Abstract
Background
Psychological problems are common complications following stroke that can cause stroke survivors to lack the motivation to take part in activities of daily living. Motivational interviewing provides a specific way for enhancing intrinsic motivation, which may help to improve activities of daily living for stroke survivors.
Objectives
To investigate the effect of motivational interviewing for improving activities of daily living after stroke.
Search methods
We searched the Cochrane Stroke Group's Trials Register (November 2014), the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 1), MEDLINE (1948 to March 2015), EMBASE (1980 to March 2015), CINAHL (1982 to March 2015), AMED (1985 to March 2015), PsycINFO (1806 to March 2015), PsycBITE (March 2015) and four Chinese databases. In an effort to identify further published, unpublished and ongoing trials, we searched ongoing trials registers and conference proceedings, checked reference lists, and contacted authors of relevant studies.
Selection criteria
Randomised controlled trials (RCTs) comparing motivational interviewing with no intervention, sham motivational interviewing or other psychological therapy for people with stroke were eligible.
Data collection and analysis
Two review authors independently selected studies for inclusion, extracted eligible data and assessed risk of bias. Outcome measures included activities of daily living, mood and death.
Main results
One study involving a total of 411 participants, which compared motivational interviewing with usual care, met our inclusion criteria. The results of this review did not show significant differences between groups receiving motivational interviewing or usual stroke care for participants who were not dependent on others for activities of daily living, nor on the death rate after three‐month and 12‐month follow‐up, but participants receiving motivational interviewing were more likely to have a normal mood than those who received usual care at three‐months and 12‐months follow‐up.
Authors' conclusions
There is insufficient evidence to support the use of motivational interviewing for improving activities of daily living after stroke. Further well designed RCTs are needed.
Plain language summary
Motivational interviewing for improving recovery after stroke
Review question
We reviewed the evidence about the effect of motivational interviewing in people with stroke. We found one study.
Background
Psychological problems such as depression and anxiety are common complications following stroke that can cause stroke survivors to lack the motivation to take part in activities of daily living or rehabilitation. Motivational interviewing is a counselling method that is designed to help people to change their behaviour through discovering and resolving their conflicts by a standardised communication skill. It provides a specific way for enhancing their expectations and beliefs of recovery following stroke. We wanted to know whether motivational interviewing was an effective treatment to improve activities of daily living after stroke.
Study characteristics
The evidence is current to March 2015. Only one study met our criteria: it involved a total of 411 stroke patients aged 18 years and over who had received either motivational interviewing or usual care between five and 28 days after stroke; the follow‐up period was 12 months. Motivational interviewing consisted of one session per week for four individual sessions, with each session lasting for 30 to 60 minutes.
Key results
The evidence we found from a single study was insufficient to support the use of motivational interviewing for improving activities of daily living after stroke, but participants receiving motivational interviewing were more likely to have a normal mood than those who received usual care.
Quality of the evidence
We assessed the included study to be at some risk of bias in methodological quality, as blinding of investigators and participants was impossible.
Background
Description of the condition
Stroke is defined as a neurological deficit due to an acute focal injury of the central nervous system by a vascular cause, and is one of the most common causes of morbidity and long‐term disability in the world (Sacco 2013). Most strokes (87%) are ischaemic, 10% are intracerebral haemorrhages, and 3% are subarachnoid haemorrhages (AHA 2014). Each year, approximately 795,000 people in the USA experience a stroke, of which around 610,000 are first attacks; it is estimated that around 6.4 million people in the USA are stroke survivors. Approximately half of stroke survivors are left dependent on others for everyday activities (AHA 2014; AHA/ASA 2014; Gibbon 2012). Unsurprisingly, stroke imposes a substantial economic burden on individuals and society. The estimated direct and indirect annual costs resulting from stroke have reached USD 36.5 billion in the USA and GBP 8.9 billion in the UK (AHA 2014; Demaerschalk 2010; Saka 2009). At present, no single standard intervention has been identified that is effective for the recovery of function after stroke. Nonetheless, functional recovery after stroke is still a high priority for health care (McArthur 2011).
Description of the intervention
The concept of motivational interviewing evolved from experiences of treating alcoholism, and was first described in 1983 by Miller (Miller 1983). Motivational interviewing is defined as "a collaborative, goal‐oriented style of communication with particular attention to the language of change", and is designed to strengthen personal motivation for, and commitment to, a specific goal by eliciting and exploring the person's own reasons for change within an atmosphere of acceptance and compassion (Miller 2013). The practice of motivational interviewing comprises four key processes and involves the flexible and strategic use of five core communication skills. The four processes seek to help people resolve ambivalence through: 1) engaging them in a working relationship, 2) focusing on particular change, 3) evoking intrinsic motivations for change, and 4) planning a reasonable next step toward change (Miller 2002; Miller 2013).
As mentioned above, five core communication skills underlie motivational interviewing: 1) asking open questions to help understand the patient's internal frame of reference, strengthen a collaborative relationship, and find a clear direction; 2) affirming the patient's particular strengths, abilities, good intentions and efforts; 3) reflective listening, which emphasises the importance of listening carefully to the patient; 4) summarising the situation, which promotes understanding and shows the patient that the practitioner has been listening carefully; and 5) informing and advising, which is useful to help patients reach their own conclusions about the relevance of any information the practitioner provides (Miller 2002; Miller 2013; Purath 2014).
Usually, the intervention of motivational interviewing ranges from a brief 20‐minute motivational consultation to motivation enhancement therapy, a standardised course of treatment that includes an intake assessment, personalised feedback of testing results, and a follow‐up interview of outcome evaluation (Lai 2010; Lawendowski 1998).
How the intervention might work
The exact way in which motivational interviewing may improve recovery after stroke is still unclear. Psychological problems such as depression, anxiety, emotionalism and post‐traumatic stress disorder are common complications following stroke and have an impact on all aspects of recovery (Gurr 2011). Early psychological problems interfere with recovery and cause stroke survivors to lack the motivation to take part in rehabilitation, leading to decreased participation in activities of daily living and a reduction in survival time (Gibbon 2012; Gurr 2011). However, motivational interviewing provides a specific way for enhancing intrinsic motivation to help people with poor motivation.
Why it is important to do this review
Motivational interviewing is a specific talk‐based therapy that has been used in a wide range of conditions including alcohol dependence, smoking cessation, drug addiction, HIV‐risk behaviours, treatment adherence, exercise, and eating disorders (Rubak 2005). Furthermore, very recent controlled studies have shown possible effects of motivational interviewing in improving patients' activities of daily living and mood, and in reducing mortality in people who have had a stroke (Byers 2010; Watkins 2011), but these studies have not been systematically reviewed. Therefore, this review focused on investigating the use of motivational interviewing for improving activities of daily living after stroke by summarising the evidence from randomised controlled trials (RCTs).
Objectives
To investigate the effect of motivational interviewing for improving activities of daily living after stroke.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs with a parallel group design that evaluated motivational interviewing compared with no intervention, or sham motivational interviewing, or other psychological therapy, for improving function after stroke.
