Abstract
Background
Recent studies have attempted to disaggregate therapeutic intervention packages by looking at the impact of structure and process characteristics of environment upon outcome. However, what is commonly referred to as the 'black box' of therapy has yet to be comprehensively unpacked. This failure to analyse the components of therapy means that it remains unclear how much therapy should be provided, who should provide it, and which patients should be targeted to ensure that functional outcomes are maximized. This review, therefore, seeks to assess the effectiveness of specific therapeutic interventions in the rehabilitation of the paretic upper limb post stroke.
Objectives
To identify if specific hands‐on therapeutic interventions enhance motor activity and function of the upper limb post stroke.
Search methods
We searched the trials registers of the Cochrane Stroke Group (March 2010), the Cochrane Complementary Medicine Field (March 2010) and the Cochrane Rehabilitation and Related Therapies Field (March 2010); MEDLINE (1966 to March 2010); AMED (1985 to March 2010); EMBASE (1980 to March 2010); CINAHL (1982 to March 2010); the Physiotherapy Evidence Database (PEDro) (March 2010); and the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 1). In an effort to identify other published, unpublished and ongoing trials we planned to handsearch journals, searched ongoing trials registers, reviewed reference lists, and contacted relevant professional organizations.
Selection criteria
Randomized controlled trials (RCTs) involving adults aged 18 years or over and including descriptions of specific hands‐on interventions and techniques, rather than packages or approaches to treatment.
Data collection and analysis
Following completion of the searches, two review authors independently assessed the trials and extracted data using a data extraction pro forma. The same two review authors independently recorded and documented the methodological quality of the trials.
Main results
Three studies, involving a total of 86 participants, met all the selection criteria and were included in the review. However, extreme levels of heterogeneity were evident. Therefore, we could not undertake a meta‐analysis of the results and completed a narrative synthesis instead.
Authors' conclusions
Overall, the review demonstrated that the limited evidence of benefit of stretching, passive exercises and mobilization, when applied to the hemiplegic upper limb following stroke, merits further research.
Keywords: Adult, Humans, Physical Therapy Modalities, Stroke Rehabilitation, Upper Extremity, Electric Stimulation Therapy, Electric Stimulation Therapy/methods, Motor Activity, Motor Activity/physiology, Muscle Stretching Exercises, Muscle Stretching Exercises/methods, Paresis, Paresis/etiology, Paresis/rehabilitation, Randomized Controlled Trials as Topic, Stroke, Stroke/complications
Hands‐on therapy interventions for upper limb motor dysfunction following stroke
Therapists use a variety of techniques to help the arm to get better following stroke. However, the details of what they doing are unclear, are not well described in studies, and are used in various combinations. It is not known which elements of these techniques are effective. This review therefore attempted to identify which, if any, of the techniques used are beneficial. However, we found only three studies giving clear descriptions of the techniques used. In addition, each of these studies used different techniques with different types of patients in different environments and the success of these interventions was measured differently. It was therefore difficult to draw clear conclusions. In light of this, it is suggested that this review has demonstrated that the limited evidence of benefit of stretching, passive exercises and mobilization, when applied to the hemiplegic upper limb following stroke, merits further research.
Background
The World Health Organization describes stroke as a "clinical syndrome, characterized by rapidly developing clinical symptoms and/or signs of focal, and at times global, loss of cerebral function, with symptoms lasting more than twenty‐four hours or leading to death, with no apparent cause other than that of vascular origin" (Hatano 1976). Stroke affects 110,000 people in the UK each year (DOH 2001) and, after coronary heart disease, is the second most common cause of death (BHF 1999) and the leading cause of disability in the UK (Murray 1997). The impact of stroke illness is, therefore, major.
It has been noted that studies looking at the economic sequelae of stroke are minimal and outdated (Kings College 2005). Estimates suggest that 7.4% of community healthcare spending and 5.5% of hospital care expenditure is consumed by illness caused by stroke (NHSE 1996). It is clear, therefore, that both the financial and human impact of stroke are significant. Furthermore, a recent audit highlighted that the average length of an in‐patient hospital stay was 28 days (Sentinel Audit 2004). In the current climate of healthcare provision, one of the key challenges facing therapists and rehabilitation service providers "is to provide services to more patients in less time without diminishing outcomes" (Bode 2004). This is clearly of great significance when considering the provision of stroke rehabilitation services. It follows that therapists must understand how much therapy they should be providing, determine at whom this therapy should be targeted, and identify which are the key successful interventions in improving functional outcomes.
Recent studies have attempted to disaggregate therapeutic intervention packages by looking at the impact of structure and process characteristics of environment upon outcome (Duncan 2002; Hoenig 2001; Reker 2000; Reker 2002). However, what is commonly referred to as the 'black box' of therapy (DeJong 2005) has yet to be comprehensively unpacked. This failure to analyse the components of therapy makes it impossible to succeed in the challenge outlined by Bode et al (Bode 2004), in that it remains unclear how much therapy should be provided, who should provide it and which patients should be targeted to ensure that functional outcomes are maximized.
Most recovery is reported to take place in the first three months following stroke (Wade 1983a). However, there is evidence that recovery is not limited to this time period and hand and upper limb recovery has been reported many years after stroke (Carey 1993; Dannenbaum 1983; Yekutiel 1993). It has also been proposed that recovery of upper limb function is intrinsic and that little can be done by therapists to influence it (Heller 1987; Wade 1983b). Carr and Shepherd suggested that poor upper limb recovery may be due not only to the direct impact of the stroke itself but also to insufficient and inadequate or inappropriate therapeutic interventions (Carr 1998). Little information is available, however, to describe what best represents 'optimum treatment' (Ballinger 1991) or what the components of these treatments are (Ashburn 1993).
It has previously been proposed that a classification is needed to support our understanding as to which activities or interventions benefit recovery and in which types and groups of patients (Bode 2004). DeJong et al describe the great intellectual focus and energy used to understand and devise models of disability, focusing on outcomes and outputs, whereas little energy has been spent on the systems of care and treatments used in rehabilitation (DeJong 2004). Furthermore, they suggest that this 'input' side of intervention has not been exposed to the same standard of methodological critique as the outcomes and outputs (DeJong 2004). Therapists have a tendency to found their clinical practice on a treatment 'approach'. In the UK, Switzerland and Australia, the Bobath approach is used most widely (Pollock 2007). Therapists often seek evidence relating to global approaches to stroke rather than seeking descriptions of, and evidence to support, specific interventions (Pollock 2007). The evaluation and application of such evidence and the replication of the research is fraught with difficulty due to poor descriptions and documentation of the actual content of the therapy being investigated (Pollock 2007). Frequently, several terms are used for the same intervention or approach. Conversely, they may also be used to mean different things (Stern 1970). In this way, research looks at aggregated packages of care (DeJong 2004). It is uncommon for the entire array of inter‐disciplinary interventions, as alluded to by Wade (Wade 2001), to be subjected to review.
There have been several recent Cochrane Reviews of interventions for the upper limb following stroke, such as constraint‐induced movement therapy (Sirtori 2009), repetitive task training (French 2007), bilateral arm training (Coupar 2010) and electrostimulation (Pomeroy 2006); however, none of the reviews have focused upon clearly described hands‐on interventions. Furthermore, therapeutic approaches and interventions in stroke rehabilitation are rarely described by their constituent components; earlier studies (Keith 1995; Kramer 1997) focused on the totality of the package of rehabilitation. One method of improving and developing the evidence base to support clinical practice is to first systematically describe current stroke interventions and then code them into treatment schedules (Pomeroy 2001). Indeed, this was recommended over 15 years ago by Edwards et al in their article describing treatment schedules for research (Edwards 1990). Subsequent treatment schedules for specific hands‐on therapeutic interventions have been published, but are limited to single modules of current hands‐on therapy (Hunter 2006) or to general therapy for the upper limb (Donaldson 2009; Tyson 2004). This systematic review, therefore, seeks to address the gap in reviews of the evidence and to assess the effectiveness of specific hands‐on therapeutic interventions in the rehabilitation of the paretic upper limb post stroke.
Objectives
The objective of the review was to identify if specific hands‐on therapeutic interventions enhance motor activity and function of the upper limb post stroke.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials (RCTs) in this review. We included trials with or without blinding of the participants, therapists and assessors. We excluded cross‐over trials.
Types of participants
We included adults aged 18 years and over, male and female, with upper limb dysfunction as a result of stroke. We included participants with motor impairment, with or without the presence of sensory impairment.
Types of interventions
We included all trials evaluating the effectiveness of a clearly described hands‐on physical intervention (manual therapy techniques), or treatment component schedules, for the upper limb following stroke, either as the experimental intervention or as the control group. We did not include pharmacological, electrical or psychological (for example, mental imagery or relaxation) techniques. We only reviewed trials with interventions that address physical impairment. We included interventions delivered during the acute and chronic stages of rehabilitation. Furthermore, we excluded task‐oriented and occupation‐based interventions, constraint‐induced movement therapy and repetitive task training. This review focused on studies that included descriptions of specific hands‐on interventions and techniques rather than packages or approaches to treatment. A previous Cochrane Review highlighted the problems relating to approach‐based reviews and recommended that further systematic reviews be completed to determine the effectiveness of clearly detailed and described individual techniques (Pollock 2007). It was intended that, if they were described in the literature, this review would also investigate the effect of dose of intervention, the location of delivery (for example in‐patient, out‐patient community‐based) and the mode of delivery of the intervention (for example, by qualified or non‐qualified staff, by physiotherapists, occupational therapists, nurses, carers).
Types of outcome measures
The primary outcome reviewed was improvement in upper limb function as measured by validated tests of upper limb function, such as the Action Research Arm Test (Lyle 1981).
Secondary outcomes were improvement in motor impairment (measured by validated tests such as the Motricity Index) and improvement in functional independence (as measured by validated tests of functional independence such as the Barthel Index (Mahoney 1965)); we have also included differences in death rates and differences in adverse events.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module.
