Abstract
Background
The suggestion that garlic may lower blood pressure, inhibit platelet aggregation, and reduce oxidative stress has led to the hypothesis that it may have a role in preventing pre‐eclampsia and its complications.
Objectives
To assess the effects of garlic on prevention of pre‐eclampsia and its complications.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (January 2010).
Selection criteria
Studies were included if they were randomised trials evaluating the effects of garlic on prevention of pre‐eclampsia and its complications.
Data collection and analysis
Two review authors independently selected trials for inclusion and extracted data. Data were entered on Review Manager software for analysis, and double checked for accuracy.
Main results
One trial (100 women) of uncertain quality compared garlic with placebo. Another study was excluded as 29% of women were lost to follow up. There was no clear difference between the garlic and control groups in the risk of developing gestational hypertension (relative risk (RR) 0.50, 95% confidence interval (CI) 0.25 to 1.00) or pre‐eclampsia (RR 0.78, 95% CI 0.31 to 1.93). Women allocated garlic were more likely to report odour than those allocated placebo (RR 8.50, 95% CI 2.07 to 34.88), but there were no significant differences in other reported side‐effects. The only other outcomes reported were caesarean section (RR 1.35, 95% CI 0.93 to 1.95), and perinatal mortality. There were no perinatal deaths in the study.
Authors' conclusions
There is insufficient evidence to recommend increased garlic intake for preventing pre‐eclampsia and its complications. Although garlic is associated with odour, other more serious side‐effects have not been reported. Further large randomised trials evaluating the effects of garlic are needed before any recommendations can be made to guide clinical practice.
Plain language summary
Garlic for preventing pre‐eclampsia and its complications
Insufficient evidence to say if taking garlic can help to reduce the risk of pre‐eclampsia and its complications for pregnant women and their babies.
Pre‐eclampsia is a serious complication of pregnancy occurring in about 2% to 8% of women. It is identified by increased blood pressure and protein in the urine, but women often suffer no symptoms initially. It can, through constriction of the blood vessels in the placenta, interfere with food and oxygen passing to the baby, thus inhibiting the baby's growth and causing the baby to be born too soon. Women can be affected through problems in their kidneys, liver, brain, and clotting system. Garlic may lower blood pressure (one of the problems with pre‐eclampsia) and so may have a role in helping to reduce the risk of pre‐eclampsia. The review of trials found just one study of 100 women which showed no differences between dried garlic tablets and dummy tablets. However, the study was small and there are many varying forms of garlic, some with odour and some without, fresh cloves, powdered garlic, garlic capsules etc., and there may be differences between these preparations. Further trials are needed to assess any possible effect of garlic on pre‐eclampsia and to assess any potential adverse effects.
Background
Hypertension (high blood pressure) is common during pregnancy. Around 10% of women will have raised blood pressure at some point before delivery. The hypertensive disorders of pregnancy comprise a spectrum of conditions that is usually classified into four categories: (i) gestational hypertension, a rise in blood pressure during the second half of pregnancy; (ii) pre‐eclampsia, usually hypertension with proteinuria (protein in urine) during the second half of pregnancy; (iii) chronic hypertension, a rise in blood pressure prior to pregnancy or before 20 weeks' gestation, and (iv) pre‐eclampsia superimposed on chronic hypertension (NHBPEP 2000). For women with uncomplicated mild to moderate hypertension, pregnancy outcome is similar to that for women with normal blood pressure. Outcome deteriorates if the blood pressure is very high, or if pre‐eclampsia develops. Pre‐eclampsia is a multisystem disorder involving the liver, kidneys, brain, and placenta. It affects 2% to 8% of pregnancies (WHO 1988), and is associated with a substantive increase in morbidity and mortality for both the woman and her baby (DH 2002). Complications for the mother may include eclampsia (seizures), stroke, liver or kidney failure, and abnormal blood clotting, and problems for the baby include poor growth and preterm birth.