Types of participants
We included participants regardless of age, gender and severity of disease after stroke. Stroke was defined according to the World Health Organization (WHO) criteria (WHO 1989), and confirmed by computerised tomography or magnetic resonance imaging.
Types of interventions
The interventions should consist of the primary principles of motivational interviewing (e.g. engaging, focusing, evoking and planning) as described in Miller 2002 and Miller 2013. We included brief motivational therapy and other interventions labelled as 'motivational interventions' if they conformed to the motivational interviewing principles and skills listed above. We assessed motivational interviewing regardless of number of sessions or duration. We included only studies of face‐to face interventions and excluded studies of interventions delivered by computer or telephone that were not given by a person. We included studies in which the control group received no intervention, or sham motivational interviewing, or other psychological therapy (e.g. cognitive behavioural therapy).
Types of outcome measures
Outcomes were assessed at the end of the treatment and at the end of follow‐up.
Primary outcomes
Activities of daily living, using the following scales.
Barthel Index (Mahoney 1965).
Functional Independence Measure (Keith 1987).
Modified Rankin Scale (van Swieten 1988).
Katz Index of Activities of Daily Living (Katz 1970).
Rehabilitation Activities Profile (van Bennekom 1995).
Secondary outcomes
Changes of mood, measured using scales such as the 28‐item General Health Questionnaire (GHQ‐28) (Goldberg 1978).
Death.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for trials in all languages and arranged for the translation of relevant articles when necessary.
Electronic searches
We searched the Cochrane Stroke Group Trials Register (November 2014) and the following electronic databases.
The Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 1) (Appendix 1).
MEDLINE (1948 to March 2015) (Appendix 2).
EMBASE (1980 to March 2015) (Appendix 3).
CINAHL (Cumulative Index to Nursing and Allied Health Literature; 1982 to March 2015) (Appendix 4).
AMED (Allied and Complementary Medicine Database; 1985 to March 2015) (Appendix 5).
PsycINFO (1806 to March 2015) (Appendix 6).
PsycBITE (http://www.psycbite.com/) (March 2015).
China Biological Medicine Database (CBM‐disc; 1979 to March 2015) (Appendix 7).
Chinese National Knowledge Infrastructure Database (CNKI; www.cnki.net; 1979 to March 2015) (Appendix 8).
VIP Chinese Science and Technique Journals Database (1979 to March 2015) (Appendix 8).
Wanfang Data (http://www.wanfangdata.com/) (1979 to March 2015) (Appendix 8).
The Cochrane Stroke Group Trials Search Co‐ordinator developed the search strategies for MEDLINE, EMBASE, CINAHL, AMED and PsycINFO and we adapted the MEDLINE search strategy (Appendix 2) for the other databases.
We also searched the following ongoing trials registers using 'stroke', 'motivational interviewing' and 'counselling' as key words (March 2015).
ClinicalTrials.gov (www.clinicaltrials.gov/).
Stroke Trials Registry (www.strokecenter.org/trials/).
ISRCTN Registry (www.isrctn.com/)
The WHO International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/search/en/).
Searching other resources
In an effort to identify further published, unpublished and ongoing trials, we also:
screened reference lists of retrieved studies;
contacted authors of included studies;
searched the motivational interviewing website (http://www.motivationalinterview.org/) (1 March 2015);
used Science Citation Index Cited Reference Search for forward tracking of relevant trials; and
handsearched the proceedings of the 22nd and 23rd European Stroke Conferences (2013 and 2014).
Data collection and analysis
Selection of studies
Two review authors (Cheng D, Qu Z) independently screened titles and abstracts of the references obtained as a result of our searching activities and excluded reports that were obviously irrelevant. We retrieved the full‐text articles for the remaining references and both review authors (Cheng D, Qu Z) independently screened the full‐text articles to identify studies for inclusion, and recorded the reasons for exclusion of the ineligible studies. We resolved any disagreements through discussion or, if required, we consulted a third review author (Wang J). We collated multiple reports of the same study so that each study, not each reference, was the unit of interest in the review. We recorded the selection process and completed a PRISMA (Moher 2009) flow diagram (Figure 1).
1.
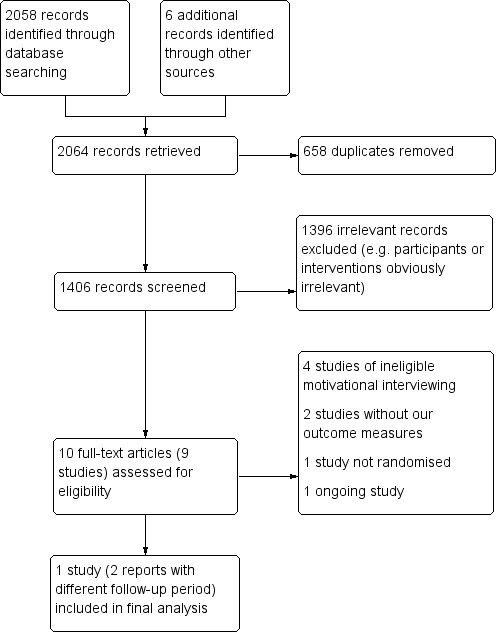
Study flow diagram.
Data extraction and management
Two review authors (Cheng D, Qu Z) independently extracted the following data from the included studies using a data extraction form.
Participants: diagnostic criteria, number in each group, age, gender, baseline comparability between two groups, withdrawals or losses to follow‐up.
Methods: study design, randomisation method, allocation concealment method, blinding methods.
Interventions: details of motivational interviewing treatment, such as treatment session, duration and co‐intervention(s).
Outcomes: primary and secondary outcomes.
Other: country and setting, publication year, sources of funding, intention‐to‐treat analysis (ITT).
We resolved disagreements by discussion between review authors.
Assessment of risk of bias in included studies
Two review authors (Cheng D, Qu Z) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another author (Wang J). We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded the risk of bias for each domain as 'high', 'low' or 'unclear', and provided information from the study report, together with a justification for our judgement in the 'Risk of bias' tables.
Measures of treatment effect
We managed data according to the intention‐to‐treat principle. For dichotomous outcomes, we planned to use the risk ratio (RR) with 95% confidence interval (CI) to express the effect size. For continuous data, we planned to use mean differences (MDs) with 95% CIs to analyse the outcomes. For outcomes measured with different scales, we planned to use standardised mean differences (SMDs) with 95% CIs for analyses.
Unit of analysis issues
We dealt with any unit of analysis issues using the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We attempted to obtain additional information from the authors of included studies through personal communication.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity of included trials by comparing the characteristics of participants, interventions, and study designs. We planned to use the I² statistic to measure heterogeneity among the trials in each analysis. We used the random‐effects model only, regardless of the level of heterogeneity, because if the heterogeneity was 0% then the results produced by a random‐effects model would be the same as the results for a fixed‐effect model; however, if the I² statistic was more than 50%, which indicated substantial heterogeneity (Higgins 2011), we then examined the sources of potential clinical and methodological heterogeneity.
