Abstract
Background
Sleep disturbances, including reduced nocturnal sleep time, sleep fragmentation, nocturnal wandering, and daytime sleepiness are common clinical problems in dementia, and are associated with significant caregiver distress, increased healthcare costs, and institutionalisation. Drug treatment is often sought to alleviate these problems, but there is significant uncertainty about the efficacy and adverse effects of the various hypnotic drugs in this vulnerable population.
Objectives
To assess the effects, including common adverse effects, of any drug treatment versus placebo for sleep disorders in people with dementia, through identification and analysis of all relevant randomised controlled trials (RCTs).
Search methods
We searched ALOIS (www.medicine.ox.ac.uk/alois), the Cochrane Dementia and Cognitive Improvement Group's Specialized Register, in March 2013 and again in March 2016, using the terms: sleep, insomnia, circadian, hypersomnia, parasomnia, somnolence, rest‐activity, sundowning.
Selection criteria
We included RCTs that compared a drug with placebo, and that had the primary aim of improving sleep in people with dementia who had an identified sleep disturbance at baseline. Trials could also include non‐pharmacological interventions, as long as both drug and placebo groups had the same exposure to them.
Data collection and analysis
Two review authors independently extracted data on study design, risk of bias, and results from the included study reports. We obtained additional information from study authors where necessary. We used the mean difference as the measure of treatment effect, and where possible, synthesized results using a fixed‐effect model.
Main results
We found six RCTs eligible for inclusion for three drugs: melatonin (222 participants, four studies, but only two yielded data on our primary sleep outcomes suitable for meta‐analysis), trazodone (30 participants, one study), and ramelteon (74 participants, one study, no peer‐reviewed publication, limited information available).
The participants in the trazodone study and almost all participants in the melatonin studies had moderate‐to‐severe dementia due to Alzheimer's disease (AD); those in the ramelteon study had mild‐to‐moderate AD. Participants had a variety of common sleep problems at baseline. All primary sleep outcomes were measured using actigraphy. In one study of melatonin, drug treatment was combined with morning bright light therapy. Only two studies made a systematic assessment of adverse effects. Overall, the evidence was at low risk of bias, although there were areas of incomplete reporting, some problems with participant attrition, related largely to poor tolerance of actigraphy and technical difficulties, and a high risk of selective reporting in one trial that contributed very few participants. The risk of bias in the ramelteon study was unclear due to incomplete reporting.
We found no evidence that melatonin, at doses up to 10 mg, improved any major sleep outcome over 8 to 10 weeks in patients with AD who were identified as having a sleep disturbance. We were able to synthesize data for two of our primary sleep outcomes: total nocturnal sleep time (mean difference (MD) 10.68 minutes, 95% CI ‐16.22 to 37.59; N = 184; two studies), and the ratio of daytime sleep to night‐time sleep (MD ‐0.13, 95% CI ‐0.29 to 0.03; N = 184; two studies). From single studies, we found no difference between melatonin and placebo groups for sleep efficiency, time awake after sleep onset, or number of night‐time awakenings. From two studies, we found no effect of melatonin on cognition or performance of activities of daily living (ADL). No serious adverse effects of melatonin were reported in the included studies. We considered this evidence to be of low quality.
There was low‐quality evidence that trazodone 50 mg given at night for two weeks improved total nocturnal sleep time (MD 42.46 minutes, 95% CI 0.9 to 84.0; N = 30; one study), and sleep efficiency (MD 8.53%, 95% CI 1.9 to 15.1; N = 30; one study) in patients with moderate‐to‐severe AD, but it did not affect the amount of time spent awake after sleep onset (MD ‐20.41, 95% CI ‐60.4 to 19.6; N = 30; one study), or the number of nocturnal awakenings (MD ‐3.71, 95% CI ‐8.2 to 0.8; N = 30; one study). No effect was seen on daytime sleep, cognition, or ADL. No serious adverse effects of trazodone were reported.
Results from a phase 2 trial investigating ramelteon 8 mg administered at night were available in summary form in a sponsor's synopsis. Because the data were from a single, small study and reporting was incomplete, we considered this evidence to be of low quality in general terms. Ramelteon had no effect on total nocturnal sleep time at one week (primary outcome) or eight weeks (end of treatment) in patients with mild‐to‐moderate AD. The synopsis reported few significant differences from placebo for any sleep, behavioural, or cognitive outcomes; none were likely to be of clinical significance. There were no serious adverse effects from ramelteon.
Authors' conclusions
We discovered a distinct lack of evidence to help guide drug treatment of sleep problems in dementia. In particular, we found no RCTs of many drugs that are widely prescribed for sleep problems in dementia, including the benzodiazepine and non‐benzodiazepine hypnotics, although there is considerable uncertainty about the balance of benefits and risks associated with these common treatments. From the studies we identified for this review, we found no evidence that melatonin (up to 10mg) helped sleep problems in patients with moderate to severe dementia due to AD. There was some evidence to support the use of a low dose (50 mg) of trazodone, although a larger trial is needed to allow a more definitive conclusion to be reached on the balance of risks and benefits. There was no evidence of any effect of ramelteon on sleep in patients with mild to moderate dementia due to AD. This is an area with a high need for pragmatic trials, particularly of those drugs that are in common clinical use for sleep problems in dementia. Systematic assessment of adverse effects is essential.
Plain language summary
Medicines for sleep problems in dementia
What difficulties are caused by sleep problems associated with dementia?
People with dementia frequently suffer from sleep disturbances. These can include a reduction in the time spent asleep at night, frequent wakening after falling asleep, wandering at night, waking early, and sleeping excessively during the day.
These behaviours cause a lot of stress to carers, and may be associated with earlier admission to institutional care for people with dementia. These behaviours can also be difficult for care‐home staff to manage well.
Can medicines help?
Treatment with medicines is often used to try to improve the sleep of people with dementia. Since the source of the sleep problems may be changes in the brain caused by dementia, it is not clear whether normal sleeping pills are effective for people with dementia, and there are worries that the medicines could cause significant side effects (harms).
The purpose of this review
In this updated Cochrane review, we tried to identify the benefits and common harms of any medicine used to treat sleep problems in people with dementia.
Findings of this review
We searched the medical literature up to March 2016 for all randomised trials that compared any medicine used for treating sleep problems in people with dementia with a fake medicine (placebo). We found six trials (326 participants) that investigated three medicines: melatonin (four trials), trazodone (one trial), and ramelteon (one trial). The ramelteon trial and one melatonin trial were commercially funded; the other trials had non‐commercial funding sources. Limited information was available for the ramelteon trial, and it came from the trial's sponsor. Overall, the evidence was of low quality, meaning that further research is very likely to affect the results.
Participants in the melatonin and trazodone trials almost all had moderate to severe dementia, while those in the ramelteon trial had mild to moderate dementia.
The four melatonin trials had a total of 222 participants. From the evidence we found, we could be moderately confident that melatonin did not improve sleep in patients with dementia due to Alzheimer's disease. No serious harms were reported.
The trazodone trial had 30 participants. Because it was so small, we could only have limited confidence in its results. It showed that a low dose of the sedative anti‐depressant trazodone, 50 mg, given at night for two weeks, increased the total time spent asleep each night by an average of 43 minutes. This medicine improved sleep efficiency (the percentage of time in bed spent sleeping), but had no effect on the time spent awake after falling asleep, or on the number of times the participants woke up at night. No serious harms were reported.
The ramelteon trial had 74 participants. The limited information available did not provide any evidence that ramelteon was better than placebo. There were no serious harms caused by ramelteon.
The trials did not report on some of the outcomes which interested us, including quality of life and the impact on carers.
Shortcomings of this review
Although we searched for them, we were unable to find any trials of other sleeping medications that are commonly prescribed to people with dementia. All of the participants had dementia due to Alzheimer's disease, although sleep problems are also common in other forms of dementia.
We concluded that there is very little evidence to guide decisions about medicines for sleeping problems in dementia. Any medicine used should be used cautiously, with a careful assessment of how well it works, and its side effects in individual patients. More trials are needed to inform medical practice, with a particular need for trials investigating medicines that are commonly used for sleep problems in dementia. It is essential that trials include careful assessment of side effects.
Summary of findings
Background
Description of the condition
Dementia is a syndrome of global cognitive decline, usually due to one or more neurodegenerative conditions in old age. The most frequent subtypes are dementia in Alzheimer's disease (AD), vascular dementia (VaD) and the related conditions of dementia with Lewy bodies (DLB) and dementia in Parkinson's disease (PDD). The dementia syndrome is characterised by progressive impairment in multiple cognitive domains and in the ability to carry out the usual activities of daily life, so that people with dementia become increasingly dependent on others. Over the course of the illness, other behavioural and psychological symptoms occur very frequently. Common among these are apathy, anxiety, agitation, and sleep disturbances.
Common sleep problems in dementia are increased wakefulness and fragmented sleep at night, increased sleep latency (time taken to go to sleep), and increased daytime sleepiness. These are examples of circadian abnormalities ‒ that is, abnormalities of 24‐hour biological rhythms. In the two‐process model of sleep regulation, the timing of sleep and wakefulness is controlled by homeostatic sleep pressure, which is the accumulating drive for sleep during time spent awake, and by the brain's circadian timekeeping system. Circadian misalignment with the desired sleep‐wake schedule and low amplitude circadian rhythms lead to mistimed and poorly consolidated bouts of wakefulness and sleep. Sleep disorders in dementia may be due directly to neurodegeneration of the sleep‐wake circuitry. Consequences of sleep disruption or institutional care, such as artifical light exposure at night, can also shift the timing of the circadian pacemaker, and further perpetuate abnormal circadian patterns.
Alzheimer’s disease (AD) is associated with sleep disturbances in at least 25% to 35% of affected individuals (Dauvilliers 2007). The sleep problems in AD may result from various factors, including neuronal loss in the suprachiasmatic nuclei (SCN), decreased SCN melatonin receptor expression, and other neuropathological changes to the sleep‐wake circuits (Swaab 1985; Van Someren 2000; Vitiello 1991). Changes in sleep architecture that have been documented in AD include a reduction in stage N3 (slow wave) sleep, reduced fast spindle activity (a component of stage N2), and reduced rapid eye movement (REM) sleep. However, the extent to which these changes are associated with subjective sleep problems or impaired daytime functioning is not known (Montplaisir 1995; Montplaisir 1998).
Vascular dementia (VaD) is caused by a variety of cerebrovascular pathologies. It can be subdivided into cortical, subcortical, and mixed types. The overall prevalence of sleep disturbances in VaD is probably similar to that in AD, although one large consecutive series of patients with AD or VaD found the highest prevalence of sleep disturbance in the cortical VaD group (Fuh 2005).
Sleep disorders are especially prevalent in DLB and PDD. In a large multi‐centre study, Bliwise 2011 found that patients with DLB were significantly more likely than those with probable AD to have nocturnal sleep disturbance on the Neuropsychiatric Inventory (NPI; odds ratio = 2.93, 95% confidence interval = 2.22 to 3.86). In a more fine‐grained analysis of the sleep problems of patients with mild dementia, Rongve 2010 reported that compared to patients with AD, patients with DLB or PDD had higher rates of all sleep disorders examined, including insomnia (AD 24%, DLB or PDD 47.2%), and excessive daytime sleepiness (AD 16.7%, DLB or PDD 40.6%). They also had higher rates of a variety of dyssomnias and parasomnias (REM sleep behaviour disorder (REM‐BD), periodic leg movements of sleep (PLMS), restless legs syndrome (RLS), sleep‐related leg cramps, and obstructive sleep apnoea).
Sleep problems in dementia result in significant caregiver distress, healthcare costs, and increased rates of institutionalisation (Donaldson 1998; Gaugler 2000). In a study of behavioural and psychological symptoms in mid‐ and late‐phase dementia due to Alzheimer's disease, sleep disturbance was notable as the only symptom rated by carers as being among the most distressing to both people with dementia and the carers themselves (Hart 2003).
Description of the intervention
Both pharmacotherapies (drugs) and non‐pharmacological treatments have been used to alleviate sleep problems in patients with dementia (Cynthia 2009). Non‐pharmacological treatment approaches are an attractive alternative to medical drug treatments and may have fewer adverse effects. However, studies of these options, other than bright light treatment, have been limited (David 2010). They are the subject of another Cochrane review (in preparation, Wilfling 2015).
Drugs which have been used to manage sleep disorders in patients with dementia include atypical antipsychotics, benzodiazepines, other GABAergic drugs (such as zolpidem, zopiclone, and zaleplon), melatonin, sedating antidepressants, and antihistamines.
Among the hypnotic agents, the most commonly used are the benzodiazepines and non‐benzodiazepine hypnotics, which are also agonists at the benzodiazepine receptor (the Z‐drugs). These have effects on sleep, but they are also associated with a considerable number of side effects, including daytime sleepiness, worsened insomnia after discontinuation (rebound insomnia), confusion, amnesia, and increased frequency of falling (Closser 1991; Cumming 2003).
Sedating antidepressants, particularly trazodone, and antihistamines are prescribed for sleep problems in patients with dementia, but have significant potential for similar adverse effects (Coupland 2011; Oderda 2012).
Antipsychotics with sedative effects, such as quetiapine or olanzapine, may also sometimes be given to dementia patients with disturbed sleep, especially if accompanied by agitated behaviour at night, but their use in dementia has been linked with serious adverse events including increased mortality, and they are not recommended except in situations of very high risk. The anti‐cholinergic effects of a number of these drugs may lead to worsened cognition.
Melatonin and melatonin‐receptor agonists, such as ramelteon, are used to treat insomnia in healthy people, and there has been considerable interest in their use in patients with dementia. Melatonin is categorized as a dietary supplement in the USA, but in Europe, a sustained‐release form is licensed as a medicine for the short‐term treatment (up to 13 weeks) of primary insomnia in adults aged 55 and over, at a dose of 2 mg each night. Ramelteon has a license in the USA for the long‐term treatment of sleep‐onset insomnia.
How the intervention might work
Drugs that are used to treat sleep disturbances work in a variety of ways. The particular disease processes underlying sleep disturbances in dementia may render some of these more or less effective in people with dementia than in neurologically healthy people.
There is some evidence that sleep itself may contribute to keeping in step with the circadian pacemaker (Buxton 2000). Hence, interventions to improve sleep at night may also improve circadian timing and potentially minimize other circadian symptoms such as sundowning, which is a phenomenon of increased motor activity and agitation in the late afternoon or evening.
Why it is important to do this review
Sleep problems in people with dementia are common, and are associated with high burdens to carers and to healthcare and social support systems. However, consensus on the safest and most effective ways to manage them is lacking. The aim of this review was to identify and appraise evidence from randomised controlled trials of drug treatments for sleep problems in dementia to inform clinical practice and to identify research needs. This version of the review updates the previous version published in 2014.
Objectives
To assess the effects, including common adverse effects, of any drug treatment versus placebo for sleep disorders in people with dementia, through identification and analysis of all relevant randomised controlled trials (RCTs).
