Abstract
Background
Macular oedema (MO) is the accumulation of extracellular fluid in the central retina (the macula). It may occur after cataract surgery and may give rise to poor visual outcome, with reduced visual acuity and distortion of the central vision. MO is often self‐limiting with spontaneous resolution, but a small proportion of people with chronic persistent MO may be difficult to treat. Chronic oedema may lead to the formation of cystic spaces in the retina termed 'cystoid macular oedema' (CMO). Non‐steroidal anti‐inflammatory drugs (NSAIDs) are commonly used in cataract surgery and may reduce the chances of developing MO.
Objectives
The aim of this review is to answer the question: is there evidence to support the prophylactic use of topical NSAIDs either in addition to, or instead of, topical steroids postoperatively to reduce the incidence of macular oedema (MO) and associated visual morbidity.
Search methods
We searched a number of electronic databases including CENTRAL, MEDLINE and Embase. Date last searched 2 September 2016.
Selection criteria
We included randomised controlled trials (RCTs) in which adult participants had undergone surgery for age‐related cataract. We included participants irrespective of their baseline risk of MO, in particular we included people with diabetes and uveitis. We included trials of preoperative and/or postoperative topical NSAIDs in conjunction with postoperative topical steroids. The comparator was postoperative topical steroids alone. A secondary comparison was preoperative and/or postoperative topical NSAIDs alone versus postoperative topical steroids alone.
Data collection and analysis
Two review authors independently selected studies for inclusion, assessed risk of bias and extracted data using standard methods expected by Cochrane. We pooled data using a random‐effects model. We graded the certainty of the evidence using GRADE and considered the following: risk of bias of included studies, precision of the effect estimate, consistency of effects between studies, directness of the outcome measure and publication bias.
Main results
We identified 34 studies that were conducted in the Americas, Europe, the Eastern Mediterranean region and South‐East Asia. Over 5000 people were randomised in these trials. The majority of studies enrolled one eye per participant; a small subset (4 trials) enrolled a proportion of people with bilateral surgery. Twenty‐eight studies compared NSAIDs plus steroids with steroids alone. Six studies compared NSAIDs with steroids. A variety of NSAIDs were used, including ketorolac, diclofenac, nepafenac, indomethacin, bromfenac, flurbiprofen and pranopfen. Follow‐up ranged from one to 12 months. In general, the studies were poorly reported. We did not judge any of the studies at low risk of bias in all domains. Six studies were funded by industry, seven studies were funded from non‐industry sources, and the rest of the studies did not report the source of funding.
There was low‐certainty evidence that people receiving topical NSAIDs in combination with steroids may have a lower risk of poor vision due to MO at three months after cataract surgery compared with people receiving steroids alone (risk ratio (RR) 0.41, 95% confidence interval (CI) 0.23 to 0.76; eyes = 1360; studies = 5; I2 = 5%). We judged this to be low‐certainty evidence because of risk of bias in the included studies and indirectness, as the extent of visual loss was not always clear. Only one study reported poor vision due to MO at 12 months and we judged this to be very low‐certainty evidence as there were only two events. Quality of life was only reported in one of the 34 studies comparing NSAIDs plus steroids versus steroids alone, and it was not fully reported, other than to comment on lack of differences between groups. There was evidence of a reduced risk of MO with NSAIDs at three months after surgery, but we judged this to be low‐certainty due to risk of bias and publication bias (RR 0.40, 95% CI 0.32 to 0.49; eyes = 3638; studies = 21). There was inconsistent evidence on central retinal thickness at three months (I2 = 87%). Results ranged from ‐30.9 µm in favour of NSAIDs plus steroids to 7.44 µm in favour of steroids alone. Similarly, data on best corrected visual acuity (BCVA) were inconsistent, but nine out of 10 trials reporting this outcome found between‐group differences in visual acuity of less than 0.1 logMAR.
None of the six studies comparing NSAIDs alone with steroids reported on poor vision due to MO at three or 12 months. There was low‐certainty evidence that central retinal thickness was lower in the NSAIDs group at three months (mean difference (MD) ‐22.64 µm, 95% CI ‐38.86 to ‐6.43; eyes = 121; studies = 2). Five studies reported on MO and showed a reduced risk with NSAIDs, but we judged this evidence to be of low‐certainty (RR 0.27, 95% CI 0.18 to 0.41; eyes = 520). Three studies reported BCVA at three months and the results of these trials were inconsistent, but all three studies found differences of less than 0.1 logMAR between groups.
We did not note any major adverse events ‐ the main consistent observation was burning or stinging sensation with the use of NSAIDs.
Authors' conclusions
Using topical NSAIDs may reduce the risk of developing macular oedema after cataract surgery, although it is possible that current estimates as to the size of this reduction are exaggerated. It is unclear the extent to which this reduction has an impact on the visual function and quality of life of patients. There is little evidence to suggest any important effect on vision after surgery. The value of adding topical NSAIDs to steroids, or using them as an alternative to topical steroids, with a view to reducing the risk of poor visual outcome after cataract surgery is therefore uncertain. Future trials should address the remaining clinical uncertainty of whether prophylactic topical NSAIDs are of benefit, particularly with respect to longer‐term follow‐up (at least to 12 months), and should be large enough to detect reduction in the risk of the outcome of most interest to patients, which is chronic macular oedema leading to visual loss.
Plain language summary
Prophylactic non‐steroidal anti‐inflammatory drugs (NSAIDs) for the prevention of macular oedema after cataract surgery
What is the aim of this review? The aim of this Cochrane Review was to find out if NSAID eye drops can prevent a sight‐threatening complication of cataract surgery (swelling at the back of the eye, known as macular oedema). Cochrane researchers collected and analysed all relevant studies to answer this question and found 34 studies.
Key messages There is only low‐certainty evidence to support the use of NSAID eye drops to prevent macular oedema affecting vision after cataract surgery.
What was studied in the review? There is a clear lens in the eye that focuses the light on the back of the eye. As people get older this lens can become cloudy. A cloudy lens is known as a cataract. Doctors can remove the cataract and replace it with an artificial lens. This is usually a very successful operation. Occasionally, people having cataract surgery can get swelling at the back of the eye after the operation. This swelling is known as macular oedema. It usually gets better on its own accord, but if it persists it can result in poor vision.
NSAIDs are a medication that can treat inflammation. They may be able to reduce the chances of this swelling happening. The NSAIDs studied in this review were eye drops.
What are the main results of the review? The review authors found 34 relevant studies. These studies were conducted in all parts of the world including the Americas, Europe, the Eastern Mediterranean region and South‐East Asia. Most (28) of these studies compared NSAIDs combined with steroids against steroids alone. Some of the studies (6) compared NSAIDs with steroids alone. A variety of NSAIDs were used, including ketorolac, diclofenac, nepafenac, indomethacin, bromfenac, pranopfen and flurbiprofen. People taking part in these trials were followed up from between one and 12 months. Most studies only followed up to two months or less. Six studies were funded by industry; seven studies were funded from non‐industry sources and the rest of the studies did not report the source of funding.
There was low‐certainty evidence that NSAIDs reduce the chance of poor vision due to macular oedema three months after cataract surgery. Only one study reported on poor vision due to macular oedema at 12 months and we judged this to have very low‐certainty of evidence.
Using NSAIDs was associated with a reduced risk of macular oedema but the review authors judged this to be low‐certainty.
Inconsistent results were seen for some measurements of macular oedema, such as the thickness of the tissue at the back of the eye (central retinal thickness) at three months after surgery. This measurement was not reported by any studies at 12 months after surgery.
Similarly, inconsistent results were seen for vision measurement (visual acuity) but most studies found small differences between people given NSAIDs and people not given NSAIDs.
Only one study reported quality of life, and this suggested little impact of NSAIDs on quality of life.
Adverse events mainly consisted of a burning or stinging sensation.
How up‐to‐date is this review? The review authors searched for studies that had been published up to 2 September 2016.
Summary of findings
Summary of findings for the main comparison. NSAIDS plus steroids compared with steroids for the prevention of macular oedema after cataract surgery.
| NSAIDs plus steroids compared with steroids for the prevention of macular oedema after cataract surgery | ||||||
|
Patient or population: people having cataract surgery Setting: eye hospital Intervention: NSAIDs plus steroids Comparison: steroids | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with steroids | Risk with NSAIDs plus steroids | |||||
| Poor vision due to MO at 3 months after surgery | 74 per 1000 | 30 per 1000 (17 to 56) | RR 0.41 (0.23 to 0.76) | 1360 (5 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ‐ |
| Poor vision due to MO at 12 months after surgery | 20 per 1000 | 26 per 1000 (2 to 407) | RR 1.32 (0.09 to 20.37) | 88 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 | ‐ |
| Quality of life at 3 months after surgery | See comment | ‐ | ‐ | 74 (1 RCT) |
‐ | Reported in 1 study only using COMTOL questionnaire. Data not fully reported but no significant differences in terms of quality of life, compliance and satisfaction scores. |
| Central retinal thickness at 3 months after surgery; assessed with OCT | See comment | ‐ | ‐ | 1021 (8 RCTs) | ‐ | Trial results were inconsistent (I2 = 87%). Results ranged from ‐30.9 microns in favour of NSAIDs plus steroids to +7.44 microns in favour of steroids alone. |
| Adverse effects | See comment | ‐ | ‐ | (18 RCTs) | ‐ | In general, no major adverse effects were noted. The main consistent observation was burning or stinging sensation with use of NSAID drops. |
| MO at 3 months after cataract surgery, clinically symptomatic, assessed with OCT | 130 per 1000 | 52 per 1000 (42 to 64) | RR 0.40 (CI 0.32 to 0.49) | 3638 (21 RCTs) |
⊕⊕⊝⊝ LOW 1 4 5 | |
| BCVA at 3 months after surgery; assessed with logMAR scale from: ‐1.3 to 1.3 | See comment | ‐ | ‐ | 1158 (10 RCTs) | ‐ | Trial results were inconsistent (I2 = 70%), but all except one study found differences less than 0.1 logMAR, i.e. clinically indistinguishable from no difference. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BCVA: best corrected visual acuity; CI: confidence interval; MO: macular oedema; NSAID: non‐steroidal anti‐inflammatory drug; OCT: optical coherence tomography; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded 1 level for risk of bias: studies at unclear or high risk of bias. 2 Downgraded 1 level for indirectness: extent of visual loss not always clearly defined. 3 Downgraded 2 levels for imprecision: Only 2 events. 4 Downgraded 1 level for publication bias: asymmetric funnel plot suggestive of publication bias.
5 We considered downgrading an additional 1 level for indirectness as the MO was not always OCT‐verified and it was not always clear if the MO was clinically symptomatic. However, we did not do so partly because the size of the effect was quite strong.
Summary of findings 2. NSAIDS compared with steroids for the prevention of macular oedema after cataract surgery.
| NSAIDscompared with steroids for the prevention of macular oedema after cataract surgery | ||||||
| Patient or population: people having cataract surgery Setting: eye hospital Intervention: NSAIDs Comparison: steroids | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with steroids | Risk with NSAIDs | |||||
| Poor vision outcome due to MO at 3 months after surgery | ‐ | ‐ | ‐ | ‐ | ‐ | No data were available for this outcome. |
| Poor vision outcome due to MO at 12 months after surgery | ‐ | ‐ | ‐ | ‐ | ‐ | No data were available for this outcome. |
| Quality of life at 3 months after surgery | No data were available for this outcome. | |||||
| Central retinal thickness at 3 months after surgery; assessed with OCT | The mean central retinal thickness at 3 months after surgery was 228 microns | MD 22.64 microns lower (38.86 lower to 6.43 lower) | ‐ | 121 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 4 | ‐ |
| Adverse effects | ‐ | ‐ | ‐ | 488 (4 RCTs) | ‐ | 1 study had 2 unspecified complications in 142 participants, 2 studies reported that no adverse events were noted in either group, 1 study (55 people) mentioned 15 mild adverse effects but unclear if related to treatment. |
| MO at 3 months after cataract surgery; clinically symptomatic assessed with OCT | 130 per 1000 | 35 per 1000 (23 to 53) |
RR 0.27 (0.18 to 0.41) | 520 (5 RCTs) |
⊕⊕⊝⊝ LOW 1 2 3 | |
| BCVA at 3 months after surgery; assessed with logMAR scale from: ‐1.3 to 1.3 | See comment | ‐ | ‐ | 220 (3 RCTs) | ‐ | Trial results were inconsistent (I2 = 84%), but all studies found differences less than 0.1 logMAR, i.e. clinically indistinguishable from no difference. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BCVA: best corrected visual acuity; CI: confidence interval; MD: mean difference; MO: macular oedema; NSAID: non‐steroidal anti‐inflammatory drug; OCT: optical coherence tomography; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded 1 level for risk of bias: studies at unclear or high risk of bias. 2 Downgraded 1 level for publication bias: asymmetric funnel plot suggestive of publication bias.
3 We considered downgrading 1 level for indirectness as the MO was not always OCT‐verified and not always clear if it was clinically symptomatic however we did not do so, partly because the effect was strong. 4 Downgraded 1 level for imprecision: confidence intervals include clinically unimportant effect. 5 Downgraded 1 level for inconsistency.
Background
Description of the condition
Cataract refers to the clouding of the natural crystalline lens of the eye. It is the leading cause of avoidable visual impairment and blindness in the world. The World Health Organization (WHO) estimates that unoperated cataract alone accounts for 33% of visual impairment, an estimated 94 million cases worldwide (Pascolini 2012). In many parts of the world, particularly higher‐income countries, availability of cataract surgery at a relatively early stage of visual impairment in the disease process has led to this procedure being one of the most commonly performed surgical procedures worldwide.
Macular oedema (MO) is the accumulation of extracellular fluid in the central retina (the macula) which may present following cataract surgery with lens implantation (pseudophakic macular oedema) or without (aphakic macular oedema) and may give rise to poor visual outcome with reduced visual acuity and distortion of the central vision. The diagnosis of this condition is made both clinically using slit lamp biomicroscopic examination of the macula and with the aid of fundus fluorescein angiography or optical coherence tomography (OCT) (Choi 2005).
The incidence of MO varies with type of surgery, intraoperative complications and pre‐existing risk factors. Reported risk of MO varies between 0.9% and 5% for modern uncomplicated phacoemulsification cataract surgery (Spaide 1993), but can be as high as 10% in the presence of surgical complications such as vitreous loss (Blomquist 2002). Vision is not always affected, and the incidence of MO with decrease in visual acuity is reported at 1% (Ahmed 2013), and is associated with increasing retinal thickness (Hee 1995). A multicentre audit of 55,567 cataract operations in the UK's National Health Service (NHS) showed a risk of 1.62%, at a median postoperative review time of 31 days (Jaycock 2009). This was based on surgeons' reports rather than systematic examination of the macula and was defined as poor visual outcome attributed to MO.
Other risk factors for MO include ocular inflammatory diseases such as uveitis, retinal ischaemic conditions such as central and branch retinal vein conditions, retinal vascular diseases and dystrophies, for example retinitis pigmentosa and retinal telangiectasia, as well as degenerative causes such as age related macular degeneration and diabetic retinopathy while the use of topical prostaglandin analogue therapy in glaucoma remains a theoretical risk (Nelson 2003). The use of topical adrenaline 2% (epinephrine) in aphakic patients has also been described to be associated with macular oedema. Other factors may include cerebrovascular and cardiovascular disease (Jain 2001) but the pathogenesis is unclear.
MO is often self‐limiting with spontaneous resolution (Ahmed 2013). The small proportion of patients with chronic persistent MO may be difficult to treat (Yannuzzi 1995), and they may experience permanent reduction in vision from atrophy of the photoreceptor layer of the retina (Ahmed 2013). Chronic oedema may lead to the formation of cystic spaces in the retina, termed 'cystoid macular oedema' (CMO).
Description of the intervention
The intervention is the topical use of non‐steroidal anti‐inflammatory drugs (NSAIDs), in this case, eyedrops, in addition to topical steroid eyedrops after cataract surgery. They may also be used preoperatively, primarily to reduce the risk of pupil constriction during surgery, but this may potentially also reduce the risk of MO. Non‐steroidal anti‐inflammatory agents are a group of drugs which are in common use orally as over‐the‐counter treatments for the reduction of pain, redness and swelling associated with systemic inflammation. Some of these are also available in eyedrop form as prescription medicines for the reduction of ocular inflammation.
The comparative intervention is the use of topical steroids on the eye after cataract surgery, which is current standard therapy, and may in itself reduce the risk of MO. Steroids are a group of prescription‐only drugs which are used systemically to suppress the symptoms, signs and sequelae of inflammation. They are also used in their topical eyedrop form for the reduction of ocular inflammation.
In the last decade or so, several clinical trials have examined the use of topical NSAIDs in the treatment and prevention of postoperative inflammation and pseudophakic macular oedema, without the adverse effects of topical corticosteroids (Ballonzoli 2010; Carnahan 2000; Heier 1999; Polanski 1992; Solomon 2001). NSAIDs such as ketorolac and indomethacin are cyclo‐oxygenase inhibitors which suppress breakdown of the blood‐aqueous barrier that may occur in the early postoperative period (Flach 1987; Flach 1988; Miyake 1984; Sanders 1984).
Jain 2001 recommended the use of prophylactic NSAIDs in patients with predisposing factors to developing postsurgical MO, irrespective of cause. Other clinical studies suggest that topical NSAIDs may be more effective than topical steroids in re‐establishing the blood‐aqueous barrier postoperatively, suggesting an important role in MO prevention (Flach 1989; Kraff 1990; Ursell 1999).
The meta‐analysis conducted in Rossetti 1998 of the use of NSAIDs suggested possible beneficial effects of NSAIDs for both the prophylaxis and treatment of MO, but concluded that the overall quality of the evidence was insufficient to justify recommendation of its widespread use in prophylaxis. A Cochrane Review on treatment of MO following cataract surgery, found that two out of seven included randomised controlled trials (RCTs) showed a beneficial effect of NSAIDs on chronic MO (Sivaprasad 2004), although problems with trial quality and heterogeneity prevented valid meta‐analysis.
A recent randomised, placebo‐controlled trial looking at the adjunctive effect of topical NSAIDs in addition to intravitreal steroids (triamcinolone) and intravitreal anti‐vascular endothelial growth factor (bevacizumab) in chronic MO, found a statistically significant improvement with the use of topical nepafenac in reduction of retinal thickness and improvement in visual acuity at 16 weeks (Warren 2010). NSAIDs have also been used with good tolerance and efficacy, as an alternate treatment for patients with MO of mixed origin who are steroid responders, and therefore cannot be treated with steroids (Warren 2008).
How the intervention might work
NSAIDs are cyclo‐oxygenase inhibitors and may work by reducing the production of pro‐inflammatory prostaglandins. Inflammation within tissue is caused by the production of pro‐inflammatory products by several pathways. NSAIDs act to suppress the cyclo‐oxygenase pathway of inflammation, inhibiting production of prostaglandins (Eisenach 2010).
Why it is important to do this review
As cataract surgery is the second most commonly performed operation worldwide, and MO occurs in between 1% and 10% of all cataract surgeries (depending on risk and complications) and leads to poor visual outcome, there is a significant volume of visual morbidity which can be potentially prevented if it is found that NSAIDs are effective in its prophylaxis. NSAIDs are relatively inexpensive, easily obtainable and carry the potential to significantly improve the outcome of cataract surgery worldwide.
Despite some evidence in favour of the beneficial effects of NSAIDs in MO, uncertainty remains about whether it has significant benefit in the prevention of MO when used perioperatively in addition to steroids. A recent editorial posed the question as to how prescribing NSAIDs for routine cataract surgery became so popular in the USA without compelling evidence of a visual benefit to patients (Kim 2016a). This uncertainty is reflected in widespread variation in clinical practice. For example, NSAIDs are much less frequently used in the UK for this indication. This review attempts either to resolve the persisting clinical uncertainty or to identify the need for further research to achieve such resolution.
This review is confined to addressing the use of NSAIDs in the prophylaxis of MO. A separate Cochrane Review on treatment of established cystoid macular oedema (CMO) has already been published (Sivaprasad 2004), but the effectiveness of NSAIDs in treatment remains uncertain. MO can lead to permanent structural damage in the central retina, therefore a prevention strategy may be more effective than treatment after the damage has been done.
Objectives
The aim of this review is to answer the question: is there evidence to support the prophylactic use of topical NSAIDs either in addition to, or instead of, topical steroids postoperatively to reduce the incidence of macular oedema (MO) and associated visual morbidity.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs) in this review. We excluded within‐person studies i.e. studies where eyes are randomly allocated to the intervention and comparator due to the possibility that the effect of non‐steroidal anti‐inflammatory drugs (NSAIDs) in one eye may affect the outcome in the other.
Types of participants
We included trials in which adult participants had undergone standard surgery for age‐related cataract. We included participants irrespective of their baseline risk of MO, in particular, we included people with diabetes and uveitis.
Types of interventions
The primary comparison of this review was topical NSAIDs in addition to topical steroids versus topical steroids alone in cataract surgery. Surgery can include extracapsular cataract extraction (ECCE; large incision with sutures), manual small incision cataract surgery (MSICS; small incision without sutures), phacoemulsification cataract surgery (mechanised small incision extracapsular extraction) and intracapsular cataract extraction (ICCE; planned and unplanned intracapsular procedures).
We included trials of preoperative and/or postoperative topical NSAIDs in conjunction with postoperative topical steroids. The comparator was postoperative topical steroids alone.
A secondary comparison was preoperative and/or postoperative topical NSAIDs alone versus topical postoperative steroids alone.
We included studies irrespective of whether incident MO was subsequently treated.
Types of outcome measures
Primary outcomes
The proportion of people with a poor vision outcome due to MO in the study eye at three months after surgery.
We defined poor vision outcome as best corrected visual acuity (BCVA) not improving to 6/9 or better (or equivalent with other notations of vision) attributed to a diagnosis of MO (detected clinically, angiographically or on optical coherence tomography (OCT)). This included participants who developed MO and required and received treatment.
Our primary outcome was measured at three months after surgery, which we took as any observation between one month and six months after surgery. We also examined poor visual outcome due to MO at 12 months after surgery, which we took as any observation between six and 18 months after surgery.
Secondary outcomes
Any quality of life or patient satisfaction measure relating to the patient's experience of surgery on the study eye., at three months and 12 months after surgery
Change in central retinal thickness from preoperative assessment in the study eye, at three months and 12 months after surgery, as measured by OCT scan. If change in central retinal thickness was not available we used the final value.
Adverse effects
We looked at known harms of NSAIDs including respiratory effects and gastrointestinal disturbance, in addition to intolerance of medication and allergic reactions. We recorded any other harms such as liver toxicity, as has been reported with some NSAIDs.
Resource use and costs
In our protocol (Abeysiri 2011) we planned to look at economic evaluations of the cost‐effectiveness and cost per quality‐adjusted life year (QALY)/disability‐adjusted life year (DALY) modelling. We amended this to look at resource use and costs more generally.
Additional outcomes (National Institute for Health and Care Excellence (NICE))
We collected data on the following additional outcomes as part of our collaboration with NICE.
Macular oedema (MO) (clinically symptomatic, OCT‐verified).
Inflammation.
BCVA.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 8), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to September 2016), Embase (January 1980 to September 2016), Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to September 2016), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 2 September 2016.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), Embase (Appendix 3), LILACS (Appendix 4), ISRCTN (Appendix 5), ClinicalTrials.gov (Appendix 6), and the ICTRP.
Searching other resources
We searched the reference lists of the studies included in the review. We used the Science Citation Index to find studies that have cited the individual trials. We did not handsearch conference proceedings or journals specifically for the review.
Data collection and analysis
Selection of studies
Three review authors (CL, BL, DL) screened the titles and abstracts resulting from the searches independently. We obtained full copies of the potentially relevant trials. Three review authors (CL, BL, DL) independently assessed full copies for inclusion according to the 'Criteria for considering studies for this review.' We resolved disagreements by discussion.
We listed all excluded studies and provided a brief justification for exclusion (See Characteristics of excluded studies).
Data extraction and management
Four review authors (JE, CL, DL, BL) independently extracted data using a pre‐piloted data extraction template in Covidence (Covidence 2016). A fifth review author (CB) generated a random sample of 20% of studies and checked data input for these. We resolved discrepancies by discussion.
We collected the following information on study characteristics (Appendix 8).
Study design: parallel group RCT, one or both eyes included and/or reported.
Participants: country, total number of participants, age, sex, inclusion and exclusion criteria.
Intervention and comparator details: including number randomised to each.
Primary and secondary outcomes as measured and reported in the trials, adverse events, methods of measurement (e.g. which chart is used for visual acuity assessment, which OCT scanner was used).
Length of follow‐up.
Date study conducted.
Funding and conflicts of interest reported.
Trial registration number.
We collected data on our predefined outcomes separately for intervention and comparator groups. For multi‐arm studies we planned to use data relevant to our intervention and comparator groups. If two groups contain relevant data (for example, if pre/postoperative application of NSAIDs) we combined groups using the RevMan calculator (RevMan 2014).
As far as possible, we extracted data for an intention‐to‐treat (ITT) analysis. We contacted trial investigators as needed. Data were imported directly from Covidence into Review Manager 5 by JE (RevMan 2014), and checked by the other review authors (CL, DL, BL). CB then conducted a final random assessment.
Assessment of risk of bias in included studies
We used Cochrane's 'Risk of bias' tool for assessing risk of bias in each included study. Four review authors (JE, CL, DL, BL) independently assessed risk of bias according to methods set out in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We followed the specific rules as set out in Table 3 and resolved disagreements by discussion.
1. 'Risk of bias' assessment.
| Domain | Risk of bias | ||
| Low | Unclear | High | |
| Sequence generation | Computer‐generated list, random table, other method of generating random list. | Not reported how list was generated. Trial may be described as “randomised” but with no further details. | Alternate allocation, date of birth, records (these RCTs should be excluded). |
| Allocation concealment | Central centre (web/telephone access), sealed opaque envelopes. | Not reported how allocation administered. Trial may be described as “randomised” but with no further details. | Investigator involved in treatment allocation or treatment allocation clearly not masked. |
| Blinding of participants and personnel | Clearly stated that participants and personnel not aware of which treatment received. | Described as “double‐blind” with no information on who was masked. | Open‐label or no information on masking. We assume that in absence of reporting on this outcome, patients and personnel were not masked. |
| Blinding of outcome assessors | Clearly stated that outcome assessors were masked. | Described as “double‐blind” with no information on who was masked. | Open‐label or no information on masking. We assume that in absence of reporting on this outcome, assessors were not masked. |
| Incomplete outcome data | Missing data less than 20% (i.e. more than 80% follow‐up) and equal follow‐up in both groups and no obvious reason why loss to follow‐up should be related to outcome. | Follow‐up not reported or missing data > 20% (i.e. follow‐up < 80%) but follow‐up equal in both groups. | Follow‐up different in each group and/or related to outcome. |
| Selective outcome reporting | All outcomes in protocol and/or trial registry entry are reported. | No access to protocol or trial registry entry. | Outcomes in protocol and/or trial registry entry selectively reported. |
| Other sources of bias Note: we did not identify any important sources of other bias so this domain is omitted from the risk of bias tables. |
No other source of bias. | Trial stopped early due to poor recruitment. Baseline imbalance, but not clear that it is important. |
Trial stopped early because of outcome. Important baseline imbalance that might have an effect on the results. |
We contacted trial investigators for Miyake 2011 for clarification of random allocation.
