Abstract
Background
Simultaneous bilateral training, the completion of identical activities with both arms simultaneously, is one intervention to improve arm function and reduce impairment.
Objectives
To determine the effects of simultaneous bilateral training for improving arm function after stroke.
Search methods
We searched the Cochrane Stroke Trials Register (last searched August 2009) and 10 electronic bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 3, 2009), MEDLINE, EMBASE, CINAHL and AMED (August 2009). We also searched reference lists and trials registers.
Selection criteria
Randomised trials in adults after stroke, where the intervention was simultaneous bilateral training compared to placebo or no intervention, usual care or other upper limb (arm) interventions. Primary outcomes were performance in activities of daily living (ADL) and functional movement of the upper limb. Secondary outcomes were performance in extended activities of daily living and motor impairment of the arm.
Data collection and analysis
Two authors independently screened abstracts, extracted data and appraised trials. Assessment of methodological quality was undertaken for allocation concealment, blinding of outcome assessor, intention‐to‐treat, baseline similarity and loss to follow up.
Main results
We included 18 studies involving 549 relevant participants, of which 14 (421 participants) were included in the analysis (one within both comparisons). Four of the 14 studies compared the effects of bilateral training with usual care. Primary outcomes: results were not statistically significant for performance in ADL (standardised mean difference (SMD) 0.25, 95% confidence interval (CI) ‐0.14 to 0.63); functional movement of the arm (SMD ‐0.07, 95% CI ‐0.42 to 0.28) or hand (SMD ‐0.04, 95% CI ‐0.50 to 0.42). Secondary outcomes: no statistically significant results. Eleven of the 14 studies compared the effects of bilateral training with other specific upper limb (arm) interventions. Primary outcomes: no statistically significant results for performance of ADL (SMD ‐0.25, 95% CI ‐0.57 to 0.08); functional movement of the arm (SMD ‐0.20, 95% CI ‐0.49 to 0.09) or hand (SMD ‐0.21, 95% CI ‐0.51 to 0.09). Secondary outcomes: one study reported a statistically significant result in favour of another upper limb intervention for performance in extended ADL. No statistically significant differences were found for motor impairment outcomes.
Authors' conclusions
There is insufficient good quality evidence to make recommendations about the relative effect of simultaneous bilateral training compared to placebo, no intervention or usual care. We identified evidence that suggests that bilateral training may be no more (or less) effective than usual care or other upper limb interventions for performance in ADL, functional movement of the upper limb or motor impairment outcomes.
Keywords: Adult, Humans, Recovery of Function, Stroke Rehabilitation, Activities of Daily Living, Arm, Motor Activity, Paresis, Paresis/rehabilitation, Randomized Controlled Trials as Topic, Stroke, Stroke/physiopathology
Simultaneous bilateral training for improving arm function after stroke
After a stroke, arm problems are common and their recovery is often limited. This review of 18 studies with 549 relevant participants looked at whether performing identical activities with both arms at the same time (simultaneous bilateral training) could improve performance in daily (or extended daily) activities, movement of the arm and/or reduce arm impairments. In comparison with usual care, bilateral training had no effect on performance in activities of daily living, functional movement of the arm or hand, performance in extended activities of daily living or motor impairment outcomes. In comparison with other arm interventions, bilateral training had no effect on performance in activities of daily living, functional movement of the arm or hand or motor impairment outcomes. One study found that people who undertook bilateral training showed less improvement in performance in extended activities of daily living than people doing another arm intervention. The evidence in this area is limited. Further research is needed to determine the effects of bilateral training.
Background
Stroke is the main cause of permanent and complex long‐term disability in adults and has implications for patients, caregivers, health professionals and society in general (Feigin 2003; Kwon 2004; Langhorne 2003; van der Lee 1999). At present there is no routinely available curative treatment for stroke patients and therefore rehabilitation interventions are relied upon to maximise patient outcomes (Langhorne 2003).
Upper limb (arm) hemiparesis is widely reported in the literature as one of the primary impairments following stroke (Johnson 2001; Page 2002; van der Lee 2001). While many patients recover ambulatory function after dense hemiplegia, restoration of arm motor skills is often incomplete (Johnson 2001; Page 2001). It has been reported that the paretic arm remains without function in between 30% (Heller 1987) to 66% (Sunderland 1989; Wade 1983) of hemiplegic stroke patients, when measured six months post‐stroke. Furthermore, only 5% (Heller 1987) to 20% (Nakayama 1995) of individuals achieve complete functional recovery. Nevertheless, return of voluntary arm movements is one of the most important goals during stroke rehabilitation in order to avoid long‐term disability in activities of daily living (ADL), social and occupational activities, and depression (Broeks 1999).
The aim of rehabilitation is to reduce impairment and minimise disability (Page 2001) and a number of interventions to achieve these aims and improve arm function after stroke have been suggested (Barreca 2003; van der Lee 2001). The effectiveness of some of these interventions has been, or is in the process of being reviewed within other Cochrane systematic reviews: electromyographic (EMG) biofeedback (Woodford 2004), electrostimulation (Pomeroy 2006), electromechanical and robotic‐assisted arm training (Merholz 2008), constraint‐induced movement therapy (Sirtori 2003) and repetitive task training (French 2006). However, rigorous systematic evaluation is still required to investigate the effectiveness of simultaneous bilateral training.
Simultaneous bilateral training involves the execution of identical activities with both arms simultaneously but independently (Mudie 2000). Beneficial effects of bilateral training are assumed to arise from an interlimb coupling effect, in which movement of the non‐paretic arm facilitates movements in the impaired limb (Kelso 1979; Morris 2008; Swinnen 2002). Cauraugh 2008 and Stinear 2008 further suggest that bilateral practice of synchronous movements with the paretic and non‐paretic limbs allows activation of the intact hemisphere to facilitate activation of the damaged hemisphere through enhanced interhemispheric inhibition. Bilateral training is often combined with other interventions, such as electrostimulation or assistive technology, to assist the affected arm to undertake the simultaneous movements.
Two reviews (Cauraugh 2005; Stewart 2006) report favourable effects of bilateral training. These reviews, however, included studies other than randomised controlled trials (RCTs) and both acknowledge that there are inconsistent findings across bilateral movement studies. A further, more recent narrative review of bilateral training (McCombe Waller 2008) acknowledges that bilateral studies have not shown improvements in all patients and that bilateral training has not been shown to be more beneficial than other training approaches. However this review was not systematic and included a range of study designs, including single case studies. We therefore sought to undertake a complete, up‐to‐date, systematic review of randomised controlled trials to determine the effects of bilateral training compared to no treatment, placebo or other interventions for improving arm function after stroke.
Objectives
To determine the effects of simultaneous bilateral training for improving arm function after stroke compared with:
placebo or no intervention;
usual care;
other specific upper limb (arm) interventions or programmes.
Methods
Criteria for considering studies for this review
Types of studies
We included controlled trials where participants had been randomly assigned (that is, each participant had an equal chance of being allocated a particular treatment as another participant). Random allocation could have been completed by having computer‐generated random numbers, or using sequentially‐numbered opaque sealed envelopes. We only included the first phase of cross‐over studies to exclude any carry‐over or learning effects. We excluded quasi‐randomised trials from this review. We included trials with or without blinding of participants, treating therapist(s) and assessor(s). One of the intervention groups must have included simultaneous bilateral training (see definition in Types of interventions) and another group either a no‐treatment group, a placebo group, usual ('conventional' or 'traditional') care, or another specific upper limb (arm) intervention or programme.
Types of participants
We included trials of participants with a clinical diagnosis of stroke ‐ 'a syndrome of rapidly developing symptoms and signs of focal, and at times, global, loss of cerebral function lasting more than 24 hours or leading to death, with no apparent cause other than that of vascular origin' (WHO 1989) ‐ regardless of time since onset, initial arm impairment, ability to follow instructions, co‐morbidities, previous strokes or location of stroke. We included studies that enrolled participants with other neurological disorders if more than 75% of participants were stroke patients.
Types of interventions
The included studies had to include simultaneous bilateral training. The definition of simultaneous bilateral training used was; 'when a motor activity is completed at the same time by both upper limbs independently' (Mudie 2000). We excluded trials that investigated simultaneous bilateral training in conjunction with another intervention (e.g. assistive technology such as machine, a robot or electrical stimulation) and compared to a control group, for example simultaneous bilateral training and electrical stimulation compared to a control group. This was to ensure that the treatment effect under investigation was bilateral training. However, we did include studies where assistive technology was given to both an intervention (bilateral training) and control (unilateral training) group, as in these cases it is the bilateral component of the training which is the active treatment under investigation, and not the assistive technology. Similarly, we also included trials which investigated bilateral training completed using assistive technology which was compared with a control intervention, also completed using assistive technology.
We included any duration or intensity of programme.
For studies comparing simultaneous bilateral training with 'usual care', we accepted any control intervention which was considered by the original trial authors to be a normal or usual component of stroke rehabilitation. We documented the description of 'usual care', where this was provided by the authors.
Types of outcome measures
The primary or initial aim of many upper limb interventions (including bilateral training) is often to improve functional movement and reduce impairment. However, it is debatable how meaningful these aspects are to individual patients. A more important goal for patients is likely to be to improve their ability to participate in and achieve independence with activities of daily living. Additionally, this is the over‐arching aim of most rehabilitation interventions. Since the key motivation of this review is to improve patient care and ensure meaningful outcomes, we therefore felt it was it appropriate to have two primary outcomes of interest: (1) performance in activities of daily living, and (2) functional movement of the upper limb.
We anticipated that the studies would use and report a large variety of different outcome measures relevant to the primary and secondary outcomes of this review. Therefore, for each outcome of interest (primary and secondary) we attempted to identify and list all the common, specific measurement tools or scales that could be included. If we identified a study which reported more than one measurement tool or scale which addressed the same outcome, we used the scale listed earliest in our lists. If a study did not use any of the measures in the list, but measured the outcome using a different measurement tool or scale we included and documented this. These hierarchical lists are given below.
Primary outcomes
Performance in activities of daily living (ADL) (including feeding, dressing, bathing, toileting, simple mobility and transfers). Common outcome measures: global measures of activities of daily living, such as the Barthel ADL Index (Mahoney 1965), Rivermead ADL assessment (Whiting 1980), Rivermead Motor Ability scale (Collen 1991), Rankin Scale (Bonita 1988), Functional Independence Measure (FIM) (Keith 1987), Katz Index of Activities of Daily Living (Katz 1970), and Rehabilitation Activities Profile (Van Bennekom 1995).
Functional movement of the upper limb (such as measures of active movement, co‐ordination, dexterity, manipulation, grasp/grip/pinch). Common outcome measures: Action Research Arm Test (Lyle 1981), Motor Assessment Scale ‐ upper arm function or combined arm score (Carr 1985), Frenchay Arm Test (Heller 1987), Wolf Motor Function Test (Wolf 2001), Upper Extremity Function Test (Carroll 1967), Functional Test of the Hemiparetic Upper Extremity (Wilson 1984), Box and Block Test (Mathiowetz 1985), Upper extremity performance test for the elderly (TEMPA) (Desrosiers 1993), Chedoke Arm and Hand Activity Inventory (Barreca 2005), Sodring Motor Evaluation of Stroke Patients ‐ arm section (Sodring 1995), University of Maryland Arm Questionnaire for Stroke (Whitall 2000), Motor Activity Log (Taub 1993), Motor Assessment Scale ‐ hand movement or advanced hand movement scores (Carr 1985), Jebsen Hand Function Test (Jebsen 1969), Nine Hole Peg Test (Kellor 1971) and Purdue Peg Test (Tiffin 1948).
Secondary outcomes
Performance in extended activities of daily living (including shopping, household tasks). Common outcome measures: Nottingham Extended Activities of Daily Living (Nouri 1987), Rivermead Extended Activities of Daily Living (Rossier 2001), Frenchay Activities Index (Holbrook 1983).
Motor impairment of the arm (measures/scales of upper limb impairment, muscle strength, muscle tone). Common outcome measures: Fugl‐Meyer Assessment of Sensorimotor Recovery after Stroke (upper limb section) (Fugl‐Meyer 1975), Motricity Index (Demeurisse 1980), Rivermead Motor Assessment (arm section) (Lincoln 1979), Motor Club Assessment (Ashburn 1982), Ashworth Scale (Ashworth 1964)/Modified Ashworth Scale (Bohannon 1987), MRC scale (MRC 1975), dynamometer scores (including Jamar) (Bohannon 1987), kinematic measures (e.g. movement time, movement efficiency, movement speed, spatial accuracy, velocity).
Additional outcomes
Adverse events (e.g. death, shoulder pain/subluxation).
We used outcomes from the end of the intervention period for analysis.
Data collected at follow‐up points after the end of the intervention period are important for assessing whether any treatment effects are sustained. However, for this review the primary aim was to determine whether bilateral training had any immediate beneficial treatment effect. If bilateral training is found to have a beneficial treatment effect we will consider including follow‐up data within a future update of this review.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module.
We searched the Cochrane Stroke Group Trials Register, which was last searched by the Managing Editor in August 2009. In addition, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 3, 2009), MEDLINE (1966 to August 2009 ) (Appendix 1), EMBASE (1980 to August 2009) (Appendix 2), CINAHL (1982 to August 2009) (Appendix 3) and AMED (1985 to August 2009) (Appendix 4). We also searched the following occupational therapy and physiotherapy databases: OTseeker (http://www.otseeker.com/) (August 2009), Physiotherapy Evidence database (PEDro, http://www.pedro.org.au), Chartered Society of Physiotherapy Research Database (August 2009) and REHABDATA (http://www.naric.com/research/rehab/default.cfm) (August 2009).
We developed search strategies in consultation with the Cochrane Stroke Group's Trials Search Co‐ordinator to avoid duplication of effort.
In an effort to identify further published, unpublished and ongoing trials we:
checked reference lists of all included studies and review papers;
searched ClinicalTrials.gov (http://www.clinicaltrials.gov/) and the National Research Register Archive (http://portal.niht.ac.uk/Pages/NRRArchive.aspx) (last searched February 2009);
used Science Citation Index Reference Search to track relevant papers (last searched February 2009);
searched ProQuest Dissertations and Theses (PQDT) dissertation abstracts (last searched February 2009); and
searched Index to Theses ‐ dissertation abstracts (last searched September 2009).
Data collection and analysis
Identification of relevant trials
One review author (FC) read the titles of the identified references and eliminated any obviously irrelevant studies. We obtained the abstracts for the remaining studies and then, based on the inclusion criteria (types of studies, types of participants, aims of interventions and outcome measures), two review authors (FC and FvW or AP) independently ranked these as 'possibly relevant' or 'definitely irrelevant'. If both review authors identified a trial as 'definitely irrelevant' we excluded it at this point, but included all other trials at this stage. We then held consensus discussions, with the assistance of additional review authors where appropriate (FvW, AP and JM), concerning the inclusion of the remaining studies, based on the abstracts, and excluded further studies. We then retrieved the full text of the remaining studies, which two authors (FC and FvW or AP) independently reviewed and classified as 'include' or 'exclude'. We excluded trials classified as 'exclude' by both review authors. Where disagreement occurred between the two review authors, or a decision could not be made, the authors reached consensus through discussion and, where necessary, sought the opinion of a third review author.
Documentation of methodological quality
Two review authors independently assessed the methodological quality of the studies using a standard critical appraisal assessment form. Assessment of the quality of studies focused on potential areas of bias within the studies, as this has been shown to affect the estimation of effectiveness of interventions. We considered and documented, where the information was provided, the following:
methods, including method of randomisation;
allocation concealment;
blinding of outcome assessor;
intention‐to‐treat;
baseline similarity;
number of patients lost to follow up;
other sources of bias.
Consideration of blinding of participants and therapists led to the conclusion that blinding would not be possible in these types of trials; consequently we did not document this information.
The two review authors resolved any disagreements through discussion, involving a third review author if necessary.
Data extraction
Two review authors independently performed the data extraction using a standard data extraction form. Where the information was provided in the studies we documented:
the trial setting;
participant details (including age, gender, type of stroke, time since stroke);
the inclusion and exclusion criteria;
the duration and/or intensity of the intervention;
a brief description of the bilateral training intervention (including movement activities completed, number of repetitions, feedback, goals);
the comparison intervention;
the outcomes.
Comparisons to be made
Simultaneous bilateral training versus placebo or no intervention.
Simultaneous bilateral training versus usual care.
Simultaneous bilateral training versus other specific upper limb interventions or programmes.
Where studies included another intervention as an adjunct to bilateral training, which was also delivered to the control group, we included these studies in the appropriate comparison groups as listed above, regardless of the adjunct intervention. For example, comparisons of (i) robot‐assisted simultaneous bilateral training versus robot‐assisted unilateral training or (ii) simultaneous bilateral training plus electrical stimulation versus unilateral training plus electrical stimulation would both be included in comparison 3 (Simultaneous bilateral training versus other specific upper limb interventions). We completed a sensitivity analysis to explore the effect of including studies where the simultaneous bilateral training was combined with another intervention.
Data analysis
For each comparison we used the study results for performance in activities of daily living, measures of functional movement, measures of motor impairment, and adverse effects if documented. We used the Cochrane Review Manager software, RevMan 5, for all analyses (RevMan 2008).
We presented all outcome measures analysed as continuous data. We calculated standardised mean differences (SMD) and 95% confidence intervals (CI). We determined heterogeneity using the I2 statistic (we considered I2 greater than 50% as substantial heterogeneity). If I2 was less than or equal to 50% we used a fixed‐effect meta‐analysis. If I2 was greater than 50%, we explored the individual trial characteristics to identify potential sources of heterogeneity. We then performed meta‐analysis using both fixed‐effect and random‐effects modelling to assess sensitivity to the choice of modelling approach.
We planned to complete subgroup analyses (following the Deeks method; Deeks 2001) on differences between acute (time at entry to trials less than three months post‐stroke) and chronic (time at entry to trials equal to or more than three months) patients (at entry to the trials) and duration and number of repetitions of the programme (intervention for less than four weeks and intervention equal to or more than four weeks, intervention less than five days per week or equal to or more than five days per week). We planned to undertake these subgroup analyses where data permitted (sufficient data were considered to be more than five trials reporting the information) and undertaken on the primary outcome only. We also planned to complete sensitivity analysis based on methodologicaI quality of studies (i.e. method of randomisation, concealment of randomisation, blinding of outcome assessor, intention‐to‐treat analysis).
Results
Description of studies
Results of the search
Our search strategy identified 6809 titles. After elimination of duplicates and obviously irrelevant studies we were left with 296 'possibly relevant' abstracts. We obtained these 296 abstracts and two review authors (FC and FvW or AP) independently assessed them for inclusion. Where disagreements or uncertainties arose, we held consensus discussions involving additional authors (FvW, AP or JM) where required. We assessed 82 abstracts as 'include' and we obtained the full papers for these 82 studies. Of these 82 full papers, we excluded 61 (see Excluded studies for further details); there was insufficient information to determine inclusion eligibility for five papers (referring to four studies) (listed in Characteristics of studies awaiting classification); leaving 16 studies for inclusion.
