
Comparison 2 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with mild cognitive impairment, Outcome 1 Progression to Alzheimer's disease (AD) (number of people progressing to AD by 36 months).
Analysis 2.2.
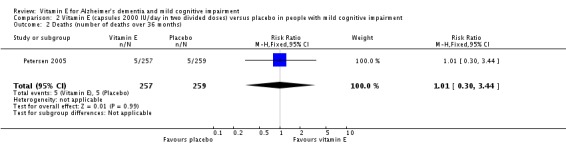
Comparison 2 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with mild cognitive impairment, Outcome 2 Deaths (number of deaths over 36 months).
What's new
Last assessed as up‐to‐date: 22 April 2016.
| Date | Event | Description |
|---|---|---|
| 25 January 2017 | New citation required and conclusions have changed | An updated search was performed for this review on 22 April 2016. One additional study was included (Dysken 2014); the results and conclusions have changed. The authors of the review have changed. |
| 25 January 2017 | New search has been performed | Update search performed 22 April 2016. One additional study included;results and conclusions changed. Author team has changed. |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 25 June 2012 | New citation required and conclusions have changed | An update search was performed for this review on 15 July 2011 and 25 June 2012. One additional study was included (Lloret 2009); the results and conclusions have changed. The authors of the review have changed. |
| 15 July 2011 | New search has been performed | An update search was performed for this review on 15 July 2011. |
| 18 March 2009 | New search has been performed | Update search of 5 March 2009 retrieved new studies for consideration for inclusion |
| 25 April 2008 | New citation required but conclusions have not changed | In 2007, the scope of this review was broadened to include both Alzheimer's disease patients and patients with mild cognitive impairment |
| 16 January 2007 | New search has been performed | Update 2007: one new study was included (Petersen 2005). The included study did not affect the results as it also showed that vitamin E has no more significant effect than placebo for mild cognitive impairment. The overall conclusion of the review has not changed as there is not enough evidence to support the use of vitamin E in the treatment of Alzheimer's disease or mild cognitive impairment. |
| 2 November 2006 | New citation required and conclusions have changed | Substantive amendment |
Differences between protocol and review
We included 'Summary of findings' tables and GRADE evaluation.
The methods of the review were updated to comply with the most recent version of the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1; Higgins 2011).
Since the June 2012 update, one new author DL has been added. AC and JR have been removed as authors.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
Design: multicentre, randomised, double‐blind, placebo‐controlled trial Duration: 6 months to 4 years |
|
| Participants |
Diagnosis (including criteria used): possible or probable AD (NINCDS‐ADRDA) Number of participants: 613 total randomised (completers: 140 in vitamin E group, 140 in placebo group, 142 in memantine group, 139 in the vitamin E + memantine group) Age: 53 to 96 years, mean age 78.8 years Gender: 97% men Cognitive status (e.g. MMSE): 21 Ethnicity: 86% white Inclusion criteria: diagnosis of possible or probable AD (NINCDS‐ADRDA); presence of a carer (friend or relative); informed consent; MMSE 12 to 26; administration of maintenance dosage of acetylcholinesterase inhibitors for at least 4 weeks; agreement not to take vitamin E/memantine outside the study. Exclusion criteria: non‐Alzheimer's primary dementia; current major depression, delirium, alcohol or psychoactive substance abuse or dependency; schizophrenia, or delusional disorder as defined by DSM‐IV; presence of any uncontrolled systemic illness that would interfere with participation in the study or life expectancy of < 1 year; pregnant or intention to become pregnant; enrolment in another interventional clinical trial; current prescription with more than one acetylcholinesterase inhibitor; current prescription for warfarin; use of vitamin E supplements in the past 2 weeks; use of memantine in the past 4 weeks or known intolerance; estimated creatinine clearance < 5 mL/minute; use of amantadine in the past 2 weeks. |
|
| Interventions |
Treatment 1: vitamin E (2000 IU total daily dose divided into 2 doses) Treatment 2: memantine (20 mg/day) Treatment 3: vitamin E + memantine Treatment 4: placebo |
|
| Outcomes | ADCS‐ADL (months 0, 6, 12, 18, 24, 30, 36, 42, 48) MMSE (months 0, 6, 12, 18, 24, 30, 36, 42, 48) ADAS‐Cog (months 0, 6, 12, 18, 24, 30, 36, 42, 48) DS (months 0, 6, 12, 18, 24, 30, 36, 42, 48) NPI (months 0, 6, 12, 18, 24, 30, 36, 42, 48) Caregiver Activity Survey (months 0, 6, 12, 18, 24, 30, 36, 42, 48) Adverse events |
|
| Notes | On average, carers reported that vitamin E was taken on 65% of the days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised centrally using "...a random permuted block design of randomly varying sizes between 4 and 12." |
| Allocation concealment (selection bias) | Low risk | "…participants were randomized centrally into one of the four treatment groups…" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The patient, caregivers, and all site investigators were blinded to the treatment assignment." Participants received "matching placebos for vitamin E were hard‐gelatin, liquid‐filled capsules containing soybean oil." The authors did not describe whether blinding was successful. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The patient, caregivers, and all site investigators were blinded to the treatment assignment." The authors did not describe how blinding was maintained and whether it was successful. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data was comparable between the vitamin E group and placebo group (12 participants excluded in each due to a lack of follow‐up data). |
| Selective reporting (reporting bias) | Low risk | Study protocol was published, which included the primary and secondary outcomes as included in the main paper. |
| Other bias | Low risk | No other sources of bias noted. |
| Methods |
Design: randomised, placebo‐controlled, double‐blind study Duration: 6 months |
|
| Participants |
Country: Spain Number of centres: 1 Diagnosis: probable AD according to NINCDS‐ADRDA Number of participants: 57 participants total randomised (completers: 19 in vitamin E group, 14 in placebo group), 18 healthy controls Age: not described Gender: not described Ethnicity: not described Cognitive status: severity of participants based on the Geriatric Dementia Scale ‐ mild (n = 25), moderate (n = 26) and severe (n = 6) Inclusion criteria: not described Exclusion criteria: people taking antioxidant supplements or any medication other than cholinesterase inhibitors |
|
| Interventions |
Treatment 1: vitamin E 800 IU/day Treatment 2: placebo daily |
|
| Outcomes | Clock Drawing Test (months 0 and 6) BDS (months 0 and 6) MMSE (months 0 and 6) Blood total glutathione levels and oxidised glutathione (months 0 and 6) Plasma malondialdehyde (months 0 and 6) |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed using a randomized list of numbers." |
| Allocation concealment (selection bias) | Unclear risk | No reference made to how allocation concealment was ensured. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study described as 'double blind' but authors did not describe how blinding was maintained and whether it was successful. It is unclear whether the placebo was identical in appearance to the vitamin E treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study described as 'double blind' but authors did not describe how blinding was maintained and whether it was successful. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 24/57 AD participants did not complete the research. "Of the patients who finished the study, 14 had been treated with placebo and 19 with vitamin E." The reasons for participant dropout were not described. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported, though the level of detail lacking. For example, means and SDs not described. |
