Abstract
Background
Circuit class therapy (CCT) offers a supervised group forum for people after stroke to practise tasks, enabling increased practise time without increasing staffing.
Objectives
To examine the effectiveness and safety of CCT on mobility in adults with stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched October 2009), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 2, 2009), MEDLINE (1950 to November 2008), EMBASE (1980 to November 2008), CINAHL (1982 to November 2008) and 14 other electronic databases (to November 2008). We also searched proceedings from relevant conferences, reference lists and unpublished theses; contacted authors of published trials and other experts in the field; and searched relevant clinical trials and research registers.
Selection criteria
Randomised or quasi‐randomised controlled trials including people over 18 years old diagnosed with stroke of any severity, at any stage, or in any setting, receiving CCT.
Data collection and analysis
Two review authors independently selected trials for inclusion, assessed methodological quality and extracted data.
Main results
We included six trials involving 292 participants. Participants were long‐term stroke survivors living in the community or receiving inpatient rehabilitation. Most could walk 10 metres without assistance. Four studies measured walking capacity and three measured gait speed, demonstrating that CCT was superior to the comparison intervention (Six Minute Walk Test: mean difference (MD), fixed 76.57 metres, 95% confidence interval (CI) 38.44 to 114.70, P < 0.0001; gait speed: MD, fixed 0.12 m/s, 95% CI 0.00 to 0.24, P = 004). Two studies measured balance, showing a superior effect in favour of CCT (Step Test: MD, fixed 3.00 steps, 95% CI 0.08 to 5.91, P = 0.04; activities‐specific balance and confidence: MD, fixed 7.76, 95% CI 0.66 to 14.87, P = 0.03). Studies also measured other balance items showing no difference in effect. Length of stay (two studies) showed a significant effect in favour of CCT (MD, fixed ‐19.73 days, 95% CI ‐35.43 to ‐4.04, P = 0.01). Only two studies measured adverse events (falls during therapy): all were minor.
Authors' conclusions
CCT is safe and effective in improving mobility for people after moderate stroke and may reduce inpatient length of stay. Further research is required, investigating quality of life, participation and cost‐benefits, that compares CCT to standard care and that also investigates the differential effects of stroke severity, latency and age.
Keywords: Adult, Humans, Stroke Rehabilitation, Arm, Arm/physiology, Exercise Therapy, Exercise Therapy/methods, Gait, Gait/physiology, Postural Balance, Postural Balance/physiology, Randomized Controlled Trials as Topic, Recovery of Function, Walking, Walking/physiology
Circuit class therapy for improving mobility after stroke
Stroke is a major cause of increased dependence for survivors in many activities of daily life, including the ability to walk and negotiate our usual environments. Intensive rehabilitation, with time spent practising specific tasks or functions under supervision, is very beneficial but achieving the sufficient amount of therapy time can be difficult if there is always a one staff to one client ratio. Circuit class therapy offers people with stroke the chance to practise meaningful functions in a group setting with the supervision of staff to give feedback and to progress the training. We found six studies involving 292 participants that compared this kind of rehabilitation to usual care or sham rehabilitation. All the trials reported benefits of circuit classes for improving the person's mobility. More specifically, we combined the results from the studies and found that the classes were more effective in improving the person's ability to walk further, longer or faster and to balance more easily and confidently when compared to other types of exercise. Also, people receiving the classes went home from inpatient rehabilitation earlier than the comparison groups. There were no increased risks of falling related to participating in the circuit classes. We are recommending people can attend circuit class therapy after stroke to achieve benefits in their ability to walk and balance. However, more research is needed to see if it works for all people at any stage or severity after stroke and if some tasks are better to practise than others.
Background
Description of the condition
Stroke is a leading cause of death and disability in many Western nations. In Australia, the United Kingdom and the United States of America it is within the top 10 causes of long‐term physical disability (Begg 2007; Muntner 2002; Wolfe 2000). Stroke care is costly: in Australia an estimated 50% of stroke survivors require a period of inpatient rehabilitation at an estimated cost of 14,000 AUD per person (Dewey 2003). The setting in which stroke survivors receive rehabilitative care (inpatient, hospital‐based versus community outpatient‐based) may vary between countries. However, the costs involved in delivering the care are consistently high, thereby placing pressure on rehabilitation services to provide evidence‐based therapies that are also cost effective.
Description of the intervention
Group circuit class therapy (CCT) describes a model of therapy delivery that utilises active exercises and activities which are task specific (practising the functional task itself or part thereof) and provided in an intensive manner. The key components of CCT are that therapy is provided in a group setting with more than two participants per therapist, and there is a focus on repetitive practise of functional tasks and continual progression of exercises (English 2007; Wevers 2009). Participants may complete a series of workstations arranged in a circuit (Wevers 2009) or may complete a series of individualised exercises within a group setting (English 2007). Circuit class therapy differs from physiological exercise programs, which aim to effect improvements in strength or aerobic fitness. While many of the activities and exercises may have a strength or fitness component, the primary focus is on repetitive practise of task‐specific training of everyday motor tasks. Circuit class therapy also differs from the conventional one‐therapist‐to‐one‐patient model for the provision of physical therapy for rehabilitation. The group nature of the intervention potentially allows a greater amount of therapy to be provided to patients for the same cost.
Circuit class therapy is usually directed at either improving mobility (walking ability, functional balance ability) or improving use of the hemiparetic upper limb, although one study included both mobility and upper limb training within the one intervention (English 2007). The majority of studies have investigated the use of circuit class therapy for improving mobility, thus this will be the focus of this review.
How the intervention might work
The strongest evidence to date suggests that therapy after stroke should focus on practise of functional tasks (van Peppen 2004) and be intensive in terms of the time spent engaged in practise (Kwakkel 2004). In the context of this literature the term 'intensive' refers to the time spent engaged in rehabilitation rather than the physiological meaning of 'intensity', which refers to the rate of energy expenditure/overload. There is strong evidence for repetitive task training for improving walking distance, speed and sit‐to‐stand ability (French 2007) and for improving walking speed and activities of daily living ability if provided within the first six months after stroke onset (Kwakkel 2004). Yet very low levels of physical activity have been reported for stroke survivors both in hospital and community settings. In the acute hospital settings stroke survivors spend less than 38 minutes per day engaged in meaningful physical activity (Bernhardt 2004) and estimates of activity levels of community‐dwelling stroke survivors (Michael 2005; Michael 2007) are less than half of that reported for sedentary adults (Tudor‐Locke 2002). Circuit class therapy may work by increasing the amount of time stroke survivors spend engaged in meaningful physical activity both within hospital settings and in the community.
The type of therapy provided is also important. Previous meta‐analyses have shown that for physical therapy interventions to be effective in improving functional ability for stroke survivors they must focus on training of functional tasks (van Peppen 2004). Interventions focused at the impairment level (such as strength, aerobic fitness training or tone management programs) may lead to improvements in strength and range of motion, but do not translate into improved functional abilities (van Peppen 2004). Circuit class therapy may improve functional ability, particularly walking ability by increasing time spent practicing this task as well as its subcomponents.
At a neurophysiological level it is well established that physical activity drives positive cortical plasticity after stroke (Johansen‐Berg 2002; Liepert 2000). Animal studies have consistently demonstrated that the engagement in physical activity after stroke induces positive neuroplastic changes within the motor cortex (Nudo 1996; Plautz 2000). A recent systematic review (Richards 2008) found a large, significant effect size in favour of positive neuroplastic changes within the lesioned motor cortex after stroke related to intensive, activity‐based rehabilitation of the paretic upper limb. The majority of studies in this area have examined the effect of upper limb rehabilitation therapies as opposed to lower limb and mobility training. However, there is emerging evidence that treadmill exercise training promotes similar positive cortical plastic changes (Enzinger 2009; Forrester 2008).
The format in which circuit class therapy is delivered also provides for optimal motor learning. In contrast to the provision of home exercise programs, exercise with a therapist present allows for the provision of extrinsic feedback which is essential for optimal motor learning (McNevin 2000; Sidaway 2001). Furthermore, practise in groups or pairs has been shown to facilitate motor learning by providing the opportunity to combine observation of others learning a new motor task with physical practise time (Shea 2000).
