Abstract
Background
This represents the first update of this review, which was published in 2012. Atorvastatin is one of the most widely prescribed drugs and the most widely prescribed statin in the world. It is therefore important to know the dose‐related magnitude of effect of atorvastatin on blood lipids.
Objectives
Primary objective
To quantify the effects of various doses of atorvastatin on serum total cholesterol, low‐density lipoprotein (LDL)‐cholesterol, high‐density lipoprotein (HDL)‐cholesterol and triglycerides in individuals with and without evidence of cardiovascular disease. The primary focus of this review was determination of the mean per cent change from baseline of LDL‐cholesterol.
Secondary objectives
• To quantify the variability of effects of various doses of atorvastatin.
• To quantify withdrawals due to adverse effects (WDAEs) in placebo‐controlled randomised controlled trials (RCTs).
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 11, 2013), MEDLINE (1966 to December Week 2 2013), EMBASE (1980 to December Week 2 2013), Web of Science (1899 to December Week 2 2013) and BIOSIS Previews (1969 to December Week 2 2013). We applied no language restrictions.
Selection criteria
Randomised controlled and uncontrolled before‐and‐after trials evaluating the dose response of different fixed doses of atorvastatin on blood lipids over a duration of three to 12 weeks.
Data collection and analysis
Two review authors independently assessed eligibility criteria for studies to be included and extracted data. We collected information on withdrawals due to adverse effects from placebo‐controlled trials.
Main results
In this update, we found an additional 42 trials and added them to the original 254 studies. The update consists of 296 trials that evaluated dose‐related efficacy of atorvastatin in 38,817 participants. Included are 242 before‐and‐after trials and 54 placebo‐controlled RCTs. Log dose‐response data from both trial designs revealed linear dose‐related effects on blood total cholesterol, LDL‐cholesterol, HDL‐cholesterol and triglycerides. The Summary of findings table 1 documents the effect of atorvastatin on LDL‐cholesterol over the dose range of 10 to 80 mg/d, which is the range for which this systematic review acquired the greatest quantity of data. Over this range, blood LDL‐cholesterol is decreased by 37.1% to 51.7% (Summary of findings table 1). The slope of dose‐related effects on cholesterol and LDL‐cholesterol was similar for atorvastatin and rosuvastatin, but rosuvastatin is about three‐fold more potent. Subgroup analyses suggested that the atorvastatin effect was greater in females than in males and was greater in non‐familial than in familial hypercholesterolaemia. Risk of bias for the outcome of withdrawals due to adverse effects (WDAEs) was high, but the mostly unclear risk of bias was judged unlikely to affect lipid measurements. Withdrawals due to adverse effects were not statistically significantly different between atorvastatin and placebo groups in these short‐term trials (risk ratio 0.98, 95% confidence interval 0.68 to 1.40).
Authors' conclusions
This update resulted in no change to the main conclusions of the review but significantly increases the strength of the evidence. Studies show that atorvastatin decreases blood total cholesterol and LDL‐cholesterol in a linear dose‐related manner over the commonly prescribed dose range. New findings include that atorvastatin is more than three‐fold less potent than rosuvastatin, and that the cholesterol‐lowering effects of atorvastatin are greater in females than in males and greater in non‐familial than in familial hypercholesterolaemia. This review update does not provide a good estimate of the incidence of harms associated with atorvastatin because included trials were of short duration and adverse effects were not reported in 37% of placebo‐controlled trials.
Plain language summary
Effect of atorvastatin on cholesterol
This represents the first update of this review, which was published in 2012 (Adams 2012). Atorvastatin is one of the most widely prescribed drugs and the most widely prescribed statin in the world. It is an HMG‐CoA reductase inhibitor that is prescribed to prevent adverse cardiovascular events and to lower blood total cholesterol and LDL‐cholesterol. It is therefore important to know the magnitude of the effect that atorvastatin has on cholesterol. We searched for all evidence obtained from three‐ to 12‐week trials reporting the effect of atorvastatin on blood cholesterol. This update found 42 additional trials and reports on 296 trials in 38,817 participants. Atorvastatin showed a consistent effect in lowering blood cholesterol over the dose range of 2.5 to 80 mg daily. The effect was greater with higher doses than with lower doses. Atorvastatin works similarly to rosuvastatin in lowering cholesterol but is about three‐fold less potent. Risk of bias for all assessed trials was high. Review authors were unable to assess harms of atorvastatin because the included trials were too short, and because only 34 included trials assessed harms.
Summary of findings
Summary of findings for the main comparison. LDL‐cholesterol‐lowering efficacy of atorvastatin.
| LDL‐cholesterol‐lowering efficacy of atorvastatin | ||||
|
Patient or population: individuals with normal or abnormal lipid profiles Settings: outpatient clinics Intervention: atorvastatin Comparison: placebo or baseline | ||||
| Outcomes | Per cent change (95% CI)a | Number of participants (studies) | Quality of the evidence (GRADE) | Comments |
|
LDL‐cholesterol atorvastatin 10 mg/d |
‐37.1 (‐37.3 to ‐36.9) |
21,941 (188) | ⊕⊕⊕⊕ Highd | Effect predicted from log dose‐response equation is ‐37.2% |
|
LDL‐cholesterol atorvastatin 20 mg/d |
‐42.3 (‐42.6 to ‐42.0) |
9,310 (80) | ⊕⊕⊕⊕ Highd | Effect predicted from log dose‐response equation is ‐42.1% |
|
LDL‐cholesterol atorvastatin 40 mg/d |
‐47.4 (‐48.0 to ‐46.9) |
3,296 (37) |
⊕⊕⊕⊕ Highd | Effect predicted from log dose‐response equation is ‐47.0% |
|
LDL‐cholesterol atorvastatin 80 mg/d |
‐51.7 (‐52.2 to ‐51.2) |
4,281 (37) |
⊕⊕⊕⊕ Highd | Effect predicted from log dose‐response equation is ‐52.0% |
|
WDAEb all doses |
RRc (0.98) (0.68 to 1.40) |
3,688 (34) |
⊕⊝⊝⊝ Very lowe |
Only 34 out of 54 placebo‐controlled trials reported withdrawals due to adverse effects |
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
aCI: confidence interval.
bWDAE: withdrawal due to adverse effect.
cRR: risk ratio.
dNot downgraded despite mostly unclear risk of selection and detection bias because of large quantity of data, narrow confidence intervals, consistency of effect estimate with that predicted from the log dose‐response equation (shown in comments) and the fact that lipid parameters were measured primarily in independent laboratories, not by investigators.
eHigh risk of loss of blinding bias and selective reporting bias plus wide confidence intervals.
Background
This represents the first update of this review, which was published in 2012 (Adams 2012).
Description of the condition
Cardiovascular disease is a major cause of death and disability in the developed world, accounting for more than one‐third of total deaths (Kreatsoulas 2010). In the United States, cardiovascular disease causes one in three reported deaths each year (CDC 2011; Roger 2011). Existing evidence shows a weak association between adverse cardiovascular events and blood concentrations of low‐density lipoprotein (LDL)‐cholesterol in adults (Grundy 2004). The current recommended treatment for secondary prevention of adverse cardiovascular events consists of diet and lifestyle changes plus drug therapy with the drug class widely known as 'statins'.
Description of the intervention
Atorvastatin is the statin most widely prescribed in the world (IMS 2012). Atorvastatin and the five other available statins are prescribed to prevent adverse cardiovascular events and to lower blood total cholesterol and LDL‐cholesterol. Atorvastatin is rapidly absorbed, reaching peak plasma concentration within 2.3 hours. The lipid‐lowering effect of atorvastatin is not influenced by the time of day the drug is administered, probably because of its relatively long half‐life of 20 hours. Atorvastatin is metabolised by cytochromes P‐450 3A4 and P‐450 3A5 to ortho‐hydroxy atorvastatin and para‐hydroxy atorvastatin. These two active metabolites extend the effect of atorvastatin on 3‐hydroxy‐3‐methylglutaryl‐CoA (HMG‐CoA) reductase, resulting in a half‐life of enzyme inhibition of 20 to 30 hours (Lins 2003; Schachter 2004;Goodman 2011). Atorvastatin and statins as a class have been shown in individual randomised controlled trials (RCTs), systematic reviews and meta‐analyses of RCTs to reduce mortality and major vascular events in people with occlusive vascular disease (CTT 2005). They have been reported to reduce major vascular events in primary prevention populations; however, whether they reduce mortality remains controversial (Mills 2008; Taylor 2013; Therapeutics Initiative 2010; Abramson 2013). Determining the effects of statins on morbidity and mortality is not the objective of this systematic review. The purpose of this review is to learn more about the pharmacology of atorvastatin by characterising the dose‐related effect and the variability of the effect of atorvastatin on four surrogate markers: blood total cholesterol, low‐density lipoprotein (LDL)‐cholesterol, high‐density lipoprotein (HDL)‐cholesterol and triglycerides.
How the intervention might work
Atorvastatin acts in the liver by inhibiting the rate‐limiting enzyme for cholesterol synthesis, HMG‐CoA reductase. This enzyme irreversibly converts 3‐hydroxy‐3‐methylglutaryl CoA to mevalonate (Moghadasian 1999). This reaction is the third step in a sequence of reactions resulting in the production of many compounds including cholesterol and its circulating blood derivatives, LDL‐cholesterol and very low‐density lipoprotein (VLDL)‐cholesterol (Gaw 2000). The prevailing hypothesis is that statins reduce mortality and morbidity in patients with occlusive vascular disease by reducing liver production of cholesterol and thus causing a reduction in blood LDL‐cholesterol and a resulting decrease in atherogenesis. However, the HMG Co‐A reductase enzyme is also responsible for the production of ubiquinone (co‐enzyme Q10), heme a, vitamin D, steroid hormones and many other compounds. It remains possible that the beneficial effects of statins are due to actions other than the reduction of cholesterol. These other actions have been referred to as the pleiotropic effects of statins (Liao 2005). Independent of how the drug works, it is important to know the average per cent reduction in the lipid parameters associated with doses commonly taken by patients. The advantage of expressing the effect as per cent reduction as compared with absolute reduction from baseline is that the per cent reduction is a pure number, is independent of the unit of measurement and is independent of baseline parameters. For this review it was established that there was no correlation between the effect expressed as per cent reduction and the baseline value. Furthermore the per cent reduction from baseline in blood LDL‐cholesterol at the present time represents the best available pharmacological marker of the magnitude of the effects of statins on the enzyme, HMG Co‐A reductase.
Why it is important to do this review
Statins are the most widely prescribed class of drugs in the world. Statin prescribing and average prescribing doses are increasing. Clinicians have an approximate sense of the different potencies of the various statins, but a systematic assessment of the potency, the slope of the dose‐response relationship and the variability of the effect has not been published for any of the statins. It is possible that in addition to differences in potency, the slope of the dose‐response relationship or the variability of the response is different for different statins. A small number of previous systematic reviews have assessed the effects of statins on serum lipids (Bandolier 2004; Edwards 2003;Law 2003;Naci 2013). These review authors have demonstrated that various statins have different potency in terms of lipid lowering, and that higher doses of statins cause greater lowering of blood total cholesterol, LDL‐cholesterol and triglycerides than are seen with lower doses. However, none of these systematic reviews have calculated the slope of the dose response or the variability of effect, and none are up‐to‐date. The limitation of the most comprehensive systematic review to date is that it presents data that are based on the average absolute reduction in LDL‐cholesterol concentration ‐ a parameter that is dependent in part on the magnitude of the baseline value (Law 2003). The purpose of our systematic review is to expand Law's work. As atorvastatin is the most widely prescribed statin in the world, we have chosen it as the first drug for study in this class. We used surrogate markers to measure the pharmacological effects of statins, which we defined as per cent change from baseline to describe the dose‐response relationship of the effects of atorvastatin on blood total cholesterol, LDL‐cholesterol, HDL‐cholesterol and triglycerides. We plan to use this established methodology to study the other drugs in this class (cerivastatin, fluvastatin, lovastatin, pravastatin, simvastatin, rosuvastatin and pitavastatin) in subsequent reviews to permit comparison of those results with the findings documented here for atorvastatin.
Objectives
Primary objective
To quantify the effects of various doses of atorvastatin on serum total cholesterol, LDL‐cholesterol, HDL‐cholesterol and triglycerides in individuals with and without evidence of cardiovascular disease. The primary focus of this review was determination of the mean per cent change from baseline of LDL‐cholesterol.
Secondary objectives
To quantify the variability of effects of various doses of atorvastatin.
To quantify withdrawals due to adverse effects (WDAEs) in placebo‐controlled RCTs.
Methods
Criteria for considering studies for this review
Types of studies
We included placebo‐controlled RCTs and uncontrolled before‐and‐after trials. We included the latter because it has been shown that there is no placebo effect of statins on lipid parameters (Tsang 2002). We included cross‐over trials when data were provided for each separate phase of the trial, and when wash‐out periods of at least three weeks were provided at the cross‐over points. This duration of wash‐out ensures that no carry‐over effect will occur and allows lipid values to stabilise.
Types of participants
Study participants may or may not have evidence of cardiovascular disease. They may have normal lipid parameters or any type of hyperlipidaemia or dyslipidaemia. Investigators applied no age restrictions and included individuals with various co‐morbid conditions including type 2 diabetes mellitus, hypertension, metabolic syndrome, chronic renal failure or cardiovascular disease.
Types of interventions
Atorvastatin was administered at a constant daily dose for a period of three to 12 weeks. This administration time window was chosen to allow at least three weeks for a steady state effect of atorvastatin to occur, and to keep the window short enough to minimise loss of participants to follow‐up. We accepted data from studies in which atorvastatin was administered in the morning or in the evening, or in which this was not specified. Trials required a wash‐out baseline dietary stabilisation period of at least three weeks during which all previous lipid‐altering medication was withdrawn. This baseline phase ensured that participants followed a standard lipid‐regulating diet and helped to stabilise baseline lipid values before initiation of treatment. The baseline wash‐out phase was not required for trials in which participants were not receiving lipid‐altering medications or dietary supplements before they were given atorvastatin.
Types of outcome measures
Primary outcomes
Placebo‐controlled RCTs: mean per cent change in LDL‐cholesterol from baseline of different doses of atorvastatin minus per cent change from baseline with placebo.
Before‐and‐after trials: mean per cent change in LDL‐cholesterol from baseline of different doses of atorvastatin.
Secondary outcomes
Placebo‐controlled RCTs: mean per cent change in total cholesterol from baseline of different doses of atorvastatin minus mean per cent change from baseline with placebo.
Before‐and‐after trials: mean per cent change in total cholesterol from baseline of different doses of atorvastatin. It is recognised that effects on total cholesterol are due primarily to effects on LDL‐cholesterol, which is the reason that this is a secondary outcome.
Placebo‐controlled RCTs: mean per cent change in HDL‐cholesterol from baseline of different doses of atorvastatin minus mean per cent change from baseline with placebo.
Before‐and‐after trials: mean per cent change in HDL‐cholesterol from baseline of different doses of atorvastatin.
Placebo‐controlled RCTs: mean per cent change in triglycerides from baseline of different doses of atorvastatin minus mean per cent change from baseline with placebo.
Before‐and‐after trials: mean per cent change in triglycerides from baseline of different doses of atorvastatin.
End‐of‐treatment variability (standard deviation (SD)) and coefficient of variation of LDL‐cholesterol measurements for each dose of atorvastatin. It is important to know whether atorvastatin has an effect on the variability of lipid measures, and ultimately to compare this with the effects of other statins.
Placebo‐controlled RCTs: WDAEs. This important measure of harm can be assessed only in placebo‐controlled trials.
Search methods for identification of studies
Electronic searches
We identified relevant trials of atorvastatin through a search of the Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 11), MEDLINE (1966 to December Week 2 2013), EMBASE (1980 to December Week 2 2013), the Institute for Scientific Information (ISI) Web of Science (1899 to December Week 2 2013) and BIOSIS Previews (1969 to December Week 2 2013). We checked the bibliographies of identified papers and applied no language restrictions to our search. See Appendix 1 for details of the search strategies.
Searching other resources
In cases of incomplete reports, we conducted further searches to look for connected papers. We used previously published meta‐analyses on the efficacy of HMG‐CoA reductase inhibitors to help us identify references to trials (CTT 2005; Edwards 2003; Law 2003). We included grey literature by searching other resources.
SciFinder Scholar (scifinder.cas.org/scifinder/view/scifinder/scifinderExplore.jsf).
ClinicalTrials.gov (www.clinicaltrials.gov/).
International Pharmaceutical Abstracts database.
ProQuest Dissertations & Theses (search.proquest.com/pqdtft/advanced?accountid=14656).
Pfizer web site (www.pfizer.ca/en/our_products/).
US Food and Drug Administration web site (www.fda.gov/).
European Patent Office web site (worldwide.espacenet.com).
Data collection and analysis
Selection of studies
Initial selection of trials involved retrieving and reading the titles and abstracts of all papers found in electronic search databases or bibliographic citations. We have provided a PRISMA flow diagram. Two review authors independently analysed the full‐text papers to determine which trials should be included. Review authors turned to a third party to resolve disagreements. Two review authors independently extracted the data from each of the included trials. In cases of disagreement regarding a value, review authors reached consensus by recalculating data to determine the correct value.
Data extraction and management
Review authors directly extracted the mean per cent change from the data or calculated this value using baseline and endpoint data. We extracted standard deviations (SDs) and standard errors (SEs) from the report or calculated SDs and SEs when possible. We entered data from placebo‐controlled and uncontrolled before‐and‐after trials into Review Manager 5 as continuous and generic inverse variance data, respectively. We conducted all meta‐analyses using RevMan 5 (RevMan 2011).
Assessment of risk of bias in included studies
We assessed all trials using the 'Risk of bias' tool under the following categories: sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and other biases as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We have reported this information in the 'Risk of bias' tables associated with each included trial.
Measures of treatment effect
We analysed treatment effects for each dose in placebo‐controlled RCTs and in uncontrolled before‐and‐after trials separately. As the effects were similar and placebo was shown not to have an effect, we combined all efficacy study data from placebo and before‐and‐after trials and re‐analysed them using the generic inverse variance fixed‐effect model outside of this review to determine overall weighted effects and 95% confidence intervals (CIs).
Unit of analysis issues
Before‐and‐after trials are not RCTs. All placebo‐controlled RCTs used a parallel‐group design whereby participants were individually randomly assigned to atorvastatin or to placebo, and investigators reported and entered the mean measurement for each outcome for all participants. Therefore this review reflects no unit of analysis issues.
Dealing with missing data
Most commonly, the data not reported consisted of SDs of the change. For studies in which SDs were not provided, imputation was done. The imputed value used is the average weighted SD of the change from other trials in the review (Furukawa 2006). We contacted study authors to retrieve missing data.
Assessment of heterogeneity
The Chi2 test is not appropriate for identifying heterogeneity because it has low power when few studies are included but has excessive power to detect clinically unimportant heterogeneity when many studies are included. A better statistic is the I2 statistic. I2 = between‐study variance/(between‐study variance + within‐study variance). This test measures the proportion of the total variation in the estimate of the treatment effect that is due to heterogeneity between studies. This statistic is independent of the number of studies included in the analysis (Higgins 2002). If I2 ≥ 50%, we used the random‐effects model to assess whether the pooled effect was statistically significant and to estimate conservatively the measure of the effect.
Assessment of reporting biases
We used funnel plots for all outcomes with more than 10 RCTs to assess whether publication bias was evident in this review.
Data synthesis
We synthesised data using the mean difference in continuous outcome effect measures for the lipid data. We entered WDAEs as dichotomous Mantel‐Haenszel risk ratio (RR) data from placebo‐controlled RCTs with RevMan 5.1.6 (RevMan 2011).
For before‐and‐after trials, we synthesised data using per cent change from baseline generic inverse variance data with RevMan 5.1.6.
We entered mean per cent reduction in lipid parameters from all trials into GraphPad Prism 4 to yield a weighted least‐squares analyses based on the inverse of the square of the standard error for each lipid parameter to generate weighted log dose response curves.
Subgroup analysis and investigation of heterogeneity
The main subgroup analyses include the different doses of atorvastatin. We assessed heterogeneity using the I2 statistic (Higgins 2002). We tried to identify possible causes of significant heterogeneity by carrying out several planned subgroup analyses, provided that sufficient numbers of trials were identified.
We analysed subgroups based on the following factors.
Placebo‐controlled RCTs versus uncontrolled before‐and‐after trials (described above).
Efficacy of atorvastatin in males versus females.
Efficacy of atorvastatin in individuals with familial versus non‐familial hypercholesterolaemia.
Morning administration time versus evening administration time analysis was not done because only one trial provided appropriate data.
Results
Description of studies
Database searching identified 38,622 citations and 64 other resource citations, providing a total of 38,686 records. After we had removed duplicates, 25,745 records remained. Of these citations, review authors obtained 518 as full‐text articles and assessed them for eligibility. For this update, 428 citations to 296 trials met the inclusion criteria and provided extractable data for use in evaluating the dose‐related blood lipid‐lowering effect of atorvastatin (Figure 1). This update includes 42 additional trials (five placebo‐controlled and 37 before‐and‐after).
1.
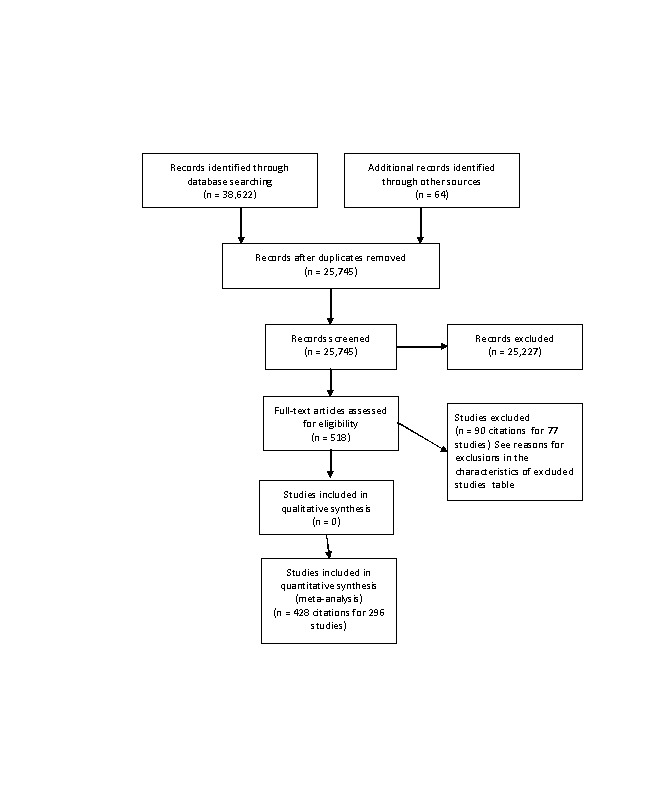
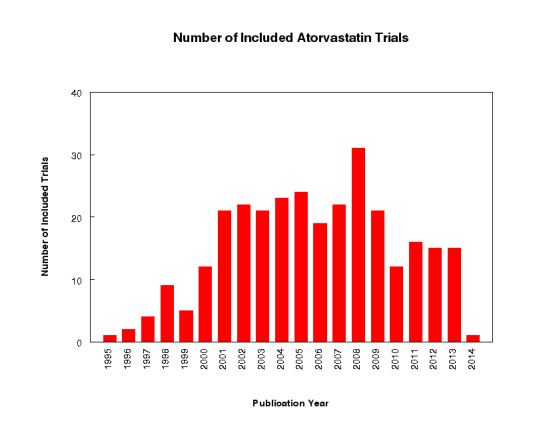
We have summarised each included study in the Characteristics of included studies table. Of the 296 included studies, 282 (95%) were published in English, four (1.4%) in Russian, three (1.0%) in Chinese, two (0.7%) in Japanese, two (0.7%) in Polish, two (0.7%) in Spanish and one (0.3%) in Portuguese. Of the 54 placebo‐controlled trials, 46 (85.2%) were double‐blind, two (3.7%) were single‐blind and three (5.6%) were open‐label; in three trials (5.6%), blinding information was not reported. We contacted study authors to ask about the method of blinding used in these three trials but received no replies. Trials evaluating the lipid‐altering efficacy of atorvastatin were first published in 1995. Between 1995 and 2014, the number of available studies increased and then decreased. Most available studies were published in 2008 (see Figure 2).
2.
We excluded 75 studies because they did not meet the inclusion criteria. Reasons for exclusion included failure to report the number of participants, an inappropriate treatment period, inappropriate dosing, values expressed as ranges and for cross‐over trials, no pre‐cross‐over data and dosing bias. We have listed the reasons for excluding each trial in the Characteristics of excluded studies table.
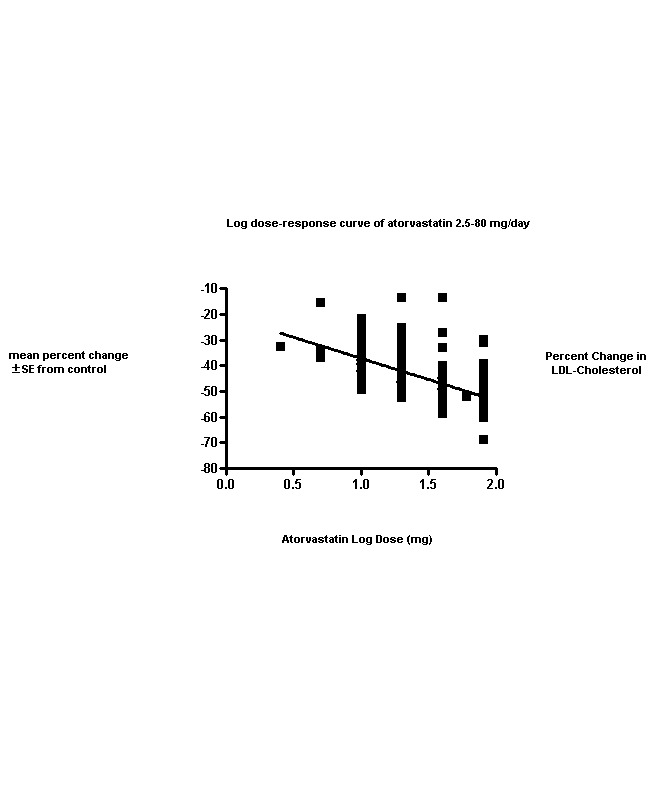
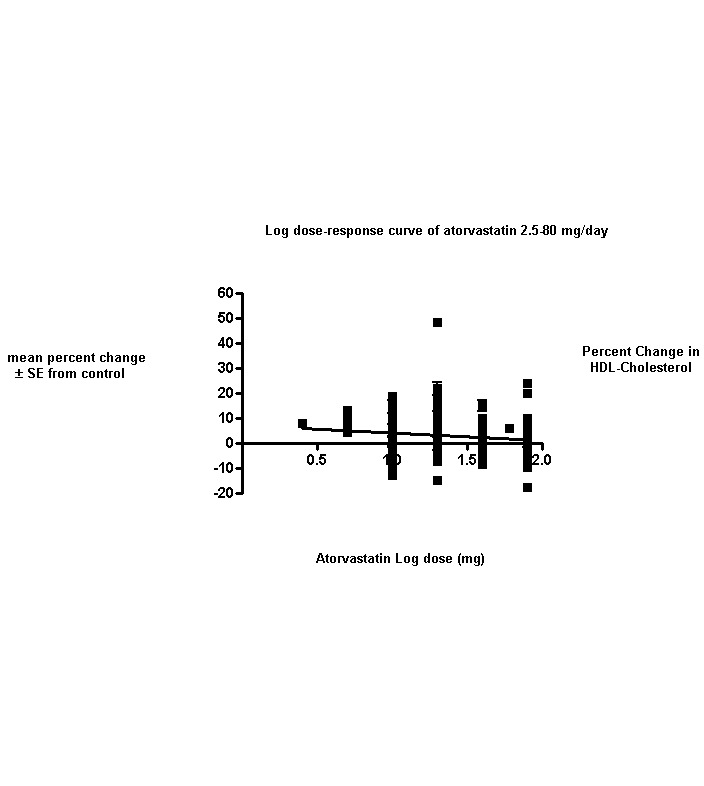
This updated review includes 296 trials involving 38,817 participants. These consist of 242 before‐and‐after trials and 54 placebo‐controlled trials. The numbers of placebo participants and atorvastatin participants were 1929 and 36,888, respectively. The numbers of male and female participants reported in 269 of the 296 trials were 20,228 and 16,454, respectively. Baseline mean (range) lipid parameters were as follows: total cholesterol, 6.65 (4.19‐11.90) mmol/L, 257 (162‐460) mg/dL; LDL‐cholesterol, 4.50 (2.38‐9.60) mmol/L, 174 (92‐371) mg/dL; HDL‐cholesterol, 1.25 (0.67‐4.30) mmol/L, 48.4 (25.9‐166.3) mg/dL; and triglycerides, 2.10 (0.76‐7.44) mmol/L, 186 (67‐659) mg/dL. Trials were available over the dose range of atorvastatin from 2.5 to 80 mg daily and were sufficient to generate dose‐response regression lines for each of these lipid parameters (Figure 3; Figure 4; Figure 5; Figure 6).
3.

Values represent the results of each trial for each dose comparison.
The standard error bars cannot be seen because they all lie within the points.
4.
Values represent the results of each trial for each dose comparison.
The standard error bars cannot be seen because they all lie within the points.
5.
Values represent the results of each trial for each dose comparison.
The standard error bars cannot be seen because they all lie within the points.
6.
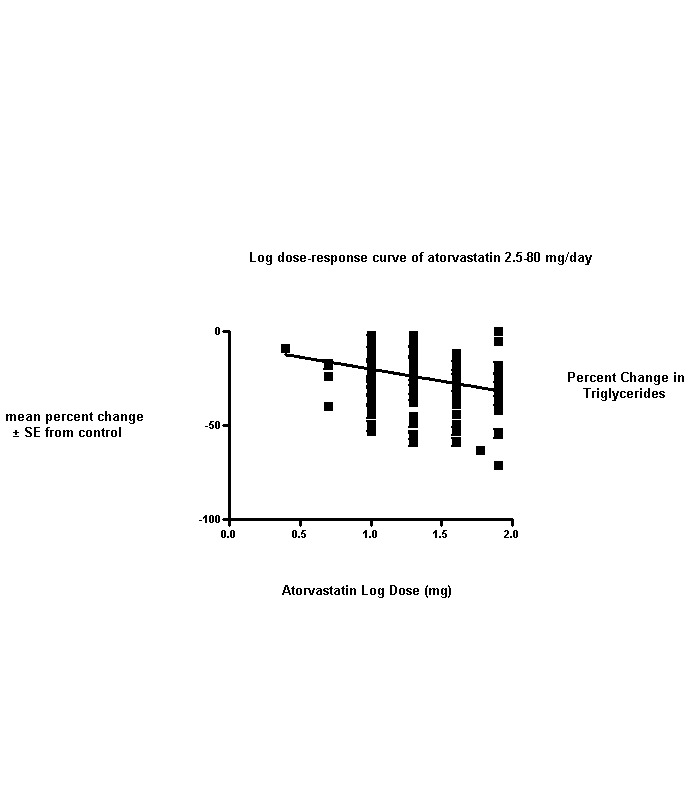
Values represent the results of each trial for each dose comparison.
The standard error bars cannot be seen because they all lie within the points.
Risk of bias in included studies
Sequence generation and allocation concealment could not be applied to the 242 before‐and‐after trials. Of the 54 placebo‐controlled trials, seven (13%) reported adequate sequence generation and 10 (18.5%) reported adequate allocation concealment. Thus risk of bias for these two categories was high. Risk of blinding bias was high for all before‐and‐after trials plus the three open‐label placebo‐controlled RCTs, the three trials in which blinding was not mentioned and the two single‐blind, placebo‐controlled RCTs. However, lack of blinding probably had little effect on primary outcomes, which included laboratory measurements of lipid parameters. Lack of blinding could have had an effect on the ascertainment of WDAEs. Incomplete outcome reporting leading to attrition bias was not a problem in this review, as few participants were lost to follow‐up and > 95% of participants completed treatment. Of 296 trials, 230 (77.7%) reported all lipid parameters; thus this was not a source of bias for the primary outcomes. See 'Risk of bias' tables in Characteristics of included studies, and for the overall risk of bias, see Figure 7 and Figure 8.
7.
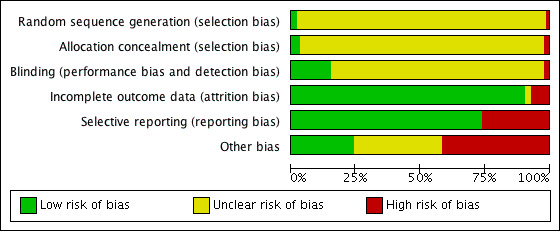
Risk of bias graph: authors' judgements about each risk of bias item presented as percentages across all included studies.
8.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
The other main potential source of bias is industry funding. Of the 296 trials, 140 (47%) reported funding by industry, 68 (23%) reported no funding by industry and 88 (29.8%) did not report the source of funding. Of the 140 industry‐funded trials, 77 (55.4%) were funded by Pfizer, the manufacturer of atorvastatin, and 63 (45%) were funded by other pharmaceutical companies.
Effects of interventions
See: Table 1
Overall efficacy of atorvastatin
Doses of 2.5 and 60 mg were provided in only one trial each, so we did not include the lipid data in the Data and analyses but did include the WDAE data (Data and analyses). We also included these two trials in calculations of log dose‐response curve equations. The efficacy of atorvastatin in lowering lipid parameters separately in placebo‐controlled trials and in before‐and‐after trials is shown in the Data and analyses section. This demonstrates that the two trial designs provide similar estimates of the lipid‐lowering efficacy of atorvastatin. In addition, we performed two‐tailed one‐sample t‐tests from the placebo‐controlled trials to test for differences between placebo mean effects and zero for total cholesterol, LDL‐cholesterol, HDL‐cholesterol and triglycerides. Results of these tests show that the placebo means were not statistically significantly different from zero: total cholesterol, ‐0.4 (95% CI ‐0.84 to 0.04), LDL‐cholesterol, 0.2 (95% CI ‐0.04 to 0.44), HDL‐cholesterol, 0.9 (95% CI 0.035 to 1.765) and triglycerides, 4.1 (95% CI 0.05 to 8.15). Evidence of lack of a placebo effect justified combining all trials to determine the overall efficacy of atorvastatin. This was done by entering all data into RevMan 5 using the generic inverse variance model outside of this review (data and analysis not shown). We have summarised the mean parameters from this analysis in Table 2.
1. Atorvastatin overall efficacy.
| Atorvastatin dose (mg/d) | 5 | 10 | 20 | 40 | 80 |
| Mean per cent change from control of total cholesterol |
‐26.0 | ‐27.0 | ‐30.7 | ‐34.1 | ‐37.9 |
| 95% confidence Interval | (‐29.6 to ‐22.4) | (‐27.2 to ‐26.8) | (‐31.0 to ‐30.4) | (‐34.6 to ‐33.6) | (‐38.4 to ‐37.5) |
| Mean per cent change from control of LDL‐C1 | ‐33.4 | ‐37.1 | ‐42.3 | ‐47.4 | ‐51.7 |
| 95% confidence Interval | (‐37.3 to ‐29.5) | (‐37.3 to ‐36.9) | (‐42.6 to ‐42.0) | (‐48.0 to ‐46.9) | (‐52.2 to ‐51.2) |
| Mean per cent change from control of HDL‐C2 | 8.0 | 4.8 | 3.7 | 3.7 | 1.6 |
| 95% confidence Interval | (3.7 to 12.3) | (4.6 to 5.0) | (3.3 to 4.0) | (3.1 to 4.2) | (1.1 to 2.1) |
| Mean per cent change from control of triglycerides | ‐27.7 | ‐18.0 | ‐20.5 | ‐29.4 | ‐28.3 |
| 95% confidence Interval | (‐38.6 to ‐16.8) | (‐18.4 to ‐17.5) | (‐21.2 to ‐19.8) | (‐30.8 to ‐28.0) | (‐29.6 to ‐27.0) |
aLDL‐C: low‐density lipoprotein cholesterol.
bHDL‐C: high‐density lipoprotein cholesterol.
Dose‐ranging effects of atorvastatin on blood lipids as calculated from the slopes of the log dose‐response curve equations
We entered data from all trials into GraphPad Prism 4 to yield a weighted least squares analysis based on the inverse of the square of the standard error for each lipid parameter to generate weighted log dose‐response curves for each of the lipid parameters.
Total cholesterol
The effects of different doses of atorvastatin on total cholesterol are shown in the Data and analyses section (Analysis 1.1; Analysis 2.1; Analysis 2.2; Analysis 3.1; Analysis 3.2; Analysis 4.1; Analysis 4.2; Analysis 5.1; Analysis 5.2). The updated analysis for total cholesterol yielded a log dose‐response straight‐line equation, y = ‐12.02 log(x) ‐ 15.01, which is similar to that provided in the original review and uses all data for atorvastatin doses ranging from 2.5 mg/d to 80 mg/d. When this formula was used, calculated reductions in total blood cholesterol were 19.8% for 2.5 mg and 37.9% for 80 mg. For every two‐fold dose increase, a 3.6% (95% CI 3.2 to 4.0) decrease in blood total cholesterol was noted (Figure 3).
1.1. Analysis.

Comparison 1 Atorvastatin 5.0 mg vs control, Outcome 1 Total cholesterol.
2.1. Analysis.
Comparison 2 Atorvastatin 10 mg vs control, Outcome 1 Total cholesterol.
2.2. Analysis.
Comparison 2 Atorvastatin 10 mg vs control, Outcome 2 Total cholesterol.
3.1. Analysis.
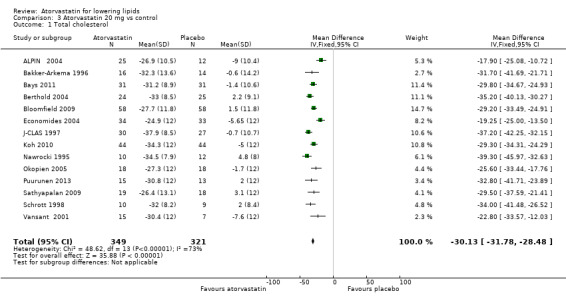
Comparison 3 Atorvastatin 20 mg vs control, Outcome 1 Total cholesterol.
3.2. Analysis.

Comparison 3 Atorvastatin 20 mg vs control, Outcome 2 Total cholesterol.
4.1. Analysis.
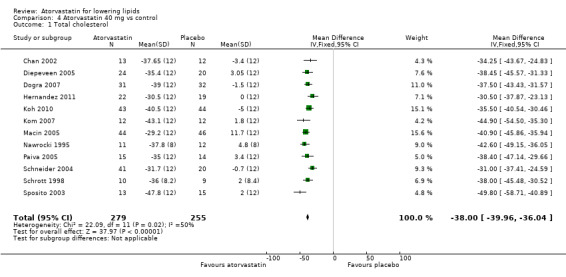
Comparison 4 Atorvastatin 40 mg vs control, Outcome 1 Total cholesterol.
4.2. Analysis.
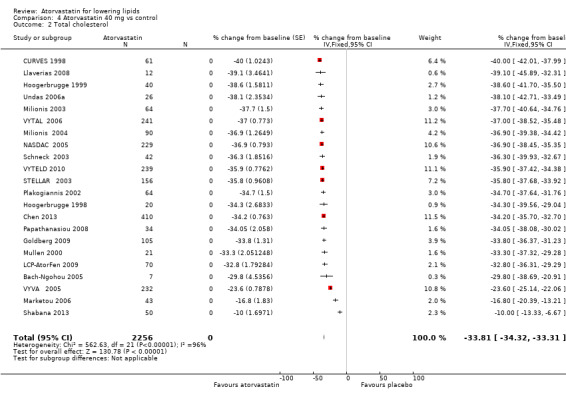
Comparison 4 Atorvastatin 40 mg vs control, Outcome 2 Total cholesterol.
5.1. Analysis.
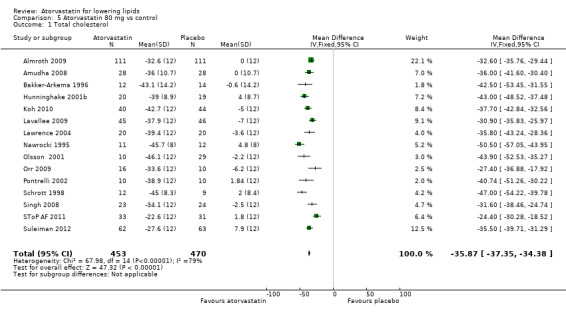
Comparison 5 Atorvastatin 80 mg vs control, Outcome 1 Total cholesterol.
5.2. Analysis.
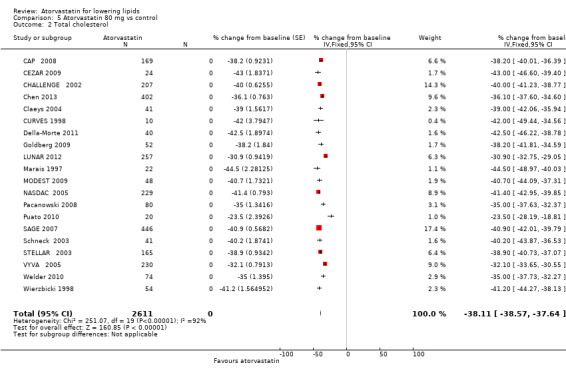
Comparison 5 Atorvastatin 80 mg vs control, Outcome 2 Total cholesterol.
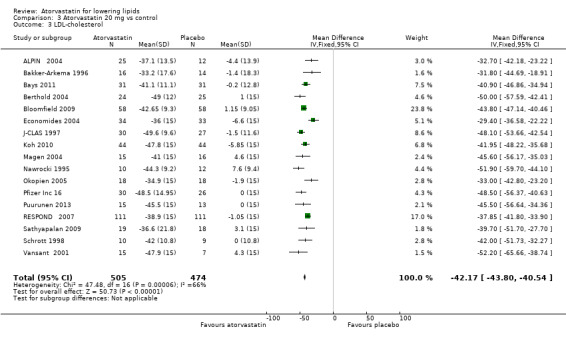
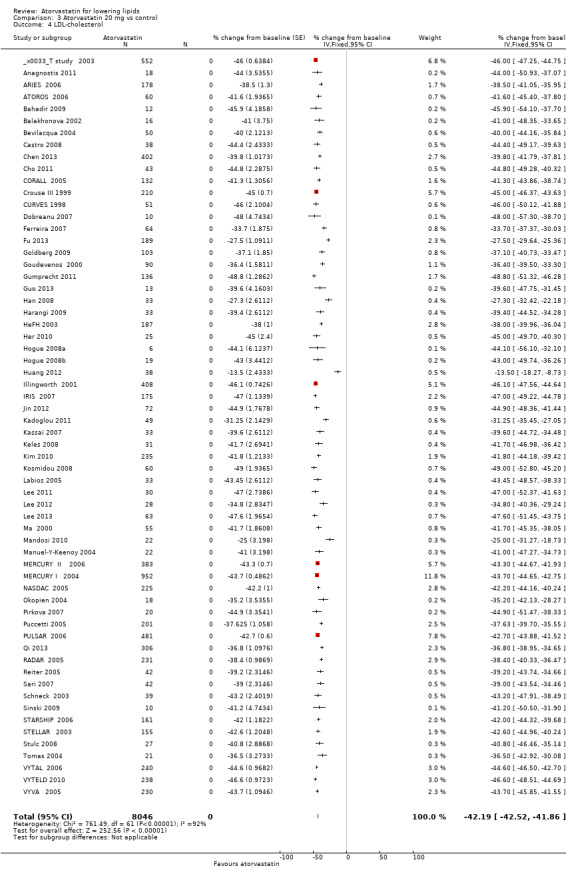
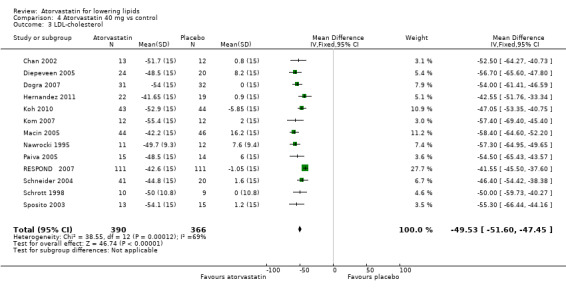
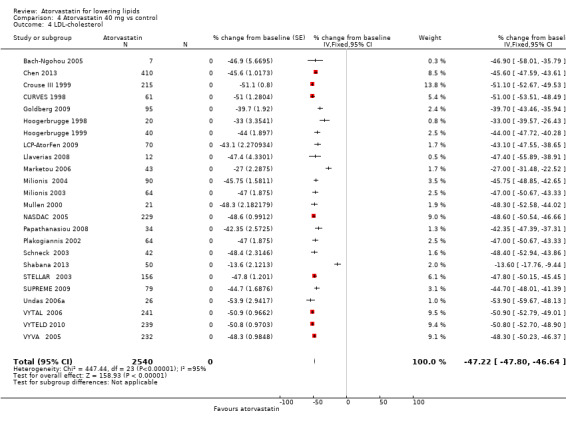
LDL‐cholesterol
The effects of different doses of atorvastatin on LDL‐cholesterol are shown in the Data and analyses section (Analysis 1.2; Analysis 2.3; Analysis 2.4; Analysis 3.3; Analysis 3.4; Analysis 4.3; Analysis 4.4; Analysis 5.3; Analysis 5.4). The updated analysis for LDL‐cholesterol yielded the log dose‐response straight‐line equation, y = ‐16.41 log(x) ‐ 20.74, which is a numerically but not statistically significant lower slope than was provided in the original review, ‐18.16. This analysis uses all available data for atorvastatin doses ranging from 2.5 mg/d to 80 mg/d. When this formula was used, calculated reductions in total blood LDL‐cholesterol were 27.3% for 2.5 mg/d and 52.0% for 80 mg/d. For every two‐fold dose increase, a 4.9% (95% CI 4.5 to 5.4) decrease in blood LDL‐cholesterol was noted (Figure 4).
1.2. Analysis.
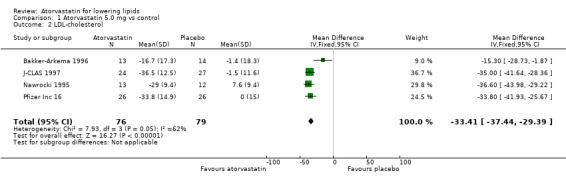
Comparison 1 Atorvastatin 5.0 mg vs control, Outcome 2 LDL‐cholesterol.
2.3. Analysis.
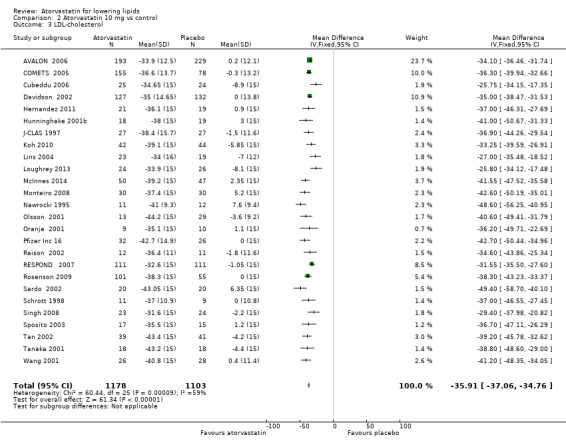
Comparison 2 Atorvastatin 10 mg vs control, Outcome 3 LDL‐cholesterol.
2.4. Analysis.
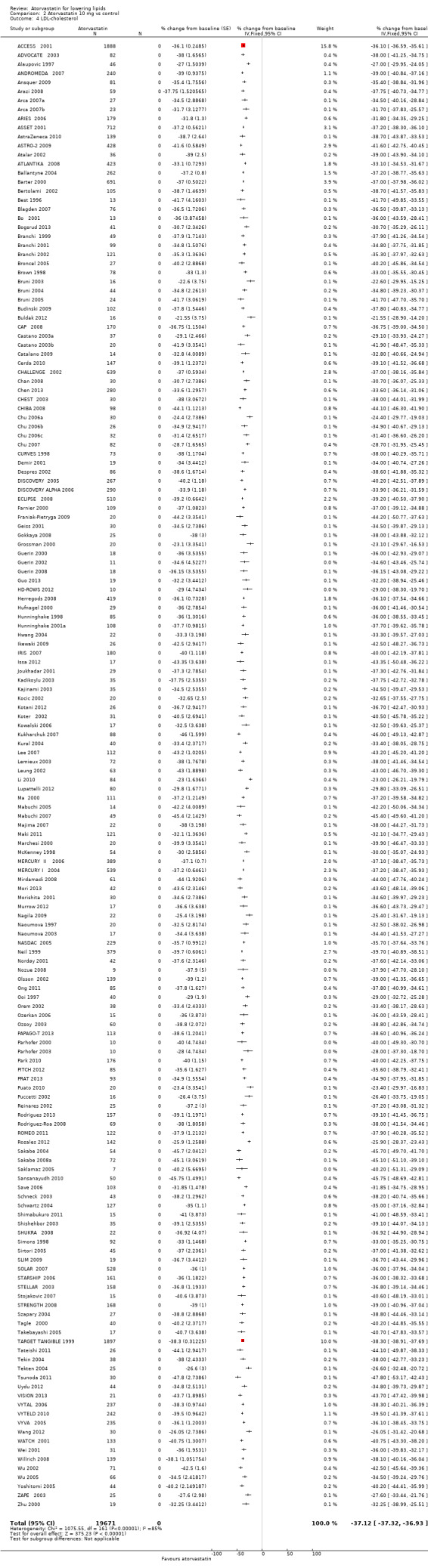
Comparison 2 Atorvastatin 10 mg vs control, Outcome 4 LDL‐cholesterol.
3.3. Analysis.
Comparison 3 Atorvastatin 20 mg vs control, Outcome 3 LDL‐cholesterol.
3.4. Analysis.
Comparison 3 Atorvastatin 20 mg vs control, Outcome 4 LDL‐cholesterol.
4.3. Analysis.
Comparison 4 Atorvastatin 40 mg vs control, Outcome 3 LDL‐cholesterol.
4.4. Analysis.
Comparison 4 Atorvastatin 40 mg vs control, Outcome 4 LDL‐cholesterol.
5.3. Analysis.
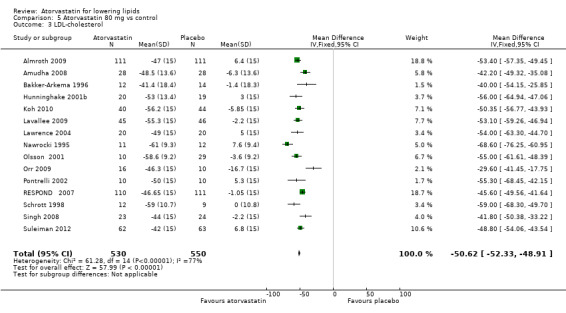
Comparison 5 Atorvastatin 80 mg vs control, Outcome 3 LDL‐cholesterol.
5.4. Analysis.
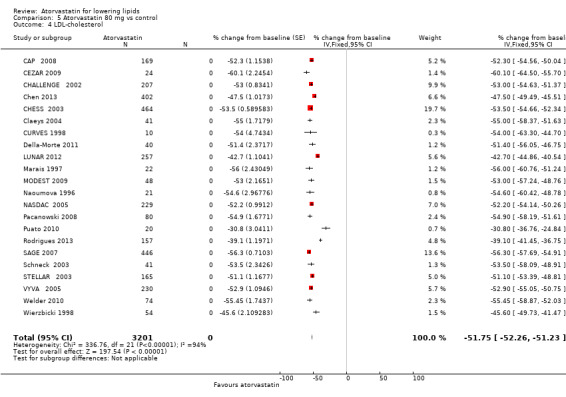
Comparison 5 Atorvastatin 80 mg vs control, Outcome 4 LDL‐cholesterol.
HDL‐cholesterol
The effects of different doses of atorvastatin on HDL‐cholesterol are shown in the Data and analyses section (Analysis 1.3; Analysis 2.5; Analysis 2.6; Analysis 3.5; Analysis 3.6; Analysis 4.5; Analysis 4.6; Analysis 5.5; Analysis 5.6). The updated analysis for HDL‐cholesterol yielded a significant log dose‐response straight‐line equation, y = ‐3.049 log(x) + 7.288. This represents a change from the original review, in which no significant log dose relation was seen for HDL. This analysis uses all available data for atorvastatin doses ranging from 2.5 mg/d to 80 mg/d. When this formula was used, calculated reductions in total blood HDL‐cholesterol were 6.1% for 2.5 mg/d and 1.5% for 80 mg/d, suggesting that lower doses of atorvastatin increase HDL and higher doses cause this effect to diminish. For every two‐fold dose increase, the HDL‐cholesterol increasing effect of atorvastatin diminished by 0.9% (95% CI 0.3 to 1.5) (Figure 5).
1.3. Analysis.
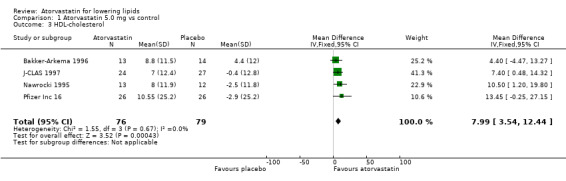
Comparison 1 Atorvastatin 5.0 mg vs control, Outcome 3 HDL‐cholesterol.
2.5. Analysis.
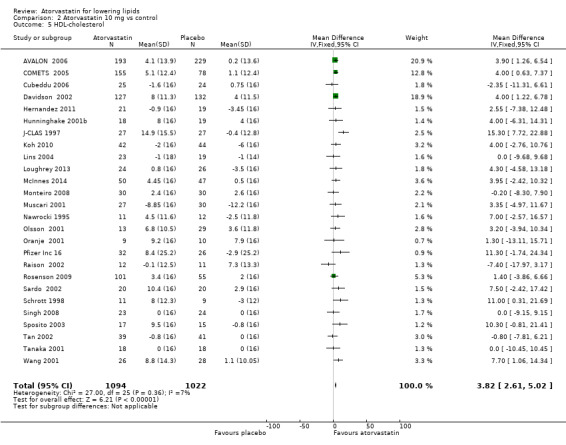
Comparison 2 Atorvastatin 10 mg vs control, Outcome 5 HDL‐cholesterol.
2.6. Analysis.
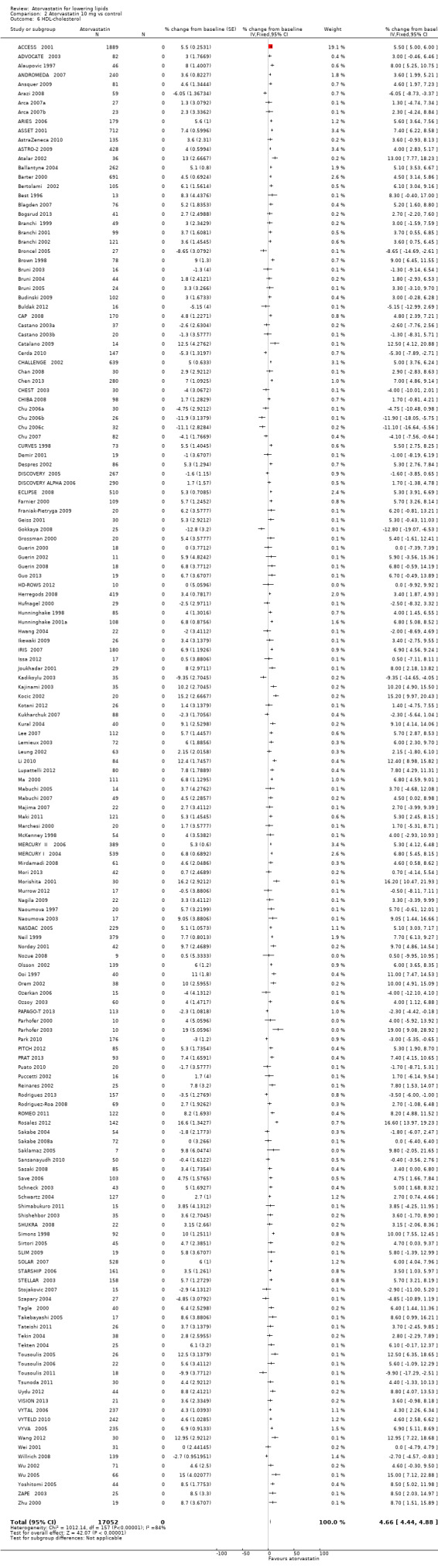
Comparison 2 Atorvastatin 10 mg vs control, Outcome 6 HDL‐cholesterol.
3.5. Analysis.
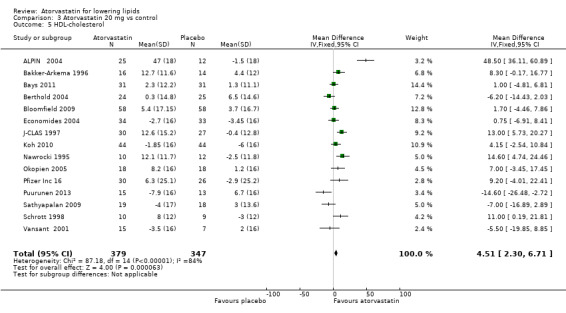
Comparison 3 Atorvastatin 20 mg vs control, Outcome 5 HDL‐cholesterol.
3.6. Analysis.
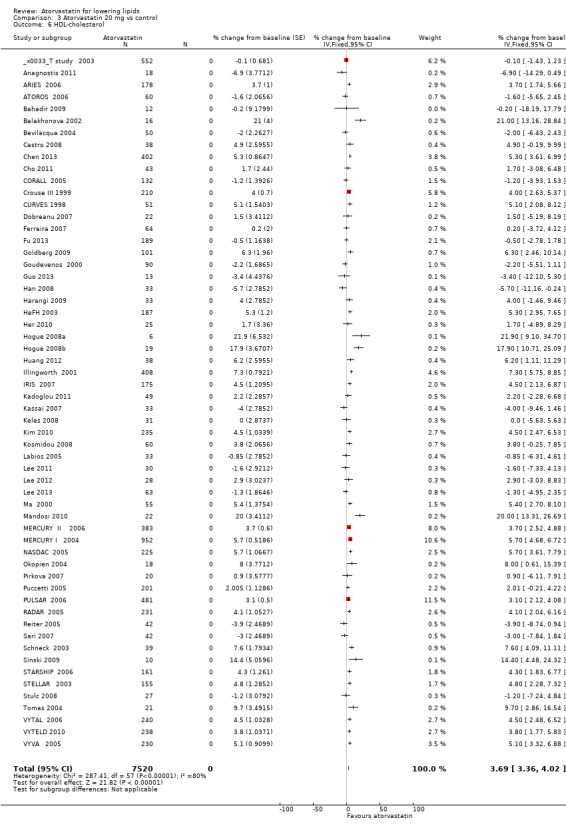
Comparison 3 Atorvastatin 20 mg vs control, Outcome 6 HDL‐cholesterol.
4.5. Analysis.

Comparison 4 Atorvastatin 40 mg vs control, Outcome 5 HDL‐cholesterol.
4.6. Analysis.
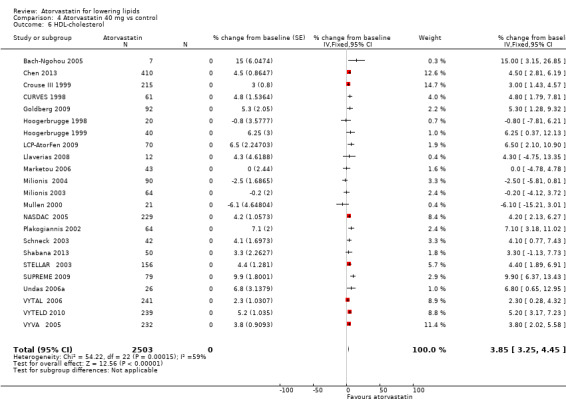
Comparison 4 Atorvastatin 40 mg vs control, Outcome 6 HDL‐cholesterol.
5.5. Analysis.
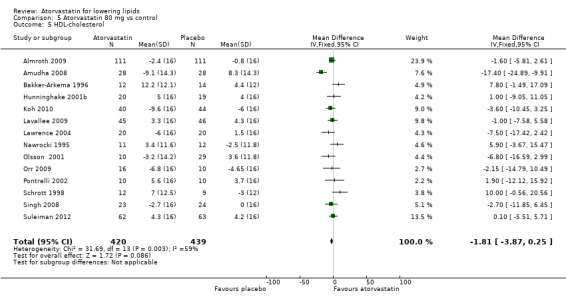
Comparison 5 Atorvastatin 80 mg vs control, Outcome 5 HDL‐cholesterol.
