Abstract
Background
It is generally accepted that taxanes are among the most active chemotherapy agents in the management of metastatic breast cancer. This is an update of a Cochrane review first published in 2003.
Objectives
The objective of this review was to compare taxane‐containing chemotherapy regimens with regimens not containing a taxane in the management of women with metastatic breast cancer.
Search methods
In this review update, we searched the Cochrane Breast Cancer Group Specialised Register, MEDLINE, EMBASE, the World Health Organization's International Clinical Trials Registry Platform (WHO ICTRP), and ClinicalTrials.gov on 14 February 2013 using keywords such as 'advanced breast cancer' and 'chemotherapy'. We searched reference lists of articles, contacted study authors, and did not apply any language restrictions.
Selection criteria
Randomised controlled trials comparing taxane‐containing chemotherapy regimens to regimens without taxanes in women with metastatic breast cancer. We included published and unpublished studies.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. We derived hazard ratios (HRs) for overall survival, time to progression, and time to treatment failure where possible, and used a fixed‐effect model for meta‐analysis. We represented objective tumour response rates and toxicity as risk ratios (RRs). We extracted quality of life data where present.
Main results
This review included 28 studies. The updated analysis included 6871 randomised women, while the original review had 3643 women. Of the 28 included studies, we considered 19 studies to be at low risk of bias overall; however, some studies failed to report details on allocation concealment and methods of outcome assessment for those outcomes that are more likely to be influenced by a lack of blinding (for example tumour response rate). Studies varied in the taxane‐containing chemotherapy backbone, and the comparator arms and were categorised into three groups: Regimen A plus taxane versus Regimen A (2 studies); Regimen A plus taxane versus Regimen B (14 studies); and single‐agent taxane versus Regimen C (13 studies). Thirteen studies used paclitaxel, 14 studies used docetaxel, and 1 study allowed the investigator to decide on the type of taxane; the majority of studies delivered a taxane every 3 weeks. Twenty studies administered taxanes as first‐line treatment, and 21 studies involved anthracycline naïve women in the metastatic setting. The combined HR for overall survival and time to progression favoured the taxane‐containing regimens (HR 0.93, 95% confidence interval (CI) 0.88 to 0.99, P = 0.002, deaths = 4477; and HR 0.92, 95% CI 0.87 to 0.97, P = 0.002, estimated 5122 events, respectively) with moderate to substantial heterogeneity across trials. If the analyses were restricted to studies of first‐line chemotherapy, this effect persisted for overall survival (HR 0.93, 95% CI 0.87 to 0.99, P = 0.03) but not for time to progression (HR 0.96, 95% CI 0.90 to 1.02, P = 0.22). Tumour response rates appeared to be better with taxane‐containing chemotherapy in assessable women (RR 1.20, 95% CI 1.14 to 1.27, P < 0.00001) with substantial heterogeneity across studies. Taxanes were associated with an increased risk of neurotoxicity (RR 4.84, 95% CI 3.18 to 7.35, P < 0.00001, 24 studies) and hair loss (RR 2.37, 95% CI 1.45 to 3.87, P = 0.0006, 11 studies) but less nausea/vomiting compared to non‐taxane‐containing regimens (RR 0.62, 95% CI 0.46 to 0.83, P = 0.001, 26 studies). Leukopaenia and treatment‐related death did not differ between the two groups (RR 1.07, 95% CI 0.97 to 1.17, P = 0.16, 28 studies; and RR 1.00, 95% CI 0.63 to 1.57, P = 0.99, 23 studies, respectively). For quality of life measures, none of the individual studies reported a difference in overall or any of quality of life subscales between taxane‐containing and non‐taxane chemotherapy regimens.
Authors' conclusions
Taxane‐containing regimens appear to improve overall survival, time to progression, and tumour response rate in women with metastatic breast cancer. Taxanes are also associated with an increased risk of neurotoxicity but less nausea and vomiting compared to non‐taxane‐containing regimens. The considerable heterogeneity encountered across studies probably reflects the varying efficacy of the comparator regimens used in these studies and indicates that taxane‐containing regimens are more effective than some, but not all, non‐taxane‐containing regimens.
Keywords: Female; Humans; Antineoplastic Agents, Hormonal; Antineoplastic Agents, Hormonal/therapeutic use; Antineoplastic Agents, Phytogenic; Antineoplastic Agents, Phytogenic/therapeutic use; Breast Neoplasms; Breast Neoplasms/drug therapy; Breast Neoplasms/mortality; Breast Neoplasms/pathology; Bridged‐Ring Compounds; Bridged‐Ring Compounds/therapeutic use; Disease Progression; Paclitaxel; Paclitaxel/therapeutic use; Randomized Controlled Trials as Topic; Tamoxifen; Tamoxifen/therapeutic use; Taxoids; Taxoids/therapeutic use
Plain language summary
Taxane‐containing regimens for metastatic breast cancer
Review question
We reviewed the evidence about the effect of taxane‐containing chemotherapy regimens in women with metastatic breast cancer. This is an update of a Cochrane review first published in 2003.
Background
Treatment for women with metastatic breast cancer (that is, cancer that has spread beyond the breast) usually involves chemotherapy to try to shrink or slow the growth of the cancer. Chemotherapy can involve a single drug or a combination of drugs. Paclitaxel and docetaxel are chemotherapy drugs known as taxanes. Taxanes can inhibit cancer cells from dividing and reproducing, and their adverse effects can include nausea, vomiting, and hair loss, as well as allergic reactions, which can be reduced by premedication. We wanted to examine whether or not taxane‐containing chemotherapy improves survival and extends time to disease progression in women with metastatic breast cancer.
Study characteristics
The evidence is current to February 2013. We included 28 studies that randomised 6871 women. Women were assigned to receive either a taxane‐containing chemotherapy regimen (single taxane or in combination with other chemotherapy drugs) or a non‐taxane chemotherapy regimen. There were variations in the taxane‐containing chemotherapy regimen and the non‐taxane treatments. Approximately half of the studies used paclitaxel and the other half used docetaxel, and in the majority of cases, taxanes were administered every three weeks. Of the 28 studies, 20 studies included women who received taxanes as their first treatment after their diagnosis of metastatic breast cancer, and 21 studies involved women who had not been previously treated with anthracyclines in the metastatic setting. From those studies reporting median follow‐up, this ranged from 9 months to 69 months.
Key results
This review showed that chemotherapy regimens including taxanes improved survival and decreased the progression of metastatic breast cancer. If the analyses were restricted to those studies where women received taxanes as their first treatment after their diagnosis of metastatic breast cancer, the survival benefit persisted. Taxanes also appeared to cause tumours to shrink more than chemotherapy regimens without taxanes. However, there were differences in side effects. The risk of experiencing neurotoxicity (tingling of hands and feet) with taxanes increased compared to non‐taxane chemotherapy. Hair loss also seemed to be more likely with taxane than with non‐taxane‐containing regimens. However, less nausea/vomiting was observed with taxanes. There was no difference in the rates of leukopaenia (low white blood cells) or treatment‐related deaths between taxane and non‐taxane chemotherapy. Of the studies that reported quality of life measures, there did not appear to be any differences (overall or on subscales) in quality of life between the two groups.
Quality of the evidence
We considered 19 out of the 28 studies to be at low risk of bias overall. However, some studies failed to report details on concealing drug treatments and methods of outcome assessment for those outcomes more likely to be at risk of bias (for example tumour response rate). The degree of variability seen across the included studies probably reflects the varying efficacy of the non‐taxane chemotherapy regimens used in these studies and indicates that taxane‐containing chemotherapies are more effective than some, but not all, non‐taxane‐containing regimens.
Background
Description of the condition
Breast cancer is the most common type of cancer in women, with more cases being diagnosed in less developed compared to more developed regions (Ferlay 2015). It is the most common cause of cancer death among women in less developed regions and the second most frequent cause of cancer death in more developed regions (Ferlay 2015). In 2012 there were an estimated 1.67 million new cases and approximately 522,000 deaths from breast cancer worldwide; an age standardised death rate of 12.9 (per 100,000) (Ferlay 2015).
The stage of breast cancer at the time of diagnosis is an important indicator of prognosis. Once breast cancer becomes metastatic it is rarely curable, with reported median survival of 18 to 24 months from the time of recurrence, although some women do experience long‐term survival (Hayes 1995; NCI 2003). Although there is no randomised evidence comparing chemotherapy with observation in women with metastatic breast cancer, it is widely accepted that women with metastatic disease should receive some form of systemic therapy at some time during the course of their disease.
Description of the intervention
Chemotherapy is considered by many to be the appropriate first treatment option for women with multiple sites of recurrence or where visceral disease is not easily treated by local modalities (Beslija 2009; Hayes 1995; NCI 2003). Chemotherapy is also considered to be useful in women whose cancer is hormone refractory, or expected to be hormone resistant (Hortobagyi 1996).
It is generally accepted that taxanes are among the most active chemotherapy agents in the management of metastatic breast cancer. The term 'taxanes' describes a group of drugs used in the treatment of cancer, specifically paclitaxel (Taxol® Bristol‐Myers Squibb) and docetaxel (Taxotere® Rhone‐Poulenc Rorer). The first taxane, paclitaxel, was identified in 1971 as part of a National Cancer Institute program that screened medicinal plants for potential anti‐cancer activity. It was originally isolated from the bark of the Pacific yew tree (Taxus brevifolia, native to western North America), but now a semisynthetic form is derived from the needles and twigs of the more common Himalayan or European yew (Taxus bacatta). Paclitaxel was first used in clinical trials in 1983 (BMS 1996). Docetaxel was first synthesised in 1986 and is similar, although not identical, to paclitaxel in its mechanism of action.
How the intervention might work
Taxanes are unique as they affect cell structures known as microtubules (or spindle fibres). In normal cell growth, microtubules are formed when a cell starts dividing and when the cell stops dividing, the microtubules are broken down or destroyed. Taxanes work by blocking the microtubules from breaking down. Cancer cells then become blocked with microtubules and stop dividing hence potentially slowing the growth of the cancer or killing the cells. The known side effects of paclitaxel include hypersensitivity reactions (such as shortness of breath or skin rash), myelosuppression (neutropaenia), peripheral neuropathy, cardiac rhythm disturbances, joint or muscle pain, diarrhoea, nausea and vomiting, or hair loss. Patients often receive premedication before receiving taxanes to prevent possible allergic reactions. The side‐effect profile of docetaxel is similar to that of paclitaxel, although docetaxel causes less neuropathy and more myelotoxicity (Vasey 2001).
Why it is important to do this review
In the previous version of this review, the primary aim was to assess taxane use in the first‐line setting, but the follow‐up data were insufficient for this to be adequately assessed. In this review update, data on an additional 3000 participants were available, with time‐to‐event data for 87% of the participants randomised, compared to 57% in the original review. Only one other systematic review and meta‐analysis appears to have been published, in 2008, but the focus was on taxanes alone or in combination with anthracyclines (refer to Piccart‐Gebhart 2008). The Piccart‐Gebhart 2008 review included first‐line therapy using individual participant data but did not conduct assessments of trial conduct and reporting or drug side effects. An update of the efficacy and safety of taxanes overall and in the first‐line setting in the form of an updated Cochrane review seemed warranted given the availability of mature follow‐up data and new trial data.
Objectives
The original review was conducted as part of a series of reviews comparing more intense (or more active) chemotherapy with less intense (or less active) chemotherapy in women with advanced (metastatic) breast cancer.
The objective of this review and review update was to compare taxane‐containing chemotherapy regimens with regimens not containing a taxane in the management of women with metastatic breast cancer. Subquestions within the review were:
subquestion A: regimen A plus taxane versus regimen A (e.g. doxorubicin plus docetaxel versus doxorubicin alone)
subquestion B: regimen A plus taxane versus regimen B (e.g. doxorubicin plus docetaxel versus doxorubicin plus cyclophosphamide)
subquestion C: single‐agent taxane versus regimen C (e.g. docetaxel versus doxorubicin plus cyclophosphamide)
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Women with advanced (metastatic) breast cancer, either newly diagnosed or recurrent. Trials that included both women with metastatic disease and women with locoregionally recurrent disease were only eligible for inclusion if it was possible to distinguish between the two groups (that is the data were reported separately) or if women with isolated locoregional recurrence were less than 20% of the total group. We applied no age restrictions.
In the protocol for this review we proposed to include trials in which the women randomised to receive chemotherapy were receiving first‐line treatment (that is no previous chemotherapy given except as adjuvant therapy). In the original review, as very few completed trials involved first‐line treatment, all trials meeting the remaining eligibility criteria were included in the review. Results were presented separately for all trials (that is all lines) and first‐line only. In the 2013 review update, the number of first‐line trials increased, but for completeness, we still reported all lines and analysed as per the original review.
Types of interventions
Intervention group: Any chemotherapy regimen containing a taxane.
Comparator: Any chemotherapy regimen not containing a taxane.
Participants may also have received endocrine therapy if the study planned to give it to both treatment groups.
Trials may or may not have specified recommended treatment upon disease progression/initial treatment failure. This treatment may have included cross‐over to the alternative treatment arm of the trial. We did not include trials where the primary intention was to investigate sequencing of treatment regimens, including, for example:
trials where participants received a given number of cycles of one regimen, followed by a given number of cycles of another regimen (randomisation being to which regimen commenced first);
trials where regimens were alternated (e.g. one cycle of regimen A followed by one cycle of regimen B followed by a second cycle of regimen A, etc.).
Types of outcome measures
Primary outcomes
1. Overall survival 2. Time to progression
Secondary outcomes
3. Time to treatment failure 4. Objective tumour response rate 5. Toxicity 6. Health related quality of life
For the purpose of this review, the following outcome definitions apply: 1. Overall survival: time from date randomised to date of death (any cause). 2. Time to progression: time from date randomised to date of progression or death (any cause). May also be referred to as progression‐free survival. 3. Time to treatment failure: time from date randomised to date of progression, death (any cause), withdrawal due to adverse event, participant refusal, or further anticancer therapy for documented progression. 4. Objective tumour response rate: the proportion of participants with a complete or partial response.
This review also attempted to investigate treatment‐related death which, for the purpose of this review, was defined as death due to the toxicity of the drug and not to disease progression. If an individual trial did not include the definition used by that trial but used the terms "toxic death" or "lethal toxicity", then we included the information in the review.
Search methods for identification of studies
Electronic searches
For the review update, we searched the following databases or registries.
Cochrane Breast Cancer Group (CBCG) Specialised Register on 14 February 2013. Details of the search strategy applied by the Group to create the register, and the procedure used to code references, are described in the Group's module on the Cochrane Library. The register includes both published and unpublished (including ongoing) trials. The CBCG codes 'advanced' and 'chemotherapy' were applied to the specialised register and combined with the keywords (imported with the references from MEDLINE) 'Taxol', 'docetaxel', or 'paclitaxel', and a search of all non‐indexed fields for the following text words: taxane, taxanes, taxol, taxotere, paclitaxel, paxene, nsc‐125973, docetaxel, or anzatax.
MEDLINE (via OvidSP) from 2008 to February 2013, see Appendix 1
EMBASE (via Embase.com) from 2008 to 14 February 2013, see Appendix 2
World Health Organization International Clinical Trials Registry Platform search portal (http://apps.who.int/trialsearch/) for all prospectively registered and ongoing trials on 14 February 2013, see Appendix 3
ClinicalTrials.gov register (http://clinicaltrials.gov/ct2/search) on 14 February 2013 for additional unpublished and ongoing studies, see Appendix 4
Searching other resources
We also searched the reference lists of other related literature reviews. In the original Cochrane Review, the systematic reviews searched included Fossati 1998 and Stockler 2000 as well as review articles identified by the search strategy. In the 2013 review update, we screened the references in the systematic review by Piccart‐Gebhart 2008.
We obtained a copy of the full article for each reference reporting a potentially eligible trial. In the 2013 review update, the review authors contacted the trial authors to provide additional information if data were available in abstract form only.
Data collection and analysis
Selection of studies
In the original review and 2013 review update, two review authors (original review: DG, ED; 2013 review update: MC, MW) applied the selection criteria to each reference identified by the search strategy. A third review author (NW) resolved any discrepancies regarding eligibility. We recorded studies deemed ineligible in the 'Characteristics of excluded studies' table. Articles in languages other than English were translated where required. Only one study required translation, from Hungarian into English (Szanto 2001).
Data extraction and management
In the original review and 2013 review update, two review authors (original review: DG, ED; 2013 review update: MC, MW) independently extracted the data and resolved queries through discussion with a third review author (NW), National Health and Medical Research Council Clinical Trial Centre statisticians, and the CBCG's Statistical Editor. We extracted data on study accrual, randomisation methods, participants' baseline characteristics (that is age, first‐line/second‐line, prior anthracyclines/anthracycline naïve), chemotherapy regimens (number of cycles and duration), outcome definitions, follow‐up, and analyses conducted. We collected multiple publications on the same study and assigned the most complete report (that is the one with the outcomes most relevant to the review or the most recent outcomes) as the primary reference.
Assessment of risk of bias in included studies
We used The Cochrane Collaboration's 'Risk of bias' assessment tool to assess potential sources of bias in the included studies (Higgins 2011). In the 2013 review update, two review authors (MC, MW) independently assessed the potential risk of bias for each study; any differences in judgement were resolved through discussion. The domains assessed were random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. We assigned ratings of 'high', 'low', or 'unclear' risk of bias to each domain for each included study following the criteria outlined in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For phase III oncology studies, open‐label studies are common owing to difficulty in concealing different chemotherapy schedules, toxicities, etc. The blinding of outcome assessment domain was therefore lumped into those outcome measures most unlikely or most likely to be influenced by a lack of blinding. The outcomes were segregated into (a) overall survival (b) progression‐free survival, time to treatment failure, response rates, and toxicity and (c) quality of life.
Measures of treatment effect
We analysed overall survival, progression‐free survival, and time to treatment failure as time‐to‐event outcomes for which the hazard ratio (HR) is the most appropriate statistic. When possible, the HR and associated variances were extracted directly from the trial publication(s). If it was not reported, we obtained it indirectly employing the methods described by Tierney et al using either other available summary statistics (Tierney 2007), or from data extracted from published Kaplan‐Meier curves (Parmar 1998; Tierney 2007). In studies that did not report the relevant effect estimates and required curve extraction, we adjusted the numbers at risk based on estimated minimum and maximum follow‐up times. If these were not reported in any of the available reports, we estimated minimum follow‐up using the estimated time taken to complete treatment, and estimated maximum follow‐up using the last event reported in the relevant time‐to‐event curve (as per methods in Tierney 2007). We have recorded these follow‐up estimates in the 'Characteristics of included studies' table under 'Notes'. A HR less than 1.0 favoured regimens containing taxanes.
We analysed response rates as dichotomous variables (complete or partial versus stable disease or no response) and derived a pooled risk ratio (RR) with 95% confidence interval (CI). As trialists usually report RRs for both randomised and assessable participants, the same was done in the original and updated review. A RR larger than 1.0 favoured regimens containing taxanes.
We analysed toxicity data as dichotomous outcomes and added up the total number of grade 3 and 4 events and number at risk across trials. In the original review, a single odds ratio (with 95% CIs) was calculated in assessable and randomised participants. In the 2013 review update, we calculated the total number of grade 3 and 4 events and the number of assessable participants in each treatment arm and derived a pooled RR with 95% CI. The denominator was the number of assessable (not randomised) participants to ensure that toxicity outcomes were only measured in those participants who actually received the treatment. In those studies where the number assessable was not provided, we used the number of participants randomised to each treatment group. We have outlined any deviations from assessable number of participants as the denominator in the 'Characteristics of included studies' 'Notes' section. We extracted the total number of toxic events for treatment‐related death, leukopaenia, nausea or vomiting, neurotoxicity, and alopecia. If grade 3/4 nausea and vomiting were reported separately, we used data for vomiting.
We collected quality of life data using a variety of instruments across trials. These data were not statistically synthesised but were summarised and evaluated qualitatively.
Unit of analysis issues
Three studies were three‐arm trials (ECOG E1193: split into ECOG E1193 (A) and ECOG E1193 (B); JCOG9802; Rugo). The three treatment regimens in the ECOG E1193 study were eligible for both subquestions A (ECOG E1193 (A)) and C (ECOG E1193 (B)). This was taken into account when the overall effect of taxanes was calculated by halving the control group each time the trial was used (which was twice). In JCOG9802, data from two arms were used. We excluded the alternating treatment regimen of doxorubicin plus cyclophosphamide versus alternating doxorubicin plus cyclophosphamide and docetaxel. We included the treatment comparison doxorubicin plus cyclophosphamide versus docetaxel. In Rugo, there was one experimental arm (that is taxane‐containing regimen) and two control arms (ixabepilone plus bevacizumab, with different schedules for both drugs). In this case, the treatment arm where participants were randomised to receive bevacizumab 10 mg/kg every 14 days was the most appropriate control comparator arm for the taxane‐containing arm. We did not include the third control arm in this analysis.
Dealing with missing data
In the original review, no attempt had been made to contact most trial investigators for additional information. Many trials were in active follow‐up and others were still recruiting participants. The UK Medical Research Council had been contacted in relation to the UKCCCR AB01 trial, but additional information on this trial was not yet available at that time. Aventis was also contacted regarding the Nabholtz trial, but further details were not yet available.
In the 2013 review update, we contacted a number of trialists to obtain time‐to‐event data and clarification on whether or not analyses had been adjusted in the trial publication. The following trial reports provided additional information, or, in some cases, unpublished manuscripts: Blohmer, EU‐93011, JCOG9802, Lyman, TOG, and Yardley. We have provided further details of the data obtained in the 'Characteristics of included studies' table 'Notes' section.
Assessment of heterogeneity
We used the Chi2 test and the I2 statistic to test for heterogeneity over all trials, as well as visual inspection of forest plots (Higgins 2011). For the Chi2 test, a P value of 0.10 indicated evidence of heterogeneity. We used the I2 statistic as a rough guide to assess heterogeneity: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and 75% to 100% considerable heterogeneity. We evaluated the value of the I2 statistic alongside the magnitude and direction of effects, and the P value for the Chi2 test (Higgins 2011).
When appropriate, we used a fixed‐effect model for the primary analysis. We considered and discussed heterogeneity between results in the Discussion section of the review and tested where appropriate (see Subgroup analysis and investigation of heterogeneity and Sensitivity analysis sections).
Assessment of reporting biases
We assessed reporting bias using Cochrane's 'Risk of bias' tool (Higgins 2011). We used trial registers (WHO ICTRP and ClinicalTrials.gov) and published protocols (where available) to cross‐check the reporting of outcomes in the trial publications.
Data synthesis
For time‐to‐event outcome data, we obtained a pooled HR from the derived observed (O) ‐ expected (E) number of events and the variance for each trial using the fixed‐effect model (Yusuf 1985). The pooled HR represents the overall risk of an event on taxane‐containing chemotherapy versus non‐taxane‐containing chemotherapy.
For objective tumour response rates and treatment‐related death, we obtained a pooled RR using the fixed‐effect model (Mantel‐Haenszel analysis).
For leukopaenia, nausea/vomiting, neurotoxicity, and alopecia, we obtained a pooled RR using the random‐effects model (Mantel‐Haenszel analysis).
We have narratively described and presented quality of life results in Table 1.
1. Quality of life (QoL).
| Trial ID | Instruments used | Summary of findings |
| 303 Study Group | Participants completed EORTC QLQ‐C30 and physicians completed KPS | 80% of assessable participants completed QoL assessments in both groups for the first 4 cycles, but was higher in the docetaxel group from cycle 6. There was no statistically significant difference between the 2 groups in mean decreases in global health and physical functioning scores from baseline |
| 304 Study Group | Participants completed EORTC QLQ‐C30 | 72% of questionnaires returned for docetaxel and 68% for MV for baseline and cycle 2, but deteriorated to 59% for docetaxel and 61% for MV by cycle 8. Attrition higher in MV compared to docetaxel, and did not occur at random. Significantly higher proportion of participants in MV discontinued treatment due to deterioration in condition; trial authors concluded that participants in the poorest health did not complete QoL questionnaires, hence QoL may be underestimated in both groups. Groups similar at baseline for global health, physical functioning, and symptoms except for role functioning and diarrhoea (imbalance in favour of docetaxel). Results: No significant difference in global health status. Significant difference in favour of docetaxel for nausea/vomiting and loss of appetite, and in favour of MV for role and social functioning |
| 306 Study Group | Participants completed EORTC QLQ‐C30 and QLQ‐BR23 (Breast cancer module) 3 days before first infusion then before every alternate cycle and at each visit during follow‐up until progression; and physicians completed KPS | Overall compliance was high through to cycle 6 (> 70%), then decreased during follow‐up (< 30%), although rates in both groups comparable. At cycle 8, more data were missing in AC group than in AT group. Baseline scores were comparable and remained constant during the study. There was no significant difference between groups in global health status/QoL score |
| ANZ TITG | Participants completed linear analog scales, and physicians completed Spitzer Quality of Life Index | Most QoL measures (physical well‐being, mood, nausea and vomiting, appetite, overall quality of life, and physician‐rated quality of life) were slightly better in the taxane arm. The exception is pain which was slightly better in the non‐taxane arm. Differences were not statistically significant |
| ECOG E1193: ECOG E1193 (A) and ECOG E1193 (B) | Participants completed FACT‐B | 93% (687/738) of randomised participants and 94% (640/683) of eligible participants completed the baseline survey. 70% (451/683) of eligible participants completed the survey at week 16. There was no statistically significant difference in overall QoL score or in any of the subscales between any of the treatment groups |
| EORTC 10923 | Participants completed EORTC QLQ‐C30 and Rotterdam Symptom Checklist | 64% of randomised participants completed baseline EORTC QLQ‐C30 and 61% completed baseline Rotterdam Symptom Checklist. QoL comparisons were only performed for the first 3 cycles. There was no difference in global health status/QoL between the 2 groups. Doxorubicin was associated with significantly more nausea/vomiting, loss of appetite, and burden of disease and treatment, but less bone pain and rash than paclitaxel |
| EORTC 10961 | Participants completed EORTC QLQ‐C30 and QLQ‐BR23 (Breast cancer module) | 79% of participants completed the baseline questionnaire. Overall compliance (over 4 assessments) was 66%. There was no significant difference in health‐related QoL between the 2 treatment groups |
| Jassem | Participants completed EORTC QLQ‐C30 and QLQ‐BR23 (Breast cancer module) | 81% of questionnaires returned for AT patients and 77% for FAC patients (throughout study and follow‐up), although compliance deteriorated over time. Information on non‐compliers not reported. No statistically significant differences in changes from baseline in functional scales for role, emotional, cognitive, social, global health status, body image, sexual enjoyment, or future perspective. Significant difference in favour of FAC for physical and sexual functioning scales, pain, fatigue, insomnia, and diarrhoea. Significant difference in favour of AT for nausea and vomiting. There was no significant difference in other symptoms. |
| JCOG9802 | Participants completed FACT‐B | 99% of the first 150 participants (i.e. 148/150) returned completed questionnaires at baseline, 89% at 6 weeks, and 87% at 18 weeks. There was no statistically significant difference between the 2 treatment arms of interest (for this review) at baseline, 6 weeks, or 18 weeks |
| Meier | Participants completed EORTC QLQ‐C30 | 102/120 participants completed QoL questionnaires. There was no significant difference between treatment groups at baseline. Compliance declined (54% at cycle 4), thereby making QoL comparisons difficult. A non‐significant trend for better scores in participants continuing on original docetaxel treatment was noted |
| Sjostrom | Participants completed EORTC QLQ‐C30 | 82% of questionnaires were returned over the entire study (overall compliance). Physical deterioration was greater in the methotrexate + fluorouracil group, hence possible bias in its favour. No statistically significant difference at baseline or by cycle 4 in any functional or symptom scale. No significant difference in median values of mean changes in QoL scores from baseline to cycle 6. |
| UKCCCR AB01 | Participants completed FACT‐B | Abstract available in 2001 reported that QoL was "similar for both arms during treatment". No other results were available in the 2001 abstract or full trial publication in 2005 |
FAC: 5‐fluorouracil, doxorubicin, cyclophosphamide FACT‐B: Functional Assessment of Cancer Therapy ‐ Breast KPS: Karnofsky Performance Status MV: mitomycin C/vinblastine
We performed all analyses using Review Manager software (RevMan) in accordance with the Cochrane Handbook for Systematic Reviews of Interventions.
Subgroup analysis and investigation of heterogeneity
Several subgroup analyses had been pre‐specified (refer to Table 2). In the original review and the 2013 review update, the following subgroup analyses were possible:
2. Possible subgroups.
| Question | Subgroups |
| Subgroups within Question B: |
|
| Subgroups within Question C: |
|
type of taxane (docetaxel/paclitaxel)
prior exposure to anthracyclines
single‐agent taxane versus single‐agent anthracycline, and single‐agent taxane versus non‐anthracycline combination
We applied Chi2 tests for interaction to these subgroup analyses.
Sensitivity analysis
In this review update, we conducted a sensitivity analysis to assess the impact of high or unclear risk of bias on the primary outcomes overall survival and time to progression. Each study was categorised overall as having low, unclear or high risk of bias based on assessing each risk of bias domain. If the majority of the eight or nine domains (that is, those studies reporting quality of life measures) were considered at unclear or high risk of bias, the study was assessed as being at risk of bias.
Results
Description of studies
Results of the search
In the review update, searching the Cochrane Breast Cancer Group Specialised Register, MEDLINE, and EMBASE on the 14 February 2013 yielded 1077 records. Searching the WHO ICTRP and ClinicalTrials.gov on 14 February 2013 retrieved eight potential ongoing studies. After removing duplicates, we screened the titles and abstracts of the remaining 903 records for review inclusion. Of these, we discarded 855 records and further assessed 48 records relating to full‐text articles or ongoing trial records. After full‐text review, we excluded nine records, with reasons provided in the 'Characteristics of excluded studies' table.
Of the remaining 39 records, 18 records related to 12 new studies (Blohmer; CECOG BM1; EU‐93011; HERNATA; JCOG9802; Lyman; Meier; Rugo; TRAVIOTA; Yardley; TIPP; Xu), 6 records related to updated data for 6 previously included studies (Bonneterre; Bontenbal; EORTC 10961; Jassem; TOG; UKCCCR AB01), 4 records were supplementary records of 3 previously included studies (AGO; EORTC 10923; Nabholtz), and 11 records were classified as 'ongoing' studies (EUCTR2012‐003530‐16‐ES; EUCTR2012‐003743‐30‐SE; ISRCTN97330959; JPRN‐C000000416; NCT00321633; NCT00490646; NCT00600340; NCT01126138; NCT01303679; NTR1349; Pegram).
The original Cochrane review identified 21 eligible studies: 18 included studies and 3 ongoing studies (refer to Ghersi 2003). When we combined studies from the original review and the review update, there were 41 eligible studies involving 28 included studies (referring to 29 treatment comparisons), 2 studies awaiting classification, and 11 ongoing studies (refer to the PRISMA flowchart, Figure 1). The PRISMA flowchart for the original review can be found in the previously published version of this review (Ghersi 2003).
1.