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant randomised placebo‐controlled trials, including cross‐over trials. We excluded quasi‐randomised trials from the review.
Types of participants
We included people who had both: (a) dementia of any sub‐type, diagnosed using any well‐validated criteria, such as the Diagnostic and Statistical Manual (DSM) or International Classification of Disease (ICD) criteria current at the time of the study, and (b) a sleep problem identified on the basis of subjective or objective measures.
We did not exclude participants on the basis of gender, age, or clinical setting (inpatient or outpatient). We included studies only if at least 80% of the participants were suffering from dementia. Sleep problems included any well‐defined disturbances in the sleep process, such as difficulty initiating sleep, problems with sleep maintenance, and excessive daytime napping. We excluded studies in which participants had obstructive sleep apnoea syndrome, because this is treated primarily as a respiratory disorder.
Types of interventions
Active intervention: any drug primarily intended to improve participants' sleep.
Control intervention: placebo.
Studies including non‐pharmacological interventions were acceptable if the drug and placebo groups were exposed to identical non‐pharmacological interventions.
Types of outcome measures
Where possible, outcomes were divided into short‐term (following treatment of up to six weeks) and long‐term (following treatment of more than six weeks).
Primary outcomes
-
Any or all of the following objective sleep outcomes measured with polysomnography or – more feasibly for dementia patients – actigraphy:
total nocturnal sleep time (TNST; i.e. total time spent asleep between 8.00 PM and 8.00 AM);
sleep efficiency (%; i.e. TNST/time in bed x 100);
nocturnal time awake (WASO; after sleep onset and before final awakening);
number of nocturnal awakenings;
sleep latency;
ratio of daytime sleep to night‐time sleep, or of night‐time sleep to total sleep over 24 hours.
Adverse events.
Secondary outcomes
Carer ratings of patient's sleep using sleep diaries or validated observer scales.
Cognition measured with any validated scale.
Activities of daily living (ADLs) measured with any validated scale.
Quality of life.
Carer burden.
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois), the Cochrane Dementia and Cognitive Improvement Group (CDCIG) Specialized Register on 31 March 2016. The search terms used were: sleep, insomnia, circadian, hypersomnia, parasomnia. We ran a supplementary search using some additional search terms on 21 May 2016. The additional terms were: somnolence, rest‐activity, sundowning.
ALOIS is maintained by the Cochrane Dementia and Cognitive Improvement Group (CDCIG) Information Specialist, and contains dementia and cognitive improvement studies identified from the following.
Monthly searches of a number of major healthcare databases: MEDLINE, Embase, CINAHL, PsycINFO and LILACS.
Monthly searches of a number of trial registers: meta Register of Controlled Trials; Umin Japan Trial Register; WHO portal (which covers ClinicalTrials.gov, ISRCTN, Chinese Clinical Trials Register, German Clinical Trials Register, Iranian Registry of Clinical Trials, Netherlands National Trials Register, and others).
Quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL).
Six‐monthly searches of a number of grey literature sources: ISI Web of Knowledge with Conference Proceedings; Index to Theses; Australasian Digital Theses.
To view a list of all sources searched for ALOIS, see About ALOIS on the website.
Additional separate searches were run in many of the above sources to ensure that the most up‐to‐date results were retrieved. The search strategy we used can be seen in Appendix 1.
Where necessary, we had abstracts translated into English. We did not require any full‐text translations.
Searching other resources
Two review authors independently searched reference lists of selected studies to find possible additional articles.
Data collection and analysis
Selection of studies
Two review authors independently examined the abstracts of references identified in the search process. We retrieved the full texts of all studies that appeared to meet our inclusion criteria, those for which an abstract was not available, and those whose eligibility either of the review authors considered uncertain. The full author team discussed disagreements about study eligibility until consensus was achieved. In some cases, we contacted study authors for further information to reach a final decision on inclusion.
Data extraction and management
Two authors independently extracted the data from each study using data collection forms. We resolved disagreements by discussion.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias in accordance with Cochrane's tool for assessing methodological quality and risk of bias (Higgins 2011). This tool assesses how the randomisation sequence was generated, how allocation was concealed, the integrity of blinding (participants, raters, and personnel), the completeness of outcome data, selective reporting, and other biases. Where inadequate details of randomisation and other characteristics of the trials were provided, we contacted authors of the studies to obtain further information.
We assessed the risk of bias in each domain and categorized them as follows.
Low risk of bias: plausible bias that is unlikely to seriously alter the results (categorized as ‘low’ in the 'Risk of bias' table).
High risk of bias: plausible bias that seriously weakens confidence in the results (categorized as ‘high’ in the 'Risk of bias' table).
Unclear risk of bias: plausible bias that raises some doubts about the results, or inadequate information with which to make a decision (categorized as ‘unclear’ in the risk of bias table).
If sequence generation was considered to be inadequate (high risk of bias), the study was considered to be quasi‐randomised and excluded from the review.
Where the two review authors disagreed on 'Risk of bias' decisions, the final rating was made by consensus discussion involving the third member of the review team.
Measures of treatment effect
All included outcomes were continuous. We used the mean difference to measure the size of the treatment effect. Where available, end point data were preferred over change data.
Unit of analysis issues
We included one cross‐over study, but it was not possible to extract paired data. We discussed this study in a narrative format only.
There were three relevant treatment groups in one included study: a placebo group and two groups receiving different formulations of the active treatment. In this case, we entered the two active treatment groups in analyses as separate subgroups and divided the shared placebo group. We chose this method to allow an approximate investigation of heterogeneity due to the drug formulation.
Dealing with missing data
We used intention‐to‐treat (ITT) data where these were available, reporting any imputation methods used in the primary studies. We used completers' data if these were all that were available.
Assessment of heterogeneity
We used visual inspection of forest plots and the I² statistic to assess heterogeneity.
Assessment of reporting biases
We found too few studies to allow assessment of possible publication bias.
Data synthesis
We grouped studies according to drug intervention. For each drug, we performed meta‐analyses if we considered the studies to be sufficiently clinically and methodologically homogeneous.
For the comparison of melatonin with placebo, one study presented data after eight weeks of treatment and one after 12 weeks (for some outcomes) and 24 weeks. When 12‐week data were provided, we preferred to pool these with 8‐week data from the other study. For the outcomes for which 12‐week data were not reported, we used the end‐of‐study (24‐week) data and pooled these with the 8‐week data.
We used a fixed‐effect model for all analyses.
Subgroup analysis and investigation of heterogeneity
Due to the small number of identified studies and low numbers of participants, there was little heterogeneity to be investigated. We found more than one study for melatonin only, and conducted subgroup analyses of immediate‐release and slow‐release melatonin formulations. Planned subgroup analyses based on treatment duration and drug dose were not indicated.
Sensitivity analysis
We conducted sensitivity analyses on the results for our primary sleep outcomes to assess the impact of the strategy we had adopted to deal with multiple groups in a single study (see Unit of analysis issues above). In the sensitivity analyses, we used the alternative strategy of combining the two active treatment groups into a single group.
Presentation of Results: GRADE and Summary of Findings
We used GRADE methods to rate the quality of the evidence (high, moderate or low) that supported each effect estimate in the review (Guyatt 2011). This rating referred to our level of confidence that the estimate reflected the true effect, taking into account the risk of bias in the included studies, inconsistency between studies, imprecision in the effect estimate, indirectness in addressing our review question, and the risk of publication bias. We produced 'Summary of findings' tables for the comparisons of trazodone and melatonin with placebo to show the effect estimate and the quantity and quality of supporting evidence for the following outcomes, which we considered the most clinically important:
total nocturnal sleep time
nocturnal time awake
number of nocturnal awakenings
ratio of daytime to night‐time sleep
adverse effects
cognitive function
caregiver burden
We had insufficient detail about the Ramelteon trial to construct a 'Summary of findings' table.
Results
Description of studies
Results of the search
We ran an initial search for eligible RCTs in November 2011, and updated the searches in March 2013, May 2013 (a supplementary search with some additional search terms), and March 2016. After de‐duplication and first assessment by the Information Specialist of the CDCIG, these searches resulted in 341 records being passed to the authors for closer scrutiny. We reviewed full texts if at least one of two authors thought that a study might meet the inclusion criteria on the basis of its abstract, or if an abstract was not available. If no full text was available (e.g. abstract in conference proceedings), we attempted to reach a decision on the basis of the abstract alone. In total, we examined 84 full text papers or trial registry entries.
In 2013, we identified one conference abstract from reference lists, which appeared to refer to a small RCT eligible for inclusion (Tozawa 1998). This study was also referred to by one of its authors in a later paper (Mishima 2000). It was described as a double‐blind RCT of exogenously administered melatonin, 6 mg daily for four weeks, in seven inpatients with Alzheimer's‐type dementia and rest‐activity rhythm disorder. It was reported that melatonin was associated with a significant reduction in night‐time, but not total, activity. We were unable to retrieve the conference abstract and found no evidence of a subsequent full publication. We identified a current address for one of the authors, but received no reply to a request for further information. Given the very small size of the trial and the low probability now of ever retrieving any data, we decided to classify it as an excluded trial.
We found one trial registry entry which referred to a phase 2, commercially‐sponsored, multicentred trial of ramelteon that was completed in 2007 (NCT00325728). A brief account of the results of this trial was contained in a clinical trial synopsis on the website of the sponsor (Takeda Global Research & Development), but there have been no associated peer‐reviewed publications, and we were unable to obtain more detailed results from the sponsor. We decided to include this trial. One possibly eligible trial of zolpidem was due to complete data collection for the primary outcome in June 2013; we classed this as ongoing in 2013. In 2016, we could not locate any published data related to this trial and did not receive a reply to our request for information from the named contact; it is now classed as awaiting assessment (NCT00814502). The second trial classed as ongoing in 2013 was EUCTR2009‐014388‐38‐GB. We considered it probable that this was the same trial described in the published paper Wade 2014, identified in the updated search of 2016, although there were some discrepancies between the trial registry entry and the paper. For example, EUCTR2009‐014388‐38‐GB indicated an intention to collect actigraphic sleep data; this was not reported in Wade 2014. We contacted the sponsor for further information, but did not receive a reply. We decided to include this as a single trial (Wade 2014).
In 2013, we included three studies of melatonin (Dowling 2008; Serfaty 2002; Singer 2003), one study of trazodone (Camargos 2014), and one study of ramelteon, for which minimal data were available. In 2016, we included one additional study of melatonin (Wade 2014). The study selection process is summarized in Figure 1.
1.
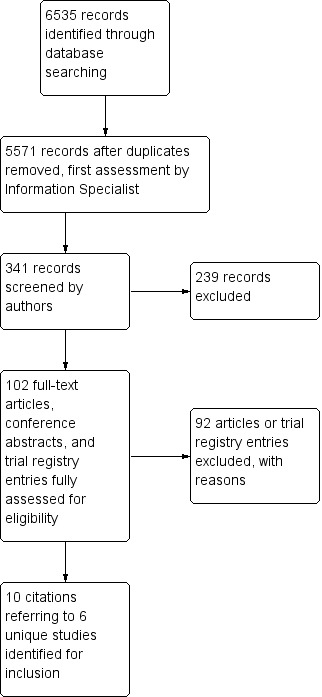
Study flow diagram
Included studies
All studies are described in detail in the Characteristics of included studies table. All of the studies for which full information was available included participants similar to patients seen in clinical practice, mostly with moderate to severe dementia. Sleep‐related inclusion criteria varied somewhat between studies, but all included participants with night‐time behaviours that were associated with disturbance or distress to caregivers, including frequent wakening, nocturnal agitation, wandering, wakening and thinking it was day, early wakening, and excessive daytime sleepiness.
Studies of melatonin
Dowling 2008 compared melatonin 5 mg to placebo in a parallel‐group design, with a treatment period of 10 weeks. The 33 participants had moderate to severe dementia and were resident in long‐term care facilities. All participants also received bright light exposure for an hour in the morning. Sleep outcomes were measured using actigraphy for continuous periods of 108 hours at baseline and end of treatment.
Serfaty 2002 used a cross‐over design to compare melatonin slow‐release (SR) 6 mg with placebo. Treatment was shorter (two weeks for each treatment period with a one week washout). Forty‐four participants were randomised, but results were only reported on the 25 who completed the study. They were similar to Dowling's participants, with the majority being resident in long‐term care facilities. Again, sleep outcomes were measured actigraphically, but only at night, for three nights at the end of each treatment and washout period.
Singer 2003 was the largest study, reporting on 151 participants. There were three parallel groups receiving either melatonin 10 mg, melatonin SR 2.5 mg, or placebo for eight weeks. Participants were broadly similar to those in the other two studies, although it was not clear how many were resident at home and how many in institutions. Participants wore actigraphs throughout the study, and outcomes were derived from a single actigraph record covering the entire eight weeks of treatment.
Wade 2014 was a parallel‐group study comparing melatonin SR 2 mg with placebo over 24 weeks, in outpatients with mild to moderate Alzheimer's disease. Seventy‐three participants were randomised, but only the 13 participants who met the study criteria for comorbid insomnia at baseline were relevant to this review. No actigraphic sleep outcomes were reported, but we were able to extract data for some of our secondary outcomes.
Study of trazodone
Camargos 2014 compared trazodone 50 mg at night to placebo over two weeks in a parallel group study of 30 out‐patients with moderate to severe dementia. Sleep outcomes were again derived from an actigraph record of the whole treatment period.
Study of ramelteon
NCT00325728 was a phase 2, multicentred, parallel‐group trial that randomised 74 participants with mild or moderate AD to eight weeks of treatment with either ramelteon 8 mg or placebo. The only account of the methods and results we could obtain was a clinical trial synopsis on the sponsor's website. Sleep outcomes were measured actigraphically at one, two, four, six, and eight weeks. The trial registry entry named the primary outcome as night‐time total sleep time (nTST); in the trial synopsis, the primary outcome was further specified as nTST at one week.
Excluded studies
We excluded several RCTs because the participants, although suffering from AD, were not selected for inclusion on the basis of a sleep problem at baseline, as would be the case for treatment of sleep disturbances in clinical practice. Some of these studies examined a wide range of neuropsychiatric outcomes, one of which was sleep, although several did have a primary aim of improving sleep (Asayama 2003; Gehrmann 2009; Magnus 1978; Valtonen 2005).
A number of older studies were excluded because the diagnostic status of the participants was not certain by modern standards; although they probably included some participants with AD, they also included participants with a variety of other types of dementia or functional psychiatric illnesses (Linnoila 1980a; Linnoila 1980b; Magnus 1978).
Other studies were excluded on the basis of the study design (not RCTs).
Studies excluded, with reasons, are listed in the Characteristics of excluded studies section below.
Risk of bias in included studies
The risk of bias in the included studies was generally low, although there were areas of incomplete reporting, particularly for NCT00325728, for which the overall risk was unclear. We considered Wade 2014 to be at overall high risk of bias, primarily due to a high risk of selective reporting, but it contributed very little data to the analyses. The assessments for each study are detailed in the Characteristics of included studies section, and the risk of bias across all included studies is summarized in Figure 2 and Figure 3.
