Abstract
Background
Prelabour rupture of membranes (PROM) at term is managed expectantly or by planned early birth. It is not clear if waiting for birth to occur spontaneously is better than intervening, e.g. by inducing labour.
Objectives
The objective of this review is to assess the effects of planned early birth (immediate intervention or intervention within 24 hours) when compared with expectant management (no planned intervention within 24 hours) for women with term PROM on maternal, fetal and neonatal outcomes.
Search methods
We searched Cochrane Pregnancy and Childbirth's Trials Register (9 September 2016) and reference lists of retrieved studies.
Selection criteria
Randomised or quasi‐randomised controlled trials of planned early birth compared with expectant management (either in hospital or at home) in women with PROM at 37 weeks' gestation or later.
Data collection and analysis
Two review authors independently assessed trials for inclusion, extracted the data, and assessed risk of bias of the included studies. Data were checked for accuracy.
Main results
Twenty‐three trials involving 8615 women and their babies were included in the update of this review. Ten trials assessed intravenous oxytocin; 12 trials assessed prostaglandins (six trials in the form of vaginal prostaglandin E2 and six as oral, sublingual or vaginal misoprostol); and one trial each assessed Caulophyllum and acupuncture. Overall, three trials were judged to be at low risk of bias, while the other 20 were at unclear or high risk of bias.
Primary outcomes: women who had planned early birth were at a reduced risk of maternal infectious morbidity (chorioamnionitis and/or endometritis) than women who had expectant management following term prelabour rupture of membranes (average risk ratio (RR) 0.49; 95% confidence interval (CI) 0.33 to 0.72; eight trials, 6864 women; Tau² = 0.19; I² = 72%; low‐quality evidence), and their neonates were less likely to have definite or probable early‐onset neonatal sepsis (RR 0.73; 95% CI 0.58 to 0.92; 16 trials, 7314 infants;low‐quality evidence). No clear differences between the planned early birth and expectant management groups were seen for the risk of caesarean section (average RR 0.84; 95% CI 0.69 to 1.04; 23 trials, 8576 women; Tau² = 0.10; I² = 55%; low‐quality evidence); serious maternal morbidity or mortality (no events; three trials; 425 women; very low‐quality evidence); definite early‐onset neonatal sepsis (RR 0.57; 95% CI 0.24 to 1.33; six trials, 1303 infants; very low‐quality evidence); or perinatal mortality (RR 0.47; 95% CI 0.13 to 1.66; eight trials, 6392 infants; moderate‐quality evidence).
Secondary outcomes: women who had a planned early birth were at a reduced risk of chorioamnionitis (average RR 0.55; 95% CI 0.37 to 0.82; eight trials, 6874 women; Tau² = 0.19; I² = 73%), and postpartum septicaemia (RR 0.26; 95% CI 0.07 to 0.96; three trials, 263 women), and their neonates were less likely to receive antibiotics (average RR 0.61; 95% CI 0.44 to 0.84; 10 trials, 6427 infants; Tau² = 0.06; I² = 32%). Women in the planned early birth group were more likely to have their labour induced (average RR 3.41; 95% CI 2.87 to 4.06; 12 trials, 6945 women; Tau² = 0.05; I² = 71%), had a shorter time from rupture of membranes to birth (mean difference (MD) ‐10.10 hours; 95% CI ‐12.15 to ‐8.06; nine trials, 1484 women; Tau² = 5.81; I² = 60%), and their neonates had lower birthweights (MD ‐79.25 g; 95% CI ‐124.96 to ‐33.55; five trials, 1043 infants). Women who had a planned early birth had a shorter length of hospitalisation (MD ‐0.79 days; 95% CI ‐1.20 to ‐0.38; two trials, 748 women; Tau² = 0.05; I² = 59%), and their neonates were less likely to be admitted to the neonatal special or intensive care unit (RR 0.75; 95% CI 0.66 to 0.85; eight trials, 6179 infants), and had a shorter duration of hospital (‐11.00 hours; 95% CI ‐21.96 to ‐0.04; one trial, 182 infants) or special or intensive care unit stay (RR 0.72; 95% CI 0.61 to 0.85; four trials, 5691 infants). Women in the planned early birth group had more positive experiences compared with women in the expectant management group.
No clear differences between groups were observed for endometritis; postpartum pyrexia; postpartum antibiotic usage; caesarean for fetal distress; operative vaginal birth; uterine rupture; epidural analgesia; postpartum haemorrhage; adverse effects; cord prolapse; stillbirth; neonatal mortality; pneumonia; Apgar score less than seven at five minutes; use of mechanical ventilation; or abnormality on cerebral ultrasound (no events).
None of the trials reported on breastfeeding; postnatal depression; gestational age at birth; meningitis; respiratory distress syndrome; necrotising enterocolitis; neonatal encephalopathy; or disability at childhood follow‐up.
In subgroup analyses, there were no clear patterns of differential effects for method of induction, parity, use of maternal antibiotic prophylaxis, or digital vaginal examination. Results of the sensitivity analyses based on trial quality were consistent with those of the main analysis, except for definite or probable early‐onset neonatal sepsis where no clear difference was observed.
Authors' conclusions
There is low quality evidence to suggest that planned early birth (with induction methods such as oxytocin or prostaglandins) reduces the risk of maternal infectious morbidity compared with expectant management for PROM at 37 weeks' gestation or later, without an apparent increased risk of caesarean section. Evidence was mainly downgraded due to the majority of studies contributing data having some serious design limitations, and for most outcomes estimates were imprecise.
Although the 23 included trials in this review involved a large number of women and babies, the quality of the trials and evidence was not high overall, and there was limited reporting for a number of important outcomes. Thus further evidence assessing the benefits or harms of planned early birth compared with expectant management, considering maternal, fetal, neonatal and longer‐term childhood outcomes, and the use of health services, would be valuable. Any future trials should be adequately designed and powered to evaluate the effects on short‐ and long‐term outcomes. Standardisation of outcomes and their definitions, including for the assessment of maternal and neonatal infection, would be beneficial.
Keywords: Female; Humans; Pregnancy; Fetal Membranes, Premature Rupture; Term Birth; Watchful Waiting; Cesarean Section; Cesarean Section/statistics & numerical data; Labor, Induced; Labor, Induced/methods; Misoprostol; Misoprostol/administration & dosage; Obstetric Labor Complications; Oxytocics; Oxytocics/administration & dosage; Oxytocin; Oxytocin/administration & dosage; Pregnancy Outcome; Prostaglandins; Prostaglandins/administration & dosage; Randomized Controlled Trials as Topic; Time Factors
Plain language summary
Is it better for a baby to be born immediately or to wait for labour to start spontaneously when waters break at or after 37 weeks?
What is the issue?
If a pregnant woman's waters break without onset of contractions (prelabour rupture of membranes – PROM) at 37 weeks of pregnancy or more, there are two options: the first is for induction of labour so that the baby is born as soon as possible (planned early birth); or secondly, to wait for labour to start naturally (expectant management).
Why is this important?
In a previous version of this review we found that planned early birth may reduce the risk of maternal infection without increasing the risk of caesarean section, compared with waiting. Fewer infants went to the neonatal intensive care unit with planned early birth, though there were no differences seen in rates of neonatal infection. While there are some benefits of early induction of labour, it is important to have a more complete picture of what happens with planned early birth compared with waiting for labour to start naturally.
What evidence did we find?
This review included data from 23 randomised controlled trials involving 8615 pregnant women at 37 weeks of pregnancy or more. Only three trials were at overall low risk of bias, and the evidence in the review was very low to moderate quality. For planned early birth, 10 trials used intravenous oxytocin for induction of labour, 12 trials used prostaglandins, and one trial each assessed Caulophyllum and acupuncture.
The findings showed that planned early birth for PROM at term reduced the risk of infection for pregnant women (including infection of the membranes surrounding the baby and the amniotic fluid (known as chorioamnionitis)) compared with expectant management (eight trials, 6864 women; this was rated low‐quality evidence), Planned early birth also reduced the risk of definite or possible infections for the babies (16 trials, 7314 babies, low‐quality evidence). However, no differences were seen in the rates of caesarean births (23 trials, 8576 women, low‐quality evidence), serious illness or death for the women (three trials, 425 women, very low‐quality evidence), definite infection for the babies (six trials, 1303 babies, very low‐quality evidence), or death for the babies (eight trials, 6392 babies, moderate‐quality evidence). Babies born after planned early birth were less likely to be admitted to the intensive care unit (eight trials, 6179 babies), and both women (two trials, 748 women) and their babies (four trials, 5691 babies) had a shorter stay in hospital after planned early birth. Women had a more positive experience of planned early birth compared with expectant management (two trials, 5134 women).
What does this mean?
Planned early birth (compared with expectant management) after PROM at term may help to reduce infection for women without increasing the need for a caesarean section, and neonatal infection may also be reduced. However, evidence about longer‐term effects on children is needed.
Summary of findings
Summary of findings for the main comparison. Planned early birth versus expectant management for prelabour rupture of the membranes at term.
| Planned early birth versus expectant management for prelabour rupture of the membranes at term | ||||||
| Patient or population: women with prelabour rupture of membranes at term (37 weeks' gestation or later) Setting: hospital settings Intervention: planned early birth Comparison: expectant management | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with expectant management | Risk with planned early birth | |||||
| Maternal infectious morbidity (chorioamnionitis and/or endometritis) | Study population | average RR 0.49 (0.33 to 0.72) | 6864 (8 RCTs) | ⊕⊕⊝⊝ LOW1,2 | ||
| 110 per 1000 | 54 per 1000 (36 to 79) | |||||
| Caesarean section | Study population | average RR 0.84 (0.69 to 1.04) | 8576 (23 RCTs) | ⊕⊕⊝⊝ LOW1,3 | ||
| 150 per 1000 | 126 per 1000 (104 to 156) | |||||
| Serious maternal morbidity or mortality (e.g. death, cardiac arrest, respiratory arrest, admission to intensive care unit) | Study population | Not estimable | 425 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW4,5 | No events | |
| no events | no events | |||||
| Definite early‐onset neonatal sepsis | Study population | RR 0.57 (0.24 to 1.33) | 1303 (6 RCTs) | ⊕⊝⊝⊝ VERY LOW5,6 | ||
| 22 per 1000 | 12 per 1000 (5 to 29) | |||||
| Definite or probable early‐onset neonatal sepsis | Study population | RR 0.73 (0.58 to 0.92) | 7314 (16 RCTs) | ⊕⊕⊝⊝ LOW1,7 | ||
| 41 per 1000 | 30 per 1000 (24 to 38) | |||||
| Perinatal mortality (stillbirth or neonatal mortality) | Study population | RR 0.47 (0.13 to 1.66) | 6392 (8 RCTs) | ⊕⊕⊕⊝ MODERATE3 | ||
| 2 per 1000 | 1 per 1000 (0 to 4) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Study limitations (‐1): Most of the studies contributing data had design limitations, dominated by a study with low risk of bias
2 Inconsistency (‐1): Substantial heterogeneity
3 Imprecision (‐1): Wide 95% CI crossing the line of no effect
4 Imprecision (‐2): No events; three trials with relatively small sample sizes
5 Study limitations (‐2): Most of the studies contributing data had serious design limitations (‐2)
6 Imprecision (‐2): Low event rate and wide 95% CI crossing the line of no effect
7 Indirectness (‐1): Substantial variation in outcome measurement and reporting of neonatal sepsis
Background
Description of the condition
Prelabour rupture of membranes (PROM) is defined as rupture of membranes (ROM) prior to the onset of labour (Duff 1998). PROM most frequently occurs at term (37 weeks or more of gestation) (Duff 1998), with the overall incidence of PROM at term being approximately 8% (Cammu 1990). Spontaneous onset of labour after term PROM usually follows within 24 hours (Cammu 1990), with 79% of women labouring spontaneously within 12 hours, and 95% within 24 hours (Conway 1984; Zlatnik 1992). Even when the state of the cervix is unfavourable, the majority of women labour spontaneously within 24 hours (Hannah 1998). However, if the woman does not labour within 24 hours, labour may be delayed up to seven days after membrane rupture (Hannah 1998), with longer latent periods in nulliparous women (Zlatnik 1992).
PROM at term is known to be associated with overdistension of the uterus due to multiple pregnancy or polyhydramnios (abnormally high levels of amniotic fluid), cigarette smoking, altered mechanical properties of the amniotic membranes, frequent digital examinations, coitus and infection (Duff 1998; Hannah 1998), although it is not clear if these are causally related to PROM (Hannah 1998).
Description of the intervention
PROM at term may be managed expectantly or by elective birth, usually by induction of labour. Planned elective early birth is usually termed active or planned early birth. Expectant management involves waiting for labour to occur and then making management decisions (such as inducing labour) if labour does not happen spontaneously after a specified period.
PROM may result in immediate risks such as cord prolapse, cord compression and placental abruption; and later problems including maternal or neonatal infection, as well as the use of interventions such as caesarean section and instrumental vaginal birth (Alexander 1996; Kong 1992; Merenstein 1996). Expectant management of term PROM has been associated with maternal infections including chorioamnionitis (inflammation of the membranes) or endometritis (generally a postpartum infection). These infections may result in neonatal infection and mortality, chronic lung disease and cerebral palsy (Cammu 1990;Gonen 1989;Merenstein 1996; Robson 1990; Zlatnik 1992), as well as serious morbidity for the mother. Some reports have suggested that the risk of maternal and fetal infection increases proportionally with the time between membrane rupture and birth (Gafni 1997; Zlatnik 1992), while others refute this (Hannah 1998; Seaward 1997). Whether or not to induce labour may depend on the state of the cervix, with an insufficiently ripe cervix resulting in increased length of labour and failed induction requiring caesarean section (Cammu 1990; Duff 1996; Duff 1998; Yawn 2001). Uterine rupture has been reported, but only rarely.
How the intervention might work
There are conflicting conclusions from literature reviews assessing PROM at term. Hallak 1999 found that with a longer interval from admission to the onset of labour, there is an increased incidence of neonatal intensive care unit admission, caesarean section and more frequent maternal diarrhoea and use of analgesia or anaesthesia. Induction of labour is supported by a retrospective study (Johnson 1981), which reported increased perinatal mortality and intrapartum fever in women at term when there was delay of more than 72 hours between PROM and birth. Oxytocin infusion was recommended as the gold standard management of PROM at term in a review (Crane 2003). These results are in contrast to the findings of Guise 1992, who reported that induction of labour results in increased frequency of chorioamnionitis, neonatal sepsis, caesarean section and longer duration of hospitalisation. Mozurkewich 1997 highlighted the risks and benefits of induction of labour, with reduced rates of chorioamnionitis, endometritis and neonatal infection, and an increased rate of caesarean section. Induction of labour for women with PROM at term may incur fewer costs than expectant management (Gafni 1997). Women may be more satisfied with care when there is a short time between PROM and birth (Hannah 1999).
Why it is important to do this review
This review updates a previously published Cochrane review on planned early birth versus expectant management (waiting) for PROM at term (Dare 2006), which included 12 randomised controlled trials, and found that planned early birth may reduce the risk of maternal infectious morbidity without increasing the risk of caesarean section or operative vaginal birth (Dare 2006). The review found that fewer infants went to the neonatal intensive care unit with planned early birth, though no clear differences were seen in neonatal infection rates (Dare 2006). It was concluded that while there may be some benefits of planned early birth, since the differences in outcomes may not be substantial, women need to be able to access the appropriate information to make an informed choice, and further research is required to assess outcomes such as maternal satisfaction, maternal and neonatal infectious morbidity and longer‐term child development/disability (Dare 2006).
Another Cochrane review has evaluated the management of women with preterm PROM between 24 and 37 weeks' gestation and found insufficient evidence to guide clinical practice, with methodological weaknesses in the clinical trials conducted to date (Buchanan 2010). Our review focuses on women with PROM at term (37 weeks' gestation or later).
Objectives
The objective of this review is to assess the effects of planned early birth (immediate intervention or intervention within 24 hours) when compared with expectant management (no planned intervention within 24 hours) for women with term PROM on maternal, fetal and neonatal outcomes.
Methods
Criteria for considering studies for this review
Types of studies
All randomised and quasi‐randomised trials that compared planned early birth with expectant management at term. We planned to exclude studies using a cross‐over design, and planned to include cluster‐randomised trials and trials only reported as abstracts.
Types of participants
Women with PROM of at least 37 weeks' gestation with no specific maternal or fetal contraindications to expectant management.
Types of interventions
Planned early birth was compared with expectant management (either in hospital or at home).
For an intervention to be considered 'planned early birth', a decision must have been made to expedite birth after PROM through some form of induction of labour or by caesarean section. The planned intervention must have been implemented (or was intended to be implemented) within 24 hours of randomisation.
Conversely, 'expectant management' must have been associated with an intended delay of at least 24 hours.
Types of outcome measures
We aimed to examine the effect of planned early birth or expectant management on clinically important outcome measures of maternal and infant morbidity and mortality. We also explored health service utilisation.
Primary outcomes
Primary outcomes were chosen to be most representative of the clinically important measures of effectiveness and complications. We assessed the primary outcome of maternal infectious morbidity (chorioamnionitis and/or endometritis), and explored its individual components as secondary outcomes. Primary outcomes were as follows.
For the mother
Maternal infectious morbidity (chorioamnionitis and/or endometritis).
Caesarean section.
Serious maternal morbidity or mortality (e.g. death, cardiac arrest, respiratory arrest, admission to intensive care unit).
For the fetus/neonate
Definite early‐onset neonatal sepsis.
Definite or probable early‐onset neonatal sepsis.
Perinatal mortality (stillbirth or neonatal mortality).
Secondary outcomes
Secondary outcomes included other measures of morbidity and mortality, effectiveness, complications, and health service utilisation.
For the mother
Chorioamnionitis (either suspected or proven).
Endometritis.
Postpartum pyrexia.
Postpartum septicaemia.
Postpartum antibiotic usage.
Caesarean section for fetal distress.
Induction of labour.
Operative vaginal birth.
Uterine rupture.
Epidural analgesia.
Postpartum haemorrhage.
Adverse effects.
Views of care.
Breastfeeding, including initiated in hospital and on discharge from hospital.
Postnatal depression.
For the fetus/neonate/child
Time from rupture of membranes to birth.
Gestational age at birth.
Birthweight.
Cord prolapse.
Stillbirth.
Neonatal mortality.
Meningitis.
Pneumonia.
Antibiotic usage.
Apgar score less than seven at five minutes.
Respiratory distress syndrome.
Use of mechanical ventilation.
Abnormality on cerebral ultrasound (cystic periventricular leukomalacia; intraventricular haemorrhage).
Necrotising enterocolitis.
Neonatal encephalopathy.
Disability at childhood follow‐up.
Health services
Duration of maternal antenatal or postnatal stay in hospital.
Admission to neonatal special or intensive care unit.
Duration of neonatal stay in hospital.
Duration of neonatal stay in special or intensive care unit.
Outcome definitions
Where possible, we aimed to employ the below definitions; however acknowledging the likelihood of variable reporting by the included trials, we also included definitions as per the trialists themselves (and have reported these in the results).
Suspected or proven chorioamnionitis: uterine infection prior to birth of the baby diagnosed on clinical signs, including pyrexia, with or without a positive culture result or haematological signs of infection.
Endometritis: clinical signs of uterine infection following labour and birth.
Postpartum pyrexia: maternal temperature of 38°C or higher.
Postpartum septicaemia: maternal positive blood culture in the presence of pyrexia following birth.
-
Definite or probable infection within the first seven days of life/early‐onset sepsis.
Definite infection: positive culture from a normally sterile site.
Probable infection: clinical signs and blood count suggestive of infection and a possible causative organism identified (i.e. gastric aspirate, urine antigen).
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (9 September 2016).
The Register is a database containing over 22,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
[For details of additional searching carried in out an earlier version of the review (Dare 2006), see: Appendix 1.]
Searching other resources
We searched reference lists of retrieved articles.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeDare 2006.
For this update, the following methods were used for assessing the 14 new reports (and the nine new included studies) that were identified as a result of the updated search. Where required, information pertaining to the previously included 12 studies was updated according to methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
At least two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we contacted authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the study authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review were reported);
high risk of bias (where not all the study’s pre‐specified outcomes were reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (6) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
For this update, we evaluated the quality of the evidence using the GRADE approach as outlined in the GRADE handbook. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for specific outcomes. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, inconsistency, imprecision of effect estimates or publication bias. In this review we used the GRADE approach to assess the primary outcomes, as follows.
For the mother
Maternal infectious morbidity (chorioamnionitis and/or endometritis).
Caesarean section.
Serious maternal morbidity or mortality (e.g. death, cardiac arrest, respiratory arrest, admission to intensive care unit).
For the fetus/neonate
Definite early‐onset neonatal sepsis.
Definite or probable early‐onset neonatal sepsis.
Perinatal mortality (stillbirth or neonatal mortality).
'Summary of findings' table
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create a 'Summary of findings’ table. A summary of the intervention effect and a measure of quality according to the GRADE approach is presented in the 'Summary of findings' table for the primary outcomes.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials for inclusion in this review.
If cluster‐randomised trials are included in future updates of this review, we plan to include these trials in the analyses along with individually‐randomised trials. Their sample sizes will be adjusted using the methods described in the Handbook (Higgins 2011) using an estimate of the intra‐cluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources are used, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We plan to also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation units.
Other unit of analysis issues
Cross‐over trials
Trials with cross‐over designs were not eligible for inclusion.
Multiple pregnancies
We did not identify any eligible studies that included multiple pregnancies. If multiple pregnancies are included in trials included in future updates of this review, we will adjust for clustering in the analyses wherever possible, and use the inverse variance method for adjusted analyses, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and in Yelland 2011.
Multi‐armed trials
We included trials with more than one treatment groups (e.g. multi‐arm studies). Where appropriate, we created a single pair‐wise comparison. We used methods described in the Handbook (Higgins 2011) to ensure that we did not double count participants.
Dealing with missing data
For included studies, levels of attrition were noted. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analyses.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Where we identified substantial heterogeneity (above 30%), we explored it using pre‐specified subgroup analyses.
Assessment of reporting biases
Where there were 10 or more studies in a meta‐analysis we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we planned to perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where studies were examining the same intervention, and the studies' populations and methods were judged sufficiently similar.
Where there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or where substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary has been treated as the average range of possible treatment effects and we have discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we planned to not combine trials. Where we used random‐effects analyses, the results have been presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Where we identified substantial heterogeneity, we investigated it using subgroup and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it.
We carried out the following subgroup analyses.
Method of induction of labour (e.g. intravenous oxytocin versus vaginal prostaglandin).
Parity (e.g. multiparous women versus nulliparous women).
Cervical status at baseline (e.g. women with an unfavourable cervix versus women with a favourable cervix).
Maternal antibiotic prophylaxis for PROM (e.g. antibiotic prophylaxis versus no antibiotic prophylaxis).
Digital vaginal examination at baseline (e.g. women who had digital vaginal examination versus women who did not have vaginal examination).
The rationale for these subgroup analyses was as follows.
(1) Method of induction of labour ‐ some studies have found differences between various methods (such as oxytocin and prostaglandin) and any such differences would be expected to be operating in women with PROM at term.
(2) and (3) Differences in outcomes according to parity and state of cervix would be expected ‐ for example, nulliparous women and those with an unfavourable cervix are likely to have longer labours and this in turn may increase the risk of infection of infection and other adverse outcomes.
(4) Maternal antibiotic prophylaxis for PROM may be more likely to reduce maternal and neonatal infection than no maternal antibiotic prophylaxis.
(5) Women who have digital examination at baseline may be prone to more infections than those who did not have digital vaginal examination.
Primary outcomes were used in subgroup analyses.
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We carried out sensitivity analyses to explore the effect of trial quality assessed by adequate sequence generation and concealment of allocation, and blinding of outcome assessment. We excluded studies with 'unclear' or 'high' risk of bias ratings for sequence generation and detection bias from the analyses for primary outcomes.
Results
Description of studies
Results of the search
The updated search of the Cochrane Pregnancy and Childbirth Group’s Trials Register identified 17 new records, relating to 15 studies. Of these 15 studies, we included eight trials (Ayaz 2008; Cheung 2006; Fatima 2015; Javaid 2008; Maqbool 2014; Selmer‐Olsen 2007; Shah 2012; Tasnim 2000), excluded six studies (Cararach 1996; Chaudhuri 2006; Doungtone 1999; Levy 2005; Levy 2007; Poornima 2011), and one study is ongoing (Walfisch 2014); the final report related to a trial already included (Hannah 1996). We have also included three additional trials in this update ‐ two that were excluded in the previous version of this review (Sperling 1993; Tamsen 1990), and one that was awaiting classification in the previous version of this review (Krupa 2005). See Figure 1.
1.

