Abstract
Background
Women with a septate uterus are at increased risk for subfertility, recurrent miscarriage, and preterm birth. Restoration of the anatomy of the uterus by hysteroscopic septum resection is an established intervention. This treatment has been assessed mainly in retrospective cohort studies, which suggested a positive effect on pregnancy outcomes. The major flaw in these studies is the before/after design, which will always favour the tested intervention.
Objectives
To determine whether hysteroscopic septum resection in women of reproductive age with a septate uterus improves live birth rates and to assess the safety of this procedure.
Search methods
We searched the Cochrane Gynaecology and Fertility Group Specialised Register (inception to May 2016), the Cochrane Central Register of Controlled Trials (CENTRAL CRSO) (inception to May 2016), MEDLINE (1946 to May 2016), Embase (1974 to May 2016), PsycINFO (1806 to May 2016), and CINAHL database (1982 to May 2016). We also searched trial registers for ongoing and registered trials, reference lists, the Cochrane Library, unpublished dissertations and theses, conference abstracts, OpenGrey, LILACS, PubMed, and Google.
Selection criteria
We planned to include randomised controlled trials that assessed the effect on reproductive outcomes and the safety of hysteroscopic septum resection in women of reproductive age with a septate uterus.
Data collection and analysis
If there had been studies to include, two review authors would have independently selected studies, assessed trial risk of bias, and extracted data. They would also have contacted study authors for additional information.
Main results
As in the 2011 version of this review, we identified no randomised controlled trials for inclusion in this update.
Authors' conclusions
Hysteroscopic septum resection in women of reproductive age with a septate uterus is performed worldwide to improve reproductive outcomes. At present, there is no evidence to support the surgical procedure in these women. Randomised controlled trials are urgently needed. Two trials are currently underway.
Plain language summary
Septum resection for women of childbearing age with a septate uterus
Review question
Cochrane authors wanted to know whether hysteroscopic septum resection (surgical removal of the septum) improves the chances of a live birth in women with a septate uterus, and whether these benefits outweigh the possible complications of the procedure.
Background
A septate uterus is an inborn abnormality of the uterus (womb), where the womb is divided into two cavities. Women with a septate uterus are at risk for subfertility, recurrent miscarriage, and preterm birth. Surgical removal of the septum is thought to improve these outcomes, but the effectiveness of this surgical procedure is unknown.
Study characteristics
We examined the research published up to May 2016. Randomised controlled trials that assessed the effect on reproductive outcomes of hysteroscopic septum resection in women of childbearing age with a septate uterus were eligible for inclusion. In these trials, women would be randomised to either septum resection or expectant management (no surgery). There were no studies to include so we cannot report on funding sources.
Key results
As in the 2011 version of this review, we identified no published randomised controlled trials to include in this update.
Background
Description of the condition
The uterus originates from the paramesonephric, or Müllerian ducts. In a septate uterus, the Müllerian ducts have not fused during the period of embryologic development. A septate uterus is defined as a uterus with a division of the uterine cavity (septum) without any restrictions to the length of the septum, according to the new ESHRE (European Society of Human Reproduction and Embryology)/ESGE (European Society for Gynaecological Endoscopy) classification system for female genital tract congenital anomalies. The external contour of the uterus should not have an indentation (Grimbizis 2013) (Figure 1). Septate uterus is the most common uterine anomaly, accounting for 35% of all identified uterine anomalies.
1.
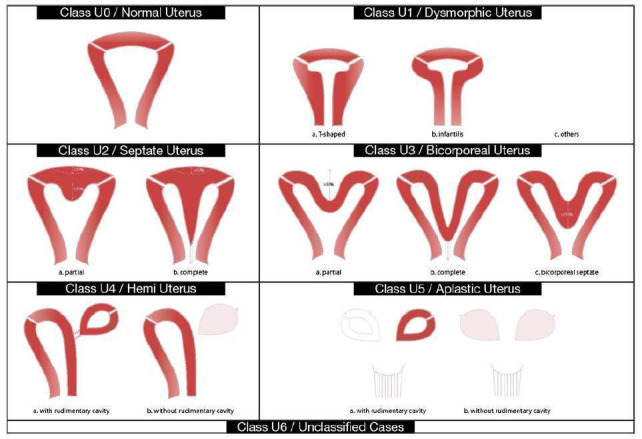
The ESHRE/ESGE classification system for female genital congenital anomalies (Grimbizis 2013).
