Abstract
Background
Rupture of the anterior cruciate ligament (ACL) is a common injury, mainly affecting young, physically active individuals. The injury is characterised by joint instability, leading to decreased activity, which can lead to poor knee‐related quality of life. It is also associated with increased risk of secondary osteoarthritis of the knee. It is unclear whether stabilising the knee surgically via ACL reconstruction produces a better overall outcome than non‐surgical (conservative) treatment.
Objectives
To assess the effects of surgical versus conservative interventions for treating ACL injuries.
Search methods
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (18 January 2016), the Cochrane Central Register of Controlled Trials (2016, Issue 1), MEDLINE (1946 to January Week 1 2016), MEDLINE In‐Process & Other Non‐Indexed Citations (18 January 2016), EMBASE (1974 to 15 January 2016), trial registers (February 2016) and reference lists.
Selection criteria
We included randomised controlled trials that compared the use of surgical and conservative interventions in participants with an ACL rupture. We included any trial that evaluated surgery for ACL reconstruction using any method of reconstruction, type of reconstruction technique, graft fixation or type of graft.
Data collection and analysis
Three review authors independently screened all titles and abstracts for potentially eligible studies, for which we then obtained full‐text reports. Two authors then independently confirmed eligibility, extracted data and assessed the risk of bias using the Cochrane 'Risk of bias' tool. We used the GRADE approach to assess the overall quality of the evidence.
Main results
We identified one study in which 141 young, active adults with acute ACL injury were randomised to either ACL reconstruction followed by structured rehabilitation (results reported for 62 participants) or conservative treatment comprising structured rehabilitation alone (results reported for 59 participants). Built into the study design was a formal option for subsequent (delayed) ACL reconstruction in the conservative treatment group, if the participant requested surgery and met pre‐specified criteria.
This study was deemed at low risk of selection and reporting biases, at high risk of performance and detection biases because of the lack of blinding and at unclear risk of attrition bias because of an imbalance in the post‐randomisation exclusions. According to GRADE methodology, the overall quality of the evidence was low across different outcomes.
This study identified no difference in subjective knee score (measured using the average score on four of the five sub‐scales of the KOOS score (range from 0 (extreme symptoms) to 100 (no symptoms)) between ACL reconstruction and conservative treatment at two years (difference in KOOS‐4 change from baseline scores: MD ‐0.20, 95% confidence interval (CI) ‐6.78 to 6.38; N = 121 participants; low‐quality evidence), or at five years (difference in KOOS‐4 final scores: MD ‐2.0, 95% CI ‐8.27 to 4.27; N = 120 participants; low‐quality evidence). The total number of participants incurring one or more complications in each group was not reported; serious events reported in the surgery group were predominantly surgery‐related, while those in conservative treatment group were predominantly knee instability. There were also incomplete data for total participants with treatment failure, including subsequent surgery. In the surgical group at two years, there was low‐quality evidence of far fewer ACL‐related treatment failures, when defined as either graft rupture or subsequent ACL reconstruction. This result is dominated by the uptake by 39% (23/59) of the participants in the conservative treatment group of ACL reconstruction for knee instability at two years and by 51% (30/59) of the participants at five years. There was low‐quality evidence of little difference between the two groups in participants who had undergone meniscal surgery at anytime up to five years. There was low‐quality evidence of no clinically important between‐group differences in SF‐36 physical component scores at two years. There was low‐quality evidence of a higher return to the same or greater level of sport activity at two years in the ACL reconstruction group, but the wide 95% CI also included the potential for a higher return in the conservative treatment group. Based on an illustrative return to sport activities of 382 per 1000 conservatively treated patients, this amounts to an extra 84 returns per 1000 ACL‐reconstruction patients (95% CI 84 fewer to 348 more). There was very low‐quality evidence of a higher incidence of radiographically‐detected osteoarthritis in the surgery group (19/58 (35%) versus 10/55 (18%)).
Authors' conclusions
For adults with acute ACL injuries, we found low‐quality evidence that there was no difference between surgical management (ACL reconstruction followed by structured rehabilitation) and conservative treatment (structured rehabilitation only) in patient‐reported outcomes of knee function at two and five years after injury. However, these findings need to be viewed in the context that many participants with an ACL rupture remained symptomatic following rehabilitation and later opted for ACL reconstruction surgery. Further research, including the two identified ongoing trials, will help to address the limitations in the current evidence, which is from one small trial in a young, active, adult population.
Plain language summary
Surgical versus conservative interventions for treating anterior cruciate ligament injuries
Background
Rupture of the anterior cruciate ligament (ACL) in the knee is a common injury in young, active individuals. It often results in an unstable knee that increases the risk of further knee damage, such as to the knee meniscii. Anterior cruciate ligament injuries in athletic individuals are often treated surgically. Surgery usually entails ACL reconstruction, that involves removing the torn ligament and replacing it with a tendon graft, often taken from another part of the patient's knee. Conservative (non‐surgical) interventions are also used as treatment for this injury. This usually takes the form of a progressive rehabilitation programme that includes exercises aimed at improving strength and balance. We aimed to assess the effects of surgical versus conservative interventions for treating ACL injuries.
Results of the search
We performed a systematic literature search (up to 18 January 2016) for studies that compared surgery and conservative interventions for treating ACL injuries. This review identified one study of 121 young, active adults with an ACL injury in the preceding four weeks. The study compared surgery (ACL reconstruction followed by structured rehabilitation) with conservative treatment (structured rehabilitation alone).
Key results
The study found there was no difference between surgery and conservative treatment in patient‐reported knee scores at two or five years. The study failed to report on the number of participants in each group who had any type of serious or non‐serious complications. However, surgery‐related complications included three cases of graft rupture in the surgery group and several participants of the conservative treatment group had unstable knees. Twenty‐three of the 59 participants in the conservative treatment group (39%) had either reconstruction of the ACL or repair of a meniscus tear within two years and 30 (51%) underwent surgery within five years. There was some evidence that similar numbers of participants in the two groups had surgical treatment of knee meniscal injuries at five years. There was very low‐quality evidence that more participants in the surgery group had damage to the knee that could mean that they were at greater risk of developing osteoarthritis.
Quality of the evidence
The quality of the evidence was limited by the availability of data from only one study. The study was also at high risk of bias because the clinicians and participants were not blinded to their treatment. Overall, the quality of the evidence was low, which means that we are uncertain of the study findings and further research may provide evidence that could change our conclusions.
Conclusions
In young, active adults being treated for acute ACL injury, we found no difference between surgery and conservative treatment in patient‐reported outcomes of knee function at two and five years. However, many participants with an ACL rupture had unstable knees after structured rehabilitation and opted to have surgery later on.
Summary of findings
for the main comparison.
| ACL reconstruction followed by structured rehabilitation compared with conservative treatment comprising structured rehabilitation alone for treating anterior cruciate ligament (ACL) injuries | ||||||
|
Patient or population: Adults with acute anterior cruciate ligament (ACL) injuries (young, active individuals) Settings: Presentation to hospital emergency department Intervention: ACL reconstruction (within 10 weeks of injury) followed by structured rehabilitation Comparison: Conservative treatment (comprising structured rehabilitation)1 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conservative treatment1 | ACL reconstruction2 | |||||
| Subjective knee score ‐ KOOS‐4 score (mean score of 4 components) at 2 years: score from 0 (extreme symptoms) to 100 (no symptoms) | The mean change in KOOS‐4 score from baseline in the conservative treatment group was 39.4 | The mean change from baseline in KOOS‐4 score in the ACL reconstruction group was 0.2 lower (6.78 lower to 6.38 higher) | 121 (1 study) | ⊕⊕⊝⊝ low3 | Knee injury and Osteoarthritis Outcome Score (KOOS). A similar lack of statistical and clinically important difference was found in the final KOOS‐4 scores at five years (MD ‐2.00, 95% CI ‐8.27 to 4.27) |
|
| Serious adverse events (such as donor site morbidity, failure of graft including re‐rupture) at 2 years | Not estimable4 | 121 (1 study) | Total participants with adverse events not reported. | |||
| Treatment failure (graft rupture or ACL reconstruction) at 2 years5 | 390 per 1000 |
47 per 1000 (16 to 133) |
RR 0.12 (0.04 to 0.34) |
121 (1 study) | ⊕⊕⊝⊝ low6 | Total participants with treatment failure (including re‐operation) not reported. In terms of applicability, consideration should be given to the study design of the trial with its inbuilt option of delayed ACL reconstruction for instability. |
| Treatment failure (meniscal surgery) at 5 years5,7 | 542 per 1000 | 477 per 1000 (11 to 678) | RR 0.88 (0.62 to 1.25) | 120 (1 study) | ⊕⊕⊝⊝ low8 | Meniscal surgery may or may not be carried out during the same operation as ACL reconstruction. |
| General health‐related quality of life: SF‐36 physical component score (range 0 to 100; higher score = better health state) at 2 years | The mean SF‐36 physical component score in the conservative group of the study was 78.0 | The mean SF‐36 physical component score in the ACL reconstruction group was 4.1 higher (2.76 lower to 10.96 higher) | 121 (1 study) | ⊕⊕⊝⊝ low3 | ||
| Return to activity or level of sport at 2 years | 356 per 1000 | 435 per 1000 (278 to 680) | RR 1.22 (0.78 to 1.91) | 121 (1 study) | ⊕⊕⊝⊝ low9 | There was also no difference in Tegner activity scores reported10 |
| Osteoarthritis at 5 years | 182 per 1000 | 328 per 1000 (168 to 641) | RR 1.80 (0.92 to 3.52) | 113 (1 study) | ⊕⊕⊕⊝ very low11 | Radiographic assessment |
|
*The assumed risk is based on data for the conservative treatment group of the only included study. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Conservative treatment comprising structured rehabilitation alone. Built into the study design was a formal option for subsequent (delayed) ACL reconstruction, if chosen by the participant and if they met pre‐specified criteria. Surgery (partial resection or fixation) for meniscal injuries when indicated by MRI and clinical findings.
