Abstract
Background
Mild cognitive impairment is hypothesised to represent a pre‐clinical stage of dementia but forms a heterogeneous group with variable prognosis.
Objectives
To assess the safety and efficacy of cholinesterase inhibitors in people with mild cognitive impairment.
Search methods
Trials were identified from the Cochrane Dementia and Cognitive Improvement Group's Specialised Register, which is frequently updated from the major healthcare databases (MEDLINE, EMBASE, CINAHL, PsycINFO and Lilacs) as well as trial registers and grey literature.
Selection criteria
Double‐blind, placebo‐controlled randomised trials of any cholinesterase inhibitor in people with mild cognitive impairment.
Data collection and analysis
Data were extracted from the published reports of the included studies, combined by meta‐analysis where appropriate, and treatment efficacy and risk of adverse events were estimated.
Main results
Nine studies (from eight published reports) of 5149 individuals with mild cognitive impairment (however defined) were included in the review. Limited pooling of results was possible owing to different lengths of trials. Meta‐analysis of the three studies reporting conversion to dementia gives no strong evidence of a beneficial effect of cholinesterase inhibitors on the progression to dementia at one, two or three years. The risk ratio (RR) for conversion at two years was significantly different from unity (0.67; 95% confidence interval (CI) 0.55 to 0.83), but this is based on only two studies reported in the same article. There was essentially no effect of cholinesterase inhibitors on cognitive test scores.
Based on the results from 4207 individuals, there were significantly more adverse events in the cholinesterase inhibitor groups (RR 1.09; 95% CI 1.02 to 1.16), but no more serious adverse events or deaths. Gastrointestinal side effects were much more common (diarrhoea: RR 2.10; 95% CI 1.30 to 3.39; nausea: RR 2.97; 95% CI 2.57 to 3.42; vomiting: RR 4.42; 95% CI 3.23 to 6.05). Cardiac problems were no more likely in either group (RR 0.71; 95% CI 0.25 to 2.02). Other side effects reported significantly more often in the cholinesterase inhibitor group were muscle spasms/leg cramps (RR 7.52; 95% CI 4.34 to 13.02), headache (RR 1.34; 95% CI 1.05 to 1.71), syncope or dizziness (RR 1.62; 95% CI 1.36 to 1.93), insomnia (RR 1.66; 95% CI 1.36 to 2.02) and abnormal dreams (RR 4.25; 95% CI 2.57 to 7.04).
Authors' conclusions
There is very little evidence that cholinesterase inhibitors affect progression to dementia or cognitive test scores in mild cognitive impairment. This weak evidence is overwhelmed by the increased risk of adverse events, particularly gastrointestinal. Cholinesterase inhibitors should not be recommended for mild cognitive impairment.
Keywords: Humans, Cholinesterase Inhibitors, Cholinesterase Inhibitors/adverse effects, Cholinesterase Inhibitors/therapeutic use, Cognitive Dysfunction, Cognitive Dysfunction/drug therapy, Dementia, Dementia/etiology, Diarrhea, Diarrhea/chemically induced, Disease Progression, Nausea, Nausea/chemically induced, Randomized Controlled Trials as Topic, Vomiting, Vomiting/chemically induced
Anti‐dementia drugs for people with memory problems but without dementia
Dementia is a very common condition and, with ageing populations, will only increase in importance in the coming years. Early diagnosis and treatment help people with dementia stay independent and living at home for longer. Cholinesterase inhibitor ('anti‐dementia') drugs are used to treat people with Alzheimer's disease (the most common cause of dementia) and can be started as soon as dementia is diagnosed. However, it is not clear whether they are helpful, or indeed safe, in people who have some memory problems but who do not have dementia. It is extremely difficult to predict who will go on to develop dementia from this group of people and some will even get better and their memory return to normal. There is very little evidence that these drugs prevent the development of dementia over three years and people taking them experience a number of side effects including nausea, vomiting and diarrhoea, as well as muscle spasms/leg cramps and abnormal dreams.
Background
Description of the condition
Dementia is a major public health concern and will only increase in importance in the future with ageing populations. Ferri 2005 estimated that there were around 24 million people with dementia on the planet in 2001 and 4.6 million new cases each year. Furthermore huge increases in prevalence are anticipated ‐ between 84% and 393% by 2040 in different areas of the world (Ferri 2005).
Dementia‐related changes develop over time as a spectrum of pathology and impairment, at least in Alzheimer's disease (Braak 1991). Therefore it is self‐evident that a pre‐clinical stage must exist when the pathological changes of dementia are present but the condition is yet to overtly manifest itself and this has become accepted in the latest consensus diagnostic criteria for Alzheimer's disease (McKhann 2011). The latest in a long line of attempts to classify people in this state is 'mild cognitive impairment' (for an historical review of the concepts see Heinik 2006; Heinik 2010), which describes a condition in a group of people who have memory problems but do not meet the diagnostic criteria for dementia. Specifically the most common criteria require that they have a memory complaint, have normal general cognitive function but show abnormal memory for their age and are not demented (Petersen 1999). Winblad 2004 defined mild cognitive impairment as someone who was "not normal, not demented," but who demonstrated cognitive decline, either from self‐ or informant‐report or objectively demonstrated by serial cognitive testing, and whose basic activities of daily living were preserved or whose complex instrumental functions were only minimally impaired. Winblad 2004 is the only set of criteria to include this longitudinal perspective. Newer consensus criteria (Albert 2011) have appeared but have not yet passed into wide usage and were published after all the trials included in this review. These new criteria require concern regarding a change in cognition, impairment in one or more cognitive domains, preservation of independence in functional abilities, and that the individual is not demented.
Despite the lack of strict consensus, the syndrome seems to be reliably diagnosable and may be a high‐risk state for the development of dementia (O'Brien 2008; Winblad 2004). However estimated rates of conversion to dementia vary greatly, with between 1% and 25% of people with mild cognitive impairment (or analogous conditions) converting to dementia per year (Dawe 1992) and between 11% and 33% developing dementia within two years (Ritchie 2004). Most importantly, the prognosis is highly heterogeneous; some patients remain the same and some even improve so that they no longer meet the criteria for the syndrome. Estimates of the number of individuals said to have mild cognitive impairment subsequently returning to normal cognition have been as high as 44% (Ritchie 2004). Further confusion results from the suggestion that there might be subtypes of mild cognitive impairment, for example amnestic mild cognitive impairment, which is hypothesised to have a more specific link with Alzheimer's disease (Petersen 2006). However this more specific condition is not uniformly referred to as amnestic mild cognitive impairment (e.g. Albert 2011).
If mild cognitive impairment is indeed a precursor or 'high‐risk state' for dementia, the amount of interest in mild cognitive impairment as a therapeutic target is understandable. Since the majority of the progression of dementia is irreversible, it would be a great advance with significant public health implications if people at risk and delay could be targeted or even if the onset of frank dementia could be prevented.
Description of the intervention
Cholinesterase inhibitors (donepezil, galantamine and rivastigmine) are usually taken orally once or twice daily, although other preparations, such as transdermal patches, are available. They act by inhibiting the enzyme acetylcholinesterase, which degrades acetylcholine, a major neurotransmitter. This has the effect of enhancing the cholinergic function of the brain. Common side effects associated with these medications are primarily gastrointestinal (nausea, vomiting, anorexia and diarrhoea) but other side effects include fatigue, insomnia, headache, dizziness, syncope, abnormal dreams, hallucinations, agitation, aggression, muscle cramps, urinary incontinence, rash and pruritus. Less common side effects include gastric and duodenal ulcers, gastrointestinal haemorrhage, bradycardia and seizures. Rare side effects include sino‐atrial block, AV block, hepatitis and extrapyramidal symptoms (BNF 2012).
How the intervention might work
The efficacy of cholinesterase inhibition on the progression of mild and moderate Alzheimer's disease is well established (Birks 2006a; Birks 2006b; Birks 2009; Loy 2006). Similarly, amnestic mild cognitive impairment is thought to be characterised by a central cholinergic deficit (Gauthier 2006). Given the progression of mild cognitive impairment to dementia (and amnestic mild cognitive impairment to Alzheimer's disease) and the cholinergic deficit in both disorders, it has been suggested that cholinesterase inhibitors might slow this process of progression.
Why it is important to do this review
Epidemiological studies worldwide have found the prevalence of mild cognitive impairment to be between 3% and 19% of the older population (Ritchie 2004). Given that the UK is estimated to have an over‐65 year old population of 9.7 million people (Office for National Statistics 2006), potentially 1.8 million people in the UK could have mild cognitive impairment. Given the above estimates, this equates to approximately 100,000 to 300,000 people progressing to dementia each year.
Three systematic reviews have previously reviewed the evidence for the use of cholinesterase inhibitors in mild cognitive impairment but produced conflicting results. Raschetti 2007 found no effect of cholinesterase inhibitors in delaying the onset of dementia in people with mild cognitive impairment whereas Diniz 2009 found the risk ratio (RR) of progression to dementia to be 0.75 (95% confidence interval (CI) 0.66 to 0.87) in those treated with a cholinesterase inhibitor. Sobów 2007, examining the same studies as Diniz 2009, similarly concluded that cholinesterase inhibitors were associated with a reduction of risk of conversion to dementia of approximately 24% but also added that this comes with a substantially increased risk of adverse events, drug discontinuation and, possibly, in the case of galantamine, mortality.
Of relevance is that progression to dementia was a primary focus of Diniz 2009 and Sobów 2007 whereas Raschetti 2007 included studies looking at progression to dementia or change in cognition. These different focuses resulted in a slightly different selection of papers being included by Raschetti 2007 compared to Diniz 2009 and Sobów 2007. All studies highlighted the side‐effect profile of these medications but Sobów 2007 was the most explicit. Furthermore none of these reviews took account of the different time points reported by different trials. The present review sought to build on the work of these systematic reviews, applying Cochrane methodology to randomised controlled trials (RCTs) conducted before these reviews and in the years since their literature search.
Objectives
To assess the efficacy, safety and tolerability of cholinesterase inhibitors for mild cognitive impairment in adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomised, double‐blind, placebo‐controlled trials.
Types of participants
Adults with mild cognitive impairment as defined by each study. Different definitions of mild cognitive impairment were acceptable but they had to be in line with the generally accepted criteria of a subjective memory complaint and relatively preserved daily functioning (e.g. Petersen 1999).
Types of interventions
Cholinesterase inhibitors of all types, at all doses, and in any formulation for a minimum of one month. We will specify no maximum duration of treatment.
The comparator group is to be placebo.
Types of outcome measures
Primary outcomes
Progression to dementia, either in general or specific subtypes: Alzheimer's disease defined by the criteria from the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS‐ARDRA; McKhann 1984); vascular dementia defined by consensus criteria (Roman 1993); or dementia with Lewy bodies defined by consensus criteria (McKeith 2005), measured at the time points of 12, 24 and 36 months. Criteria from the fourth edition of the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV) or the tenth revision of the World Health Organization's (WHO) International Statistical Classification of Diseases and Related Health Problems (ICD‐10) for the dementia syndrome in general or specific subtypes will also be acceptable. Cognition will have been measured with standardised cognitive tests.
Side effects, including gastrointestinal and cardiac.
Secondary outcomes
Change in cognitive test scores.
Mortality.