Measures of treatment effect
We calculated the risk ratio for outcome measures reported as dichotomous data (for example, poor visual acuity attributed to MO within three months). We calculated the mean difference for measures of retinal thickness. We planned to analyse ordinal outcome data as dichotomous data if an established defensible cut‐off point is available, such as quality of life measures. We did not plan to meta‐analyse adverse effects.
Unit of analysis issues
Trials included may randomise one or both eyes to the intervention or comparator. If both eyes were allocated to the same treatment, we planned to analyse as 'clustered data' if data were available. In the event four trials included data on both eyes, but this was generally a small proportion of the total participants. We have analysed as reported. We excluded studies which allocated different eyes to different treatments as there may be a confounding cross‐over effect due to systemic absorption.
Dealing with missing data
We assessed all included trials for number of participants excluded or lost to follow‐up. We documented reasons for loss to follow‐up by treatment group, if reported. We aimed to do an ITT analysis for included trials using imputed data; if computed by the trialists we did not plan to impute missing data on their behalf.
Assessment of heterogeneity
Where heterogeneity was observed between individual study results we did not combine studies but present a tabulated summary of results. We did not rely on statistical significance of a Chi2 test to indicate heterogeneity but examined the forest plot of the study results and the overall characteristics of the studies. We looked at the consistency between studies by examining the I2 statistic value. We considered I2 values over 50% to indicate substantial inconsistency, but we also considered the direction of effects.
Assessment of reporting biases
We considered selective outcome reporting under the risk of bias assessment (Table 3). We planned to look at funnel plots and consider tests for asymmetry for bias assessment in the event of 10 or more trials contributing data to a meta‐analysis.
Data synthesis
We aimed to use a random‐effects model provided we did not detect substantial inconsistency between individual study results. If there were fewer than three trials in a comparison we planned to use the fixed‐effect model. Where heterogeneity was observed between studies (see Assessment of heterogeneity) we did not combine studies but presented a narrative summary of results.
'Summary of findings' table
We prepared a 'Summary of findings' table presenting relative and absolute risks. We graded the overall certainty of the evidence for each outcome using the GRADE classification (Atkins 2004). We considered the following: risk of bias of included studies, precision of the effect estimate, consistency of effects between studies, directness of the outcome measure and publication bias. JE did the assessment and this was checked by other authors. We included the following outcomes in the 'Summary of findings' tables.
Poor vision outcome due to MO at three months after surgery.
Poor vision outcome due to MO at 12 months after surgery.
Quality of life at three months after surgery.
Central retinal thickness at three months after surgery.
Adverse effects.
MO (clinically symptomatic, OCT‐verified) at three months after surgery
BCVA at three months after surgery.
Subgroup analysis and investigation of heterogeneity
We planned to conduct a subgroup analysis on the primary outcome comparing the effect of treatment on people with higher baseline risk of MO (diabetes/uveitis) with people with lower risk of MO (no diabetes/uveitis), but we did not do them as planned as there were not enough data on the primary outcome.
Sensitivity analysis
We planned to perform three sensitivity analyses on the primary outcome, but we did not do them as planned as there were not enough data on the primary outcome.
Excluding studies at high risk of bias in one or more domains.
Excluding industry‐funded studies.
Comparing fixed‐effect and random‐effects models (if three or more trials).
Results
Description of studies
Results of the search
The electronic searches yielded a total of 928 references (Figure 1). The Cochrane Information Specialist removed 337 duplicate records and we screened the remaining 591 reports. We rejected 526 records after reading the abstracts and obtained the full‐text reports of 65 references for further assessment. We identified 43 reports of 34 studies which met the inclusion criteria (see Characteristics of included studies for details), and excluded 18 reports of 18 studies (see Characteristics of excluded studies for details). One unpublished trial is currently awaiting assessment (CTRI/2009/091/001078). We identified three ongoing studies (NCT01694212; NCT01774474; NCT02646072).
1.
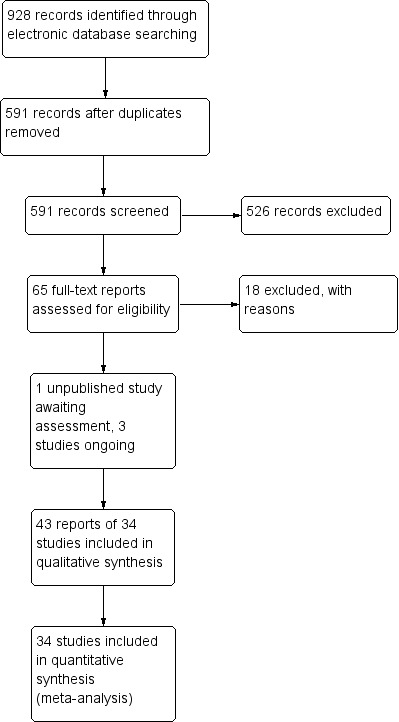
Study flow diagram.
Included studies
We have summarised the characteristics of the 34 included studies below. Details for individual studies can be found in the Characteristics of included studies. The information is also summarised in Table 4; Table 5; Table 6; Table 7; Table 8
2. Studies.
| Study | Country | Open‐label | Funding sources | Declaration of interest | Trial registration | Abstract only | |
| 1 | Almeida 2008 | Canada | Yes | Non‐industry | Reported; no CoI | NCT00335439 | No |
| 2 | Almeida 2012 | Canada | No | Non‐industry | Reported; no CoI | NCT01395069 | No |
| 3 | Asano 2008 | Japan | No | Not reported | Reported; no CoI | Not registered | No |
| 4 | Brown 1996 | USA | No | Industry | Not reported | Not registered | No |
| 5 | Cervantes‐Coste 2009 | Mexico | No | Not reported | Reported; no CoI | Not registered | No |
| 6 | Chatziralli 2011 | Greece | No | Not reported | Not reported | Not registered | No |
| 7 | Donnenfeld 2006 | USA | No | Industry/Non‐Industry | CoI | Not registered | No |
| 8 | Elsawy 2013 | Egypt | No | Not reported | Reported; no CoI | Not registered | No |
| 9 | Endo 2010 | Japan | Yes | Not reported | Reported; no CoI | Not registered | No |
| 10 | Italian Diclofenac Study Group 1997 | Italy | No | Not reported | CoI | Not registered | No |
| 11 | Jung 2015 | South Korea | No | Non‐industry | Reported; no CoI | Not registered | No |
| 12 | Kraff 1982 | USA | No | Non‐industry | Not reported | Not registered | No |
| 13 | Li 2011 | China | No | Not reported | Not reported | Not registered | No |
| 14 | Mathys 2010 | USA | No | Non‐industry | Reported; no CoI | NCT00494494 | No |
| 15 | Miyake 2007 | Japan | No | Not reported | Reported; no CoI | Not registered | No |
| 16 | Miyake 2011 | Japan | No | Not reported | CoI | Not registered | No |
| 17 | Miyanaga 2009 | Japan | No | Not reported | Not reported | Not registered | No |
| 18 | Moschos 2012 | Greece | No | Not reported | Reported; no CoI | Not registered | No |
| 19 | Quentin 1989 | Germany | No | Not reported | Not reported | Not registered | No |
| 20 | Rossetti 1996 | Italy | No | Not reported | Reported; no CoI | Not registered | No |
| 21 | Singh 2012 | USA | No | Not reported | CoI | NCT00782717 | No |
| 22 | Solomon 1995 | Canada (8 sites) and Germany (2 sites) | No | Industry | Reported; no CoI | Not registered | No |
| 23 | Tauber 2006 | USA | No | Industry | CoI | Not registered | Yes |
| 24 | Ticly 2014 | Brazil | No | Not reported | Reported; no CoI | Not registered | No |
| 25 | Tunc 1999 | Turkey | No | Not reported | Not reported | Not registered | No |
| 26 | Tzelikis 2015 | Brazil | No | Not reported | Reported; no CoI | NCT02084576 | No |
| 27 | Umer‐Bloch 1983 | Switzerland | No | Not reported | Not reported | Not registered | No |
| 28 | Wang 2013 | China | Yes | Non‐industry | Not reported | Not registered | No |
| 29 | Wittpenn 2008 | USA | No | Industry | CoI | NCT00348244 | No |
| 30 | Yannuzzi 1981 | USA | No | Non‐industry | Not reported | Not registered | No |
| 31 | Yavas 2007 | Turkey | No | Not reported | Reported; no CoI | Not registered | No |
| 32 | Yung 2007 | USA | No | Not reported | Not reported | Not registered | No |
| 33 | Zaczek 2014 | Sweden | No | Industry/Non‐industry | Reported; no CoI | Not registered | No |
| 34 | Zhang 2008 | China | No | Not reported | Not reported | Not registered | No |
CoI: conflict of interest
3. Participant numbers.
| Study | Number of people randomised | Number of people randomised (missing data imputed)* | Number of eyes | Number of eyes estimated (missing data imputed)* | Number of people followed up | Number of people followed up (missing data imputed)* | Percentage follow‐up | Eyes per person enrolled in the trial | |
| 1 | Almeida 2008 | 98 | 98 | 106 | 106 | ‐ | 74 | 75% | 106 eyes of 98 people |
| 2 | Almeida 2012 | 193 | 193 | ‐ | 193 | 162 | 162 | 84% | Probably one |
| 3 | Asano 2008 | 150 | 150 | 150 | 150 | 142 | 142 | 95% | One eye |
| 4 | Brown 1996 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Probably one | |
| 5 | Cervantes‐Coste 2009 | 60 | 60 | 60 | 60 | 60 | 60 | 100% | One eye |
| 6 | Chatziralli 2011 | 145 | 145 | 145 | 145 | 138 | 138 | 95% | Probably one |
| 7 | Donnenfeld 2006 | 100 | 100 | ‐ | 100 | ‐ | 100 | ‐ | Unclear |
| 8 | Elsawy 2013 | 70 | 70 | 86 | 86 | ‐ | 86 | ‐ | 86 eyes of 70 patients |
| 9 | Endo 2010 | 75 | 75 | 75 | 75 | 62 | 62 | 83% | One eye |
| 10 | Italian Diclofenac Study Group 1997 | 281 | 281 | 281 | 281 | 229 | 229 | 81% | One eye |
| 11 | Jung 2015 | 91 | 91 | 91 | 91 | Not reported | 91 | Not reported | One eye |
| 12 | Kraff 1982 | 500 | 500 | ‐ | 500 | 492 | 492 | 98% | Unclear |
| 13 | Li 2011 | 217 | 217 | 217 | 217 | ‐ | 217 | ‐ | One eye |
| 14 | Mathys 2010 | 84 | 84 | 84 | 84 | 79 | 79 | 94% | One eye |
| 15 | Miyake 2007 | 62 | 62 | 62 | 62 | 50 | 50 | 81% | Probably one |
| 16 | Miyake 2011 | 60 | 60 | 60 | 60 | 55 | 55 | 92% | One eye |
| 17 | Miyanaga 2009 | 72 | 72 | 72 | 72 | ‐ | 72 | ‐ | One eye |
| 18 | Moschos 2012 | 79 | 79 | 79 | 79 | ‐ | 79 | ‐ | One eye |
| 19 | Quentin 1989 | 179 | 179 | 179 | 179 | 112 | 112 | 63% | One eye |
| 20 | Rossetti 1996 | 88 | 88 | 88 | 88 | ‐ | 88 | ‐ | Probably one |
| 21 | Singh 2012 | 263 | 263 | 263 | 263 | 251 | 251 | 95% | One eye |
| 22 | Solomon 1995 | 681 | 681 | 681 | 681 | 364 | 364 | 53% | Probably one |
| 23 | Tauber 2006 | ‐ | 32 | ‐ | 32 | 32 | 32 | ‐ | Unclear |
| 24 | Ticly 2014 | 91 | 91 | 91 | 91 | 81 | 81 | 89% | Probably one |
| 25 | Tunc 1999 | 75 | 75 | 75 | 75 | 75 | 75 | ‐ | One eye |
| 26 | Tzelikis 2015 | 142 | 142 | 142 | 142 | 126 | 126 | 89% | One eye |
| 27 | Umer‐Bloch 1983 | ‐ | 73 | ‐ | 73 | 73 | 73 | ‐ | Unclear |
| 28 | Wang 2013 | 240 | 240 | ‐ | 240 | 167 | 167 | 70% | Unclear |
| 29 | Wittpenn 2008 | 546 | 546 | 546 | 546 | 478 | 478 | 88% | One eye |
| 30 | Yannuzzi 1981 | ‐ | 201 | 231 | 231 | ‐ | 231 | 59% | 231 eyes of 210 people |
| 31 | Yavas 2007 | 189 | 189 | 189 | 189 | 179 | 179 | 95% | One eye; right eye only |
| 32 | Yung 2007 | 37 | 37 | ‐ | 37 | ‐ | 37 | ‐ | Unclear |
| 33 | Zaczek 2014 | 160 | 160 | 160 | 160 | 152 | 152 | 95% | One eye |
| 34 | Zhang 2008 | ‐ | 198 | 220 | 220 | ‐ | 220 | 100% | 220 eyes of 198 people |
*For studies that did not report the number randomised, we have estimated this from the number followed up. For studies that did not report the number followed up, we have estimated this from the numbers randomised. Number of eyes estimated assuming one eye per person, if not clearly stated otherwise.
4. Participant characteristics.
| Study | Average age | Age range | % female | % with diabetes | % with uveitis | |
| 1 | Almeida 2008 | 72 | 45 to 92 | 61% | 21% | 1% |
| 2 | Almeida 2012 | 72 | 50 to 88 | 54% | ‐ but low risk population | "low risk population" |
| 3 | Asano 2008 | 66 | ‐ | 56% | 0% people with diabetes excluded | 0% people with uveitis excluded |
| 4 | Brown 1996 | ‐ | ‐ | ‐ | ‐ but people with DR excluded | 0% people with uveitis excluded |
| 5 | Cervantes‐Coste 2009 | 72 | 51 to 88 | 64% | 20% | 0% people with uveitis excluded |
| 6 | Chatziralli 2011 | 74 | ‐ | 40% | 10% | 0% people with uveitis excluded |
| 7 | Donnenfeld 2006 | 73 | ‐ | 55% | 0% people with diabetes excluded | 0% people with uveitis excluded |
| 8 | Elsawy 2013 | ‐ | ‐ | 37% | 100% | ‐ |
| 9 | Endo 2010 | 69 | 37 to 84 | 45% | 100% | 0% people with uveitis excluded |
| 10 | Italian Diclofenac Study Group 1997 | 68 | ‐ | 52% | ‐ | ‐ |
| 11 | Jung 2015 | 67 | ‐ | 55% | 26% | ‐ |
| 12 | Kraff 1982 | 69 | 37 to 97 | 57% | ‐ | ‐ |
| 13 | Li 2011 | 72 | ‐ | 63% | 100% | ‐ |
| 14 | Mathys 2010 | 72 | 44 to 90 | 54% | 0% people with diabetes excluded | 0% people with uveitis excluded |
| 15 | Miyake 2007 | 66 | ‐ | 54% | 0% people with diabetes excluded | 0% people with uveitis excluded |
| 16 | Miyake 2011 | 65 | 48 to 82 | 46% | 9% | 0% people with uveitis excluded |
| 17 | Miyanaga 2009 | 72 | 41 to 86 | 71% | 0% people with diabetes excluded | 0% people with uveitis excluded |
| 18 | Moschos 2012 | 77 | ‐ | 66% | 0% people with diabetes excluded | 0% people with uveitis excluded |
| 19 | Quentin 1989 | 73 | ‐ | 55% | ‐ but people with DR excluded | 0% people with uveitis excluded |
| 20 | Rossetti 1996 | 74 | ‐ | 64% | 0% people with diabetes excluded | ‐ |
| 21 | Singh 2012 | 67 | 32 to 87 | 63% | 100% | 0% people with uveitis excluded |
| 22 | Solomon 1995 | 68 | 39 to 100 | 53% | ‐ | 0% people with uveitis excluded |
| 23 | Tauber 2006 | ‐ | ‐ | ‐ | ‐ | ‐ |
| 24 | Ticly 2014 | 67 | ‐ | 47% | 0% people with diabetes excluded | 0% people with uveitis excluded |
| 25 | Tunc 1999 | 61 | ‐ | 39% | 0% people with diabetes excluded | 0% people with uveitis excluded |
| 26 | Tzelikis 2015 | ‐ | ‐ | ‐ | ‐ | ‐ |
| 27 | Umer‐Bloch 1983 | 69 | ‐ | 52% | ‐ but people with DR excluded | 0% people with uveitis excluded |
| 28 | Wang 2013 | 73 | 46 to 92 | 54% | 0% people with diabetes excluded | 0% people with uveitis excluded |
| 29 | Wittpenn 2008 | 70 | ‐ | 53% | ‐ | ‐ |
| 30 | Yannuzzi 1981 | ‐ | ‐ | ‐ | ‐ | ‐ |
| 31 | Yavas 2007 | 65 | ‐ | 40% | 0% people with diabetes excluded | 0% people with uveitis excluded |
| 32 | Yung 2007 | ‐ | ‐ | ‐ | 100% | ‐ |
| 33 | Zaczek 2014 | 69 | ‐ | 65% | ‐ | ‐ |
| 34 | Zhang 2008 | ‐ | ‐ | ‐ | ‐ | ‐ |
DR: diabetic retinopathy
5. Interventions.
| Study | Type of cataract surgery | Comparison | NSAIDs | Steroid | Placebo in comparator group | Type of placebo | |
| 1 | Almeida 2008 | Phacoemulsification | NSAIDs plus steroids versus steroids | Ketorolac 0.5% | Prednisolone 1% | No | ‐ |
| 2 | Almeida 2012 | Phacoemulsification | NSAIDs plus steroids versus steroids | Ketorolac 0.5%, Nepafenac 0.1% | Prednisolone 1% | Yes | Sterile saline drops |
| 3 | Asano 2008 | Phacoemulsification | NSAIDs versus steroids | Diclofenac 0.1% | Betamethasone 0.1% | No | ‐ |
| 4 | Brown 1996 | Phacoemulsification | NSAIDs versus steroids | Diclofenac 0.1% | Prednisolone 1% | No | ‐ |
| 5 | Cervantes‐Coste 2009 | Phacoemulsification | NSAIDs plus steroids versus steroids | Nepafenac 0.1% | Dexamethasone (combined with tobramycin) | No | ‐ |
| 6 | Chatziralli 2011 | Phacoemulsification | NSAIDs plus steroids versus steroids | Ketorolac 0.5% | Dexamethasone 0.1% (combined with tobramycin 0.3%) | No | ‐ |
| 7 | Donnenfeld 2006 | Phacoemulsification | NSAIDs plus steroids versus steroids | Ketorolac 0.4% | Prednisolone 1% | Yes | Vehicle |
| 8 | Elsawy 2013 | Phacoemulsification | NSAIDs plus steroids versus steroids | Ketorolac 0.4% | Dexamethasone 0.1%, | No | ‐ |
| 9 | Endo 2010 | Phacoemulsification | NSAIDs versus steroids | Bromfenac | Betamethasone (with fradiomycin sulfate) followed by fluorometholone | No | ‐ |
| 10 | Italian Diclofenac Study Group 1997 | ECCE | NSAIDs versus steroids | Diclofenac 0.1% | Dexamethasone 0.1% | Yes | Not specified |
| 11 | Jung 2015 | Phacoemulsification | NSAIDs versus steroids | Bromfenac 0.1%, Ketorolac 0.4% |
Prednisolone acetate 1% | No | ‐ |
| 12 | Kraff 1982 | ECCE and phacoemulsification | NSAIDs plus steroids versus steroids | Indomethacin | Dexamethasone (in combination with neomycin sulfate, polymyxin B sulfate) for 4 days followed by dexamethasone alone for 4 weeks followed by fluorometholone for at least 6 months | Yes | Vehicle |
| 13 | Li 2011 | Phacoemulsification | NSAIDs plus steroids versus steroids | Diclofenac 1% | Dexamethasone (combined with tobramycin) | No | ‐ |
| 14 | Mathys 2010 | Phacoemulsification | NSAIDs plus steroids versus steroids | Nepafenac 0.1% | Prednisolone 1% | No | ‐ |
| 15 | Miyake 2007 | Phacoemulsification | NSAIDs versus steroids | Diclofenac 0.1% | Fluorometholone 0.1% | No | ‐ |
| 16 | Miyake 2011 | Phacoemulsification | NSAIDs versus steroids | Nepafenac 0.1% | Fluorometholone 0.1% | No | ‐ |
| 17 | Miyanaga 2009 | Phacoemulsification | NSAIDs plus steroids versus steroids/NSAIDs versus steroids | Bromfenac 0.1% | Betamethasone 0.1%, fluorometholone | No | ‐ |
| 18 | Moschos 2012 | Phacoemulsification | NSAIDs plus steroids versus steroids | Diclofenac 0.1% | Dexamethasone 0.1% (combined with chloramphenicol 0.5%) | No | ‐ |
| 19 | Quentin 1989 | ICCE | NSAIDs plus steroids versus steroids | Diclofenac 0.1% | Dexamethasone | Yes | Not specified |
| 20 | Rossetti 1996 | ECCE | NSAIDs plus steroids versus steroids | Diclofenac | Dexamethasone (combined with tobramycin) | Yes | Not specified |
| 21 | Singh 2012 | Phacoemulsification | NSAIDs plus steroids versus steroids | Nepafenac 1% | Prednisolone | Yes | Vehicle |
| 22 | Solomon 1995 | ECCE | NSAIDs plus steroids versus steroids | Flurbiprofen 0.03% Indomethacin 1% |
Prednisolone | Yes | Vehicle |
| 23 | Tauber 2006 | Phacoemulsification | NSAIDs plus steroids versus steroids | Ketorolac 0.4% | Prednisolone 1% | No | ‐ |
| 24 | Ticly 2014 | Phacoemulsification | NSAIDs plus steroids versus steroids | Ketorolac 0.4% | Prednisolone 1% | Yes | Dextran 70/hypromellose |
| 25 | Tunc 1999 | ECCE | NSAIDs plus steroids versus steroids | Diclofenac 0.1% | Dexamethasone 1% | No | ‐ |
| 26 | Tzelikis 2015 | Phacoemulsification | NSAIDs plus steroids versus steroids | Ketorolac 0.4%, Nepafenac 0.1% | Prednisolone 1% | Yes | Artificial tears |
| 27 | Umer‐Bloch 1983 | ECCE/ICCE | NSAIDs plus steroids versus steroids | Indomethacin 1% | Dexamethasone (combined with either chloramphenicol or neomycin) | Yes | Vehicle |
| 28 | Wang 2013 | Phacoemulsification | NSAIDs plus steroids versus steroids | Bromfenac 0.1% | fluorometholone 0.1% and dexamethasone 0.1% | No | ‐ |
| 29 | Wittpenn 2008 | Phacoemulsification | NSAIDs plus steroids versus steroids | Ketorolac 0.4% | Prednisolone 1% | Yes | Artificial tears |
| 30 | Yannuzzi 1981 | ICCE | NSAIDs plus steroids versus steroids | Indomethacin 1% | Steroids given as part of standard care, not specified exactly what | Yes | Vehicle |
| 31 | Yavas 2007 | Phacoemulsification | NSAIDs plus steroids versus steroids | Indomethacin 0.1% | Prednisolone 1% | No | ‐ |
| 32 | Yung 2007 | Phacoemulsification | NSAIDs plus steroids versus steroids | Ketorolac 0.5% | Prednisolone 1% | Yes | Artificial tears |
| 33 | Zaczek 2014 | Phacoemulsification | NSAIDs plus steroids versus steroids | Nepafenac 0.1% | Dexamethasone 0.1% | Yes | Artifical tears |
| 34 | Zhang 2008 | Phacoemulsification | NSAIDs plus steroids versus steroids | Pranoprofen | Dexamethasone (combined with tobramycin) | No | ‐ |
ECCE: extracapsular cataract extraction ICCE: intracapsular cataract extraction NSAIDs: non‐steroidal anti‐inflammatory drugs
6. Outcomes.
| Poor vision outcome due to MO | Quality of life/patient satisfaction | Central retinal thickness | Adverse effects reported | CMO | Inflammation | BCVA | Additional outcomes | ||
| Study | Follow‐up | Analysis 1.1 | No analysis; only one study reported this | Analysis 1.2; Analysis 2.1 | Table 9 | Analysis 1.4; Analysis 2.2 | Analysis 1.5; Analysis 1.6; Analysis 2.3 | Analysis 1.7; Analysis 2.4 | Analysis 1.3 |
| Almeida 2008 | 1 month | Yes | OCT used but CMO not defined | Change in total macular volume | |||||
| Almeida 2012 | 1 month | COMTOL questionnaire | Mean change reported but not possible to calculate SD | LogMAR | Change in total macular volume; change in average macular cube thickness | ||||
| Asano 2008 | 8 weeks | Yes | Fluorescein angiography using Miyake 1977 classification (at 5 weeks only) | Laser flare‐cell photometry, mean value of anterior chamber flare (photons/millisecond) | LogMAR, final value | ||||
| Brown 1996 | 1 month | Laser flare‐cell photometry, mean value of anterior chamber flare reported (photons) but was not possible to calculate SD | |||||||
| Cervantes‐Coste 2009 | 6 weeks | Quote: "None of the patients developed clinically significant macular oedema associated with vision loss" | Final value | Yes | Only reported CMO associated with vision loss | "Inflammatory cells greater than 1+ during first week of postoperative visits" | Total macular volume | ||
| Chatziralli 2011 | 6 weeks | Fundoscopy and Amsler grid test Quote: "no evidence of clinically significant CME" |
Yes | "No evidence of clinically significant CME was detected in any patient via fundoscopy and the Amsler grid test" | Corneal oedema or Tyndall reaction or conjunctival hyperaemia | LogMAR, final value | |||
| Donnenfeld 2006 | 3 months | Yes | "Clinically significant CME" but otherwise not defined, at 2 weeks only | "Mean inflammation score" but was not possible to calculate SD | LogMAR, final value but could not extract data on SD | ||||
| Elsawy 2013 | 12 weeks | Clinical examination, unclear if OCT‐verified | |||||||
| Endo 2010 | 6 weeks | Final value | Yes | Anterior chamber flare values, photon count per millisecond | LogMAR, final value | ||||
| Italian Diclofenac Study Group 1997 | 140 days | Yes | Fluorescein angiography using Miyake 1977 classification | ||||||
| Jung 2015 | 1 month | Change | Yes | "Inflammatory score" (sum of anterior chamber cells and flare grade" | Change in macular volume | ||||
| Kraff 1982 | Between 2.5 and 12 months | Yes | Fluorescein angiography using Miyake 1977 classification | Snellen acuity only, not included in analyses | |||||
| Li 2011 | 1 month | Final value | OCT, "clinically apparent" CME otherwise not defined | Snellen acuity only, not included in analyses | |||||
| Mathys 2010 | 2 months | Change from baseline | Yes | LogMAR | Change in foveal thickness, change in macular volume | ||||
| Miyake 2007 | 5 weeks | Fluorescein angiography using Miyake 1977 classification | Unit of measurement unclear | Snellen acuity only, not included in analyses | |||||
| Miyake 2011 | 5 weeks | Final value | Yes | Fluorescein angiography using Miyake 1977 classification | Flare (photons/millisec), final value | Change in logMAR BCVA, categorical 3+, 2, 1 lines increase and no change | |||
| Miyanaga 2009 | 2 months | Yes | "Obvious CMO confirmed by OCT" | Aqueous flare (photons/millisecond) | LogMAR, final value | ||||
| Moschos 2012 | 1 month | Final value | LogMAR, final value | ||||||
| Quentin 1989 | 180 days | Yes | Fluorescein angiography using Miyake 1977 classification | Snellen acuity only, not included in analyses | |||||
| Rossetti 1996 | 6 months | Yes | Fluorescein angiography using Miyake 1977 classification | Snellen acuity only, not included in analyses | |||||
| Singh 2012 | 90 days | Change from baseline | Yes | ">= 30% increase in central subfield macular thickness from baseline" | Flare mentioned but data not reported | Corrected BCVA loss of more than 5 letters from day 7 postop | |||
| Solomon 1995 | 6 months | Days 21 to 60, MO = positive angiography and visual acuity <= 20/40 | Yes | Fluorescein angiography using classification*** | Snellen acuity but not reported by treatment group | ||||
| Tauber 2006 | 30 days (3 months mentioned but not reported) | Reported but no mean/SD | Proportion with > 10% increase in retinal thickness | ||||||
| Ticly 2014 | 5 weeks | Final value | Yes | Fluorescein angiography using Miyake 1977 classification | LogMAR | ||||
| Tunc 1999 | 2 months | Fluorescein angiography 0 no leakage (CME absent),1 oedema less than perifoveal, 2 mild perifoveal oedema, 3 moderate perifoveal oedema (approx. 1 disc diameter), 4 severe perifoveal oedema plus drop of 1 line of Snellen acuity since second postoperative week defined as "clinically significant" | |||||||
| Tzelikis 2015 | 12 weeks | Final value | Yes | LogMAR, final value (at 30 days only) | |||||
| Umer‐Bloch 1983 | 12 weeks | Yes | Fluorescein angiography using Miyake 1977 classification | Snellen acuity only, not included in analyses | |||||
| Wang 2013 | 2 months | OCT‐confirmed CMO with "visual impairment" (not specified cutpoint) | Final value | Yes | "CME was defined as central retinal thickness > 250 μm and the presence of intraretinal cystoid space beneath the foveal, with the diagnosis confirmed by the same retinal specialist" | Mean photon count values | LogMAR, final value | ||
| Wittpenn 2008 | 4 weeks | OCT‐confirmed CMO with visual acuity < 6/9 | Yes | Clinical and OCT‐based | |||||
| Yannuzzi 1981 | 1 year | CMO on fluorescein angiography with visual acuity < 6/60 | Yes | Fluorescein angiography, evidence but not defined | |||||
| Yavas 2007 | 3 months | "Slight fluorescein leakage into the cystic space without enclosing the entire central fovea or complete fluorescein accumulation in the cystic space was diagnosed as angiographic CME" | LogMAR, final value | ||||||
| Yung 2007 | 12 weeks | ||||||||
| Zaczek 2014 | 6 weeks | Yes | OCT‐verified but not defined | Mean anterior chamber flare reported in figure but no SD | LogMAR, final value | Change in total macular volume | |||
| Zhang 2008 | 1 month | OCT‐verified but not defined | Tyn granule + |
BCVA: best corrected visual acuity CME: cystoid macular oedema (edema) CMO: cystoid macular oedema COMTOL: Comparison of Ophthalmic Medications for Tolerability (questionnaire) MO: macular oedema OCT: ocular coherence tomography SD: standard deviation
Setting and conduct of Study
See Table 4.