There may also be additional benefits of circuit class therapy related to the peer support and social interaction provided by the group environment. Studies have suggested that the social support provided within a group environment may be a predictive factor for long‐term exercise compliance in sedentary older adults both with (Fraser 2002) or without (Cox 2003) chronic diseases.
Why it is important to do this review
Within the fiscal constraints of healthcare systems it is difficult to increase intensity by simply increasing the amount of therapy provided in one‐to‐one therapy sessions as this involves significant increases in cost. Instead, it is important that novel means of providing increased intensity of therapy, in a cost‐effective manner, are developed and researched. Circuit class therapy has the potential to be a more effective means of providing a greater amount of physical therapy for people both in the hospital setting and in the community, outpatient setting. Once overall effectiveness has been established then cost implications can be investigated.
Objectives
To examine the effectiveness and safety of CCT on mobility in adults with stroke.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing circuit class therapy with no therapy, sham therapy or another therapy modality. Due to the low number of suitable trials we also included controlled clinical trials that were quasi‐randomised.
Types of participants
We included studies of adults (18 years and older) with stroke (all types, severity and stages of stroke/rehabilitation).
Types of interventions
We defined circuit class therapy as an intervention that involves participants being treated in a group environment, with a staff to client ratio of no greater than 1:3 (that is, no more than one staff member per three clients). We included studies which provided a minimum of once weekly CCT sessions for a minimum of four weeks. We only included studies which reported interventions with a focus on repetitive (within session) practise of functional tasks arranged in a circuit, with the aim of improving mobility. We excluded studies of interventions that included exercises solely aimed at improving impairment (such as strengthening, range of motion, or cardiovascular fitness).
Types of outcome measures
We evaluated outcome measures at post‐intervention and at follow‐up wherever available (e.g. three to six months post‐intervention). We did not consider outcomes taken after a single circuit class.
Primary outcome
Measures of mobility, such as the Six Minute Walk Test (distance walked in six minutes) (6mWT), which is a clinically sensitive measure with demonstrated functional benefit for the person with stroke.
Secondary outcomes
Measures of impairment, such as:
lower limb strength; and
range of motion.
Measures of activity limitation, such as:
instrumental activities of daily living; and
personal care.
Measures of participation restriction, such as:
health‐related quality of life.
Other measures, such as:
length of hospital stay;
adverse events;
self‐reported satisfaction;
locus of control;
economic indicators.
Summary of inclusion criteria
Human participants diagnosed with stroke (haemorrhage or infarct), of any severity/stage/setting (e.g. early: less than six months; or later: more than six months).
Eighteen years of age or older.
Receiving circuit class therapy as defined.
Outcomes evaluated in domains as defined.
Randomised or quasi‐randomised controlled trial.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module.
We searched the Cochrane Stroke Group Trials Register, which was last searched by the Managing Editor in October 2009. In addition, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 2, 2009), MEDLINE (1950 to November 2008) (Appendix 1), EMBASE (1980 to November 2008), CINAHL (1982 to November 2008), Science Citation Index and Social Science Citation Index (1956 to November 2008), AGELINE (1978 to November 2008), PsycLIT (1940 to November 2008), AMED (1985 to November 2008), SPORTDiscus (1949 to November 2008), Current Contents (November 2008), Australasian Medical Index (AMI, 1968 to November 2008), NLM GATEWAY (http://gateway.nlm.nih.gov, November 2008), Latin American & Caribbean Health Sciences Literature (LILACS, 1982 to November 2008), IndMed (1985 to November 2008), SOCIOFILE (1974 to November 2008), Educational Resources Information Center (ERIC,1967 to November 2008), Dissertation Abstracts International (DAI, 1997 to November 2008), and the Physiotherapy Evidence Database (PEDro, http://www.pedro.org.au/, to November 2008).
In an effort to identify further published, unpublished and ongoing studies, we:
searched for proceedings from stroke‐related conferences that were peer‐reviewed and published in the above databases until 2008;
searched reference lists (from salient articles, journals and books) and unpublished theses;
contacted authors of published trials and other experts in the field;
-
searched the following relevant clinical trials and research registers:
CenterWatch Clinical Trials Listing Service (http://www.centerwatch.com/);
ClinicalTrials.gov (http://www.clinicaltrials.gov/);
Computer Retrieval of Information on Scientific Projects (http://crisp.cit.nih.gov/);
Current Controlled Trials (www.controlled‐trials.com);
National Institute of Health Clinical Trials Database (http://www.clinicaltrials.gov/);
National Institute of Neurological Disorders and Stroke (http://www.ninds.nih.gov/);
National Rehabilitation Information Centre (Naric) (including REHABDATA);
Stroke Trials Directory (www.strokecenter.org/trials/).
We included all languages, and there were no date limits. To improve sensitivity we did not include a trials filter.
Data collection and analysis
Selection of studies
We retrieved papers from the identified lists on the basis of title/abstract, reviewing them against the established criteria for inclusion. If all criteria were met (that is, answers to the five criteria were 'yes' or 'unsure') we retrieved the study in full and reviewed it for final inclusion and then for methodological quality and data extraction. If we disagreed on any aspect of study inclusion we reached consensus through discussion and had a third person available for consultation if consensus could not be reached.
Data extraction and management
We independently entered data into the Review Manager software, RevMan 5.0 (RevMan 2008) and included full citation details of the study, objectives, design, length, assessment time points, number and characteristics of participants (inclusion and exclusion criteria), description of intervention, outcome measures, intention‐to‐treat analysis, withdrawals and loss to follow up, and adverse events. If we disagreed on any aspect of data extraction or quality evaluation, we reached consensus through discussion and had a third person available for consultation if consensus could not be reached.
Assessment of risk of bias in included studies
We independently assessed the quality of the studies to be included. We assessed the methodological quality of the included studies for risk of bias using criteria recommended in section 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008) in six domains: sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data, selective reporting and other (sample size). Adequate sample size was based on supplied power calculations. We gave studies an overall summary of the risk of bias for each important outcome (across domains), as well as within and across studies using three levels: low, unclear or high risk of bias. We also gave a descriptive report on the overall risk of bias in relation to the findings from the meta‐analyses.
Measures of treatment effect
We extracted and analysed data to calculate relative risk (RR) or mean difference (MD) and 95% confidence intervals (CI). This required the identification of the number of participants in each group in each trial and the total number (for dichotomous data), and the number of participants plus the mean and standard deviations for each group (for continuous data).
Unit of analysis issues
We considered studies with non‐standard designs; e.g. cluster randomised trials if they were assessed as having a low risk of bias. We only considered randomised cross‐over trials prior to cross over (irrespective of wash‐out periods as the changes are assumed to be permanent) and if the study authors provided an analysis of results for the first phase.
Dealing with missing data
We contacted study authors to request appropriate data for meta‐analyses if these were not adequately reported in the retrieved paper. We considered intention‐to‐treat analysis as part of the risk of bias assessment and recorded loss to follow up.
Assessment of heterogeneity
We assessed statistical heterogeneity both visually and using the I‐squared (I2) statistic. We also evaluated clinical heterogeneity (clinical and methodological diversity).
Assessment of reporting biases
We minimised reporting biases by the comprehensive search strategies, which had no date or language limits. However, where appropriate we could also examine this statistically via funnel plots and tests for asymmetry if there were sufficient studies.
Data synthesis
We conducted a meta‐analysis with appropriate data. We considered the degree of heterogeneity to determine whether to use fixed‐effect or random‐effects analyses.
Subgroup analysis and investigation of heterogeneity
Where appropriate we considered performing subgroup analyses to establish effectiveness relative to gender, chronicity, age, severity or CCT content (respectively males versus females, acute versus chronic stroke, young adults versus older, mild/moderate versus severe stroke, purely task specific versus combination of impairment and task specific exercise) using the independent variables for meta‐regression wherever the appropriate data were available.
Sensitivity analysis
We conducted sensitivity analyses to determine if particular studies skewed results, e.g. RCT versus non‐RCTs.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
We retrieved 29 potential trials in full from the search, of which six were included in this review.
Included studies
The six included trials were all conducted between 2000 and 2009; two in Australia (Blennerhassett 2004; English 2007), one in New Zealand (Mudge 2009a) and three in Canada (Dean 2000; Marigold 2005; Pang 2005). Five were RCTs (Blennerhassett 2004; Dean 2000; Marigold 2005; Mudge 2009a; Pang 2005) and one was a controlled trial with participants allocated by date of admission (English 2007).