2 ACL reconstruction followed by structured rehabilitation. Surgery (partial resection or fixation) for meniscal injuries when indicated by MRI, clinical and surgical findings.
3 The quality of the evidence was downgraded one level because of risk of bias reflected by lack of blinding of outcome assessment and one level for imprecision because there was just one small trial.
4 Total numbers of participants incurring serious or non‐serious adverse events were not reported. In addition, low frequency events (< 5% overall or < 3% in a treatment group) were not included. The largest groups of listed complications relating to the index knee were 'subjective or clinical instability' (2/62 versus 19/59) and 'meniscal signs and symptoms' (2/62 versus 13/59); both of these were less frequent in the ACL reconstruction group. Other complications including non‐serious adverse events and mainly surgery‐related, were more common in the ACL reconstruction group; e.g. graft rupture (3/62 versus 1/59).
5 Definition of treatment failure split into two, both are knee stability‐related. The first links with ACL surgery and the second with meniscal surgery. Of the 3 participants in the ACL reconstruction group with ACL graft rupture, 2 underwent revision ACL reconstruction. At two years, 23/59 participants in the conservative group underwent ACL reconstruction; at 5 years, 30/59 had undergone ACL reconstruction.
6 The quality of the evidence was downgraded one level because of risk of bias reflected by lack of blinding of outcome assessment and one level for imprecision because there was just one small trial. We have not downgraded for indirectness, but these results need to be considered in the context of participants in the conservative treatment group who had the option of ACL reconstruction for instability.
7 Surgery for meniscal injuries was resection or fixation. There was a) initial surgery, when indicated by MRI findings and clinical signs in both groups, and during surgery in the ACL reconstruction group; and b) subsequently during follow‐up. Only data for the number of participants having meniscal surgery by 5 years were available. Fifteen participants of the conservative treatment group had meniscal surgery only.
8 The quality of the evidence was downgraded one level because of risk of bias reflected by lack of blinding of outcome assessment and one level for imprecision because there was just one small trial. We have not downgraded for indirectness, but these results need to be considered in the context of the potential for knee damage to the other knee and decisions for surgery.
9 The quality of the evidence was downgraded one level because of risk of bias reflected by lack of blinding of outcome assessment and one level for imprecision because there was just one small trial. We have not downgraded for indirectness, but these results are based on the Tegner activity scale, which may not quite represent what actually happened in practice.
10 There was no difference in return to activity level at two years (measured using the Tegner Activity Scale) between ACL reconstruction (median 6.5; IQR 3 to 8) and conservative treatment (median 5; IQR 4 to 7).
11 The quality of the evidence was downgraded one level because of risk of bias reflected by lack of blinding of outcome assessment, one level for imprecision because there was just one small trial and one level for indirectness, given the uncertain link between symptomatic and radiographic OA.
Background
Description of the condition
The anterior cruciate ligament (ACL) of the knee joint plays an essential role in both static (standing or squatting) and dynamic (walking or running) knee stability. Primarily, the ACL prevents anterior translation (forward movement) of the tibia relative to the femur in the sagittal (antero‐posterior) plane, aiding stabilisation of the joint from a flexed (bent) to a near full extension (straight) position of the knee.
Rupture of the ACL is a common injury, mainly affecting young, physically active individuals, with an estimated 200,000 ACL ruptures per year in the United States (Spindler 2008). The ACL is often injured during sporting activities, such as football, skiing and basketball (Bahr 2003). In over 70% of cases, the injury is caused by a non‐contact mechanism, such as sudden deceleration combined with changing direction or pivoting or landing with the knee in nearly full extension after a jump (Hernandez 2006). Contact (traumatic) mechanisms of injury usually involve a translational force applied to the anterior aspect of a fixed lower leg (Hewett 2006). The acute injury is frequently characterised by knee pain and an audible ‘popping’ sound at the time of injury. The injured person presents with knee pain, swelling, haemarthrosis (bleeding into the joint space), instability with further activity and painful range of motion.
In approximately 10% of cases, the ACL injury occurs in isolation. In the majority of cases, however, it is combined with other injuries, typically to the collateral ligaments, subchondral bone and meniscii (Bowers 2005; Hernandez 2006; Miyasaka 1991).
Diagnostic imaging, including magnetic resonance imaging (MRI), is used to confirm the diagnosis of ACL injury or rupture, and evaluate associated pathology such as articular cartilage injury, and meniscal and associated ligamentous tears; all of which play a role in maintaining stability of the knee (Crawford 2007).
Chronic ACL injury can have a profound effect on the knee kinematics (movements) of those affected. Common problems include recurrent knee instability (giving way) and symptoms of associated meniscal or articular cartilage damage, such as intermittent swelling or a locking sensation (Hernandez 2006). Furthermore, the injury can lead to poor reported quality of life (Spindler 2008), and decreased activity levels (Thorstensson 2009). It is also associated with increased risk of secondary osteoarthritis of the knee, irrespective of treatment (Øiestad 2009; Rout 2013). These related morbidities have been shown to be associated with high healthcare expenditure (Frobell 2010a).
Description of the intervention
Surgical treatment for ACL rupture has evolved from simple repair using suturing or suturing with some sort of augmentation to ACL reconstruction, which involves reconstruction of the ligament using a substitute graft of tendon or ligament, fixed into position in pre‐prepared drill holes. ACL reconstruction is increasingly performed as an arthroscopic procedure. Of those who undergo surgical reconstruction, 94% are performed within one year of the initial injury (Collins 2013). Anterior cruciate ligament reconstruction is the predominant method of surgery in current practice and hundreds of thousands of these operations are carried out each year.
Three types of grafts are commonly used: those from the patient's body (autograft), cadaveric human donors (allograft) or a synthetic ligament substitute. Commonly, the hamstring tendons of semitendinosus and gracilis are harvested from the limb of the ruptured ACL; this graft is removed during the reconstruction operation. Alternatively, a bone‐patella tendon‐bone (BPTB) construct uses a section of the middle of the patella (kneecap) tendon with bone at either end. The relative merits of hamstring and BPTB grafts have been reviewed (Mohtadi 2011).
Conservative (non‐surgical) treatment for people with an ACL rupture can include the use of cryotherapy (ice), continuous passive motion (movement of the joint by a machine), restrictive bracing, electrotherapy (muscle stimulation) and exercises aimed at strengthening and balance. The use of plaster casts for initial immobilisation of the knee is rare nowadays (Linko 2009).
Rehabilitation regimens used for both treatment options commonly use a three‐stage progressive programme: acute, recovery and functional phases (Micheo 2010). The acute stage following injury, or immediately after surgery, aims to restore range of motion and resolve inflammation. The recovery phase is from approximately three to six weeks, with the aim of improving lower limb muscle strength and functional stability. Finally, the functional stage of rehabilitation (from six weeks onwards) concentrates on returning the individual to previous levels of activity and decreasing the risk of re‐injury (Kvist 2004). There is little consensus over the most effective rehabilitation protocol for achieving these aims (Negus 2012).
Whilst surgical interventions have become commonplace for athletic individuals, initial non‐operative (conservative) treatments, based on physiotherapy, are used more commonly in the general population (Linko 2009).
How the intervention might work
All treatments aim to reduce knee pain and instability and restore function. Reconstructive surgery aims to restore stability to the knee by replacing the torn ACL. In comparison, conservative treatments, such as rehabilitation, aim to improve the muscle function around the knee and to substitute the function of the missing ACL. However, the optimal management strategy following rupture of the ACL remains controversial. In the short term, reconstructive surgery may improve knee function for those experiencing severe instability in activity or repeated episodes of ‘giving way’, or both. However, all surgery involves an increased risk of complications, such as infection. In particular, for reconstruction using autograft, significant donor site morbidity can occur, including anterior knee pain with BPTB grafts and pain and weakness of knee flexors with hamstring grafts (Mohtadi 2011; Spindler 2004).
Although studies of conservative treatment have demonstrated satisfactory results with patients returning to pre‐injury activity level (Frobell 2013; Kostogiannis 2007; Linko 2009;), the long‐term results, in particular relating to the development of early onset osteoarthritis, are still debatable. Controversy exists about ongoing instability and the possibility of secondary joint damage and early osteoarthritis (Smith 2014). Radiographically diagnosed osteoarthritis has been reported in 20% to 50% of ACL‐deficient knees at 10 years post injury compared with 5% in uninjured knees (Ajuied 2013; Lohmander 2007; Øiestad 2009). However, surgery has not been shown to offer protection against long‐term degenerative change (Øiestad 2009; Rout 2013). Moreover, recent studies have suggested structured neuromuscular rehabilitation might provide effective recovery following ACL rupture without increasing the risks of long‐term degenerative change (Delincé 2012).
Why it is important to do this review
The management of ACL injuries includes both reconstructive surgery and conservative treatments. It is unclear whether stabilising the knee surgically produces any benefit for the knee compared with conservative intervention. The previous Cochrane review in this area found that there was insufficient evidence from two trials to determine whether surgery, involving repair, or conservative management was superior for the treatment of ACL rupture, and highlighted the need for good quality randomised controlled trials (RCTs) of current practice, particularly ACL reconstruction (Linko 2009). Surgical practice has also changed in terms of the population, with an increasing number of ACL reconstructions being performed on a young athletic (adolescent) cohort (Ramski 2013). These point to the need for a systematic review of the evidence from randomised trials that compare the effects of current surgical and conservative treatment methods for ACL rupture.