We searched the trials registers of the Cochrane Stroke Group (last searched March 2010), the Cochrane Complementary Medicine Field (last searched March 2010), and the Cochrane Rehabilitation and Related Therapies Field (last searched March 2010). In addition, we searched the following general and specialist electronic bibliographic databases: MEDLINE (Ovid) 1966 to March 2010 (Appendix 1), AMED (Ovid) 1985 to March 2010 (Appendix 2), EMBASE (Ovid) 1980 to March 2010 (Appendix 3), CINAHL (EBSCO) 1982 to March 2010 (Appendix 4), the Physiotherapy Evidence Database (PEDro) March 2010 (Appendix 5) and the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 1) (Appendix 6). We developed search strategies in consultation and discussion with the Cochrane Stroke Group Trials Search Co‐ordinator.
We identified further published, unpublished and ongoing trials as follows.
In March 2010, we searched the National Research Register (NRR) Archive (http://www.nihr.ac.uk/Pages/NRRArchive.aspx) and Current Controlled Trials (http://www.controlled‐trials.com/).
We had planned to handsearch journals, but all those relevant had already been searched on behalf of The Cochrane Collaboration and the results were available to us through CENTRAL.
We searched the reference lists of all relevant papers.
We reviewed the Physiotherapy Researchers Register, complied by the Chartered Society of Physiotherapy, and identified and contacted any physiotherapists with a stroke rehabilitation interest.
We contacted members of the Chartered Society of Physiotherapy who were subscribed to the mailing lists via the PHYSIO email discussion board, the College of Occupational Therapists, to establish if any members knew of any unpublished or ongoing trials, and the authors of relevant studies to identify further trials and, as necessary, to request additional information for relevant trials.
We searched for relevant trials in all languages and arranged translation of trial reports published in languages other than English.
Data collection and analysis
Selection
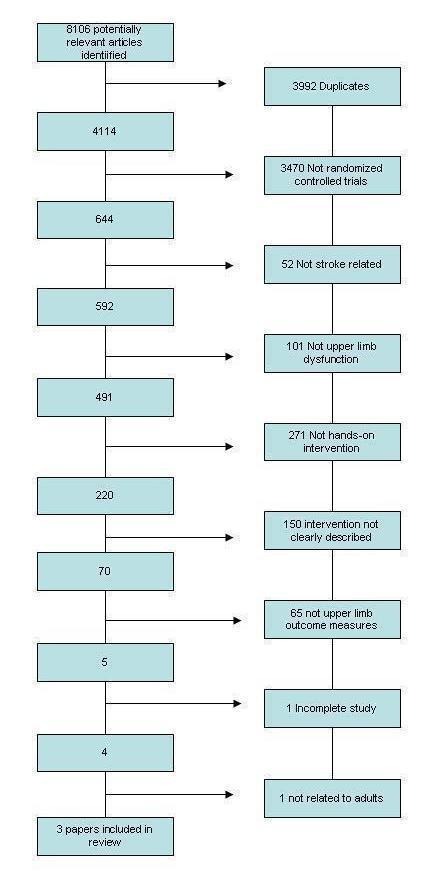
Following completion of the searches, two review authors assessed the trials independently. They initially screened trial titles and abstracts according to the inclusion criteria; each trial was assigned as either 'potentially relevant' or 'definitely not relevant'. We immediately excluded any trial rated by both assessors in the latter category. The same two review authors subsequently reviewed full copies of the remaining trials and independently graded these papers as 'relevant', 'not relevant' or 'unclear'. We excluded any trials rated 'not relevant' by both review authors at this stage. We included all trials reviewed as 'relevant' by both review authors. Discussion between the review authors and, as appropriate, the rest of the review team resolved any disagreement between the authors and assisted with decisions regarding any trials rated as 'unclear'. This process is summarized in Figure 1 and Figure 2.
Figure 1.
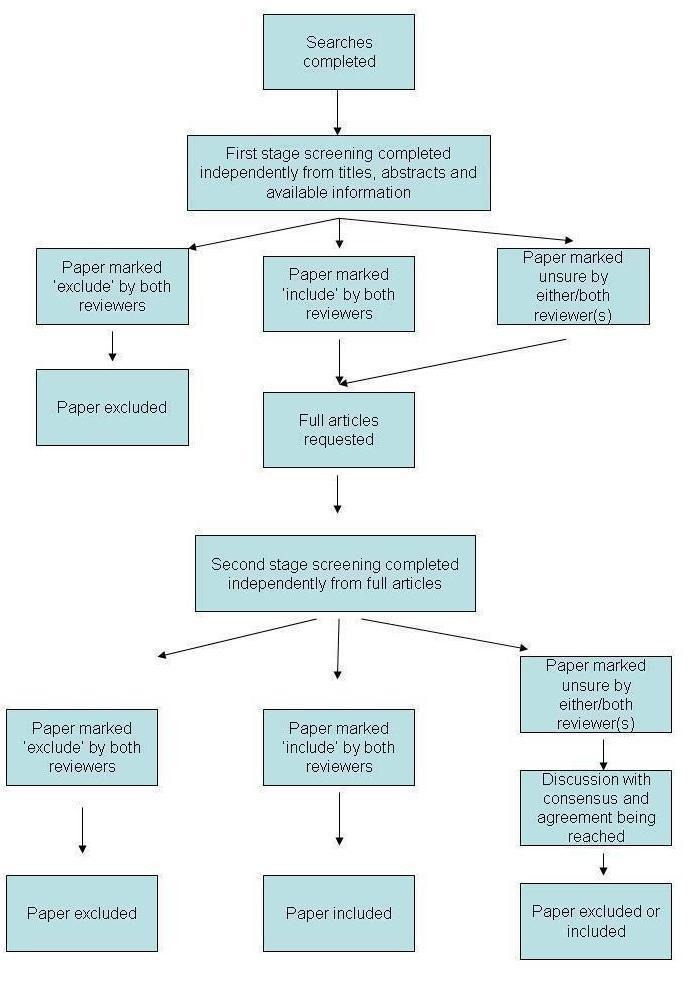
Overall screening process
Figure 2.
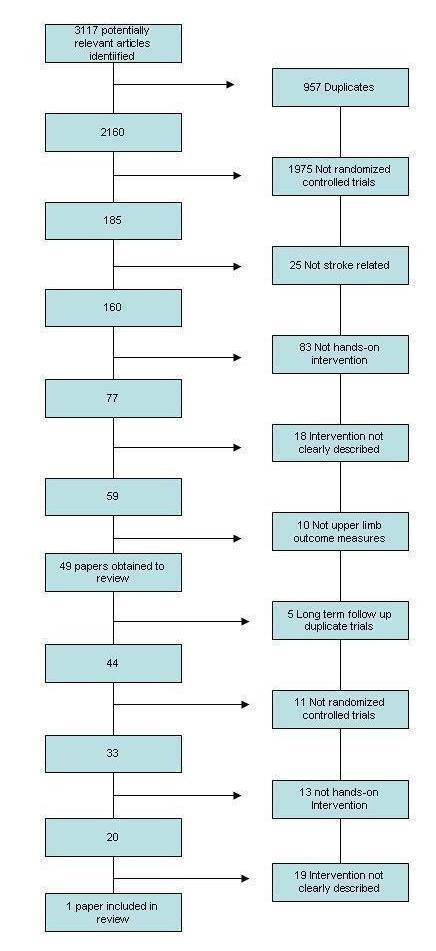
MEDLINE search results
Data extraction
The two review authors undertook data extraction independently using a data extraction form (Appendix 7). We contacted trial authors as necessary to request missing information. We documented the following information where possible:
participants (e.g. age, gender, site of lesion, length of time post stroke, stroke classification);
trial inclusion and exclusion criteria; and
assessed outcomes.
We resolved any disagreements by discussion and by contacting trial authors for clarification as appropriate.
Methodological quality
Two review authors independently recorded and documented the methodological quality of the trials following the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). We recorded the following indicators on the data extraction form (Appendix 7):
method of randomization;
concealment;
blinding of participants;
blinding of providers of care to the intervention group;
blinding of outcome assessor;
reliability and validity of outcome measures used;
any potentially confounding factors; and
statistical analysis performed (if any).
We used these indicators in the review as an indicator of overall quality of the trials, and we have reported the information gained in the results.
Comparisons to be made
We made primary comparisons as possible in relation to studies of:
intervention versus control; or
intervention versus other therapy.
For each of these comparisons we recorded improvements in measures of upper limb function such as the Action Research Arm Test, measures of motor impairment such as the Motricity Index, and measures of functional independence such as the Barthel Index, for the purpose of making comparisons. We planned to use the Cochrane Review Manager software (RevMan 2008) to calculate any weighted treatment effects across trials; we planned to base the primary analysis upon a mean difference as it was anticipated that trials would use continuous outcomes but that scales of measurement would vary between studies.
Dealing with missing data
Where possible, analysis was on an intention‐to‐treat basis. We had planned to undertake sensitivity analysis to investigate the effect of including trials with participants who did not complete the intervention as trials were not excluded on this basis; however, this was not possible. We used descriptions of the methodological quality of the study to aid the interpretation of any analyses.
Assessment of heterogeneity
We determined clinical heterogeneity based upon the characteristics of populations, interventions, settings and outcomes. We determined methodological heterogeneity based upon study design and quality. We did not undertake formal assessment of statistical heterogeneity using the I2 statistic as we did not find homogeneity across any of the study populations, interventions and settings. Therefore, as we identified such high levels of heterogeneity, we did not pool the data but completed a narrative synthesis instead. It was, therefore, not possible to conduct a random‐effects meta‐analysis.
Assessment of reporting biases
As anticipated, the studies identified were few in number and had diverse interventions; therefore, we decided that the planned use of funnel plots to address publication and related biases would not be helpful.
Subgroup analysis
We had planned to perform the following subgroup analyses, using the method described by Deeks 2001:
the effect of dose of intervention;
the effect of the location of the intervention;
any impact of the mode of intervention (e.g. qualified therapist (including experience), trained helper, carer or family member, etc).
