Abstract
Background
Many patients experience depression, social isolation and anxiety post stroke. These are associated with a poorer outcome. Ameliorating these problems may improve patient wellbeing.
Objectives
To evaluate the impact of a healthcare worker or volunteer whose multi‐dimensional roles have been grouped under the title 'stroke liaison worker'.
Search methods
We searched the Cochrane Stroke Group Trials Register (searched February 2009), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 1, 2009), MEDLINE (1966 to 2009), EMBASE (1980 to 2009) and four other databases. We performed a cited reference search, searched conference proceedings and trials registers, checked reference lists and contacted authors and trial investigators.
Selection criteria
Randomised controlled trials investigating the impact of a stroke liaison worker versus usual care.
Data collection and analysis
We invited trialists to participate in a review of individual patient data. Primary outcomes for patients were subjective health status and extended activities of daily living. Primary outcomes for carers were subjective health status including measures of carer strain.
Main results
We included 16 trials involving 4759 participants. Analysis did not show a significant overall difference for subjective health status (standardised mean difference (SMD) ‐0.03, 95% confidence interval (CI) ‐0.11 to 0.04, P = 0.34) or extended activities of daily living (SMD 0.04, 95% CI ‐0.03 to 0.11, P = 0.22). There was no overall significant effect for the outcome of carer subjective health status (SMD 0.04, 95% CI ‐0.05 to 0.14, P = 0.37). Patients with mild to moderate disability (Barthel 15 to 19) had a significant reduction in dependence (odds ratio (OR) 0.62, 95% CI 0.44 to 0.87, P = 0.006). This would equate to 10 fewer dependent patients (95% CI 17 fewer to 4 fewer) for every 100 patients seen by the stroke liaison worker. Similar results were seen for the outcome of death or dependence for the subgroup with Barthel 15 to 19 (OR 0.55, 95% CI 0.38 to 0.81, P = 0.002). This risk difference equates to 11 fewer dead or dependent patients (95% CI 17 fewer to 4 fewer) for every 100 patients seen by the stroke liaison worker.
Authors' conclusions
There is no evidence for the effectiveness of this multifaceted intervention in improving outcomes for all groups of patients or carers. Patients with mild to moderate disability benefit from a reduction in death and disability. Patients and carers do report improved satisfaction with some aspects of service provision.
Keywords: Adult, Humans, Activities of Daily Living, Anxiety, Anxiety/prevention & control, Caregivers, Caregivers/psychology, Counseling, Depression, Depression/prevention & control, Health Status, Outcome Assessment (Health Care), Patient Education as Topic, Randomized Controlled Trials as Topic, Social Isolation, Social Isolation/psychology, Social Support, Social Work, Stroke, Stroke/psychology, Stroke Rehabilitation, Volunteers
Stroke liaison workers for stroke patients and carers
Many patients experience depression, anxiety and isolation after a stroke. These post‐stroke problems can lead to subsequent poor health, low mood or increased caring burden. It seems reasonable to expect that providing more emotional and psychological support in addition to appropriate information about stroke and services available might help to reduce anxiety, improve mood and improve health or satisfaction. In this review, we evaluated 16 studies (involving 4759 participants) of healthcare workers or volunteers (a 'stroke liaison worker') providing education and social support (including counselling) and liaison with services. Overall, there do not appear to be any significant benefits for patients in terms of their perceived health, mood, activities or participation. Patients appeared to be more satisfied that someone had really listened to them, and carers appeared to be more satisfied with aspects of the care provided. It also appears that patients with mild to moderate disability may benefit from a reduction in disability and death as a result of the input from the stroke liaison worker. The reason for this is not yet clear and further research is required.
Background
Stroke is a major cause of disability and handicap, with an incidence of three to five per 1000 in people aged 45 to 84 years old (Sudlow 1997). The majority of people survive their stroke, but a third to a half remain functionally dependent after one year (Bamford 1990; Taub 1994). This is associated with significant psychosocial problems for both patients and their carers, which may also occur independent of physical disability (Dennis 1998; Kotila 1998; Scholte 1998). The burden of such problems may increase as a result of demographic change and reductions in age‐specific stroke case fatality (Barker 1997; Bonita 1993).
A number of different approaches have been evaluated to try to lessen these psychosocial problems. These include provision of therapy (OSST 2003; Wade 1992a), information leaflets (Mant 1998), antidepressants (Hackett 2008), counselling, education (Evans 1988; Forster 2001; Rogers 1999) and social support (Friedland 1992). While some studies did find positive effects of these interventions, none were found to have a significant impact on psychological outcomes or quality of life, though this may have been due to type II statistical error.
Support following discharge from hospital, information about stroke and available resources, and practical help have been identified by patients and carers as services that they would value (Greveson 1991). A stroke liaison worker can be defined as someone whose aim is to increase participation and improve wellbeing for patients and carers. Typically they provide emotional and social support and information to stroke patients and their families and liaise with services with the aim of improving aspects of participation and quality of life for patients with stroke, their carers, or both (Lilley 2003). This multifaceted role distinguishes stroke liaison workers from therapists whose aim is to treat a single problem such as improving activities (rehabilitation) or knowledge (information provision). A stroke liaison worker may be a health or social care professional, or be from the voluntary sector. Such services have been evaluated under a range of different names, such as 'social work' (Christie 1984; Towle 1989), 'specialist nurse support' (Forster 1996), 'stroke family care worker' (Dennis 1997) and 'stroke family support organiser' (Mant 1999). For the purposes of this review, such services have been grouped under the generic title of 'stroke liaison worker'. There has been one descriptive review of published trials of 'support workers' within the context of a broader review of non‐drug strategies aimed at reducing psycho‐social problems after stroke (Knapp 2000). No meta‐analysis of these studies has yet been attempted.
Objectives
To determine the efficacy of stroke liaison workers for patients with stroke and their carers in increasing participation and improving wellbeing for patients and carers, as measured by improving social activities, participation and mental health.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials comparing allocation to stroke liaison worker with normal care or alternative service.
Types of participants
Survivors of acute stroke or their closest informal carer or both. A clinical definition of stroke was used: rapidly developing clinical symptoms and/or signs of focal, and at times global loss of cerebral function (Bamford 1988). We included studies that included transient ischaemic attack (TIA) patients since TIA is part of the same disease spectrum and patients with TIAs, while seldom having reduced activities, may have reduced participation and a high level of anxiety regarding stroke recurrence (van Veenendaal 1996). Participants must be adult (aged 16 years or over). We did not include trials that address carer needs alone (and do not include patients).
Types of interventions
Referral to a stroke liaison worker. Such a worker would typically provide a multifaceted service including more than one of: education and information provision, social support and liaison with other services (OSST 2003). Often this intervention is provided from the point of patient discharge from hospital. We considered trials assessing workers of any professional background relevant, including health or social care professionals or volunteers. We excluded studies where the intervention was judged to be single‐faceted. This distinction is to separate the stroke liaison worker interventions from trials of, for example, information provision alone (Forster 2001). Similarly, we excluded trials of therapist‐delivered physical rehabilitation or psychological interventions on their own. The control group received usual care.
Types of outcome measures
Outcomes for patients
Primary outcomes: subjective health status (e.g. GHQ 12, SF36, Euroqol), extended activities of daily living (including social activities, e.g. Nottingham Extended Activities of Daily Living (EADL), Frenchay Activities Index (AI)).
Secondary outcomes: death, place of residence, activities of daily living, dependency, mental health (including anxiety and depression), knowledge about stroke, use of services, satisfaction with services, participation.
Outcomes for carers
Primary outcome: subjective health status (including measures of carer strain).
Secondary outcomes: extended activities of daily living, mental health, knowledge about stroke, satisfaction with services.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module.
We searched the Cochrane Stroke Group Trials Register, which was last searched by the Managing Editor on 2 February 2009. The register was searched for trials coded in the following categories: counselling, social support, therapists, support workers, carer training and information giving.
In addition, we searched the following bibliographic databases: the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 1, 2009), MEDLINE (from 1966 to 2009) (Appendix 1), EMBASE (1980 to 2009), CINAHL (1982 to 2009), ASSIA (1987 to 2009), PsycINFO (1967 to 2009) and Social Science Citation Index (1956 to 2009).
In order to identify further published and unpublished studies, we carried out a Cited Reference Search using Web of Science, checked the reference lists of identified relevant trials and contacted authors of relevant papers and investigators in this area of stroke services trials. We also searched the following relevant conference proceedings and trials registers: European Stroke Conference proceedings (1999 to 2008) and The Stroke Center (www.strokecenter.org) (December 2008).
Data collection and analysis
Methods of the review: selection of studies
The lead review author (GE) first excluded obviously irrelevant articles. Two review authors (GE, PL) then independently reviewed the retrieved abstracts of papers identified. We excluded papers that clearly did not meet the inclusion criteria at this stage, and recorded the reason. We obtained a full‐text copy of all possibly relevant papers, and the same review authors (GE and PL) also independently assessed these according to pre‐defined inclusion criteria. It was our intention that Simon Winner and Martin Dennis would moderate if there was disagreement: this was not required.
Contact with trialists and data collection
We contacted the contact trialist or lead investigator and invited them to join a collaborative review process. We requested individual patient data and invited trialists to meet in Glasgow, UK to discuss the development of the review. (The members of this collaborative group are listed in the Acknowledgements).
Prior to the meeting, we asked trialists for additional information including information on design characteristics, the study population, the intervention, outcome measures used and length of follow up, as well as additional information regarding the intervention that may not have been apparent in the published papers. If we could not contact any trialists, the lead review author (GE) and supervising review author (PL) completed these trial detail grids independently. We then used details about the intervention to construct subgroups according to the apparent primary emphasis of the intervention (liaison, education and information provision, social support).
At the trialists meeting, held on 4 March 2005, we discussed the intentions of the review process and methods. We invited trialists to comment on the classification system developed for grouping trials. The trialists felt that the initial system developed (analysis by primary emphasis as suggested in the original protocol) did not adequately describe the similar and differing studies. This discussion led to the development of a subsequent classification (Figure 1) which we used for the primary analysis.
Figure 1.

Subgroup definitions
The group also suggested additional subgroup analyses. These included analysis of the intervention by the profession of the individual providing the intervention. We also planned to present the results stratified by timing of referral to a stroke liaison worker (less than six months after stroke, more than six months after stroke).
Analysis by patient characteristics included subgroups defined by sex, age (less than 65 years old, and 65 years or older), the presence or absence of a main carer and patient functional status at baseline. We considered this last subgroup because one trial in particular had suggested that patients with mild to moderate functional dependence had the most to gain from the intervention (Bradford). For this reason, we used the same definitions of dependence as in the original trial (severe dependence: Barthel score less than 15; mild to moderate dependence: Barthel score 15 to 19; independent: Barthel score 20). We made all subgroup definitions prior to data analysis and blind to review data.
Assessment of quality
We did not use a quality score for eligible trials (Juni 1999). Nevertheless, we coded the trials with regard to quality of randomisation procedure, method of consent, concealment of treatment allocation, blinding of patients and carers, blinding of outcome assessor, and handling of withdrawals and drop‐outs (see Characteristics of included studies table).
Analysis
We classified outcome measures according to which domain they were assessing (activities of daily living, extended activities of daily living, participation, dependency, mental health, subjective health status, knowledge about stroke, use of services and satisfaction). Satisfaction was assessed across 17 domains for patients and 16 domains for carers. Most of the scales used were ordinal or continuous. If the same measure had been used in different studies, we calculated a mean difference (MD) across trials using the Review Manager software, RevMan 5.0 (RevMan 2008). We did not perform a meta‐analysis where grossly differing outcome measures precluded combination. Where it was possible to dichotomise the data, we calculated odds ratios (OR) and 95% confidence intervals (CI) for each study. Where it was not possible to dichotomise the data, we summarised the effects on outcome in terms of direction of effect (in favour of intervention or control), and the size of the effect (in terms of standardised mean differences (SMD)). We have presented results separately for patients and carers for each domain of outcome.
Prior to data analysis, we constructed an analysis plan which included the a priori selection of outcome measures that we would use in the review process. We used this pre‐planned method where trialists had evaluated an outcome with more than one outcome measure. Where one measure (e.g. Frenchay Activities Index) was used by several trials, we selected the most commonly used measure. There was the potential risk of the review author selecting an outcome measure that had achieved more positive results, thus influencing the results, and it was hoped that the prior planning of analysis would reduce that risk.
In the analysis of satisfaction, due to differences between trials in the questions that were asked, it became necessary to select questions for comparison. We selected questions for analysis only if they appeared in two or more studies. All satisfaction questions involved a Likert scale with four separate categories of satisfaction (highly satisfied, satisfied, dissatisfied and highly dissatisfied). We contracted the results into a dichotomised outcome (satisfied or dissatisfied) and entered data into an analysis to produce an OR for reporting being satisfied.
We cross‐checked individual patient data for completeness on receipt. In addition, we cross‐checked them with published data. For subgroup analysis, we split data into separate databases and analysed them separately. We used double data entry to enter data into RevMan to ensure the avoidance of simple errors. We checked the direction of effect for each outcome measure in each trial and cross‐checked all tables on completion for errors and completeness.
Results
Description of studies
Review process results
The search strategy identified 17,734 titles to be reviewed (search date July 2009). From these titles we selected 71 studies as being of possible relevance to the review. Where possible, we sought the full text of these studies or the abstract where a full paper was not available. Of these studies, we considered 57 to be unsuitable for inclusion: eight were not randomised controlled trials, eight evaluated interventions for caregivers only, a further 18 evaluated education interventions only, 10 evaluated some form of physical rehabilitation, six evaluated a single‐faceted intervention only such as social support alone, five evaluated only a sub‐population (such as post‐surgical subarachnoid haemorrhages only), and two evaluated the impact of chronic disease management programmes (Characteristics of excluded studies). Trial selection is illustrated in Figure 2.
Figure 2.
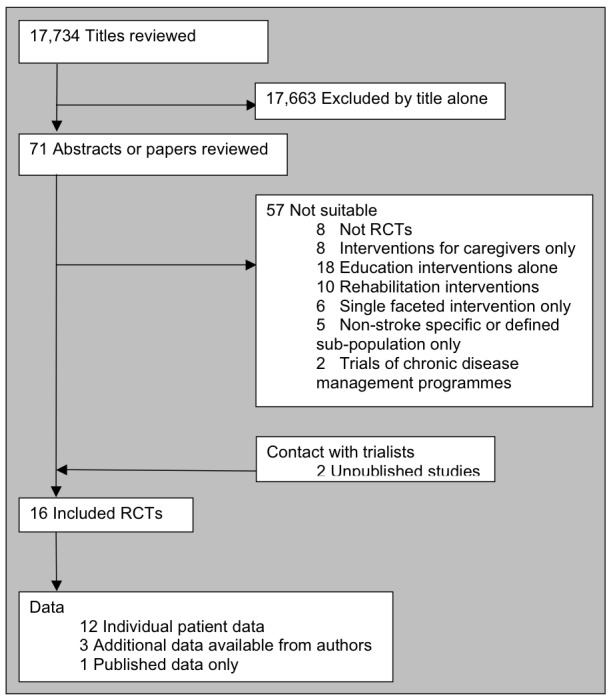
Trial flow
Selected trials
The search strategy identified 14 published randomised trials, and we identified a further two unpublished trials following contact with trialists. One of the unpublished studies evaluated two interventions in separate arms (the input of a volunteer or a psychologist for problem‐solving therapy compared to usual care) (Leeds). The other unpublished study evaluated three separate interventions, alone or combined. These included a stroke family support worker (social), a psychology intervention (psychology) and an occupational therapy intervention (physical). We considered only the stroke family support worker and the psychology arms of the study to be relevant for inclusion and where these elements were combined, we excluded the data (Liverpool).
We obtained individual patient data for 12 studies, and obtained additional tabulated data for two trials (Adelaide; Philadelphia (STAIR)). Limited information from one unpublished trial was available from correspondence with the author (Melbourne (SHIPS)). Published data only were available for one trial (Melbourne).
Risk of bias in included studies
The 16 trials came from four countries (Australia, Netherlands, UK and USA). Most were based in city hospitals and evaluated services in urban populations. Thirteen studies described adequate allocation concealment: eleven studies performed blinding of the final outcome assessor and two studies performed additional patient blinding by a means of delayed or modified consent (Edinburgh; Utrecht), the justification of which is described separately (Boter 2003). The intervention characteristics for the primary analysis are shown in Figure 1. Four interventions were classified as employing a proactive and structured approach to the intervention. Eight interventions were reactive and flexible, while six interventions employed a proactive but focused approach.
Available data for some outcomes were very limited and not combinable. For instance, knowledge was only apparently evaluated in two studies (Mansfield; Oxford). Neither study used validated methods to test that knowledge, and data were only available for one study (Oxford). For this reason, we did not attempt to perform a meta‐analysis of knowledge as an outcome. Similarly, data on caregiver knowledge were only available for one study (Oxford). Resource or service usage were reported in several studies (Edinburgh; Liverpool; Melbourne (SHIPS); Oxford; Philadelphia (STAIR); Utrecht). Data were only available for two of these studies (Oxford; Utrecht). Due to variations in service provision, and differences in the available data, it was felt to be too difficult to combine and dichotomise these data. For this reason, we did not perform a meta‐analysis of service usage.
After receipt of data, it became apparent that analysis of mental health under one single heading was potentially inaccurate where some general measures of mental health (such as GHQ 28) were being combined with more specific measures of depression (such as the Hospital Anxiety and Depression Scale (HADS)‐Depression scale) or anxiety (HADS‐Anxiety scale). In addition, since it was apparent that some studies had used the HADS‐Anxiety scale, we felt it was of potential additional value to split mental health outcomes into depression and anxiety in addition to analysis under a generic mental health domain. We considered it plausible that the intervention might reduce anxiety without necessarily reducing depression. We made this decision post hoc, and therefore we have presented the results with that caution alongside generic mental health results as originally planned.
Effects of interventions
We have presented these data in two stages for simplicity. We have presented overall data here with subgroups as pre‐planned by the collaborative group according to intervention characteristic (Figure 1).
