Abstract
Background
Sensory impairments significantly limit the ability to use the upper limb after stroke. However, little is known about the effects of interventions used to address such impairments.
Objectives
To determine the effects of interventions that target upper limb sensory impairment after stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched 8 October 2009), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2009, Issue 1), MEDLINE (1966 to January 2009), EMBASE (1980 to January 2009), and six further electronic databases to January 2009. We also handsearched relevant journals, contacted authors in the field, searched doctoral dissertation databases, checked reference lists, and completed citation tracking.
Selection criteria
Randomized controlled trials and controlled trials comparing interventions for sensory impairment after stroke with no treatment, conventional treatment, attention placebo or with other interventions for sensory impairment.
Data collection and analysis
Two review authors selected studies, assessed quality and extracted data. We analyzed study data using mean differences and odds ratios as appropriate. The primary outcome we considered was sensory function and secondary outcomes examined included upper limb function, activities of daily living, impact of stroke and quality of life as well as adverse events.
Main results
We included 13 studies, with a total 467 participants, testing a range of different interventions. Outcome measures included 36 measures of sensory impairment and 13 measures of upper limb function. All but two studies had unclear or high risk of bias. While there is insufficient evidence to reach conclusions about the effects of interventions included in this review, three studies provided preliminary evidence for the effects of some specific interventions, including mirror therapy for improving detection of light touch, pressure and temperature pain; a thermal stimulation intervention for improving rate of recovery of sensation; and intermittent pneumatic compression intervention for improving tactile and kinesthetic sensation. We could not perform meta‐analysis due to a high degree of clinical heterogeneity in both interventions and outcomes.
Authors' conclusions
Multiple interventions for upper limb sensory impairment after stroke are described but there is insufficient evidence to support or refute their effectiveness in improving sensory impairment, upper limb function, or participants' functional status and participation. There is a need for more well‐designed, better reported studies of sensory rehabilitation.
Keywords: Adult, Humans, Recovery of Function, Upper Extremity, Outcome Assessment (Health Care), Randomized Controlled Trials as Topic, Somatosensory Disorders, Somatosensory Disorders/etiology, Somatosensory Disorders/rehabilitation, Stroke, Stroke/complications, Stroke Rehabilitation
Interventions for sensory impairment in the upper limb after stroke
Up to 80% of people who have a stroke experience sensory loss in their affected arm. This sensory loss puts the arm at risk for injury and impacts functional use of the arm and the survivors' level of independence during daily activities. We found 13 studies involving 467 participants that tested different treatments for sensory loss. There is limited evidence that these treatments may be effective. No more than one study examined each particular intervention, frequently the studies were of poor quality and lacked sufficient information. Further research is needed before clear recommendations can be made.
Background
Stroke is the leading cause of disability and the third or fourth leading cause of death both in the USA and many developed countries around the world (Eaves 2000; HSAO 2001; HSFC 2005; NSFA 2005). Pendlebury et al concluded that approximately one million strokes occur each year in Europe, making it the most common neurological disorder (Pendlebury 2004). There are three million permanently disabled stroke survivors in the USA. In the USA in 2006 the American Stroke Association estimated the costs of stroke (both direct and indirect) to be USD 57.9 billion (ASA 2006). In the United Kingdom, stroke accounts for approximately 6% of total National Health Service and social services expenditure, with most of the cost associated with the resulting chronic disability (Pendlebury 2004). Approximately 25% of chronic disability in Australia is due to stroke, costing the Australian economy over AUD 1.3 billion annually (ASPSC 2004). There are 300,000 Canadians living with the effects of stroke costing the Canadian economy about CAD 2.7 billion per year (HSFC 2005). Recognition of the importance of rehabilitation services in the management of conditions that affect people's functional abilities is growing. In Canada and the USA, those who require rehabilitation after stroke make up the largest category of rehabilitation patients and have the third longest length of inpatient stay (Hopman 2003). The cost of stroke is expected to continue to grow worldwide over the next two decades due to the increasing age of the population (Pendlebury 2004).
The most common deficit after stroke is hemiparesis of the contralateral upper limb, with more than 80% of those with stroke experiencing it acutely and more than 40% chronically (Cramer 1997). Upper limb impairments continue to limit the functional independence and satisfaction for 50% to 70% of stroke survivors, and only 5% of survivors who initially experienced complete paralysis achieve functional use of their arm (HSAO 2001). Exploration of the environment and mastery and participation in daily occupations are intimately associated with both movement and sensation. Deficits in somatic sensations (body senses such as touch, temperature, pain and proprioception) after stroke are common with prevalence rates variously reported to be 11% to 85% (Yekutiel 2000), 65% (Carey 1993), 60% to 74% (Hunter 2002), and 100% ( Rand 2001). This variability among the studies is thought to be related to differences in assessment and definition of sensory impairment, and study design (Yekutiel 2000). The sensory deficits do not appear to be confined to the contralateral upper limb, with several studies noting significant impairment in the ipsilateral upper limb after stroke (Carey 1995; Kim 1996; Nowak 2007). While the level of impairment in the ipsilateral upper limb is generally considered less than that of the contralateral upper limb, in some cases moderate to severe deficits have been reported and deficits have also been noted to persist for a period of years after stroke. The incidence of ipsilateral impairment generally cited varies from 12% to 26% (Carey 1995).
There are many different sensory modalities affected by stroke. The loss of detection of touch sensation has been noted in up to 65% to 94% of all stroke survivors (Acerra 2007; Carey 1993). Impairment in proprioception (ability to sense the position and orientation of parts of the body) (17% to 52%), vibration (44%), light touch (32% to 89%), and loss of pinprick sensation (35% to 71%) have also been noted (Acerra 2007; Hunter 2002; Tyson 2008). Disturbance of other sensory modalities including two‐point discrimination, stereognosis (recognition or identification of objects by use of touch), kinesthesia (detection of bodily position, weight, or movement of the muscles, tendons, and joints), graphesthesia (recognition of writing on the skin by the sensation of touch) and pain are found (Connell 2008; Kim 1996). Tactile extinction (where people with unilateral damage do not detect touch given to the contralateral side when a symmetrical touch stimulus is given to the ipsilateral side) has been considered to be attentional in nature (tactile neglect) by some authors but is described by other authors as a higher order or cortical tactile sensation along with two point discrimination, stereognosis and graphesthesia and as such is often reported with sensory modalities in medical texts (Blumenfeld 2002; Bohannon 2003; Campbell 2005; Gilroy 2000). It is therefore included in this review but considered separately.The quality of sensory deficits experienced after stroke include delayed perception, uncertainty of responses, changes in sensory thresholds, fatigue, altered time for sensory adaptation, sensory persistence, and altered nature of the sensation (Hunter 2002; Robertson 1994).
Functionally, the problems resulting from sensory deficits after stroke can be summarized as (1) impaired detection of sensory information, (2) disturbed performance of motor tasks that require somatosensory information, and (3) diminished rehabilitation outcomes for the upper limb (Hunter 2002). Sensation is essential for safety even if there is adequate motor recovery (Yekutiel 2000). The development of secondary complications such as sores, abrasions, and shoulder‐hand syndrome has been associated with the impairment of sensation (Rand 2001). Sensory impairment has also been found to be directly associated with the development of shoulder pain and subluxation (Chang 1995; Gamble 2000; Suethanapornkul 2008).
When impairment in the ability to detect and process sensory data occurs, the stroke survivor will have difficulty exploring and relating to his environment (Dannenbaum 1993; Yekutiel 2000). It was postulated by van der Lee et al that stroke survivors who have sensory impairments do not use the affected limb to their fullest motor potential (van der Lee 1999). The spontaneous use of the upper limb has been noted to significantly decrease when cutaneous sensory processing is impaired (Carey 1993; Rand 2001). This continued disuse of the affected extremity leads to a further decrease in skilled movement, particularly for functional skills that require a constant sustained muscle contraction (Dannenbaum 1993). This further contributes to the pattern of learned non‐use. The quality of upper limb movements is also impaired in the presence of sensory impairments (Nowak 2007; Rand 2001). Stroke survivors were found to have impairments in force control, fine motor manipulation of objects, sensory ataxia, decreased grasp, and changes in prehension patterns, all of which have been found to be associated with sensory impairment (Aruin 2005; Blennerhassett 2007; Carey 1995; Nowak 2007; Robertson 1994; Welmer 2008; Yekutiel 2000).
Sensory deficits have been shown to predict poor functional outcome after stroke, including increased length of hospitalization, lower levels of discharge home, lower numbers of home discharges, and increased mortality rates (Carey 1995; Rand 2001; Yekutiel 2000). Tyson et al found that impairment of sensation was significantly associated with mobility, independence in activities of daily living and recovery (Tyson 2008) while Desrosiers et al found a significant association with long‐term participation (Desrosiers 2006). While proprioceptive status soon after stroke has been reported to be a reliable predictor of long‐term motor recovery, other studies have shown no association between functional status at discharge and somatosensory impairment (Carey 1995). For example, Rand et al found no significant difference in functional outcomes six weeks post‐stroke between individuals with both motor and proprioceptive deficits and those with pure motor deficits (Rand 1999). Tactile extinction on the left side of the body (of double simultaneous stimulation) was shown to be the single most important predictor of functional outcome (Rose 1994). Many factors contribute to the varied outcomes among these studies, including how sensory impairment is defined and measured, and the time post‐stroke and stage of recovery (Carey 1995).
Although sensory impairments significantly limit the ability to use the upper limb after stroke and increase the risk of secondary complications, to date little is known about the effectiveness of interventions that address this issue. This systematic review examines the effectiveness of interventions for sensory impairment after stroke.
Objectives
The objectives of this review were to determine if interventions for upper limb sensory impairment are more effective at improving:
sensory function than no treatment, control or placebo interventions;
upper limb function than no treatment, control or placebo interventions;
activity limitations than no treatment, control or placebo interventions; and
participation than no treatment, control or placebo interventions.
Methods
Criteria for considering studies for this review
Types of studies
We included controlled trials of sensory interventions to improve function or remediate sensory impairments of the upper limb following stroke where participants were randomly or quasi‐randomly assigned to one of two or more treatment groups. We also included trials with or without blinding of the participants, therapists, or evaluators. We also included randomized cross‐over trials that met the above criteria.
Types of participants
We included adults (18 years and over) with a clinical diagnosis of stroke, either hemorrhagic or ischemic, that did not necessarily need to be confirmed using imaging studies. The stroke resulted in the participants initially experiencing a disturbance in sensory function of the upper limb. We defined a disturbance in sensory function as any impairment that impacted on sensory registration, perception, or discrimination, resulting from a cerebral vascular accident and where the primary sensory receptors are intact. We included studies with mixed etiology groups if at least 50% of participants were diagnosed with stroke.
Types of interventions
Included studies addressed the recovery of function or remediation of sensory impairments in the upper limb by specifically focusing on interventions hypothesized to remediate sensory impairments after stroke, or both.These interventions included: sensory re‐education, tactile kinesthetic guiding, repetitive sensory practice, or desensitization. We also examined studies that explored novel intervention strategies if they were relevant to upper limb functional use and included an outcome measure for sensory function or impairment. These interventions were delivered as stand‐alone or as an adjunct to conventional therapy.
Types of outcome measures
The primary outcome of interest was sensation. There are many distinct sensory modalities identified in the literature and defined in the Background above. We considered measures of the following sensory modalities in this review (and will consider these in review updates):
light touch;
mechanical sensation;
temperature detection;
two‐point discrimination;
depth sense;
vibration sense;
sustained pressure;
kinesthesia;
position sense;
stereognosis;
graphesthesia;
pain (pressure pain, temperature pain, pain intensity);
combined sensory modality assessment.
Some controversy surrounds the inclusion of tactile/proprioceptive extinction as a sensory modality. We have included it in this review and will look at the data separately. In addition, we considered somatosensory evoked potentials. Measurement of these modalities may be from modality specific measures, global sensory measures, or sensory subscales of larger scales such as the Motor Assessment Scale and the Fugl‐Meyer, which are impairment‐based measures and address sensation. We also included the perceived level of impairment or discomfort by the stroke survivor.
The secondary outcomes of interest were upper limb functional use, activity limitations, and participation. Each of these is explained in more detail below.
-
Functional use of the upper limb. This outcome included:
specific components of upper limb function such as dexterity or hand function (using measures such as the Jebsen Taylor Hand Function Test);
upper limb motor functioning (using measures such as the Fugl Meyer, Modified Motor Assessment scale);
upper limb functioning (using measures such as Chedoke or the Motor Activity Log);
scales that identified the survivors' perceived level of use and satisfaction with level and quality of upper limb use.
-
Activity limitations measures focus on performance of activities of daily living. This outcome included:
basic activities of daily living (using measures such as the Barthel Index or the Functional Independence Measure);
instrumental activities of daily living (using measures such as the Frenchay Activites Index);
global dependency scales.
-
Participation measures focus the level of participation in life roles and satisfaction levels with that participation. This outcome included:
measures of the impact of a stroke on participation (using measures such as the Stroke Impact Scale);
quality of life measures.
We also included death from any cause during the treatment, adverse effects and economic data, if available.
We recorded outcome measures based on these categories, extracted the appropriate data from the studies, and came to a consensus as to which to include in the final analysis.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module.
We searched the Cochrane Stroke Group Trials Register, which was last searched by the Managing Editor on 8 October 2009. In addition, we searched the following electronic bibliographic databases; the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2009, Issue 1), MEDLINE (1966 to January 2009), EMBASE (1980 to January 2009), CINAHL (1982 to January 2009), AMED (1985 to January 2009), PsycLIT (1974 to January 2009), Science Citation Index (1945 to January 2009), Social Science Citation Index (1956 to January 2009) and LILACS (Latin American and Caribbean Health Sciences Literature) (1982 to January 2009). The MEDLINE Search strategy (Appendix 1), developed with assistance from the Cochrane Stroke Group Trials Search Co‐ordinator, was used as the basis for the above literature searches (with the last updated search completed 19 January 2009). We also searched the following specialist occupational therapy and physiotherapy databases: PEDro (http://www.pedro.org.au/) and OTseeker (www.otseeker.com) (last searched January 2009).
Current awareness
We searched the Excerpta Medica abstract journal Rehabilitation and Physical Medicine (Section 19 EMBASE) and the Index Medicus monthly bibliographic index.
Citation tracking and reference lists
Using key references, we used the cited reference search in Science Citation Index to identify further studies. We also searched the reference lists of all relevant articles identified.
