Abstract
Background
Recruitment manoeuvres involve transient elevations in airway pressure applied during mechanical ventilation to open (‘recruit’) collapsed lung units and increase the number of alveoli participating in tidal ventilation. Recruitment manoeuvres are often used to treat patients in intensive care who have acute respiratory distress syndrome (ARDS), but the effect of this treatment on clinical outcomes has not been well established. This systematic review is an update of a Cochrane review originally published in 2009.
Objectives
Our primary objective was to determine the effects of recruitment manoeuvres on mortality in adults with acute respiratory distress syndrome.
Our secondary objective was to determine, in the same population, the effects of recruitment manoeuvres on oxygenation and adverse events (e.g. rate of barotrauma).
Search methods
For this updated review, we searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (OVID), Embase (OVID), the Cumulative Index to Nursing and Allied Health Literature (CINAHL, EBSCO), Latin American and Caribbean Health Sciences (LILACS) and the International Standard Randomized Controlled Trial Number (ISRCTN) registry from inception to August 2016.
Selection criteria
We included randomized controlled trials (RCTs) of adults who were mechanically ventilated that compared recruitment manoeuvres versus standard care for patients given a diagnosis of ARDS.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. We contacted study authors for additional information.
Main results
Ten trials met the inclusion criteria for this review (n = 1658 participants). We found five trials to be at low risk of bias and five to be at moderate risk of bias. Six of the trials included recruitment manoeuvres as part of an open lung ventilation strategy that was different from control ventilation in aspects other than the recruitment manoeuvre (such as mode of ventilation, higher positive end‐expiratory pressure (PEEP) titration and lower tidal volume or plateau pressure). Six studies reported mortality outcomes. Pooled data from five trials (1370 participants) showed a reduction in intensive care unit (ICU) mortality (risk ratio (RR) 0.83, 95% confidence interval (CI) 0.72 to 0.97, P = 0.02, low‐quality evidence), pooled data from five trials (1450 participants) showed no difference in 28‐day mortality (RR 0.86, 95% CI 0.74 to 1.01, P = 0.06, low‐quality evidence) and pooled data from four trials (1313 participants) showed no difference in in‐hospital mortality (RR 0.88, 95% CI 0.77 to 1.01, P = 0.07, low‐quality evidence). Data revealed no differences in risk of barotrauma (RR 1.09, 95% CI 0.78 to 1.53, P = 0.60, seven studies, 1508 participants, moderate‐quality evidence).
Authors' conclusions
We identified significant clinical heterogeneity in the 10 included trials. Results are based upon the findings of several (five) trials that included an "open lung ventilation strategy", whereby the intervention group differed from the control group in aspects other than the recruitment manoeuvre (including co‐interventions such as higher PEEP, different modes of ventilation and higher plateau pressure), making interpretation of the results difficult. A ventilation strategy that included recruitment manoeuvres in participants with ARDS reduced intensive care unit mortality without increasing the risk of barotrauma but had no effect on 28‐day and hospital mortality. We downgraded the quality of the evidence to low, as most of the included trials provided co‐interventions as part of an open lung ventilation strategy, and this might have influenced results of the outcome.
Plain language summary
Recruitment manoeuvres as a ventilation strategy for adults with acute respiratory failure due to lung injury
Background: Acute respiratory failure is a common condition amongst adults admitted to intensive care units (ICUs) worldwide. Although respiratory failure has many causes, it may be due to a condition known as acute respiratory distress syndrome (ARDS). This term describes a condition in which both of the lungs have become injured and inflamed from one of various causes, and they do not work as they normally would to provide oxygen to and remove carbon dioxide from the body. This leads to a reduced amount of oxygen in the blood. Patients may require connection to a ventilator (breathing machine) to support their breathing. This therapy is known as mechanical ventilation. Supportive care with mechanical ventilation is an important pillar of standard treatment for patients with ARDS.
Although it may be life‐saving, mechanical ventilation may further contribute to lung injury by expanding and collapsing the lungs or overstretching lung tissue. To minimize damage to injured lungs, smaller volumes of air at lower pressures have been used in conjunction with a positive opening pressure at the end of expiration (PEEP). This ventilation strategy has been shown to shorten the time that patients require mechanical ventilation while improving survival; it has been adopted as standard care for patients with ARDS who are in intensive care.
Along with this strategy, additional ventilation techniques have been developed. One such technique is known as a recruitment manoeuvre; when combined with higher PEEP, it is called the open lung ventilation strategy. A recruitment manoeuvre uses sustained deep breaths to assist in the recruitment ‐ or re‐opening ‐ of collapsed lung units. This may increase the number of lung units available for breathing and may improve patient outcomes. Effects of recruitment manoeuvres have not been well established.
Search date: Evidence is current to August 2016.
Study characteristics: We included 10 trials in this review, which included a total of 1658 participants with acute respiratory distress syndrome.
Key results: Low‐quality evidence suggests that recruitment manoeuvres improve ICU survival but not 28‐day or hospital survival. Recruitment manoeuvres have no effect on the risk of air leakage from the lungs.
Quality of the evidence: We found the evidence for most outcomes to be of low to moderate quality, primarily because of the design of included trials. Many trials used the recruitment manoeuvre in conjunction with other ventilation techniques or strategies, and this might have influenced outcomes. Caution should be applied when conclusions are drawn about the effectiveness of the recruitment manoeuvre alone.
Summary of findings
for the main comparison.
| Recruitment manoeuvres compared with standard care for adults with acute respiratory distress syndrome who were mechanically ventilated | ||||||
|
Patient or population: mechanically ventilated adults with acute respiratory distress syndrome. Participants were recruited from ICUs internationally, including Australia, Brazil, China, Europe, Canada, Korea, Seoul, Taiwan and the United States. Settings: intensive care unit Intervention: recruitment manoeuvres Comparison: standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Intervention | |||||
| 28‐Day mortality | Risk for the population | RR 0.86 (0.74 to 1.01) | 1450 (5 studies) | ⊕⊕⊝⊝ Lowa | Four of the 5 trials include co‐interventions that may influence the result of the outcome. | |
| 347 per 1000 | 294 per 1000 | |||||
| ICU mortality | Risk for the population | RR 0.83 (0.72 to 0.97) | 1370 (5 studies) | ⊕⊕⊝⊝ Lowa | Four of the 5 trials include co‐interventions that may influence the result of the outcome. | |
| 362 per 1000 | 303 per 1000 | |||||
| In‐hospital mortality | Risk for the population | RR 0.88 (0.77 to 1.01) | 1313 (4 studies) | ⊕⊕⊝⊝ Lowa | Three of the 4 trials include co‐interventions that may influence the result of the outcome. | |
| 405 per 1000 | 356 per 1000 | |||||
| Rate of barotrauma | Risk for the population | RR 1.09 (0.78 to 1.51) | 1508 (7 studies) | ⊕⊕⊕⊝ Moderateb | ||
| 90 per 1000 | 86 per 1000 | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aLung recruitment manoeuvres were used with co‐interventions that may affect the result of the outcome (Hodgson 2011; Meade 2008; Huh 2009; Kacmarek 2016; Liu 2011). We downgraded the quality of the evidence by two levels for indirectness of evidence. We made this decision a priori.
bWe noted no heterogeneity among trial effect estimates but observed that reported confidence intervals around effect estimates were wide. We downgraded the quality of the evidence by one level for imprecision in results.
Background
Description of the condition
Acute respiratory distress syndrome (ARDS) is an inflammatory condition of the lung parenchyma that causes impaired gas exchange (ARDS Definition Task Force 2012). According to the Berlin definition, each stage of mild, moderate and severe ARDS are associated with increasing mortality (ARDS Definition Task Force 2012). Patients with ARDS present with bilateral lung infiltrates on chest radiograph and hypoxaemia with concomitant systemic inflammatory mediator release, frequently causing multiple organ failure and death (Gattinoni 2006). In a recent international, multi‐centre observational study, ARDS accounted for 10.4% of all intensive care unit (ICU) beds, with a mortality rate of 34.9% for mild ARDS, 40.3% for moderate ARDS and 46.1% for severe ARDS (Bellani et al, 2016).
Description of the intervention
Patients with ARDS in the ICU may require mechanical ventilation to survive (Amato 1998; ARDS Definition Task Force 2012; Sevransky 2004). However, mechanical ventilation can injure lungs through alveolar distension, cyclical collapse and reopening of alveolar units and failure to expand collapsed alveolar units (Gattinoni 2006). To minimize damage to injured lungs, small ventilatory tidal volumes and low plateau pressures have been used. Additionally, higher positive end‐expiratory pressure (PEEP) reduces collapse of alveoli at the end of expiration and decreases atelectrauma (Briel 2010). These techniques when combined are known as protective lung ventilation; they may reduce mortality and the duration of mechanical ventilation (Amato 1998; ARDSnet 2000) and have become standard care.
Recruitment manoeuvres (RMs) have been used in the ventilatory treatment of patients with ARDS (Fan 2008). Recruitment manoeuvres re‐inflate collapsed regions of the lungs by briefly raising intrapulmonary pressure to levels higher than those achieved during tidal ventilation (Brower 2003). They may be used intermittently throughout the day, or on a single occasion, to re‐inflate collapsed alveoli. A variety of RMs have been described, including prolonged continuous positive airway pressure (30 to 40 cm H2O) and stepwise or staircase RMs, which are based on progressive incremental increases in PEEP at constant driving pressure. Recruitment manoeuvres (usually) are associated with short‐term physiological benefits such as reduced intrapulmonary shunt and increased pulmonary compliance. They remain controversial because they may be harmful (Fan 2008). Recruitment manoeuvres increase intrathoracic pressure and can transiently reduce venous return and cardiac output (Odenstedt 2005). The increase in intrapulmonary pressure that results may cause barotrauma (Brower 2003; Levy 2005).
How the intervention might work
Use of a ventilation strategy that included recruitment manoeuvres and higher positive end‐expiratory pressure based on the pressure‐volume curve, which was higher than in the control group, improved survival in patients with acute respiratory distress syndrome (Amato 1998). Recruitment manoeuvres have been investigated in animal models (Funk 2004; Lim 2004) and in ventilated patients (Amato 1998; Brower 2003; Levy 2005) with variable outcomes. The reason for variability in response is not well understood.
Why it is important to do this review
This is an update of a Cochrane review that was first published in 2009 (Hodgson 2009). We have included eight new trials in this updated version (Cavalcanti et al, 2013; Hodgson 2011; Huh 2009; Kacmarek 2016; Liu 2011; Wang 2009; Xi 2010; Yang 2011). Techniques used to apply a recruitment manoeuvre vary in duration, maximum pressure and end‐expiratory pressure (Fan 2008; Suzumura 2014). This variation has made it difficult to extrapolate research findings to clinical practice. Effects of a recruitment manoeuvre may vary with the cause of lung injury (Borges 2006; Brower 2003; Kacmarek 2007). A small number of papers have reported reviews on the safety and efficacy of recruitment manoeuvres in ventilated patients (Fan 2008; Fan 2012; Lapinsky 2005; Piacentini 2004; Richard 2004), including a recent meta‐analysis (Suzumura 2014) and a Scandanavian clinical practice guideline for managing mechanical ventilation for patients with ARDS (Laake et al, 2015).
Objectives
Our primary objective was to determine the effects of recruitment manoeuvres on mortality in adults with acute respiratory distress syndrome.
Our secondary objective was to determine, in the same population, the effects of recruitment manoeuvres on oxygenation and adverse events (e.g. rate of barotrauma).
Methods
Criteria for considering studies for this review
Types of studies
We included prospective, randomized controlled trials (RCTs).
In this updated review, we excluded cross‐over trials, as they are not appropriate for assessing the primary outcome (mortality).
Types of participants
We included adults (at least 18 years of age) with acute respiratory distress (ARDS Definition Task Force 2012; Bernard 1994) who were intubated and mechanically ventilated in intensive care for at least 24 hours.
We excluded studies that enrolled children younger than 18 years of age or animals.
Types of interventions
We included RCTs that compared recruitment manoeuvres versus standard care. We defined a recruitment manoeuvre as any technique that transiently increased alveolar pressure above normal tidal ventilation (which may have included an increase in any pressure, such as plateau, peak or end‐expiratory pressure) and sustained that pressure beyond the normal time. We defined standard care as protective lung ventilation including tidal volume and pressure limitation (ARDSnet 2000) without recruitment manoeuvres. We excluded any trial that did not use standard care ventilation, as the ventilation strategy in the control group may have caused a difference in outcomes.