In addition, we identified four ongoing trials from searching additional databases. Contact with the principal investigator led to the identification of a relevant publication from one of these trials (Stoykov 2009). We identified published data relating to a further ongoing study (Lin 2009b) from a journal online (ahead of print). We assessed these studies as relevant for inclusion. Thus, we included a total of 18 studies in this review.
Contact with authors identified that two of the included studies (Lin 2009a; Lin 2009b) are still recruiting participants. However, there are published data available on both these ongoing studies, and we therefore decided that it was appropriate to include these preliminary data within this review. Future updates of this review may therefore need to include new data and information pertaining to these studies. As we have included preliminary data from these trials, they are listed as 'included studies' and are not included in the Characteristics of ongoing studies table.
Included studies
Eighteen studies (549 randomised stroke participants; 530 participants relevant to this review (some stroke participants were randomised to additional groups not relevant to this review)) met the inclusion criteria for this review (Cauraugh 2002; Cauraugh 2003a; Cauraugh 2005; Cauraugh 2008; Chang 2006; Desrosiers 2005; Dickstein 1993; Harris‐Love 2005; Kilbreath 2006; Lin 2009a; Lin 2009b; Luft 2004; Lum 2006; Morris 2008; Mudie 2001: Acute; Platz 2001; Stoykov 2009; Summers 2007). Mudie 2001 reports data divided into two groups ‐ acute and chronic. These are presented as Mudie 2001: Acute and Mudie 2001: Chronic.
A brief overview of the studies is presented below. Full descriptions of the included studies can be found in the Characteristics of included studies table and in Table 3 (Demographics of included participants).
Table 1.
Demographics of included participants
| Study | Number of participants | Age | Gender (F/M) | Time since stroke | Side of stroke (L/R) | Type of stroke |
| Cauraugh 2002 | 25 (only 20 relevant to this review) | Mean: 63.7 years (SD not stated) | 4/21 | 39.1 months | 13/12 | Not stated |
| Cauraugh 2005 | 21 | Unilateral group mean: 63.29 years (SD 10.81 years) Bilateral group mean: 69.37 years (SD 10.14 years) | Unilateral: 4/6 Bilateral: 6/5 | Unilateral: 3.57 years (SD 2.42 years) Bilateral: 4.73 years (SD 3.52 years) | Unilateral: 4/6 Bilateral: 2/9 | Not stated |
| Cauraugh 2008 | 16 | Unilateral: group mean: 66.6 years (SD 12.35 years) Bilateral group mean: 65.04 years (SD 12.47 years) | Unilateral: 3/5 Bilateral: 3/5 | Unilateral: 4.2 years (SD 9.13 years) Bilateral: 1.41 years (SD 0.89 years) | Unilateral: 5/3 Bilateral: 3/5 | Not stated |
| Cauraugh 2003a | 20 | Mean: 63.03 years | 4/16 | 33.86 months | 11/9 | Not stated |
| Chang 2006 | 20 | Mean: 56 years (SD 10.54 years) | 3/17 | 404.7 days (SD 565.06 days) 12 days to 6 years | 11/9 | 3 haemorrhagic, 17 infarct |
| Desrosiers 2005 | 41 | Usual care: 74.3 years (SD 10.1 years) Bilateral group: 72.2 years (SD 10.8 years) | Usual care: 11/10 Bilateral: 11/9 | Usual care: 35.4 days (SD 33.7 days)* Bilateral: 34.2 days (SD 34.4 days)* *times reported are times from acute admission to admission to rehabilitation unit, not time since stroke | Usual care: 10/11 Bilateral: 13/7 | Usual care: 21 ischaemic, 11 lacunar, 8 sylvian, 2 vertebrobasilar Bilateral: 19 ischaemic, 1 haemorrhagic, 9 lacunar, 7 sylvian, 3 vertebrobasilar |
| Dickstein 1993 | 25 | Mean: 73 years (SD 1.45 years) | 11/14 | 2.5 months (SD 2.22 months) | 12/13 | 24 thromboembolic brain infarction in territory of internal carotid artery 1 head trauma |
| Harris‐Love 2005 | 32 | Mean: 57 years (SD 14 years) | 17/15 | Median: 1.95 years | 19/13 | Unilateral ischaemic stroke |
| Kilbreath 2006 | 13 | Mean : 67.9 years (SD 8.3 years) | 5/8 | 36.1 months (SD 18.0 months) | 11/2 | Not stated |
| Lin 2009a | 60 | Usual care: 50.70 years (SD 13.93 years) Other upper limb intervention (CIT): 55.28 years (SD 9.34 years) Bilateral : 51.58 years (SD 8.67 years) | Usual care: 9/11 CIT: 9/11 Bilateral: 8/12 | Usual care: 21.90 months (SD 20.51 months) CIT: 21.25 months (SD 21.59 months) Bilateral: 18.50 months (SD 17.40 months) | Usual care: 8/12 CIT: 12/8 Bilateral: 9/11 | Not stated |
| Lin 2009b | 33 | Usual care: 55.5 years (SD 13.17 years) Bilateral : 52.08 years (SD 9.60 years) | Usual care: 8/9 Bilateral: 6/10 | 13.12 months (SD 8.13 months) 13.94 months (12.73 months) | Usual care: 8/9 Bilateral: 9/7 | Not stated |
| Luft 2004 | 21 | DMTE: 59.6 years (SD 10.5 years) BATRAC : 63.3 years (SD 15.3 years) | DMTE: 7/5 BATRAC: 2/7 | DMTE: median: 45.5 months (IQR 22.6 to 66.3 months) BATRAC: median: 75 months (IQR 37.9 to 84.5 months) | DMTE: 4/8 BATRAC: 3/6 | DMTE: 6 cortical, 4 subcortical, 2 brainstem BATRAC: 6 cortical, 2 subcortical, 1 brainstem |
| Lum 2006 | 30 (only 14 relevant to this review) | Unilateral mean: 69.8 years (SEM 4.0 years) Bilateral mean: 72.2 years (SEM 11.7 years) | Unilateral: 4/5 Bilateral: 3/2 | Unilateral: 10 weeks (SEM 1.9 weeks) Bilateral: 6.2 weeks (SEM 1.0 weeks) | Unilateral: 4/5 Bilateral: 2/3 | Not stated |
| Morris 2008 | 106 | Unilateral mean: 67.8 years (SD 9.9 years) Bilateral mean: 67.9 years (SD 13.1 years) | Unilateral: 23/27 Bilateral: 22/34 | Unilateral: 23.2 days (SD 5.7 days) Bilateral: 22.6 days (5.6 days) | Unilateral: 23/27 Bilateral: 29/27 | Unilateral: 6 ischaemic, 44 haemorrhagic; 2 TACS, 28 PACs, 18 lacunar, 2 posterior circulation Bilateral: 3 ischaemic, 53 haemorrhagic: 3 TACs, 31 PACs, 21 lacunar, 1 posterior circulation |
| Mudie 2001: Acute | 18 | Unilateral mean: 77.9 years (SD 9.2 years) Bilateral mean: 71.9 years (SD 5.8 years) | Unilateral: 5/4 Bilateral: 3/6 | Unilateral: 1.8 months (SD 0.6 months) Bilateral: 1.9 months (SD 1.1 months) | Unilateral: 4/5 Bilateral: 3/6 | Unilateral: 5 MCA, 1 basal ganglia, 3 lacunar infarct Bilateral: 3 MCA, 1 tumour, 2 cortical, 1 pontine, 1 lacunar, 1 occluded internal carotid artery |
| Mudie 2001: Chronic | 18 | Unilateral mean: 65.7 years (SD 13.1 years) Bilateral mean: 64.6 years (SD 10.9 years) | Unilateral: 2/7 Bilateral: 0/9 | Unilateral: 90.0 months (SD 117 months) Bilateral: 34.2 months (SD 37.2 months) | Unilateral: 5/4 Bilateral: 3/6 | Unilateral: 7 MCA, 2 lacunar Bilateral: 6 MCA, 1 subcortical, 1 occluded internal carotid artery, 1 after clipped aneurysm |
| Platz 2001 | 14 | Mean: 55.9 years (SD 11.6 years) | 5/9 | Not stated | 7/7 | 14 ischaemic in the territory of the MCA: ‐ 6 basal ganglia and/or internal capsule ‐ 2 pure subcortical infarct ‐ 4 cortical and subcortical involvement ‐ 2 cortical, subcortical and basal ganglia |
| Stoykov 2009 | 24 | Unilateral mean: 64.75 years (SD 11.1 years) Bilateral mean: 63.8 years (SD 12.6 years) | Unilateral: 5/7 Bilateral: 3/9 | Unilateral: 10.2 years (SD 10.1 years) Bilateral: 9.5 years (SD 5.4 years) | Not stated | Unilateral: 2 cortical, 4 subcortical, 6 both Bilateral: 5 cortical, 2 subcortical, 5 both |
| Summers 2007 | 12 | Unilateral mean: 60 years (SD 14 years) Bilateral mean: 63.16 years (SD 16 years) | Unilateral: 3/3 Bilateral: 4/2 | Unilateral: 4.0 years (SD 3.1 years) Bilateral: 6.3 years (SD 5.2 years) | Unilateral: 1/4/1 bilateral MCA Bilateral: 2/4 | Unilateral: 1 lacunar infarct, 1 cerebellar intracerebral, 1 ischaemic, 1 frontal/temporal, 2 MCA Bilateral: 2 MCA, 1 cortical lesion, 1 internal capsule, 2 ischaemic |
BATRAC: bilateral training with auditory cueing CIT: constraint‐induced therapy DMTE: dose‐matched therapeutic exercises F: female IQR: interquartile range L: left M: male MCA: middle cerebral artery PACS: partial anterior circulation syndrome R: right SD: standard deviation SEM: standard error of the mean TACS: total anterior circulation syndrome
Design
Fourteen of the 18 included studies were randomised controlled trials (Cauraugh 2002; Cauraugh 2003a; Cauraugh 2005; Cauraugh 2008; Desrosiers 2005; Lin 2009a; Lin 2009b; Luft 2004; Lum 2006; Morris 2008; Mudie 2001: Acute; Platz 2001; Stoykov 2009; Summers 2007). Four of the 18 included studies (Chang 2006 (20 participants); Dickstein 1993 (25 participants); Harris‐Love 2005 (32 participants); Kilbreath 2006 (13 participants)) were randomised cross‐over design studies with random allocation to the order of treatment sequence. These studies are not traditional RCTs in the sense that participants are randomly allocated to one (or more) groups. Within these studies the participants were randomised to different treatment orders. No data were available for the first phases only, therefore these four studies are not incorporated in any of the analyses. Despite not being appropriate for incorporation in the data analysis these studies met the inclusion criteria for this review. Details of these four cross‐over studies are included within the Characteristics of included studies table, Table 3 (Demographics of included participants) and Figure 1 (Methodological quality summary). However, in order to avoid any confusion, these four cross‐over studies are not discussed within the following text.
Figure 1.
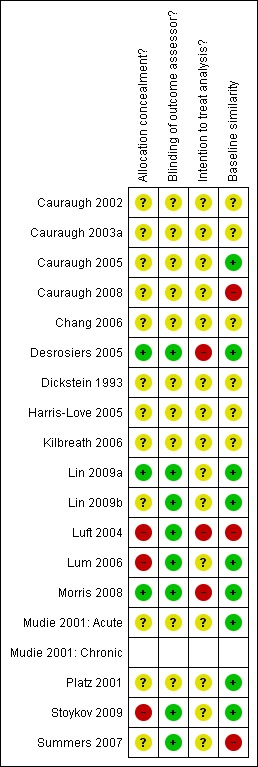
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
All following text descriptions therefore apply to the 14 included RCTs (421 participants) for which we have extracted and analysed data.
Comparison groups
Four of the 14 studies included in the analyses compared the effects of bilateral training with usual care (Desrosiers 2005; Lin 2009a; Lin 2009b; Luft 2004).
Eleven of the 14 studies included in the analyses (Cauraugh 2002; Cauraugh 2003a; Cauraugh 2005; Cauraugh 2008; Lin 2009a; Lum 2006; Morris 2008; Mudie 2001: Acute; Mudie 2001: Chronic; Platz 2001; Stoykov 2009; Summers 2007) compared the effects of bilateral training with another upper limb intervention. All of these studies except Lin 2009a compared bilateral training to unilateral training, which we classed as another upper limb intervention. Lin 2009a compared bilateral training to constraint‐induced therapy.
Lin 2009a is included in both of these analyses, as it compared three groups and reports data relevant to bilateral training compared to usual care and bilateral training compared to other upper limb programme or intervention.
Follow up
All 14 studies assessed participants after intervention completion and these follow‐up data are used in the analysis. Two of the 14 studies (Lum 2002; Morris 2008) additionally completed follow up after this point (18 weeks and six months respectively), but these data have not been used in the analyses.
Sample sizes
On average, included studies randomised 30 stroke patients into their trial prior to attrition. This ranges from just 12 participants (Summers 2007) to 106 (Morris 2008). All studies except Lin 2009a and Morris 2008 included less than 50 participants.
Setting
Of the 14 included studies, three were carried out in Australia (Desrosiers 2005; Mudie 2001: Acute; Summers 2007), one in Germany (Platz 2001), one in the UK (Morris 2008), seven in the USA (Cauraugh 2002; Cauraugh 2003a; Cauraugh 2005; Cauraugh 2008; Lum 2006; Luft 2004; Stoykov 2009) and two in Taiwan (Lin 2009a; Lin 2009b).
Participants
We have provided demographics of included participants in Table 3. Of the randomised participants 39% were female. The lowest reported mean age was 52.14 years (Lin 2009a) and the highest mean age was 74.9 years (Mudie 2001: Acute). Across the studies time since stroke varied from a mean of 22.9 days (Morris 2008) to a mean of 9.85 years (Stoykov 2009). One study did not report time since stroke (Platz 2001). Side of stroke was reported in all studies except Stoykov 2009; 257 participants had a left hemisphere stroke and 267 participants had a right hemisphere stroke. We were unable to extract information relating to initial upper limb impairment due to the limited information provided by some of the studies.
Interventions
The interventions investigated in the included studies varied in terms of types of bilateral tasks completed, duration of interventions and use of a combination of interventions. We provide details of the individual interventions, including types of tasks and durations in the Characteristics of included studies table. Some of the key differences are summarised below.
The interventions of 12 of the 14 included studies each concentrated on one specific upper limb movement or task: four studies (Cauraugh 2002; Cauraugh 2003a; Cauraugh 2005; Cauraugh 2008) were aimed at wrist/finger extension, and one trial (Mudie 2001: Acute) was specific to isometric contractions of wrist extension. In one study (Lum 2006) the intervention involved some form of bilateral reaching, while in one trial (Luft 2004) the intervention involved bilateral pushing and pulling, and in another trial (Summers 2007) the intervention was a bilateral dowel placement task.
The interventions of six of the 14 included studies involved more than one upper limb movement or task: Morris 2008, Platz 2001 and Stoykov 2009 completed four, three and six separate bilateral tasks respectively, and Desrosiers 2005 assessed a package of interventions, which included bilateral tasks in addition to unilateral and bimanually different tasks. Lin 2009a investigated simultaneous movements during a number of functional tasks in symmetric or alternating patterns. Lin 2009b focused on simultaneous bilateral completion of functional tasks with symmetric patterns.
Thirteen of the 14 included studies investigated the effect of training over a training period (rather than single training and evaluation sessions); the training period varied from four days (Cauraugh 2008) to eight weeks (Stoykov 2009). The remaining RCT (Mudie 2001: Acute) did not have a training period as they used one single training and evaluation session.
Five of the 14 studies provided a further intervention as an adjunct to treatment in both the bilateral training and control groups. Four studies (Cauraugh 2002; Cauraugh 2003a; Cauraugh 2005; Cauraugh 2008) included EMG‐triggered neuromuscular stimulation, delivered to both the bilateral training and control group. One trial (Lum 2006) used a robot to assist movement of the affected limb in both the bilateral training and control (unilateral) groups.
Luft 2004 evaluated bilateral training in conjunction with auditory cueing; auditory cueing was not provided to the control group. This study was included as auditory cueing was not assessed by the authors to be an 'assistive technology', but to be a mode of delivery of an intervention. Stoykov 2009 also used rhythmic auditory cueing as an adjunct, however, this was not used for completion of all tasks and was provided to both groups.
Outcome measures
As anticipated, a variety of outcome measures were used by the included studies. All of the studies included a measure of motor impairment. It was apparent to us that, due to differences in the measures, it would be inappropriate to combine some of the outcomes together within analyses. Therefore, following data extraction we further categorised functional movement of the upper limb into the following subgroups: (1) arm functional movement, and (2) hand functional movement, and categorised motor impairment of the upper limb into the following subgroups: (1) motor impairment scales, (2) temporal outcomes, (3) spatial outcomes and (4) strength outcomes.
The outcome measures selected from each of the 14 individual studies included in the analysis, for each outcome category are detailed below.
Primary outcomes
Performance in activities of daily living (ADL)
Functional Independence Measure (Desrosiers 2005; Lin 2009a; Lin 2009b; Lum 2006) and Barthel Index (Morris 2008).
Functional movement of the upper limb
Arm function: Box and Block Test (Cauraugh 2002; Cauraugh 2008; Desrosiers 2005), Wolf Motor Function Test (time to complete) (Luft 2004), Action Research Arm Test (Morris 2008), Motor Assessment Scale (upper arm score) (Stoykov 2009; Summers 2007) and Motor Activity Log (Amount of Use scale) (Lin 2009a;Lin 2009b).
Hand function: Purdue Peg Test (Desrosiers 2005), Stroke Impact Scale (hand function subscale) (Lin 2009a), Nine Hole Peg Test (Morris 2008) and Motor Assessment Scale (hand movements) (Stoykov 2009; Summers 2007).
Secondary outcomes
Performance in extended ADL
Stroke Impact scale (ADL/IADL section) (Lin 2009a).
Motor impairment
Motor impairment scales: Fugl‐Meyer Assessment (upper limb section) (Desrosiers 2005; Lin 2009a; Lin 2009b; Luft 2004; Lum 2006), Rivermead Motor Assessment (upper limb section) (Morris 2008) and Motor Status Score (Stoykov 2009).
Temporal outcomes: movement time for completion of various tasks (Cauraugh 2005; Cauraugh 2008; Lin 2009b; Platz 2001; Summers 2007). Cauraugh 2002 reported simple reaction premotor time. Desrosiers 2005 reported finger to nose co‐ordination (number of movements executed in 20 seconds).
Spatial outcomes: normalised total distance (Lin 2009b), spatial error for single aiming movement (Platz 2001) and elbow angle (Summers 2007).
Strength outcomes: grip strength (Desrosiers 2005), Wolf Motor Function Test (strength of hemiparetic limb) (Luft 2004), EMG activity (Cauraugh 2002; Cauraugh 2003a; Mudie 2001: Acute), maximal muscle contraction task (Cauraugh 2008), motor power examination (Lum 2006) and dynamometer (Stoykov 2009).
