Abstract
Background
Ocular herpes is a viral infection of the eye caused by the herpes simplex virus (HSV), a double‐stranded DNA virus. Corneal scarring caused by herpes simplex keratitis (HSK) is the leading infectious cause of penetrating corneal graft in high‐income countries. Acyclovir is an antiviral drug known to have a protective effect against recurrences in herpetic eye disease. While there are some studies which have evaluated the effects of intervention with oral antiviral in preventing such recurrences in people with corneal grafts, a systematic review of all comparative clinical trials has not been previously undertaken.
Objectives
To assess the efficacy of oral antivirals such as acyclovir in any dosage when taken for six months or more, in preventing recurrence of herpetic keratitis in people having corneal graft surgery for herpetic keratitis.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 5), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to June 2016), Embase (January 1980 to June 2016), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 1 June 2016. We handsearched conference proceedings and contacted authors of the included studies and researchers active in the field.
Selection criteria
We included randomised controlled trials (RCTs). People enrolled in these trials had corneal grafts for HSK. The intervention was oral antivirals for six months or more following the corneal graft surgery, and this was compared to no treatment or placebo.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. We contacted trial investigators for any clarification or missing information. We graded the certainty of the evidence using GRADE.
Main results
We included three trials, involving 126 participants, comparing the use of oral acyclovir to no treatment or placebo. Two studies were conducted in single centres in Turkey and the USA, and one was multi‐centred in the Netherlands. In general, the studies were poorly reported and it was difficult to judge the extent to which bias had been avoided.
Oral acyclovir may reduce the risk of recurrence of herpetic keratitis (risk ratio (RR) 0.29, 95% confidence interval (CI) 0.13 to 0.64, 126 people, low‐certainty evidence). Based on data from the included trials, this corresponds to approximately 23 fewer cases of HSK recurrence (95% CI 29 fewer cases to 12 fewer cases) per 100 corneal graft operations if oral acyclovir is used.
Oral acyclovir may reduce the risk of graft failure (RR 0.40, 95% CI 0.16 to 0.97, 126 people, low‐certainty evidence). Based on data from the included trials, this corresponds to approximately 13 fewer cases of graft failure (95% CI 18 fewer cases to 1 fewer cases) per 100 corneal graft operations if oral acyclovir is used.
None of the studies reported any serious side effects of the antivirals necessitating stoppage or change. None of the trials reported outcomes over the long term (more than two years) or any data on quality of life.
Authors' conclusions
Compared to placebo or to no treatment, oral antiviral (acyclovir) may reduce the risk of recurrence of herpetic keratitis in the first 12 months in eyes that have undergone corneal graft surgery.
Plain language summary
Oral antivirals for preventing recurrent herpes simplex keratitis in people with corneal grafts
What is the aim of this review? Cochrane researchers aimed to find out if treatment with an antiviral medication at the time of the corneal graft, for example acyclovir, lowers the chance of herpes simplex virus (HSV) returning and causing inflammation (keratitis). They were also interested in whether this lowered the chance of the graft failing. They collected and analysed all relevant studies to answer this question and found three studies.
Key messages Low‐certainty evidence suggests that treatment with antiviral medication may lower the chances of HSV keratitis in people having a graft due to HSV infection. It may also lower the chance of the graft failing.
What was studied in the review? If herpes simplex virus (HSV) infects the cornea (clear front part of the eye) then it can cause inflammation (keratitis) and scarring which can cause loss of vision. Doctors can replace the cornea with a clear cornea from a donor. This is known as a corneal graft. Sometimes the corneal graft does not work well because the HSV comes back and reinfects the cornea. One option is to give antiviral medication (for example, acyclovir) at the time of corneal graft surgery to prevent the virus returning and further keratitis. This may also improve the chances of the graft surgery being successful.
What are the main results of the review? The review authors found three relevant studies from the Netherlands, Turkey and the USA.
People in the studies either took antiviral medication for six months after the corneal graft surgery or they took a placebo (or no treatment). The antiviral medication was acyclovir (oral ‐ taken by mouth) in all three studies but the dose varied from 200 to 800 mg/day. One of the studies reported support from a pharmaceutical company: the tablets were provided by the company and one of the authors worked with the company. The other two studies did not report any links with pharmaceutical companies.
The main results were as follows:
∙ Oral acyclovir may lower the chance of herpetic keratitis (low‐certainty evidence). It may also reduce the risk of graft failure (low‐certainty evidence). ∙ None of the studies reported any data on quality of life. ∙ None of the studies reported any serious side effects that meant the treatment had to be stopped or changed. ∙ None of the studies followed up for more than two years.
How up‐to‐date is this review? The Cochrane researchers searched for studies that had been published up to 1st June 2016.
Summary of findings
for the main comparison.
| Oral acyclovir compared to control/placebo for preventing recurrence of HED in people with corneal graft | ||||||
|
Patient or population: People with HED who had corneal graft(s) Settings: Hospital or clinics Intervention: Oral acyclovir Comparison: Placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo/No treatment | Oral acyclovir | |||||
| HSK recurrence in people with corneal graft | 33 per 100 | 10 per 100 (4 to 21) | RR 0.29 (0.13 to 0.64) |
126 (3) | ⊕⊕⊝⊝ low1 2 | |
| Graft failure | 21 per 100 | 8 per 100 (3 to 20) | RR 0.40 (0.16 to 0.97) |
126 (3) | ⊕⊕⊝⊝ low1 2 | |
| Adverse outcomes | None of the studies reported any serious side effects of the antivirals necessitating stoppage or change. | |||||
| Quality of life | None of the trials reported any quality of life data | |||||
| * The assumed risk was calculated from the number of events in the control groups of the 3 studies combined. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HED: Herpetic eye disease; HSK: Herpes simplex keratitis; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High‐certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: We are very uncertain about the estimate. | ||||||
1 We downgraded 1 level for risk of bias as the studies were poorly reported and it was not possible to judge the extent to which bias was avoided.
2 We downgraded 1 level for imprecision as there were a total of 126 people included in these studies and there were less than 25 events.