However, this information was not clearly detailed in the trial reports hence such subgroup analysis was not possible.
Results
Description of studies
1.0 Search strategy results
We identified a total of 8106 articles as possibly relevant during the initial searches of all sources. However, we found the majority not to be relevant during the initial stage of screening of the study title and abstracts. We obtained copies of all relevant trials following a second stage of screening and we finally identified three articles for inclusion.
The three articles we identified for inclusion were:
'Differentiated manual treatment of the hand and forearm in early rehabilitation of stroke patients (a controlled study)' (published in Czech) (Mikulecka 2005);
'Manual stretch: effect on finger movement control and force control in stroke subjects with spastic extrinsic finger flexor muscles' (Carey 1990);
'A pilot study to investigate the effects of electrical stimulation on recovery of hand function and sensation in subacute stroke patients' (Mann 2005).
A flow chart of this overall process is provided in Figure 1. This section will now go on to describe details of the results of the individual searches.
1.1. General and specialist electronic bibliographic database search results
1.1.1 MEDLINE ((Ovid) 1966 to March 2010) search results
We identified a total of 3117 potentially relevant studies from the search. Following initial screening we obtained 49 papers for assessment. Following the second stage of screening, we included one paper (Carey 1990) in the review. This process is shown in Figure 2.
1.1.2 EMBASE ((Ovid) 1980 to March 2010), CINAHL ((EBSCO) records published 1982 to 2010) and AMED ((Ovid) 1985 to March 2010) combined database search results
We identified a total of 4528 potentially relevant studies from the search. Following initial screening we obtained 15 papers for assessment. Following the second stage of screening we included two papers (Mann 2005; Mikulecka 2005) in the review. This process is shown in Figure 3.
Figure 3.
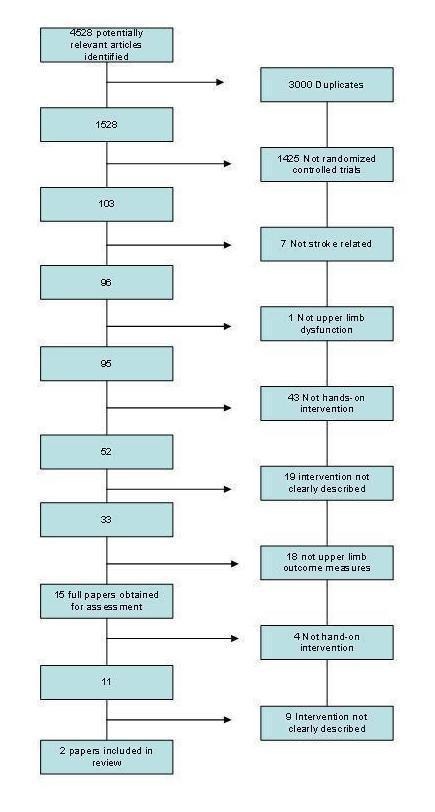
EMBASE/AMED/CINAHL combined search results
1.1.3 The Cochrane Stroke Group Trials Register search results
We identified a total of 358 potentially relevant studies from the search. Following initial screening we did not identify any papers for inclusion in the review.
1.1.4 The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 1) search results
We identified a total of 90 potentially relevant studies from the search. Following initial screening we obtained two papers for assessment. Following the second stage of screening we did not identify any papers for inclusion in the review. This process is shown in Figure 4.
Figure 4.
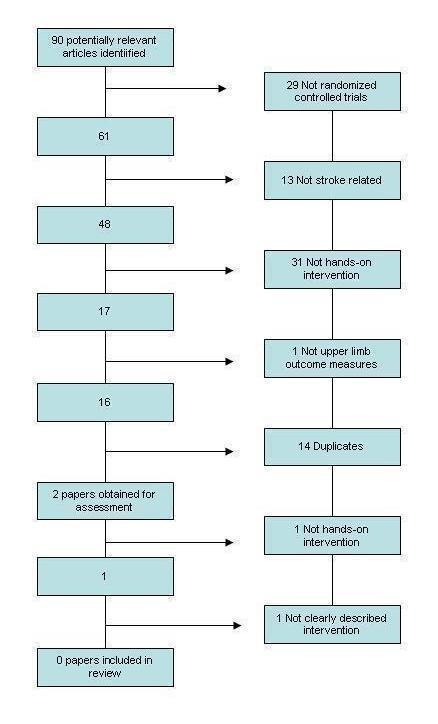
CENTRAL search results
1.1.5 The Physiotherapy Evidence Database (PEDro) search results (March 2010)
Searching the Physiotherapy Evidence Database (PEDro) identified seven possible papers for inclusion. On assessment five were excluded as they were not related to stroke, one was excluded as it reported results in children under 18 years of age, and one was excluded as the intervention reported was not a hands‐on intervention. Therefore no papers were included from this search.
The total search results from all electronic database searches is shown in Figure 5.
Figure 5.
Total search scores
1.2 Searches of ongoing trials results
Our searches of ongoing trials and research registers identified no studies for inclusion in the review.
1.3 Handsearching results
Handsearching of relevant conference proceedings that had not already been searched by The Cochrane Collaboration identified no further studies for inclusion in the review. We did not identify any relevant journals, beyond those already reviewed by The Cochrane Collaboration, to search.
1.4 Reference lists results
Our review of all reference lists from obtained papers identified one possible paper for inclusion in the review. After assessment, however, we excluded it as it did not have a clearly defined or discrete intervention. Therefore, we did not include any papers from this search.
1.5 The Physiotherapy Researchers Register results
Contacting physiotherapists via the Physiotherapy Researchers Register identified no studies for inclusion in the review.
1.6 Members of the Chartered Society of Physiotherapy results
Contacting the members of the Chartered Society of Physiotherapy identified no studies for inclusion in the review.
1.7 The College of Occupational Therapists results
Contacting the College of Occupational Therapists identified two possible unpublished research theses for inclusion in the review. After assessment, we excluded both studies as neither of them included a clearly described hands‐on therapeutic intervention.
1.8 Members of the College of Occupational Therapists results
Contacting the members of the College of Occupational Therapists via their journal identified three possible unpublished studies for inclusion in the review. After assessment, we excluded all three studies as none of them included a clearly described hands‐on therapeutic intervention.
2.0 Study assessment results
Following identification of the three trials for inclusion in the review, we undertook the two processes of data extraction and quality assessment. The results of these two processes are described in the following section.
2.1 Participant information
In total, 86 participants were investigated across the three studies included in the review: 51 males and 35 females. A summary of the participant information is shown in the Characteristics of included studies table.
Each of the three studies provided information on mean age. When assessed collectively across all of the studies, this was 62.17 years. Two studies (Mann 2005; Mikulecka 2005) reported age ranges ranging from 43 to 87 years; however, Carey 1990 presented unclear age range data, reporting the mean age ± between 2 and 15.3 years. Standard deviations were not reported consistently, with only Carey 1990 including standard deviation data.
The studies provided inconsistent information regarding the site of lesion. One study made no report (Mann 2005), one reported side of hemiparesis (Carey 1990), and one reported 'carotid artery' as a location (Mikulecka 2005).
In total, the mean time post stroke across the three studies was 3.5 years, with reported ranges from one week to six years.
Stroke classification was only clearly reported by Mann 2005, with 22 infarcts and one haemorrhagic stroke being reported, and by Mikulecka 2005 as 'anterior circulation stroke'.
2.2 Assessed outcomes
A number of different primary outcome measures to assess the impact of the intervention under investigation were used by each of the three studies. Carey 1990 used a Joint Movement Tracking Test (JMTT) and the Force Tracking Test (FTT); Mann 2005 used the Action Research Arm Test (ARAT) (Lyle 1981) and two‐point discrimination; whilst Mikulecka 2005 used the Jebson‐Taylor Test (Jebson 1969) and a visual ranking score.
Whilst some of these outcome measures, (e.g. ARAT (Lyle 1981) and Jebson‐Taylor Test (Jebson 1969)), have previously demonstrated validity and reliability, not all the tests used have been reported as valid and reliable, e.g. static two‐point discrimination. A summary of the outcome measures used is included in the Characteristics of included studies table.
The secondary outcomes of interest to this review were not reported in the three included studies.
2.3 Trial exclusions
There was little commonality of trial exclusions across the studies reviewed. Mikulecka 2005 gave no details of exclusions, Carey 1990 reported only medication for spasticity as an exclusion, whilst Mann 2005 reported cognitive, psychiatric and cardiac problems as exclusions as well as excluding participants with a cardiac pacemaker in situ. A summary of the exclusion criteria identified in the studies is included in the Characteristics of included studies table.
2.4 Trial inclusions
The inclusion criteria varied across the three studies with only limited homogeneity of these factors evident. A summary of the inclusion criteria described in the studies is included in the Characteristics of included studies table.
2.5 Interventions
All three studies utilized widely different interventions. These are discussed below and summarized in the Characteristics of included studies table.
2.5.1 Carey 1990 study
The able‐bodied group received no intervention. The measurements from this group of participants were only used for pre‐test comparisons. The control group of participants undertook pre‐tests for FTT and JMTT followed by five minutes of rest only, and then undertook FTT and JMTT post tests. The experimental group undertook pre‐tests for FTT and JMTT followed by five minutes of manual stretching exercise to: the extrinsic finger flexor muscles via slow (passive) extension of the distal interphalangeal, proximal interphalangeal and metacarpophalangeal joints of the four fingers; interphalangeal, metacarpophalangeal and carpometacarpal joints of the thumb; and to the wrist joint to full limits without producing pain. This end point was held for a maximum of 20 seconds then released for several seconds. This stretch was then repeated for five minutes, then finally the FTT and JMTT post tests were completed (Carey 1990).