5.6. Analysis.
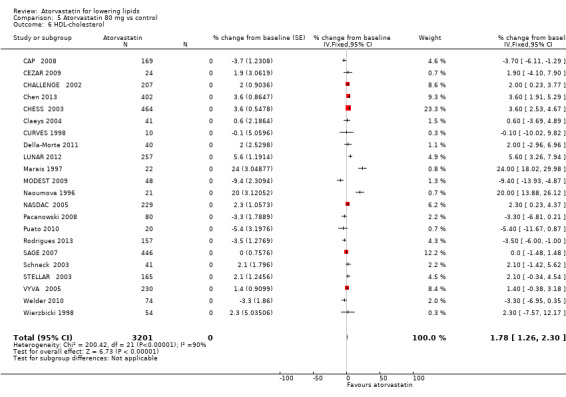
Comparison 5 Atorvastatin 80 mg vs control, Outcome 6 HDL‐cholesterol.
Triglycerides
The effects of different doses of atorvastatin on triglycerides are shown in the Data and analyses section (Analysis 1.4; Analysis 2.7; Analysis 2.8; Analysis 3.7; Analysis 3.8; Analysis 4.7; Analysis 4.8; Analysis 5.7; Analysis 5.8). The updated analysis for triglycerides yielded the log dose‐response straight‐line equation, y = ‐12.72 log(x) ‐ 7.206, which is similar to that provided in the original review and uses all data for atorvastatin doses ranging from 2.5 mg/d to 80 mg/d. When this formula was used, calculated reductions in triglycerides were 12.3% for 2.5 mg/d and 31.4% for 80 mg/d. For every two‐fold dose increase, a 3.8% (95% CI 2.7 to 5.0) decrease in blood triglycerides was noted (Figure 6).
1.4. Analysis.
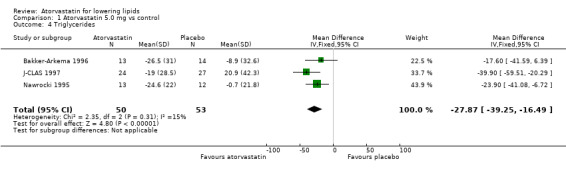
Comparison 1 Atorvastatin 5.0 mg vs control, Outcome 4 Triglycerides.
2.7. Analysis.
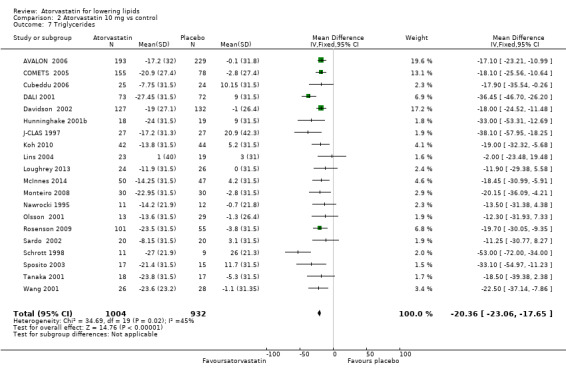
Comparison 2 Atorvastatin 10 mg vs control, Outcome 7 Triglycerides.
2.8. Analysis.
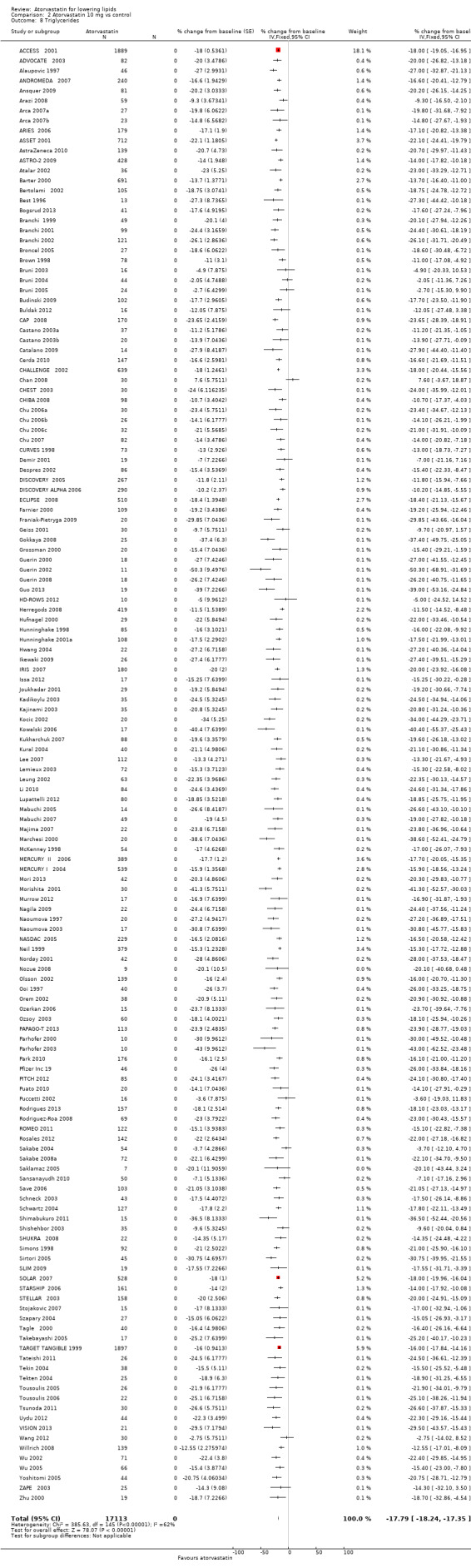
Comparison 2 Atorvastatin 10 mg vs control, Outcome 8 Triglycerides.
3.7. Analysis.
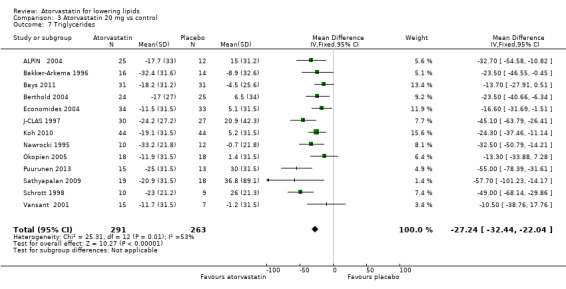
Comparison 3 Atorvastatin 20 mg vs control, Outcome 7 Triglycerides.
3.8. Analysis.
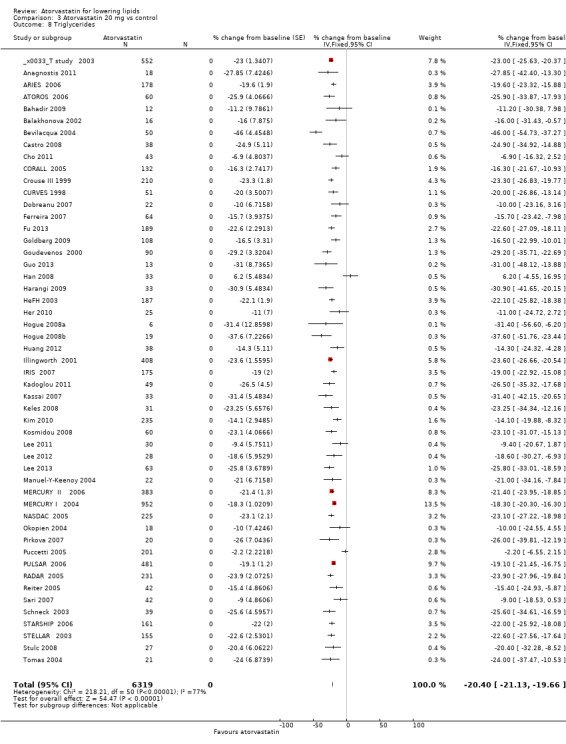
Comparison 3 Atorvastatin 20 mg vs control, Outcome 8 Triglycerides.
4.7. Analysis.
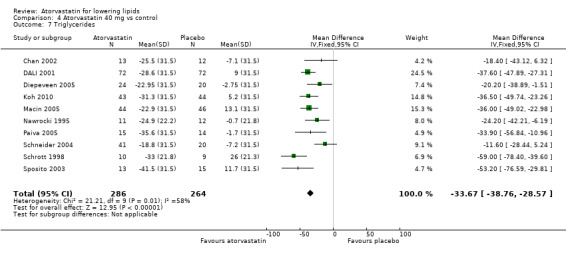
Comparison 4 Atorvastatin 40 mg vs control, Outcome 7 Triglycerides.
4.8. Analysis.
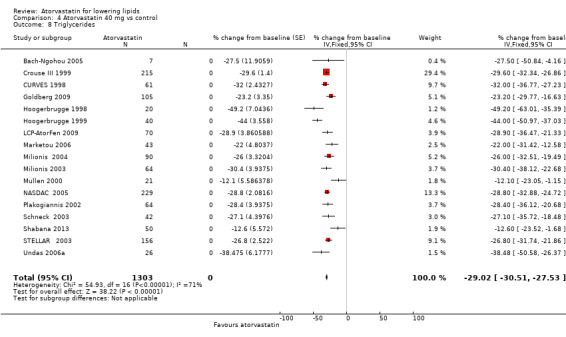
Comparison 4 Atorvastatin 40 mg vs control, Outcome 8 Triglycerides.
5.7. Analysis.
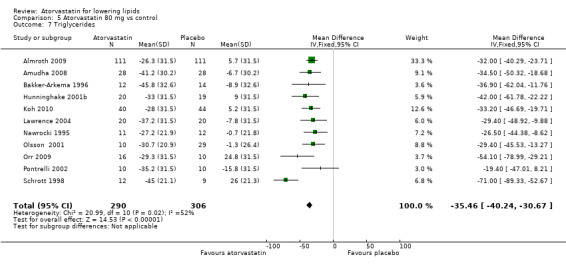
Comparison 5 Atorvastatin 80 mg vs control, Outcome 7 Triglycerides.
5.8. Analysis.
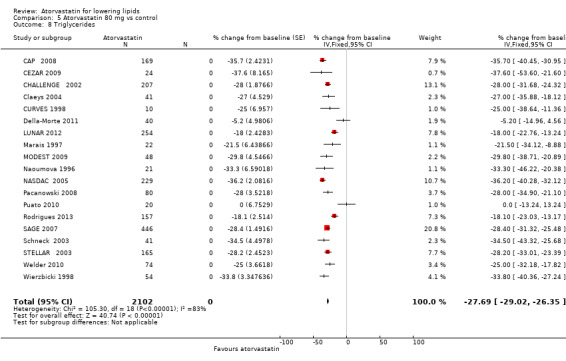
Comparison 5 Atorvastatin 80 mg vs control, Outcome 8 Triglycerides.
End‐of‐treatment variability
In 35 of the 54 placebo‐controlled trials, it was possible to compare end‐of‐treatment variability expressed as the co‐efficient of variation of atorvastatin 5, 10, 20, 40 and 80 mg/d versus placebo. One‐way analysis of variance showed a statistically significant increase in end‐of‐treatment variability of atorvastatin at all doses compared with placebo for total cholesterol (19.7 vs 14.7) and LDL‐cholesterol (31.6 vs 22.0). No statistically significant differences between all doses of atorvastatin compared with placebo were noted in the end‐of‐treatment HDL‐cholesterol co‐efficient of variation and in the triglyceride co‐efficient of variation.
Withdrawal data
Thirty‐four (63%) of the 54 placebo‐controlled trials reported WDAEs during the three‐ to 12‐week treatment period. No atorvastatin dose‐response relationship was observed for WDAEs; therefore a pooled estimate for all doses compared with placebo was done, providing a risk ratio (RR) of 0.98 (95% CI 0.68 to 1.40), suggesting no effect of atorvastatin on WDAEs in these short‐term trials, as was the case in the original review (Analysis 6.1).
6.1. Analysis.
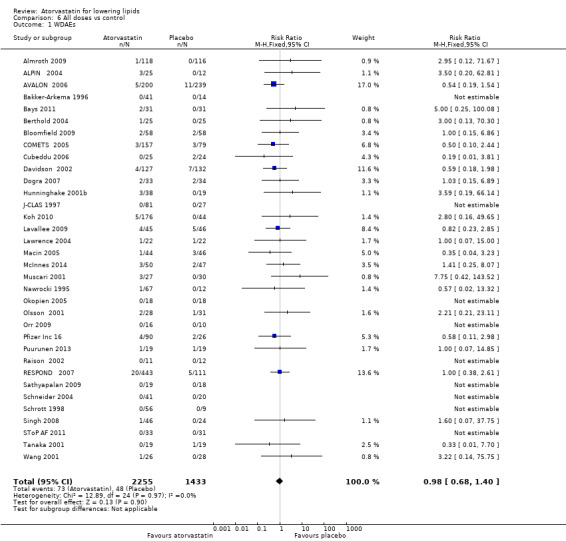
Comparison 6 All doses vs control, Outcome 1 WDAEs.
Overall completeness and applicability of evidence
For the male versus female comparison, sufficient participant data (> 100 in each group) were available only for the dose of 10 mg/d. These data were analysed for LDL‐cholesterol lowering efficacy using the generic inverse variance fixed‐effect model in RevMan 5 outside of this review. For 10 mg/d, LDL‐cholesterol lowering efficacy was ‐39.2% for male and ‐41.8% for female participants (P value < 0.05).
For the familial versus non‐familial comparison, sufficient participant data (> 100 in each group) were available for doses of 10 mg/d and 20 mg/d. These data were analysed for LDL‐cholesterol lowering efficacy using the generic inverse variance fixed‐effect model in RevMan 5. This subgroup analysis revealed that efficacy in familial participants was less than in non‐familial participants: 10 mg/d, ‐34.7% and ‐36.3% (P value 0.12) and 20 mg/d, ‐38.0% and ‐43.6% (P value < 0.00001), respectively.
Pfizer‐funded versus non‐Pfizer‐funded LDL‐cholesterol efficacy data were available for the doses 10, 20, 40 and 80 mg/d. These data were analysed separately using the generic inverse variance fixed‐effect model in RevMan 5. This sensitivity analysis revealed that the lipid‐lowering efficacy of atorvastatin in Pfizer‐funded trials was lower at 10 mg/d (‐36.8% vs ‐37.6%; P value 0.0004) but higher at 20 mg/d (‐44.0% vs ‐42.9%; P value 0.03), was not different at 40 mg/d (‐48.6% vs ‐48.8%; P value 0.78) and was higher at 80 mg/d (‐53.3% vs ‐51.2%; P value < 0.0001).
Review authors assessed publication bias by reviewing the funnel plots for all lipid outcomes with 10 or more trials. None of these funnel plots showed significant asymmetry.
Discussion
Summary of main results
Daily atorvastatin intake is highly effective in lowering blood low‐density lipoprotein (LDL)‐cholesterol concentrations, and it does so in a predictable dose‐related manner. The Table 1 documents the effect of daily atorvastatin intake on LDL‐cholesterol over the manufacturer‐recommended dose range of 10 to 80 mg/d, which is also the range for which this systematic review obtained the greatest quantity of data. Over this range, LDL‐cholesterol is decreased by 37.1% to 51.7%. These large reductions reflect a reduction in synthesis of cholesterol by the liver and indicate that liver 3‐hydroxy‐3‐methylglutaryl‐co‐enzyme A (HMG‐CoA) reductase is inhibited by one‐third to one‐half over this dose range. This has significant implications beyond circulating cholesterol, as LDL‐cholesterol is only one of many important biochemical products that are produced by the HMG‐CoA reductase pathway. For example, a systematic review has shown that statins reduce plasma co‐enzyme Q10 by 19% to 52% (Marcoff 2007). In addition, statins have been shown to reduce serum dolichol by 15.7% (Elmberger 1991) and serum squalene by 35.5% to 42.7% (Uusitupa 1992). This inhibitory effect occurs not only in the liver but also in other cells throughout the body (Holmqvist 2008). It is important to recognise that long‐term consequences of inhibition of these other compounds are presently unknown.
In the updated Data and analyses section, it can be seen that more trials used a before‐and‐after design than a placebo‐controlled design, and more data were derived from before‐and‐after trials than from placebo‐controlled trials. For doses for which large numbers of trials and participants were included, it can be seen that estimates of effect of atorvastatin on lipid parameters are similar with the two different trial designs. This, plus the demonstration that the placebo effect was not different from zero, justified use of generic inverse variance and display of the combined estimates in Table 2. In addition, all trial data were entered into GraphPad Prism 4 to calculate the regression lines shown in Figure 3, Figure 4, Figure 5 and Figure 6. Overall efficacy results from GraphPad Prism 4 provide the best estimate of treatment effect because it is based on a regression line calculated from all data for all doses. Estimates of average treatment effect derived from regression lines are similar to those shown in Table 2. The only exception is the estimate for atorvastatin 5 mg, for which estimates are based on only three trials and most likely represent an overestimation of the lipid‐lowering effect for that dose, as can be seen in Figure 3.
What is the effect of atorvastatin on end‐of‐treatment variability?
End‐of‐treatment variabilities of atorvastatin and placebo were compared to determine the effect of atorvastatin on variability of blood lipids when expressed as a co‐efficient of variation. Compared with placebo, atorvastatin at all doses increased the co‐efficient of variation of blood total cholesterol and LDL‐cholesterol. Atorvastatin did not significantly affect the variability of high‐density lipoprotein (HDL) and triglyceride measurements. The significance of this effect of atorvastatin of increasing variability of cholesterol and LDL‐cholesterol is unknown at this time.
Does atorvastatin increase withdrawals due to adverse effects?
Of 54 placebo‐controlled trials, 34 (63%) reported WDAEs. This analysis represented only 3688 participants, 2256 of whom received atorvastatin and 1432 of whom received placebo. Results are similar to those of the original review (Adams 2012) and show no dose‐response relationship of atorvastatin with WDAEs. The pooled estimate for all doses provided a similar risk ratio (RR) of 0.98 (95% CI 0.68 to 1.40), demonstrating uncertainty, but the possibility of a reduction or an increase in risk remains. As 20 (37%) of 54 placebo‐controlled trials did not report WDAEs, risk of selective reporting bias for this outcome is high, and the null effect may be a result of that bias. Furthermore, this analysis was limited to trials of three to 12 weeks' duration and thus does not reflect adverse effects of atorvastatin that occur after intake of longer duration. Risk of participant selection bias is probably high in these trials, as many of the participants studied probably were known to tolerate statins at baseline.
Overall completeness and applicability of evidence
This updated review included 296 trials with 38,817 participants ‐ representing a 16% increase in the quantity of data over data provided in the original review (Adams 2012). As such, this updated review has increased the certainty of evidence for the dose‐related lipid‐lowering effect of atorvastatin. Practitioners can use this evidence to calculate the expected effects of doses of atorvastatin commonly utilised in society. It is unlikely that further research will change these estimates appreciably. However, a fair amount of heterogeneity was noted in many of the estimates, and it is possible that this was due to differences in the populations studied (e.g. gender or genetic differences) (Thompson 2005). To explore this, we compared when possible the lipid‐lowering efficacy of atorvastatin between male and female trial participants and between participants with familial versus non‐familial hypercholesterolaemia.
Subgroup analyses in male and female participants were limited to 14 trials at a dose of 10 mg/d of atorvastatin. Analysis showed a small statistically significant increase in effect in females as compared with males, which is consistent with an effect that is 7% greater in females than in males. This is a new conclusion that did not appear in the original review but that moves in the direction that would be anticipated, as females weigh less on average than males. It is also consistent with the effect of rosuvastatin 10 mg/d, which was greater in females than in males (Adams 2014). Additional data are needed for this comparison. It is important for study authors to report data separately by sex; if this had been done in more of these trials, we could have been more confident in putting forth this conclusion.
Subgroup analyses comparing the effects of atorvastatin in familial versus non‐familial hypercholesterolaemia showed decreased efficacy of atorvastatin in lowering LDL‐cholesterol among individuals with familial hypercholesterolaemia. This difference could not be demonstrated in the original review.
The profound and relatively consistent effect of atorvastatin on lipid parameters shown in this review is probably appreciated by clinicians who treat patients with these drugs. Whether or not a patient is taking a statin is also most likely evident to investigators involved in placebo‐controlled randomised controlled trials (RCTs). Knowledge of lipid parameters almost certainly leads to loss of blinding in statin RCTs. The present review calls attention to this problem and suggests that efforts to prevent this loss of blinding are needed in future statin RCTs.
Quality of the evidence
The summary of all 'Risk of bias' tools for lipid effects suggests high risk of bias (Figure 7). However lipid parameter outcomes are probably relatively resistant to bias. If anything, high risk of bias would lead to an overestimation rather than an underestimation of lipid‐lowering effects. However, because of the objectivity of the lipid parameters, we believe that the estimates of effects are reasonably accurate. This view is strengthened by the fact that we could not show evidence consistent with a funding bias (see below), and review of funnel plots did not suggest evidence of publication bias.
That is not true for the outcome of assessing harm ‐ withdrawals due to adverse effects (WDAEs). This assessment could be performed only in placebo‐controlled trials, and this outcome was not reported in 20 (37%) of the 54 placebo‐controlled trials. Therefore risk of selective reporting bias is high, and this, combined with high risk of other biases, means that we cannot be confident that the suggested lack of increase in WDAEs is correct.
Other potential sources of bias
The most likely way that evidence of funding bias would be detected involved comparison of Pfizer‐funded trials, in which an overestimation of effect associated with industry might be expected, versus non‐Pfizer‐funded trials, in which a bias towards underestimation of the effect of atorvastatin might be expected. The fact that this comparison did not show a consistent effect one way or the other suggests that lipid measurements are relatively resistant to bias.
Potential biases in the review process
One limitation of this review is that many trials did not report standard deviations (SDs) for lipid‐lowering effects. In those trials, SDs of the per cent change from baseline of blood lipid parameters were imputed as the average of this parameter from trials that reported it. These values were determined by the method of Furukawa 2006. Such imputation might weight some studies more or less; however, in other reviews this has been shown to have little effect on the estimate of effect size (Heran 2008). Another limitation is that in this review, few studies were available that could demonstrate the effects of atorvastatin at very low and very high doses.
Agreements and disagreements with other studies or reviews
The best estimate of the mean percent reduction in blood LDL‐cholesterol for any dose of atorvastatin can be calculated from our log dose response equation. Using this equation y = ‐16.41 log(x) ‐20.74 an atorvastatin dose of 80 mg/day reduces LDL‐cholesterol by an average of 52.0%. This is within the range of 46.3% to 55.4% reduction in LDL‐cholesterol from the 11 comparative trials from the Drug Effectiveness Review Project (DERP) (Smith 2009), but significantly lower than the manufacturers prescribing information estimate of 60% (Lipitor Prescribing Information 2012) and the Adult Treatment Panel III estimate of 57% (NCEP 2002).
At the present time there is nothing to suggest that one statin is different than another statin in terms of the benefit in reduction of atherosclerotic‐related events: myocardial infarction and ischaemic stroke (Taylor 2013). It will be useful to complete the reviews of the other statins, cerivastatin, fluvastatin, lovastatin, pravastatin and simvastatin, to know how they compare in terms of the dose‐related effects on the lipid surrogate outcomes.
Authors' conclusions
Implications for practice.
Specific findings of the review
Atorvastatin 2.5 to 80 mg/d causes a linear dose‐response reduction in per cent change from control of blood total cholesterol and LDL‐cholesterol. Manufacturer‐recommended atorvastatin doses of 10 to 80 mg/d resulted in 37.1% to 51.7% decreases in LDL‐cholesterol. From the slope of the lines, it can be seen that for every two‐fold increase, a 3.6% and 4.9% decrease in blood total cholesterol and LDL‐cholesterol, respectively, was noted.
Atorvastatin has a dose‐response effect similar to that of rosuvastatin, but it is at least three‐fold less potent than rosuvastatin in reducing total and LDL‐cholesterol.
Subgroup analyses suggest that the LDL‐cholesterol‐lowering effect of atorvastatin is greater in females than in males and is lesser in people with familial hypercholesterolaemia than in people with non‐familial hypercholesterolaemia. These findings require confirmation by future trials.
All doses of atorvastatin did not change WDAEs as compared with placebo (risk ratio (RR) 0.98, 95% confidence interval (CI) 0.68 to 1.40). However, risk of bias for this outcome is high; thus this cannot be considered a reliable estimate.
Implication of these findings
This systematic review provides the best available evidence on dose‐related efficacy of atorvastatin for blood total cholesterol, LDL‐cholesterol, HDL‐cholesterol and triglycerides.
Implications for research.
More RCTs are needed of atorvastatin at higher and lower doses to expand the estimate of dose‐response efficacy of atorvastatin over a wider dose range.
All placebo‐controlled RCTs must accurately report withdrawals due to adverse effects and all serious adverse events.
All trials should report effects separately in males and in females, so it is possible to better determine whether any sex differences are present.
All trials should report effects separately for patients with familial and non‐familial hypercholesterolaemia to confirm whether efficacy is less in people with familial hypercholesterolaemia.
What's new
| Date | Event | Description |
|---|---|---|
| 24 January 2017 | Amended | corrected minor errors in citations in the additional references section; corrected citation Adams 2012a to Adams 2014; added reference to the atorvastatin protocol. |
History
Protocol first published: Issue 1, 2010 Review first published: Issue 12, 2012
| Date | Event | Description |
|---|---|---|
| 1 August 2014 | New citation required and conclusions have changed | Addition of 42 new trials allowed expansion of conclusions of the original review |
| 1 August 2014 | New search has been performed | Search was updated and 42 new trials were identified for inclusion |
| 4 March 2014 | Amended | Data in 'Summary of findings' table were corrected |
Acknowledgements
The review authors would like to acknowledge assistance provided by Gavin Wong, Dr Benji Heran, Dr David Godin and Alexandra Laugerotte, who assisted with validation of the data provided by included studies.
Appendices
Appendix 1. Search strategies
CENTRAL
1 Atorvastatin
2 Lipitor
3 CI‐981
4 1 or 2 or 3
MEDLINE (Ovid Sp)
1 Atorvastatin.af
2 Lipitor.tw
3 CI‐981.tw
4 1 or 2 or 3
5 Animals/
6 4 not 5
EMBASE (Ovid Sp)
1 Atorvastatin/
2 Atorvastatin.tw
3 Lipitor.tw
4 CI‐981.tw
5 1 or 2 or 3 or 4
6 Exp animals/not humans.sh
7 5 not 6
ISI Web of Science
1 Atorvastatin
2 Lipitor
3 Atorvas
4 "CI‐981"
5 CI‐981
6 1 or 2 or 3 or 4 or 5
BIOSIS Previews
1 Atorvastatin
2 lipitor
3 (1 or 2) AND TaxaNotes =(HUMANS)
Data and analyses
Comparison 1. Atorvastatin 5.0 mg vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total cholesterol | 3 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐26.04 [‐29.81, ‐22.28] |
| 2 LDL‐cholesterol | 4 | 155 | Mean Difference (IV, Fixed, 95% CI) | ‐33.41 [‐37.44, ‐29.39] |
| 3 HDL‐cholesterol | 4 | 155 | Mean Difference (IV, Fixed, 95% CI) | 7.99 [3.54, 12.44] |
| 4 Triglycerides | 3 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐27.87 [‐39.25, ‐16.49] |
Comparison 2. Atorvastatin 10 mg vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total cholesterol | 24 | 1902 | Mean Difference (IV, Fixed, 95% CI) | ‐25.44 [‐26.38, ‐24.50] |
| 2 Total cholesterol | 153 | 18009 | % change from baseline (Fixed, 95% CI) | ‐27.05 [‐27.21, ‐26.89] |
| 3 LDL‐cholesterol | 26 | 2281 | Mean Difference (IV, Fixed, 95% CI) | ‐35.91 [‐37.06, ‐34.76] |
| 4 LDL‐cholesterol | 162 | 19671 | % change from baseline (Fixed, 95% CI) | ‐37.12 [‐37.32, ‐36.93] |
| 5 HDL‐cholesterol | 26 | 2116 | Mean Difference (IV, Fixed, 95% CI) | 3.82 [2.61, 5.02] |
| 6 HDL‐cholesterol | 158 | 17052 | % change from baseline (Fixed, 95% CI) | 4.66 [4.44, 4.88] |
| 7 Triglycerides | 20 | 1936 | Mean Difference (IV, Fixed, 95% CI) | ‐20.36 [‐23.06, ‐17.65] |
| 8 Triglycerides | 146 | 17113 | % change from baseline (Fixed, 95% CI) | ‐17.79 [‐18.24, ‐17.35] |
Comparison 3. Atorvastatin 20 mg vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total cholesterol | 14 | 670 | Mean Difference (IV, Fixed, 95% CI) | ‐30.13 [‐31.78, ‐28.48] |
| 2 Total cholesterol | 58 | 7055 | % change from baseline (Fixed, 95% CI) | ‐30.66 [‐30.94, ‐30.39] |
| 3 LDL‐cholesterol | 17 | 979 | Mean Difference (IV, Fixed, 95% CI) | ‐42.17 [‐43.80, ‐40.54] |
| 4 LDL‐cholesterol | 62 | 8046 | % change from baseline (Fixed, 95% CI) | ‐42.19 [‐42.52, ‐41.86] |
| 5 HDL‐cholesterol | 15 | 726 | Mean Difference (IV, Fixed, 95% CI) | 4.51 [2.30, 6.71] |
| 6 HDL‐cholesterol | 58 | 7520 | % change from baseline (Fixed, 95% CI) | 3.69 [3.36, 4.02] |
| 7 Triglycerides | 13 | 554 | Mean Difference (IV, Fixed, 95% CI) | ‐27.24 [‐32.44, ‐22.04] |
| 8 Triglycerides | 51 | 6319 | % change from baseline (Fixed, 95% CI) | ‐20.40 [‐21.13, ‐19.66] |
Comparison 4. Atorvastatin 40 mg vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total cholesterol | 12 | 534 | Mean Difference (IV, Fixed, 95% CI) | ‐36.00 [‐39.96, ‐36.04] |
| 2 Total cholesterol | 22 | 2256 | % change from baseline (Fixed, 95% CI) | ‐33.81 [‐34.32, ‐33.31] |
| 3 LDL‐cholesterol | 13 | 756 | Mean Difference (IV, Fixed, 95% CI) | ‐49.53 [‐51.60, ‐47.45] |
| 4 LDL‐cholesterol | 24 | 2540 | % change from baseline (Fixed, 95% CI) | ‐47.22 [‐47.80, ‐46.64] |
| 5 HDL‐cholesterol | 11 | 471 | Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐3.45, 2.19] |
| 6 HDL‐cholesterol | 23 | 2503 | % change from baseline (Fixed, 95% CI) | 3.85 [3.25, 4.45] |
| 7 Triglycerides | 10 | 550 | Mean Difference (IV, Fixed, 95% CI) | ‐33.67 [‐38.76, ‐28.57] |
| 8 Triglycerides | 17 | 1303 | % change from baseline (Fixed, 95% CI) | ‐29.02 [‐30.51, ‐27.53] |
Comparison 5. Atorvastatin 80 mg vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total cholesterol | 15 | 923 | Mean Difference (IV, Fixed, 95% CI) | ‐35.87 [‐37.35, ‐34.38] |
| 2 Total cholesterol | 20 | 2611 | % change from baseline (Fixed, 95% CI) | ‐38.11 [‐38.57, ‐37.64] |
| 3 LDL‐cholesterol | 15 | 1080 | Mean Difference (IV, Fixed, 95% CI) | ‐50.62 [‐52.33, ‐48.91] |
| 4 LDL‐cholesterol | 22 | 3201 | % change from baseline (Fixed, 95% CI) | ‐51.75 [‐52.26, ‐51.23] |
| 5 HDL‐cholesterol | 14 | 859 | Mean Difference (IV, Fixed, 95% CI) | ‐1.81 [‐3.87, 0.25] |
| 6 HDL‐cholesterol | 22 | 3201 | % change from baseline (Fixed, 95% CI) | 1.78 [1.26, 2.30] |
| 7 Triglycerides | 11 | 596 | Mean Difference (IV, Fixed, 95% CI) | ‐35.46 [‐40.24, ‐30.67] |
| 8 Triglycerides | 19 | 2102 | % change from baseline (Fixed, 95% CI) | ‐27.69 [‐29.02, ‐26.35] |
Comparison 6. All doses vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 WDAEs | 34 | 3688 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.68, 1.40] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
3T study 2003.
| Methods | 4‐Week wash‐out period 52‐Week multi‐centre randomised double‐blind double‐dummy 8‐Week treatment period evening dose Randomisation code prepared by study statistician | |
| Participants | 1087 men and women from Scandinavia aged 35 to 75 years with CVD and dyslipidaemia; 552 participants received atorvastatin, 535 received simvastatin; LDL‐C ≥ 4.0 mmol/L (155 mg/dL) Exclusion criteria: TG ≥ 4.0 mmol/L (354 mg/dL), TC ≥ 10.0 mmol/L (387 mg/dL), secondary hypercholesterolaemia, unstable CVD, FH, lipid‐altering or antiarrhythmic drugs, hepatic dysfunction, statin hypersensitivity drugs associated with rhabdomyolysis in combination with statins Atorvastatin baseline TC: 7.25 mmol/L (280 mg/dL) Atorvastatin baseline LDL‐C: 5.19 mmol/L (201 mg/dL) Atorvastatin baseline HDL‐C: 1.21 mmol/L (46.79 mg/dL) Atorvastatin baseline TG: 1.87 mmol/L (166 mg/dL) | |
| Interventions | Atorvastatin 20 mg/d Atorvastatin conditional titration of 40 mg/d for 12 to 52 weeks Simvastatin 20 mg/d Simvastatin conditional titration of 40 mg/d for 12 to 52 weeks |
|
| Outcomes | % change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer Inc funded the study; Pfizer manufactures and markets atorvastatin |
ACCESS 2001.
| Methods | 4‐Week dietary lead‐in phase 54‐Week open‐label randomised parallel multi‐centre study |
|
| Participants | 3916 participants throughout the USA with type IIA and IIB hypercholesterolaemia were randomly assigned in a 4:1:1:1 ratio, but 131 participants were excluded from the ITT group because they did not take at least 1 dose of study medication or had a valid efficacy evaluation at baseline and post baseline, that is, 3785 participants formed the basis for the ITT efficacy analysis. 1902 received atorvastatin, 477 received fluvastatin, 476 received lovastatin, 462 received pravastatin and 468 received simvastatin LDL‐C 130 to 350 mg/dL (3.36‐9.05 mmol/L), TG < 400 mg/dL (4.52 mmol/L) Atorvastatin baseline TC: 6.83 mmol/L (264 mg/dL) Atorvastatin baseline LDL‐C: 4.60 mmol/L (178 mg/dL) Atorvastatin baseline HDL‐C: 1.24 mmol/L (48 mg/dL) Atorvastatin baseline TG: 2.15 mmol/L (190 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 6 weeks Atorvastatin conditional titration of 20 mg/d for 6 to 12 weeks Atorvastatin conditional titration of 40 mg/d for 12 to 18 weeks Atorvastatin conditional titration of 80 mg/d for 18 to 54 weeks Fluvastatin 10 mg/d for 0 to 6 weeks Fluvastatin conditional titration of 20 mg/d for 6 to 12 weeks Fluvastatin conditional titration of 40 mg/d for 12 to 18 weeks Fluvastatin conditional titration of 80 mg/d for 18 to 54 weeks Lovastatin 10 mg/d for 0 to 6 weeks Lovastatin conditional titration of 20 mg/d for 6 to 12 weeks Lovastatin conditional titration of 40 mg/d for 12 to 18 weeks Lovastatin conditional titration of 80 mg/d for 18 to 54 weeks Pravastatin 10 mg/d for 0 to 6 weeks Pravastatin conditional titration of 20 mg/d for 6 to 12 weeks Pravastatin conditional titration of 40 mg/d for 12 to 54 weeks Simvastatin 10 mg/d for 0 to 6 weeks Simvastatin conditional titration of 20 mg/d for 6 to 12 weeks Simvastatin conditional titration of 40 mg/d for 12 to 54 weeks |
|
| Outcomes | Per cent change from baseline in serum TC, LDL‐C, HDL‐C and TG at 6 weeks | |
| Notes | First atorvastatin dose was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13/1902 had no TC and TG data 14/1902 had no LDL‐C and HDL‐C data 0.7% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer Inc funded the study; Pfizer manufactures and sells atorvastatin |
ADVOCATE 2003.
| Methods | 6‐Week wash‐out period 16‐Week multi‐centre randomised open‐label study Evening dose | |
| Participants | 315 men and women from the USA; mean age 51.6 years (18‐70) 82 participants received atorvastatin, 157 received niacin ER/lovastatin, 76 received simvastatin; LDL‐C ≥ 130 mg/dL (3.36 mmol/L), TG < 300 mg/dL (3.39 mmol/L), HDL‐C < 50 mg/dL (1.29 mmol/L) Exclusion criteria: unstable CVD, alcohol abuse, drug abuse, uncontrolled mental disease, gall bladder disease, uncontrolled HTN, renal dysfunction, hepatic dysfunction, gout, peptic ulcer disease, fibromyalgia, cancer or other signs that could affect the study procedure or medications Atorvastatin baseline TC: 6.95 mmol/L (269 mg/dL) Atorvastatin baseline LDL‐C: 5.07 mmol/L (196 mg/dL) Atorvastatin baseline HDL‐C: 0.98 mmol/L (37.90mg/dL) Atorvastatin baseline TG: 1.99 mmol/L (176 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 8 weeks Atorvastatin 20 mg/d for 8 to 12 weeks Atorvastatin 40 mg/d for 12 to 16 weeks Simvastatin 10 mg/d for 0 to 8 weeks Simvastatin 20 mg/d for 8 to 12 weeks Simvastatin 40 mg/d for 12 to 16 weeks Niacin ER/lovastatin groups |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed SDs were imputed. TG data were not included because per cent change from baseline was expressed as a median |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | No TC lipid data were reported |
| Other bias | Unclear risk | Kos Pharmaceuticals funded the study; Kos markets simvastatin/niacin extended release; data may be biased against atorvastatin |
Alaupovic 1997.
| Methods | 6‐Week wash‐out period 24‐Week multi‐centre open‐label treatment period | |
| Participants | 46 men and women from Canada with elevated serum cholesterol and TG levels, aged 50 years; BMI < 33 (18‐80), TC > 5.2 mmol/L (201 mg/dL), TG > 2.3 mmol/L (203.7 mg/dL) Exclusion criteria: women who are likely to become pregnant, active liver disease, kidney disease, uncontrolled HTN, diabetes, > 10 alcoholic drinks/wk, drug abuse, E2/E2 phenotype Baseline TC: 7.45 mmol/L (288 mg/dL) Baseline LDL‐C: 4.60 mmol/L (178 mg/dL) Baseline HDL‐C: 0.9 mmol/L (34.8 mg/dL) Baseline TG: 4.53 mmol/L (401 mg/dL) | |
| Interventions | Atorvastatin 10 mg/d for 0 to 12 weeks Atorvastatin 20 mg/d for 12 to 24 weeks |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may be biased for atorvastatin |
Almroth 2009.
| Methods | Wash‐out period was not required because individuals receiving ongoing treatment with lipid‐lowering drugs were excluded 1‐Month multi‐centre double‐blind randomised placebo‐controlled trial |
|
| Participants | 234 men and women from Sweden with atrial fibrillation; cardioversion was performed on participants during the study; mean age 65 years (18‐80) Exclusion criteria: patients with paroxysmal atrial fibrillation, atrial flutter, contraindications against atorvastatin, ongoing treatment with Class I or Class III antiarrhythmics, oral amiodarone < 6 months before inclusion, known liver disease or a myopathy, patients with previous electrical CV < 1 year Placebo: Baseline TC: 5.35 mmol/L (207 mg/dL) Baseline LDL‐C: 3.13 mmol/L (121 mg/dL) Baseline HDL‐C: 1.32 mmol/L (51 mg/dL) Baseline TG: 1.58 mmol/L (140 mg/dL) Atorvastatin: Baseline TC: 5.21 mmol/L (201 mg/dL) Baseline LDL‐C: 3.19 mmol/L (123 mg/dL) Baseline HDL‐C: 1.26 mmol/L (48.7 mg/dL) Baseline TG: 1.67 mmol/L (150 mg/dL) |
|
| Interventions | Placebo Atorvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 1 month of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed; WDAEs were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Made a computer‐generated randomization list using blocks of six" |
| Allocation concealment (selection bias) | Low risk | "Participating centres received medical preparations distributed from the hospital pharmacy in Huddinge that was not involved in the randomization and packing procedure" "Placebo tablets were identical in size, taste, and weight to the atorvastatin tablets" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Assigned therapy was fully blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/116 discontinued placebo (4.3%) 7/118 discontinued atorvastatin (6%); participants were not analysed for efficacy |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured WDAEs were reported |
| Other bias | High risk | Pfizer funded the study through an unrestricted grant; data may support bias for the drug |
ALPIN 2004.
| Methods | 4‐Week run‐in phase 8‐Week multi‐centre double‐blind randomised parallel placebo‐controlled study |
|
| Participants | 41 men and women aged > 35 and ≤ 75 years with type 2 diabetes who had never had a major adverse cardiac event diagnosed or who had experienced a major adverse cardiac event that had been diagnosed at least 6 months before the study; participants had not received any hyperlipidaemic therapy. For 4 participants, the concentration of apoB in small dense LDL was not available at visit 4; therefore the full analysis set consisted of 37 participants, 25 in the atorvastatin group and 12 in the placebo group, for the efficacy analysis. 41 participants were included in the safety analysis Exclusion criteria: none No baseline lipid values |
|
| Interventions | Placebo Atorvastatin 20 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | WDAEs were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Placebo group: 1/13 were excluded Atorvastatin group: 3/28 were excluded 4/41 (9.8%) participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | The study was terminated prematurely because of difficulties with participant recruitment |
Amudha 2008.
| Methods | Wash‐out period was not required because individuals receiving lipid‐lowering agents were excluded from the study 4‐Week double‐blind randomised placebo‐controlled trial |
|
| Participants | 56 men and women from Malaysia aged 18 to 30 years; BMI < 25 and a history of type 2 diabetes mellitus in 1 or both parents Exclusion criteria: individuals with severe dyslipidaemia, CVD, chronic renal disease; individuals who smoked during the previous 6 months; childbearing potential; individuals receiving antihypertensives, glucocorticoids, antineoplastic agents, psychoactive agents and bronchodilators Placebo: Baseline TC: 4.9 mmol/L (189 mg/dL) Baseline LDL‐C: 3.2 mmol/L (124 mg/dL) Baseline HDL‐C: 1.2 mmol/L (46.4 mg/dL) Baseline TG: 1.5 mmol/L (133 mg/dL) Atorvastatin: Baseline TC: 5.0 mmol/L (193 mg/dL) Baseline LDL‐C: 3.3 mmol/L (128 mg/dL) Baseline HDL‐C: 1.1 mmol/L (42.5 mg/dL) Baseline TG: 1.7 mmol/L (151 mg/dL) |
|
| Interventions | Placebo Atorvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 1 month of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed WDAEs were not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Anagnostis 2011.
| Methods | No participant received lipid‐altering agents; no wash‐out period was required 12‐Week randomised open‐label trial |
|
| Participants | 36 men and women with dyslipidaemia 18 participants received atorvastatin, 18 received rosuvastatin Exclusion criteria: diabetes mellitus, cancer, thyroid dysfunction, any lipid‐lowering agents or anti‐obesity agents Atorvastatin baseline TC: 7.03 mmol/L (272 mg/dL) Atorvastatin baseline LDL‐C: 4.73 mmol/L (183 mg/dL) Atorvastatin baseline HDL‐C: 1.50 mmol/L (58 mg/dL) Atorvastatin baseline TG: 1.885 mmol/L (167 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d Rosuvastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 4‐12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin treatment arm was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | The source of funding was not reported |
ANDROMEDA 2007.
| Methods | 4‐Week wash‐out period 8‐Week multi‐centre randomised double‐blind parallel‐group treatment period | |
| Participants | 509 men and women from the UK at least 18 years of age with type 2 diabetes mellitus; 255 participants received atorvastatin, 254 received rosuvastatin; TG < 6.1 mmol/L Exclusion criteria: type 1 diabetes, glycated haemoglobin > 9.0%, CVD history or FH, ALT or AST > 1.4 (ULN), resting DBP or SBP > 95 mmHg or 200 mmHg, CK level > 3 × ULN Atorvastatin baseline TC: 5.5 mmol/L (213 mg/dL) Atorvastatin baseline LDL‐C: 3.4 mmol/L (132 mg/dL) Atorvastatin baseline HDL‐C: 1.2 mmol/L (46.4 mg/dL) Atorvastatin baseline TG: 4.53 mmol/L (186 mg/dL) | |
| Interventions | Atorvastatin 10 mg/d for 0 to 8 weeks Atorvastatin 20 mg/d for 8 to 16 weeks Rosuvastatin 10 mg/d for 0 to 8 weeks Rosuvastatin 20 mg/d for 8 to 16 weeks |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed TG SD was imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was available for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 240/255 were included in the ITT efficacy analysis at Week 8. 15/255 were not included. 6% of participants were not evaluated |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | AstraZeneca funded the study; data may support bias against atorvastatin |
Ansquer 2009.
| Methods | 4‐Week to 6‐week wash‐out dietary stabilisation period 12‐Week open‐label randomised parallel‐group study |
|
| Participants | 165 men and women aged 18 to 79 years with type II primary dyslipidaemia; LDL‐C ≥ 160 mg/dL (4.14 mmol/L), TG 150 to 400 mg/dL (1.69‐4.52 mmol/L) 81 participants received atorvastatin, 84 received fenofibrate Exclusion criteria: type I, III, IV, V dyslipidaemia; diabetes mellitus, pancreatitis, gall bladder disease, peptic ulcer disease, MI, cerebrovascular accident, unstable angina pectoris history or any severe life‐threatening disease, AST or ALT > 2 × ULN, gamma‐glutamyl transpeptidase > 2 × ULN, CK > 2 × ULN, creatinine > 133 μmol/L (1.5 mg/dL), TSH > 4 μIU/L, HRT; use of thiazides, steroids, retinoids, anti‐vitamin K, cyclosporine, erythromycin, digoxin or antifungal drugs; pregnancy or breastfeeding Atorvastatin baseline TC: 7.54 mmol/L (292 mg/dL) Atorvastatin baseline LDL‐C: 5.19 mmol/L (201 mg/dL) Atorvastatin baseline HDL‐C: 1.20 mmol/L (46 mg/dL) Atorvastatin baseline TG: 2.50 mmol/L (221 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Fenofibrate 200 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | 3 of the investigators were employees of Solvay Pharmaceuticals Company, which manufactures fenofibrate |
Arazi 2008.
| Methods | 4‐Week wash‐out dietary baseline stabilisation period 4‐Week open‐label study |
|
| Participants | 59 unrelated Brazilian men and women outpatients aged 31 to 77 years with hypercholesterolaemia and LDL‐C > 4 mmol/L (154.7 mg/dL) were treated with atorvastatin Exclusion criteria: gastrointestinal, thyroid, liver or renal disease; diabetes; familial dyslipidaemia TG > 4.4 mmol/L (389.7 mg/dL); under treatment with lipid‐lowering drugs, HRT or oral contraceptives Baseline TC: 6.76 mmol/L (261 mg/dL) Baseline LDL‐C: 4.55 mmol/L (176 mg/dL) Baseline HDL‐C: 1.46 mmol/L (56.5 mg/dL) Baseline TG: 1.60 mmol/L (142 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; treatment intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study seems to be free of other bias |
Arca 2007a.
| Methods | 6‐Week dietary wash‐out phase 24‐Week randomised open‐label study |
|
| Participants | 56 men and women with familial combined hyperlipidaemia, between 30 and 75 years old; TC and/or TG levels ≥ those of age‐ and sex‐specific 90th Italian population percentiles, hyperapobetalipoproteinaemia (apo B >130 mg/dL). Only families with at least 2 affected members presenting different lipid phenotypes were enrolled 27 participants received atorvastatin, 29 received fenofibrate Exclusion criteria: type III hyperlipidaemia, apo E2/E2 genotype, obesity, poorly controlled diabetes mellitus, those taking lipid‐affecting drugs Atorvastatin baseline TC: 6.68 mmol/L (258 mg/dL) Atorvastatin baseline LDL‐C: 4.30 mmol/L (166 mg/dL) Atorvastatin baseline HDL‐C: 1.16 mmol/L (45 mg/dL) Atorvastatin baseline TG: 2.70 mmol/L (239 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 6 weeks Atorvastatin conditional titration of 20 mg/d for 6 to 12 weeks Atorvastatin conditional titration of 40 mg/d for 12 to 18 weeks Atorvastatin conditional titration of 80 mg/d for 18 to 24 weeks Fenofibrate 200 mg/d for 0 to 24 weeks |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer partially funded the study; data may support bias for atorvastatin |
Arca 2007b.
| Methods | 6‐Week dietary wash‐out phase 24‐Week randomised open‐label study |
|
| Participants | 45 men and women with familial combined hyperlipidaemia 23 participants received atorvastatin, 22 received fenofibrate Exclusion criteria: thyroid and liver disease, renal dysfunction, obesity, poorly controlled diabetes mellitus, those taking lipid‐affecting drugs Atorvastatin baseline TC: 6.66 mmol/L (258 mg/dL) Atorvastatin baseline LDL‐C: 4.42 mmol/L (171 mg/dL) Atorvastatin baseline HDL‐C: 1.26 mmol/L (49 mg/dL) Atorvastatin baseline TG: 2.13 mmol/L (189 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d at 0 to 6 weeks Atorvastatin conditional titration of 20 mg/d for 6 to 12 weeks Atorvastatin conditional titration 40 mg/d for 12 to 18 weeks Atorvastatin conditional titration 80 mg/d for 18 to 24 weeks Fenofibrate 200 mg/d for 0 to 24 weeks |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin 10 mg/d at 0 to 6 weeks; treatment arm was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer partially funded the study; data may support bias for atorvastatin |
ARIES 2006.
| Methods | 6‐Week dietary wash‐out stabilisation period 6‐Week randomised open‐label multi‐centre treatment period | |
| Participants | 774 African American men and women > 17 years with type IIa and IIb hypercholesterolaemia; LDL‐C 160 to 300 mg/dL (4.14‐7.76 mmol/L), TG < 400 mg/dL (4.5 mmol/L) 383 participants received atorvastatin, 391 received rosuvastatin Exclusion criteria: homozygous type I, III or V hyperlipoproteinaemia; active arterial disease, uncontrolled HTN, poorly controlled diabetes, liver and renal dysfunction Atorvastatin baseline TC: 6.97 mmol/L (269 mg/dL) Atorvastatin baseline LDL‐C: 4.89 mmol/L (189 mg/dL) Atorvastatin baseline HDL‐C: 1.35 mmol/L (52.2 mg/dL) Atorvastatin baseline TG: 1.57 mmol/L (139 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 6 weeks Atorvastatin 20 mg/d for 0 to 6 weeks Rosuvastatin 10 mg/d for 0 to 6 weeks Rosuvastatin 20 mg/d for 0 to 6 weeks |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin groups were analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d and 20 mg/d; treatment arms were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d and 20 mg/d; treatment arms were analysed, and as no placebo group was included for comparison assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d and 20 mg/d; treatment arms were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Atorvastatin 10 mg group: 179/191 were included in the ITT efficacy analysis Atorvastatin 20 mg group: 178/192 were included in the ITT efficacy analysis Atorvastatin group: 357/383 were included in the ITT efficacy analysis 6.8% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | AstraZeneca funded the study; data may support bias against atorvastatin |
ASSET 2001.
| Methods | 8‐Week wash‐out period 54‐Week multi‐centre randomised open‐label parallel‐arm treat‐to‐target study | |
| Participants | 1424 men and women from the USA with mixed dyslipidaemia; mean age 61 years (18‐80) with and without CHD/peripheral vascular disease and with or without type 2 diabetes; TG 2.26 to 6.77 mmol/L (200‐600 mg/dL), LDL‐C 3.36 to 9.05 mmol/L (130‐350 mg/dL) Exclusion criteria: hepatic and renal dysfunction, uncontrolled hypothyroidism, statin hypersensitivity, lipid‐altering medications, MI, revascularisation procedures, unstable angina, significant medical or psychological disorders. 730 received atorvastatin and 694 received simvastatin Atorvastatin baseline TC: 7.36 mmol/L (285 mg/dL) Atorvastatin baseline LDL‐C: 4.69 mmol/L (181 mg/dL) Atorvastatin baseline HDL‐C: 1.11 mmol/L (42.92 mg/dL) Atorvastatin baseline TG: 3.43 mmol/L (304 mg/dL) | |
| Interventions | Atorvastatin 10 mg/d Simvastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 weeks: 18/730 were excluded for reasons such as "not safely evaluable, missing LDL cholesterol data at baseline or after randomization, study medication was discontinued for >48 hours before blood withdrawal" 2.5% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer Inc funded the study; Pfizer manufactures and sells atorvastatin; data may be biased towards atorvastatin |
AstraZeneca 2010.
| Methods | 4‐Week dietary wash‐out period 6‐Week before‐and‐after study |
|
| Participants | 146 men and women with hypercholesterolaemia aged 18 years or older LDL‐C ≥ 3.36 mmol/L (130 mg/dL) and < 6.50 mmol/L (250 mg/dL), fasting TG < 4.52 mmol/L (400 mg/dL) and a history of CHD or a CHD risk equivalent, or clinical evidence of atherosclerosis or 10‐year CHD risk ≥ 10% Exclusion criteria: none Atorvastatin baseline LDL‐C: 4.21 mmol/L (169 mg/dL) Atorvastatin baseline TG: 2.06 mmol/L (182.5 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Rosuvastatin 5 mg/d for 0 to 6 weeks Rosuvastatin 5 to 10 mg/d for 6 to 12 weeks |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/146 = 4.8% were not included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | Total cholesterol and HDL‐cholesterol data were not reported |
| Other bias | High risk | AstraZeneca funded the trial |
ASTRO‐2 2009.
| Methods | No wash‐out was required because participants were not receiving any lipid‐altering agents for at least 2 months 8‐Week open‐label randomised study |
|
| Participants | 877 men and women aged > 20 years with hypercholesterolaemia 442 randomly assigned to rosuvastatin 5 mg/d 435 randomly assigned to atorvastatin 10 mg/d Exclusion criteria: severe hypertension, type 1 diabetes mellitus, familial hypercholesterolaemia, fasting TG > 400 mg/dL, MI or cerebrovascular disorder within 3 months before the start of the study, serious cardiac insufficiency, revascularisation during the study period, active hepatic disease, renal dysfunction, pregnancy or possible pregnancy, hypothyroidism, muscle disease, drug and alcohol abuse Atorvastatin baseline LDL‐C: 4.38 mmol/L (169 mg/dL) Atorvastatin baseline HDL‐C: 1.57 mmol/L (61 mg/dL) Atorvastatin baseline TG: 1.46 mmol/L (129 mg/dL) |
|
| Interventions | Rosuvastatin 5 mg/d Atorvastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 4 to 8 weeks of plasma LDL‐C, HDL‐C and triglycerides | |
| Notes | Rosuvastatin 5 mg/d data were not analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/435 (0.7%) participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | TC was not included in the efficacy analysis |
| Other bias | Low risk | Industry did not fund the trial |
Atalar 2002.
| Methods | 6‐Week dietary stabilisation period 12‐Week open‐label study |
|
| Participants | 36 men and women with hyperlipidaemia with stable CAD; mean age 53 years; TC > 200 mg/dL, LDL‐C > 130 mg/dL Exclusion criteria: MI within 12 weeks, CABG surgery within 6 months, unstable angina, ongoing infection, diabetes mellitus, cancer, chronic liver disease, renal insufficiency, hypothyroidism, connective tissue disease, obesity, childbearing potential, treatment with anti‐inflammatory or anticoagulant drugs Baseline TC: 6.70 mmol/L (259 mg/dL) Baseline LDL‐C: 4.58 mmol/L (177 mg/dL) Baseline HDL‐C: 1.22 mmol/L (47 mg/dL) Baseline TG: 1.91 mmol/L (169 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
ATLANTIKA 2008.
| Methods | 4‐Week dietary baseline stabilisation period 24‐Week randomised open‐label trial |
|
| Participants | 697 men and women 18 to 75 years of age with CHD, dyslipidaemia and type 2 diabetes mellitus; LDL‐C > 2.5 mmol/L (97 mg/dL); HDL‐C < 1 mmol/L (39 mg/dL) in men and < 1.3 mmol/L (50.3 mg/dL) in women; TG ≥ 1.7 mmol/L (151 mg/dL); BP ≥ 130/85 mmHg 237 participants received atorvastatin 10 mg/d for 0 to 24 weeks; 234 received atorvastatin 10 mg/d for 0 to 4 weeks, then titrated atorvastatin from 20 mg/d to 80 mg/d for 4 to 24 weeks; 226 received common therapy, which could include lipid‐lowering drugs Exclusion criteria: TG > 4.5 mmol/L (399 mg/dL), TC > 8.0 mmol/L (309 mg/dL); secondary hyperlipidaemia due to uncontrolled hypothyroidism, nephrotic syndrome, type 1 diabetes mellitus, gall bladder disease, biliary disease, pancreatitis, active liver disease, renal dysfunction; AST, ALT and γ‐glutarylaminopeptidase are 2 × ULN, CK is 5 × ULN; acute illness within 1 month of screening, type III and IV hypercholesterolaemia; women who were pregnant or lactating; participants who were participating in another trial or were taking medication that affects serum lipids Group A atorvastatin TC: 6.47 mmol/L (250 mg/dL) Group A atorvastatin LDL‐C: 4.30 mmol/L (166 mg/dL) Group A atorvastatin HDL‐C: 1.35 mmol/L (52 mg/dL) Group A atorvastatin TG: 1.76 mmol/L (156 mg/dL) Group B atorvastatin TC: 6.30 mmol/L (244 mg/dL) Group B atorvastatin LDL‐C: 4.18 mmol/L (162 mg/dL) Group B atorvastatin HDL‐C: 1.29 mmol/L (50 mg/dL) Group B atorvastatin TG: 1.82 mmol/L (161 mg/dL) Groups A + B atorvastatin baseline TC: 6.39 mmol/L (247 mg/dL) Groups A + B atorvastatin baseline LDL‐C: 4.24 mmol/L (164 mg/dL) Groups A + B atorvastatin baseline HDL‐C: 1.32 mmol/L (51 mg/dL) Groups A + B atorvastatin baseline TG: 1.79 mmol/L (159 mg/dL) |
|
| Interventions | Group A atorvastatin 10 mg for 0 to 12 weeks Group B atorvastatin 10 mg for 0 to 4 weeks Atorvastatin 20 mg for 4 to 8 weeks Atorvastatin 40 mg for 8 to 12 weeks Atorvastatin 80 mg for 12 to 24 weeks Group C common therapy, which could include lipid‐lowering drugs |
|
| Outcomes | Per cent change from baseline at 4 to 12 weeks of LDL‐C | |
| Notes | First atorvastatin dose was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Group A atorvastatin 10 mg for 0 to 12 weeks and Group B atorvastatin 10 mg for 0 to 4 weeks; interventions were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Group A atorvastatin 10 mg for 0 to 12 weeks and Group B atorvastatin 10 mg for 0 to 4 weeks; interventions were analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Group A atorvastatin 10 mg for 0 to 12 weeks and Group B atorvastatin 10 mg for 0 to 4 weeks; interventions were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Group A: 21/237 were missing Group B: 27/234 were missing 48/471 were not included in the efficacy analysis 10% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | High risk | No data for TC, HDL‐C and TG at 4 to 12 weeks |
| Other bias | Low risk | The study appears to be free of other sources of bias |
ATOROS 2006.
| Methods | 6‐Week wash‐out period 24‐Week single‐centre open‐label randomised parallel‐group study | |
| Participants | 120 men and women from Greece with primary hyperlipidaemia; mean age 53 years; TC > 240 mg/dL (6.2 mmol/L), TG < 350 mg/dL (4.0 mmol/L) 60 participants received atorvastatin 20 mg/d, 60 received rosuvastatin 20 mg/d Exclusion criteria: hepatic dysfunction, renal dysfunction, diabetes mellitus, hyperthyroidism, medical conditions that threaten study protocol completion Atorvastatin baseline TC: 7.37 mmol/L (285 mg/dL) Atorvastatin baseline LDL‐C: 5.28 mmol/L (204 mg/dL) Atorvastatin baseline HDL‐C: 1.24 mmol/L (48 mg/dL) Atorvastatin baseline TG: 1.77 mmol/L (157 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d for 0 to 6 weeks Atorvastatin conditional titration of 40 mg/d for weeks 6 to 24 Rosuvastatin 20 mg/d for 0 to 6 weeks Rosuvastatin conditional titration of 40 mg/d for weeks 6 to 24 |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d for 0 to 6 weeks; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d for 0 to 6 weeks; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d for 0 to 6 weeks; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
AVALON 2006.