Handsearching
We handsearched the following journals (the years selected represent the timeframe of increased interest and research publications in this topic area and accessibility to the journals):
American Journal of Occupational Therapy (1980 to 2008);
American Journal of Physical Medicine & Rehabilitation (1988 to 2009);
Archives of Physical Medicine and Rehabilitation (1995 to 2008);
Australian Journal of Physiotherapy (1980 to 2008);
Australian Occupational Therapy Journal (1999 to 2008);
British Journal of Occupational Therapy (1998 to 2008);
Canadian Journal of Occupational Therapy (1997 to 2008);
Head Trauma Rehabilitation (1986 to 2008);
International Journal of Therapy and Rehabilitation (1996 to 2009);
NeuroRehabilitation (1999 to 2009);
Occupational Therapy in Health Care (1984 to 2008);
OTJR: Occupation, Participation and Health (2002 to 2009);
Physical and Occupational therapy in Geriatrics (1982 to 2008);
Physical Therapy (1980 to 2008);
Physiotherapy (1995 to 2008);
Physiotherapy Canada (1997 to 2009);
Stroke (1980 to 2008).
To avoid duplication, we checked the Cochrane Master List of journals handsearched on behalf of The Cochrane Collaboration (http://apps1.jhsph.edu/cochrane/masterlist.asp) to identify handsearching already completed.
In an effort to identify further published, unpublished and ongoing trials we have:
searched for Doctoral and Masters' theses on the OT Search bibliographic database, the AOTA website, Dissertation Abstracts and Physical Therapy theses indexes;
contacted research and professional associations or foundations (such as the Medicine and Stroke Foundations in USA, UK, Canada, and Australia) to identify any other research that they know of;
identified key researchers in the area and contacted them with regard to unpublished research;
searched the following international clinical trials and research registers: the National Research Register Archive (https://portal.nihr.ac.uk/Pages/NRRArchive.aspx), Current Controlled Trials (http://www.controlled‐trials.com/), and REHABDATA (http://www.naric.com/research/rehab/).
We did not impose any language or date restrictions on the electronic searches for trials.
Data collection and analysis
Selection of studies
The primary review author reviewed the titles identified and eliminated obviously irrelevant studies; we then obtained the abstracts for the remaining studies. Using the titles and abstracts obtained from the searches, two review authors independently completed the study selection form to determine if a study should be included or excluded or to state that they were unsure of this decision. We resolved disagreements by discussion based on the inclusion criteria.
Data extraction and management
Two review authors then reviewed the articles that were considered appropriate for inclusion in the review and completed the data extraction form with the following information.
Retrieval characteristics: source and date of publication, and authors.
Sample characteristics: sex, age, sample size, diagnosis (right or left cerebrovascular accident areas specified), and other reported clinical variables listed as inclusion or exclusion characteristics.
Time since stroke
Intervention: specific intervention technique: detail the specific intervention technique used in the study.
Frequency (dosage): detail the specific intervention frequency.
Follow‐up time period stated.
Outcome measures.
Adverse effects or side effects.
Results: means, standard deviations, significance test, t, f, P values and directions of findings.
Assessment of risk of bias in included studies
Two review authors rated the risk of bias of the studies using the Cochrane Risk of Bias Assessment as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008) and in the Review Manager software RevMan 5 (RevMan 2008). If there was disagreement, we asked the third review author to rate the study and used the rating that two of the three review authors selected. Where this did not occur all three review authors discussed the decision further until we reached agreement. Where there were items that were unclear, we attempted to contact the study authors by email or telephone to obtain the information needed.
Data analysis
We entered data into RevMan 5 (RevMan 2008) using the double data entry facility to allow for error checking. We used RevMan 5 for data entry, analysis, and display.
We undertook the following analyses:
specific treatment for sensory impairment versus no treatment (or with conventional treatment in both study arms);
specific treatment for sensory impairment versus conventional upper limb therapy;
specific treatment for sensory impairment versus placebo sensory treatment or attention placebo;
comparisons between different types of treatments for sensory impairment.
We placed studies in which both the treatment and control group received conventional therapy and the only difference was that the treatment group also received specific treatment for sensory impairment in the first group above. We defined attention placebo as a type of comparison group in which the participants met with the clinician for the similar duration and frequency as those in the experimental group but did not receive the intervention (Nock 2007), or received some input designed to provide attention.
Assessment of heterogeneity and subgroup analysis
We planned to undertake a meta‐analysis for this review and to examine heterogeneity prior to completing a meta‐analysis. We planned to do this for all included studies and then for each individual subgroup, following the recommendation in the Cochrane Handbook for Systematic Reviews of Interventions that there should be at least 10 studies in a group for analysis (Higgins 2008). If there were fewer than 10 studies, we would not do further analysis and would provide narrative information. We planned to calculate heterogeneity using the I2 test. If I2 was greater than 50%, indicating that significant heterogeneity was detected, we would do a sensitivity analysis. We planned to test the sensitivity of the review to key decisions made by recalculating the analysis in the following manner: (1) excluding studies of lower methodological quality, and (2) excluding unpublished studies. We were to undertake subgroup analysis based on the intervention technique used. We also planned to do intention‐to‐treat analysis for all studies included in the review if possible. However, due to significant clinical diversity amongst the studies found in terms of both interventions and outcomes, and in many instances, lack of available data, it was not possible to undertake a meta‐analysis or sensitivity analyses for this review.
We have expressed dichotomous outcomes as odds ratios (OR) with 95% confidence intervals (CI). We have expressed continuous outcomes, if possible, as mean differences (MD) with 95% CIs.
Results
Description of studies
See the Characteristics of included studies table, the Characteristics of excluded studies table, and the Characteristics of ongoing studies table for details.
We identified 1554 references in initial searches. Initial screening by one of the review authors reduced this to 662 references whose abstracts were then screened by two review authors to see if they met the inclusion criteria. Two review authors reviewed full copies of the references to 48 studies. At the end of this process 13 studies met the inclusion criteria and study design requirements (randomized controlled trial or controlled clinical trial) (Acerra 2007; Burridge 2002; Byl 2003; Cambier 2003; Chen 2005; Feys 1998; Heldman 2000; Jongbloed 1989; Miller 2004; Poole 1990; Posteraro 2001; Wolny 2003; Yozbatiran 2006). We limited excluded studies listed in the review in accordance with section 7.2.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008) and 32 studies that met all the inclusion criteria except study design were not included. We included five studies as excluded studies (Brogardh 2006; Carey 1993; Liu 2002; Van Vliet 2005; Yekutiel 1993) and three as ongoing studies (Ben‐Shabat 2005; Carey 2004; Carey 2005).
Sample sizes in the included studies ranged from 10 (Posteraro 2001) to 100 (Feys 1998) for a total of 467 participants. The participants ranged in age from 22 to 87 years. The sample included 173 females and 184 males. Four studies (Miller 2004; Poole 1990; Posteraro 2001; Wolny 2003) did not provide information on the gender of their participants. All studies required that the participants had had one stroke that impacted their upper limb with the exception of Byl 2003, which included three participants with more than one stroke. Each study varied in the specific definition of sensory impairments, how they were measured, and the level of impairments experienced by their participants. Not all the studies consistently reported if the upper limb impaired was right or left, or dominant or non‐dominant. Time since stroke varied between participants and between studies, with eight studies including participants within three months of their stroke (Acerra 2007; Cambier 2003; Chen 2005; Feys 1998; Jongbloed 1989; Miller 2004; Posteraro 2001; Yozbatiran 2006), three studies including participants from four to 20 months (Heldman 2000), one to 12 months (Burridge 2002), and one with a mean of 5.1 years post‐stroke (Byl 2003). Two studies (Poole 1990; Wolny 2003) did not provide any data related to the time post stroke. The exact setting of included studies was only described for six of the studies: inpatient rehabilitation (Acerra 2007; Cambier 2003; Chen 2005), combined inpatient and outpatient rehabilitation (Burridge 2002; Cambier 2003), and acute care (Jongbloed 1989; Yozbatiran 2006). The 13 studies were completed in 11 different countries as outlined in the Characteristics of included studies table.
In general interventions could be considered as taking either a sensory retraining approach or sensory stimulation approach. Only three studies had a sensory retraining focus (Acerra 2007; Byl 2003; Posteraro 2001). The remaining studies provided some sort of stimulation intervention including compression, electrical stimulation, thermal stimulation, sensory integrative treatment, magnetic stimulation, or tensive mobilizations. Many different intervention techniques were examined by the studies with most providing clear descriptions of the interventions. Two studies (Miller 2004; Wolny 2003) provided few details of the intervention even when we contacted the authors.
A sensory retraining program was used by three studies (Acerra 2007; Byl 2003; Posteraro 2001). Acerra 2007 used mirror therapy in addition to therapy as normal, asking participants to complete sensory motor tasks inside a mirror box that provided visual feedback of bilateral simultaneous hand movements. Byl 2003 compared two different sequences for fine motor and sensory retraining programs. They had clear guidelines for a fine motor program that included stress free hand activities, practising repetitive specific fine motor tasks, general aerobic, strengthening and flexibility training, and reinforcement with mental rehearsal. The sensory component involved using stress free hand strategies, graded and repetitive sensory discrimination activities, nervous and sensory system quieting activities, and reinforcement with mental imagery. Posteraro 2001 used a graded program that focused on tactile recognition starting with simple recognition and progressing through simultaneous stimuli recognition and progressing to complex stimuli recognition.
Electrical stimulation was used by two studies (Burridge 2002; Yozbatiran 2006). Burridge 2002 used a two channel neuromuscular electrical stimulation unit to stimulate the triceps brachialis and the second channel to stimulate extensor digitorum communis, extensor carpi radialis, and if possible extensor pollicis longus. This was a synchronized contraction with a duty cycle of eight seconds on and eight seconds rest and a ramp up and down time of two seconds. The pulse width was set at 300 μs and had a frequency of 40 Hz. Yozbatiran 2006 used a transcutaneous electrical nerve stimulator (TENS) machine and 2.5 cm electrodes placed on the extensor digitorum communis and extensor carpi radialis muscles at 2 HZ, pulse width 260 μs symmetrical biphase square pulse, with the amplitude adjusted to elicit wrist and finger extension.
Cambier 2003 used intermittent pneumatic compression with an automatic intermittent pattern over three minutes, with a 90‐second inflation and 90‐second deflation cycle at 40 mmHg pressure while the patient was positioned in supine with 45 degrees shoulder vertical abduction and forearm, wrist and fingers extended. The sham treatment consisted of the same positioning with a shortwave machine positioned over the hemiplegic shoulder but not turned on. Chen 2005 used thermal stimulation via monitored hot and cold packs. After 15 and 30 seconds application, respectively, the participants were encouraged to actively withdraw from the stimulus. This was repeated 10 times per cycle with at least a 30‐second pause between applications and two alternating cycles of hot and cold for each session.
Feys 1998 used sensory motor stimulation that involved pushing a rocking chair with the affected arm in an inflatable splint, designed to provide motor, proprioceptive and exteroceptive (pressure) stimulation. Heldman 2000 used repetitive peripheral magnetic stimulation produced by a figure of eight coil placed over the innervation zone for forearm and finger movement. The stimulator was able to generate instantaneous intensity of 1500 J and at a rate of 40/s‐1. Miller 2004 used early intensive task training emphasizing unimanual and bimanual functional activities, while Poole 1990 used an inflatable pressure splint with positioning at 90 degrees shoulder flexion, full elbow extension and as much external rotation as possible. Wolny 2003 examined tensive mobilizations of the peripheral nerves of the affected upper limb. Jongbloed 1989 compared sensory motor integrative treatment with functional treatment. The sensory motor integrative treatment focused on patient preparation, tone normalization, functional activity, giving verbal and visual cues and following a developmental sequence while the functional treatment emphasized compensation and adaptation.
The duration of the studies ranged from one treatment (Heldman 2000) to 12 weeks, though five studies used a six to eight‐week intervention period (Byl 2003; Chen 2005; Feys 1998; Jongbloed 1989; Posteraro 2001) and the others less. Only two studies had long‐term follow‐up time periods, Feys 1998 with six and 12‐month follow‐ups and Miller 2004 with a three‐month follow‐up. The dose frequency varied considerably with Heldman 2000 providing a single dose. The most common dose frequency was 30 to 40 minutes five days per week (Cambier 2003; Chen 2005; Feys 1998; Jongbloed 1989; Poole 1990) while Yozbatiran 2006 was 60 minutes and Byl 2003 was 90 minutes daily. Burridge 2002 was the only study to use a dose frequency of 30 minutes two times daily. Miller 2004, Posteraro 2001, and Wolny 2003 did not provide details of their dose frequency, though Miller 2004 did state daily.
Sensory impairment modalities tested in the studies included: light touch, mechanical sensation, two‐point discrimination, sustained pressure, kinesthesia, position sense, form perception, stereognosis, graphesthesia, pain (pressure pain, temperature pain, pain intensity) and combined sensory modality assessment. Tactile/proprioceptive extinction was also tested. The 13 studies in this review used 36 different outcome measures for sensory impairment. Two‐point discrimination and the Nottingham Sensory Assessment two‐point discrimination subtest were used the most frequently (Burridge 2002; Cambier 2003; Wolny 2003). Kinesthesia was measured by Byl 2003 and Yozbatiran 2006. Cambier 2003 used the Nottingham Sensory Assessment Kinesthesia subtest. Other standardized outcome measures for sensory impairment included the Nottingham Sensory Assessment (Cambier 2003), Semmes Weinstein Monofilaments (Chen 2005), Bickerstaff Sensory Protocol (Feys 1998), Sensory Motor Integration Tests (Jongbloed 1989), the QST (Acerra 2007), Byl‐Cheney Boczai Sterognosis Test (Byl 2003), and components of the Brunnstrom Fugl Meyer Assessment (Cambier 2003; Feys 1998; Poole 1990). Two forms of extinction tests were also employed: the Quality Extinction Test used by Heldman 2000, and the Tactile Extinction Test that included both tactile and proprioceptive extinction used by Posteraro 2001.
Eleven of the 13 studies addressed functional use of the upper limb, using 13 different outcome measures. The most frequently used measures were components of the Brunnstrom Fugl Meyer Assessment (Cambier 2003; Feys 1998; Poole 1990) and the Action Research Arm Test (Burridge 2002; Feys 1998). Other tests used included the Hand Function Test (Yozbatiran 2006), Hand Movement Scale (Yozbatiran 2006), Motricity Scale (Posteraro 2001), Modified Motor Assessment Scale (Chen 2005), Brunnstrom Stage Score (Chen 2005), Manual Dexterity (Miller 2004), Chedoke McMaster Stroke Assessment (Miller 2004), Digit Reaction Time (Byl 2003), Purdue Pegboard (Byl 2003), and Wolf Motor Function Test (Byl 2003).
Functional performance and participation outcomes were addressed in only 50% of the studies. The Barthel Index was the most frequently used outcome measure at this level (Feys 1998; Jongbloed 1989; Posteraro 2001). Other outcome measures at this level were the Katz Index of Independence in Activities of Daily Living (KATZ ADL) and Instrumental Activities of Daily Living (IADL) scales (Posteraro 2001), Stroke Adapted 30 Item Sickness Impact Profile (Miller 2004), Meal Preparation (Jongbloed 1989), and the California Functional Evaluation (Byl 2003).
