Abstract
Background
Acute hypoxaemic respiratory failure (AHRF) is an important cause of mortality and morbidity in children. Positive pressure ventilation is currently the standard care, however, it does have complications. Continuous negative extrathoracic pressure (CNEP) ventilation or continuous positive airway pressure (CPAP) ventilation delivered via non‐invasive approaches (Ni‐CPAP) have shown certain beneficial effects in animal and uncontrolled human studies.
Objectives
To assess the effectiveness of CNEP or Ni‐CPAP compared to conventional ventilation in children (at least one month old and less than 18 years of age) with AHRF due to non‐cardiogenic causes for improving the mortality or morbidity associated with AHRF.
Search methods
We searched CENTRAL 2013, Issue 6, MEDLINE (January 1966 to June week 3, 2013), EMBASE (1980 to July 2013) and CINAHL (1982 to July 2013).
Selection criteria
Randomised or quasi‐randomised clinical trials of CNEP or Ni‐CPAP versus standard therapy (including positive pressure ventilation) involving children (from one month old to less than 18 years at time of randomisation) who met the criteria for diagnosis of AHRF with at least one of the outcomes reported.
Data collection and analysis
We assessed risk of bias of the included studies using allocation concealment, blinding of intervention, completeness of follow‐up and blinding of outcome measurements. We abstracted data on relevant outcomes and estimated the effect size by calculating risk ratio (RR) and risk difference (RD) with 95% confidence intervals (CI).
Main results
We identified two eligible studies: one of CPAP and one of CNEP (published as an abstract). Both were unblinded studies with mainly unclear risk of bias due to lack of adequate information to assess this. The CPAP study enrolled 37 children to oxygen mask and CPAP and reported improvement in respiratory rate and oxygen saturation in both arms after 30 minutes of application. The CNEP study was published as an abstract and included 33 infants with bronchiolitis. In the CNEP study there was a reduction in the fraction of inspired oxygen (FiO2) (less than 30% within one hour of initiation of therapy) in four participants in the CNEP group compared to none in the control group (RR 10.7, 95% CI 0.6 to 183.9). One infant required CPAP and mechanical ventilation in the control group while all infants in the CNEP group were managed without intubation (RR for both outcomes 0.40, 95% CI 0.02 to 9.06). None of the trials reported on mortality. No adverse events were reported in ether of the included trials.
Authors' conclusions
There is a lack of well‐designed, controlled trials of non‐invasive modes of respiratory support in children with AHRF. Studies assessing the outcomes mortality, avoidance of intubation and its associated complications, hospital stay and patient comfort are needed.
Keywords: Humans; Infant; Infant, Newborn; Bronchiolitis; Bronchiolitis/therapy; Continuous Positive Airway Pressure; Continuous Positive Airway Pressure/methods; Dengue; Dengue/complications; Hypoxia; Hypoxia/therapy; Respiration, Artificial; Respiration, Artificial/methods; Respiratory Insufficiency; Respiratory Insufficiency/therapy; Ventilators, Negative‐Pressure
Continuous negative extrathoracic pressure or continuous positive airway pressure for children with acute respiratory failure and shortage of oxygen
Children develop respiratory failure and shortage of oxygen when they have infectious or non‐infectious respiratory illnesses. Continuous negative extrathoracic pressure (CNEP) which keeps lungs open by creating negative pressure on the chest or continuous positive airway pressure (CPAP) which keeps lungs open by delivering positive pressure in the lungs during all phases of breathing are used to help increase blood oxygen levels in respiratory failure and thereby reduce organ damage and risk of death. However, the safety and efficacy of these methods of respiratory support are uncertain. The searches for this review were updated in July 2013.
We included two studies in the review: one study of CNEP included 33 participants younger than one year old who had bronchiolitis and one study of CPAP included 37 participants who had dengue fever related illness. Both studies reported short‐term improvements but no reports of clinically significant outcomes are available. With a small number of patients in both studies, the safety of either approach could not be evaluated. Both studies have methodological issues and were under‐powered (had too few patients to detect a significant difference). No adverse events were reported in ether of the included trials. Well‐designed, multicentre, controlled studies with adequate numbers of infants and which assess clinically important outcomes are needed, as we cannot comment on the safety of the intervention as it was not evaluated in the current studies. The major limitation of this review is that it has a very limited number of studies which include a very small sample of children.
Summary of findings
Summary of findings for the main comparison.
Continuous negative extrathoracic pressure ventilation compared with control for acute hypoxaemic respiratory failure in children
| Continuous negative extrathoracic pressure ventilation compared with control for acute hypoxaemic respiratory failure in children | |||
|
Patient or population: children with acute hypoxaemic respiratory failure Settings: hospital Intervention: continuous negative extrathoracic pressure Comparison: control | |||
| Outcomes | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) |
| Less than 30% FiO2 after 1 hour of therapy | RR 10.69 (0.62 to 183.85) | 33 (1) | ⊕⊝⊝⊝ very low 1,2 |
| Intermittent positive pressure ventilation during hospital stay | RR 0.40 (0.02 to 9.06) | 33 (1) | ⊕⊝⊝⊝ very low 1,3 |
| CPAP during hospital stay | RR 0.40 (0.02 to 9.06) | 33 (1) | ⊕⊝⊝⊝ very low 1,3 |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
CPAP = continuous positive extrathoracic pressure FiO2 = fraction of inspired oxygen RR = randomised controlled trial
1 = Very wide confidence intervals
2= The outcome is only reported after one hour of treatment which is a very short period for any clinically meaningful assessment
3= Limitation in study design as the results are reported in an abstract and it was not possible to assess the study adequately (see assessment of risk of biases)
Background
Description of the condition
Acute hypoxaemic respiratory failure (AHRF) is associated with high mortality and morbidity (Peters 1998). The underlying cause of AHRF is the major determinant of mortality rather than the severity of AHRF itself (Peters 1998). Major causes of AHRF in children include respiratory infection, non‐infectious respiratory diseases and interstitial pneumonitis and associated multi‐system failure. Vulnerable children may be immunodeficient, born prematurely or those with neuromuscular disorders.
Description of the intervention
Management of AHRF requires treatment of the primary cause. It also requires respiratory support, for which there are various methods including oxygen administration, continuous distending pressure (CDP) ventilation or both. CDP can be applied via positive or negative pressure ventilation. Positive pressure support can be achieved by non‐invasive or invasive continuous positive airway pressure (Ni‐CPAP or iCPAP) or positive pressure ventilation (PPV). CPAP is achieved with a face mask, nasal prongs, nasopharyngeal tube or endotracheal tube using a conventional ventilator or CPAP driver. PPV is achieved via an endotracheal tube attached to a conventional ventilator.
The purpose of CPAP is to avoid airway collapse even during the expiration phase in order to improve oxygenation (Alexander 1979). It reduces mortality and the rate of intubation in patients with cardiogenic pulmonary oedema (Pang 1998). Earlier use of CPAP was shown to be effective in increasing oxygenation in preterm infants with hyaline membrane disease (Allen 1977). Delivered via an endotracheal tube, CPAP became the most widely used form of respiratory support. More recently, with modern, often micro‐processor‐controlled, continuous negative pressure ventilators, CPAP has been delivered without endotracheal intubation.
How the intervention might work
Continuous negative extrathoracic pressure ventilation (CNEP) is an alternative, non‐invasive form of respiratory support. It is applied externally to the thorax using a negative pressure chamber with a seal around the neck to produce lung distension. CNEP was the only way of providing respiratory support prior to the advent of modern ventilators (Woollam 1976a; Woollam 1976b). In a piglet model of acute lung injury, both CNEP and positive end expiratory pressure (PEEP) were found to have similar effects on pulmonary function and the cardiovascular system (Easa 1994). Skaburskis 1987 demonstrated in a dog model of acute lung injury that CNEP provided similar improvements to positive end expiratory pressure ventilation in oxygenation without cardiac depression. Adams 1992 showed that CNEP improved oxygenation with maintenance of cardiac output in piglets with normal lungs. CNEP reduced the risks associated with intubation such as the introduction of pathogens into the lungs, impedance to venous return to heart and pulmonary circulation, and the subsequent increase in pulmonary vascular resistance (Raine 1993). Successful use of CNEP has been reported in patients with central hypoventilation (Hartmann 1994b), cystic fibrosis (Klonin 2000), postcardiac surgery (Penny 1991) and patients with phrenic nerve palsy (Raine 1992). The disadvantages of CNEP include technical difficulties, obstructed venous flow from the upper half of the body due to the neck seal and difficult access for care of the patient (McGettigan 1998).