Primary patient outcomes
Subjective health status
Analysis of data for 3112 participants (13 interventions) did not show a significant overall difference between the intervention and control groups for this outcome (SMD ‐0.03, 95% CI ‐0.11 to 0.04, P = 0.34). Tests for heterogeneity were borderline (Chi2 heterogeneity P = 0.08); however, no single subgroup showed a significant effect.
Extended activities of daily living
Analysis of data for 3051 patients (15 interventions) did not show any benefit of stroke liaison workers over control group for an improvement in extended activities of daily living (SMD 0.05, 95% CI ‐0.02 to 0.12, P = 0.16). No significant subgroup interaction was present (Chi2 P = 0.42).
Secondary patient outcomes
Death
There was no significant effect of the stroke liaison worker intervention on the outcome of death (3891 participants, 16 interventions, OR 0.88, 95% CI 0.72 to 1.08, P = 0.23). There was no subgroup interaction (Chi2 P = 0.78).
Place of residence (institutionalisation)
Data were more limited for analysis of institutionalisation (983 participants, six interventions). However, there was no overall effect (OR 0.82, 95% CI 0.48 to 1.38, P = 0.44) and no significant subgroup effect (Chi2 P = 0.99).
Activities of daily living
No significant benefit was seen on activities of daily living for the intervention group compared to the control group (3221 participants, 15 interventions, SMD ‐0.01, 95% CI ‐0.08 to 0.06, P = 0.39). No subgroup interaction existed (Chi2 P = 0.39).
Dependency
Data on dependency were more limited (1468 participants, four trials) and were not available for one subgroup (Proactive and focused). No overall benefit was seen for the stroke liaison worker intervention, with the direction of benefit favouring the control group (SMD 0.03, 95% CI ‐0.07 to 0.14, P = 0.47). There was no significant heterogeneity seen between the two remaining subgroups (Chi2 P = 0.47). Few outcome measures for dependency were used, providing only limited outcome data for dependency (four trials, 1468 participants). In contrast a larger number of studies (13 interventions, 2817 participants) used the Barthel measure. We therefore decided to dichotomise the Barthel measure as an outcome for dependency. We discussed the potential 'cut‐point' for dependency with trialists and set it at 19/20 (i.e. dependent in one or more activities of daily living). We made this decision prior to analysis of the data without prior knowledge of the results. Analysis using this outcome for dependency yielded greater participant involvement across a greater range of studies. For this reason, we conducted subsequent analyses for the outcome of dependency using this definition of dependency. Overall, there was no significant difference between the treatment and control groups for a reduction in dependence (OR 0.89, 95% CI 0.75 to 1.05, P = 0.16). There was no subgroup interaction (Chi2 P = 0.17).
Mental health (generic)
Data were available for 3081 participants from 15 interventions. Overall results did not suggest any beneficial effect of stroke liaison workers compared to control for an improvement in mental health score (SMD ‐0.01, 95% CI ‐0.08 to 0.07, P = 0.87). There was no subgroup interaction (Chi2 P = 0.31).
Mental health (depression)
Analysis of data from 15 interventions (2743 participants) did not show any evidence of a beneficial effect from the input of a stroke liaison worker when compared to the control group, despite the direction of effect favouring the treatment group (SMD ‐0.04, 95% CI ‐0.12 to 0.04, P = 0.30). There was no subgroup interaction (Chi2 P = 0.14).
Mental health (anxiety)
Data were available for two subgroups (Proactive and structured; Reactive and flexible) involving five interventions (1200 participants). No significant benefit was seen for the intervention group (WMD ‐0.20, 95% CI ‐0.66 to 0.26, P = 0.39). Tests for heterogeneity were borderline overall (Chi2 P > 0.05), predominantly because of one study (Adelaide) that showed a positive treatment effect (‐1.7, 95% CI ‐2.89 to ‐0.51); however, this study reported differences in the groups at baseline that may have accounted for the effect.
Participation
Results for participation were more limited in the number of participants (860) and only available for one subgroup (Reactive and flexible). Overall, there were no significant differences between the groups (SMD ‐0.04, 95% CI ‐0.17 to 0.10, P = 0.59) and there was no heterogeneity within the available subgroup (Chi2 P = 0.50).
Primary caregiver outcome
Caregiver subjective health status
Data for 1775 caregivers were available from 15 interventions. The predominant measure used was the Carer Strain Index (9/13 trials). For this reason, this measure was used in the Oxford study, rather than the more positive published Carer SF 36. Although the direction of effect was in favour of the control group, there was no overall significant effect (SMD 0.04, 95% CI ‐0.05 to 0.14, P = 0.37) and no significant subgroup interaction (Chi2 P = 0.96).
Secondary caregiver outcomes
Caregiver extended activities of daily living
Only two subgroups (Proactive and structured; Reactive and flexible) had adequate data from five trials (752 participants). There was a trend to an improvement in extended activities of living in the control group (SMD ‐0.13, 95% CI ‐0.28 to 0.01, P = 0.07). No significant subgroup interaction existed (Chi2 P = 0.78).
Caregiver mental health
Data for 1629 caregivers were analysed across 13 intervention arms. No significant overall effect or subgroup effect existed (SMD ‐0.02, 95% CI ‐0.12 to 0.08, P = 0.67, heterogeneity Chi2 P = 0.58).
Patient satisfaction data
In summary, only one domain of patient satisfaction reached a statistically significant result with data from three trials (905 participants). Patients in the intervention group were significantly more satisfied that 'someone has really listened' (OR 1.58, 95% CI 1.14 to 2.19, P = 0.006). Subgroup results suggested borderline heterogeneity (Chi2 P = 0.07), with one subgroup (Reactive and flexible) strongly positive (two trials, 439 participants, P = 0.0005) and one (Proactive and structured) being neutral (one trial, 470 participants, P = 0.61).
Carer satisfaction data
Four questions for carers yielded statistically significant results.
'I received all the information I needed about the nature and causes of the patient's illness' was included in three trials (459 caregivers) from one subgroup (Reactive and flexible). The question was positive in favour of the stroke liaison worker arm of the trial (OR 1.72, 95% CI 1.04 to 2.85, P = 0.03).
'I have received enough information about recovery and rehabilitation' was answered favourably by the treatment group in three trials (457 caregivers, OR 1.98, 95% CI 1.25 to 3.14, P = 0.004).
'Someone has really listened' was answered in two trials (Edinburgh; London), both in the same subgroup, with a smaller number of caregivers (300 participants, OR 2.56, 95% CI 1.52 to 4.31, P = 0.0004).
Caregivers in the stroke liaison workers' group were more likely to report that they felt that they were not neglected (OR 2.62, 95% CI 1.44 to 4.77, P = 0.002) than in the control group.
Subgroup analyses
In order to find out which aspects of the stroke liaison worker intervention might be effective and which might not, we attempted to form meaningful intervention subgroups. This subgrouping was based on external or pragmatic factors rather than on a known mechanism of effect for the intervention. Because individual trials were conflicting, it could not be clearly established which aspects of the intervention might be resulting in a positive benefit and which might result in harm. These subgroups were agreed by the trialists at the meeting. They are presented here with their rationale and results. Satisfaction data are presented for subgrouping according to intervention type rather than for patient characteristics.
Prior to the meeting of trialists, we attempted to form a classification based on the dominant emphasis of an intervention (education and information provision, social support, liaison). We chose this method of subgroup analysis recognising that although the interventions may have had a multifaceted approach, they may have been created with a primary concern in mind (for example, counselling for psychological difficulties post stroke). In order to acquire more detailed information about the types of intervention, we asked trialists to complete a 'grid' detailing different aspects of the stroke liaison workers' role and function. We then allocated trials to a subgroup (education and information provision, social support, liaison) according to the dominant emphasis of a particular trial. Where trialists could not be contacted, PL and GE completed trial grids independently.
Primary emphasis of intervention
Primary patient outcome
Subjective health status
Analysis of data for 3114 participants (14 interventions) did not suggest an overall benefit from stroke liaison worker (as before). The subgroup providing education and information provision (two interventions) as the dominant emphasis of the service showed a positive subgroup result (SMD ‐0.24, 95% CI ‐0.44 to ‐0.04, P = 0.02). Similarly the group providing liaison as the dominant emphasis (one intervention) suggested a trend towards benefit in the treatment group (SMD ‐0.24, 95% CI ‐0.47 to 0.04, P = 0.09). There was no benefit seen for the larger subgroup (11 interventions) whose dominant emphasis was on social support (SMD 0.00, 95% CI ‐0.07 to 0.08, P = 0.94). Overall, there was significant subgroup heterogeneity (Chi2 P = 0.02) suggesting that the contrast between education and information provision and the other aspects of the stroke liaison role reflected a real difference in the intervention.
Primary carer outcome
Carer subjective health status
Analysis of data for 1775 carers did not suggest any benefit for carers from stroke liaison worker intervention (SMD 0.04, 95% CI ‐0.05 to 0.14, P = 0.37). There was no subgroup interaction (Chi2 P > 0.05).
Profession of stroke liaison worker
The term 'stroke liaison worker' describes a role that spans different professions including nursing, psychology, social work, other allied health professions or the voluntary sector. It could be argued that differing levels of knowledge and skill rather than attitude alone may differentiate between otherwise externally comparable roles. This knowledge or skill may influence patient education and information provision, or provide more focused patient and carer counselling. For this reason dividing the results by professional grouping seems a legitimate method of analysing the overall results.
We asked trialists to provide information on the professional background of the stroke liaison worker evaluated in each trial where this was not clear from published data. We grouped the professions into four distinct subgroups: nurse, psychologist, social worker and a final grouping of generic health care worker or volunteer. This last subgroup included trials where the stroke liaison worker was from an allied health profession, the voluntary sector or (in the case of some trials with more than one worker) no specific background but had been trained in the stroke liaison worker role.
Primary patient outcome
Subjective health status
Analysis of data for 3112 participants did not demonstrate any overall impact from stroke liaison workers as before (SMD ‐0.03, 95% CI ‐0.11 to 0.04, P = 0.34) and there was no subgroup interaction (Chi2 P > 0.05).
Primary carer outcome
Carer subjective health status
Analysis of data from 1775 carers did not show any benefit from stroke liaison worker (SMD 0.04, 95% CI ‐0.05 to 0.14, P = 0.37) and there was no subgroup interaction for the profession of the stroke liaison worker (Chi2 P > 0.05).
Patient characteristics: subgroup analysis
Patient age
We dichotomised patient data where possible into two subgroups: under 65 years old and 65 years or older. It was postulated that younger patients may have differing or greater psychosocial problems to older patients and may respond differently to the intervention. Results are described here where a significant subgroup exists along with a significant subgroup interaction.
Primary patient outcome
Subjective health status
Analysis of 2503 participants by age did not demonstrate any overall benefit from the stroke liaison worker intervention (SMD ‐0.06, 95% CI ‐0.14 to 0.01, P = 0.11) and there was no subgroup interaction for the subgroups under 65 years and 65 years and older (Chi2 P > 0.05).
Primary carer outcome
Carer subjective health status
Analysis of carer data for 1483 carers did not show any overall benefit from stroke liaison worker input (SMD ‐0.01, 95% CI ‐0.11 to 0.09, P = 0.87) and there was no subgroup interaction for the subgroups under 65 years and 65 years and older (Chi2 P > 0.05).
Patient gender
We dichotomised data on the basis of sex. It seemed reasonable to explore this subgrouping as men and women may respond differently to an intervention with a significant psychological and social component. It should be noted that for analysis of carer data, we analysed these according to the sex of the patient and may not reflect the sex of the carer. For instance, a carer may well be a daughter looking after a mother or a wife looking after a husband. Data on carer sex were inadequate across the trials to analyse according to carer sex.
Primary patient outcome
Subjective health status
Analysis of data from 2491 participants showed no overall benefit from stroke liaison worker input and there was no subgroup interaction for either male of female patient groups (SMD ‐0.05, 95% CI ‐0.13 to 0.03, P = 0.20, Chi2 heterogeneity P > 0.05).
Primary carer outcome
Carer subjective health status
Analysis of data for 1483 carers dichotomised into subgroups according to patient sex did not show any significant benefit from stroke liaison worker input, and there was no significant subgroup interaction (SMD ‐0.05, 95% CI ‐0.13 to 0.03, P = 0.20, Chi2 heterogeneity P > 0.05).
Presence of a carer
It is theoretically possible that patients without identified carer support might be at higher risk of poorer health. It therefore follows that this subgroup might benefit the most from social support aspects of the intervention. Within the trials there was varied involvement of carers. Some trials (Adelaide; Rhode Island) specifically recruited only patients who had an identified primary caregiver. Other studies did not include or record caregiver involvement at all (Glasgow). Clearly these trials do not allow us to test the hypothesis that the presence or absence of an identified primary caregiver results in different responses to the stroke liaison worker intervention. For this reason, we excluded these trials from this subgroup analysis.
The quality of recording of caregiver presence and proximity of involvement with the patient was variable. Two trials reported carer relationship (Oxford; Utrecht). Other trials did not collect these data. To analyse these trials adequately, we hypothesised that the presence of carer data recorded by trialists formed a proxy for the clear identification of a caregiver. This proxy has its limitations, as the proximity and level of involvement of a caregiver with a patient may vary considerably and not be sensitively measured by this dichotomisation. Nevertheless, it could be postulated that a closely involved carer is more likely to be contactable and therefore available for simple data collection. We therefore dichotomised data according to whether a carer was identified (Oxford; Utrecht) or whether carer outcome data were collected. Results are presented here for these two subgroups. Clearly there can be no comparison of carer outcomes for this subgroup analysis. We observed no significant subgroup or subgroup interaction.
Primary patient outcome
Subjective health status
We dichotomised data from 2296 participants according to the presence or absence of a main carer. Analysis did not reveal any benefit from stroke liaison worker input (SMD ‐0.05, 95% CI ‐0.13 to 0.03, P = 0.26) and there was no subgroup interaction according to the presence or absence of a main carer (Chi2 P > 0.05).
Patient functional status
It has been assumed in most trials that the intervention of a stroke liaison worker should be applied to all patients regardless of their level of disability or functional dependence. Data from one trial (Bradford) have previously suggested that patients with mild to moderate disability (as measured by the Barthel Index 15 to 19) make the most gains from stroke liaison worker input. We therefore decided to evaluate this question by functional status according to Barthel measurement. Patients were divided according to their Barthel index at recruitment (which usually equated to their Barthel at discharge from hospital). They were divided into three subgroups: Barthel 20 (independent), Barthel 15 to 19 (mild to moderately dependent) and Barthel less than 15 (dependent). Barthel indices at recruitment varied across the trials as might be expected and are illustrated in Figure 3. Recruitment appeared highest in the more independent groups as might be expected for a trial evaluating psychosocial interventions in a population returning to the community. Interestingly the patient population appears to trichotomise relatively equally. Where a significant subgroup effect was seen in conjunction with a significant subgroup interaction, the results are discussed here.
Figure 3.
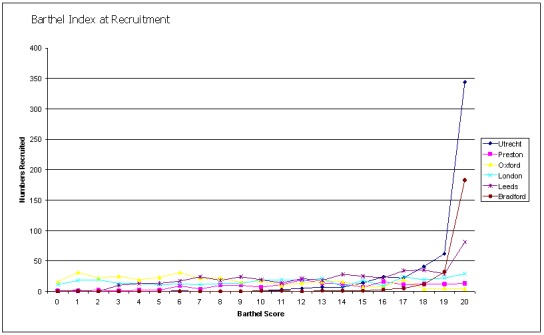
Barthel Index at recruitment
Primary patient outcome
Subjective health status
Analysis of 2268 participants subgrouped according to their Barthel score at recruitment did not show any overall benefit on subjective health status from stroke liaison worker input and no significant subgroup effect was seen for this outcome (SMD ‐0.03, 95% CI ‐0.11 to 0.05, P = 0.46, Chi2 heterogeneity P > 0.05).
Patient secondary outcomes
Dependence (Barthel)
We analysed data for 2494 participants in 12 interventions of 10 trials. The subgroup Barthel 15 to 19 had a significant reduction in dependence (OR 0.62, 95% CI 0.44 to 0.87, P = 0.006). This effect size would equate to 10 fewer dependent patients (95% CI 17 fewer to four fewer) for every 100 patients with mild to moderate disability that were seen by the stroke liaison worker. Significant subgroup heterogeneity existed suggesting that this subgroup was responding differently to the intervention than the others (Chi2 P < 0.05). The other subgroups did not show a significant effect of the stroke liaison worker intervention however (Barthel less than 15 OR 1.15, 95% CI 0.82 to 1.62, P = 0.43; Barthel 20 OR 0.98, 95% CI 0.68 to 1.42, P = 0.92).
Death or dependence
We decided after analysis of dependence data to look at this combined outcome. The concern was that there might appear to be a reduction in dependence at the expense of increased mortality. We combined data using the same dependence data. Overall, data for this new outcome did not show a significant benefit from the stroke liaison worker for a reduction in death or dependence (OR 0.89, 95% CI 0.72 to 1.11, P = 0.32). A significant subgroup effect was again seen (Barthel 15 to 19) as well as subgroup heterogeneity suggesting that this group responded differently to the stroke liaison worker input (OR 0.55, 95% CI 0.38 to 0.81, P = 0.002). This risk difference equates to 11 fewer dead or dependent patients per 100 treated cases (95% CI 17 fewer to four fewer) as a result of stroke liaison worker input for the group with mild to moderate disability. Results for the other subgroups were not significant (Barthel less than 15 OR 1.38, 95% CI 0.93 to 2.04, P = 0.11; Barthel 20 OR 0.98, 95% CI 0.68 to 1.43, P = 0.93; Chi2 P < 0.05).
Primary carer outcome
Carer subjective health status
Analysis of data for 1509 carers divided according to patient Barthel at recruitment did not show any significant effect for the primary carer outcome of carer subjective health and there was no significant subgroup effect (SMD 0.01, 95% CI ‐0.10 to 0.11, P = 0.9, Chi2 heterogeneity P > 0.05).