Review update: study flow diagram.
Since the publication of the original review, two studies categorised as ongoing studies have become included studies (CECOG BM1; EU‐93011), while one previously eligible study that was withdrawn by outcome in the original review, due to being written in Hungarian, has now been transferred to the excluded studies list (Szanto 2001). The Szanto article was translated in 2012 and reported results from one trial site from the international study referred to as the 306 Study Group in this review.
Included studies
Question A: regimen A plus taxane versus regimen A
Two included studies, ECOG E1193 (A) and EU‐93011, and two ongoing studies, NTR1349 and SAKK, addressed question A. The taxane used in ECOG E1193 (A) was paclitaxel, while EU‐93011 used docetaxel. Both ECOG E1193 (A) and EU‐93011 recruited anthracycline naïve women receiving first‐line chemotherapy for metastatic breast cancer.
Question B: regimen A plus taxane versus regimen B
Fourteen included studies (306 Study Group; AGO; Blohmer; Bonneterre; Bontenbal; CECOG BM1; EORTC 10961; HERNATA; Jassem; Lyman; Nabholtz; Rugo; TRAVIOTA; UKCCCR AB01), one study awaiting classification (Xu), and five potential ongoing studies (NCT00490646; NCT00600340; NCT01126138; NCT01303679; Xu) addressed question B. All 14 studies recruited women who were receiving first‐line chemotherapy for metastatic breast cancer, and the majority of participants in all of these trials were anthracycline naïve in the metastatic setting. Paclitaxel was the taxane used in seven studies (AGO; CECOG BM1; EORTC 10961; Jassem; Lyman; Rugo; UKCCCR AB01), and docetaxel was the taxane used in six studies (306 Study Group; Blohmer; Bonneterre; Bontenbal; HERNATA; Nabholtz). In the TRAVIOTA study, women were randomised to receive taxane therapy, which could be paclitaxel or docetaxel, at the investigator's choice. We categorised the Xu study as awaiting classification while we sought further details on the data presented in the trial publication from the trialists.
Question C: single‐agent taxane versus regimen C
Thirteen included studies (303 Study Group; 304 Study Group; ANZ TITG; Dieras; ECOG E1193 (B); EORTC 10923; JCOG9802; Meier; Sjostrom; Talbot; TOG; TXT; Yardley), one study awaiting classification (TIPP), and six potential ongoing studies (EUCTR2012‐003530‐16‐ES; EUCTR2012‐003743‐30‐SE; ISRCTN97330959; JPRN‐C000000416; NCT00321633; Pegram) addressed question C. Paclitaxel was used in six studies (ANZ TITG; Dieras; ECOG E1193 (B); EORTC 10923; Talbot; TOG), and docetaxel was used in seven studies (303 Study Group; 304 Study Group; JCOG9802; Meier; Sjostrom; TXT; Yardley). The majority of participants in 5 of the 13 included studies received first‐line chemotherapy (ANZ TITG; ECOG E1193 (B); EORTC 10923; JCOG9802; Yardley), and 6 of the 13 studies were anthracycline naïve (303 Study Group; ANZ TITG; ECOG E1193 (B); EORTC 10923; JCOG9802; Yardley). We categorised the TIPP study as awaiting classification because we could not use the data in its present abstract form.
In this review update, there were 28 included studies containing 29 treatment comparisons: 2 for question A, 14 for question B, and 13 for question C. Of the 28 included studies, 26 were fully published in peer‐reviewed journals (303 Study Group; 304 Study Group; 306 Study Group; ANZ TITG; Blohmer; Bonneterre; Bontenbal; CECOG BM1; Dieras; ECOG: ECOG E1193 (A) and ECOG E1193 (B); EORTC 10923; EORTC 10961; HERNATA; Jassem; JCOG9802; Lyman; Meier; Nabholtz; Rugo; Sjostrom; Talbot; TOG; TRAVIOTA; TXT; UKCCCR AB01; Yardley), 2 had been reported only in abstract form (AGO; Nabholtz); and 1 study was provided as an unpublished manuscript from the trialists (EU‐93011).
Thirteen studies reported time to progression (or similar definition) as the primary outcome (303 Study Group; 304 Study Group; 306 Study Group; Blohmer; EORTC 10923; EORTC 10961; EU‐93011; HERNATA; Jassem; Meier; TOG; TXT; UKCCCR AB01). In the 2013 review update, we combined studies using slight variations on the 'time to progression' definition in the same analysis (refer to Table 3). Time to progression data from the TXT study has therefore been added in this review update where it had been previously excluded in the original review. Seven studies reported objective tumour response rate as the primary outcome (Bonneterre; Bontenbal; EORTC 10923; Rugo; Talbot; TRAVIOTA; Yardley). Two studies had two primary outcomes: EU‐93011: time to progression and overall survival; and EORTC 10923: time to progression and objective response rate. Eight studies did not make a distinction between primary and secondary outcomes (AGO; ANZ TITG; CECOG BM1; Dieras; ECOG E1193: ECOG E1193 (A) and ECOG E1193 (B); Lyman; Nabholtz; Sjostrom).
3. Definitions of time to progression.
| Study | Variation in definitions and reporting of 'time to progression' used in 2013 Cochrane review update |
| 303 Study Group | TTP: date randomised to date of progression or death |
| 304 Study Group | TTP: date randomised to date of progression or death |
| 306 Study Group | TTP: date randomised to date of first progression |
| AGO | PFS: no definition provided in the abstract. Data for this outcome were not included in the review due to an inadequate amount of information presented in the abstract |
| ANZ TITG | PFS: date randomised to date of progression or death without progression |
| Blohmer | TTP: time from registration until disease progression |
| Bonneterre | TTP: not defined in the trial publication |
| Bontenbal | TTP: date of random assignment to the date of progression, death, or withdrawal |
| CECOG BM1 | TTP: dates of randomisation until disease progression or death, whichever occurred first |
| Dieras | TTP: not defined in trial publication |
| ECOG E1193: ECOG E1193 (A) and ECOG E1193 (B) | TTF: date randomised to date of progression, toxic death, death attributed to breast cancer within 6 weeks of date last known alive with stable disease |
| EORTC 10923 | PFS: date randomised to date of progression or death if it occurred before documentation of progressive disease |
| EORTC 10961 | PFS: randomisation to date of progression or death or whichever occurred first |
| EU‐93011 | TTP: i.e. progression‐free survival, the duration from randomisation until progressive disease, death, or last contact. Data presented for this outcome was incomplete (i.e. the number of events was not provided in the manuscript) |
| HERNATA | TTP: date of randomisation to date of documented progression with censoring for participants alive at last visit/date of death |
| Jassem | TTP: not defined in trial publication |
| JCOG9802 | PFS: date of randomisation to the date of the first documentation of disease progression or death from any cause |
| Meier | TTP: not defined in trial publication. Inadequate information presented in the trial publication to allow accurate data extraction. Trial authors were contacted for additional information |
| Nabholtz | TTP: no definition provided in the abstract. Data for this outcome were not included in the review due to an inadequate amount of information presented in the abstract |
| Rugo | PFS: time from randomisation to disease progression or death |
| Sjostrom | TTP: date randomised to date of progression or death or last follow‐up visit |
| Talbot | TTP: interval between first day of treatment and first recording of disease progression or death. Data for this outcome were not included in the review as limited information in the trial publication owing to premature discontinuation of the trial |
| TOG | TTP: duration between the first day of study treatment and date of progression |
| TRAVIOTA | TTP: defined according to the RECIST criteria. Data for this outcome were not included in the review as we were unable to accurately estimate the length of follow‐up |
| TXT | TTP: time of first treatment infusion to first objective evidence of tumour progression |
| UKCCCR AB01 | PFS: time from random assignment to first appearance of progressive disease or death from any cause |
| Yardley | PFS: interval from first study treatment until the date that the first progression of breast cancer was documented |
PFS: progression‐free survival RECIST: Response Evaluation Criteria in Solid Tumors TTF: time to treatment failure TTP: time to progression
Overall, paclitaxel was used in 13 studies, docetaxel was used in 14 studies, and the investigator could decide which taxane was used in 1 study. Twenty studies included first‐line taxane treatment, and 21 studies administered taxanes in anthracycline naïve women in the metastatic setting. Median follow‐up ranged from 36 weeks to 69 months in studies that reported this information.
Not all of the included studies collected data on all of the outcomes investigated in this review, or reported information on all of the outcomes that would have been expected (owing to immature follow‐up or incomplete presentation of the data in the trial publication). The number of studies with reported and useable information by outcome are as follows.
Overall survival: 22 included studies involving 23 treatment comparisons
Time to progression: 21 included studies involving 22 treatment comparisons
Time to treatment failure: 5 included studies
Response rate: 28 included studies involving 29 treatment comparisons
Toxicity: treatment‐related toxicity: 22 studies; grade 3/4 leukopaenia: 27 studies with 28 treatment comparisons; grade 3/4 nausea/vomiting: 25 studies with 26 treatment comparisons; grade 3/4 neurotoxicity: 23 studies with 24 treatment comparisons; grade 3/4 alopecia: 11 studies
Quality of life: 12 studies with 13 treatment comparisons
We have provided details on trials withdrawn only for certain outcomes in Table 4. The study ECOG E1193, that is ECOG E1193 (A) and ECOG E1193 (B), contributed data towards question A and question C.
4. Included RCTs, withdrawn by outcome, with reasons.
| Trial ID | Outcome | Reason not included |
| 303 Study Group | Toxicity: alopecia | Data reported but not as grade 3 or 4 toxicity, therefore it was not possible to calculate |
| 304 Study Group | Toxicity: alopecia | Reported but no numerical data provided |
| AGO | Time to progression | Inadequate amount of information presented in abstract form; we contacted trialists but received no reply |
| Bonneterre | Time to progression (sensitivity analysis undertaken) | The number of events in each group for time to progression were not provided in the trial publication; individual participant data from the Piccart‐Gebhart 2008 systematic review were used instead |
| CECOG BM1 | Overall survival | The trial publication stated that the data for this outcome are not yet mature |
| Nabholtz | Overall survival, time to progression | Inadequate amount of information presented in abstract form; we contacted trialists but received no reply |
| Rugo | Overall survival | Data for arms A and C (comparable control arm) were immature at the time of analysis |
| Talbot | Overall survival, time to progression | Data for outcome were not provided in the trial publication owing to premature discontinuation of the trial |
| TRAVIOTA | Time to progression, time to treatment failure | Duration of follow‐up not provided in the trial publication, therefore it was not possible to estimate the number of events in the taxane‐containing or non‐taxane‐containing arms, or hazard ratio |
| EU‐93011 | Time to progression, toxicity: alopecia | Time to progression: unpublished manuscript did not provide the number of events in each treatment arm. Alopecia: reported but no numerical data provided |
Excluded studies
We excluded six records from the review update (Brufsky 2012; Gennari 2001; Ghosn 2011; Hamberg 2011; Huang 2011; Sakurai 2007; Schmid 2005); reasons are provided in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
Refer to Figure 2 for a summary of the 'Risk of bias' judgements for each 'Risk of bias' domain of the included studies.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The 28 studies, relating to 29 treatment comparisons, were described as randomised. The method of random sequence generation was described adequately (that is with low risk of bias) in 17 studies (303 Study Group; 304 Study Group; 306 Study Group; ANZ TITG; Blohmer; Bonneterre; Bontenbal; CECOG BM1; Dieras; EORTC 10961; EU‐93011; Jassem; JCOG9802; Rugo; Talbot; TXT; UKCCCR AB01). It was not possible to accurately assess the method of random sequence generation in 11 studies owing to the lack of information presented in the published trial report or abstract. We classified these 11 studies as having an unclear risk of bias: AGO, ECOG E1193 (ECOG E1193 (A) and ECOG E1193 (B)), EORTC 10923, HERNATA, Lyman, Meier, Nabholtz, Sjostrom, TOG, TRAVIOTA, Yardley.
Seventeen of the 28 studies were at low risk of bias for allocation concealment. These studies described central randomisation systems (computer or telephone) as their method for randomisation of treatment assignment (303 Study Group; 304 Study Group; 306 Study Group; ANZ TITG; Blohmer; Bonneterre; Bontenbal; CECOG BM1; Dieras; EORTC 10923; EORTC 10961; EU‐93011; HERNATA; Jassem; JCOG9802; Meier; TOG). The remaining 11 studies did not describe methods of concealment either in the trial publication (ECOG E1193: ECOG E1193 (A) and ECOG E1193 (B); Lyman; Rugo; Sjostrom; Talbot; TRAVIOTA; TXT; UKCCCR AB01; Yardley) or available abstract (AGO; Nabholtz); we judged these studies as having unclear risk of bias.
Blinding
Eleven studies were described as "non‐blinded" or "open‐label" (303 Study Group; 304 Study Group; 306 Study Group; Blohmer; Bonneterre; Bontenbal; Jassem; JCOG9802; Sjostrom; Talbot; TOG). We could not rule out performance bias owing to the lack of blinding of participants and personnel; we judged these 11 studies as at high risk on this domain. We judged the remaining 17 studies as at unclear risk of bias as the information needed to make a firm conclusion about whether or not they were 'open‐label' studies was not presented in the trial publication (ANZ TITG; CECOG BM1; Dieras; ECOG E1193: ECOG E1193 (A) and ECOG E1193 (B); EORTC 10923; EORTC 10961; HERNATA; Lyman; Meier; Rugo; TRAVIOTA; TXT; UKCCCR AB01; Yardley), abstract (AGO; Nabholtz), or unpublished manuscript (EU‐93011).
We assessed detection bias by grouping outcomes with similar risks of bias: (a) overall survival (b) time to progression, time to treatment failure, objective tumour response rate, and toxicity, and (c) quality of life. For overall survival, we perceived a lack of blinding as being unlikely to have an impact on this outcome assessment, therefore we assessed all studies as at low risk of bias. For outcome measures that were more likely to be influenced by a lack of blinding, that is time to progression, objective tumour response rate, and toxicity, we assessed whether outcome assessments were confirmed through imaging and biochemical tests and reviewed by independent panels/adjudication committees (especially for tumour response rates) in each study. We assessed 11 studies to be at low risk of bias due to these outcomes being measured through formal assessments including scans, blood tests, and an independent clinical or radiological review group, or both (303 Study Group; 304 Study Group; 306 Study Group; Bonneterre; Bontenbal; EORTC 10923; Jassem; JCOG9802; TOG; TRAVIOTA; TXT). Seventeen studies provided partial or minimal information on outcome assessments and were therefore classified as having an unclear risk of bias on this domain (AGO; ANZ TITG; Blohmer; CECOG BM1; Dieras; ECOG E1193: ECOG E1193 (A) and ECOG E1193 (B); EORTC 10961; EU‐93011; HERNATA; Lyman; Meier; Nabholtz; Rugo; Sjostrom; Talbot; UKCCCR AB01; Yardley). Quality of life measures were likely to be affected by a lack of blinding. Twelve out of the 28 studies collected data on quality of life completed by participants and in some cases questionnaires completed by physicians (303 Study Group; 304 Study Group; 306 Study Group; ANZ TITG; ECOG E1193: ECOG E1193 (A) and ECOG E1193 (B); EORTC 10923; EORTC 10961; Jassem; JCOG9802; Meier; Sjostrom; UKCCCR AB01); we therefore considered these studies to be at high risk of bias.
Incomplete outcome data
Twenty‐five of the 28 studies outlined that data analyses were conducted according to intention‐to‐treat or provided information, or both for participant exclusions (if these occurred) in their analyses. We judged the following 25 studies as at low risk of bias: 303 Study Group; 304 Study Group; 306 Study Group; ANZ TITG; Blohmer; Bonneterre; Bontenbal; CECOG BM1; Dieras; ECOG E1193: ECOG E1193 (A) and ECOG E1193 (B); EORTC 10923; EORTC 10961; EU‐93011; HERNATA; Jassem; JCOG9802; Lyman; Meier; Rugo; Sjostrom; Talbot; TOG; TXT; UKCCCR AB01; Yardley). We judged three studies as having unclear risk of bias due to no reporting of attrition or exclusions in the abstract (AGO; Nabholtz) or an analysis plan (TRAVIOTA).
Selective reporting
One study, TRAVIOTA, did not report outcome results (that is quality of life data) in the trial publication, yet the clinical trials registration record listed quality of life as a secondary outcome. In two studies, AGO and Nabholtz, results were available only in abstract form, and it was difficult to assess whether selective reporting had occurred; as their most recent abstract publications were in 2000 and 2002, respectively, we ranked these studies as at unclear risk of bias. All other studies had either (i) outcomes listed in the methods section of the trial publication reported in the results section of the same publication (303 Study Group; 304 Study Group; 306 Study Group; ANZ TITG; Blohmer; Bonneterre; Bontenbal; CECOG BM1; Dieras; ECOG E1193: ECOG E1193 (A) and ECOG E1193 (B); EORTC 10923; EORTC 10961; EU‐93011; Jassem; Lyman; Meier; Sjostrom; Talbot; TXT; UKCCCR AB01), or (ii) had a trial registration record with the listed outcomes found in the methods and results section of the trial publication (HERNATA; JCOG9802; Rugo; TOG; Yardley).
Other potential sources of bias
We considered differences in baseline characteristics and trials prematurely stopped due to poor accrual (for example) under this domain. Six studies were prematurely stopped owing to either recruitment issues (Blohmer; EU‐93011; TRAVIOTA; Yardley), the chance of finding a difference in an outcome so low that the data monitoring committee recommended early trial closure (Bontenbal), or results reported from another trial meant the discontinuation of the trial (Talbot). Five studies reported some baseline imbalances or did not provide sufficient information to discount that baseline differences may have influenced results (ANZ TITG; Bonneterre; CECOG BM1; Lyman; Rugo). We therefore classified 11 studies as at unclear risk of bias. We judged the remaining 17 studies as at low risk of bias, as we identified no other biases.
Effects of interventions
It should be noted that 6871 women were randomised to the 28 included studies (involving 29 treatment comparisons), and that time‐to‐event data (that is overall survival and time to progression) were available for 87% of the participants randomised.
All trials included for questions A and B were of first‐line chemotherapy for metastatic breast cancer: a total of 3984 randomised women. Three studies did not report time‐to‐event data (question B: CECOG BM1; Nabholtz; Rugo). All five trials of first‐line chemotherapy eligible for question C reported time‐to‐event data.
Readers can refer to Figure 3 when interpreting the plots, particularly given the variety of regimens used in the control group.
3.

Summary of chemotherapy regimens used in the included studies
One study was a three‐armed trial eligible for both questions A and C (ECOG E1193 (A)). This was taken into account when the overall effect of taxanes was calculated (by halving the control group each time the trial was used (which was twice)). We labelled the plots for the overall effect of taxane‐containing regimens versus non‐taxane‐containing regimens 'Overall effect of taxanes' for overall survival, time to progression, objective tumour response rate and toxicity.
Overall survival
Overall effect
Data from 22 studies (23 treatment comparisons) of the 26 studies reporting on overall survival were available to enable a hazard ratio (HR) calculation for overall survival for taxane‐containing versus non‐taxane containing regimens. There were an estimated 4477 deaths in 6008 women randomised. There was a statistically significant improvement in overall survival in favour of taxane‐containing regimens with a HR of 0.93 (95% confidence interval (CI) 0.88 to 0.99; P = 0.002; participants = 6008; treatment comparisons = 23; Analysis 1.1; Figure 4). There was moderate heterogeneity across trials (I2 = 52%; P = 0.002).
1.1. Analysis.

Comparison 1 Overall Survival, Outcome 1 Overall effect: Taxane‐containing regimens vs. not.
4.
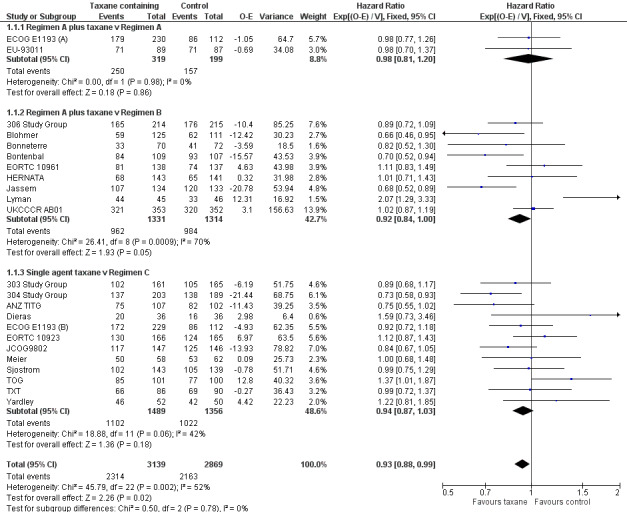
Forest plot of comparison: 1 Overall Survival, outcome: 1.1 Overall effect: Taxane‐containing regimens vs. not.
First‐line trials only (overall)
If we limited the analysis to the 15 studies (16 treatment comparisons; involving an estimated 3352 deaths in 4439 women) using first‐line chemotherapy for metastatic breast cancer, the difference remained statistically significant (HR 0.93; 95% CI 0.87 to 0.99; P = 0.03; participants = 4439; treatment comparisons = 16; Analysis 1.2). There was moderate heterogeneity across the trials (I2 = 55%; P = 0 .004).
1.2. Analysis.

Comparison 1 Overall Survival, Outcome 2 First‐line trials only: overall.
Subquestions: types of regimens
Question A: regimen A plus taxane versus regimen A
Two included studies provided information on survival (ECOG E1193 (A); EU‐93011). There were 493 deaths in 630 women randomised. The HR was 1.00 (95% CI 0.84 to 1.18; P = 0.97; Analysis 1.3), and there was no significant heterogeneity (I2 = 0%; P = 0.91).
1.3. Analysis.

Comparison 1 Overall Survival, Outcome 3 Subquestions A, B & C.
Question B: regimen A plus taxane versus regimen B
Nine studies provided adequate information on survival (306 Study Group; Blohmer; Bonneterre; Bontenbal; EORTC 10961; HERNATA; Jassem; Lyman; UKCCCR AB01). There were 1946 deaths in 2645 women randomised. The HR was 0.92 (95% CI 0.84 to 1.00; P = 0.05; Analysis 1.3), and there was substantial heterogeneity (I2 = 70%; P = 0.0009).
Question C: single‐agent taxane versus regimen C
Twelve studies provided sufficient information on survival (303 Study Group; 304 Study Group; ANZ TITG; Dieras; ECOG E1193 (B); EORTC 10923; JCOG9802; Meier; Sjostrom; TOG; TXT; Yardley). There were 2210 deaths in 2957 women randomised. The HR was 0.95 (95% CI 0.87 to 1.03; P = 0.19; Analysis 1.3), and there was moderate heterogeneity (I2 = 42%; P = 0.06). Variability in the efficacy of the comparator is of potential concern in this subquestion. If we excluded the three trials with potentially suboptimal comparators (mitomycin, vinblastine, and fluorouracil with vinorelbine: 304 Study Group;Dieras; TXT), there remains no benefit for taxane‐containing regimens (HR 0.97; 95% CI 0.89 to 1.07; P = 0.55) and moderate heterogeneity (I2 = 34%; P = 0.14).
Single taxane versus single anthracycline:
Four studies comparing single‐agent taxane with single‐agent anthracycline (involving an estimated 900 deaths in 1212 women randomised) were available to enable us to calculate a HR for overall survival (303 Study Group; ECOG E1193 (B); EORTC 10923; Yardley). There was no difference in favour of either regimen with a HR of 1.02 (95% CI 0.90 to 1.16; P = 0.72; Analysis 1.4). There was no significant heterogeneity (I2 = 0%; P = 0.52).
1.4. Analysis.

Comparison 1 Overall Survival, Outcome 4 Chemotherapy regimens.
Single taxane versus non‐anthracycline combination:
Sufficient data from all eight studies comparing single‐agent taxane with a non‐anthracycline‐containing regimen (involving an estimated 1208 deaths in 1736 women randomised) were available to enable us to calculate a HR for overall survival (304 Study Group; ANZ TITG; Dieras; HERNATA; Meier; Sjostrom; TOG; TXT). There was no detectable difference in overall survival with a HR of 0.94 (95% CI 0.84 to 1.06; P = 0.31; Analysis 1.4), and there was significant heterogeneity across these trials (I2 = 52%; P = 0.04).
Type of taxane
We conducted post‐hoc subgroup analyses to investigate the treatment effect within the types of taxane (paclitaxel or docetaxel). Nine studies (10 treatment comparisons) used paclitaxel, and there were 2232 deaths in 2834 women (ANZ TITG; Dieras; ECOG E1193 (A); ECOG E1193 (B); EORTC 10923; EORTC 10961; Jassem; Lyman; TOG; UKCCCR AB01). There was no detectable difference between the paclitaxel‐containing versus non‐taxane‐containing regimens for overall survival with a HR of 1.01 (95% CI 0.93 to 1.10; P = 0.84; Analysis 1.5). There was significant heterogeneity (I2 = 67%; P = 0.001) for this outcome across studies.
1.5. Analysis.

Comparison 1 Overall Survival, Outcome 5 Type of taxane.
Thirteen studies used docetaxel in the taxane‐containing arm, and there were 2245 deaths in 3174 women randomised (303 Study Group; 304 Study Group; 306 Study Group; Blohmer; Bonneterre; Bontenbal; EU‐93011; HERNATA; JCOG9802; Meier; Sjostrom; TXT; Yardley). There was a statistically significant difference in favour of docetaxel‐containing regimens compared to non‐taxane‐containing regimens for overall survival. The HR was 0.87 (95% CI 0.80 to 0.94; P = 0.0008; Analysis 1.5), and there was minimal heterogeneity across studies (I2 = 2%; P = 0.43).
Although the test for differences between type of taxane subgroups was statistically significant (P = 0.01), this was considered weak evidence given the variability in the comparator arms and taxane schedules (weekly versus three weekly) in these studies.
Prior anthracyclines
We conducted post‐hoc subgroup analyses to investigate the treatment effect in women who had or had not received previous anthracyclines for advanced disease. Six studies included women who had received prior anthracyclines, and there were 918 deaths in 1243 women (304 Study Group; Dieras; Meier; Sjostrom; TOG; TXT). There was no detectable difference between taxane‐containing and non‐taxane‐containing regimens for overall survival (HR 0.97; 95% CI 0.85 to 1.11; P = 0.66; Analysis 1.6), and there was significant heterogeneity for this outcome across trials (I2 = 58%; P = 0.04).
1.6. Analysis.

Comparison 1 Overall Survival, Outcome 6 Prior anthracyclines.
Sixteen studies (17 treatment comparisons) included women with no prior anthracyclines in the advanced setting, and there were 3359 deaths in 4765 women (303 Study Group; 306 Study Group; ANZ TITG; Blohmer; Bonneterre; Bontenbal; ECOG E1193 (A); ECOG E1193 (B); EORTC 10923; EORTC 10961; EU‐93011; HERNATA; Jassem; JCOG9802; Lyman; UKCCCR AB01; Yardley). There was a significance in favour of taxane‐containing regimens for overall survival (HR 0.93; 95% CI 0.87 to 0.99; P = 0.02; Analysis 1.6), but there was significant heterogeneity (I2 = 52%; P = 0.007).
A test of differences between prior and no prior exposure to anthracyclines revealed no significant interaction (P = 0.51).
Time to progression
Overall effect
Data from 21 studies (22 treatment comparisons) reporting on time to progression (involving an estimated 5122 events in 5960 women) were available to enable us to calculate a HR for taxane‐containing versus non‐taxane‐containing regimens. Six studies did not provide adequate information to calculate HRs (AGO; EU‐93011; Meier; Nabholtz; Talbot; TRAVIOTA).
There was a statistically significant difference in favour of taxane‐containing regimens with a HR of 0.92 (95% CI 0.87 to 0.97; P = 0.002; participants = 5960; treatment comparisons = 22; Analysis 2.1; Figure 5), but there was significant heterogeneity across trials (I2 = 73%; P < 0.00001). We did a sensitivity analysis by removing Bonneterre (that is the study where only individual participant data were available for time to progression from a published meta‐analysis by Piccart‐Gebhart 2008), which showed that the benefit in favour of taxane‐containing regimens persisted (HR 0.92; 95% CI 0.87 to 0.97; P = 0.002).
2.1. Analysis.

Comparison 2 Time to Progression, Outcome 1 Overall effect: Taxane‐containing regimens vs not.
5.

Forest plot of comparison: 2 Time to Progression, outcome: 2.1 Overall effect: Taxane‐containing regimens vs not.
First‐line trials only (overall)
If the analysis was limited to the 15 studies (16 treatment comparisons) in women receiving first‐line chemotherapy for metastatic breast cancer, the difference was no longer statistically significant (HR 0.96; 95% CI 0.90 to 1.02; P = 0.22; Analysis 2.2), and there was substantial heterogeneity (I2 = 62%; P = 0.0005).
2.2. Analysis.

Comparison 2 Time to Progression, Outcome 2 First‐line trials only: overall.
Subquestions: type of regimens
Question A: regimen A plus taxane versus regimen A
One study provided adequate information on time to progression (ECOG E1193 (A)). Three hundred and forty women progressed out of 454 randomised, and there was no detectable difference between the use of chemotherapy with or without the addition of a taxane (HR 0.99; 95% CI 0.81 to 1.21; P = 0.94; Analysis 2.3).
2.3. Analysis.