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois): the Cochrane Dementia and Cognitive Improvement Group's (CDCIG) Specialised Register. We searched for all treatment of mild cognitive impairment studies in which any of the following interventions had been used: donepezil, rivastigmine, galantamine and tacrine. ALOIS is maintained by the Trials Search Co‐ordinator for CDCIG and contains dementia and cognitive impairment studies identified from the following:
monthly searches of a number of major healthcare databases: MEDLINE, EMBASE, CINAHL, PsycINFO and Lilacs;
monthly searches of a number of trial registers: metaRegister of Controlled Trials (mRCT); Umin Japan Trial Register; ICTRP/WHO portal (which covers ClinicalTrials.gov; ISRCTN; Chinese Clinical Trials Register; German Clinical Trials Register; Iranian Registry of Clinical Trials and the Netherlands National Trials Register, plus others);
quarterly searches of The Cochrane Library’s Central Register of Controlled Trials (CENTRAL);
six‐monthly searches of a number of grey literature sources: ISI Web of Knowledge Conference Proceedings; Index to Theses; Australasian Digital Theses.
To view a list of all sources searched for ALOIS see About ALOIS on the ALOIS website.
We ran additional separate searches in the following sources to ensure that the most up‐to‐date results were retrieved: MEDLINE, EMBASE, PsycINFO, Web of Science, CINAHL, LILACS, ALOIS, CENTRAL, Clinicaltrials.gov and ICTRP. The search strategy that we used for the retrieval of reports of trials from MEDLINE (via the Ovid SP platform) can be seen in Appendix 1.
Searching other resources
We searched reference lists of relevant studies for additional trials.
Data collection and analysis
We included relevant publications based on the title of the publication and its abstract. In the presence of any suggestion that an article could be relevant, we retrieved it for further assessment.
Selection of studies
The two review authors (TR, JM) independently selected trials for relevance using the criteria defined in the current Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Data extraction and management
TR and JM extracted data regarding the primary and secondary outcomes from the published reports independently using a data extraction form. Any disagreement over inclusion or exclusion of trials, risk of bias or data extraction was settled by discussion. The data extracted were the number of patients progressing to dementia at the time points of 12, 24 and 36 months. We also collected information about the definition of mild cognitive impairment used, drug side effects and the secondary outcomes listed above.
Assessment of risk of bias in included studies
We commented on the methodological quality of trials. Specifically, TR undertook assessment of the risk of bias of the included trials in accordance with the current Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and JM checked these.
Five key domains were examined for bias: selection bias, performance bias, attrition bias, detection bias and reporting bias. These were assessed and classified as either a low risk of bias or as a high risk of bias. Where insufficient detail was reported in a study to assess the risk this was reported as 'unclear'. In addition, any other form of bias noted in the study was reported.
We used the Cochrane 'Risk of bias' tool in RevMan 5.1 (RevMan 2011).
Measures of treatment effect
We expressed the treatment effects as RR for dichotomous outcomes. All of the outcomes for this review were considered as dichotomous (present/absent).
Unit of analysis issues
No unit of analysis issues arose. All included studies were parallel‐group, individually randomised trials. Some trials did report repeated observations but results from more than one time point in a single study were not included in the same meta‐analysis.
Dealing with missing data
Apart from loss to follow‐up, there were no significant problems with missing data in the included studies.
Assessment of heterogeneity
Heterogeneity was explored by examining factors that may be influential such as used definition of mild cognitive impairment, care setting, duration of follow‐up and incidence of conversion to dementia. In the absence of clinical heterogeneity we tested for statistical heterogeneity by forest plot and Chi2 test.
Assessment of reporting biases
We assessed reporting bias by funnel plot.
Data synthesis
We used RevMan 5.1 to perform meta‐analysis where studies were sufficiently similar to allow this (RevMan 2011). Mantel‐Haenzel random‐effects analyses were planned since the differing definitions of mild cognitive impairment used were likely to result in substantial between‐studies heterogeneity.
Subgroup analysis and investigation of heterogeneity
Studies were not sufficiently detailed to allow comparison of different subtypes of mild cognitive impairment.
Sensitivity analysis
There were not sufficient studies reporting data for each outcome to allow a meaningful sensitivity analysis to be carried out.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies for details of the studies considered for this review.
Nine trials, reported in eight articles (Doody 2009; Feldman 2007; Koontz 2005; Narasimhalu 2010; Peters 2012; Petersen 2005a; Salloway 2004; Winblad 2008 (combined)) were included. The primary outcome in two studies was progression to dementia using NINCDS‐ADRDA criteria (McKhann 1984) with or without DSM‐IV (Feldman 2007; Petersen 2005a) and another intended to report conversion to dementia without specifying diagnostic criteria (Peters 2012). A further two trials reported conversion as a secondary end point (Winblad 2008 (Study 1); Winblad 2008 (Study 2)). The remainder studied reported changes in cognitive test scores rather than diagnoses: five examined the effect of cholinesterase inhibitors on Clinical Dementia Rating (CDR) score and Alzheimer's Disease Assessment Scale‐Cognitive subscale (ADAS‐Cog) (Doody 2009; Peters 2012; Salloway 2004; Winblad 2008 (Study 1); Winblad 2008 (Study 2)), and the remaining two examined change in other cognitive tests (Koontz 2005; Narasimhalu 2010). Three studies used donepezil (Doody 2009; Petersen 2005a; Salloway 2004), four used galantamine (Koontz 2005; Peters 2012; Winblad 2008 (Study 1); Winblad 2008 (Study 2)) and two used rivastigmine (Feldman 2007; Narasimhalu 2010).
The following cognitive tests were used as primary outcomes in one or more studies:
Mini Mental State Examination (Folstein 1975);
ADAS‐Cog, either in its original 11‐item form (Rosen 1984) or the 13‐item modified version (Mohs 1997);
CDR, either sum of boxes or individual scores (Morris 1993);
Cambridge Automated Neuropsychiatric Test Assessment Battery (CANTAB; Sahakian 1988);
Ten‐Point Clock test (Agrell 1998);
Color Trails tests (D'Elia 1996);
New York University Paragraph Test Delayed Recall (Kluger 1999);
Alzheimer's Disease Co‐operative Study's Clinical Global Impression of Change (ADCS‐CGIS; Schneider 1997);
Digit Symbol Substitution Test (Lezak 2004);
Alzheimer's Disease Cooperative Study's Activities of Daily Living (ADCS‐ADL; Galasko 1997).
Results of the search
Searching identified 3749 records, short‐listed to 259 from which 43 were identified for closer attention. When the full text of these articles had been examined, 35 articles were excluded (see Characteristics of excluded studies) and eight included in the review (see Characteristics of included studies). The process of the search is summarised in Figure 1.
Figure 1.
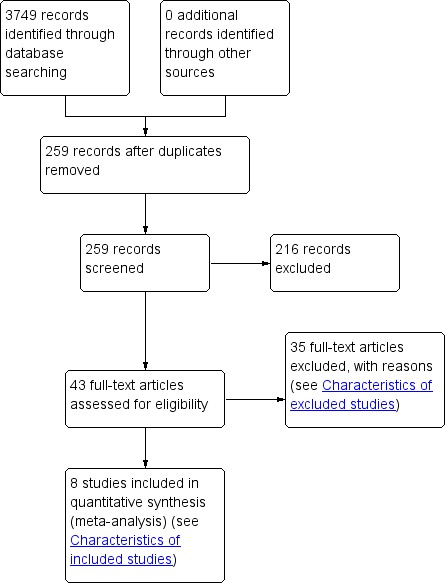
Study flow diagram.
Included studies
Doody 2009 reported a 48‐week placebo‐controlled RCT of donepezil in mild cognitive impairment.
Feldman 2007 reported a 48‐month placebo‐controlled RCT of rivastigmine in mild cognitive impairment.
Koontz 2005 reported a relatively small 16‐week placebo‐controlled RCT of galantamine in mild cognitive impairment.
Narasimhalu 2010 reported a 24‐week placebo‐controlled RCT of rivastigmine in mild cognitive impairment.
Peters 2012 reported a truncated two‐year placebo‐controlled RCT of donepezil (and memantine) in mild cognitive impairment.
Petersen 2005a reported a three‐year placebo‐controlled RCT of donepezil (and vitamin E) in mild cognitive impairment.
Salloway 2004 reported a 24‐week placebo‐controlled RCT of donepezil in mild cognitive impairment.
Winblad 2008 (combined) reported two placebo‐controlled RCTs of galantamine, both lasting 24 months. These have been referred to in this review as Winblad 2008 (Study 1) and Winblad 2008 (Study 2). When combined data were reported (e.g. for adverse events) we refer to Winblad 2008 (combined).
Excluded studies
Thirteen studies were excluded because they were duplicate reports of included studies. Seven were excluded because they were not RCTs. Six were excluded because they reported the wrong outcome, for example a proxy end point in a scanning study. Five were excluded because they were not conducted in people with mild cognitive impairment. One study was excluded for each of the following reasons: not placebo‐controlled, not human research, merely reported a protocol (the results of the study were not available anywhere), and was a case report.
Risk of bias in included studies
Allocation
All studies allocated participants to experimental groups by adequate randomisation.
Blinding
All studies were double blind.
Incomplete outcome data
All trials, particularly those of longer duration, suffered attrition, and most used an intention‐to‐treat (ITT) analysis to minimise the effect of this bias. Some reported a number of different populations that were used in the analyses, for example ITT, modified‐ITT and those 'dropouts' who were 'retrieved' in Feldman 2007, which made the exact conduct of the study difficult to ascertain.
Selective reporting
There was no evidence of selective reporting in any of the studies.
Other potential sources of bias
Doody 2009, Petersen 2005a and Salloway 2004 were funded by Esai Inc. and Pfizer Inc. (manufacturers of donepezil). Feldman 2007 was funded by Novartis Pharma AG (manufacturer of rivastigmine). Koontz 2005 and Narasimhalu 2010 were supported by Janssen Pharmaceuticals (manufacturer of galantamine). Winblad 2008 (combined) was sponsored by Janssen‐Cilag and Johnson & Johnson Pharmaceutical Research and Development (the former is a subsidiary of Johnson & Johnson and manufactures galantamine).
Effects of interventions
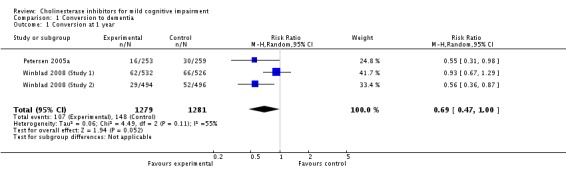
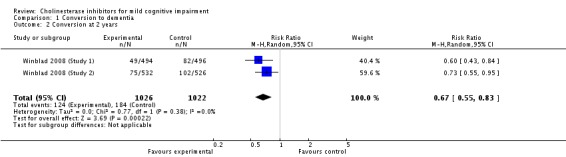
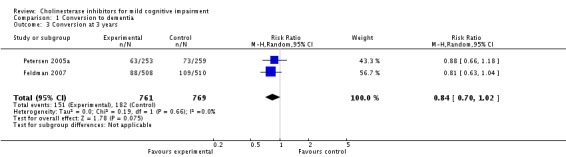
When the results of the studies were considered together, there was very little evidence that cholinesterase inhibitors reduce the risk of progression to dementia. There was no effect at one year (Figure 2) and the reduction of risk by 33% at two years (RR 0.67; 95% CI 0.55 to 0.83; Figure 3) was based on the results of two studies published in the same report (Winblad 2008 (Study 1); Winblad 2008 (Study 2)). There was also no effect on progression to dementia at three years (Figure 4). There were no significant effects on cognitive scores reported with the exception of the CDR sum of boxes at one year (mean difference ‐0.10; 95% CI ‐0.11 to ‐0.09). This difference is small and unlikely to be of any clinical significance. It was not possible to include data from Peters 2012 but their reported median change in ADAS‐Cog scores of 1 (interquartile range (IQR) ‐1 to 1.75) in the galantamine group and ‐1 (IQR ‐4.5 to 0.5) in the placebo group on a 70‐point scale is unlikely to alter Analysis 3.1 substantially or be of clinical significance.