Objectives
To assess the effects (benefits and harms) of surgical versus conservative interventions for treating anterior cruciate ligament injuries.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials that compared surgical and conservative interventions for treating anterior cruciate ligament injuries.
Types of participants
We included participants of any age (thus, including children) with anterior cruciate ligament rupture. Ideally, diagnosis had been made with positive clinical examination and either a positive MRI or a positive examination under anaesthesia (EUA).
We excluded studies whose prime focus was on the management of ACL and a concomitant knee ligament rupture (e.g. medial collateral ligament). We excluded people with inflammatory arthropathy or end stage osteoarthritis (Grade 4 Kellgren and Lawrence). However, if identified, we would have included mixed population trials if they included only a small proportion (preferably less than 10% and preferably balanced between intervention groups) of participants with other major knee ligament or cartilage lesions, or if separate data were provided for participants without these additional injuries.
Types of interventions
The interventions being compared were surgery and conservative treatment for ACL rupture. We included any trial that evaluated surgery that involved ACL reconstruction. Thus, we included any method of reconstruction (e.g. open or arthroscopic), any type of reconstruction technique (e.g. single or double bundle) or graft fixation and any type of graft. Direct repair of the ACL is increasingly rare, so we only planned to include this if we found any new, recently conducted trials (that is, since 2000). Otherwise, we did not repeat the analyses provided in Linko 2009. We included any method of conservative treatment; which was likely to include bracing, physiotherapy, or both.
Types of outcome measures
Primary outcomes
Subjective knee scores, e.g. Knee Injury and Osteoarthritis Outcome Score (KOOS; Roos 1998), anterior cruciate ligament quality of life score (Mohtadi 1998) and International Knee Documentation Committee (IKDC), subjective part (Irrgang 2001).
Adverse events (such as donor site morbidity, failure of graft including re‐rupture, infection, deep vein thrombosis and pulmonary embolism).
Treatment failure including re‐operation (for surgery) or subsequent operation (for conservative treatment).
Secondary outcomes
General health‐related quality of life, preferably measured using validated scales such as SF‐36 (Ware 1992), and EQ‐5D (Brooks 1996).
Return to activity or level of sports participation, including Lysholm (Lysholm 1982), or Tegner (Tegner 1985) scores.
Functional assessments (e.g. single‐hop test).
Composite clinical examination outcomes (IKDC, objective part; Hefti 1993).
Knee stability (assessed using manual methods (e.g. Lachman or pivot shift tests) or using knee ligament testing devices (e.g. KT 1000)).
Objective measure of muscle strength (isokinetic muscle torque).
Resource and economic outcomes
Resource and economic outcomes, such as those that measured service utilisation, including cost of surgery, length of inpatient stay, outpatient attendance, duration of sick leave.
Timing of outcome measurement
Assessments were made at short‐ (less than one year), intermediate‐ (one to three years) and long‐term (greater than three years) follow‐up, where data were available.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (18 January 2016), the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 1), MEDLINE (1946 to January Week 1 2016), MEDLINE In‐Process & Other Non‐Indexed Citations (18 January 2016) and EMBASE (1974 to 15 January 2016), using tailored search strategies. In MEDLINE, we combined subject‐specific terms with the sensitivity‐ and precision‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011; seeAppendix 1). The search strategies for CENTRAL and EMBASE are also shown in Appendix 1. We also searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) and ClinicalTrials.gov for ongoing and recently completed trials (4 February 2016). We did not apply any language or publication status restrictions.
Searching other resources
We checked the reference lists of relevant articles and contacted individuals and organisations for further data where necessary.
Data collection and analysis
Selection of studies
All review authors, divided into two teams (PM, LD, DB and SH, KH, AP), independently screened all titles and abstracts for potentially eligible studies, for which we then obtained full‐text reports. The same teams then independently performed study selection. Any disagreements regarding the inclusion or exclusion of individual studies were resolved by discussion among the review team.
Data extraction and management
We developed a data collection form to include all relevant variables for the study, including details of methods, participants, setting, interventions, outcomes, results and funding sources. Two review authors (PM and LD) piloted the data collection form in order to identify any missing or unclear items. After finalising the form, the same two review authors independently performed data extraction. Any disagreement was resolved by discussion between the two authors and, in cases where no consensus was achieved, a third review author (SH) acted as an arbitrator.
Assessment of risk of bias in included studies
Two review authors (PM and LD) independently assessed the risk of bias in each included study using the Cochrane 'Risk of bias' tool. Any disagreement was resolved by consensus between the two review authors, and in cases where no consensus was achieved, a third review author (SH) acted as an arbitrator. We assessed the risk of bias for the following domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data and selective outcome reporting, as well as other sources of bias, such as major differences in rehabilitation. Assessors rated the risk of bias as low, high or unclear for each domain.
Measures of treatment effect
We calculated the risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes and presented the mean differences (MDs) with 95% CIs for continuous outcomes. If data had been available, we had planned to use the mean differences (MDs) with 95% CIs to pool the results of individual trials of continuous outcomes where the same outcome measure was used, or the standardised mean differences (SMDs) with 95% CIs for outcomes measured using different scales. We used results based on change scores where final values were not available.
Unit of analysis issues
Bilateral involvement of the ACL is rare and the use of cluster randomisation for these trials was unlikely. Thus, we anticipated that the units of randomisation and analysis would be the individual participant in the included studies. Should either situation have arisen, we had planned, where possible, to make appropriate adjustments to the analyses according to guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), or to perform sensitivity analyses to explore the impact of incorrectly analysed data. One unit of analysis issue that arose was in the reporting of adverse events, whereby data were presented for the total complications rather than the total number of participants with a complication. Thus, we were only able to present descriptive data on the frequency of individual adverse events, such as graft rupture.
Dealing with missing data
If necessary, we had planned to contact trial investigators for any key missing or unclear data or information on their trial. To avoid the risk of overly positive answers, we had planned to ask open‐ended questions (e.g. "Please describe all measures used"), followed up by more focused questions if further clarification was required. If standard deviations were not reported for continuous outcomes, we had planned to calculate these from standard errors, confidence intervals or exact P values where possible, using the inbuilt calculator in the Review Manager software. We did not impute missing standard deviations. Where possible, we aimed to conduct intention‐to‐treat analyses but decided that we would base our primary analysis on the available data. If data had been available, we had planned to conduct sensitivity analyses to explore the effects of missing data and inclusion of 'per‐protocol' data.
Assessment of heterogeneity
Due to a lack of data, it was not possible to perform a meta‐analysis or assess heterogeneity across studies. If data had been available, the decision about whether or not to combine the results of individual studies would have depended on an assessment of clinical and methodological heterogeneity. Where studies were considered sufficiently homogeneous in their study design, we had planned to carry out a meta‐analysis and assess the statistical heterogeneity. We had planned to assess statistical heterogeneity of treatment effects between trials using a Chi² test, with a significance level at P less than 0.1, and the I² statistic. We had planned to base our interpretation of the I² results on that suggested by Higgins 2011: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and 75% to 100% may represent very substantial ('considerable') heterogeneity.
Assessment of reporting biases
If there had been more than 10 studies included the meta‐analysis, we had planned to explore potential publication bias by generating a funnel plot and statistically testing this using a linear regression test. A probability (P) value of less than 0.1 could be an indication of a publication bias or small study effects.
Data synthesis
We had planned, but did not perform, a meta‐analysis, due to a lack of available data. Therefore, we present data for our primary and secondary outcomes in the analyses for illustrative purposes and report the findings descriptively in the text.
When considered appropriate (e.g. in a future update of this review, and if more data become available), we would pool results of comparable groups of trials using both fixed‐effect and random‐effects models. The choice of the model would be guided by careful consideration of the extent of heterogeneity and whether it could be explained by known factors, such as the number and size of included studies; 95% CIs would be used throughout. We would consider not pooling data where there was considerable heterogeneity (I² greater than 75%) that could not be explained by the diversity of methodological or clinical features among trials. If included, we would analyse cluster randomised trials using the generic inverse variance method.
Subgroup analysis and investigation of heterogeneity
We had planned, but did not perform because of insufficient data, the following subgroup analyses:
Age: younger than 18 years, 18 to 30 years, older than 30 years;
Sex;
Type of graft used (hamstring autograft, bone‐patella‐bone autograft, allograft constructs or synthetic graft);
Time from index injury to entry to trial (immediate (up to 10 weeks) versus later; acute (less than six months) versus chronic (over six months));
Participants with and without meniscal injury.
We had planned to investigate whether the results of subgroups were significantly different by inspecting overlapping CIs and by performing the test for subgroup differences available in Review Manager (RevMan 2012).
Sensitivity analysis
We had planned, but did not perform, to assess the robustness of our findings by conducting sensitivity analyses. These would have included examining the effects of: a) missing or inappropriately analysed data, such as trials including participants treated for bilateral ACL injury; b) including trials at high or unclear risk of selection bias from inadequate concealment of allocation; c) including trials with mixed population groups, such as collateral ligament injuries; d) including trials with incomplete description of the diagnosis of the ACL injury; and e) the choice of statistical model for pooling (fixed‐effect versus random‐effects).