Comparison 2 Time to Progression, Outcome 3 Subquestions A, B & C.
Question B: regimen A plus taxane versus regimen B
Ten studies provided adequate information on time to progression (306 Study Group; Blohmer; Bonneterre; Bontenbal; CECOG BM1; EORTC 10961; HERNATA; Jassem; Rugo; UKCCCR AB01) and 2422 women progressed out of 2891 randomised. Data suggested a benefit in terms of time to progression in favour of taxanes with a HR of 0.90 (95% CI 0.83 to 0.98; P = 0.01; Analysis 2.3). There was moderate heterogeneity (I2 = 45%; P = 0.06). We did a sensitivity analysis by removing Bonneterre (that is the study where only individual participant data were available for time to progression from a published meta‐analysis by Piccart‐Gebhart 2008), which did not affect the benefit in favour of taxanes for time to progression (HR 0.90; 95% CI 0.83 to 0.98; P = 0.01). Similarly, by omitting CECOG BM1 (that is the one study where the chemotherapy backbone in the taxane arm was not the same in the comparator arm), the benefit in favour of the taxane‐containing regimen persisted (HR 0.91; 95% CI 0.83 to 0.98; P = 0.02).
Question C: Single‐agent taxane versus regimen C
Ten studies involving 11 treatment comparisons provided adequate information on time to progression (303 Study Group; 304 Study Group; ANZ TITG; Dieras; ECOG E1193 (B); EORTC 10923; JCOG9802; Sjostrom; TOG; TXT; Yardley) and 2431 women progressed out of 2839 randomised. The HR was 0.93 (95% CI 0.86 to 1.00; P = 0.05; Analysis 2.3) with substantial heterogeneity (I2 = 84%; P < 0.00001) across trials. If we excluded the three trials with potentially suboptimal comparators (mitomycin, vinblastine, and fluorouracil with vinorelbine: 304 Study Group;Dieras; TXT), the HR was 1.00 (95% CI 0.91 to 1.09) with substantial heterogeneity persisting (I2 = 85%; P < 0.00001). If the analysis was limited to the five trials in women receiving first‐line chemotherapy for metastatic breast cancer (ANZ TITG; Dieras; ECOG E1193 (B); EORTC 10923; JCOG9802; Yardley), there was no detectable difference between taxane‐containing and non‐taxane‐containing regimens (HR 1.03; 95% CI 0.93 to 1.14; P = 0.59; Analysis 2.4) with substantial heterogeneity (I2 = 80%; P = 0.0004).
2.4. Analysis.

Comparison 2 Time to Progression, Outcome 4 Subquestions A, B & C: first‐line only.
Single taxane versus single anthracycline:
Four studies comparing single‐agent taxane with single‐agent anthracycline (involving an estimated 1000 women who had progressed out of 1212 randomised) were available to enable us to calculate a HR for progression‐free survival (303 Study Group; ECOG E1193 (B); EORTC 10923; Yardley). There was no difference in time to progression between the two arms (HR 1.08; 95% CI 0.96 to 1.22; P = 0.20; Analysis 2.5) with substantial heterogeneity (I2 = 80%; P = 0.002).
2.5. Analysis.

Comparison 2 Time to Progression, Outcome 5 Chemotherapy Regimens.
Single taxane versus non‐anthracycline combination:
Seven studies comparing single taxane versus non‐anthracycline regimen, involving an estimated 1333 women who had progressed out of 1618 randomised, were available (304 Study Group; ANZ TITG; Dieras; HERNATA; Sjostrom; TOG; TXT). There was a statistically significant difference in favour of taxane‐containing regimens with a HR of 0.85 (95% CI 0.76 to 0.94; P = 0.002; Analysis 2.5) with substantial heterogeneity (I2 = 84%; P < 0.00001).
Type of taxane
Ten studies involving 11 treatment comparisons used paclitaxel, and 2679 women progressed out of 3080 randomised (ANZ TITG; CECOG BM1; Dieras; ECOG E1193 (A); ECOG E1193 (B); EORTC 10923; EORTC 10961; Jassem; Rugo; TOG; UKCCCR AB01). There was no significant difference between paclitaxel‐containing versus non‐taxane‐containing regimens (HR 1.04; CI 0.96 to 1.12; P = 0.32; Analysis 2.6) with substantial heterogeneity (I2 = 73%; P < 0.0001).
2.6. Analysis.

Comparison 2 Time to Progression, Outcome 6 Type of taxane.
Eleven studies used docetaxel in the taxane‐containing regimen, and 2348 women progressed out of 2880 randomised (303 Study Group; 304 Study Group; 306 Study Group; Blohmer; Bonneterre; Bontenbal; HERNATA; JCOG9802; Sjostrom; TXT; Yardley). There was a significant difference in favour of docetaxel‐containing regimens (HR 0.80; 95% CI 0.74 to 0.86; P < 0.00001; Analysis 2.6) with moderate heterogeneity across studies (I2= 48%; P = 0.04).
There was a significant interaction between subgroups for time to progression, suggesting that the effect of taxanes is greater in studies randomising women to docetaxel than to paclitaxel (P < 0.00001) for this outcome. However, there was significant and substantial heterogeneity (I2 = 95.5%; P < 0.00001) in both docetaxel and paclitaxel studies, and variability may relate to the differences in the comparator arms and taxane schedule (that is weekly versus three weekly) in these studies.
Prior anthracyclines
Five studies included women who had had prior anthracyclines in the advanced setting, and 940 women progressed out of 1125 randomised (304 Study Group; Dieras; Sjostrom; TOG; TXT). There was a detectable difference between taxane‐containing and non‐taxane‐containing regimens for time to progression (HR 0.76; 95% CI 0.67 to 0.86; P < 0.0001; Analysis 2.7) with moderate heterogeneity (I2 = 85%; P < 0.0001).
2.7. Analysis.

Comparison 2 Time to Progression, Outcome 7 Prior anthracyclines.
Sixteen studies (17 treatment comparisons) included anthracycline‐naive women, and there were 4087 progression‐free survival events out of 4835 randomised (303 Study Group; 306 Study Group; ANZ TITG; Blohmer; Bonneterre; Bontenbal; CECOG BM1; ECOG: ECOG E1193 (A) and ECOG E1193 (B); EORTC 10923; EORTC 10961; HERNATA; Jassem; JCOG9802; Rugo; UKCCCR AB01; Yardley). There was no detectable difference for time to progression (HR 0.96; 95% CI 0.90 to 1.02; P = 0.16; Analysis 2.7) and moderate heterogeneity (I2 = 60%; P = 0.0009).
There was significant heterogeneity between subgroups for time to progression, suggesting the effect of taxanes is greater in studies randomising women who had prior anthracyclines (P = 0.001).
Time to treatment failure
Overall effect
Five studies reported on time to treatment failure, two addressing subquestion B, that is 306 Study Group and HERNATA, and three addressing subquestion C (303 Study Group; 304 Study Group; JCOG9802). Although ECOG E1193 (ECOG E1193 (A) and ECOG E1193 (B)) reported this outcome, the definition of failure used in the study was more aligned with progression‐free survival (as defined by this review). Data suggested a benefit in favour of taxanes with a HR of 0.90 (95% CI 0.82 to 0.98; P = 0.02; participants = 1724; studies = 5; Analysis 3.1). There was substantial heterogeneity (I2 = 91%; P < 0.00001).
3.1. Analysis.

Comparison 3 Time to Treatment Failure, Outcome 1 Subquestions A, B & C.
First‐line trials only (overall)
When we restricted analysis to the three first‐line studies (that is 306 Study Group; HERNATA; JCOG9802), this difference was no longer statistically different (HR 1.01; 95% CI 0.89 to 1.13; P = 0.92).
Objective tumour response rate
Overall effect
Data from all 28 included studies involving 29 treatment comparisons were available to enable us to calculate a risk ratio (RR) for response rate. It is recognised that there are some differences in the definition of response across (but not within) trials. There was a significant difference in favour of taxane‐containing regimens with an RR of 1.20 (95% CI 1.14 to 1.27; P < 0.00001; Analysis 4.1: assessable participants; Figure 6). There was substantial heterogeneity across trials (I2 = 69%; P < 0.00001). We observed the same result based on randomised women (Analysis 4.2).
4.1. Analysis.

Comparison 4 Overall Response Rate, Outcome 1 Overall effect: assessable patients.
6.

Forest plot of comparison: 4 Overall Response Rate, outcome: 4.1 Overall effect: assessable patients.
4.2. Analysis.

Comparison 4 Overall Response Rate, Outcome 2 Overall effect: randomised patients.
First‐line trials only (overall)
If we limited the analysis to the 20 studies (21 treatment comparisons) of first‐line treatment involving a total of 5512 assessable women, the difference persisted in favour of taxane‐containing regimens (RR 1.16; 95% CI 1.10 to 1.23; P < 0.00001; Analysis 4.3). However, there was substantial heterogeneity (I2 = 63%; P < 0.0001). This result was reproduced for women randomised (Analysis 4.4).
4.3. Analysis.

Comparison 4 Overall Response Rate, Outcome 3 First‐line trials only: assessable patients.
4.4. Analysis.

Comparison 4 Overall Response Rate, Outcome 4 Overall effect: randomised patients ‐ firstline only.
Subquestions: type of regimens
Question A: regimen A plus taxane versus regimen A
Two studies, ECOG E1193 (A) and EU‐93011, reported on 627 assessable participants and suggested a difference in favour of taxanes (RR 1.47; 95% CI 1.21 to 1.79; P = 0.0001; Analysis 4.5). There was substantial heterogeneity (I2 = 77%; P = 0.04). We observed a similar result using women randomised (Analysis 4.6).
4.5. Analysis.

Comparison 4 Overall Response Rate, Outcome 5 Subquestions A, B & C: assessable patients.
4.6. Analysis.

Comparison 4 Overall Response Rate, Outcome 6 Subquestions A, B & C: randomised patients.
Question B: regimen A plus taxane versus regimen B
Fourteen studies involving 3740 assessable participants provided data on response rate (306 Study Group; AGO; Blohmer; Bonneterre; Bontenbal; CECOG BM1; EORTC 10961; HERNATA; Jassem; Lyman; Nabholtz; Rugo; TRAVIOTA; UKCCCR AB01). There was a statistically significant difference in favour of taxane‐containing regimens with an RR of 1.19 (95% CI 1.12 to 1.26; P < 0.00001; Analysis 4.5). However, there was a moderate level of heterogeneity (I2 = 49%; P = 0.02). This result was reproduced for women randomised (Analysis 4.6). A sensitivity analysis done by omitting CECOG BM1 (that is the one study where the chemotherapy backbone in the taxane arm was not the same in the comparator arm) did not affect the benefit of taxanes for objective tumour response rate (RR 1.19; 95% CI 1.12 to 1.27; P < 0.00001).
Question C: single‐agent taxane versus regimen C
Twelve studies, 13 treatment comparisons, involving 2856 assessable participants, provided data on response rate (303 Study Group; 304 Study Group; ANZ TITG; Dieras; ECOG E1193 (B); EORTC 10923; JCOG9802; Meier; Sjostrom; Talbot; TOG; TXT; Yardley). There was a statistically significant difference in favour of taxanes with an RR of 1.14 (95% CI 1.03 to 1.27; P = 0.01; Analysis 4.5). There was substantial heterogeneity across trials (I2 = 78%; P < 0.00001). We observed a similar result using women randomised (Analysis 4.6).
When we restricted the analysis to first‐line chemotherapy (that is five studies: ANZ TITG; ECOG E1193 (B); EORTC 10923; JCOG9802; Yardley), the difference was no longer present with a RR of 0.90 (95% CI 0.77 to 1.05; P = 0.18; Analysis 4.7) with substantial heterogeneity (I2 = 62%; P = 0.03). We observed a similar result using women randomised (Analysis 4.8).
4.7. Analysis.

Comparison 4 Overall Response Rate, Outcome 7 Subquestions A, B & C: assessable patients ‐ first‐line only.
4.8. Analysis.

Comparison 4 Overall Response Rate, Outcome 8 Subquestions A, B & C: randomised patients ‐ firstline only.
Type of taxane
Thirteen studies involving 14 treatment comparisons used a paclitaxel‐containing regimen (AGO; ANZ TITG; CECOG BM1; Dieras; ECOG E1193: ECOG E1193 (A) and ECOG E1193 (B); EORTC 10923; EORTC 10961; Jassem; Lyman; Rugo; Talbot; TOG; UKCCCR AB01). There was no detectable difference between the paclitaxel‐containing and non‐taxane‐containing regimens (RR 1.06; 95% CI 0.99 to 1.14; P = 0.12; Analysis 4.9) with moderate heterogeneity (I2 = 57%; P = 0.004).
4.9. Analysis.

Comparison 4 Overall Response Rate, Outcome 9 Type of taxane: assessable patients.
Fourteen studies used a docetaxel‐containing regimen (303 Study Group; 304 Study Group; 306 Study Group; Blohmer; Bonneterre; Bontenbal; EU‐93011; HERNATA; JCOG9802; Meier; Nabholtz; Sjostrom; TXT; Yardley). There was a significant difference in favour of docetaxel‐containing regimens (RR 1.40; 95% CI 1.29 to 1.51; P < 0.00001; Analysis 4.9) with substantial heterogeneity (I2 = 63%; P = 0.0008).
There was a significant interaction between subgroups for response rate, suggesting that the effect of taxanes is greater in studies randomising women to docetaxel than to paclitaxel (P < 0.00001). However, caution is required in considering this result owing to the variability in the control arms and taxane schedules (that is weekly versus three weekly) in these studies.
Prior anthracyclines
Seven studies included women who had prior anthracyclines in the advanced setting (304 Study Group; Dieras; Meier; Sjostrom; Talbot; TOG; TXT). There was a detectable difference between taxane‐containing and non‐taxane containing regimens for response rate (RR 1.43; 95% CI 1.20 to 1.72; P < 0.0001; Analysis 4.10) with substantial heterogeneity (I2 = 79%; P < 0.0001).
4.10. Analysis.

Comparison 4 Overall Response Rate, Outcome 10 Prior anthracyclines: assessable patients.
Twenty‐one studies (22 treatment comparisons) included anthracycline‐naive women (303 Study Group; 306 Study Group; AGO; ANZ TITG; Blohmer; Bonneterre; Bontenbal; CECOG BM1; ECOG E1193 (A); ECOG E1193 (B); EORTC 10923; EORTC 10961; EU‐93011; HERNATA; Jassem; JCOG9802; Lyman; Nabholtz; Rugo; TRAVIOTA; UKCCCR AB01; Yardley). Taxane‐containing regimens were associated with a higher objective tumour response rate compared to non‐taxane‐containing regimens with a RR of 1.17 (95% CI 1.11 to 1.24; P < 0.00001; Analysis 4.10), but there was substantial heterogeneity (I2 = 64%; P < 0.0001).
A test for interaction between subgroups (that is prior use versus naive) for objective tumour response rate was significant (P = 0.04).
Toxicity
Treatment‐related death
Twenty‐two studies reported on treatment‐related deaths (303 Study Group; 304 Study Group; 306 Study Group; Bonneterre; Bontenbal; CECOG BM1; Dieras; EORTC 10923; EORTC 10961; HERNATA; Jassem; JCOG9802; Lyman; Meier; Nabholtz; Rugo; Sjostrom; Talbot; TOG; TXT; UKCCCR AB01; Yardley). Sixty‐six treatment‐related deaths were reported: 33 on taxane‐containing regimens and 33 on non‐taxane‐containing regimens in an estimated 5517 women (assessable). There was no statistically significant difference between taxane‐containing and non‐taxane‐containing regimens (RR 1.00; 95% CI 0.63 to 1.57; P = 0.99; Analysis 5.1). No heterogeneity was present (I2 = 0%; P = 0.75).
5.1. Analysis.

Comparison 5 Toxicity, Outcome 1 Treatment‐related death: overall effect.
Grade 3/4 leukopaenia
Overall effect
Data from 27 studies (involving 28 treatment comparisons) were available for this outcome. Only one study, UKCCCR AB01, did not collect such data. Overall, there was no difference in the risk of leukopaenia (RR 1.07; 95% CI 0.97 to 1.17; P = 0.16; participants = 6564; Analysis 5.2) with significant heterogeneity across the studies (I2 = 90%; P < 0.00001).
5.2. Analysis.

Comparison 5 Toxicity, Outcome 2 Leukopaenia: overall effect.
Subquestions: type of regimens
Question A: regimen A plus taxane versus regimen A
Two studies provided data on leukopaenia. The taxane‐containing regimen was associated with an increased risk of leukopaenia (RR 1.76; 95% CI 1.11 to 2.80; P = 0.02; participants = 624), and there was substantial heterogeneity (I2 = 89%; P = 0.003).
Question B: regimen A plus taxane versus regimen B
Thirteen out of the 14 studies collected data on leukopaenia. The taxane‐containing regimen was associated with an increased risk of leukopaenia (RR 1.11; 95% CI 1.02 to 1.20; P = 0.01; participants = 3209) but substantial heterogeneity (I2 = 74%; P < 0.00001).
Question C: single‐agent taxane versus regimen C
All 13 studies provided data on leukopaenia. There was no difference in the risk for leukopaenia (RR 1.07; 95% CI 0.86 to 1.34; P = 0.55; participants = 2955) and substantial heterogeneity (I2 = 95%; P < 0.00001).
Grade 3/4 nausea or vomiting
Overall effect
Data from 25 studies (involving 26 treatment comparisons) were available for this outcome. Only two studies for subquestion B, AGO and Nabholtz, and one study for subquestion C, Meier, did not collect such data. When we combined all studies, the taxane‐containing regimen appeared to be associated with significantly less nausea or vomiting (RR 0.62; 95% CI 0.46 to 0.83; P = 0.001; participants = 6245) with moderate heterogeneity across the studies (I2 = 46%; P = 0.005).
Subquestions: type of regimens
Question A: regimen A plus taxane versus regimen A
Two studies provided data on nausea or vomiting, and there was no statistically significant difference between the taxane‐containing and non‐taxane‐containing regimens (RR 1.13; 95% CI 0.55 to 2.34; P = 0.74; participants = 624) with no heterogeneity (I2 = 0%).
Question B: regimen A plus taxane versus regimen B
Twelve studies reported on nausea and vomiting, and there was no significant difference between the taxane‐containing and non‐taxane‐containing regimens for this outcome (RR 0.79; 95% CI 0.57 to 1.11; P = 0.17; participants = 2990) with moderate heterogeneity (I2 = 38%; P = 0.09).
Question C: single‐agent taxane versus regimen C
Twelve studies reported on nausea and vomiting. The taxane‐containing regimens appeared to be associated with significantly less nausea or vomiting (RR 0.46; 95% CI 0.27 to 0.78; P = 0.004; participants = 2855) with moderate heterogeneity (I2 = 47%; P = 0.04).
Grade 3/4 neurotoxicity
Overall effect
Data from 23 studies (involving 24 treatment comparisons) were available for this outcome. One study for subquestion A (EU‐93011) and two studies for subquestion B (AGO; Nabholtz) and subquestion C (JCOG9802; Meier) did not collect data on this outcome. The taxane‐containing regimens were associated with an increased risk of neurotoxicity (RR 4.84; 95% CI 3.18 to 7.35; P < 0.00001; participants = 5783) with minimal heterogeneity (I2 = 8%; P = 0.36).
Subquestions: type of regimens
Question A: regimen A plus taxane versus regimen A
Only ECOG E1193 (A) provided data on neurotoxicity with an increased risk in the taxane‐containing arm (RR 12.17; 95% CI 2.92 to 50.79; P = 0.0006; participants = 454).
Question B: regimen A plus taxane versus regimen B
Twelve studies reported on neurotoxicity, and the taxane‐containing regimens were associated with greater neurotoxicity (RR 4.89; 95% CI 2.55 to 9.38; P < 0.00001; participants = 2991) and minimal heterogeneity (I2 = 28%; P = 0.19).
Question C: single‐agent taxane versus regimen C
Eleven studies provided data, and the taxane‐containing regimens appeared to be associated with significantly greater neurotoxicity (RR 5.99; 95% CI 2.91 to 12.31; P < 0.00001; participants = 2562) with no heterogeneity (I2 = 0%; P = 0.60).
Grade 3/4 alopecia
Overall effect
Data from 11 studies were available for this outcome. The two studies for subquestion A (ECOG E1193 (A); EU‐93011) and eight studies aligned to subquestions B (AGO; Blohmer; Bontenbal; EORTC 10961; HERNATA; Jassem; Lyman; Nabholtz) and C (303 Study Group; 304 Study Group; Dieras; ECOG E1193 (B); EORTC 10923; JCOG9802; Meier; TOG) did not collect data on this outcome. Overall, the taxane‐containing regimens appeared to be associated with greater hair loss (RR 2.37; 95% CI 1.45 to 3.87; P = 0.0006; participants = 2437). There was substantial heterogeneity (I2 = 94%; P < 0.00001).
Subquestions: type of regimens
Question A: regimen A plus taxane versus regimen A
Neither study provided data on grade 3/4 alopecia.
Question B: regimen A plus taxane versus regimen B
Based on data from six studies, the taxane‐containing regimens were associated with greater hair loss (RR 1.17; 95% CI 1.02 to 1.34; P = 0.02; participants = 1634). There was no significant heterogeneity (I2 = 28%; P = 0.24).
Question C: single‐agent taxane versus regimen C
Based on data from five studies, the taxane‐containing regimens were associated with greater hair loss (RR 4.12; 95% CI 2.94 to 5.77; P < 0.00001; participants = 803). There was no significant heterogeneity (I2 = 30%; P = 0.23).
Quality of life
We have summarised details of quality of life data reported in 12 studies in Table 1. Compliance with completion of baseline and follow‐up quality of life instruments varied across studies, ranging from 61% to 99% for baseline and approximately 30% to 87% for follow‐up. Some studies reported problems with participants in poorer health not completing questionnaires (for example 304 Study Group). None of the individual studies reported a statistically significant difference in overall quality of life or in any of the subscales between taxane‐containing and non‐taxane‐containing chemotherapy regimens.
Low versus high or unclear risk of bias
We conducted post‐hoc subgroup analyses to investigate the treatment effect in studies with low risk of bias compared to unclear/high risk of bias. Of the 28 studies, we considered 19 studies to be at low risk of bias overall. Nine studies, involving 10 treatment comparisons, were grouped as having unclear or high risk of bias overall: AGO; ECOG E1193 (A); ECOG E1193 (B); Lyman; Nabholtz; Rugo; Sjostrom; Talbot; TRAVIOTA; Yardley.
Overall survival
Eighteen of the 19 low risk of bias studies had data available for this outcome. For these studies, there was a statistically significant difference in favour of taxane‐containing regimens with a HR of 0.91 (95% CI 0.85 to 0.97; P = 0.004; Analysis 6.1) with moderate heterogeneity (I2 = 48%; P = 0.01).
6.1. Analysis.

Comparison 6 Risk of bias, Outcome 1 Overall survival.
Data for five of the nine studies (10 treatment comparisons) with an unclear/high risk of bias were available. When combining these studies, there was no difference in overall survival with a HR of 1.05 (95% CI 0.92 to 1.20; P = 0.50; Analysis 6.1) with moderate heterogeneity (I2 = 59%; P = 0.04).
Time to progression
Seventeen of the 19 low risk of bias studies had data available for time to progression. In these studies with a low risk of bias, there was an improvement in women who received the taxane‐containing regimens however it did not reach the threshold for statistical significance (HR 0.95, 95% CI 0.89 to 1.00; P = 0.07; Analysis 6.2). There was substantial heterogeneity (I2 = 74%; P < 0.00001).
6.2. Analysis.