2.5.2 Mann 2005 study
Any of the control group who were participating in other rehabilitative therapy whist participating in the study continued to receive that 'routine' therapy. In addition, however, they also received passive extension exercises of elbow, wrist and fingers daily for 30 minutes, applied twice daily for 12 weeks followed by 12 weeks without treatment. This is considered to be the 'hands‐on' therapy and, therefore, is of interest and importance to this review. Participants allocated to the experimental treatment group, who were participating in other rehabilitative therapy whilst participating in the study, continued to receive that routine therapy. In addition, however, they received two‐channel neuromuscular stimulation to stimulate elbow, wrist and finger extension for 30 minutes, twice per day for 12 weeks. This was followed by 12 weeks of no treatment (Mann 2005).
2.5.3 Mikuleka 2005 study
The control group received standard physiotherapy based on the Bobath approach (Bobath 1990) and proprioceptive neuromuscular facilitation (PNF) techniques (Knott 1968). Participants allocated to the experimental group received standard physiotherapy based on the Bobath approach and PNF techniques, as well as a clearly described intervention of differentiated manual treatment of the hand and forearm including soft tissue stretch, joint mobilization and acupressure applied daily for eight to 12 days for 20 minutes. The average number of treatments received by each participant was six (Mikulecka 2005).
2.6 Assessment of methodological quality
We adopted a narrative approach to assessment of methodological assessment. This revealed a number of methodological weaknesses in the literature reviewed, which are described in the following section and summarized in the 'Risk of bias' tables (Characteristics of included studies).
2.6.1 Power calculation
None of the studies reviewed included details of a power calculation to determine an appropriate sample size.
2.6.2 Method of randomization
All three studies were described as randomized controlled trials; however, only two included descriptions of the method of randomization. Mann 2005 used computer‐generated codes whilst Carey 1990 reported the use of a random numbers table, but gave no detail as to how this was generated.
2.6.3 Concealment
A process of allocation concealment ensures that participants and clinicians have no knowledge of impending allocations (Schulz 2002a). Correct randomization relies upon appropriate concealment and without it there is a risk of the process of randomization being inappropriately influenced (Schulz 2002a). Only one of the studies reviewed (Mann 2005) reported concealment, via codes sealed in envelopes. The other two studies made no reference to concealment, which may mean there was a potential for selection bias to occur in the allocation of participants to groups.
2.6.4 Blinding of participants
If participants are not blinded, knowledge of their group allocation may impact upon their responses to any interventions they receive (Schulz 2002b). Those who are aware that they are receiving a new intervention may have positive expectations that may influence their response and, conversely, those allocated to well known or standard treatments may feel disadvantaged (Schulz 2002b). None of the studies reviewed reported blinding of the participants. Whilst it cannot be assumed that no blinding took place, such a lack of blinding may not be unusual, given the nature of the interventions under investigation. For example, it would be obvious if one was not receiving neuromuscular stimulation as no placebo was applied. However, no comment or discussion was provided about this in the studies.
2.6.5 Blinding of intervention providers
None of the studies discussed blinding of the intervention providers. Again, given the nature of the interventions under investigation this may not be unusual; however, there was no comment or discussion on this in the studies.
2.6.6 Blinding of outcome assessors
None of the studies reported blinding of the outcome assessors. This may mean that there was a risk of experimenter bias.
2.6.7 Follow‐up period
The study by Carey 1990 provided no follow‐up period beyond the end of the intervention period. The follow up by Mikulecka 2005 was unclear. Both of these may be considered inadequate. However, Mann 2005 assessed participants 12 weeks after the completion of the 12‐week intervention period in an attempt to assess whether any treatment effects were maintained.
2.6.8 Additional sources of bias
The study by Carey 1990 had two further potential sources of bias of note. Firstly, the mean time since onset of stroke in the treatment group was three months less than in the control group. Secondly, some participants were also in receipt of additional therapy to that provided as the study intervention. This can be considered as a potential confounder to the results obtained.
2.6.9 Statistical tests employed
All three studies showed limitations with the statistical tests that they employed. Carey 1990 used change scores for the analysis but a covariate analysis may be considered to have been better, as the latter but not the former controls for regression to the mean (Vickers 2001). In addition, there were two randomized groups and one pre‐determined group (of normal participants). Randomization seeks to ensure statistical comparability of treatment groups (Sim 2000). Therefore, the statistical tests between one of the randomized groups and the normal participants would be more susceptible to bias than those between the two randomized groups.
Confidence intervals allow one to assess the precision of a sample estimate of a population treatment effect (Sim 1999). In Mann 2005, some of the confidence intervals were not presented clearly, whilst in Carey 1990 no confidence intervals were reported.
Mikulecka 2005 presented a confused account of the statistical analyses undertaken. Inappropriate baseline tests appear to have been performed (Altman 1990) and there was no indication of the size of between‐group effects. Furthermore, some of the P values quoted do not appear to accord with some of the conclusions drawn as the P values were above the cut‐off defined for significance, yet the associated effects were described as significant. Finally, some of the cited tables do not appear to be included in the article. However, it is worth reiterating that this article was published in Czech and was translated into English for the purpose of this review. Some interpretation and meaning, therefore, may have been lost in translation. We made repeated attempts to contact the authors of the article, both in English and in Czech, in an attempt to obtain the missing tables and to seek clarification; however, all attempts were unsuccessful.
2.6.10 Summary of assessment of methodological quality
Overall, the three studies reviewed demonstrated a number of significant methodological weaknesses, both individually and collectively. In addition, there was no homogeneity in respect of interventions, participants, outcome measures or statistical analyses performed; hence, pooling of data was not possible and we therefore undertook a narrative approach to assessment. This makes recommending the use of particular interventions difficult.
Risk of bias in included studies
Risks of bias for each study are detailed in the 'Risk of bias' tables (Characteristics of included studies).
Effects of interventions
This systematic review aimed to ascertain the effectiveness of clearly‐described hands‐on therapeutic interventions applied to the hemiplegic upper limb following stroke. However, from what initially appeared to be a large number of citations (8106 articles), only three met all the criteria described in the review protocol and were able to be included. On assessment, all three articles showed significant methodological limitations. Despite this, all the studies provide useful clinical messages which are discussed in the following section.
Carey 1990 study
The purpose of this study was to evaluate the effects of manual stretch of the extrinsic finger flexor muscles on finger extension movement control and force control in stroke patients with upper limb spasticity (Carey 1990). Despite the identified methodological weaknesses in this study, the inclusion and exclusion criteria appear appropriate and attempts were made to describe the randomization process via the generation of a random numbers table. In addition, clear attempts were made to demonstrate the validity of the outcome measures used and intraclass correlation coefficients were used to demonstrate the reliability of Accuracy Index scores.
The results reported in the study showed a non‐significant change in the Force Tracking Test (FTT) Accuracy Index scores in the experimental group (difference 3.57, SD 18.69). However, in the absence of a power calculation it is hard to determine the extent to which a Type 2 error may be the basis for this non‐significant finding. Conversely, however, these within‐group changes may be suggested to be of only secondary interest, as they do not relate to the central hypothesis of an anticipated between‐groups effect. Furthermore, any power calculation, if it had been included, would probably only be related to such between‐group changes. The results did, however, show a greater increased change in the Accuracy Index scores for the Joint Movement Tracking Test (JMTT) in the experimental group (difference 11.50, SD 9.16) compared to the control group (difference 0.95, SD 12.17) which was significant (P < 0.05). These results suggest that careful manual stretch applied to the hemiparetic hand can be used to improve controlled finger extension movement. It remains unclear, however, if this improvement is long‐term.
As this conclusion arises from just one study investigating manual stretch, the results should be viewed cautiously; however, the probable positive impact of manual stretch of finger flexors upon finger extension must be shared with clinicians and researchers alike. Furthermore, ongoing investigation of this type of intervention is clearly warranted.
Mann 2005 study
The purpose of this study was to investigate the effect of electrical stimulation of the wrist and elbow extensor muscles on recovery of hand function and sensation within a year following stroke (Mann 2005). Of the three studies included in the review, this was the strongest from a methodological point of view. The process of randomization was described, at least one of the outcome measures used had previously demonstrated validity and reliability and, despite no power calculation being used to inform the sample size, the results from this study were reported to have been used to inform a sample size calculation for a future, further randomized controlled trial.
The application of the intervention of electrical stimulation to the experimental group was not of interest to this review, which was focused on hands‐on interventions. However, the control group in this study received an intervention based upon passive exercises applied and practised for the same period each day, initially by a physiotherapist and, subsequently, by the participants themselves or a carer. The outcomes from this group of participants were, therefore, clearly of interest to the review; hence, the inclusion of this article.
The results reported focused upon the outcomes of the experimental group, with attention focusing on between‐group differences in improvement in ARAT and sensation scores. However, looking at the two groups individually, both the experimental group and the control group demonstrated improved scores. The ARAT scores for 10 out of the 11 participants in the control group had increased at the end of the 12‐week intervention period. The score for the 12th participant decreased. At the end of the subsequent 12‐week non‐treatment period, nine of these control group score improvements had been maintained at an unchanged level and an improved, but a smaller, score was shown by the remaining control participant. In the 'grasp' category, seven of the control group scores improved and four remained unchanged; six control group 'grip' category scores improved, with five remaining unchanged; six control group 'pinch' category scores remained the same in two and were worse in the remaining three; and five control group 'gross' movement category scores improved, with scores staying the same for the remaining six control group participants.
Within‐group improvements were achieved in overall ARAT scores by the control group, following the application of passive exercises (mean score at week 0 = 14.3, at week 12 = 24.4 and at week 24 = 24.7); this may indicate early evidence of the potential for passive exercise to have a positive impact upon the functional recovery of the hand. These improvements appear to be least in the 'gross' category (mean score at week 0 = 4.6, at week 12 = 6.3 and at week 24 = 6.3) and greatest in the 'grasp' category (mean score at week 0 = 4.6, at week 12 = 8.2 and at week 24 = 8.6). This is perhaps not unexpected, as the control intervention focused upon the hand only.