Why it is important to do this review
A previous systematic review showed that continuous distending pressure (CPAP or CNEP) for respiratory distress syndrome in neonates reduced mortality in preterm infants despite an increased rate of pneumothoraces (Ho 2010). They included three studies of CNEP (pressure varied between 4 and 14 cm) and three studies of CPAP (applied via face mask) in their review. Uncontrolled clinical trials have shown beneficial effects of CNEP with or without assisted ventilation (Chernick 1972; Cvetnic 1990; Outerbridge 1972). A cross‐over trial of intermittent mandatory ventilation with CNEP compared with PEEP for neonatal hypoxaemia showed that rescue therapy with CNEP was effective in infants with refractory hypoxaemia and may avoid extracorporeal life support therapy (Cvetnic 1992). Gappa 1994 observed improvements in pulmonary mechanics in infants recovering from neonatal respiratory distress syndrome. CNEP may avoid the need for extracorporeal life support in infants with severe lung disease and pulmonary hypertension (Sills 1989). In adults, CNEP was shown to be associated with side effects such as apnoea or hypopnoea (Levy 1992), lower oesophageal sphincter dysfunction (Marino 1992), predisposition to the risk of aspiration and increased venous return (Borelli 1998). These side effects can be harmful to children with certain disorders such as Duchenne muscular dystrophy.
We undertook this review because the use of CNEP or Ni‐CPAP compared to PPV for AHRF has not previously been systematically evaluated in terms of relative effectiveness in children.
Objectives
To assess the effectiveness of CNEP or Ni‐CPAP compared to conventional ventilation in children (at least one month old and less than 18 years of age) with AHRF due to non‐cardiogenic causes for improving the mortality or morbidity associated with AHRF.
AHRF was defined by any of the following definitions:
alveolar‐arterial oxygenation gradient more than 100; or
arterial oxygen tension (PaO2)/fraction of inspired oxygen (FiO2) ratio less than 200 in the presence of respiratory symptoms; or
PaO2 less than 10 kPa with an FiO2 more than 0.5 with bilateral diffuse infiltrates on chest X‐ray in the absence of cardiogenic causes.
Methods
Criteria for considering studies for this review
Types of studies
We included prospective randomised controlled trials (RCTs) or quasi‐RCTs comparing CNEP (all forms of CNEP: constant pressure, variable pressure or either) or Ni‐CPAP with standard therapy (PPV with endotracheal intubation) in AHRF. We also intended to include studies with multiple cross‐over arms. We included results from published abstracts.
Types of participants
Children (at least one month old and less than 18 years) at the time of onset of therapy who had AHRF as outlined above.
Types of interventions
CNEP via a chamber enclosing the thorax and lower body with or without associated assisted PPV or Ni‐CPAP by mask, nasal prong or nasopharyngeal tube (without assisted PPV) compared with standard care (including PPV with endotracheal intubation). We included all studies with a comparison of CNEP and PPV irrespective of pressure applied.
Types of outcome measures
Primary outcomes
Mortality (both early and late, defined as less than and greater than 30 days after the diagnosis).
Improvement in oxygenation (at 24‐hourly intervals up to one week) as measured by oxygenation index (mean airway pressure x fractional concentration of oxygen x 100)/arterial oxygen tension (PaO2)) and hypoxia score (arterial oxygen tension/fractional inspired oxygen concentration ratio (PaO2/FiO2)) or improvement in PaO2 with reduction of FiO2 after starting the therapy.
Failure (use of any additional form of assisted ventilation).
Secondary outcomes
Improvement in partial pressure of carbon dioxide in arterial blood (PaCO2).
Pulmonary morbidity as judged by pulmonary air leak (any air leak, gross air leak including pneumothorax) and duration of oxygen therapy.
Number of apnoeic episodes.
Episodes of aspiration pneumonia.
Development of cardiovascular compromise leading to congestive heart failure.
Skin ulceration or destruction of nasal columella associated with CPAP with nasal prongs.
Duration of stay in the intensive care unit (ICU).
Duration of stay in the hospital.
Long‐term survival, neurodevelopmental outcomes and health‐related quality of life as defined by the primary authors.
These outcomes included all the possible and clinically relevant outcomes associated with AHRF in children. Oxygenation index and hypoxia score (PaO2/FiO2) were used as outcome measurements, as the former takes into account the extent of mechanical ventilation support needed, while the latter indicates the degree of intrapulmonary oxygen exchange.
Search methods for identification of studies
Electronic searches
For this 2013 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 6) which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (January 2011 to June week 3, 2013), EMBASE (January 2011 to July 2013) and CINAHL (January 2011 to July 2013). The previous update was in January 2011.
We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We also combined the search with the search strategy developed by Boluyt 2008 to identify child studies. See Appendix 1 for details of the CENTRAL and MEDLINE search strategy used in this update. We adapted the search strategy for EMBASE (Appendix 2) and CINAHL (Appendix 3). The MEDLINE search strategy used prior to the 2011 update is in Appendix 4.
Searching other resources
We searched ClinicalTrials.gov and WHO ICTRP for completed and ongoing trials (2 July 2013). We also searched published abstracts from the meetings of the American Thoracic Society and Pediatric Critical Care Meetings (1992 to 2010) and bibliographies of identified articles, and we contacted field experts. We imposed no language or publications restrictions. Two review authors (PS, AO) independently reviewed the searches, evaluated the titles and abstracts of all identified studies, and retrieved full texts for assessment. We reached agreement by consensus, or the third review author (JS) resolved the disagreement. All three review authors verified data entry into the Review Manager software (RevMan 2012).
Data collection and analysis
Selection of studies
We reached agreement about trial inclusion by consensus. One author (PS) selected articles from an initial list of articles for detailed evaluation. Two authors (PS, AO) performed a detailed evaluation of selected studies and resolved discrepancies by involving the third author (JS).
Data extraction and management
Two review authors (PS, JS) independently extracted data using custom‐designed data collection forms. A third review author (AO) resolved discrepancies.
Assessment of risk of bias in included studies
Each review author performed an independent assessment of trials for methodological quality according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We independently assessed each identified trial for methodological quality with respect to: random sequence generation, allocation concealment, blinding of participants and personnel, incomplete outcome data, selective reporting and other bias. We included this information in the Characteristics of included studies table and completed the 'Risk of bias' tables, addressing the above methodological issues.
1. Random sequence generation. For each included study, we described the method used to generate the allocation sequence as:
low risk of bias (any truly random process, for example, random number table, computer random number generator);
high risk of bias (any non‐random process, for example, odd or even date of birth, hospital or clinic record number); or
unclear risk of bias (insufficient information about the sequence generation process to permit judgement of 'low risk' or 'high risk').
2. Allocation concealment. For each included study, we described the method used to conceal the allocation sequence as:
low risk of bias (for example, telephone or central randomisation, consecutively numbered, sealed, opaque envelopes);
high risk of bias (open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth); or
unclear risk of bias (insufficient information to permit judgement of 'low risk' or 'high risk').
3. Blinding of participants, personnel and outcome assessors. For each included study, we described the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed the methods as:
low risk of bias, high risk of bias or unclear risk of bias for participants;
low risk of bias, high risk of bias or unclear risk of bias for study personnel; and
low risk of bias, high risk of bias or unclear risk of bias for outcome assessors and specific outcomes assessed.
4. Incomplete outcome data. For each included study and for each outcome we described the completeness of data including attrition and exclusions from the analysis. We addressed whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We assessed methods as:
low risk of bias (< 20% missing data);
high risk of bias (> 20% missing data); or
unclear risk of bias.
5. Selective outcome reporting. For each included study, we assessed the possibility of selective outcome reporting bias as:
low risk of bias (where it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study's pre‐specified outcomes have been reported, one or more reported primary outcomes were not pre‐specified, outcomes of interest are reported incompletely and so cannot be used, study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk of bias.
6. Other sources of bias. For each included study, we noted any important concerns regarding other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design). We assessed whether each study was free of other problems that could put it at risk of bias as follows:
yes (low risk of bias);
no (high risk of bias); or
unclear risk of bias.
Measures of treatment effect
We used RevMan 5.2 (RevMan 2012) for statistical analysis. We used the statistical parameters risk ratio (RR), risk difference (RD), number needed to treat for an additional beneficial outcome (NNTB), number needed to treat for an additional harmful outcome (NNTH) and mean difference (MD) when appropriate. We used 95% confidence intervals (CIs) for estimates of treatment effects.
Unit of analysis issues
We used data from one patient only once, even if the patient received the intervention more than once, to avoid dependency of data.