Comparison 6 Risk of bias, Outcome 2 Time to progression.
Data for five of the nine studies (10 treatment comparisons) with an unclear/high risk of bias were available. For these studies, there was statistically significant improvement in women who received the taxane‐containing regimens with a HR of 0.80 (95% CI 0.70 to 0.90; P = 0.0005; Analysis 6.2). There was moderate heterogeneity (I2 = 65%; P = 0.02).
Discussion
Summary of main results
This is a comprehensive review of the available evidence with overall survival data available from 22 of the 26 studies, contributing information on over 6000 women. This review update shows a statistically significant survival advantage of taxane‐containing regimens, a finding that is consistent with the findings of the previous version of this review. It is reassuring that this benefit has remained since the publication of results from an additional 10 studies. This review update also confirmed the improvements in objective tumour response rate and time to progression associated with the use of taxane‐containing regimens. Results for overall survival limited to the available first‐line treatment studies showed a benefit in favour of taxane‐containing regimens that was statistically significant. This was not statistically significant in the previous version of the review due to the limited number of completed first‐line studies. This is consistent with the observed significant benefit in objective tumour response rate among the first‐line trials. Taxane‐containing regimens were associated with a greater degree of leukopaenia and neurotoxicity, but less nausea and vomiting than the comparator group, and the overall impact on quality of life did not appear to differ in any of the trials.
Overall completeness and applicability of evidence
A limitation when interpreting the results of this review relates to the statistical and clinical heterogeneity of the studies. A certain amount of heterogeneity is to be expected given the different drugs, dosages, and schedules being used across the included studies, and the different patient groups and treatment settings. However, there was substantial statistical evidence of heterogeneity among the trials when examining the effect of treatment on time to progression and objective tumour response rate (P less than 0.00001). One explanation for this is the varying efficacy of the comparator regimens. In particular, the regimens of mitomycin, vinblastine, and fluorouracil with vinorelbine could be regarded as suboptimal chemotherapy for breast cancer. If these regimens are excluded, the advantages for single‐agent taxane when compared to a non‐taxane‐containing regimen are no longer statistically significant. Consequently it is reasonable to conclude that taxanes are more effective than some, but not all, regimens to which they have been compared, and are at least as effective as the other regimens.
The two subgroup analyses of most relevance to clinical practice are the relative merits of the different taxanes and the context in which they are used (that is, in anthracycline‐naïve patients or not). The available data suggested that docetaxel may be more active than paclitaxel, at least when given in three‐weekly schedules. It is important to note that this is based on an indirect comparison of these two drugs and, as already discussed, the various comparator regimens used also need to be considered when interpreting these results. Furthermore, paclitaxel has since been shown to be more effective in the adjuvant and metastatic settings when given as a weekly schedule (Mauri 2010).
The benefit of taxanes also appears to be less apparent in participants who have not had previous anthracyclines. While subset analyses may be useful for informing clinical practice, caution is warranted when interpreting such analyses given the smaller number of participants available to address each subgroup, and the potential effect of confounding. When interpreting the indirect comparison of paclitaxel and docetaxel, for example, we did not consider the relative efficacy of the comparators used in the included trials. We also did not state our intention to investigate some of these subgroups a priori. Such analyses should therefore primarily be considered as hypothesis generating. Interestingly, the Piccart individual patient meta‐analysis found that taxanes did worse than anthracyclines for progression‐free survival; however, overall survival and response were similar (Piccart‐Gebhart 2008).
Quality of the evidence
This review included studies that were generally well‐conducted, multicentre phase 3 trials. Overall, we considered 19 out of the 28 included studies to be at low risk of bias. However, as some studies (that is 11 out of 28 studies) failed to report details on the methods related to random sequence generation or allocation concealment, it was not possible to adequately judge whether or not these aspects of trial conduct had been done. We categorised such studies as at unclear risk of bias based on the information presented in the trial publication, unpublished manuscript, or conference proceeding abstract, and this may be perceived as a hard judgement. In addition, for outcomes assessments more likely to be influenced by a lack of blinding (that is time to progression, objective tumour response rate, and toxicity), only 11 of the 28 studies involved formal outcome assessments through scans, blood tests, and independent clinical and/or radiological adjudication committees. Future studies might consider using independent committees for those outcomes more prone to detection bias. We only encountered selective reporting of outcomes in one instance, and this was for a secondary outcome (that is quality of life). Six studies were closed prematurely owing to recruitment issues, data monitoring committee recommendation, or results being published by another trial group.
Only two studies were designed with overall survival being the primary outcome. In 13 out of the 28 included studies, time to progression (or similar definition) was the primary outcome, while in 7 studies objective tumour response rate was the primary outcome. The remaining studies made no distinction between primary or secondary outcomes.
Potential biases in the review process
The concern about reporting bias raised in the previous version of this review is lessened now that an additional 10 trials have reported time‐to‐event data for the outcome overall survival, with only 4 studies yet to report data on this outcome. We did not undertake extensive grey literature searching, so there may still be unpublished trials not included in this review. It is therefore possible that the size of the treatment effects reported may be overestimated.
The definition of time to progression varied slightly across those studies that reported data on this outcome. Of those included studies that contributed time‐to‐progression data, three studies gave no definition for this outcome and seven studies reported progression‐free survival, which in this review update we deemed to be relatively synonymous with time to progression. The medical literature has previously noted slight differences in the definition of time to progression and progression‐free survival (Mauri 2010; Saad 2009). As we combined data for this outcome irrespective of such differences, time‐to‐progression findings should be viewed with a degree of caution.
Agreements and disagreements with other studies or reviews
We identified one other systematic review using individual participant data investigating taxanes (anthracycline‐taxane versus anthracycline‐based regimen or single‐agent taxane versus single‐agent anthracycline) for women with metastatic breast cancer (Piccart‐Gebhart 2008). The chemotherapy regimens assessed in the Piccart et al review did not entirely overlap (only a subset) with those included in this Cochrane review. The Piccart‐Gebhart 2008 review examined first‐line treatment only, and overall their results generally confirmed an observed benefit of taxanes in shrinking tumours. It was difficult to compare such outcomes as overall survival and time to progression across the two reviews; the Cochrane review included data from new studies published in the last few years (Blohmer; HERNATA; Lyman), and we did not have access to some data in abstract form. More recent systematic reviews are examining the efficacy of weekly versus three‐weekly taxane regimens (Belfiglio 2012; Mauri 2010).
Authors' conclusions
Implications for practice.
When we consider all trials, we have sufficient evidence to determine the effects of taxane‐containing chemotherapy regimens in women with metastatic breast cancer. Taxane‐containing regimens appear to improve overall survival, time to progression, and overall response in women with metastatic breast cancer. The degree of heterogeneity encountered indicates that taxane‐containing regimens are more effective than some, but not all, non‐taxane‐containing regimens.
Implications for research.
Breast cancer management has evolved considerably since the first version of this review. Specifically, there is an increasing emphasis on the different biological subtypes of breast cancer and a rapidly developing array of targeted therapies to be used in place of or as adjuncts to cytotoxic chemotherapy. Thus the results of this review, which was confined to trials of chemotherapy alone, are unlikely to change, and further updates are not planned. However, if future trials examine either the role of taxanes in specific subtypes of breast cancer, or the role of taxanes together with or versus targeted therapies, then a new review would be warranted.
A meta‐analysis examining docetaxel versus paclitaxel trials suggests that it is unlikely that there is a clinically significant difference in efficacy between the two agents (Qi 2013).
What's new
| Date | Event | Description |
|---|---|---|
| 14 February 2013 | Review declared as stable | Breast cancer management has evolved considerably since the first version of this Cochrane Review. There is now an emphasis on the different biological subtypes of breast cancer and there is a rapidly developing array of targeted therapies to be used in place of or as adjuncts to cytotoxic chemotherapy. Therefore, the results of this review confined to trials of chemotherapy alone are unlikely to change and further updates of this review are not planned |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 14 February 2013 | New citation required but conclusions have not changed | Ten new studies were included, adding 3228 participants. A further two studies 'awaiting classification' and 11 'ongoing studies' have been identified |
| 14 February 2013 | New search has been performed | Performed search for new studies on 14 February 2013 |
| 13 August 2008 | Amended | Converted to new review format. |
| 3 February 2005 | New citation required and conclusions have changed | Substantive amendment |
Notes
18 February 2005 Error corrected: There was a data entry error for the value of O‐E for the TOG trial for the outcomes overall survival and time to progression, which resulted in the direction of the treatment effect being in the wrong direction for those outcomes for that trial. Although the correction does change the pooled estimate and confidence interval, it does not change the conclusions of the review. 16 May 2005 Error corrected: In Table 4 the denominator for alopecia for single‐agent taxane versus regimen C was incorrect due to a misinterpretation of the data in the primary paper. The numerator remains the same. The odds ratio changes but does not change direction, and the interpretation remains the same.
Acknowledgements
We would like to thank Libby Weir and Fergus Tai for their work in searching for studies across multiple databases. We also acknowledge the contribution made to the original concept for this review by I. Craig Henderson, Kathleen Pritchard, Martin Tattersall, Martin Stockler, Christine Brunswick, Roldano Fossati, and Alessandro Liberati.
We gratefully acknowledge the statistical and methodological advice provided by Kristy Mann, Mark Donoghue, and Val Gebski (NHMRC Clinical Trial Centre statisticians) and Dianne O'Connell (Cancer Council NSW) during the data extraction phase.
We thank John Kis‐Rugo, Cochrane Consumers and Communication Review Group, for translating Szanto's paper.
We acknowledge the financial support provided by the National Breast Cancer Foundation (Australia) that allowed the completion of this review update.
Appendices
Appendix 1. MEDLINE search strategy
| 1 | randomized controlled trial.pt. |
| 2 | controlled clinical trial.pt. |
| 3 | randomized.ab. |
| 4 | randomised.ab. |
| 5 | placebo.ab. |
| 6 | randomly.ab. |
| 7 | trial.ab. |
| 8 | groups.ab. |
| 9 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 |
| 10 | advanced breast cancer$.tw,sh. |
| 11 | advanced breast carcinoma$.tw,sh. |
| 12 | advanced breast tumour$.tw,sh. |
| 13 | advanced breast tumor$.tw,sh. |
| 14 | advanced breast neoplasm$.tw,sh. |
| 15 | metastatic breast cancer$.tw,sh. |
| 16 | metastatic breast carcinoma$.tw,sh. |
| 17 | metastatic breast tumour$.tw,sh. |
| 18 | metastatic breast tumor$.tw,sh. |
| 19 | metastatic breast neoplasm$.tw,sh. |
| 20 | 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 |
| 21 | taxane containing regimen$.tw,sh. |
| 22 | taxane containing chemotherapy regimen$.tw,sh. |
| 23 | exp Taxoids/ |
| 24 | exp Paclitaxel/ |
| 25 | docetaxel.tw,sh. |
| 26 | taxane$.tw,sh. |
| 27 | taxol.tw,sh. |
| 28 | taxotere.tw,sh. |
| 29 | paxene.tw,sh. |
| 30 | nsc‐125973.tw,sh. |
| 31 | anzatax.tw,sh. |
| 32 | 4alpha.tw,sh. |
| 33 | 7‐epi‐taxol.tw,sh. |
| 34 | 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 |
| 35 | 9 and 20 and 34 |
| 36 | limit 35 to (humans and yr="2010 ‐Current") |
Appendix 2. EMBASE (via EMBASE.com) search strategy
#41 #40 AND [humans]/lim AND [embase]/lim AND [2010‐2011]/py #40 #8 AND #19 AND #39 #39 #37 AND #38 #38 #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #35 #37 #20 OR #21 OR #22 OR #23 OR #24 OR #25 #36 '7 epi taxol' #35 4alpha #34 'anzatax'/exp OR 'anzatax' #33 'nsc 125973'/exp OR 'nsc 125973' #32 'paxene'/exp OR 'paxene' #31 'taxotere'/exp OR 'taxotere' #30 taxol* #29 taxane* #28 'docetaxel'/exp OR 'docetaxel' #27 'paclitaxel'/exp OR 'paclitaxel' #26 'taxoids'/exp OR taxoids #25 taxane* AND contain* AND chemotherap* AND regimen* #24 'taxane containing chemotherapy regimens' #23 'taxane containing chemotherapy regimen' #22 'taxane containing regimens' #21 'taxane containing regimen' #20 taxane AND containing AND regimens #19 #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 #18 metastatic NEAR/6 breast AND tumor* #17 metastatic NEAR/6 breast AND tumour* #16 metastatic NEAR/6 breast AND carcinoma* #15 metastatic NEAR/6 breast AND neoplasm* #14 metastatic NEAR/6 breast AND cancer* #13 advance* NEAR/6 breast AND tumor* #12 advance* NEAR/6 breast AND tumour* #11 advance* NEAR/6 breast AND carcinoma* #10 advance* NEAR/6 breast AND neoplasm #9 advance* NEAR/6 breast AND cancer* #8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 #7 groups:ab #6 trial:ab #5 randomly:ab #4 placebo:ab #3 randomi*ed:ab #2 controlled AND clinical AND trial #1 randomised AND controlled AND trial
Appendix 3. WHO ICTRP search strategy
Basic Search:
1. Taxane containing regimens for metastatic breast cancer 2. Metatstatic breast cancer AND taxane 3. Advanced breast cancer AND taxane 4. (chemotherapy AND taxane) AND metastatic breast cancer 5. (chemotherapy AND taxane) AND advanced breast cancer
Advanced Search:
1. Title: Taxane containing regimens for metastatic breast cancer Recruitment Status: ALL
2. Condition: advance breast cancer OR advanced breast cancer OR advance breast cancers OR advanced breast cancers OR metastatic breast cancer OR metastatic breast cancers Intervention: chemotherapy AND taxane containing regimen Recruitment Status: ALL
3. Condition: advance breast cancer OR advanced breast cancer OR advance breast cancers OR advanced breast cancers OR metastatic breast cancer OR metastatic breast cancers Intervention: chemotherapy AND taxane containing regimens Recruitment Status: ALL
4. Title: chemotherapy AND taxane containing regimen Condition: advance breast cancer OR advanced breast cancer OR advance breast cancers OR advanced breast cancers OR metastatic breast cancer OR metastatic breast cancers Recruitment Status: ALL
5. Title: chemotherapy AND taxane containing regimens Condition: advance breast cancer OR advanced breast cancer OR advance breast cancers OR advanced breast cancers OR metastatic breast cancer OR metastatic breast cancers Recruitment Status: ALL
6. Condition: advance breast cancer OR advanced breast cancer OR advance breast cancers OR advanced breast cancers OR metastatic breast cancer OR metastatic breast cancers Intervention: taxane OR taxol OR taxotere OR paclitaxel OR paxene OR nsc‐125973 OR docetaxel OR anzatax OR taxanes OR taxane containing regimen OR taxane containing regimens Recruitment Status: ALL
7. Condition: metastatic breast cancer Intervention: taxane OR taxol OR taxotere OR paclitaxel OR paxene OR nsc‐125973 OR docetaxel OR anzatax OR taxanes Recruitment Status: ALL
Appendix 4. ClinicalTrials.gov
Basic Search:
1. Taxane containing regimens for metastatic breast cancer 2. Metastatic breast cancer AND taxane 3. Advanced breast cancer AND taxane 4. (chemotherapy AND taxane) AND metastatic breast cancer 5. (chemotherapy AND taxane) AND advanced breast cancer
Advanced Search:
1. Title Acronym/Titles: Taxane containing regimens for metastatic breast cancer Recruitment: All Studies Study Results: All Studies Study Type: All Studies Gender: All Studies
2. Condition: (advanced OR metastatic) AND breast cancer Intervention: chemotherapy AND taxane Recruitment: All Studies Study Results: All Studies Study Type: All Studies Gender: All Studies
3. Condition: (advanced OR metastatic) AND breast cancer Intervention: chemotherapy AND taxane containing regimen Recruitment: All Studies Study Results: All Studies Study Type: All Studies Gender: All Studies
4. Condition: (advanced OR metastatic) AND breast cancer Intervention: taxane OR taxol OR taxotere OR paclitaxel OR paxene OR nsc‐125973 OR docetaxel OR anzatax OR taxanes Recruitment: All Studies Study Results: All Studies Study Type: All Studies Gender: All Studies
Data and analyses
Comparison 1. Overall Survival.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall effect: Taxane‐containing regimens vs. not | 23 | 6008 | Hazard Ratio (95% CI) | 0.93 [0.88, 0.99] |
| 1.1 Regimen A plus taxane v Regimen A | 2 | 518 | Hazard Ratio (95% CI) | 0.98 [0.81, 1.20] |
| 1.2 Regimen A plus taxane v Regimen B | 9 | 2645 | Hazard Ratio (95% CI) | 0.92 [0.84, 1.00] |
| 1.3 Single agent taxane v Regimen C | 12 | 2845 | Hazard Ratio (95% CI) | 0.94 [0.87, 1.03] |
| 2 First‐line trials only: overall | 16 | 4439 | Hazard Ratio (95% CI) | 0.93 [0.87, 0.99] |
| 2.1 Regimen A plus taxane v Regimen A | 2 | 518 | Hazard Ratio (95% CI) | 0.98 [0.81, 1.20] |
| 2.2 Regimen A plus taxane v Regimen B | 9 | 2645 | Hazard Ratio (95% CI) | 0.92 [0.84, 1.00] |
| 2.3 Single agent taxane v Regimen C | 5 | 1276 | Hazard Ratio (95% CI) | 0.93 [0.83, 1.05] |
| 3 Subquestions A, B & C | 23 | Hazard Ratio (95% CI) | Subtotals only | |
| 3.1 Regimen A plus taxane v Regimen A | 2 | 630 | Hazard Ratio (95% CI) | 1.00 [0.84, 1.18] |
| 3.2 Regimen A plus taxane v Regimen B | 9 | 2645 | Hazard Ratio (95% CI) | 0.92 [0.84, 1.00] |
| 3.3 Single agent taxane v Regimen C | 12 | 2957 | Hazard Ratio (95% CI) | 0.95 [0.87, 1.03] |
| 4 Chemotherapy regimens | 12 | Peto Odds Ratio (95% CI) | Subtotals only | |
| 4.1 Single agent taxane vs single agent anthracycline | 4 | 1212 | Peto Odds Ratio (95% CI) | 1.02 [0.90, 1.16] |
| 4.2 Single agent taxane vs non‐anthracycline combination | 8 | 1736 | Peto Odds Ratio (95% CI) | 0.94 [0.84, 1.06] |
| 5 Type of taxane | 23 | 6008 | Hazard Ratio (95% CI) | 0.93 [0.88, 0.99] |
| 5.1 Paclitaxel containing | 10 | 2834 | Hazard Ratio (95% CI) | 1.01 [0.93, 1.10] |
| 5.2 Docetaxel containing | 13 | 3174 | Hazard Ratio (95% CI) | 0.87 [0.80, 0.94] |
| 6 Prior anthracyclines | 23 | Hazard Ratio (95% CI) | Subtotals only | |
| 6.1 Prior anthracyclines | 6 | 1243 | Hazard Ratio (95% CI) | 0.97 [0.85, 1.11] |
| 6.2 Anthracyclines naive | 17 | 4765 | Hazard Ratio (95% CI) | 0.93 [0.87, 0.99] |
Comparison 2. Time to Progression.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall effect: Taxane‐containing regimens vs not | 22 | 5960 | Hazard Ratio (95% CI) | 0.92 [0.87, 0.97] |
| 1.1 Regimen A plus taxane v Regimen A | 1 | 342 | Hazard Ratio (95% CI) | 0.97 [0.76, 1.25] |
| 1.2 Regimen A plus taxane v Regimen B | 10 | 2891 | Hazard Ratio (95% CI) | 0.90 [0.83, 0.98] |
| 1.3 Single agent taxane v Regimen C | 11 | 2727 | Hazard Ratio (95% CI) | 0.92 [0.85, 1.00] |
| 2 First‐line trials only: overall | 16 | 4509 | Hazard Ratio (95% CI) | 0.96 [0.90, 1.02] |
| 2.1 Regimen A plus taxane v Regimen A | 1 | 342 | Hazard Ratio (95% CI) | 0.97 [0.76, 1.25] |
| 2.2 Regimen A plus taxane v Regimen B | 10 | 2891 | Hazard Ratio (95% CI) | 0.90 [0.83, 0.98] |
| 2.3 Single agent taxane v Regimen C | 5 | 1276 | Hazard Ratio (95% CI) | 1.08 [0.97, 1.21] |
| 3 Subquestions A, B & C | 22 | Hazard Ratio (95% CI) | Subtotals only | |
| 3.1 Regimen A plus taxane v Regimen A | 1 | 454 | Hazard Ratio (95% CI) | 0.99 [0.81, 1.21] |
| 3.2 Regimen A plus taxane v Regimen B | 10 | 2891 | Hazard Ratio (95% CI) | 0.90 [0.83, 0.98] |
| 3.3 Single agent taxane v Regimen C | 11 | 2839 | Hazard Ratio (95% CI) | 0.93 [0.86, 1.00] |
| 4 Subquestions A, B & C: first‐line only | 16 | Hazard Ratio (95% CI) | Subtotals only | |
| 4.1 Regimen A plus taxane v Regimen A | 1 | 454 | Hazard Ratio (95% CI) | 0.99 [0.81, 1.21] |
| 4.2 Regimen A plus taxane v Regimen B | 10 | 2891 | Hazard Ratio (95% CI) | 0.90 [0.83, 0.98] |
| 4.3 Single agent taxane v Regimen C | 5 | 1388 | Hazard Ratio (95% CI) | 1.03 [0.93, 1.14] |
| 5 Chemotherapy Regimens | 11 | Peto Odds Ratio (95% CI) | Subtotals only | |
| 5.1 Single agent taxane vs single agent anthracycline | 4 | 1212 | Peto Odds Ratio (95% CI) | 1.08 [0.96, 1.22] |
| 5.2 Single agent taxane vs non‐anthracycline combination | 7 | 1618 | Peto Odds Ratio (95% CI) | 0.85 [0.76, 0.94] |
| 6 Type of taxane | 22 | Hazard Ratio (95% CI) | Subtotals only | |
| 6.1 Paclitaxel containing | 11 | 3080 | Hazard Ratio (95% CI) | 1.04 [0.96, 1.12] |
| 6.2 Docetaxel containing | 11 | 2880 | Hazard Ratio (95% CI) | 0.80 [0.74, 0.86] |
| 7 Prior anthracyclines | 22 | Hazard Ratio (95% CI) | Subtotals only | |
| 7.1 Prior anthracyclines | 5 | 1125 | Hazard Ratio (95% CI) | 0.76 [0.67, 0.86] |
| 7.2 Anthracycline naive | 17 | 4835 | Hazard Ratio (95% CI) | 0.96 [0.90, 1.02] |
Comparison 3. Time to Treatment Failure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subquestions A, B & C | 5 | 1724 | Hazard Ratio (95% CI) | 0.90 [0.82, 0.98] |
| 1.1 Regimen A plus taxane v Regimen B | 2 | 713 | Hazard Ratio (95% CI) | 1.07 [0.93, 1.23] |
| 1.2 Single agent taxane v Regimen C | 3 | 1011 | Hazard Ratio (95% CI) | 0.78 [0.69, 0.88] |
Comparison 4. Overall Response Rate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall effect: assessable patients | 29 | 6999 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.14, 1.27] |
| 1.1 Regimen A plus taxane v Regimen A | 2 | 515 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.20, 1.92] |
| 1.2 Regimen A plus taxane v Regimen B | 14 | 3740 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.12, 1.26] |
| 1.3 Single agent taxane v Regimen C | 13 | 2744 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.05, 1.31] |
| 2 Overall effect: randomised patients | 29 | 7416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.14, 1.27] |
| 2.1 Regimen A plus taxane v Regimen A | 2 | 543 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.22, 1.97] |
| 2.2 Regimen A plus taxane v Regimen B | 14 | 3953 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.12, 1.26] |
| 2.3 Single agent taxane v Regimen C | 13 | 2920 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.05, 1.31] |
| 3 First‐line trials only: assessable patients | 21 | 5512 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.10, 1.23] |
| 3.1 Regimen A plus taxane v Regimen A | 2 | 515 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.20, 1.92] |
| 3.2 Regimen A plus taxane v Regimen B | 14 | 3740 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.12, 1.26] |
| 3.3 Single agent taxane v Regimen C | 5 | 1257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.75, 1.05] |
| 4 Overall effect: randomised patients ‐ firstline only | 21 | 5796 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.10, 1.23] |
| 4.1 Regimen A plus taxane v Regimen A | 2 | 543 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.22, 1.97] |
| 4.2 Regimen A plus taxane v Regimen B | 14 | 3953 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.12, 1.26] |
| 4.3 Single agent taxane v Regimen C | 5 | 1300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.76, 1.06] |
| 5 Subquestions A, B & C: assessable patients | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Regimen A plus taxane v Regimen A | 2 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.21, 1.79] |
| 5.2 Regimen A plus taxane v Regimen B | 14 | 3740 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.12, 1.26] |
| 5.3 Single agent taxane v Regimen C | 13 | 2856 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.03, 1.27] |
| 6 Subquestions A, B & C: randomised patients | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Regimen A plus taxane v Regimen A | 2 | 665 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.23, 1.84] |
| 6.2 Regimen A plus taxane v Regimen B | 14 | 3953 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.12, 1.26] |
| 6.3 Single agent taxane v Regimen C | 13 | 3042 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.04, 1.29] |
| 7 Subquestions A, B & C: assessable patients ‐ first‐line only | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Regimen A plus taxane v Regimen A | 2 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.21, 1.79] |
| 7.2 Regimen A plus taxane v Regimen B | 14 | 3740 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.12, 1.26] |
| 7.3 Single agent taxane v Regimen C | 5 | 1369 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.77, 1.05] |
| 8 Subquestions A, B & C: randomised patients ‐ firstline only | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Regimen A plus taxane v Regimen A | 2 | 665 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.23, 1.84] |
| 8.2 Regimen A plus taxane v Regimen B | 14 | 3953 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.12, 1.26] |
| 8.3 Single agent taxane v Regimen C | 5 | 1422 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.77, 1.06] |
| 9 Type of taxane: assessable patients | 28 | 6932 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.15, 1.27] |
| 9.1 Paclitaxel | 14 | 3499 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.99, 1.14] |
| 9.2 Docetaxel | 14 | 3433 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.29, 1.51] |
| 10 Prior anthracyclines: assessable patients | 29 | 6999 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.14, 1.26] |
| 10.1 Prior anthracyclines | 7 | 1192 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.20, 1.72] |
| 10.2 Anthracyclines naive | 22 | 5807 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.11, 1.24] |
Comparison 5. Toxicity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment‐related death: overall effect | 22 | 5517 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.63, 1.57] |
| 1.1 Regimen A plus taxane v Regimen A | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Regimen A plus taxane v Regimen B | 11 | 3195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.61, 2.33] |
| 1.3 Single agent taxane v Regimen C | 11 | 2322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.46, 1.59] |
| 2 Leukopaenia: overall effect | 28 | 6564 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.97, 1.17] |
| 2.1 Regimen A plus taxane v Regimen A | 2 | 512 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.96, 1.66] |
| 2.2 Regimen A plus taxane v Regimen B | 13 | 3209 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.02, 1.20] |
| 2.3 Single agent taxane v Regimen C | 13 | 2843 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.82, 1.21] |
| 3 Leukopaenia: subquestions A, B & C | 28 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Regimen A plus taxane v Regimen A | 2 | 624 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [1.11, 2.80] |
| 3.2 Regimen A plus taxane v Regimen B | 13 | 3209 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.02, 1.20] |
| 3.3 Single agent taxane v Regimen C | 13 | 2955 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.86, 1.34] |
| 4 Nausea or vomiting: overall effect | 26 | 6245 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.46, 0.83] |
| 4.1 Regimen A plus taxane v Regimen A | 2 | 512 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.35, 1.50] |
| 4.2 Regimen A plus taxane v Regimen B | 12 | 2990 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.57, 1.11] |
| 4.3 Single agent taxane v Regimen C | 12 | 2743 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.26, 0.71] |
| 5 Nausea or vomiting: subquestions A, B & C | 26 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Regimen A plus taxane v Regimen A | 2 | 624 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.55, 2.34] |
| 5.2 Regimen A plus taxane v Regimen B | 12 | 2990 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.57, 1.11] |
| 5.3 Single agent taxane v Regimen C | 12 | 2855 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.27, 0.78] |
| 6 Neurotoxicity: overall effect | 24 | 5783 | Risk Ratio (M‐H, Random, 95% CI) | 4.84 [3.18, 7.35] |
| 6.1 Regimen A plus taxane v Regimen A | 1 | 342 | Risk Ratio (M‐H, Random, 95% CI) | 6.09 [1.47, 25.24] |
| 6.2 Regimen A plus taxane v Regimen B | 12 | 2991 | Risk Ratio (M‐H, Random, 95% CI) | 4.89 [2.55, 9.38] |
| 6.3 Single agent taxane v Regimen C | 11 | 2450 | Risk Ratio (M‐H, Random, 95% CI) | 5.14 [2.50, 10.58] |
| 7 Neurotoxicity: subquestions A, B & C | 24 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Regimen A plus taxane v Regimen A | 1 | 454 | Risk Ratio (M‐H, Random, 95% CI) | 12.17 [2.92, 50.79] |
| 7.2 Regimen A plus taxane v Regimen B | 12 | 2991 | Risk Ratio (M‐H, Random, 95% CI) | 4.89 [2.55, 9.38] |
| 7.3 Single agent taxane v Regimen C | 11 | 2562 | Risk Ratio (M‐H, Random, 95% CI) | 5.99 [2.91, 12.31] |
| 8 Alopecia: overall effect | 11 | 2437 | Risk Ratio (M‐H, Random, 95% CI) | 2.37 [1.45, 3.87] |
| 8.1 Regimen A plus taxane v Regimen A | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Regimen A plus taxane v Regimen B | 6 | 1634 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.02, 1.34] |
| 8.3 Single agent taxane v Regimen C | 5 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 4.12 [2.94, 5.77] |
5.3. Analysis.

Comparison 5 Toxicity, Outcome 3 Leukopaenia: subquestions A, B & C.
5.4. Analysis.

Comparison 5 Toxicity, Outcome 4 Nausea or vomiting: overall effect.
5.5. Analysis.

Comparison 5 Toxicity, Outcome 5 Nausea or vomiting: subquestions A, B & C.
5.6. Analysis.
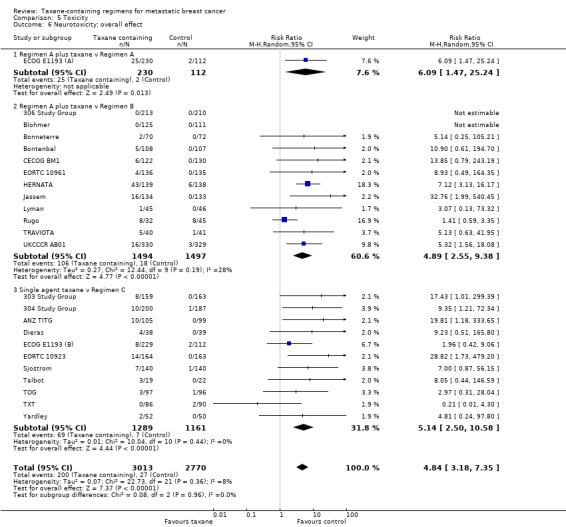
Comparison 5 Toxicity, Outcome 6 Neurotoxicity: overall effect.
5.7. Analysis.

