Abstract
Background
Cochrane systematic reviews show that systemic postnatal corticosteroids reduce the risk of bronchopulmonary dysplasia (BPD) in preterm infants. However, corticosteroids have also been associated with an increased risk of neurodevelopmental impairment. It is unknown whether these beneficial and adverse effects are modulated by differences in corticosteroid treatment regimens.
Objectives
To assess the effects of different corticosteroid treatment regimens on mortality, pulmonary morbidity, and neurodevelopmental outcome in very low birth weight (VLBW) infants.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review group to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 2) in the Cochrane Library (searched 21 March 2016), MEDLINE via PubMed (1966 to 21 March 2016), Embase (1980 to 21 March 2016), and CINAHL (1982 to 21 March 2016). We also searched clinical trials' databases, conference proceedings, and the reference lists of retrieved articles for randomized controlled trials.
Selection criteria
Randomized controlled trials (RCTs) comparing two or more different treatment regimens of systemic postnatal corticosteroids in preterm infants at risk for BPD, as defined by the original trialists. Studies investigating one treatment regimen of systemic corticosteroids to a placebo or studies using inhalation corticosteroids were excluded.
Data collection and analysis
Two authors independently assessed eligibility and quality of trials and extracted data on study design, participant characteristics and the relevant outcomes. We asked the original investigators to verify if data extraction was correct and, if possible, to provide any missing data. The primary outcomes to be assessed were: mortality at 36 weeks' postmenstrual age (PMA) or at hospital discharge; BPD defined as oxygen dependency at 36 weeks' PMA; long‐term neurodevelopmental sequelae, including cerebral palsy, measured by the Bayley Mental Developmental Index (MDI); and blindness or poor vision. Secondary outcomes were: duration of mechanical ventilation and failure to extubate at day 3 and 7 after initiating therapy; rescue treatment with corticosteroids outside the study period; and the incidence of hypertension, sepsis and hyperglycemia during hospitalizations. Data were analyzed using Review Manager 5 (RevMan 5). We used the GRADE approach to assess the quality of evidence.
Main results
Fourteen studies were included in this review. Only RCTs investigating dexamethasone were identified. Eight studies enrolling a total of 303 participants investigated the cumulative dosage administered; three studies contrasted a high versus a moderate and five studies a moderate versus a low cumulative dexamethasone dose.
Analysis of the studies investigating a moderate dexamethasone dose versus a high‐dosage regimen showed an increased risk of BPD (typical risk ratio (RR) 1.50, 95% confidence interval (CI) 1.01 to 2.22; typical risk difference (RD) 0.26, 95% CI 0.03 to 0.49; number needed to treat for an additional harmful outcome (NNTH) 4, 95% CI 1.9 to 23.3; I² = 0%, 2 studies, 55 infants) as well as an increased risk of abnormal neurodevelopmental outcome (typical RR 8.33, 95% CI 1.63 to 42.48; RD 0.30, 95% CI 0.14 to 0.46; NNTH 4, 95% CI 2.2 to 7.3; I² = 68%, 2 studies, 74 infants) when using a moderate cumulative‐dosage regimen. The composite outcomes of death or BPD and death or abnormal neurodevelopmental outcome showed similar results although the former only reached borderline significance.
There were no differences in outcomes between a moderate‐ and a low‐dosage regimen.
Four other studies enrolling 762 infants investigated early initiation of dexamethasone therapy versus a moderately early or delayed initiation and showed no significant differences in the primary outcomes. The two RCTs investigating a continuous versus a pulse dexamethasone regimen showed an increased risk of the combined outcome death or BPD when using the pulse therapy. Finally, two trials investigating a standard regimen versus a participant‐individualized course of dexamethasone showed no difference in the primary outcome and long‐term neurodevelopmental outcomes.
The quality of evidence for all comparisons discussed above was assessed as low or very low, because the validity of all comparisons is hampered by small samples of randomized infants, heterogeneity in study population and design, non‐protocolized use of ‘rescue’ corticosteroids and lack of long‐term neurodevelopmental data in most studies.
Authors' conclusions
Despite the fact that some studies reported a modulating effect of treatment regimens in favor of higher‐dosage regimens on the incidence of BPD and neurodevelopmental impairment, recommendations on the optimal type of corticosteroid, the optimal dosage, or the optimal timing of initiation for the prevention of BPD in preterm infants cannot be made based on current level of evidence. A well‐designed large RCT is urgently needed to establish the optimal systemic postnatal corticosteroid dosage regimen.
Plain language summary
Which corticosteroid regimen should be used to prevent bronchopulmonary dysplasia?
Review question: Are the effects of corticosteroids on the outcomes 'mortality, pulmonary morbidity and neurodevelopmental outcome' in preterm infants modulated by the dosage regimen administrated?
Background: Preterm infants have an increased risk of developing chronic lung disease (CLD) or bronchopulmonary dysplasia (BPD). Inflammation in the lung seems to play a central role in the development of BPD, and for this reason studies have investigated the anti‐inflammatory drugs called corticosteroids. These studies showed that corticosteroid treatment reduces the risk of BPD, but is also associated with serious adverse effects on neurodevelopment outcome. To reduce these side effects, clinicians have looked for alternative regimens such as postponing corticosteroid administration, lowering its cumulative dose, giving pulse rather than continuous doses, or individualizing the dose according to the respiratory condition of the infant.
Study characteristics: Searching all electronic databases to 21 March 2016 revealed 14 studies investigating two or more different corticosteroid regimens in preterm infants. The investigated regimens differed in the used cumulative dose, timing of initiation and duration of therapy.
Key results: Those studies comparing a high versus a lower‐dosage regimen showed an increased risk of BPD and adverse neurodevelopmental outcome for infants receiving a lower cumulative dose. Those studies investigating an early versus later administration of steroids did not show any difference in outcome. Furthermore, pulse regimens showed inferior results for the outcome BPD compared with continuous treatment. An individualized dosage regimen showed no differences compared to the standard tapering course.
Quality of evidence: Most of the studies had important methodological weaknesses, preventing any recommendations on the optimal corticosteroid dosage regimen for preterm infants at risk of BPD. More studies are urgently needed.
Summary of findings
Background
Description of the condition
The first description of bronchopulmonary dysplasia (BPD) by Northway and colleagues in 1967 was one of severe lung injury in relatively mature preterm infants who were ventilated with high pressures and high concentrations of oxygen before the advent of surfactant therapy (Northway 1967). This so‐called 'classical' BPD is characterized by profound lung parenchymal inflammation, fibrosis, muscle hypertrophy and diffuse airway damage (O'Brodovich 1985). Treatment and survival of the very young has led to a new pattern of lung injury (Jobe 1999; Coalson 2006). This so‐called 'new' BPD is mainly seen in very preterm infants with gestational ages less than 30 weeks. It is characterized by an arrest in lung development with fewer and larger alveoli, and less striking fibrosis and inflammation (Husain 1998). As a result of changes in infant and histological characteristics, the timing at which BPD is diagnosed has shifted from 28 days' postnatal age (PNA) to 36 weeks' postmenstrual age (PMA) (Bancalari 2006). Cohort studies have shown that, compared with 28 days' PNA, diagnosing BPD at 36 weeks' PMA provides a better identification of infants at risk for long‐term pulmonary and neurological sequelae (Ehrenkranz 2005).
BPD, defined as oxygen dependency at 36 weeks' PMA, remains an important complication of preterm birth with a reported incidence ranging from 23% to 73%, depending on the gestational age (Stoll 2010). BPD is characterized by prolonged respiratory support and recurrent respiratory infections during the first years, and compromised lung function lasting into adulthood. Furthermore, BPD is an independent risk factor for neurodevelopmental impairment (Walsh 2005; Short 2007).
BPD is considered a multifactorial disease. Besides genetic susceptibility, intrauterine growth restriction, nutritional deficits, direct mechanical injury caused by artificial ventilation and oxygen toxicity, pulmonary inflammation has been identified as a key factor in the development of BPD (Carlton 1997; Ferreira 2000; Jobe 2001). Corticosteroids have a strong anti‐inflammatory effect, making them an ideal candidate to attenuate this inflammatory response associated with BPD.
Description of the intervention
Since the 1980s, several randomized controlled trials (RCTs) have investigated the use of corticosteroids, in particular dexamethasone, as a means to reduce the incidence of BPD. Some of these trials started corticosteroid therapy in the first week of life (early), with the aim of preventing progression of the initial acute inflammatory response to BPD (Yeh 1997). Others used corticosteroid therapy in infants who had evolving BPD, starting administration either moderately early (7 to 14 days) or delayed (> 3 weeks) after birth (CDTG 1991; Durand 1995).
Current Cochrane reviews of placebo‐controlled RCTs clearly show that systemic corticosteroids, mainly dexamethasone, significantly reduce the incidence of BPD and the combined outcome of death or BPD in ventilated preterm infants, independent of the time of postnatal administration (Doyle 2014a; Doyle 2014b). However, at the end of the 1990s the first reports on long‐term neurodevelopmental outcome were published, showing that early postnatal systemic dexamethasone treatment is associated with an increased risk of abnormal neurological development (Yeh 1998; O'Shea 1999).
In response to these reports, the American Academy of Pediatrics, the Canadian Paediatric Society and the European Association of Perinatal Medicine concluded that routine use of systemic dexamethasone in the treatment of evolving BPD can no longer be recommended until further research has established the optimal type, dose and timing of corticosteroid therapy (Halliday 2001a; AAP 2002; Watterberg 2010). Following these statements, observational reports have shown a sharp decline in the use of postnatal corticosteroids, a reduction in its cumulative dose, a delay in starting treatment, and a switch to alternative corticosteroids such as hydrocortisone (Kaempf 2003; Shinwell 2003; Walsh 2006).
How the intervention might work
To date, most studies have used a placebo‐controlled design to study the effects of postnatal corticosteroid treatment in preterm infants at risk for BPD. These studies have shown both benefits and harms of corticosteroid treatment. Adjusting the dosage regimen might improve the benefit‐to‐risk ratio of postnatal corticosteroid use. This review identifies and analyses the available randomized trials, using a head‐to‐head comparative design, on five possible treatment regimens.
Alternative corticosteroids: The association between systemic dexamethasone treatment and long‐term neurodevelopmental impairment has resulted in the use of alternative anti‐inflammatory corticosteroids, such as hydrocortisone. Animal studies have suggested that, in contrast to dexamethasone, hydrocortisone has no detrimental effect on the brain (Huang 2007). Historical cohort studies have suggested that hydrocortisone treatment is equally effective in reducing death or BPD compared with dexamethasone‐treated infants without increasing the risk of adverse neurological outcome (van der Heide‐Jalving 2003; Lodygensky 2005; Karemaker 2006; Rademaker 2007). To date, pooled data on placebo‐controlled trials investigating a low hydrocortisone dose initiating at an early treatment onset (< 7 days' PNA) showed no reduction in the incidence of death or BPD (Doyle 2010). Only one of these trials reported long‐term follow‐up, showing no differences in adverse neurodevelopmental sequelae (Watterberg 2007). No placebo‐controlled randomized trials have investigated the use of hydrocortisone after the first week of life in ventilator‐dependent preterm infants.
Lowering the corticosteroid dose and duration: In line with the current opinion of postnatal corticosteroids being 'misguided rockets', clinicians have started to use lower dosage schedules of dexamethasone. The available reviews on placebo‐controlled trials of postnatal corticosteroids stacked information from trials with tremendous heterogeneity in their cumulative dose and duration of therapy (Doyle 2014b). Subgroup analyses using this heterogeneity by dividing the different trials according to the used cumulative dexamethasone dose showed that higher dexamethasone doses reduce the typical risk ratio (RR) for the combined outcome of death or BPD, with the largest treatment effect in trials using a cumulative dose above 4 mg/kg (Onland 2009). No overall effect was found of dosing on the risk of neurodevelopmental sequelae, but in the moderately early treatment studies the risk of death or cerebral palsy (CP) significantly decreased when using a higher cumulative dose (Onland 2009).
Postponing initiation of therapy: Besides lowering the cumulative dose, clinicians limited the use of corticosteroids to those infants that do not respond to other supportive therapies and spontaneous improvement over time. As a result, administration of postnatal corticosteroids in those infants is often postponed until the third or fourth week of life. Placebo‐controlled trials administrating dexamethasone after the first week of life differ in their timing of onset. Meta‐analysis dividing the different placebo‐controlled studies according to the timing of initiation used seems to suggest that moderately early administration is more effective in reducing BPD than delayed administration (Schmidt 2008; Onland 2009).
Pulse dose administration: To minimize the possible adverse effects associated with continuous corticosteroid use, some have suggested prescribing dexamethasone in a pulse regimen using dexamethasone‐free intervals to minimize the risk of direct toxic effects of dexamethasone, while maintaining the beneficial effects on the lung. One placebo‐controlled trial showed that such a pulse regimen resulted in improved pulmonary outcome without clinically relevant side effects (Brozanski 1995).
Individualized tailored regimen: Another approach is to reduce the risk of possible adverse effects of corticosteroids by tailoring the administered cumulative dose to the infant's pulmonary response. For instance, a rapid and clear improvement in respiratory status will allow for a rapid reduction in corticosteroid dose or duration (Bloomfield 1998). To date, there are no placebo‐controlled trials on individualized regime.
Why it is important to do this review
The international neonatal community has discarded the use of early postnatal corticosteroids completely for the reasons stated above. Regarding the use of moderately early or late postnatal systemic corticosteroids, clinicians encounter a dilemma facing those infants at high risk of BPD, since BPD itself is associated with an increased risk of adverse neurological outcome (Ehrenkranz 2005).
It is unknown whether both the beneficial and adverse treatment effects of postnatal corticosteroids can be modulated by the various different dosing regimens described above. Despite all the aforementioned concerns on the long‐term neurodevelopmental sequelae, corticosteroids are still used in approximately 16% of preterm infants (Costeloe 2012). Clinicians remain in doubt as to what the correct drug, cumulative dose, duration and timing of therapy are in terms of the optimal balance between beneficial and adverse effects. Addressing these questions is also important since studies have suggested that restricted use of postnatal corticosteroids resulted in an increased incidence of BPD (Shinwell 2007; Yoder 2009; Cheong 2013).
Objectives
To assess the effects of different corticosteroid treatment regimens on mortality, pulmonary morbidity and neurodevelopmental outcome in very low birth weight (VLBW) infants.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled or quasi‐randomized and cluster‐randomized trials comparing two or more different regimens of systemic corticosteroids in preterm infants at risk for BPD. Studies investigating the effects of one regimen of systemic corticosteroids versus a placebo arm or studies using inhalation corticosteroids were excluded.
Types of participants
Preterm infants at risk for BPD, as defined by the original trialists.
Types of interventions
Trials including infants randomized to treatment with two different regimens of systemic corticosteroids. The following types of intervention were eligible.
An alternative corticosteroid (e.g. hydrocortisone) as the experimental arm versus another type of corticosteroid (e.g. dexamethasone) as the control arm. Any type of corticosteroid in either arms was allowed.
Lower cumulative corticosteroid dosage (experimental arm) versus higher cumulative corticosteroid dosage (control arm). Both arms of the identified trials were categorized according to the cumulative dosage investigated, 'low' being less than 2 mg/kg, 'moderate' being between 2 and 4 mg/kg, and 'high' using a cumulative dosage greater than 4 mg/kg. For inclusion, all comparisons of low‐, moderate‐ or high‐dosage regimens were allowed. Although arbitrary, these cut‐off values were chosen given the results of a systematic review of placebo‐controlled trials (Onland 2009).
Later (experimental arm) versus earlier (control arm) initiation of therapy. We categorized both arms of the identified trials according to the investigated timing of initiation, 'early' being less than 8 days' PNA, 'moderately early' being between 8 and 21 days' PNA, and 'delayed' being greater than 21 days' PNA. Similar to the dosing analyses, all comparisons were allowed. This arbitrary cut‐off point was chosen according to the original Cochrane reviews on placebo‐controlled trials (Halliday 2003a; Halliday 2003b; Halliday 2003c).
Pulse‐dosage regimen (experimental arm) versus continuous‐dosage regimen (control arm). During pulse therapy, the administration of corticosteroids is interrupted for a period longer than the normal interval between corticosteroid doses. Any period of interruption was allowed.
Individually tailored regimens (experimental arm) based on the pulmonary response defined by the original trialists versus a standardized (a pre‐determined schedule administered to every infant) dosage regimen independent of the pulmonary response (control arm).
Types of outcome measures
Two review authors (WO and ADJ) independently extracted the following outcome parameters for each study.
Primary outcomes
Combined outcome of death or BPD at 36 weeks' PMA (BPD defined as oxygen dependency at 36 weeks' PMA).
Secondary outcomes
Mortality at 28 days' PNA, 36 weeks' PMA, hospital discharge and during the first year of life.
BPD (defined by the need for supplemental oxygen) at 28 days' PNA and 36 weeks' PMA.
Failure to extubate at days three and seven after initiating therapy and at the latest reported time point.
Days of mechanical ventilation.
Days of supplemental oxygen.
Hypertension, defined as more than two standard deviations (SD) according to local protocols.
Hyperglycemia, defined as greater than 8.3 mmol/L or requiring insulin therapy, or both.
Culture‐confirmed and clinically suspected infection.
Gastrointestinal bleeding or perforation (spontaneous intestinal perforation (SIP)).
Necrotizing enterocolitis (NEC), following Bell's stages.
Patent ductus arteriosus (PDA), according to trial protocol and requiring therapy.
Intraventricular hemorrhage (IVH), any and severe grades.
Periventricular leukomalacia (PVL).
Cardiac hypertrophy.
Rescue treatment with open‐label corticosteroids within or outside the study period.
Retinopathy of prematurity (ROP), any and severe stages.
Long‐term neurodevelopmental sequelae, assessed after at least one year corrected gestational age (CGA) and before a CGA of four years, and at the latest reported time point, including cerebral palsy and Bayley Scales of Infant Development (Mental Development Index, MDI).
Blindness.
Deafness.
Search methods for identification of studies
Electronic searches
We used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register). We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 2) in the Cochrane Library (searched 21 March 2016); MEDLINE via PubMed (1966 to 21 March 2016); Embase (1980 to 21 March 2016); CINAHL (1982 to 21 March 2016) using the MeSH terms and text words: ('adrenal cortex hormones' OR 'dexamethasone' OR 'betamethasone' OR 'hydrocortisone' OR 'prednisolone' OR 'methylprednisolone' OR 'steroids' OR 'corticosteroids' OR 'glucocorticoids'), and Limits: randomized controlled trials AND infant, newborn (see Appendix 1 for standard search terms for each database). We applied no language restrictions in the search strategy. We contacted original authors of all studies to confirm details of reported follow‐up studies or to obtain information about long‐term follow‐up where none are reported. We searched clinical trials' registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization’s International Trials Registry and Platform www.whoint/ictrp/search/en/; and the ISRCTN Registry).
Searching other resources
We handsearched reference lists of published trials, review articles, and the abstracts of the Pediatric Academic Societies and the European Society for Paediatric Research (from 1990 onwards).
Data collection and analysis
Selection of studies
Two review authors (WO and ADJ) classified the relevant citations found by the database searches into three groups: 'clearly an RCT', 'clearly not an RCT' and 'possibly an RCT'. A full‐text review was done on all except those classified as 'clearly not an RCT'. Any disagreements were resolved by consensus.
Data extraction and management
In addition to the pre‐defined outcome measurements, two review authors (WO and ADJ) independently extracted the following data for each study using a pre‐defined data sheet: infant's characteristics (such as birth weight, gestational age, gender); number of participants randomized; treatment with antenatal corticosteroids and postnatal surfactant; type of corticosteroid and regimens (PNA at start, duration of therapy, cumulative dose; dosing interval (fixed or variable); dose adjustments according to infant's characteristics); and the incidence of open‐label (outside the study protocol) use of corticosteroids in both arms of the studies. The original investigators of the included RCTs were asked to confirm whether the data extraction was accurate and, where necessary, to provide additional (unpublished) data.
Assessment of risk of bias in included studies
Two review authors (WO and ADJ) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool in Higgins 2011 for the following domains.
Selection bias.
Performance bias.
Detection bias.
Attrition bias.
Reporting bias.
Any other bias.
Any disagreements were resolved by discussion or by a third assessor. See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
Data management was conducted using the Cochrane statistical package, Review Manager 5 (RevMan 2012). Treatment effect estimates were calculated, where possible, for dichotomous outcomes in all individual trials expressed as typical risk ratio (RR) and typical risk difference (RD), all with a 95% confidence interval (CI). For continuous outcomes reported in individual studies the mean values for treatment and control groups were used with the SD. If median and range were given in individual studies, and the study authors were not able to provide the mean value and variance from the original data set, they were calculated according to the method described by Hozo 2005. We calculated the number needed to treat for an additional beneficial outcome (NNTB) and number needed to treat for an additional harmful outcome (NNTH) for each different outcome in case of statistical significance.
