Abstract
Background
Unilateral spatial neglect causes difficulty attending to one side of space. Various rehabilitation interventions have been used but evidence of their benefit is lacking.
Objectives
To assess whether cognitive rehabilitation improves functional independence, neglect (as measured using standardised assessments), destination on discharge, falls, balance, depression/anxiety and quality of life in stroke patients with neglect measured immediately post‐intervention and at longer‐term follow‐up; and to determine which types of interventions are effective and whether cognitive rehabilitation is more effective than standard care or an attention control.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched June 2012), MEDLINE (1966 to June 2011), EMBASE (1980 to June 2011), CINAHL (1983 to June 2011), PsycINFO (1974 to June 2011), UK National Research Register (June 2011). We handsearched relevant journals (up to 1998), screened reference lists, and tracked citations using SCISEARCH.
Selection criteria
We included randomised controlled trials (RCTs) of cognitive rehabilitation specifically aimed at spatial neglect. We excluded studies of general stroke rehabilitation and studies with mixed participant groups, unless more than 75% of their sample were stroke patients or separate stroke data were available.
Data collection and analysis
Two review authors independently selected studies, extracted data, and assessed study quality. For subgroup analyses, review authors independently categorised the approach underlying the cognitive intervention as either 'top‐down' (interventions that encourage awareness of the disability and potential compensatory strategies) or 'bottom‐up' (interventions directed at the impairment but not requiring awareness or behavioural change, e.g. wearing prisms or patches).
Main results
We included 23 RCTs with 628 participants (adding 11 new RCTs involving 322 new participants for this update). Only 11 studies were assessed to have adequate allocation concealment, and only four studies to have a low risk of bias in all categories assessed. Most studies measured outcomes using standardised neglect assessments: 15 studies measured effect on activities of daily living (ADL) immediately after the end of the intervention period, but only six reported persisting effects on ADL. One study (30 participants) reported discharge destination and one study (eight participants) reported the number of falls.
Eighteen of the 23 included RCTs compared cognitive rehabilitation with any control intervention (placebo, attention or no treatment). Meta‐analyses demonstrated no statistically significant effect of cognitive rehabilitation, compared with control, for persisting effects on either ADL (five studies, 143 participants) or standardised neglect assessments (eight studies, 172 participants), or for immediate effects on ADL (10 studies, 343 participants). In contrast, we found a statistically significant effect in favour of cognitive rehabilitation compared with control, for immediate effects on standardised neglect assessments (16 studies, 437 participants, standardised mean difference (SMD) 0.35, 95% confidence interval (CI) 0.09 to 0.62). However, sensitivity analyses including only studies of high methodological quality removed evidence of a significant effect of cognitive rehabilitation.
Additionally, five of the 23 included RCTs compared one cognitive rehabilitation intervention with another. These included three studies comparing a visual scanning intervention with another cognitive rehabilitation intervention, and two studies (three comparison groups) comparing a visual scanning intervention plus another cognitive rehabilitation intervention with a visual scanning intervention alone. Only two small studies reported a measure of functional disability and there was considerable heterogeneity within these subgroups (I² > 40%) when we pooled standardised neglect assessment data, limiting the ability to draw generalised conclusions.
Subgroup analyses exploring the effect of having an attention control demonstrated some evidence of a statistically significant difference between those comparing rehabilitation with attention control and those with another control or no treatment group, for immediate effects on standardised neglect assessments (test for subgroup differences, P = 0.04).
Authors' conclusions
The effectiveness of cognitive rehabilitation interventions for reducing the disabling effects of neglect and increasing independence remains unproven. As a consequence, no rehabilitation approach can be supported or refuted based on current evidence from RCTs. However, there is some very limited evidence that cognitive rehabilitation may have an immediate beneficial effect on tests of neglect. This emerging evidence justifies further clinical trials of cognitive rehabilitation for neglect. However, future studies need to have appropriate high quality methodological design and reporting, to examine persisting effects of treatment and to include an attention control comparator.
Plain language summary
Cognitive rehabilitation for spatial neglect following stroke
The benefit of cognitive rehabilitation (therapy) for unilateral spatial neglect, a condition that can affect stroke survivors, is unclear. Unilateral spatial neglect is a condition that reduces a person's ability to look, listen or make movements in one half of their environment. This can affect their ability to carry out many everyday tasks such as eating, reading and getting dressed, and restricts a person's independence. Our review of 23 studies involving 628 participants with stroke found insufficient high quality evidence to tell us the effect of therapy designed for treating neglect. We did find some limited evidence which suggested that such therapy might be helpful, but the quality of this evidence was poor and more research is needed to confirm this finding. People with neglect should continue to receive general stroke rehabilitation services and to have the opportunity to take part in high‐quality research.
Background
Description of the condition
Stroke can affect cognitive as well as physical and sensory abilities (Wade 1985). Cognitive deficits include a disorder of spatial awareness known as unilateral spatial neglect. The most widely quoted definition of neglect is a description of the resulting behavioural disabilities: "fails to report, respond, or orient to novel or meaningful stimuli presented to the side opposite a brain lesion" (Heilman 1993). This definition does not describe the causal mechanism of neglect but indicates that it is not simply due to sensory or motor defects. Neglect is a disorder which can reduce a person's ability to look, listen or make movements towards one half of their environment. This can also affect their ability to carry out many everyday tasks, such as eating, reading and getting dressed (Katz 1999). Stroke may differentially affect our ability to direct our attention in the visual, auditory or tactile modalities. Since different types of neglect can occur, several terms are used in clinical practice, such as visual neglect, motor neglect, hemineglect, and inattention (Bailey 1999). Although people do sometimes neglect their ipsilesional (same) side, most researchers and clinicians focus on the far more common neglect of contralesional (opposite) side space.
The reported incidence of neglect in stroke patients has varied from as high as 90% (Massironi 1988) to as low as 8% (Sunderland 1987). The figures depend on the operational definition, selection criteria for patients and method of assessment employed (Bailey 1999; Bowen 1999; Ferro 1999). A previous systematic review found that, in 16 of the 17 studies making the comparison, contralesional neglect occurred more often after right than left hemisphere stroke (Bowen 1999). Cognitive dysfunction, such as neglect, can determine the outcome of rehabilitation by adversely affecting mobility, discharge destination, length of hospital stay, meal preparation and independence in self‐care skills (Barer 1990; Bernspang 1987; Neistadt 1993). In the light of these functional implications, it is not surprising that the rehabilitation of neglect is an important aim in stroke rehabilitation.
Description of the intervention
Cognitive rehabilitation is the collective label for a wide range of therapeutic interventions (Lincoln 2012). These share a common purpose, to reduce the adverse effects that cognitive impairments have on a person’s ability to perform everyday activities, their social role participation and quality of life. There are two very different approaches known as restitutive and compensatory. Techniques using the restitutive approach aim to alter the underlying cognitive impairment. Compensatory techniques include teaching strategies to make behavioural adjustments. The emphasis in compensatory strategies is on coping with and finding ways of adapting to existing impairments. Restitution approaches are more often used in the early stage of the stroke pathway when plasticity is thought to be greatest, and compensatory strategies are typically used later. However, this is not a hard and fast rule. In most countries cognitive rehabilitation is provided by psychologists and occupational therapists or their assistants, although other professionals are also involved, for example orthopists for prisms.
It is common to categorise neglect interventions as involving either bottom‐up or top‐down processing (Parton 2004). Top‐down approaches aim to train the person to voluntarily compensate for their neglect and require awareness of the disorder. Methods include training in scanning and usually provide feedback (Pizzamiglio 2004). Top‐down approaches focus on the level of disability rather than impairment. Bottom‐up approaches do not require awareness of the disorder. They aim to modify underlying factors, i.e. to alter the impaired representation of space. Prism‐wearing and prism adaptation training are popular recent examples of a bottom‐up approach (Rossetti 1998). By wearing base‐left wedge prisms in spectacles visual space is perturbed to the right making it more likely to be seen. Other examples of bottom‐up processing approaches include eye patching and the use of devices to stimulate the neglected side. We included both bottom‐up and top‐down approaches, and categorised each intervention within this framework.
Why it is important to do this review
The two main reasons for this review are, first, that neglect is a major problem for people with stroke and secondly, there is clinical uncertainty about the effectiveness of cognitive rehabilitation. Neglect affects long‐term outcome. It can impede active participation in stroke rehabilitation programs and decrease independence in activities of daily living (ADL) and quality of life (Jehkonen 2006). Several recent reviews have argued that cognitive rehabilitation is effective for people with various cognitive impairments including neglect after a stroke (Cicerone 2005; Jutai 2003) or a traumatic brain injury (Cicerone 2009). Some of the non‐randomised evidence included in these reviews may introduce bias. This updated review aimed to systematically consider the evidence from RCTs on the effectiveness of cognitive rehabilitation aimed at spatial neglect.
Objectives
To assess whether cognitive rehabilitation improves functional independence, neglect (measured using standardised assessments), destination on discharge, falls, balance, depression/anxiety and quality of life in stroke patients with neglect measured immediately post‐intervention and at longer‐term follow‐up; to determine which types of intervention are effective and whether cognitive rehabilitation is more effective than standard care or an attention control.
Methods
Criteria for considering studies for this review
Types of studies
For the first version of this review we sought all controlled trials in which cognitive rehabilitation was compared with a control treatment. In addition to well‐designed randomised controlled trials (RCTs), we considered other studies (such as those described as quasi‐random) for inclusion but, if selected, we assigned these a lower methodological quality score. However, in the previous version (2006) and this update, we excluded all non‐randomised studies to reduce selection bias. These are listed in the Characteristics of excluded studies table.
Types of participants
This review was confined to trials that included participants with neglect following stroke. Stroke was confirmed by neurological examination or brain scanning, or both, and neglect by neuropsychological assessment. Thus, we excluded studies that included participants whose deficits were the result of head trauma, brain tumour or any other brain damage unless a subgroup of those with stroke could be identified for which there were separate results, or more than 75% of participants in the sample were stroke patients. We excluded studies of people with general perceptual problems unless a subgroup with neglect could be identified. A separate review has been published on cognitive rehabilitation for people with perceptual problems (Bowen 2011).
Types of interventions
To be included in the review, a clinical trial had to report a comparison between an active treatment group that received one of various cognitive rehabilitation programs for neglect versus a control group that received either an alternative form of treatment or none. Cognitive rehabilitation was broadly defined to include therapy activities designed to directly reduce the level of the neglect impairment or the resulting disability. We excluded drug treatments. Cognitive rehabilitation could include structured therapy sessions, computerised therapy, prescription of aids and modification of the participants' environment as long as these were specific to neglect. The aim was to directly target the neglect rather than to examine whether people with neglect happened to benefit from general rehabilitation services.
Types of outcome measures
We were interested in outcomes at two timepoints: (1) immediately after the end of an intervention, and (2) persisting beyond the end of intervention (i.e. follow‐up outcome).
Primary outcomes
Ratings on measures of functional disability: we included the following scales: Catherine Bergego Scale, Everyday Neglect Questionnaire, Nottingham Extended Activities of Daily Living scale, Lawton Instrumental Activities of Daily Living, Frenchay Activities Index, Rivermead ADL, Edmans EADL, Modified Rankin Scale, Barthel ADL Index, Functional Independence Measure, Katz Index of Activities of Daily Living, and Rehabilitation Activities Profile. When more than one of these scales was reported, we used the scale listed first above. We excluded functional measures designed for a specific study e.g. obstacle avoidance, observation of an ADL task.
For the 2006 update of the review the primary outcome was defined as 'Ratings on measures of functional disability: activities of daily living (ADL) scales: Barthel Index (BI), Functional Independence Measure (FIM), Frenchay Activities Index (FAI), or neglect‐specific ADL measures'. As this was not a comprehensive list of functional disability measures, we extended the scales listed in order to be more comprehensive and to avoid having to make decisions after the identification of studies. We checked back through the studies included in the 2006 update and this clarification did not lead to any changes to the outcomes included from those studies.
Secondary outcomes
Performance on standardised neglect assessments: target cancellation (single letter, double letter, line, shape), line bisection. In addition to a conventional subtest score (such as letter cancellation) the behavioural summary score from the Behavioural Inattention Test (BIT) was used when available. In this updated review we removed outcomes of attention and drawing tests to reduce the number of outcomes being reviewed and to concentrate on those most relevant to neglect.
Discharge destination: whether a person was discharged to live in their own home or to a care facility was included where available, with deaths before discharge treated as not discharged to their own home.
Balance: Berg balance scale, Functional Reach, Get up and go test, Standing Balance test, Step Test or other standardised balance measure. We did not include measures of weight distribution or postural sway during standing, as the relationship between ability to maintain balance and these outcomes is not established.
Falls: number of reported falls, Falls Efficacy Scale.
Depression/anxiety: e.g. Hospital Anxiety and Depression Scale, Beck Depressive Inventory, General Health Questionnaire, Geriatric Depression Scale.
Quality of life and social isolation: EQ5D (Health‐related quality of life scale, Quality of Well Being scale, SF36).
Adverse events: (any reported adverse events, excluding falls).
The 2006 update of the review only included secondary outcomes (1) and (2). For the 2013 update we extended these to reflect outcomes of importance to people with stroke. No studies had been excluded on the basis of not having suitable outcome measures and we were therefore confident that this change did not require us to repeat any previous searches. We re‐appraised all studies included in the 2006 update of the review and extracted data on any of the additional secondary outcomes.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for relevant trials in all languages and arranged translation of trial reports published in languages other than English.
We searched the Cochrane Stroke Group Trials Register, which was last searched by the Managing Editor in June 2012. In addition, we searched the following electronic databases: MEDLINE (1998 to June 2011; Appendix 1), EMBASE (1998 to June 2011; Appendix 2), CINAHL (1998 to June 2011; Appendix 3), PsycINFO (1998 to June 2011; Appendix 4), and the National Research Register (June 2011). We developed the search strategies with the help of the Cochrane Stroke Group Trials Search Co‐ordinator.
For the purpose of this and other reviews (Lincoln 2001; Das Nair 2007), we originally searched simultaneously for trials in four areas of stroke rehabilitation (cognitive rehabilitation, occupational therapy, speech therapy, and treatment for mood disorders) using online computerised bibliographic databases: MEDLINE (1966 to 1998), BIDS EMBASE (1980 to 1998), CINAHL (1983 to 1998), PsycLIT (1974 to 1998) and CLINPSYCH (1980 to November 1994). We conducted these computerised searches using combinations of the following descriptors/key words: stroke/cerebrovascular accidents/neurological disability and randomised controlled/clinical trials/random allocation/double blind method and rehabilitation/remedial therapy/treatment/intervention and cognitive/unilateral neglect/visuospatial/visuoperceptual/memory/attention span/concentration/hemianopia/attentional deficits/activities of daily living/occupational therapy/leisure/dressing/self‐care/domiciliary rehabilitation.
-
To ensure that studies not listed in the above databases were not overlooked, in 1999 we handsearched all volumes of the journals listed below. The 1999 handsearch included a broad range of journals as it covered studies in four areas of rehabilitation, only one of which (neglect) was relevant to this specific review. Therefore, for the 2006 update we checked the Master List of journals that is searched by The Cochrane Collaboration (www.cochrane.us/masterlist.asp). We found that the journals relevant to neglect had been handsearched. The resulting studies would be found from the search of the Cochrane Central Register of Controlled Trials (CENTRAL) carried out quarterly by the Cochrane Stroke Group and we did not wish to duplicate effort:
American Journal of Occupational Therapy (1947 to 1998);
Aphasiology (1987 to 1998);
Australian Occupational Therapy Journal (1965 to 1998);
British Journal of Occupational Therapy (1950 to 1998);
British Journal of Therapy and Rehabilitation (1994 to 1998);
Canadian Journal of Occupational Therapy (1970 to 1998);
Clinical Rehabilitation (1987 to 1998);
Disability Rehabilitation (1992 to 1998), formerly International Disability Studies (1987 to 1991), formerly International Rehabilitation Medicine (1979 to 1986);
International Journal of Language & Communication Disorders (1998), formerly European Journal of Disorders of Communication (1985 to 1997), formerly British Journal of Disorders of Communication (1977 to 1984);
Journal of Clinical Psychology in Medical Settings (1994 to 1998), formerly Journal of Clinical Psychology (1944 to 1994);
Journal of Developmental and Physical Disabilities (1992 to 1998), formerly Journal of the Multihandicapped Person (1989 to 1991);
Journal of Rehabilitation (1963 to 1998);
International Journal of Rehabilitation Research (1977 to 1998);
Journal of Rehabilitation Science (1989 to 1996);
Neuropsychological Rehabilitation (1987 to 1998);
Neurorehabilitation (1991 to 1998);
Occupational Therapy International (1994 to 1998);
Physiotherapy Theory and Practice (1990 to 1998), formerly Physiotherapy Practice (1985 to 1989);
Physical Therapy (1988 to 1998);
Rehabilitation Psychology (1982 to 1998);
The Journal of Cognitive Rehabilitation (1988 to 1998), formerly Cognitive Rehabilitation (1983 to 1987).
We screened reference lists of all relevant articles.
We used the three citation index databases Science Citation Index (SCI), Social Sciences Citation Index (SSCI) and Arts and Humanities Citation Index (A&HCI) for citation tracking of relevant included studies.
Data collection and analysis
The pre‐1999 searching and selection activities were carried out simultaneously for four reviews. We carried out the most recent updated searches for this review in 2012.
Selection of studies
For this update one review author (CH or AP) screened the titles of records obtained from searches of the electronic databases and excluded irrelevant papers. Two authors (CH and AB or AP) independently assessed the abstracts of the remaining papers and we obtained the full text of papers that were considered possibly relevant. At least two review authors (NBL, AB for 1999 and 2006 versions; NBL, AB, CH and AP for 2013 version) independently selected studies to be included in this review using the four inclusion criteria (types of trials, participants, interventions and outcome measures). We independently assessed the methodological quality of the studies, with reference to the Cochrane Handbook for Systematic Reviews of Interventions (Cochrane Handbook), selected, entered, and cross‐checked data for analysis. We resolved disagreements by discussion.
Data extraction and management
We extracted study characteristics and outcome data. We recorded the following information: method of participant assignment, adequacy of concealment, adequacy of matching at baseline, description of intervention, sample size, numbers lost to follow‐up, types of dependent variable(s), blinding at outcome assessment, reported results and publication details. If these data were not available or unclear from the reports then we contacted the study authors for further information or clarification. We used intention‐to‐treat analyses where possible. We categorised the type of intervention as either a bottom‐up or top‐down processing rehabilitation approach; two review authors categorised each intervention independently and resolved any differences through discussion involving another review author.
Assessment of risk of bias in included studies
For the 2006 update we assessed all studies for quality of allocation concealment and blinding of outcome assessor, and rated those assessed to be at low risk of bias as 'A'. We rated those assessed to be of unclear risk of bias or high risk of bias as 'B'. For this version of the review two review authors independently documented risk of bias for all new studies, classifying each as being at 'high risk', 'low risk' or 'unclear risk' for the following potential biases, using the Cochrane Collaboration's tool for assessing risk of bias (Cochrane Handbook, Chapter 8).
Allocation concealment (selection bias): studies with adequate concealment included those that used either central randomisation at a site remote from the study, computerised allocation in which records were in a locked readable file that could be assessed only after entering participant details, or the drawing of opaque envelopes. Studies with inadequate concealment included those using open list or table of random numbers, open computer systems, or drawing of non‐opaque envelopes. Studies with unclear concealment included those with no or inadequate information.
Blinding of outcome assessment (detection bias): adequate masking included studies that stated that a masked (blinded) outcome assessor was used, and did not identify any 'unmasking'. Inadequate blinding included studies that did not use a masked outcome assessor, or where the report clearly identified that 'unmasking' occurred during the study. We documented blinding as unclear if there was no or insufficient information to judge whether or not an outcome assessor was masked.
Incomplete outcome data (attrition bias): studies adequately addressing incomplete outcome data either had: no missing outcome data; missing outcome data that were unlikely to be related to true outcome; missing outcome data that were balanced in numbers across intervention groups, with similar reasons for missing data across groups; a reported effect size (difference in means or standardised difference in means) among missing outcomes that were not enough to have a clinically relevant impact on observed effect size; or missing data that had been imputed using appropriate methods. Studies inadequately addressing incomplete outcome data either had: missing outcome data that were likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; a reported effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; or as‐treated analysis done with substantial departure of the intervention received from that assigned at randomisation. We documented the addressing of incomplete outcome data as unclear if there was insufficient information to allow us to assess this.
Free of systematic differences in baseline characteristics of groups compared: we assessed a study to be at low risk of bias if there were no differences between groups at baseline; at high risk of bias if there were systematic differences in baseline characteristics of the groups; and at unclear of bias if baseline data were not reported, or if it was unclear whether differences were systematic or random.
Adjustment for baseline differences in the analyses: we assessed a study to be at low risk of bias if either there were no baseline differences (i.e. adjustment is not required) or if appropriate adjustment for the baseline differences had been computed. We assessed a study to be at high risk of bias if there were baseline differences and no adjustment had been computed. We reported this as unclear if there was insufficient information to allow us to assess this.
For the 2006 version of the review, we only documented information on allocation concealment and blinding of outcome assessor. We systematically assessed risk of bias relating to other methodological features in the earlier studies during this update.
Measures of treatment effect
Where a cross‐over design was used we only included data from the first treatment period. Where initial participants were randomised but later allocations were non‐randomised, we only included the study if we could extract the data on those randomised. If not we excluded the study.
Unit of analysis issues
We treated activities of daily living (ADL) data, such as the Barthel Index (BI), as continuous measures and we requested or calculated the mean and standard deviation (SD) data. We are aware that there is a difference of opinion regarding how to deal with ordinal level ADL scales. We have treated them as interval level measures, as in practice it makes relatively little difference. This is supported by a study of parametric versus nonparametric methods in stroke trials, which recommended that means and SDs should be reported (Song 2005). We analysed outcomes as the standardised mean difference (SMD) and 95% confidence interval (CI). We used random‐effects models.
For all analyses of continuous data, we entered data so that a higher score represented a favourable outcome, and the right label of the graph favoured the experimental group. For some of the neglect assessments studies reported outcomes for which a low score was better; for example for 'number of errors' in cancellation tests and 'line bisection'. We multiplied these outcomes, for which a low score was better, by ‐1 in order to pool them with other neglect assessments for which the direction of effect was opposite.
We used odds ratios (ORs) for 'discharge destination', comparing the numbers discharged to their own homes. We treated deaths before discharge as 'not discharged to their own home'. In this way those discharged home were compared with those not discharged home. We calculated ORs for the outcome 'falls', comparing the number of events (falls) within each group.
We used the Cochrane Review Manager 5.1 software for all analyses (RevMan 2011).
Data synthesis
We compared a rehabilitation approach with any other control for both immediate and persisting effects. The controls used were standard care, no treatment or 'attention control' (i.e. where the control group were given extra hours of contact in addition to their standard care to ensure the experimental and control groups had similar amounts of attention from a therapist). We also compared alternative neglect therapies.
Subgroup analysis and investigation of heterogeneity
We planned to carry out subgroup analysis to explore the effect of comparison with an attention control, compared with comparison with no treatment or standard care.
In this update we also added a subgroup comparison of bottom‐up or top‐down interventions (see Description of the intervention). We categorised bottom‐up approaches as either prisms, patching or other interventions, and top‐down approaches as either feedback or cueing, visual scanning training or mental imagery.
Sensitivity analysis
We carried out sensitivity analyses to explore the effect of only including studies with adequate allocation concealment or adequate blinding (i.e. studies assessed to be at low risk of bias).
Results
Description of studies
Results of the search
The results of this review are based on 23 studies involving 628 participants.
In the 2006 version of this review we included 12 randomised controlled trials (RCTs) (306 participants). We did not document details of the searching process and identification of these RCTs. Details of 22 studies that we excluded during the 2006 update, when we removed quasi‐randomised trials, are documented as excluded studies.
For the 2006 version, we identified one RCT of spatial neglect and placed it in 'Studies awaiting assessment' (Cubelli 1993). We have subsequently been unable to get further information relating to this study, and have therefore moved it to excluded studies. In the 2006 version, we listed three ongoing RCTs (Kerkhoff 2005; Rossetti 2005; Turton 2005). The latter has been completed and entered into this update as Turton 2010 (replacing Turton 2005). Multiple publications by Kerkhoff made it difficult to identify which publication corresponded with the Kerkhoff 2005 study that had been identified through personal communication in 2006. However, we identified a report published in 2006 that we have assumed refers to the previously ongoing study. This study did not randomly allocate participants to groups and we have therefore excluded Kerkhoff 2005 (see Characteristics of excluded studies for more information). We have not been able to gain further information for Rossetti 2005 and have therefore left this as an ongoing study. We did not identify any additional ongoing trials for this update.
For this update, we identified 11 additional RCTs. One review author (CH or AP) considered 4875 titles and excluded 4402 obviously irrelevant studies, leaving 473 for consideration. Two review authors (CH, AB or AP) applied selection criteria to these 473 abstracts and classified 63 as possibly relevant. Two review authors (CH, AB or AP) assessed the full papers of these 63 abstracts, leading to the inclusion of 11 RCTs. There was insufficient information for four studies: we have categorised these as studies awaiting classification. We added details of 12 of the 48 excluded studies to the Characteristics of excluded studies table; the remainder were very obviously not relevant to this review. We did not document details of searches prior to this update, but Figure 1 illustrates the results of the 2012 searches, added to the 12 studies included in 2006.
1.
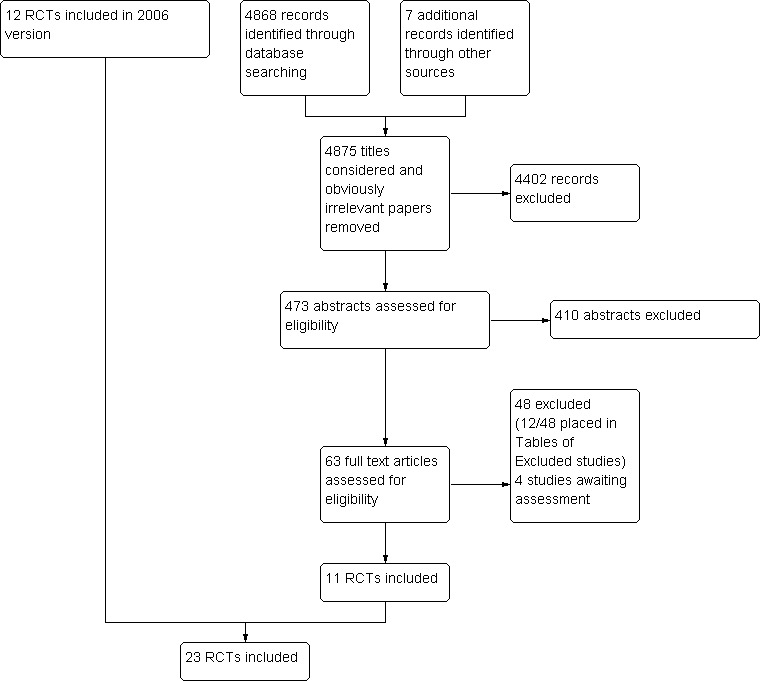
Study flow diagram, detailing results of 2012 searches added to 2006 results.