Study flow diagram.
The one ongoing trial, Walfisch 2014, plans to assess the management of PROM at 34 weeks' gestation or later for women with previous caesarean sections, comparing standard expectant management with the double‐balloon catheter device.
Included studies
This review now has a total of 23 included trials (Akyol 1999; Ayaz 2008; Beer 1999; Cheung 2006; Chung 1992; Fatima 2015; Hannah 1996; Javaid 2008; Krupa 2005; Mahmood 1992; Mahmood 1995; Maqbool 2014; McQueen 1992; Milasinovic 1998; Natale 1994; Ottervanger 1996; Selmer‐Olsen 2007; Shah 2012; Shalev 1995; Sperling 1993; Tamsen 1990; Tasnim 2000; Wagner 1989) involving 8615 women and their babies (including the large Hannah 1996 trial of 5042 women). For further details see Characteristics of included studies.
All of the trials included women with singleton pregnancies with PROM at 37 weeks' gestation or later (Akyol 1999; Ayaz 2008; Beer 1999; Cheung 2006; Chung 1992; Fatima 2015; Hannah 1996; Javaid 2008; Krupa 2005; Mahmood 1992; Mahmood 1995; Maqbool 2014; McQueen 1992; Milasinovic 1998; Natale 1994; Ottervanger 1996; Selmer‐Olsen 2007; Shah 2012; Shalev 1995; Sperling 1993; Tamsen 1990; Tasnim 2000; Wagner 1989).
Five trials were conducted in Pakistan (Ayaz 2008; Fatima 2015; Javaid 2008; Maqbool 2014; Tasnim 2000), two were conducted in China (Cheung 2006; Chung 1992), and two in Scotand (Mahmood 1992; Mahmood 1995); one trial was conducted across multiple countries (Canada, the UK, Australia, Israel, Sweden and Denmark) (Hannah 1996); the remaining trials were conducted in Brazil (Krupa 2005), Canada (Natale 1994), Denmark (Sperling 1993), Germany (Beer 1999), India (Shah 2012), Norway (Selmer‐Olsen 2007), Serbia (Milasinovic 1998), Sweden (Tamsen 1990), the Netherlands (Ottervanger 1996), Turkey (Akyol 1999), USA (Wagner 1989), and Zimbabwe (McQueen 1992).
Induction of labour methods
Ten trials assessed intravenous oxytocin; 12 assessed prostaglandins (six in the form of vaginal prostaglandin E2 and six as oral, sublingual or vaginal misoprostol); and one trial each assessed Caulophyllum and acupuncture. One trial, Hannah 1996, assessed both intravenous oxytocin and vaginal prostaglandin E2.
Oxytocin
Akyol 1999: planned early birth group: immediate induction with intravenous oxytocin ("the infusion rate titrated to contractions, according to local hospital practice"); expectant management group: induction with oxytocin if spontaneous labour had not occurred within 24 hours.
Hannah 1996: planned early birth group: labour immediately induced with intravenous oxytocin, "titrated to contractions, according to local hospital practice" expectant management group: observed for up to four days, then induced with intravenous oxytocin if spontaneous labour had not occurred, or complications occurred.
McQueen 1992: planned early birth group: oxytocin infusion (no further details provided); expectant management group: observation until birth (unless sepsis was suspected, in which case induction with oxytocin).
Natale 1994: planned early birth group: induction eight hours after PROM with intravenous oxytocin ("standard induction protocol for our hospital"); expectant management group: observation for 48 hours; induction if group B ß‐haemolytic streptococci detected on screen or culture; if a clinical diagnosis of chorioamnionitis made; or if 48 hours from PROM elapsed and spontaneous labour had not commenced.
Ottervanger 1996: planned early birth group: induction with intravenous oxytocin, starting at a dose of 2.5 mU/minute, augmented every 20 minutes until adequate contractility achieved; expectant management group: admission to hospital for 48 hours; if labour did not commence within 48 hours, induction with intravenous oxytocin offered.
Shalev 1995: planned early birth group: 12 hours of expectant management followed by oxytocin infusion (starting at 1 mU/minute and increasing as necessary by 1 mU/minute every 20 minutes); expectant management group: 72 hours of expectant management followed by oxytocin infusion.
Sperling 1993: planned early birth group: induction with oxytocin infusion six hours after ROM; expectant management group: induction with oxytocin 24 hours after ROM (initial dose of 4 mU/minutes, increased after 40 minutes by 4 mU every 20 minutes (maximum 32 mU/minute)).
Tamsen 1990: planned early birth group: induction with intravenous oxytocin, starting at a dose of 1 mU to 3 mU/minute, increased by 2 mU to 3 mU/minute every 30 minutes as required; expectant management group: admission to antenatal unit, until contractions started.
Tasnim 2000: planned early birth group: induction with oxytocin infusion following randomisation; expectant management group: monitored for signs and symptoms of chorioamnionitis; if labour had not established 24 hours after PROM, oxytocin was commenced.
Wagner 1989: planned early birth group: immediate induction with intravenous oxytocin (commenced at 3 mU/minute and increased by 3 mU/minute every 20 minutes as required); expectant management group: transferred to the antepartum ward, and returned to the labour and delivery suite: if signs of infection or fetal distress occurred; when spontaneous labour occurred; 24 hours after ROM for oxytocin if spontaneous labour did not occur.
Oral misoprostol
Ayaz 2008: planned early birth group: induction with oral misoprostol 50 µg every four hours for a maximum of four doses; expectant management group: observation for 24 hours; if vaginal birth was not achieved, labour induced with "oxytocin or prostaglandins".
Cheung 2006: planned early birth group 1: induction with oral misoprostol 50 µg every four hours until active labour was established or to a maximum of six doses; planned early birth group 2: induction with oral misoprostol 100 µg every four hours until active labour was established or to a maximum of six doses; expectant management group: oral placebo (vitamin B6 50 mg); for both groups, if there was no response (i.e. no signs of any abdominal pain) after 24 hours, oxytocin was started according to the hospital protocol.
Fatima 2015: planned early birth group: immediate induction with oral misoprostol (no further details); expectant management: observation for 24 hours; management as per departmental protocol if not in labour after 24 hours.
Javaid 2008: planned early birth group: induction with oral misoprostol (no further details given); expectant management group: left for 24 hours, unless otherwise indicated.
Sublingual misoprostol
Maqbool 2014: planned early birth group: induction with misoprostol 100 µg sublingually, up to five doses, four hours apart (as required); expectant management group: observation for uterine contractions for 24 hours.
Vaginal misoprostol
Krupa 2005: planned early birth group: induction immediately with vaginal misoprostol (tablet containing 25 µg Prostokos digitally inserted into the posterior fornix) at six hourly intervals, up to a maximum of four doses (a total of 100 µg); if no response after 24 hours, oxytocin was given; expectant management group: monitored on ward for up to 24 hours; oxytocin was commenced after 24 hours if labour had not commenced.
Vaginal prostaglandin E2
Chung 1992: planned early birth group: induction with prostaglandin E2 gel (3 mg) instilled into the posterior fornix of the vagina; expectant management group: sterile K‐Y jelly (placebo); for both groups, conservative management followed for 24 hours unless intervention was required; an oxytocin infusion for induction or augmentation "was as indicated by Departmental protocol".
Hannah 1996: planned early birth group: immediate induction with vaginal prostaglandin E2 gel (1 mg or 2 mg) inserted into the posterior vaginal fornix; repeated six hours later if labour had not started, followed oxytocin if labour had not started four or more hours later; expectant management group: observation for up to four days, then induction with vaginal prostaglandin E2 gel if spontaneous labour had not occurred, or if complications developed.
Mahmood 1992: planned early birth group: induction with prostaglandin E2 gel (2 mg) in the posterior fornix; if uterine activity did not commence, repeat treatment pf PGE2 gel (1 mg) given six hours later; expectant management group: observed for up to 24 hours; both groups received oxytocin if labour did not commence within 24 hours.
Mahmood 1995: planned early birth group: induction with prostaglandin E2 gel (1 mg) at admission, administered into the posterior fornix, repeated six hours later if labour was not established; expectant management group: observation for up to 24 hours; both groups received oxytocin if labour did not commence within 24 hours.
Milasinovic 1998: planned early birth group: induction six hours following ROM with prostaglandin E2 gel (Predipil) intracervically, followed by oxytocin three to four hours later; expectant management group: antibiotics, and monitoring every six hours.
Shah 2012: planned early birth: induction within six hours with intracervical prostaglandin E2 gel; women for whom spontaneous labour had not commenced after 10 hours were 're‐induced' with prostaglandin or oxytocin; expectant management group: expectant management for 24 hours; those who were not in labour after 24 hours were induced (with intracervical prostaglandin E2 gel or oxytocin, depending on cervical ripening).
Oral Caulophyllum
Beer 1999: planned early birth group: induction with Caulophyllum (one tablet per hour containing 250 mg Caulophyllum D4, for seven hours or until labour established); expectant management group: placebo (containing only magnesium stearate and wheat‐starch mixture).
Acupuncture
Selmer‐Olsen 2007: acupuncture group (planned early birth group): women were needled at the point CV4/Ren 4 (Guanyuan) on the "conception vessel" (midline of lower abdomen), with other points needled according to one of three main Traditional Chinese Medicine diagnostic categories; needles remained in place for 30 minutes and additional acupuncture treatment was offered the next day if labour had not commenced; expectant management group: waiting at home for 48 hours if cardiotocogram, temperature and amniotic fluid were normal; for both groups, if labour had not commenced after two days, vaginal misoprostol was administered into the posterior fornix (starting with 50 µg, and then 25 µg every six hours until contractions) (up to eight times).
Parity
The majority of the trials included both nulliparous and multiparous women (Akyol 1999; Beer 1999; Cheung 2006; Chung 1992; Fatima 2015; Hannah 1996; Javaid 2008; Krupa 2005; Maqbool 2014; McQueen 1992; Milasinovic 1998; Natale 1994; Ottervanger 1996; Shah 2012; Shalev 1995; Sperling 1993; Tamsen 1990; Tasnim 2000; Wagner 1989); though four of the trials provided data for some outcomes based on parity subgroups (Akyol 1999; Hannah 1996; Sperling 1993; Tamsen 1990).
Two trials included only nulliparous women (Mahmood 1992; Selmer‐Olsen 2007), and two trials included only multiparous women (Ayaz 2008; Mahmood 1995).
Favourable/unfavourable cervix
Nine trials included women with both favourable and unfavourable cervices: (Akyol 1999 reported on baseline 'cervix unripe' (dilated < 3 cm and < 80% effaced) and 'cervix ripe' (dilated ≥ 3cm and ≥ 80% effaced); Cheung 2006 reported on baseline modified Bishop score; Fatima 2015: mean (standard deviation) Bishop scores were 3.5 (4.9) and 3 (5.4) in the planned early birth and expectant management groups, respectively; (Hannah 1996 reported that some women had a vaginal examination with a speculum at baseline, and others a digital vaginal examination at baseline and 'cervix unripe' (dilated < 3 cm and < 80% effaced) or 'cervix ripe' (dilated ≥ 3 cm and ≥ 80% effaced) was determined; Mahmood 1992 and Mahmood 1995: reported that "All women in both groups had a cervical dilation <3 cm at entry to the trial"; though in Mahmood 1992"cervical score" at baseline was presented in Figure 1, and ranged from 1 to 8 (with favourable score defined as ≥ 6); and in Mahmood 1995 cervical score was presented in Table 1 and ranged from 2 to 9; in Natale 1994 Bishop scores were < 5 and > 5 at randomisation; Sperling 1993 presented cervical score at baseline in Table 1 which ranged from 1 to 10; Tamsen 1990 reported that women were included "regardless of cervical effacement").
Five trials included only women with unfavourable cervices at baseline (Ayaz 2008: no definition provided; Chung 1992: Bishop score of 4 or less; Milasinovic 1998: Bishop score less than 6; Tasnim 2000: Bishop score ranged from 1 to 6; Wagner 1989: "unfavorable cervix" (< 2 cm dilated and < 80% effaced) approximated by visual inspection).
In nine trials, cervical status at baseline was not reported (Beer 1999 and Shah 2012 (only reported on cervical dilation of ≤ 3 cm); Javaid 2008; Krupa 2005; Maqbool 2014; McQueen 1992; Ottervanger 1996; Selmer‐Olsen 2007; Shalev 1995).
Antibiotic prophylaxis
In five trials, all women received prophylactic antibiotics.
Ayaz 2008: "In both groups, prophylactic antibiotics were given".
Javaid 2008: "Antibiotics were prophylactically started in both groups".
Maqbool 2014: "antibiotic cover... was done in both groups".
Shah 2012: "All the patients irrespective of duration of PROM were given injectable Ampicillin 500 mg 6 hourly and injectable Gentamycin 80 mg 12 hourly by parenteral route till delivery."
Tasnim 2000: "Ampicillin is routinely given to all our patients with PROM, irrespective of duration of gestation".
In five trials, some women received antibiotics prophylaxis.
Cheung 2006: "intravenous ampicillin 1 g every 6 h was started when 24 h of PROM was reached".
Mahmood 1992: 16/220 women (eight in each group) were given prophylactic antibiotics because of a positive ß‐haemolytic streptococci test; and a further 9/220 (four in the planned early birth group and five in the expectant management group) received prophylactic antibiotics for intrapartum pyrexia.
Mahmood 1995: 9/100 women (four in the planned early birth group and five in the expectant management group) were given prophylactic antibiotics because of a positive ß‐haemolytic streptococci test.
McQueen 1992: women received antibiotics if duration of ROM reached 12 hours.
Milasinovic 1998: women in the expectant management group received antibiotics (ampicillin).
In two trials, prophylactic antibiotics for PROM did not appear to be routinely administered (Ottervanger 1996 (except in association with caesarean section); Sperling 1993: "Prophylactic antibiotic treatment in connection with caesarean section was only given when there were clinical signs of infection").
In 11 trials, the use of antibiotic prophylaxis for PROM was not clear.
Akyol 1999 did not report on clearly on prophylactic antibiotic administration; the outcome "Antibiotics before or during labour" was presented in the "Maternal Outcomes" table.
Hannah 1996: "Decisions about other aspects of... maternal care, including the use and timing of antibiotics... were made by the nurse, midwife, or attending physician".
Beer 1999; Chung 1992; Fatima 2015; Krupa 2005; Natale 1994; Selmer‐Olsen 2007; Shalev 1995; Tamsen 1990; Wagner 1989: not reported.
Digital vaginal examination
In 12 trials, it is was stated (or assumed based on descriptions provided) that all women received digital vaginal examination at baseline (Akyol 1999: baseline characteristics included 'cervix unripe' or 'cervix ripe' based on 'Digital vaginal examination'; Beer 1999; Cheung 2006; Chung 1992: investigations included calculation of "baseline Bishop score"; Fatima 2015: "Bishop score was assessed once with sterile gloves, at the time of admission and was restricted until the establishment of active labour";Mahmood 1992 and Mahmood 1995: at baseline women received "a sterile digital examination to exclude occult cord prolapse and to assess cervical score"; McQueen 1992: a single sterile vaginal examination to assess state of the cervix and obtain Bishop scores was conducted; Milasinovic 1998: to determine baseline Bishop scores; Natale 1994: "A single sterile digital examination was performed at randomization to assess cervical dilation and effacement and other parameters of the Bishop score"; Shah 2012: "To note the dilatation and effacement and to confirm the presence of membrane, vaginal examination was done"; Sperling 1993: to determine baseline cervical score (though it was also stated that "Vaginal examinations were minimized until the active phase of labor"); Tasnim 2000: "Digital vaginal examination was done for assessment of bishop score").
In three trials, some women received digital vaginal examination at baseline. Though Hannah 1996 reported that "Digital vaginal examinations were avoided," regarding baseline characteristics, cervical status was reported based on digital vaginal examination for approximately 35% of women; in Tamsen 1990, though it was reported that "To minimize the risk of iatrogenic amnionitis, no vaginal palpation was performed at time for admission..." it was also reported that "If the woman was assigned to the intervention group, a vaginal palpation was performed"; in Wagner 1989, some women were digitally examined "Our general protocol called for no digital examinations until the patients began labor or induction. However, we included those women who otherwise qualified for the study and who had received a single sterile digital examination at admission".
In three trials, it was stated (or assumed based on descriptions provided) that women did not routinely receive digital vaginal examination at baseline (Ayaz 2008: "Digital vaginal examination was avoided"; Selmer‐Olsen 2007: "To avoid infection, no digital examination is performed before onset of labour or induction"; Shalev 1995: "Women who were examined digitally were excluded from further study").
In four trials, it was not stated whether women received digital vaginal examination at baseline (Javaid 2008; Krupa 2005; Maqbool 2014; Ottervanger 1996).
Excluded studies
We excluded a total of 36 studies from this review, mostly because gestation was only reported as being at term or because some women in the trial may have not reached 37 completed weeks of gestation when their membranes ruptured (Alcalay 1996; Brosnan 1996; Cararach 1996; Chang 1997; Chaudhuri 2006; Chua 1995; Davies 1991; Doungtone 1999; Duff 1984; Freeman 1968; Gloeb 1989; Gonen 1994; Grant 1992; Hidar 2000; Hjertberg 1996; Hoffman 2001; Ladfors 1996; Levy 2005; Levy 2007; Lo 2003; Mahmood 1989; Mateos 1998; McCaul 1997; Morales 1986; Ngai 1996; Ozden 2002; Perez Picarol 1990; Poornima 2011; Ray 1992; Rydhstrom 1991; Shetty 2002; Shoaib 1994; Suzuki 2000; Thomas 2000; Van der Walt 1989; Van Heerden 1992). For further details see: Characteristics of excluded studies.
Risk of bias in included studies
For summaries of the risk of bias across all included studies see Figure 2 and Figure 3.
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Seven of the 23 trials (Cheung 2006; Chung 1992; Fatima 2015; Hannah 1996; Krupa 2005; McQueen 1992; Selmer‐Olsen 2007) used adequate methods for sequence generation. Five of the trials generated their random number sequence using a computer, and two (Fatima 2015; McQueen 1992) used a random number table.
In six trials (Ayaz 2008; Mahmood 1995; Milasinovic 1998; Shalev 1995; Tasnim 2000; Wagner 1989) risk of selection bias (due to inadequate methods to generate a random sequence) was judged to be high: Ayaz 2008: randomisation was based on choosing two types of cards; Mahmood 1995: a "randomization list" was used to assign odd and even numbers; Shalev 1995 and Wagner 1989: randomisation was based on the last digit of the medical record number (odd and even); Milasinovic 1998: randomisation was by alternation; Tasnim 2000 randomisation was based on the date of hospital visit.
In the remaining 10 trials (Akyol 1999; Beer 1999; Javaid 2008; Mahmood 1992; Maqbool 2014; Natale 1994; Ottervanger 1996; Shah 2012; Sperling 1993; Tamsen 1990) methods for random sequence generation (and thus the risk of selection bias) were unclear.
Only four of the 23 trials (Cheung 2006; Chung 1992; Hannah 1996; Krupa 2005) were judged to be at a low risk of selection bias, employing appropriate methods to conceal allocation. Chung 1992 kept the code with a third party (central randomisation), and Hannah 1996 used centrally‐controlled computer randomisation, with telephone access. Cheung 2006 and Krupa 2005 used sealed, opaque, sequentially numbered envelopes.
Six trials (Ayaz 2008; Mahmood 1995; Milasinovic 1998; Shalev 1995; Tasnim 2000; Wagner 1989) were quasi‐randomised, and thus the risk of selection bias due to lack of allocation concealment was judged to be high.
For the remaining 13 trials, the risk of selection bias due to lack of allocation concealment was judged to be unclear (largely due to inadequate information provided) (Akyol 1999; Beer 1999; Fatima 2015 ; Javaid 2008; Mahmood 1992; Maqbool 2014; McQueen 1992; Natale 1994; Ottervanger 1996; Selmer‐Olsen 2007; Shah 2012; Sperling 1993; Tamsen 1990).
Blinding
In three trials, women and personnel were blinded throughout by the use of a placebo (Beer 1999; Cheung 2006; Chung 1992), and thus the risk of performance bias was judged to be low.
In the trials that did not use a placebo, it was considered that blinding of women and personnel would not have been possible (though largely, blinding was not discussed), and thus risk of performance bias was judged to be high (Akyol 1999; Ayaz 2008; Fatima 2015; Hannah 1996; Mahmood 1992; Maqbool 2014; McQueen 1992; Milasinovic 1998; Natale 1994; Selmer‐Olsen 2007; Shah 2012; Shalev 1995; Sperling 1993; Tamsen 1990; Tasnim 2000; Wagner 1989). Three trials (Javaid 2008; Krupa 2005; Mahmood 1995) were specifically described as being "open" and Ottervanger 1996 discussed that "women, their companions, and the clinicians caring for them were all aware of group allocation".
Eight trials were judged to be at low risk of detection bias. In three trials, blinding was achieved through the use of a placebo (Beer 1999; Cheung 2006; Chung 1992). In the other five trials, blinding of outcome assessment was described (Akyol 1999; Hannah 1996; Mahmood 1992; Natale 1994; Sperling 1993) for at least some outcomes (predominately for the assessment of chorioamnionitis and neonatal infection).
In three trials the risk of detection bias in three trials was judged to be high (Javaid 2008; Krupa 2005; Mahmood 1995) with the trials described as "open".
In the remaining 12 trials, the risk of detection bias was judged to be unclear, largely due to no information provided on whether outcome assessments were able to be performed blind (Ayaz 2008; Fatima 2015; Maqbool 2014; McQueen 1992; Milasinovic 1998; Ottervanger 1996; Selmer‐Olsen 2007; Shah 2012; Shalev 1995; Tamsen 1990; Tasnim 2000; Wagner 1989).
Incomplete outcome data
In eight trials (Cheung 2006; Fatima 2015; Hannah 1996; Krupa 2005; Mahmood 1992; Milasinovic 1998; Selmer‐Olsen 2007; Shah 2012) the risk of attrition bias was judged to be low. In Cheung 2006, only one of 34 women in the planned early birth group and one of 33 women in the expectant management group were lost to follow‐up due to missing case records. One woman of 5042 in Hannah 1996 was lost to follow‐up (data not received); and for the maternal satisfaction outcome, completed questionnaires were obtained from 4129 women (81.9%). In Mahmood 1992, 4% of women (10/230, five from each group) were excluded from the final analysis, as they did not fulfil the study criteria. Milasinovic 1998 reported that only one woman (of 76) was lost to follow‐up. In Selmer‐Olsen 2007, three of 51 women in the planned early birth group and two of 55 in the expectant management group, were excluded following randomisation. In Krupa 2005 and Fatima 2015, there were no losses or exclusions.
The remaining 15 trials were judged to be at an unclear risk of attrition bias, mostly with no (or limited) information reported on losses or missing data (Akyol 1999; Ayaz 2008; Beer 1999; Chung 1992; Javaid 2008; Mahmood 1995; Maqbool 2014; McQueen 1992; Natale 1994; Ottervanger 1996; Shalev 1995; Sperling 1993; Tamsen 1990; Tasnim 2000; Wagner 1989).
Selective reporting
Only five of the 23 trials were judged to be at low risk of reporting bias (Hannah 1996; Krupa 2005; Mahmood 1992; Mahmood 1995; Shalev 1995).
The risk of reporting bias was judged to be high in seven trials. In Ayaz 2008, data for very few outcomes were reported; for some outcomes (e.g. interval between PROM and birth), only mean values were reported, and other outcomes were only mentioned in the manuscript Discussion (e.g. uterine rupture). In Fatima 2015, a number of outcomes that would be expected to be reported (including outcomes described in the abstract and/or methods of the manuscript, such as mean latency and maternal satisfaction) were not. In Javaid 2008, for many of the reported outcomes, the numbers of women in each group were unclear, or data were not reported separately for the two study groups. Further, for the outcomes chorioamnionitis and postpartum fever, only percentages were provided per group. In Natale 1994, only percentages were reported for some outcomes in the text (and for caesarean section it was unclear as to which groups of women these percentages related to); the outcome endometritis was only mentioned in the abstract, with no data reported in text. Shah 2012 did not report on a number of outcomes that would be expected, results were reported incompletely for key outcomes (such as maternal and neonatal infection: "not statistically significant"; and no measures of variance reported for others, such as interval between PROM and birth). In Sperling 1993, for many outcomes (such as birthweight and Apgar scores) results were reported incompletely ("no differences between groups"). In Tasnim 2000, there were discrepancies between data in the abstract and text (likely typographical errors); some results were also reported incompletely in text (such as regarding neonatal infection and admission to the nursery).
In Akyol 1999 the risk of reporting bias was unclear; the primary outcome pre‐defined in the Methods was definite or probable neonatal infection, however results were reported only for "neonatal antibiotics". Similarly, the risk of reporting bias was judged to be unclear in Selmer‐Olsen 2007, with median values only reported for the outcome time from PROM to birth. In a further nine trials, the risk of reporting bias was judged to be unclear due to insufficient information available to confidently determine risk (Beer 1999; Cheung 2006; Chung 1992; Maqbool 2014; McQueen 1992; Milasinovic 1998; Ottervanger 1996; Tamsen 1990; Wagner 1989).
Other potential sources of bias
There was no other obvious source of bias in four trials (Cheung 2006; Mahmood 1992; Mahmood 1995; Hannah 1996).
Five trials were judged to be at unclear risk of other bias: Chung 1992: there was a possible imbalance in the proportions of women who were nulliparous between groups (28/30 versus 21/29 women in the planned versus expectant management groups respectively) (though the authors report "There was no significant differences between the 2 groups"); Krupa 2005: there were possible baseline imbalances between groups (such as for parity: number of pregnancies, 1: 31/75 and 45/75 women in the planned early birth versus. expectant management groups respectively), though the authors state "The two groups were similar with regard to control variables"; Selmer‐Olsen 2007: 23/51 women in the expectant management group (and 15/48 women in the planned early birth group) also received acupuncture during the "active phase"; Shalev 1995: there were unbalanced group numbers (298 versus 268) and few baseline characteristics were reported; Wagner 1989: the group numbers were unbalanced (as women who were not induced after 10 hours of ROM in the planned early birth group were excluded); few baseline characteristics were reported, and the authors noted that women in the expectant management group were slightly younger ("significant difference in age").
A further 14 trials were also judged to be at an unclear risk of other potential sources of bias, mostly due to limited information regarding baseline characteristics provided and/or limited methodological detail (Akyol 1999; Ayaz 2008; Beer 1999; Fatima 2015; Javaid 2008; Maqbool 2014; McQueen 1992; Milasinovic 1998; Natale 1994; Ottervanger 1996; Shah 2012; Sperling 1993; Tamsen 1990; Tasnim 2000).
Effects of interventions
See: Table 1
Planned early birth versus expectant management
Primary outcomes (for the mother)
Maternal infectious morbidity (chorioamnionitis and/or endometritis)
There was variable reporting for this review's primary outcome of maternal infectious morbidity (chorioamnionitis and/or endometritis).
When we included only those trials reporting specifically on outcomes termed 'chorioamnionitis' or 'endometritis' a clear reduction was observed for the planned early birth group compared with the expectant management group (average risk ratio (RR) 0.49; 95% confidence interval (CI) 0.33 to 0.72; eight trials, 6864 women; Tau² = 0.19; I² = 72%, low‐quality evidence) (Analysis 1.1). We observed substantial statistical heterogeneity for this outcome, and thus a random‐effects model was used.
1.1. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 1 Maternal infectious morbidity (chorioamnionitis and/or endometritis).
The trials contributing to this meta‐analysis varied in their outcome definitions/descriptions: Hannah 1996: Clinical chorioamnionitis: "Fever before or during labor was defined as a temperature 37.5°C on two occasions 1 hour apart or a temperature of 38°C. Other signs of chorioamnionitis were a maternal white‐cell count 20,000 per cubic millimetre or foul‐smelling amniotic fluid"; Natale 1994: "pathologic diagnosis of chorioamnionitis"; Shalev 1995: chorioamnionitis "diagnosed by clinical signs and symptoms... along with microorganismic invasion of the amniotic cavity from the cultures taken at birth, and histologic evidence of placental inflammation"; Sperling 1993: "clinical signs of chorioamnionitis"; Wagner 1989: "Endometritis was defined as uterine tenderness and temperature of 38.0°C or higher on two separate occasions 4 hours apart"; Milasinovic 1998: "Clinically diagnosed chorioamnionitis"; Ayaz 2008 and Maqbool 2014: "chorioamnionitis".
A subgroup analysis, based on initial mode of induction for planned early birth was performed. While the subgroup interaction test indicated a possible effect of mode of induction on this outcome (Chi² = 12.83, P = 0.005, I² = 76.6%), all subgroups (oral misoprostol; sublingual misoprostol; vaginal prostaglandin E2; intravenous oxytocin) showed effects in favour of planned early birth, although a clear reduction in maternal infectious morbidity was seen only for the sublingual misoprostol and intravenous oxytocin subgroups (Analysis 1.1). The data contributing to the sublingual misoprostol subgroup were from only one trial, of 560 women at an overall unclear to high risk of bias; when these data were excluded from the meta‐analysis, the subgroup interaction test was no longer significant. This may suggest that there were no important differences between various modes of induction on maternal infectious morbidity.
We also conducted a meta‐analysis for this outcome including those trials that reported on intrapartum pyrexia and/or treatment with antibiotics for intrapartum pyrexia (Akyol 1999: "Fever...during labour was defined as a temperature > 37.5°C on 2 occasions 2 1 hour apart or a temperature of > 38°C."; Cheung 2006: "Pyrexia"; Chung 1992: "fever (> 37.5 "C) in the intrapartum period"; Fatima 2015: "Fever"; Mahmood 1992 and Mahmood 1995: "pyrexia if maternal temperature exceeded 37.5"C in labour").
When these data were included, a reduction in maternal infectious morbidity was also observed in favour of planned early birth (average RR 0.54; 95% CI 0.38 to 0.76; 14 trials, 7667 women; Tau² = 0.19; I² = 62%) (Analysis 1.7). Similarly in this meta‐analysis, the subgroup analysis test indicated a possible treatment effect according to mode of induction (Chi² = 16.58, P = 0.0009, I² = 81.9%), with significant benefits seen only for the oral and sublingual misoprostol and intravenous oxytocin subgroups (Analysis 1.7). The data contributing to the sublingual misoprostol subgroup were from only one trial, of 560 women, at an overall unclear to high risk of bias; when these data were excluded from the meta‐analysis, the subgroup interaction test was no longer significant, again suggesting that there were no important differences between various modes of induction on maternal infectious morbidity defined in this way.
1.7. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 7 Maternal infectious morbidity (chorioamnionitis, endometritis and/or pyrexia).
We ran a funnel plot to assess the risk of reporting bias, such as publication bias, and we found that while studies were equally distributed on either side, there was some asymmetry, which could represent the presence of bias due to smaller studies (such as Ayaz 2008 and Sperling 1993 (at unclear to high risk of bias) and Cheung 2006 (at low risk of bias)) producing exaggerated intervention effect estimates (Figure 4).
4.

Funnel plot of comparison: 1 Planned early birth versus expectant management (subgroups: method of induction), outcome: 1.7 Maternal infectious morbidity (chorioamnionitis, endometritis and/or pyrexia).
In the above meta‐analyses we did not include the following data.
Javaid 2008 reported in their abstract that there were fewer women in the misoprostol group with clinical chorioamnionitis "(3% Vs 7.8%)"; it was not clear whether there were any losses to follow‐up or missing data in this trial (and it was thus not possible to accurately/confidently calculate numbers based on these percentages).
Krupa 2005 reported in their discussion that "With regard to maternal postpartum follow up, results were also extremely favourable in both groups with minimal rates of puerperal infection, requirement for antibiotic therapy and other complications".
Shah 2012 reported that 2/50 and 2/50 women in the planned early birth and expectant management groups respectively had caesarean sections for "nonprogress of labor with chorioamnionitis;" however it was unclear whether there were additional cases of chorioamnionitis among women in the trial.
Tasnim 2000 reported that there were 0/72 and 2/80 cases of antepartum pyrexia in the planned early birth and expectant management groups, respectively.
Tamsen 1990 reported that "The only clinical infections also occurred in [the expectant management group], 1 mother and 2 babies in all".
Caesarean section
Overall, no clear difference in the risk of caesarean section birth was observed between the planned early birth and expectant management groups (average RR 0.84; 95% CI 0.69 to 1.04; 23 trials, 8576 women; Tau² = 0.10; I² = 55%, low‐quality evidence) (Analysis 1.3). Substantial statistical heterogeneity was observed for this outcome and thus a random‐effects meta‐analysis was used.
1.3. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 3 Caesarean section.
We ran a funnel plot to assess the risk of reporting bias, and we found that studies were equally distributed on either side, with no substantial asymmetry observed (Figure 5).
5.