A septate uterus is associated with reduced fertility (risk ratio (RR) 0.86, 95% confidence interval (CI) 0.77 to 0.96), increased miscarriage rates (RR 2.9, 95% CI 2.0 to 4.1), and increased preterm births (RR 2.1, 95% CI 1.5 to 3.1) (Chan 2011).
Description of the intervention
Traditionally, the uterine septum was resected by a laparotomic hysterotomy (Paradisi 2014), but since the introduction of hysteroscopic septum resection in 1970, the latter approach is considered first‐line therapy (Edström 1970). Possible complications of a hysteroscopic septum resection are bleeding, perforation of the uterus, postoperative intrauterine adhesions, and uterine rupture in subsequent pregnancies (Valle 2013). Even so, hysteroscopic septum resection is still common practice in many countries (Paradisi 2014). This procedure is assumed to be effective based on non‐randomised and mainly retrospective trials. These studies are at high risk of bias due to their mainly before/after design, with the same group of women serving as their own controls. Before‐after comparisons will always favour the intervention (Christiansen 2005; Mastenbroek 2006).
How the intervention might work
The pathophysiology behind poor reproductive outcomes in women with a septate uterus is unknown. Earlier studies asserted that the septum is avascular and mainly consists of fibrous tissue (Fayez 1986; March 1983). The main cause of impaired fertility in women with a septate uterus was considered to be a disturbed implantation. More recent studies suggest that the septum consists of normal endometrium and myometrium, and resembles the uterine wall (Candiani 1983; Sparac 2001; Zreik 1998). It is unclear whether restoring normal anatomy also restores normal function, and thereby improves fertility outcomes in women who wish to conceive.
Why it is important to do this review
Various studies (mainly retrospective) have assessed the efficacy of the hysteroscopic removal of the uterine septum and restoration of uterine anatomy. Observational studies report large improvements in likelihood of pregnancy in a before/after septum resection study design (88% miscarriages before and 5.9% after surgery) (Homer 2000). However, these results are prone to bias as the prognosis without the intervention is usually good. (Christiansen 2005). As a consequence, we currently do not know whether a septum resection increases the chances of a live birth and whether this outweighs the possible complications of the procedure.
Objectives
To determine whether hysteroscopic septum resection in women of reproductive age with a septate uterus improves live birth rates and to assess the safety of the procedure.
Methods
Criteria for considering studies for this review
Types of studies
Truly randomised controlled trials (RCTs) were eligible for inclusion. We excluded quasi‐ or pseudo‐RCTs, as they are associated with a high risk of bias (Vail 2003).
Types of participants
Women of reproductive age with a septate uterus, defined as a uterus with a division of the uterine cavity (septum), without any restrictions to the length of the septum. We excluded women diagnosed with a septate uterus, with abnormal bleeding and who might not necessarily wish to conceive.
Types of interventions
Hysteroscopic septum resection of the septate uterus versus expectant management.
Types of outcome measures
Primary outcomes
Effectiveness: live birth rate, defined as the birth of a live baby after 24 completed weeks of gestation
Safety: surgical complications following septum resection: uterine perforation, fluid overload, endometritis, and repeat surgery
Secondary outcomes
Ongoing pregnancy
Clinical pregnancy
Pregnancy complications; miscarriage, placental abruption, uterine rupture, preterm birth and mode of delivery (vaginal versus caesarean section), postoperative intrauterine adhesions, neonatal morbidity and mortality
Search methods for identification of studies
We searched for all relevant published and unpublished RCTs of hysteroscopic septum resection versus expectant management in women of reproductive age with a septate uterus using a defined search strategy, without language restriction. We searched relevant studies from inception up to 30 May 2016. We carried out all searches in consultation with the Cochrane Gynaecology and Fertility Group Information Specialist.
Electronic searches
We searched the following electronic databases.
Cochrane Gynaecology and Fertility Group (CGF) Specialised Register (from inception to 30 May 2016) (Appendix 1),
Cochrane Central Register of Studies (CRSO) (from inception May 2016) (Appendix 2)
MEDLINE,(from 1946 to 30 May 2016) (Appendix 3)
Embase,(from 1974 to 30 May 2016) (Appendix 4)
PsycINFO (from 1806 to 30 May 2016) (Appendix 5)
CINAHL from 1982 to 30 May 2016) (Appendix 6)
The MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials, described in Section 6.4.11 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The Embase and PsycINFO searches were combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/mehodology/filters.html#random).
We used no language or date restriction in these searches.