| Other bias | Low risk | No evidence of other bias. |
| Methods |
Design: randomised, placebo‐controlled, double‐blind study Duration: 36 months |
|
| Participants |
Country: US and Canada Number of centres: 69 Diagnosis: amnestic type of MCI Number of participants: 769 total randomised (ITT: 257 in vitamin E group, 259 in placebo group, 253 in donepezil group) Gender: 46% women Age: 55 to 90 years, mean 73 years Cognitive status: MMSE 24 to 30, mean MMSE 27.3 Inclusion criteria: amnestic MCI of a degenerative nature, impaired memory, a Logical Memory delayed‐recall score approximately 1.5 to 2 SD below an education adjusted‐norm, a CDR of 0.5, MMSE score 24 to 30, and 55 to 90 years of age. adequate vision and hearing for neuropsychological testing, normal vitamin B12 and thyroid function studies and non‐reactive RPR. ECG normal or no clinical significant abnormalities. Study informant available. Exclusion criteria: significant cerebral vascular disease, modified Hachinski > 4; Hamilton Depression Rating Scale > 12; central nervous system infarct, infection or focal lesions of clinical significance on CT or MRI scan. Medical diseases or psychiatric disorders that could interfere with study participation. Pregnant, lactating or of child‐bearing potential; taking vitamin supplements, other supplements or multivitamin. Restriction on concomitant medication usage, including those with significant cholinergic or anticholinergic effects or potential adverse effects on cognition. |
|
| Interventions |
Treatment 1: vitamin E group (2000 IU total daily dose divided into 2 doses, placebo donepezil and a multivitamin daily) Treatment 2: donepezil group (donepezil 10 mg, placebo vitamin E and a multivitamin daily) Treatment 3: placebo group (placebo vitamin E, placebo donepezil and a multivitamin daily) Note: the initial dose of vitamin E was 1000 IU/day, and the dose was increased to 2000 IU (1000 IU twice daily) after 6 weeks. The multivitamin contained vitamin E 15 IU |
|
| Outcomes | Time to possible or probable development of AD MMSE (months 0, 6, 12, 18, 24, 30, 36) ADAS‐Cog (months 0, 6, 12, 18, 24, 30, 36) Global CDR (months 0, 6, 12, 18, 24, 30, 36) ADCS Mild Cognitive Impairment Activities of Daily Living Scale (months 0, 6, 12, 18, 24, 30, 36) GDS (months 0, 6, 12, 18, 24, 30, 36) Neuropsychological battery including; New York University paragraph‐recall test, the Symbol Digit Modalities Test, the category‐fluency test, a number‐cancellation test, the Boston Naming Test, the digits‐backward test, the Clock Drawing Test, and a maze‐tracing task (months 0, 6, 12, 18, 24, 30, 36) Adverse events |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly assigned to treatment groups. The authors used "…an adaptive allocation scheme for the treatment assignment, with the MMSE score, age, and APOE e4 status as balancing covariates." |
| Allocation concealment (selection bias) | Unclear risk | No reference made to the method in which allocation concealment was ensured. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study described as 'double blind' but authors did not describe how blinding was maintained and whether it was successful. Unclear whether the placebo was identical in appearance to the vitamin E treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study described as 'double blind' but authors did not describe how blinding was maintained and whether it was successful. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "The primary analysis was conducted according to the intention‐to‐treat principle." During the double‐blind phase, 72 participants discontinued in the vitamin E group, and 66 in placebo group. Sensitivity analyses suggested no impact of dropouts on the results regarding vitamin E and placebo groups. 76 participants in the vitamin E group and 73 in the placebo group developed AD and switched to open label donepezil. It is not clear how subsequent data from these participants was handled. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in accordance with the methods section. |
| Other bias | Low risk | No evidence of other bias. |
| Methods |
Design: randomised, placebo‐controlled, double‐blind study Duration: 24 months |
|
| Participants |
Country: US Multicentre: 23 sites Diagnosis: probable AD according to NINCDS‐ADRDA Number of participants: 341 total randomised (completers: 85 in vitamin E group, 84 in placebo group, 87 in selegiline group, 85 in selegiline + vitamin E group) Gender: 65% women Age: mean 73 years Ethnicity: not described Cognitive status: mean MMSE 12.6 Inclusion criteria: diagnosis of probable AD, aged ≥ 45 years, fluent in English or Spanish, CDR score 2, modified Hachinski score 4, supervised by a responsible carer. Exclusion criteria: other central nervous system or psychiatric diagnosis; achieving end point on any primary outcome measure at time of entry; participation in any other investigational drug study or treatment with a psychoactive medication within 2 months of initiation of this trial; treatment with antiparkinsonian medications; recent initiated treatment for a non‐psychiatric condition with an agent to have psychoactive properties (e.g. beta‐blockers, calcium channel blockers). Entry permitted if medication and dose had been stable for 3 months prior to entry into the protocol; antilipaemic drugs specifically contradicted with selegiline (i.e. cholestyramine or colestipol) within 2 weeks of enrolment; some narcotics: demerol has been reported to interact with selegiline; ancillary vitamin E (alpha tocopherol) other than the 30 IU to 32 IU included in a standard multivitamin. |
|
| Interventions |
Treatment 1: placebo Treatment 2: vitamin E (2000 IU total daily dose divided into 2 doses) Treatment 3: selegiline (10 mg total daily dose divided into 2 doses) Treatment 4: vitamin E (2000 IU total daily dose divided into 2 doses) + selegiline (10 mg total daily dose divided into 2 doses) |
|
| Outcomes | Delay in any of 4 end points: death; institutionalisation; severity of dementia; loss of ADL ADAS‐Cog (1 month after enrolment and at 3‐month intervals) MMSE (1 month after enrolment and at 3‐month intervals) BDS (1 month after enrolment and at 3‐month intervals) DS (1 month after enrolment and at 3‐month intervals) BRSD (1 month after enrolment and at 3‐month intervals) Adverse events |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were "...randomly assigned (after stratification according to center with the use of a permuted‐block procedure)..." to receive vitamin E or placebo. |
| Allocation concealment (selection bias) | Unclear risk | Method in which allocation concealment was ensured was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study described as 'double blind' but authors did not describe how blinding was maintained and whether it was successful. Unclear whether the placebo and vitamin E treatment capsules were visually identical. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study described as 'double blind' but authors did not describe how blinding was maintained and whether it was successful. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was only able to be reported up until the point a participant reached 1 of the primary end points of the study. Loss to follow‐up was defined as those who did not reach an end point and did not complete the study: 6/84 for the placebo group and 8/85 for the vitamin E group. The reasons for withdrawal were not described in each treatment group. |