Funnel plot of comparison: 1 Planned early birth versus expectant management (subgroups: method of induction), outcome: 1.3 Caesarean section.
A subgroup analysis, based on initial mode of induction for planned early birth was performed. The subgroup interaction test indicated a possible effect of mode of induction on this outcome (Chi² = 25.30, P = 0.0003, I² = 76.3%). The only subgroup to show a significant reduction in the risk of caesarean section was the sublingual misoprostol subgroup (RR 0.54; 95% CI 0.45 to 0.66), however these data were from only one trial, of 560 women, at an overall unclear to high risk of bias (Analysis 1.3).
Natale 1994 reported that"The rate of caesarean births in the induction and expectant management groups and those patients who refused to participate in the study group was also similar and not statistically different, ranging between 12.6% and 13.8%". While it was somewhat unclear as to whether these percentages related to the planned early birth and expectant management groups, or the group of women that refused to participate; these data have been included in the meta‐analysis.
Serious maternal morbidity or mortality (e.g. death, cardiac arrest, respiratory arrest, admission to intensive care unit)
Only three of the 23 trials (425 women) reported data on this outcome (Krupa 2005; Ottervanger 1996; Tasnim 2000). Krupa 2005 reported that there were no maternal deaths or serious complications in either group; Ottervanger 1996 indicated that there were no maternal deaths, "All mothers and infants were well at follow up six weeks after delivery"; Tasnim 2000 reported that there were no maternal deaths in either group (Analysis 1.4). We assessed this outcome to be of very low‐quality evidence.
1.4. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 4 Serious maternal morbidity or mortality (e.g. death, cardiac arrest, respiratory arrest, admission to intensive care unit).
Primary outcomes (for the fetus/neonate)
Definite early‐onset neonatal sepsis
Only six trials contributed data to the outcome definite early‐onset neonatal sepsis, and overall, no clear difference between the planned early birth and expectant management groups was shown (RR 0.57; 95% CI 0.24 to 1.33; six trials, 1303 infants; very low‐quality evidence) (Analysis 1.5).
1.5. Analysis.
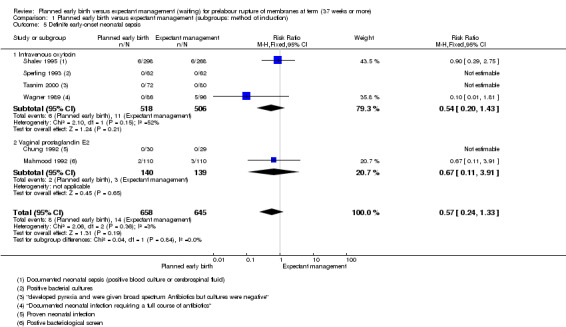
Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 5 Definite early‐onset neonatal sepsis.
A subgroup analysis, based on initial mode of induction for planned early birth was performed for this outcome, with the interaction test indicating no clear subgroup differences (Chi² = 0.04, P = 0.84, I² = 0%) (Analysis 1.5).
The trials contributing to this meta‐analysis varied in their outcome definitions/descriptions: Chung 1992: "proven neonatal infection"; Mahmood 1992: "positive bacteriological screen"; Shalev 1995: "Documented neonatal sepsis (positive blood culture or cerebrospinal fluid"; Sperling 1993: "Positive bacterial cultures"; Tasnim 2000: neonates who "developed pyrexia and were given broad spectrum Antibiotics but cultures were negative"; and Wagner 1989: "Documented neonatal infection requiring a full course of antibiotics".
Definite or probable early‐onset neonatal sepsis
Overall, a reduction in the risk of definite or probable early‐onset neonatal sepsis was observed (RR 0.73; 95% CI 0.58 to 0.92; 16 trials, 7314 infants, low‐quality evidence) (Analysis 1.6).
1.6. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 6 Definite or probable early‐onset neonatal sepsis.
A subgroup analysis, based on initial mode of induction for planned early birth was performed for this outcome, with the interaction test indicating no clear subgroup differences (Chi² = 2.66, P = 0.26, I² = 24.9%) (Analysis 1.6).
We ran a funnel plot to assess the risk of reporting bias, but we did not find pronounced asymmetry (Figure 6).
6.
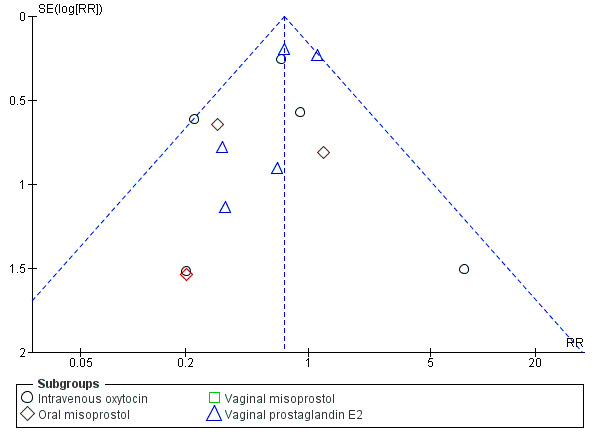
Funnel plot of comparison: 1 Planned early birth versus expectant management (subgroups: method of induction), outcome: 1.6 Definite or probable early‐onset neonatal sepsis.
The trials contributing to this meta‐analysis varied in their outcome definitions/descriptions: Hannah 1996 reported a composite outcome of definite or probable neonatal infection and did not report definite and probable infection separately: "Definite neonatal infection was defined as the presence of clinical signs of infection and one or more of the following: a positive culture of blood, cerebrospinal fluid, urine, tracheal aspirate, or lung tissue; a positive Gram’s stain of cerebrospinal fluid; a positive antigen‐detection test with blood, cerebrospinal fluid, or urine; a chest radiograph compatible with pneumonia; or a histologic diagnosis of pneumonia. Probable neonatal infection was defined as the presence of clinical signs of infection and one or more of the following: a high or low blood neutrophil count, a high immature total neutrophil ratio, a high actual immature neutrophil count, or abnormal cerebrospinal fluid findings showing an elevated white‐cell count, a high level of protein, or a low level of glucose".Ayaz 2008; Fatima 2015; Krupa 2005; Ottervanger 1996: reported "sepsis" (no further detail); Shalev 1995: "documented neonatal sepsis (positive blood culture or cerebrospinal fluid)"; Sperling 1993: "positive bacterial cultures"; Tasnim 2000: "cultures... negative";Wagner 1989: "antibiotics for infection or pending culture"; Shah 2012: "Antibiotics administered in neonates"; Chung 1992: "Proven neonatal infection"; Mahmood 1992: "Positive bacteriological screen";Cheung 2006: "Neonate sepsis" including, conjunctivitis, congenital pneumonia, septicaemia, and clinical sepsis requiring intravenous antibiotics; McQueen 1992 and Milasinovic 1998: not provided or unclear. Mahmood 1995: neonates "treated with parenteral antibiotics because of suspected infection secondary to prolonged SROM to delivery interval".
Tamsen 1990 reported that "The only clinical infections also occurred in [the expectant management group], 1 mother and 2 babies in all". These data have not been included in the meta‐analysis.
Perinatal mortality (stillbirth or neonatal mortality)
No clear difference was observed overall in the risk of perinatal mortality (stillbirth or neonatal mortality) with planned early birth compared with expectant management (RR 0.47; 95% CI 0.13 to 1.66; eight trials, 6392 infants, moderate‐quality evidence) (Analysis 1.2). There were a total of three deaths in the planned early birth group and seven in the expectant management group. The three deaths in the planned management group occurred in Hannah 1996, and were as a result of lethal congenital abnormalities; two of the seven deaths in the expectant management group were also related to lethal congenital abnormalities (these two deaths were from Hannah 1996).
1.2. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 2 Perinatal mortality (stillbirth or neonatal mortality).
Javaid 2008 reported that "One baby died of multiple congenital abnormalities that was undiagnosed as patient was unbooked. She was in expectant group of management";Shah 2012 also reported that "There was one perinatal mortality in early induction group which was because of congenital heart disease with early onset septicemia not due to induction complications such as fetal distress or hyper‐stimulation of uterus." In both trials it was unclear whether there were any other deaths, and thus these data were not included in the above meta‐analysis.
A subgroup analysis, based on initial mode of induction for planned early birth was performed for this outcome, with the interaction test indicating no clear subgroup differences (Chi² = 0.00, P = 0.95, I² = 0%) (Analysis 1.2).
Secondary outcomes (for the mother)
Chorioamnionitis (either suspected or proven)
A significant reduction in the risk of chorioamnionitis (either suspected or proven) was observed in the planned early birth group compared with the expectant management group (average RR 0.55; 95% CI 0.37 to 0.82; eight trials, 6874 women; Tau² = 0.19; I² = 73%) (Analysis 1.8).
1.8. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 8 Chorioamnionitis (either suspected or proven).
Data from Sperling 1993 on the outcome clinical signs of chorioamnionitis were included in the meta‐analysis; Sperling 1993 also reported that 100/124 placentas were examined histologically "The degree of chorioamnionitis in the LI‐group was higher than that in the EI‐group (p < 0.05)".
As above for the outcome maternal infectious morbidity, we also conducted a meta‐analysis including data from the trials reporting on intrapartum pyrexia/fever and/or treatment with antibiotics for intrapartum pyrexia (Akyol 1999; Cheung 2006; Chung 1992; Fatima 2015; Mahmood 1992; Mahmood 1995). With inclusion of these data, a significant reduction in chorioamnionitis with planned early birth was also observed (average RR 0.60; 95% CI 0.42 to 0.85; 14 trials, 7677 women; Tau² = 0.18; I² = 62%) (Analysis 1.9).
1.9. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 9 Chorioamnionitis and/or pyrexia (either suspected or proven).
We ran a funnel plot to assess the risk of reporting bias, and we found some asymmetry, which could represent the presence of bias due to smaller studies (such as Ayaz 2008 and Sperling 1993, which were judged to be at unclear to high risk of bias) producing exaggerated intervention effect estimates (Figure 7). However the overall result is also heavily influenced by the Hannah 1996 trial.
7.

Funnel plot of comparison: 1 Planned early birth versus expectant management (subgroups: method of induction), outcome: 1.9 Chorioamnionitis and/or pyrexia (either suspected or proven).
Endometritis
Only one trial (Wagner 1989) clearly reported on the outcome 'endometritis', and although fewer cases were observed with planned early birth (2/86) compared with expectant management (8/86), no clear difference overall was observed (RR 0.25; 95% CI 0.05 to 1.14; one trial, 172 women; Analysis 1.10).
1.10. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 10 Endometritis.
Natale 1994 reported in their abstract only that "the clinical diagnosis of postpartum endometritis was not significantly different in the two groups"; however no further data on this outcome were reported.
Sperling 1993 reported that "four women had postpartum infection....as wound infection, intraamnionic infection, or postpartum endometritis"; from Figure 2 in the manuscript, it could be determined that 1/62 and 3/62 women in the planned early birth and expectant management groups respectively had postpartum infection, however it was not clear how many women had postpartum endometritis.
Postpartum pyrexia
No clear difference in the risk of postpartum pyrexia was observed between the planned early birth and expectant management groups (average RR 0.91; 95% CI 0.45 to 1.84; seven trials, 5713 women; Tau² = 0.53; I² = 74%) (Analysis 1.11).
1.11. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 11 Postpartum pyrexia.
The trials contributing to this meta‐analysis varied in their outcome definitions/descriptions: Akyol 1999: "Postpartum fever was defined as a temperature > 38°C"; Hannah 1996: "Postpartum fever was defined as a temperature 38°C"; Tasnim 2000: "pyrexia of more than > 38°C... in postpartum period"; Chung 1992: "Febrile episode puerperium" (> 37.5°C); Mahmood 1992: "maternal temperature exceeded 37.5"C... within 24 h after delivery"; McQueen 1992 and Milasinovic 1998: not clear.
Javaid 2008 reported in their abstract that there were fewer women in the misoprostol group with postpartum fever "(1% Vs 1.8%)"; it was not clear whether there were any losses to follow‐up or missing data in this trial (and it was thus not possible to accurately/confidently calculate numbers based on these percentages).
Postpartum septicaemia
A possible reduction in postpartum sepsis was observed with planned early birth compared with expectant management (RR 0.26; 95% CI 0.07 to 0.96; three trials, 263 women) (Analysis 1.12). The three trials contributing data to this outcome reported on "puerperal sepsis" (Mahmood 1995); postpartum sepsis (McQueen 1992) and "maternal... sepsis" (Ottervanger 1996).
1.12. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 12 Postpartum septicaemia.
Postpartum antibiotic usage
No clear difference in the use of maternal postpartum antibiotics was observed between the planned early birth and expectant management groups (RR 0.69; 95% CI 0.40 to 1.20; four trials, 685 women) (Analysis 1.13).
1.13. Analysis.
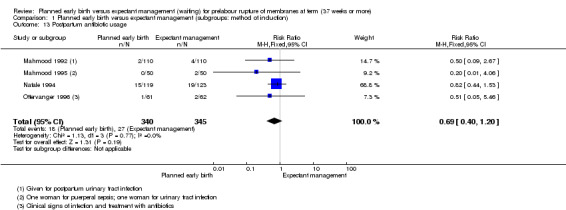
Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 13 Postpartum antibiotic usage.
Caesarean section for fetal distress
No clear difference in the risk of caesarean section for fetal distress was observed between the planned early birth and expectant management groups (RR 0.94; 95% CI 0.60 to 1.49; 11 trials, 1851 women) (Analysis 1.14). We ran a funnel plot to assess the risk of reporting bias, and we found that studies were approximately equally distributed on either side, with no substantial asymmetry observed (Figure 8).
1.14. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 14 Caesarean section for fetal distress.
8.
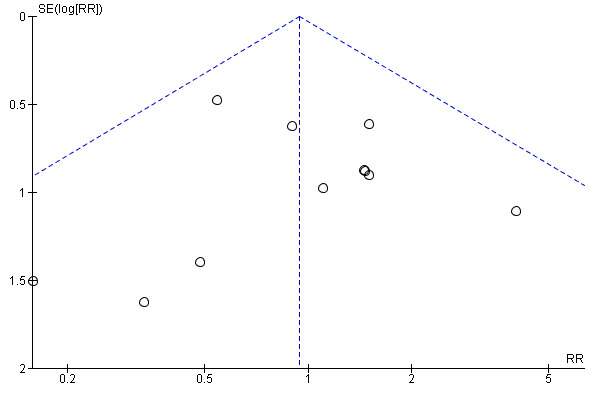
Funnel plot of comparison: 1 Planned early birth versus expectant management (subgroups: method of induction), outcome: 1.14 Caesarean section for fetal distress.
Javaid 2008 reported that "The rate of cesarean section with misoprostol was 24%, caesarean section was done for various indications, but majority i.e. 14% were due to fetal distress. But with expectant management cesarean section was slightly higher i‐e. and indications are mostly other fetal distress that is failure to progress". No further details were provided and thus these data could not be included in the meta‐analysis.
Induction of labour
Overall, a significant increase in induction of labour was observed for women in the planned early birth compared with the expectant management group (average RR 3.41; 95% CI 2.87 to 4.06; 12 trials, 6945 women; Tau² = 0.05; I² = 71%) (Analysis 1.15). We ran a funnel plot to assess the risk of reporting bias, and we found that studies were equally distributed on either side, with no substantial asymmetry observed (Figure 9).
1.15. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 15 Induction of labour.
9.

Funnel plot of comparison: 1 Planned early birth versus expectant management (subgroups: method of induction), outcome: 1.15 Induction of labour.
Operative vaginal birth
No clear difference in the risk of operative vaginal birth was observed between the planned early birth and expectant management groups (average RR 1.03; 95% CI 0.67 to 1.59; 13 trials, 6379 women; Tau² = 0.25; I² = 56%) (Analysis 1.16). We ran a funnel plot to assess the risk of reporting bias, and we found that studies were approximately equally distributed on either side, with no substantial asymmetry observed (Figure 10).
1.16. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 16 Operative vaginal birth.
10.
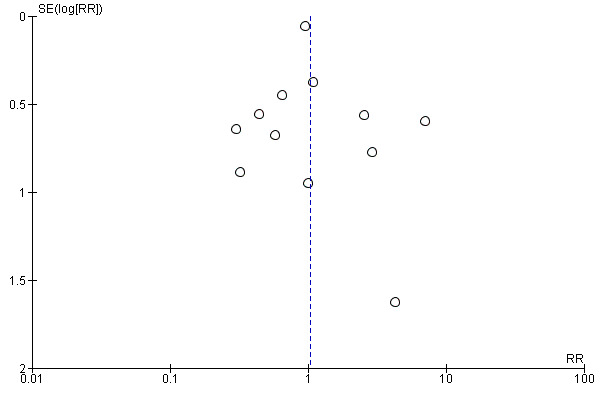
Funnel plot of comparison: 1 Planned early birth versus expectant management (subgroups: method of induction), outcome: 1.16 Operative vaginal birth.
Uterine rupture
Only two trials reported on uterine rupture, and only one case was observed in the planned early birth group in Chung 1992 (RR 2.90; 95% CI 0.12 to 68.50; two trials, 143 women; Analysis 1.17). Hannah 1996 also reported that "The frequency of other complications during labor, including... ruptured uterus... was low and did not differ significantly among the groups (data not shown)".
1.17. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 17 Uterine rupture.
Epidural analgesia
No clear difference in the use of epidural analgesia was observed between the planned early birth and expectant management groups (RR 1.07; 95% CI 0.80 to 1.42; five trials, 585 women) (Analysis 1.18).
1.18. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 18 Epidural analgesia.
Akyol 1999 did not state type of analgesia or anaesthesia (and thus the data have not been included in the meta‐analysis); however it was reported that 33/52 and 42/52 women in the planned early birth group required analgesia and anaesthesia respectively, compared with 60/74 and 58/74 in the expectant management group.
Javaid 2008 reported that "The requirement of analgesia during labour was same in induction and expectant group"; but provided no further details.
Ottervanger 1996 reported on the use of pain relief, pethidine and epidural analgesia, which was required for 31/61 (50.8%) in the planned early birth group versus 15/62 (24.2%) in the expectant management group.
Postpartum haemorrhage
Only three trials reported on postpartum haemorrhage, and no clear difference was shown between the planned early birth and expectant management groups (RR 0.43; 95% CI 0.14 to 1.28; three trials, 520 women) (Analysis 1.19).
1.19. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 19 Postpartum haemorrhage.
Javaid 2008 also reported that "PPH was experienced in 3% of the patients in the study with no difference among the two groups".
Adverse effects
Limited and varied data regarding adverse effects associated with the interventions were reported from the included trials, and thus we did not conduct a meta‐analysis for this outcome.
In regards to "complications"Ayaz 2008 reported that 2/42 women experienced uterine hyperstimulation and 1/42 uterine tachysystole in the planned early birth group, and that 1/42 women experienced nausea and vomiting in the expectant management group.
In the translation of Beer 1999, it was stated that "No unpleasant side‐effects were noted".
Cheung 2006 reported that in the planned early birth group 2, 2/33 women had uterine hyperstimulation (both having six contractions in 10 minutes), but no women required administration of tocolytics.
In Chung 1992, 1/30 and 2/29 women experienced hyperstimulation in the planned early birth and expectant management group, respectively, 2/30 and 2/29 experienced vomiting.
Fatima 2015 reported that there were no complications for 90/100 women in the planned early birth group and 23/100 in the expectant management group; however no definition was provided for "complications".
Krupa 2005 reported that 4/75 and 1/75 women experienced hyperstimulation, 4/75 and 10/75 experienced intralabour deceleration, and 8/75 and 2/75 experienced alterations of contractility (hypercontractility or tachysystoles), in the planned early birth and expectant management groups, respectively.
Selmer‐Olsen 2007 noted that "No adverse effect of acupuncture was reported, except for 1 woman reporting dizziness and discomfort after the first acupuncture treatment, and, therefore, refused another treatment day 2".
Hannah 1996 reported that "The frequency of other complications during labor, including vomiting or diarrhea, hypertonus, ruptured uterus, abruptio placentae, and shoulder dystocia, was low and did not differ significantly among the groups (data not shown)".
Shah 2012 reported that there were 2/50 and 3/50 women with "maternal morbidity" in the planned early birth group and 23/100 in the expectant management group; in the methods section of the manuscript, it was reported that "Clinical parameters considered for maternal morbidity were fever, tachycardia, abdominal tenderness, foul smelling lochia, subinvolution of uterus, and evaluation of stich (sic)line."
Views of care
Only two trials reported on measures of maternal views of care. Selmer‐Olsen 2007 asked women ‘How do you experience your plan of treatment after PROM?’, using a visual analogue scale (0 = very negative; 100 = very positive), and observed that women in the planned management group had a more positive experience compared with women in the expectant management group (mean difference (MD): 11.80 points higher; 95% CI 4.36 to 19.24; 93 women) (Analysis 1.20). Hannah 1996 observed that fewer women in the planned management group reported that there was nothing about their management that they liked (RR 0.43; 95% CI 0.36 to 0.52; 5041 women) (Analysis 1.21), and more women in the planned management group reported that there was nothing they disliked about their management (RR 1.20; 95% CI 1.10 to 1.30; 5041 women) (Analysis 1.22).
1.20. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 20 Views of care (VAS 100).
1.21. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 21 Views of care.
1.22. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 22 Views of care.
Outcomes not reported by the included trials
None of the included trials reported on the following secondary outcomes: breastfeeding, including initiation in hospital and on discharge from hospital; or postnatal depression.
Secondary outcomes (for the fetus/neonate)
Time from rupture of membranes (ROM) to birth
Overall, a significant reduction in time from ROM to birth was observed for the planned early birth group compared with the expectant management group (MD ‐10.10 hours; 95% CI ‐12.15 to ‐8.06; nine trials, 1484 women; Tau² = 5.81; I² = 60%) (Analysis 1.23). One of the trials in this meta‐analysis reported on time between recruitment and birth (Krupa 2005), and one reported on time from ROM to onset of labour (Mahmood 1995). We ran a funnel plot to assess the risk of reporting bias, and we found that studies were approximately equally distributed on either side, with no asymmetry observed (Figure 11).
1.23. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 23 Time from rupture of membranes to birth (hours).
11.

Funnel plot of comparison: 1 Planned early birth versus expectant management (subgroups: method of induction), outcome: 1.23 Time from rupture of membranes to birth (hours).
Ten other trials (Akyol 1999; Ayaz 2008; Hannah 1996; Fatima 2015; Javaid 2008; Selmer‐Olsen 2007; Shah 2012; Sperling 1993; Tamsen 1990; Tasnim 2000) reported data on this outcome that could not be included in the meta‐analysis (e.g. because medians (with 5th and 9th percentiles or ranges) were reported). These data (Analysis 1.24) were found to be consistent with the above meta‐analysis (i.e. showing significant reductions in time from ROM to birth with planned early birth), except for in one trial (Selmer‐Olsen 2007 which assessed acupuncture), where no clear difference between groups in time from ROM to birth was observed.
1.24. Analysis.
Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 24 Time from rupture of membranes to birth (hours).
| Time from rupture of membranes to birth (hours) | |||
|---|---|---|---|
| Study | Planned early birth | Expectant management | P value |
| Akyol 1999 | Group 1: Median (5th, 95th percentiles): 13.0 hours (4.0, 37.2) (N=52) | Group 2: Median (5th, 95th percentiles): 33.9 hours (25.0, 66.1) (N=25) Group 3: Median (5th, 95th percentiles): 11.0 hours (3.0, 20.8) (N=49) |
Group 2 had a significant difference compared with Group 1 and 3 (P < 0.05) |
| Ayaz 2008 | Mean: 11.6 hours (N=42) | Mean: 17.0 hours (N=42) | P < 0.001 |
| Fatima 2015 | ≤ 5 hours , N= 97 6‐10 hours , N=3 11‐15 hours, N= 0 > 15 hours. N= 0 |
≤ 5 hours, N=67 6‐10 hours, N=27 11‐15 hours, N=3 > 15 hours. N=3 |
" The induction to labour interval was significantly shorter in the misprostol group with P‐value = 7.81 " |
| Hannah 1996 | Oxytocin: Median: 17.2 hours (5th, 95th percentiles: 7.7, 47.1) (N=1258) Prostaglandin: Median: 23.0 hours (5th, 95th percentiles: 8.6, 54.1) (N=1259) |
Oxytocin: Median: 33.3 hours (5th, 95th percentiles: 10.3, 94.4) (N=1263) Prostaglandin: Median: 32.6 hours (5th, 95th percentiles: 9.9, 106.5) (N=1261) |
P < 0.001 |
| Javaid 2008 | Range: 10 to 16 hours (N=50) 'Latency period' reported |
Range: 20 to 25 hours (N=50) 'Latency period' reported |
"significantly shorter" |
| Selmer‐Olsen 2007 | Median: 31.5 hours (N=48) | Median: 25.3 hours (N=53) | P = 0.65 |
| Shah 2012 | M ean: 13 hours (N=50) | Mean: 22 hours (N=50) | P < 0.05 |
| Sperling 1993 | Primiparae: median: 15.6 hours (range: 7.7 to 35.9) (N=33) Multiparae: median: 11.2 hours (range: 8.9 to 19.0) (N=29) |
Primiparae: median: 19.6 hours (range: 12.8 to 62.1) (N=32) *Multiparae: median: 23.9 hours (8.3 to 40.2) (N=30) *Unclear if these results were for multiparae women or primiparae women who were induced |
P < 0.05 |
| Tamsen 1990 | Median: 17 hours (range: 10 to 48 hours) for nulliparous women (N=24) Median: 11 hours (range 6 to 48 hours) for parous women (N=19) |
Median: 27.5 hours (range: 9 to 117) for nulliparous women (N=26) Median: 18.5 hours (range 10 to 98) for parous women (N=24) |
Not reported |
| Tasnim 2000 | Mean: 12.8 hours (range: 7 to 22 hours) (N=72) | Mean: 19.8 hours (range: 5 to 40 hours) (N=80) | Not reported |
Birthweight
A possible lower birthweight was observed for neonates born to mothers in the planned early birth group compared with the expectant management group (MD ‐79.25 g; 95% CI ‐124.96 to ‐33.55; five trials, 1043 infants) (Analysis 1.25).
1.25. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 25 Birthweight (g).
Two additional trials reported data that could not be included in the meta‐analysis (reporting mean birthweight (and/or range) only) (Analysis 1.26); one trial suggested lower birthweight in the planned early birth group (Tamsen 1990), while the other suggested higher birthweight in the planned early birth group (Tasnim 2000); neither reported results of test of significance.
1.26. Analysis.
Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 26 Birthweight (g).
| Birthweight (g) | |||
|---|---|---|---|
| Study | Planned early birth | Expectant management | P value |
| Tamsen 1990 | Nulliparas, mean: 3340 g (N=24) Paras, mean: 3370 g (N=19) |
Nulliparas, mean: 3430 g (N=26) Paras, mean: 3470 g (N=24) |
Not reported |
| Tasnim 2000 | Mean: 3200 g (range: 2500 to 2900) (N=72) | Mean: 3100 g (range: 2500 to 2900) (N=80) | Nor reported |
Krupa 2005 did not report on birthweight, however reported that 8/75 and 4/75 neonates were born small‐for‐gestational age or large‐for‐gestational age in the planned early birth and expectant management groups, respectively (P = 0.35). Sperling 1993 reported that "There were no differences in birthweight… between the two groups".
Cord prolapse
No clear difference in risk of cord prolapse was observed between the planned early birth and expectant management groups (RR 0.51; 95% CI 0.09 to 2.75; four trials, 5740 infants) (Analysis 1.27). Two of the four trials included in this meta‐analysis reported specifically on "caesarean for cord prolapse" (Shalev 1995; Tamsen 1990).
1.27. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 27 Cord prolapse.
Stillbirth
Only three trials reported on stillbirth (Hannah 1996; Krupa 2005; Ottervanger 1996); there were no stillborn babies in the planned early birth groups of the three trials; in Hannah 1996, there were two stillborn babies in the expectant management group (RR 0.20; 95% CI 0.01 to 4.18; three trials, 5314 infants) (Analysis 1.28). These data excluded lethal congenital anomalies.
1.28. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 28 Stillbirth.
Neonatal mortality
Seven trials reported on neonatal mortality. There were no neonatal deaths in the planned early birth groups of the seven trials; in Hannah 1996, there were two neonatal deaths in the expectant management group (RR 0.20; 95% CI 0.01 to 4.18; seven trials, 6352 neonates) (Analysis 1.29). These data excluded lethal congenital anomalies.
1.29. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 29 Neonatal mortality.
Pneumonia
Only two trials reported specifically on neonatal pneumonia, and showed no clear difference between the planned early birth and expectant management groups (RR 0.62; 95% CI 0.04 to 9.09; two trials, 280 infants) (Analysis 1.30).
1.30. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 30 Pneumonia.
Antibiotic usage
Significantly fewer neonates in the planned management group compared with the expectant management group received antibiotics (average RR 0.61; 95% CI 0.44 to 0.84; 10 trials, 6427 infants; Tau² = 0.06; I² = 32%) (Analysis 1.31). We ran a funnel plot to assess the risk of reporting bias, and we found some asymmetry, which could represent the presence of bias due to smaller studies (such as Akyol 1999, Tasnim 2000 and Wagner 1989, which were judged to be at unclear to high risk of bias) producing exaggerated intervention effect estimates (Figure 12). However, the overall result is also heavily influenced by the Hannah 1996 trial.
1.31. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 31 Antibiotic usage.
12.

Funnel plot of comparison: 1 Planned early birth versus expectant management (subgroups: method of induction), outcome: 1.31 Antibiotic usage.
Javaid 2008 reported that "Only 2% of babies delivered had A/S < 5 at 5 minutes and required active resuscitation and required admission in nursery. They remained oxygen dependent for 5‐7 days. Antibiotics were given but cultures were negative"; "Remaining babies have average A/S between 7 & 9 at 5 minutes 20% were admitted in nursery to tachypnea and given antibiotics for 5 days"; and that "requirement of antibiotics were comparable". It was not possible to include these data in the meta‐analysis.
Sperling 1993 also reported on neonatal antibiotic use, however it was not clear whether the text provided described all neonates who received antibiotics (or only a subset) and thus these data have not been included in the meta‐analysis. It was reported that of the 2/62 women in the expectant management group who developed clinical signs of chorioamnionitis, one of the neonates was treated with antibiotics because of fever and tachypnoea (the bacterial cultures were negative), and the other showed no signs of infection and received no antibiotics (Sperling 1993). The characteristics of the seven neonates (2/62 and 5/62 in the planned early birth versus expectant management groups, respectively) who were transferred to the paediatric department were also provided, however it was not always clear which group the neonates were from, and/or whether antibiotics were administered. "One infant. whose mother was an EI primipara, had subarachnoid bleeding and hydro‐cephalus, was treated with antibiotics and aspiration of the hematoma, and survived... Another infant was delivered by cesarean section because of signs of intraamniotic infection... A third infant was delivered vaginally 29 hours after the membranes had ruptured... Perinatally the infant was suspected of having septicemia and was treated with antibiotics. The bacterial cultures, however, were all negative. The remaining four infants were transferred because of hyperbilirubinemia... None of the newborns had positive bacterial cultures" (Sperling 1993).
Apgar score less than seven at five minutes
No clear difference was observed for Apgar score less than seven at five minutes between the planned early birth and expectant management groups (RR 1.07; 95% CI 0.77 to 1.48; 15 trials, 7175 infants) (Analysis 1.32). We ran a funnel plot to assess the risk of reporting bias, and we found that studies were equally distributed on either side, with no substantial asymmetry observed (Figure 13).
1.32. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 32 Apgar score less than seven at five minutes.
13.