In addition, we searched the following sources, all to 5 July 2016
Trial registers for ongoing and registered trials: ClinicalTrials.gov, which is a service of the US National Institutes of Health (clinicaltrials.gov/ct2/home) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/trialsearch/Default.aspx)(Appendix 7; Appendix 8)
The Cochrane Library (www.cochrane.org/index.htm) for the Database of Abstracts of Reviews of Effects (DARE) (Appendix 9)
ProQuest Dissertations & Theses for unpublished dissertations and theses (Appendix 10)
OpenGrey for unpublished literature from Europe (http://www.opengrey.eu/) (Appendix 11)
LILACS database (regional.bvsalud.org/php/index.php?lang=en) (Appendix 12)
Google and PubMed for any recent trials that have not yet been indexed in MEDLINE (Appendix 13; Appendix 14)
Searching other resources
Reference lists from reviews and trials
Handsearching of appropriate journals (in liaison with the CGFG Information Specialist)
Conference abstracts on the Web of Science (www.wokinfo.com/)
Data collection and analysis
Selection of studies
Two review authors (JR and CK) screened titles and abstracts of all identified studies against the inclusion criteria. We retrieved all potentially relevant articles in full text. We attempted to obtain translations of non‐English language papers sufficient to judge their suitability for inclusion. A list of ongoing studies is provided in the Characteristics of ongoing studies. We communicated with the contact persons of these trials for missing information.
In future updates when eligible studies are available, two review authors will independently examine full‐text articles for compliance with the inclusion criteria and select studies that fulfil these criteria. Any difference of opinion regarding trials for inclusion will be resolved by consensus or by discussion with a third review author.
Data extraction and management
We planned that two review authors would independently extract the data from each study using a data extraction form that the review authors had designed and pilot tested. Should studies have had multiple publications, we would have used the main trial report as the reference and additional details supplemented from secondary papers. Review authors would have corresponded with study investigators in order to resolve any data queries, as required. Two review authors would have independently extracted the data. Any disagreements between the review authors would have been resolved by a third review author.
Assessment of risk of bias in included studies
We planned that two review authors (JR and CK) would independently assess the included studies for risk of bias using the Cochrane 'Risk of bias' assessment tool. Disagreements would have been resolved by discussion or by consulting a third review author (MG). We planned to assess selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other potential biases such as differences in demographic characteristics between treatment groups at baseline. We planned to describe all judgements and present the conclusions in the 'Risk of bias' table.
(1) Random sequence generation
We planned to describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We would assess the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
unclear risk of bias.
(2) Allocation concealment
We planned to describe for each included study the method used to conceal allocation to interventions prior to assignment and would assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We would assess the methods as:
low risk of bias (e.g. web or telephone randomisation; consecutively numbered, sealed, opaque envelopes);
high risk of bias (open list of random allocation; unsealed or non‐opaque envelopes; alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel
Lack of blinding is unlikely to introduce bias, so we planned to assess methods as at low risk of bias.
(3.2) Blinding of outcome assessment
We planned to describe the methods used for each included study, if any, to blind outcome assessors from knowledge of which intervention a participant received. We planned to assess blinding separately for different outcomes or classes of outcomes.
We planned to assess the methods used to blind outcome assessment as low, high, or unclear risk of bias.
(4) Incomplete outcome data
We planned to describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We planned to state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total number of randomised participants), reasons for attrition or exclusion were reported, and whether missing data were balanced across groups or were related to outcomes.
We planned to assess methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'as treated' analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting
We planned to describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We planned to assess the methods as:
low risk of bias, where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported, and the outcomes of interest have been prespecified in a prospectively registered protocol;
high risk of bias, where not all of the study’s prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so cannot be used; the study failed to include results of a key outcome that would have been expected to have been reported;
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We planned to describe for each included study any important concerns we had about other possible sources of bias.
Measures of treatment effect
We planned to perform statistical analyses in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We would report only dichotomous outcomes, and for such outcomes use the numbers of events in the control and intervention groups of each study to calculate Mantel‐Haenszel odds ratios in a fixed‐effect model. We planned to present 95% confidence intervals for all outcomes and use Review Manager 5 software (RevMan 2014) for statistical analysis. We planned to translate primary outcomes to absolute risks for reporting purposes.
We planned to use a random‐effects model in the case of an I2 statistic above 50%.
Unit of analysis issues
The primary analysis would be per woman randomised. We would have counted multiple live births (for example twins or triplets) as one live birth event.