Excluded studies
We excluded a total of 61 studies following consideration of the full papers. Reasons for exclusion were: not a simultaneous bilateral training intervention (25 studies), not stroke population (two studies), review papers (three studies), bilateral training intervention but not a randomised controlled trial (17 studies), bilateral training intervention completed with assistive technology (seven studies), no relevant outcomes (one study) and bilateral training intervention received by both groups (six studies). The studies within the latter four categories (i.e. those studies which investigate a simultaneous bilateral training intervention, but which have been excluded from this review) are listed in the Characteristics of excluded studies table.
Risk of bias in included studies
For full details of methodology and risk of bias assessments see the Characteristics of included studies table and Figure 1 (Methodological quality summary). We judged most of the included studies to be of poor or uncertain methodological quality and therefore at high risk of bias. Assessment of risk of bias was difficult due to the lack of adequate reporting of methods: for 11 of the 14 included studies at least one of the assessed components were judged to be unclear (or were not stated). Only three studies reported adequate allocation concealment (Desrosiers 2005; Lin 2009a; Morris 2008). Eight studies reported blinding of outcome assessors (Desrosiers 2005; Lin 2009a; Lin 2009b,Luft 2004; Lum 2006; Morris 2008; Stoykov 2009; Summers 2007). No studies reported the use of intention‐to‐treat analysis.
Effects of interventions
Comparison intervention
Fourteen studies are included in the analyses (Lin 2009a is included in two of the comparisons and Mudie 2001 has two subgroups: Mudie 2001: Acute and Mudie 2001: Chronic). Within these 14 studies, 459 stroke participants were randomised and data for 421 participants were available for analysis. The missing data (38 participants) relate to four studies: Cauraugh 2002 randomised participants to a control group (five participants) which were not included in the analyses and Lum 2006 randomised participants to two other groups (16 participants) which were not relevant to this review (see the Characteristics of included studies table for further details). Desrosiers 2005 and Morris 2008 had eight and nine drop‐outs respectively.
Numbers of participants given below relate to the number of participants whose data were available for inclusion in each of the analyses and not the number of randomised participants.
Simultaneous bilateral training versus placebo or no interventions
No studies compared simultaneous bilateral training with placebo or no intervention.
Simultaneous bilateral training versus usual care
Four studies compared the effects of a bilateral training with usual care (Desrosiers 2005; Lin 2009a; Lin 2009b; Luft 2004) (127 participants).
Primary outcomes
Performance in activities of daily living (ADL)
Three studies (Desrosiers 2005; Lin 2009a; Lin 2009b) (106 participants) reported performance of ADL (Functional Independence Measure); SMD 0.25 (95% CI ‐0.14 to 0.63).
Functional movement of the upper limb
Four studies (Desrosiers 2005; Lin 2009a; Lin 2009b; Luft 2004) (127 participants) reported outcomes relevant to functional movement of the upper limb.
All four studies reported arm functional movement outcomes (Box and Block Test) (Desrosiers 2005), Motor Activity Log (amount of use scale) (Lin 2009a; Lin 2009b) and Wolf Motor Function Test (time to complete) (Luft 2004). The pooled result was SMD ‐0.07 (95% CI ‐0.42 to 0.28).
Two studies (Desrosiers 2005; Lin 2009a) (73 participants) reported a hand functional movement outcome (Purdue Pegboard Test and Stroke Impact Scale (hand function subscale) respectively); SMD ‐0.04 (95% CI ‐0.50 to 0.42).
Secondary outcomes
Performance in extended ADL
One study (Lin 2009a) (40 participants) reported the effects of bilateral training on performance in extended ADL (Stroke Impact Scale; ADL/IADL section); SMD 0.15 (95% CI ‐0.47 to 0.77).
Motor impairment of the upper limb
Four studies (Desrosiers 2005; Lin 2009a; Lin 2009b; Luft 2004) (127 participants) reported outcomes of motor impairment.
All four studies reported motor impairment scale outcome (Fugl‐Meyer (upper limb section)). The pooled result was SMD 0.67 (95% CI ‐0.43 to 1.77). We used a random‐effects model as I2 = 88% (fixed‐effect result: SMD 0.43 (0.06 to 0.81).
Two studies (Desrosiers 2005; Lin 2009b) (66 participants) reported a temporal outcome (finger to nose co‐ordination (number of movements completed) and movement time for unilateral reaching task respectively). The pooled result was SMD 0.04 (95% CI ‐0.45 to 0.52).
One study reported a spatial outcome (Lin 2009b) (33 participants; normalised total distance for a unilateral reaching task); SMD 0.25 (95% CI ‐0.43 to 0.94).
Two studies (Desrosiers 2005; Luft 2004) (54 participants) reported strength outcomes (grip strength and Wolf Motor Function Test (strength of hemiparetic limb) respectively), pooled result: SMD ‐0.18 (95% CI ‐0.72 to 0.36).
Simultaneous bilateral training versus other specific upper limb interventions or programmes
Eleven studies (including one with two comparison groups) compared the effects of a bilateral intervention with another upper limb intervention (Cauraugh 2002; Cauraugh 2003a; Cauraugh 2005; Cauraugh 2008; Lin 2009a; Lum 2006; Morris 2008; Mudie 2001: Acute; Mudie 2001: Chronic; Platz 2001; Stoykov 2009; Summers 2007) (316 participants).
Primary outcomes
Performance in ADL
Three studies (Lin 2009a; Lum 2006; Morris 2008) (151 participants) reported performance of ADL (Functional Independence Measure (Lin 2009a; Lum 2006) and Barthel Index (Morris 2008) respectively): SMD ‐0.25 (95% CI ‐0.57 to 0.08).
Functional movement of the upper limb
Six studies (Cauraugh 2002; Cauraugh 2008; Lin 2009a; Morris 2008; Stoykov 2009; Summers 2007) (209 participants) reported functional movement of the upper limb outcomes.
All six studies reported arm functional movement outcomes (Box and Block Test (Cauraugh 2002; Cauraugh 2008), Motor Activity Log (Amount of Use scale) (Lin 2009a), Action Research Arm Test (Morris 2008), Motor Assessment Scale (upper arm section) (Stoykov 2009) and Modified Motor Assessment Scale (upper arm section) (Summers 2007). Data from one of the studies (Cauraugh 2002) (20 participants) were unsuitable for pooling; a graphical display was presented of means with no standard deviations (results: bilateral training 27 blocks moved at post‐test, unilateral training 22 blocks, as estimated from the graph). For the remaining five studies (189 participants) the pooled result was SMD ‐0.20 (95% CI ‐0.49 to 0.09).
Four studies (Lin 2009a; Morris 2008; Stoykov 2009; Summers 2007) (173 participants) reported hand functional movement outcomes (Stroke Impact Scale (hand function section), Nine Hole Peg Test; Motor Assessment Scale (hand movements) and Modified Motor Assessment Scale (hand movements) respectively). The pooled result was SMD ‐0.21 (95% CI ‐0.51 to 0.09).
Secondary outcomes
Performance in extended ADL
One study (Lin 2009a) (40 participants) reported the effects of bilateral training on performance in extended ADL (Stroke Impact Scale; ADL/IADL section); SMD ‐0.65 (95% CI ‐1.29 to ‐0.01).
Motor impairment of the upper limb
Eleven studies (including one with two comparison groups) (Cauraugh 2002; Cauraugh 2003a; Cauraugh 2005; Cauraugh 2008; Lin 2009a; Lum 2006; Morris 2008; Mudie 2001: Acute; Mudie 2001: Chronic; Platz 2001; Stoykov 2009; Summers 2007) (332 participants) reported motor impairment outcomes.
Four studies (Lin 2009a; Lum 2006; Morris 2008; Stoykov 2009) (175 participants) reported a motor impairment scale (Fugl‐Meyer (upper limb section) (Lin 2009a; Lum 2006), Rivermead Motor Assessment (upper limb section) and Motor Status Score (total upper limb score) respectively); SMD ‐0.25 (95% CI ‐0.55 to 0.05).
Five studies (Cauraugh 2002; Cauraugh 2005; Cauraugh 2008; Platz 2001; Summers 2007) (79 participants) reported temporal outcomes (simple reaction premotor time (Cauraugh 2002) and movement time for completion of various tasks respectively). Summers 2007 data (10 participants) were unsuitable for pooling: reported median movement time values were reported without any standard deviations (bilateral training 1.89 seconds at post‐test, unilateral training 2.74 seconds). The pooled result for the remaining four studies (69 participants) was SMD 0.46 (95% CI ‐0.03 to 0.95).
Two studies (Platz 2001; Summers 2007) (24 participants) reported spatial outcomes (single aiming movement and elbow angle respectively). Data from Summers 2007 (10 participants) were unsuitable for pooling: this study reported elbow angle means with no standard deviations (bilateral training mean 123.82° at post‐test, unilateral training mean 140.32º). The result for the remaining study (Platz 2001) (14 participants) was SMD 0.00 (95 % CI ‐1.05 to 1.05).
Six studies (Cauraugh 2002: Cauraugh 2003a; Cauraugh 2008; Lum 2006; Mudie 2001: Acute; Mudie 2001: Chronic; Stoykov 2009) (130 participants) reported strength‐related outcomes (EMG activity (Cauraugh 2002; Cauraugh 2003a; Mudie 2001: Acute; Mudie 2001: Chronic), maximal contraction time (Cauraugh 2008), motor power examination (Lum 2006) and dynamometer data (Stoykov 2009)). Data from Cauraugh 2002 (20 participants) were unsuitable for pooling; data for sustained muscle contraction and force modulation were presented in a bar graph of median root mean square error with no standard deviations (bilateral training median root mean square error 0.42 at post‐test, unilateral training 0.42; estimated from graph). Data from Cauraugh 2008 (16 participants) were also unsuitable for pooling; no means or standard deviations were presented (the authors of this study stated that analysis did not reveal any significant effects). Stoykov 2009 (24 participants) did not present means and standard deviations for the two groups, therefore data from this study could not be included in data analysis. A non‐significant result between the groups was reported. The pooled result of the remaining three studies (70 participants) was SMD 0.04 (95% CI ‐1.34 to 1.43). We used a random‐effects model because I2 = 85% (fixed‐effect result: SMD ‐0.07 (95% CI ‐0.59 to 0.46).
Other outcomes
No studies reported adverse events.
Sensitivity analyses
We carried out sensitivity analyses to investigate the effect of including the following.
Studies that had a single treatment and evaluation session (Mudie 2001: Acute; Mudie 2001: Chronic). When we removed this study (with two subgroups) the result for motor impairment: strength outcomes was SMD 0.64 (95% CI ‐2.72 to 4.00). We used a random‐effects model because I2 = 94% (fixed‐effect: SMD 0.63 (95% CI ‐0.21 to 1.48)).
Studies that investigated the effect of an adjunct therapy/assistive technology in addition to the bilateral training and control interventions (Cauraugh 2002; Cauraugh 2003a; Cauraugh 2005; Cauraugh 2008; Luft 2004; Lum 2006). In the comparison bilateral training versus usual care, removing Luft 2004 did not affect the significance of the results (arm functional outcomes: SMD ‐0.03 (95% CI ‐0.425 to 0.35); motor impairment scales: SMD 0.73 (95% CI ‐0.76 to 2.23); motor impairment, strength outcomes: SMD ‐0.17 (95% CI ‐0.85 to 0.51). For the comparison bilateral training versus other upper limb intervention, we removed six studies (Cauraugh 2002; Cauraugh 2003a; Cauraugh 2005; Cauraugh 2008; Lum 2006; Stoykov 2009) from the analysis. With these studies removed the results were: performance in activities of daily living SMD ‐0.18 (95% CI ‐0.52 to 0.16); arm functional outcomes SMD ‐0.30 (95% CI ‐0.62 to 0.03); motor impairment scales ‐0.31 (95% CI ‐0.65 to ‐0.03); motor impairment, temporal outcomes SMD ‐0.11 (‐1.16 to 0.93) and motor impairment strength outcomes SMD ‐0.51 (95% CI ‐1.18 to 0.16). Following sensitivity analysis we found a change of significance for motor impairment scales for the comparison bilateral training versus other upper limb intervention, however this significant result in favour of other upper limb intervention was based on only two studies.
The lack of information provided by the majority of studies relating to methodological quality meant that we were unable to carry out sensitivity analyses to investigate the effect of including studies with low methodological quality. If in future updates more than five studies report adequate methodological quality features then we will carry out a sensitivity analysis.
Subgroup analyses
There were insufficient data (we had pre‐defined the need for more than five studies reporting the information) to carry out planned subgroup analyses on differences between acute and chronic patients and duration and number of repetitions of the programme.
Discussion
Summary of main results
We found no studies that compared simultaneous bilateral training with placebo or no intervention.
Four of 14 included studies compared simultaneous bilateral training with usual care and found no statistically significant effect of bilateral training on any analysed outcomes (performance of activities of daily living (ADL), arm and hand functional movement, performance in extended ADL or motor impairment measures (motor impairment scales, temporal, spatial and strength outcomes). As stated in the methods we used a random‐effects model where heterogeneity was greater than 50%, therefore these conclusions are based on random‐effects analysis where appropriate. For motor impairment scales we found a significant result when we used a fixed‐effect analysis; however, due to heterogeneity (I2 = 88%) a random‐effects model was more appropriate for analysis and this result was non‐significant.
Eleven of 14 included studies compared the effects of a bilateral intervention with another upper limb intervention. We found no statistically significant effects in favour of bilateral training for any of the specified outcomes. Data from one trial (Lin 2009a) (40 participants) found a statistically significant result in favour of another upper limb intervention (constraint‐induced therapy) for a measure of performance in extended ADL. This result cannot be generalised to other outcomes and further research would be required to confirm this finding.
It must be noted that only six (Lin 2009a; Lin 2009b; Morris 2008; Mudie 2001: Acute; Platz 2001; Summers 2007) of the fourteen studies included in the analysis used a single training protocol. The other eight studies included neuromuscular stimulation (Cauraugh 2002; Cauraugh 2003a; Cauraugh 2005; Cauraugh 2008), robotic‐assistance (Lum 2006), rhythmic auditory cueing (Luft 2004; Stoykov 2009) or unilateral and bimanual tasks in combination with bilateral training (Desrosiers 2005).
In addition to the 14 studies included in the analyses, we identified four relevant cross‐over studies (Chang 2006; Dickstein 1993; Harris‐Love 2005; Kilbreath 2006). None of these four studies had data suitable for inclusion in analyses, therefore, while we have included details of these four cross‐over studies in the Characteristics of included studies table, Table 3 (Demographics of included participants) and Figure 1 (Methodological quality summary), we have not included them in analyses or referred to them within the Results or Discussion sections.
In summary, this review has identified:
insufficient high quality evidence to determine if simultaneous bilateral training is more (or less) effective than placebo, no intervention or usual care;
evidence to suggest that bilateral training is no more (or less) effective than other upper limb interventions for the specified outcomes.
Overall completeness and applicability of evidence
The evidence is currently insufficient to answer the review questions: the effects of bilateral training compared to placebo, usual care or other upper limb intervention in terms of performance in ADL, functional movement of the upper limb, performance in extended ADL, motor impairment of the upper limb and adverse events. The included studies, with the exception of Morris 2008, had small numbers of participants and reported a diverse range of outcome measures, of which many were unique to single studies or specific to certain impairments. Both these factors limit the completeness of the evidence relevant to this review.
One of the 14 included studies (Mudie 2001: Acute) had a single treatment and evaluation phase, meaning that treatment and evaluation occurred at the same time. It is debatable whether or not this method constitutes an evaluation of an intervention, or whether it is simply a test of performance under different conditions. We investigated the impact of including this study using a sensitivity analysis, and found it to have very limited impact on the results of this review.
Due to limited data we were unable to complete subgroup analysis for different participant subgroups or duration or intensity of training. The characteristics of the included studies indicate that participants within the studies varied in terms of time post‐stroke. Additionally, the type, duration and intensity of training varied between the studies.
Another key difference between the studies was the investigation of the effect of a single movement versus the effect of a series of different movements. In future updates of this review we propose to carry out subgroup analysis to explore the effect of single bilateral movements versus a series (more than one) of bilateral movements, as arguably this could have an impact.
All of the included studies had inclusion criteria specifying either minimum or maximum levels of upper limb ability, and preservation of at least some cognitive abilities (including ability to comprehend simple instructions). Therefore, the results of this review may not be generalisable to the wider population of stroke patients.
The lack of sufficient high quality evidence makes it inappropriate to draw conclusions from the results regarding the applicability of bilateral training within the context of current practice.
Quality of the evidence
The quality of most of the evidence was poor, with incomplete reporting of methodological details. The number of participants within the included studies was generally small; only Morris 2008 and Lin 2009a had more than 50 participants and seven of the studies had 20 or fewer participants. Only three of the 14 studies had adequate allocation concealment. Two studies (Lin 2009a; Lum 2006) clearly did not have allocation concealment and the remaining studies did not mention allocation concealment. Eight studies reported that a blinded assessor was used. No studies reported using an intention‐to‐treat analysis. The overall quality of the studies limits confidence in the results.
Potential biases in the review process
Through a thorough searching process we are confident that we should have identified all relevant published studies; however, it must be acknowledged that there is a small possibility that there are additional studies (published and unpublished) that we did not identify.
Four studies were categorised as comparing bilateral training with usual care. It should be noted that the intervention (categorised as usual care) in these studies was dose matched with the bilateral intervention. Therefore, it is likely that these interventions were more intensive than the typical duration of usual care. Furthermore, the interventions which we have classified as usual care differ between the four studies. However, we felt that it was more appropriate to categorise these interventions within the usual care comparison than the other upper limb intervention comparison, as the interventions completed in these four studies were not specific other upper limb interventions or programmes. Within the other upper limb interventions comparison all except one study investigated bilateral training compared to unilateral training (i.e. completing the same activities or activity with both arms compared to completing with the affected arm only). Lin 2009a compared the effects of bilateral training with constraint‐induced therapy which, in addition to undertaking of functional tasks with the affected upper limb (which was dose matched to the bilateral training), involved restraint of the unaffected limb for six hours per day. Combining these studies within these stated comparison groups further increases the heterogeneity between the included studies, limiting the conclusions that can be drawn.
The diversity of the bilateral training paradigms and the variations in reporting between studies led to the review team making some subjective decisions, which may have introduced bias. The studies within this area are heterogenous in terms of what is defined as bilateral training and there were a number of complex strands which required discussion among the review authors and consensus decisions being made. We appreciate that this could be perceived as a limitation of our review.
We used hierarchical lists (see Types of outcome measures) to select which outcome measure should be included if a study reported a number of different relevant outcome measures. There could potentially be biases in the hierarchical order developed for each outcome. However, we carefully considered the order of the hierarchy and reached consensus. Despite the potential limitations and biases of this approach, we believe that because of the large number of different outcome measures used to assess similar domains the pre‐stating of a hierarchical list provides substantial advantages in comparison to the alternative option of having to make subjective decisions about the selection of outcome measures after data collection has been completed.