Included studies
We included data from 628 participants in 23 RCTs: 12 from the 2006 version of this review: Cherney 2002; Cottam 1987; Edmans 2000; Fanthome 1995; Kalra 1997; Robertson 1990; Robertson 2002; Rossi 1990; Rusconi 2002; Weinberg 1977; Wiart 1997; Zeloni 2002, and an additional 11 RCTS identified for this update: Ferreira 2011; Fong 2007; Kerkhoff 2012a; Luukkainen‐Markkula 2009; Mizuno 2011; Nys 2008; Polanowska 2009; Schroder 2008; Tsang 2009; Turton 2010; Welfringer 2011).
Studies had small sample sizes, with a mean size of 27 participants. Four studies had 10 or fewer participants (Cherney 2002: n = 4; Ferreira 2011: n = 10; Kerkhoff 2012a: n = 6; Zeloni 2002: n = 8) and two studies had 50 or more participants (Kalra 1997: n = 50; Fong 2007: n = 60). Statistical power was rarely commented on, but some studies (such as Cherney 2002, Ferreira 2011, Kalra 1997 and Welfringer 2011) did explicitly state that they were intended as pilot or feasibility studies.
All studies were of people with neglect after stroke. However, some studies reported that participants also had visual sensory deficits. Complete hemianopia was present in three of the six participants in the experimental group and one of six participants in the control group in Luukkainen‐Markkula 2009, and in three out of 18 participants in the experimental group and in three of the 20 participants in the control group in Mizuno 2011. In Rossi 1990 some of the participants may have had visual sensory deficits (visual field defects or scanning problems) as well as or instead of neglect: there were 12 out of 18 participants with a visual sensory deficit in the experimental group and 15 of 21 participants in the control group.
Twenty of the 23 included studies only included participants with right hemisphere stroke (Cherney 2002; Cottam 1987; Fanthome 1995; Ferreira 2011; Fong 2007; Kerkhoff 2012a; Luukkainen‐Markkula 2009; Mizuno 2011; Nys 2008; Polanowska 2009; Robertson 1990; Robertson 2002; Rusconi 2002; Schroder 2008; Tsang 2009; Turton 2010; Weinberg 1977; Welfringer 2011; Wiart 1997; Zeloni 2002). The others included those with either left or right hemisphere lesions, although in each study there were more people with right hemisphere lesions.
Six of the centres contributing to the 23 RCTs were based in the UK (Edmans 2000; Fanthome 1995; Kalra 1997; Robertson 1990; Robertson 2002; Turton 2010); four were based in North America (Cherney 2002; Cottam 1987; Rossi 1990; Weinberg 1977); three in Germany (Kerkhoff 2012a; Schroder 2008; Welfringer 2011); two in Italy (Rusconi 2002; Zeloni 2002); two in Hong Kong (Fong 2007; Tsang 2009), and one each in France (Wiart 1997), Netherlands (Nys 2008), Finland (Luukkainen‐Markkula 2009), Brazil (Ferreira 2011), Poland (Polanowska 2009), and Japan (Mizuno 2011).
Many studies recruited from inpatient rehabilitation hospitals (such as Cottam 1987; Fong 2007; Mizuno 2011; Polanowska 2009; Rusconi 2002; Tsang 2009) or specialist inpatient stroke services (for example, Edmans 2000; Kalra 1997; Nys 2008; Turton 2010). In some cases it was not clear where participants were recruited, and whether or not they were inpatients (Ferreira 2011; Kerkhoff 2012a; Schroder 2008; Welfringer 2011). The average age of participants was over 60 years for most studies; it was just under 60 years in four studies (Kerkhoff 2012a; Luukkainen‐Markkula 2009; Polanowska 2009; Welfringer 2011). Three studies explicitly mentioned an age exclusion criterion: Robertson 2002 excluded participants who were aged over 80 years, Mizuno 2011 only included participants aged between 41 and 89 years, and Welfringer 2011 only included participants aged between 20 and 75 years. Many studies excluded participants on the basis of previous dementia or stroke, or current cognitive or communication problems, on the grounds that these would adversely affect responsiveness to therapy. In one study the neglect data were extracted from a larger study (Edmans 2000).
Interventions studied
A broad range of interventions were investigated (for full details see Characteristics of included studies). For 21 of the 23 included studies, the experimental intervention could be classified as either being a top‐down (12) or bottom‐up (9) rehabilitation approach. The remaining two were both classified as being a mix of top‐down and bottom‐up approaches.
Top‐down approaches
Twelve studies investigated the effect of top‐down approaches to rehabilitation (Cherney 2002; Cottam 1987; Edmans 2000; Fanthome 1995; Ferreira 2011; Kerkhoff 2012a; Luukkainen‐Markkula 2009; Robertson 1990; Rusconi 2002; Weinberg 1977; Welfringer 2011; Wiart 1997). The intervention included some sort of visual scanning training in seven studies (Cherney 2002; Cottam 1987; Ferreira 2011; Kerkhoff 2012a; Luukkainen‐Markkula 2009; Robertson 1990; Weinberg 1977); a form of feedback or cueing in four studies (Edmans 2000; Fanthome 1995; Rusconi 2002; Wiart 1997); and mental practice or imagery in two studies (Ferreira 2011; Welfringer 2011). Some approaches involved multiple strategies, for example in Wiart 1997 a therapist participated, actively guiding and giving feedback whilst the participant used the fitted pointer. Fanthome 1995 used specially adapted glasses which gave auditory feedback if the participant failed to scan the neglected side; Wiart 1997 fitted participants with a 'vest' with a metal pointer attached. Some interventions involved training with a therapist. For example, various scanning tasks were used to demonstrate the participant's deficit and show how a strategy could improve performance (Cherney 2002). A therapist was present in both arms of the Rusconi 2002 study but only provided cueing and feedback in the 'experimental' arm. This latter study is an example of cognitive rehabilitation versus an attention control, as participants in both arms received equal amounts of time/attention from a therapist. What differed was the nature of the therapy, i.e. whether or not cueing and feedback were provided by the therapist. In two studies both randomised treatment groups received a top‐down approach; Ferreira 2011 compared one group receiving a visual scanning intervention with another receiving mental practice, and Kerkhoff 2012a compared a group receiving optokinetic stimulation with another receiving standard visual scanning training. Both these studies were classified as comparisons of one cognitive rehabilitation approach versus another.
Bottom‐up approaches
Ten studies investigated the effect of bottom‐up approaches to rehabilitation (Fong 2007; Kalra 1997;Luukkainen‐Markkula 2009; Mizuno 2011; Nys 2008; Robertson 2002; Rossi 1990; Tsang 2009; Turton 2010; Zeloni 2002). Four of these studies investigated fitting prisms to spectacles in order to shift the image towards the neglected side (Mizuno 2011; Nys 2008; Rossi 1990; Turton 2010). Three studies investigated the effect of half‐field eye patching, using glasses or goggles (Fong 2007; Tsang 2009; Zeloni 2002). One study investigated a therapy‐directed intervention comprising spatio‐motor cueing aimed at integrating attention and limb movement (Kalra 1997), and another investigated an 'arm‐activation' intervention where the affected limb performed active arm exercises in the neglected part of space (Luukkainen‐Markkula 2009). The principle behind these approaches is that movements of the affected limb in the neglected part of space will result in improvements in attention skills and appreciation of spatial relationships on the affected side. Robertson 2002 provided a 'limb activation device' fitted to the wrist, leg or shoulder.
Mixed top‐down and bottom‐up approaches
Two of the 23 studies investigated interventions that comprised both top‐down and bottom‐up approaches to rehabilitation. Polanowska 2009 and Schroder 2008 both compared a visual scanning intervention (a top‐down approach) with a visual scanning intervention combined with a type of electrical stimulation (a bottom‐up approach). Both studies compared one cognitive rehabilitation approach with another. The group receiving the additional electrical stimulation was labelled the experimental group and the control was the group receiving visual scanning only (or visual scanning plus placebo electrical stimulation). Schroder 2008 included two experimental groups: one receiving visual scanning plus transcutaneous electrical stimulation (TENS) and one receiving visual scanning plus optokinetic stimulation (OKS). For analysis we have entered these as two studies (Schroder 2008 TENS and Schroder 2008 OKS) and have entered half the control group within each 'study'.
Comparison interventions
We classified 18 of the 23 studies as having a comparison of cognitive rehabilitation versus any other control (Cherney 2002; Cottam 1987; Edmans 2000; Fanthome 1995; Fong 2007; Kalra 1997; Mizuno 2011; Nys 2008; Robertson 1990; Robertson 2002; Rossi 1990; Rusconi 2002; Tsang 2009; Turton 2010; Weinberg 1977; Welfringer 2011; Wiart 1997; Zeloni 2002). Eleven of these 18 studies compared an experimental intervention with an attention control (Cherney 2002; Edmans 2000; Fong 2007; Kalra 1997; Mizuno 2011; Nys 2008; Robertson 1990; Robertson 2002; Rusconi 2002; Turton 2010; Wiart 1997). The remaining seven compared an experimental treatment with no treatment or standard care (Cottam 1987; Fanthome 1995; Rossi 1990; Tsang 2009; Weinberg 1977; Welfringer 2011; Zeloni 2002).
Five studies compared two different active treatments (Ferreira 2011; Kerkhoff 2012a; Luukkainen‐Markkula 2009; Polanowska 2009; Schroder 2008). We did consider that the 'arm‐activation' intervention investigated by Luukkainen‐Markkula 2009 could have been classified as an attention control. However, the dose of intervention given to this group was not the same as the dose given to the visual scanning intervention group (20 to 30 hours for the arm activation group and 10 hours for the visual scanning group). Furthermore, this intervention was considered to be based on principles of spatio‐motor cueing, where the affected limb is moved within the neglected part of space and this comparison intervention was therefore classified as a bottom‐up approach, and Luukkainen‐Markkula 2009 categorised as comparing one active cognitive rehabilitation intervention with another active cognitive rehabilitation intervention.
Dose of interventions
The nature of the interventions was usually well described, as were the number, frequency and duration of therapy sessions. The number of sessions varied from four (Nys 2008) to 40 (Rusconi 2002) over a duration of one to 12 weeks. Sessions ranged from daily to once a week and lasted from 30 to 75 minutes each. The Rossi 1990 study provided the highest 'dose' of rehabilitation as participants in the experimental arm wore their prisms during all daytime activities for four weeks.
Outcomes
Fifteen of the 23 included studies measured functional disability during activities of daily living, the primary outcome of interest. Five reported the Barthel Index (BI) (Edmans 2000; Kalra 1997; Robertson 2002; Rossi 1990; Rusconi 2002); four the Functional Indepedence Measure (FIM) (Ferreira 2011; Fong 2007; Tsang 2009; Wiart 1997) and one the Catherine Bergego scale (Turton 2010). Mizuno 2011 and Luukkainen‐Markkula 2009 assessed both the Catherine Bergego Scale and FIM; we used data for the Catherine Bergego Scale within meta‐analyses. Nys 2008 and Polanowska 2009 stated that the BI was administered but no useable data were provided. Similarly, one study used the Frenchay Activities Index but no data were available (Robertson 1990).
Only six studies reported ADL outcomes at follow‐up (persisting effects) (Ferreira 2011; Fong 2007; Mizuno 2011; Robertson 2002; Turton 2010; Wiart 1997). Mizuno 2011 recorded follow‐up at the time of hospital discharge, rather than at a set time following the end of the intervention.
Twenty‐two of the 23 included studies (all except Robertson 2002) reported a standardised assessment of neglect. One study reported discharge destination (Kalra 1997). In addition, one study reported that the Beck Depression Inventory was administered, but no data were provided post‐intervention (Luukkainen‐Markkula 2009); and one study reported the frequency of falls during the study period (Rossi 1990). No other relevant outcome data were reported i.e. balance, quality of life and social isolation, and adverse events (excluding falls).
Excluded studies
Thirty‐six studies are listed as excluded, with details provided in the Characteristics of excluded studies table.
The original version of this review included 22 studies that were quasi‐randomised: these were removed from the review in the 2006 update and were listed in the Characteristics of excluded studies table. Examples of non‐random methods were: allocating the first set to one arm and the second to the other (Rossetti 1998; Tham 1997); alternate allocation (Pizzamiglio 2004), allocating by bed number (Paolucci 1996), bed availability (Loverro 1988) or date of admission (Harvey 2003).
For this update, we have added a further 14 studies as excluded studies. The review authors discussed 12 of these in detail before they were excluded (Akinwuntan 2010; Bar‐Haim 2011; EEG‐NF 2009; Keller 2006; Kerkhoff 2012b; Koch 2012; Osawa 2010; Serino 2006; Serino 2009; Song 2009; Toglia 2009; Van Os 1991). One study was previously listed as ongoing (Kerkhoff 2005) and one previously listed as awaiting assessment (Cubelli 1993). The reasons for exclusion are listed in the Characteristics of excluded studies table. The review authors particularly discussed Song 2009, which was a RCT of an intervention aimed at neglect in participants with stroke. However, the intervention studied was low‐frequency repetitive transcranial magnetic stimulation (rTMS), which the review authors agreed would be inappropriate to class as a cognitive rehabilitation approach. We initially decided, based on a published English abstract, to include Keller 2006. However, during appraisal of the full German version of the published study we ascertained that the study did not appear to be randomised, and we therefore excluded it.
Risk of bias in included studies
Information on risk of bias is provided in the Characteristics of included studies table and summarised in Figure 2.
2.
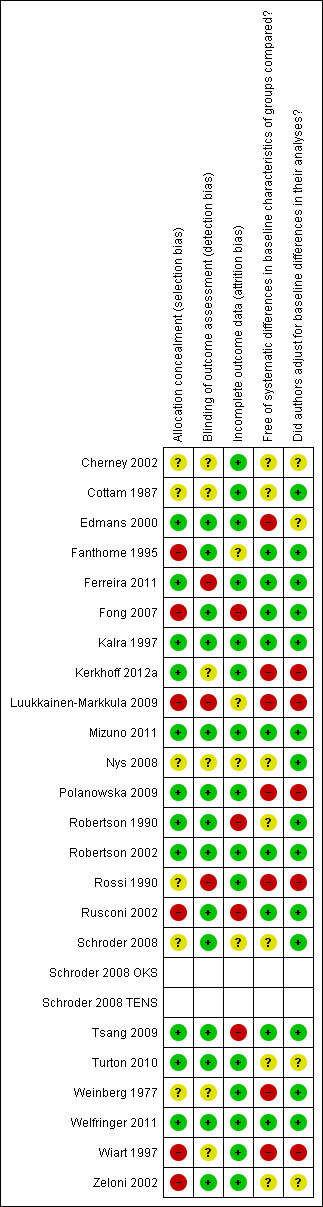
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We assessed 11 of the included RCTs as having low risk of bias with adequate allocation concealment (Edmans 2000; Ferreira 2011; Kalra 1997; Kerkhoff 2012a; Mizuno 2011; Polanowska 2009; Robertson 1990; Robertson 2002; Tsang 2009; Turton 2010; Welfringer 2011). Six studies provided insufficient details to determine adequacy of allocation concealment (Cherney 2002; Cottam 1987; Nys 2008; Schroder 2008; Rossi 1990; Weinberg 1977) and six studies had methods of allocation concealment that we assessed to be at high risk of bias (Fanthome 1995; Fong 2007; Luukkainen‐Markkula 2009; Rusconi 2002; Wiart 1997; Zeloni 2002).
Blinding
We assessed 14 of the included RCTs to have adequate blinding of outcome assessor (Edmans 2000; Fanthome 1995; Fong 2007; Kalra 1997; Mizuno 2011; Polanowska 2009; Robertson 1990; Robertson 2002; Rusconi 2002; Schroder 2008; Tsang 2009; Turton 2010; Welfringer 2011; Zeloni 2002). This information was not provided for six studies (Cherney 2002; Cottam 1987; Kerkhoff 2012a; Nys 2008; Weinberg 1977; Wiart 1997) and three studies did not have a blinded outcome assessor (Ferreira 2011; Luukkainen‐Markkula 2009; Rossi 1990).
Incomplete outcome data
We assessed 15 of the included RCTs as having dealt appropriately with incomplete outcome data (Cherney 2002; Cottam 1987; Edmans 2000, Ferreira 2011; Kalra 1997; Kerkhoff 2012a; Mizuno 2011; Polanowska 2009; Robertson 2002; Rossi 1990; Turton 2010; Weinberg 1977; Welfringer 2011; Wiart 1997; Zeloni 2002. Four were assessed to be at high risk of bias due to incomplete outcome data (Fong 2007; Robertson 1990; Rusconi 2002; Tsang 2009), and insufficient information was available to assess four studies (Fanthome 1995; Luukkainen‐Markkula 2009; Nys 2008; Schroder 2008).
Other potential sources of bias
We assessed nine of the included RCTs to be free of systematic differences in baseline characteristics of the groups compared (Fanthome 1995; Ferreira 2011; Fong 2007; Kalra 1997; Mizuno 2011; Robertson 2002; Rusconi 2002; Tsang 2009; Welfringer 2011). Seven had some baseline differences (Edmans 2000; Kerkhoff 2012a; Luukkainen‐Markkula 2009; Polanowska 2009; Rossi 1990; Weinberg 1977; Wiart 1997) and for seven studies this information was not provided (Cherney 2002; Cottam 1987; Nys 2008; Robertson 1990; Schroder 2008; Turton 2010; Zeloni 2002).
We assessed 14 of the included RCTs to have made adequate adjustments for baseline differences or have no need for adjustment (Cottam 1987; Fanthome 1995; Ferreira 2011; Fong 2007; Kalra 1997; Mizuno 2011; Nys 2008; Robertson 1990; Robertson 2002; Rusconi 2002; Schroder 2008; Tsang 2009; Weinberg 1977; Welfringer 2011). Five had not made adequate adjustments (Kerkhoff 2012a; Luukkainen‐Markkula 2009; Polanowska 2009; Rossi 1990; Wiart 1997), and for four studies this information was not provided (Cherney 2002; Edmans 2000; Turton 2010; Zeloni 2002).
Originally we planned to assess whether or not studies were free from other sources of bias. However, we found this difficult, and could not agree on our assessment of risk of bias for this category so we removed it and used the Notes section in the Characteristics of included studies table for any other potential sources of bias. We considered Luukkainen‐Markkula 2009 to be at high risk of bias because of differences between the groups in the amount of intervention and standard therapy received.
Effects of interventions
We included 23 studies in this review, involving 628 participants. However, five studies did not compare cognitive rehabilitation with a control intervention, instead comparing two active cognitive rehabilitation interventions (Ferreira 2011; Kerkhoff 2012a; Luukkainen‐Markkula 2009; Polanowska 2009; Schroder 2008). Thus, we have included 18 studies in comparisons of a cognitive rehabilitation approach versus any control, and have included five studies in a comparison of one cognitive rehabilitation approach versus another.
For the comparisons of a cognitive rehabilitation approach versus any control we pooled data within analyses of:
measures of functional disability immediately after the end of rehabilitation or on discharge (10 studies, 343 participants);
measures of functional disability persisting over time (five studies, 143 participants);
standardised neglect assessments immediately after the end of rehabilitation or on discharge (16 studies, 437 participants);
standardised neglect assessments persisting over time (eight studies, 172 participants).
For each of these comparisons and outcomes we also completed various subgroup analyses; for example for the immediate effect on measures of functional disability we explored the type of control group (attention control or other/no‐treatment control). We also explored the type of rehabilitation approach (bottom‐up or top‐down) and the various types of bottom‐up and top‐down interventions. We completed sensitivity analyses to explore the effect of studies with adequate allocation concealment and adequate blinding only.
We included data for two additional outcomes within the comparison of cognitive rehabilitation versus any control: immediate effect. These were discharge destination (one study, 50 participants) and frequency of falls (one study, 39 participants).
For the comparison of one cognitive rehabilitation approach versus another we combined data within analyses of:
measures of functional disability immediately after the end of rehabilitation or on discharge (two studies, 21 participants);
measures of functional disability persisting over time (two studies, 22 participants);
standardised neglect assessments immediately after the end of rehabilitation or on discharge (five studies (six comparisons), 98 participants);
standardised neglect assessments persisting over time (four studies (five comparisons), 86 participants).
We calculated a standardised mean difference (SMD) and 95% confidence interval (CI) using a random‐effects model for all comparisons and outcomes, with the exception of discharge destination and frequency of falls when we calculated an odds ratio (OR).
Ratings on measures of functional disability
Cognitive rehabilitation versus any control: immediate effects
For this comparison (versus any control), 10 studies (343 participants) provided usable data for a measure of disability immediately after the end of rehabilitation or on discharge, five with the BI (Edmans 2000; Kalra 1997; Robertson 2002; Rossi 1990; Rusconi 2002), three with the FIM (Fong 2007; Tsang 2009; Wiart 1997) and two with the Catherine Bergego scale (Mizuno 2011; Turton 2010). Two studies collected disability data but these were not available for the review (Robertson 1990: Frenchay Activities Index; Nys 2008: BI), and two others did not compare to a control (Ferreira 2011; Polanowska 2009).
Analyses demonstrated no statistically significant effect in favour of cognitive rehabilitation: SMD 0.23, 95% CI ‐0.02 to 0.48, heterogeneity: Tau² = 0.04; Chi² = 11.77, df = 9 (P = 0.23); I² = 24% (Analysis 1.1).
1.1. Analysis.
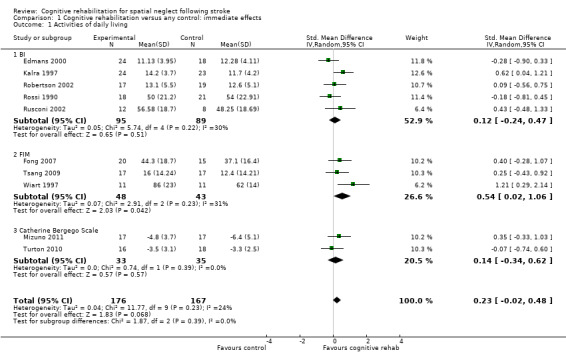
Comparison 1 Cognitive rehabilitation versus any control: immediate effects, Outcome 1 Activities of daily living.
Cognitive rehabilitation versus any control: persisting effects
For this comparison (versus any control) five studies (143 participants) provided data for a measure of disability persisting over time, one with the BI (Robertson 2002), two with the FIM (Fong 2007; Wiart 1997) and two with the Catherine Bergego Scale (Mizuno 2011; Turton 2010).
Analyses demonstrated no statistically significant effect of cognitive rehabilitation (SMD 0.31, 95% CI ‐0.10 to 0.72, heterogeneity: Chi² = 5.87, df = 4 (P = 0.21); I² = 32%) (Analysis 2.1). Subgroup analysis of the FIM data (two studies, 53 participants) showed a statistically significant effect in favour of cognitive rehabilitation (SMD 0.77, 95% CI 0.12 to 1.42, heterogeneity: Chi² = 1.27, df = 1 (P = 0.26); I² = 21%). However, the groups in Wiart 1997 were not well‐matched, with the experimental group being younger and having a higher baseline FIM score (66) than the control group (54). Outcomes assessed on the BI (Robertson 2002) and Catherine Bergego scale (Mizuno 2011; Turton 2010) favoured neither group.
2.1. Analysis.
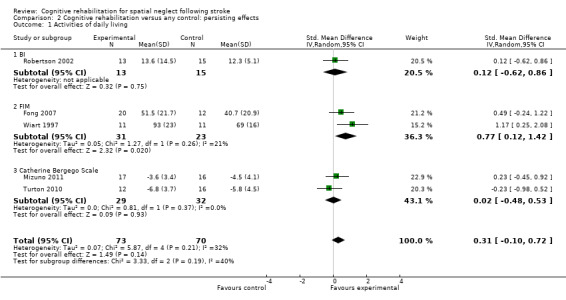
Comparison 2 Cognitive rehabilitation versus any control: persisting effects, Outcome 1 Activities of daily living.
One cognitive rehabilitation intervention versus another: immediate effects
Two studies (21 participants) compared two active cognitive rehabilitation interventions and included an immediate measure of disability (Ferreira 2011; Luukkainen‐Markkula 2009). Ferreira 2011 compared the effect of visual scanning mental practice, measuring disability using the FIM. Both interventions are based on the top‐down processing approach. Luukkainen‐Markkula 2009 compared the effect of a top‐down processing approach (visual scanning) with a bottom‐up approach (arm activation), measuring disability using the Catherine Bergego Scale.
There was no significant different between the two interventions: SMD ‐0.28, 95% CI ‐1.15 to 0.59 (Analysis 3.1). Visual scanning was entered as Approach 1 for both studies.
3.1. Analysis.
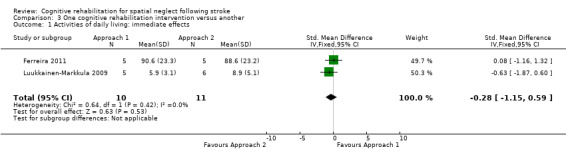
Comparison 3 One cognitive rehabilitation intervention versus another, Outcome 1 Activities of daily living: immediate effects.
One cognitive rehabilitation intervention versus another: persisting effects
Two studies (22 participants) compared two active cognitive rehabilitation interventions and included a follow‐up measure of disability (Ferreira 2011; Luukkainen‐Markkula 2009) (see above for details).
There was no significant difference between the two interventions: SMD ‐0.48, 95% CI ‐1.54 to 0.58 (Analysis 3.2).
3.2. Analysis.
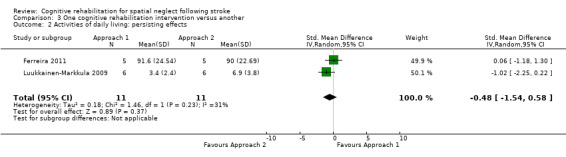
Comparison 3 One cognitive rehabilitation intervention versus another, Outcome 2 Activities of daily living: persisting effects.
Subgroup analyses
Type of control: immediate effects
Eight of the studies (170 participants) that measured disability immediately after the intervention phase had an attention control group (Edmans 2000; Fong 2007; Kalra 1997; Mizuno 2011; Robertson 2002; Rusconi 2002; Turton 2010; Wiart 1997), while two (73 participants) had a no‐treatment or non‐attention control (Rossi 1990; Tsang 2009).Tests for subgroup differences demonstrated no evidence of statistically significant differences between the groups with or without an attention control (P = 0.33) (Analysis 1.9).
1.9. Analysis.
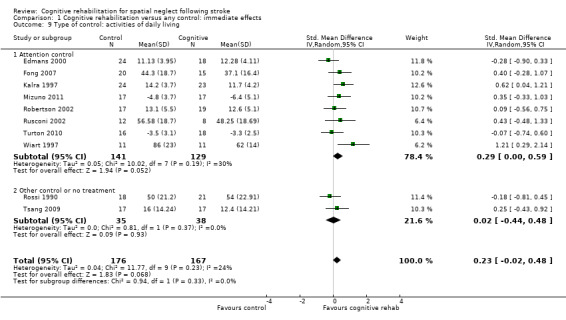
Comparison 1 Cognitive rehabilitation versus any control: immediate effects, Outcome 9 Type of control: activities of daily living.
Type of control: persisting effects
We planned to explore the effect of the type of control (attention control versus no treatment or standard care) for measures of disability persisting over time. However, all of the five studies (Fong 2007; Mizuno 2011; Robertson 2002; Turton 2010; Wiart 1997) included in Comparison 2.1 (cognitive rehabilitation versus any control for measures of disability persisting over time) had an attention control, so further subgroup analysis was not required (Analysis 2.1).