Dealing with missing data
We planned to analyse the data on an intention‐to‐treat basis. If important data were missing, we would attempt to obtain the missing data by contacting the original investigators. If this was not possible, we would impute the missing data with replacement values for the primary outcome in a sensitivity analysis.
Assessment of heterogeneity
We planned to consider whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We would assess statistical heterogeneity using the I2 statistic. We would consider an I2 measurement greater than 50% as indicating substantial heterogeneity (Higgins 2011). If we detected substantial heterogeneity, we would explore possible explanations for it in sensitivity analyses.
Assessment of reporting biases
We planned to minimise the impact of reporting biases by ensuring a comprehensive search for eligible studies while being alert to duplication of data. If there were 10 or more studies in an analysis, we would use a funnel plot to explore the possibility of small‐study effects, since there is a tendency for estimates of the intervention effect to be more beneficial in smaller studies.
Data synthesis
If trials were sufficiently similar, we would combine the data using a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We planned to perform a subgroup analysis by participant clinical characteristics (women with recurrent miscarriage, women with subfertility or other complaints). We anticipated heterogeneity between subgroups and planned to discuss possible reasons for it.
Sensitivity analysis
If data from more than four studies were available, we planned to perform sensitivity analyses. We would assess the influence of risk of bias on effect size by removing trials deemed to be at high risk of bias. Studies with high risk of bias would include those that did not use an intention‐to‐treat approach and those that had inadequate concealment of allocation. We would repeat analyses using a random‐effects model to explore whether different conclusions were reached. We would report sensitivity analyses for the primary outcomes only.
Overall quality of the body of evidence: Summary of findings table
We planned to prepare a 'Summary of findings' table using GRADEpro (GRADEpro GDT 2014) and Cochrane methods (Higgins 2011). We planned that this table would evaluate the overall quality of the body of evidence for the main review outcomes (live birth rate, ongoing pregnancy, adverse effects) for the main review comparison (septum resection versus expectant management). We planned to assess the quality of the evidence using GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness, and publication bias. We planned that two review authors would independently make judgements about evidence quality (high, moderate, low, or very low), with disagreements resolved by discussion, and that judgements would be justified, documented, and incorporated into the reporting of results for each outcome.
We planned to extract study data, format our comparisons in data tables, and prepare a 'Summary of findings' table before writing the results and conclusions of our review.
Results
Description of studies
We found no RCTs assessing whether hysteroscopic septum resection influences reproductive outcomes in women of reproductive age with a septate uterus.
Results of the search
After removal of duplicates and we identified 78 articles (Figure 2).
2.

Study flow diagram.
Included studies
None.
Ongoing studies
We found two ongoing trials in our search of the WHO ICTRP: the TRUST study, NTR1676, and the Pilot randomised controlled trial of hysteroscopic septal resection (ISRCTN28960271). Results of neither trial have been published.
Excluded studies
Not applicable.
Risk of bias in included studies
Not applicable.
Effects of interventions
Not applicable.
Discussion
Summary of main results
Consistent with the first issue of the review in 2011, we did not find any RCTs comparing hysteroscopic septum resection with expectant management in women of reproductive age with a septate uterus in this update.
Potential biases in the review process
The search was systematic and thorough, therefore the risk of introducing bias was low.
Agreements and disagreements with other studies or reviews
Over the years, nine comparative studies have been published. These studies describe miscarriage, pregnancy, or live birth rate in women with a septate uterus who consented to hysteroscopic septum resection, compared with women who chose expectant management. Three of these studies showed a significantly higher pregnancy rate in women with a septate uterus who were treated with surgery (Gaucherand 1994; Pang 2011; Tonguc 2011), while six found no significant difference between the groups (Heinonen 1997; Kirk 1993; Lin 2009; Maneschi 1991; Sugiura‐Ogasawara 2013; Valli 2004).
In conclusion, there is no evidence that hysteroscopic septum resection improves reproductive outcome in women with a septate uterus and outweighs the possible complications of the procedure.
Authors' conclusions
Implications for practice.
Hysteroscopic septum resection in women of reproductive age with a septate uterus is performed worldwide to improve reproductive outcomes. At present, there is no evidence to support this surgical intervention in these women.
Implications for research.
In this update, there were still no results from RCTs in women of reproductive age with a septate uterus. This underscores the need for properly designed RCTs. Two of these are currently underway (ISRCTN28960271; NTR1676).