Assessment of reporting biases
We did not investigate potential reporting biases as planned because only one study was included. If we identify sufficient studies in future, we will assess potential reporting biases by using funnel plots and visual inspection for asymmetry according to the approach outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Data synthesis
We did not perform a meta‐analysis by pooling the appropriate data using Review Manager 5 (RevMan 2014) because we only included one study.
Subgroup analysis and investigation of heterogeneity
We did not perform subgroup analyses, as planned, according to duration of session (e.g. within three months versus more than three months), different types of control (e.g. no intervention/placebo or other psychological therapy) and different types of stroke (e.g. ischaemic stroke versus intracranial haemorrhage) because of the limited available data.
Sensitivity analysis
We did not undertake sensitivity analyses by excluding trials of inadequate allocation concealment or without adequate blinding of outcome assessors because of the limited available data.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies and Characteristics of ongoing studies
Results of the search
We identified a total of 2064 references through the search strategies: 430 from the Cochrane Stroke Group's Trials Register, 1628 from the electronic databases, and six from other resources. After screening the titles and abstracts, we removed 2054 references as they were irrelevant (e.g. not stroke participants or not motivational interviewing intervention), or duplicate publications. We obtained the full texts of the remaining 10 references (nine studies), from which we excluded seven studies (Byers 2010; Gillham 2010; Goossensen 2014; Green 2007; Hedegaard 2014; Leistner 2013; Mitchell 2009) because they did not meet the inclusion criteria; one study is ongoing (Krishnamurthi 2014). Finally, only one study met the inclusion criteria (Watkins 2011) (see Figure 1).
Included studies
Watkins 2011 was an open‐label, RCT which recruited a total of 411 participants to investigate the efficacy of motivational interviewing versus usual stroke care for the recovery of stroke. The primary outcome was the proportion of participants with normal mood; the secondary outcomes included activities of daily living, beliefs and expectations of recovery, depression screen and death. The three‐month and 12‐month follow‐up results were reported separately as two parts of the study. Details of the study are outlined in the Characteristics of included studies table.
Excluded studies
We excluded seven studies (Byers 2010; Gillham 2010; Goossensen 2014; Green 2007; Hedegaard 2014; Leistner 2013; Mitchell 2009) as they did not meet our inclusion criteria. The reasons for exclusion are provided in the Characteristics of excluded studies table.
Risk of bias in included studies
Overall results of all the risk of bias assessments are summarised in Figure 2 and Figure 3.
2.
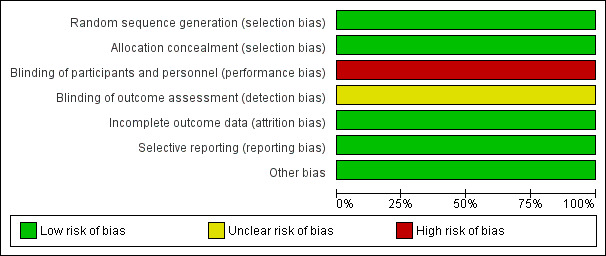
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
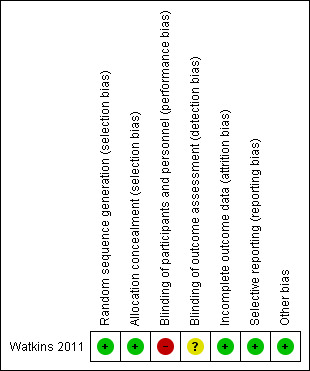
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The random list was generated using a computer program, and the allocation sequence was stored in opaque sealed envelopes by an independent investigator.
Blinding
The study was an open‐label study, the blinding of investigators and participants was impossible as the control group received usual care; the outcomes were collected through patient self report questionnaires, though the data entry person was blinded to group allocation, so we judged this study at unclear risk of detection bias.
Incomplete outcome data
The study provided information about withdrawals: 12.9% attrition rate (53/411) at 12‐month follow‐up was acceptable. The management of missing data was well described.
Selective reporting
All the prespecified outcomes were reported in the study reports.
Other potential sources of bias
We did not identify any other potential sources of bias.
Effects of interventions
Primary outcomes
Activities of daily living
Activities of daily living were measured using the Barthel Index. The authors categorised the severity of dependence as four levels: mild/no dependence (scored 18 to 20); moderate dependence (scored 11 to 17); high dependence (scored 0 to 10); and dead. We only analysed the proportion of participants with mild or no dependence after treatment. There were no significant differences between the groups receiving motivational interviewing or usual stroke care for participants who had mild, or no dependence on others, in activities of daily living after the three‐month and 12‐month follow‐up periods (risk ratio (RR) 1.01, 95% confidence interval (CI) 0.84 to 1.23; and RR 1.11, 95% CI 0.89 to 1.37, respectively) (See Analysis 1.1).
1.1. Analysis.
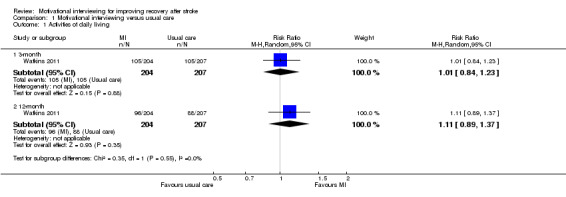
Comparison 1 Motivational interviewing versus usual care, Outcome 1 Activities of daily living.
Secondary outcomes
Changes of mood
Changes of mood were measured using the 28‐item General Health Questionnaire (GHQ‐28). The scores were dichotomised as normal (less than 5) and low (5 or more). Participants receiving motivational interviewing were more likely to have a normal mood than those participants who received usual stroke care at the three‐month and 12‐month follow‐ups (RR 1.36, 95% CI 1.07 to 1.73; and RR 1.35, 95% CI 1.05 to 1.74, respectively) (See Analysis 1.2).
1.2. Analysis.
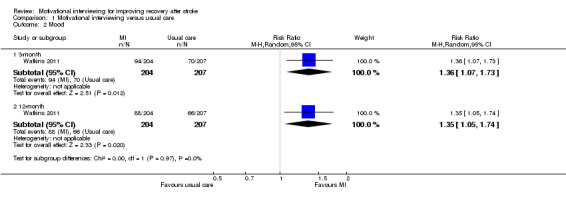
Comparison 1 Motivational interviewing versus usual care, Outcome 2 Mood.
Death
There was a lower rate of death in the motivational interviewing group than in the usual stroke care group after the three‐month and 12‐month follow‐up periods, although we detected no significant differences (RR 0.34, 95% CI 0.11 to 1.03; and RR 0.53, 95% CI 0.28 to 1.00, respectively) (see Analysis 1.3).
1.3. Analysis.
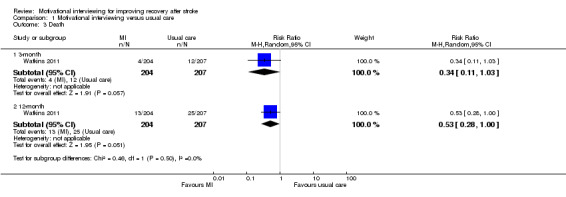
Comparison 1 Motivational interviewing versus usual care, Outcome 3 Death.