Unit of analysis issues
If cluster‐randomized trials had been included in the analyses, we would have adjusted their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We asked the study author of the included RCT to confirm whether the data extraction was accurate and, where necessary, to provide additional (unpublished) data.
Assessment of heterogeneity
We assessed heterogeneity between trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I² statistic, using the following categories as defined by the Cochrane Neonatal Review Group.
Less than 25%: no heterogeneity.
25% to 49%: low heterogeneity.
50% to 74%: moderate heterogeneity.
75% or greater: high heterogeneity.
We explored possible causes of statistical heterogeneity using pre‐specified subgroup analysis (e.g. differences in intervention regimens).
Assessment of reporting biases
We used funnel plots to assess possible reporting or publication biases.
Data synthesis
We performed meta‐analysis of the extracted data using standard Cochrane methods and Review Manager 5 (RevMan 2012). Treatment effects for dichotomous outcomes were expressed as typical RR with a 95% CI, typical RD, and NNTBs or NNTHs in case of significance. We used mean differences (MD) for continuous outcomes. In case of variance of outcome measures (with different SD) measuring the same outcome, we calculated standardized mean differences (SMD) in the meta‐analysis. We used the fixed‐effect model for all meta‐analyses.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: the combined outcome of BPD or death at 36 weeks' PMA, as well as the combined outcomes of death or cerebral palsy, and death or abnormal neurodevelopmental outcome.
Two authors independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomized controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro GDT 2016 Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades:
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
In case of substantial heterogeneity, we performed subgroup analyses and sensitivity analyses, and, if not appropriate, reconsidered whether an overall summary was meaningful at all. We planned to carry out the following subgroup analyses.
Gestational age using an arbitrary cut‐off point of 26 weeks.
The degree of illness at the start of treatment as defined by mean respiratory index or fractional inspired oxygen, if available, at trial entry.
Ventilated versus non‐ventilated neonates at study entry.
Trials allowing use of open‐label corticosteroids during the study period, by dividing the individual trials according to the percentage of infants treated with open‐label corticosteroids in the experimental arm, using arbitrary cut‐off points of less than 30%, 30% to 50%, and greater than 50% of the included infants; and trials investigating two (or more) of the main comparisons analyzed in both comparisons in subgroups. For example, if a study investigates hydrocortisone at an early initiation versus a dexamethasone regimen at a later treatment onset, this study would be analyzed in both the main comparison type of corticosteroids, as well as the comparison timing of initiation.
Sensitivity analysis
We performed sensitivity analyses when trials were judged at high risk of bias, to assess the effect of the bias on the meta‐analysis.
Results
Description of studies
Results of the search
The electronic PubMed search revealed 2961 potential citations using the search strategy described above (Figure 1). Additional electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL), Embase and CINAHL did not identify any additional RCTs. Combined with the handsearch, and after exclusion of the clearly irrelevant titles, a total of 119 abstracts were retrieved and assessed for eligibility. A total of 24 studies were deemed eligible. After reading the full reports, three studies were excluded, leaving 21 eligible for this review. Of these 21 studies, three were follow‐up reports of included RCTs (Cummings 1989; Bloomfield 1998; Halliday 2001), two were reports of additional outcome parameters of the original RCT (Papile 1998; Odd 2004), and one was an abstract found in the Pediatric Academic Societies conference proceedings, which was later published as a full report (Malloy 2005). The original author of one publication could not provide any clinical outcome data and this study was therefore excluded from the quantitative analyses (Groneck 1993). Thus, the search strategy revealed 14 original RCTs to be included in this review.
1.
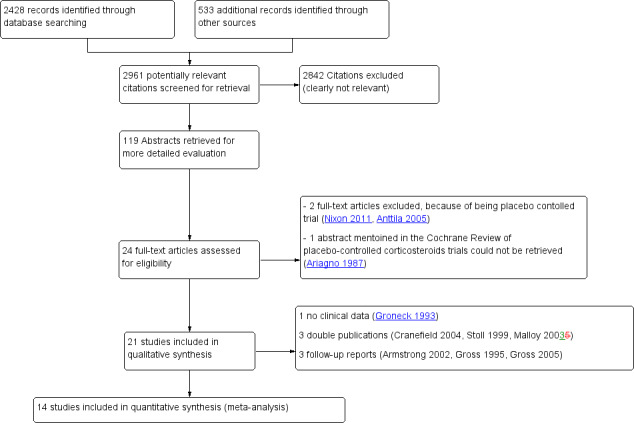
Study flow diagram.
Included studies
The 14 studies meeting the inclusion criteria for this review randomized a total of 1219 infants. Detailed description of participant characteristics of the individual trials can be found in Table 5. Most studies included preterm infants with similar ranges of gestational age and birth weight, yet there was considerable variation in the use of antenatal corticosteroids and exogenous surfactant. Pulmonary illness, assessed by the amount of supplemental oxygen and the level of mean airway pressure at study entry, differed considerably across the trials. Only three studies reported no late rescue treatment with dexamethasone in both treatment groups. The investigated regimens differed in the used cumulative dose, timing of initiation and duration of therapy.
1. Patient characteristics of individual trials.
| Allocation arm | Patients (N) | BWa (grams) | GAb (weeks) | ANSc (%) | SFd (%) | SDa (mg/kg/d) | CDb (mg/kg) | Mean Age Initiation | TDc (days) | LRGd (%) | Entry FiO₂ (%) | Entry MAP (cmH₂0) |
|
| Lower cumulative dosage (experimental arm) versus higher cumulative dosage (control arm) | |||||||||||||
| Cummings | High | 13 | 818 ± 145 | 26 ± 2 | 38 | 0 | 0.5 | 7.9 | 14 | 42 | 0 | 0.60 ± 0.27 | 1.02 ± 0.59 |
| Moderate | 12 | 810 ± 208 | 26 ± 2 | 25 | 0 | 0.5 | 3.0 | 18 | 0 | 0.51 ± 0.23 | 0.86 ± 0.26 | ||
| DeMartini | High | 16 | 741 ± 142 | 25.5 ± 1.7 | 62 | 100 | 0.5 | 4.1 | ? | 21 | 0 | 0.61 ± 26.9 | ? |
| Moderate | 14 | 848 ± 224 | 26.4 ± 1.6 | 64 | 100 | 0.5 | 2.7 | 7 | 0 | 0.60 ± 25.2 | |||
| Marr | High | 28 | 747 ± 129 | 25.0 ± 1.1 | 60 | ? | 0.5 | 7.9 | 14 ± 4 | 42 | 0 | 0.72 ± 0.12 | 10.2 ± 2.0 |
| Moderate | 28 | 790 ± 169 | 25.2 ± 1.1 | 64 | 0.5 | ? | 13 ± 4 | 9 | 37 | 0.77 ± 0.16 | 10.4 ± 1.7 | ||
| Malloy | Moderate | 9e | 767 ± 149 | 25.8 ± 0.9 | 75 | 100 | 0.5 | 2.7 | 14.8 ± 6.5 | 7 | 88 | 0.57 ± 0.08 | ? |
| Low | 8 | 773 ± 182 | 26.1 ± 1.8 | 63 | 100 | 0.08 | 0.6 | 16.8 ± 5.7 | 7 | 50 | 0.52 ± 0.16 | ||
| Durand | Moderate | 23 | 932 ± 182 | 27.1 ± 1.8 | 52 | 87 | 0.5 | 2.4 | 11.5 ± 2.2 | 7 | 22 | 0.43 ± 0.11 | 7.8 ± 2.2 |
| Low | 24 | 858 ± 186 | 26.9 ± 1.6 | 50 | 88 | 0.2 | 1.0 | 11.3 ± 2.7 | 7 | 29 | 0.41 ± 0.10 | 7.0 ± 1.2 | |
| McEvoy | Moderate | 29 | 839 ± 229 | 26.1 ± 2.0 | 34 | 97 | 0.5 | 2.4 | 10.7 ± 3.7 | 7 | 55 | 0.44 ± 0.13 | 6.8 ± 1.8 |
| Low | 33 | 830 ± 248 | 26.3 ± 1.8 | 48 | 82 | 0.2 | 1.0 | 11.6 ± 4.3 | 7 | 39 | 0.42 ± 0.13 | 7.4 ± 2.2 | |
| Ramanathan | Moderate | 15 | 850 ± 290 | 27 ± 2 | ? | 67 | 0.4 | 1.9e | 10 to 14 | 7 | 67 | ? | ? |
| Low | 13 | 817 ± 186 | 27 ± 2 | 62 | 0.2 | 1.0e | 7 | 54 | |||||
| da Silva | Moderate | 17 | 821 ± 160 | 25.4 ± 0.9 | ? | ? | 0.5 | ? | ? | 7 | ? | ? | ? |
| Low | 21 | 851 ± 465 | 25.7 ± 1.8 | 0.1 | 0.7 | 7 | |||||||
| Later initiation (experimental arm) versus earlier (control arm) | |||||||||||||
| Papile | ME | 182 | 808 ± 187 | 25.7 ± 1.9 | 29 | 91 | 0.5 | 3.7 | 14 | 14 | 12 | 0.54 ± 0.18 | 8 ± 2 |
| L | 189 | 801 ± 182 | 25.6 ± 1.6 | 27 | 89 | 28 | 16 | 0.54 ± 0.19 | 8 ± 2 | ||||
| Merz | E | 15 | 980 (710 to 1250) | 27 (25 to 29) | 87 | 87 | 0.5 | 3.1 | 7 | 16 | 0 | 0.3 (0.25 to 0.5) | ? |
| ME | 15 | 938 (680 to 1250) | 27.5 (24 to 29) | 73 | 73 | 14 | 0 | 0.3 (0.25 to 0.55) | |||||
| Halliday | E | 135 | 1017 ± 290 | 27.4 ± 1.9 | 61 | 95 | 0.5 | 2.7 | 3 | 12 | ? | ? | ? |
| ME | 150 | 1007 ± 283 | 27.1 ± 1.9 | 55 | 92 | 16 | |||||||
| Pulse dosage regimen (experimental arm) versus continuous dosage regimen (control arm) | |||||||||||||
| Bloomfield | Pulse/Ef | 39 | 776 ± 25 | 25.8 ± 0.3 | 95 | ? | 0.5 | 5.3 (1.5 to 11.8) | 7 | 34 (11 to 73) | ? | 0.30 ± 0.02 | 8.0 ± 0.3 |
| Cont/ME | 37 | 793 ± 28 | 25.8 ± 0.3 | 73 | 0.5 | 7.1 (4.5 to 7.6) | 14 | 42 (42 to 51) | 0.30 ± 0.01 | 7.8 ± 0.3 | |||
| Barkemeyer | Pulse | 58 | 816 | 26.1 | 84 | 92 | 0.5 | 4.5 | 7 to 21 | 23 | 41 | ? | ? |
| Cont | 63 | 842 | 26.2 | 78 | 88 | 36 | |||||||
| Individualized tailored (experimental arm) versus standard dosage regimen (control arm) | |||||||||||||
| Odd | Indiv | 17 | 669 ± 113 | 24 (23 to 27) | ? | ? | 0.5 | 3.8 (2.0 to 5.7) | 12 (7 to 16) | 42 (5 to 73) | 0.40 (0.25 to 1.0) | 9 (7 to 14) | |
| Cont | 16 | 720 ± 130 | 24 (23 to 26) | 0.5 | 7.9 | 10 (7 to 23) | 42 | 0.40 (0.21 to 1.0) | 9 (7 to 13) | ||||
a BW: Birth weight (grams ± SD); b GA: Gestational age (weeks ± SD); c ANS: antenatal steroids; d SF: surfactant;e Including 1 patient in high dose group who died on the second day of treatment, a SD: Starting dose (mg/kg/day); b CD: Cumulative dose; c TD: Total days of therapy; d LRG: Late rescue treatment with corticosteroids; e Estimated cumulative dose based on abstract data; f Bloomfield not only pulse versus continuous comparison, but also in early versus later initiation; E: Early initiation (≤ 7 days' PNA); ME: Moderately early initiation (7 to 14 days' PNA); L: Late initiation (> 14 days' PNA); Pulse: Pulse dosage regimen; Cont: Continuous tapered dosage regimen; Indiv: Individual tailored regimen.
The trial by Bloomfield 1998 allocated infants to a group receiving a pulse dose of corticosteroids initiated early or a group receiving a continuous tapering dose of corticosteroids started moderately early. In addition, the duration of the pulse dose, but not the continuous tapering dose, was dependent on the pulmonary response of the infant. Based on this design, the trial was used for three comparisons in this review: earlier versus later initiation of corticosteroid treatment, pulse versus continuous dosing, and individualized versus standardized dosing.
Alternative corticosteroids: No studies were identified investigating two or more different types of corticosteroids. In fact, all studies included in this review used dexamethasone in both treatment arms.
Lowering the corticosteroid dose and duration: The timing of the eight eligible studies investigating this comparison was moderately early (7 to 21 days). The cumulative dexamethasone doses ranged from 0.6 to 3.0 mg/kg in the lower‐dosage regimens (experimental arm) to 1.9 to 7.9 mg/kg in the high‐dosage regimens (control arm). Only two dosage comparisons were identified during this review, high (> 4 mg/kg cumulative dose) versus moderate dose (between 2 and 4 mg/kg cumulative dose) and moderate‐ versus low‐ (< 2 mg/kg cumulative dose) dosage regimens. Three studies compared a high dose (control arm) to a moderate dose (Cummings 1989; DeMartini 1999; Marr 2011); and five studies a moderate dose to a low dose (Ramanathan 1994; Da Silva 2002; Durand 2002; McEvoy 2004; Malloy 2005). These two comparisons were analyzed separately.
Postponing initiation of therapy: Four RCTs investigated the effect of timing on the dexamethasone treatment effects in preterm infants (Bloomfield 1998; Papile 1998; Merz 1999; Halliday 2001). Only two comparisons were identified, namely delayed versus moderately early initiation, and moderately early versus early initiation of corticosteroid therapy. Papile 1998 compared delayed (> 21 days' PNA (experimental arm)) to moderately early (between 8 and 21 days (control arm)) initiation of treatment. The other three trials contrasted early (≤ 7 days' PNA) to moderately early (experimental arm) initiation of treatment. These two comparisons were analyzed separately. The comparison of moderately early versus early initiation included the trial performed by Halliday 2001. This RCT used a factorial design with four allocation arms. Two arms administered corticosteroids by inhalation, and these data were therefore excluded for this review. The other two arms administered dexamethasone systemically starting either early or moderately early, and were therefore included in the analysis.
Pulse dose administration: Two studies compared pulse therapy of dexamethasone (experimental arm) with a continuous tapering dosage regimen (control arm) (Bloomfield 1998; Barkemeyer 2000). Both trials used a pulse dexamethasone therapy (0.5 mg/kg/day) for three consecutive days followed by seven days of no corticosteroid therapy. One trial administered similar cumulative doses of dexamethasone in both allocation arms (Barkemeyer 2000). However, in the other study the duration of the pulse‐dosage regimen varied, depending on the infant’s pulmonary condition and level of respiratory support (Bloomfield 1998). The continuous tapering dosage regimen in this study, however, was the same for every infant allocated to this arm.
Individualized tailored regimen: Two studies allocated the infants to either an individualized dosage regimen (experimental arm), or a tapering dosage regimen. One study initiated the intervention at the same postnatal age (Odd 2004), whereas the other study initiated the pulse therapy at day 7 of life, comparing it to a tapering continuous dosage regimen commencing at day 14 of life (Bloomfield 1998).
Seven of the 14 original investigators provided the authors with additional data on methodology, intervention, infant characteristics or missing outcome parameters.
Excluded studies
The review authors excluded two RCTs after reading the full text, because they investigated the effect of corticosteroid in preterm infants using placebo‐controlled study design, which is not the topic of this review (Anttila 2005; Nixon 2011). The unpublished study by Ariagno 1987, reported in the Cochrane Review by Halliday 2003b, could not be retrieved. One publication was excluded, because no clinical outcomes were published and the original author could not provide those data (Groneck 1993).
Risk of bias in included studies
The overall risk of bias of the 14 studies was deemed fair to good (Figure 2; Figure 3). Four trials were only published as abstracts, and therefore had insufficient data to make a proper methodological assessment (Ramanathan 1994; DeMartini 1999; Da Silva 2002; Marr 2011).
2.
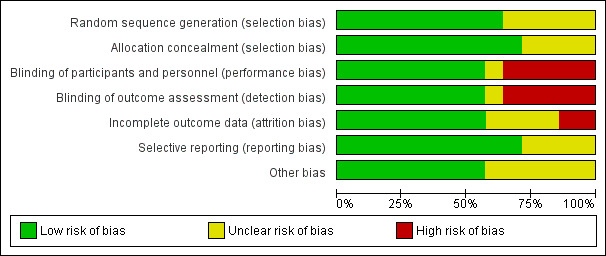
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
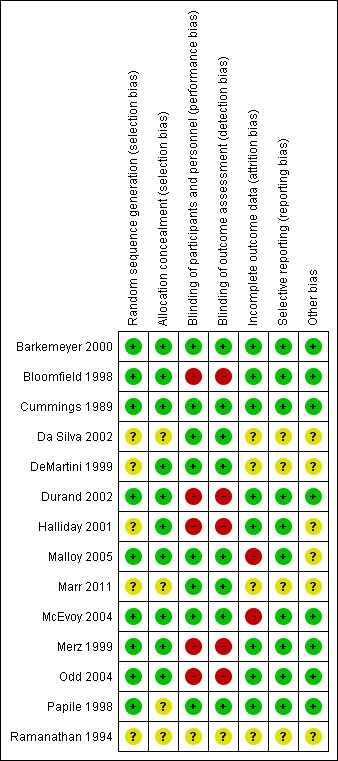
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In five studies the random sequence generation was insufficiently described, whereas the method of allocation concealment was not mentioned in four trials. Therefore, eight trials described and addressed these items properly and were judged as having low risk.
Blinding
Five trials did not attempt to blind the intervention; thus caregivers, parents and outcome assessors were not blinded. These trials were judged as being at high risk for performance and detection bias. In one trial no information on blinding was available making it impossible to assess bias (Ramanathan 1994).
Incomplete outcome data
All bar one trial reported data on 'lost to follow‐up' or participant selection, or both, and therefore these RCTs were at low risk of attrition bias. Malloy 2005 excluded one infant who died during the study course, and for this reason was assessed as being at high risk of attrition bias. However, this infant was included in the current analyses.
Selective reporting
None of the included trials published a study protocol. Except for the RCTs only published as abstracts, in which this item could not be assessed, all studies reported sufficiently on the predefined outcome parameters.