What's new
| Date | Event | Description |
|---|---|---|
| 25 February 2020 | Amended | The authors' Declarations of interest have been updated to reflect the review's compliance with the Cochrane conflict of interest policy, which includes the relevant parts of the Cochrane Commercial Sponsorship Policy. Additional information; declaration of interest by Mark Emanuel. |
History
Protocol first published: Issue 7, 2010 Review first published: Issue 6, 2011
| Date | Event | Description |
|---|---|---|
| 24 November 2016 | New citation required but conclusions have not changed | New searches and extended scope did not identify any studies to include. |
| 24 November 2016 | New search has been performed | The scope of the review has been extended from women with recurrent miscarriage and a septate uterus to all women of reproductive age with a septate uterus. The searches have been updated. |
| 21 September 2014 | Amended | The search has been updated. |
| 21 July 2010 | New citation required but conclusions have not changed | Correction of some grammatical errors in the text |
Acknowledgements
M Showell, CGF Group Information Specialist
H Nagels, CGF Group Managing Editor
We acknowledge the contributions of Professor Maas Jan Heineman, Professor Marlies Bongers, Dr Jan de Kruif, and Dr Taeke Spinder to previous versions of this review.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group specialised register
From inception to 30 May 2016
Procite platform
Keywords CONTAINS "uterine anomalies" or "uterine malformation" or "uterine septa" or "uterine septum"or"septate uterus"or "metroplasty" or Title CONTAINS "uterine anomalies" or "uterine malformation" or "uterine septa" or "uterine septum" or "septate uterus"or "metroplasty" (22 hits)
Appendix 2. Cochrane CENTRAL Register of Controlled Studies (CRSO)
From inception to 30 May 2016 Web platform
#1 (arcuate* adj2 uter*):TI,AB,KY 5
#2 (subseptate* adj2 uter*):TI,AB,KY 2
#3 (sept* adj3 uter*):TI,AB,KY 23
#4 #1 OR #2 OR #3 28
#5 metroplast*:TI,AB,KY 26
#6 septoplast*:TI,AB,KY 184
#7 resect*:TI,AB,KY 11819
#8 #5 OR #6 OR #7 12007
#9 #4 AND #8 18
Appendix 3. MEDLINE
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) From 1946 to 30 May 2016 1 (arcuate$ adj2 uter$).tw. (150) 2 (subseptate$ adj2 uter$).tw. (29) 3 (sept$ adj3 uter$).tw. (819) 4 or/1‐3 (935) 5 metroplast$.tw. (343) 6 septoplast$.tw. (1317) 7 resect$.tw. (276683) 8 or/5‐7 (278118) 9 8 and 4 (318) 10 randomized controlled trial.pt. (417272) 11 controlled clinical trial.pt. (90753) 12 randomized.ab. (354957) 13 placebo.tw. (177246) 14 clinical trials as topic.sh. (176805) 15 randomly.ab. (254384) 16 trial.ti. (154430) 17 (crossover or cross‐over or cross over).tw. (69036) 18 or/10‐17 (1057533) 19 (animals not (humans and animals)).sh. (4215056) 20 18 not 19 (973611) 21 9 and 20 (23)
Appendix 4. Embase
Ovid platform
From 1974 to 30 May 2016 1 (arcuate$ adj2 uter$).tw. (239) 2 (subseptate$ adj2 uter$).tw. (61) 3 (sept$ adj3 uter$).tw. (1246) 4 exp uterus bicornis/ or exp uterus didelphys/ (1327) 5 or/1‐4 (2474) 6 metroplast$.tw. (514) 7 septoplast$.tw. (1527) 8 resect$.tw. (365616) 9 or/6‐8 (367336) 10 9 and 5 (664) 11 Clinical Trial/ (857876) 12 Randomized Controlled Trial/ (401880) 13 exp randomization/ (70488) 14 Single Blind Procedure/ (22118) 15 Double Blind Procedure/ (128476) 16 Crossover Procedure/ (47128) 17 Placebo/ (275055) 18 Randomi?ed controlled trial$.tw. (135640) 19 Rct.tw. (20261) 20 random allocation.tw. (1519) 21 randomly allocated.tw. (24616) 22 allocated randomly.tw. (2106) 23 (allocated adj2 random).tw. (756) 24 Single blind$.tw. (17315) 25 Double blind$.tw. (161691) 26 ((treble or triple) adj blind$).tw. (549) 27 placebo$.tw. (232442) 28 prospective study/ (334005) 29 or/11‐28 (1571138) 30 case study/ (37887) 31 case report.tw. (305578) 32 abstract report/ or letter/ (960457) 33 or/30‐32 (1296915) 34 29 not 33 (1530120) 35 34 and 10 (79)