Discussion
Summary of main results
This systematic review assessed the effect of motivational interviewing for improving activities of daily living in people who had suffered a stroke. Only one study comparing motivational interviewing versus usual care met our inclusion criteria. There was insufficient evidence provided by the study to make any conclusions about the use of motivational interviewing for improving activities of daily living after stroke.
Overall completeness and applicability of evidence
This review provided limited information on the effect of motivational interviewing in patients with stroke due to a single included study. What is more, the primary outcome measurement of activities of daily living in our review did not show any significant differences between the groups receiving motivational interviewing or usual stroke care, which was a major concern that needs to be taken into consideration. For this reason, there is insufficient evidence to support the use of motivational interviewing for improving activities of daily living after stroke.
In addition, the definition of recovery, relating only to activities of daily living, was relatively narrow. As a result, we excluded two studies assessing changes of lifestyle risk factors (e.g. diet, exercise, smoking and obesity) after minor stroke or transient ischaemic attack from this review.
The results of this review did not show significant differences between groups receiving motivational interviewing or usual stroke care for the death rate after three‐month and 12‐month follow‐up, but participants receiving motivational interviewing were more likely to have a normal mood than those who received usual care at three‐months and 12‐months follow‐up.
Quality of the evidence
The major limitation of the study was that blinding of the investigators and participants was impossible, as the control group received usual care. In addition, blinding of outcome assessors was also difficult as all outcome data were collected through patient self report questionnaires. Lack of blinding is associated with an overestimation of efficacy (Schulz 1995). Another limitation was that the outcome measures mainly depended on the mailed questionnaires, the validity of this method for collecting outcome data needs further exploration compared with face‐to‐face interviews. Furthermore, there is a discrepancy in the numbers of participants who had normal mood between the abstract and the tables in the article (Watkins 2011). After we checked this discrepancy with the corresponding author, Prof Watkins, she told us the values in the abstract were those from the imputed analysis, and the data presented in the tables reflected the responses of participants whose results are known. We therefore managed the data according to the intention‐to‐treat principle based on available case analysis, but not using an imputation approach because it is generally not recommended for dichotomous data (Higgins 2011).
It is worth noting that the motivational interviewing was provided by well trained personnel, and closely monitored via a standard skill code to ensure treatment consistency across investigators (Watkins 2011).
Potential biases in the review process
Though we minimised potential biases in the review process by undertaking an extensive and comprehensive search, we still missed a subsequently excluded study (Mitchell 2009) which was identified by a referee who is familiar with this topic. This raises the concern that other publications might also have been missed. In preparing this review, two review authors independently completed screening, data extraction and quality assessment of the included studies to reduce potential bias.
Agreements and disagreements with other studies or reviews
We did not find any specific systematic reviews on the effect of motivational interviewing for improving activities of daily living after stroke. What should be noted is that the three‐month results of the included study (Watkins 2011) were also presented as a part of another Cochrane review (Hackett 2008), where the focus on investigating pharmaceutical or psychological interventions for preventing depression after stroke was somewhat different from our main objective. Another point to note is the difference between our review and the original study report at 12‐month follow‐up regarding whether motivational interviewing improves mortality after stroke. Watkins 2011 found a significantly low risk of death in favour of the motivational interviewing group while our results also showed a lower rate of death in the motivational interviewing group, but without significant differences when compared with the usual care group. A reasonable explanation for this inconsistency might be the two different statistical methods used. The study authors analysed the rate of death based on a logistic regression analysis method, whereas we used a meta‐analytic approach. It is not surprising that different statistical methods may generate discrepancies even when using the identical results for all individuals (Bland 1986).
Authors' conclusions
Implications for practice.
The evidence we found from a single study was insufficient to support the use of motivational interviewing for improving activities of daily living after stroke or reducing death, but there is some limited evidence that participants receiving motivational interviewing were more likely to have a normal mood than those who received usual care at three‐months and 12‐months follow‐up.
Implications for research.
There is a need for well designed randomised controlled trials (RCTs) to assess the effect of motivational interviewing for improving activities of daily living after stroke. Providers of motivational interviewing should be well trained and closely monitored via a standard skill code to ensure treatment consistency.
Acknowledgements