Funnel plot of comparison: 1 Planned early birth versus expectant management (subgroups: method of induction), outcome: 1.32 Apgar score less than seven at five minutes.
Javaid 2008 reported that "Only 2% of babies delivered had A/S < 5 at 5 minutes... The outcome was comparable in both induction as well as expectant group". No further details were provided.
Use of mechanical ventilation
Only two trials reported on the need for mechanical ventilation, and showed no clear difference between planned early birth and expectant management groups (average RR 0.90; 95% CI 0.33 to 2.47; two trials, 5158 infants; Tau² = 0.36; I² = 66%) (Analysis 1.33).
1.33. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 33 Use of mechanical ventilation.
Abnormality on cerebral ultrasound (cystic periventricular leukomalacia; intraventricular haemorrhage)
Krupa 2005 reported that no neonates in either group had cerebral haemorrhage (Analysis 1.34).
1.34. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 34 Abnormality on cerebral ultrasound (cystic periventricular leukomalacia; intraventricular haemorrhage);.
Outcomes not reported by the included trials
None of the included trials reported on the following secondary outcomes: gestational age at birth; meningitis; respiratory distress syndrome; necrotising enterocolitis; neonatal encephalopathy; disability at of childhood follow‐up. Shah 2012 reported on "neonatal morbidity" (a non pre‐specified outcome) ("considered in cases of neonatal septicemia, convulsions, or with birth asphyxia") for 3/50 and 3/50 infants in the planned early birth and expectant management groups respectively.
Secondary outcomes (use of health services)
Duration of maternal antenatal or postnatal stay in hospital
Two trials reported on length of maternal hospitalisation (admission to discharge time (Shalev 1995);"Length of maternal hospitalization" (Wagner 1989)). A significant reduction in length of hospitalisation was observed for the planned early birth group compared with the expectant management group (MD: ‐0.79 days; 95% CI ‐1.20 to ‐0.38; two trials, 748 women; Tau² = 0.05; I² = 59%) (Analysis 1.35).
1.35. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 35 Duration of maternal antenatal or postnatal stay in hospital (days).
Four other trials reported relevant data that could not be included in the meta‐analysis (e.g. reported medians (5th, 95th percentiles only) (Akyol 1999; Hannah 1996; Krupa 2005; McQueen 1992; Shah 2012). Data from these trials were shown to be mostly consistent with the above meta‐analysis (i.e. showing significantly shorter maternal hospitalisation with planned early birth) (Analysis 1.36).
1.36. Analysis.
Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 36 Duration of maternal antenatal or postnatal stay in hospital (days).
| Duration of maternal antenatal or postnatal stay in hospital (days) | |||
|---|---|---|---|
| Study | Planned early birth | Expectant management | P value |
| Akyol 1999 | Time in hospital before birth Group 1: Median (5th, 95th percentiles): 20.5 hours (3.0, 4.8) (N=52) |
Time in hospital before birth Group 2: Median (5th, 95th percentiles): 22.0 (4.9, 45.8) (N=25) Group 3: Median (5th, 95th percentiles): 6.0 hours (1.3, 19.0) (N=49) |
Group 2 had a significant difference compared with Group 1 and 3 (P<0.05) |
| Hannah 1996 | Time in hospital before birth Oxytocin: Median (5th, 95th percentiles): 12.0 hours (4.6, 32.1) (N=1258) Prostaglandin: Median (5th, 95th percentiles): 17.0 hours (4.8, 38.9) (N=1259) |
Time in hospital before birth Oxytocin: Median (5th, 95th percentiles): 16.5 hours (2.9, 66.8) (N=1263) Prostaglandin: Median (5th, 95th percentiles): 16.9 hours (2.0, 69.7) (N=1261) |
P<0.001 |
| Krupa 2005 | "Woman stay > 3 days" 23/75 (30.7%) |
"Woman stay > 3 days" 37/75 (49.3%) |
P=0.03 |
| McQueen 1992 | Maternal hospitalisation Mean (range): 1.65 days (1‐7) (N=20) |
Maternal hospitalisation Mean (range): 2.9 days (1‐6) (N=20) |
Not reported |
| Shah 2012 | Hospital stay Mean: 3 days (N=50) |
Hospital stay Mean: 5 days (N=50) |
Not reported |
Javaid 2008 reported that women in the misoprostol group had a "shorter period of hospitalization".
Admission to neonatal special or intensive care unit
A significant reduction in the risk of admission to the neonatal special or intensive care unit was observed with planned early birth compared with expectant management (RR 0.75; 95% CI 0.66 to 0.85; eight trials, 6179 infants) (Analysis 1.37).
1.37. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 37 Admission to neonatal special or intensive care unit.
Javaid 2008 that "Neonatal admissions were 20% in our study," and that "No significant difference was observed in neonatal morbidity and nursery admission between both groups".
Duration of neonatal stay in hospital
Wagner 1989 reported on duration of neonatal hospitalisation, and observed a shorter duration for neonates born to mothers in the planned early birth group compared with expectant management group (MD ‐11.00 hours; 95% CI ‐21.96 to ‐0.04; 182 infants) (Analysis 1.38).
1.38. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 38 Duration of neonatal stay in hospital (hours).
Krupa 2005 reported on "Neonatal stay >3 days", which occurred in 2/75 (2.6%) and 5/75 (6.7%) neonates in the planned early birth versus expectant management groups respectively (P = 0.44).
Hannah 1996 reported on stay in the postpartum ward, with data reported as medians and 5th, 95th percentiles: induction oxytocin group: 62.97 hours (22.40, 130.78) (N = 1258) versus induction prostaglandin group: 62.50 hours (20.03, 136.88) (N = 1259) versus expectant oxytocin group: 63.02 hours (23.05, 137.18) (N = 1263) versus expectant prostaglandin group: 62.97 hours (23.03, 134.22) (N = 1261).
Neonatal stay in special or intensive care unit
A significantly shorter duration of neonatal stay in special or intensive care unit was observed for neonates born to mothers in the planned early birth group (RR 0.72; 95% CI 0.61 to 0.85; four trials, 5691 infants) (Analysis 1.39). We combined in a meta‐analysis the following outcomes: Akyol 1999 and Hannah 1996 reported on stay in the neonatal intensive care unit of more than 24 hours; Mahmood 1992 reported on stay in special care baby unit 25 to 48 hours and 49 hours; and Tamsen 1990 reported on "Treatment at neonatal ward > 7 days".
1.39. Analysis.

Comparison 1 Planned early birth versus expectant management (subgroups: method of induction), Outcome 39 Neonatal stay in special or intensive care unit.
Subgroup analyses
The subgroup analyses for method of induction have been integrated into the main structure of the graphs and comments relating to these subgroups have been made above, throughout the main analysis of primary outcomes.
Parity
We conducted subgroup analyses based on parity (comparing outcomes for studies including nulliparous women with those including multiparous women and those including both nulliparous and multiparous women). We did not observe any clear subgroup differences based on parity for any of our primary outcomes: maternal infectious morbidity (Chi² = 1.65, P = 0.44, I² = 0%) (Analysis 2.1); caesarean section (Chi² = 2.47, P = 0.29, I² = 19.0%) (Analysis 2.2); definite early‐onset neonatal infection (Chi² = 0.04, P = 0.84, I² = 0%) (Analysis 2.3); definite or probable early‐onset neonatal infection (Chi² = 1.22, P = 0.54, I² = 0%) (Analysis 2.4); or perinatal mortality (test for subgroup differences: not applicable) (Analysis 2.5). Similarly, no clear subgroup differences were observed for these outcomes when we excluded the mixed 'nulliparous and multiparous women' subgroup from these analyses.
2.1. Analysis.

Comparison 2 Planned early birth versus expectant management (subgroups: parity), Outcome 1 Maternal infectious morbidity (chorioamnionitis and/or endometritis).
2.2. Analysis.

Comparison 2 Planned early birth versus expectant management (subgroups: parity), Outcome 2 Caesarean section.
2.3. Analysis.

Comparison 2 Planned early birth versus expectant management (subgroups: parity), Outcome 3 Definite early‐onset neonatal sepsis.
2.4. Analysis.

Comparison 2 Planned early birth versus expectant management (subgroups: parity), Outcome 4 Definite or probable early‐onset neonatal sepsis.
2.5. Analysis.

Comparison 2 Planned early birth versus expectant management (subgroups: parity), Outcome 5 Perinatal mortality (stillbirth or neonatal mortality).
Cervical status
We conducted subgroup analyses based on cervical status at baseline (comparing outcomes for studies including women with unfavourable cervices only with those including women with both favourable and unfavourable cervices, and those where cervical status was not clear). We did not observe any clear subgroup differences based on cervical status for any of our primary outcomes: maternal infectious morbidity (Chi² = 2.93, P = 0.23, I² = 31.7%) (Analysis 3.1); caesarean section (Chi² = 0.61, P = 0.74, I² = 0%) (Analysis 3.2); definite early‐onset neonatal infection (Chi² = 1.92, P = 0.38, I² = 0%) (Analysis 3.3); definite or probable early‐onset neonatal infection (Chi² = 3.00, P = 0.22, I² = 33.4%) (Analysis 3.4); or perinatal mortality (Chi² = 0.05, P = 0.82, I² = 0%) (Analysis 3.5).
3.1. Analysis.

Comparison 3 Planned early birth versus expectant management (subgroups: cervical status), Outcome 1 Maternal infectious morbidity (chorioamnionitis and/or endometritis).
3.2. Analysis.

Comparison 3 Planned early birth versus expectant management (subgroups: cervical status), Outcome 2 Caesarean section.
3.3. Analysis.

Comparison 3 Planned early birth versus expectant management (subgroups: cervical status), Outcome 3 Definite early‐onset neonatal sepsis.
3.4. Analysis.

Comparison 3 Planned early birth versus expectant management (subgroups: cervical status), Outcome 4 Definite or probable early‐onset neonatal sepsis.
3.5. Analysis.

Comparison 3 Planned early birth versus expectant management (subgroups: cervical status), Outcome 5 Perinatal mortality (stillbirth or neonatal mortality).
Similarly, no clear subgroup differences were observed for these outcomes when we excluded the 'cervical status: not clear' subgroup from these analyses.
Maternal antibiotic prophylaxis
We conducted subgroup analyses based on maternal antibiotic prophylaxis (comparing outcomes for studies where all women, some women, or no women received prophylaxis for PROM (or where it was unclear/not stated). The test for subgroup differences for the outcome maternal infectious morbidity was significant (Chi² = 16.19, P = 0.001, I² = 81.5%), indicating a possible differential effect based on use of antibiotic prophylaxis (Analysis 4.1). A significant benefit in favour of planned early birth was shown in the 'all women' subgroup; while no clear differences were observed for the other subgroups. When we excluded the subgroup 'not clear' from the analysis, however, the test for subgroup differences was no longer significant (Chi² = 0.70, P = 0.70, I² = 0%).
4.1. Analysis.

Comparison 4 Planned early birth versus expectant management (subgroups: antibiotic prophylaxis), Outcome 1 Maternal infectious morbidity (chorioamnionitis and/or endometritis).
No clear subgroup differences were shown for the outcomes: caesarean section (Chi² = 1.11, P = 0.77, I² = 0%) (Analysis 4.2); definite early‐onset sepsis (Chi² = 0.04, P = 0.84, I² = 0%) (Analysis 4.3); definite or probable early‐onset sepsis (Chi² = 1.02, P = 0.60, I² = 0%) (Analysis 4.4); or perinatal mortality (Chi² = 0.05, P = 0.82, I² = 0%) (Analysis 4.5). For these outcomes, this was also the case when we excluded the 'routine antibiotic prophylaxis: not clear' subgroup from the analyses.
4.2. Analysis.

Comparison 4 Planned early birth versus expectant management (subgroups: antibiotic prophylaxis), Outcome 2 Caesarean section.
4.3. Analysis.

Comparison 4 Planned early birth versus expectant management (subgroups: antibiotic prophylaxis), Outcome 3 Definite early‐onset neonatal sepsis.
4.4. Analysis.

Comparison 4 Planned early birth versus expectant management (subgroups: antibiotic prophylaxis), Outcome 4 Definite or probable early‐onset neonatal sepsis.
4.5. Analysis.

Comparison 4 Planned early birth versus expectant management (subgroups: antibiotic prophylaxis), Outcome 5 Perinatal mortality (stillbirth or neonatal mortality).
Digital vaginal examination
We conducted subgroup analyses based on whether women received digital vaginal examination at baseline (comparing outcome for studies where all women, some women, or no women received digital vaginal examination (or where it was unclear/not stated). The test for subgroup differences for the outcome maternal infectious morbidity was significant (Chi² = 9.05, P = 0.03, I² = 66.9%), indicating a possible differential effect based on receipt of digital vaginal examination (Analysis 5.1). A significant benefit in favour of planned early birth was shown for the 'all women' and 'not clear' subgroups; while no clear differences were observed for the other subgroups. When we excluded the subgroup 'not clear' from the analysis, however, the test for subgroup differences was no longer significant (Chi² = 0.05, P = 0.982, I² = 0%).
5.1. Analysis.
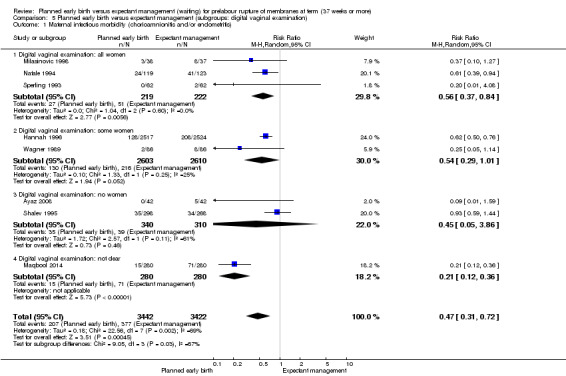
Comparison 5 Planned early birth versus expectant management (subgroups: digital vaginal examination), Outcome 1 Maternal infectious morbidity (chorioamnionitis and/or endometritis).
Similarly, for the outcome caesarean section (Analysis 5.2), the subgroup interaction test was significant (Chi² = 16.14, P = 0.001, I² = 81.4%), though the only subgroup to show a significant benefit in favour of planned early birth was the 'not clear' subgroup, and when we excluded this subgroup from the analysis, the interaction test no longer suggested a clear difference (Chi² = 0.77, P = 0.68, I² = 0%).
5.2. Analysis.
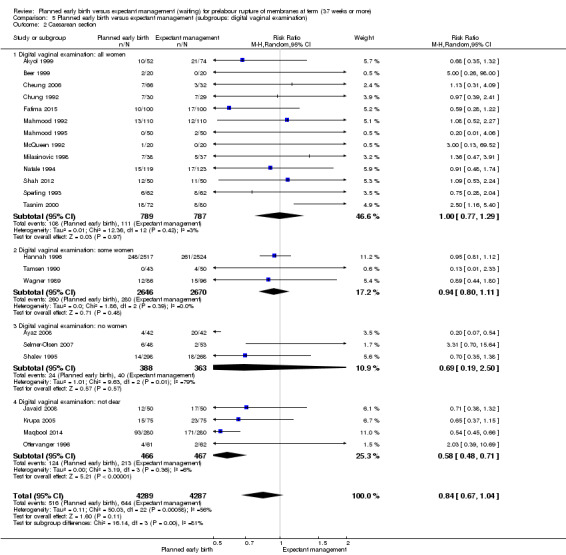
Comparison 5 Planned early birth versus expectant management (subgroups: digital vaginal examination), Outcome 2 Caesarean section.
No clear subgroup differences were shown for the outcomes: definite early‐onset sepsis (Chi² = 1.92, P = 0.38, I² = 0%) (Analysis 5.3); definite or probable early‐onset sepsis (Chi² = 0.67, P = 0.71, I² = 0%) (Analysis 5.4); or perinatal mortality (Chi² = 0.05, P = 0.82, I² = 0%) (Analysis 5.5). For these outcomes, this was also the case when we excluded the 'digital vaginal examination: not clear' subgroup from the analyses.
5.3. Analysis.

Comparison 5 Planned early birth versus expectant management (subgroups: digital vaginal examination), Outcome 3 Definite early‐onset neonatal sepsis.
5.4. Analysis.

Comparison 5 Planned early birth versus expectant management (subgroups: digital vaginal examination), Outcome 4 Definite or probable early‐onset neonatal sepsis.
5.5. Analysis.

Comparison 5 Planned early birth versus expectant management (subgroups: digital vaginal examination), Outcome 5 Perinatal mortality (stillbirth or neonatal mortality).
Sensitivity analysis based on trial quality
A sensitivity analysis based on trial quality was performed for the primary outcomes, by omitting all trials at high or unclear risk of selection bias (considering both allocation concealment and random sequence generation), and trials at high or unclear risk of detection bias. Only three trials contributed data to the sensitivity analyses (Cheung 2006; Chung 1992; Hannah 1996).
Results of the sensitivity analysis were consistent with those of the main analysis, with a reduction in maternal infectious morbidity observed with planned early birth compared with expectant management RR 0.62; 95% CI 0.50 to 0.76; one trial, 5041 women) (Analysis 6.1), and no clear differences between groups for the other outcomes; caesarean section (RR 0.96; 95% CI 0.81 to 1.12; three trials, 5198 women) (Analysis 6.2); definite early‐onset neonatal sepsis (no events; one trial, 59 infants) (Analysis 6.3); definite or probable early‐onset neonatal sepsis (RR 0.91; 95% CI 0.66 to 1.27; three trials, 5198 infants) (Analysis 6.4); and perinatal mortality (RR 0.50; 95% CI 0.13 to 2.00; one trial, 5041 infants) (Analysis 6.5).
6.1. Analysis.

Comparison 6 Planned early birth versus expectant management (sensitivity analysis based on trial quality), Outcome 1 Maternal infectious morbidity (chorioamnionitis and/or endometritis).
6.2. Analysis.

Comparison 6 Planned early birth versus expectant management (sensitivity analysis based on trial quality), Outcome 2 Caesarean section.
6.3. Analysis.

Comparison 6 Planned early birth versus expectant management (sensitivity analysis based on trial quality), Outcome 3 Definite early‐onset neonatal sepsis.
6.4. Analysis.

Comparison 6 Planned early birth versus expectant management (sensitivity analysis based on trial quality), Outcome 4 Definite or probable early‐onset neonatal sepsis.
6.5. Analysis.