As the conclusions drawn arise from just one study investigating passive exercises and from within‐group changes, the results should be viewed cautiously, as this is not conclusive evidence that passive exercise is effective. However, the possible positive impact of passive movements upon hand function should be shared with clinicians and researchers alike. Furthermore, ongoing investigation of the role of passive movement and exercise and the impact of sensory stimulation of the hand must be undertaken. In addition, consideration must be given as to how exploratory work that is not confounded by the continuation of regular therapeutic interventions can be undertaken.
Mikulecka 2005 study
The purpose of this study was to investigate the effects of manual treatment and manual sensory stimulation of the hand and forearm in the early stages of stroke rehabilitation (Mikulecka 2005). This was the only non‐English language paper included in the review and was originally published in Czech. The translation of this article from its native tongue to English may account for some of the many methodological weakness of this paper, which were described earlier.
The article included no power calculation, gave unclear detail of the method of randomization, and gave no details of blinding. However, the paper gave a very clear and focused description of the comprehensive hands‐on intervention utilized and reported the dose of application. Descriptions to this degree were not found in other articles screened for possible inclusion in the review.
Participants in the experimental group continued to receive their standard physiotherapy and in addition received the intervention treatment every day, with each of these additional sessions lasting 20 minutes. The average number of treatments was six. The intervention applied was a clearly defined programme of sensory stimulation, stretching, compression and joint mobilization. The control group received standard physiotherapy alone.
The results reported show a significant improvement in dice test results in the treatment group (reported as P = 0.05). There is no indication of the size of between‐group effects, confidence interval data are not presented and, furthermore, mean difference data are stated to be reported in the tables which accompany the text. However, despite repeated efforts by the review team, copies of these tables have not been located. It is, therefore, impossible to reflect further upon these results. Finally, the dice test was not identified as an outcome measure. It is unclear if this test is a sub‐test of one of the outcome measures used, such as the ARAT. However, this lack of clarity may be due to a loss of meaning in translation from Czech to English language. In the other tests completed, there was an improvement but in each case this was not statistically significant.
The results reported suggest that the application of a clearly described programme of stretching, mobilization and sensory stimulation can improve hand function in the early stages following stroke. The sustained impact of this intervention is not known and requires further investigation.
Discussion
This review has highlighted that there are only a limited number of randomized controlled trials of clearly described hands‐on therapeutic interventions for the hemiplegic upper limb. Furthermore, of those identified, all have limitations in terms of methodological quality. There are other studies that have used other research methodologies, such as Hunter 2004, Hunter 2006 and Hunter 2008, investigating the application of Mobilization and Tactile Stimulation (MTS) but which have not yet moved beyond Phase 2 work to a randomized controlled trial; therefore we excluded these. There was, in addition, a lack of studies in which the intervention had been clearly described, or with sufficient detail of the intervention for replication in research or in clinical practice. These are the key reasons believed to explain the very small number of studies identified by this review.
The review itself has clear strengths in that it used a wide search strategy, contacted trial authors for clarification where necessary and accepted papers in languages other than English. However, the review also has some limitations. In line with the protocol, two independent assessors screened and reviewed studies. These two assessors strictly applied the inclusion and exclusion criteria during the selection process. Despite this, there was inevitably an element of subjective assessment in this process which may have led to selection bias (Petrie 2000). This could have been reduced if more independent review authors screened the articles, but this was prohibited by the large time commitment required to complete the selection process.
A large amount of the searches were computer‐generated. This may be seen as a further study limitation as we cannot claim that these searches are complete and may have led to articles being missed. However, this is considered to be unlikely, as we designed the searches to be broad and comprehensive in order to maximize opportunities for relevant articles to be included.
It is suggested, therefore, that this study has both strengths and limitations in its design and in its completion and that these will need to be considered when future systematic reviews are undertaken.
The hands‐on interventions that have been included in these three studies ‐ passive extension exercises of elbow, wrist and fingers (Mann 2005); manual stretching to fingers, thumb and wrist (Carey 1990); soft tissue stretch, joint mobilization and pressure to the hand and forearm (Mikulecka 2005) ‐ can each be considered to be a small component of routine interventions currently used by neurological therapists, all of which have been identified to be aspects of a single module of therapy, known as Mobilization and Tactile Stimulation (MTS) for the hand (Hunter 2004; Hunter 2006). Since MTS has been described by expert clinical therapists as part of current routine practice (Hunter 2006), this limited review adds to the body of evidence around routine hands‐on therapy for the hemiplegic upper limb. In particular, the intervention described by Mikulecka 2005 shows many similarities to MTS. The conclusions drawn from that study, however, should be treated with some caution as they arise from a single study. We excluded quasi‐experimental designs, such as single system studies, from this review; consequently, additional evidence to support the effects of MTS could not be considered. Despite this, the three trials together do provide an indication that these interventions are promising and merit further investigation.
Authors' conclusions
This systematic review identified three heterogeneous research articles to include in its report. The level of heterogeneity was such that pooling of data was not possible and we provided narrative descriptions of the articles instead. The findings of the review demonstrated that the limited evidence of benefit of stretching, passive exercises and mobilization when applied to the hemiplegic upper limb following stroke merits further research. Despite the limitations of the studies reviewed, this is useful and new knowledge to inform clinical practice.
This review has demonstrated that the limited evidence of benefit of stretching, passive exercises and mobilization, when applied to the hemiplegic upper limb following stroke, merits further research. It remains unclear if any effects are the same if applied in the acute, sub‐acute and chronic stages of stroke rehabilitation. It is essential, therefore, given the methodological limitations of the existing studies, that further high quality randomized trials investigating clearly described hands‐on interventions and current practice are carried out.
Acknowledgements
The team would like to thank Hazel Fraser and Brenda Thomas for all their help and support
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
We used the following strategy, which uses a combination of controlled vocabulary (MeSH) and free text terms, for MEDLINE and modified it, as appropriate, to suit other databases.
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or cerebrovascular accident/ or exp brain infarction/ or exp cerebrovascular trauma/ or exp hypoxia‐ischemia, brain/ or exp intracranial arterial diseases/ or intracranial arteriovenous malformations/ or exp "Intracranial Embolism and Thrombosis"/ or exp intracranial hemorrhages/ or vasospasm, intracranial/ or vertebral artery dissection/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiplegia/ or exp paresis/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. 1 or 2 or 3 or 4 or 5 or 6 8. *cerebrovascular disorders/rh or exp *basal ganglia cerebrovascular disease/rh or exp *brain ischemia/rh or exp *carotid artery diseases/rh or *cerebrovascular accident/rh or exp *brain infarction/rh or exp *cerebrovascular trauma/rh or exp *hypoxia‐ischemia, brain/rh or exp *intracranial arterial diseases/rh or *intracranial arteriovenous malformations/rh or exp *"Intracranial Embolism and Thrombosis"/rh or exp *intracranial hemorrhages/rh or *vasospasm, intracranial/rh or *vertebral artery dissection/rh 9. *hemiplegia/rh or exp *paresis/rh 10. 8 or 9 11. exp Upper Extremity/ 12. (upper adj3 (limb$ or extremity)).tw. 13. (arm or shoulder or elbow or forearm or hand or wrist or finger or fingers).tw. 14. 11 or 12 or 13 15. rehabilitation/ or "recovery of function"/ 16. musculoskeletal manipulations/ or manipulation, orthopedic/ or manipulation, osteopathic/ 17. physical therapy modalities/ or "Physical Therapy (Specialty)"/ 18. exercise movement techniques/ or exercise/ or exercise therapy/ 19. range of motion, articular/ or exp posture/ 20. massage/ or therapeutic touch/ or reflexotherapy/ or acupressure/ 21. splints/ 22. (lymph/ or lymphedema/) and drainage/ 23. (rehabilitation or recovery of function).tw. 24. (manipulat$ or manual therap$ or physiotherap$ or physical therapy$ or exercise$ or movement$ or mobilization or mobilisation).tw. 25. (postur$ or position or positions or positioning or splint$).tw. 26. (massag$ or stroking or efflurage or effleurage or petrissage or knead$ or lymph drain$ or therapeutic touch or reflexotherap$ or acupressure or rolfing or shiatsu or myofascial release or reiki).tw. 27. or/15‐26 28. 10 and 14 29. 7 and 14 and 27 30. 28 or 29 31. limit 30 to humans
Appendix 2. AMED (Ovid) search strategy
AMED Hands‐on therapy upper limb
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiplegia/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp arm/ 9. (upper adj3 (limb$ or extremity)).tw. 10. (arm or shoulder or elbow or forearm or hand or wrist or finger or fingers).tw. 11. 8 or 9 or 10 12. rehabilitation speciality/ or rehabilitation techniques/ 13. "recovery of function"/ 14. exp manipulation/ 15. physical therapy modalities/ or exp exercise therapy/ or massage/ or exp mobilisation/ 16. physiotherapists/ or physiotherapy specialty/ 17. exercise/ 18. range of motion/ or exp posture/ 19. complementary therapies/ or acupressure/ or reflexology/ or exp postural therapies/ or therapeutic touch/ 20. splints/ or shiatsu/ 21. lymphedema/ and drainage/ 22. (rehabilitation or recovery of function).tw. 23. (manipulat$ or manual therap$ or physiotherap$ or physical therapy$ or exercise$ or movement$ or mobilization or mobilisation).tw. 24. (postur$ or position or positions or positioning or splint$).tw. 25. (massag$ or stroking or efflurage or effleurage or petrissage or knead$ or lymph drain$ or therapeutic touch or reflexotherap$ or acupressure or rolfing or shiatsu or myofascial release or reiki).tw. 26. or/12‐25 27. 7 and 11 and 26
