Abstract
Background
Stroke is one of the leading causes of disability worldwide. Functional impairment, resulting in poor performance in activities of daily living (ADLs) among stroke survivors is common. Current rehabilitation approaches have limited effectiveness in improving ADL performance, function, muscle strength and cognitive abilities (including spatial neglect) after stroke, but a possible adjunct to stroke rehabilitation might be non‐invasive brain stimulation by transcranial direct current stimulation (tDCS) to modulate cortical excitability, and hence to improve ADL performance, arm and leg function, muscle strength and cognitive abilities (including spatial neglect), dropouts and adverse events in people after stroke.
Objectives
To assess the effects of tDCS on ADLs, arm and leg function, muscle strength and cognitive abilities (including spatial neglect), dropouts and adverse events in people after stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register (February 2015), the Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library; 2015, Issue 2), MEDLINE (1948 to February 2015), EMBASE (1980 to February 2015), CINAHL (1982 to February 2015), AMED (1985 to February 2015), Science Citation Index (1899 to February 2015) and four additional databases. In an effort to identify further published, unpublished and ongoing trials, we searched trials registers and reference lists, handsearched conference proceedings and contacted authors and equipment manufacturers.
Selection criteria
This is the update of an existing review. In the previous version of this review we focused on the effects of tDCS on ADLs and function. In this update, we broadened our inclusion criteria to compare any kind of active tDCS for improving ADLs, function, muscle strength and cognitive abilities (including spatial neglect) versus any kind of placebo or control intervention.
Data collection and analysis
Two review authors independently assessed trial quality and risk of bias (JM and MP) and extracted data (BE and JM). If necessary, we contacted study authors to ask for additional information. We collected information on dropouts and adverse events from the trial reports.
Main results
We included 32 studies involving a total of 748 participants aged above 18 with acute, postacute or chronic ischaemic or haemorrhagic stroke. We also identified 55 ongoing studies. The risk of bias did not differ substantially for different comparisons and outcomes.
We found nine studies with 396 participants examining the effects of tDCS versus sham tDCS (or any other passive intervention) on our primary outcome measure, ADLs after stroke. We found evidence of effect regarding ADL performance at the end of the intervention period (standardised mean difference (SMD) 0.24, 95% confidence interval (CI) 0.03 to 0.44; inverse variance method with random‐effects model; moderate quality evidence). Six studies with 269 participants assessed the effects of tDCS on ADLs at the end of follow‐up, and found improved ADL performance (SMD 0.31, 95% CI 0.01 to 0.62; inverse variance method with random‐effects model; moderate quality evidence). However, the results did not persist in a sensitivity analysis including only trials of good methodological quality.
One of our secondary outcome measures was upper extremity function: 12 trials with a total of 431 participants measured upper extremity function at the end of the intervention period, revealing no evidence of an effect in favour of tDCS (SMD 0.01, 95% CI ‐0.48 to 0.50 for studies presenting absolute values (low quality evidence) and SMD 0.32, 95% CI ‐0.51 to 1.15 (low quality evidence) for studies presenting change values; inverse variance method with random‐effects model). Regarding the effects of tDCS on upper extremity function at the end of follow‐up, we identified four studies with a total of 187 participants (absolute values) that showed no evidence of an effect (SMD 0.01, 95% CI ‐0.48 to 0.50; inverse variance method with random‐effects model; low quality evidence). Ten studies with 313 participants reported outcome data for muscle strength at the end of the intervention period, but in the corresponding meta‐analysis there was no evidence of an effect. Three studies with 156 participants reported outcome data on muscle strength at follow‐up, but there was no evidence of an effect.
In six of 23 studies (26%), dropouts, adverse events or deaths that occurred during the intervention period were reported, and the proportions of dropouts and adverse events were comparable between groups (risk difference (RD) 0.01, 95% CI ‐0.02 to 0.03; Mantel‐Haenszel method with random‐effects model; low quality evidence; analysis based only on studies that reported either on dropouts, or on adverse events, or on both). However, this effect may be underestimated due to reporting bias.
Authors' conclusions
At the moment, evidence of very low to moderate quality is available on the effectiveness of tDCS (anodal/cathodal/dual) versus control (sham/any other intervention) for improving ADL performance after stroke. However, there are many ongoing randomised trials that could change the quality of evidence in the future. Future studies should particularly engage those who may benefit most from tDCS after stroke and in the effects of tDCS on upper and lower limb function, muscle strength and cognitive abilities (including spatial neglect). Dropouts and adverse events should be routinely monitored and presented as secondary outcomes. They should also address methodological issues by adhering to the Consolidated Standards of Reporting Trials (CONSORT) statement.
Plain language summary
Direct electrical current to the brain to improve rehabilitation outcomes
Review question
We reviewed the evidence about the effect of direct electrical current to the brain (transcranial direct current stimulation, tDCS) to reduce impairment in activities of daily living (ADLs), arm and leg function, muscle strength and cognitive abilities (including spatial neglect), dropouts and adverse events in people after stroke.
Background
Stroke is one of the leading causes of disability worldwide. Most strokes take place when a blood clot blocks a blood vessel leading to the brain. Without a proper blood supply, the brain quickly suffers damage, which can be permanent. This damage often causes impairment of ADLs and motor function among stroke survivors. Current rehabilitation strategies have limited effectiveness in improving these impairments. One possibility for enhancing the effects of rehabilitation might be the addition of non‐invasive brain stimulation through a technique known as transcranial direct current stimulation (tDCS). This technique can alter how the brain works and may be used to reduce impairment of ADLs and function. However, the effectiveness of this intervention for improving rehabilitation outcomes is still unknown.
Search date
The review is current to February 2015.
Study characteristics
We included 32 studies involving a total of 748 participants aged above 18 with acute, postacute or chronic ischaemic or haemorrhagic stroke. The mean age in the experimental groups ranged from 43 years up to 70 years and from 45 years up to 75 years in the control groups. The level of participants' impairment ranged from severe to moderate. The majority of studies were conducted in an inpatient setting. Different stimulation types (anodal, cathodal, dual) of tDCS with different stimulation durations and dosages were administered and compared with sham tDCS or an active control intervention. Sham tDCS means that the stimulation is switched off covertly in the first minute of the intervention.
Key results
This review found that tDCS might enhance ADLs, but it is still uncertain if arm and leg function, muscle strength and cognitive abilities may be improved. Proportions of adverse events and people discontinuing the treatment were comparable between groups. Included studies differed in terms of type, location and duration of stimulation, amount of current delivered, electrode size and positioning as well as type and location of stroke. Future research is needed in this area to foster the evidence base of these findings, especially regarding arm and leg function, muscle strength and cognitive abilities (including spatial neglect).
Quality of the evidence
The quality of evidence for tDCS for improving ADLs was very low to moderate. It was low for upper extremity function and low for adverse events and people discontinuing the treatment.
Summary of findings
Background
Description of the condition
Every year, 15 million people worldwide suffer from stroke (WHO 2011), and of those, nearly six million die (Mathers 2011). Another five million people are left permanently disabled every year (WHO 2011). Hence, stroke is one of the leading causes of death worldwide and has a considerable impact on disease burden (WHO 2011). Stroke affects function and many activities of daily living (ADLs). Three out of four patients have an impairment in performing ADLs at hospital admission, and only about one‐third of patients who have completed rehabilitation have achieved normal neurological function (Jørgensen 1999). Every second patient does not regain function of the affected arm six months after stroke (Kwakkel 2003). Three out of four people with stroke suffer from working memory impairment and may thus experience executive dysfunction (Riepe 2004). Based on the rating by people with stroke, carers and health professionals, improving cognition after stroke is the number one research priority after stroke (Pollock 2012). Therefore, neurological rehabilitation, including effective training strategies, is needed (especially therapies tailored to patients' and carers' needs) to facilitate recovery and to reduce the burden of stroke (Barker 2005).
Description of the intervention
Transcranial direct current stimulation (tDCS) is a non‐invasive method used to modulate cortical excitability by applying a direct current to the brain (Bindman 1964; Nowak 2009; Purpura 1965). Stimulation of the central nervous system by tDCS is inexpensive when compared with repetitive transcranial magnetic stimulation (rTMS) and epidural stimulation (Hesse 2011).
How the intervention might work
Transcranial direct current stimulation (tDCS) usually is delivered via saline‐soaked surface sponge electrodes, which are connected to a direct current stimulator of low intensity (Lang 2005). Three different applications might be used: 1) the anodal electrode may be placed over the presumed area of interest of the brain with the cathodal electrode placed above the contralateral orbit (anodal stimulation, A‐tDCS); 2) the cathodal electrode may be placed over the presumed area of interest of the brain with the anodal electrode placed above the contralateral orbit (cathodal stimulation, C‐tDCS) (Hesse 2011); or 3) anodal stimulation and cathodal stimulation may be applied simultaneously (dual‐tDCS) (Lindenberg 2010). Primarily resulting from a shift of the resting potential of the brain's neurons, tDCS using anodal stimulation might lead to increased cortical excitability, whereas cortical stimulation might lead to decreased excitability (Bindman 1964; Floel 2010; Purpura 1965). Stimulation lasting for longer than five minutes might induce significant after‐effects (which probably are mainly due to changes in synaptic mechanisms), which could last up to several hours (Nitsche 2001; Nitsche 2003). These effects probably are 1) anatomically specific (referring to how the electrodes are positioned and which way the current takes to reach the targeted brain areas); 2) activity selective and task specific (meaning that neuronal networks active during a certain activity are preferentially stimulated by tDCS); and 3) input selective (meaning that tDCS would alter the neuronal system's input and thereby enhance information processing) (Bikson 2013). The facilitating effect of tDCS could be used to facilitate motor learning in healthy people (Boggio 2006; Jeffery 2007; Nitsche 2001; Nitsche 2003; Reis 2009) and appears to be a promising option in rehabilitation after stroke.
Why it is important to do this review
The previous version of this review suggested that, among people with stroke, tDCS with or without simultaneous upper extremity training, has led to greater improvement in arm motor function when compared with sham tDCS alone (Elsner 2013). Some pilot studies have even reported improvement in ADLs, such as, turning over playing cards, picking up beans with a spoon, and manipulating light and heavy objects with the arm (Fregni 2005; Hummel 2005; Kim 2009). However, these findings were not supported by a large‐scale multicentre randomised controlled trial (RCT), which did not find any effects on measures of ADL (Hesse 2011). There is contradictory evidence on the additional effect of tDCS on lower extremity function and gait (Cha 2014; Fusco 2014; Geroin 2011; Tahtis 2012). There are indications that tDCS might also improve working memory or neglect by modulating excitability of the corresponding brain areas (Au‐Yeung 2014; Jo 2008; Kang 2008a; Ko 2008; Park 2013; Sunwoo 2013). However, in a systematic review of RCTs about the effects of tDCS on aphasia, no evidence of an effect was found (Elsner 2015). Despite the fact that adverse effects associated with the application of tDCS have been reported rarely so far, concerns about the safety of tDCS regarding its impact on cerebral autoregulation have recently emerged (List 2015; Nitsche 2015).
To date, studies of tDCS have tended to include small sample sizes. Currently, no systematic review has comprehensively synthesised the findings of available RCTs. Therefore, a systematic review of RCTs investigating the effectiveness and acceptability of tDCS for improving ADLs, motor function and cognitive abilities (including spatial neglect) in people with stroke is required.
Objectives
To assess the effects of transcranial direct current stimulation (tDCS) on activities of daily living (ADLs), arm and leg function, muscle strength and cognitive abilities (including spatial neglect), dropouts and adverse events in people after stroke.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and randomised controlled cross‐over trials, from which we analysed only the first period as a parallel‐group design. We did not include quasi‐RCTs.
Types of participants
We included adult participants (over 18 years of age) who had experienced a stroke. We used the World Health Organization (WHO) definition of stroke (Hatano 1976), or a clinical definition, if not specifically stated (i.e. signs and symptoms persisting longer than 24 hours). We included participants regardless of initial level of impairment, duration of illness, or gender.
Types of interventions
This is the update of an existing review. In the previous version of this review we focused on the effects of transcranial direct current stimulation (tDCS) on activities of daily living (ADLs) and function. In this update, we broadened our inclusion criteria to compare any kind of active tDCS for improving ADLs, function, muscle strength and cognitive abilities (including spatial neglect) versus any kind of placebo or control intervention (i.e. sham tDCS, no intervention or conventional motor rehabilitation). We defined active tDCS as the longer‐lasting (lasting longer than one minute) application of a direct current to the brain to stimulate the affected hemisphere, or to inhibit the healthy hemisphere. We defined sham tDCS as short‐term direct current stimulation (lasting less than one minute; this is approximately the time it usually takes to fade in and fade out the current in sham‐controlled tDCS trials in order to produce perceivable sensations on the skin similar to active tDCS (Gandiga 2006), or placement of electrodes with no direct current applied. If more than one active or sham or control group investigated the same content, we combined these into one group each (e.g. if two sham control groups were included, we combined them into a single sham group for comparison with the active group).
Types of outcome measures
Primary outcomes
The primary outcome was activities of daily living (ADLs), regardless of their outcome measurement. However, we prioritised generally accepted outcome measures in the following order to facilitate quantitative pooling.
Frenchay Activities Index (FAI) (Schuling 1993).
Barthel ADL Index (BI) (Mahoney 1965).
Rivermead ADL Assessment (Whiting 1980).
Modified Rankin Scale (mRS) (Bonita 1988).
Functional Independence Measure (FIM) (Hamilton 1994).
We analysed primary outcomes according to their time point of measurement as follows: 1) at the end of the study period; and 2) at follow‐up: from three to 12 months after the study end. In cases where included studies reported ADLs in other measures than those mentioned above, all review authors discussed and reached consensus about the outcome measures to be included in the primary outcome analysis.
Secondary outcomes
In this update we defined secondary outcomes as upper limb function, lower limb function, muscle strength, cognitive abilities (including spatial neglect), dropouts and adverse events (including death from all causes), with appropriate measures as reported in the studies. We preferred interval‐scaled outcome measures rather than ordinal‐scaled or nominal‐scaled ones. We prioritised secondary outcome measures as follows.
For upper limb function:
Action Research Arm Test (ARAT) (Lyle 1981);
Fugl‐Meyer Score (Fugl‐Meyer 1975);
Nine‐Hole Peg Test (NHPT) (Sharpless 1982); and
Jebsen Taylor Hand Function Test (JTT) (Jebsen 1969).
For lower limb function:
walking velocity (in metres per second);
walking capacity (metres walked in six minutes); and
Functional Ambulation Categories (FAC) (Holden 1984).
For muscle strength:
grip force (measured by handheld dynamometer) (Boissy 1999); and
Motricity Index Score (Demeurisse 1980).
For cognitive abilities, such as working memory, attention and spatial neglect:
Montreal Cognitive Assessment (Nasreddine 2005);
Clock Drawing Test (Goodglass 1983);
Executive Function (Assessments have been described in Chung 2013);
target cancellation (Molenberghs 2011);
line bisection (Molenberghs 2011);
other measures of cognitive abilities; and
other measures of spatial neglect.
Depending on the measurements provided in the included trials, all review authors discussed and reached consensus about which outcome measures should be included in the analysis of secondary outcomes.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for relevant trials in all languages and arranged translation of trial reports where necessary.
Electronic searches
According to the increased scope of this update we re‐ran our searches with updated search strategies of the Cochrane Stroke Group Trials Register (March 2015) and the following electronic bibliographic databases.
Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library; 2015, Issue 2) (Appendix 1).
MEDLINE (1948 to February 2015) (Appendix 2).
EMBASE (1980 to February 2015) (Appendix 3).
CINAHL (1982 to February 2015) (Appendix 4).
AMED (1985 to February 2015) (Appendix 5).
Science Citation Index (Web of Science) (1899 to February 2015) (Appendix 6).
Physiotherapy Evidence Database (PEDro) at http://www.pedro.org.au/ (March 2015) (Appendix 7).
Rehabdata at www.naric.com/?q=REHABDATA (1956 to March 2015) (Appendix 8).
Compendex (1969 to May 2013) (Appendix 9).
Inspec (1969 to March 2015) (Appendix 10).
We developed the MEDLINE search strategy with the help of the Cochrane Stroke Group Trials Search Co‐ordinator and adapted it for the other databases.
We also searched the following ongoing trials and research registers (June 2015).
Stroke Trials Registry (www.strokecenter.org/trials/).
Current Controlled Trials (www.controlled‐trials.com/).
ClinicalTrials.gov (http://clinicaltrials.gov).
EU Clinical Trials Register (www.clinicaltrialsregister.eu/).
WHO International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/).
Searching other resources
We carried out the following additional searches to identify further published, unpublished and ongoing trials not available in the aforementioned databases.
-
Handsearched the following relevant conference proceedings, which had not already been searched by the Cochrane Stroke Group.
3rd, 4th, 5th, 6th and 7th World Congress of NeuroRehabilitation (2002, 2006, 2008, 2010, 2012 and 2014).
1st, 2nd, 3rd, 4th, 5th and 6th World Congress of Physical and Rehabilitation Medicine (2001, 2003, 2005, 2007, 2009, 2011 and 2013).
Deutsche Gesellschaft für Neurotraumatologie und Klinische Neurorehabilitation (2001 to 2014).
Deutsche Gesellschaft für Neurologie (2000 to 2014).
Deutsche Gesellschaft für Neurorehabilitation (1999 to 2014).
1st, 2nd and 3rd Asian Oceania Conference of Physical and Rehabilitation Medicine (2008, 2010, 2012 and 2014).
Screened reference lists from relevant reviews, articles and textbooks.
Contacted authors of identified trials and other researchers in the field.
Used Science Citation Index Cited Reference Search for forward tracking of important articles.
-
Contacted the following equipment manufacturers (June 2015).
Activatek, Salt Lake City, USA (www.activatekinc.com).
Changsha Zhineng Electronics, Changsha City, Hunan, China (www.cszhineng.diytrade.com).
DJO Global, Vista, USA (www.djoglobal.com).
Grindhouse (www.grindhousewetware.com).
Magstim, Spring Gardens, UK (www.magstim.com).
Neuroconn, Ilmenau, Germany (www.neuroconn.de).
Neuroelectrics, Barcelona, Spain (www.neuroelectrics.com).
Newronika, Milano, Italy (www.newronika.it).
Soterix Medical, New York City, USA (www.soterixmedical.com).
Trans Cranial Technologies, Hong Kong (www.trans‐cranial.com).
Searched Google Scholar (http://scholar.google.com/) (June 2015).
Data collection and analysis
Selection of studies
One review author (BE) read the titles and abstracts of records identified by the electronic searches and eliminated obviously irrelevant studies. We retrieved the full‐text of the remaining studies, and two review authors (JK and BE) independently ranked the studies as relevant, possibly relevant or irrelevant according to our inclusion criteria (types of studies, participants and aims of interventions). Two review authors (JM and MP) then examined whether the possibly relevant publications fit the population, intervention, comparison, outcome (PICO) strategy of our study question. We included all trials rated as relevant, or possibly relevant, and excluded all trials ranked as irrelevant. We resolved disagreements by discussion with all review authors. If we needed further information to resolve disagreements concerning including or excluding a study, we contacted the trial authors and requested the required information. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), and listed in the Characteristics of excluded studies table all studies that did not match our inclusion criteria regarding types of studies, participants and aims of interventions.
Data extraction and management
Two review authors (BE and JM) independently extracted trial and outcome data from the selected trials. If one of the review authors was involved in an included trial, another review author extracted trial and outcome data from that trial. In accordance with the 'Risk of bias' tool implemented in Review Manager 5 (RevMan 2014), we used checklists to independently assess:
methods of random sequence generation;
methods of allocation concealment;
blinding of assessors;
use of an intention‐to‐treat (ITT) analysis;
adverse effects and dropouts;
important differences in prognostic factors;
participants (country, number of participants, age, gender, type of stroke, time from stroke onset to study entry and inclusion and exclusion criteria);
comparison (details of interventions in treatment and control groups, duration of treatment and details of cointerventions in the groups);
outcomes; and
their time point of measurement.
Further, we extracted data on initial ADL ability or initial functional ability, or both.
BE and JM checked the extracted data for agreement. If necessary, we contacted trialists to obtain more information.
Assessment of risk of bias in included studies
Two review authors (JM and MP) independently assessed the risk of bias in the included trials according to Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We judged each potential source of bias as high, low or unclear and provide a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the risk of bias judgements across different studies for each of the domains listed. We resolved disagreements in methodological assessment by reaching consensus through discussion by all review authors. We contacted trialists to ask for clarification and to request missing information.
Measures of treatment effect
For all outcomes that were continuous data, we entered means and standard deviations (SDs). We calculated a pooled estimate of the mean difference (MD) with 95% confidence intervals (CIs). If studies did not use the same outcomes, we calculated standardised mean differences (SMDs) instead of MDs. For all binary outcomes, we calculated risk differences (RDs) with 95% CIs. In case different scales measured the same outcome but in some scales a higher value indicated better performance and in other scales a lower value indicated better performance, we multiplied the values of the corresponding scales by ‐1 to ensure a consistent direction of the effect across all outcome measurements.
For all statistical comparisons we used the current version of Review Manager 5 (RevMan 2014).
Assessment of heterogeneity
We used the I² statistic to assess heterogeneity. We used a random‐effects model, regardless of the level of heterogeneity. Thus, when heterogeneity occurred, we could not violate the preconditions of a fixed‐effect model approach.
Data synthesis
GRADE and 'Summary of findings' table
We created two 'Summary of findings' tables using the following outcomes.
Primary outcome measure: ADLs at the end of the intervention period ‐ absolute values. Measures of activities of daily living. Scale from: 0 to infinity
Primary outcome measure: ADLs at the end of the intervention period ‐ change scores. Measures of activities of daily living. Scale from: 0 to infinity.
Primary outcome measure: ADLs until the end of follow‐up. Measures of activities of daily living. Scale from: 0 to infinity. Follow‐up: mean 3 months.
Secondary outcome measure: upper extremity function at the end of the intervention period ‐ absolute values. Clinical measures of upper extremity function. Scale from: 0 to infinity.
Secondary outcome measure: upper extremity function at the end of the intervention period ‐ change scores. Clinical measures of upper extremity function. Scale from: 0 to infinity.
Secondary outcome measure: upper extremity function to the end of follow‐up ‐ absolute values. Clinical measures of upper extremity function. Scale from: 0 to infinity. Follow‐up: mean 3 months.
Secondary outcome measure: dropouts, adverse events and deaths during the intervention period. Number of adverse events, dropouts and deaths during the intervention period.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c) using GRADEproGDT software (GRADEpro). We justified all decisions to down‐ or up‐grade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
If at least two studies were available for each group (tDCS/sham), we conducted planned analyses of the following subgroups for our primary outcome of ADL.
Duration of illness: acute/subacute phase (the first week after stroke and the second to the fourth week after stroke, respectively) versus the postacute phase (from the first to the sixth month after stroke) versus the chronic phase (more than six months after stroke).
Type of stimulation: cathodal versus anodal and position of electrodes/location of stimulation.
Type of control intervention: active (e.g. conventional therapy) versus passive (sham tDCS or no intervention).
All stratified (subgroup) analyses were accompanied by appropriate tests for interaction (statistical tests for subgroup differences as described in the Cochrane Handbook for Systematic Review of Interventions (Higgins 2011b), as implemented in Review Manager 5 (RevMan 2014).
Sensitivity analysis
We incorporated a post hoc sensitivity analysis for methodological quality to test the robustness of our results. We analysed concealed allocation, blinding of assessors, and ITT.
Results
Description of studies
Results of the search
2013 version
For the 2013 version of this review, we identified 6226 potentially relevant trials through electronic searching; we considered 92 full papers and included 15 trials with 455 participants (Boggio 2007a; Bolognini 2011; Fregni 2005a; Fusco 2013a; Geroin 2011; Hesse 2011; Khedr 2013; Kim 2009; Kim 2010; Lindenberg 2010; Mahmoudi 2011; Nair 2011; Qu 2009; Rossi 2013; Wu 2013a).
2015 version
In this update, we identified a total of 2295 records through the searches. After screening titles and abstracts, we obtained the full‐text of 52 articles. After further assessment, we determined that 17 new studies met the review inclusion criteria, and three studies are awaiting classification, as more information is required. We identified 55 ongoing pilot and large‐scale randomised trials with a cumulative estimated enrolment of 3339 participants (mean (SD) sample size: 65 (53); median sample size: 45; range of sample size: 6‐250). The majority of ongoing studies are performed in the USA, Brazil, Belgium, France, the Netherlands, and Germany.
The flow of references is shown in Figure 1.
1.