Comparison 5 Toxicity, Outcome 7 Neurotoxicity: subquestions A, B & C.
5.8. Analysis.
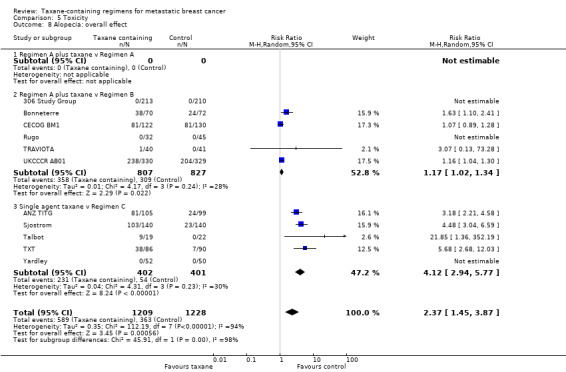
Comparison 5 Toxicity, Outcome 8 Alopecia: overall effect.
Comparison 6. Risk of bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 23 | 6008 | Hazard Ratio (95% CI) | 0.93 [0.88, 0.99] |
| 1.1 Low risk of bias | 18 | 4850 | Hazard Ratio (95% CI) | 0.91 [0.85, 0.97] |
| 1.2 Unclear or high risk of bias | 5 | 1158 | Hazard Ratio (95% CI) | 1.05 [0.92, 1.20] |
| 2 Time to progression | 22 | 5960 | Hazard Ratio (95% CI) | 0.92 [0.87, 0.97] |
| 2.1 Low risk of bias | 17 | 4815 | Hazard Ratio (95% CI) | 0.95 [0.89, 1.00] |
| 2.2 Unclear or high risk of bias | 5 | 1145 | Hazard Ratio (95% CI) | 0.80 [0.70, 0.90] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
303 Study Group.
| Methods | Accrual: 04/1994 to 01/1997 Multicentre, international Centralised randomisation, method not specified Slight imbalance in some baseline characteristics (see 'Risk of bias' table) Median follow‐up: 23 months | |
| Participants | Female Age range 25 to 74 years, median age 52.0 years in both arms 100% metastatic breast cancer 100% > first‐line All participants anthracycline naïve | |
| Interventions | Arm 1: docetaxel 100 mg/m2 Arm 2: doxorubicin 75 mg/m2 Both arms q21 days for maximum 7 cycles |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Follow‐up details not reported
All randomised participants included in time‐to‐event analyses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned randomly" and they used stratified randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation. Quote: "The randomization was centralized and stratified for treatment arm by institution" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | A non‐blinded study. Unlikely that assessment of overall survival would be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Low risk | Imaging and tumour evaluation at the end of cycles 2, 4, and 7 or at least every 3 months. CR had to be confirmed by a second evaluation more than 28 days later. Tumour assessments were reviewed by an independent panel of 2 radiologists and an oncologist. Blood tests and scans (MUGA) or echocardiography conducted. Data "analysed directly from reported laboratory parameters" |
| Blinding of outcome assessment (detection bias) QoL | High risk | EORTC QLQ‐C30 questionnaire completed by participants and Karnofsky Performance Status completed by physicians |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study reported 159/161 participants in the docetaxel group and 163/165 participants in the doxorubicin group received treatment. All participants were included in the efficacy (survival) and safety analyses |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the methods are reported in the results section of the trial publication |
| Other bias | Low risk | Similar baseline characteristics in groups except for a slight imbalance in participants with bone metastases (docetaxel 55%, doxorubicin 63%; P = 0.012) |
304 Study Group.
| Methods | Accrual: 07/1994 to 02/1997 Multicentre, international Randomisation "centralised ... with a block design by institution" Baseline comparability: no significant imbalance apparent or reported except for number of organs involved Median follow‐up: 19 months | |
| Participants | Female Age range 30 to 78 years, median age 51.0 (docetaxel) and 52.0 (mitomycin) 100% metastatic breast cancer 19% first‐line, 81% > first‐line All women had failed previous anthracycline‐containing regimens | |
| Interventions | Arm 1: docetaxel 100 mg/m2 q21 days Arm 2: mitomycin 12 mg/m2 q6 weeks + vinblastine 6 mg/m2 q21 days Both arms for a maximum of 10 3‐week cycles | |
| Outcomes | Primary:
Secondary:
|
|
| Notes | Follow‐up details not reported:
All randomised participants included in time‐to‐event analyses Estimate for time‐to‐event outcomes obtained using exact P value and total events |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned" and block randomisation by institution was used |
| Allocation concealment (selection bias) | Low risk | Central allocation. Quote: "The randomization was centralized at Rhone‐Pulene Rorer, Antony, France" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | A non‐blinded study. Unlikely that assessment of overall survival would be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Low risk | Imaging and tumour evaluation at the end of cycles 3, 6, 8, and 10 or at discontinuation or at least every 3 months. CR had to be confirmed by a second evaluation more than 28 days later. Tumour assessments were reviewed by an independent panel of 2 radiologists and an oncologist in 10% of participants Blood tests and scans. "Drug safety was analysed directly from reported laboratory parameters" |
| Blinding of outcome assessment (detection bias) QoL | High risk | EORTC QLQ‐C30 questionnaire completed by participants and Karnofsky Performance Status used to assess participant's condition from physician's perspective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Paper reported that 200/203 participants in docetaxel group and 187/189 participants in mitomycin group received treatment, and efficacy analyses used ITT principle. Safety analyses were conducted on all treated participants |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the methods were reported in the results section of the trial publication |
| Other bias | Low risk | Baseline characteristics of both groups were comparable except for the number of organs involved (i.e. ≥ 3 organs affected), however it was not mentioned if the difference was significant |
306 Study Group.
| Methods | Accrual: 06/1996 to 03/1998 Multicentre, international Randomisation centralised (block design) Baseline comparability: well balanced Median follow‐up: 49 months | |
| Participants | Median age 52.5 in doxorubicin + docetaxel group and 54 years in doxorubicin + cyclophosphamide group 100% metastatic breast cancer 100% first‐line Anthracycline naïve | |
| Interventions | Arm 1: doxorubicin + docetaxel (50/75 mg/m2) Arm 2: doxorubicin + cyclophosphamide (60/600 mg/m2) Both arms q21 days, maximum 8 cycles | |
| Outcomes | Primary:
Secondary:
|
|
| Notes | Follow‐up details not reported Report numbers at risk on survival curve All randomised participants included in time‐to‐event analyses Estimate for time to progression obtained from reported hazard ratio and 95% confidence intervals. Estimate for overall survival obtained from P value and total events. Estimate for time to treatment failure obtained from time‐to‐event curve | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from abstract: "Patients were randomly assigned to receive doxorubicin". Randomisation was centralised with block design |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization was centralized..." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | A non‐blinded study. Unlikely that assessment of overall survival would be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Low risk | Imaging and tumour evaluation after cycles 3, 6, 8 or study treatment discontinuation and then every 2 months until disease progression or death. "All tumour assessments from patients with radiologically assessable disease were reviewed by an independent expert panel" (3 radiologists and 1 medical oncologist) Weekly blood counts performed. Measurement of LVEF performed after cycles 3, 6, 8 and as clinically indicated |
| Blinding of outcome assessment (detection bias) QoL | High risk | EORTC C30 and QLQ‐BR23 completed by participants and Karnofsky Performance Status completed by physicians |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 213/214 participants on AT and 210/215 participants on AC received treatment. Efficacy analyses performed on assessable and ITT populations. Safety analyses on all treated participants |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the methods are reported in the results section of the trial publication |
| Other bias | Low risk | Quote: "Baseline characteristics were well balanced and major negative prognostic factors were similar in both groups" |
AGO.
| Methods | Accrual: 10/1996 to 05/1999 Multicentre, national (Germany) Randomisation method not specified Baseline comparability: no significant imbalance reported Median follow‐up: 36 weeks | |
| Participants | Median age 56 years in both arms 26% had "locally metastatic disease" In the remainder the main site of metastases were liver or lung (although proportions do not add up) 100% first‐line Anthracycline naïve (except for 3% of participants in epirubicin + paclitaxel group) | |
| Interventions | Arm 1: epirubicin 60 mg/m2 + paclitaxel 175 mg/m2)
Arm 2: epirubicin 60 mg/m2 + cyclophosphamide 600 mg/m2) Both arms q21 days for at least 6 cycles |
|
| Outcomes | Outcomes were not separated into primary and secondary:
|
|
| Notes | Abstract only available. Full article has not yet been published | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to receive either ...". No additional details were provided in the abstract |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described in the abstract |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided in the abstracts available |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Assessment of overall survival is unlikely to be influenced by no or incomplete blinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Unclear risk | No information provided in the abstracts available. NCI‐CTC used. Comment: blood tests were assumed to be conducted |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reporting of attrition or exclusions in the available abstract |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess from the abstract |
| Other bias | Low risk | Quote: "Prognostic factors were balanced between treatment arms" |
ANZ TITG.
| Methods | Accrual: 30 September 1993 to 30 September 1995 Multi‐centre, international Centralised, computer‐generated randomisation Baseline comparability: no significant imbalance reported Median follow‐up: 26 months | |
| Participants | Age range 32 to 80 years, median age 54 years in both arms Approximately 90% metastatic breast cancer 100% first‐line Did not exclude prior anthracycline use, however only 1% of participants on CMFP and 0 participants on paclitaxel had received anthracyclines | |
| Interventions | Arm 1: paclitaxel 200 mg/m2 Arm 2: cyclophosphamide 100 mg/m2+ methotrexate 40 mg/m2+ fluorouracil 600 mg/m2+ prednisone 40 mg/m2 (CMFP) Both arms q21 days for 8 courses | |
| Outcomes | Outcomes were not separated into primary or secondary:
|
|
| Notes | Follow‐up details reported
All randomised participants included in time‐to‐event analyses Estimate for time‐to‐event outcomes obtained using exact P value and total events |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were stratified by institution and randomized to receive either...". Statistical methods section states "randomization was based on an adaptive biased coin procedure with a bias of 3n at each allocation in favor of the arm with n fewer patients" |
| Allocation concealment (selection bias) | Low risk | Quote: "Computer‐generated randomization charts were prepared for each center and held at the Statistical Centre at Peter MacCallum Cancer Institute, Melbourne, Australia" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information in trial publication |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Assessment of overall survival is unlikely to be influenced by no or incomplete blinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Unclear risk | Weekly blood tests. Scans to evaluate disease repeated after 12 weeks and 24 weeks on therapy. Tests also repeated at time of suspected relapse or progression and at intervals no less than 4 weeks apart from PR or CR |
| Blinding of outcome assessment (detection bias) QoL | High risk | QoL linear analog scales completed by participants and Spitzer QoL index completed by physicians (p. 2356) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 105/107 participants received paclitaxel and 99/102 participants received CMFP. "All major endpoints were compared using ITT analysis that included all randomised patients" |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the methods are reported in the results section of the trial publication |
| Other bias | Unclear risk | Baseline characteristics (e.g. ECOG performance status) provided by treatment arm but no indication whether there were any significant differences between groups. 3 of 7 potential prognostic factors in this study (includes ECOG performance status) "were shown to have a significant influence on survival" |
Blohmer.
| Methods | Accrual: 02/2000 to 11/2003 Multicentre, open‐label, randomised phase III trial at 49 centres in Germany Randomisation “centralised with a block design by study centre” Imbalance in the number of participants randomised to each arm (that is epirubicin + docetaxel group, n = 125; epirubicin + cyclophosphamide group, n = 111) Median follow‐up: 24 months | |
| Participants | Age range 31 to 73 years, median age 57 years (epirubicin + docetaxel group) and 56 years (epirubicin + cyclophosphamide group) 100% metastatic breast cancer First‐line No previous chemotherapy/anthracyclines allowed | |
| Interventions | Arm 1: epirubicin 75 mg/m2 (IV bolus or infusion for 10 min) + docetaxel 75 mg/m2 (IV infusion over 1 hour) Arm 2: epirubicin 90 mg/m2 (IV) + cyclophosphamide 600 mg/m2 (IV over 30 min) Treatment (both arms) q21 days, 6 cycles and in some cases 8 cycles if “maximum benefit had not been reached” |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Trial prematurely stopped due to inadequate accrual and unlikelihood of reaching primary end point. We contacted the trialists (Peter Schmid) who provided the number of events in each treatment arm for the outcomes OS and PFS. Method 4 (Tierney 2007) was then used to estimate O‐E, and V. For toxicity, the number of randomised women was used as the denominator. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “patients were randomly assigned to receive either...”. Randomisation was centralised with a block design |
| Allocation concealment (selection bias) | Low risk | Quote: “The randomisation was centralized with a block design by study centre” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Open‐label study. Unlikely that assessment of overall survival would be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Unclear risk | Tumour lesions were assessed at end of treatment cycles 2, 4, 6, 8 or at study discontinuation and then every 2 months. No description of how evaluations were done Adverse events and toxicity were assessed weekly and recorded for every cycle. Scans were used to assess cardiac function at baseline and after cycles 3 and 6, and at the end of the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 240 participants enrolled; 4 participants (1.7%) did not receive study medication and were excluded from ITT and safety analyses |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the methods were reported in the results section of the trial publication |
| Other bias | Unclear risk | Trial prematurely terminated due to inadequate accrual and results from the interim analysis |
Bonneterre.
| Methods | Accrual: 09/1998 to 11/2000 Multicentre, national (France) Randomisation centralised and stratified according to centre Baseline comparability: no significant imbalance reported, although ET arm had a higher proportion of participants with an original diagnosis of stage IV disease and more advanced disease than FEC arm Median follow‐up: 23.8 months | |
| Participants | Female Age range 23 to 73 years, median age 54 years for both arms 100% metastatic breast cancer 100% first‐line Slightly higher number of participants had ER or PR positive tumours in FEC group | |
| Interventions | Arm1:
ET: epirubicin 75 mg/m2 over 10 min + docetaxel 75 mg/m2 Arm 2: FEC: fluorouracil 500 mg/m2 over 1 hour + epirubicin 75 mg/m2 over 10 min + cyclophosphamide 500 mg/m2 All agents given once q21 days for up to 8 cycles |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Abstract was only available in the previous version of this Cochrane review. The full article was published in 2004. For overall survival, hazard ratios were estimated using methods outlined by Parmar 1998; for TTP, individualised participant data from Piccart‐Gebhart 2008 was used and sensitivity analysis conducted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “centralised predefined schedule with randomisation stratified according to centre..”. |
| Allocation concealment (selection bias) | Low risk | Quote: “...centralised predefined schedule with randomisation stratified according to centre to one of two treatment arms” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Unlikely that overall survival assessment was influenced by unblinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Low risk | Physical examinations and blood tests run at the beginning of each treatment cycle. Target lesions assessed every 2 cycles. Responses reviewed by an independent committee of radiologists Scans were performed every 4 cycles and then every 2 cycles once maximum cumulative dose reached (anthracyclines) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 65/70 participants received ET and 67/72 participants FEC. ITT analysis undertaken on all (142) participants. For response rates, analyses used ITT population and assessable population |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the methods were reported in the results section of the trial publication |
| Other bias | Unclear risk | Baseline characteristics generally comparable; however ET group had greater metastatic involvement of lung, liver, skin, bone or > 3 organs; FEC group had a higher proportion of participants with oestrogen or progesterone receptor positive tumours |
Bontenbal.
| Methods | Accrual: 03/1997 to 04/2002 Multicentre, national (Netherlands) Randomisation (by central telephone number) stratified for centre, previous chemotherapy, WHO performance status, and presence of bone metastases Baseline comparability: no significant imbalance reported Median follow‐up: 8.7 months | |
| Participants | Age range 30 to 70 years, median age 53 years in AT group and 54 years in FAC group 100% metastatic breast cancer 100% first‐line | |
| Interventions | Arm 1:
AT: doxorubicin + docetaxel 50/75 mg/m2
Arm 2:
FAC: fluorouracil, doxorubicin + cyclophosphamide 500/50/500 mg/m2 Both arms q21 days for a maximum of 6 cycles |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Phase III trial prematurely closed due to an unplanned interim analysis that indicated that the chance of finding a statistical difference in TTP between treatment arms had become too low. For TTP, method 3 was used to estimate O‐E and V (Tierney 2007). For toxicity, the number of randomised women was used as the denominator. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from abstract: “patients were randomly assigned to either...” and involved stratification randomisation based on centre, prior chemotherapy, WHO performance status, and presence of bone metastases |
| Allocation concealment (selection bias) | Low risk | Quote: “randomisation performed by calling a central telephone number who stratified for center.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Unlikely that overall survival assessment was influenced by unblinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Low risk | X‐ray, ultrasonography, and MRI, etc. used to assess tumour status. Up to a max of 8 representable lesions were evaluated after cycles 2, 4, 6 then every 2 months for the first year, etc. Physical examination and biochemical tests and scans were performed weekly before each cycle |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 108/109 received AT treatment and 107/107 received FAC treatment. ITT analysis, and a separate per‐protocol analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the methods were reported in the results section of the trial publication |
| Other bias | Unclear risk | Early study closure date: “An independent Data Monitoring Committee determined that the chance of finding a statistical difference in TTP between the treatment arms had become so low that they recommended study closure”. No differences in baseline characteristics |
CECOG BM1.
| Methods | Accrual: 10/1999 to 11/2002 Multicentre phase III study, 29 centres in 12 countries Centralised randomisation based on a minimising algorithm and stratified by prior adjuvant chemotherapy and centre Baseline characteristics balanced between treatment arms except for menopausal status; FEC arm had a higher percentage of pre‐menopausal participants compared to GET arm (P = 0.024) Median follow‐up: 24 months | |
| Participants | Age range 29 to 74 years, median 53 years (GET) and 54 years (FEC)
100% metastatic breast cancer
100% first‐line All participants anthracycline naïve |
|
| Interventions | Arm 1:
GET: gemcitabine (1000 mg/m2 day 1 and 4), epirubicin (90 mg/m2 day 1), and paclitaxel (175 mg/m2 day 1) Arm 2: FEC: fluorouracil (500 mg/m2 day 1), epirubicin (90 mg/m2 day 1), and cyclophosphamide (500 mg/m2 day 1) Both arms: q21 days for a maximum of 8 cycles No other anticancer drugs were allowed during the study including hormonal agents or immunotherapy or both |
|
| Outcomes | Outcomes were not separated into primary or secondary:
|
|
| Notes | Data were not mature for overall survival. However, curve extraction using method 10 was undertaken to estimate TTP (Tierney 2007) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “patients were randomly assigned to GET or FEC...based on a minimizing algorithm" |
| Allocation concealment (selection bias) | Low risk | Quote: “...via a centralised randomisation system based on a minimising algorithm”; stratified by centre and prior adjuvant chemotherapy |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information in trial publication |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Unlikely that overall survival assessment was influenced by unblinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Unclear risk | Tumour imaging and response assessment carried out every cycle Blood tests weekly, so too ECG, echocardiogram (before cycles 5 & 7) and toxicity (after each cycle) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 259 randomised participants; 124/124 on GET and 132/135 on FEC received study treatment. ITT was not used, instead assessable participants, for response and toxicity analyses. Survival data not yet mature |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the methods were reported in the results section of the trial publication |
| Other bias | Unclear risk | Baseline characteristics mainly balanced between groups except for menopausal status |
Dieras.
| Methods | Accrual dates cannot be determined Multicentre, national, phase II Randomisation centralised "performed by computerized log, without direct access of the investigator" Baseline comparability: no significant imbalance reported Median follow‐up: not reported | |
| Participants | Female Pre‐, peri‐, and post‐menopausal Age range 29 to 69 years, median 52 years (arm 1) and 52.5 years (arm 2) 100% metastatic breast cancer 100% > first‐line 98% had prior anthracycline treatment | |
| Interventions | Arm 1: paclitaxel 175 mg/m2 by 3‐hour infusion q21 days Arm 2: mitomycin 12 mg/m2 in slow bolus q6 weeks Both arms given for a minimum of 2 cycles (total cumulative dose of mitomycin limited to 60 mg/m2) | |
| Outcomes | Outcomes were not separated into primary or secondary in trial report:
|
|
| Notes | Cross‐over to alternate regimen on progression. Many more cycles of paclitaxel were received than mitomycin. Follow‐up details not reported
Efficacy data available for 72/81 randomised participants. (4 participants did not receive allocated treatment and 5 received hormonal treatment while on study) Estimate for time‐to‐event outcomes obtained from time‐to‐event curves |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Central randomization was performed by computerized log, without direct access of the investigator". Comment: random assignments were probably generated by computer |
| Allocation concealment (selection bias) | Low risk | Quote: “centralized randomization was performed by computerised log, without direct access of the investigator” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information in trial publication |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Unlikely that overall survival assessment was influenced by unblinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Unclear risk | No information provided in the trial publication about tumour evaluations Blood tests conducted prior to new course |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data; all randomised participants included in analyses of baseline characteristics and efficacy, objective response rates also were analysed for evaluable participants |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the methods were reported in the results section of the trial publication |
| Other bias | Low risk | No baseline differences noted between treatment arms |
ECOG E1193 (A).
| Methods | Accrual: 2/1993 to 9/1995 Multicentre Cross‐over (on progression) Randomisation method not described Baseline comparability: no significant imbalance reported Median follow‐up: not reported | |
| Participants | Female Age range 25 to 79 years, median age 58 years (arm 1), 56 years (arm 2), and 56 years (arm 3) Progressing regional disease (13% to 19% of participants) or metastatic disease 100% first‐line All participants anthracycline naïve | |
| Interventions | Arm 1: doxorubicin 60 mg/m2 Arm 2: paclitaxel 175 mg/m2/24 hour Arm 3: doxorubicin 50 mg/m2 + paclitaxel 150 mg/m2/24 hour + granulocyte‐colony stimulating factor q21 days. Doxorubicin for a maximum 8 cycles; paclitaxel until disease progression | |
| Outcomes | Outcomes were not separated into primary and secondary:
|
|
| Notes | Single agents cross‐over to alternate single agent on progression. Did not report as ITT 739 randomised (8 cancelled). Of 731 remaining, 33 excluded as ineligible (reasons explained). Data on 683 participants included in time‐to‐event analyses (reasons for exclusion of additional 15 participants not explained). Follow‐up details not reported, therefore
The definition of TTF used in this trial was date of study entry to date progressive disease, toxic death, or death due to breast cancer within 6 weeks of date participant last known alive with stable disease. This meets the definition of PFS as used in this review. The number of participants who received treatment cannot be determined Estimate for time‐to‐event outcomes obtained from time‐to‐event curves ECOG E1193(A) compared arms 1 & 3 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients were randomized to receive either...”. No additional details were provided on how random assignment was achieved |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described in the trial publication |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information in trial publication |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Unlikely that overall survival assessment was influenced by unblinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Unclear risk | No information provided in the trial publication about tumour evaluations. NCI toxicity criteria used. Comment: standard blood tests probably done |
| Blinding of outcome assessment (detection bias) QoL | High risk | FACT‐B completed by participants at baseline and at week 16 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 33/731 randomised participants were excluded from analysis with ineligibility reasons provided. 70% of eligible participants completed the follow‐up assessment for QoL at week 16. Method of analyses not provided |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the methods were reported in the results section of the trial publication |
| Other bias | Low risk | Baseline characteristics well‐matched across the 3 arms |
ECOG E1193 (B).
| Methods | See E1193 (A) | |
| Participants | See E1193 (A) | |
| Interventions | See E1193 (A) | |
| Outcomes | See E1193 (A) | |
| Notes | See E1193 (A) ECOG E1193(B) compared arms 1 & 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients were randomized to receive either...”. No additional details were provided on how random assignment was achieved |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described in the trial publication |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information in trial publication |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Unlikely that overall survival assessment was influenced by unblinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Unclear risk | No information provided in the trial publication about tumour evaluations. NCI toxicity criteria used. Comment: standard blood tests probably done |
| Blinding of outcome assessment (detection bias) QoL | High risk | FACT‐B completed by participants at baseline and at week 16 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 33/731 randomised participants were excluded from analysis with ineligibility reasons provided. 70% of eligible participants completed the follow‐up assessment for QoL at week 16. Method of analyses not provided |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the methods were reported in the results section of the trial publication |
| Other bias | Low risk | Baseline characteristics well‐matched across the 3 arms |
EORTC 10923.
| Methods | Accrual: 8/1993 to 5/1996 Multicentre, international Cross‐over Randomisation "performed centrally...by telephone, fax or computer" Baseline comparability: no significant imbalance apparent or reported Median follow‐up: not reported | |
| Participants | Female Age range 26 to 75 years, median age 54 years (paclitaxel group) and 55 years (doxorubicin group) 100% metastatic breast cancer in overt progression (73% had 2 or more metastatic sites) 100% first‐line All participants anthracycline naïve | |
| Interventions | Arm 1: paclitaxel 200 mg/m2 Arm 2: doxorubicin 75 mg/m2 Both arms q21 days for 7 cycles | |
| Outcomes | Primary:
Secondary:
|
|
| Notes | Cross‐over to alternate regimen on progression Follow‐up details not reported
All randomised participants included in time‐to‐event analyses Estimate for time‐to‐event outcomes obtained using exact P value and total events |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients were randomly assigned to receive...”. No details on the random component were described |
| Allocation concealment (selection bias) | Low risk | Central allocation. Quote: “randomization was performed centrally at the EORTC Data Center located in Brussels (Belgium) by telephone, fax or computer” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information in trial publication |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Assessment of overall survival is unlikely to be influenced by no or incomplete blinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Low risk | Tumour response assessed per Union for International Cancer Control criteria. Serial evidence was documented by radiology or photography and assessed by external review. "All case report forms were reviewed by local investigators and to two independent radiologists who were blinded to treatment arm" Weekly blood tests and toxicity assessed using NCI‐CTC. MUGA or echocardiography for evaluating LVEF conducted at study entry and at completion of 5th and 7th course |
| Blinding of outcome assessment (detection bias) QoL | High risk | EORTC QLQ‐C30 and Rotterdam Symptom Checklist completed by participants at baseline and at completion of cycles 3, 5, 7 and during follow‐up (every 2 months) until disease progression |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 164/166 randomised participants received paclitaxel; 163/165 randomised participants received doxorubicin. All randomly assigned participants were evaluated according to ITT |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the methods were reported in the results section of the trial publication |
| Other bias | Low risk | Baseline characteristics similar between the two groups; “no significant imbalance in classical prognostic factors” |
EORTC 10961.
| Methods | Accrual: 11/1996 to 02/1999 Multicentre, international Centrally randomised at EORTC data centre Baseline comparability: no significant imbalance reported Median follow‐up: 29.2 months | |
| Participants | Pre‐ and post‐menopausal women aged 18 to 70 years 100% metastatic breast cancer 100% first‐line All participants anthracycline and taxane naive | |
| Interventions | Arm 1: doxorubicin 60 mg/m2 + paclitaxel 175 mg/m2 Arm 2: doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 Both arms q21 days to a maximum of 6 cycles | |
| Outcomes | Primary:
Secondary:
|
|
| Notes | Follow‐up details not reported
All randomised participants included in time‐to‐event analyses Estimate for time‐to‐event outcomes obtained from reported hazard ratio and 95% confidence intervals |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “...random assignment was undertaken using a minimization technique..." |
| Allocation concealment (selection bias) | Low risk | Quote: “patients were centrally randomized at the EORTC Data Center in Brussels” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information in trial publication |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Assessment of overall survival is unlikely to be influenced by no or incomplete blinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Unclear risk | Tumour measurement performed every 6 weeks until tumour progression and assessed using WHO criteria. Comment: scans would have been done to use WHO criteria Physical exam and blood tests repeated before each cycle, hematological monitoring weekly, and MUGA scan or echocardiography at study entry, before cycles 3, 5, 6 and 3 months after last chemotherapy. NCI‐CTC used |