2.
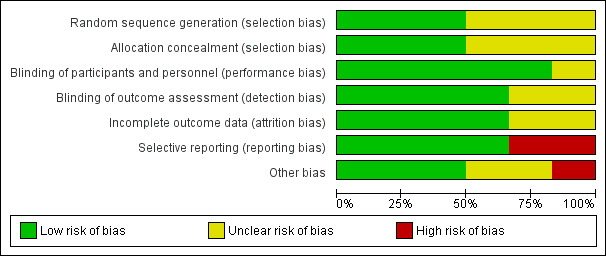
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across included studies.
3.
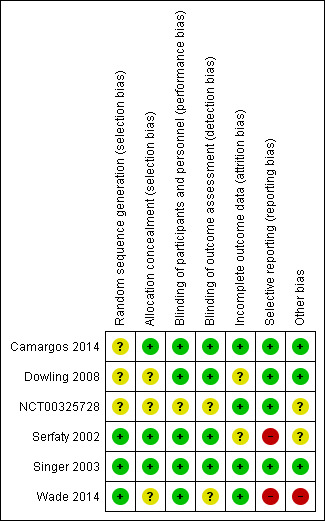
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The method of sequence generation was incompletely reported for Dowling 2008 and Camargos 2014, although information obtained from the authors of the latter study clearly indicated adequate allocation concealment. Singer 2003 did not report details of the randomisation procedure either, but as a large, multicentred study with centralised randomisation, we judged that the risk of selection bias was likely to have been low. The report of NCT00325728 gave no details of the methods used for participant allocation. Wade 2014 used an adequate randomisation method, but did not report how allocation concealment was assured.
Blinding
No information on blinding was available for NCT00325728. Wade 2014 took measures to blind participants and personnel, but did not mention the blinding of outcome assessors, so we judged risk of bias here to be unclear. Adequate measures appeared to have been taken to blind participants, personnel, and outcome assessors in the other studies, although only Serfaty 2002 reported any assessment of the success of blinding. Our primary sleep outcomes were measured with actigraphy, which is highly objective, thus reducing the potential for performance and detection bias.
Incomplete outcome data
Dowling 2008 failed to make any mention of dropouts and reported only on participants completing the study. Serfaty 2002 also provided completers' data only, having had a very high dropout rate of 43% after randomisation, albeit for reasons that were not clearly related to group allocation (predominantly poor tolerance of actigraphy). We judged there was some potential for attrition bias in these two studies. Camargos 2014 and Singer 2003 reported fully on participant flow; both studies lost some participants due to technical difficulties with actigraphy, but we judged the risk of attrition bias to be low. The report of NCT00325728 described attrition by group, giving reasons, and we judged it also to have a low risk of attrition bias. Wade 2014 lost only one participant from each treatment group in their insomnia subpopulation; we also judged this to be at low risk of attrition bias.
Selective reporting
Serfaty 2002 reported sleep outcomes that differed from those listed in the study methods, and we judged it to be at high risk of bias for this item. We found trial registry entries in clinicaltrials.gov and in the European clinical trial registry that we considered highly likely to refer to the study reported as Wade 2014, although there were differences between both trial registry entries and the published data, including differences in the number and location of sites, and in primary and secondary outcomes. Wade 2014 reported data on a subgroup with insomnia at baseline; although we included this subgroup as relevant to the review, it was not clear that the insomnia threshold had been defined prospectively. Quality of life was measured in Wade 2014, but the data were not reported beyond a statement of no significant difference. We considered this study to be at very high risk of reporting bias. The other studies reported on all of their planned outcomes.
Other potential sources of bias
We identified no other major sources of bias. NCT00325728 and Wade 2014 were commercially sponsored.
Effects of interventions
Summary of findings for the main comparison. Melatonin compared to placebo for sleep disturbances in dementia.
| Melatonin compared to placebo for sleep disturbances in dementia | ||||||
| Patient or population: sleep disturbances in dementia Setting: community and long‐term care facilities Intervention: melatonin Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with melatonin | |||||
| Total nocturnal sleep time assessed with: actigraphy follow‐up: range 8 weeks to 10 weeks | The mean total nocturnal sleep time across placebo groups was 397 minutes | The total nocturnal sleep time was, on average, 10.68 minutes higher across the melatonin groups (16.22 lower to 37.59 higher) | ‐ | 184 (2 RCTs) | ⊕⊕⊝⊝ LOW 1, 2 | |
| Nocturnal time awake after sleep onset assessed with: actigraphy follow‐up: 8 weeks | The mean nocturnal time awake after sleep onset in the placebo group was 156 minutes | The nocturnal time awake after sleep onset was, on average, 9.08 minutes higher in the melatonin group (7.51 lower to 25.66 higher) | ‐ | 151 (1 RCT) | ⊕⊕⊝⊝ LOW 1, 2 | |
| Number of nocturnal awakenings assessed with: actigraphy follow‐up: 10 weeks | The mean number of nocturnal awakenings in the placebo group was 34 | The number of nocturnal awakenings was, on average, 6 fewer in the melatonin group (2.65 more to 14.65 fewer) | ‐ | 33 (1 RCT) | ⊕⊕⊝⊝ LOW 1, 2 | |
| Ratio of daytime to night‐time sleep assessed with: actigraphy follow‐up: range 8 weeks to 10 weeks | The mean ratio of daytime to night‐time sleep across placebo groups was 0.72 | The ratio of daytime to night‐time sleep was, on average, 0.13 lower across the melatonin groups (0.29 lower to 0.03 higher) | ‐ | 184 (2 RCTs) | ⊕⊕⊝⊝ LOW 1, 2 | |
| Reporting at least one adverse event assessed with: spontaneous patient/carer report follow‐up: 8 weeks | Assumed risk for the placebo group was 70 per 100 |
Comparative risk for the melatonin group was 75 per 100 (60 to 93) |
RR 1.07 (0.86 to 1.33) | 151 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | |
| Cognitive function assessed with: MMSE, change from baseline. Scale from: 0 (worse) to 30 follow‐up: range 8 weeks to 24 weeks | The mean MMSE score across the placebo groups was 14.3 | The MMSE score was, on average, 0.09 points higher across the melatonin groups (0.85 lower to 1.03 higher) | ‐ | 162 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 3 4 | Minimum clinically important difference is uncertain but has been estimated at 1.4 points in moderate to severe AD (Howard 2011). |
| Caregiver burden ‐ not measured | Not measured | ‐ | ‐ | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 95% CI includes no effect and a difference likely to be of clinical importance.
2 Small number of participants.
3 One study had high risk of bias but result not downgraded because it contributed very few participants.
4 High level of heterogeneity
Summary of findings 2. Trazodone compared to placebo for sleep disturbances in dementia.
| Trazodone compared to placebo for sleep disturbances in dementia | ||||||
| Patient or population: sleep disturbances in dementia Setting: community Intervention: trazodone Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with trazodone | |||||
| Total nocturnal sleep time assessed with: actigraphy follow‐up: 2 weeks | The mean total nocturnal sleep time in the placebo group was 281.9 minutes | The total nocturnal sleep time was, on average, 42.5 minutes more in the trazodone group (0.9 more to 84 more) | ‐ | 30 (1 RCT) | ⊕⊕⊝⊝ LOW 1, 2 | |
| Nocturnal time awake after sleep onset assessed with: actigraphy follow‐up: 2 weeks | The mean nocturnal time awake after sleep onset in the placebo group was 203.4 minutes | The nocturnal time awake after sleep onset was, on average, 20.41 minutes less in the trazodone group (60.4 less to 19.6 more) | ‐ | 30 (1 RCT) | ⊕⊕⊝⊝ LOW 1, 2 | |
| Number of nocturnal awakenings assessed with: actigraphy follow‐up: 2 weeks | The mean number of nocturnal awakenings in the placebo group was 26 | The number of nocturnal awakenings was, on average, 3.71 fewer in the trazodone group (8.2 fewer to 0.8 more) | ‐ | 30 (1 RCT) | ⊕⊕⊝⊝ LOW 1, 2 | |
| Daytime total sleep time assessed with: actigraphy follow‐up: 2 weeks | The mean daytime total sleep time in the placebo group was 144.7 minutes | The daytime total sleep time was, on average, 5.12 minutes more in the trazodone group (28.2 fewer to 38.4 more) | ‐ | 30 (1 RCT) | ⊕⊕⊝⊝ LOW 1, 2 | Ratio of daytime to night‐time sleep not measured in this study. Total daytime sleep time included as an alternative measure of daytime somnolence. |
| Reporting at least one adverse event assessed with: spontaneous patient/carer report follow‐up: 2 weeks | Assumed risk for the placebo group was 40 per 100 |
Comparative risk for the trazodone group was 27 per 100 (9 to 76) |
RR 0.67 (0.23 to 1.89) | 30 (1 RCT) | ⊕⊕⊝⊝ LOW 1, 2 | All adverse events were described as mild. |
| Cognitive function assessed with: MMSE Scale from: 0 (worse) to 30 follow‐up: 2 weeks | The mean MMSE score in the placebo group was 10.5 | The mean MMSE score was, on average, 0.1 higher in the trazodone group (0.9 lower to 1.1 higher) | ‐ | 30 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | Minimum clinically important difference is uncertain, but has been estimated at 1.4 points in moderate‐to‐severe AD (Howard 2011). |
| Caregiver burden ‐ not measured | Not measured | ‐ | ‐ | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Single small study
2 95% CI includes no effect and effect likely to be of clinical importance.
Melatonin
We were unable to extract the intended data from Serfaty 2002, as data were presented as medians and interquartile ranges. The study had a cross‐over design, and while the authors reported using an analysis that took account of this, the analysis method was not specified in any detail. No paired data were presented. No data were presented for several outcomes. The authors reported no significant effect of treatment on total time asleep, number of awakenings, or sleep efficiency. Without presenting any data, they also reported no significant effect of treatment on carers' visual analogue scale (VAS) ratings of participants' sleep quality, or on Mini‐Mental State Examination (MMSE) scores. They stated that there were no adverse effects of treatment, but this was not listed as an outcome, and no details were provided on how the information was gathered.
We judged Singer 2003, Wade 2014 and Dowling 2008 to be sufficiently similar to justify the synthesis of data, despite some methodological differences, notably the concurrent morning bright light treatment in Dowling 2008, and the differences in melatonin dose. We were able to perform meta‐analyses for two actigraphic sleep outcomes. We found no evidence of an effect of melatonin on total nocturnal sleep time (MD 10.68 minutes, 95% CI ‐16.22 to 37.59; N = 184; two studies; Analysis 1.1; Figure 4), or on the ratio of daytime sleep to night‐time sleep (MD ‐0.13, 95% CI ‐0.29 to 0.03; N = 184; two studies; Analysis 1.5; Figure 5). Heterogeneity was low (I² = 0% for both analyses), and inspection of the forest plots did not suggest any difference between the immediate‐release and slow‐release melatonin formulations. We considered this evidence to be of low quality, downgraded due to very serious imprecision.
1.1. Analysis.
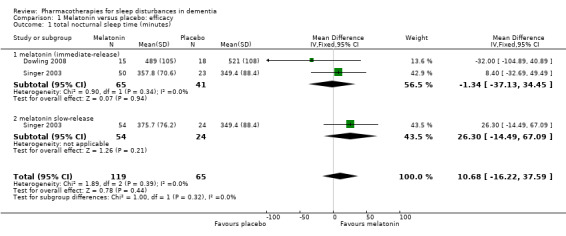
Comparison 1 Melatonin versus placebo: efficacy, Outcome 1 total nocturnal sleep time (minutes).
4.
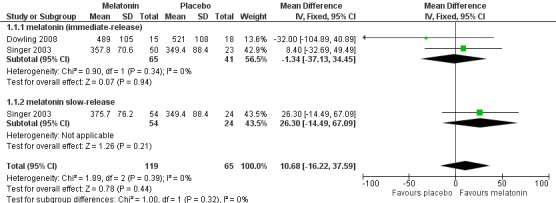
Forest plot of comparison: 1 Melatonin versus placebo, outcome: 1.1 total night‐time sleep time (minutes)
1.5. Analysis.
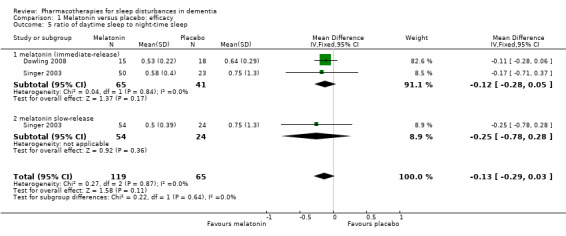
Comparison 1 Melatonin versus placebo: efficacy, Outcome 5 ratio of daytime sleep to night‐time sleep.
5.
Forest plot of comparison: 1 Melatonin versus placebo, outcome: 1.5 ratio of daytime sleep to night‐time sleep
Our other primary sleep outcomes were reported in only one of the two studies. There was no significant effect of melatonin on sleep efficiency (MD ‐0.01%, 95% CI ‐0.04 to 0.03; N = 151; one study; Analysis 1.2), nocturnal time awake after sleep onset (MD 9.08 minutes, 95% CI ‐7.51 to 25.66; N = 151; one study; Singer 2003; Analysis 1.3), or number of night‐time awakenings (MD 6.00, 95% CI ‐2.65 to 14.65; N = 33; one study; Analysis 1.4). Neither study measured sleep latency. Again, we considered the evidence supporting these results to be of low quality, downgraded due to imprecision (wide confidence intervals and small numbers of participants).
1.2. Analysis.
Comparison 1 Melatonin versus placebo: efficacy, Outcome 2 sleep efficiency.
1.3. Analysis.
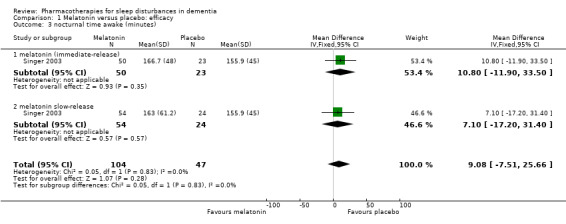
Comparison 1 Melatonin versus placebo: efficacy, Outcome 3 nocturnal time awake (minutes).
1.4. Analysis.
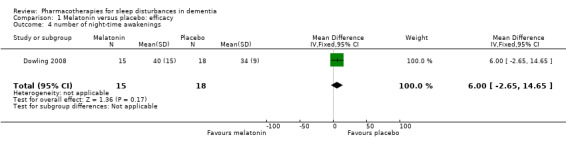
Comparison 1 Melatonin versus placebo: efficacy, Outcome 4 number of night‐time awakenings.