Assessing the quality of the evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the quality of the body of evidence for each outcome listed in Types of outcome measures (Schünemann 2011). The quality rating 'high' is reserved for a body of evidence based on randomised controlled trials. We downgraded the quality rating to 'moderate', 'low' or 'very low' depending on the presence and extent of five factors: study limitations, inconsistency of effect, imprecision, indirectness and publication bias.
'Summary of findings' table
We have presented the results and the quality assessment for the main comparison, and for the primary outcomes and the top two secondary outcomes listed in Types of outcome measures in a 'Summary of findings' table (Schünemann 2011). We also included radiographically‐assessed osteoarthritis, which would come under 'adverse events', a primary outcome.
Results
Description of studies
Results of the search
Searches identified a total of 1273 citations from the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (N = 16); CENTRAL (N = 431), MEDLINE (N = 358) and EMBASE (N = 468). We also searched the WHO ICTRP (N = 247) and ClinicalTrials.gov (N = 186). Of these, 61 full‐text reports were obtained for further examination and 49 articles were initially excluded for not being randomised. Only one study (Frobell 2010b), reported in a trial registration document and two separate publications with different follow‐up periods, was deemed eligible for inclusion in this review. We excluded two studies (Andersson 1991; Sandberg 1987) for not meeting the eligibility criteria (surgery involved repair rather than reconstruction) and we identified two ongoing studies (ACL SNNAP; NTR2746). Full details are reported in Figure 1.
1.
Study flow diagram
Included studies
We identified one study (Frobell 2010b) in which 141 young, active adults (aged 18 to 35 years) with an acute (rotational trauma within the last four weeks) ACL injury were randomised to either ACL reconstruction followed by structured rehabilitation (N = 69) or conservative treatment comprising structured rehabilitation alone (N = 72). Built into the study design was a formal option for subsequent (delayed) ACL reconstruction in the conservative treatment group, if chosen by the participant and if pre‐specified criteria were met. The study was carried out at two hospital sites in Lund, Sweden, recruiting between February 2002 and June 2006.
All participants had to have a score of five to nine on the Tegner activity scale before their injury, and of those followed‐up, all but two participants had been participating in sports when injured. Anterior cruciate ligament injury was determined by clinical examination. An MRI was performed at the time of randomisation; however, the results were not available until several days later. Twelve participants (four versus eight) were excluded because of MRI findings; of these eight (two versus six) had an intact ACL. A further eight (three versus five) were excluded at the time of surgery; seven because of extensive meniscal fixation. Thus, 121 participants were included in the primary analysis of the trial (ACL reconstruction (N = 62) and conservative treatment (N = 59)). Operative treatment involved surgical reconstruction using either the patellar‐tendon or hamstring‐tendon procedure. Participants were evaluated at two and five years after randomisation. The primary outcome was the change from baseline at two years in subjective knee score, measured using the average score of four (pain, symptoms, function in sports and recreation, and knee‐related quality of life) of the five individual components of the KOOS scores, ranging from 0 (extreme symptoms) to 100 (no symptoms). See Characteristics of included studies.
Excluded studies
Two trials were excluded from this review following full‐text eligibility assessment (Andersson 1991; Sandberg 1987). Both trials are reviewed in Linko 2009. They were excluded from this review because they were evaluating surgery that involved direct repair of the ACL and not ACL reconstruction: seeCharacteristics of excluded studies.
Ongoing studies
We identified two ongoing randomised controlled trials; see Characteristics of ongoing studies. NTR2746 is examining the clinical and cost‐effectiveness of early surgery versus conservative management (with the option for delayed surgery) for ACL rupture. The criteria for inclusion in this study is broader than Frobell 2010b in terms of age (18 to 65 years) and time since injury (trauma within two months of injury). The study aimed to recruit 188 participants and completed recruitment in February 2015. The other study is designed to examine the clinical and cost effectiveness of surgery versus conservative management (again with the option for delayed surgery) in patients with non‐acute (longer than four months) ACL deficiency (ACL SNNAP). The planned sample size is 320 participants. This study is in set‐up phase and due to start recruitment in July 2016.
Risk of bias in included studies
Information on potential risk of bias for Frobell 2010b is included in a 'Risk of bias' table (see Characteristics of included studies) and summarised in Figure 2.
2.

Risk of bias summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
The method of random sequence generation and concealment of the allocation sequence were assessed at low risk of bias. The method used to generate the random sequence was computer generated, using block randomisation with a block size of 20, and concealed using sequentially numbered, sealed, opaque envelopes.
Blinding
Due to the nature of the interventions it was not possible to blind the participants or study personnel. However, because of the subjective and self‐reported nature of the outcomes being assessed, we judged this as a potential for high risk of bias. Blinding of outcome assessment was also not performed as assessors were aware of treatment assignment; we also judged this as a high risk of bias.
Incomplete outcome data
Twenty (14%) randomised participants were not included in the primary analysis of the study. All 20 were post‐randomisation exclusions, 12 because of MRI findings and eight at time of surgery, based on their clinical diagnosis. Although the reasons for attrition were clearly reported by the intervention group, there was some imbalance in the losses between the two groups (7/69 = 10% in the surgery group; 13/72 = 18% in the conservative group) and there is some question whether it was appropriate to exclude for findings, usually resulting in extensive meniscal fixation (two versus five), at surgery. However, noting the comparability between the two groups in the baseline characteristics of those who remained in the trial, we judged the trial to be at unclear risk of attrition bias. Only one participant in the ACL reconstruction group was lost to follow‐up at five years.
Selective reporting
All outcomes pre‐specified in the clinical trial register (ISRCTN 84752559) were reported in the results section of the study article(s). Therefore, we judged there was a low risk of reporting bias.
Other potential sources of bias
We did not identify other potential sources of bias.
Effects of interventions
See: Table 1
We identified one study reporting results for 121 young, active adults with acute ACL injury who had been randomised to either ACL reconstruction followed by structured rehabilitation (N = 62) or conservative treatment comprising structured rehabilitation alone (N = 59; Frobell 2010b).
Primary outcomes
Subjective knee scores
There was no difference in subjective knee score (measured using the KOOS‐4 score (range from 0 (extreme symptoms) to 100 (no symptoms)) between participants with ACL reconstruction and conservative treatment in terms of change scores from baseline at two years (MD ‐0.20, 95% CI ‐6.78 to 6.38; N = 121 participants; low‐quality evidence; see Analysis 1.1), or final scores at five years (MD ‐2.0, 95% CI ‐8.27 to 4.27; N = 120 participants; low‐quality evidence; see Analysis 1.1). This finding of no statistically significant or clinically important between‐group differences was consistent across all five individual components of the final KOOS score at two years (ACL reconstruction versus conservative treatment (mean values): Pain, 87.2 versus 87.7; reported P = 0.87; Symptoms, 78.7 versus 83.0; reported P = 0.16; Function in activities of daily living, 93.5 versus 94.7; reported P = 0.68; Function in sports and recreation, 71.8 versus 71.2; reported P = 0.95; Knee‐related quality of life, 67.3 versus 63.0; reported P = 0.28). Similar findings of no between‐group differences also applied at five years.
1.1. Analysis.

Comparison 1 ACL reconstruction versus conservative treatment, Outcome 1 Patient‐rated knee function (KOOS‐4 score).
Adverse events
A range of serious and non‐serious adverse events were reported in Frobell 2010b; however, data were presented as the total number of complications rather than the total number of participants with a complication. In addition, only those events that occurred in 5% or more of participants in the trial or 3% or more participants in one treatment group were reported. Serious adverse events were classified as those having the potential to significantly compromise clinical outcome or result in significant disability or incapacity, requiring inpatient or outpatient care. Overall, fewer serious adverse events involving the index knee occurred in the ACL reconstruction group than in the conservative treatment group at two years: 26 versus 40 serious adverse events respectively (reported P = 0.06). Results for the different types of serious adverse events at two years are shown in Analysis 1.2. These show that the excess of adverse events in the conservative treatment group related to subjective or clinical knee instability (2/62 versus 19/59) and meniscal signs and symptoms (1/62 versus 13/59). The other serious adverse events, which were less frequent, favoured the conservative treatment group; e.g. graft rupture (3/62 versus 1/59). The overall frequency of non‐serious adverse events involving the index knee was higher in the ACL reconstruction group at two years (87 versus 44; reported P < 0.001). The three largest categories of these events, all of which favoured the conservative treatment group, were: subjective or clinical knee instability (25/62 versus 17/59), pain, swelling or both (16/62 versus 14/59) and decreased range of motion (12/62 versus 2/59).
1.2. Analysis.

Comparison 1 ACL reconstruction versus conservative treatment, Outcome 2 Serious adverse events relating to the index knee at 2 years.
The overall frequency of adverse events was not given at five years, except for graft rupture (none had occurred between two and five years follow‐up) and radiographically diagnosed osteoarthritis (19/55 versus 10/55; see Analysis 1.2).
Treatment failure including re‐operation (for surgery) or subsequent operation (for conservative treatment).
Within two years, 23 (39%) out of 59 participants in the conservative treatment group underwent ACL reconstruction; subsequently, another seven participants in the conservative group underwent ACL reconstruction. Thus, 30 (51%) participants had opted for ACL reconstruction within five years. Defining treatment failure specifically in terms of graft rupture, whether surgically treated or not, in the ACL reconstruction group, or subsequent ACL reconstruction in the conservative treatment group, produced results that strongly favoured the ACL reconstruction group at both follow‐up times: two years, 3/62 versus 23/59; RR 0.12, 95% CI 0.04 to 0.34; and five years, 3/61 versus 30/59; low‐quality evidence; see Analysis 1.3. Two of the three participants with graft rupture had revision ACL reconstruction.