The total of 292 participants were all adults post‐stroke, and sample sizes varied from 12 to 68. Reported age ranges averaged between 53.9 years and 69.1 years. Stroke latency varied, with two studies investigating people in the first three months after stroke (Blennerhassett 2004; English 2007) and the other four studies one to five years post‐stroke (Dean 2000; Marigold 2005; Mudge 2009a; Pang 2005). The settings for the intervention reflected the stroke latency, with the two early studies investigating people in an inpatient rehabilitation setting and the subsequent four trials being conducted in a community, outpatient setting. When reported, the stroke aetiology was predominantly infarction rather than haemorrhage. Stroke severity was similar across studies with English 2007 having the broadest spectrum (minimal ability able to stand with assistance) and the remainder requiring participants being able to walk 10 metres with or without aid.
All studies investigated the effects of circuit class therapy (station‐based, task‐specific practise in a group with a ratio of staff to client of 1:3) with the aim of improving mobility in people poststroke. Intensity varied slightly, with Blennerhassett 2004 and English 2007 investigating one or two hours per day respectively, five days a week for four weeks, compared to one hour three times a week for four (Dean 2000; Mudge 2009a),10 (Marigold 2005) or 19 weeks (Pang 2005).
Comparison groups all involved an alternate 'other' intervention with one study comparing CCT to usual care (one‐to‐one therapy) (English 2007), three studies comparing CCT for mobility tasks with CCT for upper limb tasks (Blennerhassett 2004; Dean 2000; Pang 2005), one comparing CCT with non‐task specific exercises (such as stretching tasks) (Marigold 2005) and one comparing CCT with group social and education sessions (Mudge 2009a).
All studies used a composite of measures related to mobility including tests of walking ability (gait speed and capacity) and balance (Timed Up and Go (TUG) Test, Berg Balance Scale (BBS), Step test). Some studies used measures of upper limb function, balance self‐efficacy, tests for impairment (strength, VO2max, kinematic data), free‐living walking ability (steps per day using activity monitor), numbers of adverse events (falls during therapy), satisfaction and length of stay. No studies investigated measures of quality of life or economic indicators.
Excluded studies
We excluded the remaining 23 studies for a variety of reasons including inappropriate methodology (non‐controlled), or interventions that were either not task‐specific (that is to say the interventions addressed impairments not functional tasks), not in a group (staff‐to‐client ratio was less than 1:3) or not in a circuit. See Characteristics of excluded studies for individual reasons for each excluded study.
Risk of bias in included studies
We assessed the overall risk of bias as low. Figure 1 shows the trials together achieved between 70% and 80% methodological quality, with the worst performance in the area of incomplete reporting of outcome data. Figure 2 shows again that the majority of studies, this time considered individually, achieved a low risk of bias.Three studies achieved all criteria (Blennerhassett 2004; Mudge 2009a; Pang 2005) although two of these lacked clarity in one area each: where the actual technique to generate the random sequences was not stated (Blennerhassett 2004) or selective reporting was suspected because the same trial was reported in two separate articles but with different sets of outcome data in each paper (Pang 2005). Dean 2000 had the highest risk of bias with issues around small sample size (total N = 10), potential unblinding of the assessor, and no intention‐to‐treat analysis with higher rates of drop outs. English 2007 did not have adequate sequence generation or concealed allocation and Marigold 2005 also failed to use appropriate reporting for drop outs.
Figure 1.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Figure 2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Effects of interventions
Sufficient clinical homogeneity allowed us to pool study data, comparing CCT for mobility versus 'other' intervention(s). Five measures of mobility were used across the studies (primary outcomes) and one secondary outcome measure (length of stay) was reported in the two inpatient studies (Blennerhassett 2004; English 2007).
Four studies (157 participants) measured walking capacity using the 6mWT (Blennerhassett 2004; Dean 2000; Mudge 2009a: Pang 2005). Meta‐analysis demonstrated that overall CCT was superior to the comparison intervention (MD, fixed 76.57 m, 95% CI 38.44 to 114.70, P < 0.0001) (Analysis 1.1).
Analysis 1.1.

Comparison 1 Circuit class therapy versus other, Outcome 1 6mWT.
Three studies (130 participants) measured gait speed (Dean 2000; English 2007; Mudge 2009a), with meta‐analysis showing a difference between the two groups that reached significance in favour of CCT (MD, fixed 0.12 m/s, 95% CI 0.00 to 0.24, P = 0.04) (Analysis 1.2).
Analysis 1.2.

Comparison 1 Circuit class therapy versus other, Outcome 2 Gait speed.
Two studies used the Step Test to measure balance (39 participants) (Blennerhassett 2004; Dean 2000) with the meta‐analysis showing a superior effect in favour of CCT (MD, fixed 3.00 steps, 95% CI 0.08 to 5.91, P = 0.04) (Analysis 1.3).
Analysis 1.3.
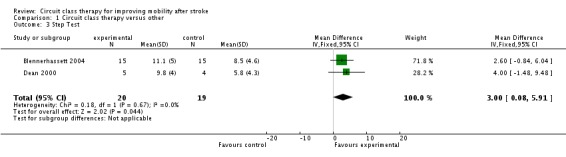
Comparison 1 Circuit class therapy versus other, Outcome 3 Step Test.
Three studies (89 participants) used the Timed Up and Go Test to measure the ability to stand up, walk and turn around (Blennerhassett 2004; Dean 2000; Marigold 2005) and meta‐analysis showed no significant effects in favour of either intervention (MD, fixed ‐3.08 seconds, 95% CI ‐7.59 to 1.43, P = 0.18) (Analysis 1.4).
Analysis 1.4.
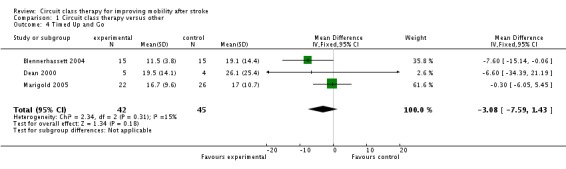
Comparison 1 Circuit class therapy versus other, Outcome 4 Timed Up and Go.
Three studies applied the Berg Balance Scale (177 participants) (English 2007; Marigold 2005; Pang 2005) with meta‐analysis showing no difference in effect (MD, fixed 0.86, 95% CI ‐1.02 to 2.74, P = 0.37) (Analysis 1.5).
Analysis 1.5.
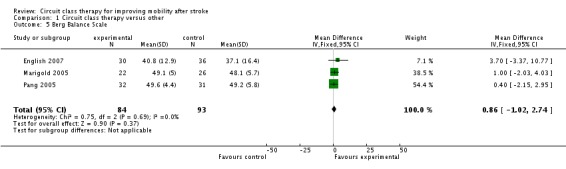
Comparison 1 Circuit class therapy versus other, Outcome 5 Berg Balance Scale.
Two studies (103 participants) (Marigold 2005; Mudge 2009a) measured balance self‐efficacy using the Activities‐specific Balance Confidence Scale (ABC) with meta‐analysis showing a significant effect in favour of CCT (MD, fixed 7.76 points, 95% CI 0.66 to 14.87, P = 0.03) (Analysis 1.6).
Analysis 1.6.

Comparison 1 Circuit class therapy versus other, Outcome 6 Activities‐specific Balance Confidence Scale.
Length of stay was the only common secondary measure with the two inpatient studies (96 participants) recording these data (Blennerhassett 2004; English 2007). Meta‐analysis showed a significant effect in favour of CCT (MD, fixed ‐19.73 days, 95% CI ‐35.43 to ‐4.04, P = 0.01) (Analysis 1.7).
Analysis 1.7.
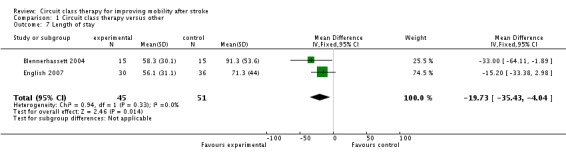
Comparison 1 Circuit class therapy versus other, Outcome 7 Length of stay.