Study flow diagram. Please note that the number of full‐texts is not necessarily equal to the number of studies (e.g. The studies Di Lazzaro 2014a and Di Lazzaro 2014b have been presented in a single full‐text. Moreover there often are several full‐texts of a single trial (e.g. as is the case for Hesse 2011 or Nair 2011).
Included studies
Design
We included 32 studies involving a total of 748 participants in the qualitative analysis (see Characteristics of included studies). All studies investigated the effects of transcranial direct current stimulation (tDCS) versus sham tDCS, except Cha 2014 and Qu 2009, which compared tDCS with physical therapy alone. Eleven trials with 105 participants were randomly assigned cross‐over trials (Au‐Yeung 2014; Boggio 2007a; Fregni 2005a; Fusco 2013a; Jo 2008; Kang 2008a; Kim 2009; Ko 2008; Mahmoudi 2011; Sohn 2013; Sunwoo 2013), whereas the remaining 21, with 643 participants, were RCTs (Ang 2012; Bolognini 2011; Cha 2014; Di Lazzaro 2014a; Di Lazzaro 2014b; Fusco 2014; Geroin 2011; Hesse 2011; Khedr 2013; Kim 2010; Lee 2014; Lindenberg 2010; Nair 2011; Park 2013; Qu 2009; Rossi 2013; Tahtis 2012; Tedesco Triccas 2015b; Viana 2014; Wang 2014; Wu 2013a).
Sample sizes
The sample sizes of included studies ranged from four in Boggio 2007a to 96 in Hesse 2011, with a mean (SD) sample size of 24 (23). The median sample size was 14.
Setting
Ten of the included studies were conducted in the Republic of Korea, six in Italy, three in the USA, three in China, two in Brazil, one in Iran, one in Egypt, one in the UK, one in Singapore, and one in Germany/Italy. In three studies, the country was not stated clearly.
Participants
The proportion of participants with ischaemic stroke ranged from 36% in Sohn 2013 to 100% in Fusco 2014. The mean age in the experimental groups ranged from 43 years in Bolognini 2011 to 70 years in Kang 2008a, and from 45 years in Qu 2009 to 75 years in the control groups (Boggio 2007a). The proportion of women participating in the included studies ranged from 0% in Au‐Yeung 2014 and Boggio 2007a to 71% in Bolognini 2011. See Table 6 for a comprehensive summary of participant characteristics.
1. Patient characteristics.
| Study ID | Experimental: age, mean (SD) | Control: age, mean (SD) | Experimental: time poststroke, mean (SD) | Control: time poststroke, mean (SD) | Experimental: sex, n (%) | Control: sex, n (%) | Experimental: lesioned hemisphere, n (%) | Control: lesioned hemisphere, n (%) | Experimental: severity, mean (SD) |
Control: severity, mean (SD) |
Experimental: lesion cause/ location, n (%) | Control: lesion cause/ location, n (%) | Handedness, n (%) |
| Ang 2012 | 52 (12) years | 56 (10) years | 3 (2) years | 3 (1) years | 4 (40) female | 1 (11) female | 5 (50) | 6 (67) | UE‐FM 35 (8) | UE‐FM 33 (8) | 6 (60) ischaemic; 1 (10) cortical, 9 (90) subcortical | 7 (78) ischaemic; 9 (100) subcortical | Not stated |
| Au‐Yeung 2014 | 63 (6) years | 8 (3) years | 0 female | 5 (50) left | UE‐FM 58 (8); MMSE 29 (2) | 8 (80) ischaemic | 10 (100) right‐handed | ||||||
| Boggio 2007a | 56 (11) years | 75 (NA) years | 33 (34) months | 39 months | 3 (100) male | 1 (100) male | 2 (67) left | 1 (100) left | MRC 4.2 (0.53) | MRC 4.7 (NA) | 3 (100) ischaemic and subcortical | 1 (100) ischaemic and subcortical | 12 (100) right‐handed |
| Bolognini 2011 | 43 (13) years | 51 (15) years | 44 (31) months |
26 (18) months | 4 (57) female | 5 (71) female | 4 (57) left | 4 (57) left | BI 18.13 (2.42) | BI 14.33 (5.46) | 2 (29) haemorrhagic, 5 (71) ischaemic | 7 (100) ischaemic | 14 (100) right‐handed |
| Cha 2014 | 60 (11) years | 58 (10) years | 14 (5) months | 15 (4) months | Not stated | Not stated | 4 (40) left | 5 (50) left | Brunnstrom 5 (1) | Brunnstrom 5 (1) | Not stated | Not stated | Not stated |
| Di Lazzaro 2014a | 66 (16) years | 71 (14) years | 3 (1) days | 3 (1) days | 2 (29) female | 3 (43) female | 3 (43) left | 3 (43) left | NIHSS 7 (5) | NIHSS 7 (4) | 7 (100) ischaemic; 3 (43) subcortical; 4 (57) corticosubcortical | 7 (100) ischaemic; 2 (29) subcortical, 5 (71) corticosubcortical | Not stated |
| Di Lazzaro 2014b | 61 (16) years | 69 (12) years | 3 (2) days | 3 (1) days | 4 (40) female | 6 (60) male | 2 (20) left | 6 (60) left | NIHSS 6 (3) | NIHSS 6 (2) | 10 (100) ischaemic; 4 (40) subcortical, 6 (60) corticosubcortical | 10 (100) ischaemic; 4 (40) subcortical, 6 (60) corticosubcortical | Not stated |
| Fregni 2005a | 54 (17) years | 27 (24) months | 2 (33) female | 3 (50) left | MRC 4.18 (0.37) | Cause not clearly stated by the authors | 6 (100) right‐handed | ||||||
| Fusco 2013a | 44 (16) years | 65 (22) years | 31 (13) days | 25 (5) days | 3 (60) female | 1 (25) female | 3 (60) left | 2 (50) left | Grasp force 17.83 (7.45) kg | 5 (100) ischaemic | 3 (75) ischaemic, 1 (25) haemorrhagic | 9 (100) right‐handed | |
| Fusco 2014 | 56 (15) years | 60 (12) years | 19 (8) days | 3 (60) female | 3 (50) female | 2 (40) left | 2 (33) left | BI 33 (22) | BI 51 (34) | 5 (100) ischaemic | 6 (100) ischaemic | 9 (73) right‐handed | |
| Geroin 2011 | 64 (7) years | 63 (6) years | 26 (6) months | 27 (5) months | 2 (20) female | 4 (40) female | Not stated by the authors | Not stated by the authors | ESS 79.6 (4.1) | ESS 79.6 (2.7) | 10 (100) ischaemic; 4 (40) cortical, 3 (30) corticosubcortical, 3 (30) subcortical |
10 (100) ischaemic; 5 (50) cortical, 3 (30) corticosubcortical, 2 (20) subcortical | Not stated by the authors |
| Hesse 2011 | 65 (10) years | 66 (10) years | 4 (2) weeks | 4 (2) weeks | 26 (41) female | 11 (34) female | 35 (55) left | 16 (50) left | BI 34.15 (6.97); UE‐FM 7.85 (3.58) | BI 35.0 (7.8); UE‐FM 8.2 (4.4) | 64 (100) ischaemic; 29 (45) TACI, 20 (31) PACI, 15 (23) LACI | 32 (100) ischaemic; 13 (41) TACI, 13 (41) PACI, 6 (18) LACI | Not stated by the authors |
| Jo 2008 | 48 (9) years | 2 (1) months | 3 (30) female | 10 (100) right | Not reported | 4 (40) ischaemic | Not stated by the authors | ||||||
| Kang 2008a | 70 (3) years | 544 (388) days | 4 (40) female | 7 (70) right | 21 (1) MMSE | 7 (70) ischaemic | Not stated by the authors | ||||||
| Khedr 2013 | 59 (9) years | 57 (8) years | 13 (5) days | 13 (5) days | 9 (33) female | 5 (38) female | 12 (44) left | 6 (46) left | BI 32.76 (10.75) | BI 31.1 (12.6) | 27 (100) ischaemic; 12 (44) cortical, 5 (19) corticosubcortical, 10 (37) subcortical | 13 (100) ischaemic; 6 (42) cortical, 3 (23) corticosubcortical, 4 (31) subcortical | Not stated by the authors |
| Kim 2009 | 63 (13) years | 6 (3) weeks | 7 (70) female | 8 (80) left | MRC between 3 and 5 for the all paretic finger flexors and extensors | 8 (80) infarction, 2 (20) haemorrhage | Not stated by the authors | ||||||
| Kim 2010 | 54 (15) years | 63 (9) years | 27 (21) days | 23 (8) days | 2 (18) female | 3 (43) female | 7 (64) left | 2 (29) left | BI 71.77 (23.86) UE‐FM 34.7 (15.0) | BI 67.9 (22.4) UE‐FM 41.0 (13.0) | 11 (100) ischaemic; 3 (27) cortical, 3 (27) corticosubcortical, 5 (71) subcortical |
7 (100) ischaemic; 2 (29) cortical, 1 (14) corticosubcortical, 4 (57) subcortical | Not stated by the authors |
| Ko 2008 | 62 (9) years | 29‐99 days | 5 (33) female | 15 (100) right | 19 per cent deviation (11) | 10 (66) ischaemic | 15 (100) right‐handed | ||||||
| Lee 2014 | 62 (11) years | 61 (14) years | 18 (8) days | 17 (6) days | 17 (44) female | 9 (45) female | 19 (49) left | 13 (65) | UE‐FM 37 (23) | UE‐FM 35 (22) | 21 (54) ischaemic; 21 (54) cortical; 18 (46) subcortical | 14 (70) ischaemic; 10 (50) cortical; 10 (50) subcortical | Not stated by the authors |
| Lindenberg 2010 | 62 (15) years | 56 (13) years | 31 (21) months | 40 (23) months | 2 (20) female | 3 (30) female | 6 (60) left | 7 (70) left | UE‐FM 38.2 (13.3) | UE‐FM 39.8 (11.5) | 10 (100) ischaemic | 10 (100) ischaemic | 19 (95) right‐handed, 1 (5) both‐handed |
| Mahmoudi 2011 | 61 (14) years | 8 (5) months | 3 (33) female | 6 (60) left, 3 (30) right, 1 (10) brainstem | JTT (without handwriting): 12.3 (7.3) s | 10 (100) ischaemic | Not stated by the authors | ||||||
| Nair 2011 | 61 (12) years | 56 (15) years | 33 (20) months | 28 (28) months | 2 (29) female | 3 (43) female | 3 (43) left | 5 (71) left | UE‐FM 30 (11) | UE‐FM 31 (10) | 7 (100) ischaemic; 5 (71) cortical and corticosubcortical, 2 (29) subcortical | 7 (100) ischaemic; 4 (56) cortical and corticosubcortical, 3 (43) subcortical | 14 (100) right‐handed |
| Park 2013 | 65 (14) years | 66 (11) years | 29 (19) days | 25 (17) days | 6 (67) female | 2 (40) female | 2 (33) left | 2 (40) left | NIHSS 8 (3) | NIHSS 10 (3) | 4 (67) ischaemic | 3 (60) ischaemic | Not stated by the authors |
| Qu 2009 | 45 (11) years | 45 (14) years | 6 (range 3 to 36) months | 4 (range 3 to 12) months | 4 (16) female | 3 (12) female | 14 (56) left | 13 (52) left | BI 64 (17) | BI 72 (22) | 10 (40) haemorrhagic, 15 (60) infarction | 10 (40) haemorrhagic, 15 (60) infarction | Not stated by the authors |
| Rossi 2013 | 66 (14) years | 70 (14) years | 2 days | 2 days | 13 (52) female | 11 (44) female | 18 (72) left | 16 (64) left | UE‐FM 4.1 (6.4) | FM 4.6 (7.8) | 25 (100) ischaemic; 1 (4) cortical, 17 (68) corticosubcortical, 7 (28) subcortical | 25 (100) ischaemic; 2 (8) cortical, 18 (72) corticosubcortical, 5 (20) subcortical | Not stated by the authors |
| Sohn 2013 | 58 (15) years | 63 (17) days | 2 (18) female | 6 (55) left | Not stated by the authors | 4 (36) ischaemic | Not stated by the authors | ||||||
| Sunwoo 2013 | 63 (13) years | 28 (60) months | 6 (60) female | 10 (100) left | MMSE 28 (2) | 7 (70) ischaemic | 10 (100) right‐handed | ||||||
| Tahtis 2012 | 67 (12) years | 56 (12) years | 20 (5) days | 25 (11) days | 2 (29) female | 1 (14) female | 3 (43) left | 3 (43) left | MRS 2 (1) | MRS 3 (1) | 7 (100) ischaemic; 4 (57) cortical, 3 (43) subcortical | 7 (100) ischaemic; 3 (43) cortical; 4 (57) subcortical | Not stated by the authors |
| Tedesco Triccas 2015b | 64 (10) years | 63 (14) years | 25 (31) months | 13 (16) months | 5 (42) female | 4 (33) female | 6 (50) left | 5 (45) left | UE‐FM 28 (19) | UE‐FM 37 (14) | 3 (25) ischaemic; 3 (25) cortical, 9 (75) subcortical | 9 (81) ischaemic; 4 (36) cortical; 7 (64) subcortical | 22 (96) right‐handed |
| Viana 2014 | 56 (10) years | 55 (12) years | 32 (18) months | 35 (20) months | 1 (10) female | 3 (30) female | 5 (50) left | 3 (30) left | UE‐FM 41 (16) | UE‐FM 39 (17) | 9 (90) ischaemic | 10 (100) ischaemic | 19 (95) right‐handed |
| Wang 2014 | 54 (14) years | 52 (9) years | Not explicitly stated, but all participants were enrolled between 1 and 4 weeks poststroke | 1 (16) female | 1 (33) female | 2 (33) left | 0 left | FIM 59 (18) | FIM 74 (8) | 6 (100) ischaemic | 3 (100) ischaemic | Not stated by the authors | |
| Wu 2013a | 46 (11) years | 49 (13) years | 5 (3) months | 5 (3) months | 11 (24) female | 10 (22) female | 24 (53) left | 23 (51) left | BI 55 (range 0 to 85) UE‐FM 12.3 (5.5) | BI 55 (range 25 to 95) UE‐FM 11.8 (8.2) | 27 (60) ischaemic, 18 (40) haemorrhagic | 26 (58) ischaemic, 19 haemorrhagic (42) | Not stated by the authors |
BBT: Box and Block Test BI: Barthel Index ESS: European Stroke Scale JTT: Jebsen Taylor Hand Function Test LACI: lacunar stroke MRC: Medical Research Council NA: not applicable NIHSS: National Institute of Health Stroke Scale PACI: partial anterior circulation stroke SD: standard deviation TACI: total anterior circulation stroke UE‐FM: Upper Extremity Fugl‐Meyer Score
Interventions
The experimental groups received anodal stimulation (A‐tDCS) (Au‐Yeung 2014; Boggio 2007a; Bolognini 2011; Fregni 2005a; Fusco 2013a; Geroin 2011; Hesse 2011; Jo 2008; Kang 2008a; Ko 2008; Khedr 2013; Kim 2009; Kim 2010; Mahmoudi 2011; Park 2013; Rossi 2013; Sohn 2013; Sunwoo 2013; Tedesco Triccas 2015b; Viana 2014; Wang 2014), cathodal stimulation (C‐tDCS) (Au‐Yeung 2014; Boggio 2007a; Fregni 2005a; Fusco 2013a; Fusco 2014; Hesse 2011; Khedr 2013; Kim 2010; Lee 2014; Mahmoudi 2011; Nair 2011; Qu 2009; Wu 2013a) or dual‐tDCS (anodal plus cathodal stimulation simultaneously) (Ang 2012; Di Lazzaro 2014a; Di Lazzaro 2014b; Fusco 2013a; Lindenberg 2010; Mahmoudi 2011; Tahtis 2012), and the control groups of all included studies except Cha 2014, Qu 2009 and Lee 2014 received sham tDCS or physical therapy or virtual reality, respectively, as a control intervention. See Table 7 for a comprehensive summary of intervention characteristics, dropouts and adverse events.
2. Demographics of studies, including dropouts and adverse events.
| Study ID | Type of intervention/ stimulation (polarity) | Electrode position and size | Reference electrode position | Treatment intensity | Base treatment | Dropouts | Adverse events | Source of information | |
| Ang 2012 | Dual‐tDCS | Saline‐soaked sponge electrodes with the anode placed over M1 of the affected hemisphere and the cathode placed over M1 the unaffected hemisphere (size not stated) | 1 mA for 20 minutes | 20 minutes of Dual‐tDCS or sham tDCS followed by 8 minutes of evaluation prior to base treatment | 60 minutes of therapy using EEG‐based MI‐BCI with robotic feedback with the MIT‐Manus 5 times a week for 2 weeks | None | Not described by the authors | Published | |
| Sham tDCS | 1 mA for 30 seconds | ||||||||
| Au‐Yeung 2014 | A‐tDCS | Saline‐soaked 35 cm² sponge electrodes over the M1 of the lesioned hemisphere | Over the contralateral supraorbital forehead | 1 mA for 20 minutes | A‐tDCS, C‐tDCS and sham tDCS once in random order with at least 5 days wash‐out period | None | None | Not described by the authors | Published |
| C‐tDCS | Saline‐soaked 35 cm² sponge electrodes over the M1 of the non‐lesioned hemisphere | 1 mA for 20 minutes | |||||||
| Sham tDCS | Saline‐soaked 35 cm² sponge electrodes over M1 of both hemispheres | 1 mA for 10 seconds | |||||||
| Boggio 2007a | A‐tDCS | Saline‐soaked 35 cm² sponge electrodes over the M1 of the lesioned hemisphere | Over the contralateral supraorbital forehead | 1 mA for 20 minutes | A‐tDCS, C‐tDCS or sham tDCS 4 days once a day | None | None | None | Published |
| C‐tDCS | Saline‐soaked 35 cm² sponge electrodes over the M1 of the non‐lesioned hemisphere | ||||||||
| Sham tDCS | Not described by the authors | 1 mA for 30 seconds | |||||||
| Bolognini 2011 | A‐tDCS | Saline‐soaked 35 cm² sponge electrodes; with the anode placed over M1 of the lesioned hemisphere and the cathode over M1 of the non‐lesioned hemisphere | 2 mA for 40 minutes | Base treatment + A‐tDCS or sham tDCS 5 days a week for 2 consecutive weeks | CIMT up to 4 hours/day for 5 days a week for 2 consecutive weeks | 7 (33%) due to frustration and tiredness during assessments (Bolognini 2013 [pers comm]); these participants have been excluded from analysis and presentation of results | None | Published and unpublished | |
| Sham tDCS | 2 mA for 30 seconds | ||||||||
| Cha 2014 | A‐tDCS | Water‐soaked 35 cm² sponge electrodes over M1 of the lesioned hemisphere | Over the contralateral supraorbital forehead | 1 mA for 20 minutes | Base treatment + A‐tDCS for 20 minutes | Basic training for improving function of upper and lower extremities for 30 minutes per day, 5 times a week for four weeks | None | Not reported | Published |
| PT | NA | NA | NA | ||||||
| Di Lazzaro 2014a | Dual‐tDCS | anode over M1 of the lesioned hemisphere and cathode over M1 of the non‐lesioned hemisphere, | 2 mA for 40 min | Dual‐tDCS or sham tDCS on 5 continuous days | None | None | Not reported | Published | |
| Sham tDCS | 2 mA for 30 sec | ||||||||
| Di Lazzaro 2014b | Dual‐tDCS | anode over M1 of the lesioned hemisphere and cathode over M1 of the non‐lesioned hemisphere, | 2 mA for 40 min | Base treatment + dual‐tDCS or sham tDCS on 5 continuous days | CIMT for at least 90% of waking hours, including 1.5 hours per day arm training | None | Not reported | Published | |
| Sham tDCS | 2 mA for 30 sec | ||||||||
| Fusco 2013a | A‐tDCS | Saline‐soaked 35 cm² sponge electrodes over the M1 of the lesioned hemisphere | Over the contralateral supraorbital forehead | 1.5 mA for 15 minutes | 1 active tDCS (A‐tDCS, C‐tDCS, dual‐tDCS) and 1 sham tDCS session in 2 consecutive days | None | None | None | Published and unpublished |
| C‐tDCS | Saline‐soaked 35 cm² sponge electrodes over the M1 of the non‐lesioned hemisphere | 1.5 mA for 15 minutes | |||||||
| Dual‐tDCS | Saline‐soaked 35 cm² sponge electrodes with the anode over M1 of the lesioned hemisphere and the cathode over M1 of the non‐lesioned hemisphere | 1.5 mA for 15 minutes | |||||||
| Sham tDCS | Not described by the authors | ||||||||
| Fusco 2014 | C‐tDCS | Saline‐soaked 35 cm² gel‐sponge electrodes with the cathode over M1 of the non‐lesioned hemisphere | Above the right shoulder | 1.5 mA for 10 min | Each participant underwent C‐tDCS and sham tDCS on 5 consecutive days each week for 2 weeks prior to a rehabilitative session in randomised order | Patient‐tailored motor rehabilitation focusing on recovery of upper limb for 45 minutes twice a day | 2 (14%); reasons not described by the authors | Not reported | Published |
| Sham tDCS | not described | 1 (7%); emergency transfer to another hospital | |||||||
| Fregni 2005a | A‐tDCS | Saline‐soaked 35 cm² sponge electrodes over the M1 of the lesioned hemisphere | Over the contralateral supraorbital forehead | 1 mA for 20 minutes | Each participant underwent A‐tDCS, C‐tDCS and sham tDCS once, separated by at least 48 hours of rest | None | None | None | Published |
| C‐tDCS | Saline‐soaked 35 cm² sponge electrodes over the M1 of the non‐lesioned hemisphere | 1 mA for 20 minutes | |||||||
| Sham tDCS | Not described by the authors | 1 mA for 30 seconds | |||||||
| Geroin 2011 | A‐tDCS | Saline‐soaked 35 cm² sponge electrodes over M1 of the lesioned hemisphere | Over the contralateral supraorbital forehead | 1.5 mA for 7 minutes | Base treatment + A‐tDCS or sham tDCS 5 days a week for 2 consecutive weeks | 50‐minute training sessions 5 days a week for 2 consecutive weeks, consisting of 20 minutes of robot‐assisted gait training and 30 minutes of lower limb strength and joint mobilisation training | None | None | Published |
| Sham tDCS | 0 mA for 7 minutes | ||||||||
| Hesse 2011 | A‐tDCS | Saline‐soaked 35 cm² sponge electrodes over M1 of the lesioned hemisphere | Over the contralateral supraorbital forehead | 2 mA for 20 minutes | Base treatment + A‐tDCS, C‐tDCS or sham tDCS 5 days a week for 6 consecutive weeks | 20 minutes of robot‐assisted arm training 5 days a week for 6 consecutive weeks | 11 (11%); 7 dropouts in the EXP‐groups: 1 (14%) during intervention period due to pneumonia and 6 (86%) until 3 months of follow‐up (2 deaths due to myocardial infarction and stent surgery, 3 due to being unavailable and 1 due to refusal of further enrolment); 4 dropouts in the CTL group: 3 (75%) due to being not available and 1 (25%) due to refusal of further enrolment | None | Published |
| C‐tDCS | Saline‐soaked 35 cm² sponge electrodes over M1 of the non‐lesioned hemisphere | 2 mA for 20 minutes | |||||||
| Sham tDCS | As in the A‐tDCS or the C‐tDCS group (changing consecutively) | 0 mA for 20 minutes | |||||||
| Jo 2008 | A‐tDCS | Saline‐soaked 25 cm² sponge electrodes over DLPFC of the non‐lesioned hemisphere | Over the contralateral supraorbital forehead | 2 mA for 30 minutes | A‐tDCS once and sham tDCS once or vice versa, separated by at least 48 hours of resting period | None | None | 6 Quote: "Transient aching or burning sensations were reported in six cases, and transient skin redness at the electrode contact site was reported in three cases." | Published |
| Sham tDCS | 2 mA for 10 seconds | ||||||||
| Kang 2008a | A‐tDCS | 25 cm² electrodes over the left DLPFC | Over the contralateral supraorbital forehead | 2 mA for 20 minutes | A‐tDCS and sham tDCS or vice versa, separated by at least 48 hours of resting period | None | Not described | Not described | Published |
| Sham tDCS | 25 cm² electrodes over the left DLPFC | Over the contralateral supraorbital forehead | 2 mA for 1 minute | ||||||
| Khedr 2013 | A‐tDCS | Saline‐soaked 35 cm² sponge electrodes, anode over M1 of the lesioned hemisphere | Over the contralateral supraorbital forehead | 2 mA for 25 minutes | Base treatment + A‐tDCS, C‐tDCS or sham tDCS for 6 consecutive days after | Rehabilitation program within 1 hour after each tDCS session, starting with passive movement and range of motion exercise up to active resistive exercise | None | None | Published |
| C‐tDCS | Saline‐soaked 35 cm² sponge electrodes, cathode over M1 of the non‐lesioned hemisphere | Over the contralateral supraorbital forehead | 2 mA for 25 minutes | ||||||
| Sham tDCS | Saline‐soaked 35 cm² sponge electrodes, anode over M1 of the lesioned hemisphere | Over the contralateral supraorbital forehead | 2 mA for 2 minutes | ||||||
| Kim 2009 | A‐tDCS | Saline‐soaked 25 cm² sponge electrodes, anode over M1 of the lesioned hemisphere | Over the contralateral supraorbital forehead | 1 mA for 20 minutes | Each participant underwent A‐tDCS and sham tDCS, separated by at least 24 hours of rest | None | None | None | Published and unpublished |
| Sham tDCS | 1 mA for 30 seconds | ||||||||
| Kim 2010 | A‐tDCS | Saline‐soaked 25 cm² sponge electrodes over M1 of the lesioned hemisphere (as confirmed by MEP) | Over the contralateral supraorbital forehead | 2 mA for 20 minutes | Base treatment + A‐tDCS, C‐tDCS or sham tDCS 5 days a week for 2 consecutive weeks at the beginning of each therapy session | Occupational therapy according to a standardised protocol aimed at improving paretic hand function for 30 minutes 5 days a week for 2 consecutive weeks | 2 of 20; 1 participant discontinued treatment because of dizziness and another because of headache (authors did not state corresponding groups) | 2 | Published |
| C‐tDCS | Saline‐soaked 25 cm² sponge electrodes over M1 of the non‐lesioned hemisphere (confirmed by MEP) | Over the contralateral supraorbital forehead | 2 mA for 20 minutes | ||||||
| Sham tDCS | Saline‐soaked 25 cm² sponge electrodes over M1 of the lesioned hemisphere (confirmed by MEP) | Over the contralateral supraorbital forehead | 2 mA for 1 minutes | ||||||
| Ko 2008 | A‐tDCS | Saline‐soaked 25 cm² surface sponge electrodes over right (lesioned) PPC | Over the contralateral supraorbital forehead Over the contralateral supraorbital forehead |
2 mA for 20 minutes | A‐tDCS once and sham tDCS once or vice versa, separated by at least 48 hours of resting period | None | Not described | None | Published |
| Sham tDCS | 2 mA for 10 seconds | ||||||||
| Lee 2014 | C‐tDCS | Saline‐soaked 25 cm² surface sponge electrodes over hand area of M1 of the non‐lesioned hemisphere | Over the contralateral supraorbital forehead | 2 mA for 20 minutes | 20 minutes per day, 5 times per week for 3 weeks | Occupational therapy for 30 minutes per day, 5 times per week for 3 weeks | 3 of 42 (7%); 2 medical problems; 1 refused to participate | No major adverse events | Published |
| Virtual reality therapy for 30 minutes per day, 5 times per week for 3 weeks | |||||||||
| Virtual Reality | NA | NA | NA | Virtual reality only for 30 minutes per day, 5 times per week for 3 weeks | 2 of 22 (9%); 1 refused to participate; 1 early discharge | ||||
| Lindenberg 2010 | Dual‐tDCS | Saline‐soaked 16.3 cm² sponge electrodes with the anode over M1 of the lesioned hemisphere and the cathode over M1 of the non‐lesioned hemisphere | 1.5 mA for 30 minutes | Base treatment + dual‐tDCS or sham tDCS at 5 consecutive sessions on 5 consecutive days | Physical and occupational therapy sessions at 5 consecutive sessions on 5 consecutive days for 60 minutes, including functional motor tasks | None | None | Published | |
| Sham tDCS | 1.5 mA for 30 seconds | ||||||||
| Mahmoudi 2011 | A‐tDCS1 | Saline‐soaked 35 cm² sponge electrodes, anode over M1 of the lesioned hemisphere | Over the contralateral orbit | 1 mA for 20 minutes | Each participant underwent A‐tDCS1, A‐tDCS2, C‐tDCS, dual‐tDCS and sham tDCS once with a wash‐out period of at least 96 hours | None | None | Not clearly stated, most likely none | Published |
| A‐tDCS2 | Saline‐soaked 35 cm² sponge electrodes, anode over M1 of the lesioned hemisphere | On the contralateral deltoid muscle | 1 mA for 20 minutes | ||||||
| C‐tDCS | Saline‐soaked 35 cm² sponge electrodes, cathode over M1 of the non‐lesioned hemisphere | Over M1 of the lesioned hemisphere | 1 mA for 20 minutes | ||||||
| Dual‐tDCS | Saline‐soaked 35 cm² sponge electrodes with the anode over M1 of the lesioned hemisphere and the cathode over M1 of the non‐lesioned hemisphere | 1 mA for 20 minutes | |||||||
| Sham tDCS | Not described by the authors | 1 mA for 30 seconds | |||||||
| Nair 2011 | C‐tDCS | Saline‐soaked sponge electrodes with the cathode over M1 of the lesioned hemisphere | Over the contralateral supraorbital forehead | 1 mA for 30 minutes | Base‐treatment + C‐tDCS or sham tDCS for 5 consecutive daily sessions, each at the beginning of the base treatment sessions | Occupational therapy (PNF; shoulder abduction, external rotation, elbow extension, forearm pronation) for 5 consecutive daily sessions (60 minutes each) | None | None | Published |
| Sham tDCS | Not described by the authors | For 30 minutes | |||||||
| Park 2013 | A‐tDCS | Sponge electrodes with the anode positioned over the bilateral prefrontal cortex | at the non‐dominant arm | 2 mA for 30 minutes | Base‐treatment + A‐tDCS or sham tDCS for 5 days a week for approximately 18 days | Computer‐assisted cognitive rehabilitation (CACR) with the ComCog program (15 minute attention and 15 minute memory training) | Unclear | None | Published |
| Sham tDCS | 2 mA for 30 seconds | ||||||||
| Qu 2009 | C‐tDCS | Saline‐soaked 18 cm² sponge electrodes over primary sensorimotor cortex of the lesioned hemisphere | Unclear | 0.5 mA for 20 minutes, once a day for 5 consecutive days for 4 weeks | NA | None | None | Published | |
| PT | NA | Physical therapy according to the Bobath, Brunnstrom and Rood approaches for 40 minutes twice a day for 5 consecutive days for 4 weeks | |||||||
| Rossi 2013 | A‐tDCS | Saline‐soaked 35 cm² sponge electrodes over M1 of the lesioned hemisphere | Over the contralateral supraorbital forehead | 2 mA for 20 minutes | Once a day for 5 consecutive days | Not described by the authors | None | None | Published |
| Sham tDCS | 2 mA for 30 seconds | ||||||||
| Sohn 2013 | A‐tDCS | 25 cm² sponge electrodes over M1 of the affected hemisphere | Not described | 2 mA for 10 minutes | A‐tDCS or sham tDCS once | None | Unclear | Unclear | Published |
| Sham tDCS | 2 mA for 20 seconds | ||||||||
| Sunwoo 2013 | Dual‐tDCS | Saline‐soaked 25 cm² sponge electrodes over the right posterior parietal cortex (PPC) plus cathodal tDCS over the left PPC | Over the contralateral supraorbital forehead | 1 mA for 20 minutes | Each participant underwent dual‐tDCS, A‐tDCS and sham tDCS once with a wash‐out period of at least 24 hours | None | None | 3 (30%) suffered from mild headache after dual‐tDCS, which disappeared spontaneously | Published |
| A‐tDCS | Saline‐soaked 25 cm² sponge electrodes over the right PPC plus sham tDCS over the left PPC | For A‐tDCS: 1 mA for 20 minutes For sham tDCS: 1 mA for 10 seconds |
|||||||
| Sham tDCS | Saline‐soaked 25 cm² sponge electrodes over the right PPC plus sham tDCS over the left PPC | 1 mA for 10 seconds | |||||||
| Tahtis 2012 | Dual‐tDCS | Saline‐soaked 25 cm² electrodes with the anode placed over the leg area of the lesioned hemisphere and the cathode placed over leg area of the non‐lesioned hemisphere | Not described | 2 mA for 15 minutes | Dual‐tDCS or sham tDCS once | None | Unclear | None | Published |
| Sham tDCS | 2 mA for < 30 seconds | ||||||||
| Tedesco Triccas 2015b | A‐tDCS | Saline‐soaked 35 cm² sponge electrodes with the anode placed over M1 of the affected hemisphere | Over the contralateral supraorbital forehead | 1 mA for 20 minutes | Base therapy plus tDCS or sham tDCS for 18 sessions during 8 weeks (approximately 2 to 3 sessions per week) | Robotic arm training with the ArmeoSpring device (60 minutes per session) for 18 sessions during 8 weeks (approximately 2 to 3 sessions per week) | 1 out of 12 (8%) in the A‐tDCS group due to a skin reaction after receiving four sessions of A‐tDCS | 6 out of 12 (50%) in the A‐tDCS group reported adverse events such as pain, burning or headache after receiving A‐tDCS | Published/unpublished |
| Sham tDCS | 1 mA for 20 seconds | ||||||||
| Viana 2014 | A‐tDCS | Saline‐soaked 35 cm² sponge electrodes with the anode placed over M1 of the affected hemisphere | Over the contralateral supraorbital forehead | 2 mA for 13 minutes | Base therapy + A‐tDCS or sham tDCS 3 times a week for 5 weeks | Virtual reality training using Nintendo Wii (Games used: Wii Sports resort, Wii Play Motion, Let's Tap) aiming at movements of shoulder, elbow, wrist, hand and fingers; each game was played for 15 minutes (total time per training session: 60 minutes); passive stretching exercises were performed before and after each training session | None | None | Published |
| Sham tDCS | 2 mA for 30 seconds | ||||||||
| Wang 2014 | Dual‐tDCS | 35 cm² electrodes with the anode placed over M1 of the affected hemisphere | Over contralateral M1 | 1 mA for 20 minutes | Dual‐tDCS or sham‐tDCS once | Placebo methylphenidate 1 hour prior to stimulation | Unclear | No major adverse events; 3 participants (50%) from the dual‐tDCS group reported mild tingling sensation with tDCS stimulation | Published |
| 20 mg MP 1 hour prior to stimulation | |||||||||
| Sham‐tDCS | 1 mA for 10 seconds | ||||||||
| Wu 2013a | C‐tDCS | Saline‐soaked 24.75 cm² sponge electrodes over primary sensorimotor cortex of the lesioned hemisphere | Over the shoulder on the unaffected side | 1.2 mA for 20 minutes | Once daily 5 days a week for 4 weeks | Quote: "Both groups received a conventional physical therapy program for 30 minutes twice daily, including maintaining good limb position, chronic stretching via casting or splinting, physical modalities and techniques, and movement training" | None | None | Published |
| Sham tDCS | 1.2 mA for 30 seconds | ||||||||
A‐tDCS: anodal direct current stimulation C‐tDCS: cathodal direct current stimulation CIMT: constraint‐induced movement therapy Dual‐tDCS: A‐tDCS and C‐tDCS simultaneously EEG: electroencephalography M1: primary Motor Cortex MEP: motor‐evoked potentials MI‐BCI: motor imagery brain‐computer interface MP: methylphenidate NA: not applicable PNF: proprioceptive neuromuscular facilitation PPC: posterior parietal cortex PT: physical therapy SD: standard deviation tDCS: transcranial direct current stimulation
Outcomes
A widely used outcome was the Barthel Index (BI) and the Upper Extremity Fugl‐Meyer Score (UE‐FM). Twenty‐two out of 32 studies (69%) have reported data on dropouts and 13 out of 32 studies (41%) have reported data on adverse events.
We had to exclude nine of the included trials from quantitative syntheses (meta‐analyses) because of missing information regarding the first intervention period of the cross‐over trial (Au‐Yeung 2014; Fregni 2005a; Jo 2008; Kang 2008a; Kim 2009; Ko 2008; Mahmoudi 2011; Sohn 2013; Sunwoo 2013).
Excluded studies
We excluded 29 trials from qualitative assessment (Boggio 2007b; Bradnam 2012; Byblow 2011; Celnik 2009; Danzl 2012; Edwards 2009; Gandiga 2006; Giacobbe 2013; Goh 2015; Gurchin 1988; Hummel 2005a; Hummel 2005b; Jayaram 2009; Kasashima 2012; Kharchenko 2001; Kitisomprayoonkul 2012; Kumar 2011; Kwon 2012; Lee 2012; Lefebvre 2013; Lefebvre 2015; Madhavan 2011; Manganotti 2011; Ochi 2013; Paquette 2011; Sheliakin 2006; Stagg 2012a; Takeuchi 2012; Zimerman 2012), mainly because they were not RCTs, or because their outcomes did not measure function or activities of daily living (ADLs) (see Characteristics of excluded studies).
Risk of bias in included studies
We provided information about the risk of bias in Characteristics of included studies. To complete the rating of methodological quality, we contacted all principal investigators of the included trials and of trials awaiting classification to request further information about methodological issues, if necessary. We made contact via letter and email, including email reminders once a month if we received no response. Some trialists provided all requested information, and some did not answer our requests. We used the 'Risk of bias' tool, as implemented in Review Manager 5, to assess risk of bias according to the aspects listed under Methods. Two review authors (BE and JM) independently assessed risk of bias of the included trials, and two other review authors (JK and MP) checked the extracted data for agreement. All review authors discussed disagreements and, if necessary, sought arbitration by another review author. A detailed description of risk of bias can be found in Characteristics of included studies. Information on risk of bias on study level and outcome level is provided in Figure 2 and in Figure 3.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sixteen of the 32 included studies (50%) described a low risk of bias for sequence generation, whereas eight studies (25%) described a low risk of bias for allocation concealment.
Blinding
Twenty‐three of the 32 included studies (72%) described low risk of bias for blinding of participants and personnel for subjective outcomes and 29 studies (91%) for objective outcomes, respectively. Twenty‐eight studies (88%) described low risk of bias for blinding of outcome assessment for subjective and objective outcomes, whereas two studies were determined to have high risk of bias (Fusco 2013a; Kim 2009).
Incomplete outcome data
Twenty‐three of the 32 included studies (72%) were at low risk of bias for incomplete outcome data for subjective outcomes, whereas 18 (56%) were at low risk of bias for subjective outcomes, and one was at high risk of bias (Kim 2010).
Selective reporting
Four of the 32 included studies (13%) were at low risk of bias for selective outcome reporting (Hesse 2011; Khedr 2013; Rossi 2013; Wu 2013a), and three studies (9%) were at high risk (Di Lazzaro 2014a; Di Lazzaro 2014b; Nair 2011).
Effects of interventions
Summary of findings for the main comparison. tDCS versus any type of placebo or passive control intervention for improving function, and activities of daily living, cognitive abilities and neglect in people after stroke.
| tDCS versus any type of placebo or passive control intervention for improving function, and activities of daily living, cognitive abilities and neglect in people after stroke | ||||||
| Patient or population: people with improving function, and activities of daily living, cognitive abilities and neglect after stroke Settings: unspecified Intervention: tDCS versus any type of placebo or passive control intervention | ||||||
| Outcomes |
Illustrative comparative risks* (95% CI): Corresponding risk tDCS versus any type of placebo or passive control intervention |
No. of participants (studies) | Quality of the evidence (GRADE) | Comments | ||
| Primary outcome measure: ADLs at the end of the intervention period ‐ absolute values Measures of activities of daily living. Scale from: 0 to infinity | The mean primary outcome measure: ADLs at the end of the intervention period ‐ absolute values in the intervention groups was 0.24 standard deviations higher (0.03 to 0.44 higher) | 396 (9 studies) | ⊕⊕⊕⊝ moderate1 | SMD 0.24 (0.03 to 0.44); however, this effect was not sustained when including only studies with adequate allocation concealment (Table 8) | ||
| Primary outcome measure: ADLs at the end of the intervention period ‐ change scores Measures of activities of daily living. Scale from: 0 to infinity. | The mean primary outcome measure: ADLs at the end of the intervention period ‐ change scores in the intervention groups was 0.46 standard deviations higher (0.75 lower to 1.67 higher) | 11 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | SMD 0.46 (‐0.75 to 1.67) | ||
|
Primary outcome measure: ADLs until the end of follow‐up
Measures of activities of daily living. Scale from: 0 to infinity Follow‐up: mean 3 months |
The mean primary outcome measure: ADLs until the end of follow‐up in the intervention groups was 0.31 standard deviations higher (0.01 to 0.62 higher) | 269 (6 studies) | ⊕⊕⊕⊝ moderate4 | SMD 0.31 (0.01 to 0.62); however, this effect was not sustained when including only studies with adequate allocation concealment (Table 9) | ||
|
Secondary outcome measure: upper extremity function at the end of the intervention period ‐ absolute values Clinical measures of upper extremity function. Scale from: 0 to infinity |
The mean secondary outcome measure: upper extremity function at the end of the intervention period ‐ absolute values in the intervention groups was 0.11 standard deviations higher (0.17 lower to 0.39 higher) | 431 (12 studies) | ⊕⊕⊝⊝ low1,2 | SMD 0.11 (‐0.17 to 0.39) | ||
|
Secondary outcome measure: upper extremity function at the end of the intervention period ‐ change scores Clinical measures of upper extremity function. Scale from: 0 to infinity |
The mean secondary outcome measure: upper extremity function at the end of the intervention period ‐ change scores in the intervention groups was 0.32 standard deviations higher (0.51 lower to 1.15 higher) | 53 (4 studies) | ⊕⊕⊝⊝ low1,2 | SMD 0.32 (‐0.51 to 1.15) | ||
|
Secondary outcome measure: upper extremity function to the end of follow‐up ‐ absolute values Clinical measures of upper extremity function. Scale from: 0 to infinity Follow‐up: mean 3 months |
The mean secondary outcome measure: upper extremity function to the end of follow‐up ‐ absolute values in the intervention groups was 0.01 standard deviations higher (0.48 lower to 0.50 higher) | 187 (4 studies) | ⊕⊕⊝⊝ low2,3 | SMD 0.01 (‐0.48 to 0.50) | ||
|
Secondary outcome measure: dropouts, adverse events and deaths during the intervention period Number of adverse events, dropouts and deaths during the intervention period |
Study population | See comment | 664 (23 studies) | ⊕⊕⊝⊝ low1,2 | Risks were calculated from pooled risk differences | |
| 20 per 1000 | 48 per 1000 (10 to 80) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADL: activities of daily living; CI: Confidence interval; SMD: standardised mean difference; tDCS: transcranial direct current stimulation | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded due to several ratings with 'unclear' or 'high' risk of bias. 2 Downgraded because 95% CI contains effect size of no difference and the minimal important difference. 3 Downgraded because the total sample size is less than 400 (as a rule of thumb). 4 Downgraded because the results did not persist, when only studies of high methodological quality were included.
Summary of findings 2. tDCS versus any type of active control intervention for improving function, activities of daily living, cognitive abilities and neglect in people after stroke.
| tDCS versus any type of active control intervention for improving function activities of daily living, cognitive abilities and neglect in people after stroke | ||||||
| Patient or population: people with improving function, activities of daily living, cognitive abilities and neglect after stroke Settings: unspecified Intervention: tDCS versus any type of active control intervention | ||||||
| Outcomes |
Illustrative comparative risks* (95% CI) Corresponding risk tDCS versus any type of active control intervention |
No. of participants (studies) | Quality of the evidence (GRADE) | Comments | ||
| Primary outcome measure: ADLs at the end of the intervention period, absolute values Measures of activities of daily living. Scale from: 0 to 100 | The mean primary outcome measure: ADLs at the end of the intervention period, absolute values in the intervention groups was 0 higher (10.04 lower to 10.04 higher) | 50 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | |||
| Secondary outcome measure: upper extremity function at the end of the intervention period Clinical measures of upper extremity function. Scale from: 0 to 66 | The mean secondary outcome measure: upper extremity function at the end of the intervention period in the intervention groups was 19 higher (9.38 to 28.62 higher) | 20 (1 study) | ⊕⊕⊝⊝ low1,2 | |||
| Secondary outcome measure: lower extremity function at the end of the intervention period Clinical measures of lower extremity function. Scale from: 0 to 34 | The mean secondary outcome measure: lower extremity function at the end of the intervention period in the intervention groups was 5.3 higher (0.75 to 9.85 higher) | 20 (1 study) | ⊕⊕⊝⊝ low1,2 | |||
| Secondary outcome measure: dropouts, adverse events and deaths during the intervention period Adverse events, dropouts and deaths during the intervention period | Study population | See comment | 70 (2 studies) | See comment | Risks were calculated from pooled risk differences | |
| See comment | See comment | |||||
| Moderate | ||||||
| 0 per 1000 | ‐2147483648 per 1000 (‐2147483648 to ‐2147483648) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADL: activities of daily living; CI: Confidence interval; tDCS: transcranial direct current stimulation | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded due to several ratings with 'unclear' or 'high' risk of bias. 2 Downgraded due to total sample size being less than 400 (as a rule of thumb). 3 Downgraded because 95% CI contains effect size of no difference and the minimal important difference.
Twenty‐three of the 32 included studies (72%) were included within the meta‐analysis (Ang 2012; Boggio 2007a; Bolognini 2011; Cha 2014; Di Lazzaro 2014a; Di Lazzaro 2014b; Fusco 2013a; Fusco 2014; Geroin 2011; Hesse 2011; Khedr 2013; Kim 2010; Lee 2014; Lindenberg 2010; Nair 2011; Park 2013; Qu 2009; Rossi 2013; Tahtis 2012; Tedesco Triccas 2015b; Viana 2014; Wang 2014; Wu 2013a).
Comparison 1. tDCS versus any type of placebo or passive control intervention
Comparison 1.1 Primary outcome measure: ADLs at the end of the intervention period
1.1.1 Studies presenting absolute values
We found nine studies with 396 participants examining the effects of tDCS on ADLs (Bolognini 2011; Di Lazzaro 2014a; Di Lazzaro 2014b; Hesse 2011; Khedr 2013; Kim 2010; Lee 2014; Tedesco Triccas 2015b; Wu 2013a). We found evidence of effect regarding ADL performance when we analysed the data with combined intervention groups, as stated in Methods (i.e. A‐tDCS and/or C‐tDCS versus sham tDCS; standardised mean difference (SMD) 0.24, 95% confidence interval (CI) 0.03 to 0.44; inverse variance method with random‐effects model; moderate quality evidence; Analysis 1.1; Table 1).
1.1. Analysis.

Comparison 1 tDCS versus any type of placebo or passive control intervention, Outcome 1 Primary outcome measure: ADLs at the end of the intervention period.
1.1.2 Studies presenting change scores
One study with 11 participants reported the effects of tDCS on ADLs as change values relative to baseline (Fusco 2014). There is very low quality evidence that there is no evidence of an effect (SMD 0.46, 95% CI ‐0.75 to 1.67; inverse variance method with random‐effects model; very low quality evidence; Analysis 1.1; Table 1).
The funnel plot of Analysis 1.1 can be found in Figure 4. By visual inspection, we concluded that there were no indications of funnel plot asymmetry indicating a small‐study effect or publication bias.
4.

Funnel plot of comparison: 1 Primary outcome measure: tDCS for improvement of ADLs versus any type of placebo or control intervention, outcome: 1.1 ADLs at the end of the intervention period, absolute values (BI points).
Comparison 1.2 Primary outcome measure: ADLs until the end of follow‐up, absolute values (at least three months after the end of the intervention period)
We included six studies with 269 participants (Di Lazzaro 2014b; Hesse 2011; Khedr 2013; Kim 2010; Rossi 2013; Tedesco Triccas 2015b); investigators measured the effects of tDCS on ADLs at the end of follow‐up. We found evidence of effect regarding ADL performance when we analysed the data with combined intervention groups (SMD 0.31, 95% CI 0.01 to 0.62; inverse variance method with random‐effects model; moderate quality evidence; Analysis 1.2; Table 1).
1.2. Analysis.

Comparison 1 tDCS versus any type of placebo or passive control intervention, Outcome 2 Primary outcome measure: ADLs until the end of follow‐up.
Because there were less than 10 included studies in this comparison, we omitted the visual inspection of the funnel plot of this comparison.
Comparison 1.3 Secondary outcome measure: upper extremity function at the end of the intervention period
1.3.1 Studies presenting absolute values
Twelve trials with a total of 431 participants examined upper limb function at the end of the intervention period and provided absolute values for the outcome (Bolognini 2011; Di Lazzaro 2014a; Di Lazzaro 2014b; Fusco 2013a; Hesse 2011; Kim 2010; Lee 2014; Lindenberg 2010; Rossi 2013; Tedesco Triccas 2015b; Viana 2014; Wu 2013a). There was no evidence of effect of tDCS when we analysed the data with combined intervention groups (SMD 0.11, 95% CI ‐0.17 to 0.39; inverse variance method with random‐effects model; low quality evidence; Analysis 1.3; Table 1).
1.3. Analysis.

Comparison 1 tDCS versus any type of placebo or passive control intervention, Outcome 3 Secondary outcome measure: upper extremity function at the end of the intervention period.
1.3.2 Studies presenting change scores
We included four studies with 53 participants (Ang 2012; Fusco 2013a; Nair 2011; Wang 2014); investigators measured the effects of tDCS on upper limb function at the end of the intervention period and provided absolute values for the outcome. There was no evidence of effect of tDCS when we analysed the data with combined intervention groups (SMD 0.32, 95% CI ‐0.51 to 1.15; inverse variance method with random‐effects model; low quality evidence; Analysis 1.3; Table 1).
Upon graphical inspection of the funnel plot of Analysis 1.3, we found no evidence of small‐study effects.
Comparison 1.4 Secondary outcome measure: upper extremity function to the end of follow‐up (at least three months after the end of the intervention period)
1.4.1 Studies presenting absolute values
Four studies with a total of 187 participants examined upper extremity function at the end of follow‐up and reported absolute values for this outcome (Di Lazzaro 2014b; Hesse 2011; Rossi 2013; Tedesco Triccas 2015b). We found no evidence of effect regarding upper extremity function when we analysed the data with combined intervention groups (i.e. A‐tDCS and/or C‐tDCS versus sham tDCS; SMD 0.01, 95% CI ‐0.48 to 0.50; inverse variance method with random‐effects model; low quality evidence; Analysis 1.4; Table 1).
1.4. Analysis.

Comparison 1 tDCS versus any type of placebo or passive control intervention, Outcome 4 Secondary outcome measure: upper extremity function to the end of follow‐up.
1.4.2 Studies presenting change scores
We included one study with 18 participants (Kim 2010); the investigators measured the effects of tDCS on upper limb function at the end of follow‐up and provided change values for the outcome. There was evidence of effect of tDCS when we analysed the data with combined intervention groups (SMD 1.49, 95% CI 0.40 to 2.59; inverse variance method with random‐effects model; low quality evidence; Analysis 1.4).
Because there were less than 10 included studies in this comparison, we omitted the visual inspection of the funnel plot of this comparison.
Comparison 1.5 Secondary outcome measure: lower extremity function at the end of the intervention period
1.5.1 Studies presenting absolute values
Two studies with a total of 50 participants examined lower extremity function at the end of the intervention period and reported absolute values for this outcome (Cha 2014; Geroin 2011). We found no evidence of effect regarding lower extremity function when we analysed the data with combined intervention groups (i.e. A‐tDCS and/or C‐tDCS versus sham tDCS; SMD 0.20, 95% CI ‐1.26 to 1.67; inverse variance method with random‐effects model; low quality evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1 tDCS versus any type of placebo or passive control intervention, Outcome 5 Secondary outcome measure: lower extremity function at the end of the intervention period.
1.5.2 Studies presenting change scores
Two studies with a total of 25 participants examined lower extremity function at the end of the intervention period and reported change values for this outcome (Fusco 2014; Tahtis 2012). We found no evidence of effect regarding lower extremity function when we analysed the data with combined intervention groups (i.e. A‐tDCS and/or C‐tDCS versus sham tDCS; SMD 0.81, 95% CI ‐0.02 to 1.65; inverse variance method with random‐effects model; Analysis 1.5.
Because there were less than 10 included studies in this comparison, we omitted the visual inspection of the funnel plot of this comparison. We did not identify any study examining the effects of tDCS on lower extremity function at follow‐up.
Comparison 1.6 Secondary outcome measure: muscle strength at the end of the intervention period
1.6.1 Studies presenting absolute values
We included eight studies with 272 participants (Bolognini 2011; Di Lazzaro 2014a; Di Lazzaro 2014b; Fusco 2013a; Hesse 2011; Khedr 2013; Lee 2014; Viana 2014); investigators measured the effects of tDCS on muscle strength at the end of the intervention period and provided absolute values for the outcome. There was no evidence of effect of tDCS when we analysed the data with combined intervention groups (SMD 0.14, 95% CI ‐0.11 to 0.38; inverse variance method with random‐effects model; Analysis 1.6).
1.6. Analysis.