Figure 2.
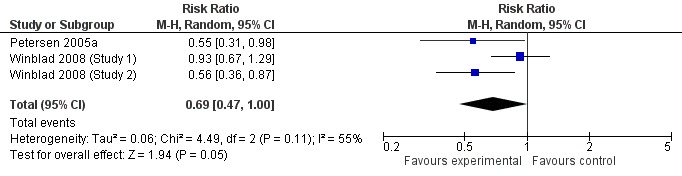
Conversion to dementia at one year.
Figure 3.
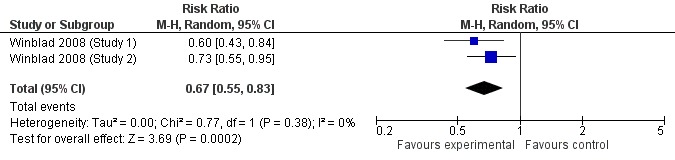
Conversion to dementia at two years.
Figure 4.
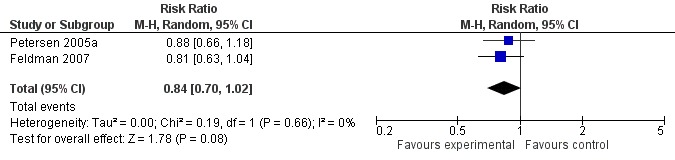
Conversion to dementia at three years.
Analysis 3.1.
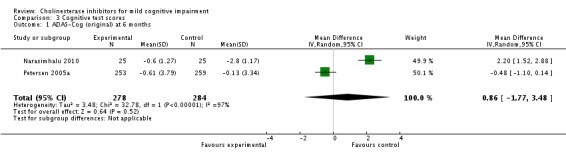
Comparison 3 Cognitive test scores, Outcome 1 ADAS‐Cog (original) at 6 months.
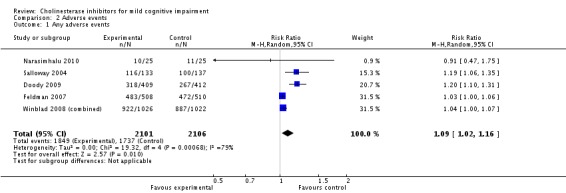
However, even if this is accepted as weak evidence of an effect, there was clear evidence, based on 4207 people, of increased adverse events in the drug groups compared to placebo (RR 1.09; 95% CI 1.02 to 1.16; Figure 5). There was no evidence of an increased risk of serious adverse events (Figure 6) but people taking cholinesterase inhibitors reported more gastrointestinal side effects including diarrhoea (RR 2.10; 95% CI 1.30 to 3.39), nausea (RR 2.97; 95% CI 2.57 to 3.42) and vomiting (RR 4.42; 95% CI 3.23 to 6.05). No studies reported arrhythmias directly but there was no excess of cardiac problems, including bradycardia, in the drug group (RR 0.71; 95% CI 0.25 to 2.02). Other significant adverse events occurring more often in people taking cholinesterase inhibitors were muscle spasms or leg cramps (RR 7.52; 95% CI 4.34 to 13.02), abnormal dreams (RR 4.25; 95% CI 2.57 to 7.04), insomnia (RR 1.66; 95% CI 1.36 to 2.02), syncope or dizziness (RR 1.62; 85% CI 1.36 to 1.93) and headache (RR 1.34; 95% CI 1.05 to 1.71). There was no evidence of increased rates of depression in the drug group (RR 1.14; 95% CI 0.83 to 1.57).
Figure 5.
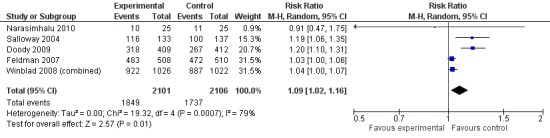
Any adverse events.
Figure 6.
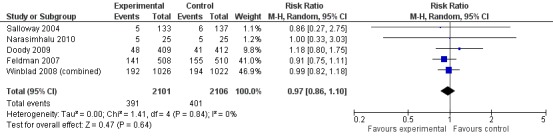
Serious adverse events.
There was also no evidence of increased mortality associated with cholinesterase inhibitors in individuals with mild cognitive impairment. However an excess of deaths in the treatment arm was noted in Winblad 2008 (Study 1) and Winblad 2008 (Study 2) (13/1026 galantamine vs 1/1022 placebo) with the possibility that further participants might have withdrawn and subsequently died. This led to a retrieved drop‐out study (Johnson & Johnson 2005b; reported in Winblad 2008 (combined)) investigating the vital status and, where relevant, cause of death of all patients randomised in the trials. Preliminary conclusions showed an increased, though statistically non‐significant, risk of death during the study period if treated with galantamine (RR 1.54; 95% CI 0.78 to 3.04) but they noted that, over a period of 1539 days from the start of the double‐blind portion of the trial (all available data), the RR was lower at 1.24 (95% CI 0.84 to 1.83).
Discussion
Summary of main results
Since the studies were conducted over such a range of time periods and report such different outcomes, synthesis of these results was a challenge. Of the three studies that reported conversion to dementia, a random‐effects meta‐analysis based on between 1530 and 2650 individuals, gave no strong evidence of a beneficial effect of cholinesterase inhibitors on the progression to dementia at one, two or three years (Figure 2; Figure 3; Figure 4). The RR for conversion at two years was significantly different from unity but this was based on only two studies that were conducted in parallel with each other (Winblad 2008 (Study 1); Winblad 2008 (Study 2)). Each of these studies had different inclusion criteria and so comparing them was difficult.
The only cognitive test that showed a different outcome in the cholinesterase inhibitor group compared to placebo, based on a meta‐analysis of two studies, was the CDR sum of boxes, which showed a 0.1 point worsening in the placebo group at one year (Analysis 3.4). On an 18‐point scale, it is unlikely that this is of any clinical significance.
Analysis 3.4.
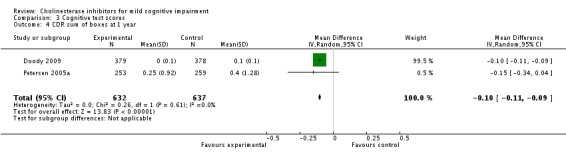
Comparison 3 Cognitive test scores, Outcome 4 CDR sum of boxes at 1 year.
There were significantly more adverse events in the cholinesterase inhibitor groups but no more serious adverse events or deaths. However there was a suggestion that galantamine may be associated with increased mortality in mild cognitive impairment and Peters 2012 was terminated early in response to the report of increased mortality in Johnson & Johnson 2005b and Winblad 2008 (combined). Gastrointestinal side effects were much more common but cardiac problems were no more likely in either group; however, arrhythmias were not reported separately. Other side effects reported significantly more often in the cholinesterase inhibitor group were muscle spasms or leg cramps, headache, syncope or dizziness, insomnia and abnormal dreams.
Overall completeness and applicability of evidence
While mild cognitive impairment is itself a heterogeneous category, the definitions used in different studies add to the heterogeneity. Few studies used a standard definition for mild cognitive impairment and none differentiated between amnestic and non‐amnestic mild cognitive impairments. Three studies used the Petersen criteria, or a version of them (Doody 2009; Koontz 2005; Petersen 2005a) and the remainder used their own operationalised criteria. Therefore it is difficult to be certain that samples from different studies are truly comparable. Given the appropriate enthusiasm for early diagnosis of dementia, cholinesterase inhibitors are indeed the intervention that would be offered to these people.
Because of the different durations of trials, definitions of mild cognitive impairment, inclusion criteria and outcomes reported, there are some objectives of this review that cannot be definitively answered: (1) it is not possible to be certain that cholinesterase inhibitors do not affect conversion to dementia up to three years and we cannot comment on their effects over a longer period of time; and (2) we cannot comment on the effect of cholinesterase inhibitors specifically on arrhythmias which, since bradyarrhythmias are one of the main contraindications for these medications, should be an important consideration.
Quality of the evidence
All studies report well‐conducted, robust RCTs. The main difficulties are the definitions of mild cognitive impairment, which varied widely between studies, thus limiting the validity of comparisons and loss to follow‐up potentially introducing selection bias. In such a chronic, slowly progressive condition as dementia, following individuals up for three years to see if they developed dementia seems to be inadequate.
Potential biases in the review process
ALOIS: the CDCIG Specialised Register is a comprehensive and weekly updated register of all publications relating to cognitive function. As a result when searched in we believe that all trials with the potential for inclusion will have been accessed. The search strategy was developed in conjunction with experts in the field to maximise study identification. It was not possible to contact any mild cognitive impairment specialists to recover any additional unpublished or ongoing studies. Data collection and analysis methods were robust.
Because of the small number of trials, a decision was taken to combine trials at similar time points, even if this meant combining trials measuring conversion to Alzheimer's disease (e.g. Petersen 2005a) with trials investigating conversion to dementia (e.g. Winblad 2008 (combined), which defined dementia by the CDR scale). This decision was partly pragmatic but since a clinical diagnosis of dementia can be no more than 'probable' (e.g. McKhann 1984) pre‐mortem and because Alzheimer's disease is the most common cause of dementia, it is unlikely that this will have had a major effect on the results.
Agreements and disagreements with other studies or reviews
This review agrees with the results of Raschetti 2007 in concluding that there is no evidence of an effect of cholinesterase inhibitors on progression to dementia in people with mild cognitive impairment and that adverse events associated with these medications are common and significant. The current review included data from the same trials as Raschetti 2007 in addition to those identified by Doody 2009 and Narasimhalu 2010, published subsequently. Diniz 2009 and Sobów 2007, both of which only included four studies (Feldman 2007; Petersen 2005a; Winblad 2008 (Study 1); Winblad 2008 (Study 2)) concluded that cholinesterase inhibitors protected against progression to dementia (hazard ratio (HR) 0.75; 95% CI 0.66 to 0.87 in Diniz 2009). However they combined progression to dementia at two and three years. As can be seen from the current review, there was no effect at one or three years and the effect at two years results from two studies reported in the same paper (Winblad 2008 (combined)).
Authors' conclusions
There is no convincing evidence that cholinesterase inhibitors have an effect on progression to dementia in mild cognitive impairment. In addition they are associated with a number of adverse events, outlined above. Therefore there is no basis for suggesting that these medications be prescribed for individuals presenting with memory complaints who do not meet the diagnostic criteria for dementia. However, it is questionable how the participants in these studies compare to individuals in the community who might attract the label 'mild cognitive impairment' in different contexts such as primary care or specialist memory clinics. In primary care, where the prevalence of dementia is relatively low (approximately 5% of people over the age of 65 years), an individual presenting with a memory complaint has a low chance of being in the early stages of dementia and not every individual presenting in this way should be considered to have mild cognitive impairment. In fact, subjective memory complaints are not associated with current cognitive impairment but may be associated with future cognitive decline, though there is complex confounding by depression and personality traits (Reid 2006). In contrast, in secondary care or specialist memory clinics, when individuals have been carefully filtered by primary care and a longitudinal component has been introduced by the delay of referral, it would be much more likely that the individual who still reports impaired memory has an underlying dementing condition and thus, watchful waiting of individuals who do not currently meet the criteria for a diagnosis of dementia is often advised, as tends to happen in clinical practice.