The cause of pre‐eclampsia is uncertain. Current belief is that reduced blood supply to the placenta leads to abnormal function of endothelial cells that line the woman's blood vessels, possibly as a result of oxidative stress (oxidative damage to cells caused by increased reactive oxygen species and lipid peroxides). Endothelial cell dysfunction results in generalised vasoconstriction, platelet activation and thrombosis, and decreased plasma volume, with subsequent reduction of blood supply to multiple organs. Pre‐eclampsia is discussed in more detail in the generic protocol for this review (Generic Protocol 05).
Garlic (Allium sativum) is part of the Allium, or onion, family. A hardy perennial herb, it probably originated in central Asia. Garlic is the most pungent of all the alliums, and has been widely used in many cultures for both medicinal and culinary purposes. It is also one of the most ancient herbs, recorded in Babylonian times (circa 3000 BC), found in the tomb of Tutankhamen, consumed in large quantities by the ancient Greeks and Romans, and used in both traditional Chinese and Ayurvedic medicines. There are also many superstitions related to garlic, such as that it wards off evil spirits and vampires, or that chewing it will prevent competitors from getting ahead in races. The traditional medicinal uses of garlic include prevention of infection and treatment of colds, influenza, bronchitis, whooping cough, gastroenteritis, dysentery and skin problems.
Garlic's main active ingredient is thought to be allicin, a strong smelling sulphide. When raw garlic cloves are crushed or chewed, allicin is formed from garlic's main sulphur compound, alliin, by the action of enzyme alliinase. Although other constituents of garlic have been shown to be biologically active, the importance of different constituents in explaining health benefits of garlic is unclear (Amagase 2001).
More recently, it has been suggested that garlic may have lipid lowering properties (Silagy 1994b; Stevinson 2000; Warshafsky 1993) which may be beneficial for the treatment of arteriosclerosis and diabetes, and for prevention of myocardial infarction. There is also some evidence that certain garlic preparations also inhibit lipid oxidation (Ide 1997; Lau 2001) and may thus prevent development of oxidative stress (Borek 2001). A meta‐analysis of eight trials (415 participants) reported reductions in both systolic and diastolic blood pressure associated with garlic treatment in the form of dried powder (Silagy 1994a). Later trials failed to confirm these effects, however, and a subsequent systematic review reported no significant effect of garlic on blood pressure (AHRQ 2000). Possible explanations for the more positive results seen in earlier studies are that recent trials may have used garlic preparations with poor delivery of garlic's active ingredient (Lawson 2001), or publication bias with failure to publish early negative studies, or the poor concealment of allocation in early studies may have led to overestimates of the effects of garlic (Silagy 1994a). Experimental studies have demonstrated that garlic inhibits platelet aggregation (Ali 1999), and may also increase the production of nitric oxide (Das 1995), which is itself a platelet inhibitor and vasodilator.
The suggestions that garlic may lower blood pressure, reduce oxidative stress or inhibit platelet aggregation, or both, have led to the hypothesis that garlic may have a role in prevention of pre‐eclampsia.
Many commercial preparations of garlic are available, falling into one of the following four categories: dehydrated garlic powder, garlic oil, garlic oil macerate, and aged garlic extract (Amagase 2001). These are available as a syrup, tablets, or capsules. As garlic's active compound allicin is unstable, and its formation depends on the presence of alliin and alliinase, commercial preparations must be prepared with considerable care. Processing of garlic may eliminate allicin or alliin, and decrease alliinase activity substantially. Moreover, stomach acid may also destroy alliinase, and digestion may be unpredictable despite the use of protective coatings (Lawson 2001). The bioavailability of allicin therefore remains uncertain. There are no recommendations for standard intake of garlic. In early studies that used raw garlic, the dosage required to obtain health benefits was relatively high, ranging from 7 to 28 cloves per day. In more recent studies that have used commercial preparations of garlic, the daily dosage has ranged from 600 mg to 1000 mg.