Comparison 1 tDCS versus any type of placebo or passive control intervention, Outcome 6 Secondary outcome measure: muscle strength at the end of the intervention period.
1.6.2 Studies presenting change scores
Two studies with a total of 41 participants examined muscle strength at the end of the intervention period and reported change values for this outcome (Fusco 2014; Geroin 2011). We found no evidence of effect regarding muscle strength when we analysed the data with combined intervention groups (i.e. A‐tDCS and/or C‐tDCS versus sham tDCS; SMD 0.05, 95% CI ‐2.12 to 2.23; inverse variance method with random‐effects model; Analysis 1.6).
By visual inspection, the authors concluded that there were no indications of funnel plot asymmetry indicating a small‐study effect or publication bias.
Comparison 1.7 Secondary outcome measure: muscle strength at the end of follow‐up (at least three months after the end of the intervention period), absolute values
We included three studies with 156 participants (Di Lazzaro 2014b; Hesse 2011; Khedr 2013); investigators measured the effects of tDCS on muscle strength at the end of follow‐up and provided absolute values for the outcome. There was no evidence of effect of tDCS when we analysed the data with combined intervention groups (SMD 0.07, 95% CI ‐0.26 to 0.41; inverse variance method with random‐effects model; Analysis 1.7).
1.7. Analysis.
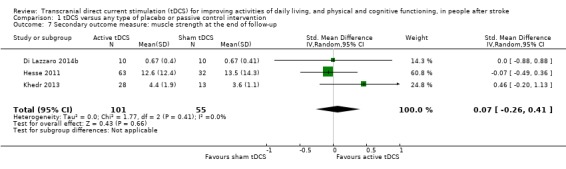
Comparison 1 tDCS versus any type of placebo or passive control intervention, Outcome 7 Secondary outcome measure: muscle strength at the end of follow‐up.
Because there were less than 10 included studies in this comparison, we omitted the visual inspection of the funnel plot of this comparison.
Comparison 1.8 Secondary outcome measure: cognitive abilities at the end of the intervention period
There was one study with eleven participants that examined the effects of tDCS on cognitive abilities (Park 2013). We did not perform statistical pooling. We identified three randomised cross‐over trials that examined the effects of tDCS on cognitive abilities, but data extraction was not possible due to missing information regarding the first intervention period (Au‐Yeung 2014; Jo 2008; Kang 2008a). However, each of the studies reported evidence of an effect in favour of tDCS regarding measures of attention. We did not identify any studies examining the effects of tDCS on cognitive abilities at follow‐up.
Empty comparison: Secondary outcome measure: spatial neglect
We identified a randomised cross‐over trial with 15 participants that examined the effects of tDCS on neglect, but data extraction was not possible due to missing information regarding the first intervention period (Ko 2008). This study reported significant improvement in neglect tests. We did not identify any randomised studies examining the effects of tDCS on spatial neglect at follow‐up.
Comparison 1.9 Secondary outcome measure: dropouts, adverse events and deaths during the intervention period
Twenty‐two out of 32 studies (69%) reported data on dropouts, and 13 out of 32 studies (41%) reported data on adverse events. In six of 23 studies (26%), dropouts, adverse events or deaths that occurred during the intervention period were reported (Bolognini 2011; Fusco 2014; Hesse 2011; Kim 2010; Lee 2014; Tedesco Triccas 2015b), whereas the remaining studies reported no dropouts, adverse events or deaths. We found no evidence of effect regarding differences in dropouts, adverse effects and deaths between intervention and control groups (risk difference (RD) 0.01, 95% CI ‐0.02 to 0.03; Mantel‐Haenszel method with random‐effects model; analysis based only on studies that reported either on dropouts or on adverse events or on both; low quality evidence; Analysis 1.9; Table 1). A detailed description of dropouts, adverse events and deaths during the intervention period can be found in Table 7.
1.9. Analysis.

Comparison 1 tDCS versus any type of placebo or passive control intervention, Outcome 9 Secondary outcome measure: dropouts, adverse events and deaths during the intervention period.
Comparison 2. tDCS versus any type of active control intervention
Comparison 2.1 Primary outcome measure: ADLs at the end of the intervention period, absolute values
There was one study with 50 participants that examined the effects of tDCS on ADLs at the end of the intervention period and provided absolute values on this outcome (Qu 2009). This study did not show any evidence of effect of tDCS on ADLs at the end of the intervention period. We did not identify any study examining the effects of tDCS versus any type of active control intervention on ADLs at follow‐up. We gave a GRADE rating of very low quality evidence (Table 2).
Comparison 2.2 Secondary outcome measure: upper extremity function at the end of the intervention period
There was one study with 20 participants that examined the effects of tDCS on upper extremity function at the end of the intervention period (Cha 2014). This study reported evidence of effect of tDCS on upper extremity function at the end of the intervention period. We did not identify any study examining the effects of tDCS versus any type of active control intervention on upper extremity function at follow‐up. We gave a GRADE rating of low quality evidence (Table 2).
Comparison 2.3 Secondary outcome measure: upper extremity function at the end of the intervention period
There was one study with 20 participants that examined the effects of tDCS on lower extremity function at the end of the intervention period (Cha 2014). This study reported evidence of effect of tDCS on lower extremity function at the end of the intervention period. We could not identify any study examining the effects of tDCS versus any type of active control intervention on lower extremity function at follow‐up. We gave a GRADE rating of low quality evidence (Table 2).
Comparison 2.4 Secondary outcome measure: dropouts, adverse events and deaths during the intervention period
Neither of the two included studies reported dropouts, adverse events, or deaths that occurred during the intervention period (Cha 2014; Qu 2009). We found no evidence of effect regarding differences in dropouts, adverse effects and deaths between intervention and control groups (RD 0.00, 95% CI ‐0.07 to 0.07; Mantel‐Haenszel method with random‐effects model; analysis based only on studies which reported either on dropouts or on adverse events or on both; Analysis 2.4).
2.4. Analysis.

Comparison 2 tDCS versus any type of active control intervention, Outcome 4 Secondary outcome measure: dropouts, adverse events and deaths during the intervention period.
Comparison 3. Subgroup analyses
Outcome 3.1. Planned analysis: duration of illness ‐ acute/subacute versus postacute versus chronic phase for ADLs at the end of the intervention period
In a planned subgroup analysis, we analysed the effects of tDCS on the primary outcome of ADLs in the acute/subacute and postacute phases (Analysis 3.1). We found no evidence for different effects of tDCS between subgroups Chi² = 1.06, df = 2 (P = 0.59), I² = 0%.
3.1. Analysis.

Comparison 3 Subgroup analyses for primary outcome measure: ADLs at the end of the intervention period, Outcome 1 Planned analysis: duration of illness ‐ acute/subacute phase versus postacute phase for ADLs at the end of the intervention period.
Subgroup 3.1.1 Acute/subacute phase (the first week after stroke and the second to the fourth week after stroke)
We included four studies with 213 participants (Hesse 2011; Khedr 2013; Kim 2010; Lee 2014). We found no evidence of effect regarding differences in ADL performance between intervention and control groups when we analysed the data with combined intervention groups, as stated in the protocol (i.e. A‐tDCS or C‐tDCS or dual‐tDCS versus sham tDCS; SMD 0.22, 95% CI ‐0.07 to 0.51; inverse variance method with random‐effects model).
Subgroup 3.1.2 Postacute phase (from the first to the sixth month after stroke)
We included two studies with 140 participants (Qu 2009; Wu 2013a). We found evidence of differences in effect of tDCS regarding ADL performance between tDCS‐ and control groups when we analysed the data with combined intervention groups, as stated in the protocol (i.e. A‐tDCS or C‐tDCS or dual‐tDCS versus sham tDCS; SMD 0.30, 95% CI ‐0.22 to 0.82; inverse variance method with random‐effects model).
Subgroup 3.1.2 Chronic phase (from the sixth month after stroke)
We included four studies with 93 participants (Bolognini 2011; Di Lazzaro 2014a; Di Lazzaro 2014b; Tedesco Triccas 2015b). We found no evidence of effect regarding differences in ADL performance between intervention and control groups when we analysed the data with combined intervention groups, as stated in the protocol (i.e. A‐tDCS or C‐tDCS or dual‐tDCS versus sham tDCS; SMD ‐0.01, 95% CI ‐0.41 to 0.40); inverse variance method with random‐effects model).
Outcome 3.2. Planned analysis: effects of type of stimulation (A‐tDCS/C‐tDCS/dual‐tDCS) and location of stimulation (lesioned/non‐lesioned hemisphere) on ADLs at the end of the intervention period
We performed a planned subgroup analysis regarding the location of electrode positioning and hence of stimulation (Analysis 3.2). No studies investigated the effects of A‐tDCS over the non‐lesioned hemisphere. We found no evidence of differences in effects of location and type of stimulation regarding ADL performance between subgroups (Chi² = 0.67, df = 2 (P = 0.72), I² = 0%).
3.2. Analysis.

Comparison 3 Subgroup analyses for primary outcome measure: ADLs at the end of the intervention period, Outcome 2 Planned analysis: effects of type of stimulation (A‐tDCS/C‐tDCS/dual‐tDCS) and location of stimulation (lesioned/non‐lesioned hemisphere) on ADLs at the end of the intervention period (study groups collapsed).
Subgroup 3.2.1 A‐tDCS over the lesioned hemisphere
We included five studies with 164 participants (Bolognini 2011; Hesse 2011; Khedr 2013; Kim 2010; Tedesco Triccas 2015b). We found no evidence of differences in effects regarding ADL performance between A‐tDCS and sham tDCS groups (SMD ‐0.04, 95% CI ‐0.35 to 0.27; inverse variance method with random‐effects model).
Subgroup 3.2.2 C‐tDCS over the non‐lesioned hemisphere
We included six studies with 301 participants (Hesse 2011; Khedr 2013; Kim 2010; Lee 2014; Qu 2009; Wu 2013a). We found evidence of differences in effect regarding ADL performance between C‐tDCS and the sham tDCS groups, but the confidence intervals were wide (SMD 0.33, 95% CI 0.10 to 0.57; inverse variance method with random‐effects model).
Subgroup 3.2.3 Dual‐tDCS (A‐tDCS over the lesioned and C‐tDCS over the non‐lesioned hemisphere)
We included two studies with 33 participants (Di Lazzaro 2014a; Di Lazzaro 2014b). We did not find evidence of differences in effect regarding ADL performance between dual‐tDCS and sham tDCS groups (SMD 0.30, 95% CI ‐0.39 to 0.99; inverse variance method with random‐effects model).
Outcome 3.3. Planned sensitivity analysis regarding types of control interventions (sham tDCS/conventional therapy/no intervention)
Eight studies with 337 participants comparing active tDCS versus sham tDCS revealed evidence of an effect in favour of active tDCS (SMD 0.23, 95% CI 0.01 to 0.46; inverse variance method with random‐effects model; Analysis 3.3). Two studies with 109 participants comparing active tDCS versus active control interventions did not reveal evidence of an effect (SMD 0.14, 95% CI ‐0.24 to 0.53; inverse variance method with random‐effects model; Analysis 3.3). The subgroups did not differ statistically significantly (test for subgroup differences: Chi² = 0.14, df = 1 (P = 0.71), I² = 0%).
Sensitivity analyses
We conducted a sensitivity analysis of methodological quality to test the robustness of our results. We repeated the analysis of our primary outcome, ADL performance at the end of the intervention period and at the end of follow‐up, and considered only studies with correctly concealed allocation, blinding of assessors and ITT. The evidence of an effect of tDCS disappeared when we analysed only those studies with correct allocation concealment. See Table 8 and Table 9.
3. Sensitivity analyses for comparison 1.1: primary outcome of ADL performance at the end of the intervention period.
| Sensitivity analysis | Studies included in analysis | Effect estimate |
| All studies with proper allocation concealment | Hesse 2011; Khedr 2013; Kim 2010; Tedesco Triccas 2015b; Wu 2013a | (SMD 0.24, 95% CI ‐0.07 to 0.56; participants = 290; studies = 5; I2 = 36%; inverse variance method with random‐effects model) |
| All studies with proper blinding of outcome assessor for primary outcome absolute values | Bolognini 2011; Di Lazzaro 2014a; Di Lazzaro 2014b; Hesse 2011; Khedr 2013; Kim 2010; Lee 2014; Tedesco Triccas 2015b; Wu 2013a | (SMD 0.24, 95% CI 0.03 to 0.44; participants = 396; studies = 9; I2 = 0%; inverse variance method with random‐effects model) |
| All studies with proper blinding of outcome assessor for primary outcome change values | Fusco 2014 | (SMD 0.46, 95% CI ‐0.75 to 1.67; participants = 11; studies = 1; I2 = 0%; inverse variance method with random‐effects model) |
| All studies with intention‐to‐treat analysis | Di Lazzaro 2014a; Di Lazzaro 2014b; Hesse 2011; Khedr 2013; Wu 2013a | (SMD 0.31, 95% CI 0.05 to 0.56; participants = 259; studies = 5; I2 = 0%; inverse variance method with random‐effects model) |
CI: confidence interval SMD: standardised mean difference
4. Sensitivity analyses for comparison 1.2: primary outcome of ADL performance at the end of follow‐up at least 3 months after the end of the intervention period.
| Sensitivity analysis | Studies included in analysis | Effect estimate |
| All studies with proper allocation concealment | Hesse 2011; Khedr 2013; Kim 2010; Tedesco Triccas 2015b | (SMD 0.30, 95% CI ‐0.15 to 0.75; participants = 199; studies = 4; I2 = 51%; inverse variance method with random‐effects model) |
| All studies with proper blinding of outcome assessor for primary outcome | Di Lazzaro 2014b; Hesse 2011; Khedr 2013; Kim 2010; Rossi 2013; Tedesco Triccas 2015b | (SMD 0.31, 95% CI 0.01 to 0.62; participants = 269; studies = 6; I2 = 27%; inverse variance method with random‐effects model) |
| All studies with intention‐to‐treat analysis | Di Lazzaro 2014b; Hesse 2011; Khedr 2013; Rossi 2013 | (SMD 0.38, 95% CI 0.05 to 0.70; participants = 205; studies = 4; I2 = 16%; inverse variance method with random‐effects model) |
CI: confidence interval SMD: standardised mean difference
Discussion
Summary of main results
This review focused on evaluating the effectiveness of transcranial direct current stimulation (tDCS) (anodal stimulation (A‐tDCS)/cathodal stimulation (C‐tDCS)/(anodal plus cathodal stimulation simultaneously (dual‐tDCS)) versus any passive control intervention (sham tDCS or no intervention) and tDCS versus any active control intervention (any other approach) for improving activities of daily living (ADLs), arm and leg function, muscle strength and cognitive abilities (including spatial neglect), dropouts and adverse events in people after stroke. We included 32 trials with a total of 748 participants.
Comparison 1: tDCS versus any type of placebo or passive control intervention
We found nine studies with 396 participants examining the effects of tDCS on our primary outcome measure, ADLs, after stroke. In addition to these studies presenting absolute values of the outcome, we found one study with 11 participants, presenting change values for the outcome. We found evidence of effect regarding ADL performance at the end of the intervention period for nine studies presenting absolute values (standardised mean difference (SMD) 0.24, 95% confidence interval (CI) 0.03 to 0.44; inverse variance method with random‐effects model). The funnel plot shows no evidence of a small‐study effect. Six studies with 269 participants assessed the effects of tDCS on ADLs at the end of follow‐up. Evidence suggested an effect regarding ADL performance (SMD 0.31, 95% CI 0.01 to 0.62; inverse variance method with random‐effects model). However, this effect was not sustained when including only studies with adequate allocation concealment (Table 8; Table 9).
One of our secondary outcome measures was upper extremity function: 12 trials with a total of 431 participants measured upper extremity function at the end of the intervention period, revealing no evidence of an effect in favour of tDCS (SMD 0.01, 95% CI ‐0.48 to 0.50 for studies presenting absolute values, and SMD 0.32, 95% CI ‐0.51 to 1.15 for studies presenting change values; inverse variance method with random‐effects model). Regarding the effects of tDCS on upper extremity function at the end of follow‐up, we identified four studies with a total of 187 participants (absolute values) that showed no evidence of an effect (SMD 0.01, 95% CI ‐0.48 to 0.50; inverse variance method with random‐effects model). Four studies with 75 participants examined the effect of tDCS on lower extremity function, but did not show evidence of an effect. Ten studies with 313 participants reported outcome data for muscle strength at the end of the intervention period, but in the corresponding meta‐analysis there was no evidence of an effect. Three studies with 156 participants reported outcome data on muscle strength at follow‐up, but there was no evidence of an effect. Four studies with 41 participants examined the effects of tDCS on cognitive abilities (including spatial neglect), however, statistical pooling was not possible due to missing information (three out of the four studies reported evidence of an effect). We identified a randomised cross‐over trial with 15 participants that examined the effects of tDCS on neglect (which reported evidence of an effect of tDCS on neglect).
Twenty‐two out of 32 studies (69%) reported data on dropouts and 13 out of 32 studies (41%) have reported data on adverse events. In six of 23 studies (26%), dropouts, adverse events or deaths occurred during the intervention period. We found no evidence of an effect regarding differences in dropouts, adverse effects and deaths between intervention and control groups (risk difference (RD) 0.03, 95% CI ‐0.01 to 0.06; Mantel‐Haenszel method with random‐effects model; analysis based only on studies that reported either on dropouts or on adverse events or on both).
A summary of this review's main findings can be found in Table 1.
Comparison 2: tDCS versus any type of active control intervention
We identified two studies with 70 participants comparing active tDCS with an active control intervention (physiotherapy or virtual reality). A summary of this review's main findings can be found in Table 2.
Overall completeness and applicability of evidence
The results of this review appear seem to be generalisable to other settings in industrialised countries. However, some factors suggest uncertainty in generalisations. These include the following.
Most of the studies included participants with first‐time ever stroke.
Most participants suffered from ischaemic stroke.
Hence, the results may be of limited applicability for people with recurrent and haemorrhagic strokes. Moreover, completeness of evidence is lacking regarding studies on the effects of tDCS on lower limb function, cognitive abilities (including spatial neglect), and the reporting of adverse events.
The physiological mechanisms of tDCS are not fully understood yet. Included studies are heterogeneous in terms of type, location and duration of stimulation, amount of direct current delivered, electrode size and positioning, and participants with cortical and subcortical stroke. For example, recent research suggests that the effectiveness of cathodal stimulation (C‐tDCS) over the contralesional M1 depends on corticospinal tract integrity, thus implicating that this is not a "one size fits all" intervention (Byblow 2011). Hence, it could be that this heterogeneity ‐ even in the absence of excess statistical heterogeneity in our analyses ‐ produces a false‐negative finding (Antal 2015).
Twenty‐two out of 32 studies (69%) reported data on dropouts, and 13 out of 32 studies (41%) reported data on adverse events. We therefore decided to include only studies that reported either on dropouts, or on adverse events, or on both, in our analyses of adverse events. However, it could be that the real risk of dropouts or adverse events is underestimated in our analysis, since the analysis could be prone to reporting bias.
Quality of the evidence
Based on our assessments of the quality of evidence provided in Table 1 and Table 2, we downgraded risk of bias and the imprecision of effect estimates that included no difference in the comparators, and concurrently failed to exclude clinically unimportant differences between them. We also found heterogeneity regarding trial design (parallel‐group or cross‐over design, two or three intervention groups), therapy variables (type of stimulation, location of stimulation, dosage of stimulation) and participant characteristics (age, time poststroke, severity of stroke/initial functional impairment).
Potential biases in the review process
The methodological rigour of Cochrane reviews minimises bias during the process of conducting systematic reviews. However, some aspects of this review represent an 'open door' to bias, such as eliminating obviously irrelevant publications according to titles and abstracts on the determination of only one review author (BE). This encompasses the possibility of unintentionally ruling out relevant publications. Another possibility is that publication bias could have affected our results. With the funnel plot for our main outcome of ADLs (at the end of the intervention period) showing no asymmetry, a small‐study effect or publication bias nevertheless could exist, resulting in overestimation of the effects (Figure 4) (Sterne 2011).
Another potential source for the introduction of bias is that two of the review authors (JM and MP) were involved in conducting and analysing the largest of the included trials (Hesse 2011). However, they did not participate in extracting outcome data and determining risk of bias of this trial. They were replaced by another review author (JK), so that the introduction of bias is unlikely in this case.
We had to exclude nine trials from quantitative synthesis (meta‐analysis) because of missing information regarding treatment order (i.e. the first intervention period of the cross‐over trial) (Au‐Yeung 2014; Fregni 2005a; Jo 2008; Kang 2008a; Kim 2009; Ko 2008; Mahmoudi 2011; Sohn 2013; Sunwoo 2013). However, the results of these trials regarding upper and lower extremity function, cognitive abilities (including spatial neglect) are mostly consistent with the results of comparisons made in our meta‐analyses, and it is therefore unlikely that the results of these studies would have substantially altered our results.
Agreements and disagreements with other studies or reviews
As far as we know, there are several systematic reviews on the effects of tDCS on function after stroke: Tedesco Triccas 2015a included genuine RCTs with multiple sessions of tDCS. They included nine studies with 371 participants and showed no evidence of effect at the end of the intervention period (SMD 0.11, 95% CI ‐0.17 to 0.38; inverse variance method with fixed‐effect model) or at long‐term follow‐up (SMD 0.23, 95% CI ‐0.17 to 0.62; inverse variance method with fixed‐effect model). These results are similar to the results of our analyses regarding the effects of tDCS (combined) on upper limb function.
Another systematic review of quasi‐randomised and properly randomised controlled trials has examined the effects of A‐tDCS on upper limb motor recovery in stroke patients (Butler 2013). The review authors included eight trials with 168 participants, and their analysis revealed evidence of an effect of tDCS on upper limb function (SMD 0.49, 95% CI 0.18 to 0.81), mainly measured by the Jebsen Taylor Hand Function Test (JTT).
In another systematic review on the effects of tDCS, Adeyemo 2012 included 50 non‐randomised and RCTs with 1314 participants (1282 people with stroke and 32 healthy volunteers) on the pooled effects of tDCS and repetitive transcranial magnetic stimulation (rTMS) on motor outcomes after stroke. With their analysis based on change values they revealed an effect of SMD 0.59, 95% CI 0.42 to 0.76; inverse variance method with random‐effects model). These results differ from the results of our analyses, maybe because of 1) the inclusion of non‐randomised studies that tend to overestimate treatment effects (Higgins 2011a), and 2) the statistical pooling with trials examining the effects of rTMS on motor outcomes after stroke.
Two other systematic reviews included meta‐analyses dealing with the topic of tDCS for improving function after stroke (Bastani 2012; Jacobson 2012). Bastani 2012 examined the effects of A‐tDCS on cortical excitability (as measured by transcranial magnet stimulation (TMS)) and upper extremity function (mainly measured by JTT) in healthy volunteers and people with stroke. Their analysis of the effects of A‐tDCS over the lesioned hemisphere, based mainly on results of randomised cross‐over studies, yielded no evidence of effect (SMD 0.39, 95% CI ‐0.17 to 0.94; inverse variance method with fixed‐effect model). Jacobson 2012, a review about the effects of A‐tDCS and C‐tDCS on healthy volunteers, stated that the anodal‐excitation and cathodal‐inhibition (AeCi) dichotomy is relatively consistent regarding the effects of tDCS on function in healthy volunteers. However, in our analysis on people with stroke, we found evidence of an effect of C‐tDCS over the non‐lesioned as well as over the lesioned hemisphere, which seems to be contradictory to the AeCi dichotomy for healthy volunteers, but is in accordance with the findings of another trial, aimed at comparing the lesion‐ and stimulation‐specific effects of tDCS in healthy volunteers and stroke patients (Suzuki 2012). However, we found no evidence of effect for A‐tDCS over the lesioned hemisphere in our planned subgroup analysis, which is consistent with the findings of Bastani 2012, but not with the findings of Suzuki 2012. In contrast to that we found evidence of an effect of tDCS on ADLs for C‐tDCS over the non‐lesioned hemisphere, which in turn is consistent with the findings of Suzuki 2012. However, when compared with the subgroups, A‐tDCS over the lesioned hemisphere and dual‐tDCS, the subgroup C‐tDCS over the non‐lesioned hemisphere has the highest statistical power.
Another systematic review found evidence of an effect of tDCS on motor‐evoked potentials (MEP), but not on physiologic parameters, which is not in accordance with our findings (Horvath 2015). Most of the published systematic reviews to date have focused on the effects of tDCS on function and ADLs. To our knowledge, our review, including 32 genuine RCTs with a total of 748 participants is the most comprehensive review about the effects of tDCS on ADLs, function, muscle strength and cognitive abilities (including spatial neglect) in stroke.
Authors' conclusions
Implications for practice.
Currently, evidence of very low to moderate quality suggests that transcranial direct current stimulation (tDCS) (anodal stimulation (A‐tDCS)/cathodal stimulation (C‐tDCS)/(anodal plus cathodal stimulation simultaneously (dual‐tDCS)) versus control (sham tDCS or any other approach or no intervention) might improve activities of daily living (ADLs) after stroke. Evidence of low quality suggests that there is no effect of tDCS on arm and leg function, muscle strength and cognitive abilities (including spatial neglect) in people after stroke. Evidence of low quality indicates that no effect regarding dropouts and adverse events can be seen between tDCS and control groups. However, this effect may be underestimated due to reporting bias.
Implications for research.
Currently the quality of evidence is still of very low to moderate quality, but there are many ongoing randomised trials on this topic that could change the quality of evidence in the future. Future studies should focus on those who may benefit most from tDCS after stroke and include outcomes of upper and lower limb function, muscle strength and cognitive abilities (including spatial neglect). Furthermore, dropouts and adverse events should be routinely monitored and presented as secondary outcomes. Methodological quality of future studies, particularly in relation to allocation concealment and intention‐to‐treat analysis, needs to be improved, along with adhering to the CONSORT statement, particularly for reporting dropouts and adverse events. Information on treatment order in randomised cross‐over trials also should be routinely presented in future publications.
What's new
| Date | Event | Description |
|---|---|---|
| 28 September 2015 | New citation required and conclusions have changed | The conclusions have changed: there is evidence of an effect of transcranial direct current stimulation for improving activities of daily living, but not for arm function. |
| 28 September 2015 | New search has been performed | The scope of the updated review has broadened since the previous version. This was in response to a request from the Cochrane Stroke Group to incorporate evidence relating to cognitive function (including neglect) into this update. We have rerun and expanded the searches to February 2015 and revised the text as appropriate. We have included 32 trials involving 748 participants in this update compared with 15 trials with 455 participants in the last version of this review from 2013. |
Acknowledgements
We thank Brenda Thomas for assistance in developing the search strategy and Hazel Fraser for giving us helpful support. We thank Shunjie Chua, Dee Shneiderman and Christine Fyfe for their valuable contribution as Consumer Reviewers. We also thank all researchers, who answered our requests and provided additional information.
Appendices
Appendix 1. CENTRAL search strategy
#1 [mh ^"cerebrovascular disorders"] or [mh "basal ganglia cerebrovascular disease"] or [mh "brain ischemia"] or [mh "carotid artery diseases"] or [mh "intracranial arterial diseases"] or [mh "intracranial embolism and thrombosis"] or [mh "intracranial hemorrhages"] or [mh ^stroke] or [mh "brain infarction"] or [mh ^"vertebral artery dissection"]
#2 (stroke or poststroke or "post‐stroke" or cerebrovasc* or brain next vasc* or cerebral next vasc* or cva* or apoplex* or SAH):ti,ab
#3 ((brain* or cerebr* or cerebell* or intracran* or intracerebral) near/5 (isch*emi* or infarct* or thrombo* or emboli* or occlus*)):ti,ab
#4 ((brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) near/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)):ti,ab
#5 [mh ^hemiplegia] or [mh paresis]
#6 (hemipleg* or hemipar* or paresis or paretic or hemineglect or "hemi‐neglect" or ((unilateral or spatial or hemi*spatial or visual) near/5 neglect)):ti,ab
#7 #1 or #2 or #3 or #4 or #5 or #6
#8 [mh ^"Electric Stimulation Therapy"]
#9 [mh ^"Electric Stimulation"]
#10 [mh ^Electrodes]
#11 (transcranial near/5 direct current near/5 stimulation):ti,ab
#12 (transcranial near/5 DC near/5 stimulation):ti,ab
#13 (transcranial near/5 electric* near/5 stimulation):ti,ab
#14 (tDCS or A‐tDCS or C‐tDCS or S‐tDCS or electrode* or anode or anodes or anodal or cathode or cathodes or cathodal):ti,ab
#15 #8 or #9 or #10 or #11 or #12 or #13 or #14
#16 #7 and #15
Number of records retrieved: 484
Appendix 2. MEDLINE (Ovid SP) search strategy
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vertebral artery dissection/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. hemiplegia/ or exp paresis/
6. (hemipleg$ or hemipar$ or paresis or paretic or hemineglect or hemi‐neglect or ((unilateral or spatial or hemi?spatial or visual) adj5 neglect)).tw.
7. or/1‐6
8. Electric Stimulation Therapy/
9. Electric Stimulation/
10. Electrodes/
11. (transcranial adj5 direct current adj5 stimulation).tw.
12. (transcranial adj5 DC adj5 stimulation).tw.
13. (transcranial adj5 electric$ adj5 stimulation).tw.
14. (tDCS or A‐tDCS or C‐tDCS or S‐tDCS or electrode$ or anode or anodes or anodal or cathode or cathodes or cathodal).tw.
15. or/8‐14
16. Randomized Controlled Trials as Topic/
17. random allocation/
18. Controlled Clinical Trials as Topic/
19. control groups/
20. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/
21. double‐blind method/
22. single‐blind method/
23. Placebos/
24. placebo effect/
25. cross‐over studies/
26. randomized controlled trial.pt.
27. controlled clinical trial.pt.
28. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt.
29. (random$ or RCT or RCTs).tw.
30. (controlled adj5 (trial$ or stud$)).tw.
31. (clinical$ adj5 trial$).tw.
32. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
33. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
34. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
35. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
36. (cross‐over or cross over or crossover).tw.
37. (placebo$ or sham).tw.
38. trial.ti.
39. (assign$ or allocat$).tw.
40. controls.tw.
41. or/16‐40
42. 7 and 15 and 41
43. exp animals/ not humans.sh.
44. 42 not 43
45. limit 44 to ed=20130501‐20150227
Number of hits: 193
Appendix 3. EMBASE (Ovid SP) search strategy
1. cerebrovascular disease/ or exp basal ganglion hemorrhage/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or cerebral artery disease/ or exp cerebrovascular accident/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or stroke patient/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. hemiparesis/ or hemiplegia/ or paresis/
6. (hemipleg$ or hemipar$ or paresis or paretic or hemineglect or hemi‐neglect or ((unilateral or spatial or hemi?spatial or visual) adj5 neglect)).tw.
7. or/1‐6
8. transcranial direct current stimulation/
9. electrostimulation therapy/ or nerve stimulation/ or electrostimulation/
10. electrode/
11. (transcranial adj5 direct current adj5 stimulation).tw.
12. (transcranial adj5 DC adj5 stimulation).tw.
13. (transcranial adj5 electric$ adj5 stimulation).tw.
14. (tDCS or A‐tDCS or C‐tDCS or S‐tDCS or electrode$ or anode or anodes or anodal or cathode or cathodes or cathodal).tw.
15. or/8‐14
16. Randomized Controlled Trial/ or "randomized controlled trial (topic)"/
17. Randomization/
18. Controlled clinical trial/ or "controlled clinical trial (topic)"/
19. control group/ or controlled study/
20. clinical trial/ or "clinical trial (topic)"/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/
21. Crossover Procedure/
22. Double Blind Procedure/
23. Single Blind Procedure/ or triple blind procedure/
24. placebo/ or placebo effect/
25. (random$ or RCT or RCTs).tw.
26. (controlled adj5 (trial$ or stud$)).tw.
27. (clinical$ adj5 trial$).tw.
28. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
29. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
30. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
31. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
32. (cross‐over or cross over or crossover).tw.
33. (placebo$ or sham).tw.
34. trial.ti.
35. (assign$ or allocat$).tw.
36. controls.tw.
37. or/16‐36
38. 7 and 15 and 37
39. (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/)
40. 38 not 39
41. limit 40 to dd=20130501‐20150227
Number of records retrieved: 496
Appendix 4. CINAHL search strategy (EBSCO)
S1 .(MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR (MH "Intracranial Embolism and Thrombosis") OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections")
S2 .(MH "Stroke Patients") OR (MH "Stroke Units")
S3 .TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH )
S4 .TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral )
S5 .TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* )
S6 .S4 and S5
S7 .TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid )
S8 .TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )
S9 .S7 and S8
S10 .(MH "Hemiplegia")
S11 .TI ( hemipleg* or hemipar* or paresis or paretic ) or AB ( hemipleg* or hemipar* or paresis or paretic )
S12 .(MH "Unilateral Neglect") OR (MH "Unilateral Neglect (Saba CCC)") OR (MH "Unilateral Neglect (NANDA)")
S13 .TI ((unilateral or spatial or hemispatial or hemi‐spatial or visual) N5 neglect) or AB ((unilateral or spatial or hemispatial or hemi‐spatial or visual) N5 neglect)
S14 .S1 OR S2 OR S3 OR S6 OR S9 OR S10 OR S11 OR S12 OR S13
S15 .(MH "Electric Stimulation") OR (MH "Electrical Stimulation, Functional") OR (MH "Electrical Stimulation, Neuromuscular") OR (MH "Electrodes")
S16 .TI (transcranial N5 direct current N5 stimulation) OR AB (transcranial N5 direct current N5 stimulation)
S17 .TI (transcranial N5 electric N5 stimulation) OR AB (transcranial N5 electric N5 stimulation)
S18 .TI (tDCS or A‐tDCS or C‐tDCS or S‐tDCS or electrode* or anode or anodes or anodal or cathode or cathodes or cathodal) OR AB (tDCS or A‐tDCS or C‐tDCS or S‐tDCS or electrode* or anode or anodes or anodal or cathode or cathodes or cathodal)
S19 .S15 OR S16 OR S17 OR S18
S20 .(MH "Randomized Controlled Trials") or (MH "Random Assignment") or (MH "Random Sample+")
S21 .(MH "Clinical Trials") or (MH "Intervention Trials") or (MH "Therapeutic Trials")
S22 .(MH "Double‐Blind Studies") or (MH "Single‐Blind Studies") or (MH "Triple‐Blind Studies")
S23 .(MH "Control (Research)") or (MH "Control Group") or (MH "Placebos") or (MH "Placebo Effect")
S24 .(MH "Crossover Design") OR (MH "Quasi‐Experimental Studies")
S25 .PT (clinical trial or randomized controlled trial)
S26 .TI (random* or RCT or RCTs) or AB (random* or RCT or RCTs)
S27 .TI (controlled N5 (trial* or stud*)) or AB (controlled N5 (trial* or stud*))
S28 .TI (clinical* N5 trial*) or AB (clinical* N5 trial*)
S29 .TI ((control or treatment or experiment* or intervention) N5 (group* or subject* or patient*)) or AB ((control or treatment or experiment* or intervention) N5 (group* or subject* or patient*))
S30 .TI ((control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*)) or AB ((control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*))
S31 .TI ((singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*)) or AB ((singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*))
S32 .TI (cross‐over or cross over or crossover) or AB (cross‐over or cross over or crossover)
S33 .TI (placebo* or sham) or AB (placebo* or sham)
S34 .TI trial
S35 .TI (assign* or allocat*) or AB (assign* or allocat*)
S36 .TI controls or AB controls
S37 .TI (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*) or AB (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*)
S38 .S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37
S39 .S14 AND S19 AND S38
S40 .EM 201305‐
S41 .S39 AND S40
Number of records retrieved: 73
Appendix 5. AMED (OvidSP) search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ or stroke/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. hemiplegia/
6. (hemipleg$ or hemipar$ or paresis or paretic or hemineglect or hemi‐neglect or ((unilateral or spatial or hemi?spatial or visual) adj5 neglect)).tw.
7. or/1‐6
8. electric stimulation/ or functional electric stimulation/ or electrotherapy/
9. (transcranial adj5 direct current adj5 stimulation).tw.
10. (transcranial adj5 DC adj5 stimulation).tw.
11. (transcranial adj5 electric$ adj5 stimulation).tw.
12. (tDCS or A‐tDCS or C‐tDCS or S‐tDCS or electrode$ or anode or anodes or anodal or cathode or cathodes or cathodal).tw.
13. or/8‐12
14. 7 and 13
15. limit 14 to up=201305‐201503
Number of hits: 42
Appendix 6. Web of Science search strategy
#1.TS=(stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc* or cva* or apoplex* or SAH)
#2.TS=((brain* or cerebr* or cerebell* or intracran* or intracerebral) NEAR/5 (isch$emi* or infarct* or thrombo* or emboli* or occlus*))
#3.TS=((brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) NEAR/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*))
#4.TS=(hemipleg* or hemipar* or paresis or paretic or hemineglect or hemi‐neglect)
#5.TS=((unilateral or spatial or hemi$spatial or visual) NEAR/5 neglect)
#6.#5 OR #4 OR #3 OR #2 OR #1
#7.TS=(transcranial NEAR/5 "direct current" NEAR/5 stimulation)
#8.TS=(transcranial NEAR/5 "DC" NEAR/5 stimulation)
#9.TS=(transcranial NEAR/5 electric* NEAR/5 stimulation)
#10.TS=(tDCS or A‐tDCS or C‐tDCS or S‐tDCS or electrode* or anode or anodes or anodal or cathode or cathodes or cathodal)
#11.#10 OR #9 OR #8 OR #7
#12.TS=(random* or RCT or RCTs)
#13.TS=(controlled NEAR/5 (trial* or stud*))
#14.TS=(clinical* NEAR/5 trial*)
#15.TS=((control or treatment or experiment* or intervention) NEAR/5 (group* or subject* or patient*))
#16.TS=(quasi‐random* or quasi random* or pseudo‐random* or pseudo random*)
#17.TS=((control or experiment* or conservative) NEAR/5 (treatment or therapy or procedure or manage*))
#18.TS=((singl* or doubl* or tripl* or trebl*) NEAR/5 (blind* or mask*))
#19.TS=(cross‐over or cross over or crossover)
#20.TS=(placebo* or sham)
#21.TI=trial
#22.TS=(assign* or allocat*)
#23.TS=controls
#24.#23 OR #22 OR #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12
#25.#24 AND #11 AND #6
Number of records retrieved: 996
Appendix 7. PEDro search strategy
Abstract & Title: stroke Therapy: electrotherapies, heat, cold Subdiscipline: neurology Method: clinical trial (Search terms matched with AND)
New records added since: 01/05/2013
Number of records retrieved: 67
Appendix 8. RehabDATA search strategy
Find results with all of the words: stroke Where Abstract OR Title contains transcranial OR tDCS
Year of publication between 2013 and 2015
Number of records retrieved: 29
Appendix 9. COMPENDEX search strategy (Dialog)
s1 s ((vertebral (w) artery (w) dissection?)) or brain or carotid or intracran? s2 s stroke? or poststroke? or cerebr? or cva? or apoplex? or sah s3 s cerebell? or intracerebral or subarachnoid s4 s hemipleg? or hemipar? or paresis or paretic s5 s s1‐s4 s6 s ((electric (w) stimulation?)) or electrode? s7 s transcranial (5n) direct (5n) current (5n) stimulation? s8 s transcranial (5n) DC (5n) stimulation? s9 s transcranial (5n) electric? (5n) stimulation? s10 s tdcs or electrode? or anod? or cathod? s11 s s6‐s10 s12 s randomized (w) controlled (w) trial? s13 s random (w) allocation s14 s control (w) group? s15 s clinical (w) trial? s16 s blind (w) method? s17 s placebo? s18 s investigat? and therap??? s19 s research (5n) design s20 s evaluation (w) stud??? s21 s ((evaluation (w) stud???)) or ((comparative (w) stud???)) s22 s random? s23 s ((controlled (5n) trial?)) or ((controlled (5n) stud???)) s24 s (control or treatment or experiment? or intervention?) (5n) (group? or subject? or patient?) s25 s (multicent??? or therapeutic) (5n) (trial? or stud???) s26 s (control or experiment$ or conservative) (5n) (treatment or therap??? or procedure? or manage?) s27 s (singl? or doubl? or tripl? or trebl?) (5n) (blind? or mask?) s28 s (flip??? or toss?) (5n) coin s29 s versus s30 s ((cross (w) over)) or crossover) s31 s sham s31 s ((multiple (w) baseline)) or assign? or alternate or allocate? or counterbalance? s33 s control? ? s34 s s12‐s33 s35 s s5 and s11 and s34
Number of records retrieved (until March 2013): 1024
Appendix 10. INSPEC via TecFinder
Advanced search: Stroke AND tDCS
Number of hits: 11
Data and analyses
Comparison 1. tDCS versus any type of placebo or passive control intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome measure: ADLs at the end of the intervention period | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Absolute values | 9 | 396 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [0.03, 0.44] |
| 1.2 Change scores | 1 | 11 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [‐0.75, 1.67] |
| 2 Primary outcome measure: ADLs until the end of follow‐up | 6 | 269 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [0.01, 0.62] |
| 3 Secondary outcome measure: upper extremity function at the end of the intervention period | 16 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Absolute values | 12 | 431 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.17, 0.39] |
| 3.2 Change scores | 4 | 53 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.51, 1.15] |
| 4 Secondary outcome measure: upper extremity function to the end of follow‐up | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Absolute values | 4 | 187 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.48, 0.50] |
| 4.2 Change scores | 1 | 18 | Std. Mean Difference (IV, Random, 95% CI) | 1.49 [0.40, 2.59] |
| 5 Secondary outcome measure: lower extremity function at the end of the intervention period | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Absolute values | 2 | 50 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.26, 1.67] |
| 5.2 Change scores | 2 | 25 | Std. Mean Difference (IV, Random, 95% CI) | 0.81 [‐0.02, 1.65] |
| 6 Secondary outcome measure: muscle strength at the end of the intervention period | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Absolute values | 8 | 272 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.11, 0.38] |
| 6.2 Change values | 2 | 41 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐2.12, 2.23] |
| 7 Secondary outcome measure: muscle strength at the end of follow‐up | 3 | 156 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.26, 0.41] |
| 8 Secondary outcome measure: cognitive abilities at the end of the intervention period | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9 Secondary outcome measure: dropouts, adverse events and deaths during the intervention period | 23 | 664 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.03] |
1.8. Analysis.