Background
Description of the condition
Herpes simplex virus (HSV) is a DNA virus commonly affecting humans. Infection is spread by direct contact of infectious secretions (e.g. saliva and tears) and lesions. There are two types of HSV: Type 1 (HSV‐1) primarily affects the face and eyes, and type 2 (HSV‐2) is generally transmitted sexually and causes genital infections. Herpes simplex virus‐2 may rarely infect the eye via direct contact with infectious genital secretions and occasionally is transmitted to neonates as they pass through the birth canal of a mother with genital HSV‐2 infection. Approximately 20,000 new cases of herpetic eye disease (HED) and more than 28,000 reactivations occur annually in the USA. Within the US population, a history of external ocular HSV infection is present in 0.15%. Herpes simplex virus keratitis (HSK) is one of the most frequent causes of corneal blindness in the USA with 500,000 people experiencing HSV‐related ocular disease, and is a leading indication for corneal transplantation. Herpetic eye disease is one of the major infectious causes of corneal scarring and secondary visual loss in high‐income countries (Liesegang 1991; Liesegang 2001).
Primary HSV‐1 infection most commonly involves the skin and mucus membrane in the distribution of any of the three branches of the trigeminal nerve. In the eyes, the initial infection can be asymptomatic or may cause typical branching (dendritic) corneal ulcer(s). After the primary infection, the virus spreads from the infected epithelial cells to nearby sensory nerve endings and is transported along the nerve axon to the cell body located in the trigeminal ganglion. The virus genome then enters the nucleus of a neuron, where it can go into a latent state and persist indefinitely. Interneuronal spread of HSV within the ganglion allows the infected person to develop ocular disease without ever having had primary ocular HSV infection (Liesegang 2001).
Recurrent HSV‐1 infection is traditionally thought to be due to reactivation of the virus in the sensory ganglion, which migrates down the nerve axon to produce a lytic infection in ocular tissue. Recent evidence suggests that the virus may subsist latently within corneal tissue, serving as a potential source of recurrent disease and also donor‐derived HSV in transplanted corneas (Shimomura 2007).
Herpes simplex virus keratitis encompasses a number of disease processes that HSV can cause in the human cornea. A variety of clinical manifestations of both infectious and immune disease, such as infectious epithelial keratitis, neurotrophic keratopathy, necrotising stromal keratitis, immune stromal keratitis (ISK), and endotheliitis, can affect all levels of the cornea.
Description of the intervention
Numerous studies indicate that HSV‐1 infection induces an array of cellular and humoral immune responses (Kohl 1985; Pepose 1991). Various forms of physical or emotional stress can cause transient suppression of these immune defences and trigger virus reactivation, which occurs apparently in the face of pre‐existing immunity. In people with corneal grafts due to HED, topical steroids are used for up to a year. In the corneal microenvironment, the role of topical steroids is to modulate the immune response which, in its attempt to clear virus infection or re‐infection of the cornea, in fact causes incidental damage to surrounding tissue that is largely responsible for the pathology associated with HSK. Herpetic eye disease recurrences and graft rejection threaten the survival of corneal grafts (Moyes 1994). A previous study has demonstrated that the incidence of HSV epithelial keratitis after corneal transplantation was six times higher than in the general population, even if there was no history of ocular HSV (Remeijer 2004). The surgery, the latency of the virus in the region, or the donor tissue may explain this recurrence rate. Both HED recurrences and graft rejection are probably related to HSV reactivation before or during many of the rejection episodes, and graft survival is improved when antivirals are added to the steroid treatment (Ficker 1989). Graft survival can probably be further enhanced by effectively preventing HSV reactivation after corneal transplantation in HED (Ficker 1988;Moyes 1994). Our review looks at the effect of oral antivirals in such people with corneal grafts.
Keratoplasty or corneal graft is the replacement of the recipient’s cornea with a healthy donor cornea. It can be lamellar (partial thickness: the top 80% to 90% of the cornea is replaced, leaving a very small layer of deep stroma and Descemet's membrane (DM) with endothelium of the host cornea) or penetrating (full thickness, including DM and endothelium). Keratoplasty is done for tectonic support (in corneal thinning and perforation) or improvement in visual outcome, such as in replacement of scarred cornea. Corneal graft in people with HED is mostly performed to improve the visual impairment resulting from corneal scarring caused by the stromal/endothelial disease. Although the rate of success of corneal graft surgery is excellent, especially when it is done for keratoconus, bullous keratopathy or Fuchs endothelial dystrophy, the chances of rejection increase significantly in the presence of active or recurrent infection, inflammation, corneal vascularisation, or previous graft rejection. In cases of HED, recurrences and graft rejection threaten the survival of corneal grafts. Both HED recurrences and graft rejection are probably related to HSV reactivation before or during many of the rejection episodes, and graft survival is improved when antivirals are added to the steroid treatment during rejection episodes. Graft survival can probably be further enhanced by effectively preventing HSV reactivation after corneal transplantation in HED.
How the intervention might work
Acyclovir is an acyclic purine nucleoside analogue, effective against HSV by inhibiting its DNA replication. Its use as a prophylactic agent was first studied in herpetic genito‐labial infections and was shown to diminish the frequency and severity of HSV recurrences. More recently, its use has been extended to ocular infections. A topical antiviral is potentially toxic and has minimal systemic absorption but offers modest prophylaxis (Cobo 1980; Ficker 1989). One landmark study demonstrated reduced recurrence of HSK with the oral antiviral, acyclovir (HEDS 1998). Oral antivirals have also been reported to decrease the rate of HSV recurrence in corneal transplantation (Akova 1999; Tambasco 1999; Van Rooij 2003). The highest frequency of HSV recurrence occurs in the first year after corneal transplantation and therefore oral antivirals (acyclovir) therapy is likely to prevent recurrence if continued during this period (Ficker 1989; Foster 1992; Moyes 1994; Tambasco 1999; Van Rooij 1995). It has been shown that the herpes virus genome is found in high numbers in the corneas of people with herpetic keratitis (Shimomura 2007), and therefore the continued use of oral antivirals may help in preventing the recurrence of the disease after corneal grafts.
Why it is important to do this review
Although there is some evidence in the literature suggesting a benefit of using oral antivirals (e.g. acyclovir) in preventing the recurrence of HSK, the guidelines are not robust. This review examines the published evidence on this particular topic.
Objectives
To assess the efficacy of oral antivirals such as acyclovir in any dosage when taken for six months or more, in preventing recurrence of herpetic keratitis in people having corneal graft surgery for herpetic keratitis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) with a minimum follow‐up duration of six months.
Types of participants
We included trials in which participants (of any sex, gender or ethnicity) had corneal grafts for herpetic keratitis and were on oral antivirals following corneal graft surgery.