Garlic is generally well tolerated. In some communities, the breath and body odour may make it less acceptable. Other side‐effects include minor gastrointestinal disturbances such as nausea and diarrhoea, and allergic reactions. Several reports suggest that there may be an increased tendency to bleed with concurrent use of anticoagulants (AHRQ 2000).
The purpose of this review was to assess the effects of garlic therapy for preventing pre‐eclampsia and its complications.
Objectives
To assess the effects of garlic on prevention of pre‐eclampsia and its complications.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials evaluating the effects of garlic on prevention of pre‐eclampsia were included. Trials with quasi‐random design were excluded.
Types of participants
Pregnant women were included, regardless of gestation at trial entry. Whenever possible and relevant, women were grouped on the basis of their risk of developing pre‐eclampsia at trial entry as follows.
(1) Normotensive women
(a) High risk: defined as having one or more of the following: diabetes, renal disease, thrombophilia, autoimmune disease, previous severe or early onset pre‐eclampsia, or multiple pregnancy. (b) Moderate risk: defined as none of the above, but having either previous pre‐eclampsia that was not severe or early onset (or severity unspecified), or a first pregnancy and at least one of the following: teenager or over 35 years age, family history of pre‐eclampsia, obesity (body mass index of 30 or more), increased sensitivity to Angiotensin II, positive roll‐over test, abnormal uterine artery doppler scan. (c) Low risk: defined as pregnancy that does not qualify as either high or moderate risk. (d) Undefined risk: when the risk is unclear or not specified.
(2) Hypertensive women, without proteinuria
These women are all at high risk of developing pre‐eclampsia. They fall into two groups. (a) Gestational hypertension: hypertension detected for the first time after 20 weeks' gestation, in the absence of proteinuria. (b) Chronic hypertension: essential or secondary hypertension detected prior to pregnancy or before 20 weeks' gestation. Some women with chronic hypertension may have longstanding proteinuria due to their underlying disease. We planned to include these women as their proteinuria is not due to pre‐eclampsia.
(3) Undefined
When it is unclear, or not specified, whether or not the women have hypertension.
Women were excluded if they had established pre‐eclampsia.
If a trial included women with pre‐eclampsia as well as those with hypertension alone (gestational or chronic), where possible, we planned to include only the women with hypertension alone in the review. For trials that did not report results separately for the two categories, we planned to include them in the review, but present them as a separate subgroup. However, we did not find any such trials.
Types of interventions
The following comparisons were included:
garlic versus placebo or no intervention; and
garlic versus any other intervention for prevention of pre‐eclampsia, where appropriate.
All types of garlic preparation were included. All dosage regimens and routes of delivery were included. Studies were excluded if the intended duration of therapy at trial entry was less than seven days. This cut off was taken because it is unlikely that such short therapy could influence pregnancy outcomes, based on what is known of the pathophysiology of pre‐eclampsia.
Types of outcome measures
The following outcomes were included. The definitions used for each outcome are summarised below. Trials that used acceptable variations of these definitions, or that did not define their outcomes were still included, and definitions, where available, were described in the table 'Characteristics of included studies'. If an important outcome was not reported, we attempted to contact authors.
For the woman
Main outcome
(1) Pre‐eclampsia: defined as hypertension (blood pressure at least 140 mmHg systolic or 90 mmHg diastolic) with proteinuria (at least 300 mg protein in a 24 hour urine collection or 30 mg/dL in a single sample or 1+ on dipstick or 30 mg/mmol urine protein/creatinine ratio). For a woman with chronic hypertension and proteinuria at trial entry, pre‐eclampsia is defined as sudden worsening of proteinuria or hypertension, or both, or other signs and symptoms of pre‐eclampsia after 20 weeks' gestation.