Given the significant clinical and methodological diversity in the studies and the incomplete data for some of the studies, we did not attempt a meta‐analysis.
Risk of bias in included studies
Of the 13 included studies only three (Acerra 2007; Burridge 2002; Chen 2005) had adequate random sequence generation and concealment. Yozbatiran 2006 used 'controlled clinical trial with alternate allocation' for the allocation to groups and the other studies did not provide enough information to make a judgement though they did say that participants were randomly allocated to the control and experimental groups. Fifty per cent of the studies (Acerra 2007; Byl 2003; Cambier 2003; Chen 2005; Feys 1998; Jongbloed 1989; Poole 1990) included in the review reported blinding of at least outcome assessment personnel and key personnel where possible, while some reported blinding of participants as well. Burridge 2002, Posteraro 2001, and Yozbatiran 2006 did not blind any study personnel or participants and Heldman 2000, Miller 2004, and Wolny 2003 did not provide adequate information to know if blinding occurred. All participants were accounted for in all of the studies except for Byl 2003, Feys 1998, Miller 2004, and Wolny 2003 who did not provide adequate information, with only one study (Yozbatiran 2006) providing the CONSORT flow chart. All of the studies were free from selective reporting of the outcomes except for Wolny 2003 where some outcomes were not mentioned in the abstracts provided, and Miller 2004 who did not provide adequate information. All of the studies appeared free of other biases except for Byl 2003, Miller 2004, and Wolny 2003 who did not provide adequate information to allow judgement on this criteria. See Figure 1 and Figure 2.
Figure 1.
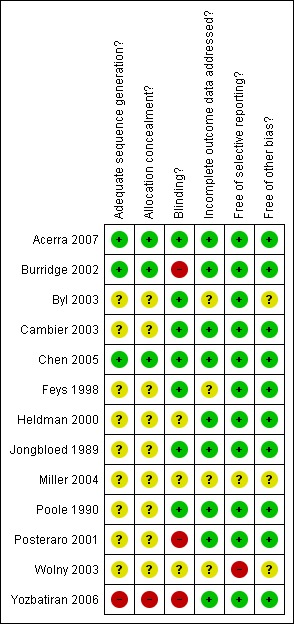
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Figure 2.
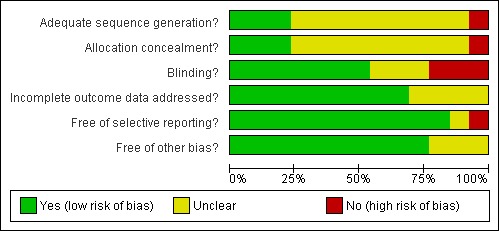
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
In summary, most of the information in this review is from studies that have unclear or high risk of bias. The following studies were classified as having unclear risk of bias for one or more domains: Byl 2003, Cambier 2003, Feys 1998, Heldman 2000, Jongbloed 1989, Miller 2004, and Poole 1990. The following studies were classified as having high risk of bias for one or more domains: Burridge 2002, Posteraro 2001, Wolny 2003, and Yozbatiran 2006. Only two studies (Acerra 2007; Chen 2005) had a low risk of bias.
Effects of interventions
Comparision 1: Specific treatment for sensory impairment versus no treatment (or with conventional treatment in both study arms): sensory impairment measures
Seven studies (Chen 2005; Heldman 2000; Miller 2004; Poole 1990; Posteraro 2001; Wolny 2003; Yozbatiran 2006) compared a specific treatment for sensory impairment with no treatment (or with conventional treatment in both study arms) and used sensory impairment outcomes with a total of 162 participants. Three studies (Chen 2005; Poole 1990; Yozbatiran 2006) provided adequate data to allow for calculations of effect size. They were as follows.
A trial of electrical stimulation of wrist and fingers in addition to neurodevelopment exercise compared with neurodevelopment exercise alone (Yozbatiran 2006) used clinical assessment of kinesthesia and position sense of wrist and fingers but found no differences between experimental and control groups ( Analysis 1.1; Analysis 1.2; Analysis 1.3).
In a comparison of inflatable pressure splinting intervention and no splinting Poole 1990 reported upper limb sensation (combined light touch and position sense) and pain at the end of scheduled follow‐up using subscales of the Fugl‐Meyer upper limb assessment as an outcome measure. An individual analysis of 18 participants from this study found no difference in scores between the intervention and control group. A difference between control and experimental group was found for pain with the experimental group having lower pain scores (MD ‐2.40, 95% CI ‐4.65 to ‐0.15) (Analysis 1.4).
One trial compared repetitive thermal stimulation (heating alternating with cooling) of the hand (with participants being encouraged to move their hand away from the stimulus on discomfort), in addition to standard therapy with standard therapy alone (Chen 2005). This study tested mechanical sensation using the Semmes‐Weinstein monofilament and reported a greater rate of recovery of sensation over six weeks in favor of the experimental group (MD 0.21, 95% CI 0.10 to 0.32) (Analysis 1.5).
Analysis 1.1.
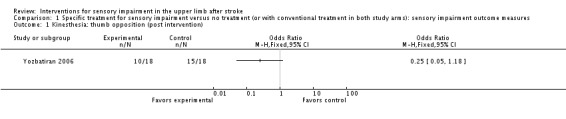
Comparison 1 Specific treatment for sensory impairment versus no treatment (or with conventional treatment in both study arms): sensory impairment outcome measures, Outcome 1 Kinesthesia: thumb opposition (post intervention).
Analysis 1.2.
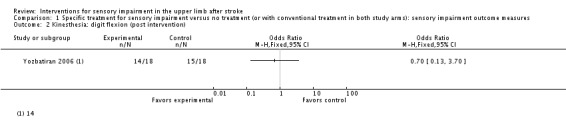
Comparison 1 Specific treatment for sensory impairment versus no treatment (or with conventional treatment in both study arms): sensory impairment outcome measures, Outcome 2 Kinesthesia: digit flexion (post intervention).
Analysis 1.3.
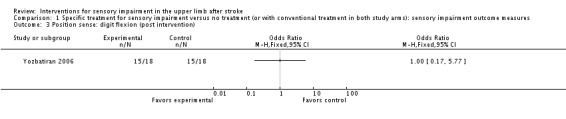
Comparison 1 Specific treatment for sensory impairment versus no treatment (or with conventional treatment in both study arms): sensory impairment outcome measures, Outcome 3 Position sense: digit flexion (post intervention).
Analysis 1.4.
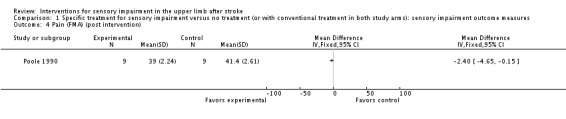
Comparison 1 Specific treatment for sensory impairment versus no treatment (or with conventional treatment in both study arms): sensory impairment outcome measures, Outcome 4 Pain (FMA) (post intervention).
Analysis 1.5.
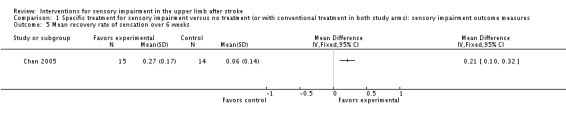
Comparison 1 Specific treatment for sensory impairment versus no treatment (or with conventional treatment in both study arms): sensory impairment outcome measures, Outcome 5 Mean recovery rate of sensation over 6 weeks.
The remaining four studies (Heldman 2000; Miller 2004; Posteraro 2001; Wolny 2003) did not provide adequate data to calculate an effect size. Wolny 2003 measured two‐point discrimination and thermesthesia to test the effect of tensive mobilizations of the peripheral nerves. Although the authors reported a significant improvement in discrimination sense for the treatment group, between‐group results were not reported. Miller 2004 reported a significant difference in hand sensation in favor of the early, intensive task‐oriented training over the control group that had postural and concentration exercises but did not provide adequate data to calculate effect size.
Two trials focused on tactile extinction (Heldman 2000; Posteraro 2001). Heldman 2000 compared a single dose of repetitive peripheral magnetic stimulation with no intervention. Using the Quality Extinction Test as the outcome measure they reported significant reduction in left‐side tactile extinctions but no impact on ipsilateral extinctions. Attentional cueing did not impact left‐side extinction errors but did increase ipsilateral errors. However, this study did not provide adequate data to calculate effect size. A trial of a graded sensory rehabilitation program (Posteraro 2001) reported significant differences for their outcome measures of tactile and proprioceptive sensation in favor of the treatment condition but did not provide adequate data to calculate effect size.
Comparision 2: Specific treatment for sensory impairment versus no treatment (or with conventional treatment in both study arms): upper limb functional use outcome measures
Five studies (Chen 2005; Miller 2004; Poole 1990; Posteraro 2001; Yozbatiran 2006) compared a specific treatment for sensory impairment with no treatment (or with conventional treatment in both study arms) and utilized upper limb function outcome measures with a total of 108 participants. Three studies (Chen 2005; Poole 1990; Yozbatiran 2006) provided adequate data to allow for calculations of effect size.They were as follows.
The trial of thermal stimulation (Chen 2005) demonstrated a greater rate of recovery of arm function over a six‐week period in the experimental group than the control group using the Modified Motor Assessment Scale (MD 1.58, 95% CI 0.98 to 2.18) (Analysis 2.4) and a greater recovery rate using the Brunstrom Stage Score over six weeks (MD 0.19, 95% CI 0.09 to 0.29) (Analysis 2.5).
Poole 1990 used the Fugl‐Meyer Assessment upper arm and hand and wrist outcome measures to assess the effect of using an air splint on upper limb function. No between‐group differences were demonstrated for Fugl‐Meyer Assessment upper limb function (MD ‐6.00, 95% CI ‐16.58 to 4.58) (Analysis 2.1) or for Fugl‐Meyer Assessment hand and wrist function (MD ‐0.12, 95% CI ‐9.06 to 8.82) (Analysis 2.2).
Yozbatiran 2006 used the Hand Function Test to measure the effectiveness of electrical stimulation on upper limb function and found a significant difference in favor of the control group (MD ‐1.16, 95% CI ‐2.10 to ‐0.22) (Analysis 2.3).
Analysis 2.4.
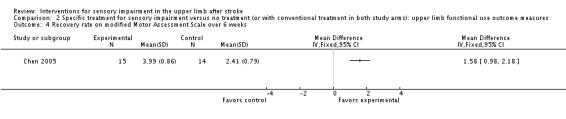
Comparison 2 Specific treatment for sensory impairment versus no treatment (or with conventional treatment in both study arms): upper limb functional use outcome measures, Outcome 4 Recovery rate on modified Motor Assessment Scale over 6 weeks.
Analysis 2.5.
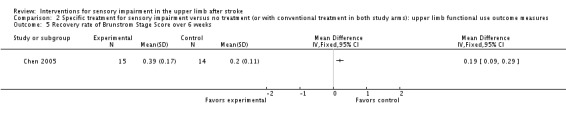
Comparison 2 Specific treatment for sensory impairment versus no treatment (or with conventional treatment in both study arms): upper limb functional use outcome measures, Outcome 5 Recovery rate of Brunstrom Stage Score over 6 weeks.
Analysis 2.1.
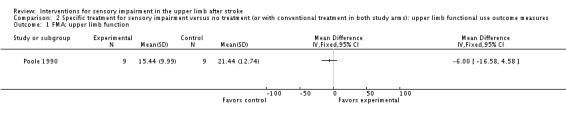
Comparison 2 Specific treatment for sensory impairment versus no treatment (or with conventional treatment in both study arms): upper limb functional use outcome measures, Outcome 1 FMA: upper limb function.
Analysis 2.2.
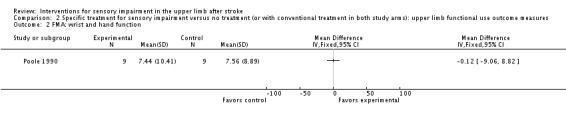
Comparison 2 Specific treatment for sensory impairment versus no treatment (or with conventional treatment in both study arms): upper limb functional use outcome measures, Outcome 2 FMA: wrist and hand function.
Analysis 2.3.
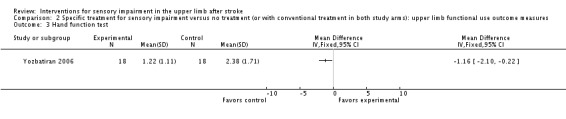
Comparison 2 Specific treatment for sensory impairment versus no treatment (or with conventional treatment in both study arms): upper limb functional use outcome measures, Outcome 3 Hand function test.
There were insufficient data to calculate effect sizes for the study of an early, intensive task‐related training program (Miller 2004) although the authors reported significantly greater gains in motor recovery of the arm for the experimental group compared with control on the Chedoke McMaster Stroke Assessment (P < 0.001), but not for dexterity.
A trial of tactile extinction (Posteraro 2001) with only 10 participants had no data reported but the authors stated there was no difference between groups for the outcome of motricity.
Activities limitations and participation outcome measures
Two studies considered effects of their interventions on functional performance or participation. Miller 2004 used the Barthel Index and Stroke‐Adapted Sickness Impact Profile and Posteraro 2001 used the Katz Index of Activities of Daily Living, Katz Index of Instrumental Activities of Daily Living, and Barthel Index. Neither study presented sufficient data to determine effect sizes but reported between‐group differences in favor of the experimental groups.
Specific treatment for sensory impairment versus conventional upper limb therapy
No studies met this categorization.
Comparison 3: Specific treatment for sensory impairment versus placebo/attention placebo: sensory impairment outcome measures
Four studies (Acerra 2007; Burridge 2002; Cambier 2003; Feys 1998) compared a specific treatment for sensory impairment with either a placebo or attention control and used sensory impairment outcomes with a total of 144 participants. Three of these studies provided enough data to calculate effect sizes for the individual studies. They were as follows.
A study of mirror therapy compared with sham treatment measured light touch, thermal (hot pain) and pressure pain thresholds using the Quanitative Sensory Test and shoulder and arm pain intensity at rest using a 0 to 10 Visual Analogue Scale (Acerra 2007). Between‐group differences in favor of the experimental group were found for light touch on the volar side of the hand (dorsal side had similar results) (MD ‐2.05, 95% CI ‐2.42 to ‐1.68) (Analysis 3.1), thermal (hot) pain measured on the hand (MD ‐1.20, 95% CI ‐1.42 to ‐0.98) (Analysis 3.9), and pressure pain (MD ‐41.30, 95% CI ‐56.57 to ‐26.03) (Analysis 3.10). No between‐group differences were found for pain intensity at rest (Analysis 3.8).
A trial of neuromuscular electrical stimulation compared with passive stretching (Burridge 2002) found no differences between groups in mean change of two point discrimination at the end of treatment (MD 5.18, 95% CI ‐1.50 to 11.86) (Analysis 3.3).