Types of interventions
We included trials where participants who underwent corneal graft surgery and were given oral antiviral (e.g. acyclovir, at any dosage) for at least six months postoperatively were compared to people who had grafts but either did not receive any oral antiviral or received placebo. We also planned to include participants with lamellar corneal grafts.
Types of outcome measures
Primary outcomes
The proportion of participants having recurrence of HSK after corneal graft surgery within one year. We defined 'recurrence' as the re‐appearance of signs/symptoms of the disease, diagnosed clinically or microbiologically, or both.
Secondary outcomes
Primary or secondary corneal graft failure attributable to the herpetic infection.
Adverse outcomes
Any serious side effects of the antivirals used, necessitating stoppage or change.
Economic data
We planned to assess economic data if available.
Quality‐of‐life data
We planned to assess quality‐of‐life data if available in the included studies.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 5), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to June 2016), Embase (January 1980 to June 2016), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 1 June 2016.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), Embase (Appendix 3), ISRCTN (Appendix 4), ClinicalTrials.gov (Appendix 5) and the ICTRP (Appendix 6).
Searching other resources
We searched The Association for Research in Vision and Ophthalmology (ARVO) conference for abstracts related to the use of antivirals in corneal graft patients from 1992 to 2016. We also looked at conference abstracts of The Royal College of Ophthalmologists, Asia‐ARVO, American Academy of Ophthalmology (AAO), European Association for Vision and Eye Research (EVER), International Conference on Herpetic Eye Disease and International Conference on Antiviral Research from 1992 to August 2010. We also referred to the reference lists of identified trials for further trials. We did not handsearch journals or contact pharmaceutical companies.
Data collection and analysis
Selection of studies
Two review authors (UB, MN) independently scanned the titles, abstracts and keywords of every citation retrieved in the searches. We obtained full‐text copies of any report referring to definitely or possibly relevant trials. For any citation where we could not reach a decision based on the information given in the title and abstract, we retrieved a full‐text copy for clarification. We assessed all the full‐text copies of all the potential citations according to the definitions in the 'Criteria for considering studies for this review' section. We included only those trials meeting these criteria. We contacted the authors of trials that appeared to meet the inclusion criteria but did not report sufficient data. The review authors were unmasked to the trial authors, institution and results during the assessment. We resolved any discrepancies by discussion.
Data extraction and management
Two review authors (UB, MN) independently extracted data using a data extraction form adapted from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). The results were compared and discrepancies were resolved. We contacted the authors of reports to obtain missing data. One review author entered data into Review Manager 5 (RevMan 2014) and the second review author checked the entered data for any errors or discrepancies. We resolved any such discrepancies by discussion and by reference to the trial report.
We were not masked to the study authors and trial results during the assessment. We resolved disagreements by discussion.
Assessment of risk of bias in included studies
We considered the following parameters and assessed each one as high, low or unclear risk of bias, as in Cochrane’s tool for assessing risk of bias specified in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b):
Selection bias: We graded all selected studies according to their concealment of treatment allocation. We put studies into one of three categories as follows.
-
Category A: adequate concealment
Centralised randomisation
Sequential administration of prenumbered or coded containers.
On‐site computer system.
Serially‐numbered sealed opaque envelopes.
Other approaches appearing to offer adequate concealment.
-
Category B: unclear
Randomisation used but method not stated.
List or table used.
Envelopes, but no qualifying statement.
An apparently adequate concealment but other information in trial indicates concealment may not have been adequate.
-
Category C: inadequate
Alternation.
Case record numbers.
Dates of birth.
Days of the week.
We included in this review only trials coded as category A or B.
Performance bias
Were the recipients of care unaware of their assigned treatment?
Were persons providing care unaware of the assigned treatment?
Detection bias
Were persons responsible for outcome assessments unaware of the assigned treatment?
Reporting bias
Was there any selective reporting?
Attrition bias
Were rates of follow‐up similar in the comparison groups?
Was the analysis 'intention‐to‐treat'?
Measures of treatment effect
We calculated the risk ratio (RR) for outcome measures reported as dichotomous data (graft failure due to recurrence of herpetic keratitis). We have not reported any continuous data (e.g. visual acuity, pain scores, other quality‐of‐life data).
Unit of analysis issues
We included data from each eye individually for the analysis. None of the studies had one eye randomised to the treatment group and the other eye in the control group, to cause any kind of conflict in the analysis.
Dealing with missing data
We planned to contact trial investigators for any missing data. However this was not required for two of the included studies (Akova 1999; Van Rooij 2003) and for the third one (Foster 1992) investigators could not be reached (possibly due to the age of the trial).
Assessment of heterogeneity
We checked for heterogeneity of individual studies by examining:
The characteristics of the study.
The forest plot of the results of the studies.
The results of the Chi2 and I2 measures for statistical heterogeneity.
Data synthesis
Data synthesis was performed following the guidelines in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). We pooled data using a fixed‐effect model because there were only three included trials.
Summary of findings
We prepared a Summary of findings table using the GRADE approach (Langenham 2013) for the main comparison: oral acyclovir compared to control/placebo for preventing recurrence of HED in people with corneal graft.
Results
Description of studies
See the Included studies and Excluded studies sections below.
Results of the search
The electronic searches yielded 337 references (Figure 1). After 82 duplicate were removed the Cochrane Information Specialist (CIS) screened the remaining 255 records and removed 211 references which were not relevant to the scope of the review. We screened the remaining 44 references and obtained the full‐text reports of five references for further assessment. We identified four reports of three studies which met the inclusion criteria (Akova 1999; Foster 1992; Van Rooij 2003) and excluded one study (Jansen 2009). Despite handsearching, contacting authors of identified trials and researchers active in the field, we have not identified any other relevant studies for inclusion in the review. As there were only three trials included in this review we used a fixed‐effect model for all analyses.
1.