Other potential sources of bias
Two trials were judged as having an unclear risk for other potential sources of bias. Malloy 2005 was terminated prematurely; and in Halliday 2001, a large proportion of the infants randomized to delayed selective treatment either died or did not fulfill the entry criteria. The other trials were at low risk.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Higher versus lower cumulative dosage regimens of dexamethasone to prevent BPD in preterm infants.
| Higher versus lower cumulative dosage regimens of dexamethasone to prevent BPD in preterm infants | |||||
|
Patient or population: preterm infants Settings: neonatal intensive care unit Intervention: lower dosage Comparison: higher dosage | |||||
| Outcomes | № of participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with higher cumulative dose dexamethasone regimen | Risk difference with Lower | ||||
| Death or bronchopulmonary dysplasia at 36 weeks' PMA ‐ Moderate versus high cumulative dose regimen | 55 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | RR 1.35 (1.00 to 1.82) | Study population | |
| 19/29 (65.5%) | 229 more per 1000 (0 fewer to 537 more) | ||||
| Moderate | |||||
| 65.1% | 228 more per 1000 (0 fewer to 534 more) | ||||
| Death or bronchopulmonary dysplasia at 36 weeks' PMA ‐ Low versus moderate cumulative dose regimen | 154 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 4 | RR 0.83 (0.50 to 1.40) | Study population | |
| 19/76 (25.0%) | 43 fewer per 1000 (125 fewer to 100 more) | ||||
| Moderate | |||||
| 18.7% | 32 fewer per 1000 (94 fewer to 75 more) | ||||
| Death or cerebral palsy ‐ Moderate versus high cumulative dose regimen | 25 (1 RCT) | ⊕⊕⊝⊝ LOW 2 3 | RR 2.17 (0.87 to 5.37) | Study population | |
| 4/13 (30.8%) | 360 more per 1000 (40 fewer to 1.345 more) | ||||
| Moderate | |||||
| 30.8% | 360 more per 1000 (40 fewer to 1.345 more) | ||||
| Death or cerebral palsy ‐ Low versus moderate dose regimen | 109 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 5 | RR 0.78 (0.28 to 2.18) | Study population | |
| 7/52 (13.5%) | 30 fewer per 1000 (97 fewer to 159 more) | ||||
| Moderate | |||||
| 13.4% | 30 fewer per 1000 (97 fewer to 158 more) | ||||
| Death or abnormal neurodevelopmental outcome (various definitions) ‐ Moderate versus high cumulative dose regimen | 81 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 6 | RR 3.37 (1.42 to 7.99) | Study population | |
| 5/41 (12.2%) | 289 more per 1000 (51 more to 852 more) | ||||
| Moderate | |||||
| 17.2% | 407 more per 1000 (72 more to 1.200 more) | ||||
| Death or abnormal neurodevelopmental outcome (various definitions) ‐ Low versus moderate cumulative dose regimen | 16 (1 RCT) | ⊕⊕⊝⊝ LOW 2 7 | RR 0.43 (0.12 to 1.51) | Study population | |
| 6/9 (66.7%) | 380 fewer per 1000 (587 fewer to 340 more) | ||||
| Moderate | |||||
| 66.7% | 380 fewer per 1000 (587 fewer to 340 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 In the study by DeMartini selection, attrition and reporting bias could not be ruled out
2 Total number of included patients less than OIS calculation
3 Study by Marr has not reached full publication
4 Ramanathan methodology could not be assessed, Durand not blinded, Malloy and McEvoy attrition bias. Malloy study was terminated prematurely
5 Study by Durand had performance and detection bias, McEvoy study had attrition bias
6 In the study by Marr, selection, attrition and reporting bias could not be ruled out
7 Attrition bias was detected in the study by Malloy
Summary of findings 2. Earlier versus later initiation of dexamethasone therapy to prevent BPD in preterm infants.
| Earlier versus later initiation of dexamethasone therapy to prevent BPD in preterm infants | |||||
|
Patient or population: preterm infants Settings: neonatal intensive care unit Intervention: later initiation Comparison: earlier initiation | |||||
| Outcomes | № of participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with earlier initiation of dexamethasone therapy | Risk difference with Late | ||||
| Death or bronchopulmonary dysplasia at 36 weeks PMA ‐ Moderate early versus early initiation | 391 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | RR 1.06 (0.87 to 1.29) | Study population | |
| 90/189 (47.6%) | 29 more per 1000 (62 fewer to 138 more) | ||||
| Moderate | |||||
| 46.1% | 28 more per 1000 (60 fewer to 134 more) | ||||
| Death or cerebral palsy ‐ Moderate early versus early initiation | 86 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 3 4 | RR 1.12 (0.68 to 1.84) | Study population | |
| 14/34 (41.2%) | 49 more per 1000 (132 fewer to 346 more) | ||||
| Moderate | |||||
| 41.2% | 49 more per 1000 (132 fewer to 346 more) | ||||
| Death or abnormal neurodevelopmental outcome (various definitions) ‐ Moderate early versus early | 167 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 3 5 | RR 0.87 (0.63 to 1.21) | Study population | |
| 38/75 (50.7%) | 66 fewer per 1000 (187 fewer to 106 more) | ||||
| Moderate | |||||
| 50.4% | 66 fewer per 1000 (187 fewer to 106 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Performance and detection bias in study by Bloomfield, Merz and Halliday
2 Unclear selection bias in Halliday
3 Total number of included patients less than OIS calculation
4 Performance and detection bias in study by Halliday
5 Performance and detection bias in Bloomfield and Halliday studies
Summary of findings 3. Pulse versus tapered continuous dosage regimens to prevent BPD in preterm infants.
| Pulse versus tapered continuous dosage regimens to prevent BPD in preterm infants | |||||
|
Patient or population: preterm infants Settings: neonatal intensive care unit Intervention: pulse therapy Comparison: tapered continuous dosage | |||||
| Outcomes | № of participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with continuous dexamethasone therapy | Risk difference with Pulse | ||||
| Death or bronchopulmonary dysplasia at 36 weeks PMA | 197 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | RR 1.38 (1.02 to 1.88) | Study population | |
| 39/100 (39.0%) | 148 more per 1000 (8 more to 343 more) | ||||
| Moderate | |||||
| 38.2% | 145 more per 1000 (8 more to 336 more) | ||||
| Death or abnormal neurodevelopmental outcome (various definitions) | 76 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | RR 1.23 (0.79 to 1.92) | Study population | |
| 17/37 (45.9%) | 106 more per 1000 (96 fewer to 423 more) | ||||
| Moderate | |||||
| 46.0% | 106 more per 1000 (96 fewer to 423 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Peformance and detection bias in Bloomfield study
2 Total number of included patients less than OIS calculation
3 Barkemeyer could provide long‐term outcomes
Summary of findings 4. Individually tailored versus tapered continuous dosage regimens to prevent BPD in preterm infants.
| Individually tailored versus tapered continuous dosage regimens to prevent BPD in preterm infants | |||||
|
Patient or population: preterm infants Settings: neonatal intensive care unit Intervention: individualized dosage regimen Comparison: tapered dosage regimen | |||||
| Outcomes | № of participants (studies) Follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with continuous regimen | Risk difference with Individual tailored | ||||
| Death or bronchopulmonary dysplasia at 36 weeks PMA | 109 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | RR 1.17 (0.83 to 1.66) | Study population | |
| 31/53 (58.5%) | 99 more per 1000 (99 fewer to 386 more) | ||||
| Moderate | |||||
| 75.0% | 127 more per 1000 (128 fewer to 495 more) | ||||
| Death or abnormal neurodevelopmental outcome (various definitions) | 109 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | RR 1.06 (0.55 to 2.06) | Study population | |
| 8/53 (15.1%) | 9 more per 1000 (68 fewer to 160 more) | ||||
| Moderate | |||||
| 50.0% | 30 more per 1000 (225 fewer to 530 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Performance and detection bias in Odd and Bloomfield studies
2 Total number of included patients less than OIS calculation
Lower (experimental arm) versus higher (control arm) cumulative dosage regimens of dexamethasone (Comparison 1)
Primary outcome
Combined outcome of death or BPD at 36' weeks PMA
Compared to the infants who were allocated to a moderate cumulative dosage regimen of dexamethasone, the infants allocated to the low dexamethasone dosage regimens showed no difference in the incidence in the combined outcome of death or BPD at 36 weeks' PMA (Analysis 1.1). However, in the comparison of studies investigating a high‐ versus moderate‐dosage regimen a borderline significance was found. Compared to the infants who were allocated to a high dose of dexamethasone, the infants allocated to a moderate dose regimen had a higher incidence of the composite outcome of death or BPD (typical RR 1.35, 95% CI 1.00 to 1.82; NNTH 5, 95% CI 3 to 375; (Analysis 1.1)). The quality of evidence was graded very low because of the small number of events, publication bias and the risk of selection, attrition and reporting bias (Table 1).
1.1. Analysis.
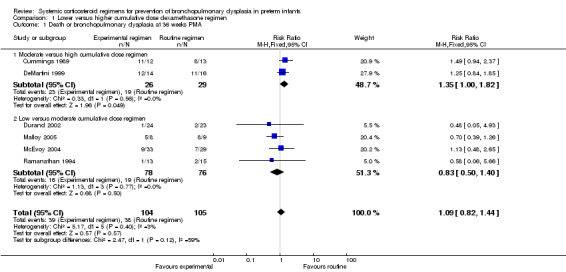
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 1 Death or bronchopulmonary dysplasia at 36 weeks PMA.
Secondary outcomes
Mortality at 28 days' PNA, at 36 weeks' PMA and at hospital discharge.
No data were retrieved on mortality at 28 days' PNA. Compared to the infants who were allocated to a higher‐dosage regimen, the infants who were allocated to lower‐dosage regimens had no significant difference in the incidence of the outcome of death at 36 weeks' PMA and at hospital discharge (Analysis 1.2; Analysis 1.3).
1.2. Analysis.
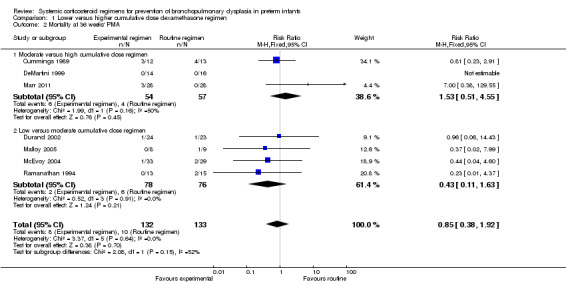
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 2 Mortality at 36 weeks' PMA.
1.3. Analysis.
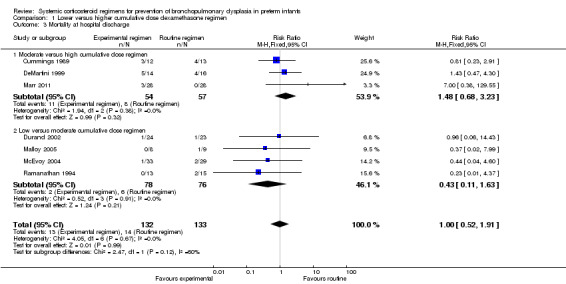
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 3 Mortality at hospital discharge.
BPD at 28 days' PNA and 36 weeks' PMA
No data were retrieved on the outcome of BPD at 28 days' PNA. Compared to infants who were allocated to a high dexamethasone dose, infants who were allocated to a moderate dexamethasone dose had a significantly higher incidence of BPD (typical RR 1.50, 95% CI 1.01 to 2.22; NNTH 4, 95% CI −3 to 197) (Analysis 1.4). Compared to infants who were allocated to a moderate dose, the infants allocated to a low dose had no significant difference in the outcome of BPD (Analysis 1.4).
1.4. Analysis.
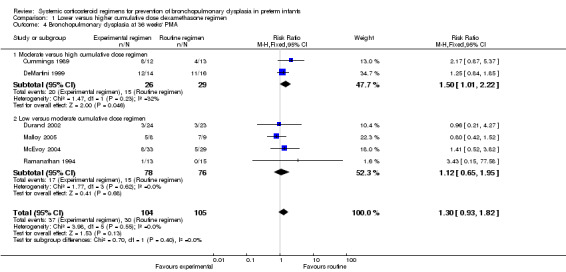
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 4 Bronchopulmonary dysplasia at 36 weeks' PMA.
Short‐term outcomes
The cumulative dexamethasone dose did not impact the outcome of 'failure to extubate at day 3 of life' (Analysis 1.5). However, compared to the infants allocated to the high‐dosage regimen, the infants allocated to the moderate‐dose regimen had a significantly higher incidence of failing extubation at day 7 of life (typical RR 1.33, 95% CI 1.05 to 1.68; NNTH 7, 95% CI 4 to 58) (Analysis 1.6). The duration of mechanical ventilation was significantly shorter in the high‐dose regimen compared to the moderate‐dosage regimen (MD 7.41, 95% CI 1.43 to 13.39 (Analysis 1.7)), whereas no difference was seen in the outcome 'days of supplemental oxygen'. Compared to the infants allocated to the moderate‐dosage regimen, the infants allocated to the low‐corticosteroid regimen showed a significantly lower incidence of the short‐term adverse effects of hypertension (typical RR 0.31, 95% CI 0.11 to 0.87; NNTB 7, 95% CI 3.6 to 29.4) (Analysis 1.9) and hyperglycemia (typical RR 0.40, 95% CI 0.17 to 0.93; NNTB 7, 95% CI 3.5 to 41.2) (Analysis 1.10), but no differences were seen between the high‐ and moderate‐dosage comparison. The incidence of late ‘rescue’ therapy with open label corticosteroids, sepsis, gastrointestinal hemorrhage or perforation, NEC, severe IVH, PVL, or severe ROP was not significantly different between the different dosage regimens. No data were retrieved on the outcomes PDA and cardiac hypertrophy.
1.5. Analysis.
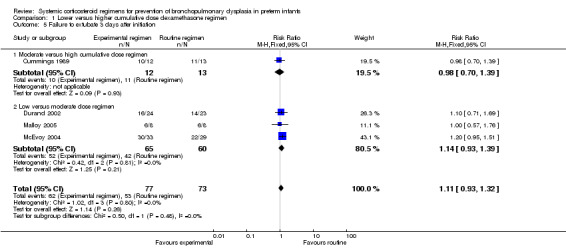
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 5 Failure to extubate 3 days after initiation.
1.6. Analysis.
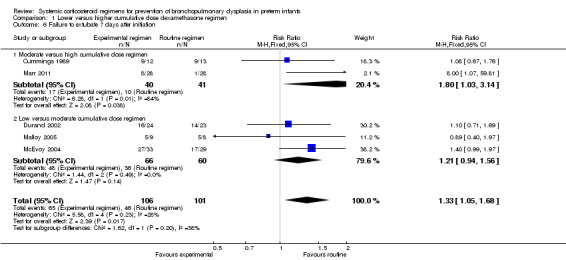
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 6 Failure to extubate 7 days after initiation.
1.7. Analysis.
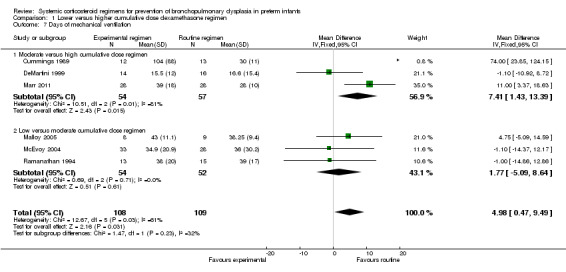
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 7 Days of mechanical ventilation.
1.9. Analysis.
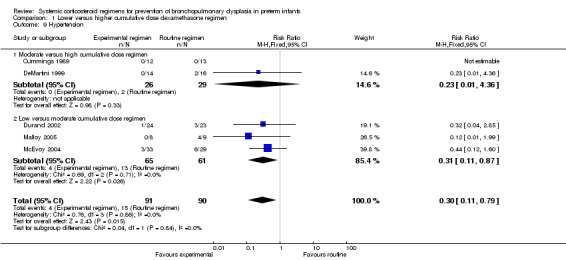
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 9 Hypertension.
1.10. Analysis.
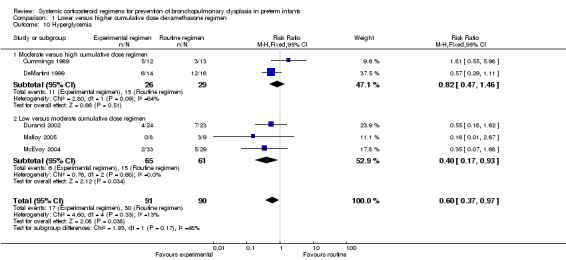
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 10 Hyperglycemia.
Neurodevelopmental sequelae
Four studies reported the long‐term neurodevelopmental outcomes of cerebral palsy, visual impairment or the Bayley MDI in survivors, including 66% to 100% of their randomized infants. Malloy 2005 performed long‐term neurodevelopmental assessment, but used the modified Gesell Developmental Appraisal, which was deemed not to be comparable with the Bayley MDI reported in the other studies. Analysis showed no significant differences in the incidence of cerebral palsy, or the composite outcome of death or cerebral palsy between both allocation arms (Analysis 1.20; Analysis 1.21). There were no significant differences in the number of infants with Bayley MDI less than 2 SD, or with visual impairment (Analysis 1.22; Analysis 1.23). Three studies reported on the incidence of abnormal neurodevelopmental outcome as defined by the trialists. The meta‐analyses of the moderate versus the low dosage regimens did not reveal any differences. However, compared to the infants allocated to a high‐dosage regimen, a significant higher incidence of abnormal neurodevelopmental outcome was seen in group of infants allocated to the moderate‐dosage regimen (typical RR 8.33, 95% CI 1.63 to 42.48; NNTH 4, 95% CI 3 to 8) (Analysis 1.24). The composite outcome of abnormal neurodevelopmental outcome or death showed the same benefits in favor of the high‐dosage group (Analysis 1.25). The quality of evidence was graded low to very low because of the small number of events, publication bias and the risk of performance, detection and attrition bias (Table 1).
1.20. Analysis.
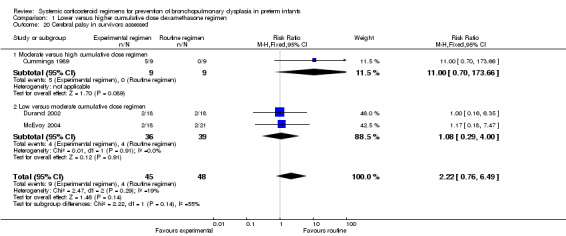
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 20 Cerebral palsy in survivors assessed.
1.21. Analysis.
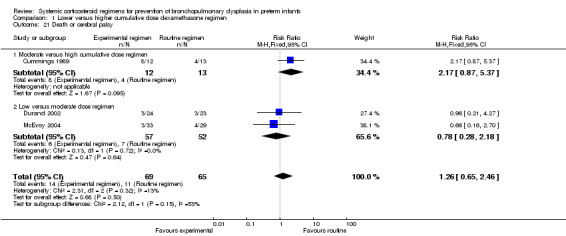
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 21 Death or cerebral palsy.
1.22. Analysis.
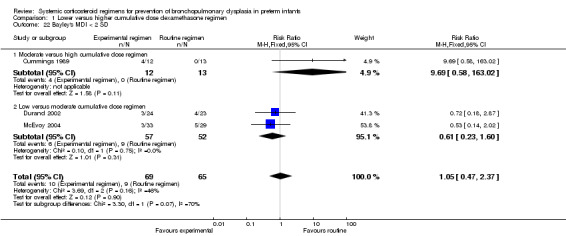
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 22 Bayley's MDI < 2 SD.
1.23. Analysis.
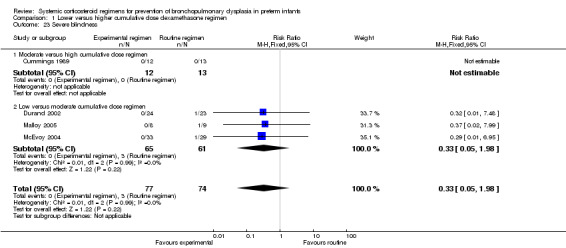
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 23 Severe blindness.
1.24. Analysis.
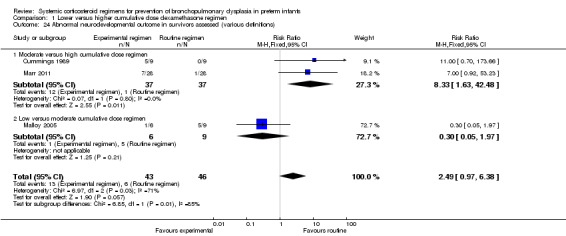
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 24 Abnormal neurodevelopmental outcome in survivors assessed (various definitions).
1.25. Analysis.
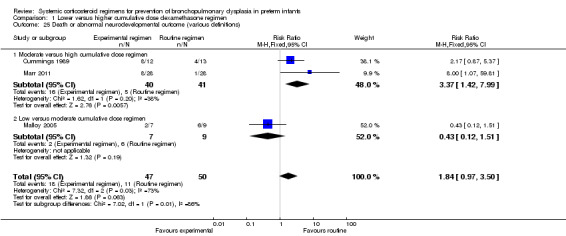
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 25 Death or abnormal neurodevelopmental outcome (various definitions).
Later (experimental arm) versus earlier (control arm) initiation of dexamethasone (Comparison 2)
Primary outcome
Combined outcome of death or BPD at 36 weeks' PMA
The combined outcome of death or BPD at 36 weeks' PMA showed no difference between the allocation arms. The quality of evidence was graded very low because of the small number of events, and the risk of performance and detection bias in all three trials and unclear selection bias in one trial (Table 2).
Secondary outcomes
Mortality at 28 days' PNA, 36 weeks' PMA and at hospital discharge
No differences were found on mortality at 28 days' PNA and 36 weeks' PMA. No data were retrieved for the outcome of mortality at hospital discharge.
BPD at 28 days' PNA and 36 weeks' PMA
Compared to the infants who were allocated to moderately early initiation, the infants allocated to delayed initiation had a higher incidence of the outcome BPD at 28 days' PNA (typical RR 1.15, 95% CI 1.05 to 1.26; NNTH 9, 95% CI 5 to 26) (Analysis 2.5). Furthermore, compared to the infants allocated in the early administration, the infants who were allocated in the moderately early group had a higher incidence of BPD at 36 weeks' PMA (typical RR 1.38, 95% CI 1.01 to 1.90; NNTH 11, 95% CI 6 to 333) (Analysis 2.6).