| Selective reporting (reporting bias) | Unclear risk | All outcome measures were reported as per the protocol paper. No SDs reported for change scores. |
| Other bias | Low risk | No evidence of other bias. |
AD: Alzheimer's dementia; ADAS‐Cog: Alzheimer's Disease Assessment Scale ‐ Cognitive subsection; ADCS: Alzheimer's Disease Cooperative Study; ADL: activities of daily living; APoE: apolipoprotein E; BDS: Blessed Dementia Scale; BRSD: Behavior Rating Scale for Dementia; CDR: Clinical Dementia Rating; CT: computer tomography; DS: Dependence Scale; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; ECG: electrocardiography; GDS: Global Deterioration Scale; ITT: intention to treat; MCI: mild cognitive impairment; MMSE: Mini‐Mental State Examination; MRI: magnetic resonance imaging; n: number of participants; NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association; NPI: Neuropsychiatric Inventory; RPR: rapid plasmin reagin; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alavi 2013 | Not suitable placebo |
| Alavi 2014 | Control group did not receive a suitable placebo. |
| Alzoubi 2012 | Sample population did not have a diagnosis of MCI or AD. |
| Anand 2011 | Sample population did not have a diagnosis of MCI or AD. |
| Anonymous 2014 | Newsletter |
| Apostolova 2013 | Not an RCT |
| Arlt 2012 | Not an RCT |
| Biesalski 2010 | Review |
| Bittner 2009 | Retrospective design |
| Brewer 2010 | Review |
| Carlsson 2002 | Sample population did not have a diagnosis of MCI or AD. |
| Chan 2008‐2009 | Used a nutriceutical formulation and there was no placebo. |
| Chan 2010 | Used a nutriceutical formulation. Placebo was just inert ingredients. |
| Chapman 2014 | Editorial |
| Clarke 2003 | Vitamin E was used in conjunction with vitamin C. It examined the association of cognitive impairment with platelet activation and reactive oxygen species and total homocysteine levels. |
| Corbett 2014 | Commentary |
| Cornelli 2010 | Used a nutriceutical formulation, not vitamin E separately. |
| de Oliveira 2012 | In vitro study |
| Dunham 2013 | Review |
| Dysken 2009 | No vitamin E intervention, just a questionnaire |
| Evans 2014 | Editorial |
| Galasko 2012 | Not suitable placebo |
| Geldmacher 2011 | Pioglitazone was the primary intervention. Placebo was administered alongside vitamin E. |
| Gopalan 2014 | Sample population did not have a diagnosis of MCI or AD. |
| Grodstein 2013 | Sample population did not have a diagnosis of MCI or AD. |
| Guan 2012 | No relevant outcome measures |
| Gutierrez 2009 | Sample population did not have a diagnosis of MCI or AD. |
| Han 2011 | Review |
| Hermann 2014 | Newsletter |
| Jae 2006 | Vitamin E intervention given to healthy older women. |
| Joshi 2012 | Review |
| Jyvakorpi 2012 | Not an RCT |
| Kamat 2008 | Literature review about antioxidants |
| Kesse‐Guyot 2011a | Sample population did not have a diagnosis of MCI or AD. |
| Kesse‐Guyot 2011b | Sample population did not have a diagnosis of MCI or AD. |
| Kryscio 2013a | Sample population did not have a diagnosis of MCI or AD. |
| Kryscio 2013b | Sample population did not have a diagnosis of MCI or AD. |
| Lee 2010 | Literature review about antioxidants |
| Lehtisalo 2013 | No vitamin E intervention group |
| Lehtisalo 2014 | No vitamin E intervention group |
| Lewis 2014 | Sample population did not have a diagnosis of MCI or AD. |
| Li 2015 | No placebo group |
| Lott 2011 | Sample population had a diagnosis of Down's syndrome and dementia. |
| Lu 2009 | Focus of intervention was towards depressed vs non‐depressed MCI population. |
| MacPherson 2012 | Sample population did not have a diagnosis of MCI or AD. |
| Mecocci 2012 | Review |
| Mishra 2012 | Survey |
| Morris 2012a | Review |
| Morris 2012b | Review |
| Muss 2015 | Sample population did not have a diagnosis of MCI or AD. |
| Naeini 2014 | No co‐administration of other compounds to controls |
| Onofrj 2002 | Vitamin E compared to donepezil not placebo. |
| Pavlik 2009 | Retrospective in nature. No controls or placebo group |
| Podea 2012 | Not an RCT |
| Polidori 2012 | Review |
| Pribis 2012 | Did not use vitamin E as an intervention. |
| Péneau 2011 | Not a randomised double‐blinded study; investigated the intake of fruits and vegetables. |
| Remington 2009 | Used a nutriceutical formulation and the placebo was inert. |
| Remington 2015 | Used a nutriceutical formulation. |
| Rijpma 2014a | Systematic review |
| Rijpma 2014b | Used a nutriceutical formulation. |
| Satalich 2014 | Secondary analysis (modelling) of intervention data |
| Schmitt 2009 | Used a healthy population. |
| Schneider 2009 | Same data as Petersen 2005 |
| Shea 2013 | Used a nutriceutical formulation, not vitamin E separately. |
| Srour 2013 | Vitamin C taken by treatment arm but not controls, no relevant outcomes. |
| Stough 2012 | Sample population did not have a diagnosis of MCI or AD. |
| Suominen 2013 | Vitamin E not administered |
| Takahashi 2009 | Case study, no placebo and no controls |
| Usoro 2010 | Review |
| Vinyoles‐Bargallo 2010 | Not an original paper, just a personal comment |
| von Arnim 2013 | Vitamin E not administered |
| Whitehair 2010 | Same data as Petersen 2005 |
| Zanotta 2014 | Not an RCT |
AD: Alzheimer's dementia; MCI: mild cognitive impairment; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by year of study]
| Trial name or title | Effects of Vitamin E on Cognition and Measures of Activities of Daily Living in Patients with Moderately Severe Alzheimer's Disease |
| Methods | Unknown |
| Participants | People with moderately severe Alzheimer's disease |
| Interventions | Vitamin E 500 mg twice daily with cholinesterase inhibitors |
| Outcomes | Changes in cognition and activities of daily living |
| Starting date | 1 November 2002 |
| Contact information | Dr Vishnu Gopal; Argyll House, 9 Williamson Road, Nether Edge, Sheffield S11 9AR; tel: (+44) 114 2718656; email: vgopal@doctors.org.uk |
| Notes | Study topic and author searched online, no evidence of trial or published data. Author no longer at contact address, emailed for an update but no response. |
Contributions of authors
Original: NT drew up the protocol. M Isaac (MI) planned the review and protocol, selected studies, critiqued the trials, advised on analyses, revised drafts of protocol and review and supervised the review process.
Update 2006: MI and Rebecca Quinn (RQ) reviewed and selected the included studies. MI extracted the data, performed the analysis and wrote the draft. NT contributed to the text and supervised. The search was carried out by Dymphna Hermans.
Update 2012: NF and MI reviewed and selected the included studies. NF extracted the data and wrote the draft. Annalie R Clark (AC), Jennifer Rusted (JR) and NT contributed to the text. JR and NT supervised. The search was carried out by Anna‐Noel Storr.
Update 2016: NF and DL independently reviewed and extracted descriptive data from included studies. NF, NT and DL independently extracted outcome data. NF wrote the initial draft. DL, MI and NF all contributed to the text. NT made the final decision on any disagreements between authors. The search was carried out by Anna‐Noel Storr.
Sources of support
Internal sources
Centre for Dementia Studies, Brighton & Sussex Medical School, UK.
External sources
-
NIHR, UK.