Type of rehabilitation approach: immediate effects
Seven studies of bottom‐up approaches (259 participants) and three studies of top‐down approaches (84 participants) included a measure of disability immediately at the end of rehabilitation. Test for subgroup differences demonstrated no evidence of statistically significant differences between the group comparing bottom‐up approaches with control and the group comparing top‐down approaches with control (P = 0.70) (Analysis 1.11).
1.11. Analysis.
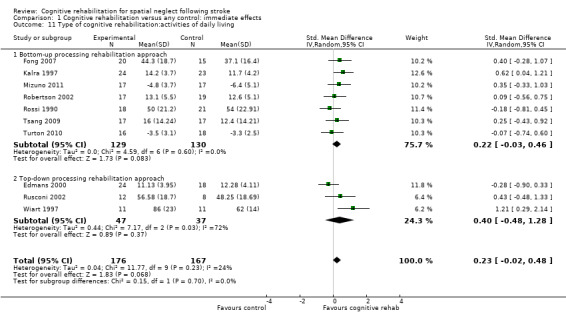
Comparison 1 Cognitive rehabilitation versus any control: immediate effects, Outcome 11 Type of cognitive rehabilitation:activities of daily living.
Type of rehabilitation approach: persisting effects
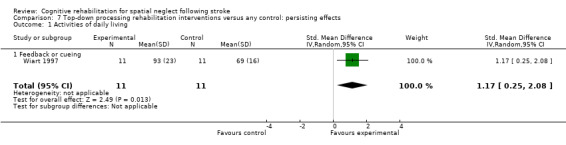
Four studies of bottom‐up approaches (121 participants) and one study of a top‐down approach (22 participants) included a measure of disability persisting over time. Tests for subgroup differences between bottom‐up and top‐down interventions approached statistical significance (P = 0.05). There were no significant differences between bottom‐up approaches and control: SMD 0.16, 95% CI ‐0.20 to 0.52 (Analysis 2.8.1). A statistically significant effect in favour of the single top‐down approach was found (SMD 1.17, 95% CI 0.25 to 2.08). However, there were baseline differences between groups in this study.
2.8. Analysis.
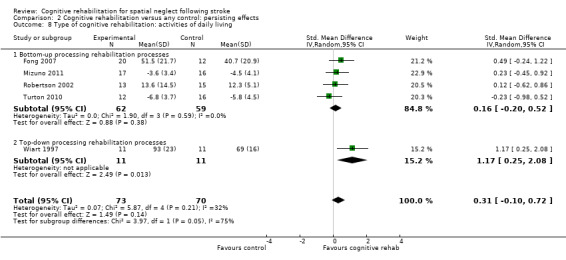
Comparison 2 Cognitive rehabilitation versus any control: persisting effects, Outcome 8 Type of cognitive rehabilitation: activities of daily living.
Types of rehabilitation interventions (Comparisons 4.1, 5.1, 6.1 and 7.1)
Comparison 4.1 explores the effects of different types of bottom‐up interventions (e.g. prisms, eye‐patching) on immediate effects of disability (Analysis 4.1), and Comparison 5.1 the effects on persisting effects of disability (Analysis 5.1). Comparison 6.1 explores the effects of different types of top‐down interventions (e.g. feedback, visual scanning training) on immediate effects of disability (Analysis 6.1), and Comparison 7.1 the effects of persisting effects of disability (Analysis 7.1). These analyses demonstrated no evidence of any significant differences between any of the subgroups (P > 0.05).
4.1. Analysis.
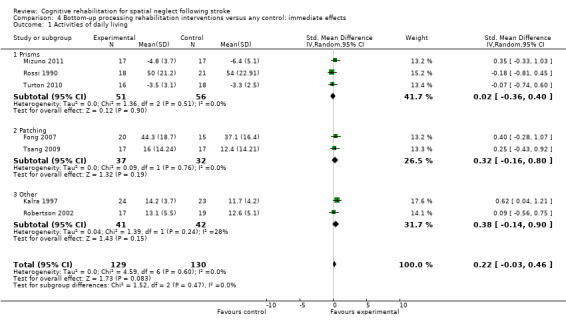
Comparison 4 Bottom‐up processing rehabilitation interventions versus any control: immediate effects, Outcome 1 Activities of daily living.
5.1. Analysis.
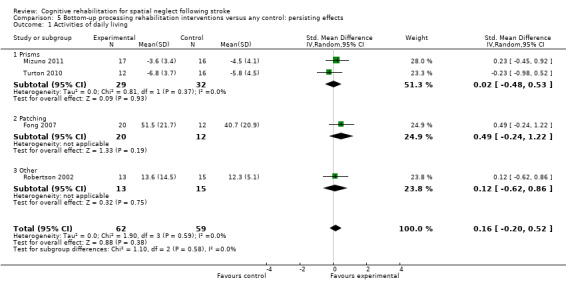
Comparison 5 Bottom‐up processing rehabilitation interventions versus any control: persisting effects, Outcome 1 Activities of daily living.
6.1. Analysis.
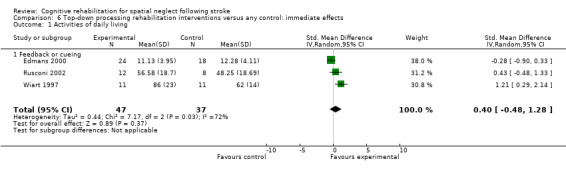
Comparison 6 Top‐down processing rehabilitation interventions versus any control: immediate effects, Outcome 1 Activities of daily living.
7.1. Analysis.
Comparison 7 Top‐down processing rehabilitation interventions versus any control: persisting effects, Outcome 1 Activities of daily living.
Sensitivity analyses
We carried out sensitivity analyses to explore the effect of high or unclear risk of bias due to inadequate allocation concealment and blinding of outcome assessor on the activities of daily living (ADL) outcome.
Cognitive rehabilitation versus any control: adequate allocation concealment only
For immediate effect on ADL: including only the six out of 11 studies that clearly had adequate allocation concealment (low risk of bias) removed the significant effect that was found when including all studies (Comparison 1.1): SMD 0.17, 95% CI ‐0.09 to 0.44, heterogeneity: Chi² = 4.99, df = 5 (P = 0.42); I² = 0% (Analysis 1.5). The significant effect on the FIM was no longer present, with only one study that measured the FIM having adequate allocation concealment (Tsang 2009).
1.5. Analysis.
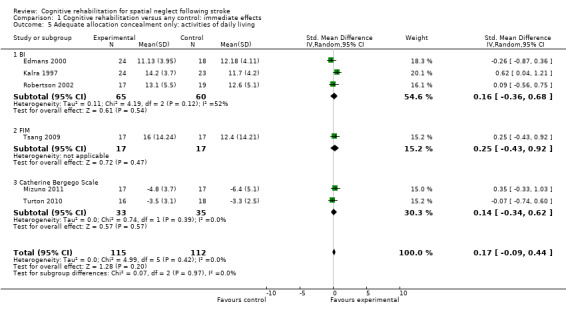
Comparison 1 Cognitive rehabilitation versus any control: immediate effects, Outcome 5 Adequate allocation concealment only: activities of daily living.
For persisting effect on ADL: only three of the five studies had adequate allocation concealment so a sensitivity analysis including only these studies resulted in a reduced effect size and reduced heterogeneity but did not alter the overall result of no significant effect (SMD 0.05, 95% CI ‐0.37 to 0.47, heterogeneity: Chi² = 0.85, df = 2 (P = 0.65); I² = 0%) (Analysis 2.3).
2.3. Analysis.
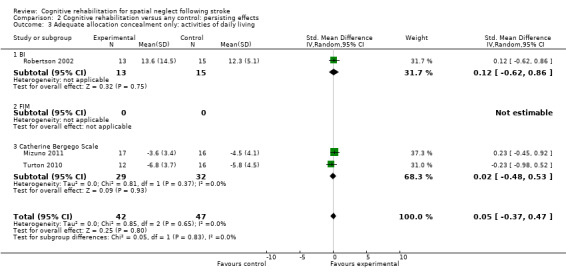
Comparison 2 Cognitive rehabilitation versus any control: persisting effects, Outcome 3 Adequate allocation concealment only: activities of daily living.
Cognitive rehabilitation versus any control: adequate blinding only
For immediate effect on ADL: including only the eight of 11 studies with blinded outcome assessor (low risk of bias) removed the significant effect which was found when including all studies (Comparison 1.1): SMD 0.22, 95% CI ‐0.02 to 0.45, heterogeneity: Chi² = 5.58, df = 7 (P = 0.59); I² = 0% (Analysis 1.6). The significant effect on the FIM was no longer present, but only two studies which used the FIM had blinded outcome assessment (Fong 2007; Tsang 2009).
1.6. Analysis.
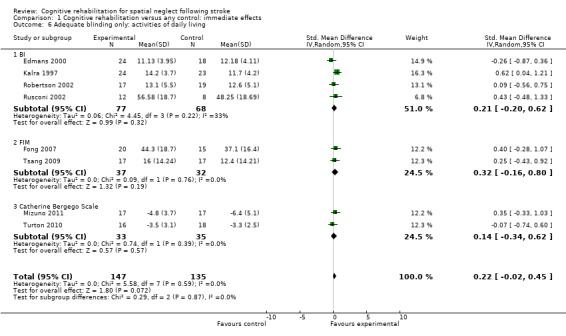
Comparison 1 Cognitive rehabilitation versus any control: immediate effects, Outcome 6 Adequate blinding only: activities of daily living.
For persisting effect on ADL: including only studies with blinded outcome assessor removed one study from the analysis (Wiart 1997); this did not alter the overall result of no significant effect (SMD 0.16, 95% CI ‐0.20 to 0.52, heterogeneity: Chi² = 1.90, df = 3 (P = 0.59); I² = 0%) (Analysis 2.4) but did remove the significant effect of the FIM.
2.4. Analysis.
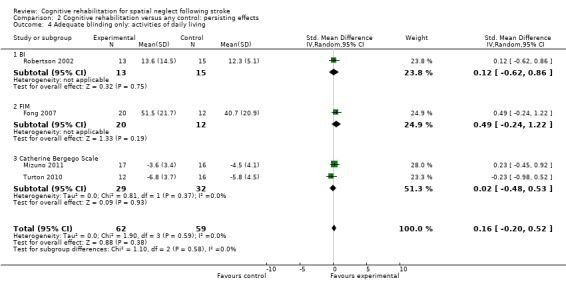
Comparison 2 Cognitive rehabilitation versus any control: persisting effects, Outcome 4 Adequate blinding only: activities of daily living.
Performance on standardised neglect assessments
All except one of the 23 included studies (Robertson 2002) provided data on standardised tests of neglect that were suitable for inclusion, although there was no one measure common to all studies and some used more than one measure. We used a pre‐specified hierarchical system to select one neglect test outcome from each study. Thus 19 of the 20 studies comparing cognitive rehabilitation versus any control and all three studies comparing one cognitive rehabilitation approach with another have data pooled in meta‐analyses. Two studies provided data at follow‐up but not immediately after treatment (Cottam 1987; Fanthome 1995), and many reported immediate but not persisting effects (detailed below).
Cognitive rehabilitation versus any control: immediate effects
Sixteen studies (437 participants) included standardised neglect assessment data for the immediate effect of a comparison of cognitive rehabilitation versus any control. Nine studies had measures of target cancellation (Edmans 2000; Fanthome 1995; Kalra 1997; Nys 2008; Rusconi 2002; Tsang 2009; Weinberg 1977; Welfringer 2011; Zeloni 2002); two had measures of line bisection (Rossi 1990; Wiart 1997); and five had measures of BIT behavioural subtests (Cherney 2002; Fong 2007; Mizuno 2011; Robertson 1990; Turton 2010).
The combined analysis found a significant effect in favour of cognitive rehabilitation (SMD 0.35, 95% CI 0.09 to 0.62, heterogeneity: Chi² = 25.80, df = 15 (P = 0.04); I² = 42%) (Analysis 1.2). There was a statistically significant effect in favour of cognitive rehabilitation for the nine studies using cancellation tests (SMD 0.39, 95% CI 0.03 to 0.74, heterogeneity: Chi² = 13.44, df = 8 (P = 0.10); I² = 40%) and two studies using line bisection (SMD 1.00, 95% CI 0.46 to 1.54, heterogeneity: Chi² = 0.71, df = 1 (P = 0.40); I² = 0%) but not for the studies using the BIT.
1.2. Analysis.
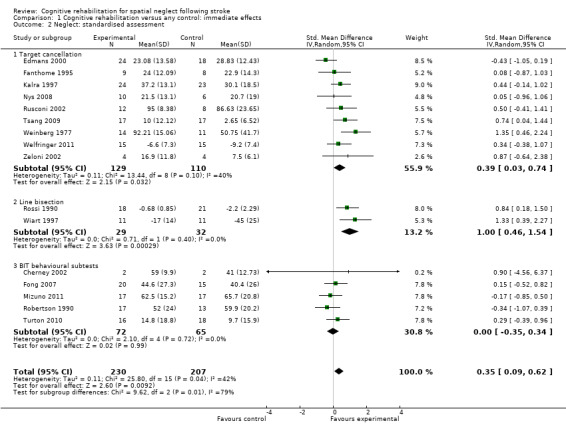
Comparison 1 Cognitive rehabilitation versus any control: immediate effects, Outcome 2 Neglect: standardised assessment.
Cognitive rehabilitation versus any control: persisting effects
Eight of the studies (172 participants) included standardised neglect assessment data for the persisting effect of a comparison of cognitive rehabilitation versus any control. One study had a measure of star cancellation (Nys 2008); one had line bisection (Wiart 1997), and five provided the BIT behavioural subscale (Fanthome 1995; Fong 2007; Mizuno 2011; Robertson 1990; Turton 2010). In addition, one study (Cottam 1987) reported the number of errors during cancellation: as this was the only neglect measure for this study it was entered as a cancellation outcome (with data multiplied by ‐1 to ensure direction of effect was consistent with other outcomes).
The combined analysis found no significant effect of cognitive rehabilitation (SMD 0.28, 95% CI ‐0.03 to 0.59, with no heterogeneity (Chi² = 5.55, df = 7 (P = 0.59); I² = 0%)) (Analysis 2.2).
2.2. Analysis.
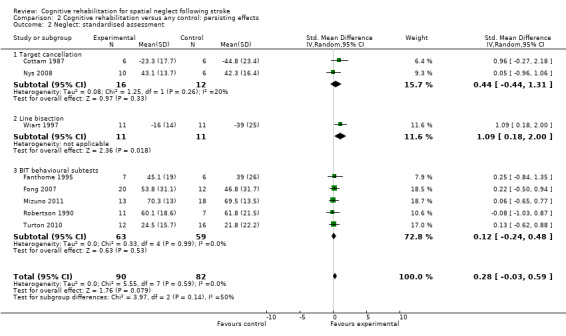
Comparison 2 Cognitive rehabilitation versus any control: persisting effects, Outcome 2 Neglect: standardised assessment.
One cognitive rehabilitation intervention versus another: immediate effect
Five studies comparing different cognitive rehabilitation interventions reported neglect test outcomes (Ferreira 2011; Kerkhoff 2012a; Luukkainen‐Markkula 2009; Polanowska 2009; Schroder 2008). Three of the studies compared visual scanning training with another cognitive rehabilitation intervention; the other interventions comprised a top‐down approach (mental practice) for Ferreira 2011, and a bottom‐up approach for Kerkhoff 2012a (optokinetic stimulation, OKS) and Luukkainen‐Markkula 2009 (arm activation). For each of these studies the visual scanning training was entered as Approach 1 and the other intervention as Approach 2. Pooling data within this subgroup demonstrated no statistically significant differences between Approach 1 and Approach 2 (SMD 0.09, 95% CI ‐0.71 to 0.89, heterogeneity: Chi² = 3.61, df = 2 (P = 0.16); I² = 45%) (Analysis 3.3).
3.3. Analysis.
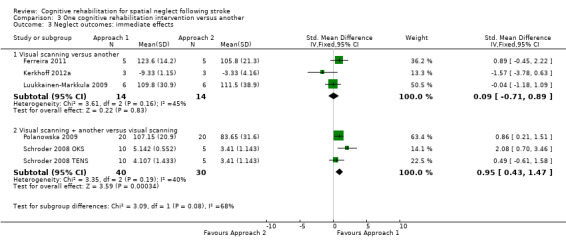
Comparison 3 One cognitive rehabilitation intervention versus another, Outcome 3 Neglect outcomes: immediate effects.
The other two studies investigated the addition of other interventions to visual scanning training. Schroder 2008 compared visual scanning training with the addition of either transcutaneous electrical nerve stimulation (TENS) or OKS. We have entered these as Schroder 2008 OKS and Schroder 2008 TENS and we have entered the OKS and TENS groups as Approach 1 and the control visual scanning training intervention as Approach 2. Polanowska 2009 compared visual scanning training plus electrical somatosensory stimulation (Approach 1) with visual scanning training plus placebo electrical stimulation (Approach 2). Pooling data within this subgroup demonstrated a statistically significant effect in favour of Approach 1 (SMD 0.95, 95% CI 0.43 to 1.47, heterogeneity: Chi² = 3.35, df = 2 (P = 0.19); I² = 40%) (Analysis 3.3.2), suggesting that a combination of visual scanning training plus bottom‐up interventions such as OKS or electrical stimulation may have added benefit. However, there is considerable heterogeneity.
One cognitive rehabilitation intervention versus another: persisting effects
Four of the five studies comparing different cognitive rehabilitation interventions (see descriptions in preceding paragraphs) reported persisting neglect test outcomes (Ferreira 2011; Kerkhoff 2012a; Luukkainen‐Markkula 2009; Schroder 2008). No statistically significant effect in favour of either Approach 1 or 2 was found, for either the subgroup comparing a visual scanning intervention with another cognitive rehabilitation intervention (SMD ‐0.12, 95% CI ‐1.20 to 0.96) or for the subgroup comparing a visual scanning intervention plus another cognitive rehabilitation intervention with a visual scanning intervention alone (SMD 1.13, 95% CI ‐0.33 to 2.60) (Analysis 3.4). There was considerable heterogeneity within these subgroup analyses (heterogeneity: Tau² = 0.38; Chi² = 3.42, df = 2 (P = 0.18); I² = 41% and Tau² = 0.73; Chi² = 2.88, df = 1 (P = 0.09); I² = 65% respectively).
3.4. Analysis.
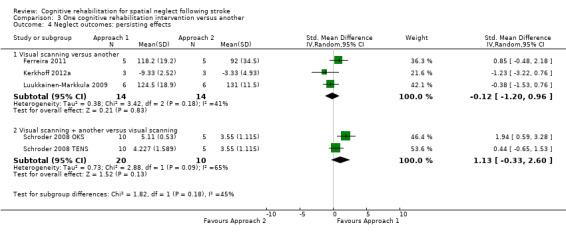
Comparison 3 One cognitive rehabilitation intervention versus another, Outcome 4 Neglect outcomes: persisting effects.
Subgroup analyses
Type of control: immediate effects
Ten of the studies (284 participants) that measured immediate effect on neglect tests had an attention control group (Cherney 2002; Edmans 2000; Fong 2007; Kalra 1997; Mizuno 2011; Nys 2008; Robertson 1990; Rusconi 2002; Turton 2010; Wiart 1997), while six (153 participants) had a no‐treatment or non‐attention control (Fanthome 1995; Rossi 1990; Tsang 2009; Weinberg 1977; Welfringer 2011; Zeloni 2002). Testing for subgroup differences demonstrated some evidence of a statistically significant difference between the group with an attention control group and the group without (P = 0.04). There was no statistically significant effect in favour of cognitive rehabilitation (SMD 0.15, 95% CI ‐0.16 to 0.46) for the studies with an attention control, but there was a statistically significant effect for the studies without an attention control (SMD 0.69, 95% CI 0.36 to 1.02) (Analysis 1.10).
1.10. Analysis.
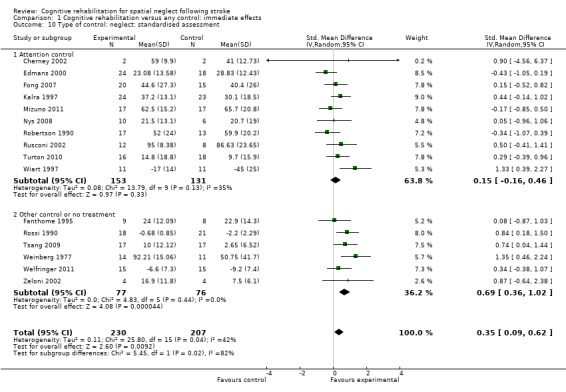
Comparison 1 Cognitive rehabilitation versus any control: immediate effects, Outcome 10 Type of control: neglect: standardised assessment.
Type of control: persisting effects
Six of the studies (147 participants) that measured persisting effect on neglect tests had an attention control group (Fong 2007; Mizuno 2011; Nys 2008; Robertson 1990; Turton 2010; Wiart 1997), while two (25 participants) had a no‐treatment or non‐attention control (Cottam 1987; Fanthome 1995). Tests for subgroup differences demonstrated that there were no statistically significant differences between the groups with or without an attention control (P = 0.45) (Analysis 2.7).
2.7. Analysis.
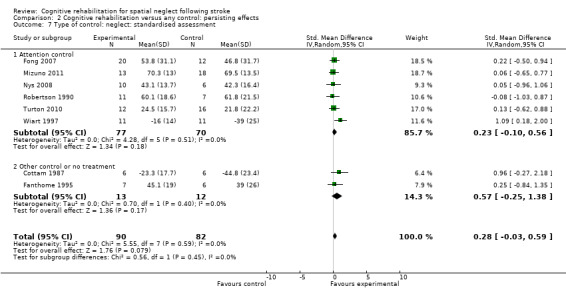
Comparison 2 Cognitive rehabilitation versus any control: persisting effects, Outcome 7 Type of control: neglect: standardised assessment.
Type of rehabilitation approach: immediate effects
Eight studies of bottom‐up approaches (244 participants) and eight studies of top‐down approaches (190 participants) included a standardised neglect assessment immediately at the end of rehabilitation. Tests for subgroup differences demonstrated that there were no statistically significant differences between the group comparing bottom‐up approaches with control and the group comparing top‐down approaches with control (P = 0.96) (Analysis 1.12).
1.12. Analysis.
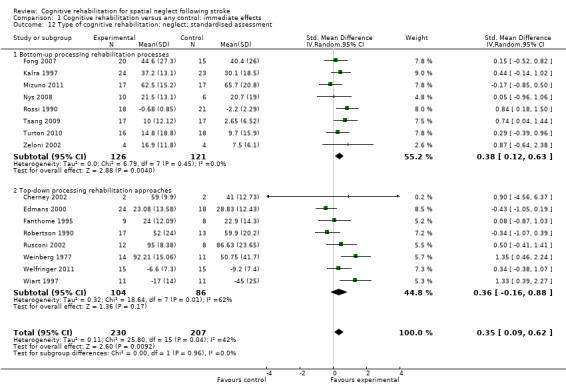
Comparison 1 Cognitive rehabilitation versus any control: immediate effects, Outcome 12 Type of cognitive rehabilitation: neglect: standardised assessment.
Type of rehabilitation approach: persisting effects
Four studies of bottom‐up approaches (107 participants) and four studies of top‐down approaches (65 participants) included a standardised neglect assessment persisting over time. Tests for subgroup differences demonstrated that there were no statistically significant differences between the group comparing bottom‐up approaches with control and the group comparing top‐down approaches with control (P = 0.24) (Analysis 2.9).
2.9. Analysis.
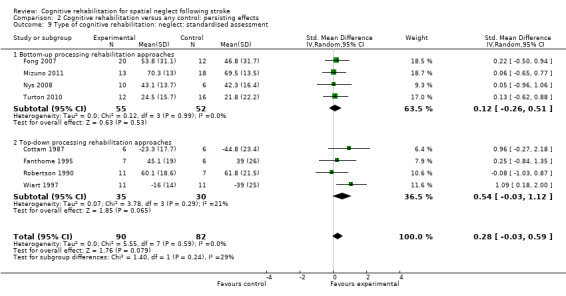
Comparison 2 Cognitive rehabilitation versus any control: persisting effects, Outcome 9 Type of cognitive rehabilitation: neglect: standardised assessment.
Types of rehabilitation interventions
Comparison 4.2 explores the effects of different types of bottom‐up interventions (e.g. prisms, eye‐patching) on immediate effects on neglect outcomes (Analysis 4.2), and Comparison 5.2 the effects on persisting effects on neglect (Analysis 5.2). Comparison 6.2 explores the effects of different types of top‐down interventions (feedback, visual scanning training) on immediate effects on neglect outcomes (Analysis 6.2), and Comparison 7.2 the effects of persisting effects on neglect outcomes (Analysis 7.2). These analyses demonstrated no evidence of significant differences between any of the subgroups (P > 0.05).
4.2. Analysis.
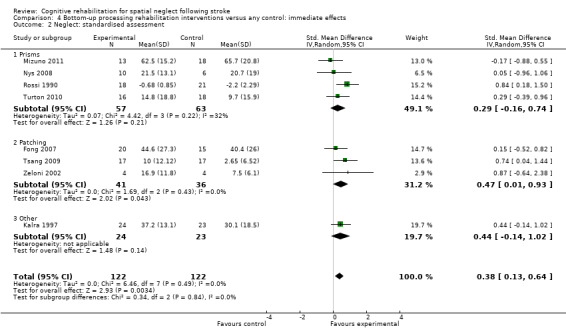
Comparison 4 Bottom‐up processing rehabilitation interventions versus any control: immediate effects, Outcome 2 Neglect: standardised assessment.
5.2. Analysis.
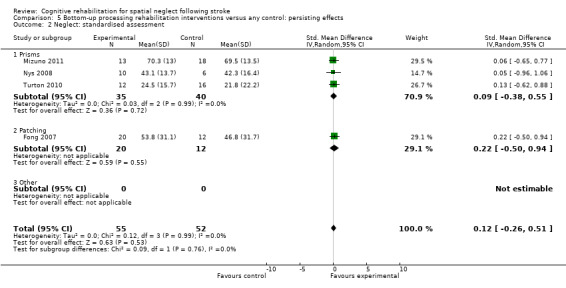
Comparison 5 Bottom‐up processing rehabilitation interventions versus any control: persisting effects, Outcome 2 Neglect: standardised assessment.
6.2. Analysis.
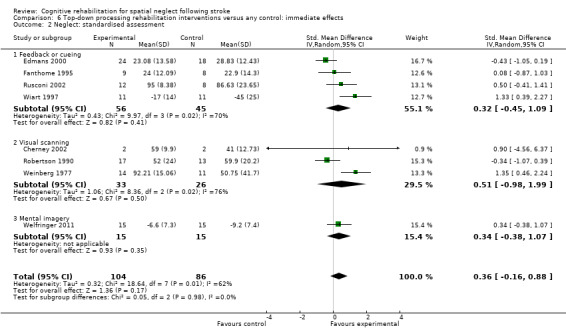
Comparison 6 Top‐down processing rehabilitation interventions versus any control: immediate effects, Outcome 2 Neglect: standardised assessment.