2.5. Analysis.
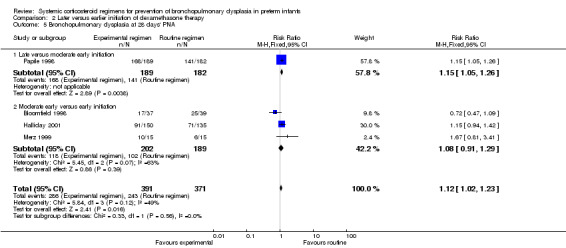
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 5 Bronchopulmonary dysplasia at 28 days' PNA.
2.6. Analysis.
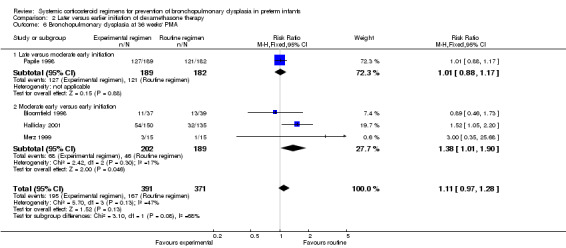
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 6 Bronchopulmonary dysplasia at 36 weeks' PMA.
Short‐term outcomes
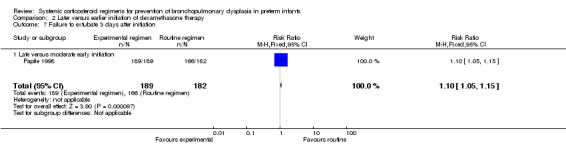
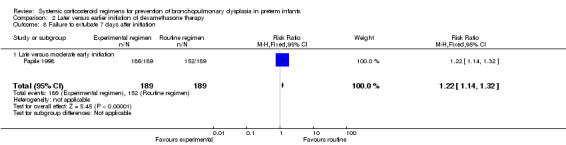
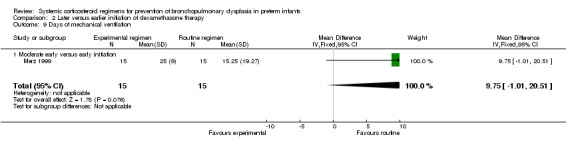
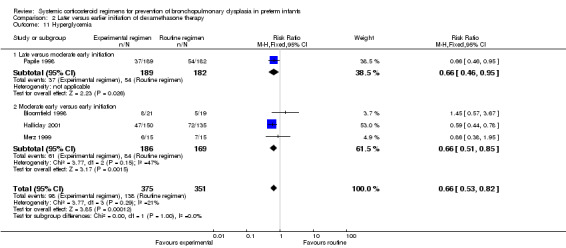
Compared to moderately early initiation, delayed initiation resulted in a significant reduction in the number of infants failing extubation at day 3 and day 7 in the only trial reporting this outcome (Analysis 2.7; Analysis 2.8). The single trial publishing data on the duration of mechanical ventilation showed no significant difference between early administration and moderately early administration (Analysis 2.9). No data were reported on the outcome of supplemental days of oxygen, PVL and clinically suspected infections. The incidence of hypertension, gastrointestinal perforation, NEC, IVH, or ROP was not significantly different between any of the allocation arms. Compared to the infants allocated to the earlier administration arm, the infants allocated to the later initiation arm had a lower incidence of hyperglycemia (typical RR 0.66, 95% CI 0.53 to 0.82; NNTB 8, 95% CI 5.0 to 15.7) (Analysis 2.11). Compared to the infants allocated to the moderate early dexamethasone initiation, the infants allocated to delayed initiation showed a lower incidence of the outcomes of culture‐proven infection (typical RR 0.67, 95% CI 0.54 to 0.84; NNTH 6, 95% CI 3.50 to 12.00) and gastrointestinal hemorrhage (typical RR 0.60, 95% CI 0.38 to 0.95; NNTH 12, 95% CI 6.0 to 98.5) (Analysis 2.12; Analysis 2.13). Compared to the infants allocated to the early initiation group, the infants allocated to the moderately early initiation arm had an increased risk of a PDA requiring therapy (typical RR of 1.74, 95% CI 1.32 to 22.29; NNTH 5, 95% CI 2.80 to 7.60) (Analysis 2.16). Furthermore, more open label rescue therapy was given in case of delayed initiation (typical RR 1.71, 95% CI 1.04 to 2.81; NNTH 25, 95% CI 12.50 to 462.4) (Analysis 2.18)).
2.7. Analysis.
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 7 Failure to extubate 3 days after initiation.
2.8. Analysis.
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 8 Failure to extubate 7 days after initiation.
2.9. Analysis.
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 9 Days of mechanical ventilation.
2.11. Analysis.
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 11 Hyperglycemia.
2.12. Analysis.
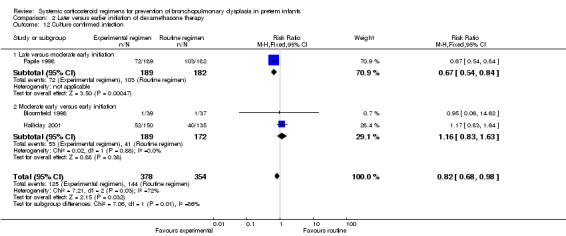
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 12 Culture confirmed infection.
2.13. Analysis.
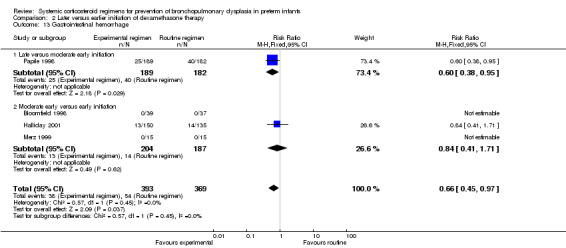
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 13 Gastrointestinal hemorrhage.
2.16. Analysis.
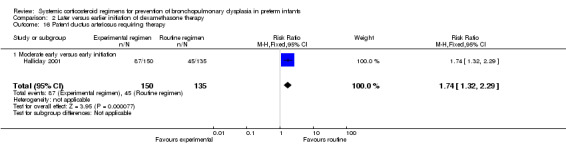
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 16 Patent ductus arteriosus requiring therapy.
2.18. Analysis.
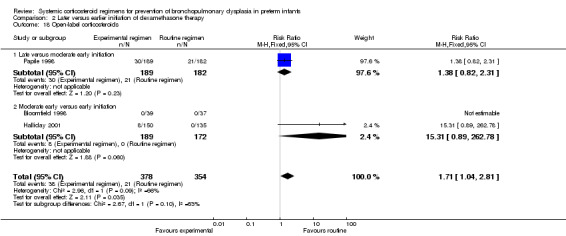
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 18 Open‐label corticosteroids.
Neurodevelopmental sequelae
Two studies investigating early versus moderately early initiation of dexamethasone reported long‐term neurodevelopmental outcomes using various definitions. Analysis showed no significant differences in the incidence in these outcomes between both allocation arms. No data were reported on the Mental Developmental Index of the Bayley Scales of Infant Development in these trials. The composite outcome of death or long‐term neurodevelopmental outcomes showed no difference. The quality of evidence was graded very low because of the small number of events, and the risk of performance and detection bias and unclear selection bias (Table 2).
Pulse therapy (experimental arm) versus continuous tapered (control arm) dosage regimens of dexamethasone (Comparison 3)
Primary outcome
Combined outcome death or BPD at 36 weeks' PMA
Compared to the infants allocated to the continuous tapered dosage regimen, the infants allocated to pulse therapy showed a significant increase in the incidence of the combined outcome of death or BPD at 36 weeks' PMA (typical RR 1.38, 95% CI 1.02 to 1.88; NNTH 7, 95% CI 4, 155) (Analysis 3.1). The quality of evidence was graded low because of the small number of events, and the risk of performance and detection bias in one trial and potential publication bias of one trial (Table 3).
3.1. Analysis.
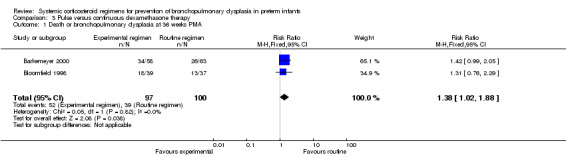
Comparison 3 Pulse versus continuous dexamethasone therapy, Outcome 1 Death or bronchopulmonary dysplasia at 36 weeks PMA.
Secondary outcomes
Mortality at 28 days, 36 weeks' PMA and at hospital discharge
No significant differences were found between the two allocation arms in the outcome of mortality at any time point.
BPD at 28 days' PNA and at 36 weeks' PMA
Compared to the infants allocated to the continuous tapered dosage therapy, infants who were allocated to the pulse‐dosage regimen had no significant difference in the outcomes of BPD at 28 days' PNA or 36 weeks' PMA.
Short‐term outcomes
No data could be retrieved on the outcomes of failure to extubate, days of mechanical ventilation or supplemental oxygen, IVH (any grade), PVL, gastrointestinal perforation, cardiac hypertrophy or adrenal suppression. No differences between the two allocation arms were found for the outcomes hyperglycemia, hypertension, culture‐proven or clinically suspected infection, gastrointestinal hemorrhage, NEC, IVH above grade II, and ROP. The use of open label was similar in both groups in the trial providing this information.
Neurodevelopmental sequelae
Follow‐up was only performed in one trial, which showed no difference in abnormal neurodevelopmental outcome alone or combined with death. No data were reported on Bayley Scales of Infant Development or cerebral palsy outcomes in this trial. The quality of evidence was graded very low because of the small number of events, and the risk of performance and detection bias in one trial and potential publication bias of one trial (Table 3).
Individual tailored (experimental arm) versus continuous tapered (control arm) dosage regimens of dexamethasone (Comparison 4)
Primary outcome
Combined outcome death or BPD at 36 weeks' PMA
Compared to the infants who were allocated to the continuous tapered regimen, the infants who were allocated to the individual tailored dosage regimen had no significant difference in the incidence of the outcome of combined death or BPD at 36 weeks' PMA. The quality of evidence was graded very low because of the small number of events, and the risk of performance and detection bias (Table 4).
Secondary outcomes
Mortality at 28 days' PNA, 36 weeks' PMA and at hospital discharge
No differences were found in mortality at 28 days' PNA and 36 weeks' PMA in this comparison of individual tailored versus continuous tapered dosage regimens.
BPD at 28 days' PNA and 36 weeks' PMA
Compared to the infants who were allocated to the continuous tapered regimens, the infants who were allocated to the individual tailored dosage regimens showed no significant difference in the incidence of the outcome BPD at 28 days' PNA or 36 weeks' PMA.
Short‐term outcomes
The predefined outcomes of failure to extubate, days of supplemental oxygen, clinically suspected infection, PDA, cardiac hypertrophy or PVL were not reported in these studies. Compared to the infants who were allocated to the continuous tapered regimen, the infants who were allocated to the individualized tailored dosage regimen had no significant difference in the incidence of the outcomes of culture‐proven infection and IVH above grade II. The only reported short‐term outcome showing a difference was mechanical ventilation. Compared to the infants allocated to the continuous tapered regimen, the infants who were allocated to the individualized tailored dosage regimen had a significantly decreased duration of mechanical ventilation (MD 7.50, 95% CI 2.20 to 12.80) (Analysis 4.9).
4.9. Analysis.
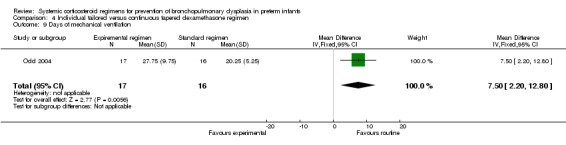
Comparison 4 Individual tailored versus continuous tapered dexamethasone regimen, Outcome 9 Days of mechanical ventilation.
Neurodevelopmental sequelae
The included studies reporting in this comparison did not show any difference in the outcomes of abnormal neurodevelopmental outcome, defined as either a Bayley mental score greater than 2 SD below the mean, bilateral blindness, sensorineural deafness requiring hearing aids or the presence of severe cerebral palsy alone or in combination with death. The quality of evidence was graded very low because of the small number of events, and the risk of performance and detection bias (Table 4).
Discussion
It has been proven in RCTs that corticosteroids reduce the combined outcome of death or BPD at 36 weeks' PMA. However, concerns have risen about negative long‐term neurodevelopmental effects of this therapy. Despite the firm recommendations of several pediatric societies to stop using postnatal systemic dexamethasone outside the realm of randomized clinical trials, clinicians are still using dexamethasone to treat ventilator‐dependent preterm infants. Therefore, attempts to identify the optimal corticosteroid treatment regimen remain clinically relevant and important. Questions that need to be answered are: 1) what is the optimal time to start corticosteroid treatment; 2) what is optimal cumulative dose; 3) what is the optimal duration of therapy; 4) what is the optimal corticosteroid to use? This systematic review summarizes all published studies that have investigated the impact of various corticosteroid treatment regimens on the incidence of the combined outcome of death or BPD and the risk of adverse effects on neurodevelopment.
Summary of main results
Four types of interventions are summarized in this review. The first intervention summarized eight RCTs (n = 303) investigating a lower versus a higher dose of dexamethasone. The absolute dexamethasone dose used to contrast a higher versus a lower dose varied considerably between the included trials. This heterogeneity in dose contrast precluded a pooled analysis of all available trials. For this reason, the studies were divided into a high‐range contrast subgroup, comparing a high cumulative dose (> 4 mg/kg) to a moderate dose (2 to 4 mg/kg) and a low‐range contrast subgroup, comparing a moderate to a low cumulative dose (< 2 mg/kg). We would like to emphasize that the terms 'high', 'moderate', and 'low' should be interpreted from a relative perspective, because compared to the physiological levels of corticosteroids all reported doses are supraphysiological (i.e. 'high'). The analyses showed no outcome differences when contrasting a moderate to a low dexamethasone dose. However, compared to a moderate dose, a high dexamethasone dose significantly reduced risk of failure to extubate, prolonged duration of mechanical ventilation, BPD at 36 weeks' PMA, and the combined outcome of death or BPD at 36 weeks' PMA.
This finding is consistent with a previous meta‐analysis assessing the impact of (different) cumulative dexamethasone doses used in placebo‐controlled trials (Onland 2009). We can only speculate on the possible explanations for this finding. First, the a priori risk of BPD might have been different between the comparisons, considering that one of the studies in the high‐range contrast comparison was performed in the pre‐surfactant era, and another study in this comparison included infants with a quite low birth weight and gestational age. Both factors are known BPD risk factors. Second, the use of additional ('rescue') dexamethasone treatment outside the study protocol by infants in both allocation arms was only observed in the studies comparing a moderate to low cumulative dose. This could well have resulted in an underestimation of the true treatment effect in these trials (Onland 2010). Finally, these results may also suggest that a relatively low cumulative dexamethasone dose as used in the low‐range contrast comparison is, in a pharmacodynamic sense, not sufficient to change the rate of BPD and hence any contrast in this dosing range will not result in a group difference in BPD.
This review also suggests that the benefit of high‐dose dexamethasone on pulmonary outcome is not outweighed by an increased risk of neurodevelopmental impairment. It even suggests that, compared to a moderate cumulative dose, neurodevelopment might be improved in the infants treated with a high dose, although this finding should be interpreted cautiously for the following reasons. First, the improvement was not seen in the outcomes of cerebral palsy, Bayley MDI, and visual impairment. Second, the low a priori chance of adverse neurodevelopmental outcomes in combination with the relatively small number of included infants in this review might not be sufficient to detect small but clinically relevant treatment effects on these outcomes. Third, the number of infants lost to follow‐up was more than 10% in two of the three studies, which might have biased the results, since children with cerebral palsy are especially difficult to follow up. A possible benefit of high‐dose dexamethasone on neurodevelopmental outcome might be mediated by the reduced duration of mechanical ventilation and the reduced risk of BPD. Both these outcomes are associated with an increased risk of neurodevelopmental impairment and may, in the high‐risk infant, override a possible direct toxic effect of dexamethasone on the brain (Doyle 2005; Ehrenkranz 2005; Walsh 2005; Doyle 2014).
The second intervention in this review, contrasting an earlier versus a later initiation of therapy, showed conflicting results. The subgroup analyses comparing trials that started corticosteroids within the first week to trials starting after the first week of life showed a decreased risk of BPD when treatment was initiated earlier. This beneficial effect of early treatment did not come at the expense of an increased risk of adverse neurodevelopmental outcome, as reported in the meta‐analysis of placebo‐controlled trials starting corticosteroids in the first week of life (Doyle 2014a). However, it is important to emphasize that only two studies performed a head‐to‐head comparison of early versus moderately early dexamethasone treatment, and included a small number of participants.
Analyses of primary comparisons including trials investigating late‐initiated dexamethasone versus initiation in the moderately early period revealed no benefits on long‐term pulmonary outcomes. Although postponing the start of dexamethasone treatment did reduce the risk of hypertension and culture‐proven sepsis, data on long‐term neurodevelopmental outcomes were not reported. These results are in contrast with the meta‐analyses of the placebo‐controlled trials, showing a lower number needed to treat to benefit (NNTB) for reducing BPD when starting treatment moderately early compared to delayed administration (Schmidt 2008; Onland 2009).
The third intervention summarized in this review involved studies exploring the effect of a pulse‐dosing regimen on both the beneficial and adverse effects of dexamethasone treatment. These analyses showed a pulse‐dosing regimen increased the risk of the combined outcome death or BPD compared with a continuous‐dosing regimen. Although speculative, it might be that the ongoing inflammatory response causing the development of BPD will not be suppressed by a pulse therapy regimen, which incorporated a seven‐day treatment pause.
Finally, tailoring the dexamethasone dose to the individual pulmonary response of the infant seems a logical approach, since there is a wide spectrum of lung damage in preterm infants. More inflamed and damaged lungs could theoretically benefit from a higher cumulative corticosteroid dose. To date, only two trials including a small number of infants have investigated this contrast, with no difference in the primary or secondary outcomes.
Overall completeness and applicability of evidence
Although the funnel plot analyses of the primary outcomes did not reveal potential publication bias, we cannot rule out that other small RCTs were performed, but not published. Several studies were only published as abstracts, limiting methodological assessment and data on the primary and secondary outcomes. Another major problem of this review is that even when the full text was published, not every trial reported on our stated primary and secondary outcomes. Specifically, few studies reported on neurodevelopmental outcome parameters, and those that did used various definitions or assessed neurodevelopment at different points in time. Although we pooled the data as if they were homogeneous, this clinical heterogeneity might compromise the validity of the results of our meta‐analysis. It remains unclear how this influences the conclusions of this review. We could not perform the previously mentioned subgroup analyses, i.e. according to gestational age and respiratory status at trial entry, due to lack of data or heterogeneity between the trials on these clinical characteristics.
Quality of the evidence
Except for the trials only published as abstract for which assessment of potential biases was not possible, the risk of bias in the trials was fair to good and will probably not influence the results. However, the overall quality of the evidence provided by the meta‐analyses using the GRADE approach for each outcome was assessed as low to very low due to several severe study limitations, such as risk of bias, potential publication bias, and imprecision of effect estimates. First, as discussed earlier, the sample size of these analyses was small, resulting in inadequate power to detect small but clinically relevant differences in some of the important outcome parameters. Second, although most studies contrasted two dosing regimens of dexamethasone, there was considerable diversity in the study designs, like the cumulative dexamethasone dose used in both arms, the starting dose and the duration of therapy. It remains unclear if and how these differences affect the observed treatment effect in the different interventions. Third, the use of late 'rescue' corticosteroids outside the study protocol was considerable in the majority of the trials and this may have confounded the true dexamethasone treatment effect. However, the fact that contamination was not present in the high‐range contrast subgroup indicates that the observed reduction in BPD in this subgroup in favor of the higher dexamethasone dose was indeed a true dose‐dependent treatment effect.
Potential biases in the review process
None to report.
Agreements and disagreements with other studies or reviews
The previous systematic review investigating the effect of different dosage regimens on the outcome BPD was published in 2008 (Onland 2008). The conclusion on the pulmonary outcome remains unchanged. However, that review did not include the abstract of Marr 2011. Including that study into the current review changed the long‐term neurodevelopmental outcome, showing a significantly reduced risk when administrating a higher‐dosage regimen. These results do not support the recommendation of international guidelines proclaiming that steroids should be dosed as short and low as possible (AAP 2002; Watterberg 2010). No previous reviews are published investigating the differences in the initiation of therapy, and the use of alternative dosage regimens or drugs.