1.3. Analysis.

Comparison 1 ACL reconstruction versus conservative treatment, Outcome 3 Treatment failure (graft rupture or ACL reconstruction).
In both groups of Frobell 2010b, meniscal tears were treated with partial resection or fixation, initially when indicated by MRI findings and clinical signs in both groups, and when found during ACL reconstruction in the surgery group. More participants in the ACL reconstruction group had initial meniscal surgery, with results provided for meniscii, not knees (34/62 versus 21/59). Conversely, fewer participants in the ACL reconstruction group had meniscal surgery during the two‐year follow‐up (6/62 versus 29/59; RR 0.02, 95% CI 0.09 to 0.44). At five years, there was little difference between the two groups in the numbers of participants who had undergone meniscal surgery, whether initially or during follow‐up: 29/61 versus 32/59; RR 0.88, 95% CI 0.02 to 1.25; see Analysis 1.4). Fifteen participants of the conservative treatment group had meniscal surgery only.
1.4. Analysis.

Comparison 1 ACL reconstruction versus conservative treatment, Outcome 4 Meniscal surgery.
Secondary outcomes
General health‐related quality of life
There was no difference in health‐related quality of life (measured using the SF‐36 physical and mental components (range from 0 (worst health state) to 100 (best health state)) between ACL reconstruction and conservative treatments at two years (physical: MD 4.1, 95% CI ‐2.76 to 10.96; mental: MD 4.50, 95% CI ‐0.66 to 9.66; N = 121 participants, low‐quality evidence; see Analysis 1.5) or five years (physical: MD 1.00, 95% CI ‐4.54 to 6.54; mental: MD 2.00, 95% CI ‐5.06 to 9.06; N = 120 participants, low‐quality evidence; see Analysis 1.5).
1.5. Analysis.

Comparison 1 ACL reconstruction versus conservative treatment, Outcome 5 General health‐related quality of life (SF‐36 Physical and Mental scores).
Return to activity or level of sport participation
There was no difference between the two groups in the return to pre‐injury activity level or higher, based on Tegner activity scale (range from 1 to 10, where 1 is least strenuous activity level and 10 corresponds to high knee‐demanding activities on a professional level) at either two years (27/62 versus 21/59; RR 1.22, 95% CI 0.78 to 1.91; low‐quality evidence) or five years (14/61 versus 12/59; RR 1.13, 95% CI 0.57 to 2.23; low‐quality evidence); see Analysis 1.6.
1.6. Analysis.
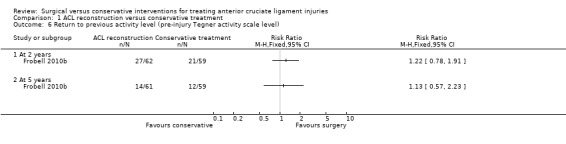
Comparison 1 ACL reconstruction versus conservative treatment, Outcome 6 Return to previous activity level (pre‐injury Tegner activity scale level).
There was also no between‐group differences in Tegner activity scale results at two years (ACL reconstruction: median 6.5, interquartile range (IQR) 3 to 8; conservative treatment: median 5, IQR 4 to 7; N = 121 participants; reported P = 0.82; low‐quality evidence) or at five years (ACL reconstruction: median 4, IQR 2.5 to 7; conservative treatment: median 4.0, IQR 2 to 7; N = 120 participants; reported P = 0.74; low‐quality evidence).
Functional assessment
This outcome was not reported.
Composite clinical examination outcomes
This outcome was not reported.
Knee stability
All three objective measures of knee stability favoured the ACL reconstruction. Anterior sagittal translation of the tibia, measured using a knee‐ligament testing device, the KT 1000 arthrometer performed at 134 Newtons, was lower in the ACL reconstruction group (MD ‐1.70 mm, 95% CI ‐2.68 to ‐0.72 mm; N = 121 participants; low‐quality evidence; see Analysis 1.7). This outcome measurement was not reported at five years. This result was consistent with the finding of greater numbers of normal knees in the surgery group when testing the anteroposterior laxity of the knee at rest, measured using the Lachman's test (scores range from zero to three, with zero indicating normal stability and three indicating severely increased laxity). Knees with normal stability (score zero) at two years: RR 2.22, 95% CI 1.43 to 3.45; N = 118 participants; and five years: RR: 2.37, 95% CI 1.60 to 3.51; N = 116 participants; low‐quality evidence; see Analysis 1.8. A similar finding applied when testing rotational stability at rest, using the Pivot shift test (scores range from zero to three, with zero indicating normal stability and three indicating severely increased laxity). Knees with normal stability (score zero) at two years: RR 1.61, 95% CI 1.18 to 2.20; N = 118 participants; and five years: RR 1.96, 95% CI 1.38 to 2.77; N = 116 participants; low‐quality evidence; see Analysis 1.8).
1.7. Analysis.
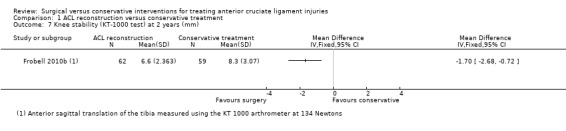
Comparison 1 ACL reconstruction versus conservative treatment, Outcome 7 Knee stability (KT‐1000 test) at 2 years (mm).
1.8. Analysis.
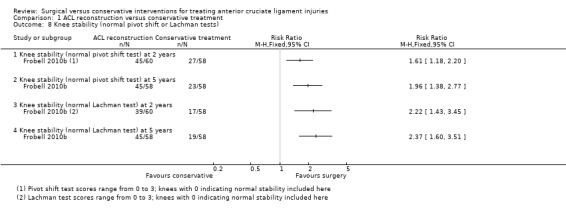
Comparison 1 ACL reconstruction versus conservative treatment, Outcome 8 Knee stability (normal pivot shift or Lachman tests).
Objective measure of muscle strength
This outcome was not reported.
Resource and economic outcomes
These outcomes were not reported.
Discussion
Summary of main results
Despite a rigorous search of the literature, we only identified one randomised trial that met the inclusion criteria of this review (Frobell 2010b). This contrasts with the substantial literature on the different surgical techniques for anterior cruciate ligament (ACL) reconstruction. The trial compared ACL reconstruction followed by structured rehabilitation and conservative treatment comprising structured rehabilitation alone, reporting results for 121 participants at two years and 120 participants at five years. Built into the study design was a formal option for subsequent (delayed) ACL reconstruction in the conservative treatment group.
The Table 1 presents a summary of the current evidence from randomised controlled trials for surgical versus conservative interventions for treating ACL injuries. Overall, there was low‐quality evidence indicating no difference between the two groups in subjective knee score at either two or five years for the KOOS‐4 outcome measure or the five separate components of the KOOS score. The total number of participants incurring one or more adverse events in each group was not reported in Frobell 2010b. The majority of individual categories of serious adverse events were surgery‐related in the ACL reconstruction group and knee instability and meniscal signs and symptoms in the conservative treatment group. Although there were also no data for total participants with treatment failure, including subsequent surgery, we devised two separate categories, one relating to ACL surgery (graft rupture and subsequent ACL reconstruction) and the other relating to meniscal surgery. There was low‐quality evidence of far fewer ACL‐related treatment failures in the surgical treatment group at two years. This result is dominated by the uptake by 39% (23/59) of participants of ACL reconstruction for knee instability at two years and by 51% (30/59) of participants at five years. There was low‐quality evidence of little difference between the two groups in participants who had undergone meniscal surgery at anytime up to five years. There was low‐quality evidence of no clinically important between‐group differences in SF‐36 physical component scores at two years. There was low‐quality evidence of a higher return to the same level or greater sport activity at two years in the ACL reconstruction group, but the wide 95% confidence interval also included the potential for a higher return in the conservative treatment group. Based on an illustrative return of 382 per 1000 conservatively treated patients, this amounts to an extra 84 returns per 1000 ACL reconstruction patients (95% CI 84 fewer to 348 more). There was very low‐quality evidence of a higher incidence of radiographically‐detected osteoarthritis in the surgery group (19/58 (35%) versus 10/55 (18%)).
Overall completeness and applicability of evidence
Considering ACL reconstruction is relatively commonplace in some countries (Gianotti 2009 reported 37 per 100,000 had undergone ACL reconstruction surgery per year in New Zealand) and choosing surgery is a major treatment decision, it is surprising that we only found one trial that compared ACL reconstruction and conservative treatment. This small trial, which was carried out in Scandinavia, included only young (mean age 26 years), active individuals with an acute injury (not more than four weeks since injury). Further potential limitations to applicability reside in the study design. This included the formal option for subsequent (delayed) ACL reconstruction as well as separate surgery for meniscal injuries in the conservative treatment group. Of interest, is that, of the participants analysed in the conservative treatment group, 39% elected to undergo ACL reconstruction within two years and 51% elected to undergo ACL reconstruction at five years. In our review, it was not possible to further distinguish the outcome of the true conservatively managed patients, due to the pragmatic aspects of the trial design. The evidence suggests that the present management options for ACL injury (in the acutely injured knee) are either 1) immediate surgery (within four weeks) or 2) initial conservative care, followed by surgery if and when conservative care fails. Notably, the definition of 'failure' was open to interpretation and was related to participant preference and expectation. The trial did not provide full data for participants with adverse events or treatment failure; the definition needs careful consideration for further trials.
Quality of the evidence
Frobell 2010b was judged to be at low risk of selection and reporting biases, at high risk of performance and detection biases because of the lack of blinding and at unclear risk of attrition bias because of an imbalance in the post‐randomisation exclusions.