The studies were conducted in Brazil (Ticly 2014; Tzelikis 2015), Canada (Almeida 2008; Almeida 2012; Solomon 1995), China (Li 2011; Wang 2013; Zhang 2008), Egypt (Elsawy 2013), Germany (Quentin 1989; Solomon 1995), Greece (Chatziralli 2011; Moschos 2012) , Italy (Italian Diclofenac Study Group 1997; Rossetti 1996), Japan (Asano 2008; Endo 2010; Miyake 2007; Miyake 2011; Miyanaga 2009), Mexico (Cervantes‐Coste 2009), South Korea (Jung 2015), Sweden (Zaczek 2014), Switzerland (Umer‐Bloch 1983), Turkey (Tunc 1999; Yavas 2007) and the USA (Brown 1996; Donnenfeld 2006; Kraff 1982; Mathys 2010; Singh 2012; Tauber 2006; Wittpenn 2008; Yannuzzi 1981; Yung 2007).
They were all parallel group RCTs, i.e. participants were randomly allocated to intervention or comparator. Three of the studies were described as “open‐label” (Almeida 2008;; Endo 2010; Wang 2013).
Four studies were funded by industry alone (Brown 1996; ; Solomon 1995; Tauber 2006; Wittpenn 2008; ); seven studies reported only non‐industry funding (Almeida 2008; Almeida 2012; Jung 2015; Kraff 1982; Mathys 2010; Wang 2013; Yannuzzi 1981); two studies had funding from both industry and non‐industry sources (Donnenfeld 2006; Zaczek 2014) and the rest of the studies did not report the source of funding.
Declarations of interest were not reported in 12 studies; 17 studies reported that they had no conflicts of interest and six studies reported conflicts of interest for one or more investigators (Donnenfeld 2006; Italian Diclofenac Study Group 1997; Miyake 2011; Singh 2012; Tauber 2006; Wittpenn 2008).
Six trials were registered on a publicly available database. For three of these trials the registration was probably prospective as the month of registration was the same, or before, the month the study started (Almeida 2008; Mathys 2010; Singh 2012).Three trials were registered retrospectively (Almeida 2012; Tzelikis 2015; Wittpenn 2008).
Two trials were reported in abstract form only (Tauber 2006; Yung 2007). However, we contacted the first authors of Tauber 2006 and Yung 2007 and we received additional information in the form of a poster from Yung 2007.
Participants
There were variations in the reporting of recruited and randomised participants. As such it is difficult to establish definitively the total number of people that were randomised in these trials. We estimate that there were 5532 people (5608 eyes) enrolled in these 34 studies and 4476 followed up. (Table 5).
Five studies did not report the number of people randomised (Brown 1996; Tauber 2006; Umer‐Bloch 1983; Yannuzzi 1981; Zhang 2008). For four of these five studies we estimated the number of people in the trial from the number analysed. One study provided no information on the number of participants (Brown 1996).
For those studies that did not report follow‐up clearly we have assumed the number randomised and number followed up was the same.
The majority of the studies (n = 24) enrolled one eye person in the trial, although this was not always clearly described. In six studies the number of eyes/people was not reported in enough detail to be confident how many eyes per person had been enrolled (Donnenfeld 2006; Kraff 1982; Tauber 2006; Umer‐Bloch 1983; Wang 2013; Yung 2007), although it is likely that they too largely performed unilateral surgery.
Four studies performed bilateral surgery on a subset of patients, and so had more eyes than people in the trial (Almeida 2008; Elsawy 2013; Yannuzzi 1981; Zhang 2008). The proportion of people with bilateral surgery was 1% (Yannuzzi 1981), 8% (Almeida 2008), 11% (Zhang 2008) and 23% (Elsawy 2013). None of the studies adjusted for within‐person correlation. We have analysed the data as reported.
For the studies that reported average age, the median average age of participants was 70 years (Table 6). Ages ranged from 37 to 100 years. For the studies that reported gender, the median percentage of women was 54%.
Fifteen studies reported that they excluded patients with diabetes or diabetic retinopathy, or were a “low risk population”. Nine studies did not report the diabetes status of their participants. Nine studies included people with diabetes and reported the percentage of the participants with diabetes. The percentage with diabetes was 10%/9% (Chatziralli 2011; Miyake 2011), 21%/20% (Almeida 2008; Cervantes‐Coste 2009) and 26% (Jung 2015). Five studies only included people with diabetes (Elsawy 2013; Endo 2010; Li 2011; Singh 2012; Yung 2007).
The majority of studies either excluded people with uveitis (n = 19) or had a “low risk population” (Almeida 2012), or very low proportion with uveitis (1/56 people) (Almeida 2008). Thirteen studies did not report uveitis and it was not included in the exclusion criteria.
Interventions
See Table 7
Type of surgery
Twenty‐four of the 34 studies reported that only phacoemulsification was performed for cataract extraction (Table 7). In one study both extracapsular cataract extraction (ECCE) and phacoemulsification were performed (Kraff 1982). Four studies reported that they performed ECCE (Italian Diclofenac Study Group 1997; Rossetti 1996; Solomon 1995; Tunc 1999), two studies performed ICCE (Quentin 1989; Yannuzzi 1981) and one study performed a mixture of ECCE/intracapsular cataract extraction (ICCE) (Umer‐Bloch 1983). In two studies that were reported in abstract form only there was no information on type of surgery but we have assumed that they used phacoemulsification because of the date published and location of the study (Tauber 2006; Yung 2007).
Comparison
Twenty‐eight of the 34 studies compared non‐steroidal anti‐inflammatory drugs (NSAIDs) with steroids versus steroids. In 14 of these 28 studies, a placebo (for the NSAIDs) was used in the comparator group. This placebo was not specified in two trials (Quentin 1989; Rossetti 1996;); was artificial tears in five trials (Ticly 2014; Tzelikis 2015; Wittpenn 2008; Yung 2007; Zaczek 2014); a vehicle in six studies (Donnenfeld 2006; Kraff 1982; Singh 2012; Solomon 1995; Umer‐Bloch 1983; Yannuzzi 1981); and sterile saline drops in Almeida 2012. .
Six of the 34 studies compared NSAIDs (on their own) with steroids (Asano 2008; Brown 1996; Endo 2010; Italian Diclofenac Study Group 1997; Miyake 2007; Miyake 2011). Only one of these studies used a placebo in the steroid group; the contents of this placebo were not specified. (Italian Diclofenac Study Group 1997).
NSAIDs
The most frequently used NSAID was ketorolac (11 studies) followed by diclofenac (9 studies), nepafenac (7 studies), indomethacin (5 studies), bromfenac (4 studies), pranoprofen (1 study) and flurbiprofen (1 study). Four studies had two different NSAID groups ‐ ketorolac and nepafenac (Almeida 2012; Tzelikis 2015), ketorolac and bromfenac (Jung 2015) and flurbiprofen and indomethacin (Solomon 1995). We combined these groups for the analysis.
The ketorolac concentration was either 0.4% or 0.5%. Diclofenac was largely used at a concentration of 0.1% (7 studies) but also used at 1% in Li 2011 and concentration was not specified in one study (; Rossetti 1996). Nepafenac was used at 0.1% in six studies and 1% in one study (Singh 2012). Indomethacin 1% was used in three studies (Solomon 1995; Umer‐Bloch 1983; Yannuzzi 1981), 0.1% in Yavas 2007 while the concentration used was not specified in Kraff 1982. Bromfenac 0.1% was used in Miyanaga 2009, Jung 2015 and Wang 2013; it was not specified in Endo 2010. Flubiprofen was used at 0.03% (Solomon 1995). Pranopfen concentration was not specified (Zhang 2008).
Steroids
Prednisolone was used in 13 studies, usually at 1%.
Dexamethasone was used in 15 studies, at a concentration of 0.1% in eight studies and 1% in one study (Tunc 1999). The concentration used was not specified in 6 studies. It was combined with tobramycin in four studies (Cervantes‐Coste 2009; Li 2011; Rossetti 1996; Zhang 2008) and other antibiotics (Kraff 1982; Moschos 2012; Umer‐Bloch 1983).
Betamethasone was used at 0.1% in two studies (Asano 2008; Miyanaga 2009) and not specified in one study (Endo 2010).
Fluorometholone 0.1% was used as the sole topical corticosteroid therapy in three studies (Miyake 2007; Miyake 2011; Wang 2013) and used as part of a tapering regimen in one study (Kraff 1982).
The type of steroids used in Yannuzzi 1981 were not specified.
Other medications
Most studies reported the use of additional antibiotics. See Characteristics of included studies.
Outcomes
Maximum follow‐up ranged from one month (8 studies) to 12 months postoperatively (Kraff 1982; Yannuzzi 1981) (Table 8).
The majority of trials followed up to two months or less (23 studies). Five studies followed up to three months (Elsawy 2013; Singh 2012; Umer‐Bloch 1983; Yavas 2007; Yung 2007) and six studies followed up longer: 140 days (Italian Diclofenac Study Group 1997), six months (Quentin 1989; Rossetti 1996; Solomon 1995) and 12 months (Kraff 1982; Yannuzzi 1981). Kraff 1982 had a low follow‐up of 10 % at 12 months
Table 8 shows the outcomes reported in the studies.
Excluded studies
Risk of bias in included studies
See Figure 2
2.
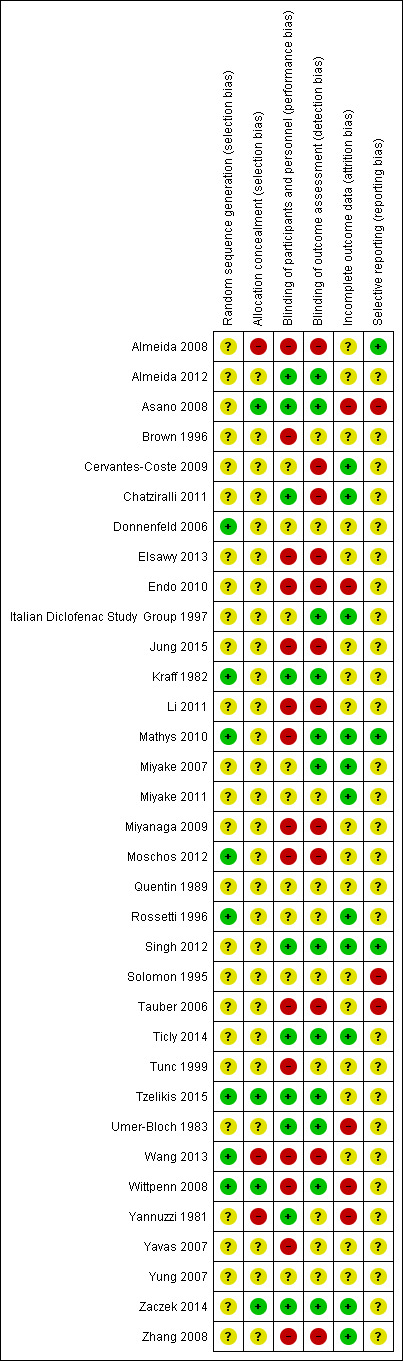
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The majority of trialists did not report sufficient information to judge selection bias. These trials were marked as unclear for sequence generation and allocation concealment. Only two trials were judged at low risk of bias on both sequence generation and allocation concealment (Tzelikis 2015; Wittpenn 2008).
Eight trials reported a method of sequence generation judged to be likely to generate an unpredictable sequence. Some trials used random number tables (Kraff 1982; Rossetti 1996; Wang 2013), other reports suggested computer‐generated random numbers or allocation schedules (Mathys 2010; Tzelikis 2015), others referred to random numbers or randomly generated lists but did not specify how these were created (Donnenfeld 2006; Moschos 2012; Wittpenn 2008).
Four trials reported a convincing method of allocation concealment (Asano 2008; Tzelikis 2015; Wittpenn 2008; Zaczek 2014). In Asano 2008 the assignment code was kept secret by a named individual until the end of the study; in Tzelikis 2015 all investigators were masked to treatment group; Wittpenn 2008 used a central co‐ordination centre for allocation and in Zaczek 2014 the allocation was prepared in such a way that neither investigators nor participants could identify the group.
In three studies, we judged that the allocation was probably not concealed adequately (Almeida 2008, Wang 2013; Yannuzzi 1981).
Blinding
Ten studies were not masked and we judged them to be at high risk of both performance and detection bias (Almeida 2008; Elsawy 2013; Endo 2010; Jung 2015; Li 2011; Miyanaga 2009; Moschos 2012; Tauber 2006; Wang 2013; Zhang 2008).
Eight studies were masked and we judged them to be at low risk of both performance and detection bias (Almeida 2012; Asano 2008; Kraff 1982; Singh 2012; Ticly 2014; Tzelikis 2015; Umer‐Bloch 1983; Zaczek 2014).
Two studies that did not mask participants, stated explicitly that outcome assessors were masked (Mathys 2010; Wittpenn 2008).
For six studies, there was not enough information to judge the risk of either performance or detection bias (Donnenfeld 2006; Miyake 2011; Quentin 1989; Rossetti 1996; Solomon 1995; Yung 2007).
Incomplete outcome data
We judged five studies to be at high risk of attrition bias. In Asano 2008, there was variable follow‐up by outcome, and it was not clearly explained why. Some of the stated exclusion criteria for the study, such as inflammation after surgery, would have been related to the outcome. In Endo 2010, follow‐up was unequal between study groups and reason for loss to follow‐up was not clearly reported. In Umer‐Bloch 1983, 35 people withdrew before the end of the study because of intraoperative complications or they had, as only later recognised, an exclusion criteria as defined as maculopathy, diabetic retinopathy, prior uveitis or a systemic steroid therapy. It was not reported to which groups these patients belonged. In Wittpenn 2008, there was very low follow‐up at six weeks, with 77/546 (14%) people followed‐up. In Yannuzzi 1981 there was a high loss to follow‐up at 12 months: 38/100 (38%) in the NSAIDs group and 50/131 (38%) in the control group were followed‐up.
We judged 11 studies to be at low risk of attrition bias. For the other studies there was not enough information to judge.
Selective reporting
For most studies there was little information to judge selective outcome reporting because we did not have access to a trial registry entry or study protocol. We judged three studies to be at low risk of selective outcome reporting on the basis that the trial was prospectively registered and all outcomes prespecified on the clinical trials registry entry were reported (Almeida 2008; Mathys 2010; Singh 2012). For three studies it was clear that some outcomes were not fully reported and so we judged them to be at high risk of selective outcome reporting bias (Asano 2008; Solomon 1995; Tauber 2006).
Effects of interventions
Non‐steroidal anti‐inflammatory drugs plus steroids versus steroids
Primary outcome
Poor vision due to macular oedema
Five studies reported this outcome at three months (eyes = 1360) (Analysis 1.1). Follow‐up ranged from four weeks to two months. Two studies reported optical coherence tomography (OCT)‐confirmed macular oedema (MO) with visual acuity < 6/9 in one study (Wittpenn 2008) but the level of visual impairment not defined in the other (Wang 2013). Solomon 1995 defined the presence of clinical MO as visual acuity <=20/40 and angiographic evidence of CMO. Cervantes‐Coste 2009 reported that none of the participants developed clinically significant macular oedema nor vision loss. Chatziralli 2011 reported that none of the participants developed clinically significant CMO as assessed via fundoscopy and the Amsler grid test. There was some evidence of selective reporting in Solomon 1995, which provided most of the information for the meta‐analysis. Data were only reported for the earlier follow‐up at days 21 to 60. Quote: "By day 121‐240 the incidence of clinical CME [cystoid macular edema] was less than 2% in all three groups and no significant differences were seen."
1.1. Analysis.

Comparison 1 NSAIDs plus steroids versus steroids, Outcome 1 Poor vision due to MO.
People receiving non‐steroidal anti‐inflammatory drugs (NSAIDs) combined with steroids had a lower risk of poor vision due to macular oedema (MO) at three months after surgery compared with people receiving steroids alone. The pooled risk ratio (RR) was 0.41, 95% confidence interval (CI) 0.23 to 0.76; eyes = 1360; studies = 5. There was no evidence of any major inconsistency (I2 = 5%). We judged this to be low‐certainty evidence (Table 1). We downgraded for risk of bias, as the trials were poorly reported and were largely at high or unclear risk of bias. We downgraded for indirectness, as the outcomes reported by the trials only approximated the outcome which we wished to collect, which was poor vision (best corrected visual acuity (BCVA) < 6/9) due to MO.
One study reported this outcome at 12 months (Yannuzzi 1981). There was high attrition in this study (only 38% of eyes followed up) and only two events (RR 1.32, 95% CI 0.09 to 20.37; eyes = 88). We judged this to be very low‐certainty evidence, downgrading for risk of bias and imprecision (2 levels; Table 1).
Secondary outcomes
Quality of life/patient satisfaction
One study reported quality of life at 1 month after surgery using the Comparison of Ophthalmic Medications for Tolerability (COMTOL) questionnaire (Almeida 2012), No differences in the impact upon quality of life measures were identified between the treatment and control groups. The use of topical NSAIDs was also reported to have good tolerability and comparable side‐effect profile to placebo. However the data in this study were not fully reported and a response rate of only 60% was achieved with significant attrition with 65 out of 162 patients declining to answer the interview after surgery for "logistical reasons".
Quote: "The global [health‐related quality of life] HRQOL questions showed no difference in the extent to which quality of life was affected by medication side effects between “not at all” and any reported effect (question 6; P = 0.8476). Regarding the extent quality of life was affected by activity limitations, there was no difference between “not at all” and any reported limitations (question 9; P = 0.8584). According to the COMTOL questionnaire, there was no difference in compliance between the 3 study groups (question 10; P = 0.3801). Most patients in all 3 groups reported being satisfied with the medication, and there was no difference between satisfied responses and dissatisfied responses (question 11; P = 0.4777)").
Central retinal thickness
Nine studies reported this outcome (eyes = 1112) (Analysis 1.2). Follow‐up ranged from one to two months. Six studies reported central retinal thickness at the end of the follow‐up period, three studies reported change in thickness from baseline. Trial results were inconsistent (I2 = 87%). Results ranged from ‐30.9 µm in favour of NSAIDs plus steroids to +7.44µm in favour of steroids alone (Table 1).
1.2. Analysis.
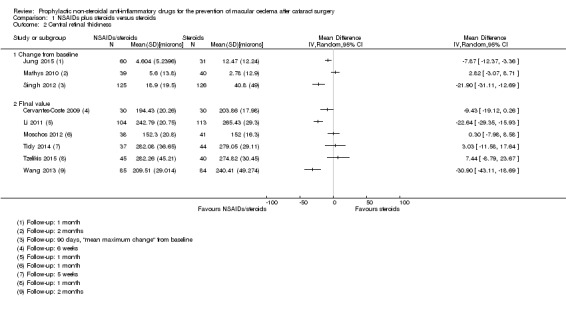
Comparison 1 NSAIDs plus steroids versus steroids, Outcome 2 Central retinal thickness.
Six studies reported change in macular volume (eyes = 570) (Analysis 1.3). The pooled mean difference (MD) was ‐0.14 mm3 (95% CI ‐0.21 to ‐0.07). There was some inconsistency (I2 = 50%), mainly attributable to Mathys 2010.
1.3. Analysis.

Comparison 1 NSAIDs plus steroids versus steroids, Outcome 3 Total macular volume.
Adverse effects
See Table 9. In the studies that reported adverse effects, no evidence of serious adverse events were seen. The most notable adverse effect associated with NSAID use was burning or stinging sensation.