Authors' conclusions
Implications for practice.
The present review includes all studies that to date have investigated two different steroid treatment regimens. All of these studies used the corticosteroid dexamethasone. Despite the fact that some studies reported a modulating effect of treatment regimens in favor of higher‐dosage regimens on the incidence of BPD and neurodevelopmental impairment, recommendations on the optimal type of corticosteroid, the optimal dosage, or the optimal timing of initiation for the prevention of BPD in preterm infants cannot be made based on the current level of evidence. Furthermore, the results of this review do not justify a change in the recommendation published in international guidelines of corticosteroid use. A well‐designed large RCT is urgently needed to establish the optimal systemic postnatal corticosteroid dosage regimen.
Implications for research.
In light of the ongoing use of dexamethasone in the clinical setting, we feel that an RCT on dexamethasone dose and timing is justified and urgently needed. A large multicenter study with a factorial design is needed to provide evidence on the optimal use of dexamethasone. This trial should compare a higher cumulative dexamethasone dose with a lower dose, as well as timing of initiation using a factorial study design. Although the current evidence prevents firm recommendations, the present review suggests contrasting the dexamethasone dose in the higher ranges. The trial should be adequately powered to detect small but clinically relevant treatment effects and interaction between dose and timing of initiation. It should include ventilated preterm infants with a high risk for BPD based on the known determinants in the development of BPD. The time window to initiate dexamethasone treatment between 7 days and 14 days after birth should be compared with initiation after that time period. We recommend that data on the following primary outcome parameters be collected in any future comparative study: BPD at 36 weeks' PMA, mortality at 36 weeks' PMA and at discharge, and neurodevelopmental outcome using predefined definitions, standardized diagnostic tests and time points. In addition, short‐term benefits (time of extubation, ventilation time) and adverse effects (hypertension, sepsis, and hyperglycemia) should be reported as secondary outcomes. Various threats to the internal validity of the trial should be recognized and contained. For example, dilution of treatment effect due to the use of 'rescue' corticosteroids outside the study protocol, or crossing over between trial arms should be avoided as much as possible. In any event, additional treatments should be adequately reported in order to assess the possibility of confounding.
Notes
Part of this systematic review on one of the comparisons has been published before (Onland 2008).
Acknowledgements
Colleen Ovelman and Yolanda Brosseau kindly assisted with the literature search of the different databases. The authors thank Dr JK Muraskas, Loyola University Medical Center, Dr M Durand, Los Angeles County‐University of Southern California Medical Center, Dr C McEvoy, Oregon Health Sciences University, Dr CA Malloy, Children’s Memorial Hospital, Northwestern University's Feinberg School of Medicine, Dr BM Barkemeyer, LSU Health Sciences Center, New Orleans and Dr JJ Cummings, Brody School of Medicine, East Carolina University, for providing us with additional data and thoughtful review of the draft.
Appendices
Appendix 1. Standard search methodology
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh])) Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial) CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) Cochrane Library: (infant or newborn or neonate or neonatal or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. Risk of bias tool
The following issues were evaluated and entered into the risk of bias table:
Adequate sequence generation? For each included study, we categorized the risk of selection bias as
low risk ‐ adequate (any truly random process, e.g. random number table; computer random number generator
high risk ‐ inadequate (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number)
unclear risk ‐ no or unclear information provided.
Allocation concealment? For each included study, we categorized the risk of bias regarding allocation concealment as
low risk ‐ adequate (e.g. telephone or central randomizations; consecutively numbered sealed opaque envelopes);
high risk ‐ inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk ‐ no or unclear information provided.
-
Blinding?
-
Performance bias? For each included study, we categorized the methods used to blind study personnel from knowledge of which intervention a participant received (as our study population consists of neonates, they are all blinded to the study intervention).
low risk ‐ adequate for personnel (a placebo that could not be distinguished from the active drug was used in the control group);
high risk ‐ inadequate ‐ personnel aware of group assignment;
unclear risk ‐ no or unclear information provide.
-
Detection bias? For each included study, we categorized the methods used to blind outcome assessors from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorized the methods used with regards to detection bias as:
low risk ‐ adequate; follow‐up was performed with assessors blinded to group assignment;
high risk ‐ inadequate; assessors at follow‐up were aware of group assignment;
unclear risk ‐ no or unclear information provided.
-
Incomplete data addressed (attrition bias)? For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods with respect to the risk attrition bias as:
low risk ‐ adequate (< 10% missing data);
high risk ‐ inadequate (>10% missing data);
unclear risk ‐ no or unclear information provided
-
Free of selective reporting (reporting bias)? For each included study, we investigated the risk of selective outcome reporting bias and what we found. We assessed the methods as:
low risk ‐ adequate (where it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk ‐ inadequate (where not all the study's pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so cannot be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk ‐ no or unclear information provided (the study protocol was not available).
-
Free of other bias? For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk ‐ no concerns of other bias raised;
high risk ‐ concerns raised about multiple looks at the data with the results made known to the investigators, difference in number of patients enrolled in abstract and final publications of the paper;
unclear ‐ concerns raised about potential sources of bias that could not be verified by contacting the authors
Overall risk of bias
Explicit judgments were made about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The magnitude and direction of the bias was assessed and the possible impact on the findings. The impact of the level of bias was explored through undertaking sensitivity analyses ‐ see Sensitivity analysis. If necessary, the original investigators were asked to provide additional information.
Data and analyses
Comparison 1. Lower versus higher cumulative dose dexamethasone regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or bronchopulmonary dysplasia at 36 weeks PMA | 6 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.82, 1.44] |
| 1.1 Moderate versus high cumulative dose regimen | 2 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.00, 1.82] |
| 1.2 Low versus moderate cumulative dose regimen | 4 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.50, 1.40] |
| 2 Mortality at 36 weeks' PMA | 7 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.38, 1.92] |
| 2.1 Moderate versus high cumulative dose regimen | 3 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.51, 4.55] |
| 2.2 Low versus moderate cumulative dose regimen | 4 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.11, 1.63] |
| 3 Mortality at hospital discharge | 7 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.52, 1.91] |
| 3.1 Moderate versus high cumulative dose regimen | 3 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.68, 3.23] |
| 3.2 Low versus moderate cumulative dose regimen | 4 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.11, 1.63] |
| 4 Bronchopulmonary dysplasia at 36 weeks' PMA | 6 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.93, 1.82] |
| 4.1 Moderate versus high cumulative dose regimen | 2 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.01, 2.22] |
| 4.2 Low versus moderate cumulative dose regimen | 4 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.65, 1.95] |
| 5 Failure to extubate 3 days after initiation | 4 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.93, 1.32] |
| 5.1 Moderate versus high cumulative dose regimen | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.70, 1.39] |
| 5.2 Low versus moderate dose regimen | 3 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.93, 1.39] |
| 6 Failure to extubate 7 days after initiation | 5 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.05, 1.68] |
| 6.1 Moderate versus high cumulative dose regimen | 2 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.03, 3.14] |
| 6.2 Low versus moderate cumulative dose regimen | 3 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.94, 1.56] |
| 7 Days of mechanical ventilation | 6 | 217 | Mean Difference (IV, Fixed, 95% CI) | 4.98 [0.47, 9.49] |
| 7.1 Moderate versus high cumulative dose regimen | 3 | 111 | Mean Difference (IV, Fixed, 95% CI) | 7.41 [1.43, 13.39] |
| 7.2 Low versus moderate cumulative dose regimen | 3 | 106 | Mean Difference (IV, Fixed, 95% CI) | 1.77 [‐5.09, 8.64] |
| 8 Days on supplemental oxygen | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐25.29, 21.29] |
| 8.1 Low versus moderate cumulative dose regimen | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐25.29, 21.29] |
| 9 Hypertension | 5 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.11, 0.79] |
| 9.1 Moderate versus high cumulative dose regimen | 2 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 4.36] |
| 9.2 Low versus moderate cumulative dose regimen | 3 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.11, 0.87] |
| 10 Hyperglycemia | 5 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.37, 0.97] |
| 10.1 Moderate versus high cumulative dose regimen | 2 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.47, 1.46] |
| 10.2 Low versus moderate cumulative dose regimen | 3 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.17, 0.93] |
| 11 Culture confirmed infection | 6 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.60, 1.38] |
| 11.1 Moderate versus high cumulative dose regimen | 2 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.62, 2.42] |
| 11.2 Low versus moderate cumulative dose regimen | 4 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.46, 1.32] |
| 12 Clinical suspected infection | 2 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.62, 1.70] |
| 12.1 Moderate versus high cumulative dose regimen | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.71, 2.09] |
| 12.2 Low versus moderate cumulative dose regimen | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.32, 2.08] |
| 13 Gastrointestinal hemorrhage | 2 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Moderate versus high cumulative dose regimen | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Low versus moderate cumulative dose regimen | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Gastrointestinal perforation | 3 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.13, 6.28] |
| 14.1 Low versus moderate cumulative dose regimen | 3 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.13, 6.28] |
| 15 Necrotizing enterocolitis | 3 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.18, 1.56] |
| 15.1 Moderate versus high cumulative dose regimen | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.23, 3.19] |
| 15.2 Low versus moderate cumulative dose regimen | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.03, 1.97] |
| 16 Intraventricular hemorrhage (> grade II) | 2 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.48, 3.67] |
| 16.1 Moderate versus high cumulative dose regimen | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.27, 4.37] |
| 16.2 Low versus moderate cumulative dose regimen | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.37, 7.67] |
| 17 Periventricular leukomalacia (PVL) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.13, 5.85] |
| 17.1 Low versus moderate cumulative dose regimen | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.13, 5.85] |
| 18 Open‐label corticosteroids | 6 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.57, 1.14] |
| 18.1 Moderate versus high cumulative dose regimen | 2 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Low versus moderate cumulative dose regimen | 4 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.57, 1.14] |
| 19 Severe retinopathy of prematurity | 4 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.26, 1.23] |
| 19.1 Moderate versus high cumulative dose regimen | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.04, 2.10] |
| 19.2 Low versus moderate cumulative dose regimen | 3 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.28, 1.57] |
| 20 Cerebral palsy in survivors assessed | 3 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [0.76, 6.49] |
| 20.1 Moderate versus high cumulative dose regimen | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.0 [0.70, 173.66] |
| 20.2 Low versus moderate cumulative dose regimen | 2 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.29, 4.00] |
| 21 Death or cerebral palsy | 3 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.65, 2.46] |
| 21.1 Moderate versus high cumulative dose regimen | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.87, 5.37] |
| 21.2 Low versus moderate dose regimen | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.28, 2.18] |
| 22 Bayley's MDI < 2 SD | 3 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.47, 2.37] |
| 22.1 Moderate versus high cumulative dose regimen | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.69 [0.58, 163.02] |
| 22.2 Low versus moderate cumulative dose regimen | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.23, 1.60] |
| 23 Severe blindness | 4 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.05, 1.98] |
| 23.1 Moderate versus high cumulative dose regimen | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Low versus moderate cumulative dose regimen | 3 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.05, 1.98] |
| 24 Abnormal neurodevelopmental outcome in survivors assessed (various definitions) | 3 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.49 [0.97, 6.38] |
| 24.1 Moderate versus high cumulative dose regimen | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.33 [1.63, 42.48] |
| 24.2 Low versus moderate cumulative dose regimen | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.05, 1.97] |
| 25 Death or abnormal neurodevelopmental outcome (various definitions) | 3 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.97, 3.50] |
| 25.1 Moderate versus high cumulative dose regimen | 2 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.37 [1.42, 7.99] |
| 25.2 Low versus moderate cumulative dose regimen | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.12, 1.51] |
1.8. Analysis.
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 8 Days on supplemental oxygen.
1.11. Analysis.
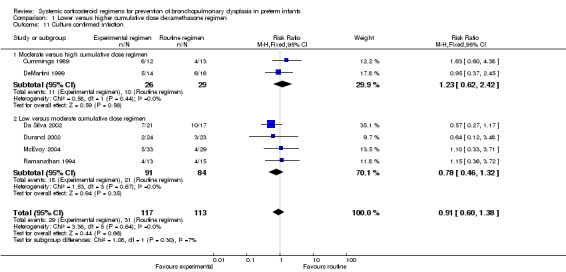
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 11 Culture confirmed infection.
1.12. Analysis.
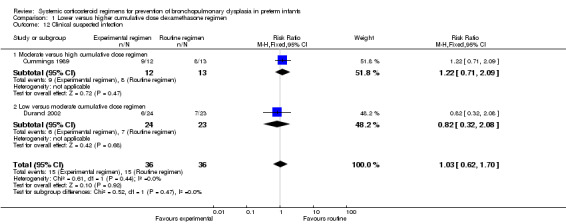
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 12 Clinical suspected infection.
1.13. Analysis.
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 13 Gastrointestinal hemorrhage.
1.14. Analysis.
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 14 Gastrointestinal perforation.
1.15. Analysis.
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 15 Necrotizing enterocolitis.
1.16. Analysis.
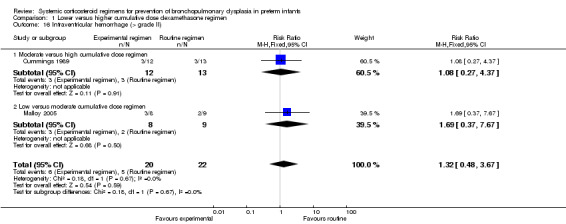
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 16 Intraventricular hemorrhage (> grade II).
1.17. Analysis.
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 17 Periventricular leukomalacia (PVL).
1.18. Analysis.
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 18 Open‐label corticosteroids.
1.19. Analysis.
Comparison 1 Lower versus higher cumulative dose dexamethasone regimen, Outcome 19 Severe retinopathy of prematurity.
Comparison 2. Later versus earlier initiation of dexamethasone therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or bronchopulmonary dysplasia at 36 weeks' PMA | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.87, 1.29] |
| 1.1 Late versus moderate early initiation | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Moderate early versus early initiation | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.87, 1.29] |
| 2 Mortality at 28 days' PNA | 4 | 762 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.69, 1.47] |
| 2.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.93, 5.23] |
| 2.2 Moderate early versus early initiation | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.51, 1.20] |
| 3 Mortality at 36 weeks' PMA | 4 | 762 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.68, 1.28] |
| 3.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.83, 2.62] |
| 3.2 Moderate early versus early initiation | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.49, 1.07] |
| 4 Mortality at hospital discharge | 4 | 762 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.72, 1.31] |
| 4.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.83, 2.62] |
| 4.2 Moderate early versus early initiation | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.56, 1.14] |
| 5 Bronchopulmonary dysplasia at 28 days' PNA | 4 | 762 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [1.02, 1.23] |
| 5.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.05, 1.26] |
| 5.2 Moderate early versus early initiation | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.91, 1.29] |
| 6 Bronchopulmonary dysplasia at 36 weeks' PMA | 4 | 762 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.97, 1.28] |
| 6.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.88, 1.17] |
| 6.2 Moderate early versus early initiation | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.01, 1.90] |
| 7 Failure to extubate 3 days after initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [1.05, 1.15] |
| 7.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [1.05, 1.15] |
| 8 Failure to extubate 7 days after initiation | 1 | 378 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.14, 1.32] |
| 8.1 Late versus moderate early initiation | 1 | 378 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.14, 1.32] |
| 9 Days of mechanical ventilation | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 9.75 [‐1.01, 20.51] |
| 9.1 Moderate early versus early initiation | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 9.75 [‐1.01, 20.51] |
| 10 Hypertension | 4 | 762 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.67, 1.47] |
| 10.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.72, 2.36] |
| 10.2 Moderate early versus early initiation | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.47, 1.34] |
| 11 Hyperglycemia | 4 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.53, 0.82] |
| 11.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.46, 0.95] |
| 11.2 Moderate early versus early initiation | 3 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.51, 0.85] |
| 12 Culture confirmed infection | 3 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.68, 0.98] |
| 12.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.54, 0.84] |
| 12.2 Moderate early versus early initiation | 2 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.83, 1.63] |
| 13 Gastrointestinal hemorrhage | 4 | 762 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.45, 0.97] |
| 13.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.38, 0.95] |
| 13.2 Moderate early versus early initiation | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.41, 1.71] |
| 14 Gastrointestinal perforation | 2 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.23, 2.40] |
| 14.1 Moderate early versus early initiation | 2 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.23, 2.40] |
| 15 Necrotizing enterocolitis | 4 | 725 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.82, 2.55] |
| 15.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.59, 5.07] |
| 15.2 Moderate early versus early initiation | 3 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.68, 2.61] |
| 16 Patent ductus arteriosus requiring therapy | 1 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.32, 2.29] |
| 16.1 Moderate early versus early initiation | 1 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.32, 2.29] |
| 17 Intraventricular hemorrhage (> grade II) | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.49, 11.48] |
| 17.1 Moderate early versus early initiation | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.49, 11.48] |
| 18 Open‐label corticosteroids | 3 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.04, 2.81] |
| 18.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.82, 2.31] |
| 18.2 Moderate early versus early initiation | 2 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.31 [0.89, 262.78] |
| 19 Retinopathy of prematurity (any) | 2 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.52, 1.23] |
| 19.1 Moderate early versus early initiation | 2 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.52, 1.23] |
| 20 Severe retinopathy of prematurity | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.63, 3.53] |
| 20.1 Moderate early versus early initiation | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.63, 3.53] |
| 21 Cerebral palsy in survivors assessed | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.43, 8.86] |
| 21.1 Moderate early versus early initiation | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.43, 8.86] |
| 22 Death or cerebral palsy | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.68, 1.84] |
| 22.1 Moderate early versus early initiation | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.68, 1.84] |
| 23 Severe blindness | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 Moderate early versus early initiation | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Abnormal neurodevelopmental outcome in survivors assessed (various definitions) | 2 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.66, 1.69] |
| 24.1 Moderate early versus early initiation | 2 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.66, 1.69] |
| 25 Death or abnormal neurodevelopmental outcome (various definitions) | 2 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.63, 1.21] |
| 25.1 Moderate early versus early | 2 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.63, 1.21] |
2.1. Analysis.
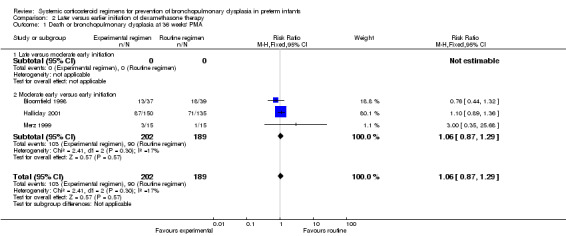
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 1 Death or bronchopulmonary dysplasia at 36 weeks' PMA.
2.2. Analysis.
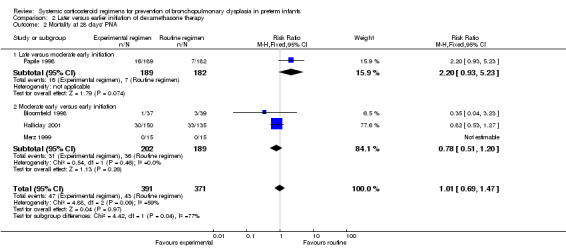
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 2 Mortality at 28 days' PNA.
2.3. Analysis.
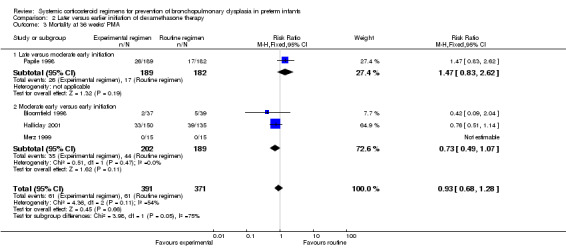
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 3 Mortality at 36 weeks' PMA.
2.4. Analysis.
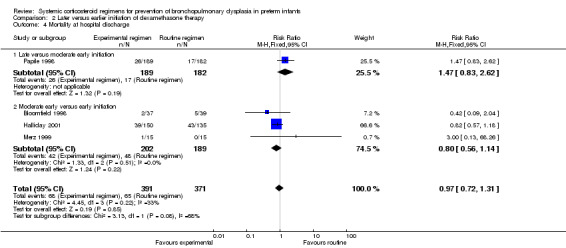
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 4 Mortality at hospital discharge.
2.10. Analysis.
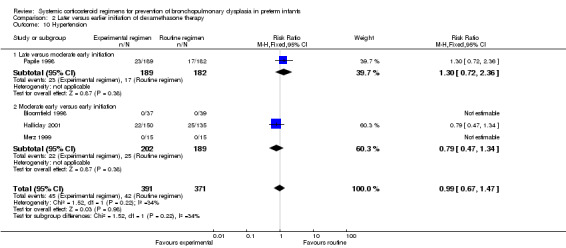
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 10 Hypertension.