Adverse effects were reported by Singer 2003 and Wade 2014, although Wade 2014 reported adverse event data for the whole trial population, not for the insomnia subgroup, so we did not use these data (for consistency with our exclusion of other trials that did not meet our inclusion criterion of sleep problems at baseline). There was low‐quality evidence, downgraded due to imprecision, that melatonin and placebo groups did not differ in the number of adverse event reports per person (MD 0.20, 95% CI ‐0.72 to 1.12; N = 151; one study; Analysis 2.1), in the severity of adverse events (3‐point scale from 1 = mild to 3 = severe, MD 0.10, 95% CI ‐0.06 to 0.26; N = 151; one study; Analysis 2.2), or in the likelihood of reporting any adverse event (74% in the melatonin group versus 69% in the placebo group; RR 1.07, 95% CI 0.86 to 1.33; N = 151; one study; Analysis 2.3).
2.1. Analysis.
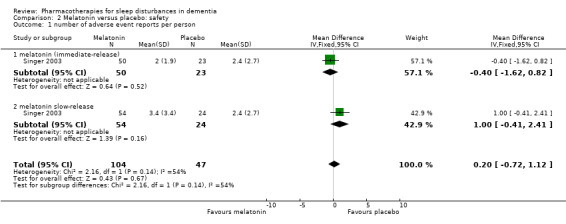
Comparison 2 Melatonin versus placebo: safety, Outcome 1 number of adverse event reports per person.
2.2. Analysis.
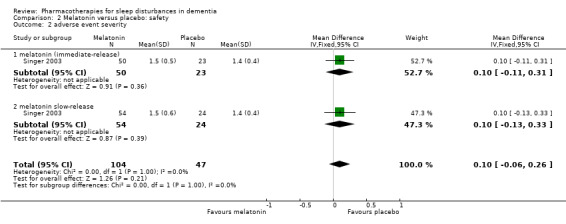
Comparison 2 Melatonin versus placebo: safety, Outcome 2 adverse event severity.
2.3. Analysis.
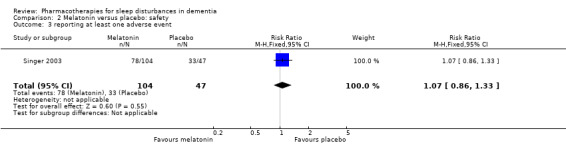
Comparison 2 Melatonin versus placebo: safety, Outcome 3 reporting at least one adverse event.
Singer 2003 and Wade 2014 reported on our secondary outcomes of carer‐rated sleep quality, cognition (measured with MMSE and the Alzheimer's Disease Assessment Scale, cognitive subscale (ADAS‐cog)), and activities of daily living (ADLs). For carer‐rated sleep quality, Wade 2014 reported outcomes after 12 and 24 weeks; we preferred to pool the 12‐week data with the 8‐week data reported by Singer 2003. However, Wade 2014 reported cognitive and ADL data from the 24‐week time point only, so for these outcomes, we pooled these data with the 8‐week data from Singer 2003. There was no evidence of a difference between melatonin and placebo groups for any of these outcomes (Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9). The effect estimates for the cognitive and ADL outcomes were reasonably precise, but there were few participants, and for MMSE, there was inconsistency between the two included trials, so we considered this evidence to be of low quality.
1.6. Analysis.
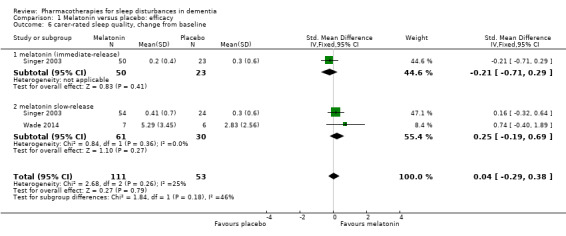
Comparison 1 Melatonin versus placebo: efficacy, Outcome 6 carer‐rated sleep quality, change from baseline.
1.7. Analysis.
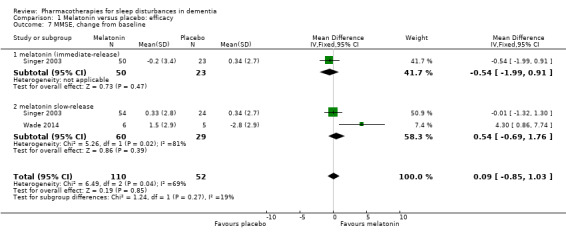
Comparison 1 Melatonin versus placebo: efficacy, Outcome 7 MMSE, change from baseline.
1.8. Analysis.
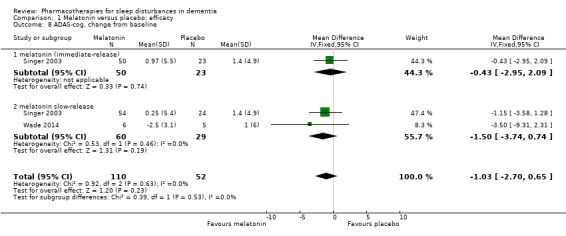
Comparison 1 Melatonin versus placebo: efficacy, Outcome 8 ADAS‐cog, change from baseline.
1.9. Analysis.
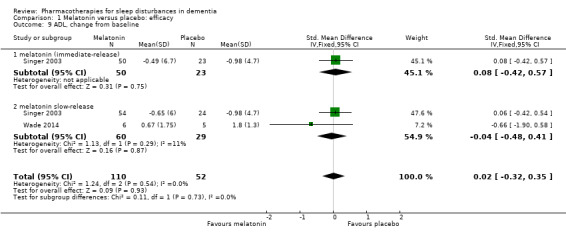
Comparison 1 Melatonin versus placebo: efficacy, Outcome 9 ADL, change from baseline.
Patients' quality of life was measured by Wade 2014, but the data were not reported; the paper stated that there was no significant difference between treatment groups.
Carer burden was not assessed in these trials.
Our sensitivity analysis (in which the two active treatment groups in Singer 2003 were combined to form a single group) had no effect on the results for our primary sleep outcomes (not shown).
Trazodone
In Camargos 2014, results were presented as the mean difference (with 95% confidence interval) between trazodone and placebo groups post‐treatment, adjusted for baseline values using ANCOVA. We considered the evidence related to all the sleep outcomes to be of low quality, downgraded due to very serious imprecision (wide confidence intervals and a very small number of participants). We found evidence that trazodone may increase nocturnal total sleep time (MD 42.46 minutes, 95% CI 0.9 to 84.0) and sleep efficiency (MD 8.53, 95% CI 1.9 to 15.1). However, we found no evidence of an effect of trazodone on the amount of time spent awake after sleep onset (MD ‐20.41, 95% CI ‐60.4 to 19.6), the number of nocturnal awakenings (MD ‐3.71, 95% CI ‐8.2 to 0.8), the amount of time spent asleep during the day (MD 5.12 minutes, 95% CI ‐28.2 to 38.4), or the number of daytime naps (MD 0.84, 95% CI ‐2.6 to 4.3).
Data on adverse events were collected by spontaneous report only; 4/15 participants in the trazodone group and 6/15 in the placebo group reported an adverse event; all were mild.
The study included several of our secondary outcomes. Carers of 10/15 trazodone‐treated patients and 9/15 placebo‐treated patients rated the participant's sleep as better, or much better. There was no evidence of a difference between groups in ADL performance on the Katz Index (MD 0.5, 95% CI ‐0.8 to 1.8; low‐quality evidence), or on any of a variety of cognitive measures, including the MMSE (MD 0.1, 95% CI ‐0.9 to 1.1; moderate‐quality evidence). Quality of life and carer burden were not assessed.
Ramelteon
The sponsor's clinical trial synopsis for NCT00325728 gave most results in brief narrative form. The synopsis named the primary outcome as night‐time total sleep time at one week, despite the fact that the study duration was eight weeks. In all other included studies, the primary outcome was the end of the treatment period. Therefore, we sought data from both the one‐week and eight‐week time points. Results were derived from an ANCOVA model with effects for treatment, pooled centre and baseline disease severity (mild or moderate), and with baseline as a covariate. The trial synopsis reported no significant difference between groups for the primary outcome of night‐time total sleep time at week one (MD 18.2 minutes, 95% CI ‐7.8 to 44.3). There was also no significant difference between groups after one week in the percentage of participants whose night‐time sleep time increased by 30 minutes or more, in time awake after sleep onset, in sleep efficiency, or in the number of daytime naps. Daytime total sleep time was significantly higher in the ramelteon group after one week (MD (standard error) 43.1 (16.19) minutes, P = 0.010), although this was not seen at later time points. The ramelteon group also had a significantly higher ratio of daytime to night‐time sleep at weeks one, four, and eight, or early termination (P = 0.014, P = 0.019, and P = 0.029, respectively). No other sleep outcomes differed significantly between groups at week eight. The exploratory (non‐sleep) outcomes appeared to have been assessed at weeks four and eight only. Among these, the only significant group difference in efficacy was a lower Neuropsychiatric Inventory (NPI) disinhibition score at week eight or early termination in the ramelteon group (MD (SE) ‐0.9 ± 0.44, P = 0.039); the authors of the report did not consider the results to be clinically significant.
Adverse events that were considered possibly, probably, or definitely related to the study drug occurred in 21/74 participants, with a similar incidence in placebo and ramelteon treatment groups (27.9% placebo, 29.0% ramelteon). Four participants in the placebo group experienced at least one serious adverse event; there were no such events in the ramelteon group. There were no deaths.
In general terms, the evidence related to ramelteon was of low quality. It was from a single trial with no peer‐reviewed publication; the sponsor's report lacked important details on trial methods and results.
Discussion
Summary of main results
We found no evidence from four RCTs, reporting data on 222 participants, that melatonin had either beneficial or harmful effects on any major sleep outcome in sleep‐disordered patients with moderate to severe dementia due to AD. No serious adverse events were reported in the trials.
One RCT of trazodone (30 participants) found that trazodone 50 mg at night improved total nocturnal sleep time and sleep efficiency in patients with moderate to severe Alzheimer Disease (AD) and disturbed sleep. It did not find evidence of an effect on daytime sleep, nor on cognition or activities of daily living (ADL). There were no serious adverse events.
A phase 2 trial of ramelteon (74 participants), reported in summary form on the sponsor's website, found no clear evidence of benefit or harm from ramelteon, used for the treatment of sleep disturbances in patients with mild to moderate AD. There was weak evidence, incompletely reported, of more daytime sedation in the ramelteon group. There were no serious adverse events among participants taking ramelteon.
Overall completeness and applicability of evidence
We discovered a distinct lack of evidence to help guide drug treatment of sleep problems in dementia. All of the data was on participants with dementia due to AD, although sleep problems may be even more common in patients with vascular dementia and, especially, dementia with Lewy bodies (DLB) or dementia in Parkinson's disease (PDD).
We found adequately‐reported data from RCTs for only two drugs: trazodone and melatonin. Trazodone is a drug in common clinical use for this indication, but the one study of trazodone was very small (30 participants), so its results must be regarded as preliminary. Of the four published trials of melatonin, only two yielded data on our primary sleep outcomes suitable for meta‐analysis. A fifth RCT of melatonin was probably conducted, but we were unable to locate any data (Tozawa 1998). This trial purportedly found a beneficial effect of melatonin, but given its very small size (seven participants), it was unlikely to have had a substantial impact on our results.
No peer‐reviewed data were available from a commercially‐sponsored trial of ramelteon that had been completed, and we found no other RCTs of melatonin agonists in a population with dementia.
We found no RCTs of other drugs that are widely prescribed for sleep problems in dementia, including the benzodiazepine and non‐benzodiazepine hypnotics, although there is considerable uncertainty about the balance of benefits and risks associated with these common treatments.
One of the included trials studied melatonin in combination with morning bright light; although heterogeneity between studies was low, on the basis of the small amount of included data, we could not confidently exclude any differential effects of melatonin in combination with light therapy. The doses of melatonin used in these studies were at or above the dose licensed for healthy elderly people in Europe, but were nevertheless low in comparison to doses of melatonin and melatonin analogues which have been used or studied in other populations (without dementia). We found no evidence relating to high dose melatonin treatment in patients with dementia, but it is possible that effects could be different. Several different mechanisms are likely to cause sleep disturbances in dementia, some of which may relate to circadian misalignment, and achieving the full chronobiotic effect of melatonin in these circumstances may take up to several months. Therefore, it is possible that some patients might respond to longer periods of treatment with melatonin. Until patients can be well‐characterised on the basis of the mechanisms underlying their sleep disorders, differential effects on different subgroups cannot be excluded.
Small RCTs are limited in their ability to detect adverse effects of treatment, but they can provide important evidence on common adverse events. One of the included trials made no mention at all of adverse effects of treatment, and only three studies reported adverse events in any detail. This was a gap in the evidence, given the potential for all sedative drugs to cause significant adverse events in patients with dementia.
On the positive side, the studies in this review all included patients similar to those seen in clinical practice, with predominantly moderate to severe dementia and a variety of common sleep problems at baseline, so the results should be widely applicable. However, they cannot be taken to apply to sleep problems occurring in the early stages of Alzheimer's Disease, when less severe pathology in the brain's circadian systems may be associated with a better response to drug treatments.
Quality of the evidence
Sleep outcomes in the included studies were measured using actigraphy. Although polysomnography is considered to be the gold standard in sleep studies, its use in this patient group is infeasible. However, there is evidence of a good correlation between actigraphy and polysomnography measures in patients with dementia (Ancoli‐Israel 1997).
No study was at overall high risk of selection, performance or detection bias. There were significant problems with attrition in a couple of the studies, largely reflecting poor tolerance of actigraphy among participants with AD and technical difficulties with the procedures. We thought that one trial of melatonin was at very high risk of selective reporting bias, but it contributed only 13 participants. We did not consider the results of this review to be at significant risk from bias in the included trials.
Overall, we considered the quality of evidence for trazodone to be of low quality for almost all outcomes due to imprecision (wide confidence intervals and data from a single, small study). We rated the evidence for melatonin to be of low quality for all outcomes, again downgraded due to imprecision; although there were several trials, many outcomes were reported in only one trial, the number of participants was low, and again, the confidence intervals were wide. Other than for the MMSE, where estimates of a minimum clinically important difference have been published (e.g. Howard 2011), we had to base our judgements about precision, in part, on what we considered were likely to be clinically important differences.
Potential biases in the review process
We identified no potential biases in the review process.
Agreements and disagreements with other studies or reviews
Cardinali 2010 included a non‐systematic review of studies of melatonin in AD, covering case reports and non‐randomised studies as well as RCTs. The authors identified two RCTs with sleep outcomes, which we excluded because participants were not selected for a sleep problem at baseline. Following a narrative synthesis, they found the evidence for efficacy to be inconclusive.
Xu 2015 conducted a systematic review and meta‐analysis of RCTs of melatonin in dementia, examining sleep efficiency, total sleep time, and cognitive outcomes. The authors included seven RCTs. We had excluded three of these because participants had not been identified as having a sleep disorder at baseline (Asayama 2003; Gehrmann 2009; Riemersma‐van der Lek2008); a fourth study included by Xu (Gao 2009) had no primary aim to improve sleep and Xu and colleagues were able to extract only a cognitive outcome. In contrast to our findings, these authors reported marginal improvement in sleep efficiency and an increase in total sleep time on melatonin. However, we considered that there were important methodological errors in their review, including a unit of analysis error which led to excessive weight being given to the 'positive' results of Singer 2003.