Appendix 3. EMBASE (Ovid) search strategy
EMBASE Hands‐on therapy for upper limb
1. exp cerebrovascular disease/ or stroke patient/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiparesis/ or hemiplegia/ or paresis/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp *Cerebrovascular Disease/rh [Rehabilitation] 9. *hemiparesis/rh or *hemiplegia/rh or *paresis/rh 10. 8 or 9 11. exp arm/ 12. (upper adj3 (limb$ or extremity)).tw. 13. (arm or shoulder or elbow or forearm or hand or wrist or finger or fingers).tw. 14. 11 or 12 or 13 15. rehabilitation/ or convalescence/ 16. exp manipulative medicine/ 17. physiotherapy/ or physiotherapy practice/ 18. exp kinesiotherapy/ or exp exercise/ 19. passive movement/ or "range of motion"/ or exp body position/ 20. massage/ or alternative medicine/ 21. splint/ 22. lymphatic drainage/ 23. (rehabilitation or recovery of function).tw. 24. (manipulat$ or manual therap$ or physiotherap$ or physical therapy$ or exercise$ or movement$ or mobilization or mobilisation).tw. 25. (postur$ or position or positions or positioning or splint$).tw. 26. (massag$ or stroking or efflurage or effleurage or petrissage or knead$ or lymph drain$ or therapeutic touch or reflexotherap$ or acupressure or rolfing or shiatsu or myofascial release or reiki).tw. 27. or/15‐26 28. 10 and 14 29. 7 and 14 and 27 30. 28 or 29 31. limit 30 to human
Appendix 4. CINAHL (EBSCO) search strategy
S32 ..S16 and S31 S31 ..S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 S30 ..TI ( massag* or stroking or efflurage or effleurage or petrissage or knead* or lymph drain* or therapeutic touch or reflexotherap* or acupressure or rolfing or shiatsu or myofascial release or reiki ) or AB ( massag* or stroking or efflurage or effleurage or petrissage or knead* or lymph drain* or therapeutic touch or reflexotherap* or acupressure or rolfing or shiatsu or myofascial release or reiki ) S29 ..TI ( postur* or position or positions or positioning or splint* ) or AB ( postur* or position or positions or positioning or splint* ) S28 ..TI ( manipulat* or manual therap* or physiotherap* or physical therapy* or exercise* or movement* or mobilization or mobilisation ) or AB ( manipulat* or manual therap* or physiotherap* or physical therapy* or exercise* or movement* or mobilization or mobilisation ) S27 ..TI ( rehabilitation or "recovery of function" ) or AB ( rehabilitation or "recovery of function" ) S26 ..( (MH "lymph") or (MH "lymphedema") ) and (MH "drainage") S25 ..(MH "reiki") or (MH "therapeutic touch") or (MH "rolfing") or (MH "alternative therapies") or (MH "splints") S24 ..(MH "massage+") or (MH "acupressure+") or (MH "reflexology") S23 ..(MH "Range of Motion") or (MH "posture+") S22 ..(MH "Exercise+") or (MH "Therapeutic Exercise+") S21 ..(MH "physical therapy") or (MH "hand therapy") or (MH "joint mobilization") or (MH "physical therapists") S20 ..(MH "Myofascial Release") S19 ..(MH "Manipulation, Orthopedic") or (MH "Manipulation, Osteopathic") or (MH "Manual Therapy") S18 ..(MH "Recovery") S17 ..(MH "Rehabilitation") S16 ..S11 and S15 S15 ..S12 or S13 or S14 S14 ..TI ( upper limb* or upper extremit* ) or AB ( upper limb* or upper extremit* ) S13 ..TI ( arm or shoulder or elbow or forearm or hand or wrist or finger or fingers ) or AB ( arm or shoulder or elbow or forearm or hand or wrist or finger or fingers ) S12 ..(MH "Upper Extremity+") S11 ..S1 or S2 or S5 or S8 or S9 or S10 S10 ..TI ( hemipleg* or hemipar* or paresis or paretic ) or AB ( hemipleg* or hemipar* or paresis or paretic ) S9 ..(MH "Hemiplegia") S8 ..S6 and S7 S7 ..TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) S6 ..TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) S5 ..S3 and S4 S4 ..TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) S3 ..TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) S2 ..TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) S1 ..(MH "Cerebrovascular Disorders+") or (MH "stroke patients") or (MH "stroke units")
Appendix 5. PEDro search strategy
The Physiotherapy Evidence Database (PEDro) search strategy
| Search number | Therapy | Problem | Body part | Sub‐category | Type |
| 1 | Stretch/Mobilisation/ Manipulation/massage |
No appropriate value in this field | Upper arm/ shoulder/ shoulder girdle | Neuro | Clinical trial |
| 2 | Stretch/Mobilisation/ Manipulation/massage |
No appropriate value in this field | Forearm or elbow | Neuro | Clinical trial |
| 3 | Stretch/Mobilisation/ Manipulation/massage |
No appropriate value in this field | Hand and wrist | Neuro | Clinical trial |
| 4 | Neurodevelopmental | No appropriate value in this field | Upper arm/ shoulder/ shoulder girdle | Neuro | Clinical trial |
| 5 | Neurodevelopmental | No appropriate value in this field | Forearm or elbow | Neuro | Clinical trial |
| 6 | Neurodevelopmental | No appropriate value in this field | Hand and wrist | Neuro | Clinical trial |
| 7 | Stretch/Mobilisation/ Manipulation/massage |
No appropriate value in this field | Upper arm/ shoulder/ shoulder girdle | Gerontology | Clinical trial |
| 8 | Stretch/Mobilisation/ Manipulation/massage |
No appropriate value in this field | Forearm or elbow | Gerontology | Clinical trial |
| 9 | Stretch/Mobilisation/ Manipulation/massage |
No appropriate value in this field | Hand and wrist | Gerontology | Clinical trial |
| 10 | Neurodevelopmental | No appropriate value in this field | Upper arm/ shoulder/ shoulder girdle | Gerontology | Clinical trial |
| 11 | Neurodevelopmental | No appropriate value in this field | Forearm or elbow | Gerontology | Clinical trial |
| 12 | Neurodevelopmental | No appropriate value in this field | Hand and wrist | Gerontology | Clinical trial |
| 13 | No appropriate value in this field | No appropriate value in this field | Upper arm/ shoulder/ shoulder girdle | Neuro | Clinical trial |
| 14 | No appropriate value in this field | No appropriate value in this field | Forearm or elbow | Neuro | Clinical trial |
| 15 | No appropriate value in this field | No appropriate value in this field | Hand and wrist | Neuro | Clinical trial |
| 16 | No appropriate value in this field | No appropriate value in this field | Upper arm/ shoulder/ shoulder girdle | Gerontology | Clinical trial |
| 17 | No appropriate value in this field | No appropriate value in this field | Forearm or elbow | Gerontology | Clinical trial |
| 18 | No appropriate value in this field | No appropriate value in this field | Hand and wrist | Gerontology | Clinical trial |
| 19 | Stretch/Mobilisation/ Manipulation/massage |
No appropriate value in this field | Upper arm/ shoulder/ shoulder girdle | No appropriate value in this field | Clinical trial |
| 20 | Stretch/Mobilisation/ Manipulation/massage |
No appropriate value in this field | Forearm or elbow | No appropriate value in this field | Clinical trial |
| 21 | Stretch/Mobilisation/ Manipulation/massage |
No appropriate value in this field | Hand and wrist | No appropriate value in this field | Clinical trial |
| 19 | No appropriate value in this field | No appropriate value in this field | Upper arm/ shoulder/ shoulder girdle | Gerontology | Clinical trial |
| 20 | No appropriate value in this field | No appropriate value in this field | Forearm or elbow | Gerontology | Clinical trial |
| 21 | No appropriate value in this field | No appropriate value in this field | Hand and wrist | Gerontology | Clinical trial |
| 22 | Neurodevelopmental | No appropriate value in this field | Upper arm/ shoulder/ shoulder girdle | No appropriate value in this field | Clinical trial |
| 23 | Neurodevelopmental | No appropriate value in this field | Forearm or elbow | No appropriate value in this field | Clinical trial |
| 24 | Neurodevelopmental | No appropriate value in this field | Hand and wrist | No appropriate value in this field | Clinical trial |
| 25 | No appropriate value in this field | No appropriate value in this field | Upper arm/ shoulder/ shoulder girdle | No appropriate value in this field | Clinical trial |
| 26 | No appropriate value in this field | No appropriate value in this field | Forearm or elbow | No appropriate value in this field | Clinical trial |
| 27 | No appropriate value in this field | No appropriate value in this field | Hand and wrist | No appropriate value in this field | Clinical trial |
Appendix 6. CENTRAL search strategy
#1..MeSH descriptor Cerebrovascular Disorders explode all trees #2..(stroke* in Title, Abstract or Keywords or cva* in Title, Abstract or Keywords or cerebrovascular in Title, Abstract or Keywords or "cerebral vascular" in Title, Abstract or Keywords or poststroke in Title, Abstract or Keywords or "post stroke" in Title, Abstract or Keywords or apoplexy in Title, Abstract or Keywords) #3..(cerebral in Title, Abstract or Keywords or cerebellar in Title, Abstract or Keywords or brain* in Title, Abstract or Keywords or vertebrobasilar in Title, Abstract or Keywords) #4..(infarct* in Title, Abstract or Keywords or ischemi* in Title, Abstract or Keywords or ischaemi* in Title, Abstract or Keywords or thrombo* in Title, Abstract or Keywords or emboli* in Title, Abstract or Keywords or apoplexy in Title, Abstract or Keywords) #5..(#3 and #4) #6..(cerebral in Title, Abstract or Keywords or brain* in Title, Abstract or Keywords or subarachnoid in Title, Abstract or Keywords) #7..(haemorrhage in Title, Abstract or Keywords or hemorrhage in Title, Abstract or Keywords or haematoma in Title, Abstract or Keywords or hematoma in Title, Abstract or Keywords or bleed* in Title, Abstract or Keywords) #8..(#6 and #7) #9..MeSH descriptor hemiplegia explode all trees #10..(hemipar* in Title, Abstract or Keywords or hemipleg* in Title, Abstract or Keywords or paresis in Title, Abstract or Keywords or paretic in Title, Abstract or Keywords) #11..(#1 or #2 or #5 or #8 or #9 or #10) #12..MeSH descriptor Upper Extremity explode all trees #13..("upper limb*" in Title, Abstract or Keywords or "upper extremit*" in Title, Abstract or Keywords) #14..(arm in Title, Abstract or Keywords or shoulder in Title, Abstract or Keywords or elbow in Title, Abstract or Keywords or forearm in Title, Abstract or Keywords or hand in Title, Abstract or Keywords or wrist in Title, Abstract or Keywords or finger in Title, Abstract or Keywords or fingers in Title, Abstract or Keywords) #15..(#12 or #13 or #14) #16..(#11 and #15)
Appendix 7. Data extraction form
‘Hands‐on therapy for the hemiplegic upper limb’ ‐ DATA EXTRACTION FORM
Study ID:
Researcher ID:
Participant information:
Mean (SD) age:
Age range:
Gender ratio:
Site of lesion:
Time post stroke:
Stroke classification:
Trial inclusion / exclusion criteria:
Assessed outcomes:
Methodological quality:
Randomization method:
Concealment:
Blinding of participants:
Blinding of providers of care to intervention group:
Blinding of outcome assessor:
Reliability and validity of outcome measures:
Follow up and exclusions; selective reporting; other sources of bias:
Appropriate statistical analysis performed:
Data and analyses
This review has no analyses.