Comparison 1 tDCS versus any type of placebo or passive control intervention, Outcome 8 Secondary outcome measure: cognitive abilities at the end of the intervention period.
Comparison 2. tDCS versus any type of active control intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome measure: ADLs at the end of the intervention period, absolute values | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome measure: upper extremity function at the end of the intervention period | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Secondary outcome measure: lower extremity function at the end of the intervention period | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Secondary outcome measure: dropouts, adverse events and deaths during the intervention period | 2 | 70 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
2.1. Analysis.

Comparison 2 tDCS versus any type of active control intervention, Outcome 1 Primary outcome measure: ADLs at the end of the intervention period, absolute values.
2.2. Analysis.

Comparison 2 tDCS versus any type of active control intervention, Outcome 2 Secondary outcome measure: upper extremity function at the end of the intervention period.
2.3. Analysis.

Comparison 2 tDCS versus any type of active control intervention, Outcome 3 Secondary outcome measure: lower extremity function at the end of the intervention period.
Comparison 3. Subgroup analyses for primary outcome measure: ADLs at the end of the intervention period.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Planned analysis: duration of illness ‐ acute/subacute phase versus postacute phase for ADLs at the end of the intervention period | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Acute/subacute phase (the first week after stroke and the second to the fourth week after stroke | 4 | 213 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.07, 0.51] |
| 1.2 Postacute phase (from the first to the sixth month after stroke) | 2 | 140 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.22, 0.82] |
| 1.3 Chronic phase (from the sixth month after stroke) | 4 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.41, 0.40] |
| 2 Planned analysis: effects of type of stimulation (A‐tDCS/C‐tDCS/dual‐tDCS) and location of stimulation (lesioned/non‐lesioned hemisphere) on ADLs at the end of the intervention period (study groups collapsed) | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 A‐tDCS over the lesioned hemisphere | 5 | 164 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.35, 0.27] |
| 2.2 C‐tDCS over the lesioned hemisphere | 6 | 301 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [0.10, 0.57] |
| 2.3 Dual‐tDCS (A‐tDCS over the lesioned and C‐tDCS over the non‐lesioned hemisphere) | 2 | 33 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.39, 0.99] |
| 3 Planned analysis: type of control intervention (sham tDCS, conventional therapy or nothing) | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Sham tDCS | 8 | 337 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [0.01, 0.46] |
| 3.2 Active control intervention | 2 | 109 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.24, 0.53] |
3.3. Analysis.