7. Adverse effects.
| Study | Follow‐up | Number of people followed up | Adverse effects |
| Almeida 2008 | 1 month | 74 | Quote: "There were 3 dropouts in the treatment group related to ketorolac corneal toxicity, most notably pain attributed to the drops." |
| Almeida 2012 | 1 month | 162 | Quote: “One patient in the ketorolac group was hospitalized with a cardiovascular event and could not complete the follow‐up. Finally, 1 patient on nepafenac had side effects of ocular redness and irritation and could not continue with the study.” |
| Asano 2008 | 8 weeks | 142 | 2 "complications" not specified. |
| Brown 1996 | 1 month | NR | Adverse effects not reported. |
| Cervantes‐Coste 2009 | 6 weeks | 60 | Quote: "There were no serious treatment‐related adverse events or toxicity related to the use of nepafenac 0.1%." |
| Chatziralli 2011 | 6 weeks | 138 | Quote: "All patients reported pain and ocular discomfort lower than 1/10 on the visual analog scale at all time points." |
| Donnenfeld 2006 | 2 weeks | 100 | Quote: "Use of ketorolac 0.4% for 1 or 3 days provided decreased levels of patient discomfort intraoperatively and postoperatively. Intraoperatively, 3 days of ketorolac 0.4% provided significantly lower discomfort scores than with 1‐hour and placebo dosing (P < 0.001). One day of ketorolac 0.4% also provided significantly reduced intraoperative discomfort scores than with 1‐hour dosing (P = 0.001) and placebo dosing (P < 0.001). Postoperatively, 3 days of ketorolac 0.4% provided significantly lower discomfort scores than 1‐hour dosing or control dosing (P < 0.001) (Figure 5). In addition, patients randomised to 1 or 3 days of ketorolac 0.4% were significantly less likely to require additional intravenous anesthesia (8% in each group) than patients in the control group (40%) (P = 0.008). Twenty percent of patients in the 1‐hour group required additional anesthesia for pain control." |
| Elsawy 2013 | 12 weeks | 86 | Adverse effects not reported. |
| Endo 2010 | 6 weeks | 62 | Quote: "No adverse events were noted in either group." |
| Italian Diclofenac Study Group 1997 | 140 days | 229 | Quote: "No major adverse effects were noted in either group." "Subjective tolerance of the two treatments was good and remained similar throughout the study, although a trend towards increased burning was seen in the diclofenac group." |
| Jung 2015 | 1 month | 91 | Quote: "There were no adverse events except for a mild burning sensation in one patient in the ketorolac group; the symptom was tolerable and did not lead to discontinuation of the medication." |
| Kraff 1982 | between 2.5 and 12 months | 492 | Quote: "There were no complications that could be ascribed to the use of topical indomethacin other than minor stinging and burning noted by the patients." |
| Li 2011 | 1 month | 217 | Adverse effects not reported. |
| Mathys 2010 | 2 months | 79 | Quote: "There were no adverse events reported by patients using nepafenac." |
| Miyake 2007 | 5 weeks | 50 | Adverse effects not reported. |
| Miyake 2011 | 5 weeks | 55 | NSAIDs: 6 adverse effects: decreased lacrimation, conjunctivitis allergic, abnormal sensation in eye, vomiting (2), constipation. Steroid group: 9 adverse effects: decreased lacrimation, conjunctivitis allergic, retinal haemorrhage, keratoconjunctivitis sicca, chorioretinopathy, influenza, insomnia, diarrhoea, humeral fracture. |
| Miyanaga 2009 | 2 months | 72 | Adverse effects not reported. |
| Moschos 2012 | 1 month | 79 | Adverse effects not reported. |
| Quentin 1989 | 180 days | 112 | Quote: "Diclofenac group: two patients were feeling burning after application of eye drops during the stationary care, for placebo: none. In both groups burning was reported later on in the examinations." |
| Rossetti 1996 | 6 months | 88 | Quote: "Treatment regimens were well tolerated with no evidence of relevant side effects." |
| Singh 2012 | 90 days | 251 | Quote: "No patient deaths were reported during the study. Overall, 13 patients reported other serious adverse events, none of which were related to treatment. Three of the serious adverse events reported in the vehicle group (cardiac failure congestive, coronary artery occlusion, and pancreatitis) led to patient discontinuation; no other serious adverse events led to discontinuation in either treatment group. Separate from the three patients who discontinued due to serious adverse events, four other patients discontinued study participation due to nonserious adverse events. Of these nonserious events, two reported instances of punctate keratitis (one in each treatment group) were assessed as being related to the study drugs. No instances of targeted adverse events (defined as corneal erosions) were reported during the study. Two reports of punctate keratitis and a single report of corneal epithelium defect were assessed as being related to treatment with nepafenac. A single report of punctate keratitis was assessed as being related to treatment with vehicle. No other ocular or nonocular adverse events reported in the study were assessed as being related to the study drugs. In both treatment groups, corneal staining and intraocular pressure were each generally similar at the presurgical baseline and at the day 90 visit (or early exit). Additionally, no safety issues or trends were identified based upon changes from baseline in fundus parameters (retina/macula/choroid and optic nerve) and ocular signs (inflammatory cells, aqueous flare, corneal oedema, and bulbar conjunctival injection). The study results indicate no new clinically relevant risks associated with increasing the dosing of nepafenac from 14 days to 90 days, even in the higher‐risk diabetic patient population." |
| Solomon 1995 | 6 months | 364 | Quote: "During the study, the mean severity of foreign‐body sensation, pain, photophobia, and tearing did not become more than mild (1 +) in any treatment group. This was also true of burning and stinging following treatment instillation (Figure 4). The severity of burning and stinging was significantly greater in the flurbiprofen group on days 4‐20 and 21‐60 and in the indomethacin group on days 1‐3, 4‐20, 21‐60, and 61‐120 than in the vehicle group. At day 1‐3, moderate to severe burning and stinging were reported by 7.0% (16/230) of the patients treated with flurbiprofen, 9.7% (23/237) of the patients treated with indomethacin, and 3.1% (7/224) of the patients treated with vehicle." |
| Tauber 2006 | 30 days (3 months mentioned but not reported) | 32 | Adverse effects not reported. |
| Ticly 2014 | 5 weeks | 81 | One patient withdrew because of burning. |
| Tunc 1999 | 2 months | 75 | Adverse effects not reported. |
| Tzelikis 2015 | 1 month | 126 | Quote: "There were no adverse side effects in either group." |
| Umer‐Bloch 1983 | 12 weeks | 73 | Quote from translation: "40% reported a short burning after using indomethacin eye drops, only rare in patients of the placebo group. One patient had 6 weeks after treatment an allergic blepharitis due to indomethacin. Long‐term: 52 patients were followed for 6 months and 34 patients one year. 4 patients with indomethacin had visual acuity reduction because of a clinically new cystoid edema; 2 of these patients had spontaneous healing after 4‐6 weeks, the other 2 edema cases did not resolve. 2 patients had a new senile macula pathology, and 2 patients had a retinal detachment due to aphakia. Placebo: 2 patients still had an edema after 12 weeks, while one patient developed a new edema later." |
| Wang 2013 | 2 months | 167 | Quote: "No drug‐related adverse events were identified." |
| Wittpenn 2008 | 4 weeks | 478 | Quote: "The most commonly reported adverse events (investigator self‐report) in the ketorolac/steroid group were burning/stinging/tearing (4/268). Transient elevations in intraocular pressure (IOP) were the most commonly reported adverse event in the steroid group (3/278). There were two serious adverse events, both in the steroid group: one patient developed endophthalmitis and one patient died (cause determined to be unrelated to the study medication)." |
| Yannuzzi 1981 | 1 year | 231 | Adverse effects not reported. |
| Yavas 2007 | 3 months | 179 | Adverse effects not reported. |
| Yung 2007 | 12 weeks | 37 | Adverse effects not reported. |
| Zaczek 2014 | 6 weeks | 152 | Quote: "Mild to moderate punctuate epithelial defects of the cornea were found in both groups 3 weeks after treatment.Statistically significantly more patients in the nepafenac group than in the control group had corneal fluorescein staining (20 [26.7%] versus 8 [10.4%]) (PZ.0119). Headache was reported by 3 patients (4.0%) in the nepafenac group and 2 patients (2.6%) in the control group (PZ.9750). No other systemic or local untoward effects were recorded during 3 weeks of treatment in either study group." |
| Zhang 2008 | 1 month | 220 | Adverse effects not reported. |
Resource use and costs
None of the studies commented on this.
Additional National Institute for Health and Care Excellence (NICE) outcomes
Macular oedema (MO) (clinically symptomatic, optical coherence tomography‐verified)
Twenty‐one studies reported this outcome (eyes = 3638) (Analysis 1.4). Follow‐up ranged from two weeks to just less than six months.
1.4. Analysis.
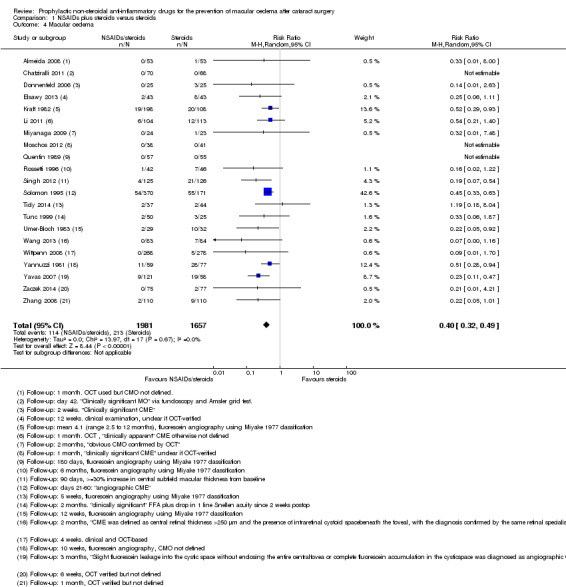
Comparison 1 NSAIDs plus steroids versus steroids, Outcome 4 Macular oedema.
Most studies reported "cystoid" macular oedema but it was not always clearly defined nor was it clear that it was clinically significant. Nine studies used OCT, although it was not always clear if the OCT was used to verify the MO; nine studies used fluorescein angiography, often using the Miyake 1977 classification; clinical assessment for the presence of MO was made in two studies.
There was an asymmetric funnel plot, suggesting that publication bias might be an issue (Figure 3).
3.
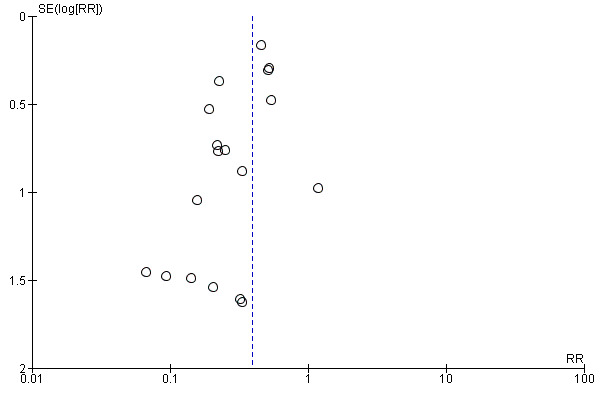
Funnel plot of comparison: 1 NSAIDs plus steroids versus steroids, outcome: 1.4 Macular oedema.
People receiving NSAIDs combined with steroids had a lower risk of MO after surgery compared with people receiving steroids alone. The pooled RR was 0.40, 95% CI 0.32 to 0.49; I2 = 0%. We judged this to be low‐certainty evidence (Table 1). We downgraded one level for risk of bias, as the studies were at unclear or high risk of bias and we downgraded one level for publication bias as an asymmetric funnel plot was suggestive of publication bias. We considered downgrading one level for indirectness, as the MO was not always OCT‐verified and it was not always clear if the MO was clinically significant but in the event did not as the size of the effect was strong.
Inflammation
Three studies reported inflammation as a dichotomous outcome (Analysis 1.5). In Cervantes‐Coste 2009 there were no cases of "inflammatory cells greater than 1+ during first week of postoperative visits." In Chatziralli 2011, at day 28, inflammation, which was defined as corneal oedema or Tyndall reaction or conjunctival hyperemia was seen in two participants in the NSAIDs plus steroid group (RR 4.86, 95% CI 0.24 to 99.39); by day 35 this had disappeared. In Zhang 2008, 20 participants in the steroids group had inflammation defined as "Tyn granule +" compared to 0 participants in the NSAIDs plus steroids group at one month (RR 0.02, 95% CI 0.00 to 0.38). In view of such different results, we did not pool the data from these trials.
1.5. Analysis.
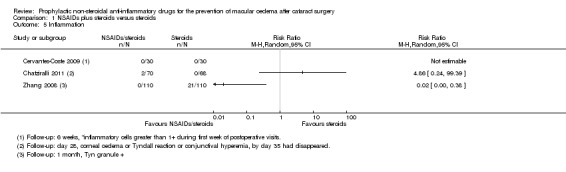
Comparison 1 NSAIDs plus steroids versus steroids, Outcome 5 Inflammation.
Two studies reported flare in photons/millisecond (eyes = 216) (Analysis 1.6). The MD was ‐1.41 photons/millisecond in favour of NSAIDs plus steroids (95% CI ‐2.30 to ‐0.52), but there was some inconsistency between the two studies (I2 = 49%). There was some evidence of skew for the control group of Miyanaga 2009 (mean/standard deviation (SD) < 2).
1.6. Analysis.

Comparison 1 NSAIDs plus steroids versus steroids, Outcome 6 Inflammation (flare).
Jung 2015 reported "summed ocular inflammation score" which was the sum of the scores of cells and flare, scored against a maximum total score of 9. The inflammatory score at one month was 0.21 ± 0.42 in the bromfenac group and 0.32 ± 0.48 in the ketorolac group (P = 0.853). The score in the control group was 0.84 ± 0.76.
Best corrected visual acuity
Ten studies reported BCVA (eyes = 1158) (Analysis 1.7). For Mathys 2010 change in BCVA was reported in letters. We converted this to logMAR score by multiplying by ‐0.02 and we estimated the SD from the P value.
1.7. Analysis.
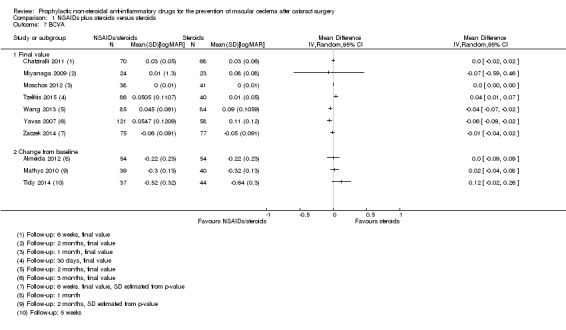
Comparison 1 NSAIDs plus steroids versus steroids, Outcome 7 BCVA.
There was statistical heterogeneity (I2 = 70%), and not all effect estimates were in the same direction, so we did not provide a pooled estimate. However, we note that most studies found differences clinically indistinguishable from no difference.
Non‐steroidal anti‐inflammatory drugs versus steroids
Primary outcome
Poor vision due to macular oedema
None of the studies reported this outcome.
Secondary outcomes
Quality of life/patient satisfaction
None of the studies reported this outcome.
Central retinal thickness
Two studies reported central retinal thickness (Analysis 2.1). The pooled MD was ‐22.64 µm (95% CI ‐38.86 to ‐6.43; I2 = 0%) in favour of NSAIDs. We judged this to be low‐certainty evidence (Table 2). We downgraded one level for risk of bias, as the studies were at unclear or high risk of bias, and we downgraded one level for imprecision as the confidence intervals include a clinically unimportant effect.
2.1. Analysis.
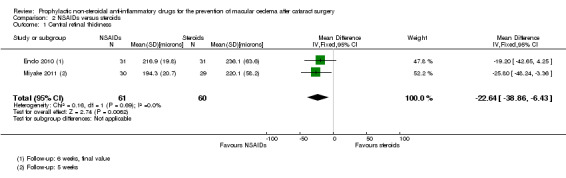
Comparison 2 NSAIDs versus steroids, Outcome 1 Central retinal thickness.
Adverse effects
See Table 9. In the studies that reported adverse effects, no evidence of serious adverse events were seen. The most notable adverse effect associated with NSAID use was burning or stinging.
Resource use and costs
None of the studies commented on this.
Additional NICE outcomes
Macular oedema (clinically symptomatic, optical coherence tomography‐verified)
Five studies reported this outcome (eyes = 520) (Analysis 2.2). All studies assessed MO using fluorescein angiography. The pooled RR was 0.27 (95% CI 0.18 to 0.41) in favour of NSAIDs. We note that for Asano 2008 there may have been selective reporting ‐ data on MO were reported only at five weeks, but were not reported at the end of eight weeks follow‐up in that study.
2.2. Analysis.
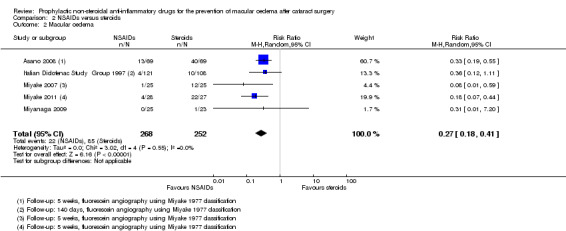
Comparison 2 NSAIDs versus steroids, Outcome 2 Macular oedema.
We judged this to be low‐certainty evidence (Table 2). We downgraded one level for risk of bias, as the studies were at unclear or high risk of bias and we downgraded one level for publication bias because of an asymmetric funnel plot suggestive of publication bias (Figure 4). We would not usually do a funnel plot with so few studies, but as the funnel plot for this outcome, for the comparison NSAIDs plus steroids versus steroids alone was asymmetric (Figure 3), we felt that publication bias may be an issue here as well.
4.
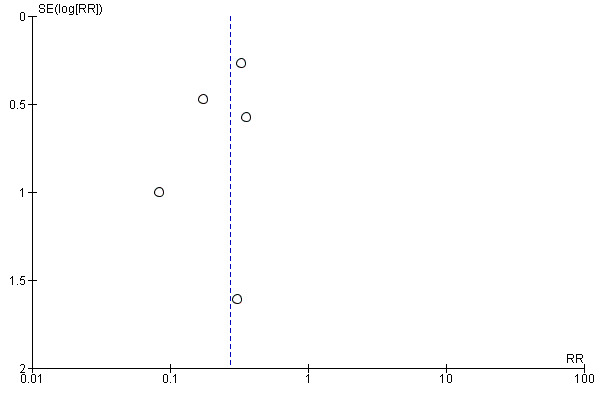
Funnel plot of comparison: 2 NSAIDs versus steroids, outcome: 2.2 Macular oedema.
Inflammation
Five studies reported aqueous flare (eyes = 346) (Analysis 2.3). There was substantial inconsistency ( I2 = 68%) and some evidence of skewed data so we did not report a pooled value.
2.3. Analysis.

Comparison 2 NSAIDs versus steroids, Outcome 3 Inflammation (flare).
Best corrected visual acuity
Three studies reported BCVA (eyes = 220) (Analysis 2.4). There was statistical heterogeneity (I2 = 84%) so we did not report a pooled value, but we note that all three studies found between group differences that were clinically indistinguishable from no difference.
2.4. Analysis.
Comparison 2 NSAIDs versus steroids, Outcome 4 BCVA.
Discussion
Summary of main results
We identified 34 studies that were conducted in the Americas, Europe, the Eastern Mediterranean region and South‐East Asia.
Over 5000 people were randomised in these trials. The majority of studies probably enrolled one eye per participant, a small subset (4 trials) enrolled a proportion of people with bilateral surgery. Twenty‐eight of these 34 studies compared non‐steroidal anti‐inflammatory drugs (NSAIDs) plus steroids with steroids alone. Six studies compared NSAIDs (on their own or with placebo) with steroids. A variety of NSAIDs were used, including ketorolac, diclofenac, nepafenac, indomethacin, bromfenac and pranopfen. Follow‐up ranged from one month to 12 months. The majority of studies (n = 23) followed up to two months or less. In general, the studies were poorly reported. We did not judge any of the studies at low risk of bias in all domains.
There was low‐certainty evidence that people receiving topical NSAIDs in combination with steroids may have a lower risk of poor vision due to macular oedema (MO) at three months after cataract surgery compared with people receiving steroids alone (risk ratio (RR) 0.40, 95% confidence interval (CI) 0.27 to 0.61; eyes = 1360; studies = 5; I2 = 5%). There were very little data for 12 months (only one study reported poor vision due to MO at this time point) and we judged this to have very low‐certainty evidence. Similarly, we judged the evidence on 'clinically symptomatic MO' to be low‐certainty. There was evidence on central retinal thickness at three months, but this was inconsistent (I2 = 87%). Results ranged from ‐30.9 microns in favour of NSAIDs plus steroids to 7.44 microns in favour of steroids alone. Similarly, data on best corrected visual acuity (BCVA) were inconsistent. Nine out of 10 trials reporting this outcome found between‐group differences of less than 0.1 logMAR.
None of the six studies comparing NSAIDs alone with steroids reported on poor vision due to MO at three months or 12 months. We judged the evidence on MO to be low‐certainty. There was low‐certainty evidence that mean central retinal thickness was lower in the NSAIDs group at three months (mean difference (MD) ‐22.64 microns, 95% CI ‐38.86 to ‐6.43; eyes = 121; studies = 2; I2 = 0%). Two studies reported BCVA at three months, and the results of these trials were inconsistent, but both found differences of less than 0.1 logMAR between groups.
Quality of life was only reported in one of the 34 studies, and it was not fully reported other than to comment on lack of differences between groups. In general, no major adverse events were noted ‐ the main consistent observation was burning or stinging.
Overall completeness and applicability of evidence
There were a relatively large number of trials, and these studies have a wide global range which means their results will be globally applicable.
The included studies compared NSAIDs and steroids in cataract surgery using phacoemulsification, extracapsular cataract extraction (ECCE) and intracapsular cataract extraction (ICCE) surgical techniques. However, the more recent trials exclusively used phacoemulsification, which may make their findings less applicable to parts of the world where resources are less available and ECCE is standard.
The aim of this review was to assess whether the use of NSAIDs had an impact on visual loss due to MO in the long‐term. The evidence is very sparse with respect to that question, with only one study with high attrition, reporting on visual loss due to MO at 12 months after surgery. This is clearly an important gap in the evidence.
There are many trials looking at the short‐term effects of NSAIDs, but there is considerable variation in terms of types, doses and regimens of NSAIDs and steroids used. One aspect that we have not highlighted in this review, but has been discussed elsewhere (Kim 2016a), is the potency of the steroid used in the comparison group. Use of low potency steroids, such as fluorometholone 0.1%, may lead to an overestimate of the relative effect of NSAIDs.
Certainty of the evidence
We graded the evidence as low‐ to very low‐certainty. In general, the trials were poorly reported and it was difficult to judge the extent to which bias had been avoided. We did not judge any of the studies at low risk of bias for all domains. Many trials were not properly masked and, in a few studies, there were problems with attrition bias and selective outcome reporting. For outcomes that had more data we identified the possibility of publication bias with an asymmetric funnel plot. There were also problems with directness. For example, many studies reported "CMO" but were not clear whether or not this was 'clinically significant', or indeed what this meant in terms of whether it caused both symptoms and signs. And in many of the older studies this could not be verified by optical coherence tomography (OCT).
Potential biases in the review process
We have made several modifications to the original protocol (see Differences between protocol and review), but these were made before the data extraction and analysis phases of the review.
Agreements and disagreements with other studies or reviews
A recent systematic review and meta‐analysis has been published (Wielders 2015). This review included 17 trials. The reason why they had fewer trials than the current review was because they only included studies of phacoemulsification cataract surgery and they excluded studies that did not report the incidence of cystoid macular oedema (CMO).
The review by Wielders 2015 reported effect measures in the same order of magnitude as that suggested by this review, but because they reported odds ratios (ORs), rather than risk ratios (RRs), these effect estimates are exaggerated (further away from null). The authors concluded that the odds of CMO were reduced in people who were given NSAIDs, but they did not incorporate a judgement on the overall certainty (or quality) of the evidence in their conclusions, even though they had assessed the risk of bias in the included trials using two different methods. It is also notable that, although the abstract highlights the fact that 17 trials were included in the review, it is less clearly pointed out that the effect estimates were based on a relatively small subset of these trials. This review was subsequently criticised because it did not fully incorporate an assessment of visual loss due to CMO, because the conclusions were based on so few trials, and because of the likely exclusion of studies that did not report any events (Kim 2016).
A report by the American Academy of Ophthalmology, also published in 2015, was more conservative in its conclusions (Kim 2015). This was a narrative review of the literature with no meta‐analysis, nor any assessment of the quality of the evidence. They concluded that NSAIDs reduced the incidence of CMO, and may increase visual recovery, depending on the treatment of the comparator group, however, they concluded that the use of NSAIDs did not alter long‐term (3 months) visual outcomes, a finding which is supported by the current review.
One slightly older systematic review published in 2014, included 15 trials and did include an overall GRADE assessment of the certainty of the evidence, which they judged to be low‐ to moderate‐certainty for inflammation, low‐certainty for visual acuity and high‐certainty for CMO (Kessel 2014). This review again focused on phacoemulsification. It was restricted to the comparison of NSAIDs (on their own or with placebo) versus steroids alone. They cited the previously published protocol of this review justifying theirs as being different for these two reasons. They evaluated inflammation within one week of surgery and MO at any time point. There are some differences between the current review and Kessel 2014 in terms of the included studies. This is because the searches for the current review were restricted to evidence relating to MO. However, the trials contributing data to the analysis of MO are similar in the two reviews. Kessel 2014 included one study that we judged was probably not a randomised controlled trial (RCT) (Miyake 2000), and one study that we have included in the NSAIDs plus steroids comparison (Wang 2013). The estimates of effect for MO reported in Kessel 2014 and reported in this review are of a similar order of magnitude, although Kessel 2014 reports a stronger effect. This can be attributed to the fact that, when extracting data from studies using the Miyake 1977 classification, Kessel 2014 considered Grades 2 to 3 as MO, whereas in the current review we considered Grades 1 to 3. The main difference between the reviews is in the grading of the certainty of the evidence. Kessel 2014 considered the evidence to be high‐certainty. It is not clearly stated why, but the footnote refers to a RR of 6, which we understand to mean that it is a strong effect, therefore they have not downgraded. We have considered the evidence on MO to be low‐certainty, downgrading for risk of bias and publication bias (Table 2).
Authors' conclusions
Implications for practice.
Using topical NSAIDs may reduce the risk of developing macular oedema after cataract surgery, although it is possible that current estimates as to the size of this reduction are exaggerated due to selective non‐reporting of negative studies. It is unclear the extent to which this reduction has an impact on the visual function and quality of life of patients. There is little evidence to suggest any important effect on vision after surgery
The value of adding topical NSAIDs to steroids, or using them as an alternative to topical steroids with a view to reducing the risk of poor visual outcome after cataract surgery is uncertain. This is reflected in wide variations in modern practice. The role of the relative effectiveness and safety of NSAIDs as an alternative to steroids in the control of post operative inflammation is being addressed in another Cochrane Review (Gonzales 2013).
Implications for research.
Future trials should address the remaining clinical uncertainty of whether prophylactic topical NSAIDs are of benefit, particularly with respect to longer‐term follow‐up (at least to 12 months), and should be large enough to detect to detect reduction in the risk of the outcome of most interest to patients, which is chronic macular oedema leading to visual loss. They should be rigorously conducted and double‐masked.
History
Protocol first published: Issue 3, 2007 Review first published: Issue 11, 2016
| Date | Event | Description |
|---|---|---|
| 10 July 2008 | Amended | Converted to new review format. |
Notes
The protocol for this review question was first published in 2007 (Goh 2007). The original review team were unable to complete the review and therefore a new review team was found. The latest protocol for this review was published in 2011 (Abeysiri 2011).