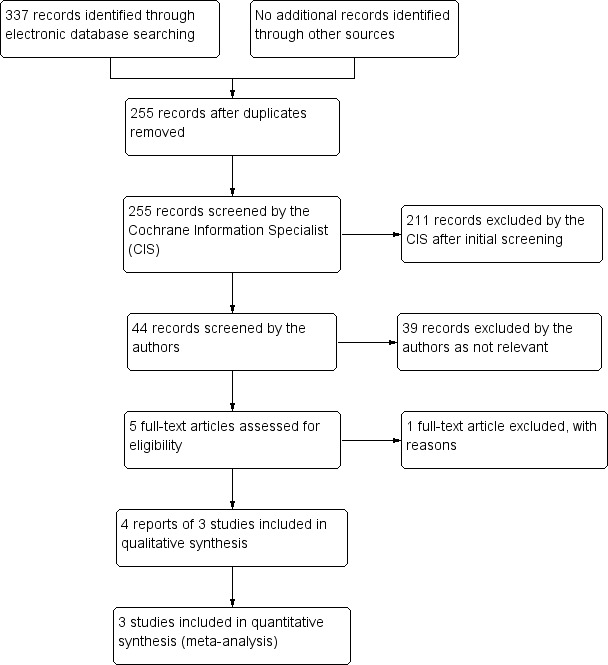
Study flow diagram.
Included studies
See the 'Characteristics of included studies' table for further information.
We included three RCTs in this review: Akova 1999; Foster 1992; Van Rooij 2003.
In Van Rooij 2003 participants were given oral antiviral for only six months; although this trial did not fulfil one of the inclusion criteria (of oral antiviral for 12 months) the trial investigators have given the incidence of recurrences after 12 months. This study is methodologically very sound and therefore included for analysis. A follow‐up report of this trial at five years (Jansen 2009) was excluded from the analysis; however the results of this report are important for the scope of this review and have therefore been discussed separately.
Design
Two of the included studies were single‐centre RCTs. In one of them it was not clear whether it was an RCT, but this was confirmed when we contacted the main author (Akova 1999). However, the method of randomisation was not confirmed, even after correspondence with the author. All three trials sought to determine the effectiveness of acyclovir in preventing further episodes of herpetic keratitis in participants with corneal graft. Two trials (Akova 1999; Foster 1992) compared an intervention to no treatment, while one (Van Rooij 2003) compared it to placebo. There was no significant heterogeneity among the studies.
Participants
Diagnosis of previous HED was clinical in all included studies. Van Rooij 2003 included positive HSV culture as a confirmation of HED but they also included clear and recurrent clinical history of dendritic keratitis or herpetic keratouveitis as means of proven HED.
The three studies were conducted in different countries, i.e. the Netherlands, Turkey and the USA. The number of participants randomised ranged from 23 to 68. Only one study defined the age range of participants (15 to 73 years) and the ratio of men to women (Van Rooij 2003).
Of the three included studies, only one (Van Rooij 2003) had clear information about two participants who dropped out at three and six months.
Details on the study populations are given below (see ’Results’ section).
Types of interventions
Oral antiviral (acyclovir) was started before or just after corneal graft surgery in any dosage of 200 mg to 800 mg once/twice/three times daily for at least six months following the surgery. In all the included studies the participants had herpetic keratitis. People who had received oral acyclovir for HED were also included. The dose and duration of treatment varied.
Types of outcome measure
All three trials reported incidences of recurrence of herpetic keratitis during the 12‐months or more follow‐up period. Exact definitions of recurrence varied across trials. In the earliest included study (Foster 1992), recurrence of HSK was determined by culture or clinically by the typical findings of epithelial dendrites or stromal keratitis. Graft rejection was determined clinically by the presence of anterior chamber reaction with keratic precipitates on donor endothelium or endothelial rejection line. In the second study (Akova 1999), recurrent HSK was diagnosed clinically by the typical findings of epithelial dendrites or stromal keratouveitis. Herpetic keratouveitis was diagnosed based on keratic precipitates distributed over both graft and host endothelium; it may also be associated with epithelial dendrite. Corneal allograft rejection episodes were defined as anterior chamber reactions with keratic precipitates on the donor endothelium, or endothelial rejection line with or without localised or diffuse corneal oedema. In the most recent included study (Van Rooij 2003), an event was defined as any clinically suspicious rejection, HED recurrence, or a combination of the two. For any such event, clinical parameters for the type of event were recorded, and polymerase chain reaction (PCR) and culture samples were taken. In the event of a visible epithelial lesion or suture infiltrate, with or without any anterior chamber reaction, a sample for viral cell culture and PCR was taken by swabbing the lesion. In the event of a graft rejection episode without an epithelial lesion, aqueous humor was drawn for PCR and viral cell culture.
Other outcomes reported in the trials included graft failure, raised intraocular pressure and microbial keratitis.
Excluded studies
See the 'Characteristics of excluded studies' table for further details.
We excluded one study (Jansen 2009) as this was a five‐year follow‐up of a subset of the same group of participants from the Van Rooij 2003 study already included in the review. In addition, only a portion of those assessed in this five‐year follow up received further oral acyclovir thereby making them quite heterogenous groups. It is therefore impossible to include the results of this report in the analysis.
Risk of bias in included studies
The general quality of the reporting of studies was low. Of the three studies included, we graded only one initially at low risk of bias (Van Rooij 2003). See Figure 2 for additional information on our assessments of each parameter for each study.
2.
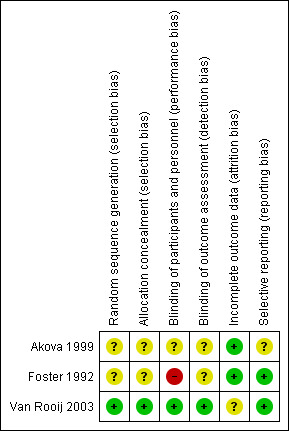
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Of the three trials included, only Van Rooij 2003 had allocated participants using appropriate methodology (a 2 x 2 list). We had a response from the lead author of one of the trials (Akova 1999) that allowed us to upgrade in the quality assessment, although we still could not assign the level of risk due to the lack of clarity. Foster 1992 did randomise the participants but we still graded it at high risk, as no clear methodology of randomisation was described.
Blinding
Only one study (Van Rooij 2003) was double‐masked. For the other two there was no obvious masking attempted.
Incomplete outcome data
The risk was low for Foster 1992 and Akova 1999, as both trials had all the enrolled participants completing the study. In the third trial (Van Rooij 2003), 68 participants entered the study but five did not complete for various reasons, although in statistical calculations all participants (including those five) were included. This attrition did not appear to have been taken into account. However, personal communication with the senior author clarified that they had performed an 'intention‐to‐treat' analysis, which means that all participants who entered the trial were analysed regardless of treatment adherence in a survival analysis model. When any participant had reached an 'event' (HED recurrence in this trial) or was no longer available for follow‐up, the data of this particular participant were 'censored'. This censoring corrected for the participants not reaching 24 months of follow‐up.