The included studies used a wide range of outcome measures, methodologies and time intervals for follow up making statistical pooling difficult. To overcome the variations in outcome measures and to maximise statistical pooling we categorised the outcomes of functional movement and motor impairment of the upper limb into subgroups. For four studies, mean values were not available (for at least some of the outcomes) and we therefore imputed median values (where these were provided instead of mean values) as mean values and calculated standard deviations from reported standard error (SD = SE √n). Where data were presented in graphical form two review authors independently estimated values from the graphs. This may have introduced some bias into the review process. However, we believe that including imputed and estimated data from these studies is preferable to excluding the data.
Inclusion of single training and evaluation studies
We included one randomised controlled trial (Mudie 2001: Acute) and four cross‐over studies (Chang 2006; Dickstein 1993; Harris‐Love 2005; Kilbreath 2006) (not included in analyses) which involved a single evaluation session. Within these studies there was no training period and it is questionable if these studies constitute intervention studies or merely a test of performance. In addition, the four cross‐over studies were not designed or presented as traditional RCTs, but rather participants were randomly allocated to different treatment orders (using a randomised cross‐over design). We debated the suitability of including these studies. Only one of these five studies (Mudie 2001) was incorporated in any analysis; therefore including these other studies in the review does not alter the results or conclusions of this review.
For this version of the review we decided to include these studies (although the cross‐over studies were included in tables only and not included in analyses or described in the text), however we would appreciate any feedback on this, and may revise this decision in subsequent updates. Options for future updates of this review could therefore be either the exclusion of randomised cross‐over studies or the exclusion of any study which only has a single evaluation session. If randomised cross‐over studies are to be included in updates of this review, we must first identify appropriate methods of obtaining and including data within analyses.
Agreements and disagreements with other studies or reviews
The results of this review vary from the results presented in the review by Stewart 2006, which reported a significant overall effect in favour of bilateral movement training alone or in combination with auxiliary sensory feedback for improving motor recovery post‐stroke (Fugl‐Meyer, Box and Block Test or kinematic variables). This review was systematic in terms of its methods, however it had a more limited search strategy than our review and included studies that were not randomised controlled trials. The authors did assess trials for randomisation, which was defined as either randomly placed in a treatment or control group or if the treatment was randomly assigned to the participants. Eleven studies were included in the Stewart 2006 meta‐analysis, seven of which were not included in our review (Cauraugh 2003b; Lewis 2004a; Mudie 1996; Mudie 2000; McCombe Waller 2004; Stinear 2004; Whitall 2000). Five of these studies (Mudie 1996; Mudie 2000; Cauraugh 2003b; Lewis 2004a; Stinear 2004) were assessed to have some form of random assignment within the Stewart 2006 review, however we disagreed with this decision. Many of these studies were considered not to have an appropriate control group and these types of studies will give an inflated effect of the intervention. Reasons for excluding the above studies from our review are stated in the Characteristics of excluded studies table. We included two studies (Dickstein 1993; Mudie 2001: Acute) which were identified by the Stewart 2006 review, but not included in the meta‐analysis due to not having a functional outcome measure (Dickstein 1993) and not involving bilateral movements as a treatment (Mudie 2001: Acute) respectively. In contrast, we included Dickstein 1993 as it included other outcomes relevant to our criteria, and we assessed that Mudie 2001: Acute did involve some element of bilateral intervention. Ten studies included in this current review were published after the searching for the Stewart 2006 review was completed (2005) (Cauraugh 2008; Chang 2006; Desrosiers 2005; Harris‐Love 2005; Kilbreath 2006; Lin 2009a; Lin 2009b; Lum 2006; Morris 2008; Stoykov 2009). Therefore, our review presents more up‐to‐date data. Additionally, we included a further two studies (Cauraugh 2003a; Platz 2001) which were not acknowledged in the Stewart 2006 review.
A narrative review by Cauraugh 2005 reported the findings from a number of studies, including non‐randomised studies, and concluded that favourable effects of bilateral training protocols have been found. However, Cauraugh 2005 made no attempt to discuss the quality of the reviewed studies and the potential impact this could have on the individual study results. However, it also acknowledged that some studies have not reported enhanced performance following bilateral training. This review differs from our review as it was not systematic and did not attempt to combine studies.
Authors' conclusions
This review has identified that there is currently insufficient evidence to make any recommendations about the relative effect of bilateral training compared to placebo, no intervention or usual care. It has also identified evidence from studies of varied methodological quality that suggests that bilateral training may be no more (or less) effective than other upper limb interventions for performance in activities of daily living (ADL), functional movement of the upper limb, or motor impairment outcomes.
Specific implications for research, based on the findings of this review, are outlined below.
Are further randomised controlled trials required?
Randomised controlled trials (RCTs) are required to determine the effect of:
simultaneous bilateral training compared to no treatment, placebo or usual care;
simultaneous bilateral training compared to other upper limb interventions.
Such randomised controlled trials must:
have adequate power (i.e. with an appropriate power calculation undertaken based on existing trial evidence);
have adequate allocation concealment, blinding of outcome assessor and intention‐to‐treat analysis;
clearly define trial participants (e.g. time since stroke, initial upper limb deficits);
clearly define types, frequency, durations and intensities of bilateral training;
include global measures of functioning (i.e. performance of ADL measures) and upper limb function (e.g. Motor Assessment Scale, ARAT);
report clear and usable data.
We recommend that future RCTs concentrate on answering the specific question relating to the effectiveness of bilateral training and do not confound the answer to this question by introducing adjunct interventions such as robotics or electrical stimulation. We believe that until such time as the benefits of bilateral training as a single intervention have been established (or refuted) it is not beneficial to investigate the combined effects of bilateral training plus adjunct interventions.
We recommend that future RCTs should have a defined training period, and should not have a single treatment and intervention session, in order to establish the effects of actual skill acquisition (rather than mere performance). Further, we recommend that standard RCT methodology is followed, i.e. random allocation of participants to one of two groups and not random allocation to treatment order.
A number of RCTs are currently ongoing (see the Characteristics of ongoing studies table). Once these trials are completed it will be important to update this review, and to re‐evaluate the need for further RCTs of bilateral training. If there continues to be no evidence of beneficial effects attributable to bilateral training, we would recommend that no further RCTs are carried out.
Are other primary research studies required?
We do not recommend other study designs aimed at comparing the effectiveness of bilateral training. This review has highlighted the difficulties associated with the large number of outcome measures, which are associated with upper limb function and impairment. There is a need for further research to identify optimal outcome measures for use within future RCTs in this area.
Are further systematic reviews required?
We do not recommend any further systematic reviews aimed at addressing the effectiveness of bilateral training. However, future updates of this review ought to consider longer‐term follow‐up outcomes. In addition, future updates need to consider whether the inclusion of randomised cross‐over trials, or trials with only a single evaluation session, are beneficial to this evidence base.
Summary of findings
Methodological quality of studies is in general very poor, providing insufficient high quality evidence on which to reach generalisable conclusions.
Limited evidence suggests bilateral training is no more or less effective than usual care or other upper limb interventions (unilateral interventions) for functional outcomes.
Very limited evidence shows that bilateral training is no less effective than other upper limb interventions for motor impairment outcomes.
There is not enough evidence to recommend bilateral training as clinical intervention.
Good quality RCTs are needed to compare bilateral and unilateral training.
Acknowledgements
Brenda Thomas for developing the search strategy. Stroke Therapy Evaluation Project colleagues for support and advice throughout the completion of this project. Greater Glasgow Health Board Managed Clinical Network for Stroke for funding and supporting this project.
Appendices
Appendix 1. MEDLINE search strategy
We used the following search strategy, using a combination of controlled vocabulary (MeSH) and free‐text terms, for MEDLINE. This was modified to suit other databases (see Appendix 2; Appendix 3; Appendix 4).
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp cerebrovascular trauma/ or exp intracranial arterial diseases/ or exp intracranial arteriovenous malformations/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vasospasm, intracranial/ or vertebral artery dissection/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiplegia/ or exp paresis/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. 1 or 2 or 3 or 4 or 5 or 6 8.*cerebrovascular disorders/rh or exp *basal ganglia cerebrovascular disease/rh or exp *brain ischemia/rh or exp *carotid artery diseases/rh or exp *cerebrovascular trauma/rh or exp *intracranial arterial diseases/rh or exp *intracranial arteriovenous malformations/rh or exp *"intracranial embolism and thrombosis"/rh or exp *intracranial hemorrhages/rh or *stroke/rh or exp *brain infarction/rh or *vasospasm, intracranial/rh or *vertebral artery dissection/rh 9. *hemiplegia/rh or exp *paresis/rh 10. 8 or 9 11. exp Upper Extremity/ 12. (upper adj3 (limb$ or extremity)).tw. 13. (arm or shoulder or elbow or forearm or hand or wrist or finger or fingers).tw. 14. 11 or 12 or 13 15. rehabilitation/ or "recovery of function"/ 16. physical therapy modalities/ or "Physical Therapy (Specialty)"/ 17. exercise movement techniques/ or exercise/ or exercise therapy/ 18. range of motion, articular/ or movement/ or motor activity/ or kinesiology, applied/ 19. "task performance and analysis"/ 20. occupational therapy/ or activities of daily living/ 21. "Physical Education and Training"/ or motor skills/ 22. (rehabilitation or recovery of function or physiotherap$ or physical therap$ or exercise$ or movement$ or motor activit$ or occupational therap$ or activities of daily living or adl).tw. 23. ((bilateral or bimanual) adj5 (train$ or retrain$ or facilitat$ or function$ or activit$)).tw. 24. ((mirror$ or coupled) adj5 movement$).tw 25. or/15‐24 26. 10 and 14 27. 7 and 14 and 25 28. 26 or 27 29. limit 28 to humans
Appendix 2. EMBASE search strategy
We used the following search strategy, using a combination of controlled vocabulary (MeSH) and free‐text terms, for EMBASE.
1. cerbrovascular disease/or exp basal ganglion hemorrhage/ or exp brain ischemia/ or exp carotid artery disease/ or cerebrovascular accident/ or exp brain infarction/ or exp cerebrovascular accident/ or exp brain ischemia/ or exp cerebral artery disease/ or brain arteriovenous malformations/ or exp thromboembolism/ or exp brain hemorrhage/ or *brain vasospasm/ or artery dissection/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiplegia/ or exp paresis 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. 1 or 2 or 3 or 4 or 5 or 6 8. *cerebrovascular disease/rh or exp *basal ganglion hemorrhage /rh or exp *brain ischemia/rh or exp *carotid artery disease/rh or *cerebrovascular accident/rh or exp *brain infarction/rh or exp *cerebrovascular accident/rh or exp *brain ischemia/rh or exp *cerebral artery disease/rh or *brain arteriovenous malformations/rh or exp *thromboembolism/rh or exp *brain hemorrhage/rh or *brain vasospasm/rh or *artery dissection/rh 9. *hemiplegia/rh or exp *paresis/rh 10. 8 or 9 11. exp arm/ 12. (upper adj3 (limb$ or extremity)).tw. 13. (arm or shoulder or elbow or forearm or hand or wrist or finger or fingers).tw. 14. 11 or 12 or 13 15. rehabilitation/ or convalescence/ 16. physiotherapy/ 17. kinesiotherapy/ or exercise/ or kinesiotherapy/ 18. joint characteristics and functions/ or "movement (physiology)"/ or motor activity/ or kinesiology/ 19. task performance/ 20. occupational therapy/ or daily life activity/ 21. physical education/ or motor performance/ 22. (rehabilitation or recovery of function or physiotherap$ or physical therap$ or exercise$ or movement$ or motor activit$ or occupational therap$ or activities of daily living or adl).tw. 23. ((bilateral or bimanual) adj5 (train$ or retrain$ or facilitat$ or function$ or activit$)).tw. 24. ((mirror$ or coupled) adj5 movement$).tw 25. or/15‐24 26. 10 and 14 27. 7 and 14 and 25 28. 26 or 27 29. limit 28 to humans
Appendix 3. CINAHL search strategy
1. MH "Cerebrovascular Disorders+" 2. MH "Carotid Artery Diseases+" 3. MH "Cerebral Aneurysm" 4. MH "Cerebral Embolism and Thrombosis” 5. MH "Cerebral Ischemia+" 6. MH "Cerebral Vasospasm" 7. MH "Intracranial Hemorrhage+" 8. MH "Vertebral Artery Dissections") 9. S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 10. stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc* or cva* or apoplex* or SAH 11. brain* or cerebr* or cerebell* or intracran* or intracerebral n5 isc?emi* or infarct* or thrombo* or emboli* or occlus* 12. brain* or cerebr* pr cerebell* or intracerebral or intracranial or subarachnoid n5 haemorrhage* or hemorrhage* or haematoma* or bleed* 13. mh hemiplegia or mh stroke patients 14. hemipleg* or hemipar* or paresis or paretic.tw. 15. S9 or S10 or S11 or S12 or S13 or S14 16. mm cerebrovascular disorders/rh 17. mh carotid artery diseases+/rh 18. mm cerebral aneurysm/rh 19. mm "cerebral embolism and thrombosis"/rh 20. mh cerebral ischemia+/rh 21. mm cerebral vascular accident/rh 22. mm cerebral vasospasm/rh 23. mh intracranial hemorrhage+/rh 24. mm vertebral artery dissections/rh 25. S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 26. mm hemiplegia/rh 27. S25 or S26 28. mh upper extremity+ 29. upper n3 limb* or extremity.tw. 30. arm or shoulder or elbow or forearm or hand or wrist or finger or fingers.tw. 31. S28 or S29 or S30 32. mh rehabilitation 33. mh "activities of daily living" 34. mh home rehabilitation+ 35. mh occupational therapy+ 36. mh physical therapy+ 37. S32 or S33 or S34 or S35 or S36 38. mh occupational therapists 39. mh occupational therapy assistants 40. mh physical therapists 41. S38 or S39 or S40 42. mh exercise+ 43. mh therapeutic exercise+ 44. mh exercise intensity 45. S42 or S43 or S44 46. mh kinesiology 47. mh applied kinesiology 48. mh recovery 49. S46 or S47 or S48 50. mh movement 51. mh motor activity 52. mh range of motion 53. S50 or S51 or S52 54. mh "Task Performance and Analysis" 55. mh "Physical Education and Training" 56. mh motor skills+ 57. S55 or S56 58. rehabilitation or recovery of function or physiotherapy* or physical therap* or exercise* or movement* or motor active* or occupational therap* or activities of daily living or adl.tw. 59. bilateral or bimanual n5 train* or retrain* or facilitate* or function* or activit*.tw. 60. mirror* or coupled n5 movemen*.tw. 61. 37 or 41 or 45 or 49 or 53 or 54 or 57 or 58 or 59 or 60 62. 27 and 31 63. 15 and 31 and 61 64. 62 or 63
Appendix 4. AMED search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiplegia/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp arm/ 9. (upper adj3 (limb$ or extremity)).tw. 10. (arm or shoulder or elbow or forearm or hand or wrist or finger or fingers).tw. 11. 8 or 9 or 10 12. rehabilitation techniques/ or "activities of daily living"/ or exp occupational therapy techniques/ 13. movement/ or motor activity/ or "range of motion"/ or "recovery of function"/ 14. occupational therapists/ or physiotherapists/ 15. exp physical therapy modalities/ 16. physical therapy speciality/ or occupational therapy speciality 17. exercise/ or exercise movement techniques/ or exercise therapy/ 18. movement/ or motor activity/ or "range of motion"/ 19. "task performance and analysis"/ or applied kinesiology/ 20. exp physical education/ or exp motor skills/ 21. psychomotor performance/ 22. (rehabilitation or recovery of function or physiotherap$ or physical therap$ or exercise$ or movement$ or motor activit$ or occupational therap$ or activities of daily living or adl).tw. 23. ((bilateral or bimanual) adj5 (train$ or retrain$ or facilitat$ or function$ or activit$)).tw. 24. ((mirror$ or coupled) adj5 movement$).tw. 25. or/12‐24 26. 7 and 11 and 25
Data and analyses
Comparison 1.
Bilateral training versus usual care
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Performance in activities of daily living | 3 | 106 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.14, 0.63] |
| 2 Functional movement of the upper limb | 4 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Arm functional movement | 4 | 127 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.42, 0.28] |
| 2.2 Hand functional movement | 2 | 73 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.50, 0.42] |
| 3 Performance in extended activities of daily living | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.47, 0.77] |
| 4 Motor impairment of the upper limb | 4 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Motor impairment scales | 4 | 127 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.43 [0.06, 0.81] |
| 4.2 Temporal outcomes | 2 | 66 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.45, 0.52] |
| 4.3 Spatial outcomes | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.43, 0.94] |
| 4.4 Strength outcomes | 2 | 54 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.72, 0.36] |
Analysis 1.1.
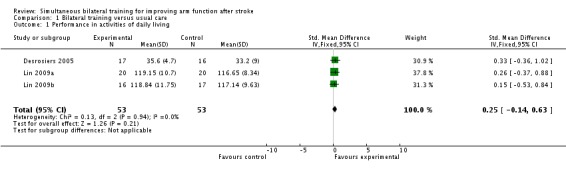
Comparison 1 Bilateral training versus usual care, Outcome 1 Performance in activities of daily living.
Analysis 1.2.
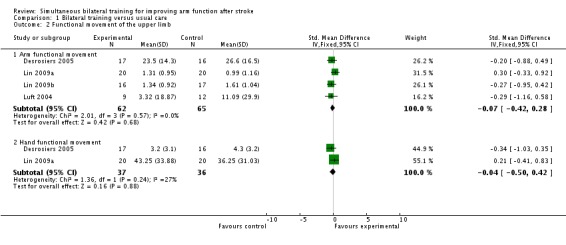
Comparison 1 Bilateral training versus usual care, Outcome 2 Functional movement of the upper limb.
Analysis 1.3.
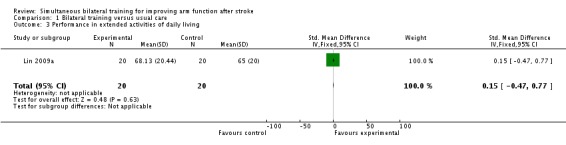
Comparison 1 Bilateral training versus usual care, Outcome 3 Performance in extended activities of daily living.
Analysis 1.4.
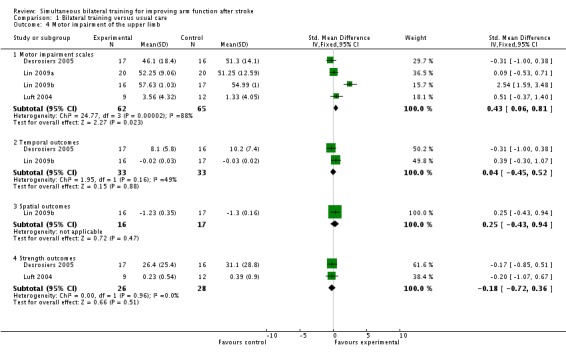
Comparison 1 Bilateral training versus usual care, Outcome 4 Motor impairment of the upper limb.