These results were based on a small number of studies of arguable comparability. The most important outcome – conversion to dementia – is a long process but the longest duration reported in a trial was three years. Further research to clarify the efficacy, or lack of it, of cholinesterase inhibitors in mild cognitive impairment could be argued for but, more urgently, much more attention should be paid to improving the identification of the pre‐clinical stages of dementia starting with a clarification (or even complete overhaul) of the concept of mild cognitive impairment itself.
Feedback
Miscellaneous, 11 April 2016
Summary
Comment written by Aaron M Tejani, BSc(Pharm), PharmD; Valerie Higgins, BSc(Pharm)
While we do agree with the author’s conclusion that there is very little evidence of benefit, we are concerned that the actual risk of harm with cholinesterase inhibitors may have been underestimated based on the method utilized to account for missing data in 4 of the 5 large trials included in the review; namely last observation carried forward (LOCF).
Across all 5 of these trials, the dropout rate in the study arm receiving active treatment was greater than 30%, with the highest rate of discontinuation occurring early on. Loss to follow‐up was not reported in the study by Petersen et al. (Petersen 2005), but was reported in the other 4 trials and ranged from 0.8‐4% (Doody 2009, Winblad 2008 (2 trials), Salloway 2004). It is not clear, however, with respect to those patients that dropped out and/or were lost to follow‐up, when the final evaluation of cognitive function was actually conducted. The criteria for inclusion in the intention‐to treat (ITT) analysis in each study, required only one evaluation after starting treatment and therefore, could have been conducted early on in the study and the result carried forward (i.e. LOCF was used).
With the exception of the study by Petersen et al., the method of ITT utilized to estimate missing patient data was LOCF. This technique assumes that the outcome measures remain stable from the time of participant dropout until the end of the study period and that the missing values are not related to any other variable (i.e. the values are missing completely at random) (Molnar 2008, Molnar 2009). Use of LOCF can lead to serious bias in degenerative conditions because attrition may be related to side effects of the drug and as a result, outcomes which would be expected to deteriorate over time could bias the effect estimate in favour of the drug (Higgins 2011). The risk of attrition bias resulting from missing data in the studies by Doody, Winblad, and Salloway is unclear, at best, as arguably, the validity of imputed outcomes using LOCF is questionable in a progressive disease.
In dementia, the assumption that this degenerative process halts at the time of study dropout, may exaggerate the effectiveness of these drugs (Molnar 2009). In particular, in studies with earlier drop out and greater drop out rates in the treatment group, last observation carried forward may overestimate the effect of the drug relative to placebo (Molnar 2009). In fact, this method of analysis would actually favour more toxic treatment modalities since a greater number of patients would be likely to drop out early on in the study due to intolerance of the intervention (Molnar 2008). Due to the high dropout rates in trials of cholinesterase inhibitors and use of LOCF, we do not know the true treatment effect, positive or negative, of these drugs on cognitive decline. We must at least consider the possibility that the effect of these drugs has been exaggerated through use of LOCF, which is resulting in the effect of the drug appearing to be similar to placebo when it is plausible that the drug may actually be worse.
One alternate approach for consideration is that used by Petersen et al. This study employed both sensitivity analyses and modelling for dropouts to analyze the effect of various modelling assumptions. This method was based on that used in an earlier study by Aisen et al. (Aisen 2003). The imputed values were calculated using an estimate of change based on individuals with complete data in the participants treatment group collected during the unobserved period and applied it to the participants last observed value. Although there is no ideal method of imputing data, this method appears to be a step in the right direction, providing a “best estimate” based on observations of those who remained in the study.
One final concern we would like to address is regarding the risk of bias in the abovementioned studies. We would suggest that the risk of bias is actually higher than that assessed by Russ et al. In the Winblad study and in the Doody study, there is no mention of how the allocation sequence was generated, which at best would provide an unclear risk of selection bias. Additionally, the justification for selection bias relating to allocation concealment is indicated as being due to the double‐blinding of the trials for Doody, Petersen and Salloway. We believe it is important to point out that blinding does not guarantee allocation concealment, as these are two separate processes each of which should be assessed individually for risk of bias. Given the elevated rates of adverse drug reaction in the active drug arm in each of these studies, we would also suggest that blinding at best, could be assessed as unclear risk of bias in each of these studies as unblinding of those involved in the study would have been highly likely.
We suggest that the risk of bias assessments be revised based on the aforementioned issues and the conclusions and implications reflect the change.
References
Aisen 2003: Aisen PS, Schafer KA, Grundman M, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA 2003;289: 2819‐26
Doody 2009: Doody RS, Ferris SH, Salloway S, Sun Y, Goldman R, Watkins WE, et al. Donepezil treatment of patients with MCI. A 48‐week randomized, placebo controlled trial. Neurology. 2009;72:1555‐61.
Higgins 2011: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Molnar 2008: Molnar FJ, Hutton B, Fergusson D. Does analysis using “last observation carried forward” introduce bias in dementia research. CMAJ. 2008; 179(8):751‐753.
Molnar 2009: Molnar FJ, Man‐Son‐Hing M, Hutton B, Fergusson D. Have last‐observation‐carried‐forward analyses caused us to favor more toxic dementia therapies over less toxic alternatives. A systematic review. Open Medicine. 2009; 3(2):31‐50.
Petersen 2005: Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment: Alzheimer’s Disease Cooperative Study Group. N Engl J Med 2005;352:2379–2388.
Salloway 2004: Salloway S, Ferris S, Kluger A, Goldman R, Griesing T, Kumar D, et al. Efficacy of donepezil in mild cognitive impairment: A randomized placebo ¬controlled trial. Neurology. 2004;63(4):651–657.
Winblad 2008: Winblad B, Gauthier S, Scinto L, Feldman H, Wilcock GK, Truyen L, et al. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology 2008;70:2024–2035.
Reply
We read this comment with interest and thank the authors for it. Our understanding is that they did not disagree with the conclusions of our review, but rather had some concerns about whether the methodology used might lead to the most accurate conclusions.
MCI is an extremely heterogeneous description and, while it will include some people with a neurodegenerative process in their brain, it will also include many people who will not decline over time. Thus, while their criticism of the use of LOCF is valid and very relevant to dementia studies, it may have less impact in a study of people with MCI where a good proportion of people could be expected to remain stable cognitively. The review is currently being updated and during this process, we will carefully consider the risk of bias related to incomplete outcome data and whether use of sensitivity analyses is feasible.
Alongside (a lack of) efficacy, the issue of side‐effects is central to the question of whether or not cholinesterase inhibitors should be prescribed in MCI and the authors point out that such adverse drug reactions may have resulted in unblinding in the treatment arms. We agree that this may have been the case and will also consider this possibility in our risk of bias assessments when updating the review.
In the meantime, we would repeat that our review does not support the prescription of cholinesterase inhibitors for patients with MCI.
Contributors
Tom Russ, Contact Author
Jenny McCleery, Co‐ordinating Editor
Rupert McShane, Co‐ordinating Editor
Acknowledgements
TR is an Alzheimer Scotland Clinical Research Fellow in the UK National Health Service (NHS) with the Scottish Dementia Clinical Research Network, funded by Alzheimer Scotland. He is a member of the Alzheimer Scotland Dementia Research Centre at the University of Edinburgh and an Associate Member of the Centre for Cognitive Ageing and Cognitive Epidemiology, University of Edinburgh. JR is funded by Diabetes UK.
Appendices
Appendix 1. MEDLINE search strategy
| MEDLINE (Ovid SP) search strategy |
| 1. "cognit* impair*".mp. 2. exp *Cognition Disorders/ 3. MCI.ti,ab. 4. ACMI.ti,ab. 5. ARCD.ti,ab. 6. SMC.ti,ab. 7. CIND.ti,ab. 8. BSF.ti,ab. 9. AAMI.ti,ab. 10. MD.ti,ab. 11. LCD.ti,ab. 12. QD.ti,ab. 13. AACD.ti,ab. 14. MNCD.ti,ab. 15. MCD.ti,ab. 16. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 17. ((cognit* or memory or cerebr* or mental*) adj3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*)).ti,ab. 18. "preclinical AD".mp. 19. "pre‐clinical AD".mp. 20. ("preclinical alzheimer*" or "pre‐clinical alzheimer*").mp. 21. (aMCI or MCIa).ti,ab. 22. ("CDR 0.5" or "clinical dementia rating scale 0.5").ti,ab. 23. ("GDS 3" or "stage 3 GDS").ti,ab. 24. ("global deterioration scale" and "stage 3").mp. 25. "Benign senescent forgetfulness".ti,ab. 26. "mild neurocognit* disorder*".ti,ab. 27. (prodrom* adj2 dement*).ti,ab. 28. or/1‐27 29. exp Cholinesterase Inhibitors/ 30. ("acetylcholinesterase inhibitor*" or "cholinesterase inhibitor*" or "anti‐cholinesteras*").mp. 31. donepezil*.mp. 32. aricept*.mp. 33. donezepil.mp. 34. exp *Galantamine/ 35. galantamin*.ti,ab. 36. galanthamin*.mp. 37. Nivalin*.mp. 38. Razadyne*.mp. 39. Reminyl*.mp. 40. rivastigmin*.mp. 41. exelon*.mp. 42. exp *Tacrine/ 43. tacrin*.ab,ti. 44. cognex*.mp. 45. ("anti‐dementia drug*" or "memory drug" or "anti‐alzheimer* ADJ2 drug*").mp. 46. or/29‐45 47. 28 and 46 48. randomized controlled trial.pt. 49. controlled clinical trial.pt. 50. random*.ab. 51. placebo.ab. 52. drug therapy.fs. 53. trial.ab. 54. groups.ab. 55. or/48‐54 56. (animals not (humans and animals)).sh. 57. 55 not 56 58. 47 and 57 |
Data and analyses
Comparison 1.
Conversion to dementia
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Conversion at 1 year | 3 | 2560 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.47, 1.00] |
| 2 Conversion at 2 years | 2 | 2048 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.55, 0.83] |
| 3 Conversion at 3 years | 2 | 1530 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.70, 1.02] |
Analysis 1.1.
Comparison 1 Conversion to dementia, Outcome 1 Conversion at 1 year.
Analysis 1.2.
Comparison 1 Conversion to dementia, Outcome 2 Conversion at 2 years.
Analysis 1.3.
Comparison 1 Conversion to dementia, Outcome 3 Conversion at 3 years.
Comparison 2.
Adverse events
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any adverse events | 5 | 4207 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [1.02, 1.16] |
| 2 Serious adverse events | 5 | 4207 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.10] |
| 3 Death | 6 | 4719 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.79, 1.46] |
| 4 Diarrhoea | 6 | 4671 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [1.30, 3.39] |
| 5 Nausea | 6 | 4671 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [2.57, 3.42] |
| 6 Vomiting | 4 | 1845 | Risk Ratio (M‐H, Random, 95% CI) | 4.42 [3.23, 6.05] |
| 7 Muscle spasms or leg cramps | 3 | 1557 | Risk Ratio (M‐H, Random, 95% CI) | 7.52 [4.34, 13.02] |
| 8 Insomnia | 6 | 4671 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.36, 2.02] |
| 9 Cardiac problems, including coronary artery disease, cardiac disorder and bradycardia | 3 | 3844 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.25, 2.02] |
| 10 Headache | 4 | 3890 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [1.05, 1.71] |
| 11 Syncope or dizziness | 4 | 3890 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [1.36, 1.93] |
| 12 Depression | 3 | 3331 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.83, 1.57] |
| 13 Abnormal dreams | 3 | 1559 | Risk Ratio (M‐H, Random, 95% CI) | 4.25 [2.57, 7.04] |
Analysis 2.1.