Types of outcome measures
Primary outcomes
We included trials that reported the primary outcome of mortality (28‐day mortality, ICU mortality and in‐hospital mortality).
Secondary outcomes
We included trials that reported the following secondary outcomes.
Barotrauma.
Hypoxaemia requiring use of rescue therapies.
Oxygenation (partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2 ratio).
In this version of the review, we excluded:
blood pressure as an outcome measure (as it was considered to be a transient outcome measure that was not measured beyond the first day); and
length of stay in ICU and in hospital, as these were not reported separately for survivors and non‐survivors.
Search methods for identification of studies
Electronic searches
We updated our previous search of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (OVID), Embase (OVID), the Cumulative Index to Nursing and Allied Health Literature (CINAHL, EBSCO), Latin American and Caribbean Health Sciences (LILACS) and the International Standard Randomized Controlled Trial Number (ISRCTN) registry, from inception to August 2016.
We adapted our MEDLINE search strategy for use with other electronic databases. Our search strategies can be found in the appendices (MEDLINE, Appendix 1; CINAHL, Appendix 2; CENTRAL, Appendix 3; EMBASE, Appendix 4; LILACS, Appendix 5).
Searching other resources
We handsearched the bibliographies of all retrieved articles to identify potentially relevant trials.
We applied no language restrictions.
We attempted to identify unpublished trials by contacting experts in the field of recruitment manoeuvre research.
We tracked the citations of authors of included studies.
Data collection and analysis
Selection of studies
We (CH, SB, EG) independently and sequentially excluded studies by reading titles, abstracts, then full papers. We resolved disagreements by discussion.
Data extraction and management
We (CH, SB, EG, MY) independently extracted relevant data from included trials. We extracted study location, population description, intervention description, intervention dosage (frequency, intensity, repetition, duration), hospital environment and participant and hospital outcome data. We resolved disagreements by discussion. When information regarding the outcomes of interest was inadequate, we contacted the study authors directly (via email).
We planned to include funnel plots for any analyses that contained at least five studies. Funnel plot asymmetry may be caused by selection bias (publication or location bias); poor methodological quality of smaller studies (design, analysis, fraud); true heterogeneity (variation in effect size); and artefact or chance (Egger 1997). We performed a meta‐analysis by using Cochrane Review Manager software (RevMan 5.3).
Assessment of risk of bias in included studies
We appraised each included study according to the criteria described below (CH, EG, MY).
Under the following criteria, the response ’Yes’ indicates low risk of bias, ’No’ represents high risk of bias and ’Unclear’ means that insufficient information was available to permit a judgement.
Adequacy of the sequence generation (randomization)
Yes: Adequate sequence generation was reported via computer‐generated random numbers or codes or sealed envelopes.
No: Inadequate sequence generation was reported.
Unclear: Investigators did not describe one of the adequate methods but mentioned randomization.
Adequacy of allocation concealment
Yes: A randomization method was described that would not allow an investigator or a participant to know or influence allocation to an intervention group before an eligible participant entered the study.
No: An inadequate method of allocation was used, such as alternate medical record numbers or unsealed envelopes; or information in the study report indicated that investigators or participants were aware of group allocation before enrolment.
Unclear: The trial report mentioned randomization but provided no information on the method used, or study authors reported a method that was not clearly adequate.
Blinding of participants
We graded this item as ’Yes’ for blinded participants, ’Unclear’ if relevant information was not stated in the trial report and ’No’ for unblinded participants.
Blinding of outcome assessors
We graded this item as ’Yes’ for blinded outcome assessment, ’Unclear’ if relevant information was not stated in the trial report and ’No’ for unblinded outcome assessment.
Free of other sources of bias
Yes (low risk of bias): The trial appears to be free of other components that could put it at risk of bias.
No (high risk of bias): Other factors in the trial could put it at risk of bias, such as inadequate size calculation, early stopping or an extreme baseline imbalance.
Measures of treatment effect
Data analysis
Dichotomous data
We calculated the risk ratio (RR) and absolute risk reduction (ARR), as well as associated 95% confidence intervals (CIs). When possible, we calculated and reported the number needed to treat for one patient to benefit compared with a control (NNTB).
Continuous data
We calculated the mean difference (MD) and the associated 95% CI. We used the standardized mean difference (SMD) for data that we could not convert to a uniform scale.
We pooled data using the random‐effects model or the fixed‐effect model, depending on the presence or absence of statistical heterogeneity.
Unit of analysis issues
The unit of analysis was the individual.
We did not include studies with a cross‐over design because this study design was not appropriate for this research question.
Dealing with missing data
We reported incomplete outcome data in the following ways.
Incomplete outcome data
Yes: Numbers of withdrawals per group were reported, with reasons provided; or it was clear from the report that no withdrawals occurred.
No: Some withdrawals were evident, but numbers per group and reasons were not provided.
Incomplete outcome data addressed (use of intention‐to‐ treat (ITT) analysis)
We defined ITT analysis as analysis conducted when all trial participants were analysed in the group to which they had been randomized, regardless of which (or how much) treatment they actually received, and regardless of other protocol irregularities, such as ineligibility.
Yes: The trial report stated that ITT was undertaken and this was confirmed on study assessment, or it was not stated but was evident from study assessment that ITT was undertaken.
No: Intention‐to‐treat analysis was not confirmed on study assessment (participants who were randomized were not included in the analysis because they did not receive the study intervention, they withdrew from the study or they had committed a protocol violation), regardless of whether the analysis was described as ITT.
Assessment of heterogeneity
Clinical and statistical heterogeneity
We used the term 'clinical heterogeneity' to describe differences between participants, interventions and outcomes that might reasonably impact the effect of recruitment manoeuvres. We measured statistical heterogeneity by using the I2 statistic (Higgins 2002), which describes the percentage of variability in effect estimates that is due to heterogeneity rather than to sampling error or chance. We considered a value greater than 50% to indicate that an outcome was significantly heterogeneous. In the absence of clinical heterogeneity, we pooled studies on a case‐by‐case basis. We assessed the interaction of study variables with effects of recruitment manoeuvres in predefined sensitivity and subgroup analyses.
Assessment of reporting biases
Reporting bias refers to systematic differences between reported and unreported findings. We planned to contact trial authors to request missing data. We extracted data regarding intention‐to‐treat. analysis. If study authors did not perform ITT analysis, and if less than 20% of participants were lost to follow‐up, but sufficient raw data were available, we planned to conduct an ITT analysis before entering data into RevMan 5.3.
We planned to use funnel plot analysis to assess publication bias when more than five studies were included in the meta‐analysis, and to use the Egger test to assess funnel plot asymmetry (Egger 1997). A thorough search of grey literature for unpublished studies and contact with known experts in the field assisted review authors in reducing the risk of publication bias.
Data synthesis
We carried out statistical analysis using Review Manager software. One review author (CH) entered data into Review Manager 5.3, and a second review author (MY) checked these data for accuracy (RevMan 5.3). If the outcome of heterogeneity was low, as indicated by an I² statistic less than 50%, we planned to use the fixed‐effect model to synthesize results. If heterogeneity was moderate or high, as indicated by an I² statistic greater than 50%, we planned to use the random‐effects model to synthesize results, or to refrain from pooling, and we restricted the analysis to a qualitative overview when I² statistical values were above 60%.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis
We planned to assess the interaction between cause of lung injury (intrapulmonary vs extrapulmonary) (Richard 2004) and effects of recruitment manoeuvres.
We planned to assess the interaction between type of recruitment manoeuvre and effect, while dichotomising studies according to the following definitions.
A manoeuvre that included a plateau pressure of 40 cm H2O or higher that was sustained for 40 seconds or longer and had a PEEP after the manoeuvre of at least 15 cm H2O, with a plan to repeat or actual repetition of the recruitment manoeuvre (Hedenstierna 2002).
All other recruitment manoeuvres.
Sensitivity analysis
We aimed to determine whether conclusions were robust with regards to decisions made during the review process, such as inclusion/exclusion of particular studies. We pooled all studies for analysis, then performed a sensitivity analysis excluding studies that used co‐interventions that may have influenced study outcomes.
Summary of findings table and GRADE
We used the principles of the GRADE system to assess the quality of the body of evidence associated with specific outcomes in our review, and we constructed Table 1 by using GRADEpro software (Guyatt 2008). We included these specific outcomes in the table.
28‐Day mortality.
ICU mortality.
In‐hospital mortality.
Barotrauma.
The GRADE approach appraises the quality of a body of evidence on the basis of the extent to which one can be confident that an estimate of effect or association reflects the item assessed. Assessment of the quality of a body of evidence considers within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias. A priori, we agreed to lower the GRADE assessment of evidence if the results of studies may have been influenced by co‐interventions.
Results
Description of studies
Results of the search
We initially identified 26,255 citations through database searches, manual searches, citation review and contact with experts (Figure 1). After screening by title and then abstract, we obtained full‐text articles for 29 citations and two abstracts that were potentially eligible for inclusion in the review. We excluded 18 of these for reasons described in the Characteristics of excluded studies table.
1.
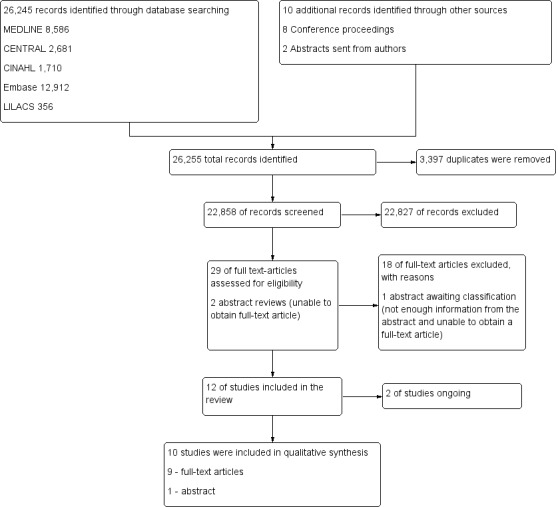
Study flow diagram.29
Included studies
We included in our review 10 trials (Cavalcanti et al, 2013; Hodgson 2011; Huh 2009; Kacmarek 2016; Liu 2011; Meade 2008; Oczenski 2004; Wang 2009; Xi 2010; Yang 2011) that enrolled 1658 participants. The number of participants in each study varied from 20 in a pilot RCT (Hodgson 2011) to 983 in a Phase III multi‐centre RCT (Meade 2008). All trials included participants with acute respiratory distress syndrome (ARDS). Most used the definition of ARDS provided by the North American‐European Consensus Conference (NAECC) (Bernard 1994). Two trials used the NAECC definition but included patients with a ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) less than 250 (Liu 2011; Meade 2008). For full details of the 10 included trials, see the Characteristics of included studies table.
The included trials fell broadly into two groups.
Effects of open lung ventilation (which included co‐interventions such as differences in mode of ventilation and titration of PEEP, as well as recruitment manoeuvres) compared with standard care (Cavalcanti et al, 2013; Hodgson 2011; Huh 2009; Kacmarek 2016; Liu 2011; Meade 2008). Participants were allocated to the treatment group (open lung ventilation including a recruitment manoeuvre) or to a control group (standard care that did not include recruitment manoeuvres). Outcome measures included mortality, oxygenation and hypoxaemia requiring use of rescue therapies and barotrauma.
Effect of recruitment manoeuvres alone (treatment and control groups received the same mode of ventilation and PEEP). Participants were allocated to receive a recruitment manoeuvre or to not receive an RM (Oczenski 2004; Xi 2010; Wang 2009; Yang 2011), and other variables were held constant (mode of ventilation, PEEP).
Recruitment manoeuvres varied between trials (Table 2). Four trials used a staircase or stepwise increment in PEEP (Cavalcanti et al, 2013; Hodgson 2011; Huh 2009; Kacmarek 2016) to a maximum peak pressure of 55 to 60 cm H2O, and four trials used an increase in PEEP to a set pressure (40 to 50 cm H2O) for a short time (30 to 40 seconds) (Meade 2008; Oczenski 2004; Xi 2010; Yang 2011). Two trials did not provide details of the recruitment manoeuvre (Liu 2011; Wang 2009).