According to GRADE methodology, the overall quality of the evidence across different outcomes was low. Thus, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate (Table 1). We downgraded the quality of the evidence for imprecision, reflecting that only one study of 121 participants was included. As with many other studies of this nature that examine surgical versus conservative interventions, it was not possible to blind participants or study personnel. Due to the subjective and self‐reported nature of outcomes in this study, we judged this item as having a potential for high risk of bias. An improvement in knee stability was reported in the ACL reconstruction group; however, as these tests were performed by unblinded outcome assessors, the results were considered to be at a high risk of bias. Therefore, we downgraded the quality of the evidence one level for methodological limitations. Hence, the results of the included trial should be interpreted with caution and viewed, at this stage, as requiring further investigation with studies of good methodological quality.
Potential biases in the review process
There were no obvious biases within the review process. We carried out a comprehensive search strategy and thorough methods of study selection. We applied no language restrictions. The matching of desired outcomes with those reported in trials does require an element of judgement. This related particularly to the incomplete data for treatment failure in Frobell 2010b, where data were provided for individual procedures but not participants undergoing subsequent surgery, relating either to ACL or new knee injury. Thus, our presentation of two categories of treatment failure, split into ACL and meniscal surgery, is a compromise but one that avoids unit of analysis issues as well as making the best use of the available evidence.
Agreements and disagreements with other studies or reviews
Our review is consistent with a recent systematic review by Smith 2014 that compared ACL reconstruction and non‐surgical treatment. They included 16 studies, only one of which was a randomised trial (Frobell 2010b). The authors also concluded that the evidence base is limited in methodological quality, with the current literature insufficient on which to base clinical decision‐making with respect to treatment options for people following ACL rupture. Our search also identified two ongoing studies with a similar study design to that of Frobell 2010b. One study is examining the clinical and cost‐effectiveness of early surgery versus conservative management (with the option for delayed surgery) for ACL rupture (NTR2746), and was due to complete enrolment in February 2015. The other study (ACL SNNAP) is designed to examine the clinical and cost‐effectiveness of surgery versus conservative management (again with the option for delayed surgery) in patients with non‐acute (longer than four months) ACL deficiency. This study is in set‐up phase and due to start recruitment in July 2016.
Authors' conclusions
Implications for practice.
We found low‐quality evidence from a single trial involving 121 young, active adults with acute ACL injuries that there was no difference between surgical management (ACL reconstruction followed by structured rehabilitation) and conservative treatment (structured rehabilitation only) in patient‐reported outcomes of knee function at two and five years after injury. However, these findings need to be viewed in the context that many participants with an ACL rupture remained symptomatic following rehabilitation and later opted for ACL reconstruction surgery.
Implications for research.
In future updates of this review, the addition of evidence from the two ongoing trials should help to inform the optimal management of anterior cruciate ligament injuries.
Further randomised trials comparing surgery with conservative management should be robust in design. Not only should such trials assess and report outcomes that are important to patients with ACL rupture (such as subjective knee function, quality of life, effects on daily activities, and return to activity and sport) but they also should consider factors such as cross‐over, standardisation of interventions and treatment preferences that create additional challenges in the design, conduct and interpretation of trials of this type.
Acknowledgements
We would like to thank Helen Handoll and Haris Vasiliadis for helpful comments about drafts of the review; and Lindsey Elstub and Joanne Elliott for support through the editorial process.
We are grateful to Helen Handoll, Nikolaos Paschos and Haris Vasiliadis for valuable comments about the protocol; and Joanne Elliott and Laura MacDonald for editorial help.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, or the UK National Health Service or Department of Health.
Appendices
Appendix 1. Search strategies
CENTRAL (Cochrane Register of Studies Online)
#1 MESH DESCRIPTOR Anterior Cruciate Ligament (688) #2 MESH DESCRIPTOR Anterior Cruciate Ligament Reconstruction EXPLODE ALL TREES (197) #3 (((anterior adj2 cruciate* adj2 ligament*) or acl)):TI,AB,KY (1488) #4 #1 OR #2 OR #3 (1489) #5 MESH DESCRIPTOR Orthopedics (293) #6 MESH DESCRIPTOR Surgical Procedures, Operative EXPLODE ALL TREES (93308) #7 MESH DESCRIPTOR Orthopedic Fixation Devices EXPLODE ALL TREES (1976) #8 MESH DESCRIPTOR Orthopedic Procedures EXPLODE ALL TREES (8745) #9 MESH DESCRIPTOR Arthroscopy EXPLODE ALL TREES (1104) #10 MESH DESCRIPTOR Suture Techniques EXPLODE ALL TREES (1590) #11 (surg* or operat* or reconstruct* or repair* or graft* or arthroscop*):TI,AB,KY (142949) #12 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 (172347) #13 MESH DESCRIPTOR Physical Therapy Modalities EXPLODE ALL TREES (16072) #14 MESH DESCRIPTOR Braces EXPLODE ALL TREES (319) #15 (non‐surg* or nonsurg* or non‐operat* or nonoperat* or conserv* or rehab* or physiotherapy or physical therapy or brace* or exercis* or cast or casts):TI,AB,KY (76067) #16 #13 OR #14 OR #15 (80623) #17 #4 AND #12 AND #16 (431)
MEDLINE (Ovid Online)
1 Anterior Cruciate Ligament/ or Anterior Cruciate Ligament Reconstruction/ (11139) 2 ((anterior adj2 cruciate* adj2 ligament*) or acl).tw. (16152) 3 or/1‐2 (17797) 4 Orthopedics/ (17890) 5 exp Surgical Procedures,Operative/ (2599718) 6 exp Orthopedic Fixation Devices/ (64256) 7 Orthopedic Procedures/ (19857) 8 Arthroscopy/ (17948) 9 Suture Techniques/ (37962) 10 su.fs. (1668419) 11 (surg* or operat* or reconstruct* or repair* or graft* or arthroscop*).tw. (2418655) 12 or/4‐11 (4264040) 13 exp Physical Therapy Modalities/ (119036) 14 Braces/ (4724) 15 (non‐surg* or nonsurg* or non‐operat* or nonoperat* or conserv* or rehab* or physiotherapy or physical therapy or brace* or exercis* or cast*1).tw. (739890) 16 or/13‐15 (816600) 17 3 and 12 and 16 (2466) 18 Randomized controlled trial.pt. (403863) 19 Controlled clinical trial.pt. (89971) 20 randomized.ab. (332231) 21 placebo.ab. (165137) 22 Clinical trials as topic/ (174213) 23 randomly.ab. (240016) 24 trial.ti. (143900) 25 18 or 19 or 20 or 21 or 22 or 23 or 24 (986765) 26 exp Animals/ not Humans/ (4171020) 27 25 not 26 (908881) 28 17 and 27 (358)
EMBASE (Ovid Online)
1 Anterior cruciate ligament/ (9112) 2 Anterior cruciate ligament rupture/ (3623) 3 Anterior cruciate ligament reconstruction/ (4521) 4 Anterior cruciate ligament injury/ (2019) 5 ((anterior adj2 cruciate* adj2 ligament*) or acl).tw. (19597) 6 or/1‐5 (22467) 7 exp Orthopedic surgery/ (365125) 8 exp Arthroscopy/ (22580) 9 Suturing method/ (28904) 10 su.fs. (1828190) 11 (surg* or operat* or reconstruct* or repair* or graft* or arthroscop*).tw. (3128145) 12 or/7‐11 (4070516) 13 exp Physiotherapy/ (65336) 14 exp Kinesiotherapy/ (55643) 15 exp Brace/ (8144) 16 (non‐surg* or nonsurg* or non‐operat* or nonoperat* or conserv* or rehab* or physiotherapy or physical therapy or brace* or exercis* or cast*1).tw. (927885) 17 or/13‐16 (981076) 18 6 and 12 and 17 (3317) 19 exp Randomized Controlled Trial/ or exp Single Blind Procedure/ or exp Double Blind Procedure/ or Crossover Procedure/ (445012) 20 (random* or RCT or placebo or allocat* or crossover* or 'cross over' or trial or (doubl* adj1 blind*) or (singl* adj1 blind*)).ti,ab. (1490757) 21 19 or 20 (1570098) 22 (exp Animal/ or animal.hw. or Nonhuman/) not (exp Human/ or Human cell/ or (human or humans).ti.) (5749662) 23 21 not 22 (1385888) 24 18 and 23 (468)
WHO ICTRP
1. Anterior cruciate ligament (N = 247)
ClinicalTrials.gov
1. Anterior cruciate ligament (N = 186)
Data and analyses
Comparison 1. ACL reconstruction versus conservative treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient‐rated knee function (KOOS‐4 score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 KOOS change score at 2 years from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 KOOS score at 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Serious adverse events relating to the index knee at 2 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Arthrofibrosis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Graft rupture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Subjective or clinical instability | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Meniscal signs and symptoms | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Pain, swelling or both | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Decreased range of motion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Extension deficit | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 'Other' | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Radiographic osteoarthritis at 5 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Treatment failure (graft rupture or ACL reconstruction) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 At 2 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 At 5 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Meniscal surgery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Meniscal surgery at 2 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Meniscal surgery at any time over 5 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 General health‐related quality of life (SF‐36 Physical and Mental scores) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 SF‐36 physical component at 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 SF‐36 physical component at 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 SF‐36 mental component at 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 SF‐36 mental component at 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Return to previous activity level (pre‐injury Tegner activity scale level) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 At 2 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 At 5 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Knee stability (KT‐1000 test) at 2 years (mm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Knee stability (normal pivot shift or Lachman tests) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Knee stability (normal pivot shift test) at 2 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Knee stability (normal pivot shift test) at 5 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Knee stability (normal Lachman test) at 2 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Knee stability (normal Lachman test) at 5 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Frobell 2010b.