Comparison 2.
Bilateral training versus other upper limb intervention
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Performance in activities of daily living | 3 | 151 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.57, 0.08] |
| 2 Functional movement of the upper limb | 6 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Arm functional movement | 6 | 209 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.49, 0.09] |
| 2.2 Hand functional movement | 4 | 173 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.51, 0.09] |
| 3 Performance in extended activities of daily living | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.65 [‐1.29, ‐0.01] |
| 4 Motor impairment | 12 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Motor impairment scales | 4 | 175 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.55, 0.05] |
| 4.2 Temporal outcomes | 5 | 79 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.46 [‐0.03, 0.95] |
| 4.3 Spatial outcomes | 2 | 24 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.05, 1.05] |
| 4.4 Strength outcomes | 7 | 130 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.59, 0.46] |
Analysis 2.1.
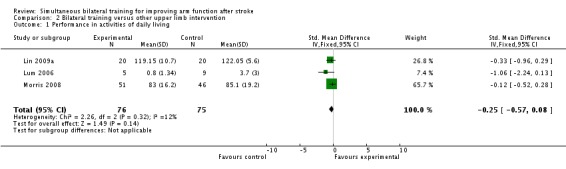
Comparison 2 Bilateral training versus other upper limb intervention, Outcome 1 Performance in activities of daily living.
Analysis 2.2.
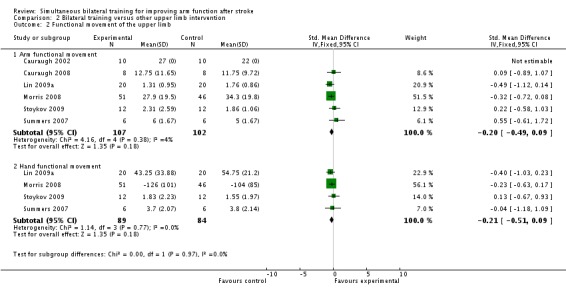
Comparison 2 Bilateral training versus other upper limb intervention, Outcome 2 Functional movement of the upper limb.
Analysis 2.3.
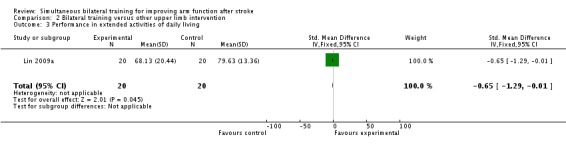
Comparison 2 Bilateral training versus other upper limb intervention, Outcome 3 Performance in extended activities of daily living.
Analysis 2.4.
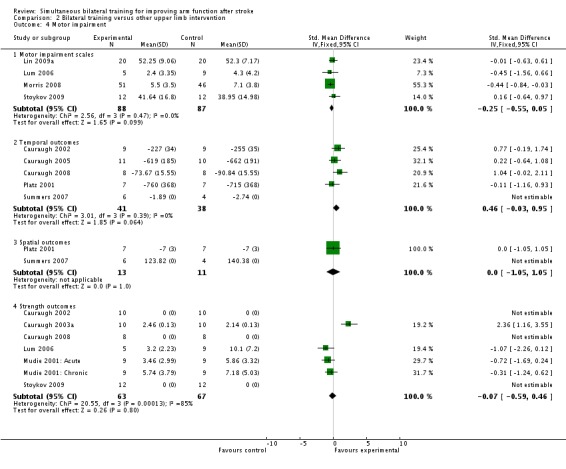
Comparison 2 Bilateral training versus other upper limb intervention, Outcome 4 Motor impairment.
Differences between protocol and review
The protocol stated that trial authors would be contacted to provide additional details relating to aspects of their studies. We did not contact authors to obtain any missing information.
The protocol stated that we would search OT Search. Following advice from the Cochrane Stroke Group Trials Search Co‐ordinator, we did not do this because this database now requires a subscription.
The protocol stated that we would identify and handsearch relevant journals and conference proceedings that had not been searched on behalf of The Cochrane Collaboration. We did not identify any relevant journals and therefore did not carry out any handsearching.
The protocol stated that we would exclude any studies that used assistive technologies such as robot‐devices. We changed this to exclude studies which investigated assistive technologies as active treatment, but include studies that used assistive technologies as an adjunct to both the bilateral training and control intervention.
At the protocol stage we did not state that we would separate upper limb functional outcomes into arm and hand outcomes or that we would separate motor impairment outcomes into motor impairment scales, temporal outcomes, spatial outcomes and strength outcomes. We have explained our reasons for doing this in the text.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cauraugh 2002
| Methods | Randomised controlled trial Random assignment with restriction that 20 participants were tested in the 2 treatment groups Method of randomisation and allocation concealment not stated | |
| Participants | 25 participants Inclusion criteria: diagnosis of CVA and no more than two CVAs on same side of brain, upper limit of 80% motor recovery (EMG activation patterns compared with non‐affected upper limb), lower limit of 10° voluntary wrist or finger extension against gravity, no other neurological deficits, no pacemaker, no use of drugs for spasticity, not enrolled in any other rehabilitation protocol | |
| Interventions | Group 1 (10 participants): unilateral + EMG‐triggered stimulation wrist/finger extension Group 2 (10 participants): bilateral + EMG‐triggered stimulation wrist/finger extension Each group completed 3 sets of 30 successful EMG‐triggered neuromuscular stimulation trials (approximately 1 hour 30 minutes); in total 6 hours of training (4 days) were completed during 2 weeks Profession of individual(s) administrating training unclear | |
| Outcomes | Primary outcome: functional movement: BBT Secondary outcome: motor impairment ‐ temporal outcomes: reaction time for speed of information processing and rapid muscle onset (simple reaction time, premotor reaction time and chronometric motor reaction time) ‐ premotor reaction time selected for use in analysis; strength outcomes: muscle activity (EMG activity of wrist/fingers extensor muscles) | |
| Notes | Control group (did not receive the neuromuscular electric stimulation or bilateral assistance for the wrist/fingers extensors); 5 participants not included in the analysis Unable to use presented data for BBT within analysis as no standard deviations presented Means from graph were estimated and presented in results section Pre‐motor reaction time was chosen for inclusion as temporal outcome as medians and standard deviations presented and therefore could be included in statistical pooling of results Medians imputed as mean values 2 participants were excluded from analyses due to extreme reaction times: it was unclear from the paper which groups these participants were in, therefore analysis for reaction time based on 18 participants (1 participant removed from bilateral and unilateral training groups respectively) For muscle activity (strength) unable to use presented data within analysis as median root mean square error presented with no standard deviations Medians from graph were estimated and presented in results section Data for this outcome based on 24 participants but unclear from which group of the 3 groups (control group not included in this review) the excluded participant was from | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Method of randomisation and allocation concealment not stated |
| Blinding of outcome assessor? | Unclear risk | Not stated |
| Intention to treat analysis? | Unclear risk | Not stated |
| Baseline similarity | Unclear risk | Demographic details between the groups not reported |
Cauraugh 2003a
| Methods | Randomised controlled trial Participants randomly assigned to 1 of 2 groups Method of randomisation and allocation concealment not stated | |
| Participants | 20 participants Inclusion criteria: absence of other neurological deficits, able to voluntarily extend wrist or fingers 10° against gravity, upper limit of 80% motor recovery (EMG activation patterns), no use of drugs for spasticity, not enrolled in any other rehabilitation protocol, diagnosis of CVA, sufficient voluntary control to activate the microprocessor, sufficient cognitive function to follow instructions | |
| Interventions | Group 1 (10 participants): unilateral wrist and finger extension + EMG‐triggered stimulation Group 2 (10 participants): bilateral wrist and finger movement + EMG‐triggered stimulation During each day of training participants completed 3 sessions of 30 successful EMG triggered stimulation trials (approximately 90 minutes) with 5‐minute break between sessions Participants completed 360 trials across 12 sessions of training over 4 days Profession of individual(s) providing training unclear | |
| Outcomes | Secondary outcome: motor impairment ‐ strength outcomes: EMG activity level (wrist and finger extensor muscles) | |
| Notes | Number of participants in each group not reported; we assumed an equal number of participants in each group Data presented in paper as a graph ‐ mean log10 and SE Means estimated from graph and standard deviation calculated from estimated standard error to allow for inclusion in statistical pooling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Method of randomisation and allocation concealment not stated |
| Blinding of outcome assessor? | Unclear risk | Not stated |
| Intention to treat analysis? | Unclear risk | Not stated |
| Baseline similarity | Unclear risk | Details of the 2 groups at baseline were not reported |
Cauraugh 2005
| Methods | Randomised controlled trial Random assignment following a randomisation schedule | |
| Participants | 21 participants Inclusion criteria: diagnosis of no more than 3 strokes, lower limit of 10° voluntary wrist/finger extension starting from 80° wrist and finger flexion, upper limit of 80% motor recovery, no other neurological deficits, not participating in another upper limb programme | |
| Interventions | Group 1 (10 participants): unilateral + EMG‐triggered stimulation wrist/finger extension Group 2 (11 participants): bilateral + EMG‐triggered stimulation wrist/finger extension Each group completed 4 days of 90 minutes training/week over 2 weeks Profession of individual(s) administrating training unclear | |
| Outcomes | Secondary outcome: motor impairment ‐ temporal outcomes: reaction time (ms), movement time (ms) deceleration time (ms), peak velocity (cm/s) and SD peak velocity (movement time selected) All measured for single aiming test and recorded by EMG | |
| Notes | Control group (5 participants), no stroke history, not included in participant numbers or analysis Median values presented in paper; this imputed as a mean value in the analysis Movement time data inverted for analysis (multiplied by ‐1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | No mention of allocation concealment |
| Blinding of outcome assessor? | Unclear risk | Not stated |
| Intention to treat analysis? | Unclear risk | Not reported; no drop‐outs reported |
| Baseline similarity | Low risk | No differences between groups reported |
Cauraugh 2008
| Methods | Randomly assigned to 1 of 2 treatment protocol orders Method of randomisation and allocation concealment not stated | |
| Participants | 16 participants Inclusion criteria: diagnosis of no more than 2 strokes, lower limit of 10° voluntary wrist/finger extension, absence of other neurological deficits, currently not participating in another rehabilitation programme | |
| Interventions | Group 1 (8 participants): unilateral + EMG‐triggered neuromuscular stimulation Group 2 (8 participants): bilateral + EMG‐triggered neuromuscular stimulation Both groups completed 5 consecutive upper limb protocols For the purposes of this review we compared the first treatment protocol from each group (unilateral wrist/finger extension + stimulation with a 5:25 stimulation/rest schedule versus bilateral wrist/finger extension + stimulation with a 5:25 stimulation/rest schedule Each training session involved 90 successful movement trials; completed in 4 days of 90 minutes training per day over 2 weeks Consecutive treatment protocols were separated on average by 4 weeks of no rehabilitation All 5 treatment protocols were administered over 12‐month period Profession of individual(s) administrating training unclear | |
| Outcomes | Primary outcome: functional movement of the upper limb: BBT Secondary outcome: motor impairment ‐ temporal outcomes: motor reaction time and total reaction time (motor reaction time selected); strength outcomes: sustained muscle contraction task ‐ maximal isometric contraction of wrist/finger extensors No suitable data were available for strength outcome Outcomes were recorded at the end of each intervention protocol (end of intervention period) | |
| Notes | Data presented in paper in graph format: mean and SE for BBT Means estimated from graph and standard deviation calculated from estimated standard error to allow for inclusion in statistical pooling 2 review authors independently estimated the values from the graphs; the average of the 2 estimates was used in the analysis Motor reaction data also presented in graph format: median and SE Median value estimated from graph imputed as mean and SD calculated from SE Motor reaction time score (m/s) inverted (multiplied by ‐1) for analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | No mention of allocation concealment |
| Blinding of outcome assessor? | Unclear risk | Not stated |
| Intention to treat analysis? | Unclear risk | Not stated |
| Baseline similarity | High risk | Group 1 mean time post‐stroke 1.41 years compared with Group 2 mean 4.22 years |
Chang 2006
| Methods | Randomised cross‐over design Participants each performed 3 tasks in randomly presented order This study was not designed or presented as a traditional RCT | |
| Participants | 20 participants Inclusion criteria: CT or MRI imaging evidence of single‐hemisphere stroke, arm reaching ability (Fugl‐Meyer assessment > 30), no perceptual‐cognitive dysfunction which limits comprehension of experimental task, no severe concurrent medical problems, no other neurological or orthopaedic conditions affecting arm/trunk movements | |
| Interventions | Each participant performed 3 movement tasks: (1) reaching forward with affected limb (unilateral); (2) reaching forward with both limbs simultaneously (bilateral); (3) reaching forward with both limbs simultaneously + load applied to non‐affected upper limb (bilateral + load) Each movement condition performed for 5 trials with 5‐minute rest between each condition Typical experimental session lasted approximately 40 minutes There was no training period ‐ movement and outcome measurement occurred simultaneously Profession of individual(s) providing training unclear | |
| Outcomes | Secondary outcome: motor impairment: kinematics on completion of elbow flexion ‐ temporal outcomes: movement time, movement velocity, number of movement units and normalised jerk score of movement (movement time selected); spatial outcomes: elbow flexion‐extension range, shoulder flexion‐extension range and trunk linear line value (elbow range selected) | |
| Notes | Data are not available for the first phase only of this study, and it is therefore not included in any analyses The unilateral and bilateral conditions would have been a suitable comparison | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | All participants completed training in each condition |
| Blinding of outcome assessor? | Unclear risk | Assessments completed at the same time as the training |
| Intention to treat analysis? | Unclear risk | Not stated |
| Baseline similarity | Unclear risk | Participants not separated into different groups |
Desrosiers 2005
| Methods | Randomly assigned by block randomisation scheme within each stratum (stratified on impairment level of hand and sensibility of the hand) Randomisation completed in blocks of 4 Allocation concealment completed through the use of sealed envelopes | |
| Participants | 41 participants Inclusion criteria: unilateral stroke > 10 days but < 2 months, cognitive functioning within normal limits, understand French or English, minimal upper extremity function (stage 2 for hand and stage 3 for arm on Chedoke‐McMaster Stroke Assessment), no severe body neglect or visual perception deficits | |
| Interventions | Group 1 (21 participants): usual care ‐ functional activities and exercises for the arm Group 2 (20 participants): bilateral ‐ package of interventions including bilateral and unilateral tasks Both groups received usual therapy interventions Both interventions provided by same occupational therapy research assistant Both groups received 4 x 45‐minute sessions per week for 5 weeks, in total receiving between 15 and 20 sessions Note: the descriptions of interventions provided in the full‐text paper are confusing; information given in the abstract has been central to the above classifications of the nature of the interventions | |
| Outcomes | Primary outcome: performance in activities of daily living: measure de l'independence fonctionelle (MIF ‐ French translation of FIM) Primary outcome: functional movement ‐ arm functional movement: BBT, TEMPA (BBT selected); hand functional outcome: Purdue Pegboard Test Secondary outcome: motor impairment: motor impairment scales: Fugl‐Meyer (upper limb section); temporal outcomes: co‐ordination (finger to nose, number of movements in 20 seconds); strength outcomes: grip strength (vigorimeter) AMPS also used as outcome measures but not relevant to this review | |
| Notes | Control group received usual care, however this may have contained some bilateral tasks; this could be a confounding factor Descriptions of interventions are unclear and definitions of symmetrical, synchronous and simultaneous are difficult to interpret 5 drop‐outs from Group 1 (lack of interest x 2, early release, fatigue, death) and 3 from Group 2 (death, fracture, refusal) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Sealed envelopes |
| Blinding of outcome assessor? | Low risk | Independent evaluator |
| Intention to treat analysis? | High risk | Only complete cases were analysed Drop‐outs were accounted for |
| Baseline similarity | Low risk | No significant differences between groups at baseline |
Dickstein 1993
| Methods | Randomised cross‐over design Participants each performed 3 movements in a randomly presented order This study was not designed or presented as a traditional RCT | |
| Participants | 25 participants Inclusion criteria: absence of cognitive impairments, unimpaired hearing, absence of movement disorders in unaffected upper extremity, ability to flex elbow on paretic side at least 30° from partial extension of 150°, not bilateral brain damage | |
| Interventions | Each participant performed 1 familiarisation set of unilateral movements with the unaffected arm, then performed 3 sets of movements presented in a random order (unilateral (unaffected), unilateral (affected) or bilateral) Each set comprised 16 elbow flexion movements which were carried out in response to an auditory signal There was no training period ‐ movement and outcome measurement occurred simultaneously Profession of individual(s) providing training unclear (assume physiotherapist) | |
| Outcomes | Secondary outcome: motor impairment ‐ temporal outcomes: reaction and movement time (movement time selected) | |
| Notes | Data are not available for the first phase only of this study and it is therefore not included in any analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | All participants completed training in each condition |
| Blinding of outcome assessor? | Unclear risk | Assessments completed at the same time as the training |
| Intention to treat analysis? | Unclear risk | Not stated |
| Baseline similarity | Unclear risk | Participants not separated into different groups |
Harris‐Love 2005
| Methods | Randomised cross‐over design Participants each performed 4 trials of 6 reaching tasks in a block randomised order This study was not designed or presented as a traditional RCT | |
| Participants | 32 participants Inclusion criteria: at least 6 months post‐stroke, at least 10° antigravity shoulder flexion and 20° of gravity minimised elbow extension, able to produce at least 5 cm of forward translation of the hand on a table without leaning forward, no orthopaedic conditions and/or pain in paretic arm or shoulder | |
| Interventions | Each participant performed 4 trials each of unilateral paretic, unilateral non‐paretic and bilateral reaching, then 4 trials of 6 reaching tasks (unilateral paretic, unilateral non‐paretic, bilateral reaching and 3 bilateral reaching tasks involving different loads added to the non‐paretic hand) completed at the fastest possible speed For all tasks participants were instructed to reach the target (box) as quickly as possible after a verbal go command and come to a complete stop There was no training period ‐ movement and outcome measurement occurred simultaneously Profession of individual(s) providing training unclear (assume physiotherapist) | |
| Outcomes | Secondary outcome: motor impairment ‐ temporal outcomes: movement time, peak velocity and peak acceleration (movement time selected) | |
| Notes | Data are not available for the first phase only of this study and it is therefore not included in any analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | All participants completed training in each condition |
| Blinding of outcome assessor? | Unclear risk | Assessments completed at the same time as the training |
| Intention to treat analysis? | Unclear risk | Not stated; no drop‐outs |