2.14. Analysis.
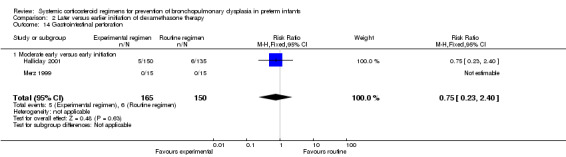
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 14 Gastrointestinal perforation.
2.15. Analysis.
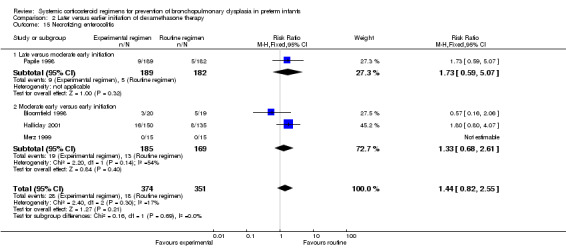
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 15 Necrotizing enterocolitis.
2.17. Analysis.
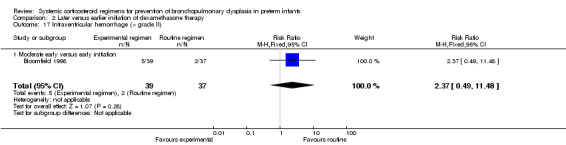
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 17 Intraventricular hemorrhage (> grade II).
2.19. Analysis.
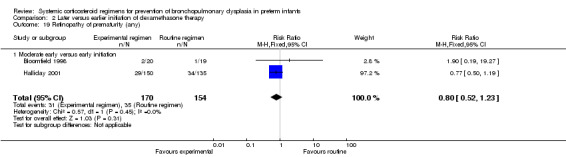
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 19 Retinopathy of prematurity (any).
2.20. Analysis.
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 20 Severe retinopathy of prematurity.
2.21. Analysis.
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 21 Cerebral palsy in survivors assessed.
2.22. Analysis.
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 22 Death or cerebral palsy.
2.23. Analysis.
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 23 Severe blindness.
2.24. Analysis.
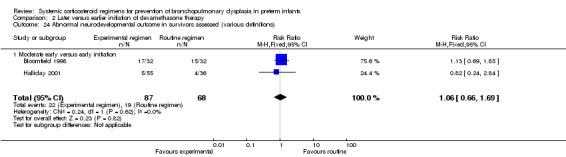
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 24 Abnormal neurodevelopmental outcome in survivors assessed (various definitions).
2.25. Analysis.
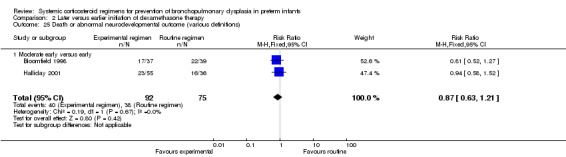
Comparison 2 Later versus earlier initiation of dexamethasone therapy, Outcome 25 Death or abnormal neurodevelopmental outcome (various definitions).
Comparison 3. Pulse versus continuous dexamethasone therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or bronchopulmonary dysplasia at 36 weeks PMA | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.02, 1.88] |
| 2 Mortality at 28 days PNA | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.31, 26.15] |
| 3 Mortality at 36 weeks PMA | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.72, 5.78] |
| 4 Mortality at hospital discharge | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.72, 5.78] |
| 5 Bronchopulmonary dysplasia at 28 days PNA | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.92, 2.13] |
| 6 Bronchopulmonary dysplasia at 36 weeks PMA | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.90, 1.83] |
| 7 Hypertension | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.20, 1.23] |
| 8 Hyperglycemia | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.71, 1.65] |
| 9 Culture confirmed infection | 1 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.87, 2.01] |
| 10 Clinical suspected infection | 1 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.70, 2.10] |
| 11 Gastrointestinal hemorrhage | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.25, 1.68] |
| 12 Necrotizing enterocolitis | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.33, 1.83] |
| 13 Intraventricular hemorrhage (> grade II) | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.49, 11.48] |
| 14 Open‐label corticosteroids | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.64, 1.47] |
| 15 Retinopathy of prematurity (any) | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.05, 5.34] |
| 16 Severe retinopathy of prematurity | 1 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.05, 1.07] |
| 17 Abnormal neurodevelopmental outcome in survivors assessed (various definitions) | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.54, 1.44] |
| 18 Death or abnormal neurodevelopmental outcome (various definitions) | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.79, 1.92] |
3.2. Analysis.
Comparison 3 Pulse versus continuous dexamethasone therapy, Outcome 2 Mortality at 28 days PNA.
3.3. Analysis.
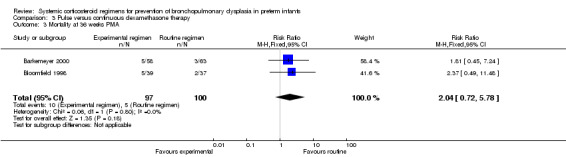
Comparison 3 Pulse versus continuous dexamethasone therapy, Outcome 3 Mortality at 36 weeks PMA.
3.4. Analysis.
Comparison 3 Pulse versus continuous dexamethasone therapy, Outcome 4 Mortality at hospital discharge.
3.5. Analysis.
Comparison 3 Pulse versus continuous dexamethasone therapy, Outcome 5 Bronchopulmonary dysplasia at 28 days PNA.
3.6. Analysis.
Comparison 3 Pulse versus continuous dexamethasone therapy, Outcome 6 Bronchopulmonary dysplasia at 36 weeks PMA.
3.7. Analysis.
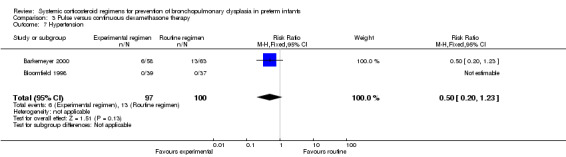
Comparison 3 Pulse versus continuous dexamethasone therapy, Outcome 7 Hypertension.
3.8. Analysis.
Comparison 3 Pulse versus continuous dexamethasone therapy, Outcome 8 Hyperglycemia.
3.9. Analysis.
Comparison 3 Pulse versus continuous dexamethasone therapy, Outcome 9 Culture confirmed infection.
3.10. Analysis.
Comparison 3 Pulse versus continuous dexamethasone therapy, Outcome 10 Clinical suspected infection.
3.11. Analysis.
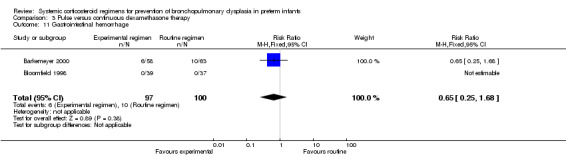
Comparison 3 Pulse versus continuous dexamethasone therapy, Outcome 11 Gastrointestinal hemorrhage.
3.12. Analysis.
Comparison 3 Pulse versus continuous dexamethasone therapy, Outcome 12 Necrotizing enterocolitis.
3.13. Analysis.
Comparison 3 Pulse versus continuous dexamethasone therapy, Outcome 13 Intraventricular hemorrhage (> grade II).
3.14. Analysis.
Comparison 3 Pulse versus continuous dexamethasone therapy, Outcome 14 Open‐label corticosteroids.
3.15. Analysis.
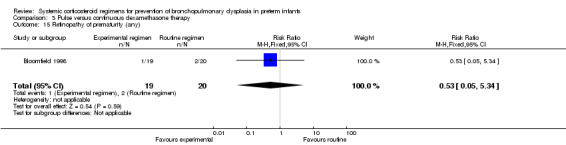
Comparison 3 Pulse versus continuous dexamethasone therapy, Outcome 15 Retinopathy of prematurity (any).
3.16. Analysis.
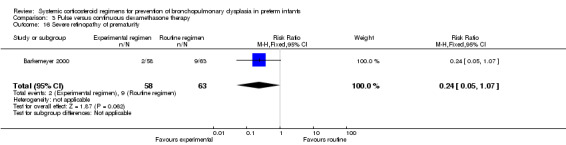
Comparison 3 Pulse versus continuous dexamethasone therapy, Outcome 16 Severe retinopathy of prematurity.
3.17. Analysis.
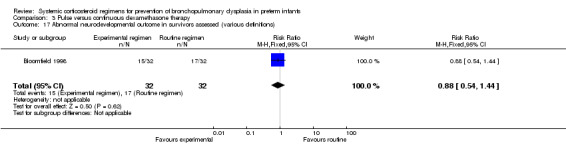
Comparison 3 Pulse versus continuous dexamethasone therapy, Outcome 17 Abnormal neurodevelopmental outcome in survivors assessed (various definitions).
3.18. Analysis.
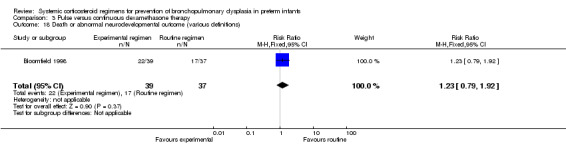
Comparison 3 Pulse versus continuous dexamethasone therapy, Outcome 18 Death or abnormal neurodevelopmental outcome (various definitions).
Comparison 4. Individual tailored versus continuous tapered dexamethasone regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or bronchopulmonary dysplasia at 36 weeks PMA | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.83, 1.66] |
| 2 Mortality at 28 days PNA | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.60, 13.32] |
| 3 Mortality at 36 weeks PMA | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.55, 3.64] |
| 4 Mortality at hospital discharge | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.63, 3.92] |
| 5 Bronchopulmonary dysplasia at 28 days PNA | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.88, 1.50] |
| 6 Bronchopulmonary dysplasia at 36 weeks PMA | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.68, 1.76] |
| 7 Hypertension | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Hyperglycemia | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.26, 1.66] |
| 9 Days of mechanical ventilation | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 7.5 [2.20, 12.80] |
| 10 Culture confirmed infection | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.12, 64.89] |
| 11 Gastrointestinal hemorrhage | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Necrotizing enterocolitis | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.48, 6.35] |
| 13 Intraventricular hemorrhage (> grade II) | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.62, 4.68] |
| 14 Open‐label corticosteroids | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Retinopathy of prematurity (any) | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.05, 5.34] |
| 16 Abnormal neurodevelopmental outcome in survivors assessed (various definitions) | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.57, 1.40] |
| 17 Death or abnormal neurodevelopmental outcome (various definitions) | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.81, 1.70] |
4.1. Analysis.
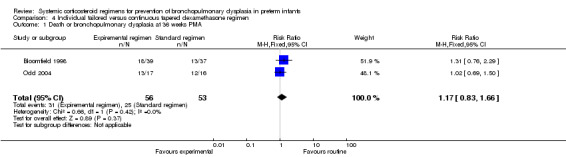
Comparison 4 Individual tailored versus continuous tapered dexamethasone regimen, Outcome 1 Death or bronchopulmonary dysplasia at 36 weeks PMA.
4.2. Analysis.
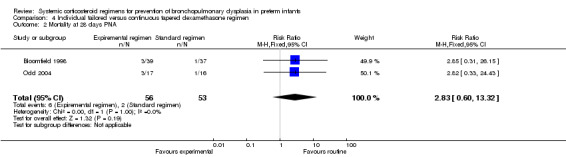
Comparison 4 Individual tailored versus continuous tapered dexamethasone regimen, Outcome 2 Mortality at 28 days PNA.
4.3. Analysis.
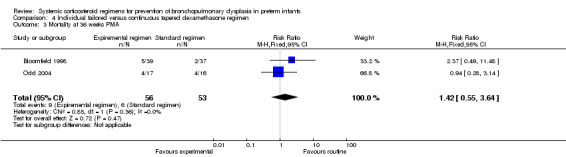
Comparison 4 Individual tailored versus continuous tapered dexamethasone regimen, Outcome 3 Mortality at 36 weeks PMA.
4.4. Analysis.
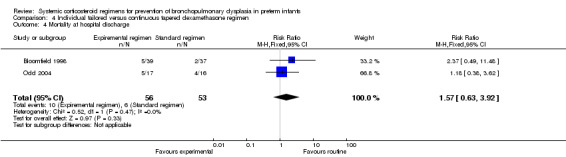
Comparison 4 Individual tailored versus continuous tapered dexamethasone regimen, Outcome 4 Mortality at hospital discharge.
4.5. Analysis.
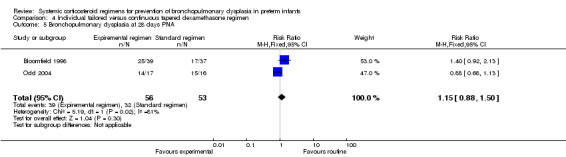
Comparison 4 Individual tailored versus continuous tapered dexamethasone regimen, Outcome 5 Bronchopulmonary dysplasia at 28 days PNA.
4.6. Analysis.
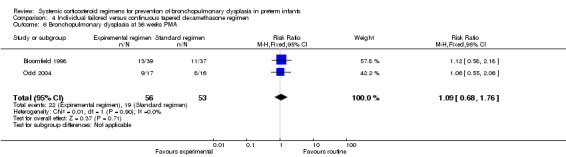
Comparison 4 Individual tailored versus continuous tapered dexamethasone regimen, Outcome 6 Bronchopulmonary dysplasia at 36 weeks PMA.
4.7. Analysis.
Comparison 4 Individual tailored versus continuous tapered dexamethasone regimen, Outcome 7 Hypertension.
4.8. Analysis.
Comparison 4 Individual tailored versus continuous tapered dexamethasone regimen, Outcome 8 Hyperglycemia.
4.10. Analysis.
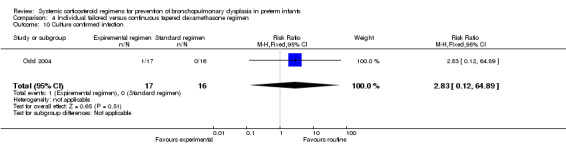
Comparison 4 Individual tailored versus continuous tapered dexamethasone regimen, Outcome 10 Culture confirmed infection.
4.11. Analysis.
Comparison 4 Individual tailored versus continuous tapered dexamethasone regimen, Outcome 11 Gastrointestinal hemorrhage.
4.12. Analysis.
Comparison 4 Individual tailored versus continuous tapered dexamethasone regimen, Outcome 12 Necrotizing enterocolitis.
4.13. Analysis.
Comparison 4 Individual tailored versus continuous tapered dexamethasone regimen, Outcome 13 Intraventricular hemorrhage (> grade II).
4.14. Analysis.
Comparison 4 Individual tailored versus continuous tapered dexamethasone regimen, Outcome 14 Open‐label corticosteroids.
4.15. Analysis.
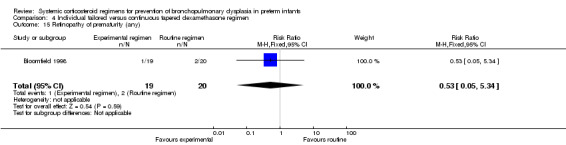
Comparison 4 Individual tailored versus continuous tapered dexamethasone regimen, Outcome 15 Retinopathy of prematurity (any).
4.16. Analysis.
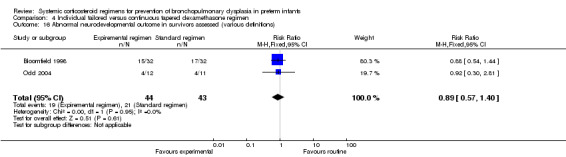
Comparison 4 Individual tailored versus continuous tapered dexamethasone regimen, Outcome 16 Abnormal neurodevelopmental outcome in survivors assessed (various definitions).
4.17. Analysis.
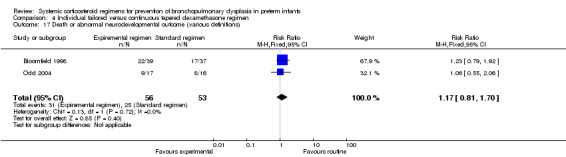
Comparison 4 Individual tailored versus continuous tapered dexamethasone regimen, Outcome 17 Death or abnormal neurodevelopmental outcome (various definitions).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barkemeyer 2000.
| Methods | Randomized controlled trial investigating a pulse‐dosage versus continuous‐dosage regimen. | |
| Participants | Infants were eligible for enrollment with birth weight < 1500 grams, a history of respiratory distress syndrome, and ventilator dependence at 7 to 21 days of life. Infants were excluded if significant anomalies of cardiac or respiratory systems, or clinically significant patent ductus arteriosus at time of enrollment. |
|
| Interventions | The infants were randomly assigned to 1 of 2 regimens.
All administrations were in 2 divided doses. |
|
| Outcomes | Primary endpoint of the study was survival of 36 weeks' PMA without the need for supplemental oxygen. Secondary endpoints included survival, days of mechanical ventilation, days of supplemental oxygen, and length of hospital stay. Potential side effects were evaluated included hyperglycemia, hypertension, infection, left ventricular hypertrophy, necrotizing enterocolitis, gastritis, abnormal head ultrasound, retinopathy of prematurity, growth delay, and leucocytosis. No long‐term neurodevelopmental outcomes were assessed (personal communication) | |
| Notes | Trial was only published as abstract. Original author provided unpublished manuscript with additional data on secondary outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator. |
| Allocation concealment (selection bias) | Low risk | Centralized random number generator program. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Only the pharmacists at the participating centers were aware of the randomization assignments, caregivers and parents were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Attending physicians were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis with no missing data. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were mentioned in the manuscript. |
| Other bias | Low risk | No concerns of other biases. |
Bloomfield 1998.
| Methods | Randomized controlled trial comparing a pulse course against high‐dosage regimen dexamethasone. | |
| Participants | Infants with a birth weight ≤ 1250 grams, and ventilated at ≥ 15 cycles/min at 7 days of age. Infants with major congenital malformations or who were ventilated for surgical reasons were excluded. |
|
| Interventions | The infants were randomly assigned to 1 of 2 regimens.
The initial dosage administration of 0.5 mg/kg/day was in 2 divided doses. |
|
| Outcomes | The primary outcome was linear growth, measured as weight gain, crown‐heel length, and head circumference. Secondary outcomes were hypertension, hyperglycemia requiring insulin therapy, necrotizing enterocolitis, retinopathy of prematurity, proven infections, myocardial hypertrophy, supplemental oxygen at 28 days' PNA and 36 weeks' PMA, BPD at 28 days' PNA and 36 weeks' PMA. In addition a Synacthen test was performed 1 week after discontinuation of the dexamethasone. The long‐term follow‐up manuscript reported on neurodevelopmental outcome with an extended inclusion rate. Infants were classified into 1 of 4 outcome categories defined and modified from Kitchen 1987. |
|
| Notes | Original authors provided additional data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By computer randomization. |
| Allocation concealment (selection bias) | Low risk | By computer randomization, no additional details. Randomizaton was balanced in blocks of 6 and stratified by sex and birth weight. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis. 1 infant was found to have a birth weight of > 1250 grams. 3 infants were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were mentioned in the manuscript. |
| Other bias | Low risk | No concerns of other biases. |
Cummings 1989.
| Methods | Single center, randomized, double‐blind, placebo‐controlled study investigating a moderate dosage versus a high dosage of dexamethasone. | |
| Participants | Preterm infants with a birth weight ≤ 1250 grams, a gestational age of ≤ 30 weeks, and a postnatal age of more than 14 days. All infants were ventilated with a rate of at least 15 cycles per minute and received more than 30% oxygen. Attempts to wean these settings failed over a period of at least 72 hours. Infants with a symptomatic PDA, renal failure or sepsis at entry were excluded. |
|
| Interventions | The included infants were randomly assigned to 1 of 3 dosage regimens.