| Methods | Randomised controlled trial Number of centres: 2 Dates of enrolment: February 2002 to June 2006 Follow‐up: 2 and 5 years |
|
| Participants | 141 participants were randomised; 121 were included in the main analysis. Assigned: 69/72 (ACL reconstruction/conservative treatment) Assessed: 62/59 (ACL reconstruction/conservative treatment) Inclusion criteria:
Exclusion criteria:
Post‐randomisation exclusion criteria: If one of the following associated injuries to the index knee were visualised on MRI, arthroscopy or both:
|
|
| Interventions | Group 1: ACL reconstruction followed by structured rehabilitation. Early ACL reconstruction was defined as surgery performed within 10 weeks after the injury. Group 2: Conservative treatment comprising structured rehabilitation alone. Built into the study design was a formal option for subsequent (delayed) ACL reconstruction in the conservative treatment group, if chosen by the participant reporting instability and if pre‐specified criteria were met. |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | The study was supported by grants from the Swedish Research Council and the Medical Faculty of Lund University, the Skåne Regional Council, the Thelma Zoegas Fund, the Stig and Ragna Gorthon Research Foundation, the Swedish National Center for Research in Sports, and Pfizer Global Research. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "..they [patients] were randomly assigned by computer‐generated random numbers in permuted blocks of 20". |
| Allocation concealment (selection bias) | Low risk | Quote: "An investigator who was not involved in the randomisation procedure prepared all sequentially numbered, opaque, sealed envelopes containing the assigned interventions to ensure that the sequence was concealed." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the interventions, it was impossible to blind the participants or personnel. |
| Blinding of outcome assessment (detection bias) Subjective knee score | High risk | Quote: ".. assessments were performed by one or two experienced clinicians, both of whom were aware of the treatment assignments". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of 20 (14% of 141) post‐randomisation exclusions, 12 were excluded because of MRI findings and 8 because of findings at surgery. There was some imbalance in the losses in the two groups (7/69 = 10% in the surgery group; 13/72 = 18% in the conservative group) and there is some question whether it was appropriate to exclude for findings, usually resulting in extensive meniscal fixation (2 versus 5), at surgery. However, there was clear comparability between the two groups in the baseline characteristics of the 121 kept in the trial. |
| Selective reporting (reporting bias) | Low risk | Outcomes pre‐specified in the clinical trial register (ISRCTN 84752559) are reported in the results. |
| Other bias | Low risk | No additional potential sources of bias were identified. |
ACL: Anterior cruciate ligament MCL: Medial collateral ligament PCL: Posterior cruciate ligament
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andersson 1991 | Quasi‐randomised trial involving 167 participants with an acute and complete ACL rupture, recruited 1980 to 1983. Reported at several follow‐up times. Included in Linko 2009. Excluded because surgery involved direct repair of the ACL and not reconstruction. |
| Sandberg 1987 | Randomised trial involving 200 participants with acute ACL, MCL or both, injuries; recruited 1982 to 1984. Included in Linko 2009. Excluded because surgery involved direct repair of the ACL and not reconstruction. |
ACL: Anterior cruciate ligament MCL: Medial collateral ligament
Characteristics of ongoing studies [ordered by study ID]
ACL SNNAP.
| Trial name or title | ACL SNNAP study ‐ Comparison of the clinical and cost‐effectiveness of two management strategies for non‐acute anterior cruciate ligament (ACL) injury: rehabilitation versus surgical reconstruction |
| Methods | Randomised controlled trial |
| Participants | Target number of participants: 320 Inclusion criteria: 1. Symptomatic ACL deficiency (instability‐episodes of frank giving way or feeling unstable) with ACL deficiency confirmed using clinical assessment and/or MRI scan; 2. Aged 18 years or above. Exclusion Criteria: 1. Less than 4 months since injury; 2. Previous knee surgery (other than diagnostic arthroscopy) to index knee or concomitant severe injury to contra‐lateral knee; 3. Meniscal pathology considered sufficiently symptomatic to require surgery, i.e. locked knee, large bucket handle cartilage tear; 4. Knee joint status greater than Grade 2 on Kellgren and Lawrence scale; 5. Grade 3 MCL/LCL injury, associated PCL/PLC injury; 6. Inflammatory arthropathy. |
| Interventions | Group 1: Conservative management group: Rehabilitation with the option for later reconstruction only if required. Group 2: Surgical management group: ACL reconstruction. |
| Outcomes | Primary:
Secondary:
|
| Starting date | July 2016 |
| Contact information | Professor David Beard Royal College of Surgeons Surgical Intervention Trials Unit (SITU) Nuffield Dept of Orthopaedics, Rheumatology and Musculoskeletal Sciences University of Oxford |
| Notes | Funding Source: National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme (14/140/63). |
NTR2746.
| Trial name or title | Cost‐effectiveness of two treatment strategies of an anterior cruciate ligament rupture. A randomized clinical study |
| Methods | Randomised controlled trial |
| Participants | Target number of participants: 188 Inclusion criteria: 1. MRI proven ACL tear, physical examination on high suspicion of ACL tear, or both; 2. Age 18 to 65 years; 3. Patient agreement with randomisation; 4. Trauma within 2 months of inclusion. Exclusion criteria: 1. Associated PCL injury or injury to the lateral or posterolateral ligament complex with significantly Increased laxity; 2. Pregnancy; 3. Patient is unlikely to complete study through 2‐year follow‐up; 4. Insufficient command of the English language, spoken, written, or both; 5. Presence of disorder(s) that affect the activity level of the lower limb; 6. Malalignment of the knee‐hip‐ankle axis that requires intervention. |
| Interventions | Group 1: ACL reconstruction: will be performed within 4 to 6 weeks after inclusion in the study, followed by an exercise program (standardised protocol) for 9 months; Group 2: Conservative management group: Rehabilitation training for 3 to 4 months, followed by assessment of knee function and quality of life. If repeated episodes of giving way, in spite of rehabilitation, occurs or the patient is not satisfied for any reason, a late reconstruction can be performed (delayed surgery). |
| Outcomes | Primary:
Secondary:
|
| Starting date | May 2011 |
| Contact information | Dr. V. Eggerding Erasmus Medical Centre, Department of Orthopaedics PO Box 2040 Rotterdam The Netherlands |
| Notes | Funding source: ZON‐MW, The Netherlands Organization for Health Research and Development. Recruitment completed in February 2015; trial registration number: NTR2746. |
ACL: Anterior cruciate ligament LCL: Lateral collateral ligament MCL: Medial collateral ligament PCL: Posterior cruciate ligament PLC: Posterior lateral complex
Differences between protocol and review
In the protocol we had planned to present the first seven outcomes listed in Types of outcome measures in the 'Summary of findings' table. We adjusted this to accommodate our splitting of treatment failure into two categories. Given data for objectively measured functional outcomes were unavailable, we replaced this with osteoarthritis at five years, even though this was radiologically assessed rather than symptomatic.
Contributions of authors
Andrew P Monk: lead author, writing of manuscript, co‐ordination of team. Sally Hopewell: development of review, writing of the manuscript, statistical expertise. Kristina Harris: statistical expertise. Loretta J Davies: physiotherapy treatment expertise. David Beard: methodological expertise. Andrew Price: clinical expertise and development of protocol.
Declarations of interest
Andrew P Monk: none known. Sally Hopewell: none known. Kristina Harris: none known. Loretta J Davies: none known. David Beard: none known. Andrew Price: none known.
Four review authors (DB, LJD, APM, AP) are member of the trial management group of an ongoing trial (ACL SNNAP); arrangements will be made for independent review of this trial when completed.
New
References
References to studies included in this review
Frobell 2010b {published data only}
- Frobell RB, Roos EM, Roos HP, Ranstam J, Lohmander LS. A randomized trial of treatment for acute anterior cruciate ligament tears. New England Journal of Medicine 2010;363(4):331‐42. [DOI] [PubMed] [Google Scholar]
- Frobell RB, Roos HP, Roos EM, Roemer FW, Ranstam J, Lohmander LS. Treatment for acute anterior cruciate ligament tear: five year outcome of randomised trial. BMJ 2013;346(7895):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmander S. Surgical versus non‐surgical treatment of anterior cruciate ligament (ACL) injuries: a randomised prospective clinical trial. http://www.controlled‐trials.com/ISRCTN84752559 (accessed 18 January 2016).