| Methods | 2‐Week to 6‐week wash‐out period 8‐Week multi‐centre double‐blind double‐dummy randomised placebo‐controlled study | |
| Participants | 848 men and women from the USA and Canada aged 18 to 75 years of any ethnicity with HTN and dyslipidaemia were randomly assigned; 847 took at least 1 dose of the study medication 239 participants received placebo, 200 received atorvastatin 10 mg/d, 201 received amlodipine 5 mg/d, 207 received atorvastatin 10 mg/d + amlodipine 5 mg/d Exclusion criteria: calcium channel blocker and statin intolerance, pregnancy or lactation, hepatic and renal dysfunction, serious cardiovascular problems within 3 to 6 months of screening, secondary dyslipidaemia or HTN of any aetiology, diabetes mellitus or disorders that would interfere with study Placebo baseline LDL‐C: 4.22 mmol/L (163 mg/dL) Atorvastatin baseline LDL‐C: 4.18 mmol/L (162 mg/dL) |
|
| Interventions | Placebo amlodipine and placebo atorvastatin for 0 to 8 weeks Placebo amlodipine and atorvastatin 10 mg/d for 0 to 8 weeks Amlodipine 5 mg/d and placebo atorvastatin for 0 to 8 weeks Amlodipine 5 mg/d + atorvastatin 10 mg/d for 0 to 8 weeks Amlodipine 5 mg/d + atorvastatin 10 mg/d for 8 to 16 weeks Amlodipine 5 to 10 mg/d + atorvastatin 10 to 80 mg/d for 16 to 28 weeks |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Placebo and atorvastatin monotherapy groups were analysed WDAEs were reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Phase one was an 8‐week, double‐blind, double‐dummy" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Placebo: 10/239 were not included in the ITT efficacy analysis Atorvastatin: 7/200 were not included in the ITT efficacy analysis 4% of participants were not included in the ITT efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer Inc funded the study; Pfizer markets atorvastatin; data may be biased towards atorvastatin |
Bach‐Ngohou 2005.
| Methods | None were taking any medication that could affect lipids for at least 2 months before the study 8‐Week open‐label study |
|
| Participants | 7 men and women with type 2 diabetes mellitus with mixed dyslipidaemia aged 47 to 65 years; 169 mg/dL < TG < 670 mg/dL (219 mg/dL < TC < 321 mg/dL) Baseline TC: 6.97 mmol/L (270 mg/dL) Baseline LDL‐C: 4.26 mmol/L (165 mg/dL) Baseline HDL‐C: 1.22 mmol/L (47 mg/dL) Baseline TG: 3.84 mmol/L (340 mg/dL) |
|
| Interventions | Atorvastatin 40 mg/d | |
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Number of participants was small (7). Pfizer funded the study; data may be biased towards atorvastatin |
Bahadir 2009.
| Methods | No wash‐out period was required; participants were not taking any medication that would affect lipid metabolism 8‐Week open‐label study |
|
| Participants | 12 men and women with hypercholesterolaemia and metabolic syndrome aged ≥ 30 years; TG ≥ 150 mg/dL, HDL‐C < 40 mg/dL Baseline TC: 6.35 mmol/L (246 mg/dL) Baseline LDL‐C: 4.17 mmol/L (161 mg/dL) Baseline HDL‐C: 1.17 mmol/L (45 mg/dL) Baseline TG: 2.37 mmol/L (210 mg/dL) Exclusion criteria: patients with severe renal disease or renal dysfunction, liver disease, inflammatory muscle disease, CVD, cancer except non‐melanoma skin cancer, antidiabetic treatment modification within the past 3 months, conditions known to influence metabolism or immunity, drug and substance dependency and a history of stroke or coronary syndrome within the past 3 months |
|
| Interventions | Atorvastatin 20 mg/d Rosuvastatin 10 mg/d Simvastatin 40 mg/d Pravastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding for the trial was not reported |
Bakker‐Arkema 1996.
| Methods | 4‐Week wash‐out period 4‐Week double‐blind randomised Placebo‐controlled parallel‐group multi‐centre study Randomisation method: stratified randomisation code based on the mean of the participant's qualifying LDL‐C levels | |
| Participants | 56 men and women from Canada and the USA with primary hypertriglyceridaemia aged 26 to 74 years; BMI ≤ 32, women of non‐childbearing potential, 2 total TG values ≥ 3.95 mmol/L (350 mg/dL) with the lower value within 30% of the higher value 14 participants received placebo, 13 received atorvastatin 5 mg/d, 16 received atorvastatin 10 mg/d, 12 received atorvastatin 80 mg/d Exclusion criteria: active liver disease, kidney disease, uncontrolled HTN, endocrine disease that affects serum lipids, > 14 alcoholic drinks per week, lipid‐altering medications Placebo baseline TC: 6.78 mmol/L (262 mg/dL) Placebo baseline LDL‐C: 2.98 mmol/L (115 mg/dL) Placebo baseline HDL‐C: 0.80 mmol/L (32 mg/dL) Placebo baseline TG: 7.04 mmol/L (624 mg/dL) Atorvastatin 5 mg/d baseline TC: 6.55 mmol/L (253 mg/dL) Atorvastatin 5 mg/d baseline LDL‐C: 3.15 mmol/L (122 mg/dL) Atorvastatin 5 mg/d baseline HDL‐C: 0.84 mmol/L (32 mg/dL) Atorvastatin 5 mg/d baseline TG: 6.14 mmol/L (544 mg/dL) Atorvastatin 10 mg/d baseline TC: 7.47 mmol/L (289 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 3.19 mmol/L (123 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 0.83 mmol/L (32 mg/dL) Atorvastatin 10 mg/d baseline TG: 7.44 mmol/L (659 mg/dL) Atorvastatin 80 mg/d baseline TC: 6.82 mmol/L (264 mg/dL) Atorvastatin 80 mg/d baseline LDL‐C: 2.79 mmol/L (108 mg/dL) Atorvastatin 80 mg/d baseline HDL‐C: 0.80 mmol/L (31 mg/dL) Atorvastatin 80 mg/d baseline TG: 6.62 mmol/L (586 mg/dL) |
|
| Interventions | Placebo Atorvastatin 5 mg/d Atorvastatin 10 mg/d Atorvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | WDAEs not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All primary objective parameters were measured |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may be biased towards atorvastatin |
Balakhonova 2002.
| Methods | 4‐Week baseline dietary stabilisation period 12‐Week open‐label trial |
|
| Participants | 16 men and women aged 32 to 63 years with type 2a hypercholesterolaemia; LDL‐C ≥ 7 mmol/L (271 mg/dL), TG ≤ 4 mmol/L (354 mg/dL) Exclusion criteria: statin hypersensitivity, diabetes mellitus, unstable angina, uncontrolled HTN with BP > 150/100 mmHg, active liver disease, active kidney disease, serum liver enzymes > 20% ULN, women receiving HRT Baseline TC: 10.7 mmol/L (415 mg/dL) Baseline LDL‐C: 8.6 mmol/L (333 mg/dL) Baseline HDL‐C: 1.4 mmol/L (54 mg/dL) Baseline TG: 1.5 mmol/L (133 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Ballantyne 2004.
| Methods | 20‐Week wash‐out period 24‐Week multi‐centre double‐blind randomised study Evening dose | |
| Participants | 788 men and women from the USA aged > 17 years with hypercholesterolaemia; LDL‐C > 130 mg/dL (3.36 mmol/L), TG < 350 mg/dL (3.95 mmol/L) 262 received atorvastatin, 526 received Vytorin + simvastatin Exclusion criteria: no active liver disease or renal disease Atorvastatin baseline TC: 6.90 mmol/L (268 mg/dL) Atorvastatin baseline LDL‐C: 4.67 mmol/L (118 mg/dL) Atorvastatin baseline HDL‐C: 1.21 mmol/L (31.71 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for weeks 0 to 6 Atorvastatin 20 mg/d for weeks 6 to 12 Atorvastatin 40 mg/d for weeks 12 to 18 Atorvastatin 80 mg/d for weeks 18 to 24 Vytorin + simvastatin 10 mg/d for weeks 0 to 6 Vytorin + simvastatin 20 mg/d for weeks 6 to 12 Vytorin + simvastatin 40 mg/d for weeks 12 to 18 Vytorin + simvastatin 80 mg/d for weeks 18 to 24 Vytorin + simvastatin 20 mg/d for weeks 0 to 6 Vytorin + simvastatin 40 mg/d for weeks 6 to 12 Vytorin + simvastatin 40 mg/d for weeks 12 to 18 Vytorin + simvastatin 80 mg/d for weeks 18 to 24 |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C and HDL‐C | |
| Notes | First atorvastatin dose was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | No TG data were reported because reported values were median values |
| Other bias | High risk | Merck funded the study; data may support bias against atorvastatin |
Barter 2000.
| Methods | 4‐Week wash‐out period 6‐Week open‐label randomised parallel‐group multi‐centre study Evening dose | |
| Participants | 1028 men and women from Australia aged 18 to 75 years with hypercholesterolaemia; TC > 5.5 mmol/L (213 mg/dL), HDL‐C < 1.0 mmol/L (39 mg/dL) 691 participants received atorvastatin, 337 received simvastatin Exclusion criteria: secondary hypercholesterolaemia, CHD symptoms in the previous 3 months, plasma TG > 4.0 mmol/L, ALT or AST > 1.5 × ULN, CK > 3 × ULN, statin hypersensitivities, other conditions precluding completion of study Atorvastatin baseline TC: 7.41 mmol/L (287 mg/dL) Atorvastatin baseline LDL‐C: 5.22 mmol/L (202 mg/dL) Atorvastatin baseline HDL‐C: 1.23 mmol/L (47.5 mg/dL) Atorvastatin baseline TG: 2.10 mmol/L (186 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 6 weeks Atorvastatin conditionally titrated to 20 mg/d for 6 to 12 weeks Atorvastatin conditionally titrated to 40 mg/d for 12 to 18 weeks Atorvastatin conditionally titrated to 80 mg/d for 18 to 24 weeks Simvastatin 10 mg/d for 6 weeks Simvastatin conditionally titrated to 20 mg/d for 6 to 12 weeks Simvastatin conditionally titrated to 40 mg/d for 12 to 18 weeks Simvastatin conditionally titrated to 80 mg/d for 18 to 24 weeks |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer funded the study; data may support bias in favour of atorvastatin |
Bays 2011.
| Methods | 5‐Week single‐blind placebo wash‐out period 8‐Week multi‐centre double‐blind randomised placebo‐controlled study |
|
| Participants | 62 dyslipidaemic obese men and women aged 18 to 75 years; LDL‐C 130 to 280 mg/dL, TG 150 to 550 mg/dL, HDL‐C no greater than 60 mg/dL 31 participants received placebo, 31 received atorvastatin 20 mg/d Exclusion criteria: patients with diabetes mellitus, CVD, secondary dyslipidaemia Placebo: Baseline TC: 6.51 mmol/L (252 mg/dL) Baseline LDL‐C: 4.16 mmol/L (161 mg/dL) Baseline HDL‐C: 1.13 mmol/L (44 mg/dL) Baseline TG: 2.87 mmol/L (254 mg/dL) Atorvastatin: Baseline TC: 6.35 mmol/L (246 mg/dL) Baseline LDL‐C: 4.27 mmol/L (165 mg/dL) Baseline HDL‐C: 1.1 mmol/L (42.5 mg/dL) Baseline TG: 2.26 mmol/L (200 mg/dL) |
|
| Interventions | Placebo Atorvastatin 20 mg/d MBX‐8025 50 mg/d, 100 mg/d MBX‐8025 50 mg/d + atorvastatin 20 mg/d MBX‐8025 100 mg/d + atorvastatin 20 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG; withdrawals due to adverse events | |
| Notes | Placebo and atorvastatin monotherapy groups were analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment process was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Metabolex funded the study; the company makes MBX‐8025; data may support bias against atorvastatin |
Berthold 2004.
| Methods | 8‐Week wash‐out period 8‐Week multi‐centre randomised assignment in blocks of 6; double‐blind placebo‐controlled trial |
|
| Participants | 50 menopausal (of at least 2 years) women > 55 years old 25 participants received placebo, 25 received atorvastatin Exclusion criteria: metabolic bone disease, non‐postmenopausal bone loss, untreated hyperthyroidism, inadequately treated hypothyroidism, liver disease, elevated serum transaminases > 2 × ULN, kidney disease, severe heart disease, muscular or neuromuscular disease and/or CK > 3 × ULN, cancer, statin intolerance, HRT, use of drugs to affect bone metabolism, diabetes mellitus, smoking, complete lack of exercise Placebo baseline TC: 6.54 mmol/L (253 mg/dL) Placebo baseline LDL‐C: 4.27 mmol/L (165 mg/dL) Placebo baseline HDL‐C: 1.76 mmol/L (68 mg/dL) Placebo baseline TG: 1.19 mmol/L (105 mg/dL) Atorvastatin baseline TC: 6.44 mmol/L (249 mg/dL) Atorvastatin baseline LDL‐C: 4.14 mmol/L (160 mg/dL) Atorvastatin baseline HDL‐C: 1.81 mmol/L (70 mg/dL) Atorvastatin baseline TG: 1.22 mmol/L (108 mg/dL) |
|
| Interventions | Placebo Atorvastatin 20 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | WDAEs were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "A multicenter, randomized, double‐blind, placebo‐controlled trial" "All investigators were blinded to the lipid measurements" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Atorvastatin 20 mg: 1/25 were missing because the study was terminated after 4 weeks as the result of viral bronchitis 2% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Bertolami 2002.
| Methods | 4‐Week wash‐out period 12‐Week multi‐centre randomised open treatment period | |
| Participants | 157 participants from Brazil with hypertriglyceridaemia and hypercholesterolaemia aged 45 to 65 years; LDL 130 to 250 mg/dL (3.36‐6.46 mmol/L), TG < 400 mg/dL (4.52 mmol/L) 107 participants received atorvastatin, 50 received simvastatin Exclusion criteria: statin hypersensitivity, uncontrolled HTN, secondary hyperlipidaemia, aged < 18 or > 80 years, major coronary events, lipid‐altering drugs Atorvastatin baseline TC: 7.14 mmol/L (276 mg/dL) Atorvastatin baseline LDL‐C: 4.93 mmol/L (191 mg/dL) Atorvastatin baseline HDL‐C: 1.27 mmol/L (49.11 mg/dL) Atorvastatin baseline TG: 2.05 mmol/L (182 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 6 weeks Atorvastatin 20 mg/d for 6 to 12 weeks Simvastatin 10 mg/d for 0 to 6 weeks Simvastatin 20 mg/d for 6 to 12 weeks |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Atorvastatin group: 2/107 were not included in the efficacy analysis Comment: very small number; 2% of participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Best 1996.
| Methods | 4‐Week wash‐out period 4‐Week open‐label Randomised multi‐centre; treatment period evening dose | |
| Participants | 25 men and women outpatients from Australia with NIDDM with elevated cholesterol; 13 received atorvastatin, 12 received simvastatin; mean age 63 years; LDL ≥ 4.1 mmol/L (159 mg/dL) Exclusion criteria: BMI < 20 or > 38, DBP > 95 mmHg; hepatic, renal or thyroid dysfunction; alcohol intake > 140 g/wk, lipid‐altering drug intake Atorvastatin baseline TC: 7.10 mmol/L (275 mg/dL) Atorvastatin baseline LDL‐C: 4.80 mmol/L (186 mg/dL) Atorvastatin baseline HDL‐C: 1.20 mmol/L (46 mg/dL) Atorvastatin baseline TG: 2.20 mmol/L (195 mg/dL) | |
| Interventions | Atorvastatin 10 mg/d Simvastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may be biased in favour of atorvastatin |
Bevilacqua 2004.
| Methods | 4‐Week wash‐out period
3‐Month single‐centre open‐label randomised study via a randomisation list Evening dose |
|
| Participants | 100 men and women from Italy with NIDDM aged 45 to 71 years; LDL‐C 150 to 300 mg/dL (3.9‐7.76 mmol/L), HDL‐C < 50 mg/dL (1.29 mmol/L), TG > 200 mg/dL (2.256 mmol/L) 50 participants received fluvastatin, 50 received atorvastatin No baseline lipid values were reported Exclusion criteria: surgery, hepatic and renal dysfunction, serious cardiovascular events within 6 months, statin hypersensitivity, poorly controlled HTN, myopathy, alcohol or drug abuse, risk of getting pregnant, oral contraceptive use at study start, lipid‐lowering drug intake within 8 weeks preceding study Atorvastatin baseline LDL‐C: 3.65 mmol/L (141 mg/dL) Atorvastatin baseline HDL‐C: 1.06 mmol/L (41 mg/dL) Atorvastatin baseline TG: 4.63 mmol/L (410 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d Fluvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 3 months of serum LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | TC data were not reported |
| Other bias | Unclear risk | The source of funding was not provided |
Blagden 2007.
| Methods | 4‐Week baseline period 6‐Week randomised open‐label parallel‐group multi‐centre study |
|
| Participants | 148 men and women with untreated primary hypercholesterolaemia and CHD aged 18 to 75 years; LDL‐C 3.3 to 5.2 mmol/L (130‐209 mg/dL), TG < 4.2 mmol/L (368 mg/dL) 76 participants received atorvastatin, 72 received ezetimibe + atorvastatin Exclusion criteria: congestive heart failure, MI, acute coronary insufficiency, coronary bypass surgery or angioplasty, unstable or severe peripheral artery disease within the past 3 months, unstable angina, poorly controlled type 1 and 2 diabetes, uncontrolled HTN, conditions that affect serum lipids or lipoproteins, renal dysfunction; blood, gastrointestinal or neurological disorders; AST, ALT, CK > 1.5 × ULN; cancer, statin hypersensitivity, individuals taking lipid‐lowering treatment, pregnancy Atorvastatin baseline TC: 5.89 mmol/L (228 mg/dL) Atorvastatin baseline LDL‐C: 4.09 mmol/L (158 mg/dL) Atorvastatin baseline HDL‐C: 1.37 mmol/L (53 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Ezetimibe 10 mg/d + atorvastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/76 were not included in the efficacy analysis because of adverse events 1.3% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Schering‐Plough funded the study; data may support bias against atorvastatin |
Bloomfield 2009.
| Methods | Visit 1 to Visit 2 period was 6 weeks if the participant needed to wash out from fibrate therapy, or 4 weeks for other lipid‐lowering therapy. Participants not requiring a wash‐out had 2 weeks between Visit 1 and Visit 2. 2‐Week to 6‐week placebo run‐in between Visits 2 and 3. Participants were randomly assigned at Visit 3 8‐Week multi‐centre randomised double‐blind placebo‐controlled parallel‐group dose‐ranging study |
|
| Participants | 589 men and women aged 18 to 75 years; LDL‐C 110 to 190 mg/dL (2.84‐4.91 mmol/L), TG > 150 mg/dL (1.69 mmol/L) 59 received placebo, 59 received atorvastatin, 236 received anacetrapib, 235 received anacetrapib + atorvastatin Exclusion criteria: CHD history, symptomatic artery disease, uncontrolled cardiac arrhythmias, HTN (> 160/90 mmHg), uncontrolled diabetes, women of childbearing potential or who were pregnant or lactating, use of drugs that affect serum lipids Placebo baseline TC: 5.78 mmol/L (224 mg/dL) Placebo baseline LDL‐C: 3.60 mmol/L (139 mg/dL) Placebo baseline HDL‐C: 1.33 mmol/L (51 mg/dL) Atorvastatin baseline TC: 5.85 mmol/L (226 mg/dL) Atorvastatin baseline LDL‐C: 3.64 mmol/L (141 mg/dL) Atorvastatin baseline HDL‐C: 1.31 mmol/L (51 mg/dL) |
|
| Interventions | Placebo Atorvastatin 20 mg/d Anacetrapib 10, 40, 150, 300 mg/d Atorvastatin 20 mg/d + anacetrapib 10, 40, 150, 300 mg/d |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C and HDL‐C | |
| Notes | Placebo and atorvastatin monotherapy groups were analysed WDAEs were reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Low risk | "Randomized via an interactive voice response system equally to one of 10 groups" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Placebo 1/59 was not included in the efficacy analysis Atorvastatin 20 mg/d 1/59 was not included in the efficacy analysis 1.7% of participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | TG data were not reported because they were expressed as median values |
| Other bias | High risk | Merck funded the study; data may support bias against atorvastatin |
Bo 2001.
| Methods | 2‐Month wash‐out period 24‐Week single‐centre randomised open‐label study Evening dose | |
| Participants | 26 men and women from Italy recruited from a lipid clinic with heterozygous FH, mean age 55 years; TC > 8.0 mmol/L (309 mg/dL), TG < 4.5 mmol/L (399 mg/dL) 13 participants received atorvastatin, 13 received simvastatin Exclusion criteria: aged < 18 and > 75 years, hepatic and renal dysfunction, neurological or endocrine disease, alcohol abuse, neoplasms, use of lipid‐lowering drugs, women who are likely to become pregnant Baseline TC, HDL‐C and TG values not given Atorvastatin baseline LDL‐C: 8.50 mmol/L (329 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 6 weeks Atorvastatin conditional titration of 20 mg/d for 6 to 12 weeks Atorvastatin conditional titration of 40 mg/d for 12 to 18 weeks Atorvastatin conditional titration of 80 mg/d for 18 to 24 weeks Simvastatin 10 mg/d for 0 to 6 weeks Simvastatin conditional titration of 20 mg/d for 6 to 12 weeks Simvastatin conditional titration of 40 mg/d for 12 to 18 weeks Simvastatin conditional titration of 80 mg/d for 18 to 24 weeks |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum LDL‐C | |
| Notes | First atorvastatin dose was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | TC, HDL‐C and TG data were not reported |
| Other bias | Unclear risk | The source of funding was not provided |
Bogsrud 2013.
| Methods | 6‐Week wash‐out period 4‐Week before‐and‐after study |
|
| Participants | 41 men and women aged 18 to 75 years with hypercholesterolaemia Exclusion criteria: serious adverse events during previous statin therapy, liver or kidney failure, individuals taking concomitant medication that would interfere with the study Atorvastatin baseline TC: 7.17 mmol/L (277 mg/dL) Atorvastatin baseline LDL‐C: 5.18 mmol/L (200 mg/dL) Atorvastatin baseline HDL‐C: 1.49 mmol/L (57.6 mg/dL) Atorvastatin baseline TG: 1.67 mmol/L (148 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; treatment intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study was funded by Pharma Nord ApS, which does not market statins of any kind |
Branchi 1999.
| Methods | 6‐Week wash‐out period 2‐Month randomised study | |
| Participants | 200 men and women outpatients from Italy, mean age 58.3 years (24‐75); LDL 160 mg/dL (4.14 mmol/L), 160‐426 mg/dL (4.14‐11.02 mmol/L); TG < 400 mg/dL (4.52 mmol/L), 52 to 398 mg/dL (1.34‐4.49 mmol/L) 50 participants received atorvastatin, 50 received fluvastatin, 50 received pravastatin, 50 received simvastatin Exclusion criteria: diabetes, hypothyroidism, renal and hepatic dysfunction Atorvastatin baseline TC: 8.29 mmol/L (320 mg/dL) Atorvastatin baseline LDL‐C: 6.05 mmol/L (233 mg/dL) Atorvastatin baseline HDL‐C: 1.29 mmol/L (49.88 mg/dL) Atorvastatin baseline TG: 2.09 mmol/L (185 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 2 months Fluvastatin 40 mg/d for 0 to 2 months Pravastatin 20 mg/d for 0 to 2 months Simvastatin 10 mg/d for 0 to 2 months |
|
| Outcomes | Per cent change from baseline at 2 months of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/50 was not included in the efficacy analysis because of loss to follow‐up 2% of participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Branchi 2001.
| Methods | 1‐Month to 2‐month wash‐out period 6‐Month randomised study Evening dose | |
| Participants | 200 men and women adult outpatients from Italy with hypercholesterolaemia, mean age 57 years; not taking any lipid‐altering drugs 100 participants received atorvastatin, 100 received simvastatin Exclusion criteria: hepatic and renal dysfunction, uncontrolled hypothyroidism, type 1 and uncontrolled type 2 diabetes Atorvastatin baseline TC: 8.19 mmol/L (317 mg/dL) Atorvastatin baseline LDL‐C: 5.90 mmol/L (228 mg/dL) Atorvastatin baseline HDL‐C: 1.31 mmol/L (50.66 mg/dL) Atorvastatin baseline TG: 1.94 mmol/L (172 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 2 months Simvastatin 20 mg/d for 2 months |
|
| Outcomes | Per cent change from baseline at 2 months of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed Per cent change from baseline for TG was expressed as median value |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 weeks: 1/100 was not included in the efficacy analysis because of loss to follow‐up Comment: very low number; 1% of participants were excluded from the analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Branchi 2002.
| Methods | Wash‐out period was not required because no participants were taking drugs known to affect lipid metabolism 2‐Month treatment period |
|
| Participants | 121 men and women outpatients from Italy with diet‐resistant hypercholesterolaemia, mean age 57 years (24‐77) Exclusion criteria: diabetes, hypothyroidism, renal failure, liver disease Baseline TC: 8.1 mmol/L (313 mg/dL) Baseline LDL‐C: 5.73 mmol/L (222 mg/dL) Baseline HDL‐C: 1.31 mmol/L (50.7 mg/dL) Baseline TG: 2.33 mmol/L (206 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 1 month of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not reported |
Broncel 2005.
| Methods | 4‐Week wash‐out period 8‐Week open‐label randomised study | |
| Participants | 49 men and women aged 40 to 65 with type 2 hyperlipidaemia; TC > 200 mg/dL (5.17 mmol/L), LDL‐C > 145 mg/dL (3.75 mmol/L), TG 400 mg/dL (4.52 mmol/L) 27 participants received atorvastatin, 22 received simvastatin Exclusion criteria: type I, III, IV, V hypercholesterolaemia; homozygous hypercholesterolaemia, BMI > 30, HTN, diabetes mellitus, hepatic or renal dysfunction, alcohol abuse, interfering drugs Atorvastatin baseline TC: 7.68 mmol/L (297 mg/dL) Atorvastatin baseline LDL‐C: 5.33 mmol/L (206 mg/dL) Atorvastatin baseline HDL‐C: 1.40 mmol/L (54 mg/dL) Atorvastatin baseline TG: 2.10 mmol/L (164 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Simvastatin 20 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Brown 1998.
| Methods | 12‐Week wash‐out period 54‐Week multi‐centre open‐label parallel‐group randomised study Randomisation code | |
| Participants | 318 men and women from the USA aged 18 to 80 years; LDL‐C > 130 to 250 mg/dL (3.36‐6.5 mmol/L) 80 participants received atorvastatin, 80 received fluvastatin, 81 received lovastatin, 77 received simvastatin Exclusion criteria: pregnancy threat, statin hypersensitivities, taking prohibited medications, secondary hyperlipidaemia, uncontrolled hypothyroidism, nephrotic syndrome, renal dysfunction, uncontrolled diabetes mellitus, hepatic dysfunction, cardiovascular incidents within 1 month of screening, taking confounding agents Atorvastatin baseline TC: 6.57 mmol/L (254 mg/dL) Atorvastatin baseline LDL‐C: 4.47 mmol/L (173 mg/dL) Atorvastatin baseline HDL‐C: 1.06 mmol/L (41 mg/dL) Atorvastatin baseline TG: 2.29 mmol/L (203 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 12 weeks Atorvastatin 20 mg/d for 12 to 24 weeks Atorvastatin 40 mg/d for 24 to 36 weeks Atorvastatin 80 mg/d for 36 to 48 weeks Atorvastatin 80 mg/d + colestipol 5 g BID for 48 to 54 weeks Simvastatin 10 mg/d for 0 to 12 weeks Simvastatin 20 mg/d for 12 to 24 weeks Simvastatin 40 mg/d for 24 to 36 weeks Simvastatin 40 mg/d + colestipol 5 g BID for 36 to 48 weeks Simvastatin 40 mg/d + colestipol 10 g BID for 48 to 54 weeks Lovastatin 20 mg/d for 0 to 12 weeks Lovastatin 40 mg/d for 12 to 24 weeks Lovastatin 40 mg BID for 24 to 36 weeks Lovastatin 40 mg BID + colestipol 5 g BID for 36 to 48 weeks Lovastatin 40 mg BID + colestipol 10 g BID for 48 to 54 weeks Fluvastatin 20 mg/d for 0 to 12 weeks Fluvastatin 40 mg/d for 12 to 24 weeks Fluvastatin 40 mg/d + colestipol 5 g BID for 24 to 36 weeks Fluvastatin 40 mg/d + colestipol 10 g BID for 36 to 54 weeks |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 weeks: 2/80 were not included in the efficacy analysis because of lack of post‐randomisation lipid measurement, or participants were off study medication 2.5% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may support bias for atorvastatin |
Bruni 2003.
| Methods | 6‐Week dietary stabilisation period 6‐Week randomised study |
|
| Participants | 64 men and women with hypercholesterolaemia aged 36 to 63 years 16 participants received atorvastatin, 16 received simvastatin, 16 received fluvastatin, 16 received pravastatin Atorvastatin baseline TC: 6.91 mmol/L (267 mg/dL) Atorvastatin baseline LDL‐C: 5.14 mmol/L (199 mg/dL) Atorvastatin baseline HDL‐C: 1.24 mmol/L (48 mg/dL) Atorvastatin baseline TG: 1.15 mmol/L (102 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Simvastatin 20 mg/d Fluvastatin 40 mg/d Pravastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 3 to 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Bruni 2004.
| Methods | 6‐Week dietary stabilisation period 2‐Month open‐label study |
|
| Participants | 44 men and women with hypercholesterolaemia aged 36 to 64 years Exclusion criteria: history of cardiovascular events, HTN; liver, renal, thyroid, infective, immunological or malignant disease Baseline TC: 6.75 mmol/L (261 mg/dL) Baseline LDL‐C: 4.89 mmol/L (189 mg/dL) Baseline HDL‐C: 1.29 mmol/L (50 mg/dL) Baseline TG: 1.23 mmol/L (109 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 2 months of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Bruni 2005.
| Methods | 6‐Week wash‐out period 6‐Week single‐centre study | |
| Participants | 72 men and women aged 38 to 65 years with hypercholesterolaemia; BMI 24.9, TC 6.58 mmol/L (254 mg/dL), HDL‐C 1.23 mmol/L (48 mg/dL), TG 1.11 mmol/L (98 mg/dL) 24 participants received atorvastatin, 24 received simvastatin, 24 received pravastatin Exclusion criteria: history of cardiovascular events, HTN, diabetes mellitus; liver, renal, thyroid infection; immunological or malignant disease, pregnancy threat Atorvastatin baseline TC: 6.56 mmol/L (254 mg/dL) Atorvastatin baseline LDL‐C: 4.84 mmol/L (187 mg/dL) Atorvastatin baseline HDL‐C: 1.21 mmol/L (47 mg/dL) Atorvastatin baseline TG: 1.11 mmol/L (98.4 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Simvastatin 20 mg/d Pravastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, this is an unblinded trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Budinski 2009.
| Methods | 6‐Week to 8‐week lead‐in dietary stabilisation period 12‐Week multi‐centre prospective randomised double‐blind double‐dummy controlled trial |
|
| Participants | 821 men and non‐pregnant, non‐lactating women aged 18 to 75 years with primary hypercholesterolaemia or combined dyslipidaemia; LDL‐C 160 to 220 mg/dL (4.1‐5.7 mmol/L), TG ≤ 400 mg/dL (4.5 mmol/L) 616 participants received pitavastatin; 102 received atorvastatin 10 mg for 0 to 12 weeks; 103 received atorvastatin 10 mg for 4 weeks, then titrated up to atorvastatin 20 mg for 4 to 12 weeks Exclusion criteria: statin intolerance, homozygous FH, familial hypoalphalipoproteinaemia, secondary dyslipidaemia, uncontrolled diabetes mellitus, pregnancy, condition affecting drug metabolism or excretion, heart failure type III or IV, significant CVD, impaired pancreatic function, liver enzyme levels > 1.5 × ULN, impaired renal function, impaired urinary tract function, uncontrolled hypothyroidism, symptomatic cerebrovascular disease, left ventricular ejection fraction < 0.25, uncontrolled HTN, muscular or neuromuscular disease, cancer, treatment with other lipid‐lowering drugs or drug that interfere with statins Atorvastatin baseline TC: 6.76 mmol/L (261 mg/dL) Atorvastatin baseline LDL‐C: 4.65 mmol/L (180 mg/dL) Atorvastatin baseline HDL‐C: 1.30 mmol/L (50 mg/dL) Atorvastatin baseline TG: 1.77 mmol/L (157 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Atorvastatin 10 mg/d for 4 weeks, then titrated to 20 mg/d for Weeks 4 to 12 Pitavastatin 2 mg/d Pitavastatin 2 mg/d for 4 weeks, then titrated to 4 mg/d for Weeks 4 to 12 |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin monotherapy group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, this is an unblinded trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Buldak 2012.
| Methods | Wash‐out period was not required because individuals receiving lipid‐lowering agents were excluded from the study 3‐Month single‐blind randomised trial |
|
| Participants | 67 men and women with mixed hyperlipidaemia aged 35 to 65 years TC > 200 mg/dL (5.17 mmol/L), LDL‐C > 130 mg/dL (3.36 mmol/L), TG > 200 mg/dL (2.26 mmol/L), fasting glycaemia (100‐125 mg/dL), glycaemia at 2 hours of oral glucose tolerance test < 140 mg/dL, BMI 25 to 35 kg/m2, postmenopausal state or effective methods of mechanical contraception Exclusion criteria: secondary hyperlipidaemia, significant heart failure, unstable coronary artery disease, moderate or severe hypertension, cancer within 5 years, chronic kidney disease (Stage III‐V), hepatic dysfunction, malnutrition syndrome, diabetes or glucose intolerance, oral contraceptive or HRT, inflammatory disease, non‐compliance, abnormal safety lab findings Atorvastatin baseline TC: 6.67 mmol/L (258 mg/dL) Atorvastatin baseline LDL‐C: 3.93 mmol/L (152 mg/dL) Atorvastatin baseline HDL‐C: 1.13 mmol/L (43.7 mg/dL) Atorvastatin baseline TG: 2.71 mmol/L (240 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Fenofibrate 267 mg/d Atorvastatin fenofibrate 10/267 mg/d combination therapy |
|
| Outcomes | Per cent change from baseline at 30 to 90 days of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin monotherapy group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, this is an unblinded trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | University‐funded trial |
CAP 2008.
| Methods | 6‐Week wash‐out period 26‐Week multi‐centre prospective randomised double‐blind double‐dummy design |
|
| Participants | 340 men and women aged < 80 years with documented CAD; low‐grade inflammation, LDL‐C 1.29 to 3.87 mmol/L (50‐150 mg/dL), TG ≤ 4.56 mmol/L (400 mg/dL) 170 received 10 mg/d, 169 received 80 mg/d Exclusion criteria: presence of secondary hyperlipidaemia or type 1 diabetes mellitus or type 2 with insulin therapy, inadequately controlled diabetes mellitus, alcohol or drug abuse, inadequate compliance, progressive or life‐threatening disease with life expectancy < 1 year, statin intolerance, active hepatic disease or hepatic dysfunction, use of a potent cytochrome P45 3A4 inhibitor, women who were pregnant or breastfeeding Atorvastatin 10 mg/d baseline TC: 5.41 mmol/L (209 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 3.26 mmol/L (126 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.24 mmol/L (48 mg/dL) Atorvastatin 10 mg/d baseline TG: 2.05 mmol/L (182 mg/dL) Atorvastatin 80 mg/d baseline TC: 5.34 mmol/L (206 mg/dL) Atorvastatin 80 mg/d baseline LDL‐C: 3.26 mmol/L (126 mg/dL) Atorvastatin 80 mg/d baseline HDL‐C: 1.24 mmol/L (48 mg/dL) Atorvastatin 80 mg/d baseline TG: 1.85 mmol/L (164 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Atorvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 5 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Atorvastatin 10 mg/d: All 170 were included in the analysis Atorvastatin 80 mg/d: 1/170 was not included in the ITT analysis because of lack of post‐baseline data 0.3% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer funded the study; data may support bias for atorvastatin |
Castano 2003a.
| Methods | 4‐Week wash‐out period 8‐Week single‐centre randomised single‐blind parallel‐treatment period Randomly assigned by a fixed randomisation method using a block size of 10 and an allocation ratio of 1:1 Evening dose | |
| Participants | 75 men and women from Cuba with type II hypercholesterolaemia, mean age 65 years (60 to 80); Veterans House, LDL‐C ≥ 3.4 mmol/L (131 mg/dL), TC ≥ 5.2 mmol/L (201 mg/dL), TG < 4.52 mmol/L (400 mg/dL) 37 participants received atorvastatin, 38 received policosanol Exclusion criteria: renal and hepatic dysfunction, severe HTN, neoplastic disease and uncontrolled diabetes, unstable cardiovascular condition Atorvastatin baseline TC: 6.34 mmol/L (245 mg/dL) Atorvastatin baseline LDL‐C: 4.27 mmol/L (165 mg/dL) Atorvastatin baseline HDL‐C: 1.27 mmol/L (49.11 mg/dL) Atorvastatin baseline TG: 2.04 mmol/L (181 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Policosanol 10 mg/d |
|
| Outcomes | Per cent change from baseline at 4 to 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Castano 2003b.
| Methods | 6‐Week run‐in period 8‐Week single‐centre randomised single‐blind parallel‐group comparative study Randomly assigned by a fixed randomisation method using a block size of 10 and an allocation ratio of 1:1 Evening dose | |
| Participants | 40 men and women with dyslipidaemia and NIDDM from Cuba aged 40 to 75 years; LDL‐C > 3.0 mmol/L (116 mg/dL), TG < 4.52 mmol/L (401 mg/dL) 20 participants received atorvastatin, 20 received policosanol Exclusion criteria: renal and hepatic dysfunction, cancer, severe HTN, uncontrolled diabetes mellitus, cardiovascular events within 3 months of study Atorvastatin baseline TC: 6.31 mmol/L (244 mg/dL) Atorvastatin baseline LDL‐C: 4.42 mmol/L (171 mg/dL) Atorvastatin baseline HDL‐C: 1.09 mmol/L (42 mg/dL) Atorvastatin baseline TG: 2.14 mmol/L (190 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Policosanol 10 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Castro 2008.
| Methods | 4‐Week placebo wash‐out period 8‐Week before‐and‐after study |
|
| Participants | 38 men and women with heart failure, mean age 58 years; TC ≤ 200 mg/dL Exclusion criteria: ACS in the past 6 months, CABG surgery or coronary angioplasty in the past 6 months, uncontrolled arterial HTN, hypertrophic cardiomyopathy and congenital cardiopathy, antioxidant or stain use in the previous 2 months, presence of other conditions that affect determination of oxidative stress status Baseline TC: 4.60 mmol/L (178 mg/dL) Baseline LDL‐C: 3.03 mmol/L (117 mg/dL) Baseline HDL‐C: 1.06 mmol/L (41 mg/dL) Baseline TG: 2.09 mmol/L (185 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Catalano 2009.
| Methods | 6‐Week wash‐out period 12‐Week before‐and‐after study |
|
| Participants | 14 males 36 to 59 years of age with type IIB combined hyperlipidaemia, TC ≥ 230 mg/dL (5.95 mmol/L), TG ≥ 150 mg/dL (1.69 mmol/L) Exclusion criteria: dysbetalipoproteinaemia, diabetes mellitus, secondary hyperlipidaemia, uncontrolled HTN, history of a major cardiovascular event Baseline TC: 6.52 mmol/L (252 mg/dL) Baseline LDL‐C: 4.50 mmol/L (174mg/dL) Baseline HDL‐C: 1.03 mmol/L (40 mg/dL) Baseline TG: 2.15 mmol/L (190 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Torcetrapib/atorvastatin 60/10 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer partially funded the study; data may support bias for atorvastatin |
Cerda 2010.
| Methods | 4‐Week wash‐out dietary stabilisation period 4‐Week before‐and‐after study |
|
| Participants | 147 men and women with hypercholesterolaemia with LDL‐C > 4.14 mmol/L (160 mg/dL) Exclusion criteria: diabetes mellitus, hypertriglyceridaemia; liver, renal or thyroid disease; pregnant women or women under treatment with oral contraceptive, other causes of secondary dyslipidaemia Baseline TC: 7.24 mmol/L (280 mg/dL) Baseline LDL‐C: 4.97 mmol/L (192 mg/dL) Baseline HDL‐C: 1.47 mmol/L (57 mg/dL) Baseline TG: 1.77 mmol/L (157 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
CEZAR 2009.
| Methods | Participants were not receiving lipid‐altering substances within 3 months of the study; wash‐out was not required 8‐Week double‐blind trial |
|
| Participants | 24 men and women with CAD aged 57 to 75 years; LDL‐C > 100 mg/dL Exclusion criteria: ACS, initiation of some antihypertensive agents within 4 weeks of the study, serum creatinine > 2.0 mg/dL, elevated liver enzymes > 1.5 × ULN, elevated CK 3 × ULN or overt heart failure Baseline TC: 6.03 mmol/L (233 mg/dL) Baseline LDL‐C: 3.83 mmol/L (148 mg/dL) Baseline HDL‐C: 1.34 mmol/L (52 mg/dL) Baseline TG: 1.86 mmol/L (165 mg/dL) |
|
| Interventions | Atorvastatin 80 mg/d Atorvastatin + ezetimibe 10/10 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin 80 mg/d group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
CHALLENGE 2002.
| Methods | 4‐Week wash‐out period 6‐Week multi‐centre open‐label randomised study | |
| Participants | 1732 men and women from the USA with dyslipidaemia with and without CHD aged 18 to 80 years who had discontinued any lipid‐lowering medication; TG ≤ 6.8 mmol/L (602 mg/dL), LDL‐C 3.4 to 4.9 mmol/L (131‐189 mg/dL) 650 participants received atorvastatin 10 mg/d, 216 received atorvastatin 40 mg BID, 650 received simvastatin 20 mg/d, 216 received simvastatin 40 mg BID Exclusion criteria: women who are likely to become pregnant, BMI > 32, statin hypersensitivity, uncontrolled hypothyroidism, type 1 diabetes and uncontrolled type 2 diabetes, renal and hepatic dysfunction, MI, revascularisation procedures, unstable angina, use of lipid‐altering drugs Atorvastatin 10 mg/d baseline TC: 6.9 mmol/L (267 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 4.65 mmol/L (180 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.24 mmol/L (48 mg/dL) Atorvastatin 10 mg/d baseline TG: 2.10 mmol/L (187 mg/dL) Atorvastatin 40 mg BID baseline TC: 6.85 mmol/L (265 mg/dL) Atorvastatin 40 mg BID baseline LDL‐C: 4.63 mmol/L (179 mg/dL) Atorvastatin 40 mg BID baseline HDL‐C: 1.24 mmol/L (48 mg/dL) Atorvastatin 40 mg BID baseline TG: 2.13 mmol/L (189 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Atorvastatin 40 mg BID Simvastatin 20 mg/d Simvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin groups were analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6‐week 10‐mg dose: 11/650 were missing 6‐week 80‐mg dose: 9/216 were missing Due to "no study medication, no follow‐up information" "invalid or missing follow‐up efficacy data" 1.5% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer Inc funded the study; data may support bias for atorvastatin |
Chan 2002.
| Methods | 3‐Week wash‐out period 6‐Week single‐centre randomised double‐blind placebo‐controlled study Evening doses | |
| Participants | 25 obese men from Australia recruited from the community with dyslipidaemia, mean age 52 years; TG > 1.2 mmol/L (106 mg/dL), TC > 5.2 mmol/L (201 mg/dL) 12 participants received placebo, 13 received atorvastatin Exclusion criteria: diabetes E2/E2 genotype, macroproteinuria; liver, renal dysfunction; hypothyroidism, CVD, > 30 g alcohol/d Placebo baseline TC: 5.82 mmol/L (225 mg/dL) Placebo baseline LDL‐C: 3.80 mmol/L (147 mg/dL) Placebo baseline HDL‐C: 1.05 mmol/L (41 mg/dL) Placebo baseline TG: 1.69 mmol/L (150 mg/dL) Atorvastatin baseline TC: 5.81 mmol/L (225 mg/dL) Atorvastatin baseline LDL‐C: 3.81 mmol/L (147 mg/dL) Atorvastatin baseline HDL‐C: 1.01 mmol/L (39 mg/dL) Atorvastatin baseline TG: 1.88 mmol/L (167 mg/dL) |
|
| Interventions | Placebo Atorvastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed WDAEs were not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind, placebo‐controlled intervention" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | All lipid parameters were measured; withdrawals due to adverse events were not reported |
| Other bias | High risk | Pfizer partially funded the study; data may support bias for atorvastatin |
Chan 2008.
| Methods | Wash‐out period was not required because individuals who took any antihyperlipidaemic drugs were excluded from the study 3‐Month before‐and‐after study |
|
| Participants | 60 men and women from Taiwan with CAD with stable angina and normal lipid profile aged 66 years; BMI 26 Exclusion criteria: individuals who took thiazolidinediones, angiotensin II receptor blockers or angiotensin‐converting enzyme inhibitors Baseline TC: 5.05 mmol/L (195 mg/dL) Baseline LDL‐C: 3.33 mmol/L (129 mg/dL) Baseline HDL‐C: 1.06 mmol/L (41 mg/dL) Baseline TG: 1.85 mmol/L (164 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 1 month of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Chen 2013.
| Methods | 4‐Week placebo run‐in wash‐out period 12‐Week randomized double‐blind trial |
|
| Participants | Men and women aged 18 to 80 years with mixed hyperlipidaemia; LDL‐C 130 to 190 mg/dL (3.36‐4.91 mmol/L), TG 150 to 500 mg/dL (1.69‐5.65 mmol/L) Exclusion criteria: creatinine > 2.0 mg/dL, ALT or AST > 1.5 ULN, creatine kinase > 2 ULN, abnormal thyroid‐stimulating hormone, endocrine or metabolic disease, significant renal disease, cardiovascular event or procedure within 3 months, hepatic disease, peptic ulcer disease within 3 months of study, gout within 1 year unless taking allopurinol, cancer within 5 years, gastric bypass surgery and HIV, pregnancy or breastfeeding women, those about to get pregnant, medications or supplements that affect lipid metabolism and HRT |
|
| Interventions | ERN/LRPT 1 g + simvastatin 10 mg, then titrated to ERN/LRPT 2 g + simvastatin 20 mg at 4 weeks ERN/LRPT 1 g + simvastatin 20 mg, then titrated to ERN/LRPT 2 g + simvastatin 40 mg at 4 weeks Atorvastatin 10 mg Atorvastatin 20 mg Atorvastatin 40 mg Atorvastatin 80 mg |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C and HDL‐C | |
| Notes | Atorvastatin monotherapy groups were analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin treatment arms were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin treatment arms were analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin treatment arms were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18/298 (6.0%) of atorvastatin 10‐mg/d arm were not included in the efficacy analysis 37/439 (8.4%) of atorvastatin 20‐mg/d arm were not included in the efficacy analysis 27/437 (6.2%) of atorvastatin 40‐mg/d arm were not included in the efficacy analysis 31/433 (7.2%) of atorvastatin 80‐mg/d arm were not included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | Serum triglycerides were not measured |
| Other bias | High risk | Merck funded the trial |
CHESS 2003.
| Methods | 6‐Week run‐in period 24‐Week multi‐centre randomised double‐blind parallel‐group study | |
| Participants | 917 men and women from the USA with hypercholesterolaemia aged 21 to 75 years; LDL‐C > 130 mg/dL (3.36 mmol/L), HDL‐C < 40 mg/dL (1.03 mmol/L) in men; HDL‐C < 50 mg/dL (1.29 mmol/L) in women; TG > 150 mg/dL (1.69 mmol/L) 464 participants received atorvastatin, 453 received simvastatin Exclusion criteria: drugs known to interfere with statin metabolism, renal dysfunction, secondary hypercholesterolaemia, diabetes mellitus, hepatic dysfunction, CK 50% > ULN Atorvastatin baseline LDL‐C: 4.85 mmol/L (187.5 mg/dL) Atorvastatin baseline HDL‐C: 1.21 mmol/L (47 mg/dL) |
|
| Interventions | Atorvastatin 80 mg/d Simvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum LDL‐C and HDL‐C | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Atorvastatin group: 95/464 were missing (without valid baseline and post‐baseline measurements and withdrawal due to adverse events) 20.4% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | High risk | TC and TG data were not reported |
| Other bias | High risk | Merck funded the study; data may support bias against atorvastatin |
CHEST 2003.
| Methods | No use of lipid‐lowering agents in the 6 months before enrolment 12‐Week before‐and‐after study | |
| Participants | 80 men and women from the USA aged > 18 years 2 participants failed to return for follow‐up blood testing; therefore 78 participants were included in the analysis 30 participants received atorvastatin, 21 received simvastatin, 27 received pravastatin Exclusion criteria: CHD history, hepatic dysfunction, statin intolerance history, alcohol or drug abuse, major illness, surgery, malignancy, pregnancy threat Atorvastatin baseline TC: 6.15 mmol/L (238 mg/dL) Atorvastatin baseline LDL‐C: 4.08 mmol/L (158 mg/dL) Atorvastatin baseline HDL‐C: 1.22 mmol/L (47 mg/dL) Atorvastatin baseline TG: 2.05 mmol/L (182 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Simvastatin 20 mg/d Pravastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
CHIBA 2008.
| Methods | 4‐Week dietary lead‐in period 12‐Week open randomised study |
|
| Participants | 204 men and women aged ≥ 20 years; TC ≥ 220 mg/dL, TG < 400 mg/dL including FH 103 participants received atorvastatin, 101 received pitavastatin Exclusion criteria: statin hypersensitivity, hepatic dysfunction, ALT ≥ 100 IU/L, suspected hepatic metabolism disorders or biliary obstruction, renal dysfunction, pregnancy or those who may become pregnant, poorly controlled diabetes Atorvastatin baseline TC: 6.90 mmol/L (267 mg/dL) Atorvastatin baseline LDL‐C: 4.60 mmol/L (178 mg/dL) Atorvastatin baseline HDL‐C: 1.55 mmol/L (60 mg/dL) Atorvastatin baseline TG: 1.61 mmol/L (143 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Pitavastatin 2 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/103 were excluded from the efficacy analysis because of loss to follow‐up 103 were included in the safety analysis 5% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Cho 2011.
| Methods | 4‐Week wash‐out dietary stabilisation period 6‐Week before‐and‐after study |
|
| Participants | 43 men and women with CAD and hypercholesterolaemia aged 20 to 79 years; TG ≥ 200 mg/dL but < 400 mg/dL, HDL‐C < 40 mg/dL Exclusion criteria: congestive heart failure type III or IV, poorly controlled HTN, uncontrolled endocrine or metabolic disease known to influence serum lipid profile and concomitant excluded drug use Baseline TC: 5.13 mmol/L (198 mg/dL) Baseline LDL‐C: 3.42 mmol/L (132 mg/dL) Baseline HDL‐C: 1.19 mmol/L (46 mg/dL) Baseline TG: 1.47 mmol/L (130 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d Ezetimibe/simvastatin 10/20 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not stated |
Chu 2006a.
| Methods | 4‐Week wash‐out period 3 months of rosiglitazone monotherapy, then 3 more months of atorvastatin 10 mg/d added |
|
| Participants | 30 men and women with type 2 diabetes and hypercholesterolaemia; TC ≥ 200 mg/dL, LDL‐C ≥ 160 mg/dL Exclusion criteria: renal or hepatic disease, jaundice, severe hypertriglyceridaemia, anaemia, acute illness, leukocytosis, thrombocytosis, chronic inflammatory disease, connective tissue disorder, Class III or IV congestive heart failure, ACS within 6 months of screening, SBP > 180 mmHg, DBP > 110 mmHg, drug or alcohol abuse, use of cytochrome P450 3A inducers or inhibitors Baseline TC: 5.90 mmol/L (228 mg/dL) Baseline LDL‐C: 3.49 mmol/L (135 mg/dL) Baseline HDL‐C: 1.09 mmol/L (42 mg/dL) Baseline TG: 2.31 mmol/L (205 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d + rosiglitazone 4 mg/d | |
| Outcomes | Per cent change from baseline at 3 months of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d + rosiglitazone 4 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d + rosiglitazone 4 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d + rosiglitazone 4 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Chu 2006b.
| Methods | 4‐Week dietary stabilisation period 3‐Month before‐and‐after study |
|
| Participants | 26 consecutive men and women outpatients with hypercholesterolaemia; TC > 200 mg/dL, LDL‐C > 160 mg/dL Exclusion criteria: active hepatic or renal disease, any acute illness, leukocytosis, thrombocytosis, chronic inflammatory disease, cancer, connective tissue disease, corticosteroid therapy, ACS within 6 months of enrolment, women of childbearing potential, pregnant women Baseline TC: 6.72 mmol/L (260 mg/dL) Baseline LDL‐C: 4.89 mmol/L (189 mg/dL) Baseline HDL‐C: 1.09 mmol/L (42 mg/dL) Baseline TG: 1.45 mmol/L (128 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 3 months of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Chu 2006c.
| Methods | 4‐Week dietary stabilisation period 3‐Month before‐and‐after study |
|
| Participants | 32 men and women with hypercholesterolaemia; TC > 200 mg/dL (5.17 mmol/L), LDL‐C > 130 mg/dL (3.36 mmol/L) Exclusion criteria: any acute illness, leukocytosis, thrombocytosis, chronic inflammatory disease, connective tissue disease, individuals with ACS within 6 months of enrolment Baseline TC: 6.60 mmol/L (255 mg/dL) Baseline LDL‐C: 4.61 mmol/L (178 mg/dL) Baseline HDL‐C: 1.09 mmol/L (42 mg/dL) Baseline TG: 1.76 mmol/L (156 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 3 months of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Chu 2007.
| Methods | 4‐Week wash‐out period 3‐Month before‐and‐after study |
|
| Participants | 82 men and women with hypercholesterolaemia; TC ≥ 200 mg/dL (5.17 mmol/L) or LDL‐C ≥ 160 mg/dL (4.13 mmol/L) Exclusion criteria: renal or hepatic disease, severe hypertriglyceridaemia, anaemia, acute illness, leukocytosis, thrombocytosis, chronic inflammatory disease, Class III or IV heart failure, MI history, mitral valve prolapse, heart block, psychotropic drugs, use of antiarrhythmics, SBP > 180 mmHg, DBP > 110mm Hg, drug of alcohol abuse, use of cytochrome P450 3A inducers or inhibitors Baseline TC: 6.41 mmol/L (248 mg/dL) Baseline LDL‐C: 4.06 mmol/L (157 mg/dL) Baseline HDL‐C: 1.27 mmol/L (49 mg/dL) Baseline TG: 1.85 mmol/L (1642 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Claeys 2004.
| Methods | 4‐Week placebo run‐in period 4‐Week before‐and‐after multi‐centre study |
|
| Participants | 41 individuals with stable ischaemic heart disease; LDL‐C > 130 mg/dL (3.36 mmol/L) Exclusion criteria: ACS within 1 month of study entry, with PTCA/CABG within 3 months of study entry; severe organic disease, baseline ECG abnormalities, treatment with lipid‐lowering therapy within 2 months of study, treatment with medications interfering with statin metabolism Baseline TC: 6.52 mmol/L (252 mg/dL) Baseline LDL‐C: 4.19 mmol/L (162 mg/dL) Baseline HDL‐C: 1.58mmol/L (61 mg/dL) Baseline TG: 1.69 mmol/L (150 mg/dL) |
|
| Interventions | Atorvastatin 80 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer funded the study; data may support bias for atorvastatin |
COMETS 2005.
| Methods | 4‐Week wash‐out period 12‐Week multi‐centre randomised double‐blind placebo‐controlled double‐dummy parallel‐group study | |
| Participants | 401 men and women recruited internationally with metabolic syndrome aged > 17 years; TG > 1.70 mmol/L (150 mg/dL), HDL‐C < 1.04 mmol/L (40 mg/dL) for men, HDL‐C < 1.30 mmol/L (50 mg/dL) for women, LDL‐C > 3.36 mmol/L (130 mg/dL) 79 participants received placebo, 157 received atorvastatin, 165 received rosuvastatin Exclusion criteria: use of lipid‐lowering agents within past 6 months, CVD, FH, statin hypersensitivity, uncontrolled hypothyroidism and HTN, acute liver disease and hepatic dysfunction, unexplained serum CK increase, use of prohibited concomitant medications Placebo baseline TC: 6.60 mmol/L (255 mg/dL) Placebo baseline LDL‐C: 4.42 mmol/L (171 mg/dL) Placebo baseline HDL‐C: 1.20 mmol/L (46 mg/dL) Placebo baseline TG: 5.40 mmol/L (478 mg/dL) Atorvastatin baseline TC: 6.47 mmol/L (250 mg/dL) Atorvastatin baseline LDL‐C: 4.35 mmol/L (168 mg/dL) Atorvastatin baseline HDL‐C: 1.16 mmol/L (45 mg/dL) Atorvastatin baseline TG: 5.30 mmol/L (470 mg/dL) |
|
| Interventions | Placebo 0 to 6 weeks Placebo 6 to 12 weeks Atorvastatin 10 mg/d for 0 to 6 weeks Atorvastatin 20 mg/d for 6 to 12 weeks Rosuvastatin 10 mg/d for 0 to 6 weeks Rosuvastatin 20 mg/d for 6 to 12 weeks |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | 0‐Week to 6‐week placebo and atorvastatin groups were analysed WDAEs were reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "A double‐blind, double‐dummy" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Placebo: 1/79 were not included in the efficacy analysis Atorvastatin 10 mg/d: 2/157 were not included in the efficacy analysis 1.3% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | AstraZeneca funded the study. Data may support bias against atorvastatin Conflict of Interest: Some study authors had received consultant fees from several pharmaceutical companies |
CORALL 2005.
| Methods | 6‐Week wash‐out period 18‐Week multi‐centre open randomised study | |
| Participants | 263 men and women from the Netherlands with NIDDM aged > 18 years; LDL‐C 2.99 to 5.00 mmol/L (115.6‐193.3 mg/dL), TG < 4.52 mmol/L (400.7 mg/dL) 132 participants received atorvastatin, 131 received rosuvastatin Exclusion criteria: statin hypersensitivity, active CVD, pregnancy threat, renal and hepatic disease, homozygous FH, uncontrolled hypothyroidism, unexplained CK elevations > 3 × ULN Atorvastatin baseline TC: 6.53 mmol/L (252.5 mg/dL) Atorvastatin baseline LDL‐C: 4.43 mmol/L (171 mg/dL) Atorvastatin baseline HDL‐C: 1.27 mmol/L (49 mg/dL) Atorvastatin baseline TG: 1.84 mmol/L (163 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d Atorvastatin conditional titrated dose of 40 mg/d for 6 to 12 weeks Atorvastatin conditional titrated dose of 80 mg/d for 12 to 18 weeks Rosuvastatin 10 mg/d Rosuvastatin conditional titrated dose of 20 mg/d for 6 to 12 weeks Rosuvastatin conditional titrated dose of 40 mg/d for 12 to 18 weeks |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group as included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | AstraZeneca funded the study; data may be biased against atorvastatin |
Crouse III 1999.
| Methods | 4‐Week wash‐out period 12‐Week open randomised multi‐centre treatment period | |
| Participants | 842 individuals with hypercholesterolaemia 425 participants received atorvastatin, 417 received simvastatin Exclusion criteria: none reported No baseline TC value given Atorvastatin baseline LDL‐C: 5.5 mmol/L (213 mg/dL) Atorvastatin baseline HDL‐C: 1.2 mmol/L (46.4 mg/dL) Atorvastatin baseline TG: 2.1 mmol/L (186 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d Atorvastatin 40 mg/d Simvastatin 40 mg/d Simvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin groups were analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d and atorvastatin 40 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d and atorvastatin 40 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d and atorvastatin 40 mg/d; interventions were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | TC data were not reported |
| Other bias | High risk | Merck and Co funded the study; data may support bias against atorvastatin |
Cubeddu 2006.
