Abstract
Background
Inhaled nitric oxide (iNO) is effective in term infants with hypoxic respiratory failure. The pathophysiology of respiratory failure and the potential risks of iNO differ substantially in preterm infants, necessitating specific study in this population.
Objectives
To determine effects of treatment with inhaled nitric oxide (iNO) on death, bronchopulmonary dysplasia (BPD), intraventricular haemorrhage (IVH) or other serious brain injury and on adverse long‐term neurodevelopmental outcomes in preterm newborn infants with hypoxic respiratory failure.
Owing to substantial variation in study eligibility criteria, which decreases the utility of an overall analysis, we divided participants post hoc into three groups: (1) infants treated over the first three days of life because of defects in oxygenation, (2) preterm infants with evidence of pulmonary disease treated routinely with iNO and (3) infants treated later (after three days of age) because of elevated risk of BPD.
Search methods
We used standard methods of the Cochrane Neonatal Review Group. We searched MEDLINE, Embase, Healthstar and the Cochrane Central Register of Controlled Trials in the Cochrane Library through January 2016. We also searched the abstracts of the Pediatric Academic Societies.
Selection criteria
Eligible for inclusion were randomised and quasi‐randomised studies in preterm infants with respiratory disease that compared effects of iNO gas versus control, with or without placebo.
Data collection and analysis
We used standard methods of the Cochrane Neonatal Review Group and applied the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the quality of evidence.
Main results
We found 17 randomised controlled trials of iNO therapy in preterm infants. We grouped these trials post hoc into three categories on the basis of entry criteria: treatment during the first three days of life for impaired oxygenation, routine use in preterm babies along with respiratory support and later treatment for infants at increased risk for bronchopulmonary dysplasia (BPD). We performed no overall analyses.
Eight trials providing early rescue treatment for infants on the basis of oxygenation criteria demonstrated no significant effect of iNO on mortality or BPD (typical risk ratio (RR) 0.94, 95% confidence interval (CI) 0.87 to 1.01; 958 infants). Four studies examining routine use of iNO in infants with pulmonary disease reported no significant reduction in death or BPD (typical RR 0.94, 95% CI 0.87 to 1.02; 1924 infants), although this small effect approached significance. Later treatment with iNO based on risk of BPD (three trials) revealed no significant benefit for this outcome in analyses of summary data (typical RR 0.92, 95% CI 0.85 to 1.01; 1075 infants).
Investigators found no clear effect of iNO on the frequency of all grades of IVH nor severe IVH. Early rescue treatment was associated with a non‐significant 20% increase in severe IVH.
We found no effect on the incidence of neurodevelopmental impairment.
Authors' conclusions
iNO does not appear to be effective as rescue therapy for the very ill preterm infant. Early routine use of iNO in preterm infants with respiratory disease does not prevent serious brain injury or improve survival without BPD. Later use of iNO to prevent BPD could be effective, but current 95% confidence intervals include no effect; the effect size is likely small (RR 0.92) and requires further study.
Plain language summary
Inhaled nitric oxide for respiratory failure in preterm infants
Review question: Does giving inhaled nitric oxide gas to preterm infants with pulmonary disease improve survival without long‐term brain or lung injury?
Background: Breathing failure in preterm newborn babies may be complicated by raised pressure within the vessels that carry blood to the lungs (pulmonary hypertension). Mechanically assisted ventilation is used to support such infants, and sedative medications may be given. Inhaled nitric oxide gas (iNO) helps regulate muscle tone in the arteries of the lungs and decreases pulmonary hypertension; therefore, it may reduce the need for assisted ventilation, leading to less lung injury. However, iNO has effects on platelet function and is believed to potentially increase bleeding (haemorrhage), in particular bleeding into the brain.
Study characteristics: Review authors found 17 randomised controlled trials of iNO in the preterm newborn through searches updated until January 2016. These trials studied preterm babies with very different baseline characteristics; therefore, we decided to divide them into three groups: (1) trials of babies treated in the first few days of life with severe lung disease, (2) studies providing treatment after the first few days of life to babies who were at increased risk of chronic lung disease and (3) trials providing routine early treatment for babies who experienced respiratory distress.
Key results: In none of the three groups of trials did iNO improve survival, and no consistent evidence suggests that iNO decreases lung injury. Studies in group 1 (early rescue treatment) reported a 20% increase in severe bleeding into the brain. This finding was close to statistically significant. The quality of the evidence was moderate to high.
This review of studies found that inhaled nitric oxide therapy does not appear to improve the chances of improved outcomes for preterm infants with pulmonary disease. When given to babies who were very ill, iNO did not seem to help and may have contributed to increased risk of intracranial haemorrhage.
Summary of findings
Summary of findings for the main comparison. Inhaled NO compared with control for respiratory failure in preterm infants.
| Inhaled NO compared with control for respiratory failure in preterm infants | ||||||
| Patient or population: respiratory failure in preterm infants Setting: neonatal intensive care units Intervention: inhaled NO Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with Inhaled NO | |||||
| Death before discharge ‐ Studies with entry before 3 days based on oxygenation | Study population | RR 1.02 (0.89 to 1.18) | 1066 (10 RCTs) | ⊕⊕⊕⊕ High | ||
| 394 per 1000 | 402 per 1000 (351 to 465) | |||||
| Death before discharge ‐ Studies with entry after 3 days based on BPD risk | Study population | RR 1.18 (0.81 to 1.71) | 1075 (3 RCTs) | ⊕⊕⊕⊕ High | ||
| 83 per 1000 | 98 per 1000 (67 to 141) | |||||
| Death before discharge ‐ Studies of routine use in preterm infants on respiratory support | Study population | RR 0.90 (0.74 to 1.10) | 1924 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | ||
| 170 per 1000 | 153 per 1000 (126 to 187) | |||||
| Death or bronchopulmonary dysplasia at 36 weeks ‐ Studies with entry before 3 days based on oxygenation | Study population | RR 0.94 (0.87 to 1.01) | 958 (8 RCTs) | ⊕⊕⊕⊕ High | ||
| 743 per 1000 | 698 per 1000 (646 to 750) | |||||
| Death or bronchopulmonary dysplasia at 36 weeks ‐ Studies with entry after 3 days based on BPD risk | Study population | RR 0.92 (0.85 to 1.01) | 1075 (3 RCTs) | ⊕⊕⊕⊕ High | ||
| 667 per 1000 | 614 per 1000 (567 to 674) | |||||
| Death or bronchopulmonary dysplasia at 36 weeks ‐ Studies of routine use in preterm infants on respiratory support | Study population | RR 0.94 (0.87 to 1.02) | 1924 (4 RCTs) | ⊕⊕⊕⊕ High | ||
| 548 per 1000 | 515 per 1000 (477 to 559) | |||||
| Intraventricular haemorrhage (grade 3 or 4) ‐ Studies with entry before 3 days based on oxygenation | Study population | RR 1.20 (0.98 to 1.47) | 773 (6 RCTs) | ⊕⊕⊕⊕ High | ||
| 231 per 1000 | 278 per 1000 (227 to 340) | |||||
| Intraventricular haemorrhage (grade 3 or 4) ‐ Studies of routine use in preterm infants on respiratory support | Study population | RR 0.89 (0.73 to 1.09) | 1913 (4 RCTs) | ⊕⊕⊕⊝ Moderateb | ||
| 127 per 1000 | 113 per 1000 (93 to 139) | |||||
| Neurodevelopmental disability ‐ Studies with entry before 3 days based on oxygenation | Study population | RR 1.05 (0.78 to 1.40) | 208 (2 RCTs) | ⊕⊕⊕⊝ Moderatec | ||
| 455 per 1000 | 477 per 1000 (355 to 636) | |||||
| Neurodevelopmental disability ‐ Studies with entry after 3 days based on BPD risk | Study population | RR 0.90 (0.74 to 1.09) | 498 (2 RCTs) | ⊕⊕⊕⊝ Moderatec | ||
| 480 per 1000 | 432 per 1000 (355 to 523) | |||||
| Neurodevelopmental disability ‐ Studies of routine use in preterm infants on respiratory support | Study population | RR 0.91 (0.74 to 1.13) | 1223 (3 RCTs) | ⊕⊕⊕⊕ High | ||
| 195 per 1000 | 178 per 1000 (144 to 220) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aHighly variable risk ratio in individual trials (I2 = 50%).
bHighly variable risk ratio in individual trials (I2 = 33%).
cBased on 2 studies, wide confidence intervals.
Background
Description of the condition
Respiratory failure in the preterm newborn may be complicated by pulmonary hypertension (Walther 1992). Conventional therapy for pulmonary hypertension involves respiratory support, which includes assisted ventilation and continuous distending pressure, administration of surfactant and sedation and muscle relaxation if necessary. The systemic circulation is often supported with inotropic agents in the sickest infants. Since the time surfactant was introduced, mortality from respiratory failure in preterm infants has fallen significantly (Soll 2001). However, some infants do not show adequate improvement in oxygenation after receiving surfactant treatment. Preterm infants receiving mechanical respiratory support are at increased risk of bronchopulmonary dysplasia (BPD) as the result of lung injury, which may have a significant long‐term impact.
Description of the intervention
Regulation of vascular muscle tone at the cellular level occurs via nitric oxide (NO) (Abman 1990). Nitric oxide is generated enzymatically from L‐arginine by one of three NO synthases. NO activates guanylyl cyclase by binding to its heme component, leading to the production of cyclic guanosine monophosphate (cGMP), which causes vasodilation (Finer 1998). NO gas can be mixed with inspired gases and delivered directly to the peripheral airways, where it may cause local vasodilation.
How the intervention might work
Pulmonary vascular resistance of foetal animals is regulated by endogenous (Cornfield 1992) or exogenous NO (Kinsella 1992). In several newborn animal models, pulmonary hypertension was reversed by inhaled exogenous nitric oxide (Frostell 1991; Roberts 1993). In general, researchers have noted little or no effect of inhaled nitric oxide (iNO) on the systemic circulation (Finer 1998). Studies in adults and in full‐term human infants have confirmed that iNO causes selective pulmonary vasodilation, reducing pulmonary arterial pressure and improving ventilation/perfusion mismatch. In adult respiratory distress syndrome (ARDS), reduced pulmonary arterial pressure and decreased intrapulmonary shunting occur within 40 minutes of iNO treatment (Rossaint 1993). In these patients, the major benefit was likely due to improvement in ventilation/perfusion matching because iNO causes vasodilation only in ventilated lung units.
Preterm animals with hyaline membrane disease have elevated pulmonary vascular resistance, and pulmonary artery pressure and oxygenation may be improved by iNO (Kinsella 1994; Skimming 1995). These animal models are very similar to preterm human neonates with respiratory failure. In the term neonate with hypoxic respiratory failure, iNO decreases the requirement for extracorporeal membrane oxygenation (ECMO) but does not decrease overall mortality (Finer 2000).
Owing to differences in pathophysiology, different entry criteria and different outcomes, treatment results in term infants cannot be extrapolated to preterm infants. Entry criteria used for studies of iNO in the term neonate have different implications for the preterm neonate because, at the same levels of the oxygenation index (OI), mortality is much higher among preterm than term infants.
Although pulmonary artery pressure is increased in preterm infants with respiratory failure, this elevation in pulmonary artery pressure is rarely sufficient to cause reversal of the ductal shunt. Therefore, the haemodynamic profile differs somewhat from that of the term neonate. ECMO is not used for preterm infants owing to concerns regarding haemorrhagic complications; therefore, the requirement for ECMO cannot be used as an outcome criterion.
Animal models of BPD suggest that prolonged treatment with iNO may modify lung injury (Bland 2005). Inhaled nitric oxide has effects that may reduce pulmonary inflammation and improve vascular remodeling in the preterm lung with acute injury.
Preterm infants are at risk of long‐term pulmonary disability associated with BPD. The importance of preventing or ameliorating BPD is related to the association between BPD and subsequent development of neurodevelopmental impairment (Vohr 2005; Wood 2005), chronic medical illness and rehospitalisation. If iNO therapy leads to a decrease in the need for ventilator support, reduction in lung injury may lead to prevention or amelioration of BPD. Nitric oxide participates in both production of and protection from oxidative injury (McAndrew 1997). Therefore, effects of iNO therapy on the developing lung deserve careful evaluation before iNO is introduced into clinical practice.
Why it is important to do this review
Of particular concern in the preterm infant is the effect of iNO on coagulation. Inhaled nitric oxide has been shown to increase bleeding time in adult volunteers (Hogman 1993) as well as in adults with ARDS (Samama 1995). This appears to occur via cGMP‐dependent mechanisms; presumably, intraplatelet cGMP is increased during the passage of platelets through the lung. Preterm infants are at high risk of developing intraventricular haemorrhage (IVH), which has substantial effects on long‐term developmental outcome. Therefore, it is important for investigators to evaluate iNO in terms of its effect on IVH rates among preterm infants.
The few case reports and case series published before randomised controlled trials (RCTs) were conducted demonstrate that preterm infants with severe respiratory failure who have not responded to conventional management, including surfactant and high‐frequency ventilation, may have improved oxygenation with iNO (Abman 1993; Peliowski 1995; Van Meurs 1997). These reports describe mortality and IVH.
In Skimming 1997, researchers randomised 23 surfactant‐treated preterm infants requiring conventional mechanical ventilation with at least 50% oxygen to 5 ppm or 20 ppm of iNO for 15 minutes (non‐blinded). Twelve infants received 20 ppm and 11 received 5 ppm. Mean gestational age was 28 ± 0.6 weeks, and mean postnatal age was 49.8 ± 8.1 hours. Results show significant increases in partial pressure of oxygen in arterial blood (PaO2) and peripheral capillary oxygen saturation (SpO2), a significant fall in the spontaneous respiratory rate (all P < 0.01) and no differences in response between the two doses of iNO, suggesting that if iNO is to be used in preterm infants, a dose of 5 ppm would be adequate.
More recent animal studies showing direct effects of iNO in protecting the preterm lung from adverse effects of hyperoxia (Bland 2005) and supporting microvascular development (McCurnin 2005) suggest possible direct benefits for reduction of BPD. Other published systematic reviews of iNO in the preterm are inconclusive (Hoehn 2006; Wirbelauer 2010).
Objectives
To determine effects of treatment with inhaled nitric oxide (iNO) on death, bronchopulmonary dysplasia (BPD), intraventricular haemorrhage (IVH) or other serious brain injury and on adverse long‐term neurodevelopmental outcomes in preterm newborn infants with hypoxic respiratory failure.
Owing to substantial variation in study eligibility criteria, which decreases the utility of an overall analysis, we divided participants post hoc into three groups: (1) infants treated over the first three days of life because of defects in oxygenation, (2) preterm infants with evidence of pulmonary disease treated routinely with iNO and (3) infants treated later (after three days of age) because of elevated risk of BPD.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised clinical trials.
Types of participants
Preterm infants (less than 35 weeks' gestation) with respiratory failure after adequate treatment with surfactant.
Types of interventions
Inhaled nitric oxide compared with placebo or control in addition to conventional treatment for respiratory failure.
Types of outcome measures
Primary outcomes
Death before hospital discharge
Bronchopulmonary dysplasia (oxygen dependence at 36 weeks' postmenstrual age)
Death or bronchopulmonary dysplasia (at 36 weeks' postmenstrual age)
Intraventricular haemorrhage (any grade and more severe, grades 3 and 4)
Secondary outcomes
Periventricular leukomalacia
Neurodevelopmental disability (proportion of survivors with neurological abnormality sufficient to affect quality of life or developmental index more than two standard deviations below the mean based on a validated scale at greater than 12 months of age; or mean developmental index among survivors greater than 12 months of age)
Retinopathy of prematurity (any stage and ≥stage 3)
Post hoc outcomes (reported in trials but not prespecified) include the following.