Acknowledgements
This work was undertaken in collaboration with the National Institute for Health and Care Excellence (NICE). The views expressed in this publication are those of the authors and not necessarily those of NICE. We thank:
David Goh, Natasha Lim, Natalie Attreed and Poorna Abeysiri for their contributions to earlier versions of the protocol and/or review;
Tianjing Li, Jod Mehta and Gerry Clare for comments on versions of the protocol or review;
Hsin‐wen Wu for translating Chinese reports of trials;
Iris Gordon for creating and executing the electronic searches and Anupa Shah for her assistance throughout the review process; and
Professor Yung and Professor Miyake for supplying further information on their trials.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Macular Edema, Cystoid #2 macula* near/3 (edema* or odema*) #3 (cme or cmo) #4 (#1 OR #2 OR #3) #5 MeSH descriptor Anti‐Inflammatory Agents, Non‐Steroidal #6 nsaid* #7 nonsteroidal anti‐inflammator* #8 non‐steroidal anti‐inflammator* #9 MeSH descriptor Diclofenac #10 diclofenac* OR fenoprofen* OR flurbiprofen* #11 MeSH descriptor Indomethacin #12 indometacin* #13 MeSH descriptor Ketoprofen #14 ketoprofen* #15 ketorolac #16 piroxicam #17 (#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15) #18 (#4 AND #17)
Appendix 2. MEDLINE (Ovid) search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp macular edema cystoid/ 14. exp macula lutea/ 15. (macula$ adj3 oedema).tw. 16. (macula$ adj3 edema).tw. 17. (CME or CMO).tw. 18. or/13‐17 19. exp anti inflammatory agents non steroidal/ 20. nsaid$.tw. 21. nonsteroidal anti‐inflammator$.tw. 22. non‐steroidal anti‐inflammator$.tw. 23. exp diclofenac/ 24. diclofenac$.tw. 25. fenoprofen$.tw. 26. flurbiprofen$.tw. 27. exp indometacin/ 28. indometacin$.tw. 29. exp ketoprofen/ 30. ketoprofen$.tw. 31. ketorolac$.tw. 32. piroxicam$.tw. 33. or/19‐32 34. 18 and 33 35. 12 and 34
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase (Ovid) search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or propspectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp retina macula cystoid edema/ 34. exp eye edema/ 35. exp retina macula lutea/ 36. (macula$ adj3 oedema).tw. 37. (macula$ adj3 edema).tw. 38. (CME or CMO).tw. 39. or/33‐38 40. exp nonsteroidal antiinflammatory agent/ 41. nsaid$.tw. 42. nonsteroidal anti‐inflammator$.tw. 43. non‐steroidal anti‐inflammator$.tw. 44. exp diclofenac/ 45. diclofenac$.tw. 46. fenoprofen$.tw. 47. flurbiprofen$.tw. 48. exp indometacin/ 49. indometacin$.tw. 50. exp ketoprofen/ 51. ketoprofen$.tw. 52. ketorolac$.tw. 53. exp piroxicam/ 54. piroxicam$.tw. 55. or/40‐54 56. 39 and 55 57. 32 and 56
Appendix 4. LILACS search strategy
Anti‐Inflammatory Agents, Non‐Steroidal [Subject descriptor] or nonsteroidal antiinflammator$ or nonsteroidal anti inflammator$ or non steroidal anti inflammator$ or NSAID$ and macula$ edema or macula$ oedema or CMO or CME
Appendix 5. ISRCTN search strategy
"( Condition: macular edema OR macular oedema AND Interventions: NSAID OR nonsteroidal anti‐inflammatory OR non‐steroidal anti‐inflammatory )"
Appendix 6. ClinicalTrials.gov search strategy
macular edema OR macular oedema OR CMO OR CME | NSAID OR nonsteroidal anti‐inflammatory OR non‐steroidal anti‐inflammatory
Appendix 7. WHO ICTRP search strategy
macular edema OR macular oedema OR CMO OR CME = Condition AND NSAID OR nonsteroidal anti‐inflammatory OR non‐steroidal anti‐inflammatory = Intervention
Appendix 8. Data for characteristics of included studies
| Mandatory items | Optional items | |
| Methods | ||
| Study design | · Parallel group RCTi.e. people randomised to treatment · Within‐person RCTi.e. eyes randomised to treatment · Cluster‐RCTi.e. communities randomised to treatment · Cross‐over RCT · Other, specify |
Exclusions after randomisation Losses to follow‐up Number randomised/analysed How were missing data handled? e.g. available case analysis, imputation methods Reported power calculation (Y/N), if yes, sample size and power Unusual study design/issues |
| Eyes or unit of randomisation/ unit of analysis |
· One eye included in study, specify how eye selected · Two eyes included in study, both eyes received same treatment, briefly specify how analysed (best/worst/average/both and adjusted for within‐person correlation/both and not adjusted for within‐person correlation) and specify if mixture one eye and two eyes · Two eyes included in study, eyes received different treatments,specify if correct pair‐matched analysis done |
|
| Participants | ||
| Country | Setting Ethnic group Equivalence of baseline characteristics (Y/N) |
|
| Total number of participants | This information should be collected for total study population recruited into the study. If these data are only reported for the people who were followed up, please indicate. | |
| Number (%) of men and women | ||
| Average age and age range | ||
| Inclusion criteria | ||
| Exclusion criteria | ||
| Interventions | ||
| Intervention (n= ) Comparator (n= ) See MECIR 65 and 70 |
· Number of people randomised to this group · Drug (or intervention) name · Dose · Frequency · Route of administration |
|
| Outcomes | ||
| Primary and secondary outcomes as defined in study reports See MECIR R70 |
List outcomes Adverse effects reported (Y/N) Length of follow‐up and intervals at which outcomes assessed |
Planned/actual length of follow‐up |
| Notes | ||
| Date conducted | Specify dates of recruitment of participants mm/yr to mm/yr | Full study name: (if applicable) Reported subgroup analyses (Y/N) Were trial investigators contacted? |
| Sources of funding | ||
| Declaration of interest See MECIR 69 |
||
Data and analyses
Comparison 1. NSAIDs plus steroids versus steroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Poor vision due to MO | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 3 months | 5 | 1360 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.23, 0.76] |
| 1.2 12 months | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.09, 20.37] |
| 2 Central retinal thickness | 9 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Change from baseline | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 FInal value | 6 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Total macular volume | 6 | 570 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.21, ‐0.07] |
| 4 Macular oedema | 21 | 3638 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.32, 0.49] |
| 5 Inflammation | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Inflammation (flare) | 2 | 216 | Mean Difference (IV, Fixed, 95% CI) | ‐1.41 [‐2.30, ‐0.52] |
| 7 BCVA | 10 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Final value | 7 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Change from baseline | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. NSAIDs versus steroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Central retinal thickness | 2 | 121 | Mean Difference (IV, Fixed, 95% CI) | ‐22.64 [‐38.86, ‐6.43] |
| 2 Macular oedema | 5 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.18, 0.41] |
| 3 Inflammation (flare) | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 BCVA | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Almeida 2008.
| Methods |
Study design: Parallel group RCT Open‐label |
|
| Participants |
Country: Canada Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Inclusion criteria: Clinic patient having phacoemulsification with IOL implantation in their first eye; agreed to participate. Exclusion criteria: Hypersensitivity to the NSAID drug class; aspirin/NSAID‐induced asthma; pregnancy in the third trimester. Pretreatment: More women in control group (70%) versus ketorolac group (51%), but unclear of importance of this difference. Eyes: 106 eyes of 98 patients enrolled but clinical trials registry specifies first eye surgery only. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Comparator: Steroids alone
All participants also received gatifloxacin 0.3% (Zymar) 4 times a day for 1 week Type of surgery: phacoemulsification |
|
| Outcomes |
Follow‐up: 1 month
|
|
| Contact details |
Authors name: Sherif El‐Defrawy Institution: Queen’s University, Ontario, Canada Email: eldefras@hdh.kari.net Address: Department of Ophthalmology, Queen’s University, Hotel Dieu Hospital, Brock Wing 230A, 166 Brock Street, Kingston, Ontario K7L 5G2, Canada |
|
| Notes |
Funding sources: "Funded by a Queen’s University grant, Kingston, Ontario, Canada" Declaration of interest: "No author has a financial or proprietary interest in any material or method mentioned." Date study conducted: June 2006 to May 2007 (from clinical trials registry entry) Trial registration number: NCT00335439 Contacting study investigators: Not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. |
| Allocation concealment (selection bias) | High risk | Quote: "open‐label non‐masked." Judgement comment: High risk of bias, given open‐label nature of trial. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: Open‐label study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "98 were assessed at 1 week and 80 at 1 month." Judgement comment: 38/53 (72%) in ketorolac group seen at 1 month versus 42/53 (79%) of non‐treated group. One case of CMO excluded in non‐treated group; 3 ketorolac‐related AEs excluded. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: Only one outcome specified on clinical trials registry and this outcome was the main focus of the published report. |
Almeida 2012.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: Canada Setting: Eye hospital Intervention: NSAIDs plus steroids
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Inclusion criteria: 18 years of age or older; cataract and were expected to have phacoemulsification with implantation of a posterior chamber IOL. Exclusion criteria: Pre‐existing retinal disease (e.g. diabetic retinopathy, vein occlusion, exudative macular degeneration); previous uveitis, previous intraocular surgery; allergy or hypersensitivity to NSAIDs. "Enrolled patients who had complicated cataract surgery (e.g. significant corneal edema, posterior capsule rupture, vitreous loss, dropped nuclear material, retained cortical material, or an IOL not placed in the capsular bag) were subsequently excluded." Pretreatment: "There were no differences in age, sex, or operative eye between the 3 groups." Eyes: Probably one eye only included in the trial but not clearly reported and unclear how selected. |
|
| Interventions |
Intervention 1: NSAIDs plus steroids
Intervention 2: NSAIDs plus steroids
Comparator: Steroids plus placebo
All participants received gatifloxacin 0.3% drops 4 times a day starting 3 days before surgery and continued for 1 week after surgery. Type of surgery: Phacoemulsification |
|
| Outcomes |
Follow‐up: 1 month
|
|
| Contact details |
Authors name: David RP Almeida Institution: Queen's University, Ontario, Canada Email: dalmeida@evolation‐medical.com Address: Department of Ophthalmology, Queen’s University, Hotel Dieu Hospital, 166 Brock Street, Eye Centre (Johnson 6), Kingston, Ontario K7L 5G2, Canada |
|
| Notes |
Funding sources: "Funded by an unrestricted Queen’s University educational research grant." Declaration of interest: "No author has a financial or proprietary interest in any material or method mentioned." Date study conducted: March 2010 to May 2011 Trial registration number: NCT01395069 Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to receive a placebo (sterile saline drops), nepafenac 0.1%, or ketorolac 0.5%." Judgement comment: Not reported how list was generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The placebo, nepafenac, and ketorolac suspensions were supplied in identical generic drop bottles that were individually made by the Kingston General Hospital Investigational Pharmacy division. Bottles concealed medication information and were labelled with study identification number, patient identification number, expiration date, and emergency contact information only." Judgement comment: Unclear if investigators involved in the treatment allocation were masked. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The placebo, nepafenac, and ketorolac suspensions were supplied in identical generic drop bottles that were individually made by the Kingston General Hospital Investigational Pharmacy division. Bottles concealed medication information and were labelled with study identification number, patient identification number, expiration date, and emergency contact information only." Judgement comment: Placebo‐controlled study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The placebo, nepafenac, and ketorolac suspensions were supplied in identical generic drop bottles that were individually made by the Kingston General Hospital Investigational Pharmacy division. Bottles concealed medication information and were labelled with study identification number, patient identification number, expiration date, and emergency contact information only." Judgement comment: Placebo‐controlled study which probably means that the outcome assessors were masked. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "One hundred sixty‐two patients, 54 in each arm, made up the intent‐to‐treat data set." Quote: "Ninety‐seven patients (35 placebo, 32 ketorolac, 30 nepafenac) completed the COMTOL interview questionnaire (60.0% response rate)." Judgement comment: 84% follow‐up. Not clearly reported but no evidence for any differential drop out by intervention group. 31 patients out of 193 lost to follow‐up (16%). However, only 97 patients (60%) completed the COMTOL interview questionnaire and no further breakdown of losses to follow‐up in each group provided. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: Outcomes on clinical trial registry entry (NCT01395069) were reported but the trial was retrospectively registered. |
Asano 2008.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: Japan Setting: 5 Eye hospitals Intervention: NSAIDs alone
Comparator: Steroids alone
Inclusion criteria: Age 55 to 75 years of age; nuclear hardness of Emery‐Little grade IV or less; surgery in 1 eye only. Exclusion criteria: Acute infection or inflammation within 1 month after initiation of the study; allergy to NSAIDs, steroids, or fluorescein; history of eye trauma or intraocular disease other than cataract; pseudoexfoliation syndrome; uveitis; glaucoma; diabetes and related complications; kidney disease;asthma or chronic airway disease; uncontrolled hypertension;severe heart failure; myocardial infarction or cerebrovascular disorders; intraoperative complications such as posterior capsule rupture, vitreous loss, retained lens nucleus, or lens fragments in the vitreous. Pretreatment: None noted. Compared age, gender, duration of surgery, ultrasound time, irrigating solution and hardness of crystalline lens. Eyes: One eye, unclear how selected. |
|
| Interventions |
Intervention: NSAIDs alone
Comparator: Steroids alone
Concomitant mydriatic and antibiotic agents were permitted. Type of surgery: Phacoemulsification |
|
| Outcomes |
Follow‐up: 8 weeks
|
|
| Contact details |
Authors name: Kensaku Miyake Institution: Shohzankai Medical Foundation, Miyake Eye Hospital Email: miyake@spice.or.jp Address: Shohzankai Medical Foundation, Miyake Eye Hospital, 3‐15‐68, Ozone, Kita‐ku, Nagoya, 462‐0825, Japan |
|
| Notes |
Funding sources: NR Declaration of interest: "No author has a financial or proprietary interest in any material or method mentioned." Date study conducted: April 2004 to September 2005 Trial registration number: NR Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The test drugs were assigned to patients at random after the controller validated that the assigned therapy was indistinguishable from the alternative therapy." Judgement comment: Not reported how list was generated. |
| Allocation concealment (selection bias) | Low risk | Quote: "The controller kept the assignment code until completion of the study." Judgement comment: This probably means that the allocation was concealed from the investigators although it was not clearly reported who the controller was exactly. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The test drugs were assigned to patients at random after the controller validated that the assigned therapy was indistinguishable from the alternative therapy. The controller kept the assignment code until completion of the study. The controller created an emergency code, which was given to the principal investigator in an envelope. The investigator could open the envelope if severe adverse effects developed. The test drugs were administered to each patient 3 hours, 2 hours, 1 hour, and 30 minutes before surgery and 3 times a day for 8 weeks after surgery." Judgement comment: Although not clearly stated that participants and personnel were unaware of which treatment received, the study was placebo‐controlled and efforts made to keep the allocation away from investigators so we assume that masking was done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The test drugs were assigned to patients at random after the controller validated that the assigned therapy was indistinguishable from the alternative therapy. The controller kept the assignment code until completion of the study. The controller created an emergency code, which was given to the principal investigator in an envelope. The investigator could open the envelope if severe adverse effects developed. The test drugs were administered to each patient 3 hours, 2 hours, 1 hour, and 30 minutes before surgery and 3 times a day for 8 weeks after surgery." Judgement comment: Although not clearly stated that outcome assessors were unaware of which treatment received, the study was placebo‐controlled and efforts made to keep the allocation away from investigators so we assume that masking was done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of the 150 eyes initially included in this study, 75 were assigned to the diclofenac group and 75 to the betamethasone group. Four patients in each group dropped out of the study: 1 in each group due to complications; 3 in the diclofenac group and 2 in the betamethasone group due to a discontinuation proposal (there were patients who withdrew their consent during the course of this study); 1 in the betamethasone group for not returning to the hospital 2 weeks after surgery. Seventy‐one eyes in each group completed the study." Judgement comment: In the results text quoted follow‐up appeared to be high (95%) and equal between groups but in table 3 visual acuity results follow‐up was lower 58/75 (77%) versus 52/75 (69%) and unclear why. Judgement comment: Some of the exclusion criteria may have lead to bias if they occurred differently between two treatment groups: "acute infection or inflammation within 1 month after initiation of the study" and "intraoperative complications such as posterior capsule rupture, vitreous loss, retained lens nucleus, or lens fragments in the vitreous", however these exclusions were not reported. |
| Selective reporting (reporting bias) | High risk | Judgement comment: No access to protocol or trials registry entry but noted that data on CMO were reported only at 5 weeks, but other data available at 8 weeks follow‐up. |
Brown 1996.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: USA Setting: Eye hospital Intervention group: NSAIDs alone
Comparator: Steroids alone
Inclusion criteria: Undergoing phacoemulsification with posterior capsular opacification after lens (PCOL) implantation. Exclusion criteria: History of systemic or ocular inflammation (iritis, uveitis); taking oral or ophthalmic steroids or NSAIDs; other ocular disease such as glaucoma, corneal disease, or diabetic retinopathy. Pretreatment: Group differences not reported. Eyes: Unclear if one or both eyes included. |
|
| Interventions |
Intervention group: NSAIDs alone
Comparator: Steroids alone
All patients had gentamicin drops for 7 days postoperative. Type of surgery: Phacoemulsification |
|
| Outcomes |
Follow‐up: 1 month
|
|
| Contact details |
Authors name: Rose Marie Brown Institution: New York Hospital ‐ Cornell Medical Center Email: NR Address: Cornell University Medical College, 520 E. 70th St, Starr 817, New York, NY 10021 |
|
| Notes |
Funding sources: "Supported in part from a grant from Ciba Vision Ophthalmics, Duluth, Ga." Declaration of interest: NR Date study conducted: 1991 Trial registration number: NR Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "We conducted a prospective, randomised study." "The patients were randomly assigned to receive..." Judgement comment: Not reported how list was generated. Study was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Study was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this, patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: For measurement of inflammation ‐ Quote: "Neither examiner knew which of the study groups the patient was enrolled in." But for other outcomes, masking not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: Follow‐up not reported. Unclear how many people seen at 1 month. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trials registry entry. |
Cervantes‐Coste 2009.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: Mexico Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Inclusion criteria: Adult patients 40 years of age or older; diagnosed with senile and/or metabolic cataract (according to the Lens Opacities Classification System LOCS III, with classification NO and NC 2–3); scheduled for surgery by phacoemulsification and IOL implantation inside the capsular bag; normal fundoscopy exam (if observance was possible). Exclusion criteria: Pregnancy or breastfeeding; history of ocular inflammatory or infectious eye disease; treatment for eye infection within 30 days prior to inclusion in the study;alterations on the eye surface (including dry eye); history of ocular surgery and/or trauma; knowledge or suspicion of allergy or hypersensitivity to the preservatives, steroids, topical NSAIDs, or any other component of the study medication; use of eye medications, including prostaglandin analogues; use of topical or systemic steroids within 30 days prior to inclusion in the study; use of topical or systemic NSAIDs within 14 days prior to inclusion in the study; non‐controlled diabetes mellitus, based on clinical history and blood glucose level (126 mg); proliferative diabetic retinopathy, and/or macular oedema; preoperative mydriasis less than 6 mm prior to the study; synechiae; ocular alteration preventing adequate mydriasis such as iris atrophy; macular alteration documented by OCT, including macular oedema of any etiology, macular holes, epiretinal membrane, macular degeneration related to age, and central serous chorioretinopathy; the use of contact lens in the eye involved during the study. Pretreatment: No differences noted; compared age, gender, operated eye, ocular and systemic pathology. Eyes: One eye, unclear how selected. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Type of surgery: Phacoemulsification |
|
| Outcomes |
Follow‐up: 6 weeks
Subgroup analysis by diabetes reported. |
|
| Contact details |
Authors name: Guadalupe Cervantes‐Coste Institution: Asociación Para Evitar la Ceguera en México I.A.P. Hospital Email: gpecervantes@hotmail.com Address: Av. México 85‐5, México City, 06100 México |
|
| Notes |
Funding sources: NR Declaration of interest: The authors have no conflicts of interest to disclose. Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This was a prospective, randomised, single‐masked, single‐center, longitudinal, experimental and comparative study in patients undergoing phacoemulsification cataract surgery." Judgement comment: Not reported how list was generated. Trial described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The identity of patients receiving preoperative mydriatic or preoperative mydriatic and nepafenac was concealed from the surgeons." Judgement comment: Only the surgeons appeared to be masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement Comment: The study compared nepafenac versus no treatment so is essentially open‐label. No information was provided on masking. We assume that in absence of reporting on this outcome, assessors were not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients completed the follow‐up visits over a 6‐week period." Judgement comment: No patients appeared to have been excluded or lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
Chatziralli 2011.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: Greece Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Inclusion criteria: NR Exclusion criteria: History of intraocular surgery on the eye to be operated; any previous episode of uveitis in the eye to be operated; severe systemic disease (heart failure of the New York Heart Association stage III of IV, endstage renal failure, pulmonary failure, receiving chemotherapy); regular, systemic use of steroid or NSAIDs during the last 3 months. Pretreatment: None noted; compared age, gender, baseline visual acuity, education, marital status, smoking, and various systemic ocular factors. Eyes: Probably one eye only included in the trial but not clearly reported and unclear how selected. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Type of surgery: phacoemulsification |
|
| Outcomes |
Follow‐up: 6 weeks
|
|
| Contact details |
Authors name: Irini Chatziralli Institution: Department of Ophthalmology, Veroia General Hospital Email: eirchat@yahoo.gr Address: Department of Ophthalmology, Veroia General Hospital, 28, Papanastasiou Street, GR–17342 Athens (Greece) |
|
| Notes |
Funding sources: NR Declaration of interest: NR Date study conducted: October 2009 to January 2010 Trial registration number: NR Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomised to 1 of the 2 postoperative treatment arms." Judgement comment: Not reported how list was generated. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study was masked to the patients, i.e. they received unmarked bottles so as to be unaware of which treatment they received." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: No information on masking of outcome assessors. We assume that in absence of reporting on this outcome, assessors were not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: Follow‐up high and reasonably equal between groups: 70/73 (96%) in NSAIDs group versus 68/72 (94%) in steroid group. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
Donnenfeld 2006.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: USA Setting: Eye hospital Intervention: NSAIDs plus steroids
Intervention: NSAIDs plus steroids
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Inclusion criteria: Scheduled for phacoemulsification. Exclusion criteria: Known sensitivity to any ingredient in the study medications; monocular status; a history of previous intraocular surgery;diabetes mellitus; a history of uveitis, iritis, or intraocular inflammation; use of a systemic NSAID during the study or the week before surgery; or pupils that did not dilate to more than 5.0 mm before surgery or requiring mechanical pupil stretching; pregnant, nursing an infant, or planning a pregnancy. Pretreatment: "There were no significant between‐group differences in any demographic variable or baseline value." Eyes: Unclear if one or both eyes included. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Intervention: NSAIDs plus steroids
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
All participants received topical gatifloxacin 0.3% 4 times a day for 3 days before cataract surgery and for 1 week after surgery. Type of surgery: Phacoemulsification |
|
| Outcomes |
Follow‐up: 3 months
|
|
| Contact details |
Authors name: Eric D. Donnenfeld Institution: Ophthalmic Consultants of Long Island Email: eddoph@aol.com Address: Ophthalmic Consultants of Long Island, Ryan Medical Arts Building, 2000 North Village Avenue, Suite 402, Rockville Centre, New York 11570, USA |
|
| Notes |
Funding sources: "Supported in part by an unrestricted grant from Allergan Inc., Irvine, California, and the Lions Eye Bank for Long Island, Long Island, New York, USA" Declaration of interest: "Drs. Donnenfeld, Perry, and Wittpenn are consultants to Allergan Pharmaceuticals. No other author has a financial or proprietary interest in any material or method mentioned." Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Group assignment was based on a random‐number‐generated protocol that was created before initiation of the study." |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: Placebo‐controlled, but not clear if masking was successful ‐ some of the groups had different schedules. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: Placebo‐controlled but not clear if masking was successful ‐ some of the groups had different schedules. Corneal endothelial cell counts and OCT scans were evaluated by masked specialists. It was unclear whether assessors of other outcomes were aware of the treatment allocation, or if only the specialists were affected. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: Follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
Elsawy 2013.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: Egypt Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Some inconsistencies in the data. Not clearly stated exactly number of people (eyes) randomly allocated to each group and followed up. Inclusion criteria: High risk characteristics for the postoperative development of CME, one of the risk factors for CME (beside diabetic retinopathy). History of retinal vein occlusion or presence of epiretinal membrane or preoperative use of prostaglandin analogues eye drops. Exclusion criteria: NR Pretreatment: Compared age, gender, type of diabetes, duration of diabetes, retinal vein occlusion, epiretinal membrane and prostaglandin drops. Some imbalances, e.g. more prostaglandin eye drop use in control group. Eyes: 86 eyes of 70 people. Type of surgery: Phacoemulsification |
|
| Interventions |
Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Type of surgery: Phacoemulsification |
|
| Outcomes |
Follow‐up: 12 weeks
|
|
| Contact details |
Authors name: Moataz F Elsawy Institution: Menoufia University Hospital Email: mfelsawy@yahoo.co.uk Address: Ophthalmology Department, Menoufia University Hospital, Menoufia, 53211, Egypt |
|
| Notes |
Funding sources: NR Declaration of interest: "The authors report no conflicts of interest in this work." Date study conducted: January 2011 to March 2012 Trial registration number: NR Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomisation process used four opaque envelopes in two containers. The first container had (1) for dexamethasone drops only, and (2) for combined drops, and the second container had the name of patients listed for cataract surgery on that day. Patients were randomised to one of the regimes by asking an independent person to choose one envelope from each container." Judgement comment: Unusual random allocation process. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomisation process used four opaque envelopes in two containers. The first container had (1) for dexamethasone drops only, and (2) for combined drops, and the second container had the name of patients listed for cataract surgery on that day. Patients were randomised to one of the regimes by asking an independent person to choose one envelope from each container. All patients underwent phacoemulsification (divide and conquer)." Judgement comment: Unusual allocation process. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this, patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this, outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: Follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
Endo 2010.
| Methods |
Study design: Parallel group RCT Open‐label |
|
| Participants |
Country: Japan Setting: Eye hospital Intervention: NSAIDs alone
Comparator: Steroids alone
Inclusion criteria: Patients with diabetes undergoing small incision phacoemulsification with IOL implantation. Exclusion criteria: foveal thickness of 250 microns or more; severe diabetic retinopathy for which ocular surgery (including photocoagulation) indicated;use of topical medications for glaucoma, uveitis and other diseases that cause CMO; ocular allergies to bromfenac or steroids (steroid group); use of systemic steroids or NSAIDs; serious cardiac, cerebral or renal disease. Pretreatment: No major imbalances; compared age, gender, hypertension, blood urea nitrogen. HbA1c slightly higher in NSAIDs group. Eyes: One eye, unclear how selected. |
|
| Interventions |
Intervention: NSAIDs alone
Comparator: Steroids alone
Preoperatively, all participants received gatifloxacin (four times daily for 1 day preoperatively; on the day of surgery, they received 0.5% tropicamide, 0.5% phenylephrine hydrochloride every 30 mins 2 hours preoperatively. Postoperatively, gatifloxacin four times daily until week 6, and 0.5% tropicamide and 0.5% phenylephrine hydrochloride once daily for 1 week. Type of surgery: Phacoemulsification |
|
| Outcomes |
Follow‐up: 6 weeks
|
|
| Contact details |
Authors name: Naoko Endo Institution: Tokyo Women’s Medical University Diabetes Centre Email: 51026745@mail.goo.ne.jp Address: Tokyo Women’s Medical University Diabetes Centre, 8‐1 Kawada‐cho, Shinjuku‐ku, Tokyo 162‐0054, Japan |
|
| Notes |
Funding sources: NR Declaration of interest: "The authors have no financial interest in any aspect of this article." Date study conducted: March 2005 to May 2007 Trial registration number: NR Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A prospective open‐label trial was conducted using the envelope method." Judgement comment: Not reported how list was generated. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Although mentioned "envelope method", not enough information on how the allocation was administered. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: Open‐label study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: 17% (13/75) of patients were excluded. Vague reasons were provided. Three were excluded because of difficulty with the OCT measurement. Ten people (10 eyes) dropped out of the study for the following reasons: poor health (8), posterior capsular rupture (1) and epidemic keratoconjunctivitis (1). No details were provided about the 'difficulties with OCT measurements' and 'poor health'. 31/40 (78%) in NSAIDs group and 31/35 (89%) in steroids group were followed‐up but reasons for dropout by group were not clearly reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
Italian Diclofenac Study Group 1997.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: Italy Setting: Eye hospital Intervention: NSAIDs alone
Comparator: Steroids plus placebo
Inclusion criteria: 45 to 75 years of age; age‐related cataract. Exclusion criteria: Ocular malformations; dry‐eye syndrome (Schirmer I < 5 mm); glaucoma or ocular hypertension (lOP > 22 mmHg); vitreoretinal pathology; surgical complications (posterior capsule rupture, Descemet's membrane detachment, vitreous loss, significant intraocular haemorrhage, IOL dislocation); severe systemic affections; ocular surgery in the previous 2 months or had had bilateral surgery; hypersensitive to one or more of the study compounds; pregnant or nursing woman. Pretreatment: No major imbalances in age, sex, IOP and operated eye. Eyes: One eye, unclear how selected. |
|
| Interventions |
Intervention: NSAIDs alone
Comparator: Steroids plus placebo
Type of surgery: ECCE |
|
| Outcomes |
Follow‐up: 140 days
|
|
| Contact details |
Authors name: Lucio Lobefalo Institution: NR Email: NR Address: via Gran Sasso 100, 1‐66100 Chieti, Italy |
|
| Notes |
Funding sources: NR Declaration of interest: "S. Bianco, MD, is a Ciba Vision Ophthalmics officer. None of the other authors has a proprietary or financial interest in diclofenac." Date study conducted: October 1992 to February 1994 Trial registration number: NR Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: Placebo‐controlled but masking of participants not described specifically. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "In each center, all patients were observed by the same examiner; surgeons and examiners were masked at all postoperative visits." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: Follow‐up: 118/140 (84%) in diclofenac group and 111/141 (79%) in dexamethasone group followed up. Follow‐up reasonably high and not very different between the two groups. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trials registry entry. |
Jung 2015.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: South Korea Setting: Eye hospital Intervention: NSAIDs plus steroids
Intervention: NSAIDs plus steroids
Comparator: Steroids
Inclusion criteria: Males or non‐pregnant females aged between 20‐ to 80‐years‐old. Exclusion criteria: Poor general condition, including high blood pressure, poor blood glucose control, or renal failure; history of ocular trauma or disease; history of intraocular surgery; systemic or topical NSAIDs or corticosteroids use within 4 weeks of enrolment; known hypersensitivity to salicylates or other NSAIDs; and use of alpha‐1 adrenergic antagonist or other analogous systemic medications that may increase the tendency for miosis during the operation (intraoperative floppy iris syndrome). Pretreatment: No major imbalances, age, sex, hypertension, diabetes, macular thickness and volume and ocular surface status compared. Eyes: One eye, unclear how selected. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Intervention: NSAIDs plus steroids
Comparator: Steroids alone
All patients received topical gatifloxacin 0.3% 4 times a day for 28 days. Type of surgery: Phacoemulsification |
|
| Outcomes |
Follow‐up: 1 month
|
|
| Contact details |
Authors name: Dr. Tae‐im Kim Institution: Yonsei University College of Medicine Email: tikim@yuhs.ac Address: Department of Ophthalmology, Yonsei University College of Medicine, 50‐1 Yonsei‐ro, Seodaemun‐gu, Seoul 03722, Korea |
|
| Notes |
Funding sources: "This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology 2013R1A1A2058907)." Declaration of interest: "The authors have no financial conflicts of interest." Date study conducted: November 2013 to June 2014 Trial registration number: NR Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: Open‐label or no information on masking. We assume that in absence of reporting on this outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: Follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
Kraff 1982.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: USA Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Included criteria: Eligible for extracapsular cataract extraction with implantation of a Shearing posterior chamber lens. Excluded criteria: NR Pretreatment: None noted; age, gender, follow‐up and endothelial cell density preoperative compared. Eyes: Unclear if one or both eyes included. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Type of surgery: ECCE and phacoemulsification (unplanned ICCE n = 19 were excluded). |
|
| Outcomes |
Follow‐up: between 2.5 and 12 months. Quote: "The mean interval between surgery and angiography was 4.1 months, with a range of 2.5 to 12 months. Ninety percent of the angiograms were performed between 2.5 and 5 months after surgery, and 10% between 6 and 12 months after surgery."