Two studies reported adverse events for a combined 131 participants (falls in therapy). English 2007 reported two falls in the control group and four in the intervention group and Pang 2005 reported one fall in the control group and five falls in the intervention group. All falls were minor with no reported injuries in either study.
We did not retrieve any studies using a cross‐over or cluster randomisation procedure. We considered missing data as part of the overall evaluation of risk of bias. We evaluated heterogeneity using the I2 statistic ‐ for the analyses reported above this was between 0% and 23% and therefore acceptable. In light of the low heterogeneity we applied fixed rather than random effects in the analyses. We could not evaluate reporting biases by funnel plots due to the low number of studies, nor could we undertake subgroup analyses for the same reason. One study was not randomised (English 2007), therefore we carried out sensitivity analyses on all meta‐analyses involving this trial. These did not change the results for any of the outcome measures. Gait speed remained significant (Analysis 1.8), the Berg Balance Scale remained non‐significant (Analysis 1.9) and length of stay remained in favour of the CCT group (Analysis 1.10).
Analysis 1.8.
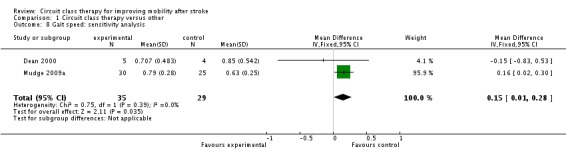
Comparison 1 Circuit class therapy versus other, Outcome 8 Gait speed: sensitivity analysis.
Analysis 1.9.
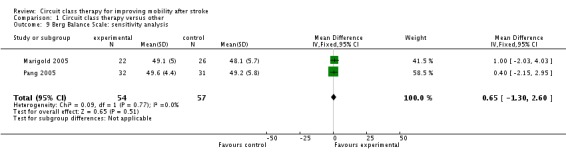
Comparison 1 Circuit class therapy versus other, Outcome 9 Berg Balance Scale: sensitivity analysis.
Analysis 1.10.
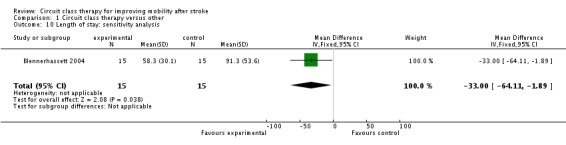
Comparison 1 Circuit class therapy versus other, Outcome 10 Length of stay: sensitivity analysis.
Discussion
Summary of main results
The primary aim of this review was to investigate the effectiveness of group circuit class therapy (CCT) for improving mobility after stroke. For our primary outcome measure of gait capacity (measured by 6mWT) a meta‐analysis revealed that CCT was effective at improving the distance walked. The minimal clinically meaningful improvement on the 6mWT has been estimated at 13% (Flansbjer 2005), which equates to a distance of between 32.5 and 52.5 metres based on the data from included studies. Thus, we can be confident that the mean improvement found in the meta‐analysis of 76.57 metres represents a real clinical change. The positive finding for the 6mWT is of functional relevance as it has been shown to be a stronger predictor of the community walking ability than measures of walking speed (Mudge 2009b; Rand 2009), which may overestimate community ambulatory ability (Taylor 2006). Furthermore, the 6mWT has been shown to correlate significantly with quality of life after stroke (Muren 2008).
We also found a favourable effect of CCT in regards to improvements in walking speed, although this only just reached significance. While gait speed is a valid and responsive measure of improvement in walking ability in persons post‐stroke (Kollen 2006; Salbach 2001), it has been shown to overestimate walking capacity (Dean 2001; Taylor 2006), meaning that the 6mWT is perhaps a more clinically meaningful measure.
The reported positive walking results in favour of CCT may be explained in light of the content of the intervention. The intervention in all the included studies involved a strong emphasis on continuous walking practise, which is likely to have led to the improvements in the 6mWT. By contrast, none of the studies reported specifically training gait speed as part of the intervention and others have shown that for gait speed to increase after stroke, it must be specifically and aggressively trained (Pohl 2002). Perhaps greater improvements in walking speed may have been seen if a greater emphasis on walking speed training was provided in the classes.
On measures of balance ability, results were mixed. There were no significant effects found for the TUG or BBS scales, but there was a significant effect for the Step Test and for balance confidence. These differences may be due to the relative sensitivity of the measures. The BBS has been demonstrated as having a ceiling effect when used with community dwelling, ambulant stroke survivors (Mao 2002). Two studies investigated the impact of CCT on balance self‐efficacy using the ABC scale (Marigold 2005; Mudge 2009a) and our meta‐analysis revealed a significant positive effect for this outcome. This outcome is clinically meaningful as the mean difference between groups of 7.76 points is greater than the standard error of measurement (5.05 points) (Salbach 2006b) and improvements in balance self‐efficacy, in addition to improvements in walking ability, lead to greater improvements in both physical functioning and perceived health status (Salbach 2006a).
Only two studies investigated the effectiveness of CCT in stroke survivors receiving inpatient rehabilitation with both studies including length of hospital stay as an outcome measure. When these results were pooled a significant effect was found in favour of a reduction in length of stay for those participants who received CCT. The mean difference of 19.6 days is highly clinically relevant as it represents the potential for significant savings to the healthcare system. There are many factors that influence length of hospital stay, so a direct causal relationship between CCT and length of stay cannot be established. Furthermore, the participant numbers were relatively small and one study (English 2007) did not have adequate sequence generation or concealed allocation. However, the magnitude of the finding and the potential benefits for both the individuals concerned and the healthcare system suggests that this benefit of CCT deserves further investigation.
Only two studies reported adverse events (falls during therapy) (English 2007; Pang 2005). While both these studies reported a greater number of falls in the intervention group compared to the control group, all falls were minor with none resulting in injury. Any intervention aimed at improving mobility and balance after stroke carries an inherent risk of causing falls because it is necessary for participants to undertake activities at the limits of their abilities for the interventions to be effective. The slightly greater falls rate in the intervention group is perhaps not surprising considering that the control group were either undertaking interventions that did not expose the participant to an increased risk of falls (a seated upper extremity exercise program) (Pang 2005) or had significantly less risk exposure because they spent significantly less time engaged in physical therapy sessions (English 2007). Nevertheless, it would be pertinent for future studies to more closely examine the link between CCT and falls in therapy.
Overall completeness and applicability of evidence
The content of the intervention provided was remarkably similar across all six studies with many of the same exercises and activities included (see Characteristics of included studies). The participants in the included studies can be divided into two clear groups with respect to stroke latency: those less than three months (Blennerhassett 2004; English 2007) and those between one and five years post‐stroke (Dean 2000; Marigold 2005; Mudge 2009a; Pang 2005). The characteristics of participants included in these four studies involving people later after stroke were homogeneous. Thus, the results can be extrapolated to community dwelling stroke survivors, able to walk at least 10 metres independently and free from significant co‐morbidities, as well as stroke survivors receiving inpatient rehabilitation who are commencing mobility training or are able to walk 10 metres. Importantly, results cannot be extrapolated to stroke survivors who have not regained the ability to walk 10 metres, or those living in residential care. The benefits may also be restricted to those people with sufficient motivation and social support to enable them to regularly attend an exercise class.
There is some evidence that CCT has an immediate effect on improving walking capacity (as measured by 6mWT) both in persons in the first months after stroke (Blennerhassett 2004) and later after stroke. Only two of the later stage studies (Dean 2000; Marigold 2005) included follow‐up measures taken one and two months after the intervention ceased, respectively. These studies did not include common outcome measures meaning that results could not be pooled. Therefore, there is insufficient evidence on whether benefits of CCT provided to people later after stroke are maintained over time.
The evidence for the effectiveness of CCT on balance ability for people after stroke is less clear with a significant benefit found for the step test and balance confidence (ABC Scale), but not the BBS or TUG. Ultimately, the reason for including exercises aimed at improving balance within the intervention is to improve a person's ability to participate in the community and to reduce the risk of falls. One study prospectively tracked falls for one year after the intervention and found no significant difference between groups (Marigold 2005); however, those receiving CCT did have less falls in response to perturbations on a force platform compared to the control group. Thus, there is evidence to draw modest conclusions about the effectiveness of CCT for improving balance and balance self‐efficacy (confidence when performing activities requiring balance), as well as reducing falls after stroke.