This update was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health
Declarations of interest
Nicolas Farina ‐ None known David Llewellyn ‐ None known Mokhtar Gad El Kareem Nasr Isaac‐ None known Naji Tabet ‐ None known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
- Dysken MW, Guarino PD, Vertrees JE, Asthana S, Sano M, Llorente M, et al. Vitamin E and memantine in Alzheimer's disease: clinical trial methods and baseline data. Alzheimer's & Dementia: the Journal of the Alzheimer's Association 2014;10(1):36‐44. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; Dysken MW, Sano M, Asthana S, Vertrees JE, Pallaki M, Llorente M, et al. Effect of vitamin E and memantine on functional decline in Alzheimer. JAMA 2014;311(11):1161. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; Dysken MW, Sano M, Asthana S, Vertrees JE, Pallaki M, Llorente M, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM‐AD VA cooperative randomized trial. JAMA 2014;311(1):33‐44. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 3262292
- Lloret A, Badía MC, Mora NJ, Pallardó FV, Alonso MD, Viña J. Vitamin E paradox in Alzheimer's disease: it does not prevent loss of cognition and may even be detrimental. Journal of Alzheimer's Disease 2009;17(1):143‐9. [] [DOI] [PubMed] [Google Scholar]; 3262296
- Petersen R, Grundman R, Thomas R, Thal L. Donepezil and vitamin E as treatments for mild cognitive impairment. NeuroBiology of Aging 2004;25(S2):20. [] [Google Scholar]; Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. for the Alzheimer's Disease Cooperative Study Group. Vitamin E and donepezil for the treatment of mild cognitive impairment. New England Journal of Medicine 2005;352(23):2379‐88. [] [DOI] [PubMed] [Google Scholar]; 3262298
- Sano M, Ernesto C, Klauber MR, Schafer K, Woodbury P, Thomas R, et al. and members of the Alzheimer's Disease Cooperative Study. Rationale and design of a multicenter study of selegiline and a‐tocopherol in the treatment of Alzheimer's disease using novel clinical outcomes. Alzheimer Disease and Associated Disorders 1996;10(3):132‐40. [] [DOI] [PubMed] [Google Scholar]; Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, et al. Effects of selegiline and alpha‐tocopherol on cognitive and functional outcome measures in moderately impaired patients with Alzheimer's disease. Neurology 1997;48(3):A377‐8. [] [Google Scholar]; Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, et al. for the members of the Alzheimer's Disease Cooperative Study. A controlled trial of selegiline, alpha‐tocopherol, or both as treatment for Alzheimer's disease. New England Journal of Medicine 1997;336(17):1216‐22. [] [DOI] [PubMed] [Google Scholar]; Sano M, Growdon J, Klauber M, Ernesto C, Schafer P, Woodbury P, et al. Expanding the severity range of patients in clinical trials for Alzheimer's disease: a multicentre clinical trial of selegiline and a‐tocopherol. Neurology 1996;45 Suppl 4:289. [] [Google Scholar]; 3262301
References to studies excluded from this review
- Alavi Naeini AM, Elmadfa I, Djazayeri A, Barekatain M, Aghayeghazvini MR, Jalali M, et al. Effect of vitamin E and C supplementation on elderly with mild cognitive impairment (MCI) in Isfahan, Iran. Annals of Nutrition & Metabolism 2013:1145. [] [Google Scholar]; 3262306
- Alavi Naeini AM, Elmadfa I, Djazayery A, Barekatain M, Aghaye Ghazvini MR, Djalali M, et al. The effect of antioxidant vitamins E and C on cognitive performance of the elderly with mild cognitive impairment in Isfahan, Iran: a double‐blind, randomized, placebo‐controlled trial. European Journal of Nutrition 2014;53(5):1255‐62. [] [DOI] [PubMed] [Google Scholar]; 3262308
- Alzoubi KH, Khabour OF, Abu Rashid B, Damaj IM, Salah HA. The neuroprotective effect of vitamin E on chronic sleep deprivation‐induced memory impairment: the role of oxidative stress. Behavioural Brain Research 2012;226(1):205‐10. [] [DOI] [PubMed] [Google Scholar]; 3262310
- Anand VPR, Kumar BJ, Varghese JM, Das SK. Supplementation of vitamin E improves cognitive status and oxidative stress in type 2 diabetes mellitus. International Research Journal of Pharmacy 2011;2(11):169‐72. [] [Google Scholar]; 3262312
- Anonymous. High dose vitamin E may slow Alzheimer's decline. Harvard Men's Health Watch 2014;18(8):8. [] [PubMed] [Google Scholar]; 3262316
- Apostolova LG, Babakchanian S, Hwang KS, Green AE, Zlatev D, Chou Y‐Y, et al. Ventricular enlargement and its clinical correlates in the imaging. Alzheimer Disease and Associated Disorders 2013;27(2):174‐81. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 3262318
- Arlt S, Muller‐Thomsen T, Beisiegel U, Kontush A. Effect of one‐year vitamin C‐ and E‐supplementation on cerebrospinal fluid oxidation parameters and clinical course in Alzheimer's disease. Neurochemical Research 2012;37(12):2706‐14. [] [DOI] [PubMed] [Google Scholar]; 3262320
- Biesalski HK, Grune T, Tinz J, Zollner I, Blumberg JB. Reexamination of a meta‐analysis of the effect of antioxidant supplementation on mortality and health in randomized trials. Nutrients 2010;2(9):929‐49. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 3262322
- Bittner DM. Combination therapy of acetylcholinesterase inhibitor and vitamin E in Alzheimer disease. Journal of Clinical Psychopharmacology 2009;29(5):511‐3. [] [DOI] [PubMed] [Google Scholar]; 3262324
- Brewer GJ. Why vitamin E therapy fails for treatment of Alzheimer's disease. Journal of Alzheimer's Disease 2010;19(1):27‐30. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 3262326
- Carlsson CM, Papcke‐Benson K, Carnes M, McBride PE, Stein JH. Health‐related quality of life and long‐term therapy with pravastatin and tocopherol (vitamin E) in older adults. Drugs and Aging 2002;19(10):793‐805. [] [DOI] [PubMed] [Google Scholar]; 3262328
- Chan A, Paskavitz J, Remington R, Rasmussen S, Shea TB. Efficacy of a vitamin/nutriceutical formulation for early‐stage Alzheimer's disease: a 1‐year, open‐label pilot study with an 16‐month caregiver extension. American Journal of Alzheimer's Disease & Other Dementias 2008‐2009;23(6):571‐85. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 3262330
- Chan A, Remington R, Kotyla E, Lepore A, Zemianek J, Shea TB. A vitamin/nutriceutical formulation improves memory and cognitive performance in community‐dwelling adults without dementia. Journal of Nutrition, Health & Aging 2010;14(3):224‐30. [] [DOI] [PubMed] [Google Scholar]; 3262332
- Chapman J. Vitamin E and Alzheimer's disease. Australian Journal of Pharmacy 2014;95(1126):92‐3. [] [Google Scholar]; 3262334
- Clarke R, Harrison G, Richards S, Vital Trial Collaborative Group. Effects of vitamins and aspirin on markers of platelet activation, oxidative stress and homocysteine in people at high risk of dementia. Journal of Internal Medicine 2003;245:67‐75. [] [DOI] [PubMed] [Google Scholar]; Jacoby RJ. A pilot study for the VITAL trial (Vitamins and Aspirin for the treatment of Dementia). National Research Register2002. [] ; 3262336