Discussion
Individual patient data meta‐analysis confers considerable advantage in unpacking the potential benefits or harms of an intervention. At a statistical level it is less likely to overestimate the effect of an intervention, but also gives narrower confidence intervals, reducing the chance of missing a real benefit or harm. It permits a greater understanding of the studies involved, which is vital where the interventions and trials are complex, as in this case. Additionally, they allow greater flexibility in exploring subgroups. This is important in this case as little is understood regarding the underlying mechanism of potential benefit for stroke liaison workers and therefore it is hard to identify which intervention characteristics are most important and additionally which patient subgroups, if any, are most likely to benefit. However, individual patient data meta‐analysis is considerably more complex. Meta‐analysis is secondary analysis of research where ethics approval has already been obtained. Despite that, however, some trialists did run into difficulties in sharing their data. Two studies (Adelaide; Boston) described having to have permission to share their data with a third party. In one case (Adelaide) the ethics committee would not give permission to share anonymised individual patient data with the collaborative group. Instead, data for subgroup analysis were provided in aggregate form on request from the lead author. In the other case (Boston), approval from the data review board took approximately one year and represented a considerable delay for the review process.
It is important to state that in the planning of this analysis, the lack of a theoretical or pathophysiological rationale for stroke liaison workers affected the selection of appropriate primary outcome measures. Given that this is a broad or comprehensive intervention for a wide range of problems, it was unclear from the published trials (Bradford; Edinburgh; Oxford; Utrecht) which outcome might be expected to be impacted most. As a result, the published trials evaluated different primary outcome measures from activities of daily living (Boston), satisfaction (London; Utrecht), subjective health status (Oxford; Preston) and extended activities of daily living (Bradford) or none at all (Edinburgh). For this reason, two primary outcome measures were chosen, as it was argued that if the stroke liaison workers' aim was to return patients to normal roles that this might be measured in activities and perceived health. It remains a relevant potential criticism that we chose the wrong outcome measure to evaluate effectiveness and that stroke liaison workers might have an unanticipated impact in an entirely different domain. The intervention of a stroke liaison worker did not affect the primary outcomes of subjective health status or extended activities of daily living. The implication is that patients who were seen by the stroke liaison worker did not feel their health or quality of life to be better than controls, and they were not more independent. There are two potential explanations for failing to demonstrate an impact. The first concerns the intervention itself. The second concerns matters of methodology.
The criticism of the intervention might appropriately be that it is poorly focused and too broad or diffuse to impact a single, specific outcome. Because these interventions were developed on a practical and intuitive basis, as has already been said, they lack a clear underlying mechanism of action. This development of services to meet a wide range of problems post stroke may have been too ambitious and as a consequence poorly focused. The provision of information and education is a real need with a wider evidence base than in this review alone (Forster 2001). The impact of information might reasonably be expected to be measured in satisfaction first, rather than in behaviour change (Education Group 1999). Liaison as a service offered for patients may be difficult to measure and is perhaps poorly addressed in the stroke liaison worker trials. It might be reasonable to expect that liaison between the patient and community services or health services might be measured in resource use. Unfortunately, we were unable to assess resource use in this review due to a lack of standardised measures. In addition, the meaning of increased resource use must be evaluated. It is unclear whether increased uptake of community services for instance means increased access and enhanced resources, or conversely an increasing dependence. Similarly, in a health care context it would be difficult to tell from crude data whether increased use of medical and therapy resources meant an attitude of active and self‐motivated health care or simply increasing ill health. Social support, whilst being potentially hardest to define, may in fact be the one outcome that has the most measures of effectiveness. Many of these outcomes are surrogates (e.g. measures of mental health, self‐efficacy, satisfaction, participation) and poorly correlated with what is a diffuse and poorly defined entity. Some trials have tried to be more specific about social support and have included counselling as part of the intervention (Boston; Philadelphia (STAIR)). Whilst it might be expected that this would more directly impact some measures (e.g. depression, anxiety, etc.) it has not been so clear in either the trials or the review process.
It has been assumed that all patients have information needs and share many of the psychological and social problems post stroke. Similarly, it has been assumed that the intervention should be targeted at all patients regardless of age, sex or stroke severity. This assumption does not adequately take into consideration differences in risk between patients in terms of, for example, the onset of post‐stroke depression, social isolation, anxiety, etc. Patient subgroup analysis was only possible for those identifiers that allow division of patients into groups (e.g. age, sex, Barthel at recruitment, etc.). These patient descriptors may not adequately correlate with the risks of a poor outcome (e.g. age and depression) and therefore may not subdivide patient subgroups according to meaningful groups. Despite these shortfalls, and because of the existing limitations with the data, the subgroup analysis by age, sex, Barthel and the presence of a carer seems a robust and plausible exploration of the data. Only one study (Melbourne) recruited patients more than six months after stroke. Data for this study were only obtained from published data for limited numbers of outcomes. For this reason subgroup analysis for this description was felt to be unhelpful. Subgroup analysis within the review process highlights some interesting areas of differences that may be important and may identify important aspects of either the intervention or the target population. It is recognised in observational studies and trials that multiple analyses carry the risk of false positive results. This risk should be lessened in meta‐analysis, nevertheless it still remains a risk with multiple subgroup analyses. For this reason we have only considered subgroup results where there is both a subgroup with a significant difference and a significant subgroup interaction.
Analysis of the intervention effect by emphasis of the intervention highlights some differences between the intervention types that may be important. In the analysis of subjective health status, for instance, two subgroups (education and information provision as well as liaison) were significant in favour of the intervention, whilst one subgroup (social support) was non‐significant. Importantly, the subgroups varied in size, with the education and information provision subgroup containing two studies, and the liaison group containing only one. The social support group contained 10 interventions from eight trials. The differences between the groups could be potentially accounted for by 'regression to the mean' where the larger subgroup has more neutral results. Heterogeneity tests were positive, suggesting that a subgroup interaction exists and that the subgroups are behaving differently for this outcome. There is a risk of over interpreting subgroup data and, given the overall non‐significant result for stroke liaison workers, we cannot necessarily conclude that any one subgroup is effective. Despite this it is possible to conclude that interventions with a strong emphasis on social support are less effective at impacting subjective health status. Analysis of the same data by the profession of the stroke liaison worker reveals a few interesting findings. Patients whose stroke liaison worker was a nurse by professional background appear to have a significant reduction in depression score when compared to controls. This effect also differed significantly from the other subgroups suggesting that the intervention, when delivered by a nurse, differed in nature from interventions delivered by other professions.
If the intervention itself does not appear to be effective for all patients, it is reasonable to explore patient subgroups to establish which groups of patients, if any, benefit, and which do not. Analysis by age suggested differences between the two subgroups in how they responded to the stroke liaison worker in the area of activities of daily living (ADL). Younger patients appeared to benefit in improvement in ADL score in the group reviewed by the stroke liaison worker. In addition, heterogeneity tests were positive suggesting that the younger (less than 65 years) and older (65 years or older) patients respond differently to the intervention of a stroke liaison worker. It is not possible to say whether these differences relate to differences in the way the stroke liaison workers treated patients who were younger, or more probably because there are important differences in the patient group examined. Analysis of data by the presence or absence of a carer could be criticised for our choice of a surrogate for carer involvement. The absence of data for a primary carer does not mean the absence of a good caregiving network. This may be a reason that no significant differences were found between the two subgroups. Equally, the impact of a stroke liaison worker's social support on the effects of social isolation may be small. Arguably, several short visits from a stroke liaison worker may not be enough to mitigate against the risks of social isolation, depression and mortality that appear to be associated with reduced social support.
The final patient subgrouping is the functional status of the patient at recruitment. This subgrouping was suggested by one of the trials (Bradford) as identifying patients who would benefit most. This trial had suggested that patients with mild‐moderate dependence (Barthel 15 to 19) benefited most. For this reason, we have used the same definitions of dependence. It is plausible that patients with severe dependence may make minimal gains with information provision. Similarly, patients who are dependent may already have established connections with services (such as carers, social work, primary care, etc.) and may have little to gain from liaison input. Alternatively, you might expect that caregivers of patients who are dependent would need the most support and might be the most satisfied. By contrast, independent patients might be expected to have the least risk of depression or morbidity, and the least to gain from social support or liaison. The finding of a reduction in dependence (or an increase in the number who were independent in activities of daily living) was in some respects surprising. The intervention was primarily a psychosocial one and was not expected to impact physical outcomes. As has already been said, there was concern that the reduction in dependence could be at the expense of an increase in mortality. For this reason we considered a post hoc analysis of a combined outcome of death or dependency necessary in order to rule out this concern. This result was also considered surprising. The effect size of this reduction in death or dependence is considerable, equating to 11 fewer dead or dependent patients for every 100 patients treated. The mechanism of this effect is not clear. It is interesting to note that the review of early supported discharge after stroke also found that patients with mild to moderate disability (Barthel 10 to 20) made the most gains with early supported discharge, resulting in reductions in death or disability (ESDT 2005). It is interesting to postulate whether the improvement seen in this group reflects the impact of the stroke liaison worker per se (as opposed to any other form of rehabilitation intervention) or whether it reflects the sensitivity of this particular patient group to rehabilitation and to potential gains in independence. None of the studies in the stroke liaison worker review appears to have had input from an early supported discharge service. There may be potential benefit from further research exploring the rehabilitation potential of this patient subgroup and the most appropriate design of rehabilitation interventions.
Data for the outcome of satisfaction appear to provide the only overall statistically significant results for the intervention. Patients responded significantly to only one question: 'someone has really listened'. This response would potentially fit well with the social support intentions of the intervention. It is interesting to note, however, that there are a number of patient responses that show a trend towards significance in favour of the control group ('I have been treated with kindness and respect', 'I was able to talk to the staff about any problems I had' and 'I am satisfied with outpatient services'). There may be more than one explanation for these findings (although in the absence of statistical significance we must be cautious about drawing conclusions). One possible explanation is that in simple terms patients were as satisfied with the intervention as those who did not receive it. One additional potential explanation reflects the complexity of using satisfaction as an outcome measure. Satisfaction, it might be argued, is based in part on a participant's expectations. If expectations are low, participants may express high satisfaction with what is delivered. If expectations are raised, for example by a stroke liaison worker educating patients on the importance of treatment and investigation, it might be expected that patients become less satisfied with service provision (e.g. outpatient services). In addition, it is recognised that satisfaction questionnaires have a ceiling effect and that they may not be sensitive to discriminate between groups if overall satisfaction is high.
Interestingly, carers appear to express satisfaction more frequently than patients. The responses that reach significance appear to be related to the nature of the intervention and are therefore plausible. For example the response 'I have received enough information about recovery and rehabilitation' and the response 'I have received all the information I needed about the causes of the patient's illness' would plausibly fit with the education and information provision aspects of the intervention. Similarly, the response 'someone has really listened' would fit with the social support aspects of the intervention and the response 'I have not felt neglected' would fit with the liaison aspects of the service. In some cases, however, the positive results are drawn from only two studies studying approximately 300 carers. The results, although statistically significant, are not robust and the addition of as few as 10 positive responses to the control group could result in a change to a non‐significant result ('someone has really listened').
Authors' conclusions
There is little evidence from this meta‐analysis to support stroke liaison workers for all groups of patients and carers. There appears to be significant benefit from stroke liaison worker input to patients with mild to moderate disability (e.g. Barthel 15 to 19) and it seems reasonable to ensure that services do not neglect this group. Patients and carers do report improved satisfaction with aspects of service provision. Healthcare providers need to evaluate the value of this outcome.
Research into this multifaceted intervention must be re‐evaluated. Research should consider evaluating more closely the relationship between more focused interventions for specific impairments (e.g. counselling for depression) and their wider impact on participation (e.g. extended activities of daily living). Multifaceted interventions with broad intentions also need to be linked more closely to evidenced therapeutic methods. Further work on the model of interventions for psychosocial problems is warranted. In addition, it must be noted that most trials were conducted in well‐developed healthcare settings with established stroke services. Extrapolation cannot be made to all settings or healthcare organisations.
The impact of stroke liaison worker interventions on patients with mild to moderate disability needs to be explored. Further work should concentrate on establishing if this is a real effect of the intervention or simply reflects the ability of patients with mild to moderate disability to improve with continued healthcare input. The implications are significant. Potential overlap exists with the well‐documented effects of early supported discharge after stroke. No such services existed in the trials evaluated in this review.
Acknowledgements
We would like to thank Brenda Thomas of the Cochrane Stroke Group for developing the search strategy.
The Stroke Liaison Workers Collaboration are (in study alphabetical order): Michael Clark (Adelaide), Martha Fay, Thomas Glass (Boston, Anne Forster, John Young (Bradford), Martin Dennis, Suzanne O'Rourke (Edinburgh), Graham Ellis, Peter Langhorne (Glasgow), Allan House (Leeds), Michael Leathley, Anil Sharma, Caroline Watkins (Liverpool), Kate Tilling, Catherine Coshall, Charles Wolfe (London), Nadina Lincoln (Mansfield), Judith Frayne (Melbourne (SHIPS)), Jonathan Mant (Oxford), Arthur Gershkoff (Philadelphia (STAIR)), Chris Burton (Preston), Ivan Miller, Duane Bishop (Rhode Island), Han Boter, Thora Hafsteinsdottir (Utrecht).
Appendices
Appendix 1. MEDLINE search strategy
The search strategy for MEDLINE is shown below. This was adapted for the other databases.
1. stress, psychological/ 2. psychosocial$.tw. 3. social adjustment/ 4. adaptation, psychological/ 5. activities of daily living/ 6. exp interpersonal relations/ 7. morale/ 8. (cope or coping).tw. 9. patient satisfaction/ 10. exp emotions/ 11. ((psychological or social) and (problem$ or difficult$)).tw. 12. exp social isolation/ 13. emotion$.tw. 14. stress/ 15. knowledge, attitudes, practice/ 16. exp motivation/ 17. quality of life/ 18. anxiety/ 19. caregivers/ 20. life change events/ 21. depression/ 22. life style/ 23. social behavior/ 24. mental health/ 25. knowledge/ 26. psychomotor performance/ 27. exp family relations/ 28. or/1‐27 29. patient care management/ 30. continuity of patient care/ 31. needs assessment/ 32. rehabilitation nursing/ 33. home nursing/ 34. "referral and consultation"/ 35. social support/ 36. exp professional‐patient relations/ 37. ((patient$ or carer or caregiver$ or famil$) adj10 support$).tw. 38. patient education/ 39. exp social work/ 40. community health services/ 41. (home or in‐home or home‐based).tw. 42. health services for the aged/ 43. ((patient$ or carer or caregiver$ or famil$) adj10 information$).tw. 44. family health/ 45. family care$.tw. 46. outreach.tw. 47. advice.tw. 48. counseling/ 49. counsel?ing.tw. 50. nursing assessment/ 51. aftercare/ 52. volunteer$.tw. 53. exp rehabilitation/ 54. communit$.tw. 55. empathy/ 56. visitor$.tw. 57. patient‐centered care/ 58. health education/ 59. interview, psychological/ 60. exp patient care planning/ 61. domiciliary.tw. 62. (liaison or link or contact).tw. 63. Home care services/ 64. ambulatory care/ 65. or/29‐64 66. exp cerebrovascular disorders/ 67. (stroke$ or cva$ or cerebrovascular or cerebral vascular or post‐stroke or transient isch$ or TIA).tw. 68. (cerebral or cerebellar or brain$ or vertebrobasilar).tw. 69. (infarct$ or isch?emi$ or thrombo$ or emboli$ or apoplexy).tw. 70. 68 and 69 71. (cerebral or brain or subarachnoid).tw. 72. (haemorrhage or hemorrhage or haematoma or hematoma or bleed$ or aneurysm).tw. 73. 71 and 72 74. hemiplegia/ or exp aphasia/ or hemianopsia/ 75. (aphasi$ or dysphasi$ or hemianop$ or hemipleg$ or hemipar$).tw. 76. 66 or 67 or 70 or 73 or 74 or 75 77. 28 and 65 and 76 78. limit 77 to human
Data and analyses
Comparison 1.