| Blinding of outcome assessment (detection bias) QoL | High risk | EORTC QLQ‐C30 & BR23 completed by participants at baseline and before cycles 2, 4, and 6 and at first follow‐up |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 217/273 randomised participants received study treatment. Quote: “all randomized patients were evaluated for Response Rate, PFS and OS according to intention‐to‐treat principle”. Sensitivity analyses were performed to investigate missing data for QoL. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported in 2 separate publications |
| Other bias | Low risk | Participant characteristics between the 2 groups at baseline were balanced |
EU‐93011.
| Methods | Accrual: 06/1997 to 12/2001. Observed until the end of 2003 Multicentre study (Germany) Randomisation carried out centrally using block sizes of various lengths Participants stratified by age, treatment centre, disease‐free interval (> or < 18 months), hormone receptor status, prior adjuvant therapy with anthracyclines, presence of liver metastases and lung metastases No baseline characteristics reported Median follow‐up: 43.6 months | |
| Participants | Participants < 80 years, no further details provided 100% metastatic breast cancer First‐line Anthracycline naïve | |
| Interventions | Arm 1: mitoxantrone (12 mg/m2 q21 days). Administered until complete response (plus 2 cycles) or cumulative dose 160 mg/m2 Arm 2: mitoxantrone (12 mg/m2 q21 days) for up to 6 cycles plus docetaxel (80 mg/m2 q21 days) until progressive disease for up to 6 cycles | |
| Outcomes | Primary:
Secondary:
|
|
| Notes | We contacted the trialists, and Martin Schumacher forwarded data from an unpublished manuscript for inclusion into this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned using blocks of various lengths" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization was carried out centrally by fax or telephone in blocks of variable length" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information in unpublished manuscript |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Unlikely that overall survival assessment was influenced by unblinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Unclear risk | Assessed MBS that was composed of time to progression, PS/WHO (WHO performance status during chemotherapy as compared to that before the start of treatment), SUJ (Patient's rating of the treatment benefit) and TOX (toxicity component based on alopecia and nausea/vomiting during therapy). Comment: scans to assess tumour response probably done Toxicity graded per WHO criteria. Comment: standard blood tests probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 176/179 randomised participants and included in ITT analysis for primary outcomes. 3 participants excluded (1.7%) due to cerebral metastases and insufficient data (reasons provided in PRISMA flowchart) |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the methods were reported in the results section of the unpublished manuscript |
| Other bias | Unclear risk | Recruitment stopped early because of poor accrual rate |
HERNATA.
| Methods | Accrual: 05/2004 to 08/2008 Multicentre, phase III trial, conducted in Denmark, Sweden, and Norway Randomisation occurred centrally Baseline characteristics were balanced Median follow‐up: 34 months |
|
| Participants | Age range 29 to 72 years, median age 56 years (docetaxel + trastuzumab arm) and 57 years (vinorelbine + trastuzumab) 96.1% metastatic breast cancer; 3.9% locally advanced breast cancer
HER2 status: IHC 3+ = > 81%, FISH + = 35.2%, unknown 1.4% First‐line for metastatic breast cancer or locally advanced disease |
|
| Interventions | Arm 1: docetaxel 100 mg/m2 IV over 60 minutes q21 days Arm 2: vinorelbine 30 mg/m2 or 35 mg/m2 IV as bolus injections days 1 and 8 q21 days Both arms q21 days for a median of 8 cycles (0 to 26 for docetaxel + trastuzumab) and 10.5 cycles (2 to 42 for vinorelbine + trastuzumab) Trastuzumab given before chemotherapy as IV infusion over 90 minutes with 8 mg/kg in the first cycle and subsequent cycles over 30 minutes with 6 mg/kg |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | All randomised participants included in ITT analysis For OS and TTP, method 3 used to estimate O‐E and V (Tierney 2007) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned". No further details provided in the trial report or retrieved case report form |
| Allocation concealment (selection bias) | Low risk | Central allocation. Quote: "...patients were, unstratified, randomly assigned centrally by the Danish Breast Cancer Cooperative Group Secretariat" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information in trial publication |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Assessment of overall survival is unlikely to be influenced by no or incomplete blinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Unclear risk | Imaging and tumour evaluation every 3 months Lab tests (blood counts) repeated at each cycle |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At time of analysis, 128/139 in the docetaxel group and 120/138 in the vinorelbine group discontinued therapy, including 11 and 15 participants, respectively, due to "other reasons" including lost to follow‐up. ITT analysis |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes in ClinicalTrials.gov record (http://clinicaltrials.gov/ct2/show/NCT00430001?term=HERNATA&rank=1) and the methods section of the trial publication are the same. All outcomes were reported in the results section |
| Other bias | Low risk | Quote: "baseline demographics and other variables were well balanced between the treatment groups" |
Jassem.
| Methods | Accrual: 11/1996 to 4/1998 Multicentre, international "central randomisation" Baseline comparability: no significant imbalance reported Median follow‐up: 69 months | |
| Participants | Age range 24 to 72 years, median age 50 years (in both groups) 100% metastatic breast cancer 100% first‐line All participants anthracycline naïve | |
| Interventions | Arm 1: doxorubicin 50 mg/m2 followed 24 hour later by paclitaxel 220 mg/m2 Arm 2: fluorouracil 500 mg/m2 + doxorubicin 50 mg/m2 IV + cyclophosphamide 500 mg/m2 Both arms q21 days for up to 8 cycles |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | For OS and TTP, method 3 was used to estimate O‐E and V (Tierney 2007) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “...patients were randomized in a 1:1 ratio to receive therapy with either...”. Involved stratified randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: “Before central randomization, patients were stratified according to...” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Unlikely that overall survival assessment was influenced by unblinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Low risk | All efficacy data was subjected to a blinded clinical and radiological independent review. Physical examination every cycle and imaging studies every other cycle Blood tests repeated before each cycle; LVEF assessed before cycles 5 and 7 and at end of study; toxicity assessed using WHO criteria |
| Blinding of outcome assessment (detection bias) QoL | High risk | EORTC QLQ‐C30 and EORTC QLQ‐BR23 completed by participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 267 participants were enrolled and randomised, with 264 receiving treatment and 259 assessable for response. TTP and OS analyses based on ITT population. 264/267 (98.9%) of participants available for toxicity analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the methods were reported in the results section of the trial publication |
| Other bias | Low risk | Quote: “patient characteristics were well‐balanced between two treatment arms” |
JCOG9802.
| Methods | Accrual: 01/1999 to 05/2003 Randomised, multicentre, non‐blinded phase III study that took place at 29 institutions (Japan) Participants were randomly assigned to 1 of 3 treatment groups by the minimisation method, balancing the arms according to disease status (stage IV versus recurrent disease), prior anthracyclines, liver metastasis, and institution All prognostic factors well balanced between the 3 treatment groups Median follow‐up: not reported |
|
| Participants | Participant age range 26 to 75 years, median age 54 years (AC), 54 years (D), and 56 years (AC+D) 100% metastatic breast cancer No previous anthracyclines or taxanes were allowed |
|
| Interventions | Arm 1: AC: doxorubicin 40 mg/m2 + cyclophosphamide 500 mg/m2 q21 days for 6 cycles Arm 2: D: docetaxel 60 mg/m2 Arm 3 (not used in review): AC+D: 3 cycles of AC and 3 cycles of D Treatment was administered q21 days |
|
| Outcomes | Primary:
Secondary:
Pilot study: quality of life |
|
| Notes | Only 2 arms of this 3‐arm trial were used. The sequential treatment comparison (i.e. AC vs AC+D) was excluded from analysis as it was not the focus of this review. This review compared AC vs D only (arm 1 vs arm 2) We contacted the trialists, and Noriyuki Katsumata provided the number of events in each group, the hazard ratio, confidence intervals, and P values for OS, TTP, and TTF. Method 3 was used to estimate O‐E and V (Tierney 2007) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...patients were then randomly assigned to one of the three treatment groups by the minimization method..." |
| Allocation concealment (selection bias) | Low risk | Quote: “The randomisation of treatment assignments was centralized. After confirming that candidate subjects met all the inclusion and exclusion criteria, the Japan Clinical Oncology Group (JCOG) Data Center was informed by telephone or fax” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Unlikely that overall survival assessment was influenced by unblinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Low risk | Objective responses were assessed by central review at regular meetings. Response was classified using criteria similar to WHO criteria. Comment: scans would have been done to use WHO‐like criteria Blood tests conducted. Toxicity assessed using criteria similar to NCI‐CTC |
| Blinding of outcome assessment (detection bias) QoL | High risk | FACT‐B questionnaire completed by first 50 participants in each treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | TTF, PFS, and OS were analysed using ITT population; response and safety used assessable participants. 68% of participants in the AC arm, 76% in the D arm, and 77% in alternating AC+D arm completed 6 cycles mainly due to disease progression |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes in ClinicalTrials.gov record (https://clinicaltrials.gov/show/NCT00193037) and the methods section of the trial publication are similar; the trial publication adds QoL. All outcomes were reported in the results section |
| Other bias | Low risk | All prognostic factors well balanced between the 3 treatment groups |
Lyman.
| Methods | Accrual: 02/1996 to 01/1997 Multi‐institutional cooperative group trial Randomised phase II study Baseline characteristics were balanced between treatment arms except that performance status was “somewhat better on the doxorubicin/paclitaxel arm” (p.146) Median follow‐up: not reported | |
| Participants | Age range 30 to 79 years, median age 53.7 years (doxorubicin and paclitaxel) and 55.9 years (doxorubicin and cyclophosphamide) Metastatic breast cancer or locally advanced breast cancer (number of participants with locally advanced breast cancer not provided) No prior therapy with anthracyclines, anthracenediones, paclitaxel, or docetaxel permitted |
|
| Interventions | Arm 1: AT: doxorubicin 60 mg/m2 IV over 30 min followed by paclitaxel 200 mg/m2 IV over 3 hour q21 days. Combined treatment was stopped after 6 cycles Arm 2: AC: doxorubicin 60 mg/m2 IV over 30 min followed by cyclophosphamide 600 mg/m2 IV. After 6 cycles, participants could be treated with any appropriate regimen |
|
| Outcomes | No distinction between primary and secondary outcomes provided:
|
|
| Notes | We contacted the trialists, and William Barlow provided the unadjusted hazard ratio, confidence interval, and P value for OS. Method 3 used to estimate O‐E and V | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients randomized between the two treatment arms in this study”. No additional details were provided on how random assignment was achieved |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described in the trial publication |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information in trial publication |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Assessment of overall survival is unlikely to be influenced by no or incomplete blinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Unclear risk | Responses assessed according to SWOG criteria, using markers and other lab values LVEF assessed on study and after 6 cycles. Comment: scans/tests probably done to grade toxicity |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in overall survival and objective complete response |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the methods were reported in the results section of the trial publication |
| Other bias | Unclear risk | Baseline characteristics for performance status differed between treatment groups with doxorubicin/paclitaxel arm somewhat better, but no statistics provided |
Meier.
| Methods | Accrual: 11/1998 to 01/2004 Central randomisation Baseline characteristics appear to be balanced; no mention of any significant differences Cross‐over (on disease progression or intolerable toxicity) Median follow‐up: not reported | |
| Participants | Age range 31 to 78 years, median age 60 years in both treatment arms 100% metastatic breast cancer Pre‐treated with anthracyclines | |
| Interventions | Arm 1: vinorelbine 30 mg/m2 weekly x6 q8 weeks
Arm 2: docetaxel 35 mg/m2 weekly x6 q8 weeks For both arms: up to 4 consecutive cycles From cycle 2, participants had the option to cross‐over to the alternate treatment arm |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | For OS, hazard ratios were estimated using methods outlined by Parmar 1998 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (abstract): “patients were randomized to receive...”. No additional details were provided on how random assignment was achieved |
| Allocation concealment (selection bias) | Low risk | Quote: “Eligible and consenting patients were centrally randomized” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information in trial publication |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Assessment of overall survival is unlikely to be influenced by no or incomplete blinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Unclear risk | No information provided regarding criteria or tests used to evaluate response or progression NCI‐CTC v2 was used. Comment: blood tests and scans were probably done |
| Blinding of outcome assessment (detection bias) QoL | High risk | EORTC QLQ‐C30 completed by participants at the start of each treatment cycle |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for, including cross‐overs |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the methods were reported in the results section of the trial publication |
| Other bias | Low risk | Baseline characteristics seemed to be comparable |
Nabholtz.
| Methods | Accrual: 1/1998 to 12/1999 Multicentre, international Randomisation method not specified Baseline comparability: no significant imbalance reported Median follow‐up: 30 months | |
| Participants | Median age: 54 years (no further details provided in abstract) 100% metastatic breast cancer 100% first‐line | |
| Interventions | Arm 1: docetaxel 75 mg/m2 + doxorubicin 50 mg/m2 + cyclophosphamide 500 mg/m2 Arm 2: fluorouracil 500 mg/m2 + doxorubicin 50 mg/m2 + cyclophosphamide 500 mg/m2 Both arms q21 days for maximum of 8 cycles | |
| Outcomes | No distinction made between primary or secondary outcomes:
|
|
| Notes | Abstract only available Reported response and toxicity as percentages; assumed to be percentage of participants receiving treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “A phase III randomized trial...”. No additional details were provided in the abstract |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described in the abstract |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information in the abstract |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Assessment of overall survival is unlikely to be influenced by no or incomplete blinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Unclear risk | No information provided regarding criteria or tests used to evaluate response or progression NCI criteria used. Comment: blood tests and scans were probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reporting of attrition or exclusions in the available abstract |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess from the abstract |
| Other bias | Low risk | Baseline characteristics comparable |
Rugo.
| Methods | Accrual dates not provided Participants randomised in a 3:3:2 ratio to treatment arms 1, 2, and 3 respectively Baseline characteristics were balanced between treatment arms except for site of metastasis; fewer participants had liver or lung metastasis or both in arm 3 than in arms 1 and 2 Median follow‐up: not reported | |
| Participants | Age range 27 to 83 years, median age 60 years (arm 1), 59 years (arm 2), and 59 years (arm 3) Locally advanced or metastatic breast cancer First‐line No prior chemotherapy | |
| Interventions | Participants randomised in a 3:3:2 ratio to arms 1, 2, 3
Arm 1: ixabepilone 16 mg/m2 IV on days 1, 8, and 15 q28 days + bevacizumab 10 mg/kg IV q14 days Arm 2: ixabepilone 40 mg/m2 IV q21 days + bevacizumab 15 mg/kg IV q21 days Arm 3: paclitaxel 90 mg/m2 IV + bevacizumab 10 mg/kg IV q14 days Treatment continued until disease progression or unacceptable toxicity Median of 6 cycles in arm 1, 7 cycles in arm 2, and 6.5 cycles in arm 3 |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Only data from arms 1 and 3 were included in this review. Arm 1 was chosen as the most appropriate control comparator (i.e. bevacizumab 10 mg/kg q14 days) to the paclitaxel arm (with the same bevacizumab dose) ClinicalTrials.gov record: http://www.clinicaltrials.gov/ct2/show/NCT00370552 For OS, data immature at the time of trial publication. For TTP, method 6 was used to estimate O‐E and V (Tierney 2007) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Women...randomized in a 3:3:2 ratio...” and "randomisation was stratified" |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described in the trial publication |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information in trial publication |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Assessment of overall survival is unlikely to be influenced by no or incomplete blinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Unclear risk | RECIST used. Comment: scans and tests probably done NCI‐CTC used, and blood tests completed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | PFS and OS analysed using randomised participants, while response rate and toxicity on evaluable‐assessable participants. 95% of participants discontinued treatment at time of analysis with reasons and number of participants provided |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes in ClinicalTrials.gov record (http://clinicaltrials.gov/show/NCT00370552) and the methods section of the trial publication are the same. All outcomes were reported in the results section |
| Other bias | Unclear risk | Differences reported in baseline characteristics |
Sjostrom.
| Methods | Accrual: 12/1994 to 10/1997 Multicentre, international Cross‐over allowed after relapse Randomisation method not specified Baseline comparability: no significant imbalance apparent or reported Median follow‐up: 11 months | |
| Participants | Pre‐ and post‐menopausal Age range 26 to 69 years, median age 50 years (arm 1) and 51 years (arm 2) 100% metastatic breast cancer 15% first‐line 85% > first‐line All participants had failed prior anthracycline therapy | |
| Interventions | Arm 1: docetaxel 100 mg/m2
Arm 2: sequential methotrexate 200 mg/m2 ‐‐> fluorouracil 600 mg/m2
Both arms q21 days for at least 6 cycles (responding and stable participants only) Median number of cycles: 6 |
|
| Outcomes | Outcomes were not reported as primary or secondary:
|
|
| Notes | 1 participant in the methotrexate‐fluorouracil arm did not have breast cancer recurrence and was not included by the trialists or in the current meta‐analysis Follow‐up details reported
Time‐to‐event analyses based on 282/283 randomised Estimate for time‐to‐event outcomes obtained from time‐to‐event curves |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (from Hakamies‐Blomqvist 2000): “...were randomised into this study...". No additional details were provided on how random assignment was achieved |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described in the trial publication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Unlikely that overall survival assessment was influenced by unblinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Unclear risk | Lesions assessed every third course of treatment and according to WHO criteria Blood tests conducted during every course of treatment; assessment based on WHO criteria |
| Blinding of outcome assessment (detection bias) QoL | High risk | EORTC QLQ‐C30 completed by participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Efficacy analyses done on ITT population. 19/283 (6.7%) participants were excluded from TTP analysis with reasons provided |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the methods were reported in the results section of the trial publication |
| Other bias | Low risk | Baseline characteristics were similar between treatment groups |
Talbot.
| Methods | Accrual: 05/1996 to 03/1997 Multicentre, international Randomisation method not specified Baseline comparability: reported that well‐balanced Median follow‐up: not reported | |
| Participants | Age range 27 to 73 years, median age 52 years in both groups 95% metastatic breast cancer Majority (% unclear) > first‐line for metastatic breast cancer All participants were either anthracycline resistant or failing |
|
| Interventions | Arm 1: paclitaxel 175 mg/m2 q21 days Arm 2: intermittent oral capecitabine (1255 mg/m2 twice daily, 2 weeks plus 1 week's rest, minimum 2 cycles) OR Arm 3: arm closed (recruited only 2 participants). Continuous oral capecitabine (666 mg/m2 twice daily) |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | No Kaplan‐Meier curves were provided for OS and TTP. Hazard ratio and confidence intervals could not be calculated as limited information provided in trial report | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomised to three treatment arms in a 1:1:1 ratio” and "patients were stratified" |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described in the trial publication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Unlikely that overall survival assessment was influenced by unblinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Unclear risk | Tumour response assessed using WHO criteria. Comment: scans were probably done for this assessment Assessed using the NCI‐CTC. Blood tests undertaken |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT population consisted of 41/44 participants; reasons for non‐inclusion were provided |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the methods were reported in the results section of the trial publication |
| Other bias | Unclear risk | Quote: “baseline demographic and tumour characteristics, which were well balanced between treatment arms”. Premature discontinuation of the trial due to results from other trial data |
TOG.
| Methods | Accrual: 12/1997 to 08/2002 Multicentre, national, non‐blinded Cross‐over allowed at progression or if no response at end of 2 cycles Randomisation performed centrally by the data centre of TOG (Turkish Oncology Group) Baseline comparability: no significant imbalance apparent or reported except for receptor status (paclitaxel/cisplatin + etoposide: ER/PR positive 30/26, ER/PR negative 13/25, unknown 53/46) Median follow‐up: not reported | |
| Participants | Age range 24 to 70 years, median age 49 years (paclitaxel) and 47 years (cisplatin + etoposide) 100% metastatic breast cancer Approximately 20% first‐line, 80% > first‐line All participants had been previously treated with anthracyclines | |
| Interventions | Arm 1: paclitaxel 175 mg/m2 IV on day 1
Arm 2: cisplatin 70 mg/m2 IV on day 1 + etoposide 50 mg PO twice daily for 7 days Both arms q21 days for up to 6 cycles At least 2 cycles of study treatment were planned unless there was clear evidence of progression following the first cycle Median number of treatment cycles was 4 (range 1 to 8) for both arms Cross‐over was allowed at the discretion of the physician |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | We contacted the trialists, and Fikri Icli provided the number of events in each group, the hazard ratio, confidence intervals, and P values for OS and TTP. Method 3 was used to estimate O‐E and V (Tierney 2007) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients were randomised, 100 to EoP and 101 to paclitaxel arms”. No additional details were provided on how random assignment was achieved |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomisation was carried out centrally by the data centre of TOG” (Turkish Oncology Group) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Unlikely that overall survival assessment was influenced by unblinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Low risk | Blood tests repeated during each cycle, X‐rays every 6 weeks. WHO criteria used to assess responses. Responses reviewed by 2 independent experts. Grade III and IV toxicity reported. Comment: blood tests and scans probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 91/100 participants and 95/101 participants randomised to cisplatin + etoposide and paclitaxel, respectively, reasons for exclusions provided. Survival data analysed on ITT principle. |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes in ClinicalTrials.gov record (http://clinicaltrials.gov/show/NCT00370552) and the methods section of the trial publication are the same. All outcomes were reported in the results section of the trial publication |
| Other bias | Low risk | Baseline characteristics were similar between treatment groups |
TRAVIOTA.
| Methods | Accrual: 09/2001 to 12/2003 Multicentre (34), national (USA) Randomisation: method not specified Baseline comparability: not uniform, differences in hormone receptor‐positive tumours, performance status of 0, liver metastases, prior adjuvant chemotherapy, and likelihood of having had metastatic disease > 2 years Median follow‐up: not reported | |
| Participants | Age range 36 to 83 years, median age 55 years (arm 1) and 50 years (arm 2) 100% metastatic breast cancer 100% first‐line | |
| Interventions | Arm 1: trastuzumab (2 mg/kg q7 days, after 1 loading dose of 4 mg/kg) + vinorelbine (25 mg/m2 q7 days) Arm 2: trastuzumab (2 mg/kg q7 days, after 1 loading dose of 4 mg/kg) + taxane (paclitaxel 80 mg/m2 or docetaxel 35 mg/m2 q7 days or paclitaxel 175 mg/m2 + carboplatin AUC6 q21 days) | |
| Outcomes | Primary:
Secondary:
|
|
| Notes | The study was closed early because of poor accrual Data values for TTP or TTF could not be calculated because length of follow‐up was unclear in paper; when follow‐up was estimated it did not contribute to censoring on the Kaplan‐Meier curve. Curve extraction of data points only (no censoring) leads to "erroneously precise values" and therefore was not conducted (Tierney 2007) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised 1:1 to receive...". No further details were provided |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described in the trial publication |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information in trial publication |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Unlikely that overall survival assessment was influenced by unblinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Low risk | Participants underwent re‐assessment (blood tests and scans): tests every 8 weeks for the first 6 months and then every 12 weeks. An independent data and safety board reviewed accrual, toxicity, and efficacy data Blood tests conducted every week. An independent data and safety board reviewed accrual, toxicity, and efficacy data |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 85 participants were randomised; 4 participants (4.7%) did not contribute baseline or follow‐up data, did not start treatment on trial, or went off study within 2 days of randomisation. Reasons for participant exclusions in response rate were provided. No description of analyses method |
| Selective reporting (reporting bias) | High risk | Trial registration details stated QoL as a secondary outcome (https://clinicaltrials.gov/ct2/show/NCT00146549), but no QoL data presented in trial publication |
| Other bias | Unclear risk | Early closure of study due to poor accrual. Baseline characteristics between groups were not uniform |
TXT.
| Methods | Accrual: 06/1995 to 07/1997 Multicentre Randomisation method not specified ("patients were randomly assigned on a one‐to‐one basis to 1 of 2 groups, stratified by accruing centre") Baseline comparability: no significant imbalance apparent or reported Median follow‐up: 30.3 months | |
| Participants | Age range 27 to 79 years, median age 54.9 years (docetaxel) and 54.55 years (FUN) 32% first‐line 68% > first‐line All participants had been pre‐treated with anthracyclines regimen | |
| Interventions | Arm 1: docetaxel 100 mg/m2
Arm 2: fluorouracil 750 mg/m2 + vinorelbine 25 mg/m2 (FUN) Both arms q21 days for a median of 6 (range 1 to 12) cycles |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Follow‐up details reported
Time‐to‐event analyses based on 176/178 randomised (2 in docetaxel arm excluded). Estimate for time‐to‐event outcomes obtained from time‐to‐event curve for OS. TTP data were added to this review update (unlike the original review). Variations in TTP definitions were accepted in the 2013 review update (see Table 3) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomly assigned on a one‐to‐one basis to 1 of 2 groups, stratified by accruing centre.” |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described in the trial publication |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information in trial publication |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Assessment of overall survival is unlikely to be influenced by no or incomplete blinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Low risk | Before each cycle, biochemical and blood tests and physical examination were conducted. "Tumour response and time‐related parameters assessed according to WHO criteria. Before each cycle, biochemical and blood tests conducted" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analyses performed on 176 out of 178 randomised participants. Number of participants censored provided |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the methods were reported in the results section of the trial publication |
| Other bias | Low risk | Baseline characteristics were similar between treatment groups |
UKCCCR AB01.
| Methods | Accrual: 1996 to 1999 Multicentre, UK and Republic of Ireland Random assignment was performed using a minimisation process stratified by centre, previous anthracyclines, site of disease, measurable/assessable disease, and WHO performance status Baseline comparability: no significant imbalance apparent and reported as well balanced Median follow‐up: not provided | |
| Participants | Age range 32 to 83 years, median 55 years (epirubicin and paclitaxel) and 54 years (epirubicin and cyclophosphamide) 100% metastatic breast cancer 100% first‐line | |
| Interventions | Arm 1: EP: epirubicin 75 mg/m2 (bolus or short infusion) followed by paclitaxel 200 mg/m2 as a 3‐hour infusion Arm 2: EC: epirubicin 75 mg/m2 followed by cyclophosphamide 600 mg/m2 (both as bolus or short infusion) Both arms q21 days for 6 cycles | |
| Outcomes | Primary:
Secondary:
|
|
| Notes | Data updated with 2005 published results Method 3 was used to estimate O‐E and V (Tierney 2007) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomly assigned to receive either EP or EC intravenously...” “Random assignment was performed using a minimization procedure stratified by centre...” |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described in the trial publication |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information in trial publication |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Assessment of overall survival is unlikely to be influenced by no or incomplete blinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Unclear risk | Blood counts monitored during and before each cycle. Radiological assessments of known disease were performed after the 3rd and 6th chemotherapy cycle and 3 months thereafter Blood counts and toxicity assessed during treatment and before each cycle |
| Blinding of outcome assessment (detection bias) QoL | High risk | FACT‐B used; a QoL result briefly mentioned in the 2001 abstract but not in the full trial publication |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analyses used ITT population for efficacy but not for toxicity or response. 71% of participants in both arms received 6 cycles of treatment; progressive disease was the main reason for participants not receiving treatment. |
| Selective reporting (reporting bias) | Low risk | Trial registration details outline outcomes as "activity and toxicity" (see http://clinicaltrials.gov/show/NCT00002953). Toxicity and other time‐to‐event outcomes reported in methods and results sections of trial publication |