1. Description of recruitment manoeuvre procedure.
| Study | Mode |
Peak pressure (cm H2O) |
Time (sec) |
PEEP titration differed between groups |
Mean PEEP after RM (cm H2O) |
Repetitions |
| Cavalcanti et al, 2013 | PCV | 60 (delivered incrementally) | 240 | Yes | 16.1 | Daily (+ after desaturation or disconnection) |
| Hodgson 2011 | PCV | 55 (delivered incrementally) | 360 | Yes | 17.4 | Daily (+ after desaturation or disconnection) |
| Huh 2009 | VCV | ≤ 55 (delivered incrementally to 25 cm H2O PEEP with decremental tidal volume setting) | NS | Yes | 10 | Daily (+ after desaturation or disconnection) |
| Kacmarek 2016 | PCV | ≤ 60 depending on the participant's response (delivered incrementally to PEEP 35 to 45 cm H2O) | 120 | Yes | 15.8 | NS |
| Liu 2011 | NS | NS | NS | Yes | NS | NS |
| Meade 2008 | PCV | 40 | 40 | Yes | 14.6 | Frequently after disconnection |
| Oczenski 2004 | PCV | 50 | 30 | No | 15.1 | Once |
| Wang 2009 | BIPAP | NS | NS | N/A | NS | Eight‐hourly |
| Xi 2010 | CPAP | 40 (cm H2O CPAP) | 40 | No | 10.5 | Eight‐hourly |
| Yang 2011 | CPAP | 40 (cm H2O CPAP) | 30 | No | NS | Eight‐hourly |
Huh 2009 ‐ RM with incremental and decremental titration cycled twice over 10 minutes.
BIPAP = bi‐level positive airway pressure; CPAP = continuous positive airway pressure; NS = not stated; PCV = pressure‐cycled ventilation; sec = seconds; VCV = volume‐cycled ventilation.
Outcome measures varied (Table 3). Five trials assessed mortality at 28 days (Huh 2009; Kacmarek 2016; Liu 2011; Meade 2008; Xi 2010) (Figure 2); five trials assessed mortality at intensive care discharge (Hodgson 2011; Huh 2009; Kacmarek 2016; Meade 2008; Xi 2010) (Figure 3); and four in‐hospital (Hodgson 2011; Kacmarek 2016; Meade 2008; Xi 2010). Mortality was also reported during mechanical ventilation (Meade 2008). Eight trials reported the rate of radiological evidence of barotrauma (Cavalcanti et al, 2013; Hodgson 2011; Huh 2009; Kacmarek 2016; Liu 2011; Meade 2008; Xi 2010; Yang 2011). Three trials reported severe hypoxaemia requiring the use of rescue therapies (Hodgson 2011; Huh 2009; Meade 2008), and six trials reported changes in oxygenation (PaO2/FiO2 ratio) at 24 hours or at 48 hours (Cavalcanti et al, 2013; Hodgson 2011; Huh 2009; Meade 2008; Wang 2009; Xi 2010).
2. Outcomes considered for this review.
| Study | Mortality | Oxygenation | Adverse events |
| Cavalcanti et al, 2013 | N/A | PaO2/FiO2 | Barotrauma |
| Hodgson 2011 | 1. in hospital | PaO2/FiO2 | Barotrauma Rescue therapies |
| Huh 2009 | 1. 28‐day 2. in ICU |
PaO2/FiO2 | Barotrauma Rescue therapies |
| Kacmarek 2016 | 1. in hospital 2. in ICU |
Barotrauma | |
| Liu 2011 | 28‐day | PaO2/FiO2 | Barotrauma |
| Meade 2008 |
|
PaO2/FiO2 | Barotrauma Rescue therapies |
| Oczenski 2004 | N/A | PaO2/FiO2 | N/A |
| Wang 2009 | N/A | PaO2/FiO2 | N/A |
| Xi 2010 |
|
PaO2/FiO2 | Barotrauma |
| Yang 2011 | N/A |
|
Barotrauma Pneumonia |
CO = cardiac output; FiO2/PEEP step = changes in level of inspired oxygen at set levels of positive end‐expiratory pressure; HR = heart rate; ICU = intensive care unit; MAP = mean arterial pressure; N/A = not available; PaO2/FiO2 = fraction of arterial oxygen to inspired oxygen; SBP = systolic blood pressure; SpO2 = oxygen saturation from pulse oximetry.
2.
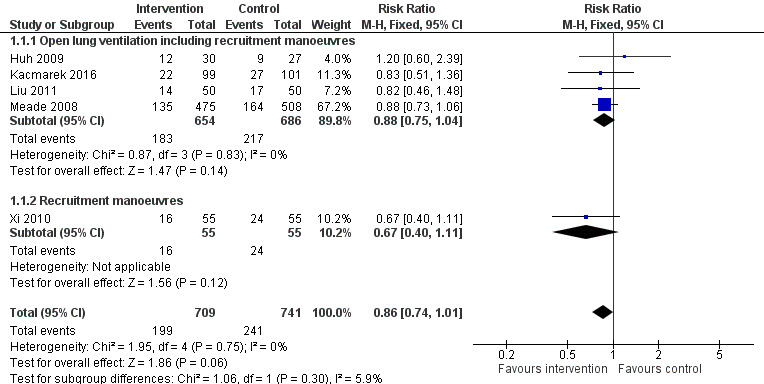
Forest plot of comparison: 1 Recruitment manoeuvres versus no recruitment manoeuvres, outcome: 1.1 28‐Day mortality.
3.
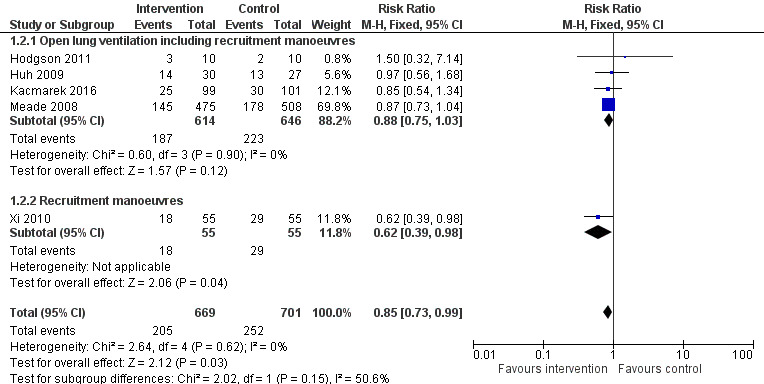
Forest plot of comparison: 1 Recruitment manoeuvres versus no recruitment manoeuvres, outcome: 1.7 ICU mortality.
Awaiting classification
One study is awaiting classification (Wang 2007), as the full article was written in Mandarin and we were unable to contact the study author or the journal for further information. For details, see Characteristics of studies awaiting classification.
Ongoing studies
Two studies are ongoing (ART STudy Investigators; PHARLAP Group Investigators); see Characteristics of ongoing studies for details.
Excluded studies
We excluded from the review 18 full‐text articles (Amato 1995; Amato 1998; Barker 2002; Bollen 2005; Brower 2003; Constantin 2010; Derdak 2002; Dolinay 2011; Dyhr 2003; Foti 2000; Gattinoni 2006; Holzapfel 1987; Hurst 1990; Lasocki 2005; Lim 2003; Lowhagen 2011; Meade 2002; Stewart 2002) (Figure 1). For details of excluded trials, see the Characteristics of excluded studies table.
Risk of bias in included studies
The 10 trials included in this systematic review had low to moderate risk of bias (Figure 4). Five trials demonstrated low risk of bias (Cavalcanti et al, 2013; Hodgson 2011; Kacmarek 2016; Meade 2008; Oczenski 2004), and five demonstrated moderate risk (Huh 2009; Liu 2011; Wang 2009; Xi 2010; Yang 2011) (Figure 5).
4.
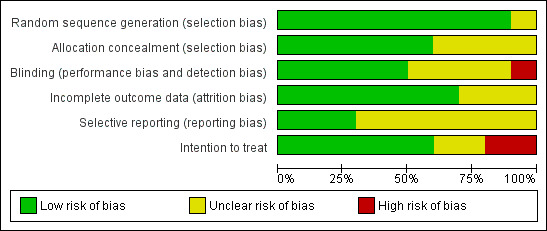
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
5.
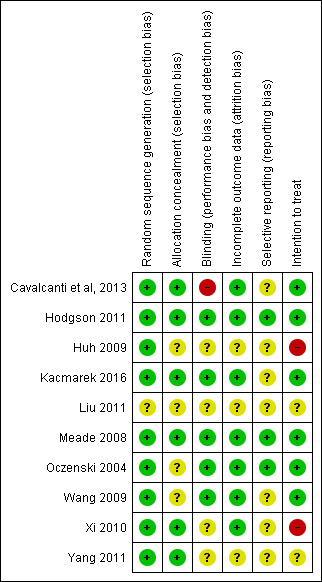
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Five trials (Cavalcanti et al, 2013; Hodgson 2011; Kacmarek 2016; Meade 2008; Xi 2010) clearly used adequate randomization and allocation schemes (see Characteristics of included studies table). One trial reported a programming error in the allocation procedure that occurred late in the study and disrupted specific randomization blocks; this may have accounted for modest baseline imbalances in age and presence of sepsis (Meade 2008). We addressed this by performing secondary analysis with adjustments for age, sepsis, acute physiology and duration of hospitalization.
Blinding
The intervention did not allow investigators or bedside staff to be blinded to group allocation. We assumed that participants were unaware of group allocation because they were critically ill, and consent for participation in the study was gained from the next of kin. No trials described blinding of the outcome assessor, and one trial described the data analysis as blinded (Meade 2008).
Incomplete outcome data
All trials completely reported mortality, except one trial, which excluded 15 participants for failure to follow the study protocol ‐ three in the control group and 12 in the intervention group ‐ and did not analyse these participants as intention‐to‐treat (Xi 2010).
Selective reporting
One trial excluded 15 participants for failure to follow the study protocol ‐ three in the control group and 12 in the intervention group ‐ and did not analyse these participants as intention‐to‐treat (Xi 2010).
Other potential sources of bias
We observed significant clinical heterogeneity. Recruitment manoeuvres varied between trials in terms of maximum pressure achieved, duration of maximum pressure, mode of delivery and PEEP after the recruitment manoeuvre (Table 2). Five trials included recruitment manoeuvres as part of an open lung ventilation strategy that was different from the control ventilation in aspects other than the recruitment manoeuvre (such as PEEP titration) (Hodgson 2011; Huh 2009; Kacmarek 2016; Liu 2011; Meade 2008).
Effects of interventions
See: Table 1
Primary outcome: mortality
One trial assessed effects of recruitment manoeuvres alone on the primary outcome of mortality (Xi 2010). This trial had a moderate risk of bias and did not use intention‐to‐treat analysis (Analysis 1.1).
1.1. Analysis.
Comparison 1 Recruitment manoeuvres versus no recruitment manoeuvres, Outcome 1 28‐Day mortality.
Five trials assessed effects of open lung ventilation that included recruitment manoeuvres (along with other co‐interventions such as differences in PEEP) and reported primary outcomes (Hodgson 2011; Huh 2009; Kacmarek 2016; Liu 2011; Meade 2008) (see Table 3). As these trials were randomized controlled trials that included recruitment manoeuvres, we have provided the results below, but we acknowledge that effects of recruitment manoeuvres cannot be isolated from co‐interventions of the open lung ventilation strategy, as outlined in the discussion (Analysis 1.1). For this reason, we downgraded mortality outcomes from high to low quality.
28‐Day mortality
Analysis 1.1: Huh 2009, Kacmarek 2016, Liu 2011, Meade 2008 and Xi 2010 examined 28‐day mortality (five trials; N = 1450). We used the fixed‐effect model to pool data from these trials, as the funnel plot demonstrated minimal asymmetry (I2 = 0%). Ventilatory strategies that included recruitment manoeuvres did not appear to reduce 28‐day mortality (RR 0.86, 95% CI 0.74 to 1.01, P = 0.06) (Figure 2). Effects on 28‐day mortality were not different for trials of open lung ventilation that included recruitment manoeuvres (Huh 2009; Kacmarek 2016; Liu 2011; Meade 2008) compared with the one trial that provided recruitment manoeuvres alone (Xi 2010).