Authors' conclusions
Implications for practice.
Due to lack of primary evidence, the implications of this review for practice are limited. The evidence reviewed suggested that melatonin was unlikely to be of benefit to patients with Alzheimer's disease (AD) and moderate to severe dementia and sleep problems. There was some evidence that a low dose (50 mg) of trazodone improved sleep in people with AD, although a larger trial is needed to be able to draw a more definitive conclusion on the balance of risks and benefits. Until further evidence emerges, this and other hypnotic drugs should be used with particular caution in patients with AD or other dementias, with careful assessment of the risks (of treatment and of no treatment), efficacy, and adverse effects in individuals.
Implications for research.
There is a need for pragmatic trials in this area, particularly to study those drugs that are in common clinical use; these should include sequential strategies for non‐responders. It is important that trials include systematic assessment of adverse events.
Attention to consistency of trial design, actigraphy techniques, and reporting would aid further synthesis and interpretation of research in this area. Helpful suggestions regarding this are made by Camargos (Camargos 2013).
What's new
| Date | Event | Description |
|---|---|---|
| 14 November 2016 | New citation required but conclusions have not changed | One new study added. Conclusions unchanged. |
| 14 November 2016 | New search has been performed | One new study added. Conclusions unchanged. |
Acknowledgements
We gratefully acknowledge the contribution of the author team, led by Dr A Modabbernia, who produced the original protocol for this review. Thank you to Anna Noel‐Storr, Information Specialist, CDCIG, for her work on the search strategy and for locating papers, and to Sue Marcus, Managing Editor, CDCIG, for her support in producing the review.
We are grateful to all the researchers who responded to our requests for further information about their work, and in particular to Dr Camargos, who allowed us access to data prior to publication while we were working on the first version of this review.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy (including additional terms from supplementary search) | Hits retrieved |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) | sleep OR circadian OR insomnia OR sleeplessness OR somatic OR somnolence OR “rest activity” | 271 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE Ovid SP (1950 to 31 March 2016) | 1. exp Dementia/ 2. Delirium/ 3. Wernicke Encephalopathy/ 4. Delirium, Dementia, Amnestic, Cognitive Disorders/ 5. dement*.mp. 6. alzheimer*.mp. 7. (lewy* adj2 bod*).mp. 8. deliri*.mp. 9. (chronic adj2 cerebrovascular).mp. 10. ("organic brain disease" or "organic brain syndrome").mp. 11. ("normal pressure hydrocephalus" and "shunt*").mp. 12. "benign senescent forgetfulness".mp. 13. (cerebr* adj2 deteriorat*).mp. 14. (cerebral* adj2 insufficient*).mp. 15. (pick* adj2 disease).mp. 16. (creutzfeldt or jcd or cjd).mp. 17. huntington*.mp. 18. binswanger*.mp. 19. korsako*.mp. 20. "cognit* impair*".mp. 21. neurodegenerat*.mp. 22. cerebrovascular.mp. 23. neuropsychiatric.mp. 24. neurobehavioral.mp. 25. or/1‐24 26. exp Sleep/ 27. sleep*.ti,ab. 28. "Sleep Initiation and Maintenance Disorders"/ 29. insomnia.mp. 30. exp sleep disorders, circadian rhythm/ or "disorders of excessive somnolence"/ 31. (hypersomnia or parasomnia).mp. 32. circadian.mp. 33. “rest‐activity”.ti,ab. 34. somnolence.ti,ab. 35. sundowning.ti,ab. 36. or/26‐35 37. 25 and 36 38. randomized controlled trial.pt. 39. controlled clinical trial.pt. 40. randomized.ab. 41. placebo.ab. 42. drug therapy.fs. 43. randomly.ab. 44. trial.ab. 45. groups.ab. 46. or/38‐45 47. (animals not (humans and animals)).sh. 48. 46 not 47 49. 37 and 48 |
2176 |
| 3. Embase Ovid SP (1980 to 2016 week 12) | 1. exp dementia/ 2. Lewy body/ 3. delirium/ 4. Wernicke encephalopathy/ 5. cognitive defect/ 6. dement*.mp. 7. alzheimer*.mp. 8. (lewy* adj2 bod*).mp. 9. deliri*.mp. 10. (chronic adj2 cerebrovascular).mp. 11. ("organic brain disease" or "organic brain syndrome").mp. 12. "supranuclear palsy".mp. 13. ("normal pressure hydrocephalus" and "shunt*").mp. 14. "benign senescent forgetfulness".mp. 15. (cerebr* adj2 deteriorat*).mp. 16. (cerebral* adj2 insufficient*).mp. 17. (pick* adj2 disease).mp. 18. (creutzfeldt or jcd or cjd).mp. 19. huntington*.mp. 20. binswanger*.mp. 21. korsako*.mp. 22. CADASIL.mp. 23. or/1‐22 24. *sleep/ 25. sleep*.ti,ab. 26. insomnia.ti,ab. 27. circadian rhythm*.ti,ab. 28. (hypersomnia or parasomnia).ti,ab. 29. “rest‐activity”.ti,ab. 30. somnolence.ti,ab. 31. sundowning.ti,ab. 32. or/24‐31 33. 23 and 32 34. randomized controlled trial/ 35. randomly.ab. 36. placebo*.ti,ab. 37. "double‐blind*".ti,ab. 38. trial.ti,ab. 39. RCT.ti,ab. 40. or/34‐39 41. 33 and 40 |
832 |
| 4. PsycINFO Ovid SP (1806 to March week 4 2016) | 1. exp Dementia/ 2. exp Delirium/ 3. exp Huntingtons Disease/ 4. exp Kluver Bucy Syndrome/ 5. exp Wernickes Syndrome/ 6. exp Cognitive Impairment/ 7. dement*.mp. 8. alzheimer*.mp. 9. (lewy* adj2 bod*).mp. 10. deliri*.mp. 11. (chronic adj2 cerebrovascular).mp. 12. ("organic brain disease" or "organic brain syndrome").mp. 13. "supranuclear palsy".mp. 14. ("normal pressure hydrocephalus" and "shunt*").mp. 15. "benign senescent forgetfulness".mp. 16. (cerebr* adj2 deteriorat*).mp. 17. (cerebral* adj2 insufficient*).mp. 18. (pick* adj2 disease).mp. 19. (creutzfeldt or jcd or cjd).mp. 20. huntington*.mp. 21. binswanger*.mp. 22. korsako*.mp. 23. ("parkinson* disease dementia" or PDD or "parkinson* dementia").mp. 24. or/1‐23 25. Sleep/ 26. sleep*.ti,ab. 27. insomnia.ti,ab. 28. Sleep Disorders/ 29. (hypersomnia or parasomnia).mp. 30. circadian.mp. 31. “rest‐activity”.ti,ab. 32. somnolence.ti,ab. 33. sundowning.ti,ab. 34. or/25‐33 35. 24 and 34 36. trial.ti,ab. 37. placebo*.ti,ab. 38. "double‐blind*".ti,ab. 39. RCT.ti,ab. 40. randomi?ed.ab. 41. randomly.ab. 42. groups.ab. 43. or/36‐42 44. 35 and 43 |
440 |
| 5. CINAHL EBSCOhost (1974 to 31 March 2016) | S1 (MH "Dementia+") S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S3 (MH "Wernicke's Encephalopathy") S4 TX dement* S5 TX alzheimer* S6 TX lewy* N2 bod* S7 TX deliri* S8 TX chronic N2 cerebrovascular S9 TX "organic brain disease" or "organic brain syndrome" S10 TX "normal pressure hydrocephalus" and "shunt*" S11 TX "benign senescent forgetfulness" S12 TX cerebr* N2 deteriorat* S13 TX cerebral* N2 insufficient* S14 TX pick* N2 disease S15 TX creutzfeldt or jcd or cjd S16 TX huntington* S17 TX binswanger* S18 TX korsako* S19 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S20 (MH "Sleep") OR (MH "Sleep Disorders, Circadian Rhythm") S21 TX sleep* S22 TX circadian S23 TX insomnia S24 TX hypersomnia S25 TX parasomnia S26. “rest‐activity”.ti,ab. S27. somnolence.ti,ab. S28. sundowning.ti,ab. S29 S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 S27 S19 and S29 S30 (MH "Randomized Controlled Trials") S31 TX placebo* S32 TX "double‐blind*" OR "single‐blind*" S33 AB groups S34 AB RCT OR CCT S35 AB random* S36 S30 or S31 or S32 or S33 or S34 or S35 S37 AND S27 |
401 |
| 6. ISI Web of Knowledge – all databases (includes: Web of Science (1945 to 31 March 2016); BIOSIS Previews (1926 to 31 March 2016); MEDLINE (1950 to 31 March 2016); Journal Citation Reports (31 March 2016)) | Topic=(sleep* OR insomnia* OR circadian OR hypersomnia OR parasomnia OR “rest‐activity” OR somnolence OR sundowning) AND Topic=(alzheimer* OR AD) AND Topic=(randomly OR placebo OR groups OR trial OR RCT OR randomized OR randomised) Timespan=All Years. |
1798 |
| 7. LILACS BIREME (31 March 2016) | sueño OR sleep OR arrastar OR insomnia OR insomina [Words] and alzheimer OR alzheimers OR alzheimer's OR dementia OR demenc$ [Words] | 65 |
| 8. CENTRAL; 2016, Issue 3 in the Cochrane Library (searched 31 March 2016) |
#1 MeSH descriptor Dementia explode all trees #2 MeSH descriptor Delirium, this term only #3 MeSH descriptor Wernicke Encephalopathy, this term only #4 MeSH descriptor Delirium, Dementia, Amnestic, Cognitive Disorders, this term only #5 dement* #6 alzheimer* #7 "lewy* bod*" #8 deliri* #9 "chronic cerebrovascular" #10 "organic brain disease" or "organic brain syndrome" #11 "normal pressure hydrocephalus" and "shunt*" #12 "benign senescent forgetfulness" #13 "cerebr* deteriorat*" #14 "cerebral* insufficient*" #15 "pick* disease" #16 creutzfeldt or jcd or cjd #17 huntington* #18 binswanger* #19 korsako* #20 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19) #21 MeSH descriptor Sleep, this term only #22 sleep* #23 circadian #24 insomnia #25 hypersomnia #26 parasomnia #27 “rest‐activity” OR insomnolence OR sundowning #28 (#22 OR #23 OR #24 OR #25 OR #26 OR #27) #29 (#20 AND #28) |
455 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov; searched 31 March 2016) | sleep OR insomnia OR circadian OR hypersomnia OR parasomnia OR insomnia OR somnolence OR sundowning OR “rest‐activity” | Interventional Studies | alzheimer OR alzheimer's OR alzheimers OR dementia | 68 |
| 10. ICTRP Search Portal (apps.who.int/trialsearch; includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register; searched 31 March 2016) | (sleep OR insomnia OR somnolence OR sundowning) AND alzheimer | 29 |
| TOTAL before de‐duplication | 6535 | |
| TOTAL after software de‐duplication | 5571 | |
| TOTAL after first assess and sent to authors | 241 | |
| Full‐text assessment | 67 | |
Data and analyses
Comparison 1. Melatonin versus placebo: efficacy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 total nocturnal sleep time (minutes) | 2 | 184 | Mean Difference (IV, Fixed, 95% CI) | 10.68 [‐16.22, 37.59] |
| 1.1 melatonin (immediate‐release) | 2 | 106 | Mean Difference (IV, Fixed, 95% CI) | ‐1.34 [‐37.13, 34.45] |
| 1.2 melatonin slow‐release | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 26.30 [‐14.49, 67.09] |
| 2 sleep efficiency | 1 | 151 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.04, 0.03] |
| 2.1 melatonin (immediate‐release) | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 2.2 melatonin slow‐release | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 3 nocturnal time awake (minutes) | 1 | 151 | Mean Difference (IV, Fixed, 95% CI) | 9.08 [‐7.51, 25.66] |
| 3.1 melatonin (immediate‐release) | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | 10.80 [‐11.90, 33.50] |
| 3.2 melatonin slow‐release | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 7.10 [‐17.20, 31.40] |
| 4 number of night‐time awakenings | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 6.00 [‐2.65, 14.65] |
| 5 ratio of daytime sleep to night‐time sleep | 2 | 184 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.29, 0.03] |
| 5.1 melatonin (immediate‐release) | 2 | 106 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.28, 0.05] |
| 5.2 melatonin slow‐release | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.78, 0.28] |
| 6 carer‐rated sleep quality, change from baseline | 2 | 164 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.29, 0.38] |
| 6.1 melatonin (immediate‐release) | 1 | 73 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.71, 0.29] |
| 6.2 melatonin slow‐release | 2 | 91 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.19, 0.69] |
| 7 MMSE, change from baseline | 2 | 162 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.85, 1.03] |
| 7.1 melatonin (immediate‐release) | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐1.99, 0.91] |
| 7.2 melatonin slow‐release | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | 0.54 [‐0.69, 1.76] |
| 8 ADAS‐cog, change from baseline | 2 | 162 | Mean Difference (IV, Fixed, 95% CI) | ‐1.03 [‐2.70, 0.65] |
| 8.1 melatonin (immediate‐release) | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐2.95, 2.09] |
| 8.2 melatonin slow‐release | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐3.74, 0.74] |
| 9 ADL, change from baseline | 2 | 162 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.32, 0.35] |
| 9.1 melatonin (immediate‐release) | 1 | 73 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.42, 0.57] |
| 9.2 melatonin slow‐release | 2 | 89 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.48, 0.41] |
Comparison 2. Melatonin versus placebo: safety.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 number of adverse event reports per person | 1 | 151 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.72, 1.12] |
| 1.1 melatonin (immediate‐release) | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.62, 0.82] |
| 1.2 melatonin slow‐release | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.41, 2.41] |
| 2 adverse event severity | 1 | 151 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.06, 0.26] |
| 2.1 melatonin (immediate‐release) | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.11, 0.31] |
| 2.2 melatonin slow‐release | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.13, 0.33] |
| 3 reporting at least one adverse event | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.86, 1.33] |
Comparison 3. trazodone AEs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 reporting at least one adverse event | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.23, 1.89] |
3.1. Analysis.
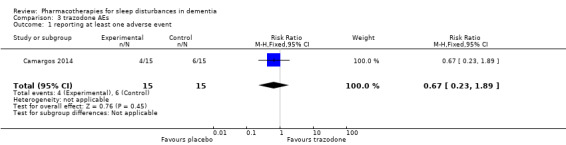
Comparison 3 trazodone AEs, Outcome 1 reporting at least one adverse event.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Camargos 2014.