Oxygenation within two hours of therapy.
Pulmonary artery pressure.
Duration of assisted ventilation.
Search methods for identification of studies
We used criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (see the Cochrane Neonatal search strategy for specialized register).
We used paper indices from 1995 to 2006 to search for abstracts of annual Pediatric Academic Societies meetings, and subsequently searched on‐line abstracts.
We updated this search in January 2016.
Electronic searches
For the 2016 update, we conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 1) in The Cochrane Library; MEDLINE via PubMed (1966 to 15 January 2016); EMBASE (1980 to 15 January 2016); and CINAHL (1982 to 15 January 2016) using the following search terms: (nitric oxide), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). We did not apply language restrictions. We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization’s International Trials Registry and Platform www.whoint/ictrp/search/en/, and the ISRCTN Registry http://www.isrctn.com/).
Data collection and analysis
We used Cochrane standard methods. Review authors independently assessed trials for inclusion and methodological quality and resolved differences by discussion. We planned to contact study authors to request additional information, if needed.
Selection of studies
All three review authors screened titles and abstracts of all studies identified by the above search strategy. We assessed the full text of potentially eligible reports and excluded studies that did not meet all of the inclusion criteria. We discussed disagreements until we achieved consensus.
Data extraction and management
All three review authors extracted data separately and discussed disagreements until achieving consensus. We contacted trial authors to request further information if trial reports provided insufficient data.
Assessment of risk of bias in included studies
We used criteria and standard methods of the Cochrane Neonatal Group. We assessed the methodological quality of studies by using the following key criteria: allocation concealment (blinding of randomisation), blinding of intervention, completeness of follow‐up and blinding of outcome measurement/assessment. We assessed each criterion as 'yes', 'no' or 'cannot determine'. We requested additional information from trial authors to clarify methods and results as necessary. We included this information in the Characteristics of included studies table.
Review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We assessed the methodological quality of studies by using the following criteria.
Sequence generation (evaluating possible selection bias). For each included study, we described the method used to generate the allocation sequence as adequate (any truly random process, e.g. random number table; computer random number generator), inadequate (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number) or unclear.
Allocation concealment (evaluating possible selection bias). For each included study, we described the method used to conceal the allocation sequence as adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes), inadequate (open random allocation; unsealed or non‐opaque envelopes; alternation; date of birth) or unclear.
Blinding (evaluating possible performance bias). For each included study, we described the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes. We assessed these methods as adequate, inadequate or unclear for participants; adequate, inadequate or unclear for study personnel; and adequate, inadequate or unclear for outcome assessors.
Incomplete outcome data (evaluating possible attrition bias through withdrawals, drop‐outs, protocol deviations). For each included study and for each outcome, we described the completeness of data including attrition and exclusions from analysis. We stated whether attrition and exclusions were reported, numbers included in the analysis at each stage (compared with the total number of randomised participants), reasons for attrition or exclusion when reported and whether missing data were balanced across groups or were related to outcomes. We assessed methods as adequate (< 20% missing data), inadequate (≥ 20% missing data) or unclear.
Selective reporting bias. For each included study for which the protocol was available (through trials registers), we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed these methods as adequate (when it was clear that all of the study's prespecified outcomes and all expected outcomes of interest for the review had been reported), inadequate (when not all of the study's prespecified outcomes had been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; the study failed to include results of a key outcome that would have been expected to have been reported) or unclear.
Other sources of bias. We noted other possible sources of bias (e.g. whether a potential source of bias was related to the specific study design, whether the trial was stopped early owing to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as yes, no or unclear.
Funding sources. When reported, we divided funding sources into funding from local funds (hospital, research centre or university), charitable foundations, government agencies or industry. If funding was obtained from industry, and industry had substantial input into study design, conduct and/or analysis, we determined that the study was at high risk of bias. We considered funding from sources that would derive no potential financial benefit from the results to introduce low risk of bias. We considered unclear or partial funding to involve unclear risk of bias.
Measures of treatment effect
We calculated risk ratio (RR) and risk difference (RD) for dichotomous data, and mean difference (MD) for continuous data, with respective 95% confidence intervals (CIs). When it was deemed appropriate to combine two or more study arms, we obtained treatment effects from data combined according to methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We determined the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH) when we noted a statistically significant difference in RD.
Assessment of heterogeneity
We examined treatment effects of individual trials and heterogeneity between trial results by inspecting forest plots. We calculated the I² statistic for each analysis to quantify inconsistency across studies and to describe the percentage of variability in effect estimates that may be due to heterogeneity rather than to sampling error. If we detected substantial heterogeneity (I² statistic > 50%), we explored possible causes (e.g. differences in study design, participants, interventions or completeness of outcome assessments) by performing sensitivity analyses.
Assessment of reporting biases
We planned to investigate publication bias by using funnel plots if we included at least 10 clinical trials in the systematic review (Higgins 2011).
Data synthesis
We used the standard methods of the Cochrane Neonatal Review Group. For categorical outcomes, we calculated typical estimates for risk ratio (RR) and risk difference (RD), along with their 95% confidence intervals. For significant results, we planned on reporting the number needed to treat for an additional beneficial outcome or the number needed to treat for an additional harmful outcome (NNTB/NNTH), assuming a fixed‐effect model. We analysed continuous outcomes using weighted mean difference, also assuming a fixed‐effect model.
Quality of evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: death before discharge (studies with entry before three days based on oxygenation), death before discharge (studies with entry after three days based on BPD risk), death before discharge (studies of routine use in preterm infants on respiratory support), death or bronchopulmonary dysplasia at 36 weeks (studies with entry before three days based on oxygenation), death or bronchopulmonary dysplasia at 36 weeks (studies of routine use in preterm infants on respiratory support), intraventricular haemorrhage (grade 3 or 4; studies with entry before three days based on oxygenation), intraventricular haemorrhage (grade 3 or 4; studies of routine use in preterm infants on respiratory support), neurodevelopmental disability (studies with entry before three days based on oxygenation), neurodevelopmental disability (studies with entry after three days based on BPD risk) and neurodevelopmental disability (studies of routine use in preterm infants on respiratory support).
Two review authors independently assessed the quality of evidence for each of the outcomes listed above. We considered evidence from RCTs as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations on the basis of the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates and presence of publication bias. We used the GRADEpro Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of evidence.
Use of the GRADE approach results in assessment of the quality of a body of evidence and assignment to one of four grades.
High: We are very confident that the true effect lies close to the estimate of effect.
Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different.
Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect.
Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
In view of variable eligibility criteria for these trials, we divided them post hoc into three groups of trials.
In one group of trials, enrolment occurred early, over the first few days of life, for infants who were eligible because they had significantly impaired oxygenation. Investigators considered these studies to explore early 'rescue' therapy and designed them to determine effects on survival and early complications of prematurity.
Another group of trials enrolled infants after three days on the basis of clinical characteristics that predicted increased risk of BPD.
The third group of trials enrolled infants shortly after birth to provide routine 'prophylactic' use of iNO.
Review authors analysed these studies in three separate groups because study findings had very different implications for clinical practice. Within each subgroup, we examined heterogeneity by using the I2 statistic, as outlined above.
Results
Description of studies
Results of the search
The previous version of this review included 14 trials. The latest updated search in January 2016 revealed 463 titles after removal of duplicates. Screening of these articles led to inclusion of three new trials (Figure 1).
1.
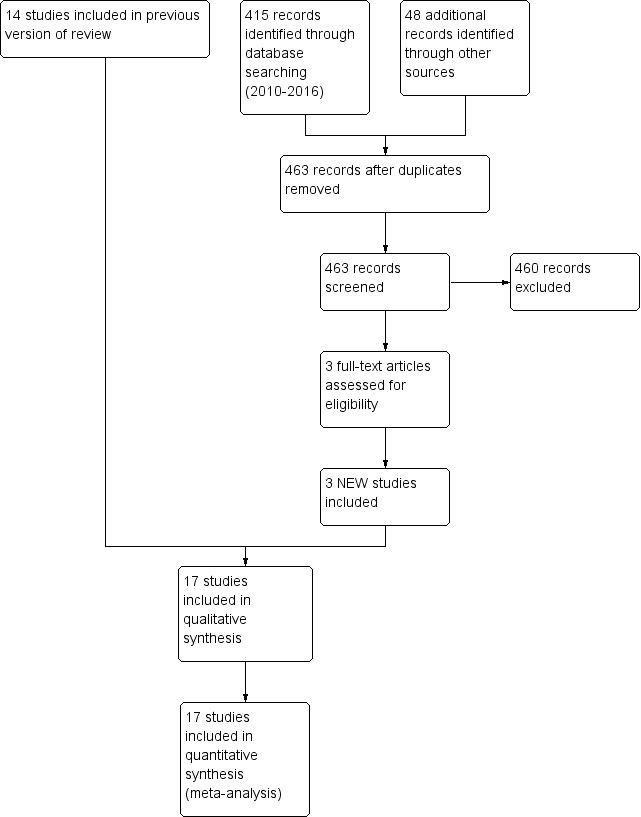
Study flow diagram: review update.
Included studies
We identified 17 published RCTs that compared inhaled nitric oxide (iNO) versus control in preterm infants (Ballard 2006; Dani 2006; EUNO 2009;Hascoet 2005; INNOVO 2005; Kinsella 1999; Kinsella 2006; Kinsella 2014;Mercier 1999; Schreiber 2003; Srisuparp 2002; Su 2007; Subhedar 1997; Van Meurs 2005; Van Meurs 2007; Wei 2014; Yoder 2013).
Trials of iNO in preterm infants eligible within the first three days because they met oxygenation criteria (early rescue therapy)
Studies were eligible to be considered in this first group if they included acutely ill ventilated preterm infants, most of whom were enrolled within the first three days of life, if infants satisfied a severity of illness criterion. We included in this group 10 trials of iNO in preterm infants (Dani 2006; Hascoet 2005; INNOVO 2005; Kinsella 1999; Mercier 1999; Srisuparp 2002; Su 2007;Van Meurs 2005; Van Meurs 2007; Wei 2014). Mean oxygenation indices of infants enrolled in these "rescue" studies ranged from 12 to 32 (32 for INNOVO 2005, 23 for Van Meurs 2005, approximately 25 for Van Meurs 2007,18 to 20 in Mercier 1999, 12 to 15 for Hascoet 2005, 30 for Su 2007 and 24 for Wei 2014).
Kinsella and colleagues randomised 80 preterm infants to blinded administration of 5 ppm iNO or no additional gas (79 had received surfactant). Infants underwent baseline cranial ultrasonography before administration of the study gas, results of which were noted but did not constitute an exclusion criterion. Investigators randomised infants if the ratio of arterial to alveolar partial pressure of oxygen (PO2) was less than 0.1 on two successive blood gases in the first seven days of life. They chose this criterion to enrol infants with expected mortality of 50%. The study was powered to detect a 30% reduction in mortality with iNO, which led to a planned sample size of 210 infants. Researchers planned to perform an interim analysis after 2.5 years and found no detectable difference in the main outcome (survival to discharge). They excluded seven participants from the analysis of acute oxygenation response because of early protocol violations but included them in all other outcome data. Forty‐eight infants received iNO, and 32 infants served as controls. Baseline characteristics of the groups were similar, except that a greater number of infants treated with iNO had no intracranial haemorrhage at the start of the study (73% vs 59%). The ratio of partial pressure of oxygen in arterial blood (PaO2) to fraction of inspired oxygen (FiO2) was 42 (standard deviation (SD) 18) in the iNO group and 42 (SD 16) in the control group. Researchers continued treatment for seven days, after which they made attempts to wean iNO. They then restarted study gas for an increase in oxygenation index of 15% or more. The maximum duration of treatment was 14 days.
Mercier 1999 The Franco‐Belgium Collaborative NO trial group randomised near‐term and preterm infants to separate strata and reported most results for the two strata separately. The cutoff between strata for this study occurred at 33 weeks' gestation. Admission criteria included postnatal age less than seven days and oxygenation index between 12.5 and 30. All except one of the 85 preterm infants received surfactant, and most (75%) received high‐frequency ventilation. A majority of the infants were enrolled on the first or second day of life. Researchers randomised infants to iNO at 10 ppm or to control. If the oxygenation index (OI, mean airway pressure × FiO2 × 100)/PaO2) exceeded 30 during the two‐hour study period, then iNO at 20 ppm was used. The median baseline OI was 18 in the control group and 20.2 in the iNO group. The primary outcome variable was OI two hours after the start of therapy; after two hours, if a response was noted, iNO was reduced to 5 ppm, then was weaned 'slowly', but the maximum duration of therapy is not clear.
Srisuparp 2002 Srisuparp and colleagues in a single‐institution study randomised intubated preterm infants weighing less than 2000 grams who had received surfactant, had clinical respiratory distress syndrome (RDS) and were less than 72 hours of age. OI entry criteria varied with birth weight: OI had to be > 4 for infants < 1000 grams, > 6 for infants 1001 to 1250 grams, > 8 for infants 1251 to 1500 grams, > 10 for infants 1501 to 1750 grams and > 12 for infants 1751 to 2000 grams. Infants had central arterial catheters in place and were without major malformations. Treatment consisted of open‐label iNO at 20 ppm or control. iNO was weaned by protocol and was limited to seven days. This was a pilot study for Schreiber 2003. Although no hypothesis was clearly stated, study authors indicated that they were assessing effects on oxygenation and potential adverse effects of iNO.
Van Meurs 2005 The study of Van Meurs and colleagues was a multi‐centre US study. Initial entry criteria restricted enrolment to infants less than 34 weeks' gestation with birth weight between 401 and 1500 grams (with three birth weight strata: 401 to 750 grams, 751 to 1000 grams and 1001 to 1500 grams). Eligible infants were receiving assisted ventilation at least four hours after surfactant therapy and were considered at high risk because they had an OI of at least 10 on two consecutive blood gases. Investigators revised this criterion (after initial interim analysis showed an unexpectedly high mortality rate) to an OI of at least five followed by an OI of at least 7.5 after at least 30 minutes. The primary outcome was the combined outcome of BPD (oxygen dependence at 36 weeks) or death. The actual oxygenation indices of enrolled participants were 23 (SD 17) in the intervention group and 22 (SD 17) in the control group. Investigators in the study of Hintz 2007 reported neurological and developmental outcomes (Bayley Scales of Infant Development) at 18 to 22 months' corrected age.
Hascoet 2005 Hascoet and colleagues conducted a multi‐centre study at 10 European centres. They enrolled and randomised intubated preterm infants but revealed randomisation only if infants developed hypoxic respiratory failure, defined as an arterial‐alveolar oxygen ratio (a/AO2 ratio = PaO2/713 × FiO2 ‐ partial pressure of carbon dioxide in arterial blood (PaCO2)) less than 0.22 at between six and 48 hours of age. Unfortunately, a large quantity of reported data pertain to the overall group of 860 infants, many of whom were not eligible to receive the assigned intervention. A total of 61 iNO infants and 84 control infants were actually exposed to the study intervention. Infants who developed refractory hypoxaemia at any time (defined as PO2 < 50 and PCO2 < 50 mmHg for FiO2 = 1.0) were defined as "failures" with regard to the primary outcome measure, and iNO was given according to French Drug Agency recommendations. All consented infants started to receive the study intervention when they met hypoxic respiratory failure criteria, defined as the need for mechanical ventilation, FiO2 > 40% and a/AO2 ratio < 0.22. The first hour of iNO treatment consisted of a dose of 5 ppm iNO. Researchers determined subsequent dosages according to the a/AO2 response. As soon as the response was positive (defined as an a/AO2 increase > 0.22), investigators decreased iNO to 2 ppm for two hours, then weaned participants according to the results of blood gas evaluations. The primary outcome variable was survival without respiratory support, oxygen supplementation or IVH > grade 1 to 28 days of age.