| Methods | 8‐Week wash‐out period 12‐Week double‐blind randomised placebo‐controlled double‐dummy parallel study Randomly assigned by a double‐dummy design to 4 groups | |
| Participants | 99 men and women from the USA aged > 20 years; LDL‐C 140 to 190 mg/dL (3.62‐4.91 mg/dL) 25 participants received policosanol, 25 received atorvastatin, 25 received policosanol plus atorvastatin, 24 received placebo Exclusion criteria: individuals with CVD, hepatic dysfunction, renal dysfunction, uncontrolled diabetes mellitus, alcohol or drug abuse, oral hypoglycaemic therapy within 4 weeks of study, cancer, hyperthyroidism, women who might become pregnant, HRT Placebo baseline TC: 6.23 mmol/L (241 mg/dL) Placebo baseline LDL‐C: 4.08 mmol/L (158 mg/dL) Placebo baseline HDL‐C: 1.31 mmol/L (51 mg/dL) Placebo baseline TG: 1.80 mmol/L (159 mg/dL) Atorvastatin baseline TC: 6.54 mmol/L (253 mg/dL) Atorvastatin baseline LDL‐C: 4.34 mmol/L (168 mg/dL) Atorvastatin baseline HDL‐C: 1.38 mmol/L (53 mg/dL) Atorvastatin baseline TG: 1.82 mmol/L (161 mg/dL) |
|
| Interventions | Placebo
Atorvastatin 10 mg/d Policosanol 20 mg/d Policosanol 20 mg/d + atorvastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Placebo and atorvastatin monotherapy group was analysed SDs were imputed WDAEs were reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | "A noninvestigator pharmacist provided to the study coordinator the medications according to the randomization code" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Atorvastatin and dummy atorvastatin were provided in identical bottles" "A randomized, parallel, double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | PHARMED Group funded the study; PHARMED Group markets policosanol; data may support bias against atorvastatin |
CURVES 1998.
| Methods | 6‐Week wash‐out period 8‐Week multi‐centre randomised open‐label parallel‐group study | |
| Participants | 534 men and women from the USA with hypercholesterolaemia, 55 years old (20‐80); LDL > 161 mg/dL (4.16 mmol/L), TG < 401 mg/dL (4.53 mmol/L) ITT analysis included 522 participants who provided post‐treatment efficacy data 195 participants received atorvastatin, 80 received pravastatin, 180 received simvastatin, 43 received lovastatin, 24 received fluvastatin Exclusion criteria: primary hypothyroidism, nephrotic syndrome, uncontrolled diabetes, HTN hepatic dysfunction, BMI > 32, MI, coronary bypass, unstable angina, HMG‐CoA reductase inhibitor hypersensitivities, participants receiving lipid‐altering drugs Atorvastatin 10 mg/d baseline TC: 7.72 mmol/L (299 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 5.52 mmol/L (213 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.37 mmol/L (53 mg/dL) Atorvastatin 10 mg/d baseline TG: 1.91 mmol/L (169 mg/dL) Atorvastatin 20 mg/d baseline TC: 7.68 mmol/L (297 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 5.51 mmol/L (213 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.28 mmol/L (49 mg/dL) Atorvastatin 20 mg/d baseline TG: 1.94 mmol/L (172 mg/dL) Atorvastatin 40 mg/d baseline TC: 7.40 mmol/L (286 mg/dL) Atorvastatin 40 mg/d baseline LDL‐C: 5.32 mmol/L (206 mg/dL) Atorvastatin 40 mg/d baseline HDL‐C: 1.29 mmol/L (50 mg/dL) Atorvastatin 40 mg/d baseline TG: 1.73 mmol/L (153 mg/dL) Atorvastatin 80 mg/d baseline TC: 7.65 mmol/L (296 mg/dL) Atorvastatin 80 mg/d baseline LDL‐C: 5.51 mmol/L (213 mg/dL) Atorvastatin 80 mg/d baseline HDL‐C: 1.37 mmol/L (53 mg/dL) Atorvastatin 80 mg/d baseline TG: 1.69 mmol/L (150 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Atorvastatin 20 mg/d Atorvastatin 40 mg/d Atorvastatin 80 mg/d Simvastatin 10 mg/d Simvastatin 20 mg/d Simvastatin 40 mg/d Pravastatin 10 mg/d Pravastatin 20 mg/d Pravastatin 40 mg/d Lovastatin 20 mg/d Lovastatin 40 mg/d Lovastatin 80 mg/d Fluvastatin 20 mg/d Fluvastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin groups were analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d, atorvastatin 20 mg/d, atorvastatin 40 mg/d and atorvastatin 80 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d, atorvastatin 20 mg/d, atorvastatin 40 mg/d and atorvastatin 80 mg/d; interventions were analysed, and as no placebo group was included for assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d, atorvastatin 20 mg/d, atorvastatin 40 mg/d and atorvastatin 80 mg/d; interventions were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may support bias for atorvastatin |
DALI 2001.
| Methods | Lipid‐lowering drugs were withdrawn at least 8 weeks before the start of the 2‐week placebo run‐in phase 30‐Week double‐blind randomised placebo‐controlled trial |
|
| Participants | 217 men and women with diabetic dyslipidaemia aged 45 to 75 years; TC 4.0 to 8.0 mmol/L (155‐309 mg/dL) TG 1.5 to 6.0 mmol/L (133‐531 mg/dL) 72 participants received placebo 73 participants received atorvastatin 10 mg/d 72 participants received atorvastatin 40 mg/d Exclusion criteria: MI history, coronary angioplasty, bypass, coronary artery disease Unstable with severe angina pectoris, heart failure, severe cardiac arrhythmia, renal or hepatic dysfunction, intestinal bypass surgery, any surgical procedure or systemic inflammatory disease within 3 months of trial, cancer, vasculitis, rheumatoid arthritis, lung fibrosis, ulcerative colitis, Crohn's disease, drug known to interfere with lipid metabolism Placebo baseline TG: 2.62 mmol/L (232 mg/dL) Atorvastatin 10 mg/d baseline TG: 2.54 mmol/L (225 mg/dL) Atorvastatin 40 mg/d baseline TG: 2.85 mmol/L (252 mg/dL) |
|
| Interventions | Placebo for 0 to 4 weeks Atorvastatin 10 mg/d for 0 to 4 weeks Atorvastatin 40 mg/d for 0 to 4 weeks Atorvastatin 80 mg/d for 4 to 30 weeks |
|
| Outcomes | Per cent change from baseline in serum triglycerides | |
| Notes | Atorvastatin 80 mg/d for 4 to 30 weeks; intervention was not analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | Triglycerides were included in the efficacy analysis |
| Other bias | High risk | Parke‐Davis funded the study; efficacy data could be biased towards atorvastatin |
Davidson 2002.
| Methods | 6‐Week wash‐out period 12‐Week multi‐centre double‐blind randomised Placebo‐controlled treatment period | |
| Participants | 519 men and women from Canada and the USA aged ≥ 18 years with type IIa or IIb hypercholesterolaemia; LDL 4.14 to 6.47 mmol/L (160‐250 mg/dL), TG ≤ 4.52 mmol/L (175 mg/dL) 132 participants received placebo, 259 received rosuvastatin, 128 received atorvastatin Exclusion criteria: unstable CVD, FH, uncontrolled HTN and diabetes, hepatic dysfunction Placebo baseline TC: 7.06 mmol/L (273 mg/dL) Placebo baseline LDL‐C: 4.83 mmol/L (187 mg/dL) Placebo baseline HDL‐C: 1.26 mmol/L (49 mg/dL) Placebo baseline TG: 2.11 mmol/L (187 mg/dL) Atorvastatin baseline TC: 7.04 mmol/L (272 mg/dL) Atorvastatin baseline LDL‐C: 4.80 mmol/L (186 mg/dL) Atorvastatin baseline HDL‐C: 1.30 mmol/L (50 mg/dL) Atorvastatin baseline TG: 2.06 mmol/L (182 mg/dL) |
|
| Interventions | Placebo for 0 to 12 weeks Atorvastatin 10 mg/d for 0 to 12 weeks Rosuvastatin 10 mg/d for 0 to 12 weeks Rosuvastatin 20 mg/d for 0 to 12 weeks |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Placebo and atorvastatin monotherapy group was analysed WDAEs were reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind study, placebo‐controlled trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Atorvastatin group at 12 weeks: 1/128 was not included in the efficacy analysis because participant did not take any medication. All 132 participants receiving placebo were included in the efficacy analysis 0.4% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | AstraZeneca funded the study; data may support bias against atorvastatin |
Della‐Morte 2011.
| Methods | No wash‐out period was required because no participant received lipid medications within 6 months of the trial 1 month before and after study |
|
| Participants | 40 men and women stroke free and statin naive with coronary heart disease or equivalent ≥ 2 risk factors for CHD and an LDL ≥ 130 mg/dL (3.36 mmol/L) < 2 risk factors and an LDL ≥ 160 mg/dL (4.14 mmol/L) Men ≥ 45 years, women ≥ 55 years; NCEP ATP III risk factors for CHD Exclusion criteria: carotid stenosis ≥ 60%, hospitalisation for acute coronary syndrome within the past 6 months, hepatic or renal dysfunction, connective tissue or chronic inflammatory disease, cancer history, any acute illness, leukocytosis, thrombocytosis, anaemia, corticosteroid use, pregnancy or breastfeeding Atorvastatin baseline TC: 5.715 mmol/L (221 mg/dL) Atorvastatin baseline LDL‐C: 3.72 mmol/L (144 mg/dL) Atorvastatin baseline HDL‐C: 1.267 mmol/L (49 mg/dL) Atorvastatin baseline TG: 1.51 mmol/L (134 mg/dL) |
|
| Interventions | Atorvastatin 80 mg/d | |
| Outcomes | Per cent change from baseline at 30 days of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 80 mg/d; treatment intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 80 mg/d; treatment intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 80 mg/d; treatment intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer funded the study |
Demir 2001.
| Methods | No wash‐out was required because participants were not treated pharmacologically or participants receiving other hypolipidaemic drugs were excluded from the study 12‐Week prospective open‐label non‐comparative study |
|
| Participants | 19 men and women with hypercholesterolaemia from Turkey aged ≥ 30 years; LDL‐C ≥ 130 mg/dL or TC ≥ 200 mg/dL or both Exclusion criteria: history of acute coronary events or clinical instability, severe congestive heart failure, TG > 600 mg/dL, uncontrolled HTN, statin hypersensitivity, active liver disease or hepatic dysfunction, secondary hypercholesterolaemia, CK > 3 × ULN, alcoholism, any medication that could possibly interfere with haemostatic parameters Baseline TC: 6.71 mmol/L (259 mg/dL) Baseline LDL‐C: 4.59 mmol/L (177 mg/dL) Baseline HDL‐C: 1.29 mmol/L (50 mg/dL) Baseline TG: 1.78 mmol/L (158 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Despres 2002.
| Methods | 4‐Week wash‐out period 12‐Week multi‐centre randomised open‐label study | |
| Participants | 181 men and women from Canada and the UK with dyslipidaemia, mean age 50 years (18‐75); HDL‐C < 1.1 to 1.2 mmol/L (42.5‐46.4 mg/dL), LDL‐C > 125 mg/dL (3.23 mmol/L), TG < 400 mg/dL (4.52 mmol/L) 87 participants received fenofibrate, 94 received atorvastatin Exclusion criteria: type 1 and 2 diabetes, pancreatitis, gall bladder disease, ulcers, alcohol abuse Atorvastatin baseline TC: 6.15 mmol/L (238 mg/dL) Atorvastatin baseline LDL‐C: 4.18 mmol/L (162 mg/dL) Atorvastatin baseline HDL‐C: 0.94 mmol/L (36.35 mg/dL) Atorvastatin baseline TG: 2.26 mmol/L (200 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Fenofibrate 200 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Atorvastatin group: 8/94 were not included in the efficacy analysis according to the ITT principle, using the population of the full analysis set, that is, all treated participants with a baseline value and at least 1 value on treatment 8.5% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Diepeveen 2005.
| Methods | Wash‐out was not required because no participants used lipid‐lowering drugs 12‐Week double‐blind placebo‐controlled trial |
|
| Participants | 44 clinically stable non‐diabetic men and women from the Netherlands receiving dialysis therapy without manifest CVD, mean age 49 years; BMI 24.7 Exclusion criteria: none Groups 1 and 4 Placebo: Baseline TC: 4.8 mmol/L (186 mg/dL) Baseline LDL‐C: 2.7 mmol/L (104 mg/dL) Baseline HDL‐C: 0.92 mmol/L (35.6 mg/dL) Baseline TG: 2.6 mmol/L (230 mg/dL) Atorvastatin: Baseline TC: 5.1 mmol/L (197 mg/dL) Baseline LDL‐C: 2.6 mmol/L (100.5 mg/dL) Baseline HDL‐C: 0.97 mmol/L (37.5 mg/dL) Baseline TG: 3.8 mmol/L (337 mg/dL) Groups 2 and 3 Placebo: Baseline TC: 4.8 mmol/L (186 mg/dL) Baseline LDL‐C: 2.7 mmol/L (104 mg/dL) Baseline HDL‐C: 0.95 mmol/L (36.7 mg/dL) Baseline TG: 2.5 mmol/L (221 mg/dL) Atorvastatin: Baseline TC: 4.9 mmol/L (189 mg/dL) Baseline LDL‐C: 3.0 mmol/L (116 mg/dL) Baseline HDL‐C: 1.46 mmol/L (56.5 mg/dL) Baseline TG: 1.7 mmol/L (151 mg/dL) |
|
| Interventions | Group 1: atorvastatin 40 mg/d + placebo vitamin E Group 2: placebo atorvastatin + vitamin E Group 3: atorvastatin 40 mg/d + vitamin E Group 4: placebo atorvastatin + placebo vitamin E |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin data from groups 1 and 3 were combined, and placebo data from groups 2 and 4 were combined No WDAEs were reported SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | All lipid parameters were measured; WDAEs were not reported |
| Other bias | Unclear risk | The source of funding was not reported |
DISCOVERY 2005.
| Methods | 6‐Week dietary stabilisation period 12‐Week randomised multi‐national multi‐centre open‐label 2‐arm parallel‐group phase 3b/4 study |
|
| Participants | 2159 men and women were enrolled in the study, of whom 1482 aged ≥ 18 years with primary hypercholesterolaemia were randomly assigned with LDL‐C > 3.5 mmol/L (135 mg/dL), cardiovascular risk > 20%/10 y, type 2 diabetes, history of CHD or other established atherosclerotic disease Exclusion criteria: none 995 participants received rosuvastatin (950 in the ITT efficacy analysis set), 487 received atorvastatin (472 in the ITT efficacy analysis set) Atorvastatin baseline TC: 6.50 mmol/L (251 mg/dL) Atorvastatin baseline LDL: 4.38 mmol/L (169 mg/dL) Atorvastatin baseline HDL: 1.33 mmol/L (51 mg/dL) Atorvastatin baseline TG: 1.74 mmol/L (154 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Rosuvastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 205/472 were not analysed because no dietary wash‐out baseline stabilisation period was provided for the 204 switched participants and 1 participant had Week 12 lipid data provided by a non‐study laboratory |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | AstraZeneca funded the study; data may support bias against atorvastatin |
DISCOVERY ALPHA 2006.
| Methods | No lipid‐lowering therapies within the previous 6 months if lipid‐lowering therapy‐naive; 12‐week randomised open‐label study | |
| Participants | 1506 men and women aged ≥ 18 years with primary hypercholesterolaemia; LDL‐C > 3.5 mmol/L (135 mg/dL), TG < 4.52 mmol/L (400 mg/dL) and 10‐year CHD risk > 20%, history of CHD, atherosclerosis 1002 participants received rosuvastatin, 504 received atorvastatin Exclusion criteria: FH, dysbetalipoproteinaemia, secondary dyslipidaemia of any cause, uncontrolled diabetes mellitus or HTN, unstable CVD, active hepatic disease or hepatic dysfunction, AST or ALT ≥ 1.5 × ULN, CK > 3 × ULN, childbearing potential, pregnant or breastfeeding, use of lipid‐modifying agents, agents known to interact with statins and increase risk for muscular adverse events Naive atorvastatin baseline TC: 6.75 mmol/L (261 mg/dL) Naive atorvastatin baseline LDL‐C: 4.57 mmol/L (177 mg/dL) Naive atorvastatin baseline HDL‐C: 1.25 mmol/L (48 mg/dL) Naive atorvastatin baseline TG: 2.06 mmol/L (182 mg/dL) |
|
| Interventions | Naive atorvastatin 10 mg/d Switched atorvastatin 10 mg/d Naive rosuvastatin 10 mg/d Switched rosuvastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Naive atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Naive atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Naive atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Naive atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 214/504 were not analysed because 185 participants in the non‐naive group did not have a wash‐out dietary stabilisation period and 29 participants did not receive at least 1 dose of atorvastatin |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | AstraZeneca partially funded the study; data may support bias against atorvastatin |
Dobreanu 2007.
| Methods | 4‐Week wash‐out baseline period 8‐Week before‐and‐after study |
|
| Participants | 22 participants with unstable angina and 10 with stable CAD 130 ≤ LDL‐C ≤ 160 Exclusion criteria: TG ≥ 400 mg/dL (4.52 mmol/L), CPK and alanine aminotransferase ≥ 3 × ULN, creatinine ≥ 2 mg/dL, myopathy, nephrotic syndrome, diabetes, pancreatitis, smoking, obesity. Initial C‐reactive protein ≤ 8 mg/dL and a marked increase after 1 month (≥ 25 mg/L) suggest the possibility of acute infection and were excluded from the statistical analysis Baseline TC: 6.10 mmol/L (236 mg/dL) Baseline LDL‐C: 3.87 mmol/L (150 mg/dL) Baseline HDL‐C: 1.27 mmol/L (49 mg/dL) Baseline TG: 1.86 mmol/L (165 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks and 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12/22 were not included in the efficacy analysis (TC and LDL‐C were not measured in unstable angina patients); 55% of participants were excluded from the efficacy analysis Risk of bias was high |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Dogra 2007.
| Methods | 4‐Week run‐in period 6‐Week double‐blind randomised placebo‐controlled parallel study |
|
| Participants | 119 men and women with stage 3 to 5 chronic kidney disease 40 participants received placebo, 39 received atorvastatin, 40 received gemfibrozil Exclusion criteria: individuals with nephrotic range proteinuria, bilateral arteriovenous fistulas, abnormal liver function test results, CK > 2 × ULN, alcohol abuse, active upper gastrointestinal dyspepsia, a clinical cardiovascular event within the preceding 6 months, anticoagulant or immunosuppressant use, statin of fibrate intolerance Placebo baseline TC: 5.09 mmol/L (197 mg/dL) Placebo baseline LDL‐C: 2.90 mmol/L (112 mg/dL) Atorvastatin baseline TC: 5.90 mmol/L (229 mg/dL) Atorvastatin baseline LDL‐C: 3.59 mmol/L (139 mg/dL) |
|
| Interventions | Placebo Atorvastatin 40 mg/d Gemfibrozil 600 mg BID |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC and LDL‐C | |
| Notes | Placebo and atorvastatin monotherapy groups were analysed SDs were imputed WDAEs were reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Block randomization using random number tables" |
| Allocation concealment (selection bias) | Low risk | "Performed by a clinical trials pharmacist" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "A double‐blind" "All investigators, patients, renal staff, and the vascular function technician were blinded to treatment allocation" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Placebo: 8/40 were not included in the efficacy analysis because of adverse events Atorvastatin: 8/39 were not included in the efficacy analysis because of adverse events 20% of participants were excluded from the efficacy analysis Risk of bias was high |
| Selective reporting (reporting bias) | High risk | HDL‐C and TG data were not reported |
| Other bias | High risk | Pfizer partially funded the study; data may support bias for atorvastatin |
ECLIPSE 2008.
| Methods | 6‐Week dietary lead‐in period 24‐Week open‐label randomised parallel‐group study |
|
| Participants | 1036 men and women ≥ 18 years; hypercholesterolaemia and a history of CHD, clinical evidence of atherosclerosis or a 10‐year CHD risk score > 20%, 160 ≤ LDL‐C < 250 mg/dL (4.14 ≤ LDL‐C < 6.47 mmol/L and within 15% of each other), TG < 400 mg/dL (4.52 mmol/L) 514 participants received atorvastatin, 522 received rosuvastatin Atorvastatin baseline TC: 7.15 mmol/L (276 mg/dL) Atorvastatin baseline LDL‐C: 4.89 mmol/L (189 mg/dL) Atorvastatin baseline HDL‐C: 1.34 mmol/L (52 mg/dL) Atorvastatin baseline TG: 2.00 mmol/L (177 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 6 weeks Atorvastatin 20 mg/d for 6 to 12 weeks Atorvastatin 40 mg/d for 12 to 18 weeks Atorvastatin 80 mg/d for 18 to 24 weeks Rosuvastatin 10 mg/d for 0 to 6 weeks Rosuvastatin 20 mg/d for 6 to 12 weeks Rosuvastatin 40 mg/d for 12 to 24 weeks |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/514 were not included in the efficacy analysis because no post‐treatment lipid value was provided 0.7% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | AstraZeneca funded the study; data may support bias against atorvastatin |
Economides 2004.
| Methods | Wash‐out was not required because participants were excluded if they received lipid‐lowering drugs within 3 months of the study 3‐Month randomised double‐blind placebo‐controlled trial |
|
| Participants | 77 men and women from USA with diabetes or at risk of diabetes aged 51 years (21‐80); BMI 29.65 Exclusion criteria: cardiac arrhythmia, congestive heart failure, uncontrolled HTN, recent stroke, chronic renal disease, severe dyslipidaemia or other serious chronic disease requiring active treatment, participants taking glucocorticoids, antineoplastic agents, psychoactive drugs and bronchodilators, type 2 diabetes and at risk of type 2 diabetes groups combined Placebo: Baseline TC: 5.48 mmol/L (212 mg/dL) Baseline LDL‐C: 3.28 mmol/L (127 mg/dL) Baseline HDL‐C: 1.61 mmol/L (62 mg/dL) Baseline TG: 1.25 mmol/L (111 mg/dL) Atorvastatin: Baseline TC: 5.16 mmol/L (200 mg/dL) Baseline LDL‐C: 3.10 mmol/L (120 mg/dL) Baseline HDL‐C: 1.51 mmol/L (58 mg/dL) Baseline TG: 1.29 mmol/L (114 mg/dL) |
|
| Interventions | Placebo Atorvastatin 20 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | No WDAEs were reported SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind fashion" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10/77 participants were not analysed 19% of participants were excluded from the efficacy analysis Risk of bias is quite high |
| Selective reporting (reporting bias) | High risk | All lipid parameters were measured; WDAEs were not reported |
| Other bias | High risk | Pfizer partially funded the study; data may support bias for the drug |
Farnier 2000.
| Methods | 6‐Week wash‐out period 6‐Week multi‐centre randomised open‐label study Randomisation was unbalanced, 2:2:1 ratio Evening dose | |
| Participants | 272 men and women from France recruited from lipid clinics and general practitioner centres with hypercholesterolaemia, mean age 50 years (18‐70); LDL ≥ 4.1 mmol/L (159 mg/dL), TG ≤ 3.4 mmol/L (301 mg/dL) 109 participants received atorvastatin, 163 received simvastatin Exclusion criteria: pregnancy threat, secondary hyperlipidaemia, major CVD within 6 months of study, uncontrolled HTN, BMI > 30, statin hypersensitivities, alcohol abuse Atorvastatin baseline TC: 8.25 mmol/L (319 mg/dL) Atorvastatin baseline LDL‐C: 6.39 mmol/L (247 mg/dL) Atorvastatin baseline HDL‐C: 1.14 mmol/L (44.08 mg/dL) Atorvastatin baseline TG: 1.69 mmol/L (1 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Simvastatin 10 mg/d Simvastatin 20 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may support bias for atorvastatin |
Ferreira 2007.
| Methods | No current or past use of hypolipidaemic drugs in the past 6 months 8‐Week before‐and‐after trial |
|
| Participants | 91 women with systemic lupus erythematosus; three were excluded for reasons not related to the therapeutic protocol; 88 participants completed the study 64 participants received atorvastatin, 24 control participants received no atorvastatin Exclusion criteria: menopausal status, diabetes mellitus, serum creatinine > 1.2 mg/dL, pregnancy, smoking status in the past 12 months, family history of CHD, skeletal myopathic disease, elevated CK, hepatic disease, use of cyclosporine Atorvastatin baseline TC: 4.20 mmol/L (162 mg/dL) Atorvastatin baseline LDL‐C: 2.38 mmol/L (92 mg/dL) Atorvastatin baseline HDL‐C: 1.22 mmol/L (47 mg/dL) Atorvastatin baseline TG: 1.30 mmol/L (50 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Franiak‐Pietryga 2009.
| Methods | 8‐Week wash‐out dietary stabilisation period 12‐Week before‐and‐after trial |
|
| Participants | 20 menopausal women with metabolic syndrome aged 55 years; TG > 150 mg/dL (1.7 mmol/L), HDL‐C < 50 mg/dL (1.3 mmol/L) Exclusion criteria: none Atorvastatin baseline TC: 7.25 mmol/L (280 mg/dL) Atorvastatin baseline LDL‐C: 4.80 mmol/L (186 mg/dL) Atorvastatin baseline HDL‐C: 1.11 mmol/L (43 mg/dL) Atorvastatin baseline TG: 2.76 mmol/L (244 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 4 and 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not stated |
Fu 2013.
| Methods | 4‐Week lead‐in dietary phase 4‐Week open study |
|
| Participants | 363 men and women with hyperlipidaemia aged 41 to 78 years TG > 1.78 mmol/L (158 mg/dL), total cholesterol > 6.00 mmol/L (232 mg/dL), LDL‐C > 3.36 mmol/L (130 mg/dL) 189 participants received atorvastatin, 174 received simvastatin Exclusion criteria: unstable or uncontrolled clinically significant disease, uncontrolled hypothyroidism or diabetes, hepatic and renal dysfunction Atorvastatin baseline TC: 7.09 mmol/L (274 mg/dL) Atorvastatin baseline LDL‐C: 3.62 mmol/L (140 mg/dL) Atorvastatin baseline HDL‐C: 1.65 mmol/L (63.8 mg/dL) Atorvastatin baseline TG: 1.93 mmol/L (171 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d Simvastatin 20 mg/d |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | Industry did not fund the study |
Geiss 2001.
| Methods | 6‐Week wash‐out period 4‐Week before‐and‐after study | |
| Participants | 9 hypercholesterolaemic individuals from Germany, LDL ≥ 200 mg/dL (5.17 mmol/L); 11 type 2 diabetes mellitus, LDL‐C ≥ 125 mg/dL (3.23 mmol/L); 10 normolipidaemic controls LDL‐C < 150 mg/dL (3.88 mmol/L) Exclusion criteria: none Baseline TC: 6.40 mmol/L (247 mg/dL) Baseline LDL: 4.30 mmol/L (166 mg/dL) Baseline HDL: 1.22 mmol/L (47 mg/dL) Baseline TG: 2.03 mmol/L (180 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may support bias for atorvastatin |
Gokkaya 2008.
| Methods | 1‐Month wash‐out period 1‐Month before‐and‐after study |
|
| Participants | 25 men with hypercholesterolaemia, TC > 200 mg/dL Exclusion criteria: individuals with hormonal or neurogenic pathologies, previous treatment with sildenafil and a normal penile vascular system after penile duplex ultrasonography investigation, cardiac instability Baseline TC: 6.28 mmol/L (243 mg/dL) Baseline LDL‐C: 4.55 mmol/L (176 mg/dL) Baseline HDL‐C: 1.31 mmol/L (50.6 mg/dL) Baseline TG: 2.45 mmol/L (217 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Atorvastatin 10 mg/d + sildenafil 200 mg/wk |
|
| Outcomes | Per cent change from baseline at 1 month of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin 10 mg/d + sildenafil 200 mg/wk group was not analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Goldberg 2009.
| Methods | 6‐Week diet run‐in period 12‐Week phase 3 multi‐centre double‐blind active‐controlled study |
|
| Participants | 613 men and women aged ≥ 18 years with mixed dyslipidaemia; TG ≥ 150 mg/dL (1.69 mmol/L), HDL‐C < 40 mg/dL (1.03 mmol/L) for men and < 50 mg/dL (1.29 mmol/L) for women, LDL‐C ≥ 130 mg/dL (3.36 mmol/L) 113 participants received atorvastatin 20 mg/d, 110 received atorvastatin 40 mg/d, 56 received atorvastatin 80 mg/d, 113 received ABT‐335, 110 received ABT‐335 + atorvastatin 20 mg/d, 111 received ABT‐335 + atorvastatin 40 mg/d Exclusion criteria: pregnancy, unstable CHD, type 1 diabetes mellitus, history of diabetic ketoacidosis, unstable type 2 diabetes mellitus with haemoglobin A1c > 8.5% or history of diagnosed myopathy, use of prohibited medications Baseline atorvastatin TC: 6.73 mmol/L (260 mg/dL) Baseline atorvastatin LDL‐C: 4.11 mmol/L (159 mg/dL) Baseline atorvastatin HDL‐C: 1.00 mmol/L (39 mg/dL) Baseline atorvastatin TG: 3.09 mmol/L (274 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d Atorvastatin 40 mg/d Atorvastatin 80 mg/d ABT‐335 for 0 to 12 weeks ABT‐335 + atorvastatin 20 mg/d ABT‐335 + atorvastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks for serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin groups were analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d, atorvastatin 40 mg/d and atorvastatin 80 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d, atorvastatin 40 mg/d and atorvastatin 80 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d, atorvastatin 40 mg/d and atorvastatin 80 mg/d; interventions were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d: TC 4/113 were not included in the efficacy analysis, resulting in 109 in the full analysis set LDL‐C: 6/109 values were not reported HDL‐C: 8/109 values were not reported TG: 1/109 value was not reported Atorvastatin 40 mg/d: TC and TG 4/109 were not included in the efficacy analysis, resulting in 105 in the full analysis set LDL‐C: 10/105 values were not reported HDL‐C: 13/105 values were not reported Atorvastatin 80 mg/d: TC 4/56 were not included in the efficacy analysis, resulting in 52 in the full analysis set |
| Selective reporting (reporting bias) | High risk | All lipid parameters were reported for atorvastatin 20 and 40 mg/d LDL‐C, HDL‐C and TG were not reported for atorvastatin 80 mg/d |
| Other bias | High risk | Abbott funded the study; data may support bias against atorvastatin |
Goudevenos 2000.
| Methods | 6‐Week wash‐out period 24‐Week open‐label single‐centre study Evening dose | |
| Participants | 90 men and women from Greece were recruited from a lipid clinic with dyslipidaemia, mean age 48 years; BMI ≤ 32, LDL‐C > 4.16 mmol/L (161 mg/dL), TG > 2.26 mmol/L (200 mg/dL) Exclusion criteria: smokers, hepatic and renal dysfunction, proteinuria, diabetes, raised TSH, drugs that affect lipid parameters Baseline TC: 8.07 mmol/L (312 mg/dL) Baseline LDL‐C: 5.69 mmol/L (220 mg/dL) Baseline HDL‐C: 1.16 mmol/L (44.86 mg/dL) Baseline TG: 2.71 mmol/L (240 mg/dL) | |
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Grossman 2000.
| Methods | 12‐Week dietary run‐in baseline stabilisation period 12‐Week prospective open‐label pilot study |
|
| Participants | 30 men and women > 18 years of age; type 2 diabetes and no documented CHD, LDL‐C ≥ 130 mg/dL Exclusion criteria: women of childbearing age, individuals who were successful in reducing LDL‐C < 130 mg/dL on diet alone, individuals with CHD, serum creatinine > 1.4 mg/dL in women and > 1.5 mg/dL in men, AST and ALT > 2 × ULN, hepatic dysfunction, drugs that could interact with atorvastatin Baseline TC: 6.70 mmol/L (259 mg/dL) Baseline LDL‐C: 4.27 mmol/L (165 mg/dL) Baseline HDL‐C: 1.36 mmol/L (53 mg/dL) Baseline TG: 2.31 mmol/L (205 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10/30 were not included in the efficacy analysis because of 6 losses to follow‐up ‐ 3 to dyspepsia, 1 for personal reasons 33% of participants were excluded from the efficacy analysis Risk of bias is high |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Guerin 2000.
| Methods | 6‐Week placebo run‐in period 6‐Week before‐and‐after study |
|
| Participants | 18 men and women with hypercholesterolaemia and hypertriglyceridaemia aged 35 to 75 years; TG > 150 mg/dL (1.69 mmol/L), TC > 230 mg/dL (5.95 mmol/L) Exclusion criteria: dysbetalipoproteinaemia, diabetes mellitus, secondary hyperlipidaemia, uncontrolled HTN or major cardiovascular event Baseline TC: 6.90 mmol/L (267 mg/dL) Baseline LDL‐C: 4.53 mmol/L (175 mg/dL) Baseline HDL‐C: 1.19 mmol/L (46 mg/dL) Baseline TG: 2.22 mmol/L (197 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Parke‐Davis partially funded the study; data may support bias for atorvastatin |
Guerin 2002.
| Methods | 6‐Week wash‐out period 12‐Week single‐centre study Evening dose | |
| Participants | 11 men from France with type IIb hyperlipidaemia, mean age 51 years (35‐66); TC > 6.5 mmol/L (251 mg/dL), TG 1.71 to 4.57 mmol/L (151‐405) mg/dL Exclusion criteria: dysbetalipoproteinaemia, diabetes, secondary hypercholesterolaemia, uncontrolled HTN, major cardiovascular event, BMI > 30, alcohol abuse Baseline TC: 7.5 mmol/L (290 mg/dL) Baseline LDL‐C: 5.14 mmol/L (199 mg/dL) Baseline HDL‐C: 0.85 mmol/L (33 mg/dL) Baseline TG: 3.28 mmol/L (290.5 mg/dL) | |
| Interventions | Atorvastatin 10 mg/d for 0 to 6 weeks Atorvastatin 40 mg/d for 6 to 12 weeks |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer partially funded the study; data may support bias for atorvastatin |
Guerin 2008.
| Methods | 6‐Week wash‐out dietary baseline stabilisation period 12‐Week before‐and‐after study |
|
| Participants | 18 males aged 36 to 59 years; type IIB hyperlipidaemia, TC 205 to 316 mg/dL (5.30‐8.17 mmol/L), TG 117 to 366 mg/dL (1.32‐4.13 mmol/L) Exclusion criteria: dysbetalipoproteinaemia; diabetes mellitus; secondary causes of hyperlipidaemia such as uncontrolled hyperthyroidism, renal impairment or nephrotic syndrome; liver or muscle disease; uncontrolled HTN; major cardiovascular event history Baseline TC: 6.67 mmol/L (258 mg/dL) Baseline LDL‐C: 4.58 mmol/L (177 mg/dL) Baseline HDL‐C: 1.14 mmol/L (44 mg/dL) Baseline TG: 2.16 mmol/L (191 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 6 weeks Torcetrapib/atorvastatin 60/10 mg/d for 6 to 12 weeks |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Torcetrapib/atorvastatin 60/10 mg/d for 6 to 12 weeks; group was not analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer partially funded the study; data may support bias for atorvastatin |
Gumprecht 2011.
| Methods | 6‐Week to 8‐week wash‐out dietary stabilisation period 4‐Week before‐and‐after study |
|
| Participants | Eligible individuals aged 18 to 73 years; type 2 diabetes and combined dyslipidaemia, LDL‐C ≥ 100 and ≤ 220 mg/dL (≥ 2.6 and ≤ 5.7 mmol/L), TG ≥ 150 mg/dL (≥ 1.7 mmol/L) Exclusion criteria: homozygous FH, secondary dyslipidaemia, significant cardiovascular and cerebrovascular disease, neoplastic disease within 10 years, SBP/DBP > 160/90 mmHg, muscular or neuromuscular disease, supplement use that affects lipid metabolism Atorvastatin baseline LDL‐C: 3.77 mmol/L (146 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d Pitavastatin 4 mg/d |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum LDL‐C | |
| Notes | Atorvastatin group was analysed SD was imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | Only LDL‐C was measured |
| Other bias | Unclear risk | The study was funded by Kowa Research Europe Ltd |
Guo 2013.
| Methods | Participants were not taking any lipid medications within 4 weeks of the trial 8‐Week before‐and‐after trial |
|
| Participants | 32 participants with atherosclerosis aged 18 to 70 years 19 participants received atorvastatin 10 mg/d, 13 received atorvastatin 20 mg/d Exclusion criteria: TG ≥ 500 mg/dL (5.6 mmol/L), previous coronary syndrome within 1 month, serious heart failure or arrhythmia, infectious disease within 1 month, hepatic and renal dysfunction, autoimmune disease, cancer, pregnancy or lactation, psychiatric disorders, ALT or AST > 3 × ULN, creatine phosphokinase > 5 × ULN Atorvastatin 10 mg/d baseline TC: 4.93 mmol/L (191 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 3.03 mmol/L (117 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.19 mmol/L (46.0 mg/dL) Atorvastatin 10 mg/d baseline TG: 2.18 mmol/L (193 mg/dL) Atorvastatin 20 mg/d baseline TC: 4.73 mmol/L (183 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 3.17 mmol/L (123 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.02 mmol/L (39.4 mg/dL) Atorvastatin 20 mg/d baseline TG: 2.13 mmol/L (189 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Atorvastatin 20 mg/d |
|
| Outcomes | Per cent change from baseline at 4 to 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d and 20 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d and 20 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d and 20 mg/d; interventions were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | Industry did not fund the trial |
Han 2008.
| Methods | 4‐Week run‐in dietary stabilisation period 4‐Week single‐blind randomised trial |
|
| Participants | 67 aged hypercholesterolaemic individuals with LDL‐C 4.14 to 6.50 mmol/L (160‐251 mg/dL), TG ≤ 4.52 mmol/L (400 mg/dL) 34 participants received atorvastatin, 33 received rosuvastatin Exclusion criteria: cardiovascular or cerebrovascular events within 3 months before randomisation, hypersensitivity to statins, liver disease, kidney disease, radiotherapy or chemotherapy, drug or alcohol abuse, serious medical conditions Atorvastatin baseline TC: 5.2 mmol/L (201 mg/dL) Atorvastatin baseline LDL‐C: 3.3 mmol/L (128 mg/dL) Atorvastatin baseline HDL‐C: 1.23 mmol/L (48 mg/dL) Atorvastatin baseline TG: 1.6 mmol/L (142 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d Rosuvastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/34 participants was not included in the efficacy analysis 3% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Harangi 2009.
| Methods | 6‐Week dietary baseline stabilisation period 3‐Month before‐and‐after study |
|
| Participants | 33 non‐smoking men and women aged 21 to 70 years; untreated type IIa and IIb hyperlipidaemia Exclusion criteria: hepatic, endocrine or renal disorders; type 2 diabetes mellitus; alcohol and drug abuse; gallstones; cancer; pregnancy or lactation; anticoagulant or use of lipid‐lowering therapy Baseline TC: 6.68 mmol/L (258 mg/dL) Baseline LDL‐C: 4.39 mmol/L (170 mg/dL) Baseline HDL‐C: 1.49 mmol/L (58 mg/dL) Baseline TG: 1.75 mmol/L (155 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 3 months of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
HD‐ROWS 2012.
| Methods | No wash‐out was required because participants were not receiving any lipid‐altering agents 8‐Week randomised double‐blind parallel‐group study |
|
| Participants | 23 men and women aged 18 to 65 years with documented dyslipidaemia LDL‐C > 100 mg/dL (2.59 mmol/L) and TG < 200 mg/dL (2.26 mmol/L) Exclusion criteria: HMG‐CoA reductase sensitivity or intolerance, coronary heart disease, diabetes mellitus, hypothyroidism, concurrent use of interacting agents, hepatic disease, renal disease, any medical or psychological condition that would interfere with the study, pregnancy or threat of pregnancy Baseline TC: 5.79 mmol/L (224 mg/dL) Baseline LDL‐C: 3.67 mmol/L (142 mg/dL) Baseline HDL‐C: 1.267 mmol/L (49 mg/dL) Baseline TG: 1.48 mmol/L (131 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Rosuvastatin 80 mg/wk |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3/13 = 23.1% of participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not reported |
HeFH 2003.
| Methods | 6‐Week wash‐out period 18‐Week multi‐centre randomised double‐blind parallel‐group study | |
| Participants | 623 men and women from North America with heterozygous FH aged 19 to 79 years; LDL‐C > 190 mg/dL (4.91 mmol/L), TC > 290 mg/dL (7.5 mmol/L), TG < 400 mg/dL (4.5 mmol/L) 187 participants received atorvastatin, 436 received rosuvastatin Exclusion criteria: hepatic impairment, active arterial disease, uncontrolled HTN, uncontrolled hypothyroidism, CK increase > 3 × ULN, renal dysfunction, HRT, any medication that affects serum lipids or with potential safety issue with regard to drug‐drug interactions Atorvastatin baseline TC: 9.49 mmol/L (367 mg/dL) Atorvastatin baseline LDL‐C: 7.45 mmol/L (288 mg/dL) Atorvastatin baseline HDL‐C: 1.22 mmol/L (47 mg/dL) Atorvastatin baseline TG: 1.79 mmol/L (159 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d for 0 to 6 weeks Atorvastatin conditional titration to 40 mg/d for 6 to 12 weeks Atorvastatin conditional titration to 80 mg/d for 12 to 18 weeks Rosuvastatin 20 mg/d for 0 to 6 weeks Rosuvastatin conditional titration 40 mg/d for 6 to 12 weeks Rosuvastatin conditional titration 80 mg/d for 12 to 18 weeks |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | AstraZeneca funded the study; data may support bias against atorvastatin |
Her 2010.
| Methods | 4‐Week wash‐out dietary stabilisation period 8‐Week before‐and‐after study |
|
| Participants | Men and women aged 20 to 79 years; LDL‐C > 130 mg/dL (3.36 mmol/L), TG < 400 mg/dL (4.52 mmol/L) Exclusion criteria: FH, diabetes mellitus, pregnant or breastfeeding women, stroke or MI within 3 months, thyroid dysfunction, cancer Atorvastatin baseline TC: 6.49 mmol/L (251 mg/dL) Atorvastatin baseline LDL‐C: 4.34 mmol/L (168 mg/dL) Atorvastatin baseline HDL‐C: 1.30 mmol/L (50 mg/dL) Atorvastatin baseline TG: 1.87 mmol/L (166 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d Rosuvastatin 10 mg/d Atorvastatin/ezetimibe 5 mg/5 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Hernandez 2011.
| Methods | Wash‐out was not required because participants were not receiving lipid‐lowering therapy within the past 3 months 4‐Week double‐blind randomised placebo‐controlled trial |
|
| Participants | 63 men and women with hypercholesterolaemia aged 45 to 75 years Exclusion criteria: receiving anti‐inflammatory agents, pregnancy, acute or chronic infection, diabetes mellitus, cancer, liver disease, connective tissue disease, renal failure Placebo: Baseline TC: 6.59 mmol/L (255 mg/dL) Baseline LDL‐C: 4.34 mmol/L (168 mg/dL) Baseline HDL‐C: 1.42 mmol/L (55 mg/dL) Atorvastatin 10 mg/d: Baseline TC: 6.31 mmol/L (244 mg/dL) Baseline LDL‐C: 4.19 mmol/L (162 mg/dL) Baseline HDL‐C: 1.4 mmol/L (54 mg/dL) Atorvastatin 40 mg/d: Baseline TC: 6.7 mmol/L (259 mg/dL) Baseline LDL‐C: 4.5 mmol/L (174 mg/dL) Baseline HDL‐C: 1.47 mmol/L (57 mg/dL) |
|
| Interventions | Placebo Atorvastatin 10 mg/d Atorvastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C and HDL‐C | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information about the sequence generation process was insufficient to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | Information about the allocation concealment process was insufficient to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/66 = 6.1% did not finish the trial |
| Selective reporting (reporting bias) | High risk | TG data and withdrawal due to adverse events data were not reported |
| Other bias | High risk | Pfizer partially funded the study |
Herregods 2008.
| Methods | 4‐Week dietary wash‐out period 24‐Week open‐label randomised 2‐arm parallel‐group multi‐centre study |
|
| Participants | 938 men and women aged ≥ 18 years; type IIa and type IIb hypercholesterolaemia and 10‐year cardiovascular risk > 20% or type 2 diabetes or history of CHD or other established atherosclerotic disease; LDL‐C > 3.5 mmol/L (> 135 mg/dL) for naive participants 459 participants received atorvastatin, 479 received rosuvastatin Exclusion criteria: history of a serious adverse event with another HMG‐CoA reductase inhibitor, active liver disease, unstable CVD, severe renal or hepatic impairment, use of cyclosporine or any disallowed drug Atorvastatin baseline TC: 6.61 mmol/L (256 mg/dL) Atorvastatin baseline LDL‐C: 4.38 mmol/L (169 mg/dL) Atorvastatin baseline HDL‐C: 1.38 mmol/L (53 mg/dL) Atorvastatin baseline TG: 1.86 mmol/L (165 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Rosuvastatin 10 mg/d Atorvastatin 10 mg/d for 0 to 12 weeks, then switched to rosuvastatin for 12 to 24 weeks Rosuvastatin 10 mg/d Rosuvastatin 20 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Unswitched atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 72/479 were not included in the efficacy analysis (72 were treated with usual Belux starting dose) 15% of participants were excluded from the efficacy analysis Risk of bias is high |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | Dr Vandenhoven and Dr Vissers are employees of AstraZeneca The source of funding was not provided |
Hogue 2008a.
| Methods | 6‐Week wash‐out period 6‐Week randomised double‐blind study |
|
| Participants | 11 men with type 2 diabetes mellitus TG (2.3‐8.1 mmol/L) 6 participants received atorvastatin, 5 received fenofibrate Exclusion criteria: CVD, microalbuminuria, genetic conditions that affect serum lipids, uncontrolled hypothyroidism, nephrotic syndrome, anorexia nervosa, statin hypersensitivity, alcohol or drug abuse, uncontrolled diabetes mellitus; persistent elevation of ALT, AST, CPK; uncontrolled endocrine or metabolic disease, mental health issues, HIV positive Atorvastatin baseline TC: 6.25 mmol/L (242 mg/dL) Atorvastatin baseline LDL‐C: 2.61 mmol/L (101 mg/dL) Atorvastatin baseline HDL‐C: 0.73 mmol/L (28 mg/dL) Atorvastatin baseline TG: 5.07 mmol/L (449 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d Fenofibrate 200 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer funded the study; data may support bias for atorvastatin |
Hogue 2008b.
| Methods | 6‐Week wash‐out period 6‐Week randomised double‐blind study |
|
| Participants | 40 men and women with type 2 diabetes mellitus and hypertriglyceridaemia 19 participants received atorvastatin, 19 received fenofibrate Exclusion criteria: CVD, microalbuminuria, genetic conditions that affect serum lipids, uncontrolled hypothyroidism, nephrotic syndrome, anorexia nervosa, statin hypersensitivity, alcohol or drug abuse, uncontrolled diabetes mellitus; persistent elevation of ALT, AST, CPK; uncontrolled endocrine or metabolic disease, mental health issues, HIV positive Atorvastatin baseline TC: 6.24 mmol/L (241 mg/dL) Atorvastatin baseline LDL‐C: 2.70 mmol/L (104 mg/dL) Atorvastatin baseline HDL‐C: 0.67 mmol/L (26 mg/dL) Atorvastatin baseline TG: 5.40 mmol/L (478 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d Fenofibrate 200 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer funded the study; data may support bias for atorvastatin |
Hoogerbrugge 1998.
| Methods | 6‐Week wash‐out period 12‐Week open‐label single‐centre study | |
| Participants | 41 men and women from the Netherlands with FH from an outpatient lipid clinic of a tertiary referral centre, LDL‐C > 5.0 mmol/L (193 mg/dL) Exclusion criteria: none reported Baseline TC: 10.2 mmol/L (394 mg/dL) Baseline LDL‐C: 7.69 mmol/L (297 mg/dL) Baseline HDL‐C: 1.22 mmol/L (47 mg/dL) Baseline TG: 2.52 mmol/L (223 mg/dL) |
|
| Interventions | Atorvastatin 40 mg/d for 0 to 6 weeks Atorvastatin 80 mg/d for 6 to 12 weeks |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed SD was imputed for HDL‐C |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Hoogerbrugge 1999.
| Methods | 6‐Week wash‐out period 12‐Week before‐and‐after study | |
| Participants | 40 men and women from the Netherlands, mean age 42 years; hypercholesterolaemia, LDL‐C > 6 mmol/L (232 mg/dL), tendon xanthomas in participant or first‐degree relative Exclusion criteria: none reported Baseline TC: 10.23 mmol/L (396 mg/dL) Baseline LDL‐C: 8.07 mmol/L (312 mg/dL) Baseline HDL‐C: 1.31 mmol/L (50.66 mg/dL) Baseline TG: 2.14 mmol/L (190 mg/dL) |
|
| Interventions | Atorvastatin 40 mg/d for 0 to 6 weeks Atorvastatin 80 mg/d for 6 to 12 weeks |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may support bias for atorvastatin |
Huang 2012.
| Methods | Participants were not receiving lipid‐altering medications within 1 month of the study; no wash‐out was required 3‐Month quasi‐randomised trial |
|
| Participants | 80 elderly participants with type 2 diabetes mellitus ‐ 60 years old 40 participants received atorvastatin 20 mg/d, 40 received pitavastatin 2 mg/d Exclusion criteria: type 1 diabetes, statin allergies, homozygous familial hypercholesterolaemia, active liver disease, AST or ALT 3 × ULN, individuals receiving immunosuppressants, severe kidney disease, severe cardiovascular and cerebrovascular diseases, acute heart failure, critically ill individuals, severe infection, hyperthyroidism, cancer, psychiatric problems Baseline atorvastatin TC: 4.37 mmol/L (169 mg/dL) Baseline atorvastatin LDL‐C: 2.96 mmol/L (114 mg/dL) Baseline atorvastatin HDL‐C: 1.13 mmol/L (43.7 mg/dL) Baseline atorvastatinTG: 1.61 mmol/L (143 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d Pitavastatin 2 mg/d |
|
| Outcomes | Per cent change from baseline at 3 months of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin 20 mg/d; intervention was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/40 (5%) participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not reported |
Hufnagel 2000.
| Methods | No wash‐out period was required because participants did not receive hypolipidaemic treatment in the previous 3 months 4‐Week before‐and‐after study |
|
| Participants | 31 individuals from France with hypercholesterolaemia aged < 80 years Exclusion criteria: statin allergy, liver disease, alcohol abuse, hypothyroidism, severe progressive disease, cachexia Baseline TC: 7.65 mmol/L (296 mg/dL) Baseline LDL‐C: 5.22 mmol/L (202 mg/dL) Baseline HDL‐C: 1.16 mmol/L (45 mg/dL) Baseline TG: 2.96 mmol/L (262 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/31 were not analysed because they withdrew as the result of adverse effects 6.5% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not reported |
Hunninghake 1998.
| Methods | 4‐Week wash‐out period 54‐Week multi‐centre open‐label randomised drug parallel‐group study | |
| Participants | 344 men and women from the USA with risk factors for CHD from primary, lipid and cardiology centres, aged 18 to 80 years; BMI ≤ 32 Exclusion criteria: hypersensitivities to reductase inhibitors, drugs that affect lipid metabolism, pregnancy or lactation, secondary hyperlipoproteinaemia, active liver disease or hepatic dysfunction, cardiovascular events or interventions within 1 month of screening, participation in another clinical study, significant abnormalities that the investigator judged could compromise the individual's safety or successful participation in the study Atorvastatin baseline TC: 7.70 mmol/L (286 mg/dL) Atorvastatin baseline LDL‐C: 5.30 mmol/L (205 mg/dL) Atorvastatin baseline HDL‐C: 1.09 mmol/L (42.15 mg/dL) Atorvastatin baseline TG: 2.14 mmol/L (190 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 12 weeks Atorvastatin 20 mg/d for 12 to 24 weeks Atorvastatin 40 mg/d for 24 to 36 weeks Atorvastatin 80 mg/d for 36 to 48 weeks Atorvastatin 80 mg/d + colestipol 5 g BID for 48 to 54 weeks Simvastatin 10 mg/d for 0 to 12 weeks Simvastatin 20 mg/d for 12 to 24 weeks Simvastatin 40 mg/d for 24 to 36 weeks Simvastatin 40 mg/d + colestipol 5 g BID for 36 to 48 weeks Simvastatin 40 mg/d + colestipol 10 g BID for 48 to 54 weeks Lovastatin 20 mg/d for 0 to 12 weeks Lovastatin 40 mg/d for 12 to 24 weeks Lovastatin 80 mg/d for 24 to 36 weeks Lovastatin 80 mg/d + colestipol 5 g BID 36 to 48 weeks Lovastatin 80 mg/d + colestipol 10 g BID 48 to 54 weeks Fluvastatin 20 mg/d for 0 to 12 weeks Fluvastatin 40 mg/d for 12 to 24 weeks Fluvastatin 40 mg/d + colestipol 5 g BID for 24 to 36 weeks Fluvastatin 40 mg/d + colestipol 10 g BID for 36 to 54 weeks |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/86 participants was not included in the efficacy analysis 1.1% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may support bias for atorvastatin |
Hunninghake 2001a.
| Methods | 8‐Week wash‐out period 6‐Week multi‐centre randomised open‐label study Evening dose | |
| Participants | 215 men and women from the USA with primary hypercholesterolaemia, mean age 56.5 years (18‐80); BMI ≤ 32, LDL ≥ 4.14 mmol/L (160 mg/dL), TG ≤ 4.52 mmol/L (400 mg/dL) 108 participants received atorvastatin, 107 received cerivastatin Exclusion criteria: women who are likely to become pregnant, secondary hyperlipoproteinaemia, renal or hepatic dysfunction, type 1 diabetes, uncontrolled type 2 diabetes, uncontrolled HTN, alcohol abuse, unstable CVD, lipid‐altering drug usage, statin hypersensitivity, immunosuppressants Atorvastatin baseline TC: 7.48 mmol/L (289 mg/dL) Atorvastatin baseline LDL‐C: 5.25 mmol/L (203 mg/dL) Atorvastatin baseline HDL‐C: 1.3 mmol/L (50.27 mg/dL) Atorvastatin baseline TG: 2.03 mmol/L (180 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Cerivastatin 0.3 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer Inc funded the study; data may support bias for atorvastatin |
Hunninghake 2001b.
| Methods | 4‐Week wash‐out period 4‐Week multi‐centre double‐blind randomised placebo‐controlled parallel‐group study | |
| Participants | 94 men and women from the USA with moderate hypercholesterolaemia aged 28 to 79 years; LDL ≥ 160 mg/dL (4.14 mmol/L) 19 participants received placebo, 17 received colesevelam, 19 received atorvastatin 10 mg/d, 20 received atorvastatin 80 mg/d, 19 received colesevelam + atorvastatin Exclusion criteria: women who were likely to become pregnant, dysphagia, swallowing or intestinal motility disorders, any medically unstable condition Placebo baseline TC: 6.80 mmol/L (263 mg/dL) Placebo baseline LDL‐C: 4.77 mmol/L (184 mg/dL) Placebo baseline HDL‐C: 1.20 mmol/L (46 mg/dL) Placebo baseline TG: 1.70 mmol/L (151 mg/dL) Atorvastatin 10 mg/d baseline TC: 6.94 mmol/L (268 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 4.71 mmol/L (182 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.27 mmol/L (49 mg/dL) Atorvastatin 10 mg/d baseline TG: 2.00 mmol/L (177 mg/dL) Atorvastatin 80 mg/d baseline TC: 6.84 mmol/L (265 mg/dL) Atorvastatin 80 mg/d baseline LDL‐C: 4.71 mmol/L (182 mg/dL) Atorvastatin 80 mg/d baseline HDL‐C: 1.21 mmol/L (47 mg/dL) Atorvastatin 80 mg/d baseline TG: 1.73 mmol/L (153 mg/dL) |
|
| Interventions | Placebo for 0 to 4 weeks
Atorvastatin 10 mg/d for 0 to 4 weeks Atorvastatin 80 mg/d for 0 to 4 weeks Atorvastatin 10 mg/d + colesevelam 3.8 g/d for 0 to 4 weeks Colesevelam 3.8 g/d for 0 to 4 weeks |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Placebo and atorvastatin monotherapy groups were analysed HDL‐C and TG were not analysed because the per cent change from baseline was expressed as median WDAEs were not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Low risk | Placebo capsules were identical in appearance to active medications |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Placebo for colesevelam or atorvastatin was administered to monotherapy groups in order to satisfy the double‐blind nature of the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Placebo: All 19 participants were included in the efficacy analysis Atorvastatin 10 mg/d: 1/19 was not included in the efficacy analysis because the participant was excluded from the ITT analysis Atorvastatin 80 mg/d: All 20 participants were included in the efficacy analysis 1.7% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | High risk | HDL‐C and TG were not analysed; WDAEs were not measured |
| Other bias | High risk | GelTex Pharmaceuticals funded the study; data may result in bias against atorvastatin |
Hunninghake 2003.
| Methods | 4‐Week wash‐out period 36‐Week multi‐centre double‐blind randomised study | |
| Participants | Of the 826 randomly assigned participants, 813 men and women from the USA with or without metabolic syndrome in hypercholesterolaemia aged 21 to 70 years, were included in the ITT population. Information on 808 participants was sufficient to reveal metabolic syndrome status at baseline. 212 in the simvastatin group and 113 in the atorvastatin group met the criteria for metabolic syndrome. 193 in the simvastatin group and 295 in the atorvastatin group did not meet the criteria for metabolic syndrome. LDL ≥ 160 mg/dL (4.14 mmol/L); TG < 350 mg/dL (3.95 mmol/L) Exclusion criteria: types 1, 3‐5 hyperlipidaemia, homozygous FH, type 1 or uncontrolled type 2 diabetes, renal and hepatic dysfunction, uncontrolled HTN and unstable cardiovascular conditions Atorvastatin baseline TC: 7.58 mmol/L (293 mg/dL) | |
| Interventions | Atorvastatin 20 mg/d for 0 to 6 weeks Atorvastatin 40 mg/d for 6 to 12 weeks Atorvastatin 80 mg/d for 12 to 36 weeks Simvastatin 20 mg/d for 0 to 6 weeks Simvastatin 40 mg/d for 6 to 12 weeks Simvastatin 80 mg/d for 12 to 36 weeks |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC | |
| Notes | First atorvastatin dose was analysed LDL‐C, HDL‐C and TG results from the original study by Illingworth 2001 were analysed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Atorvastatin group of 408 from the Illingworth paper: 295/408 participants were missing because of non‐metabolic syndrome 72.3% of participants were excluded from the efficacy analysis Risk of bias was high |
| Selective reporting (reporting bias) | Low risk | LDL‐C, HDL‐C and TG data were obtained from the Illingworth 2001 trial |
| Other bias | High risk | Merck funded the study; data may support bias against atorvastatin |
Hwang 2004.
| Methods | Wash‐out was not required because no participants were receiving any drug treatments at baseline 12‐Week before‐and‐after study |
|
| Participants | 22 patients from Taiwan with hypercholesterolaemia, TC > 220 mg/dL, LDL‐C > 130 mg/dL Exclusion criteria: none Baseline TC: 6.65 mmol/L (257 mg/dL) Baseline LDL‐C: 4.65 mmol/L (180 mg/dL) Baseline HDL‐C: 1.06 mmol/L (41 mg/dL) Baseline TG: 1.59 mmol/L (141 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Ikewaki 2009.
| Methods | 4‐Week to 8‐week run‐in period 4‐Week before‐and‐after study |
|
| Participants | 26 men and women aged 40 to 75 years with hypercholesterolaemia; LDL‐C ≥ 140 mg/dL (3.62 mmol/L) Exclusion criteria: none Baseline TC: 7.45 mmol/L (288 mg/dL) Baseline LDL‐C: 5.04 mmol/L (195 mg/dL) Baseline HDL‐C: 1.60 mmol/L (62 mg/dL) Baseline TG: 1.80 mmol/L (159 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Astellas Pharmaceutical Co, a subsidiary of Pfizer, funded the study; data may support bias for atorvastatin |
Illingworth 2001.