ICU mortality
Analysis 1.2: Hodgson 2011, Huh 2009, Kacmarek 2016, Meade 2008 and Xi 2010 examined ICU mortality (five trials; N = 1370). We used the fixed‐effect model to pool data from these trials, as the funnel plot demonstrated minimal asymmetry (I2 = 0%). Recruitment manoeuvres significantly reduced mortality in intensive care (RR 0.83, 95% CI 0.72 to 0.97, P = 0.02) (Figure 3). Effects on ICU mortality were different for trials of open lung ventilation that included recruitment manoeuvres (RR 0.86, 95% CI 0.74 to 1.01, P = 0.07) (Huh 2009; Kacmarek 2016; Liu 2011; Meade 2008) compared with the one trial that provided recruitment manoeuvres alone (RR 0.62, 95% CI 0.39 to 0.98, P = 0.04) (Xi 2010).
1.2. Analysis.
Comparison 1 Recruitment manoeuvres versus no recruitment manoeuvres, Outcome 2 ICU mortality.
In‐hospital mortality
Analysis 1.3: Hodgson 2011, Kacmarek 2016, Meade 2008 and Xi 2010 examined in‐hospital mortality (four studies; N = 1313). We used the fixed‐effect model to pool data from these trials, as the funnel plot demonstrated minimal asymmetry (I2 = 0%). Recruitment manoeuvres did not reduce mortality in‐hospital (RR 0.88, 95% CI 0.77 to 1.01, P = 0.07). Effects on hospital mortality were not different for trials of open lung ventilation that included recruitment manoeuvres (Huh 2009; Kacmarek 2016; Liu 2011; Meade 2008) compared with the one trial that provided recruitment manoeuvres alone (Xi 2010).
1.3. Analysis.
Comparison 1 Recruitment manoeuvres versus no recruitment manoeuvres, Outcome 3 In‐hospital mortality.
Secondary outcomes
Many of the secondary outcomes described in this section were measured at different time points. When possible, we pooled results based on outcomes with similar time points of measurement.
Barotrauma
Analysis 1.4: Eight trials reported rates of barotrauma (Cavalcanti et al, 2013; Hodgson 2011; Huh 2009; Kacmarek 2016; Liu 2011; Meade 2008; Xi 2010; Yang 2011). We used the fixed‐effect model to pool data, as the funnel plot demonstrated minimal asymmetry for seven studies (I2 = 0%). Recruitment manoeuvres did not significantly affect the risk of barotrauma (RR 1.09, 95% CI 0.78 to 1.53, P = 0.60). Three trials reported no barotrauma in intervention or control groups (Hodgson 2011; Xi 2010; Yang 2011). One study reported no differences in the rate of barotrauma between groups and did not report specific numbers of participants (RR 0.78, 95% CI 0.19 to 3.30; Cavalcanti et al, 2013).
1.4. Analysis.
Comparison 1 Recruitment manoeuvres versus no recruitment manoeuvres, Outcome 4 Rate of barotrauma.
Rescue therapies
Analysis 1.5: Three trials reported use of rescue therapies for participants with severe hypoxaemia (Hodgson 2011; Huh 2009; Meade 2008). We used the random‐effects model to pool data (I2 = 74%). An open lung ventilation strategy that included recruitment manoeuvres had no effect on the use of rescue therapies for participants with severe hypoxaemia (RR 0.64, 95% CI 0.27 to 1.51, P = 0.31).
1.5. Analysis.
Comparison 1 Recruitment manoeuvres versus no recruitment manoeuvres, Outcome 5 Use of rescue therapies.
Oxygenation
Analysis 1.6: Six trials reported changes in oxygenation (PaO2/FiO2 from baseline to 24 or 48 hours after randomization) with a recruitment manoeuvre (Cavalcanti et al, 2013; Hodgson 2011; Huh 2009; Meade 2008; Wang 2009; Xi 2010); however, four of these trials included open lung ventilation along with other changes in the intervention group such as increased PEEP (Cavalcanti et al, 2013; Hodgson 2011; Huh 2009; Meade 2008).
1.6. Analysis.
Comparison 1 Recruitment manoeuvres versus no recruitment manoeuvres, Outcome 6 PaO2/FiO2 ratio at 24 to 48 hours.
Several trials measured oxygenation at more than one time point. For inclusion of these trials, we chose the time point closest to 24 hours after the recruitment manoeuvre. We used a random‐effects model to analyse study results (I2 = 88%). Recruitment manoeuvres improved oxygenation 24 to 48 hours after randomization compared with standard care (MD ‐39.10, 95% CI ‐57.64 to ‐20.56, P < 0.0001). The funnel plot demonstrated significant asymmetry indicating selection bias (publication or location bias); poor methodological quality of smaller studies (design, analysis, fraud); true heterogeneity (variation in effect size); and artefact or chance differences in study samples.
One trial reported a positive response to a recruitment manoeuvre that was maintained for only a few minutes (Oczenski 2004) and was not included in the analysis. Oczenski 2004 randomly assigned 30 participants from a positive end‐expiratory (PEEP) trial who had low tidal volumes and high PEEP to receive a recruitment manoeuvre or to not receive an RM. Compared with control, a recruitment manoeuvre significantly increased the PaO2/FiO2 ratio (139 ± 46 vs 246 ± 111, P < 0.001) and the shunt fraction (30.8 ± 5.8 vs 29.2 ± 7.4) three minutes later. In both groups, values returned to baseline within 30 minutes, and no significant differences between groups were noted.
Two trials investigated recruitment manoeuvres alone compared with standard care (with no other changes in ventilation strategy such as PEEP or plateau pressure) and found no differences in oxygenation at 24 to 48 hours (Wang 2009; Xi 2010).
Subgroup analyses
We found insufficient data and clinical heterogeneity in the included trials, and we were unable to perform planned subgroup analyses.
Discussion
Summary of main results
We included 10 trials in this systematic review (Cavalcanti et al, 2013; Hodgson 2011; Huh 2009; Kacmarek 2016; Liu 2011; Meade 2008; Oczenski 2004; Wang 2009; Xi 2010; Yang 2011). We pooled data from eight of the nine randomized controlled trials that provided mechanical ventilation for participants with acute respiratory distress syndrome (ARDS) with an intervention that included recruitment manoeuvres (RMs) compared with standard care (without RMs) (Cavalcanti et al, 2013; Hodgson 2011; Huh 2009; Kacmarek 2016; Liu 2011; Meade 2008; Xi 2010; Yang 2011). Trials varied with regards to the RM used (duration, maximum pressure, mode of delivery) and with regards to risk of bias. Six trials included an "open lung ventilation strategy" whereby the intervention group differed from the control group in providing co‐interventions other than the RM (such as higher positive end‐expiratory pressure (PEEP)) (Cavalcanti et al, 2013; Hodgson 2011; Huh 2009; Kacmarek 2016; Liu 2011; Meade 2008). Clinical heterogeneity of these studies makes it difficult to interpret results.
Six trials reported on events for our primary outcome (mortality) (Hodgson 2011; Huh 2009; Kacmarek 2016; Liu 2011; Meade 2008; Xi 2010). The intervention group in five of these trials received open lung ventilation with co‐interventions, such as increased PEEP, as well as RMs (Hodgson 2011; Huh 2009; Kacmarek 2016; Liu 2011; Meade 2008). Recruitment manoeuvres were not allowed in control participants. Results demonstrated statistically significant differences in intensive care unit (ICU) mortality, but not in 28‐day or hospital mortality. The composite design of these interventions means that any difference in outcomes between control and intervention groups could be due to any of the components of the intervention, or, as likely, to the combined synergistic effects of two or more of these components. A recent individual participant data meta‐analysis reported an association between improved survival in participants with acute respiratory distress who were mechanically ventilated and reduced driving pressure during mechanical ventilation (Amato 2015). It is possible that trials included in this review that provided an intervention with open lung ventilation including RMs may have also used reduced driving pressure (as a result of increased PEEP combined with protective lung ventilation), and this may have had an effect on mortality. Only one trial isolated the effects of RMs on our primary outcome and showed no difference between intervention and control groups (Xi 2010); however, this trial had a moderately high risk of bias (Figure 5).
Recruitment manoeuvres were performed at different time points following randomization in the included trials; they were performed in response to both disconnection and desaturation in several trials, further complicating interpretation of results. In the largest trial (Meade 2008), the intervention group (n = 423) received an RM at the start of the trial, and 366 received at least one additional RM following ventilator disconnection (but 57 did not receive the additional RM). Hodgson 2011 applied a daily staircase RM in the intervention group that lasted up to six minutes but performed brief RMs throughout the day for disconnection or desaturation. Three trials performed RMs eight‐hourly (Wang 2009; Xi 2010; Yang 2011). As a result of the clinical heterogeneity associated with dose and repetition of RMs, as well as the use of co‐interventions, it is not clear whether one or multiple RMs might have caused the effect. Additionally, we found limited information on who was involved in ventilatory management within the ICU, so we can draw no conclusions about international differences in ventilatory management for patients with ARDS.
We pooled the results of seven randomized trials and found that RMs did not affect the rate of barotrauma (Hodgson 2011; Huh 2009; Kacmarek 2016; Liu 2011; Meade 2008; Xi 2010; Yang 2011). These results support a recent systematic review of RMs and clinical outcomes (Suzumura 2014) and may indicate that RMs are safe; however, clinical heterogeneity between studies was significant. Evidence previously obtained by computerized tomography (Gattinoni 2006) indicated that response to high PEEP is heterogeneous in patients with ARDS and may lead to over‐distension rather than to lung recruitment.Study authors reported that some participants with ARDS might benefit from an RM and high PEEP, although for others, this approach may be harmful. Future individual participant data meta‐analyses may answer specific questions regarding response to RMs (i.e. responders vs non‐responders).
This review reported an increase in oxygenation from baseline to 24 to 48 hours after RMs were commenced in six pooled trials (Cavalcanti et al, 2013; Hodgson 2011; Huh 2009; Meade 2008; Wang 2009; Xi 2010). However, the isolated effect of RMs in Oczenski 2004 increased the fraction of arterial oxygen to inspired oxygen (PaO2/FiO2) for three minutes (returning to baseline levels within 30 minutes). Similarly, two trials of RMs (in isolation from other components of open lung ventilation) reported that oxygenation was unchanged compared with control at 24 or 48 hours after RMs (Wang 2009; Xi 2010). Additional studies are needed to determine effects of RMs in isolation from other co‐interventions on sustained changes in oxygenation.
Overall completeness and applicability of evidence
In two trials comparing open lung ventilation (Hodgson 2011; Meade 2008), sample size was calculated before the study commenced. The target sample was reached in Meade 2008, which reported no loss to follow‐up. The Hodgson 2011 pilot study recruited 20 participants at a single centre as planned, which limits the external validity of the study. Kacmarek 2016 was stopped early owing to slow recruitment, although it was a multi‐centre study.
Quality of the evidence
Although we judged the included trials to be at varying risks of bias overall, we drew evidence for our main outcomes from studies at low risk of bias. Effects of additional ventilatory co‐interventions were not controlled (Cavalcanti et al, 2013; Hodgson 2011; Huh 2009; Kacmarek 2016; Liu 2011; Meade 2008). Recruitment manoeuvres were applied inconsistently and in some cases were linked to ventilatory disconnection and desaturation. Investigators did not blind treatment or assessor, and only one trial reported blinding of the analysis (Meade 2008). One trial did not use intention‐to‐treat analysis (Xi 2010) and excluded 15 participants from the final analysis for failure to adhere to the study protocol; this limits the applicability of the evidence, and we rated this study as having high risk of bias.
Potential biases in the review process
We identified several potential biases in the review process. First, we excluded studies with cross‐over design, as we could not assess the primary outcome. However, other outcomes (barotrauma, oxygenation) may have been relevant to the review. Second, we excluded length of stay as an outcome measure (hospital length of stay; ICU length of stay), as survivors and non‐survivors were not reported separately. In the future, we recommend that randomized controlled trials should report length of stay for both survivors and non‐survivors separately. Third, we included studies with a transient increase in pressure from baseline as an RM. In doing so, we excluded studies of airway pressure release ventilation, which is a mode of ventilation that is used to recruit alveoli and may be considered a type of RM. The study by Kacmarek was completed in 2007 but was not published until 2014. Finally, one study not included in the review is awaiting classification, as we were unable to contact the study author (Wang 2009).