|
|
| Contact details |
Authors name: Manus C Kraff Institution: Abraham Lincoln School of Medicine, University of Illinois Email: NR Address: 5600 W. Addison Street, Chicago, IL 60634 |
|
| Notes |
Funding sources: Core Grant EY 1792 NEI Bethesda Maryland Declaration of interest: NR Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: Randomisation was using a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: Quote: "The study was double‐masked; neither the physician nor the patient knew what drops the patient was receiving." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: Quote: "The study was double‐masked; neither the physician nor the patient knew what drops the patient was receiving." Quote: "The angiograms were read in a masked fashion by a retired specialist (LMJ) who had no knowledge of either the drug regimen or the type of surgical procedure." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: Some patients were excluded (n = 19) and not reported: two with vitreous loss, two with vitreous pressure and a shallow anterior chamber and 15 with possible rupture of the posterior capsule. Unclear which groups these were in. Follow‐up high for visual acuity (> 95%) but lower for CMO (60% in indomethacin group versus 64% in placebo). |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
Li 2011.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: China Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Included criteria: Diabetes mellitus type 2 patients who received phacoemulsification together with artificial lens implants intervention. Excluded criteria: Diabetic retinopathy, age‐related macular degeneration, epiretinal membrane and retinal vascular disorders. Pretreatment: Unclear if group differences. Eyes: One eye, unclear how selected. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Type of surgery: Phacoemulsification |
|
| Outcomes |
Follow‐up: 1 month
|
|
| Contact details |
Authors name: Min‐Chao Li Institution: Department of Ophthalmology, Affiliated Nanhai Hospital of Southern Medical University, Foshan Email: liminchao@126.com Address: Department of Ophthalmology, Affiliated Nanhai Hospital of Southern Medical University, Foshan 528200, Guangdong Province, China |
|
| Notes |
Funding sources: NR Declaration of interest: NR Date study conducted: January 2009 to December 2010 Trial registration number: NR Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: As per translation: "Unclear, not specified if there was any participant withdrawal or lost during the study period." |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
Mathys 2010.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: USA Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Inclusion criteria: Planning to have cataract surgery by KLC at the Ambulatory Care Center, the University of North Carolina Hospitals. Exclusion criteria: Medically treated diabetes mellitus; history of uveitis;use of topical prostaglandin analogues for glaucoma; history of earlier intraocular surgery in the same eye; retinal vascular disease; macular degeneration;abnormal preoperative OCT measurements. Pretreatment: Nepafenac group were slightly older, similar gender, preoperative VA, follow‐up time, slightly longer phaco time. Eyes: One eye, unclear how selected. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Comparator: Steroids alone
All participants received nepafenac 0.01% drops in the operated eye thrice, 5 mins apart, immediately before surgery to maintain pupillary dilation and postoperatively, moxifloxacin 0.5% four times a day for 10 days. Type of surgery: Phacoemulsification |
|
| Outcomes |
Follow‐up: 2 months
|
|
| Contact details |
Authors name: KL Cohen Institution: School of Medicine, University of North Carolina Email: klc@med.unc.edu Address: Department of Ophthalmology, School of Medicine, University of North Carolina at Chapel Hill, 5100 Bioinformatics Building, 130 Mason Farm Road, CB no. 7040, Chapel Hill, NC 27599–7040, USA |
|
| Notes |
Funding sources: "This work was supported in part by Research to Prevent Blindness, Inc., New York, NY." Declaration of interest: "Kenneth C Mathys and Kenneth L Cohen have no financial interest." Date study conducted: June 2007 to April 2008 Trial registration number: NCT00494494 Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomised according to the even/odd subject identification number, using computer‐generated random numbers, to the control group (standard of care only) or the treatment group (standard of care plus nepafenac)." |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "were consecutively enrolled in this randomised, non‐masked, parallel‐group clinical trial." Judgement comment: Participants were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "At the 2 months visit, technicians, who were masked to treatment, measured ETDRS BCVA, and OCT scans were performed." Judgement comment: Experienced ophthalmic photographers, who were masked to treatment, obtained Stratus OCT (Carl Zeiss Meditec, Inc., San Francisco, CA, USA) scans using the fast macular thickness protocol. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The mean time to follow‐up was 73.31 days ( ± 21.58 SD, range 55‐146) in the treatment group and 68.98 days ( ± 13.98, range 50‐120) in the standard‐of‐ care group." Judgement comment: 39/42 (93%) of intervention group and 40/42 (95%) of comparator group followed‐up. Missing data less than 20% (i.e. more than 80% follow‐up) and equal follow‐up in both groups and no obvious reason why loss to follow‐up should be related to outcome. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: Outcomes on trial registry entry were reported. |
Miyake 2007.
| Methods | Study design: Randomised control trial | |
| Participants |
Country: Japan Setting: Eye hospital Intervention: NSAIDs alone
Comparator: Steroids alone
Inclusion criteria: Age 50 to 70 years; subjected for unilateral surgery or to have 6 months’ span between surgeries in patients with bilateral cataract. Exclusion criteria: Eyes encountering acute ocular infection or inflammation during the first month of the study; eyes showing sensitivity to diclofenac or fluorometholone; eyes showing sensitivity to fluorescein sodium; eyes with insufficient dilation, (pupil diameter 4 mm) and with hazy media affecting laser Doppler flowmetry (LDF); eyes with history of other ocular surgeries; eyes with pseudoexfoliation syndrome; history of trauma; uveitis, glaucoma or other disorders; complication of diabetes and kidney disorders; heart failure, cardiac infarction, and cerebrovascular disease; uncontrollable hypertension; rupture of the posterior capsule, vitreous loss, and other complications during a cataract/IOL implantation procedure. Pretreatment: No major imbalances; compared age and sex. Eyes: One eye, unclear how selected. |
|
| Interventions |
Intervention: NSAIDs alone
Comparator: Steroids alone
Quote "Other topical drugs used before and after surgery included mydriatics and antibiotics only." Type of surgery: Phacoemulsification |
|
| Outcomes |
Follow‐up: 5 weeks
|
|
| Contact details |
Authors name: Kensaku Miyake Institution: Shohzankai Medical Foundation, Miyake Eye Hospital Email: miyake@spice.or.jp Address: Miyake Eye Hospital, 3‐15‐68, Ozone, Kita‐ku, Nagoya 462‐0825, Japan |
|
| Notes |
Funding sources: NR Declaration of interest: Reported none for all authors. Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each patient was randomly assigned to one of the two groups by one of the authors (SA), using the envelope method." Judgement comment: Not reported how list was generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Each patient was randomly assigned to one of the two groups by one of the authors (SA), using the envelope method." Judgement comment: Reported that envelopes used but unclear if they were sequentially numbered, sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: Study described as being "conducted in a prospective, double‐masked, randomised manner." Patients probably masked not clearly described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: Fluorescein angiography and laser flarimetry assessed by masked observers and analysis was masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: 25/31 (80%) of eyes in both groups were followed up and reasons for loss to follow‐up did not appear to be related to outcome. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
Miyake 2011.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: Japan Setting: Eye hospital Intervention: NSAIDs alone
Comparator: Steroids alone
Inclusion criteria: Aged over 20 years; phacoemulsification cataract extraction and IOL implantation between October 2007 and April 2008 at Shohzankai Medical Foundation, Miyake Eye Hospital. Exclusion criteria: Systemic, topical, or ointment steroidal agents within 14 days of surgery; had had an intraocular or periocular injection of steroidal agents within 90 days of surgery; had taken systemic or topical NSAIDs within 7 days of surgery; had a history of ophthalmic surgery (including laser surgery) or of ocular trauma that could affect the study results; had pseudoexfoliation syndrome; had a history of chronic or recurring ocular inflammation (e.g. uveitis or scleritis); had diabetic retinopathy; had an ocular anomaly (e.g. aniridia, congenital cataract); had iris atrophy; had disorders that would preclude improvement in visual function; had macular oedema; had severe corneal epithelial disorder (e.g. corneal ulcer); had no visual function in the contralateral eye; were scheduled to have other ocular surgery from baseline to 5 weeks after cataract surgery; had secondary IOL implantation, were allergic to or might have been sensitive to NSAIDs, amfenac, or fluorometholone; had a positive skin reaction to fluorescein; had a tendency to bleed or were currently on anticoagulants; had had prostaglandin‐type treatment for glaucoma within 4 days of surgery; had been included in a previous study of prostaglandin type antiglaucoma drugs; had joined another clinical study within 30 days of the study; had ocular infection, had uncontrollable diabetes mellitus; had severe liver, kidney, or heart disorder; might have been pregnant or were currently breastfeeding; had other factors determined to be unsuitable for the study. Pretreatment: No major imbalances. Eyes: One eye, unclear how selected. |
|
| Interventions |
Intervention: NSAIDs alone
Comparator: Steroids alone
Levofloxacin ophthalmic solution 0.5% (Cravit) was applied to each eye 5 times before surgery and 3 times a day after surgery for 2 weeks. Type of surgery: Phacoemulsification |
|
| Outcomes |
Follow‐up: 5 weeks
|
|
| Contact details |
Authors name: K Miyake Institution: Shohzankai Medical Foundation, Miyake Eye Hospital (K.Miyake, Ota, G.Miyake), Nagoya, and TokyoMetropolitan Geriatric Hospital (Numaga), Tokyo, Japan Email: miyake@spice.or.jp Address: Shohzankai Medical Foundation, Miyake Eye Hospital, 3‐15‐68, Ozone, Kita‐ku, Nagoya, 462‐0825, Japan |
|
| Notes |
Funding sources: NR Declaration of interest: "Drs. Miyake and Numaga are consultants to Alcon Japan Ltd." Date study conducted: October 2007 to April 2008 Trial registration number: NR Contacting study investigators: Primary investigator emailed to confirm how patients allocated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The 2 drugs had identical outer appearances and could not be differentiated. The same physician (J.N.) served as the medical monitor and assigned 1 of the drugs to each patient." Judgement comment: Unclear if allocation concealed from person recruiting participants. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: Described as “double‐blind” with no information on who was masked. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: Described as “double‐blind” with no information on who was masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: Missing data less than 20% (i.e. more than 80% follow‐up) and equal follow‐up in both groups and no obvious reason why loss to follow‐up should be related to outcome: 28/30 (93%) in nepafenac group and 27/30 (90%) in the fluorometholone group. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
Miyanaga 2009.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: Japan Setting: Eye hospital Intervention: NSAIDs plus steroids
Intervention: NSAIDs alone
Comparator: Steroids alone
Inclusion criteria: Scheduled to undergo routine phacoemulsification combined with IOL. Exclusion criteria: Corneal disease; glaucoma; uveitis; pseudoexfoliation syndrome; diabetes; other pathologies that might affect treatment responses or evaluations; systemic or topical anti‐inflammatory therapy within 1 month prior to surgery. Pretreatment: Quote: "There were no significant differences between groups in gender or age." Eyes: Probably one eye only included in the trial but not clearly reported and unclear how selected. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Intervention: NSAIDs alone
Comparator: Steroids alone
All participants received 0.5% levofloxacin eyedrops four times daily until 2 months after surgery, and 0.5% tropicamide and 0.5% phenylephrinehydrochloride once daily for 2 weeks. Type of surgery: Phacoemulsification |
|
| Outcomes |
Follow‐up: 2 months
|
|
| Contact details |
Authors name: Masaru Miyanaga Institution: Miyata Eye Hospital Email: miyanaga@miyata‐med.ne.jp Address: Miyata Eye Hospital, 6‐3 Kurahara, Miyakonojo, Miyazaki 885‐0051, Japan |
|
| Notes |
Funding sources: NR Declaration of interest: NR Date study conducted: February 2006 to August 2006 Trial registration number: NR Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: Only 1 patient was withdrawn from the study from the steroid only group due to CMO 1 month postop. Otherwise follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
Moschos 2012.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: Greece Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Inclusion criteria: Patients requiring phacoemulsification cataract surgery. Exclusion criteria: Presence of corneal abnormalities; history of intraocular surgery; preoperative ECC < 1500 cells/mm2; history of uveitis, diabetes, and age‐related macular degeneration; regular, systemic use of steroid or NSAIDs during the previous 3 months; and intraoperative complications, such as posterior capsule rupture, vitreous loss, lost nucleus, zonule dehiscence, and wound leak. Pretreatment: No major imbalances noted. Eyes: One eye, unclear how selected. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Type of surgery: Phacoemulsification |
|
| Outcomes |
Follow‐up: 1 month
|
|
| Contact details |
Authors name: Irini P. Chatziralli Institution: Department of Ophthalmology University of Athens Email: eirchat@yahoo.gr Address: Department of Ophthalmology, University of Athens, 28 Papanastasiou street 17342 Athens, Greece |
|
| Notes |
Funding sources: NR Declaration of interest: "No competing financial interests exist." Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised (through random number generation) to 1 of the 2 postoperative treatment arms." |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: Follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
Quentin 1989.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: Germany Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Inclusion criteria: No complication during surgery; fluorescein angiography can be done; compliance of the patient is very probable. Exclusion criteria: Exudative maculopathy; diabetic retinopathy; prior uveitis; glaucoma; allergic reaction on fluorescein angiography; systemic steroid treatment; therapy with non‐steroid antiphlogistics; treatment with anticoagulation. Pretreatment: Age and gender comparable. Eyes: One eye, unclear how selected. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
All participants received antibiotic eye drops for the first 4 days after surgery. Type of surgery: ICCE |
|
| Outcomes |
Follow‐up: not reported, assume 180 days as this is duration of treatment.
|
|
| Contact details |
Authors name: CD Quentin Institution: Uni Augenklinik Göttingen Email: NR Address: Uni Augenklinik GöttingenRobert‐Koch‐Straße 40, D‐3400 Göttingen, Germany |
|
| Notes |
Funding sources: NR Declaration of interest: NR Date study conducted: NR Trial registration number: NR Contacting study investigators: Not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: Described as “double‐blind” with no information on who was masked. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: Described as “double‐blind” with no information on who was masked. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: Follow‐up missing data > 20% but follow‐up equal in both groups: 57/90 (63%) followed up in diclofenac group and 55/89 (62%) in the placebo group. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
Rossetti 1996.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: Italy Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Inclusion criteria: Extracapsular cataract extraction (ECCE) with implantation of an IOL. Exclusion criteria: Diabetes; glaucoma; maculopathy; on systemic steroids, acetazolamide, or NSAIDs. Pretreatment: Age, gender and preoperative visual acuity were compared. Higher proportion of women in the diclofenac group (71%) compared with the placebo group (57%). Otherwise groups were similar. Eyes: Probably one eye only included in the trial but not clearly reported and unclear how selected. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Type of surgery: ECCE |
|
| Outcomes |
Follow‐up: 6 months
|
|
| Contact details |
Authors name: Nicola Orzalesi Institution: Clinica Oculistica Universitti di Milano, Istituto di Scienze Biomediche, Ospedale San Paolo Email: NR Address: Clinica Oculistica Universitti di Milano, Istituto di Scienze Biomediche, Ospedale San Paolo, Via di Rudini 8,20142 Milano, Italy |
|
| Notes |
Funding sources: NR Declaration of interest: None of the authors has a proprietary interest in the instruments or materials mentioned. Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: Randomisation was obtained using a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: Not reported how allocation administered. Trial described as “double‐masked” but with no further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: anterior chamber cell and flare and fluorescein angiography was performed by masked evaluations. No indication if the rest of the exam (visual acuity assessment (Snellen chart), slit‐ lamp biomicroscopy, lOP measurement by applanation tonometry, and ophthalmoscopic evaluation was performed by masked evaluators. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: Follow‐up not explicitly reported. However, demonstrated in several tables (such as in Table 7 (% of patients in the calculation of mean (SD) postoperative VA)). None of these were < 80%. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
Singh 2012.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: USA Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Inclusion criteria: Diabetic (type 1 or type 2); 18 years and older; existing diagnosis of nonproliferative diabetic retinopathy that required cataract extraction with planned implantation of a posterior chamber IOL; at least 50% of all enrolled patients were required to have moderate to severe nonproliferative diabetic retinopathy, as defined by the International Clinical Diabetic Retinopathy Disease Severity Scale 2. Exclusion criteria: Significant corneal staining scores at baseline; history of dry eye syndrome; other conditions that may have caused macular oedema, including pre‐existing histories of retinal vein occlusions, ocular surgeries, inflammatory eye diseases, ocular infections, congenital ocular anomalies, and ocular traumas; central subfield macular thickness 250 microns or more; baseline cysts, and the presence of macular traction and epiretinal membranes; use of concomitant medications such as topical or systemic NSAIDs and steroids. Pretreatment: No major group differences. Compared age, gender, ethnic group, iris colour, NPDR classification. visual acuity. Eyes: One eye, unclear how selected. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Interventions Approximately one‐third of the patients were instructed, based on the opinion of the investigator, to use steroids for more than 2 weeks postsurgery. Type of surgery: NR but presumably was phacoemulsification as USA study conducted 2008. |
|
| Outcomes |
Follow‐up: 90 days
|
|
| Contact details |
Authors name: Rishi Singh Institution: Cole Eye Institute, Cleveland Clinic Foundation, Email: drrishisingh@gmail.com Address: Cole Eye Institute, Cleveland Clinic Foundation, 9500 Euclid Avenue, i‐32 Cleveland, OH 44195, USA |
|
| Notes |
Funding sources: NR Declaration of interest: "RS, LA, GJJ, RPL, JL, HJR, KS, and TW are paid consultants for Alcon Research Ltd (Fort Worth, TX). DS is an employee of Alcon Research, Ltd. Medical writing support, which was funded by Alcon Research Ltd, was provided by Cullen T Vogelson and Usha Sivaprasad, of Illuminated Research LLC (Fort Worth, TX)." Date study conducted: November 2008 and July 2010 Trial registration number: NCT00782717 Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This was a multicenter, randomised, double‐masked, vehicle‐controlled, parallel‐group study" Judgement comment: Not reported how list was generated. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: Study was double‐masked with a placebo consisting of vehicle only. It was not clearly stated whether the masking was likely to have been effective but we have assumed that it was. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: Study was double‐masked with a placebo consisting of vehicle only. It was not clearly stated whether the masking was likely to have been effective but we have assumed that it was. Quote: "Total macular volume was determined from a 6 mm diameter circle centered on the foveal center. Morphological features, including intraretinal cysts, were analyzed by the reading center in a masked fashion." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: 125/133 (94%) in nepafenac group included in the analysis compared with 126/130 (97%) in control group. Missing data less than 20%. 95%‐96% of patients enrolled included in final analysis. However, 8 patients in the Nepafenac group and 4 patients in the Vehicle group excluded from final analysis. Reasons not clearly explained. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: Outcomes on trial registry entry were reported. |
Solomon 1995.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: Canada (8 sites) and Germany (2 sites) Setting: Eye hospital Intervention: NSAIDs plus steroids
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Inclusion criteria: Unilateral extracapsular cataract extraction (by manual nuclear expression) with posterior chamber lens implantation. Exclusion criteria: Taking aspirin, topical epinephrine, systemic or topical cyclo‐oxygenase inhibitors, or oral corticosteroid; allergic to cyclo‐oxygenase inhibitors; history of chronic intraocular inflammation; pre‐existing macular pathology; history of herpetic keratitis; corneal or vitreous opacity; non‐compliant patients. Pretreatment: No major imbalances in age, gender, ethnic group. Eyes: One eye, this was the eye scheduled for unilateral extracapsular cataract extraction. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Duration postoperative: days ‐ the investigator had the option of extending the treatment for an additional 3 months. This option was chosen for 10.9% (25/230) of vehicle‐treated patients, 8.4% (20/238) of flurbiprofen‐treated patients, and 9.7% (22/227) of indomethacin‐treated patients. Concomitant medications included aminoglycoside antibiotics (100% of patients) and topical corticosteroids (prednisolone acetate 1% or dexamethasone sodium phosphate 0.1%) in 88.7% (204/230) of vehicle treated patients, 87.8% (209/238) of flurbiprofen treated patients, and 88.1% (200/227) of indomethacin‐treated patients. Type of surgery: ECCE |
|
| Outcomes |
Follow‐up: 6 months
|
|
| Contact details |
Authors name: Leon D Solomon Institution: NR Email: NR Address: NR |
|
| Notes |
Funding sources: Supported by Allergan, Inc., Irvine California Declaration of interest: None of the Flurbiprofen‐CME Study Group members has a commercial or proprietary interest in 0.03% flurbiprofen or 1% indomethacin. Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial was described as “randomised, double‐masked” but with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: Described as “double‐masked”. Medications were masked and fluorescein angiograms were read in a masked fashion by 2 retinal specialists. Uncertain if the operating surgeons or clinicians involved in follow‐up were masked to the allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: Each fluorescein angiogram was read in a masked fashion by two retinal specialists. Unclear if treating ophthalmologists involved in other aspects of patient care were also masked. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: Follow‐up: 177/226 (78%) in flurbiprofen group, 177/234 (76%) in indomethacin group, 160/221 (72%) in placebo group. Reasons for loss to follow‐up not described. |
| Selective reporting (reporting bias) | High risk | Judgement comment: No access to protocol or trials registry entry. Not all follow‐up points were reported fully. |
Tauber 2006.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: USA Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Inclusion criteria: NR Exclusion criteria: NR Pretreatment: Groups differences not reported. Eyes: Unclear if one or both eyes included. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Type of surgery: NR |
|
| Outcomes |
Follow‐up: 30 days (3 month follow‐up mentioned but not reported)
|
|
| Contact details |
Authors name: S Tauber Institution: Ophthalmology, St. John's Hospital and Clinics, Springfield, MO Email: NR Address: Ophthalmology, St. John's Hospital and Clinics, Springfield, MO |
|
| Notes |
Funding sources: Alcon Laboratories, Inc. Declaration of interest: "Commercial Relationships S. Tauber, Alcon, F; Alcon, R; J. Gessler, None; W. Scott, None; C. Peterson, None; P. Hamlet, None." Date study conducted: NR Trial registration number: NR Contacting study investigators: Abstract only, authors contacted by email regarding publication of full study results but no reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: Follow‐up not reported. |
| Selective reporting (reporting bias) | High risk | Judgement comment: Some outcomes not reported including 3‐month OCT outcomes. |
Ticly 2014.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: Brazil Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Included criteria: Nuclear cataract density of 2 and 3 determined by LOCS II; ( > 50 years old); indication for cataract surgery with IOL implantation under local anaesthesia. Excluded criteria: Diabetes; NSAID use; use of topical eye drops (including antiglaucoma drugs); uveitis; macular disease; pseudoexfoliation syndrome; congenital ocular abnormalities; cataract density of 1 and 4 determined by LOCS II; previous intraocular surgery; previous injections; complications during cataract surgery (e.g. posterior capsule rupture, vitreous loss, retained cortical material, or an IOL not placed in the capsular bag); not follow instructions or if they did not show up for appointments. Pretreatment: No major imbalances in age, gender and visual acuity. Eyes: Probably one eye only included in the trial but not clearly reported and unclear how selected. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Type of surgery: Phacoemulsification |
|
| Outcomes |
Follow‐up: 5 weeks
|
|
| Contact details |
Authors name: Dr. Flavia G. Ticly Institution: Department of Ophthalmology, University of Campinas (UNICAMP), Campinas, Sao Paulo, Brazil Email: flaviaticly@gmail.com Address: Department of OphthalmologyUniversity of Campinas (UNICAMP)P.O. Box 6111Campinas 13083‐970, Sao Paulo, Brazil |
|
| Notes |
Funding sources: NR Declaration of interest: Reported no competing financial interests exist. Date study conducted: February 2011 to March 2012 Trial registration number: NTC01542190 Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Each of the 2 intervention groups received 50 different numbers from a random number table. These numbers were transferred to small individual envelopes and also affixed to one of the relabeled eye drop bottles. Unclear how this would work. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Numbers were transferred to small individual envelopes and also affixed to one of the relabeled eye drop bottles. Unclear how this concealed the allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: Placebo‐controlled study. We assume the masking was effective. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: Placebo‐controlled study. We assume the masking was effective. It was stated that the surgeon and the ophthalmologist who collected the data were not aware of the group assignment of the patients. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: 89% follow‐up. Five patients (10%) did not complete the trial in the placebo group while five patients (11%) did not complete the study in the ketorolac group. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
Tunc 1999.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: Turkey Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Inclusion criteria: Patients with unilateral cataracts. Exclusion criteria: Diabetes; rheumatoid disease; immunological disease; uveitis; glaucoma; ARMD; retinitis pigmentosa; retinal detachment; NSAIDs use; corticosteroid use; diuretic use; antihistaminics; previous eye surgery; surgical complications (e.g.. posterior capsular tear, vitreous loss, iatrogenic iridodialysis); combined surgery; postoperative complications (e.g.. iris capture, retinal detachment, choroidal detachment); non‐compliance with medications; use of systemic steroids or NSAIDs during the follow‐up period; definite posterior capsule opacification. Pretreatment: No differences in age sex, and hypertension. Eyes: One eye, people with unilateral cataracts recruited. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Comparator: Steroids alone
At the end of surgery all participants had subconjunctival injection of dexamethasone and gentamicin. All participants used 0.03% tobramycin eye drops postoperatively 4 times a day for 14 days. Type of surgery: ECCE |
|
| Outcomes |
Follow‐up: 2 months
|
|
| Contact details |
Authors name: Murat Tunc Institution: Dokuz Eylul University Medical School Email: NR Address: Dokuz Eylul University Cumhuriyet Blv No:144, 35210 Alsancak/İzmir, Turkey |
|
| Notes |
Funding sources: NR Declaration of interest: NR Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information on masking. We assume that in absence of reporting on this participants and personnel were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The angiograms were read by the retina unit (Dr Saatchi); the patients' names and treatment protocols were kept hidden. Judgement quote: No other information on other outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement Comment: Follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement Comment: No access to protocol or trial registry entry. |
Tzelikis 2015.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: Brazil Setting: Eye hospital Intervention: NSAIDs plus steroids
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Inclusion criteria: Older than 40 years; age‐related cataract; normal ophthalmological exam. Exclusion criteria: Previous ocular surgery; central endothelial cell count < 2000 cells/mm2; glaucoma or IOP > 21 mmHg; amblyopia; retinal abnormalities; steroid or immunosuppressive treatment; connective tissue diseases; allergy or hypersensitivity to NSAIDs; enrolled patients with complicated cataract surgery (e.g. posterior capsule rupture, vitreous loss or an IOL not placed in the capsular bag). Pretreatment: Group differences at baseline not reported. Eyes: One eye, unclear how selected. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
All participants received moxifloxacin 0.5% 4 times a day 2 days before surgery and 7 days postoperatively. Type of surgery: Phacoemulsification |
|
| Outcomes |
Follow‐up: 12 weeks for some outcomes, 30 days for others
|
|
| Contact details |
Authors name: Patrick F Tzelikis Institution: Brasilia Ophthalmologic Hospital Email: tzelikis@gmail.com Address: Brasilia Ophthalmologic Hospital, HOB, SQN 203, bloco K, apart 502, Brasilia, DF 70833‐110, Brazil |
|
| Notes |
Funding sources: NR Declaration of interest: Reported no competing interests Date study conducted: June 2013 to October 2013 Trial registration number: NCT02084576. Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned in a 1:1:1 ratio to one of three treatment groups using a computer‐generated randomisation list." |
| Allocation concealment (selection bias) | Low risk | Quote: "All investigators were masked with regard to treatment group." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: Placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All investigators were masked with regard to treatment group." Judgement comment: Placebo‐controlled. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Follow‐up by intervention group not reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: Trial study protocol registered at NCT02084576 but does not clearly define outcomes. |
Umer‐Bloch 1983.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: Switzerland Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Included criteria: Intracapsular cataract extraction (124 persons); 40 patients with IOL implantation after cataract extraction. Excluded criteria: Maculopathy; diabetic retinopathy; prior uveitis; systemic steroid therapy. Pretreatment: Unclear if groups comparable. Eyes: Unclear if one or both eyes included. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Additional for all participants: cycloplegics (atropine 1%); if necessary timoptic or diamox to lower eye pressure. Type of surgery: ECCE (40) ICCE (124) |
|
| Outcomes |
Follow‐up: 12 weeks
|
|
| Contact details |
Authors name: U Umer‐Bloch Institution: University Augenklink Zurich Email: NR Address: University Augenklinik, Ramistrasse 100, CH‐8091 Zurich |
|
| Notes |
Funding sources: NR Declaration of interest: NR Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation was administered. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: Medication placed by nurses in a bottle with suspension: one with indomethacin another with vehicle. Neither the examiner nor the patient knew the contents of the bottle. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: Placebo‐controlled using vehicle only. Patients, nurses, physician analysing fluorescein angiography were masked. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: For 35 patients the study was stopped before the end of the study because of intra‐operative complications or they had, as only later recognized, an exclusion criteria as defined as maculopathy, diabetic retinopathy, prior uveitis or a systemic steroid therapy. Not reported to which groups these patients belonged. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
Wang 2013.