We thank the Cochrane Stroke Group Editorial Team and peer reviewers for their help in developing this review.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1. MeSH descriptor Cerebrovascular Disorders explode all trees #2. MeSH descriptor Basal Ganglia Cerebrovascular Disease explode all trees #3. MeSH descriptor Brain Ischemia explode all trees #4. MeSH descriptor Carotid Artery Diseases explode all trees #5. MeSH descriptor Intracranial Arterial Diseases explode all trees #6. MeSH descriptor Intracranial Embolism and Thrombosis explode all trees #7. MeSH descriptor Intracranial Hemorrhages explode all trees #8. MeSH descriptor Stroke explode all trees #9. MeSH descriptor Brain Infarction explode all trees #10. MeSH descriptor Vertebral Artery Dissection explode all trees #11. (stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc* or cva* or apoplex* or SAH) #12. (brain* or cerebr* or cerebell* or intracran* or intracerebral) near/5 (isch?emi* or infarct* or thrombo* or emboli* or occlus*) #13. (brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) near/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*) #14. MeSH descriptor Paresis explode all trees #15. MeSH descriptor Hemiplegia explode all trees #16. (hemipleg* or hemipar* or paresis or paretic) #17. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 #18. MeSH descriptor motivational interviewing explode all trees #19. MeSH descriptor counseling explode all trees #20. MeSH descriptor directive counseling explode all trees #21. MeSH descriptor motivation explode all trees #22. (motivat*) near/5 (interview* or counsel* or therap* or consult* or intervention* or instruct* or advice or guidance or recommend* or elicit* or enhance* or improve* or task*) #23. (prescriptive or directive or behav* or goal‐oriented) near/5 (counsel*) #24. (motivat*) near/5 (lack* or poor or reduced) #25. #18 or #19 or #20 or #21 or #22 or #23 or #24 #26. #17 AND #25
Appendix 2. MEDLINE (Ovid) search strategy
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vertebral artery dissection/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiplegia/ or exp paresis/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. or/1‐6 8. motivational interviewing/ or counseling/ or directive counseling/ 9. exp motivation/ 10. (motivat$ adj5 (interview$ or counsel$ or therap$ or consult$ or intervention$ or instruct$ or advice or guidance or recommend$ or elicit$ or enhance$ or improve$ or task$)).tw. 11. ((prescriptive or directive or behav$ or goal‐oriented) adj5 counsel$).tw. 12. (motivat$ adj5 (lack$ or poor or reduced)).tw. 13. 8 or 9 or 10 or 11 or 12 14. Randomized Controlled Trials as Topic/ 15. random allocation/ 16. Controlled Clinical Trials as Topic/ 17. control groups/ 18. clinical trials as topic/ 19. double‐blind method/ 20. single‐blind method/ 21. Placebos/ 22. placebo effect/ 23. randomized controlled trial.pt. 24. controlled clinical trial.pt. 25. clinical trial.pt. 26. (random$ or RCT or RCTs).tw. 27. (controlled adj5 (trial$ or stud$)).tw. 28. (clinical$ adj5 trial$).tw. 29. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 30. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 31. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 32. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 33. (placebo$ or sham).tw. 34. trial.ti. 35. (assign$ or allocat$).tw. 36. controls.tw. 37. or/14‐36 38. 7 and 13 and 37 39. exp animals/ not humans.sh. 40. 38 not 39
Appendix 3. EMBASE (Ovid) search strategy
1. cerebrovascular disease/ or exp basal ganglion hemorrhage/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or cerebral artery disease/ or exp cerebrovascular accident/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or stroke unit/ or stroke patient/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiparesis/ or hemiplegia/ or paresis/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. or/1‐6 8. motivational interviewing/ or counseling/ or directive counseling/ 9. motivation/ 10. (motivat$ adj5 (interview$ or counsel$ or therap$ or consult$ or intervention$ or instruct$ or advice or guidance or recommend$ or elicit$ or enhance$ or improve$ or task$)).tw. 11. ((prescriptive or directive or behav$ or goal‐oriented) adj5 counsel$).tw. 12. (motivat$ adj5 (lack$ or poor or reduced)).tw. 13. 8 or 9 or 10 or 11 or 12 14. Randomized Controlled Trial/ or "randomized controlled trial (topic)"/ 15. Randomization/ 16. Controlled clinical trial/ or "controlled clinical trial (topic)"/ 17. control group/ or controlled study/ 18. clinical trial/ or "clinical trial (topic)"/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ 19. Crossover Procedure/ 20. Double Blind Procedure/ 21. Single Blind Procedure/ or triple blind procedure/ 22. placebo/ or placebo effect/ 23. (random$ or RCT or RCTs).tw. 24. (controlled adj5 (trial$ or stud$)).tw. 25. (clinical$ adj5 trial$).tw. 26. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 27. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 28. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 29. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 30. (cross‐over or cross over or crossover).tw. 31. (placebo$ or sham).tw. 32. trial.ti. 33. (assign$ or allocat$).tw. 34. controls.tw. 35. or/14‐34 36. 7 and 13 and 35 37. (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/) 38. 36 not 37
Appendix 4. CINAHL (Ebsco) search strategy
S1 .(MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR (MH "Intracranial Embolism and Thrombosis") OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections") S2 .(MH "Stroke Patients") OR (MH "Stroke Units") S3 .TI ( stroke* or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc* or cva* or apople* or SAH ) or AB ( stroke* or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc* or cva* or apople* or SAH) S4 .TI ( brain or cerebr* or cerebell* or intracran* or intracerebral) or AB ( brain or cerebr* or cerebell* or intracran* or intracerebral ) S5 .TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus*) S6 .S4 and S5 S7 .TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) S8 .TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) S9 .S7 and S8 S10 .(MH "Hemiplegia") S11 .TI ( hemipleg* or hemipar* or paresis or paretic ) or AB ( hemipleg* or hemipar* or paresis or paretic) S12 .S1 OR S2 OR S3 OR S6 OR S9 OR S10 OR S11 S13 .(MH "Motivational Interviewing") OR (MH "Counseling") S14 .(MH "Motivation+") OR (MH "Self‐Awareness Enhancement (Iowa NIC)") S15 .TI ( (motivat* N5 (interview* or counsel* or therap* or consult* or intervention* or instruct* or advice or guidance or recommend* or elicit* or enhance* or improve* or task*)) ) OR AB ( (motivat* N5 (interview* or counsel* or therap* or consult* or intervention* or instruct* or advice or guidance or recommend* or elicit* or enhance* or improve* or task*)) ) S16 .TI ( ((prescriptive or directive or behav* or goal‐oriented) N5 counsel*) ) OR AB ( ((prescriptive or directive or behav* or goal‐oriented) N5 counsel*) ) S17 .TI ( (motivat* N5 (lack* or poor or reduced)). ) OR AB ( (motivat* N5 (lack* or poor or reduced)). ) S18 .S13 OR S14 OR S15 OR S16 OR S17 S19 .(MH "Randomized Controlled Trials") or (MH "Random Assignment") or (MH "Random Sample+") S20 .(MH "Clinical Trials") or (MH "Intervention Trials") or (MH "Therapeutic Trials") S21 .