7.2. Analysis.
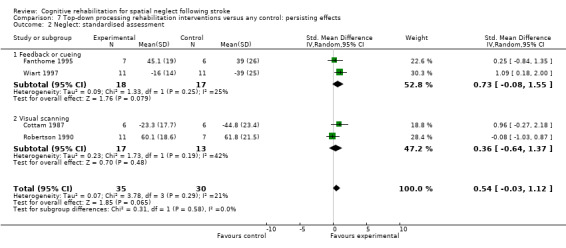
Comparison 7 Top‐down processing rehabilitation interventions versus any control: persisting effects, Outcome 2 Neglect: standardised assessment.
Sensitivity analyses
We carried out sensitivity analyses to explore the effect of high or unclear risk of bias due to inadequate allocation concealment and blinding of outcome assessor on the neglect test outcomes.
Cognitive rehabilitation versus any control: adequate allocation concealment only
For immediate effect on neglect: including only studies that clearly had adequate allocation concealment (low risk of bias) resulted in an analysis of seven studies (242 participants). The results of the analysis changed from showing an effect in favour of cognitive rehabilitation to showing no effect of cognitive rehabilitation (SMD 0.17, 95% CI ‐0.23 to 0.58). There was substantial heterogeneity: Chi² = 14.38, df = 6 (P = 0.03); I² = 58% (Analysis 1.7).
1.7. Analysis.
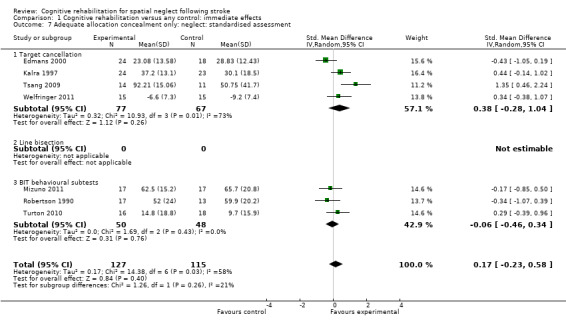
Comparison 1 Cognitive rehabilitation versus any control: immediate effects, Outcome 7 Adequate allocation concealment only: neglect: standardised assessment.
For persisting effect on neglect: only three studies (77 participants) with data had adequate allocation concealment; there remained no effect of cognitive rehabilitation (SMD 0.05, 95% CI ‐0.40 to 0.51, heterogeneity: Chi² = 0.12, df = 2 (P = 0.94); I² = 0%) (Analysis 2.5).
2.5. Analysis.
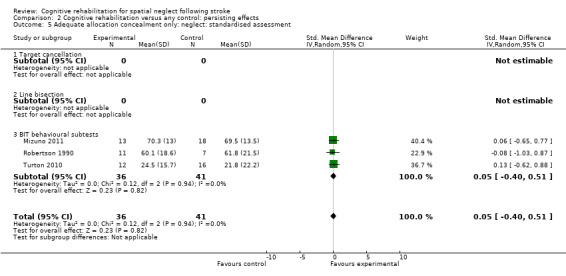
Comparison 2 Cognitive rehabilitation versus any control: persisting effects, Outcome 5 Adequate allocation concealment only: neglect: standardised assessment.
Cognitive rehabilitation versus any control: adequate blinding only
For immediate effect on neglect: including only studies with blinded outcome assessors resulted in an analysis including 11 studies (336 participants), changing the result from showing an effect in favour of cognitive rehabilitation to showing no effect of cognitive rehabilitation (SMD 0.24, 95% CI ‐0.06 to 0.54, heterogeneity: Chi² = 17.67, df = 10 (P = 0.06); I² = 43%) (Analysis 1.8).
1.8. Analysis.
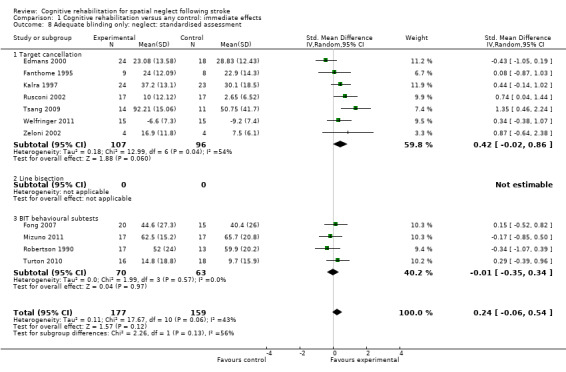
Comparison 1 Cognitive rehabilitation versus any control: immediate effects, Outcome 8 Adequate blinding only: neglect: standardised assessment.
For persisting effect on neglect: including only studies with blinded outcome assessors included four studies (122 participants); there remained no effect of cognitive rehabilitation (SMD 0.12, 95% CI ‐0.24 to 0.48, heterogeneity: Chi² = 0.33, df = 4 (P = 0.99); I² = 0%) (Analysis 2.6).
2.6. Analysis.
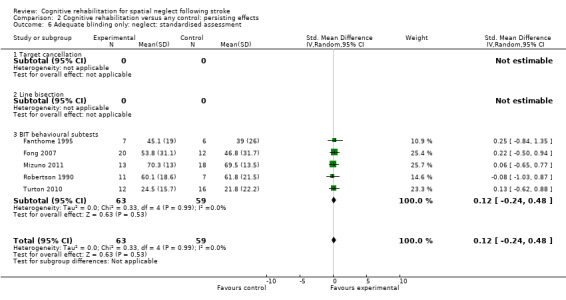
Comparison 2 Cognitive rehabilitation versus any control: persisting effects, Outcome 6 Adequate blinding only: neglect: standardised assessment.
Discharge destination
Only one RCT (50 participants), assessed as being at low risk of bias for allocation concealment and blinding of outcome assessment, investigated discharge destination as an outcome (Kalra 1997). The odds of being discharged home were not significantly higher for the experimental group (OR 1.40, 95% CI 0.45 to 4.35, P = 0.56) (Analysis 1.3).
1.3. Analysis.
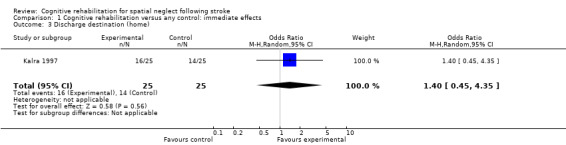
Comparison 1 Cognitive rehabilitation versus any control: immediate effects, Outcome 3 Discharge destination (home).
Falls
One study (39 participants) reported the number of falls during the study period (Rossi 1990). There were no significant differences in the odds of falling between groups (OR 1.21, 95% CI 0.26 to 5.76, P = 0.81) (Analysis 1.4).
1.4. Analysis.
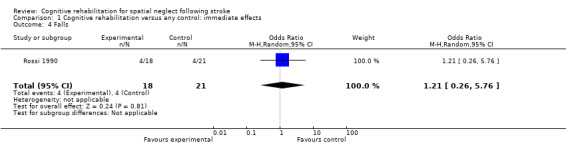
Comparison 1 Cognitive rehabilitation versus any control: immediate effects, Outcome 4 Falls.
Discussion
In this updated version of the review, we included 11 new randomised controlled trials (RCTs) bringing the total to 23 trials (628 participants).
Summary of main results
A summary of the results of the key analyses is shown in Table 8. Meta‐analyses demonstrated no statistically significant effect of cognitive rehabilitation compared with control for persisting effects on measures of functional disability (five studies, 143 participants) or standardised neglect assessments (eight studies, 172 participants), or for immediate effects on activities of daily living (ADL) (10 studies, 343 participants). In contrast, we found a statistically significant effect in favour of cognitive rehabilitation compared with control for immediate effects on standardised neglect assessments (16 studies, 437 participants). However, sensitivity analyses (including only studies of high methodological quality) removed evidence of a significant effect of cognitive rehabilitation. Thus, while the studies included within this review appear to provide some evidence of an immediate effect of cognitive rehabilitation standardised neglect assessments, this finding is not sustained when only studies of the highest quality are examined.
1. Summary of key results.
| ADL: immediate | ADL: persisting | Neglect test: immediate | Neglect test: persisting | |
| Cognitive rehabilitation versus control | 10 studies, n = 343 No significant difference (SMD 0.23, 95% CI ‐0.02 to 0.48) |
5 studies, n = 143 No significant difference (SMD 0.31, 95% CI ‐0.10 to 0.72) |
16 studies, n = 437 Significant difference favours cognitive rehabilitation (SMD 0.35, 95% CI 0.09 to 0.62) |
8 studies, n = 172 No significant difference (SMD 0.23, 95% CI ‐0.03 to 0.59) |
| Subgroup: type of control | Attention control: 8 studies n = 170 Non‐attention control: 2 studies n = 73 No significant differences between subgroups (P = 0.33) |
Attention control: 5 studies, n = 143 (No subgroup analysis required) |
Attention control: 10 studies, n = 284 Non‐attention control: 6 studies, n = 153 Significant difference between subgroup with and without an attention control group (P = 0.04) |
Attention control: 6 studies, n = 147 Non‐attention control: 2 studies, n = 25 No significant differences between subgroups (P = 0.45) |
| Subgroup: type of rehabilitation approach (bottom‐up or top‐down) | Bottom‐up: 7 studies, n = 259 Top‐down: 3 studies, n = 84 No significant differences between subgroups (P = 0.70) |
Bottom‐up: 4 studies, n = 121 Top‐down: 1 study, n = 22 Differences between bottom‐up and top‐down interventions approached statistical significance (P = 0.05) |
Bottom‐up: 8 studies, n = 244 Top‐down: 8 studies, n = 190 No significant differences between subgroups (P = 0.96) |
Bottom‐up: 4 studies, n = 107 Top‐down: 4 studies, n = 65 No significant differences between subgroups (P = 0.24) |
|
Sensitivity: high‐quality studies only (variety of quality criteria explored) |
No significant difference | No significant difference | No significant difference | No significant difference |
CI: confidence interval SMD: standardised mean difference
Subgroup analyses exploring the effect of having an attention control (as opposed to a control that did not equalise the time participants spent with a therapist) demonstrated that there was some evidence of a statistically significant difference between the subgroup comparing cognitive rehabilitation with attention control and the subgroup comparing cognitive rehabilitation with another control or a no‐treatment group (i.e. for immediate effects on measures of neglect). This indicates that part of the effect of these interventions could be the time spent with the therapist, and demonstrates the importance of attention control groups within trials of therapist‐led interventions.
In addition to the studies comparing bottom‐up and top‐down cognitive rehabilitation approaches each against a control, we identified five studies comparing one cognitive rehabilitation intervention with another. These included three studies comparing a visual scanning intervention with another cognitive rehabilitation intervention, and two studies (three comparison groups) comparing a visual scanning intervention plus another cognitive rehabilitation intervention with a visual scanning intervention alone. Only two small studies reported a measure of functional disability and there was considerable heterogeneity within these subgroups (I² > 40%) when we pooled standardised neglect assessment data, limiting our ability to draw generalised conclusions.
Key findings from this updated review
23 RCTs (628 participants) evaluated a range of cognitive rehabilitation interventions for people with neglect after stroke. Most studies measured outcomes using standardised neglect assessments. Many reported immediate effects on ADL, but few reported persisting effects on ADL. Other meaningful outcomes such as discharge destination, falls, mood, quality of life or adverse events were rarely or never reported.
Methodological quality was generally poor or poorly described, and sample sizes small. Interventions were generally well‐described and trialists were helpful in providing additional unpublished methodological details.
There was some limited evidence that cognitive rehabilitation may have an immediate effect on neglect impairment. However, there was considerable heterogeneity and evidence that this effect was not sustained when studies with high risk of bias were removed.
There was some evidence of subgroup differences between studies with and without an attention control group, highlighting the need for attention control in rehabilitation research.
Despite 23 completed studies, there is still insufficient evidence to draw generalised conclusions about the effect of cognitive rehabilitation interventions on functional ability in ADL or on standardised neglect assessments. This is largely because adequately powered, appropriately designed trials do not yet exist. Further research must ensure low risk of bias, evaluate persisting effects on ADL measures and ideally include an attention control.
Patients' and carers' views on the acceptability of interventions and on appropriate outcome measurement were strikingly absent from the literature.
Overall completeness and applicability of evidence
Methods
Both included and excluded study authors were helpful in providing unpublished data. This review therefore presents a considerable amount of unpublished data and previously unpublished clarification of the methods used by the original authors. In contrast to the problems of methodological reporting, the reporting quality of the rehabilitation approach used has generally improved. This enabled us to add comparisons of the two main theoretical approaches to cognitive rehabilitation, bottom‐up and top‐down.
Participants
Almost all participants in the included studies had right hemisphere stroke, and the majority of the studies were completed in inpatient settings. It is, therefore, appropriate only to generalise from the results of these studies to the population of inpatients with neglect following right hemisphere stroke. Rehabilitation for people with long‐term persisting neglect may be different for those in the earlier stages of recovery.
Studies generally had small sample sizes, limiting our ability to make generalisations. We observed no trends in the sample size over years, with the majority failing to address issues of statistical power. The available studies should provide sufficient data to enable power calculations for future studies, and we urge researchers to design appropriately powered studies.
Interventions
The included studies investigated a variety of bottom‐up and top‐down rehabilitation interventions and some may challenge our treatment of them as a single entity. However, this review was designed to establish whether cognitive rehabilitation interventions were more beneficial than control, and to that end pooling the studies was appropriate. We also carried out subgroup comparisons according to mode of intervention (bottom‐up or top‐down) and subdivided into specific treatments (e.g. visual scanning, prisms, patching). Although these studies differed in the number, frequency and duration of therapy sessions, these were generally well‐described and the interventions were similar, providing evidence that is applicable to clinical settings.
Comparisons
Eleven of the 23 included studies had an attention control group, and six had either a no‐treatment or a standard care control. These comparisons are appropriate to evaluate the effectiveness of cognitive rehabilitation, providing results that should be generalisable. Our subgroup analyses comparing studies with and without an attention control group provided some evidence of a significant difference between these subgroups for immediate effect on neglect, demonstrating the importance of attention control groups for future trials of cognitive rehabilitation interventions.
Five of the included studies compared two cognitive rehabilitation approaches. Considerable heterogeneity and variations in the approaches studied make it difficult to draw generalised conclusions from these studies. Further research is clearly required to identify the relative effectiveness of different cognitive rehabilitation approaches.
Outcomes
The majority (15 out of 23) of studies reported our primary outcome of interest, a measure of functional disability during activities of daily living, but only six reported these outcomes at follow‐up. This lack of follow‐up data on functional disability limited our ability to determine the persistence or maintenance of functional recovery.
Almost all (22 out of 23) of the studies reported a standardised neglect assessment. In previous versions of this review, we extracted and analysed all reported neglect assessments separately. However, for this update, we decided it was more clinically relevant to extract results of only one neglect assessment from each study, according to a pre‐defined hierarchy, and to combine these assessments in order to determine a pooled estimate of effect on neglect.
There were very few data on other outcomes, such as discharge destination, falls or depression. These are known to be of importance to stroke survivors and including these in future research would improve the completeness of the evidence base.
Quality of the evidence
The method of randomisation was generally poorly described and the published papers were often not sufficiently detailed to determine whether allocation concealment was adequate. We assessed six of the included studies to be at high risk of bias due to inadequate allocation concealment, and there was insufficient information for a further six studies. We assessed the majority of the studies (14 out of 23) to have adequately blinded outcome assessment, although three studies did not, and six failed to provide this information.
Authors' conclusions
Implications for practice.
As the effectiveness of cognitive rehabilitation for reducing the disabling effects of neglect and increasing independence remains unproven, no rehabilitation approach can be supported or refuted from current randomised controlled trials. However, there is some very limited evidence that cognitive rehabilitation may have an immediate effect on performance of tests of neglect. Until robust evidence is available, clinical practice should follow national clinical guidelines and clinicians are strongly encouraged to participate in high quality trials. People with neglect should continue to receive general stroke rehabilitation services and to have the opportunity to take part in high quality research.
Implications for research.
There is sufficiently compelling evidence to encourage further trials of cognitive rehabilitation for neglect. However, future studies need to improve on methodological and reporting issues and should describe the different types of neglect. Key procedural aspects, such as randomisation, concealment, completeness of follow‐up, and blinding of assessors, must be sufficiently described. In fact the process of random allocation appears to be misunderstood. We found several studies that were described as randomised but which had instead used alternate allocation or other methods that risked selection bias. Trialists are referred to the Cochrane Handbook for a description of acceptable methods of randomisation. Concealment and blinding appear to be confused with each other but again are well described in the Cochrane Handbook. By its nature, cognitive rehabilitation is likely to be restricted to single‐blind trials (of outcome assessors) as blinding of participants and therapists is not usually achievable. Cross‐over trials are not appropriate for cognitive rehabilitation as the effects of one approach may contaminate the next. As rehabilitation aims to promote independence and the maintenance of treatment effects it is not logical to expect the 'washout' effect that is possible with some drug therapies.
Furthermore, trials need to have adequate statistical power to detect a clinically meaningful difference. Power was very rarely mentioned in neglect trials and the small sample sizes used were unlikely to be adequate. Sample specification and the description of selection methods could also be improved. Neglect is a heterogeneous condition and it is unlikely that a single rehabilitation approach is appropriate for all types and severities. Future trials should provide adequate sample description, theoretical justification, and consider using stratified randomisation to avoid imbalance of any factors likely to confound the trial. Future studies must avoid using non‐random allocation methods (such as matching) as this risks introducing bias. Trials should assess both functional activities of daily living and neglect at a follow‐up assessment (i.e. persisting effects) as the maintenance of function is of key importance. Trialists should also consider other outcomes that are of importance to stroke survivors, including falls and quality of life. Part of the effect of therapist‐led interventions may well be the time spent with a therapist and future trials should include an attention control.
There is scope for both pragmatic and explanatory RCTs. Explanatory trials provide evidence on efficacy, examining whether a single rehabilitation approach (such as prism adaptation) can work in an optimum situation. These typically involve more homogeneous samples with little co‐morbidity, treated by research therapists with protected time in a controlled environment. There is also a need for pragmatic RCTs to provide evidence on effectiveness and, ideally, cost effectiveness of rehabilitation in a realistic clinical setting. Finally, completeness of follow‐up and intention‐to‐treat analysis are necessary. Previous analyses tended to be per protocol and therefore do not indicate the acceptability of rehabilitation to service users. A high drop‐out rate may be an important indication of effectiveness and future neglect trialists are recommended to consult the Cochrane Handbook for a good discussion of intention‐to‐treat analysis.
This review is ongoing and the authors would be grateful to receive information on ongoing studies for a future update.
What's new
| Date | Event | Description |
|---|---|---|
| 17 April 2013 | New citation required but conclusions have not changed | Despite the addition of 11 further trials, the key conclusions of this review have not changed greatly since the 2006 version: The effectiveness of cognitive rehabilitation for reducing the disabling effects of neglect and increasing independence remains unproven. No rehabilitation approach can be supported or refuted from current randomised controlled trials. |
| 23 September 2012 | New search has been performed | We added 11 new trials to the 12 trials that we included in the previous version. Twenty‐three trials (628 participants) are now included. We have re‐written the Discussion section using standard Cochrane sub‐headings. We have expanded the outcomes: previous versions of the review had functional disability, neglect assessments and discharge destination as outcomes. In this update we added a number of secondary outcomes that had been identified as important to stroke survivors. This brings this review into line with other reviews of visual problems after stroke. We have changed the comparisons: for this version of the review we changed the presentation of the statistical comparisons. In particular, we amended the subgroup comparisons of bottom‐up and top‐down approaches so that analyses included subgroups of types of treatment. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 2, 2002
| Date | Event | Description |
|---|---|---|
| 4 August 2008 | Amended | Converted to new review format. |
| 26 April 2006 | New search has been performed | In this updated review we excluded several previously included non‐randomised trials to reduce bias. We added several new, or newly identified, randomised controlled trials (RCTs), resulting in a review of 306 participants from 12 RCTs. |
Acknowledgements
We would like to thank the principal investigators of many of the included and excluded studies who provided additional information to that published and to Clare Starmer for helping with early searches. We are especially indebted to Brenda Thomas and Hazel Fraser at the Cochrane Stroke Group for their continued support and specialist guidance. Thanks also to the Editorial team and external peer reviewer who provided useful suggestions for improving the clarity and focus of this review. We would also like to thank Editorial team members Jan Mehrholz and Frederike van Wijck for helping us with translation and appraisal of non‐English language papers.
The searches for the first version of this review were funded by grants to Nadina Lincoln from the Stroke Association and the UK NHS Research and Development Programme for Physical and Complex Disabilities. Michael Dewey provided statistical input to the first published version of this review, for which we are very grateful. The RNIB (Royal National Institute for Blind people) funded Christine Hazelton's time on this update.