INNOVO 2005 Investigators in this European multi‐centre study planned to enrol 200 infants at less than 34 weeks' gestation and less than 28 days old. Eligibility was limited to "severe respiratory failure requiring assisted ventilation if the responsible physician was uncertain about whether an infant might benefit from iNO". The protocol suggested that iNO should be started at 5 ppm and could be doubled up to a maximum of 40 ppm. Study authors listed two primary outcome criteria in the main publication, but they calculated the sample size on the basis of a reduction in frequency of the combined outcome of death or severe disability at one year corrected postnatal age. Researchers had enrolled 108 infants when the study was terminated. The primary publication of the trial included data on neurological and developmental outcomes to one year of corrected age (obtained from a questionnaire completed by the local paediatrician); investigators in Huddy 2008 reported further follow‐up to four to five years' corrected age (British Ability Scales).
Dani 2006 Dani and colleagues planned to enrol 52 infants at less than 30 weeks' gestation and less than one week of age in a single‐centre study. Infants ventilated with severe respiratory distress were eligible if they had FiO2 > 0.5 and an a/AO2 ratio < 0.15 despite surfactant treatment. Forty infants had been enrolled at termination of the study. Mean age at the start of the intervention was 43 hours in the iNO group. Infant treatment began at 10 ppm of iNO, which was decreased to 6 ppm after four hours; treatment then continued until extubation or until weaning criteria were reached.
At the same time that they enrolled preterm infants with birth weight less than 1500 grams into the Van Meurs 2005 study, the same group of investigators enrolled larger preterm infants at less than 34 weeks' gestation and weighing more than 1500 grams into a pilot RCT. They applied the same eligibility criteria of severe hypoxic respiratory failure, which was defined as an OI of 15 or more on two consecutive measurements of arterial blood gases (ABGs) between 30 minutes and 12 hours apart. Study authors expected the sample size to be small, and indeed they were able to randomise only 29 infants. They started infant treatment with iNO or placebo gas at a dose of 5 ppm, with an option to increase to 10 ppm depending on response. Maximum treatment duration was 14 days.
This single‐centre trial performed in Taiwan included a short‐term primary outcome variable of oxygenation response 24 hours after enrolment. Investigators randomised 65 babies at less than 31 weeks' gestation and weighing less than 1500 grams with OI of at least 25. They commenced iNO at 5 ppm and could vary dosage according to oxygenation response, up to a maximum of 20 ppm. The maximum duration of therapy was not described. The actual OI before treatment averaged just over 30 in each group.
This single‐centre trial in China enrolled 60 infants at ≤ 34 weeks' gestation. Admission criteria included postnatal age less than seven days and OI of at least 11, which persisted after two hours of surfactant therapy. Treatment for infants in the NO group started at 5 ppm and could be varied according to oxygenation response. Researchers continued iNO inhalation for at least seven days or until ventilation withdrawal. Study authors did not report time of enrolment but stated that the short‐term primary outcome variable was OI before treatment and at one, 12, 24, 48 and 72 hours post treatment.
Trials of iNO in preterm infants eligible after three days of age because of elevated risk of BPD
Review authors included studies in the second group if they enrolled preterm infants who were more than three days old after using a risk score or other method to determine that infants were at increased risk for BPD. This group consisted of three trials of iNO in preterm infants (Ballard 2006; Subhedar 1997; Yoder 2013). Ballard 2006 enrolled infants after seven days of age if they remained ventilated or, for the smallest infants, were receiving continuous positive airway pressure (CPAP). Subhedar 1997 enrolled infants at 96 hours of age who met the main eligibility criterion of a score predicting high risk of BPD. Yoder 2013 included infants who were five to 14 days of age and still required respiratory support. Thus, infants in the three studies (Ballard 2006; Subhedar 1997; Yoder 2013) were enrolled after the major risk period for development of IVH. Ballard 2006 reported a respiratory severity score (which cannot be directly converted to the OI, as it does not take into account the PaO2 ‐ few eligible infants would have had arterial lines) calculated as FiO2 multiplied by mean airway pressure in cmH2O. The median score was 3.5 in each group (mean 4.1), which suggests mild illness. In Yoder 2013, the mean score on the same scale was 2.9.
Subhedar 1997 Subhedar and colleagues performed an open randomised trial of iNO in preterm infants at less than 32 weeks' gestation who were enrolled at 96 hours of age if still intubated. Entry criteria included "high" risk for developing BPD, as determined by a previously published risk score (Ryan 1996). The actual score required for eligibility was not given. Investigators randomised nfants in a 2 × 2 factorial design to receive iNO only (n = 10), iNO plus dexamethasone (n = 10), dexamethasone only (n = 11) or neither (n = 11). Treatment consisted of 20 ppm of iNO, reduced to 5 ppm according to treatment response. The dexamethasone dose was 1 mg/kg/d for three days, then 0.5 mg/kg/d for three additional days. The study included 42 infants with a mean birth weight of 882 grams (range 416 to 1354 grams) and a mean gestational age of 27 weeks (range 24 to 30 weeks) for the 20 iNO infants compared with a mean birth weight of 762 grams (range 520 to 1320 grams) and a mean gestational age of 27 weeks (range 22 to 31 weeks) for the 22 non‐iNO infants. Study authors did not present results separately for each of the four groups randomised in this study. They presented almost all data as iNO versus no iNO, regardless of whether dexamethasone was given. Therefore, it is not possible to assess possible interactions or to compare the effects of iNO solely among infants who did not receive steroids. Investigators performed developmental assessments (Bayley Scales of Infant Development) and neurological examinations at 30 months' corrected age and reported results in Bennett 2001. Ballard 2006 Ballard and colleagues studied 582 infants at ≤ 32 weeks' gestation with birth weight of 500 to 1250 grams who were receiving mechanical ventilation for lung disease (not apnoea) between seven and 21 days of age. Infants with birth weight of 500 to 799 grams who were being treated with nasal CPAP were also eligible. Investigators initially treated infants with 20 ppm of study gas (iNO or nitrogen placebo) for 48 to 96 hours, and subsequently decreased doses to 10, 5 and 2 ppm at weekly intervals. Minimum treatment duration was 24 days. The median respiratory severity score (calculated as FiO2 multiplied by mean airway pressure in cmH2O) was 3.5 in each group, which suggests, on average, relatively mild respiratory disease. Study authors calculated that a severity score of 3.5 is equivalent to an OI between 5 and 9. Twelve percent of participants in the iNO group had a score > 10, as did 13% in the control group. Walsh 2010 reported neurological and developmental outcomes (Bayley Scales of Infant Development) at 24 months' corrected age.
This multi‐centre trial (funded by Ikaria) enrolled 451 infants at less than 30 weeks' gestation with birth weight less than 1250 grams if they still required assisted ventilation at five to 14 days of age. Infants weighing less than 800 grams could be enrolled if on CPAP; larger infants ‐ 800 to 1249 grams ‐ were eligible only if intubated on positive‐pressure ventilation. The dosing regimen was 20 ppm given for 72 to 96 hours, then reduced to 10 ppm for 10 days, then to 5 ppm to complete 24 total days of therapy. The mean respiratory severity score was 2.9, compared with 4.1 in Ballard 2006. The primary outcome variable was survival without BPD; data presented to this point include respiratory outcomes out to one year of corrected age. Data on neurological and developmental outcomes to 18 months of age are awaited.
Trials of routine prophylactic use of iNO in preterm infants
Studies were eligible to be included in this third group if they enrolled infants early in life (at less than three days of age) and required no minimum severity of illness criteria other than the need for respiratory support. We included four trials in this category (EUNO 2009; Kinsella 2006; Kinsella 2014; Schreiber 2003).
Schreiber 2003 This single‐centre study enrolled intubated preterm infants at less than 34 weeks' gestation with birth weight less than 2 kg at less than 72 hours of age. Study authors stated no specific oxygenation criteria. The study used a 2 × 2 factorial design and examined seven days of iNO or oxygen placebo with high‐frequency oscillatory ventilation provided by the SensorMedics device (SensorMedics Corporation, Yorba Linda, California, USA) or conventional ventilation. Treatment began as iNO given at 10 ppm the first day, followed by 5 ppm for six days. The iNO intervention was blinded. Entry criteria did not require a prespecified disease severity. The median OI was 7.3 for the iNO group and 6.8 for the control group. The primary outcome was survival without chronic lung disease (oxygen requirement at 36 weeks' postmenstrual age). Mestan 2003 reported neurological outcomes and developmental progress (Bayley Scales of Infant Devleopment) of infants at two years' corrected age.
Kinsella 2006 Kinsella and colleagues conducted one of the largest multi‐centre trials completed to date. They achieved the planned sample size of 792 infants and enrolled infants at < 34 weeks' gestation who were ventilated for respiratory failure in the first 48 hours and were expected to remain intubated for longer than 48 hours. They included no further requirement for severity of illness. Investigators administered 5 ppm of iNO or nitrogen placebo to infants until extubation or for up to 21 days. The primary outcome variable was survival without BPD; the main secondary outcomes were severe IVH, periventricular leukomalacia and cerebral ventricular dilation. The OI at baseline was 5.4 (SD 5.2) in the treatment group and 5.8 (SD 6.7) in the placebo group. At one year of corrected age, researchers performed neurological examination and developmental evaluation (Bayley Scales of Infant Development); Watson 2009 reported these results.
Mercier and colleagues completed EUNO (European trial of iNO) in 2009. This multi‐centre trial randomised 800 infants within the first 26 hours of life to 5 ppm of iNO for a minimum of seven days (up to 21 days if still on respiratory support) or to placebo. Infants were eligible if they were between 24 weeks' gestation and 28 weeks and six days. Investigators applied two entry criteria. Infants were eligible if they were intubated and received surfactant before 24 hours of age, or if they required ≥ 30% oxygen and were supported by CPAP. Researchers excluded infants in either group if they required more than 50% oxygen to maintain arterial saturation greater than 85%. The primary outcome variable was survival without BPD, defined according to Walsh's physiological criteria at 36 weeks' postmenstrual age. Secondary outcome criteria were survival without serious brain injury defined according to findings of head ultrasonography. Durrmeyer 2013 reported follow‐up neurological and developmental evaluations. Follow‐up of this study was based on the third version of the Bayley Scales of Infant Development, and results are not directly comparable with scores reported by the other two trials. The absolute proportion of children with developmental delay was much lower when the third version of the Bayley Scales of Infant Development was used; as developmental delay is the largest component of the outcome of neurodevelopmental Impairment, fewer infants had this outcome in the EUNO follow‐up trial. iNO Therapeutics funded this trial.
This multi‐centre trial enrolled infants with birth weight between 50 and 1250 grams only if they were receiving non‐invasive respiratory support along with some additional oxygen within the first 72 hours of life. Investigators added iNO at 10 ppm to the inspired gases, to achieve an effective concentration of at least 5 ppm. The study was masked, and controls received placebo nitrogen gas. The primary outcome was the combined outcome of death or BPD. Researchers continued to provide study gas until 30 weeks' postmenstrual age, for a minimum of two weeks.
Excluded studies
Skimming 1997 Skimming and colleagues conducted a randomised comparison of response to 5 ppm and 20 ppm of iNO. All infants received iNO.
Lindwall 2005 This short‐term (30‐minute) cross‐over study of preterm infants receiving CPAP for RDS examined effects on gas exchange. All infants received iNO as the first or second gas administered.
Risk of bias in included studies
Trials of iNO in preterm infants eligible within the first three days because they met oxygenation criteria (early rescue therapy)
Kinsella 1999 This multi‐centre study reported masked allocation and masked intervention. Investigators stratified randomisation by centre and gestational age (≤ 28 weeks and > 28 weeks) and performed intention‐to‐treat (ITT) analysis. The study was terminated after 80 of the planned 210 infants were enrolled, when an interim analysis suggested that significant benefit was not likely to be detected "within a reasonable time frame". Researchers planned the interim analysis at the start of the study but did not state whether slow enrolment was a predesignated stopping criterion.
Mercier 1999 This multi‐centre international trial provided the unmasked intervention and adequately concealed randomisation at the co‐ordinating centre by using sealed envelopes. Investigators designed the study to assess the primary outcome after two hours. They permitted later treatment with iNO if the infant's oxygenation worsened such that the OI exceeded 30. Five of the control infants eventually received iNO. The availability of back‐up iNO treatment for control infants limited the ability of the study to address long‐term outcomes. Researchers planned to enrol a total of 360 infants across both gestational age strata, but the study was terminated because of slowing enrolment after two years. It was not stated whether early analysis or cessation criteria were predesignated. Researchers performed ITT analysis. All baseline characteristics were similar between groups.
Srisuparp 2002 This single‐centre trial included ventilated preterm infants with birth weight less than 2000 grams. Enrolled infants had received surfactant, were less than 72 hours old and had an arterial line in place. Oxygenation criteria allowed randomisation of the smallest babies (≤ 1000 grams) with only mild disease (OI ≥ 4) and required increased OI for enrolment of infants with increasing birth weight (≥ 12 with birth weight 1751 to 2000 grams). The adequacy of masking is uncertain, as allocation was completed according to a "card‐picking scheme". Researchers did not mask the intervention. The primary outcome variable was severe IVH. Investigators weaned iNO at 20 ppm within six to 12 hours; a subsequent weaning protocol planned weaning within 72 hours unless deterioration was evident. The maximum duration of treatment was seven days.
Van Meurs 2005 This multi‐centre study used a telephone system to mask randomisation. Investigators masked the intervention by using simulated flow of gas as a placebo, performed ITT analysis and masked the outcome assessment. Researchers assessed all infants for the primary outcome. They initially planned to recruit 440 infants; the study was terminated after two‐thirds of the infants had been assessed for the primary outcome because an increase in severe IVH was apparent with no benefit in terms of the primary outcome. By the time the analysis was completed, researchers had enrolled 420 infants, and the study was terminated.
This study followed the same design as the primary Van Meurs 2005 trial. Investigators determined sample size by progression of the primary trial; masked randomisation, intervention and outcome assessment; and performed ITT analysis.
Hascoet 2005 This multi‐centre trial conducted at 10 tertiary perinatal centres in France and Belgium achieved masked randomisation with a call‐in telephone system and did not mask the intervention. Refractory hypoxaemia before six hours of age occurred in 20 infants, who therefore were not entered into the study. An additional 20 iNO infants and 28 control infants received open‐label iNO for refractory hypoxaemia, further complicating analyses of results through significant contamination of control infants. Researchers achieved the initially planned sample size and the approximately expected incidence of hypoxic respiratory failure.
INNOVO 2005 This multi‐centre trial attained masked allocation with a telephone system. Investigators assigned treatment by minimisation ("with a probabilistic element") rather than by strict randomisation and did not mask the intervention. Researchers performed ITT analysis and reported that follow‐up was complete for all except one infant. Follow‐up was not formally blinded. Investigators enrolled 55 iNO infants and 53 controls. The study was stopped at the end of the calendar year 2001; apparently, this had been planned, although it was not mentioned in the on‐line version of the trial protocol nor in the register of controlled trials.
Dani 2006 This study randomised infants by using sealed opaque envelopes and, therefore, was presumably masked. The intervention was not masked. The study was terminated after 40 of the initially planned 52 infants had been enrolled, after a previously unplanned interim analysis confirmed the investigators' impression that BPD was reduced. This early termination provided insufficient protection from type 1 errors.
Researchers did not describe the randomisation process used in this study; therefore, masking is uncertain. The intervention was not masked, nor was the evaluation of outcomes. Investigators pre‐planned and achieved the planned sample size and performed ITT analysis.
Study authors did not describe the randomisation process; therefore, masking of allocation is uncertain. They reported no masking of the intervention and provided no information about sample size determination.