Agreements and disagreements with other studies or reviews
Some issues remain open for debate. In particular, do recruitment manoeuvres, in isolation from other co‐interventions, have an impact on outcomes such as survival? Data are currently insufficient to answer this question. It is plausible that RMs alone are not sufficient to improve longer‐term outcomes, but they add value when used with co‐interventions as part of an open lung ventilation strategy that includes low tidal volumes, high PEEP and limited plateau pressures (Amato 2015; ARDSnet 2000; Kacmarek 2007; Meade 2008).
Also, if RMs have a beneficial effect on outcomes, what would be the optimal inspiratory pulmonary pressure, length of time and level of PEEP needed to maintain such effects? All of the included studies used different inspiratory pressures for different lengths of time, with varying levels of PEEP (Table 2). The mode of ventilation used to achieve an RM also varied widely. Any given inspiratory pressure might be effective in some patients, ineffective in some and harmful in others (for instance, by overdistending lung units). It may be important to determine the minimum PEEP that sustains the benefits of an RM (Briel 2010; Kacmarek 2007; Lapinsky 2005).
Evidence is insufficient to show the optimal frequency of delivering an RM in patients with acute respiratory distress syndrome.
Authors' conclusions
Implications for practice.
Evidence in our review on delivery of recruitment manoeuvres for participants with acute ARDS shows considerable heterogeneity. Available evidence suggests that recruitment manoeuvres may improve intensive care unit survival, and that they are used predominantly as part of an open lung ventilation strategy. Oxygenation was increased from baseline to 24 to 48 hours after a recruitment manoeuvre, and no evidence suggests increased barotrauma. At this stage, recruitment manoeuvres delivered once per day as part of an open lung strategy during moderate to severe acute respiratory distress syndrome may be beneficial. The largest studies to date have used a plateau pressure of 40 to 50 cm H2O, with recent (Kacmarek 2016) and incomplete trials (ART STudy Investigators; PHARLAP Group Investigators) increasing inspiratory pressure stepwise to 55 to 60 cm H2O. Ongoing studies in this area may have an influence on future practice (ART STudy Investigators; PHARLAP Group Investigators).
Implications for research.
Recruitment manoeuvres generally are used as part of an open lung ventilation strategy, with positive end‐expiratory pressure (PEEP) titrated following the recruitment manoeuvre on an individual basis to maintain lung recruitment. Further research is required to determine the ideal recruitment manoeuvre with regards to duration, peak pressure and titration of PEEP. An individual participant data meta‐analysis may reveal whether effects of recruitment manoeuvres vary with severity and cause of acute respiratory distress syndrome and with the type of recruitment manoeuvre provided. In addition, future research on recruitment manoeuvres may be required to separate effects of recruitment manoeuvres from those of co‐interventions such as increased PEEP, although clinically, recruitment manoeuvres are rarely used in isolation, and a separate analysis of open lung ventilation (including limited tidal volumes and inspiratory pressure with recruitment manoeuvres and titrated PEEP) may be pertinent.
What's new
| Date | Event | Description |
|---|---|---|
| 14 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 3, 2007 Review first published: Issue 2, 2009
| Date | Event | Description |
|---|---|---|
| 1 August 2016 | New citation required and conclusions have changed | We added Meredith Young and Ewan Goligher as review authors. The conclusions of this review have changed with the inclusion of new studies. |
| 1 August 2016 | New search has been performed | We updated the title to match the Berlin definition of acute respiratory distress syndrome (ARDS) (ARDS Definition Task Force 2012). We updated the search from May 2008 to August 2016. We found 15 new studies; we included 8 and excluded 4, 2 are ongoing and 1 is awaiting classification. We added a new risk of bias table. We updated methods and outcome measures. We removed length of stay (hospital and ICU) as an outcome, as it is not reported for both survivors and non‐survivors. We removed blood pressure as an outcome, as it was reported generally as a short‐term outcome. We added use of rescue therapies as an outcome. We redefined the control group standard of care as "protective lung ventilation". We excluded the Amato 1998 study, as investigators used 12 mL/kg in the control group, which is not standard care. We excluded cross‐over trials from the included studies. We assessed the strength of the evidence by using GRADE. |
| 9 May 2012 | Amended | We updated contact details. |
| 23 November 2010 | Amended | We updated contact details. |
| 2 September 2008 | Amended | We converted this review to the new review format. |
Acknowledgements
We would like to acknowledge the support of the ANZIC‐RC, the NHMRC (CH fellowship) and Alfred Health. We would also like to acknowledge and thank Andrew R Davies for his contribution to the original review (Hodgson 2009).
We would like to thank Sue Huckson, Thomas Bein, Davide Chiumello, and Todd Rice (Peer Referees); Cathal D Walsh (Statistical Editor); Corrie Billedeau and Janet L Wale (Consumer Referee and Editor); and Harald Herkner (Content Editor) for help and advice provided during preparation of the updated review.
We would like to thank John Carlisle (Content Editor), Nathan Pace (Statistical Editor) and Rafael Fernandez, Malcolm G Booth and David Pestaña (Peer Reviewers) for help and editorial advice provided during preparation of the previous review (Hodgson 2009).
Appendices
Appendix 1. MEDLINE OVID (January 1966 to August 2016)
#1 (recruit$ and (manoeuv$ or manouev$ or maneuv$ or manuev$)).af. #2 (recruitment or derecruitment).ti,ab. #3 exp respiration, artificial/ or exp positive pressure respiration/ or ventilat$.ti,ab. #4 (recruit$ and (respirat$ or lung or pulmon$ or airway$)).af. #5 #2 or #3 or #4 #6. exp Lung/ or exp Respiratory Distress Syndrome, Adult/ or exp Atelectasis/ #7 ((lung adj injury) or (lung adj collapse$) or (alveoli adj collapse$) or atelecta$ or hypox?emia or hypoxic or oxygenation).ti,ab. #8 #6 or #7 #9 #5 and #8 #10 #1 or #9 #11 clinical trial$.af. #12 randomi?ed.ti,ab. #13 placebo.ti,ab. #14 dt.fs. #15 (random or randomly).ti,ab. #16 (trial or trials).ti,ab. #17. groups.ti,ab. #18 #11 or #12 or #13 or #14 or #15 or #16 or #17 #19 Animals/ #20 Humans/ #21 #19 and #20 #22 #19 not #21 #23 #18 not #22 #24 #10 and #23
Appendix 2. CINAHL OVID (January 1982 to August 2016)
#1 (recruit$ and (manoeuv$ or manouev$ or maneuv$ or manuev$)).af. #2 (recruitment or derecruitment).ti,ab. #3 exp Ventilation, Mechanical/ or exp Positive Pressure Ventilation/ or ventilat$.ti,ab. #4 (recruit$ and (respirat$ or lung or pulmon$ or airway$)).af. #5 #2 or #3 or #4 #6 exp Lung/ or exp Respiratory Distress Syndrome, Acute/ or exp Atelectasis/ #7 ((lung adj injury) or (lung adj collapse$) or (alveoli adj collapse$) or atelecta$ or hypox?emia or hypoxic or oxygenation).ti,ab. #8 #6 or #7 #9 #5 and #8 #10 #1 or #9 #11 clinical trial$.af #12 randomi?ed.ti,ab #13 placebo.ti,ab. #14 dt.fs. #15 (random or randomly).ti,ab. #16 (trial or trials).ti,ab #17 groups.ti,ab. #18 #11 or #12 or #13 or #14 or #15 or #16 or #17 #19 #10 and #18
Appendix 3. CENTRAL, the Cochrane Library (2016, Issue 7)
#1 (recruit* and (manoeuv* or manouev* or maneuv* or manuev*)) #2 (recruitment or derecruitment):ti,ab #3 MeSH descriptor Respiration, Artificial explode all trees #4 MeSH descriptor Positive‐Pressure Respiration explode all trees #5 (ventilat*):ti,ab #6 (recruit* and (respirat* or lung or pulmon* or airway*)) #7 (#2 or #3 or #4 or #5 or #6) #8 MeSH descriptor Lung explode all trees #9 MeSH descriptor Respiratory Distress Syndrome, Adult explode all trees #10 MeSH descriptor Atelectasis explode all trees #11 (lung NEXT injury):ti,ab or (lung NEXT collaps*):ti,ab or (alveoli NEXT collaps*):ti,ab or (atelecta* OR hypox?emia OR hypoxic OR oxygenation):ti,ab #12 (#8 or #9 or #10 or #11) #13 (#7 and #12) #14 (#1 or #13)
Appendix 4. Embase OVID (January 1980 to August 2016)
#1 (recruit$ and (manoeuv$ or manouev$ or maneuv$ or manuev$)).af. #2 (recruitment or derecruitment).ti,ab. #3 exp Artificial Ventilation/ or exp Positive End Expiratory Pressure/ or ventilat$.ti,ab. #4 (recruit$ and (respirat$ or lung or pulmon$ or airway$)).af. #5 #2 or #3 or #4 #6 exp atelectasis/ or exp acute lung injury/ or exp adult respiratory distress syndrome/ or exp Lung Injury/ #7 ((lung adj injury) or (lung adj collapse$) or (alveoli adj collapse$) or atelecta$ or hypox?emia or hypoxic or oxygenation).ti,ab. #8 #6 or #7 #9 #5 and #8 #10 #1 or #9 #11 clinical trial$.af. #12 random?ed.ti,ab. #13 placebo.ti,ab. #14 dt.fs. #15 (random or randomly).ti,ab. #16 (trial or trials).ti,ab. #17 groups.ti,ab. #18 #11 or #12 or #13 or #14 or #15 or #16 or #17 #19 exp Animal/ #20 Human/ #21 #19 and #20 #22 #19 not #21 #23 #18 not #22 #24 #10 and #23
Appendix 5. LILACS OVID (January 1982 to August 2016)
(("recruit$" or "derecruit$" or "respiration, artificial" or "artificial respiration" or "respiration, artificial/" or "recruitment" or "positive‐pressure respiration" or "ventilat$")) and (("oxygenation" or "hypoxic" or "hypoxaemia" or "hypoxemia" or "atelecta$" or "alveoli collapse$" or "lung collapse$" or "lung injury" or "lung" or "respiratory distress syndrome, acute/" or "respiratory distress syndrome, adult/" or "atelectasis"))
Data and analyses
Comparison 1. Recruitment manoeuvres versus no recruitment manoeuvres.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 28‐Day mortality | 5 | 1450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.74, 1.01] |
| 1.1 Open lung ventilation including recruitment manoeuvres | 4 | 1340 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.75, 1.04] |
| 1.2 Recruitment manoeuvres | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.40, 1.11] |
| 2 ICU mortality | 5 | 1370 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.73, 0.99] |
| 2.1 Open lung ventilation including recruitment manoeuvres | 4 | 1260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.75, 1.03] |
| 2.2 Recruitment manoeuvres | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.39, 0.98] |
| 3 In‐hospital mortality | 4 | 1313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.77, 1.01] |
| 3.1 Open lung ventilation including recruitment manoeuvres | 3 | 1203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.78, 1.04] |
| 3.2 Recruitment manoeuvres | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.50, 1.09] |
| 4 Rate of barotrauma | 7 | 1508 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.78, 1.51] |
| 4.1 Open lung ventilation including recruitment manoeuvres | 6 | 1398 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.78, 1.51] |
| 4.2 Recruitment manoeuvres | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Use of rescue therapies | 3 | 1060 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.27, 1.51] |
| 6 PaO2/FiO2 ratio at 24 to 48 hours | 6 | 1270 | Mean Difference (IV, Random, 95% CI) | ‐39.10 [‐57.64, ‐20.56] |
| 6.1 Open lung ventilation including recruitment manoeuvres | 5 | 1160 | Mean Difference (IV, Random, 95% CI) | ‐44.76 [‐66.29, ‐23.22] |
| 6.2 Recruitment manoeuvres | 1 | 110 | Mean Difference (IV, Random, 95% CI) | ‐17.0 [‐37.19, 3.19] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cavalcanti et al, 2013.
| Methods | Report of the first 100 participants in a prospective, multi‐centre, parallel‐group RCT | |
| Participants | n = 100 participants with moderate to severe ARDS (< 72 hours in ICU) No inclusion/exclusion criteria stated in the abstract but full details of the study available in the published protocol |
|
| Interventions | Maximal stepwise alveolar recruitment manoeuvre followed by ventilation at optimal PEEP | |
| Outcomes | Preliminary results of a larger study: oxygenation (PaO2/FiO2 ratio daily for 7 days) and barotrauma during first 7 days | |
| Notes | Conference abstract only, direct contact with study authors July 2016 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized random block schedule |
| Allocation concealment (selection bias) | Low risk | Web‐based, centralized, automated randomization schedule |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label design Blinding of treatment: no Blinding of assessor: no Blinding of data analysis: not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were reported for all participants. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes listed in the methods were reported in the results. |
| Intention to treat | Low risk | Primary analysis was by intention‐to‐treat. |
Hodgson 2011.