| Methods | RCT, 2 parallel treatment groups | |
| Participants | Number of participants: 36 randomised, data available for 30 Country: Brazil Setting: outpatients of a Geriatric Medical Centre Diagnosis: probable AD (NINCDS‐ADRDA criteria) Sleep‐related inclusion criteria: insomnia ‐ complained of by patient or observed by caregiver; researcher judged the insomnia to be due to the dementia; the sleep disorder caused emotional distress to the caregiver (NPI 2 or more) Gender: 20 women, 10 men Age: 81.0 ± 7.5 Severity of dementia: MMSE 11.2 ± 6.2 |
|
| Interventions | Trazodone 50 mg, or placebo | |
| Outcomes | Single actigraph records were created for the 7 to 9 day screening/baseline and 2‐week treatment periods Primary:
Secondary:
|
|
| Notes | Adherence to treatment was > 85% in all participants Non‐commercial funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Online random number generator (www.random.org) was used by one investigator to produce random alphanumeric, 3‐digit codes. These then were used by external pharmacist to label pill bottles. Bottles were handed "in scrambled order" to clinical pharmacist to dispense. |
| Allocation concealment (selection bias) | Low risk | Allocation sequence known only to one investigator who took no further part in study, inaccessible to recruiting clinicians or clinical pharmacist |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Both the medication pills and the equivalent placebos were received in bulk from the sole manufacturer of trazodone in Brazil (Apsen Laboratory®), and the placebos were prepared to be indistinguishable in appearance with trazodone prepared as 50‐mg pills. The bottles of trazodone or placebo had the same size." "All patients and geriatricians were blinded to the treatment assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All patients and geriatricians" [outcome assessors] "were blinded to the treatment assignment, and the final randomization list was not accessed until the clinical database was completed" (confirmed by author to mean after actigraphic analysis was completed) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After randomisation, 1 participant was excluded from each group for clinical reasons (heart failure secondary to noncompliance with other medication, episode of agitation leading to arm fracture), and 4 participants (3 trazodone, 1 placebo) due to technical failure of actigraphy. We judged these exclusions to be unlikely to lead to bias |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Dowling 2008.
| Methods | RCT, 3 parallel treatment groups | |
| Participants | Number of participants: 50 completed study (33 contributed data to this review) Country: USA Setting: 2 large long‐term care facilities Diagnosis: probable AD (NINCDS‐ADRDA criteria) Sleep‐related inclusion criteria: rest‐activity rhythm disruptions including insomnia, frequent night‐time awakenings, wandering at night, unusually early morning awakenings, sundowning, excessive daytime sleepiness. Gender: 43 women, 7 men Age: 86 ± 8 years (range 60 to 100) Severity of dementia: MMSE 9.3 ± 7.9, no significant difference between groups |
|
| Interventions | Duration of treatment: 10 weeks Treatment group 1 (15 participants): 5 mg melatonin in the evening combined with bright light exposure in the morning (light for 1 h, > 2500 lux, at gaze height, 5 days a week) Treatment group 2 (18 participants): lactose placebo in the evening combined with bright light exposure in the morning Both treatment groups: Route of administration: oral Time of administration: 5 to 6 PM (bedtime at 8 PM) Additional treatment groups, not included in review: Usual light only, no drug or placebo, 17 participants |
|
| Outcomes | All outcomes measured using actigraphy over a 108‐h monitoring period (Monday 8 PM to Saturday 8 AM) Outcome time points: baseline and end of treatment (10 weeks) Sleep/wake: Night‐time outcomes
Daytime outcomes
Subsidiary measures: Additional circadian outcomes were computed to quantify each participant’s 24‐h rest‐activity rhythm, and this was then used to assess:
|
|
| Notes | Placebo and melatonin groups did not differ significantly in bright light received No mention was made of adverse events Non‐commercial funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomly assigned to one of the three groups." No information on randomisation method provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The University of California, San Francisco Drug Product Services Laboratory provided melatonin (5 mg) and identically appearing lactose placebo capsules." "Study staff, nursing home staff, and subjects were all blinded to melatonin treatment group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Study staff . . . were all blinded to melatonin treatment group assignment." Outcomes were actigraphy data (objective) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Fifty subjects completed the study." No mention of how many participants were randomised, so unclear if there were any dropouts. Those completing the study were reported to have tolerated the procedures well. There was a target of 108 h of data at each time point. Valid data at baseline: mean 105 h (range 75 to 108); valid data at the end of intervention: mean 107 h (range 90 to 108); "no significant differences between the groups." |
| Selective reporting (reporting bias) | Low risk | Results reported for all listed outcome measures |
| Other bias | Low risk | None identified |
NCT00325728.
| Methods | RCT Stratified by severity of dementia (mild AD (MMSE 19 to 28) or moderate AD (MMSE 8 to 18)) |
|
| Participants | Number of participants: 74 randomised, 66 completed study Country: USA (38 sites) Diagnosis: diagnosis of dementia of the Alzheimer's type (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition Revised), or probable AD (NINCDS‐ADRDA criteria) Sleep‐related inclusion criteria: History of 2 or more sleep disorder behaviours occurring at least once weekly in the 2 weeks prior to the first screening visit and actigraphy evidence of a night‐time total sleep time < 7 h/night, based on at least 4/7 nights of complete actigraphy data collected over the single‐blind, placebo run‐in period Gender: 32 women, 42 men Age: mean age 76 years (minimum for inclusion 55 years) Severity of dementia: mild or moderate, defined as MMSE of 8 to 28, inclusive |
|
| Interventions | Ramelteon (Rozerem) 8 mg once a day at bedtime, or placebo | |
| Outcomes | Actigraphic data collected at 1, 2, 4, 6, and 8 weeks Primary outcome: Mean nTST as determined by actigraphy (further specified in trial synopsis as nTST at 1 week) Secondary outcomes:
Exploratory:
|
|
| Notes | Phase 2 study of efficacy, safety and tolerability. Study completed in 2007. Sponsor: Takeda Global Research & Development Center, Inc. No peer reviewed publications identified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "This was a double‐blind, randomized, placebo‐controlled, parallel‐group, proof‐of‐concept study." No further information provided. 43/74 (58%) participants received placebo |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "This was a double‐blind, randomized, placebo‐controlled, parallel‐group, proof‐of‐concept study." No further information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "This was a double‐blind, randomized, placebo‐controlled, parallel‐group, proof‐of‐concept study." No further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8/74) participants (10.8%) discontinued prematurely (4 placebo, 4 ramelteon 8 mg). Of these, 5 participants withdrew due to an AE (3 placebo, 2 ramelteon). 2 participants withdrew voluntarily, and 1 participant was terminated for other reasons. ITT and per protocol analyses conducted |
| Selective reporting (reporting bias) | Low risk | Although reporting of results in the clinical trial synopsis was cursory, mention was made of all planned outcomes |
| Other bias | Unclear risk | No information available |
Serfaty 2002.
| Methods | Randomised, 2‐period cross‐over design | |
| Participants | Number of participants: 44 randomised, 25 completed study Country: UK Setting: 16 participants resident in nursing home, 4 in hospital, 5 at home (25 study completers) Diagnosis: dementia (DSM‐IV criteria). 35/44 (80%) of those randomised and 21/25 (84%) of those completing the study had AD or mixed (AD + vascular) pathology Sleep‐related inclusion criteria: sleep disturbance identified by the main carer, defined as shouting, agitated behaviour, wandering, or both, on at least 2 nights/week Gender: 16 women, 9 men (25 study completers) Age: 84.2 ± 7.6 years (25 study completers) Severity of dementia: MMSE 13.4 ± 8.5 (25 study completers) Other information: 9/25 were regularly taking other sleep medication (stable for at least 4 weeks prior to trial entry) |
|
| Interventions | Duration of treatment: 1‐week baseline period, then 2 treatment periods of 2 weeks, each followed by a 1‐week washout period Oral 6 mg melatonin slow‐release, or placebo, at usual bedtime |
|
| Outcomes | Outcomes measured for 3 nights at each of: baseline, the end of both treatment periods, and the end of both washout periods. Outcome was the mean of the 3 measurements at each time point 'Main' outcomes (measured with wrist actigraphy):
'Subsidiary' outcomes:
|
|
| Notes | Pill count was used to assess compliance: 4.9% doses missed in first treatment period, 10.6% in second; no differences between placebo and melatonin groups.
Paper reported "no adverse effects", but this was not a listed outcome and it is not clear how data were gathered. 308/352 patients who met inclusion criteria had to be excluded (207 due to absence of suitable carer, other reasons not given) Results were presented as medians and interquartile ranges Analysis method was not described in any detail Non‐commercial funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatment order was random and determined individually by a computer‐generated algorithm as soon as consent was obtained." |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Both the pharmacist dispensing the trial medication and the researchers were blind to the treatment received. The code for treatment allocation was only broken once the trial was completed." "Melatonin and placebo were identical with respect to preparation and packaging." Blindness of participants, carers, and researchers was rated with a VAS. None of the participants could remember taking medication. Neither carers nor researchers could distinguish between treatments |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above. Main outcomes were actigraphic (objective) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10/44 randomised participants excluded from analysis because no actigraph data. Further 9 participants excluded due to insufficient actigraph data (7), stroke (1), or poor compliance with medication (2). Results reported on only 25/44 randomised. (ITT analysis conducted on 34 participants, but not reported: "as this did not alter the results"). In main analysis, group median baseline scores used in 4 cases, and LOCF in 7 cases. |
| Selective reporting (reporting bias) | High risk | Sleep results reported (total time asleep, number of awakenings, and sleep efficiency) differed from those described in methods section. Compliance with diary sheets poor and data not analysed. Participants' sleep quality by VAS and cognitive function reported as "unchanged with melatonin", but no data presented. Carers' sleep not reported because only 5 participants lived with informal carer. |
| Other bias | Unclear risk | Authors reported conducting an analysis that excluded carry‐over effects on sleep outcomes. "In order to detect carry‐over effects the ‘summed’ scores for each participant were compared across the two groups (Mel/Plac and Plac/Mel; Everitt 1994). No significant carry‐over effects were observed." |
Singer 2003.
| Methods | RCT, 3 parallel treatment groups | |
| Participants | Number of participants: 157 randomised (data for ITT analysis available for only 151 due to technical difficulties with actigraphy) Country: USA Setting: recruited through 36 AD research centres. Broad recruitment strategy including long‐term care facilities; number in institutional care not specified Diagnosis: probable AD (NINCDS‐ADRDA) Sleep‐related inclusion criteria: a night‐time sleep disturbance defined as averaging < 7 h of total time immobile between 8 PM and 8 AM during the screening period of at least 1 week plus 2 or more episodes/week of night‐time behaviours as reported by the caregiver on the SDI. SDI is derived from the NPI. The SDI items included difficulty falling asleep, getting up during the night (other than for toileting), night‐time wandering, awakening the caregiver, awakening and thinking it is daytime, awakening too early, and excessive daytime sleeping Gender: 88 women, 69 men Age: 77.4 ± 8.9 years Severity of dementia: MMSE 13.9 ± 8.8, ADAS‐cog 38.5 ± 18.9 |
|
| Interventions | Duration of treatment: 2 to 3‐week screening and/baseline period, 8‐week treatment period, 2‐week placebo washout period Treatment group 1 (54 participants; 54 included in ITT analysis): 2.5 mg melatonin SR Treatment group 2 (51 participants; 51 included in ITT analysis): 10 mg melatonin (immediate‐release) Treatment group 3 (52 participants; 47 included in ITT analysis): Placebo All treatment groups: Route of administration: oral Time of administration: 1 h before habitual bedtime |
|
| Outcomes | Sleep outcomes all measured with actigraphy. Single actigraph records were created for the 2 to 3‐week screening and baseline and 8‐week treatment periods Primary outcome:
Other actigraphic sleep outcomes:
Secondary outcomes:
|
|
| Notes | Other psychotropic or sleep medications were allowed if use was stable Non‐commercial funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomly assigned (blocked by study site) in a double‐blind fashion to 1 of 3 groups." Randomized centrally. Likely to have been adequate |
| Allocation concealment (selection bias) | Low risk | No details of process given, but centrally randomised, so effective allocation concealment highly likely |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated to be doubleblind. "Placebo and both melatonin preparations were supplied by Genzyme Limited (Boston, Mass) in identical capsules." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Actigraphic analysis conducted off site. SIte staff conducting other assessments stated to be blinded. "Sealed code breakers were distributed to each site and recovered at the end of the trial. In no instance was it necessary to break the blind." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 randomised participants excluded from sleep analyses due to technical difficulties with actigraphy. Group allocations not reported, but judged to be unlikely to introduce bias. 12 participants discontinued treatment but were included in ITT analysis, using a LOCF method. Group allocation of these participants also not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Wade 2014.