Trials of iNO in preterm infants eligible after three days of age because of elevated risk of BPD
Subhedar 1997 This study provided unmasked intervention but adequately concealed randomisation with the use of sealed envelopes. Researchers initially planned a sample size of 88 participants. However, the study was terminated at a sample size of 42 because at a predesignated 12‐month review, the incidence of the primary outcome of death or BPD was much higher than planned, "which would have enabled the planned outcome to be detected with a much smaller group." It was not stated whether this was a predesignated stopping criterion. Investigators performed ITT analysis. Despite randomisation, oxygenation was not well matched between groups at baseline. The median OI in control infants was 3.9 (range 1.2 to 11.5) and in iNO infants 7.9 (range 1.6 to 46.7). The iNO group included a greater proportion of males (12/20 vs 5/22); other baseline characteristics were similar.
Ballard 2006 This multi‐centre trial reported masked allocation. Investigators masked study gas (iNO or nitrogen) to all except the study respiratory therapist. Follow‐up to assessment of the primary outcome was complete, and investigators remained masked. Investigators performed ITT analysis. A data safety monitoring committee oversaw the study, and researchers performed interim analyses according to preplanned rules. The study was allowed to proceed to the initially planned sample size.
This study reported masked allocation and masked intervention. Follow‐up to assessment of the primary outcome was complete, and researchers performed ITT analysis. They achieved the initially planned sample size.
Trials of routine use of iNO in preterm infants on respiratory support
Schreiber 2003 This single‐centre study reported masked randomisation. Investigators masked the iNO intervention by using an oxygen placebo and performed ITT analysis. Follow‐up of enrolled infants was complete. Researchers assessed the primary outcome in a masked fashion and did not state whether long‐term neurodevelopmental follow‐up was also masked. They achieved the planned sample size.
Kinsella 2006 This multi‐centre trial described masked allocation. Investigators masked study gas (iNO or nitrogen). Follow‐up was complete with respect to assessment of the primary outcome, which researchers assessed in a masked fashion. They performed ITT analysis. A data safety monitoring committee oversaw the study, and study authors performed interim analyses according to preplanned rules. The study was allowed to proceed to the initially planned sample size.
This multi‐centre trial reported masked allocation and intervention. Investigators administered placebo study gas to controls. Follow‐up was complete with respect to assessment of the primary outcome, which researchers assessed in a masked fashion. They performed ITT analysis. A data safety monitoring committee oversaw the study, and investigators conducted interim analyses according to preplanned rules. The study was allowed to proceed to the initially planned sample size.
This multi‐centre trial reported masked allocation and intervention. Follow‐up was complete to hospital discharge or death, and investigators performed ITT analysis. They planned sample size for a reduction in the primary outcome variable and achieved this size.
We have provided summaries of risk of bias evaluations in Figure 2 and Figure 3.
2.
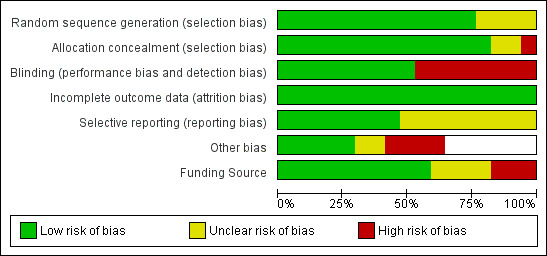
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
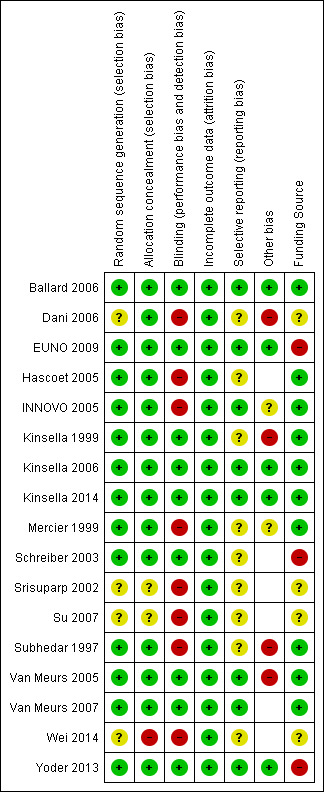
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
Review authors considered the usefulness of analyses that included all trials of iNO in preterm infants to be limited because of differing entry criteria for these studies. Severity of illness criteria and age at entry varied so greatly that pooling of results was not considered appropriate. Control group mortality also varied substantially (6% to 64%), further emphasising differences among eligible patients; we have reported only subgroup analyses.
As noted above, we grouped trials post hoc into three categories that created groups of studies, each of which was fairly homogeneous in terms of age of entry and severity of illness criteria (and control group mortality).
Entry in the first three days of life based on oxygenation criteria.
Routine use in preterm babies receiving respiratory support.
Later enrolment based on BPD risk.
The post hoc group of studies that randomised ventilated preterm infants with an oxygenation defect in the first few days of life were fairly similar. All studies randomised infants to low‐dose iNO. INNOVO 2005 did not provide precise criteria for entry but stated that the criterion was "if the responsible physician was uncertain about whether an infant might benefit from iNO". Despite this difference, mortality and BPD frequencies are not very dissimilar from those reported by the other studies in this group. Methods used for calculation of the oxygenation defect in remaining studies were different and were not all directly comparable. Several studies reported the OI. In this group of studies, most participants were entered before three days of age, although some studies allowed enrolment up to seven days of age. Srisuparp 2002 was a pilot study of 34 infants that was designed primarily to evaluate changes in oxygenation. Srisuparp 2002 did not report on BPD. Of note, both Hascoet 2005 and Mercier 1999 allowed back‐up treatment of controls with iNO if their condition worsened to a prespecified degree. This may have led to underestimation of both benefit and risk. Most of the studies in this group reported comparable mortality in control groups, at between 30% and 44%; the single exception was INNOVO, which reported mortality of 64% among controls. Van Meurs 2007, which enrolled somewhat larger babies, reported slightly lower mortality among controls, at four of 15. Wei 2014 reported a lower mortality rate in the NO group than in the control group (3% vs 13%, P < 0.05).
Three studies evaluated infants at more than three days of age on the basis of elevated risk of BPD. Subhedar 1997 was quite different from the other trials; investigators examined both iNO and dexamethasone therapy using a factorial design. They selected infants at 96 hours of age on the basis of high risk of BPD. Indeed, investigators found an almost universal incidence of BPD at 36 weeks. Ballard 2006 enrolled infants who were still ventilator dependent at seven to 21 days of age (or, in the case of the smallest infants, at birth weight 500 to 799 grams and requiring CPAP) with no other criteria for increased BPD risk; enrolled infants received 24 days of iNO starting at 20 ppm. Yoder 2013, which shared many features with Ballard 2006, enrolled infants between five and 14 days of age if they were still requiring mechanical respiratory support, without requiring other criteria for increased risk of BPD; and using prolonged treatment with iNO starting at 20 ppm. These trials were not similar to Subhedar 1997, which applied a unique entry criterion and a factorial design. For this reason, we performed a sensitivity analysis with and without Subhedar 1997. As the Subhedar trial enrolled very small numbers of infants, results of analyses with and without this study are identical. The mortality of control groups in Ballard 2006 and Yoder 2013 was only 6% and 7%, respectively, reflecting older age at entry compared with the other two groups of studies, as well as lesser severity of illness than in early rescue studies.
Four studies enrolled infants without specific minimum criteria for disease severity. Schreiber 2003 randomised preterm infants who were ventilator dependent after receiving surfactant, without requiring a specific disease severity. Similarly, Kinsella 2006 enrolled infants at less than 34 weeks who were ventilated and were expected to be ventilated for longer than 48 hours. They applied no other severity of illness criteria. Eighty percent had received surfactant. EUNO 2009 enrolled infants who were intubated for surfactant or were on CPAP with a modest oxygen requirement. This study excluded infants who required more than 50% oxygen. Infants in this group of trials were substantially less sick as demonstrated by lower OIs than were reported for infants in the first group of studies. Control group mortalities were comparable in the first two studies, at 23% and 25%, and were lower, at 11%, in the most recent study (EUNO 2009. Kinsella 2014 enrolled children who were receiving non‐invasive support with some supplemental oxygen within the first 72 hours of age; infants were less sick again than in the other trials, with three deaths among 124 infants, and with 40% developing BPD.
We have provided below the results for each subgroup.
Death before hospital discharge (Outcome 1.1)
All trials assessed survival to discharge. None of the individual trials noted a significant effect, except Wei 2014. The three subgroups showed no effect on hospital mortality (Early studies with entry before three days based on oxygenation criteria: typical risk ratio (RR) 1.02, 95% confidence interval (CI) 0.89 to 1.18; 10 studies, 1066 infants; typical RD 0.01, 95% CI 0.05 to 0.07; Entry after three days of age based on BPD risk: typical RR 1.18, 95% CI 0.81 to 1.71; three studies, 1075 infants; typical RD 0.01, 95% CI ‐0.02 to 0.05; Studies of early routine use: typical RR 0.90, 95% CI 0.74 to 1.10; four studies, 1924 infants; typical RD ‐0.02, 95% CI ‐0.05 to 0.02).
Death before 36 weeks' postmenstrual age (Outcome 1.2)
Nine studies reported this outcome, five of which included 458 infants and were grouped under early entry on the basis of oxygenation criteria. For infants entered early (before three days) on the basis of oxygenation criteria, investigators reported no significant effect of iNO on this outcome (typical RR 0.89, 95% CI 0.72 to 1.11; typical RD ‐0.05, 95% CI ‐0.13 to 0.04). Subhedar 1997, with entry after three days of age based on BPD risk, also reported this result and did not show a significant effect. EUNO 2009 showed no significant effect.
Bronchopulmonary dysplasia (oxygen dependence among survivors at 36 weeks' postmenstrual age) (Outcome 1.3)
All published studies except Hascoet 2005 and Srisuparp 2002 reported BPD rates at 36 weeks; the principal investigator supplied data from Hascoet to review authors (for four of the iNO infants and for 10 controls, data on oxygen dependency at 36 weeks were missing, although these infants were known to have survived). None of the individual trials reported a significant effect. Heterogeneity for each of the subgroups was substantial, and no subgroups noted a statistically significant difference in BPD. Results included the following.
Studies with entry before three days of age based on oxygenation criteria: typical RR 0.89, 95% CI 0.76 to 1.04; eight studies, 681 infants; I2 = 29%; typical RD ‐0.05, 95% CI ‐0.12 to 0.01.
Studies with entry after three days of age based on BPD risk: typical RR 0.91, 95% CI 0.83 to 1.01; three studies, 990 infants; I2 = 11%; typical RD ‐0.06, 95% CI ‐0.12 to 0.00.
Studies of routine use in intubated preterm infants: typical RR 0.95, 95% CI 0.85 to 1.05; four studies, 1782 infants; I2 = 10%; typical RD ‐0.02, 95% CI ‐0.07 to 0.02.
Death or bronchopulmonary dysplasia (Outcome 1.4)
The combined outcome of death or bronchopulmonary dysplasia (or its converse, survival without bronchopulmonary dysplasia) was available for all studies.
None of the individual trials with entry based on an oxygenation deficit found a significant effect, and this subgroup of studies showed no effect (typical RR 0.94, 95% CI 0.87 to 1.01; eight studies, 958 infants; typical RD ‐0.05, 95% CI ‐0.10 to 0.01). Similarly, studies with entry after three days of age based on BPD risk did not individually show a significant effect, and group results were not significant (typical RR 0.92, 95% CI 0.85 to 1.01; three studies, 1075 infants; typical RD ‐0.05, 95% CI ‐0.11 to 0.01). The group of studies of routine early use also showed no significant effect (typical RR 0.94, 95% CI 0.87 to 1.01; four studies, 1924 infants; typical RD ‐0.03, 95% CI ‐0.08 to 0.01).
Of note, the published report of Ballard 2006 showed a modest but statistically significant improvement in survival without BPD, from 36.8% to 43.9% (P = 0.042). This finding is different from that of the analysis performed for this review because study authors used a statistical method that cannot be replicated from published summary data ‐ the multiple outputation technique (see below).
Any intraventricular haemorrhage (Outcome 1.5)
Four studies with entry in the first three days of life based on oxygenation criteria reported this outcome. No evidence suggested an effect of iNO on overall IVH frequency (typical RR 0.94, 95% CI 0.69 to 1.28; 314 infants; typical RD ‐0.02, 95% CI ‐.012 to 0.08). Among routine early treatment studies, Kinsella 2006 reported a small reduction and EUNO 2009 reported a barely significant increase. Overall researchers noted no effect (typical RR 1.04, 95% CI 0.88 to 1.23; two studies, 1573 infants; typical RD 0.01, 95% CI ‐0.03 to 0.05).
Severe intraventricular haemorrhage (grade 3 or 4) (Outcome 1.6)
Six of the studies reporting entry in the first three days of life based on oxygenation criteria reported on severe IVH. Meta‐analysis of these studies showed an almost significant increase in the incidence of severe IVH (typical RR 1.20, 95% CI 0.98 to 1.47; 773 infants; typical RD 0.05, 95% CI ‐0.00 to 0.10).
Most cases of IVH occur in the first three days of life; therefore, studies with later entry would not be expected to report an effect on IVH. Evolution of preexisting abnormalities, development of hydrocephalus and occurrence of periventricular leukomalacia were reported as a single variable by Ballard 2006 and were not different between groups.
Studies of routine use of iNO in intubated preterm infants reported a small and non‐significant reduction in severe haemorrhage (typical RR 0.89, 95% CI 0.73 to 1.09; four studies, 1913 infants; typical RD ‐0.01, 95% CI ‐0.04 to 0.01). Heterogeneity for this result is evident (I2 = 52%): EUNO 2009 reported an increase, and Schreiber 2003 and Kinsella 2006 described a decrease.
Severe intraventricular haemorrhage or periventricular leukomalacia (Outcome 1.7)
Studies with entry in the first three days of age based on oxygenation criteria showed no significant effect but noted a small non‐significant increase in this adverse outcome (typical RR 1.08, 95% CI 0.88 to 1.33; eight studies, 901 infants; typical RD 0.02, 95% CI ‐0.04 to 0.08).
Studies of routine use of iNO in intubated preterm infants showed a small and non‐significant reduction in this outcome (typical RR 0.90, 95% CI 0.73 to 1.12; three studies, 1747 infants; typical RD ‐0.02, 95% CI ‐0.05 to 0.02). This result shows major heterogeneity (I2 = 80%): EUNO 2009 showed a small increase, whereas the other two trials (Kinsella 2006; Schreiber 2003) described a decrease in this outcome.
Periventricular leukomalacia
This outcome was not reported.
Neurodevelopmental outcome (Outcomes 1.8, Neurodevelopmental disability; and 1.9, Bayley Mental Development Index or Physical Development Index < ‐2 SD)
All results are expressed among the number of survivors evaluated.
To date, studies reporting neurodevelopmental outcomes include Subhedar 1997; Schreiber 2003; INNOVO 2005; Van Meurs 2005; Kinsella 2006; Van Meurs 2007; and Ballard 2006.
Subhedar 1997 (Bennett 2001): Twenty‐two children were still alive at 30 months of age, and 21 were formally examined (seven iNO infants and 14 control infants). Findings revealed no significant differences in outcomes. The definition of "severe neurodisability" in the outcome manuscript was very similar to our definition of neurodevelopmental disability. All five infants with severe neurodisability (mental development index (MDI) or physical development index (PDI) < 71, cerebral palsy or sensorineural impairment) were control infants.
Schreiber 2003 (Mestan 2005): At two years' corrected age, the original study included 168 survivors, 139 of whom (82%) were evaluated. This study showed a significant reduction in the frequency of a composite outcome of neurodevelopmental disability (cerebral palsy, bilateral blindness, bilateral hearing loss or a score on the Bayley Scales of Infant Development > 2 SD below the mean). This improvement was largely the result of a decrease in the incidence of a Bayley score greater than two SDs below the mean. The occurrence of cerebral palsy was similar between groups.