Appendix 5. PsycINFO
Ovid platform
From 1806 to 30 May 2016 1 (arcuate$ adj2 uter$).tw. (0) 2 (subseptate$ adj2 uter$).tw. (0) 3 (sept$ adj3 uter$).tw. (8) 4 or/1‐3 (8) 5 metroplast$.tw. (0) 6 septoplast$.tw. (6) 7 resect$.tw. (2514) 8 or/5‐7 (2519) 9 4 and 8 (4)
Appendix 6. CINAHL
Ebsco platform
From 1982 to 30 May 2016
| # | Query | Results |
| S10 | S5 AND S9 | 26 |
| S9 | S6 OR S7 OR S8 | 16,377 |
| S8 | TX resect* | 16,209 |
| S7 | TX septoplast* | 152 |
| S6 | TX metroplast* | 28 |
| S5 | S1 OR S2 OR S3 OR S4 | 164 |
| S4 | TX(uter* N3 anomal*) | 95 |
| S3 | TX(sept* N3 uter*) | 82 |
| S2 | TX (subseptate* N3 uter*) | 2 |
| S1 | TX (arcuate* N3 uter*) | 16 |
Appendix 7. ClinicalTrials.gov
Date searched: from inception to 5 July 2016
Search key words:
Uterus anomaly
Septate uterus
Septum
Metroplasty
(0 hits)
Appendix 8. WHO ICTRP
Date searched: from inception to 5 July 2016
Search key words:
Uterus anomaly
Septate uterus
Septum
Metroplasty
(2 hits)
Appendix 9. Cochrane Library
Date searched: from inception to 5 July 2016
Search key words:
Uterus anomaly
Septate uterus
Septum
Metroplasty
(1 hit)
Appendix 10. ProQuest Dissertations & Theses
Date searched: from inception to 5 July 2016
Search key words:
Uterus anomaly
Septate uterus
Septum
Metroplasty
(0 hits)
Appendix 11. OpenGrey
Date searched: from inception to 5 July 2016
Search key words:
Uterus anomaly
Septate uterus
Septum
Metroplasty
(0 hits)
Appendix 12. LILACS
Date searched: from inception to 5 July 2016
Search key words:
Septate uterus
Septum AND uterus
Metroplasty
(0 hits)
Appendix 13. Google
Date searched: from inception to 5 July 2016
Search key words:
Septate uterus
Septum AND uterus
Metroplasty
(0 hits)
Appendix 14. PubMed
Date searched: from inception to 5 July 2016
(subseptat*[tw] or sub‐septat*[tw] OR ((uterine[tw] or uterus[tw]) AND septat*[tw]) OR uterine sept*[tw]) NOT MEDLINE[sb]
(74 hits)
Characteristics of studies
Characteristics of ongoing studies [ordered by study ID]
ISRCTN28960271.
| Trial name or title | Pilot randomised controlled trial of hysteroscopic septal resection |
| Methods | Randomised controlled trial |
| Participants | Women with septate uteri and a history of miscarriage or preterm birth |
| Interventions | Women will be randomised to septum resection or expectant management. |
| Outcomes | Primary outcome: live birth surviving until discharge from hospital Secondary outcome measures:
|
| Starting date | October 2013 (date of ethical approval) |
| Contact information | Matthew Prior: mprior@me.com |
| Notes | Pilot/feasibility RCT, so power calculation determined. Currently 2 women have been included. Protocol/serial number 13GY007 |
NTR1676.
| Trial name or title | TRUST (The Randomised Uterine Septum Transsection Trial) |
| Methods | Multicentre randomised controlled trial |
| Participants | Women with a septate uterus and a history of (recurrent) miscarriage, subfertility, or preterm birth |
| Interventions | Women will be randomised to septum resection or expectant management. |
| Outcomes | The primary outcome is live birth, defined as the birth of a living foetus beyond 24 weeks of gestational age. Secondary outcomes are ongoing pregnancy, clinical pregnancy, miscarriage, and complications following metroplasty. Based on retrospective studies, an improvement in the live birth rate is anticipated from 35% without surgery to 70% with surgery. |
| Starting date | October 2008 |
| Contact information | Judith Rikken: j.f.rikken@amc.uva.nl |
| Notes | The sample size of this RCT is 68, and currently 43 women have been included. The expected final inclusion is December 2017. |
RCT: randomised controlled trial
Differences between protocol and review
In this updated review, we changed the inclusion criteria to include all women of reproductive age with a septate uterus.