| Methods | RCT, 2 parallel treatment groups | |
| Participants | Number of participants: 73 patients were randomised; 13 of these were in the subpopulation with comorbid insomnia at baseline and hence were relevant to this review. Country: 1 centre in UK; 4 centres in USA Setting: outpatients Diagnosis: AD (diagnostic criteria not specified) Sleep‐related inclusion criteria: a subset of participants had insomnia at baseline, defined as Pittsburgh Sleep Quality Index (PSQI) score ≥ 6 Gender: data available for whole study population only, 36 women, 37 men Age: data available for whole study population only, range 52 to 85 years Severity of dementia: data available for whole study population only, MMSE had to be ≥15 for inclusion Other information: all participants were on stable doses of acetylcholinesterase inhibitor with or without memantine for 2 months prior to recruitment. Patients were instructed to spend 2 hours a day in outdoor daylight. |
|
| Interventions | Duration of treatment: 2‐week single‐blind placebo run‐in phase, 24‐week double‐blind randomised treatment phase, 2‐week placebo run‐out phase Treatment group 1: 7 participants from insomnia subpopulation: 2 mg melatonin SR, once daily, 1 to 2 hours before bedtime. Treatment group 2: 6 participants from insomnia subpopulation: placebo |
|
| Outcomes |
|
|
| Notes | The study was funded by Neurim Pharmaceuticals. Four of eight authors were paid employees of Neurim Pharmaceuticals. The other four authors have acted as paid consultants to Neurim Pharmaceuticals. The study used the Pittsburgh Sleep Questionnaire (PSQ) as a carer‐reported sleep measure. No evidence was cited for its validity in this context. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Selection for treatment group was determined by a computer‐generated randomization list in a 1:1 ratio using the randomized permuted blocks method." Note: No stratification by baseline sleep score was used to identify insomnia subgroup. |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "To prevent bias, matching placebo tablets, which were identical in appearance, taste and odor, were used. The treatment was double‐blinded." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant lost from each group. |
| Selective reporting (reporting bias) | High risk | Relation of this report to NCT00940589 and to EUCTR2009‐014388‐38‐GB is uncertain. It seems likely that these all refer to the same study, but we have been unable to get confirmation of this from Neurim Pharmaceuticals. The trial registry entries and this report differ in several respects, including sites and outcome measures. |
| Other bias | High risk | Not clear that insomnia subgroup was defined prospectively. Possible conflict of interest |
Abbreviations AD = Alzheimer's disease ADCS‐ADL = Alzheimer’s Disease Cooperative Study Activities of Daily Living ADAS‐cog = Alzheimer's Disease Assessment Scale ‐ cognitive subscale AEs = adverse events CAS = Caregiver Burden via the Caregiver Activity Survey CGGI = Caregiver Global Impression of sleep CGI = Clinical Global Impression of sleep DSM‐IV = Diagnostic and Statistical Manual of Mental Disorders, 4th edition dTST = daytime total sleep time ECG = electrocardiogram h = hour(s) ITT = intention‐to‐treat LOCF = last observation carried forward MMSE = Mini‐Mental State Examination NINCDS‐ADRDA = National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association NPI = Neuropsychiatric Inventory nTST = night‐time total sleep time SDI = Sleep Disorder Inventory SR = slow release VAS = visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Asano 2013 | Not an RCT |
| Asayama 2003 | Participants had no identified sleep problem at baseline |
| Bergener 1968 | Geriatric population with mixed diagnoses. No primary sleep aim. No identified sleep problem at baseline |
| Bergonzi 1973 | Not an RCT |
| Cardinali 2014 | Not an RCT |
| Cohen‐Mansfield 2000 | Not an RCT |
| Cruz‐Aguilar 2013 | Not an RCT |
| Eeles 2003 | Not an RCT (group assignment by birth date) |
| Gehrmann 2009 | Participants had no identified sleep problem at baseline |
| Gillman 1997 | Not an RCT |
| GInsburg 1976 | Not an RCT (incomplete Latin square design). Uncertain diagnoses |
| Haworth 2001 | Trial register entry in 2001 for an investigator‐led trial of melatonin planned for 2001 to 2002. Described in the trial register as 'a pilot study'. We found no evidence that this trial was ever completed and were unable to obtain any information from the author. |
| Howcroft 2005 | Not an RCT |
| Kittur 1999 | Not an RCT |
| Linnoila 1980a | Participants were 19 psychogeriatric inpatients, 7 of whom were identified as having 'senile dementia' |
| Linnoila 1980b | Participants were 20 psychogeriatric inpatients, 10 of whom were identified as having 'senile dementia' |
| Magnus 1978 | Participants were 17 elderly patients with unspecified organic dementia. No identified sleep problems at baseline |
| Mahlberg 2004 | Not an RCT |
| Markowitz 2003 | No identified sleep problem at baseline. Aimed to assess effect of galantamine on sleep but no primary aim to improve sleep. |
| Meguro 2004 | Not an RCT. Participants selected for wandering behaviour |
| Mishima 1992 | Not an RCT. Participants had multi‐infarct dementia |
| Moraes 2003 | No identified sleep problems at baseline |
| Moraes 2006 | No identified sleep problems at baseline. (Patients excluded for severe sleep disturbance) |
| NCT00035204 | No primary aim to improve sleep. No placebo control (comparison of two cholinesterase inhibitors) |
| NCT00480870 | No identified sleep problems at baseline. No primary aim to improve sleep |
| NCT00626210 | Not an RCT |
| NCT00706186 | Study terminated July 2010. Withdrawn by investigator |
| NCT00940589 | No identified sleep problems at baseline |
| NCT01548287 | No identified sleep problems at baseline (confirmed by representative of sponsor) |
| Petit 1993 | No mention of randomised allocation. No identified sleep problems at baseline |
| Riemersma‐van der Lek2008 | No identified sleep problems at baseline |
| Riley McCarten 1995 | Not an RCT |
| Ruths 2004 | No identified sleep problems at baseline. Drug withdrawal study |
| Schubert 1984 | Not an RCT |
| Scoralick 2016 | Only 10/24 included participants were randomised; majority of control data were drawn from an earlier study. |
| Scripnikov 2007 | No primary sleep aim. No identified sleep problems at baseline |
| Shaw 1992 | Participants were elderly psychiatric inpatients, < 50% had dementia |
| Shinno 2008 | Not an RCT |
| Simon Padilla 2009 | Not an RCT |
| Singer 2007 | Not an RCT. |
| Stahl 2004 | No primary sleep aim. (Investigated sleep‐related adverse events in RCTs of galantamine) |
| Stemmelin 2013 | No identified sleep problems at baseline |
| Tozawa 1998 | Identified from Conference proceedings and other literature. Probably a small RCT (7 participants). Minimal information and no results obtainable |
| UMIN000006388 | Not an RCT |
| UMIN000016374 | No placebo control, comparing hypnotic, antidepressant and herbal medicine |
| Valtonen 2005 | No identified sleep problems at baseline |
| Walther 2011 | Planned trial (Swissmedic 2007DR2217) was not completed. Only 2 patients could be randomised, published as case reports |
| Yehuda 1996 | No primary sleep aim. No identified sleep problems at baseline |
Abbreviations
AD = Alzheimer's disease MCI = mild cognitive impairment
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00814502.
| Methods | RCT |
| Participants | AD or vascular dementia, aged 60 to 99 years |
| Interventions | Zolpidem CR, or placebo |
| Outcomes | Primary: sleep efficiency and other actigraphy‐derived sleep parameters |
| Notes | Unclear what proportion of participants have AD. May not have been selected for sleep problem at baseline |
Differences between protocol and review
In the original review (2012)
Three of the original authors left the team and a new author (AS) was added.
We changed outcomes from those specified in the published protocol. This change was made prior to conduct of the search and identification of studies.
The protocol stated that cross‐over studies would be excluded if it was not possible to extract paired data from the study report. However, we decided that Serfaty 2002 should be included in order to give a fuller account of the evidence available.
In the first review update (2016)
The following changes were planned before the updated search was conducted.
We extended the scope of the review to include participants with any subtype of dementia (not just dementia due to Alzheimer's disease).
We planned additional subgroup analyses, based on the subtype of dementia.
We added GRADE ratings of the quality of the evidence supporting each effect estimate, and 'Summary of fIndings' tables that included seven pre‐defined outcomes.
Contributions of authors
All three authors contributed to screening of search results, selection of studies, extraction of data, design and interpretation of the analyses. The review was written largely by JMcC with contributions from DC and AS.
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR, UK.
This update was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health
Declarations of interest
Jenny McCleery: nothing to declare Daniel A Cohen: nothing to declare Ann L Sharpley: nothing to declare
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Camargos 2014 {published data only}
- Camargos EF, Louzada LL, Quintas JL, Naves JOS, Louzada FM, Nobrega OT. Trazodone improves sleep parameters in Alzheimer disease patients: a randomized, double‐blind and placebo‐controlled study. American Journal of Geriatric Psychiatry 2014 [Epub 2014 Jan 4];22(12)(12):1565‐74. [DOI: 10.1016/j.jagp.2013.12.174] [DOI] [PubMed] [Google Scholar]
- Camargos EF, Quintas JL, Louzada LL, Naves JOS, Furioso ACT, Nobrega OT. Trazodone and cognitive performance in Alzheimer disease. Journal of clinical psychopharmacology 2015;35(1):88‐9. [DOI] [PubMed] [Google Scholar]
Dowling 2008 {published data only}
- Dowling GA, Burr RL, Someren EJW, Hubbard EM, Luxenberg JS, Mastick J, et al. Melatonin and bright light treatment for rest‐activity disruption in institutionalized patients with Alzheimer's disease. American Journal of Geriatric Psychiatry 2008;56(2):239‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00325728 {published data only (unpublished sought but not used)}
- NCT00325728. A double‐blind, randomized, placebo‐controlled study of the efficacy, safety and tolerability of 8 week treatment of Rozerem 8 mg (QHS) in sleep disturbed, mild to moderately severe Alzheimer's disease subjects. clinicaltrials.gov/ct2/show/NCT00325728?.
- NCT00325728. Clinical Trial Synopsis 01‐05‐TL‐375‐061, NCT#00325728: A double‐blind, randomized, placebo‐controlled study of the efficacy, safety and tolerability of 8 week treatment of Rozerem 8 mg (QHS) in sleep disturbed, mild to moderately severe Alzheimer’s disease subjects. www.takeda.com/research/ct/pdf/report/15_Ramelteon_01‐05‐TL‐375‐061_ClinicalTrialSynopsis_NCT00325728_en.pdf.
Serfaty 2002 {published data only}
- Serfaty M, Kennell‐Webb S, Warner J, Blizard R, Raven P. Double blind randomised placebo controlled trial of low dose melatonin for sleep disorders in dementia. International Journal of Geriatric Psychiatry 2002;17(12):1120‐7. [DOI] [PubMed] [Google Scholar]
Singer 2003 {published data only}
- Singer C, Tractenberg RE, Kaye J, Schafer K, Gamst A, Grundman M, et al. A multicenter, placebo‐controlled trial of melatonin for sleep disturbance in Alzheimer's disease. Sleep 2003;26(7):893‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wade 2014 {published data only}
- Wade AG, Farmer M, Harari H, Fund N, Laudon M, Nir T, et al. Add‐on prolonged‐release melatonin for cognitive function and sleep in mild to moderate Alzheimer's disease: a 6‐month, randomized, placebo‐controlled, multicenter trial. Clinical Interventions in Aging 2014;9:947‐61. [10.2147/CIA.S65625. eCollection 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Asano 2013 {published data only}
- Asano M, Ishitobi M, Tanaka Y, Wada Y. Effects of ramelteon on refractory behavioral and psychological symptoms of Alzheimer's disease. Journal of Clinical Psychopharmacology 2013;33(4):579‐81. [DOI: 10.1097/JCP.0b013e3182946702] [DOI] [PubMed] [Google Scholar]
Asayama 2003 {published data only}
- Asayama K, Yamadera H, Ito T, Suzuki H, Kudo Y, Endo S. Double blind study of melatonin effects on the sleep‐wake rhythm, cognitive and non‐cognitive functions in Alzheimer type dementia. Journal of Nippon Medical School 2003;70:334‐41. [DOI] [PubMed] [Google Scholar]
Bergener 1968 {published data only}
- Bergener M, Undeutsch D. Methods and problems of clinical psychopharmacology. Results of a double blind study with Medazepam and placebo in geriatric psychiatry. Arzneimittel‐Forschung 1968;18:1563‐6. [PubMed] [Google Scholar]
Bergonzi 1973 {published data only}
- Bergonzi P, Capocchi G, Chiurulla C, Mennuni G, Tempsta E. Tryptophan and sleep in subjects with neuropsychiatric syndromes. Rivista di Neurologia 1973;43:403‐9. [PubMed] [Google Scholar]
Cardinali 2014 {published data only}
- Cardinali DP, Vigo DE, Natividad O, Vidal MF, Brusco LI. Melatonin therapy in patients with Alzheimer's disease. Antioxidants 2014;2:245‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen‐Mansfield 2000 {published data only}
- Cohen‐Mansfield J, Garfinkel D, Lipson S. Melatonin for treatment of sundowning in elderly persons with dementia – a preliminary study. Archives of Gerontology and Geriatrics 2000;31:65‐76. [DOI] [PubMed] [Google Scholar]
Cruz‐Aguilar 2013 {published data only}
- Cruz‐Aguilar MA, Ramirez‐Salado I, Cruz‐Ulloa C, Benitez‐King G. Melatonin effects on macro sleep architecture in Alzheimer's disease patients. Salud Mental 2013;36:243‐9. [Google Scholar]
Eeles 2003 {published data only}
- Eeles E. The effect of melatonin on sleep pattern and levels of agitation in patients with dementia. National Research Register 2003.
Gehrmann 2009 {published data only}
- Gehrmann PR, Donald JC, Martin JL, Shochat T, Corey‐Bloom J, Ancoli‐Israel S. Melatonin fails to improve sleep or agitation in a double‐blind randomized placebo‐controlled trial of instituitionalized patients with Alzheinmer's disease. American Journal of Geriatric Psychiatry 2009;17(2):166‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillman 1997 {published data only}
- GIllman PK. Tacrine for treatment of sleep disturbance in dementia. Journal of the American Geriatrics Society 1997;45:1286. [DOI] [PubMed] [Google Scholar]
GInsburg 1976 {published data only}
- Ginsburg R, Weintraub M. Caffeine in the 'Sundown syndrome'. Report of negative results. Journal of Gerontology 1976;31:419‐20. [DOI] [PubMed] [Google Scholar]
Haworth 2001 {published data only (unpublished sought but not used)}
- Haworth J. A pilot, double‐blind, placebo controlled, parallel group study of the effect of melatonin treatment in patients with Alzheimer's disease and sleep. National Research Register Archive 2001.
Howcroft 2005 {published data only}
- Howcroft DJ, Jones RW. Does modafinil have the potential to improve disrupted sleep patterns in patients with dementia?. International Journal of Geriatric Psychiatry 2005;20:492‐5. [DOI] [PubMed] [Google Scholar]
Kittur 1999 {published data only}
- Kittur S, Hause P. Improvement of sleep and behavior by methylphenidate in Alzheimer's disease. American Journal of Psychiatry 1999;156:1116‐7. [DOI] [PubMed] [Google Scholar]
Linnoila 1980a {published data only}
- Linnoila M, Viukari M, Numminen A, Auvinen J. Efficacy and side effects of chloral hydrate and tryptophan as sleeping aids in psychogeriatric patients. International Pharmacopsychiatry 1980;15:124‐8. [DOI] [PubMed] [Google Scholar]
Linnoila 1980b {published data only}
- Linnoila M, Viukari M, Lamminsivu U, Auvinen J. Efficacy and side effects of lorazepam, oxazepam, and temazepam as sleeping aids in psychogeriatric in‐patients. International Pharmacopsychiatry 1980;15:129‐35. [DOI] [PubMed] [Google Scholar]
Magnus 1978 {published data only}
- Magnus RV. A controlled trial of chlormethiazole in the management of symptoms of the organic dementias in the elderly. Clinical Therapeutics 1978;1(6):387‐96. [Google Scholar]
Mahlberg 2004 {published data only}
- Mahlberg R, Kunz D, Sutej I, Kuhl KP, Hellweg R. Melatonin treatment of day‐night rhythm disturbances and sundowning in Alzheimer disease: an open‐label pilot study using actigraphy. Journal of Clinical Psychopharmacology 2004;24:456‐9. [DOI] [PubMed] [Google Scholar]
Markowitz 2003 {published data only}
- Markowitz JS, Gutterman EM, Lilienfeld S, Papadopoulos G. Sleep‐related outcomes in persons with mild to moderate Alzheimer disease in a placebo‐controlled trial of galantamine. Sleep 2003;26:602‐6. [DOI] [PubMed] [Google Scholar]
Meguro 2004 {published data only}
- Meguro K, Meguro M, Tanaka Y, Akanuma K, Yamaguchi K, Itoh M. Risperidone is effective for wandering and disturbed sleep/wake patterns in Alzheimer's disease. Journal of Geriatric Psychiatry and Neurology 2004;17:61‐7. [DOI] [PubMed] [Google Scholar]
Mishima 1992 {published data only}
- Mishima K, Okawa M, Hishikawa Y. Effect of methylcobalamin (VB12) injection on sleep‐wake rhythm in demented patients. Japanese Journal of Psychiatry & Neurology 1992;46:227‐8. [DOI] [PubMed] [Google Scholar]
Moraes 2003 {published data only}
- Moraes WA, Poyares DL, Ramos LR, Bertolucci PH, Tufik S. Six‐month donepezil treatment alters REM sleep parameters in Alzheimer's disease patients: a double‐blind placebo controlled study. Sleep 2003;26:A351. [DOI] [PubMed] [Google Scholar]
Moraes 2006 {published data only}
- Moraes WA, Poyares DR, Guilleminault C, Ramos LR, Ferreira Bertolucci PH, Tufik S. The effect of donepezil on sleep and REM sleep EEG in patients with Alzheimer disease: a double‐blind placebo‐controlled study. Sleep 2006;29:199‐205. [DOI] [PubMed] [Google Scholar]
NCT00035204 {published data only}
- NCT00035204. A study of the effects on sleep, attention, and gastrointestinal tolerance of galantamine and donepezil in patients with Alzheimer's disease. clinicaltrials.gov/show/NCT00035204 (first received 2 May 2002).