Medication was given intravenously and divided into 2 dosages per day. Each infant received the same volume of medication by using different concentrations of dexamethasone. Infants in the low‐dosage regimen group received additional saline injections to complete the 42‐day course. The placebo group was excluded for the purpose of this review. No treatment with corticosteroids outside the protocol was allowed. |
|
| Outcomes | The primary outcomes were mortality, duration of mechanical ventilation and duration of oxygen dependence. Secondary outcomes were the duration of hospitalizations, ROP, bloody gastric aspirates, number of transfusions, and occurrence of clinically suspected sepsis, hypertension, hyperglycemia and hypertriglyceridemia. Growth and neurodevelopment (abnormal neurological outcome and the Bayley Scales of Infant Development) were assessed at 6 and 15 months of age corrected for prematurity. Normal neurodevelopmental outcome was defined as having Bayley Mental and Psychomotor Indexes of more than 84 and normal neurological findings (not specified). Further follow‐up studies were done at 4 years and 15 years. Neurological exams and the cognitive function using the McCarthy Scales of Children's Abilities were assessed at the age of 4, whereas at 15 years neurological examination, IQ and the need for specialized education was assessed. |
|
| Notes | The original investigator provided additional data on duration of mechanical ventilation, failure to extubate on day 7 and the total number of patients with a Bayley MDI < 2 SD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequential assignment by random number table. |
| Allocation concealment (selection bias) | Low risk | Performed by a pharmacist unaware of the clinical status of the infant. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Individual daily doses were drawn from a specific vial designated for that treatment day, ensuring the same volume of study medication every day. Infants in the moderate‐dosage regimen received placebo saline for the remaining 24 days. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All members of the medical team, including the investigators, remained blinded to group assignment throughout the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized infants were evaluated and no missing data. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were mentioned in the manuscript. |
| Other bias | Low risk | |
Da Silva 2002.
| Methods | Single center double blind randomized trial on moderate‐ versus low‐dosage regimen of dexamethasone. | |
| Participants | Extremely low birth weight infants (≤ 1500 grams), initial starting administration between 7 and 21 days. | |
| Interventions | The included infants were randomly assigned to 1 of 2 dosage regimens.
|
|
| Outcomes | Primary outcomes were growth parameters (weight, length and head circumference) at 36 weeks' corrected gestational age. Secondary outcomes were documented sepsis and long‐term growth parameters at 9 months of corrected age (actual numbers not provided). | |
| Notes | Trial was only published as an abstract and original authors could not provide any additional data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in abstract. |
| Allocation concealment (selection bias) | Unclear risk | Not described in abstract. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated in the abstract as being double blinded, actual procedure not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated in the abstract as being double blinded, actual procedure not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unknown. |
| Selective reporting (reporting bias) | Unclear risk | Unknown. |
| Other bias | Unclear risk | Unknown. |
DeMartini 1999.
| Methods | Single center randomized controlled trial. | |
| Participants | Intubated preterm infants | |
| Interventions | The infants were randomly assigned to 1 of 2 dosage regimens.
All medication was given divided into 2 dosages per day. No patients were treated with any corticosteroids outside the study protocol. |
|
| Outcomes | The primary outcomes were mortality, duration of mechanical ventilation and duration of oxygen dependence. Secondary outcomes were the occurrence of clinically suspected sepsis, NEC, hypertension, hyperglycemia and hypertriglyceridemia. No long‐term follow‐up was performed. |
|
| Notes | Only published as abstract. The original investigator provided data on the incidence of BPD, defined as oxygen dependence at 36 weeks' PMA, combined with mortality at 36 weeks. No long‐term follow‐up performed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information. |
| Allocation concealment (selection bias) | Low risk | By personal communication, no information on the methods. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | By personal communication. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | By personal communication. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified in the abstract. |
| Selective reporting (reporting bias) | Unclear risk | Unknown due to abstract form. |
| Other bias | Unclear risk | Unknown due to abstract form. |
Durand 2002.
| Methods | Single center randomized controlled trial. | |
| Participants | Infants were included when having a birth weight between 501 and 1500 grams, a gestational age between 24 weeks and 32 weeks, postnatal age between 7 and 14 days and at entry on ventilation support with a rate of 15 cycles per minute or more, and 30% supplemental oxygen or more to maintain a pulse oxymeter oxygen saturation of 90% or higher, despite weaning trials. Infants were excluded from the randomization if they had multiple congenital anomalies or chromosomal abnormalities, systemic hypertension, congenital heart disease, IVH grade IV, renal failure or sepsis at entry. |
|
| Interventions | The included infants were randomly assigned to 1 of 2 dosage regimens.
All medication was given divided into 2 dosages per day. Administration of open‐label dexamethasone was allowed after the study period at the discretion of the attending neonatologist. |
|
| Outcomes | The primary outcomes were the dynamic respiratory mechanics, measured before and on days 2, 5 and 7 of dexamethasone therapy. Secondary outcomes were ventilator settings, occurrence of CLD, defined as dependence on oxygen supplementation at 36 weeks' PMA, survival without CLD, duration of mechanical ventilation, duration of hospitalizations, hyperglycemia, hypertension, ROP, NEC, spontaneous GI perforation, sepsis and pulmonary air leaks. |
|
| Notes | Data of the long‐term follow‐up were retrieved from the original investigator. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blind drawing of random cards. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | An outside investigator blinded to the group assignment evaluated the dynamic pulmonary mechanics and graphics. However, assessment of clinical diagnosis was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 59 infants eligible, 7 parents were unavailable and 5 parents refused. 1 included participant had a few doses of dexamethasone withheld because of suspected infection. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were mentioned in the manuscript. |
| Other bias | Low risk | No concerns of other biases. |
Halliday 2001.
| Methods | Multicenter partly double‐blinded randomized controlled trial with a factorial design investigating early versus late administration of inhaled and systemic dexamethasone. | |
| Participants | Intubated infants < 30 weeks' gestational age, a postnatal age < 72 hours and with an inspired oxygen concentration > 30%. Infants with a gestational age between 30 and 31 weeks could be included if needing inspired oxygen > 50%. Infants with lethal congenital anomalies, severe IVH > III, and proven infections were excluded. When strong suspicion of infection, hypertension or hyperglycemia, inclusion was postponed until resolved. |
|
| Interventions | Eligible infants were randomized in 1 of 4 arms, of which 2 contained inhaled corticosteroids. These infants were excluded from this review. The remaining infants were randomized into 1 of 2 arms.
All medication was given divided into 2 dosages per day. |
|
| Outcomes | Primary outcome was death or oxygen dependency at 36 weeks' PMA. Secondary outcomes were death or major cerebral abnormality, death or oxygen dependency at 28 days and expected date of delivery, duration of > 40% oxygen, duration of any oxygen, duration of mechanical ventilation, and duration of hospital stay. Furthermore, complications such as pneumothorax, necrotizing enterocolitis, hypertension, hyperglycemia, sepsis, pneumonia, persistent ductus arteriosus requiring therapy, pulmonary hemorrhage, seizures, recurrent apnea, retinopathy of prematurity, gastric hemorrhage, gastrointestinal perforation were reported. The follow‐up manuscript reported on neurodevelopmental outcome at 7 years of age, including level of disability, cerebral palsy, cognitive ability using the British Ability Scales (BAS 2nd edition), behavioral difficulties using the Strengths and Difficulties Questionnaire (SDQ), competencies using the Child Behavior Checklist for Children, growth, and respiratory symptoms. Impairment was defined as BAS cluster score < 10th percentile, weight or height < 2nd percentile, head circumference < 2nd or > 98th percentile, seizures, borderline SDQ total difficulties score (14 to 16), strabismus, or nystagmus. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not mentioned. |
| Allocation concealment (selection bias) | Low risk | Supervising clinician telephoned the randomization center in Belfast. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Of the 47 participating NICUs, 11 conducted a double‐blinded study. In the remaining centers the design was open because some clinicians wanted to prescribe broad spectrum antibiotics or H2 blockers, or both. In the 11 double‐blinded centers intravenous saline was given. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analyses were on intention‐to‐treat analyses. 5 infants allocated to early treatment were not treated within 5 days, whereas 10 infants allocated to the moderately early period were treated before the 10th day. 2 infants were given the wrong drug. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were mentioned in the manuscript. |
| Other bias | Unclear risk | A large proportion of the total included infants randomized to delayed selective treatment either died or did not fulfill the entry criteria. |
Malloy 2005.
| Methods | Single center, randomized double‐blinded controlled trial | |
| Participants | 17 infants of birth weight < 1500 grams and gestational age of 34 weeks, randomized before the 28th day. | |
| Interventions | The included infants were randomly assigned to 1 of 2 dosage regimens.
|
|
| Outcomes | Clinical outcomes on the already included patients were mortality on discharge, duration of mechanical ventilation and oxygen dependence, survival without CLD, retreatment with dexamethasone, and number of days on oxygen supplementation, number of hospital days, IVH, NEC, gastrointestinal perforation, ROP requiring laser photocoagulation, hypertension, and hyperglycemia. Long‐term follow‐up was performed through 3 years of age and neurodevelopmental status was assessed by using the modified Gesell Developmental Appraisal. |
|
| Notes | Additional data on failure to extubate on day 3, days on mechanical ventilation and blindness or poor vision were retrieved from the original investigator. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By personal communication, method not specified. |
| Allocation concealment (selection bias) | Low risk | By personal communication, method not specified. Infants were stratified into 3 groups according to birth weight. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Only study pharmacist, with no clinical involvement, was aware of doses administered. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 infant in the high‐dose group died on the 2nd day, whereas an infant in the low‐dose died at 4 months of age (1 month after hospital discharge). These infants were included in the analyses of the review. 2 infants in the moderate allocation group were withdrawn from the study on the 6th day of study medication. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were mentioned in the manuscript. |
| Other bias | Unclear risk | This study was terminated prematurely due to the 2002 statement from the American Academy of Pediatrics and the Canadian Paediatric Society. |
Marr 2011.
| Methods | Single center double‐blinded randomized trial investigating a moderate versus a high dexamethasone dosage regimen. | |
| Participants | Infants with < 28 weeks' gestational age, ventilatory support (mean airway pressure > 8 cmH₂O, FiO₂ > 60%), and consistent X‐ray. | |
| Interventions | The included infants were randomly assigned to 1 of 2 dosage regimens.
|
|
| Outcomes | Broad clinical data were collected, including long‐term neurodevelopmental outcomes at 6, 15 and 24 months (not specified). | |
| Notes | This study was only published in abstract form. Original investigators were contacted and willing to provide additional data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not specified in abstract. |
| Allocation concealment (selection bias) | Unclear risk | Method not specified in abstract. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as blinded, although not specified in abstract. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as blinded, although not specified in abstract. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified in abstract. |
| Selective reporting (reporting bias) | Unclear risk | Unknown due to abstract form. |
| Other bias | Unclear risk | Unknown due to abstract form. |
McEvoy 2004.
| Methods | Single center randomized controlled trial | |
| Participants | Infants were included when between 7 and 21 days of postnatal age, with a birth weight of > 501 grams and < 1500 grams, a gestational age of > 24 weeks and < 32 weeks. The infants were dependent on ventilation support with 15 cycles per minute or more and oxygen levels of 30% or more at entry. Infants with multiple congenital anomalies, systemic hypertension, congenital heart disease, IVH grade IV, renal failure, and sepsis at entry were excluded. |
|
| Interventions | The included infants were randomly assigned to 1 of 2 dosage regimens.
All medication was given divided into 2 dosages per day. The use of open‐label dexamethasone therapy was discouraged, but could be administered at the discretion of the attending neonatologist. |
|
| Outcomes | The primary outcomes were the functional residual capacity and passive respiratory compliance before and during the 7‐day therapy. Secondary outcome measurements were the ventilator settings, the duration of mechanical ventilation, the duration of hospitalizations, CLD (defined as oxygen dependence at 36 weeks' PMA), survival without CLD, PDA, hyperglycemia, hypertension, IVH, periventricular leukomalacia, ROP, NEC, spontaneous GI perforation, sepsis, pulmonary air leaks. At 1 year of corrected age the infants were assessed for early neurodevelopmental follow‐up (cerebral palsy and Bayley Scales of Infant Development) by a developmental pediatrician, a pediatric neurologist and specialized personnel. Cerebral palsy was defined as non‐progressive motor impairment characterized by abnormal muscle tone and decreased range/control of movements. Severe cognitive delay was defined as lower than 70 on the mental developmental index (MDI) score. |
|
| Notes | Additional data on duration of mechanical ventilation, failure to extubate on day 3 and 7, were retrieved from the original investigator. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Group assignment was done by the pharmacy using a randomization table. |
| Allocation concealment (selection bias) | Low risk | Investigators and clinical staff was unaware of treatment allocation, because a staff pharmacist was in charge of randomization and study drug preparation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although method not specified in manuscripts. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although method not specified in manuscripts. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In 3 patients of the high‐dose group, 1 dose of dexamethasone was withheld due to blood in the gastric tube or hypertension. For 1 patient of the low dose group, a dose was inadvertently not given. 66% of the survivors were assessed for follow‐up. No statement on the influence on the neurodevelopmental outcome. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were mentioned in the manuscript. |
| Other bias | Low risk | |
Merz 1999.
| Methods | Single center randomized controlled study investigating moderately early versus late administration of dexamethasone. | |
| Participants | Infants with birth weight ≤ 1250 grams, gestational age between 24 and 30 weeks, ventilator dependent at 7 days of age with rate ≥ 15 cycles/min and oxygen requirement 25%. Infants with sepsis, multiple or severe congenital anomalies or evidence of hypertension were excluded. |
|
| Interventions | The included infants were randomly assigned to 1 of 2 regimens.
Both arms received a starting dose of 0.5 mg/kg/day for 3 days, followed by 0.3 days for 3 days, followed by 0.1 mg/kg/day, and followed by this dose alternatively every 2nd day until day 16. All medication was given divided into 2 dosages per day. |
|
| Outcomes | The primary outcome was the time of extubation. Secondary outcomes were duration of supplemental oxygen, the incidence of BPD at 28 days' PNA and pulmonary function tests. Side effects were collected including sepsis, hypertension, hyperglycemia, and adrenal suppression. | |
| Notes | Original investigator was not able to provide additional data. No long‐term follow‐up was performed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomization list was provided by the department of medical statistics. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes with information on timing of initiation were drawn after informed consent by opening the envelope with the lowest number. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No masked intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No masked intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In addition to predefined outcomes, data on necrotizing enterocolitis and gastrointestinal perforation were collected. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were mentioned in the manuscript. |
| Other bias | Low risk | |
Odd 2004.
| Methods | Single center randomized controlled trial investigating a continuous dosage regimen versus an individualized course tailored to the infants' respiratory status. | |
| Participants | Infants ≤ 1250 grams, ventilated between postnatal age of 7 days and 28 days for which dexamethasone was indicated. Infants with congenital anomalies and surgical problems were excluded. |
|
| Interventions | The included infants were randomly assigned to 1 of 2 regimens.
|
|
| Outcomes | The primary outcome was linear growth, measured by knemometry, weight, crown‐heel length, and head circumference. Secondary outcomes were hypertension, myocardial hypertrophy, respiratory status (mode, peak inspiratory pressure, and end expiratory pressure and FiO₂ at enrolment, study days 14, 42, 28 days' postnatal age and 36 weeks' corrected gestational age, hyperglycemia requiring insulin therapy, renal and cranial ultrasounds, proven and suspected infections. In addition a Synacthen test was performed 1 week after discontinuation of the dexamethasone. The long‐term neurodevelopmental outcome were assessed at 9 and 18 months using the Bayley Scales of Infant Development II. Infants were classified into 1 of 4 outcome categories defined and modified from Kitchen et al (J Ped 1987;283). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By computer generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Stratified by sex and birth weight. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Clinical outcome assessment was not blinded, although the primary outcome was (knemometry), as well as ultrasounds performed by staff unaware of treatment allocation. The developmental psychologist was also unaware of the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In 1 infant in the individual group, the dexamethasone treatment was stopped on day 10. Intention‐to‐treat analyses were performed. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were mentioned in the manuscript. |
| Other bias | Low risk | |
Papile 1998.
| Methods | Multicenter double‐blinded randomized controlled trial investigating dexamethasone therapy initiated moderately early versus late. | |
| Participants | Ventilator‐dependent infants with birth weight 501 to 1500 grams, at a postnatal age between 13 and 15 days, with a respiratory index of ≥ 2.4. Infants who received glucocorticoid therapy after birth, had proven or suspected sepsis, or congenital anomaly of cardiovascular, pulmonary, or central nervous system were excluded. |
|
| Interventions | The included infants were randomly assigned to 1 of 2 regimens.
Both dexamethasone regimens started with 0.5 mg/kg/day (divided in 2 doses) for 5 days, followed by 0.15 mg/kg, 0.07 mg/kg, and 0.03 mg/kg for 3 days each. |
|
| Outcomes | Primary outcome was the number of days from randomization to ventilator independence. Secondary outcomes were death before hospital discharge, duration of assisted ventilation, supplemental oxygen, and hospital stay, BPD at 36 weeks, hyperglycemia, hypertension, changes in weight and head circumference, proven sepsis, necrotizing enterocolitis, and gastric hemorrhage. | |
| Notes | No long‐term follow‐up was performed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An order form was sent to each center's pharmacy, where the infants were randomly assigned to 1 of 2 treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | To blind clinical staff, different volumes of placebo were prepared to match the various doses of dexamethasone. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 infants did not receive any of the assigned treatments. Of the 173 infants in the late dexamethasone group who were alive on treatment day 14, 31 did not meet the criteria for starting dexamethasone treatment. Results were analyzed on intention‐to‐treat method. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were mentioned in the manuscript. |
| Other bias | Low risk | |
Ramanathan 1994.
| Methods | Single center randomized controlled trial | |
| Participants | 28 infants of birth weight between 520 and 1440 grams and gestational age of 27 weeks. | |
| Interventions | The included infants were randomly assigned at 10 to 14 days of age to 1 of 2 dosage regimens.
|
|
| Outcomes | Clinical outcomes were mortality on discharge, duration of mechanical ventilation and oxygen dependence, survival without CLD, retreatment with dexamethasone, ROP > stage II, sepsis and hyperglycemia. | |
| Notes | Trial only in abstract form. No long‐term follow‐up was reported and no additional data were retrieved from the original authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information in the abstract. |
| Allocation concealment (selection bias) | Unclear risk | No information in the abstract. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information in the abstract. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information in the abstract. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information in the abstract. |
| Selective reporting (reporting bias) | Unclear risk | No information in the abstract. |
| Other bias | Unclear risk | No information in the abstract. |
BPD = bronchopulmonary dysplasia
CLD = chronic lung disease
GI = gastrointestinal
IVH = intraventricular hemorrhage
NEC = necrotizing enterocolitis
PMA = postmenstrual age
PNA = postnatal age
ROP = retinopathy of prematurity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anttila 2005 | Placebo controlled trial. |
| Ariagno 1987 | Could not be retrieved. |
| Groneck 1993 | Original investigator could not provide clinical data. |
| Nixon 2011 | Placebo‐controlled trial. |
Differences between protocol and review
A post hoc decision was made to include the trial by Bloomfield 1998 in three comparisons: the comparison of postponing initiation of therapy, the comparison of pulse dose administration, and the individually tailored regimen comparison. This trial allocated infants to a group initiating pulse therapy starting at early initiation, comparing it to a group treated with a continuous tapering dose started moderately early. Furthermore, the infants allocated to the early initiation received a pulse dose during three days which was repeated every 10 days until ventilation or supplemental oxygen was no longer required for that participant. Therefore, it was also suitable for the individually tailored comparison. Furthermore, although not mentioned in the protocol, the quality of evidence was assessed for the main comparisons at the outcome level using the GRADE approach.
Contributions of authors
Dr Onland has full access to all of the data in the study and will take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Onland, van Kaam. Acquisition of data: Onland, De Jaegere. Analysis and interpretation of data: Onland, De Jaegere, Offringa, van Kaam. Drafting of the manuscript: Onland. Critical revision of the manuscript for important intellectual content: Onland, De Jaegere, Offringa, van Kaam. Statistical analysis: Onland, De Jaegere. Study supervision: Offringa, van Kaam.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
Declarations of interest
No financial disclosure to be declared. No potential conflicts of interest known.
New
References
References to studies included in this review
Barkemeyer 2000 {published data only}
- Barkemeyer BM, Davey A, Cummings JJ, Pappagallo M, Durand M, Stevens D, et al. Pulse vs. continuous dexamethasone therapy for neonatal chronic lung disease (CLD) in very low birthweight (VLBW) infants. Pediatric Research. 2000; Vol. 47, issue 4:276A.
Bloomfield 1998 {published data only}
- Armstrong DL, Penrice J, Bloomfield FH, Knight DB, Dezoete JA, Harding JE. Follow up of a randomised trial of two different courses of dexamethasone for preterm babies at risk of chronic lung disease. Archives of Disease in Childhood. Fetal and Neonatal Edition 2002;86(2):F102‐7. [PUBMED: 11882552] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield FH, Knight DB, Harding JE. Side effects of 2 different dexamethasone courses for preterm infants at risk of chronic lung disease: a randomized trial. Journal of Pediatrics 1998;133(3):395‐400. [PUBMED: 9738724] [DOI] [PubMed] [Google Scholar]
Cummings 1989 {published data only}
- Cummings JJ, D'Eugenio DB, Gross SJ. A controlled trial of dexamethasone in preterm infants at high risk for bronchopulmonary dysplasia. New England Journal of Medicine 1989;320(23):1505‐10. [PUBMED: 2657423] [DOI] [PubMed] [Google Scholar]
- Gross SJ, Anbar RD, Mettelman BB. Follow‐up at 15 years of preterm infants from a controlled trial of moderately early dexamethasone for the prevention of chronic lung disease. Pediatrics 2005;115(3):681‐7. [PUBMED: 15741372] [DOI] [PubMed] [Google Scholar]
- Gross SJ, Cummings JJ. Four year follow‐up of a controlled trial of dexamethasone (DEX) in ventilator dependent preterm infants. Pediatric Research. 1994; Vol. 35(4):204A.