Two studies included measures of lower limb strength (Dean 2000; Pang 2005) but the measures used (laboratory‐based kinetic data versus clinical dynamometer) were not similar enough to allow pooling of data. Therefore, there is insufficient evidence for the effect of CCT at the impairment level. Similarly, there is as yet no evidence regarding the effects of CCT for improving instrumental activities of daily living, personal care, participation restriction, health‐related quality of life or self‐reported satisfaction.
The positive finding in regard to a reduction in length of hospital stay is promising though it is based on only two studies (Blennerhassett 2004; English 2007), one of which was not randomised (English 2007).
In the context of current practice, this review suggests that CCT may be an effective means of providing task‐specific programs to stroke survivors, particularly in the community. Current practice around this service delivery varies both within and between countries. Only one of the included studies directly compared CCT to another common model of physiotherapy service delivery (one‐to‐one) (English 2007), so the question of whether CCT is superior to other currently used methods is not clear.
Quality of the evidence
The overall quality of the evidence was relatively high and, therefore, the results can be considered to be strong despite the small number of trials and small numbers within trials.
Potential biases in the review process
Potential biases in the review process need to be considered in that both of the review authors are stroke rehabilitation trialists and take a pragmatic stand on trial design. For example, we did not assess trials as having a risk of bias where the therapist or the participants were not blinded, as we did not consider this possible in these kinds of clinical trials (other than to maintain the participant naive as to which arm of the trial is of interest to the researchers).
Agreements and disagreements with other studies or reviews
There has been a recent systematic review of the effectiveness of CCT for improving walking after stroke which differed slightly from our review in terms of the studies included but reached similar conclusions (Wevers 2009). The inclusion criteria and definition of CCT were similar in the two reviews; however, Wevers 2009 included two studies that we excluded on the basis that the intervention was not delivered in a group (Salbach 2004; Yang 2006). We also excluded another study (Mead 2007) included by Wevers 2009 because the intervention was primarily impairment‐based rather than task‐specific and functional in nature. Finally, we included one study that was not an RCT (English 2007) and an additional study currently in press (Mudge 2009a).
Comparing the results, Wevers 2009 reported significant effects in favour of CCT on the 6mWT, gait speed and the TUG, but not the Step Test. The magnitude of the effect size on the 6mWT (0.43 standard deviation units (SDU), equivalent to 42.5 metres) was smaller than our result of a mean effect of 76.57 metres. While Wevers 2009 found a significant effect size in favour of CCT improving gait speed, the mean difference was smaller than the result of our meta‐analysis (0.35 SDU, or 0.07 m/s compared with 0.12 m/s). The differences in results between the two reviews may also be related to the acuity of the participants. Wevers 2009 included only one study involving participants less than six months post‐stroke (Blennerhassett 2004) and found a larger effect size for the 6mWT, TUG and Step Test in this study compared to those involving participants later after stroke. Further studies into the effectiveness of CCT for people in the sub‐acute post‐stroke period are required to determine the effect of stroke latency on the effectiveness of CCT.
Authors' conclusions
Based on the existing evidence, circuit class therapy (CCT) is effective in improving gait capacity and other aspects of mobility for adults after stroke and can be implemented in the post acute and chronic stages for people with moderate stroke severity. Intensity can vary from daily to three times weekly for four weeks or more to achieve benefits. There is evidence that it can reduce length of stay in the inpatient setting. There was insufficient evidence to determine the effect of the intervention on adverse events (e.g. falls).
While evidence is strong for the effectiveness of CCT for improving mobility in people later after stroke who are able to walk independently, the evidence for CCT for people early after stroke is less clear. Further quality randomised controlled trials are required to compare CCT to standard care for people in hospital after stroke to allow service providers to make more informed choices about whether CCT should be offered as an adjunct or as an alternative to usual care. These studies should include measures of cost‐benefits as well as quality of life and participation. Future research should also investigate differential effects regarding stroke severity, age and stroke latency.
Acknowledgements
Alexis Young of the Centre for Allied Health Evidence. Staff of the Cochrane Stroke Group.
Appendices
Appendix 1. MEDLINE search strategy
We used the following search strategy for MEDLINE (Ovid) and adapted it to search the other databases. As the subject area of this review is quite specific we did not include a trials filter. This increased the sensitivity of the search.
MEDLINE (Ovid)
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vasospasm, intracranial/ or vertebral artery dissection/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiplegia/ or exp paresis/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. exp gait disorders, neurologic/ 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. exercise movement techniques/ or exercise/ or exercise therapy/ or muscle stretching exercises/ or walking/ 10. physical fitness/ or exertion/ or exp locomotion/ 11. sports/ or bicycling/ or gymnastics/ or weight lifting/ 12. "task performance and analysis"/ or athletic performance/ or mobility limitation/ 13. physical therapy modalities/ or "physical therapy (specialty)"/ 14. (physical adj3 (exercise$ or therap$ or conditioning or activit$ or fitness or endurance)).tw. 15. (exercise adj3 (train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$)).tw. 16. (fitness adj3 (train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$)).tw. 17. ((training or conditioning) adj3 (intervention$ or protocol$ or program$ or activit$ or regim$)).tw. 18. (sport$ or cycl$ or bicycl$ or treadmill$ or run$ or walk$).tw. 19. muscle strengthening.tw. 20. ((weight or strength or resistance) adj (train$ or lift$ or exercise$)).tw. 21. 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 22. fitness centers/ or sports equipment/ 23. (circuit adj3 (class or classes or therapy or training or program$ or exercise$ or arranged or arrangement)).tw. 24. (sport$ equipment or station or work station).tw. 25. (fitness adj3 (center$ or centre$ or group$ or class or classes or training or program$)).tw. 26. (exercise$ adj3 (routine$ or group$ or class or classes)).tw. 27. ((task‐related or sequential) adj3 exercise$).tw. 28. group environment.tw. 29. (repetitive pract$ or functional task$).tw. 30. 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 31. cerebrovascular disorders/rh or exp basal ganglia cerebrovascular disease/rh or exp brain ischemia/rh or exp carotid artery diseases/rh or exp intracranial arterial diseases/rh or exp "intracranial embolism and thrombosis"/rh or exp intracranial hemorrhages/rh or stroke/rh or exp brain infarction/rh or vasospasm, intracranial/rh or vertebral artery dissection/rh 32. 8 and 21 33. 31 or 32 34. 30 and 33 35. limit 34 to human
Data and analyses
Comparison 1.
Circuit class therapy versus other
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 6mWT | 4 | 157 | Mean Difference (IV, Fixed, 95% CI) | 76.57 [38.44, 114.70] |
| 2 Gait speed | 3 | 130 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [0.00, 0.24] |
| 3 Step Test | 2 | 39 | Mean Difference (IV, Fixed, 95% CI) | 3.00 [0.08, 5.91] |
| 4 Timed Up and Go | 3 | 87 | Mean Difference (IV, Fixed, 95% CI) | ‐3.08 [‐7.59, 1.43] |
| 5 Berg Balance Scale | 3 | 177 | Mean Difference (IV, Fixed, 95% CI) | 0.86 [‐1.02, 2.74] |
| 6 Activities‐specific Balance Confidence Scale | 2 | 103 | Mean Difference (IV, Fixed, 95% CI) | 7.76 [0.66, 14.87] |
| 7 Length of stay | 2 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐19.73 [‐35.43, ‐4.04] |
| 8 Gait speed: sensitivity analysis | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [0.01, 0.28] |
| 9 Berg Balance Scale: sensitivity analysis | 2 | 111 | Mean Difference (IV, Fixed, 95% CI) | 0.65 [‐1.30, 2.60] |
| 10 Length of stay: sensitivity analysis | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐33.0 [‐64.11, ‐1.89] |
What's new
Last assessed as up‐to‐date: 21 October 2009.