| Methods | 4‐Week wash‐out period 36‐Week multi‐centre double‐blind randomised parallel‐group study | |
| Participants | Of the 826 randomly assigned participants, 813 men and women from the USA aged 21 to 70 years; LDL‐C > 4.2 mmol/L (162 mg/dL), TG < 4.0 mmol/L (354 mg/dL) 405 participants received simvastatin, 408 received atorvastatin Exclusion criteria: receiving immunosuppressants or drugs affecting lipid determinations, women likely to become pregnant No baseline TC values were reported Atorvastatin baseline LDL‐C: 5.33 mmol/L (206 mg/dL) Atorvastatin baseline HDL‐C: 1.31 mmol/L (50.66 mg/dL) Atorvastatin baseline TG: 2.00 mmol/L (177 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d for 0 to 6 weeks Atorvastatin 40 mg/d for 6 to 12 weeks Atorvastatin 80 mg/d for 12 to 36 weeks Simvastatin 20 mg/d for 0 to 6 weeks Simvastatin 40 mg/d for 6 to 12 weeks Simvastatin 80 mg/d for 12 to 36 weeks |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed TC data from the Hunninghake 2003 paper were analysed SDs for imputed per cent change from baseline of TG were expressed as a median value, so this was not added to the dataset |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | TC data were obtained from the Hunninghake 2003 trial |
| Other bias | High risk | Merck & Co funded the study; data may support bias against atorvastatin |
IRIS 2007.
| Methods | 6‐Week wash‐out period 6‐Week multi‐centre randomised open‐label parallel study | |
| Participants | 740 men and women from North America of South Asian ethnicity with hypercholesterolaemia, mean age 55 years (> 17 years); LDL‐C < 300 mg/dL (7.8 mmol/L), TG < 500 mg/dL (5.6 mmol/L) 369 participants received atorvastatin, 371 received rosuvastatin Exclusion criteria: type I, III or V hyperlipoproteinaemia; active arterial disease, uncontrolled HTN, poorly controlled diabetes mellitus, hepatic or renal dysfunction Atorvastatin baseline TC: 6.18 mmol/L (239 mg/dL) Atorvastatin baseline LDL‐C: 4.07 mmol/L (157 mg/dL) Atorvastatin baseline HDL‐C: 1.12 mmol/L (43.3 mg/dL) Atorvastatin baseline TG: 2.16 mmol/L (191.5 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Atorvastatin 20 mg/d Rosuvastatin 10 mg/d Rosuvastatin 20 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin groups were analysed SDs were imputed for LDL‐C and HDL‐C |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d and atorvastatin 20 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d and atorvastatin 20 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d and atorvastatin 20 mg/d; interventions were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Atorvastatin 10 mg/d: 5/185 participants were not included in the efficacy analysis because they were not included in the ITT group Atorvastatin 20 mg/d: 9/184 participants were not included in the efficacy analysis because they were not included in the ITT group 3.8% of participants were excluded from the ITT groups |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstraZeneca funded the study; data may support bias against atorvastatin |
Issa 2012.
| Methods | 4‐Week wash‐out period and 4‐week placebo run‐in period 12‐Week randomised trial |
|
| Participants | 87 non‐smoking postmenopausal women with hypercholesterolaemia aged 50 to 65 years with no family history of coronary artery disease LDL‐C > 130 mg/dL (3.36 mmol/L) 17 received atorvastatin 10 mg/d 34 received hormone therapy 36 received combined hormone therapy and atorvastatin Exclusion criteria: thyroid, liver or renal disease; diabetes, hypertriglyceridaemia Atorvastatin baseline TC: 7.24 mmol/L (280 mg/dL) Atorvastatin baseline LDL‐C: 4.89 mmol/L (189 mg/dL) Atorvastatin baseline HDL‐C: 1.47 mmol/L (57 mg/dL) Atorvastatin baseline TG: 1.99 mmol/L (176 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Hormone therapy Hormone therapy and atorvastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin 10 mg/d; intervention was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | Industry did not fund the trial |
J‐CLAS 1997.
| Methods | 4‐Week wash‐out 8‐Week double‐blind multi‐centre randomised placebo‐controlled study Evening doses | |
| Participants | 121 individuals from Japan with primary hyperlipidaemia were randomly assigned; 13 withdrew and 108 completed the study; men and women aged 50 to 60 years; TC > 219 mg/dL (5.66 mmol/L), TG < 400 mg/dL (4.52 mmol/L) on a low‐fat diet 27 participants received placebo, 81 received atorvastatin Placebo baseline TC: 7.9 mmol/L (305 mg/dL) Placebo baseline LDL‐C: 5.9 mmol/L (228 mg/dL) Placebo baseline HDL‐C: 1.3 mmol/L (50 mg/dL) Placebo baseline TG: 1.5 mmol/L (133 mg/dL) Atorvastatin 5 mg/d baseline TC: 7.7 mmol/L (298 mg/dL) Atorvastatin 5 mg/d baseline LDL‐C: 5.7 mmol/L (220 mg/dL) Atorvastatin 5 mg/d baseline HDL‐C: 1.3 mmol/L (50 mg/dL) Atorvastatin 5 mg/d baseline TG: 1.6 mmol/L (142 mg/dL) Atorvastatin 10 mg/d baseline TC: 7.7 mmol/L (298 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 5.7 mmol/L (220 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.3 mmol/L (50 mg/dL) Atorvastatin 10 mg/d baseline TG: 1.4 mmol/L (124 mg/dL) Atorvastatin 20 mg/d baseline TC: 8.2 mmol/L (317 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 6.2 mmol/L (240 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.3 mmol/L (50 mg/dL) Atorvastatin 20 mg/d baseline TG: 1.6 mmol/L (142 mg/dL) |
|
| Interventions | Placebo Atorvastatin 5 mg/d Atorvastatin 10 mg/d Atorvastatin 20 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | WDAEs were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind randomized treatment period" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured; WDAEs were reported |
| Other bias | Unclear risk | No source of funding was provided |
Jin 2012.
| Methods | Participants were not receiving any lipid medications; therefore no wash‐out period was required 3‐Month before‐and‐after study |
|
| Participants | 72 men and women with hyperlipidaemia; LDL‐C ≥ 4.14 mmol/L (160 mg/dL) Exclusion criteria: coronary artery disease, cardiac dysfunction, fever, peripheral vascular disease, liver or renal dysfunction, autoimmune disease, cancer, surgery or stroke within 6 months, history of infection, chronic inflammation, abnormal thyroid function, electrolyte imbalance, use of anti‐inflammatory drugs excluding aspirin Atorvastatin baseline TC: 6.37 mmol/L (246 mg/dL) Atorvastatin baseline LDL‐C: 4.99 mmol/L (193 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change in serum TC and LDL‐C from baseline at 12 weeks | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | HDL‐C and triglycerides were not included in the efficacy analysis |
| Other bias | Low risk | Industry did not fund the trial |
Joukhadar 2001.
| Methods | Participants were not taking any hypolipidaemic drugs before the study; no wash‐out run‐in period was required 3‐Month double‐blind randomised study |
|
| Participants | 99 male and female participants from Austria enrolled in 3 groups, 33 per group, aged 52 to 55 years; BMI 24 to 25, TC 5.2 to 9.1 mmol/L, TG < 2.9 mmol/L Exclusion criteria: aged < 35 or > 75 years, BMI > 32, statin hypersensitivity, unstable coronary heart disease, diabetes mellitus, impaired hepatic or renal function, secondary hypercholesterolaemia, consumption of > 40 g ethanol per day, BP > 160/100 mmHg, anticoagulant, anti‐inflammatory or antihypertensive drugs, thyroid disease, pregnancy or lactation, cancer, other abnormalities that threatened participant safety or completion of the trial Atorvastatin 10 mg/d baseline TC: 6.49 mmol/L (251 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 4.29 mmol/L (166 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.63 mmol/L (63 mg/dL) Atorvastatin 10 mg/d baseline TG: 1.25 mmol/L (111 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Simvastatin 40 mg/d Pravastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4/33 (12.1%) were not included in the efficacy analysis because of problems with the assay |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | Source of funding was not reported |
Kadikoylu 2003.
| Methods | Participants were not taking lipid‐lowering drugs within 8 weeks of the study; therefore no wash‐out run‐in period was required 12‐Week double‐blind randomised prospective trial |
|
| Participants | 61 men and women from Turkey, mean age 53 years (39 to 74); LDL‐C > 130 mg/dL (3.36 mmol/L); all participants had at least 2 coronary risk factors 35 received atorvastatin 10 mg/d, 26 received simvastatin 10 mg/d Exclusion criteria: pregnancy, lactation, cancer, CHD, type 1 or uncontrolled type 2 diabetes mellitus, TG > 500 mg/dL, BMI > 35, clotting disorders, elevated CK, liver enzyme levels at ULN, thrombocytopenia or thrombocytosis, hepatitis, chronic renal failure, alcohol abuse, secondary hypercholesterolaemia due to hypothyroidism, obstructive liver disease and nephrotic syndrome, statin hypersensitivity Baseline TC: 6.82 mmol/L (264 mg/dL) Baseline LDL‐C: 4.36 mmol/L (169 mg/dL) Baseline HDL‐C: 1.39 mmol/L (54 mg/dL) Baseline TG: 2.50 mmol/L (221 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 12 weeks Atorvastatin 20 mg/d for 12 to 24 weeks in some patients Simvastatin 10 mg/d for 0 to 12 weeks Simvastatin 20 mg/d for 12 to 24 weeks in some patients |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not reported |
Kadoglou 2011.
| Methods | Participants were not receiving lipid‐altering substances within 12 weeks of the study; no wash‐out was required 12‐Week before‐and‐after study |
|
| Participants | 52 men and women with hypercholesterolaemia; LDL‐C ≥ 130 mg/dL (3.36 mmol/L) Exclusion criteria: liver dysfunction, renal dysfunction, CAD, uncontrolled hormone or metabolic disease, diabetes, autoimmune disease, cancer, infection, use of anti‐inflammatory drugs, weight loss Atorvastatin 20 mg/d baseline TC: 6.21 mmol/L (240 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 4.17 mmol/L (161 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.16 mmol/L (45 mg/dL) Atorvastatin 20 mg/d baseline TG: 2.04 mmol/L (181 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6% were not analysed |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kajinami 2003.
| Methods | ≥ 8‐Week wash‐out period 24‐Week titration study |
|
| Participants | 35 Japanese men and women with heterozygous FH; TC > 230 mg/dL (5.94 mmol/L) Exclusion criteria: acute illness, bone disease, chronic endocrine or renal disease, bone metabolism affecting drugs Baseline TC: 9.21 mmol/L (356 mg/dL) Baseline LDL‐C: 7.19 mmol/L (278 mg/dL) Baseline HDL‐C: 1.27 mmol/L (49 mg/dL) Baseline TG: 1.63 mmol/L (144 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 4 weeks Atorvastatin 20 mg/d for 4 to 12 weeks Atorvastatin 40 mg/d for 12 to 24 weeks |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Kassai 2007.
| Methods | 6‐Week drug wash‐out period 12‐Week before‐and‐after study |
|
| Participants | 33 men and women with type IIa and IIb primary hyperlipoproteinaemia, LDL‐C > 4.2 mmol/L (162 mg/dL) with or without TG > 2.2 mmol/L (195 mg/dL) Exclusion criteria: diabetes mellitus, HTN, CAD, MI, liver disease, cholelithiasis, use of anticoagulants or corticosteroids or previous lipid‐lowering therapy, cancer, microalbuminuria, serum creatinine > 130 μmol/L, pregnancy or breastfeeding, alcohol use or smoking Baseline TC: 6.68 mmol/L (258 mg/dL) Baseline LDL‐C: 4.39 mmol/L (170 mg/dL) Baseline HDL‐C: 1.49 mmol/L (58 mg/dL) Baseline TG: 1.75 mmol/L (155 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 3 months of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Keles 2008.
| Methods | No anti‐lipaemic use within the past 3 months 3‐Month prospective randomised study |
|
| Participants | 61 consecutive patients with TC > 200 mg/dL (5.17 mmol/L), LDL‐C > 130 mg/dL (3.36 mmol/L) 31 participants received atorvastatin every day, 30 received atorvastatin every other day Exclusion criteria: high levels of liver enzymes, diagnosis of ACS or hospitalisation within the past 3 months, presence of infectious or inflammatory disease Atorvastatin every day baseline TC: 6.26 mmol/L (242 mg/dL) Atorvastatin every day baseline LDL‐C: 4.19 mmol/L (162 mg/dL) Atorvastatin every day baseline HDL‐C: 1.22 mmol/L (47 mg/dL) Atorvastatin every day baseline TG: 1.80 mmol/L (159 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d every day Atorvastatin 20 mg every other day |
|
| Outcomes | Per cent change from baseline at 4 to 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin 20 mg/d every day group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Kim 2010.
| Methods | 4‐Week wash‐out period 8‐Week before‐and‐after study |
|
| Participants | Eligible patients were men and women aged 20 to 85 years with hypercholesterolaemia and CAD; LDL‐C ≥ 100 mg/dL, TG < 500 mg/dL Exclusion criteria: hepatic dysfunction, stent surgery within 12 months of study, statin hypersensitivity, uncontrolled HTN, uncontrolled diabetes mellitus, myopathy, renal impairment, confounding drugs, alcohol intake > 30 g/d, hypothyroidism, genetic defects Atorvastatin 20 mg/d baseline TC: 5.62 mmol/L (217 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 3.53 mmol/L (136 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.18 mmol/L (46 mg/dL) Atorvastatin 20 mg/d baseline TG: 1.91 mmol/L (169 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | Chong Kun Dang Pharmacy Corporation funded the study |
Kim 2013.
| Methods | 4‐Week wash‐out period 8‐Week before‐and‐after study |
|
| Participants | Eligible patients were men and women aged 20 to 79 years with documented primary hypercholesterolaemia; LDL‐C > 100 mg/dL Exclusion criteria: therapy with other investigational drugs within 30 days of randomisation, statin hypersensitivity, uncontrolled hypertension, poorly controlled diabetes mellitus, unstable angina, new‐onset MI, creatinine > 2.5 mg/dL, ALT > 2 × ULN, AST > 2 × ULN, CK 2 × ULN, history of malignancy or psychosis, chronic liver disease, drug or alcohol abuse, women who could become pregnant, HRT Atorvastatin 20 mg/d baseline TC: 5.875 mmol/L (227 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 4.03 mmol/L (156 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.24 mmol/L (48 mg/dL) Atorvastatin 20 mg/d baseline TG: 1.74 mmol/L (154 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | Dong‐A Pharmaceutical Company Co, Ltd, sponsored the study |
Kocic 2002.
| Methods | 6‐Week dietary wash‐out baseline stabilisation period 8‐Week before‐and‐after trial |
|
| Participants | 20 eligible patients were men and women aged 50 to 75 years with primary hypercholesterolaemia; TC > 6.2 mmol/L, LDL‐C > 4.2 mmol/L, TG < 4.5 mmol/L Exclusion criteria: pregnancy or breastfeeding, patients who had metabolic or endocrine disease that affected lipid levels Atorvastatin 10 mg/d baseline TC: 9.53 mmol/L (369 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 5.42 mmol/L (210 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.02 mmol/L (39 mg/dL) Atorvastatin 10 mg/d baseline TG: 2.87 mmol/L (254 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 8 weeks of plasma TC, LDL‐C, HDL‐C and TG | |
| Notes | 16 participants with NIDDM were not included in the analysis because lipid values did not add up according to the Friedewald equation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not reported |
Koh 2010.
| Methods | No participants took lipid‐altering medications or supplements within 2 months of screening; no wash‐out was required 8‐Week single‐blind randomised placebo‐controlled trial |
|
| Participants | 220 men and women with hypercholesterolaemia aged 54 to 58 years; LDL‐C ≥ 100 mg/dL Exclusion criteria: hepatic dysfunction, renal failure, hyperthyroidism, myopathy, uncontrolled diabetes, severe HTN, stroke, unstable angina, acute MI, coronary revascularisation within 3 months, alcohol abuse Placebo baseline TC: 6.21 mmol/L (240 mg/dL) Placebo baseline LDL‐C: 3.98 mmol/L (154 mg/dL) Placebo baseline HDL‐C: 1.29 mmol/L (50 mg/dL) Placebo baseline TG: 1.94 mmol/L (172 mg/dL) Atorvastatin 10 mg/d baseline TC: 6.15 mmol/L (238 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 4.03 mmol/L (156 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.32 mmol/L (51 mg/dL) Atorvastatin 10 mg/d baseline TG: 1.72 mmol/L (152 mg/dL) Atorvastatin 20 mg/d baseline TC: 6.34 mmol/L (245 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 4.11 mmol/L (159 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.40 mmol/L (54 mg/dL) Atorvastatin 20 mg/d baseline TG: 1.77 mmol/L (157 mg/dL) Atorvastatin 40 mg/d baseline TC: 6.26 mmol/L (242 mg/dL) Atorvastatin 40 mg/d baseline LDL‐C: 4.01 mmol/L (155 mg/dL) Atorvastatin 40 mg/d baseline HDL‐C: 1.32 mmol/L (51 mg/dL) Atorvastatin 40 mg/d baseline TG: 2.02 mmol/L (179 mg/dL) Atorvastatin 80 mg/d baseline TC: 6.54 mmol/L (253 mg/dL) Atorvastatin 80 mg/d baseline LDL‐C: 4.37 mmol/L (169 mg/dL) Atorvastatin 80 mg/d baseline HDL‐C: 1.34 mmol/L (52 mg/dL) Atorvastatin 80 mg/d baseline TG: 1.85 mmol/L (164 mg/dL) |
|
| Interventions | Placebo Atorvastatin 10 mg/d Atorvastatin 20 mg/d Atorvastatin 40 mg/d Atorvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG; WDAEs | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | High risk | Single‐blind; investigators were not blinded |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single‐blind study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/220 (3.2%) were not analysed |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured; WDAEs were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kom 2007.
| Methods | All participants were naive to statins or other lipid‐lowering medications; therefore no wash‐out run‐in period was required 6‐Week randomised placebo‐controlled trial |
|
| Participants | 24 men and women from Germany with hypercholesterolaemia aged 35 to 60 years; LDL‐C ≥ 160 mg/dL 12 participants received placebo, 12 received atorvastatin 40 mg/d Exclusion criteria: alcohol and drug abuse, pregnancy or breastfeeding status, liver dysfunction, renal disease, nephrotic syndrome, diabetes mellitus Placebo: Baseline TC: 7.34 mmol/L (284 mg/dL) Baseline LDL‐C: 5.22 mmol/L (202 mg/dL) Baseline HDL‐C: 1.31 mmol/L (51 mg/dL) Atorvastatin: Baseline TC: 8.18 mmol/L (316 mg/dL) Baseline LDL‐C: 5.97 mmol/L (231 mg/dL) Baseline HDL‐C: 1.53 mmol/L (59 mg/dL) |
|
| Interventions | Placebo Atorvastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C and HDL‐C | |
| Notes | SDs were imputed No WDAE data were recorded |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information about blinding was provided to permit judgement of 'yes' or 'no' "Randomized to atorvastatin 40 mg or placebo for 6 weeks" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | TG data were not reported; no withdrawals due to adverse events were reported |
| Other bias | Unclear risk | The source of funding was not reported |
Kosmidou 2008.
| Methods | 6‐Week dietary lead‐in 12‐Week before‐and‐after study |
|
| Participants | 97 men and women with hyperlipidaemia with and without HTN; LDL‐C > 160 mg/dL (4.14 mmol/L), TG < 250 mg/dL (2.82 mmol/L) 60 participants received atorvastatin, 37 received no medication Exclusion criteria: renal dysfunction, liver disease, TSH > 5 mU/L, diabetes mellitus, childbearing potential, use of lipid‐altering drugs, antihypertensive therapy Atorvastatin baseline TC: 7.3 mmol/L (282 mg/dL) Atorvastatin baseline LDL‐C: 5.1 mmol/L (197 mg/dL) Atorvastatin baseline HDL‐C: 1.3 mmol/L (50 mg/dL) Atorvastatin baseline TG: 1.9 mmol/L (168 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Kotani 2012.
| Methods | Participants were not receiving any lipid medications; therefore no wash‐out period was required 12‐Week before‐and‐after study |
|
| Participants | 26 men and women with hypercholesterolaemia, mean age 63 years; LDL‐C ≥ 3.64 mmol/L (141 mg/dL) Exclusion criteria: poor glycaemic control, history of clinically overt CVD; thyroid, kidney or liver disease; drug or alcohol abuse, drug hypersensitivity Atorvastatin baseline LDL‐C: 4.03 mmol/L (156 mg/dL) Atorvastatin baseline HDL‐C: 1.46 mmol/L (56 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 12 weeks of serum LDL‐C and HDL‐C | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | Total cholesterol and triglycerides were not included in the efficacy analysis |
| Other bias | Low risk | Industry did not fund the trial |
Koter 2002.
| Methods | 8‐Week wash‐out period 12‐Week before‐and‐after study | |
| Participants | 31 men and women from Poland, mean age 58.3 years (40‐70) with type II hyperlipidaemia; TC > 250 mg/dL (6.46 mmol/L), LDL‐C > 170 mg/dL (4.40 mmol/L), TG < 400 mg/dL (4.52 mmol/L) Exclusion criteria: homozygous hypercholesterolaemia also type 3 to 5, secondary hyperlipoproteinaemia, uncontrolled severe HTN, hepatic dysfunction, unstable coronary disease, MI, lipid‐altering drugs, BMI > 35 Baseline TC: 7.94 mmol/L (307 mg/dL) Baseline LDL‐C: 5.74 mmol/L (222 mg/dL) No baseline HDL and TG values were given | |
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 6 weeks of serum TC and LDL‐C | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | No HDL‐C nor TG data were reported |
| Other bias | Unclear risk | The source of funding was not provided |
Kowalski 2006.
| Methods | 4‐Week hypolipaemic dietary period 6‐Week randomised study |
|
| Participants | 35 men and women aged 35 to 47 years with CHD risk (mixed hyperlipidaemia, BMI > 25 kg/m2, TC > 300 mg/dL (7.76 mmol/L), LDL‐C > 170 mg/dL (4.40 mmol/L), TG > 200 mg/dL (2.26 mmol/L)) 17 participants received atorvastatin, 18 received fluvastatin; 12 healthy participants with no drug administration served as the control group Exclusion criteria: none reported No baseline parameters were reported |
|
| Interventions | Atorvastatin 10 mg/d Fluvastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | HDL‐C data were not reported |
| Other bias | Unclear risk | The source of funding was not provided |
Kukharchuk 2007.
| Methods | 1‐Month wash‐out period 24‐Week before‐and‐after study |
|
| Participants | 134 individuals aged 30 to 75 years with CHD, atherosclerosis and the presence of 2 or more risk factors for 10‐year risk for CHD of 10% to 20% Exclusion criteria: TC ≥ 9.0 mmol/L (348 mg/dL), TG > 4.0 mmol/L (354 mg/dL), AST and ALT 2 × ULN, CPK 5 × ULN Baseline TC: 6.1 mmol/L (236 mg/dL) Baseline LDL‐C: 4.2 mmol/L (162 mg/dL) Baseline HDL‐C: 1.3 mmol/L (50 mg/dL) Baseline TG: 1.7 mmol/L (151 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 24 weeks Atorvastatin 20 mg/d for 4 to 24 weeks Atorvastatin 40 mg/d for 8 to 24 weeks Atorvastatin 80 mg/d for 16 to 24 weeks |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kural 2004.
| Methods | No participants had been receiving lipid‐lowering drug treatment; therefore no wash‐out period was required 6‐Week to 12‐week before‐and‐after trial |
|
| Participants | 40 men and women from Turkey with dyslipidaemia aged 53 years (41‐65) Exclusion criteria: hypothyroidism, diabetes mellitus, nephrotic syndrome, renal insufficiency, hepatic dysfunction, cancer, immune disorder, uncontrolled HTN, smoking, CAD Baseline TC: 7.45 mmol/L (288 mg/dL) Baseline LDL‐C: 5.28 mmol/L (204 mg/dL) Baseline HDL‐C: 1.06 mmol/L (41 mg/dL) Baseline TG: 2.27 mmol/L (201 mg/dL) |
|
| Interventions | Atorvastatin 40 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Labios 2005.
| Methods | 6‐Week wash‐out period 2‐Month before‐and‐after study |
|
| Participants | 33 men and women with primary hypercholesterolaemia with or without mixed hyperlipidaemia, aged 55 years Exclusion criteria: organic underlying disease, cancer, infectious or inflammatory disorder, use of antiplatelet drugs, diabetes, SBP > 140 mmHg, DBP > 90 mmHg, obesity, pregnancy, other additional lipid‐lowering treatment Baseline TC: 6.85 mmol/L (265 mg/dL) Baseline LDL‐C: 4.53 mmol/L (175 mg/dL) Baseline HDL‐C: 1.50 mmol/L (58 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 2 months of serum TC, LDL‐C and HDL‐C | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer funded the study; data may support bias for atorvastatin |
Lavallee 2009.
| Methods | Statin or fibrate treatment had to be stopped ≥ 50 days before the study 12‐Week double‐blind randomised placebo‐controlled trial |
|
| Participants | 117 men and women with lacunar stroke within the previous 3 months aged ≥ 18 years Exclusion criteria: arterial stenosis ≥ 50% in the stroke territory, childbearing potential, unstable angina, severe respiratory insufficiency, no temporal bone window Placebo baseline TC: 5.7 mmol/L (220 mg/dL) Placebo baseline LDL‐C: 3.6 mmol/L (139 mg/dL) Placebo baseline HDL‐C: 1.4 mmol/L (54 mg/dL) Atorvastatin 80 mg/d baseline TC: 5.8 mmol/L (224 mg/dL) Atorvastatin 80 mg/d baseline LDL‐C: 3.6 mmol/L (139 mg/dL) Atorvastatin 80 mg/d baseline HDL‐C: 1.5 mmol/L (58 mg/dL) |
|
| Interventions | Placebo Atorvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C and HDL‐C; WDAEs | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 33.2% were not analysed |
| Selective reporting (reporting bias) | High risk | TG data were not analysed |
| Other bias | High risk | Pfizer funded the trial |
Lawrence 2004.
| Methods | 6‐Week baseline dietary stabilisation period 8‐Week randomised double‐blind placebo‐controlled study | |
| Participants | 44 obese men and women with NIDDM from the UK aged 45 to 80 years; TC > 5 mmol/L (193 mg/dL); 40 completed the study 20 participants received placebo, 20 received atorvastatin Exclusion criteria: statin hypersensitivity, taking insulin or a thiazolidinedione, lipid‐lowering therapy within 4 months of the study; women of childbearing age were not excluded if sterilised or using adequate contraception Placebo baseline TC: 5.87 mmol/L (227 mg/dL) Placebo baseline LDL‐C: 6.20 mmol/L (240 mg/dL) Placebo baseline HDL‐C: 1.24 mmol/L (48 mg/dL) Placebo baseline TG: 2.05 mmol/L (182 mg/dL) Atorvastatin baseline TC: 5.64 mmol/L (218 mg/dL) Atorvastatin baseline LDL‐C: 5.74 mmol/L (222 mg/dL) Atorvastatin baseline HDL‐C: 1.30 mmol/L (50 mg/dL) Atorvastatin baseline TG: 1.93 mmol/L (171 mg/dL) |
|
| Interventions | Placebo Atorvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed WDAEs were reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "A double‐blind,randomized, placebo‐controlled design" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Placebo: 2/22 were not included in the efficacy analysis because they did not complete the study Atorvastatin: 2/22 were not included in the efficacy analysis because they did not complete the study 9.1% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured; WDAEs were reported |
| Other bias | High risk | Pfizer Pharmaceuticals funded the study; data may support bias for atorvastatin |
LCP‐AtorFen 2009.
| Methods | 4‐Week to 8‐week wash‐out period 12‐Week multi‐centre randomised double‐blind study |
|
| Participants | 220 men and women ≥ 18 years with mixed lipidaemia; TG 150 to 500 mg/dL 145 participants received fenofibrate, 73 received fenofibrate‐atorvastatin combination, 74 received atorvastatin Exclusion criteria: women who were pregnant or could become pregnant, poorly controlled type 2 diabetes mellitus, type 1 diabetes mellitus, lipoprotein lipase impairment or deficiency, apo CII deficiency, familial dysbetalipoproteinaemia, history of pancreatitis, CPK > 2 × ULN, ALT or AST > 1.5 × ULN , muscle pain, myopathy or rhabdomyolysis, poorly controlled HTN, unstable CHD, TIAs, stroke, aortic aneurysm, revascularisation or resection 6 months previously; renal, pulmonary, hepatic, biliary or gastrointestinal disease; statin or fibrate allergy, food supplements that can alter serum lipids Atorvastatin baseline TC: 6.58 mmol/L (2548 mg/dL) Atorvastatin baseline LDL‐C: 4.27 mmol/L (165 mg/dL) Atorvastatin baseline HDL‐C: 1.10 mmol/L (42.5 mg/dL) Atorvastatin baseline TG: 2.99 mmol/L (2651 mg/dL) |
|
| Interventions | Atorvastatin 40 mg/d Atorvastatin/fenofibrate 40/100 mg/d Fenofibrate 145 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin monotherapy group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 40 mg/d monotherapy group; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 40 mg/d monotherapy group; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 40 mg/d monotherapy group; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 74 participants randomly assigned to atorvastatin, 70 were analysed for efficacy |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | The study was sponsored by LifeCycle Pharma, which supplies atorvastatin fenofibrate combination, so there may be bias against atorvastatin |
Lee 2007.
| Methods | 4‐Week dietary lead‐in period 8‐Week randomised open‐label multi‐centre study |
|
| Participants | 268 men and women aged 20 to 79 years with untreated hypercholesterolaemia; TG < 400 mg/dL (4.52 mmol/L), LDL‐C > 130 mg/dL (3.36 mmol/L), 222 participants completed the study 112 participants received atorvastatin, 110 received pitavastatin Exclusion criteria: pregnant and breastfeeding women, current use of lipid‐altering therapy, uncontrolled diabetes mellitus, uncontrolled HTN, history of cerebrovascular disease or MI within 3 months of enrolment, congestive heart failure, serum creatinine > 2.0 mg/dL, hepatic dysfunction, CK > 2.5 × ULN Atorvastatin baseline TC: 6.18 mmol/L (239 mg/dL) Atorvastatin baseline LDL‐C: 4.14 mmol/L (160 mg/dL) Atorvastatin baseline HDL‐C: 1.34 mmol/L (52 mg/dL) Atorvastatin baseline TG: 1.54 mmol/L (136 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 4 weeks Atorvastatin conditional titration of 20 mg/d for 4 to 8 weeks Pitavastatin 2 mg/d for 0 to 4 weeks Pitavastatin conditional titration of 4 mg/d for 4 to 8 weeks |
|
| Outcomes | Per cent change from baseline at 4 weeks for serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Choongwae Pharma Corp funded the study; data may support bias against atorvastatin |
Lee 2011.
| Methods | 4‐Week dietary wash‐out period 8‐Week before‐and‐after trial |
|
| Participants | Men and women aged 20 to 79 years; LDL‐C > 130 mg/dL, TG < 400 mg/dL Exclusion criteria: FH, pregnancy or breastfeeding, stroke history or MI within 3 months of study, renal dysfunction, thyroid dysfunction, inflammatory disease, anti‐inflammatory drug use, cancer, history of adverse reaction to test drugs Atorvastatin 20 mg/d baseline TC: 6.18 mmol/L (239 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 4.24 mmol/L (164 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.31 mmol/L (51 mg/dL) Atorvastatin 20 mg/d baseline TG: 1.45 mmol/L (128 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d Atorvastatin/ezetimibe 5 mg/5 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Lee 2012.
| Methods | 4‐Week dietary wash‐out period 8‐Week randomised open‐label study |
|
| Participants | 78 men and women aged 20 to 79 years; LDL‐C > 130 mg/dL (3.36 mmol/L), TG 150 to 499 mg/dL (1.69‐5.63 mmol/L) 39 participants received atorvastatin, 39 received atorvastatin/ezetimibe Exclusion criteria: familial hypercholesterolaemia, pregnancy, breastfeeding, history of acute cerebrovascular accident, MI within 3 months of trial entry, serum creatinine > 2.0 mg/dL, transaminase level > 2 × ULN, thyroid dysfunction, serum creatine kinase > 2.5 × ULN, infection, inflammatory disease, cancer, adverse reactions to test drugs Atorvastatin 20 mg/d baseline TC: 6.44 mmol/L (249 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 4.16 mmol/L (161 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.23 mmol/L (48 mg/dL) Atorvastatin 20 mg/d baseline TG: 2.25 mmol/L (199 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d Atorvastatin/ezetimibe 5 mg/5 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin 20 mg/d; treatment arm was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 11/39 (28.2%) participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were included in the efficacy analysis |
| Other bias | Low risk | Industry did not fund the trial |
Lee 2013.
| Methods | 4‐Week wash‐out period 12‐Week randomised open‐label parallel‐group phase 4 study |
|
| Participants | 132 men and women aged 20 to 80 years with type 2 diabetes mellitus; LDL‐C > 100 mg/dL (2.59 mmol/L) 66 participants received atorvastatin, 66 received ezetimibe/atorvastatin Exclusion criteria: hypersensitivity to drugs, chronic renal failure, hepatic dysfunction, unexplained serum creatinine kinase elevation > 2.5 × ULN, congestive heart failure, stroke, MI, coronary revascularisation within preceding 3 months, uncontrolled thyroid disease, medical condition requiring medicine that could interfere with test drugs, life expectancy < 1 year, pregnant or breastfeeding Atorvastatin 20 mg/d baseline TC: 5.6 mmol/L (217 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 3.46 mmol/L (134 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.24 mmol/L (48 mg/dL) Atorvastatin 20 mg/d baseline TG: 1.97 mmol/L (174 mg/dL) |
|
| Interventions | Atorvastaitn 20 mg/d Ezetimbe/atorvastatin 10 mg/20 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin 20 mg/d treatment arm was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | Grant from MSD Inc was provided for funding |
Lemieux 2003.
| Methods | 4‐Week wash‐out period 12‐Week multi‐centre randomised study | |
| Participants | 136 dyslipidaemic individuals from Canada aged 18 to 75 years; LDL‐C > 3.24 mmol/L (125.3 mg/dL), HDL‐C < 1.10 mmol/L (42.5 mg/dL) in men, HDL‐C < 1.20 mmol/L (46.4 mg/dL) in women, TG < 4.52 mmol/L (400.7 mg/dL) 72 participants received atorvastatin, 64 received fenofibrate Exclusion criteria: diabetes mellitus, digestive disease, PTCA or CABG within 6 months, unstable angina, alcoholism Atorvastatin baseline TC: 6.03 mmol/L (233 mg/dL) Atorvastatin baseline LDL‐C: 4.10 mmol/L (159 mg/dL) Atorvastatin baseline HDL‐C: 0.94 mmol/L (36 mg/dL) Atorvastatin baseline TG: 2.18 mmol/L (193 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 12 weeks Fenofibrate 200 mg/d for 0 to 12 weeks |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | No source of funding was provided |
Leung 2002.
| Methods | 6‐Week wash‐out period 18‐Week open‐label multi‐centre study | |
| Participants | 63 men and women from Hong Kong, mean age 64 years (44‐78) with CAD and hypercholesterolaemia; LDL 3.4 to 5.2 mmol/L (131‐201 mg/dL), TG ≤ 4.5 mmol/L (399 mg/dL)
Exclusion criteria: < 18 or > 80 years, women likely to become pregnant, heavy drinking, secondary hypercholesterolaemia, uncontrolled HTN and type 2 diabetes, BMI > 30, liver and renal dysfunction or history of CAD that required intervention Baseline TC: 5.90 mmol/L (228 mg/dL) Baseline LDL‐C: 4.04 mmol/L (156 mg/dL) Baseline HDL‐C: 1.20 mmol/L (46.4 mg/dL) Baseline TG: 1.60 mmol/L (142 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 6 to 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | No source of funding was provided |
Li 2010.
| Methods | Participants were not receiving lipid‐altering agents, so no wash‐out was required 12‐Week before‐and‐after trial |
|
| Participants | 84 men and women with CHD aged 55 to 76 years Exclusion criteria: liver or renal failure, diabetes mellitus, MI, HTN, severe or unstable angina pectoris, heart failure, severe cardiac arrhythmia, transplantation Atorvastatin 10 mg/d baseline TC: 5.43 mmol/L (210 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 3.31 mmol/L (128 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.13 mmol/L (44 mg/dL) Atorvastatin 10 mg/d baseline TG: 1.68 mmol/L (149 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Simvastatin 20 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Lins 2004.
| Methods | 4‐Week wash‐out period 12‐Week multi‐centre randomised double‐blind placebo‐controlled parallel‐group study Block randomisation with block size 4 Morning doses | |
| Participants | 42 men and women from Belgium with chronic renal failure > 17 years, TC > 210 mg/dL (5.4 mmol/L), TG > 500 mg/dL (5.6 mmol/L) 23 participants received atorvastatin, 19 received placebo Exclusion criteria: pregnant and breastfeeding women, uncontrolled diabetes, hepatic dysfunction Placebo baseline TC: 6.08 mmol/L (235 mg/dL) Placebo baseline LDL‐C: 3.54 mmol/L (137 mg/dL) Placebo baseline HDL‐C: 1.14 mmol/L (44 mg/dL) Placebo baseline TG: 2.26 mmol/L (200 mg/dL) Atorvastatin baseline TC: 6.28 mmol/L (243 mg/dL) Atorvastatin baseline LDL‐C: 3.39 mmol/L (131 mg/dL) Atorvastatin baseline HDL‐C: 1.16 mmol/L (45 mg/dL) Atorvastatin baseline TG: 2.22 mmol/L (197 mg/dL) |
|
| Interventions | Placebo Atorvastatin 10 mg/d for 0 to 4 weeks Atorvastatin 20 mg/d for 4 to 8 weeks Atorvastatin 40 mg/d for 8 to 12 weeks |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | The placebo group and the first atorvastatin dose group were analysed WDAEs were not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The study had a randomized, double‐blind, placebo‐controlled, parallel‐group design" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | All lipid parameters were measured; WDAEs were not reported |
| Other bias | High risk | Pfizer funded the study; data may support bias for atorvastatin |
Llaverias 2008.
| Methods | 4‐Week wash‐out period 4‐Week study |
|
| Participants | 12 men with type IIb FH; all individuals fulfilled the standard criteria of untreated serum cholesterol or TG levels above the sex‐ and age‐adjusted 90th percentile for the reference population Exclusion criteria: secondary causes of hyperlipidaemia, tendon acanthoma, diagnosis matching FH Baseline TC: 8.74 mmol/L (338 mg/dL) Baseline LDL‐C: 6.05 mmol/L (234 mg/dL) Baseline HDL‐C: 1.22 mmol/L (47.2 mg/dL) |
|
| Interventions | Atorvastatin 40 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C and HDL‐C | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | TG data were not reported because data were expressed as median values |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Loughrey 2013.
| Methods | Participants were not receiving any lipid medications; therefore no wash‐out period was required 6‐Week randomized double‐blind placebo‐controlled trial |
|
| Participants | 50 men and women with metabolic syndrome aged 35‐63 years with metabolic syndrome as defined by the International Diabetes Federation; central obesity plus 2 of the following: hypertension, glucose intolerance, low HDL‐C hypertriglyceridaemia 24 participants received atorvastatin; 26 received placebo Exclusion criteria: preexisting indication for lipid‐lowering therapy, history of intolerance for these agents, insulin use or HRT, liver or muscle disease, renal dysfunction, potential for pregnancy, total cholesterol > 6.5 mmol/L (251 mg/dL) or < 4 mmol/L (155 mg/dL) Atorvastatin baseline TC: 5.64 mmol/L (218 mg/dL) Atorvastatin baseline LDL‐C: 3.37 mmol/L (130 mg/dL) Atorvastatin baseline HDL‐C: 1.26 mmol/L (49 mg/dL) Atorvastatin baseline TG: 2.02 mmol/L (179 mg/dL) Placebo baseline TC: 5.52 mmol/L (213 mg/dL) Placebo baseline LDL‐C: 3.21 mmol/L (124 mg/dL) Placebo baseline HDL‐C: 1.43 mmol/L (55 mg/dL) Placebo baseline TG: 2.02 mmol/L (179 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Placebo |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | The source of funding was not reported |
LUNAR 2012.
| Methods | Participants were not receiving lipid‐lowering medications within 4 weeks of the trial; no wash‐out period was required 12‐Week randomised open‐label parallel‐group study |
|
| Participants | 825 men and women with acute coronary syndrome and coronary artery disease 18 to 75 years old; LDL‐C > 70 mg/dL (1.81 mmol/L) and TG < 500 mg/dL (5.645 mmol/L) 278 participants received atorvastatin, 547 received rosuvastatin Exclusion criteria: hormone therapy within the previous 3 months, Q‐wave MI, pulmonary oedema, moderate or severe heart failure, acute moderate to severe mitral regurgitation, acute ventricular septal defect, ventricular fibrillation or tachycardia, complete heart block, new‐onset atrial fibrillation with uncontrolled ventricular rate, paced ventricular rhythm, stroke, sepsis, acute pericarditis, systemic or pulmonary embolus within preceding 4 weeks, bypass within 3 months, PCI within 6 months, statin hypersensitivity, pregnancy or breastfeeding, uncontrolled diabetes mellitus, hypertension, hypothyroidism, systolic hypotension, hepatic dysfunction, severe anaemia, serum creatine kinase 3 × ULN not caused by myocardial injury Atorvastatin 80 mg/d baseline TC: 5.066 mmol/L (196 mg/dL) Atorvastatin 80 mg/d baseline LDL‐C: 3.445 mmol/L (133 mg/dL) Atorvastatin 80 mg/d baseline HDL‐C: 1.03 mmol/L (40 mg/dL) Atorvastatin 80 mg/d baseline TG: 1.78 mmol/L (157 mg/dL) |
|
| Interventions | Atorvastatin 80 mg/d Rosuvastatin 20 mg/d Rosuvastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 6 to 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin treatment was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 80 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 80 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 80 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 21/278 (7.6%) participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstraZeneca funded the trial; efficacy could be biased against atorvastatin |
Lupattelli 2012.
| Methods | Participants were not receiving any lipid medications; therefore no wash‐out period was required 8‐Week before‐and‐after trial |
|
| Participants | 80 men and women with polygenic hypercholesterolaemia or familial combined hyperlipaemia type IIB; individuals with polygenic hypercholesterolaemia had LDL‐C of 160 to 199 mg/dL (4.14‐5.15 mmol/L) Exclusion criteria: secondary hyperlipaemia, autosomal dominant hypercholesterolaemia, familial hypertriglyceridaemia Atorvastatin 10 mg/d baseline LDL‐C: 5.13 mmol/L (198 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.125 mmol/L (43.5 mg/dL) Atorvastatin 10 mg/d baseline TG: 1.97 mmol/L (174 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 8 weeks of serum LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | Total cholesterol was not included in the efficacy analysis |
| Other bias | Unclear risk | The source of funding was not reported |
Ma 2000.
| Methods | 10‐Week wash‐out period 8‐Week multi‐centre double‐blind randomised study Randomly assigned in a 2:2:1:1 ratio Evening doses | |
| Participants | 340 men and women from Canada with combined type IIb dyslipidaemia, mean age 56.6 years (18‐80); TG 2.8 to 9.0 mmol/L (248‐797 mg/dL), LDL‐C 2.6 to 3.4 mmol/L (100‐131 mg/dL) Exclusion criteria: women who are likely to become pregnant, unstable weight, type 1 diabetes or uncontrolled type 2 diabetes, severe HTN, MI, unstable CVD, hypothyroidism, cancer, renal and hepatic dysfunction, neuromuscular disease, gastrointestinal disease, pancreatitis, drugs affecting lipid analysis or values, alcohol and drug abuse, immunosuppressants Atorvastatin 10 mg/d baseline TC: 6.80 mmol/L (263 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 4.21 mmol/L (163 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.01 mmol/L (39 mg/dL) Atorvastatin 10 mg/d baseline TG: 3.67 mmol/L (325 mg/dL) Atorvastatin 20 mg/d baseline TC: 7.20 mmol/L (278 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 4.57 mmol/L (1773 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.06 mmol/L (41 mg/dL) Atorvastatin 20 mg/d baseline TG: 3.75 mmol/L (315 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Atorvastatin 20 mg/d Cerivastatin 0.4 mg/d Cerivastatin 0.8 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C and HDL‐C | |
| Notes | Atorvastatin groups were analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d and atorvastatin 20 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d and atorvastatin 20 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d and atorvastatin 20 mg/d; interventions were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Atorvastatin 10 mg/d: 1/112 were not included in the efficacy analysis because of absence of post‐randomisation lipid data Atorvastatin 20 mg/d: 1/56 were not included in the efficacy analysis because of absence of post‐randomisation lipid data 1.2% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | High risk | TG data reported were excluded from this review because they were expressed as median per cent change |
| Other bias | High risk | The trial was supported by Bayer Canada Inc; data may support bias against atorvastatin |
Mabuchi 2005.
| Methods | 4‐Week dietary lead‐in period 8‐Week study |
|
| Participants | 14 men and women from Japan with hypercholesterolaemia; TC > 220 mg/dL (5.69 mmol/L) Exclusion criteria: pregnant or lactating women, women of childbearing potential Baseline TC: 7.09 mmol/L (274 mg/dL) Baseline LDL‐C: 4.97 mmol/L (192 mg/dL) Baseline HDL‐C: 1.40 mmol/L (54 mg/dL) Baseline TG: 1.57 mmol/L (139 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | No source of funding was provided |
Mabuchi 2007.
| Methods | 4‐Week dietary lead‐in period 16‐Week randomised double‐blind placebo‐controlled study to examine the effects of CoQ10 and placebo in hypercholesterolaemic patients treated with atorvastatin |
|
| Participants | 49 men and women with hypercholesterolaemia 25 participants received placebo CoQ10 + atorvastatin, 24 received CoQ10 + atorvastatin Exclusion criteria: pregnancy or lactation, FH, taking other lipid‐altering medications including antioxidants Placebo CoQ10 + atorvastatin 10 mg/d baseline TC: 7.33 mmol/L (283 mg/dL) Placebo CoQ10 + atorvastatin 10 mg/d baseline LDL‐C: 4.84 mmol/L (187 mg/dL) Placebo CoQ10 + atorvastatin 10 mg/d baseline HDL‐C: 1.56 mmol/L (60 mg/dL) Placebo CoQ10 + atorvastatin 10 mg/d baseline TG: 1.85 mmol/L (164 mg/dL) CoQ10 + atorvastatin 10 mg/d baseline TC: 7.15 mmol/L (276 mg/dL) CoQ10 + atorvastatin 10 mg/d baseline LDL‐C: 4.73 mmol/L (183 mg/dL) CoQ10 + atorvastatin 10 mg/d baseline HDL‐C: 1.61 mmol/L (62 mg/dL) CoQ10 + atorvastatin 10 mg/d baseline TG: 1.39 mmol/L (123 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d + placebo CoQ10 Atorvastatin 10 mg/d + CoQ10 10 mg/d |
|
| Outcomes | Per cent change from baseline at 4 to 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d + placebo and atorvastatin 10 mg/d + CoQ10 interventions were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d + placebo and atorvastatin 10 mg/d + CoQ10 interventions were analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d + placebo and atorvastatin 10 mg/d + CoQ10 interventions were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The study was supported by Kaneka Co Osaka; Kaneka sells CoQ10 |
Macin 2005.
| Methods | No participant received a statin or other lipid‐lowering agents at any time during the preceding 1 month; therefore no wash‐out period was required 1‐Month prospective randomised double‐blind placebo‐controlled trial |
|
| Participants | 90 men and women with ACS from Argentina aged > 21 years Exclusion criteria: active liver disease, untreated endocrine disorder, systemic inflammatory disease or cancer, statin hypersensitivity, threat of poor compliance, known infectious disease within 30 days of study, lack of consent, cardiogenic shock, acute pulmonary oedema Placebo: Baseline TC: 5.02 mmol/L (194 mg/dL) Baseline LDL‐C: 3.09 mmol/L (119 mg/dL) Baseline HDL‐C: 0.96 mmol/L (37 mg/dL) Baseline TG: 2.08 mmol/L (184 mg/dL) Atorvastatin: Baseline TC: 5.02 mmol/L (194 mg/dL) Baseline LDL‐C: 3.30 mmol/L (128 mg/dL) Baseline HDL‐C: 0.95 mmol/L (37 mg/dL) Baseline TG: 2.19 mmol/L (194 mg/dL) |
|
| Interventions | 46 participants received placebo, 44 received atorvastatin 40 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG; WDAEs were reported as deaths | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind fashion" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | All lipid parameters were measured WDAEs were not reported for placebo and atorvastatin groups |
| Other bias | Unclear risk | The source of funding was not reported |
Magen 2004.
| Methods | 4‐Week run‐in phase 8‐Week single‐blind randomised placebo‐controlled trial |
|
| Participants | 31 men and women with hyperlipidaemia and essential HTN, LDL‐C > 130 mg/dL (3.36 mmol/L) Exclusion criteria: diabetes mellitus treated with medication, fasting hyperglycaemia > 150 mg/dL, liver or kidney disease, cancer, acute MI or unstable angina within 6 months, heart failure, smoking > 10 cigarettes/d, use of corticosteroids or other immunosuppressive therapy, abnormal levels of plasma aldosterone, supine and standing plasma renin activity and 24‐hour urinary catecholamines, renal artery stenosis Placebo baseline LDL‐C: 3.84 mmol/L (148 mg/dL) Atorvastatin baseline LDL‐C: 4.20 mmol/L (162 mg/dL) |
|
| Interventions | Placebo Atorvastatin 20 mg/d Ascorbic acid 500 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum LDL‐C | |
| Notes | Placebo and atorvastatin groups were analysed SDs were imputed WDAEs were not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | High risk | Single‐blind; investigators were not blinded |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Randomized in a single‐blind manner" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | TC, HDL‐C and TG data were not reported; WDAEs were not reported |
| Other bias | Unclear risk | No source of funding was provided |
Majima 2007.
| Methods | Participants had untreated hypercholesterolaemia; therefore no wash‐out period was required 3‐Month (at baseline and 3 months after the start of treatment) longitudinal trial |
|
| Participants | 22 men from Japan with hypercholesterolaemia aged 62 years; TC > 220 mg/dL, LDL‐C > 140 mg/dL, BMI 24.6 Exclusion criteria: history of fracture, type 1 diabetes mellitus, liver disease, renal dysfunction, cancer, hyperthyroidism, hyperparathyroidism, hypogonadism, drugs that affect bone metabolism, TG > 500 mg/dL Baseline TC: 6.41 mmol/L (248 mg/dL) Baseline LDL‐C: 4.21 mmol/L (163 mg/dL) Baseline HDL‐C: 1.33 mmol/L (51 mg/dL) Baseline TG: 1.87 mmol/L (166 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not reported |
Maki 2011.
| Methods | 4‐Week diet lead‐in wash‐out period 16‐Week before‐and‐after study |
|
| Participants | 245 men and women with mixed dyslipidaemia aged 18 to 79 years; non‐HDL‐C > 160 mg/dL (4.14 mmol/L) TG ≥ 250 mg/dL (2.82 mmol/L) and < 600 mg/dL (6.77 mmol/L) 123 participants received atorvastatin and POM3, 122 received atorvastatin Exclusion criteria: history of cardiovascular events, significant renal or pulmonary disease, cancer, uncontrolled hypertension, myopathy, lipoprotein lipase dysfunction, Apo CII deficiency, familial dysbetalipoproteinaemia, BMI > 40, glycosylated haemoglobin > 9%, elevated serum transaminase, creatinine or creatine kinase, drug or alcohol abuse Atorvastatin 10 mg/d baseline LDL‐C: 5.03 mmol/L (195 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 0.835 mmol/L (32 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 8 weeks + placebo POM3 Atorvastatin 20 mg/d for 8 to 12 weeks + placebo POM3 Atorvastatin 40 mg/d for 12 to 16 weeks + placebo POM3 Atorvastatin 10 mg/d for 8 weeks + POM3 Atorvastatin 20 mg/d for 8 to 12 weeks + POM3 Atorvastatin 40 mg/d for 12 to 16 weeks + POM3 |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum LDL‐C and HDL‐C | |
| Notes | Atorvastatin 10 mg/d for 8 weeks + placebo POM3; treatment arm was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/122 (0.8%) participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | Total cholesterol and triglycerides were not included in the efficacy analysis |
Mandosi 2010.
| Methods | Participants were not receiving lipid‐altering agents, so no wash‐out was required 8‐Week before‐and‐after trial |
|
| Participants | 22 men and women with type 2 diabetes mellitus aged 54 to 68 years Exclusion criteria: anti‐inflammatory use, smoking, cancer, major surgery or MI within 6 months of trial, infection, inflammatory disease, renal and hepatic dysfunction, severe retinopathy, pregnancy Atorvastatin 20 mg/d baseline TC: 5.2 mmol/L (201 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 3.2 mmol/L (124 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.0 mmol/L (29 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C and HDL‐C | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | TG data were not analysed |
| Other bias | Unclear risk | The source of funding was not revealed |
Manuel‐Y‐Keenoy 2004.
| Methods | 4‐Week to 6‐week wash‐out period 6‐Month randomised block atorvastatin study |
|
| Participants | 24 individuals with type 1 diabetes; TC > 4.9 mmol/L, LDL‐C > 3.0 mmol/L, TG < 4.5 mmol/L Baseline TC: 6.08 mmol/L (235 mg/dL) Baseline LDL‐C: 3.91 mmol/L (151 mg/dL) Baseline TG: 1.17 mmol/L (104 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d + vitamin E 250 IU Atorvastatin 20 mg/d + vitamin E placebo |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C and TG | |
| Notes | Data for both interventions were combined SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d + placebo and atorvastatin 20 mg/d + vitamin E; interventions were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d + placebo and atorvastatin 20 mg/d + vitamin E; interventions were analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d + placebo and atorvastatin 20 mg/d + vitamin E; interventions were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/24 were not included in the efficacy analysis because they did not complete the study 8.3% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | High risk | HDL‐C data were not reported |
| Other bias | Unclear risk | Omega‐Pharma NV supplied the alpha tocopherol and placebo. No source of funding was provided |
Marais 1997.
| Methods | 4‐Week wash‐out period 6‐Week open‐label randomised study | |
| Participants | 22 ambulatory men and women from South Africa with FH recruited from lipid clinics aged 38 years (21‐58); TG < 4.5 mmol/L (399 mg/dL) Exclusion criteria: consumption of lipid‐modifying drugs; significant liver, renal or endocrine disease; alcohol consumption, uncontrolled HTN, conception risk, BMI > 32 Baseline TC: 9.90 mmol/L (383 mg/dL) Baseline LDL‐C: 8.16 mmol/L (316 mg/dL) Baseline HDL‐C: 1.19 mmol/L (46.02 mg/dL) Baseline TG: 1.34 mmol/L (119 mg/dL) | |
| Interventions | Atorvastatin 40 mg/d BID Atorvastatin 80 mg/d in the evening |
|
| Outcomes | Per cent change from baseline at 4 to 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Data for both interventions were combined SDs were imputed for TC, HDL‐C and TG |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may support bias for atorvastatin |
Marchesi 2000.
| Methods | No participant was receiving lipid‐lowering drugs; therefore no wash‐out period was required 8‐Week before‐and‐after trial |
|
| Participants | 30 postmenopausal women with hypercholesterolaemia, mean age 58 years; BP 131/75 mmHg, BMI 23.8 Exclusion criteria: none reported Baseline TC: 8.25 mmol/L (319 mg/dL) Baseline LDL‐C: 5.90 mmol/L (228 mg/dL) Baseline HDL‐C: 1.53 mmol/L (59 mg/dL) Baseline TG: 1.78 mmol/L (158 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Marketou 2006.
| Methods | 6‐Week wash‐out period 3‐Week open‐label parallel‐group randomised design | |
| Participants | 43 men and women from Greece aged 35 to 70 years; LDL‐C > 190 mg/dL Exclusion criteria: current or previous statin treatment, ACS history, revascularisation procedures, coronary or peripheral arterial disease symptoms, uncontrolled diabetes mellitus or HTN, left ventricular ejection fraction < 60%, liver disease, renal insufficiency, drug or alcohol abuse history, any inflammatory or other chronic infectious disease, pregnancy threat, uncontrolled hypothyroidism, immunosuppressant use Atorvastatin baseline TC: 7.21 mmol/L (279 mg/dL) Atorvastatin baseline LDL‐C: 4.60 mmol/L (178 mg/dL) Atorvastatin baseline HDL‐C: 1.03 mmol/L (40 mg/dL) Atorvastatin baseline TG: 2.56 mmol/L (227 mg/dL) | |
| Interventions | Atorvastatin 40 mg/d Simvastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 3 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed Results of the placebo group were not reported SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | No source of funding was provided |
McInnes 2014.
| Methods | 3‐Month baseline wash‐out period for all lipid‐altering agents 6‐Week randomised double‐blind placebo‐controlled trial |
|
| Participants | 98 men and women 18 years of age or older with rheumatoid arthritis Exclusion criteria: haemoglobin < 9.0 g/dL, haematocrit < 30%, white blood cell count < 3.0 × 109/L, absolute neutrophil count < 1.2 × 109/L or platelet count < 100 × 109/L, estimated glomerular filtration rate ≤ 40 mL/min, AST or ALT > 1.5 × ULN, TG > 4.52 mmol/L (400 mg/dL) and TC > 8.29 mmol/L (321 mg/dL), cancer, herpes zoster, hepatitis B or C or HIV, evidence of TB within 3 months of screening 50 participants received atorvastatin, 48 received placebo Placebo baseline TC: 6.07 mmol/L (235 mg/dL) Placebo baseline LDL‐C: 3.58 mmol/L (138 mg/dL) Placebo baseline HDL‐C: 1.83 mmol/L (71 mg/dL) Placebo baseline TG: 1.46 mmol/L (129 mg/dL) Atorvastatin 10 mg/d baseline TC: 5.91 mmol/L (229 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 3.52 mmol/L (136 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.75 mmol/L (68 mg/dL) Atorvastatin 10 mg/d baseline TG: 1.28 mmol/L (113 mg/dL) |
|
| Interventions | Placebo Atorvastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 4 to 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Fixed randomisation was accomplished using IVRS (an automated web/telephone randomisation system) |
| Allocation concealment (selection bias) | Low risk | The study drug for atorvastatin was labelled in such a manner that participants and staff were unable to determine from the dispensed packaging to which treatment arm participants were assigned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/48 ((2.1%) participants given placebo were not included in the efficacy analysis All participants given atorvastatin were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were included in the efficacy analysis |
| Other bias | High risk | Pfizer funded the trial; efficacy data could be biased towards atorvastatin |
McKenney 1998.
| Methods | 6‐Week wash‐out period 12‐Week multi‐centre open‐label randomised parallel‐group study Randomly assigned in sequential order at each site, stratified by type of dyslipidaemia Evening dose | |
| Participants | 108 men and women from the USA aged 55 years (18‐80) with combined hyperlipidaemia or isolated hypertriglyceridaemia; TC > 199 mg/dL (5.15 mmol/L), TG 201 to 799 mg/dL (2.27‐9.02 mmol/L), BMI < 33 Exclusion criteria: hepatic dysfunction, renal dysfunction, uncontrolled HTN and diabetes, TSH > 7.0 μU/mL at screening, MI, coronary angioplasty or bypass graft, unstable angina, gall bladder disease, gout or peptic ulcer disease Atorvastatin baseline TC: 7.1 mmol/L (275 mg/dL) Atorvastatin baseline LDL‐C: 4.4 mmol/L (170 mg/dL) Atorvastatin baseline HDL‐C: 0.98 mmol/L (37.9 mg/dL) Atorvastatin baseline TG: 4.50 mmol/L (399 mg/dL) | |
| Interventions | Atorvastatin 10 mg/d Nicotinic acid 1 g 3 times daily |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Atorvastatin group: 1/55 were not included in the efficacy analysis because the participant had no treatment phase measurement within 3 days of receiving the drug 1.8% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may support bias for atorvastatin |
MERCURY II 2006.
| Methods | 6‐Week wash‐out period 16‐Week multi‐centre open‐label randomised study | |
| Participants | 1993 men and women from North and South America aged > 17 years; LDL‐C 130 to 250 mg/dL (3.36‐6.46 mmol/L), TG < 400 mg/dL (4.52 mmol/L) 772 people received atorvastatin, 778 received simvastatin, 383 received rosuvastatin Exclusion criteria: pregnancy or lactation; homozygous familial type I, III, IV or V hyperlipidaemia; unstable arterial disease within 3 months of trial; uncontrolled HTN or diabetes mellitus; renal and hepatic dysfunction Atorvastatin 10 mg/d baseline TC: 6.54 mmol/L (253 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 4.37 mmol/L (169 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.22 mmol/L (47 mg/dL) Atorvastatin 10 mg/d baseline TG: 2.08 mmol/L (184 mg/dL) Atorvastatin 20 mg/d baseline TC: 6.49 mmol/L (251 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 4.35 mmol/L (168 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.21 mmol/L (47 mg/dL) Atorvastatin 20 mg/d baseline TG: 2.05 mmol/L (182 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Atorvastatin 20 mg/d Rosuvastatin 20 mg/d Simvastatin 20 mg/d Simvastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin groups were analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d and atorvastatin 20 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d and atorvastatin 20 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d and atorvastatin 20 mg/d; interventions were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Atorvastatin 10 mg/d: 14/403 were not included in the efficacy analysis because they were not in the ITT group Atorvastatin 20 mg/d: 12/395 were not included in the efficacy analysis because they were not in the ITT group 3.3% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | AstraZeneca funded the study; data may support bias against atorvastatin |
MERCURY I 2004.