INNOVO 2005 reported the incidence of major disability at one year of age among 43 of 44 survivors. Severe disability was not different between iNO and control groups. Investigators defined severe disability as no/minimal head control or inability to sit unsupported or no/minimal response to visual stimuli (equivalent to developmental quotient < 50). Results showed no differences between groups (7/55 vs 3/53), but lack of formal testing and earlier age at assessment made comparison with Schreiber difficult. Differences in definition did now allow these results to be combined in a meta‐analysis. Infants were also evaluated more formally at four to five years of age (Huddy 2008). Investigators performed a neurological examination and used the British Ability Scales to evaluate 38 of 43 survivors, noting no differences between groups.
Van Meurs 2005 (Hintz 2007) performed standardised testing (Bayley II) at 18 to 22 months of age among 193 of 213 surviving infants (90%), and reported neurodevelopmental impairment defined identically in this review. Researchers reported no differences between groups in neurodevelopmental impairment but more frequent moderate to severe cerebral palsy in the group that had received iNO (18/90 vs 11/102, adjusted RR 2.01, 95% CI 1.01 to 3.98) (adjusted data from Hintz 2007).
Kinsella 2006 (Watson 2009) reported results of neurological examination and Bayley testing at one year corrected age in 455 of 615 survivors (74%). Results showed no differences between groups in terms of neurodevelopmental impairment occurring as cerebral palsy, blindness or severe hearing loss (Bayley MDI < 70 or PDI < 70).
Van Meurs 2007 applied the same testing protocol for larger infants in this study as was applied for Van Meurs 2005. Of 20 survivors, 17 were seen, and results presented in the main publication showed no differences between groups.
Ballard 2006 (Walsh 2010) performed follow‐up examinations including a Bayley II evaluation at two years' corrected age. Researchers evaluated 477 of 535 survivors in this way (89%) and diagnosed neurodevelopmental impairment by using criteria identical to those of this review. Results showed no differences between groups.
Cerebral palsy (Outcome 1.10)
Of note in the meta‐analysis of effects of iNO on cerebral palsy, Schreiber 2003 and Subhedar 1997 presented data on all severities of CP, whereas the other trials (Ballard 2006; Kinsella 2006; Van Meurs 2005; Van Meurs 2007) provided results showing moderate/severe or disabling cerebral palsy.
Severe retinopathy of prematurity (Outcome 1.11)
Investigators expressed results among the number of survivors evaluated. Evidence did not suggest an effect on severe retinopathy of prematurity (as reported by Schreiber 2003 and by Van Meurs 2005).
Retinopathy requiring surgery (Outcome 1.12)
No evidence suggests an effect on this outcome. Results were as follows.
Studies with entry before three days of age based on oxygenation criteria: typical RR 0.87, 95% CI 0.59 to 1.29; four studies, 673 infants; typical RD ‐0.02, 95% CI ‐0.07 to 0.03.
Studies with entry after three days of age based on BPD risk: typical RR 1.04, 95% CI 0.78 to 1.38; one study, 582 infants; typical RD 0.01, 95% CI ‐0.06 to 0.08.
Studies of routine use in intubated preterm infants: typical RR 1.08, 95% CI 0.79 to 1.47; two studies, 917 infants; typical RD 0.01, 95% CI ‐0.03 to 0.06.
Other reported results
Oxygenation within two hours of therapy
Kinsella 1999 reported improvement in PaO2 after 60 minutes of about 40 mmHg compared with about 10 mmHg among controls.
Subhedar 1997 reported that treated infants had a sustained fall of OI on iNO; a 25% reduction in oxygenation index or a 0.10 reduction in FiO2 was more likely during the first two hours among treated infants (13/20) compared with control infants (4/22). This was reflected by the greater percentage decrease in OI in the treated group (16.9%) compared with the control group (no change); however, OI was higher at baseline among treated infants.
Mercier 1999 demonstrated effects of iNO on oxygenation at two hours, with a median 5.4 fall in OI for nitric oxide infants, and a median 3.6 fall in OI for control infants. Study authors presented oxygenation results in ways that were too variable to allow meta‐analysis. Overall, it appeared that improvements in oxygenation were probably more frequent when infants received iNO compared with no therapy.
Srisuparp 2002 reported significant increases in PaO2 and SpO2 from baseline for the iNO group.
Wei 2014 reported no significant changes in blood gas markers (PaO2, SpO2, OI) after 60 minutes between the NO group and the control group.
Pulmonary artery pressure
Subhedar 1997 reported a reduction in pulmonary artery pressure as assessed by echocardiography within 30 minutes of treatment compared with no change in control infants.
Duration of assisted ventilation
Kinsella 1999 found a significant reduction in ventilator days among iNO survivors (median 26, range three to 69 days among iNO infants; median 37, range eight to 395 days among control infants). However, Mercier 1999 reported no significant difference in the duration of assisted ventilation among survivors (iNO, median 12 days; control, median 16 days). As only median results were given with a different descriptor of variance (interquartile range), projecting a typical estimate was not possible. Hascoet 2005 supplied data regarding ventilator days among iNO‐treated survivors compared with controls (iNO 14.5 days, SD 11.4 (median 9 days); control 17.1 days, SD 16.4 (median 10 days)). Wei 2014 reported no significant differences in time spent when mechanical ventilation or nasal CPAP supplementary ventilation was used among survivors (iNO, median 98 days; control, median 112 days).
Discussion
This review suggests that no clear indications are known for inhaled nitric oxide (iNO) in preterm infants. Previous versions of this review suggested that early routine treatment might be beneficial, but with the addition of EUNO 2009 results, reduction in death or bronchopulmonary dysplasia (BPD) and reduction in brain injury, which appeared possible previously, are no longer significant. Heterogeneity for these results remains unexplained, and further investigation of the reasons behind this heterogeneity may be helpful.
Early rescue treatment does not appear successful and may lead to a non‐significant increase in brain injury. However, preterm infants with clear evidence of pulmonary hypertension have not been separately identified in these studies and may constitute a subgroup with a different response.
Mortality
None of the individual trials showed a reduction in mortality, except Wei 2014. Meta‐analysis of the first two trials that evaluated routine use of iNO in intubated infants suggested a potential difference, but addition of the similar EUNO 2009 trial revealed no apparent effect.
Survival without bronchopulmonary dysplasia
Studies investigating iNO as routine treatment in preterm infants showed no effect on the combined outcome of death or bronchopulmonary dysplasia. Studies with entry before three days of age based on oxygenation criteria revealed no apparent benefit from iNO.
Ballard 2006 reported a reduction in the combined outcome of death or BPD in a large multi‐centre trial upon analysis of data, as planned, with the multiple outputation technique. A preplanned subgroup analysis in the subgroup of infants who were seven to 14 days of age at enrolment, and in the subgroup of infants who were less unwell (respiratory severity score < 3.5), showed greatest benefit. Yoder 2013 conducted a further trial specifically enrolling such children and showed, in contrast, no benefit of iNO. The summary overall effect noted in trials of later treatment to prevent BPD shows a risk ratio of "death or BPD" of 0.92, with 95% confidence intervals (CIs) just including the possibility of no effect (0.85 to 1.01).
Brain injury
Three studies of early routine use of iNO in intubated preterm infants showed no effect on serious brain injury diagnosed by ultrasonography ‐ severe intraventricular haemorrhage or the combined outcome of severe haemorrhage or periventricular leukomalacia. Early rescue studies showed a possible effect on this outcome; the 20% increase in severe haemorrhage is important, given that researchers demonstrated no benefit.
Neurological and developmental outcomes
Data regarding long‐term neurodevelopmental outcomes are accumulating. Early rescue studies that reported neurodevelopmental outcomes reported no improvement.
As for early routine use, Schreiber 2003 demonstrated a reduction in abnormal neurodevelopmental outcome at two years of age, largely due to improved Bayley scores of mental development. The other study that showed improvement in appearances of the brain on ultrasonography (Kinsella 2006) has not yet reported longer‐term outcomes.
Early routine use of iNO in intubated preterm infants previously appeared to decrease the incidence of severe brain injury as demonstrated on ultrasonography. Although the criteria for study entry for Kinsella 2006 required only that infants must be less than or equal to 34 weeks' gestation, in fact, the mean gestational age was 25.6 weeks in each group, and mean birth weight less than 800 grams, demonstrating that a higher‐risk group was enrolled. Similarly, for Schreiber 2003, the actual birth weight was approximately 1 kilogram for the two groups and gestational age was less than 28 weeks. In addition, this hospital served a somewhat deprived inner city neighbourhood, with a low rate of antenatal steroid use (< 60%). However, the addition of EUNO 2009 abolished this apparent benefit, and no significant change in the overall frequency of severe intraventricular haemorrhage or periventricular leukomalacia is apparent. Of note, only Kinsella 2006 carefully reported head abnormalities on ultrasonography before enrolment and described a lower frequency of new lesions in the group treated with iNO than in the control group.
Overall
Further analysis of patient characteristics associated with a possible beneficial response would be helpful. An editorial accompanying published results of Schreiber 2003 suggested that maternal ethnicity may have influenced findings; however, the somewhat similar study of Kinsella 2006 showed no effect of ethnicity.
One particular finding of this review is our inability to replicate the analysis performed by authors of the Ballard trial. The authors of this study randomised multiples (twins or triplets from the same pregnancy) as clusters. The analysis technique that they utilised (multiple outputation) cannot be replicated without access to the original data; therefore, results of the RevMan analysis differ from those reported in the original publication. We did not find a significant effect, whereas multiple outputation revealed a statistically significant result. Ballard and associates used this technique in part because significant evidence of a genetic effect may influence outcomes such as BPD, and thus infants from multiple gestations may be more likely to show coherent effects of an intervention than non‐related infants.
Various approaches are being utilised by investigators, including generalised estimating equations (GEEs) with sensitivity analysis and the multiple outputation techniques mentioned above. We recently performed an individual participant meta‐analysis using data from all but one of the relevant trials and confirmed the original authors' conclusions based on these techniques (Askie 2010). We advise caution when attempts are made to combine trial results because some trials used specific techniques to account for multiples.
Overall results of meta‐analysis when individual participant data were combined confirm overall results of RevMan analyses and should allow further in‐depth analysis of subgroups of infants who may benefit and who should be entered into future trials. In future studies, investigators need to be specific regarding techniques used to account for outcomes of multiple pregnancies.
Authors' conclusions
Implications for practice.
In very sick preterm infants who meet the criteria for poor oxygenation, rescue therapy with iNO does not improve survival, survival without BPD or brain injury, even though oxygenation may be improved in the short term. In fact, some evidence suggests a potential increase in severe intracranial haemorrhage and in the combined outcome of severe intraventricular haemorrhage or periventricular leukomalacia. In view of these findings, iNO should not be routinely used for preterm infants as rescue therapy in cases of hypoxic respiratory failure.
Early routine use of iNO in ventilated preterm infants who are not severely ill but nevertheless are at risk for serious brain injury or BPD does not appear to be effective. Although some trials have shown potential benefit, meta‐analysis of the three trials in this grouping did not confirm an overall benefit. Only a single study in this group has reported longer‐term neurodevelopmental outcomes, and this study has suggested beneficial effects. Current data do not support widespread routine treatment with iNO to prevent BPD or to prevent brain injury.
Use of iNO in small preterm infants who remain on respiratory support at five to 21 days does not seem to reduce BPD. The effect appears to be most marked in infants who are less sick, as determined by subgroup analyses, and in infants treated earlier, that is, between seven and 14 days of age. The risk difference is 0.07, and, if this effect is confirmed, the number needed to treat for an additional beneficial outcome (NNTB) is approximately 14. Owing to inability to replicate the original analysis, the 95% CI of the risk difference includes 1; therefore, we calculated the 95% CI of the NNTB to include 7 and infinity.
Implications for research.
Additional studies on the use of inhaled nitric oxide in preterm infants are warranted. Further analysis of the results of Kinsella 2006,Schreiber 2003 and EUNO 2009 may delineate groups that are more likely to benefit from early routine iNO with reduction in both brain injury and BPD for inclusion in future studies. Long‐term follow‐up should be provided in future studies.
Later use at between seven and 14 days of age for infants who continue to require respiratory support also warrants further investigation. Lack of apparent effect as determined by subgroup analysis of sicker infants is disappointing, and other approaches should be considered for these infants, including investigation of other doses.
What's new
| Date | Event | Description |
|---|---|---|
| 18 December 2016 | New search has been performed | This updates the review, "Inhaled nitric oxide for respiratory failure in preterm infants", which was published in the Cochrane Database of Systematic Reviews. Three new trials have been added. |
| 15 January 2016 | New search has been performed | This updates again the review, "Inhaled nitric oxide for respiratory failure in preterm infants", which was published in the Cochrane Database of Systematic Reviews (Barrington 2006). The search was updated in January 2016. One new trial has been added (Wei 2014). |
| 15 January 2016 | New citation required but conclusions have not changed | Conclusions remain unchanged. |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 1, 1999
| Date | Event | Description |
|---|---|---|
| 13 October 2010 | New citation required and conclusions have changed | This review suggests that no clear indications are known for iNO in preterm infants. Previous versions of this review suggested that early routine treatment might be beneficial, but with addition of the EUNO 2009 results, reduction in death or BPD and reduction in brain injury, which appeared possible previously, are no longer significant. |
| 13 October 2010 | New search has been performed | This updates the review, "Inhaled nitric oxide for respiratory failure in preterm infants", which was published in the Cochrane Database of Systematic Reviews (Barrington 2006). The search was updated in June 2010. Three new trials have been added, along with follow‐up data from other studies. |
| 28 October 2009 | Amended | This review has been converted to new review format. |
| 1 March 2007 | New citation required but conclusions have not changed | Substantive amendments have been made. |
| 1 March 2007 | New search has been performed | This review is an update of the existing review, "Inhaled nitric oxide for respiratory failure in preterm infants" (Barrington 2006). This update includes additional information regarding completed trials by Ballard, Kinsella and Srisuparp. Overall analyses have been deleted, as study characteristics differ too much for these to be useful. Subgroup analyses are presented instead. |
Acknowledgements
The Cochrane Neonatal Review Group has been funded in part with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Appendices
Appendix 1. Search strategy January 2016
(nitric oxide OR nitric) and database specific terms:
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh])) Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomised controlled trial or controlled clinical trial or randomised or placebo or clinical trials as topic or randomly or trial or clinical trial) CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomised controlled trial OR controlled clinical trial OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Clinicaltrials.gov: (infant)
Controlled‐trials.com: (infant)
WHO trials database: (infant OR neonate)
Data and analyses
Comparison 1. Inhaled NO compared with control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death before discharge | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Studies with entry before 3 days based on oxygenation | 10 | 1066 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.18] |
| 1.2 Studies with entry after 3 days based on BPD risk | 3 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.81, 1.71] |
| 1.3 Studies of routine use in preterm infants on respiratory support | 4 | 1924 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.10] |
| 2 Death before 36 weeks' postmenstrual age | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Studies with entry before 3 days based on oxygenation | 5 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.72, 1.11] |
| 2.2 Studies with entry after 3 days based on BPD risk | 2 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.81, 2.20] |
| 2.3 Studies of routine use in preterm infants on respiratory support | 2 | 924 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.90, 1.89] |
| 3 Bronchopulmonary dysplasia among survivors at 36 weeks | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Studies with entry before 3 days based on oxygenation | 8 | 681 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.76, 1.04] |
| 3.2 Studies with entry after 3 days based on BPD risk | 3 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.83, 1.01] |
| 3.3 Studies of routine use in preterm infants on respiratory support | 4 | 1782 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.85, 1.05] |
| 4 Death or bronchopulmonary dysplasia at 36 weeks | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Studies with entry before 3 days based on oxygenation | 8 | 958 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.87, 1.01] |
| 4.2 Studies with entry after 3 days based on BPD risk | 3 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.85, 1.01] |
| 4.3 Studies of routine use in preterm infants on respiratory support | 4 | 1924 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.87, 1.02] |
| 5 Intraventricular haemorrhage (all grades) | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Studies with entry before 3 days based on oxygenation | 4 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.69, 1.28] |
| 5.2 Studies with entry after 3 days based on BPD risk | 1 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.48, 2.54] |
| 5.3 Studies of routine use in preterm infants on respiratory support | 2 | 1573 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.88, 1.23] |
| 6 Intraventricular haemorrhage (grade 3 or 4) | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Studies with entry before 3 days based on oxygenation | 6 | 773 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.98, 1.47] |
| 6.2 Studies of routine use in preterm infants on respiratory support | 4 | 1913 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.73, 1.09] |
| 7 Intraventricular haemorrhage (grade 3 or 4) or periventricular leukomalacia | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Studies with entry before 3 days based on oxygenation | 8 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.88, 1.33] |
| 7.2 Studies of routine use in preterm infants on respiratory support | 3 | 1747 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.73, 1.12] |
| 8 Neurodevelopmental disability | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Studies with entry before 3 days based on oxygenation | 2 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.78, 1.40] |
| 8.2 Studies with entry after 3 days based on BPD risk | 2 | 498 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.09] |
| 8.3 Studies of routine use in preterm infants on respiratory support | 3 | 1223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.74, 1.13] |
| 9 Bayley MDI or PDI < ‐2 SD | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Studies of routine use in preterm infants on respiratory support | 2 | 768 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.36, 0.90] |
| 10 Cerebral palsy | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Studies with entry before 3 days based on oxygenation | 2 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.93, 3.71] |
| 10.2 Studies with entry after 3 days based on BPD risk | 2 | 498 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.54, 2.23] |
| 10.3 Studies of routine use in preterm infants on respiratory support | 3 | 1223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.69, 1.47] |
| 11 Severe retinopathy of prematurity (≥stage 3) | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Studies with entry before 3 days based on oxygenation | 3 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.64, 1.47] |
| 11.2 Studies of routine use in preterm infants on respiratory support | 2 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.29, 1.46] |
| 12 Retinopathy of prematurity requiring surgery | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Studies with entry before 3 days based on oxygenation | 4 | 673 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.59, 1.29] |
| 12.2 Studies with entry after 3 days based on BPD risk | 1 | 582 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.78, 1.38] |
| 12.3 Studies of routine use in preterm infants on respiratory support | 2 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.79, 1.47] |