| Date | Event | Description |
|---|---|---|
| 9 July 2010 | Amended | Minor correction made to the participant characteristics in the Results section of the Abstract and under Included studies in the main Results section of the review. |
Differences between protocol and review
None.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Blennerhassett 2004
| Methods | RCT Mobility CCT versus upper limb CCT | |
| Participants | 30 participants (15 each group) receiving inpatient rehabilitation (mean of 43 days post‐stroke), mean age 55.1 years, able to walk 10 metres with close supervision with or without gait aids | |
| Interventions | Group 1: mobility‐related CCT Group 2: upper limb‐related CCT Therapy provided in addition to usual care | |
| Outcomes | 6mWT, Step Test, TUG, LOS, MAS upper arm and hand items, JTHFT | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Card draw: unclear how cards were constructed |
| Allocation concealment? | Low risk | Sealed opaque envelopes, independent person |
| Blinding? All outcomes | Low risk | Assessor blinded |
| Incomplete outcome data addressed? All outcomes | Low risk | 100% data at 4 weeks |
| Free of selective reporting? | Low risk | All outcome measures were reported on |
| Free of other bias? | Low risk | Adequate sample size |
Dean 2000
| Methods | RCT Mobility CCT versus upper limb CCT | |
| Participants | 9 participants (Group 1 = 5, Group 2 = 4), mean 1.3 years post‐stroke, mean age 62.3 years, able to walk 10 metres independently with or without gait aid | |
| Interventions | Group 1: mobility‐related CCT Group 2: upper limb‐related CCT | |
| Outcomes | 6mWT, Step Test, TUG, gait speed, peak vertical ground reaction force through affected lower limb during sit‐to‐stand, laboratory measures of gait kinematics and kinetics | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation by lottery: "drawing two cards, one with subject's name and one with group allocation from two separate boxes" |
| Allocation concealment? | Low risk | Cards drawn by a person independent of the study |
| Blinding? All outcomes | High risk | Clinical assessments, with exception of 6mWT, conducted by independent rater; however, this blinding may have been unmasked as the result of this observer inadvertently observing 1 training session |
| Incomplete outcome data addressed? All outcomes | High risk | Missing data balanced across groups (1 in experimental and 2 in control) for transport or unrelated illness reasons, but no intention‐to‐treat analysis undertaken |
| Free of selective reporting? | Low risk | All outcome measures reported |
| Free of other bias? | High risk | Very small sample size |
English 2007
| Methods | Controlled trial (allocation by time of admission to rehabilitation) CCT (mobility and upper limb related) versus individual therapy | |
| Participants | 68 participants (Group 1 = 31, Group 2 = 37) receiving inpatient rehabilitation, mean of 26.8 days post stroke, mean age 61.6 years, able to stand with one person assisting | |
| Interventions | Group 1: mobility and upper limb‐related CCT Group 2: individual 'usual care' physiotherapy Group 1 participants received only CCT care, i.e. no individual physiotherapy sessions | |
| Outcomes | Gait speed, BBS, LOS, MAS upper limb items, ILAS, patient satisfaction | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Sequence generated by time of admission to rehabilitation |
| Allocation concealment? | High risk | Allocation performed by researcher |
| Blinding? All outcomes | Low risk | An examiner who was unaware of the design and aim of study and was blinded to participants' group allocation |
| Incomplete outcome data addressed? All outcomes | Low risk | Similar loss of participants in both groups for reasons unrelated to outcome Intention‐to‐treat analysis undertaken |
| Free of selective reporting? | Low risk | All outcome measures reported |
| Free of other bias? | Low risk | Adequate sample size |
Marigold 2005
| Methods | RCT Mobility‐related CCT versus general balance class | |
| Participants | 59 participants (Group 1 = 28, Group 2 = 31), mean 3.7 years post‐stroke, mean age 67.8 years, able to walk 10 metres independently with or without gait aid | |
| Interventions | Group 1: mobility‐related CCT Group 2: stretching and reaching tasks | |
| Outcomes | BBS, TUG, ABC, NHP, standing postural reflexes using force platform, self‐reported prospective falls diary | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated codes |
| Allocation concealment? | Low risk | Person independent of the study |
| Blinding? All outcomes | Low risk | All assessors were blinded to group assignment, study design and purpose |
| Incomplete outcome data addressed? All outcomes | High risk | Total of 11 lost before post‐intervention testing, another 6 lost before follow‐up No intention‐to‐treat analysis or imputation of missing data |
| Free of selective reporting? | Low risk | All outcomes reported |
| Free of other bias? | Low risk | Adequate sample size |
Mudge 2009a
| Methods | RCT Mobility‐related CCT versus education or social groups | |
| Participants | 58 participants (Group 1 = 31, Group 2 = 27), mean 4.9 years post‐stroke, mean age 69.1 years, able to walk 10 metres independently with or without gait aid | |
| Interventions | Group 1: mobility‐related CCT Group 2: education or social group events of equivalent dosage | |
| Outcomes | Gait speed, 6mWT, RMI, ABC, steps per day using activity monitor, PADS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random numbers |
| Allocation concealment? | Low risk | Person independent of the study matched the participants to the codes |
| Blinding? All outcomes | Low risk | Unmasking occurred for 3 out of 58 participants (5%) |
| Incomplete outcome data addressed? All outcomes | Low risk | 2 lost before randomisation, 3 withdrew before post‐intervention assessment and a further 5 lost before follow‐up assessment; losses balanced across groups Intention‐to‐treat analysis undertaken with imputation of missing data using carry forward method |
| Free of selective reporting? | Low risk | All outcomes reported |
| Free of other bias? | Low risk | Adequate sample size |
Pang 2005
| Methods | RCT Mobility CCT versus upper limb CCT | |
| Participants | 63 participants (Group 1 = 32, Group 2 = 31), mean 5.1 years post‐stroke, mean age 65.3 years, able to walk 10 metres independently with or without gait aids | |
| Interventions | Group 1: mobility‐related CCT and fitness training Group 2: upper limb‐related CCT | |
| Outcomes | 6mWT, BBS, VO2max, knee extension strength (dynamometer), PASIPD, proximal femur BMD | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Drawing ballots |
| Allocation concealment? | Low risk | Ballots drawn by person not involved with enrolment, screening or outcome assessments |
| Blinding? All outcomes | Low risk | Research personnel who performed outcome assessments were blinded to group assignment |
| Incomplete outcome data addressed? All outcomes | Low risk | Similar small amount of missing data across groups Missing data imputed from baseline values and intention‐to‐treat analysis used |
| Free of selective reporting? | Unclear risk | This study was reported in at least 3 separate papers all including different outcome measures |
| Free of other bias? | Low risk | Adequate sample size |
6mWT: 6 Minute Walk Test ABC: Activities‐specific Balance and Confidence Scale BBS: Berg Balance Scale BMD: bone mineral density CCT: circuit class therapy ILAS: Iowa Level of Assistance Scale JTHFT: Jebsen Taylor Hand Function Test LOS: length of hospital stay MAS: Motor Assessment Scale NHP: Nottingham Health Profile PADS: Physical Activity and Disability Scale PASIPD: Physical Acitivity Scale for Individuals with Physical Disabilities RCT: randomised controlled trial RMI: Rivermead Mobility Index TUG: Timed Up and Go VO2max: maximum oxygen volume
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Blennerhassett 2008 | Method: not a trial |
| Brogårdh 2006 | Method: no control group |
| Chu 2004 | Intervention: not task‐specific training |
| English 2008 | Outcome: upper limb/pain |
| French 2008 | Method: systematic review Intervention: not CCT exclusively |
| Kowalczewski 2007 | Intervention: not group format |
| Langhammer 2008 | Intervention: not task‐specific, not circuit, not group |
| Lynch 2008 | Method: no control group |
| Mead 2007 | Intervention: not task‐specific |
| Olney 2006 | Intervention: not task‐specific |
| Purton J | Method: not a trial |
| Pyöriä 2007 | Intervention: not group format |
| Rimmer 2000 | Intervention: not task‐specific |
| Salbach 2004 | Intervention: not group format |
| Shepherd 2001 | Method: not a trial |
| Sherrington 2008 | Intervention: not task‐specific |
| Sullivan 2007 | Intervention: not circuit format |
| Sunnerhagen 2007 | Intervention: not task‐specific |
| Tanne 2008 | Intervention: not task‐specific |
| Taskinen 1999 | Method: no control group |
| Teixeira‐Salmela 1999 | Intervention: not task‐specific |
| Yang 2006 | Intervention: not group format |
CCT: circuit class therapy
Contributions of authors
Both authors were involved in all stages of the review. Coralie English has experience in the clinical use of circuit class therapy and Susan Hillier has experience as a review author.