| Methods | 6‐Week wash‐out period 16‐Week multi‐centre open‐label randomised parallel‐group study | |
| Participants | 3140 men and women from Europe, Canada and Australia with hypercholesterolaemia aged > 17 years; LDL‐C > 2.99 mmol/L (116 mg/dL), TG < 4.52 mmol/L (401 mg/dL) 1491 participants received atorvastatin, 559 received simvastatin, 538 received pravastatin, 552 received rosuvastatin Exclusion criteria: pregnancy threat, homozygous familial or type III hypercholesterolaemia, active CVD within 2 months of screening, uncontrolled HTN, renal and hepatic dysfunction Atorvastatin 10 mg/d baseline LDL‐C: 4.19 mmol/L (162 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 4.33 mmol/L (167 mg/dL) No baseline TC, HDL‐C or TG values |
|
| Interventions | Atorvastatin 10 mg/d Atorvastatin 20 mg/d Rosuvastatin 10 mg/d Simvastatin 20 mg/d Pravastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin groups were analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d and atorvastatin 20 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d and atorvastatin 20 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d and atorvastatin 20 mg/d; interventions were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | AstraZeneca funded the study; data may support bias against atorvastatin |
Milionis 2004.
| Methods | 6‐Week wash‐out period
12‐Week randomised study Evening dose |
|
| Participants | 180 men and women from Greece with primary hyperlipidaemia from outpatient lipid clinic, mean age 58.5 years; TC > 240 mg/dL (6.21 mmol/L), TG < 350 mg/dL (3.95 mmol/L) 90 participants received atorvastatin, 90 received simvastatin Exclusion criteria: hepatic dysfunction, renal dysfunction, diabetes, TSH > 5.0 μU/L, any medical conditions that interfere with study protocol Atorvastatin baseline TC: 7.84 mmol/L (303 mg/dL) Atorvastatin baseline LDL‐C: 5.77 mmol/L (223 mg/dL) Atorvastatin baseline HDL‐C: 1.22 mmol/L (47.18 mg/dL) Atorvastatin baseline TG: 1.89 mmol/L (167 mg/dL) |
|
| Interventions | Atorvastatin 40 mg/d Simvastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 6 to 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Milionis 2003.
| Methods | 6‐Week wash‐out period
12‐Week randomised single‐centre study Evening dose |
|
| Participants | 128 men and women from Greece with dyslipidaemia from an outpatient clinic; TC > 240 mg/dL (6.21 mmol/L), TG < 300 mg/dL (3.39 mmol/L) 64 participants received atorvastatin, 42 received simvastatin, 22 received fenofibrate Exclusion criteria: impaired hepatic function, alcohol abuse, impaired renal function, diabetes mellitus, thyroid dysfunction, any medical condition that might preclude successful study completion, drugs that interfere with lipid determination Atorvastatin baseline TC: 7.84 mmol/L (303 mg/dL) Atorvastatin baseline LDL‐C: 5.77 mmol/L (223 mg/dL) Atorvastatin baseline HDL‐C: 1.22 mmol/L (47.18 mg/dL) Atorvastatin baseline TG: 1.89 mmol/L (167 mg/dL) |
|
| Interventions | Atorvastatin 40 mg/d Simvastatin 40 mg/d Fenofibrate 200 mg/d |
|
| Outcomes | Per cent change from baseline at 6 to 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | No source of funding was provided |
Mirdamadi 2008.
| Methods | 6‐Week diet run‐in period 12‐Week randomised study |
|
| Participants | 164 men and women aged 21 to 70 years with untreated type IIb hyperlipidaemia 61 participants received atorvastatin, 46 received simvastatin, 57 received fluvastatin Exclusion criteria: hepatic disorders, endocrine or renal disorders, diabetes mellitus, impaired glucose tolerance, alcoholism, drug abuse, gallstones, cancer, pregnancy or lactation, use of anticoagulants or lipid‐lowering therapy Atorvastatin baseline TC: 6.98 mmol/L (270 mg/dL) Atorvastatin baseline LDL‐C: 4.70 mmol/L (182 mg/dL) Atorvastatin baseline HDL‐C: 1.31 mmol/L (51 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Simvastatin 10 mg/d Simvastatin 20 mg/d Fluvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C and HDL‐C | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | TG data were not reported because data were expressed as median values |
| Other bias | Low risk | The study appears to be free of other sources of bias |
MODEST 2009.
| Methods | No participant received lipid‐altering medication; no wash‐out was required 12‐Week before‐and‐after study |
|
| Participants | 60 men and women with type 2 diabetes mellitus with clinically evident CHD Exclusion criteria: statin hypersensitivity, liver disease, overt nephropathy, pregnancy, breastfeeding, increased CK levels, postmenopausal women Baseline TC: 5.72 mmol/L (221 mg/dL) Baseline LDL‐C: 3.77 mmol/L (146 mg/dL) Baseline HDL‐C: 1.19 mmol/L (46 mg/dL) Baseline TG: 1.81 mmol/L (160 mg/dL) |
|
| Interventions | Atorvastatin 80 mg/d Ezetimibe/atorvastatin 10 mg/10 mg/d |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12/60 (20%) were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Monteiro 2008.
| Methods | Participants were not receiving lipid‐altering substances within 30 days of enrolment, so no wash‐out was required 6‐Week double‐blind randomised placebo‐controlled trial |
|
| Participants | 60 men and women with metabolic syndrome and coronary syndromes aged 30 to 75 years; LDL‐C < 130 mg/dL Exclusion criteria: diabetes mellitus, Class III or IV heart failure, coronary revascularisation procedures within the next 6 weeks Placebo baseline TC: 4.94 mmol/L 191 mg/dL) Placebo baseline LDL‐C: 3.00 mmol/L (116 mg/dL) Placebo baseline HDL‐C: 1.01 mmol/L (39 mg/dL) Placebo baseline TG: 2.02 mmol/L (179 mg/dL) Atorvastatin 10 mg/d baseline TC: 4.94 mmol/L (191 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 2.97 mmol/L (115 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.06 mmol/L (41 mg/dL) Atorvastatin 10 mg/d baseline TG: 2.07 mmol/L (183 mg/dL) |
|
| Interventions | Placebo Atorvastatin 10 mg/d Fenofibrate 200 mg/d Atorvastatin/fenofibrate 10 mg/200 mg/d |
|
| Outcomes | Per cent change from baseline at 6 to 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Placebo and atorvastatin monotherapy groups were analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information about the sequence generation process was insufficient to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | Information about allocation concealment was insufficient to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | All lipid parameters were measured; no WDAEs were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Mori 2013.
| Methods | 1 month or longer wash‐out period 3‐Month randomised trial |
|
| Participants | 128 men and women age 20 to 80 years with hypercholesterolaemia and type 2 diabetes mellitus LDL‐C ≥ 100 mg/dL (2.59 mmol/L) 44 participants received atorvastatin, 42 received rosuvastatin, 42 received pravastatin Exclusion criteria: stroke or ischaemic heart disease, history within previous 6 months, liver disease, renal dysfunction, pregnancy, possibility of becoming pregnant Atorvastatin 10 mg/d baseline TC: 6.24 mmol/L (241 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 4.18 mmol/L (162 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.55 mmol/L (60 mg/dL) Atorvastatin 10 mg/d baseline TG: 1.52 mmol/L (135 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Rosuvastatin 5 mg/d Pravastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 3 months of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin treatment arm was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/42 (4.8%) participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | The trial was partially funded by a government grant |
Morishita 2001.
| Methods | 4‐Week dietary baseline stabilisation period 12‐Week before‐and‐after study | |
| Participants | 30 men and women from Japan with primary hyperlipidaemia, mean age 55 years; TC ≥ 5.7 mmol/L (220 mg/dL), TG ≥ 1.7 mmol/L (151 mg/dL) Exclusion criteria: women likely to become pregnant, severe hepatic or renal disease, hypothyroidism, MI or stroke, blood coagulation drugs Baseline TC: 6.89 mmol/L (266 mg/dL) Baseline LDL‐C: 4.36 mmol/L (169 mg/dL) Baseline HDL‐C: 1.36 mmol/L (52.59 mg/dL) Baseline TG: 2.47 mmol/L (219 mg/dL) | |
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | No source of funding was provided |
Mullen 2000.
| Methods | Participants were not taking cholesterol‐lowering medication; therefore no wash‐out period was required 6‐Week randomised double‐blind placebo‐controlled 2 × 2 factorial trial |
|
| Participants | 84 men and women aged 18 to 45 years with type 1 diabetes mellitus; LDL‐C < 4.5 mmol/L Exclusion criteria: none reported Baseline TC: 4.92 mmol/L (190 mg/dL) Baseline LDL‐C: 3.08 mmol/L (119 mg/dL) Baseline HDL‐C: 1.47 mmol/L (57 mg/dL) Baseline TG: 0.83 mmol/L (74 mg/dL) |
|
| Interventions | Placebo + placebo Arginine + placebo Arginine + atorvastatin 40 mg/d Placebo + atorvastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | No lipid data provided for the 3 groups titled:
Data were provided for the group titled placebo + atorvastatin 40 mg/d This last group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Murrow 2012.
| Methods | Participants were not receiving lipid‐lowering medication within 8 weeks of the trial; no wash‐out period was required 12‐Week randomized double‐blind trial |
|
| Participants | 36 men and women with hypercholesterolaemia and metabolic syndrome or diabetes 21 to 80 years old; LDL‐C > 120 mg/dL (3.10 mmol/L) Exclusion criteria: oral antioxidants, pregnancy potential, initiation or change in medication within 2 months of study, uncontrolled hypertension, smoking, statin allergy, acute infection in previous 4 weeks, substance abuse, cancer, renal dysfunction, acute coronary syndrome, liver failure, heart failure, stroke, aortic stenosis, hypertrophic cardiomyopathy 17 participants received atorvastatin, 19 received pravastatin Atorvastatin 10 mg/d baseline TC: 6.24 mmol/L (241 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 4.09 mmol/L (158 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.055 mmol/L (41 mg/dL) Atorvastatin 10 mg/d baseline TG: 1.795 mmol/L (159 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Pravastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin treatment arm was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were included in the efficacy analysis |
| Other bias | High risk | Pfizer funded the trial; efficacy could be biased for atorvastatin |
Muscari 2001.
| Methods | Participants did not receive lipid‐lowering drugs for 3 months before randomisation 3‐Month randomised double‐blind placebo‐controlled trial |
|
| Participants | 60 men aged 55 to 64 years with C3 > 1.19 g/L, TC ≥ 5.56 mmol/L Exclusion criteria: none reported Placebo: Baseline TC: 6.68 mmol/L (258 mg/dL) Baseline HDL‐C: 1.35 mmol/L (52 mg/dL) Atorvastatin: Baseline TC: 6.47 mmol/L (250 mg/dL) Baseline HDL‐C: 1.36 mmol/L (53 mg/dL) |
|
| Interventions | Placebo for vitamin E low cholesterol Vitamin E low cholesterol Placebo for atorvastatin high cholesterol Atorvastatin 10 mg/d high cholesterol Atorvastatin 10 mg/d + vitamin E high cholesterol |
|
| Outcomes | % change from baseline of TC and HDL‐C | |
| Notes | Placebo for atorvastatin high cholesterol and atorvastatin 10 mg/d high cholesterol were analysed SDs were imputed WDAEs were not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blindly" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3/30 participants who received atorvastatin only were not analysed (10%); some bias is possible |
| Selective reporting (reporting bias) | High risk | LDL‐C and TG data were not reported; WDAEs were not reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Nagila 2009.
| Methods | Participants were not receiving lipid‐altering agents, so no wash‐out was required 12‐Week before‐and‐after trial |
|
| Participants | 22 men and women with hypercholesterolaemia, mean age of 58 years; LDL‐C 3.4 to 6.2 mmol/L Exclusion criteria: CVD, diabetes mellitus, HTN, hepatic and renal dysfunction, hyperthyroidism, cancer, alcoholism, smoking, drug addiction, pregnancy and lactation in women |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Naoumova 1996.
| Methods | 4‐Week wash‐out period
6‐Week single evening dose of 80 mg or 40 mg BID; participants were analysed as 1 group for the atorvastatin group 18‐Week multi‐centre double‐blind dose‐titration comparison of simvastatin vs pravastatin |
|
| Participants | 35 men and women from South Africa with FH, aged 21 to 53 years; BMI 19 to 32 21 participants received atorvastatin, 7 received pravastatin, 7 received simvastatin Atorvastatin baseline TC: value not given Atorvastatin baseline LDL‐C: 7.77 mmol/L (300 mg/dL) Atorvastatin baseline HDL‐C: 1.25 mmol/L (48.34 mg/dL) Atorvastatin baseline TG: 1.32 mmol/L (117 mg/dL) |
|
| Interventions | Atorvastatin 80 mg/d Pravastatin 10 mg/d Pravastatin 20 mg/d Pravastatin 40 mg/d Simvastatin 10 mg/d Simvastatin 20 mg/d Simvastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed TC data were not reported SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | Total cholesterol‐lowering efficacy was not reported |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may support bias for atorvastatin |
Naoumova 1997.
| Methods | 1‐Month wash‐out period
1‐Month study Evening dose |
|
| Participants | 21 men and women from the UK, mean age 51 years (< 65) with heterozygous FH; LDL‐C > 5.1 mmol/L (197 mg/dL) 21 participants received simvastatin for 1 month, then were washed out for 1 month, then received atorvastatin for 1 month Exclusion criteria: hepatic dysfunction, cyclosporine, BMI > 35 Atorvastatin baseline TC: 11.84 mmol/L (458 mg/dL) Atorvastatin baseline LDL‐C: 9.56 mmol/L (370 mg/dL) Atorvastatin baseline HDL‐C: 1.08 mmol/L (42 mg/dL) Atorvastatin baseline TG: 2.38 mmol/L (211 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 1 month Simvastatin 40 mg/d for 0 to 1 month |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/21 was not included in the efficacy analysis because of difficulty in obtaining blood samples 4.8% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may support bias for atorvastatin |
Naoumova 2003.
| Methods | 4‐Week placebo wash‐out period 12‐Week single‐blind titration study |
|
| Participants | 17 men and women with heterozygous FH aged 51 years Baseline TC: 11.9 mmol/L (460 mg/dL) Baseline LDL‐C: 9.6 mmol/L (371 mg/dL) Baseline HDL‐C: 1.1 mmol/L (43 mg/dL) Baseline TG: 2.6 mmol/L (230 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 4 weeks Atorvastatin 40 mg/d for 4 to 12 weeks |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed SDs were imputed TG data were not analysed because they were expressed as geometric means |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | No source of funding was provided |
NASDAC 2005.
| Methods | 8‐Week wash‐out period 8‐Week multi‐centre randomised double‐blind parallel‐group before‐and‐after study | |
| Participants | 919 men and women with dyslipidaemia from USA, aged 18 to 80 years; LDL‐C > 100 mg/dL (2.6 mmol/L), TG < 600 mg/dL (6.8 mmol/L)
Exclusion criteria: statin hypersensitivity, gastrointestinal disease, hepatic dysfunction, uncontrolled HTN, alcohol or drug abuse, pregnancy threat, renal dysfunction, uncontrolled hypothyroidism, severe disease within 3 months of screening Atorvastatin 10 mg/d baseline TC: 6.56 mmol/L (254 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 4.44 mmol/L (172 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.23 mmol/L (48 mg/dL) Atorvastatin 10 mg/d baseline TG: 1.96 mmol/L (174 mg/dL) Atorvastatin 20 mg/d baseline TC: 6.56 mmol/L (254 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 4.33 mmol/L (167 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.16 mmol/L (45 mg/dL) Atorvastatin 20 mg/d baseline TG: 2.32 mmol/L (205 mg/dL) Atorvastatin 40 mg/d baseline TC: 6.56 mmol/L (254 mg/dL) Atorvastatin 40 mg/d baseline LDL‐C: 4.44 mmol/L (172 mg/dL) Atorvastatin 40 mg/d baseline HDL‐C: 1.21 mmol/L (47 mg/dL) Atorvastatin 40 mg/d baseline TG: 1.97 mmol/L (174 mg/dL) Atorvastatin 80 mg/d baseline TC: 6.79 mmol/L (263 mg/dL) Atorvastatin 80 mg/d baseline LDL‐C: 4.57 mmol/L (177 mg/dL) Atorvastatin 80 mg/d baseline HDL‐C: 1.18 mmol/L (46 mg/dL) Atorvastatin 80 mg/d baseline TG: 2.28 mmol/L (202 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Atorvastatin 20 mg/d Atorvastatin 40 mg/d Atorvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; atorvastatin 20 mg/d, atorvastatin 40 mg/d and atorvastatin 80 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d, atorvastatin 20 mg/d, atorvastatin 40 mg/d and atorvastatin 80 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d, atorvastatin 20 mg/d, atorvastatin 40 mg/d and atorvastatin 80 mg/d interventions were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Atorvastatin 20 mg/d: 3/228 were not included in the efficacy analysis because participants did not have at least 1 post‐baseline observation Atorvastatin 40 mg/d: 2/231 were not included in the efficacy analysis because participants did not have at least 1 post‐baseline observation Atorvastatin 80 mg/day: 2/231 were not included in the efficacy analysis because participants did not have at least 1 post‐baseline observation 1% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer Inc funded the study; data may support bias for atorvastatin |
Nawrocki 1995.
| Methods | 8‐Week dietary wash‐out period 6‐Week double‐blind randomised placebo‐controlled parallel‐group multi‐centre study Evening doses | |
| Participants | 81 outpatients from Canada and the USA with primary hypercholesterolaemia; men and women aged 54 years (18‐70); LDL‐C 4.15 to 6.20 mmol/L (160‐240 mg/dL), TG < 3.39 mmol/L (300 mg/dL) 12 participants received placebo, 67 received atorvastatin Exclusion criteria: uncontrolled HTN, diabetes, endocrine disease, liver and renal dysfunction, drugs that affect serum lipids > 14 oz/wk of ethanol equivalents No baseline lipid values were given |
|
| Interventions | Placebo Atorvastatin 2.5 mg/d Atorvastatin 5 mg/d Atorvastatin 10 mg/d Atorvastatin 20 mg/d Atorvastatin 40 mg/d Atorvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | WDAEs were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Randomization code prepared by the Parke‐Davis Biometrics Department" |
| Allocation concealment (selection bias) | Unclear risk | Information about allocation concealment was insufficient to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Patients received either one bottle containing 2.5, 5, 10, 20, 40 mg atorvastatin capsules and one bottle with matching placebo capsules, two bottles with atorvastatin 40 mg capsules; or two bottles with placebo capsules. The appearance of the capsules did not change throughout the baseline and double‐blind phases of the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/81 were not included in the efficacy analysis; the participants withdrew after 2 days because they had been incorrectly entered 2.4% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may support bias for atorvastatin |
Neil 1999.
| Methods | 5‐Week wash‐out period 17‐Week open‐label non‐comparative study | |
| Participants | 399 men and women from Ireland and the UK with CHD, mean age 64 years (18 to 80); LDL‐C > 3.4 mmol/L (131 mg/dL), TG < 5.5 mmol/L (487 mg/dL) Exclusion criteria: women likely to become pregnant or breastfeeding, active liver disease or hepatic dysfunction, MI, inhibitor hypersensitivity, alcohol abuse, lipid‐altering drugs, long‐term immunosuppressant use Baseline TC: 6.41 mmol/L (248 mg/dL) Baseline LDL‐C: 4.37 mmol/L (169 mg/dL) Baseline HDL‐C: 1.23 mmol/L (48 mg/dL) Baseline TG: 1.76 mmol/L (156 mg/dL) | |
| Interventions | Atorvastatin 10 mg/d for 0 to 5 weeks Atorvastatin conditional titration doses of 20 to 80 mg/d for 5 to 17 weeks |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20/399 were not included in the efficacy analysis because of premature withdrawal 5% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may support bias for atorvastatin |
Nordøy 2001.
| Methods | 12‐Week to 16‐week wash‐out dietary stabilisation period 10‐Week before‐and‐after study |
|
| Participants | 42 men and women aged 28 to 61 years; TC ≥ 5.3 mmol/L (205 mg/dL), TG 2.0 to 15.0 mmol/L (177‐1329 mg/dL) Baseline TC: 7.99 mmol/L (309mg/dL) Baseline LDL‐C: 5.09 mmol/L (197 mg/dL) Baseline HDL‐C: 1.01 mmol/L (39 mg/dL) Baseline TG: 4.02 mmol/L (356 mg/dL) |
|
| Interventions | All participants were given atorvastatin 10 mg/d for 5 weeks After 5 weeks, all participants were randomly assigned to 2 groups:
|
|
| Outcomes | Per cent change from baseline at 10 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Both groups were analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | No source of funding was provided |
Nozue 2008.
| Methods | 8‐Week wash‐out period 12‐Week randomised study |
|
| Participants | 17 men and women with heterozygous FH; TC > 230 mg/dL (5.95 mmol/L) 9 participants received atorvastatin, 8 received pitavastatin Atorvastatin baseline TC: 8.22 mmol/L (318 mg/dL) Atorvastatin baseline LDL‐C: 6.05 mmol/L (234 mg/dL) Atorvastatin baseline HDL‐C: 1.60 mmol/L (62 mg/dL) Atorvastatin baseline TG: 1.59 mmol/L (141 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 12 weeks Pitavastatin 2 mg/d for 0 to 12 weeks |
|
| Outcomes | Per cent change from baseline at 4 to 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | No source of funding was provided |
Okopien 2004.
| Methods | 3‐Month dietary baseline stabilisation period 30‐Day before‐and‐after study |
|
| Participants | 18 individuals with primary isolated hypercholesterolaemia; TC > 200 mg/dL, LDL‐C > 135 mg/dL, TG < 200 mg/dL Exclusion criteria: other types of primary dyslipidaemia, secondary dyslipidaemia in the course of diabetes mellitus, autoimmune disorder, thyroid disorder, chronic pancreatitis, nephrotic syndrome, liver and biliary tract disease, obesity, alcohol abuse, acute or chronic inflammatory process, symptomatic congestive heart failure, unstable coronary artery disease, MI or stroke within 6 months preceding the study, moderate and severe arterial HTN, impaired renal or hepatic function, malabsorption syndrome, treatment with drugs that affect serum lipids, HRT, oral contraception, poor patient compliance Baseline TC: 7.50 mmol/L (290mg/dL) Baseline LDL‐C: 5.38 mmol/L (208 mg/dL) Baseline HDL‐C: 1.24 mmol/L (48 mg/dL) Baseline TG: 1.74 mmol/L (154 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 30 days of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Okopien 2005.
| Methods | 6‐Week wash‐out period 4‐Week double‐blind randomised, placebo‐controlled study Evening dose | |
| Participants | 36 men and women from Poland with primary mixed dyslipidaemia, aged 41 to 64 years, TC > 200 mg/dL (5.17 mmol/L), LDL‐C > 135 mg/dL (3.49 mmol/L), TG > 200 mg/dL ( 2.26 mmol/L) 18 participants received placebo, 18 received atorvastatin, 16 received fenofibrate Exclusion criteria: secondary dyslipidaemia, acute or chronic inflammatory disease, congestive heart failure, uncontrolled HTN, cardiovascular events within 6 months of study, renal and hepatic dysfunction, use of other hypolipaemic drugs within 3 months of study, malabsorption syndrome, confounding factors, HRT, poor patient compliance Placebo baseline TC: 7.33 mmol/L (283 mg/dL) Placebo baseline LDL‐C: 4.97 mmol/L (192 mg/dL) Placebo baseline HDL‐C: 0.87 mmol/L (34 mg/dL) Placebo baseline TG: 3.06 mmol/L (271 mg/dL) Atorvastatin baseline TC: 7.50 mmol/L (290 mg/dL) Atorvastatin baseline LDL‐C: 5.38 mmol/L (208 mg/dL) Atorvastatin baseline HDL‐C: 1.01 mmol/L (39 mg/dL) Atorvastatin baseline TG: 2.83 mmol/L (251 mg/dL) |
|
| Interventions | Placebo Atorvastatin 20 mg/d Fenofibrate 267 mg/d |
|
| Outcomes | Per cent change from baseline at 30 days of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Placebo and atorvastatin groups were analysed SDs were imputed WDAEs were reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind fashion" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Olsson 2001.
| Methods | 6‐Week wash‐out period
6‐Week open‐label randomised parallel‐group trial for the atorvastatin study 6‐Week randomised double‐blind placebo‐controlled study for the rosuvastatin trial |
|
| Participants | 206 men and women from Northern Europe aged 18 to 70 years with hypercholesterolaemia; BMI ≤ 30, LDL‐C 4.14 to 6.21 mmol/L (160‐240 mg/dL), TG < 3.39 mmol/L (300 mg/dL) 31 participants received placebo, 28 received atorvastatin, 137 received rosuvastatin Exclusion criteria: active arterial disease, active cancer, uncontrolled HTN, diabetes, hypothyroidism, homozygous FH, hepatic dysfunction Placebo baseline TC: 7.00 mmol/L (271 mg/dL) Placebo baseline LDL‐C: 5.10 mmol/L (197 mg/dL) Placebo baseline HDL‐C: 1.40 mmol/L (54 mg/dL) Placebo baseline TG: 1.40 mmol/L (124 mg/dL) Atorvastatin 10 mg/d baseline TC: 6.80 mmol/L (263 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 4.90 mmol/L (189 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.30 mmol/L (50 mg/dL) Atorvastatin 10 mg/d baseline TG: 1.40 mmol/L (124 mg/dL) Atorvastatin 80 mg/d baseline TC: 6.90 mmol/L (267 mg/dL) Atorvastatin 80 mg/d baseline LDL‐C: 5.00 mmol/L (193 mg/dL) Atorvastatin 80 mg/d baseline HDL‐C: 1.20 mmol/L (46 mg/dL) Atorvastatin 80 mg/d baseline TG: 1.40 mmol/L (124 mg/dL) |
|
| Interventions | Placebo Atorvastatin 10 mg/d Atorvastatin 80 mg/d Rosuvastatin 1 mg/d Rosuvastatin 2.5 mg/d Rosuvastatin 5 mg/d Rosuvastatin 10 mg/d Rosuvastatin 20 mg/d Rosuvastatin 40 mg/d Rosuvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Placebo and atorvastatin groups were analysed WDAEs were not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Open‐label study |
| Allocation concealment (selection bias) | High risk | Open‐label study |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Placebo: 2/31 were not included in the efficacy analysis: 1 because of an adverse event and 1 because of major protocol violations Atorvastatin 10 mg/d: 2/15 were not included in the efficacy analysis: 1 because of an adverse event and 1 because of major protocol violations Atorvastatin 80 mg/d: 3/13 were not included in the efficacy analysis: 1 because of an adverse event and 2 because of major protocol violations 12% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | High risk | All lipid parameters were measured; WDAEs were not reported |
| Other bias | High risk | AstraZeneca funded the study; data may support bias against atorvastatin |
Olsson 2002.
| Methods | 6‐Week wash‐out period 52‐Week multi‐centre double‐blind randomised study | |
| Participants | 412 men and women from Northern Europe, mean age 58 years (29‐79); LDL‐C 160 to 249 mg/dL (4.14‐6.44 mmol/L), TG ≤ 400 mg/dL (4.52 mmol/L) 140 participants received atorvastatin, 272 received rosuvastatin Exclusion criteria: use of lipid‐modifying drugs Atorvastatin baseline TC: 7.08 mmol/L (274 mg/dL) Atorvastatin baseline LDL‐C: 4.86 mmol/L (188 mg/dL) Atorvastatin baseline HDL‐C: 1.39 mmol/L (54 mg/dL) Atorvastatin baseline TG: 1.81 mmol/L (160 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Rosuvastatin 5 mg/d Rosuvastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for analysis, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for analysis, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for analysis, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Atorvastatin group: 1/140 was not included in the efficacy analysis because this participant had not received trial medication or because baseline or post‐baseline efficacy assessment was not performed 0.7% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | AstraZeneca funded the study; data may support bias against atorvastatin |
Ong 2011.
| Methods | Participants were not receiving lipid‐lowering medications within 6 weeks of the trial; no wash‐out was required 52‐Week multi‐centre open‐label single‐arm study |
|
| Participants | 114 men and women with primary hypercholesterolaemia > 18 years old; LDL‐C 100 to 290 mg/dL (2.6‐7.5 mmol/L) 85 participants received atorvastatin Exclusion criteria: hypersensitivity or muscle toxicity to statins, hereditary muscle disease, uncontrolled diabetes mellitus, treatment with lipid‐lowering drugs within 6 weeks of the trial, elevated liver enzymes > 1.5 × ULN, CPK > 5 × ULN, serum creatinine > 1.2 × ULN, serum TG > 5.56 mmol/L (492 mg/dL) Atorvastatin baseline TC: 4.46 mmol/L (172 mg/dL) Atorvastatin baseline LDL‐C: 4.37 mmol/L (169 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 4 weeks Atorvastatin 20 mg/d for 4 to 8 weeks Atorvastatin 20 mg/d for 8 to 52 weeks |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC and LDL‐C | |
| Notes | Atorvastatin 10 mg/d for 0 to 4 week group was included in the efficacy analysis HDL‐C and triglycerides per cent change from baseline data were not included because given and calculated values were different by more than 10% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 29/114 were not included in the efficacy analysis because of follow‐up loss, pregnancy, adverse events, transfer to other centres, lack of consent 25% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | High risk | HDL‐C and triglycerides were not included in the efficacy analysis |
| Other bias | High risk | Ranbaxy Sdn Bhd funded the study; data may support bias against atorvastatin |
Ooi 1997.
| Methods | 6‐Week wash‐out period 24‐Week open‐label randomised multi‐centre study | |
| Participants | 84 outpatients from Canada with combined hyperlipidaemia aged 18 to 80 years; BMI < 33
Exclusion criteria: pregnancy, liver and kidney dysfunction, uncontrolled HTN, hypothyroidism, diabetes, consuming > 10 alcoholic drinks per week, drug abuse, TC 5.2 to 9.0 mmol/L (201‐348 mg/dL), LDL‐C > 3.5 mmol/L (135 mg/dL), TG > 2.3 mmol/L (204 mg/dL) 41 participants received atorvastatin, 43 received fenofibrate Atorvastatin baseline TC: 7.65 mmol/L (296 mg/dL) Atorvastatin baseline LDL‐C: 4.84 mmol/L (187 mg/dL) Atorvastatin baseline HDL‐C: 0.96 mmol/L (37.12 mg/dL) Atorvastatin baseline TG: 4.34 mmol/L (384 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 12 weeks Atorvastatin 20 mg/d for 12 to 24 weeks Fenofibrate 100 mg TID for 0 to 24 weeks |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Atorvastatin group: 1/41 were not included in the efficacy analysis 2.4% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may support bias for atorvastatin |
Oranje 2001.
| Methods | 6‐Week wash‐out period 3‐Month single‐centre double‐blind randomised placebo‐controlled study Evening doses | |
| Participants | Type 2 diabetic individuals from the Netherlands were recruited from an outpatient endocrinology clinic, BMI < 35, HbA1c < 10%, TC < 6.5 mmol/L (251 mg/dL) Exclusion criteria: none reported Placebo baseline TC: 5.33 mmol/L (206 mg/dL) Placebo baseline LDL‐C: 2.76 mmol/L (107 mg/dL) Placebo baseline HDL‐C: 1.39 mmol/L (54 mg/dL) Atorvastatin baseline TC: 5.62 mmol/L (217 mg/dL) Atorvastatin baseline LDL‐C: 3.22 mmol/L (125 mg/dL) Atorvastatin baseline HDL‐C: 0.87 mmol/L (34 mg/dL) |
|
| Interventions | Placebo Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 3 months of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed. TG data were not analysed because per cent change from baseline was expressed as a median value WDAEs were not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "In this double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | All lipid parameters were measured; WDAEs were not reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Orem 2002.
| Methods | No participants were receiving lipid‐lowering therapy before entry into the study; therefore no wash‐out baseline stabilisation period was required 12‐Week before‐and‐after trial |
|
| Participants | 38 men and women from Turkey with dyslipidaemia aged 54 years (35‐71); BMI 26.4 Exclusion criteria: hypothyroidism, diabetes mellitus, nephrotic syndrome, renal insufficiency, hepatic dysfunction, cancer, immune disorder, uncontrolled HTN, smoking, CAD Baseline TC: 7.37 mmol/L (285 mg/dL) Baseline LDL‐C: 5.24 mmol/L (203 mg/dL) Baseline HDL‐C: 1.06 mmol/L (41 mg/dL) Baseline TG: 2.20 mmol/L (195 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Orr 2009.
| Methods | Participants were not taking any medications; no wash‐out was required 12‐Week double‐blind randomised placebo‐controlled trial |
|
| Participants | 26 obese men and women aged 40 to 65 years; BP < 160/100 mmHg, TC < 300 mg/dL, TG < 450 mg/dL Exclusion criteria: elevated transaminase levels, BMI ≥ 25 kg/m2, brachial arterial BP ≤ 159/99 mmHg Placebo baseline TC: 5.87 mmol/L (227 mg/dL) Placebo baseline LDL‐C: 4.19 mmol/L (162 mg/dL) Placebo baseline HDL‐C: 1.11 mmol/L (43 mg/dL) Placebo baseline TG: 1.41 mmol/L (125 mg/dL) Atorvastatin 80 mg/d baseline TC: 5.46 mmol/L (211 mg/dL) Atorvastatin 80 mg/d baseline LDL‐C: 3.85 mmol/L (149 mg/dL) Atorvastatin 80 mg/d baseline HDL‐C: 1.14 mmol/L (44 mg/dL) Atorvastatin 80 mg/d baseline TG: 1.39 mmol/L (123 mg/dL) |
|
| Interventions | Placebo Atorvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, TG There were no adverse events reported during the study |
|
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information about sequence generation was insufficient to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | Information about allocation concealment was insufficient to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured; WDAEs were reported |
| Other bias | High risk | Pfizer funded the trial |
Ozerkan 2006.
| Methods | 12‐Week dietary stabilisation period 12‐Week prospective non‐randomised open‐label study |
|
| Participants | 15 men and women aged ≥ 18 years with hyperlipidaemia with insulin resistance; TC > 240 mg/dL (6.21 mmol/L), TG 200 to 400 mg/dL (2.26‐4.52 mmol/L) Exclusion criteria: obese, SBP/DBP ≥ 130/85 mmHg or receiving antihypertensive medication, impaired glucose tolerance, hepatic insufficiency, renal insufficiency, CK ≥ 3 × ULN, secondary hyperlipidaemia, alcohol abuse; use of medication affecting insulin, glucose or lipid metabolism; psychiatric problems, pregnant, could become pregnant, breastfeeding Baseline TC: 7.01 mmol/L (271 mg/dL) Baseline LDL‐C: 4.48 mmol/L (173 mg/dL) Baseline HDL‐C: 1.18 mmol/L (46 mg/dL) Baseline TG: 3.04 mmol/L (269 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | No source of funding was provided |
Ozsoy 2003.
| Methods | 6‐Week wash‐out period 6‐Week before‐and‐after study | |
| Participants | 150 men and women from the Netherlands with dyslipidaemia or early renal failure, mean age 45 years (18‐75); outpatient nephrology clinic, LDL‐C > 2.6 mmol/L (100 mg/dL), HDL‐C > 1.1 mmol/L (42.5 mg/dL), TG < 1.7 mmol/L (151 mg/dL) 60 participants received atorvastatin Exclusion criteria: none Baseline TC: 6.13 mmol/L (237 mg/dL) Baseline LDL‐C: 3.94 mmol/L (152 mg/dL) Baseline HDL‐C: 1.43 mmol/L (55.3 mg/dL) Baseline TG: 1.72 mmol/L (152 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 weeks: 90/150 were not included in the selection process: "the first 60 consecutive patients with elevated LDL‐C were treated uniformly with a cholesterol‐lowering diet and atorvastatin 10 mg daily" 60% of participants were excluded from the efficacy analysis Risk of bias is high |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | No source of funding was provided |
Pacanowski 2008.
| Methods | No participant received lipid‐lowering therapy; therefore no wash‐out period was required 8‐Week before‐and‐after trial |
|
| Participants | 84 healthy men and women from the USA Exclusion criteria: coronary disease, carotid artery disease, peripheral vascular disease, diabetes, aneurysm, dyslipidaemia, Framingham 10‐year risk > 20%, cancer, pregnancy, hepatic dysfunction, alcohol abuse, muscle pain Baseline TC: 4.73 mmol/L (183 mg/dL) Baseline LDL‐C: 2.64 mmol/L (102 mg/dL) Baseline HDL‐C: 1.58 mmol/L (61 mg/dL) Baseline TG: 1.13 mmol/L (100 mg/dL) |
|
| Interventions | Atorvastatin 80 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Paiva 2005.
| Methods | Participants received no lipid‐altering medications before randomisation; therefore no wash‐out period was required 8‐Week randomised double‐blind placebo‐controlled trial |
|
| Participants | 48 men and women aged 31 to 69 years; TC 5.9 mmol/L, TG < 4.5 mmol/L Exclusion criteria: TC > 7.0 mmol/L, individuals with FH, women of childbearing potential, use of antioxidant vitamins, renal or hepatic dysfunction, medications that alter statin metabolism Placebo: Baseline TC: 5.90 mmol/L (228 mg/dL) Baseline LDL‐C: 3.68 mmol/L (142 mg/dL) Baseline HDL‐C: 1.41 mmol/L (54.5 mg/dL) Baseline TG: 1.79 mmol/L (159 mg/dL) Atorvastatin: Baseline TC: 5.80 mmol/L (224 mg/dL) Baseline LDL‐C: 3.65 mmol/L (141 mg/dL) Baseline HDL‐C: 1.27 mmol/L (49 mg/dL) Baseline TG: 1.94 mmol/L (172 mg/dL) |
|
| Interventions | Placebo Atorvastatin 40 mg/d Simvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Placebo and atorvastatin groups were analysed SDs were imputed WDAEs were not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Low risk | All study drugs were supplied in sealed, identical, numbered containers |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind; all investigators and participants were blinded until analyses were done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/16 in the placebo group were not analysed (12.5%) 1/16 in the atorvastatin group was not analysed (6.25%) 3/32 were not included in the efficacy analysis (9.4%) |
| Selective reporting (reporting bias) | High risk | All lipid parameters were measured; WDAEs were not reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
PAPAGO‐T 2013.
| Methods | 4‐Week dietary lead‐in period 12‐Week randomised double‐blind study |
|
| Participants | 225 men and women aged 20 or older with LDL‐C > 100 mg/dL (2.59 mmol/L) with hypercholesterolaemia with and without type 2 diabetes mellitus HDL‐C < 40 mg/dL (1.03 mmol/L) Exclusion criteria: statin hypersensitivity, hepatic dysfunction, renal dysfunction, pregnancy, possible pregnancy or breastfeeding, poorly controlled diabetes mellitus 113 participants received atorvastatin 112 participants received pitavastatin Atorvastatin 10 mg/d baseline TC: 5.53 mmol/L (214 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 3.91 mmol/L (151 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.25 mmol/L (48 mg/dL) Atorvastatin 10 mg/d baseline TG: 1.73 mmol/L (153 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Pitavastatin 2 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin 10 mg/d treatment arm was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; treatment arm was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | Kowa Ltd funded the trial |
Papathanasiou 2008.
| Methods | No participants were receiving any medication; therefore no wash‐out period was required 1‐Month trial |
|
| Participants | 83 men and women with ACS from Greece: Group A consisted of 34 people aged 58 years (42‐78); BMI 26.9 Exclusion criteria: individuals who did not meet the criteria for non‐ST‐segment elevation; acute coronary syndrome, Lpa ≥ 0.8 mg/dL, angiographically normal coronary arteries, individuals who did not undergo any revascularisation procedure, individuals who underwent CABG Baseline TC: 6.43 mmol/L (249 mg/dL) Baseline LDL‐C: 4.28 mmol/L (166 mg/dL) |
|
| Interventions | Group A atorvastatin 40 mg/d for 3 months Group B atorvastatin 40 mg/d for 3 to 5 days |
|
| Outcomes | Per cent change from baseline from 1 to 3 months of serum TC and LDL‐C | |
| Notes | Group A was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 40 mg/d for 3 months; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 40 mg/d for 3 months; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 40 mg/d for 3 months; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | Serum HDL‐C and TG were not measured |
| Other bias | Unclear risk | The source of funding was not provided |
Parhofer 2000.
| Methods | 4‐Week wash‐out period 4‐Week before‐and‐after study | |
| Participants | 10 healthy men from Germany, mean age 30 years; BMI 22 Baseline TC: 4.84 mmol/L (187 mg/dL) Baseline LDL‐C: 3.00 mmol/L (116 mg/dL) Baseline HDL‐C: 1.17 mmol/L (45.24 mg/dL) Baseline TG: 1.47 mmol/L (130 mg/dL) | |
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Parke‐Davis Pharmaceuticals partially funded the study; data may support bias for atorvastatin |
Parhofer 2003.
| Methods | No participants were taking any medication; therefore no wash‐out period was required 4‐Week before‐and‐after trial |
|
| Participants | 10 healthy hypertriglyceridaemic individuals, 8 men and 2 women, aged 40 years; BMI 27 Exclusion criteria: none reported Baseline TC: 5.74 mmol/L (222 mg/dL) Baseline LDL‐C: 3.18 mmol/L (123 mg/dL) Baseline HDL‐C: 0.85 mmol/L (33 mg/dL) Baseline TG: 3.90 mmol/L (345 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of plasma TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer partially funded the study; data results may be biased towards atorvastatin |
Park 2010.
| Methods | 6‐Week wash‐out dietary baseline stabilisation period 6‐Week multi‐centre randomised open‐label trial |
|
| Participants | 178 men and women with metabolic syndrome and hypercholesterolaemia aged ≥ 18 years; LDL‐C ≥ 130 to < 220 mg/dL, TG ≥ 150 mg/dL (1.70 mmol/L) or < 400 mg/dL (4.52 mmol/L), HDL‐C men < 40 mg/dL, HDL‐C women < 50 mg/dL, BP ≥ 130/85 mmHg Exclusion criteria: pregnant, cancer, diabetes mellitus, unstable angina, MI, stroke, coronary artery bypass surgery or angioplasty within 2 months of enrolment Baseline TC: 6.17 mmol/L (239 mg/dL) Baseline LDL‐C: 4.24 mmol/L (164 mg/dL) Baseline HDL‐C: 1.03 mmol/L (40 mg/dL) Baseline TG: 1.97 mmol/L (174 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Rosuvastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of plasma TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1% of participants were not analysed |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | AstraZeneca funded the trial |
Pfizer Inc 16.
| Methods | 8‐Week dietary baseline wash‐out stabilisation period 12‐Week multi‐centre double‐blind placebo‐controlled randomised parallel‐group dose‐response study for torcetrapib and torcetrapib‐atorvastatin combination doses 12‐Week multi‐centre open‐label placebo‐controlled randomised parallel‐group dose‐response study for atorvastatin doses |
|
| Participants | 614 men and women were screened 459 men and women with hypercholesterolaemia aged 20 to 64 years inclusive; BMI < 30 kg/m2, LDL‐C ≥ 140 mg/dL (3.62 mmol/L), randomly assigned 26 participants received placebo‐atorvastatin/placebo‐torcetrapib 28 participants received atorvastatin 5 mg/d / placebo‐torcetrapib 32 participants received atorvastatin 10 mg/d / placebo‐torcetrapib 30 participants received atorvastatin 20 mg/d / placebo‐torcetrapib 31 participants received placebo‐atorvastatin / torcetrapib 30 mg/d 29 participants received atorvastatin 5 mg/d / torcetrapib 30 mg/d 28 participants received atorvastatin 10 mg/d / torcetrapib 30 mg/d 29 participants received atorvastatin 20 mg/d / torcetrapib 30 mg/d 31 participants received placebo‐atorvastatin / torcetrapib 60 mg/d 28 participants received atorvastatin 5 mg/d / torcetrapib 60 mg/d 29 participants received atorvastatin 10 mg/d / torcetrapib 60 mg/d 23 participants received atorvastatin 20 mg/d / torcetrapib 60 mg/d 29 participants received placebo‐atorvastatin / torcetrapib 90 mg/d 28 participants received atorvastatin 5 mg/d / torcetrapib 90 mg/d 28 participants received atorvastatin 10 mg/d / torcetrapib 90 mg/d 30 participants received atorvastatin 20 mg/d / torcetrapib 90 mg/d Exclusion criteria: prolonged QTc interval, history of uterine cancer No baseline lipid parameters were reported |
|
| Interventions | Placebo‐atorvastatin / placebo‐torcetrapib for 0 to 12 weeks Atorvastatin 5 mg/d / placebo‐torcetrapib for 0 to 12 weeks Atorvastatin 10 mg/d / placebo‐torcetrapib for 0 to 12 weeks Atorvastatin 20 mg/d / placebo‐torcetrapib for 0 to 12 weeks Placebo‐atorvastatin / torcetrapib 30 mg/d for 0 to 12 weeks Placebo‐atorvastatin / torcetrapib 60 mg/d for 0 to 12 weeks Placebo‐atorvastatin / torcetrapib 90 mg/d for 0 to 12 weeks Atorvastatin 5 mg/d / torcetrapib 30 mg/d for 0 to 12 weeks Atorvastatin 5 mg/d / torcetrapib 60 mg/d for 0 to 12 weeks Atorvastatin 5 mg/d / torcetrapib 90 mg/d for 0 to 12 weeks Atorvastatin 10 mg/d / torcetrapib 30 mg/d for 0 to 12 weeks Atorvastatin 10 mg/d / torcetrapib 60 mg/d for 0 to 12 weeks Atorvastatin 10 mg/d / torcetrapib 90 mg/d for 0 to 12 weeks Atorvastatin 20 mg/d / torcetrapib 30 mg/d for 0 to 12 weeks Atorvastatin 20 mg/d / torcetrapib 60 mg/d for 0 to 12 weeks Atorvastatin 20 mg/d / torcetrapib 90 mg/d for 0 to 12 weeks |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum LDL‐C and HDL‐C | |
| Notes | Placebo and atorvastatin monotherapy groups were analysed WDAEs were reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Open‐label |
| Allocation concealment (selection bias) | High risk | Open‐label |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Atorvastatin 5 mg/d / placebo‐torcetrapib: 2/28 were not included in the efficacy analysis 1.7% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | High risk | TC and TG data were not reported |
| Other bias | High risk | Pfizer funded the study; data may support bias for atorvastatin |
Pfizer Inc 19.
| Methods | 6‐Week dietary lead‐in phase 24‐Week open‐label randomised parallel‐arm multi‐centre study |
|
| Participants | 99 men and women, TC > 200 mg/dL (5.17 mmol/L), TG < 800 mg/dL (9.03 mmol/L) 47 participants received atorvastatin, 52 received fenofibrate Exclusion criteria: women of childbearing potential or lactation, > 14 alcoholic drinks per week, taking insulin or oral hypoglycaemic agents, renal or hepatic dysfunction, conditions that affected serum lipids, HTN, participating in another study within 30 days of screening Atorvastatin baseline TC: 7.45 mmol/L (288 mg/dL) Atorvastatin baseline TG: 4.54 mmol/L (402 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 12 weeks Atorvastatin 20 mg/d for 12 to 24 weeks Fenofibrate 100 mg TID for 0 to 24 weeks |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC and TG | |
| Notes | First atorvastatin dose was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/47 was not included in the efficacy analysis because of an adverse event allergic response 2.1% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | High risk | LDL‐C and HDL‐C data were not reported |
| Other bias | High risk | Pfizer funded the study; data may support bias for atorvastatin |
Pirkova 2007.
| Methods | 3‐Week dietary baseline stabilisation period 12‐Week before‐and‐after study |
|
| Participants | 20 men aged 57 years with documented CAD, history of MI, Class II or III angina, HTN, CHD with plasma TC > 5.2 mmol/L (201 mg/dL), LDL‐C > 3.0 mmol/L (116 mg/dL), TG > 4.5 mmol/L (399 mg/dL) Exclusion criteria: unstable angina, MI within 6 months of study enrolment, familial hyperlipidaemia, severe liver and kidney disease, diabetes mellitus, congestive heart failure, cardiac arrhythmia, severe HTN, surgery within the previous 6 months with accompanying acute inflammatory disease Baseline TC: 6.36 mmol/L (246mg/dL) Baseline LDL‐C: 4.50 mmol/L (174 mg/dL) Baseline HDL‐C: 1.14 mmol/L (44 mg/dL) Baseline TG: 2.00 mmol/L (177 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
PITCH 2012.
| Methods | No wash‐out period was required because no participants were receiving any lipid‐altering agents 12‐Week randomised open‐label dose‐titration study |
|
| Participants | 202 men and women aged 25 to 75 years with elevated ALT (≥ 1.25 times and ≤ 2.5 times ULN), 40 IU/L LDL‐C ≥ 3.36 mmol/L (≥ 130 mg/dL) 99 participants received atorvastatin, 103 received pitavastatin Exclusion criteria: overt and irreversible liver cirrhosis, viral hepatitis, serum bilirubin > 2 × ULN TG ≥ 4.52 mmol/L (400 mg/dL), acute or unstable conditions such as recent MI, advanced heart failure, renal dysfunction, uncontrolled hypertension, thyroid dysfunction Atorvastatin baseline TC: 5.81 mmol/L (225 mg/dL) Atorvastatin baseline LDL‐C: 3.85 mmol/L (149 mg/dL) Atorvastatin baseline HDL‐C: 1.13 mmol/L (43.7 mg/dL) Atorvastatin baseline TG: 2.53 mmol/L (224 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 4 weeks Atorvastatin 20 mg/d for 4 to 12 weeks Pitavastatin 2 mg/d for 4 weeks Pitavastatin 4 mg/d for 4 to 12 weeks |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin 10 mg/d for 4 weeks; intervention was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14/99 (14%) participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | JW Pharmaceutical Co Korea funded the study |
Plakogiannis 2002.
| Methods | No participant received lipid‐altering medication 4 months before the trial; therefore no wash‐out period was required 8‐Week retrospective and prospective trial |
|
| Participants | 64 men with hyperlipidaemia Exclusion criteria: newly diagnosed with a disease known to affect serum lipoproteins Retrospective study morning dosing: Baseline TC: 8.31 mmol/L (321 mg/dL) Baseline LDL‐C: 4.87 mmol/L (188 mg/dL) Baseline HDL‐C: 1.20 mmol/L (46 mg/dL) Baseline TG: 4.90 mmol/L (434 mg/dL) Retrospective study evening dosing: Baseline TC: 8.51 mmol/L (329 mg/dL) Baseline LDL‐C: 5.04 mmol/L (195 mg/dL) Baseline HDL‐C: 1.06 mmol/L (41 mg/dL) Baseline TG: 5.29 mmol/L (469 mg/dL) Prospective study morning dosing: Baseline TC: 8.22 mmol/L (318 mg/dL) Baseline LDL‐C: 4.99 mmol/L (193 mg/dL) Baseline HDL‐C: 1.09 mmol/L (42 mg/dL) Baseline TG: 4.69 mmol/L (415 mg/dL) Prospective study evening dosing: Baseline TC: 8.25 mmol/L (319 mg/dL) Baseline LDL‐C: 4.96 mmol/L (192 mg/dL) Baseline HDL‐C: 1.01 mmol/L (39 mg/dL) Baseline TG: 4.97 mmol/L (440 mg/dL) |
|
| Interventions | Atorvastatin 40 mg/d for 8 weeks retrospective study morning dosing Atorvastatin 40 mg/d for 8 weeks retrospective study evening dosing Atorvastatin 40 mg/d for 8 weeks prospective study morning dosing Atorvastatin 40 mg/d for 8 weeks prospective study evening dosing |
|
| Outcomes | Per cent change from baseline at 4 weeks of plasma TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 40 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 40 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 40 mg/d; interventions were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Pontrelli 2002.
| Methods | 8‐Week wash‐out period 8‐Week double‐blind randomised placebo‐controlled cross‐over study | |
| Participants | 20 men and women from Canada with type 2 diabetes and combined dyslipidaemia, mean age 59 years; TG > 2.5 mmol/L (221 mg/dL), HDL‐C < 0.9 mmol/L (35 mg/dL) 10 participants received placebo, 10 received atorvastatin Exclusion criteria: statin hypersensitivity, use of lipid‐altering drugs, women who are likely to become pregnant, type 1 diabetes, TSH > 5.5 mU/mL, renal and hepatic dysfunction, > 14 alcoholic drinks/wk Placebo baseline TC: 5.97 mmol/L (231 mg/dL) Placebo baseline LDL‐C: 3.74 mmol/L (145 mg/dL) Placebo baseline HDL‐C: 0.81 mmol/L (31 mg/dL) Placebo baseline TG: 4.95 mmol/L (438 mg/dL) Atorvastatin baseline TC: 6.60 mmol/L (255 mg/dL) Atorvastatin baseline LDL‐C: 4.08 mmol/L (158 mg/dL) Atorvastatin baseline HDL‐C: 0.89 mmol/L (34 mg/dL) Atorvastatin baseline TG: 3.44 mmol/L (305 mg/dL) |
|
| Interventions | Placebo Atorvastatin 80 mg/d | |
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | For the second period after the cross‐over point, data were not analysed SDs were imputed WDAEs not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Then randomized in a double‐blind manner into 2 treatment groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | All lipid parameters were measured; no withdrawals due to adverse events were reported |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may support bias for atorvastatin |
PRAT 2013.
| Methods | 4‐Week wash‐out period if participants were receiving lipid‐lowering medication before enrolment 1‐Year randomised open‐label parallel‐group study |
|
| Participants | 202 men and women with dyslipidaemia and glucose intolerance, men ≥ 20 years old or postmenopausal women; LDL‐C ≥140 mg/dL (3.62 mmol/L) HDL‐C < 80 mg/dL (2.07 mmol/L), TG < 500 mg/dL (5.645 mmol/L) 101 participants received atorvastatin, 101 received pravastatin Exclusion criteria: poorly controlled diabetes mellitus, hepatic dysfunction, renal dysfunction, secondary hyperlipidaemia, steroid use, severe hypertension, cerebrovascular disease, MI, coronary artery reconstruction within 3 months, heart failure Class III or higher, statin hypersensitivity Atorvastatin baseline LDL‐C: 4.161 mmol/L (161 mg/dL) Atorvastatin baseline HDL‐C: 1.329 mmol/L (51 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Pravastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum LDL‐C and HDL‐C | |
| Notes | Pravastatin treatment arm was not analysed Efficacy was determined at 1 month only SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/101 (6.9%) participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | Total cholesterol and triglycerides were not included in the efficacy analysis |
| Other bias | Low risk | Industry did not fund this trial |
Puato 2010.
| Methods | Participants were never treated with lipid‐lowering agents, so no wash‐out was required 12‐Week randomised trial |
|
| Participants | 40 men and women with hypercholesterolaemia aged 78 to 79 years; TC 5.83 to 7.64 mmol/L Exclusion criteria: none Atorvastatin 10 mg/d baseline TC: 6.15 mmol/L (238 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 4.03 mmol/L (156 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.32 mmol/L (51 mg/dL) Atorvastatin 10 mg/d baseline TG: 1.72 mmol/L (152 mg/dL) Atorvastatin 80 mg/d baseline TC: 6.54 mmol/L (253 mg/dL) Atorvastatin 80 mg/d baseline LDL‐C: 4.37 mmol/L (169 mg/dL) Atorvastatin 80 mg/d baseline HDL‐C: 1.34 mmol/L (52 mg/dL) Atorvastatin 80 mg/d baseline TG: 1.85 mmol/L (164 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Atorvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer partially funded the trial |
Puccetti 2002.
| Methods | No participants were taking hypolipidaemic drugs; therefore no wash‐out period was required. Also participants were on a 6‐week AHA step II diet regimen before therapy allocation 4‐Week trial |
|
| Participants | 64 men and women from Italy with hypercholesterolaemia aged 49 years (36‐64); TC 6.86 mmol/L, HDL‐C 1.24 mmol/L, TG 1.13 mmol/L, BMI 24.7 Exclusion criteria: cardiovascular events in the clinical history, HTN, diabetes; liver, renal, thyroid, infective, immunological or malignant disease Baseline TC: 6.56 mmol/L (254 mg/dL) Baseline LDL‐C: 4.84 mmol/L (179 mg/dL) Baseline HDL‐C: 1.21 mmol/L (47 mg/dL) Baseline TG: 1.11 mmol/L (98 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Simvastatin 20 mg/d Fluvastatin 40 mg/d Pravastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 4 weeks of plasma TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Puccetti 2005.
| Methods | 6‐Week dietary stabilisation period 6‐Week study |
|
| Participants | 201 men and women with hypercholesterolaemia aged 37 to 61 years Exclusion criteria: history of cardiovascular events, HTN, diabetes; liver, renal, thyroid, infective, immunological or malignant disease Baseline TC: 6.57 mmol/L (254 mg/dL) Baseline LDL‐C: 4.84 mmol/L (187 mg/dL) Baseline HDL‐C: 1.22 mmol/L (47 mg/dL) Baseline TG: 1.10 mmol/L (97 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | No source of funding was provided |
PULSAR 2006.
| Methods | 6‐Week wash‐out period 6‐Week multi‐centre open‐label randomised study | |
| Participants | 996 men and women with hypercholesterolaemia aged ≥ 18 years; LDL‐C 130 to 220 mg/dL (3.4‐5.7 mmol/L), TG < 400 mg/dL (4.5 mmol/L) 505 participants received rosuvastatin, 491 received atorvastatin Exclusion criteria: unstable CVD, history of statin‐induced myopathy, severe congestive heart failure, history of malignancy, homozygous FH, uncontrolled hypothyroidism, alcohol or drug abuse within 5 years, women who could become pregnant Atorvastatin baseline TC: 6.5 mmol/L (251 mg/dL) Atorvastatin baseline LDL‐C: 4.3 mmol/L (166 mg/dL) Atorvastatin baseline HDL‐C: 1.3 mmol/L (50.3 mg/dL) Atorvastatin baseline TG: 2.3 mmol/L (204 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d Rosuvastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11/491 were not included in the efficacy analysis because of unmet criteria, adverse event, unwillingness to continue, loss to follow‐up, other 2.2% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | AstraZeneca funded the study; data may support bias against atorvastatin |
Puurunen 2013.
| Methods | Participants were not receiving any lipid‐lowering agents; wash‐out period was not required 3‐Month randomised double‐blind placebo‐controlled trial |
|
| Participants | 38 women with polycystic ovary syndrome aged 29 to 50 years; BMI 19.9 to 53.8 19 participants received atorvastatin, 19 received placebo Exclusion criteria: type 2 diabetes mellitus; medication affecting glucose tolerance, lipid metabolism or steroid synthesis in the preceding 3 months; menopause, smoking, alcohol abuse, previous ovarian drilling, oophorectomy or hysterectomy, contraindications regarding the use of atorvastatin Placebo baseline TC: 4.9 mmol/L (189 mg/dL) Placebo baseline LDL‐C: 3.0 mmol/L (116 mg/dL) Placebo baseline HDL‐C: 1.5 mmol/L (58 mg/dL) Placebo baseline TG: 1.0 mmol/L (89 mg/dL) Atorvastatin 20 mg/d baseline TC: 5.2 mmol/L (201 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 3.3 mmol/L (128 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.52 mmol/L (58.8 mg/dL) Atorvastatin 20 mg/d baseline TG: 1.2 mmol/L (106 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d Placebo |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG WDAEs: One subject from the placebo group withdrew due to myalgia and one from the atorvastatin group due to arthralgia. |
|
| Notes | SDs were imputed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list in blocks of 6 |
| Allocation concealment (selection bias) | Low risk | Medication was provided in closed envelopes that were sequentially numbered |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6/19 (31.5%) participants given placebo and 4/19 (21%) given atorvastatin were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were included in the efficacy analysis; WDAEs were reported |
| Other bias | Low risk | Industry did not fund the trial |
Qi 2013.
| Methods | Participants were not receiving any lipid‐lowering agents; wash‐out period was not required 1‐Month before‐and‐after trial |
|
| Participants | 306 men and women with high risk of coronary heart disease aged 30 to 70 years LDL‐C ≥ 2.6 mmol/L (101 mg/dL) with a history of CHD and diabetes mellitus or LDL‐C ≥ 4.1 mmol/L (159 mg/dL) with 2 or more risk factors All participants received atorvastatin Exclusion criteria: acute MI within 3 months, serious heart failure, autoimmunity disease, severe arrhythmia, cancer, hypothyroidism, nephrotic syndrome, serious trauma or major surgery within past 3 months, hepatic and renal dysfunction, drugs that interact with statins, lack of compliance with medication Atorvastatin 20 mg/d baseline LDL‐C: 3.72 mmol/L (144 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 1 month of serum LDL‐C | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | LDL‐C was included in the efficacy analysis |
| Other bias | Unclear risk | The source of funding was not reported |
RADAR 2005.
| Methods | 6‐Week wash‐out dietary run‐in period 18‐Week multi‐centre open‐label randomised parallel‐group study Randomly assigned sequentially in blocks of 4 | |
| Participants | 461 men and women from the Netherlands with CVD aged 40 to 80 years; HDL‐C < 1.0 mmol/L (40 mg/dL), TG < 4.5 mmol/L (400 mg/dL) 231 participants received atorvastatin, 230 received rosuvastatin Exclusion criteria: use of lipid‐altering drugs, statin hypersensitivity, pregnancy threat, active arterial disease within 2 months of trial, therapeutics intervention within 6 months, uncontrolled HTN, cancer, homozygous FH or type III hyperproteinaemia, alcohol or drug abuse, active liver disease, unexplained CK increase, serious medical or unstable psychological conditions Atorvastatin baseline TC: 5.7 mmol/L (220 mg/dL) Atorvastatin baseline LDL‐C: 3.7 mmol/L (143 mg/dL) Atorvastatin baseline HDL‐C: 0.8 mmol/L (31 mg/dL) Atorvastatin baseline TG: 2.7 mmol/L (239 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d for 0 to 6 weeks Atorvastatin 40 mg/d for 6 to 12 weeks Atorvastatin 80 mg/d for 12 to 18 weeks Rosuvastatin 10 mg/d for 0 to 6 weeks Rosuvastatin 20 mg/d for 6 to 12 weeks Rosuvastatin 40 mg/d for 12 to 18 weeks |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | AstraZeneca funded the study; data may support bias against atorvastatin |
Raison 2002.
| Methods | 1‐Month wash‐out period 12‐Week single‐centre double‐blind randomised placebo‐controlled study Evening doses | |
| Participants | 28 individuals from France, mean age 57 years (32‐70), with HTN and hypercholesterolaemia were included in the study; 23 participants entered the treatment period; SBP 160 to 209 mmHg, DBP 95 to 109 mmHg, LDL plasma levels were chosen to be either >4.9 or >3.4 mmol/l, depending in each individual on the number of associated CV risk factors or the presence of personal history of coronary heart disease. Exclusion criteria: uncontrolled HTN, stroke, major cardiac or renal disease, arteritis, stage IV retinopathy, FH, hepatic dysfunction, diabetes, severe obesity, ECG faults or therapeutic contraindications to statins.