| Methods |
Study design: Parallel group RCT Open label |
|
| Participants |
Country: China Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Inclusion criteria: Age‐related cataract patients undergoing phacoemulsification with posterior chamber IOL implantation. Exclusion criteria: Any ocular diseases that might affect treatment responses or evaluations, such as corneal disease, glaucoma, uveitis, retinal detachment, optic neuropathy or amblyopia; any systemic diseases that might affect treatment responses or evaluations, such as diabetes mellitus; potentially pregnant women; systemic or topical anti‐inflammatory therapy within 1 month prior to surgery and contraindication of oral steroids, such as patients with peptic ulcer, cancer and tuberculosis; surgical complications, such as posterior capsule rupture or hyphema; special diseases which might affect surgery in the eyes, such as limitation of pupil dilation. Pretreatment: Groups were not compared. Eyes: Not clearly reported but probably one eye per person, unclear how selected. |
|
| Interventions |
Intervention: NSAIDs plus (oral) steroids
Comparator: Steroids alone
All participants received levofloxacin eye drops (Santen PharmaceuticalCo., Ltd) 4 times a day for 1 day preoperatively and 7 days postoperatively. Type of surgery: Phacoemulsification |
|
| Outcomes |
Follow‐up: 2 months
|
|
| Contact details |
Authors name: Ke Yao Institution: Medical College of Zhejiang University Email: xlren@zju.edu.cn Address: Eye Center, 2nd Affiliated Hospital Medical College of Zhejiang University Hangzhou 310009 (China) |
|
| Notes |
Funding sources: "This study was supported by grants from Zhejiang Key Innovation Team Project of China (grant no. 009R50039) and Zhejiang Key Laboratory Fund of China (No.2011E10006)." Declaration of interest: NR Date study conducted: October 2010 to December 2011 Trial registration number: NR Contacting study investigators: Trial authors not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomly and prospectively assigned into four groups (OBS1, OBS2, OFM and ODM) by a random‐numbers table." |
| Allocation concealment (selection bias) | High risk | Judgement comment: The drugs were applied topically to the assigned patients open‐label. The same physician served as the medical monitor and assigned one of the drugs to each patient. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: The drugs were applied topically to the assigned patients open‐label. The same physician served as the medical monitor and assigned one of the drugs to each patient. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: The drugs were applied topically to the assigned patients open‐label. The same physician served as the medical monitor and assigned one of the drugs to each patient. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: Follow‐up was 83/120 (69%) in NSAIDs group and 84/120 (70%) in the steroid group. Significant loss to follow‐up but similar in both groups. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
Wittpenn 2008.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: USA Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Inclusion criteria: Scheduled to undergo cataract surgery; 20/20 BCVA potential without any evidence of macular abnormality, including age‐related macular changes, epiretinal membranes, or other retinal‐vascular anomalies. Exclusion criteria: Systemic diseases with ocular manifestations of the disease (e.g. diabetic patients with normal retinal exams were not excluded); vitreous loss or capsular disruption/rupture occurred during surgery; postoperative day 1, the surgeon felt the amount of inflammation was greater than expected and, in his best clinical judgment, more aggressive anti‐inflammatory treatment was indicated. Pretreatment: Quote: "There were no statistically significant between‐group differences in any demographic variable." But no data reported. Eyes: One eye, unclear how selected. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
The comparator group: "...also received four drops of ketorolac 0.4% one hour prior to cataract surgery." Type of surgery: Phacoemulsification |
|
| Outcomes |
Follow‐up: 4 weeks
|
|
| Contact details |
Authors name: John R. Wittpenn Institution: State University of New York at Stony Brook Email: jrwittpenn@aol.com Address: State University of New York at Stony Brook, 2500 Route 347, Building 24, Stony Brook, NY 11790 |
|
| Notes |
Funding sources: "This study was supported by an unrestricted education grant from Allergan Inc, Irvine, Calfiornia." Declaration of interest: "The authors indicate no financial conflict of interest." Date study conducted: June 2005 to August 2006 Trial registration number: NCT00348244 Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised in a 1:1 ratio using a randomly generated list of patient identification numbers." |
| Allocation concealment (selection bias) | Low risk | Quote: "A central coordination center (IMEDS Inc, Riverside, California, USA; [M.E.]) generated the allocation sequence, enrolled participants, and assigned participants to their treatment groups." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The patients and technical staff were unmasked because regulations prevented the medications from being repackaged into similar, unmarked bottles. The labels were covered but the technicians were capable of recognizing the bottle color and shape. Patients, however, would only have been unmasked if they researched the type and shape of the different bottles." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All investigators were masked with regard to treatment group." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: Very low follow‐up at 6 weeks. "Of the 546 patients who entered the study, 77 patients also returned for the week‐6 visit, 35 in the ketorolac/steroid group and 42 in the steroid group." |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol and trial registry entry did not include outcomes. |
Yannuzzi 1981.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: USA Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Included criteria: Patients undergoing intracapsular cataract extraction. Excluded criteria: Undergone procedures other than conventional ICCE; pre‐existing macular disease predisposing to macular oedema, such as neovascular age‐related macular degeneration. Pretreatment: Baseline comparisons not reported. Eyes: 21 people had bilateral cataract surgery ‐ the second eye was randomised separately. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Quote: "Routine postoperative drops such as cycloplegics, antibiotics and steroids were also given as was the custom of the operating ophthalmologist." Type of surgery: ICCE |
|
| Outcomes |
Follow‐up: 1 year
|
|
| Contact details |
Authors name: Lawrence A Yannuzzi Institution: Manhattan Eye, Ear and Throat Hospital Email: NR Address: Manhattan Eye, Ear and Throat Hospital 210 E 64th St, New York, NY 10021, United States |
|
| Notes |
Funding sources: LuEster Mertz Retinal Research Fund of the Eye, Ear and Throat Hospital Declaration of interest: NR Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Allocation was described as being done “in a random fashion" but with no further details. |
| Allocation concealment (selection bias) | High risk | Judgement comment: Pharmacist involved in giving treatment did not appear to be masked to treatment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: Placebo‐controlled study described as "double‐masked". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: Placebo‐controlled study described as "double‐masked". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: Follow‐up 59% in both groups. High loss to follow‐up at 1 year 38/100 (38%) in NSAIDs group and 50/131 (38%) in the control group. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
Yavas 2007.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: Turkey Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Inclusion criteria: NR Exclusion criteria: History of intraocular surgery; any complication during cataract surgery; glaucoma; uveitis; vitreoretinal pathology; history of diabetes mellitus, hypertension, or cardiac disease; or topical or systemic drug use. Pretreatment: Some imbalances in age and sex but unclear if important. Eyes: Right eye only included. |
|
| Interventions |
Intervention: NSAIDsplus steroids
Comparator: Steroids alone
All participants received 1 drop of topical antibiotic (ofloxacin 0.3%) 4 times a day daily for 1 week. Type of surgery: Phacoemulsification |
|
| Outcomes |
Follow‐up: 3 months
|
|
| Contact details |
Authors name: Guliz Yavas Institution: Afyon Kocatepe University Email: gkumbar@ttnet.net.tr Address: P.K. 25, 06502 Bahcelievler, Ankara, Turkey |
|
| Notes |
Funding sources: NR Declaration of interest: "No author has a financial or proprietary interest in any material or method mentioned." Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised into 3 groups." Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Fluorescein angiography was performed in all patients, and fluorescein leakage to diagnose angiographic CME was evaluated by a masked observer." Judgement comment: Unclear if other outcomes were masked. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: Follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
Yung 2007.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: USA Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Inclusion criteria: Diabetic patients having cataract surgery. Exclusion criteria: NR Pretreatment: Group differences not reported. Eyes: Unclear if one or both eyes included. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Type of surgery: NR |
|
| Outcomes |
Follow‐up: 12 weeks
|
|
| Contact details |
Authors name: C Yung Institution: Indiana University Email: NR Address: Indiana University107 S Indiana Ave, Bloomington, IN 47405, United States |
|
| Notes |
Funding sources: NR Declaration of interest: NR Date study conducted: NR Trial registration number: NR Contacting study investigators: Abstract only, tried to contact authors but could not find email address. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: Placebo‐controlled but no information on who was masked. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: Placebo‐controlled but no information on who was masked. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: Follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
Zaczek 2014.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: Sweden Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Inclusion criteria: 45 and 85 years of age; cataract surgery under local anaesthesia; translucent cataract for good‐quality OCT scans of the macular at baseline. Exclusion criteria: Small pupils (< 5.0 mm after pharmacologic dilation); dark brown irides; exfoliation syndrome, history of uveitis; glaucoma; macular degeneration; vision impairing eye disorder except cataract; diabetic patients; pregnant women; patients using topical or systemic anti‐inflammatory treatment; hypersensitivity to any of the given study treatments; intraoperative difficulties (e.g. loose zonular fibres, extended operating time, residual cortical material); intraoperative complications (e.g. posterior capsule rupture and vitreous loss). Pretreatment: No major imbalances, age, gener and operated eye compared. Eyes: One eye, unclear how selected. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Comparator: Steroids plus placebo
Type of surgery: Phacoemulsification |
|
| Outcomes |
Follow‐up: 6 weeks
|
|
| Contact details |
Authors name: Anna Zaczek Institution: Scanloc Healthcare AB Email: anna. zaczek@scanloc.se Address: Scanloc Healthcare AB, Lilla Bommen 6, 411 04 Gothenburg, Sweden |
|
| Notes |
Funding sources: Supported by Alcon Research Ltd, Fort Worth, Texas, USA, and S.A. Alcon‐Couvreur N.V. Puurs, Belgium, which produced and provided the masked eyedrop bottles. Partially supported by Alcon, Inc. Sweden. Financial support was also provided through the regional agreement on Medical training and Clinical research (ALF) between Stockholm County Council and Karolinska Institutet (20120623). Declaration of interest: "No author has a financial or proprietary interest in any material or method mentioned." Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Low risk | Quote: "All products used in this clinical trial were produced, labelled, packaged, and released by S.A. Alcon‐Couvreur N.V. Puurs, Belgium. Nepafenac and placebo suspensions were supplied in identical bottles labelled with a protocol and a patient number so neither the investigators nor the patients were able to identify their contents." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All products used in this clinical trial were produced, labelled, packaged, and released by S.A. Alcon‐Couvreur N.V. Puurs, Belgium. Nepafenac and placebo suspensions were supplied in identical bottles labelled with a protocol and a patient number so neither the investigators nor the patients were able to identify their contents." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All products used in this clinical trial were produced, labelled, packaged, and released by S.A. Alcon‐Couvreur N.V. Puurs, Belgium. Nepafenac and placebo suspensions were supplied in identical bottles labelled with a protocol and a patient number so neither the investigators nor the patients were able to identify their contents". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: Missing data less than 20% (i.e. more than 80% follow‐up) and equal follow‐up in both groups and no obvious reason why loss to follow‐up should be related to outcome. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
Zhang 2008.
| Methods | Study design: Parallel group RCT | |
| Participants |
Country: China Setting: Eye hospital Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Included criteria: NR Excluded criteria: NR Pretreatment: No information on pretreatment differences. Eyes: 220 eyes of 198 people. |
|
| Interventions |
Intervention: NSAIDs plus steroids
Comparator: Steroids alone
Type of surgery: Phacoemulsification |
|
| Outcomes |
Follow‐up: 1 month
|
|
| Contact details |
Authors name: Zhang HY Institution: Beijing Tongren Eye Center Email: NR Address: Beijing Tongren Eye Centre, Beijing Tongren Hospital, Capital Medical University; Beijing Ophthalmology and Visual Science Key Laboratory, Beijing 100730, China |
|
| Notes |
Funding source: NR Declaration of interest: NR Date study conducted: NR Trial registration number: NR Contacting study investigators: Trial authors not contacted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Not reported how list was generated. Trial described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: Not reported how allocation administered. Trial described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this patients and personnel were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: No information on masking. We assume that in absence of reporting on this outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: Missing data less than 20% (i.e. more than 80% follow‐up) and equal follow‐up in both groups and no obvious reason why loss to follow‐up should be related to outcome. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: No access to protocol or trial registry entry. |
AE: adverse events BCVA: best corrected visual acuity CMO: cystoid macular oedema CRT: corneal retinal thickness DR: diabetic retinopathy ECCE: extracapsular cataract extraction IOL: intraocular lens IOP: intraocular pressure NR: not reported NSAID: non‐steroidal anti‐inflammatory drug OCT: optical coherence tomography RCT: randomised controlled trial SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abelson 1989 | Not topical treatment. |
| Carenini 1993 | Not RCT. |
| Chen 2015 | Study only performed follow‐up for 2 weeks in total. |
| Dehgan 1992 | Not able to source paper. |
| Duong 2015 | Not RCT. |
| Hendrikse 1982 | Not able to source paper. |
| Hollwich 1983 | Not relevant comparator. |
| ISRCTN02628492 | Study was terminated due to lack of funding. |
| Miyake 2000 | Probably not random allocation, unclear response from study author. |
| Nishino 2009 | Not relevant intervention. |
| Riley 2006 | Not relevant intervention. |
| Sanders 1982 | Not able to source paper. |
| Sellares 1992 | Not able to source paper. |
| Sholiton 1979 | Not topical treatment. |
| Tang 2015 | Not relevant intervention. |
| Wolf 2007 | Not RCT. |
| Yamaaki 1984 | Not RCT. |
| Yilmaz 2012 | Not RCT. |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
CTRI/2009/091/001078.
| Methods | Parallel group RCT |
| Participants | Country: India 704 people aged 50 to 70 years within 40 kms of Vellore town. Exclusion criteria: Inabiltity to visualise the macula preoperatively in the eye to be operated. Ocular disease that can affect macular function. Uncontrolled diabetics defined by RBS/PP Sugars > 200 mg/dl. Diabetic maculopathy with oedema in eye to be operated. Past history of intraocular surgery in the eye under consideration. History of use of topical steroid drops or NSAID drops within the past 30 days prior to enrolment. Current use of Oral steroids. Known NSAIDs allergy. |
| Interventions | Intervention: ketorolac tromethamine Comparator: polyvinyl Alcohol |
| Outcomes | Primary outcome:
|
| Notes | September 2016: Study investigator confirms that this study is unpublished. We are awaiting a response to request for unpublished data. |
NSAID: non‐steroidal anti‐inflammatory drug
Characteristics of ongoing studies [ordered by study ID]
NCT01694212.
| Trial name or title | Preoperative topic diclofenac as a prevention of postoperative macular edema in patients with diabetic retinopathy |
| Methods | Parallel group RCT |
| Participants | Country: Croatia 120 people aged 60 to 90 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: diclofenac Comparator: placebo |
| Outcomes | Primary Outcome:
Secondary Outcome:
|
| Starting date | October 2012 End date: December 2016 |
| Contact information | Ljubo Znaor, MD PhD, Clinical Hospital Center, Split |
| Notes |
NCT01774474.
| Trial name or title | PRevention of Macular EDema After Cataract Surgery (PREMED) |
| Methods | Parallel group RCT |
| Participants | Country: Netherlands 1135 people aged 21 years and older Inclusion criteria:
Exclusion criteria will be different for non‐diabetic and diabetic patients. All ophthalmic exclusion criteria are applicable to the study eye only, unless stated otherwise. General exclusion criteria for participation in this study are:
Non‐diabetic patients with a history of CME will be excluded from participation in the study. Additionally, diabetic patients will be excluded from participation in case of:
|
| Interventions | Intervention: bromfenac Intervention: bromfenac and dexamethasone Comparator: dexamethasone |
| Outcomes | Primary outcome:
Secondary outcomes:
Other outcome measures at 6 and 12 weeks see clinicaltrials.gov/ct2/show/NCT01774474 |
| Starting date | July 2013 End date: October 2016 |
| Contact information | Prof. Rudy MM Nuijts, MD, PhD rudy.nuijts@0mumc.nl Laura HP Wielders, MD laura.wielders@mumc.nl |
| Notes |
NCT02646072.
| Trial name or title | Effect of preoperative topical ketorolac on aqueous cytokine levels and macular thickness in cataract surgery patients |
| Methods | Parallel group RCT |
| Participants | Country: Malaysia 80 participants aged 18 to 90 years Inclusion criteria: Diabetic patient group
Non‐diabetic patient group
Exclusion criteria
|
| Interventions | Intervention: ketorolac tromethamine Comparator: no intervention |
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | August 2014 End date: June 2015 |
| Contact information | Yin Peng Lai, Univerisity of Malaya |
| Notes |
CME: cystoid macular oedema (edema) DR: diabetic retinopathy ETDRS: early treatment diabetic retinopathy study IOP: intraocular pressure NSAID: non‐steroidal anti‐inflammatory drug OCT: optical coherence tomography RCT: randomised controlled trial
Differences between protocol and review
In the protocol we had planned to contact pharmaceutical companies for more information (Goh 2007). We did not do this because since the protocol was written, the role of clinical trial registries have meant that it is much easier to identify potentially unpublished trials.
We had planned to use confidence intervals for the I2 value, but as this is not routinely implemented in RevMan 5 as yet, we have not done this. We felt the extra effort required to analyse the data in a software package that could provide these confidence intervals, such as Stata, was not worth it.
We added some additional outcomes as a result of our collaboration with the National Institute for Health and Care Excellence (NICE). These are clearly identified in the text. We have clarified our definition of macular oedema to include all 3 levels of the Miyake classification and whether or not cystic spaces are detectable on imaging which we have termed simply macular oedema (MO). Cystoid has been removed from the title.
Contributions of authors
Conceiving the review: Cochrane Eyes and Vision (CEV)
Designing the review: JE
Co‐ordinating the review: JE
-
Data collection for the review
designing search strategies: CEVG Information Specialist
undertaking electronic searches: CEVG Information Specialist
screening search results: BL, CL, DL
organising retrieval of papers: CEVG Information Specialist
screening retrieved papers against inclusion criteria: BL, CL, DL
appraising quality of papers: BL, CL, DL, JE
extracting data from papers: BL, CL, DL, JE
writing to authors of papers for additional information: BL, JE
providing additional data about papers: BL, JE
obtaining and screening data on unpublished studies: JE, BL
-
Data management for the review
entering data into RevMan 5: JE
analysis of data: JE, CB
-
Interpretation of data
providing a methodological perspective: JE, CB, RW
providing a clinical perspective: BL, CL, DL, RW
providing a policy perspective: RW
Writing the review: BL, CL, DL, JE, RW
Providing general advice on the review: RW
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEV Editorial Base in London which funds part of Jennifer Evan's salary.
- Cochrane Incentive Scheme awarded in 2015.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
JE: None known BL: Noneknown CL: None known DL: None known CB: None known RW: None known.