Comparison 6 Planned early birth versus expectant management (sensitivity analysis based on trial quality), Outcome 5 Perinatal mortality (stillbirth or neonatal mortality).
Discussion
Summary of main results
This review included 23 randomised controlled trials (involving 8615 women and their babies; including the large Hannah 1996 trial of 5042 women) assessing planned early birth, compared with expectant management for prelabour rupture of membranes (PROM) at term. On meta‐analysis, we observed a reduction in the risk of maternal infectious morbidity (chorioamnionitis and/or endometritis) (eight trials, 6864 women) for women in the planned early birth group; the absolute risk reduction was 5.01% (from 11.02% (377/3422) in the expectant management group to 6.01% (207/3442) in the planned early birth group). We also observed a reduction in the risk of definite or probable early‐onset neonatal sepsis (from 4.10% (149/3637) in the expectant management group to 2.99% (110/3677) in the planned early birth group. We did not observe any clear differences between the planned early birth and expectant management groups when considering the other primary outcomes of caesarean section, serious maternal morbidity or mortality (no events in the three trials reporting on this outcome), definite early‐onset neonatal sepsis, or perinatal mortality (stillbirth and/or neonatal death).
Similarly, we did not observe clear differences between groups for many of the secondary review outcomes, including the maternal outcomes: endometritis; postpartum pyrexia; postpartum antibiotic usage; caesarean section for fetal distress; operative vaginal birth; uterine rupture; epidural analgesia; and postpartum haemorrhage; and the fetal/neonatal outcomes: cord prolapse; stillbirth; neonatal mortality; pneumonia; Apgar score less than seven at five minutes; use of mechanical ventilation; and abnormality on cerebral ultrasound.
We did, however, observe significant reductions with planned early birth in the risk of chorioamnionitis (either suspected or proven) (eight trials, 6874 women), postpartum septicaemia (three trials, 263 women), and neonatal antibiotic usage (10 trials, 6427 infants). In relation to the use of health services, women in the planned early birth group, compared with the expectant management group had a shorter length of hospitalisation, of 0.79 days on average (two trials, 748 women); their neonates also were less likely to be admitted to the neonatal special or intensive care unit (eight trials, 6179 infants), and were more likely to have a shorter neonatal stay in hospital of 11 hours on average (one trial, 182 infants), and a shorter stay in the special or intensive care unit (four trials, 5691 infants).
Women who had a planned early birth also viewed their care more favourably in two trials (93 women (Selmer‐Olsen 2007); and 5041 women (Hannah 1996)). In Selmer‐Olsen 2007, women rated their PROM management more positively in the planned early birth group; in Hannah 1996, fewer women in the planned early birth group disliked aspects of their management. While these findings are from single studies, they are valuable (particularly the results from Hannah 1996, which was a large, high‐quality trial), and contribute to the understanding of women's preferences.
As may have been expected, women in the planned early birth group were over three times more likely to have their labour induced (12 trials, 6945 women) and had a shorter duration of rupture of membranes (ROM) to birth, on average of 10 hours (nine trials, 1484 women). Babies born to women in the planned early birth group weighed, on average, 79 g less (five trials 1043 infants).
No information was available from the 23 included trials on the maternal outcomes: breastfeeding and postnatal depression; and fetal/neonatal outcomes: gestational age at birth; meningitis; respiratory distress syndrome; necrotising enterocolitis; neonatal encephalopathy. No trial reported on disability at childhood follow‐up.
There was substantial heterogeneity for some outcomes. The subgroup analyses (based on initial method of induction, parity, cervical status, antibiotic prophylaxis, digital vaginal examination) and sensitivity analyses (based on trial quality) performed in this review, however largely revealed no clear differential treatment effects according to characteristics of the women or trials. The different methods of outcome measurement and definitions, particularly for review outcomes relating to maternal and neonatal infection, also likely contributed to the heterogeneity observed.
Considering the mode of induction of labour, no clear subgroup differences were seen for definite early‐onset neonatal sepsis, definite or probable early‐onset neonatal sepsis or perinatal mortality, and we could not conduct a subgroup analysis for serious maternal morbidity or mortality (with no events occurring). While the subgroup analyses for maternal infectious morbidity and caesarean section suggested possible differential treatment effects (for maternal infectious morbidity: with a significant benefit seen only for the intravenous oxytocin and sublingual misoprostol subgroups; and for caesarean section: with a significant reduction seen for the sublingual misoprostol subgroup), no clear conclusions can be based on these data; the sublingual misoprostol subgroup included data from only one trial (560 women; unclear to high risk of bias) (Maqbool 2014), and when this subgroup was removed from the analyses, the interaction tests were no longer significant. Considering the subgroup analyses based on parity, cervical status, maternal antibiotic prophylaxis and digital vaginal examination, largely no differential effects were observed for any of the primary outcomes. Where we did observe possible differential effects (interaction tests were significant), these were mostly not sustained when the mixed/unclear subgroups were removed from analyses; thus the poor reporting or limited methodological detail provided by many of the included studies limited our ability to assess subgroup effects.
The sensitivity analyses, including only those high‐quality trials (judged at low risk of selection and detection bias) (including only three trials: Cheung 2006; Chung 1992; Hannah 1996) revealed similar findings to the main analysis ‐ a reduction in maternal infectious morbidity (chorioamnionitis and/or endometritis) with planned early birth, and no clear differences between groups for the other primary outcomes.
Overall completeness and applicability of evidence
Although 23 trials were able to be included in this review, over 30 other studies were excluded, and some of these may have had relevant data. Most of these trials reported outcomes for women less than, as well as more than, 37 weeks' gestation at PROM and it was not possible to extract only the information relating to women 37 weeks' gestation or later at PROM; nor were trial authors able to provide this information when it was requested from them. This strict inclusion criterion was applied because women at full term may represent a different clinical group than women with PROM at less than 37 weeks' gestation (which is the topic of another Cochrane review (Buchanan 2010)).
Many important outcomes were not assessed at all by the included trials (including breastfeeding; postnatal depression; gestational age at birth; meningitis; respiratory distress syndrome; necrotising enterocolitis; neonatal encephalopathy; and disability at childhood follow‐up). While all 23 included trials reported on caesarean section, many did not report on the other primary outcomes (for example, with only three trials reporting on serious maternal morbidity or mortality), and for a number of secondary outcomes (such as endometritis; uterine rupture; views of care; neonatal pneumonia; use of mechanical ventilation; and abnormality on cerebral ultrasound), only one or two trials contributed outcome data for the meta‐analyses.
The included trials were conducted in China (Cheung 2006; Chung 1992), Scotland (Mahmood 1992; Mahmood 1995), Brazil (Krupa 2005), Canada (Natale 1994), Denmark (Sperling 1993), Germany (Beer 1999), India (Shah 2012), Norway (Selmer‐Olsen 2007), Pakistan (Ayaz 2008; Fatima 2015; Javaid 2008; Maqbool 2014; Tasnim 2000), Serbia (Milasinovic 1998), Sweden (Tamsen 1990), the Netherlands (Ottervanger 1996), Turkey (Akyol 1999), USA (Wagner 1989), Zimbabwe (McQueen 1992), and multiple countries (Canada, the UK, Australia, Israel, Sweden and Denmark) (Hannah 1996). The results of the review may therefore be applicable to a variety of settings or countries across the world.
Quality of the evidence
The methodological quality of the 23 trials included in this review was low to moderate; overall, most of the trials were judged to be at unclear risk of bias. We were only able to include three 'high‐quality' trials in our sensitivity analyses (Cheung 2006; Chung 1992; Hannah 1996). Only four of the 23 trials were judged to be at low risk of selection bias (with adequate methods used to generate the random sequence and conceal allocation) (Cheung 2006; Chung 1992; Hannah 1996; Krupa 2005). The potential for performance bias was common across the included trials, due to the nature of the intervention. All trials were judged to be at high risk of performance bias, with the exception of three, which used a placebo (Beer 1999; Cheung 2006; Chung 1992). Eight of the trials were judged to be low risk of detection bias, with blinding of outcome assessment (Akyol 1999; Beer 1999; Cheung 2006; Chung 1992; Hannah 1996; Mahmood 1992; Natale 1994; Sperling 1993). Very few trials reported clearly on losses to follow‐up or missing data (with only eight judged to be low risk of attrition bias (Cheung 2006; Fatima 2015; Hannah 1996; Krupa 2005; Mahmood 1992; Milasinovic 1998; Selmer‐Olsen 2007; Shah 2012)), and only fives trials were considered to be at low risk of reporting bias (Hannah 1996; Krupa 2005; Mahmood 1992; Mahmood 1995; Shalev 1995). In many cases, poor reporting (and limited methodological detail provided) in the included trials led to 'unclear' judgements across one or more of the seven pre‐specified domains for assessing risk of bias.
GRADE profiler was used to assess the quality of the evidence for our primary outcomes. The evidence was of moderate quality for perinatal mortality, low quality for maternal infectious morbidity, caesarean section and definite or probable early‐onset neonatal sepsis; and of very low quality for definite early‐onset neonatal sepsis and serious maternal morbidity or mortality. Evidence was mainly downgraded due to the majority of studies contributing data having some serious or very serious design limitations, and for most outcomes estimates were imprecise (seeTable 1 for a full explanation of reasons for downgrading for each outcome).
Potential biases in the review process
We were aware of the possibility of introducing bias through the review process, and thus tried to minimise bias in a number of ways. Two review authors independently assessed eligibility for inclusion, carried out data extraction and assessed risk of bias. As we included only those trials with reported data for women at 37 weeks' gestation or later at PROM, the evidence we have presented may not represent the total available evidence (given that some trials have been excluded based on also including women at earlier gestations at PROM). However, we developed and applied a priori criteria to enable us to select trials for inclusion in a consistent manner, and all trial selection and assessment steps were performed by two review authors to maximise consistency of judgement.
To reduce the potential for publication bias, we conducted a detailed, systematic search process without language or publication status restrictions. It is possible that additional trials comparing planned early birth versus expectant management for PROM at term have been published but not identified. It is also possible that there are other studies that have been conducted but are not yet published. Should any such studies be identified, we will include them in future updates of this review.
We explored the potential for publication bias using funnel plots for the review's primary outcomes maternal infectious morbidity, caesarean section and definite or probable early‐onset neonatal sepsis, and secondary outcomes, chorioamnionitis and/or pyrexia, caesarean section for fetal distress, induction of labour, operative vaginal birth, time from ROM to birth, neonatal antibiotic usage and Apgar score less than seven at five minutes. While there was no clear indication of publication bias, there was some asymmetry, possibly associated with small‐study effects (i.e. with small studies of low methodological quality producing exaggerated intervention effect estimates) for three outcomes (maternal infectious morbidity, chorioamnionitis, and neonatal antibiotic usage).
Agreements and disagreements with other studies or reviews
Mozurkewich 2009, has assessed the evidence supporting different indications for induction, including for PROM at term, and identified three systematic reviews (including the previous version of this review, Dare 2006; Lin 2005 and Mozurkewich 1997). Two reviews compared early birth (such as with oxytocin, prostaglandin E2 or Caulophyllum) with conservative management (Mozurkewich 1997: 23 trials, 7493 women; Dare 2006: 12 trials, 6814 women), and the third assessed misoprostol for induction following PROM at term (Lin 2005: six trials, 452 women). Mozurkewich 2009 concluded, based on these three reviews, that "Expedited induction of labour after PROM reduces chorioamnionitis, endometritis, and admissions to a neonatal intensive care unit. Quality of evidence: high, grade of recommendation for induction of labour: strong". While the general findings of the Mozurkewich 2009 'best evidence' review are similar to those of our review, the assessments of the quality of the evidence vary. In Mozurkewich 2009, while the GRADE system was also used, it appears that it was applied differently (i.e. across all outcomes in the three reviews) "In evaluating the strength of the evidence for each indication for induction, we adhered to the GRADE system that classifies the overall quality of evidence as high, moderate, low, and very low"; Mozurkewich 2009 then assigned one of four categories to the evidence: 'net benefits', 'trade‐offs', 'uncertain trade‐offs' or 'net harm'; and subsequently classified a recommendation as 'strong' where the quality of the evidence was considered high, and there was a 'net benefit' for induction of labour.
Mishanina 2014 conducted a broader review of whether the number of caesarean births were higher with induction of labour compared with expectant management and found that overall the number of caesarean births at term were lower when labour was induced (this included women with and without PROM).
A number of other current Cochrane reviews are of relevance to our review.
Buchanan 2010 assessed planned early birth compared with expectant management for women with preterm PROM. While this review showed no clear differences between groups for neonatal sepsis and perinatal mortality, increases in endometritis and caesarean birth with planned early birth were observed (Buchanan 2010). The review, however, included only seven trials (690 women), all with methodological weaknesses, and concluded that there is insufficient evidence to guide clinical practice on the benefits and harms of planned early birth compared with expectant management for women with preterm PROM (Buchanan 2010). The next update of this review will consider the large PPROMT trial of 1839 women (Morris 2016), which has recently shown expectant management to be a more optimal strategy than immediate birth in women with ruptured membranes close to term.
Gülmezoglu 2012 assessed induction of labour compared with expectant management for women at or beyond term. This review, including 22 trials (9383 women) showed reductions in perinatal mortality and caesarean section birth with induction of labour (Gülmezoglu 2012). These benefits were however predominately associated with post‐term induction (41 weeks' gestation or later), and the review concluded that as the absolute risk of perinatal mortality is small, women should be appropriately counselled in order to make an informed choice between being scheduled for induction post‐term or monitored without induction (Gülmezoglu 2012).
Wojcieszek 2014 assessed the routine use of antibiotics for PROM at or near term. This review, including four trials (2639 women), similarly identified no differences in neonatal sepsis and perinatal mortality. In contrast to our review, Wojcieszek 2014 did not show a difference in maternal infectious morbidity, and an increase in caesarean section with the use of antibiotics was observed (which was largely attributed to one study of 1640 women, in which repeat caesarean section, increased baseline hypertension and pre‐eclampsia were more common in the antibiotic group, despite randomisation processes) (Wojcieszek 2014). The review concluded that there is no convincing evidence of benefit for mothers or neonates from the routine use of antibiotics for PROM at or near term (Wojcieszek 2014).
Authors' conclusions
Implications for practice.
There is low‐quality evidence to suggest that planned early birth (with induction methods such as oxytocin or prostaglandins) reduces the risk of maternal infectious morbidity (chorioamnionitis and/or endometritis) and definite or probable neonatal sepsis compared with expectant management for prelabour rupture of membranes (PROM) at 37 weeks' gestation or later, without increasing the risk of caesarean birth. Very low‐ to moderate‐quality evidence suggests no clear differences between planned early birth and expectant management for serious maternal morbidity or mortality, perinatal mortality and definite neonatal sepsis. Women should be appropriately counselled in order to make an informed choice between planned early birth and expectant management for PROM at 37 weeks' gestation or later.
Implications for research.
Although the 23 included trials in this review involved a large number of women and babies, the quality of the trials and evidence was not high overall, and there was very limited reporting of data for a number of important outcomes. Thus, further evidence assessing the benefits or harms of planned early birth compared with expectant management, considering maternal, fetal, neonatal and longer‐term childhood outcomes, and the use of health services, would be valuable. Any future trials should be adequately designed (including appropriate randomisation processes and blinding of outcome assessment) and powered to evaluate the effects on short‐ and longer‐term outcomes. Standardisation of outcomes and their definitions, including for the assessment of maternal and neonatal infection, would be beneficial.
Feedback
Kripke, March 2006
Summary
There appears to be an inconsistency between the abstract and text. In the abstract it says, "However, fewer infants under planned early birth went to neonatal intensive or special care compared with expectant management (RR 0.72, 95% CI 0.57 to 0.92)"
Then the main text of results states,"Overall, there were fewer admissions to the neonatal intensive care unit or special care nursery for planned early birth compared with expectant management (RR 0.73, 95% CI 0.58 to 0.91; 5 trials, 5679 infants)."
Which relative risk and confidence interval are correct?
(Summary of comment from Clarissa Kripke, March 2006)
Reply
Thank you for your comment. We have checked the figures and confirm that the relative risk and the confidence interval in the Abstract are correct. We have corrected the figures in the text.
(Reply from Philippa Middleton, February 2007)
Contributors
Clarissa Kripke
What's new
| Date | Event | Description |
|---|---|---|
| 9 September 2016 | New search has been performed | Searched updated. Methods updated. Two new co‐authors (Emily Shepherd and Rosemary McBain) were involved in this update. |
| 9 September 2016 | New citation required but conclusions have not changed | Eleven new trials have been incorporated. The conclusions of this review remain unchanged. |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 1, 2006
| Date | Event | Description |
|---|---|---|
| 30 July 2008 | Amended | Converted to new review format. |
| 1 October 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to acknowledge the support from the Cochrane Pregnancy and Childbirth editorial team in Liverpool, and the Australia and New Zealand Satellite of Cochrane Pregnancy and Childbirth (funded by the Australian National Health and Medical Research Council (NHMRC)).
We thank Therese Dowswell (TD) (Cochrane Pregnancy and Childbirth Group) for providing assistance in data extraction and entry and in grading the evidence for the 'Summary of findings' table. TD is supported by the NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines.
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
We thank Ruth Martis for translating the German language study into English.
Appendices
Appendix 1. Searches carried in out previous versions of the review
In the previous version of the review (Dare 2006), authors also searched the Cochrane Central Register of Controlled Trials (the Cochrane Library, Issue 4, 2004), MEDLINE (1966 to November 2004) and Embase (1974 to November 2004) using the following terms: (term) and [('rupture near membranes') or 'PROM'] and ('induction' and 'labo*r') and ('randomi*ed controlled trial').
Data and analyses
Comparison 1. Planned early birth versus expectant management (subgroups: method of induction).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal infectious morbidity (chorioamnionitis and/or endometritis) | 8 | 6864 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.33, 0.72] |
| 1.1 Intravenous oxytocin | 5 | 3625 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.40, 0.85] |
| 1.2 Oral misoprostol | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 1.59] |
| 1.3 Sublingual misoprostol | 1 | 560 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.12, 0.36] |
| 1.4 Vaginal prostaglandin E2 | 2 | 2595 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.39, 1.23] |
| 2 Perinatal mortality (stillbirth or neonatal mortality) | 8 | 6392 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.13, 1.66] |
| 2.1 Intravenous oxytocin | 5 | 3402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.10, 2.02] |
| 2.2 Vaginal misoprostol | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Vaginal prostaglandin E2 | 3 | 2840 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.52] |
| 3 Caesarean section | 23 | 8576 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.69, 1.04] |
| 3.1 Acupuncture | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 3.31 [0.70, 15.64] |
| 3.2 Intravenous oxytocin | 10 | 4169 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.76, 1.27] |
| 3.3 Oral Caulophyllum | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.26, 98.00] |
| 3.4 Oral misoprostol | 4 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.30, 1.00] |
| 3.5 Sublingual misoprostol | 1 | 560 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.45, 0.66] |
| 3.6 Vaginal misoprostol | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.37, 1.15] |
| 3.7 Vaginal prostaglandin E2 | 6 | 3074 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.75, 1.13] |
| 4 Serious maternal morbidity or mortality (e.g. death, cardiac arrest, respiratory arrest, admission to intensive care unit) | 3 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Intravenous oxytocin | 2 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Vaginal misoprostol | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Definite early‐onset neonatal sepsis | 6 | 1303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.24, 1.33] |
| 5.1 Intravenous oxytocin | 4 | 1024 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.20, 1.43] |
| 5.2 Vaginal prostaglandin E2 | 2 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 3.91] |
| 6 Definite or probable early‐onset neonatal sepsis | 16 | 7314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.58, 0.92] |
| 6.1 Intravenous oxytocin | 7 | 3708 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.43, 0.96] |
| 6.2 Oral misoprostol | 3 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.18, 1.08] |
| 6.3 Vaginal misoprostol | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 Vaginal prostaglandin E2 | 6 | 3074 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.64, 1.15] |
| 7 Maternal infectious morbidity (chorioamnionitis, endometritis and/or pyrexia) | 14 | 7667 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.38, 0.76] |
| 7.1 Intravenous oxytocin | 6 | 3751 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.43, 0.92] |
| 7.2 Oral misoprostol | 3 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.08, 0.70] |
| 7.3 Sublingual misoprostol | 1 | 560 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.12, 0.36] |
| 7.4 Vaginal prostaglandin E2 | 5 | 2974 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.49, 1.22] |
| 8 Chorioamnionitis (either suspected or proven) | 8 | 6874 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.37, 0.82] |
| 9 Chorioamnionitis and/or pyrexia (either suspected or proven) | 14 | 7677 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.42, 0.85] |
| 10 Endometritis | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.05, 1.14] |
| 11 Postpartum pyrexia | 7 | 5713 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.45, 1.84] |
| 12 Postpartum septicaemia | 3 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.07, 0.96] |
| 13 Postpartum antibiotic usage | 4 | 685 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.40, 1.20] |
| 14 Caesarean section for fetal distress | 11 | 1851 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.60, 1.49] |
| 15 Induction of labour | 12 | 6945 | Risk Ratio (M‐H, Random, 95% CI) | 3.41 [2.87, 4.06] |
| 16 Operative vaginal birth | 13 | 6379 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.67, 1.59] |
| 17 Uterine rupture | 2 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 68.50] |
| 18 Epidural analgesia | 5 | 585 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.80, 1.42] |
| 19 Postpartum haemorrhage | 3 | 520 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.14, 1.28] |
| 20 Views of care (VAS 100) | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | 11.80 [4.36, 19.24] |
| 20.1 ‘How do you experience your plan of treatment after PROM?’ | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | 11.80 [4.36, 19.24] |
| 21 Views of care | 1 | 5041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.36, 0.52] |
| 21.1 Nothing liked about treatment | 1 | 5041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.36, 0.52] |
| 22 Views of care | 1 | 5041 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.10, 1.30] |
| 22.1 Nothing disliked about treatment | 1 | 5041 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.10, 1.30] |
| 23 Time from rupture of membranes to birth (hours) | 9 | 1484 | Mean Difference (IV, Random, 95% CI) | ‐10.10 [‐12.15, ‐8.06] |
| 24 Time from rupture of membranes to birth (hours) | Other data | No numeric data | ||
| 25 Birthweight (g) | 5 | 1043 | Mean Difference (IV, Fixed, 95% CI) | ‐79.25 [‐124.96, ‐33.55] |
| 26 Birthweight (g) | Other data | No numeric data | ||
| 27 Cord prolapse | 4 | 5740 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.09, 2.75] |
| 28 Stillbirth | 3 | 5314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.18] |
| 29 Neonatal mortality | 7 | 6352 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.18] |
| 30 Pneumonia | 2 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.04, 9.09] |
| 31 Antibiotic usage | 10 | 6427 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.44, 0.84] |
| 32 Apgar score less than seven at five minutes | 15 | 7175 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.77, 1.48] |
| 33 Use of mechanical ventilation | 2 | 5158 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.33, 2.47] |
| 34 Abnormality on cerebral ultrasound (cystic periventricular leukomalacia; intraventricular haemorrhage); | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Duration of maternal antenatal or postnatal stay in hospital (days) | 2 | 748 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.20, ‐0.38] |
| 36 Duration of maternal antenatal or postnatal stay in hospital (days) | Other data | No numeric data | ||
| 37 Admission to neonatal special or intensive care unit | 8 | 6179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.66, 0.85] |
| 38 Duration of neonatal stay in hospital (hours) | 1 | 182 | Mean Difference (IV, Fixed, 95% CI) | ‐11.0 [‐21.96, ‐0.04] |
| 39 Neonatal stay in special or intensive care unit | 4 | 5691 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.61, 0.85] |
Comparison 2. Planned early birth versus expectant management (subgroups: parity).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal infectious morbidity (chorioamnionitis and/or endometritis) | 8 | 6864 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.31, 0.72] |
| 1.1 Nulliparous women | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.89] |
| 1.2 Multiparous women | 2 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 1.59] |
| 1.3 Nulliparous and multiparous women | 6 | 6656 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.32, 0.76] |
| 2 Caesarean section | 23 | 8576 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.69, 1.03] |
| 2.1 Nulliparous women | 6 | 3519 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.76, 1.18] |
| 2.2 Multiparous women | 6 | 2370 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.21, 1.10] |
| 2.3 Nulliparous and multiparous women | 15 | 2687 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.67, 1.15] |
| 3 Definite early‐onset neonatal sepsis | 6 | 1303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.24, 1.33] |
| 3.1 Nulliparous women | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 3.91] |
| 3.2 Nulliparous and multiparous women | 5 | 1083 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.20, 1.43] |
| 4 Definite or probable early‐onset neonatal sepsis | 16 | 7314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.58, 0.92] |
| 4.1 Nulliparous women | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 3.91] |
| 4.2 Multiparous women | 2 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.05, 1.62] |
| 4.3 Nulliparous and multiparous women | 13 | 6910 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.59, 0.95] |
| 5 Perinatal mortality (stillbirth or neonatal mortality) | 8 | 6392 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.13, 1.66] |
| 5.1 Nulliparous women | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Multiparous women | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Nulliparous and multiparous women | 6 | 6072 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.13, 1.66] |
Comparison 3. Planned early birth versus expectant management (subgroups: cervical status).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal infectious morbidity (chorioamnionitis and/or endometritis) | 8 | 6864 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.31, 0.72] |
| 1.1 Unfavourable cervices | 3 | 331 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.11, 0.69] |
| 1.2 Favourable and unfavourable cervices | 3 | 5407 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.51, 0.74] |
| 1.3 Cervical status: not clear | 2 | 1126 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.10, 1.93] |
| 2 Caesarean section | 23 | 8576 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.67, 1.04] |
| 2.1 Unfavourable cervices | 5 | 552 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.42, 2.02] |
| 2.2 Favourable and unfavourable cervices | 9 | 6244 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.79, 1.05] |
| 2.3 Cervical status: not clear | 9 | 1780 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.56, 1.10] |
| 3 Definite early‐onset neonatal sepsis | 6 | 1303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.24, 1.33] |
| 3.1 Unfavourable cervices | 3 | 393 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.81] |
| 3.2 Favourable and unfavourable cervices | 2 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 3.91] |
| 3.3 Cervical status: not clear | 1 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.29, 2.75] |
| 4 Definite or probable early‐onset neonatal sepsis | 16 | 7314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.58, 0.92] |
| 4.1 Unfavourable cervices | 5 | 552 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.19, 0.84] |
| 4.2 Favourable and unfavourable cervices | 6 | 5783 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.60, 1.11] |
| 4.3 Cervical status: not clear | 5 | 979 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.50, 1.06] |
| 5 Perinatal mortality (stillbirth or neonatal mortality) | 8 | 6392 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.13, 1.66] |
| 5.1 Unfavourable cervices | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Favourable and unfavourable cervices | 3 | 5361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.13, 2.00] |
| 5.3 Cervical status: not clear | 4 | 879 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
Comparison 4. Planned early birth versus expectant management (subgroups: antibiotic prophylaxis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal infectious morbidity (chorioamnionitis and/or endometritis) | 8 | 6864 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.31, 0.72] |
| 1.1 Routine antibiotic prophylaxis: all women | 2 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.12, 0.35] |
| 1.2 Routine antibiotic prophylaxis: some women | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.10, 1.27] |
| 1.3 Routine antibiotic prophylaxis: no women | 1 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.08] |
| 1.4 Routine antibiotic prophylaxis: not clear | 4 | 6021 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.51, 0.85] |
| 2 Caesarean section | 23 | 8576 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.67, 1.04] |
| 2.1 Routine antibiotic prophylaxis: all women | 5 | 996 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.40, 1.38] |
| 2.2 Routine antibiotic prophylaxis: some women | 5 | 533 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.66, 1.92] |
| 2.3 Routine antibiotic prophylaxis: no women | 2 | 247 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.41, 2.34] |
| 2.4 Routine antibiotic prophylaxis: not clear | 11 | 6800 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.78, 1.03] |
| 3 Definite early‐onset neonatal sepsis | 6 | 1303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.24, 1.33] |
| 3.1 Routine antibiotic prophylaxis: all women | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Routine antibiotic prophylaxis: some women | 2 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 3.91] |
| 3.3 Routine antibiotic prophylaxis: not clear | 3 | 872 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.20, 1.43] |
| 4 Definite or probable early‐onset neonatal sepsis | 16 | 7314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.58, 0.92] |
| 4.1 Routine antibiotic prophylaxis: all women | 3 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.54, 1.16] |
| 4.2 Routine antibiotic prophylaxis: some women | 5 | 533 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.23, 1.12] |
| 4.3 Routine antibiotic prophylaxis: no women | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Routine antibiotic prophylaxis: not clear | 7 | 6322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.56, 1.00] |
| 5 Perinatal mortality (stillbirth or neonatal mortality) | 8 | 6392 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.13, 1.66] |
| 5.1 Routine antibiotic prophylaxis: all women | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Routine antibiotic prophylaxis: some women | 3 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 5.3 Routine antibiotic prophylaxis: no women | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Routine antibiotic prophylaxis: not clear | 3 | 5757 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.13, 2.00] |
Comparison 5. Planned early birth versus expectant management (subgroups: digital vaginal examination).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal infectious morbidity (chorioamnionitis and/or endometritis) | 8 | 6864 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.31, 0.72] |
| 1.1 Digital vaginal examination: all women | 3 | 441 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.37, 0.84] |
| 1.2 Digital vaginal examination: some women | 2 | 5213 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.29, 1.01] |
| 1.3 Digital vaginal examination: no women | 2 | 650 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.05, 3.86] |
| 1.4 Digital vaginal examination: not clear | 1 | 560 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.12, 0.36] |
| 2 Caesarean section | 23 | 8576 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.67, 1.04] |
| 2.1 Digital vaginal examination: all women | 13 | 1576 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.77, 1.29] |
| 2.2 Digital vaginal examination: some women | 3 | 5316 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.80, 1.11] |
| 2.3 Digital vaginal examination: no women | 3 | 751 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.19, 2.50] |
| 2.4 Digital vaginal examination: not clear | 4 | 933 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.48, 0.71] |
| 3 Definite early‐onset neonatal sepsis | 6 | 1303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.24, 1.33] |
| 3.1 Digital vaginal examination: all women | 4 | 555 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 3.91] |
| 3.2 Digital vaginal examination: some women | 1 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.81] |
| 3.3 Digital vaginal examination: no women | 1 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.29, 2.75] |
| 4 Definite or probable early‐onset neonatal sepsis | 16 | 7314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.58, 0.92] |
| 4.1 Digital vaginal examination: all women | 10 | 1168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.46, 0.91] |
| 4.2 Digital vaginal examination: some women | 2 | 5223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.57, 1.08] |
| 4.3 Digital vaginal examination: no women | 2 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.25, 1.94] |
| 4.4 Digital vaginal examination: not clear | 2 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Perinatal mortality (stillbirth or neonatal mortality) | 8 | 6392 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.13, 1.66] |
| 5.1 Digital vaginal examination: all women | 4 | 512 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 5.2 Digital vaginal examination: some women | 1 | 5041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.13, 2.00] |