References to studies excluded from this review
Andersson 1991 {published data only}
- Andersson C, Odensten M Gillquist J. Knee function after surgical or nonsurgical treatment of acute rupture of the anterior cruciate ligament: A randomized study with a long‐term follow up period. Clinical Orthopaedics and Related Research 1991;(264):255‐63. [PubMed] [Google Scholar]
- Andersson C, Odensten M, Good L, Gillquist J. Surgical or non‐surgical treatment of acute rupture of the anterior cruciate ligament. A randomized study with long‐term follow‐up. Journal of Bone and Joint Surgery ‐ American Volume 1989;71(7):965‐74. [PubMed] [Google Scholar]
- Meunier A, Odensten M, Good L. Long‐term results after primary repair or non‐surgical treatment of anterior cruciate ligament rupture: a randomized study with a 15‐year follow‐up. Scandinavian Journal of Medicine and Science in Sports 2007;17:230‐7. [DOI] [PubMed] [Google Scholar]
- Odensten M, Hamberg P, Nordin M, Lysholm J, Gillquist J. Surgical or conservative treatment of the acutely torn anterior cruciate ligament. Clinical Orthopaedics and Related Research 1985;(198):87‐93. [PubMed] [Google Scholar]
- Odensten M, Hamberg P, Nordin M, Lysholm J, Gillquist J. Treatment of the acute torn anterior cruciate ligament: A randomized study with a short‐term follow‐up [abstract]. Acta Orthopaedica Scandinavica 1984;55(4):474. [Google Scholar]
Sandberg 1987 {published data only}
- Sandberg R, Balkfors B, Edwards P, Nilsson B, Westlin N. Surgical or non‐surgical treatment of ligamentous knee injuries. A randomized controlled study ‐ early results [abstract]. Acta Orthopaedica Scandinavica 1986;57:254. [Google Scholar]
- Sandberg R, Balkfors B, Nilsson B, Westlin N. Operative versus non‐operative treatment of recent injuries to the ligaments of the knee. Journal of Bone and Joint Surgery ‐ American Volume 1987;69(8):1120‐6. [PubMed] [Google Scholar]
References to ongoing studies
ACL SNNAP {published data only}
- Beard D. The ACL SNNAP trial: Comparison of the clinical and cost effectiveness of two management strategies for non‐acute anterior cruciate ligament (ACL) injury: rehabilitation versus surgical reconstruction. http://www.nets.nihr.ac.uk/projects/hta/1414063 (accessed 18 January 2016).
NTR2746 {unpublished data only}
- Eggerding V. Cost‐effectiveness of two treatment strategies of an anterior cruciate ligament rupture. A randomized clinical study. www.trialregister.nl/trialreg/admin/rctview.asp?TC=2746 (accessed 30 March 2015). [Trial Registration number: NTR2746]
Additional references
Ajuied 2013
- Ajuied A, Wong F, Smith C, Norris M, Earnshaw P, Back D, et al. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta‐analysis. American Journal of Sports Medicine 2014;42(9):2242‐52. [DOI] [PubMed] [Google Scholar]
Bahr 2003
- Bahr R, Holme I. Risk factors for sports injuries: a methodological approach. British Journal of Sports Medicine 2003;37(5):384‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowers 2005
- Bowers AL, Spindler KP, McCarty EC, Arrigain S. Height, weight, and BMI predict intra‐articular injuries observed during ACL reconstruction: evaluation of 456 cases from a prospective ACL database. Clinical Journal of Sport Medicine 2005;15(2):9‐13. [DOI] [PubMed] [Google Scholar]
Brooks 1996
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53‐72. [DOI] [PubMed] [Google Scholar]
Collins 2013
- Collins JE, Katz JN, Donnell‐Fink LA, Martin SD, Losina E. Cumulative incidence of ACL reconstruction after ACL injury in adults: role of age, sex and race. American Journal of Sports Medicine 2013;41(3):544‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crawford 2007
- Crawford R, Walley G, Bridgman S. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: A systematic review. British Medical Bulletin 2007;84:5‐23. [DOI] [PubMed] [Google Scholar]
Delincé 2012
- Delincé P, Ghafil D. Anterior cruciate ligament tears: conservative or surgical treatment? A critical review of the literature. Knee Surgery, Sports Traumatology, Arthroscopy 2012;20(1):48‐61. [DOI] [PubMed] [Google Scholar]
Frobell 2010a
- Frobell RB, Roos EM, Roos HP, Ranstam J, Lohmander LS. A randomized trial of treatment for acute anterior cruciate ligament tears. New England Journal of Medicine 2010;363:331‐42. [DOI] [PubMed] [Google Scholar]
Frobell 2013
- Frobell RB, Roos HP, Roos EM, Roemer FW, Ranstam J, Lohmander LS. Treatment for acute anterior cruciate ligament tear: five year outcome of randomised trial. BMJ 2013;346:f232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gianotti 2009
- Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population‐based study. Journal of Science and Medicine in Sport 2009;12(6):622‐7. [DOI] [PubMed] [Google Scholar]
Hefti 1993
- Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surgery, Sports Traumatology, Arthroscopy 1993;1(3‐4):226‐34. [DOI] [PubMed] [Google Scholar]
Hernandez 2006
- Hernandez L, Micheo W, Amy E. Rehabilitation update for the anterior cruciate ligament injured patient: Current concepts. Boletin de la Asociacion Medica de Puerto Rico 2006;98(1):62‐72. [PubMed] [Google Scholar]
Hewett 2006
- Hewett TE, Myer GD, Ford KR. Anterior cruciate ligament injuries in female athletes: Part 1, mechanisms and risk factors. American Journal of Sports Medicine 2006;34(2):299‐311. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Irrgang 2001
- Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P, et al. Development and validation of the international knee documentation committee subjective knee form. American Journal of Sports Medicine 2001;29(5):600‐13. [DOI] [PubMed] [Google Scholar]
Kostogiannis 2007
- Kostogiannis I, Ageberg E, Neuman P, Dahlberg L, Friden T, Roos H. Activity level and subjective knee function 15 years after anterior cruciate ligament injury: a prospective, longitudinal study of nonreconstructed patients. American Journal of Sports Medicine 2007;25(7):1135‐43. [DOI] [PubMed] [Google Scholar]
Kvist 2004
- Kvist J. Rehabilitation following anterior cruciate ligament injury: current recommendations for sports participation. Sports Medicine 2004;34(4):269‐80. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Linko 2009
- Linko E, Harilainen A, Malmivaara A, Seitsalo S. Surgical versus conservative interventions for anterior cruciate ligament ruptures in adults. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001356.pub3] [DOI] [PubMed] [Google Scholar]
Lohmander 2007
- Lohmander S, Englund PM, Dahl LL, Roos EW. The long‐term consequence of anterior cruciate ligament and meniscus injuries. American Journal of Sports Medicine 2007;35(10):1756‐69. [DOI] [PubMed] [Google Scholar]
Lysholm 1982
- Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. American Journal of Sports Medicine 1982;10(3):150‐4. [DOI] [PubMed] [Google Scholar]
Micheo 2010
- Micheo W, Hernandez L, Seda C. Evaluation, management, rehabilitation, and prevention of anterior cruciate ligament injury: Current concepts. American Academy of Physical Medicine and Rehabilitation 2010;2(10):935‐44. [DOI] [PubMed] [Google Scholar]
Miyasaka 1991
- Miyasaka KC, Daniel DM, Stone ML, Hirschman P. The incidence of knee ligament injuries in the general population. American Journal of Knee Surgery 1991;4:43‐8. [Google Scholar]
Mohtadi 1998
- Mohtadi N. Development and validation of the quality of life outcome measure (questionnaire) for chronic anterior cruciate ligament deficiency. American Journal of Sports Medicine 1998;3(26):350‐9. [DOI] [PubMed] [Google Scholar]
Mohtadi 2011
- Mohtadi NG, Chan DS, Dainty KN, Whelan DB. Patellar tendon versus hamstring tendon autograft for anterior cruciate ligament rupture in adults. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD005960.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Negus 2012
- Negus J, Fransen M, Chen JS, Parker DA, March L. Exercise‐based interventions for conservatively or surgically treated anterior cruciate ligament injuries in adults. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD010128] [DOI] [Google Scholar]
Ramski 2013
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager. Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Roos 1998
- Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)‐‐development of a self‐administered outcome measure. Journal of Orthopaedic & Sports Physiotherapy 1998;28(2):88‐96. [DOI] [PubMed] [Google Scholar]
Rout 2013
- Rout R, McDonnell S, Hulley P, Jayadev C, Khan T, Carr A, et al. The pattern of cartilage damage in antero‐medial osteoarthritis of the knee and its relationship to the anterior cruciate ligament. Journal of Orthopaedic Research 2013;31(6):908‐13. [DOI] [PubMed] [Google Scholar]
Smith 2014
- Smith TO, Postle K, Penny F, McNamara I, Mann CJV. Is reconstruction the best management strategy for anterior cruciate ligament rupture? A systematic review and meta‐analysis comparing anterior cruciate ligament reconstruction versus non‐operative treatment. Knee 2014;21(2):462–70. [DOI] [PubMed] [Google Scholar]
Spindler 2004
- Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone‐tendon‐bone versus hamstring: does it really matter? A systematic review. American Journal of Sports Medicine 2004;32(8):1986‐95. [DOI] [PubMed] [Google Scholar]
Spindler 2008
- Spindler KP, Wright RW. Clinical practice. Anterior cruciate ligament tear. New England Journal of Medicine 2008;359(20):135‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tegner 1985
- Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clinical Orthopaedics and Related Research 1985;(198):43‐9. [PubMed] [Google Scholar]
Thorstensson 2009
- Thorstensson CA, Lohmander LS, Frobell RB, Roos EM, Gooberman‐Hill R. Choosing surgery: patients' preferences within a trial of treatments for anterior cruciate ligament injury. A qualitative study. BMC Musculoskeletal Disorders 2009;10:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1992
- Ware JE, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PubMed] [Google Scholar]
Øiestad 2009
- Øiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury. American Journal of Sports Medicine 2009;37(7):1434‐43. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Monk 2014
- Monk AP, Hopewell S, Harris K, Davies LJ, Beard D, Price A. Surgical versus conservative interventions for treating anterior cruciate ligament injuries. Cochrane Database of Systematic Reviews 14, Issue 6. [DOI: 10.1002/14651858.CD011166] [DOI] [PMC free article] [PubMed] [Google Scholar]