Joint first author
Joint first author
Joint first author
New
References
References to studies included in this review
Almeida 2008 {published data only}
- Almeida DR, Johnson D, Hollands H, Smallman D, Baxter S, Eng KT, et al. Effect of prophylactic nonsteroidal antiinflammatory drugs on cystoid macular edema assessed using optical coherence tomography quantification of total macular volume after cataract surgery. Journal of Cataract and Refractive Surgery 2008;34(1):64‐9. [DOI: 10.1016/j.jcrs.2007.08.034] [DOI] [PubMed] [Google Scholar]
Almeida 2012 {published data only}
- Almeida DR, Khan Z, Xing L, Bakar SN, Rahim K, Urton T, et al. Prophylactic nepafenac and ketorolac versus placebo in preventing postoperative macular edema after uneventful phacoemulsification. Journal of Cataract and Refractive Surgery 2012;38(9):1537‐43. [DOI: 10.1016/j.jcrs.2012.04.034] [DOI] [PubMed] [Google Scholar]
- El‐Defrawy SR, Almeida DR. Randomized controlled trial comparing nepafenac, ketorolac and placebo in preventing macular oedema after uncomplicated cataract extraction. West Indian Medical Journal 2000;61:29. [Google Scholar]
Asano 2008 {published data only}
- Asano S, Miyake K, Ota I, Sugita G, Kimura W, Sakka Y, et al. Reducing angiographic cystoid macular edema and blood‐aqueous barrier disruption after small‐incision phacoemulsification and foldable intraocular lens implantation: multicenter prospective randomized comparison of topical diclofenac 0.1% and betamethasone 0.1%. Journal of Cataract and Refractive surgery 2008;34(1):57‐63. [DOI: 10.1016/j.jcrs.2007.08.030] [DOI] [PubMed] [Google Scholar]
Brown 1996 {published data only}
- Brown RM, Roberts CW. Preoperative and postoperative use of nonsteroidal antiinflammatory drugs in cataract surgery. Insight (American Society of Ophthalmic Registered Nurses) 1996;21(1):13‐6. [DOI] [PubMed] [Google Scholar]
Cervantes‐Coste 2009 {published data only}
- Cervantes‐Coste G, Sánchez‐Castro YG, Orozco‐Carroll M, Mendoza‐Schuster E, Velasco‐Barona C. Inhibition of surgically induced miosis and prevention of postoperative macular edema with nepafenac. Clinical Ophthalmology 2009;3(1):219‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chatziralli 2011 {published data only}
- Chatziralli IP, Papazisis L, Sergentanis TN. Ketorolac plus tobramycin/dexamethasone versus tobramycin/dexamethasone after uneventful phacoemulsification surgery: a randomized controlled trial. Ophthalmologica 2011;225(2):89‐94. [DOI: 10.1159/000317067] [DOI] [PubMed] [Google Scholar]
Donnenfeld 2006 {published data only}
- Donnenfeld ED, Perry HD, Wittpenn JR, Solomon R, Nattis A, Chou T. Preoperative ketorolac tromethamine 0.4% in phacoemulsification outcomes: pharmacokinetic‐response curve. Journal of Cataract and Refractive Surgery 2006;32(9):1474‐82. [DOI: 10.1016/j.jcrs.2006.04.009] [DOI] [PubMed] [Google Scholar]
Elsawy 2013 {published data only}
- Elsawy MF, Badawi N, Khairy HA. Prophylactic postoperative ketorolac improves outcomes in diabetic patients assigned for cataract surgery. Clinical Ophthalmology 2013;7:1245‐9. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Endo 2010 {published data only}
- Endo N, Kato S, Haruyama K, Shoji M, Kitano S. Efficacy of bromfenac sodium ophthalmic solution in preventing cystoid macular oedema after cataract surgery in patients with diabetes. Acta Ophthalmologica 2010;88(8):896‐900. [DOI: 10.1111/j.1755-3768.2009.01582.x] [DOI] [PubMed] [Google Scholar]
- Kitano S, Endo N, Shoji M, Haruyama K, Kato S. Effect of bromfenac sodium ophthalmic solution on preventing cystoids macular edema and inflammation after phacoemulsification and implantation of intraocular lens in diabetic patients. Investigative Ophthalmology and Visual Science 2008;49(3):ARVO E‐abstract 3470. [Google Scholar]
Italian Diclofenac Study Group 1997 {published data only}
- Italian Diclofenac Study Group. Efficacy of diclofenac eyedrops in preventing postoperative inflammation and long‐term cystoid macular edema. Italian Diclofenac Study Group. Journal of Cataract and Refractive Surgery 1997;23(8):1183‐9. [DOI] [PubMed] [Google Scholar]
Jung 2015 {published data only}
- Jung JW, Chung BH, Kim EK, Seo KY, Kim TI. The effects of two non‐steroidal anti‐inflammatory drugs, bromfenac 0.1% and ketorolac 0.45%, on cataract surgery. Yonsei Medical Journal 2015;56:1671‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kraff 1982 {published data only}
- Kraff MC, Sanders DR, Jampol LM, Peyman GA, Lieberman HL. Prophylaxis of pseudophakic cystoid macular edema with topical indomethacin. Ophthalmology 1982;89(8):885‐90. [DOI] [PubMed] [Google Scholar]
Li 2011 {published data only}
- Li M‐C, Yang X‐R, Liu F, Shao D‐P, Li Y‐B. Effect of topical NSAIDs in preventing macular edema after phacoemulsification in diabetes. International Journal of Ophthalmology 2011;11(9):1614‐6. [DOI: 10.3969/j.issn.1672-5123.2011.09.039] [DOI] [Google Scholar]
Mathys 2010 {published data only}
- Mathys KC, Cohen KL. Impact of nepafenac 0.1% on macular thickness and postoperative visual acuity after cataract surgery in patients at low risk for cystoid macular oedema. Eye 2010;24(1):90‐6. [DOI: 10.1038/eye.2009.10] [DOI] [PubMed] [Google Scholar]
Miyake 2007 {published data only}
- Miyake K, Nishimura K, Harino S, Ota I, Asano S, Kondo N, et al. The effect of topical diclofenac on choroidal blood flow in early postoperative pseudophakias with regard to cystoid macular edema formation. Investigative Ophthalmology & Visual Science 2007;48(12):5647‐52. [DOI: 10.1167/iovs.07-0262] [DOI] [PubMed] [Google Scholar]
Miyake 2011 {published data only}
- Miyake K, Ota I, Miyake G, Numaga J. Nepafenac 0.1% versus fluorometholone 0.1% for preventing cystoid macular edema after cataract surgery. Journal of Cataract and Refractive Surgery 2011;37(9):1581‐8. [DOI: 10.1016/j.jcrs.2011.03.052] [DOI] [PubMed] [Google Scholar]
Miyanaga 2009 {published data only}
- Miyanaga M, Miyai T, Nejima R, Maruyama Y, Miyata K, Kato S. Effect of bromfenac ophthalmic solution on ocular inflammation following cataract surgery. Acta Ophthalmologica 2009;87(3):300‐5. [DOI: 10.1111/j.1755-3768.2008.01433.x] [DOI] [PubMed] [Google Scholar]
Moschos 2012 {published data only}
- Moschos MM, Chatziralli IP, Pantazis P, Rouvas AA, Sergentanis TN. Is topical diclofenac essential before and after uneventful phacoemulsification cataract surgery?. Journal of Ocular Pharmacology and Therapeutics 2012;28(4):335‐9. [DOI: 10.1089/jop.2011.0256] [DOI] [PubMed] [Google Scholar]
Quentin 1989 {published data only}
- Quentin CD, Behrens‐Baumann W, Gaus W. Prevention of cystoid macular edema with diclofenac eyedrops in intracapsular cataract extraction using the Choyce Mark IX anterior chamber lens. Fortschritte der Ophthalmologie 1989;86(6):546‐9. [PubMed] [Google Scholar]
Rossetti 1996 {published data only}
- Rossetti L, Bujtar E, Castoldi D, Torrazza C, Orzalesi N. Effectiveness of diclofenac eyedrops in reducing inflammation and the incidence of cystoid macular edema after cataract surgery. Journal of Cataract and Refractive Surgery 1996;22 Suppl 1:794‐9. [DOI] [PubMed] [Google Scholar]
- Torrazza C, Rossetti L, Castoldi D, Bujtar E, Orzalesi N. Effectiveness of diclofenac eyedrops in reducing postoperative inflammation angiographic cme after cataract surgery. Investigative Ophthalmology & Visual Science 1995;36:ARVO E‐Abstract 3769. [Google Scholar]
Singh 2012 {published data only}
- Pollack A, Narvekar A, Adewale A, Singh R. A post‐hoc analysis comparing nepafenac (0.1%) to vehicle treatment post‐cataract surgery in patients with diabetic retinopathy. Ophthalmologica 2014;232:20‐1. [Google Scholar]
- Pollack A, Sager D, Singh R. Risk reduction of macular edema with nepafenac (0.1%) post‐cataract surgery in patients with diabetic retinopathy: Efficacy and safety from 2 multicenter trials. Ophthalmologica 2014;232:20. [Google Scholar]
- Singh R, Alpern L, Jaffe GJ, Lehmann RP, Lim J, Reiser HJ, et al. Evaluation of nepafenac in prevention of macular edema following cataract surgery in patients with diabetic retinopathy. Clinical Ophthalmology 2012;6(1):1259‐69. [DOI: 10.2147/OPTH.S31902] [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomon 1995 {published data only}
- Solomon LD. Efficacy of topical flurbiprofen and indomethacin in preventing pseudophakic cystoid macular edema. Flurbiprofen‐CME Study Group I. Journal of Cataract and Refractive Surgery 1995;21(1):73‐81. [DOI] [PubMed] [Google Scholar]
- Solomon LD, Boyaner D, Breslin CW, Demco TA, Fabian E, LeBlanc RP, et al. Topical flurbiprofen and indomethacin inhibit postoperative cystoid macular edema (CME). American Academy of Ophthalmology 1990:120. [Google Scholar]
Tauber 2006 {published data only}
- Tauber S, Gessler J, Scott W, Peterson C, Hamlet P. The effect of topical ketorolac 0.4% on cystoid macular edema following routine cataract surgery. Investigative Ophthalmology & Visual Science 2006;47:ARVO E‐abstract 683. [Google Scholar]
Ticly 2014 {published data only}
- Ticly FG, Lira RP, Zanetti FR, Machado MC, Rodrigues GB, Arieta CE. Prophylactic use of ketorolac tromethamine in cataract surgery: a randomized trial. Journal of Ocular Pharmacology and Therapeutics 2014;30(6):495‐501. [DOI: ] [DOI] [PubMed] [Google Scholar]
Tunc 1999 {published data only}
- Tunc M, Saatci OA, Ergin MH, Ergin S. Cystoid macular edema early after uncomplicated cataract surgery: The efficacy of diclofenac in prophylaxis. Annals of Ophthalmology ‐ Glaucoma 1999;31(1):27‐32. [Google Scholar]
Tzelikis 2015 {published data only}
- Tzelikis PF, Vieira M, Hida WT, Motta AF, Nakano CT, Nakano EM, et al. Comparison of ketorolac 0.4% and nepafenac 0.1%for the prevention of cystoid macular oedema after phacoemulsification: prospective placebo‐controlled randomised study. British Journal of Ophthalmology 2015;99:654‐8. [DOI] [PubMed] [Google Scholar]
Umer‐Bloch 1983 {published data only}
- Umer‐Bloch U. Prevention of cystoid macular edema following cataract extraction using local indomethacin application. Klinische Monatsblätter für Augenheilkunde 1983;183(6):479‐84. [DOI: 10.1055/s-2008-1054988] [DOI] [PubMed] [Google Scholar]
- Umer‐Bloch U. Should indomethacin be used topically to prevent aphakic cystoid macular edema? [Indocid Lokal Zur Pravention Des Postoperativen Zystoiden Makulaodems?]. Klinische Monatsblätter für Augenheilkunde 1983;182(5):495‐6. [Google Scholar]
Wang 2013 {published data only}
- Wang QW, Yao K, Xu W, Chen PQ, Shentu XC, Xie X, et al. Bromfenac sodium 0.1%, fluorometholone 0.1% and dexamethasone 0.1% for control of ocular inflammation and prevention of cystoid macular edema after phacoemulsification. Ophthalmologica 2013;229(4):187‐94. [DOI: 10.1159/000346847] [DOI] [PubMed] [Google Scholar]
Wittpenn 2008 {published data only}
- Rocha G. A randomized, masked comparison of topical ketorolac 0.4% plus steroid versus steroid alone in low‐risk cataract surgery patients. Evidence‐Based Ophthalmology 2009;10(2):92‐3. [DOI] [PubMed] [Google Scholar]
- Wittpenn J, Silverstein SM, Hunkeler JD, Schechter B, Price FW, Chu YR, et al. A masked comparison of acular LS plus steroid vs. steroid alone for the prevention of macular leakage in cataract patients. American Academy of Ophthalmology 2006:197. [Google Scholar]
- Wittpenn JR, Silverstein S, Heier J, Kenyon KR, Hunkeler JD, Earl M, et al. A randomized, masked comparison of topical ketorolac 0.4% plus steroid vs steroid alone in low‐risk cataract surgery patients. American Journal of Ophthalmology 2008;146(4):554‐60. [DOI: ] [DOI] [PubMed] [Google Scholar]
Yannuzzi 1981 {published data only}
- Yannuzzi LA, Landau AN, Turtz AI. Incidence of aphakic cystoid macular edema with the use of topical indomethacin. Ophthalmology 1981;88(9):947‐54. [DOI] [PubMed] [Google Scholar]
Yavas 2007 {published data only}
- Yavas GF, Oztürk F, Küsbeci T. Preoperative topical indomethacin to prevent pseudophakic cystoid macular edema. Journal of Cataract and Refractive Surgery 2007;33(5):804‐7. [DOI: 10.1016/j.jcrs.2007.01.033] [DOI] [PubMed] [Google Scholar]
Yung 2007 {published data only}
- Yung CW, Hui SL, Wang J, Gao H, Peracha MO, Pratt LM, et al. The effect of topical ketorolac tromethamine 0.5% on macular thickness in diabetic patients after cataract surgery. American Academy of Ophthalmology 2007:208. [Google Scholar]
Zaczek 2014 {published data only}
- Zaczek A, Artzen D, Laurell CG, Stenevi U, Montan P. Nepafenac 0.1% plus dexamethasone 0.1% versus dexamethasone alone: effect on macular swelling after cataract surgery. Journal of Cataract and Refractive Surgery 2014;40(9):1498‐505. [DOI: ] [DOI] [PubMed] [Google Scholar]
Zhang 2008 {published data only}
- Zhang H‐Y, Zhu S‐Q. Clinical study of Pranoprofen eyedrops on prophylaxis of cystoids macular edema after cataract surgery. International Journal of Ophthalmology 2008;8(7):1370‐2. [Google Scholar]
References to studies excluded from this review
Abelson 1989 {published data only}
- Abelson MB, Smith LM, Ormerod LD. Prospective, randomized trial of oral piroxicam in the prophylaxis of postoperative cystoid macular edema. Journal of Ocular Pharmacology 1989;5(2):147‐53. [DOI] [PubMed] [Google Scholar]
Carenini 1993 {published data only}
- Carenini BB, Brancato R, Molfetta V, Frezzotti R, Balestrazzi E, Cardia L, et al. Indomethacin ophthalmic suspension in the prevention of cystoid macular oedema in cataract extraction patients [L'indometacina, sospensione oftalmica, nella prevenzione dell'edema maculare cistoide in pazienti sottoposti ad estrazione di cataratta]. Annali di Ottalmologia e Clinica Oculistica 1993;119(9):681‐7. [Google Scholar]
Chen 2015 {published data only}
- Chen J, Wang G‐Q. Clinical observation of bromfenac sodium 0.1% eye drops on cataract surgery. International Eye Science 2015;15(12):2102‐4. [Google Scholar]
Dehgan 1992 {published data only}
- Dehgan JA, Ustuner A, Aras C, Ozdamar A. Prophylaxis of pseudophakic cystoid macular edema with topical indomethacin. Turk Oftalmoloji Gazetesi 1992;22(2):168‐73. [Google Scholar]
Duong 2015 {published data only}
- Duong HV, Westfield KC, Singleton IC. Treatment paradigm after uncomplicated cataract surgery: a prospective evaluation. Acta Ophthalmologica 2015;93(4):e314‐5. [DOI: 10.1111/aos.12221] [DOI] [PubMed] [Google Scholar]
Hendrikse 1982 {published data only}
- Hendrikse F, Yamaaki H, Deutman AF. Local administration of indomethacin to prevent postoperative cystoid macular edema. Nederlands Tijdschrift voor Geneeskunde 1982;126(3):134. [Google Scholar]
Hollwich 1983 {published data only}
- Hollwich F, Jacobi K, Küchle HJ, Lerche W, Reim M, Straub W. Prevention of cystoid macular edema using indomethacin eye drops. Klinische Monatsblätter für Augenheilkunde 1983;183(6):477‐8. [DOI: 10.1055/s-2008-1054987] [DOI] [PubMed] [Google Scholar]
ISRCTN02628492 {published data only}
- ISRCTN02628492. Prevention of post‐cataract surgery macula oedema with prophylactic ketorolac. isrctn.com/ISRCTN02628492 (first received 6 July 2008).
Miyake 2000 {published data only}
- Miyake K, Masuda K, Shirato S, Oshika T, Eguchi K, Hoshi H, et al. Comparison of diclofenac and fluorometholone in preventing cystoid macular edema after small incision cataract surgery: a multicentered prospective trial. Japanese Journal of Ophthalmology 2000;44:58‐67. [DOI] [PubMed] [Google Scholar]
Nishino 2009 {published data only}
- Nishino M, Eguchi H, Iwata A, Shiota H, Tanaka M, Tanaka T. Are topical steroids essential after an uneventful cataract surgery?. Journal of Medical Investigation 2009;56(1‐2):11‐5. [DOI] [PubMed] [Google Scholar]
Riley 2006 {published data only}
- Riley AF, Wakely LA, Patel HY, Neveldsen B, Purdie GL, Wells AP. Use of a cyclo‐oxygenase 2 inhibitor for prophylaxis of cystoid macular oedema following cataract surgery: a randomized placebo‐controlled trial. Clinical and Experimental Ophthalmology 2006;34(4):299‐304. [DOI: 10.1111/j.1442-9071.2006.01213.x] [DOI] [PubMed] [Google Scholar]
Sanders 1982 {published data only}
- Sanders DR, Kraff MC, Peyman GA. Effects of indomethacin on postoperative inflammation after intraocular lens surgery. Afro‐Asian Journal of Ophthalmology 1982;1(4):149‐52. [Google Scholar]
Sellares 1992 {published data only}
- Sellares MT, Maseras M, Roque A, Duch F. Effect of topical indomethacin in prevention of aphakic cystoid macular edema [Efectividad de la indometacina tópica en la prevención del edema macular quístico del afaquico]. Archivos de la Sociedad Española de Oftalmología 1992;62(6):469‐76. [Google Scholar]
Sholiton 1979 {published data only}
- Sholiton DB, Reinhart WJ, Frank KE. Indomethacin as a means of preventing cystoid macular edema following intracapsular cataract extraction. Journal ‐ American Intra‐Ocular Implant Society 1979;5(2):137‐40. [DOI] [PubMed] [Google Scholar]
Tang 2015 {published data only}
- Tang H‐Y, Lu M, Hong D‐M, Ming G‐Y, Zeng Z‐R, Ling H‐J. Effect and safety of intrachamberal triamcinolone acetonide injection during cataract surgery in diabetic patients. International Eye Science 2015;15(3):474‐7. [DOI: ] [Google Scholar]
Wolf 2007 {published data only}
- Wolf EJ, Braunstein A, Shih C, Braunstein RE. Incidence of visually significant pseudophakic macular edema after uneventful phacoemulsification in patients treated with nepafenac. Journal of Cataract and Refractive Surgery 2007;33(9):1546‐9. [DOI] [PubMed] [Google Scholar]
Yamaaki 1984 {published data only}
- Yamaaki H, Hendrikse F, Deutman AF. Iris angiography after cataract extraction and the effect of indomethacin eyedrops. Ophthalmologica 1984;188(2):82‐6. [DOI] [PubMed] [Google Scholar]
Yilmaz 2012 {published data only}
- Yilmaz T, Cordero‐Coma M, Gallagher MJ. Ketorolac therapy for the prevention of acute pseudophakic cystoid macular edema: a systematic review. Eye 2012;26(2):252‐8. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
CTRI/2009/091/001078 {unpublished data only}
- CTRI/2009/091/001078. Randomised, triple blinded, placebo controlled, clinical trial to compare the effect of Ketorolac tromethamine 0.4% in prophylactically preventing cystoid macula edema following cataract surgery. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=1207 (accessed 5 October 2016).
References to ongoing studies
NCT01694212 {published data only}
- NCT01694212. Effect of perioperative topical diclofenac on intraocular inflammation after cataract surgery and the incidence of postoperative macular edema in patients with diabetic retinopathy. clinicaltrials.gov/ct2/show/NCT01694212 (accessed 5 October 2016).
NCT01774474 {published data only}
- NCT01774474. PRevention of Macular EDema after cataract surgery (PREMED). clinicaltrials.gov/ct2/show/NCT01774474 (accessed 5 October 2016).
NCT02646072 {published data only}
- NCT02646072. The effect of preoperative topical ketorolac 0.45% on aqueous cytokine levels and macular thickness in diabetic and non diabetic patients undergoing cataract surgery. clinicaltrials.gov/ct2/show/NCT02646072 (accessed 5 October 2016).
Additional references
Ahmed 2013
- Ahmed I, Everett A. Cystoid macular edema. In: Yanoff M, Duker JS editor(s). Ophthalmology. 4th Edition. Mosby, 2013:Section 6, page 34. [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ballonzoli 2010
- Ballonzoli L, Bourcier T. Ocular side effects of steroids and other immunosuppressive agents. Therapie 2010;65(2):115‐20. [DOI] [PubMed] [Google Scholar]
Blomquist 2002
- Blomquist PH, Rugwani RM. Visual outcomes after vitreous loss during cataract surgery performed by residents. Journal of Cataract and Refractive Surgery 2002;28(5):847‐52. [DOI] [PubMed] [Google Scholar]
Carnahan 2000
- Carnahan MC, Goldstein DA. Ocular complications of topical, peri‐ocular, and systemic corticosteroids. Current Opinion in Ophthalmology 2000;11(6):478‐83. [DOI] [PubMed] [Google Scholar]
Choi 2005
- Choi J, Buzney SM, Weiter JJ. Cystoid macular edema: current modes of therapy. International Ophthalmology Clinics 2005;45(4):143‐51. [DOI] [PubMed] [Google Scholar]
Covidence 2016 [Computer program]
- Veritas Health Innovation. Covidence systematic review software. Melbourne: Veritas Health Innovation, (accessed prior to 30 September 2016).
Eisenach 2010
- Eisenach JC, Curry R, Tong C, Houle TT, Yaksh TL. Effects of intrathecal ketorolac on human experimental pain. Anesthesiology 2010;112(5):1216‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flach 1987
- Flach AJ, Dolan BJ, Irvine AR. Effectiveness of ketorolac tromethamine 0.5% ophthalmic solution for chronic aphakic and pseudophakic cystoid macular edema. American Journal of Ophthalmology 1987;103(4):479‐86. [DOI] [PubMed] [Google Scholar]
Flach 1988
- Flach AJ, Kraff MC, Sanders DR, Tanenbaum L. The quantitative effect of 0.5% ketorolac tromethamine solution and 0.1% dexamethasone sodium phosphate solution on postsurgical blood‐aqueous barrier. Archives of Ophthalmology 1988;106(4):480‐3. [DOI] [PubMed] [Google Scholar]
Flach 1989
- Flach AJ, Jaffe NS, Akers WA. The effect of ketorolac tromethamine in reducing postoperative inflammation: double‐mask parallel comparison with dexamethasone. Annals of Ophthalmology 1989;21(11):407‐11. [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Gonzales 2013
- Gonzales JA, Gritz DC, Channa R, Quinto GG, Kim A, Chuck RS. Non‐steroidal anti‐inflammatory drugs versus corticosteroids for controlling inflammation after uncomplicated cataract surgery. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD010516] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hee 1995
- Hee MR, Puliafito CA, Wong D, Duker JS, Reichel E, Rutledge B, et al. Quantitative assessment of macular edema with optical coherence tomography. Acta Ophthalmologica 1995;113(8):1019‐29. [DOI] [PubMed] [Google Scholar]
Heier 1999
- Heier J, Cheetham JK, Degryse R, Dirks MS, Caldwell DR, Silverstone DE, et al. Ketorolac tromethamine 0.5% ophthalmic solution in the treatment of moderate to severe ocular inflammation after cataract surgery: a randomized, vehicle‐controlled clinical trial. American Journal of Ophthalmology 1999;127(3):253‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jain 2001
- Jain R, Stevens JD, Bunce CV, Garrett C, Hykin PG. Ischaemic heart disease may predispose to pseudophakic cystoid macular oedema. Eye 2001;15(Pt 1):34‐8. [DOI] [PubMed] [Google Scholar]
Jaycock 2009
- Jaycock P, Johnston RL, Taylor H, Adams M, Tole DM, Galloway P, et al. The national cataract dataset electronic multi‐centre audit of 55,567 operations: updating the benchmark standards of care in the UK and internationally. Eye 2009;23(1):38‐49. [DOI] [PubMed] [Google Scholar]
Kessel 2014
- Kessel L, Tendal B, Jorgensen KJ, Erngaard D, Flesner P, Andresen JL, et al. Post‐cataract prevention of inflammation and macular edema by steroid and nonsteroidal anti‐inflammatory eye drops: a systematic review. Ophthalmology 2014;121:1915‐24. [DOI] [PubMed] [Google Scholar]
Kim 2015
- Kim SJ, Schoenberger SD, Thorne JE, Ehlers JP, Yeh S, Bakri SJ. Topical nonsteroidal anti‐inflammatory drugs and cataract surgery: a report by the American Academy of Ophthalmology. Ophthalmology 2015;122:2159‐68. [DOI] [PubMed] [Google Scholar]
Kim 2016
- Kim SJ, Jampel H. Prevention of cystoid macular edema after cataract surgery in non‐diabetic and diabetic patients: A systematic review and meta‐analysis. American Journal of Ophthalmology 2016;161:221‐2. [DOI] [PubMed] [Google Scholar]
Kim 2016a
- Kim SJ, Patel SN, Sternberg P Jr. Routine use of nonsteroidal anti‐inflammatory drugs with corticosteroids in cataract surgery: Beneficial or redundant?. Ophthalmology 2016;123(3):444‐6. [DOI] [PubMed] [Google Scholar]
Kraff 1990
- Kraff MC, Sanders DR, McGuigan L, Raanan MG. Inhibition of blood‐aqueous humor barrier breakdown with diclofenac. A fluorophotometric study. Archives of Ophthalmology 1990;108(3):380‐3. [DOI] [PubMed] [Google Scholar]
Miyake 1977
- Miyake K. Prevention of cystoid macular edema after lens extraction by topical indomethacin (I). A preliminary report. Albrecht von Graefes Archiv fur Klinische und Experimentelle Ophthalmologie 1977;203:81‐8. [DOI] [PubMed] [Google Scholar]
Miyake 1984
- Miyake K. Indomethacin in the treatment of postoperative cystoid macular edema. Survey of Ophthalmology 1984;28(Suppl):554‐68. [DOI] [PubMed] [Google Scholar]
Nelson 2003
- Nelson MS, Martidis A. Managing cystoid macular edema after cataract surgery. Current Opinion in Ophthalmology 2003;14(1):39‐43. [DOI] [PubMed] [Google Scholar]
Pascolini 2012
- Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. British Journal of Ophthalmology 2012;96(5):614‐8. [DOI] [PubMed] [Google Scholar]
Polanski 1992
- Polansky JR. Side effects of topical therapy with anti‐inflammatory steroids. Current Opinion in Ophthalmology 1992;3(2):259‐72. [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rossetti 1998
- Rossetti L, Chaudhuri J, Dickersin K. Medical prophylaxis and treatment of cystoid macular edema after cataract surgery. The results of a meta‐analysis. Ophthalmology 1998;105(3):397‐405. [DOI] [PubMed] [Google Scholar]
Sanders 1984
- Sanders DR, Kraff M. Steroidal and nonsteroidal anti‐inflammatory agents. Effect on postsurgical inflammation and blood‐aqueous humor barrier breakdown. Archives of Ophthalmology 1984;102(10):1453‐6. [DOI] [PubMed] [Google Scholar]
Sivaprasad 2004
- Sivaprasad S, Bunce C, Patel N. Non‐steroidal anti‐inflammatory agents for treating cystoid macular oedema following cataract surgery. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004239.pub2] [DOI] [PubMed] [Google Scholar]
Solomon 2001
- Solomon KD, Cheetham JK, DeGryse R, Brint SF, Rosenthal A. Topical ketorolac tromethamine 0.5% ophthalmic solution in ocular inflammation after cataract surgery. Ophthalmology 2001;108(2):331‐7. [DOI] [PubMed] [Google Scholar]
Spaide 1993
- Spaide RF, Yannuzzi LA, Sisco LJ. Chronic cystoid macular edema and predictors of visual acuity. Ophthalmic Surgery 1993;24(4):262‐7. [PubMed] [Google Scholar]
Ursell 1999
- Ursell PG, Spalton DJ, Whitcup SM, Nussenblatt RB. Cystoid macular edema after phacoemulsification: relationship to blood‐aqueous barrier damage and visual acuity. Journal of Cataract and Refractive Surgery 1999;25(11):1492‐7. [DOI] [PubMed] [Google Scholar]
Warren 2008
- Warren KA, Fox JE. Topical nepenafac as an alternate treatment for cystoid macular edema in steroid responsive patients. Retina 2008;28(10):1427‐34. [DOI] [PubMed] [Google Scholar]
Warren 2010
- Warren K, Bahrani H, Fox JE. NSAIDs in combination therapy for the treatment of chronic pseudophakic cystoid macular edema. Retina 2010;30(2):260‐6. [DOI] [PubMed] [Google Scholar]
Wielders 2015
- Wielders LH, Lambermont VA, Schouten JS, Biggelaar FJ, Worthy G, Simons RW, et al. Prevention of cystoid macular edema after cataract surgery in nondiabetic and diabetic patients: a systematic review and meta‐analysis. American Journal of Ophthalmology 2015;160:968‐81. [DOI] [PubMed] [Google Scholar]
Yannuzzi 1995
- Yannuzzi LA, Guyer DR, Green WR. The Retina Atlas. 1st Edition. Elsevier Health Sciences, 1995. [Google Scholar]
References to other published versions of this review
Abeysiri 2011
- Abeysiri P, Wormald R, Bunce C. Prophylactic non‐steroidal anti‐inflammatory agents for the prevention of cystoid macular oedema after cataract surgery. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD006683.pub2] [DOI] [Google Scholar]
Goh 2007
- Goh D, Lim N. Prophylactic non‐steroidal anti‐inflammatory agents for the prevention of cystoid macular oedema after cataract surgery. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD006683] [DOI] [Google Scholar]