Other outcomes
(2) Death during pregnancy or up to 42 days after end of pregnancy. (3) Severe morbidity including eclampsia, liver or renal failure, haemolysis elevated liver enzymes and low platelets syndrome, disseminated intravascular coagulation, stroke and pulmonary oedema. These outcomes will be reported individually, and as a composite measure where the information is available. (4) Severe pre‐eclampsia: pre‐eclampsia with features of severe disease such as severe hypertension, severe proteinuria, reduced urinary volume, visual disturbances, upper abdominal pain, pulmonary oedema, impaired liver and renal function tests, low platelets, intrauterine growth restriction or reduced liquor volume (for full definition, seeGeneric Protocol 05). (5) Early onset of pre‐eclampsia, at or before 33 completed weeks. (6) Severe hypertension: blood pressure at least 160 mmHg systolic or 110 mmHg diastolic. (7) Gestational hypertension: blood pressure at least 140 mmHg systolic or 90 mmHg diastolic after 20 weeks' gestation. (8) Use of antihypertensive drugs or need for additional antihypertensive drugs. (9) Abruption of the placenta or antepartum haemorrhage. (10) Elective delivery: induction of labour or caesarean section. (11) Caesarean section: emergency and elective. (12) Postpartum haemorrhage: blood loss of 500 ml or more. (13) Side‐effects such as odour, gastrointestinal disturbances, and bleeding; garlic stopped due to side‐effects. (14) Use of health resources: visit to day care unit, antenatal hospital admission, intensive care (admission to intensive care unit, length of stay, ventilation, dialysis). (15) Women's experiences and views of garlic.
For the child
Main outcomes
(1) Death: including all deaths before birth and up to discharge from hospital. (2) Preterm birth: birth at or before 37 completed weeks' gestation. (3) Small‐for‐gestational age: growth below the 3rd centile, or lowest centile reported.
Other outcomes
(4) Apgar score at five minutes: low (less than seven) and very low (less than four) or lowest reported. (5) Endotracheal intubation or use of mechanical ventilation. (6) Neonatal morbidity: respiratory distress syndrome, chronic lung disease, sepsis, necrotizing enterocolitis, retinopathy of prematurity, and intraventricular haemorrhage. (7) Long‐term growth and development: blindness, deafness, seizures, poor growth, neurodevelopmental delay and cerebral palsy. (8) Side‐effects associated with garlic. (9) Use of hospital resources: admission to neonatal intensive care unit, duration of hospital stay after delivery.
Economic outcomes
Costs to health service resources: short term and long term for both mother and baby.
Costs to the woman, her family, and society associated with exercise.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (January 2010).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
For details of the additional searching we undertook for the initial version of the review, seeAppendix 1.
Searching other resources
When possible, we also contacted experts and trialists in this field through personal communications.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Both review authors independently assessed potentially eligible studies for inclusion. We resolved any differences in opinion by discussion.
Assessment of study quality
Two review authors independently assessed the quality of each trial using the criteria outlined in the Cochrane Reviewers' Handbook (Clarke 2003). Methods used for generation of the randomisation sequence are described for each trial, wherever possible. Each study was assessed for quality of concealment of allocation, completeness of follow up, and blinding. If important data were missing, we contacted trialists when possible to obtain additional information.
(1) Allocation concealment
A quality score for concealment of allocation was assigned to each trial, using the following criteria: (A) adequate concealment of allocation, such as telephone randomisation, consecutively numbered sealed opaque envelopes; (B) unclear whether concealment of allocation was adequate; (C) inadequate concealment of allocation such as open random number tables, sealed envelopes that were not numbered and opaque.
(2) Completeness of follow up
Completeness of follow up was assessed using the following criteria: (A) less than 5% of participants excluded from analysis; (B) 5% to 10% of participants excluded from analysis; (C) more than 10% and up to and including 20% of participants excluded from analysis.