| 5.3 Digital vaginal examination: no women | 1 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Digital vaginal examination: not clear | 2 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Planned early birth versus expectant management (sensitivity analysis based on trial quality).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal infectious morbidity (chorioamnionitis and/or endometritis) | 1 | 5041 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.50, 0.76] |
| 2 Caesarean section | 3 | 5198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.81, 1.12] |
| 3 Definite early‐onset neonatal sepsis | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Definite or probable early‐onset neonatal sepsis | 3 | 5198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.66, 1.27] |
| 5 Perinatal mortality (stillbirth or neonatal mortality) | 1 | 5041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.13, 2.00] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akyol 1999.
| Methods | Randomised controlled trial. | |
| Participants | 126 women were randomised. Setting: Dr Zekai Tahir Burak Women's Hospital, Ankara, Turkey, October 1997 to February 1998. Inclusion criteria: women with PROM, at least 37 weeks' gestation, and had a single fetus in cephalic presentation. PROM was determined clinically and confirmed by positive litmus or ferning tests. If necessary, a vaginal exam was performed with a speculum; no further vaginal exam was done in the conservative (control) group until labour started spontaneously, or was induced. Exclusion criteria: women in active labour, previous failed attempt to induce labour, contraindication to either induction of labour (such as placenta praevia) or expectant management (such as meconium staining of amniotic fluid or chorioamnionitis). Characteristics for planned subgroup analyses: Method of induction: intravenous oxytocin. Parity: mixed. Both nulliparous and multiparous women included (% presented in Table 1 of manuscript) (34/52 (65%) nulliparous in planned early birth group versus 49/74 (66%) in expectant management group). Cervix: mixed. Mixture of ‘cervix unripe’ (< 3 cm dilated and < 80% effaced) and ‘cervix ripe’ (% presented in Table 1 in manuscript); (26/52 (50%) unripe cervix in planned early birth group versus 36/74 (49%) unripe cervix in expectant management group). Antibiotic prophylaxis: not stated for prophylaxis. Some women received antibiotics before or during labour (Table 2 in manuscript) (23/52 (44%) versus 34/74 (46%)). Digital vaginal examination: all women. Cervical status at baseline was assessed from 'Digital vaginal examination' (Table 1 in manuscript). |
|
| Interventions |
Planned early birth (n = 52): immediate induction of labour with intravenous oxytocin. The infusion was initiated and the infusion rate was titrated to contractions according to local hospital practice. Expectant management (n = 74): labour induced with oxytocin after 24 hours (n = 25) or labour began spontaneously within 24 hours (n = 49). |
|
| Outcomes | Outcome data in meta‐analyses for: maternal infectious morbidity; caesarean section; chorioamnionitis; postpartum pyrexia; induction of labour; operative vaginal birth; time from ROM to birth; antibiotic usage; Apgar score < 7 at 5 minutes; use of mechanical ventilation; duration of maternal antenatal or postnatal stay in hospital; duration of neonatal stay in special or intensive care unit. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participating women were randomly assigned (simple randomization)…". |
| Allocation concealment (selection bias) | Unclear risk | As above, no further details given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding, and considered unfeasible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An adjudication committee, unaware of the women’s group assignments and of whether labour was induced or spontaneous, determine whether neonatal infection was present". Low risk for neonatal infection; unclear risk for other outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Results reported in tables with group numbers reflecting no losses (i.e. 52 and 74); however not clearly specified that there were no losses or that there was no attrition. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome pre‐defined in methods as definite or probable neonatal infection; however results are reported for ‘neonatal antibiotics’ only. No access to trial protocol to further assess reporting bias. |
| Other bias | Unclear risk | Limited information regarding baseline characteristics provided; limited methodological detail provided. |
Ayaz 2008.
| Methods | Quasi‐randomised controlled trial. | |
| Participants | 84 women were randomised. Setting: Bahawal Victoria Hospital, Bahawalpur, Pakistan, June 2004 to November 2004. Inclusion criteria: women aged between 25 and 35 years, multi‐gravid (parity ≤ 5), demonstrated PROM (< 4 hours; confirmed by detection of a pool of amniotic fluid on sterile speculum and using a nitrazine test; digital vaginal exam was avoided), at term (at least 37 weeks' gestation), who were not in labour, had a singleton pregnancy with cephalic presentation, a normal cardiotocogram and an adequate pelvis on clinical pelvimetry. Exclusion criteria: women in established labour at the time of presentation, signs and symptoms suggestive of chorioamnionitis (maternal fever, tachycardia, uterine pain/tenderness, purulent vaginal discharge, fetal tachycardia), primigravid status, fetal distress (meconium), malpresentation, postdate pregnancy, cord prolapse, inadequate pelvis on clinical pelvimetry, previous uterine surgery, sensitivity to misoprostol, and other medical problems (vaginal growth retardation, diabetes mellitus). Characteristics for planned subgroup analyses: Method of induction: oral misoprostol. Parity: multigravid women (parity < 5) were included in the trial. Cervix: all women had an unfavourable cervix (no definition provided). Antibiotic prophylaxis: all women. "In both groups, prophylactic antibiotics were given". Digital vaginal examination: not stated at baseline. "Digital vaginal examination was avoided" for PROM diagnosis; when uterine activity suggested the onset of labour "vaginal assessment was performed". |
|
| Interventions |
Planned early birth (n = 42): oral misoprostol 50 μg was given every 4 hours for a maximum of 4 doses (doses were repeated if there were no uterine contractions or less than 2 mild contractions in 10 minutes). When uterine activity suggested the onset of labour, vaginal assessment was performed and the women were moved to the labour ward. (Failed induction of labour: vaginal delivery not achieved within 24 hours of initiating induction.) The indications for caesarean section were uncontrolled hyperstimulation, chorioamnionitis and/or fetal distress. Expectant management (n = 42): women were observed for 24 hours (continuous maternal and fetal monitoring was performed). Detailed records of progress were maintained with a partogram. After failed conservative management of labour (a vaginal delivery not achieved/any intervention required within 24 hours) further options were discussed and labour augmented with oxytocin or prostaglandins. |
|
| Outcomes | Outcome data in meta‐analyses for: maternal infectious morbidity; caesarean section; definite early‐onset neonatal sepsis; chorioamnionitis; uterine rupture; time from ROM to birth; Apgar score < 7 at 5 minutes. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Trial described as "quasi‐experimental study"; quote "Each subject chose one of type types of cards… and they were divided into the two groups according to these cards". |
| Allocation concealment (selection bias) | High risk | As above. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding, and considered unfeasible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding for outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Results reported in tables with group numbers reflecting no losses (i.e. 42 and 42); however not clearly specified that there were no losses/was no attrition. |
| Selective reporting (reporting bias) | High risk | Very few outcomes with reported data; for some outcomes (e.g. interval between ROM and birth) only mean values are reported; for others, results reported narratively in Discussion (uterine rupture). |
| Other bias | Unclear risk | Maternal age was the online baseline characteristic reported; lack of methodological detail to assess other risk of bias. |
Beer 1999.
| Methods | Randomised controlled trial. | |
| Participants | 40 women were randomised. Setting: Luisenhospital, Aachen, Germany. Inclusion criteria: women with PROM between 38 and 42 weeks' gestation, and cervical dilation ≤ 3 cm, with no regular uterine contractions. Exclusion criteria: risky pregnancies, mothers under 18 years. Characteristics for planned subgroup analyses: Method of induction:Caulophyllum. Parity: mixed; 70% in the planned early birth group and 60% in the expectant management group were nulliparous. Cervix: all women had cervical dilation ≤ 3. Antibiotic prophylaxis: not stated. Digital vaginal examination: all women (stated that the Bishop score was used). |
|
| Interventions |
Planned early birth (n = 20):Caulophyllum (D4) was given for 7 hours (1 tablet per hour, containing 250 mg Caulophyllum D4 and added magnesium stearate and wheat‐starch mixture) or until labour was established. Expectant management (n = 20): women were given a placebo (1 tablet per hour for 7 hours, containing magnesium stearate and wheat‐starch mixture). |
|
| Outcomes | Outcome data in meta‐analyses for: caesarean section; induction of labour; operative vaginal birth; epidural analgesia; time from ROM to birth. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding through the use of an identical placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Results reported in tables with group Ns reflecting no losses (i.e. 20 and 20); however not clearly specified that there were no losses/was no attrition. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information available to confidently assess selective reporting. |
| Other bias | Unclear risk | Insufficient information available from translation to confidently assess other potential sources of bias. |
Cheung 2006.
| Methods | Randomised controlled trial. | |
| Participants | 100 women were randomised. Setting: labour ward of the Department of Obstetrics and Gynaecology of Kwong Wah Hospital, Hong Kong, China, January 2002 to July 2004. Inclusion criteria: 1) confirmed gestational age of 37 to 42 weeks; 2) a singleton pregnancy with normal fetus in cephalic presentation; 3) PROM confirmed by visualising a pool of amniotic fluid at a sterile speculum examination; 4) absence of other indications for urgent induction of labour; 5) PROM for < 6 hours; 6) reassuring fetal heart rate tracing; 7) no signs of labour, no abdominal pain on admission. Exclusion criteria: 1) known hypersensitivity or any contraindications to prostaglandins (e.g. glaucoma or sickle cell disease); 2) aged less than 18 years old; 3) Group B streptococcus carrier; 4) multiple pregnancy; 5) non‐reassuring cardiotocograph or meconium‐stained liquor; 6) previous uterine surgery; 7) contraindication to vaginal birth; 8) estimated fetal weight of > 4 kg or < 2 kg; 9) placenta praevia or unexplained vaginal bleeding; 10) evidence of chorioamnionitis; 11) grand multipara (parity ≧ 4); 12) active medication at time of PROM or presence of any pre‐existing medical disease, e.g. cardiovascular disease or chronic renal failure. Characteristics for planned subgroup analyses: Method of induction: oral misoprostol. Parity: mixed. 75%, 79% and 70% were primipara in the control, treatment 1 and treatment 2 groups. Cervix: mixed. The mean baseline modified Bishop scores were 4.3 (1.61) (control), 5.1 (1.68) (treatment 1) and 5.0 (1.7) (treatment 2). Antibiotic prophylaxis: some women. "Intravenous ampicillin 1 g every 6 h was started when 24 h of PROM was reached." Digital vaginal examination: all women at baseline. "Cervical assessment was performed 4 h after onset of regular uterine contractions or earlier if any nonreassuring CTG was detected." |
|
| Interventions |
Planned early birth group 1 (n = 34): oral misoprostol 50 µg every 4 hours until active labour was established or to a maximum of 6 doses. Planned early birth group 2 (n = 33): oral misoprostol 100 µg every 4 hours until active labour was established or to a maximum of 6 doses. Expectant management group (n = 33): oral placebo (vitamin B6 50 mg). For all women, if no response (i.e. no signs of any abdominal pain at all) after 24 hours of treatment, the patient had an oxytocin infusion started for induction of labour according to usual protocol used in the hospital. |
|
| Outcomes | Outcome data in meta‐analyses for: maternal infectious morbidity; caesarean section; definite early‐onset neonatal sepsis; chorioamnionitis; caesarean for fetal distress; operative vaginal birth; time from ROM to birth; birthweight; pneumonia; Apgar score < 7 at 5 minutes. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "were assigned by a computerized random‐number generator...". |
| Allocation concealment (selection bias) | Low risk | Quote: "Group allocation was predetermined and placed in consecutively numbered and sealed opaque envelopes...". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women and study personnel blinded through use of a placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded through the use of a placebo; quote: "Everyone was blinded to which treatment each subject received, until the end of the study, when the enveloped number code was deciphered". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "There was one case record missing in both the control group and treatment group 1, making the total number of cases analysed 98." |
| Selective reporting (reporting bias) | Unclear risk | No access to trial protocol; not possible to confidently assess selective reporting. |
| Other bias | Low risk | No other obvious risk of bias identified. |
Chung 1992.
| Methods | Randomised controlled trial. | |
| Participants | 59 women were randomised. Setting: Department of Obstetrics and Gynaecology, The Prince of Wales Hospital, Hong Kong, China, from August 1988 to July 1990. Inclusion criteria: women with a singleton pregnancy with cephalic presentation, at least 37 weeks' gestation, with a history highly suggestive of PROM, confirmed by visualisation of a pool of amniotic fluid in the vagina on speculum exam and a positive nitrazine text, with a Bishop score of 4 or less (unfavourable cervix), with a 20 minute cardiotocogram showing no evidence of fetal distress, and no evidence of uterine contractions. Exclusion criteria: evidence of uterine contractions, maternal tachycardia, medical or obstetric complications. Characteristics for planned subgroup analyses: Method of induction: vaginal prostaglandin E2 gel. Parity: mixed. 28/30 women in the planned early birth group and 21/29 in the expectant management group were nulliparous. Cervix: all women had an unfavourable cervix (Bishop score of 4 or less). Antibiotic prophylaxis: not stated. Digital vaginal examination: all women (Bishop score was determined at baseline). |
|
| Interventions |
Planned early birth (n = 30): prostaglandin E2 (3 mg) gel instilled into the posterior fornix of the vagina. Expectant management (n = 29): placebo ‐ sterile K‐Y jelly instilled into the posterior fornix of the vagina. Conservative management was followed in the next 24 hours for both groups unless the clinical situation demanded intervention. The use of oxytocin infusion for induction or augmentation was indicated by departmental protocol. |
|
| Outcomes | Outcomes data in meta‐analyses for: maternal infectious morbidity; caesarean section; definite early‐onset neonatal sepsis; chorioamnionitis; postpartum pyrexia; caesarean section for fetal distress; operative vaginal birth; uterine rupture; time from ROM to birth; birthweight; Apgar score < 7 at 5 minutes; admission to neonatal special or intensive care unit. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were allocated "according to a computer‐generated set of random numbers". |
| Allocation concealment (selection bias) | Low risk | Quote: "The code identifying the type of gel the woman received was kept by the trial coordinator and not released to the obstetrician in charge of the case". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women and study personnel were blinded with the use of pre‐packed syringes containing either prostaglandin gel or K‐Y jelly (placebo); both syringes were unmarked except for the trial number. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above; blinding through the use of an identical placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Results reported suggest no losses; however not clearly specified that there were no losses/was no attrition. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information (i.e. no trial protocol) to confidently assess selective reporting. |
| Other bias | Unclear risk | 28/30 versus 21/29 women in the planned versus expectant management groups respectively were nulliparous (though authors note "no significant differences between the 2 groups"). No other obvious sources of bias identified. |
Fatima 2015.
| Methods | Randomised controlled trial. | |
| Participants | 200 women were randomised. Setting: Department of Obs & Gynae, Allamalqbal Medical College, Jinnah Hospital, Lahore, Pakistan, from 1 January 2013 to 30 June 2013. Inclusion criteria: women with ROM at or > 37 weeks gestation. Exclusion criteria: women with previous caesarean section; not willing to be part of the study. Characteristics for planned subgroup analyses: Method of induction: oral misoprostol. Parity: mixed."The subjects were similar with respect to… parity." Results report that 63% (63) of planned early birth group and 70% of expectant management group were primigravidas, Cervix: mixed. Mean (standard deviation) Bishop score in planned early birth group: 3.5 (4.9); expectant management group: 3 (5.4) Antibiotic prophylaxis: not reported. Digital vaginal examination: all women."On speculum examination cervical dilatation…was assessed. Bishop score was assessed once with sterile gloves, at the time of admission and was restricted until the establishment of active labour." |
|
| Interventions |
Planned early birth (n = 100): women were induced immediately at presentation with oral misoprostol. Expectant management (n = 100): women were watched for spontaneous occurrence of labour within 24 hours after ROM; if they were not in labour after 24 hours they were managed as per departmental protocol. |
|
| Outcomes | Outcomes data in meta‐analyses for: maternal infectious morbidity ("fever"); caesarean section; definite or probable early‐onset neonatal sepsis; chorioamnionitis and/or pyrexia; caesarean section for fetal distress; operative vaginal birth; postpartum haemorrhage; time from ROM to birth ('other data'); Apgar score < 7 at 5 minutes (reported Apgar 6‐7); admission to neonatal special or intensive care unit. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using random number table." |
| Allocation concealment (selection bias) | Unclear risk | As above; no further detail provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated, however considered unlikely due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail provided regarding blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses or exclusions. |
| Selective reporting (reporting bias) | High risk | A number of outcomes which would be expected to be reported were not; no access to trial protocol to further assess selective reporting. Abstract reports the aim is to assess the effects of active versus expectant management on outcomes including mean latency period and chorioamnionitis – which are not subsequently reported. The methods also mention maternal satisfaction; which was not reported. |
| Other bias | Unclear risk | Limited detail regarding baseline characteristic reported "The subjects were similar with respect to mean age, parity and estimated gestational age at entry"; limited methodological detail reported |
Hannah 1996.
| Methods | Randomised controlled trial. | |
| Participants | 5042 women were randomised from January 1992 to May 1995. Setting: women were recruited in 72 hospitals in Canada, the UK, Australia, Israel, Sweden and Denmark. Inclusion criteria: women at least 37 weeks' gestation, with PROM with a single fetus in a cephalic presentation, with no contraindications for induction of labour or expectant management. PROM was determined clinically and confirmed by positive litmus (nitrazine) or ferning tests. If necessary a vaginal exam was performed with a speculum; digital vaginal exams were avoided. Exclusion criteria: women in active labour, if there had been a previous failed attempt to induce labour, or if there was a contraindication to either induction of labour (such as placenta praevia) or expectant management (such as meconium staining of the amniotic fluid or chorioamnionitis). Characteristics for planned subgroup analyses: Method of induction: 2 interventions: intravenous oxytocin or vaginal prostaglandin E2 gel. Parity: mixed. Nulliparous: 59.1% induction/oxytocin; 59.4% expectant/oxytocin; 59.7% induction/prostaglandin; 60.0% expectant/prostaglandin. Cervix: mixed. Vaginal examination with a speculum unripe/ripe: 49.4/14.6% induction/oxytocin; 50.8/14.5% expectant/oxytocin; 54.0/12.8% induction/prostaglandin; 52.2/12.4% expectant/prostaglandin. Antibiotic prophylaxis: some women."Decisions about other aspects of... maternal care, including the use and timing of antibiotics... were made by the nurse, midwife, or attending physician". Digital vaginal examination: mixed at baseline. None: 61.1% induction/oxytocin; 62.6% expectant/oxytocin; 64.6% induction/prostaglandin; 63.04% expectant/prostaglandin. |
|
| Interventions |
Planned early birth (n = 2517): either: 1) immediate induction of labour with intravenous oxytocin, with the infusion rate titrated to contractions according to local hospital practice (n = 1258) or 2) immediate induction of labour with vaginal prostaglandin E2 gel, with 1 mg or 2 mg inserted into the posterior vaginal fornix, repeated 6 hours later if labour had not started, and followed by intravenous oxytocin if labour still had not started 4 hours later (n = 1259). Expectant management (n = 2524): expectant management for up to 4 days (either admitted to the hospital or cared for as outpatients), then induced with intravenous oxytocin (n = 1263) or vaginal prostaglandin E2 gel (n = 1261) if spontaneous labour had not occurred. |
|
| Outcomes | Outcome data in meta‐analyses for: maternal infectious morbidity; caesarean section; definite or probable early‐onset neonatal sepsis; perinatal mortality; chorioamnionitis; postpartum pyrexia; induction of labour; operative vaginal birth; views of care; time from ROM to birth; cord prolapse; stillbirth; neonatal mortality; antibiotic usage; Apgar score < 7 at 5 minutes; antibiotic usage; use of mechanical ventilation; duration or maternal antenatal or postnatal hospital stay; admission to neonatal special or intensive care unit; duration of neonatal stay in special or intensive care unit. | |
| Notes | Power of 80% to detect a reduction of 50% or more, from = 4% to = 2% in the rate of neonatal infection in each treatment group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally controlled computerised randomisation, with telephone access. |
| Allocation concealment (selection bias) | Low risk | Quote ‐ "randomisation was centrally controlled at the Perinatal Clinical Epidemiology Unit at Women's College Hospital in Toronto with the use of a computerized randomisation program, accessible by means of a touch‐tone telephone". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated, however considered unlikely due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An adjunction committee, unaware of the woman's group assignment and of whether labour was induced or spontaneous determined whether neonatal infection was present. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were not received for 1/5042 women. Complete questionnaires obtained from 4129 women (81.9%). |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported; outcomes clearly pre‐specified. |
| Other bias | Low risk | No other obvious risk of bias identified. |
Javaid 2008.
| Methods | Randomised controlled trial. | |
| Participants | 100 women were randomised. Setting: Gynae Unit‐II Services Hospital, Lahore, Pakistan, from April to September 2007. Inclusion criteria: women with PROM at ≥ 37 weeks' gestation, with a singleton pregnancy in cephalic presentation. PROM was confirmed by "clinical examination". Exclusion criteria: women at less than 37 weeks, with indication for elective caesarean section. Characteristics for planned subgroup analyses: Method of induction: oral misoprostol. Parity: mixed. "Parity ranged from primigravida to para four". Cervix: not stated. Antibiotic prophylaxis: all women. "Antibiotics were prophylactically started in both group but the requirement of antibiotics in induction group was less…". Digital vaginal examination: not stated. |
|
| Interventions |
Planned early birth (n = 50): labour was induced with misoprostol (oral route). Expectant management (n = 50): women were left for 24 hours. |
|
| Outcomes | Outcome data in meta‐analyses for: caesarean section; time from ROM to birth. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"It was an open randomized comparative study". |
| Allocation concealment (selection bias) | Unclear risk | As above; no further detail provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial described as "open". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | As above; assumed there was no blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | See below – appears there may have been some losses/incomplete data, however this is not clear/not reported. |
| Selective reporting (reporting bias) | High risk | For many of the reported outcomes, the number of women in each group was unclear, or data were not reported separately for the 2 study groups. For chorioamnionitis and postpartum fever only percentages were reported ‐ these percentages do not allow calculation of number of women based on the total number of women in each group (i.e. some women must have been lost to follow‐up, but this was not reported). For other outcomes, only results across both study groups are presented in text. |
| Other bias | Unclear risk | Very limited methodological detail provided to assess other potential sources of bias. |
Krupa 2005.
| Methods | Randomised controlled trial. | |
| Participants | 150 women were randomised from January 2000 to May 2003. Setting: Department of Obstetrics of the Centre for Integral Care to Woman's Health, Sao Paulo, Brazil. Inclusion criteria: PROM confirmed up to 6 hours after the occurrence; gestational age at least 37 weeks, cephalic presentation and a live fetus showing no signs of fetal compromise as evaluated by CTG. Diagnosis of PROM was performed based on clinical history, speculum exam and if necessary nitrazine and fern test, as well as ultrasound. Exclusion criteria: past caesarean section or uterine surgery; being in labour at admission as characterised by regular painful uterine contractions (2 contractions in 10 minutes and gradually shortening); presence of fetal malformations incompatible with life; twin pregnancy or strongly suspected or confirmed chorioamnionitis. Characteristics for planned subgroup analyses: Method of induction: vaginal misoprostol. Parity: mixed. planned early birth: 31 (41.3%) were primiparae; expectant management: 45 (60.0%) were primiparae. Cervix: not stated. Antibiotic prophylaxis: not stated. Digital vaginal examination: not stated. |
|
| Interventions |
Planned early birth group (n = 75): immediate induction of labour with vaginal misoprostol. The vaginal misoprostol tablet (25 µg (Prostokos)) was digitally inserted into the posterior fornix at 6‐hourly intervals, up to a maximum of 4 doses (100 µg). Women who did not respond to induction using misoprostol within a 24‐hour period with a cumulative dose of 100 µg were also given an intravenous infusion of oxytocin. Expectant management group (n = 75): monitoring of temperature, fetal heart rate and uterine activity on the ward for up to 24 hours. If labour occurred within the 24 hour period since recruitment, the woman was admitted to the delivery ward and had routine care. If 24 hours had passed since recruitment and the woman had not yet begun labour, she was taken to the delivery ward where she received an intravenous infusion of oxytocin. Oxytocin infusion for labour induction consisted of 5 units mixed into 500 mL of lactate Ringer solution, resulting in an oxytocin concentration of 10 mU/mL. A starting dose of 6 mU/minute was increased 3 mU/minute at 30‐minute intervals to a maximum of 42 mU/minute or stabilised labour. |
|
| Outcomes | Outcome data in meta‐analyses for: caesarean section; serious maternal morbidity or mortality; definite early‐onset neonatal sepsis; perinatal mortality; caesarean section for fetal distress; induction of labour; time from ROM to birth; stillbirth; neonatal mortality; antibiotic usage; Apgar score < 7 at 5 minutes; abnormality on cerebral ultrasound; duration of maternal antenatal or postnatal stay in hospital; admission to neonatal special or intensive care unit. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote ‐ "Randomisation was carried out by computer prior to initiation of the study, specifying the same number of cases per group". |
| Allocation concealment (selection bias) | Low risk | Quote ‐ "information regarding the assigned intervention was contained on the forms, which were kept inside sealed opaque envelopes, sequentially numbered according to randomisation. Then, each case enrolled in the study had the next sequential numbered envelope assigned and the correspondent intervention was known only after envelope was opened". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Described as "open, randomised, controlled trial". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | As above; assumed there was no blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote ‐ "There were no dropouts and no woman was discontinued for any reason after enrolment to the study". All analyses were by "intention to treat". |
| Selective reporting (reporting bias) | Low risk | Although there was no access to a trial protocol, most expected outcomes were reported. |
| Other bias | Unclear risk | Some baseline imbalances (parity). No other obvious risk of bias identified. |
Mahmood 1992.
| Methods | Randomised controlled trial. | |
| Participants | 230 women were randomised. Setting: Labour Ward, Aberdeen Maternity Hospital, Scotland, UK, from January 1988 to May 1990. Inclusion criteria: primigravid women with PROM in an uncomplicated singleton pregnancy with gestation confirmed by early pregnancy ultrasound, cephalic presentation, with no uterine activity. The diagnosis of PROM was confirmed by sterile speculum exam to demonstrate the presence of amniotic fluid. Exclusion criteria: women with previous significant antepartum haemorrhage, intrauterine growth retardation, diabetes mellitus, Rhesus disease, moderate pre‐eclampsia, a history of venereal disease, a temperature > 37.5 C on admission, PROM > 12 hours, or meconium stained amniotic fluid on admission. Characteristics for planned subgroup analyses: Method of induction: vaginal prostaglandin E2 gel. Parity: all women were primigravid. Cervix: mixed (all women had a cervical dilation < 3 cm at trial entry); cervical scores ranged from 1 to 8 at baseline (Figure 1). Antibiotic prophylaxis: some women: 8 women in each group were given prophylactic antibiotics because of a positive ß‐haemolytic streptococci test; 4 women in the planned early birth group and 5 in the expectant management group received prophylactic antibiotics because of intrapartum pyrexia. Digital vaginal examination: all women. A sterile digital examination to exclude occult cord prolapse and assess cervical score was conducted. |
|
| Interventions |
Planned early birth (n = 115): 2 mg prostaglandin E2 gel (Upjohn) in posterior fornix; if uterine activity did not ensue (after 1 hour), then a repeat treatment with prostaglandin E2 gel (1 mg) was given 6 hours later. Expectant management (n = 115): remained for up to 24 hours in the observation ward. Women had their blood pressure, pulse and temperature checked 6 hourly; if labour did not ensue after 24 hours, women were treated with intravenous oxytocin using an escalating scale of 1‐32 mU/min. In both groups, intravenous oxytocin was started 24 hours after hospital admission, if labour had not begun, or sooner if augmentation of established labour was required. |
|
| Outcomes | Outcome data in meta‐analyses for: maternal infectious morbidity; caesarean section; definite early‐onset neonatal sepsis; perinatal mortality; chorioamnionitis; postpartum pyrexia; postpartum antibiotic usage; caesarean section for fetal distress; epidural analgesia; postpartum haemorrhage; time from ROM to birth; birthweight; neonatal mortality; antibiotic usage; Apgar score < 7 at 5 minutes; admission to neonatal special or intensive care unit; duration of neonatal stay in special or intensive care unit. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Once the patient had consented to enter the study, a numbered sealed randomization envelope was opened which allocated her to one of the two study groups". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated, however considered unlikely due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Each newborn was seen and examined by a paediatrician resident, who was unaware of the woman's participation in the study". Blinding of outcome assessment for other outcomes not detailed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Excluded outcome data balanced in numbers across groups. Of the 230 women randomised, 10 were excluded from the final analysis (5 from each group), as they did not fulfil the study criteria – 2 were parous, 4 had undiagnosed breech presentation; 2 had no definite fluid pool in the vagina; 2 had case notes that could not be traced. |
| Selective reporting (reporting bias) | Low risk | Although there was no access to a trial protocol, most of the expected outcomes were reported. |
| Other bias | Low risk | No other obvious risk of bias identified. |
Mahmood 1995.
| Methods | Quasi‐randomised controlled trial. | |
| Participants | 100 women were randomised. Setting: Labour Ward, Aberdeen Maternity Hospital, Scotland, UK. Inclusion criteria: healthy, parous women with PROM and singleton uncomplicated pregnancies, cephalic presentation and no uterine activity. On admission, each patient had a sterile speculum exam to confirm the presence of amniotic fluid. Exclusion criteria: previous serious antepartum haemorrhage, fetal growth retardation, diabetes mellitus, Rhesus immunisation, moderate pre‐eclampsia, history of venereal disease, previous caesarean birth, temperature above 37.5 C on admission, PROM for longer than 12 hours, or meconium‐stained amniotic fluid on admission. Characteristics for planned subgroup analyses: Method of induction: vaginal prostaglandin E2 gel. Parity: all women were multiparous; median parity (range): planned early birth: 2 (1‐4); expectant management: 2 (1‐4). Cervix: mixed (all women had a cervical dilation < 3 cm at trial entry); cervical scores ranged from 2 to 9 at baseline. Antibiotic prophylaxis: some women. At entry, 9 women were treated prophylactically with antibiotics (due to positive endocervical swab for ß‐haemolytic streptococci). Digital vaginal examination: all women. A sterile digital examination was performed to exclude occult cord prolapse and assess cervical score. |
|
| Interventions |
Planned early birth (n = 50): prostaglandin E2 gel, 1 mg administered at admission to posterior fornix and repeated 6 hours later if labour was not established. Expectant management (n = 50): conservative management; women remained in the observation ward for up to 24 hours. Women had 6 hourly check‐ups for blood pressure, pulse and temperature. If clinically significant uterine activity was not established after 24 hours they were treated with oxytocin. Both groups received intravenous oxytocin if labour did not start within 24 hours of admission, or sooner if augmentation of ineffective labour was required, using an escalating scale of 1‐32 µ/min. |
|
| Outcomes | Outcome data in meta‐analyses for: maternal infectious morbidity; caesarean section; probable early‐onset neonatal sepsis; perinatal mortality; chorioamnionitis; postpartum septicaemia; postpartum antibiotic usage; caesarean section for fetal distress; epidural analgesia; postpartum haemorrhage; time from ROM to birth; birthweight; neonatal mortality; antibiotic usage; Apgar score < 7 at 5 minutes. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised trial; quote: "Before the start of the study, a randomization list was prepared, using a random‐numbers list to assign odd and even numbers for the two treatments". |
| Allocation concealment (selection bias) | High risk | As above; quote: "The instructions for individual patients were stored in separate envelopes… After a woman consented to enter the study, she opened a sealed and numbered envelope that allocated her to one of the two study groups". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial was described as "open". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | As above; assumed there was no blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided on losses/missing data. |
| Selective reporting (reporting bias) | Low risk | Although there was no access to a trial protocol, most of the expected outcomes were reported. |
| Other bias | Low risk | No other obvious risk of bias identified. |
Maqbool 2014.
| Methods | Randomised controlled trial. | |
| Participants | 560 women were randomised. Setting: Obstetrics and Gynaecology Department, Sir Ganga Ram Hospital, Lahore, Pakistan. Inclusion criteria: women between 18‐35 years, primigravida to gravida 4, with a term pregnancy (≥ 37 weeks' gestation), singleton pregnancy, cephalic presentation, and ROM for less than 4 hours duration. Exclusion criteria: women with evidence of chorioamnionitis, in labour with regular uterine contraction < 10 minutes apart, women with gestational diabetes and hypertension, scarred uterus, fetal distress and fetal malformation. Characteristics for planned subgroup analyses: Method of induction: sublingual misoprostol. Parity: mixed. "Primigravida to gravida four". Cervix: not stated. Antibiotic prophylaxis: all women: "sterile pad, antibiotic cover and fetal heart rate monitoring was done in both groups". Digital vaginal examination: not stated. |
|
| Interventions |
Planned early birth (n = 280): women were inducted with misoprostol (100 micrograms) sublingually up to 5 doses, 4 hours apart (as required). Expectant management (n = 280): women were observed for uterine contractions for 24 hours. Both groups: sterile pad, antibiotic cover and fetal heart rate monitoring was carried out in both groups. |