A study of intermittent pneumatic compression of the hemiplegic upper limb compared with sham short‐wave therapy (Cambier 2003) demonstrated between‐group differences in favor of the experimental group on the Nottingham Sensory Assessment overall (MD 37.10, 95% CI 8.16 to 66.04) (Analysis 3.11) and for the subscales of tactile sensation (MD 26.20, 95% CI 6.99 to 45.41) (Analysis 3.2) and kinesthetic sensation (MD 5.00, 95% CI 0.05 to 9.95) (Analysis 3.5), but not for two‐point discrimination (MD 0.31, 95% CI ‐0.43 to 1.05) (Analysis 3.4) or stereognosis (MD 5.60, 95% CI ‐0.54 to 11.74) (Analysis 3.6). No difference between groups was found for pain (MD ‐5.00, 95% CI ‐31.82 to 21.82) (Analysis 3.7).
Analysis 3.1.
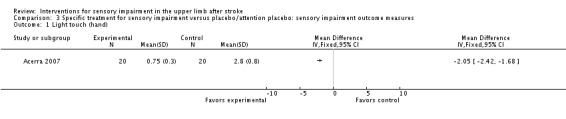
Comparison 3 Specific treatment for sensory impairment versus placebo/attention placebo: sensory impairment outcome measures, Outcome 1 Light touch (hand).
Analysis 3.9.
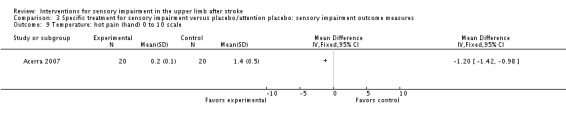
Comparison 3 Specific treatment for sensory impairment versus placebo/attention placebo: sensory impairment outcome measures, Outcome 9 Temperature: hot pain (hand) 0 to 10 scale.
Analysis 3.10.
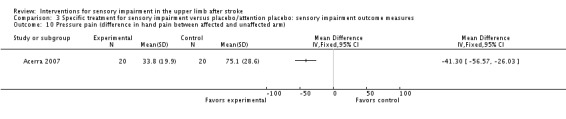
Comparison 3 Specific treatment for sensory impairment versus placebo/attention placebo: sensory impairment outcome measures, Outcome 10 Pressure pain (difference in hand pain between affected and unaffected arm).
Analysis 3.8.
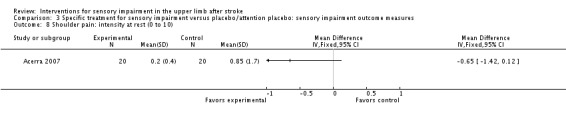
Comparison 3 Specific treatment for sensory impairment versus placebo/attention placebo: sensory impairment outcome measures, Outcome 8 Shoulder pain: intensity at rest (0 to 10).
Analysis 3.3.
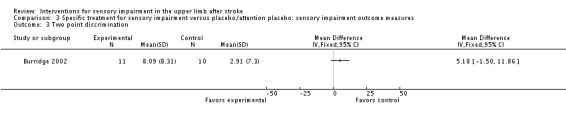
Comparison 3 Specific treatment for sensory impairment versus placebo/attention placebo: sensory impairment outcome measures, Outcome 3 Two point discrimination.
Analysis 3.11.
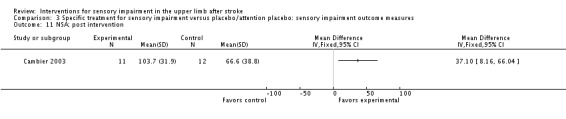
Comparison 3 Specific treatment for sensory impairment versus placebo/attention placebo: sensory impairment outcome measures, Outcome 11 NSA: post intervention.
Analysis 3.2.
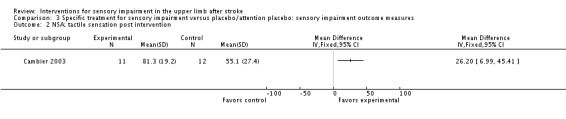
Comparison 3 Specific treatment for sensory impairment versus placebo/attention placebo: sensory impairment outcome measures, Outcome 2 NSA: tactile sensation post intervention.
Analysis 3.5.
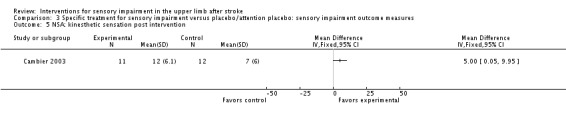
Comparison 3 Specific treatment for sensory impairment versus placebo/attention placebo: sensory impairment outcome measures, Outcome 5 NSA: kinesthetic sensation post intervention.
Analysis 3.4.
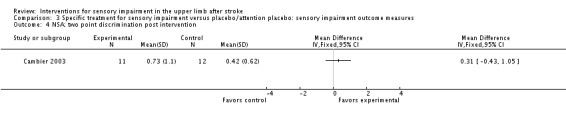
Comparison 3 Specific treatment for sensory impairment versus placebo/attention placebo: sensory impairment outcome measures, Outcome 4 NSA: two point discrimination post intervention.
Analysis 3.6.
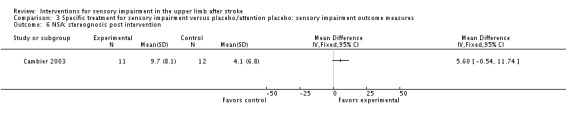
Comparison 3 Specific treatment for sensory impairment versus placebo/attention placebo: sensory impairment outcome measures, Outcome 6 NSA: stereognosis post intervention.
Analysis 3.7.
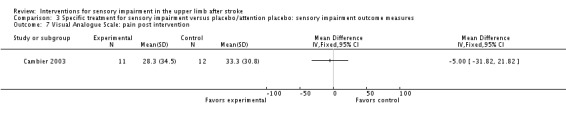
Comparison 3 Specific treatment for sensory impairment versus placebo/attention placebo: sensory impairment outcome measures, Outcome 7 Visual Analogue Scale: pain post intervention.
A novel intervention required participants to push themselves in a rocking chair with the hemiplegic limb in an inflatable splint compared with sham short‐wave therapy while in a rocking chair (Feys 1998). It tested exteroceptive and proprioceptive sensory function but did not provide data sufficient for calculating an effect size. The authors reported no significant differences between the groups.
Comparison 4: Specific treatments for sensory impairments versus placebo/attention placebo: upper limb function outcome measures
Four studies compared a specific treatment for sensory versus placebo or attentional control and measued upper limb functional outcomes (Acerra 2007; Burridge 2002; Cambier 2003; Feys 1998).
The study of mirror therapy compared with sham treatment measured grip strength. Post‐intervention between‐group differences were found in favor of the experimental group for grip strength (MD 4.10, 95% CI 1.06 to 7.14) (Acerra 2007).
The trial of neuromuscular electrical stimulation compared with passive stretching (Burridge 2002) measured upper limb function using the Action Research Arm Test (ARAT) and found a between‐group difference in favor of the experimental group (MD 12.90, 95% CI 5.65 to 20.15) (Analysis 4.2).
The study of intermittent pneumatic compression of the hemiplegic upper limb compared with sham short‐wave therapy (Cambier 2003) found no difference using the Brunnstrom Fugl‐Meyer assessment of motor recovery (MD 11.50, 95% CI ‐5.45 to 28.45) (Analysis 4.3).
Feys 1998 study of participants with their hemiplegic arm in an inflatable splint while in a rocking chair compared with sham short‐wave therapy demonstrated a higher proportion of participants achieving a greater than 10% gain on the Brunnstrom Fugl‐Meyer assessment in the experimental group compared with controls (OR 6.05, 95% CI 2.00 to 18.31) but did not provide adequate data to calculate an effect size for the use of the ARAT.
Analysis 4.2.
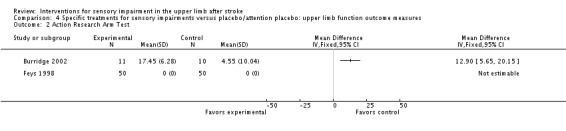
Comparison 4 Specific treatments for sensory impairments versus placebo/attention placebo: upper limb function outcome measures, Outcome 2 Action Research Arm Test.
Analysis 4.3.
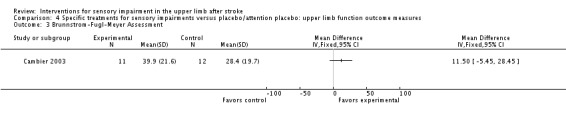
Comparison 4 Specific treatments for sensory impairments versus placebo/attention placebo: upper limb function outcome measures, Outcome 3 Brunnstrom‐Fugl‐Meyer Assessment.
Activities limitations and participation outcome measures
Only the study by Feys 1998 measured the effects of the intervention on functional performance using the Barthel Index but it did not provide adequate data to calculate an effect size. The authors reported no significant differences between the groups.
Comparisons between different types of treatments for sensory impairment
Sensory impairment outcome measures
Two studies (Byl 2003; Jongbloed 1989) compared different types of treatments for sensory impairment using sensory impairment outcomes with a total of 108 participants. In a cross‐over trial of sensory discrimination retraining followed by fine motor retraining Byl 2003 measured graphesthesia, kinesthesia and stereognosis but only means were presented so that effect sizes could not be calculated. The authors concluded that both groups made significant gains in sensory discrimination. The study by Jongbloed 1989 compared the effects of an occupational therapy sensorimotor integrative treatment with a functional approach using the Sensorimotor Integration Test Battery (including finger identification, form perception, wire shape recognition, imitation and sequencing of postures) but did not provide sufficient data to calculate an effect size. The authors reported significant between‐group differences for finger identification and posture imitation in favor of the functional approach group.
Upper limb function outcome measures
Byl 2003 used Digit reaction, the Purdue Pegboard, and Wolf Motor Function Test to measure upper limb function but provided insufficient data to determine effect sizes. The authors reported significant improvements in both groups with no significant differences between them except the group that had motor then sensory retraining had significantly higher fine motor outcomes at the end of the follow‐up period than the other group (sensory followed by motor retraining).
Activities limitations and participation outcome measures
Jongbloed 1989 measured functional performance using the Barthel Index and assessment of meal preparation but did not provide sufficient data to calculate an effect size. The authors reported no significant between‐group differences. Byl 2003 used the California Functional Evaluation to measure functional performance and participation but provided insufficient data to determine effect sizes.
Two of the 13 studies addressed adverse effects (Chen 2005; Feys 1998). Chen 2005 reported no physical damage or adverse effectsalthough their observations were limited to increased muscle tone, which showed no difference between the groups and the fact that assessment took place during and after thermal stimulation. Feys 1998 assessed participants for soft tissue lesions, shoulder‐hand syndrome, subluxation, and shoulder pain before and after the intervention and at follow‐up. They concluded that there were no significant differences between the two groups at the end of the study.
Discussion
Summary of results
The aim of this review was to examine the effects of interventions for sensory impairment on upper limb sensation, upper limb function, activities limitations and participation in participants who have experienced a stroke. We included 13 studies. Meta‐analyses were not possible due to considerable clinical and methodological diversity and lack of data. Lack of data also limited the calculation of individual study effect size for a large number of the studies.
In summary there is insufficient evidence to reach conclusions on the effectiveness of any interventions for sensory impairment of the upper limb. Only preliminary evidence exists from individual studies for the effectiveness of some specific interventions for sensory impairment in the upper limb. With respect to the primary outcome of interest, upper limb sensation, there was some limited evidence for:
the effects of mirror therapy for improving detection of light touch, pressure and temperature pain;
a thermal stimulation intervention for improving rate of recovery of sensation; and
intermittent pneumatic compression for improving tactile and kinesthetic sensation.
It is possible that other interventions reporting statistically significant results may be beneficial (repetitive peripheral magnetic stimulation, early intensive task‐orientated training and graded sensory rehabilitation) but data were not available to determine effect sizes. Similarly there is insufficient evidence to reach conclusions on the effectiveness of any interventions for sensory impairment to make a difference to upper limb function, activity limitations, and participation.
Overall there were limited studies on each of the interventions, inadequate data available in many instances to determine effect sizes, and unclear or high risk of bias for most of the studies, limiting the ability to draw significant conclusions.
Overall completeness and availability of the evidence
Most studies did not provide adequate descriptions of the study design to allow for accurate assessment of risk of bias. It was difficult to obtain adequate data to complete statistical analysis of the results. It was difficult to track down several of the authors to try to obtain adequate data. Several authors did assist with providing further data when contacted though one set did state they were going to publish the study and did not want to release any of the data.
Quality of the evidence
Overall the sample size for the studies was small, with no mention of power calculations for sample size in most of the studies. There were some exceptions with a larger sample size of 100 and 90 used by Feys 1998 and Jongbloed 1989; otherwise, all other samples sizes were under 40 with some as low as 10 participants. The considerable clinical and methodological diversity impacted on the study conclusions. The risk of bias was unclear or high for all but two studies.
Potential biases in the review process
When designing this review, we made the decision to include only studies that were directly aimed at improving sensory impairments. We found several studies that were focused on motor outcomes but used sensory motor stimulation and had some sensory outcome measures. These were not included. It is possible that these studies may have added to the evidence available. Tactile extinction was included in this review as it remains contentious in the literature as to how to separate sensation from the attention (Yekutiel 1993) and is included as a disorder of sensation in a number of medical texts.
Agreements or disagreements with other studies or reviews
There was one other review of sensory retraining after stroke found in the literature search during completion of this review, carried out by Schabrun and Hillier (Schabrun 2009) titled Evidence for the retraining of sensation after stroke: a systematic review. Our review is different from Schabrun's review in several ways. Schabrun 2009 included both sensory retraining for the upper and lower limb in the review. They also included non‐randomized studies. Schabrun 2009 also included some studies where the stated aim was not to improve sensory function but to improve motor function although sensory outcome measures were used. Our review focused clearly on studies that were aimed specifically at improving sensory function.
Schabrun 2009 (page 36) concluded that 'the results of this meta‐analysis suggest that there is some evidence to support the use of passive sensory training to improve hand function and dexterity in those with stroke.'. Schabrun 2009’s term passive sensory training referred to electrical stimulation interventions. Our results for the effectiveness of studies that involved electrical stimulation are mixed. Yozbatiran 2006 compared electrical stimulation with NDT‐Bobath therapy with NDT‐Bobath therapy alone and found no differences on the sensory impairment outcome measures of kinesthesia and position sense though they did find an effect in favor of the control group on the Hand Function Test. Burridge 2002 compared electrical stimulation with a placebo of passive stretching. In this study no effect was found on the sensory impairment outcome of two‐point discrimination but upper limb function as measure by the Action Research Arm Test demonstrated an effect in favor of the treatment group. In Schabrun 2009's review the electrical stimulation was compared to sham or low current electrical stimulation. This may explain the differences in the results from the Yozbatiran 2006 study in which the comparison was with a more active and dynamic treatment that espoused to incorporate active and guided movement that incorporates sensory input.