Comparison 3 Subgroup analyses for primary outcome measure: ADLs at the end of the intervention period, Outcome 3 Planned analysis: type of control intervention (sham tDCS, conventional therapy or nothing).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ang 2012.
| Methods | Study design: RCT Number of dropouts: none Number of adverse effects: none (Ang 2015 [pers comm]) Deaths: none ITT: yes |
|
| Participants | Country: Singapore Sample size: 19 participants; mean age (SD) 54 (10) years; mean UE‐FM (SD) 34 (8) Inclusion criteria: not explicitly stated Exclusion criteria: history of seizures; major depression; implants that interfered with tDCS; being able to operate an EEG‐based motor imagery brain‐computer interface (MI‐BCI); further therapy aiming at improving function in the affected upper limb |
|
| Interventions | 2 arms:
|
|
| Outcomes | Outcomes were measured at baseline, at the end of intervention period at 2 weeks and at 2 week follow‐up:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | People were randomised by "A randomization stratification generated using a computer‐generated random sequence" (Ang 2015 [pers comm]) |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Interventions of the subjects were applied by an engineer and a research assistant respectively. For tDCS, the research assistant was the only person who knew the randomization sequence for the subjects allocation" (Ang 2015 [pers comm]) |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Participants were blinded; personnel were not blinded (Ang 2015 [pers comm]) |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Quote: "Yes, the outcome assessors for Fugl‐Meyer were blinded to group allocation" (Ang 2015 [pers comm]) |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Incomplete outcome data (attrition bias) Objective outcome measures | Low risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | Unclear risk | Not all of the secondary outcome measures listed in the published trial protocol have been reported, but will be presented in further publications (RMT of affected M1; grip strength; BBT; MRI parameters) |
Au‐Yeung 2014.
| Methods | Study design: randomised controlled cross‐over trial Number of dropouts: none Number of adverse effects: not described Deaths: none ITT: yes |
|
| Participants | Country: China Sample size: 10 participants; mean age (SD) 63 (6) years; mean UE‐FM (SD) 58 (8) Inclusion criteria: not explicitly stated; participants were recruited from a convenience sample from two patient self help groups for stroke; participants were < 80 years of age; had a single stroke more than a year prior to enrolment and had weakness in the affected upper limb and could perform a pincer grip with the index finger Exclusion criteria: not explicitly stated, but people excluded were either illiterate in Chinese, had a history of other neurologic disorders, metal in the head, musculoskeletal pathologies affecting movements in the upper limbs, had aphasia or < 18 points on the MMSE |
|
| Interventions | Each participant underwent all of the following conditions:
|
|
| Outcomes | Outcomes were measured at baseline and at the end of intervention period Primary outcome measures:
Secondary outcome measures:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The sequence was determined in advance for each subject by drawing lots from an envelope" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The sequence was determined in advance for each subject by drawing lots from an envelope" |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Unclear risk | Participants were blinded, but personnel were not. Quote: "It was the third investigator (C.Y.) who set the tDCS parameters for both channels and operated the machine behind the subject throughout the experimental procedure" |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Participants were blinded, but personnel were not. Quote: "It was the third investigator (C.Y.) who set the tDCS parameters for both channels and operated the machine behind the subject throughout the experimental procedure" |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | Outcome assessors were blinded. Quote: "Two other investigators (J.W. and E.C.) who were blinded to the allocated tDCS conditions then assessed the baseline motor status of the subjects’ paretic upper limb" |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Outcome assessors were blinded. Quote: "Two other investigators (J.W. and E.C.) who were blinded to the allocated tDCS conditions then assessed the baseline motor status of the subjects’ paretic upper limb" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Unclear risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Incomplete outcome data (attrition bias) Objective outcome measures | Unclear risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the methods section reported |
Boggio 2007a.
| Methods | Study design: randomised sham‐controlled cross‐over trial Dropouts: none Adverse effects: none Deaths: none ITT: yes Duration: 16 weeks |
|
| Participants | Country: Brazil Number of participants: 4 Age: (mean ± SD) 60.75 ± 13.15 years Gender: 0 female Type of stroke: not described, most likely ischaemic stroke Time poststroke: (mean ± SD) 34.5 ± 27.74 months Severity: mean muscle strength of the finger flexors (MRC) 3.8; mean ASS 0.5 Inclusion criteria: not clearly stated, but all participants had chronic, subcortical stroke, were right‐handed and had their stroke at least 12 months before study enrolment Exclusion criteria: not stated |
|
| Interventions | Characteristics: 4 weekly sessions of A‐tDCS (1 mA) over the hand area of M1 of the lesioned hemisphere, or C‐tDCS (1 mA) over the hand area of M1 of the non‐lesioned hemisphere or sham tDCS over the hand area of M1 of the lesioned hemisphere for 20 minutes with at least 2 weeks of rest between stimulation conditions | |
| Outcomes | Outcomes used: duration of JTT in seconds Time point(s) of measurement: at baseline, after the first and after the fourth session of each treatment condition |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Procedure not described, quote: "The order of these conditions was counterbalanced and randomised across subjects" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Participants were blinded; blinding of personnel was not described |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Quote: "A blinded rater evaluated motor function using the Jebsen‐Taylor Hand Function Test" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Incomplete outcome data (attrition bias) Objective outcome measures | Low risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the methods section reported |
Bolognini 2011.
| Methods | Study design: randomised controlled multicentre trial Dropouts: 7 Adverse effects: none Deaths: not stated ITT: no |
|
| Participants | Country: not stated Number of participants: 14 participants from the outpatient population of 3 neurological research units Age: (mean ± SD) 46.71 ± 14.08 years Gender: 9 female (64%) Type of stroke: 2 haemorrhagic (14%) Time poststroke: (mean ± SD) 35.21 ± 26.45 months Severity: moderate to severe hemiparesis, as indexed by UE‐FM (mean score 26, range 8 to 50) Inclusion criteria: ischaemic or haemorrhagic first‐ever stroke, stroke onset > 6 months before the study, functional inclusion criteria as defined by the EXCITE trial Exclusion criteria: pre stroke motor impairment affecting the upper limbs, moderate to severe major depression, previous CIMT and/or tDCS and contraindications regarding CIMT and/or tDCS |
|
| Interventions | Number of arms: 2
|
|
| Outcomes | Outcomes used:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list (Bolognini 2013 [pers comm]) |
| Allocation concealment (selection bias) | Unclear risk | The principal investigator, who took no part in data collection, nor in participants' evaluations, nor in treatment, knew the randomisation list and performed allocation (Bolognini 2013 [pers comm]) |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Unclear risk | Participants were blinded; blinding of personnel was not described |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Participants were blinded; blinding of personnel was not described |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | Quote: "The assessment of motor functions and the administration of the functional scales and questionnaires were performed by a trained staff, blinded to group assignment" |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Quote: "The assessment of motor functions and the administration of the functional scales and questionnaires were performed by a trained staff, blinded to group assignment" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | Dropouts due to frustration and tiredness during assessment, quote: "Five patients (2 in the active group and 3 in the sham group) did not complete the JHFT. Two patients (1 in the active group and 1 in the sham group) did not complete the HS task." These participants have been excluded from analysis and presentation of results" |
| Incomplete outcome data (attrition bias) Objective outcome measures | Unclear risk | Dropouts due to frustration and tiredness during assessment, quote: "Five patients (2 in the active group and 3 in the sham group) did not complete the JHFT. Two patients (1 in the active group and 1 in the sham group) did not complete the HS task." These participants have been excluded from analysis and presentation of results" |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the methods section reported |
Cha 2014.
| Methods | Study design: RCT Number of dropouts: none Number of adverse effects: not reported Deaths: none ITT: yes |
|
| Participants | Country: Republic of Korea Sample size: 20 (10 in experimental and 10 in control group) Inclusion criteria: hemiplegia due to stroke; gait disturbances Exclusion criteria: not stated |
|
| Interventions | 2 arms:
|
|
| Outcomes | Outcomes were measured at baseline and at the end of intervention period
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The subjects were assigned to the treatment groups by having each of the subjects take out one card from a box containing two types of card representing both of the treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | Not described by the authors |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Unclear risk | Not described by the authors |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Unclear risk | Not described by the authors |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Incomplete outcome data (attrition bias) Objective outcome measures | Unclear risk | Not described by the authors |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the methods section reported |
Di Lazzaro 2014a.
| Methods | Study design: RCT Number of dropouts: none Number of adverse effects: not reported Deaths: none ITT: yes |
|
| Participants | Country: Italy Sample size: 14 (7 in the experimental and 7 in the control group) Inclusion criteria: first ischaemic cerebral infarction confirmed by MRI; admitted to Stroke Unit; aged between 18 to 90 years; acute phase of stroke Exclusion criteria: prestroke disability; not understanding instructions for motor testing; excessive pain in any joint of the paretic limbs; contraindications to single‐pulse TMS; advanced diseases of inner organs; concurrent neurologic or psychiatric diseases; history of substance abuse; use of neuropsychotropic drugs |
|
| Interventions | 2 arms
|
|
| Outcomes | Outcomes were measured at baseline and at the end of intervention period
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized to real or sham tDCS treatment through a block randomization stratification approach" |
| Allocation concealment (selection bias) | Unclear risk | Not described by the authors |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | Participants and personnel were blinded; quote "The investigators who applied real/sham tDCS were kept blind to the intervention by using the pre‐programmed stimulation mode in the stimulator settings" |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Participants and personnel were blinded; quote "The investigators who applied real/sham tDCS were kept blind to the intervention by using the pre‐programmed stimulation mode in the stimulator settings" |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | Quote: "An evaluator, blinded to the treatment, assessed the effects of the intervention" |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Quote: "An evaluator, blinded to the treatment, assessed the effects of the intervention." |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Incomplete outcome data (attrition bias) Objective outcome measures | Low risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | High risk | All outcomes listed in the methods section reported except 'Adverse events', which was not reported clearly |
Di Lazzaro 2014b.
| Methods | Study design: RCT Number of dropouts: none Number of adverse effects: not reported Deaths: none ITT: yes |
|
| Participants | Country: Italy Sample size: 20 (10 in the experimental and 10 in the control group) Inclusion criteria: first ischaemic cerebral infarction confirmed by MRI; admitted to Stroke Unit; aged between 18 to 90 years; acute phase of stroke Exclusion criteria: prestroke disability; not understanding instructions for motor testing; excessive pain in any joint of the paretic limbs; contraindications to single‐pulse TMS; advanced diseases of inner organs; concurrent neurologic or psychiatric diseases; history of substance abuse; use of neuropsychotrophic drugs |
|
| Interventions | 2 arms
|
|
| Outcomes | Outcomes were measured at baseline, at the end of intervention period and at 3‐month follow‐up
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized to real or sham tDCS treatment through a block randomization stratification approach" |
| Allocation concealment (selection bias) | Unclear risk | Not described by the authors |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | Participants and personnel were blinded; quote "The investigators who applied real/sham tDCS were kept blind to the intervention by using the pre‐programmed stimulation mode in the stimulator settings" |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Participants and personnel were blinded; quote "The investigators who applied real/sham tDCS were kept blind to the intervention by using the pre‐programmed stimulation mode in the stimulator settings" |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | Quote: "An evaluator, blinded to the treatment, assessed the effects of the intervention" |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Quote: "An evaluator, blinded to the treatment, assessed the effects of the intervention" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Incomplete outcome data (attrition bias) Objective outcome measures | Low risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | High risk | All outcomes listed in the methods section reported except "Adverse events", which was not reported clearly |
Fregni 2005a.
| Methods | Study design: randomised double‐blind sham‐controlled cross‐over trial Dropouts: none Adverse effects: none Deaths: none ITT: yes |
|
| Participants | Country: not clearly stated Number of participants: 6 participants with chronic stroke neuroimaging‐proofed diagnosis; all were right‐handed and all had their strokes at least 12 months before the study Age: (mean ± SD) 53.7 ± 16.6 years Gender: 4 women (66%) Type of stroke: not stated Time poststroke: 27.1 months (range 12 to 72 months) Severity: motor strength (mean ± SD) 4.18 ± 0.37; ASS (mean ± SD) 0.83 ± 0.75 Inclusion criteria: not clearly stated Exclusion criteria: not clearly stated, but the authors referred to Hummel 2005, where the exclusion criteria were as follows: severe depression, history of severe alcohol or drug abuse, severe language disturbances, particularly of a receptive nature, or serious cognitive deficits (MMSE < 23/30 points) |
|
| Interventions | Characteristics: each participant underwent 3 different conditions for 20 minutes, separated by at least 48 hours of rest
|
|
| Outcomes | Outcomes used: duration of JTT in seconds Time point of measurement: at baseline after familiarisation session, during stimulation and directly after stimulation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Participants were blinded; blinding of personnel was not described |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Outcome assessor was blinded; quote: "A blinded neuropsychologist—instructed not to communicate with the patient during the task—evaluated patients’ performance" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Incomplete outcome data (attrition bias) Objective outcome measures | Low risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the methods section reported |
Fusco 2013a.
| Methods | Study design: double‐blinded, sham‐controlled, randomised cross‐over study Dropouts: none Adverse effects: none Deaths: none ITT: yes |
|
| Participants | Country: Italy Number of participants: 9 Age (mean ± SD): 53.5 ± 20.7 years Gender: 4 (57%) female Type of stroke: 8 (89%) ischaemic, 1 (11%) haemorrhagic Time poststroke (mean ± SD): 28.3 ± 10.4 days Severity (mean ± SD): grip strength 17.83 ± 7.45 kg Inclusion criteria: cortical or subcortical first‐ever stroke (radiologically confirmed), possibility to perform pinch/grip test Exclusion criteria: history of chronic disabling pathologies of the upper limb; spasticity; presence of pacemaker or severe cardiovascular conditions; a history of tumour, prior neurosurgical brain intervention, severe cardiovascular conditions (including the presence of a pacemaker), a diagnosis of epilepsy or major psychiatric disorders |
|
| Interventions | Each participant underwent 1 of the following different stimulation conditions in 2 consecutive sessions on 2 consecutive days in random order (sham tDCS was obligatory)
|
|
| Outcomes | Outcomes were measured at baseline and at the end of intervention period
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For the random sequence generation, we used the RAND function in Matlab" |
| Allocation concealment (selection bias) | Low risk | Quote: "Specifically, patients were asked to take a sealed envelope from a box, containing a piece of paper with the assignment, which was concealed until the envelope was opened" |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Unclear risk | Quote: "Patients were blinded while physicians and assessors knew the treatment (real or sham)" |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Quote: "Patients were blinded while physicians and assessors knew the treatment (real or sham)" |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | High risk | Quote: "Patients were blinded while physicians and assessors knew the treatment (real or sham)" |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Unclear risk | Quote: "Patients were blinded while physicians and assessors knew the treatment (real or sham)" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Incomplete outcome data (attrition bias) Objective outcome measures | Low risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the methods section reported |
Fusco 2014.
| Methods | Method: RCT Number of dropouts: 3 (2 (14%) in the experimental group, 1 (7%) in the control group) Number of adverse effects: not reported Deaths: not described ITT: no |
|
| Participants | Country: Italy Sample size: 11 participants (5 in the experimental and 6 in the control group) Inclusion criteria: admission to stroke unit; age between 18 and 83 years; ischaemic stroke in the MCA area confirmed by MRI or CT; time since stroke less than 30 days; no history of severe cognitive impairment; written informed consent Exclusion criteria: inability to perform a motor rehabilitation training; haemorrhagic stroke or multiple foci of ischaemia; previous stroke; diagnosis of major psychiatric disorders; epilepsy; history of tumour; pacemaker; uncontrolled arrhythmias; non‐stabilised heart diseases; dementia or severe aphasia |
|
| Interventions | 2 arms
|
|
| Outcomes | Outcomes were measured at baseline, after the end of intervention period, 1 month after the intervention period and at the end of inpatient rehabilitation (75 to 110 days)
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was created in accordance with a binary sequence previously generated using MATLAB R2007b Software (TheMatworks Inc., USA)" |
| Allocation concealment (selection bias) | Unclear risk | Not described by the authors |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Unclear risk | Quote: "The patient was blind to the type of stimulation. An unblinded investigator administered the stimulation" |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Quote: "The patient was blind to the type of stimulation. An unblinded investigator administered the stimulation" |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | Quote: "The patient was blind to the type of stimulation, as well as the physician performing the assessments" |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Quote: "The patient was blind to the type of stimulation, as well as the physician performing the assessments" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | High risk | Quote: "Two patients of EG dropped out from the study (one at the first and the other one at the second session). Also one patient of control group dropped out for an emergency transfer to another hospital." These participants have not been analysed |
| Incomplete outcome data (attrition bias) Objective outcome measures | High risk | Quote: "Two patients of EG dropped out from the study (one at the first and the other one at the second session). Also one patient of control group dropped out for an emergency transfer to another hospital." These participants have not been analysed |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the methods section reported |
Geroin 2011.
| Methods | Study design: pilot RCT Dropouts: none Adverse effects: none Deaths: none ITT: yes |
|
| Participants | Country: Italy Number of participants: 30 outpatients Age: (mean ± SD) 62.7 ± 6.4 years Gender: 7 females (23%) Type of stroke: unilateral ischaemic stroke Time poststroke: (mean ± SD) 26.4 ± 5.5 months Severity: mean ESS score 79.93 (minimum score: 0, maximum score: 100; a completely healthy person would have a score of 100) Inclusion criteria: at least 12 months from first unilateral ischaemic stroke, age < 75 years, ESS score ≥ 75 and ≤ 85, MMSE‐score ≥ 24, ability to maintain standing position without aid for at least 5 minutes, ability to walk independently for at least 15 minutes with the use of walking aids Exclusion criteria: history of seizures, EEG suspect of elevated cortical excitability, metallic implants within the brain and previous brain neurosurgery, medications altering cortical excitability or with a presumed effect of brain plasticity, posterior circulation stroke, deficits of somatic sensations involving the paretic lower limb, presence of vestibular disorders/paroxysmal vertigo, severe cognitive or communicative disorders, cardiovascular comorbidity, rehabilitation treatment 3 months before study enrolment |
|
| Interventions | Number of arms: 3; all participants underwent 50‐minute training sessions 5 times a week for 2 consecutive weeks and 1 of the following interventions
|
|
| Outcomes | Primary outcomes: six‐minute walking test, 10‐metre walking test Secondary outcomes: GAITRite system, FAC, RMI, MI leg subscore and MAS Time point of measurement: at baseline, after treatment and at two weeks follow‐up |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After baseline evaluation, patients were allocated to one of three treatment groups according to a simple software‐generated randomisation scheme" |
| Allocation concealment (selection bias) | Low risk | Quote: "We allocated patients to one of the three treatment arms according to a restricted randomisation scheme. One of the investigators checked correct patient allocation according to the randomisation list. After unmasking at the end of the study, we checked that no errors had been made in allocation" (Smania 2013 [pers comm]) |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Unclear risk | Quote: "Asking the assessor to make an educated guess tested the success of blinding. The therapists were aware of the type of treatment received by the patients. Patients were aware of the type of treatment who underwent but they were not aware about the type of stimulation (Group 1 stimulation vs Group 2 sham stimulation)" (Smania 2013 [pers comm]) |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Quote: "Asking the assessor to make an educated guess tested the success of blinding. The therapists were aware of the type of treatment received by the patients. Patients were aware of the type of treatment who underwent but they were not aware about the type of stimulation (Group 1 stimulation vs Group 2 sham stimulation)" (Smania 2013 [pers comm]) |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | Quote: "All patients were evaluated by the same examiner (an experienced internal coworker) who was not aware of the treatment received by the patients" |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Quote: "All patients were evaluated by the same examiner (an experienced internal coworker) who was not aware of the treatment received by the patients" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Incomplete outcome data (attrition bias) Objective outcome measures | Low risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in the methods section were reported, except muscle tone as measured by MAS |
Hesse 2011.
| Methods | Study design: double‐blind randomised sham‐controlled multicentre trial Dropouts: 11 (11%) Adverse effects: none Deaths: 2 (2%) due to heart infarction and during stent surgery ITT: yes, 85 participants completed the study (89%) |
|
| Participants | Country: Germany/Italy Number of participants: 96 stroke participants from 3 study centres Mean age: 65.0, range 39 to 79 years Gender: 37 female (39%) Type of stroke: all ischaemic, 45 of 96 (47%) right‐hemispheric stroke Time poststroke: (mean ± SD) A‐tDCS group: 3.4 ± 1.8 weeks; C‐tDCS group: 3.8 ± 1.4 weeks; sham tDCS group: 3.8 ± 1.5 weeks Severity: at least wheelchair‐mobile participants, who had severe flaccid upper limb paresis with no (MRC 0) or minimal (MRC 1) volitional hand and finger extensor activity. 24 had an upper limb UE‐FM (range 0 to 66) < 18 and were unable to transfer 3 wooden blocks from 1 compartment to the other in the Box and Block test Inclusion criteria: age 18 to 79 years, first supratentorial ischaemic stroke with a stroke interval of 3 to 8 weeks' duration, and with participation in a comprehensive inpatient rehabilitation programme Exclusion criteria: history of epileptic seizures, EEG suspect of elevated cortical excitability, metallic implants in the brain, preceding brain surgery, sensitive scalp skin, anticonvulsant or neuroleptic medications |
|
| Interventions | Number of arms: 3; each participant practiced for 6 weeks every working day for 20 minutes with the arm robot (AT) and simultaneously received one of the following interventions:
|
|
| Outcomes | Primary outcome: sensory and motor integrity, degree of synergy as assessed by UE‐FM assessment score (0 to 66, 0 = no movement, 66 = full motion) Secondary outcomes: upper limb muscle strength (MRC; 0 to 5, 0 = plegic, 5 = full power), muscle tone (MAS; 0 to 5, 0 = no increase, 5 = affected part rigid in flexion or extension), BI, upper limb function (as assessed by Box and Block test, the transfer of as many wooden blocks as possible with the affected hand from 1 compartment to the other within 1 minute, with a high value indicating good function) Time point of measurement: study onset, end of the 6‐week intervention and 3 months of follow‐up |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Following a telephone call, an independent person randomly allocated eligible patients to 1 of the 3 groups by drawing a lot out of an envelope containing 96 lots, indicating A, B, and C" |
| Allocation concealment (selection bias) | Low risk | Quote: "Following a telephone call, an independent person randomly allocated eligible patients to 1 of the 3 groups by drawing a lot out of an envelope containing 96 lots, indicating A, B, and C. He then informed the locally responsible person about the group assignment and the study started the next day" |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | Participants and personnel were blinded |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | Quote: "To ensure blinded evaluation of the FMS, videos of the assessment, where the patients sat on a chair and a mirror was placed 45° behind them, were sent to an experienced therapist off site" and "Two experienced physiotherapists, blinded with respect to group assignment, assessed the secondary parameters together" and "The blinding was maintained at all measurement points" |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Quote: "To ensure blinded evaluation of the FMS, videos of the assessment, where the patients sat on a chair and a mirror was placed 45° behind them, were sent to an experienced therapist off site" and "Two experienced physiotherapists, blinded with respect to group assignment, assessed the secondary parameters together" and "The blinding was maintained at all measurement points" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | 1 dropout occurred during the study period as the result of pneumonia, and 10 after the end of the intervention period until follow‐up (6 were caused by being unavailable, 2 resulted from refusal to further participate and 2 were caused by cardiac conditions). ITT analysis was performed |
| Incomplete outcome data (attrition bias) Objective outcome measures | Low risk | 1 dropout occurred during the study period as the result of pneumonia, and 10 after the end of the intervention period until follow‐up (6 were caused by being unavailable, 2 resulted from refusal to further participate and 2 were caused by cardiac conditions). ITT analysis was performed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the methods section and in the published trial protocol reported |
Jo 2008.
| Methods | Method: Randomised cross‐over trial Number of dropouts: none Number of adverse effects: 6 Deaths: none ITT: yes |
|
| Participants | Country: Republic of Korea Sample size: 10 participants Inclusion criteria: Unilateral right hemispheric stroke, younger than 70 years; noticeable cognitive disorder after stroke; written informed consent Exclusion criteria: Seizures; metal implants in the head, cardiac pacemaker; history of neuropsychiatric diseases |
|
| Interventions | Each participant underwent one of the following treatments:
|
|
| Outcomes | Outcomes were measured at baseline and at the end of intervention period
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The order of stimulation was randomly assigned for all participants" |
| Allocation concealment (selection bias) | Unclear risk | Not described by the authors |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Participants have been blinded by sham tDCS; blinding of personnel not stated |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Not described by the authors, however all outcome data were acquired by a computerised assessment during cognitive tasks |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Incomplete outcome data (attrition bias) Objective outcome measures | Low risk | All randomised participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the methods section reported |
Kang 2008a.
| Methods | Method: randomised cross‐over trial Dropouts: none Adverse effects: none (Paik 2015 [pers comm]) Deaths: none ITT: yes, all participants completed the study |
|
| Participants | Country: Republic of Korea Sample size: 10 people with stroke aged 48 to 84 years Inclusion criteria: not explicitly stated; written informed consent Exclusion criteria: cerebellar or brainstem lesion; metallic body implant; pacemaker; cochlear implant; history of seizure; unstable medical condition; inability to perform outcome tasks; Na+ or Ca++ channel blockers |
|
| Interventions | Each participant underwent one of the following treatments
|
|
| Outcomes | Outcomes were measured at baseline, at the end of intervention period and at 3 hours postintervention
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We applied random order using computerized program. Randominzation program is freely available in the Internet." (Paik 2015 [pers comm]) Comment: However, Patient‐ID and first session stimulation type were continuously alternated, as can be seen in Table 6 |
| Allocation concealment (selection bias) | Unclear risk | Not described by the authors |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Participants were blinded; blinding of personnel not stated |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Outcome assessor was blinded; quote: "Both patients and the investigator that carried out the behavioral measurements were unaware of the type of intervention, because tDCS and sham were administered by another investigator who did not participate in the behavioral task or data analysis" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Incomplete outcome data (attrition bias) Objective outcome measures | Low risk | All randomised participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the methods section reported. There was no published a priori trial protocol (Paik 2015 [pers comm]) |
Khedr 2013.
| Methods | Study design: RCT (parallel assignment) Dropouts: none Adverse effects: none Deaths: none ITT: yes, all participants completed the study |
|
| Participants | Country: Egypt Number of participants: 40 outpatients Age: (mean ± SD) years Gender: 14 females (35%) Type of stroke: acute single thromboembolic non‐haemorrhagic infarction, documented by MRI Time poststroke: (mean ± SD) 17.1 ± 3.6 days Severity: (range) 7 to 13 on NIHSS Exclusion criteria: extensive infarction (all territories of MCA), severe flaccid hemiplegia, head injury, neurological disease other than stroke, renal or hepatic impairment, previous administration of tranquilliser, inability to give informed consent, no MEP recorded from FDI muscle of the affected hand |
|
| Interventions | 3 arms:
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each patient was given a serial number from a computer‐generated randomisation table" |
| Allocation concealment (selection bias) | Low risk | Quote: "Group allocations (Anodal, Cathodal, or Sham) were placed in serially numbered, opaque closed envelopes ... and each patient was placed in the appropriate group after opening the corresponding sealed envelope" |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | Participants and therapists were blinded |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Participants and therapists were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | Outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Outcome assessor was blinded |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | All randomised participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Incomplete outcome data (attrition bias) Objective outcome measures | Low risk | All randomised participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the study protocol and listed in the methods section of the publication have been reported |
Kim 2009.
| Methods | Study design: single‐blinded, sham‐controlled, randomised cross‐over study Dropouts: none Adverse effects: none Deaths: none ITT: yes |
|
| Participants | Country: Republic of Korea Number of participants: 10 subacute participants Age: (mean) 62.8 years Gender: seven female (70%) Type of stroke: first‐ever stroke, as confirmed by MRI; 2 had haemorrhagic stroke (20%) Time poststroke: (mean) 6.4 weeks, range 3 to 12 weeks Severity: participants could grasp and release independently; degree of strength according to MRC was ≥ 3 but < 5 for all paretic finger flexors and extensors. Participants did not have a family history of seizure, could understand the purpose of the study and did not have any deformities or contractures of the fingers, hands, elbows and shoulders Inclusion criteria: not explicitly stated Exclusion criteria: not explicitly stated |
|
| Interventions | Each participant underwent 2 different stimulation conditions, each for 20 minutes, separated by at least 24 hours of rest
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A doctor who works in tDCS's room, he randomised patients on his own sequence" (Kim 2013 [pers comm]) |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A doctor who works in tDCS's room, he randomised patients on his own sequence" (Kim 2013 [pers comm]) |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | Both participants and personnel were blinded (Kim 2013 [pers comm]) |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Both participants and personnel were blinded (Kim 2013 [pers comm]) |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | High risk | No blinding of outcome assessors. Quote: "An examiner who was aware of the stimulation method used was instructed not to communicate with patients during the task and evaluated patients’ performances" |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding. Quote . Quote: "An examiner who was aware of the stimulation method used was instructed not to communicate with patients during the task and evaluated patients’ performances" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | All randomised participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Incomplete outcome data (attrition bias) Objective outcome measures | Low risk | All randomised participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the methods section reported |
Kim 2010.
| Methods | Study design: double‐blind sham‐controlled multicentre randomised trial Dropouts: 1 participant discontinued treatment because of dizziness and another because of headache (2 out of 20) during follow‐up Adverse effects: none Deaths: none ITT: no |
|
| Participants | Country: Republic of Korea Number of participants: 20 participants from neurorehabilitation units at 2 tertiary university hospitals Age: (mean ± SD) 57.27 ± 4.95 Gender: 7 female (35%) Type of stroke: first‐ever cortical or subcortical ischaemic stroke Time poststroke: (mean ± SD) A‐tDCS group: 34 ± 27.1 days; C‐tDCS: 19.4 ± 9.3 days; sham tDCS: 22.9 ± 7.5 days Severity: mild to moderate motor deficits (MRC score ≥ 2) Inclusion criteria: first‐ever ischaemic strokes in the cortical or subcortical area within the previous 2 months and mild to moderate motor deficits (MRC score ≥ 2) Exclusion criteria: cerebellar or brainstem lesions; presence of a metallic foreign body implant, such as a pacemaker or an artificial cochlea; history of seizure or another unstable medical condition; severe language disturbance; neglect, depression or cognitive deficits (based on the MMSE, 10 of 30 points) that would limit participation; history of severe alcohol or drug abuse; previous stroke that resulted in residual disability; premorbid arm impairment; and hemiplegic shoulder pain; use Na+ or Ca2+channel blockers or NMDA receptor antagonists |
|
| Interventions | Number of arms: 3 Each participant received 10 sessions (5 times per week for 2 weeks during conventional occupational therapy aiming at improving the co‐ordination and strength of the paretic hand) of 1 of the following interventions:
|
|
| Outcomes | Outcomes used: FMA 0 to 66 (with higher scores indicating better function) for assessing upper limb motor function and MBI 0 to 100 (with higher scores indicating better global function) Time point of measurement: at baseline, 1 day and 6 months after intervention |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to one of the three groups (atDCS, ctDCS or Sham treatment) using a stratified randomisation procedure with permuted block size of 3 and an algorithm that balanced Brunnstrom stages" |
| Allocation concealment (selection bias) | Low risk | Quote: "Sealed opaque envelopes were used for randomisation" |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | Participants and personnel were blinded |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | Quote: "Two independent raters blinded to the type of intervention performed outcome measurements" |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Quote: "Two independent raters blinded to the type of intervention performed outcome measurements" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Unclear risk | 1 participant of each interventional arm (14% each) discontinued intervention; we excluded these participants from analysis |
| Incomplete outcome data (attrition bias) Objective outcome measures | Unclear risk | 1 participant of each interventional arm (14% each) discontinued intervention; we excluded these participants from analysis |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the methods section reported |
Ko 2008.
| Methods | Method: randomised cross‐over trial Number of dropouts: not described Number of adverse effects: none Deaths: none ITT: yes, all participants completed the study |
|
| Participants | Country: Republic of Korea Sample size: 15 people with stroke and neglect Baseline characteristics: 10 men and 5 women; mean age (SD): 62 (9) years; time since stroke (range) 29‐99 days; right‐hemispheric stroke; right‐handed Inclusion criteria: not explicitly described; written informed consent Exclusion criteria: metal in the head or skin lesions in the electrode area; uncontrolled medical problems; severe cognitive impairments |
|
| Interventions | Each participant underwent one of the following conditions
|
|
| Outcomes | Outcomes were measured at baseline and at the end of intervention period
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All of patients participated in both anodal and sham DC brain polarization with counterbalanced and randomized order and 48 hour interval between two sessions" |
| Allocation concealment (selection bias) | Unclear risk | Not described by the authors |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Unclear risk | Participants were blinded, whereas blinding of personnel was not stated; however the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Unclear risk | Not described by the authors |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Incomplete outcome data (attrition bias) Objective outcome measures | Low risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the methods section reported |
Lee 2014.
| Methods | Method: RCT Number of dropouts: 5 (3 out of 42 in the experimental groups (7%) and 2 out of 22 in the control group (9%)) Number of adverse effects: no major adverse events Deaths: none ITT: no |
|
| Participants | Country: Republic of Korea Sample size: 59 people with stroke (39 in the experimental groups and 20 in the control group) Inclusion criteria: unilateral hemiparesis caused by stroke; first stroke within 1 month prior to enrolment; shoulder motor strength Medical Research Council grade ≤ 2 Exclusion criteria: contraindications to brain stimulation; previous history of brain neurosurgery or epilepsy; metallic implants in the brain; severe cognitive impairment; aphasia interfering with understanding study instructions; poor sitting balance; impaired vision; hemispatial neglect |
|
| Interventions | 3 arms
|
|
| Outcomes | Outcomes were measured at baseline and at the end of intervention period
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All of the enrolled patients were randomly assigned to 1 of 3 groups using a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not described by the authors |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | Participants and personnel providing the base treatment were blinded |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Participants and personnel providing the base treatment were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | Quote: "All evaluations were performed before and immediately after treatment by a single experienced occupational therapist who was not aware of the treatment allocation" |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Quote: "All evaluations were performed before and immediately after treatment by a single experienced occupational therapist who was not aware of the treatment allocation" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | High risk | 3 participants out of 42 (7%) in the experimental groups and 2 out of 22 (9%) were lost to follow‐up and excluded from the analysis. 2 out of the 3 losses to follow‐up in the experimental group dropped out due to "medical problem(s)" |
| Incomplete outcome data (attrition bias) Objective outcome measures | High risk | 3 participants out of 42 (7%) in the experimental groups and 2 out of 22 (9%) were lost to follow‐up and excluded from the analysis. 2 out of the 3 losses to follow‐up in the experimental group dropped out due to "medical problem(s)" |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the methods section reported |
Lindenberg 2010.
| Methods | Study design: sham‐controlled double‐blinded randomised trial Dropouts: not stated Adverse effects: none Deaths: not stated, likely none ITT: not stated |
|
| Participants | Country: USA Number of participants: 20 chronic stroke participants Age: (mean ± SD) 55.8 ± 12.9 years Gender: 5 female (25%) Type of stroke: first and only ischaemic stroke Time poststroke: (mean ± SD) 40.3 ± 23.4 months Severity: UE‐FM Score (mean ± SD) 39.8 ± 11.5 Inclusion criteria: ischaemic stroke in the territory of the medial cerebral artery at least 5 months before enrolment; no previous or subsequent strokes; MRC strength grade of 3/5 in extensor muscles of the lesioned upper extremity in the acute phase with at least 15 degrees of active wrist dorsiflexion at enrolment Exclusion criteria: additional neurological or psychiatric disorders; concurrent use of CNS‐affecting drugs |
|
| Interventions | Number of arms: 2, each participant underwent 5 consecutive sessions of physical therapy/occupational therapy and 1 of the following interventions
|
|
| Outcomes | Primary outcome measure: UE‐FM scores (0 to 66, with higher scores reflecting better motor performance) Secondary outcome measure: WMFT (with lower scores indicating better motor performance) Time point of measurement: at baseline and at 3 and 7 days after the last intervention session |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to one of two groups ... using a block randomisation with 3 strata of impairment" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Quote: "Each patient underwent motor impairment assessments and MRI at baseline and after the intervention, conducted by trained individuals who were blinded to the type of intervention the patients received" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Incomplete outcome data (attrition bias) Objective outcome measures | Unclear risk | All randomised participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in the methods section were reported |
Mahmoudi 2011.
| Methods | Study design: sham‐controlled cross‐over randomised trial Dropouts: not stated, most likely none Adverse effects: none Deaths: not stated, most likely none ITT: not stated |
|
| Participants | Country: Iran Number of participants: 10 right‐handed stroke participants with no sensory deficits Age: (mean ± SD) 60.8 ± 14.1 years Gender: 3 female (30%) Type of stroke: ischaemic Time poststroke: (mean ± SD) 8.3 ± 5.45, range 1 to 16 months Severity: median Brunnstrom stage 6 Inclusion criteria: single ischaemic stroke with more than 1 month's duration of mild to moderate motor deficit (to ensure that all participants could perform all items on the JTT Exclusion criteria: clinically significant or unstable medical or psychiatric disorder with history of substance abuse, any neuropsychiatric comorbidity other than stroke and contraindications to tDCS |
|
| Interventions | Each participant underwent 5 different treatments with at least 4 days of each of the following
|
|
| Outcomes | Outcomes used: JTT (with familiarisation sessions) Time points of measurement: at baseline and after stimulation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The order of these conditions was counterbalanced and randomised across patients" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Participants probably were blinded; blinding of personnel was not described. Quote: "Patients were then randomised to the double‐blinded, sham‐controlled cross over part of the experiment" |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Quote: "A blinded physiatrist—instructed not to communicate with the patients during the task—evaluated patients' performance" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Incomplete outcome data (attrition bias) Objective outcome measures | Unclear risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in the methods section were reported |
Nair 2011.
| Methods | Study design: randomised double‐blind sham‐controlled trial Dropouts: none Adverse effects: none Deaths: none ITT: yes |
|
| Participants | Country: USA Number of participants: 14 right‐handed Age: (mean) 55.8, range of 40 to 76 years Gender: 5 female (36%) Type of stroke: first‐ever unihemispheric stroke, 6 (43%) had right‐hemispheric stroke, 9 (64%) had predominantly cortical stroke, 5 (36%) had predominantly subcortical stroke Time poststroke: (mean ± SD) Severity: moderate to severe upper extremity impairment, UE‐FM (mean ± SD) 30.1 ± 10.4 Inclusion criteria: not clearly stated Exclusion criteria: previous history of stroke, bilateral infarcts, haemorrhage, arthritis, chronic pain, other neurological diseases |
|
| Interventions | Number of arms: 2 participants underwent occupational therapy + 1 of the following conditions
|
|
| Outcomes | Primary outcomes: mean ROM for shoulder abduction, elbow extension and wrist extension (3J‐ROM; calculated as active ROM∗100/passive ROM for each joint, 0 to 100, with higher values indicating better function) and proportional change in UE‐FM (0 to 66, with higher scores indicating better motor performance) Time point of measurement: at baseline, after the intervention and at 1‐week follow‐up |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described, quote: "Patients were randomised to either the cathodal group or the sham group" |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | There were no subjective outcome measures. |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Quote: "The 3J‐ROM and the FM assessments were done by an investigator who was blind with regard to whether real tDCS or sham tDCS was applied" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Incomplete outcome data (attrition bias) Objective outcome measures | Low risk | All randomised participants completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | High risk | Results of Wolf Motor Function Test, Modified Ashworth Scale and Motor Activity Log Rating Scale were not reported, as intended by the protocol (http://ClinicalTrials.gov/show/NCT00792428) |
Park 2013.
| Methods | Method: RCT Number of dropouts: unclear Number of adverse effects: none Deaths: none ITT: unclear |
|
| Participants | Country: Republic of Korea Sample size: 11 participants Inclusion criteria: not explicitly stated; newly diagnosed with radiologically confirmed stroke; written informed consent Exclusion criteria: patients with metal in the head or with skin lesions in the electrode area; significant aphasia |
|
| Interventions | 2 arms
|
|
| Outcomes | Outcomes were measured at baseline and at study end
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned to two groups" |