Stroke liaison workers versus usual care: intervention type
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subjective health status | 14 | 3112 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.11, 0.04] |
| 1.1 Proactive and structured | 4 | 1033 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.17, 0.07] |
| 1.2 Reactive and flexible | 5 | 1085 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.09, 0.15] |
| 1.3 Proactive and focused | 5 | 994 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.22, 0.04] |
| 2 Extended activities of daily living | 15 | 3051 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.02, 0.12] |
| 2.1 Proactive and structured | 4 | 1048 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.03, 0.21] |
| 2.2 Reactive and flexible | 6 | 1254 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.04, 0.18] |
| 2.3 Proactive and focused | 5 | 749 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.18, 0.11] |
| 3 Death | 16 | 3891 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.72, 1.08] |
| 3.1 Proactive and structured | 3 | 952 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.66, 1.95] |
| 3.2 Reactive and flexible | 7 | 1820 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.66, 1.08] |
| 3.3 Proactive and focused | 6 | 1119 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.55, 1.36] |
| 4 Place of residence | 6 | 983 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.48, 1.38] |
| 4.1 Proactive and structured | 1 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.34, 2.94] |
| 4.2 Reactive and flexible | 2 | 275 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.34, 2.47] |
| 4.3 Proactive and focused | 3 | 468 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.32, 1.47] |
| 5 Activities of daily living | 15 | 3221 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.08, 0.06] |
| 5.1 Proactive and structured | 3 | 904 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.06, 0.20] |
| 5.2 Reactive and flexible | 7 | 1474 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.15, 0.06] |
| 5.3 Proactive and focused | 5 | 843 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.18, 0.10] |
| 6 Dependence | 4 | 1468 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.07, 0.14] |
| 6.1 Proactive and structured | 2 | 728 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.15, 0.14] |
| 6.2 Reactive and flexible | 2 | 740 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.07, 0.21] |
| 7 Dependence (defined as Barthel less than or equal to 19) | 13 | 2817 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.75, 1.05] |
| 7.1 Proactive and structured | 3 | 820 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.52, 1.01] |
| 7.2 Reactive and flexible | 6 | 1278 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.74, 1.23] |
| 7.3 Proactive and focused | 4 | 719 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.69, 1.29] |
| 8 Mental health: generic | 15 | 3081 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.08, 0.07] |
| 8.1 Proactive and structured | 3 | 856 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.12, 0.15] |
| 8.2 Reactive and flexible | 6 | 1201 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.07, 0.16] |
| 8.3 Proactive and focused | 6 | 1024 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.21, 0.04] |
| 9 Mental health: depression | 15 | 2743 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.12, 0.04] |
| 9.1 Proactive and structured | 3 | 716 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.28, 0.02] |
| 9.2 Reactive and flexible | 6 | 1104 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.07, 0.17] |
| 9.3 Proactive and focused | 6 | 923 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.21, 0.05] |
| 10 Mental health: anxiety | 5 | 1200 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.66, 0.26] |
| 10.1 Proactive and structured | 1 | 459 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐1.18, 0.42] |
| 10.2 Reactive and flexible | 4 | 741 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.67, 0.45] |
| 11 Participation | 4 | 860 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.17, 0.10] |
| 11.1 Reactive and flexible | 4 | 860 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.17, 0.10] |
| 12 Caregiver subjective health status | 15 | 1775 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.05, 0.14] |
| 12.1 Proactive and structured | 4 | 645 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.10, 0.21] |
| 12.2 Reactive and flexible | 7 | 776 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.12, 0.17] |
| 12.3 Proactive and focused | 4 | 354 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.17, 0.27] |
| 13 Caregiver extended activities of daily living | 5 | 752 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.28, 0.01] |
| 13.1 Proactive and structured | 2 | 251 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.35, 0.15] |
| 13.2 Reactive and flexible | 3 | 501 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.32, 0.03] |
| 14 Caregiver mental health | 13 | 1629 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.12, 0.08] |
| 14.1 Proactive and structured | 3 | 521 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.15, 0.20] |
| 14.2 Reactive and flexible | 6 | 767 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.22, 0.07] |
| 14.3 Proactive and focused | 4 | 341 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.18, 0.26] |
| 15 'I have been treated with kindness and respect': patient | 6 | 1558 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.28, 1.00] |
| 15.1 Proactive and structured | 1 | 483 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.08, 1.10] |
| 15.2 Reactive and flexible | 2 | 536 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.28, 1.45] |
| 15.3 Proactive and focused | 3 | 539 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.16, 3.20] |
| 16 'The staff attended well to my personal needs': patient | 6 | 1555 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.47, 1.24] |
| 16.1 Proactive and structured | 1 | 483 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.10, 1.02] |
| 16.2 Reactive and flexible | 2 | 533 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.46, 1.86] |
| 16.3 Proactive and focused | 3 | 539 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.40, 2.52] |
| 17 'I was able to talk to the staff about any problems': patient | 6 | 1547 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.51, 1.02] |
| 17.1 Proactive and structured | 1 | 478 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.33, 1.09] |
| 17.2 Reactive and flexible | 2 | 530 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.40, 1.19] |
| 17.3 Proactive and focused | 3 | 539 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.50, 1.87] |
| 18 'I received all the information I want about the causes and nature of my disease': patient | 8 | 1914 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.81, 1.30] |
| 18.1 Proactive and structured | 1 | 482 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.46, 1.17] |
| 18.2 Reactive and flexible | 4 | 893 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.87, 1.68] |
| 18.3 Proactive and focused | 3 | 539 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.59, 1.69] |
| 19 'The staff have done everything to make me well': patient | 6 | 1552 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.54, 1.44] |
| 19.1 Proactive and structured | 1 | 483 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.24, 1.47] |
| 19.2 Reactive and flexible | 2 | 531 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.42, 2.07] |
| 19.3 Proactive and focused | 3 | 538 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.49, 2.87] |
| 20 'I am happy with my recovery': patient | 6 | 1557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.67, 1.18] |
| 20.1 Proactive and structured | 1 | 483 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.57, 1.85] |
| 20.2 Reactive and flexible | 2 | 536 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.40, 0.98] |
| 20.3 Proactive and focused | 3 | 538 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.75, 1.86] |
| 21 'I am satisfied with the type of treatment I have received': patient | 5 | 1226 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.70, 1.47] |
| 21.1 Proactive and structured | 1 | 408 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.52, 1.73] |
| 21.2 Reactive and flexible | 2 | 472 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.54, 1.88] |
| 21.3 Proactive and focused | 2 | 346 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.55, 2.32] |
| 22 'I was given all the information I needed about allowances': patient | 8 | 1724 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.82, 1.31] |
| 22.1 Proactive and structured | 1 | 435 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.68, 1.48] |
| 22.2 Reactive and flexible | 4 | 766 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.79, 1.62] |
| 22.3 Proactive and focused | 3 | 523 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.56, 1.52] |
| 23 'Things were well prepared for my return home': patient | 5 | 1223 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.70, 1.28] |
| 23.1 Proactive and structured | 1 | 387 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.57, 1.35] |
| 23.2 Reactive and flexible | 2 | 500 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.52, 1.55] |
| 23.3 Proactive and focused | 2 | 336 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.62, 2.30] |
| 24 'I get all the services I need': patient | 6 | 1256 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.75, 1.36] |
| 24.1 Proactive and structured | 1 | 334 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.54, 1.31] |
| 24.2 Reactive and flexible | 3 | 591 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.80, 2.10] |
| 24.3 Proactive and focused | 2 | 331 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.45, 1.96] |
| 25 'I am satisfied with outpatient services': patient | 5 | 1244 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.49, 1.06] |
| 25.1 Proactive and structured | 1 | 463 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.37, 1.09] |
| 25.2 Reactive and flexible | 1 | 254 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.55, 3.63] |
| 25.3 Proactive and focused | 3 | 527 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.33, 1.25] |
| 26 'I am satisfied with the practical help I have received': patient | 2 | 655 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.62, 1.34] |
| 26.1 Proactive and structured | 1 | 403 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.47, 1.14] |
| 26.2 Reactive and flexible | 1 | 252 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.82, 4.22] |
| 27 'I have had enough information about recovery: patient | 4 | 1089 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.91, 1.57] |
| 27.1 Proactive and structured | 1 | 476 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.65, 1.44] |
| 27.2 Reactive and flexible | 3 | 613 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.00, 2.09] |
| 28 'Someone has really listened': patient | 3 | 905 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.14, 2.19] |
| 28.1 Proactive and structured | 1 | 470 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.72, 1.75] |
| 28.2 Reactive and flexible | 2 | 435 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.36 [1.45, 3.83] |
| 29 'I have had enough emotional support': patient | 6 | 1363 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.82, 1.51] |
| 29.1 Proactive and structured | 1 | 471 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.52, 1.23] |
| 29.2 Reactive and flexible | 2 | 363 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.95, 4.25] |
| 29.3 Proactive and focused | 3 | 529 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.81, 2.40] |
| 30 'I know who to contact': patient | 4 | 1112 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.78, 1.50] |
| 30.1 Proactive and structured | 1 | 469 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.49, 1.16] |
| 30.2 Reactive and flexible | 2 | 451 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.96, 2.86] |
| 30.3 Proactive and focused | 1 | 192 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.59, 15.25] |
| 31 'I have not felt neglected': patient | 3 | 923 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.62, 1.26] |
| 31.1 Proactive and structured | 1 | 473 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.53, 1.33] |
| 31.2 Reactive and flexible | 2 | 450 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.55, 1.63] |
| 32 'The patient has been treated with kindness and respect': carer | 4 | 725 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.46, 1.74] |
| 32.1 Reactive and flexible | 2 | 460 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.51, 2.27] |
| 32.2 Proactive and focused | 2 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.07, 2.31] |
| 33 'The staff have attended to my needs': carer | 4 | 720 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.66, 1.69] |
| 33.1 Reactive and flexible | 2 | 459 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.82, 2.56] |
| 33.2 Proactive and focused | 2 | 261 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.16, 1.22] |
| 34 'I received all the information I needed about the nature and causes of the patient's illness': carer | 3 | 459 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.04, 2.85] |
| 34.1 Reactive and flexible | 3 | 459 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.04, 2.85] |
| 35 'The staff have done everything to make the patient well': carer | 4 | 720 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.58, 1.91] |
| 35.1 Reactive and flexible | 2 | 455 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.60, 2.47] |
| 35.2 Proactive and focused | 2 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.22, 2.34] |
| 36 'I am satisfied with the type of treatment the patient received': carer | 2 | 429 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.74, 2.07] |
| 36.1 Reactive and flexible | 2 | 429 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.74, 2.07] |
| 37 'They have had enough therapy': carer | 2 | 437 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.89, 1.93] |
| 37.1 Reactive and flexible | 2 | 437 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.89, 1.93] |
| 38 'I was given enough information about allowances available': carer | 6 | 891 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.92, 1.71] |
| 38.1 Reactive and flexible | 4 | 628 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.01, 2.10] |
| 38.2 Proactive and focused | 2 | 263 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.50, 1.57] |
| 39 'Things were well prepared for their return home': carer | 4 | 682 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.69, 1.47] |
| 39.1 Reactive and flexible | 2 | 424 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.69, 1.77] |
| 39.2 Proactive and focused | 2 | 258 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.45, 1.61] |
| 40 'I get all the support I need from services': carer | 5 | 717 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.70, 1.41] |
| 40.1 Reactive and flexible | 3 | 466 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.71, 1.69] |
| 40.2 Proactive and focused | 2 | 251 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.43, 1.51] |
| 41 'I have received enough practical help': carer | 2 | 305 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.78, 2.85] |
| 41.1 Reactive and flexible | 2 | 305 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.78, 2.85] |
| 42 'I have received enough information about recovery and rehabilitation': carer | 3 | 457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.98 [1.25, 3.14] |
| 42.1 Reactive and flexible | 3 | 457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.98 [1.25, 3.14] |
| 43 'Someone has really listened': carer | 2 | 300 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.56 [1.52, 4.31] |
| 43.1 Reactive and flexible | 2 | 300 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.56 [1.52, 4.31] |
| 44 'I have not felt neglected': carer | 2 | 306 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.62 [1.44, 4.77] |
| 44.1 Reactive and flexible | 2 | 306 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.62 [1.44, 4.77] |
| 45 'I have received enough emotional support': carer | 4 | 519 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.81, 1.87] |
| 45.1 Reactive and flexible | 2 | 259 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.98 [1.01, 3.88] |
| 45.2 Proactive and focused | 2 | 260 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.52, 1.54] |
| 46 'I have received enough special equipment': carer | 2 | 250 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.91, 3.64] |
| 46.1 Reactive and flexible | 2 | 250 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.91, 3.64] |
| 47 'I know who to contact': carer | 2 | 305 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.69, 2.20] |
| 47.1 Reactive and flexible | 2 | 305 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.69, 2.20] |
Analysis 1.1.
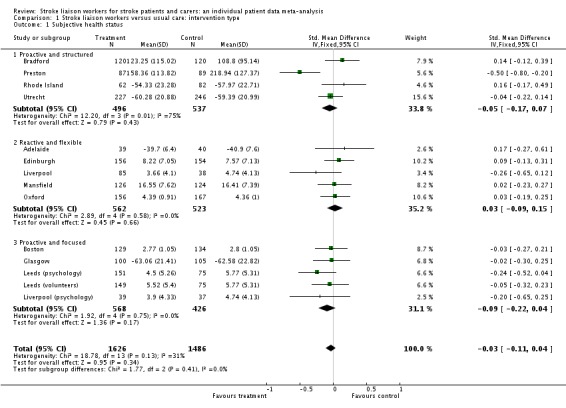
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 1 Subjective health status.
Analysis 1.2.
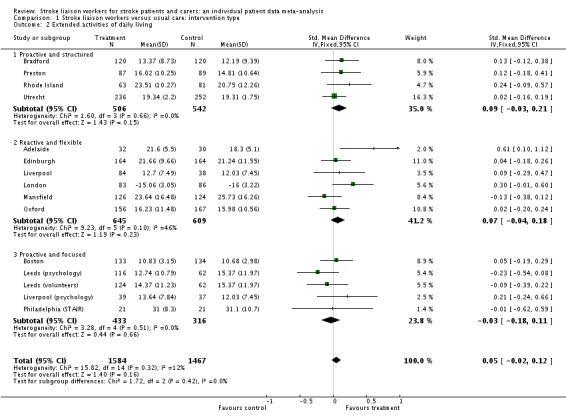
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 2 Extended activities of daily living.
Analysis 1.3.
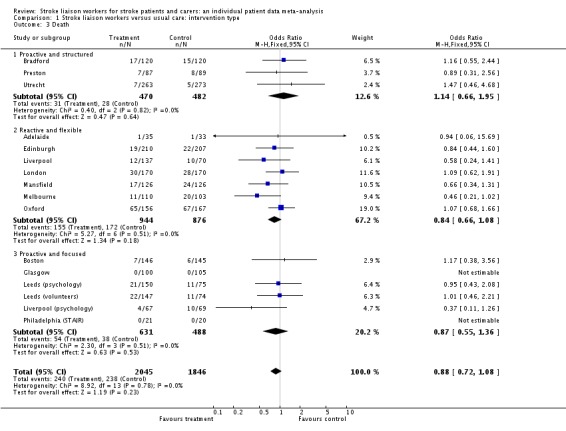
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 3 Death.
Analysis 1.4.
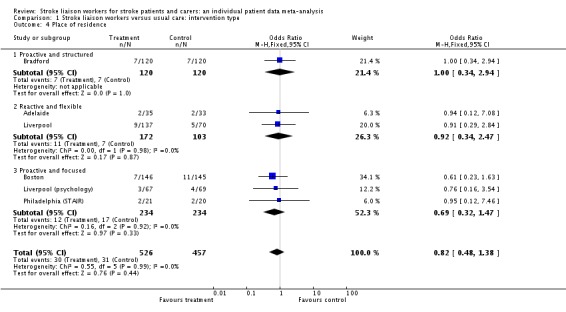
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 4 Place of residence.
Analysis 1.5.
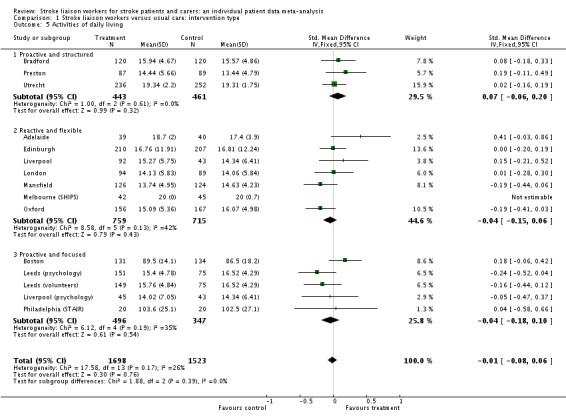
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 5 Activities of daily living.
Analysis 1.6.
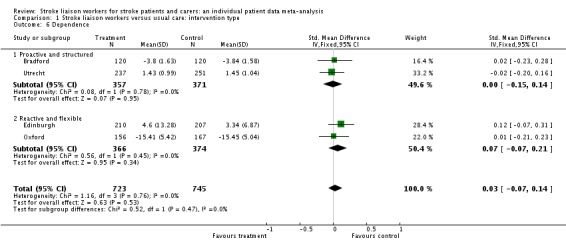
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 6 Dependence.
Analysis 1.7.
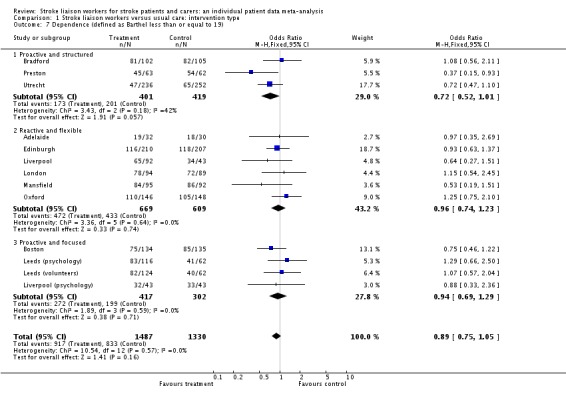
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 7 Dependence (defined as Barthel less than or equal to 19).
Analysis 1.8.
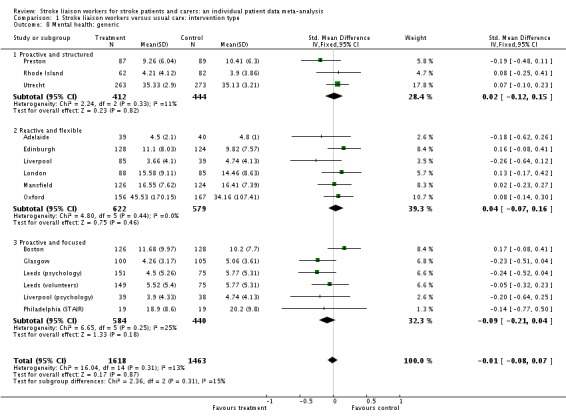
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 8 Mental health: generic.
Analysis 1.9.
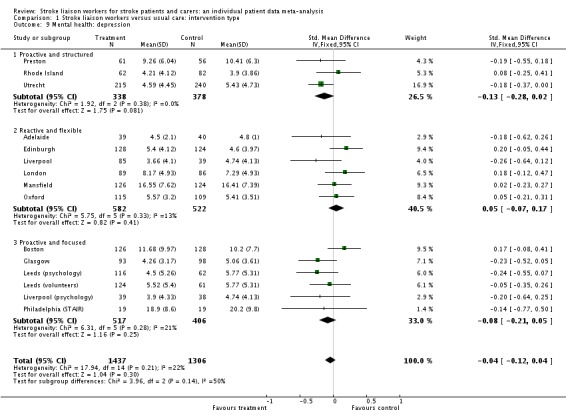
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 9 Mental health: depression.
Analysis 1.10.
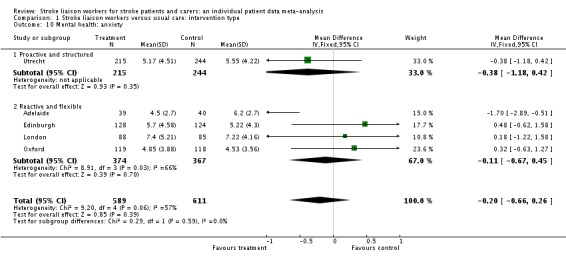
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 10 Mental health: anxiety.
Analysis 1.11.
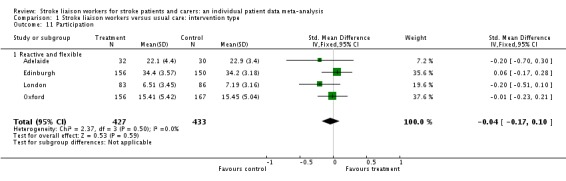
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 11 Participation.