Selective reporting
Reporting bias was unclear for Akova 1999. In Van Rooij 2003 such bias was low, as all successive participants with corneal opacities attributed to HED who were scheduled for penetrating keratoplasty (PK) were considered for enrolment in the study. In Foster 1992 there was low risk of reporting bias, as all participants receiving acyclovir were included.
Other potential sources of bias
We found no further obvious biases.
Effects of interventions
See: Table 1
See: 'Summary of findings' for the main comparison
Primary outcomes
Recurrence of HSK: A total of 126 participants (68 cases and 58 controls) were randomised in three trials (Akova 1999; Foster 1992; Van Rooij 2003). The effect of treatment on recurrent HSK was strongly influenced by Van Rooij 2003, which reported results for participants with recurrence of herpetic keratitis over 24 months post‐corneal graft when treated with oral acyclovir for six months as 3/35 (8.5%) in comparison with the 9/33 (27%) control group (risk ratio (RR) of recurrence 0.31, 95% confidence interval (CI) 0.09 to 1.06). Akova 1999 reported recurrent lesions in 3/19 in study group and 6/16 in control group participants after follow‐up for two years (RR 0.42, 95% CI 0.12 to 1.42). Similarly, Foster 1992 reported 0/14 recurrence in the treatment group versus 4/9 in the control group (RR 0.07, 95% CI 0.00 to 1.23). The overall results show a statistically significant benefit of oral antiviral (acyclovir) in preventing recurrence of HSK in participants with corneal grafts at 12 months (RR 0.29, 95% CI 0.13 to 0.64; I2= 0%) (Analysis 1.1). The direction of effect is same in all three studies, indicating a beneficial effect with oral acyclovir. We graded the certainty of evidence as moderate (Table 1).
1.1. Analysis.
Comparison 1 Oral acyclovir versus control (no treatment or placebo), Outcome 1 Herpes simplex keratitis (HSK) recurrence in corneal graft participants: oral acyclovir versus control.
Secondary outcomes
Graft failure: Among three studies, there were 6/68 cases in the oral antiviral group and 12/58 cases in the control group. This indicates a lower rate of graft failure in the oral antiviral group at 12 months after surgery (RR 0.40, 95% CI 0.16 to 0.97; I2 = 0%) (Analysis 1.2). One trial (Foster 1992) reports that the 4/5 failed grafts in the control group had both an episode of HSK recurrence and rejection either sequentially or separated by time, suggesting HSK recurrence as an important cause of rejection and graft failure sequence. We graded the certainty of evidence as moderate (Table 1).
1.2. Analysis.
Comparison 1 Oral acyclovir versus control (no treatment or placebo), Outcome 2 Graft failure in oral acyclovir versus control group.
Graft rejection: Graft rejection as an outcome was defined in two (Akova 1999; Foster 1992) out of the three included studies and reported reduced graft rejection in the treated group compared with the untreated/control group participants. In all, 7/33 (21%) in the oral acyclovir group had graft rejection episodes compared to 12/25 (48%) in the control group. One of the studies reported a 2/9 (14%) of the participants receiving oral acyclovir compared to 5/9 (56%) in those who were not.
Other complications: Only one of the three included trials (Akova 1999) reported any other secondary complications, such as secondary glaucoma and bacterial keratitis. There were 2/19 cases of secondary glaucoma in the study population compared to 3/16 in the control group. Only one participant in the study group had bacterial keratitis and none in the control group.
Adverse outcomes
Side effect(s): None of the included trials reported any serious side effects of oral acyclovir treatment.
Economic data
Only Van Rooij 2003 has commented on the cost of the prophylactic therapy and also that the cost of repeating a corneal graft would be much more than the prophylactic treatment and would have a worse prognosis, clearly favouring the prophylactic treatment.
Quality of life
None of the trials reported any quality of life data.
Discussion
Although episodes of herpes simplex viral (HSV) epithelial keratitis can be treated effectively, it has a natural ability to establish life long latency by means of the viral genome entering the host cell without disturbing the metabolic functioning of the host cell or causing lysis (Cook 1991). Reactivation of latent infection in the trigeminal ganglion or the cornea or both leads to recurrent diseases. Such recurrences may lead to corneal stromal scarring and decreased visual acuity, and not unsurprisingly a common indication for corneal transplantation in many published reports. Acyclovir, an antiviral when used orally has the potential to prevent the recurrence as evidenced by the HEDS Acyclovir Prevention Trial (HEDS 1998). This review suggests that beneficial effect of oral acyclovir can be extended to prevent similar recurrences in people with corneal graft.
Summary of main results
The main finding of this review is that compared to placebo or no treatment, oral acyclovir reduces the risk of recurrence of herpes simplex keratitis (HSK) following corneal graft and therefore favours its use. This review also suggests a protective effect of oral acyclovir treatment against graft failure. However, it is important to note that the definition of graft failure is not standardised in the three studies included in this review. Only Van Rooij 2003 has clearly defined graft failure as reduced best‐corrected visual acuity due to persistent oedema or to a corneal opacification.
Overall completeness and applicability of evidence
It must be pointed out that for all three studies included in the review diagnostic criteria are not standardised, postoperative medications were different and the duration of follow up was also variable. In Foster 1992, they have included participants with previous penetrating keratoplasty while the other two studies did not. The duration and dosages of acyclovir are different in all three studies: Foster 1992 used 800 mg to 1000 mg in four to five divided doses, Akova 1999 used 400 mg/day and Van Rooij 2003 used 400 mg twice daily. Oral acyclovir’s bioavailability is poor, as only 15% to 30% of the formulation is absorbed. Peak serum concentration of about 0.5 μg/ml is attained at approximately 1½ to 2½ hours following a 200 mg dose. Higher doses of acyclovir result in higher serum concentrations. The half‐life of acyclovir is two to three hours in adults with normal renal function (Kimberlin 2007). Clearly, this would cause a huge variation in the actual effective drug levels across the participants in the three studies.