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp intracranial arteriovenous malformations/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vasospasm, intracranial/ or vertebral artery dissection/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiplegia/ or exp paresis/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp Perceptual disorders/ 9. exp perception/ 10. Attention/ 11. "Extinction (psychology)"/ 12. (hemineglect or hemi‐neglect).tw. 13. ((unilateral or spatial) adj5 neglect).tw. 14. (perception or inattention or hemi‐inattention or attention or extinction).tw. 15. ((perceptual or visuo?spatial or visuo?perceptual or attentional) adj5 (disorder$ or deficit$ or impairment$ or abilit$)).tw. 16. ((perceptual or visuo?spatial or visuo?perceptual or attention$ or cognit$ or scanning$) adj5 (training or re‐training or rehabilitation or intervention or therapy)).tw. 17. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 18. 7 and 17 19. Randomized Controlled Trials as Topic/ 20. random allocation/ 21. Controlled Clinical Trials as Topic/ 22. control groups/ 23. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/ 24. double‐blind method/ 25. single‐blind method/ 26. Placebos/ 27. placebo effect/ 28. cross‐over studies/ 29. Multicenter Studies as Topic/ 30. Therapies, Investigational/ 31. Drug Evaluation/ 32. Research Design/ 33. Program Evaluation/ 34. evaluation studies as topic/ 35. randomized controlled trial.pt. 36. controlled clinical trial.pt. 37. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt. 38. multicenter study.pt. 39. (evaluation studies or comparative study).pt. 40. random$.tw. 41. (controlled adj5 (trial$ or stud$)).tw. 42. (clinical$ adj5 trial$).tw. 43. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 44. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 45. ((multicenter or multicentre or therapeutic) adj5 (trial$ or stud$)).tw. 46. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 47. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 48. (coin adj5 (flip or flipped or toss$)).tw. 49. latin square.tw. 50. versus.tw. 51. (cross‐over or cross over or crossover).tw. 52. placebo$.tw. 53. sham.tw. 54. (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw. 55. controls.tw. 56. (treatment$ adj6 order).tw. 57. or/19‐56 58. 18 and 57 59. exp child/ or exp infant/ 60. (neonat$ or child or children or childhood or juvenile or infant or toddler).tw. 61. exp neoplasms/ 62. (cancer$ or carcinoma$ or tumor$ or tumour$ or neoplasm$).tw. 63. case reports.pt. or case report$.tw. 64. 59 or 60 or 61 or 62 or 63 65. 58 not 64 66. limit 65 to humans
Appendix 2. EMBASE search strategy
1. cerebrovascular disease/ or basal ganglion hemorrhage/ or cerebral artery disease/ or cerebrovascular accident/ or stroke/ or exp carotid artery disease/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp cerebrovascular malformation/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or stroke unit/ or stroke patient/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiparesis/ or hemiplegia/ or paresis/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp perception disorder/ 9. exp perception/ 10. exp attention/ 11. visual deprivation/ 12. (hemineglect or hemi‐neglect).tw. 13. ((unilateral or spatial or hemi?spatial) adj5 neglect).tw. 14. (perception or inattention or hemi‐inattention or attention or extinction).tw. 15. ((perceptual or visuo?spatial or visuo?perceptual or attentional) adj5 (disorder$ or deficit$ or impairment$ or abilit$ or dysfunction)).tw. 16. ((perceptual or visuo?spatial or visuo?perceptual or attention$ or cognit$ or scanning$) adj5 (training or retraining or rehabilitation or intervention or therapy)).tw. 17. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 18. 7 and 17 19. Randomized Controlled Trial/ 20. Randomization/ 21. Controlled Study/ 22. control group/ 23. clinical trial/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ or controlled clinical trial/ 24. Crossover Procedure/ 25. Double Blind Procedure/ 26. Single Blind Procedure/ or triple blind procedure/ 27. latin square design/ 28. Parallel Design/ 29. placebo/ 30. Multicenter Study/ 31. experimental design/ or experimental study/ or quasi experimental study/ 32. experimental therapy/ 33. drug comparison/ or drug dose comparison/ 34. drug screening/ 35. Evaluation/ or "Evaluation and Follow Up"/ or evaluation research/ or clinical evaluation/ 36. Methodology/ 37. "types of study"/ 38. research subject/ 39. Comparative Study/ 40. random$.tw. 41. (controlled adj5 (trial$ or stud$)).tw. 42. (clinical$ adj5 trial$).tw. 43. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 44. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 45. ((multicenter or multicentre or therapeutic) adj5 (trial$ or stud$)).tw. 46. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 47. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 48. (coin adj5 (flip or flipped or toss$)).tw. 49. latin square.tw. 50. versus.tw. 51. (cross‐over or cross over or crossover).tw. 52. placebo$.tw. 53. sham.tw. 54. (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw. 55. controls.tw. 56. (treatment$ adj6 order).tw. 57. or/19‐56 58. 18 and 57 59. exp child/ or exp newborn/ 60. (neonat$ or child or children or childhood or juvenile or infant or toddler).tw. 61. exp Neoplasm/ 62. (cancer$ or carcinoma$ or tumor$ or tumour$ or neoplasm$).tw. 63. case report/ or case study/ 64. 59 or 60 or 61 or 62 or 63 65. 58 not 64 66. limit 65 to human
Appendix 3. CINAHL (Ebsco) search strategy
S1.. (MH "Cerebrovascular Disorders+") or (MH "stroke patients") or (MH "stroke units") S2.. TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc* or cva or apoplex* or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc* or cva or apoplexy* or SAH ) S3.. TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) S4.. TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) S5.. S3 and S4 S6.. TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) S7.. TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) S8.. S6 and S7 S9.. (MH "Hemiplegia") S10.. TI ( hemipleg* or hemipar* or paresis or paretic ) or AB ( hemipleg* or hemipar* or paresis or paretic ) S11.. S1 or S2 or S5 or S8 or S9 or S10 S12. (MH "Perceptual Disorders+") S13. (MH "Perception+") S 14. (MH "attention") S 15. (MH "Unilateral Neglect (Saba CCC)") or (MH "Unilateral Neglect (NANDA)") S 16. TI (hemineglect or hemi‐neglect) or AB (hemineglect or hemi‐neglect) S 17. TI (unilateral or spatial) or AB (unilateral or spatial) S 18. TI (neglect) or AB (neglect) S19. S17 and S18 S 20. TI (perception or inattention or hemi‐inattention or attention or extinction) or AB (perception or inattention or hemi‐inattention or attention or extinction) S 21. TI (perceptual or visuo#spatial or visuo#perceptual or attentional) or AB (perceptual or visuo#spatial or visuo#perceptual or attentional) S 22. TI (disorder* or deficit* or impairment* or abilit*) or AB (disorder* or deficit* or impairment* or abilit*) S 23. S21 and S22 S 24. TI (perceptual or visuo#spatial or visuo#perceptual ot attention* or cognit* or scanning*) or AB (perceptual or visuo#spatial or visuo#perceptual ot attention* or cognit* or scanning*) S 25. TI ( training or re‐training or rehabilitation or intervention or therapy) or AB (training or re‐training or rehabilitation or intervention or therapy) S26. S24 and S25 S27. S12 or S13 or S14 or S15 or S16 or S19 or S20 or S23 or S26 S28. S11 and S27 S29.. (MH "Random Assignment") or (MH "Random Sample+") S30.. (MH "Crossover Design") or (MH "Clinical Trials+") or (MH "Comparative Studies") S31.. (MH "Control (Research)") or (MH "Control Group") S32.. (MH "Factorial Design") or (MH "Quasi‐Experimental Studies") or (MH "Nonrandomized Trials") S33.. (MH "Placebo Effect") or (MH "Placebos") or (MH "Meta Analysis") S34.. (MH "Clinical Research") or (MH "Clinical Nursing Research") S35.. (MH "Community Trials") or (MH "Experimental Studies") or (MH "One‐Shot Case Study") or (MH "Pretest‐Posttest Design+") or (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") or (MH "Study Design") S36.. PT clinical trial S37.. PT systematic review S38.. TI random* or AB random* S39.. TI ( singl* or doubl* or tripl* or trebl* ) or AB ( singl* or doubl* or tripl* or trebl* ) S40.. TI ( blind* or mask*) or AB ( blind* or mask* ) S41.. S39 and S40 S42.. TI ( crossover or cross‐over or placebo* or control* or factorial or sham ) or AB ( crossover or cross‐over or placebo* or control* or factorial or sham ) S43.. TI ( clin* or intervention* or compar* or experiment* or preventive or therapeutic ) or AB ( clin* or intervention* or compar* or experiment* or preventive or therapeutic ) S44.. TI trial* or AB trial* S45.. S43 and S44 S46.. TI ( counterbalance* or multiple baseline* or ABAB design ) or AB ( counterbalance* or multiple baseline* or ABAB design ) S47.. TI ( meta analysis* or metaanlaysis or meta‐anlaysis or systematic review* ) or AB ( meta analysis* or metaanlaysis or meta‐anlaysis or systematic review*) S48.. S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S41 or S42 or S45 or S46 or S47. S49.. S48 and S28
Appendix 4. PsycINFO (Ovid) search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral ischemia/ or cerebrovascular accidents/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiplegia/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp perceptual disturbances/ 9. exp perception/ 10. sensory neglect/ 11. exp attention/ 12. "Extinction (learning)"/ 13. (hemineglect or hemi‐neglect).tw. 14. ((unilateral or spatial) adj5 neglect).tw. 15. (perception or inattention or hemi‐inattention or attention or extinction).tw. 16. ((perceptual or visuo?spatial or visuo?perceptual or attentional) adj5 (disorder$ or deficit$ or impairment$ or abilit$)).tw. 17. ((perceptual or visuo?spatial ro visuo?perceptual or attention$ or cognit$ or scanning$) adj5 (training or re‐training or rehabilitation or intervention or therapy)).tw. 18. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 19. 7 and 18 20. clinical trials/ 21. quasi experimental methods/ 22. empirical methods/ 23. experiment controls/ 24. experimental methods/ 25. meta analysis/ 26. placebo/ 27. program evaluation/ 28. treatment outcomes/ 29. treatment effectiveness evaluation/ 30. random sampling/ 31. experimental design/ 32. experiment volunteers/ 33. experimentation/ 34. random$.tw. 35. (controlled adj5 (trial$ or stud$)).tw. 36. (clinical$ adj5 trial$).tw. 37. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 38. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 39. ((multicenter or multicentre or therapeutic) adj5 (trial$ or stud$)).tw. 40. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 41. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 42. (coin adj5 (flip or flipped or toss$)).tw. 43. latin square.tw. 44. versus.tw. 45. (cross‐over or cross over or crossover).tw. 46. placebo$.tw. 47. sham.tw. 48. (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw. 49. controls.tw. 50. (treatment$ adj6 order).tw. 51. (meta analysis or "systematic review" or treatment outcome clinical trial).md. 52. or/20‐51 53. 19 and 52 54. (childhood birth 12 yrs or infancy 2 23 mo or neonatal birth 1 mo or preschool age 2 5 yrs or school age 6 12 yrs).ag. 55. (neonat$ or child or children or childhood or juvenile or infant or toddler).tw. 56. exp neoplasms/ 57. (cancer$ or carcinoma$ or tumor$ or tumour$ or neoplasm$).tw. 58. (clinical case study or nonclinical case study).md 59. case report$.tw. 60. 54‐59 61.53 not 60 62. limit 61 to human
Data and analyses
Comparison 1. Cognitive rehabilitation versus any control: immediate effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activities of daily living | 10 | 343 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.02, 0.48] |
| 1.1 BI | 5 | 184 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.24, 0.47] |
| 1.2 FIM | 3 | 91 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [0.02, 1.06] |
| 1.3 Catherine Bergego Scale | 2 | 68 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.34, 0.62] |
| 2 Neglect: standardised assessment | 16 | 437 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [0.09, 0.62] |
| 2.1 Target cancellation | 9 | 239 | Std. Mean Difference (IV, Random, 95% CI) | 0.39 [0.03, 0.74] |
| 2.2 Line bisection | 2 | 61 | Std. Mean Difference (IV, Random, 95% CI) | 1.00 [0.46, 1.54] |
| 2.3 BIT behavioural subtests | 5 | 137 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.35, 0.34] |
| 3 Discharge destination (home) | 1 | 50 | Odds Ratio (M‐H, Random, 95% CI) | 1.40 [0.45, 4.35] |
| 4 Falls | 1 | 39 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.26, 5.76] |
| 5 Adequate allocation concealment only: activities of daily living | 6 | 227 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.09, 0.44] |
| 5.1 BI | 3 | 125 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.36, 0.68] |
| 5.2 FIM | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.43, 0.92] |
| 5.3 Catherine Bergego Scale | 2 | 68 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.34, 0.62] |
| 6 Adequate blinding only: activities of daily living | 8 | 282 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.02, 0.45] |
| 6.1 BI | 4 | 145 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.20, 0.62] |
| 6.2 FIM | 2 | 69 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.16, 0.80] |
| 6.3 Catherine Bergego Scale | 2 | 68 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.34, 0.62] |
| 7 Adequate allocation concealment only: neglect: standardised assessment | 7 | 242 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.23, 0.58] |
| 7.1 Target cancellation | 4 | 144 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.28, 1.04] |
| 7.2 Line bisection | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 BIT behavioural subtests | 3 | 98 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.46, 0.34] |
| 8 Adequate blinding only: neglect: standardised assessment | 11 | 336 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.06, 0.54] |
| 8.1 Target cancellation | 7 | 203 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.02, 0.86] |
| 8.2 Line bisection | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 BIT behavioural subtests | 4 | 133 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.35, 0.34] |
| 9 Type of control: activities of daily living | 10 | 343 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.02, 0.48] |
| 9.1 Attention control | 8 | 270 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.00, 0.59] |
| 9.2 Other control or no treatment | 2 | 73 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.44, 0.48] |
| 10 Type of control: neglect: standardised assessment | 16 | 437 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [0.09, 0.62] |
| 10.1 Attention control | 10 | 284 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.16, 0.46] |
| 10.2 Other control or no treatment | 6 | 153 | Std. Mean Difference (IV, Random, 95% CI) | 0.69 [0.36, 1.02] |
| 11 Type of cognitive rehabilitation:activities of daily living | 10 | 343 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.02, 0.48] |
| 11.1 Bottom‐up processing rehabilitation approach | 7 | 259 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.03, 0.46] |
| 11.2 Top‐down processing rehabilitation approach | 3 | 84 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.48, 1.28] |
| 12 Type of cognitive rehabilitation: neglect: standardised assessment | 16 | 437 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [0.09, 0.62] |
| 12.1 Bottom‐up processing rehabilitation processes | 8 | 247 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [0.12, 0.63] |
| 12.2 Top‐down processing rehabilitation approaches | 8 | 190 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.16, 0.88] |
Comparison 2. Cognitive rehabilitation versus any control: persisting effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activities of daily living | 5 | 143 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.10, 0.72] |
| 1.1 BI | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.62, 0.86] |
| 1.2 FIM | 2 | 54 | Std. Mean Difference (IV, Random, 95% CI) | 0.77 [0.12, 1.42] |
| 1.3 Catherine Bergego Scale | 2 | 61 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.48, 0.53] |
| 2 Neglect: standardised assessment | 8 | 172 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.03, 0.59] |
| 2.1 Target cancellation | 2 | 28 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.44, 1.31] |
| 2.2 Line bisection | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | 1.09 [0.18, 2.00] |
| 2.3 BIT behavioural subtests | 5 | 122 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.24, 0.48] |
| 3 Adequate allocation concealment only: activities of daily living | 3 | 89 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.37, 0.47] |
| 3.1 BI | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.62, 0.86] |
| 3.2 FIM | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Catherine Bergego Scale | 2 | 61 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.48, 0.53] |
| 4 Adequate blinding only: activities of daily living | 4 | 121 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.20, 0.52] |
| 4.1 BI | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.62, 0.86] |
| 4.2 FIM | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | 0.49 [‐0.24, 1.22] |
| 4.3 Catherine Bergego Scale | 2 | 61 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.48, 0.53] |
| 5 Adequate allocation concealment only: neglect: standardised assessment | 3 | 77 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.40, 0.51] |
| 5.1 Target cancellation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Line bisection | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 BIT behavioural subtests | 3 | 77 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.40, 0.51] |
| 6 Adequate blinding only: neglect: standardised assessment | 5 | 122 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.24, 0.48] |
| 6.1 Target cancellation | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Line bisection | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 BIT behavioural subtests | 5 | 122 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.24, 0.48] |
| 7 Type of control: neglect: standardised assessment | 8 | 172 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.03, 0.59] |
| 7.1 Attention control | 6 | 147 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.10, 0.56] |
| 7.2 Other control or no treatment | 2 | 25 | Std. Mean Difference (IV, Random, 95% CI) | 0.57 [‐0.25, 1.38] |
| 8 Type of cognitive rehabilitation: activities of daily living | 5 | 143 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.10, 0.72] |
| 8.1 Bottom‐up processing rehabilitation processes | 4 | 121 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.20, 0.52] |
| 8.2 Top‐down processing rehabilitation processes | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | 1.17 [0.25, 2.08] |
| 9 Type of cognitive rehabilitation: neglect: standardised assessment | 8 | 172 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.03, 0.59] |
| 9.1 Bottom‐up processing rehabilitation approaches | 4 | 107 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.26, 0.51] |
| 9.2 Top‐down processing rehabilitation approaches | 4 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [‐0.03, 1.12] |
Comparison 3. One cognitive rehabilitation intervention versus another.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activities of daily living: immediate effects | 2 | 21 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐1.15, 0.59] |
| 2 Activities of daily living: persisting effects | 2 | 22 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.54, 0.58] |
| 3 Neglect outcomes: immediate effects | 6 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Visual scanning versus another | 3 | 28 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.71, 0.89] |
| 3.2 Visual scanning + another versus visual scanning | 3 | 70 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.95 [0.43, 1.47] |
| 4 Neglect outcomes: persisting effects | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Visual scanning versus another | 3 | 28 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐1.20, 0.96] |
| 4.2 Visual scanning + another versus visual scanning | 2 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 1.13 [‐0.33, 2.60] |
Comparison 4. Bottom‐up processing rehabilitation interventions versus any control: immediate effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activities of daily living | 7 | 259 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.03, 0.46] |
| 1.1 Prisms | 3 | 107 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.36, 0.40] |
| 1.2 Patching | 2 | 69 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.16, 0.80] |
| 1.3 Other | 2 | 83 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.14, 0.90] |
| 2 Neglect: standardised assessment | 8 | 244 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [0.13, 0.64] |
| 2.1 Prisms | 4 | 120 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.16, 0.74] |
| 2.2 Patching | 3 | 77 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.01, 0.93] |
| 2.3 Other | 1 | 47 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.14, 1.02] |
Comparison 5. Bottom‐up processing rehabilitation interventions versus any control: persisting effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activities of daily living | 4 | 121 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.20, 0.52] |
| 1.1 Prisms | 2 | 61 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.48, 0.53] |
| 1.2 Patching | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | 0.49 [‐0.24, 1.22] |
| 1.3 Other | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.62, 0.86] |
| 2 Neglect: standardised assessment | 4 | 107 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.26, 0.51] |
| 2.1 Prisms | 3 | 75 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.38, 0.55] |
| 2.2 Patching | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.50, 0.94] |
| 2.3 Other | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Top‐down processing rehabilitation interventions versus any control: immediate effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activities of daily living | 3 | 84 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.48, 1.28] |
| 1.1 Feedback or cueing | 3 | 84 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.48, 1.28] |
| 2 Neglect: standardised assessment | 8 | 190 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.16, 0.88] |
| 2.1 Feedback or cueing | 4 | 101 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.45, 1.09] |
| 2.2 Visual scanning | 3 | 59 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [‐0.98, 1.99] |
| 2.3 Mental imagery | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.38, 1.07] |
Comparison 7. Top‐down processing rehabilitation interventions versus any control: persisting effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activities of daily living | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | 1.17 [0.25, 2.08] |
| 1.1 Feedback or cueing | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | 1.17 [0.25, 2.08] |
| 2 Neglect: standardised assessment | 4 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [‐0.03, 1.12] |
| 2.1 Feedback or cueing | 2 | 35 | Std. Mean Difference (IV, Random, 95% CI) | 0.73 [‐0.08, 1.55] |
| 2.2 Visual scanning | 2 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.64, 1.37] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cherney 2002.
| Methods | RCT: no further information provided Setting: USA | |
| Participants | 4 right hemisphere stroke survivors with clinical evidence of neglect at least 6 months post‐onset Experimental: n = 2, control: n = 2 Mean age (SD): experimental 69.5 years (23.3), control 62.0 years (5.7) Sex (male): experimental 2, control 1 Side of damage (RBD): experimental 2, control 2 Mean months post‐onset (SD): experimental 16 (12.7), control 7.5 (0.7) Inclusion: right‐handed, right hemisphere stroke, persisting neglect after 6 months, spoke English as a primary language, passed pure tone audiometry in their better ear, corrected visual acuity was sufficient to read newsprint | |
| Interventions | Visual scanning training, practising letter and word cancellation tasks (to address the assumed underlying impairment of selective visual attention) versus repetitive practice of a functional task: oral reading (to represent an approach commonly used in rehabilitation) Both groups received 20 sessions. The frequency of sessions is not known Both scanning and reading training included the use of visual, verbal and tactile cues to attend to the left. In both training conditions the task difficulty gradually increased if the participant achieved 90% success (scanning) or 100% success (reading). In reading training the cues were gradually removed (NB. Scanning is coded as 'experimental' in this review) For analysis of bottom‐up and top‐down rehabilitation approaches this review coded the experimental condition as top down | |
| Outcomes | The study collected 4 types of outcomes, pre‐ and post‐training:
The latter was to identify 5 names from a local telephone book; there was a time limit of 3 minutes per name. The BIT was scored in 3 ways: conventional subtests; behavioural subtests; and total. It is assumed this was measured immediately post‐training For comparability with other studies this review used only the BIT behavioural subtests post‐training |
|
| Notes | A comparison of 2 treatments. Intended as a small preliminary study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No details of randomisation provided. Paper states "randomly assigned" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None reported |
| Free of systematic differences in baseline characteristics of groups compared? | Unclear risk | Insufficient details provided and sample size very small but seem comparable |
| Did authors adjust for baseline differences in their analyses? | Unclear risk | Not clear if it was needed |
Cottam 1987.
| Methods | RCT: no further information provided Setting: USA | |
| Participants | 12 stroke rehabilitation inpatients with left hemispatial neglect Experimental n = 6, control = 6 Mean age: experimental 66.2 years, control 71.3 years Sex (male/female): 7/5 Side of damage: all had right middle cerebral artery lesions Time post‐onset (mean weeks): experimental 6, control 16.3 Inclusion: right‐handed, visual acuity > 20/100 corrected on Snellen's, orientated in person, place and time, evidence of left hemispatial neglect on at least 3 of the tests used, either WAIS‐R VIQ > 80 or minimum scaled score of 8 on 4/6 verbal subtests, arm and leg able to propel wheelchair | |
| Interventions | 3‐phase intervention, each phase consisting of 5 half‐hour sessions per day
versus no information other than participants were inpatients at a rehabilitation facility and were assessed after same periods as experimental group For analysis of bottom‐up and top‐down rehabilitation approaches this review coded the experimental condition as top down |
|
| Outcomes | The study collected 3 types of outcomes:
Assessed pre‐intervention, after each phase (5 days) and at follow‐up 6 weeks post‐discharge from hospital This review used only the cancellation data, immediate and persisting effects |
|
| Notes | Single letter cancellation outcome data are entered as left‐sided omissions (i.e. low score is better outcome) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No details of randomisation provided. States "randomly assigned" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. Not mentioned so unlikely to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 control lost to follow‐up |
| Free of systematic differences in baseline characteristics of groups compared? | Unclear risk | Controls were older and later after onset |
| Did authors adjust for baseline differences in their analyses? | Low risk | |
Edmans 2000.
| Methods | RCT Setting: UK | |
| Participants | 42 (see Notes) stroke patients with visual neglect from those with general perceptual problems admitted to an inpatient SU Experimental n = 24, control n = 18 Mean age (SD): experimental 69.17 years (11.35), control 66.61years (14.5) Sex (male/female): experimental 10/14, control 8/10 Mean time post‐onset: 37 days Inclusion: a subset of those with neglect from those with general perceptual problems from those consecutive admissions to a stroke unit trial. SU trial criteria were: medically stable, able to transfer with maximum 2 nurses, no discharge date planned, able to tolerate 30‐minute treatments, able to carry out some independent ADLs pre‐stroke | |
| Interventions | ToT approach to treat the 'cause of the perceptual problem'. The underlying assumption is that practising a perceptual task will treat the underlying impairment and if successful will improve performance of other tasks which depend on the skills. Personal communication suggested that cueing and feedback were used to teach participants to compensate versus FA to treat the 'symptom rather than the cause' and involved practising ADL tasks Both groups received 2.5 hours per week for 6 weeks in addition to standard OT (NB: ToT is coded as experimental in this review) For analysis of bottom‐up and top‐down rehabilitation approaches this review coded the experimental condition as top‐down | |
| Outcomes | The broader study of perceptual problems completed the following measures by different assessors immediately after the 6 weeks treatment: an independent blinded assessor completed the BI, Edmans ADL Scale, and RPAB. This assessor completed the ADL scales following interviews with unblinded nursing staff. The unblinded ward OT also completed the BI and Edmans ADL Scale. An unblinded physiotherapist completed the RMA gross motor score. Additionally assessments by other clinical staff were analysed: speech and language therapists, psychologists, physiotherapists For comparability with other studies this review used only the RPAB letter cancellation subtest score (number correctly cancelled) and the blinded assessor's BI | |
| Notes | Personal communication supplied further data and clarification of method. Authors provided unpublished data on 42 neglect patients from a larger RCT of 80 left and right (35) hemisphere strokes with perceptual problems which was itself taken from the stroke unit admission arm (n = 158) of a RCT of stroke unit versus general medical care. No pre‐randomisation differences between groups except that the ToT group were a little longer post‐stroke (40/33 days) than the FA group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | The researcher used random number tables to prepare sequentially numbered opaque sealed envelopes. The random number tables were then returned and due to the large number randomised (80 to the full perception trial) it was unlikely that the sequence would be remembered. The envelopes were only opened in the presence of a witness. Random number tables. Concealment was highly likely to have been achieved, although it could not be guaranteed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "No patients withdrew from the study but one patient (in the functional approach group) died before completing his six weeks of perceptual treatment." Data from this patient are included in analyses |
| Free of systematic differences in baseline characteristics of groups compared? | High risk | "There was a significant difference between groups using t‐test on time post‐stroke to entry to the study (t = 2.12, p < 0.05) with the transfer of training group patients being slightly longer post stroke that the functional group" |
| Did authors adjust for baseline differences in their analyses? | Unclear risk | Not stated |
Fanthome 1995.
| Methods | RCT Setting: UK |
|
| Participants | 18 (see Notes) RH stroke patients admitted to hospital Experimental n = 9, control n = 9 (The following data describe the 18 initial participants: see Notes) Mean age (SD): experimental 66.3 years (10.7), control 71.1 years (7.6) Sex (male/female): experimental 6/3, control 6/3 Time post‐onset (mean months): experimental 1.0, control 0.6 Inclusion: not blind; < 80 years of age; no history of dementia or psychiatric problems; not ill; right‐handedness; score > 6 on Abbreviated Mental Test; RH stroke; score < 130 on BIT | |
| Interventions | 4 weeks (2 hours 40 minutes per week) feedback of eye movements (wearing specially adapted glasses with auditory signal) versus 4 weeks no treatment For analysis of bottom‐up and top‐down rehabilitation approaches this review coded the experimental condition as top‐down | |
| Outcomes | The study collected 3 types of outcomes: eye movements, conventional BIT subtests and behavioural BIT subtests, immediately post‐treatment (4 weeks) and 4 weeks later (8 weeks) For this review we used the 4‐week single letter cancellation test (for immediate outcomes), and the 8‐week BIT summary behavioural subtest scores (for persisting outcomes) | |
| Notes | Personal communication supplied group data on BIT subtests for all but 1 control participant at 4 weeks (missing data, therefore n = 18 ‐ 1), and the information that assessor blinded to allocation. BIT behavioural data are for all 18 at 4 weeks but only 13 at 8 weeks. 8 weeks = post‐start of treatment, i.e. is a 4‐week follow‐up post‐end of treatment Single letter cancellation data are for number cancelled, i.e. higher numbers indicate better outcome Experimental and control groups appeared adequately matched on demographic and clinical data although control group slightly older than experimental, no baseline BIT data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Sealed opaque envelopes prepared from random number tables. Concleament of allocation can not be guaranteed as it was not done by a third party. The combination of a small sample size with no external randomisation meant that there was a potential risk to concealment Random number tables |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 participant was recruited but not included as "he could not move his eyes to the fixation points". 1 participant from the control group was excluded as he was discharged home outside the area of the hospital |
| Free of systematic differences in baseline characteristics of groups compared? | Low risk | Groups appear similar at baseline, and no significant differences were found |
| Did authors adjust for baseline differences in their analyses? | Low risk | N/A – no baseline differences |
Ferreira 2011.
| Methods | RCT | |
| Participants | n = 10 Right hemisphere stroke |
|
| Interventions | Group 1: visual scanning Group 2: mental practice Visual scanning was classified as top‐down Mental practice was classified as top‐down This comparison was classified as one cognitive rehabilitation approach versus another cognitive rehabilitation approach. Visual scanning was defined as Approach 1 and mental practice as Approach 2 |
|
| Outcomes |
Intervention group (visual scanning) was assessed at end of intervention period and at 3 months Control group (mental practice) were "evaluated twice and two months between evaluations" |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Randomisation (information from authors): "Concealed envelopes for every patients (0 or 1). Then patients as they were recruited/included and subsequently randomised by the same method." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | States "The evaluations were always done by a physical therapist not directly involved in patients’ treatment." However, correspondence with authors confirm: "There were two therapists involved, each one directly responsible for a different treatment strategy (mental practice or visual scanning). For instance, whenever a patient was randomised to mental practice, treatment was done by one and assessments by the other therapist. Hence, the assessor was always the therapist who would not be involved in treatment but he always knew the treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All complete |
| Free of systematic differences in baseline characteristics of groups compared? | Low risk | 3 groups compared at baseline. Paper reports no significant differences on age, formal schooling, initial BIT and FIM scores . Sex distribution looks similar and all were ischaemic stroke (see Table 8) Raw scores are provided in Table 1 so means SD can be computed |
| Did authors adjust for baseline differences in their analyses? | Low risk | None present |
Fong 2007.
| Methods | 3‐arm RCT Setting: Hong Kong |
|
| Participants | 60 participants
Experimental 1: n = 20; experimental 2: n = 20; control: n = 20 Number lost to follow‐up: immediate post‐treatment (day 30) assessments on 19, 20 and 15 respectively Also lost 5, 0 and 3 respectively to follow‐up (day 60) so final analysis of 14, 20 and 12 Adequacy of matching at baseline? yes: P values are reported for all demographics and baseline data ‐ there are no significant differences Mean age (mean (SD)): experimental 1 = 69.9 (11.0), experimental 2 = 69.9 (9.8), control = 73.8 (9.9) Sex (male/female): 34/20 Side of damage: all had right brain damage Time post‐onset: experimental 1 = 12.1 (9.4), experimental 2 = 11.6 (5.1), control = 12.1 (7.1) days Inclusion criteria: first or second unilateral right lesion stroke confirmed by imaging and examination, admitted to rehabilitation hospital, < 8 weeks since stroke onset, right‐handed, left visual inattention or neglect diagnosed by < 51/54 on Star Cancelled of BIT and GCS = 15 at recruitment Exclusion criteria: severe aphasia, significantly impaired visual acuity, hemianopia Visual sensory deficit: hemianopia and visual acuity assessed (method of assessment not stated) |
|
| Interventions | Experimental 1: voluntary trunk rotation 1 hour per day, 5 days per week for 30 days = 30 hours, OT present throughout Each hour composed of 15 minutes ADLs + 45 voluntary trunk rotation with set up equipment (supine, unsupported sitting and in standing frame) reaching with ipsilateral hand into contralateral space and therefore rotating upper body/trunk by 15 to 35 degrees from midline. Used set up apparatus (peg board or shoulder arc). Voluntary or if necessary therapist provided verbal or motor prompting for 15 minutes Experimental 2: voluntary trunk rotation and half field eye‐patching Same amount and content as experimental group 1 but wearing half field eye patches to ipsilesional (right) hemifield wearing patches on plastic goggles (over own glasses if necessary) Control: same amount of time as experimental groups 1 and 2. Conventional OT for hemiplegia (15 minutes ADLs + 45 minutes training upper extremity). No mention of any neglect‐specific treatment implying treated as if had only hemiplegia For analysis of bottom‐up and top‐down rehabilitation approaches this review coded the experimental condition as bottom‐up For analysis voluntary trunk rotation and half field eye‐patching was classed as the experimental condition and control as the control condition Profession of outcome provider: OT |
|
| Outcomes | Used 3 (some with multiple subtests) at 2 follow‐up timepoints (day 30 immediately post‐therapy + day 60)
Did not measure serious adverse events, excluded anyone rehospitalised or with deteriorating health |
|
| Notes | "Recruitment hypothesis" target both spatial representation and motor intentional deficits of personal and peripersonal space ‐ this is the voluntary rotation plus eye patches "Inexpensive and easily integrated into use in day‐to‐day rehabilitation" Lack of intention‐to‐treat analysis, no baseline data on those allocated, baseline data on those followed up suggests pre‐therapy differences described in 'Risk of bias' table below |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Personal communication with authors: "we didn't have concealment of allocation of participants from the person who was recruiting" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Two independent blinded assessors, who were blinded to group membership, were responsible for all repeated measures throughout the duration of the study" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Although reasons for post‐randomisation exclusions are stated, it would have been preferable if all participants had been included in intention‐to‐treat analysis |
| Free of systematic differences in baseline characteristics of groups compared? | Low risk | P values reported for all demographics and baseline data ‐ no significant differences |
| Did authors adjust for baseline differences in their analyses? | Low risk | No adjustment was required as there were no baseline differences |
Kalra 1997.