| Other bias | Low risk | Baseline characteristics were similar between treatment groups |
Yardley.
| Methods | Accrual: 03/2001 to 07/2007 Randomised phase II, multicentre, national, cross‐over trial Baseline characteristics of treatment arms similar with no statistical differences Cross‐over at progression Median follow‐up: not reported | |
| Participants | Age range 31 to 87 years, median 62 years (doxorubicin) and 63 years (docetaxel) 100% metastatic breast cancer First‐line | |
| Interventions | Arm 1: liposomal doxorubicin 40 mg/m2 by 1‐hour IV infusion repeated q28 days Arm 2: docetaxel 36 mg/m2 by 30‐minute IV infusion on days 1, 8, and 15. Cycles were repeated q28 days Participants eligible at time of tumour progression to cross‐over to the other treatment arm Median treatment duration of 4 cycles in both groups |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Trial prematurely stopped at 102 participants instead of the planned 120 participants due to slow accrual For OS, we contacted the trialists, and John Hainsworth kindly provided the number of events, hazard ratio, confidence interval, and P value for OS. Method 3 was used to estimate O‐E and V (Tierney 2007). For PFS, data were extracted from the Kaplan‐Meier curve (Method 10, Tierney 2007) by 2 authors. For each time point, an average was taken. Estimated minimum follow‐up: 2 months Estimated maximum follow‐up: 48.75 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients underwent a 1:1 randomization to receive either...”. No additional details were provided on how random assignment was achieved |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described in the trial publication |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information in trial publication |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Assessment of overall survival is unlikely to be influenced by no or incomplete blinding |
| Blinding of outcome assessment (detection bias) TTP, TTF, Response rate & Toxicity | Unclear risk | RECIST used. Comment: scans and tests probably done Monitoring either by scan or echocardiogram for LVEF. Toxicity graded in line with NCI‐CTC |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. 48/50 participants on doxorubicin and 44/52 participants on docetaxel were evaluable for response to treatment; numbers and reasons for attrition were provided |
| Selective reporting (reporting bias) | Low risk | Trial registration details outline overall response rate as primary endpoint and PFS as the secondary endpoint (see: https://clinicaltrials.gov/show/NCT00193037). Both endpoints were reported in the methods and results sections of the trial publication, as well as additional endpoints OS and toxicity |
| Other bias | Unclear risk | Trial prematurely stopped |
CR: complete response ER/PR: oestrogen receptor/progesterone receptor FACT‐B: Functional Assessment of Cancer Therapy ‐ Breast HER2: human epidermal growth factor receptor 2 ITT: intention to treat LVEF: left ventricular ejection fraction MBS: Modified Brunner's score NCI‐CTC: National Cancer Institute Common Terminology Criteria OS: overall survival O‐E: observed and expected events PFS: progression‐free survival PO: oral administration PR: partial response q: every/each QoL: quality of life RECIST: Response Evaluation Criteria in Solid Tumors TTF: time to treatment failure TTP: time to progression V: variance
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brufsky 2012 | Taxane may not be in the treatment arm as investigator could choose chemotherapy regimen |
| Gebbia 2003 | This 3‐armed trial compared epidoxorubicin/cyclophosphamide (arm A), doxorubicin/paclitaxel (arm B) and epidoxorubicin/paclitaxel (arm C) as first‐line chemotherapy for metastatic breast cancer. The authors describe the trial as "centrally registered and randomised in a 1:2 fashion to arm A or arm B with stratification for previous exposure to anthracycline drugs", however all participants who had previously received anthracyclines received a taxane‐containing regimen. The authors also describe the trial as "not comparative", but it is not clear what this means. As there were clearly questions regarding the effectiveness of the randomisation process used in this trial, we decided not to include it in the review. |
| Gennari 2001 | All participants received paclitaxel as part of first‐line treatment for metastatic breast cancer and were then randomised to additional paclitaxel vs no further treatment. |
| Ghosn 2011 | All participants received vinorelbine and capecitabine (Navcap); participants with no disease progression were then randomised to Navcap or docetaxel. |
| Hamberg 2011 | Docetaxel in both arms |
| Huang 2011 | Not a randomised controlled trial |
| Sakurai 2007 | The trial included trastuzumab alone (group A) vs trastuzumab plus taxanes (group B); after 6 months, group A received taxane therapy. The primary question was the action of trastuzumab plus taxane rather than taxane versus other chemotherapeutic regimens. |
| Schmid 2005 | An atypical chemotherapy regimen (i.e. high‐dose chemotherapy) was used in only one arm. |
| Szanto 2001 | Study reported in Hungarian and translated in 2012. The paper reported the results from a single country (i.e. Hungary) of the multicentre, multinational 306 Study Group |
Characteristics of studies awaiting assessment [ordered by study ID]
TIPP.
| Methods | Accrual period not specified 2‐centre, randomised phase II study No information provided concerning baseline characteristics of treatment arms Median follow‐up: not provided |
| Participants | Age range unknown 100% metastatic breast cancer No prior chemotherapy for metastatic disease |
| Interventions | Arm 1: docetaxel 100 mg/m2 with standard oral dexamethasone premedication Arm 2: epirubicin 90 mg/m2 as IV bolus + cyclophosphamide 600 mg/m2 IV infusion For both arms, cycles repeated q21 days for a maximum of 6 cycles |
| Outcomes | Outcomes were not reported as primary or secondary:
|
| Notes | Only abstracts from conference proceedings were available Wrote to author on 10 January 2014 to ask for further details about the number of participants in each group and end results; no reply |
Xu.
| Methods | Accrual: 03/2005 to 12/2007, follow‐up completed 11/2009 Randomised phase II, open‐label, multicentre trial Conducted at 21 sites in China, Brazil, India, Mexico, South Korea, and Turkey 3‐arm trial Baseline characteristics of treatment arms were generally similar, except that gemcitabine/cisplatin arm had a longer disease‐free interval of > 24 months compared to the other 2 treatment arms Follow‐up continued until death or 24 months postrandomisation |
| Participants | Median age 49 years (paclitaxel/gemcitabine), 45 years (gemcitabine/carboplatin), and 48 years (gemcitabine/cisplatin) 100% metastatic breast cancer First‐line |
| Interventions | Arm 1: paclitaxel 150 mg/m2 3‐hour infusion followed by 2500 mg/m2 gemcitabine on day 1 repeated q14 days Arm 2: gemcitabine 2500 mg/m2 30‐60 min IV infusion followed by carboplatin 30‐60 min IV (AUC 2.5 mg/mL x min) repeated q14 days Arm 3: gemcitabine 2500 mg/m2 30‐60 min IV infusion followed by cisplatin 50 mg/m2 60 min IV q14 days Maximum of 8 cycles Median number of cycles received were: Arm 1: 8 (range 1 to 12) Arm 2: 8 (range 3 to 8) Arm 3: 7 (range 1 to 8) |
| Outcomes | Primary:
Secondary:
|
| Notes | ClinicalTrials.gov record: http://clinicaltrials.gov/show/NCT00191854 We contacted the trialists on 28 May 2014 regarding the number of events in each arm for progression‐free survival, overall survival, time to treatment failure, and clarification on adjusted hazard ratios. No reply received as yet |
NCI‐CTC: National Cancer Institute Common Terminology Criteria
Characteristics of ongoing studies [ordered by study ID]
EUCTR2012‐003530‐16‐ES.
| Trial name or title | Study evaluating weekly oral vinorelbine versus weekly paclitaxel in a population of people with advanced breast cancer |
| Methods | Randomised study, open label |
| Participants | Locally recurrent or metastatic breast cancer First‐line |
| Interventions | Weekly vinorelbine 20 mg vs vinorelbine 30 mg vs weekly paclitaxel No indication of dose of paclitaxel provided in trial record |
| Outcomes |
|
| Starting date | Date of registration: 12 November 2012 |
| Contact information | Gustavo Villanova, Pierre Fabre Medicament, email: Gustavo.villanova@pierre‐fabre.com |
| Notes | Source of support: Pierre Fabre Medicament |
EUCTR2012‐003743‐30‐SE.
| Trial name or title | A randomised trial to identify markers for personalised treatment in participants treated with bevacizumab and paclitaxel for advanced breast cancer |
| Methods | Randomised study, open label |
| Participants | Age 18‐70 years Stage IV or recurrent HER‐2 negative breast cancer First‐line treatment |
| Interventions | Bevacizumab vs paclitaxel No indication of dose and frequency provided in trial record |
| Outcomes |
|
| Starting date | Date of registration: 30 September 2012 |
| Contact information | Clinical Trials Unit, Radiumhemmet, Karolinska University Hospital, Stockholm, Sweden; email: pia.schonbeck@karolinska.se |
| Notes | Sponsor: Roche AB |
ISRCTN97330959.
| Trial name or title | Triple‐negative trial: a randomised phase III trial of carboplatin compared to docetaxel for people with advanced oestrogen receptor‐progesterone receptor‐human epidermal growth factor receptor 2 breast cancer |
| Methods | Phase III Multicentre, randomised trial |
| Participants | Women aged 18 years or older Histologically confirmed ER, PR, and HER2 negative breast cancer Measurable confirmed metastatic or recurrent locally advanced disease |
| Interventions | Arm 1: carboplatin AUC 6 q21 days for 6 cycles (18 weeks) Arm 2: docetaxel 100 mg/m2 q21 days for 6 cycles (18 weeks) |
| Outcomes |
|
| Starting date | 16 January 2008 Estimated completion date: January 2014 Accrual target: 370 to 450 participants |
| Contact information | Andrew Tutt (Principal Investigator), King's College London, Guy's and St Thomas' Hospital NHS Foundation Trust, London, United Kingdom, SE1 9RT |
| Notes | Sponsor(s): Institute of Cancer Research, United Kingdom; King's College London; Cancer Research UK; Breakthrough Breast Cancer |
JPRN‐C000000416.
| Trial name or title | Randomised study of taxane vs TS‐1 in people with metastatic or recurrent breast cancer |
| Methods | Randomised method |
| Participants | Women aged 20 to 75 years old Histologically confirmed breast cancer, distant metastasis (stage IV) in first diagnosis At least 1 assessable lesion No prior taxane administration or at least 6 months ago No prior fluorouracil administration or last administration over 6 months ago No hormonal therapy over the last 7 days |
| Interventions | Arm 1: taxane
(a) docetaxel 60 to 75 mg/m2 (1 cycle: 3 or 4 week interval)
(b) paclitaxel 175 mg/m2 (1 cycle: 3 or 4 week interval)
(c) paclitaxel 175 mg/m2 (1 cycle: every 3 weeks continuously followed by 1‐week rest period) Arm 2: TS‐1 40 to 60 mg/m2, twice a day (AM and PM) for 28 days continuously followed by 14 days rest. Total 6 weeks as 1 cycle and 4 cycles repeated or unless cancer progresses |
| Outcomes |
|
| Starting date | 1 January 2006 Estimated completion date: unknown Accrual target: 600 participants |
| Contact information | Yasuo Ohashi, 7‐3‐1, Hongo, Bunkyo‐ku, Tokyo, 113‐0033 Japan ohashi@epistat.m.u‐tokyo.ac.jp |
| Notes | Sponsor: Public Health Research Foundation |
NCT00321633.
| Trial name or title | Carboplatin or docetaxel in treating women with metastatic genetic breast cancer |
| Methods | Randomised study Multicentre, pilot study Participants stratified according to gene mutation (BRCA1, BRCA2), prior adjuvant taxane chemotherapy (yes vs no), liver or lung metastasis (yes vs no), Jewish ancestry (yes vs no), first‐line vs second‐line treatment |
| Participants | Women with histologically confirmed BRCA1 or BRAC2 mutation carrier Measurable disease defined as > 1 unidimensionally measurable lesion Patients with bone metastasis or brain metastasis are eligible Patients who have not received anthracycline chemotherapy in adjuvant setting may receive a non‐taxane, anthracycline regimen as first‐line metastatic treatment |
| Interventions | Arm 1: carboplatin IV over 1 hour on day 1
Arm 2: docetaxel IV over 1 hour on day 1 For both arms, repeat treatment q21 days for up to 6 courses in the absence of disease progression or unacceptable toxicity. If disease progresses, treatment cross‐over can occur |
| Outcomes |
|
| Starting date | January/September 2005 Estimated completion date: September 2009 Accrual target: 148 participants |
| Contact information | James Mackay, North East Thames Clinical Genetics Service, Great Ormond Street Hospital & the Institute of Child Health, 30 Guilford Street WC1 1EH, London, United Kingdom |
| Notes | Sponsor(s): University College London (UK), Breakthrough Breast Cancer, Cancer Research UK (via Clinical Trials Awards and Advisory Committee) |
NCT00490646.
| Trial name or title | A phase II combination of trastuzumab and ixabepilone vs trastuzumab and docetaxel in people with advanced or metastatic breast cancer, or both |
| Methods | Phase II, randomised study Multicentre, international |
| Participants | Women aged 18 years or older Locally advanced or metastatic HER2+ breast cancer |
| Interventions | Arm 1: ixabepilone 40 mg/m2 + trastuzumab 2 mg/kg (loading dose 4 mg/kg) q21 days, duration of combination approximately 10 cycles
Arm 2: docetaxel 100 mg/m2 + trastuzumab 2 mg/kg (loading dose 4 mg/kg) q21 days, duration of combination approximately 10 cycles Trastuzumab can continue up to 38 months in both arms. |
| Outcomes |
|
| Starting date | February 2008 Estimated completion date: November 2011 Accrual target: 80 participants |
| Contact information | Bristol‐Myers Squibb noted as study director |
| Notes | Primary sponsor: Bristol‐Myers Squibb |
NCT00600340.
| Trial name or title | A randomised phase III 2‐arm trial of paclitaxel plus bevacizumab vs capecitabine plus bevacizumab for the first‐line treatment of HER2‐negative locally recurrent or metastatic breast cancer |
| Methods | Randomised (non‐inferiority) method Multicentre, international |
| Participants | Women and men aged > 18 years Histologically or cytologically confirmed HER2‐negative adenocarcinoma of the breast with locally recurrent or metastatic breast cancer Permitted prior (neo)adjuvant chemotherapy, last dose more than 6 months prior to randomisation Permitted prior adjuvant radiotherapy, last fraction at least 6 months prior to randomisation |
| Interventions | Arm 1: bevacizumab 10 mg/kg IV days 1 and 15 q28 days + paclitaxel 90 mg/m2 days 1, 8, and 15 q28 days Arm 2: bevacizumab 15 mg/kg IV day 1 q21 days + capecitabine 1000 mg/m2 BD day 1 to 14 q21 days Treatment given until first disease progression, unacceptable toxicity, or withdrawal of patient consent |
| Outcomes | Overall survival (assessed from date of randomisation until date of death) |
| Starting date | April 2008 Estimated study completion date November 2013 Accrual target: 560 participants |
| Contact information | Christoph C Zielinski (Principal Investigator), Department of Internal Medicin I, Oncology, Medical University of Vienna, Austria |
| Notes | Sponsors/collaborators: Central European Cooperative Oncology Group |
NCT01126138.
| Trial name or title | NX vs TX as 1‐line chemotherapy on metastatic breast cancer (MBC) A randomised phase III study to investigate the efficacy and safety of vinorelbine plus capecitabine (NX) and docetaxel plus capecitabine (TX) as first‐line treatment followed by capecitabine alone as first‐line therapy on people with locally advanced and metastatic breast cancer (BOOG 2008‐03) |
| Methods | Participant recruitment underway Randomisation (non‐inferiority) method Recruiting centre in China |
| Participants | Pathologically confirmed and documented metastatic or locally advanced breast cancer (at least 1 lesion measured by radiological method) Women aged 18 years and older Permitted adjuvant or neoadjuvant chemotherapy (including anthracyclines) Permitted hormone therapy if HER2 positive Prior radiation therapy concluded 14 days before enrolment |
| Interventions | Arm 1: vinorelbine plus capecitabine for 6 cycles followed by capecitabine. Capecitabine 1000 mg/m2 PO BD (day 1 to 14); vinorelbine 25 mg/m2 IV over 3 hours on day 1 and 8, q21 days as 1 cycle and 6 cycles are required Arm 2: docetaxel plus capecitabine for 6 cycles, followed by capecitabine. Capecitabine 1000 mg/m2 PO BD (day 1 to 14); docetaxel 75 mg/m2 IV over 3 hours on day 1, q21 days as 1 cycle and 6 cycles are required. Followed by capecitabine 1000 mg/m2 PO BD (day 1 to 14), 21 days as 1 cycle until progression or unacceptable toxicity |
| Outcomes |
|
| Starting date | May 2010 (as stated on ClinicalTrials.gov) Target accrual: 200 participants |
| Contact information | Binghe Xu (Principal Investigator), Cancer Institute and Hospital, Chinese Academy of Medical Sciences, Beijing, China, 100021 |
| Notes |
NCT01303679.
| Trial name or title | First‐line treatment of bevacizumab‐taxane vs bevacizumab‐exemestane in metastatic breast cancer |
| Methods | Randomised controlled trial Open label |
| Participants | Metastatic or locally advanced breast cancer
ER‐positive; HER2‐negative Patients receiving paclitaxel‐bevacizumab first‐line chemotherapy |
| Interventions | Arm 1: paclitaxel 80 mg/m2 d1, d8, d15 + bevacizumab 10 mg/kg at d1, d15 Arm 2: exemestane 25 mg daily + bevacizumab 15 mg/kg q21 days |
| Outcomes | Primary free survival (time frame: 24 months for recruitment and 18 months for follow‐up) |
| Starting date | June 2010
Estimated completion date: May 2018
Estimated primary completion date: June 2014 (final data collection date for primary outcome measures) Target accrual: 117 participants |
| Contact information | Thomas Bachelot (Principal Investigator), ARCAGY/GINECO Group, France |
| Notes | The ClinicalTrials.gov record stated that the study has been terminated; and did not reveal any significant difference between the 2 arms |
NTR1349.
| Trial name or title | A randomised phase II study of concomitant trastuzumab, bevacizumab with paclitaxel vs trastuzumab and bevacizumab followed by the combination of trastuzumab, bevacizumab, and paclitaxel at progression as first‐line treatment of people with metastatic breast cancer with HER2/neu overexpression |
| Methods | Randomised study |
| Participants | People 18 years or older Histologically confirmed breast cancer, locally recurrent or metastatic lesions in pre‐ or post‐menopausal women Measurable lesions have at least 1 dimension as > 1 cm HER2 protein overexpression Permitted trastuzumab in the adjuvant setting as long as they received at least 10 months of therapy with trastuzumab and > 6 months have elapsed since last adjuvant administration Permitted anthracyclines in adjuvant or neoadjuvant setting if their last dose was > 6 months prior to randomisation |
| Interventions | Arm 1: trastuzumab 8 mg/kg loading dose 90 minutes IV then 6 mg/kg 30 minutes IV q21 days until progression + bevacizumab 15 mg/kg in 90 minutes on day 1 q21 days until progression + paclitaxel 90 mg/m2; day 1, 8, 15 q28 days for 6 cycles Arm 2: trastuzumab 8 mg/kg loading dose 90 minutes IV then 6 mg/kg 30 minutes IV q21 days until progression + bevacizumab 15 mg/kg IV in 90 minutes on day 1 q21 days until progression. At progression followed by trastuzumab 6 mg/kg and bevacizumab 15 mg/kg q21 days until further progression + paclitaxel 90 mg/m2 at days 1, 8, and 15 of a 4‐week cycle for 6 cycles |
| Outcomes |
|
| Starting date | 1 April 2009 Expected closing date: 1 September 2011 Target accrual: 84 participants |
| Contact information | Dr S Sleijfer, Erasmus Medical Center ‐ Daniel den Hoed, Department of Medical Oncology, Groene Hilledijk 301, 3075 EA Rotterdam, The Netherlands |
| Notes | Primary sponsor(s): Breast Cancer Study Group (BOOG) and Roche Nederland BV |
Pegram.
| Trial name or title | Phase III randomised study of XRP9881 vs capecitabine in people with locally recurrent inoperable or metastatic breast cancer that progressed after prior taxane‐ and anthracycline‐based therapy |
| Methods | Randomised study |
| Participants | Confirmed metastatic breast cancer or locally recurrent disease and inoperable with curative intent HER2/neu positive disease allowed Received prior anthracycline‐ or taxane‐based treatment in the adjuvant or metastatic setting |
| Interventions | Arm 1: XRP9881 IV over 1 hour on day 1 Arm 2: capecitabine twice daily on days 1‐14 |
| Outcomes |
|
| Starting date | Registered/published in March 2005 |
| Contact information | Mark Pegram, Protocol Chair, Jonsson Comprehensive Cancer Centre, UCLA |
| Notes | Projected accrual: 800 participants |
SAKK.
| Trial name or title | SWS‐SAKK‐22/99 Phase III randomised study of first‐line trastuzumab (Herceptin) alone followed by combination trastuzumab and paclitaxel vs first‐line combination trastuzumab and paclitaxel in women with HER2‐overexpressing metastatic breast cancer |
| Methods | |
| Participants | Women aged 18‐70 with HER2‐overexpressing metastatic breast cancer |
| Interventions | Arm 1: trastuzumab (followed by trastuzumab and paclitaxel at progression) Arm 2: trastuzumab and paclitaxel |
| Outcomes |
|
| Starting date | Not available |
| Contact information | Aron Goldhirsch, Chair, Swiss Institute for Applied Cancer Research |
| Notes | Projected accrual: 170‐250 women |
AUC: area under the curve BD: twice a day BRCA1/2: Breast Cancer (mutation) gene 1 or 2 d: day ER: oestrogen receptor HER2: human epidermal growth factor receptor 2 ITT: intention to treat NCI‐CTCAE: National Cancer Institute Common Terminology Criteria for Adverse Events PR: progesterone receptor PO: oral administration q: every/each QoL: quality of life RECIST: Response Evaluation Criteria in Solid Tumors
Differences between protocol and review
Differences between original review and review update:
'Risk of bias' assessments: In the original review, risk of bias was assessed on three domains (quality of randomisation, comparability between groups (treatment arms) at the baseline, and inclusion of all randomised participants in the analysis). In the review update, we assessed risk of bias for all the domains of the Cochrane risk of bias tool for previously included studies and new studies.
The forest plot labels for objective tumour response rates: In the original review, a pooled risk ratio was derived for tumour response rates, but the default mode in Review Manager labelled the summary statistic as odds ratio in the forest plots. In the review update, we have changed the forest plot labels to reflect the appropriate summary statistic (i.e. risk ratios for response rates).
The forest plot labels for time‐to‐event outcomes: In the original review, overall survival, time to progression, and time to treatment failure were analysed as time‐to‐event outcomes with the effect measure being the hazard ratio, however the forest plots were labelled as odds ratio plots as part of Review Manager's default mode. In the review update, we have changed the labels to reflect the appropriate summary statistic, that is hazard ratios.
We added data from the TXT study for the outcome time to progression into the review update. In the original review, the definition of time to progression did not exactly match the pre‐specified definition in the review and was therefore withdrawn by outcome.
Effect measure for toxicity: In the original review, toxicity data was narratively presented as odds ratio. In the review update, we have presented such data as risk ratios and pooled.
Subgroup analysis: A number of subgroups were specified in the original review and updated in 2013 (refer to Table 2). In the review update, some of these subgroups were possible, e.g. single taxane versus single anthracycline regimens, however subgroups related to subquestion B (e.g. substitution fluorouracil and cyclophosphamide) were still not possible because very few studies were available.
Sensitivity analysis: To test the robustness of the results for the outcomes overall survival and time to progression, we conducted a sensitivity analysis by separating high/unclear risk of bias studies from low risk of bias studies
We have removed Table 1 and Figure 3 in the original review from the review update; they can be viewed in Ghersi 2003.
Contributions of authors
DG designed the review; developed the protocol; identified, selected, and critically appraised the studies; applied eligibility criteria; extracted and entered data; analysed the data; and wrote the first draft of the 2003 review. DG reviewed and approved the final version of the review update. MW identified, selected, and critically appraised the studies; applied eligibility criteria; extracted and entered data; assessed risk of bias; analysed the data; and drafted the manuscript for the review update. MC identified, selected, and critically appraised the studies; applied eligibility criteria; extracted and entered data; assessed risk of bias; analysed the data; and drafted the manuscript for the review update. JS commented on the design of the review and the protocol and contributed to and approved the 2003 review. ED critically appraised the studies to be included in the review; applied eligibility criteria; extracted data; and reviewed the draft and final versions of the 2003 review. NW collaborated in the design of the review and the development of the protocol and reviewed the draft and final versions of the 2003 review. NW screened studies for the review update, reviewed the drafts and approved the final version of the review update.
Sources of support
Internal sources
NHMRC Clinical Trials Centre, Australia.
External sources
U.S. Army Medical Research Acquisition Activity, USA.
-
National Breast Cancer Foundation (Australia), Australia.
Provided financial support to update this priority review topic
Declarations of interest
DG: none known MW: none known MC: no relevant conflict of interest JS: no relevant conflict of interest ED: none known NW: has received honoraria from Aventis
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
303 Study Group {published data only}
- Aapro M. Docetaxel versus doxorubicin in patients with metastatic breast cancer who have failed alkylating chemotherapy: a preliminary report of the randomized phase III trial. 303 Study Group. Seminars in Oncology 1998;25(5 Supplement 12):7‐11. [PubMed] [Google Scholar]
- Chan S. Docetaxel vs doxorubicin in metastatic breast cancer resistant to alkylating chemotherapy. Oncology (Huntington) 1997;11(8 Supplement 8):19‐24. [PubMed] [Google Scholar]
- Chan S, Friedrichs K, Noel D, Duarte R, Vorobiof D, Pinter T, et al. Docetaxel (Taxotere) vs doxorubicin in patients with metastatic breast cancer (MBC) who have failed alkylating chemotherapy. Randomized multicenter phase III trial. Proceedings of American Society of Clinical Oncology; 1997. 1997; Vol. 16:Abstract 540.
- Chan S, Friedrichs K, Noel D, Pinter T, Belle S, Vorobiof D, et al. A phase III study of taxotere vs doxorubicin in patients with metastatic breast cancer who have failed an alkylating containing regimen. Breast Cancer Research and Treatment; San Antonio Breast Cancer Symposium; 1997; San Antonio. 1997; Vol. 46:Abstract 1. [MEDLINE: ]
- Chan S, Friedrichs K, Noel D, Pinter T, Belle S, Vorobiof D, et al. Prospective randomized trial of docetaxel versus doxorubicin in patients with metastatic breast cancer. John Crown for the 303 Study Group. Journal of Clinical Oncology 1999;17(8):2341‐54. [DOI] [PubMed] [Google Scholar]
- Nabholtz JM, Crown J. Phase III studies of single‐agent docetaxel in patients with metastatic breast cancer who have progressed despite previous chemotherapy regimens: preliminary results. Seminars in Oncology 1998;25(6 Supplement 13):4‐9. [PubMed] [Google Scholar]
304 Study Group {published data only}
- Nabholtz JM. Docetaxel (Taxotere) vs mitomycin C + vinblastine in patients with metastatic breast cancer (MBC) who have failed an anthracycline‐containing regimen. Preliminary evaluation of a randomized phase III study. Proceedings of American Society of Clinical Oncology; 1997. 1997; Vol. 16:Abstract 148a.
- Nabholtz JM, Crown J. Phase III studies of single‐agent docetaxel in patients with metastatic breast cancer who have progressed despite previous chemotherapy regimens: preliminary results. Seminars in Oncology 1998;25(6 Supplement 13):4‐9. [PubMed] [Google Scholar]
- Nabholtz JM, Senn HJ, Bezwoda WR, Melnychuk D, Deschenes L, Douma J, et al. Prospective randomized trial of docetaxel versus mitomycin plus vinblastine in patients with metastatic breast cancer progressing despite previous anthracycline‐containing chemotherapy. 304 Study Group. Journal of Clinical Oncology 1999;17(5):1413‐24. [DOI] [PubMed] [Google Scholar]
- Nabholtz JM, Thuerlimann B, Beswoda WR, Melnychuk D, Deschenes L, Douma J, et al. Taxotere (T) improves survival over mitomycin C vinblastine (mv) in patients (pts) with metastatic breast cancer (mbc) who have failed an anthracycline (ant) containing regimen: final results of a phase III randomized trial. Proceedings of American Society of Clinical Oncology; 1998. 1998; Vol. 17:Abstract 390.
- Nabholtz JM, Thuerlimann B, Bezwoda WR, Melnychuk D, Deschenes L, Douma J, et al. Taxotere vs mitomycin c‐vinblastine in patients with metastatic breast cancer who have failed an anthracycline containing regimen. Breast Cancer Research and Treatment; San Antonio Breast Cancer Symposium; 1997; San Antonio. 1997; Vol. 46:Abstract 401.
- Nabholtz JM, Thuerlimann B, Bezwoda WR, Melnychuk D, Deschenes L, Douma, J, et al. Docetaxel vs mitomycin plus vinblastine in anthracycline‐resistant metastatic breast cancer. Oncology (Huntington) 1997;11(8 Supplement 8):32‐7. [PubMed] [Google Scholar]
306 Study Group {published data only}
- Nabholtz JM, Falkson C, Campos D, Szanto J, Martin M, Chan S, et al. Docetaxel and doxorubicin compared with doxorubicin and cyclophosphamide as first‐line chemotherapy for metastatic breast cancer: results of a randomized, multicenter, phase III trial. Journal of Clinical Oncology 2003;21(6):968‐75. [DOI] [PubMed] [Google Scholar]
- Nabholtz JM, Falkson G, Campos D, Szanto J, Martin M, Chan S, et al. A Phase III trial comparing doxorubicin (A) and docetaxel (T) (AT) to doxorubicin and cyclophosphamide (AC) as firstline chemotherapy for MBC. Proceedings of American Society of Clinical Oncology; 1999. 1999; Vol. 18:Abstract 485.
AGO {published data only}
- Konecny G, Kuhn W, Sattler D, Thomssen C, Bauknecht T, Eidtmann H, et al. Epirubicin/paclitaxel (ET) vs epirubicin/cyclophosphamide (EC) as first line chemotherapy (CRx) in metastatic breast cancer (MBC): toxicity data and overall response rates of a randomized multicenter trial. Breast Cancer Research and Treatment; San Antonio Breast Cancer Symposium; 1998; San Antonio. 1998; Vol. 50:Abstract 222.
- Konecny G, Thomssen C, Pegram M, Luck H, Untch M, Pauletti G, et al. HER‐2/neu Gene amplification and response to paclitaxel in patients with metastatic breast cancer. Proceedings of the American Society of Clinical Oncology. 2001; Vol. 20:Abstract 88.
- Luck HJ, Thomssen C, Untch M, Kuhn W, Eidtmann H, du Bois A, et al. Multicentric Phase III Study in firstline treatment of advanced metastatic breast cancer (ABC). Epirubicin/paclitaxel (ET) vs epirubicin/cyclophosphamide (EC). A study of the Ago Breast Cancer Group. Proceedings of American Society of Clinical Oncology; 2000. 2000; Vol. 19:Abstract 280.
- Müller V, Bauknecht T, du Bois A, Eidtmann H, Jackisch C, Köhler G, et al. A randomized multicenter phase III trial comparing epirubicin and paclitaxel as first line chemotherapy in patients with metastatic breast cancer. Breast Cancer Research and Treatment; San Antonio Breast Cancer Symposium; 1999; San Antonio. 1999; Vol. 57:Abstract 331.
- Schroeder W, Luck HJ, Thomssen C, Mobus V, Kuhn W, Minckwitz G. Phase III multicenter trial of epirubicin‐paclitaxel vs epirubicin‐cyclophosphamide first‐line therapy of metastatic breast cancer. An AGO Study protocol. European Society for Medical Oncology Congress Proceedings; 1998. 1998; Vol. 9 (Supplement 4):Abstract 560.
- Minckwitz G, Kuhn W, Thomssen C, Untch M, Bauknecht T, Eidtmann D, et al. Phase III trial comparing epirubicin/paclitaxel vs epirubicin/cyclophosphamide as first line treatment in metastatic breast cancer: preliminary results of a German AGO trial. European Journal of Cancer 1998;34 (Supplement 5):S90. [Google Scholar]
ANZ TITG {published data only}
- Bishop JF, Australia and New Zealand Taxol Investigators Trials Group. Taxol versus CMFP combination therapy in advanced untreated patients with breast cancer (protocol). Trial protocol 1995.
- Bishop JF, Dewar J, Tattersall MH, Smith J, Olver I, Ackland S, et al. A randomized phase III study of Taxol (paclitaxel) vs CMFP in untreated patients with metastatic breast cancer. Proceedings of American Society of Clinical Oncology; 1996; Chicago. 1996; Vol. 15:Abstract 107.
- Bishop JF, Dewar J, Toner G, Tattersall MH, Olver I, Ackland S, et al. A randomized study of paclitaxel versus cyclophosphamide/methotrexate/5‐fluorouracil/prednisone in previously untreated patients with advanced breast cancer: preliminary results. Taxol Investigational Trials Group, Australia/New Zealand. Seminars in Oncology 1997;24(5 Supplement 17):9. [PubMed] [Google Scholar]
- Bishop JF, Dewar J, Toner GC, Smith J, Tattersall MH, Olver IN, et al. Initial paclitaxel improves outcome compared with CMFP combination chemotherapy as front‐line therapy in untreated metastatic breast cancer. Journal of Clinical Oncology 1999;17(8):2355‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bishop JF, Dewar J, Toner GC, Tattersall MH, Olver IN, Ackland S, et al. Paclitaxel as first‐line treatment for metastatic breast cancer. Oncology (Huntington) 1997;11(4 Supplement 3):19‐23. [PubMed] [Google Scholar]
Blohmer {published data only}