Sources of support
Internal sources
Centre for Allied Health Evidence, University of South Australia, Australia.
External sources
No sources of support supplied
Declarations of interest
Both authors have published a trial investigating the use of CCT with people with stroke (English 2007).
Edited (no change to conclusions)
References
References to studies included in this review
- Blennerhassett J, Dite W. Additional task‐related practice improves mobility and upper limb function early after stroke: a randomised controlled trial. Australian Journal of Physiotherapy 2004;50:219‐24. [DOI] [PubMed] [Google Scholar]
- Dean C, Richards C, Malouin F. Task‐related circuit training improves performance of locomotor tasks in chronic stroke: a randomised controlled pilot trial. Archives of Physical Medicine and Rehabilitation 2000;81:409‐17. [DOI] [PubMed] [Google Scholar]
- English C, Hillier S, Stiller K, Warden‐Flood A. Circuit class therapy versus individual physiotherapy sessions during inpatient stroke rehabilitation: a controlled trial. Archives of Physical Medicine and Rehabilitation 2007;88:955‐63. [DOI] [PubMed] [Google Scholar]
- Marigold D, Eng J, Dawson A, Inglis J, Harris J, Gylfadóttir S. Exercise leads to faster postural reflexes, improved balance and mobility, and fewer falls in older persons with chronic stroke. Journal of the American Geriatrics Society 2005;53:426‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge S, Stott N, Barber P. Circuit‐based rehabilitation improves gait endurance but not usual walking activity in chronic stroke: a randomised clinical trial. Archives of Physical Medicine and Rehabilitation 2009;90(12):1989‐96. [DOI] [PubMed] [Google Scholar]
- Pang M, Eng J, Dawson A, McKay H, Harris J. A community‐based fitness and mobility exercise program for older adults with chronic stroke: a randomised controlled trial. Journal of the American Geriatrics Society 2005;53:1667‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pang MY, Harris JE, Eng JJ. A community‐based upper‐extremity group exercise program improves motor function and performance of functional activities in chronic stroke: a randomised controlled trial. Archives of Physical Medicine and Rehabilitation 2006;87:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Blennerhassett J. The value of circuit classes in stroke rehabilitation. International Journal of Therapy and Rehabilitation 2008;15(6):272. [Google Scholar]
- Brogårdh C, Sjölund B. Constraint‐induced movement therapy in patients with stroke: a pilot study on effects of small group training and of extended mitt use. Clinical Rehabilitation 2006;20:218‐27. [DOI] [PubMed] [Google Scholar]
- Chu KS, Eng JJ, Dawson AS, Harris JE, Ozkaplan A, Gylfadóttir S. Water‐based exercise for cardiovascular fitness in people with chronic stroke: a randomised controlled trial. Archives of Physical Medicine and Rehabilitation 2004;85:870‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English C, Hillier S, Stiller K. Incidence and severity of shoulder pain does not increase with the use of circuit class therapy during inpatient stroke rehabilitation: a controlled trial. Australian Journal of Physiotherapy 2008;54:41‐6. [DOI] [PubMed] [Google Scholar]
- French B, Leathley M, Sutton C, McAdam J, Thomas A, Forster A, et al. A systematic review of repetitive functional task practice with modelling of resource use, costs and effectiveness. Health Technology Assessment 2008;12(30):1‐117. [DOI] [PubMed] [Google Scholar]
- Kowalczewski J, Gritsenko V, Ashworth N, Ellaway P, Prochazka A. Upper‐extremity functional electric stimulation‐assisted exercises on a workstation in the sub‐acute phase of stroke recovery. Archives of Physical Medicine and Rehabilitation 2007;88:833‐9. [DOI] [PubMed] [Google Scholar]
- Langhammer B, Stanghelle JK, Lindmark B. Exercise and health‐related quality of life during the first year following acute stroke. A randomised controlled trial. Brain Injury 2008;22(2):135‐45. [DOI] [PubMed] [Google Scholar]
- Lynch E, Harling R, English C, Stiller K. Patient satisfaction with circuit class therapy and individual physiotherapy. International Journal of Therapy and Rehabilitation 2008;15(4):167‐73. [Google Scholar]
- Mead GE, Greig CA, Cunningham I, Lewis SJ, Dinan S, Saunders DH, et al. Stroke: a randomised trial of exercise or relaxation. Journal of the American Geriatrics Society 2007;55:892‐9. [DOI] [PubMed] [Google Scholar]
- Olney SJ, Nymark J, Brouwer B, Culham E, Day A, Heard J, et al. A randomised controlled trial of supervised versus unsupervised exercise programs for ambulatory stroke survivors. Stroke 2006;37:476‐81. [DOI] [PubMed] [Google Scholar]
- Purton J, Golledge J. Establishing an effective quantity of physiotherapy after stroke: a discussion. International Journal of Therapy and Rehabilitation 2007;14(7):318‐23. [Google Scholar]
- Pyöriä O, Talvitie U, Nyrkkö H, Kautiainen H, Pohjolainen T, Kasper V. The effect of two physiotherapy approaches on physical and cognitive functions and independent coping at home in stroke rehabilitation. A preliminary follow‐up study. Disability and Rehabilitation 2007;29(6):503‐11. [DOI] [PubMed] [Google Scholar]
- Rimmer J, Riley B, Creviston T, Nicola T. Exercise training in a predominantly African‐American group of stroke survivors. Medicine and Science in Sports and Exercise 2000;32(12):1990‐6. [DOI] [PubMed] [Google Scholar]
- Salbach NM, Mayo NE, Wood‐Dauphinee S, Hanley JA, Richards CL, Côté R. A task‐orientated intervention enhances walking distance and speed in the first year post stroke: a randomised controlled trial. Clinical Rehabilitation 2004;18:509‐15. [DOI] [PubMed] [Google Scholar]
- Shepherd RB. Exercise and training to optimise functional motor performance in stroke: driving neural reorganization?. Neural Plasticity 2001;8(1‐2):121‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington C, Pamphlett PI, Jacka JA, Olivetti LM, Nugent JA, Hall JM, et al. Group exercise can improve participants' mobility in an outpatient rehabilitation setting: a randomised controlled trial. Clinical Rehabilitation 2008;22:493‐502. [DOI] [PubMed] [Google Scholar]
- Sullivan KJ, Brown DA, Klassen T, Mulroy S, Ge T, Azen SP, et al. Effects of task‐specific locomotor and strength training in adults who were ambulatory after stroke: results of the STEPS randomised clinical trial. Physical Therapy 2007;87:1580‐602. [DOI] [PubMed] [Google Scholar]
- Sunnerhagen S. Circuit training in community‐living "younger" men after stroke. Journal of Stroke and Cerebrovascular Diseases 2007;16(3):122‐9. [DOI] [PubMed] [Google Scholar]
- Tanne D, Tsabari R, Chechik O, Toledano A, Orion D, Schwammenthal Y, et al. Improved exercise capacity in patients after minor Ischaemic stroke undergoing a supervised exercise training program. Israeli Medical Association Journal 2008;10:113‐6. [PubMed] [Google Scholar]
- Taskinen P. The development of health enhancing exercise groups adapted for hemiplegic patients. A pilot study. Neurorehabilitation 1999;13:35‐43. [Google Scholar]
- Teixeira‐Salmela LF, Olney SJ, Nadeau S, Brouwer B. Muscle strengthening and physical conditioning to reduce impairment and disability in chronic stroke survivors. Archives of Physical Medicine and Rehabilitation 1999;80:1211‐8. [DOI] [PubMed] [Google Scholar]
- Yang Y‐U, Wang R‐Y, Lin K‐H, Chu M‐Y, Chan R‐C. Task‐oriented progressive resistance strength training improves muscle strength and functional performance in individuals with stroke. Clinical Rehabilitation 2006;20(20):860‐70. [DOI] [PubMed] [Google Scholar]
Additional references
- Begg S, Vos T, Barker B, Stevenson C, Stanley L, Lopez AD. The burden of disease and injury in Australia 2003. Australian Institute of Health and Welfare. Canberra2007.