Studies were excluded if:
more than 20% of participants were excluded from analysis.
more than 10% of participants were not analysed in their randomised groups and it was not possible to restore participants to the correct group.
there was more than 10% difference in loss of participants between groups.
Data were analysed based on the group to which the participants were randomised, regardless of whether or not they received the allocated intervention. If data were missing, whenever possible, we sought clarification from the authors.
(3) Blinding
Blinding was assessed using the following criteria:
blinding of participants (yes/no/unclear or unspecified);
blinding of caregiver (yes/no/unclear or unspecified);
blinding of outcome assessment (yes/no/unclear or unspecified).
Data extraction and data entry
Both review authors independently extracted data, entered it onto the Review Manager software (RevMan 2003), and double checked it for accuracy.
Statistical analyses
Statistical analyses were carried out using Review Manager (RevMan 2003). Results are presented as summary relative risk with 95% confidence intervals. At present, only one trial is included, but when sufficient data become available, we will assess heterogeneity between trials using the I‐squared statistic. In the absence of significant heterogeneity, results will be pooled using a fixed‐effect model. If substantial heterogeneity is detected (I‐squared more than 50%), we will explore possible causes and perform subgroup analyses for the main outcomes. Heterogeneity that is not explained by subgroup analyses may be modelled using random‐effects analysis, if appropriate.
Sensitivity analyses
We planned to undertake a sensitivity analysis to explore the effects of trial quality based on concealment of allocation, by excluding studies with clearly inadequate allocation concealment (rated C). This analysis has not been done due to insufficient data.
Subgroup analyses
We planned to do subgroup analyses for the main outcomes by:
risk of women at trial entry: high, moderate, low, or unspecified;
preparations of garlic: fresh, different commercial preparations, unspecified.
This analysis has not been done due to insufficient data, but will be performed when more data become available.
Results
Description of studies
This review includes one study with 100 primigravid women at 28 to 32 weeks. The participants were women at moderate risk of pre‐eclampsia, as determined by a positive roll‐over test. This test is positive when there is a rise in diastolic blood pressure of at least 20 mmHg between two blood pressure readings, the first taken with the woman lying on her left side, the second taken with her in the supine position. The study was conducted in Iran. Details of the study can be found in the 'Characteristics of included studies' table.
One study was excluded because 29% of women were excluded from the analysis (Mose 2000).
Risk of bias in included studies
The one included study was of uncertain methodological quality as there was no information on the exact methods used for allocation generation or concealment. Follow up was reported for all women. A placebo was used for blinding of participants, although garlic odour was reported by one third of the women in the active group. There was no blinding of caregivers or outcome assessment.
Effects of interventions
Comparison: garlic versus placebo
There was no clear difference between the garlic and control groups in the relative risk (RR) of gestational hypertension (one trial, 100 women; RR 0.50, 95% confidence interval (CI) 0.25 to 1.00) or pre‐eclampsia (RR 0.78, 95% CI 0.31 to 1.93).
Women allocated garlic were more likely to report odour than those allocated placebo (RR 8.50, 95% CI 2.07 to 34.88), but there was no clear difference in other reported side‐effects.
The only other outcomes reported were caesarean section (RR 1.35, 95% CI 0.93 to 1.95), and perinatal mortality. There were no perinatal deaths in the study.
Discussion
The aim of this review was to assess the effects of garlic for prevention of pre‐eclampsia and its complications. As only one trial with 100 women was identified for inclusion in the review, there is insufficient evidence for any reliable conclusions about the potential benefits or harms of garlic.
A potential methodological problem for future studies is the issue of how to adequately blind participants. In the Iranian study reported here, garlic was given in tablet form, and the tablets were claimed to be 'odour controlled'. Nevertheless, one third of women taking the active treatment reported odours, compared to only 4% on placebo. Such odours may be acceptable in communities with a high dietary intake of garlic, but may be unacceptable where garlic intake is lower. Moreover, pregnant women may be particularly sensitive to odour, which may influence compliance with the intervention in communities with low dietary intake.