Analysis 1.12.
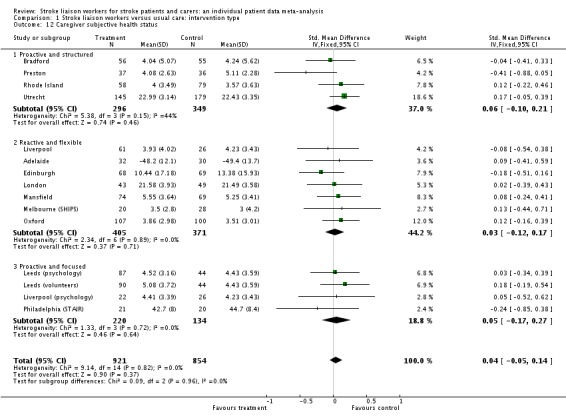
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 12 Caregiver subjective health status.
Analysis 1.13.
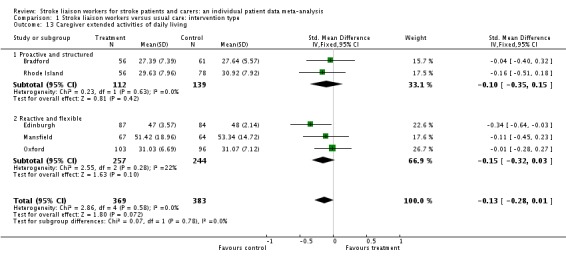
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 13 Caregiver extended activities of daily living.
Analysis 1.14.
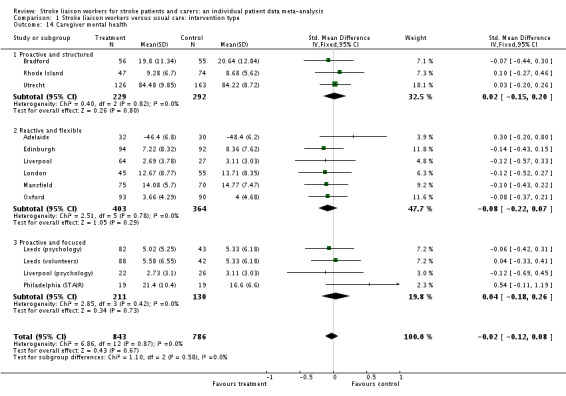
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 14 Caregiver mental health.
Analysis 1.15.
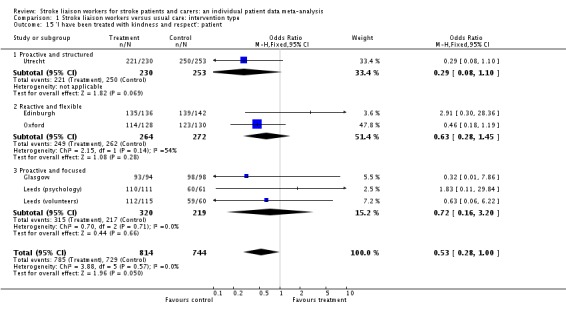
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 15 'I have been treated with kindness and respect': patient.
Analysis 1.16.
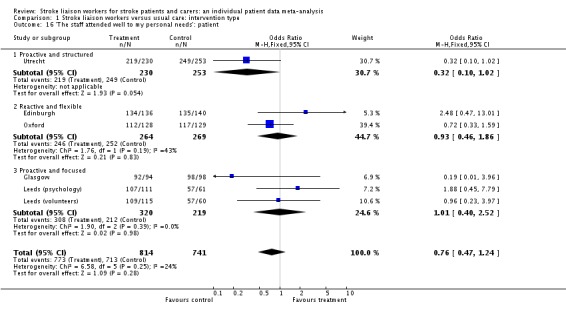
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 16 'The staff attended well to my personal needs': patient.
Analysis 1.17.
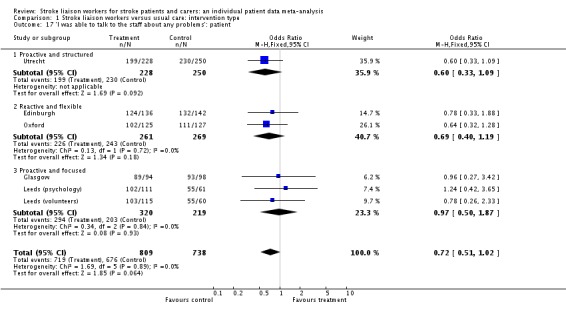
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 17 'I was able to talk to the staff about any problems': patient.
Analysis 1.18.
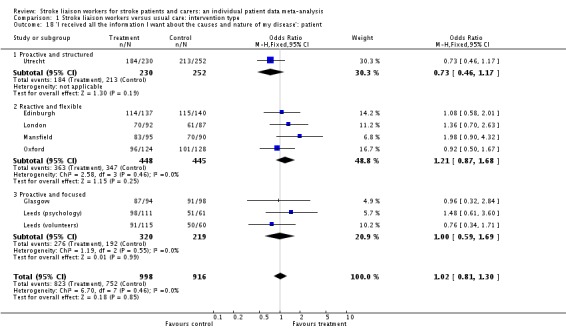
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 18 'I received all the information I want about the causes and nature of my disease': patient.
Analysis 1.19.
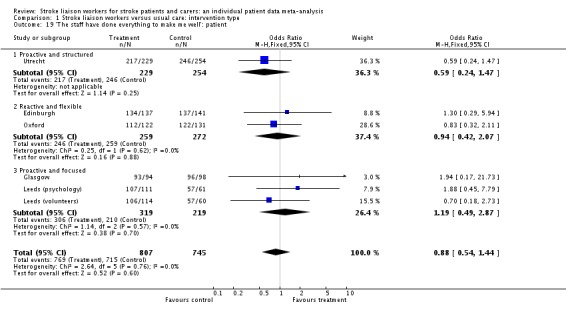
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 19 'The staff have done everything to make me well': patient.
Analysis 1.20.
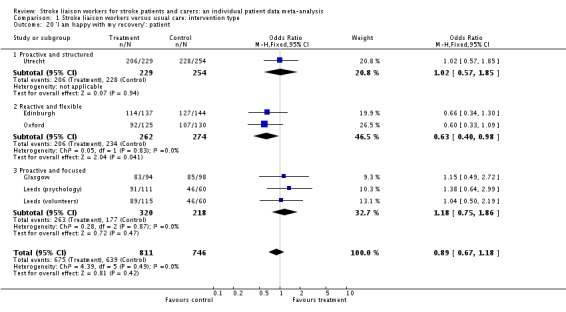
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 20 'I am happy with my recovery': patient.
Analysis 1.21.
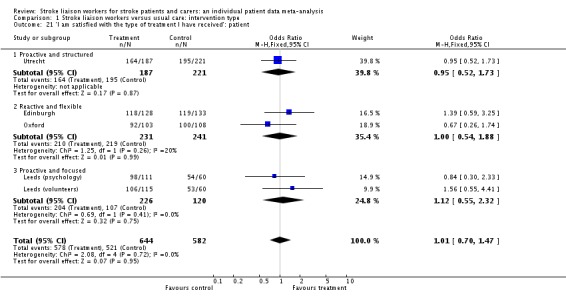
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 21 'I am satisfied with the type of treatment I have received': patient.
Analysis 1.22.
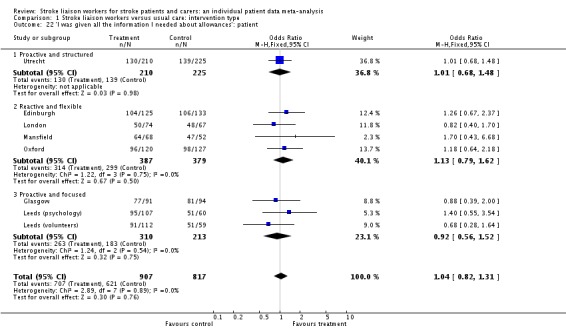
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 22 'I was given all the information I needed about allowances': patient.
Analysis 1.23.
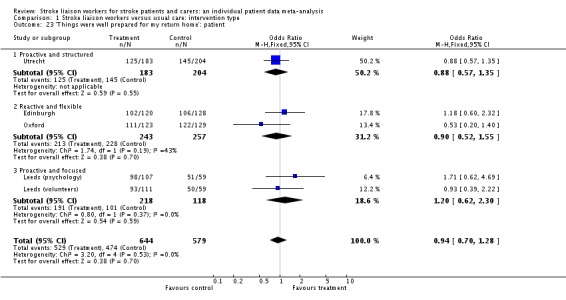
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 23 'Things were well prepared for my return home': patient.
Analysis 1.24.
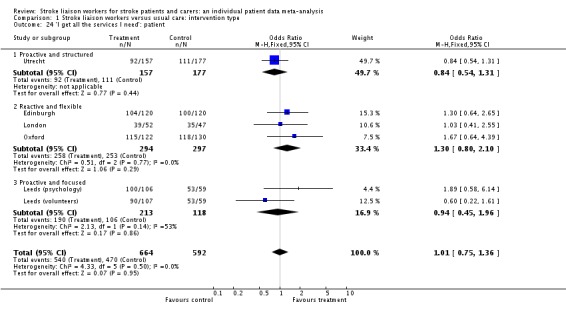
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 24 'I get all the services I need': patient.
Analysis 1.25.
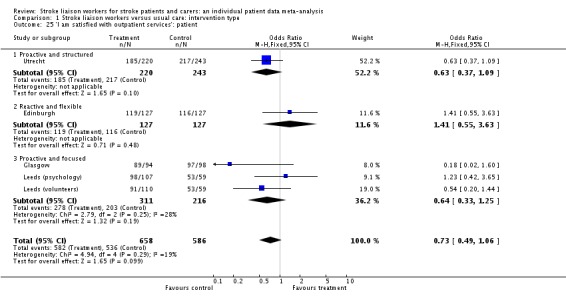
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 25 'I am satisfied with outpatient services': patient.
Analysis 1.26.
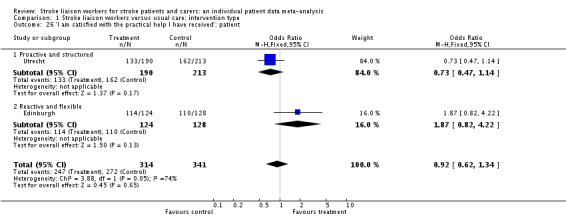
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 26 'I am satisfied with the practical help I have received': patient.
Analysis 1.27.
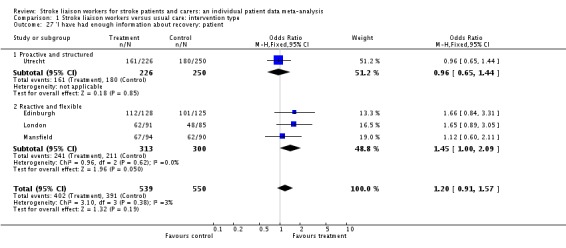
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 27 'I have had enough information about recovery: patient.
Analysis 1.28.
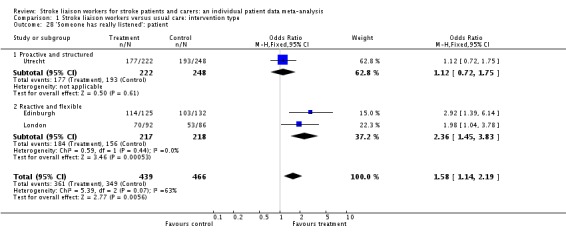
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 28 'Someone has really listened': patient.
Analysis 1.29.
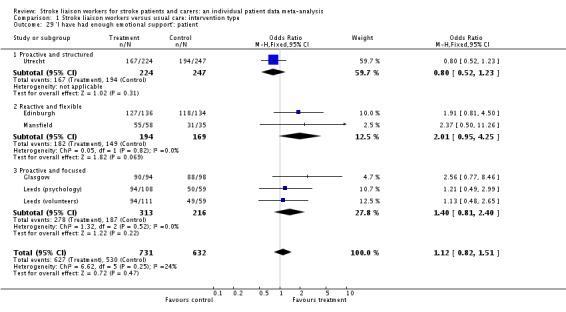
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 29 'I have had enough emotional support': patient.
Analysis 1.30.
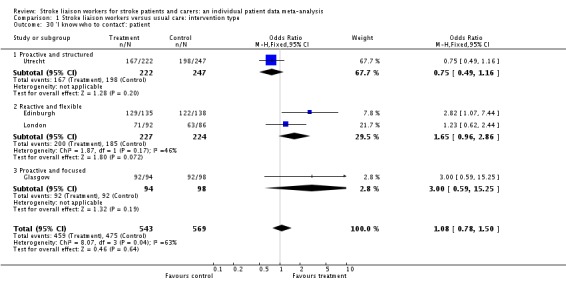
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 30 'I know who to contact': patient.
Analysis 1.31.
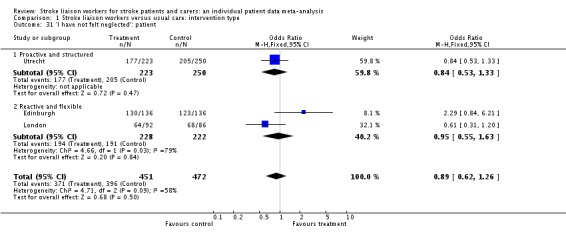
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 31 'I have not felt neglected': patient.
Analysis 1.32.
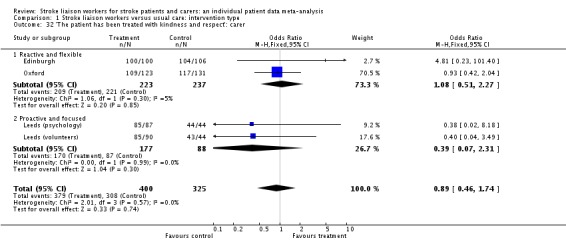
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 32 'The patient has been treated with kindness and respect': carer.
Analysis 1.33.
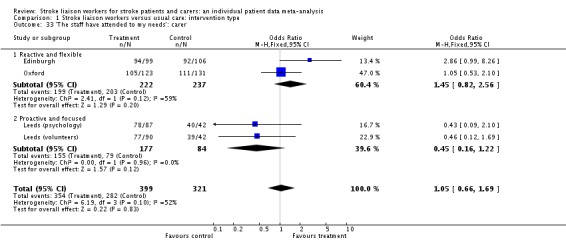
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 33 'The staff have attended to my needs': carer.
Analysis 1.34.
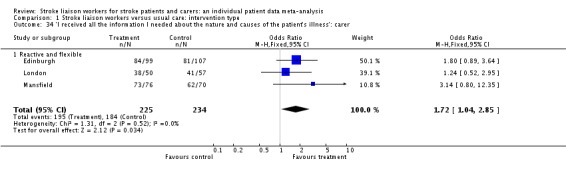
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 34 'I received all the information I needed about the nature and causes of the patient's illness': carer.
Analysis 1.35.
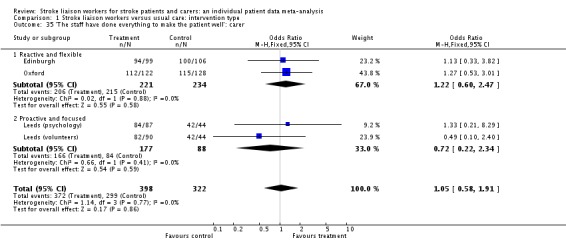
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 35 'The staff have done everything to make the patient well': carer.
Analysis 1.36.
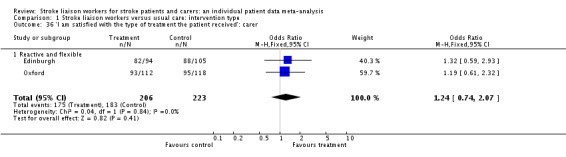
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 36 'I am satisfied with the type of treatment the patient received': carer.
Analysis 1.37.
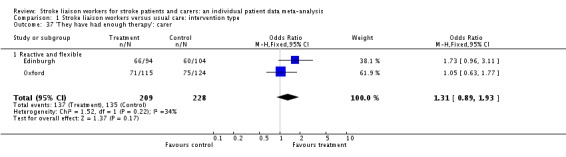
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 37 'They have had enough therapy': carer.
Analysis 1.38.
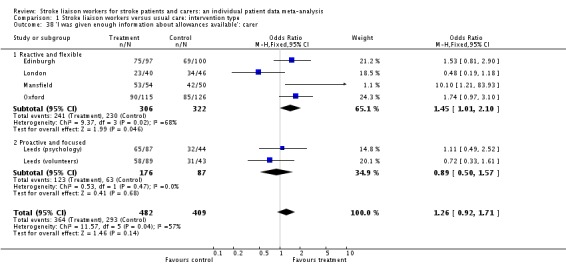
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 38 'I was given enough information about allowances available': carer.
Analysis 1.39.
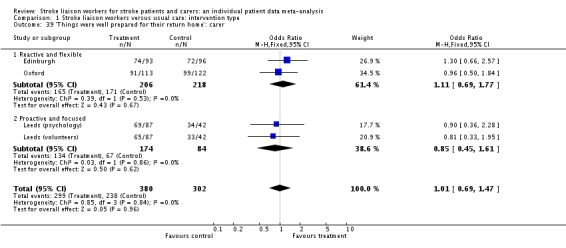
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 39 'Things were well prepared for their return home': carer.
Analysis 1.40.
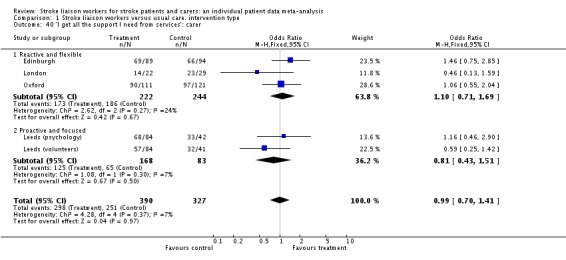
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 40 'I get all the support I need from services': carer.
Analysis 1.41.
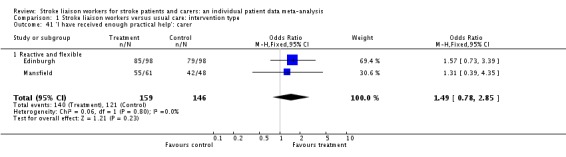
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 41 'I have received enough practical help': carer.