- Corbett A, Ballard C. The value of vitamin E as a treatment for Alzheimer's disease remains unproven despite functional improvement, due to a lack of established effect on cognition or other outcomes from RCTs. Evidence‐based Medicine 2014;19(4):140. [] [DOI] [PubMed] [Google Scholar]; 3262339
- Cornelli U. Treatment of Alzheimer's disease with a cholinesterase inhibitor combined with antioxidants. Neuro‐degenerative Diseases 2010;7(1‐3):193‐202. [] [DOI] [PubMed] [Google Scholar]; 3262341
- Oliveira BF, Veloso CA, Nogueira‐Machado JA, Moraes EN, dos Santos RR, Cintra MTG, et al. Ascorbic acid, alpha‐tocopherol, and betacarotene reduce oxidative stress and proinflammatory cytokines in mononuclear cells of Alzheimer's disease patients. Nutritional Neuroscience 2012;15(6):244‐51. [] [DOI] [PubMed] [Google Scholar]; 3262343
- Dunham A, Johnson EJ. Fruits, vegetables, and their components and mild cognitive impairment. Food Reviews International 2013;29(4):409‐40. [] [Google Scholar]; 3262345
- Dysken MW, Kirk LN, Kuskowski M. Changes in vitamin E prescribing for Alzheimer patients. American Journal of Geriatric Psychiatry 2009;17(7):621‐4. [] [DOI] [PubMed] [Google Scholar]; 3262347
- Evans DA, Morris MC, Rajan KB. Vitamin E, memantine, and Alzheimer disease. JAMA 2014;311(1):29‐30. [] [DOI] [PubMed] [Google Scholar]; 3262349
- Galasko DR, Peskind E, Clark CM, Quinn JF, Ringman JM, Jicha GA, et al. Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Archives of Neurology 2012;69(7):836‐41. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 3262351
- Geldmacher DS, Fritsch T, McClendon MJ, Landreth G. A randomized pilot clinical trial of the safety of pioglitazone in treatment of patients with Alzheimer disease. Archives of Neurology 2011;68(1):45‐50. [] [DOI] [PubMed] [Google Scholar]; 3262353
- Gopalan Y, Shuaib IL, Magosso E, Ansari MA, Abu BMR, Wong JW, et al. Clinical investigation of the protective effects of palm vitamin E tocotrienols on brain white matter. Stroke 2014;45(5):1422‐8. [] [DOI] [PubMed] [Google Scholar]; 3262355
- Grodstein F, O'Brien J, Kang JH, Dushkes R, Cook NR, Okereke O, et al. Long‐term multivitamin supplementation and cognitive function in men: a randomized trial. Annals of Internal Medicine 2013;159(12):806‐14. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 3262357
- Guan JZ, Guan WP, Maeda T, Makino N. Effect of vitamin E administration on the elevated oxygen stress and the telomeric and subtelomeric status in Alzheimer's disease. Gerontology 2012;58(1):62‐9. [] [DOI] [PubMed] [Google Scholar]; 3262359
- Gutierrez AD, Serna DG, Robinson I, Schade DS. The response of gamma vitamin E to varying dosages of alpha vitamin E plus vitamin C. Metabolism: Clinical and Experimental 2009;58(4):469‐78. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 3262361
- Han SH, Li H, Liu LT. Therapeutic efficacy assessment of Chinese medicine on mild cognitive impairment [in Chinese]. Zhongguo Zhong xi yi jie he za zhi [Chinese Journal of Integrated Traditional and Western Medicine] 2011;31(5):608‐17. [] [PubMed] [Google Scholar]; 3262363
- Hermann DM. Vitamin E delays Alzheimer disease progression only slightly [in German]. MMW Fortschritte der Medizin 2014;156(11):36. [] [PubMed] [Google Scholar]; 3262365
- Jae HK, Cook N, Manson J, Buring JE, Grodstein F. A randomized trial of vitamin E supplementation and cognitive function in women. Archives of Internal Medicine 2006;166(22):2462‐8. [] [DOI] [PubMed] [Google Scholar]; 3262369
- Joshi YB, Pratico D. Vitamin E in aging, dementia, and Alzheimer's disease. Biofactors 2012;38(2):90‐7. [] [DOI] [PubMed] [Google Scholar]; 3262371
- Jyvakorpi S, Puranen T, Suominen MH. Quality of life, cognition and physical functioning between higher and lower intakes of nutrients of home‐dwelling older AD patients. Clinical Nutrition, Supplement 2012:275. [] [Google Scholar]; 3262375
- Kamat CD, Gadal S, Mhatre M, Williamson KS, Pye QN, Hensley K. Antioxidants in central nervous system diseases: preclinical promise and translational challenges. Journal of Alzheimer's Disease 2008;15(3):473‐93. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 3262377
- Kesse‐Guyot E, Amieva H, Castetbon K, Henegar A, Ferry M, Jeandel C, et al. Adherence to nutritional recommendations and subsequent cognitive performance: findings from the prospective Supplementation with Antioxidant Vitamins and Minerals 2 (SU.VI.MAX 2) study. American Journal of Clinical Nutrition 2011;93(1):200‐10. [] [DOI] [PubMed] [Google Scholar]; 3262379
- Kesse‐Guyot E, Fezeu L, Jeandel C, Ferry M, Andreeva V, Amieva H, et al. French adults' cognitive performance after daily supplementation with antioxidant vitamins and minerals at nutritional doses: a post hoc analysis of the Supplementation in Vitamins and Mineral Antioxidants (SU.VI.MAX) trial. American Journal of Clinical Nutrition 2011;94(3):892‐9. [] [DOI] [PubMed] [Google Scholar]; 3262381
- Kryscio RJ, Abner EL, Schmitt FA, Goodman PJ, Mendiondo M, Caban‐Holt A, et al. A randomized controlled Alzheimer's disease prevention trial's evolution. Journal of Nutrition, Health & Aging 2013;17(1):72‐5. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 3262383
- Kryscio R, Abner E, Caban‐Holt A, Mathews M, Schmitt F. Baseline memory problems associate with clinical impairments eight years later: centralized follow‐up in the preadvise trial. Journal of Nutrition, Health & Aging 2013:782‐3. [] [Google Scholar]; 3262385
- Lee HP, Zhu X, Casadesus G, Castellani RJ, Nunomura A, Smith MA, et al. Antioxidant approaches for the treatment of Alzheimer's disease. Expert Review of Neurotherapeutics 2010;10(7):1201‐8. [] [DOI] [PubMed] [Google Scholar]; 3262387
- Lehtisalo J, Lindstrom J, Ngandu T, Laatikainen T, Soininen H, Strandberg T, et al. Diet and cognition: baseline associations in the Finnish geriatric intervention study to prevent cognitive impairment and disability (FINGER). Alzheimer's & Dementia 2013:P604‐5. [] [DOI] [PubMed] [Google Scholar]; 3262389
- Lehtisalo J, Lindstrom J, Ngandu T, Honninen T, Laatikainen T, Strandberg T, et al. Feasibility of dietary intervention among individuals with elevated risk of dementia: 1st‐year results from the Finnish geriatric intervention study to prevent cognitive impairment and disability (finger). Alzheimer's & Dementia 2014:P166‐7. [] [Google Scholar]; 3262391
- Lewis JE, Melillo AB, Tiozzo E, Chen L, Leonard S, Howell M, et al. A double‐blind, randomized clinical trial of dietary supplementation on cognitive and immune functioning in healthy older adults. BMC Complementary and Alternative Medicine 2014;14:43. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 3262393
- Li Y, Liu S, Man Y, Li N, Zhou Y. Effects of vitamins E and C combined with β‐carotene on cognitive function in the elderly. Experimental and Therapeutic Medicine 2015;9(4):1489‐93. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 3262395