Quality of the evidence
Variable formulations and dosages of acyclovir among the trials included in this review could potentially cause heterogeneity. In a study of an interventional case series (Simon 1996), the efficacy of oral acyclovir prophylaxis at different doses was investigated in 13 participants with a history of recurrent HSV keratitis. It concluded that recurrences were more likely in participants on relatively lower doses (less than 800 mg per day) and in those who had recent ocular surgery in the past six weeks. Among the participants experiencing recurrent HSV keratitis following ocular surgery, those on higher doses of oral acyclovir (an average dose of 1321 mg/day) experienced fewer recurrences compared to those on lower doses (average of 1000 mg/day). In terms of the duration of the effect, one of the included trials (Akova 1999) reported some evidence of rebound recurrence after cessation of treatment in one subgroup (necrotising keratitis with corneal perforation), while Van Rooij 2003 did not notice any such rebound in the first year, despite cessation of the treatment in the study group after six months. In a further extension of this study, the same group found a statistically significant lower monthly event rate in the acyclovir group over five years follow‐up (Jansen 2009). Although this report suggests that the effect of oral acyclovir used over the first six months after corneal graft surgeries can last up to five years, it does not provide any explanation of the mechanism of how the effect might persist for that long. In HEDS 1998, oral acyclovir was used for a year, based on this and also the findings of investigators that the highest rates of epithelial recurrences and rejections occur during the first 12 months, it is reasonable to suggest that acyclovir can be safely used for this duration. However, in the review authors' opinion, the quality of the evidence to support this argument is moderate at best.
Potential biases in the review process
We reduced the risk of bias during the review process by conducting a thorough literature search of various sources and by not limiting studies by language. Two review authors independently assessed the search results for inclusion and also for biased reporting. As we strictly followed the protocol criteria, we have little reason to believe that there would be any risk of any bias in the review process.
Agreements and disagreements with other studies or reviews
There is no other systematic review analysing the effect of oral antiviral in preventing recurrence of HSK in transplanted corneas. HEDS 1998 showed that acyclovir at a dosage of 400 mg twice a day is effective in reducing recurrent HSV keratitis during one year of treatment. This study was in participants without corneal grafts. However, there is a relatively high risk of graft failure in the participant group with corneal grafts for herpes eye disease (HED). Although the results of HEDS 1998 may be applicable to people with grafts, it is important to realise that the participants with corneal graft differed from those in this trial in two ways: the presence of a corneal transplant and also the use of topical steroids after the corneal graft. Moreover, the participants with multiple recurrences of HSK developed scarring and loss of vision necessitating corneal graft. The fact that a corneal graft is required in these people indicates the severe end of the spectrum of HED. It is therefore, difficult to apply the results of HEDS 1998 directly to this population, although it would be fair to say that if oral acyclovir is beneficial to those with less severe HED, it is also likely to benefit those requiring grafts. This is supported by a recently published clinical guideline from the American Academy of Ophthalmology (AAO), which recommends that oral acyclovir in doses of 800 mg/three times a day initially, and later tapered to 400 mg/twice a day for at least one year, reduces the recurrence rate of HSV keratitis in people after penetrating keratoplasty. It is reasonable to continue prophylaxis as long as the person remains on a topical corticosteroid (White 2014). A rational basis for management of people with visually significant herpetic keratitis is therefore eagerly awaited and this review aims to provide the evidence base for this.
Authors' conclusions
Implications for practice.
For eyes that have undergone corneal graft surgery due to herpetic eye disease, compared with placebo or no treatment, people taking oral acyclovir may have a reduced risk of recurrence of the disease. In addition, it may also reduce the risk of rejection and subsequent graft failure in these people. Although the data suggest positive effects of the use of oral acyclovir to prevent recurrences of herpes eye disease following corneal grafts, the quality of evidence supporting these conclusions was low. Although there is some evidence of the effect lasting much longer after cessation of the treatment, there is no consensus regarding the most effective dosage or duration of treatment necessary to achieve optimum benefit without significant risks or side effects.
Implications for research.
There have been some concerns about the development of resistance to acyclovir, particularly in immune‐compromised people due to the widespread use of acyclovir for the treatment of genital, orofacial, and other herpetic diseases, augmented by over‐the‐counter availability of the drug in certain countries. Some studies have sought to determine the prevalence of either acyclovir‐resistant strains or clinical resistance (Garcia 2007).
The dose of oral acyclovir and duration of treatment is not standardised across the included studies in this review. Use of acyclovir after penetrating keratoplasty began In the early 1990s and surgeons varied in how they used it. It seems that when two of the included studies (Akova 1999; Foster 1992) were conducted, there was no 'standard care’ to be followed for antiviral prophylaxis. Despite some debate on the correct dosage, the benefits of its use can still clearly be seen in terms of the decreased number of recurrences and improved overall graft survival compared with the prior decade.
Given the significant prophylactic effect of oral acyclovir on preventing recurrence of herpes simplex keratitis after corneal graft surgery shown in individual trials and in this review, it might be useful to consider designing future trials that involve using oral acyclovir for various durations in different subsets of participants (with disease at different stages, such as stromal keratitis with or without inflammation and endotheliitis) and also at different dosages, to answer the question of the risks and benefits of oral acyclovir prophylaxis. However, conducting such a trial would require a large number of participants in a multi‐centre approach and would be extremely difficult to administer. It is worth pointing out here that the evidence for the conclusions drawn in this review is not of high quality and therefore not compelling, especially for the link between oral acyclovir and its effect on preventing graft rejection and failure. Future studies should be directed to quantify this effect. People with corneal graft survive for a long time following the graft and can potentially suffer recurrence of the herpes eye disease and consequent graft rejection. It is therefore important to know how long the protective effect of oral antiviral would be beneficial. A longer follow up would therefore be useful to ascertain whether this beneficial effect is sustained.