1.1. Analysis.
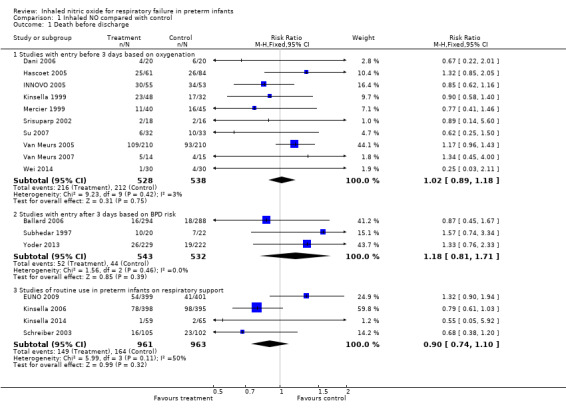
Comparison 1 Inhaled NO compared with control, Outcome 1 Death before discharge.
1.2. Analysis.
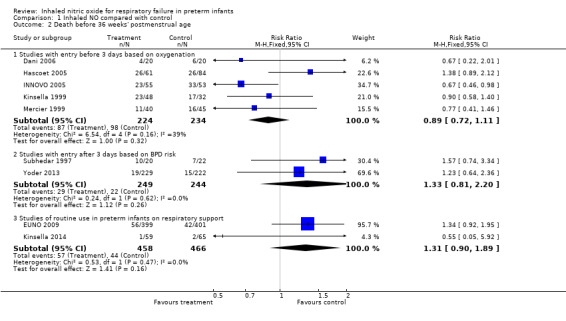
Comparison 1 Inhaled NO compared with control, Outcome 2 Death before 36 weeks' postmenstrual age.
1.3. Analysis.
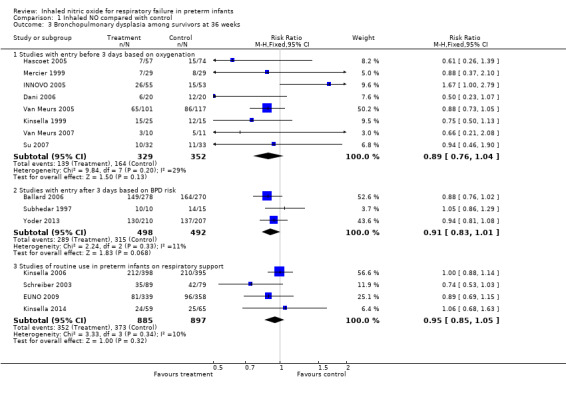
Comparison 1 Inhaled NO compared with control, Outcome 3 Bronchopulmonary dysplasia among survivors at 36 weeks.
1.4. Analysis.
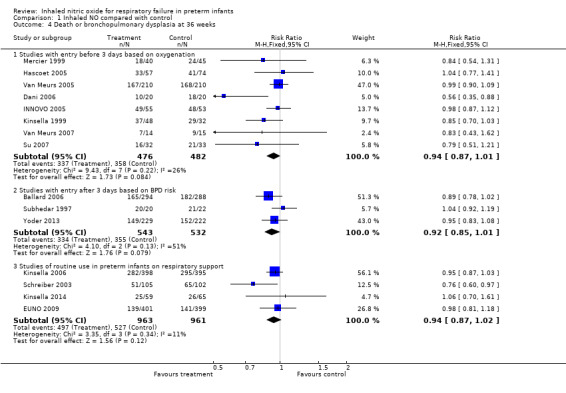
Comparison 1 Inhaled NO compared with control, Outcome 4 Death or bronchopulmonary dysplasia at 36 weeks.
1.5. Analysis.
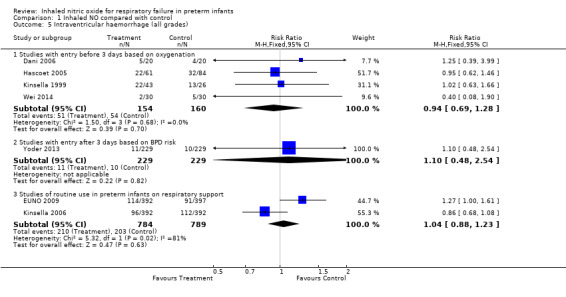
Comparison 1 Inhaled NO compared with control, Outcome 5 Intraventricular haemorrhage (all grades).
1.6. Analysis.
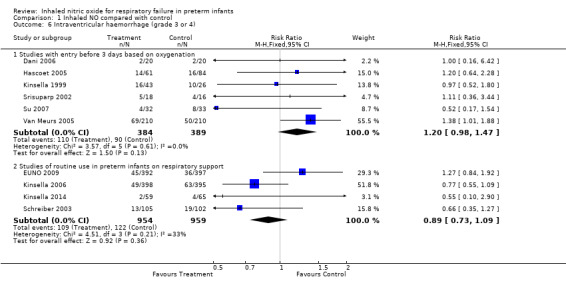
Comparison 1 Inhaled NO compared with control, Outcome 6 Intraventricular haemorrhage (grade 3 or 4).
1.7. Analysis.
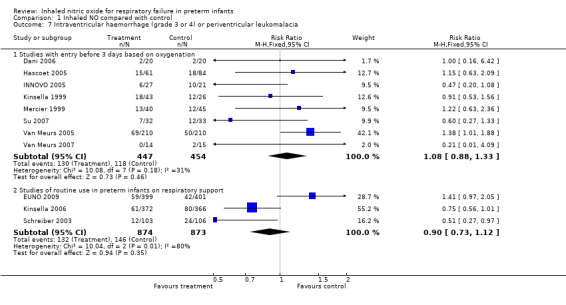
Comparison 1 Inhaled NO compared with control, Outcome 7 Intraventricular haemorrhage (grade 3 or 4) or periventricular leukomalacia.
1.8. Analysis.
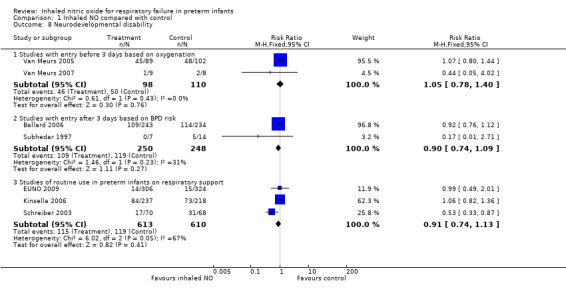
Comparison 1 Inhaled NO compared with control, Outcome 8 Neurodevelopmental disability.
1.9. Analysis.
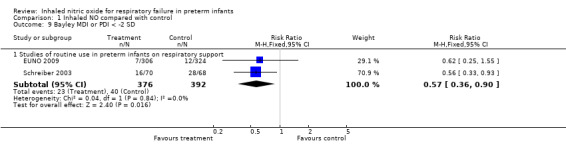
Comparison 1 Inhaled NO compared with control, Outcome 9 Bayley MDI or PDI < ‐2 SD.
1.10. Analysis.
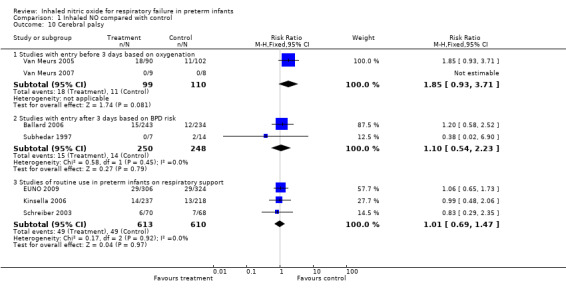
Comparison 1 Inhaled NO compared with control, Outcome 10 Cerebral palsy.
1.11. Analysis.
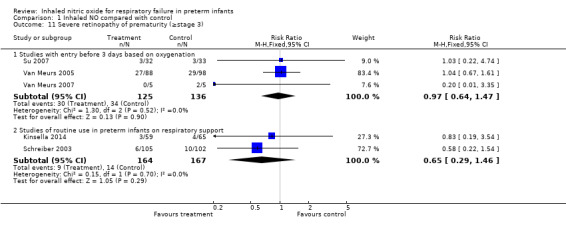
Comparison 1 Inhaled NO compared with control, Outcome 11 Severe retinopathy of prematurity (≥stage 3).
1.12. Analysis.
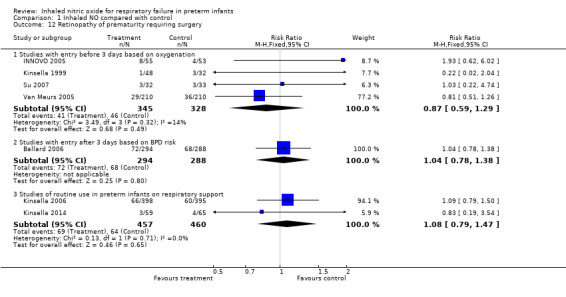
Comparison 1 Inhaled NO compared with control, Outcome 12 Retinopathy of prematurity requiring surgery.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ballard 2006.
| Methods | Multi‐centre trial | |
| Participants | 582 infants < 1250 grams on assisted ventilation at 7‐21 days (or, if < 800 grams, on CPAP) | |
| Interventions | Inhaled NO at 20 ppm initial dose for 48‐96 hours; the dose was subsequently decreased to 10, 5 and 2 ppm at weekly intervals, with a minimum treatment duration of 24 days | |
| Outcomes | Survival without BPD at 36 weeks' postmenstrual age Secondary outcomes included duration of oxygen therapy and duration of hospitalisation. In addition, investigators prospectively evaluated the need for hospitalisation and respiratory support, including mechanical ventilation, continuous positive airway pressure and oxygen supplementation at 40, 44, 52 and 60 weeks' postmenstrual age. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in permuted blocks at study centre |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masking of intervention: yes Masking of outcome assessment: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completeness of follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
| Funding Source | Low risk | Funded by a government agency (NICHD); study gas provided by industry (Ikaria) |
Dani 2006.
| Methods | Single‐centre trial | |
| Participants | 40 preterm infants ventilated with severe RDS with FiO2 > 0.5 and arterial‐alveolar oxygen ratio < 0.15, despite surfactant treatment | |
| Interventions | iNO at 10 ppm for 4 hours followed by 6 ppm compared with no treatment. Weaning started at 72 hours or when the infant was extubated, or when FiO2 was < 0.3 with mean airway pressure < 8 cmH2O | |
| Outcomes | The primary endpoint was death or BPD. Bronchopulmonary dysplasia was defined as oxygen requirement at 36 weeks' postmenstrual age. Secondary endpoints were evaluation of ventilation changes during iNO therapy, duration of oxygen treatment, NCPAP and mechanical ventilation, incidence of patent ductus arteriosus (PDA), pulmonary hypertension, intraventricular haemorrhage (IVH), periventricular leukomalacia (PVL), retinopathy of prematurity (ROP), necrotising enterocolitis (NEC), sepsis and length of stay in the intensive care unit and in hospital. |
|
| Notes | Study terminated after 40 infants enrolled. Initially planned to include 26 per group. Unplanned interim analysis was performed because of an impression that the results were significant. No evidence indicated that the analysis was adjusted to account for potential multiple looks at the data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Masking of allocation: yes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting, but protocol not available |
| Other bias | High risk | Early termination of trial due to impression of an effect |
| Funding Source | Unclear risk | Funding source unclear |
EUNO 2009.
| Methods | Multi‐centre trial | |
| Participants | 800 infants between 24 weeks' and 28 weeks' gestation and 6 days enrolled at less than 24 hours of age. If intubated, they had to have received surfactant and could be enrolled if on CPAP requiring > 30% oxygen. Patients were ineligible if they required more than 50% O2 to maintain saturation over 85% on a mean airway pressure ≥ 8 cmH2O. | |
| Interventions | Inhaled NO at 5 ppm for at least 7 and a maximum of 21 days | |
| Outcomes | Primary outcome was survival without BPD at 36 weeks' postmenstrual age. Secondary outcome was survival without severe brain injury on head ultrasonography. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Centralised interactive Web‐based enrolment and randomisation system |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masking of intervention: yes Masking of outcome assessment: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completeness of follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | Registered trial, primary outcomes reported |
| Other bias | Low risk | |
| Funding Source | High risk | Funded by industry (Ikaria), initiated by investigators |
Hascoet 2005.
| Methods | Multi‐centre trial | |
| Participants | 860 infants < 32 weeks enrolled but not eligible for study gas unless they developed hypoxic respiratory failure (i.e. need for mechanical ventilation, FiO2 > .40, and arterial alveolar O2 ratio < 0.22 at 6 to 48 hours of age; n = 145 | |
| Interventions | Inhaled NO was started at 5 ppm, with adjustments allowed depending on response up to a maximum of 10 ppm. Eventually, 61 were treated with iNO and 84 were given the control intervention. Participants were allowed to receive iNO in either group if they developed refractory hypoxaemia. | |
| Outcomes | Primary outcome was survival to 28 days without death, need for oxygen, IVH > grade 1 or refractory hypoxaemia defined as need for 100% oxygen with PaO2 < 50. Secondary outcomes included incidence and severity of IVH and periventricular leukomalacia (PVL), BPD or steroid treatment and pulmonary haemorrhage, patent ductus arteriosus (PDA), necrotising enterocolitis and nosocomial infection. |
|
| Notes | Open‐label iNO provided to all infants when they met refractory hypoxaemia criteria ‐ 20 infants received treatment before 6 hours and were not included; 28 control infants received open‐label iNO after the randomised intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified, blocked central randomisation |
| Allocation concealment (selection bias) | Low risk | Masking of allocation: yes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Masking of intervention: no Masking of outcome assessment: no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completeness of follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | Registration documents or protocol not found |
| Funding Source | Low risk | Supported by local sources (University of Nantes) and industry (Air Liquide) |
INNOVO 2005.