Dealing with missing data
We contacted primary trial authors for missing information; however, we did not receive any response.
Assessment of heterogeneity
Clinical heterogeneity is described in the Characteristics of included studies table. We tested for study heterogeneity using the I2 statistic, to assess the appropriateness of combining studies. Because of significant heterogeneity between the two trials with respect to the intervention, we did not combine them in meta‐analyses.
Assessment of reporting biases
We planned to use funnel plots to assess for publication bias if appropriate.
Data synthesis
We synthesised data using the standard methods of the Cochrane Acute Respiratory Infections Group (ARI Group). We included the RR, RD, NNTB and NNTH, derived from 1/RD. We calculated the MD for continuous outcomes. We analyzed studies using the fixed‐effect model with the Cochrane RevMan 5.2 software (RevMan 2012). As studies were heterogeneous, we did not undertake meta‐analyses but descriptively summarised the results.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses on the basis of aetiology of AHRF and according to types of therapy, i.e. CNEP and Ni‐CPAP. We planned to undertake a sensitivity analysis by trial quality (randomised or quasi‐randomised). We planned a priori subgroup analyses as follows:
children with acute respiratory failure with no underlying illness;
children with respiratory failure with underlying conditions (we planned to divide this group into further groups if sufficient numbers of children were found in any group, such as children with neuromuscular diseases, restrictive or obstructive lung diseases, and children with immunodeficiency);
if a sufficient number of studies using a similar pressure of CNEP or PPV were available, we planned a subgroup analysis with an arbitrary cut‐off level of studies comparing CPAP greater than 5 and CPAP less than or equal to 5, and CNEP greater than 15 cm of water and less than or equal to 15 cm of water; and
Ni‐CPAP given via various methods.
Sensitivity analysis
If required, we planned a sensitivity analysis based on indication and method of non‐invasive support.
Results
Description of studies
Results of the search
From the initial search we screened 197 titles and for further determination of eligibility, we read their abstracts. We retrieved 18 studies for detailed evaluation and of these two studies met the eligibility criteria (Cam 2002; Hartmann 1994a). In this review update we retrieved 182 records from the searches of the electronic literature. However, no trials were eligible for inclusion.
Included studies
The Cam 2002 study included 37 children (18 received CPAP and 18 received oxygen via mask) who had dengue shock syndrome. The inclusion criteria were infants with a clinical diagnosis of dengue shock syndrome who had acute respiratory failure, defined as failure to respond to 40% oxygen given via nasal cannula, evidenced by (a) cyanosis, oxygen saturation < 93 or PaO2 < 70 mm Hg; (b) respiratory rate > 50 breaths/minute; or (c) severe chest retraction and nasal flaring. CPAP was started at 6 cm water pressure delivered through a Beneviste valve connected to binasal prongs at a FiO2 of 60%. The oxygen mask group received oxygen via a face mask with reservoir bag at a flow rate of 6 to 8 l/min resulting in a FiO2 of 60% to 80%. The management of infants in both groups remained similar except for the intervention. The outcome reported was stabilisation of the participant with a PaO2 > 80 mm Hg after 30 minutes of treatment.
The Hartmann 1994a study was published in abstract form and included 33 infants (15 received CNEP and 18 were controls) under one year of age. The inclusion criteria were a clinical diagnosis of bronchiolitis and oxygen requirement of greater than or equal to 40% to maintain oxygen saturation between 96% and 99%. CNEP was started at ‐6 cm water pressure. Weaning from CNEP was left at the discretion of the clinicians. The management of infants in the control group was unclear from the abstract. The outcomes reported were reduction in oxygen requirement within one hour of initiating the intervention, need for intubation or CPAP, and duration of CNEP.
Excluded studies
We identified two additional relevant reports (Linney 1997; Samuels 1989); these were reports from a single institution. The Linney 1997 study was published in abstract format. This was a report of the use of CNEP ventilation in 27 infants with bronchiolitis. The study was performed in an uncontrolled manner without randomisation. The Samuels 1989 study reported on 88 infants and young children with respiratory failure due to various causes. These patients received negative pressure ventilation via a purpose‐built respirator in a non‐randomised fashion. Neither study met the entry criteria specified a priori for this review. Neither of these reports were of randomised or non‐randomised studies.
In an updated search we identified one RCT of non‐invasive positive pressure ventilation in children with lower airway obstruction (Thill 2004). The children in this study did not meet the criteria for acute hypoxaemic respiratory failure. In the subsequent review update (Shah 2008) we identified a further eight studies relevant to the objectives of the review (Codazzi 2006; Katz 2004; Padman 2004; Palombini 2004; Prado 2005; Rodriguez 2002; Shime 2006; Thia 2007). Of these eight, five studies were single‐centre and single‐arm studies (Codazzi 2006; Padman 2004; Palombini 2004; Prado 2005; Shime 2006) and one (Katz 2004) was a retrospective case‐control study. Rodriguez 2002 performed a RCT of CPAP in patients with post‐extubation laryngitis and Thia 2007 performed a RCT of children with bronchiolitis and hypercapnia. Neither of these two studies met the criteria for hypoxaemic respiratory failure. In this current review update we identified another four single‐arm studies (Carretero 2008; Chidini 2010; Essouri 2008; Stucki 2009) and one double‐arm study (Sanabria 2008) comparing CPAP delivery by two methods (helmet versus mask). We have provided the reasons for exclusion of these studies in the Characteristics of excluded studies table.
Risk of bias in included studies
Both included studies in this review were open‐label studies. The risk of bias is summarised in Figure 1 and Figure 2. One was a study of CPAP (Cam 2002) whereas the other was a study of CNEP (Hartmann 1994a).
Figure 1.
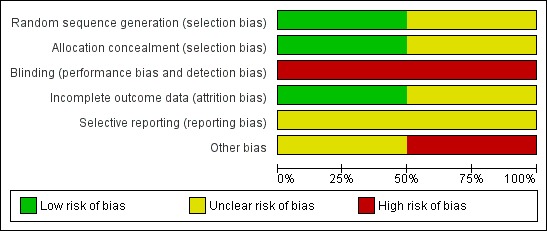
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 2.
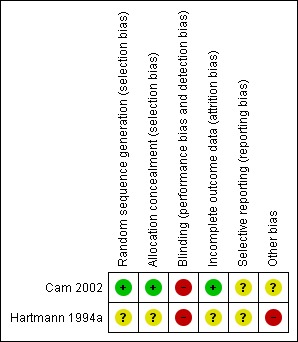
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
In Cam 2002 randomisation was performed using sealed envelopes which were randomly numbered. This was an open‐label trial. Forty‐eight participants were enrolled but 11 were later excluded (two with pneumonia, three were comatose and six had negative serodiagnosis of dengue fever). We reported outcomes for the remaining 37 infants.
In Hartmann 1994a the randomisation procedure was not reported. Masking of the intervention was practically not possible, and information regarding masking of assessment of outcome was not provided in the abstract. Outcomes were reported for all infants enrolled in the study but only some of the outcomes specified in this review were reported.
Allocation
Cam 2002 stated that envelopes were randomly numbered, whereas Hartmann 1994a did not provide information regarding allocation generation.
Blinding
Neither studies masked the intervention.
Incomplete outcome data
Both studies reported data on all eligible infants.
Selective reporting
Cam 2002 reported data in the first 30 minutes after the intervention for some of the outcomes. Hartmann 1994a reported data on completion of the trial for some of the outcomes.
Other potential sources of bias
Data from Hartmann 1994a have not been published in full.
Effects of interventions
See: Table 1
Primary outcomes
1. Mortality
This outcome was not reported in either included trial.
2. Improvement in oxygenation
In the Cam 2002 study 37 children were enrolled (19 in the oxygen mask group and 18 in the CPAP group). After 30 minutes of starting the intervention, the respiratory rate decreased significantly in the CPAP group (a mean of 55 breaths/minute at the start decreasing to a mean of 48 breaths/minute at the end of 30 minutes, compared to a mean of 60 breaths/minute at the start decreasing to a mean of 58 breaths/minute at the end of 30 minutes in the oxygen mask group; P < 0.05). Oxygen saturation increased significantly in both groups (mean of 93% at the start improving to a mean of 96% at the end of 30 minutes in the CPAP group compared to a mean of 94% at the start in the oxygen mask group improving to a mean of 98% at the end of 30 minutes in the oxygen mask group; P < 0.05). PaO2 improved significantly in both groups (a mean of 65 mm Hg at the start increasing to a mean of 120 mm Hg at the end of 30 minutes in the CPAP group, compared to a mean of 91 mm Hg at the start increasing to a mean of 146 mm Hg at the end of 30 minutes in the oxygen mask group; P < 0.01).