| Methods | Prospective, single‐centre, parallel‐group RCT | |
| Participants | n = 20. Inclusion criteria: ARDS, PaO2/FiO2< 200, age > 15 years in 1 ICU in Australia (2007 to 2009) Excluded if chest trauma, intercostal catheter with air leak, pneumothorax, bronchospasm, raised ICP, MAP ≤ 60 mmHg, significant arrhythmias or MV > 72 hours |
|
| Interventions | Treatment: staircase recruitment manoeuvre to peak of 55 cm H2O airway pressure and decremental PEEP titration to determine optimal PEEP, performed daily with PCV, VT < 6 mL/kg Pplat < 30 mmHg, permissive hypercapnia. Additionally, RM with PEEP = 40 cm H2O for 1 minute was performed after oxygen desaturation or circuit disconnection Control: ARDSnet protocol, with ACVC and FiO2/PEEP titration, VT < 6 mL/kg, plateau pressure < 30 mmHg |
|
| Outcomes |
|
|
| Notes | The first author of this Cochrane review declares a conflict of interest, as she is the first study author on this publication. Data were extracted by independent researchers (see Declarations of interest). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized random block schedule, stratified by diagnosis of severe sepsis |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open‐label design Blinding of treatment: no Blinding of assessor: not stated Blinding of data analysis: not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were reported for all participants. |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were reported in the results. |
| Intention to treat | Low risk | Primary analysis was by intention‐to‐treat. |
Huh 2009.
| Methods | Prospective, single‐centre, parallel‐group RCT | |
| Participants | n = 57. Inclusion criteria: ARDS, PaO2/FiO2 < 200 in Korea and Seoul | |
| Interventions | Treatment: extended sigh method of recruitment manoeuvre to peak of 55 cm H2O airway pressure and VCV with VT decreased by 25% from baseline, performed daily for 7 days. PCV, VT = 6 mL/kg, optimal PEEP setting determined during decremental PEEP titration. RM was also performed after oxygen desaturation or circuit disconnection. Control: ARDSNet protocol, with table‐based FiO2/PEEP strategy, PCV, VT = 6 mL/kg |
|
| Outcomes | Primary: oxygenation measured with PaO2/FiO2 daily for 7 days Secondary: PEEP and dynamic compliance (daily for 7 days), ICU LOS, duration of sedatives and paralysing agents, duration of MV, 28‐day mortality, 60‐day mortality and 90‐day mortality |
|
| Notes | Exclusion criteria not clearly stated, but 4 participants with fulminant hepatitis or terminal cancer were excluded. Contacted study author directly by email for additional details and received a response on 19.03.2015 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a randomization scheme |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participant follow‐up was incomplete. |
| Selective reporting (reporting bias) | Unclear risk | 4 participants who withdrew from the study were excluded from the analysis. It is unclear on what bases these participants were excluded. |
| Intention to treat | High risk | 4 participants who withdrew from the study were excluded from the analysis. It is unclear on what bases these participants were excluded. |
Kacmarek 2016.
| Methods | Prospective, multi‐centre, parallel‐group RCT | |
| Participants | n = 200. Inclusion criteria: ARDS, PaO2/FiO2< 200 at FiO2 ≥ 0.5 and PEEP ≥ 10 in North America, Brazil, Spain and Chile, from 2007 to 2013 Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment: staircase recruitment manoeuvre in PCV to peak of ≤ 60 cm H2O airway pressure for 2 minutes and decremental PEEP titration to determine optimal PEEP. Ongoing ventilation with peak pressure ≤ 30 mmHg to VT 3‐5 mL/kg of PBW, RR ≤ 35/min to PaCO2 40‐50 Control: ARDSnet protocol, VCV, VT < 6 mL/kg of PBW, RR ≤ 35/min to maintain pH of 7.30‐7.45, plateau pressure ≤ 30 mmHg, I:E ratio 1:1‐1:3 |
|
| Outcomes | ICU and hospital, 28‐day and 60‐day mortality, ICU length of stay, ventilator‐free days, adverse events (hypotension, hypoxaemia, pneumothorax, arrhythmias, cardiac arrest) | |
| Notes | Contacted the first study author. Trial ceased early owing to low rate of enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Contacted co‐investigators to clarify the procedure. Awaiting full publication |
| Allocation concealment (selection bias) | Low risk | Contacted co‐investigators to clarify the procedure. Awaiting full publication |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open‐label design |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome was reported for all enrolled participants. |
| Selective reporting (reporting bias) | Unclear risk | Not yet fully reported. Awaiting full publication |
| Intention to treat | Low risk | Yes |
Liu 2011.
| Methods | Prospective parallel‐group RCT | |
| Participants | n = 100. Early ARDS, with 91 meeting ARDS criteria in Taiwan Inclusion criteria
Exclusion criteria
|
|
| Interventions | Intervention group: lung recruitment manoeuvre conducted with PEEP 35 cm H2O and peak inspiration pressure up to 50 cm H2O maintained for 2 minutes, then PEEP was set higher at 2 cm H2O above closing pressure (no description of how closing pressure was defined) Control group: ventilated with lung protective ventilatory strategy only |
|
| Outcomes | Primary: 28‐day mortality Secondary: duration of mechanical ventilation, ICU length of stay |
|
| Notes | Abstract only. No address for correspondence. Registered on clinicaltrials.gov and conference abstract citing results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Intention to treat | Unclear risk | Not stated |
Meade 2008.
| Methods | Prospective, multi‐centre, parallel‐group RCT | |
| Participants | n = 983 Participants were given diagnosis of ARDS with PaO2/FiO2 < 250 Excluded if left atrial hypertension, anticipated MV < 48 hours, inability to wean from experimental strategies, severe chronic respiratory disease, neuromuscular disease, intracranial hypertension, morbid obesity, pregnancy, conditions with expected 6‐month mortality risk > 50% | |
| Interventions | Treatment: RM = after allocation to treatment group CPAP 40 cm H2O for 40 seconds with FiO2 1.0. Subsequent RMs after circuit disconnection (up to 4 each day) were not defined. PCV, VT 6 mL/kg, Pplat < 40, high PEEP (mean 14.6 cm H2O) Control: VT 6 mL/kg, Pplat < 30, standard PEEP (mean 9.8 cm H2O) |
|
| Outcomes | Primary: hospital mortality Secondary: ICU mortality, 28‐day mortality, time to independent breathing, refractory hypoxaemia, barotrauma, use of rescue therapies |
|
| Notes | Recruitment manoeuvres were part of a package of ventilation. The other differences between intervention group and control group ventilation packages were tidal volume, plateau pressure, fraction of inspired oxygen, inspiratory:expiratory ratio and PEEP. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐stratified enrolment by site via variable permutated blocks |
| Allocation concealment (selection bias) | Low risk | Central computerized telephone system used for allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open‐label design Blinding of treatment: no Blinding of assessor: not stated Blinding of data analysis: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods were reported in the results. |
| Intention to treat | Low risk | Primary analysis was by intention‐to‐treat. |
Oczenski 2004.
| Methods | Prospective, single‐centre RCT | |
| Participants | n = 30 Extrapulmonary ARDS for < 72 hours (NAECC PaO2/FiO2 < 200, PEEP ≥ 5 cm H2O, PCWP 18 mmHg and/or LVEF < 50%) Excluded if direct lung injury (pulmonary ARDS), SBP < 100, arrhythmias, APO, barotrauma | |
| Interventions | Treatment: RM = CPAP 50 cm H2O for 30 seconds once only, VT 6 mL/kg, Pplat < 30, PEEP determined by incremental PEEP trial Control: VT 6 mL/kg, Pplat < 30, PEEP determined by incremental PEEP trial, no RMs |
|
| Outcomes | Oxygenation measured with PaO2/FiO2, HR, MAP 3 minutes and 30 minutes post RM | |
| Notes | Only short‐term outcomes reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of treatment: no Blinding of assessor: not stated Blinding of data analysis: not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were reported in the results. |
| Intention to treat | Low risk | Primary analysis was by intention‐to‐treat. |
Wang 2009.
| Methods | Prospective single‐centre RCT | |
| Participants | n = 20 participants with ARDS | |
| Interventions | Treatment: RM in BIPAP mode, performed 8‐hourly for 7 days or until weaning of MV Both groups: lung protective ventilation |
|
| Outcomes | EVLW, EVLWI after RM, respiratory mechanics, oxygenation parameters, CVP, plasma COP, dosage of corticosteroid and adrenergic drugs, 24‐hour net fluid balance at 0, 48 and 72 hours post randomization | |
| Notes | Full article in Mandarin. Abstract only in English. Study author contacted 1 Dec 2014 and again in April 2015. Use of Chinese students to determine risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelope method |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open‐label Blinding of treatment: no Blinding of assessor: not stated Blinding of data analysis: not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary analysis was by intention‐to‐treat. |
| Selective reporting (reporting bias) | Unclear risk | Information was insufficient to permit a judgement. |
| Intention to treat | Low risk | Primary analysis was by intention‐to‐treat. |
Xi 2010.
| Methods | Prospective, multi‐centre RCT | |
| Participants | n = 110 Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment: RM (CPAP 40 cm H2O for 40 seconds) performed 8‐hourly for first 5 days, unless weaning standard was reached Both groups: VT 6‐8 mL/kg; Pplat < 30 cmH2O; VCV or PCV for first 24 hours, with any mode thereafter; PEEP titrated to target PaO2 or FiO2 or both; permissive hypercapnia |
|
| Outcomes | Primary: ICU mortality Secondary: number of ventilator‐free days; non‐pulmonary organ dysfunction‐free days from day 1 to day 28; 28‐day mortality; percentage of unassisted breathing hours for at least 48 consecutive hours; incidence of barotrauma or pneumatocoele > 2 cm |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15 participants were excluded for failure to follow the study protocol. |
| Selective reporting (reporting bias) | Unclear risk | 15 participants were excluded for failure to follow the study protocol ‐ 3 in the control group and 12 in the intervention group (not analysed as intention‐to‐ treat). |
| Intention to treat | High risk | 15 participants were excluded from final analysis for failure to adhere to study protocol. |
Yang 2011.