Analysis 1.42.
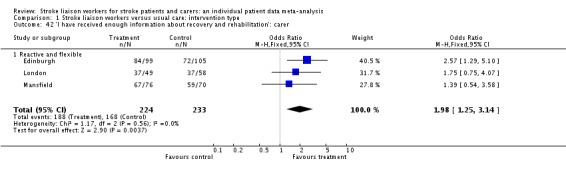
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 42 'I have received enough information about recovery and rehabilitation': carer.
Analysis 1.43.
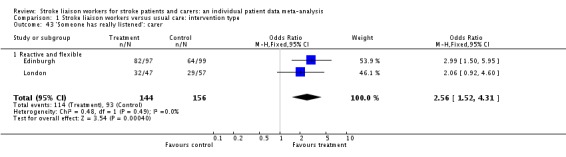
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 43 'Someone has really listened': carer.
Analysis 1.44.
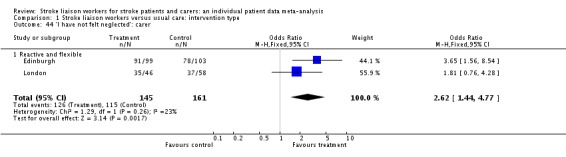
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 44 'I have not felt neglected': carer.
Analysis 1.45.
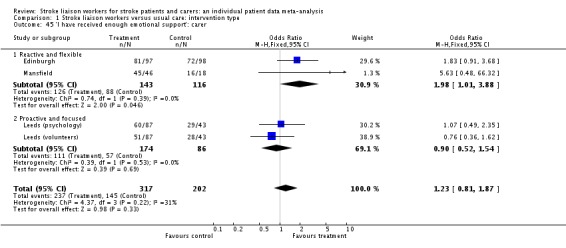
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 45 'I have received enough emotional support': carer.
Analysis 1.46.
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 46 'I have received enough special equipment': carer.
Analysis 1.47.
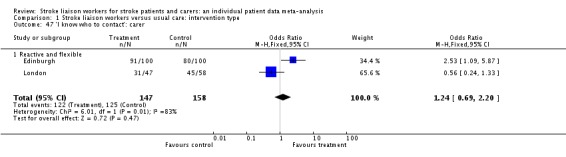
Comparison 1 Stroke liaison workers versus usual care: intervention type, Outcome 47 'I know who to contact': carer.
Comparison 2.
Stroke liaison workers versus usual care: emphasis
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subjective health status | 14 | 3114 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.11, 0.04] |
| 1.1 Education and information provision | 2 | 381 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.44, ‐0.04] |
| 1.2 Social support | 11 | 2507 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.07, 0.09] |
| 1.3 Liaison | 1 | 226 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.52, 0.04] |
Analysis 2.1.
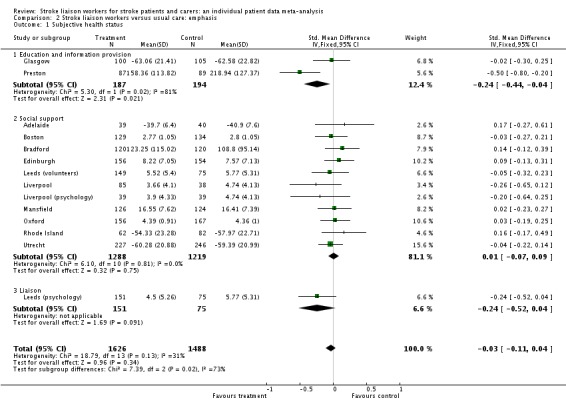
Comparison 2 Stroke liaison workers versus usual care: emphasis, Outcome 1 Subjective health status.
Comparison 3.
Stroke liaison workers versus usual care: patient functional status
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Barthel dependency | 11 | 2280 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.72, 1.08] |
| 1.1 Barthel less than 15 | 11 | 923 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.82, 1.62] |
| 1.2 Barthel 15 to 19 | 11 | 670 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.44, 0.87] |
| 1.3 Barthel 20 | 11 | 687 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.68, 1.42] |
| 2 Death or dependence | 10 | 2011 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.72, 1.11] |
| 2.1 Barthel less than 15 | 10 | 758 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.93, 2.04] |
| 2.2 Barthel 15 to 19 | 10 | 579 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.38, 0.81] |
| 2.3 Barthel 20 | 10 | 674 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.68, 1.43] |
Analysis 3.1.
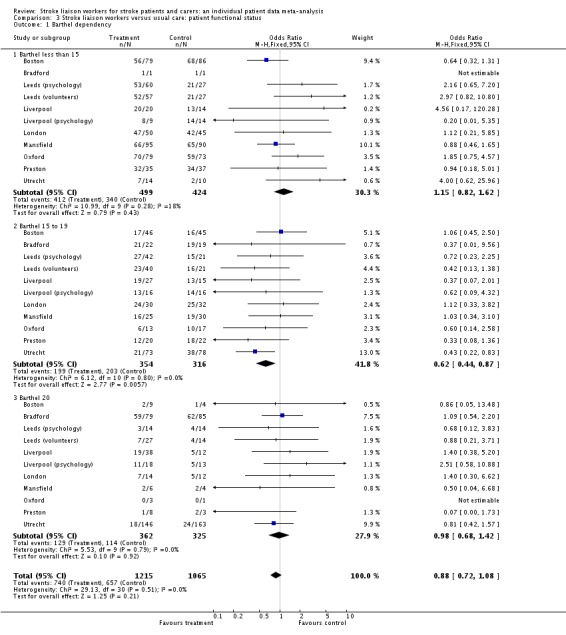
Comparison 3 Stroke liaison workers versus usual care: patient functional status, Outcome 1 Barthel dependency.
Analysis 3.2.
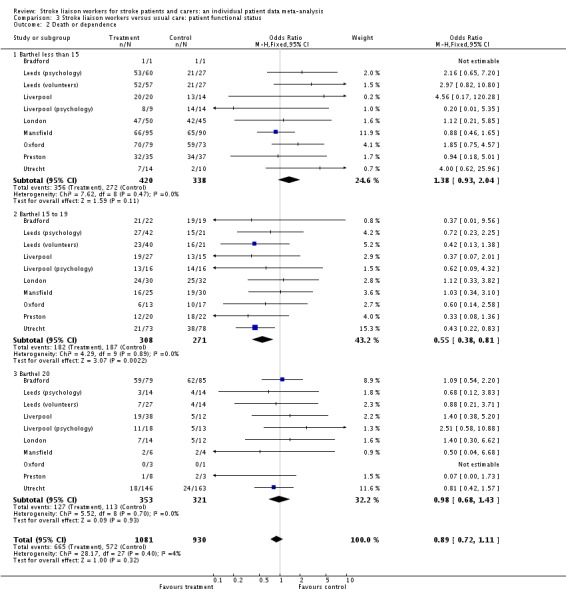
Comparison 3 Stroke liaison workers versus usual care: patient functional status, Outcome 2 Death or dependence.
History
Protocol first published: Issue 1, 2005 Review first published: Issue 5, 2010
| Date | Event | Description |
|---|---|---|
| 3 July 2008 | Amended | Converted to new review format. |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adelaide
| Methods | Randomised controlled trial Centralised randomisation procedure No blinding of outcome assessment | |
| Participants | Stroke patients and carers recruited from a stroke rehabilitation unit in Adelaide within 2 weeks of stroke onset (62 patients and 62 carers) Exclusion criteria: severe communication problems, poor English language, cognitive impairment and ongoing care or rehabilitation needs Inclusion criteria: confirmed stroke, co‐resident with spouse and returning to the community Mean age: 71 years (SD 9) controls, 73 (SD 9) experiment Approximately 60% male | |
| Interventions | Intervention: an information package and 3 visits from a social worker trained in family counselling techniques; visits lasted on average 1 hour; final visits were conducted at 5 months Control: control group patients and their spouses did not receive the information pack or the visits from the social worker | |
| Outcomes | Follow up was at 6 months, conducted by a research nurse independent of the interventions No blinding is described Patient outcomes assessed were: subjective health status (SF 36), extended activities of daily living (Adelaide activities profile), activities of daily living (Barthel), mood (Geriatric Depression Score, Hospital Anxiety and Depression Scale ‐ Anxiety component); McMaster Family Assessment Device Global Function Scale‐Mastery Scale) Carer outcomes were: subjective health status (SF 36; McMaster Family Assessment Device Global Functioning Scale) | |
| Notes | Involvement of a spouse was compulsory for entry into the study Aggregated data were available on request | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Boston
| Methods | Randomised controlled trial Centralised randomisation by computer‐generated random numbers and remote telephone allocation | |
| Participants | Stroke patients recruited from inpatient stroke unit care within 30 days of event (291 participants) Exclusion criteria: age less than 45, resident out with area, terminally ill, severe communication problems, cognitive impairment, poor English language, institutional care, social isolation Inclusion criteria: confirmed stroke of mild or moderate severity, competent to consent | |
| Interventions | Intervention: intervention was provided by a clinical psychologist or a social worker who were formally trained; the emphasis of the intervention was on recruiting families and naturally occurring social networks rather than formal or community‐care based services; 15 intervention visits were made according to protocol (approximately 90 minutes in duration) Control: the control group received usual care (not defined) | |
| Outcomes | Outcome assessment was conducted at 3 and 6 months by a blinded outcome assessor Patient outcome measures: included activities of daily living (Barthel, instrumental activities of daily living), dependency (physical performance test), mood (Centre for Epidemiological Studies Depression Scale CESD), cognition (a cognitive summary score), perceived health status (self‐rated health and quality of life) | |
| Notes | Involvement of a carer was not compulsory | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Bradford
| Methods | Randomised controlled trial Allocation by random number tables and carried out by assistant not connected to study | |
| Participants | Patients were obtained from hospital and primary care within 6 weeks of stroke (240 participants) Exclusion criteria: cognitive impairment, poor prognosis or placement in institutional care Inclusion criteria: acute stroke with some disability; aged over 60 and able to give informed consent Mean age: 73 years (SD 7) Male 53% | |
| Interventions | Intervention: the intervention was delivered by senior nurses who visited 7 times according to a protocol and provided information, advice and support Control: the control group received no visits | |
| Outcomes | The outcomes were assessed at 3, 6 and 12 months No blinding was attempted Patient outcomes: extended activities of daily living (Nottingham extended ADL), activities of daily living (Barthel), dependency (Functional Ambulatory Category), subjective health status (Nottingham Health Profile) Carer outcomes: mental health and subjective health status (GHQ 28); extended activities of daily living (Frenchay Activities Index) | |
| Notes | Involvement of a carer was not compulsory | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Edinburgh
| Methods | Randomised controlled trial Allocation by remote computer‐generated random numbers | |
| Participants | Patients were recruited from both inpatient and outpatient settings within 30 days of stroke onset Patient blinding was achieved through a process of delayed consent 417 participants Mean age: 68 years (SD 13) 50% male | |
| Interventions | Intervention: the intervention was delivered by a social worker, who contacted patients on average 4 times to provide social support, counselling and to identify unmet needs requiring services Control: control patients received usual care which did not include contact with the stroke family care worker until after final follow up had been completed at 6 months | |
| Outcomes | Outcomes were recorded by a research psychologist blinded to treatment allocation at 6 months Patient outcomes: extended activities of daily living (Frenchay Activities Index), activities of daily living (Barthel), dependency (Oxford Handicap Scale), mental/health (GHQ 30, Hospital Anxiety and Depression Scale, Mental Adjustment to Stroke Scale, medical coping modes questionnaire), satisfaction (Pound Satisfaction Scale) Carer outcomes: subjective health status (Caregiver Hassles Scale), extended activities of daily living (Frenchay Activities Index, social adjustment scale), mental health (GHQ 28), satisfaction (Pound Satisfaction Scale) | |
| Notes | Involvement of a carer was not compulsory | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Glasgow
| Methods | Randomised controlled trial Allocation by remote random number generation and sequentially numbered opaque envelopes | |
| Participants | Patients were recruited on discharge from outpatient clinics and rehabilitation facilities Exclusion criteria: major illness, cognitive impairment, severe communication disorder Inclusion criteria: clinical diagnosis of stroke or TIA, presence of at least 1 modifiable risk factor, able to give informed consent 205 participants Mean age: 65 years (SD 9) 51% male | |
| Interventions | Intervention: intervention patients received 3 appointments (30 minutes each) with a nurse to discuss lifestyle, risk factors and recovery from stroke; in addition, patients were given written information specific to them regarding risk factor targets, etc Control: control patients received 1 meeting with the stroke nurse to discuss risk factors prior to recruitment | |
| Outcomes | Outcomes were assessed at 5 months by a research nurse blinded to treatment allocation Outcomes: cumulative risk factor control, individual risk factor control, subjective health status (Euroqol), mood (Geriatric Depression Scale), satisfaction (Pound Satisfaction Scale) | |
| Notes | Carers were not involved in this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Leeds
| Methods | Randomised controlled trial Remote random number sequence generation and telephone allocation | |
| Participants | Patients were identified from admissions to hospital 450 participants Mean age: 71 years (SD 12) 54% male Consent was obtained after randomisation Patients were blinded to other treatment or control arms Exclusion criteria: subarachnoid haemorrhage, too ill, poor communication, poor English language ability, cognitive impairment, serious concurrent illness Inclusion criteria: first or recurrent stroke, local to area and able to give consent | |
| Interventions | The trial tested 2 separate interventions (Leeds (psychology); Leeds (volunteers)) and 1 control group Control: patients in the control group received usual care, although this was not standardised | |
| Outcomes | Outcomes were measured at 6 and 12 months by an outcome assessor blinded to patient allocation | |
| Notes | Involvement of a carer was not compulsory This study remains unpublished at this time | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Leeds (psychology)
| Methods | As above | |
| Participants | As above | |
| Interventions | Intervention: the psychology arm of this trial was delivered by psychiatric nurses who aimed to improve patients' problem solving skills by working with patients at fortnightly visits; the psychiatric nurses were supervised fortnightly by a senior psychiatrist | |
| Outcomes | As above | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Leeds (volunteers)
| Methods | As above | |
| Participants | As above | |
| Interventions | Intervention: volunteers were recruited through local charities and self‐help groups; all attended a training session on the consequences of stroke | |
| Outcomes | As above | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Liverpool
| Methods | Randomised controlled trial Allocation by remote computer‐generated random numbers and telephone randomisation | |
| Participants | 828 participants were recruited at discharge from hospital following admission with stroke to a larger multicentre study (Life after stroke) | |
| Interventions | Three separate interventions were evaluated singly or in combination for this study; they include the evaluation of a stroke family support worker (social intervention), a psychologist (psychological) and an occupational therapist (physical); only the social and psychological arms of this study have been used for analysis where these were conducted alone and in comparison to the control group Social intervention: this involved the input of a stroke family support worker to provide verbal and written information, informal counselling and social support as well as liaison with other services; on average the stroke family support worker made 4 contacts per patient by telephone or visit Control group: the control group received usual care which did not include either the family support worker, psychologist or the occupational therapist | |
| Outcomes | Outcomes were assessed at 12 months | |
| Notes | Involvement of a carer was not compulsory This study remains unpublished at this time | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Liverpool (psychology)
| Methods | As above | |
| Participants | As above | |
| Interventions | Psychology intervention: the intervention was delivered by a psychology assistant working under the supervision of an experienced clinical psychologist; it included assessing a patient's mental and emotional state and delivering cognitive behavioural therapy for the patient as well as problem‐solving for the whole family; on average the psychologists made 10 visits per patient | |
| Outcomes | As above | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
London
| Methods | Randomised controlled trial Allocation by fax to remote centre with computer‐generated random number table | |
| Participants | 340 participants were recruited from admissions to hospital with first‐in‐a‐lifetime stroke Mean age: 78 years (SD 10) 42% male Exclusion criteria: unable to consent due to poor prognosis, cognitive impairment or poor communication and where assent was not available Inclusion criteria: first stroke and resident in area | |
| Interventions | Intervention: a UK Stroke Association family support organiser offering emotional support, information and liaison to their services and voluntary agencies; they made contact, primarily through visits on average 15 times Control: the control group received no input from the family support organiser but could receive usual care including other agency involvement | |
| Outcomes | Outcomes were evaluated at 12 months by a blinded outcome assessor Patient outcomes: extended Activities of Daily Living (Frenchay Activities Index), death, residence, activities of daily living (Barthel), mood (Hospital Anxiety and Depression Scale), participation (Reintegration to Normal Living Index), satisfaction (Pound Satisfaction Scale), Hope and Acceptance scale Carer outcomes: Subjective Health Status (Caregiver Strain Index), extended activities of daily living (Reintegration to Normal Living Index), mental health (Hospital Anxiety and Depression Scale), Hope and Acceptance scale | |
| Notes | Involvement of a carer was not compulsory | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Mansfield
| Methods | Randomised controlled trial Telephone allocation from remote, computer‐generated list of random numbers | |
| Participants | 250 participants admitted to hospital with an acute stroke Mean age: 69 years (SD 11) 54% male Exclusion criteria: unconscious on admission, institutional care, severe disability, resident out with local area Inclusion criteria: confirmed stroke within 4 weeks of onset | |
| Interventions | Intervention: a psychologist with training by the UK Stroke Association as a family support organiser delivered an information pack and identified unmet information needs, concerns and emotional needs; in addition they acted as liaison to the stroke team; the family support organiser visited on average twice with additional telephone liaison; the intervention was provided for up to 9 months Control: control group patients received no contact from the family support organiser | |
| Outcomes | Outcomes were recorded at 4 months and 9 months by an independent assessor who was blinded to treatment allocation Patient outcomes: subjective health status (GHQ 12), extended activities of daily living (Nottingham extended ADL), activities of daily living (Barthel), mental health (GHQ 12), satisfaction (Modified Pound Satisfaction Scale) Carer outcomes: subjective health status (Carer Strain Index), mental health (GHQ 12), extended activities of daily living (Nottingham extended ADL) | |
| Notes | Involvement of a carer was not compulsory | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Melbourne
| Methods | Randomised controlled trial Methods of allocation and randomisation are not defined | |
| Participants | 213 participants were recruited from a previous population incidence study, 2 years after the onset of stroke | |
| Interventions | Intervention: this was provided by a social worker who provided liaison with health and community services and social support; on average 7 visits to a clients home were carried out Control: the control group had no social work input | |
| Outcomes | Outcomes were recorded at 1 year No attempt was made to blind outcome assessment Patient outcomes: death, days in institutional care, undefined measures of activities of daily living and dependency | |
| Notes | We obtained no individual patient data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Melbourne (SHIPS)
| Methods | Randomised controlled trial No information was available on methods of randomisation or allocation concealment | |
| Participants | 96 participants were recruited from hospital | |
| Interventions | Intervention: regular visits and phone calls from a nurse who provided support, education and liaison to patients and carers Control: patients and caregivers in the control group received no home visits until after the end of the 9‐month trial follow up | |
| Outcomes | Patient outcomes: subjective health status (assessment of quality of life scores), activities of daily living (Barthel), dependency (Modified Rankin Score), health service usage | |