Another problem with research into the effects of garlic is assessing the bioavailability of active constituents after ingestion. It has been recommended that commercial garlic preparations should be systematically tested for their ability to release allicin (Lawson 2001). It was not reported whether the garlic preparation used for the trial in this review was tested.
Authors' conclusions
Implications for practice.
There is insufficient evidence to recommend increased garlic intake for preventing pre‐eclampsia and its complications.
Implications for research.
Garlic is a common constituent of food, especially in some parts of the world. There are reasonable theoretical grounds for suggesting garlic may help prevent pre‐eclampsia and its complications. Although garlic is associated with odour, other more serious side‐effects have not been reported. Further randomised trials to evaluate the effects of garlic for prevention of pre‐eclampsia are justified, particularly in parts of the world where garlic intake is common. For reliable conclusions any such trials should be large. If garlic products are used, it is important to determine the amount of main constituents released in various garlic preparations. Further research is needed to clarify if potential health benefits are specific to particular preparations, constituents, or doses.
What's new
| Date | Event | Description |
|---|---|---|
| 13 January 2010 | New search has been performed | Search updated. No new trials found. |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 3, 2006
| Date | Event | Description |
|---|---|---|
| 10 November 2008 | Amended | Edited contact details |
| 12 February 2008 | Amended | Converted to new review format. |
| 29 October 2007 | New search has been performed | Seach updated. No new trials found. |
| 6 March 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Our thanks to Dr JC Mose for providing us with unpublished data for his trial (Mose 2000).
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), one or more members of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. Additional searching for initial version of review
| Sources searched | Search strategy |
| The Cochrane Central Register of Controlled Trials (The Cochrane Library 2005, Issue 2) and EMBASE (1974 to April 2005). | We used combinations of the term 'garlic', 'allium sativum', 'alliin', 'allicin', and 'alliinase' with the CENTRAL and EMBASE search strategies listed in the generic protocol (see 'Generic Protocol 05' in 'Additional references' ). |
Data and analyses
Comparison 1. Garlic versus placebo/no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pre‐eclampsia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.31, 1.93] |
| 2 Gestational hypertension | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.25, 1.00] |
| 3 Caesarean section | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.93, 1.95] |
| 4 Maternal side‐effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Odour | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.5 [2.07, 34.88] |
| 4.2 Nausea | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.89, 17.91] |
| 5 Perinatal mortality | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
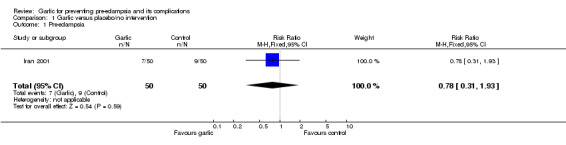
Comparison 1 Garlic versus placebo/no intervention, Outcome 1 Pre‐eclampsia.
1.2. Analysis.
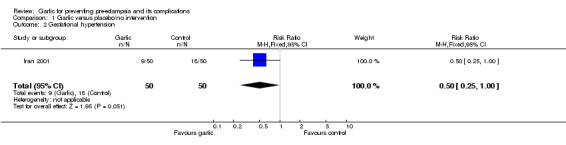
Comparison 1 Garlic versus placebo/no intervention, Outcome 2 Gestational hypertension.
1.3. Analysis.
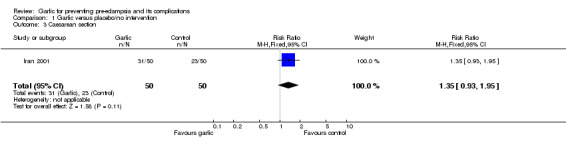
Comparison 1 Garlic versus placebo/no intervention, Outcome 3 Caesarean section.