Comparison 2 Adverse events, Outcome 1 Any adverse events.
Analysis 2.2.
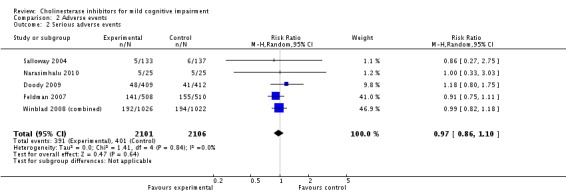
Comparison 2 Adverse events, Outcome 2 Serious adverse events.
Analysis 2.3.
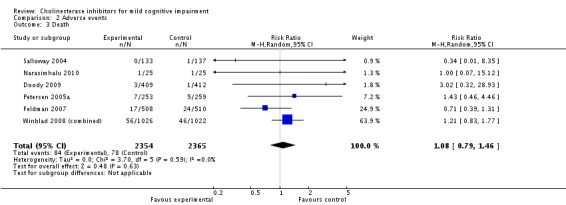
Comparison 2 Adverse events, Outcome 3 Death.
Analysis 2.4.
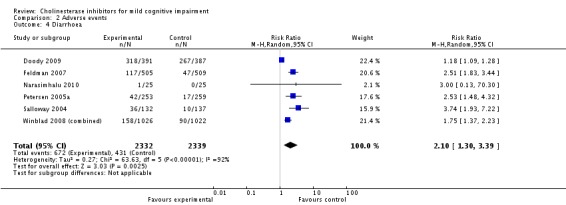
Comparison 2 Adverse events, Outcome 4 Diarrhoea.
Analysis 2.5.
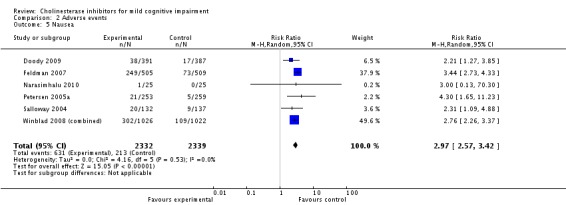
Comparison 2 Adverse events, Outcome 5 Nausea.
Analysis 2.6.
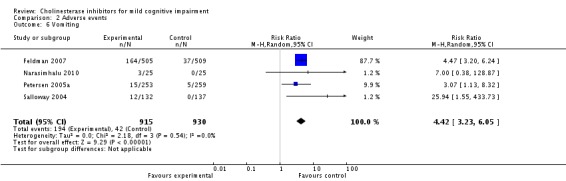
Comparison 2 Adverse events, Outcome 6 Vomiting.
Analysis 2.7.
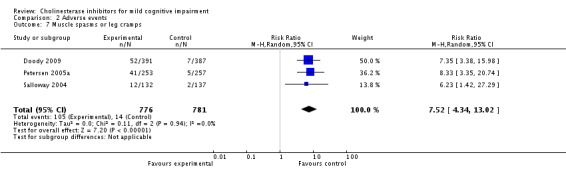
Comparison 2 Adverse events, Outcome 7 Muscle spasms or leg cramps.
Analysis 2.8.
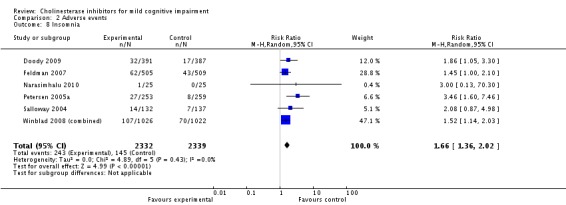
Comparison 2 Adverse events, Outcome 8 Insomnia.
Analysis 2.9.
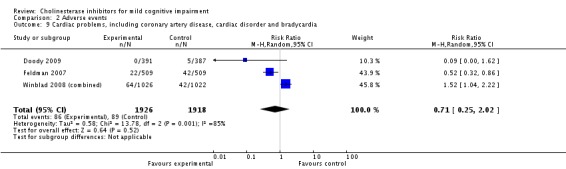
Comparison 2 Adverse events, Outcome 9 Cardiac problems, including coronary artery disease, cardiac disorder and bradycardia.
Analysis 2.10.
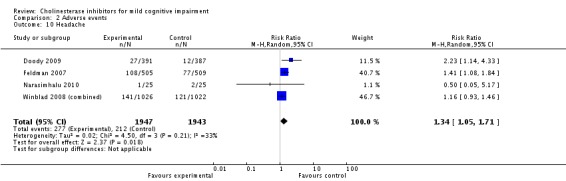
Comparison 2 Adverse events, Outcome 10 Headache.
Analysis 2.11.
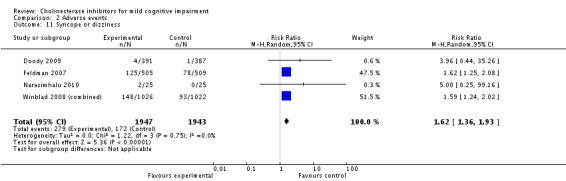
Comparison 2 Adverse events, Outcome 11 Syncope or dizziness.
Analysis 2.12.
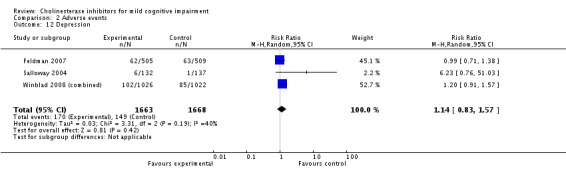
Comparison 2 Adverse events, Outcome 12 Depression.
Analysis 2.13.
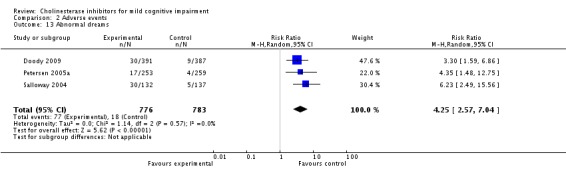
Comparison 2 Adverse events, Outcome 13 Abnormal dreams.
Comparison 3.
Cognitive test scores
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADAS‐Cog (original) at 6 months | 2 | 562 | Mean Difference (IV, Random, 95% CI) | 0.86 [‐1.77, 3.48] |
| 2 ADAS‐Cog (modified) at 1 year | 4 | 3170 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.30, ‐0.01] |
| 3 ADAS‐Cog (modified) at 2 years | 4 | 2675 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.92, 0.35] |
| 4 CDR sum of boxes at 1 year | 2 | 1269 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.11, ‐0.09] |
| 5 Symbol Digit Modalities Test at 6 months | 2 | 312 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐2.87, 3.21] |
| 6 MMSE at 1 year | 2 | 1269 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.13, 0.61] |
| 7 ADCS‐ADL at 6 months | 2 | 562 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐3.35, 2.24] |
| 8 ADCS‐ADL at 1 year | 3 | 2408 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.27, 0.57] |
Analysis 3.2.
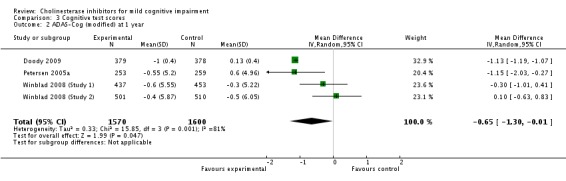
Comparison 3 Cognitive test scores, Outcome 2 ADAS‐Cog (modified) at 1 year.
Analysis 3.3.
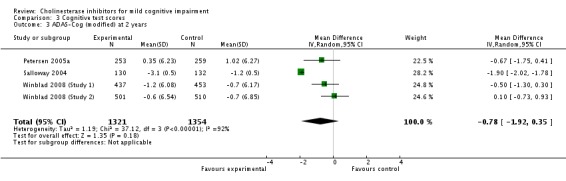
Comparison 3 Cognitive test scores, Outcome 3 ADAS‐Cog (modified) at 2 years.
Analysis 3.5.
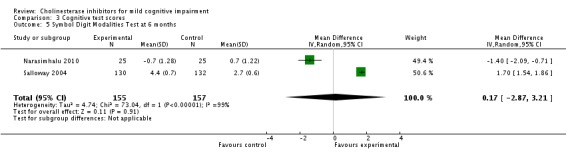
Comparison 3 Cognitive test scores, Outcome 5 Symbol Digit Modalities Test at 6 months.
Analysis 3.6.
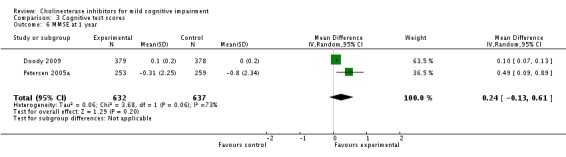
Comparison 3 Cognitive test scores, Outcome 6 MMSE at 1 year.
Analysis 3.7.
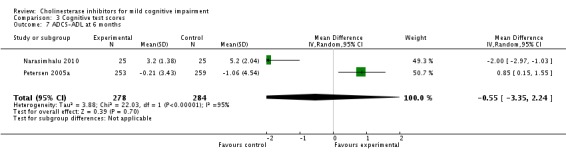
Comparison 3 Cognitive test scores, Outcome 7 ADCS‐ADL at 6 months.
Analysis 3.8.
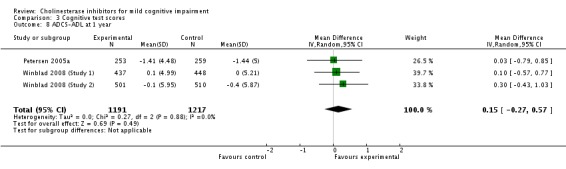
Comparison 3 Cognitive test scores, Outcome 8 ADCS‐ADL at 1 year.