In the Hartmann 1994a study 15 infants were enrolled in the CNEP group and 18 infants were enrolled in the control group. After one hour of intervention the oxygen requirement was reduced to less than or equal to 30% in four infants in the CNEP group, while none of the infants in the control group had such an improvement (typical RR 10.7, 95% CI 0.6 to 183.9 and RD 0.27, 95% CI 0.02 to 0.51) (Analysis 1.1). No further respiratory parameters were reported in the study.
Analysis 1.1.
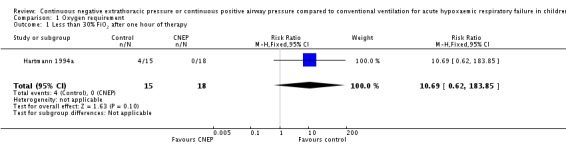
Comparison 1 Oxygen requirement, Outcome 1 Less than 30% FiO2 after one hour of therapy.
3. Failure (use of any additional form of assisted ventilation)
In the Cam 2002 study all participants in the CPAP group met the success criteria, whereas one participant in the oxygen mask group failed to respond and subsequently responded when given CPAP. After the initial 30 minutes, 12 out of 18 participants in the oxygen mask group worsened and then improved after CPAP institution. Four participants in the CPAP group worsened in the later phase and required mechanical ventilation.
In the Hartmann 1994a study none of the infants in the CNEP group required assisted ventilation or nasal CPAP, while one infant each required assisted ventilation and nasal CPAP in the control group (typical RR for both outcomes 0.40, 95% CI 0.02 to 9.06 and RD ‐0.06, 95% CI ‐0.21 to 0.10) (Analysis 2.1; Analysis 2.2). The median duration of CNEP was five (range one to seven) days.
Analysis 2.1.
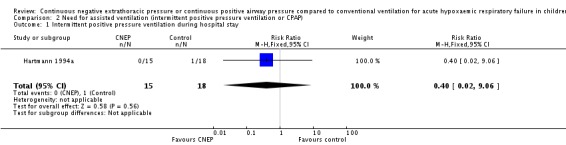
Comparison 2 Need for assisted ventilation (intermittent positive pressure ventilation or CPAP), Outcome 1 Intermittent positive pressure ventilation during hospital stay.
Analysis 2.2.
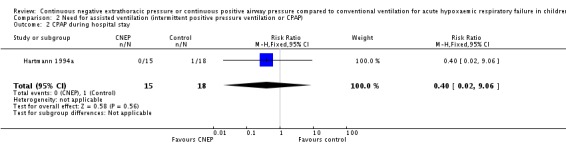
Comparison 2 Need for assisted ventilation (intermittent positive pressure ventilation or CPAP), Outcome 2 CPAP during hospital stay.
Secondary outcomes
1. Improvement in partial pressure of carbon dioxide in arterial blood
This outcome was not reported in either included trial.
2. Pulmonary morbidity as judged by pulmonary air leak (any air leak, gross air leak including pneumothorax) and duration of oxygen therapy
This outcome was not reported in either included trial.
3. Number of apnoeic episodes
This outcome was not reported in either included trial.
4. Episodes of aspiration pneumonia
This outcome was not reported in either included trial.
5. Development of cardiovascular compromise leading to congestive heart failure
This outcome was not reported in either included trial.
6. Skin ulceration or destruction of nasal columella associated with CPAP with nasal prongs
This outcome was not reported in either included trial.
7. Duration of stay in the intensive care unit (ICU)
This outcome was not reported in either included trial.
8. Duration of stay in the hospital
This outcome was not reported in either included trial.
9. Long‐term survival, neurodevelopmental outcomes and health‐related quality of life as defined by primary authors
This outcome was not reported in either included trial.
Discussion
Summary of main results
The effectiveness of either continuous negative extrathoracic pressure (CNEP) or non‐invasive continuous positive airway pressure (Ni‐CPAP) in acute hypoxaemic respiratory failure (AHRF) in children has not been evaluated in detail. We included two studies in this review, one of which is published in an abstract form only (Hartmann 1994a). Cam 2002 studied the effectiveness of CPAP over a 30‐minute period in children with dengue fever and Hartmann 1994a studied the effectiveness of CNEP in children with AHRF over one hour. Short‐term improvements in outcomes were reported in both studies with improvement in haemodynamic parameters. The severity of the underlying illness in both studies is difficult to assess from the data provided because in one study (Cam 2002) only four participants required mechanical ventilation whereas in the second study (Hartmann 1994a) only one of the control group infants required assisted ventilation.
Overall completeness and applicability of evidence
Samuels 1989 was the first to report on the use of CNEP in this population. The participants were not randomised. Reduction in oxygen requirements was evident in 75 out of 88 participants after two hours and in 74 out of 88 participants after 48 hours. There was no comparison group so it is difficult to assess the effectiveness of CNEP on mortality or other significant outcomes. Linney 1997 published a report from the same institution on a subgroup of patients with bronchiolitis. The authors showed that CNEP helped in avoiding intubation in the majority of participants (26 out of 27). This was a non‐randomised study. Samuels 1996 reported on the use of CNEP in neonatal respiratory distress syndrome in a randomised controlled trial (RCT). Infants were randomised to standard therapy or standard therapy and CNEP. CNEP resulted in a reduction in the number of infants needing intubation (5%), reduced duration of oxygen therapy and subsequent reduction in the incidence of chronic lung disease of preterm infants. However, there was a statistically insignificant increase in mortality, cranial ultrasound abnormalities and pneumothoraces in the CNEP group.
Corrado 1998, in a retrospective case‐control study, compared CNEP and conventional ventilation in the treatment of acute respiratory failure in chronic obstructive pulmonary disease (COPD) patients and found negative pressure ventilation to be equally efficacious in reducing in‐hospital mortality. Corrado 2002, in a retrospective case‐control study, compared negative pressure ventilation and non‐invasive positive pressure ventilation in the treatment of acute chronic respiratory failure in adult patients with COPD in four intermediate respiratory intensive care units (ICUs) in Italy. Both ventilatory techniques were found to be equally effective in avoiding endotracheal intubation and death.
Gorini 2001 studied seven adult patients who had acute exacerbation of COPD using a microprocessor‐based negative pressure ventilator and found that CNEP in association with negative pressure ventilation improved ventilatory patterns, arterial blood gases and reduced the work of inspiratory muscles. Negative pressure ventilation has a limitation of triggering capability. Gorini 2002 studied a microprocessor‐based negative pressure ventilator capable of thermistor triggering in four normal participants and six patients with COPD. The authors found that this technique improved the synchrony between the patient and the negative pressure ventilator. Thus, in these uncontrolled studies negative pressure ventilation has been found to be equally as efficacious as conventional mechanical ventilation in adult patients with respiratory failure, however prospective studies comparing these interventions are lacking.
Soong 1993 studied 10 infants with bronchiolitis and impending respiratory failure in a non‐randomised study. They reported improvement in symptoms, signs and physiologic parameters (heart rate, respiratory rate, PaCO2 and oxygenation index) after two hours of application of CPAP.
Hilbert 2000 evaluated adult patients who were neutropenic and had AHRF treated by CPAP. They reported that CPAP was successful in avoiding intubation in 25% of the patients.
Use of CPAP in neonates has shown some benefits but the trials were conducted in the pre‐surfactant era (Ho 2010). Recently, Declaux 2000 in a RCT of CPAP in AHRF adults showed that after one hour of therapy the subjective response to treatment and PaO2/FiO2 ratio were better in the CPAP group compared to the standard therapy group, although this was not associated with a reduced rate of intubation or in‐hospital mortality.
The use of CPAP in paediatric AHRF is increasing. Several pilot studies (Essouri 2008; Sanabria 2008; Stucki 2009) were identified recently which have used different forms of CPAP devices such as helmet, nasal prongs or high‐flow nasal cannula to generate CPAP and effectively help patients to breath easily and support respiration during acute phases. The relative ease of application prior to invasive forms of ventilation has led clinicians to use CPAP without sufficient evidence. RCTs should be conducted in this population.