| Baseline similarity | Unclear risk | Participants not separated into different groups |
Kilbreath 2006
| Methods | Randomised cross‐over design Participants each performed 3 tasks in randomly presented order | |
| Participants | 13 participants Inclusion criteria: no significant musculotendinous or bony restrictions of upper limbs, no chronic disease independently causing significant disability or significant weakness of the upper limbs, sufficient strength in affected arm to move the arm forward at the shoulder and elbow and grasp with affected hand, score ≧ 1 on Frenchay upper limb test, comprehend simply instructions Note: it is unclear whether or not these were pre‐stated inclusion criteria, or whether these criteria are descriptors of the participants who were eventually included | |
| Interventions | Each participant performed 2 bimanual and 1 unimanual task Each task involved participant reaching, grasping and transporting a tray with either affected arm (unimanual task), reaching for a large tray with both arms or 2 small trays (bimanual tasks) There was no training period ‐ movement and outcome measurement occurred simultaneously Each task was performed 5 times Profession of individual(s) providing training unclear (assume physiotherapist) | |
| Outcomes | Secondary outcome: motor impairment ‐ kinematics of movement: the average of trials 3 to 5 for each condition; temporal outcomes: movement duration for hand to reach tray and for tray transport (hand to reach tray time selected (movement time)); spatial outcomes: lateral deviation of the hands, synchrony of hand movements and relative phase angle (lateral deviation of the hands selected) | |
| Notes | Study included another 13 participants with no stroke history; not included in participant numbers or analysis Data are not available for the first phase only of this study and it is therefore not included in any analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | All participants completed training in each condition |
| Blinding of outcome assessor? | Unclear risk | Assessments completed at the same time as the training |
| Intention to treat analysis? | Unclear risk | Not reported |
| Baseline similarity | Unclear risk | Participants not separated into different groups |
Lin 2009a
| Methods | Randomised controlled trial using a stratified block allocation scheme Computerised (block) randomisation, with pre‐stratification according to participating hospital Allocation concealment ensured by use of opaque, numbered envelopes (each hospital site had a pre‐prepared set of envelopes with cards indicating allocation) | |
| Participants | 60 participants Inclusion criteria: > 6 months post CVA, > Stage III Brunnstrom stage for proximal and distal parts of upper limb, considerable non‐use of the affected upper limb (Motor activity log, amount of use < 2.5), no serious cognitive deficits (≥ 24 on MMSE), no excessive spasticity in any joints of upper limb (Modified Ashworth Scale ≤ 2), lack of participation in any experimental rehabilitation or drug study within past 6 months, no balance problems sufficient to compromise safety when wearing constraint mitt | |
| Interventions | Group 1 (20 participants): usual care ‐ training for hand function, co‐ordination, balance and movements of the affected upper limb and compensatory practice with affected or both upper limbs Group 2 (20 participants): other upper limb intervention ‐ constraint‐induced therapy: restriction of movement of the unaffected hand by placement in a mitt for 6 hours/day and intensive training of the affected upper limb in functional tasks; level of ability adapted based on patient ability and improvement during training Group 3 (20 participants): bilateral training ‐ simultaneous movements of both affected and unaffected upper limb in functional tasks in symmetric or alternating patterns All groups completed therapy for 2 hours/day, 5 days per week for 3 weeks All other interdisciplinary rehabilitation continued Occupational therapists undertook the training in each group | |
| Outcomes | Primary outcome: performance in activities of daily living: Functional Independence Measure Primary outcome: functional movement ‐ Motor Activity Log: amount of use and quality of movement scales (amount of use scale selected); Stroke Impact Scale ‐ hand function section Secondary outcome: performance in extended activities of daily living: Stroke Impact Scale (ADL/IADL section); motor impairment ‐ motor impairment scales: Fugl‐Meyer scale | |
| Notes | Overall and sub‐scores for the Fugl‐Meyer and Functional Independence measure were presented We only entered the overall scores into the data analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Opaque, numbered envelopes |
| Blinding of outcome assessor? | Low risk | Occupational therapists blinded to group assignment completed outcome assessments |
| Intention to treat analysis? | Unclear risk | Not reported; no drop‐outs from study |
| Baseline similarity | Low risk | No significant differences between groups in terms of demographic and clinical characteristics |
Lin 2009b
| Methods | Randomised controlled trial Randomisation procedure and allocation concealment not reported | |
| Participants | 33 participants Inclusion criteria: clinical diagnosis of a first or recurrent unilateral stroke; ability to reach Brunnstrom stage III or above in the proximal and distal part of the arm; no serious cognitive deficits (MMSE ≥ 24); no excessive spasticity in the affected arm (Modified Ashworth Scale score ≤ 2 in any joint); no other neurologic, neuromuscular or orthopaedic disease; lack of participation in any experimental rehabilitation or drug studies | |
| Interventions | Group 1 (17 participants): usual care (dose‐matched standard occupational therapy that also focused on upper extremity training and included neurodevelopmental techniques, trunk‐arm control, weight bearing by the affected arm, fine motor tasks practice and practice on compensatory strategies) Group 2 (16 participants): bilateral training; both upper extremities moving simultaneously in functional tasks with symmetric patterns Both groups received training for 2 hours per day, 5 days a week for 3 weeks Occupational therapists provided the interventions | |
| Outcomes | Primary outcome: performance in activities of daily living: Functional independence measure Primary outcome: functional movement ‐ Motor Activity Log: amount of use and quality of movement scales (amount of use scale selected) Secondary outcome: motor impairment ‐ motor impairment scales: Fugl‐Meyer scale; temporal outcomes: movement time and percentage of movement time at which peak velocity occurs for unilateral and bilateral reaching task (movement time for unilateral task selected); spatial outcomes: normalised total distance Sub‐categories of the Functional Independence Measure are presented We only used the total score as most relevant to this review | |
| Notes | Adjusted means (controlling for pre‐treatment differences) and post‐treatment means were presented. We used adjusted means for all outcomes Standard deviation was taken from the post‐treatment columns Movement time and spatial outcome data inverted for analysis (multiplied by ‐1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding of outcome assessor? | Low risk | Occupational therapists blind to group assignment were trained to complete the outcome assessments |
| Intention to treat analysis? | Unclear risk | Not reported; no drop‐outs |
| Baseline similarity | Low risk | Baseline characteristics were comparable No significant differences between the groups for age, months since stroke, side of stroke lesion, or initial upper limb impairment |
Luft 2004
| Methods | Randomised controlled trial using a stratified block allocation scheme (variable block size, allocation 1:1) | |
| Participants | 21 participants Inclusion criteria: residual upper extremity spastic hemiparesis following single cortical or subcortical ischaemic stroke; ability to move affected limb (at least partial range movement against gravity); completed 3 to 6 months of rehabilitation therapy; adequate language and neurocognitive function to understand instructions; no multiple strokes, history of other neurological disease, chronic pain or emotional disorders | |
| Interventions | Group 1 (12 participants): usual care (DMTE based on neurodevelopmental principles) Group 2 (9 participants): BATRAC BATRAC consisted of pushing and pulling bilaterally, either in synchrony or alternation, 2 independent handles sliding in the traverse plane. It consisted of hour‐long therapy sessions (4 x 5‐minute movement periods interspersed with 10‐minute rest periods) 3 times per week for 6 weeks DMTE was based on neurodevelopmental principles and included mobilisation, weight‐bearing and opening a closed fist; the exercises were administered in a standard format and equal to the time used for BATRAC Profession of individual(s) providing training unclear (assume physiotherapist) | |
| Outcomes | Primary outcome: functional movement ‐ arm functional movement: WMAT (time to complete 14 functional tasks with affected arm and hand and University of Maryland Arm Questionnaire for Stroke) (WMAT selected) Secondary outcome: motor impairment ‐ Fugl‐Meyer Motor Performance Test (upper limb section); strength outcomes: WMAT (strength) and dynamometry (elbow and shoulder strength) (WMAT strength selected) fMRI and EMG variables also recorded ‐ these were not relevant to this review | |
| Notes | Bilateral training group also received rhythmic auditory cueing, to guide the speed of the movements Discussion amongst review authors led to the conclusion that the rhythmic auditory cueing could be viewed as an adjunct or guide to the bilateral training and that therefore this study was relevant to this review (i.e. the rhythmic auditory cueing has not been considered as another intervention) This study is a substudy of a larger study designed to investigate the effect of BATRAC SEM presented in paper, this was converted into SD units and entered into the analysis Change scores presented in paper and used in analysis WMFT (time) data inverted for analysis (multiplied by ‐1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | No mention of allocation concealment |
| Blinding of outcome assessor? | Low risk | ‐ |
| Intention to treat analysis? | High risk | No mention of intention‐to‐treat analysis; reasons for drop‐out reported |
| Baseline similarity | High risk | No difference in terms of age, time since stroke, or baseline scores but significantly more women in Group 1 (unilateral group) |
Lum 2006
| Methods | Randomly allocated Patients were stratified by initial Fugl‐Meyer score and side of stroke and randomly assigned to 1 of 4 groups Following interim analysis the randomisation schedule was changed from providing the same number of participants to each group so that subsequent participants could only be allocated to 2 of the groups, therefore participants did not have an equal chance of entering 1 of the 4 groups The change in randomisation during the trial may have introduced bias | |
| Participants | 30 participants (only 2 groups of participants ‐ 14 participants ‐ relevant to this review) Inclusion criteria: single CVA, 1 to 5 months post‐stroke, no upper‐limb joint pain or ROM limitations that would limit ability to complete training, no unstable cardiovascular, orthopaedic or neurological conditions, > 21 on MMSE | |
| Interventions | Group 1 (9 participants): robot‐unilateral group, 12 reaching tasks progressing from easiest robotic‐mode to most challenging mode Group 2 (5 participants): robot‐bilateral group, practised same 12 reaching tasks but in bilateral mode rhythmic circular movements also performed Training lasted 1 hour per session for 15 sessions over 4 weeks Training was supervised by an occupational therapist | |
| Outcomes | Primary outcome: performance in activities of daily living: Functional Independence Measure (self‐care and transfer sections only) Secondary outcome: motor impairment ‐ motor impairment scales: Fugl‐Meyer (proximal and distal upper limb sections), Motor Status Score (movement scale and synergy scale) and Modified Ashworth scale (proximal and distal scores) (Fugl‐Meyer (proximal upper limb section) selected for analysis); strength outcomes: Motor power examination (several joints across proximal upper limb) | |
| Notes | This study included assistive technology, however it compared a bilateral and unilateral group both receiving robotic assistance, therefore we decided that this was relevant to include as bilateral training versus unilateral training 4 groups were included in this trial: robot‐unilateral, robot‐bilateral, robot‐combined and control Only robot‐unilateral and robot‐bilateral relevant to this review Participants in the other 2 groups (16 participants) not included in any analysis Average gains data presented in paper and used in analysis Standard deviations calculated from presented standard error of the mean to allow for inclusion in statistical pooling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Initially allocation concealment was possible, however an interim analysis was carried out to compare the groups and, as a result the randomisation process was changed so that later participants could only be entered into 1 of the 2 groups, not 1 of the 4 |
| Blinding of outcome assessor? | Low risk | An occupational therapist blinded to group assignment completed the assessments |
| Intention to treat analysis? | Unclear risk | Not stated; all participants completed the training and post‐treatment evaluations |
| Baseline similarity | Low risk | No significant differences at baseline |
Morris 2008
| Methods | Randomised controlled trial Randomly allocated using concealed web‐based randomisation Stratified according to side of hemiplegia, stroke classification and baseline ARAT | |
| Participants | 106 participants Inclusion criteria: acute unilateral stroke confirmed by CT; persistent upper limb impairment (< 6 on each upper limb sections of Motor Assessment Scale); ability to participate in 30‐minute physiotherapy sessions; ability to sit unsupported for 1 minute; no severe neglect, aphasia or cognitive impairment that would limit participation; no previous stroke resulting in residual disability; no pre‐morbid arm impairment; no hemiplegic shoulder pain; ability to provide informed consent | |
| Interventions | Group 1 (50 participants): unilateral; performed 4 tasks (moving dowelling peg, moving block, grasp empty glass and take to mouth and point to targets) with affected arm Group 2 (56 participants): bilateral; performed same 4 tasks with each arm simultaneously The intervention protocol was progressive and standardised Systematic feedback was provided on performance Training lasted 20 minutes a session 5 weekdays a week over 6 weeks in addition to usual therapy As many trials as possible were completed in each session with a maximum of 30 trials of each task, maximum of 120 trials per session 2 senior stroke rehabilitation physiotherapists with 15 years experience conducted the intervention | |
| Outcomes | Primary outcome: performance in activities daily living: Barthel Index Primary outcome: functional movement ‐ arm functional movement: ARAT; hand functional movement: Nine Hole Peg Test Secondary outcome: motor impairment ‐ motor impairment scales: Rivermead Motor Assessment (upper limb section) Hospital Anxiety and Depression Scale and Nottingham Health Profile also used as outcome measures but not relevant to this review | |
| Notes | End of intervention outcome assessment (6 weeks) used in analysis Outcome measures also recorded after 18 weeks (97 participants) At 6 weeks: 4 drop‐outs from Group 1 (died, moved away, requested withdrawal) and 5 drop‐outs from Group 2 (died, moved away, requested withdrawal) Change and final outcome scores presented Outcome scores used in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Concealed, remote web‐based randomisation |
| Blinding of outcome assessor? | Low risk | Blinded to treatment allocation and otherwise not involved in trial |
| Intention to treat analysis? | High risk | Analysis done on complete data sets only. Statistical comparison did show drop‐outs in both groups to be similar |
| Baseline similarity | Low risk | ‐ |
Mudie 2001: Acute
| Methods | Random assignment to 1 of 2 groups Method of randomisation not stated | |
| Participants | 36 participants Inclusion criteria: dense hemiplegia (less than or equal to 2 on Motor Assessment Scale items 6 and 7), able to understand instructions, produce a response with non‐hemiplegic arm during bilateral trials, no other strokes or confounding co‐morbidities | |
| Interventions | Group 1 (18 participants): unilateral Group 2 (18 participants): bilateral Each group completed 5 trials, including 5 repetitions of 5 seconds each (of isometric contractions for 2 tasks (shoulder abduction and wrist extension)) 15 seconds rest between each of the 5 trials, and 5 minutes rest between the 2 tasks There was no training period: movement and outcome measurement occurred simultaneously Profession of individual(s) providing training unclear (assume occupational therapist) For Group 1, trials 1, 2, 3 and 5 were performed unilaterally and trial 4 bilaterally For Group 2, trials 1, 3 and 5 were performed unilaterally and trials 2 and 4 bilaterally Therefore, data from trial 2 only was extracted for this review | |
| Outcomes | Secondary outcomes: motor impairment ‐ strength outcomes: muscle activity (EMG) for shoulder abduction and wrist extension were reported (data from wrist extension activity only was extracted for analysis) | |
| Notes | Results for acute and chronic patients presented separately, therefore 2 subgroups of this trial are included in the relevant analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Method of randomisation and allocation concealment not stated |
| Blinding of outcome assessor? | Unclear risk | Assessment completed at the same time as the training |
| Intention to treat analysis? | Unclear risk | Not stated |
| Baseline similarity | Low risk | No significant differences between groups |
Mudie 2001: Chronic
| Methods | (as Mudie 2001: Acute) | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Platz 2001
| Methods | Random allocation to 1 of 2 groups, with blocked randomisation according to side of stroke Details of allocation concealment not stated | |
| Participants | 14 participants Inclusion criteria: CT‐proven stroke in middle cerebral artery territory, sub‐acute phase, clinically complete or almost complete recovery from hemiparesis, no cognitive impairment Note: it is unclear whether or not these were pre‐stated inclusion criteria, or whether these criteria are descriptors of the included participants written following patient assessment | |
| Interventions | Group 1: unilateral training Group 2: bilateral training Each group completed 3 training tasks (fast and accurate aiming movements, fast tapping movements with index finger, picking up and placing small wooden sticks) Each participant completed training comprising of 10 practice blocks, each lasting 2.5 minutes Tasks were completed in a repetitive way and serial order Total training time was approximately 30 minutes per session, performed on 5 consecutive weekdays Training was supervised by an occupational therapist | |
| Outcomes | Secondary outcome: motor impairment ‐ temporal outcomes: total movement time (ms), MT/first phase, MT/second phase, MT coefficient of variation (total movement time selected); spatial outcomes: spatial error (mm), spatial error/first phase (spatial error selected) All outcomes assessed for aiming movements during single task and dual task Outcome data for single task aiming movement used for analysis | |
| Notes | Data extracted comprised least square means Standard deviation for outcome not provided Baseline standard deviation used as estimated value for both groups and imputed for the analysis Number of participants in each group not stated; assumed 50% (7 participants) assigned to each group 14 healthy controls were also recruited; numbers not included in participant numbers or in analysis Movement time and spatial error data inverted for analysis (multiplied by ‐1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding of outcome assessor? | Unclear risk | Not stated |