Da Silva 2002 {published data only}
- Silva OP, Kumaran VS, Knoppert DC. Randomized Controlled Trial Comparing Two Regimens of Dexamethasone in the Neonate with Chronic Lung Disease. Pediatric Research. 2002; Vol. 53:369A.
DeMartini 1999 {published data only}
- DeMartini TJ, Muraskas JK. Pulse versus tapered dosing dexamethasone for evolving bronchopulmonary dysplasia (BPD). Pediatric Research 1999;45(4):300A. [Google Scholar]
Durand 2002 {published data only}
- Durand M, Mendoza ME, Tantivit P, Kugelman A, McEvoy C. A randomized trial of moderately early low‐dose dexamethasone therapy in very low birth weight infants: dynamic pulmonary mechanics, oxygenation, and ventilation. Pediatrics 2002;109(2):262‐8. [PUBMED: 11826205] [DOI] [PubMed] [Google Scholar]
Halliday 2001 {published data only}
- Halliday HL, Patterson CC, Halahakoon CW. A multicenter, randomized open study of early corticosteroid treatment (OSECT) in preterm infants with respiratory illness: comparison of early and late treatment and of dexamethasone and inhaled budesonide. Pediatrics 2001;107(2):232‐40. [PUBMED: 11158452] [DOI] [PubMed] [Google Scholar]
- Wilson TT, Waters L, Patterson CC, McCusker CG, Rooney NM, Marlow N, et al. Neurodevelopmental and respiratory follow‐up results at 7 years for children from the United Kingdom and Ireland enrolled in a randomized trial of early and late postnatal corticosteroid treatment, systemic and inhaled (the Open Study of Early Corticosteroid Treatment). Pediatrics 2006;117(6):2196‐205. [PUBMED: 16740865] [DOI] [PubMed] [Google Scholar]
Malloy 2005 {published data only}
- Malloy CA, Hilal K, Weiss MG, Rizvi Z, Muraskas JK. A prospective, randomized, double‐masked trial comparing low dose to conventional dose dexamethasone in neonatal chronic lung disease. Internet Journal of Pediatrics and Neonatology 2005; Vol. 5, issue 1:10473.
- Malloy CA, Hilal K, Weiss MG, Rizvi Z, Muraskas JK. Randomized controlled trial comparing standard vs. lower dose dexamethasone therapy in neonates with chronic lung disease. E‐PAS. 2003:2776.
Marr 2011 {published data only}
McEvoy 2004 {published data only}
- McEvoy C, Bowling S, Williamson K, McGaw P, Durand M. Randomized, double‐blinded trial of low‐dose dexamethasone: II. Functional residual capacity and pulmonary outcome in very low birth weight infants at risk for bronchopulmonary dysplasia. Pediatric Pulmonology 2004;38(1):55‐63. [PUBMED: 15170874] [DOI] [PubMed] [Google Scholar]
Merz 1999 {published data only}
- Merz U, Peschgens T, Kusenbach G, Hornchen H. Early versus late dexamethasone treatment in preterm infants at risk for chronic lung disease: a randomized pilot study. European Journal of Pediatrics 1999;158(4):318‐22. [PUBMED: 10206132] [DOI] [PubMed] [Google Scholar]
Odd 2004 {published data only}
- Cranefield DJ, Odd DE, Harding JE, Teele RL. High incidence of nephrocalcinosis in extremely preterm infants treated with dexamethasone. Pediatric Radiology 2004;34(2):138‐42. [PUBMED: 14624322] [DOI] [PubMed] [Google Scholar]
- Odd DE, Armstrong DL, Teele RL, Kuschel CA, Harding JE. A randomized trial of two dexamethasone regimens to reduce side‐effects in infants treated for chronic lung disease of prematurity. Journal of Paediatrics and Child Health 2004;40(5‐6):282‐9. [PUBMED: 15151582] [DOI] [PubMed] [Google Scholar]
Papile 1998 {published data only}
- Papile LA, Tyson JE, Stoll BJ, Wright LL, Donovan EF, Bauer CR, et al. A multicenter trial of two dexamethasone regimens in ventilator‐dependent premature infants. New England journal of medicine 1998;338(16):1112‐8. [PUBMED: 9545359] [DOI] [PubMed] [Google Scholar]
- Stoll BJ, Temprosa M, Tyson JE, Papile LA, Wright LL, Bauer CR, et al. Dexamethasone therapy increases infection in very low birth weight infants. Pediatrics 1999;104(5):e63. [PUBMED: 10545589] [DOI] [PubMed] [Google Scholar]
Ramanathan 1994 {published data only}
- Ramanathan R, Siassi B, Sardesai S, deLemos RA. Comparison of two dosage regimens of dexamethasone for early treatment of chronic lung disease in very low birth weight (VLBW). Pediatric Research 1994;34:250A. [Google Scholar]
References to studies excluded from this review
Anttila 2005 {published data only}
- Anttila E, Peltoniemi O, Haumont D, Herting E, ter Horst H, Heinonen K, et al. Early neonatal dexamethasone treatment for prevention of bronchopulmonary dysplasia. Randomised trial and meta‐analysis evaluating the duration of dexamethasone therapy. European Journal of Pediatrics 2005;164(8):472‐81. [PUBMED: 15864643] [DOI] [PubMed] [Google Scholar]
Ariagno 1987 {published data only}
- Ariagno RL, Sweeney TE, Baldwin RB, Inguillo D, Martin D. Controlled trial of dexamethasone in preterm infantsat risk for bronchopulmonary dysplasia: lung function, clinical course and outcome at three years. Unpublished manuscript as stated in Cochrane review (Halliday et al.).
Groneck 1993 {published data only}
- Groneck P, Oppermann M, Speer CP. Levels of complement anaphylatoxin C5a in pulmonary effluent fluid of infants at risk for chronic lung disease and effects of dexamethasone treatment. Pediatric Research 1993;34(5):586‐90. [PUBMED: 8284093] [DOI] [PubMed] [Google Scholar]
- Groneck P, Reuss D, Gotze‐Speer B, Speer CP. Effects of dexamethasone on chemotactic activity and inflammatory mediators in tracheobronchial aspirates of preterm infants at risk for chronic lung disease. Journal of Pediatrics 1993;122(6):938‐44. [PUBMED: 8388949] [DOI] [PubMed] [Google Scholar]
Nixon 2011 {published data only}
- Nixon PA, Washburn LK, Mudd LM, Webb HH, O'Shea TM. Aerobic fitness and physical activity levels of children born prematurely following randomization to postnatal dexamethasone. Journal of Pediatrics 2011;158(1):65‐70. [PUBMED: 20732688] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
AAP 2002
- Committee on Fetus and Newborn. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics 2002;109(2):330‐8. [PUBMED: 11826218] [DOI] [PubMed] [Google Scholar]
Bancalari 2006
- Bancalari E, Claure N. Definitions and diagnostic criteria for bronchopulmonary dysplasia. Seminars in Perinatology 2006;30(4):164‐70. [PUBMED: 16860155] [DOI] [PubMed] [Google Scholar]
Brozanski 1995
- Brozanski BS, Jones JG, Gilmour CH, Balsan MJ, Vazquez RL, Israel BA, et al. Effect of pulse dexamethasone therapy on the incidence and severity of chronic lung disease in the very low birth weight infant. Journal of Pediatrics 1995;126(5 Pt 1):769‐76. [PUBMED: 7752005] [DOI] [PubMed] [Google Scholar]
Carlton 1997
- Carlton DP, Albertine KH, Cho SC, Lont M, Bland RD. Role of neutrophils in lung vascular injury and edema after premature birth in lambs. Journal of Applied Physiology 1997;83(4):1307‐7. [PUBMED: 9338441] [DOI] [PubMed] [Google Scholar]
CDTG 1991
- Collaborative Dexamethasone Trial Group. Dexamethasone therapy in neonatal chronic lung disease: an international placebo‐controlled trial. Pediatrics 1991;88(3):421‐7. [PUBMED: 1881718] [PubMed] [Google Scholar]
Cheong 2013
- Cheong JL, Anderson P, Roberts G, Duff J, Doyle LW. Postnatal corticosteroids and neurodevelopmental outcomes in extremely low birthweight or extremely preterm infants: 15‐year experience in Victoria, Australia. Archives of Disease in Childhood. Fetal and Neonatal Edition 2013;98(1):F32‐6. [PUBMED: 22684163] [DOI] [PubMed] [Google Scholar]
Coalson 2006
- Coalson JJ. Pathology of bronchopulmonary dysplasia. Seminars in Perinatology 2006;30(4):179‐84. [PUBMED: 16860157] [DOI] [PubMed] [Google Scholar]
Costeloe 2012
- Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ (Clinical research ed.) 2012;345:e7976. [PUBMED: 23212881] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doyle 2005
- Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. Impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk for chronic lung disease. Pediatrics 2005;115(3):655‐61. [PUBMED: 15741368] [DOI] [PubMed] [Google Scholar]
Doyle 2010
- Doyle LW, Ehrenkranz RA, Halliday HL. Postnatal hydrocortisone for preventing or treating bronchopulmonary dysplasia in preterm infants: a systematic review. Neonatology 2010;98(2):111‐7. [PUBMED: 20150750] [DOI] [PubMed] [Google Scholar]
Doyle 2014
- Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. An update on the impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk of bronchopulmonary dysplasia. Journal of Pediatrics 2014;165(6):1258‐60. [PUBMED: 25217197] [DOI] [PubMed] [Google Scholar]
Doyle 2014a
- Doyle LW, Ehrenkranz RA, Halliday HL. Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD001146.pub4] [DOI] [PubMed] [Google Scholar]
Doyle 2014b
- Doyle LW, Ehrenkranz RA, Halliday HL. Late (> 7 days) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD001145.pub3] [DOI] [PubMed] [Google Scholar]
Durand 1995
- Durand M, Sardesai S, McEvoy C. Effects of early dexamethasone therapy on pulmonary mechanics and chronic lung disease in very low birth weight infants: a randomized, controlled trial. Pediatrics 1995;95(4):584‐90. [PUBMED: 7700763] [PubMed] [Google Scholar]
Ehrenkranz 2005
- Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005;116(6):1353‐60. [PUBMED: 16322158] [DOI] [PubMed] [Google Scholar]
Ferreira 2000
- Ferreira PJ, Bunch TJ, Albertine KH, Carlton DP. Circulating neutrophil concentration and respiratory distress in premature infants. Journal of Pediatrics 2000;136(4):466‐72. [PUBMED: 10753244] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2016 [Computer program]
- on www.gradepro.org. GRADEpro. www.gradepro.org. Version 2014. McMaster University, 06‐03‐2016.
Halliday 2001a
- Halliday HL. Guidelines on neonatal steroids. Prenatal Neonatal Medicine 2001;6:371‐3. [Google Scholar]
Halliday 2003a
- Halliday HL, Ehrenkranz RA, Doyle LW. Early postnatal (< 96 hours) corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD001146] [DOI] [PubMed] [Google Scholar]
Halliday 2003b
- Halliday HL, Ehrenkranz RA, Doyle LW. Moderately early (7‐14 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD001144] [DOI] [PubMed] [Google Scholar]
Halliday 2003c
- Halliday HL, Ehrenkranz RA, Doyle LW. Delayed (> 3 weeks) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD001145.pub2] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [PUBMED: 15840177] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2007
- Huang CC, Lin HR, Liang YC, Hsu KS. Effects of neonatal corticosteroid treatment on hippocampal synaptic function. Pediatric Research 2007;62(3):267‐70. [PUBMED: 17622955] [DOI] [PubMed] [Google Scholar]
Husain 1998
- Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in post surfactant bronchopulmonary dysplasia. Human Pathology 1998;29(7):710‐7. [PUBMED: 9670828] [DOI] [PubMed] [Google Scholar]
Jobe 1999
- Jobe AJ. The new BPD: an arrest of lung development. Pediatric Research 1999;46(6):641‐3. [PUBMED: 10590017] [DOI] [PubMed] [Google Scholar]
Jobe 2001
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1723‐9. [PUBMED: 11401896] [DOI] [PubMed] [Google Scholar]
Kaempf 2003
- Kaempf JW, Campbell B, Sklar RS, Arduza C, Gallegos R, Zabari M, et al. Implementing potentially better practices to improve neonatal outcomes after reducing postnatal dexamethasone use in infants born between 501 and 1250 grams. Pediatrics 2003;111(4 Pt 2):e534‐41. [PUBMED: 12671173] [PubMed] [Google Scholar]
Karemaker 2006
- Karemaker R, Heijnen CJ, Veen S, Baerts W, Samsom J, Visser GH, et al. Differences in behavioral outcome and motor development at school age after neonatal treatment for chronic lung disease with dexamethasone versus hydrocortisone. Pediatric Research 2006;60(6):745‐50. [PUBMED: 17065571] [DOI] [PubMed] [Google Scholar]
Kitchen 1987
- Kitchen WH, Ford GW, Rickards AL, Lissenden JV, Ryan MM. Children of birth weight less than 1000 g: changing outcome between ages 2 and 5 years. Journal of Pediatrics 1987;110(2):283‐8. [PUBMED: 2433422] [DOI] [PubMed] [Google Scholar]
Lodygensky 2005
- Lodygensky GA, Rademaker K, Zimine S, Gex‐Fabry M, Lieftink AF, Lazeyras F, et al. Structural and functional brain development after hydrocortisone treatment for neonatal chronic lung disease. Pediatrics 2005;116(1):1‐7. [PUBMED: 15995023] [DOI] [PubMed] [Google Scholar]
Northway 1967
- Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respiratory therapy of hyaline‐membrane disease. Bronchopulmonary dysplasia. New England Journal of Medicine 1967;276(7):357‐68. [PUBMED: 5334613] [DOI] [PubMed] [Google Scholar]
O'Brodovich 1985
- O'Brodovich HM, Mellins RB. Bronchopulmonary dysplasia. Unresolved neonatal acute lung injury. American Review of Respiratory Disease 1985;132(3):694‐709. [PUBMED: 3898946] [DOI] [PubMed] [Google Scholar]
O'Shea 1999
- O'Shea TM, Kothadia JM, Klinepeter KL, Goldstein DJ, Jackson BG, Weaver RG 3rd, et al. Randomized placebo‐controlled trial of a 42‐day tapering course of dexamethasone to reduce the duration of ventilator dependency in very low birth weight infants: outcome of study participants at 1‐year adjusted age. Pediatrics 1999;104(1 Pt 1):15‐21. [PUBMED: 10390254] [DOI] [PubMed] [Google Scholar]
Onland 2009
- Onland W, Offringa M, Jaegere AP, Kaam AH. Finding the optimal postnatal dexamethasone regimen for preterm infants at risk of bronchopulmonary dysplasia: a systematic review of placebo‐controlled trials. Pediatrics 2009;123(1):367‐77. [PUBMED: 19117904] [DOI] [PubMed] [Google Scholar]
Onland 2010
- Onland W, Kaam AH, Jaegere AP, Offringa M. Open‐label glucocorticoids modulate dexamethasone trial results in preterm infants. Pediatrics 2010;126(4):e954‐64. [PUBMED: 20837588] [DOI] [PubMed] [Google Scholar]
Rademaker 2007
- Rademaker KJ, Uiterwaal CS, Groenendaal F, Venema MM, Bel F, Beek FJ, et al. Neonatal hydrocortisone treatment: neurodevelopmental outcome and MRI at school age in preterm‐born children. Journal of Pediatrics 2007;150(4):351‐7. [PUBMED: 17382109] [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Schmidt 2008
- Schmidt B, Roberts R, Millar D, Kirpalani H. Evidence‐based neonatal drug therapy for prevention of bronchopulmonary dysplasia in very‐low‐birth‐weight infants. Neonatology 2008;93(4):284‐7. [PUBMED: 18525211] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GWG. GRADE handbook for grading quality of evidence and strength of recommendations. Available from www.guidelinedevelopment.org/handbook Updated October 2013.
Shinwell 2003
- Shinwell ES, Karplus M, Bader D, Dollberg S, Gur I, Weintraub Z, et al. Neonatologists are using much less dexamethasone. Archives of Disease in Childhood. Fetal and Neonatal Edition 2003;88(5):F432‐3. [PUBMED: 12937052] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shinwell 2007
- Shinwell ES, Lerner‐Geva L, Lusky A, Reichman B. Less postnatal steroids, more bronchopulmonary dysplasia: a population‐based study in very low birth weight infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2007;92(1):F30‐3. [PUBMED: 16769711] [DOI] [PMC free article] [PubMed] [Google Scholar]
Short 2007
- Short EJ, Kirchner HL, Asaad GR, Fulton SE, Lewis BA, Klein N, et al. Developmental sequelae in preterm infants having a diagnosis of bronchopulmonary dysplasia: analysis using a severity‐based classification system. Archives of Pediatrics and Adolescent Medicine 2007;161(11):1082‐7. [PUBMED: 17984411] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoll 2010
- Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126(3):443‐56. [PUBMED: 20732945] [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Heide‐Jalving 2003
- Heide‐Jalving M, Kamphuis PJ, Laan MJ, Bakker JM, Wiegant VM, Heijnen CJ, et al. Short‐ and long‐term effects of neonatal glucocorticoid therapy: is hydrocortisone an alternative to dexamethasone?. Acta Paediatrica (Oslo, Norway: 1992) 2003;92(7):827‐35. [PUBMED: 12892163] [DOI] [PubMed] [Google Scholar]
Walsh 2005
- Walsh MC, Morris BH, Wrage LA, Vohr BR, Poole WK, Tyson JE, et al. Extremely low birthweight neonates with protracted ventilation: mortality and 18‐month neurodevelopmental outcomes. Journal of Pediatrics 2005;146(6):798‐804. [PUBMED: 15973322] [DOI] [PubMed] [Google Scholar]
Walsh 2006
- Walsh MC, Yao Q, Horbar JD, Carpenter JH, Lee SK, Ohlsson A. Changes in the use of postnatal steroids for bronchopulmonary dysplasia in 3 large neonatal networks. Pediatrics 2006;118(5):e1328‐35. [PUBMED: 17079534] [DOI] [PubMed] [Google Scholar]
Watterberg 2007
- Watterberg KL, Shaffer ML, Mishefske MJ, Leach CL, Mammel MC, Couser RJ, et al. Growth and neurodevelopmental outcomes after early low‐dose hydrocortisone treatment in extremely low birth weight infants. Pediatrics 2007;120(1):40‐8. [PUBMED: 17606560] [DOI] [PubMed] [Google Scholar]
Watterberg 2010
- Watterberg KL. Policy statement ‐ postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics 2010;126(4):800‐8. [PUBMED: 20819899] [DOI] [PubMed] [Google Scholar]
Yeh 1997
- Yeh TF, Lin YJ, Hsieh WS, Lin HC, Lin CH, Chen JY, et al. Early postnatal dexamethasone therapy for the prevention of chronic lung disease in preterm infants with respiratory distress syndrome: a multicenter clinical trial. Pediatrics 1997;100(4):E3. [PUBMED: 9310536] [DOI] [PubMed] [Google Scholar]
Yeh 1998
- Yeh TF, Lin YJ, Huang CC, Chen YJ, Lin CH, Lin HC, et al. Early dexamethasone therapy in preterm infants: a follow‐up study. Pediatrics 1998;101(5):E7. [DOI] [PubMed] [Google Scholar]
Yoder 2009
- Yoder BA, Harrison M, Clark RH. Time‐related changes in steroid use and bronchopulmonary dysplasia in preterm infants. Pediatrics 2009;124(2):673‐9. [PUBMED: 19620192] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Onland 2008
- Onland W, Jaegere AP, Offringa M, Kaam AH. Effects of higher versus lower dexamethasone doses on pulmonary and neurodevelopmental sequelae in preterm infants at risk for chronic lung disease: a meta‐analysis. Pediatrics 2008;122(1):92‐101. [PUBMED: 18595991] [DOI] [PubMed] [Google Scholar]