(MH "Double‐Blind Studies") or (MH "Single‐Blind Studies") or (MH "Triple‐Blind Studies") S22 .(MH "Control (Research)") or (MH "Control Group") or (MH "Placebos") or (MH "Placebo Effect") S23 .(MH "Crossover Design") OR (MH "Quasi‐Experimental Studies") S24 .PT (clinical trial or randomized controlled trial) S25 .TI (random* or RCT or RCTs) or AB (random* or RCT or RCTs) S26 .TI (controlled N5 (trial* or stud*)) or AB (controlled N5 (trial* or stud*)) S27 .TI (clinical* N5 trial*) or AB (clinical* N5 trial*) S28 .TI ((control or treatment or experiment* or intervention) N5 (group* or subject* or patient*)) or AB ((control or treatment or experiment* or intervention) N5 (group* or subject* or patient*)) S29 .TI ((control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*)) or AB ((control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*)) S30 .TI ((singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*)) or AB ((singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*)) S31 .TI (cross‐over or cross over or crossover) or AB (cross‐over or cross over or crossover) S32 .TI (placebo* or sham) or AB (placebo* or sham) S33 .TI trial S34 .TI (assign* or allocat*) or AB (assign* or allocat*) S35 .TI controls or AB controls S36 .TI (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*) or AB (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*) S37 .S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 S38 .S12 AND S18 AND S37
Appendix 5. AMED (Ovid) search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ or stroke/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiplegia/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. or/1‐6 8. counseling/ or interviews/ 9. exp motivation/ 10. (motivat$ adj5 (interview$ or counsel$ or therap$ or consult$ or intervention$ or instruct$ or advice or guidance or recommend$ or elicit$ or enhance$ or improve$ or task$)).tw. 11. ((prescriptive or directive or behav$ or goal‐oriented) adj5 counsel$).tw. 12. (motivat$ adj5 (lack$ or poor or reduced)).tw. 13. 8 or 9 or 10 or 11 or 12 14. clinical trials/ or randomized controlled trials/ or random allocation/ 15. research design/ or comparative study/ 16. double blind method/ or single blind method/ 17. placebos/ 18. (random$ or RCT or RCTs).tw. 19. (controlled adj5 (trial$ or stud$)).tw. 20. (clinical$ adj5 trial$).tw. 21. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 22. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 23. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 24. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 25. (cross‐over or cross over or crossover).tw. 26. (placebo$ or sham).tw. 27. trial.ti. 28. (assign$ or allocat$).tw. 29. controls.tw. 30. or/14‐29 31. 7 and 13 and 30
Appendix 6. PsycINFO (Ovid) search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or exp cerebral ischemia/ or cerebral small vessel disease/ or cerebrovascular accidents/ or subarachnoid hemorrhage/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiparesis/ or hemiplegia/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. or/1‐6 8. motivational interviewing/ or counseling/ 9. exp motivation/ 10. (motivat$ adj5 (interview$ or counsel$ or therap$ or consult$ or intervention$ or instruct$ or advice or guidance or recommend$ or elicit$ or enhance$ or improve$ or task$)).tw. 11. ((prescriptive or directive or behav$ or goal‐oriented) adj5 counsel$).tw. 12. (motivat$ adj5 (lack$ or poor or reduced)).tw. 13. 8 or 9 or 10 or 11 or 12 14. clinical trials/ or treatment effectiveness evaluation/ or placebo/ 15. (random$ or RCT or RCTs).tw. 16. (controlled adj5 (trial$ or stud$)).tw. 17. (clinical$ adj5 trial$).tw. 18. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 19. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 20. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 21. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 22. (cross‐over or cross over or crossover).tw. 23. (placebo$ or sham).tw. 24. trial.ti. 25. (assign$ or allocat$).tw. 26. controls.tw. 27. or/14‐26 28. 7 and 13 and 27
Appendix 7. CBM search strategy
#1 卒中 (MeSH) #2 中风 (MeSH) #3 脑梗死 (MeSH) #4 脑出血 (MeSH) #5 蛛网膜下腔出血(MeSH) #6 颅内栓塞 (MeSH) #7 颅内血栓形成 (MeSH) #8 颅内栓塞和血栓形成 (MeSH) #9 卒中 (Ti/Ab/Kw/Tx) #10 中风 (Ti/Ab/Kw/Tx) #11 脑梗死 (Ti/Ab/Kw/Tx) #12 脑出血 (Ti/Ab/Kw/Tx) #13 蛛网膜下腔出血 (Ti/Ab/Kw/Tx) #14 脑栓塞 (Ti/Ab/Kw/Tx) #15 脑血栓 (Ti/Ab/Kw/Tx) #16 脑血管意外 (Ti/Ab/Kw/Tx) #17 动机 (MeSH) #18 咨询 (MeSH) #19 动机性访谈 (Ti/Ab/Kw/Tx) #20 动机 (Ti/Ab/Kw/Tx) #21 咨询 (Ti/Ab/Kw/Tx) #22 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #13 OR #14 OR #15 OR #16 #23 #17 OR #18 OR #19 OR #20 OR #21 #24 #22 AND #23
Appendix 8. CNKI, VIP and Wanfang Data search strategy
#1卒中 (Ti/Ab/Kw/Tx) AND 动机性访谈 (Ti/Ab/Kw/Tx) #2卒中(Ti/Ab/Kw/Tx) AND 咨询(Ti/Ab/Kw/Tx) #3卒中(Ti/Ab/Kw/Tx) AND 动机(Ti/Ab/Kw/Tx) #4中风(Ti/Ab/Kw/Tx) AND 动机性访谈 (Ti/Ab/Kw/Tx) #5中风(Ti/Ab/Kw/Tx) AND 咨询(Ti/Ab/Kw/Tx) #6中风(Ti/Ab/Kw/Tx) AND 动机(Ti/Ab/Kw/Tx) #7脑梗死(Ti/Ab/Kw/Tx) AND 动机性访谈 (Ti/Ab/Kw/Tx) #8脑梗死(Ti/Ab/Kw/Tx) AND 咨询(Ti/Ab/Kw/Tx) #9脑梗死(Ti/Ab/Kw/Tx) AND 动机(Ti/Ab/Kw/Tx) #10脑出血(Ti/Ab/Kw/Tx) AND 动机性访谈 (Ti/Ab/Kw/Tx) #11脑出血(Ti/Ab/Kw/Tx) AND 咨询(Ti/Ab/Kw/Tx) #12脑出血(Ti/Ab/Kw/Tx) AND 动机(Ti/Ab/Kw/Tx) #13蛛网膜下腔出血 (Ti/Ab/Kw/Tx) AND 动机性访谈 (Ti/Ab/Kw/Tx) #14蛛网膜下腔出血 (Ti/Ab/Kw/Tx) AND 咨询 (Ti/Ab/Kw/Tx) #15蛛网膜下腔出血 (Ti/Ab/Kw/Tx) AND 动机 (Ti/Ab/Kw/Tx) #16脑栓塞(Ti/Ab/Kw/Tx) AND 动机性访谈 (Ti/Ab/Kw/Tx) #17脑栓塞(Ti/Ab/Kw/Tx) AND 咨询(Ti/Ab/Kw/Tx) #18脑栓塞(Ti/Ab/Kw/Tx) AND 动机(Ti/Ab/Kw/Tx) #19脑血栓(Ti/Ab/Kw/Tx) AND 动机性访谈 (Ti/Ab/Kw/Tx) #20脑血栓(Ti/Ab/Kw/Tx) AND 咨询(Ti/Ab/Kw/Tx) #21脑血栓(Ti/Ab/Kw/Tx) AND 动机(Ti/Ab/Kw/Tx) #22脑血管意外 (Ti/Ab/Kw/Tx) AND 动机性访谈 (Ti/Ab/Kw/Tx) #23脑血管意外 (Ti/Ab/Kw/Tx) AND 咨询 (Ti/Ab/Kw/Tx) #24脑血管意外 (Ti/Ab/Kw/Tx) AND 动机 (Ti/Ab/Kw/Tx) #25#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24
Data and analyses
Comparison 1. Motivational interviewing versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activities of daily living | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 3‐month | 1 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.84, 1.23] |
| 1.2 12‐month | 1 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.89, 1.37] |
| 2 Mood | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 3‐month | 1 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.07, 1.73] |
| 2.2 12‐month | 1 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [1.05, 1.74] |
| 3 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 3‐month | 1 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.11, 1.03] |
| 3.2 12‐month | 1 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.28, 1.00] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Watkins 2011.