Contributions of authors
All authors contributed to the 2016 update of this review, and approved the final version.
Sources of support
Internal sources
No sources of support supplied
External sources
New Source of support, Other.
Declarations of interest
The authors of this review are conducting a trial: TRUST (The Randomised Uterine Septum Transsection Trial). In this study, women with recurrent miscarriage or subfertility and a septate uterus are being randomised between surgical intervention (hysteroscopic metroplasty) and expectant management.
Mark Emanuel has received consultancy fees and royalties from Smith & Nephew Endoscopy and holds patents for hysteroscopic morcellation and tubal patency foam ultrasonography testing.
For the version of this Cochrane review published in 2017, Mark Emanuel declared that he holds patents for hysteroscopic morcellation and tubal patency foam ultrasonography testing. These conflicts were declared prior to publication and applied during the period that the review was in preparation.
Clarification statement added from the Co‐ordinating Editor Professor Cindy Farquhar on 25 February 2020: this review was found by the Cochrane Funding Arbiters, post‐publication, to be noncompliant with theCochrane conflict of interest policy, which includes the relevant parts of theCochrane commercial sponsorship policy.In line with the policy, Dr Emanuel cannot be an author on the review while holding patents relevant to the topic of this review. We expect it will be updated by 25 February 2021. The update will have a majority of authors and lead author free of conflicts."
Edited (no change to conclusions)
References
References to ongoing studies
ISRCTN28960271 {unpublished data only}
- ISRCTN28960271. Pilot randomised controlled trial of hysteroscopic septal resection [Assessment of hysteroscopic metroplasty in women with a uterine septum and a history of miscarriage: a randomised controlled trial]. isrctn.com/ISRCTN28960271 (date applied 6 January 2015).
NTR1676 {unpublished data only}
- NTR1676. The Randomised Uterine Septum Transsection Trial [The Randomised Uterine Septum Transsection Trial]. trialregister.nl/trialreg/admin/rctview.asp?TC=1676 (date registered 18 February 2009).
Additional references
Candiani 1983
- Candiani GB, Fedele L, Zamberletti D, Virgiliis D, Carinelli S. Endometrial patterns in malformed uteri. Acta Europaea Fertilitatis 1983;14(5):311‐8. [PubMed] [Google Scholar]
Chan 2011
- Chan YY, Jayaprakasan K, Tan A, Thornton JG, Coomarasamy A, Raine‐Fenning NJ. Reproductive outcomes in women with congenital uterine anomalies: a systematic review. [Review]. Ultrasound in Obstetrics & Gynecology 2011;38:371‐82. [DOI] [PubMed] [Google Scholar]
Christiansen 2005
- Christiansen OB, Nybo Andersen AM, Bosch E, Daya S, Delves PJ, Hvijd TV, et al. Evidence‐based investigations and treatments of recurrent pregnancy loss. Fertility and Sterility 2005;83(4):821‐39. [DOI] [PubMed] [Google Scholar]
Edström 1970
- Edström K, Fernström I. The diagnostic possibilities of a modified hysteroscopic technique. Acta Obstetricia et Gynecologica Scandinavica 1970;49:327‐30. [DOI] [PubMed] [Google Scholar]
Fayez 1986
- Fayez JA. Comparison between abdominal and hysteroscopic metroplasty. Obstetrics & Gynecology 1986;68:399‐403. [DOI] [PubMed] [Google Scholar]
Gaucherand 1994
- Gaucherand P, Awada A, Rudigoz RC, Dargent D. Obstetrical prognosis of the septate uterus: a plea for treatment of the septum. European Journal of Obstetrics & Gynecology and Reproductive Biology 1994;54(2):109‐12. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version Accessed prior to 26 October 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Grimbizis 2013
- Grimbizis GF, Gordts S, Spiezio Sardo A, Brucker S, .De Angelis C, Gergolet M, et al. The ESHRE/ESGE consensus on the classification of female genital tract congenital anomalies. Gynecological Surgery 2013; Vol. 10, issue 3:199‐212. [DOI] [PMC free article] [PubMed]
Heinonen 1997