| Allocation concealment (selection bias) | Unclear risk | Not described by the authors |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Unclear risk | Patients were blinded; whereas blinding of personnel was not clearly described by the authors: "The tDCS and the cognitive function test were performed by two independent personnel" |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Patients were blinded; whereas blinding of personnel was not clearly described by the authors: "The tDCS and the cognitive function test were performed by two independent personnel" |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Unclear risk | Quote: "The tDCS and the cognitive function test were performed by two independent personnel" |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Quote: "The tDCS and the cognitive function test were performed by two independent personnel." |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Unclear risk | All randomised participants apparently completed the study; no treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Incomplete outcome data (attrition bias) Objective outcome measures | Unclear risk | All randomised participants apparently completed the study; no treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | Unclear risk | Not all of the 10 dimensions of the Seoul Computerized Neuropsychological Test (SCNT), as stated in the methods section, have been reported |
Qu 2009.
| Methods | Study design: RCT Dropouts: none Adverse effects: not reported Deaths: none ITT: yes Duration: 1 month |
|
| Participants | Country: China Number of participants: 50 Age: tDCS (mean ± SD): 45 (11), control: 45 (14) years Gender: tDCS: 21 (84%) male, control: 22 (88%) male Type of stroke: 15 (60%) ischaemic Time poststroke: tDCS: 6 months (3 to 36), control: 4 months (3 to 12) Severity: tDCS: FMA 12 (5 to 44), BI 64 (17), control: FMA 5 (2 to 35), BI: 72 ± 22 Inclusion criteria: admitted to hospital between June 2008 and June 2009 and MRI‐confirmed stroke Exclusion criteria: not stated |
|
| Interventions | 2 arms
|
|
| Outcomes | Outcomes used: MAS, FMA, BI Time points of measurement: at baseline and at the end of the intervention period |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned using a computer‐generated randomisation list by a single investigator" (Wu 2013b [pers comm]) |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The assigned random number was inputted into the stimulator device by the same investigator. She did not participate in other parts of the study" (Wu 2013b [pers comm]) |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Unclear risk | Quote: "All other investigators, subjects, and outcome assessors remained blinded to group allocation until the completion of the final statistical analyses" (Wu 2013b [pers comm]) |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Quote: "All other investigators, subjects, and outcome assessors remained blinded to group allocation until the completion of the final statistical analyses" (Wu 2013b [pers comm]) |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Unclear risk | See "Blinding of participants and personnel" |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | See "Blinding of participants and personnel" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Incomplete outcome data (attrition bias) Objective outcome measures | Low risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from the methods section were reported |
Rossi 2013.
| Methods | Study design: single‐centre, randomised, double‐blind, sham‐controlled trial Dropouts: none Adverse effects: none Deaths: none ITT: yes, all participants completed the study |
|
| Participants | Country: Italy Number of participants: 50 Inclusion criteria: age between 18 and 80 years and an acute ischaemic lesion in the territory of the MCA, a score between 6 and 20 at the NIHSS and a UE‐FM score between 15 and 55 Exclusion criteria: pre stroke mRS > 1, thrombolysis, history of seizure, advanced systemic diseases coexistent neurological/psychiatric diseases, current treatment with antidepressants, antipsychotics or benzodiazepines Age: (mean ± SD) tDCS‐group: 66.1 (± 14.3); sham group: 70.3 (± 13.5) years Gender: tDCS group: 12 male (48%), sham group: 14 male (56%) Time poststroke: 2 days Severity according NIHSS at baseline: tDCS‐group: 15.4 (± 4.9); sham group: 14.1 (± 3.5) |
|
| Interventions | Number of arms: 2; each participant underwent 1 of the following conditions
|
|
| Outcomes | Primary outcomes: UE‐FM at baseline, at the end of intervention and at 3 month follow‐up Secondary outcomes: NIHSS at baseline, at the end of intervention and at 3 month follow‐up; mRS at baseline, at the end of intervention and at 3‐month follow‐up | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation scheme was generated by a computer program (Koch 2013 [pers comm]) |
| Allocation concealment (selection bias) | Unclear risk | Allocation was performed by a third person via telephone (Koch 2013 [pers comm]) |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | Personnel were blinded to the type of treatment (Koch 2013 [pers comm]) |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Personnel were blinded to the type of treatment (Koch 2013 [pers comm]) |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | Evaluators were blinded (Koch 2013 [pers comm]) |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Evaluators were blinded (Koch 2013 [pers comm]) |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | All participants completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Incomplete outcome data (attrition bias) Objective outcome measures | Low risk | All participants completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | Low risk | All outcomes were stated as mentioned in preceding conference papers |
Sohn 2013.
| Methods | Study design: randomised sham‐controlled cross‐over trial Number of dropouts: not stated Number of adverse effects: not stated Deaths: not stated ITT: unclear |
|
| Participants | Country: Republic of Korea Sample size: 11 (age in years (mean (SD)): 58 (15); time since stroke in days (mean (SD)): 63 (17)) Inclusion criteria: not explicitly stated, undergoing rehabilitation following acute treatment Exclusion criteria: history of previous stroke; history of previous epilepsy/seizure; family history of epilepsy/seizure; metal in the cranial cavity; permanent pacemaker; previous or persistent other neurological disorders; stroke lesion in the cerebellum; contracture of the lower limb on the affected side |
|
| Interventions | Each participant underwent one of the following two conditions
|
|
| Outcomes | Outcomes were measured at baseline and at study end
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The two stimulation experiments were performed in random order for each patient" |
| Allocation concealment (selection bias) | Unclear risk | Not described by the authors |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Unclear risk | Participants were blinded, quote: "Patients were unlikely to be aware of any difference between real and sham stimulation", whereas personnel were probably not; quote: "Second, a double‐blind design was not used for experiments" |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Outcome assessor probably was not blinded, however the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; quote: "Second, a double‐blind design was not used for experiments" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Unclear risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Incomplete outcome data (attrition bias) Objective outcome measures | Unclear risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the methods section reported |
Sunwoo 2013.
| Methods | Study design: randomised controlled cross‐over trial Number of dropouts: not stated Number of adverse effects: 3 (mild headache after real dual‐tDCS) Deaths: not stated ITT: unclear |
|
| Participants | Country: Republic of Korea Sample size: 10 chronic stroke patients (mean age 63 years) with left unilateral visuospatial neglect after stroke Inclusion criteria: not explicitly stated except written informed consent Exclusion criteria: metallic implants in the head; skull defect; history of seizure; uncontrolled medical problems; severe cognitive impairment |
|
| Interventions | Each participant underwent all of the following conditions (separated by a resting period of at least 24 hours)
|
|
| Outcomes | Outcomes were measured at baseline and at the end of stimulation
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All patients participated in dual, single, and sham tDCSsessions at intervals of at least 24 hours between sessions in a randomized order" |
| Allocation concealment (selection bias) | Unclear risk | Not described by the authors |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | Participants were blinded, whereas blinding of personnel was not stated. However, the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Participants were blinded, whereas blinding of personnel was not stated. However, the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | Outcome assessor was blinded; quote: "Both tests were performed by a single examiner who was blinded to the type of stimulation" |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Outcome assessor was blinded; quote: "Both tests were performed by a single examiner who was blinded to the type of stimulation" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Unclear risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Incomplete outcome data (attrition bias) Objective outcome measures | Unclear risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | Unclear risk | All outcome measures listed in the methods section have been reported |
Tahtis 2012.
| Methods | Study design: RCT Number of dropouts: not stated Number of adverse effects: none Deaths: not stated ITT: unclear |
|
| Participants | Country: not stated 14 subacute stroke patients (2 to 8 weeks after stroke) Inclusion criteria: mobile stroke survivors with focal, ischaemic stroke; walking difficulties after stroke (self reported) Exclusion criteria: previous neurological conditions, seizure; musculoskeletal insult; pacemaker |
|
| Interventions | 2 arms
|
|
| Outcomes | Outcomes were measured at baseline and at study end
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomised to either the treatment group or to placebo" |
| Allocation concealment (selection bias) | Unclear risk | Not described by the authors |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Unclear risk | Participants were blinded, whereas blinding of personnel was not stated |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Participants were blinded, whereas blinding of personnel was not stated |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | Outcome assessors were blinded; quote: "Two independent assessors blindly assessed the POMA" and "Three consecutive recordings of the TUG were taken by the same blinded assessor" |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Outcome assessors were blinded; quote: "Two independent assessors blindly assessed the POMA" and "Three consecutive recordings of the TUG were taken by the same blinded assessor" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Unclear risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Incomplete outcome data (attrition bias) Objective outcome measures | Unclear risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the methods section reported |
Tedesco Triccas 2015b.
| Methods | Study design: RCT Number of dropouts: 1 in the A‐tDCS group (skin reaction due to tDCS) Number of adverse effects: 1 in the A‐tDCS group (skin reaction due to tDCS) Deaths: none ITT: no |
|
| Participants | Country: UK Sample size: 22 participants Inclusion criteria: aged 18 and above; clinical diagnosis of first‐ever stroke, confirmed by a neurologist/stroke specialist; time since stroke > 2 weeks prior to enrolment; upper and fore‐arm and hand paresis (MRC > 2); minimal spasticity (MAS ≤ 2); partial shoulder flexion with gravity; good sitting balance; informed consent Exclusion criteria: MMSE < 24; other neurological conditions; shoulder pain resulting from shoulder flexion > 90°; epilepsy; metal implants in the skull or brain; previous brain neurosurgery; medications that influence cortical excitability; previous adverse effects when stimulated with tDCS; pregnancy |
|
| Interventions | 2 arms
|
|
| Outcomes | Outcomes were measured at baseline and at the end of intervention and at 3 months follow‐up Primary outcomes:
Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Block randomisation was used with a computer program called 'random allocation software'" |
| Allocation concealment (selection bias) | Low risk | Quote: "To conceal allocation, an independent person placed the printed papers of sham/real in sealed opaque envelopes according to block randomisation. As soon as a participant enrolled in the study, the researcher made a telephone call to the independent person who then stated whether ‘real’ or ‘sham’ was to be administered to the participant" |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Unclear risk | Participants apparently were blinded, but blinding of personnel not stated |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Participants apparently were blinded, but blinding of personnel not stated |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | Quote: "Three blinded assessors, trained qualified physiotherapists with experience in stroke assessment and neurological rehabilitation carried out clinical assessments. In addition to the clinical assessor, video recorded FMA and ARAT assessments were also scored by an additional blinded clinical assessor" |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Quote: "Three blinded assessors, trained qualified physiotherapists with experience in stroke assessment and neurological rehabilitation carried out clinical assessments. In addition to the clinical assessor, video recorded FMA and ARAT assessments were also scored by an additional blinded clinical assessor" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Unclear risk | 1 participant in the A‐tDCS group dropped out (1 out of 23; 4%) because of a skin reaction due to tDCS, whereas in the sham group there were no dropouts. Quote: "After four intervention sessions, a participant with chronic stroke dropped out of the trial due to a skin reaction after receiving four real tDCS sessions" |
| Incomplete outcome data (attrition bias) Objective outcome measures | Unclear risk | 1 participant in the A‐tDCS group dropped out (1 out of 23; 4 %) because of a skin reaction due to tDCS, whereas in the sham group there were no dropouts. Quote: "After four intervention sessions, a participant with chronic stroke dropped out of the trial due to a skin reaction after receiving four real tDCS sessions" |
| Selective reporting (reporting bias) | Unclear risk | All outcome measures listed in the methods section have been reported. All outcome measures from the published study protocol have been reported, except measures of cortical excitability |
Viana 2014.
| Methods | Study design: RCT Number of dropouts: none Number of adverse effects: none Deaths: none ITT: yes |
|
| Participants | Country: Brazil Sample size: 20 participants Inclusion criteria: unilateral stroke within 6 months prior to enrolment; age above 21 years; residual weakness/spasticity of the affected upper limb; being able to hold a Nintendo Wii controller with paretic hand; no cognitive deficits as measured by MMSE; being able to follow instructions and interact with the games; informed consent Exclusion criteria: history of seizure; cerebral aneurysm; prior surgery involving metallic implants |
|
| Interventions | 2 arms
|
|
| Outcomes | Outcomes were measured at baseline and at the end of intervention and at 5‐week follow‐up Primary outcomes
Secondary outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly assigned to the experimental or control groups by using sealed opaque envelopes" |
| Allocation concealment (selection bias) | Low risk | Quote: "Participants were randomly assigned to the experimental or control groups by using sealed opaque envelopes" |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Quote: "The participants and the researchers involved in the VRT interventions and evaluations were blind to group allocations for the duration of the trial" |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Quote: "The participants and the researchers involved in the VRT interventions and evaluations were blind to group allocations for the duration of the trial" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Incomplete outcome data (attrition bias) Objective outcome measures | Low risk | All participants completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the methods section reported |
Wang 2014.
| Methods | Study design: RCT Number of dropouts: not stated Number of adverse effects: 3 (mild tingling) Deaths: none ITT: unclear |
|
| Participants | Country: USA Sample size: 9 participants Inclusion criteria: aged between 18 and 90 years; first time clinical ischaemic or haemorrhagic stroke, radiologically confirmed; > 20° wrist extension and > 10° finger extension (all fingers); time since stroke more than 1 month prior to study enrolment Exclusion criteria: significant prestroke disability; advanced or terminal disease; substantial decrease in alertness, language reception or attention interfering with understanding instructions; contraindications to TMS; history of alcohol/drug abuse; participation in another study targeting stroke recovery; use of neuropsychotropic drugs (monoamine oxidase‐inhibitors); epilepsy; marked agitation/anxiety; having already received MP or tDCS treatment; pregnancy |
|
| Interventions | 3 arms
|
|
| Outcomes | Outcomes were measured at baseline, immediately after the intervention and 30 minutes after the end of intervention
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned to 1 of 3 groups" |
| Allocation concealment (selection bias) | Unclear risk | Not described by the authors |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Participants were blinded; blinding of personnel not described, however the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Quote: "A blinded rater measured safety, hand function, and cortical excitability before and after treatment" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | There were no subjective outcome measures |
| Incomplete outcome data (attrition bias) Objective outcome measures | Unclear risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the methods section reported |
Wu 2013a.
| Methods | Study design: RCT with parallel‐group design Dropouts: none Adverse effects: none Deaths: none ITT: yes Duration: 1 month |
|
| Participants | Country: China Number of participants: 90 Age: mean (SD) C‐tDCS: 45.9 (11.2), sham tDCS 49.3 (12.6) years Gender: C‐tDCS: 34 (76%) male, sham tDCS: 35 (78%) male Type of stroke: C‐tDCS: 27 (60%) ischaemic, sham tDCS: 26 (58%) ischaemic Time poststroke in months: mean (SD) C‐tDCS: 4.9 (3.0); sham tDCS 4.9 (2.9) Severity: FMA for C‐tDCS: 12 (4 to 26) and 8 (3 to 34), BI for C‐tDCS 55 (0 to 85) and 55 (25 to 95) for sham tDCS Inclusion criteria: time since stroke > 2 months, first‐ever stroke, muscle tone at wrist and elbow with MAS score ≥ 1 and ≤ 3, no history of Botox or other invasive treatment in the previous 6 months, use of spasmolytics resulting in an adverse event or maximised dosing without effect and no severe cognitive or mood disorders Exclusion criteria: unstable vital signs or unstable, progressive or severe neurological disease, heart condition or hypertension |
|
| Interventions | 2 arm
|
|
| Outcomes | Outcomes used: MAS (range from 0 to 4, with a score of 4 reflecting the highest possible muscle tone), UE‐FM (0 to 66, with higher scores reflecting better motor performance) and MBI (0 to 105, with higher scores reflecting better ADL performance) Time points of measurement: at baseline, at the end of the intervention period and at 4‐week follow‐up |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomly assigned using a computer‐generated randomisation list by a single investigator" |
| Allocation concealment (selection bias) | Low risk | Quote: "The assigned random number was inputted into the stimulator device by the same investigator. She did not participate in other parts of the study. The device automatically generated active or sham tDCS according to the parity of the random number" |
| Blinding of participants and personnel (performance bias) Subjective outcome measures | Low risk | Quote: "All other investigators, subjects, and outcome assessors remained blinded to group allocation until the completion of the final statistical analyses" |
| Blinding of participants and personnel (performance bias) Objective outcome measures | Low risk | Quote: "All other investigators, subjects, and outcome assessors remained blinded to group allocation until the completion of the final statistical analyses" |
| Blinding of outcome assessment (detection bias) Subjective outcome measures | Low risk | Quote: "All other investigators, subjects, and outcome assessors remained blinded to group allocation until the completion of the final statistical analyses" |
| Blinding of outcome assessment (detection bias) Objective outcome measures | Low risk | Quote: "All other investigators, subjects, and outcome assessors remained blinded to group allocation until the completion of the final statistical analyses" |
| Incomplete outcome data (attrition bias) Subjective outcome measures | Low risk | All participants completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Incomplete outcome data (attrition bias) Objective outcome measures | Low risk | All participants completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were stated |
| Selective reporting (reporting bias) | Low risk | All outcomes from the methods section and from the published trial protocol were reported |
A‐tDCS: anodal transcranial direct current stimulation AMT: active motor threshold ARAT: Action Research Arm Test ASS: Ashworth Spasticity Score AT: arm robotic training BBT: Box and Block Test BI: Barthel Index C‐tDCS: cathodal transcranial direct current stimulation CIMT: constraint‐induced movement therapy DLPFC: Dorsolateral prefrontal cortex EEG: electroencephalography ESS: European Stroke Scale FAC: Functional Ambulation Category FDI: first dorsal interosseous muscle FMA: Fugl‐Meyer Assessment iTBS: intermittent theta burst stimulation ITT: intention‐to‐treat analysis JTT: Jebsen Taylor Hand Function Test LTP: Long‐term potentiation M1: primary motor cortex mA: milliampere MAL: Motor Activity Log Rating Scale MAS: Modified Ashworth Scale MBI: Modified Barthel Index MCA: middle cerebral artery MEP: motor‐evoked response MI: Motricity Index MI‐BCI: motor imagery brain‐computer interface MIT: Massachusetts Institute of Technology MMSE: Mini Mental State Examination MP: methylphenidate MRC: Medical Research Council MRI: magnetic resonance imaging NIHSS: National Institute of Health Stroke Scale NMDA: N‐methyl‐D‐aspartate NRS: Numerical Rating Scale OMCASS: Orgogozo MCA scale PPC: posterior parietal cortex PPT: Purdue Pegboard Test RCT: randomised controlled trial ROM: range of motion RMI: Rivermead Mobility Index RMT: resting motor threshold SD: standard deviation SIS: Stroke Impact Scale tDCS: transcranial direct current stimulation TUG: Timed Up and Go Test UE‐FM: Upper Extremity Fugl‐Meyer Score WMFT: Wolf Motor Function Test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Boggio 2007b | Not a genuine RCT |
| Bradnam 2012 | Not a genuine RCT |
| Byblow 2011 | Not a genuine RCT, irrelevant outcome: motor evoked potential |
| Celnik 2009 | Outcome number of correct key presses clinically not relevant |
| Danzl 2012 | Sham group was stimulated more than 1 minute |
| Edwards 2009 | Not a genuine RCT |
| Gandiga 2006 | Not a genuine RCT |
| Giacobbe 2013 | Irrelevant outcome measure movement kinematics |
| Goh 2015 | Irrelevant Outcome: motor evoked potential |
| Gurchin 1988 | Irrelevant intervention: transcranial alternating current stimulation |
| Hummel 2005a | Not a genuine RCT |
| Hummel 2005b | Not a genuine RCT |
| Jayaram 2009 | Irrelevant outcome for review question 'motor evoked potentials' |
| Kasashima 2012 | Irrelevant outcome for review question 'event‐related desynchronisation' |
| Kharchenko 2001 | Irrelevant Intervention for review question 'transcranial alternating current stimulation' |
| Kitisomprayoonkul 2012 | Irrelevant outcome for review question 'sensation' |
| Kumar 2011 | Irrelevant intervention for review question: study did not evaluate impact of tDCS on upper limb/lower limb function and/or ADLs |
| Kwon 2012 | Not a genuine RCT |
| Lee 2012 | Irrelevant patients |
| Lefebvre 2013 | Not a genuine randomised controlled cross‐over trial |
| Lefebvre 2015 | Not a genuine randomised controlled cross‐over trial |
| Madhavan 2011 | Irrelevant outcome for review question 'accuracy index' |
| Manganotti 2011 | Not a genuine RCT |
| Ochi 2013 | Irrelevant comparison for review question: A‐tDCS versus C‐tDCS with no control group |
| Paquette 2011 | Irrelevant intervention for review question: tDCS was contaminated with rTMS at each stimulation session |
| Sheliakin 2006 | Not a genuine RCT |
| Stagg 2012a | Irrelevant outcome for review question 'response time' |
| Takeuchi 2012 | Irrelevant outcome for review question 'bimanual co‐ordination', as measured by tapping task |
| Zimerman 2012 | Not a genuine randomised controlled cross‐over trial |
A‐tDCS: anodal transcranial direct current stimulation ADLs: activities of daily living C‐tDCS: cathodal transcranial direct current stimulation RCT: randomised controlled trial rTMS: repetitive transcranial magnetic stimulation tDCS: transcranial direct current stimulation
Characteristics of studies awaiting assessment [ordered by study ID]
Brem 2010.
| Methods | Not clearly stated by the study authors |
| Participants | 3 right‐handed participants with acute stroke (< 5 weeks) |
| Interventions | A‐tDCS at 1 mA for 20 minutes twice a day on 5 consecutive days |
| Outcomes | UE‐FM, NHPT |
| Notes | Conference abstract only |
Miller 2013.
| Methods | Randomised sham‐controlled cross‐over trial |
| Participants | 20 chronic stroke patients with residual upper limb motor deficits |
| Interventions | Each participant underwent either A‐tDCS, C‐tDCS or sham tDCS separated by a two week resting period |
| Outcomes | Outcomes were assessed at baseline and after every treatment session
|
| Notes | Conference abstract only |
Park 2014.
| Methods | Randomised sham‐controlled cross‐over study |
| Participants | 17 chronic stroke patients (5 (29%) female; mean age 59 years; 12 (71%) had ischaemic stroke) |
| Interventions | Each participant underwent all of the following conditions
|
| Outcomes | Outcome measures
|
| Notes | Conference abstract only |
A‐tDCS: anodal transcranial direct current stimulation C‐tDCS: cathodal transcranial direct current stimulation Hz: hertz JTT: Jebsen–Taylor test M1: primary motor cortex
mA: milliampere MEP: Motor Evoked Potentials NHPT: Nine‐Hole Peg Test rTMS: repetitive transcranial magnetic stimulation UE‐FM: Upper Extremity Fugl‐Meyer Assessment
Characteristics of ongoing studies [ordered by study ID]
ACTRN12613000109707.
| Trial name or title | A pilot investigation of the effect of cathodal transcranial direct current stimulation (ctDCS) plus standard upper limb rehabilitation to augment motor recovery post acute stroke |
| Methods | RCT with blinded outcome assessor ITT analysis: yes |
| Participants | 37 to 40 people between 18 and 80 years of age with acute first‐ever ischaemic stroke (in the first week) and moderate to severe hemiparesis (UE‐FM ≤ 52) with MEPs detectable by TMS, stable blood pressure parameters and MMSE > 24 Exclusion criteria: pre‐existing upper limb impairment causing functional limitation, hemiplegic shoulder pain, metallic implants (pacemaker or artificial cochlea), history of seizure or another unstable medical condition, pregnancy, severe language disturbance, English as a second language, severe neglect (score < 44 out of 54 points on the Star Cancellation test), history of depression, alcohol or drug abuse, coexistent neurological or psychiatric disease, current treatment with antidepressants, antipsychotics or benzodiazepines or current treatment with Na+ or Ca2+ Channel blockers or NMDA receptor antagonists |
| Interventions | 10 rehabilitation sessions (30 minutes each) to the affected arm over a period of 2 weeks (i.e. 5 days of treatment, 2 days rest, 5 days of treatment) + 1 of the following interventions:
|
| Outcomes | All assessments are to be completed at baseline and at 1 day, 2 weeks and 3 months after the end of the intervention Primary outcome measure: UE‐FM change scores Secondary outcome measures: MEP as measured by TMS, NIHSS, Tardieu Spasticity Assessment, FIM, PostStroke Depression Scale |
| Starting date | 4 February 2013 |
| Contact information | Ms Jimena Garcia‐Vega, jimena.garcia‐vega@health.wa.gov.au |
| Notes |
Chelette 2012.
| Trial name or title | Not stated by the authors |
| Methods | Randomised sham‐controlled double‐blind trial |
| Participants | Estimated enrolment: 44 participants with severe upper extremity motor deficit due to chronic stroke |
| Interventions | Every participant is randomly assigned to 1 of 4 arms, consisting of 10 tDCS‐treatment sessions, followed by 3 hours of OT:
|
| Outcomes | UE‐FM at baseline and postintervention ARAT SIS |
| Starting date | Not stated by the study authors |
| Contact information | University of Kentucky, Lexington, KY |
| Notes |
ChiCTR‐TRC‐11001398.
| Trial name or title | Effect of transcranial direct current stimulation on recovery of upper limb function after stroke |
| Methods | Randomised controlled pilot trial in parallel‐group design Random sequence generation: computer software Blinding: participants, study staff and outcome assessors are blinded |
| Participants | 120 people with first‐time ever stroke and upper limb hemiplegia in the first 3 months after stroke, spasticity at the wrist and elbow (MAS ≤ 1) and no history of spasmolytics |
| Interventions | Experimental 1: physical therapy + active tDCS Sham comparator: physical therapy + sham tDCS |
| Outcomes | Brunnstrom stages, FMA, BI, MAS, ARAT |
| Starting date | 1 July 2011 |
| Contact information | Dongyu Wu, wudongyu73@yahoo.com.cn |
| Notes |
ChiCTR‐TRC‐11001490.
| Trial name or title | Using transcranial direct current stimulation to treat ataxia and balance impairment after stroke |
| Methods | Randomised controlled pilot trial in parallel‐group design Random sequence generation: computer software Blinding: participants, study staff and outcome assessors are blinded |
| Participants | 40 people with first‐time ever stroke and upper limb hemiplegia in the first 3 months after stroke and lesions involving the cerebellum without obvious cerebral edema Exclusion criteria: unstable vital signs; depression after stroke; severe aphasia; obvious cognition dysfunction (MMSE < 24); serious vision or vision correction anomalies; or history of vertigo attack; hearing impairment or otitis media |
| Interventions | Experimental 1: balance and intervention training + active tDCS Sham comparator: balance and intervention training + sham tDCS |
| Outcomes | Biodex Balance System, International Cooperative Ataxia Rating Scale, BBS, BI |
| Starting date | 1 August 2011 |
| Contact information | Dongyu Wu, wudongyu73@yahoo.com.cn |
| Notes |
NCT00542256.
| Trial name or title | Effects of transcranial direct current stimulation coupled with constraint‐induced movement therapy on motor function in stroke patients |
| Methods | Double‐blind RCT |
| Participants | 50 people 18 to 80 years of age with radiologically confirmed first‐time ever ischaemic or haemorrhagic stroke; at least 6 months prior to study enrolment, demonstrating adequate balance with the non‐lesioned arm restraint and the ability to stand up from sitting and to stand without help of the upper extremity Exclusion criteria: significant prestroke disability, neuropsychological impairments that hinder motor testing, considerable joint pain in the paretic extremity, life expectancy less than 1 year because of terminal medical diagnosis, advanced disease of viscera, considerable neurological or psychiatric disease, history of substance abuse, use of neuropsychotropic drugs, inability to enrol in another study targeting stroke recovery, prior admittance of CIMT or tDCS |
| Interventions | Experimental group: 40 minutes of tDCS over M1 at the beginning of 10 of 14 consecutive up to 6 hours lasting CIMT training sessions Control group: 30 seconds of tDCS over M1 at the beginning of 10 of 14 consecutive up to 6 hours lasting CIMT training sessions |
| Outcomes | Primary outcome measures: Jebsen Taylor Hand Function Test at baseline, training days 1, 5, and 10 and follow‐up; Motor Activity Log Rating Scale at baseline, training days 1, 5, and 10 and follow‐up; Beck Depression Inventory at baseline, training days 1, 5, and 10 and follow‐up; Visual Analogue Scale for Anxiety at baseline, training days 1, 5, and 10 and follow‐up Secondary outcome measures: Fugl‐Meyer Assessment of Motor Recovery at baseline; Barthel Index Score at baseline; Modified Ashworth Scale at baseline |
| Starting date | September 2007 |
| Contact information | Julie A Williams, MSc 617‐667‐5261 jawillia@bidmc.harvard.edu |
| Notes | Last updated: 9 May 2008 |
NCT00783913.
| Trial name or title | Enhancing the beneficial effects of upper extremity visuomotor training with tDCS |
| Methods | Double‐blind RCT in a parallel‐group design |
| Participants | 18 people 18 to 85 years of age with ability to sit and be active for an hour on a chair/wheelchair without cardiac, respiratory and/or pain disturbances as assessed during the screening visit; willingness to commit to participate in the long‐term follow‐up study (up to 3 months); willingness to give written informed consent; diagnosis of a first clinically apparent unilateral cortical or subcortical stroke at least 3 months before study entry Exclusion criteria: history of severe neurological illness, severe cognitive impairment (MMSE < 23); MRI contraindications; history of alcohol or drug abuse; active depression with psychoactive medication changes in the last 2 months, active psychosis, disruptive or violent behavior, poor motivational capacity; aphasia or language disturbances that would interfere with performance of study tasks; uncontrolled medical problems; increased intracranial pressure; severe neglect or ataxia that would interfere with completion of study tasks; history of more than one stroke or a stroke that affects both sides of the brain, the brainstem or the cerebellum; inflammation of the tissue, severe rheumatoid arthritis or abnormal function of the joints due to arthritis in the affected arm used most often; pregnancy |
| Interventions | Baseline intervention: 1‐hour computerised movement training and tDCS sessions twice a day, 5 days a week, for 3 weeks. Participants will sit in front of a computer screen that shows a target (round dots) and a cursor (a line). Participants will be instructed to move the cursor to various targets on the computer screen as fast and as accurately as possible, while controlling the position of the cursor by moving their arm, which will rest on a mechanical device Experimental: A‐tDCS stimulation during the first 20 minutes of each training session; electrode sponges soaked in tap water are placed on the scalp and forehead Control: sham tDCS |
| Outcomes | Primary outcome measures: accuracy (defined as the difference between the straight line connecting the origin and the target and the line followed by the participant) during reaching. 1 of the additional outcomes is the time to complete a reaching task Secondary outcome measures: UE‐FM |
| Starting date | October 2008 |
| Contact information | National Institutes of Health Clinical Center, 9000 Rockville Pike, Bethesda, Maryland, USA |
| Notes |
NCT00853866.
| Trial name or title | Enhancement of motor function with reboxetine and transcranial direct current stimulation (STIMBOX) |
| Methods | Randomised sham‐controlled double‐blind cross‐over trial |
| Participants | 12 people with stroke between 18 and 86 years of age, able to give informed consent, with first‐ever ischaemic stroke at least 6 months before study enrolment and paresis of arm/hand muscles above 3 on MRC scale Exclusion criteria: multiple cerebral lesions with associated residual deficits, severe head trauma, seizures, ferromagnetic implants in the head/neck region, pacemaker, other psychiatric or neurological diseases, substance abuse, inability to give informed consent, contraindications for reboxetine (seizures, glaucoma, prostate hyperplasia with urinary retention, cardiac arrhythmias, potential interactions with comedication), pregnancy and breast‐feeding |
| Interventions | Experimental group 1: reboxetine + active tDCS: single dose of reboxetine/edrona × 4 mg 80 minutes before assessment of JTT + 20 minutes of 1 mA tDCS during JTFHT assessment with the active electrode over M1 of the lesioned hemisphere Experimental group 2: reboxetine + sham tDCS: single dose of reboxetine/edrona × 4 mg 80 minutes before assessment of JTT + 30 seconds of 1 mA tDCS during JTFHT assessment with the active electrode over M1 of the lesioned hemisphere Experimental group 3: placebo drug + active tDCS: placebo 80 minutes before assessment of JTT + 20 minutes of 1 mA tDCS during JTFHT assessment with the active electrode over M1 of the lesioned hemisphere Experimental group 4: placebo drug + sham tDCS: placebo 80 minutes before assessment of JTT + 30 s of 1 mA tDCS during JTFHT assessment with the active electrode over M1of the lesioned hemisphere |
| Outcomes | Primary outcome measures: Jebsen Taylor Test at 4 different sessions with 4 different interventions Secondary outcome measures: maximum grip force at 4 different sessions with 4 different interventions; Nine‐Hole Peg Test at 4 different sessions with 4 different interventions |
| Starting date | January 2009 |
| Contact information | Contact: GianpieroLiuzzi, MD +49 40 7410 ext 59278 g.liuzzi@uke.de Contact: Christian Gerloff, MD + 49 40 7410 ext 53770 gerloff@uke.de |
| Notes | Last updated: 1 December 2010 |
NCT00909714.
| Trial name or title | Neuroregeneration enhanced by tDCS in stroke |
| Methods | Double‐blind RCT (parallel assignment) |
| Participants | 250 people aged 18 years and older with subacute stroke (5 to 21 days after stroke), ischaemic subcortical or cortical first‐ever strokes and moderate to moderately severe upper extremity hemiparesis (UE‐FM between 28 and 50) Exclusion criteria: more than 1 stroke; progressive stroke; completely lesioned hand knob area of M1 affected, cerebellar lesions, history of severe alcohol or drug abuse, psychiatric illnesses such as severe depression, poor motivational capacity or severe language disturbances, or with serious cognitive deficits; severe uncontrolled medical problems; rheumatological or traumatic diseases affecting the upper extremities; other neurological diseases; severe microangiopathy, polyneuropathy, ischaemic peripheral disease; pregnancy; contraindication for MRI or TMS |
| Interventions | Baseline intervention: standardised upper extremity rehabilitative training; A‐tDCS (20 minutes) or sham tDCS will be applied once a day in combination with standardised upper extremity rehabilitative training Experimental: tDCS once a day for 20 minutes + baseline (polarity and dosage not stated) Control: sham tDCS + baseline |
| Outcomes | Primary outcome measures: UE‐FM at 12 months after the end of the intervention period Secondary outcome measures: JTT, ARAT, 9‐HPT, SIS, UE‐FM at days 11, 40, 100 and 190 after the end of intervention period and at 12 months after the end of the intervention period |
| Starting date | July 2009 |
| Contact information | Friedhelm Hummel, Dr f.hummel@uke.uni‐hamburg.de Christian Gerloff, Prof Dr gerloff@uke.uni‐hamburg.de |
| Notes |
NCT01007136.
| Trial name or title | TDCS‐enhanced stroke recovery and cortical reorganisation |
| Methods | Double‐blind randomised controlled trial in parallel‐group design |
| Participants | 150 people with single ischaemic stroke between 18 and 80 years of age with arm weakness between 5 and 15 days poststroke and no other neurological or psychiatric diseases Exclusion criteria: people with bilateral motor impairment, with poor motivational capacity or history of severe alcohol or drug abuse, people with severe aphasia, MMSE Score < 23; people with severe uncontrolled medical problems (e.g. seizures, progressive stroke syndromes, severe rheumatoid arthritis, active joint deformity of arthritic origin, active cancer or renal disease, end‐stage pulmonary or cardiovascular disease, a deteriorated condition due to age or others); people with unstable thyroid disease; people with increased intracranial pressure; people with unstable cardiac arrhythmia; people with contraindication to TMS or tDCS stimulation (pacemaker, an implanted medication pump, a metal plate in the skull, or metal objects inside the eye or skull, patients who had a craniotomy, skin lesions at the site of stimulation); people who are not available for follow‐up at 3 and 12 months; pregnancy; people with contraindication to MRI will not participate in MRI |
| Interventions | Experimental: tDCS and occupational therapy: 1 mA electrical current will be delivered over M1 of the lesioned hemisphere for the first 20 minutes during the 1‐hour physical therapy Sham comparator: sham and occupational therapy: electrical current will be ramped up and down over M1 of the lesioned hemisphere for the first seconds during the 1 hour physical therapy |
| Outcomes | Primary outcome measures: UE‐FM at 2 weeks, 3 months and 1 year after stroke Secondary outcome measures: JTT at 2 weeks, 3 months and 1 year after stroke; WMFT at 2 weeks, 3 months and 1 year after stroke; MRC grading scale at 2 weeks, 3 months and 1 year after stroke; BI at 2 weeks, 3 months and 1 year after stroke; Abilhand questionnaire at 2 weeks, 3 months and 1 year after stroke; Ashworth Spasticity Scale at 2 weeks, 3 months and 1 year after stroke; Beck Depression Inventory at 2 weeks, 3 months and 1 year after stroke; Visual Analog Pain Scale at 2 weeks, 3 months and 1 year after stroke; Mini Mental Status Scale at 2 weeks, 3 months and 1 year after stroke; NIHSS at 2 weeks, 3 months and 1 year after stroke; Motor Activity Log at 2 weeks, 3 months and 1 year after stroke; fMRI overactivation in motor cortex: voxel count and intensity at 2 weeks, 3 months and 1 year after stroke |
| Starting date | March 2009 |
| Contact information | Timea Hodics, MD Timea.Hodics@UTSouthwestern.edu Charlotte Bentley Charlotte.Bentley@UTSouthwestern.edu |
| Notes |
NCT01014897.
| Trial name or title | tDCS in chronic stroke recovery—pilot |
| Methods | Double‐blind randomised sham‐controlled cross‐over trial |
| Participants | 45 people between 18 and 80 years of age with single symptomatic stroke more than 3 months ago with hand/arm weakness and ability to perform required tests and provide consent; Modified Ashworth scale < 3; ROM functional at shoulder, elbow, wrist and hand Exclusion criteria: more than 1 symptomatic stroke in MCA territory or bilateral involvement; severe medical or psychiatric conditions, drug abuse, seizure disorder; pregnancy/breast‐feeding; SAH, lobar haemorrhage; people who cannot have tDCS (prior head surgery, pacemakers, metallic implants in the head, etc); people taking antiadrenergic medications |
| Interventions | Experimental: subcortical: subcortical stroke participants will receive tDCS stimulation and sham in random order; tDCS and sham will be applied in random order during standardised occupational therapy Experimental: cortical: participants will receive active and sham tDCS in random order; tDCS and sham will be applied in random order during standardised occupational therapy |
| Outcomes | Primary outcome measures: WMFT at baseline and after the end of the intervention period; UE‐FM at baseline and after the end of the intervention period Secondary outcome measures: adverse events during the intervention period |
| Starting date | April 2009 |
| Contact information | Timea Hodics, MD Timea.Hodics@UTSouthwestern.edu |
| Notes |
NCT01127789.
| Trial name or title | Use of transcranial direct current stimulation (tDCS) to study implicit motor learning on people with brain injury |
| Methods | Double‐blind RCT (parallel assignment) |
| Participants | Enrolment: 0 People 18 to 65 years of age with TBI or stroke participants with partially preserved fine motor function Exclusion criteria: with metal clips in head or device (e.g. pacemaker); active CNS drugs |
| Interventions | Experimental: non‐invasive brain stimulation (both anodal and C‐tDCS will be used) |
| Outcomes | Primary outcome measures: reaction time (millisecond) of a serial reaction time task at 24 hours postintervention Secondary outcome measures: error rate (percentage) of a serial reaction time task at 24 hours postintervention |
| Starting date | March 2010 |
| Contact information | Wen‐Shiang Chen, MD, PhD Department of Physical Medicine and Rehabilitation, NTUH, Taipei, Taiwan, 100 |
| Notes | Withdrawn prior to enrolment |
NCT01143649.
| Trial name or title | Effects of transcranial DC stimulation coupled with constraint induced movement therapy on motor function in stroke patients |
| Methods | Double‐blind RCT (parallel‐group design) |
| Participants | 120 people between 18 and 90 years of age: 40 of whom have first‐time ever clinical ischaemic or haemorrhagic cerebrovascular accident confirmed by a radiological or physician's report, with weakness less than 55 (out of 66) on the UE‐FM scale; stroke onset > 6 months before study enrolment. The remaining 80 people are healthy volunteers Exclusion criteria: significant prestroke disability, major depression; any substantial decrease in alertness, language reception, or attention that might interfere with understanding instructions for motor testing; excessive pain in any joint of the paretic extremity (not applicable to severe stroke patients), contraindications to single pulse TMS (TMS will be used to measure cortical excitability); contraindications to tDCS, advanced liver, kidney, cardiac or pulmonary disease; terminal medical diagnosis consistent with survival < 1 year; coexistent major neurological or psychiatric disease; history of significant alcohol or drug abuse in the prior 6 months; use of carbamazepine and amitriptyline; patients may not be actively enrolled in a separate intervention study targeting stroke recovery and prior CIMT and/or tDCS treatment for stroke; history of epilepsy before stroke; patients with global aphasia and deficits of comprehension; pregnancy |
| Interventions | Experimental 1: tDCS + CIMT in stroke participants (40 people), tDCS over M1; intensity 1 mA, for the first 40 minutes of 10 consecutive sessions of CIMT (Monday to Friday) Experimental 2: tDCS + motor training in healthy participants (40 people); 1 day of treatment (when the order in which they receive sham or active tDCS stimulation will be randomly assigned). Each stimulation day will include up to 6 hours of training termed "shaping" in the non‐dominant hand, while the dominant hand is restrained in a resting hand splint and is secured in a sling. At the start of this training, participants will undergo 40 minutes of real tDCS at 1 mA or sham tDCS Active comparator: tACS 40 healthy participants, 1 day of treatment (when the order in which they receive sham or active transcranial alternating current stimulation (tACS) stimulation will be randomly assigned), stimulated at 1 mA for 40 minutes |
| Outcomes | Primary outcome measures: motor function as measured by JTT, MAS, UE‐FM, BI at 2 weeks after the end of the intervention period Secondary outcome measures: cortical excitability measured by MEP and the resting motor threshold, intracortical excitability by paired‐pulse and also transcallosal inhibition to measure interhemispheric differences |
| Starting date | April 2010 |
| Contact information | Location: Spaulding Rehabilitation Hospital, Boston, Massachusetts, 02114, USA Investigator: Felipe Fregni, PhD |
| Notes |
NCT01169181.
| Trial name or title | AMES + brain stimulation: treatment for profound plegia in stroke |
| Methods | Not clearly stated |
| Participants | Estimated enrolment: 6 Inclusion criteria: age 18 to 75 years; stroke more than 1 year prior to enrolment; hemispheric stroke; residual upper‐extremity weakness without the ability to activate finger extension volitionally Exclusion criteria: significant upper‐extremity proprioceptive deficit; cortical stroke involving M1; unstable epilepsy; Botox injections less than 5 months prior to enrolment; use of intrathecal Baclofen; residual pain in the affected arm; significant neglect involving the affected limb; exercise intolerance; uncontrolled hypertension or angina; cognitive or behavioural inability to follow instructions; terminal illness; severe apraxia; circumference of arm incompatible with the AMES device; contractures, decreased range of motion, or skin condition preventing tolerance of the AMES device (Assisted Motion with Enhanced Sensation); spinal cord injury; arthritis or fractures of affected limbs, decreasing range of motion; peripheral nerve injury or neuropathy in the affected arm resulting in significant motor or sensory loss; other neurological comorbidities; implanted devices; previous vascular surgery on brain or heart blood vessels; pregnancy |
| Interventions | 2 arms
|
| Outcomes | Outcomes will be recorded at baseline Primary outcome
Secondary outcome
|
| Starting date | July 2010 |
| Contact information | Jau‐Shin Lou, MD PhD Oregon Health and Science University Portland, Oregon, United States, 97239 |
| Notes |
NCT01201629.
| Trial name or title | Does transcranial direct current stimulation (tDCS) improve functional motor recovery in the affected arm‐hand in patients after an acute ischemic stroke? Pilot study |
| Methods | Double‐blind RCT (parallel‐group design) |
| Participants | 50 people 19 to 90 years of age with unilateral, first‐time ever acute ischaemic stroke within 4 weeks of admission to an inpatient rehabilitation facility and severe upper limb weakness (MRC < 2), medically stable from a cardiorespiratory standpoint so that they can participate in daily therapies, with ability to give informed consent Exclusion criteria: haemorrhagic stroke, patients with an episode of poststroke seizure or history of epilepsy; medically unstable, demented, or terminally ill patients; spasmolytics and medications known to enhance motor recovery such as d‐amphetamine, implanted pacemakers and defibrillators and refusal to provide informed consent |
| Interventions | Experimental: tDCS + OT, 1 mA of tDCS will be delivered through surface electrodes (25 to 35 cm2) to the unaffected motor cortex for 30 minutes before a participant's scheduled OT Sham comparator: tDCS + OT, stimulation for 30 seconds only |
| Outcomes | Primary outcome measures: total Functional Independence Measure after 4 weeks of therapy Secondary outcome measures: ARAT after 4 weeks of therapy |
| Starting date | January 2009 |
| Contact information | Meheroz H Rabadi, MD, MRCPI rabadimh@gmail.com |
| Notes |
NCT01207336.
| Trial name or title | Effect of combined anodal tDCS and peripheral nerve stimulation on motor recovery in acute stroke |
| Methods | Double‐blind RCT (parallel assignment) |
| Participants | 20 people 35 to 85 years of age with first‐ever ischaemic stroke within 5 to 30 days; paresis of the arm/hand with NIHSS < 15 Exclusion criteria: pregnancy, psychiatric history, history of substance abuse or severe depression, severe language disturbances, patients with increased intracranial pressure or serious cardiac disease, patients with contraindication to TMS |
| Interventions | Experimental: 1 session of A‐tDCS (1.2 mA for 13 minutes) to the ipsilesional primary motor cortex (M1) combined with peripheral radial nerve electrical stimulation (rEPNS) to the paretic hand repeated on 5 successive days, rEPNS (at radial nerve 5 Hz), 0.7* motor threshold Sham: the same rEPNS regimen as in the experimental group but combined with sham tDCS |
| Outcomes | Primary outcome measures: Jebsen Taylor test at 5, 15 and 30 days Secondary outcome measures: grip and wrist force at 5, 15 and 30 days; Nine‐Hole Peg Test at 5, 15 and 30 days; cortical excitability of ipsilesional M1 (as measured by TMS) at 5, 15 and 30 days |
| Starting date | September 2010 |
| Contact information | Marion Simonetta‐Moreau, MD, PhD simonetta.m@chu‐toulouse.fr |
| Notes |
NCT01356654.
| Trial name or title | The use of transcranial direct current stimulation in the recovery of postural control in stroke |
| Methods | Double‐blind randomised controlled cross‐over trial |