|
| Outcomes | Outcome data in meta‐analyses for: maternal infectious morbidity; caesarean section; chorioamnionitis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All of them were divided into two groups, randomly using lottery method". |
| Allocation concealment (selection bias) | Unclear risk | As above, no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated however considered unfeasible for women and study personnel due to the nature of the interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail provided regarding blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information of losses to follow‐up or missing data reported. |
| Selective reporting (reporting bias) | Unclear risk | No access to trial protocol to confidently assess selective reporting; very few outcomes are reported in the manuscript. |
| Other bias | Unclear risk | Short report, with very few details provided regarding methodology. |
McQueen 1992.
| Methods | Randomised controlled trial. | |
| Participants | 40 women were randomised. Setting: Hospital in Harare, Zimbabwe. Inclusion criteria: PROM confirmed by speculum examination and the presence of ferning. No contractions felt or observed after half hour of admission (therefore early ROM). Gestation of 37 weeks or more confirmed by the women's dates, by clinical assessments at antenatal visits, by ultrasound. Exclusion criteria: evidence of fetal distress, e.g. meconium staining of the liquor, sepsis, manifested by fetal or maternal tachycardia, pyrexia or uterine tenderness. Other risk factors in pregnancy, e.g. medical complication, abnormal lie, multiple pregnancy, previous caesarean section, etc. Characteristics for planned subgroup analyses: Method of induction: intravenous oxytocin. Parity: mixed. 25% nulliparous (5 in each group). Cervix: not stated. Antibiotic prophylaxis: some women. Antibiotics were administered once duration of ROM reached 12 hours. Digital vaginal examination: all women. A single examination to assess state of the cervix and obtain Bishop score was conducted. |
|
| Interventions |
Planned early birth (n = 20): an oxytocin infusion was commenced. Sterile speculum examination to confirm ROM; single sterile vaginal examination to assess the state of the cervix and obtain Bishop score; antibiotics administered once duration of ROM reached 12 hours. Progress of labour followed by regular observation; further management decided according to the progress of labour and maternal and fetal conditions. Expectant management (n = 20): sterile speculum examination only to confirm ROM and the absence of meconium or offensive liquor. Women were admitted to early labour ward then antenatal ward for continuing observation of pulse, temperature, uterine tenderness, state of liquor and daily white blood cell counts. Women fell into 3 categories: a) once progressive contractions occurred after study admission, woman was assumed to be in labour and managed as for the active management group (spurious prelabour was excluded by close observation to ensure definite palpable and increasing contractions); b) if no contractions occurred in the first instance, the woman was observed as above, in an endeavour to allow the pregnancy to continue until ripening of the cervix and onset of contractions (this waiting period is referred to as ‘latency’); c) if sepsis was suspected at any time during the latent period, antibiotics were started and the woman was induced with oxytocin. |
|
| Outcomes | Outcome data in meta‐analyses for: caesarean section; definite or probable early‐onset neonatal sepsis; perinatal mortality; postpartum pyrexia; postpartum septicaemia; operative vaginal birth; cord prolapse; Apgar score < 7 at 5 minutes; duration of maternal antenatal or postnatal stay in hospital. | |
| Notes | Personal communication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A table of random numbers was used. |
| Allocation concealment (selection bias) | Unclear risk | Not detailed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated, however considered unlikely due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to make judgement. Losses to follow‐up: not stated (although not clear if 7/47 exclusions were before randomisation). |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make judgement. |
| Other bias | Unclear risk | Insufficient information to make judgement. |
Milasinovic 1998.
| Methods | Quasi‐randomised controlled trial. | |
| Participants | 76 women were randomised. Setting: Novi Sad, Serbia. Inclusion criteria: women with PROM post 258 days since the first day of LMP (= 37 weeks). Characteristics for planned subgroup analyses: Method of induction: vaginal prostaglandin E2 gel (and oxytocin). Parity: mixed. Parity ranged from 1‐3. Primigravidas and primiparous women made up 55% to 60% of all patients. Cervix: unfavourable: Bishop scores were < 6 in all patients. Antibiotic prophylaxis: some women (expectant management group). Digital vaginal examination: all women (assumed; to determine Bishop score). |
|
| Interventions |
Planned early birth (n = 38 analysed): labour was induced 6 hours post ROM with prostaglandin E2 (Predipil) gel intracervically and oxytocin infusion 3‐4 hours later. Expectant management (n = 37 analysed): women were given antibiotic prophylaxis (ampicillin, 500 mg) and were monitored every 6 hours. |
|
| Outcomes | Outcome data in meta‐analyses for: maternal infectious morbidity; caesarean section; definite early‐onset neonatal sepsis; chorioamnionitis; postpartum pyrexia; caesarean section for fetal distress; time from ROM to birth. | |
| Notes | Partial translation of manuscript. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised trial with patients alternately allocated to treatment/control. |
| Allocation concealment (selection bias) | High risk | As above. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated, however considered unlikely due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/76 lost to follow‐ up in planned early birth group. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine; no access to trial protocol; paper only partially translated. |
| Other bias | Unclear risk | Insufficient information to determine; paper only partially translated. Some additional information from personal communication. |
Natale 1994.
| Methods | Randomised controlled trial. | |
| Participants | 262 women were randomised. Setting: St Joseph’s Health Centre, London, Ontario, Canada. Inclusion criteria: all women diagnosed with PROM with a confirmed gestational age greater than or equal to 37 completed weeks. PROM was confirmed by obvious pooling of amniotic fluid on sterile speculum examination. Women with no risks other than previous caesarean birth or breech presentation (frank or complete) were included. Exclusion criteria: meconium staining of the amniotic fluid, diabetes (gestational or overt), pre‐eclampsia, malpresentation (footling or incomplete breech, not frank breech), intrauterine growth restriction, women transferred from other centres, known placenta praevia or active vaginal bleeding, cervical dilation > 3 cm and effacement > 80%, active herpes and known group B streptococci‐positive women. Characteristics for planned subgroup analyses: Method of induction: intravenous oxytocin. Parity: mixed. Cervix: mixed. Women were excluded who had cervical dilation > 3 cm and effacement > 80%; 89/119 women in the planned early birth group and 84/123 in the expectant management group had a Bishop score < 5. Antibiotic prophylaxis: not stated. Digital vaginal examination: all women. A single sterile digital examination was performed at randomisation to asses cervical dilation and effacement and other parameters of the Bishop score. For women in expectant management group, no digital examinations were performed until the woman was deemed to be clinically in active labour. |
|
| Interventions |
Planned early birth (n = 129 randomised): induction of labour 8 hours after PROM with intravenous oxytocin. Expectant management (n = 133 randomised): expectant management for 48 hours. Patients had a non‐stress test – if the test was reactive but they were not in labour, they were transferred to the antepartum ward. The women were followed up closely for evidence of infection and maternal/fetal health. Care strategies included: white blood cell counts daily; 4 hourly temperature; daily non‐stress test; no digital examinations until woman deemed to be clinically in active labour; induction if group B beta‐haemolytic streptococci were detected on screen or culture; if a clinical diagnosis of chorioamnionitis was made; if 48 hours from PROM had elapsed and spontaneous labour had not ensued. |
|
| Outcomes | Outcome data in meta‐analyses for: maternal infectious morbidity; caesarean section; chorioamnionitis; postpartum antibiotic usage; induction of labour; antibiotic usage; admission to neonatal special or intensive care unit. | |
| Notes | Pre‐determined sample size was 275 per group. Quote:"Unfortunately, the accrual rate was so low that the trial could not be carried out and therefore sample size required was not achievable". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The investigators realized that this study could not be performed in a blinded manner". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Neonatal treatment was prescribed by physicians who were blinded as to which arm the neonate was in. Pathologists assigning diagnoses of chorioamnionitis and funisitis were also blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 20/262 women dropped out after randomisation (10 from each group); reasons for drop‐ out were not reported. Analysis was based on 242 women. |
| Selective reporting (reporting bias) | High risk | Quote: "Neonatal sepsis was not considered an outcome measure because we recognized that we would not be able to accrue a large enough patient group". Outcome data for caesarean section were reported as percentages in text (somewhat unclear whether they related to the induction and expectant groups, or also the patients who "refused to participate in the study" who were also mentioned in text). Endometritis mentioned in abstract; no data reported in text. Very few outcomes reported; no access to trial protocol. |
| Other bias | Unclear risk | Baseline characteristics were incompletely reported as "no difference" between group (age, weight, height, gestational age and so on). |
Ottervanger 1996.
| Methods | Randomised controlled trial. | |
| Participants | 123 women were randomised.
Inclusion criteria: women with a singleton pregnancy with cephalic presentation and PROM for at least 8 hours at a gestational age between 37 and 42 weeks. ROM was diagnosed from the history, loss of amniotic fluid, and occasionally, by sterile speculum examination.
Exclusion criteria: women with obstetric problems judged to require direct intervention, such as signs of intrauterine infection, abnormal cardiotocograph registration or hypertensive disorders. Characteristics for planned subgroup analyses: Method of induction: intravenous oxytocin. Parity: mixed "Groups were comparable in terms of … parity". Cervix: not stated. Antibiotic prophylaxis: no women. "Prophylactic antibiotics were not administered except in association with caesarean section". Digital vaginal examination: not stated. |
|
| Interventions |
Planned early birth (n = 61): intravenous oxytocin, starting at a dose of 2.5 mU/minute and augmented every 20 minutes until adequate contractility was obtained. Expectant management (n = 62): admission to hospital for 48 hours; if labour had not ensued within 48 hours, women were offered induction of labour by intravenous oxytocin. |
|
| Outcomes | Outcome data in meta‐analyses for: caesarean section; definite early‐onset neonatal sepsis; serious maternal morbidity or mortality; perinatal mortality; postpartum septicaemia; postpartum antibiotic usage; induction of labour; operative vaginal birth; stillbirth; neonatal mortality; antibiotic usage. | |
| Notes | The trial was ended, following interim analysis, after 123 women had been randomised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported, described as "randomized controlled trial". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "allocation concealment was by means of sealed opaque envelopes". Unclear how envelopes were numbered (given random sequence generation was not reported). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | In the discussion authors note "women, their companions, and the clinicians caring for them were all aware of group allocation". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcome assessment was able to be blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to make judgement. No losses to follow‐up stated. |
| Selective reporting (reporting bias) | Unclear risk | No access to trial protocol; not possible to confidently assess selective reporting. |
| Other bias | Unclear risk | Limited methodological detail; regarding baseline characteristics, authors report "Groups were comparable…" without providing group data. |
Selmer‐Olsen 2007.
| Methods | Randomised controlled trial. | |
| Participants | 106 women were randomised. Setting: St. Olavs Hospital, Trondheim University Hospital, Norway, from January 2004 to January 2006. Inclusion criteria: nulliparity and an uneventful singleton cephalic pregnancy between 37 and 42 weeks, with confirmed PROM without contractions of the uterus. No details of exclusions. Characteristics for planned subgroup analyses: Method of induction: acupuncture. Parity: nulliparous women were included. Cervix: not stated. Mixed cervix dilation at first exam (cm): planned early birth (< 3 (n = 24); 3‐6 (n = 18); 7‐10 (n = 6)); expectant management (< 3 (n = 25); 2; 3‐6 (n = 21); 7‐10 (n = 4)) Antibiotic prophylaxis: not stated. Digital vaginal examination: no women at baseline. "To avoid infection, no digital examination is performed before onset of labour or induction." |
|
| Interventions |
Planned early birth (acupuncture group) (n = 51): women were needled at the point CV4/Ren 4 (Guanyuan) on the conception vessel, with other points needled according to 1 of 3 main TCM diagnostic categories. The needles remained in place for 30 minutes and the women not in labour the following day were offered an additional acupuncture treatment. Midwives giving acupuncture had attended a 120‐hour acupuncture course for midwives, with a 6‐hour refresher. Time from ROM to acupuncture ranged from 1 to 30 hours (median: 2.8 hours); 3 women received ‘late’ acupuncture – more than 24 hours after PROM. Expectant management (n = 55): women in waited at home for approximately 48 hours, if cardiotocogram (CTG), temperature and amniotic fluid were normal; these observations were performed on a daily basis. To avoid infection, no digital examination was performed before onset of labour or induction. For all women, if labour was absent after 2 days, they were induced by the following regimen (not reported in the published paper, and was requested from author): a vaginal misoprostol capsula in the posterior fornix, starting with 50 micrograms misoprostol, and then 25 micrograms every 6 hours until contractions commenced (repeated up to 8 times). |
|
| Outcomes | Outcome data in meta‐analyses for: caesarean section; caesarean section for fetal distress; induction of labour; operative vaginal birth; epidural analgesia; views of care; time from ROM to birth; Apgar score < 7 at 5 minutes. | |
| Notes | According to a power calculation, to give 80% power and significance level 5%, the required sample size was 208 with 104 in each group; with an anticipated recruitment time of 1 year. This study only involved 106 women, with recruitment terminated after 2 years. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote ‐ "using an Internet‐based block randomisation". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote ‐ "After randomisation, [women] were instructed not to state which group they belonged to on their return". Considered unlikely that this was successful. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated whether outcome assessors were blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/51 in acupuncture group lost to follow‐up (1 refused further participation, 1 had meconium‐stained water (exclusion criteria), and 1 woman did not return the questionnaire). 2/55 in standard care group excluded after randomisation (both due to intact membranes). |
| Selective reporting (reporting bias) | Unclear risk | Time from PROM to birth was reported as a median value only. No access to trial protocol to further assess selective reporting. |
| Other bias | Unclear risk | Table 1 in manuscript indicates that 23/51 women in the control group (and 15/48 women in the acupuncture group) received acupuncture during 'active phase'. |
Shah 2012.
| Methods | Randomised controlled trial. | |
| Participants | 100 women were randomised. Setting: authors affiliated to Department of Obstetrics and Gynaecology, B J Medical College, Ahmedabad, India. Inclusion criteria: singleton pregnancy with cephalic presentation; gestational age between 37 and 41 completed weeks; spontaneous PROM confirmed by history, examination and specific test; admission to labour room within 6 hours of PROM; cervical dilatation < 3 cm; no evidence of immediate uterine contractions. Exclusion criteria: PROM before 37 weeks; features of chorioamnionitis; meconium‐stained liquor; medical or obstetric complications indicating prompt delivery; multiple pregnancies. Characteristics for planned subgroup analyses: Method of induction: intracervical prostaglandin E2 gel. Parity: mixed; "groups were similar with respect to… parity." Cervix: not stated; cervical dilatation < 3 cm. Antibiotic prophylaxis: all women. "All the patients irrespective of duration of PROM were given injectable Ampicillin 500 mg 6 hourly and injectable Gentamycin 80 mg 12 hourly by parenteral route till Delivery." Digital vaginal examination: all women. "To note the dilatation and effacement and to confirm the presence of membrane, vaginal examination was done." |
|
| Interventions |
Planned early birth (n = 50): early induction within 6 hours with intracervical prostaglandin E2 gel. Women were "subdivided" into groups: 1) successful induction; 2) re‐induction with prostaglandin or oxytocin required because of primary induction failure (who did not commence labour after 10 hours). Women were monitored for uterine contractions and fetal heart rate activity following induction until birth; per vaginal examination was done to confirm labour progress or induction failure after 6 hours; emergency caesareans performed for fetal distress, non‐progress of labour; failure of induction with/without chorioamnionitis. Expectant management (n = 50): Women received expectant management for 24 hours. Women were "subdivided" into groups: 1) spontaneous labour started within 24 hours; 2) induction was required after 24 hours (with prostaglandin or oxytocin, depending on cervical ripening). Women were monitored for uterine contractions for 24 hours; per vaginal examination was done only if uterine contractions were good to decide labour progress. |
|
| Outcomes | Outcomes data in meta‐analyses for: caesarean section; definite or probable early‐onset neonatal sepsis; caesarean section for fetal distress; induction of labour; time from ROM to birth (other data); antibiotic usage; duration of antenatal or postnatal stay (other data). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study patients were randomly allocated to one of the two groups." |
| Allocation concealment (selection bias) | Unclear risk | Not detailed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported and considered unlikely/unfeasible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses or exclusions. |
| Selective reporting (reporting bias) | High risk | No measures of variance reported for outcomes (e.g. time from ROM to birth); a number of outcomes which would be expected to be reported were not. Abstract reports "Increases in maternal‐neonatal infection rate…were noted in expectant group; however, this was not statistically significant", though no clear results are reported in text for maternal and neonatal infection/sepsis. No access to trial protocol to further assess selective reporting. |
| Other bias | Unclear risk | No baseline characteristics table presented;"Expectant and early induction groups were similar with respect to age, parity, previous history of PROM, and previous history of abortions." |
Shalev 1995.
| Methods | Quasi‐randomised controlled trial. | |
| Participants | 566 women were randomised. Setting: Central Emek Hosptial, Afula, Israel, November 1990 to October 1993. Inclusion criteria: women between 37‐42 weeks' gestation (as defined by the last menstrual period and confirmed by ultrasound). All had presented with PROM followed by at least 6 hours without uterine contractions. PROM confirmed by single, sterile, speculum exam and nitrazine test. Exclusion criteria: women with uncertain dating, maternal diseases (gestational diabetes and hypertension), maternal fever, previous caesarean, nonvertex presentation, suspected fetal malformation or fetal distress. Women who were examined digitally were excluded from further study. Characteristics for planned subgroup analyses: Method of induction: intravenous oxytocin. Parity: mixed. Median parity in both groups was 2; 99/298 nulliparas in the 12‐hour group, and 79/268 in the 72‐hour group. Cervix: not stated (though in discussion "we excluded women with obvious cervical effacement and dilation on presentation"). Antibiotic prophylaxis: not stated. Digital vaginal examination: no women. Women who were examined digitally were excluded from further study. |
|
| Interventions |
Planned early birth (n = 298): 12 hours of expectant management, followed by an oxytocin infusion. Expectant management (n = 268): 72 hours of expectant management. All women were managed with bed rest unless signs of chorioamnionitis or uterine contractions developed. Intrapartum management was similar for both groups. Women who had not entered labour at the end of the assigned period were induced with oxytocin; starting at 1 mU/minute and increasing as necessary by 1 mU/minute every 20 minutes. Prostaglandins were not used. |
|
| Outcomes | Outcome data in meta‐analyses for: maternal infectious morbidity; caesarean section; definite early‐onset neonatal sepsis; perinatal mortality; chorioamnionitis; caesarean section for fetal distress; induction of labour; time from ROM to birth; birthweight; cord prolapse; neonatal mortality; Apgar score < 7 at 5 minutes; duration of maternal antenatal or postnatal stay in hospital. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Women agreeing to participate were assigned… according to a system known only by the attending physicians. This used the last digit of each patient’s identification number (even for the 12‐hour group and off for the 72‐hour group". |
| Allocation concealment (selection bias) | High risk | As above; trial was quasi‐randomised. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The alternation system of allocation was known only to the attending physicians ‐ women, nurses and other medical staff members were not told of the assignment method; however, it was considered unlikely that blinding was feasible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up not stated. Insufficient information to make judgement. |
| Selective reporting (reporting bias) | Low risk | Although there was no access to a trial protocol, most expected outcomes were reported. |
| Other bias | Unclear risk | Slightly unbalanced group numbers (298 versus 268); limited methodological detail (short report), and few baseline characteristics reported. |
Sperling 1993.
| Methods | Randomised controlled trial. | |
| Participants | 124 women were randomised. Setting: Herlev Hospital, Copenhagen and Hillerrad Hospital, Denmark, from December 1986 to April 1990. Inclusion criteria: 1) completely normal singleton pregnancy 2) spontaneous PROM confirmed by history and sterile vaginal examination by a midwife 3) gestational age of 36 completed weeks or more 4) no evidence of spontaneous onset of labour during the first 6 hours 5) normal cardiotocograph recording on admission 6) aged 18 years or over. Exclusion criteria: malpresentation, uncertain gestational age, multiple pregnancy, vaginal bleeding, signs of intrauterine growth restriction, diabetes mellitus, rhesus immunisation, meconium‐stained liquor and temperature > 37.7 (rectal) on admission. Characteristics for planned subgroup analyses: Method of induction: intravenous oxytocin. Parity: mixed. early induction: 33/62 were primiparae; late induction: 32/62 were primiparae. Cervix: mixed. cervical score: median (range): early induction: 5 (2‐8) primiparae; 5 (2‐10) pluriparae; late induction: 5 (1‐8) primiparae; 4 (1‐6) pluriparae. Antibiotic prophylaxis: not stated; quote: "Prophylactic antibiotic treatment in connection with caesarean section was only given when there were clinical signs of infection". Digital vaginal examination: all women (to determine cervical score); then quote: "Vaginal examinations were minimized until the active phase of labor". |
|
| Interventions |
Planned early birth (n = 62): labour was induced with an oxytocin infusion 6 hours after spontaneous ROM. Expectant management (n = 62) labour was induced with oxytocin infusion 24 hours after spontaneous ROM. Oxytocin regimen for induction in both groups was: an initial dose of 4 mU/minute, which was increased after 40 minutes by 4 mU every 20 minutes until concentrations were acceptable (max 32 mU/minute). |
|
| Outcomes | Outcome data in meta‐analyses for: maternal infectious morbidity; caesarean section; definite early‐onset neonatal sepsis; chorioamnionitis; induction of labour; operative vaginal birth; epidural analgesia; time from ROM to birth; Apgar score < 7 at 5 minutes; admission to neonatal special or intensive care unit. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Allocation was done by drawing a sealed envelope according to parity after admission to the labor ward". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported and considered unlikely/unfeasible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind assessment of placenta/membranes (histological chorioamnionitis); not reported whether any other outcomes were assessed blind. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 362 women were originally eligible, and 238 declined: "The study group thus comprised 124 women". No information provided on losses/exclusions post‐randomisation, except for the outcome histologic chorioamnionitis ‐ 100/124 placentas assessed. |
| Selective reporting (reporting bias) | High risk | For many outcomes (i.e. birthweight, Apgar scores) results reported incompletely: "no differences between groups". For the outcome "time from rupture of membranes to birth" there were no data reported for pluriparae women in the late induction group (or the Table II was formatted incorrectly). |
| Other bias | Unclear risk | Limited methodological detail provided; unable to confidently assess other potential sources of bias. |
Tamsen 1990.
| Methods | Randomised controlled trial. | |
| Participants | 93 women were randomised. Setting: The University Hospital, Uppsala, Sweden, from May 1986 to September 1987. Inclusion criteria: 1) uneventful pregnancy; 2) term pregnancy (> 36 competed weeks); 3) singleton pregnancy with cephalic presentation; 4) PROM less than 4 hours before admission to the hospital (confirmed on fern test via sterile speculum examination); 5) normal CTG for 0.5 hours after admission; oral temperature 37.5 or less; 6) no evidence of spontaneous contractions 4 hours after PROM. Exclusion criteria: 1) pregnancies with complications such as diabetes mellitus, hypertension, proteinuric pre‐eclampsia, rhesus iso‐immunisation, etc. Characteristics for planned subgroup analyses: Method of induction: intravenous oxytocin. Parity: mixed. 24 nulliparas 19 paras in planed management group; 26 nulliparas and 24 paras in expectant management group. Cervix: mixed ("regardless of cervical effacement"). Antibiotic prophylaxis: not stated. Digital vaginal examination: some women."To minimize the risk of iatrogenic amnionitis, no vaginal palpation was performed at time for admission… If the woman was assigned to the intervention group, a vaginal palpation was performed… [for] women assigned for an expectant treatment… no vaginal palpation was carried out until contractions started." |
|
| Interventions |
Planned early birth (n = 42): oxytocin infusion intravenously was commenced at a dose of 1‐3 mU/min; the infusion was increased by 2‐3mU/minutes every 30th minute until the desired effect was obtained. Expectant management (n = 50): women were admitted to the antenatal unit. Once contractions were established, the women were treated as per the hospital routine. |
|
| Outcomes | Outcome data in meta‐analyses for: caesarean section; operative vaginal birth; time from ROM to birth; birthweight; cord prolapse; Apgar score < 7 at 5 minutes; duration of neonatal stay in special or intensive care unit. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not detailed; quote ‐ "she was randomly assigned to the expectant group or the intervention group". |
| Allocation concealment (selection bias) | Unclear risk | Not detailed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated, however considered unfeasible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided on blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No losses to follow‐up/attrition reported. Insufficient detail to judge attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes were not clearly pre‐defined; difficult to confidently assess selective reporting. For birthweight, only mean values are presented (no measure of variance). |
| Other bias | Unclear risk | Limited methodological detail provided to assess risk of other potential sources of bias. |
Tasnim 2000.
| Methods | Quasi‐randomised controlled trial. | |
| Participants | 152 women were randomised. Setting: Pakistan Institute of Medical Sciences, Islamabad, Pakistan from October 1993 to November 1996. Inclusion criteria: women with gestational ages between 37 and 42 completed weeks of gestation and a history of PROM (confirmed by sterile speculum examination). Exclusion criteria: grand multipara; multiple pregnancy; malpresentation; previous caesareans section; duration of PROM of more than 12 hours; attempted induction at another place; ultrasound evidence of severe oligohydramnios; biophysical profile of less than 6/10; pregnancies complicated by pre‐eclampsia; diabetes; heart disease and intrauterine growth restriction. Characteristics for planned subgroup analyses: Method of induction: intravenous oxytocin. Parity: mixed. The baseline data re: "parity" was reported as comparable between 2 groups (and presented in a Figure: primipara versus multipara). Cervix: unfavourable. Bishop score: planned early birth group (mean, range): 3.13 (1‐6); expectant management group (mean, range): 2.6 (2‐6). Antibiotic prophylaxis: all women. "Ampicillin is routinely given to all our patients with PROM." Digital vaginal examination: all women. "Digital vaginal examination was done for assessment of bishop score… Digital vaginal examination (DVE), although not favoured by many in cases of PROM due to risk of infection, was routinely performed in our patients." |
|
| Interventions |
Planned early birth (n = 72): an oxytocin infusion was commenced following randomisation. Expectant management (n = 80): group were monitored for signs and symptoms of chorioamnionitis; oxytocin infusion was commenced 24 hours after PROM if labour did not start spontaneously. |
|
| Outcomes | Outcome data in meta‐analyses for: caesarean section; serious maternal morbidity or mortality; probable early‐onset neonatal sepsis; definite early‐onset neonatal sepsis; perinatal mortality; postpartum pyrexia; induction of labour; operative vaginal birth; time from ROM to birth; birthweight; antibiotic usage; neonatal mortality; admission to neonatal special or intensive care unit. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi randomised; quote:"Those presenting on odd days of calendar month were allocated to active group… While women presenting on even days were managed conservatively". |
| Allocation concealment (selection bias) | High risk | As above. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated, however considered unfeasible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail of blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided on losses/attrition. The "induction of labour" outcome appeared to be based on 78/80 in expectant management group, no information was given on the remaining 2 women. |
| Selective reporting (reporting bias) | High risk | Some discrepancies between data in abstract and text (typographical errors); some results reported incompletely in text (i.e. text regarding neonatal infection and admission to the nursery). The outcome of "APGAR score at 5 min" was reported as mean and range, and the range included both Apgar score above 7 and less than 7. |
| Other bias | Unclear risk | Insufficient information to determine other risk of bias. |
Wagner 1989.
| Methods | Quasi‐randomised controlled trial. | |
| Participants | 182 women were randomised. Setting: Kaiser Permanente Medical Center, Oakland, California, United States, from April 1985 to December 1987. Inclusion criteria: healthy pregnant women with low‐risk pregnancies at 37‐42 weeks' gestation, seen within 6 hours of spontaneous ROM, who had an unfavourable cervix and were not in labour. ROM had to be documented by sterile speculum examination with positive ferning and nitrazine tests. Cervix had to appear dilated less than 2 cm and effaced less than 80%. Exclusion criteria: women with spontaneous labour (1 hour of regular painful contractions at least every 5 minutes) within 6 hours of spontaneous ROM; malpresentation; uncertain dates; previous caesarean section; history of gonorrhoea, herpes or a positive beta‐haemolytic streptococcal culture of the cervix during the current pregnancy; multiple gestation; toxaemia; vaginal bleeding; fetal distress; meconium‐stained fluid; insulin‐dependent diabetes; Rhesus factor disease; temperature above 37.8 degrees celsius; an elevated left‐shifted leukocyte count > 20 x 10^9/L; uterine tenderness. Characteristics for planned subgroup analyses: Method of induction: intravenous oxytocin. Parity: mixed: early induction: 64% nulliparas (55/86); delayed induction: 77% nulliparas (74/96). Cervix: all women had an unfavourable cervix. Antibiotic prophylaxis: not stated. Digital vaginal examination: some women."Our general protocol called for no digital examinations until the patients began labor or induction. However, we included those women who otherwise qualified for the study and who had received a single sterile digital examination at admission." All patients had a digital cervical examination at the beginning of labour or induction. |
|
| Interventions |
Planned early birth (n = 86): immediate induction with oxytocin. Expectant management (n = 96): transferred to antepartum floor, to await the onset of labour; returned to labour and delivery suite if: 1) if signs of infection or fetal distress occurred; 2) when spontaneous labour occurred; 3) 24 hours after spontaneous ROM for oxytocin labour if labour did not occur spontaneously (3 mU/minute and was increased by 3 mU/minute every 20 minutes until the desired contraction pattern). |
|
| Outcomes | Outcome data in meta‐analyses for: maternal infectious morbidity; caesarean section; probable early‐onset neonatal sepsis; definite early‐onset neonatal sepsis; chorioamnionitis; endometritis; caesarean section for fetal distress; induction of labour; operative vaginal birth; time from ROM to birth; pneumonia; antibiotic usage; Apgar score < 7 at 5 minutes; duration of maternal antenatal or postnatal stay in hospital; duration of neonatal stay in hospital. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Women were randomised "by means of the last digit of the medical record number" (those with an even number were placed in the delayed group; those with an odd number, in the early group). |
| Allocation concealment (selection bias) | High risk | As above; study was quasi‐randomised. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding, and considered unfeasible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding for outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up were not stated but women in the planned early birth group were excluded if they had not gone into labour within 10 hours of ROM (likely reason for fewer women in planned early birth group compared with the expectant management group). |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information (i.e. no trial protocol) to confidently assess selective reporting. |
| Other bias | Unclear risk | As above, the authors note that the numbers in the 2 groups differ, as conditions in the labour and delivery unit meant that at times early induction of labour was not possible (and therefore, some participants randomised to the early group were not induced by 10 hours after ROM, and were excluded); no further mention of how many participants were excluded, etc. and whether baseline characteristics differed for these patients. Limited information provided on baseline characteristics, however authors note that women in the delayed group were slightly younger. |
CTG: cardiotocography LMP: last menstrual period PROM: prelabour rupture of membranes ROM: rupture of membranes TCM: Traditional Chinese Medicine
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alcalay 1996 | Could not establish that all women had gestations of at least 37 weeks; paper stated "greater than 36 weeks". |
| Brosnan 1996 | Plan for a study that appears not to have been carried out. |
| Cararach 1996 | Could not establish that all women had gestations of at least 37 weeks; abstract stated greater than or equal to 34 weeks. |
| Chang 1997 | Could not establish that all women had gestations of at least 37 weeks; abstract stated "at term". |
| Chaudhuri 2006 | Expectant management lasted less than 24 hours. |
| Chua 1995 | Could not establish that all women had gestations of at least 37 weeks; paper stated "after 36 weeks of pregnancy" plus labour was induced after only 12 hours in the expectant management group. |
| Davies 1991 | Could not establish that all women had gestations of at least 37 weeks; paper stated "after 36 weeks of pregnancy". |
| Doungtone 1999 | Expectant management lasted less than 24 hours. |
| Duff 1984 | Could not establish that all women had gestations of at least 37 weeks; paper stated "greater than or equal to 36 weeks". |
| Freeman 1968 | Could not establish that all women had gestations of at least 37 weeks; paper stated "36 weeks or greater". |