- Lott IT, Doran E, Nguyen VQ, Tournay A, Head E, Gillen DL. Down syndrome and dementia: a randomized, controlled trial of antioxidant supplementation. American Journal of Medical Genetics, Part A 2011;155(8):1939‐48. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 3262397
- Lu P, Edland S, Teng E, Tingus K, Petersen R, Cummings J, et al. Donepezil delays progression to AD in MCI subjects with depressive symptoms. Neurology 2009;72(24):2115‐21. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 3262399
- MacPherson H, Ellis KA, Sali A, Pipingas A. Memory improvements in elderly women following 16 weeks treatment with a combined multivitamin, mineral and herbal supplement. A randomized controlled trial. Psychopharmacology 2012;220(2):351‐65. [] [DOI] [PubMed] [Google Scholar]; 3262401
- Mecocci P, Polidori MC. Antioxidant clinical trials in mild cognitive impairment and Alzheimer's disease. Biochimica et Biophysica Acta ‐ Molecular Basis of Disease 2012;1822(5):631‐8. [] [DOI] [PubMed] [Google Scholar]; 3262403
- Mishra V, Mishra M, Kashaw V, Kashaw S. A survey on nutritional and other natural therapies for Alzheimer's disease. Alzheimer's & Dementia 2012:P404. [] [Google Scholar]; 3262405
- Morris MC. Nutritional determinants of cognitive aging and dementia. Proceedings of the Nutrition Society 2012;71(1):1‐13. [] [DOI] [PubMed] [Google Scholar]; 3262407
- Morris MC. Symposium 1: vitamins and cognitive development and performance. Proceedings of the Nutrition Society 2012, (1):1‐13. [] [Google Scholar]; 3262409
- Muss C, Mosgoeller W, Endler T. Neuroprotective impact of a vitamin trace element composition ‐ a randomized, double blind, placebo controlled clinical trial with healthy volunteers. Neuroendocrinology Letters 2015;36(1):31‐40. [] [PubMed] [Google Scholar]; 3262411
- Naeini AM, Elmadfa I, Djazayery A, Barekatain M, Ghazvini MR, Djalali M, et al. The effect of antioxidant vitamins E and C on cognitive performance of the elderly with mild cognitive impairment in Isfahan, Iran: a double‐blind, randomized, placebo‐controlled trial. European Journal of Nutrition 2014;53(5):1255‐62. [] [DOI] [PubMed] [Google Scholar]; 3262413
- Onofrj M, Thomas A, Luciano AL, Iacono D, Rollo A, D'Andreamatteo G, et al. Donepezil versus vitamin E in Alzheimer's disease: part 2: mild versus moderate‐severe Alzheimer's disease. Clinical Neuropharmacology 2002;25:207‐15. [] [DOI] [PubMed] [Google Scholar]; 3262415
- Pavlik VN, Doody RS, Rountree SD, Darby EJ. Vitamin E use is associated with improved survival in an Alzheimer's disease cohort. Dementia & Geriatric Cognitive Disorders 2009;28(6):536‐40. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 3262417
- Péneau S, Galan P, Jeandel C, Ferry M, Andreeva V, Hercberg S, et al. Fruit and vegetable intake and cognitive function in the SU.VI.MAX 2 prospective study. American Journal of Clinical Nutrition 2011;94(5):1295‐303. [] [DOI] [PubMed] [Google Scholar]; 3262419
- Podea D, Mila C. Therapeutically strategies and outcome in mild cognitive impairment. European Psychiatry 2012;27:1. [] 22153731 [Google Scholar]; 3262421
- Polidori MC, Spirt S, Stahl W, Pientka L. Conflict of evidence: carotenoids and other micronutrients in the prevention and treatment of cognitive impairment [Review]. BioFactors (Oxford, England) 2012;38(2):167‐71. [] [DOI] [PubMed] [Google Scholar]; 3262423
- Pribis P, Bailey RN, Russell AA, Kilsby MA, Hernandez M, Craig WJ, et al. Effects of walnut consumption on cognitive performance in young adults. British Journal of Nutrition 2012;107(9):1393‐401. [] [DOI] [PubMed] [Google Scholar]; 3262425
- Remington R, Chan A, Paskavitz J, Shea TB. Efficacy of a vitamin/nutriceutical formulation for moderate‐stage to later‐stage Alzheimer's disease: a placebo‐controlled pilot study. American Journal of Alzheimer's Disease and Other Dementias 2009;24(1):27‐33. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 3262427
- Remington R, Bechtel C, Larsen D, Samar A, Doshanjh L, Fishman P, et al. A phase II randomized clinical trial of a nutritional formulation for cognition and mood in Alzheimer's disease. Journal of Alzheimer's Disease 2015;45(2):395‐405. [] [DOI] [PubMed] [Google Scholar]; 3262429
- Rijpma A, Meulenbroek O, Hees AM, Sijben JW, Scheltens P, Olde Rikkert MG. Effects of a medical food on plasma micronutrient and fatty acid levels in mild to moderate Alzheimer's disease. Clinical Nutrition (Edinburgh, Scotland) 2014:S193. [] [Google Scholar]; 3262431
- Rijpma A, Meulenbroek O, Olde Rikkert MG. Cholinesterase inhibitors and add‐on nutritional supplements in Alzheimer's disease: a systematic review of randomized controlled trials. Ageing Research Reviews 2014;16:105‐12. [] [DOI] [PubMed] [Google Scholar]; 3262433
- Satalich TA, Shankle WR, Alexander GE, Batchelder WH. Markov models detect vitamin E and donepezil treatment effects in ADCS MCI trial. Alzheimer's & Dementia 2014:P776. [] [Google Scholar]; 3262435
- Schmitt FA, Kryscio RJ, Xu L, Mendiondo M, Caban‐Holt A, Abner E, et al. Effects of repeated screening and potential impact on AD prevention trial design: the antioxidant AD prevention (PREADVISE) trial. Alzheimer's and Dementia 2009;5 Suppl 1(4):258. [] [Google Scholar]; 3262437
- Schneider LS, Raman R, Schmitt FA, Doody RS, Insel P, Clark CM, et al. Characteristics and performance of a modified version of the ADCS‐CGIC CIBIC+ for mild cognitive impairment clinical trials. Alzheimer Disease & Associated Disorders 2009;23(3):260‐7. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 3262439
- Shea T, Remington R, Bechtel C, Sammar AM, Larsen D, Lortie JJ, et al. A nutritional formulation for cognitive performance and mood in Alzheimer's disease and mild cognitive impairment: a phase II multisite randomized trial with an open‐label extension. Alzheimer's & Dementia 2013:P658‐9. [] [Google Scholar]; 3262441
- Srour M, Alavi Naeini AM, Elmadfa I. Status of serum 25 (OH)‐vitamin d in elderly Iranian subjects after one year supplementation with vitamin E and C. Annals of Nutrition & Metabolism 2013:1506. [] [Google Scholar]; 3262443
- Stough C, Downey L, Silber B, Lloyd J, Kure C, Wesnes K, et al. The effects of 90‐day supplementation with the omega‐3 essential fatty acid docosahexaenoic acid (DHA) on cognitive function and visual acuity in a healthy aging population. Neurobiology of Aging 2012;33(4):e1‐3. [] [DOI] [PubMed] [Google Scholar]; 3262445
- Suominen MH, Puranen TM, Eloniemi‐Sulkava U, Jyvakorpi SK, Siljamaki‐Ojansuu U, Kautiainen H, et al. Effectiveness of a tailored nutrition intervention on nutrient intake and quality of life of aged persons with Alzheimer disease living at home with their spouses. A randomized, controlled trial. European Geriatric Medicine 2013:S96. [] [Google Scholar]; 3262447
- Takahashi T, Murata T, Kosaka H, Wada Y, Yoneda M. Effect of vitamin E treatment on progressive cognitive impairment in a patient with adult‐onset ataxia: a case report. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 2009;33(1):150‐2. [] [DOI] [PubMed] [Google Scholar]; 3262449