Acknowledgements
The electronic search strategies were developed and executed by the Cochrane Eyes and Vision (CEV) editorial team. We thank Kirk Wilhelmus and Catey Bunce for their peer review comments on the protocol of this review and Jenny Evans for her comments on the methodology section of the final review. We also thank Anupa Shah of CEV for her constructive comments at various stages of writing this review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Keratitis, Herpetic #2 MeSH descriptor Keratitis, Dendritic #3 (herpe* or simplex) near (cornea* or kerati* or dendr* or epithel* or endothel* or stroma* or uveiti* or ocular) #4 (#1 OR #2 OR #3) #5 MeSH descriptor Corneal Transplantation #6 cornea* near transplant* #7 cornea* near graft* #8 keratoplast* #9 (#5 OR #6 OR #7 OR #8) #10 MeSH descriptor Antiviral Agents #11 oral* near antiviral* #12 MeSH descriptor Acyclovir #13 ac?clovir* #14 MeSH descriptor Inosine Pranobex #15 isoprinosin* #16 (#10 OR #11 OR #12 OR #13 OR ##14 OR #15) #17 (#4 AND #9 AND #16)
Appendix 2. MEDLINE (Ovid) search strategy
1. randomised controlled trial.pt. 2. (randomised or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp keratitis herpetic/ 14. exp keratitis dendritic/ 15. ((herpe$ or simplex) adj4 (cornea$ or kerati$ or dendr$ or epithel$ or endothel$ or stroma$ or uveiti$ or ocular)).tw. 16. or/13‐14 17. exp corneal transplantation/ 18. (cornea$ adj3 transplant$).tw. 19. (cornea$ adj3 graft$).tw. 20. keratoplast$.tw. 21. or/17‐20 22. exp antiviral agents/ 23. (oral adj3 antiviral$).tw. 24. exp acyclovir/ 25. ac?clovir$.tw. 26. exp inosine pranobex/ 27. isoprinosin$.tw. 28. or/22‐27 29. 16 and 21 and 28 30. 12 and 29 The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase (Ovid) search strategy
1. exp randomised controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp herpes simplex keratitis/ 34. ((herpe$ or simplex) adj4 (cornea$ or kerati$ or dendr$ or epithel$ or endothel$ or stroma$ or uveiti$ or ocular)).tw. 35. or/33‐34 36. exp cornea transplantation/ 37. (cornea$ adj3 transplant$).tw. 38. (cornea$ adj3 graft$).tw. 39. keratoplast$.tw. 40. or/36‐39 41. exp antivirus agent/ 42. (oral adj3 antiviral$).tw. 43. exp aciclovir/ 44. ac?clovir$.tw. 45. exp Methisoprinol/ 46. isoprinosin$.tw. 47. or/41‐46 48. 35 and 40 and 47 49. 32 and 48
Appendix 4. ISRCTN search strategy
Herpetic Keratitis
Appendix 5. ClinicalTrials.gov search strategy
Herpetic Keratitis
Appendix 6. WHO ICTRP search strategy
Herpetic Keratitis
Data and analyses
Comparison 1. Oral acyclovir versus control (no treatment or placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Herpes simplex keratitis (HSK) recurrence in corneal graft participants: oral acyclovir versus control | 3 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.13, 0.64] |
| 2 Graft failure in oral acyclovir versus control group | 3 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.16, 0.97] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akova 1999.
| Methods | Study design: randomised controlled trial* Number randomised: 35 total; 19 acyclovir; 16 control (no treatment) Exclusions after randomisation: None Number analysed: at 12 months: 35 total; 19 acyclovir; 16 control (no treatment) Unit of analysis: Individuals Losses to follow up: None Note: Study design was not clear on reading the article. *Confirmed randomised through communication with the lead author |
|
| Participants | Country: Turkey, single centre Mean age: 33.8 years overall; 34.6 years for acyclovir group; 31.5 years for control group Gender: 13/19 (68%) men in acyclovir group; M:F ratio not given for the control group Inclusion criteria: Participants undergoing penetrating keratoplasty for HSK Exclusion criteria: None specified |
|
| Interventions | Intervention: 400 mg/day acyclovir orally Comparator: No oral acyclovir Length of follow‐up: 25 months average |
|
| Outcomes | Primary outcome: Recurrence of HK in the same eye Adverse events reported: Yes, rejection and graft failures |
|
| Notes | Funding sources: None reported Disclosures of interest: None Study period: 1990‐1996 Reported subgroup analyses: Yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation is not mentioned |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None indicated |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol and trial was not registered |
Foster 1992.
| Methods | Study design: Randomised controlled trial Number randomised: 23 total; 14 acyclovir; 9 control (no treatment) Exclusions after randomisation: None Number analysed: at 12 months: 23 total; 14 acyclovir; 9 control (no treatment) Unit of analysis: Eyes Losses to follow up: None Note: 2 participants from the control group received acyclovir when needed penetrating keratoplasty for a recurrence. |
|
| Participants | Country: USA, single centre Mean age: 57 years overall; 60 years in acyclovir group; 54 years in control group Gender: Not specified Inclusion criteria: 1. Any participant undergoing PK for HSK, 2. Participants had to be free of HSK for at least 3 months Exclusion criteria: 1. Pregnant and lactating women; 2. Women of childbearing age not taking contraception |
|
| Interventions | Intervention: 800 or 1000 mg acyclovir orally each day in 4‐5 divided doses Comparator: No oral acyclovir Length of follow‐up: 16.5 months |
|
| Outcomes | Primary outcome: Recurrence of HK in the same eye Adverse events reported: Yes, rejection and Irreversible graft failures |
|
| Notes | Funding sources: Heed Ophthalmic Foundation, Cleveland, Ohio Disclosures of interest: None Study period: 3 years; 1 January 1987 to 31 December 1990 Reported subgroup analyses: Yes For analysis an 'Intention‐to‐treat' strategy was followed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report mentions randomisation: "patients were randomly assigned to one of two groups" but have not clarified the way it was done |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither the surgeon nor the participants were masked in this study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | One of the authors was masked for data tabulation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants with HK who received acyclovir in the unit during the study period were included |
| Selective reporting (reporting bias) | Low risk | All participants with HK who received acyclovir in the unit during the study period were included |
Van Rooij 2003.