- Heinonen PK. Reproductive performance of women with uterine anomalies after abdominal or hysteroscopic metroplasty or no surgical treatment. Journal of the American Association of Gynecologic Laparoscopists 1997;4(3):311‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Homer 2000
- Homer HA, Li TC, Cooke ID. The septate uterus: a review of management and reproductive outcome. Fertility and Sterility 2000;73(1):1‐14. [DOI] [PubMed] [Google Scholar]
Kirk 1993
- Kirk EP, Chuong CJ, Coulam CB, Williams TJ. Pregnancy after metroplasty for uterine anomalies. Fertility and Sterility 1993;59(6):1164‐8. [DOI] [PubMed] [Google Scholar]
Lin 2009
- Lin K, Zhu X, Xu H, Liang Z, Zhang X. Reproductive outcome following resectoscope metroplasty in women having a complete uterine septum with double cervix and vagina. International Journal of Gynecology & Obstetrics 2009;105(1):25‐8. [DOI] [PubMed] [Google Scholar]
Maneschi 1991
- Maneschi F, Parlato M, Incandela S, Maneschi M. Reproductive performance in women with complete septate uteri. Journal of Reproductive Medicine 1991;36(10):741‐4. [PubMed] [Google Scholar]
March 1983
- March CM. Hysteroscopy as an aid to diagnosis in female infertility. Clinical Obstetrics & Gynecology 1983;26:302‐12. [DOI] [PubMed] [Google Scholar]
Mastenbroek 2006
- Mastenbroek S, Twisk M, Goddijn M, Veen F, Repping S, Bossuyt PM, et al. PGD ‐ a model to evaluate efficacy?. Fertility and Sterility 2006;85:534. [DOI] [PubMed] [Google Scholar]
Pang 2011
- Pang LH, Li MJ, Li M, Xu H, Wei ZL. Not every subseptate uterus requires surgical correction to reduce poor reproductive outcome. International Journal of Gynecology & Obstetrics 2011;115:260‐3. [DOI] [PubMed] [Google Scholar]
Paradisi 2014
- Paradisi R, Barzanti R, Fabbri R. The techniques and outcomes of hysteroscopic metroplasty. Current Opinion in Obstetrics and Gynecology 2014;26:295‐301. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Cochrane Collaboration. Review Manager 5 (RevMan 5), Version 5.3. Copenhagen: Nordic Cochrane Centre: The Cochrane Collaboration, 2014.
Sparac 2001
- Sparac V, Kupesic S, Ilijas M, Zodan T, Kurjak A. Histologic architecture and vascularization of hysteroscopically excised intrauterine septa. Journal of the American Association of Gynecologic Laparoscopists 2001;8(1):111‐6. [DOI] [PubMed] [Google Scholar]
Sugiura‐Ogasawara 2013
- Sugiura‐Ogasawara M, Ozaki Y, Suzumori N. Mullerian anomalies and recurrent miscarriage. [Review]. Current Opinion in Obstetrics & Gynecology 2013;25:293‐8. [DOI] [PubMed] [Google Scholar]
Tonguc 2011
- Tonguc EA, Var T, Batioglu S. Hysteroscopic metroplasty in patients with a uterine septum and otherwise unexplained infertility. International Journal of Gynecology & Obstetrics 2011;113(2):128‐30. [DOI] [PubMed] [Google Scholar]
Vail 2003
- Vail A, Gardener E. Common statistical errors in the design and analysis of subfertility trials. Human Reproduction 2003;18(5):1000‐4. [DOI] [PubMed] [Google Scholar]
Valle 2013
- Valle RF, Ekpo GE. Hysteroscopic metroplasty for the septate uterus: review and meta‐analysis. Journal of Minimally Invasive Gynecology 2013;20(1):22‐42. [DOI] [PubMed] [Google Scholar]
Valli 2004
- Valli E, Vaquero E, Lazzarin N, Caserta D, Marconi D, Zupi E. Hysteroscopic metroplasty improves gestational outcome in women with recurrent spontaneous abortion. Journal of the American Association of Gynecologic Laparoscopists 2004;11(2):240‐4. [DOI] [PubMed] [Google Scholar]
Zreik 1998
- Zreik TG, Troiano RN, Ghoussoub RAD, Olive DL, Arici A, McCarthy SM. Myometrial tissue in uterine septa. Journal of the American Association of Gynecologic Laparoscopists 1998;5(2):155‐60. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kowalik 2011
- Kowalik CR, Goddijn M, Emanuel MH, Bongers MY, Spinder T, Kruif JH, et al. Metroplasty versus expectant management for women with recurrent miscarriage and a septate uterus. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD008576.pub3] [DOI] [PubMed] [Google Scholar]