| Methods | Prospective, single‐centre RCT | |
| Participants | n = 38. ARDS | |
| Interventions | Treatment: RM (PEEP 40 cm H2O, support pressure set to 0, FiO2 1.0 for 30 seconds), repeated 8‐hourly for 5 days Both groups: PSV, Pplat ≤ 30 cm H2O, minimum PEEP with FiO2 < 0.6 and PaO2 60‐80 mmHg |
|
| Outcomes | Oxygenation status for 5 days, lung injury indexes, adverse effects of RM and incidence of barotrauma | |
| Notes | Full article in Mandarin. Abstract only in English. Study author contacted 1 Dec 2014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelope method |
| Allocation concealment (selection bias) | Low risk | "The envelope method" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information was insufficient to permit a judgement. |
| Selective reporting (reporting bias) | Unclear risk | Information was insufficient to permit a judgement. |
| Intention to treat | Unclear risk | Information was insufficient to permit a judgement. |
ABG = arterial blood gases; ACVC = assist control; APO = acute pulmonary oedema; ARDS = acute respiratory distress syndrome; BIPAP = bi‐level positive airway pressure; CO = cardiac output; COP = colloid osmotic pressure; COPD = chronic obstructive pulmonary disease; CPAP = continuous positive airway pressure; Crs = respiratory system compliance; CVP = central venous pressure; EELV = end‐expiratory lung volume; EVLW = extravascular lung water; EVLWI = extravascular lung water index; FiO2 = fraction of inspired oxygen; FRC = functional residual capacity; HR = heart rate; ICP = intracranial pressure; ICU = intensive care unit; IL = interleukin; LIP = lower inflection point; LISS = lung injury severity score; LOS = length of stay; LVEF = left ventricular ejection fraction; MAP = mean arterial pressure; MV = mechanical ventilation; NAECC = North American‐European Consensus Conference; PaO2 = arterial oxygen partial pressure; PaCO2 = arterial carbon dioxide partial pressure; PAP = pulmonary artery pressure; PBW = predicted body weight; PCV = pressure control ventilation; PCWP = pulmonary capillary wedge pressure; PEEP = positive end‐expiratory pressure; P/F = PaO2/FiO2; PIP = peak inspiratory pressure; Ppeak = peak pressure; Pplat = plateau pressure; PSV = pressure support ventilation; Qva/Qt = venous admixture; RCT = randomized controlled trial; RM = recruitment manoeuvre; RR = respiratory rate; SBP = systolic blood pressure; SpO2 = arterial oxygen saturation from pulse oximeter; TNF = tumour necrosis factor; VCV = volume‐cycled ventilation; Vd/Vt = dead space; VT = tidal volume.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amato 1995 | Participants in this study were included from the subsequent larger study published in 1998 (see Amato 1998). |
| Amato 1998 | The control group strategy included a tidal volume of 12 mL/kg, not protective lung ventilation. The intervention group included children. |
| Barker 2002 | Not a recruitment manoeuvre using the ventilator, but a manual breath using a rebreathing bag |
| Bollen 2005 | RCT of high‐frequency oscillatory ventilation that is not covered as part of this review |
| Brower 2003 | Cross‐over study, not a RCT |
| Constantin 2010 | Participants with acute hypoxaemic respiratory failure (not ARDS) |
| Derdak 2002 | RCT of high‐frequency oscillatory ventilation (Sud 2013) |
| Dolinay 2011 | Not an RCT of recruitment manoeuvres vs no recruitment manoeuvres |
| Dyhr 2003 | Cross‐over study, not an RCT |
| Foti 2000 | Cross‐over study, not an RCT |
| Gattinoni 2006 | Not an RCT of recruitment manoeuvres vs no recruitment manoeuvres |
| Holzapfel 1987 | RCT of high‐frequency oscillatory ventilation (Sud 2013) |
| Hurst 1990 | RCT of high‐frequency oscillatory ventilation (Sud 2013) |
| Lasocki 2005 | Cross‐over study, not an RCT |
| Lim 2003 | Not an RCT |
| Lowhagen 2011 | Comparison of 2 different recruitment manoeuvre techniques |
| Meade 2002 | Not an RCT of recruitment manoeuvres vs no recruitment manoeuvres |
| Stewart 2002 | Not an RCT of recruitment manoeuvres vs no recruitment manoeuvres |
ARDS = acute respiratory distress syndrome; RCT = randomized controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Wang 2007.
| Methods | Prospective, single‐centre RCT |
| Participants | n = 28 participants with ARDS |
| Interventions | Intervention groups: BIPAP ventilation with lung recruitment manoeuvres Control group: low tidal volume assist control ventilation |
| Outcomes | PaO2/FiO2, respiratory system compliance and CVP (at 0, 48 and 72 hours); duration of ventilation, lung injury and 28‐day mortality |
| Notes | Full article in Mandarin. Abstract only in English. Unable to contact study author as we found no email address. Emailed the journal that published the manuscript, but the email address was no longer working in April 2015. Attempted to download full article in Mandarin, but the site was blocked. |
ARDS = acute respiratory distress syndrome; BIPAP = bi‐level positive airway pressure; CVP = central venous pressure; FiO2 = fraction of inspired oxygen; PaO2 = arterial oxygen partial pressure; RCT = randomized controlled trial.
Characteristics of ongoing studies [ordered by study ID]
ART STudy Investigators.
| Trial name or title | Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial |
| Methods | Prospective, multi‐centre, event‐guided RCT |
| Participants | Participants considered for this trial are those in mechanical ventilation with diagnosis of ARDS < 72 hours Partcipants will be excluded if they are younger than 18 years old, in use of vasopressor drugs in increasing doses over the past 2 hours or mean arterial blood pressure < 65 mmHg, presence of any contraindication to hypercapnia, undrained pneumothorax or subcutaneous emphysema and lack of consent from participant's surrogate for participation |
| Interventions | Intervention: maximum alveolar recruitment manoeuvre in association with PEEP titrated by static compliance of respiratory system Control: conventional mechanical ventilation strategy |
| Outcomes | Primary: survival in 28 days Secondary
|
| Starting date | June 2011 |
| Contact information | |
| Notes | ClinicalTrials.gov Identifier: NCT01374022 |
PHARLAP Group Investigators.
| Trial name or title | A Multi‐centre Trial of an Open Lung Strategy Including Permissive Hypercapnia, Alveolar Recruitment and Low Airway Pressure in Patients With Acute Respiratory Distress Syndrome |
| Methods | Prospective, multi‐centre, parallel‐group RCT |
| Participants | Participants considered for this trial are those in mechanical ventilation with diagnosis of ARDS < 72 hours Participants will be excluded if they are younger than 18 years old, in use of vasopressor drugs in increasing doses over the past 2 hours or mean arterial blood pressure < 65 mmHg, presence of any contraindication to hypercapnia, undrained pneumothorax or subcutaneous emphysema and lack of consent from participant's surrogate for participation |
| Interventions | Intervention group: pressure control ventilation to maintain tidal volume 4‐6 mL/kg and plateau pressure ≤ 30 cm H2O while tolerating respiratory acidosis if pH > 7.15; daily staircase recruitment manoeuvre and individualized PEEP titration Control group: mechanical ventilation based on the ARDSnet protocol using volume control ventilation with tidal volume 6 mL/kg, plateau pressure ≤ 30 cm H2O and FiO2/PEEP titration according to FiO2/PEEP/oxygen saturation combination chart. This has been modified for Australian and New Zealand practice to allow pressure control and pressure support ventilation |
| Outcomes | Primary: number of ventilator‐free days at day 28 post randomization Secondary
|
| Starting date | August 2012 |
| Contact information | carol.hodsgon@monash.edu |
| Notes | ClinicalTrials.gov Identifier: NCT01667146 |
AKI = acute kidney injury; ARDS = acute respiratory distress syndrome; ECMO = extracorporeal membrane oxygenation; FiO2 = fraction of inspired oxygen; ICU = intensive care unit; IL = interleukin; PaO2 = partial pressure of oxygen; PEEP = positive end‐expiratory pressure; RCT = randomized controlled trial.
Differences between protocol and review
We made the following changes to the protocol (Hodgson 2007) in August 2016.
One author, Andrew R Davies, was not involved in the update. Two new additional co‐authors were added (EG and MY).
We changed the title and content to reflect the Berlin definition of ARDS and removed the term 'acute lung injury' (ARDS Definition Task Force 2012).
We updated the search to August 2016. We found 15 new studies; we included three of these and excluded nine, two are ongoing and one is awaiting classification.
We added new risk of bias tables.
We updated methods and outcome measures. We removed length of stay (hospital and ICU) as an outcome, as it is not reported for survivors and non‐survivors. We removed blood pressure as an outcome, as generally it was reported as a short‐term outcome. We added use of rescue therapies as an outcome.
We redefined the control group standard of care as "protective lung ventilation"
We excluded Amato 1998, as investigators used 12 mL/kg in the control group, which is not standard care.
We excluded cross‐over trials from the included studies, as we could not assess the primary outcome with this type of study.
Contributions of authors
Carol Hodgson (CH), Ewan C Goligher (EG), Meredith E Young (MY), Jennifer L Keating (JK), Anne E Holland (AH) Lorena Romero (LR), Scott J Bradley (SB), David Tuxen (DT).
Conceiving of the review: CH, JK, DT. Co‐ordinating the review: CH, JK. Undertaking manual searches: CH, LR. Screening search results: CH, SB, EG. Organizing retrieval of papers: CH, LR, MY. Screening retrieved papers against inclusion criteria: CH, SB, EG, MY. Appraising the quality of papers: CH, SB, EG, MY. Abstracting data from papers: CH, SB, EG, MY. Writing to authors of papers for additional information: CH, MY. Providing additional data about papers: CH. Obtaining and screening data from unpublished studies: CH, MY. Managing data for the review: CH, JK. Entering data into Review Manager (RevMan 5.3): CH, JK, MY. Analysing RevMan statistical data: CH, JK, AH. Performing other statistical analysis not using RevMan: CH, JK, AH. Performing double entry of data: data entered by person one, CH; data entered by person two, JK. Interpreting data: CH, JK, AH, EG, DT. Making statistical inferences: CH, JK, EG. Writing the review: CH, AH, DT, JK, SB, LR. Securing funding for the review: CH, DT, AH, JK. Performing previous work that provided the foundation for the present study: CH, DT, EG. Serving as guarantor for the review (one review author): CH. Taking responsibility for reading and checking the review before submission: DT.
Sources of support
Internal sources
No sources of support, Australia.
External sources
No sources of support, Australia.
Declarations of interest
Carol Hodgson is a co‐author of an included study (Hodgson 2011) and is co‐chair of the ongoing study (PHARLAP Group Investigators). EG and MY provided critical appraisal and data extraction for this included study.
Ewan C Goligher: none known.
Meredith E Young: none known.
Jennifer L Keating: co‐author of an included study (Hodgson 2011). EG and MY provided critical appraisal and data extraction for this included study.
Anne E Holland: co‐author of an included study (Hodgson 2011). EG and MY provided critical appraisal and data extraction for this included study.
Lorena Romero: none known.
Scott J Bradley: none known.
David Tuxen: co‐author of an included study (Hodgson 2011) and member of the management committee for the ongoing study (PHARLAP Group Investigators). EG and MY provided critical appraisal and data extraction for this included study.