| Intention to treat analysis? | Unclear risk | Not stated |
| Baseline similarity | Low risk | Reported as comparable with regards to age, gender, cortical versus subcortical/basal ganglia stroke and severity of residual paresis |
Stoykov 2009
| Methods | Randomised controlled trial Stratified into 2 impairment levels based on Fugl‐Meyer upper extremity scores (19 to 28 or 29 to 40) Within each group of 12 participants a randomised computer‐generated list provided group assignment | |
| Participants | 24 participants Inclusion criteria: Fugl‐Meyer upper extremity score 19 to 40, ≥ 6 months post‐stroke, cortical or subcortical lesion, ability to follow 2‐step commands, 18 to 80 years of age, no evidence of cerebellum or brainstem involvement, no evidence of field cut, no evidence of neglect, ability to give informed consent, no symptomatic cardiac failure or unstable angina, no uncontrolled hypertension, no significant orthopaedic or pain conditions in affected upper extremity, no severe obstructive pulmonary disease | |
| Interventions | Group 1 (12 participants): unilateral training Group 2 (12 participants): bilateral training Training consisted of 6 training tasks that incorporated both discrete movements (2 tasks) and rhythmic movements (4 tasks), paced by a metronome Initially most tasks completed for 20 repetitions, which was gradually increased to 40 repetitions Therapeutic challenge was increased throughout the training period 3 training sessions of 1 hour duration completed each week for 8 weeks were completed Profession of individual administering training not reported | |
| Outcomes | Primary outcome: functional movement ‐ arm functional movement: Motor Assessment Scale (upper arm function and combined upper limb movements; upper arm function scores used for analysis); hand functional movement: Motor Assessment Scale (hand movements and advanced hand movements; hand movement scores used for analysis) Secondary outcome: motor impairment ‐ motor impairment scales: Motor Status Score (total scale, shoulder/elbow scale and wrist/hand scale; total scale selected for use in analysis); strength outcomes: muscle strength comparator dynamometer for arm strength and Jamar dynamometer for grip strength (arm strength outcome selected for use in analysis) | |
| Notes | Data presented in paper in graph format ‐ mean and SE for Motor Assessment Scale and Motor Status Score Means estimated from graph and standard deviation calculated from estimated standard error to allow for inclusion in statistical pooling 2 review authors independently estimated the values from the graphs; the average of the 2 estimates was used in the analysis Unable to include strength outcome in analysis as separate results for the 2 groups (unilateral and bilateral) not presented A non‐significant result between the groups reported in the paper on these measures and this indicated in the results section | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Computer‐generated list provided group assignment but first author enrolled participants and provided both treatment interventions |
| Blinding of outcome assessor? | Low risk | Single rater completed outcome assessments blinded to group allocation and study methodology |
| Intention to treat analysis? | Unclear risk | Not reported; no drop‐outs reported |
| Baseline similarity | Low risk | No differences between groups evident No significant differences reported between groups in terms of age, years post‐stroke or baseline Fugl‐Meyer upper extremity score |
Summers 2007
| Methods | Randomised controlled trial Participants randomly allocated to 1 of 2 groups Method of randomisation and allocation concealment not stated | |
| Participants | 12 participants Inclusion criteria: first stoke at least 3 months prior to intervention, no multiple infarctions, most components of movement present in the affected extremity but impairment of function relative to unaffected side, intact cognitive functions, no other neurological disorders | |
| Interventions | Group 1 (6 participants): unilateral Group 2 (6 participants): bilateral Participants performed 50 training trials of a dowel placement task (lifting a wooden dowel from table and placing it on a shelf) and 2 warm‐up reaching trials during each session 6 sessions completed over a period of 6 days Profession of individual administering training not reported | |
| Outcomes | Primary outcome: functional movement ‐ arm functional movement: Modified Motor Assessment Scale upper arm function and combined upper limb movements (upper arm function scores used for analysis); hand functional movement: Modified Motor Assessment scale hand movements and advanced hand movements (hand movement scores used for analysis) Secondary outcome: motor impairment ‐ temporal outcomes: movement time and velocity profile (movement time selected); spatial outcomes: elbow angle and curvature of arm trajectories (elbow angle selected) TMS recorded but not relevant to this review | |
| Notes | SD for bilateral group equals 0 for upper arm function on Modified Motor Assessment Scale, therefore effect size not estimable Imputed control group SD to allow for statistical pooling No SD presented for movement kinematics and therefore unsuitable for inclusion in statistical pooling 2 participants excluded from movement time and elbow angle analysis due to technical difficulties within the trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding of outcome assessor? | Low risk | Blinded to assignment of participants (for completion of motor assessment scale) Unclear if blinded for assessment of movement time and elbow angle outcomes |
| Intention to treat analysis? | Unclear risk | No mention of ITT |
| Baseline similarity | High risk | Similar on variables of age, sex and affected side Group 2 mean 6.3 years post‐stroke, compared with Group 1 mean of 4.0 years |
AMPS: Assessment of Motor and Process Skills ARAT: Action Research Arm Test BATRAC: bilateral training with auditory cueing BBT: box and block test CT: computerised tomography CVA: cerebrovascular accident DMTE: dose matched therapeutic exercises EMG: electromyogram fMRI: functional MRI ITT: intention‐to‐treat MMSE: Mimi Mental State Examination MRI: magnetic resonance imaging ms: metres per second RCT: randomised controlled trial ROM: range of movement SD: standard deviation SE: standard error SEM: standard error of the mean TEMPA: upper extremity performance test for the elderly TMS: transcranial magnetic stimulation WMAT: Wolf Motor Arm Test WMFT: Wolf Motor Function Test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Altschuler 1998 | All groups received bilateral training Intervention of interest was method of completing bilateral training (mirror or transparent plastic) |
| Barnes 2006 | Bilateral training but not RCT |
| Cauraugh 2003b | All groups received bilateral training Intervention of interest was active neuromuscular stimulation |
| Cauraugh 2009 | Bilateral training completed in 2 groups; however, both groups also received neuromuscular stimulation and only difference between groups was load added to unimpaired arm or not Control group did not receive bilateral training or neuromuscular stimulation |
| Chan 2009 | Both groups received bilateral training Intervention of interest was functional electric stimulation |
| Chang 2007 | Bilateral training but completed with assistive technology |
| Cunningham 2002 | Bilateral training but not RCT |
| Dohle 2009 | All groups received a form of bilateral training Intervention of intervention was mirror therapy (mirror in situ or not while both limbs moved) |
| Garry 2005 | Bilateral training but not RCT |
| Hesse 2003 | Bilateral training but completed with assistive technology |
| Hesse 2005 | Bilateral training but completed with assistive technology |
| Hesse 2008 | Bilateral training but completed with assistive technology |
| Lewis 2004a | Bilateral training but not RCT |
| Lewis 2004b | Randomised order trial of bilateral training but no relevant outcomes |
| Lum 2002 | Bilateral training but completed with assistive technology |
| McCombe Waller 2004 | Bilateral training but not RCT |
| McCombe Waller 2005 | Bilateral training but not RCT |
| McCombe Waller 2006 | Bilateral training but not RCT |
| Messier 2005 | Bilateral training but not RCT |
| Messier 2006 | Bilateral training but not RCT |
| Mudie 1996 | Bilateral training but not RCT |
| Mudie 2000 | Bilateral training but not RCT |
| Richards 2008 | Bilateral training but not RCT |
| Rose 2004 | Bilateral training but not RCT |
| Rose 2005 | Bilateral training but not RCT |
| Stevens 2004 | Bilateral training but not RCT |
| Stinear 2004 | Bilateral training, however unaffected arm assisted hemiplegic arm using a device (assistive technology) |
| Stinear 2008 | Bilateral training, however unaffected arm assisted hemiplegic arm using a device (assistive technology) |
| Tijs 2006 | Bilateral training but not RCT |
| Whitall 2000 | Bilateral training but not RCT |
| Yavuzer 2008 | Both groups completed a form of bilateral training Intervention of interest was mirror therapy (mirror in situ or not) During session patients were asked to try and attempt the same movements with the paretic had while moving non‐paretic hand |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Aimet 2003
| Methods | 2 groups but unclear how participants allocated to each group |
| Participants | 22 stroke patients |
| Interventions | Group 1 trained both the arm extensor muscles of the paretic arm and both arms in one exercise unit Group 2 trained only the extensors of the paretic arm on the arm press machine |
| Outcomes | Arm extensor strength |
| Notes | Poster abstracts only; attempting to contact authors regarding full methods of the review |
Miller 2000
| Methods | Blocked randomisation |
| Participants | Ongoing study |
| Interventions | Control intervention: aimed at improving postural control and concentration Treatment group: task‐specific training of affected upper limb emphasising unilateral and bilateral functional activities |
| Outcomes | Sensation, dexterity and motor recovery |
| Notes | Poster abstract of an ongoing study; attempting to contact authors regarding details of intervention |
NINDS 2006
| Methods | Cross‐over study |
| Participants | 40 chronic stroke patients |
| Interventions | Group 1: single session unilateral paretic arm training Group 2: single session bilateral arm training |
| Outcomes | Reaching test, TMS |
| Notes | Ongoing study |
Whitall 2002
| Methods | RCT |
| Participants | 72 participants |
| Interventions | Group 1: dose equivalent conventional OT/PT Group 2: bilateral arm training with rhythmic auditory cueing |
| Outcomes | Motor function, upper‐limb daily use, quality of life, TMS |
| Notes | Larger study of Luft 2004 Ongoing study Contacted author who indicated that the study is in the process of being written up and will hopefully be published by the end of 2009 This publication will be reviewed for inclusion in updates of the review |
OT: occupational therapy PT: physiotherapy RCT: randomised controlled trial TMS: transcranial magnetic stimulation
Characteristics of ongoing studies [ordered by study ID]
Cauraugh 2006
| Trial name or title | Subacute stroke recovery (upper extremity motor function): bimanual co‐ordination training |
| Methods | Treatment, randomised, double‐blind (participant, outcomes assessor), dose comparison, parallel assignment, efficacy study |
| Participants | 44 participants Inclusion criteria: ability to complete 10º of wrist or finger extension from a 60 to 65º flexed position; score less than 56 on the upper extremity subscale of the Fugl‐Meyer Assessment; ability to voluntarily activate slight movements in the wrist and fingers so that the EMG activity reaches a minimal level on the microprocessor for electrical stimulation to be activated; unilateral, first stroke of ischaemic or haemorrhagic origin in the carotid artery distribution; free of major post‐stroke complications; able to attend therapy 2 days/week or 4 days/week for 2 weeks; score at least 16 on the MMSE; able to discriminate sharp from dull and light touch using traditional sensation tests Exclusion criteria: hemiparetic arm is insensate; motor impairments from stroke on opposite side of body; pre‐existing neurological disorders such as Parkinson's disease, multiple sclerosis, or dementia; legal blindness or severe visual impairment; life expectancy less than 1 year; severe arthritis or orthopaedic problems that limit passive range of motion of upper extremity joints (passive finger extension < 40º; passive wrist extension < 40º; passive elbow extension < 40º; shoulder flexion/abduction < 80º); history of sustained alcoholism or drug abuse in the last 6 months; has pacemaker or other implanted device; pregnant |
| Interventions | Behavioural: bilateral movement practice + neuromuscular electrical stimulation Behavioural: bilateral motor practice + neuromuscular electrical stimulation Behavioural: sham electrical stimulation + bilateral motor practice |
| Outcomes | Fugl‐Meyer Upper Extremity Motor Test Box and Block Test Wolf Motor Function Test Fractionated Reaction Time and Sustained Muscle Contraction |
| Starting date | August 2006 |
| Contact information | James H Cauraugh PhD jcaura@hhp.ufl.edu |
| Notes | Trial due to complete July 2009 We have contacted the investigators of this trial to identify if this study is the same as reported Cauraugh 2009 (excluded trial) |
Thonnard 2009
| Trial name or title | Effect of rehabilitation of patients with a central nervous system lesion |
| Methods | Supportive care, single‐blind (outcomes assessor), cross‐over assignment |
| Participants | 15 participants Inclusion criteria: adult minimum 6 months after first stroke, minimal prehension of both hands, not diabetic, no other upper limb pathologies, more than MMSE 26 |
| Interventions | Experimental: bilateral and unilateral prehension‐oriented rehabilitation to enhance prehension |
| Outcomes | Prehension functionality |
| Starting date | September 2006 |
| Contact information | Jean‐Louis Thonnard jean‐louis.thonnard@uclouvain.be |
| Notes | Trial due to complete September 2009 |
EMG: electromyogram MMSE: Mini Mental Status Examination
Contributions of authors
Fiona Coupar (FC) co‐ordinated the review process and managed searching and main data extraction input. Fiona Coupar, Alex Pollock and Frederike van Wijck (FvW) undertook searching for trials and decided upon trial inclusion/exclusion. Jacqui Morris (JM) provided assistance with this process. All review authors assisted with data extraction and assessment of methodological quality. Peter Langhorne (PL) provided methodological advice and reviewed major drafts to ensure review quality.
Sources of support
Internal sources
Greater Glasgow Health Board Managed Clinical Network for Stroke, UK.
-
Chief Scientist Office, Scotland, UK.
Fiona Coupar is currently in receipt of a CSO Research Training Fellowship
-
NMAHP Research Unit, Glasgow Caledonian University, UK.
Alex Pollock is employed by NMAHP Research Unit, which is funded by the Chief Scientist Office.
External sources
Big Lottery Fund, UK.
Declarations of interest
Jacqui Morris and Frederike van Wijck were authors of one of the studies included in this review. Methodological quality for this study was assessed by Fiona Coupar and Alex Pollock.
The work presented here represents the view of the author(s) and not necessarily those of the funding bodies.
New
References
References to studies included in this review
- Cauraugh JH, Kim S. Two coupled motor recovery protocols are better than one. Electromyogram ‐triggered neuromuscular stimulation and bilateral movements. Stroke 2002;33:1589‐94. [DOI] [PubMed] [Google Scholar]
- Cauraugh JH, Kim S. Progress toward motor recovery with active neuromuscular stimulation: muscle activation pattern evidence after a stroke. Journal of the Neurological Sciences 2003;207:25‐9. [DOI] [PubMed] [Google Scholar]
- Cauraugh JH, Kim AB, Duley A. Coupled bilateral movements and active neuromuscular stimulation: intralimb transfer evidence during bimanual aiming. Neuroscience Letters 2005;382:39‐44. [DOI] [PubMed] [Google Scholar]
- Cauraugh JH, Kim S‐B, Summers JJ. Chronic stroke longitudinal motor improvements: cumulative learning evidence found in the upper extremity. Cerebrovascular Diseases 2008;25:115‐21. [DOI] [PubMed] [Google Scholar]
- Chang JJ, Tung WL, Wu WL, Su FC. Effect of bilateral reaching on affected arm motor control in stroke ‐ with and without loading on unaffected arm. Disability and Rehabilitation 2006;28(24):1507‐16. [DOI] [PubMed] [Google Scholar]
- Desrosiers J, Bourbonnais D, Corriveau H, Gosselin S, Bravo G. Effectiveness of unilateral and symmetrical bilateral task training for arm during the subacute phase of stroke: a randomised controlled trial. Clinical Rehabilitation 2005;19:581‐93. [DOI] [PubMed] [Google Scholar]
- Dickstein R, Hocherman S, Amdor G, Pillar T. Reaction and movement times in patients with hemiparesis for unilateral and bilateral elbow flexion. Physical Therapy 1993;73(6):374‐85. [DOI] [PubMed] [Google Scholar]
- Harris‐Love M, McCombe Waller S, Whitall J. Exploiting interlimb coupling to improve paretic arm reaching performance in people with chronic stroke. Archives of Physical Medicine and Rehabilitation 2005;86:2131‐7. [DOI] [PubMed] [Google Scholar]
- Kilbreath SL, Crosbie J, Canning G, Lee M‐J. Inter‐limb coordination in bimanual reach‐to‐grasp following stroke. Disability and Rehabilitation 2006;28(23):1435‐43. [DOI] [PubMed] [Google Scholar]
- Lin K‐C, Chang Y‐F, Wu C‐Y, Chen Y‐A. Effects of constraint induced therapy versus bilateral arm training on motor performance, daily functions and quality of life in stroke survivors. Neurorehabilitation and Neural Repair 2009;23(5):441‐8. [DOI] [PubMed] [Google Scholar]
- Lin K‐C, Chen Y‐A, Chen C‐L, Wu C‐Y, Chang Y‐F. The effects of bilateral arm training on motor control and functional performance in chronic stroke: a randomized controlled study. Neurorehabilitation and Neural Repair 2010;24(1):42‐51. [DOI] [PubMed] [Google Scholar]
- Luft AR, McCombe‐Waller S, Whitall J, Forrester LW, Macko R, Sorkin JD, et al. Repetitive bilateral arm training and motor cortex activation in chronic stroke. JAMA 2004;292:1853‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]; McCombe Waller S, Liu W, Whitall J. Temporal and spatial control following bilateral versus unilateral training. Human Movement Science 2008;27:749‐58. [DOI] [PubMed] [Google Scholar]
- Lum PS, Burgar CG, Loos M, Shor PC, Majmundar M, Yap R. MIME robotic device for upper‐limb neurorehabilitation in subacute stroke subjects: a follow‐up study. Journal of Rehabilitation Research and Development 2006;43(5):631‐42. [DOI] [PubMed] [Google Scholar]
- MacWalter RS. An investigation of bilateral simultaneous upper limb task training in acute stroke: a randomised controlled trial. www.controlled‐trials.com/2003. ; Morris J. Does bilateral simultaneous upper limb task training improve motor performance in acute stroke patients more than unilateral task training?. Proceedings of the Chartered Society of Physiotherapists Congress, 13‐14 October 2006, Birmingham: UK. Chartered Society of Physiotherapists. 2006. [Google Scholar]; Morris J, MacWalter RS, Ogston S, Scottish Executive Health Department Chief Scientist Office Focus on Research. Investigating bilateral simultaneous upper limb task training in acute stroke: a randomised controlled trial. Scottish Executive Health Department Chief Scientist Office Focus on Research. www.show.scot.nhs.uk/cso/index.htm.2006. ; Morris J, Wijck F, Joice S, Cole I, Ogston S, MacWalter R. Responses to bilateral training compared to unilateral tasks training in acute stroke: a randomised controlled trial. Cerebrovascular Diseases 2008;25:143 (Abst 2). [Google Scholar]; Morris JH, Wijck F, Joice S, Ogston SA, Cole I, MacWalter ARS. A comparison of bilateral and unilateral upper‐limb task training in early poststroke rehabilitation: a randomised controlled trial. Archives of Physical Medicine and Rehabilitation 2008;89:1237‐45. [DOI] [PubMed] [Google Scholar]