- Bernhardt J, Dewey H, Thrift A, Donnan G. Inactive and alone. Physical activity within the first 14 days of acute stroke unit care. Stroke 2004;35:1005‐9. [DOI] [PubMed] [Google Scholar]
- Cox KL, Burke V, Gorley TJ, Beilin LJ, Puddy IB. Controlled comparison of retention and adherence in home‐ vs center‐initiated exercise interventions in women ages 40‐65 years: the SWEAT study (Sedentary Women Exercise Adherence Trial). Preventive Medicine 2003;36:17‐29. [DOI] [PubMed] [Google Scholar]
- Dean C, Richards C, Malouin F. Walking speed over 10 metres overestimates locomotor capacity after stroke. Clinical Rehabilitation 2001;15:415‐21. [DOI] [PubMed] [Google Scholar]
- Dewey H, Sturm J, Donnan G, Macdonell R, McNeil J, Thrift A. Incidence and outcome of subtypes of ischaemic stroke: initial results from the North East Melbourne Stroke Incidence Study (NEMESIS). Cerebrovascular Diseases 2003;15:133‐9. [DOI] [PubMed] [Google Scholar]
- Enzinger C, Dawes H, Johansen‐Berg H, Wade D, Bogdanovic M, Collet J, et al. Brain activity changes associated with treadmill training after stroke. Stroke 2009;40(7):2460‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flansbjer UB, Holmback AM, Downham D, Patten C, Lexell J. Reliability of gait performance in men and women with hemiparesis after stroke. Journal of Rehabilitation Medicine 2005;37:75‐82. [DOI] [PubMed] [Google Scholar]
- Forrester LW, Wheaton LA, Luft AR. Exercise‐mediated locomotor recovery and lower‐limb neuroplasticity after stroke. Journal of Rehabilitation Research and Development 2008;45(2):205‐20. [DOI] [PubMed] [Google Scholar]
- Fraser N, Spink K. Examining the role of social support and group cohesion in exercise compliance. Journal of Behavioral Medicine 2002;25(3):233‐49. [DOI] [PubMed] [Google Scholar]
- French B, Thomas LH, Sutton CJ, McAdam J, Forster A, Langhorne P, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database of Systematic Reviews 2007, Issue 4. [Art. No.: CD006073. DOI: 10.1002/14651858.CD006073.pub2.] [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org2008.
- Johansen‐Berg H, Dawes H, Guy C, Smith S, Wade D, Matthews P. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain 2002;125:2731‐42. [DOI] [PubMed] [Google Scholar]
- Kollen B, Kwakkel G, Lindeman E. Time dependency of walking classification in stroke. Physical Therapy 2006;86(5):618‐25. [PubMed] [Google Scholar]
- Kwakkel G, Peppen R, Wagenaar R, Wood‐Dauphinee S, Richards C, Ashburn A, et al. Effects of augmented exercise therapy time after stroke. A meta‐analysis. Stroke 2004;35:2529‐36. [DOI] [PubMed] [Google Scholar]
- Liepert J, Bauder H, Miltner W, Taub E, Weiller C. Treatment‐induced cortical reorganization after stroke in humans. Stroke 2000;31:1210‐6. [DOI] [PubMed] [Google Scholar]
- Mao H, Hseuh I, Tang P, Sheu C, Hsieh C. Analysis and comparison of the psychometric properties of three balance measures for stroke patients. Stroke 2002;33:1022‐7. [DOI] [PubMed] [Google Scholar]
- McNevin N, Wulf G, Carlson C. Effects of attentional focus, self‐control and dyad training on motor learning. Implications for physical rehabilitation. Physical Therapy 2000;80:373‐85. [DOI] [PubMed] [Google Scholar]
- Michael K, Allen J, Macko R. Reduced ambulatory activity after stroke: the role of balance, gait, and cardiovascular fitness. Archives of Physical Medicine and Rehabilitation 2005;86:1552‐6. [DOI] [PubMed] [Google Scholar]
- Michael K, Macko R. Ambulatory activity intensity profiles, fitness, and fatigue in chronic stroke. Topics in Stroke Rehabilitation 2007;14:5‐12. [DOI] [PubMed] [Google Scholar]
- Mudge S, Stott S. Timed walking tests correlate with daily step activity in person with stroke. Archives of Physical Medicine and Rehabilitation 2009;90:296‐301. [DOI] [PubMed] [Google Scholar]
- Muntner P, Garrett E, Klag M, Coresh J. Trends in stroke prevalence between 1973 and 1991 in the US population 25 to 74 years of age. Stroke 2002;33:1209‐13. [DOI] [PubMed] [Google Scholar]
- Muren MA, Hutler M, Hooper J. Functional capacity and health‐related quality of life in individuals post‐stroke. Topics in Stroke Rehabilitation 2008;15(1):51‐8. [DOI] [PubMed] [Google Scholar]
- Nudo R, Milliken G, Jenkins W, Merzenich M. Use‐dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. Journal of Neuroscience 1996;16:785‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plautz E, Milliken G, Nudo R. Effects of repetitive motor training of movement representation on adult squirrel monkeys. Role of use versus learning. Neurobiology of Learning and Memory 2000;74:27‐55. [DOI] [PubMed] [Google Scholar]
- Pohl M, Mehrholz J, Ritschel C, Rükreim S. Speed‐dependent treadmill training in ambulatory hemiparetic stroke patients. A randomised controlled trial. Stroke 2002;33:553‐8. [DOI] [PubMed] [Google Scholar]
- Rand D, Eng J, Tang P‐F, Jeng J‐S, Hung C. How active are people with stroke? Use of accelerometers to assess physical activity. Stroke 2009;40:163‐8. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
- Richards LG, Stewart KC, Woodbury MCL, Senesac C, Cauraugh JH. Movement‐dependent stroke recovery: a systematic review and meta‐analysis of TMS and fMRI evidence. Neuropsychologia 2008;46:3‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salbach N, Mayo N, Higgins J, Ahmed S, Finch L, Richards C. Responsiveness and predictability of gait speed and other disability measures in acute stroke. Archives of Physical Medicine and Rehabilitation 2001;82(9):1204‐12. [DOI] [PubMed] [Google Scholar]
- Salbach N, Mayo N, Robichaud‐Ekstrand S, Hanley J, Richards C, Wood‐Dauphinee S. Balance self‐efficacy and its relevance to physical function and perceived health status after stroke. Archives of Physical Medicine and Rehabilitation 2006;87(3):364‐70. [DOI] [PubMed] [Google Scholar]
- Salbach N, Mayo N, Hanley J, Richards C, Wood‐Dauphinee S. Psychometric evaluation of the original and Canadian French version of the activities‐specific balance confidence scale among people with stroke. Archvies of Physical Medicine and Rehabilitation 2006;87(12):1597‐604. [DOI] [PubMed] [Google Scholar]
- Shea C, Wulf G, Whitacre C. Physical and observational practice afford unique learning opportunities. Journal of Motor Behavior 2000;32:27‐36. [DOI] [PubMed] [Google Scholar]
- Sidaway B, Yook D, Fairweather M. Visual and verbal guidance in the learning of a novel motor skill. Journal of Human Movement Studies 2001;40:43‐63. [Google Scholar]
- Taylor DW, Stretton C, Mudge S, Garrett N. Does clinic measured gait speed differ from gait speed measured in the community in people with stroke. Clinical Rehabilitation 2006;20:438‐44. [DOI] [PubMed] [Google Scholar]
- Tudor‐Locke C, Jones R, Myers AM, Paterson DH, Ecclestone NA. Contribution of structured exercise class participation and informal walking for exercises to daily physical activity in community‐dwelling older adults. Research Quarterly for Exercise and Sport 2002;73:350‐6. [DOI] [PubMed] [Google Scholar]
- Peppen R, Kwakkel G, Wood‐Dauphinee S, Hendriks H, Wees P, Dekker J. The impact of physical therapy on functional outcomes after stroke: what's the evidence?. Clinical Rehabilitation 2004;18:833‐62. [DOI] [PubMed] [Google Scholar]
- Wevers L, Port I, Vermue M, Mead G, Kwakkel G. Effects of a task‐oriented circuit class training on walking competency after stroke. Stroke 2009;40:2450‐9. [DOI] [PubMed] [Google Scholar]
- Wolfe C. The impact of stroke. British Medical Bulletin 2000;56:275‐86. [DOI] [PubMed] [Google Scholar]