Placebo baseline TC: 7.11 mmol/L (275 mg/dL)
Placebo baseline LDL‐C: 5.18 mmol/L (200 mg/dL)
Placebo baseline HDL‐C: 1.31 mmol/L (51 mg/dL) Atorvastatin baseline TC: 7.23 mmol/L (280 mg/dL) Atorvastatin baseline LDL‐C: 4.97 mmol/L (192 mg/dL) Atorvastatin baseline HDL‐C: 1.36 mmol/L (53 mg/dL) No baseline TG values were given |
|
| Interventions | Placebo Atorvastatin 10 mg/d |
|
| Outcomes | Percent change from baseline at 12 weeks of serum TC, LDL‐C and HDL‐C | |
| Notes | WDAEs were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind, two‐parallel‐group study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | TG data were not reported; WDAEs were not reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Reinares 2002.
| Methods | 6‐Week wash‐out period 6‐Week before‐and‐after study |
|
| Participants | 25 men and women aged 43 to 73 years with CHD and hypercholesterolaemia; LDL‐C ≥ 130 mg/dL (3.36 mmol/L) Exclusion criteria: acute and chronic infections, cancer, inflammatory processes Baseline LDL‐C: 4.25 mmol/L (164 mg/dL) Baseline HDL‐C: 1.36 mmol/L (53 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 6 weeks of serum LDL‐C and HDL‐C SDs were imputed |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | TC and TG lipid data were not reported |
| Other bias | High risk | Pfizer partially funded the study; data may support bias for atorvastatin |
Reiter 2005.
| Methods | Participants were not receiving lipid‐lowering therapy within the past year; therefore no baseline wash‐out period was required 6‐Week prospective randomised trial |
|
| Participants | 129 men and women aged 63.5 years Exclusion criteria: < 18 years old, pregnancy, psoriasis or eczema on either hand, use of topical medication within 24 hours of testing, chronic liver disease, inflammatory muscle disease or CK 3 × ULN, statin hypersensitivity, conditions leading to incomplete follow‐up Baseline TC: 6.44 mmol/L (249 mg/dL) Baseline LDL‐C: 4.01 mmol/L (155 mg/dL) Baseline HDL‐C: 1.44 mmol/L (56 mg/dL) Baseline TG: 2.26 mmol/L (200 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d Simvastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 4 weeks of plasma TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
RESPOND 2007.
| Methods | ≥ 6‐Week wash‐out for lipid‐lowering therapy 8‐Week double‐blind double‐dummy randomised placebo‐controlled trial, 3 × 5 factorial design |
|
| Participants | 1660 men and women with concomitant HTN and dyslipidaemia; LDL‐C 182 mg/dL (4.7 mmol/L) 111 participants received atorvastatin‐placebo / amlodipine‐placebo 111 participants received atorvastatin 10 mg/d / amlodipine‐placebo 111 participants received atorvastatin 20 mg/d / amlodipine‐placebo 111 participants received atorvastatin 40 mg/d / amlodipine‐placebo 110 participants received atorvastatin 80 mg/d / amlodipine‐placebo 110 participants received atorvastatin‐placebo / amlodipine 5 mg/d 111 participants received atorvastatin 10 mg/d / amlodipine 5 mg/d 111 participants received atorvastatin 20 mg/d / amlodipine 5 mg/d 110 participants received atorvastatin 40 mg/d / amlodipine 5 mg/d 111 participants received atorvastatin 80 mg/d / amlodipine 5 mg/d 111 participants received atorvastatin‐placebo / amlodipine 10 mg/d 110 participants received atorvastatin 10 mg/d / amlodipine 10 mg/d 110 participants received atorvastatin 20 mg/d / amlodipine 10 mg/d 111 participants received atorvastatin 40 mg/d / amlodipine 10 mg/d 111 participants received atorvastatin 80 mg/d / amlodipine 10 mg/d Exclusion criteria: calcium channel blocker or statin intolerance, any serious disease or condition that could affect safety or study results All groups baseline LDL‐C: 4.70 mmol/L (182 mg/dL) |
|
| Interventions | Atorvastatin‐placebo / amlodipine‐placebo for 0 to 8 weeks Atorvastatin 10 mg/d / amlodipine‐placebo for 0 to 8 weeks Atorvastatin 20 mg/d / amlodipine‐placebo for 0 to 8 weeks Atorvastatin 40 mg/d / amlodipine‐placebo for 0 to 8 weeks Atorvastatin 80 mg/d / amlodipine‐placebo for 0 to 8 weeks Atorvastatin‐placebo / amlodipine 5 mg/d for 0 to 8 weeks Atorvastatin‐placebo / amlodipine 10 mg/d for 0 to 8 weeks Atorvastatin 10 mg/d / amlodipine 5 mg/d for 0 to 8 weeks Atorvastatin 10 mg/d / amlodipine 10 mg/d for 0 to 8 weeks Atorvastatin 20 mg/d / amlodipine 5 mg/d for 0 to 8 weeks Atorvastatin 20 mg/d / amlodipine 10 mg/d for 0 to 8 weeks Atorvastatin 40 mg/d / amlodipine 5 mg/d for 0 to 8 weeks Atorvastatin 40 mg/d / amlodipine 10 mg/d for 0 to 8 weeks Atorvastatin 80 mg/d / amlodipine 5 mg/d for 0 to 8 weeks Atorvastatin 80 mg/d / amlodipine 10 mg/d for 0 to 8 weeks |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum LDL‐C | |
| Notes | Placebo and atorvastatin groups were analysed SDs were imputed WDAEs were reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomization code" |
| Allocation concealment (selection bias) | Low risk | "Randomization code obtained by telephone (ClinPhone, Inc., Princeton, New Jersey)" "Placebo capsules were similar in size, color, smell, taste and appearance to the corresponding active tablets" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blinded therapy" "randomization schedule was protected, and the study remained blinded throughout" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | TC, HDL‐C and TG data results were not reported |
| Other bias | High risk | Pfizer funded the study; data may support bias for atorvastatin |
Rodrigues 2013.
| Methods | Participants were not receiving any lipid‐lowering agents; wash‐out period was not required 4‐Week before‐and‐after trial |
|
| Participants | 157 men and women with dyslipidaemia; TC ≥ 200 mg/dL (5.17 mmol/L), LDL‐C ≥ 160 mg/dL (4.14 mmol/L) with or without TG ≥ 150 mg/dL (1.69 mmol/L) and low levels of HDL‐C (men < 40 mg/dL and women > 50 mg/dL) Exclusion criteria: diabetes mellitus, thyroid disease, triglycerides > 400 mg/dL (4.52 mmol/L), renal or hepatic disease, hormone treatment, pregnancy Atorvastatin 10 mg/d baseline LDL‐C: 4.965 mmol/L (192 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.47 mmol/L (57 mg/dL) Atorvastatin 10 mg/d baseline TG: 1.81 mmol/L (160 mg/dL) |
|
| Interventions | Atorvastaitn 10 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of serum LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | Total cholesterol was not included in the efficacy analysis |
| Other bias | Low risk | Industry did not fund the trial |
Rodriguez‐Roa 2008.
| Methods | 4‐Week placebo wash‐out period 8‐Week double‐blind randomised study |
|
| Participants | 69 men and women aged 18 to 60 years with FH or dietary hypercholesterolaemia; LDL‐C > 160 mg/dL (4.14mmol/L), TG < 400 mg/dL (4.52 mmol/L) 37 participants received atorvastatin, 32 received Tarimyl Exclusion criteria: uncontrolled diabetes mellitus, active liver disease, AST or ALT 3 × ULN, hypothyroidism, uncontrolled HTN, coronary events within 3 months of study enrolment, CK 3 × ULN, statin hypersensitivity, drugs that affect serum lipids, pregnancy or lactation, BMI > 32, drug or alcohol abuse Atorvastatin baseline TC: 7.42 mmol/L (287 mg/dL) Atorvastatin baseline LDL‐C: 5.37 mmol/L (208 mg/dL) Atorvastatin baseline HDL‐C: 1.08 mmol/L (42 mg/dL) Atorvastatin baseline TG: 1.97 mmol/L (174 mg/dL) Tarimyl baseline TC: 7.66 mmol/L (296 mg/dL) Tarimyl baseline LDL‐C: 5.43 mmol/L (210 mg/dL) Tarimyl baseline HDL‐C: 1.09 mmol/L (42 mg/dL) Tarimyl baseline TG: 2.11 mmol/L (187 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 4 weeks Atorvastatin conditional titration of 20 mg/d for 4 to 8 weeks Tarimyl 10 mg/d for 0 to 4 weeks Tarimyl conditional titration of 20 mg/d for 4 to 8 weeks |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin and Tarimyl doses were analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d and Tarimyl 10 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d and Tarimyl 10 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d and Tarimyl 10 mg/d; interventions were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | No source of funding was provided |
ROMEO 2011.
| Methods | 6‐Week dietary run‐in baseline wash‐out period 6‐Week randomised open‐label parallel‐group study |
|
| Participants | 258 men and women with metabolic syndrome with LDL‐C ≥130 mg/dL (3.36 mmol/L) < 220 mg/dL (5.69 mmol/L) and triglycerides < 500 mg/dL (5.645 mmol/L) 126 participants received atorvastatin, 132 received rosuvastatin |
|
| Interventions | Atorvastatin 10 mg/d Rosuvastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Rosuvastatin treatment arm was not analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/126 (3.2%) participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were included in the efficacy analysis |
| Other bias | High risk | AstraZeneca funded the trial; efficacy results could be biased against atorvastatin |
Rosales 2012.
| Methods | Participants were not receiving any lipid‐lowering agents; wash‐out period was not required 4‐Week before‐and‐after study |
|
| Participants | 142 men and women with hypercholesterolaemia, mean age 56 years Exclusion criteria: diabetes, kidney disease, endocrinological disorder, cancer or concomitant lipid‐lowering therapy, medication that could affect lipid profile, familial hypercholesterolaemia Atorvastatin baseline TC: 7.096 mmol/L (274 mg/dL) Atorvastatin baseline LDL‐C: 4.794 mmol/L (185 mg/dL) Atorvastatin baseline HDL‐C: 1.2 mmol/L (46 mg/dL) Atorvastatin baseline TG: 2.403 mmol/L (213 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were included in the efficacy analysis |
| Other bias | Low risk | Government grants funded the trial |
Rosenson 2009.
| Methods | 4‐Week dietary stabilisation period 12‐Week randomised double‐blind placebo‐controlled double‐dummy parallel‐group study |
|
| Participants | 318 men and women aged ≥18 years with metabolic syndrome; LDL‐C 3.36 to 6.48 mmol/L (130‐250 mg/dL), 10‐year CHD risk score > 10% 119 participants received atorvastatin in ITT, 136 received rosuvastatin in ITT, 63 received placebo in ITT 55 participants received placebo in per‐protocol participant population because a complete laboratory dataset was required for each participant 101 participants received atorvastatin 10 mg/d in per‐protocol participant population because a complete laboratory dataset was required for each participant 122 participants received rosuvastatin 10 mg/d in per‐protocol participant population because a complete laboratory dataset was required for each participant Exclusion criteria: TG ≥ 5.65 mmol/L (500 mg/dL), use of lipid‐lowering therapy within 6 months, CHD or other atherosclerotic disease, diabetes, liver dysfunction Placebo TG < 2.26 mmol/L group baseline LDL‐C: 4.18 mmol/L (162 mg/dL) Placebo TG < 2.26 mmol/L group baseline HDL‐C: 1.3 mmol/L (50 mg/dL) Placebo TG < 2.26 mmol/L group baseline TG: 1.7 mmol/L (151 mg/dL) Placebo TG > 2.26 mmol/L group baseline LDL‐C: 4.47 mmol/L (173 mg/dL) Placebo TG > 2.26 mmol/L group baseline HDL‐C: 1.1 mmol/L (43 mg/dL) Placebo TG > 2.26 mmol/L group baseline TG: 3.0 mmol/L (266 mg/dL) Atorvastatin TG < 2.26 mmol/L group baseline LDL‐C: 4.34 mmol/L (168 mg/dL) Atorvastatin TG < 2.26 mmol/L group baseline HDL‐C: 1.2 mmol/L (46 mg/dL) Atorvastatin TG < 2.26 mmol/L group baseline TG: 1.7 mmol/L (151 mg/dL) Atorvastatin TG > 2.26 mmol/L group baseline LDL‐C: 4.55 mmol/L (176 mg/dL) Atorvastatin TG > 2.26 mmol/L group baseline HDL‐C: 1.1 mmol/L (43 mg/dL) Atorvastatin TG > 2.26 mmol/L group baseline TG: 2.9 mmol/L (257 mg/dL) |
|
| Interventions | Placebo for 0 to 6 weeks Atorvastatin 10 mg/d for 0 to 6 weeks Atorvastatin 20 mg/d for 6 to 12 weeks Rosuvastatin 10 mg/d for 0 to 6 weeks Rosuvastatin 20 mg/d for 6 to 12 weeks |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum LDL‐C, HDL‐C and TG | |
| Notes | Placebo and first atorvastatin dose were analysed SDs were imputed WDAEs were not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "A double‐blind study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | TC data were not reported; WDAEs were not reported |
| Other bias | High risk | AstraZeneca funded the study; data may support bias against atorvastatin |
SAGE 2007.
| Methods | ≥ 6‐Week wash‐out period 12‐Month multi‐centre double‐blind randomised double‐dummy parallel study | |
| Participants | 893 men and women with CHD aged 65 to 85 years; LDL‐C 100 to 250 mg/dL (2.6‐6.5 mmol/L) 446 participants received atorvastatin, 447 received pravastatin Exclusion criteria: atrial fibrillation and type III and IV heart failure Atorvastatin baseline TC: 5.84 mmol/L (226 mg/dL) Atorvastatin baseline LDL‐C: 3.81 mmol/L (147 mg/dL) Atorvastatin baseline HDL‐C: 1.17 mmol/L (45 mg/dL) Atorvastatin baseline TG: 1.85 mmol/L (164 mg/dL) |
|
| Interventions | Atorvastatin 80 mg/d Pravastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 3 months of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer funded the study; data may support bias for atorvastatin |
Sakabe 2004.
| Methods | Participants were not taking any cholesterol‐lowering medication; therefore no wash‐out period was required 1‐Year prospective trial |
|
| Participants | 54 men and women aged 40 to 76 years with primary hypercholesterolaemia; LDL‐C ≥ 160 mg/dL, TG ≤ 400 mg/dL Exclusion criteria: smoking, diabetes, HTN, previous vascular events, revascularisation procedures, CAD, active liver disease, HRT, any drug known to influence measured parameters of interest Baseline TC: 7.03 mmol/L (272 mg/dL) Baseline LDL‐C: 4.76 mmol/L (184 mg/dL) Baseline HDL‐C: 1.42 mmol/L (55 mg/dL) Baseline TG: 1.52 mmol/L (135 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of plasma TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Sakabe 2008a.
| Methods | No participants took any cholesterol‐lowering medication; therefore no wash‐out period was required 3‐Month prospective trial |
|
| Participants | 72 men and women with primary hypercholesterolaemia from Japan; LDL‐C ≥ 160 mg/dL, TG ≤ 400 mg/dL Exclusion criteria: diabetes, HTN, smoking, previous vascular event, revascularisation procedure, CAD, active liver disease, any drug that would affect measured parameters, HRT Baseline TC: 7.06 mmol/L (273 mg/dL) Baseline LDL‐C: 4.59 mmol/L (177 mg/dL) Baseline HDL‐C: 1.46 mmol/L (56 mg/dL) Baseline TG: 1.90 mmol/L (168 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of plasma TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Saklamaz 2005.
| Methods | 6‐Week wash‐out period 8‐Week randomised study | |
| Participants | 21 men and women from Turkey with type IIa and IIb hyperlipidaemia aged 52 years; LDL‐C > 160 mg/dL (4.14 mmol/L) 7 participants received atorvastatin, 7 received pravastatin, 7 received fenofibrate Exclusion criteria: endocrine problems, hepatic and renal dysfunction, BMI < 30, alcohol abuse Atorvastatin baseline TC: 6.78 mmol/L (262 mg/dL) Atorvastatin baseline LDL‐C: 4.50 mmol/L (174 mg/dL) Atorvastatin baseline HDL‐C: 1.32 mmol/L (51 mg/dL) Atorvastatin baseline TG: 2.13 mmol/L (189 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 8 weeks Pravastatin 20 mg/d for 0 to 8 weeks Fenofibrate 250 mg/d for 0 to 8 weeks |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | No source of funding was provided |
Sansanayudh 2010.
| Methods | Participants were not receiving lipid‐altering agents, so wash‐out was not required 8‐Week randomised open‐label parallel trial |
|
| Participants | 50 participants > 18 years; LDL‐C ≥ 100 mg/dL Exclusion criteria: active liver disease, renal dysfunction, hepatic dysfunction, pregnancy, TG > 400 mg/dL, statin hypersensitivity Atorvastatin baseline TC: 6.60 mmol/L (255 mg/dL) Atorvastatin baseline LDL‐C: 4.47 mmol/L (173 mg/dL) Atorvastatin baseline HDL‐C: 1.39 mmol/L (54 mg/dL) Atorvastatin baseline TG: 1.60 mmol/L (142 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Pitavastatin 1 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not reported |
Sardo 2002.
| Methods | 4‐Week to 6‐week run‐in period 12‐week single‐centre randomised placebo‐controlled study Randomised 1:1 ratio (atorvastatin:placebo) Evening doses | |
| Participants | 40 men and women from Italy recruited from a medical centre, mean age 45 years with hypercholesterolaemia; TC > 7.0 mmol/L (271 mg/dL), LDL‐C > 4.1 mmol/L (159 mg/dL), TG < 2.0 mmol/L (177 mg/dL) Exclusion criteria: arterial HTN, BMI > 27, thyroid disease, renal and hepatic dysfunction, smoking, diabetes, infection, inflammatory or autoimmune disease, arterial and cardiovascular disease Placebo baseline TC: 7.61 mmol/L (294 mg/dL) Placebo baseline LDL‐C: 5.34 mmol/L (206 mg/dL) Placebo baseline HDL‐C: 1.38 mmol/L (53 mg/dL) Placebo baseline TG: 1.14 mmol/L (101 mg/dL) Atorvastatin baseline TC: 7.52 mmol/L (291 mg/dL) Atorvastatin baseline LDL‐C: 5.41 mmol/L (209 mg/dL) Atorvastatin baseline HDL‐C: 1.35 mmol/L (52 mg/dL) Atorvastatin baseline TG: 1.17 mmol/L (104 mg/dL) |
|
| Interventions | Placebo Atorvastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 4 to 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed WDAEs were not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information about blinding was provided to permit judgement of 'yes' or 'no' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | All lipid parameters were measured; WDAEs were not reported |
| Other bias | Unclear risk | The source of funding was not provided |
Sari 2007.
| Methods | No participants were receiving lipid‐lowering agents; therefore no wash‐out period was required 3‐Month trial |
|
| Participants | 42 men and women from Turkey with hypercholesterolaemia; LDL‐C > 160 mg/dL, aged 53 years, BMI 28.3 Exclusion criteria: kidney or liver disease, type 2 diabetes mellitus, hypothyroidism Baseline TC: 6.88 mmol/L (266 mg/dL) Baseline LDL‐C: 4.81 mmol/L (186 mg/dL) Baseline HDL‐C: 1.41 mmol/L (54.5 mg/dL) Baseline TG: 1.41 mmol/L (125 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of plasma TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Sasaki 2008.
| Methods | 2‐Week to 4‐week run‐in period 52‐Week multi‐centre open‐label randomised parallel‐group study |
|
| Participants | 85 men and women aged ≥ 20 years; LDL‐C ≥ 140 mg/dL (3.62 mmol/L), HDL‐C < 80 mg/dL (2.07 mmol/L), TG < 500 mg/dL (5.645 mmol/L), with glucose intolerance Exclusion criteria: contraindication to statin use, severe renal impairment or dysfunction, hypothyroidism, Cushing's syndrome, use of steroid hormones, severe HTN, cerebrovascular disease in the past 3 months, MI or coronary artery reconstruction in the past 3 months, Class III or higher heart failure, statin hypersensitivity, type 1 diabetes Atorvastatin baseline HDL‐C: 1.33 mmol/L (51.4 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Pitavastatin 2 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum HDL‐C | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | HDL‐C data were reported at 8 weeks, LDL‐C and TG data were reported at 12 months and TC data were not reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Sathyapalan 2009.
| Methods | Participants were not taking any medication that would affect blood lipid within the past 6 months; therefore no wash‐out period was required 3‐Month randomised double‐blind placebo‐controlled trial |
|
| Participants | 40 women from the UK with polycystic ovary syndrome, BMI 33 to 34 Exclusion criteria: individuals taking oral contraceptives and metformin within the past 6 months; those with non‐classical 21 hydroxylase deficiency, hyperprolactinaemia, Cushing's disease; individuals with androgen‐secreting tumours Placebo: Baseline TC: 4.5 mmol/L (174 mg/dL) Baseline LDL‐C: 2.7 mmol/L (104 mg/dL) Baseline HDL‐C: 1.1 mmol/L (42.5 mg/dL) Baseline TG: 1.39 mmol/L (123 mg/dL) Atorvastatin: Baseline TC: 4.6 mmol/L (178 mg/dL) Baseline LDL‐C: 2.9 mmol/L (112 mg/dL) Baseline HDL‐C: 1.07 mmol/L (41.4 mg/dL) Baseline TG: 1.34 mmol/L (119 mg/dL) |
|
| Interventions | Placebo Atorvastatin 20 mg/d |
|
| Outcomes | Per cent change from baseline at 4 weeks of plasma TC, LDL‐C, HDL‐C and TG | |
| Notes | WDAEs were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Drug labelling was done by personnel not involved in the trial |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/18 in the placebo group were not analysed because of non‐compliance (11%) 1/19 in the atorvastatin group was not analysed because of non‐compliance (5.2%) |
| Selective reporting (reporting bias) | High risk | All lipid parameters were measured; WDAEs were not reported |
| Other bias | High risk | Pfizer funded the trial; data results may be biased for atorvastatin |
Save 2006.
| Methods | 6‐Week dietary stabilisation period 24‐Week study |
|
| Participants | 110 consecutive men and women aged 30 to 70 years with hyperlipidaemia and type 2 diabetes; LDL‐C ≥ 130 mg/dL (3.57 mmol/L), TG ≤ 400 mg/dL (4.51 mmol/L) Exclusion criteria: primary hypothyroidism, nephrotic syndrome, type 1 diabetes, hepatic dysfunction, BMI > 30 kg/m2 , uncontrolled HTN, MI, coronary angioplasty, unstable angina pectoris within 3 months before the study, active liver and renal disease Baseline TC: 6.52 mmol/L (252 mg/dL) Baseline LDL‐C: 4.60 mmol/L (178 mg/dL) Baseline HDL‐C: 1.11 mmol/L (43 mg/dL) Baseline TG: 1.78 mmol/L (158 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 6 to 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/110 were not included in the efficacy analysis because of loss to follow‐up 6.4% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | No source of funding was provided |
Schneck 2003.
| Methods | 6‐Week dietary baseline stabilisation period 6‐Week multi‐centre randomised double‐blind parallel‐group trial | |
| Participants | 374 men and women from Canada and the USA, mean age 56.5 years (> 17 years) with hypercholesterolaemia and without active arterial disease; LDL‐C 4.14 to 6.46 mmol/L (160‐250 mg/dL), TG < 4.52 mmol/L (400 mg/dL) 209 participants received rosuvastatin, 165 received atorvastatin Exclusion criteria: women who may become pregnant, FH and type III hyperlipoproteinaemia Atorvastatin 10 mg/d baseline TC: 7.24 mmol/L (280 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 4.91 mmol/L (190 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.40 mmol/L (54 mg/dL) Atorvastatin 10 mg/d baseline TG: 2.03 mmol/L (180 mg/dL) Atorvastatin 20 mg/d baseline TC: 7.03 mmol/L (272 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 4.78 mmol/L (185 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.27 mmol/L (49 mg/dL) Atorvastatin 20 mg/d baseline TG: 2.13 mmol/L (189 mg/dL) Atorvastatin 40 mg/d baseline TC: 7.08 mmol/L (274 mg/dL) Atorvastatin 40 mg/d baseline LDL‐C: 4.86 mmol/L (188 mg/dL) Atorvastatin 40 mg/d baseline HDL‐C: 1.27 mmol/L (49 mg/dL) Atorvastatin 40 mg/d baseline TG: 2.05 mmol/L (182 mg/dL) Atorvastatin 80 mg/d baseline TC: 7.19 mmol/L (278 mg/dL) Atorvastatin 80 mg/d baseline LDL‐C: 4.91 mmol/L (190 mg/dL) Atorvastatin 80 mg/d baseline HDL‐C: 1.24 mmol/L (48 mg/dL) Atorvastatin 80 mg/d baseline TG: 2.18 mmol/L (193 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Atorvastatin 20 mg/d Atorvastatin 40 mg/d Atorvastatin 80 mg/d Rosuvastatin 5 mg/d Rosuvastatin 10 mg/d Rosuvastatin 20 mg/d Rosuvastatin 40 mg/d Rosuvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin groups were analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d, atorvastatin 20 mg/d, atorvastatin 40 mg/d and atorvastatin 80 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d, atorvastatin 20 mg/d, atorvastatin 40 mg/d and atorvastatin 80 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d, atorvastatin 20 mg/d, atorvastatin 40 mg/d and atorvastatin 80 mg/d; interventions were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | AstraZeneca funded the study; data may be biased against atorvastatin |
Schneider 2004.
| Methods | No lipid‐lowering therapy was received in the past 3 months 8‐Week open placebo‐controlled randomised clinical trial |
|
| Participants | 61 men and women with type 2 diabetes mellitus Exclusion criteria: intravenous or subcutaneous heparin treatment in the 72 hours before the study, severe kidney or liver disease, TG ≥ 11.4 mmol/L, statin or heparin intolerance Placebo baseline TC: 5.58 mmol/L (216 mg/dL) Placebo baseline LDL‐C: 3.65 mmol/L (141 mg/dL) Placebo baseline HDL‐C: 1.32 mmol/L (51 mg/dL) Placebo baseline TG: 1.66 mmol/L (147 mg/dL) Atorvastatin baseline TC: 6.06 mmol/L (234 mg/dL) Atorvastatin baseline LDL‐C: 4.11 mmol/L (159 mg/dL) Atorvastatin baseline HDL‐C: 1.09 mmol/L (42 mg/dL) Atorvastatin baseline TG: 2.24 mmol/L (198 mg/dL) |
|
| Interventions | Placebo Atorvastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed WDAEs were not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Open‐label |
| Allocation concealment (selection bias) | High risk | Open‐label |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Open" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | All lipid parameters were measured; WDAEs were not reported |
| Other bias | High risk | Pfizer funded the study; data may support bias for atorvastatin |
Schrott 1998.
| Methods | 6‐Week placebo baseline period 6‐Week multi‐centre randomised parallel‐group placebo‐controlled trial | |
| Participants | 65 men and women from the USA, mean age 58 (18‐75) years; type IIa and IIb hypercholesterolaemia, LDL 4.1 to 6.5 mmol/L (159‐251 mg/dL), TG ≤ 4.5 mmol/L (399 mg/dL)
Exclusion criteria: women likely to become pregnant, hepatic dysfunction, renal dysfunction, uncontrolled HTN, diabetes, metabolic endocrine disease, alcohol consumption > 14‐oz/wk equivalents, taking lipid‐altering drugs Placebo baseline TC: 7.10 mmol/L (275 mg/dL) Placebo baseline LDL‐C: 4.93 mmol/L (191 mg/dL) Placebo baseline HDL‐C: 1.24 mmol/L (48 mg/dL) Placebo baseline TG: 2.09 mmol/L (185 mg/dL) Atorvastatin 10 mg/d baseline TC: 7.17 mmol/L (277 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 4.95 mmol/L (191 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.11 mmol/L (43 mg/dL) Atorvastatin 10 mg/d baseline TG: 2.38 mmol/L (211 mg/dL) Atorvastatin 20 mg/d baseline TC: 7.07 mmol/L (273 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 4.85 mmol/L (188 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.21 mmol/L (47 mg/dL) Atorvastatin 20 mg/d baseline TG: 2.07 mmol/L (183 mg/dL) Atorvastatin 40 mg/d baseline TC: 6.94 mmol/L (268 mg/dL) Atorvastatin 40 mg/d baseline LDL‐C: 4.77 mmol/L (184 mg/dL) Atorvastatin 40 mg/d baseline HDL‐C: 1.42 mmol/L (55 mg/dL) Atorvastatin 40 mg/d baseline TG: 1.60 mmol/L (142 mg/dL) Atorvastatin 60 mg/d baseline TC: 6.94 mmol/L (268 mg/dL) Atorvastatin 60 mg/d baseline LDL‐C: 4.88 mmol/L (189 mg/dL) Atorvastatin 60 mg/d baseline HDL‐C: 1.21 mmol/L (47 mg/dL) Atorvastatin 60 mg/d baseline TG: 1.85 mmol/L (164 mg/dL) Atorvastatin 80 mg/d baseline TC: 7.40 mmol/L (286 mg/dL) Atorvastatin 80 mg/d baseline LDL‐C: 5.08 mmol/L (196 mg/dL) Atorvastatin 80 mg/d baseline HDL‐C: 1.42 mmol/L (55 mg/dL) Atorvastatin 80 mg/d baseline TG: 2.02 mmol/L (179 mg/dL) |
|
| Interventions | Placebo
Atorvastatin 10 mg/d Atorvastatin 20 mg/d Atorvastatin 40 mg/d Atorvastatin 60 mg/d Atorvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | WDAEs were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | High risk | Open trial |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may support bias for atorvastatin |
Schwartz 2004.
| Methods | 6‐Week wash‐out period 24‐Week multi‐centre randomised double‐blind parallel‐group study Randomisation was performed by balanced block scheme for each centre Evening dose | |
| Participants | 383 participants from USA with hypercholesterolaemia aged > 17 years; LDL‐C 160 to 250 mg/dL (4.13‐6.465 mmol/L), TG < 400 mg/dL (4.51 mmol/L) 128 participants received atorvastatin, 255 received rosuvastatin Exclusion criteria: pregnancy threat, taking lipid‐altering drugs, active arterial disease, FH, uncontrolled HTN, hypothyroidism, diabetes, cancer history, renal and hepatic dysfunction Atorvastatin baseline TC: 7.11 mmol/L (275 mg/dL) Atorvastatin baseline LDL‐C: 4.86 mmol/L (188 mg/dL) Atorvastatin baseline HDL‐C: 1.22 mmol/L (47 mg/dL) Atorvastatin baseline TG: 2.28 mmol/L (202 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 12 weeks Atorvastatin conditional titration of 40 mg/d for 12 to 18 weeks Atorvastatin conditional titration of 80 mg/d for 18 to 24 weeks Rosuvastatin 5 mg/d for 0 to 12 weeks Rosuvastatin conditional titration of 20 mg/d for 12 to 18 weeks Rosuvastatin conditional titration of 80 mg/d for 18 to 24 weeks Rosuvastatin 10 mg/d for 0 to 12 weeks Rosuvastatin conditional titration of 40 mg/d for 12 to 18 weeks Rosuvastatin conditional titration of 80 mg/d for 18 to 24 weeks |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | AstraZeneca funded the study; data may support bias against atorvastatin |
Shabana 2013.
| Methods | Participants were not receiving any lipid‐lowering agents; wash‐out period was not required 4‐Week before‐and‐after study |
|
| Participants | 50 men and women, mean age 55 years, with hypercholesterolaemia Exclusion criteria: pregnancy, familial hypercholesterolaemia, acute coronary syndrome, hepatic and renal disease, endocrinological disorder, cancer, medications known to affect lipid metabolism Atorvastatin baseline TC: 5.612 mmol/L (217 mg/dL) Atorvastatin baseline LDL‐C: 3.641 mmol/L (141 mg/dL) Atorvastatin baseline HDL‐C: 1.019 mmol/L (39 mg/dL) Atorvastatin baseline TG: 2.212 mmol/L (196 mg/dL) |
|
| Interventions | Atorvastatin 40 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | The source of funding was not reported |
Shimabukuro 2011.
| Methods | 4‐Week wash‐out dietary baseline stabilisation period 4‐Week before‐and‐after trial |
|
| Participants | 15 individuals with type 2 diabetes and hypercholesterolaemia aged 30 to 79 years; TC ≥ 220 mg/dL, TG 150 to 350 mg/dL Exclusion criteria: statin hypersensitivity, hepatic dysfunction, renal dysfunction, pregnancy, poorly controlled diabetes, recent stroke, CHD, congestive heart failure, FH, secondary hyperlipidaemia Atorvastatin baseline TC: 6.58 mmol/L (254 mg/dL) Atorvastatin baseline LDL‐C: 4.23 mmol/L (164 mg/dL) Atorvastatin baseline HDL‐C: 1.42 mmol/L (55 mg/dL) Atorvastatin baseline TG: 1.96 mmol/L (174 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Pitavastatin 2 mg/d |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | No source of funding was provided |
Shishehbor 2003.
| Methods | 6‐Week to 8‐week dietary baseline stabilisation period 12‐Week study |
|
| Participants | 35 consecutive patients out of 200 individuals from 2 venues in Boston, MA, USA, ≥ 21 years of age; LDL‐C ≥ 130 mg/dL (3.3 mmol/L); no clinical evidence of CAD, peripheral artery disease or diabetes mellitus; naive to statin therapy Exclusion criteria: liver disease, renal insufficiency, changes in medical therapy during the treatment period Baseline TC: 6.54 mmol/L (253 mg/dL) Baseline LDL‐C: 4.37 mmol/L (169 mg/dL) Baseline HDL‐C: 1.45 mmol/L (56 mg/dL) Baseline TG: 1.65 mmol/L (146 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
SHUKRA 2008.
| Methods | 6‐Week dietary run‐in period 6‐Week randomised double‐blind comparative multi‐centre parallel‐group study |
|
| Participants | 686 men and women aged ≥ 18 years; LDL‐C 3.5 to 5.7 mmol/L (135‐220 mg/dL), TG < 4.52 mmol/L (400 mg/dL), 55 participants were randomly assigned 25 participants received atorvastatin, 30 received rosuvastatin Atorvastatin baseline TC: 6.45 mmol/L (249 mg/dL) Atorvastatin baseline LDL‐C: 4.43 mmol/L (171 mg/dL) Atorvastatin baseline HDL‐C: 1.27 mmol/L (49 mg/dL) Atorvastatin baseline TG: 1.63 mmol/L (144 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Rosuvastatin 5 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3/25 were not included in the efficacy analysis because end‐of‐treatment data were missing 12% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | AstraZeneca funded the study; data may support bias against atorvastatin |
Simons 1998.
| Methods | 5‐Week wash‐out period
30‐Week multi‐centre open‐label randomised study
Randomly assigned two‐thirds of participants received atorvastatin, one‐third simvastatin with or without cholestyramine Atorvastatin dose was doubled every 6 weeks if LDL‐C was ≥ 3.5 mmol/L |
|
| Participants | 136 men and women from Australia and New Zealand, mean age 51 years, with primary hypercholesterolaemia; LDL ≥ 5.0 mmol/L (193 mg/dL), TG < 4.0 mmol/L (354 mg/dL) 92 participants received atorvastatin, 44 received simvastatin with or without resin Atorvastatin baseline TC: 11.00 mmol/L (425 mg/dL) Atorvastatin baseline LDL‐C: 8.80 mmol/L (340 mg/dL) Atorvastatin baseline HDL‐C: 1.10 mmol/L (42.54 mg/dL) Atorvastatin baseline TG: 2.50 mmol/L (221 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 6 weeks Atorvastatin conditional titration to 20 mg/d for 6 to 12 weeks Atorvastatin conditional titration to 40 mg/d for 12 to 18 weeks Atorvastatin conditional titration to 80 mg/d for 18 to 30 weeks Simvastatin 10 mg/d for 0 to 6 weeks Simvastatin with or without resin conditional titration to 20 mg/d for 6 to 12 weeks Simvastatin with or without resin conditional titration to 40 mg/d for 12 to 30 weeks |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may support bias for atorvastatin |
Singh 2008.
| Methods | Participants received no lipid‐altering drugs, so wash‐out period was not required 12‐Week double‐blind randomised placebo‐controlled trial |
|
| Participants | 70 individuals with metabolic syndrome aged 50 to 51 years Exclusion criteria: smoking, diabetes, aspirin use > 81 mg/d, anti‐inflammatory drugs, infection, cancer, recent major surgery, illness, liver, renal or uncompensated metabolic/hormonal disorders, high‐sensitivity C‐reactive protein > 10 mg/L Placebo baseline TC: 5.25 mmol/L (203 mg/dL) Placebo baseline LDL‐C: 3.56 mmol/L (138 mg/dL) Placebo baseline HDL‐C: 0.98 mmol/L (38 mg/dL) Atorvastatin 10 mg/d baseline TC: 5.35 mmol/L (207 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 3.52 mmol/L (136 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.03 mmol/L (40 mg/dL) Atorvastatin 80 mg/d baseline TC: 5.46 mmol/L (211 mg/dL) Atorvastatin 80 mg/d baseline LDL‐C: 3.65 mmol/L (141 mg/dL) Atorvastatin 80 mg/d baseline HDL‐C: 0.96 mmol/L (37 mg/dL) |
|
| Interventions | Placebo Atorvastatin 10 mg/d Atorvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C; WDAEs were reported | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information about the sequence generation process was insufficient to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | Information about allocation concealment was insufficient to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | TGs were not measured |
| Other bias | Low risk | The trial was not funded by pharmaceutical companies |
Sinski 2009.
| Methods | No participant was receiving lipid‐altering agents, so wash‐out was not required 8‐Week before‐and‐after trial |
|
| Participants | 10 men aged 31 to 55 years with hypercholesterolaemia and with mild to moderate essential HTN Exclusion criteria: secondary forms of HTN, diabetes Atorvastatin 20 mg/d baseline TC: 6.53 mmol/L (252.5 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 4.16 mmol/L (161 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.20 mmol/L (46 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C and HDL‐C | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | TGs were not measured |
| Other bias | Unclear risk | The source of funding was not reported |
Sirtori 2005.
| Methods | 4‐Week wash‐out period 12‐Week multi‐centre double‐blind double‐dummy parallel randomised study | |
| Participants | 86 men and women from Italy with familial combined hyperlipidaemia aged 55 years; LDL‐C > 160 mg/dL (4.14 mmol/L), TG > 200 mg/dL (2.26 mmol/L) 45 participants received atorvastatin, 41 received pravastatin Exclusion criteria: none Atorvastatin baseline TC: 7.91 mmol/L (306 mg/dL) Atorvastatin baseline LDL‐C: 5.45 mmol/L (211 mg/dL) Atorvastatin baseline HDL‐C: 1.18 mmol/L (45.6 mg/dL) Atorvastatin baseline TG: 3.26 mmol/L (289 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 12 weeks Pravastatin 20 mg/d for 0 to 12 weeks |
|
| Outcomes | Per cent change from baseline at 6 to 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer funded the study; data may support bias for atorvastatin |
SLIM 2009.
| Methods | Participants were not receiving lipid‐altering medications within 1 month of screening, so wash‐out was not required 12‐Week before‐and‐after trial |
|
| Participants | Men and women with combined hyperlipidaemia aged 21 to 75 years; LDL‐C > 130 mg/dL, HDL‐C < 45 mg/dL in men and < 55 mg/dL in women Exclusion criteria: medications that affect lipid metabolism, statin hypersensitivity, liver or gastrointestinal disease, type III hyperlipidaemia, TG > 500 mg/dL, BP > 150/95 mmHg, alcoholism, gout |
|
| Interventions | Atorvastatin 10 mg/d Slo‐Niacin 250 mg BID Slo‐Niacin 500 mg BID Slo‐Niacin 750 mg BID Atorvastatin 10 mg/d + Slo‐Niacin 250 mg BID Atorvastatin 10 mg/d + Slo‐Niacin 500 mg BID Atorvastatin 10 mg/d + Slo‐Niacin 750 mg BID |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
SOLAR 2007.
| Methods | 6‐Week wash‐out period 12‐Week multi‐centre open‐label randomised study | |
| Participants | 1632 men and women from the USA aged ≥ 18 years; LDL‐C 130 to 250 mg/dL (3.36‐6.46 mmol/L), TG < 400 mg/dL (4.5 mmol/L) 544 participants received atorvastatin, 542 received rosuvastatin, 546 received simvastatin Exclusion criteria: unstable CVD, uncontrolled HTN, uncontrolled diabetes mellitus, hepatic or renal dysfunction Atorvastatin baseline TC: 6.49 mmol/L (251 mg/dL) Atorvastatin baseline LDL‐C: 4.32 mmol/L (167mg/dL) Atorvastatin baseline HDL‐C: 1.22 mmol/L (47 mg/dL) Atorvastatin baseline TG: 2.09 mmol/L (185 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 6 weeks Atorvastatin 20 mg/d for 6 to 12 weeks Rosuvastatin 10 mg/d for 0 to 6 weeks Rosuvastatin 20 mg/d for 6 to 12 weeks Simvastatin 20 mg/d for 0 to 6 weeks Simvastatin 400 mg/d for 6 to 12 weeks |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16/544 were not included in the efficacy analysis because of adverse events or withdrawal of consent 2.9% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | AstraZeneca funded the study; data may support bias against atorvastatin |
Sposito 2003.
| Methods | No lipid‐lowering therapy in the 6 months preceding the study 3‐Month dietary stabilisation period 6‐Week randomised placebo‐controlled trial |
|
| Participants | 45 men and women aged 30 to 76 years; LDL‐C > 100 mg/dL (2.59 mmol/L), TG < 400 mg/dL (4.52 mmol/L) Exclusion criteria: premenopausal women, diabetes mellitus, alcohol abuse; liver, renal and thyroid disease; cancer Placebo baseline TC: 6.34 mmol/L (245 mg/dL) Placebo baseline LDL‐C: 4.34 mmol/L (168 mg/dL) Placebo baseline HDL‐C: 0.98 mmol/L (38 mg/dL) Placebo baseline TG: 2.28 mmol/L (202 mg/dL) Atorvastatin 10 mg/d baseline TC: 6.49 mmol/L (251 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 4.37 mmol/L (169 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 0.98 mmol/L (38 mg/dL) Atorvastatin 10 mg/d baseline TG: 2.37 mmol/L (210 mg/dL) Atorvastatin 40 mg/d baseline TC: 6.00 mmol/L (232 mg/dL) Atorvastatin 40 mg/d baseline LDL‐C: 4.40 mmol/L (170 mg/dL) Atorvastatin 40 mg/d baseline HDL‐C: 0.91 mmol/L (35 mg/dL) Atorvastatin 40 mg/d baseline TG: 2.18 mmol/L (193 mg/dL) |
|
| Interventions | Placebo Atorvastatin 10 mg/d Atorvastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed WDAEs were not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information about blinding was provided to permit judgement of 'yes' or 'no' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | All lipid parameters were measured; WDAEs were not reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
STARSHIP 2006.
| Methods | 6‐Week wash‐out period 6‐Week multi‐centre open‐label randomised trial | |
| Participants | 696 Hispanic men and women from the USA with hypercholesterolaemia aged > 17 years; LDL‐C 130 to 300 mg/dL (3.36‐7.76 mmol/L), TG < 400 mg/dL (4.51 mmol/L) 339 participants received atorvastatin, 357 received rosuvastatin Exclusion criteria: homozygous familial type I, III or V hypercholesterolaemia; active arterial disease, uncontrolled HTN, poorly controlled diabetes mellitus, active liver disease of hepatic dysfunction, unexplained CK increase > 3 × ULN Atorvastatin 10 mg/d baseline TC: 6.41 mmol/L (247 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 4.24 mmol/L (164mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.24 mmol/L (48 mg/dL) Atorvastatin 10 mg/d baseline TG: 2.02 mmol/L (179 mg/dL) Atorvastatin 20 mg/d baseline TC: 6.44 mmol/L (249 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 4.27 mmol/L (165 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.21 mmol/L (47 mg/dL) Atorvastatin 20 mg/d baseline TG: 2.10 mmol/L (186 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Atorvastatin 20 mg/d Rosuvastatin 10 mg/d Rosuvastatin 20 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin groups were analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d and atorvastatin 20 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d and atorvastatin 20 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d and atorvastatin 20 mg/d; interventions were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Atorvastatin 10 mg/d: 7/168 were not included in the efficacy analysis because of withdrawal of consent, adverse events, loss to follow‐up Atorvastatin 20 mg/d: 10/171 were not included in the efficacy analysis because of withdrawal of consent, adverse events, loss to follow‐up 5% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | AstraZeneca funded the study; data may support bias against atorvastatin. |
STELLAR 2003.
| Methods | 6‐Week wash‐out period 6‐Week multi‐centre randomised open‐label study Evening doses | |
| Participants | 2431 men and women from the USA, mean age 58 years (21‐86); LDL‐C 160 to 250 mg/dL (4.14‐6.46 mmol/L), TG < 400 mg/dL (4.52 mmol/L) 641 participants received atorvastatin, 655 received simvastatin, 492 received pravastatin, 643 received rosuvastatin Exclusion criteria: women likely to become pregnant, statin sensitivity, serious or unstable medical condition, FH, lipid‐altering drug use, drug and alcohol abuse Atorvastatin 10 mg/d baseline TC: 7.09 mmol/L (274 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 4.89 mmol/L (189 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.29 mmol/L (50 mg/dL) Atorvastatin 10 mg/d baseline TG: 1.96 mmol/L (174 mg/dL) Atorvastatin 20 mg/d baseline TC: 7.11 mmol/L (275 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 4.91 mmol/L (190 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.29 mmol/L (50 mg/dL) Atorvastatin 20 mg/d baseline TG: 2.00 mmol/L (177 mg/dL) Atorvastatin 40 mg/d baseline TC: 7.11 mmol/L (275 mg/dL) Atorvastatin 40 mg/d baseline LDL‐C: 4.89 mmol/L (189 mg/dL) Atorvastatin 40 mg/d baseline HDL‐C: 1.29 mmol/L (50 mg/dL) Atorvastatin 40 mg/d baseline TG: 2.01 mmol/L (178 mg/dL) Atorvastatin 80 mg/d baseline TC: 7.21 mmol/L (279 mg/dL) Atorvastatin 80 mg/d baseline LDL‐C: 4.91 mmol/L (190 mg/dL) Atorvastatin 80 mg/d baseline HDL‐C: 1.32 mmol/L (51 mg/dL) Atorvastatin 80 mg/d baseline TG: 2.04 mmol/L (181 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Atorvastatin 20 mg/d Atorvastatin 40 mg/d Atorvastatin 80 mg/d Rosuvastatin 10 mg/d Rosuvastatin 20 mg/d Rosuvastatin 40 mg/d Rosuvastatin 80 mg/d Simvastatin 10 mg/d Simvastatin 20 mg/d Simvastatin 40 mg/d Simvastatin 80 mg/d Pravastatin 10 mg/d Pravastatin 20 mg/d Pravastatin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin groups were analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d, atorvastatin 20 mg/d, atorvastatin 40 mg/d and atorvastatin 80 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d, atorvastatin 20 mg/d, atorvastatin 40 mg/d and atorvastatin 80 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d, atorvastatin 20 mg/d, atorvastatin 40 mg/d and atorvastatin 80 mg/d; interventions were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Atorvastatin 20 mg/d: 1/156 was not included in the efficacy analysis Atorvastatin 40 mg/d: 4/160 were not included in the efficacy analysis Atorvastatin 80 mg/d: 2/167 were not included in the efficacy analysis 1.4% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | AstraZeneca funded the study; data may support bias against atorvastatin |
Stojakovic 2007.
| Methods | 4‐Week placebo run‐in period 20‐Week prospective single‐centre single‐blinded study |
|
| Participants | 15 women with primary biliary cirrhosis and incomplete biochemical response to ursodeoxycholic acid Exclusion criteria: primary biliary cirrhosis stage III to IV at the time of liver biopsy, > 75 years old, decompensated liver disease, AST or ALT > 3 × ULN, CPK > 5 × ULN; CPK > 5 × ULN or 3 × ULN with muscle pain, tenderness or weakness; severe renal dysfunction, nephrotic syndrome, statin hypersensitivity, pregnancy or breastfeeding, premenopausal women without safe contraception Baseline TC: 6.13 mmol/L (237 mg/dL) Baseline LDL‐C: 4.65 mmol/L (180 mg/dL) Baseline HDL‐C: 1.78 mmol/L (69 mg/dL) Baseline TG: 1.20 mmol/L (106 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 4 weeks Atorvastatin 20 mg/d for 4 to 8 weeks Atorvastatin 40 mg/d for 8 to 12 weeks Atorvastatin discontinuation for 12 to 20 weeks |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3/18 were not included in the efficacy analysis for personal reasons and because of gastrointestinal side effects during the placebo run‐in period 16.7% of participants were excluded from the efficacy analysis Risk of bias is high |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | No source of funding was provided |
SToP AF 2011.
| Methods | Participants had a normal lipid profile, so no wash‐out was required 4‐Week double‐blind randomised placebo‐controlled trial |
|
| Participants | 64 men and women with atrial fibrillation Exclusion criteria: aged < 18 years, paroxysmal atrial fibrillation, haemodynamic instability, atrial fibrillation ablation within 6 months of study, anticoagulation contraindication, severe valvular heart disease, unstable angina, implantable defibrillator, Class IV heart failure, hyperthyroidism, uncontrolled HTN, significant CAD, illicit drug use, alcoholism, atorvastatin hypersensitivity, pregnancy threat, nursing mothers, liver and renal dysfunction, inflammatory muscle disease Placebo baseline TC: 4.22 mmol/L (163 mg/dL) Atorvastatin baseline TC: 4.74 mmol/L (183 mg/dL) |
|
| Interventions | Placebo Atorvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC; WDAEs were reported | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information about sequence generation was insufficient to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Low risk | Active drug and placebo were administered in identical appearing tablets |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | LDL‐C, HDL‐C and TG data were not reported |
| Other bias | High risk | Pfizer funded the trial |
STRENGTH 2008.
| Methods | 6‐Week wash‐out dietary baseline period 8‐Week before‐and‐after trial |
|
| Participants | 168 men and women aged 18 to 75 years with type IIa or IIb hypercholesterolaemia Exclusion criteria: LDL‐C > 240 mg/dL, TG > 400 mg/dL, history of MI within 6 months, unstable angina, stroke, transient ischaemic attack or coronary revascularisation within the past year Baseline LDL‐C 4.42 mmol/L (171 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Simvastatin 20 mg/d Pravastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum LDL‐C | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | TC, HDL‐C and TG data were not analysed |
| Other bias | Unclear risk | Genaissance Pharmaceuticals funded the trial |
Stulc 2008.
| Methods | 4‐Week dietary wash‐out period 12‐Week before‐and‐after trial |
|
| Participants | 27 individuals with primary hypercholesterolaemia aged > 18 years; TC > 7.0 mmol/L (271 mg/dL) Exclusion criteria: hypertriglyceridaemia, secondary hyperlipidaemia, manifest vascular disease, diabetes, malignancy, other major disease Baseline TC: 8.59 mmol/L (332 mg/dL) Baseline LDL‐C: 6.20 mmol/L ( 240 mg/dL) Baseline HDL‐C: 1.63 mmol/L (63 mg/dL) Baseline TG: 1.67 mmol/L (148 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Suleiman 2012.
| Methods | Participants were not receiving any lipid‐lowering agents; wash‐out period was not required 3‐Month randomised double‐blind placebo‐controlled trial |
|
| Participants | 125 men and women with atrial fibrillation ≥ 18 years old 62 participants received atorvastatin; 63 received placebo Exclusion criteria: cancer, inflammatory disease, surgery, trauma, MI in the previous month, contraindication to statin therapy, elevated liver enzymes > 2 × ULN Placebo baseline TC: 4.888 mmol/L (189 mg/dL) Placebo baseline LDL‐C: 3.05 mmol/L (118 mg/dL) Placebo baseline HDL‐C: 1.24 mmol/L (48 mg/dL) Atorvastatin 80 mg/day baseline TC: 4.78 mmol/L (185 mg/dL) Atorvastatin 80 mg/day baseline LDL‐C: 2.896 mmol/L (112 mg/dL) Atorvastatin 80 mg/day baseline HDL‐C: 1.215 mmol/L (47 mg/dL) |
|
| Interventions | Placebo Atorvastatin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C and HDL‐C | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | Triglycerides were not included in the efficacy analysis; no WDAEs were reported |
| Other bias | High risk | Pfizer funded the trial; efficacy data could be biased favourably towards atorvastatin |
SUPREME 2009.
| Methods | 4‐Week wash‐out period 12‐Week before‐and‐after trial |
|
| Participants | 80 men and women with mixed dyslipidaemia or hyperlipidaemia aged ≥ 21 years; LDL‐C ≥ 130 and < 250 mg/dL, HDL‐C < 40 mg/dL for men or < 50 mg/dL for women, TG < 350 mg/dL Exclusion criteria: niacin or statin intolerance, excessive alcohol consumption or substance abuse, drugs that interfere with lipid metabolism, psychiatric disease, unstable endocrine disease, uncontrolled hypothyroidism, HTN or cardiac arrhythmia, peripheral artery disease occlusion, Class III or IV congestive heart failure, unstable angina, uncontrolled diabetes, heart surgery, stomach or liver disease, pancreatitis, cancer Baseline LDL‐C: 4.32 mmol/L (167 mg/dL) Baseline HDL‐C: 0.97 mmol/L (37.5 mg/dL) |
|
| Interventions | Atorvastatin 40 mg/d Niacin/simvastatin 1000/40 mg/d titrated to 2000/40 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum LDL‐C and HDL‐C | |
| Notes | Atorvastatin group was analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 40 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1.2% of participants were not analysed |
| Selective reporting (reporting bias) | High risk | TC and TG data were not analysed |
| Other bias | High risk | Abbott funded the study |
Szapary 2004.
| Methods | Participants did not receive lipid‐lowering drug treatment before the study; therefore a wash‐out period was not required 12‐Week before‐and‐after trial |
|
| Participants | 27 men and women from Hungary with cerebrovascular disease and hyperlipidaemia, mean age 61 years; TC > 5.2 mmol/L, LDL‐C > 3.4 mmol/L Exclusion criteria: none reported Baseline TC: 7.03 mmol/L (272 mg/dL) Baseline LDL‐C: 4.32 mmol/L (167 mg/dL) Baseline HDL‐C: 1.55 mmol/L (60 mg/dL) Baseline TG: 2.32 mmol/L (205 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of plasma TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Tagle 2000.
| Methods | 4‐Week wash‐out 8‐Week single‐centre before‐and‐after study Evening dose | |
| Participants | 40 men and women from Ecuador aged 18 to 80 years with hypercholesterolaemia; LDL‐C > 100 mg/dL (2.59 mmol/L) Exclusion criteria: hypothyroidism, type 1 and 2 diabetes, hepatic dysfunction, BMI > 34, uncontrolled HTN, MI, unstable CVD, statin hypersensitivity, lipid‐altering drugs Baseline TC: 6.97 mmol/L (270 mg/dL) Baseline LDL: 4.90 mmol/L (189 mg/dL) Baseline HDL: 1.00 mmol/L (38.67 mg/dL) Baseline TG: 2.08 mmol/L (184 mg/dL) | |
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 4 to 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | No source of funding was provided |
Takebayashi 2005.
| Methods | No individuals were being treated with anti‐lipidaemic drugs; therefore no wash‐out period was required 3‐Month before‐and‐after trial |
|
| Participants | 30 individuals with type 2 diabetes with hyperlipidaemia from Japan aged 61 years; TC > 220 mg/dL, TG > 150 mg/dL Exclusion criteria: liver or renal dysfunction, infectious or autoimmune disease Baseline TC: 6.74 mmol/L (261 mg/dL) Baseline LDL‐C: 4.41 mmol/L (171 mg/dL) Baseline HDL‐C: 1.48 mmol/L (57 mg/dL) Baseline TG: 1.41 mmol/L (125 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 3 months of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3/20 in atorvastatin group were not analysed for efficacy because of non‐compliance 15% of participants were not included in the efficacy analysis Risk of bias is high |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Tan 2002.
| Methods | 8‐Week wash‐out period 6‐Month single‐centre double‐blind randomised placebo‐controlled study | |
| Participants | 80 men and women from Hong Kong with NIDDM, mean age 55 years; LDL‐C > 3.4 mmol/L (131 mg/dL), TG < 4.0 mmol/L (355 mg/dL)
Exclusion criteria: current use of lipid‐modifying agents, secondary hyperlipidaemia, renal and hepatic dysfunction, major cardiovascular event within 6 months Placebo baseline TC: 6.12 mmol/L (237 mg/dL) Placebo baseline LDL‐C: 4.29 mmol/L (166 mg/dL) Placebo baseline HDL‐C: 1.12 mmol/L (43 mg/dL) Atorvastatin baseline TC: 6.35 mmol/L (246 mg/dL) Atorvastatin baseline LDL‐C: 4.45 mmol/L (172 mg/dL) Atorvastatin baseline HDL‐C: 1.19 mmol/L (46 mg/dL) |
|
| Interventions | Placebo for 0 to 3 months Atorvastatin 10 mg/d for 0 to 3 months Placebo for 3 to 6 months Atorvastatin 20 mg/d for 3 to 6 months |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First placebo and atorvastatin dose groups were analysed SDs were imputed WDAEs were not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind, placebo‐controlled study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | TGs were not analysed because data were expressed as median values; WDAEs were not reported |
| Other bias | High risk | This study was partially funded by Pfizer Inc; data may support bias for atorvastatin |
Tanaka 2001.