| Participants | 34 people 18 to 75 years of age, suffering from a stroke in the MCA region, during subacute phase (4 to 24 weeks after onset), hospitalised in rehabilitation Hospital Hof Ter Schelde, Antwerp, Belgium, capable of understanding and giving informed consent Exclusion criteria: cerebellum or brainstem lesions, recent multiple lesions and older lesions manifested clinically, history of severe substance abuse (alcohol, drugs, benzodiazepines), cardiac diseases that in the opinion of the clinician preclude participation in the trial (e.g. severe dyspnoea in rest, severe rhythm disturbances), history of epileptic insults not caused by the stroke, severe organic comorbidity, history of psychiatric disorders, pacemaker/internal defibrillator, pregnancy |
| Interventions | Experimental: tDCS, 20 minutes, 4 times a week for 4 weeks Sham comparator: sham TDCS, 20 minutes, 4 times a week for 4 weeks |
| Outcomes | Primary outcome measures (at baseline, after one month and after two months): Trunk Impairment Scale (change score); RMAB; Tinetti test |
| Starting date | March 2010 |
| Contact information | Wim Saeys, MSc, wim.saeys@hotmail.com |
| Notes |
NCT01414582.
| Trial name or title | Transcranial direct current stimulation (tDCS) as a potential adjunct intervention in stroke rehabilitation |
| Methods | Double‐blind RCT (parallel assignment) |
| Participants | 80 people 18 to 80 years of age who are willing and able to give informed consent for participation in the study and who should be at least 6 months post first symptomatic stroke affecting motor function of the hand Exclusion criteria: no adequate understanding of verbal and written information in English, sufficient to complete any of the safety screening forms, previous history of epilepsy, history of drug abuse or a previous history of a neurological or psychiatric illness, or a history of neurosurgical procedure; prescription of medications such as antidepressants, took or taking of antimalarial treatment in the last 72 hours, pregnancy, metallic implant in the neck, head, or eye; any implanted electrical devices, claustrophobia, more than one stroke, limited communication in the form of aphasia or a history of dementia |
| Interventions | Baseline intervention: standardised motor training intervention for the upper paretic limb Experimental group: baseline Intervention and A‐tDCS over the M1 of the ipsilesional hemisphere, stimulation intensity of 1 mA for the first 20 minutes of motor training (9 consecutive sessions from Monday to Friday) Sham comparator: baseline Intervention and sham tDCS over M1 of the ipsilesional hemisphere for the first 20 minutes of motor training (9 consecutive sessions from Monday to Friday) |
| Outcomes | Primary outcome measures: UE‐FM, WMFT, ARAT, Nine‐Hole Peg Test Secondary outcome measures: Reaction Time Test, SIS All assessed at 2 separate baseline sessions (at least 1 week apart), and then again immediately after the end of the intervention period (day 10), 1 week, 1 month and 3 months after the end of the intervention period |
| Starting date | January 2011 |
| Contact information | Heidi Johansen‐Berg, Prof, heidi@fmrib.ox.ac.uk |
| Notes |
NCT01500564.
| Trial name or title | Functional Interest of non invasive brain stimulation during physiotherapy at a subacute phase post stroke (anodal protocol) |
| Methods | Double‐blind RCT (parallel‐group design) |
| Participants | 20 people 18 to 80 years of age; participants volunteer to participate in the study, with written informed consent, affiliation with a national health insurance program, first‐time ever clinical ischaemic or haemorrhagic cerebrovascular accident as evidenced by a radiological (or physician's) report, contralesional motor deficit with a lesion sparing M1, stroke onset > 1 month and < 6 months before study enrolment Exclusion criteria: coexistent major neurological or psychiatric disease, history of epilepsy before stroke, substantial decrease in alertness, language reception, or attention that might interfere with understanding instructions for motor testing; patients with global aphasia and deficits of comprehension, excessive pain in any joint of the paretic extremity (VAS > 4), contraindications to tDCS such as metal in the head, implanted brain medical devices, history of significant substance abuse in the prior 6 months, antimalarial treatment in the last 72 hours, no prior CIMT/tDCS treatment for stroke; pregnancy |
| Interventions | Baseline intervention: 20 minutes of motor training during physiotherapy in 10 consecutive sessions (Monday to Friday) during 2 weeks Experimental: baseline intervention + A‐tDCS over M1 of the ipsilesional hemisphere; stimulation intensity of 1 mA Sham comparator: baseline intervention + sham tDCS over the M1 of the ipsilesional hemisphere |
| Outcomes | Primary outcome measures: UE‐FM (change score from baseline to 2 weeks after the end of the intervention period) Secondary outcome measures (change score from baseline to 2 weeks after the end of the intervention period, 2 weeks, 1 month, 3 months and 6 months later): FIM, MAL, JTT, BBT, MAS, muscle strength as measured by MRC |
| Starting date | December 2011 |
| Contact information | Sophie Jacquin‐Courtois, MD, sophie.courtois@chu‐lyon.fr |
| Notes |
NCT01503073.
| Trial name or title | Noninvasive brain stimulation for stroke improvement |
| Methods | Double‐blind RCT cross‐over trial |
| Participants | 200 persons 18 to 90 years of age with acute or chronic stroke (and with a slight deficit at least) Exclusion criteria: epilepsy, contraindication to tDCS and/or to fMRI, inability to understand/complete behavioural tasks, history of substance abuse, major health condition, presence of pacemaker, pregnancy |
| Interventions | Active comparator: tDCS Sham comparator: sham tDCS |
| Outcomes | Primary outcome measures: change in function before/after tDCS, any brain function impaired by stroke Secondary outcome measures: change in neuroimaging and neurophysiological outcome measures before/after tDCS: (1) noninvasive neuroimaging: brain activity studied by means of fMRI, (2) noninvasive neurophysiological measure: TMS, EEG, evoked potentials, EMG Time points of their measurement: before intervention, immediately after intervention, 10, 20, 30, 40, 50, 60 minutes after intervention; long‐term after intervention: 1, 2, 3 and 4 weeks and 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 months after the end of the intervention period |
| Starting date | January 2008 |
| Contact information | Yves Vandermeeren, MD, PhD, yves.vandermeeren@uclouvain.be |
| Notes |
NCT01519843.
| Trial name or title | Post‐stroke procedural learning: from neural substrates to therapeutic modulation by non‐invasive brain stimulation |
| Methods | Double‐blind randomised controlled cross‐over trial |
| Participants | 200 people 18 to 95 years of age with chronic stroke with an at least slight deficit Exclusion criteria: epilepsy, contraindication to tDCS and/or to fMRI, inability to understand/complete behavioural tasks, history of substance abuse, major health condition, presence of pacemaker, pregnancy |
| Interventions | Active comparator: tDCS Placebo comparator: sham tDCS |
| Outcomes | Primary outcome measures: motor learning improvement with tDCS from baseline to 4 weeks after the end of the intervention period as measured by a motor skill learning task and by Purdue Pegboard, hand dynamometer, pinch dynamometer, 9‐HPT Secondary outcome measures: neuroimaging before motor learning task, during motor learning and after (immediately, 30 minutes, 60 minutes) motor learning; neurophysiological outcome measure (of brain excitability and connectivity with TMS (single and paired pulse)) 5 minutes before motor learning, just at the end of motor learning, after 30 minutes of motor learning, after 60 minutes of motor learning and at 1, 2, 3, and 4 weeks after the day of intervention |
| Starting date | September 2010 |
| Contact information | Yves Vandermeeren, MD, PhD, yves.vandermeeren@uclouvain.be |
| Notes |
NCT01539096.
| Trial name or title | Brain stimulation‐aided stroke rehabilitation: neural mechanisms of recovery |
| Methods | Double‐blind RCT (parallel‐group design) |
| Participants | 30 people 21 years of age and older diagnosed with a stroke that occurred at least 6 months ago Exclusion criteria: pregnancy, ongoing use of CNS‐activating medications, presence of an electrically, magnetically or mechanically activated implant, including cardiac pacemaker or cochlear implants, metal in the head, history of medication‐resistant epilepsy in the family, history of seizures or unexplained spells of loss of consciousness |
| Interventions | Baseline intervention: CIMT for 3 days a week for 5 weeks for 1 hour each day. Participants will also be asked to use their affected hand in daily activities at home for 5 hours a day while wearing a mitt on the unaffected hand Experimental: baseline intervention + tDCS to areas of the brain responsible for movement of the affected hand Sham comparator: baseline intervention + sham tDCS with a similar setup to that for the active tDCS |
| Outcomes | Primary outcome measures: change in upper limb function at baseline during intervention (on average 2.5 weeks from baseline) and at 5 weeks at the end of the intervention period Secondary outcome measures: study of change in neural mechanisms that underlie the complementary association of cortical stimulation and CIMT |
| Starting date | July 2011 |
| Contact information | Ela B Plow, PhD, PT, plowe2@ccf.org Alexandria Wyant, wyanta@ccf.org |
| Notes |
NCT01544699.
| Trial name or title | Impact of non‐invasive brain stimulation on motor recuperation |
| Methods | Double‐blind randomised controlled cross‐over trial |
| Participants | 200 people 18 to 90 years of age with chronic stroke (> 6 months after stroke) and at least a slight deficit in upper or lower limb Exclusion criteria: epilepsy, contraindication to tDCS and/or to fMRI, inability to understand/complete behavioural tasks, history of substance abuse, major health condition, presence of pacemaker, pregnancy |
| Interventions | Active comparator: tDCS Sham comparator: sham tDCS |
| Outcomes | Primary outcome measures: change in motor function of upper/lower limb before/after tDCS from baseline to immediately after intervention (30 minutes of tDCS) to 10, 20, 30, 40, 50, 60 minutes after intervention and long‐term after intervention: 1, 2, 3, and 4 weeks |
| Starting date | January 2012 |
| Contact information | Yves Vandermeeren, MD, PhD, yves.vandermeeren@uclouvain.be |
| Notes |
NCT01574989.
| Trial name or title | Effects of repetitive transcranial magnetic stimulation and transcranial DC stimulation on motor function in stroke patients |
| Methods | Double‐blind randomised controlled cross‐over trial |
| Participants | 26 people 18 to 90 years of age Additional inclusion criteria for stroke participants: first‐time ever clinical ischaemic or haemorrhagic cerebrovascular events as evidenced by a radiological (or physician's) report; weakness, defined as score of less than 55 (out of 66) on UE‐FM scale; stroke onset > 6 months before study enrolment Exclusion criteria: history of major depression, BDI > 30, any substantial decrease in alertness, language comprehension, or attention that might interfere with understanding instructions for motor testing; contraindications to TMS/tDCS; advanced liver, kidney, cardiac or pulmonary disease; terminal medical diagnosis consistent with survival < 1 year; coexistent major neurological or psychiatric disease, history of significant substance abuse in the prior 6 months, patients may not be actively enrolled in a separate intervention study targeting stroke recovery and any other clinical trials, patients with global aphasia and deficits of comprehension, pregnancy, neuropsychotropic medication (healthy people only) Additional exclusion criteria for stroke patients: patients may not have already received TMS and/or tDCS stimulation for stroke, history of epilepsy before stroke or episodes of seizures within the last 6 months |
| Interventions | Participants will receive 5 sessions of stimulation. They will undergo (1) active low‐frequency rTMS (1 Hz continuous), (2) active high‐frequency rTMS (10 Hz, 2‐second trains with intertrain interval of 28 seconds) or (3) sham rTMS (using a sham coil). Each session will last 20 minutes and will be conducted at 100% of the motor threshold. Each tDCS session will last 20 minutes and will be conducted using 1 mA with 35 cm² electrodes Experimental 1: single session of active low‐frequency rTMS/sham tDCS on the scalp during the 20‐minute session Experimental 2: single session of active high‐frequency rTMS/sham tDCS on the scalp during the 20‐minute session Experimental 3: single session of sham rTMS/active anodal tDCS on the scalp during the 20‐minute session Experimental 4: single session of sham rTMS/active C‐tDCS on the scalp during the 20‐minute session Sham comparator: single session of sham rTMS/sham tDCS on the scalp during the 20‐minute session |
| Outcomes | Primary outcome measures: changes in cortical excitability measures using single‐ and paired‐pulse TMS before and after each single stimulation session Secondary outcome measures: changes in motor function as measured by behavioural tasks (e.g. Purdue pegboard, JTT, ROM) both before and after the stimulation sessions Time frame: measured for approximately 6 weeks |
| Starting date | May 2011 |
| Contact information | Felipe Fregni, MD, PhD, MPH, ffregni@partners.org Kayleen M Weaver, BA, kmweaver@partners.org |
| Notes |
NCT01644929.
| Trial name or title | Rehabilitation combined with bihemispheric transcranial direct current stimulation in subacute ischemic stroke to increase upper limb motor recovery: a randomised, controlled, double‐blind study (RECOMBINE) |
| Methods | Double‐blind randomised controlled cross‐over trial (multicentre) |
| Participants | 36 people 18 years of age or older with subcortical or subcortical/cortical ischaemic lesions in the territory of MCA, as confirmed by neuroimaging in the subacute phase (2 to 4 weeks after stroke) with persistent hemiparesis (score of 1 to 3 on the motor arm item of the NIH Stroke Scale (NIHSS) but wrist and finger movement is not required) and no upper extremity injury or conditions that limited its use before the stroke; subscription of informed consent Exclusion criteria: history of epilepsy, brain tumour, major head trauma, learning disorder, severe cognitive impairment, drug or alcohol abuse, major psychiatric illness. Use of medications that may lower seizure threshold (e.g. metronidazole, fluoroquinolones), severe pain in the affected upper limb (≥ 8 on the shoulder item of the "joint pain during passive motion" of the UE‐FM); recurrent stroke or other significant medical complications during the study; evidence of severe leucoencephalopathy (grade IV according to Fazeka's scale); significant aphasia that would impair understanding and performance on assessment scales |
| Interventions | Each participant receives standardised physical/occupational treatment according to the Impairment‐Oriented Training, plus 1 of the following treatment schemes:
|
| Outcomes | Primary outcome measures: UE‐FM at the end of the intervention period Secondary outcome measures: UE‐FM at 3 weeks and at 6 months; BI at 3 weeks, at 6 weeks and at 6 months; Ashworth scale at 3 weeks, at 6 weeks and at 6 months; Test of Upper Limb Apraxia (TULIA) at 6 weeks and at 6 months; grip strength at 3 weeks, at 6 weeks and at 6 months; Hamilton Depression Rating Scale at 6 weeks and at 6 months |
| Starting date | September 2012 |
| Contact information | Carlo Cereda, MD, Carlo.Cereda@eoc.ch René Müri, MD, rene.mueri@insel.ch |
| Notes |
NCT01726673.
| Trial name or title | Effects of transcranial direct current stimulation paired with robotic arm therapy on recovery of upper extremity motor function in stroke patients |
| Methods | Double‐blind RCT (parallel assignment) |
| Participants | 66 people 18 years of age or older with first single focal unilateral lesion as verified by brain imaging at least 6 months after stroke, with cognitive function sufficient to understand experiments and follow instructions; FMA of 7 to 58 out of 66 (neither hemiplegic nor fully recovered motor function in the muscles of the shoulder, elbow and wrist) Exclusion criteria: Botox treatment within 6 weeks of enrolment, fixed contraction of the affected limb, complete flaccid paralysis of the affected limb, history of haemorrhagic stroke, ongoing use of CNS active or psychoactive medications, presence of additional potential tDCS/TMS risk factors, including damaged skin at the site of stimulation, presence of a magnetically/mechanically active implant, metal in the head, family history of epilepsy and personal history of seizures |
| Interventions | Experimental arm: tDCS + robotic arm therapy, 2 mA for 20 minutes over M1 in the lesioned hemisphere, followed by robotic arm therapy for 60 minutes, 3 times per week for 12 weeks Placebo comparator arm: sham tDCS + robotic arm therapy (0 mA) for 20 minutes over M1 in the lesioned hemisphere, followed by robotic arm therapy for 60 minutes, 3 times per week for 12 weeks |
| Outcomes | Primary outcome measures: change from baseline in UE‐FM at the end of the intervention period and at 6 months of follow‐up Secondary outcome measures: change from baseline in kinematic data (upper extremity mobility as measured by Interactive Motion Technologies planar (shoulder/elbow) robot and wrist (wrist flexion/extension and pronation/supination) robots during therapy and evaluations) at the end of the intervention period and at 6 months of follow‐up; change from baseline in WMFT at the end of the intervention period and at 6 months of follow‐up; change from baseline Motor Power Manual Muscle Test at the end of the intervention period and at 6 months of follow‐up; change from baseline NIH stroke scale at the end of the intervention period and at 6 months of follow‐up; change from baseline SIS at the end of the intervention period and at 6 months of follow‐up |
| Starting date | September 2012 |
| Contact information | Bruce T Volpe, MD, bvolpe1@nshs.edu Johanna Chang, MS, jchang14@nshs.edu |
| Notes |
NCT01807637.
| Trial name or title | Using transcranial direct current stimulation to jump start gait training in chronic stroke patients |
| Methods | RCT with parallel‐group design |
| Participants | Estimated enrolment: 88 Inclusion criteria: stroke > 3 months prior to enrolment; unilateral stroke; MRI‐confirmed; age > 30 years; complete NIHSS; sufficient endurance motor ability and balance to ambulate at least 10 meters; ankle dorsiflexion passive ROM > 0°; demonstrating foot‐drop during ambulation such that gait instability or inefficient gait patterns are exhibited; pass the TMS Adult Safety Screen (TASS) Exclusion criteria: oedema; skin breakdown; absent sensation of the affected lower limb, which interferes with the peroneal nerve stimulator; serious cardiac arrhythmia; pacemakers or any other implanted electronic systems; pregnancy; uncontrolled seizures; Parkinson's Disease; spinal cord injury, traumatic brain injury; multiple sclerosis; fixed ankle plantar flexor contracture; history of dementia, severely impaired cognition, communication or comprehension; severe or frequent headaches; history of BOTOX injection within 3 months prior to enrolment; receiving other forms of electrical stimulation; other medical conditions or medications that compromise ambulation or balance; PI's or Medical Monitor's discretion not to include a participant |
| Interventions | 2 arms
|
| Outcomes | Outcomes will be recorded at baseline, at 1 week, 1 month and at 6 months postintervention Primary outcome measures
Secondary outcome measures
|
| Starting date | March 2013 |
| Contact information | Chad I Lairamore, PhD; chadl@uca.edu University of Central Arkansas Conway, Arkansas, United States, 72035 |
| Notes |
NCT01828398.
| Trial name or title | tDCS and robotic therapy in stroke |
| Methods | RCT with parallel‐group design |
| Participants | Estimated enrolment: 40 Inclusion criteria: age > 18 years; first‐ever ischaemic stroke; impairment of the upper limb; TCT score > 50 Exclusion criteria: insufficient understanding in Italian to complete any test; MMSE‐score < 24; contraindications to single‐pulse TMS; history of epilepsy; frequent headaches or neck pain; implanted devices; contraindications to tDCS; neurological or psychiatric pathology; severe cardio‐pulmonary, renal, hepatic diseases; pregnancy |
| Interventions | 2 arms
|
| Outcomes | Outcomes will be recorded at baseline (further time points not stated) Primary outcome measure:
Secondary outcome measures
|
| Starting date | November 2011 |
| Contact information | Sofia Straudi, MD University Hospital of Ferrara Ferrara, Italy |
| Notes |
NCT01879787.
| Trial name or title | Impact of transcranial direct current stimulation (tDCS) on the effects of mental practice and modified constraint‐induced movement therapy (mCIMT) in the rehabilitation of chronic stroke patients |
| Methods | RCT with parallel‐group design |
| Participants | Estimated enrolment: 84 Inclusion criteria: aged between 40 and 80 years; stroke > 6 months prior to enrolment; MMSE ≥ 20; ≤ 3 Ashworth Scale; ≤ 4 Visual Analogue Pain Scale Exclusion criteria: multiple brain lesions; medication for treatment of spasticity; attention deficit; inability to follow instructions; pregnancy; pacemaker; metal implant in the head; history of convulsion; epilepsy |
| Interventions | 7 arms
|
| Outcomes | Outcomes will be recorded at baseline and at 1 and 2 months Primary outcome measures
Secondary outcome measures
|
| Starting date | January 2011 |
| Contact information | Kátia K Monte Silva, PhD Universidade Federal de Pernambuco Recife, Pernambuco, Brazil, 50740‐560 |
| Notes |
NCT01883843.
| Trial name or title | Efficacy of a task‐oriented circuit training associated with transcranial direct current stimulation (tDCS) for gait improvement in chronic stroke patients. A randomised controlled trial |
| Methods | RCT with parallel‐group design |
| Participants | Estimated enrolment: 21 Inclusion criteria: aged between 18 and 75 years; diagnosis of first‐ever ischaemic stroke > 6 months prior to enrolment; MMSE > 24; FAC ≥ 4 Exclusion criteria: contraindications to tDCS; neurological or psychiatric pathology; severe cardio‐pulmonary, renal or hepatic disease; pregnancy |
| Interventions | 2 arms
|
| Outcomes | Outcomes will be recorded at baseline, at 1 week after treatment end and at 3 months follow‐up Primary outcome measure:
Secondary outcome measures:
|
| Starting date | May 2013 |
| Contact information | Sofia Straudi, MD Ferrara Rehabilitation Hospital Ferrara, Italy, 44124 |
| Notes |
NCT01897025.
| Trial name or title | Combined transcranial direct current stimulation and motor imagery‐based robotic arm training for stroke rehabilitation ‐ a feasibility study |
| Methods | RCT with parallel‐group design |
| Participants | Estimated enrolment: 32 Inclusion criteria: first‐ever stroke more than 9 months prior to study enrolment; upper extremity impairment of 11 to 45 on the Fugl‐Meyer assessment scale Exclusion criteria: epilepsy; neglect; cognitive impairment; other neurological or psychiatric diseases; severe arm pain; spasticity score > 2 MAS in shoulder/elbow joint; contraindications to TMS or tDCS; grip strength < 10 kg as measured by dynamometer; participation in other interventions or trials targeting motor recovery |
| Interventions | 2 arms
|
| Outcomes | Outcomes will be recorded at baseline, at the end of intervention period and 4 weeks after the end of intervention period Primary outcome measure
Secondary outcome measures
|
| Starting date | January 2011 |
| Contact information | Effie Chew, MD National University Hospital Singapore, Singapore, 119074 |
| Notes |
NCT01945515.
| Trial name or title | Robotic‐assisted gait training combined with transcranial direct current stimulation to maximize gait recovery after stroke: a double‐blind, randomised, controlled trial |
| Methods | RCT with parallel‐group design |
| Participants | Estimated enrolment: 34 Inclusion criteria: aged above 18 years; stroke diagnosis confirmed by CT or MRI; hemiplegia due to unilateral lesion; time since stroke from 1 to 8 weeks; inability to ambulate independently Exclusion criteria: unstable vital signs; history of seizure or cranial operation; premorbid inability to ambulate; bilateral hemispheric stroke; metallic implants; MMSE < 10; severe aphasia |
| Interventions | 2 arms
|
| Outcomes | Outcomes will be recorded at baseline Primary outcome measures: not stated Secondary outcome measures: not stated |
| Starting date | September 2013 |
| Contact information | Han Gil Seo, MD Seoul National University Hospital Seoul, Korea, Republic of, 110‐744 |
| Notes |
NCT01969097.
| Trial name or title | Efficacy basics of bihemispheric motorcortex stimulation after stroke |
| Methods | RCT with parallel‐group design |
| Participants | Estimated enrolment: 50 Inclusion criteria: aged between 18 and 80 years; chronic stroke (> 6 months after stroke) Exclusion criteria: more than 1 stroke; severe alcohol disease or drug abuse; severe psychiatric disease like depression or psychosis; severe cognitive deficits; severe untreated medical conditions; other neurologic diseases; severe microangiopathy; pregnancy |
| Interventions | 3 arms
|
| Outcomes | Outcomes will be recorded at baseline and at the end of intervention period Primary outcome measures
Secondary outcome measures
|
| Starting date | May 2012 |
| Contact information | Robert Lindenberg, M.D. Charite Universitätsmedizin Berlin Berlin, Germany, 10117 |
| Notes |
NCT01983319.
| Trial name or title | Transcranial direct current stimulation combined with constraint induced movement therapy and role of GABA activity in stroke recovery |
| Methods | RCT with parallel‐group design |
| Participants | Estimated enrolment: 64 Inclusion criteria: age between 18 and 80 years; stroke > 3 months prior to enrolment; > 10° mobility in the wrist, thumb and fingers of the affected side; ability to move, stand up and stand firmly with constraint healthy hand; ability to perform training 6 hours daily in 2 weeks; being able to understand instructions and to co‐operate Exclusion criteria: contraindication to MRI of the brain; pregnancy; epilepsy, major psychiatric diseases; excessive pain, preventing treatment; history of other diseases resulting in decreased mobility of affected upper limb |
| Interventions | 3 arms
|
| Outcomes | Primary outcome measures (measured at baseline and at the end of intervention)
Secondary outcome measures
|
| Starting date | September 2013 |
| Contact information | Krystian Figlewski, MD Regionhospital Hammel Neurocenter, Research Unit Hammel, Denmark, 8450 |
| Notes |
NCT02031107.
| Trial name or title | Randomized controlled trial of transcranial theta‐burst stimulation and transcranial direct current stimulation in subacute stroke |
| Methods | RCT with parallel‐group design |
| Participants | Estimated enrolment: 45 Inclusion criteria: unilateral stroke with resulting deficits in motor function and significantly impaired activities in daily living at enrolment Exclusion criteria: epilepsy; metal in the head; implants; pregnancy; sleep deprivation; recent traumatic brain injury; delirium or disturbed vigilance; inability to participate in 1 hour treatment sessions; severe language comprehension deficits; skull breach; recurrent stroke during rehabilitation; medical complications |
| Interventions | 3 arms
|
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | September 2013 |
| Contact information | Adrian G Guggisberg, MD Service de Neurorééducation, Unversity Hospital Geneva, Switzerland, 1211 |
| Notes |
NCT02080286.
| Trial name or title | Boosting the therapeutic benefits of prism adaptation by combining it with tDCS |
| Methods | Randomised cross‐over trial |
| Participants | Estimated enrolment: 40 Inclusion criteria: aged 18 to 85 years; right‐hemispheric stroke at least 1 month prior to enrolment;diagnosis of neglect confirmed by the Behavioural Inattention Test (BIT) Exclusion criteria: adequate understanding of English, sufficient to give informed consent; limited verbal communication in the form of dysphasia; history of drug abuse; history of dementia or other psychiatric conditions |
| Interventions | 3 arms
|
| Outcomes | Primary outcome measures
Secondary outcome measure:
|
| Starting date | February 2014 |
| Contact information | Jacinta O'Shea FMRIB Centre, John Radcliffe Hospital, University of Oxford Oxford, United Kingdom, OX3 9DU |
| Notes |
NCT02109796.
| Trial name or title | A controlled, randomised study evaluating the immediate effect of one tDCS session on quadriceps strength in hemiparetic patients |
| Methods | Randomised cross‐over trial |
| Participants | Estimated enrolment: 30 Inclusion criteria: written informed consent; stroke > 6 months prior to enrolment; hemiparesis; ability to walk with or without technical assistance; following rehabilitation program for lower limbs Exclusion criteria: patient with bilateral brain lesion; cerebellar syndrome; apraxia; aphasia; previous orthopedic surgery in paretic lower limb (< 6 months); usual tDCS contraindications; pregnancy |
| Interventions | No detailed information provided except the following quotation: "We test a new electrode configuration: a anodal stimulation opposite to the cortical representation area of the injured hemisphere and a simultaneous stimulation opposite to the homonyme the cortical representation area of the healthy hemisphere. We hypothesis that one session of tDCS with this electrode configuration allow to improve paretic quadriceps strength in hemiparetic patients after stroke." |
| Outcomes | Outcomes will be recorded at baseline and 2 hours after the end of intervention Primary outcome measure
Secondary outcome measures
|
| Starting date | February 2015 |
| Contact information | Roche Nicolas, MD PH Raymond Poincare Hospital Garches, France, 92380 |
| Notes |
NCT02156635.
| Trial name or title | A double‐blind, sham‐controlled, randomised clinical trial on stroke treatment using transcranial direct current stimulation |
| Methods | RCT with parallel‐group design |
| Participants | Estimated enrolment: 40 Inclusion criteria: aged between 18 and 65 years; acute ischaemic stroke; informed consent Exclusion criteria: NIHSS between 25 and 32; Rankin ≥ 5; MMSE ≤ 24; use of drugs changing CNS excitability; metallic implants; seizures; pregnancy; other conditions interfering with CIMT criteria; inability to voluntarily execute wrist flexion, 10° of finger extension and 20° of wrist extension |
| Interventions | 2 arms
|
| Outcomes | Primary outcome measure
Secondary outcome measures
|
| Starting date | June 2014 |
| Contact information | Suellen Marinho Andrade, MSc Federal University of Paraíba, Department of Psychology João Pessoa, Paraíba, Brazil, 58051‐900 |
| Notes |
NCT02166619.
| Trial name or title | Transcranial direct current stimulation in rehabilitation of chronic stroke patients: multicenter clinical trial |
| Methods | Randomised controlled trial with parallel‐group design |
| Participants | Estimated enrolment: 24 Inclusion criteria: age between 40 and 80 years; primary or recurrent stroke, confirmed by CT or MRI; stroke > 12 months prior to enrolment; upper limb impairment due to stroke; MMSE ≥ 18; Ashworth Scale ≥ 4; minimal active wrist movement (flexion and extension); at least one pinch movement Exclusion criteria: prior neurological diseases; multiple brain lesions; metal implant in the head; pacemaker; history of seizures; epilepsy; pregnancy; haemodynamic instability; cointervention of physical therapy elsewhere during the study; initial UE‐FM > 59; traumatic or orthopaedic lesion limiting the range of motion of the upper limb |
| Interventions | 2 arms
|
| Outcomes | Outcomes will be recorded at baseline and at days 30 and 90 Primary outcome measure:
Secondary outcome measures:
|
| Starting date | December 2013 |
| Contact information | Kátia Monte‐Silva, PhD Déborah Marques, PT Applied Neuroscience Laboratory, Universidade Federal de Pernambuco Recife, PE, Brazil, 50670‐900 |
| Notes |
NCT02209922.
| Trial name or title | The effects of tDCS combined with balance training on postural control and spasticity in chronic stroke patients (a randomised controlled trial) |
| Methods | RCT with parallel‐group design |
| Participants | Estimated enrolment: 40 Inclusion criteria: age between 18 and 80 years; first ischaemic MCA stroke > 6 months prior to enrolment; Romberg test > 30 seconds Exclusion criteria: haemorrhagic stroke; other neurological conditions affecting balance |
| Interventions | 2 arms
|
| Outcomes | Outcomes will be recorded at baseline and 1 week after the end of intervention Primary outcome measures
Secondary outcome measures
|
| Starting date | December 2014 |
| Contact information | Fariba Yadolahi ShahidBeheshti Univesity of Medical sciences Tehran, Iran, Islamic Republic of, 1616931111 |
| Notes |
NCT02210403.
| Trial name or title | The Influence of tDCS on the arm and hand function in stroke patients |
| Methods | Randomised cross‐over trial |
| Participants | Estimated enrolment: 26 Inclusion criteria: age above 18 years; stroke onset > 6 months prior to enrolment; first‐ever stroke; decreased hand and arm function; MMSE > 24 Exclusion criteria: depression; pregnancy; alcohol abuse; aneurysm clips; pacemaker; neurostimulator; implemented defibrillator; magnetically activated implant or device; implemented pump; spinal cord stimulator; implemented hearing aid; artificial or prosthetic limb; metal parts in the body; any external or internal metal; artificial heart valve; other implants; history of brain surgery migraine; family history of epilepsy |
| Interventions | 2 arms
|
| Outcomes | Outcomes will be recorded at baseline, at the 3rd intervention day and at 1 week postintervention Primary outcome measure
Secondary outcome measure
Other outcome measure
|
| Starting date | April 2013 |
| Contact information | Xue Zhang K U Leuven Leuven, Belgium, 3000 |
| Notes |
NCT02213640.
| Trial name or title | Potentiation of the effects of prismatic adaptation by transcranial direct current stimulation (tDCS): evaluation of functional interest in negligence rehabilitation |
| Methods | RCT with parallel‐group design |
| Participants | Estimated enrolment: 24 Inclusion criteria: age between 18 and 80; right‐handedness; unilateral neglect due to right‐hemispheric stroke, radiologically confirmed; hospitalised in the Department of Physical Medicine and Rehabilitation or external monitoring; diagnosis of negligence as indicated by BIT score ≤ 129; stroke > 1 month prior to enrolment Exclusion criteria: degenerative neurological condition; uncontrolled epilepsy; temporo‐spatial disorientation; language disorders or psychiatric disorders interfering with understanding instructions; history of prior stroke; multiple stroke; unstable medical condition; pregnancy; implanted materials; unweaned alcoholism |
| Interventions | 2 arms
|
| Outcomes | Outcomes will be recorded at baseline, at the end of intervention (5 weeks) and 2, 6 and 15 weeks after the end of intervention Primary outcome measure
Secondary outcome measures:
|
| Starting date | September 2014 |
| Contact information | Sophie Jacquin‐Courtois, MD‐PhD Laurent Villeneuve, CRA Hospices Civils de Lyon |
| Notes |
NCT02254616.
| Trial name or title | Hybrid approach to mirror therapy and transcranial direct current stimulation for stroke recovery: a follow up study on brain reorganisation, motor performance of upper extremity, daily function, and activity participation |
| Methods | Randomised controlled trial with parallel‐group design |
| Participants | Estimated enrolment: 80 Inclusion criteria: first stroke in cortical regions; time since stroke > 6 months prior to enrolment; initial UE‐FM score between 24 to 52; MAS ≤ 2 in any joints of the affected arm; MMSE ≥ 24; willing to sign the informed consent Exclusion criteria: aphasia interfering with understanding instructions; visual/attention impairments that might interfere with the seeing of mirror illusion, including hemineglect/hemianopsia; currently participation in any other research; previous brain neurosurgery; metallic implants within the brain |
| Interventions | 4 arms
|
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | August 2014 |
| Contact information | Ching‐Yi Wu, ScD Chang Gung Memorial Hospital Kwei‐Shan, Tao‐Yuan, Taiwan, 333 |
| Notes |
NCT02292251.
| Trial name or title | Study to enhance motor acute recovery with intensive training after stroke |
| Methods | RCTwith factorial assignment |
| Participants | Estimated enrolment: 72 Inclusion criteria: age over 21 years; first or recurrent ischaemic stroke < 5 weeks prior to enrolment, confirmed by CT or MRI; residual unilateral arm weakness with UE‐FM between 6 and 40; informed consent; ability to understand the tasks involved Exclusion criteria: prior stroke with resulting motor deficits; space‐occupying haemorrhagic transformation or associated intracranial haemorrhage; recent Botox injection to upper limb or planned Botox injection over the course of the 7‐month study duration; MoCA ≤ 20; history of physical or neurological condition that interferes with study procedures or assessment of motor function; contraindications to tDCS; inability to sit in a chair and perform upper limb exercises for one hour at a time; participation in another upper extremity rehab study or tDCS study during the study period; terminal illness; social or personal circumstances that interfere with the ability to return for therapy sessions and follow‐up assessments |
| Interventions | 3 arms
|
| Outcomes | Primary outcome measure
Secondary outcome measure
|
| Starting date | May 2015 |
| Contact information | John Krakauer, MD Johns Hopkins University Baltimore, Maryland, United States, 21205 |
| Notes |
NCT02308852.
| Trial name or title | Improving bi‐manual activities in stroke patients with application of neurostimulation |
| Methods | Randomised cross‐over trial |
| Participants | Estimated enrolment: 100 Inclusion criteria: age between 18 and 95 years; stroke with at least slight deficit Exclusion criteria: epilepsy; contraindications to tDCS and fMRI; presence of metal in the head; inability to understand/complete behavioural tasks; chronic substance abuse; major health condition; pacemaker; pregnancy |
| Interventions | 2 arms
|
| Outcomes | Primary outcome measure
Secondary outcome measures
|
| Starting date | October 2014 |
| Contact information | Yves Vandermeeren, MD,PhD University Hospital CHU Dinant Godinne UcL Namur Yvoir, Belgium, 5530 |
| Notes |
NCT02325427.
| Trial name or title | Changes in cortical excitability associated with upper limb motor recovery ‐ a study of neural strategies employed in motor recovery |
| Methods | RCT with parallel‐group design |
| Participants | Estimated enrolment: 119 Inclusion criteria: age 21 to 80 years; first‐ever hemiplegic stroke < 2 weeks prior to study enrolment; UE‐FM between 0 and 45; MMSE ≥ 24; ability to provide informed consent Exclusion criteria: pregnancy; cardiac pacemakers; metal implants; history of epilepsy; sensorimotor impairments due to other causes than stroke; uncontrolled medical conditions; diabetes mellitus and unstable angina; major depression and history of psychotic disorders |
| Interventions | 3 arms
|
| Outcomes | Primary outcome measure
Secondary outcome measures
|
| Starting date | November 2014 |
| Contact information | Effie Chew, MD National University Hospital Singapore, Singapore, 119074 |
| Notes |
NCT02389608.
| Trial name or title | The immediate effect of electrical stimulation transcranial direct current (tDCS) associated with the use of FES, in muscle activity of the tibialis anterior muscle, balance and plantar pressure distribution of individuals with hemiparesis due to stroke ‐ randomised, double blind |
| Methods | Randomised cross‐over trial |
| Participants | Estimated enrolment: 30 Inclusion criteria: age above 20 years; hemiparesis due to stroke; ability to maintain a standing position without an assistance device for at least 60 seconds; signed informed consent Exclusion criteria: other health condition or use of medication affecting balance; positive cutoff point for cognitive deficit (MMSE); illiteracy; Wernicke's aphasia; reduced ankle mobility due to history of ankle fracture and use of pins in ankle; strength less than grade 1 in the tibialis anterior muscle; tDCS contraindication; skin infection at the tDCS/FES site; anaesthesia/hyperaesthesia at FES site |
| Interventions | Each participant will undergo all of the following conditions
|
| Outcomes | Outcomes will be recorded at baseline and at 1 year after the end of intervention period Primary outcome measure
|
| Starting date | January 2015 |
| Contact information | Aline M.A Fruhauf University Nove de Julho São Paulo, SP, Brazil |
| Notes |
NCT02393651.
| Trial name or title | Late LTP‐like plasticity effects of tDCS in subacute stroke patients |
| Methods | RCT with parallel‐group design |
| Participants | Estimated enrolment: 48 Inclusion criteria: age 18 to 79 years; single ischaemic stroke, documented by a neurologist; subacute stroke within 1 to 3 weeks poststroke; acute hemiparesis with Fugl‐Meyer Stage < IV Exclusion criteria: absence of MEPs; absence of voluntarily movement (Fugl‐Meyer Stage < III); head injury or metal in the head; history of cranial irradiation; history of epilepsy; pacemaker; anticonvulsant or neuroleptic medication; substance abuse; inability to understand instructions history of psychiatric disorders |
| Interventions | 2 arms
|
| Outcomes | Primary outcome measure
Secondary outcome measures
|
| Starting date | March 2015 |
| Contact information | Rick van der Vliet, MSc Rijndam Rotterdam, Zuid‐Holland, Netherlands, 3015LJ |
| Notes |
NCT02399540.
| Trial name or title | Late LTP‐like plasticity effects of tDCS in chronic stroke patients |
| Methods | RCT with parallel‐group design |
| Participants | Estimated enrolment: 80 Inclusion criteria: aged 18 to 79 years; stroke onset > 6 months prior to enrolment; motor deficit in the upper limb due to the stroke Exclusion criteria: absence of MEPs; absence of voluntarily movement (Fugl‐Meyer Stage < III); head injury or metal in the head; history of cranial irradiation; history of epilepsy; pacemaker; anticonvulsant or neuroleptic medication; substance abuse; inability to understand instructions; history of psychiatric disorders |
| Interventions | 4 arms
|
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | March 2015 |
| Contact information | Contact: Rick van der Vliet, MSc Rijndam Rotterdam, Zuid‐Holland, Netherlands, 3015LJ |
| Notes |
NCT02401724.
| Trial name or title | A randomised trial of non‐invasive brain stimulation (NIBS) in stroke survivors |
| Methods | RCT with parallel‐group design |
| Participants | Estimated enrolment: 60 Inclusion criteria: aged between 18 and 90 years; ischaemic stroke affecting right hemisphere, radiologically confirmed; persistent neglect > 1 months after stroke, confirmed by BIT; prestroke functional independence (mRS 0 to 2) Exclusion criteria: patients who do not understand English; bilateral infarcts, radiologically confirmed; MoCA < 26; other neurological diseases; significant morbidity; alcohol excess; exclusion criteria for tDCS |
| Interventions | 4 arms
|
| Outcomes | Primary outcome measure
Secondary outcome measures:
|
| Starting date | March 2015 |
| Contact information | Monika Harvey, BSc (Hons), MSc, PhD NHS Greater Glasgow and Clyde |
| Notes |
NCT02416791.
| Trial name or title | Robotic therapy and transcranial direct current stimulation in patients with stroke |
| Methods | RCT with parallel‐group design |
| Participants | Estimated enrolment: 51 Inclusion criteria: stroke onset 3 to 9 weeks prior to enrolment, radiologically confirmed; UE‐FM between 7 and 38; ability to provide informed consent; ability to comply with the schedule of interventions and evaluation of the protocol Exclusion criteria: MAS > 3 in the paretic arm; upper limb plegia; uncontrolled medical conditions; pregnancy; seizures; pacemakers; other neurological disorders; psychiatric illnesses; aphasia compromising comprehension of the experimental protocol; MMSE < 23 for patients with > 1 year of education and MMSE < 19 for patients with > 1 year of education; hemineglect |
| Interventions | 2 arms
|
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | June 2015 |
| Contact information | Thais Midori K Tokuno Hospital das Clínicas São Paulo, SP, Brazil, 05403900 |
| Notes |
NCT02422173.
| Trial name or title | Effects of different montages of transcranial direct current stimulation on the risk of falls and lower limb function for acute stroke patients: a randomised controlled trial |
| Methods | RCT with parallel‐group design |
| Participants | Estimated enrolment: 60 Inclusion criteria: clinical diagnosis of acute stroke; ability to walk 10 metres independently; high risk of falling Exclusion criteria: severe functional limitations; cognitive impairment |
| Interventions | 4 arms
|
| Outcomes | Primary outcome measure
Secondary outcome measures
|
| Starting date | January 2015 |
| Contact information | Suellen Marinho Andrade Federal University of Paraíba |
| Notes |
NCT02455427.
| Trial name or title | Safety of transcranial direct current stimulation in the subacute phase after stroke |
| Methods | RCT with parallel‐group design |
| Participants | Estimated enrolment: 40 Inclusion criteria: age between 18 and 80 years; ischaemic stroke, radiologically confirmed; onset between 72 hours and 6 weeks prior to enrolment; unilateral paresis of upper limb; NIHSS between 5 and 15; NIHSS score of at least 1 point in items 5a or 5b; written informed consent Exclusion criteria: lesions affecting the corticomotor pathway in the hemisphere contralateral to the stroke; use of neuroleptics or other psychoactive drugs; except antidepressants; advanced systemic diseases; other neurologic diseases except migraine; mRS < 2 prior to stroke; advanced systemic diseases; uncontrolled medical conditions; contraindications for physical therapy; pregnancy; absolute or relative contraindications for tDCS |
| Interventions | 2 arms
|
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | May 2015 |
| Contact information | Adriana B Conforto, MD Phd Hospital Israelita Albert Einstein Brazil |
| Notes |
NTR3315.
| Trial name or title | The effect of noninvasive brain stimulation on lower limb motor skill acquisition |
| Methods | Randomised controlled double‐blind trial with parallel assignment |
| Participants | 60 participants 18 years of age or older with hemiparesis due to a first‐time ever ischaemic subcortical stroke at least 6 months before study enrolment, good vision on 2 metre distance, being able to stand and to make stepping movements for 42 minutes, independent walkers with clear walking impairment Exclusion criteria: metallic implants in the brain, presence of severe or frequent headache, other neurological disorders or orthopaedic problems, history of cardiac conditions that interfere with physical load |
| Interventions | 3 training sessions with 3 different interventions of tDCS during the first 10 minutes of each training session
|
| Outcomes | Primary outcome measure
Secondary outcome measure
|
| Starting date | 1 March 2012 |
| Contact information | Edwin van Asseldonk, e.h.f.vanasseldonk@utwente.nl |
| Notes |
Paquette 2013.
| Trial name or title | Not stated by the authors |
| Methods | Not clearly stated by the authors |
| Participants | Estimated enrolment: not stated by the authors Inclusion criteria: not stated by the authors Exclusion criteria: not stated by the authors |
| Interventions | 4 arms
|
| Outcomes | CAHAI |
| Starting date | Not stated by the authors |
| Contact information | None known |
| Notes | Conference abstract only |
Sattler 2012.
| Trial name or title | Not stated by the authors |
| Methods | Study design: randomised double‐blind sham‐controlled trial (parallel‐group design) |
| Participants | Estimated enrolment: 20 patients within the first month of a cortical or subcortical stroke |
| Interventions | 2 arms
|
| Outcomes | Motor performance as measured by JTT at baseline, after the intervention period and at 5, 15 and 30 days of follow‐up Cortical excitability at baseline |
| Starting date | Not stated by the authors |
| Contact information | None known |
| Notes | Conference abstract only |
6MWT: Six minute walking test 9‐HPT: Nine‐Hole Peg Test 10MWT: 10‐Meter Walk Test A‐tDCS: anodal transcranial direct current stimulation AAP: Adelaide Activities Profile
AMES: Assisted Motion with Enhanced Sensation device ARAT: Action Research Arm Test BBS: Berg Balance Scale BBT: Box and Block Test BDI: Beck Depression Inventory BI: Barthel Index BIT: Behavioural Inattention Test BTN: Negligence Battery Test C‐tDCS: cathodal transcranial direct current stimulation CIMT: constraint‐induced movement therapy
CMSA: Chedoke‐McMaster Stroke Assessment CNS: central nervous system COP: centre of pressure DTI: Diffusion Tensor Imaging EEG: electroencephalography EMG: electromyography FBCSP: Filter Bank Common Spatial Pattern FIM: Functional Independence Measure FMA: Fugl‐Meyer Assessment fMRI: functional magnetic resonance imaging FSS: Fatigue Severity Scale GABA: gamma‐aminobutyric acid ITT: intention‐to‐treat JTT: Jebsen Taylor Hand Function Test M1: primary motor cortex
mA: milliampere MEP: motor‐evoked potentials MAL: Motor Activity Log MAS: Motor Assessment Scale MCA: middle cerebral artery mCIMT: modified constraint‐induced movement therapy MI‐BCI: motor imagery brain‐computer interface MoCA: Montreal Cognitive Assessment MMSE: Mini Mental State Examination MRC: Medical Research Council MRI: magnetic resonance imaging NIHSS: National Institutes of Health Stroke Scale NMDA: N‐methyl‐D‐aspartate OT: occupational therapy
PPT: Purdue Pegboard Test RCT: randomised controlled trial ROM: range of motion RMAB: Rivermead Motor Assessment Battery rEPNS: repetitive peripheral nerve stimulation rNSA: revised Nottingham Sensory Assessment rTMS: SAH: subarachnoidal haemorrhage SIS: Stroke Impact Scale SS‐QOL: Stroke Specific Quality of Life STST: Sit to Stand Test TBI: traumatic brain injury TCT: Trunk Control Test tDCS: transcranial direct current stimulation TMS: transcranial magnetic stimulation TUG: Timed Up and Go Test UBS: Unified Balance Scale UE‐FM: Upper Extremity Fugl‐Meyer VAS: Visual Analogue Scale WMFT: Wolf Motor Function Test
Differences between protocol and review
We calculated risk differences (RDs) instead of risk ratios (RRs) for binary outcomes because of the low rates of dropouts and adverse events.
Contributions of authors
All review authors contributed to the conception and design of the protocol and approved the final draft of the review.
All review authors participated in all stages of the review. BE was involved in screening titles and abstracts of publications identified by the searches; BE and JM extracted trial and outcome data from the selected trials and analysed outcome data. JM and MP were involved in assessing the methodological quality of the studies. All review authors participated in interpreting the results.
Sources of support
Internal sources
Gesundheitswissenschaften/Public Health, Medizinische Fakultät Carl Gustav Carus der TU Dresden, Fetscherstr. 74, 01307 Dresden, Germany.
Wissenschaftliches Institut, Private Europäische Medizinische Akademie der Klinik Bavaria in Kreischa GmbH, An der Wolfsschlucht 1‐2, 01731 Kreischa, Germany.
Lehrstuhl Therapiewissenschaften, SRH Fachhochschule für Gesundheit Gera gGmbH, Hermann‐Drechsler‐Str. 2, 07548 Gera, Germany.
External sources
No sources of support supplied
Declarations of interest
Two review authors (Jan Mehrholz and Marcus Pohl) were involved in conducting and analysing the largest of the included trials (Hesse 2011). Bernhard Elsner: none known. Joachim Kugler: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Ang 2012 {published and unpublished data}
- Ang KK, Guan C, Phua KS, Wang C, Teh I, Chen CW, et al. Transcranial direct current stimulation and EEG‐based motor imagery BCI for upper limb stroke rehabilitation. Proceedings of the 34th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 2012 Aug28 ‐ Sept 1; San Diego. 2012. [1557‐170X] [DOI] [PubMed]