| Notes | This study finished early due to funding shortages No individual patient data were available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Oxford
| Methods | Randomised controlled trial Allocation by telephone randomisation to remote individual, with sequentially numbered opaque envelopes | |
| Participants | 323 participants were recruited from hospital presenting within 6 weeks of stroke Mean age: 74 years (SD 13) 52% male Exclusion criteria: institutional care, dominant medical problems or severe illness Inclusion criteria: confirmed stroke, aged 18 or over, local in area and with close family carer | |
| Interventions | Intervention: patients assigned to the intervention group were visited by the family support organiser (trained by the UK Stroke Association) who provided written and verbal information and advice, support and liaison with services; on average patients received 2 visits and 3 telephone contacts Control: the control group did not receive any input from the family support organiser | |
| Outcomes | Outcomes were assessed at 6 months by a researcher blinded to treatment allocation Patient outcomes: subjective health status (Dartmouth CO‐OP chart), death, place of residence, activities of daily living (Barthel, Rivermead Mobility Index), dependency (London Handicap Scale), mental health (Hospital Anxiety and Depression Scale), satisfaction (Local Satisfaction Scale) Carer outcomes: subjective health status (Carer Strain Index, SF 36), extended activities of daily living (Frenchay Activities Index), mental health (GHQ 28), satisfaction | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Philadelphia (STAIR)
| Methods | Randomised controlled trial Randomisation by random number table but allocation concealment unclear | |
| Participants | 55 participants were recruited from in‐patient wards within 3 months of stroke onset Exclusion criteria: severe co‐morbidity, cognitive impairment, communication problems, institutional care Inclusion criteria: aged over 65, recent stroke, returning to community, caregiver identified, able to give informed consent | |
| Interventions | Intervention: patients were assigned a case manager who visited monthly and telephoned weekly; they provided access to information, identified psychosocial stresses and provided liaison to community or hospital resources Control: the control group did not receive visits from the case manager or input from the multidisciplinary team | |
| Outcomes | Outcomes were recorded at 12 months by a researcher blinded to treatment allocation Patient outcomes: extended activities of daily living (Frenchay Activities Index, Social Functioning Examination, Older American Resources and Services Scales‐Social Resources (OARS‐SR)), activities of daily living (Functional Independence Measure, OARS‐ADL, OARS‐Physical health, Social Functioning Examination, Frenchay Activities Index); OARS‐Economic resources Carer outcomes: subjective health status (Questionnaire on Resources and Stress (QRS)), mental health (Centre for Epidemiological Studies‐Depression Scale (CESD)) | |
| Notes | Carer involvement was necessary for the study Some aggregated data were available in addition to the paper, but not individual patient data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Preston
| Methods | Randomised controlled trial Allocation by telephone to remote centre with concealed random number sequence | |
| Participants | 176 participants with a clinical diagnosis of stroke were recruited from admission to hospital Mean age: 75 years (SD 10) 52% male Exclusion criteria: depression prior to stroke, cognitive impairment, poor prognosis, substance addiction Inclusion criteria: clinical diagnosis of stroke | |
| Interventions | Intervention: patients were visited by a stroke nurse who visited on average 3 times over 2 months and provided information and advice, emotional support and liaison with services Control: the control group received inpatient case management and multidisciplinary rehabilitation, but no home visits on discharge | |
| Outcomes | Outcomes were recorded at 3 and 12 months by a researcher blinded to treatment allocation Patient outcomes: subjective health status (Nottingham Health Profile), extended activities of daily living (Frenchay Activities Index), activities of daily living (Barthel), mental health (Beck Depression Inventory) Carer outcomes: subjective health status (Carer Strain Index). | |
| Notes | Involvement of a carer was not compulsory | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Rhode Island
| Methods | Randomised controlled trial There was no information on the method of randomisation or allocation concealment | |
| Participants | 215 patients and carers were recruited in hospital following stroke Mean age: 65 years (SD 13) 55% male Exclusion criteria: subarachnoid haemorrhage, institutional care, no caregiver Inclusion criteria: age over 35 with confirmed stroke, competent to consent and caregiver present | |
| Interventions | Intervention: patients received on average 13 telephone calls (lasting 15 to 20 minutes) from the stroke liaison worker who provided education, social and emotional support and counselling Control group: control group patients were allocated to usual care | |
| Outcomes | Outcomes were recorded at 3, 6, 12 and 18 months by staff blinded to treatment assignment Patient outcomes: subjective health status (SF 36), extended activities of daily living (Frenchay Activities Index, Functional Independence Measure), mental health (Geriatric Depression Scale), family function (Family Assessment Device) Carer outcomes: subjective health status (Caregiver Strain Index, SF 36), mental health (CES‐D) | |
| Notes | Involvement of a carer was compulsory This trial is unpublished at present | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Utrecht
| Methods | Randomised controlled trial Randomisation was performed by telephone to a remote centre Patient blinding was achieved by a process of delayed consent | |
| Participants | 536 participants were recruited from 12 hospitals prior to discharge following a first‐in‐a‐lifetime stroke Mean age: 63 years (SD 15) 49% male Exclusion criteria: recurrent stroke, age under 18 years, poor prognosis, severe dependency, lives out of area, institutional care Inclusion criteria: age 18 years or over with first ever stroke, resident in area with no or only mild dependency, discharged to community and expected to live more than 1 year | |
| Interventions | Intervention: senior nurses made 3 telephone contacts and visited the patient in their homes; they provided information, support and liaison to primary care Control group: the control group received the same in‐patient care but were not contacted on discharge | |
| Outcomes | Outcomes were recorded at 6 months by postal questionnaire and telephone interview by an assessor blinded to treatment allocation Patient outcomes: subjective health status (SF 36), activities of daily living (Barthel), dependency (Modified Rankin Scale), mental health (Hospital Anxiety and Depression Scale), use of services, satisfaction (Satisfaction‐with‐stroke‐care questionnaire SASC 19) Carer outcomes: subjective health status (Carer Strain Index), extended activities of daily living (Social Support List ‐ Discrepancies (SSL‐D)), mental health (Sense of Competence Questionnaire (SCQ)) | |
| Notes | Involvement of a carer was not compulsory | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
ADL: activities of daily living SD: standard deviation TIA: transient ischaemic attack
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allen 2002 | Trial of nurse assessment only and multidisciplinary treatment |
| Banet 1997 | Shared medical records |
| Blake 2001 | Not RCT |
| Boquan 2004 | Trial of psychological therapy for anxiety after stroke |
| Carter 1998 | Trial of an information pack post stroke |
| Chang 2000 | Trial of social support only |
| Clairborne 2006 | Trial of co‐ordinated multidisciplinary chronic disease management post discharge |
| Cohen 1986 | Trial of counselling only post‐stroke |
| Corr 2004 | Trial of a day centre post‐stroke |
| Dow 2001 | Trial of rehabilitation |
| Downes 1993 | Trial of counselling |
| Draper 2007 | Trial of a psycho‐education programme for caregivers of aphasic patients only |
| Evans 1988 | Group counselling and education for carers only |
| Feng 2003 | Trial of nursing delivered rehabilitation at home |
| Folden 1993 | Not RCT |
| Frank 2000 | Workbook‐based intervention |
| Friedland 1992 | Trial of social support only |
| Geddes 1994 | Not RCT |
| Grant 1999 | Trial of social problem solving therapy for caregivers only |
| Grasel 2005 | CCT of training seminars and discharge support |
| Gu 1998 | Carer support and training in rehabilitation |
| Hartke 2003 | Intervention for carers only |
| Hoffman 2007 | RCT of information provision only |
| Johnson 2000 | Not RCT |
| Johnstone 2007 | Trial of education only |
| Kalra 2004 | Trial of training for caregivers only |
| Katayama 1986 | Study of multidisciplinary team therapy |
| Kendall 2007 | Trial of group education programme |
| Kent 2003 | Patient‐held communication and information booklet |
| King 2007 | Non‐RCT of psychological support for carers only |
| Larson 2005 | RCT of social support and education for carers only |
| Li 2003 | Impact of information provision on rehabilitation |
| Logan 1997 | Enhanced social service occupational therapy after stroke |
| Lorenc 1992 | Education and information provision only |
| Louie 2006 | Trial of group education |
| Lowe 2007 | Trial of information provision |
| Mackenzie 2001 | Trial of protocol to improve in‐patient rehabilitation outcomes |
| Matsumoto 1985 | Education intervention only |
| Mitchell 2008 | RCT of psychosocial intervention for depressed post‐stroke patients only |
| Morrison 1998 | Not RCT |
| Nir 2006 | Information provision during inpatient rehabilitation |
| Ostwald 2002 | Nurse‐led rehabilitation teams |
| Overs 1971 | Evaluation of social work only |
| Pain 1990 | Information provision only, not RCT |
| Pitkanen 1999 | Counselling and multidisciplinary team intervention |
| Pritchard 2004 | Trial in subarachnoid patients postoperatively |
| Rawl 1998 | Rehabilitation intervention |
| Rodgers 1999 | Trial of stroke education package for groups of patients and carers |
| Smith 2003 | Trial designed to improve communication and information provision |
| Stewart 1998 | Trial of peer support for caregivers |
| Stop Stroke 2008 | Secondary prevention programme |
| Towle 1989 | Trial of social work intervention for depressed patients only |
| Tsutsumishita 1985 | Before and after study design evaluating information provision |
| van de Heuvel 2002 | Group support therapy and home visits for caregivers only |
| Watkins 2007 | Trial of motivational interviewing to achieve goal setting and problem solving No education or liaison |
| Williams 2007 | Education, counselling and treatment of post‐stroke depression |
| Zhengyi 2003 | Trial of nursing‐based physical rehabilitation at home post discharge |
CCT: case‐controlled trial RCT: randomised controlled trial
Contributions of authors
The protocol was written by JM, GE and PL. The lead review author (GE) was responsible for conducting the literature search, assessing trials for inclusion, analysing and checking data, conducting meta‐analysis and writing the review paper. PL was responsible for assessing trials for inclusion. All trialists in the collaborative group were responsible for submitting data.
Sources of support
Internal sources
No sources of support supplied
External sources
Chest Heart and Stroke Scotland, UK.
Declarations of interest
Graham Ellis: has been involved in trials evaluated in this review.
Jonathan Mant: received research funding from The Stroke Association, UK and was an investigator in one of the trials included in this review.
Peter Langhorne: none known.
Martin Dennis: was the chief investigator of one of the included randomised controlled trials. He is also a member of the Council, Executive and Research Committee of Chest Heart and Stroke Scotland, a charity which employs stroke liaison workers.
Simon Winner: has been involved in the design, conduct and publication of an eligible study for this review, resulting in the following publications:
Mant J, Carter J, Wade D, Winner S. Family support for stroke: a randomised controlled trial. Lancet 2000;356:808‐13.
Mant J, Winner S, Roche J, Wade DT. Family support for stroke: one year follow up of a randomised controlled trial. Journal of Neurology, Neurosurgery & Psychiatry 2005;76:1006‐8.
He has no other competing interests to declare.
New
References
References to studies included in this review
- Clark MS, Rubenach S, Winsor A. A randomized controlled trial of an education and counselling intervention for families after stroke. Clinical Rehabilitation 2003;17:703‐12. [DOI] [PubMed] [Google Scholar]
- Glass TA, Berkman LF. Psychosocial intervention in stroke: a progress report from the FIRST study. Gerontologist 1998;38:138. [Google Scholar]; Glass TA, Berkman LF, Hiltunen EF, Furie K, Glymour MM, Fay ME, et al. The families in recovery from stroke trial (FIRST): primary study results. Psychosomatic Medicine 2004;66:889‐97. [DOI] [PubMed] [Google Scholar]
- Forster A, Young J. Specialist nurse support for patients with stroke in the community: a randomised controlled trial. BMJ 1996;312:1642‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, O'Rourke S, Slattery J, Staniforth T, Warlow C. Evaluation of a stroke family care worker: results of a randomised controlled trial. BMJ 1997;314:1071‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]; O'Rourke S, MacHale S, Signorini D, Dennis M. Detecting psychiatric morbidity after stroke: comparison of the GHQ and HAD scale. Stroke 1998;29:980‐5. [DOI] [PubMed] [Google Scholar]
- Ellis G, Rodger J, McAlpine C, Langhorne P. The impact of stroke nurse specialist input on risk factor modification: a randomised controlled trial. Age and Ageing 2005;34(4):389‐92. [DOI] [PubMed] [Google Scholar]; McManus JA, Craig A, McAlpine C, Langhorne P, Ellis G. Does behaviour modification affect post‐stroke risk factor control? Three year follow‐up of a randomized controlled trial. Clinical Rehabilitation 2009;23:99‐105. [DOI] [PubMed] [Google Scholar]
- House A. Problem‐solving therapy improves psychological outcome after stroke: a randomised controlled trial. Unpublished2007.
- House A. Problem‐solving therapy improves psychological outcome after stroke: a randomised controlled trial. Unpublished2007.
- House A. Problem‐solving therapy improves psychological outcome after stroke: a randomised controlled trial. Unpublished2007.
- Leathley M, Fall S, Sharma C, Watkins C, Barer D. Life after stroke: multicentre trial of psychosocial interventions after stroke. Cerebrovascular Diseases 2003;16 Suppl 4:70. [Google Scholar]
- Leathley M, Fall S, Sharma C, Watkins C, Barer D. Life after stroke: multicentre trial of psychosocial interventions after stroke. Cerebrovascular Diseases 2003;16 Suppl 4:70. [Google Scholar]
- Tilling K, Coshall C, McKevitt C, Daneski K, Wolfe C. A family support organiser for stroke patients and their carers: a randomised controlled trial. Cerebrovascular Diseases 2005;20:85‐91. [DOI] [PubMed] [Google Scholar]
- Lilley SA, Lincoln NB, Francis VM. A qualitative study of stroke patients' and carers' perceptions of the stroke family support organiser service. Clinical Rehabilitation 2003;17:540‐7. [DOI] [PubMed] [Google Scholar]; Lincoln NB, Francis VM, Lilley SA, Sharma JC. Evaluation of a stroke family support organiser: a randomised controlled trial. Stroke 2003;34:116‐21. [DOI] [PubMed] [Google Scholar]
- Christie D, Weigall D. Social work effectiveness in two year stroke survivors: a randomised controlled trial. Community Health Studies 1984;8:26‐32. [DOI] [PubMed] [Google Scholar]
- Frayne J. Study of Home Intervention Post Stroke (SHIPS). Unpublished2002.
- Mant J, Carter J, Wade DT, Winner S. Family support for stroke: a randomised controlled trial. Lancet 2000;356:808‐13. [DOI] [PubMed] [Google Scholar]
- Goldberg G, Segal ME, Berk SN, Schall RR, Gershkoff AM. Stroke transition after inpatient rehabilitation. Topics in Stroke Rehabilitation 1997;4(1):64‐79. [DOI] [PubMed] [Google Scholar]
- Burton C, Gibbon B. Expanding the role of the stroke nurse: a pragmatic clinical trial. Journal of Advanced Nursing 2005;52(6):640‐50. [DOI] [PubMed] [Google Scholar]
- Miller IW, Bishop DS, Epstein‐Lubow G. Treatment of stroke patients and caregivers: the FITT study. Unpublished2007.
- Boter H, the HESTIA Study Group. Multicenter randomized controlled trial of an outreach nursing support program for recently discharged stroke patients. Stroke 2004;35:2867‐72. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Allen KR, Hazelett S, Jarjoura D, Wickstrom GC, Hua K, Weinhardt J, et al. Effectiveness of a postdischarge care management model for stroke and transient ischaemic attack: a randomised controlled trial. Journal of Stroke and Cerebrovascular Diseases 2002;11:88‐98. [DOI] [PubMed] [Google Scholar]
- Banet GA, Felclia MA. The potential utility of a shared medical record in a 'first time' stroke population. Journal of Vascular Nursing 1997;15:29‐33. [DOI] [PubMed] [Google Scholar]
- Blake K. Evaluation of stroke family support services. National Research Register2001.
- Boquan Z, Xiqing B, Zhaofu C. Effect of supported psychological intervention on anxiety after stroke: a controlled prospective study. Chinese Mental Health Journal 2001;15(6):415‐8. [Google Scholar]
- Carter J, Wade D, Mant J, Winner S. The impact of an information pack on patients with stroke and their carers: a randomised controlled trial. Clinical Rehabilitation 1998;12(2):161‐2. [DOI] [PubMed] [Google Scholar]
- Chang T, Li L. The effect of social support intervention on home‐bound stroke patients' physical and mental health in the Ilan area. Journal of Nursing Research 2000;8(4):423‐34. [Google Scholar]
- Clairborne N. Effectiveness of a care coordination model for stroke survivors: a randomized study. Health and Social Work 2006;31(2):87‐96. [DOI] [PubMed] [Google Scholar]
- Cohen DD, Harbin C, Collis L, Greenberger JS. Counselling the stroke patient and family. Journal of the Medical Association of Georgia 1986;75(2):93‐7. [PubMed] [Google Scholar]
- Corr S, Phillips CJ, Walker M. Evaluation of a pilot service designed to provide support following stroke: a randomised cross‐over design study. Clinical Rehabilitation 2004;18(1):69‐75. [DOI] [PubMed] [Google Scholar]
- Dow L. The development and evaluation of a community‐based reintegration service for people who have experienced stroke and their main carers. National Research Register2001.