The role of non‐invasive ventilation in modern clinical practice was evaluated at an international consensus conference (Evans 2001). Important issues were raised in terms of study designs. These included matching of patients in a non‐randomised fashion, the small sample size of available studies and the influence of this on confounding variables, the potential for missing undetected adverse effects in a subgroup population, practical problems associated with unblinded studies of increased care and surveillance of study participants and non‐standardised assessment of qualitative endpoints. Recommendations were made for identifying means of rapidly assessing patients who will benefit from non‐invasive ventilatory modes. In adult patients with AHRF, evidence was pointing towards the effectiveness of non‐invasive PPV in avoiding intubation, complications and mortality. However, the need for larger controlled trials was identified (Evans 2001). Recently there have been reports on the use of CNEP and CPAP. However, these were single‐centre studies with no comparative cohort or, if a comparative cohort was included, the participants did not meet the criteria for hypoxaemic respiratory failure. In light of these reports, proper studies are warranted as this practice continues to be implemented in a random fashion.
For future studies it is important to decide the importance of various outcomes. Reduction of in‐hospital mortality is a very important outcome and even a small reduction would be beneficial. However, as patients with the most severe disease are not going to be candidates for these experimental interventions, such trials would require very large sample sizes to achieve any significant reduction. Other outcomes of interest may include reduction in intubation rates, associated complications, duration of hospital stay and increased patient comfort. Standardised interventions in the control group and assessments are warranted to assess these outcomes. CNEP and Ni‐CPAP are perceived to be potentially useful in certain clinical situations where avoidance of intubation or early extubation and management by CNEP or Ni‐CPAP may be useful, but further studies are warranted.
Thus, there is insufficient evidence from randomised studies to conclude that CNEP is beneficial in AHRF in paediatric patients. Potential advantages, such as reduction in the intubation rate, intubation‐associated complications and patient comfort (less restlessness, discomfort or dyspnoea), make this modality appealing. However, properly conducted RCTs are needed to assess the benefits and risks.
Quality of the evidence
The quality of the CPAP study (Cam 2002) was relatively good, whereas the quality of the CNEP study (Hartmann 1994a) was difficult to assess as it was only published in abstract form. Both studies reported on short‐term outcomes only and had small sample sizes.
Potential biases in the review process
None.
Agreements and disagreements with other studies or reviews
Guidelines for non‐invasive management of respiratory failure have been published. However, most of the evidence stems from non‐randomised studies.
Authors' conclusions
There is a lack of well‐designed, large, controlled trials comparing the use of non‐invasive modes of respiratory support in children with acute hypoxaemic respiratory failure (AHRF). Uncontrolled evidence of a reduction in intubation and hospital stay with the use of continuous negative extrathoracic pressure (CNEP) and continuous positive airway pressure (CPAP) needs to be confirmed in well‐designed studies that evaluate the risks involved with this practice, such as pneumothorax or air leak. Studies should also stratify groups based on the severity of respiratory failure and age, and report on long‐term outcomes. It is not possible to provide any recommendations, given the lack of evidence.
There is a need for good‐quality, multicentre RCTs. Reduction in mortality is an important, albeit difficult, outcome to achieve following the use of CNEP or non‐invasive CPAP in paediatric AHRF. Even a small reduction in mortality is clinically very important and should be the aim of future studies. Studies assessing other outcomes, such as avoidance of intubation and its associated complications, reduction in hospital stay and improvement in patient comfort, are valuable in gauging the overall impact of these strategies.
Acknowledgements
The authors are sincerely grateful to Dr Edmund Hey for providing feedback on the initial draft of this review. The authors wish to thank Chris Del Mar, Nelcy Rodriguez, Edmund Hey and Marilyn Oates for commenting on the 2005 updated review and Hayley Edmonds, Anne Greenough, Max Bulsara and Nick Matheson for commenting on the 2007 updated review. Finally, we wish to thank the following for commenting on this 2011 update: Lisa Banirian, Anne Greenough, Mark Jones and Carl Heneghan.
Appendices
Appendix 1. CENTRAL and MEDLINE search strategy
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 1) which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (June 2007 to January week 3, 2011), Embase.com from (July 2007 to January 2011) and CINAHL (2007 to December 2010).
1 exp Respiratory Insufficiency/ 2 respiratory failure.tw. 3 Anoxia/ 4 hypoxia.tw. 5 hypoxem*.tw. 6 hypercapnia.tw. 7 ahrf.tw. 8 or/1‐7 9 exp Respiratory Therapy/ 10 exp Pulmonary Ventilation/ 11 Ventilators, Negative‐Pressure/ 12 positive airway pressure.tw. 13 ((positive pressure or positive‐pressure) adj2 ventilat*).tw. 14 ((negative pressure or negative‐pressure) adj2 ventilat*).tw. 15 (continuous distending pressure or cdp).tw. 16 (continuous negative extrathoracic pressure or cnep).tw. 17 (ppv or cpap).tw. 18 or/9‐17 19 8 and 18
Appendix 2. EMBASE (Elsevier) search strategy
29 #27 OR #28 835154 31 Jan 2011 28 random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR 'cross‐over':ab,ti OR volunteer*:ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR ((singl* OR doubl*) NEAR/1 blind*):ab,ti AND [embase]/lim 796108 31 Jan 2011 27 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp AND [embase]/lim 235923 31 Jan 2011 26 #20 AND #25 4070 31 Jan 2011 25 #22 OR #23 OR #241347258 31 Jan 2011 24 ((nursery OR primary OR secondary OR elementary OR high) NEAR/1 school*):ab,ti AND [embase]/lim 21451 31 Jan 2011 23 adoles*:ab,ti OR teen*:ab,ti OR boy*:ab,ti OR girl*:ab,ti OR minor*:ab,ti OR pubert*:ab,ti OR pubescen*:ab,ti OR pediatric*:ab,ti OR paediatric*:ab,ti OR kindergar*:ab,ti OR highschool*:ab,ti AND [embase]/lim 544636 31 Jan 2011 22 infant*:ab,ti OR infancy:ab,ti OR newborn*:ab,ti OR baby*:ab,ti OR babies:ab,ti OR neonat*:ab,ti OR preterm*:ab,ti OR prematur*:ab,ti OR child*:ab,ti OR schoolchild*:ab,ti OR 'school age':ab,ti OR 'school ages':ab,ti OR 'school aged':ab,ti OR preschool*:ab,ti OR kid:ab,ti OR kids:ab,ti OR toddler*:ab,ti AND [embase]/lim 1017192 31 Jan 2011 21 'infant'/exp OR 'child'/exp OR 'adolescent'/exp OR 'puberty'/exp OR 'pediatrics'/exp AND [embase]/lim 1100582 31 Jan 2011 20 #5 AND #19 20578 31 Jan 2011 19 #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 102237 31 Jan 2011 18 cdp:ab,ti OR cpap:ab,ti OR cnep:ab,ti OR ppv:ab,ti AND [embase]/lim 13142 31 Jan 2011 17 'continuous negative extrathoracic pressure':ab,ti AND [embase]/lim 26 31 Jan 2011 16 'continuous distending pressure':ab,ti AND [embase]/lim 42 31 Jan 2011 15 'positive airway pressure':ab,ti AND [embase]/lim 5393 31 Jan 2011 14 'intermittent positive pressure breathing':ab,ti OR 'intermittent positive pressure ventilation':ab,ti AND [embase]/lim 1276 31 Jan 2011 13 'negative pressure ventilation':ab,ti AND [embase]/lim 220 31 Jan 2011 12 'positive pressure respiration':ab,ti OR 'positive pressure ventilation':ab,ti AND [embase]/lim 3601 31 Jan 2011 11 'negative pressure ventilator':ab,ti OR 'negative pressure ventilators':ab,ti AND [embase]/lim 27 31 Jan 2011 10 'ventilator'/de AND [embase]/lim 7142 31 Jan 2011 9 'respiratory therapy':ab,ti OR 'artificial respiration':ab,ti AND [embase]/lim 1469 31 Jan 2011 8 'artificial ventilation'/exp AND [embase]/lim 70355 31 Jan 2011 7 'lung ventilation':ab,ti OR 'pulmonary ventilation':ab,ti AND [embase]/lim 3413 31 Jan 2011 6 'lung ventilation'/exp AND [embase]/lim 20589 31 Jan 2011 5 #1 OR #2 OR #3 OR #4 136290 31 Jan 2011 4 hypoxia:ab,ti OR hypoxem*:ab,ti OR hypercapnia:ab,ti OR anoxia:ab,ti OR ahrf:ab,ti AND [embase]/lim 75160 31 Jan 2011 3 'hypoxia'/de OR 'hypoxemia'/de OR 'anoxia'/de OR 'hypercapnia'/exp AND [embase]/lim 69416 31 Jan 2011 2 'respiratory insufficiency':ab,ti OR 'respiratory failure':ab,ti AND [embase]/lim 21461 31 Jan 2011 1 'respiratory failure'/exp AND [embase]/lim 35513 31 Jan 2011
Appendix 3. CINAHL (EBSCO) search strategy