| Methods | RCT Setting: UK |
|
| Participants | 50 (see Notes) stroke patients with visual neglect admitted to a SU The following data are for the 47 surviving patients Experimental n = 24, control n = 23 Mean age (SD): experimental 78 years (9), control 76 years (10) Sex (male): experimental 11, control 9 Side of damage (RBD): experimental 16, control 17 Median time post‐onset (range): 6 days (2 to 14) Inclusion: infarcts partial anterior circulation, known to be sensitive to rehabilitation on basis of impairments of power, balance, proprioception and cognition at 1 to 2 weeks after stroke Exclusion: TIAs, reversible neurological deficits, hemianopsia or severe dysphasia | |
| Interventions | Spatio‐motor cueing based on 'attentional‐motor integration' model and early emphasis on restoration of function versus conventional therapy input concentrating on restoration of tone, movement pattern and motor activity before addressing skilled functional activity For analysis of bottom‐up and top‐down rehabilitation approaches this review coded the experimental condition as bottom‐up | |
| Outcomes | The study collected 6 types of outcomes:
This review used only the BI, RPAB letter cancellation subtest, and discharge home. These were all analysed as immediate effects |
|
| Notes | Principle behind approach: movements of affected limb in the deficit hemispace led to summation of activation of affected receptive fields of 2 distinct but linked spatial systems for personal and extrapersonal space resulting in improvements in attention skills and appreciation of spatial relationships on the affected side. Personal communication supplied further data and clarification of method No difference between groups on demographic variables or initial impairment or disability including BI Outcome data on 47 of 50 stroke patients with visual neglect admitted to a SU: experimental n = 24 (+ 1 died), control n = 23 (+ 2 died). For the 'destination discharge' outcome the total figure of 50 was used in this review as deaths were entered as not going home | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | External randomisation, using random permuted block technique in groups of 10, allocated by telephone by clerical staff using computer‐generated random numbers |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 lost to follow‐up, 1 intervention and 2 control. All died so low risk of bias |
| Free of systematic differences in baseline characteristics of groups compared? | Low risk | Groups compared on main characteristics and very similar |
| Did authors adjust for baseline differences in their analyses? | Low risk | Not needed |
Kerkhoff 2012a.
| Methods | RCT. Setting: Germany |
|
| Participants | 6 stroke patients with left‐sided visual and auditory neglect, who were "enrolled in our clinic" Optokinetic stimulation (OKS) group: mean age 62.3 years; SCAN group: mean age 56.3 years Inclusion criteria: single right‐hemispheric lesion due to stroke (infarction or haemorrhage); evidence of left‐sided visual neglect in at least 2 out of the 4 screening tests, and a pathological rightward shift in the ASMP |
|
| Interventions | Group 1 ‐ OKS: repetitive leftward OKS stimulation with active pursuit eye movements. Participants were instructed to look at a computer screen (17") and make pursuit eye movements to the left (contralesional) side while looking at moving dot displays of 100 to 200 stimuli (mean velocity: 5 to 30◦) Group 2 ‐ visual scanning training: participants viewed identical visual stimuli on the same computer monitor as the OKS group, but these patterns were always static. These participants were instructed to make systematic scanning eye movements to the left side and explore the visual stimuli on the screen, just as in conventional visual scanning therapy Both groups received 20 treatment sessions of around 50 minutes, 5 sessions per week, 1 session per work‐day OKS was classified as bottom‐up Visual scanning was classified as top‐down This comparison was classified as 1 cognitive rehabilitation approach versus another cognitive rehabilitation approach. For analyses; scanning training was defined as Approach 1 and OKS as Approach 2 |
|
| Outcomes | Auditory neglect: ASMP Visual neglect: visual neglect was measured by the following 3 tests: number cancellation, horizontal line bisection and paragraph reading |
|
| Notes | NB. Data presented as single‐subjects in graph form | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | "The patients were randomly allocated to either an OKS (N = 3) or a SCAN (N = 3) treatment group by having a person neither involved in the study nor associated with the clinic draw concealed papers from an envelope containing 6 sheets of paper stating either 'OKS' or 'SCAN'." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Does not stated if outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 participants recruited and none lost |
| Free of systematic differences in baseline characteristics of groups compared? | High risk | A difference was found in the ASMP baseline measure between the 2 groups, but not for other baseline measures |
| Did authors adjust for baseline differences in their analyses? | High risk | No adjustments appear to have been made |
Luukkainen‐Markkula 2009.
| Methods | RCT, single site, comparing 2 active interventions Setting: Finland |
|
| Participants | 12 participants with left hemispatial neglect, due to a first single right hemisphere stroke occurring a maximum of 6 months previously Experimental 1: n = 6 , experimental 2: n = 6 Number lost to follow‐up: none, however 1 person's data was missing from both groups for some measures and timepoints There were baseline differences in the CBS OT score ‐ arm activation group, mean 9.4 (SD 2.3) and visual scanning group, mean 13.5 (SD 7.8), based on data from 10 participants Age: (mean (SD)): experimental 1 = 59.5 (8.4), experimental 2 = 57.8 (11.8) Sex (male/female): experimental 1 = 3/3, experimental 2 = 2/4 Side of damage:right hemisphere stroke Method of diagnosing stroke: CT or MRI, neurologist and radiologist Method of diagnosing neglect: For acute phase (< 3 months post‐stroke) – at least 2 of: score of 100 or less on the BIT conventional subtests (BIT C); at least 2 of the BIT subtests under the cut‐off point; or a CBS OT score of 10 to 30 points For sub‐acute phase (3 to 6 months post‐stroke) – at least 2 of: score of 129 or less on the BIT C subtests; at least one BIT C subtest under the cut‐off; or CBS OT score of 2 or more Time post‐onset: experimental 1 = 81.0 (64.6) , experimental 2 = 95.5 (63.2) days Inclusion and exclusion criteria: diagnosis of single right hemisphere stroke within 6 months, right‐handed with no other co‐existing diseases causes cognitive decline or a lack of co‐operation Visual sensory deficit: (method of assessment): experimental 1 = 1 complete hemianopia, experimental 2 = 3 with complete hemianopia |
|
| Interventions | Arm activation training 20 to 30 hours of left arm activation – amount determined by observing subjective needs of individuals Content determined by individual WMFT performance: 1 patient had constraint‐induced movement therapy (intensive exercise of affected arm while unaffected was restrained with a sling). 5 patients without sufficient left arm mobility had modified arm activation therapy all with left arm in left space and right arm resting on right side (50% passive arm activation FES with a glove/or for spasticity stretching by a therapist + 50% voluntary shoulder motor training in push‐pull equipment in left hemispace) versus Visual scanning training: 10 hours traditional visual scanning training – aimed for 1 hour, 4 x week of visual scanning combined with 2 daily physiotherapy sessions + 1 hour per day of OT/group therapy Was achieved 1 hour, 5 x week during 3 weeks Content: 3 procedures (half‐hour on 1 then half‐hour on 2 or 3):
This comparison was classified as 1 cognitive rehabilitation approach versus another cognitive rehabilitation approach For analysis of bottom‐up and top‐down rehabilitation approaches this review coded the arm activation training as bottom‐up and the visual scanning training as top‐down. For analyses, visual scanning training was called 'Approach 1' and arm activation training 'Approach 2' Profession of intervention provider: arm‐activation ‐ constraint‐induced movement therapist Visual scanning ‐ clinical psychologist |
|
| Outcomes | A number of outcomes were measured. These are given along with details (where provided) of the time point of measurement, and the profession of the person performing the measure
Also reported 1 person with recurrent stroke |
|
| Notes | "Sufficient amount of active or passive left arm activation in the left half space combined with simultaneous visual tasks or while doing daily activities is likely to ameliorate visual and behavioural neglect" Confounding factors may possibly be due to baseline imbalance on CBS and possibly also Beck Depression Inventory (effect on engagement in therapy) and the multiple assessments Group 1 received a lot more arm activation than Group 2 received visual scanning training. Group 2 received more OT and group therapy than group 1. Correspondence with the author states this difference is to keep the total hours of therapy received by the participants in each group comparable. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Personal communication with author: "The method of randomization was carried out as follows: A clerk of the rehabilitation ward offered a pair of brown envelopes to an entering patient. One envelope included the AA group and the other envelope contained the VS group. The first patient picked one of the envelopes and the following patient entering the study was randomized automatically into the other group. This arrangement of paired randomization was necessary for the resources of the ward." Consequently the allocation of the second patient would be known to researchers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Of the 13 assessments a number were carried out by those who did not participate in the rehabilitation. These outcome measures were visual and behavioural neglect, BIT C and CBS. However, although the assessments were carried out by someone who did not participate in the rehabilitation, these people were not blinded to the treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 person's data were missing from both groups for some measures and time points |
| Free of systematic differences in baseline characteristics of groups compared? | High risk | There were baseline differences between groups, with CBS and Beck Despression Inventory scores appearing higher and more variable for visual scanning group |
| Did authors adjust for baseline differences in their analyses? | High risk | There was no adjustment for baseline differences for the CBS |
Mizuno 2011.
| Methods | RCT. Multicentre, double‐blind. Comparing training using prisms with training without prisms Setting: Japan | |
| Participants | 38 participants (444 screened) Experimental group (prisms): n = 20; control group: n = 18 Recruited from rehabilitation departments from 8 hospitals in Japan Age ‐ mean (SD): experimental: 66.0 (11.5), control 66.6 (7.7) years Time from stroke ‐ mean (SD): experimental 67.1 (18.4); control: 64.4 (20.9) days Inclusion criteria: within 3 months of first ever right hemisphere stroke, 42 to 89 years old, neglect as assessed by BIT behavioural test Exclusion criteria: unable to sit in wheelchair, aphasia or cognitive impairment resulting in inability to understand task, unable to understand Japanese, impaired vision or hearing, impaired right upper limb, previous brain injury 34 participants completed intervention and follow‐up; 4 drop‐outs (1 control, 3 prisms) ‐ 2 stroke relapse, 1 refused, 1 developed delirium 31 participants completed follow‐up BIT |
|
| Interventions | 2 daily training sessions, lasting 20 minutes, 5 days per week for 2 weeks; for a total of 20 sessions Training ‐ pointing at targets, whilst sitting at a table Experimental group: prisms (shifting visual field 12° to right, Fresnel lens). Pointing task ‐ 30 times without prisms; 90 times with; 60 times without Control group: neutral plastic glasses. Pointing task as for experimental group Routine stroke rehabilitation provided as usual For analysis of bottom‐up and top‐down rehabilitation approaches this review coded the experimental condition as bottom‐up |
|
| Outcomes |
Outcomes were recorded at baseline, after the 2‐week intervention and immediately prior to hospital discharge |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Computerised block randomisation, with pre‐stratification according to BIT behavioural test (dichotomised to above or equal to 55 or below 55) and participating hospital |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was masked to treatment allocation and otherwise uninvolved in the participant's treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A small number of participants did not complete follow‐up assessments, but there were no significant differences between those who did and did not complete the follow‐up evaluation |
| Free of systematic differences in baseline characteristics of groups compared? | Low risk | No significant differences were found at baseline between the prism and the control groups with regard to the mean days from onset to intervention, mean hospital stay, MMSE score, and SIAS motor score |
| Did authors adjust for baseline differences in their analyses? | Low risk | No differences at baseline |
Nys 2008.
| Methods | RCT "single‐blind randomised controlled design" Setting: Netherlands | |
| Participants | 16 participants with neglect from 3 stroke units Experimental: n = 10 , control: n = 6 Adequacy of matching at baseline? Yes Number lost to follow‐up: not clear ‐ only those who completed were included in the report. Also excluded 1 patient with deterioration of neurological condition during treatment phase , which probably should have been a loss to follow‐up rather than exclusion Mean age (mean (SD)): experimental = 63.6 (13.8), control = 61.5 (11.0) years Sex (male/female): experimental = 7/3, control = 3/3 Time post‐onset: experimental 1 = 8.8 (5.3), control = 11.2 (6.4) days Side of damage: right Method of diagnosing stroke: not stated, was based on the referral by a stroke physician on their admitting SU Method of diagnosing neglect: 2 or more subtests (out of 4): BIT subtests below cut‐off. The four tests were Star Cancellation (cut‐off ≤ 51), line bisection (cut‐off ≤ 7), figure copying (cut‐off ≤ 2) and representational drawing (cut‐off ≤ 2) Inclusion criteria: inpatient in SU with neglect, within 4 weeks post‐stroke. All participants had to demonstrate an after‐effect of at least 3 visual degrees to the left of the landing position after the first prism adaptation; this would only apply to the active treatment group, but none were excluded for this reason Exclusion criteria: ocular problems, a disturbed consciousness or a too limited attention span (participants excluded during screening) Visual sensory deficit: 2 in the experimental group had hemianopia, diagnosed by confrontation comparing cueing and non‐cueing conditions by a stroke neurologist |
|
| Interventions | Prism adaptation: "an extended version of that used by Rosetti et al 1998". While wearing goggles with prisms inducing a rightward optical shift of 10°, participants made 100 fast pointing movements to 2 visual targets presented 10° to the left and right of the body midline. Sessions of 30 minutes were conducted 4 days in a row versus placebo ‐ as above but wearing goggles with no optical shift. Sessions of 30 minutes were conducted 4 days in a row
For analysis of bottom‐up and top‐down rehabilitation approaches this review coded the experimental condition as bottom‐up Profession of intervention provider not stated |
|
| Outcomes |
|
|
| Notes | Postulated mechanism of action: not clear but stated there was a "neural basis for the therapeutic effect" and treated early because the brain is most sensitive to rehabilitative treatment early after stroke | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details "according to a randomisation procedure in SPSS" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Only 1 measure, scene copying, appears to have been scored retrospectively by an independent rater. Not stated if outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear ‐ only those who completed were included in the report |
| Free of systematic differences in baseline characteristics of groups compared? | Unclear risk | Removed 1 person who failed to complete treatment but no indication of which group this was from |
| Did authors adjust for baseline differences in their analyses? | Low risk | Not necessary |
Polanowska 2009.
| Methods | RCT. Single site and double‐blinded Setting: Poland | |
| Participants | 40 participants with first ever stroke and hemineglect Experimental: n = 20. control: n = 20 Adequacy of matching at baseline? No, although age, sex, BI, MMSE are well balanced the baseline, accuracy on neglect tests seems lower in the experimental versus control group Number lost to follow‐up: authors confirm no losses to follow‐up Mean age (mean (SD)): experimental 61.6 (8.3), control 58.3 (12.9) years Sex (male/female): experimental 11/9, control 14/6 Side of damage: right Method of diagnosing stroke: confirmed by neuroimaging and neurological exam (CT) Method of diagnosing neglect: confirmed by neuropsychological exam as fulfilling 2 of 3 criteria: at least 4 omissions of left‐sided targets in subtest A of Balloons Test; marked rightward bias (cut‐off score 7) on line bisection; spontaneous behaviours specific to neglect e.g. ipsilesional deviation of head, eyes, trunk; attending to ipsilesional side; neglect dyslexia and dysgraphia with tendency to initiate search on right of stimulus sheet Time post‐onset: experimental 44.4 (27.3), control 46.6 (26.2) days Inclusion criteria: first right hemisphere stroke, left visuospatial neglect, recruited from single rehabilitation unit, 2 to 12 weeks post‐stroke, right‐handed, 25 to 75 years, informed consent obtained Reasons for exclusion: if electrical stimulation contraindicated, history of dementia, neurological or psychiatric disorders, if communication or other problems meant were unable to co‐operate Visual sensory deficit: 'visual sensory deficit': experimental 13/20, control 13/20; and 'hemianopia': experimental 6/20, control 9/20 as assessed by "standard neurological assessment" |
|
| Interventions | Electrical somatosensory stimulation of the left hand combined with conventional visual scanning training, 1 month duration of 20 session of 45 minutes duration each, 5 days per week. This stimulation lasted for the first 30 of the 45 minutes. Electrical stimulation was provided by 2 electrodes on the hand giving a maximum intensity of 15 mA. Visual scanning used 2 programs from RehaCom computerised system to get active purposeful exploration of visual field (1. saccadic training ‐ seek stimuli within detailed background, 2. attention and concentration ‐ detected and identify stimuli then seek their counterpart on the opposite side within a detailed background). Visual scanning also used some paper and pencil tasks to improve scanning when reading and writing; drawing and copying; analysing form and content of complex visual stimuli. Verbal and visual cues and instructions given as was feedback on achievements and errors versus visual scanning training as above, with placebo stimulation where electrodes were applied to the hand but without "current intensity" The visuospatial scanning training was conducted by a neuropsychologist and electrostimulation was supervised by a neurologist For this review we classified this as a comparison of one cognitive rehabilitation approach to another cognitive rehabilitation approach. For analyses; visual scanning training plus electrical somatosensory stimulation was classed as Approach 1 and the visual scanning training plus placebo stimulation as Approach 2. For analysis of bottom‐up and top‐down rehabilitation approaches this review coded the experimental condition as bottom‐up, but combined with top‐down visual scanning training |
|
| Outcomes |
Also measured after only 1 day of stimulation but excluded those results in favour of the more meaningful 1 month results which are immediate post‐rehabilitation – so no maintenance/follow‐up outcomes were measured |
|
| Notes | Postulated mechanism of action: visual scanning training aims to remind and motivate participants to scan to the left to build the habit of voluntarily scanning their neglected space. Requires awareness by the participant which is not always present. Hence the use of passive (non‐volitional) physiological approaches such as sensory stimulation. Assumes manipulated sensory inputs are linked to auto levels of orientation behaviour. But effects seem transitory so this study attempts to combine active training of visual scanning with passive stimulation to enhance activation of right hemisphere attention system and improve visual exploration of extra‐personal space All participants received visual scanning training ‐ the only difference was the electrical stimulation Authors state in the paper that 11 participants reported a tingling sensation during a trial electrostimulation period. During the study itself, however, only 1 participant noted such a sensation; afterwards it was noted this person was in the sham group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | "Blocked randomisation was performed by 1 person unblinded to group allocations and was based on random number tables. For each 10 subjects, numbers 1‐5 meant that patients would be in group E, the numbers 6‐10 meant that patients would be in Group C with the constraint that in each block of 10 there would be 5 in group E and 5 in group C. Allocations were stored in sealed, numbered envelopes that were opened only at the time of recruitment and the author has confirmed all envelopes were prepared before recruitment began by someone other than the recruiter." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors advise that all randomised participants were followed up on all variables and there were no post‐randomisation exclusions |
| Free of systematic differences in baseline characteristics of groups compared? | High risk | The control group had significantly better scanning accuracy at baseline |
| Did authors adjust for baseline differences in their analyses? | High risk | No adjustment made for differences is scanning accuracy |
Robertson 1990.
| Methods | RCT Setting: UK |
|
| Participants | 30 (see Notes) inpatients of Edinburgh hospitals who showed left visual field neglect on BIT Experimental: n = 17, control: n = 13 (The following data describe the 36 initial participants: see Notes) Mean age (SD): experimental 64.2 years (12.6), control 63.1 years (9.6) Sex (male/female): experimental 9/11, control 10/6 Onset of neglect (SD): experimental 19.2 weeks (21.1), control 10.8 weeks (6.3) Inclusion: presence of neglect (failure on at least 3/9 behavioural tests), oriented for time and place, ability to consent, ability to concentrate sufficiently to sit at computer‐based task for at least 15 minutes | |
| Interventions | 15½ hours (14 sessions of 75 minutes each, 2 x week for 7 weeks) computerised scanning and attention training (intensive briefing about nature of participant's problems, feedback on left and right latencies, trainer reinforcement and encouragement) versus 11.4 hours recreational computing (to minimise scanning and timed attention tasks, without any potential neuropsychological mechanism to improve cognitive function, but exposed to computer activities such as games, quizzes and simple logical games) For analysis of bottom‐up and top‐down rehabilitation approaches this review coded the experimental condition as top‐down | |
| Outcomes | The study collected several types of outcomes:
The BIT was the principal outcome measure. (Although not explicitly stated it is assumed from the description on page 664 and the low scores in Table 2 that only the BIT behavioural subtests were given.) The outcomes were given immediately after training and after 6 months. The study also collected data on several other tests including the GHQ and the FAI to ensure matching of groups (see Notes).These were collected at each time point This review used the BIT, immediately and after 6 months |
|
| Notes | This review entered n = 30 of initial 36 (33 with CVA, 2 HI, 1 had surgery for excision of meningioma). 3/36 not followed up immediately and 9/36 not seen at 6 months but no information on which group these were from so data entered to this review subtracted 3 and 9 from each group at first (n = 30) and second assessments respectively. Information on allocation concealment provided by personal communication. 6 months follow‐up Exclusion: participants with BIT score > 70 Cancellation data reported as errors rather than correct performance The review could not include the FAI data as these were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | External randomisation.Randomisation restricted in blocks of patients with severe or mild neglect, therefore stratifies by severity. Randomisation was carried out by a third party |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3/36 not followed up immediately and 9/36 not seen at 6 months but no information on which group these were |
| Free of systematic differences in baseline characteristics of groups compared? | Unclear risk | Slight difference in letter cancellation errors and Wisconsin at baseline |
| Did authors adjust for baseline differences in their analyses? | Low risk | Not necessary |
Robertson 2002.
| Methods | RCT Setting: UK |
|
| Participants | 40 randomised but 36 seen for immediate outcome assessment (see Notes), recruited from London hospital and community rehabilitation teams, had left visual neglect on cancellation or bisection tests (The following data describe the initial 40 participants: see Notes) Experimental: n = 19, control: n = 21 Mean age (SD): experimental 69.3 years (9), control 67 years (9.4) Sex (male/female): experimental 13/6, control 16/5 Onset of neglect (SD): experimental 152.8 days (142.4), control 152.1 days (117.9) Inclusion: right hemisphere stroke, aged under 80, right‐handed, no history of major psychiatric/disease/disability that would prevent participation or contaminate results | |
| Interventions | LAT wearing (on the wrist/leg/shoulder) an active limb activation device during perceptual training. The device emitted an auditory tone if no left‐sided movement was made, versus perceptual training wearing an inactive (no tone) limb activation device Both groups received training at their residence (usually own home) for 12 weeks for approximately 45 minutes per week The perceptual training for both groups involved working on visuoperceptual puzzles and reading tasks which implicitly but not explicitly involved advice to scan to the left For analysis of bottom‐up and top‐down rehabilitation approaches this review coded the experimental condition as bottom‐up | |
| Outcomes | The study collected 3 types of outcomes:
For comparability with other studies this review used only the following outcome/time points: BI immediate and 6 months |
|
| Notes | Attrition: 36/40 followed up immediately (experimental 17, control 19); 32 at 6 months, 26 at 18 to 24 months Groups appeared appropriately matched for demographic and clinical baseline variables No information on number per group at 6 months. Know 4 lost but not whether all were from a single group so assumed worst case and subtracted 4 per group, i.e. conservative sample estimate of 28 not 32 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Robertson 2002 confirmed that the recruiters were unaware of and unable to predict allocation concealment. Authors confirmed randomisation but did not specify the method used. Concealment was highly likely to have been achieved, although it could not be guaranteed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 (2 from each group) dropped out during treatment. 36 participants were followed up at 3 months, and 32 at 6 months. Of the 4 who dropped out at 6 month follow‐up, 2 had a further CVA, 1 died, and 1 refused. Low risk, loss to follow‐up unlikely to affect outcomes |
| Free of systematic differences in baseline characteristics of groups compared? | Low risk | Groups comparable on baseline characteristics. A slight difference on verbal memory but unlikely to be relevant to outcomes |
| Did authors adjust for baseline differences in their analyses? | Low risk | Not needed |
Rossi 1990.
| Methods | RCT Setting: USA |
|
| Participants | 39 stroke patients from an inpatient stroke rehabilitation unit with HHA or VN Experimental: n = 18, control n = 21 Mean age: experimental 72.6 years, control 63.3 years Sex (male/female): experimental 10/8, control 9/12 Mean weeks post‐stroke: experimental 4.4, control 4.7 Side of stroke (right/left): experimental 16/2, control 13/8 Lesion type (infarct/haemorrhage): experimental 15/3, control 18/3 Inclusion: participants free of disabling cardiac pulmonary or rheumatological problems, HHA determined by inability to detect 1 cm red target on tangent screen examination, VN defined as inability to detect bilateral tachistoscopically presented targets using HFVS HHA/VN: experimental 12/6, Control 15/6 Exclusion: people with best‐corrected visual acuity worse than 20/200; inability to comprehend and co‐operate with assessments | |
| Interventions | 15‐diopter plastic press‐on fresnel prisms (cut to a half circle, to fit on the inside of spectacle lenses, overlaying the affected hemi‐field with the base of the prism towards the affected field to produce an intended effect of shifting a peripheral image more towards the centre) worn for all daytime activities versus no prism treatment Both groups received routine rehabilitation programs including ADL training and table‐top visual perception retraining tasks For analysis of bottom‐up and top‐down rehabilitation approaches this review coded the experimental condition as bottom‐up | |
| Outcomes | The study collected eight types of outcomes:
Outcomes were assessed at baseline, 2 weeks and 4 weeks. This review used line bisection, BI and falls. The 4‐week outcome data were used. However, as prisms were still being used at that time this review analysed them as 'immediate' rather than 'persisting' effects |
|
| Notes | Clarification of randomisation procedure sought but not obtained Control group younger but otherwise groups were similar on demographic and clinical background factors including BI Data for VN subgroup not reported separately to HHA subgroup therefore all outcome data in this review are for VN and HHA combined. The authors report that the HHA diagnosis precluded a diagnosis of neglect and that participants with either HHA or VN who were treated with prisms showed equal improvement The prism group wore their prisms during outcome assessments Cancellation data reported as errors rather than correct performance. Line bisection scores are errors in cms from the middle SEM data converted to SD for analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No details of randomisation provided |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessor not blinded, apart from Tangent screen examinations which were judged by an observer who was unaware of group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 treatment and 5 control not retested on tangent screen examination because of scheduling problems, but outcomes of interest seem to be complete |
| Free of systematic differences in baseline characteristics of groups compared? | High risk | Difference in age, control group younger |
| Did authors adjust for baseline differences in their analyses? | High risk | |
Rusconi 2002.