- Mudie MH, Matyas TA. Responses of the densely hemiplegic upper extremity to bilateral training. Neurorehabilitation and Neural Repair 2001;15:129‐40. [DOI] [PubMed] [Google Scholar]
- Mudie MH, Matyas TA. Responses of the densely hemiplegic upper extremity to bilateral training. Neurorehabilitation and Neural Repair 2001;15:129‐40. [DOI] [PubMed] [Google Scholar]
- Platz T, Bock S, Prass K. Reduced skillfulness of arm motor behaviour among motor stroke patients with good clinical recovery: does it indicate reduced automaticity? Can it be improved by unilateral or bilateral training? A kinematic motion analysis study. Neuropsychologia 2001;39:687‐98. [DOI] [PubMed] [Google Scholar]
- Stoykov ME, Lewis GN, Corcos DM. Comparison of bilateral and unilateral training for upper extremity hemiparesis in stroke. Neurorehabilitation and Neural Repair 2009;23:945. [DOI] [PubMed] [Google Scholar]
- Summers JJ, Kagere FA, Garry MI, Hiraga CY, Loftus A, Cauraugh JH. Bilateral and unilateral movement training on upper limb function in chronic stroke patients: a TMS study. Journal of the Neurological Sciences 2007;252:76‐82. [DOI] [PubMed] [Google Scholar]; Summers JJ, Kagerer FA, Garry MI, Loftus A. Unilateral and bilateral movement training after stroke: effects on motor function and cortical organisation. Internal Medicine Journal 2005;35 Suppl 2:A10. [Google Scholar]
References to studies excluded from this review
- Altschuler EL, Wsidon SB, Stone L, Foster C, Galasko D, Llewellyn DME, et al. Rehabilitation of hemiparesis after stroke with a mirror. Lancet 1998;353:2035‐6. [DOI] [PubMed] [Google Scholar]
- Barnes CL, Smith MB, Harriet E, Kunisch A, Little C, Modica J, et al. A pilot study of bilateral arm training with repetitive auditory cueing in subjects with low functioning upper limb hemiparesis as a result of chronic stroke. Journal of Neurologic Physical Therapy 2006;4:221. [Google Scholar]
- Cauraugh JH, Kim SB. Chronic stroke motor recovery: duration of active neuromuscular stimulation. Journal of the Neurological Sciences 2003;215:13‐9. [DOI] [PubMed] [Google Scholar]
- Cauraugh JH, Coombes SA, Lodha N, Naik SK, Summers JJ. Upper extremity improvements in chronic stroke: coupled bilateral load training. Restorative Neurology and Neuroscience 2009;27:17‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MK, Tong RK, Chung KY. Bilateral upper limb training with functional electrical stimulation in patients with chronic stroke. Neurorehabilitation and Neural Repair 2009;23(4):357‐65. [DOI] [PubMed] [Google Scholar]
- Chang JJ, Tung WL, Wu WL, Huang MH, Su FC. Effects of robot‐aided bilateral force‐induced isokinetic arm training with conventional rehabilitation on arm motor function in patients with chronic stroke. Archives of Physical Medicine and Rehabilitation 2007;88:1332‐8. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Stoykov ME, Walter CB. Bilateral facilitation of motor control in chronic hemiplegia. Acta Psychologica 2002;110:321‐37. [DOI] [PubMed] [Google Scholar]
- Dohle C, Pullen J, Nakaten A, Kust J Reitz C, Karbe H. Mirror therapy promotes recovery form severe hemiparesis: a randomised controlled trial. Neurorehabilitaiton and Neural Repair 2009;23:209‐17. [DOI] [PubMed] [Google Scholar]
- Garry MI, Steenis RE, Summers JJ. Interlimb coordination following stroke. Human Movement Science 2005;24:849‐64. [DOI] [PubMed] [Google Scholar]
- Hesse S, Schulte‐Tigges G, Konrad M, Bardeleben A, Werner C. Robot‐assisted arm trainer for the passive and active practice of bilateral forearm and wrist movements in hemiparetic subjects. Archives of Physical Medicine and Rehabilitation 2003;84:915‐20. [DOI] [PubMed] [Google Scholar]
- Hesse S, Werner C, Pohl M, Rueckriem S, Mehrholz J, Lingnau ML. Computerized arm training improves the motor control of the severely affected arm after stroke: a single‐blinded randomized trial in two centers. Stroke 2005;36:1960‐6. [DOI] [PubMed] [Google Scholar]
- Hesse S, Werner C, Pohl M, Merholz J, Puzich U, Krebs HI. Mechanical arm trainer for the treatment of the severely affected arm after a stroke; a single‐blinded randomized trial in two centers. American Journal of Physical Medicine and Rehabilitation 2008;87:779‐88. [DOI] [PubMed] [Google Scholar]
- Lewis GN, Byblow WD. Neurophysiological and behavioural adaptations to a bilateral training intervention in individuals following stroke. Clinical Rehabilitation 2004;18:48‐59. [DOI] [PubMed] [Google Scholar]
- Lewis GN, Byblow WD. Bimanual coordination dynamics in poststroke hemiparetics. Journal of Motor Behavior 2004;36(2):174‐88. [DOI] [PubMed] [Google Scholar]
- Lum PS, Burgar CG, Shor PC, Majmundar M, Loos M. Robot‐assisted movement training compared with conventional therapy techniques for the rehabilitation of upper‐limb motor function after stroke. Archives of Physical Medicine and Rehabilitation 2002;83:952‐9. [DOI] [PubMed] [Google Scholar]
- McCombe Waller S, Whitall J. Fine motor control in adults with and without chronic hemiparesis: baseline comparison to nondisabled adults and effects of bilateral arm training. Archives of Physical Medicine and Rehabilitation 2004;85:1076‐83. [DOI] [PubMed] [Google Scholar]
- McCombe Waller S, Whitall J. Hand dominance and side of stroke affect rehabilitation in chronic stroke. Clinical Rehabilitation 2005;19:544‐51. [DOI] [PubMed] [Google Scholar]
- McCombe Waller S, Harris‐Love M, Liu W, Whitall J. Temporal coordination of the arms during bilateral simultaneous and sequential movements in patients with chronic hemiparesis. Experimental Brain Research 2006;168:450‐4. [DOI] [PubMed] [Google Scholar]
- Messier Sl, Bourbonnais D, Desrosiers J, Roy Y. Weight‐bearing on the lower limbs in a sitting position during bilateral movement of the upper limbs in post‐stroke hemiparetic subjects. Journal of Rehabilitation Medicine 2005;37:242‐6. [DOI] [PubMed] [Google Scholar]
- Messier S, Bourbonnais D, Desrosiers J, Roy Y. Kinematic analysis of upper limbs and trunk movement during bilateral movement after stroke. Archives of Physical Medicine and Rehabilitation 2006;87:1463‐70. [DOI] [PubMed] [Google Scholar]
- Mudie HM, Matyas T. Upper extremity retraining following stroke: effects of bilateral practice. Neurorehabilitation and Neural Repair 1996;10(3):167‐84. [Google Scholar]
- Mudie MH, Matyas TA. Can simultaneous bilateral movement involve the undamaged hemisphere in reconstruction of neural networks damaged by stroke?. Disability and Rehabilitation 2000;22(1/2):23‐37. [DOI] [PubMed] [Google Scholar]
- Richards LG, Senesac CR, Davis SB, Woodbury ML, Nadeau SE. Bilateral arm training with rhythmic auditory cueing in chronic stroke: not always efficacious. Neurorehabilitation and Neural Repair 2008;22:180‐4. [DOI] [PubMed] [Google Scholar]
- Rose DK, Winstein CJ. Bimanual training after stroke: are two hands better than one?. Topics in Stroke Rehabilitation 2004;11(4):20‐30. [DOI] [PubMed] [Google Scholar]
- Rose DK, Winstein CJ. The co‐ordination of bimanual rapid aiming movements following stroke. Clinical Rehabilitation 2005;19:452‐62. [DOI] [PubMed] [Google Scholar]
- Stevens JA, Stoykov MEP. Simulation of bilateral training through mirror reflection: a case report demonstrating an occupational therapy technique for hemiparesis. Topics in Stroke Rehabilitation 2004;11(1):59‐66. [DOI] [PubMed] [Google Scholar]
- Stinear JW, Byblow WD. Rhythmic bilateral movement training modulates corticomotor excitability and enhances upper limb motricity poststroke: a pilot study. Journal of Clinical Neurophysiology 2004;21(2):124‐31. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Coxon JP, Fleming MK, Byblow WD. Priming the motor system enhances the effects of upper limb therapy in chronic stroke. Brain 2008;131:1381‐90. [DOI] [PubMed] [Google Scholar]
- Tijs E, Matayas TA. Bilateral training does not facilitate performance of copying tasks in poststroke hemiplegia. Neurorehabilitation and Neural Repair 2006;20:473‐83. [DOI] [PubMed] [Google Scholar]
- Whitall J, McCombe Waller S, Silver KHC, Macko RF. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke 2000;31:2390‐5. [DOI] [PubMed] [Google Scholar]
- Yavuzer G, Selles R, Sexer N, Sutbeyaz S, Bussmann JB, Koseoglu F, et al. Mirror therapy improves hand function in subacute stroke: a randomised controlled trial. Archives of Physical Medicine and Rehabilitation 2008;89:393‐8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Aimet M, Pokan R, Kotzian S, Musil U, Pelikan J, Skopetz R, et al. Comparison of combined unilateral and bilateral vs an isolated unilateral strength training in stroke patients. Medicine and Science in Sports and Exercise 2003;35(5):S232. [Google Scholar]
- Miller KJ, Galea P, Kilbreath SL, Phillips BA. Early intensive task‐specific sensory and motor training of the upper limb after acute stroke: a pilot study. Neurorehabilitation and Neural Repair 2001;15(4):345‐6. [Google Scholar]
- NINDS 2006. Effects of two different kinds of exercise on stroke rehabilitation. ClinicalTrials.gov (NCT00108680)2006.
- Globas C, Lam JM, Hertler B, Becker C, Whitall J, McCombe‐Waller S, et al. Lesion localisation predicts response to bilateral training. Cerebrovascular Diseases 2008;25:Abst 2. [Google Scholar]; Whitall J. Upper extremity training for chronic stroke. CRISP database2002.
References to ongoing studies
- Cauraugh JH. Effects of different amounts of electrical stimulation + bilateral practice on improving hand function after stroke. ClinicalTrials.gov (NCT00369668)2006.
- Thonnard J‐L. Effect of rehabilitation of patients with a central nervous system lesion. ClinicalTrials.gov (NCT00837460)2009.
Additional references
- Ashburn 1982. A physical assessment for stroke patients. Physiotherapy 1982;68:109‐13. [PubMed] [Google Scholar]
- Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner 1964;192:540‐2. [PubMed] [Google Scholar]
- Barreca S, Wolf SL, Fasoli S, Bohannon R. Treatment interventions for the paretic upper limb of stroke survivors: a critical review. Neurorehabilitation and Neural Repair 2003;17:220‐6. [DOI] [PubMed] [Google Scholar]
- Barreca SR, Stratford PW, Lambert CL. Test‐retest reliability, validity, and sensitivity of the Chedoke Arm and Hand Activity Inventory: a new measure of upper limb function for survivors of stroke. Archives of Physical Medicine and Rehabilitation 2005;86:1616‐22. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Smith MB. Inter‐rater reliability of a modified Ashworth scale of muscle spasticity. Physical Therapy 1987;67:206‐7. [DOI] [PubMed] [Google Scholar]
- Bonita R, Beaglehole R. Modification of Rankin Scale: recovery of motor function after stroke. Stroke 1988;19:1497‐500. [DOI] [PubMed] [Google Scholar]
- Broeks JG, Lankhorst GJ, Rumping LK, Prevo AJ. The long‐term outcome of arm function after stroke: results of a follow‐up study. Disability and Rehabilitation 1999;21:357‐64. [DOI] [PubMed] [Google Scholar]
- Carr J, Shepherd R. Investigation of a new Motor Assessment Scale for stroke patients. Physical Therapy 1985;65:175‐80. [DOI] [PubMed] [Google Scholar]
- Carroll D. Hand function in quadriplegia. Maryland State Medical Journal 1967;16:107‐8. [PubMed] [Google Scholar]
- Cauraugh JH, Summers JJ. Neural plasticity and bilateral movements: a rehabilitation approach for chronic stroke. Progress in Neurobiology 2005;75:309‐20. [DOI] [PubMed] [Google Scholar]
- Collen FM, Wade DT, Robb GF, Bradshaw CM. The Rivermead Mobility Index: a further development of the Rivermead Motor Assessment. International Disability Studies 1991;13:50‐4. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
- Demeurisse G, Demol O, Robaye E. Motor evaluation in vascular hemiplegia. European Neurology 1980;19:382‐9. [DOI] [PubMed] [Google Scholar]
- Desrosiers J, Hebert R, Dutil E, Bravo G. Development and reliability of an upper extremity function test for the elderly: the TEMPA. Canadian Journal of Occupational Therapy 1993;60:9‐16. [Google Scholar]
- Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population‐based studies of incidence, prevalence, and case fatality in the late 20th century. Lancet Neurology 2003;2(1):43‐53. [DOI] [PubMed] [Google Scholar]
- French B, Forster A, Langhorne P, Leathley MJ, McAdam J, Price CIM, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database of Systematic Reviews 2007, Issue 4. [Art. No.: CD006073. DOI: 10.1002/14651858.CD006073.pub2] [DOI] [PubMed] [Google Scholar]
- Fugl‐Meyer AR, Jaasko L, Leyman IL, Olsson S, Steglind S. The post‐stroke hemiplegic patient. I. A method for evaluation of physical performance. Scandinavian Journal of Rehabilitation Medicine 1975;7:13‐31. [PubMed] [Google Scholar]
- Heller A, Wade DT, Wood VA, Sunderland A, Hewer RL, Ward E. Arm function after stroke: measurement and recovery over the first three months. Journal of Neurology, Neurosurgery and Psychiatry 1987;50:714‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook M, Skilbeck CE. An activities index for use with stroke patients. Age and Ageing 1983;12:166‐70. [DOI] [PubMed] [Google Scholar]
- Jebsen RH, Taylor N, Trieschmann RB, Howard LA. An objective and standardised test of hand function. Archives of Physical Medicine and Rehabilitation 1969;50:311‐9. [PubMed] [Google Scholar]
- Johnson MJ, Loos HFM, Burgar CG, Shor P, Leifer LJ. Designing a robotic stroke therapy device to motivate use of the impaired limb. Integration of Assistive Technology in the Information Age 2001;9:123‐32. [Google Scholar]
- Katz S, Down TD, Cash HR. Progress in the development of the index of ADL. Gerontologist 1970;10:20‐30. [DOI] [PubMed] [Google Scholar]
- Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. Advances in Clinical Rehabilitation 1987;1:6‐18. [PubMed] [Google Scholar]
- Kellor M, Frost J, Silberberg N. Hand strength and dexterity. American Journal of Occupational Therapy 1971;25:77‐83. [PubMed] [Google Scholar]
- Kelso JA, Southard DL, Goodman D. On the nature of human interlimb coordination. Science 1979;203:1029‐31. [DOI] [PubMed] [Google Scholar]
- Kwon S, Hartzema AG, Duncan PW, Lai S‐M. Disability measures in stroke relationship among the Barthel Index, the Functional Independence Measure, and the Modified Rankin Scale. Stroke 2004;35:918‐23. [DOI] [PubMed] [Google Scholar]
- Langhorne P, Legg L. Evidence behind stroke care. Journal of Neurology, Neurosurgery and Psychiatry 2003;74 Suppl 4:18‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln NB, Leadbetter D. Assessment of motor function in stroke function. Physiotherapy 1979;65:48‐51. [PubMed] [Google Scholar]
- Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. International Journal of Rehabilitation Research 1981;4:483‐92. [DOI] [PubMed] [Google Scholar]
- Mahoney FI, Barthel D. Functional evaluation: the Barthel Index. Maryland State Medical Journal 1965;14:56‐61. [PubMed] [Google Scholar]
- Mathiowetz V. Adult norms for the Box and Block Test of manual dexterity. American Journal of Occupational Therapy 1985;39:386‐91. [DOI] [PubMed] [Google Scholar]
- McCombe Waller S, Whitall J. Bilateral arm training: why and who benefits?. NeuroRehabiltiaiton 2008;23:29‐41. [PMC free article] [PubMed] [Google Scholar]
- Merholz J, Platz T, Kugler J, Pohl M. Electromechanical and robot‐assisted arm training for improving arm function and activities of daily living after stroke. Cochrane Database of Systematic Reviews 2008, Issue 4. [Art. No.: CD006876. DOI: 10.1002/14651858.CD006876.pub2] [DOI] [PubMed] [Google Scholar]
- Medical Research Council. Aids to the investigation of peripheral nerve injuries. Medical Research Council. London: HMSO, 1975. [Google Scholar]
- Nakayama H, Jørgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Archives of Physical Medicine and Rehabilitation 1995;76:27‐32. [DOI] [PubMed] [Google Scholar]
- Nouri FM, Lincoln NB. An Extended Activities of Daily Living Index for stroke patients. Clinical Rehabilitation 1987;1:301‐5. [Google Scholar]
- Page SJ, Sisto S, Levine P, Johnston MV, Hughes M. Modified constraint induced therapy: a randomised feasibility and efficacy study. Journal of Rehabilitation and Development 2001;38(5):583‐90. [PubMed] [Google Scholar]
- Page SJ, Sisto S, Johnston MV, Levine P. Modified constraint induced therapy after subacute stroke: a preliminary study. Neurorehabilitation and Neural Repair 2002;16(3):290‐2. [DOI] [PubMed] [Google Scholar]
- Pomeroy VM, King L, Pollock A, Baily‐Hallam A, Langhorne P. Electrostimulation for promoting recovery of movement of functional ability after stroke. Cochrane Database of Systematic Reviews 2006, Issue 2. [Art. No.: CD003241. DOI: 10.1002/14651858.CD003241.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
- Rossier P, Wade DT, Murphy M. An initial investigation of the reliability of the Rivermead Extended ADL index in patients presenting with neurological impairment. Journal of Rehabilitation Medicine 2001;33:61‐70. [DOI] [PubMed] [Google Scholar]
- Sirtori V, Gatti R, Davide C. Constraint induced movement therapy for upper extremities in stroke patients. Cochrane Database of Systematic Reviews 2003, Issue 4. [Art. No.: CD004433. DOI: 10.1002/14651858.CD004433] [DOI] [PubMed] [Google Scholar]
- Sodring KM, Bautz‐Holter E. Description and validation of a test of motor function and activities in stroke patients: the Sodring evaluation of stroke patients. Scandinavian Journal of Rehabilitation Medicine 1995;27:211‐7. [PubMed] [Google Scholar]
- Stewart KC, Cauraugh JH, Summers JJ. Bilateral movement training and stroke rehabilitation: a systematic review and meta‐analysis. Journal of the Neurological Sciences 2006;244(1‐2):89‐95. [DOI] [PubMed] [Google Scholar]
- Sunderland A, Tinson DJ, Bradley L, Langton Hewer RL. Arm function after stroke: an evaluation of grip strength as a measure of recovery and prognostic indicator. Journal of Neurology, Neurosurgery and Psychiatry 1989;52:1267‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen SP. Intermanual coordination: from behavioural principles to neural‐network interactions. Nature Reviews Neuroscience 2002;3:348‐59. [DOI] [PubMed] [Google Scholar]
- Taub E, Miller NE, Novack TA, Cook EW, Fleming WC, Nepomuceno CS, et al. Technique to improve chronic motor deficit after stroke. Archives of Physical Medicine and Rehabilitation 1993;74:347‐54. [PubMed] [Google Scholar]
- Tiffin J, Asher EJ. The Purdue pegboard: norms and studies of reliability and validity. Journal of Applied Psychology 1948;32:234‐47. [DOI] [PubMed] [Google Scholar]
- Bennekom CA, Jelles F, Lankhorst GJ, Bouter LM. The Rehabilitation Activities Profile: a validation study of its use as a disability index with stroke patients. Archives of Physical Medicine and Rehabilitation 1995;76:501‐7. [DOI] [PubMed] [Google Scholar]
- Lee JH, Wagenaar RC, Lankhorst GJ, Vogelaar TW, Devileee WL, Bouter LM. Forced use of the upper extremity in chronic stroke patients: results from a single‐blind randomised clinical trial. Stroke 1999;30:2369‐75. [DOI] [PubMed] [Google Scholar]
- Lee JH, Snels IAK, Beckerman H, Lankhorst GJ. Exercise therapy for arm function in stroke patients: a systematic review of randomised controlled trials. Clinical Rehabilitation 2001;15:20‐31. [DOI] [PubMed] [Google Scholar]
- Wade DT, Langton‐Hewer R, Wood VA, Skilbeck CE, Ismail HM. The hemiplegic arm after stroke: measurement and recovery. Journal of Neurology, Neurosurgery and Psychiatry 1983;46:521‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting S, Lincoln N. An ADL assessment for stroke patients. British Journal of Occupational Therapy 1980;43:44‐6. [Google Scholar]
- World Health Organization Task Force. Recommendations on stroke prevention, diagnosis and therapy. Report of the WHO task force on stroke and other cerebrovascular disorders. Stroke 1989;20:1407‐31. [DOI] [PubMed] [Google Scholar]
- Wilson DJ, Baker LL, Craddock JA. Functional test for the hemiparetic upper extremity. American Journal of Occupational Therapy 1984;38:159‐64. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf Motor Function Test as outcome measure for research in patients after stroke. Stroke 2001;32:1635‐9. [DOI] [PubMed] [Google Scholar]
- Woodford H, Price C. EMG biofeedback for the recovery of motor function after stroke. Cochrane Database of Systematic Reviews 2007, Issue 2. [Art. No.: CD004585. DOI: 10.1002/14651858.CD004585.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