| Methods | Multi‐centre trial | |
| Participants | 108 preterm infants (< 34 weeks) less than 28 days of age, with "severe respiratory failure" | |
| Interventions | Inhaled NO usually at 5 ppm up to 40 ppm (n = 55) or no supplemental gas (n = 53) | |
| Outcomes | Primary outcomes were (1) death or severe disability at 1 year corrected postnatal age; and (2) death or continued oxygen need at expected date of birth. Secondary outcomes included length of stay in hospital; length of time on supplemental oxygen; length of time on ventilatory support; pneumothorax; other pulmonary air leak; pulmonary haemorrhage; major cerebral abnormality; necrotising enterocolitis; patent ductus arteriosus needing medical treatment; treatment of retinopathy of prematurity; infection; and age at which full oral feeding was established. Secondary outcomes at 1 year corrected age included disability and/or impairment of neuromotor development, vision and hearing; respiratory problems; seizures; growth; and hospital admissions. |
|
| Notes | Initially planned 200 participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation with minimisation |
| Allocation concealment (selection bias) | Low risk | Masking of allocation: yes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Masking of intervention: no Masking of outcome assessment: no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completeness of follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | Registered trial, primary outcomes reported |
| Other bias | Unclear risk | Recruited half of planned sample size in the 2‐year time frame |
| Funding Source | Low risk | Funded by government agency (MRC, UK) |
Kinsella 1999.
| Methods | Multi‐centre trial | |
| Participants | 80 preterm infants (≤ 34 weeks) < 7 days of age, with a/AO2 < 0.1 on 2 blood gases after surfactant treatment | |
| Interventions | Inhaled NO at 5 ppm (n = 48) or no supplemental gas (n = 32) for 7 days, after which "trials off" were allowed. Maximum treatment duration was 14 days. | |
| Outcomes | Primary outcome was survival. Bronchopulmonary dysplasia, intraventricular haemorrhage and duration of ventilation were secondary outcomes. |
|
| Notes | Initially planned 210 participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central stratified randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol or registration document found |
| Other bias | High risk | Study terminated early after planned first interim analysis, as little difference in outcomes was apparent |
| Funding Source | Low risk | Funded by government agency (NIH) and in part by industry (iNOtherapeutics) |
Kinsella 2006.
| Methods | Multi‐centre trial | |
| Participants | 793 preterm infants < 34 weeks, respiratory failure needing assisted ventilation in first 48 hours | |
| Interventions | iNO at 5 ppm (n = 398) or no iNO (n = 395) for 21 days or until extubation | |
| Outcomes | Primary outcome was death or bronchopulmonary dysplasia. Secondary outcomes included severe intraventricular haemorrhage, periventricular leukomalacia and ventriculomegaly. |
|
| Notes | Baseline and follow‐up cranial ultrasonography was required. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central stratified, blocked randomisation |
| Allocation concealment (selection bias) | Low risk | Masking of allocation: yes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Registered trial, primary outcomes reported |
| Other bias | Low risk | |
| Funding Source | Low risk | Funded by government agency (NHLBI) and in part by industry (iNOtherapeutics) |
Kinsella 2014.
| Methods | Multi‐centre parallel‐group randomised trial | |
| Participants | 124 preterm infants with birth weight of 500 to 1250 grams, receiving oxygen by non‐invasive means at < 72 hours of age | |
| Interventions | iNO at 10 ppm (to give effective concentration ≥ 5 ppm) or placebo, for at least 2 weeks and until 30 weeks' postmenstrual age | |
| Outcomes | Death or BPD, IVH, retinopathy of prematurity, necrotising enterocolitis, treatment of infants with PDA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled trial |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Registered trial, primary outcomes reported |
| Other bias | Low risk | |
| Funding Source | Low risk | Funded by government agency (NHLBI), funded in part by industry (Ikaria supplied gases) |
Mercier 1999.
| Methods | Multi‐centre parallel‐group randomised trial | |
| Participants | 85 preterm infants (< 33 weeks) with OI of 12.5 to 30 at < 7 days | |
| Interventions | 10 ppm inhaled NO (n = 40) or control (n = 45). Open‐label treatment with NO allowed in controls if OI > 30 | |
| Outcomes | Primary outcome was decrease in OI after 2 hours of therapy. | |
| Notes | Initially planned 360 infants across both gestational age strata | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised phone randomisation |
| Allocation concealment (selection bias) | Low risk | Masking of allocation: yes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completeness of follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | No protocol or registration found |
| Other bias | Unclear risk | Stopped early because of slowing enrolment |
| Funding Source | Low risk | Funded by local agency, supported in part by industry (Air Liquide) |
Schreiber 2003.
| Methods | Single‐centre trial | |
| Participants | 207 infants < 34 weeks, < 72 hours of age, intubated and ventilated for RDS, birth weight < 2000 grams | |
| Interventions | Randomised to iNO (N = 105) (starting at 10 ppm for 1 day, then 5 ppm for 6 days; thereafter weaned by 1 ppm, stopped if extubated) vs control (N = 102); HFOV (N= 102) vs CMV (N = 105) | |
| Outcomes | Primary outcome was a decrease in death or BPD at 36 weeks. | |
| Notes | Factorial 2 × 2 design comparing high‐frequency ventilation vs conventional treatment and iNO vs placebo gas | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified blocked randomisation |
| Allocation concealment (selection bias) | Low risk | Masked allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo‐controlled trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol or registration documents found |
| Funding Source | High risk | Funded by industry (Ikaria), investigator initiated |
Srisuparp 2002.
| Methods | Single‐centre trial | |
| Participants | 34 infants weighing < 2000 grams, ventilated after surfactant with an arterial catheter at < 72 hours of age. Also required to satisfy a severity of illness criterion. OI > 4 for birth weight < 1000 grams, > 6 for birth weight 1001‐1250 grams, > 8 for 1251‐1500 grams, > 10 for 1501‐1750 grams and > 12 for 1751‐2000 grams | |
| Interventions | iNO at 20 ppm or standard care, trial of weaning after 72 hours, maximum duration 7 days | |
| Outcomes | Primary outcome was severe intraventricular haemorrhage (grade 3 or 4). | |
| Notes | Performed as a preliminary pilot study, before Schreiber 2003 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details available |
| Allocation concealment (selection bias) | Unclear risk | Masking of allocation: unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completeness of follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | No registration or protocol found |
| Funding Source | Unclear risk | Not stated |
Su 2007.
| Methods | Single‐centre randomised trial | |
| Participants | 65 preterm infants with birth weight < 1500 grams or gestational age < 32 weeks, intubated with OI ≥ 25 | |
| Interventions | iNO initially at a dose of 5ppm, could be increased to 20 ppm in cases of poor response, or decreased if good response obtained; duration of therapy not clear according to protocol, but mean duration of receipt of iNO was 4.9 days | |
| Outcomes | Primary outcome variable was OI 24 hours after randomisation. Secondary outcomes included mortality, BPD, intracranial haemorrhage, patent ductus arteriosus and retinopathy of prematurity. | |
| Notes | Not all of the infants received surfactant: 23 of 32 iNO treated and 24 of 33 controls | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details of randomisation procedures provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | Masking of intervention: no Masking of outcome assessment: no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No registration documents or protocol found |
| Funding Source | Unclear risk | Unclear |
Subhedar 1997.
| Methods | Single‐centre randomised comparison of iNO, dexamethasone, combined therapy and control, using a 2 × 2 factorial design | |
| Participants | 42 preterm infants less than 32 weeks' gestation with "high risk" of developing BPD | |
| Interventions | iNO initially administered at 20 ppm and weaned if effective (n = 20) or control (n = 22). Dexamethasone at 1 mg/kg/d for 3 days, followed by 0.5 mg/kg/d for 3 days (n = 21) (3 infants received a lower dose), or no steroids (n = 21) | |
| Outcomes | Primary outcome was survival without bronchopulmonary dysplasia. Secondary outcomes included duration of ventilation, intraventricular haemorrhage and other neonatal complications. | |
| Notes | Initially planned 88 participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Masking of allocation: yes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Masking of intervention: no Masking of outcome measurement: no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completeness of follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | No registration or protocol found |
| Other bias | High risk | Terminated early because frequency of adverse primary outcome was close to 100% in all groups |
| Funding Source | Low risk | Government agency and local funds, some support from industry |
Van Meurs 2005.
| Methods | Multi‐centre trial | |
| Participants | 420 preterm infants, < 34 weeks, OI ≥ 10 on 2 blood gases 30 minutes to 12 hours apart. ≥ 4 hours after surfactant | |
| Interventions | iNO initially at 5 ppm to 10 ppm (210) or placebo (210) (if no response at 10 ppm, study gas was stopped). Weaning ≥ 10 hours after initiation. Maximum duration was 336 hours. | |
| Outcomes | Primary outcome was reduced death or BPD at 36 weeks. Secondary outcomes were grade 3 or 4 intraventricular haemorrhage or periventricular leukomalacia, number of days of assisted ventilation and oxygen use, length of hospitalisation and threshold retinopathy of prematurity. | |
| Notes | Initially planned 220 infants per arm; stopped by the data monitoring committee because of apparent increase in severe IVH with no evidence of benefit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified blocked central randomisation |
| Allocation concealment (selection bias) | Low risk | Masking of allocation: yes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masking of intervention: yes Masking of outcome assessment: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completeness of follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | Registered trial, primary outcomes reported |
| Other bias | High risk | Study terminated a little early because of an increase in severe IVH in the intervention group |
| Funding Source | Low risk | Support from government agency (NICHHD), partial support from industry (Ikaria) |
Van Meurs 2007.
| Methods | Multi‐centre trial | |
| Participants | 29 infants at less than 34 weeks' gestation with birth weight > 1500 grams; ventilated with OI > 15 on 2 consecutive blood gases between 30 minutes and 12 hours apart | |
| Interventions | iNO initially at 5 ppm to 10 ppm (210) or placebo (210) (if no response at 10 ppm, study gas was stopped). Weaning ≥ 10 hours after initiation. Maximum duration was 14 days. | |
| Outcomes | Primary outcome was reduced death or BPD at 36 weeks. Secondary outcomes were grade 3 or 4 intraventricular haemorrhage or periventricular leukomalacia, number of days of assisted ventilation and oxygen use, length of hospitalisation and threshold retinopathy of prematurity. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central telephone randomisation, stratified and blocked |
| Allocation concealment (selection bias) | Low risk | Masked allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo‐controlled trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Funding Source | Low risk | Government agency (NICHHD), partial support from industry (Ikaria) |
Wei 2014.
| Methods | Single‐centre randomised trial of iNO vs no treatment gas | |
| Participants | 60 preterm infants at less than 34 weeks, receiving mechanical ventilation or CPAP, with OI ≥ 11 2 hours after surfactant therapy | |
| Interventions | iNO initially at 5 ppm or placebo. Duration of therapy 7 days or until ventilation withdrawal | |
| Outcomes | Primary outcome was change in OI status over different times during first 3 days of therapy. Secondary outcomes included mortality, BPD, intraventricular haemorrhage and other neonatal complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details available |
| Allocation concealment (selection bias) | High risk | No details of randomisation procedures provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No registration or protocol found |
| Funding Source | Unclear risk | Not stated |
Yoder 2013.
| Methods | Multi‐centre parallel‐group randomised trial | |
| Participants | 451 preterm infants, < 1250 grams and < 30 weeks, at 5 to 14 days of age, on invasive ventilation, or on non‐invasive support if they weighed < 800 grams | |
| Interventions | iNO at 20 ppm for 3 to 4 days, then 10 ppm for 10 days, then 5 ppm until 24 days | |
| Outcomes | Survival without BPD, duration of respiratory support and hospitalisation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised computer‐generated |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled trial |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No significant attrition |
| Selective reporting (reporting bias) | Low risk | Registered trial, primary outcomes reported |
| Other bias | Low risk | |
| Funding Source | High risk | Industry initiated and funded (Ikaria) |
a/AO2: arterial‐alveolar oxygen ratio. BPD: bronchopulmonary dysplasia. CMV: cytomegalovirus. CPAP: continuous positive airway pressure. FiO2: fraction of inspired oxygen. HVOF: high‐velocity oxygen fuel. iNO: inhaled nitric oxide. IVH: intraventricular haemorrhage. MRC: Medical Research Council. NEC: necrotising enterocolitis. NICHD: Eunice Kennedy Shriver National Institute of Child Health and Human Development. NO: nitric oxide. PDA: patent ductus arteriosus. PVL: periventricular leukomalacia. RDS: respiratory distress syndrome. ROP: retinopathy of prematurity.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Day 1996 | This was a randomised controlled trial of 50 infants with pulmonary hypertension, 16 of whom were at less than 36 weeks' gestation. The definition of prematurity is not the same as that used in this review, and in any case, it is not possible to extract data that refer solely to preterm infants. |
| Lindwall 2005 | No untreated control group was included. Short‐term randomised cross‐over trial of response to inhaled nitric oxide among infants on continuous positive airway pressure |
| Skimming 1997 | No untreated control group was included. This was a randomised comparison of 5 ppm and 20 ppm for 15 minutes in preterm infants. |
Differences between protocol and review
The initial protocol did not foresee dividing included trials into three groups according to indications for enrolment. We added methods, the plan for Summary of findings tables and GRADE recommendations, which were not included in the original protocol.
We added the following outcomes post hoc (as reported in trials but not prespecified): oxygenation within two hours of therapy, pulmonary artery pressure, duration of assisted ventilation.
Contributions of authors
KJB and NF were involved in development and writing of the review protocol, the literature search and appraisal, data extraction and completion of the final review. TP assisted in revision of the article, analysis of publications and completion of the latest update.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201600005C
Declarations of interest
Dr Barrington was Chair of the Canadian Medical Advisory Committee for iNO therapeutics for one meeting in 2004. Dr Barrington and Dr Finer were participants in a research project funded by Ikaria, an individual patient data meta‐analysis of iNO in the preterm, from which neither received any income.