Schabrun 2009 (page 36) also reported the following finding: "A number of single studies report positive effects on function, sensation and proprioception following active sensory training. However, the lack of sufficient data to perform meta‐analysis and insignificant effect sizes mean it is not yet possible to determine the effectiveness of active sensory training in stroke rehabilitation.". Schabrun 2009’s definition of "active sensory retraining" included interventions that were generally a graded sensory re‐education program. This review found similar findings in that there was a lack of sufficient data to perform a meta‐analysis. In our review we identified three studies that used a sensory retraining program. Acerra 2007 used mirror therapy and found improvements in detection of light touch and pain. Byl 2003 compared a graded sensory re‐education program for four weeks followed by a graded fine motor program for four weeks with the reverse order for the other treatment group and found no significant differences between the groups for graphesthesia, kinesthesia, and stereognosis. Significant gains were reported for upper limb function outcome measures (digit reaction time, Purdue pegboard, Wolf Motor function test) but there were insufficient data available to calculate effect sizes. Posteraro 2001 used a graded sensory re‐education program to address tactile and proprioceptive extinction. Posteraro 2001 found no difference in the tactile and proprioceptive extinction scores of the Motricity score for upper limb function between the control and the intervention group. The authors reported significant differences in favor of the intervention group on the functional performance outcomes of Katz ADL & IADL, and Barthel Scales although inadequate data were provided for effect sizes to be calculated. These findings tend to support the findings found by Schabrun.
Our review also found some single studies that reported positive effects on sensory impairment, upper limb function and functional performance and participation for interventions not addressed by Schabrun’s review such as intermittent pneumatic compression, repetitive peripheral magnetic stimulation, early, intensive task oriented training, and thermal stimulation.
Overall, our review was specifically directed at the sensory rehabilitation of the upper limb after stroke versus the more general approach of the Schabrun 2009 review. While the results were generally consistent with the findings of the Schabrun 2009 review, this review found a larger number of randomized controlled trials relevant to the upper limb, that addressed a wider range of interventions and outcomes. Similar issues related to the number and quality of the studies remain and similar conclusions related to single studies that may support specific interventions were found but there were inadequate data to allow effective analysis.
Authors' conclusions
There are a large number of techniques that show promise for addressing sensory impairments in the upper limb after stroke but we do not at this stage have adequate high quality trials to be able to make recommendations that support or refute the use of specific interventions. Since few studies mentioned adverse effects, the clinician should be conscious of monitoring adverse affects when using any interventions for sensory impairment.
This review was based on a small number of trials, generally only one, for each of the types of interventions. Most of the trials included a small number of participants and had high to unclear levels of bias. Addressing these issues should be priorities in research design in the stroke rehabilitation area. Some interventions identified in this review have potential to prove beneficial to those with sensory impairment of the upper limb after stroke but need further high quality studies to assess their effectiveness. When searching for studies for this review it was evident there are also many non‐randomized studies that addressed these and other interventions that could be investigated with randomized controlled trials to ascertain the value of these treatment techniques in this field.
The large number of outcome measures used was another significant factor that contributed to the clinical diversity of this review. Diagnostic test accuracy reviews to look at the effectiveness of these outcome measures for measuring sensory impairments, upper limb function and functional performance and participation after stroke would also be a priority.
Improved reporting of trials of rehabilitation interventions would assist with the ability to determine risk of bias and contributions of these trials. Compliance with the CONSORT guidelines is recommended. Researchers should include outcome measures that address participants’ functional performance and quality of life and any possible adverse reactions should be actively screened for in both experimental and control groups. Improved descriptions of the intervention would assist with reviewing the study and with replicating the study.
There was inadequate descriptions of the settings in which the interventions occurred and no studies addressed cost effectiveness of the different delivery options, dosages available, or the timing of the intervention after stroke. Further, no studies addressed the effectiveness of any of these interventions against usual care. These would be factors to address in further studies.
Acknowledgements
We thank Brenda Thomas for assisting with developing the search strategy and Hazel Fraser for all her multiple assistance with editing issues and the many questions that we developed along the way. Our special thanks also go to Madelyn Hall and Patsy Bacon, Medical Librarians at Southwest Washington Medical Center, for their endless assistance with obtaining and finding references. We thank all the trial authors and researchers in the area who responded to our requests for information. Thanks also to Dr Roberta Scherer from the US Cochrane Center for her support and encouragement throughout this process.
Appendices
Appendix 1. MEDLINE search strategy
We used the following search strategy, developed with assistance from the Cochrane Stroke Group Trials Search Coordinator, to search MEDLINE (Ovid) and we adapted it for the other databases.
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or cerebrovascular accident/ or exp brain infarction/ or exp cerebrovascular trauma/ or exp hypoxia‐ischemia, brain/ or exp intracranial arterial diseases/ or intracranial arteriovenous malformations/ or exp "Intracranial Embolism and Thrombosis"/ or exp intracranial hemorrhages/ or vasospasm, intracranial/ or vertebral artery dissection/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiplegia/ or exp paresis/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp Upper Extremity/ 9. (upper adj3 (limb$ or extremity)).tw. 10. (arm or shoulder or elbow or forearm or hand or wrist or finger or fingers).tw. 11. 8 or 9 or 10 12. sensation/ or proprioception/ or kinesthesis/ or touch/ 13. sensation disorders/ or exp somatosensory disorders/ 14. stereognosis/ or agnosia/ 15. Psychomotor Disorders/ 16. (sensation or sensory or somatosensory or propriocept$ or kinesthesi$ or touch or stereognosis or tactile).tw. 17. two point discrimination.tw. 18. position sense.tw. 19. 12 or 13 or 14 or 15 or 16 or 17 or 18 20. 7 and 11 and 19
Data and analyses
Comparison 1.
Specific treatment for sensory impairment versus no treatment (or with conventional treatment in both study arms): sensory impairment outcome measures
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Kinesthesia: thumb opposition (post intervention) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Kinesthesia: digit flexion (post intervention) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Position sense: digit flexion (post intervention) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Pain (FMA) (post intervention) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Mean recovery rate of sensation over 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Combined sensory modalities (FMA sensation = light touch plus position sense) (post intervention) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Analysis 1.6.
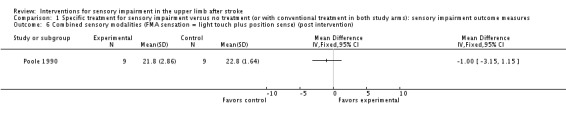
Comparison 1 Specific treatment for sensory impairment versus no treatment (or with conventional treatment in both study arms): sensory impairment outcome measures, Outcome 6 Combined sensory modalities (FMA sensation = light touch plus position sense) (post intervention).
Comparison 2.
Specific treatment for sensory impairment versus no treatment (or with conventional treatment in both study arms): upper limb functional use outcome measures
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FMA: upper limb function | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 FMA: wrist and hand function | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Hand function test | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Recovery rate on modified Motor Assessment Scale over 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Recovery rate of Brunstrom Stage Score over 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3.
Specific treatment for sensory impairment versus placebo/attention placebo: sensory impairment outcome measures
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Light touch (hand) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 NSA: tactile sensation post intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Two point discrimination | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 NSA: two point discrimination post intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 NSA: kinesthetic sensation post intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 NSA: stereognosis post intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Visual Analogue Scale: pain post intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Shoulder pain: intensity at rest (0 to 10) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Temperature: hot pain (hand) 0 to 10 scale | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Pressure pain (difference in hand pain between affected and unaffected arm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 NSA: post intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4.
Specific treatments for sensory impairments versus placebo/attention placebo: upper limb function outcome measures
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Grip strength | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Action Research Arm Test | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Brunnstrom‐Fugl‐Meyer Assessment | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Percentage achieving > n10% improvement on Brunnstrom‐Fugl‐Meyer Assesment at 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Analysis 4.1.
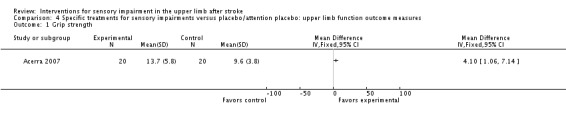
Comparison 4 Specific treatments for sensory impairments versus placebo/attention placebo: upper limb function outcome measures, Outcome 1 Grip strength.
Analysis 4.4.
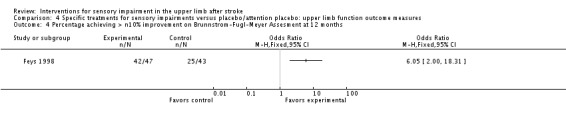
Comparison 4 Specific treatments for sensory impairments versus placebo/attention placebo: upper limb function outcome measures, Outcome 4 Percentage achieving > n10% improvement on Brunnstrom‐Fugl‐Meyer Assesment at 12 months.
History
Protocol first published: Issue 1, 2007 Review first published: Issue 6, 2010
| Date | Event | Description |
|---|---|---|
| 4 April 2008 | Amended | Converted to new review format. |
| 9 November 2006 | New citation required and major changes | Substantive amendment |
Differences between protocol and review
When completing the review we made several minor changes. We do not anticipate that these changes impacted the quality of the review or the outcomes. The changes are listed as follows.
At the end of the second paragraph under the heading 'Data collection and analysis' we have added the following: Where articles referred to the same primary study, they would be listed under one study in accordance with Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions. For this review, the authors will use one data extraction form for all the linked reports of the one study.
Under the 'Data retrieval' subheading we have deleted item 5 and adjusted the numbering accordingly. This was deleted because no information was found in any study and it was felt it did not contribute to the review quality sufficiently to leave in. '(5) Theoretical perspective: extract details the identified theoretical perspective if stated'.
Under the 'Data retrieval' subheading we have deleted item 12 and adjusted the numbering accordingly. This was deleted because no information was found in the studies and it was felt it did not contribute sufficiently to the quality of the review to leave in. '(12) Clinical reasoning decision making indicators identified, for example, movement, edema, pain, sensation level, subluxation, cognitive levels, perceptual issues, contraindications, exclusions from the study, complications reported or listed, inclusion criteria for the study, including time post stroke'.
In Item 10 of the 'Data collection and analysis' section we had originally stated that '(10) Effect size: if not reported and sufficient information is provided, this will be calculated using the methodology outlined in Rosenthal (Rosenthal 1991). These will be calculated as r values and then will be displayed in binomial effect size display (BESD).'. This was removed with description of calculations in RevMan 5 added in 'Data analysis'.
-
Under the 'Data retrieval' subheading the following item was changed from '(3) Sensory return group, as per the Heart and Stroke Association of Ontario definitions (HSAO 2001):
early stage low level return;
early stage high level return;
late stage low level return;
late stage high level return. If information was not available to make this classification with all of the studies, studies were then classified using the time period since the stroke occurred for the participants. The categories were defined by time since stroke of zero to three months, more than three months to six months, more than six months to 12 months, and more than 12 months. If groups had less than 10 participants, then groups were defined as zero to six months, and more than six months.' to 'Time since stroke'. This was changed to be consistent with the data available in the studies and does not impact the quality of the review.
Under 'Data analysis', the third comparison was altered by adding 'attentional placebo' after placebo and a definition was included in the following paragraph.