S32 S22 and S31 S31 S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 S30 (MH "Quantitative Studies") S29 TI placebo* or AB placebo* S28 (MH "Placebos") S27 TI random* or AB random* S26 TI (singl* blind* or doubl* blind* or tripl* blind* or trebl* blind* or singl* mask* or doubl* mask* or tripl* mask* or trebl* mask* ) or AB (singl* blind* or doubl* blind* or tripl* blind* or trebl* blind* or singl* mask* or doubl* mask* or tripl* mask* or trebl* mask*) S25 TI clinic* trial* or AB clinic* trial* S24 PT clinical trial S23 (MH "Clinical Trials+") S22 S12 and S21 S21 S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 S20 TI (nursery school* or primary school* or secondary school* or elementary school* or high school*) or AB (nursery school* or primary school* or secondary school* or elementary school* or high school*) S19 TI (adoles* or teen* or boy* or girl* or minor* or pubert* or pubescen* or pediatric* or paediatric* or kindergar* or highschool*) or AB ( adoles* or teen* or boy* or girl* or minor* or pubert* or pubescen* or pediatric* or paediatric* or kindergar* or highschool*) S18 TI (infant* or infancy or newborn* or baby* or babies or neonat* or preterm* or prematur* or child* or schoolchild* or school age* or preschool* or kid or kids or toddler*) or AB (infant* or infancy or newborn* or baby* or babies or neonat* or preterm* or prematur* or child* or schoolchild* or school age* or preschool* or kid or kids or toddler*) S17 (MH "Puberty+") S16 (MH "Pediatrics+") S15 (MH "Adolescence+") S14 (MH "Child+") S13 (MH "Infant+") S12 S6 and S11 S11 S7 or S8 or S9 or S10 S10 TI (cdp or cpap or cnep or ppv) or AB (cdp or cpap or cnep or ppv) S9 TI (respiratory therapy or pulmonary ventilation or negative pressure ventilator* or positive airway pressure or positive pressure ventilat* or continuous distending pressure or continuous negative extrathoracic pressure) or AB (respiratory therapy or pulmonary ventilation or negative pressure ventilator* or positive airway pressure or positive pressure ventilat* or continuous distending pressure or continuous negative extrathoracic pressure) S8 (MH "Respiration, Artificial+") S7 (MH "Respiratory Therapy+") S6 S1 or S2 or S3 or S4 or S5 S5 TI (hypoxia or hypoxem* or hypercapnia or anoxia or ahrf ) or AB (hypoxia or hypoxem* or hypercapnia or anoxia or ahrf) S4 (MH "Hypercapnia") S3 (MH "Anoxia+") S2 TI (respiratory insufficiency or respiratory failure) or AB (respiratory insufficiency or respiratory failure) S1 (MH "Respiratory Failure+")
Appendix 4. Previous MEDLINE search strategy
We searched the Cochrane Central Register of Controlled Trials (The Cochrane Library Issue 3, 2007); MEDLINE (January 1966 to July 2007); EMBASE (1980 to July 2007); and CINAHL (1982 to July 2007).
We ran the following search terms over MEDLINE and CENTRAL and adapted them for EMBASE and CINAHL. We combined the search terms with phases 1 and 2 of the Cochrane highly sensitive search strategy for identifying controlled trials as it appears in the Cochrane Reviewers' Handbook (Appendix 5b) (Alderson 2004).
MEDLINE (OVID) 1 exp Respiratory Insufficiency/ 2 respiratory failure.mp. 3 hypoxia.mp. 4 hypoxemia.mp. 5 exp Hypercapnia/ 6 or/1‐5 7 exp VENTILATION/ 8 exp Respiratory Therapy/ 9 exp Ventilators, Negative‐Pressure/ 10 exp Positive‐Pressure Respiration/ 11 exp Pulmonary Ventilation/ 12 exp Intermittent Positive‐Pressure Breathing/ 13 exp Intermittent Positive‐Pressure Ventilation/ 14 positive airway pressure.mp. 15 PPV.mp. 16 CPAP.mp. 17 CNEP.mp. 18 CDP.mp. 19 or/7‐18 20 6 and 19 21 RANDOMIZED CONTROLLED TRIAL.pt. 22 CONTROLLED CLINICAL TRIAL.pt. 23 RANDOMIZED CONTROLLED TRIALS.sh. 24 RANDOM ALLOCATION.sh. 25 DOUBLE BLIND METHOD.sh. 26 SINGLE‐BLIND METHOD.sh. 27 or/21‐26 28 Animals/ 29 Humans/ 30 28 not 29 31 27 not 30 32 CLINICAL TRIAL.pt. 33 exp Clinical Trials/ 34 (clin$ adj25 trial$).ti,ab. 35 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 36 PLACEBOS.sh. 37 placebo$.ti,ab. 38 random$.ti,ab. 39 or/32‐38 40 39 not 30 41 31 or 40 42 20 and 41 43 exp CHILD/ 44 exp INFANT/ 45 (child or children or infant$ or newborn or neonate$ or pediatric or paediatric).mp. 46 or/43‐45 47 42 and 46
Data and analyses
Comparison 1.
Oxygen requirement
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Less than 30% FiO2 after one hour of therapy | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.69 [0.62, 183.85] |
Comparison 2.
Need for assisted ventilation (intermittent positive pressure ventilation or CPAP)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Intermittent positive pressure ventilation during hospital stay | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.02, 9.06] |
| 2 CPAP during hospital stay | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.02, 9.06] |
What's new
Last assessed as up‐to‐date: 2 July 2013.
| Date | Event | Description |
|---|---|---|
| 7 November 2013 | Amended | Summary of findings table Footnote edited to inform the quality of evidence grading. |
History
Protocol first published: Issue 3, 2002 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 2 July 2013 | New search has been performed | Searches updated but no new studies were identified for inclusion. |
| 2 July 2013 | New citation required but conclusions have not changed | Review text and 'Summary of findings' table added. Our conclusions remain unchanged. |
| 31 January 2011 | New search has been performed | Searches updated and one new trial included (Cam 2002). The conclusions remain unchanged. |
| 14 March 2005 | New search has been performed | Searches conducted. |
| 12 December 2002 | New search has been performed | Searches conducted. |
Differences between protocol and review
None.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cam 2002
| Methods | Single‐centre study in Vietnam | |
| Participants | Inclusion criteria: children with dengue fever who had acute respiratory failure as defined by failure to respond to 40% oxygen given via nasal cannula as evidenced by (a) cyanosis, oxygen saturation < 93 or PaO2 < 70 mm Hg; (b) respiratory rate > 50 breaths/minute; or (c) severe chest retraction and nasal flaring. The management of infants in both groups remained similar except for the intervention. The outcomes reported were stabilisation of the patient with PaO2 > 80 mm Hg after 30 minutes of treatment | |
| Interventions | Continuous positive airway pressure (CPAP) was started at 6 cm water pressure delivered through a Beneviste valve connected to binasal prongs at a FiO2 of 60%. The oxygen mask group received oxygen via a face mask with reservoir bag at a flow rate of 6 to 8 l/min resulting in a FiO2 of 60% to 80% | |
| Outcomes | The outcome reported was stabilisation of the patient with PaO2 > 80 mm Hg after 30 minutes of treatment Secondary outcomes included: respiratory rate, PaO2 and SaO2 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly numbered sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Randomly numbered sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported outcomes on all randomised participants |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available for checking |
| Other bias | Unclear risk | Insufficient information to assess |
Hartmann 1994a
| Methods | Single‐centre study in the UK | |
| Participants | Inclusion criteria: infants less than 1 year of age. Bronchiolitis diagnosed on a clinical basis and requirement of greater than or equal to 40% oxygen to maintain SaO2 between 96% to 99% | |
| Interventions | Control group: n = 18, 9 males Post‐menstrual age at study median 48 (range 40 to 61) weeks CNEP group: n = 15, 7 males Post‐menstrual age at study median 45 (range 41 to 56) weeks CNEP was started at ‐6 cm of water in the intervention group | |
| Outcomes | FiO2 less than 0.3 within 1 hour after start of the therapy Need for IPPV Need for nasal CPAP | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only abstract is available which is lacking in information |
| Allocation concealment (selection bias) | Unclear risk | Only abstract is available which is lacking in information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Only abstract is available which is lacking in information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only abstract is available which is lacking in information |
| Selective reporting (reporting bias) | Unclear risk | Only abstract is available which is lacking in information |
| Other bias | High risk | Only abstract is available which is lacking in information |
CNEP = continuous negative extrathoracic pressure CPAP = continuous positive airway pressure FiO2 = fraction of inspired oxygen IPPV = intermittent positive pressure ventilation PaO2 = partial arterial pressure of oxygen SaO2 = oxygen saturation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Carretero 2008 | Not a RCT |
| Chidini 2010 | Not a RCT |
| Codazzi 2006 | Not a RCT |
| Essouri 2008 | Not a RCT |
| Katz 2004 | Not a RCT |
| Linney 1997 | Not a RCT |
| Padman 2004 | Not a RCT |
| Palombini 2004 | Not a RCT |
| Prado 2005 | Not a RCT |
| Rodriguez 2002 | RCT of CPAP for post‐extubation laryngitis. The patients did not have hypoxaemic respiratory failure |
| Samuels 1989 | Not a RCT |
| Sanabria 2008 | Randomised trial of 2 methods of CPAP |
| Shime 2006 | Not a RCT |
| Stucki 2009 | Not a RCT |
| Thia 2007 | RCT of CPAP versus standard treatment in children with bronchiolitis but the inclusion criteria included mild hypercapnia. There was no hypoxaemic respiratory failure |
| Thill 2004 | RCT but children included in the study did not meet the criteria for acute hypoxaemic respiratory failure specified a priori for this review |
CPAP = continuous positive extrathoracic pressure RCT = randomised controlled trial
Contributions of authors
Dr. P Shah (PS) was responsible for the literature search and identification of trials, evaluation of methodological quality of trials, verification of data and entry into RevMan 2012, and writing the text of the review. Dr. A Ohlsson (AO) was responsible for the literature search and identification of trials, evaluation of methodological quality of trials, verification of data and revision of the review. Dr. J Shah (JS) reviewed the identified studies, acted as arbitrator in the case of disagreement, edited the review and verified data entry.