NCT00480870 {published data only}
- NCT00480870. The effect of anticholinesterase drugs on sleep in Alzheimer's disease patients. clinicaltrials.gov/show/NCT00480870 (first received 30 May 2007).
NCT00626210 {published data only}
- NCT00626210. Modafinil treatment for sleep/wake disturbances in older adults. clinicaltrials.gov/show/NCT00626210 (first received 20 February 2008).
NCT00706186 {published data only}
- NCT00706186. Safety and feasibility of sodium oxybate in mild Alzheimer's disease patients. clinicaltrials.gov/show/NCT00706186 (first received 24 June 2008).
NCT00940589 {published data only}
- NCT00940589. Efficacy of Circadin® 2 mg in patients with mild to moderate Alzheimer disease treated with AChE inhibitor. clinicaltrials.gov/show/NCT00940589 (first received 15 July 2009).
NCT01548287 {published data only}
- NCT01548287. A study of the safety and tolerability of AZD5213 effect on sleep for patients with Alzheimer's/cognitive impairment. clinicaltrials.gov/show/NCT01548287 (first received 5 March 2012).
Petit 1993 {published data only}
- Petit G, Montplaisir J, Lorrain D, Gauthier S. THA does not affect sleep or EEG spectral power in Alzheimer's disease. Biological Psychiatry 1993;33:753‐4. [DOI] [PubMed] [Google Scholar]
Riemersma‐van der Lek2008 {published data only}
- Riemersma‐van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJG, Someren EJW. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA 2008;299:2642‐55. [DOI] [PubMed] [Google Scholar]
Riley McCarten 1995 {published data only}
- Riley McCarten J, Kovera C, Maddox MK, Cleary JP. Triazolam in Alzheimer's disease: pilot study on sleep and memory effects. Pharmacology, Biochemistry and Behavior 1995;52:447‐52. [DOI] [PubMed] [Google Scholar]
Ruths 2004 {published data only}
- Ruths S, Straand J, Nygaard HA, Bjorvatn B, Pallesen S. Effect of antipsychotic withdrawal on behavior and sleep/wake activity in nursing home residents with dementia: a randomized, placebo‐controlled, double‐blinded study. Journal of the American Geriatrics Society 2004;52:1737‐43. [DOI] [PubMed] [Google Scholar]
Schubert 1984 {published data only}
- Schubert DS. Hydroxyzine for acute treatment of agitation and insomnia in organic mental disorder. Psychiatric Journal of the University of Ottawa 1984;9:59‐60. [PubMed] [Google Scholar]
Scoralick 2016 {published data only}
- Scoralick FM, Louzada LL, Quintas JL, Naves JOS, Camargos EF, Nobrega OT. Mirtazapine does not improve sleep disorders in Alzheimer's disease: results from a double‐blind, placebo‐controlled pilot study. Psychogeriatrics 2016 Jan 27 [Epub ahead of print]:1‐8. [DOI: 10.1111/psyg.12191] [DOI] [PubMed] [Google Scholar]
Scripnikov 2007 {published data only}
- Scripnikov A, Khomenko A, Napryeyenko O. Effects of Ginkgo biloba extract EGb 761 on neuropsychiatric symptoms of dementia: findings from a randomised controlled trial. Wiener Medizinische Wochenschrift 2004;157:295‐300. [DOI] [PubMed] [Google Scholar]
Shaw 1992 {published data only}
- Shaw SH, Curson H, Coquelin JP. A double‐blind, comparative study of zolpidem and placebo in the treatment of insomnia in elderly psychiatric in‐patients. Journal of International Medical Research 1992;20:150‐61. [DOI] [PubMed] [Google Scholar]
Shinno 2008 {published data only}
- Shinno H, Inami Y, Inagaki T, Nakamura Y, Horiguchi J. Effect of Yi‐Gan San on psychiatric symptoms and sleep structure at patients with behavioral and psychological symptoms of dementia. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 2008;32:881‐5. [DOI] [PubMed] [Google Scholar]
Simon Padilla 2009 {published data only}
- Simon Padilla OJ, Linares Del Rio C, Godden P, Granizo E, Pinilla M, Rodriguez C. Use of combined treatment with melatonin and clomethiazole in circadian rhythm sleep disorder in the elderly with dementia. Revista Espanola de Geriatria y Gerontologia 2009;44:233‐4. [DOI] [PubMed] [Google Scholar]
Singer 2007 {published data only}
- Singer CM, Nanda F. Modafinil vs. placebo: effects on daytime sleep in Alzheimer's disease. Biological Psychiatry 2007;61:144S. [Google Scholar]
Stahl 2004 {published data only}
- Stahl SM, Markowitz JS, Papadopoulos G, Sadik K. Examination of nighttime sleep‐related problems during double‐blind, placebo‐controlled trials of galantamine in patients with Alzheimer's disease. Current Medical Research and Opinion 2004;20:517‐25. [DOI] [PubMed] [Google Scholar]
Stemmelin 2013 {published data only}
- Stemmelin J, Kirkesseli S, Martincova R, Bejuit R, Corp‐Dit‐Genti V, Morrison G, et al. A phase 2 study to investigate the effects of Sar110894 on cognition, daily function, apathy and sleep in mild to moderate Alzheimer's disease patients. Journal of Nutrition, Health and Aging: 6th Conference Clinical Trials on Alzheimer's Disease. 2013:804‐5.
Tozawa 1998 {published data only (unpublished sought but not used)}
- Tozawa T, Mishima K, Satoh K, Matsumoto Y, Echizenya M, Sasaki M, et al. Melatonin replacement therapy for rest‐activity rhythm disorders in patients with senile dementia of Alzheimer's type. 21st Collegium Internationale Neuropsychopharmacologium (CINP) Congress, 1998 Jul 12‐16; Glasgow, Scotland. 1998.
UMIN000006388 {published data only}
- JPRN‐UMIN000006388. Investigation on the effects of memantine on psychiatric symptoms and sleep disturbances in patients with Alzheimer's disease. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000007581 (disclosed on 21 September 2011).
UMIN000016374 {published data only}
- UMIN000016374. The controlled study of medical treatment for the sleep disturbance in dementia. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000019016 (date of disclosure 29 January 2015).
Valtonen 2005 {published data only}
- Valtonen M, Niskanen L, Kangas A‐P, Koskinen T. Effects of melatonin‐rich night‐time milk on sleep and activity in elderly institutionalized subjects. Nordic Journal of Psychiatry 2005;59:217‐21. [DOI] [PubMed] [Google Scholar]
Walther 2011 {published data only}
Yehuda 1996 {published data only}
- Yehuda S, Rabinovtz S, Carasso RL, Mostofsky DI. Essential fatty acids preparation (sr‐3) improves Alzheimer's patients quality of life. International Journal of Neuroscience 1996;87:141‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00814502 {published data only}
- Zolpidem CR and hospitalized patients with dementia. ClinicalTrials.gov.
Additional references
Ancoli‐Israel 1997
- Ancoli‐Israel S, Clopton P, Klauber MR, Fell R, Mason W. Use of wrist actigraphy for monitoring sleep/wake in demented nursing home patients. Sleep 1997;20:24‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bliwise 2011
- Bliwise DL, Mercaldo ND, Avidan AY, Boeve BF, Greer SA, Kukull WA. Sleep disturbance in dementia with Lewy bodies and Alzheimer's disease: a multicenter analysis. Dementia & Geriatric Cognitive Disorders 2011;31(3):239‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buxton 2000
- Buxton OM, Copinschi G, Onderbergen A, Karrison TG, Cauter E. A benzodiazepine hypnotic facilitates adaptation of circadian rhythms and sleep‐wake homeostasis to an eight hour delay shift simulating westward jet lag. Sleep 2000;23:915‐27. [PubMed] [Google Scholar]
Camargos 2013
- Camargos EF, Louzarda FM, Nobrega OT. Wrist actigraphy for measuring sleep in intervention studies with Alzheimer's disease patients: application, usefulness and challenges. Sleep Medicine Reviews 2013;17:475‐88. [DOI] [PubMed] [Google Scholar]
Cardinali 2010
- Cardinali DP, Furio AM, Brusco LI. Clinical aspects of melatonin intervention in Alzheimer's disease progression. Current Neuropharmacology 2010;8:218‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Closser 1991
- Closser MH. Benzodiazepines and the elderly: a review of potential problems. Journal of Substance Abuse Treatment 1991;8:35‐41. [DOI] [PubMed] [Google Scholar]
Coupland 2011
- Coupland CA, Dhiman P, Barton G, Morriss R, Arthur A, Sach T, et al. A study of the safety and harms of antidepressant drugs for older people: a cohort study using a large primary care database. Health Technology Assessment 2011;15(28):1‐202. [DOI] [PubMed] [Google Scholar]
Cumming 2003
- Cumming RG, Couteur DG. Benzodiazepines and risk of hip fractures in older people: a review of the evidence. CNS Drugs 2003;17:825‐37. [DOI] [PubMed] [Google Scholar]
Dauvilliers 2007
- Dauvilliers Y. Insomnia in patients with neurodegenerative conditions. Sleep Medicine 2007;4 Suppl:27‐34. [DOI] [PubMed] [Google Scholar]
David 2010
- David R, Zeitzer J, Friedman L, Noda A, O'Hara R, Robert P, et al. Non‐pharmacologic management of sleep disturbance in Alzheimer's disease. The Journal of Nutrition, Health & Aging 2010;14:203‐6. [DOI] [PubMed] [Google Scholar]
Donaldson 1998
- Donaldson C, Tarrier N, Burns A. Determinants of carer stress in Alzheimer’s disease. International Journal of Geriatric Psychiatry 1998;13:248‐56. [DOI] [PubMed] [Google Scholar]
Fuh 2005
- Fuh J‐L, Wang S‐J, Cummings JL. Neuropsychiatric profiles in patients with Alzheimer's disease and vascular dementia. Journal of Neurology, Neurosurgery and Psychiatry 2005;76(10):1337‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gao 2009
- Gao QW, LIU Y, Luo GQ, Xiang W, Peng KR. Effect of melatonin on mild Alzheimer's disease in elderly male patients. Practical Geriatrics 2009;23(1):56‐8. [Google Scholar]
Gaugler 2000
- Gaugler JE, Edwards AB, Femia EE, Zarit SH, Parris Stevens M‐A, Townsend A, et al. Predictors of institutionalization of cognitively impaired elders: family help and the timing of placement. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 2000;55:247‐55. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64:383‐94. [DOI] [PubMed] [Google Scholar]
Hart 2003
- Hart DJ, Craig D, Compton SA, Critchlow S, Kerrigan B, McIlroy SP, et al. A retrospective study of the behavioural and psychological symptoms of mid and late phase Alzheimer's disease. International Journal of Geriatric Psychiatry 2003;18(11):1037‐42. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Howard 2011
- Howard R, Phillips P, Johnson T, O'Brien J, Sheehan B, Lindesay J, et al. Determining the minimum clinically important differences for outcomes in the DOMINO trial. International Journal of Geriatric Psychiatry 2011;26(8):812‐7. [DOI] [PubMed] [Google Scholar]
Mishima 2000
- Mishima K, Okawa M, Hozumi S, Hishikawa Y. Supplementary administration of artificial bright light and melatonin as potent treatment for disorganized circadian rest‐activity and dysfunctional autonomic and neuroendocrine systems in institutionalzed demented elderly persons. Chronobiology International 2000;17(3):419‐32. [DOI] [PubMed] [Google Scholar]
Montplaisir 1995
- Montplaisir J, Petit D, Lorrain D, Gauthier S, Nielsen T. Sleep in Alzheimer's disease: further considerations on the role of brainstem and forebrain cholinergic populations in sleep‐wake mechanisms. Sleep 1995;18:145‐8. [DOI] [PubMed] [Google Scholar]
Montplaisir 1998
- Montplaisir J, Petit D, Gauthier S, Gaudreau H, Decary A. Sleep disturbances and EEG slowing in Alzheimer's disease. Sleep Research Online 1998;1(4):147‐51. [PubMed] [Google Scholar]
Oderda 2012
- Oderda LH, Young JR, Asche CV, Pepper GA. Psychotropic‐related hip fractures: meta‐analysis of first‐generation and second‐generation antidepressant and antipsychotic drugs. Annals of Pharmacotherapy 2012;46:917‐28. [DOI] [PubMed] [Google Scholar]
Rongve 2010
- Rongve A, Boeve BF, Aarsland D. Frequency and correlates of caregiver‐reported sleep disturbances in a sample of persons with early dementia. Journal of the American Geriatrics Society 2010;58(3):480‐6. [DOI] [PubMed] [Google Scholar]
Swaab 1985
- Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Research 1985;342:37‐44. [DOI] [PubMed] [Google Scholar]
Van Someren 2000
- Someren EJW. Circadian rhythms and sleep in human aging. Chronobiology International 2000;17:233‐43. [DOI] [PubMed] [Google Scholar]
Vitiello 1991
- Vitiello MV, Poceta JS, Prinz PN. Sleep in Alzheimer’s disease and other dementing disorders. Canadian Journal of Psychology 1991;45:221‐39. [DOI] [PubMed] [Google Scholar]
Wilfling 2015
- Wilfling D, Junghans A, Marshall L, Eisemann N, Meyer G, Moehler R, et al. Non‐pharmacological interventions for sleep disturbances in people with dementia. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD011881] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2015
- Xu J, Wang LL, Dammer EB, Li CB, Xu G, Chen SD, et al. Melatonin for sleep disorders and cognition in dementia: a meta‐analysis of randomized controlled trials. American Journal of Alzheiner's Disease and Other Dementias 2015;30(5):439‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]