| Methods | Open‐label RCT Random list was generated using a computer programme Allocation sequence was performed by an independent investigator using opaque sealed envelopes to ensure participants and investigators could not predict allocation Blinding: study investigators and participants were not blinded because of the nature of treatment; the outcome was assessed by mailed questionnaires ITT analysis: yes |
|
| Participants | 411 participants aged 18 years and over with a stroke between 5 to 28 days after onset were randomly assigned into the motivational interviewing group (204 participants) or the usual stroke care group (207 participants) for 4 weeks treatment and a follow‐up period of 3 and 12 months Exclusion criteria: patients with severe cognitive and communication problems; already received psychiatric or psychological interventions; known to be leaving the city after discharge The baseline clinical characteristics were similar in both groups |
|
| Interventions | Intervention group: participants received motivational interviewing 1 session per week for 4 individual sessions, each session lasted for 30 to 60 minutes Control group: participants received usual stroke care, including inpatient care and discharge planning through regular multidisciplinary team meetings | |
| Outcomes |
|
|
| Notes | At the 3‐month follow‐up, 13.7% of the participants (28/204) in the motivational interviewing group withdrew while the rate of withdrawals was 13.5% (28/207) in the usual stroke care group; 4 participants in the motivational interviewing group and 12 participants in the usual stroke care group died during the 3‐month follow‐up period At the end of the 12‐month follow‐up period, 13.2% of the participants (27/204) in the motivational interviewing group withdrew or declined to complete the mailed questionnaires, while 12.6% of the participants (26/207) withdrew in the usual stroke care group; additionally 13 participants in the motivational interviewing group and 25 participants in the usual stroke care group died during the 12‐month follow‐up period There is a discrepancy between the abstract and tables of the article regarding the number of participants who had normal mood. The corresponding author, Prof Watkins, informed us that the values in the abstract are those from the imputed analysis, whereas the data presented in the tables reflect the responses of participants whose results are known |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random programme |
| Allocation concealment (selection bias) | Low risk | An independent investigator used opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label study, blinding of the investigators and participants was not possible because of the nature of treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Results were collected through patient self report questionnaires; data entry person was blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The attrition rate at 12‐month follow‐up was acceptable; the management of missing data was well described |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | Low risk | Not identified |
AD: activities of daily living ITT: intention‐to‐treat RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Byers 2010 | 1. It was not a randomised trial because participants were assigned according to date of birth 2. Outcome measures: stroke knowledge test (20 multiple‐choice questions) and patient satisfaction survey (a 10‐item Likert scale), did not meet our predefined outcome measures 3. Enrolled participants with stroke or TIA and outcomes not reported separately |
| Gillham 2010 | The study, which compared additional advice, motivational interviewing and telephone support + conventional care versus conventional care alone in patients with minor stroke/TIA, did not meet our predefined intervention criteria. What's more, the labelled 'motivational interviewing' is not real motivational interviewing because it is a motivational interviewing style of discussion about behaviour change intentions which does not consist of the primary principles of motivational interviewing as described by Miller 2013 |
| Goossensen 2014 | An abstract presented at the 23rd European Stroke Conference: it did not include our predefined outcome measures. The study compared motivational interviewing at a nurse‐led outpatient clinic versus standard outpatient clinic in patients with minor ischaemic stroke or TIA. Changes of lifestyle behaviour (diet, exercise, smoking cession, cholesterol and glucose) are the outcome measures in this study, which do not contain our predefined outcome measures. The study is still ongoing and no further details are available; we therefore listed it as an excluded study at present |
| Green 2007 | 1. Outcome measures: stroke knowledge test (a 43‐item, self report survey questionnaire) and changes of lifestyle risk factors (e.g. smoking, exercise, obesity, alcohol intake) did not meet our predefined outcome measures 2. Enrolled participants with minor stroke or TIA and outcomes not reported separately |
| Hedegaard 2014 | The study compared a complex intervention with usual care in patients with stroke/TIA. The complex intervention consisted of a focused medication review, a brief motivational interviewing‐based consultation, and 3 follow‐up telephone supports, whereas the control group only received usual care without the same additional intervention, except motivational interviewing, as the intervention group. It did not meet our predefined intervention criteria |
| Leistner 2013 | A protocol of an ongoing study; motivational interviewing is part of an intensified secondary prevention intervention; it does not meet our predefined intervention criteria. The ongoing study plans to compare intensified secondary prevention intervention (multifactorial risk factor modifications) with usual care for preventing recurrent events after stroke/TIA. The intensified secondary prevention intervention includes a total of 8 outpatient appointments, a motivational interviewing‐based method will be used to assess and enhance patients' motivation during each appointment |
| Mitchell 2009 | The study investigated a brief psychosocial‐behavioural intervention for improving depression symptoms after stroke. Though some components (e.g. problem‐solving) of the intervention are similar to motivational interviewing, it was obvious that the intervention was modified from the 'Seattle Protocols' that did not conform to the primary principles of motivational interviewing as described by Miller 2013 |
TIA: transient ischaemic attack
Characteristics of ongoing studies [ordered by study ID]
Krishnamurthi 2014.
| Trial name or title | Motivational interviews for secondary stroke prevention: a randomised clinical trial |
| Methods | A randomised, single‐blind controlled study |
| Participants | Stroke survivors aged 16 years or older who had a first‐ever stroke and who are residents of Auckland or Waikato Region are eligible |
| Interventions | Intervention group: motivational interviewing Control group: usual care |
| Outcomes | Primary outcomes: change in systolic blood pressure and low‐density lipoprotein (LDL)‐cholesterol levels Secondary outcomes: self reported adherence; self reported barriers to adherence; cardiovascular events; quality of life; mood; change in other blood lipid levels; activities of daily living; healthcare resource consumption and cost‐effectiveness |
| Starting date | 1 March 2011 |
| Contact information | Valery L Feigin, Email: valery.feigin@aut.ac.nz |
| Notes | The trial is registered in the Australian New Zealand Clinical Trial Registry (http://www.anzctr.org.au/) (Trial Registration Number: ACTRN 12610000715077) |
Differences between protocol and review
We removed the exclusion criteria relating to patients who had cognitive and communication problems or who had already received psychiatric or psychological interventions because it was difficult to isolate these patients from enrolled participants.
We analysed continuous data (e.g. activities of daily living and mood) using RRs with 95% CIs, rather than mean differences (MDs) with 95% CIs, as stated in our protocol, because the data from the original study were categorised as dichotomous data and further data were unavailable.
We analysed the timing of outcome as reported in the original article, instead of as planned in our protocol, according to the time points at the end of the treatment and at the end of follow‐up, because we only included one study.
Contributions of authors
Daobin Cheng, Zhanli Qu, Jianyi Huang, Yousheng Xiao, Hongye Luo and Jin Wang drafted the protocol. Daobin Cheng and Zhanli Qu ran the search strategy. Daobin Cheng and Zhanli Qu selected relevant articles for inclusion. Daobin Cheng and Zhanli Qu extracted the data from included studies. Daobin Cheng, Zhanli Qu and Jin Wang assessed the risk of bias in included studies. Daobin Cheng and Zhanli Qu entered data into Review Manager. Daobin Cheng, Zhanli Qu, Jianyi Huang, Yousheng Xiao, Hongye Luo and Jin Wang carried out the analysis. Daobin Cheng, Zhanli Qu, Jianyi Huang, Yousheng Xiao, Hongye Luo and Jin Wang interpreted the results. Daobin Cheng, Zhanli Qu, Jianyi Huang, Yousheng Xiao, Hongye Luo and Jin Wang drafted the final review. Jin Wang will update the review.
Declarations of interest
Daobin Cheng: none known. Zhanli Qu: none known. Jianyi Huang: none known. Yousheng Xiao: none known. Hongye Luo: none known. Jin Wang: none known.