History
Protocol first published: Issue 3, 2007 Review first published: Issue 6, 2011
| Date | Event | Description |
|---|---|---|
| 14 April 2008 | Amended | Converted to new review format. |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Carey 1990
| Methods | Trial inclusions: living at home, adequate cognitive and visual function to perform the tests and give informed consent, evidence of spasticity of the extrinsic finger flexors, at least 20° voluntary finger extension at meta‐carpo phalangeal joint of index finger Trial exclusions: medication for spasticity Statistical analyses performed: t‐test with alpha set at < 0.05 | |
| Participants | Number of participants: 24 Mean age (years): able‐bodied group 51.5; experimental group 53.7; control group 57 Age range: unclear: able‐bodied group: ± 2 years, experimental group: ± 15.3 years, control group: ± 14.1 years Gender ratio: (male:female): able‐bodied group: 5:3, experimental group: 5:3, control group: 4:4 Site of lesion: able‐bodied group: n/a, experimental group: 4 right hemiparetic side; 4 left hemiparetic side, control group: 3 right hemiparetic side; 5 left hemiparetic side Time post stroke: able‐bodied group: n/a, experimental group: mean 6 years (± 4.7 years), control group: mean 5.9 years (± 7.3 years) Stroke classification: unclear | |
| Interventions | Able‐bodied group: none ‐ only used for pre‐test comparisons Control group: pre‐tests FTT and JMTT, 5 minutes rest, post‐tests FTT and JMTT Experimental group: pre‐tests for FTT and JMTT, 5 minutes manual stretching in patterns of 20 second holds followed by release, post‐tests for FTT and JMTT | |
| Outcomes | JMTT, FTT | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Power Calculation | High risk | None |
| Blinding of participants | Unclear risk | Unclear |
| Blinding of intervention providers | Unclear risk | Unclear |
| Blinding of outcome assessor | Unclear risk | Unclear |
| Follow up and exclusions | Unclear risk | No follow up beyond the intervention |
| Selective reporting and other sources of bias | Unclear risk | Treatment group mean time of onset of stroke 3 months less than control group. Some participants in receipt of additional therapy apart from the study intervention |
| Reliability of outcome measures used | Unclear risk | Intraclass correlation coefficients used to demonstrate reliability of Accuracy Index scores, with good intra‐individual consistency |
| Randomization method | Unclear risk | Random numbers table (unclear how generated) |
Mann 2005
| Methods | Trial inclusions: 1 to 12 months post first stroke, medically stable, over 18 years old, able to take hemiplegic hand to mouth, sensory impairment (light touch), no previous upper limb pathology, no cardiac pacemaker in situ, able to comply with treatment and assessment procedures, able to give informed consent Trial exclusions: cognitive problems, psychiatric problems, cardiac problems, cardiac pacemaker in situ Statistical analyses performed: ANCOVA on Action Research Arm Test and sensation at 12 and 24 weeks | |
| Participants | Number of participants: 22 Mean age (years): experimental group: 68, control group: 71 Age range: experimental group: 57 to 86, control group: 57 to 87 Gender ratio (male:female): experimental group: 5:6, control group: 5:6 Site of lesion: not reported Time post stroke: experimental group: mean 8.5 months (range 1 to 12 months), control group: mean 5.7 months (range 2 to 12 months) Stroke classification: experimental group: 10 infarct:1 haemorrhagic, control group: 11 infarct | |
| Interventions | Able‐bodied group: n/a Control group: ongoing therapy continued, plus passive extension exercises of elbow, wrist and fingers daily for 30 minutes, then twice per day for 12 weeks, followed by 12 weeks without treatment Experimental group: ongoing therapy continued, plus neuromuscular stimulation, 30 minutes, twice a day for 12 weeks, then 12 weeks of no treatment | |
| Outcomes | ARAT (Lyle 1981) and 2‐point discrimination | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Sealed allocation codes |
| Power Calculation | High risk | None |
| Blinding of participants | Unclear risk | Unclear |
| Blinding of intervention providers | Unclear risk | Unclear |
| Blinding of outcome assessor | Unclear risk | Unclear |
| Follow up and exclusions | Unclear risk | 24 participants recruited, however only 22 completed. 2 dropped out and lost to follow up without clear reason |
| Selective reporting and other sources of bias | Unclear risk | Consecutive sample |
| Reliability of outcome measures used | Unclear risk | Action Research Arm Test has previously been demonstrated as reliable. Reliability of 2‐point discrimination test unclear |
| Randomization method | Low risk | Computer‐generated sealed allocation codes |
Mikulecka 2005
| Methods | Trial inclusions: ischaemic cerebrovascular accident, admitted to hospital, medically stable Trial exclusions: none described Statistical analyses performed: Kruskal‐Wallis and Friedman tests | |
| Participants | Number of participants: 40 Mean age (years): experimental group: 65, control group: 69 Age range: experimental group: 43 to 80, control group: 50 to 84 Gender ratio (male:female): experimental group: 15:5, control group: 12:8 Site of lesion: experimental group: 12 right carotid; 8 left carotid, control group: 13 right carotid; 7 left carotid Time post stroke: 1 week Stroke classification: anterior circulation | |
| Interventions | Able‐bodied group: n/a Control group: standard physiotherapy approach Experimental group: standard physiotherapy approach, plus clearly‐described hands‐on treatment for 20 minutes for 8 to 12 days (average: 6 treatments) | |
| Outcomes | Jebson‐Taylor Test (Jebson 1969), Visual Ranking Score for Hand Function Test | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Power Calculation | High risk | None |
| Blinding of participants | Unclear risk | Unclear |
| Blinding of intervention providers | Unclear risk | Unclear |
| Blinding of outcome assessor | Unclear risk | Unclear |
| Follow up and exclusions | Unclear risk | Unclear |
| Selective reporting and other sources of bias | Unclear risk | Unclear |
| Reliability of outcome measures used | Unclear risk | Jebson‐Taylor Test has previously been demonstrated as reliable. Visual ranking score reliability unclear |
| Randomization method | Unclear risk | Unclear |
ARAT: Action Research Arm Test FTT: Force Tracking Test JMTT: Joint Movement Tracking Test n/a: not applicable
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Hunter 2004 | Not a randomized controlled trial |
| Hunter 2006 | Not a randomized controlled trial |
| Hunter 2008 | Not a randomized controlled trial |
Contributions of authors
J Winter, contact author: conceiving, designing and co‐ordinating the review; data collection (designing strategies, undertaking searches, screening search results, retrieving papers, quality appraisal and data extraction); data management and data entry; analysis and interpretation; writing the review.
SM Hunter, co‐author: clinical advisor to the team; academic advisor to the contact author; conceiving and designing the review; data collection (screening search results, screening retrieved papers, appraising quality of papers, data extraction); analysis and interpretation of data; general advice on the review.
J Sim, co‐author: statistical advisor to the review team; academic advisor to the contact author; conceiving and designing the review; data analysis and interpretation; general advice to the team.
P Crome, co‐author: clinical advisor to the review team; academic advisor to the contact author; conceiving and designing the review; data analysis and interpretation; general advice to the team.
Sources of support
Internal sources
The Vreeburg Bursary Fund, UK.
External sources
No sources of support supplied
Declarations of interest
P Crome is employed by North Staffordshire Combined Healthcare NHS Trust and Keele University, both of which are involved in stroke research.
New
References
References to studies included in this review
- Carey JR. Manual stretch: effect on finger movement control and force control in stroke subjects with spastic extrinsic finger flexor muscles. Archives of Physical Medicine and Rehabilitation 1990;71:888‐94. [PubMed] [Google Scholar]
- Mann GE, Burridge JH, Malone LJ, Strike PW. A pilot study to investigate the effects of electrical stimulation on recovery of hand function and sensation in subacute stroke patients. Neuromodulation 2005;8:193‐202. [DOI] [PubMed] [Google Scholar]
- Mikulecka E, Petruskava L, Mayer M, Vlachova I. Differentiated manual treatment of the hand and forearm in early rehabilitation of stroke patients (a controlled study). Rehabilitacia 2005;42:52‐61. [Google Scholar]
References to studies excluded from this review
- Hunter SM. Definition and effects of physical therapy treatment for sensorimotor dysfunction in the hemiplegic upper limb after stroke. Unpublished PhD thesis, University of Keele.