1.4. Analysis.
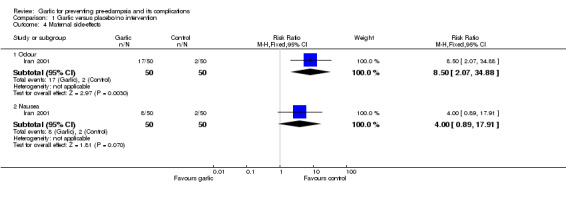
Comparison 1 Garlic versus placebo/no intervention, Outcome 4 Maternal side‐effects.
1.5. Analysis.
Comparison 1 Garlic versus placebo/no intervention, Outcome 5 Perinatal mortality.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Iran 2001.
| Methods | Randomisation: 'randomly divided into two groups'. No further information. Allocation concealment: no information. Blinding: only participants blinded. Follow up: no losses to follow up. | |
| Participants | 100 primigravid women between 28‐32 weeks' gestation with a positive roll‐over test. | |
| Interventions | Garlic: 2 garlic tablets/day ‐ total 800 mg/day (dry powder with 1000 mcg allicin in each tablet) for 8 weeks. Control: 2 placebo tablets of similar size and color for 8 weeks. | |
| Outcomes | Women: hypertension, pre‐eclampsia, weight gain (mean), caesarean section, garlic odour, side‐effects, plasma lipid levels, platelet aggregation. Babies: perinatal mortality, gestation at delivery (mean), birthweight (mean), 1 minute Apgar score (mean). | |
| Notes | 300 women screened with roll‐over test, 100 with positive test recruited. Tablets stated to be odour controlled by supplier. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Mose 2000 | 20 women (29%) were lost to follow up and so excluded from analysis after randomisation. Methods: permuted block randomisation. Participants: 70 women with a singleton pregnancy between 26‐36 weeks with normal blood pressure and a positive roll‐over test and MAP > 90 mmHg. Intervention: garlic capsules 1050 mg per day (3 x 350 mg/day) versus placebo for 2 weeks. Outcomes: pre‐eclampsia, changes in blood pressure, pregnancy duration, side‐effects, maternal and fetal morbidity and mortality, platelet count and aggregation. |
MAP: mean arterial pressure
Contributions of authors
The protocol for this review was based on the Generic Protocol of interventions for prevention pre‐eclampsia and its complications, which was drafted by Shireen Meher and Lelia Duley in consultation with the Prevention of Pre‐eclampsia Review Authors (PPRA). Shireen Meher and Lelia Duley independently assessed trials for inclusion. Shireen Meher extracted data and entered these into Review Manager software, and Lelia Duley double checked for accuracy.
Shireen Meher and Lelia Duley drafted the review.
Sources of support
Internal sources
The University of Liverpool, UK.
University of Oxford, UK.
External sources
Health Technology Assessment, UK.
Medical Research Council, UK.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Iran 2001 {published data only}
- Ziaei S, Hantoshzadeh S, Rezasoltani P, Lamyian M. The effect of garlic tablet on plasma lipids and platelet aggregation in nulliparous pregnants at high risk of pre‐eclampsia. European Journal of Obstetrics & Gynecology and Reproductive Biology 2001;99:201‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Mose 2000 {published and unpublished data}
- Mose JC. The effects of garlic (allium sativum) on platelet activity and blood pressure in pregnant women destined to develop preeclampsia. Journal of Obstetrics and Gynaecology Research 2000;26(Suppl 1):123. [Google Scholar]
Additional references
AHRQ 2000
- Agency for Healthcare Research and Quality. Garlic: effects on cardiovascular risks and disease, protective effects against cancer, and clinical adverse effects. Summary, Evidence Report/Technology Assessment: Number 20. AHRQ Publication No. 01‐E022, October 2000. Agency for Healthcare Research and Quality, Rockville, MD. http://www.ahrq.gov/clinic/epcsums/garlicsum.htm (accessed April 2005).