| Methods | RCT Setting: Italy |
|
| Participants | 24 randomised (see Notes) but outcome data collected on 20 (The following data describe the 20 participants) Experimental: n = 12, control: n = 8 (experimental is Type 1 and Control is Type 2: see Interventions) Mean age: experimental: 69.8 years, control: 65.1 years Sex (male/female): experimental 5/7, control 3/5 Mean weeks post‐stroke: experimental: 6.92, control 8.38 Inclusion: unilateral right hemisphere stroke assessed by CT scan, right‐handed, symptoms of unilateral neglect, admitted to hospital for rehabilitation 5 weeks post‐stroke Exclusion: dementia | |
| Interventions | The study compared more than 2 interventions. First there is a comparison of 2 types of cognitive training: Type 1 versus Type 2. Each 'type' is then subdivided into whether or not TENS is added (see Notes) Type 1 versus Type 2: both consist of 5 x 1‐hour sessions per week for 2 consecutive months (40 sessions) using 4 procedures requiring the participant to actively scan the visual field (reading sentences and stories, line drawing on a dot matrix, assembling 3D cubes, matching cards containing the name and the visual image of an object. Type 1 and 2 differed in that only Type 1 involved verbal and visuospatial cueing and verbal feedback. Although Type 2 used the same 4 procedures it did not involve cueing or feedback, i.e. the aspects of the training designed to improve awareness and encourage compensation For this review Type 1 was classed as a top‐down approach and Type 2 as an attention control | |
| Outcomes | Assessments were classified as 'functional and neurological' (i.e. BI, standard clinical neurological examination) or 'neuropsychological' (i.e. line cancellation, letter cancellation, line bisection, sentence reading, O'clock test, judgement of drawings, anosognosia, RCPM, facial recognition, position deficit) These were taken at 4 time points: on admission for neurorehabilitation at least 5 weeks post‐stroke (T0), 1 month later (T1) after which eligibility was determined and participants were randomised, after 1 month of intervention (T2) and after 2 months of intervention (T3) For comparability with other studies this review used only the T3 letter cancellation and BI. As intervention continued for 2 months T3 is coded in this review as immediate effects | |
| Notes | Author provided clarification and raw data by personal communication 24 people were randomised: 12 to Type 1 and 12 to Type 2. The authors excluded 4 from the final evaluation because of a "clinical worsening that prevented the conclusion of the treatment". These 4 were all allocated to Type 2 Cancellation scores were for the number correctly cancelled. Separate scores were given for left and right space but this review used the total score. Line bisection data were for mean deviation in mm left (negative) or right (positive) from the midpoint. Line cancellation data could not be used as the experimental group's SD was 0 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Paper states that "randomly assigned". No details provided Subsequent information states allocations stored in sequentially numbered, sealed, opaque, envelopes. Concealment of allocation is unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | None reported in paper but authors reported 24 people were randomised: 12 to Type 1 and 12 to Type 2. The authors excluded 4 from the final evaluation because of a "clinical worsening that prevented the conclusion of the treatment". These 4 were all allocated to Type 2. Not intention‐to‐treat analysis |
| Free of systematic differences in baseline characteristics of groups compared? | Low risk | Groups look comparable but numbers in each group are very small |
| Did authors adjust for baseline differences in their analyses? | Low risk | Used a repeated measures ANOVA |
Schroder 2008.
| Methods | RCT. 3‐arm study Setting: Germany |
|
| Participants | 30 right‐handed participants with right brain damage, moderate left neglect Experimental 1: n = 10, experimental 2: n = 10, control: n = 10 Number lost to follow‐up: none Adequacy of matching at baseline? Yes, no significant differences at baseline Mean age (mean (SD)): experimental 1: 68.4 , experimental 2: 60.6, control: 67.3 Sex (male/female): experimental 1: 7/3, experimental 2: 5/5, control: 6/4 Time post‐onset (mean (SD)): experimental 1: 43.8 (23.6), experimental 2: 24.6 (9.6), control: 36.2 (24.2) days Side of damage: right Method of diagnosing stroke: not stated Method of diagnosing neglect:
No details of cut‐offs provided Inclusion criteria: right‐handed, less than 90 days post‐stroke, left brain damage, at least moderate neglect Exclusion criteria: not stated Visual sensory deficit: not stated |
|
| Interventions | Visual Exploration and TENS: 20 therapy sessions, each lasting 25 to 40 minutes over 4 weeks (TENS: 100 Hz, over left trapezius, applied throughout exploration training) versus visual exploration and OKS 20 therapy sessions, each lasting 25 to 40 minutes over 4 weeks (OKS: small randomly spaced squares moving slowly to the left across a screen, 2 x 10 minute periods of OKS separated by 10 to 20 minutes exploration training) versus visual exploration (control) using the ELEX apparatus (stimuli patterns were presented on a screen that subtended 53° vertically and 40° horizontally: after initial fixation, participants had to shift fixation to a yellow stimulus) Profession of the intervention provider not stated For this review we classified this as a comparison of 1 cognitive rehabilitation approach versus another cognitive rehabilitation approach For analysis of bottom‐up and top‐down rehabilitation approaches this review coded the TENS and OKS as bottom‐up and the scanning training as top‐down For analyses, the groups including TENS and OKS were defined as 'Approach 1' and the visual exploration as 'Approach 2' |
|
| Outcomes |
|
|
| Notes | TENS: "a non‐specific activation of the right hemisphere or a directional effect on the egocentric coordinates of extrapersonal space" OKS "activates multiple cortical (temporoparietal and vestibular cortex, the insula) and subcortical structures (basal ganglia) involved in multisensory integration" For analyses this study was entered as 2 studies: Schroder 2008 OKS and Schroder 2008 TENS |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | "Randomly assigned", no other details |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | For the few variables given, the groups appear comparable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None reported |
| Free of systematic differences in baseline characteristics of groups compared? | Unclear risk | No information provided |
| Did authors adjust for baseline differences in their analyses? | Low risk | Not required |
Schroder 2008 OKS.
| Methods | See Schroder 2008 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Schroder 2008 TENS.
| Methods | See Schroder 2008 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Tsang 2009.
| Methods | RCT Setting: Hong Kong |
|
| Participants | 35 participants (1 drop‐out during the trial) Experimental: n = 17, control n = 17 Adequacy of matching at baseline? Yes Number lost to follow‐up: 1; no details as to which group ‐ no data included in analyses Mean age (mean (SD)): experimental 70.47 (9.30), control 71.82 (5.26) years Sex (male/female): experimental 12/5, control 9/8 Time post‐onset (mean (SD)): experimental 22.18 (15.87), control 21.50 (21.67) days Side of damage: right (experimental right 11, basal ganglia 0, other 6; control right 11, basal ganglia 2, other 4) Method of diagnosing stroke: CT or MRI Method of diagnosing neglect: BIT conventional subtest < 129 Inclusion criteria: subacute inpatients with right hemisphere stroke, undergoing rehabilitation, left visual field inattention, right‐handed, within 8 weeks after onset of stroke, Glasgow coma scale = 15 Exclusion criteria: severe dysphasia, TIA or reversible neurological deficit; significant impairment in visual acuity caused by cataracts, diabetic retinopathy, and glaucoma; history of other neurological disease, psychiatric disorder, or alcoholism Visual sensory deficit: visual acuity screened for, no other method of assessing visual fields etc noted |
|
| Interventions | Right half‐field eye patching glasses: 4 weeks of conventional OT with right half‐field eye‐patching during OT session (conventional OT = 30 minutes ADL training and 30 minutes upper limb training using neurodevelopmental therapy ‐ this seems to be the standard procedure, rather than a record of what participants actually got, there was no mention of deviation from this amount. Other standard care received was 5 physiotherapy sessions of 60 minutes/week, speech and language therapy and psychological counselling as indicated, skilled nursing care, daily medical round) versus control (4 weeks of conventional OT as described above, without patching. Other standard care received was 5 physiotherapy sessions of 60 minutes/week, speech and language therapy and psychological counselling as indicated, skilled nursing care, daily medical round) Profession of intervention provider: OT For analysis of bottom‐up and top‐down rehabilitation approaches this review coded the experimental condition as bottom‐up |
|
| Outcomes | BIT conventional subtest FIM |
|
| Notes | "Concentrates the patients' attention on the contralesional space by blocking the ipsilesional visual field, and hence lessens the disinhibition of the orienting mechanism of the ipsilesional side resulting from interhemispheric imbalance". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly assigned, by a designated person ... using consecutively numbered sealed envelopes for each group (according to random permuted blocks of four that were derived from the block of 4 randomisation table)." The designated person was the case therapist and envelopes were prepared by a different person ‐ an OT |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Corresponence with the author states "An occupational therapist, who was the blinded assessor and did not know the group allocation, was responsible for all the outcome measures, both pre and posttests" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 participant dropped out but was not included in the analysis. Both baseline and outcome assessments only include the 34 who completed the study. Therefore no intention‐to‐treat analysis |
| Free of systematic differences in baseline characteristics of groups compared? | Low risk | Study is free from systematic differences |
| Did authors adjust for baseline differences in their analyses? | Low risk | Not necessary |
Turton 2010.
| Methods | RCT. Single‐blind, pilot, 2 sites Setting: UK |
|
| Participants | 37 participants consented following screening but 1 person excluded post‐recruitment but pre‐randomisation for failing to complete assessments, so 36 randomised. Experimental: n = 17, control: n = 19 Adequacy of matching at baseline? Yes, although large variation in severity of neglect Number lost to follow‐up: overall 34 remained at 4 days and 28 at 8 weeks of the 36 randomised Mean age (mean (SD)): experimental 72 (14), control 71 (14) years Sex (male/female): experimental 8/8, control 11/7 Time post‐onset (mean (SD)): experimental 1 45 (23), control 47 (39) days Side of damage: right Method of diagnosing stroke: not specified Method of diagnosing neglect: star cancellation task and/or line bisection test of BIT Inclusion criteria: right hemisphere stroke, at least 20 days before entry to study; self‐care problems due to neglect identified by OT (from consecutive hospital admissions); ability to sit and point with the unaffected hand; ability to understand and follow instructions; medical fitness to participate Exclusion criteria: neglect prior to this stroke Visual sensory deficit: sensory score at baseline given Hemianopia: experimental 3/16, control 4/18 Assessed by Nottingham Sensory Assessment and confrontation |
|
| Interventions | Prism adaptation training (repeated pointing movements to targets using the right 'unaffected' hand while wearing prism glasses; prism power of 10 diopters that shifted the field of view 6° to the right; training once per day, each working day for 2 weeks) versus sham treatment (same pointing procedure wearing plain glasses) Once a day for each working day for 2 weeks Profession of intervention provider: OT For analysis of bottom‐up and top‐down rehabilitation approaches this review coded the experimental condition as bottom‐up |
|
| Outcomes |
|
|
| Notes | Conflict between proprioception and vision occurs when pointing wearing prisms and they mis‐point to the right and there is subsequent adaptation. "Treatment triggers a realignment of the egocentric coordinate system that is responsible for the localisation of the body in space and of object position in relation to the body" Therapy and control were well tolerated, with only 1% and 3% respectively of sessions missed by participants due to illness |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | "A minimisation method using a 4:1 element of chance was implemented and automated using Microsoft Excel for pseudo‐random allocation to groups" "A secretary who was located outside of the stroke services administered the randomisation procedure" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Outcome assessments were carried out with assessors blind to group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs accounted for |
| Free of systematic differences in baseline characteristics of groups compared? | Unclear risk | The are a number of differences in baseline demographics and variation in these demographics between the groups, and the median BIT was 21 points higher for controls |
| Did authors adjust for baseline differences in their analyses? | Unclear risk | There was no adjustment of the baseline differences, but the impact of the differences in baseline demographics are unclear |
Weinberg 1977.
| Methods | RCT Setting: USA | |
| Participants | 25 (see Notes) stroke rehabilitation inpatients Experimental: n = 14, control: n = 11 (The following data describe the 57 initial participants: see Notes) Mean age (SD): experimental: 61.5 years (9.84), control 65.7 years (10.92) Onset of testing (weeks): experimental: 9.9, control 10.53 | |
| Interventions | 20 hours visual training (1 hour each day for 4 weeks in reading, writing and calculation) versus no visual training (but received OT as part of general rehabilitation programme) For analysis of bottom‐up and top‐down rehabilitation approaches this review coded the experimental condition as top‐down |
|
| Outcomes | The study collected 3 types of outcomes:
Outcomes assessed after 1 month, i.e. immediate effects This review used only the single letter cancellation |
|
| Notes | Hypothesises that neglect underlies visual perceptual problems Experimental and control groups appeared similar in age, 2 participants in the experimental group had "aberrantly long times since onset" Groups divided into RBD severe and RBD mild No reply to request for clarification of randomisation procedure and other outcome measures 57 patients reported but outcome data reported separately for severe and mild RBD groups and only severe data (n = 25) entered in this review, experimental 14 and Control 11 Control group better than experimental on single letter cancellation at baseline. No difference in double letter cancellation or digit span | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No details of randomisation provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None reported |
| Free of systematic differences in baseline characteristics of groups compared? | High risk | Onset since testing may be different but not clear. Two cases with very long onset were excluded from the comparison of time since onset |
| Did authors adjust for baseline differences in their analyses? | Low risk | Calculated an effectiveness change index |
Welfringer 2011.
| Methods | RCT. Designed as a feasibility study Setting; Germany |
|
| Participants | 30 participants with right‐hemisphere stroke, less than 6 months previously Inclusion criteria: had a diagnosis of right‐hemispheric stroke dated less than 6 months earlier; had no history of major psychiatric problems and no other co‐existing disease/disability; showed unilateral left visuospatial neglect symptoms as defined by a score of 54 or less on the Letter Cancellation Test; had no diagnosis of hemianopia; had sufficient sensory, physical and cognitive capacities to follow instructions for more than 30 minutes and no additional verbal‐memory deficits as defined by a percentage rank above 16 in the story recall sub‐test of the Wechsler‐Memory‐Scale‐Revised (WMS‐R); were aged between 20 and 75 years; were right‐handed; and had provided informed consent Experimental: n = 15; mean age 56.3 years (SD 11.2); mean time since stroke: 3.2 months (SD 1.5) Control: n = 15; mean age 57.3 years (SD 11.3): mean time since stroke: 3.4 months (SD 2.8) |
|
| Interventions | Visuomotor‐imagery therapy (2 daily half‐hour sessions of visuomotor‐imagery therapy as an add‐on treatment over a period of 3 weeks; participants mentally practised positions and movements of the contralesional upper limb in a repetitive fashion and as vividly and intensively as possible; over the course of the 3‐week intervention period, they participated in 28 to 30 training sessions; a total of 4 positions and 6 sequences (simple and complex movements) were imagined, with 1 exercise being repeated up to 10 times per session) versus no supplementary intervention All participants received standardised rehabilitation therapies including 45 minutes of exploration training 4 times per week For analysis of bottom‐up and top‐down rehabilitation approaches this review coded the experimental condition as top‐down |
|
| Outcomes | Neglect tests: Bells Cancellation test; drawing tasks; text‐reading task Representation tests: test of mental representation of left side of body Arm function texts: sensation of left arm; Action Research Arm Test For analyses within this review we used: neglect ‐ Bells Cancellation Test |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Blocked randomisation, in blocks of 10; computer‐generated sequence, delivered by person independent of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States: "Outcome measures were assessed by a blinded tester." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Free of systematic differences in baseline characteristics of groups compared? | Low risk | No differences noted between groups |
| Did authors adjust for baseline differences in their analyses? | Low risk | Not necessary |
Wiart 1997.
| Methods | RCT Setting: France |
|
| Participants | 22 people within 3 months onset of stroke and severe left unilateral neglect, hospitalised in 2 neurorehabilitation hospitals, positive for neglect on 3 tests (see Outcomes) Experimental: n = 11, control: n = 11 Mean age: experimental: 66 years, control 72 years Sex (male/female): experimental: 6/5, control: 6/5 Time post‐onset (mean days): experimental: 35, control: 30 Exclusions: history of stroke, alteration of general status, or cognitive difficulties incompatible with rehabilitation | |
| Interventions | 1 hour per day for 20 days of experimental treatment followed by traditional rehabilitation (1 to 2 hours physiotherapy and 1 hour OT; experimental treatment is Bon Saint Come method: participant wears a thoracolumbar vest with attached metal pointer above head, participant points to target on mobile wooden panel, audible and luminous signals provide biofeedback effect when targets are touched; initially conducted when sitting, this progresses to standing, the therapist participates actively during the session, stimulating, guiding and correcting) versus 3 to 4 hours traditional rehabilitation per day For analysis of bottom‐up and top‐down rehabilitation approaches this review coded the experimental condition as top‐down | |
| Outcomes | The study collected 2 types of outcomes:
These assessments were conducted 3 times: day 0, day 30 (after therapy) and day 60 This review used only the data from line bisection and FIM. Both the 30 day (immediate) and 60 day (persisting) data were used in this review |
|
| Notes | The paper consists of 2 studies. These data refer to Study 1 only The experimental group were younger and had a higher initial FIM score (66) than the control group (54) Cancellation data reported as errors rather than correct performance. Only 1 set of cancellation data (lines not bells) were entered in this review to avoid entering the same group of participants twice into the meta‐analysis Line bisection scores are % deviation to right Control group had more, but not significantly so, omissions on line cancellation (control 16, experimental 14) and right deviations on line bisection (control 53%, experimental 50%) at baseline compared with experimental group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Information on allocation concealment unclear. Random number tables |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. Assessment done by 'one of us (LW)'. Different from person delivering therapy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Free of systematic differences in baseline characteristics of groups compared? | High risk | Control group older and more disabled |
| Did authors adjust for baseline differences in their analyses? | High risk | No correction made |
Zeloni 2002.
| Methods | RCT Setting: Italy |
|
| Participants | 8 randomised (see Notes) Experimental: n = 4, control: n = 4 Mean age: experimental: 68.8 years, control: 76.3 years Sex (male/female): experimental: 4/0, control: 2/2 Mean months post‐stroke: experimental: 11.25, control: 4.5 Inclusion: "post‐acute" patients with right hemisphere vascular lesions and neglect, admitted to hospital, right‐handed, left hemiplegic Exclusions: normally wore glasses | |
| Interventions | Wearing plastic goggles for 1 week, only removing them to go to sleep (the right side of each lens was blinded), versus no goggles All 8 participants were involved in the hospital's daily activities including the usual treatment for neglect, tasks to train compensation for faulty scanning For analysis of bottom‐up and top‐down rehabilitation approaches this review coded the experimental condition as bottom‐up | |
| Outcomes | Participants were assessed on 3 occasions: at recruitment, after the experimental group had received 1 week of hemi‐blinding goggles, and again 1 week after the goggle treatment ended. Controls were assessed at the same time points but never wore the hemi‐blinding goggles. Testing was performed without goggles. The outcomes used were: line, letter and bell cancellation, copy drawing, line bisection For this version of the review we used the single letter cancellation outcome data only. We used data from the third time point; as this was only 1 week after intervention it is coded in this review as 'immediate' effects | |
| Notes | Personal communication from the authors confirmed the methods used and provided data. The 8 randomised participants are numbers 1 to 4 in the treatment and control group as listed in the authors' Table 1, page 196. The original study recruited 11 participants. The first 8 were randomised as described above. The other 3 were non‐randomly added to the groups (1 to treatment and 2 to control). This review only used the 8 randomised participants Cancellation tests were scored as number correct. Line bisection was scored as % correct decreasing for rightward deviation. Authors provided raw data (%) for the 8 participants on line bisection. The mean (SD) were: experimental: 62.5 (35.2), control: 73.8 (22.2). These data were used in the 2006 version of this review, but for this version the number of neglect outcomes was reduced and the line bisection data removed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | For the first 8 participants group allocation was performed by randomly selecting a label from a pre‐printed set of 8 (see Notes). The label preparation was performed by a member of the trial team but the selection was performed by a student who had no previous or later involvement in the trial. Although the allocation was done externally the method used did not permit verification |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcomes |
| Free of systematic differences in baseline characteristics of groups compared? | Unclear risk | Controls seem somewhat older, though sample very small |
| Did authors adjust for baseline differences in their analyses? | Unclear risk | No adjustment made |
ADL: activities of daily living ASMP: auditory subjective median plane BI: Barthel Index BIT: Behavioural Inattention Test CBS OT: Catherine Bergego Scale occupational therapist’s evaluation score cm: centimetre CT: computerised tomography CVA: cerebrovascular accident FA: functional approach FAI: Frenchay Activities Index FIM: Functional Independence Measure GHQ: General Health Questionnaire HFVS: Harrington Flocks Visual Screener HHA: homonymous hemianopia HI: head injury LAT: limb activation training mm: millimetre MMSE: Mini Mental Status Exam MVPT: Motor Free Visual Perception Test N/A: not applicable Nottingham EADL: extended ADL index OKS: optokinetic stimulation OT: occupational therapy/therapist RBD: right brain damage RCPM: Raven's Coloured Progressive Matrices RCT: randomised controlled trial Rey CFT: Rey Osterreith Complex Figure Test RH: right hemisphere RMA: Rivermead Motor Assessment RPAB: Rivermead Perceptual Assessment Battery SD: standard deviation SEM: standard error of the mean SIAS: Social Interaction Anxiety Scale SU: stroke unit TENS: transcutaneous electrical nerve stimulation TIA: transient ischaemic attack ToT: transfer of training VN: visual neglect WAIS‐R: Revised Weschler Adult Intelligence Scale WMFT: Wolf Motor Function Test WRAT: Wide Range Achievement Test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akinwuntan 2010 | Study does not include a neglect population. It is a study of 3 types of attention problems (selective attention, divided attention, speed of processing) that would affect driving ability. Refers to visual problems but these are not neglect. No neglect measures are used. |
| Al Mahasneh 1991 | Extreme difficulties with recruitment and participant attrition. 14 participants with neglect consented. These were unevenly assigned to the experimental (9) and control (5) groups. Only 5 participants completed 3 weeks of treatment. Review authors did not feel the data were adequate for meta‐analysis, e.g. missing data and no SDs. |
| Bar‐Haim 2011 | The method of allocation was not fully randomised. Clarification from the author stated "the first individual was assigned randomly to 1 of the 3 groups (by assigning numbers to the groups, 1‐3, and drawing a number for that patient). From that on each new participant was assigned to the next group consecutively". |
| Beis 1999 | Controlled trial but not RCT: allocation by fixed order of presentation of participants i.e. first to group 1, second to group 2, etc. Outcome assessors were blinded to allocation. Personal communication provided FIM data, confirmed allocation method, and that assessments were carried out by 2 blinded researchers. |
| Butter 1992 | Clarification of randomisation sought but not obtained. Appropriate results (means and SDs) not reported. Review authors were not sure that the trial was actually evaluating a treatment for spatial neglect. |
| Carter 1980 | Clarification of randomisation sought but not obtained. Separate data for stroke patients also requested but not obtained. Appropriate data (means, SDs) not reported. |
| Cubelli 1993 | Identified as a potential RCT of spatial neglect for the 2006 version. As further information could not be obtained, this was added to studies awaiting classification. As further details have still not been obtained for this 2012 version it has been moved to Excluded studies. We will reconsider this study for inclusion if further information becomes available. |
| Diller 1974 | Reported data inadequate for review. No reply to our letter of 9 February 1999 asking for difficult‐to‐extract data. |
| EEG‐NF 2009 | Not a cognitive rehabilitation approach. |
| Frassinetti 2002 | Controlled clinical trial: non‐random allocation (n = 13). Controls at different hospital. Assessment of outcome not clear (probably non‐blind). |
| Gordon 1985 | Controlled trial: quasi‐randomisation based on rehabilitation service to which participant was assigned. Experimental and control conditions alternated every 6 months between the 2 services. Not randomised. |
| Harvey 2003 | Controlled trial: non‐randomised, initial recruits allocated by date of admission to hospital ward, later recruits allocated by attempting to match the groups on their scores on pre‐intervention neglect assessments. Author provided clarification and unpublished data by personal communication. |
| Keller 2006 | Although the English abstract states that participants were "randomly assigned", the full German paper does not state random allocation and it appears that this study is not a randomised study. |
| Kerkhoff 2005 | Not randomised. States that participants were "consecutively collected and matched for clinical and demographic variables as well as neglect severity" and "all subjects were treated in a single‐subject baseline design". |
| Kerkhoff 2012b | The intervention involved one single treatment session (i.e. this was not a rehabilitation programme). |
| Klos 2005 | Personal communication from an expert in the field reported that Klos had completed an unpublished RCT of prism adaptation therapy for neglect. Excluded from review as no reply to request for clarification of methods and data. Will reconsider for inclusion in next update if further information becomes available. |
| Koch 2012 | θ‐burst stimulation not classed as a cognitive rehabilitation approach. |
| Lincoln 1985 | RCT of patients with general perceptual problems. Problems likely to have included neglect but this subgroup could not be separately identified. |
| Loverro 1988 | Controlled trial: reported as randomly assigned but allocation based on bed availability; outcome assessors blinded to purpose of the study. |
| Niemeier 1998 | Controlled trial: not randomised, selected in order of consecutive admissions and on documented left or right neglect. No information on concealment. |
| Osawa 2010 | No mention of randomisation and appears that the group allocation was based on whether they happened to have family or not. |
| Paolucci 1996 | Controlled trial: abstract states randomly assigned but allocated on the basis of bed number (odd or even), bed number had been assigned by Hospital Administration, odd numbers got immediate training, even numbers got training after 2 months (delayed training), neglect screening assessment done after allocation by psychologist unaware of purpose of study, outcome assessor blinded to the purpose of the study, after eight weeks the delayed group received the training and the immediate group received the control treatment (broad cognitive stimulation). |
| Pizzamiglio 2004 | Non‐random controlled trial (n = 22): alternate allocation. Blind assessment of outcome on BI (functional outcome). Not clear if outcome assessed blind on impairment measures. |
| Rossetti 1998 | Controlled trial: further data from author confirms it was not randomised. First 6 consecutive cases were allocated to experimental group and next 6 to control. Outcome assessors were not blinded. The trial is the second of 2 experiments reported in the paper. |
| Schindler 2002 | Non‐randomised cross‐over controlled trial. First 10 participants were randomised to 1 of 2 groups but the data on these 10 were not available at the time of this version of this review. It would be considered for inclusion at the next update if the authors could provide the randomised data. |
| Serino 2006 | Not randomised. |
| Serino 2009 | Non‐randomised controlled trial. After the first 5 participants allocation is by alternation in blocks of 4. |
| Song 2009 | Does not fall into the categories of cognitive rehabilitation intervention included within this review. Investigates low‐frequency repetitive transcranial magnetic stimulation (rTMS). |
| Tham 1997 | Non‐random controlled trial. First 7 participants assigned to novel treatment group, second 7 participants to conventional treatment group. |
| Toglia 2009 | It is a randomised controlled trial of assessment methods ‐ dynamic versus static. |
| Trudell 2003 | A published abstract suggested this may be an eligible study. Excluded from review as no information with which to confirm methods. We will reconsider it for inclusion in next update if further information becomes available. |
| Van Os 1991 | Not randomised (confirmed by native Dutch speaker). |
| Webster 2001 | Controlled clinical trial: 40 assigned, 1 excluded and matched participant excluded, n = 38. 20 controls were from a previous study, not simultaneous. Non‐blind assessment of outcome. Wheelchair navigation (functional measure) as outcome, no impairment measures. |
| Weinberg 1979 | Clarification of randomisation procedure sought but not obtained, and unlikely to be given the age of this article. The timescale of publication (and a statement in the results) suggests the participants in this study were not in the Weinberg 1977 study; however, this has not been confirmed by the authors. On the other hand the 1979 paper does not explicitly mention 'neglect' and may instead be a trial of visual perception. Given the amount of uncertainty about this study's fit to the inclusion criteria, inability to obtain confirmation and clarification about this old study, lack of detail on randomisation and concern to avoid duplicating data by including this and the 1977 article we decided to exclude the 1979 article from this version of the review. |
| Weinberg 1982 | Confirmation regarding randomisation sought from trialist but not obtained. No SD reported. |
| Young 1983 | Controlled trial: not randomised. Divided into 3 groups matched for age, education, time since onset and degree of deficit: no further information provided other than assessor blinded to group's membership. |
BI: Barthel Index FIM: Functional Independence Measure RCT: randomised controlled trial SD: standard deviation
Characteristics of studies awaiting assessment [ordered by study ID]
Harvey 2010.
| Methods | Comparison trial |
| Participants | Participants with neglect |
| Interventions | Grasping and lifting training with or without visuomotor feedback |
| Outcomes | BIT, Stroke Impact Scale |
| Notes | Not sure if fully randomised |
Hauer 2007.
| Methods | 3‐arm trial |
| Participants | 18 participants with visuospatial neglect |
| Interventions | Prism adaptation. Group 1 had 1 daily treatment, Group 2 had 2 daily treatments and Group 3 had none |
| Outcomes | Symptoms of neglect |
| Notes | Unsure if fully random allocation ‐ awaiting translation |
Kang 2009.
| Methods | RCT |
| Participants | 16 participants with right stroke, hemiplegia and perceptual difficulties |
| Interventions | Computer‐assisted cognitive rehabilitation with motion‐tracking versus computer‐assisted cognitive rehabilitation |
| Outcomes | MMSE, motor‐free visual perceptual test, modified BI (Korean version) |
| Notes | Not clear if participants had neglect |
Van Wyk 2011.
| Methods | "Randomized matched double blinded clinical trial" |
| Participants | 24 stroke patients |
| Interventions | Visual scanning exercises, provided in addition to task‐specific training |
| Outcomes | King‐Devick test, star cancellation test, MMSE, TUG, BI, HADS, Stroke Impact Scale |
| Notes | Not clear if participants had neglect or visual deficits, or both |
BI: Bathel Index BIT: Behavioural Inattention Test HADS:Hospital Anxiety and Depression Scale MMSE: Mini Mental State Examination RCT: randomised controlled trial TUG: timed up and go
Characteristics of ongoing studies [ordered by study ID]
Rossetti 2005.
| Trial name or title | Unknown |
| Methods | |
| Participants | Unknown |
| Interventions | Prism adaptation |
| Outcomes | Unknown |
| Starting date | Unknown |
| Contact information | Yves Rossetti rossetti@lyon.inserm.fr |
| Notes | Author reported is currently running a double‐blind RCT |
RCT: randomised controlled trial
Contributions of authors
Nadina Lincoln initiated and co‐ordinated the review project, was the principal grant holder for the initial review, conducted data collection, confirmed the analysis and contributed to all final reports.