| Gloeb 1989 | Could not establish that all women had gestations of at least 37 weeks; abstract stated"34 completed to 41 weeks gestation". |
| Gonen 1994 | Could not establish that all women had gestations of at least 37 weeks; paper stated "PROM at or beyond 36 complete weeks". |
| Grant 1992 | Excluded women with gestation equal to or less than 36 weeks so trial may have included women with less than 37 weeks' gestation. |
| Hidar 2000 | Could not establish that all women had gestations of at least 37 weeks; paper stated "greater than or equal to 36 weeks". |
| Hjertberg 1996 | Could not establish that all women had gestations of at least 37 weeks; paper stated "36+0 to 42+0 weeks". |
| Hoffman 2001 | Expectant management lasted less than 24 hours. |
| Ladfors 1996 | Could not establish that all women had gestations of at least 37 weeks; paper stated"34 to 42 weeks". |
| Levy 2005 | Expectant management lasted less than 24 hours. |
| Levy 2007 | Expectant management lasted less than 24 hours. |
| Lo 2003 | Could not establish that all women had gestations of at least 37 weeks; paper stated "at least 36 0/7 to 41 6/7 weeks' gestation". |
| Mahmood 1989 | Could not establish that all women had gestations of at least 37 weeks; abstract stated "after 34 weeks' gestation". |
| Mateos 1998 | Included women > 34 weeks' gestation; figures for 37 weeks or later gestation not reported separately. |
| McCaul 1997 | Could not establish that all women had gestations of at least 37 weeks; paper stated "between 36 weeks and 42 weeks". |
| Morales 1986 | Could not establish that all women had gestations of at least 37 weeks; paper stated "greater than 36 weeks". |
| Ngai 1996 | Labour was induced after only 12 hours in the expectant management group. |
| Ozden 2002 | Could not establish that all women had gestations of at least 37 weeks; paper stated "36 weeks of completed gestation". |
| Perez Picarol 1990 | Could not establish that all women had gestations of at least 37 weeks; abstract stated "at term". |
| Poornima 2011 | Expectant management was intended for < 24 hours (after 12 hours of expectant management, oxytocin was given). |
| Ray 1992 | Could not establish that all women had gestations of at least 37 weeks; paper stated "greater than 36 weeks". |
| Rydhstrom 1991 | Could not establish that all women had gestations of at least 37 weeks; paper stated "between 36 weeks and 41 weeks". |
| Shetty 2002 | Could not establish that all women had gestations of at least 37 weeks; specified only as at or after 36 weeks. |
| Shoaib 1994 | Could not establish that all women had gestations of at least 37 weeks; specified only as "at or near term". |
| Suzuki 2000 | Not all women had PROM. |
| Thomas 2000 | Could not establish that all women had gestations of at least 37 weeks; abstract stated "at term". |
| Van der Walt 1989 | Could not establish that all women had gestations of at least 37 weeks; paper stated greater than or equal to 36 weeks. |
| Van Heerden 1992 | Included women > 34 weeks' gestation; figures for 37 weeks or later gestation not reported separately. |
PROM: prelabour rupture of membranes
Characteristics of ongoing studies [ordered by study ID]
Walfisch 2014.
| Trial name or title | Management of labor in patients with previous cesarian section and premature rupture of membranes who desire TOLAC: comparison between the use of standard expectant management and the double‐balloon catheter device. A prospective randomized study. |
| Methods | Randomised controlled trial. |
| Participants | Inclusion criteria: pregnant with PROM at > 34 weeks in last 24 hours; unripe cervix; singleton pregnancy in vertex presentation and absence of significant and regular uterine contractions; previous caesarean section; willingness to comply with protocol; signed consent. Exclusion criteria: contraindication for vaginal birth (e.g. placenta praevia; non‐vertex presentation); regular uterine contractions; ROM > 24 hours prior to study inclusion; evidence of chorioamnionitis; suspected abruption or significant haemorrhage; non‐reassuring fetal status necessitating immediate intervention. |
| Interventions | Double‐balloon catheter device versus standard expectant management. |
| Outcomes | Primary outcome: vaginal birth. Secondary outcomes: safety (fetal heart rate; uterine haemorrhage; maternal haemodynamic changes; uterine atony); maternal satisfaction. |
| Starting date | September 2014. |
| Contact information | Asnat Walfisch, Hillel Yaffe Medical Center, 050‐4492200. |
| Notes | Estimated completion: September 2018; estimated enrolment: 200 women. |
PROM: prelabour rupture of membranes ROM: rupture of membranes
Differences between protocol and review
In this update of the review:
We have separated the outcomes into primary and secondary review outcomes, and have amended these to better align with those in the Wojcieszek 2014 review.
We have updated the methods in line with those in the standard template used by the Cochrane Pregnancy and Childbirth Group.
We have used the GRADE approach to assess the quality of the body of evidence and we have included 'Summary of findings' table.
In the previous version of this review:
The title was changed to better reflect that the intervention is designed to result in early birth and to clarify that the definition of term was 37 weeks' gestation or more.
The objectives were clarified to explain the intervention and comparison, rather than using the term 'optimal management'.
The intervention and comparisons were clarified; planned intervention must have been implemented or intended to be implemented within 24 hours of randomisation and conversely, expectant management must have had an intended delay of at least 24 hours.
The definition of postpartum fever was changed from a temperature greater than 38°C on at least two occasions after the first 24 hours after birth to postpartum fever as variously defined by authors.
Rationales for subgroup analyses were included.
A random‐effects model was used throughout (the protocol specified that a random‐effects model would be used when there was a substantial amount of statistical heterogeneity).
Contributions of authors
In this update of the review, Emily Shepherd and Philippa Middleton assessed studies for eligibility and extracted data, and Rosie McBain assisted. Emily Shepherd and Philippa Middleton drafted the first version of the update and all authors made comments on subsequent drafts and contributed to the final version.
In the previous version of the review, Marianna Dare, Philippa Middleton and Bala Varatharaju carried out the data extraction and all authors worked to produce the final draft of the review.
Marianna Dare wrote the original protocol for this review and Caroline Crowther and Philippa Middleton worked with Marianna Dare to produce the final draft.
Sources of support
Internal sources
ARCH: Australian Research Centre for Health of Women and Babies, Robinson Research Institute, The University of Adelaide, Australia.
External sources
NHS Programme for Research & Development, UK.
National Health and Medical Research Council, Australia.
-
National Institute for Health Research (NIHR), UK.
NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines
Declarations of interest
Philippa Middleton: none known.
Emily Shepherd: none known.
Vicki Flenady: none known.
Rosemary D McBain: none known.
Caroline A Crowther: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Akyol 1999 {published data only}
- Akyol D, Mungan T, Unsal A, Yuksel K. Prelabour rupture of the membranes at term: no advantage of delaying induction for 24 hours. Australian and New Zealand Journal of Obstetrics and Gynaecology 1999;39(3):291‐5. [DOI] [PubMed] [Google Scholar]
Ayaz 2008 {published data only}
- Ayaz A, Saeed S, Farooq MU, Ahmad F, Bahoo LA, Amad I. Pre‐labor rupture of membranes at term in patients with an unfavorable cervix: active versus conservative management. Taiwanese Journal of Obstetrics and Gynecology 2008;47(2):192‐6. [DOI] [PubMed] [Google Scholar]
Beer 1999 {published data only}
- Beer AM, Heiliger F. Randomized, double‐blind trial of caulophyllum D4 for induction of labour after premature rupture of the membranes at term [Caulophyllum D4 zur geburtsinduktion bei vorzeitigem blasensprung: eine doppelblindstudie]. Geburtshilfe und Frauenheilkunde 1999;59:431‐5. [Google Scholar]
Cheung 2006 {published data only}
- Cheung PC, Yeo EL, Wong KS, Tang LC. Oral misoprostol for induction of labor in prelabor rupture of membranes (PROM) at term: a randomized control trial. Acta Obstetricia et Gynecologica Scandinavica 2006;85(9):1128‐33. [DOI] [PubMed] [Google Scholar]
Chung 1992 {published data only}
- Chung T, Rogers MS, Gordon H, Chang A. Prelabour rupture of the membranes at term and unfavourable cervix: a randomized placebo‐controlled trial on early intervention with intravaginal prostaglandin E2 gel. Australian and New Zealand Journal of Obstetrics and Gynaecology 1992;32(1):25‐7. [DOI] [PubMed] [Google Scholar]
Fatima 2015 {published data only}
- Fatima S, Rizvi S, Saeed G, Jafri A, Eusaph A, Haider R. Expectant vs active management of prelabour rupture of membranes at term. Pakistan Journal of Medical and Health Sciences 2015;9(4):1353‐7. [Google Scholar]
Hannah 1996 {published data only}
- Cecco R, Hannah M, Hodnett E, Foster G, Farine D, Helewa M. Prelabor rupture of the membranes (PROM) at term: expectant management at home vs. in hospital. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S30. [Google Scholar]
- Gafni A, Goeree R, Myhr TL, Hannah ME, Blackhouse G, Willan A, et al. Induction of labour versus expectant management for prelabour rupture of the membranes at term: an economic evaluation. Canadian Medical Association Journal 1997;157(11):1519‐25. [PMC free article] [PubMed] [Google Scholar]
- Hannah M, Ohlsson A, Farine D, Hewson S, Hodnett E, Myhr T, et al. International termPROM trial: a RCT of induction of labour for prelabour rupture of membranes at term. In: Proceedings of the 14th Annual Congress of the Australian Perinatal Society in conjunction with the New Zealand Perinatal Society; 1996; Adelaide, Australia. 1996:A79.
- Hannah M, Ohlsson A, Farine D, Hewson S, Hodnett E, Myhr T, et al. Vaginal prostaglandin E2 gel vs. intravenous oxytocin vs. expectant management for prelabour rupture of membranes at term: a randomised clinical trial. Proceedings of the 15th Conference of Priorities in Perinatal Care; 1996; South Africa. 1996:14.
- Hannah M, Ohlsson A, Wang E, Myhr T, Farine D, Hewson S, et al. Inducing labor with iv oxytocin may reduce the risk of neonatal infection in GBS positive women with PROM at term. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S32. [Google Scholar]
- Hannah M, TermPROM Trial Group. The term prelabour rupture of the membranes (PROM) study. International Journal of Gynecology & Obstetrics 1994;46:16. [Google Scholar]
- Hannah ME, Ohlsson A, Farine D, Hewson SA, Hodnett E, Myhr T, et al. Induction of labor compared with expectant management for prelabor rupture of the membranes at term. New England Journal of Medicine 1996;334(16):1005‐10. [DOI] [PubMed] [Google Scholar]
- Hannah ME, Ohlsson A, Wang EE, Matlow A, Foster GA, Willan AR, et al. Maternal colonization with group B Streptococcus and prelabor rupture of membranes at term: the role of induction of labor. American Journal of Obstetrics and Gynecology 1997;177(4):780‐5. [DOI] [PubMed] [Google Scholar]
- Hodnett ED, Hannah ME, Weston JA, Ohlsson A, Myhr TL, Wang EE, et al. Women's evaluations of induction of labor versus expectant management for prelabor rupture of the membranes at term. Birth 1997;24(4):214‐20. [DOI] [PubMed] [Google Scholar]
- Seaward G, Hannah M, Myhr T, Ohlsson A, Farine D, Wang E, et al. PROM at term: maternal risk factors for clinical chorioamnionitis. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S116. [DOI] [PubMed] [Google Scholar]
- Seaward PG, Hannah ME, Myhr TL, Farine D, Ohlsson A, Wang EE, et al. International multicenter term prom study: evaluation of predictors of neonatal infection in infants born to patients with premature rupture of membranes at term. American Journal of Obstetrics and Gynecology 1998;179(3 Pt 1):635‐9. [DOI] [PubMed] [Google Scholar]
- Weston J, Hannah M, Ohlsson A. Changing the study design during the recruitment phase of an international perinatal multicentre clinical trial. Controlled Clinical Trials 1993;14:401. [Google Scholar]
Javaid 2008 {published data only}
- Javaid M, Tahira T, Hassan S. Management prelabour rupture of the membranes at term; induction of labour compared with expectant. Professional Medical Journal 2008;15(2):216‐9. [Google Scholar]
Krupa 2005 {published data only}
- Krupa FG, Cecatti JG, Surita GC, Milanez HMBP, Parpinelli MA. Misoprostol versus expectant management in premature rupture of membranes at term. BJOG: an international journal of obstetrics and gynaecology 2005;112:1284‐90. [DOI] [PubMed] [Google Scholar]
Mahmood 1992 {published data only}
- Mahmood TA, Dick MJ, Smith NC, Templeton AA. Role of prostaglandin in the management of prelabour rupture of the membranes at term. British Journal of Obstetrics and Gynaecology 1992;99:112‐7. [DOI] [PubMed] [Google Scholar]
- Mahmood TA, Dick MJW, Smith NC, Templeton A. Management of spontaneous rupture of membranes at term without uterine activity in healthy primigravidae: a prospective study (PGE2 gel versus conservative treatment). The 2nd European Congress of Prostaglandins in Reproduction; 1991 April 30‐May 3; The Hague, Netherlands. 1991:95.
Mahmood 1995 {published data only}
- Mahmood TA, Dick MJW. A randomized trial of management of pre‐labor rupture of membranes at term in multiparous women using vaginal prostaglandin gel. Obstetrics & Gynecology 1995;85(1):71‐4. [DOI] [PubMed] [Google Scholar]
Maqbool 2014 {published data only}
- Maqbool S, Usmani AS, Bano B. Comparison of induction and expectant management of prelabour rupture of membranes at term for maternal outcome. Pakistan Journal of Medical and Health Sciences 2014; Vol. 8, issue 3:648‐51.
McQueen 1992 {unpublished data only}
- McQueen D. A randomized controlled trial comparing expectant management with active management in early rupture of the membranes. Personal communication 1992.
Milasinovic 1998 {published and unpublished data}
- Milasinovic L, Radeka G, Petrovic D, Orelj M, Savin A. Premature rupture of the membranes: early induction of labor versus expectant management [Rano prsnuce plodovih ovojaka: activni ili ekspektativni pristup resanvanju opstetrickog problema]. Medicinski Pregled 1998;51(7‐8):346‐9. [PubMed] [Google Scholar]
Natale 1994 {published data only}
- Natale R, Milne JK, Campbell MK, Potts PGG, Webster K, Halinda E. Management of premature rupture of membranes at term: randomized trial. American Journal of Obstetrics and Gynecology 1994;171(4):936‐9. [DOI] [PubMed] [Google Scholar]
- Natale R, Milne K, Campbell K, Webster K, Halinda E. Management of premature rupture of membranes at term: randomized trial. Proceedings of 49th Annual Clinical Meeting of the Society of Obstetricians and Gynaecologists of Canada; 1993 June 22‐26; Ottawa, Ontario, Canada. 1993:15.
- Natale R, Milne K, Campbell K, Wester K, Halinda E. Management of premature rupture of membranes at term: randomized trial. American Journal of Obstetrics and Gynecology 1994;170(1 Pt 2):285. [DOI] [PubMed] [Google Scholar]
Ottervanger 1996 {published data only}
- Ottervanger HP, Holm JP, Keirse MJNC. A randomized trial of expectant vs active management for prelabour rupture of the membranes at term. Journal of Perinatal Medicine 1992;20(1):223. [Google Scholar]
- Ottervanger HP, Holm JP, Keirse MJNC. Premature rupture of the membranes at term: induction of labour or expectant care?. Proceedings of 13th World Congress of Gynaecology and Obstetrics (FIGO); 1991 September; Singapore Vol. 1991:432.
- Ottervanger HP, Keirse MJNC, Smit W, Holm J. Controlled comparison of induction versus expectant care for prelabour rupture of the membranes at term. Journal of Perinatal Medicine 1996;24:237‐42. [DOI] [PubMed] [Google Scholar]
Selmer‐Olsen 2007 {published data only}
- Selmer‐Olsen T, Lydersen S, Morkved S. Does acupuncture used in nulliparous women reduce time from prelabour rupture of membranes at term to active phase of labour? A randomised controlled trial. Acta Obstetricia et Gynecologica Scandinavica 2007;86(12):1447‐52. [DOI] [PubMed] [Google Scholar]
Shah 2012 {published data only}
- Shah K, Doshi H. Premature rupture of membrane at term: Early induction versus expectant management. Journal of Obstetrics and Gynecology of India 2012;62(2):172‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shalev 1995 {published data only}
- Shalev E, Peleg D, Eliyahu S, Nahum Z. Comparison of 12‐ and 72‐hour expectant management of premature rupture of membranes in term pregnancies. Obstetrics & Gynecology 1995;85:766‐8. [DOI] [PubMed] [Google Scholar]
Sperling 1993 {published data only}
- Sperling LS, Schantz AL, Wahlin A, Duun S, Jaszczak P, Scherling B, et al. Management of prelabor rupture of membranes at term. Acta Obstetricia et Gynecologica Scandinavica 1993;72:627‐32. [DOI] [PubMed] [Google Scholar]
Tamsen 1990 {published data only}
- Tamsen L, Lyrenas S, Cnattingius S. Premature rupture of the membranes ‐ intervention or not. Gynecologic and Obstetric Investigation 1990;29:128‐31. [DOI] [PubMed] [Google Scholar]
Tasnim 2000 {published data only}
- Tasnim N. Spontaneous pre‐labour rupture of membranes (PROM) at term. Pakistan Journal of Medical Research 2000;39(2):66‐9. [Google Scholar]
Wagner 1989 {published data only}
- Wagner MV, Chin VP, Peters CJ, Drexler B, Newman LA. A comparison of early and delayed induction of labour with spontaneous rupture of membranes at term. Obstetrics & Gynecology 1989;74(1):93‐7. [PubMed] [Google Scholar]
- Wagner MV, Chin VP, Peters CJ, Drexler B, Newman LA. Management of spontaneous rupture of membranes at term. Proceedings of 36th Annual Clinical Meeting of the American College of Obstetricians and Gynecologists; 1988 May 2‐5; Boston, Massachusetts, USA. 1988:16.
References to studies excluded from this review
Alcalay 1996 {published data only}
- Alcalay M, Hourvitz A, Reichman B, Luski A, Quint J, Barkai G, et al. Prelabour rupture of membranes at term: early induction of labour versus expectant management. European Journal of Obstetrics & Gynecology and Reproductive Biology 1996;70:129‐33. [DOI] [PubMed] [Google Scholar]
- Alcalay M, Reichman B, Lipitz S, Hourvitz A, Chayen B, Mashiach S, et al. A prospective randomized study of premature rupture of membranes at term: early induction of labor vs expectant management. American Journal of Obstetrics and Gynecology 1993;168(1 Pt 2):433. [DOI] [PubMed] [Google Scholar]
Brosnan 1996 {published data only}
- Brosnan C. Trial of active vs conservative approach to induction of labour in patients with prelabour rupture of the membranes at term on serious fetal/neonatal infection. Personal communication 1996.
Cararach 1996 {published data only}
- Cararach V, Sentis J, Botet F, Foradada C, Manau D, Figueras F, et al. Cervical prostaglandin E1 compared with expectant management and with systematic induction in PROM near term, with bad cervical conditions. I‐maternal results. 3rd World Congress of Perinatal Medicine; 1996 Oct 20‐24; San Francisco, USA. 1996:44.
- Foradada C, Cararach V, Sentis J, Botet F, Manau D, Figueras F, et al. Cervical prostaglandin E1 compared with expectant management and with systematic induction in PROM near term, with bad cervical conditions. II‐fetal and neonatal results [abstract]. 3rd World Congress of Perinatal Medicine; 1996 Oct 20‐24; San Francisco, USA. 1996:51‐52.
Chang 1997 {published data only}
- Chang P, Langer O. Premature rupture of membranes at term; a randomized controlled trial. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S148. [Google Scholar]
Chaudhuri 2006 {published data only}
- Chaudhuri S, Mitra SN, Biswas PK, Bhattacharyya S. Premature rupture of membranes at term: immediate induction with PGE2 gel compared with delayed induction with oxytocin. Journal of Obstetrics and Gynaecology of India 2006;56(3):224‐9. [Google Scholar]
Chua 1995 {published data only}
- Chua S, Arulkumaran S, Yap C, Selamat N, Ratnam SS. Premature rupture of membranes in nulliparas at term with unfavorable cervices: a double‐blind randomized trial of prostaglandin and placebo. Obstetrics & Gynecology 1995;86:550‐4. [DOI] [PubMed] [Google Scholar]
Davies 1991 {published data only}
- Davies NJ, Martindale E, Haddad NG. Cervical ripening with oral PGE2 tablets and the effect of the latent period in patients with premature rupture of the membranes at term. Proceedings of 2nd European Congress on Prostaglandins in Reproduction; 1991 April 30‐May 3; The Hague, Netherlands. 1991:156.
- Davies NJ, Martindale E, Haddad NG. Cervical ripening with oral prostaglandin E2 tablets and the effect of the latent period in patients with premature rupture of the membranes at term. Journal of Obstetrics and Gynaecology 1991;11:405‐8. [Google Scholar]
Doungtone 1999 {published data only}
- Doungtone N, Tanaput Y. Management of premature rupture of membranes with unfavorable cervix in term pregnancy. Journal of Obstetrics and Gynaecology 1999;11(4):253. [Google Scholar]
Duff 1984 {published data only}
- Duff P, Huff RW, Gibbs RS. Management of premature rupture of membranes and unfavorable cervix in term pregnancy. Obstetrics & Gynecology 1984;63(5):697‐702. [PubMed] [Google Scholar]
Freeman 1968 {published data only}
- Freeman RK, Mishell DR. Induction of labor with sparteine sulfate for premature rupture of the fetal membranes near term. Pacific Medicine and Surgery 1968;76:43‐7. [PubMed] [Google Scholar]
Gloeb 1989 {published data only}
- Gloeb DJ, O'Sullivan MJ, Beydoun SN. Relationship of the interval between spontaneous premature rupture of the membranes and inducibility of labor. Proceedings of 9th Annual Meeting of the Society of Perinatal Obstetricians; 1989 February 1‐4; New Orleans, Louisiana, USA. 1989:493.
Gonen 1994 {published data only}
- Gonen R, Samberg I, Degani S. Intracervical prostaglandin E2 for induction of labour in patients with premature rupture of membranes and an unripe cervix. American Journal of Perinatology 1994;11(6):436‐8. [DOI] [PubMed] [Google Scholar]
- Gonen R, Samberg I, Degani S, Sharf M. Intracervical prostaglandin E2 for induction of labor in patients with premature rupture of membranes and an unfavourable cervix. American Journal of Obstetrics and Gynecology 1993;168(1 Pt 2):362. [Google Scholar]
Grant 1992 {published data only}
- Grant JM, Serle E, Mahmood T, Sarmandal P, Conway D. Management of prelabour rupture of the membranes in term primigravidae: report of a randomized prospective trial. British Journal of Obstetrics and Gynaecology 1992;99:557‐62. [DOI] [PubMed] [Google Scholar]
Hidar 2000 {published data only}
- Hidar S, Bibi M, Jerbi M, Bouguizene S, Nouira M, Mellouli R, et al. Contribution of intracervical PGE2 administration in premature rupture of the membranes at term. Prospective randomised clinical trial [Apport de l'administration intracervicale de PGE2 dans les ruptures prematurees des membranes a terme]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 2000;29(6):607‐13. [PubMed] [Google Scholar]
Hjertberg 1996 {published data only}
- Granstrom L, Hammarstrom M, Hjertberg R, Moberger B, Berg A, Norlander E. Expectant management in nulliparous term pregnant women with premature rupture of membranes and an unripe cervix. Journal of Obstetrics and Gynaecology 1995;15:366‐72. [Google Scholar]
- Hjertberg R, Berg A, Ekman G, Granstrom L, Hammarstrom M, Moberger B, et al. Twelve or 24‐hours expectancy in premature rupture of the membranes (PROM) at term. Proceedings of 14th European Congress of Perinatal Medicine; 1994 June 5‐8; Helsinki, Finland. 1994:408.
- Hjertberg R, Hammarstrom M, Moberger B, Nordlander E, Granstrom L. Premature rupture of the membranes (PROM) at term in nulliparous women with a ripe cervix: a randomized trial of 12 or 24 hours expectant management. Acta Obstetricia et Gynecologica Scandinavica 1996;75:48‐53. [DOI] [PubMed] [Google Scholar]
Hoffman 2001 {published data only}
- Hoffman R, Fawcus J. Oral misoprostol vs. placebo in the management of prelabor rupture of membranes at term. International Journal of Gynecology & Obstetrics 2001;72:215‐21. [DOI] [PubMed] [Google Scholar]
- Hoffmann R, Fawcus S, Anthony J. Oral misoprostol versus placebo in the management of prelabour rupture of membranes at term. Women's Health ‐ into the new millenium. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 October 3‐6; Cape Town South Africa. 1999:65.
Ladfors 1996 {published data only}
- Ladfors L, Mattsson LA, Eriksson M, Fall O. A randomised trial of two expectant managements of prelabour rupture of the membranes at 34 to 42 weeks. British Journal of Obstetrics and Gynaecology 1996;103:755‐62. [DOI] [PubMed] [Google Scholar]
- Ladfors L, Mattsson LA, Eriksson M, Fall O. A randomized prospective trial of two expectant managements of pre‐labor rupture of the membranes (PROM) at 34‐42 weeks. American Journal of Obstetrics and Gynecology 1994;170:344. [Google Scholar]
- Ladfors L, Tessin I, Fall O, Erikson M, Matsson L. A comparison of neonatal infectious outcome comparing two expectant managements of women with prelabor rupture of the membranes at 34‐42 weeks. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S197. [Google Scholar]
Levy 2005 {published data only}
- Levy R, Vaisbuch E, Furman B, Doitch H, Oron S, Hagay Z. Prospective randomized clinical trial of immediate induction of labor with oral misoprostol for prelabor rupture of the membranes in women with unfavorable cervix at term [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S44. [Google Scholar]
Levy 2007 {published data only}
- Levy R, Vaisbuch E, Furman B, Brown D, Volach V, Hagay ZJ. Induction of labor with oral misoprostol for premature rupture of membranes at term in women with unfavorable cervix: a randomized, double‐blind, placebo‐controlled trial. Journal of Perinatal Medicine 2007;35(2):126‐9. [DOI] [PubMed] [Google Scholar]
Lo 2003 {published data only}
- Lo J, Alexander J, McIntire D, Leveno K. Efficacy of oral misoprostol in nulliparous women with premature rupture of membranes [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S204. [Google Scholar]
- Lo JY, Alexander JM, McIntire DD, Leveno KJ. Randomized trial of oral misoprostol in nulliparous women with premature rupture of membranes at term [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S204. [Google Scholar]
- Lo JY, Alexander JM, McIntire DD, Leveno KJ. Ruptured membranes at term: randomized, double‐blind trial of oral misoprostol for labor induction. Obstetrics & Gynecology 2003;101:685‐9. [DOI] [PubMed] [Google Scholar]
Mahmood 1989 {published data only}
- Mahmood TA, Dick MJW, Smith NC. Management of spontaneous rupture of the membranes and no uterine activity in healthy primigravidae after 34 weeks' gestation. Lancet 1989;1:721. [DOI] [PubMed] [Google Scholar]
Mateos 1998 {published data only}
- Cararach V, Sentis J, Botet F, Costa J, Manau D, Arimany MC. Cervical prostaglandin E2 compared with expectant management or systematic induction in PROM with bad cervical conditions: I‐maternal results. Proceedings of 14th European Congress of Perinatal Medicine; 1994 June 5‐8; Helsinki, Finland. 1994.
- Mateos D, Cararach V, Sentis J, Botet F, Figueras F, Arimany M, et al. Cervical prostaglandin E2 compared with expectant management or systematic induction in premature rupture of the membranes with bad cervical conditions. Prenatal and Neonatal Medicine 1998;1(Suppl 1):85. [Google Scholar]
McCaul 1997 {published data only}
- McCaul JF, Rogers LW, Perry KG, Martin RW, Albert JR, Morrison JC. Premature rupture of membranes at term with an unfavorable cervix: comparison of expectant management, vaginal prostaglandin, and oxytocin induction. Southern Medical Journal 1997;90(12):1229‐33. [DOI] [PubMed] [Google Scholar]
- McCaul JF, Williams LM, Martin RW, Magann EF, Gallagher L, Morrison JC. Comparison of induction methods for premature rupture of membranes at term. American Journal of Obstetrics and Gynecology 1992;166(1 Pt 2):275. [Google Scholar]
Morales 1986 {published data only}
- Morales WJ, Lazar AJ. Expectant management of rupture of membranes at term. Southern Medical Journal 1986;79(8):955‐8. [DOI] [PubMed] [Google Scholar]
Ngai 1996 {published data only}
- Ngai C, To W, Lao T, Ho P. Cervical priming with oral misoprostol in prelabour rupture of membranes at term. 27th British Congress of Obstetrics and Gynaecology;1995 July 4‐7; Dublin. 1995.
- Ngai SW, To WK, Lao T, Ho PC. Cervical priming with oral misoprostol in pre‐labour rupture of membranes at term. Obstetrics & Gynecology 1996;87:923‐6. [DOI] [PubMed] [Google Scholar]
Ozden 2002 {published data only}
- Ozden S, Delikara MN, Avci A, Ficicioglu C. Intravaginal misoprostol vs. expectant management in premature rupture of membranes with low Bishop scores at term. International Journal of Gynecology & Obstetrics 2002;77:109‐15. [DOI] [PubMed] [Google Scholar]
Perez Picarol 1990 {published data only}
- Perez Picarol E, Gamissans D, Lecumberri J, Jimenez M, Vernet M. Ripening the cervix with intracervical PGE2 gel in term pregnancies with premature rupture of the membranes. Proceedings of the 12th European Congress of Perinatal Medicine; 1990 September 11‐14; Lyon, France. 1990:197.
Poornima 2011 {published data only}
- Poornima B, Dharma DB. Premature rupture of membranes at term: Immediate induction with PGE 2 gel compared with delayed induction with oxytocin. Journal of Obstetrics and Gynecology of India 2011;61(5):516‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ray 1992 {published data only}
- Ray DA, Garite TJ. Prostaglandin E2 for induction in term patients with premature rupture of the membranes. Proceedings of 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 January 23‐27; Houston, Texas, USA. 1990:80.
- Ray DA, Garite TJ. Prostaglandin E2 for induction of labour in patients with premature rupture of membranes at term. American Journal of Obstetrics and Gynecology 1992;166:836‐43. [DOI] [PubMed] [Google Scholar]
Rydhstrom 1991 {published data only}
- Rydhstrom H, Ingemarsson I. No benefit from conservative management in nulliparous women with premature rupture of the membranes (PROM) at term: a randomized study. Acta Obstetricia et Gynecologica Scandinavica 1991;70:543‐7. [DOI] [PubMed] [Google Scholar]
Shetty 2002 {published data only}
- Shetty A, Stewart K, Stewart G, Rice P, Danielian P, Templeton A. Active management of term prelabour rupture of membranes with oral misoprostol. BJOG: an international journal of obstetrics and gynaecology 2002;109:1354‐8. [DOI] [PubMed] [Google Scholar]
Shoaib 1994 {published data only}
- Shoaib F. Management of premature rupture of membranes with unfavourable cervix at term, by prostaglandins. Specialist 1994;10:227‐32. [Google Scholar]
Suzuki 2000 {published data only}
- Suzuki S, Otsubo Y, Sawa R, Yoneyama Y, Araki T. Clinical trial of induction of labor versus expectant management in twin pregnancy. Gynecologic and Obstetric Investigation 2000;49:24‐7. [DOI] [PubMed] [Google Scholar]
Thomas 2000 {published data only}
- Thomas N, Longo SA, Rumney PJ. Intravaginal misoprostol in prelabour rupture of membranes at term. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S136. [Google Scholar]
Van der Walt 1989 {published data only}
- Walt D, Venter PF. Management of term pregnancy with premature rupture of the membranes and unfavourable cervix. South African Medical Journal 1989;75:54‐6. [PubMed] [Google Scholar]
Van Heerden 1992 {published data only}
- Heerden J, Steyn DW. Management of premature rupture of membranes after 34 weeks gestation. Proceedings of 11th Conference on Priorities in Perinatal Care in South Africa; 1992; Caledon, South Africa. 1992.
- Heerden J, Steyn DW. Management of premature rupture of the membranes after 34 weeks' gestation ‐ early versus delayed induction of labour. South African Medical Journal 1996;86:264‐8. [PubMed] [Google Scholar]
References to ongoing studies
Walfisch 2014 {published data only}
- Walfisch A. Management of labor in patients with previous cesarian section and premature rupture of membranes who desire TOLAC: comparison between the use of standard expectant management and the double‐balloon catheter device. a prospective randomized study. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 18 August 2015] 2014.
Additional references
Alexander 1996
- Alexander J, Cox S. Clinical course of premature rupture of the membranes. Seminars in Perinatology 1996;20(5):369‐74. [DOI] [PubMed] [Google Scholar]
Buchanan 2010
- Buchanan SL, Crowther CA, Levett KM, Middleton P, Morris J. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks' gestation for improving pregnancy outcome. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD004735] [DOI] [PubMed] [Google Scholar]
Cammu 1990
- Cammu H, Verlaenen H, Derde M. Premature rupture of membranes at term in nulliparous women: a hazard?. Obstetrics & Gynecology 1990;76:671‐4. [PubMed] [Google Scholar]
Conway 1984
- Conway D, Prendiville W, Morris A, Speller D, Stirrat G. Management of spontaneous rupture of the membranes in the absence of labor in primigravid women at term. American Journal of Obstetrics and Gynecology 1984;150:947‐51. [DOI] [PubMed] [Google Scholar]
Crane 2003
- Crane J, Young D. Induction of labour with a favourable cervix and/or pre‐labour rupture of membranes. Best Practice & Research. Clinical Obstetrics & Gynaecology 2003;17(5):795‐809. [DOI] [PubMed] [Google Scholar]
Duff 1996
- Duff P. Premature rupture of the membranes in term patients. Seminars in Perinatology 1996;20(5):401‐8. [DOI] [PubMed] [Google Scholar]
Duff 1998
- Duff P. Premature rupture of the membranes in term patients: induction of labor versus expectant management. Clinical Obstetrics and Gynecology 1998;41(4):883‐91. [DOI] [PubMed] [Google Scholar]
Gafni 1997
- Gafni A, Goeree R, Myhr T, Hannah M, Blackhouse G, Willan A, et al. Induction of labour versus expectant management for prelabour rupture of membranes at term: an economic evaluation. Canadian Medical Association Journal 1997;157(11):1519‐25. [PMC free article] [PubMed] [Google Scholar]
Gonen 1989
- Gonen R, Hannah M, Milligan J. Does prolonged preterm premature rupture of the membranes predispose to abruptio placentae?. Obstetrics & Gynecology 1989;74:347‐50. [PubMed] [Google Scholar]
Guise 1992
- Guise J, Duff P, Christian J. Management of term patients with premature rupture of membranes and an unfavorable cervix. American Journal of Perinatology 1992;9(1):56‐60. [DOI] [PubMed] [Google Scholar]
Gülmezoglu 2012
- Gülmezoglu AM, Crowther CA, Middleton P, Heatley E. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD004945.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hallak 1999
- Hallak M, Bottoms S. Induction of labour in patients with term premature rupture of membranes. Fetal Diagnosis and Therapy 1999;14:128‐42. [DOI] [PubMed] [Google Scholar]
Hannah 1998
- Hannah M, Seaward G. Prelabour rupture of membranes at term: the role of induction of labour. Fetal and Maternal Medicine Review 1998;10:61‐8. [Google Scholar]
Hannah 1999
- Hannah M. Commentary: managing labor: what do women really want?. Birth 1999;26(2):97‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Johnson 1981
- Johnson JWC, Daikoku NH, Niebyl JR, Johnson TRB, Khouzami VA, Witter FR. Premature rupture of the membranes and prolonged latency. Journal of the American College of Obstetricians and Gynecologists 1981;57(5):547‐56. [PubMed] [Google Scholar]
Kong 1992
- Kong AS, Bates SJ, Rizk B. Rupture of membranes before the onset of spontaneous labour increases the likelihood of instrumental delivery. British Journal of Anaesthesia 1992;68:252‐5. [DOI] [PubMed] [Google Scholar]
Lin 2005
- Lin MG, Nuthalapaty FS, Carver AR, Case AS, Ramsey PS. Misoprostol for labour induction in women with term premature rupture of membranes: a meta‐analysis. Obstetrics and Gynecology 2005;106:593‐601. [DOI] [PubMed] [Google Scholar]
Merenstein 1996
- Merenstein G, Weisman L. Premature rupture of the membranes: neonatal consequences. Seminars in Perinatology 1996;20(5):375‐80. [DOI] [PubMed] [Google Scholar]
Mishanina 2014
- Mishanina E, Rogozinska E, Thatthi T, Uddin‐Khan R, Khan KS, Meads C. Use of labour induction and risk of cesarean delivery: a systematic review and meta‐analysis. Canadian Medical Association Journal 2014;186(9):665‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 2016
- Morris JM, Roberts CL, Bowen JR, Patterson JA, Bond DM, Algert CA, et al. Immediate delivery compared with expectant management after preterm pre‐labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet 2016;387:444‐52. [DOI] [PubMed] [Google Scholar]
Mozurkewich 1997
- Mozurkewich E, Wolf F. Premature rupture of membranes at term: a meta‐analysis of three management systems. Obstetrics and Gynecology 1997;89:1035‐43. [DOI] [PubMed] [Google Scholar]
Mozurkewich 2009
- Mozurkewich E, Chilimigras J, Koepke E, Keeton K, King V. Indications for induction of labour: a best‐evidence review. BJOG: an international journal of obstetrics and gynaecology 2009;116:626‐36. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robson 1990
- Robson MS, Turner MJ, Stronge JM, O'Herlihy C. Is amniotic fluid quantitation of value in the diagnosis and conservative management of prelabour rupture of membranes at term?. British Journal of Obstetrics and Gynaecology 1990;97(4):324‐8. [DOI] [PubMed] [Google Scholar]
Seaward 1997
- Seaward P, Hannah M, Myhr T, Farine D, Ohlsson A, Wang E, et al. International Multicentre Term Prelabour Rupture of Membranes Study: evaluation of predictors of clinical chorioamnionitis and postpartum fever in patients with prelabour rupture of membranes at term. American Journal of Obstetrics and Gynecology 1997;177:1024‐9. [DOI] [PubMed] [Google Scholar]
Wojcieszek 2014
- Wojcieszek AM, Stock OM, Flenady V. Antibiotics for prelabour rupture of membranes at or near term. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD001807.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yawn 2001
- Yawn B, Wollan P, McKeon K, Field C. Temporal changes in rates and reasons for medical induction of term labor, 1980‐1996. American Journal of Obstetrics and Gynecology 2001;184:611‐9. [DOI] [PubMed] [Google Scholar]
Zlatnik 1992
- Zlatnik FJ. Management of premature rupture of membranes at term. Obstetrics and Gynecology Clinics of North America 1992;19(2):353‐64. [PubMed] [Google Scholar]
References to other published versions of this review
Dare 2005
- Dare MR, Crowther CA, Middleton P. Induction of labour versus expectant management for prelabour rupture of membranes at term (more than 37 weeks). Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD005302] [DOI] [Google Scholar]
Dare 2006
- Dare MR, Middleton P, Crowther CA, Flenady V, Varatharaju B. Planned early birth versus expectant management (waiting) for prelabour rupture of membranes at term (37 weeks or more). Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD005302.pub2] [DOI] [PubMed] [Google Scholar]