Edited (no change to conclusions)
References
References to studies included in this review
Cavalcanti et al, 2013 {published data only}
- ART Study Investigators. Alveolar recruitment for ARDS trial: preliminary results. Critical Care 2013;17:S40‐1. [Google Scholar]
Hodgson 2011 {published data only}
- Hodgson CL, Tuxen DV, Davies AR, Bailey MJ, Higgins AM, Holland AE, et al. A randomised controlled trial of an open lung strategy with staircase recruitment, titrated PEEP and targeted low airway pressures in patients with acute respiratory distress syndrome. Critical Care 2011;15(3):R133. [PUBMED: 21635753 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huh 2009 {published data only}
- Huh JW, Jung H, Choi HS, Hong S‐B, Lim C‐M, Koh Y. Efficacy of positive end‐expiratory pressure titration after the alveolar recruitment manoeuvre in patients with acute respiratory distress syndrome. Critical Care 2009;13(1):R22. [PUBMED: 19239703] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kacmarek 2016 {published and unpublished data}
- Kacmarek RM, Villar J, Sulemanji D, Montiel R, Ferrando C, Blanco J, et al. Open lung approach for the acute respiratory distress syndrome: a pilot, randomized controlled trial. Critical Care Medicine 2016; Vol. 44, issue 1:32‐42. [DOI: ; http://www.clinicaltrials.gov/ct2/show/NCT00431158] [DOI] [PubMed]
Liu 2011 {published data only}
- Liu W‐L, Wang C‐M, Chen W‐L. Effects of recruitment maneuvers in patients with early acute lung injury and acute respiratory distress syndrome. Respirology 2011;16(Suppl 2):1‐326. [DOI: 10.1111/j.1440-1843.2011.02071.x] [DOI] [Google Scholar]
Meade 2008 {published data only}
- Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end‐expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299(6):637‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oczenski 2004 {published data only}
- Oczenski W, Hörmann C, Keller C, Lorenzl N, Kepka A, Schwarz S, et al. Recruitment maneuvers after a positive end‐expiratory pressure trial do not induce sustained effects in early adult respiratory distress syndrome. Anesthesiology 2004;101:620‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang 2009 {published data only}
- Wang Z, Zhu X, Li H, Wang T, Yao G. A study on the effect of recruitment maneuver imposed on extravascular lung water in patients with acute respiratory distress syndrome. Chinese Critical Care Medicine 2009;21(10):604‐8. [PubMed] [Google Scholar]
Xi 2010 {published data only}
- Xi X, Jiang L, Zhu B, the RM Group. Clinical efficacy and safety of recruitment maneuver in patients with acute respiratory distress syndrome using low tidal volume ventilation: a multicenter randomized controlled clinical trial. Chinese Medical Journal 2010;123(21):3100‐5. [DOI: 10.3760/cma.j.issn.0366-6999.2010.21.027] [DOI] [PubMed] [Google Scholar]
Yang 2011 {published data only}
- Yang G, Wang C, Ning R. Effects of high positive end‐expiratory pressure combined with recruitment maneuvers in patients with acute respiratory distress syndrome. Chinese Critical Care Medicine 2011;21(1):28‐31. [PubMed] [Google Scholar]
References to studies excluded from this review
Amato 1995 {published and unpublished data}
- Amato MB, Barbas CS, Medeiros DM, Schettino Gde P, Lorenzi Filho G, Kairalla RA, et al. Beneficial effects of the "open lung approach" with low distending pressures in acute respiratory distress syndrome. A prospective randomized study on mechanical ventilation. American Journal of Respiratory and Critical Care Medicine 1995;152(6 Pt 1):1835‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Amato 1998 {published data only}
- Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi‐Filho G, et al. Effect of a protective‐ventilation strategy on mortality in the acute respiratory distress syndrome. New England Journal of Medicine 1998;338:347‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Barker 2002 {published and unpublished data}
- Barker M, Adams S. An evaluation of a single chest physiotherapy treatment on mechanically ventilated patients with acute lung injury. Physiotherapy Research International 2002;7(3):157‐69. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bollen 2005 {published data only}
- Bollen CW, Well GT, Sherry T, Beale RJ, Shah S, Findlay G, et al. High frequency oscillatory ventilation compared with conventional mechanical ventilation in adult respiratory distress syndrome: a randomized controlled trial [ISRCTN24242669]. Critical Care 2005;9(4):R430‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brower 2003 {published data only}
- Brower RG, Morris A, MacIntyre N, Matthay MA, Hayden D, Thompson T, et al. ARDS Clinical Trials Network. Effects of recruitment maneuvers in patients with acute lung injury and acute respiratory distress syndrome ventilated with high positive end‐expiratory pressure. Critical Care Medicine 2003;31:2592‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Constantin 2010 {published data only}
- Constantin J‐M, Futier E, Cherprenet A‐L, Chanques G, Guerin R, Cayot‐Constantin S, et al. A recruitment maneuver increases oxygenation after intubation of hypoxemic intensive care unit patients: a randomized controlled study. Critical Care 2010;14(2):R76. [PUBMED: 20426859 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derdak 2002 {published and unpublished data}
- Derdak S, Mehta S, Stewart TE, Smith T, Rogers M, Buchman TG, et al. High‐frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. American Journal of Respiratory and Critical Care Medicine 2002;166(6):801‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dolinay 2011 {published data only}
- Dolinay T, Kyle W, Lansing C, Shah J, Gunter J, Shome S, et al. Patients with acute lung injury benefit from airway pressure release ventilation. American Journal of Respiratory and Critical Care Medicine 2011;183:A1652. [Google Scholar]
Dyhr 2003 {published data only}
- Dyhr T, Bonde J, Larsson A. Lung recruitment manoeuvres are effective in regaining lung volume and oxygenation after open endotracheal suctioning in acute respiratory distress syndrome. Critical Care 2003;7:55‐62. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Foti 2000 {published data only}
- Foti G, Cereda M, Sparacino ME, Marchi L, Villa F, Pesenti A. Effects of periodic lung recruitment maneuvers on gas exchange and respiratory mechanics in mechanically ventilated acute respiratory distress syndrome (ARDS) patients. Intensive Care Medicine 2000;26:501‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gattinoni 2006 {published and unpublished data}
- Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. New England Journal of Medicine 2006;354(17):1775‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Holzapfel 1987 {published and unpublished data}
- Holzapfel L, Robert D, Perrin F, Gaussorgues P, Giudicelli DP. Comparison of high‐frequency jet ventilation to conventional ventilation in adults with respiratory distress syndrome. Intensive Care Medicine 1987;13(2):100‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hurst 1990 {published data only}
- Hurst JM, Branson JD, Davis K Jr, Barrette RR, Adams KS. Comparison of conventional mechanical ventilation and high‐frequency ventilation. A prospective, randomized trial in patients with respiratory failure. Annals of Surgery 1990;211(4):486‐91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lasocki 2005 {published data only}
- Lasocki S, Lu Q, Sartorius A, Fouillat D, Remerand F, Rouby J. Open and closed‐circuit endotracheal suctioning in acute lung injury: efficiency and effects on gas exchange. Anesthesiology 2006;104:39‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lim 2003 {published data only}
- Lim C‐M, Jung H, Koh Y, Lee JS, Shim T‐S, Lee SD, et al. Effect of alveolar recruitment maneuver in early acute respiratory distress syndrome according to antiderecruitment strategy, etiological category of diffuse lung injury, and body position of the patient. Critical Care Medicine 2003;31(2):411‐8. [DOI: 10.1097/01.CCM.0000048631.88155.39] [DOI] [PubMed] [Google Scholar]
Lowhagen 2011 {published data only}
- Lowhagen K, Lindgren S, Odenstedt O, Lundin S. Prolonged moderate pressure recruitment manoeuvre results in lower optimal positive end‐expiratory pressure and plateau pressure. ACTA Anaesthesiologica Scandinavica 2011;55(2):175‐84. [DOI: 10.1111/j.1399-6576.2010.02337.x] [DOI] [PubMed] [Google Scholar]
Meade 2002 {published data only}
- Meade MO, Guyatt GH, Cook DJ, Lapinsky SE, Hand L, Griffith L, et al. Physiologic randomized pilot study of a lung recruitment maneuver in acute lung injury. American Journal of Respiratory and Critical Care Medicine 2002;165 Suppl 8:A682. [CENTRAL: CN‐00383680] [Google Scholar]
Stewart 2002 {published data only}
- Stewart TE, Meade MO, Slutsky AS, Hand L, Ronco J, Chittock D, et al. Complications of a lung recruitment maneuver. American Journal of Respiratory and Critical Care Medicine 2002;165 Suppl 8:A682. [Google Scholar]
References to studies awaiting assessment
Wang 2007 {published data only}
- Wang X‐Z, Lu C‐J, Gao F‐Q, Li X‐H, Hao D, Ning F‐Y. Comparison of the effects of BiPAP ventilation combined with lung recruitment maneuvers and low tidal volume A/C ventilation in patients with acute respiratory distress syndrome. Chinese Journal of Tuberculosis and Respiratory Diseases 2007;30(1):44‐7. [DOI: 10.3760/j:issn:1001-0939.2007.01.011] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ART STudy Investigators {unpublished data only}
- Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial. Ongoing study June 2011.
PHARLAP Group Investigators {unpublished data only}
- A Multi‐centre Trial of an Open Lung Strategy Including Permissive Hypercapnia, Alveolar Recruitment and Low Airway Pressure in Patients With Acute Respiratory Distress Syndrome. Ongoing study August 2012.
Additional references
Amato 2015
- Amato MBP, Meade MO, Slutsky AS, Brochard L, Costa ELV, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. New England Journal of Medicine 2015;372:747‐55. [DOI: 10.1056/NEJMsa1410639] [DOI] [PubMed] [Google Scholar]
ARDS Definition Task Force 2012
- ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307(23):2526‐33. [DOI: 10.1001/jama.2012.5669] [DOI] [PubMed] [Google Scholar]
ARDSnet 2000
- Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New England Journal of Medicine 2000;342(18):1301‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bellani et al, 2016
- Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016;315:788‐800. [DOI: 10.1001/jama.2016.0291] [DOI] [PubMed] [Google Scholar]
Bernard 1994
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American‐European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. American Journal of Respiratory and Critical Care Medicine 1994;149:818‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Borges 2006
- Borges JB, Okamoto VN, Matos GF, Caramez MP, Arantes PR, Barros F, et al. Reversibility of lung collapse and hypoxemia in early acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 2006;174(3):268‐78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Briel 2010
- Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, et al. Higher vs lower positive end‐expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta‐analysis. JAMA 2010;303(9):865‐73. [DOI: 10.1001/jama.2010.218.] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis is detected by a simple graphical test. BMJ 1997;31(5):629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fan 2008
- Fan E, Wilcox ME, Brower RG, Stewart TE, Mehta S, Lapinsky SE, et al. Recruitment maneuvers for acute lung injury: a systematic review. American Journal Respiratory and Critical Care Medicine 2008;178:1156‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fan 2012
- Fan E, Checkley C, Stewart T, Muscadere J, Lesur O, Granton J, et al. Complications from recruitment maneuvers in patients with acute lung injury: secondary analysis from the lung open ventilation study. Respiratory Care 2012;57(11):1842‐9. [DOI: 10.4187/respcare.01684] [DOI] [PubMed] [Google Scholar]
Funk 2004
- Funk DJ, Graham MR, Girling LG, Thliveris JA, McManus BM, Walker EKY, et al. A comparison of biologically variable ventilation to recruitment manoeuvres in a porcine model of acute lung injury. Respiratory Research 2004;5(1):22. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [PUBMED: 18456631 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hedenstierna 2002
- Hedenstierna G, Lattuada M. Gas exchange in the ventilated patient. Current Opinion in Critical Care 2002;8:39‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;15(21):1539‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kacmarek 2007
- Kacmarek RM, Kallet RH. Respiratory controversies in the critical care setting. Should recruitment maneuvers be used in the management of ALI and ARDS?. Respiratory Care 2007;52(5):622‐35. [MEDLINE: ] [PubMed] [Google Scholar]
Laake et al, 2015
- Laake JH, Claesson J, Freundlich M, Gunnarsson I, Varpula T, Vandvik PO, et al. Scandinavian clinical practice guideline on mechanical ventilation in adults with the acute respiratory distress syndrome. Acta Anaesthesiologica Scandinavica 2015;59:41. [DOI: ] [DOI] [PubMed] [Google Scholar]
Lapinsky 2005
- Lapinsky SE, Mehta S. Bench‐to‐bedside review: recruitment and recruiting maneuvers. Critical Care 2005;9(1):60‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Levy 2005
- Levy MM, Baylor MS, Bernard GR, Fowler R, Franks TJ, Hayden FG, et al. Clinical issues and research in respiratory failure from severe acute respiratory syndrome. American Journal of Respiratory and Critical Care Medicine 2005;171:518‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lim 2004
- Lim SC, Adams AB, Simonson DA, Dries DJ, Broccard AF, Hotchkiss JR, et al. Intercomparison of recruitment maneuver efficacy in three models of acute lung injury. Critical Care Medicine 2004;32:2371‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Odenstedt 2005
- Odenstedt H, Aneman A, Karason S, Stenqvist O, Lundin S. Acute hemodynamic changes during lung recruitment in lavage and endotoxin‐induced ALI. Intensive Care Medicine 2005;31:112‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Piacentini 2004
- Piacentini E, Villagra A, Lopez‐Aguilar J, Blanch L. Clinical review: the implications of experimental and clinical studies of recruitment maneuvers in acute lung injury. Critical Care 2004;8(2):115‐21. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richard 2004
- Richard JC, Maggiore SM, Mercat A. Clinical review: bedside assessment of alveolar recruitment. Critical Care 2004;8(3):163‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sevransky 2004
- Sevransky JE, Levy MM, Marini JJ. Mechanical ventilation in sepsis induced acute lung injury/acute respiratory distress syndrome: an evidence based review. Critical Care Medicine 2004;32(Suppl):548‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sud 2013
- Sud S, Sud M, Friedrich J, Wunsch H, Meade M, Ferguson D, et al. High‐frequency ventilation versus conventional ventilation for treatment of acute lung injury and acute respiratory distress syndrome. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD004085.pub3; PUBMED: 23450549] [DOI] [PubMed] [Google Scholar]
Suzumura 2014
- Suzumura EA, Figueiro M, Normilio‐Silva K, Laranjieira L, Oliveira C, Buehler AM, et al. Effects of alveolar recruitment maneuvers on clinical outcomes in patients with acute respiratory distress syndrome: a systematic review and meta‐analysis. Intensive Care Medicine 2014;40:1227‐40. [DOI: 10.1007/s00134-014-3413-6] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hodgson 2007
- Hodgson C, Bradley SJ, Davies AR, Holland A, Keating JL, Smirneos L, et al. Recruitment manoeuvres for adults receiving mechanical ventilation with acute lung injury. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD006667] [DOI] [PubMed] [Google Scholar]
Hodgson 2009
- Hodgson C, Keating JL, Holland AE, Davies AR, Romero L, Bradley SJ, et al. Recruitment manoeuvres for adults with acute lung injury receiving mechanical ventilation. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006667.pub2] [DOI] [PubMed] [Google Scholar]