Sources of support
Internal sources
Department of Paediatrics, Mount Sinai Hospital, University of Toronto, Toronto, Canada.
External sources
No sources of support supplied
Declarations of interest
None known.
Notes
The review title was changed slightly in 2011 from 'Continuous negative extrathoracic pressure or continuous positive airway pressure for acute hypoxaemic respiratory failure in children' to 'Continuous negative extrathoracic pressure or continuous positive airway pressure compared to conventional ventilation for acute hypoxaemic respiratory failure in children' to clarify the treatment comparisons.
Edited (no change to conclusions)
References
References to studies included in this review
- Cam BV, Tuan DT, Fonsmark L, Poulsen A, Tien NM, Tuan HM, et al. Randomized comparison of oxygen mask treatment vs. nasal continuous positive airway pressure in dengue shock syndrome with acute respiratory failure. Journal of Tropical Pediatrics 2002;48(6):335‐9. [DOI] [PubMed] [Google Scholar]
- Hartman H, Noyes JP, Wright T, Wheatley R, Spencer A, Boon A, et al. Continuous negative extrathoracic pressure ventilation in infants with bronchiolitis. European Respiratory Journal 1994;7(Suppl 18):379. [Google Scholar]
References to studies excluded from this review
- Chidini G, Calderini E, Cesana BM, Gandini C, Prandi E, Pelosi P. Noninvasive continuous positive airway pressure in acute respiratory failure: helmet versus facial mask. Pediatrics 2010;126(2):330‐6. [DOI] [PubMed] [Google Scholar]
- Chidini G, Calderini E, Pelosi P. Treatment of acute hypoxemic respiratory failure with continuous positive airway pressure delivered by a new pediatric helmet in comparison with a standard full face mask: a prospective pilot study. Pediatric Critical Care Medicine 2010;11(4):502‐8. [DOI] [PubMed] [Google Scholar]
- Codazzi D, Nacoti M, Passoni M, Bonanomi E, Sperti LR, Fumagalli R. Continuous positive airway pressure with modified helmet for treatment of hypoxemic acute respiratory failure in infants and a preschool population: a feasibility study. Pediatric Critical Care Medicine 2006;7(5):455‐60. [DOI] [PubMed] [Google Scholar]
- Essouri S, Durand P, Chevret L, Haas V, Perot C, Clement A, et al. Physiological effects of noninvasive positive ventilation during acute moderate hypercapnic respiratory insufficiency in children. Intensive Care Medicine 2008;34(12):2248‐55. [DOI] [PubMed] [Google Scholar]
- Katz S, Selvadurai H, Keilty K, Mitchell M, MacLusky I. Outcome of non‐invasive positive pressure ventilation in paediatric neuromuscular disease. Archives of Diseases of Childhood 2004;89(2):121‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linney MJ, Marinaki T, Southall DP, Samuels MP. Negative pressure ventilation in bronchiolitis. Care of the Critically Ill 1997;13:161. [Google Scholar]
- Padman R, Henry M. The use of bilevel positive airway pressure for the treatment of acute chest syndrome of sickle cell disease. Delaware Medical Journal 2004;76(5):199‐203. [PubMed] [Google Scholar]
- Palombini L, Pelayo R, Guilleminault C. Efficacy of automated continuous positive airway pressure in children with sleep‐related breathing disorders in an attended setting. Pediatrics 2004;113(5):e412‐7. [DOI] [PubMed] [Google Scholar]
- Prado F, Godoy MA, Godoy M, Boza ML. Pediatric non‐invasive ventilation for acute respiratory failure in an Intermediate Care Unit. Revista Médica de Chile 2005;133(5):525‐33. [DOI] [PubMed] [Google Scholar]
- Rodriguez JA, Dessauer B, Duffau G. Non‐invasive continuous positive airways pressure for post‐extubation laryngitis in pediatric patients. Archivos de Bronconeumología 2002;38(10):463‐7. [DOI] [PubMed] [Google Scholar]
- Samuels MP, Southall DP. Negative extrathoracic pressure in treatment of respiratory failure in infants and young children. BMJ 1989;299(6710):1253‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanabria Carretero P, Palomero Rodríguez MA, Laporta Báez Y, Suso Martínez de Bujo B, Suárez Gonzalo L, Muriel Villoria C. Evaluation of a continuous positive airway pressure system without a ventilator to treat acute respiratory failure in children. Revista Española de Anestesiología y Reanimación 2008;55(10):621‐5. [DOI] [PubMed] [Google Scholar]
- Shime N, Toida C, Itoi T, Hashimoto S. Continuous negative extrathoracic pressure in children after congenital heart surgery. Critical Care and Resuscitation 2006;8(4):297‐301. [PubMed] [Google Scholar]
- Stucki P, Perez MH, Scalfaro P, Halleux Q, Vermeulen F, Cotting J. Feasibility of non‐invasive pressure support ventilation in infants with respiratory failure after extubation: a pilot study. Intensive Care Medicine 2009;35(9):1623‐7. [DOI] [PubMed] [Google Scholar]
- Thia LP, McKenzie SA, Blyth TP, Minasian CC, Kozlowska WJ, Carr SB. Randomised controlled trial of nasal continuous positive airways pressure (CPAP) in bronchiolitis. Archives of Disease in Childhood 2008;93(1):45‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Thill PJ, McGuire JK, Baden HP, Green TP, Checchia PA. Noninvasive positive‐pressure ventilation in children with lower airway obstruction. Pediatric Critical Care Medicine 2004;5(4):337‐42. [DOI] [PubMed] [Google Scholar]
Additional references
- Adams JA, Osiovich H, Goldberg RN, Suguihara C, Banaclari E. Hemodynamic effects of continuous negative extrathoracic pressure and continuous positive airway pressure in piglets with normal lungs. Biology of the Neonate 1992;62(2‐3):69‐75. [DOI] [PubMed] [Google Scholar]
- Alexander G, Gerhardt T, Banacalari E. Hyaline membrane disease. Comparison of continuous negative pressure and nasal positive airway pressure in its treatment. American Journal of Diseases of Children 1979;133(11):1156‐9. [DOI] [PubMed] [Google Scholar]
- Allen LP, Reynolds ER, Rivers RP, Souef PM, Wimberley PD. Controlled trial of continuous positive airway pressure given by face mask for hyaline membrane disease. Archives of Disease in Childhood 1977;52(5):373‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boluyt N, Tjosvold L, Lefebvre C, Klassen TP, Offringa M. Usefulness of systematic review search strategies in finding child health systematic reviews in MEDLINE. Archives of Pediatrics & Adolescent Medicine 2008;162(2):111‐6. [DOI] [PubMed] [Google Scholar]
- Borelli M, Benini A, Denkewitz T, Acciaro C, Foti G, Pesenti A. Effects of continuous negative extrathoracic pressure versus positive end‐expiratory pressure in acute lung injury patients. Critical Care Medicine 1998;26(6):1025‐31. [DOI] [PubMed] [Google Scholar]
- Chernick V, Vidyasagar D. Continuous negative chest wall pressure in hyaline membrane disease: one year experience. Pediatrics 1972;49(5):753‐60. [PubMed] [Google Scholar]
- Corrado A, Gorini M, Ginanni R, Pelagatti C, Villella G, Buoncristiano U, et al. Negative pressure ventilation versus conventional mechanical ventilation in the treatment of acute respiratory failure in COPD patients. European Respiratory Journal 1998;12(3):519‐25. [DOI] [PubMed] [Google Scholar]
- Corrado A, Confalonieri M, Marchese S, Mollica C, Villella G, Gorini M, et al. Iron lung vs mask ventilation in the treatment of acute chronic respiratory failure in COPD patients: a multicenter study. Chest 2002;121(1):189‐95. [DOI] [PubMed] [Google Scholar]
- Cvetnic WG, Cunningham MD, Sills JH, Gluck L. Reintroduction of continuous negative pressure ventilation in neonates: two‐year experience. Pediatric Pulmonology 1990;8(4):245‐53. [DOI] [PubMed] [Google Scholar]