Ali 1999
- Ali M, Bordia T, Mustafa T. Effect of raw versus boiled aqueous extract of garlic and onion on platelet aggregation. Prostaglandins, Leukotrienes, and Essential Fatty Acids 1999;60(1):43‐7. [DOI] [PubMed] [Google Scholar]
Amagase 2001
- Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. Journal of Nutrition 2001;131:955S‐962S. [DOI] [PubMed] [Google Scholar]
Borek 2001
- Borek C. Antioxidant health effects of aged garlic extract. Journal of Nutrition 2001;131:1010‐5. [DOI] [PubMed] [Google Scholar]
Clarke 2003
- Clarke M, Oxman AD, editors. Cochrane Reviewers' Handbook 4.2.0 [updated March 2003]. In: The Cochrane Library, Issue 2, 2003. Oxford: Update Software. Updated quarterly.
Das 1995
- Das I, Khan NS, Soorana SR. Potent activation of nitric oxide synthase by garlic: a basis for its therapeutic applications. Current Medical Research and Opinion 1995;5(13):257‐63. [DOI] [PubMed] [Google Scholar]
DH 2002
- Department of Health, Scottish Executive Health Department and Department of Health, Social Services, Public Safety. Northern Ireland. Why mothers die. The sixth report on confidential enquiries into maternal deaths in the United Kingdom 2000‐2002. London: RCOG Press, 2002. [Google Scholar]
Generic Protocol 05
- Meher S, Duley L, Prevention of Pre‐eclampsia Cochrane Review authors. Interventions for preventing pre‐eclampsia and its consequences: generic protocol. Cochrane Database of Systematic Reviews 2005, Issue 2. [Art. No.: CD005301. DOI: 10.1002/14651858.CD005301] [Google Scholar]
Ide 1997
- Ide N, Lauu BHS. Aged garlic extract and its constituents inhibit Cu‐ induced oxidative modification of low density lipoprotein. Plantation Medicine 1997;63(3):263‐4. [DOI] [PubMed] [Google Scholar]
Lau 2001
- Lau BHS. Suppression of LDL oxidation by garlic. Journal of Nutrition 2001;131:985‐8. [DOI] [PubMed] [Google Scholar]
Lawson 2001
- Lawson LD, Wang ZJ, Papadimitriou D. Allicin release under simulated gastrointestinal conditions from garlic powder tablets employed in clinical trials on serum cholesterol. Plantation Medicine 2001;67:13‐8. [DOI] [PubMed] [Google Scholar]
NHBPEP 2000
- Gifford RW Jr, August PA, Cunningham G, Green LA, Lindhemier MD, McNellis D, et al. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. American Journal of Obstetrics and Gynecology 2000;183 Suppl:1‐22. [PubMed] [Google Scholar]
RevMan 2003 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2003.
Silagy 1994a
- Silagy CA, Neil HAW. A meta‐analysis of the effect of garlic on blood pressure. Journal of Hypertension 1994;12(4):463‐8. [PubMed] [Google Scholar]
Silagy 1994b
- Silagy C, Neil A. Garlic as a lipid lowering agent: a meta‐analysis. Journal of the Royal College of Physicians 1994;28(1):39‐45. [PMC free article] [PubMed] [Google Scholar]
Stevinson 2000
- Stevinson C, Pittler MH, Ernst E. Garlic for treating hypercholesterolemia: a meta‐analysis of randomized clinical trials. Annals of Internal Medicine 2000;133:420‐9. [DOI] [PubMed] [Google Scholar]
Warshafsky 1993
- Warshafsky S, Kamer RS, Sivak SL. Effect of garlic on total serum cholesterol. A meta‐analysis. Annals of Internal Medicine 1993;119(7):599‐605. [DOI] [PubMed] [Google Scholar]
WHO 1988
- World Health Organization International Collaborative Study of Hypertensive Disorders in Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. American Journal of Obstetrics and Gynecology 1988;158:80‐3. [PubMed] [Google Scholar]