What's new
Last assessed as up‐to‐date: 7 August 2012.
| Date | Event | Description |
|---|---|---|
| 4 July 2016 | Amended | This review is awaiting update |
Differences between protocol and review
The time points used for analyses of outcomes had to be modified because of the limited data presented in the published papers.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Doody 2009
| Methods | 48‐week, multicentre, double‐blind, randomised, placebo‐controlled parallel design trial | |
| Participants | Country: USA 74 sites 2037 individuals screened, aged 45 to 90 years 821 participants randomised Selection criteria: generally well, memory complaint corroborated by informant, MMSE = 24 to 28, CDR = 0.5, delayed paragraph recall (WMS‐R) below education‐matched norms, modified Hachinski ≤ 4, and CT/MRI within 12 months showing no clinically significant pathology |
|
| Interventions | 1. Donepezil 5 mg/day for 6 weeks followed by 10 mg/day 2. Placebo |
|
| Outcomes | Modified ADAS‐Cog CDR sum of boxes Symbol Digit Modalities Test MMSE Digit Span Backwards NPI Perceived Deficits Questionnaire CGIC‐MCI Patient Global Assessment |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation schedule independently generated |
| Allocation concealment (selection bias) | Low risk | Quote: "Subjects, investigators and sponsors were blinded to treatment allocation" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Subjects, investigators and sponsors were blinded to treatment allocation" Medication provided in coded containers |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Outcomes not reported for all patients randomised. 'ITT population,' 'safety population' and completers reported separately |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No additional biases |
Feldman 2007
| Methods | 48‐month, double‐blind, parallel‐group, placebo‐controlled, randomised clinical trial with 3 phases (the first being the double‐blind component) | |
| Participants | 14 countries 65 centres 1526 individuals screened 1018 individuals enrolled, aged 55 to 85 years Selection criteria: cognitive symptoms, CDR = 0.5, NYU paragraph recall < 9, modified Hachinski ≤ 4 Exclusion criteria: AD according to DSM‐IV‐TR or NINCDS‐ADRDA criteria, HAM‐D ≥13, any primary neurodegenerative disease, any advanced, severe unstable medical condition, uncontrolled seizure disorder, any history of TIA, any severe or unstable cardiovascular disease or asthma, known hypersensitivity to cholinesterase inhibitors, received cholinergic drug in the past 2 weeks, rivastigmine in the previous 4 weeks, or had previously taken part in a rivastigmine trial |
|
| Interventions | 1. Rivastigmine 0.5 mg bd increased to 1.5 mg bd after 2 weeks and subsequently increasing by a further 1.5 mg bd at a minimum of 4‐weekly intervals to a maximum of 12 mg daily (minimum 3 mg daily) 2. Placebo |
|
| Outcomes | Time to clinical diagnosis of AD Difference between groups in change from baseline in a 10‐test neuropsychological test battery: (NYU paragraph recall, delayed word list recall, letter number sequencing, Buschke selective reminding task, symbol‐digit modalities task, digit cancellation task, maze, verbal fluencies categories subtest, and clock drawing) ADAS‐Cog MMSE ADCS‐ADL BDI NPI Quality of life |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation procedure used a validated interactive voice‐response system with an automated assignment of treatment groups" |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation procedure used a validated interactive voice‐response system with an automated assignment of treatment groups" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "To preserve the blinding, the rivastigmine and placebo capsules were identical in shape, size, and colour. Study drugs were dispensed in bottles labelled with unique numbers. All personnel directly involved in the study were blind to the treatment group allocation until all patients had completed the trial and all data had been finalised for analysis" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A large number of drop‐outs were later recovered for later stages of the study after the double‐blind portion of the trial was complete |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No additional biases |
Koontz 2005
| Methods | 16‐week, single‐centre, double‐blind, randomised, placebo‐controlled, dose‐escalation study | |
| Participants | Country: USA 35 consecutive outpatients with memory problems screened 19 men, aged 51 to 87 years Selection criteria: memory complaints, normal/close to normal ADL, normal general cognitive function (MMSE ≥ 26), abnormal memory for age and no dementia (from Petersen 1995). Also: free from physical or mental conditions that could account for impaired memory, in good enough health to participate in study, living independently and not taking any cognition enhancers |
|
| Interventions | 1. Galantamine 4 mg bd increased to 8 mg bd after 1 month and 12 mg bd after 2 months 2. Placebo |
|
| Outcomes | CANTAB (delayed match to sample, paired associates learning, pattern recognition memory, spatial recognition memory, intra‐/extradimensional shift, and stockings of Cambridge tests) California Verbal learning Test FAQ |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data reported for almost all patients at third visit and 10‐12/15 at fourth visit but this is not clearly accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | High risk | No power calculation reported but likely to have been underpowered |
Narasimhalu 2010
| Methods | 24‐week investigator‐initiated, single‐centre, double‐blind, randomised placebo‐controlled trial | |
| Participants | Country: Singapore 154 individuals screened 3 months after an ischaemic stroke, aged 55 to 85 years 50 individuals randomised Selection criteria: impaired in at least 1 cognitive domain but were not considered to fulfil DSM‐IV criteria for dementia at a multidisciplinary consensus conference, neuroradiologist confirmed cerebrovascular disease was present on MRI Exclusion criteria: advanced, severe or unstable disease of any kind, major depression according to DSM‐IV, a disability that may prevent participation in the study, known sensitivity to cholinergic compounds, ingestion of drugs that could interfere with the trial in the previous 4 weeks, and ingesting metrifonate in the preceding 3 months |
|
| Interventions | 1. Rivastigmine 1.5 mg bd increasing after a minimum of 4 weeks to 3 mg bd and subsequently to 4.5 mg bd. Flexible dosing regimen apart from 4‐week minimum before increases 2. Placebo |
|
| Outcomes | Change from baseline in Clock test and Colour Trails Test Change from baseline in ADAS‐Cog and neuropsychological test battery (MMSE, cognitive battery, FAB) ADCS‐ADL scale for MCI GDS NPI |
|
| Notes | All patients had MCI following a stroke therefore are not comparable with participants in other studies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was by the pharmaceutical company's "validated system" approved by a Biostatistics Quality Assurance Group |
| Allocation concealment (selection bias) | Low risk | Quote: "A trial coordinator blind to the treatment allocation randomized each patient" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All investigators were blind to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | While only 36/50 people completed the trial, almost all were included in analyses |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No additional biases |
Peters 2012
| Methods | 2 year, multicentre, double‐blinded, placebo‐controlled study terminated early following publication of increased mortality in galantamine trials in MCI (Johnson & Johnson 2005b; Winblad 2008 (combined)) | |
| Participants | Country: Germany 12 sites 232 individuals recruited Mean (sd) age: placebo 67.2 (7.6), galantamine 67.9 (8.3), galantamine + memantine 67.3 (7.7) Selection criteria: (A) complaint about decline in cognitive ability, (B) deficits in at least one of the domains learning/memory, language, attention and visuoconstructional ability of the CERAD battery, (C) general intellectual functioning unimpaired, (D) ADLs normal (BAYER‐ADL < 4), (E) CDR = 0.5. Also: availability of a consistent informant, and sufficient visual, hearing and communication capabilities Exclusion criteria: other clinically significant medical, psychiatric, neurodegenerative or intracerebral diseases |
|
| Interventions | 1. Placebo 2. Galantamine 8 mg bd 3. Galantamine 8 mg bd + memantine 10 mg bd |
|
| Outcomes | Primary outcomes: Development of dementia ADAS‐Cog Other measures: CERAD Wechsler Memory Scale revised Clock Drawing test Trail Making Test A and B |
|
| Notes | Trial terminated early Change in ADAS‐Cog reported as median and quartiles therefore not included in meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not defined |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not outlined: "Provided all entry criteria were met, randomization occurred immediately before administration of study medication" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Investigators blind. Double‐dummy technique used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis conducted |
| Selective reporting (reporting bias) | Low risk | Does not report conversion rates to dementia but trial significantly shorter than planned |
| Other bias | Low risk | No additional biases |
Petersen 2005a
| Methods | 36‐month multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group study | |
| Participants | Countries: USA and Canada 69 sites 769 individuals enrolled, aged 55 to 90 years Selection criteria: amnestic MCI of a degenerative nature (insidious onset and gradual progression), impaired memory, logical memory delayed recall score 1.5 to 2 SD below education‐adjusted norm, CDR = 0.5, MMSE = 24 to 30 |
|
| Interventions | 1. 2000 IU vitamin E, placebo, donepezil and multivitamin (containing 15 IU vitamin E) 2. Donepezil 5 mg/day increased to 10 mg/day after 6 weeks, placebo, vitamin E and multivitamin 3. Placebo, donepezil, placebo, vitamin E and multivitamin |
|
| Outcomes | Development of possible or probably AD defined by NINCDS‐ADRDA MMSE ADAS‐Cog CDR CDR sum of boxes ADCS‐ADL for MCI GDS Neuropsychological battery (NYU paragraph recall, symbol digit modalities, category fluency, number cancellation, Boston naming, digits‐backwards, clock drawing and maze tracing tests) |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[P]atients were randomly assigned..." Quote: "We used an adaptive allocation scheme for the treatment assignment, with the MMSE score, age and APOE e4 status as balancing covariates" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "double‐blind", no additional information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double‐blind", no additional information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A large number discontinued treatment. They ascertained that the data were missing completely at random and completed a number of sensitivity analyses |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No additional biases |
Salloway 2004
| Methods | 24‐week, multi‐centre, randomised, double‐blind, placebo‐controlled, parallel group study | |
| Participants | Country: USA 22 centres 270 individual randomised, aged 55 to 89 years Selection criteria: generally healthy, living independently and capable of complying with testing procedures. Documented memory complaint, MMSE ≥ 24, CDR = 0.5, ADLs normal or only slightly impaired, delayed paragraph recall test (WMS‐R) below education‐matched norms and modified Hachinski ≤ 4 Exclusion criteria: HAM‐D > 12, possible or probably AD, drug or alcohol abuse/dependence in the last 5 years, poorly controlled diabetes or other medical conditions, or previous treatment with a cholinesterase inhibitor |
|
| Interventions | 1. Donepezil 5 mg/day increased to 10 mg after 42 days 2. Placebo |
|
| Outcomes | Change from baseline in NYU paragraph test delayed recall CGIC‐MCI Modified ADAS‐Cog WMS‐R digit span backwards test Symbol digit modalities test NYU Paragraph test immediate recall Neuropsychological test battery (Modified Boston naming, verbal fluency, maze and number cancellation tests) Patient Global Assessment |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomised in a 1:1 ratio" Quote: "double‐blind" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Efficacy analyses used last observation carried forward in 3 populations, including "observed cases" and the "fully evaluable" population |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No additional biases |
Winblad 2008 (combined)
| Methods | See below for details of study 1 and study 2 | |
| Participants | See below for details of study 1 and study 2 | |
| Interventions | See below for details of study 1 and study 2 | |
| Outcomes | See below for details of study 1 and study 2 | |
| Notes | See below for details of study 1 and study 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned, 1:1" |