Dr Barrington received an honorarium for speaking at a symposium funded by Mallinckrodt on the topic of probiotics.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ballard 2006 {published data only}
- Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, et al. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. New England Jounal of Medicine 2006;355:343‐53. [DOI] [PubMed] [Google Scholar]
- Fiore JM, Hibbs AM, Zadell AE, Merrill JD, Eichenwald EC, Puri AR, et al. The effect of inhaled nitric oxide on pulmonary function in preterm infants. Journal of Perinatology 2007;27:766‐71. [DOI] [PubMed] [Google Scholar]
- Hibbs AM, Walsh MC, Martin RJ, Truog WE, Lorch SA, Alessandrini E, et al. One‐year respiratory outcomes of preterm infants enrolled in the Nitric Oxide (to prevent) Chronic Lung Disease trial. Journal of Pediatrics 2008;153:525‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MC, Hibbs AM, Martin CR, Cnaan A, Keller RL, Vittinghoff E, et al. Two‐year neurodevelopmental outcomes of ventilated preterm infants treated with inhaled nitric oxide. Journal of Pediatrics 2010;156:556‐61.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dani 2006 {published data only}
- Dani C, Bertini G, Pezzati M, Filippi L, Cecchi A, Rubaltelli FF. Inhaled nitric oxide in very preterm infants with severe respiratory distress syndrome. Acta Paediatrica 2006;95:1116‐23. [DOI] [PubMed] [Google Scholar]
EUNO 2009 {published data only}
- Durrmeyer X, Hummler H, Sanchez‐Luna M, Carnielli VP, Field D, Greenough A, et al. Two‐year outcomes of a randomized controlled trial of inhaled nitric oxide in premature infants. Pediatrics 2013;132(3):e695‐703. [PUBMED: 23940237] [DOI] [PubMed] [Google Scholar]
- Mercier JC, Hummler H, Durrmeyer X, Sanchez‐Luna M, Carnielli V, Field D, et al. Inhaled nitric oxide for the prevention of bronchopulmonary dysplasia in premature babies (EUNO): a randomized controlled trial. Lancet 2010;376:346‐54. [DOI] [PubMed] [Google Scholar]
Hascoet 2005 {published and unpublished data}
- Hascoet JM, Fresson J, Claris O, Hamon I, Lombet J, Liska A, et al. The safety and efficacy of nitric oxide therapy in premature infants. Journal of Pediatrics 2005;146:318‐23. [DOI] [PubMed] [Google Scholar]
INNOVO 2005 {published data only}
- Ahluwalia J, Tooley J, Cheema I, Sweet DG, Curley AE, Halliday HL, et al. A dose response study of inhaled nitric oxide in hypoxic respiratory failure in preterm infants. Early Human Development 2006;82:477‐83. [DOI] [PubMed] [Google Scholar]
- Field D, Elbourne D, Truesdale A, Grieve R, Hardy P, Fenton AC, et al. Neonatal ventilation with inhaled nitric oxide versus ventilatory support without inhaled nitric oxide for preterm infants with severe respiratory failure: the INNOVO multicentre randomized controlled trial (ISRCTN 17821339). Pediatrics 2005;115:926‐36. [DOI] [PubMed] [Google Scholar]
- Huddy CL, Bennett CC, Hardy P, Field D, Elbourne D, Grieve R, et al. The INNOVO multicentre randomised controlled trial: neonatal ventilation with inhaled nitric oxide versus ventilatory support without nitric oxide for severe respiratory failure in preterm infants: follow up at 4‐5 years. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93:F430‐5. [DOI] [PubMed] [Google Scholar]
Kinsella 1999 {published data only}
- Kinsella JP, Walsh WF, Bose CL, Gerstmann DR, Labella JJ, Sardesai S. Inhaled nitric oxide in premature neonates with severe hypoxaemic respiratory failure: a randomized controlled trial. Lancet 1999;354:1061‐5. [DOI] [PubMed] [Google Scholar]
Kinsella 2006 {published and unpublished data}
- Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, et al. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. New England Journal of Medicine 2006;355:354‐64. [DOI] [PubMed] [Google Scholar]
- Watson RS, Clermont G, Kinsella JP, Kong L, Arendt RE, Cutter G, et al. Clinical and economic effects of iNO in premature newborns with respiratory failure at 1 year. Pediatrics 2009;124:1333‐43. [DOI] [PubMed] [Google Scholar]
Kinsella 2014 {published data only}
- Kinsella JP, Cutter GR, Steinhorn RH, Nelin LD, Walsh WF, Finer NN, et al. Noninvasive inhaled nitric oxide does not prevent bronchopulmonary dysplasia in premature newborns. Journal of Pediatrics 2014;165(6):1101‐8. [PUBMED: 25063725] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mercier 1999 {published data only}
- Franco‐Belgium Collaborative NO Trial Group. Early compared with delayed inhaled nitric oxide in moderately hypoxaemic neonates with respiratory failure: a randomized controlled trial. Lancet 1999;354:1066‐71. [PubMed] [Google Scholar]
- Mercier JC, Dehan M, Breart G, Clement S, O'Nody P. Inhaled nitric oxide in neonatal respiratory failure. A randomized clinical trial. Pediatric Research 1998;43:290A (Abstract). [Google Scholar]
- Truffert P, Llado‐Paris J, Mercier JC, Dehan M, Bréart G, Franco‐Belgian iNO Study Group. Early inhaled nitric oxide in moderately hypoxemic preterm and term newborns with RDS: the RDS subgroup analysis of the Franco‐Belgian iNO Randomized Trial. European Journal of Pediatrics 2003;162:646‐7. [DOI] [PubMed] [Google Scholar]
Schreiber 2003 {published data only}
- Mestan K, Marks J, Hecox K, Huo D, Schreiber MD. Neurodevelopmental outcomes of premature infants treated with inhaled nitric oxide. New England Journal of Medicine 2005;353:23‐32. [DOI] [PubMed] [Google Scholar]
- Schreiber MD, Gin‐Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. New England Journal of Medicine 2003;349:2099‐107. [DOI] [PubMed] [Google Scholar]
Srisuparp 2002 {published data only}
- Srisuparp P, Heitschmidt M, Schreiber MD. Inhaled nitric oxide therapy in premature infants with mild to moderate respiratory distress syndrome. Journal of the Medical Association of Thailand 2002;85:S469‐78. [PubMed] [Google Scholar]
Su 2007 {published data only}
- Su PH, Chen JY. Inhaled nitric oxide in the management of preterm infants with severe respiratory failure. Journal of Perinatology 2008;28:112‐6. [DOI] [PubMed] [Google Scholar]
Subhedar 1997 {published data only}
- Bennett AJ, Shaw NJ, Gregg JE, Subhedar NV. Neurodevelopmental outcome in high‐risk preterm infants treated with inhaled nitric oxide. Acta Paediatrica 2001;90:573‐6. [PubMed] [Google Scholar]
- Subhedar NV, Ryan SW, Shaw NJ. Open randomized controlled trial of inhaled nitric oxide and early dexamethasone in high risk preterm infants. Archives of Disease in Childhood. Fetal Neonatal Edition 1997;77:F185‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subhedar NV, Shaw NJ. Changes in oxygenation and pulmonary haemodynamics in preterm infants treated with inhaled nitric oxide. Archives of Disease in Childhood. Fetal Neonatal Edition 1997;77:F191‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Meurs 2005 {published data only}
- Chock VY, Meurs KP, Hintz SR, Ehrenkranz RA, Lemons JA, Kendrick DE, et al. Inhaled nitric oxide for preterm premature rupture of membranes, oligohydramnios, and pulmonary hypoplasia. American Journal of Perinatology 2009;26:317‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintz SR, Meurs KP, Perritt R, Poole WK, Das A, Stevenson DK, et al. Neurodevelopmental outcomes of premature infants with severe respiratory failure enrolled in a randomized controlled trial of inhaled nitric oxide. Journal of Pediatrics 2007;151:16‐22, 22.e1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurs KP, Wright L, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK, et al. Inhaled nitric oxide for premature infants with severe respiratory failure. New England Journal of Medicine 2005;353:13‐22. [DOI] [PubMed] [Google Scholar]
Van Meurs 2007 {published data only}
- Meurs KP, Hintz SR, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK, et al. Inhaled nitric oxide in infants >1500 g and <34 weeks gestation with severe respiratory failure. Journal of Perinatology 2007;27:347‐52. [DOI] [PubMed] [Google Scholar]
Wei 2014 {published data only}
- Wei QF, Pan XN, Li Y, Feng L, Yao LP, Liu GL, et al. Efficacy of inhaled nitric oxide in premature infants with hypoxic respiratory failure. Zhongguo Dang Dai Er Ke za Zhi [Chinese Journal of Contemporary Pediatrics] 2014;16(8):805‐9. [PUBMED: 25140772] [PubMed] [Google Scholar]
Yoder 2013 {published data only}
- Yoder BA. Inhaled NO for prevention of BPD: update on the NEWNO Trial. Hot Topics in Neonatology; 2013 December 8; Washington, DC. 2013.
References to studies excluded from this review
Day 1996 {published data only}
- Day RW, Lynch JM, White KS, Ward RM. Acute response to inhaled nitric oxide in newborns with respiratory failure and pulmonary hypertension. Pediatrics 1996;98:698‐705. [PubMed] [Google Scholar]
Lindwall 2005 {published data only}
- Lindwall R, Blennow M, Svensson M, Jonsson B, Berggren‐Bostrom E, Flanby M, et al. A pilot study of inhaled nitric oxide in preterm infants treated with nasal continuous positive airway pressure for respiratory distress syndrome. Intensive Care Medicine 2005;31:959‐64. [DOI] [PubMed] [Google Scholar]
Skimming 1997 {published data only}
- Skimming JW, Bender KA, Hutchison AA, Drummond WH. Nitric oxide inhalation in infants with respiratory distress syndrome. Journal of Pediatrics 1997;130:225‐30. [DOI] [PubMed] [Google Scholar]
Additional references
Abman 1990
- Abman SH, Chatfield BA, Hall SL, McMurtry IF. Role of endothelium‐derived relaxing factor during transition of pulmonary circulation at birth. American Journal of Physiology 1990;259:H1921‐7. [DOI] [PubMed] [Google Scholar]
Abman 1993
- Abman SH, Kinsella JP, Schaffer MS, Wilkening RB. Inhaled nitric oxide in the management of a premature newborn with severe respiratory distress and pulmonary hypertension. Pediatrics 1993;92:606‐9. [PubMed] [Google Scholar]
Askie 2010
- Askie LM, Ballard RA, Cutter G, Dani C, Elbourne D, Field D, et al. Inhaled nitric oxide in preterm infants: a systematic review and individual patient data meta‐analysis. BMC Pediatrics 2010;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bland 2005
- Bland RD, Albertine KH, Carlton DP, MacRitchie AJ. Inhaled nitric oxide effects on lung structure and function in chronically ventilated preterm lambs. American Journal of Respiratory and Critical Care Medicine 2005;172:899‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cornfield 1992
- Cornfield DN, Chatfield BA, McQueston JA, McMurtry IF, Abman SH. Effects of birth‐related stimuli on L‐arginine‐dependent pulmonary vasodilation in ovine fetus. American Journal of Physiology 1992;262:H1474‐81. [DOI] [PubMed] [Google Scholar]
Finer 1998
- Finer NN, Barrington KJ. Nitric oxide therapy for the newborn infant. Seminars in Neonatology 1998;3:127‐36. [DOI] [PubMed] [Google Scholar]
Finer 2000
- Finer NN, Barrington KJ. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD000399] [DOI] [PubMed] [Google Scholar]
Frostell 1991
- Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM. Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation 1991;83:2038‐47. [DOI] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- McMaster University, 2014. GRADEpro [www.gradepro.org]. Ontario: McMaster University, 2014.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hoehn 2006
- Hoehn T, Krause MF, Buhrer C. Meta‐analysis of inhaled nitric oxide in premature infants: an update. Klinische Padiatrie 2006;218:57‐61. [DOI] [PubMed] [Google Scholar]
Hogman 1993
- Hogman M, Frostell C, Arnberg H, Hedenstierna G. Bleeding time prolongation and NO inhalation. Lancet 1993;341:1664‐5. [DOI] [PubMed] [Google Scholar]
Kinsella 1992
- Kinsella JP, McQueston JA, Rosenberg AA, Abman SH. Hemodynamic effects of exogenous nitric oxide in ovine transitional pulmonary circulation. American Journal of Physiology 1992;263:H875‐80. [DOI] [PubMed] [Google Scholar]
Kinsella 1994
- Kinsella JP, Ivy DD, Abman SH. Inhaled nitric oxide improves gas exchange and lowers pulmonary vascular resistance in severe experimental hyaline membrane disease. Pediatric Research 1994;36:402‐8. [DOI] [PubMed] [Google Scholar]
McAndrew 1997
- McAndrew J, Patel RP, Jo H, Cornwell T, Lincoln T, Moellering D, et al. The interplay of nitric oxide and peroxynitrite with signal transduction pathways: implications for disease. Seminars in Perinatology 1997;21:351‐66. [DOI] [PubMed] [Google Scholar]
McCurnin 2005
- McCurnin DC, Pierce RA, Chang LY, Gibson LL, Osborne‐Lawrence S, Yoder BA, et al. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. American Journal of Physiology. Lung Cellular and Molecular Physiology 2005;288:L450‐9. [DOI] [PubMed] [Google Scholar]
Peliowski 1995
- Peliowski A, Finer N, Etches P, Tierney AJ, Ryan CA. Inhaled nitric oxide for premature infants after prolonged rupture of the membranes. Journal of Pediatrics 1995;126:450‐3. [DOI] [PubMed] [Google Scholar]
Roberts 1993
- Roberts JD Jr, Chen TY, Kawai N, Wain J, Dupuy P, Shimouchi A, et al. Inhaled nitric oxide reverses pulmonary vasoconstriction in the hypoxic and acidotic newborn lamb. Circulation Research 1993;72:246‐54. [DOI] [PubMed] [Google Scholar]
Rossaint 1993
- Rossaint R, Falke KJ, Lopez F, Slama K, Pison U, Zapol WM. Inhaled nitric oxide for the adult respiratory distress syndrome. New England Journal of Medicine 1993;328:399‐405. [DOI] [PubMed] [Google Scholar]
Ryan 1996
- Ryan SW, Nycyk J, Shaw BNJ. Prediction of chronic neonatal lung disease on day 4 of life. European Journal of Pediatrics 1996;155:668‐71. [DOI] [PubMed] [Google Scholar]
Samama 1995
- Samama CM, Diaby M, Fellahi JL Mdhafar A, Eyraud D, Arock M. Inhibition of platelet aggregation by inhaled nitric oxide in patients with acute respiratory distress syndrome. Anesthesiology 1995;83:56‐65. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. www.guidelinedevelopment.org/handbook. Updated October 2013.
Skimming 1995
- Skimming JW, DeMarco VG, Cassin S. The effects of nitric oxide inhalation on the pulmonary circulation of preterm lambs. Pediatric Research 1995;37:35‐40. [PubMed] [Google Scholar]
Soll 2001
- Soll R. Prophylactic natural surfactant extract for preventing morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews 1997, Issue 4. [DOI: 10.1002/14651858.CD000511] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Meurs 1997
- Meurs KP, Rhine WD, Asselin JM, Durand DJ, Premie INO Collaborative Group. Response of premature infants with severe respiratory failure to inhaled nitric oxide. Pediatric Research 1997;41:271A (Abstract). [DOI] [PubMed] [Google Scholar]
Vohr 2005
- Vohr BR, Wright LL, Poole K, McDonald SA. Neurodevelopmental outcomes of extremely low birth weight infants <32 weeks' gestation between 1993 and 1998. Pediatrics 2005;116:635‐43. [DOI] [PubMed] [Google Scholar]
Walther 1992
- Walther FJ, Benders MJ, Leighton JO. Persistent pulmonary hypertension in premature neonates with severe respiratory distress syndrome. Pediatrics 1992;90:899‐904. [PubMed] [Google Scholar]
Wirbelauer 2010
- Wirbelauer J, Speer CP. Significance of nitric oxide inhalation (NO) in preterm infants < 34 weeks of gestation [Stellenwert der Inhalation von Stickstoffmonoxid (NO) bei Fruhgeborenen<34 Gestationswochen.]. Klinische Padiatrie 2010;222:56‐61. [DOI] [PubMed] [Google Scholar]
Wood 2005
- Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90:F134‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Barrington 1999
- Barrington KJ, Finer NN. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database of Systematic Reviews 1999, Issue 1. [DOI: 10.1002/14651858.CD000509] [DOI] [Google Scholar]
Barrington 2001
- Barrington KJ, Finer NN. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD000509] [DOI] [PubMed] [Google Scholar]
Barrington 2006
- Barrington KJ, Finer NN. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD000509] [DOI] [PubMed] [Google Scholar]