- Usoro OB, Mousa SA. Vitamin E forms in Alzheimer's disease: a review of controversial and clinical experiences. Critical Reviews in Food Science & Nutrition 2010;50(5):414‐9. [] [DOI] [PubMed] [Google Scholar]; 3262451
- Vinyoles‐Bargallo E. During the first year of treatment, donepezil delays the incidence of Alzheimer's disease in patients with cognitive deterioration. Vitamin E is not efficient. FMC Formacion Medica Continuada en Atencion Primaria 2010;12(10):715. [] [Google Scholar]; 3262453
- Arnim CA, Dismar S, Ott‐Renzer CS, Noeth N, Ludolph AC, Biesalski HK. Micronutrients supplementation and nutritional status in cognitively impaired elderly persons: a two‐month open label pilot study. Nutrition Journal 2013;12(1):148. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 3262455
- Whitehair DC, Sherzai A, Emond J, Raman R, Aisen PS, Petersen RC, et al. Influence of apolipoprotein e 4 on rates of cognitive and functional decline in mild cognitive impairment. Alzheimer's and Dementia 2010;6(5):412‐9. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 3262457
- Zanotta D, Puricelli S, Bonoldi G. Cognitive effects of a dietary supplement made from extract of Bacopa monnieri, astaxanthin, phosphatidylserine, and vitamin E in subjects with mild cognitive impairment: a noncomparative, exploratory clinical study. Neuropsychiatric Disease and Treatment 2014;10:225‐30. [] [DOI] [PMC free article] [PubMed] [Google Scholar]; 3262459
References to ongoing studies
- Gopal V. A pilot study of effect of vitamin E on cognition and measures of activities of daily living in patients with moderately severe Alzheimer's disease. National Research Register2005. [] ; 3262461
Additional references
- Ahlskog JE, Uitti RJ, Low PA, Tyce GM, Nickander KK, Petersen RC, et al. No evidence for systemic oxidant stress in Parkinson's or Alzheimer's disease. Movement Disorders 1995;10:566‐73. [DOI] [PubMed] [Google Scholar]
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, et al. the Alzheimer's Disease Cooperative Study. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA 2003;289(21):2819‐26. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington DC: American Psychiatric Press, 1994. [Google Scholar]
- Behl C, Davis J, Cole GM, Schubert D. Vitamin E protects nerve cells from amyloid‐beta protein toxicity. Biochemical and Biophysical Research Communication 1992;186:944‐50. [DOI] [PubMed] [Google Scholar]
- Berg L. Clinical Dementia Rating (CDR). Psychopharmacology Bulletin 1988;24:637‐9. [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD007176] [DOI] [PubMed] [Google Scholar]
- Blessed B, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral gray matter of elderly subjects. British Journal of Psychiatry 1968;114:797‐811. [DOI] [PubMed] [Google Scholar]
- Boccardi V, Baroni M, Mangialasche F, Mecocci P. Vitamin E family: role in the pathogenesis and treatment of Alzheimer's disease. Alzheimer's & Dementia: Translational Research & Clinical Interventions 2016;2(3):182‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothby LA, Doering PL. Vitamin C and vitamin E for Alzheimer's disease. Annals of Pharmacotherapy 2005;39:2073‐9. [PMID: 16227450] [DOI] [PubMed] [Google Scholar]
- Brigelius‐Flohé R. Adverse effects of vitamin E by induction of drug metabolism. Genes & Nutrition 2007;2(3):249‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruscoli M, Lovestone S. Is MCI really just early dementia? A systematic review of conversion studies. International Psychogeriatrics 2004;16(2):129‐40. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid beta‐peptide‐induced lipid peroxidation and its sequelae in Alzheimer's disease brain contribute to neuronal death. Neurobiology of Aging 2002;23(5):655‐64. [PMID: 12392766] [DOI] [PubMed] [Google Scholar]
- Chalmers TC, Celano P, Sacks HS, Smith H Jr. Bias in treatment assignment in controlled clinical trials. New England Journal of Medicine 1983;309:1358‐61. [DOI] [PubMed] [Google Scholar]
- Cherubini A, Martin A, Andres‐Lacueva C, Iorio A, Lamponi M, Mecocci P, et al. Vitamin E levels, cognitive impairment and dementia in older persons: the InCHIANTI study. Neurobiology of Aging 2005;26(7):987‐94. [DOI: 10.1016/j.neurobiolaging.2004.09.002] [DOI] [PubMed] [Google Scholar]
- Crichton GE, Bryan J, Murphy, KJ. Dietary antioxidants, cognitive function and dementia ‐ a systematic review. Plant Foods for Human Nutrition 2013;68:279‐92. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory comprehensive assessment of psychopathology in dementia. Neurology 1994;44(12):2308. [DOI] [PubMed] [Google Scholar]
- Davis KL, Marin DB, Kane R, Patrick D, Peskind ER, Raskind MA, et al. The Caregiver Activity Survey (CAS): development and validation of a new measure for caregivers of persons with Alzheimer's disease. International Journal of Geriatric Psychiatry 1997;12(10):978‐88. [DOI] [PubMed] [Google Scholar]
- Engelhart MJ, Geerlings MI, Ruitenberg A, Swieten JC, Hofman A, Witteman JC, et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA 2002;287(24):3223‐9. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. 'Mini‐Mental State': a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189‐98. [DOI] [PubMed] [Google Scholar]
- Galasko D, Bennet D, Sano M, Marson D, Kaye J, Edland SD. Alzheimer's Disease Cooperative Study. ADCS Prevention Instrument Project: assessment of instrumental activities of daily living for community‐dwelling elderly individuals in dementia prevention clinical trials. Alzheimer Disease and Associated Disorders 2006;20:s152‐69. [DOI: 10.1097/01.wad.0000213873.25053.2b] [DOI] [PubMed] [Google Scholar]
- Ganguli M, Snitz BE, Saxton JA, Chang C‐CH, Lee C‐W, Vander Blit J, et al. Outcomes of mild cognitive impairment by definition. Archives of Neurology 2011;68(6):761‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundman M. Vitamin E and Alzheimer disease: the basis for additional clinical trials. American Journal of Clinical Nutrition 2000;71 Suppl:630S‐6S. [DOI] [PubMed] [Google Scholar]
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64:383‐94. [DOI] [PubMed] [Google Scholar]
- Hara H, Kato H, Kogure K. Protective effect of alpha‐tocopherol on ischemic neuronal damage in the gerbil hippocampus. Brain Research 1990;510:335‐8. [DOI] [PubMed] [Google Scholar]
- Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M, et al. Brain regional correspondence between Alzheimer's disease histopathology and biomarkers of protein oxidation. Journal of Neurochemistry 1995;65:2146‐56. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Hsiao KK, Borchelt DR, Olson K, Johannsdottir R, Kitt C, Yunis W, et al. Age‐related CNS disorder and early death in transgenic FVB/N mice overexpressing Alzheimer amyloid precursor proteins. Neuron 1995;15:1203‐18. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The ICD‐10 Classification of Mental and Behavioural Disorders: diagnostic criteria for research, 1993. www.who.int/entity/classifications/icd/en/GRNBOOK.pdf (accessed 10 October 2012).