- Ang KK, Guan C, Phua KS, Wang C, Zhao L, Teo WP, et al. Facilitating effects of transcranial direct current stimulation on motor imagery brain‐computer interface with robotic feedback for stroke rehabilitation. Archives of Physical Medicine and Rehabilitation 2015;96(3 Suppl):S79‐87. [1532‐821X: (Electronic)] [DOI] [PubMed] [Google Scholar]
Au‐Yeung 2014 {published data only}
- Au‐Yeung S, Wang J, Chen Y, Chua E. TDCS to primary motor area improves hand dexterity and selective attention in chronic stroke. Clinical Neurophysiology. 2014; Vol. 125:S142. [1388‐2457] [DOI] [PubMed]
- Au‐Yeung SSY, Wang J, Ye C, Chua E. Transcranial direct current stimulation to primary motor area improves hand dexterity and selective attention in chronic stroke. American Journal of Physical Medicine & Rehabilitation 2014;95(12):1057‐64. [0894‐9115] [DOI] [PubMed] [Google Scholar]
- Park E, Kwon TG, Chang WH, Kim YH. Non‐invasive brain stimulation for motor function of chronic stroke patients. International Stroke Conference Poster Abstracts. 2014. [0039‐2499]
Boggio 2007a {published data only}
- Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual‐Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restorative Neurology and Neuroscience 2007;25(2):123‐9. [PubMed] [Google Scholar]
Bolognini 2011 {published and unpublished data}
- Bolognini N, Vallar G, Casati C, Latif LA, El‐Nazer R, Williams J, et al. Neurophysiological and behavioral effects of tDCS combined with constraint‐induced movement therapy in poststroke patients. Neurorehabilitation and Neural Repair 2011;25(9):819‐29. [DOI] [PubMed] [Google Scholar]
Cha 2014 {published data only}
- Cha HK, Ji SG, Kim MK, Chang JS. Effect of transcranial direct current stimulation of function in patients with stroke. Journal of Physical Therapy Science 2014;26(3):363‐5. [0915‐5287] [DOI] [PMC free article] [PubMed] [Google Scholar]
Di Lazzaro 2014a {published data only}
- Lazzaro V, Dileone M, Capone F, Pellegrino G, Ranieri F, Musumeci G, et al. Immediate and late modulation of interhemispheric imbalance with bilateral transcranial direct current stimulation in acute stroke. Brain Stimulation 2014;7(6):841‐8. [1935‐861X] [DOI] [PubMed] [Google Scholar]
Di Lazzaro 2014b {published data only}
Fregni 2005a {published data only}
- Fregni F, Boggio PS, Mansur CG, Wagner T, Ferreira MJL, Lima MC, et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport 2005;16(14):1551‐5. [DOI] [PubMed] [Google Scholar]
Fusco 2013a {published and unpublished data}
- Fusco A, Angelis D, Morone G, Maglione L, Paolucci T, Bragoni M, et al. The ABC of tDCS: effects of anodal, bilateral and cathodal montages of transcranial direct current stimulation in patients with stroke ‐ a pilot study. Stroke Research and Treatment 2013 Jan 8 [Epub ahead of print]. [2090‐8105] [DOI] [PMC free article] [PubMed]
Fusco 2014 {published data only}
- Fusco A, Assenza F, Iosa M, Izzo S, Altavilla R, Paolucci S, et al. The ineffective role of cathodal tDCS in enhancing the functional motor outcomes in early phase of stroke rehabilitation: an experimental trial. BioMed Research International 2014 May 5 [Epub ahead of print]. [2314‐6133] [DOI] [PMC free article] [PubMed]
- Fusco A, Iosa M, Venturiero V, Angelis D, Morone G, Maglione L, et al. After vs. priming effects of anodal transcranial direct current stimulation on upper extremity motor recovery in patients with subacute stroke. Restorative Neurology and Neuroscience 2014;32(2):301‐12. [0922‐6028] [DOI] [PubMed] [Google Scholar]
Geroin 2011 {published and unpublished data}
- Geroin C, Picelli A, Munari D, Waldner A, Tomelleri C, Smania N. Combined transcranial direct current stimulation and robot‐assisted gait training in patients with chronic stroke: a preliminary comparison. Clinical Rehabilitation 2011;25(6):537‐48. [DOI] [PubMed] [Google Scholar]
- NCT01040299. Mechanized gait trainer combine transcranial galvanic stimulation (tDCS) in chronic stroke. ClinicalTrials.gov/show/NCT01040299 (accessed 4 March 2013).
Hesse 2011 {published data only}
- Hesse S, Waldner A, Mehrholz J, Tomelleri C, Pohl M, Werner C. Combined transcranial direct current stimulation and robot‐assisted arm training in subacute stroke patients: an exploratory, randomized multicenter trial. Neurorehabilitation and Neural Repair 2011;25(9):838‐46. [DOI] [PubMed] [Google Scholar]
- Hesse S, Waldner A, Mehrholz J, Tomelleri C, Pohl M, Werner C. Combined transcranial direct current stimulation and robot‐assisted arm training in subacute stroke patients: an exploratory, randomized multicenter trial. [German]. Neurologie und Rehabilitation 2012; Vol. 18, issue 4:242‐7. [0947‐2177] [DOI] [PubMed]
- NCT00407667. Transcranial galvanic stimulation after stroke. ClinicalTrials.gov/show/NCT00407667 (accessed 4 March 2013).
- Waldner A, Werner C, Mehrholz J, Tomelleri C, Pohl M, Hesse S. Combined transcranial direct current stimulation and robot‐assisted arm training in subacute stroke patients. Neurorehabilitation and Neural Repair 2012;26(4):400 (Abst 009). [DOI] [PubMed] [Google Scholar]
- Werner C, Hesse S. Transcranial direct current stimulation (tDCS) and repetitive arm training to enhance motor function of the severely affected arm after stroke: a double blind placebo RCT (TRAGAT) ‐ preliminary results. Cerebrovascular Diseases 2009;27 Suppl 6:147. [Google Scholar]
- Werner C, Hesse S, Kroczek G, Waldner A. Transcranial galvanic stimulation (tDCS) + robot‐assisted therapy to improve upper limb impairment after stroke: a double‐blind placebo controlled trial: preliminary results. Neurorehabilitation and Neural Repair 2008;22(5):550‐1. [Google Scholar]
Jo 2008 {published data only}
- Jo JM, Kim YH, Ko MH, Ohn SH, Joen B, Lee KH. Enhancing the working memory of stroke patients using tDCS. American Journal of Physical Medicine & Rehabilitation 2009; Vol. 88, issue 5:404‐9. [DOI] [PubMed]
- Jo JM, Ohn SH, Ko MH, Kim GM, Yoo WK, Woo PK, et al. Effects of transcranial direct current stimulation on verbal working memory in patients with stroke. Journal of Rehabilitation Medicine 2008;40(Suppl 46):146. [Google Scholar]
Kang 2008a {published and unpublished data}
- Kang EK, Baek MJ, Kim S, Paik NJ. Non‐invasive cortical stimulation improves post‐stroke attention decline. Restorative Neurology and Neuroscience 2009;27(6):645‐50. [DOI] [PubMed] [Google Scholar]
- Kang EK, Lim JY, Baek MJ, Kim SY, Paik NJ. The effect of anodal transcranial direct current stimulation on post‐stroke attention decline. International Journal of Stroke 2008;3(Suppl 1):351. [Google Scholar]
Khedr 2013 {published data only}
- Khedr E, Shawky O, Tohamy A, Darwish E, Hamady D. Effect of anodal versus cathodal transcranial direct current stimulation on stroke recovery: a pilot randomized controlled trial. European Journal of Neurology 2012;19:185. [1351‐5101] [Google Scholar]
- Khedr EM, Shawky OA, El‐Hammady DH, Rothwell JC, Darwish ES, Mostafa OM, et al. Effect of anodal versus cathodal transcranial direct current stimulation on stroke rehabilitation: a pilot randomized controlled trial. Neurorehabilitation and Neural Repair 2013 Apr 22 [Epub ahead of print]. [DOI: 10.1177/1545968313484808] [DOI] [PubMed]
- NCT01601392. Anodal and cathodal transcranial direct current stimulation in stroke recovery. ClinicalTrials.gov/show/NCT01601392 (accessed 4 March 2013).
Kim 2009 {published and unpublished data}
- Kim DY, Ohn SH, Yang EJ, Park C‐I, Jung KJ. Enhancing motor performance by anodal transcranial direct current stimulation in subacute stroke patients. American Journal of Physical Medicine & Rehabilitation 2009;88(10):829‐36. [DOI] [PubMed] [Google Scholar]
- Kim DY, Park CI, Ohn SH, Yang EJ. Effects of transcranial direct current stimulation on motor performance in subacute poststroke patients. Neurorehabilitation and Neural Repair 2006;20(1):166. [Google Scholar]
Kim 2010 {published data only}
- Kim DY, Lim JY, Kang EK, You DS, Oh MK, Oh BM, et al. Effect of transcranial direct current stimulation on motor recovery in patients with subacute stroke. American Journal of Physical Medicine & Rehabilitation 2010;89(11):879‐86. [DOI] [PubMed] [Google Scholar]
Ko 2008 {published data only}
- Ko MH, Han SH, Park SH, Seo JH, Kim YH. Improvement of visual scanning after DC brain polarization of parietal cortex in stroke patients with spatial neglect. Neuroscience Letters 2008;448(2):171‐4. [0304‐3940] [DOI] [PubMed] [Google Scholar]
Lee 2014 {published data only}
- Lee SJ, Chun MH. Combination transcranial direct current stimulation and virtual reality therapy for upper extremity training in patients with subacute stroke. Archives of Physical Medicine & Rehabilitation 2014;95(3):431‐8. [0003‐9993] [DOI] [PubMed] [Google Scholar]
Lindenberg 2010 {published data only}
- Lindenberg R, Nair D, Zhu LL, Renga V, Schlaug G. Non‐invasive motor cortex stimulation after stroke: the effect of number of sessions on outcome. Stroke 2011;42(3):e72. [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology 2010;75(24):2176‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Zhu LL, Schlaug G. Combined central and peripheral stimulation to facilitate motor recovery after stroke: the effect of number of sessions on outcome. Neurorehabilitation and Neural Repair 2012;26(5):479‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahmoudi 2011 {published data only}
- Mahmoudi H, Borhani HA, Petramfar P, Jahanshahi S, Salehi Z, Fregni F. Transcranial direct current stimulation: electrode montage in stroke. Disability and Rehabilitation 2011;33(15‐16):1383‐8. [DOI] [PubMed] [Google Scholar]
Nair 2011 {published data only (unpublished sought but not used)}
- NCT00792428. Non‐invasive brain stimulation and occupational therapy to enhance stroke recovery. ClinicalTrials.gov/show/NCT00792428 (accessed 4 March 2013).
- Nair D, Renga V, Hamelin S, Pascual‐Leone A, Schlaug G. Improving motor function in chronic stroke patients using simultaneous occupational therapy and TDCS. Stroke 2008;39(2):542. [Google Scholar]
- Nair DG, Hamelin S, Pascual‐Leone A, Schlaug G, Israel B. Transcranial direct current stimulation in combination with occupational therapy for 5 consecutive days improves motor function in chronic stroke patients. Stroke 2007;38(2):518. [Google Scholar]
- Nair DG, Renga V, Lindenberg R, Zhu L, Schlaug G. Optimizing recovery potential through simultaneous occupational therapy and non‐invasive brain‐stimulation using tDCS. Restorative Neurology and Neuroscience 2011;29(6):411‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renga V, Lindenberg R, Zhu L, Nair D, Schlaug G. Facilitating stroke recovery using simultaneous occupational therapy and bi‐hemispheric brain‐stimulation. Stroke 2009;40(4):e150. [Google Scholar]
- Schlaug G. TDCS+OT. Non‐invasive brain stimulation and occupational therapy to enhance stroke recovery. Stroke Trials Registry, Internet Stroke Center: www.strokecenter.org/trials/ 2006. [CENTRAL: CN‐00709259]
- Schlaug G, Betzler F, Zhu L, Lindenberg R. Plastic changes in corticospinal motor tracts are related to motor improvement in chronic stroke patients. Stroke 2010;41(4):e210. [0039‐2499] [Google Scholar]
Park 2013 {published data only}
- Kim HM, Lee SI, Chun MH. The effects of direct current brain polarization on motor recovery of lower extremity in stroke. Archives of Physical Medicine and Rehabilitation 2011, issue 10:1715.
- Park DH. The effects of direct current brain polarization on motor recovery of lower extremity in stroke. Archives of Physical Medicine and Rehabilitation 2013, issue 9 Suppl 1:S241‐S242.
- Park SH, Koh EJ, Choi HY, Ko MH. A double‐blind, sham‐controlled, pilot study to assess the effects of the concomitant use of transcranial direct current stimulation with the computer assisted cognitive rehabilitation to the prefrontal cortex on cognitive functions in patients with stroke. Journal of Korean Neurosurgical Society 2013;56(6):484‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qu 2009 {published data only (unpublished sought but not used)}
- Qu YP, Wu DY, Tu XQ, Qian L, Yang YB, Geng H. Effect of transcranial direct current stimulation on relieving upper‐limb spasticity after stroke [Chinese]. Chinese Journal of Cerebrovascular Diseases 2009;6(11):586‐9. [Google Scholar]
Rossi 2013 {published and unpublished data}
- Rossi C, Sallustio F, Legge S, Rizzato B, Stanzione P, Koch G. Anodal transcranial direct current stimulation (TDCS) of the affected hemisphere in patients with acute ischemic stroke: a preliminary study. Cerebrovascular Diseases. Proceedings of the 19th European Stroke Conference; Barcelona, Spain 2010;34:247‐8. [1015‐9770] [Google Scholar]
- Rossi C, Sallustio F, Legge S, Stanzione P, Koch G. Transcranial direct current stimulation of the affected hemisphere does not accelerate recovery of acute stroke patients. European Journal of Neurology 2013;20(1):202‐4. [DOI] [PubMed] [Google Scholar]
Sohn 2013 {published data only}
- Sohn M, Jee S, Kim W. Effect of transcranial direct current stimulation on postural stability and lower extremity strength in hemiplegic stroke patients. Annals of Rehabilitation Medicine 2013;37(6):759‐65. [2234‐0645] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sunwoo 2013 {published data only}
- Sunwoo H, Kim YH, Chang WH, Noh S, Kim EJ, Ko MH. Effects of dual transcranial direct current stimulation on post‐stroke unilateral visuospatial neglect. Neuroscience Letters 2013;554:94‐8. [DOI] [PubMed] [Google Scholar]
Tahtis 2012 {published data only}
- Kaski DM, Tahtis VM, Seemungal BM. The effect of single session bi‐cephalic transcranial direct current stimulation on gait performance in subacute stroke. Journal of Neurology 2014;261:S165. [0340‐5354] [DOI] [PubMed] [Google Scholar]
- Tahtis V, Kaski D, Seemungal BM. The effect of single session bi‐cephalic transcranial direct current stimulation on gait performance in sub‐acute stroke. Cerebrovascular Diseases 2012;33 Suppl 2:42 (Abst 3). [DOI] [PubMed] [Google Scholar]
- Tahtis V, Kaski D, Seemungal BM. The effect of single session bi‐cephalic transcranial direct current stimulation on gait performance in sub‐acute stroke: a pilot study. Restorative Neurology and Neuroscience 2014;32(4):527‐32. [0922‐6028] [DOI] [PubMed] [Google Scholar]
Tedesco Triccas 2015b {published and unpublished data}
- NCT01405378. Non‐invasive brain stimulation for people with stroke. ClinicalTrials.gov/show/NCT01405378 (accessed 4 March 2013).
- Triccas TL, Burridge J, Hughes AM, Verheyden G, Rothwell J. Combining transcranial direct current stimulation with robot therapy for the impaired upper limb after sub‐acute stroke. Clinical Neurophysiology 2011;122:S149‐50. [Google Scholar]
- Triccas TL, Burridge JH, Hughes A, Verheyden G, Desikan M, Rothwell J. A double‐blinded randomised controlled trial exploring the effect of anodal transcranial direct current stimulation and uni‐lateral robot therapy for the impaired upper limb in sub‐acute and chronic stroke. Neurorehabilitation 2015;37(2):181‐91. [DOI] [PubMed] [Google Scholar]
Viana 2014 {published data only}
- Viana RT, Laurentino GEC, Souza RJP, Fonseca JB, Silva Filho EM, Dias SN, et al. Effects of the addition of transcranial direct current stimulation to virtual reality therapy after stroke: A pilot randomized controlled trial. Neurorehabilitation 2014;34(3):437‐46. [1053‐8135] [DOI] [PubMed] [Google Scholar]
Wang 2014 {published data only}
- Wang QM, Cui H, Han SJ, Black‐Schaffer R, Volz MS, Lee YT, et al. Combination of transcranial direct current stimulation and methylphenidate in subacute stroke. Neuroscience Letters 2014;569:6‐11. [0304‐3940] [DOI] [PubMed] [Google Scholar]
Wu 2013a {published and unpublished data}
- ChiCTR‐TRC‐11001367. Effects on relieving upper‐limb spasticity after stroke using transcranial direct current stimulation. apps.who.int/trialsearch/Trial.aspx?TrialID=ChiCTR‐TRC‐11001367 (accessed 4 March 2013).
- Wu D, Qian L, Zorowitz R, Zhang L, Qu Y, Yuan Y. Effects on decreasing upper‐limb poststroke muscle tone using transcranial direct current stimulation: a randomized sham‐controlled study. Archives of Physical Medicine and Rehabilitation 2013;94:1‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Boggio 2007b {published data only}
- Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual‐Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restorative Neurology and Neuroscience 2007;25(2):123‐9. [PubMed] [Google Scholar]
Bradnam 2012 {published data only}
- Bradnam LV, Stinear CM, Barber PA, Byblow WD. Contralesional hemisphere control of the proximal paretic upper limb following stroke. Cerebral Cortex 2012;22(11):2662‐71. [1460‐2199] [DOI] [PMC free article] [PubMed] [Google Scholar]
Byblow 2011 {published data only}
- Byblow W, Bradnam L, Barber PA, Stinear C. Ipsilateral effects of direct current stimulation. Proceedings of the 14th European Congress of Clinical Neurophysiology and the 4th International Conference on Transcranial Magnetic and Direct Current Stimulation, Rome, Italy. 2011.
Celnik 2009 {published data only}
- Celnik P, Paik N‐J, Vandermeeren Y, Dimyan M, Cohen LG. Effects of combined peripheral nerve stimulation and brain polarization on performance of a motor sequence task after chronic stroke. Stroke 2009;40(5):1764‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik N, Celnik P, Cohen L. Effects of combined peripheral nerve stimulation and noninvasive anodal cortical stimulation on motor learning in chronic stroke. Archives of Physical Medicine and Rehabilitation 2006;87:E2. [Google Scholar]
Danzl 2012 {published data only}
- Danzl MM, Chelette K, Lee K, Lykins D, Sawaki L. Non‐invasive brain stimulation paired with a novel locomotor training in chronic stroke: A feasibility study. Archives of Physical Medicine and Rehabilitation 2012;93(10):E19‐20. [0003‐9993] [Google Scholar]
- Danzl MM, Chelette KC, Lee K, Lykins D, Sawaki L. Brain stimulation paired with novel locomotor training with robotic gait orthosis in chronic stroke: a feasibility study. Neurorehabilitation 2013;33(1):67‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 2009 {published data only}
- Edwards DJ, Krebs HI, Rykman A, Zipse J, Thickbroom GW, Mastaglia FL, et al. Raised corticomotor excitability of M1 forearm area following anodal tDCS is sustained during robotic wrist therapy in chronic stroke. Restorative Neurology and Neuroscience 2009;27(3):199‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gandiga 2006 {published data only}
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double‐blind sham‐controlled clinical studies in brain stimulation. Clinical Neurophysiology 2006;117:845‐50. [DOI] [PubMed] [Google Scholar]
Giacobbe 2013 {published data only}
- Giacobbe V, Krebs H, Volpe B, Pascual‐Leone A, Rykman A, Zeiarati G, et al. Transcranial direct current stimulation (tDCS) and robotic practice in chronic stroke: the dimension of timing. Neurorehabilitation 2013;33(1):49‐56. [1053: 8135] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goh 2015 {published data only}
- Goh HT, Chan HY, Abdul‐Latif L. After effects of 2 noninvasive brain stimulation techniques on corticospinal excitability in persons with chronic stroke: a pilot study. Journal of Neurologic Physical Therapy 2015;39(1):15‐22. [1557‐0576] [DOI] [PubMed] [Google Scholar]
Gurchin 1988 {published data only}
- Gurchin FA, Medvedev SV, Puzenko VI. Cortical electrostimulation in skull and brain injury [Russian]. Fiziologiia Cheloveka 1988;14(2):324‐6. [PubMed] [Google Scholar]
Hummel 2005a {published data only}
- Hummel F, Celnik P, Giraux P, Flöel A, Wu W‐H, Gerloff C, et al. Effects of non‐invasive cortical stimulation on skilled motor function in chronic stroke. Brain 2005;128(Pt 3):490‐9. [DOI] [PubMed] [Google Scholar]
- Hummel F, Wu CJ, Flöel A, Gerloff C, Cohen L. Effects of cortical stimulation on motor function in patients with chronic stroke. Neurology 2004;62 Suppl 5:A459‐60 (Abst P06.008). [Google Scholar]
Hummel 2005b {published data only}
- Hummel F, Voller B, Celnik P, Flöel A, Giraux P, Gerloff C, et al. Effects of brain polarization on reaction times and pinch force in chronic stroke. BMC Neuroscience 2006;7:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel F, Voller B, Celnik P, Flöel A, Giraux P, Gerloff C, et al. Effects of cortical stimulation on visuomotor integration and force in stroke. Cerebrovascular Diseases 2006;21:4‐5 (Abst 9). [Google Scholar]
Jayaram 2009 {published data only}
- Jayaram G, Stinear JW. The effects of transcranial stimulation on paretic lower limb motor excitability during walking. Journal of Clinical Neurophysiology 2009;26(4):272‐9. [DOI] [PubMed] [Google Scholar]
Kasashima 2012 {published data only}
- Kasashima Y, Fujiwara T, Matsushika Y, Tsuji T, Hase K, Ushiyama J, et al. Modulation of event‐related desynchronization during motor imagery with transcranial direct current stimulation (tDCS) in patients with chronic hemiparetic stroke. Experimental Brain Research 2012; Vol. 221, issue 3:263‐8. [DOI] [PubMed]
Kharchenko 2001 {published data only}
- Kharchenko EV. Transcranial microelectrostimulation activates fast mechanisms of brain plasticity. Doklady Biological Sciences 2001;378:217‐9. [DOI] [PubMed] [Google Scholar]
Kitisomprayoonkul 2012 {published data only}
- Kitisomprayoonkul W. Transcranial direct current stimulation improves hand sensation in acute stroke. Archives of Physical Medicine and Rehabilitation 2012;93(10):E33. [Google Scholar]
Kumar 2011 {published data only}
- Kumar S, Wagner CW, Frayne C, Zhu L, Selim M, Feng W, et al. Noninvasive brain stimulation may improve stroke‐related dysphagia: a pilot study. Stroke 2011;42(4):1035‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kwon 2012 {published data only}
- Kwon YH, Jang SH. Onsite‐effects of dual‐hemisphere versus conventional single‐hemisphere transcranial direct current stimulation: a functional MRI study. Neural Regeneration Research 2012;7(24):1889‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2012 {published data only}
- Lee YS, Yang HS, Jeong CJ, Yoo YD, Jeong SH, Jeon OK, et al. The effects of transcranial direct current stimulation on functional movement performance and balance of the lower extremities. Journal of Physical Therapy Science 2012;24(12):1215‐8. [0915‐5287] [Google Scholar]
Lefebvre 2013 {published data only}
- Lefebvre S, Laloux P, Peeters A, Desfontaines P, Jamart J, Vandermeeren Y. Dual‐tDCS enhances online motor skill learning and long‐term retention in chronic stroke patients. Frontiers in Human Neuroscience 2013;6:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre S, Thonnard JL, Laloux P, Peeters A, Jamart J, Vandermeeren Y. Single session of dual‐tDCS transiently improves precision grip and dexterity of the paretic hand after stroke. Neurorehabilitation and Neural Repair 2013 Mar 13 [Epub ahead of print]. [DOI] [PubMed]
- Vandermeeren Y, Laloux P, Jamart J, Peeters A, Thonnard J‐L, Lefebvre S. Dual hemisphere tDCS in chronic stroke patients improves 'simple' precision grip and digital dexterity of the paretic hand with a delayed time‐course. Cerebrovascular Diseases 2012;33 Suppl 2:63 (Abst 9). [Google Scholar]
Lefebvre 2015 {published data only}
- Lefebvre S, Dricot L, Laloux P, Gradkowski W, Desfontaines P, Evrard F, et al. Neural substrates underlying stimulation‐enhanced motor skill learning after stroke. Brain 2015;138:149‐63. [0006‐8950] [DOI] [PMC free article] [PubMed] [Google Scholar]
Madhavan 2011 {published data only}
- Madhavan S, Weber KA, Stinear JW. Non‐invasive brain stimulation enhances fine motor control of the hemiparetic ankle: implications for rehabilitation. Experimental Brain Research 2011;209(1):9‐17. [DOI] [PubMed] [Google Scholar]
Manganotti 2011 {published data only}
- Manganotti P, Daloli V, Fiaschi A. Decrease of upper limb spasticity after transcranial direct current stimulation in patients affected by stroke. Proceedings of the 14th European Congress of Clinical Neurophysiology and the 4th International Conference on Transcranial Magnetic and Direct Current Stimulation, Rome, Italy. 2011.
Ochi 2013 {published data only}
- Ochi M, Saeki S, Oda T, Matsushima Y, Hachisuka K. Effects of anodal and cathodal transcranial direct current stimulation combined with robotic therapy on severely affected arms in chronic stroke patients. Journal of Rehabilitation Medicine 2013;45(2):137‐40. [DOI] [PubMed] [Google Scholar]
- Shiraishi J, Seeki S, Ochi M, Oda T, Matsushima Y, Yoshikawa K, et al. Combined robotic therapy with transcranial direct current stimulation. Cerebrovascular Diseases 2012;34 Suppl 1:132 (Abst PP‐171). [Google Scholar]
Paquette 2011 {published data only}
- Paquette C, Radlinska B, Sidel M, Thiel A. Reducing transcallosal inhibition with non‐invasive brain stimulation to improve post‐infarct motor disorders. Movement Disorders 2011;26 Suppl 2:S40. [Google Scholar]
Sheliakin 2006 {published data only}
- Sheliakin AM, Preobrazhenskaia IG, Tiul'kin ON. [Micropolarization of the brain: a noninvasive method for correction of morphological and functional disturbances in acute focal brain lesions and their consequences]. Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova 2006;106(10):27‐37. [PubMed] [Google Scholar]
Stagg 2012a {published data only}
- Stagg C. Transcranial direct current stimulation ‐ evidence for functional improvements in conjunction with brain activation changes in chronic stroke patients. Third UK Stroke Forum Conference 2008:6.
- Stagg CJ, Bachtiar V, O'Shea J, Allman C, Bosnell RA, Kischka U, et al. Cortical activation changes underlying stimulation‐induced behavioural gains in chronic stroke. Brain 2012;135(Pt 1):276‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takeuchi 2012 {published data only}
- Takeuchi N, Tada T, Matsuo Y, Ikoma K. Low‐frequency repetitive TMS plus anodal transcranial DCS prevents transient decline in bimanual movement induced by contralesional inhibitory rTMS after stroke. Neurorehabilitation and Neural Repair 2012;26(8):988‐98. [DOI] [PubMed] [Google Scholar]
Zimerman 2012 {published data only}
- Zimerman M, Heise KF, Hoppe J, Cohen LG, Gerloff C, Hummel FC. Modulation of training by single‐session transcranial direct current stimulation to the intact motor cortex enhances motor skill acquisition of the paretic hand. Stroke 2012;43(8):2185‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Brem 2010 {published data only}
- Brem AK, Speight I, Jaencke L. Impact of tDCS on motor function in acute stroke. 6th World Congress of Neurorehabilitation 2010:123‐7.
Miller 2013 {published data only}
- Miller J, Marquez J, Vliet P, Lagopoulos J, Parsons M. Transcranial Direct Current Stimulation: A randomised controlled trial to investigate the effects on upper limb function in chronic stroke. International Journal of Stroke 2013:22.
Park 2014 {published data only}
- Park E, Kwon TG, Chang WH, Kim YH. Non‐invasive brain stimulation for motor function of chronic stroke patients. International Stroke Conference Poster Abstracts. 2014. [0039‐2499]
References to ongoing studies
ACTRN12613000109707 {published and unpublished data}
- ACTRN12613000109707. Standard upper limb therapy treatment with or without non‐invasive brain stimulation to assist recovery after stroke. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12613000109707 (accessed 5 March 2013).
Chelette 2012 {published data only}
- Chelette K, Carrico C, Nichols L, Sawaki L. Optimizing transcranial direct current stimulation for motor recovery from severe post‐stroke hemiparesis: early results from an ongoing clinical trial. Archives of Physical Medicine and Rehabilitation 2012;93(10):E20. [0003‐9993] [Google Scholar]
ChiCTR‐TRC‐11001398 {published data only}
- ChiCTR‐TRC‐11001398. Effect of transcranial direct current stimulation on recovery of upper‐limb function after stroke. http://apps.who.int/trialsearch/Trial.aspx?TrialID=ChiCTR‐TRC‐11001398 (accessed 4 March 2013).
ChiCTR‐TRC‐11001490 {published data only}
- ChiCTR‐TRC‐11001490. Using transcranial direct current stimulation to treat ataxia and balance impairment after stroke. apps.who.int/trialsearch/Trial.aspx?TrialID=ChiCTR‐TRC‐11001490 (accessed 4 March 2013).
NCT00542256 {published data only}
- NCT00542256. tDCS and physical therapy in stroke. http://clinicaltrials.gov/ct2/show/NCT00542256?term=tDCS+and+physical+therapy+in+stroke&rank=1 (accessed 4 March 2013).
NCT00783913 {published data only}
- NCT00783913. Using transcranial direct current stimulation (tDCS) to enhance the benefit of movement training in stoke patients. http://ClinicalTrials.gov/show/NCT00783913 (accessed 4 March 2013).
NCT00853866 {published data only}
- NCT00853866. Enhancement of motor function with reboxetine and transcranial direct current stimulation (STIMBOX). http://clinicaltrials.gov/ct2/show/NCT00853866 (accessed 4 March 2013).
NCT00909714 {published data only}
- NCT00909714. Neuroregeneration enhanced by transcranial direct current stimulation (TDCS) in stroke. http://ClinicalTrials.gov/show/NCT00909714 (accessed 4 March 2013).
NCT01007136 {published data only}
- Hodics T, Cohen L, Upreti B, Alex A, Kowalske K, Hart J, et al. Enrollment in early brain stimulation arm motor recovery studies is limited primarily by stimulation‐unrelated exclusions including ethnic and racial characteristics. Stroke 2012;43(2):(Abst 2439). [Google Scholar]
- Hodics T, Hidler J, Xu B, Kowalske K, Hart J, Briggs R, et al. Transcranial direct current stimulation (tDCS) enhanced stroke recovery and cortical reorganization. In: International Stroke Conference; San Antonio, Texas; February 23‐26, 2010. [CENTRAL: CN‐00747781]
- Hodics T, Upreti B, Alex A, Xu B, Hidler J, Kowalske K, et al. Transcranial direct current stimulation (tDCS) enhanced stroke recovery and cortical reorganization ‐ an ongoing clinical trial. European Journal of Neurology 2011;18(Suppl 2):127. [Google Scholar]
- Hodics TM, Dromerick AW, Pezullo JC, Xu B, Hidler J, Hart J, et al. Transcranial direct current stimulation (tDCS) enhanced stroke recovery and cortical reorganization. Proceedings of the International Stroke Conference. Los Angeles, USA, 2012:(Abst CT P25).
- NCT01007136. Transcranial direct current stimulation (tDCS)‐enhanced stroke recovery. http://ClinicalTrials.gov/show/NCT01007136 (accessed 4 March 2013).
NCT01014897 {published data only}
- NCT01014897. Transcranial direct current stimulation (tDCS) in chronic stroke recovery. http://ClinicalTrials.gov/show/NCT01014897 (accessed 4 March 2013).
NCT01127789 {published data only}
- NCT01127789. The use of transcranial direct current stimulation (tDCS) to study implicit motor learning on patients with brain injury [Withdrawn]. http://ClinicalTrials.gov/show/NCT01127789 (accessed 4 March 2013).
NCT01143649 {published data only}
- NCT01143649. Use of transcranial direct current stimulation (tDCS) coupled with constraint induced movement therapy in stroke patients. http://ClinicalTrials.gov/show/NCT01143649 (accessed 4 March 2013).
NCT01169181 {published data only}
- NCT01169181. AMES + brain stimulation: treatment for profound plegia in stroke. https://clinicaltrials.gov/show/NCT01169181 (accessed 7 September 2015).
NCT01201629 {published data only}
- NCT01201629. Transcranial direct current stimulation (tDCS). http://ClinicalTrials.gov/show/NCT01201629 (accessed 4 March 2013).
NCT01207336 {published data only}
- NCT01207336. Combined tDCS + PNS after acute stroke. http://clinicaltrials.gov/show/NCT01207336 (accessed 4 March 2013).
NCT01356654 {published data only}
- NCT01356654. Transcranial direct current stimulation in stroke rehabilitation. http://ClinicalTrials.gov/show/NCT01356654 (accessed 4 March 2013).
NCT01414582 {published data only}
- NCT01414582. Transcranial stimulation and motor training in stroke rehabilitation. http://ClinicalTrials.gov/show/NCT01414582 (accessed 4 March 2013).
NCT01500564 {published data only}
- Kandel M, Beis JM, Ganis V, Prestini M, Paysant J, Jacquin‐Courtois S. Transcranial direct current stimulation associated with physical therapy after stroke: feasability of a prospective, randomised, double blinded, sham controlled study. Annals of Physical and Rehabilitation Medicine 2011;54:e234. [Google Scholar]
- NCT01500564. Functional interest of non invasive brain stimulation during physiotherapy at a subacute phase post stroke (anodal protocol): ReSTIM. http://ClinicalTrials.gov/show/NCT01500564 (accessed 4 March 2013).
NCT01503073 {published data only}
- NCT01503073. Noninvasive brain stimulation for stroke. http://ClinicalTrials.gov/show/NCT01503073 (accessed 4 March 2012).
NCT01519843 {published data only}
- NCT01519843. Post stroke motor learning. http://ClinicalTrials.gov/show/NCT01519843 (accessed 4 March 2013).
NCT01539096 {published data only}
- NCT01539096. Brain stimulation‐aided stroke rehabilitation: neural mechanisms of recovery. http://ClinicalTrials.gov/show/NCT01539096 (accessed 4 March 2013).
- Plow EB, Cunningham DA, Beall E, Jones S, Wyant A, Bonnett C, et al. Effectiveness and neural mechanisms associated with tDCS delivered to premotor cortex in stroke rehabilitation: Study protocol for a randomized controlled trial. Trials 2013;14(1):331. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01544699 {published data only}
- NCT01544699. Impact of non‐invasive brain stimulation on motor recuperation. http://ClinicalTrials.gov/show/NCT01544699 (accessed 4 March 2013).
NCT01574989 {published data only}
- NCT01574989. Effects of rTMS and tDCS on motor function in stroke. http://ClinicalTrials.gov/show/NCT01574989 (accessed 4 March 2013).
NCT01644929 {published data only}
- NCT01644929. Rehabilitation combined with bihemispheric transcranial direct current stimulation in subacute ischemic stroke (RECOMBINE). http://ClinicalTrials.gov/show/NCT01644929 (accessed 4 March 2013).
NCT01726673 {published data only}
- NCT01726673. Robots paired with tDCS in stroke recovery. http://ClinicalTrials.gov/show/NCT01726673 (accessed 4 March 2012).
NCT01807637 {published data only}
- NCT01807637. Transcranial direct current stimulation for improving gait training in stroke. https://clinicaltrials.gov/show/NCT01807637 (accessed 7 September 2015).
NCT01828398 {published data only}
- NCT01828398. tDCS and robotic therapy in stroke. https://clinicaltrials.gov/show/NCT01828398 (accessed 7 September 2015).
NCT01879787 {published data only}
- NCT01879787. Effects of tDCS combined with mCIMT or mental practice in poststroke patients. https://clinicaltrials.gov/show/NCT01879787 (accessed 7 September 2015).
NCT01883843 {published data only}
- NCT01883843. Efficacy of TOCT and (tDCS) for gait improvement in patients with chronic stroke. https://clinicaltrials.gov/show/NCT01883843 (accessed 7 September 2015).
NCT01897025 {published data only}
- NCT01897025. Combined transcranial direct current stimulation and motor imagery‐based robotic arm training for stroke rehabilitation. https://clinicaltrials.gov/show/NCT01897025 (accessed 7 September 2015).
NCT01945515 {published data only}
- NCT01945515. Robotic‐assisted gait training combined with transcranial direct current stimulation to maximize gait recovery after stroke. https://clinicaltrials.gov/show/NCT01945515 (accessed 7 September 2015).
NCT01969097 {published data only}
- NCT01969097. Efficacy basics of bihemispheric motorcortex stimulation after stroke. https://clinicaltrials.gov/show/NCT01969097 (accessed 7 September 2015).
NCT01983319 {published data only}
- NCT01983319. Transcranial direct current stimulation combined with constraint induced movement therapy and role of GABA activity in stroke recovery. https://clinicaltrials.gov/show/NCT01983319 (accessed 7 September 2015).
NCT02031107 {published data only}
- NCT02031107. Randomized trial of transcranial theta‐burst stimulation and transcranial direct current stimulation. https://clinicaltrials.gov/show/NCT02031107 (accessed 7 September 2015).
NCT02080286 {published data only}
- NCT02080286. Transcranial stimulation (tDCS) and prism adaptation in spatial neglect rehabilitation. https://clinicaltrials.gov/show/NCT02080286 (accessed 7 September 2015).
NCT02109796 {published data only}
- NCT02109796. Effects of tDCS on quadriceps strength after stroke. https://clinicaltrials.gov/show/NCT02109796 (accessed 7 September 2015).
NCT02156635 {published data only}
- NCT02156635. Stroke treatment associate to rehabilitation therapy and transcranial DC stimulation. https://clinicaltrials.gov/show/NCT02156635 (accessed 7 September 2015).
NCT02166619 {published data only}
- NCT02166619. tDCS in poststroke on upper limb rehabilitation. https://clinicaltrials.gov/show/NCT02166619 (accessed 7 September 2015).
NCT02209922 {published data only}
- NCT02209922. The effects of tDCS combined with balance training on postural control and spasticity in chronic stroke patients. https://clinicaltrials.gov/show/NCT02209922 (accessed 7 September 2015).
NCT02210403 {published data only}
- NCT02210403. The influence of tDCS on the arm and hand function in stroke patients. https://clinicaltrials.gov/show/NCT02210403 (accessed 7 September 2015).
NCT02213640 {published data only}
- NCT02213640. Potentiation of the effects of prismatic adaptation by transcranial direct current stimulation (tDCS): evaluation of functional interest in negligence rehabilitation. https://clinicaltrials.gov/show/NCT02213640 (accessed 7 September 2015).
NCT02254616 {published data only}
- NCT02254616. Hybrid approach to mirror therapy and transcranial direct current stimulation for stroke recovery. https://clinicaltrials.gov/show/NCT02254616 (accessed 7 September 2015).
NCT02292251 {published data only}
- NCT02292251. Study to enhance motor acute recovery with intensive training after stroke. https://clinicaltrials.gov/show/NCT02292251 (accessed 7 September 2015).
NCT02308852 {published data only}
- NCT02308852. Improving bi‐manual activities in stroke patients with application of neuro‐stimulation. https://clinicaltrials.gov/show/NCT02308852 (accessed 7 September 2015).
NCT02325427 {published data only}
- NCT02325427. Changes in brain activity associated with upper limb motor recovery. https://clinicaltrials.gov/show/NCT02325427 (accessed 7 September 2015).
NCT02389608 {published data only}
- NCT02389608. The immediate effect of electrical stimulation transcranial direct current (tDCS) associated with the use of FES, in muscle activity of the tibialis anterior muscle, balance and plantar pressure distribution of individuals with hemiparesis due to stroke. https://clinicaltrials.gov/show/NCT02389608 (accessed 7 September 2015).
NCT02393651 {published data only}
- NCT02393651. Late LTP‐like plasticity effects of tDCS in subacute stroke patients. https://clinicaltrials.gov/show/NCT01807637 (accessed 7 September 2015).
NCT02399540 {published data only}
- NCT02399540. Late LTP‐like plasticity effects of tDCS in chronic stroke patients. https://clinicaltrials.gov/show/NCT02399540 (accessed 7 September 2015).
NCT02401724 {published data only}
- NCT02401724. NonInvasive brain stimulation in stroke patients. https://clinicaltrials.gov/show/NCT02401724 (accessed 7 September 2015).
NCT02416791 {published data only}
- NCT02416791. Robotic therapy and transcranial direct current stimulation in patients with stroke. https://clinicaltrials.gov/show/NCT02416791 (accessed 7 September 2015).
NCT02422173 {published data only}
- NCT02422173. Transcranial direct current stimulation on the risk of falls and lower limb function for acute stroke. https://clinicaltrials.gov/show/NCT02422173 (accessed 7 September 2015).
NCT02455427 {published data only}
- NCT02455427. Safety of transcranial direct current stimulation in the subacute phase after stroke. https://clinicaltrials.gov/show/NCT02455427 (accessed 7 September 2015).
NTR3315 {published data only}
- NTR3315. The effect of non invasive brain stimulation on lower limb motor skill acquisition. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=3315 (accessed 4 March 2013).
Paquette 2013 {published data only}
- Paquette C, Riegel M, Anglade C, Fung J, Thiel A. Early inhibitory non‐invasive brain stimulation of the unaffected hemisphere combined improves motor outcome in acute stroke. Cerebrovascular Diseases 2013;35:147‐8. [1015‐9770] [Google Scholar]
Sattler 2012 {published data only}
- Sattler V, Acket B, Gerdelat‐Mas A, Raposo N, Albucher JF, Thalamas C, et al. Effect of repeated sessions of combined anodal tDCS and peripheral nerve stimulation on motor performance in acute stroke: a behavioural and electrophysiological study [Effet sur la recuperation motrice post‐AVC, en phase aigue, de sessions repetees de tDCS anodale du cortex moteur primaire couplee a une stimulation electrique peripherique repetitive]. Annals of Physical and Rehabilitation Medicine 2012;55:e3 + e5‐e6. [1877‐0657] [Google Scholar]
Additional references
Adeyemo 2012
- Adeyemo BO, Simis M, Macea D, Fregni F. Systematic review of parameters of stimulation: clinical trial design characteristics and motor outcomes in noninvasive brain stimulation in stroke. Frontiers in Psychiatry 2012;3:88. [1664‐0640] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ang 2015 [pers comm]
- Ang KK. RE: Cochrane‐Review on tDCS on function and ADL after stroke. Email to: B Elsner 29 September 2015.
Antal 2015
- Antal A, Keeser D, Priori A, Padberg F, Nitsche MA. Conceptual and procedural shortcomings of the systematic review "Evidence that transcranial direct current stimulation (tDCS) generates little‐to‐no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: a systematic review" by Horvath and Co‐workers. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation 2015;8(4):846‐9. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O'Connell D, Oxman AD, Phillips B, Schünemann HJ, Edejer T, Varonen H, Vist GE, Williams JW Jr, Zaza S, GRADE Working Group. Grading quality of evidence and strength of recommendations. British Medical Journal 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barker 2005
- Barker RN, Brauer SG. Upper limb recovery after stroke: the stroke survivors' perspective. Disability and Rehabilitation 2005;30(20):1213‐23. [DOI] [PubMed] [Google Scholar]
Bastani 2012
- Bastani A, Jaberzadeh S. Does anodal transcranial direct current stimulation enhance excitability of the motor cortex and motor function in healthy individuals and subjects with stroke: a systematic review and meta‐analysis. Clinical Neurophysiology 2012;123(4):644‐57. [DOI] [PubMed] [Google Scholar]
Bikson 2013
- Bikson M, Name A, Rahman A. Origins of specificity during tDCS: anatomical, activity‐selective, and input‐bias mechanisms. Frontiers in Human Neuroscience 2013;7:688. [1662‐5161: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bindman 1964
- Bindman LJ, Lippold OCJ, Redfearn JW. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long‐lasting after‐effects. Journal of Physiology 1964;172(3):369‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boggio 2006
- Boggio PS, Castro LO, Savagim EA, Braite R, Cruz VC, Rocha RR, et al. Enhancement of non‐dominant hand motor function by anodal transcranial direct current stimulation. Neuroscience Letters 2006;404(1‐2):232‐6. [DOI] [PubMed] [Google Scholar]
Boissy 1999
- Boissy P, Bourbonnais D, Carlotti MM, Gravel D, Arsenault BA. Maximal grip force in chronic stroke subjects and its relationship to global upper extremity function. Clinical Rehabilitation 1999;13(4):354‐62. [DOI] [PubMed] [Google Scholar]
Bolognini 2013 [pers comm]
- Bolognini N. Cochrane Review on tDCS on function and ADL after stroke. Personal communication 8 May 2013.
Bonita 1988
- Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke 1988;19:1497‐500. [DOI] [PubMed] [Google Scholar]
Butler 2013
- Butler AJ, Shuster M, O'Hara E, Hurley K, Middlebrooks D, Guilkey K. A meta‐analysis of the efficacy of anodal transcranial direct current stimulation for upper limb motor recovery in stroke survivors. Journal of Hand Therapy 2013;26(2):162‐70; quiz 171. [PUBMED: 22964028] [DOI] [PubMed] [Google Scholar]
Chung 2013
- Chung CSY, Pollock A, Campbell T, Durward BR, Hagen S. Cognitive rehabilitation for executive dysfunction in adults with stroke or other adult non‐progressive acquired brain damage. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD008391.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Demeurisse 1980
- Demeurisse G, Demol O, Robaye E. Motor evaluation in vascular hemiplegia. European Neurology 1980;19(6):382‐9. [DOI] [PubMed] [Google Scholar]
Elsner 2015
- Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving aphasia in patients with aphasia after stroke. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD009760.pub3] [DOI] [PubMed] [Google Scholar]
Floel 2010
- Floel A, Cohen LG. Recovery of function in humans: cortical stimulation and pharmacological treatments after stroke. Neurobiology of Disease 2010;37(2):243‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fregni 2005
- Fregni F, Boggio PS, Mansur CG, Wagner T, Ferreira MJ, Lima MC, et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport 2005;16(14):1551‐5. [DOI] [PubMed] [Google Scholar]
Fugl‐Meyer 1975
- Fugl‐Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post‐stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scandinavian Journal of Rehabilitation Medicine 1975;7(1):13‐31. [PubMed] [Google Scholar]
Goodglass 1983
- Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders. Philadelphia: Lea and Febiger, 1983. [Google Scholar]
GRADEpro [Computer program]
- McMaster University. GRADEpro. Version 18 September 2015. McMaster University, 2014.
Hamilton 1994
- Hamilton BB, Laughlin JA, Fiedler RC, Granger CV. Interrater reliability of the 7‐level functional independence measure (FIM). Scandinavian Journal of Rehabilitation Medicine 1994;26:115‐9. [PubMed] [Google Scholar]
Hatano 1976
- Hatano S. Experience from a multicentre stroke register: a preliminary report. Bulletin of the World Health Organization 1976;54:541‐53. [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org, 2011. [Google Scholar]
Holden 1984
- Holden MK, Gill KM, Magliozzi MR, Nathan J, Piehl‐Baker L. Clinical gait assessment in the neurologically impaired. Reliability and meaningfulness. Physical Therapy 1984;64(1):35‐40. [DOI] [PubMed] [Google Scholar]
Horvath 2015
- Horvath JC, Forte JD, Carter O. Evidence that transcranial direct current stimulation (tDCS) generates little‐to‐no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: a systematic review. Neuropsychologia 2015;66:213‐36. [0028‐3932] [DOI] [PubMed] [Google Scholar]
Hummel 2005
- Hummel R, Celnik P, Giraux B, Floel A, Wu WH, Gerloff C, et al. Effects of non‐invasive cortical stimulation on skilled motor function in chronic stroke. Brain 2005;128:490‐9. [DOI] [PubMed] [Google Scholar]
Jacobson 2012
- Jacobson L, Koslowsky M, Lavidor M. tDCS polarity effects in motor and cognitive domains: a meta‐analytical review. Experimental Brain Research 2012;216(1):1‐10. [DOI] [PubMed] [Google Scholar]
Jebsen 1969
- Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Archives of Physical Medicine and Rehabilitation 1969;50:311‐9. [PubMed] [Google Scholar]
Jeffery 2007
- Jeffery DT, Norton JA, Roy FD, Gorassini MA. Effects of transcranial direct current stimulation on the excitability of the leg motor cortex. Experimental Brain Research 2007;182(2):281‐7. [DOI] [PubMed] [Google Scholar]
Jørgensen 1999
- Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Stroke. Neurologic and functional recovery the Copenhagen Stroke Study. Physical Medicine and Rehabilitation Clinics of North America 1999;10(4):887‐906. [PubMed] [Google Scholar]
Kim 2013 [pers comm]
Koch 2013 [pers comm]
- Koch G. Cochrane Review on tDCS on function and ADL after stroke. Personal communication 8 May 2013.
Kwakkel 2003
- Kwakkel G, Kollen BJ, Grond J, Prevo AJ. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke 2003;34(9):2181‐6. [DOI] [PubMed] [Google Scholar]
Lang 2005
- Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, et al. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain?. European Journal of Neuroscience 2005;22(2):495‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
List 2015
- List J, Lesemann A, Kübke JC, Külzow N, Schreiber SJ, Flöel A. Impact of tDCS on cerebral autoregulation in aging and in patients with cerebrovascular diseases. Neurology 2015;84(6):626‐8. [DOI] [PubMed] [Google Scholar]
Lyle 1981
- Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. International Journal of Rehabilitation Research 1981;4(4):483‐92. [DOI] [PubMed] [Google Scholar]
Mahoney 1965
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Maryland State Medical Journal 1965;14:61‐5. [PubMed] [Google Scholar]
Mathers 2011
- Mathers CD, Bernard C, Iburg KM, Inoue M, Ma Fat D, Shibuya K, et al. Global burden of disease: data sources, methods and results. www.who.int/healthinfo/bod/en/index.html (accessed 16 June 2011).
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. British Medical Journal 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Molenberghs 2011
- Molenberghs P, Sale MV. Testing for Spatial Neglect with Line Bisection and Target Cancellation: Are Both Tasks Really Unrelated?. PLoS ONE 2011;6(7):e23017. [1932‐6203] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nasreddine 2005
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatric Society 2005;53(4):695‐9. [(Print)] [DOI] [PubMed] [Google Scholar]
Nitsche 2001
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001;57:1899‐901. [DOI] [PubMed] [Google Scholar]
Nitsche 2003
- Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Journal of Physiology 2003;114:600‐4. [DOI] [PubMed] [Google Scholar]
Nitsche 2015
- Nitsche MA, Paulus W. Vascular safety of brain plasticity induction via transcranial direct currents. Neurology 2015;84(6):556‐7. [DOI] [PubMed] [Google Scholar]
Nowak 2009
- Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemsipsheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabilitation and Neural Repair 2009;23(7):641‐56. [DOI] [PubMed] [Google Scholar]
Paik 2015 [pers comm]
- Paik NJ. RE: Cochrane‐Review on tDCS on function and ADL after stroke. Email to: B Elsner 30 September 2015.
Pollock 2012
- Pollock A, George B, Fenton M, Firkins L. Top ten research priorities relating to life after stroke. Lancet Neurology 2012;11(3):209. [1474‐4422] [DOI] [PubMed] [Google Scholar]
Purpura 1965
- Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. Journal of Neurophysiology 1965;28(1):166‐85. [DOI] [PubMed] [Google Scholar]
Reis 2009
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proceedings of the National Academy of Sciences of the United States of America 2009;106(5):1590‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riepe 2004
- Riepe MW, Riss S, Bittner D, Huber R. Screening for cognitive impairment in patients with acute stroke. Dementia and Geriatric Cognitive Disorders 2004;17(1‐2):49‐53. [1420‐8008: (Print)] [DOI] [PubMed] [Google Scholar]
Schuling 1993
- Schuling J, Haan R, Limburg M, Groenier KH. The Frenchay Activities Index: assessment of functional status in stroke patients. Stroke 1993;24:1173‐7. [DOI] [PubMed] [Google Scholar]
Sharpless 1982
- Sharpless JW. The nine‐hole peg test of finger hand coordination for the hemiplegic patient. In: Mossman PL editor(s). Mossman's A Problem Oriented Approach to Stroke Rehabilitation. Vol. 2, Springfield, IL: CC Thomas, 1982:470‐3. [Google Scholar]
Smania 2013 [pers comm]
- Smania N. Cochrane Review on tDCS on function and ADL after stroke. Personal communication 5 March 2013.
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Suzuki 2012
- Suzuki K, Fujiwara T, Tanaka N, Tsuji T, Masakado Y, Hase K, et al. Comparison of the after‐effects of transcranial direct current stimulation over the motor cortex in patients with stroke and healthy volunteers. International Journal of Neuroscience 2012;122(11):675‐81. [DOI] [PubMed] [Google Scholar]
Tedesco Triccas 2015a
Whiting 1980
- Whiting S, Lincoln N. An ADL assessment for stroke patients. British Journal of Occupational Therapy 1980;43:44‐6. [Google Scholar]
WHO 2011
- World Health Organization. The atlas of heart disease and stroke. www.who.int/cardiovascular_diseases/resources/atlas/en/ (accessed 8 June 2011).
Wu 2013b [pers comm]
- Wu D. Cochrane Review on tDCS on function and ADL after stroke. Personal communication 9 March 2013.
References to other published versions of this review
Elsner 2012
- Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving function and activities of daily living in patients after stroke. Cochrane Database of Systematic Reviews 15 February 2012, Issue 2. [DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]
Elsner 2013
- Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving function and activities of daily living in patients after stroke. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD009645.pub2] [DOI] [PubMed] [Google Scholar]