| Allocation concealment (selection bias) | Low risk | Quote: "Remote interactive voice response data‐entry system" used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition dealt with by "a retrospective observational study" to identify vital status of all randomised participants: "retrieved dropout study" |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No additional biases |
Winblad 2008 (Study 1)
| Methods | 24‐month randomised, double‐blind, parallel‐group, placebo‐controlled, flexible‐dose study | |
| Participants | 16 countries 177 sites 990 individuals enrolled, aged 50 years or over Selection criteria: declining cognitive ability of gradual onset and slow progression, CDR = 0.5, CDR memory ≥ 0.5, and insufficient impairment of cognition and ADLs Delayed recall score ≤ 10 on NYU paragraph recall test |
|
| Interventions | 1. Galantamine 4 mg bd increasing to 8 mg bd after 1 month, if tolerated. At 2 months, either 8 mg bd or 12 mg bd depending on tolerance 2. Placebo |
|
| Outcomes | CDR ADAS‐Cog/MCI DSST ADCS‐ADL/MCI |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See above for comments on paper reporting studies 1 and 2 |
| Allocation concealment (selection bias) | Unclear risk | See above for comments on paper reporting studies 1 and 2 |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | See above for comments on paper reporting studies 1 and 2 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | See above for comments on paper reporting studies 1 and 2 |
| Selective reporting (reporting bias) | Unclear risk | See above for comments on paper reporting studies 1 and 2 |
| Other bias | Unclear risk | See above for comments on paper reporting studies 1 and 2 |
Winblad 2008 (Study 2)
| Methods | 24‐month randomised, double‐blind, parallel‐group, placebo‐controlled, flexible‐dose study | |
| Participants | 16 countries 177 sites 1058 individuals enrolled, aged 50 years or over Selection criteria: declining cognitive ability of gradual onset and slow progression, CDR = 0.5, CDR memory ≥ 0.5, and insufficient impairment of cognition and ADLs Delayed recall score ≤ 10 on NYU paragraph recall test |
|
| Interventions | 1. Galantamine 4 mg bd increasing to 8 mg bd after 1 month, if tolerated. At 2 months, either 8 mg bd or 12 mg bd depending on tolerance 2. Placebo |
|
| Outcomes | CDR ADAD‐Cog/MCI DSST ADCS‐ADL/MCI |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See above for comments on paper reporting studies 1 and 2 |
| Allocation concealment (selection bias) | Unclear risk | See above for comments on paper reporting studies 1 and 2 |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | See above for comments on paper reporting studies 1 and 2 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | See above for comments on paper reporting studies 1 and 2 |
| Selective reporting (reporting bias) | Unclear risk | See above for comments on paper reporting studies 1 and 2 |
| Other bias | Unclear risk | See above for comments on paper reporting studies 1 and 2 |
AD: Alzheimer's disease; ADAS‐Cog: Alzheimer's Disease Assessment Scale‐Cognitive subscale; ADCS‐ADL: Alzheimer's Disease Co‐operative Study ‐ Activities of Daily Living Inventory; ADL: activities of daily living; bd: twice daily; BDI: Beck Depression Inventory; CDR: Clinical Dementia Rating; CERAD: Consortium to Establish a Registry for Alzheimer's Disease; DSST: Digit symbol substitution test ; GDS: Geriatric Depression Scale; CGIC‐MCI: Clinical Global Impression of Change Scale for Mild Cognitive Impairment; CT: computerised tomography; DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders, 4th edition ‐ text revision; FAB: Frontal Assessment Battery; FAQ: Functional Assessment Questionnaire; GDS: Global Deterioration Scale; HAM‐D: Hamilton Depression Rating Scale; ITT: intention to treat; IU: International Units; MCI: mild cognitive impairment; MMSE: Mini Mental State Examination; MRI: magnetic resonance imaging; NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association; NPI: Neuropsychiatric Inventory; NYU: New York University; SD: standard deviation; TIA: transient ischaemic attack; WMS‐R: Wechsler Memory Scale‐Revised.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anon 2009 | Reports Doody 2009 |
| Barnes 2005 | Reports Petersen 2005a |
| Bokde 2008 | Wrong outcome |
| Chen 2006 | Wrong outcome |
| Crane 2009 | Reports Doody 2009 |
| Djalalov 2009 | Not an RCT |
| Dubois 2009 | Wrong outcome |
| E2020‐A001‐412 | Protocol for Doody 2009 |
| Ferris 2009 | Wrong outcome |
| GAL‐COG‐3002 | Not an RCT |
| GAL‐INT‐11 | Reported as Winblad 2008 (Study 1) |
| GAL‐INT‐18 | Reported as Winblad 2008 (Study 2) |
| Gold 2003 | Reports baseline characteristics of sample only. No record of results ever being published was found |
| Grundman 2000 | Published as Petersen 2005a |
| Jack 2008 | Reanalysis of Petersen 2005a |
| Johnson & Johnson 2005a | Published as Winblad 2008 (Study 1) |
| Jones 2004 | Not MCI |
| Kopchak 2010 | Author contacted: they did not use a pure sample of individuals with MCI but rather a mixture of people with 'vascular MCI' and vascular dementia |
| Lu 2009 | Reanalysis of Petersen 2005a |
| Montero‐Odasso 2009 | Wrong outcome |
| Onofrj 2003 | Not MCI |
| Ownby 2006 | Reports Petersen 2005a |
| Ozenli 2007 | Not placebo‐controlled |
| Panza 2010 | Reports Lu 2009 |
| Petersen 2005b | Reports Petersen 2005a |
| Petrella 2009 | Wrong outcome |
| Politis 2010 | Case report |
| Saykin 2004 | Not an RCT |
| Seltzer 2004 | Not MCI |
| Shua‐Haim 2001 | Not an RCT |
| Turon‐Estrada 2003 | Not an RCT |
| Vandesquille 2012 | Not human research |
| Wang 2004 | Not an RCT |
| Wang 2010 | Not an RCT |
| Yesavage 2008 | Not MCI |
MCI: mild cognitive impairment; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Study of Exelon Transdermal Patch in Amnestic Mild Cognitive Impairment Patients |
| Methods | 24‐week, Randomized, Double‐blind, Placebo‐controlled, Parallel Group Study |
| Participants | 120 APOE e4 Positive Amnestic MCI Patients 55‐85 years Men and women |
| Interventions | 1. Rivastigmine patch 2. Placebo |
| Outcomes | Change in BOLD response on Functional Magnetic Resonance Imaging |
| Starting date | June 2011 |
| Contact information | Christine Reece, B.S., CCRP (raolab@ccf.org) |
| Notes | The primary outcome of this trial ("to determine if task‐activated fMRI is sensitive to the central cholinergic deficit associated with Mild Cognitive Impairment") is not relevant to this review but they may report secondary outcomes which could be relevant. |
Contributions of authors
Tom Russ: developed and wrote the protocol, selected trials, developed data extraction tool, extracted data, assessed risk of bias, undertook analyses and wrote the review.
Joanne Morling: developed and wrote the protocol, selected trials, extracted data, assessed risk of bias and wrote the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Alzheimer Scotland, UK.
Financial support to TR
-
BBSRC, EPSRC, ESRC and MRC, UK.
TCR is a member of The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross‐council Lifelong Health and Wellbeing Initiative (G0700704/84698). Funding from the BBSRC, EPSRC, ESRC and MRC is gratefully acknowledged.
-
NIHR, UK.
This review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
- Doody RS, Ferris SH, Salloway S, Sun Y, Goldman R, Watkins WE, et al. Donepezil treatment of patients with MCI: a 48‐week randomized, placebo‐controlled trial. Neurology 2009;72:1555‐61. [DOI] [PubMed] [Google Scholar]
- Feldman HH, Ferris S, Winblad B, Sfikas N, Mancione L, He Y, et al. Effect of rivastigmine on delay to diagnosis of Alzheimer's disease from mild cognitive impairment: the InDDEx study. Lancet Neurology 2007;6:501‐12. [DOI] [PubMed] [Google Scholar]
- Koontz J, Baskys A. Effects of galantamine on working memory and global functioning in patients with mild cognitive impairment: a double‐blind placebo‐controlled study. American Journal of Alzheimer's Disease and Other Dementias 2005;20:295‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhalu K, Effendy S, Sim CH, Lee JM, Chen I, Hia SB, et al. A randomized controlled trial of rivastigmine in patients with cognitive impairment no dementia because of cerebrovascular disease. Acta Neurologica Scandinavica 2010;121:217‐24. [DOI] [PubMed] [Google Scholar]
- Peters O, Lorenz D, Fesche A, Schmidtke K, Hüll M, Perneczky R, et al. A combination of galantamine and memantine modifies cognitive function in subjects with amnestic MCI. Journal of Nutrition, Health & Aging 2012;16:544‐8. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. New England Journal of Medicine 2005;352:2379‐88. [DOI] [PubMed] [Google Scholar]
- Salloway S, Ferris S, Kluger A, Goldman R, Griesing T, Kumar D, et al. Efficacy of donepezil in mild cognitive impairment: a randomized placebo‐controlled trial. Neurology 2004;63:651‐7. [DOI] [PubMed] [Google Scholar]
- Winblad B, Gauthier S, Scinto L. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology 2008;70:2024‐35. [DOI] [PubMed] [Google Scholar]
- Winblad B, Gauthier S, Scinto L. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology 2008;70:2024‐35. [DOI] [PubMed] [Google Scholar]
- Winblad B, Gauthier S, Scinto L. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology 2008;70:2024‐35. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Anon. Study of donepezil for MCI shows only minor improvement in patients with mild cognitive impairment. Geriatric Psychopharmacology Update 2009;13(8):1. [Google Scholar]
- Barnes DE, Yaffe K, Petersen RC, Thomas RG, Thal LJ. Vitamin E and donepezil for the treatment of mild cognitive impairment. New England Journal of Medicine 2005;352:2379‐88. [DOI] [PubMed] [Google Scholar]
- Bokde A, Lopz‐Bayo P, Meindl T, Teipel SJ, Reiser M, Moller HJ, et al. Effect of rivastigmine in a double‐blind study in MCI subjects: a functional magnetic resonance imaging study. International Journal of Neuropsychopharmacology 2008;11:29‐30. [Google Scholar]
- Chen X, Magnott VA, Duff K, Boles Ponto LL, Schultz SK. Donepezil effects on cerebral blood flow in older adults with mild cognitive deficits. Journal of Neuropsychiatry & Clinical Neurosciences 2006;18(2):175‐85. [DOI] [PubMed] [Google Scholar]
- Crane PK, Doody RS. Donepezil treatment of patients with MCI: a 48‐week randomized, placebo‐controlled trial. Neurology 2009;73(18):1514‐5. [DOI] [PubMed] [Google Scholar]
- Djalalov S, Yong JHE, Saposnik G, Hoch J. Is genetic testing in combination with preventive donepezil (ARICEPT) treatment for patients with mild cognitive impairment cost‐effective? (ISPOR 14th Annual International Meeting Orlando, FL United States). Value in Health 2009;12(3):A191. [Google Scholar]
- Dubois B, Sarazin M, Lehericy S, Chupin M, Tonelli I, Garnero L, et al. Effects of donepezil on structural MRI and clinical markers in patients with amnestic mild cognitive impairment: a randomized, placebo‐controlled trial (Alzheimer's Association International Conference on Alzheimer's Disease, Vienna). Alzheimer's and Dementia 2009;5(4 Suppl 1):269. [Google Scholar]
- E2020‐A001‐412. A one year, multicenter, randomized, double‐blind, placebo‐controlled evaluation of the efficacy and safety of donepezil hydrochloride in subjects with mild cognitive impairment (MCI), 2005. www.mrw.interscience.wiley.com/cochrane/clcentral/articles/394/CN‐00519394/frame.html (accessed 3 August 2012).
- Ferris S, Darreh‐Shori T, Lane R. Effects of rivastigmine on brain white matter volume in mild cognitive impairment (MCI) (Alzheimer's Association International Conference on Alzheimer's Disease, Vienna). Alzheimer's and Dementia 2009;5(4 Suppl 1):332. [Google Scholar]
- GAL‐COG‐3002. An analysis of mortality in subjects who participated in three studies of galantamine in mild cognitive impairment (MCI), 2005. clinicaltrials.gov/ct2/show/NCT00297414 (accessed 3 August 2012).
- GAL‐INT‐11. A randomised placebo‐controlled trial to evaluate the efficacy and safety of galantamine in patients with minimal cognitive impairment (MCI) clinically at risk for development of probable Alzheimer's disease, 2003. www.mrw.interscience.wiley.com/cochrane/clcentral/articles/132/CN‐00452132/frame.html (accessed 3 August 2012).
- GAL‐INT‐18. A randomized double‐blind, placebo‐controlled trial to evaluate the efficacy and safety of galantamine in subjects with mild cognitive impairment (MCI) clinically at risk for development of clinically probable Alzheimer's disease, 2004. www.mrw.interscience.wiley.com/ cochrane/clcentral/articles/227/CN‐00744227/frame.html.
- Gold M, Wang D, Truyen L. Galantamine for the treatment of mild cognitive impairment: 2 double‐blind, placebo‐controlled studies. International Psychogeriatrics 2003;15(Suppl 2):259. [Google Scholar]
- Grundman M. MCI trial with vitamin E and donepezil. Proceedings of the 6th International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy, Stockholm, 2000. www.mrw.interscience.wiley.com/cochrane/clcentral/articles/212/CN‐00404212/frame.html (accessed 3 August 2012).