- Hunter SM, Crome P, Sim, J, Donaldson C, Pomeroy VM. Development of treatment schedules for research: a structured review to identify methodologies used and a worked example of 'Mobilisation and Tactile Stimulation' for stroke patients. Physiotherapy 2006;92:195‐207. [Google Scholar]
- Hunter SM, Crome P, Sim J, Pomeroy VM. Effects of Mobilization and Tactile Stimulation on recovery of the hemiplegic upper limb: a series of replicated single‐system studies. Archives of Physical Medicine and Rehabilitation 2008;89:2003‐10. [DOI] [PubMed] [Google Scholar]
Additional references
- Altman DG, Doré CJ. Randomisation and baseline comparisons in clinical trials. Lancet 1990;335:149‐53. [DOI] [PubMed] [Google Scholar]
- Ashburn A, Partridge C, DeSouza L. Physiotherapy in the rehabilitation of stroke: a review. Clinical Rehabilitation 1993;7:337‐45. [Google Scholar]
- Ballinger C, Ashburn A, Low J, Roderick P. Unpacking the black box of therapy ‐ a pilot study to describe occupational therapy and physiotherapy interventions for people with stroke. Clinical Rehabilitation 1991;13:301‐9. [DOI] [PubMed] [Google Scholar]
- British Heart Foundation. Coronary heart disease statistics. British Heart Foundation1999.
- Bobath B. Adult hemiplegia: evaluation and treatment. 3rd Edition. Elsevier Health Sciences, 1990. [Google Scholar]
- Bode R, Heinmann A, Semik P, Mallinson T. Patterns of therapy activities across length of stay and impairment levels: peering inside the "black box" of inpatient stroke rehabilitation. Archives of Physical Medicine and Rehabilitation 2004;85:1901‐8. [DOI] [PubMed] [Google Scholar]
- Carey LM, Matyas TA, Oke LE. Sensory loss in stroke patients: effective training of tactile and proprioceptive discrimination. Archives of Physical Medicine and Rehabilitation 1993;74:602‐11. [DOI] [PubMed] [Google Scholar]
- Carr J, Shepherd R. Neurological rehabilitation: optimizing motor performance. Butterworth‐Heinemann, 1998. [Google Scholar]
- Coupar F, Pollock A, Wijck F, Morris J, Langhorne P. Simultaneous bilateral training for improving arm function after stroke. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD006432.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenbaum RM, Dykes RW. Sensory loss in the hand after sensory stroke: therapeutic rationale. Archives of Physical Medicine and Rehabilitation 1983;69:833‐9. [PubMed] [Google Scholar]
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. Systematic Reviews in Health Care: Meta‐analysis in Context. BMJ Books, 2001. [Google Scholar]
- DeJong G, Horn S, Gassaway J, Slavin M, Dijkers M. Towards a taxonomy of rehabilitation to examine the black box of rehabilitation. Archives of Physical Medicine and Rehabilitation 2004;85:678‐86. [DOI] [PubMed] [Google Scholar]
- DeJong G, Horn S, Conroy B, Nichols D, Healton E. Opening the black box of post stroke rehabilitation: stroke rehabilitation patients, processes and outcomes. Archives of Physical Medicine and Rehabilitation 2005;86 Suppl 2:S1‐7. [DOI] [PubMed] [Google Scholar]
- Department of Health. National Service Framework for Older People. Department of Health2001.
- Donaldson C, Tallis R, Pomeroy V. A treatment schedule of conventional physical therapy provided to enhance upper limb sensori‐motor recovery after stroke: expert criterion validity and intra‐rater reliability. Physiotherapy 2009;95:110‐9. [DOI] [PubMed] [Google Scholar]
- Duncan P, Horner R, Reker D, Samsa G, Hoenig H, Hamilton B, et al. Adherence to postacute rehabilitation guidelines is associated with functional recovery in stroke. Stroke 2002;33:167‐77. [DOI] [PubMed] [Google Scholar]
- Edwards S, Partridge C, Mee R. Treatment schedules for research: a model for physiotherapy. Physiotherapy 1990;76:605‐7. [Google Scholar]
- French B, Thomas LH, Leathley MJ, Sutton CJ, McAdam J, Forster A, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006073.pub2] [DOI] [PubMed] [Google Scholar]
- Hatano S. Experience from a multicentre stroke register: a preliminary report. Bulletin of the World Health Organization 1976;54:541‐53. [PMC free article] [PubMed] [Google Scholar]
- Heller A, Wade DT, Wood VA, Sunderland A, Hewer RL, Ward E. Arm function after stroke: measurement and recovery over the first three months. Journal of Neurology, Neurosurgery and Psychiatry 1987;50:714‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
- Hoenig H, Sloane R, Zolkewitz M, Reker D. Differences in rehabilitation services and outcomes among stroke patients cared for in veterans' hospitals. Health Services Research 2001;35:1293‐318. [PMC free article] [PubMed] [Google Scholar]
- Jebson RH, Taylor N. An objective and standardised test of hand function. Archives of Physical Medicine and Rehabilitation 1969;6:311‐19. [PubMed] [Google Scholar]
- Keith R, Wilson D, Gutierrez P. Acute and sub acute rehabilitation for stroke: a comparison. Archives of Physical Medicine and Rehabilitation 1995;76:495‐500. [DOI] [PubMed] [Google Scholar]
- Kings College London. Economic burden of stroke in England. Division of Health and Social Care Research. University of London2005.
- Knott M, Voss DE. Proprioceptive Neuromuscular Facilitation: Patterns and Techniques. 2nd Edition. New York: Hoeber Medical Division, Harper & Row, 1968. [Google Scholar]
- Kramer A, Steiner J, Schlenker R, Eilertsen T, Hrincevich C, Tropea D, et al. Outcomes and costs after hip fracture and stroke. A comparison of rehabilitation settings. JAMA 1997;277:396‐404. [PubMed] [Google Scholar]
- Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. International Journal of Rehabilitation Research 1981;4:483‐92. [DOI] [PubMed] [Google Scholar]
- Mahoney FI, Barthel DW. Functional Evaluation: the Barthel Index. Maryland State Medical Journal 1965;14:61‐5. [PubMed] [Google Scholar]
- Murray C, Lopez A. Global mortality, disability and the contribution of risk factors. Global burden of the disease study. Lancet 1997;349:1436‐42. [DOI] [PubMed] [Google Scholar]
- National Health Service Executive. Burden of Disease. NHSE, London1996.
- Petrie A, Sabin S. Medical statistics at a glance. Blackwell Science, Oxford, 2000. [Google Scholar]
- Pollock A, Baer G, Pomeroy V, Langhorne P. Physiotherapy treatment approaches for the recovery of postural control and lower limb function following stroke. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD001920.pub2] [DOI] [PubMed] [Google Scholar]
- Pomeroy V, Niven D, Barrow S, Faragher E, Tallis R. Unpacking the black box of nursing and therapy practice for post‐stroke shoulder pain: a precursor to evaluation. Clinical Rehabilitation 2001;15:67‐83. [DOI] [PubMed] [Google Scholar]
- Pomeroy VM, King LM, Pollock A, Baily‐Hallam A, Langhorne P. Electrostimulation for promoting recovery of movement or functional ability after stroke. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003241.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reker D, Hoenig H, Zolkewitz, Sloane R, Horner R, Hamilton B, et al. The structure and structural effects of VA rehabilitation bed service care for stroke. Journal of Rehabilitation Research Development 2000;37:438‐91. [PubMed] [Google Scholar]
- Reker D, Duncan P, Horner R, Hoenig H, Samsa G, Hamilton B, et al. Post acute stroke guideline compliance is associated with greater patient satisfaction. Archives of Physical Medicine and Rehabilitation 2002;83:750‐6. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
- Schulz K, Grimes D. Allocation concealment in randomised trials: defending against deciphering. Lancet 2002;359:614‐8. [DOI] [PubMed] [Google Scholar]
- Schulz K, Grimes D. Blinding in randomised trials: hiding who got what. Lancet 2002;359:696‐700. [DOI] [PubMed] [Google Scholar]
- National Sentinel Audit of Stroke. National and local results for the case mix and process of stroke care. The Intercollegiate Stroke Group by the Clinical Effectiveness and Evaluation Unit. Royal College of Physicians, London2004.
- Sim J, Reid N. Statistical inference by confidence intervals: issues of interpretation and utilization. Physical Therapy 1999;79:186‐95. [PubMed] [Google Scholar]
- Sim J, Wright C. Research in health care: concepts, designs and methods. Research in health care: concepts, designs and methods. Nelson Thornes, Cheltenham, 2000. [Google Scholar]
- Sirtori V, Corbetta D, Moja L, Gatti R. Constraint‐induced movement therapy for upper extremities in stroke patients. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD004433.pub2] [DOI] [PubMed] [Google Scholar]
- Stern P, McDowell F, Miller J, Robinson M. Effects of facilitation exercise techniques in stroke rehabilitation. Archives of Physical Medicine and Rehabilitation 1970;51:526‐31. [PubMed] [Google Scholar]
- Tyson SF, Selly A. The development of the Stroke Physiotherapy Intervention Recording Tool (SPIRIT). Disability & Rehabilitation 2004;26:1184‐8. [DOI] [PubMed] [Google Scholar]
- Vickers AJ, Altman DG. Analysing controlled trials with baseline and follow up measurements. BMJ 2001;323:1123‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade D, Skilbeck CE, Hewer RL. Predicting Barthel ADL score at 6 months after an acute stroke. Archives of Physical Medicine and Rehabilitation 1983;64:24‐8. [PubMed] [Google Scholar]
- Wade DT, Langton‐Hewer R, Wood VA, Skilbeck CE, Ismail HM. The hemiplegic arm after stroke: measurement and recovery. Journal of Neurology Neurosurgery and Psychiatry 1983;46:521‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade D. Research into the black box of rehabilitation: the risks of a type III error. Clinical Rehabilitation 2001;15:1‐4. [DOI] [PubMed] [Google Scholar]
- Yekutiel M, Guttmann E. A controlled trial of retraining the sensory function of the hand in stroke patients. Journal of Neurology, Neurosurgery and Psychiatry 1993;56:241‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
- Winter J, Hunter S, Sim J, Crome P. Hands‐on therapy interventions for upper limb motor dysfunction following stroke (Protocol). Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD006609] [DOI] [PMC free article] [PubMed] [Google Scholar]