- Blohmer JU, Hauschild M, Hilfich J, Kleine‐Tebbe A, Kuemmel S, Lichtenegger W. [Safety and efficacy of first‐line epirubicin‐docetaxel versus epirubicin‐cyclophosphamide; updated results of multicentre randomised phase III trial in metastatic breast cancer]. Proceedings of American Society of Clinical Oncology; 2004. 2004; Vol. 22:Abstract 14S.
- Blohmer JU, Schmid P, Hilfich J, Friese K, Kleine‐Tebbe A, Koelbl H, et al. Epirubicin and cyclophosphamide versus epirubicin and docetaxel as first‐line therapy for women with metastatic breast cancer: final results of a randomised phase III trial. Annals of Oncology 2010;21:1430‐5. [DOI] [PubMed] [Google Scholar]
Bonneterre {published data only}
- Bonneterre J, Dieras V, Tubiana‐Hulin M, Bougnoux P, Bonneterre M, Delozier T, et al. 6 cycles of epirubicin / docetaxel (ET) versus 6 cycles of 5FU epirubicin / cyclophosphamide (FEC) as first line metastatic breast cancer (MBC) treatment. Proceedings of American Society of Clinical Oncology; 2001. 2001; Vol. 20:Abstract 42a.
- Bonneterre J, Dieras V, Tubiana‐Hulin M, Bougnoux P, Bonneterre ME, Delozier T, et al. Phase II multicentre randomised study of docetaxel plus epirubicin vs 5‐fluorouracil plus epirubicin and cyclophosphamide in metastatic breast cancer. British Journal of Cancer 2004;91(8):1466‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieras V, Tubiana‐Hulin M, Bougnoux P, Bonneterre M‐E, Mayer F, Delozier T, et al. 6 cycles of epirubicin/taxotere (ET) versus 6 cycles of 5FU/epirubicin/cyclophosphamide (FEC) as first line metastatic breast cancer (MBC) treatment: preliminary results of a randomised phase II trial. Breast Cancer Research and Treatment; San Antonio Breast Cancer Symposium; 2000; San Antonio. 2001; Vol. 64:Abstract 314.
Bontenbal {published data only}
- Bontenbal M, Braun JJ, Creemers GJ, Boer AC, Janssen JTP, Leys MBL, et al. Phase III study comparing AT (Adriamycin, Docetaxel) to FAC (Fluorouracil, Adriamycin, Cyclophosphamide) as first‐line chemotherapy (CT) in patients with metastatic breast cancer (MBC). European Journal of Cancer Supplements 2003;1(5):S201‐2. [Google Scholar]
- Bontenbal M, Creemers GJ, Braun HJ, Boer AC, Janssen JT, Leys RB, et al. Phase II to III study comparing doxorubicin and docetaxel with fluorouracil, doxorubicin, and cyclophosphamide as first‐line chemotherapy in patients with metastatic breast cancer: results of a Dutch community setting trial for the clinical trial group of the comprehensive cancer centre. Journal of Clinical Oncology 2005;23(28):7081‐8. [DOI] [PubMed] [Google Scholar]
CECOG BM1 {published data only}
- Zielinski C, Beslija S, Cervek J, Mrsic‐Krmpotic Z, Tchernozemsky I, Wiltschke C, et al. Gemcitabine/Epirubicin/Paclitaxel (GET) vs. 5‐fluorouracil/Epirubicin/Cyclophosphamide (FEC) as first‐line treatment in metastatic breast cancer: interim toxicity analysis of a randomised, multicenter phase III trial of the Central European Cooperative Oncology Group (CECOG). Proceedings of American Society of Clinical Oncology; 2001. 2001; Vol. 20:Abstract 1958.
- Zielinski C, Beslija S, Mrsic‐Krmpotic Z, Welnicka‐Jaskiewicz M, Wiltschke C, Kahan Z, et al. Gemcitabine, epirubicin, and paclitaxel versus fluorouracil, epirubicin, and cyclophosphamide as first‐line chemotherapy in metastatic breast cancer: a Central European Cooperative Oncology Group international, multicenter, prospective, randomized phase III trial. Journal of Clinical Oncology 2005;23(7):1401‐8. [DOI] [PubMed] [Google Scholar]
Dieras {published data only}
- Dieras V, Marty M, Tubiana N, Corette L, Morvan F, Serin D, et al. Phase II randomised study of paclitaxel versus mitomycin in advanced breast cancer. Seminars in Oncology 1995;22(4):33‐9. [PubMed] [Google Scholar]
ECOG E1193 (A) {published data only}
- Sledge GW. Doxorubicin/paclitaxel combination chemotherapy for metastatic breast cancer: The Eastern Cooperative Oncology Group experience. Seminars in Oncology 1995;22(5 Supplement 12):123‐9. [PubMed] [Google Scholar]
- Sledge GW, Jr, Neuberg D, Ingle J, Martino S, Wood W. Phase III trial of doxorubicin (A) vs paclitaxel (T) vs doxorubicin + paclitaxel (A + T) as first‐line therapy for metastatic breast cancer (MBC): an intergroup trial. Proceedings of American Society of Clinical Oncology; 1997. 1997; Vol. 16:16.
- Sledge GW, Neuberg D, Bernardo P, Ingle JN, Martino S, Rowinsky EK, et al. Phase III trial of doxorubicin, paclitaxel and the combination of doxorubicin and paclitaxel as front‐line chemotherapy for metastatic breast cancer: an intergroup trial (E1193). Journal of Clinical Oncology 2003;21(4):588‐92. [DOI] [PubMed] [Google Scholar]
- Sledge GWJ, Robert N, Sparano JA, Cogleigh M, Goldstein LJ, Neuberg D, et al. Eastern Cooperative Oncology Group studies of paclitaxel and doxorubicin in advanced breast cancer. Seminars in Oncology 1995;22:105‐8. [PubMed] [Google Scholar]
ECOG E1193 (B) {published data only}
- Sledge GW, Neuberg D, Bernardo P, Ingle JN, Martino S, Rowinsky EK, et al. Phase III trial of doxorubicin, paclitaxel and the combination of doxorubicin and paclitaxel as front‐line chemotherapy for metastatic breast cancer: an intergroup trial (E1193). Journal of Clinical Oncology 2003;21(4):588‐92. [DOI] [PubMed] [Google Scholar]
EORTC 10923 {published data only}
- Awada A, Paridaens R, Bruning P, Klijn J, Gamucci T, Houston S, et al. Doxorubicin or taxol as first‐line chemotherapy for metastatic breast cancer: Results of an EORTC‐IDBBC/ECSG randomised trial with crossover (EORTC 10923). Breast Cancer Research and Treatment; San Antonio Conference Proceedings; 1997; San Antonio. 1997; Vol. 46:Abstract 2.
- Bruning P, Piccart MJ, Klijn J, Gamucci T, Kusenda Z, Roy JA, et al. Paclitaxel (P) versus doxorubicin (D) as first line chemotherapy (CT) in advanced breast cancer (ABC): a randomized trial with crossover of the EORTC‐IDBBC in collaboration with EORTC‐ECSG. European Journal of Cancer (Supplement) 1996;32(2):50. [Google Scholar]
- Kramer JA, Curran D, Piccart M, Haes JC, Bruning P, Klijn J, et al. Identification and interpretation of clinical and quality of life prognostic factors for survival and response to treatment in first‐line chemotherapy in advanced breast cancer. European Journal of Cancer 2000;36(12):1498‐506. [DOI] [PubMed] [Google Scholar]
- Kramer JA, Curran D, Piccart M, Haes JC, Bruning PF, Klijn JG, et al. Randomised trial of paclitaxel versus doxorubicin as first‐line chemotherapy for advanced breast cancer: quality of life evaluation using the EORTC QLQ‐C30 and the Rotterdam symptom checklist. European Journal of Cancer 2000;36(12):1488‐97. [DOI] [PubMed] [Google Scholar]
- Paridaens R. Efficacy of paclitaxel or doxorubicin used as single agents in advanced breast cancer: a literature survey. Seminars in Oncology 1998;25(5 Supplement 12):3‐6. [PubMed] [Google Scholar]
- Paridaens R, Biganzoli L, Bruning P, Klijn JG, Gamucci T, Houston S, et al. Paclitaxel versus doxorubicin as first‐line single‐agent chemotherapy for metastatic breast cancer: a European Organization for Research and Treatment of Cancer Randomized Study with cross‐over. Journal of Clinical Oncology 2000;18(4):724‐33. [DOI] [PubMed] [Google Scholar]
- Paridaens R, Bruning P, Calabresi F, Awada A, Roy JA, Kusenda Z, et al. Taxol or doxorubicin as first line chemotherapy in advanced breast cancer (ABC). A prospective randomized phase II study with crossover. European Journal of Cancer (Supplement) 1995;31A(5):S75. [Google Scholar]
- Piccart‐Gebhart MJ, Bruning P, Gamucci T, Klijn J, Roy JA, Awada A, et al. An ongoing European organization for research and treatment of cancer crossover trial comparing single‐agent paclitaxel and doxorubicin as first‐ and second‐line treatment of advanced breast cancer. Seminars in Oncology 1996;23(5 Supplement 11):11‐5. [PubMed] [Google Scholar]
EORTC 10961 {published data only}
- Biganzoli L, Cufer T, Bruning P, Coleman R, Duchateau L, Calvert AH, et al. Doxorubicin and paclitaxel versus doxorubicin and cyclophosphamide as first‐line chemotherapy in metastatic breast cancer: EORTC 10961. Journal of Clinical Oncology 2002;20(14):3114‐21. [DOI] [PubMed] [Google Scholar]
- Biganzoli L, Cufer T, Bruning P, Coleman RE, Duchateau L, Rapoport B, et al. Doxorubicin‐paclitaxel: a safe regimen in terms of cardiac toxicity in metastatic breast carcinoma patients. Results from a European Organization for Research and Treatment of Cancer Multicenter Trial. Cancer 2003;97(1):40‐5. [DOI] [PubMed] [Google Scholar]
- Bottomley A, Biganzoli L, Cufer T, Coleman RE, Coens C, Efficace F, et al. Randomized, controlled trial investigating short‐term health‐related quality of life with doxorubicini and paclitaxel versus doxorubicin and cyclophosphamide as first‐line chemotherapy in patients with metastatic breast cancer: European Organization for Research and Treatment of Cancer Breast Cancer Group, Investigational Drug Branch for Breast Cancer and the New Drug Development Group Study. Journal of Clinical Oncology 2004;22(13):2576‐86. [DOI] [PubMed] [Google Scholar]
- Piccart‐Gebhart MJ. Phase III randomised study of first‐line chemotherapy with doxorubicin/paclitaxel vs doxorubicin/cyclophosphamide in metastatic breast cancer. Physician Data Query (NCI website) 1998.
EU‐93011 {unpublished data only}
- Heidemann E, Hollander N, Minckwitz G, Souchon R, Clemens MR, Mahike M, et al. Mitoxantrone (M) versus mitoxantrone plus docetaxel (MDoc) as first‐line therapy for patients with high‐risk metastatic breast cancer: results of a multicentre randomized trial. Unpublished manuscript (as supplied March 2011). Data on file.
- Heidemann E, Minckwitz GV, Hollander N, Souchon R, Clemens M, Mahike M, et al. Mitoxantrone plus docetaxel vs single agent mitoxantrone in metastatic breast cancer (MBC): results of a multicenter randomized trial. Proceedings of American Society of Clinical Oncology; 2004. 2004; Vol. 22, No 14S:637.
- NCT00002544. Mitoxantrone with or without docetaxel in treating women with metastatic breast cancer. http://clinicaltrials.gov/show/NCT00002544 (accessed 12 January 2011).
HERNATA {published data only}
- Andersson M, Lidbrink E, Bjerre K, Wist E, Enevoldsen K, Jensen AB, et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first‐line therapy of metastatic or locally advanced human epidermal growth factor receptor 2‐positive breast cancer: the HERNATA study. Journal of Clinical Oncology 2011;29(3):264‐71. [DOI] [PubMed] [Google Scholar]
Jassem {published data only}
- Jassem J, Pienkowski T, Pluzanska A, Jelic S, Gorbunova V, Berzins J, et al. Doxorubicin and paclitaxel versus fluorouracil, doxorubicin and cyclophosphamide as first‐line therapy for women with advanced breast cancer: long‐term analysis of the previously published trial. Onkologie 2009;32(8‐9):468‐72. [DOI] [PubMed] [Google Scholar]
- Jassem J, Pienkowski T, Pluzanska A, Jelic S, Gorbunova V, Mrsic‐Krmpotic Z, et al. Doxorubicin and paclitaxel versus fluorouracil, doxorubicin, and cyclophosphamide as first‐line therapy for women with metastatic breast cancer: final results of a randomized phase III multicenter trial. Journal of Clinical Oncology 2001;19(6):1707‐15. [DOI] [PubMed] [Google Scholar]
- Pluzanska A, Jassem J, Jelic S, Gorbunova V, Mrsic‐Krmpotic Z, Berzins J, et al. Randomized open‐label phase III multicenter trial comparing TAXOL/doxorubicin (AT) versus 5‐fluorouracil/doxorubicin and cyclophosphamide (FAC) as a first line treatment for patients with metastatic breast cancer. European Journal of Cancer 1999;35(Supplement 4):S314. [Google Scholar]
- Pluzanska A, Pienkowski T, Jelic S, Mrsic‐Krmpotic Z, Gorbunova V, Garin A, et al. Phase III multicenter trial comparing Taxol®/Doxorubicin (AT) vs 5‐fluorouracil/Doxorubicin and Cyclophosphamide (FAC) as a first line treatment for patients with metastatic breast cancer. Breast Cancer Research and Treatment; San Antonio Breast Cancer Symposium; 1999; San Antonio. 1999; Vol. 57:Abstract 21.
JCOG9802 {published data only}
- Katsumata N, Minami H, Aogi K, Tabei T, Sano M, Masuda N, et al. Phase III trial of doxorubicin (A)/cyclophosphamide (C) (AC), docetaxel (D), and alternating AC and D (AC‐D) as front‐line chemotherapy for metastatic breast cancer (MBC): Japan Clinical Oncology Group trial (JCOG9802). Journal of Clinical Oncology; Proceedings of American Society of Clinical Oncology; 2005. 2005; Vol. 23, No 16S:Abstract 521. [DOI] [PubMed]
- Katsumata N, Watanabe T, Minami H, Aogi K, Tabei T, Sano M, et al. Phase III trial of doxorubicin plus cyclophosphamide (AC), docetaxel, and alternating AC and docetaxel as front‐line chemotherapy for metastatic breast cancer: Japan Clinical Oncology Group trial (JCOG9802). Annals of Oncology 2009;20(7):1210‐5. [DOI] [PubMed] [Google Scholar]
Lyman {published data only}
- Lyman GH, Green SK, Ravdin PM, Geyer Jn CE, Russell CA, Balcerzak SP, et al. A Southwest Oncology Group randomized phase II study of doxorubicin and paclitaxel as frontline chemotherapy for women with metastatic breast cancer. Breast Cancer Research and Treatment 2004;85(2):143‐50. [DOI] [PubMed] [Google Scholar]
Meier {published data only}
- Meier CR, Illiger HJ, Steder M, Janssen J, Deertz H, Braun M, et al. Weekly vinorelbine versus docetaxel for metastatic breast cancer after failing anthracycline treatment. Onkologie 2008;31(8‐9):447‐53. [DOI] [PubMed] [Google Scholar]
Nabholtz {published data only}
- Mackey JR, Paterson A, Dirix LY, Dewar J, Chap L, Martin M, et al. [Final results of the phase III randomized trial comparing doectaxel (T), doxorubicin (A) and cyclophosphamide (C) to FAC as first line chemotherapy (CT) for patients (pts) with metastatic breast cancer (MBC)]. Proceedings of American Society of Clinical Oncology; 2002. 2002; Vol. 21:Abstract 137.
- Nabholtz JA, Paterson A, Dirix L, Dewar J, Chap L, Martin M, et al. A phase III randomized trial comparing docetaxel (T), doxorubicin (A) and cyclophosphamide (C) (TAC) to FAC as first line chemotherapy (CT) for patients (Pts) with metastatic breast cancer (MBC). Proceeding of American Society of Clinical Oncology; 2001. 2001; Vol. 20:Abstract 83.
Rugo {published data only}
- Rugo HS, Campone M, Amadori D, Aldrighetti D, Conte P, Wardley A, et al. A randomized, phase II, three‐arm study of two schedules of ixabepilone or paclitaxel plus bevacizumab as first‐line therapy for metastatic breast cancer. Breast Cancer Research and Treatment 2013;139(2):411‐9. [PUBMED: 23649189] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugo HS, Campone M, Amadori D, Wardley A, Villa E, Conte PF, et al. Randomized phase II study of weekly versus every‐3‐week ixabepilone plus bevaziumab (ixa/bev) versus paclitaxel plus bev (pac/bev) as first‐line therapy for metastatic breast cancer (MBC). Proceedings of American Society of Clinical Oncology; 2009. 2009; Vol. 27 No 15S:Abstract 1029.
- Rugo HS, Campone M, Amadori D, Wardley AM, Aldrighetti D, Conte PF, et al. Randomized phase II study of weekly versus every 3 week ixabepilone plus bevacizumab (ixa/bev) versus paclitaxel plus bev (pac/bev) as first‐line therapy for metastatic breast cancer (MBC): final results. Journal of Clinical Oncology; American Society of Clinical Oncology Annual Meeting Proceedings; 2010. 2010; Vol. 28 Supplement 15:Abstract 1040.
Sjostrom {published data only}
- Hakamies‐Blomqvist L, Luoma M, Sjostrom J, Pluzanska A, Sjodin M, Mouridsen H, et al. Quality of life in patients with metastatic breast cancer receiving either docetaxel or sequential methotrexate and 5‐fluorouracil. A multicentre randomised phase III trial by the Scandinavian Breast Group. European Journal of Cancer 2000;36(11):1411‐7. [DOI] [PubMed] [Google Scholar]
- Luoma ML, Hakamies‐Blomqvist L, Sjostrom J, Pluzanska A, Ottoson S, Mouridsen H, et al. Prognostic value of quality of life scores for time to progression (TTP) and overall survival time (OS) in advanced breast cancer. European Journal of Cancer 2003;39(10):1370‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sjostrom J, Blomqvist C, Mouridsen H, Pluzanska A, Ottosson‐Lonn S, Bengtsson NO, et al. Docetaxel compared with sequential methotrexate and 5‐fluorouracil in patients with advanced breast cancer after anthracycline failure: a randomised phase III study with crossover on progression by the Scandinavian Breast Group. European Journal of Cancer 1999;35(8):1194‐201. [DOI] [PubMed] [Google Scholar]
- Sjostrom J, Mouridsen H, Pluzanska A, Ottosson‐Lonn S, Bengtsson N‐O, Ostenstad B, et al. Taxotere (T) versus methotrexate‐5‐fluorouracil (MF) in patients with advanced anthracycline‐resistant breast cancer: preliminary results of a randomized phase III study by Scandanavian Breast Group. Proceedings of the American Society of Clinical Oncology. 1998; Vol. 17:Abstract 427.
Talbot {published data only}
- Talbot DC, Moiseyenko V, Belle S, O'Reilly SM, Albo Conejo E, Ackland S, et al. Randomised, phase II trial comparing oral capecitabine (Xeloda) with paclitaxel in patients with metastatic/advanced breast cancer pretreated with anthracyclines. British Journal of Cancer 2002;86(9):1367‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
TOG {published data only}
- Icli F, Akbulut H, Uner A, Bulent Y, Altinbas M, Baltali E, et al. Paclitaxel (T) vs cisplatin + VP‐16 (EP) in metastatic breast cancer patients treated with anthracyclines: A phase III randomized study, Turkish Oncology Group. Annals of Oncology. 2002; Vol. 13:Abstract 47.
- Icli F, Akbulut H, Uner A, Yalcin B, Altinbas M, Baltali E, et al. Paclitaxel (T) vs cisplatin + VP‐16 (EP) in advanced breast cancer patients pre‐treated with anthracyclines: A phase III randomized study, Turkish Oncology Group. Annals of Oncology; Proceedings of the 27th ESMO Congress; 2002; Nice. 2002; Vol. 13 Supplement 15:Abstract 167O.
- Icli F, Akbulut H, Uner A, Yalcin B, Baltali E, Altinbas M, et al. Cisplatin plus oral etoposide (EoP) combination is more effective than paclitaxel in patients with advanced breast cancer pretreated with anthracyclines: a randomised phase III trial of Turkish Oncology Group. British Journal of Cancer 2005;92:639‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
TRAVIOTA {published data only}
- Burstein HJ, Keshaviah A, Baron A, Hart R, Lambert‐Falls R, Marcom PK, et al. Trastuzumab and vinorelbine or taxane chemotherapy for HER2+ metastatic breast cancer: the TRAVIOTA study. Americal Society of Clinical Oncology Annual Meeting Proceedings; 2006; Chicago. 2006; Vol. 24, No 18S:Abstract 650.
- Burstein HJ, Keshaviah A, Baron AD, Hart RD, Lambert‐Falls R, Marcom PK, et al. Trastuzumab plus vinorelbine or taxane chemotherapy for HER2‐overexpressing metastatic breast cancer: the trastuzumab and vinorelbine or taxane study. Cancer 2007;110(5):965‐72. [DOI] [PubMed] [Google Scholar]
TXT {published data only}
- Bonneterre J, Roche H, Monnier A, Fargeot P, Namer M, Guastella JP, et al. Docetaxel (D) versus 5 fluorouracil‐vinorelbine (FUN) in patients (PTS) with metastatic breast cancer (MBC) as second line chemotherapy: a phase III study. Breast Cancer Research and Treatment; San Antonio Breast Cancer Symposium; 1998; San Antonio. 1998; Vol. 50:Abstract 223.
- Bonneterre J, Roche H, Monnier A, Guastalla JP, Namer M, Fargeot P, et al. Docetaxel vs 5‐fluorouracil plus vinorelbine in metastatic breast cancer after anthracycline therapy failure. British Journal of Cancer 2002;87(11):1210‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier A, Bonneterre J, Roche H, Fargeot P, Namer M, Guastalla JP. Phase III study: Taxotere (TXT) versus 5‐fluorouracil + navelbine (FUN) in patients (pts) with metastatic breast cancer (MBC) as 2nd line chemotherapy (CT) (preliminary results). Annals of Oncology. Congress of the European Society for Medical Oncology; 1998; Athens. 1998; Vol. 9:Abstract 580.
UKCCCR AB01 {published data only}
- Carmichael J. UKCCCR trial of epirubicin and cyclophosphamide (EC) vs epirubicin and taxol (ET) in the first line treatment of women with metastatic breast cancer (MBC). Proceedings of American Society of Clinical Oncology; 2001. 2001; Vol. 20:Abstract 84.
- Carmichael J, Jones A. A randomised trial of epirubicin & cyclophosphamide vs epirubicin & paclitaxel in metastatic breast cancer. http://www.cancer.gov/about‐cancer/treatment/clinical‐trials/search/view?cdrid=65426&version=healthprofessional (accessed 1997).
- Langley RE, Carmichael J, Jones AL, Cameron DA, Qian W, Uscinska B, et al. Phase III trial of epirubicin plus paclitaxel compared with epirubicin plus cyclophosphamide as first‐line chemotherapy for metastatic breast cancer: United Kingdom National Cancer Research Institute trial AB01. Journal of Clinical Oncology 2005;23(33):8322‐30. [DOI] [PubMed] [Google Scholar]
Yardley {published data only}
- Yardley DA, Burris HA, Spigel DR, Clark BL, Vazquez E, Shipley D, et al. A phase II randomized crossover study of liposomal doxorubicin versus weekly docetaxel in the first‐line treatment of women with metastatic breast cancer. Clinical Breast Cancer 2009;9(4):247‐52. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Brufsky 2012 {published data only}
- Brufsky A, Valero V, Tiangco B, Dakhil S, Brize A, Rugo HS, et al. Second‐line bevacizumab‐containing therapy in patients with triple‐negative breast cancer: subgroup analysis of the RIBBON‐2 trial. Breast Cancer Research and Treatment 2012;133:1067‐75. [DOI] [PubMed] [Google Scholar]
Gebbia 2003 {published data only}
- Gebbia V, Blasi L, Borsellino N, Caruso M, Leonardi V, Agostara B, et al. Paclitaxel and epidoxorubicin or doxorubicin versus cyclophosphamide and epidoxorubicin as first‐line chemotherapy for metastatic breast carcinoma: a randomised phase II study. Anticancer Research 2003;23(1B):765‐71. [MEDLINE: ] [PubMed] [Google Scholar]
Gennari 2001 {published data only}
- Gennari A, Amadori D, Lena M, Nanni O, Bruzzi P, Lorusso V, et al. Lack of benefit of maintenance paclitaxel in first‐line chemotherapy in metastatic breast cancer. Journal of Clinical Oncology 2006;24(24):3912‐8. [DOI] [PubMed] [Google Scholar]
- Gennari A, Manzione L, Mastro L, Amadori D, Lena M, Moretti G, et al. Paclitaxel (P) maintenance treatment following first line chemotherapy with anthracyclines plus paclitaxel in metastatic breast cancer (MBC): preliminary results from the Italian MANTA 1 study. Breast Cancer Research and Treatment. 2001; Vol. 64:Abstract 325.
Ghosn 2011 {published data only}
- Ghosn M, Aftimos P, Farhat FS, Kattan JG, Hanna C, Haddad N, et al. A phase II randomized study comparing navelbine and capecitabine (Navcap) followed either by Navcap or by weekly docetaxel in the first‐line treatment of HER‐2/neu negative metastatic breast cancer. Medical Oncology 2011;28:S142‐51. [DOI] [PubMed] [Google Scholar]
Hamberg 2011 {published data only}
- Hamberg P, Bos MMEM, Braun HJJ, Southard JML, Deijk GA, Erdkamp FLG, et al. Randomized phase II study comparing efficacy and safety of combination‐therapy trastuzumab and docetaxel vs. sequential therapy of trastuzumab followed by docetaxel alone at progression as first‐line chemotherapy in patients with HER2+ metastatic breast cancer: HERTAX trial. Clinical Breast Cancer 2011;11(2):103‐13. [DOI] [PubMed] [Google Scholar]
Huang 2011 {published data only}
- Huang HY, Jiang ZF, Wang T, Zhang SH, Bian L, Cao Y, et al. Efficacy and safety of regimens of capecitabine‐based chemotherapy in the treatment of advanced breast cancer. Chinese Journal of Oncology [Zhonghua Zhong Liu Za Zhi] 2011;33(11):850‐3. [PubMed] [Google Scholar]
Sakurai 2007 {published data only}
- Sakurai K, Enomoto K, Kitajima A, Tani M, Amano S. Efficacy of trastuzumab alone therapy was compared to trastuzumab plus taxane therapy in patients of advanced and metastatic breast cancers [article in Japanese]. Gan To Kagaku Ryoho 2007;34(12):1911‐3. [PubMed] [Google Scholar]
Schmid 2005 {published data only}
- Schmid P, Schippinger W, Nitsch T, Huebner G, Heilmann V, Schultze W, et al. Up‐front tandem high‐dose chemotherapy compared with standard chemotherapy with doxorubicin and paclitaxel in metastatic breast cancer: results of a randomized trial. Journal of Clinical Oncology 2005;23(3):432‐40. [DOI] [PubMed] [Google Scholar]
Szanto 2001 {published data only}
- Szanto J, Pinter T, Szanto J. Effectiveness of doxorubicin + docetaxel (DD) or doxorubicin + cyclophosphamide (DC) combination in advanced breast cancer with distant metastases. Orvosi Hetilap 2001;142(14):723‐6. [PubMed] [Google Scholar]
References to studies awaiting assessment
TIPP {published data only}
- Friedrich M, Wasemann C, Hollander M, Ertan K, Schmidt W. Randomized phase II study of docetaxel vs. epirubicin/cyclophosphamide to optimize first‐line therapy of metastatic breast cancer (MBC): preliminary results of the TIPP study. Proceedings of American Society of Clinical Oncology; 2002. 2002; Vol. 21:Abstract 2028.
- Wasemann C, Ertan AK, Schmidt W, Diedrich K, Friedrich M. Randomized phase II study of docetaxel vs epirubicin/cyclophoshamide to optimize first‐line therapy of metastatic breast cancer: end results of TIPP study. International Journal of Gynecological Cancer; 2005. 2005; Vol. 15, Supplement 2:Abstract 000408.
Xu {published data only}
- Xu B, Jiang Z, Kim SB, Yu S, Feng J, Malzyner A, et al. Biweekly gemcitabine‐paclitaxel, gemcitabine‐carboplatin, or gemcitabine‐cisplatin as first‐line treatment in metastatic breast cancer after anthracycline failure: a phase II randomized selection trial. Breast Cancer 2011;18(3):203‐12. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
EUCTR2012‐003530‐16‐ES {unpublished data only}
- EUCTR2012‐003530‐16‐ES. Randomised phase II study evaluating, as first‐line chemotherapy, weekly oral vinorelbine as a single‐agent versus weekly paclitaxel as a single‐agent in estrogen receptor positive, HER2 negative patients with advanced breast cancer. http://apps.who.int/trialsearch/trial.aspx?trialid=EUCTR2012‐003530‐16‐ES (accessed 22 July 2014).
EUCTR2012‐003743‐30‐SE {unpublished data only}
- EUCTR2012‐003743‐30‐SE. A prospective randomized Phase II study to identify predictive biomarkers and mechanisms of therapy resistance in patients with HER2‐negative metastatic breast cancer (MBC) treated with the combination of bevacizumab and paclitaxel. http://apps.who.int/trialsearch/trial.aspx?trialid=EUCTR2012‐003743‐30‐SE (accessed 22 July 2014).
ISRCTN97330959 {unpublished data only}
- ISRCTN97330959. Triple negative trial: a randomised phase III trial of carboplatin compared to docetaxel for patients with advanced oestrogen receptor‐progesterone receptor‐human epidermal growth factor receptor two‐breast cancer TNT. http://apps.who.int/trialsearch/Trial.aspx?TrialID=ISRCTN97330959 (accessed 23 July 2010).
JPRN‐C000000416 {unpublished data only}
- JPRN‐C000000416. Randomised study of taxane vs TS‐1 in metastatic or recurrent breast cancer patients. http://apps.who.int/trialsearch/Trial.aspx?TrialID=JPRN‐C000000416 (accessed 23 July 2010).
- Mukai H, Takashima T, Hozumi Y, Watanabe T, Murakami S, Masuda N, et al. Randomized study of taxane versus TS‐1 in women with metastatic or recurrent breast cancer (SELECT BC) [trial protocol]. Japanese Journal of Clinical Oncology 2010;40(8):811‐4. [DOI] [PubMed] [Google Scholar]
NCT00321633 {unpublished data only}
- NCT00321633. Carboplatin or docetaxel in treating women with metastatic genetic breast cancer. http://apps.who.int/trialsearch/Trial.aspx?TrialID=NCT00321633 (accessed 23 July 2010).
NCT00490646 {unpublished data only}
- NCT00490646. A phase II combination of trastuzumab and ixabepilone versus trastuzumab and docetaxel in patients with advanced and/or metastatic breast cancer. http://apps.who.int/trialsearch/Trial.aspx?TrialID=NCT00490646 (accessed 23 July 2010).
NCT00600340 {unpublished data only}
- NCT00600340. 2‐arm trial of paclitaxel plus bevacizumab vs. capecitabine plus bevacizumab. http://apps.who.int/trialsearch/Trial.aspx?TrialID=NCT00600340 (accessed 23 July 2010).
NCT01126138 {unpublished data only}
- NCT01126138. A randomised phase III study to investigate the efficacy and safety of docetaxel plus capecitabine vs vinorelbine plus capecitabine followed by capecitabine alone as first line therapy on locally advanced and metastatic breast cancer patients. http://apps.who.int/trialsearch/Trial.aspx?TrialID=NCT01126138 (accessed 23 July 2010).
NCT01303679 {unpublished data only}
- NCT01303679. Phase III randomised multicenter trial comparing continued maintenance therapy with the bevacizumab + taxane versus bevacizumab + substituting exemestane in patients with metastatic breast cancer or locally advanced with estrogen receptor positive and having at least a stable disease after 16 to 18 weeks of treatment with bevacizumab + taxane. http://clinicaltrials.gov/show/NCT01303679 (accessed 23 July 2014).
NTR1349 {unpublished data only}
- NTR1349. A randomised phase II study of concomitant trastuzumab, bevacizumab with paclitaxel versus trastuzumab and bevacizumab followed by the combination of trastuzumab, bevacizumab and paclitaxel at progression as first‐line treatment of patients with metastatic breast cancer with HER2‐neu overexpression. http://apps.who.int/trialsearch/Trial.aspx?TrialID=NTR1349 (accessed 23 July 2010).
Pegram {unpublished data only}
- NCT00081796/EUDRACT‐2004‐000618‐38. Phase III randomized study of XRP9881 versus capecitabine in patients with locally recurrent inoperable or metastatic breast cancer that progressed after prior taxane‐ and anthracycline‐based therapy. National Cancer Institute ‐ Clinical Trials (PDQ) 2005.
SAKK {unpublished data only}
- Goldhirsch A. Phase III randomised study of trastuzumab (Herceptin) alone followed by paclitaxel plus trastuzumab versus upfront combination of trastuzumab and paclitaxel in women with HER2 overexpressing metastatic breast cancer. CancerNet (http://ctnetdb.nci.nih.gov) 2000.
Additional references
Belfiglio 2012
- Belfiglio M, Fanizza C, Tinari N, Ficorella C, Iacobelli S, Natoli C, et al. Meta‐analysis of phase III trials of docetaxel alone or in combination with chemotherapy in metastatic breast cancer. Journal of Cancer Research and Clinical Oncology 2012;138(2):221‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beslija 2009
- Beslija S, Bonneterre J, Burstein HJ, Cocquyt V, Gnant M, Heinemann V, et al. Third consensus on medical treatment of metastatic breast cancer. Annals of Oncology 2009;20(11):1771‐85. [DOI] [PubMed] [Google Scholar]
BMS 1996
- Bristol‐Myers Squibb (BMS) Oncology. Breast Cancer: Taxane Clinical Perspectives. Princeton, New Jersey, USA: Bristol‐Myers Squibb, 1996. [Google Scholar]
Ferlay 2015
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 2015;136(5):E359‐86. [DOI] [PubMed] [Google Scholar]
Fossati 1998
- Fossati R, Confalonierir C, Torri V, Ghislandi E, Penna A, Pistotti V, et al. Cytotoxic and hormonal treatment for metastatic breast cancer: a systematic review of published randomized trials involving 31,510 women. Journal of Clinical Oncology 1998;16(10):3439‐60. [DOI] [PubMed] [Google Scholar]
Ghersi 2003
- Ghersi D, Wilcken N, Simes J, Donoghue E. Taxane containing regimens for metastatic breast cancer. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003366] [DOI] [PubMed] [Google Scholar]
Hakamies‐Blomqvist 2000
- Hakamies‐Blomqvist L, Luoma M, Sjostrom J, Pluzanska A, Sjodin M, Mouridsen H, et al. Quality of life in patients with metastatic breast cancer receiving either docetaxel or sequential methotrexate and 5‐fluorouracil. A multicentre randomised phase III trial by the Scandinavian Breast Group. European Journal of Cancer 2000;36(11):1411‐7. [DOI] [PubMed] [Google Scholar]
Hayes 1995
- Hayes DF, Henderson IC, Shapiro CL. Treatment of metastatic breast cancer: present and future prospects. Seminars in Oncology 1995;22(2):5‐21. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hortobagyi 1996
- Hortobagyi GN, Piccart‐Gebhart MJ. Current management of advanced breast cancer. Seminars in Oncology 1996;23(Supplement 11):1‐5. [PubMed] [Google Scholar]
Mauri 2010
- Mauri D, Kamposioras K, Tsali L, Bristianou M, Valachis A, Karathanasi I, et al. Overall survival benefit for weekly vs. three‐weekly taxanes regimens in advanced breast cancer: a meta‐analysis. Cancer Treatment Reviews 2010;36:69‐74. [DOI] [PubMed] [Google Scholar]
NCI 2003
- National Cancer Institute. Breast cancer (PDQ): treatment summary. http://www.cancer.gov/cancerinfo/pdq/treatment/breast/healthprofessional/ (accessed 01 February 2003).
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Piccart‐Gebhart 2008
- Piccart‐Gebhart MJ, Burzykowski T, Buyse M, Sledge G, Carmichael J, Luck HJ, et al. Taxanes alone or in combination with anthracyclines as first‐line therapy of patients with metastatic breast cancer. Journal of Clinical Oncology 2008;26(12):1980‐6. [DOI] [PubMed] [Google Scholar]
Qi 2013
- Qi WX, Shen Z, Lin F, Sun YJ, Min DL, Tang LN, et al. Paclitaxel‐based versus docetaxel‐based regimens in metastatic breast cancer: a systematic review and meta‐analysis of randomized controlled trials. Current Medical Research & Opinion 2013;29(2):117‐25. [DOI] [PubMed] [Google Scholar]
RevMan [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saad 2009
- Saad ED, Katz A. Progression‐free survival and time to progression as primary end points in advanced breast cancer: often used, sometimes loosely defined. Annals of Oncology 2009;20:460‐4. [DOI] [PubMed] [Google Scholar]
Stockler 2000
- Stockler M, Wilcken NR, Ghersi D, Simes RJ. Systematic reviews of chemotherapy and endocrine therapy in metastatic breast cancer. Cancer Treatment Reviews 2000;26(3):151‐68. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vasey 2001
- Vasey P on behalf of the Scottish Gynaecologic Cancer Trials Group. Preliminary results of the SCOTROC trial: A phase III comparison of paclitaxel‐carboplatin and docetaxel‐carboplatin as first‐line chemotherapy for stage IC‐IV epithelial ovarian cancer. Proceedings of the American Society of Clinical Oncology; 2001; Chicago. 2001; Vol. 20:Abstract 804.
Yusuf 1985
- Yusuf S, Peto R, Lewis J, Colins R, Sleight P. Beta blockade during and after myocardial infarction. An overview of randomized trials. Progress in Cardiovascular Disease 1985;27(5):335‐71. [DOI] [PubMed] [Google Scholar]