Characteristics of studies
Characteristics of included studies [ordered by year of study]
Jongbloed 1989
| Methods | RCT | |
| Participants | Country: Canada 46 right‐side CVA and 44 left‐side CVA 41 males, 49 females Mean age: 71.32 years (SD 9.07) Days since onset: average 40 (SD 42 days) Setting: initially acute hospital but not clear if patients stayed there for the full 8 weeks Inclusion criteria: admitted to hospital within 12 weeks after first CVA, weakness in upper and lower extremity (1 side after CVA ‐ Brunnstrom score 1 to 5), signed informed consent Exclusion criteria: residing in extended care facility prior to CVA, severe aphasia | |
| Interventions | Occupational therapy 40 minutes per day 5 days per week for 8 weeks 1. Functional treatment 2. Sensory motor treatment | |
| Outcomes | Barthel Index Meal preparation Sensory Motor Integration Test Battery (8 subtests) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "A project co‐ordinator randomly assigned..." Comment: no further description was given of how randomization was generated |
| Allocation concealment? | Unclear risk | Quote: "Subjects were unaware of the group to which they had been assigned" Comment: allocation concealment not stated though possible the project co‐ordinator was aware of assignment While participants could not foresee assignments it is unclear whether or not investigators could do so |
| Blinding? All outcomes | Low risk | Quote: "Subjects were unaware of the group to which they had been assigned" "An independent evaluator, who was unaware of the group to which the subject had been assigned, recorded subject performance on various measures before the assigned treatment was initiated, after 4 weeks of treatment, and after 8 weeks of treatment" Comment: blinding of key study personnel and participants was recorded by the study authors, who stated it was double blind Although the authors stated that participants were unaware of the group to which they were allocated (and later states the study was double blind), it could have been possible for participants to determine which group they were in due to the nature of the interventions Overall the judgement is that key assessment personnel were blind but not necessarily the participants |
| Incomplete outcome data addressed? All outcomes | Low risk | While the text does not mention participant retention throughout the study, it appears from what is in the text and tables that all 90 participants starting the study were still in the study at completion |
| Free of selective reporting? | Low risk | All outcomes were addressed in the results |
| Free of other bias? | Low risk | This study appears to be free of other sources of bias, although no clinical data were provided to allow comparison of groups at baseline on potentially important characteristics |
Poole 1990
| Methods | Randomly assigned matched pairs controlled trial | |
| Participants | Country: USA 6 participants with right hemiplegia and 12 with left hemiplegia Aged 55 to 82 years Inclusion criteria: patients with hemiplegia resulting from CVA Exclusion criteria: not stated | |
| Interventions | Intervention group: inflatable pressure splint with positioning for 30 minutes, 5 days per week for 3 weeks and daily traditional occupational therapy treatment Control group: daily traditional occupational therapy treatment | |
| Outcomes | Outcomes were recorded at baseline and within 24 hours of the 3‐week period ending Measures: upper limb sensation, pain, and motor function components of the Fugl‐Meyer Assessment (FMA) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Pairs of subjects were matched according to upper extremity motor scores ... subjects were then randomly assigned to a non‐splint or splint condition" Comment: insufficient details provided |
| Allocation concealment? | Unclear risk | Comment: no data provided |
| Blinding? All outcomes | Low risk | Quote: "All subjects received an initial evalautiaon and a final evalutation at the end of week 3 with the FMA by two therapists who were unaware of the group to which each subject had been assigned." Comment: blinding was evident for outcomes assessors but did not occur for the participants and treating therapists |
| Incomplete outcome data addressed? All outcomes | Low risk | There was complete follow up in this study |
| Free of selective reporting? | Low risk | The outcome measures outlined in the methods section were all reported in the results |
| Free of other bias? | Low risk | None noted |
Feys 1998
| Methods | Multicenter, single blind RCT with stratification based on level of motor return | |
| Participants | Country: Belgium Setting: unclear 100 participants (50 patients in each group) Age ranged from 38 to 87 years; mean 65.65 years (SD 11.81) 31 females, 59 males Inclusion criteria: within 2 to 5 weeks of onset with diagnosis of Ischemic brain injury or intracerebral hemorrhage, obvious motor deficit in the upper limb (Brunnstrom Fugl Meyer Score < 46), ability to sit independently or with minimal support, ability to perform the experimental treatment independently Exclusion criteria: too old or too frail for participation in intervention, cognitive impairment preventing participation in tervention, discharged prior to participation, significant comorbidities | |
| Interventions | Intervention group: 30 minutes 5 days per week for 6 weeks, sessions using the affected arm to push in a rocking chair with assist of an airsplint for support and usual rehabilitation procedures Control group: experienced rocking in the chair for the same time period and usual rehabilitation procedures | |
| Outcomes | Pre‐, mid‐point, and post‐intervention assessments with 6 and 12 month follow‐up Brunnstrom Fugl‐Meyer test Action Research Arm Test, Barthel Index Sensory function, exteroceptive and proprioceptive, at proximal, medial and distal sections according to Bickerstaff protocol Ashworth Scale Scores for 7 muscle groups | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "A single blind stratified randomized controlled design was used. To obtain comparable groups patients were then stratified according to their initial motor score on the Brunnstrom Fugle‐Meyer score. In addition, stratification was applied based on the type of stroke. Within these 4 strata pts were randomly allocated to either an experimental or a control group." Comment: no information provided about how the sequence was generated |
| Allocation concealment? | Unclear risk | Comment: no information provided about allocation concealment |
| Blinding? All outcomes | Low risk | Quote: "Clinical evaluations were performed by independent assessors who were blinded to group assignment and not involved in the routine treatment of the patients." Comment: no reporting of therapists or particpant blinding; unlikely to impact outcomes greatly |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Quote: "A total of 108 patients (out of approximately 1000 considered) entered the trial. Eight patients discontinued the treatment for various reasons. One patient died, another patient had a second stroke and for a third patient, the general medical condition deteriorated to the extent that the treatment was discontinued. In addition, there was 1 patient with a humerus fracture, and 1 with extreme shoulder pain. Finally 2 patients were unable to perform the treatment autonomously, and 1 patient was discharged during the intervention period. These patients were excluded from further analysis. The control and experimental groups each consisted of 50 patients. Of the 100 subjects, there were 4 and 10 defaulters, respectively, at the 6 and 12 month follow up tests. Of the defaulters at 12 months post stroke, 7 belonged to the control group and 3 to the experimental group." Comment: 16% of the participants left the study following randomization; no information is provided about the 8 who originally left the study in terms of whether they were from the control or experimental group |
| Free of selective reporting? | Low risk | Comment: text and tables include all planned outcome measures |
| Free of other bias? | Low risk | Comment: no other apparent bias noted |
Heldman 2000
| Methods | RCT with age‐matched normal control group | |
| Participants | Country: Germany Setting: unclear 14 participants (7 in each group) with left‐sided, tactile extinction folowing unilateral, right hemispheric brain lesions, 4 to 20 months post stroke Age: 22 to 67 years with normal single stimulus detection, and seven normal age matched controls 8 males, 6 female stroke patients | |
| Interventions | Intervention group: single treatment of repetitive peripheral magnetic stimulation (RPMS) which generated muscle contractions in left index finger; left‐side attentional cueing (encouraging the participants to report the left side first) Control group: no interventions | |
| Outcomes | Outcome measure recorded at baseline and 30 minutes after initial testing or stimulation Quality Extinction Test (QET) ‐ 36 trials of simultaneous sensory presentation, accuracy of detection recorded | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Seven were randomly allocated to the experimental group." Comment: no details provided |
| Allocation concealment? | Unclear risk | Quote: "Seven were allocated randomly to the experimental group ... seven served as a patient control group. Seven age‐matched normal subjects ... served as normal controls in the extinction test." Comment: no details provided |
| Blinding? All outcomes | Unclear risk | Quote: none found Comment: blinding not reported |
| Incomplete outcome data addressed? All outcomes | Low risk | All participants accounted for at short‐term assessment, no long‐term outcomes planned |
| Free of selective reporting? | Low risk | All outcome measures planned were accounted for; only 1 outcome measure (quality extinction test) reported for all participants |
| Free of other bias? | Low risk | None noted |
Posteraro 2001
| Methods | RCT | |
| Participants | Country: Italy Setting: unknown 10 participants with right brain damage after their first stroke; 5 participants in each of experimental and control groups: "There were no statistical differences between EG (experimental) and CG (control) groups as regards mean age (EG: 64 years; CG: 72.4 years; t‐value 1.861, df 8, P ns) and time from onset (A: 5.2 weeks; B: 5 weeks; t‐value 0.064, df 8, P ns)" (unpublished data from study author) | |
| Interventions | Experimental group: rehabilitation protocol for tactile extinction plus physiotherapy as normal for 2 months (no intenstity of intervention was given) Rehabilitation protocol was a series of exercises aimed at improving tactile extinction and included 3 stages: single hand single object recognition, simple bilateral simultaneous stimuli recogntion, complex bilateral simultaneous stimuli recogntion Control group: physiotherapy as normal | |
| Outcomes | Outcome measures were given at baseline and after 2 months of treatment Motor Assessment: Motricity Index ‐ Trunk Control Test and Bisiach Test Functional Assessment: the Katz ADL & IADL indices and the Barthel Index were used Tactile Extinction Test | |
| Notes | Information was obtained via email from the study author who submitted outline of paper presented to conference in Venice in 2002 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Ten RBD subjects were enrolled. All the patients suffered for their first stroke. They were randomly divided in two subgroups: (1) five RBD subjects who were submitted to rehabilitation protocol for TE plus the usual physiotherapy after vascular hemiplegia (Experimental Group ‐ EG); (2) five RBD patients who followed usual rehabilitation programs for stroke, as the Control Group (CG)." Comment: methodology for randomization is unclear |
| Allocation concealment? | Unclear risk | Comment: no reporting of allocation concealment |
| Blinding? All outcomes | High risk | Comment: no comment about blinding; unlikely that there was either blinding of participants or therapists delivering intervention; no assessor blinding mentioned |
| Incomplete outcome data addressed? All outcomes | Low risk | Comment: all participants were accounted for |
| Free of selective reporting? | Low risk | Comment: all planned outcome measures were reported |
| Free of other bias? | Low risk | Comment: no other bias apparent |
Burridge 2002
| Methods | RCT | |
| Participants | Country: UK Setting: inpatient and outpatient setting 22 participants with hemiplegia from CVA within the last 12 months (mean 5.7 and 8.5 months post stroke both groups) Age range: 57 to 87 years 10 male (5 in each group) 12 female (6 in each group) Inclusion criteria: between 1 and 12 months post first stroke resulting in hemiplegia, medically stable, at least 18 years of age, evidence of sensory impairment, no previous pathology to the upper limb, ability to comply with assessment and treatment procedures, ability to give consent Exclusion criteria: cognitive or psychiatric problems affecting the ability to comply, history of cardiac problems, implanted cardiac pacemaker | |
| Interventions | Intervention group: electrical stimulation was applied to the elbow and forearm extensor muscle groups of the hemiplegic arm for 12 weeks (10 to 30 minutes twice per day) Control group: passive range of motion to elbow, wrist, fingers | |
| Outcomes | Outcome measures: administered at baseline, after 12 weeks of treatment, 12 weeks post‐intervention completion Action Research Arm Test 2‐point discrimination | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "recruitment subjects were randomly assigned to stimulation(treatment) and passive stretching (control) groups using computer generated sealed allocation codes" Comment: used computer‐generated random sequence generation |
| Allocation concealment? | Low risk | Quote: "sealed allocation codes" Comment: details not provided but authors do indicate concealment |
| Blinding? All outcomes | High risk | Quote: none found Comment: no blinding was reported |
| Incomplete outcome data addressed? All outcomes | Low risk | 24 participants were recruited to the study 2 withdrew before completion due to unrelated problems and were lost to follow‐up Comment: all |
| Free of selective reporting? | Low risk | All planned outcome measures were reported on in the Results section |
| Free of other bias? | Low risk | No other bias apparent |
Cambier 2003
| Methods | Multicenter RCT | |
| Participants | Country: Belgium Setting: inpatient rehabilitation hospital 23 participants (11 experimental (5 males, 6 females), 12 control (9 males, 3 females) Mean age: 61.1 years (SD 11.2) (control group); 63.9 years (SD 12.8) (experimental group) Days post stroke: mean 83 (control group) and 114 (experimental group) Inclusion criteria: first ever stroke, less than 1 year post stroke, clinically impaired upper limb sensation, no other neurological or orthopedic conditions present in the upper limb prior to stroke, ability to understand oral instructions, willingness to participate | |
| Interventions | Both groups received conventional therapy based on neurodevelopmental treatment for 4 weeks and interventions as follows: Experimental group: intermittent pneumatic compression to the hemiplegic arm for 30 minutes 5 times per week Control group: sham short‐wave treatment to hemiplegic shoulder for 30 minutes 5 times per week | |
| Outcomes | Assessment at baseline, 2 weeks (10 treatments) and 4 weeks (20 treatments) Nottingham Sensory Assessment Fugle Meyer Assessment Ashworth Scale Spasticity Visual Analogue for Pain | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Some basic demographic details ... as well as data referring to the stroke of the 23 patients who were randomly divided into an experimental group (n = 11) or a control group (n = 12)" Comment: sequence generation unspecified |
| Allocation concealment? | Unclear risk | Quote: "Some basic demographic details ... as well as data referring to the stroke of the 23 patients who were randomly divided into an experimental group (n = 11) or a control group (n = 12)." Comment: allocation concealment unspecified |
| Blinding? All outcomes | Low risk | Quote: "All clinical evaluations were performed by a trained physiotherapist different from the treating group, blinded for the given treatment" Comment: personnel responsible for outcome measures blinded but treating therapists and participants were unlikely to be blinded, though no comment was made regarding this in the text; it is possible this would impact subjective outcomes |
| Incomplete outcome data addressed? All outcomes | Low risk | Quote: "Both groups were evaluated 2 times over a period of 4 weeks ... baseline, after 10 treatments or 2 weeks, and at end of trial (4 weeks or 20 treatments)." Comment: no reports of lost data; sensory scores for all participants are shown in Table 3 of the published study |
| Free of selective reporting? | Low risk | Comment: text and tables include all outcome measures |
| Free of other bias? | Low risk | Comment: no apparent bias |
Byl 2003
| Methods | Randomized controlled cross‐over trial | |
| Participants | Country: USA Setting: unclear 21 post‐stroke patients Mean time post stroke: 5.1 years Mean age: 63 years (SD 9.4 with a range of 42 to 79 years) 12 males and 6 females completed the study Inclusion criteria: stroke (either right or left hemisphere) at least 6 months in duration, able to walk 100 feet with or without a cane, partially opened and closed the hand, partially elevated the shoulder and elbow against gravity (45 to 60 degrees), can speak conversational English Exclusion criteria: tramatic brain injury, degenerative neuromuscular disease, or serious musculoskelatal injury | |
| Interventions | Based on principles of neural adaptation Group A: 4 weeks sensory discrimination retraining then 4 weeks fine motor retraining Group B: 4 weeks fine motor retraining and then 4 weeks sensory discrimination retraining Each session 90 minsutes (also combination of guided mental imagery for 15 to 20 minutes and glove use on least affected limb 7 hours per day, home program) | |
| Outcomes | Outcome measures: baseline, 4 and 8 weeks, and then 3 months post treatment Sensory discrimination (kinesthesia, graphesthesia, stereognosis) Fine motor control (digital reaction time, performance time on Purdue Peg Board) Upper extremity strength and range of motion Functional independence (Wolf motor Function Test, Califormia Functional Evaluation, gait speed) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Subjects were randomly assigned" Comments: no specifics of sequence generation provided |
| Allocation concealment? | Unclear risk | Quote: "Subjects were randomly assigned" Comments: no mention of allocation concealment |
| Blinding? All outcomes | Low risk | Quote: "The evaluators were blinded to group assignment and a different evaluator readministered the tests at each assessment period. This controlled for the bias of retesting by the same evaluator" Comment: authors further state that "Where possible parallel test forms were administered at the beginning and the end of the study to minimize the bias of patient learning with retesting." Unlikely that therapist or participant blinding occurred |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Quote: "Twenty‐one subjects were admitted to the study. Three subjects (1 from group A and 2 from Group B) were dropped from the study due to unrelated medical or personal problems." Comment: data for all measures and 18 remaining patients are presented in Tables 5 and 6 of the published study for outcomes at 4 and 8 weeks 3‐month follow‐up consisted of only 10 participants (first 10 admitted to the study) Although data for the 10 are reported in table 6, no statement as to why all 18 did not complete 3‐month follow‐up (or which patients were not included in this long‐term follow‐up) |