- Downes B, Rooney V, Roper‐Hall A, Oyebode J, Main A, Mayer P. The effectiveness of counselling stroke survivors and their carers in the community. Age and Ageing 1993;22:28. [Google Scholar]
- Draper B, Bowring G, Thompson C, Heyst J, Conroy P, Thompson J. Stress in caregivers of aphasic stroke patients: a randomized controlled trial. Clinical Rehabilitation 2007;21(2):122‐30. [DOI] [PubMed] [Google Scholar]
- Evans RL, Matlock AL, Bishop DS, Stranahan S, Pederson C. Family intervention after stroke: does counselling or education help?. Stroke 1988;19(10):1243‐9. [DOI] [PubMed] [Google Scholar]
- Feng Z, Zhang H, Hu Y, Wang B, Qian X, Lu H. The affection of home‐based rehabilitation nursing intervention on motor function of daily living in patients with stroke. Zhonghua Lichuang Kangfu Zazhi 2003;7(5):730‐1. [Google Scholar]
- Folden SL. Effect of supportive‐educative nursing interventions on older adults' perceptions of self‐care after stroke. Rehabilitation Nursing 1993;1:162‐7. [DOI] [PubMed] [Google Scholar]
- Frank G, Johnston M, Morrison V, Pollard B, MacWalter R. Perceived control and recovery from functional limitations: preliminary evaluation of a workbook‐based intervention for discharged stroke patients. British Journal of Health Psychology 2000;5:413‐20. [Google Scholar]
- Friedland JF, McColl M. Social support intervention after stroke: results of a randomised trial. Archives of Physical Medicine and Rehabilitation 1992;73(6):573‐81. [PubMed] [Google Scholar]
- Geddes J. The role of liaison health visitors in rehabilitation of stroke patients after discharge. Health Visitor 1994;1:347‐51. [Google Scholar]
- Grant JS. Social problem‐solving partnerships with family caregivers. Rehabilitation Nursing 1999;24(6):254‐60. [DOI] [PubMed] [Google Scholar]
- Grasel E, Biehler J, Schmidt R, Schupp W. Intensification of the transition between inpatient neurological rehabilitation and home care of stroke patients. Controlled clinical trial with follow‐up assessment six months after discharge. Clinical Rehabilitation 2005;19:725‐36. [DOI] [PubMed] [Google Scholar]
- Gu Y, Cao L. The influence of family intervention on the quality of life in elderlys with cerebral apoplexy. Geriatrics and Health Care 1998;4(3):29‐31. [Google Scholar]
- Hartke RJ, King RB. Telephone group intervention for older stroke caregivers. Topics in Stroke Rehabilitation 2003;9(4):65‐81. [DOI] [PubMed] [Google Scholar]
- Hoffman T, McKenna K, Worrall L, Read SJ. Randomised trial of a computer‐generated tailored written education package for patients following stroke. Age and Ageing 2007;36(3):280‐6. [DOI] [PubMed] [Google Scholar]
- Johnson J, Pearson V. The effects of a structured education course on stroke survivors living in the community. Rehabilitation Nursing 2000;25(2):59‐65. [Google Scholar]
- Johnstone M, Bonetti D, Joice S, Pollard B, Morrison V, Francis JJ, et al. Recovery from disability after stroke as a target for a behavioural intervention: results of a randomized controlled trial. Disability Rehabilitation 2007;29(14):1117‐27. [DOI] [PubMed] [Google Scholar]
- Kalra L, Evans A, Perez I, Melbourn A, Patel A, Knapp M, et al. Training caregivers of stroke patients: randomised controlled trial. BMJ 2004;328:1099‐01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama E. Assistance toward independence of the patient and preparation by the family to accept him. Kangogaku Zasshi ‐ Japanese Journal of Nursing 1986;50(5):518‐22. [PubMed] [Google Scholar]
- Kendall E, Catalano T, Kuipers P, Posner N, Buys N, Charker J. Recovery following stroke: the role of self‐management education. Social Science and Medicine 2007;64:735‐46. [DOI] [PubMed] [Google Scholar]
- Kent R. Patient held notes in stroke disease. www.controlled‐trials.com2003.
- King RB, Hartke RJ, Denby F. Problem‐solving early intervention: a pilot study of stroke caregivers. Rehabilitation Nursing 2007;32(2):68‐76. [DOI] [PubMed] [Google Scholar]
- Larson J, Franzen‐Dahlin A, Biling E, Arbin M, Murray V, Wredling R. The impact of a nurse‐led support and education programme for spouses of stroke patients: a randomized controlled trial. Journal of Clinical Nursing 2005;14:995‐1003. [DOI] [PubMed] [Google Scholar]
- Li Y, Huang Y, Liu Y, Huang J. Influence of health education on early rehabilitation in patients with stroke. Proceedings of the 4th International Conference on Research Advances in Cerebrovascular Disease. 2003:182‐4. [Google Scholar]
- Logan PA, Ahern J, Gladman JRF, Lincoln NB. A randomized controlled trial of enhanced social service occupational therapy for stroke patients. Clinical Rehabilitation 1997;11:107‐13. [DOI] [PubMed] [Google Scholar]
- Lorenc L, Sturmey P, Brittain H. Evaluation of a meta‐cognitive strategy to improve the information gained from a stroke information pack. Stress Medicine 1992;8:111‐2. [Google Scholar]
- Louie SWS, Liu PKK, Man WK. The effectiveness of a stroke education group on persons with stroke and their caregivers. International Journal of Rehabilitation Research 2006;29:123‐9. [DOI] [PubMed] [Google Scholar]
- Lowe DB, Sharma AK, Leathley MJ. The CareFile project: a feasibility study to examine the effects of an individualised information booklet on patients after stroke. Age and Ageing 2007;36(1):83‐9. [DOI] [PubMed] [Google Scholar]
- Mackenzie AE, Chang AM. The effectiveness of nursing care: use of a protocol to promote stroke rehabilitation. Research Report. Health Services Research Committee, Hong Kong.2001.
- Matsumoto I, Tabata S, Ono T, Inoue H, Kawahira K. Effects of education of families caring for apoplexy patients following hospital discharge: effects on prevention of recurrence and maintenance of ADL. Kangogaku Zasshi ‐ Japanese Journal of Nursing 1985;49(9):1022‐5. [PubMed] [Google Scholar]
- Mitchell PH, Teri L, Veith R, Buzaitis A, Tirschwell D, Becker K, et al. Living well with stroke: design and methods for a randomized controlled trial of a psychosocial behavioural intervention for poststroke depression. Journal of Stroke and Cerebrovascular Diseases 2008;17(3):109‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison VL, Johnston M, MacWalter RS, Pollard BS. Improving emotional outcomes following acute stroke: a preliminary evaluation of a work‐book based intervention. Scottish Medical Journal 1998;43(2):52‐3. [DOI] [PubMed] [Google Scholar]
- Nir Z, Weisel‐Eichler A. Improving knowledge and skills for use of medication by patients after stroke: evaluation of a nursing intervention. American Journal of Physical Medicine and Rehabilitation 2006;85:582‐92. [DOI] [PubMed] [Google Scholar]
- Ostwald SK. Interventions for stroke survivors and spousal caregivers. CRISP Database2002.
- Overs RP, Healy JR. Educating stroke patient families. Media for Rehabilitation Research Reports 1971;1:12. [Google Scholar]
- Pain HS, McLellan DL. The use of individualised booklets after a stroke. Clinical Rehabilitation 1990;4(4):265‐72. [Google Scholar]
- Pitkanen K, Sulkava R, Hamalainen R, Sivenius J. Social and physical activation of chronic stroke patients. Cerebrovascular Diseases 1999;9(Suppl 1):29 (Abst 22). [Google Scholar]
- Pritchard C, Foulkes L, Lang DA, Neil‐Dwyer G. Cost‐benefit analysis of an integrated approach to reduce psychosocial trauma following neurosurgery compared with standard care: two‐year prospective comparative study of enhanced specialist liaison nurse service for aneurysmal subarachnoid hemorrhage (ASAH) patients and carers. Surgical Neurology 2004;62(1):17‐27. [DOI] [PubMed] [Google Scholar]; Pritchard C, Foulkes L, Lang DA, Neil‐Dwyer G. Two‐year prospective study of psychosocial outcomes and a cost analysis of "treatment‐as‐usual" versus an "enhanced" (specialist liaison nurse) service for aneurysmal subarachnoid haemorrhage (ASAH) patients and families. British Journal of Neurosurgery 2004;18(4):347‐56. [DOI] [PubMed] [Google Scholar]
- Rawl SM, Easton KL, Kwaitkowski S, Zemen D, Burczyk B. Effectiveness of a nurse‐managed follow‐up program for rehabilitation patients after discharge. Rehabilitation Nursing 1998;23(4):204‐9. [DOI] [PubMed] [Google Scholar]
- Rodgers H, Atkinson C, Bond S, Suddes M, Dobson R, Curless R. Randomised controlled trial of a comprehensive stroke education program for patients and caregivers. Stroke 1999;30(12):2585‐91. [DOI] [PubMed] [Google Scholar]
- Smith J, Forster A, Young J. A randomized trial to evaluate an education programme for patients and carers after stroke. Clinical Rehabilitation 2004;18(7):726‐36. [DOI] [PubMed] [Google Scholar]; Smith JE, Forster A, Young JB. A randomised trial to evaluate improved routine communication to patients and carers after stroke. Cerebrovascular Diseases 2003;16(4):70. [Google Scholar]
- Stewart MJ, Doble S, Hart G, Langille L, MacPherson K. Peer visit support for family caregivers of seniors with stroke. Canadian Journal of Nursing Research 1998;1:87‐117. [PubMed] [Google Scholar]
- Redfern J, Rudd AD, Wolfe CD, McKevitt C. Stop Stroke: development of an innovative intervention to improve risk factor management after stroke. Patient Education and Counselling 2008;72(2):201‐9. [DOI] [PubMed] [Google Scholar]
- Towle D, Lincoln NB, Mayfield LM. Evaluation of social work on depression after stroke. Clinical Rehabilitation 1989;3:89‐96. [Google Scholar]
- Tsutsumishita H, Ikegami M. Nursing education of family members of stroke patients: a survey on their nursing knowledge before and after the training. Kangogaku Zasshi ‐ Japanese Journal of Nursing 1985;49(11):1268‐72. [PubMed] [Google Scholar]
- Heuvel ET, Witte LP, Stewart RE, Schure LM, Sanderman R, Meyboom‐de Jong B. Long‐term effects of a group support program and an individual support program for informal caregivers of stroke patients: which caregivers benefit the most?. Patient Education and Counselling 2002;47(4):291‐9. [DOI] [PubMed] [Google Scholar]
- Watkins CL, Auton MF, Deans CF, Dickinson HA, Jack CIA, Lightbody CE, et al. Motivational interviewing early after acute stroke. Stroke 2007;38:1004‐9. [DOI] [PubMed] [Google Scholar]
- Williams LS, Kroenke K, Bakas T, Plue LD, Brizzendine E, Tu W, et al. Care management of poststroke depression: a randomized, controlled trial. Stroke 2007;38(8):998‐1003. [DOI] [PubMed] [Google Scholar]
- Zhengyi F, Zhang H, Hu Y, Wang B, Qian X, Lu H. The affection of home‐based rehabilitation nursing intervention on motor function of daily living in patients with stroke. Chinese Journal of Clinical Rehabilitation 2003;7(5):730‐1. [Google Scholar]
References to ongoing studies
- Graven C. From rehabilitation to recovery: a model to optimise consumer and carer involvement in the first year post stroke. Australian New Zealand Clinical Trials Registry (ANZCTR) http://www.anzctr.org.au/2008.
- LoTS‐Care Stroke System of Care Trial Team. Cluster randomised trial evaluation of a patient and carer‐centred system of longer‐term stroke care. Proceedings of the 17th European Stroke Conference. Nice, France, 13‐16 May 2008:Abs 80. [Google Scholar]
- Mayer C, Chan R, Hachinski V. Partners: Promoting Adherence to Regimen of risk factor modification by Trained volunteers or Nurses Evaluated against Regular practice Study. International Journal of Stroke 2008;3 Suppl 1:321. [Google Scholar]
- Sveen U. Thriving, activity and social participation after stroke. ClinicalTrials.gov2007.
Additional references
- Bamford J, Sandercock P, Dennis M, Warlow C, Jones L, McPherson K, et al. A prospective study of acute cerebrovascular disease in the community: the Oxfordshire Community Stroke Project 1981‐86. Journal of Neurology Neurosurgery and Psychiatry 1988;51:1373‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. A prospective study of acute cerebrovascular disease in the community: the Oxfordshire Community Stroke Project 1981‐86 2. Incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. Journal of Neurology, Neurosurgery and Psychiatry 1990;53:16‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker WH, Mullooly JP. Stroke in a defined elderly population, 1967‐85. Stroke 1997;28:284‐90. [DOI] [PubMed] [Google Scholar]
- Bonita R, Broad JB, Beaglehole R. Changes in stroke incidence and case‐fatality in Auckland, New Zealand, 1981‐91. Lancet 1993;342:1470‐3. [DOI] [PubMed] [Google Scholar]
- Boter H, Delden JJM, Haan RJ, Rinkel GJE, the Home Evaluation of Stroke Induced Aid Study Group. Modified informed consent procedure: consent to postponed information. BMJ 2003;327:284‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie D, Weigall D. Social work effectiveness in two year stroke survivors: a randomised controlled trial. Community Health Studies 1984;8:26‐32. [DOI] [PubMed] [Google Scholar]
- Dennis M, O'Rourke S, Slattery J, Staniforth T, Warlow C. Evaluation of a stroke family care worker: results of a randomised controlled trial. BMJ 1997;314:1071‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, O'Rourke S, Lewis S, Sharpe M, Warlow C. A quantitative study of the emotional outcome of people caring for stroke survivors. Stroke 1998;29:1867‐72. [DOI] [PubMed] [Google Scholar]
- Education Group for Guidelines on Evaluation. Guidelines for evaluating papers on educational interventions. BMJ 1999;318:1265‐7. [PMC free article] [PubMed] [Google Scholar]
- Early Supported Discharge Trialists. Services for reducing duration of hospital care for acute stroke patients. Cochrane Database of Systematic Reviews 2005, Issue 2. [Art. No.: CD000443. DOI: 10.1002/14651858.CD000443.pub2] [DOI] [PubMed] [Google Scholar]
- Evans RL, Matlock A‐L, Bishop DS, Stranahan S, Pederson C. Family intervention after stroke: does counselling or education help?. Stroke 1988;19:1243‐9. [DOI] [PubMed] [Google Scholar]
- Forster A, Young J. Specialist nurse support for patients with stroke in the community: a randomised controlled trial. BMJ 1996;312:1642‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J, Forster A, House A, Knapp P, Wright J, Young J. Information provision for stroke patients and their caregivers. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD001919.pub2] [DOI] [PubMed] [Google Scholar]
- Friedland JF, McColl M. Social support intervention after stroke: results of a randomised trial. Archives of Physical Medicine and Rehabilitation 1992;73:573‐81. [PubMed] [Google Scholar]
- Greveson G, James O. Improving long term outcome after stroke ‐ the views of patients and carers. Health Trends 1991;23:161‐2. [PubMed] [Google Scholar]
- Hackett ML, Anderson CS, House A, Xia J. Interventions for treating depression after stroke. Cochrane Database of Systematic Reviews 2008, Issue 4. [Art. No.: CD003437. DOI: 10.1002/14651858.CD003437.pub3] [DOI] [PubMed] [Google Scholar]
- Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta‐analysis. JAMA 1999;282:1054‐60. [DOI] [PubMed] [Google Scholar]
- Knapp P, Young J, House A, Forster A. Non‐drug strategies to resolve psycho‐social difficulties after stroke. Age and Ageing 2000;29:23‐30. [DOI] [PubMed] [Google Scholar]
- Kotila M, Numminen H, Waltimo O, Kaste M. Depression after stroke. Results of the FINNSTROKE Study. Stroke 1998;29:368‐72. [DOI] [PubMed] [Google Scholar]
- Lilley SA, Lincoln NB, Francis VM. A qualitative study of stroke patients' and carers' perceptions of the stroke family support organiser service. Clinical Rehabilitation 2003;17:540‐7. [DOI] [PubMed] [Google Scholar]
- Mant J, Carter J, Wade DT, Winner S. The impact of an information pack on patients with stroke and their carers: a randomised controlled trial. Clinical Rehabilitation 1998;12:465‐78. [DOI] [PubMed] [Google Scholar]
- Mant J, Carter J, Wade DT, Winner S. Randomised controlled trial of a stroke family support organiser. Cerebrovascular Diseases 1999;9 Suppl 1:123. [Google Scholar]
- Outpatient Stroke Service Trialists. Therapy‐based rehabilitation services for stroke patients at home. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD002925] [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
- Rogers H, Atkinson C, Bond S, Suddes M, Dobson R, Curless R. Randomised controlled trial of a comprehensive stroke education programme for patients and caregivers. Stroke 1999;30:2585‐91. [DOI] [PubMed] [Google Scholar]
- Scholte of Reimer WJM, Haan RJ, Rijnders PT, Limburg M, ven den Bos GAM. The burden of caregiving in partners of long‐term stroke survivors. Stroke 1998;29:1605‐11. [DOI] [PubMed] [Google Scholar]
- Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke 1997;28:491‐9. [DOI] [PubMed] [Google Scholar]
- Taub NA, Wolf CDA, Richardson E, Burney PGJ. Predicting the disability of first‐time stroke sufferers at 1 year. Stroke 1994;25:352‐7. [DOI] [PubMed] [Google Scholar]
- Towle D, Lincoln NB, Mayfield LM. Evaluation of social work on depression after stroke. Clinical Rehabilitation 1989;3:89‐96. [Google Scholar]
- Veenendaal H, Grinspun DR, Adriaanse HP. Educational needs of stroke survivors and their family members, as perceived by themselves and by health professionals. Patient Education and Counselling 1996;28:265‐76. [DOI] [PubMed] [Google Scholar]
- Wade DT, Collen FM, Robb GF, Warlow CP. Physiotherapy intervention after stroke and mobility. BMJ 1992;304:609‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]