Audrey Bowen designed the 2006 updated review, conducted the data collection and analysis and prepared the 2006 final report, and contributed to the 2013 final report.
Christine Hazelton conducted the searches for the 2013 version, conducted data collection and analysis, and contributed to the final report.
Alex Pollock designed the 2013 updated review, conducted data collection and analysis and prepared the 2013 final report.
Sources of support
Internal sources
No sources of support supplied
External sources
The Stroke Association, UK.
NHS Executive Research and Development Programme Physical and Complex Disabilities, UK.
The Scottish Government's Chief Scientist Office funds the Nursing Midwifery and Allied Health Professions (NMAHP) Research Unit., UK.
Declarations of interest
Nadina Lincoln has been involved in studies included in and excluded from this review (Edmans 2000; Fanthome 1995; Lincoln 1985). The searches for the first version of this review were funded by grants to Nadina Lincoln from Stroke Association and the UK NHS Research and Development Programme for Physical and Complex Disabilities.
Christine Hazelton's position as Research Assistant on this review was funded by RNIB Scotland.
The work presented here represents the view of the authors and not necessarily those of the funding bodies.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Cherney 2002 {published data only}
- Cherney LR, Halper AS, Papachronis D. Two approaches to treating unilateral neglect after right hemisphere stroke: a preliminary investigation. Topics in Stroke Rehabilitation 2003;9(4):22‐33. [DOI] [PubMed] [Google Scholar]
Cottam 1987 {published data only}
- Cottam GL. Visual scanning training for right hemispheric stroke patients exhibiting hemispatial neglect [Dissertation]. Mississippi: University of Mississippi, 1987. [Google Scholar]
Edmans 2000 {published and unpublished data}
- Edmans JA, Webster J, Lincoln NB. A comparison of two approaches in the treatment of perceptual problems after stroke. Clinical Rehabilitation 2000;14(3):230‐43. [DOI] [PubMed] [Google Scholar]
Fanthome 1995 {published and unpublished data}
- Fanthome Y, Lincoln N, Drummond A, Walker M. The treatment of visual neglect using feedback of eye movements: a pilot study. Disability Rehabilitation 1995;17(8):413‐7. [DOI] [PubMed] [Google Scholar]
Ferreira 2011 {published data only}
- Ferreira HP, Lopes MAL, Luiz RR, Cardoso L, Andre C. Is visual scanning better than mental practice in hemispatial neglect? Results from a pilot study. Topics in Stroke Rehabilitation 2011;18(2):155‐61. [DOI] [PubMed] [Google Scholar]
Fong 2007 {published and unpublished data}
- Fong KNK, Chan MKL, Ng PPK, Tsang MHN, Chow KKY, Lau CWL, et al. The effect of voluntary trunk rotation and half‐field eye‐patching for patients with unilateral neglect in stroke: a randomized controlled trial. Clinical Rehabilitation 2007;21:729‐41. [DOI] [PubMed] [Google Scholar]
Kalra 1997 {published and unpublished data}
- Kalra L, Perez I, Gupta S, Whittink M. Effects of visual neglect on stroke rehabilitation. Proceedings of the Challenge of Stroke. The Lancet Conference 15‐16 October 1998:71 (Abst 92).
- Kalra L, Perez I, Gupta S, Wittink M. The influence of visual neglect on stroke rehabilitation. Stroke 1997;28(7):1386‐91. [DOI] [PubMed] [Google Scholar]
Kerkhoff 2012a {published data only}
- Kerkhoff G, Keller I, Artinger F, Hildebrandt H, Marquardt C, Reinhart S, et al. Recovery from auditory and visual neglect after optokinetic stimulation with pursuit eye movements ‐ transient modulation and enduring treatment effects. Neuropsychologia 2012;50(6):1164‐77. [DOI] [PubMed] [Google Scholar]
Luukkainen‐Markkula 2009 {published and unpublished data}
- Luukkainen‐Markkulaa R, Tarkkaa IM, Pitkanena K, Siveniusa J, Hamalainen H. Rehabilitation of hemispatial neglect: a randomized study using either arm activation or visual scanning training. Restorative Neurology and Neuroscience 2009;27:663‐72. [DOI] [PubMed] [Google Scholar]
Mizuno 2011 {published data only}
- Mizuno K, Tsuji T, Takebayashi T, Fujiwara T, Hase K, Liu M. Prism adaptation therapy enhances rehabilitation of stroke patients with unilateral spatial neglect: a randomized, controlled trial. Neurorehabilitation and Neural Repair 2011;25(8):711‐20. [DOI] [PubMed] [Google Scholar]
Nys 2008 {published data only (unpublished sought but not used)}
- Nys GMS, Haan EHF, Kunneman A, Kort PLM, Dijkerman HC. Acute neglect rehabilitation using repetitive prism adaptation: a randomized placebo‐controlled trial. Restorative Neurology and Neuroscience 2008;26:1‐12. [PubMed] [Google Scholar]
Polanowska 2009 {published and unpublished data}
- Polanowska K, Seniówa J, Paprota E, Leśniaka M, Członkowskaa A. Left‐hand somatosensory stimulation combined with visual scanning training in rehabilitation for post‐stroke hemineglect: a randomised,double‐blind study. Neuropsychological Rehabilitation 2009;19(3):364‐82. [DOI] [PubMed] [Google Scholar]
Robertson 1990 {published and unpublished data}
- Robertson I, Gray J, Pentland B, Waite L. Microcomputer‐based rehabilitation for unilateral left visual neglect: a randomised controlled trial. Archives of Physical Medicine and Rehabilitation 1990;71:663‐8. [PubMed] [Google Scholar]
Robertson 2002 {published data only}
- Robertson IH, McMillan TM, MacLeod E, Edgeworth J, Brock D. Rehabilitation by limb activation training reduces left‐sided motor impairment in unilateral neglect patients: a single‐blind randomised control trial. Neuropsychological Rehabilitation 2002;12(5):439‐54. [Google Scholar]
Rossi 1990 {published data only}
- Rossi P, Kheyfets S, Reding M. Fresnel prisms improve visual perception in stroke patients with homonymous hemianopia or unilateral visual neglect. Neurology 1990;40:1597‐9. [DOI] [PubMed] [Google Scholar]
Rusconi 2002 {published data only}
- Rusconi ML, Meinecke C, Sbrissa P, Bernardini B. Different cognitive trainings in the rehabilitation of visuo‐spatial neglect. Europa Medicophysica 2002;38:159‐66. [Google Scholar]
Schroder 2008 {published data only}
- Schroder A, Wist ER, Homberg V. TENS and optokinetic stimulation in neglect therapy after cerebrovascular accident: a randomized controlled study. European Journal of Neurology 2008;15:922‐7. [DOI] [PubMed] [Google Scholar]
Schroder 2008 OKS {published data only}
- Schroder A, Wist ER, Homberg V. TENS and optokinetic stimulation in neglect therapy after cerebrovascular accident: a randomized controlled study. European Journal of Neurology 2008;15:922‐7. [DOI] [PubMed] [Google Scholar]
Schroder 2008 TENS {published data only}
- Schroder A, Wist ER, Homberg V. TENS and optokinetic stimulation in neglect therapy after cerebrovascular accident: a randomized controlled study. European Journal of Neurology 2008;15:922‐7. [DOI] [PubMed] [Google Scholar]
Tsang 2009 {published and unpublished data}
- Tsang MHM, Sze KH, Fong KNK. Occupational therapy treatment with right half‐field eye‐patching for patients with subacute stroke and unilateral neglect: a randomised controlled trial. Disability and Rehabilitation 2009;31(8):630‐7. [DOI] [PubMed] [Google Scholar]
Turton 2010 {published data only}
- Turton AJ, O'Leary K, Gabb J, Woodward R, Gilchrist I. A single blinded randomised controlled pilot trial of prism adaptation for improving self‐care in stroke patients with neglect. Neuropsychological Rehabilitation 2010;20(2):180‐96. [DOI] [PubMed] [Google Scholar]
Weinberg 1977 {published data only}
- Weinberg J, Diller L, Gordon W, Gerstman L, Lieberman A, Lakin P, et al. Visual scanning training effect on reading‐related tasks in acquired right brain damage. Archives of Physical Medicine and Rehabilitation 1977;58:479‐86. [PubMed] [Google Scholar]
Welfringer 2011 {published data only}
- Welfringer A, Leifert‐Fiebach G, Babinsky R, Brandt T. Visuomotor imagery as a new tool in the rehabilitation of neglect:a randomised controlled study of feasibility and efficacy. Disability and Rehabilitation 2011;33:2033‐43. [DOI] [PubMed] [Google Scholar]
Wiart 1997 {published and unpublished data}
- Wiart L, Bon Saint Come A, Debellaix X, Petit H, Joseph PA, Mazaux JM, et al. Unilateral neglect syndrome rehabilitation by trunk rotation and scanning training. Archives of Physical Medicine and Rehabilitation 1997;78:424‐9. [DOI] [PubMed] [Google Scholar]
Zeloni 2002 {published data only}
- Zeloni G, Farne A, Baccini M. Viewing less to see better. Journal of Neurology Neurosurgery and Psychiatry 2002;73:195‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Akinwuntan 2010 {published data only}
- Akinwuntan A, Devos H, Verheyden G, Baten G, Kiekens C, Feys H, et al. Retraining moderately impaired stroke survivors in driving‐related visual attention skills. Topics in Stroke Rehabilitation 2010;17(5):328‐36. [DOI] [PubMed] [Google Scholar]
Al Mahasneh 1991 {published data only}
- Al Mahasneh SM. Nursing interventions to reduce unilateral neglect in right hemisphere stroke patients [Dissertation]. University of Michigan 1991.
Bar‐Haim 2011 {unpublished data only}
- Bar‐Haim Erez A, Soroker N, Averbuch S. Phasic alerting combined with visual search training: a novel therapeutic approach for unilateral spatial neglect. Data on file.
Beis 1999 {published and unpublished data}
- Beis J‐M, Andre JM, Baumgarten A, Challier B. Eye patching in unilateral spatial neglect : efficacy of two methods. Archives of Physical Medicine and Rehabilitation 1999;80:71‐6. [DOI] [PubMed] [Google Scholar]
Butter 1992 {published data only}
- Butter C, Kirsch N. Combined and separate effects of eye patching and visual stimulation on unilateral neglect following stroke. Archives of Physical Medicine and Rehabilitation 1992;73:1133‐9. [PubMed] [Google Scholar]
Carter 1980 {published data only}
- Carter L, Caruso J, Languirand M, Berard M. Cognitive skill remediation in stroke and non‐stroke elderly. Clinical Neuropsychology 1980;2(3):109‐13. [Google Scholar]
Cubelli 1993 {published data only}
- Cubelli R, Inzaghi MG, Tanti A. The rehabilitation of unilateral spatial neglect: experimental verification of its effectiveness [La rieducazione della negligenza spaziale unilaterale: verifica sperimentale della sua efficacia]. Europa Medicophysica 1993;29:111‐21. [Google Scholar]
Diller 1974 {published data only}
- Diller L, Ben Yishay Y, Gerstman LJ, Goodki R, Gordon W, Weinberg J. Studies in cognition and rehabilitation in hemiplegia. Rehabilitation Monograph No. 50. New York: New York University, 1974. [Google Scholar]
EEG‐NF 2009 {published data only}
- Smithard D. A novel neurofeedback‐based intervention to reduce neglect and improve function in stroke patients. UK Clinical Research Network Portfolio Database (http://public.ukcrn.org.uk/search/) 2012.
- Smithard D. Electroencephalogram‐neurofeedback (EEG‐NF) to improve neglect in stroke patients. Current Controlled Trials (http://www.controlled‐trials.com/) 2009.
Frassinetti 2002 {published data only}
- Frassinetti A, Angeli V, Meneghello F, Avanzi S, Ladavas E. Long‐lasting amelioration of visuospatial neglect by prism adaptation. Brain 2002;125:608‐23. [DOI] [PubMed] [Google Scholar]
Gordon 1985 {published data only}
- Gordon W, Hibbard M, Egelko S, Diller L. Perceptual remediation in patients with right brain damage: a comprehensive programme. Archives of Physical Medicine and Rehabilitation 1985;66:353‐9. [PubMed] [Google Scholar]
Harvey 2003 {published data only}
- Harvey M, Hood B, North A, Robertson IH. The effects of visuomotor feedback training on the recovery of hemispatial neglect symptoms: assessment of a 2‐week and follow‐up intervention. Neuropsychologia 2003;41:886‐93. [DOI] [PubMed] [Google Scholar]
Keller 2006 {published data only}
- Keller I, Beer AL, Kerkhoff G. Optokinetic stimulation in visual neglect [Optokinetische stimulation bei visuellem neglect]. Neurologie und Rehabilitation 2003;9(6):272‐9. [Google Scholar]
Kerkhoff 2005 {unpublished data only}
- Kerkhoff G. Personal communication 2005.
- Kerkhoff G, Keller I, Ritter V, Marquandt C. Repetitive optokinetic stimulation induces lasting recovery from visual neglect. Restorative Neurology and Neuroscience 2006;24:357‐68. [PubMed] [Google Scholar]
Kerkhoff 2012b {published data only}
- Kerkhoff G, Keller I, Artinger F, Hildebrandt H, Marquardt C, Reinhart S, et al. Recovery from auditory and visual neglect after optokinetic stimulation with pursuit eye movements ‐ transient modulation and enduring treatment effects. Neuropsychologia 2012;50(6):1164‐77. [DOI] [PubMed] [Google Scholar]
Klos 2005 {unpublished data only}
- Klos T. Personal communication 2005.
Koch 2012 {published data only}
- Koch G, Bonnì S, Giacobbe V, Bucchi G, Basile B, Lupo F, et al. θ‐burst stimulation of the left hemisphere accelerates recovery of hemispatial neglect. Neurology 2012;1:24‐30. [DOI] [PubMed] [Google Scholar]
Lincoln 1985 {published data only}
- Lincoln N, Whiting S, Cockburn J, Bhavnani G. An evaluation of perceptual retraining. International Rehabilitation Medicine 1985;7:99‐110. [DOI] [PubMed] [Google Scholar]
Loverro 1988 {published data only}
- Loverro J, Reding M. Bed orientation and rehabilitation outcome for patients with stroke hemianopsia or visual neglect. Journal of Neurologic Rehabilitation 1988;2:147‐50. [Google Scholar]
Niemeier 1998 {published data only}
- Niemeier JP. The Lighthouse Strategy: use of a visual imagery technique to treat visual inattention in stroke patients. Brain Injury 1998;12(5):399‐406. [DOI] [PubMed] [Google Scholar]
Osawa 2010 {published data only}
- Osawa A, Maeshima S. Family participation can improve unilateral spatial neglect in patients with acute right hemispheric stroke. European Neurology 2010;63:170‐5. [DOI] [PubMed] [Google Scholar]
Paolucci 1996 {published and unpublished data}
- Antonucci G, Guariglia C, Judica A, Magnotti L, Paolucci S, Pizzamiglio L, et al. Effectiveness of neglect rehabilitation in a randomised group study. Journal of Clinical and Experimental Neuropsychology 1995;17(3):383‐9. [DOI] [PubMed] [Google Scholar]
- Paolucci S, Antonucci G, Guariglia C, Magnotti L, Pizzamiglio L, Zoccolotti P. Facilitatory effect of neglect rehabilitation on the recovery of left hemiplegic stroke patients: a cross‐over study. Journal of Neurology 1996;243:308‐14. [DOI] [PubMed] [Google Scholar]
Pizzamiglio 2004 {published data only}
- Pizzamiglio L, Fasotti L, Jehkonen M, Antonucci G, Magnotti L, Boelen D, et al. The use of opto‐kinetic stimulation in rehabilitation of the hemi‐neglect disorder. Cortex 2004;40:441‐50. [DOI] [PubMed] [Google Scholar]
Rossetti 1998 {published and unpublished data}
- Rossetti Y, Rode G, Pisella L, Farne A, Li L, Boisson D, et al. Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature 1998;395(6698):166‐9. [DOI] [PubMed] [Google Scholar]
Schindler 2002 {published data only}
- Schindler I, Kerkhoff G, Karnath H‐O, Keller I, Goldenberg G. Neck muscle vibration induces lasting recovery in spatial neglect. Journal of Neurology, Neurosurgery and Psychiatry 2002;73:412‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Serino 2006 {published data only}
- Serino A, Angeli V, Frassinetti F, Ladavas E. Mechanisms underlying neglect recovery after prism adaptation. Neuropsychologia 2006;44:1068‐78. [DOI] [PubMed] [Google Scholar]
Serino 2009 {published data only}
- Serino A, Barbiani M, Rinaldesi ML, Ladavas E. Effectiveness of prism adaptation in neglect rehabilitation: a controlled trial study. Stroke 2009;40:1392‐8. [DOI] [PubMed] [Google Scholar]
Song 2009 {published data only}
- Song W, Du B, Xu Q, Hu J, Wang M, Luo Y. Low‐frequency transcranial magnetic stimulation for visualspatial neglect: a pilot study. Journal of Rehabilitation Medicine 2009;41:162‐5. [DOI] [PubMed] [Google Scholar]
Tham 1997 {published data only}
- Tham K, Tegner R. Video feedback in the rehabilitation of patients with unilateral neglect. Archives of Physical Medicine and Rehabilitation 1997;78:410‐3. [DOI] [PubMed] [Google Scholar]
Toglia 2009 {published data only}
- Toglia J, Cermak SA. Dynamic assessment and prediction of learning potential in clients with unilateral neglect. American Journal of Occupational Therapy 2009;63(5):569‐79. [DOI] [PubMed] [Google Scholar]
Trudell 2003 {published data only}
- Trudell C, Bodian L, Demaio JH, Cheskes BE, Reding M. Comparison of hemi‐field fresnel prisms versus patching for visual neglect after stroke. Archives of Physical Medicine and Rehabilitation 2003;84:A9. [Google Scholar]
Van Os 1991 {published data only}
- Os ES, Driessen M‐J, Wagenaar RC. Visual scanning training in stroke patients. Nederlands Tijdschrift voor Ergotherapie 1991;19:47‐53. [Google Scholar]
Webster 2001 {published data only}
- Webster JS, McFarland PT, Rapport LJ, Morrill B, Roades LA, Abadee PS. Computer‐assisted training for improving wheelchair mobility in unilateral neglect patients. Archives of Physical Medicine and Rehabilitation 2001;82:769‐75. [DOI] [PubMed] [Google Scholar]
Weinberg 1979 {published data only}
- Weinberg J, Diller L, Gordon W, Gerstman L, Lieberman A, Lakin P, et al. Training sensory awareness and spatial organisation in people with right brain damage. Archives of Physical Medicine and Rehabilitation 1979;60:491‐7. [PubMed] [Google Scholar]
Weinberg 1982 {published data only}
- Weinberg J, Piasetsky E, Diller L, Gordon W. Treating perceptual organisation deficits in nonneglecting RBD stroke patients. Journal of Clinical Neuropsychology 1982;4(1):59‐75. [DOI] [PubMed] [Google Scholar]
Young 1983 {published data only}
- Young G, Collins D, Hren M. Effect of pairing scanning training with block design training in the remediation of perceptual problems in left hemiplegics. Journal of Clinical Neuropsychology 1983;5(3):210‐2. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Harvey 2010 {published and unpublished data}
- Harvey M, Muir K, Rossit S. Long term improvements in activities of daily living in patients with hemispatial neglect. Behavioural Neurology 2010;23:249‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hauer 2007 {published and unpublished data}
- Hauer B, Qirbach A. Unilateral neglect: prism adaptation as an economic and effective therapy? [Unilateraler Neglect: Prismenadaptation als ökonomische und effefktive Therapie?]. Zeitschrift für Neuropsychologie 2007;18(3):171‐81. [Google Scholar]
Kang 2009 {published data only}
- Kang SH, Kim DY, Seo KM, Kwang C‐A, Yoo JY, Sung SY, et al. A computerized visual perception rehabilitation programme with interactive computer interface using motion tracking technology – a randomized controlled,single‐blinded, pilot clinical trial study. Clinical Rehabilitation 2009;23:434‐44. [DOI] [PubMed] [Google Scholar]
Van Wyk 2011 {published and unpublished data}
- Wyk A, Eksteen C. Effect of visual scanning exercises integrated into task‐specific activities on the functional ability in patients with visual perceptual disorders post‐stroke. World Congress of Physical Therapy, Amsterdam, Netherlands. 21‐24 June. 2011.
References to ongoing studies
Rossetti 2005 {unpublished data only}
- Rossetti Y. Personal communication 2005.
Additional references
Bailey 1999
- Bailey MJ, Riddoch MJ. Hemineglect. Part 1. The nature of hemineglect and its clinical assessment in stroke patients: an overview. Physical Therapy Reviews 1999;4:67‐75. [Google Scholar]
Barer 1990
- Barer D. The influence of visual and tactile inattention on predictions for recovery from acute stroke. Quarterly Journal of Medicine 1990;74:21‐32. [PubMed] [Google Scholar]
Bernspang 1987
- Bernspang B, Asplund K, Evikson S, Fugl‐Meyer A. Motor and perceptual impairments in acute stroke patients: effects on self‐care ability. Stroke 1987;18:1081‐6. [DOI] [PubMed] [Google Scholar]
Bowen 1999
- Bowen A, McKenna K, Tallis R. Reasons for the variability in the reported rate of occurrence of unilateral spatial neglect following stroke. Stroke 1999;30(6):1196‐202. [DOI] [PubMed] [Google Scholar]
Bowen 2011
- Bowen A, Knapp P, Gillespie D, Nicolson DJ, Vail A. Non‐pharmacological interventions for perceptual disorders following stroke and other adult‐acquired, non‐progressive brain injury. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.CD007039.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cicerone 2005
- Cicerone KC, Dahlberg C, Malec JF, Langenbahn DM, Felicetti T, Kneipp S, et al. Evidence based cognitive rehabilitation:updated review of the literature from 1998 through 2002. Archives of Physical Medicine and Rehabilitation 2005;86:1681‐92. [DOI] [PubMed] [Google Scholar]
Cicerone 2009
- Cicerone K, Azulay J, Trott C. Methodological quality of research on cognitive rehabilitation after traumatic brain injury. Archives of Physical Medicine and Rehabilitation 2009;90 Suppl 1:s52‐s59. [DOI] [PubMed] [Google Scholar]
Cochrane Handbook
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Das Nair 2007
- Das Nair R, Lincoln N. Cognitive rehabilitation for memory deficits following stroke. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD002293.pub2] [DOI] [PubMed] [Google Scholar]
Ferro 1999
- Ferro JM, Mariano G, Madureira S. Recovery from aphasia and neglect. Cerebrovascular Diseases 1999;9 Suppl 5:6‐22. [DOI] [PubMed] [Google Scholar]
Heilman 1993
- Heilman KM, Watson RT, Valenstein E. Neglect and related disorders. In: Heilman KM, Valenstein E editor(s). Clinical Neuropsychology. 2nd Edition. New York: Oxford University Press, 1993:243‐94. [Google Scholar]
Jehkonen 2006
- Jehkonen M, Laihosalo M, Kettunen JE. Impact of neglect on functional outcome after stroke: a review of methodological issues and recent research findings. Restorative Neurology and Neuroscience 2006;24:209‐15. [PubMed] [Google Scholar]
Jutai 2003
- Jutai J, Bhogal S, Foley N, Bayley M, Teasell R, Speechley M. Treatment of visual perceptual disorders post stroke. Topics in Stroke Rehabilitation 2003;10:77‐106. [DOI] [PubMed] [Google Scholar]
Katz 1999
- Katz N, Harman‐Maier A, Ring HM, Soroker NR. Functional disability and rehabilitation outcome in right hemisphere damaged patients with and without unilateral spatial neglect. Archives of Physical Medicine and Rehabilitation 1999;80:379‐84. [DOI] [PubMed] [Google Scholar]
Lincoln 2001
- Lincoln NB, Majid MJ, Weyman N. Cognitive rehabilitation for attention deficits following stroke. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD002842] [DOI] [PubMed] [Google Scholar]
Lincoln 2012
- Lincoln NB, Kneebone II, Macniven JAB, Morris RC. Cognitive rehabilitation. Psychological Management of Stroke. West Sussex: Wiley‐Blackwell, 2012:248‐65. [Google Scholar]
Massironi 1988
- Massironi M, Antonucci G, Pizzamiglio L, Vitale M, Zoccolotti P. The Wundt‐Jastrow illusion in the study of spatial hemi‐inattention. Neuropsychologia 1988;26:161‐6. [DOI] [PubMed] [Google Scholar]
Neistadt 1993
- Neistadt ME. The relationship between constructional and meal preparation skills. Archives of Physical Medicine and Rehabilitation 1993;74:144‐8. [PubMed] [Google Scholar]
Parton 2004
- Parton A, Malhotra P, Hussain M. Hemispatial neglect. Journal of Neurology, Neurosurgery and Psychiatry 2004;75:13‐21. [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Song 2005
- Song FJ, Jerosch‐Herold CJ, Harvey I, Drachler M, Holland R, Mares K. Can parametric statistical methods be used to analyse and present ordinal Barthel data in trials of post‐stroke interventions?. Proceedings of the European Stroke Conference. 2005.
Sunderland 1987
- Sunderland A, Wade D, Langton‐Hewer R. The natural history of visual neglect after stroke: indications from two assessment methods. International Disability Studies 1987;9:55‐61. [DOI] [PubMed] [Google Scholar]
Wade 1985
- Wade D, Skilbeck C, David R, Langton‐Hewer R. Stroke: a critical approach to diagnosis, treatment and management. London: Chapman and Hall, 1985. [Google Scholar]