| Methods | Study design: Randomised double‐masked placebo‐controlled trial Number randomised: 68 total; 35 acyclovir; 33 control (placebo) Exclusions after randomisation: None Number analysed: At 24 months: 63 total; 35 acyclovir; 33 control Unit of analysis: Participants; no participant with bilateral HED was included in the study Losses to follow up: 3, 1 died 14 months after PK, 2 did not attend 3 and 6 months follow up Note: For analysis, an intention‐to‐treat policy was adhered to |
|
| Participants | Country: The Netherlands, multi‐centre Mean age: 51 years overall; 53 years (15‐73) in acyclovir group; 50 (18‐74) in placebo group Gender (M:F): 20/15 in acyclovir group, 21/12 in placebo group Inclusion criteria: 1. Any participant undergoing penetrating keratoplasty for visual loss due to HED; 2. Participants had to be free of any active HED or infectious keratitis for 1 year before inclusion Exclusion criteria: 1. Compromised immune system; 2. Pregnancy (planned or actual); 3. Any contraindication to the use of acyclovir |
|
| Interventions | Intervention: 400 mg acyclovir orally twice daily for 6 months Comparator: Placebo (Identical tablets to acyclovir) Length of follow‐up: 24 months |
|
| Outcomes | Primary outcome: Recurrence of HK in the same eye Adverse events reported: Yes, corneal graft failure |
|
| Notes | Funding sources: Acyclovir and placebo tablets were provided by GlaxoWellcome BV, Zeist, The Netherlands Disclosures of interest: One of the authors worked as a consultant statistician with Glaxo‐ Wellcome BV for 1 day a week Study period: 3 years; dates not reported Reported subgroup analyses: No |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial report states: "A randomisation list was generated with the aid of 2x2 squares, and the medication was labelled and distributed by the local hospital pharmacist." |
| Allocation concealment (selection bias) | Low risk | The trial report states: "The participants were allocated to double‐masked treatment with oral acyclovir 400 mg twice daily or with identical placebo tablets." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial report states: "The participants were allocated to double‐masked treatment with oral acyclovir 400 mg twice daily or with identical placebo tablets." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial report states: "The participants were allocated to double‐masked treatment with oral acyclovir 400 mg twice daily or with identical placebo tablets." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 68 participants entered the study but 5 did not complete for various reasons. However in statistical calculations, all participants (including those 5) were taken into account. |
| Selective reporting (reporting bias) | Low risk | The trial report states: "All successive participants with corneal opacities attributed to HED who were scheduled for PK were considered for enrolment in this study." |
HED: herpes eye disease HK: herpetic keratitis HSK: herpes simplex keratitis PK: penetrating keratoplasty
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Jansen 2009 | 5‐year follow‐up of the same group of participants from a study included in the review (Van Rooij 2003). In addition, only a portion of those assessed in this 5‐year follow‐up received further oral acyclovir thereby making the participants a very heterogenous groups, making it impossible to include the results of this report in the analysis. |
Differences between protocol and review
Although originally we had planned to include participants who had received oral antiviral for at least 12 months post surgery, we found one study with only six months of oral antiviral use but very useful long follow‐up data. We have therefore changed the criteria to studies where acyclovir was used for six months or more. We have also included a Summary of findings table.
We did not do the subgroup and sensitivity analyses planned in our protocol (Bhatt 2009) due to the fact that there were only three trials identified.
Contributions of authors
Conceiving the review: UB, MN Designing the review: UB Co‐ordinating the review: UB Data collection for the review ‐ Designing search strategies: Cochrane Eyes and Vision Group ‐ Undertaking searches: UB, MN ‐ Screening search results: UB, MN, JP ‐ Organising retrieval of papers: UB ‐ Screening retrieved papers against inclusion criteria: UB, MN ‐ Appraising quality of papers: UB, MN, ‐ Extracting data from papers: UB, MN ‐ Writing to authors of papers for additional information: UB ‐ Providing additional data about papers: UB ‐ Obtaining and screening data on unpublished studies: UB, MN, UF Data management for the review ‐ Entering data into RevMan: UB, MN Analysis of data: UB, MN, UF Interpretation of data ‐ Providing a methodological perspective: UB, MN ‐ Providing a clinical perspective: UB, VSM, JP ‐ Providing a policy perspective: UB ‐ Providing a consumer perspective: UB Writing the review: UB Providing general advice on the review: VSM, JP
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute of Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEV Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
UB: None known AK: None known JP: None known SVM: None known UF: None known
New
References
References to studies included in this review
Akova 1999 {published data only}
Foster 1992 {published data only}
- Barney NP, Foster CS. A prospective randomized trial of oral acyclovir after penetrating keratoplasty for herpes simplex keratitis. Cornea 1994;13(3):232‐6. [DOI] [PubMed] [Google Scholar]
- Foster CS, Barney NP. Systemic acyclovir and penetrating keratoplasty for herpes simplex keratitis. Documenta Ophthalmologica 1992; Vol. 80, issue 4:363‐9. [DOI] [PubMed]
Van Rooij 2003 {published data only}
References to studies excluded from this review
Jansen 2009 {published data only}
- Jansen AF, Rijneveld WJ, Remeijer L, Volker‐Dieben HJ, Eggink CA, Geerards AJ, et al. Five‐year follow‐up on the effect of oral acyclovir after penetrating keratoplasty for herpetic keratitis. Cornea 2009;28(8):843‐5. [DOI] [PubMed] [Google Scholar]
Additional references
Cobo 1980
Cook 1991
- Cook SD, Hill JH. Herpes simplex virus: molecular biology and the possibility of corneal latency. Survey of Ophthalmology 1991;36(2):140‐8. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ficker 1988
Ficker 1989
Garcia 2007
- Garcia DD, Farjo Q, Musch DC, Sugar A. Effect of prophylactic oral acyclovir after penetrating keratoplasty for herpes simplex keratitis. Cornea 2007;26(8):930‐4. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
HEDS 1998
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kimberlin 2007
- Kimberlin DW, Whitley RJ. Antiviral therapy of HSV‐1 and ‐2. In: Arvin A, Campadelli‐Fiume G, Mocarski E editor(s). Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press, 2007. [PubMed] [Google Scholar]
Kohl 1985
- Kohl S. Herpes simplex virus immunology: problems, progress, and promises. Journal of Infectious Diseases 1985;152(3):435‐40. [DOI] [PubMed] [Google Scholar]
Langenham 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schünemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;2:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liesegang 1991
Liesegang 2001
Moyes 1994
Pepose 1991
Remeijer 2004
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shimomura 2007
Simon 1996
- Simon AL, Pavan‐Langstan D. Long‐term oral acyclovir therapy. Effect on recurrent infectious herpes simplex keratitis in patients with or without grafts. Ophthalmoogy 1996;103(9):404‐5. [DOI] [PubMed] [Google Scholar]
Tambasco 1999
Van Rooij 1995
White 2014
- White ML, Chodosh J. Herpes simplex virus keratitis: a treatment guideline ‐ 2014. www.aao.org/clinical‐statement/herpes‐simplex‐virus‐keratitis‐treatment‐guideline (accessed 19 January 2015).
References to other published versions of this review
Bhatt 2009
- Bhatt UK, Abdul Karim M, Prydal JI. Oral antivirals for preventing recurrent herpes simplex keratitis in people with corneal grafts. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007824] [DOI] [PMC free article] [PubMed] [Google Scholar]