- Jeandel C, Nicolas MB, Dubois F, Nabet‐Belleville F, Penin F, Cuny G. Lipid peroxidation and free radical scavengers in Alzheimer's disease. Gerontology 1989;35:275‐82. [DOI] [PubMed] [Google Scholar]
- Jimenez‐Jimenez FJ, Bustos F, Molina JA, Benito‐León J, Tallón‐Barranco A, Gasalla T, et al. Cerebrospinal fluid levels of alpha‐tocopherol (vitamin E) in Alzheimer's disease. Journal of Neural Transmission 1997;104:703‐10. [DOI] [PubMed] [Google Scholar]
- Klein EA, Thompson IM, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, et al. Vitamin E and the risk of prostate cancer. JAMA 2011;306(14):1549‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppal T, Subramaniam R, Drake J, Prasad MR, Dhillon H, Butterfield DA. Vitamin E protects against Alzheimer's amyloid peptide (25‐35)‐induced changes in neocortical synaptosomal membrane lipid structure and composition. Brain Research 1998;786(1‐2):270‐3. [DOI] [PubMed] [Google Scholar]
- Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, et al. Effects of long‐term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA 2005;293:1338‐47. [DOI] [PubMed] [Google Scholar]
- Mangialasche F, Kivipelto M, Mecocci P, Rizzuto D, Palmer K, Winblad B, et al. High plasma levels of vitamin E forms and reduced Alzheimer's disease risk in advanced age. Journal of Alzheimer's Disease 2010;20(4):1029‐37. [DOI] [PubMed] [Google Scholar]
- Mangialasche F, Xu W, Kivipelto M, Costanzi E, Ercolani S, Pigliautile M, et al. Tocopherols and tocotrienols plasma levels are associated with cognitive impairment. Neurobiology of Aging 2012;33(10):2282‐90. [DOI] [PubMed] [Google Scholar]
- Marcus DL, Thomas C, Rodriguez C, Simberkoff K, Tsai JS, Strafaci JA, et al. Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer's disease. Experimental Neurology 1998;150:40‐4. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services task Force on Alzheimer's Disease. Neurology 1984;34:939‐44. [DOI] [PubMed] [Google Scholar]
- Mecocci P, MacGarvey U, Beal MF. Oxidative damage to mitochondrial DNA is increased in Alzheimer's disease. Annals of Neurology 1994;36:747‐51. [DOI] [PubMed] [Google Scholar]
- Mecocci P. Oxidative stress in mild cognitive impairment and Alzheimer's disease: a continuum. Journal of Alzheimer's Disease 2004;6:159‐63. [PMID: 15096699] [DOI] [PubMed] [Google Scholar]
- Miller ER, Pastor‐Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta‐analysis: high‐dosage vitamin E supplementation may increase all‐cause mortality. Annals of Internal Medicine 2005;142:37‐46. [DOI] [PubMed] [Google Scholar]
- Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, et al. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA 2002;287(24):3230‐7. [DOI] [PubMed] [Google Scholar]
- Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS, Aggarwal NT, et al. Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. American Journal of Clinical Nutrition 2005;81(2):508‐14. [DOI] [PubMed] [Google Scholar]
- National Academy of Sciences. Institute of Medicine. Food and Nutrition Board. Vitamin E. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington DC: National Academy Press, 2000:186‐283. [Google Scholar]
- The Parkinson's Study Group. Effects of tocopherol and deprenyl on the progression of disability in early Parkinson's disease. New England Journal of Medicine 1993;328:176‐83. [DOI] [PubMed] [Google Scholar]
- Perrig WJ, Perrig P, Stahelin HB. The relation between antioxidants and memory performance in the old and very old. Journal of American Geriatric Society 1997;45:718‐24. [DOI] [PubMed] [Google Scholar]
- Petersen CR. Mild cognitive impairment. New England Journal of Medicine 2011;364(23):2227‐34. [DOI] [PubMed] [Google Scholar]
- Peyser CE, Folstein M, Chase GA, Starkstein S, Brandt J, Cockrell JR, et al. Trial of d‐alpha‐tocopherol in Huntington's disease. American Journal of Psychiatry 1995;152:1771‐5. [DOI] [PubMed] [Google Scholar]
- Pham DQ, Plakogiannis R. Vitamin E supplementation in Alzheimer's disease, Parkinson's disease, tardive dyskinesia and cataract: part 2. Annals of Pharmacotherapy 2005;39(12):2065‐72. [PMID: 16288072] [DOI] [PubMed] [Google Scholar]
- Polidori MC, Mecocci P. Plasma susceptibility to free radical‐induced antioxidant consumption and lipid peroxidation is increased in very old subjects with Alzheimer disease. Journal of Alzheimers Disease 2002;4(6):517‐22. [PMID: 12629261] [DOI] [PubMed] [Google Scholar]
- Prince M, Wimo A, Guerchet M, Ali G‐C, Wu Y‐T, Prina M, Alzheimer's Disease International. World Alzheimer Report 2015. London: Alzheimer's Disease International, 2015. [Google Scholar]
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. American Journal of Psychiatry 1984;141:1356‐64. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
- Schürks M, Glynn RJ, Rist PM, Tzourio C, Kurth T. Effects of vitamin E on stroke subtypes: meta‐analysis of randomised controlled trials. BMJ 2010;341:c5702. [DOI: 10.1136/bmj.c5702] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socci DJ, Crandall BM, Arendash GW. Chronic antioxidant treatment improves the cognitive performance of aged rats. Brain Research 1995;693:88‐94. [DOI] [PubMed] [Google Scholar]
- Steiner M, Glantz M, Lekos A. Vitamin E plus aspirin compared with aspirin alone in patients with transient ischemic attacks. American Journal of Clinical Nutrition 1995;62:1381S‐4S. [DOI] [PubMed] [Google Scholar]
- Stern Y, Albert SM, Sano M, Richards M, Miller L, Folstein M, et al. Assessing patient dependence in Alzheimer's disease. Journal of Gerontology 1994;49(5):M216‐22. [DOI] [PubMed] [Google Scholar]
- Sunderland T, Hill JL, Mellow AM, Lawlor BA, Gundersheimer J, Newhouse PA, et al. Clock drawing in Alzheimer's disease. A novel measure of dementia severity. Journal of the American Geriatrics Society 1989;37:725‐9. [DOI] [PubMed] [Google Scholar]
- Tabet N, Mantle D, Walker Z, Orrel M. Vitamins, trace elements, and antioxidant status in dementia disorders. International Psychogeriatrics 2001;13(3):265‐75. [DOI] [PubMed] [Google Scholar]
- Tabet N, Mantle D, Walker Z, Orrell M. Endogenous antioxidant activities in relation to concurrent vitamins A, C, and E intake in dementia. International Psychogeriatrics 2002;12(1):7‐15. [DOI] [PubMed] [Google Scholar]
- Tariot PN, Mack JL, Patterson MB, Edland SD, Weiner MF, Fillenbaum G, et al. The Behavior Rating Scale for Dementia Consortium to establish a registry for Alzheimer's disease. American Journal of Psychiatry 1995;152:1349‐57. [DOI] [PubMed] [Google Scholar]
- Tohgi H, Abe T, Nakanishi M, Hamato F, Sasaki K, Takahashi S. Concentrations of alpha‐tocopherol and its quinone derivative in cerebrospinal fluid from patients with vascular dementia of the Binswanger type and Alzheimer type dementia. Neuroscience Letters 1994;174:73‐6. [DOI] [PubMed] [Google Scholar]
- Traber MG, Stevens, JF. Vitamins C and E: beneficial effects from mechanistic perspective. Free Radical Biology & Medicine 2011;51:1000‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uneri C, Sari M, Akboga J, Yuksel M. Vitamin E‐coated tympanostomy tube insertion decrease the quantity of free radicals in tympanic membrane. Laryngoscope 2006;116(1):140‐3. [PMID: 16481827] [DOI] [PubMed] [Google Scholar]
- Vatassery GT, Brin MF, Fahn S, Kayden HJ, Traber MG. Effect of high doses of dietary vitamin E on the concentrations of vitamin E in several brain regions, plasma, liver, and adipose tissue of rats. Journal of Neurochemistry 1988;51:621‐3. [DOI] [PubMed] [Google Scholar]
- Wang J, Xiong S, Xie C, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer's disease. Journal of Neurochemistry 2005;93(4):953‐62. [PMID: 15857398] [DOI] [PubMed] [Google Scholar]
- Wang J, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in mild cognitive impairment. Journal of Neurochemistry 2006;96(3):825‐32. [PMID: 16405502] [DOI] [PubMed] [Google Scholar]
- Wortwein G, Stackman RW, Walsh TJ. Vitamin E prevents the place learning deficit and the cholinergic hypofunction induced by AF64A. Experimental Neurology 1994;125:15‐21. [DOI] [PubMed] [Google Scholar]
- Zaman Z, Roche S, Fielden P, Frost PG, Niriella DC, Cayley AC. Plasma concentrations of vitamin A and E and Carotenoids in Alzheimer's disease. Age and Ageing 1992;21:91‐4. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Gopalakrishanan V, Richardson JS. Actions of neurotoxic beta‐amyloid on calcium homeostasis and viability of PC12 cells are blocked by antioxidants but not by calcium channel antagonists. Journal of Neurochemistry 1996;67:1419‐25. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Farina N, Isaac MGEKN, Clark AR, Rusted J, Tabet N. Vitamin E for Alzheimer's dementia and mild cognitive impairment. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD002854.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac MGEKN, Quinn R, Tabet N. Vitamin E for Alzheimer's disease and mild cognitive impairment. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002854] [DOI] [PubMed] [Google Scholar]
- Isaac MGEKN, Quinn R, Taber N. Vitamin E for Alzheimer’s disease and mild cognitive impairment. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD002854.pub2] [DOI] [PubMed] [Google Scholar]