- Jack CR, Petersen RC, Grundman M, Jin S, Gamst A, Ward CP, et al. Longitudinal MRI findings from the Vitamin E and Donepezil Treatment Study for MCI. Neurobiology of Aging 2008;29(9):1285‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson & Johnson Pharmaceutical Research and Development. A randomized double blind placebo‐controlled trial to evaluate the efficacy and safety of galantamine in patients with mild cognitive impairment (MCI) clinically at risk for development of clinically probable Alzheimer's disease, 2005. clinicaltrials.gov/ct2/show/NCT00236574 (accessed 3 August 2012).
- Jones W, Soininen H, Hager K, Aarsland D, Passmore P, Murthy A, et al. A multinational, randomised, 12‐week study comparing the effects of donepezil and galantamine in patients with mild to moderate Alzheimer's disease. International Journal of Geriatric Psychiatry 2004;19(1):58‐67. [DOI] [PubMed] [Google Scholar]
- Kopchak O. Efficacy of citicoline in the treatment of patients with vascular cognitive impairment (20th Meeting of the European Neurological Society, Berlin). Journal of Neurology 2010;257:S128. [Google Scholar]
- Lu PH, Edland SD, Teng E, Tingus K, Petersen RC, Cummings JL. Donepezil delays progression to AD in MCI subjects with depressive symptoms. Neurology 2009;72(24):2115‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero‐Odasso M, Wells JL, Borrie MJ, Speechley M. Can cognitive enhancers reduce the risk of falls in older people with mild cognitive impairment? A protocol for a randomised controlled double blind trial. BMC Neurology 2009;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onofrj M, Thomas A, Iacono D, Lucino AL, Iorio A. The effects of a cholinesterase inhibitor are prominent in patients with fluctuating cognition: a part 3 study of the main mechanism of cholinesterase inhibitors in dementia. Clinical Neuropharmacology 2003;26(5):239‐51. [DOI] [PubMed] [Google Scholar]
- Ownby R. Donepezil and vitamin E for mild cognitive impairment. Current Psychiatry Reports 2006;8(1):9. [PubMed] [Google Scholar]
- Ozenli Y, Yagci D, Karaca S. Efficacy of donepezil on cognitive functions in mild cognitive impairment. Klinik Psikofarmakoloji Bulteni 2007;17(2):62‐7. [Google Scholar]
- Panza F, Frisardi V, Capurso C, D'Introno A, Colacicco AM, Chiloiro R, et al. Effect of donepezil on the continuum of depressive symptoms, mild cognitive impairment, and progression to dementia. Journal of the American Geriatrics Society 2010;58(2):389‐90. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Thomas RG, Thal LJ. "Vitamin E and donepezil for the treatment of mild cognitive impairment": the authors reply. New England Journal of Medicine 2005;353(9):951‐2. [DOI] [PubMed] [Google Scholar]
- Petrella JR, Prince SE, Krishnan S, Husn H, Kelley L, Doraiswamy PM. Effects of donepezil on cortical activation in mild cognitive impairment: a pilot double‐blind placebo‐controlled trial using functional MR imaging. American Journal of Neuroradiology 2009;30(2):411‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politis AM, Theleritis C, Passa M, Maillis A, Papadimitriou GN. Improvement of cognitive dysfunction with high‐dose rivastigmine. Psychosomatics 2010;51(1):89‐90. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, Flashman LA, McHugh TL, Mamourian AC, et al. Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain 2004;127(7):1574‐83. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Zolnouni P, Nunez M, Goldman R, Kumar D, Ieni J, et al. Efficacy of donepezil in early‐stage Alzheimer disease: a randomized placebo‐controlled trial. Archives of Neurology 2004;61(12):1852‐56. [DOI] [PubMed] [Google Scholar]
- Shua‐Haim JR, Shua‐Haim V, Comsti E. Donepezil in the treatment of age‐acquired cognitive deficit. Clinical Geriatrics 2001;9(9):35. [Google Scholar]
- Turon‐Estrada A, Lopez‐Pousa S, Gelada‐Batlle E, Garre‐Olmo J, Lozano‐Gallego M, Hernandez‐Ferrandiz M, et al. Tolerance and adverse events of treatment with acetylcholinesterase inhibitors in a clinical sample of patients with very slight and mild Alzheimer's disease over a six‐month period [Tolerancia y acontecimientos adversos del tratamiento con inhibidores de la acetilcolinesterasa en una muestra clínica de pacientes con enfermedad de Alzheimer de gravedad mínima y leve durante un perÍodo de seis meses]. Revista de Neurologia 2003;36:421‐4. [PubMed] [Google Scholar]
- Vandesquille M, Chauveau F, Decros‐Tesolin B, Pierard C, Sebban S, Mouthon F, et al. Potentiation of donepezil efficiency profile by connexin inhibitors in age‐related cognitive impairment. European Neuropsychopharmacology Conference: 2012 ECNP Workshop on Neuropsychopharmacology for Young Scientists in Europe, Nice, France. 2012. [Google Scholar]
- Wang LN, Wang W, Zhang XH, Ma L, Yin H, Li DJ. An interventional study on amnestic mild cognitive impairment with small dose donepezil. Zhonghua Nei Ke Za Zhi 2004;43(10):760‐3. [PubMed] [Google Scholar]
- Wang L, Harms MP, Staggs JM, Xiong C, Morris JC, Csernansky JG, et al. Donepezil treatment and changes in hippocampal structure in very mild Alzheimer disease. Archives of Neurology 2010;67:99‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Friedman L, Ashford JW, Kraemer HC, Mumenthaler MS, Noda A, et al. Acetylcholinesterase inhibitor in combination with cognitive training in older adults. Journals of Gerontology Series B: Psychological Sciences & Social Sciences 2008;63B(5):P288‐94. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- 24‐week, randomized, double‐blind, placebo‐controlled, parallel group study of the Exelon® [rivastigmine] transdermal patch in 120 APOE e4 positive amnestic MCI patients, 2011. http://clinicaltrials.gov/ct2/show/NCT01602198 (accessed 3 August 2012).
Additional references
- Agrell B, Dehlin O. The clock‐drawing test. Age and Ageing 1998;27:399‐403. [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois, B, Feldman, HH, Fox, NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia 2011;7:270‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD005593] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks J, Harvey RJ. Donepezil for dementia due to Alzheimer's disease. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD001190.pub2] [DOI] [PubMed] [Google Scholar]
- Birks J, Grimley Evans J, Iakovidou V, Tsolaki M. Rivastigmine for Alzheimer's disease. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD001191.pub2] [DOI] [PubMed] [Google Scholar]
- British Medical Association and the Royal Pharmaceutical Society of Great Britain. British National Formulary No. 63. British National Formulary, 63rd Edition. London: BMJ Publishing Group, March 2012. [Google Scholar]
- Braak H, Braak E. Neuropathological Stageing of Alzheimer‐related changes. Acta Neuropathologica 1991;82:239‐59. [DOI] [PubMed] [Google Scholar]
- D'Elia. Color Trails Test: Professional Manual. PAR, 1996. [Google Scholar]
- Dawe B, Procter A, Philpot M. Concepts of mild memory impairment in the elderly and their relationship to dementia ‐ a review. International Journal of Geriatric Psychiatry 1992;7(7):473‐9. [Google Scholar]
- Diniz BS, Pinto JA, Gonzaga MCG. To treat or not to treat? A meta‐analysis of the use of cholinesterase inhibitors in mild cognitive impairment for delaying progression to Alzheimer’s disease. European Archives of Psychiatry and Clinical Neuroscience 2009;259:248‐56. [DOI] [PubMed] [Google Scholar]
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005;366:2112‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini‐Mental State". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189‐98. [DOI] [PubMed] [Google Scholar]
- Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Disease and Associated Disorders 1997;11(Suppl 2):S33‐9. [PubMed] [Google Scholar]
- Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, et al. Mild cognitive impairment. Lancet 2006;367:1262‐70. [DOI] [PubMed] [Google Scholar]
- Heinik J. V. A. Kral, the Montreal Hebrew Old People's Home, and benign senescent forgetfulness. History of Psychiatry 2006;17:313‐32. [DOI] [PubMed] [Google Scholar]
- Heinik J. V. A. Kral and the origins of benign senescent forgetfulness and mild cognitive impairment. International Psychogeriatrics 2010;22:395‐402. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Johnson & Johnson Pharmaceutical Research & Development, L.L.C. An analysis of mortality in subjects who participated in three studies of galantamine in mild cognitive impairment. CR004240.
- Kluger A, Ferris SH, Golomb G, Mittelman MS, Reisberg B. Neuropsychological prediction of decline to dementia in non‐demented elderly. Journal of Geriatric Psychiatry and Neurology 1999;12:168‐79. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. Oxford University Press, 2004. [Google Scholar]
- Loy C, Schneider L. Galantamine for Alzheimer's disease and mild cognitive impairment. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD001747.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65(12):1863‐72. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34(7):939‐44. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging – Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia 2011;7:263‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohs RC, Knopman D, Petersen RC, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. The Alzheimer’s Disease Cooperative Study. Alzheimer Disease and Associated Disorders 1997;11(Suppl 2):S13‐21. [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412‐4. [DOI] [PubMed] [Google Scholar]
- O'Brien JT. Mild cognitive impairment. In: Jacoby R, Oppenheimer C, Dening T, Thomas A editor(s). Oxford Textbook of Old Age Psychiatry. Oxford: Oxford University Press, 2008. [Google Scholar]
- Office for National Statistics. Key Population and Vital Statistics. Basingstoke: Palgrave Macmillan, 2006. [Google Scholar]
- Petersen RC. Normal aging, mild cognitive impairment, and early Alzheimer's disease. Neurologist 1995;1:326‐44. [Google Scholar]
- Petersen RC, Amith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology 1999;56:303‐8. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, Boeve BF, et al. Neuropathologic features of amnestic mild cognitive impairment. Archives of Neurology 2006;63:665‐72. [DOI] [PubMed] [Google Scholar]
- Raschetti R, Albanese E, Vanacore N, Maggini M. Cholinesterase inhibitors in mild cognitive impairment: a systematic review of randomised trials. PLoS Medicine 2007;4(11):e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid LM, MacLullich AMJ. Subjective memory complaints and cognitive impairment in older people. Dementia and Geriatric Cognitive Disorders 2006;22:471‐85. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
- Ritchie K. Mild cognitive impairment: an epidemiological perspective. Dialogues in Clinical Neuroscience 2004;6:401‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS‐AIREN International Workshop. Neurology 1993;43(2):250‐60. [DOI] [PubMed] [Google Scholar]
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. American Journal of Psychiatry 1984;141(11):1356‐64. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Morris RG, Evenden JL, Heald A, Levy R, Philpot M, et al. A comparative study of visuospatial memory and learning in Alzheimer‐type dementia and Parkinson's disease. Brain 1988;111(3):695‐718. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, et al. Validity and reliability of the Alzheimer's Disease Cooperative Study‐Clinical Global Impression of Change. The Alzheimer's Disease Cooperative Study. Alzheimer Disease and Associated Disorders 1997;11(Suppl 2):S22‐32. [DOI] [PubMed] [Google Scholar]
- Sobów T, Kłoszewska I. Cholinesterase Inhibitors in mild cognitive impairment: a meta‐analysis of randomized controlled trials [Inhibitory cholinesterazy w łagodnych zaburzeniach poznawczych: metaanaliza badań z randomizacją i z grupą kontrolną otrzymującą placebo]. Neurologia i Neurochirurgia Polska 2007;41:13‐21. [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment – beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine 2004;256:240‐6. [DOI] [PubMed] [Google Scholar]