New
References
References to studies included in this review
Watkins 2011 {published data only}
- Watkins CL, Auton MF, Deans CF, Dickinson HA, Jack CI, Lightbody CE, et al. Motivational interviewing early after acute stroke: a randomized, controlled trial. Stroke 2007;38(3):1004‐9. [DOI] [PubMed] [Google Scholar]
- Watkins CL, Wathan JV, Leathley MJ, Auton MF, Deans CF, Dickinson HA, et al. The 12‐month effects of early motivational interviewing after acute stroke: a randomized controlled trial. Stroke 2011;42(7):1956‐61. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Byers 2010 {published data only}
- Byers AM, Lamanna L, Rosenberg A. The effect of motivational interviewing after ischemic stroke on patient knowledge and patient satisfaction with care: a pilot study. Journal of Neuroscience Nursing 2010;42(6):312‐22. [DOI] [PubMed] [Google Scholar]
Gillham 2010 {published data only}
- Gillham S, Endacott R. Impact of enhanced secondary prevention on health behaviour in patients following minor stroke and transient ischaemic attack: a randomized controlled trial. Clinical Rehabilitation 2010;24(9):822‐30. [DOI] [PubMed] [Google Scholar]
Goossensen 2014 {published data only}
- Brouwer‐Goossensen D, Genugten L, Lingsma HF, Dippel DWJ, Koudstaal PJ, Hertog HM. Supporting lifestyle behavior change after TIA or minor ischemic stroke with motivational interviewing at a nurse‐led outpatient clinic. Proceedings of the European Stroke Conference 2014. 6‐9 May 2014; Nice, France. 2014.
Green 2007 {published data only}
- Green T, Haley E, Eliasziw M, Hoyte K. Education in stroke prevention: efficacy of an educational counselling intervention to increase knowledge in stroke survivors. Canadian Journal of Neuroscience Nursing 2007;29(2):13‐20. [PubMed] [Google Scholar]
Hedegaard 2014 {published data only}
- Hedegaard U, Kjeldsen LJ, Pottegard A, Bak S, Hallas J. Multifaceted intervention including motivational interviewing to support medication adherence after stroke/transient ischemic attack: a randomized trial. Cerebrovascular Diseases Extra 2014;4(3):221‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leistner 2013 {published data only}
- Leistner S, Michelson G, Laumeier I, Ahmadi M, Smyth M, Nieweler G, et al. Intensified secondary prevention intending a reduction of recurrent events in TIA and minor stroke patients (INSPiRE‐TMS): a protocol for a randomised controlled trial. BMC Neurology 2013;13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 2009 {published data only}
- Mitchell PH, Veith RC, Becker KJ, Buzaitis A, Cain KC, Fruin M, et al. Brief psychosocial‐behavioral intervention with antidepressant reduces poststroke depression significantly more than usual care with antidepressant: living well with stroke: randomized, controlled trial. Stroke 2009;40(9):3073‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Krishnamurthi 2014 {published data only}
- Krishnamurthi R, Witt E, Barker‐Collo S, McPherson K, Davis‐Martin K, Bennett D, et al. Reducing recurrent stroke: methodology of the motivational interviewing in stroke (MIST) randomized clinical trial. International Journal of Stroke 2014;9(1):133‐9. [DOI] [PubMed] [Google Scholar]
Additional references
AHA 2014
- American Heart Association. Heart disease and stroke statistics‐2014 update: a report from the American Heart Association. Circulation 2014;129(3):e28‐e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
AHA/ASA 2014
- American Heart Association/American Stroke Association. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45(7):2160‐236. [DOI] [PubMed] [Google Scholar]
Bland 1986
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1(8476):307‐10. [PubMed] [Google Scholar]
Demaerschalk 2010
- Demaerschalk BM, Hwang HM, Leung G. US cost burden of ischemic stroke: a systematic literature review. American Journal of Managed Care 2010;16(7):525‐33. [PubMed] [Google Scholar]
Gibbon 2012
- Gibbon B, Gibson J, Lightbody CE, Radford K, Watkins C. Promoting rehabilitation for stroke survivors. Nursing Times 2012;108(47):12‐5. [PubMed] [Google Scholar]
Goldberg 1978
- Goldberg D. Manual of the General Health Questionnaire. Windsor: NFER‐Nelson, 1978. [Google Scholar]
Gurr 2011
- Gurr B, Muelenz C. A follow‐up study of psychological problems after stroke. Topics in Stroke Rehabilitation 2011;18(5):461‐9. [DOI] [PubMed] [Google Scholar]
Hackett 2008
- Hackett ML, Anderson CS, House A, Halteh C. Interventions for preventing depression after stroke. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD003689.pub3] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Katz 1970
- Katz S, Down TD, Cash HR, Grotz RC. Progress in the development of the index of ADL. The Gerontologist 1970;10(1):20‐30. [DOI] [PubMed] [Google Scholar]
Keith 1987
- Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. Advances in Clinical Rehabilitation 1987;1:6‐18. [PubMed] [Google Scholar]
Lai 2010
- Lai DT, Cahill K, Qin Y, Tang JL. Motivational interviewing for smoking cessation. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD006936.pub2] [DOI] [PubMed] [Google Scholar]
Lawendowski 1998
- Lawendowski LA. A motivational intervention for adolescent smokers. Preventive Medicine 1998;27(5 Pt 3):A39‐46. [DOI] [PubMed] [Google Scholar]
Mahoney 1965
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Maryland State Medical Journal 1965;14:56‐61. [PubMed] [Google Scholar]
McArthur 2011
- McArthur KS, Quinn TJ, Higgins P, Langhorne P. Post‐acute care and secondary prevention after ischaemic stroke. BMJ 2011;342:d2083. [DOI] [PubMed] [Google Scholar]
Miller 1983
- Miller W. Motivational interviewing with problem drinkers. Behavioural Psychotherapy 1983;11:141‐72. [Google Scholar]
Miller 2002
- Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 2nd Edition. New York: Guilford Press, 2002. [Google Scholar]
Miller 2013
- Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd Edition. New York: Guilford Press, 2013. [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Purath 2014
- Purath J, Keck A, Fitzgerald CE. Motivational interviewing for older adults in primary care: a systematic review. Geriatric Nursing 2014;35(3):219‐24. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rubak 2005
- Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta‐analysis. British Journal of General Practice 2005;55(513):305‐12. [PMC free article] [PubMed] [Google Scholar]
Sacco 2013
- Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44(7):2064‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saka 2009
- Saka O, McGuire A, Wolfe C. Cost of stroke in the United Kingdom. Age and Ageing 2009;38(1):27‐32. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
van Bennekom 1995
- Bennekom CA, Jelles F, Lankhorst GJ, Bouter LM. The Rehabilitation Activities Profile: a validation study of its use as a disability index with stroke patients. Archives of Physical Medicine and Rehabilitation 1995;76(6):501‐7. [DOI] [PubMed] [Google Scholar]
van Swieten 1988
- Swieten J, Koudstaal P, Visser M, Schouten H, Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19(5):604‐7. [DOI] [PubMed] [Google Scholar]
WHO 1989
- World Health Organization. Stroke ‐ 1989 recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on stroke and other cerebrovascular disorders. Stroke 1989;20:1407‐31. [DOI] [PubMed] [Google Scholar]