| Free of selective reporting? | Low risk | All planned outcomes were commented on in the Results section |
| Free of other bias? | Unclear risk | Comment: this was a cross‐over design with potential carry‐over effects across treatments (over 8 week period) Figure 4 in the published study indicates that group B made significant gains in both fine motor and sensory discrim during second 4 weeks of treatment No control group for comparison Authors appropriately report other study limitations in Discussion section |
Wolny 2003
| Methods | RCT | |
| Participants | Country: Poland Setting: unclear 40 participants with stroke Age range 32 to 82 years Inclusion criteria: not described Exclusion criteria: not described | |
| Interventions | Intervention period unclear Treatment group: routine rehabilitation procedures and tensive mobilizations to median, ulnar, and radial nerves Control group: routine rehabilitation procedures | |
| Outcomes | Measurements taken pre‐intervention and post‐intervention 2‐point discrimination Thermesthesia | |
| Notes | Only brief details provided in abstract, several attempts to contact the authors for further details were unsuccessful | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "They were randomly divided into two groups" Comment: not enough details provided |
| Allocation concealment? | Unclear risk | Quote: "They were randomly divided into two groups" Comment: not enough details provided |
| Blinding? All outcomes | Unclear risk | Comment: not enough details provided |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Comment: no details of recruited or completing participants |
| Free of selective reporting? | High risk | Comment: the abstract provided only the significant data |
| Free of other bias? | Unclear risk | Comment: not enough details were provided to make a judgement |
Miller 2004
| Methods | Block RCT | |
| Participants | Country: Australia Setting: unclear 24 participants Inclusion criteria: within 6 weeks of cortical stroke Exclusion criteria: none reported | |
| Interventions | Intervention period 3 weeks daily Treatment group: task‐related training of upper limb emphasizing uni‐manual and bi‐manual functional activities Control group: exercises to improve postural control and concentration | |
| Outcomes | Assessed pre, post, and at 3‐month follow‐up Motor Assessment Scale Chedoke McMaster Stroke Assessment Stroke Adapted 30‐item Sickness Impact Profile Manual dexterity Muscle strength Sensation hand | |
| Notes | Attempted to contact authors for further details; authors declined to supply further details as they wanted to pursue further publications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "Subjects were recruited within six weeks of their first cortical stroke and randomly allocated to treatment (T) or control (C) intervention." Comment: sequence generation unspecified |
| Allocation concealment? | Unclear risk | Quote: "Subjects were recruited within six weeks of their first cortical stroke and randomly allocated to treatment (T) or control (C) intervention." Comment: allocation concealment unspecified |
| Blinding? All outcomes | Unclear risk | Quote: none applicable Comment: no details provided of whether there was blinding or not in the study of any of participants, therapists or study peronnel |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Comment: appears all participants recruited so far have been accounted for, unclear if further participants were being recruited |
| Free of selective reporting? | Unclear risk | Comment: not all results were provided in the summaries available to the review authors, not enough information was provided |
| Free of other bias? | Unclear risk | Comment: the results of this study are interim only and results may differ on completion of study; unable to make a judgement at this time |
Chen 2005
| Methods | RCT, standard treatment and discussion with therapist versus standard treatment and thermal stimulation | |
| Participants | Country: Taiwan Setting: inpatient rehabilitation center 29 participants 1 month post stroke Age: experimental group: mean 58.5 years (SD 12.9), control group mean 59.6 years (SD 12.0) Gender: experimental group 6 males, 9 females; control group 10 males, 4 females Inclusion criteria: first ever stroke less than 1 month ago, no cardiac or orthopedic problem prior to stroke, no cognitive impairment that impairs ability to follow directions, motor deficit of the upper limb under Brunnstrom stage IV Exclusion criteria: diabetic history or sensory impairment attributable to peripheral vascular disease or neuropathy, speech disorder or global aphasia | |
| Interventions | Intervention group: thermal stimulation program (stimulation, stimulus detection, arm withdrawal) 30 minutes daily 5 times per week for 6 weeks plus standard therapy Control group: 15 to 20 minutes 3 to 5 times per week for 6 weeks reviewing progress with therapist plus standard therapy | |
| Outcomes | Outcome measures: assessed weekly starting at baseline until intervention completed Brunnstrom stage Modified Motor Assessment Scale Grasp strength Wrist extension Wrist flexion Sensation by monofilament Tone with Ashworth Scale | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "Randomization was by computer‐generated random numbers held in sealed envelopes by an individual not involved in the study." |
| Allocation concealment? | Low risk | Quote: "Randomization was by computer‐generated random numbers held in sealed envelopes by an individual not involved in the study." |
| Blinding? All outcomes | Low risk | Quote: "The outcome measures were assessed weekly by the same physical and occupational therapists who were blinded to the group of subjects." Comment: authors state in discussion that it was not feasible to blind patients to treatment allocation; it would also not be possible to blind therapists |
| Incomplete outcome data addressed? All outcomes | Low risk | Quote: "46 patients ... initially enrolled in the study. Twenty‐nine subjects completed the treatment protocol ... Seventeen patients did not finish the experiment because of discharge from hospital, pulmonary infection, transfer to home‐care settings, or searching alternative Chinese medicine therapy." Comment: large percentage of patients lost to follow‐up (37%) and small sample size put study at risk for type II error (authors cite this as potential limitation in Discussion section) |
| Free of selective reporting? | Low risk | Comment: text, graphs, and tables include all outcome measures |
| Free of other bias? | Low risk | Comment: potential limitations discussed in the study |
Yozbatiran 2006
| Methods | Controlled clinical trial with alternate allocation | |
| Participants | Country: Turkey Setting: acute inpatient medical care at a univeristy hospital 36 stroke patients (18 intervention group, 18 control group) Age: intervention group mean 69.5 years (SD 14), control group mean 66.7 years (SD 11.2) Intervention group: 15 females 3 males; control group 6 females 12 males Days since stroke: 9.5 days (SD 3.6) intervention group; 9.8 days (SD 5.9) control group Inclusion criteria: first stroke, in an acute inpatient setting Exclusion criteria: potentially fatal heart arrhythmias, prior stroke with residual motor deficits, lower motor neuron lesion of the impaired extremity, uncontrolled hypertension, significant orthopedic or chronic pain conditions | |
| Interventions | Control group: 1 hour per day of neurodevelopment exercise for 10 days Intervention group: 1 hour per day of neurodevelopment exercise for 10 days plus received additional electrical stimulation of wrist and finger extensors for 1 hour per day | |
| Outcomes | Outcome measures: recorded at baseline and post intervention Kinesthesia Position sense Hand function test Hand movement scale | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Quote: "Controlled Clinical trial with alternate allocation" Quote: "Thirty six acute stroke subjects were assigned in ranked order" |
| Allocation concealment? | High risk | Alternate allocation was not concealed |
| Blinding? All outcomes | High risk | Quote "The following parameters were recorded at initial assessment and at discharge by the same experienced physiotherapist working with neurological patients" No indication of blinding |
| Incomplete outcome data addressed? All outcomes | Low risk | Quote: "There were no drop outs in the study" |
| Free of selective reporting? | Low risk | It is clear that the published reports include all of the study's pre‐specified outcomes |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias; some differences at baseline but these were in favor of the control group |
Acerra 2007
| Methods | Assessor‐blind RCT | |
| Participants | Country: Australia 40 participants randomized to 2 groups of 20 Mean age: 68 years 57% female 100% right‐side dominant 57% right‐side CVA Mean 5 days post stroke Inclusion criteria: within 2 weeks of first ischemic stroke as documented with CT Exclusion criteria: previous stroke, trauma affecting the upper limbs, vision and hearing deficits, unable to sit in a chair for 1 hour, MMSE < 22/30, major comorbidities | |
| Interventions | 14 days with 20 to 30 minutes mirror or sham therapy and 1 to 2 hours 5 times per week of therapy as usual Intervention group: mirror therapy plus therapy as normal; completed sensory motor tasks inside mirror box that provided visual feedback of bilateral simulataneous hand movements Control group: sham therapy plus therapy as normal; completed sensory motor tasks inside sham box with no mirror providing visual feedback of unilateral activity Sensory motor tasks included: grip strengthening tasks, AROM, sensory discrimination tasks, functional hand tasks | |
| Outcomes | Blinded assessors with observations recorded at baseline, and post intervention (2 weeks and 6 weeks from baseline) Synchiria Pain Quanitative Sensory Test (light touch, punctate touch, thermal and pressure pain thresholds) Affected hand grip strength Motor Assessment Scale: upper limb portion | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "Recruited participants firstly underwent the upper limb sensorimotor assessment. Afterwards, patients were randomised into two groups with a computer‐generated randomisation." |
| Allocation concealment? | Low risk | Quote: "Randomisation was performed by an independent investigator and was concealed from the investigator at a remote site." |
| Blinding? All outcomes | Low risk | Participants not blinded even though attempts were made to reduce differences in expectation Quote: "The investigator was made aware of the patient‐group on the first day of treatment by telephone or email. Participants knew which group they were in but were under the impression that both mirror and sham therapy were expected to generate similar outcomes. Assessors were blinded. Post‐treatment outcome measures were taken by one of two experienced physiotherapists with at least five years of clinical stroke experience ... Post‐treatment outcome measures were performed by the same assessors who were blinded to the experimental group; outcome measures were taken after the intervention (i.e. after two weeks of treatment)" |
| Incomplete outcome data addressed? All outcomes | Low risk | All participants and data accounted for |
| Free of selective reporting? | Low risk | The thesis details all pre‐planned outcomes |
| Free of other bias? | Low risk | None noted |
ADL: activities of daily living AROM: active range of motion CT: computerized tomography CVA: cerebrovascular accident IADL: instrumental activities of daily living MMSE: Mini Mental State Examination RCT: randomized controlled trial SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brogardh 2006 | Focus was motor retraining although there was a sensory outcome measure |
| Carey 1993 | Not an RCT; single participant design |
| Liu 2002 | This study had fewer than 50% of participants with stroke |
| Van Vliet 2005 | RCT; focus was on motor intervention even though there was a sensory outcome measure |
| Yekutiel 1993 | Did not have randomization |
RCT: randomized controlled trial
Characteristics of ongoing studies [ordered by study ID]
Ben‐Shabat 2005
| Trial name or title | A brain activation study of limb position sense in stroke affected inviduals with and without sensory training and in healthy aged |
| Methods | fMRI will be used to study areas of brain activation During scans participants will perform wrist position tasks with varying degrees of attention Healthy participants will undergo 1 scan while stroke participants will be scanned 3 times (scans will be timed 4 weeks apart) Between the second and third scans stroke participants will be randomly allocated to receive either sensory training (treatment condition) or a sensory exposure (control condition) |
| Participants | Country: Australia Stroke patients and healthy participants No further details were available |
| Interventions | Intervention group: sensory retraining for 4 weeks Control group: sensory exposure for 4 weeks |
| Outcomes | Outcome measures: baseline, 4 weeks, 8 weeks fMRI Unclear of others |
| Starting date | 2005 |
| Contact information | Principal investigator: Ettie Ben Shabbat MPT, Center of Clinical Research Excellence, University of Melbourne, PhD Candidate, School of Occupational Therapy, and School of Psychological Sciences at La Trobe University National Stroke Research Institute, Heidelberg Repatriation Hospital. Melbourne Australia ebshabat@ nsri.org.au lcarey@nsri.org.au |
| Notes |
Carey 2004
| Trial name or title | IN‐TOUCH: brain adaptation associated with spontaneous and training‐induced recovery of touch sensation post‐stroke |
| Methods | RCT |
| Participants | Post stroke patients |
| Interventions | The aim of this project is to locate and compare areas of brain activation associated with spontaneous (study 1) and training‐induced (study 2) recovery of touch sensation following stroke, using serial fMRI Intervention involves stimulus‐specific training of touch sensation and includes graded presentation of stimuli, active exploration, feedback and calibration of sensations Training is conducted for 15 x 45 minute sessions over a 6‐week interval The control condition involves exposure to similar touch stimuli over the same number of sessions but does not include training principles |
| Outcomes | Primary outcome measures are assessed between 1 and 6‐month scans post‐stroke for study 1 and between 6 and 7.5‐month scans post‐stroke for study 2 Primary outcome measure 1: the primary outcome is change in intensity, particularly in ipsilesional primary somatosensory cortex and bilateral secondary somatosensory cortex Primary outcome measure 2: the primary outcome is the extent of activation, particularly in ipsilesional primary somatosensory cortex and bilateral secondary somatosensory cortex Secondary outcome measures are assessed at 1, 3, 4.5, 6 and 7.5 months post‐stroke for secondary measure 1 and at 1, 6 and 7.5 months for secondary measure 2 Secondary outcome measure 1: clinical measures of touch sensation, i.e texture discrimination using the Tactile Discrimination Test and detection of touch pressure using the WEST hand monofilaments Secondary outcome measure 2: neurological function will be measured using the Neurological Institute Stroke Scale and activities of daily living using the Barthel Index |
| Starting date | 1 January 2004 |
| Contact information | Professor Leeanne Carey National Stroke Research Institute, Austin Health Repatriation Campus, Neurosciences Building 300 Waterdale Road, Heidelberg Heights, VIC 3081, Australia Phone: +61 3 94962586 Email: lcarey@nsri.org.au |
| Notes |
Carey 2005
| Trial name or title | SENSE: effectiveness of training somatosensation in the hand after stroke: a randomized controlled trial |
| Methods | RCT |
| Participants | Post‐stroke patients |
| Interventions | The experimental intervention (EI) will comprise 10 sessions of generalized discrimination training of texture discrimination, limb position sense and tactual object recognition. Sessions are 60 to 90 minutes duration and are conducted 3 times per week. Group A will receive 2 phases of EI. Group B will receive 1 phase of control intervention (CI) followed by 1 phase of EI; the CI will comprise 10 sessions of exposure to sensory stimuli |
| Outcomes | Conducted at baseline, 6 weeks and 6 months Primary outcome 1: multiscale score of sensory discrimination: texture discrimination, limb position sense, tactile object recognition Primary outcome 2: hand function in self care (Sequential Occupational Dexterity Assessment) Secondary outcome 1: actual use of the upper limb in life situations will be measured using the Upper Extremity Motor Activity Log (UE/MAL) Secondary outcome 2: the Barthel Index (BI) |
| Starting date | 1 March 2002 |
| Contact information | Professor Leeanne Carey National Stroke Research Institute, Austin Health Repatriation Campus, Neurosciences Building 300 Waterdale Road, Heidelberg Heights, VIC 3081, Australia Phone: +61 3 94962586 Email: lcarey@nsri.org.au |
| Notes |
fMRI: functional magnetic resonance imaging RCT: randomized controlled trial
Contributions of authors
Susan Doyle: conceiving, designing, and co‐ordinating the review; designing search strategies; undertaking searches; screening search results; organizing the retrieval of papers; screening retrieved papers against inclusion criteria; appraising the quality of papers and extracting data; writing to study authors for additional information; providing additional data about papers; obtaining and screening data for unpublished studies; data management for the review; analysis and interpretation of the data (providing a methodological and clinical perspective); and writing the review.
Sally Bennett: screening retrieved papers against inclusion criteria, appraising the quality of papers and extracting data, interpretation of the data (providing a methodological, clinical, and policy perspective), and contributing to the writing of the review.
Susan Fasoli: designing the review, designing search strategies, screening search results, appraising quality of papers and extracting data, interpretation of the data (providing a methodological and clinical perspective), and contributing to the writing of the review.
Kryss McKenna (deceased April 2009): designing the review, designing search strategies, screening search results, screening retrieved papers against inclusion criteria. Substantive changes were made by the other authors to the analysis and interpretation of the data and writing of the review.
Sources of support
Internal sources
Southwest Washington Medical Center, USA.
University of Queensland, Australia.
External sources
No sources of support supplied
Declarations of interest
None known. Dr Kryss McKenna (deceased April 2009) had no known declarations of interest listed in the previously published protocol.