- Cvetnic WG, Shoptaugh M, Sills JH. Intermittent mandatory ventilation with continuous negative pressure compared with positive end‐expiratory pressure for neonatal hypoxemia. Journal of Perinatology 1992;12(4):316‐24. [PubMed] [Google Scholar]
- Declaux C, L'Her E, Alberti C, Mancebo J, Abroug F, Conti G, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by face mask: a randomized controlled trial. JAMA 2000;284(18):2352‐60. [DOI] [PubMed] [Google Scholar]
- Easa D, Mundie TG, Finn KC, Hashiro G, Balaraman V. Continuous negative extrathoracic pressure versus positive end‐expiratory pressure in piglets after saline lung lavage. Pediatric Pulmonology 1994;17(3):161‐8. [DOI] [PubMed] [Google Scholar]
- Evans TW. International consensus conferences in intensive care medicine: non‐invasive positive pressure ventilation in acute respiratory failure. Intensive Care Medicine 2001;27(1):166‐78. [DOI] [PubMed] [Google Scholar]
- Gappa M, Costeloe K, Southall DP, Rabbete PS, Stocks J. Effect of continuous negative extrathoracic pressure on respiratory mechanics and timings in infants recovering from neonatal respiratory distress syndrome. Pediatric Research 1994;36(3):364‐72. [DOI] [PubMed] [Google Scholar]
- Gorini M, Corrado A, Villella G, Ginanni R, Augustynen A, Tozzi D. Physiologic effects of negative pressure ventilation in acute exacerbation of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1614‐8. [DOI] [PubMed] [Google Scholar]
- Gorini M, Villella G, Ginanni R, Augustynen A, Tozzi D, Corrado A. Effect of assist negative pressure ventilation by microprocessor based iron lung on breathing effort. Thorax 2002;57(3):258‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann H, Jawad MH, Noyes J, Samuels MP, Southall DP. Negative extrathoracic pressure ventilation in central hypoventilation syndrome. Archives of Disease in Childhood 1994;70(5):418‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Hilbert G, Gruson D, Vargas F, Valentino R, Chene G, Boiron JM, et al. Noninvasive continuous positive airway pressure in neutropenic patients with acute respiratory failure requiring intensive care unit admission. Critical Care Medicine 2000;28(9):3185‐90. [DOI] [PubMed] [Google Scholar]
- Ho JJ, Henderson‐Smart DJ, Davis PG. Early versus delayed initiation of continuous distending pressure for respiratory distress syndrome in preterm infants. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD002975] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonin H, Campbell C, Hawthron J, Southall DP, Samuels MP. Negative extrathoracic pressure in infants with cystic fibrosis and respiratory failure. Pediatric Pulmonology 2000;30(3):260‐4. [DOI] [PubMed] [Google Scholar]
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org. Chichester, UK: Wiley‐Blackwell, 2011. [Google Scholar]
- Levy RD, Cosio MG, Gibbons L, Macklem PT, Martin JG. Induction of sleep apnoea with negative pressure ventilation in patients with chronic obstructive lung disease. Thorax 1992;47(8):612‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino WD, Pitchumoni CS. Reversal of negative pressure ventilation‐induced lower esophageal sphincter dysfunction with metoclopramide. American Journal of Gastroenterology 1992;87(2):190‐4. [PubMed] [Google Scholar]
- McGettigan MC, Adolph VR, Ginsberg HG, Goldsmith JP. New ways to ventilate newborns in acute respiratory failure: a systematic review. Pediatric Clinics of North America 1998;45(3):475‐509. [DOI] [PubMed] [Google Scholar]
- Outerbridge EW, Roloff DW, Stern L. Continuous negative pressure in the management of severe respiratory distress syndrome. Journal of Pediatrics 1972;81(2):384‐91. [DOI] [PubMed] [Google Scholar]
- Pang D, Keenan SP, Cook DJ, Sibbald WJ. The effect of positive pressure airway support on mortality and the need for intubation in cardiogenic pulmonary edema. Chest 1998;114(4):1185‐92. [DOI] [PubMed] [Google Scholar]
- Penny DJ, Hayek Z, Redington AN. The effects of positive and negative extrathoracic pressure ventilation on pulmonary blood flow after the total cavopulmonary shunt procedure. International Journal of Cardiology 1991;30(1):128‐30. [DOI] [PubMed] [Google Scholar]
- Peters MJ, Tasker RC, Kiff KM, Yates R, Hatch DJ. Acute hypoxemic respiratory failure in children: case mix and utility of respiratory severity indices. Intensive Care Medicine 1998;24(7):699‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine J, Samuels MP, Mok Q, Shinebourne EA, Southall DP. Negative extrathoracic pressure ventilation for phrenic nerve palsy after paediatric cardiac surgery. British Heart Journal 1992;67(4):308‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine J, Redington AN, Benatar A, Samuels MP, Southall DP. Continuous negative extrathoracic pressure and cardiac output‐a pilot study. European Journal of Pediatrics 1993;152(7):595‐8. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
- Samuels MP, Raine J, Wright T, Alexander JA, Lockyer K, Spencer SA, et al. Continuous negative extrathoracic pressure in neonatal respiratory failure. Pediatrics 1996;98(6 Pt 1):1154‐60. [PubMed] [Google Scholar]
- Sills JH, Cvetnick WG, Pietz J. Continuous negative pressure in the treatment of infants with pulmonary hypertension and respiratory failure. Journal of Perinatology 1989;9(1):43‐8. [PubMed] [Google Scholar]
- Skaburskis M, Helal R, Zidulka A. Hemodynamic effects of external continuous negative pressure ventilation compared with those of continuous positive pressure ventilation in dogs with acute lung injury. American Review of Respiratory Diseases 1987;136(4):886‐91. [DOI] [PubMed] [Google Scholar]
- Soong WJ, Hwang B, Tang RB. Continuous positive airway pressure by nasal prongs in bronchiolitis. Pediatric Pulmonology 1993;16(3):163‐6. [DOI] [PubMed] [Google Scholar]
- Woollam CH. The development of apparatus for intermittent negative pressure respiration. Anaesthesia 1976;31(4):537‐47. [DOI] [PubMed] [Google Scholar]
- Woollam CH. The development of apparatus for intermittent negative pressure respiration. (2) 1919‐1976, with special reference to the development and uses of cuirass respirators. Anaesthesia 1976;31(5):666‐85. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Shah PS, Ohlsson A, Shah JP. Continuous negative extrathoracic pressure or continuous positive airway pressure for acute hypoxemic respiratory failure in children. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003699.pub3] [DOI] [PubMed] [Google Scholar]
- Shah PS, Ohlsson A, Shah JP. Continuous negative extrathoracic pressure or continuous positive airway pressure for acute hypoxemic respiratory failure in children. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003699.pub3] [DOI] [PubMed] [Google Scholar]
- Shah PS, Ohlsson A, Shah J. Continuous negative extrathoracic pressure or continuous positive airway pressure compared to conventional ventilation for acute hypoxaemic respiratory failure in children. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD003699.pub3; PUBMED: 18254028] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PS, Ohlsson A, Shah J. Continuous negative extrathoracic pressure or continuous positive airway pressure compared to conventional ventilation for acute hypoxaemic respiratory failure in children. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD003699.pub3; PUBMED: 18254028] [DOI] [PMC free article] [PubMed] [Google Scholar]