New
References
References to studies included in this review
- Acerra NE. Sensorimotor Dysfunction in CRPS1 and Stroke: Characterisation, Prediction and Intervention. Doctor of Philosophy Degree Thesis. University of Queensland, 2007. [Google Scholar]; Acerra NE, Souvlis T, Moseley G. Does mirror‐box therapy improve sensory and motor changes in the early post‐stroke population? A randomised controlled trial. Australian Journal of Physiotherapy 2005;51(4‐e Suppl):S7. [Google Scholar]
- Burridge JH, Mann GE, Malone L, Taylor PN. A randomised controlled pilot study to investigate the effect of neuromuscular electrical stimulation on upper limb function following stroke. Proceedings of the 3rd World Congress in Neurological Rehabilitation April 2‐ 6. 2002. [Google Scholar]; Burridge JH, Mann GE, Malone L, Taylor PN. A randomized controlled pilot study to investigate the effects of neuromuscular electrical stimulation on upper limb function following stroke. Neurorehabiliation and Neural Repair 2002;16(1):11. [Google Scholar]; Mann G, Burridge JH, Malone LJ, Strike P. A pilot study to investigate the effects of electrical stimulation on recovery of hand function and sensation in subacute stroke patients. Neuromodulation 2005;3:193‐202. [DOI] [PubMed] [Google Scholar]; Mann GE, Malone LJ, Taylor PN, Burridge JH. A randomised controlled pilot study to investigate the effect of neuromuscular electrical stimulation on upper limb function and hand sensation following stroke. 1st Annual Conference of FESnet September 2‐3. 2002. [Google Scholar]
- Byl N, Roderick J, Mohamed O, Hanny M, Kotler J, Smith A, et al. Effectiveness of sensory motor rehabilitation of the upper limb following the principles of neuroplasticity: patients stable poststroke. Neurorehabilitation and Neural Repair 2003;17(3):176‐91. [DOI] [PubMed] [Google Scholar]
- Cambier DC, Corte E, Danneels LA, Witvrouw EE. Treating sensory impairments in the post‐stroke upper limb with intermittent pneumatic compression. Results of a preliminary trial. Clinical Rehabilitation 2003;17:14‐20. [DOI] [PubMed] [Google Scholar]
- Chen JC, Liang CC, Shaw FZ. Facilitation of sensory and motor recovery by thermal intervention for the hemiplegic upper limb in acute stroke patients. Stroke 2005;36(12):2665‐9. [DOI] [PubMed] [Google Scholar]
- Feys HM, Weerdt WJ, Selz BE, Cox Steck GA, Spichiger R, Vereeck LE, et al. Effect of a therapeutic intervention for the hemiplegic upper limb in the acute phase after stroke: a single‐blind, randomized, controlled multicenter trial. Stroke 1998;29(4):785‐92. [DOI] [PubMed] [Google Scholar]
- Heldman B, Kerkhoff G, Struppler A, Havel P, Jahn T. Repetitive peripheral magnetic stimulation alleviates tactile extinction. Cognitive Neuroscience and Neuropsychology: NeuroReport 2000;11(14):3193‐8. [DOI] [PubMed] [Google Scholar]
- Jongbloed L, Stacey S, Brighton C. Stroke rehabilitation: sensorimotor integrative treatment versus functional treatment. American Journal of Occupational Therapy 1989;43(6):391‐7. [DOI] [PubMed] [Google Scholar]
- Galea M, Miller K, Phillips B. A multi‐centre randomised controlled study of early task‐related training of the upper limb following stroke. Cerebrovascular Diseases 2006;21(S4):3. [Google Scholar]; Galea MP, Miller KJ, Phillips BA. A multi‐centre randomised controlled pilot study of intensive task‐related training of the upper limb following stroke. Journal of Clinical Neuroscience 2005;12(3):335. [Google Scholar]; Miller K, Galea M, Kilbreath S. Early task‐related upper limb training is effective following stroke. Stroke 2000;31(11):2816. [Google Scholar]; Miller KJ, Galea MP, Kilbreath SL, Philips BA. Early intensive task‐specific sensory and motor training of the upper limb after acute stroke: a pilot study. Neurorehabilitation and Neural Repair 2001;15(4):345‐6. [Google Scholar]; Miller KJ, Galea MP, Kilbreath SL, Phillips BA. Early intensive task‐specific sensory and motor training of the upper limb following acute stroke: a pilot study. Proceedings of the 3rd World Congress in Neurological Rehabilitation April 2‐6. 2002. [Google Scholar]; Miller KJ, Galea MP, Phillips BA. A multi‐centre randomised controlled pilot study of intensive task‐related training of the upper limb following acute stroke. Australian Journal of Physiotherapy 2003;49(4s):s16. [Google Scholar]; Miller KJ, Galea MP, Phillips BP. A multi‐center randomized controlled study of intensive task related training of the upper limb. Stroke 2004;35(6):e316. [Google Scholar]
- Poole JL, Whitney SL, Hangeland N, Baker C. The effectiveness of inflatable pressure splints on motor function in stroke patients. Occupational Therapy Journal of Research 1990;10(6):360‐6. [Google Scholar]
- Posteraro L, Corsini D, Bidini C, Bassoli M, Curti M, Grassi E. The rehabilitation of tactile extinction. NeuroRehabilitation and Neural Repair 2001;15(4):345. [Google Scholar]
- Wolny T, Saulicz E, Gnat R, Bacik B. The evaluation of the efficacy of tensive mobilisations of the upper extremity peripheral nerves in therapy of the sensory disorders in hemiplegic patients ‐ a preliminary study. 14th International Congress of The World Confederation for Physical Therapy June 7‐12. 2003. [Google Scholar]
- Yozbartiran N, Donmez B, Kayak N, Bozan O. Electrical stimulation of wrist and fingers for sensory and functional recovery in acute hemiplegia. Clinical Rehabilitation 2006;20:4‐11. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Brogardh C, Sjolund B. Constraint induced movement therapy in patients with stroke: a pilot study on effects of small group training and of extended mitt use. Clinical Rehabilitation 2006;20:218‐27. [DOI] [PubMed] [Google Scholar]
- Carey L, Matyas T, Oke L. Sensory loss in stroke patients: effective training of tacticle and proprioceptive discrimination. Archives of Physical Medicine and Rehabilitation 1993;74(6):602‐11. [DOI] [PubMed] [Google Scholar]
- Liu W, Lipsitz LA, Montero‐Odasso M, Bean J, Kerrigan DC, Collins JJ. Noise‐enhanced vibrotactile sensitivity in older adults, patients with stroke, and patients with diabetic neuropathy. Archives of Physical Medicine and Rehabilitation 2002;83:171‐6. [DOI] [PubMed] [Google Scholar]
- Vliet P, Lincoln N, Foxall A. Comparison of Bobath based and movement science based treatment for stroke: a randomized controlled trial. Journal of Neurology, Neurosurgery, and Psychiatry 2005;76:503‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekutiel M, Guttman E. A controlled trial of the retraining of the sensory function of the hand in stroke patients. Journal of Neurology, Neurosurgery, and Psychiatry 1993;56:241‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
- Ben‐Shabat E, Carey L, Brotchie PR. A brain activation study of limb position sense in stroke affected individuals with and without sensory training and in healthy aged. Royal Australian College of Physicians2006. ; Ben‐Shabat E, Carey LM, Matyas TA, Brotchie PR. A brain activation study of limb position sense in stroke affected individuals, with and without sensory training, and in healthy aged. Internal Medicine Journal 2006;36:A14. [Google Scholar]
- Carey L. Brain adaptation associated with spontaneous and training‐induced recovery of touch sensation post‐stroke. Australian New Zealand Clinical Trials Registry2004.
- Carey L. Effectiveness of training somatosensation in the hand after stroke: a randomized controlled trial. Australian New Zealand Clinical Trials Registry2002. ; Carey L. SENSE (Study of effectiveness of Neurorehabilitation on Sensation after Stroke): a randomized controlled trial. Internal Medicine Journal 2007;37 Suppl 1:A12. [Google Scholar]
Additional references
- Aruin AS. Support‐specific modulation of grip force in individuals with hemiparesis. Archives of Physical Medicine and Rehabilitation 2005;86(4):768‐75. [DOI] [PubMed] [Google Scholar]
- American Stroke Association. Impact of stroke. http://www.strokeassociation.org/presenter.jhtml?identifier=10332006.
- Aboriginal Stroke Project Steering Committee. National stroke unit program: Aboriginal stroke project. National Stroke Foundation of Australia, 2004. [Google Scholar]
- Blennerhassett JM, Matyas TA, Carey LM. Impaired discrimination of surface friction contributes to pinch grip deficit after stroke. Neurorehabilitation and Neural Repair 2007;21:263‐72. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H. Neuroanatomy Through Clinical Cases. Sunderland, MA: Sinauer Associates, 2002. [Google Scholar]
- Bohannon RW. Evaluation and treatment of sensory and perceptual impairments following stroke. Topics in Geriatric Rehabilitation 2003;19(2):87‐97. [Google Scholar]
- Campbell WW, DeJong RN, Haerer AF. DeJong's The Neurological Examination. 6th Edition. Philadelphia, PA: Lippincott, Williams, and Wilkins, 2005. [Google Scholar]
- Carey M, Matyas T, Oke L. Sensory loss in stroke patients: effective training of tactile and proprioceptive discrimination. Archives of Physical Medicine and Rehabilitation 1993;74(6):602‐11. [DOI] [PubMed] [Google Scholar]
- Carey L. Somatosensory loss after stroke. Critical Reviews in Physical and Rehabilitation Medicine 1995;7(1):51‐91. [Google Scholar]
- Chang JJ, Tsau JC, Lin YT. Predictors of shoulder subluxation in stroke patients. Gaoxiong Yi Xue Ke Xue Za Zhi 1995;11(5):250‐6. [PubMed] [Google Scholar]
- Connell LA, Lincoln NB, Radford KA. Somatosensory impairment after stroke: frequency of different deficits and their recovery. Clinical Rehabilitation 2008;22:758‐67. [DOI] [PubMed] [Google Scholar]
- Cramer S, Nelles G, Benson R, Kaplan J. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke 1997;28(12):2518. [DOI] [PubMed] [Google Scholar]
- Dannenbaum RM, Jones LA. The assessment and treatment of patients who have sensory loss following cortical lesions. Journal of Hand Therapy 1993;6(2):130‐8. [DOI] [PubMed] [Google Scholar]
- Desrosiers J, Noreau L, Rochette A, Bourbonnais D, Bravo G, Bourget A. Predictors of long term participation after stroke. Disability and Rehabilitation 2006;28(4):221‐30. [DOI] [PubMed] [Google Scholar]
- Eaves Y. 'What happened to me?': rural African American elders' experiences of stroke. Journal of Neuroscience Nursing 2000;32:37‐42. [PubMed] [Google Scholar]
- Gamble GE, Barberan E, Bowsher D, Tyrrell PJ, Jones AK. Post stroke shoulder pain: more common than previously realized. European Journal of Pain 2000;4(3):313‐5. [DOI] [PubMed] [Google Scholar]
- Gilroy J. Basic Neurology. 3rd Edition. New York: McGraw Hill, 2000. [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
- Hopman W, Verner J. Quality of life during and after inpatient stroke rehabilitation. Stroke 2003;34(3):801‐5. [DOI] [PubMed] [Google Scholar]
- Heart and Stroke Association of Ontario. Management of the post stroke arm and hand: treatment recommendations of the 2001 consensus panel. http://209.5.25.171/ClientImages/1/PostStrokeArmandHandFinal2002.pdf.
- Heart and Stroke Foundation of Canada. General information ‐ Stroke statistics. http://ww1.heartandstroke.ca/Page.asp?PageID=33&ArticleID=428&Src=strokeFrom=SubCategory 428&Src=strokeFrom=SubCategory.
- Hunter SM, Crome P. Hand function and stroke. Reviews in Clinical Gerontology 2002;12(1):66‐81. [Google Scholar]
- Kim J, Choi‐Kwon S. Discriminative sensory dysfunction after unilateral stroke. Stroke 1996;27:677‐82. [DOI] [PubMed] [Google Scholar]
- Nock M, Janis I, Wedig M. Research designs. In: Nezu A, Nezu C editor(s). Evidence‐Based Outcome Research. A Practical Guide to Conducting Randomized Controlled Trials for Psychosocial Interventions. Oxford: Oxford University Press, 2007. [Google Scholar]
- Nowak DA, Grefkes C, Dafotakis M, Kust J, Karbe H, Fink GR. Dexterity is impaired at both hands following unilateral subcortical middle cerebral artery stroke. European Journal of Neuroscience 2007;25:3173‐84. [DOI] [PubMed] [Google Scholar]
- National Stroke Foundation ‐ Australia. All about stroke. http://www.strokefoundation.com.au/.
- Pendlebury S, Rothwell P, Algra A, Ariesen M, Bakac G, Czlonkowska A, et al. Underfunding of stroke research: a Europe‐wide problem. Stroke 2004;35:2368. [DOI] [PubMed] [Google Scholar]
- Rand D, Weiss PLT, Gottlieb D. Does proprioceptive loss influence recovery of the upper extremity after stroke?. Neurorehabilitation and Neural Repair 1999;13(1):15‐21. [Google Scholar]
- Rand D, Gottlieb D, Weiss P. Recovery of patients with a combined motor and proprioception deficit during the first six weeks of post stroke rehabilitation. Physical and Occupational Therapy in Geriatrics 2001;18(3):69‐87. [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
- Robertson SL, Jones LA. Tactile sensory impairments and prehensile function in subjects with left hemisphere cerebral lesions. Archives of Physical Medicine and Rehabilitation 1994;75(10):1108‐17. [DOI] [PubMed] [Google Scholar]
- Rose L, Bakal DA, Fung TS, Farn P, Weaver LE. Tactile extinction and functional status after stroke. A preliminary investigation. Stroke 1994;25(10):1973‐6. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. Meta‐analytic procedures for social research: applied social research methods series Volume 6. Newbury Park, CA: Sage Publications, 1991. [Google Scholar]
- Schabrun SM, Hillier S. Evidence for the retraining of sensation after stroke: a systematic review. Clinical Rehabilitation 2009;23:27‐39. [DOI] [PubMed] [Google Scholar]
- Suethanapornkul S, Kuptniratsaikul PS, Kuptniratsaikul V, Uthensut P, Dajpratha P, Wongwisethkarn J. Poststroke shoulder subluxation and shoulder pain: a cohort multicenter study. Journal of the Medical Association of Thailand 2008;91(12):1885‐93. [PubMed] [Google Scholar]
- Tyson SF, Hanley M, Chillala J, Selley AB, Tallis RC. Sensory loss in hospital admitted people with stroke: characteristics, associated factors, and relationship with function. Neurorehabilitation and Neural Repair 2008;22:166‐72. [DOI] [PubMed] [Google Scholar]
- Lee JH, Wagenaar RC, Lankhorst GJ, Vogelaar TW, Deville WL, Bouter LM. Forced use of the upper extremity in chronic stroke patients: results from a single‐blind randomized clinical trial. Stroke 1999;30(11):2369‐75. [DOI] [PubMed] [Google Scholar]
- Welmer AK, Holmqvist LW, Sommerfled DK. Limited fine hand use after stroke and its association with other disabilities. Journal of Rehabilitation Medicine 2008;40:603‐8. [DOI] [PubMed] [Google Scholar]
- Yekutiel M. Sensory Re‐education of the Hand after Stroke. Philadelphia: Whurr, 2000. [Google Scholar]