| Methods | 4‐Week wash‐out period 12‐Week multi‐centre double‐blind randomised parallel‐group placebo‐controlled study Randomisation by permuted block design of block size 6 (3: atorvastatin, 3: placebo) at each institution | |
| Participants | 55 men and women from Japan with NIDDM and hypercholesterolaemia aged 20 to 70 years; TC ≥ 5.7 mmol/L (220 mg/dL)
Stable glycaemic controls were recruited to participate in the trial. 4 individuals were ineligible and 1 withdrew consent during the observation period. Of the remaining 50 individuals, 10 were excluded in a blinded review because they fulfilled exclusion criteria. 40 were defined as the full analysis set 20 participants received placebo, 20 received atorvastatin Exclusion criteria: aged < 20 or > 71 years, women taking hormone replacement therapy, HbA1c value ≥ 10%, TG ≥ 4.5 mmol/L (400 mg/dL), hepatic and renal dysfunction, MI or cerebrovascular disease within 3 months, secondary hyperlipidaemia, alcohol abuse Placebo baseline TC: 6.80 mmol/L (263 mg/dL) Placebo baseline LDL‐C: 4.5 mmol/L (174 mg/dL) Placebo baseline HDL‐C: 1.4 mmol/L (54 mg/dL) Placebo baseline TG: 1.9 mmol/L (168 mg/dL) Atorvastatin baseline TC: 6.80 mmol/L (263 mg/dL) Atorvastatin baseline LDL‐C: 4.4 mmol/L (170 mg/dL) Atorvastatin baseline HDL‐C: 1.5 mmol/L (58 mg/dL) Atorvastatin baseline TG: 2.1 mmol/L (186 mg/dL) |
|
| Interventions | Placebo Atorvastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed WDAEs were reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "A double‐blind, placebo‐controlled, parallel‐group study was performed" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12‐Week placebo: 2/20 were not included in the efficacy analysis; 1 person died during treatment: "1 in whose case the antidiabetic therapy was changed" 12‐Week atorvastatin: 2/20 were not included in the efficacy analysis: "2 were lost to follow‐up" 10% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may support bias for atorvastatin |
TARGET TANGIBLE 1999.
| Methods | 6‐Week wash‐out period 14‐Week open‐label randomised parallel‐group study Evening dose | |
| Participants | 4097 men and women from Germany with CHD aged 35 to 75 years; LDL‐C > 130 mg/dL (3.36 mmol/L), 3748 of 4097 were enrolled in the diet phase A total of 2856 participants met the lipid criteria at the end of the diet phase and were randomly assigned 1897 participants received atorvastatin, 959 received simvastatin Exclusion criteria: statin hypersensitivity, liver disease, muscle disease, active arterial disease, FH, TG > 1000 mg/dL (11.28 mmol/L), heart failure stage III or IV, uncontrolled HTN, cancer, surgery or severe medical disease, nephrotic syndrome, endocrine disorder, LDL apheresis, drug or alcohol abuse, pregnancy threat, participation in another study, lack of consent Atorvastatin baseline TC, HDL‐C and TG were not reported Atorvastatin baseline LDL‐C: 4.86 mmol/L (188 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 5 weeks Atorvastatin conditional titration 20 mg/d for 5 to 10 weeks Atorvastatin conditional titration 40 mg/d for 10 to 14 weeks Simvastatin 10 mg/d for 0 to 5 weeks Simvastatin conditional titration 20 mg/d for 5 to 10 weeks Simvastatin conditional titration 40 mg/d for 10 to 14 weeks |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C and TG | |
| Notes | First atorvastatin dose was analysed SD was imputed for LDL‐C only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | HDL‐C data were not reported |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may support bias for atorvastatin |
Tateishi 2011.
| Methods | Participants were not receiving any lipid‐altering agents; no wash‐out period was required 12‐Week randomised trial |
|
| Participants | 78 men and women with hypercholesterolaemia; LDL‐C ≥ 140 mg/dL (3.62 mmol/L) 26 participants received atorvastatin, 26 received rosuvastatin, 26 received pitavastatin Exclusion criteria: participants receiving statins Atorvastatin 10 mg/d baseline LDL‐C: 4.30 mmol/L (166 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.24 mmol/L (48 mg/dL) Atorvastatin 10 mg/d baseline TG: 1.735 mmol/L (154 mg/dL) |
|
| Interventions | Rosuvastatin 2.5 mg/d for 0 to 12 weeks Rosuvastatin 5 mg/d for 12 to 24 weeks Atorvastatin 10 mg/d Pitavastatin 2 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum LDL‐C, HDL‐C and triglycerides | |
| Notes | Rosuvastatin 2.5 mg/d for 0 to 12 weeks Rosuvastatin 5 mg/d for 12 to 24 weeks Pitavastatin 2 mg/d Groups were not included in the efficacy analysis SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | Total cholesterol was not included in the efficacy analysis |
| Other bias | Unclear risk | The source of funding was not reported |
Tekin 2004.
| Methods | ≥ 6‐Week dietary stabilisation period 3‐Month before‐and‐after study |
|
| Participants | 38 men and women with hyperlipidaemia and CHD; LDL‐C > 135 mg/dL (3.49 mmol/L), TG < 300 mg/dL (3.39 mmol/L) Exclusion criteria: unstable angina pectoris, MI, stroke, coronary angioplasty, coronary bypass surgery, major surgery, anti‐inflammatory medication or anticoagulant usage before 6 months of study, cancer; liver, kidney or thyroid disease; SBP/DBP > 160/100 mmHg Baseline TC: 5.61 mmol/L (217 mg/dL) Baseline LDL‐C: 4.09 mmol/L (158 mg/dL) Baseline HDL‐C: 0.93 mmol/L (36 mg/dL) Baseline TG: 1.76 mmol/L (156 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 3 months of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer funded the study; data may support bias for atorvastatin |
Tekten 2004.
| Methods | No participants were receiving lipid‐lowering therapy for at least 2 months; therefore no wash‐out period was required 2‐Month before‐and‐after trial |
|
| Participants | 25 men and women from Turkey with CAD aged 57 years; LDL‐C > 100 mg/dL Exclusion criteria: unstable angina, MI, stroke, anticoagulant therapy, major surgery, coronary bypass grafting or PCI within past 6 months, thrombocytopaenia, bleeding disorder Baseline TC: 5.99 mmol/L (232 mg/dL) Baseline LDL‐C: 3.59 mmol/L (139 mg/dL) Baseline HDL‐C: 1.27 mmol/L (49 mg/dL) Baseline TG: 2.27 mmol/L (201 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 3 months of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | No source of funding was provided |
Tomas 2004.
| Methods | Participants had not received hypolipidaemic treatment in the 3 months before inclusion in the study 3‐Month before‐and‐after study |
|
| Participants | 21 men and women with dyslipidaemia aged 65 years; TC > 250 mg/dL (6.465 mmol/L), LDL‐C > 160 mg/dL (4.14 mmol/L) Baseline TC: 6.50 mmol/L (251 mg/dL) Baseline LDL‐C: 4.44 mmol/L (172 mg/dL) Baseline HDL‐C: 1.36 mmol/L (53 mg/dL) Baseline TG: 1.45 mmol/L (128 mg/dL) |
|
| Interventions | Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 3 months of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 20 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | No source of funding was provided |
Tousoulis 2005.
| Methods | No use of lipid‐lowering agents during the past 6 months 4‐Week randomised trial |
|
| Participants | 26 participants with ischaemic heart failure Exclusion criteria: left ventricular hypertrophy, acute and chronic inflammatory disease involving organs other than the heart and other cardiac disease, smoking, fasting cholesterol levels > 210 mg/dL (5.43 mmol/L), use of antioxidant vitamins or other dietary supplements, patients who required medication changes Atorvastatin 10 mg/d baseline TC: 4.78 mmol/L (185 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 0.87 mmol/L (34 mg/dL) Atorvastatin 10 mg/d baseline TG: 1.61 mmol/L (143 mg/dL) Atorvastatin 10 mg/d + vitamin E 400 IU/d baseline TC: 4.89 mmol/L (189 mg/dL) Atorvastatin 10 mg/d + vitamin E 400 IU/d baseline HDL‐C: 0.85 mmol/L (33 mg/dL) Atorvastatin 10 mg/d + vitamin E 400 IU/d baseline TG: 1.72 mmol/L (152 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 3 months Atorvastatin 10 mg/d + vitamin E 400 IU/d for 0 to 3 months |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d and atorvastatin 10 mg/d + vitamin E; interventions were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d and atorvastatin 10 mg/d + vitamin E; interventions were analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d and atorvastatin 10 mg/d + vitamin E; interventions were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | LDL‐C data were not reported |
| Other bias | Unclear risk | No source of funding was provided |
Tousoulis 2006.
| Methods | No use of lipid‐lowering agents during the past 6 months 6‐Week before‐and‐after trial |
|
| Participants | 46 participants with unstable angina 22 participants received atorvastatin, 24 received no statin Exclusion criteria: left ventricular hypertrophy, acute and chronic inflammatory disease involving organs other than the heart and other cardiac disease, heart failure, overt atherosclerotic peripheral vascular disease, fasting cholesterol levels > 210 mg/dL (5.43 mmol/L) Atorvastatin baseline TC: 5.09 mmol/L (197 mg/dL) Atorvastatin baseline HDL‐C: 0.86 mmol/L (33 mg/dL) Atorvastatin baseline TG: 1.26 mmol/L (112 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, HDL‐C and TG | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | LDL‐C data were not reported |
| Other bias | Unclear risk | No source of funding was provided |
Tousoulis 2011.
| Methods | Participants were not receiving any lipid‐altering agents; no wash‐out period was required 12‐Week randomised study |
|
| Participants | 35 men and women with type 2 diabetes mellitus 17 participants received metformin; 18 received metformin and atorvastatin Exclusion criteria: previous treatment with antidiabetic or hypolipidaemic agent, cancer, liver disease, inflammatory disease Atorvastatin baseline TC: 5.635 mmol/L (218 mg/dL) Atorvastatin baseline HDL‐C: 1.15 mmol/L (44 mg/dL) |
|
| Interventions | Metformin 850 mg/d Metformin 850 mg/d and atorvastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC and HDL‐C | |
| Notes | Metformin 850 mg/d; treatment arm was not analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | LDL‐C and triglycerides were not included in the efficacy analysis |
| Other bias | Unclear risk | The source of funding was not reported |
Tsunoda 2011.
| Methods | Participants were not receiving any lipid‐altering agents; no wash‐out period was required 12‐Week randomised open‐label trial |
|
| Participants | 60 men and women with hypercholesterolaemia; LDL‐C ≥ 140 mg/dL (3.62 mmol/L), TG ≤ 400 mg/dL (4.52 mmol/L) 30 participants received atorvastatin, 30 received rosuvastatin Exclusion criteria: TG > 400 mg/dL (4.52 mmol/L) Atorvastatin baseline TC: 6.48 mmol/L (251 mg/dL) Atorvastatin baseline LDL‐C: 4.0 mmol/L (155 mg/dL) Atorvastatin baseline HDL‐C: 1.57 mmol/L (61 mg/dL) Atorvastatin baseline TG: 1.986 mmol/L (176 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Rosuvastatin 2.5 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Notes | Rosuvastatin 2.5 mg/d; treatment arm was not included in the efficacy analysis SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | The source of funding was not reported |
Undas 2006a.
| Methods | No statins were taken for at least 6 weeks before enrolment 8‐Week double‐blind randomised allocated study |
|
| Participants | 26 men with angiographically documented CAD Exclusion criteria: diabetes mellitus, any acute illness, cancer, hepatic or renal dysfunction, ACS within previous 6 months, prior CABG Baseline TC: 5.81 mmol/L (225 mg/dL) Baseline LDL‐C: 3.82 mmol/L (148 mg/dL) Baseline HDL‐C: 1.20 mmol/L (46 mg/dL) Baseline TG: 1.85 mmol/L (164 mg/dL) |
|
| Interventions | Atorvastatin 40 mg/d Atorvastatin 40 mg/d + quinapril 10 mg/d |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Data from both groups were combined SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 40 mg/d and atorvastatin 40 mg/d + quinapril 10 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 40 mg/d and atorvastatin 40 mg/d + quinapril 10 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 40 mg/d and atorvastatin 40 mg/d + quinapril 10 mg/d; interventions were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | Pfizer provided the drugs tested |
Uydu 2012.
| Methods | Participants were not receiving any lipid‐altering agents; no wash‐out period was required 12‐Week before‐and‐after study |
|
| Participants | 44 men and women aged 30 to 76 years with hypercholesterolaemia and mixed hyperlipidaemia Exclusion criteria: hypothyroidism, diabetes mellitus, nephrotic syndrome, renal dysfunction, hepatic dysfunction, cancer, immune disorder, uncontrolled hypertension, coronary artery disease, smoking Atorvastatin baseline TC: 7.36 mmol/L (285 mg/dL) Atorvastatin baseline LDL‐C: 5.25 mmol/L (203 mg/dL) Atorvastatin baseline HDL‐C: 1.1 mmol/L (42.5 mg/dL) Atorvastatin baseline TG: 2.19 mmol/L (194 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were included in the efficacy analysis |
| Other bias | Low risk | Industry did not fund the trial |
Vansant 2001.
| Methods | 4‐Week wash‐out period 4‐Week single‐centre double‐blind randomised placebo‐controlled study Randomly assigned placebo:atorvastatin ratio 1:2 Evening doses | |
| Participants | 22 obese women from Belgium selected from the obesity outpatient clinic homozygous for apo E3 aged 32 to 48 years; euthyroid, non‐diabetic, normal kidney function, not taking lipid‐altering drugs Placebo baseline TC: 5.12 mmol/L (198 mg/dL) Placebo baseline LDL‐C: 3.02 mmol/L (117 mg/dL) Placebo baseline HDL‐C: 1.29 mmol/L (50 mg/dL) Placebo baseline TG: 1.88 mmol/L (167 mg/dL) Atorvastatin baseline TC: 5.53 mmol/L (214 mg/dL) Atorvastatin baseline LDL‐C: 3.62 mmol/L (140 mg/dL) Atorvastatin baseline HDL‐C: 1.47 mmol/L (57 mg/dL) Atorvastatin baseline TG: 1.35 mmol/L (120 mg/dL) |
|
| Interventions | Placebo Atorvastatin 20 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed WDAEs were not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "As a double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | All lipid parameters were measured; WDAEs were not reported |
| Other bias | Unclear risk | No source of funding was provided |
VISION 2013.
| Methods | No participant received lipid‐altering agents; no wash‐out period was required 12‐Week randomised trial |
|
| Participants | 42 men and women with hyperlipidaemia aged 45 to 75 years 21 participants received pitavastatin; 21 received atorvastatin Exclusion criteria: age < 20 years, premenopausal females, diabetes, CVD, liver and renal dysfunction, endocrine disease, administration of agents that could affect lipid metabolism and oxidation Atorvastatin baseline TC: 6.96 mmol/L (269 mg/dL) Atorvastatin baseline LDL‐C: 4.73 mmol/L (183 mg/dL) Atorvastatin baseline HDL‐C: 1.42 mmol/L (55 mg/dL) Atorvastatin baseline TG: 1.49 mmol/L (132 mg/dL) |
|
| Interventions | Pitavastatin 2 mg/d Atorvastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Pitavastatin group was not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were included in the efficacy analysis |
| Other bias | Low risk | Industry did not fund the trial |
VYTAL 2006.
| Methods | 3‐Week to 5‐week wash‐out period 6‐Week multi‐centre randomised double‐blind study Randomly assigned using an interactive voice response system | |
| Participants | 1229 men and women from the USA with NIDDM and hypercholesterolaemia aged 18 to 80 years; LDL‐C > 100 mg/dL (2.59 mmol/L), TG < 400 mg/dL (4.51 mmol/L) 735 participants received atorvastatin, 494 received Vytorin Exclusion criteria: none Atorvastatin 10 mg/d baseline TC: 5.96 mmol/L (230 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 3.75 mmol/L (145 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.16 mmol/L (45 mg/dL) Atorvastatin 20 mg/d baseline TC: 5.97 mmol/L (231 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 3.79 mmol/L (147 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.20 mmol/L (46 mg/dL) Atorvastatin 40 mg/d baseline TC: 5.95 mmol/L (230 mg/dL) Atorvastatin 40 mg/d baseline LDL‐C: 3.77 mmol/L (146 mg/dL) Atorvastatin 40 mg/d baseline HDL‐C: 1.19 mmol/L (46 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Atorvastatin 20 mg/d Atorvastatin 40 mg/d Vytorin 10 mg/d Vytorin 20 mg/d Vytorin 40 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C and HDL‐C | |
| Notes | Atorvastatin groups were analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d, atorvastatin 20 mg/d and atorvastatin 40 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d, atorvastatin 20 mg/d and atorvastatin 40 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d, atorvastatin 20 mg/d and atorvastatin 40 mg/d; interventions were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All atorvastatin doses: 17/735 were not included in the efficacy analysis because of protocol deviation, withdrawal of consent, adverse event, relocation Comment: Number is low; 2.3% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | High risk | TGs were not analysed because data were reported as median values |
| Other bias | High risk | Merck funded the study; data may support bias against atorvastatin |
VYTELD 2010.
| Methods | 6‐Week to 8‐week wash‐out period and 3‐week single‐blind placebo run‐in period 12‐Week before‐and‐after study |
|
| Participants | 773 men and women aged ≥ 65 years with hyperlipidaemia; LDL‐C ≥ 130 mg/dL (3.36 mmol/L), TG ≤ 350 mg/dL (3.96 mmol/L) Exclusion criteria: congestive heart failure, unstable angina pectoris, MI, coronary bypass surgery, angioplasty or uncontrolled peripheral artery disease, ≤ 3 months of placebo run‐in, uncontrolled HTN, intestinal or renal disease, uncontrolled endocrine or metabolic disease, treatment with prohibited concomitant therapy, systemic corticosteroids, anti‐obesity medication with < 3 months' stabilisation Atorvastatin 10 mg/d baseline TC: 6.54 mmol/L (253 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 4.32 mmol/L (167 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.37 mmol/L (53 mg/dL) Atorvastatin 20 mg/d baseline TC: 6.46 mmol/L (250 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 4.27 mmol/L (165 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.40 mmol/L (54 mg/dL) Atorvastatin 40 mg/d baseline TC: 6.54 mmol/L (253 mg/dL) Atorvastatin 40 mg/d baseline LDL‐C: 4.34 mmol/L (168 mg/dL) Atorvastatin 40 mg/d baseline HDL‐C: 1.37 mmol/L (53 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Atorvastatin 20 mg/d Atorvastatin 40 mg/d Ezetimibe/simvastatin 10 mg/20 mg/d Ezetimibe/simvastatin 10 mg/40 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C and HDL‐C | |
| Notes | Atorvastatin groups were analysed TGs were not analysed because they were expressed as median values SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d, atorvastatin 20 mg/d and atorvastatin 40 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d, atorvastatin 20 mg/d and atorvastatin 40 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d, atorvastatin 20 mg/d and atorvastatin 40 mg/d; interventions were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 7% of participants receiving atorvastatin were not included in the analysis |
| Selective reporting (reporting bias) | High risk | TGs were not analysed because they were median values |
| Other bias | High risk | Merck funded the study |
VYVA 2005.
| Methods | 4‐Week wash‐out period 6‐Week multi‐centre randomised double‐blind parallel‐group study Participants were centrally randomly assigned with use of an interactive voice response system and were stratified according to visit 2 to achieve balance among treatment groups | |
| Participants | 1902 men and women from the USA with hypercholesterolaemia aged 18 to 79 years; LDL‐C > 130 mg/dL (3.36 mmol/L), TG < 350 mg/dL (3.95 mmol/L) 951 participants received atorvastatin, 951 received Vytorin Exclusion criteria: none Atorvastatin 10 mg/d baseline TC: 6.76 mmol/L (261 mg/dL) Atorvastatin 10 mg/d baseline LDL‐C: 4.53 mmol/L (175 mg/dL) Atorvastatin 10 mg/d baseline HDL‐C: 1.24 mmol/L (48 mg/dL) Atorvastatin 20 mg/d baseline TC: 6.86 mmol/L (265 mg/dL) Atorvastatin 20 mg/d baseline LDL‐C: 4.61 mmol/L (178 mg/dL) Atorvastatin 20 mg/d baseline HDL‐C: 1.26 mmol/L (49 mg/dL) Atorvastatin 40 mg/d baseline TC: 6.85 mmol/L (265 mg/dL) Atorvastatin 40 mg/d baseline LDL‐C: 4.65 mmol/L (180 mg/dL) Atorvastatin 40 mg/d baseline HDL‐C: 1.30 mmol/L (50 mg/dL) Atorvastatin 80 mg/d baseline TC: 6.89 mmol/L (266 mg/dL) Atorvastatin 80 mg/d baseline LDL‐C: 4.72 mmol/L (183 mg/dL) Atorvastatin 80 mg/d baseline HDL‐C: 1.24 mmol/L (48 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Atorvastatin 20 mg/d Atorvastatin 40 mg/d Atorvastatin 80 mg/d Vytorin 10 mg/d Vytorin 20 mg/d Vytorin 40 mg/d Vytorin 80 mg/d |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin groups were analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d, atorvastatin 20 mg/d, atorvastatin 40 mg/d and atorvastatin 80 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d, atorvastatin 20 mg/d, atorvastatin 40 mg/d and atorvastatin 80 mg/d; interventions were analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d, atorvastatin 20 mg/d, atorvastatin 40 mg/d and atorvastatin 80 mg/d; interventions were analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All atorvastatin groups: 24/951 were not included in the efficacy analysis because of discontinuation for adverse events, withdrawal of consent, loss to follow‐up, protocol deviation, other reasons Comment: 2.5% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | High risk | TG data were not analysed in our review because data were expressed as median values |
| Other bias | High risk | Merck funded the study; data may result in bias against atorvastatin |
Wang 2001.
| Methods | 6‐Week wash‐out baseline dietary stabilisation period 8‐Week double‐blind randomised placebo‐controlled study | |
| Participants | 54 men and women outpatients from Taiwan with elevated LDL‐C, mean age 66 years (18‐80); LDL‐C 4.2 to 6.5 mmol/L (162‐251 mg/dL), TG < 4.5 mmol/L (399 mg/dL)
Exclusion criteria: women likely to become pregnant, hypothyroidism, liver and renal dysfunction, alcohol abuse, unstable CVD, dementia, statin hypersensitivity, cancer, alcohol abuse, chronic lung disease, taking lipid‐altering medications Placebo baseline TC: 6.73 mmol/L (260 mg/dL) Placebo baseline LDL‐C: 4.84 mmol/L (187 mg/dL) Placebo baseline HDL‐C: 1.17 mmol/L (45 mg/dL) Placebo baseline TG: 1.56 mmol/L (138 mg/dL) Atorvastatin baseline TC: 6.90 mmol/L (267 mg/dL) Atorvastatin baseline LDL‐C: 4.98 mmol/L (193 mg/dL) Atorvastatin baseline HDL‐C: 1.17 mmol/L (45 mg/dL) Atorvastatin baseline TG: 1.63 mmol/L (144 mg/dL) |
|
| Interventions | Placebo Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | WDAEs were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation was provided to permit judgement of 'yes' or 'no' |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was provided to permit judgement of 'yes' or 'no' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "This study was a randomized, double‐blind, placebo‐controlled, 8‐week study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | High risk | All lipid parameters were measured; WDAEs were not reported |
| Other bias | High risk | Pfizer funded the study; data may support bias for atorvastatin |
Wang 2012.
| Methods | 1‐Month baseline wash‐out dietary stabilisation period 12‐Week randomised trial |
|
| Participants | 90 men and women with mild to moderate renal dysfunction aged 26 to 81 years; TC ≥ 5.18 mmol/L (200 mg/dL), LDL‐C ≥ 3.37 mmol/L (130 mg/dL), TG ≥ 1.7 mmol/L (151 mg/dL) 30 participants received atorvastatin, 60 received rosuvastatin Exclusion criteria: calcium allergy, familial hypercholesterolaemia, thyroid dysfunction, acute or chronic liver disease or liver dysfunction, use of lipid‐altering agents within 2 months of the study, severe infection, surgery, trauma within 1 month of the study Atorvastatin baseline TC: 6.17 mmol/L (239 mg/dL) Atorvastatin baseline LDL‐C: 3.78 mmol/L (146 mg/dL) Atorvastatin baseline HDL‐C: 0.89 mmol/L (34 mg/dL) Atorvastatin baseline TG: 2.21 mmol/L (196 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Rosuvastatin 5 mg/d Rosuvastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 4 to 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Rosuvastatin 5 mg/d and rosuvastatin 10 mg/d; treatment arms were not analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were included in the efficacy analysis |
| Other bias | Unclear risk | The source of funding was not reported |
WATCH 2001.
| Methods | 3‐Week wash‐out period 16‐Week open‐label multi‐centre non‐randomised treat‐to‐target clinical trial | |
| Participants | 318 women from Canada with severe dyslipidaemia with and without CVD aged 18 to 75 years; LDL‐C ≥ 2.6 mmol/L (100.5 mg/dL) Exclusion criteria: statin hypersensitivity, women likely to become pregnant, alcohol abuse, immunosuppressant, unstable cardiovascular condition, type 1 diabetes, untreated hypothyroidism, renal and hepatic dysfunction No baseline TC, HDL‐C and TG values were given Baseline LDL‐C: 4.63 mmol/L (179 mg/dL) | |
| Interventions | Atorvastatin 10 mg/d for 0 to 4 weeks Atorvastatin conditional titration 20 mg/d for 4 to 8 weeks Atorvastatin conditional titration 40 mg/d for 8 to 12 weeks Atorvastatin conditional titration 80 mg/d for 12 to 16 weeks |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum LDL‐C | |
| Notes | Atorvastatin conditional titration 20 mg/d for 4 to 8 weeks Atorvastatin conditional titration 40 mg/d for 8 to 12 weeks Atorvastatin conditional titration 80 mg/d for 12 to 16 weeks Interventions were not analysed SDs were imputed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Non‐CVD group: For 47/120, efficacy was not reported because they did not achieve their target value CVD group: For 138/198, efficacy was not reported because they did not achieve their target value 58% of participants were excluded from the efficacy analysis Risk of bias is high |
| Selective reporting (reporting bias) | High risk | TC, HDL‐C and TG data were not reported |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may support bias for atorvastatin |
Wei 2001.
| Methods | 4‐Week screening diet/placebo run‐in period 16‐Week open‐label active study |
|
| Participants | 33 men and women with FH Exclusion criteria: none Baseline TC: 9.1 mmol/L (352 mg/dL) Baseline LDL‐C: 7.0 mmol/L (271 mg/dL) Baseline HDL‐C: 1.33 mmol/L (51 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 4 weeks Atorvastatin 20 mg/d for 4 to 8 weeks Atorvastatin 40 mg/d for 8 to 12 weeks Atorvastatin 80 mg/d for 12 to 16 weeks |
|
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C and HDL‐C | |
| Notes | Atorvastatin 20 mg/d for 4 to 8 weeks Atorvastatin 40 mg/d for 8 to 12 weeks Atorvastatin 80 mg/d for 12 to 16 weeks Interventions were not analysed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/33 were not included in the efficacy analysis because 1 participant defaulted for personal reasons and 1 withdrew because of heartburn 6% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | High risk | TG data were not reported because they were expressed as median values |
| Other bias | Unclear risk | No source of funding was provided |
Welder 2010.
| Methods | No participants received lipid‐altering medication; therefore no wash‐out was required 8‐Week before‐and‐after trial |
|
| Participants | 84 normal participants aged ≥ 18 years Exclusion criteria: coronary disease, pregnant, hepatic dysfunction Baseline TC: 4.65 mmol/L (180 mg/dL) Baseline LDL‐C: 2.61 mmol/L (101 mg/dL) Baseline HDL‐C: 1.55 mmol/L (60 mg/dL) Baseline TG: 1.08 mmol/L (96 mg/dL) |
|
| Interventions | Atorvastatin 80 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11.9% of participants were not analysed |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Wierzbicki 1998.
| Methods | 4‐Week wash‐out period of previous lipid‐lowering treatment 12‐Week before‐and‐after trial |
|
| Participants | 54 individuals aged 18 to 70 years with severe FH; TC > 7 mmol/L (271 mg/dL) Exclusion criteria: none reported Baseline TC: 10.5 mmol/L (406 mg/dL) Baseline LDL‐C: 8.10 mmol/L (313 mg/dL) Baseline HDL‐C: 1.32 mmol/L (51 mg/dL) Baseline TG: 2.48 mmol/L (220 mg/dL) |
|
| Interventions | Atorvastatin 80 mg/d | |
| Outcomes | Per cent change from baseline at 3 months of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 80 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | The source of funding was not provided |
Willrich 2008.
| Methods | 4‐Week wash‐out baseline dietary stabilisation period 4‐Week open‐label trial |
|
| Participants | 139 men and women of African or non‐African descent with hypercholesterolaemia, mean age 57 years Exclusion criteria: hypertriglyceridaemia, TG ≥ 4.52 mmol/L (400 mg/dL), hypothyroidism, diabetes mellitus, liver or kidney disease, secondary dyslipidaemia Baseline TC: 7.27 mmol/L (281 mg/dL) Baseline LDL‐C: 4.99 mmol/L (193 mg/dL) Baseline HDL‐C: 1.45 mmol/L (56 mg/dL) Baseline TG: 1.80 mmol/L (159 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study seems to be free of other bias |
Wu 2002.
| Methods | 6‐Week wash‐out period 16‐Week multi‐centre randomised double‐blind double‐dummy study Randomisation was stratified by site, relying on a table of random numbers designed by Boston Biostatistics Inc | |
| Participants | 157 Asian individuals with elevated LDL‐cholesterol, mean age 55 years (31‐76), LDL‐C 4.2 to 6.5 mmol/L (162‐251 mg/dL), TG 4.2 to 6.5 mmol/L (372‐576 mg/dL), modified ITT population consisted of 145 participants 73 participants received atorvastatin, 72 received simvastatin Exclusion criteria: uncontrolled hypothyroidism, type 1 and uncontrolled type 2 diabetes, hepatic dysfunction, unstable CVD, cancer, dementia, taking lipid‐altering drugs, statin hypersensitivity, cerebrovascular disease, pulmonary disease, drug and alcohol abuse Atorvastatin baseline TC: 6.87 mmol/L (266 mg/dL) Atorvastatin baseline LDL‐C: 4.78 mmol/L (185 mg/dL) Atorvastatin baseline HDL‐C: 1.29 mmol/L (49.88 mg/dL) Atorvastatin baseline TG: 1.73 mmol/L (153 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 8 weeks Atorvastatin 20 mg/d for 8 to 16 weeks Simvastatin 10 mg/d for 0 to 8 weeks Simvastatin 20 mg/d for 8 to 16 weeks |
|
| Outcomes | Per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8/79 were not included in the efficacy analysis 10% of participants were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may support bias for atorvastatin |
Wu 2005.
| Methods | 3‐Month dietary stabilisation period 3‐Month multi‐centre randomised study | |
| Participants | 66 men and women from Taiwan with hypercholesterolaemia, mean age 53 years; TC > 240 mg/dL (6.21 mmol/L) 32 participants received atorvastatin in the phase 1 study, 34 received atorvastatin in the phase 2 study, 34 received simvastatin in the phase 1 study, 32 received simvastatin in the phase 2 study Exclusion criteria: pregnancy or lactation, secondary HTN, serious cardiovascular event, serious intestinal ailment, liver cirrhosis, renal disease, unstable NIDDM, elevated CK, thyroid disease, nephrotic syndrome, alcoholism, confounding medication Atorvastatin baseline TC: 6.88 mmol/L (266 mg/dL) Atorvastatin baseline LDL‐C: 4.27 mmol/L (165 mg/dL) Atorvastatin baseline HDL‐C: 1.30 mmol/L (50.3 mg/dL) Atorvastatin baseline TG: 2.11 mmol/L (187 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d Simvastatin 10 mg/d |
|
| Outcomes | Per cent change from baseline at 3 months of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | Atorvastatin group was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Yoshitomi 2005.
| Methods | 4‐Week dietary stabilisation period 12‐Week open‐label multi‐centre study |
|
| Participants | 44 men and women aged ≥ 18 years; LDL‐C > 140 mg/dL (3.62 mmol/L), TG < 400 mg/dL (4.52 mmol/L) Exclusion criteria: treated diabetes mellitus or FPG ≥ 110 mg/dL, active liver disease, hepatic or renal dysfunction, uncontrolled HTN, use of drug that interferes with lipid levels or interacts with study medication Baseline TC: 7.27 mmol/L (281 mg/dL) Baseline LDL‐C: 4.86 mmol/L (188 mg/dL) Baseline HDL‐C: 1.53 mmol/L (59 mg/dL) Baseline TG: 1.7 mmol/L (151 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | Unclear risk | No source of funding was provided |
ZAPE 2003.
| Methods | 4‐Week placebo period 1‐Year open‐label randomised parallel‐group multi‐centre study |
|
| Participants | 56 participants aged 10 to 18 years with FH and severe hypercholesterolaemia; Tanner stage II or greater at screening visit, LDL‐C ≥ 200 mg/dL (5.2 mmol/L), TG ≤ 200 mg/dL (2.25 mmol/L) 25 participants received atorvastatin, 31 received colestipol Atorvastatin baseline TC: 7.94 mmol/L (307 mg/dL) Atorvastatin baseline LDL‐C: 6.56 mmol/L (254 mg/dL) Atorvastatin baseline HDL‐C: 1.16 mmol/L (45 mg/dL) Atorvastatin baseline TG: 0.79 mmol/L (70 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d for 0 to 6 weeks Colestipol 5 mg/d for 0 to 6 weeks Atorvastatin 20 mg/d for 6 to 52 weeks Colestipol 10 mg/d for 6 to 52 weeks |
|
| Outcomes | Per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | First atorvastatin dose was analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Pfizer funded the study; data may support bias for atorvastatin |
Zhu 2000.
| Methods | 6‐Week baseline dietary stabilisation period 4‐Week single‐centre trial |
|
| Participants | 25 individuals from Canada recruited from lipid clinic with hyperlipidaemia; TC > 6.2 mmol/L (240 mg/dL); none taking lipid‐altering drugs Exclusion criteria: none reported 19 of 25 participants received atorvastatin Baseline TC: 7.62 mmol/L (295 mg/dL) Baseline LDL‐C: 5.21 mmol/L (201 mg/dL) Baseline HDL‐C: 1.38 mmol/L (53.36 mg/dL) Baseline TG: 2.19 mmol/L (194 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/d | |
| Outcomes | Per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C and TG | |
| Notes | SDs were imputed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of random sequence generation is not applicable |
| Allocation concealment (selection bias) | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, assessment of allocation concealment is not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Atorvastatin 10 mg/d; intervention was analysed, and as no placebo group was included for comparison, blinding status is not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | All lipid parameters were measured |
| Other bias | High risk | Parke‐Davis Pharmaceuticals funded the study; data may support bias for atorvastatin |
ACS: acute coronary syndrome; ALT: alanine transaminase; AST: aspartate aminotransferase; BID: twice daily; BMI: body mass index; CABG: coronary artery bypass graft; CAD: coronary artery disease; CHD: coronary heart disease; CK: creatine kinase; CPK: creatine phosphokinase; CVD: cardiovascular disease; DBP: diastolic blood pressure; ER: extended release; FH: familial hypercholesterolaemia; HDL‐C: high‐density lipoprotein cholesterol; HIV: human immunodeficiency virus; HRT: hormone replacement therapy; ITT: intention to treat; LDL‐C: low‐density lipoprotein cholesterol; MI: myocardial infarction; NIDDM: non‐insulin‐dependent diabetes; PAD: peripheral artery disease; PTCA: percutaneous transluminal coronary angioplasty; SBP: systolic blood pressure; SD: standard deviation; TC: total cholesterol; TG: triglyceride; TSH: thyroid‐stimulating hormone; ULN: upper limit of normal; WDAE: withdrawal due to adverse effect.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTFAST 2 2004 | Bias: atorvastatin dose dependent on baseline LDL‐C values |
| ADSL 2003 | Bias: atorvastatin dose dependent on baseline LDL‐C values |
| Ahmed 2008 | Location not found for this document |
| Alrasadi K 2008 | Confounding factor: niacin |
| Alvarez 1999 | Renal transplantation confounding factor: immunosuppressants to prevent rejection of transplanted kidney |
| Arad 2005 | Randomisation stratified by ratio of LDL‐C to HDL‐C |
| Argent 2003 | Renal transplantation confounding factor: immunosuppressants to prevent rejection of transplanted kidney |
| ASG 2008 | Bias: atorvastatin dosing based on LDL‐C baseline values |
| AstraZeneca 3 | Number of participants randomly assigned to atorvastatin not reported |
| AstraZeneca 4 | Baseline lipid values not reported Data expressed as absolute change from baseline; therefore per cent change from baseline could not be determined |
| ATGOAL 2005 | Bias: atorvastatin dosing based on baseline LDL‐C values |
| Athyros 2008 | Atorvastatin added to ezetimibe therapy |
| Atorvastatin 2008 | Biased trial dosing based on LDL‐C baseline values |
| Banyai 2001 | Confounding factor: LDL apheresis treatment |
| Boh 2011 | Bias: atorvastatin dose dependent on baseline LDL‐cholesterol values; participants with low LDL‐cholesterol received 10 mg/d Participants with high LDL‐cholesterol received 20 mg/d |
| Bolewski 2008 | Data expressed as median per cent change from baseline |
| Bonet 2002 | Confounding factor: cyclosporine as immunosuppressant in heart transplantation |
| Brown 2004 | Median per cent changes from baseline reported |
| CAPABLE | Bias: atorvastatin dose dependent on baseline LDL‐C values |
| Carnevale 2010 | Median per cent change in total cholesterol and LDL‐cholesterol measured |
| COMPELL 2007 | Confounding factor: niacin extended release |
| Conard 2008 | Up‐titration of atorvastatin monotherapy |
| Costa 2003 | Median per cent changes from baseline reported |
| Davis 2000 | Median per cent change from baseline for all lipid parameters |
| Di Renzo 2008 | LDL‐C values do not add up |
| DISCOVERY PENTA 2005 | Data from statin‐naive participants and from switched participants combined. No baseline dietary wash‐out stabilisation period for at least 3 weeks for switched participants |
| Dogra 2002 | Atorvastatin dose adjusted to lower serum LDL‐C to 3.4 mmol/L and TG to ≤ 1.8 mmol/L |
| Dujovne 2000 | Median per cent changes from baseline reported |
| Faludi 2004 | Exact number of participants receiving atorvastatin not reported |
| Farsang 2007 | Bias: atorvastatin dose dependent on baseline LDL‐C |
| Ferrer‐Garcia 2008 | Bias: atorvastatin dose dependent on baseline LDL‐C |
| Gandelman 2011 | Bias: only participants with sufficient LDL‐cholesterol decrease reported |
| Geiss 1999 | Confounding factor: LDL apheresis |
| Goldammer 2002 | Confounding factor: LDL‐C apheresis |
| Gupta 2004 | LDL‐C values do not add up |
| Ishigami 2003 | Participant selection bias |
| Jafari 2003 | LDL‐C values do not add up |
| Jose 2012 | Per cent change from baseline could not be calculated because baseline values and per cent change were not reported |
| Jyoti 2008 | Article not available via interlibrary loan; study author contacted; no response |
| Kaya 2009 | Lipid parameters do not add up |
| Kearns 2008 | LDL‐C values do not add up |
| Krysiak 2010 | LDL‐C numbers do not add up according to Friedewald formula |
| Lamon‐Fava 2007 | Data could not be expressed as per cent change from baseline for placebo, atorvastatin 20 mg and atorvastatin 80 mg. Data expressed as per cent change from placebo Lipid data combined for all phases of cross‐over trial; data not provided before first cross‐over point |
| Lee 2010 | Evident bias introduced; atorvastatin dosing dependent on baseline LDL‐C |
| Lundberg 2010 | Data reported as median values |
| McVey 1999 | Data are biased: Participants with higher LDL‐C baseline values received higher doses LDL‐C 4.83 mmol/L received 10 mg/d LDL‐C 5.62 mmol/L received 40 mg/d LDL‐C 8.13 mmol/L received 80 mg/d |
| Meas 2009 | Impossible outcome. TC went down from 1.98 to 1.6 g/L; TC went up from 5.12 to 6.6 mmol/L |
| Nawawi 2003 | Median values recorded |
| Nicholls 2011 | Data taken from follow‐up visit 7 after 12 weeks of treatment |
| Paolisso 2000 | SDs reported are impossible SDs for atorvastatin group are 4 times the value of the placebo group or greater; therefore mean values are also suspect |
| Perez‐Castrillon 2007 | Bias: atorvastatin dose based on TC and TG baseline values |
| Pfizer Inc 10 | Bias: atorvastatin dose dependent on baseline LDL‐C values |
| Pfizer Inc 11 | Per cent change from baseline for each group was not reported and cannot be calculated. Per cent change from baseline for T/A compared with atorvastatin was reported |
| Pfizer Inc 13 | Per cent change from baseline for each group was not reported. Mean difference between T/A vs atorvastatin was reported |
| Pfizer Inc 14 | Per cent change from baseline for each group was not reported. Mean difference between T/A vs atorvastatin was reported |
| Pfizer Inc 5 | Bias: atorvastatin dose dependent on baseline LDL‐C values |
| Puccetti 2001 | Per cent change from baseline could not be retrieved from data after 6 weeks of atorvastatin dosing |
| Puccetti 2011 | Data reported as median per cent change from baseline with interquartile ranges |
| Riahi 2006 | Lipid data combined for all phases of cross‐over trial and not provided before first cross‐over point. Data for placebo and atorvastatin groups combined |
| Riesen 2002 | Median per cent change from baseline reported |
| Roberto 2010 | Lipid data expressed as median per cent change |
| Schaefer 2002 | Per cent change from placebo, not baseline |
| Schaefer 2004 | Per cent change from placebo, not baseline |
| Schuster 1998 | Per cent change from baseline for all lipid parameters expressed as median values |
| Soedamah‐Muthu 2003 | Median per cent change from baseline reported |
| Son 2013 | Bias: atorvastatin dose dependent on baseline LDL‐cholesterol values |
| Sparks 2005 | Values from graph not reliable |
| Tutunov 2008 | Outcomes expressed as per cent change from baseline as range of values (values not specific) |
| Undas 2006b | Median per cent change from baseline reported |
| VYMET 2009 | Some participants did not have a wash‐out dietary stabilisation period "Eligible patients naive to lipid‐lowering agents (no agent within 6 weeks [8 weeks for fibrates] before the first visit) who were at high risk of CHD or those receiving lipid‐lowering therapy who were at moderately high risk of CHD were randomized to treatment" 113/686 (16.5%) atorvastatin‐treated participants had no baseline wash‐out period |
| Wanner 2005 | Median per cent change from baseline reported |
| Wierzbicki 1999 | Study bias: dosing based on theoretically matched LDL reduction protocol Participants with higher LDL‐C baseline values received higher doses LDL‐C 5.92 mmol/L received atorvastatin 10 mg/d LDL‐C 6.37 mmol/L received atorvastatin 20 mg/d LDL‐C 7.20 mmol/L received atorvastatin 40 mg/d LDL‐C 8.38 mmol/L received atorvastatin 80 mg/d |
| Winkler 2004 | Design flawed; participants received fenofibrate, then atorvastatin |
| Yamamoto 2000 | Confounding factor: probucol |
| Yang 2007 | Per cent decrease in LDL‐C 36% less than TC. Data erroneous. 33.8% decrease in TC and 12.2% decrease in LDL‐C |
| Yildiz 2007 | Bias: atorvastatin doses based on LDL‐C baseline values |
| Yilmaz 2005 | Lipid parameters do not add up according to Friedewald equation |
LDL‐C: low‐density lipoprotein cholesterol; TC: total cholesterol; TG: triglyceride.
Contributions of authors
JMW, MT and SPA contributed to the design of the protocol.
MT extracted the data.
SPA extracted and analysed the data.
Sources of support
Internal sources
University of British Columbia, Canada.
External sources
None, Canada.
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
3T study 2003 {published data only}
- Miettinen TA, Gylling H, Lindbohm N, Miettinen TE, Rajaratnam RA, Relas H, Finnish Treat‐to‐Target Study Investigators. Serum non‐cholesterol sterols during inhibition of cholesterol synthesis by statins. Journal of Laboratory & Clinical Medicine 2003;141(2):131‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Olsson AG, Eriksson M, Johnson O, Kjellstrom T, Lanke J, Larsen ML, et al. A 52‐week, multicenter, randomized, parallel‐group, double‐blind, double‐dummy study to assess the efficacy of atorvastatin and simvastatin in reaching low‐density lipoprotein cholesterol and triglyceride targets: The Treat‐to‐Target (3T) study. Clinical Therapeutics 2003;25(1):119‐38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Olsson AG, Eriksson M, Johnson O, Kjellstrom T, Lanke J, Lytken M, et al. Effect of atorvastatin and simvastatin in obtaining targets for lipids in secondary prevention. The treat‐to‐target study (3T). Atherosclerosis 2000;151(1):46. [CENTRAL: CN‐00447036] [Google Scholar]
- Seljeflot I, Tonstad S, Hjermann I, Arnesen H. Improved fibrinolysis after 1‐year treatment with HMG CoA reductase inhibitors in patients with coronary heart disease. Thrombosis Research 2002;105(4):285‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Seljeflot I, Tonstad S, Hjermann I, Arnesen H. Reduced expression of endothelial cell markers after 1 year treatment with simvastatin and atorvastatin in patients with coronary heart disease. Atherosclerosis 2002;162(1):179‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vasankari T, Ahotupa M, Viikari J, Nuotio I, Strandberg T, Vanhanen H, et al. Effect of 12‐month statin therapy on antioxidant potential of LDL and serum antioxidant vitamin concentrations. Annals of Medicine 2004;36(8):618‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ACCESS 2001 {published data only}
- Ballantyne CM, Andrews TC, Hsia JA, Kramer JH, Shear C. Correlation of non‐high‐density lipoprotein cholesterol with apolipoprotein B: effect of 5 hydroxymethylglutaryl coenzyme A reductase inhibitors on non‐high‐density lipoprotein cholesterol levels. American Journal of Cardiology 2001;88(3):265‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Campese VM, Kostis JB, Shear CL. Comparing the efficacy of statins in various patient subgroups in the atorvastatin comparative cholesterol efficacy and safety study (ACCESS). Atherosclerosis 2000;151(1):315. [CENTRAL: CN‐00444655] [Google Scholar]
- Center for Drug Evaluation and Research. ACCESS clinical trial. Drugs @ FDA 2001;Application Number: 20‐702/S025:1‐23. [Google Scholar]
- Edmundowicz D, Andrews TC, Shear CL. Comparing treatment success with statins: results from the atorvastatin comparative cholesterol efficacy and safety study (ACCESS). Atherosclerosis 2000;151(1):277. [CENTRAL: CN‐00445197] [Google Scholar]
- Smith DG, McBurney CR. An economic analysis of the Atorvastatin Comparative Cholesterol Efficacy and Safety Study (ACCESS). Pharmacoeconomics 2003;21 Suppl 1:13‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ADVOCATE 2003 {published data only}
- Bays H. Combination niacin and statin therapy compared with monotherapy. Cardiology Review 2003;20(11):34‐7. [EMBASE: 2003430296] [Google Scholar]
- Bays HE, Dujovne CA, MCGovern ME, White TE, Kashyap ML, Hutcheson AG, et al. Comparison of once‐daily, niacin extended‐release/lovastatin with standard doses of atorvastatin and simvastatin (The Advicor versus Other Cholesterol‐Modulating Agents Trial Evaluation [ADVOCATE]). American Journal of Cardiology 2003;91(6):667‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bays HE, McGovern ME. Once‐daily niacin extended release/lovastatin combination tablet has more favourable effects on lipoprotein particle size and subclass distribution than atorvastatin and simvastatin. Preventive Cardiology 2003;6(4):179‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Alaupovic 1997 {published data only}
- Alaupovic P, Heinonen T, Shurzinske L, Black DM. Effect of a new HMG‐CoA reductase inhibitor, atorvastatin, on lipids, apolipoproteins and lipoprotein particles in patients with elevated serum cholesterol and triglyceride levels. Atherosclerosis 1997;133(1):123‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Almroth 2009 {published data only}
- Almroth H, Hoglund N, Boman K, Englund A, Jensen S, Kjellman B, et al. Atorvastatin and persistent atrial fibrillation following cardioversion: a randomized placebo‐controlled multicentre study. European Heart Journal 2009;30(7):827‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ALPIN 2004 {unpublished data only}
- Pfizer Inc. Impact of Atorvastatin on the Distribution, Composition and Metabolism of LDL (Low Density Lipoprotein) and HDL (High Density Lipoprotein) Subfractions: a Double‐Blind Placebo‐Controlled Phase IV Study With Patients Suffering From Combined Hyperlipidemia and Diabetes ‐ Atorvastatin and LDL Profile In NIDDM (ALPIN Study). Protocol No. A2581040 2004. [NCT00640549]
- Wunderlich T, Hoffmann M, Friedrich I, Wieland H, Hanefeld M, Marz W, et al. Atorvastatin and LDL profile in NIDDM (Alpin study). Atherosclerosis Supplements 2006;7(3):581. [IDS Number: 064MN] [Google Scholar]
Amudha 2008 {published data only}
- Amudha K, Choy AM, Mustafa MR, Lang CC. Short‐term effect of atorvastatin on endothelial function in healthy offspring of parents with type 2 diabetes mellitus. Cardiovascular Therapeutics 2008;26(4):253‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Anagnostis 2011 {published data only}
- Anagnostis P, Selalmatzidou D, Polyzos SA, Panagiotou A, Slavakis A, Panagiotidou A, et al. Comparative effects of rosuvastatin and atorvastatin on glucose metabolism and adipokine levels in non‐diabetic patients with dyslipidaemia: a prospective randomised open‐label study. International Journal of Clinical Practice 2011;65(6):679‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ANDROMEDA 2007 {published data only}
- Betteridge DJ, Gibson JM. Effects of rosuvastatin on lipids, lipoproteins and apolipoproteins in the dyslipidaemia of diabetes. Diabetic Medicine 2007;24(5):541‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Betteridge DJ, Gibson JM, ANDROMEDA Study Investigators. Effects of rosuvastatin on lipids, lipoproteins and apolipoproteins in the dyslipidaemia of diabetes. Diabetic Medicine 2007;24(5):541‐9. [EMBASE: 2007212120] [DOI] [PubMed] [Google Scholar]
- Betteridge DJ, Gibson JM, Sager PT. Comparison of effectiveness of rosuvastatin versus atorvastatin on the achievement of combined C‐reactive protein (< 2 mg/L) and low‐density lipoprotein cholesterol (< 70 mg/dl) targets in patients with type 2 diabetes mellitus (from the ANDROMEDA study). American Journal of Cardiology 2007;100(8):1245‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ansquer 2009 {published data only}
- Ansquer JC, Corda C, Malicot K, Jessent V. Effects of atorvastatin 10 mg and fenofibrate 200 mg on the low‐density lipoprotein profile in dyslipidemic patients: a 12‐week, multicenter, randomized, open‐label, parallel‐group study. Current Therapeutic Research Clinical and Experimental 2009;70(2):71‐93. [EMBASE: 2009218875] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arazi 2008 {published data only}
- Arazi SS, Genvigir FD, Willrich MA, Hirata MH, Dorea EL, Bernik M, et al. Atorvastatin effects on SREBF1a and SCAP gene expression in mononuclear cells and its relation with lowering‐lipids response. Clinica Chimica Acta 2008;393(2):119‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arca 2007a {published data only}
- Arca M, Cambuli VM, Montali A, Sentinelli F, Filippi E, Campagna F, et al. Serum adiponectin is decreased in patients with familial combined hyperlipidemia and normolipaemic relatives and is influenced by lipid‐lowering treatment. Nutrition, Metabolism, and Cardiovascular Diseases 2009; Vol. 19, issue 9:660‐6. [EMBASE: 19632099] [DOI] [PubMed]
- Arca M, Montali A, Pigna G, Antonini R, Antonini TM, Luigi P, et al. Comparison of atorvastatin versus fenofibrate in reaching lipid targets and influencing biomarkers of endothelial damage in patients with familial combined hyperlipidemia. Metabolism: Clinical & Experimental 2007;56(11):1534‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arca 2007b {published data only}
- Arca M, Natoli S, Micheletta F, Riggi S, Angelantonio E, Montali A, et al. Increased plasma levels of oxysterols, in vivo markers of oxidative stress, in patients with familial combined hyperlipidemia: reduction during atorvastatin and fenofibrate therapy. Free Radical Biology & Medicine 2007;42(5):698‐705. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ARIES 2006 {published data only}
- AstraZeneca. A 6‐week, randomized, open‐label, comparative study to evaluate the efficacy and safety of rosuvastatin and atorvastatin in the treatment of hypercholesterolemia in African‐American subjects. Protocol No. D3560L00022/4522US0002 2003.
- Ferdinand KC, Clark LT, Watson KE, Neal RC, Brown CD, Kong BW, et al. Comparison of efficacy and safety of rosuvastatin versus atorvastatin in African‐American patients in a six‐week trial. American Journal of Cardiology 2006;97(2):229‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ASSET 2001 {published data only}
- Insull W, Kafonek S, Goldner D, Zieve F. Comparison of efficacy and safety of atorvastatin (10 mg) with simvastatin (10 mg) at six weeks. American Journal of Cardiology 2001;87(5):554‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Insull W, Kafonek S, Goldner DB, Zieve F. Efficacy and safety of atorvastatin versus simvastatin in mixed dyslipidemic patients with and without type 2 diabetes during 54 weeks. Atherosclerosis Supplement 2001;2(2):87. [CENTRAL: CN‐00445855] [Google Scholar]
AstraZeneca 2010 {unpublished data only}
- AstraZeneca. A randomised, double‐blind trial to compare the efficacy of rosuvastatin 5 and 10 mg to atorvastatin 10 mg in the treatment of high risk patients with hypercholesterolemia followed by an open label treatment period with rosuvastatin up‐titrated to the maximum dose of 20 mg for those patients who do not achieve goal. NCT00683618 D356FC00007 2010.
ASTRO‐2 2009 {published data only}
- Yamazaki T, Kurabayashi M, ASTRO‐2 Study Group. A randomized controlled study to compare the effect of rosuvastatin 5mg with atorvastatin 10mg on plasma lipids in Japanese patients with hypercholesterolemia (ASTRO‐2). Annals of Vascular Diseases 2009;2(3):159‐73. [PUBMED: 23555376] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atalar 2002 {published data only}
- Atalar E, Ozmen F, Haznedaroglu I, Acil T, Ozer N, Ovunc K, et al. Effects of short‐term atorvastatin treatment on global fibrinolytic capacity, and sL‐selectin and sFas levels in hyperlipidemic patients with coronary artery disease. International Journal of Cardiology 2002;84(2‐3):227‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ATLANTIKA 2008 {published data only}
- Mareev VI. Atorvastatin in the treatment of high risk patients with ischemic heart disease and dyslipidemia. Safety assessment in the Russian Multicenter Study ATLANTIKA [in Russian]. Kardiologiia 2010; Vol. 50, issue 9:4‐14. [EMBASE: 21118160] [PubMed]
- Mareev VI, Belenkov IN, Oganov RG, Barbik‐Zhagar B, Mareev VI, Belenkov IN, et al. Atorvastatin in treatment of patients with coronary heart disease and dyslipidemia and high general risk: efficiency and safety estimation. Design and main results of ATLANTIKA [in Russian]. Kardiologiia 2008;48(11):4‐13. [MEDLINE: ] [PubMed] [Google Scholar]
ATOROS 2006 {published data only}
- Milionis HJ, Rizos E, Kostapanos M, Filippatos TD, Gazi IF, Ganotakis ES, et al. Treating to target patients with primary hyperlipidaemia: comparison of the effects of ATOrvastatin and ROSuvastatin (the ATOROS study). Current Medical Research & Opinion 2006;22(6):1123‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
AVALON 2006 {published data only}
- Cohn JN, Wilson DJ, Neutel J, Houston M, Weinberger MH, Grimm R Jr, et al. Co‐administered amlodipine and atorvastatin produces early improvements in arterial wall compliance in hypertensive patients with dyslipidemia. American Journal of Hypertension 2009; Vol. 22, issue 2:137‐44. [MEDLINE: ] [DOI] [PubMed]
- Messerli FH, Bakris GL, Ferrera D, Houston MC, Petrella RJ, Flack JM, et al. Efficacy and safety of co‐administered amlodipine and atorvastatin in patients with hypertension and dyslipidemia: results of the AVALON trial. Journal of Clinical Hypertension 2006;8(8):571‐81. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizer inc. A Multicenter, Randomized, Double‐Blind, Placebo‐Controlled and Open‐Label Evaluation of the Safety and Efficacy of Dual Therapy With Atorvastatin Plus Amlodipine When Compared to Either Therapy Alone in the Treatment of Patients With Simultaneous Hyperlipidemia and Hypertension (The Avalon Study). Protocol No. A3841001 2003.
Bach‐Ngohou 2005 {published data only}
- Bach‐Ngohou K, Ouguerram K, Frenais R, Maugere P, Ripolles‐Piquer B, Zair Y, et al. Influence of atorvastatin on apolipoprotein E and AI kinetics in patients with type 2 diabetes. Journal of Pharmacology & Experimental Therapeutics 2005;315(1):363‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bahadir 2009 {published data only}
- Bahadir MA, Oguz A, Uzunlulu M, Bahadir O, Bahadir MA, Oguz A, et al. Effects of different statin treatments on small dense low‐density lipoprotein in patients with metabolic syndrome. Journal of Atherosclerosis & Thrombosis 2009;16(5):684‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bakker‐Arkema 1996 {published data only}
- Bakker‐Arkema RG, Davidson MH, Goldstein RJ, Davignon J, Isaacsohn JL, Weiss SR, et al. Efficacy and safety of a new HMG‐CoA reductase inhibitor, atorvastatin, in patients with hypertriglyceridemia. JAMA 1996;275(2):128‐33. [MEDLINE: ] [PubMed] [Google Scholar]
- Bakker‐Arkema RG, Davidson MH, Goldstein RJ, Davignon J, Isaacsohn JL, Weiss SR, et al. Efficacy and safety of a new HMG‐CoA reductase inhibitor, atorvastatin, in patients with hypertriglyceridemia. Perfusion 1996;9(5):163‐8. [IDS Number: UP394] [PubMed] [Google Scholar]
- Le NA, Innis‐Whitehouse W, Li X, Bakker‐Arkema R, Black D, Brown WV. Lipid and apolipoprotein levels and distribution in patients with hypertriglyceridemia: effect of triglyceride reductions with atorvastatin. Metabolism 2000;49(2):167‐77. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Balakhonova 2002 {published data only}
- Balakhonova TV, Pogorelova OA, Susekov AV, Kobylianskii AG, Kuznetsova TV, Tvorogova MG, et al. Effect of atorvastatin on endothelial function in patients with familial hypercholesterolemia [in Russian]. Kardiologiia 2002;42(1):15‐21. [MEDLINE: ] [PubMed] [Google Scholar]
Ballantyne 2004 {published data only}
- Ballantyne C, Blazing M, King T, Brady W, Palmisano J. Efficacy of co‐administered ezetimibe plus simvastatin versus atorvastatin alone in adults with hypercholesterolemia. Atherosclerosis Supplements 2004;5(1):105. [IDS Number: 823UO] [Google Scholar]
- Ballantyne CM, Blazing MA, King TR, Brady WE, Palmisano J. Efficacy and safety of ezetimibe co‐administered with simvastatin compared with atorvastatin in adults with hypercholesterolemia. American Journal of Cardiology 2004;93(12):1487‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Merck, Co Inc. A Multicenter, Double‐Blind, Randomized, Parallel Group, 28‐Week Study to Evaluate the Efficacy and Safety of Ezetimibe and Simvastatin Co‐Administration Versus Atorvastatin in Patients With Hypercholesteremia. Merck Protocol Number: 025‐0 2003.
Barter 2000 {published data only}
- Barter PJ, O'Brien RC. Achievement of target plasma cholesterol levels in hypercholesterolaemic patients being treated in general practice. Atherosclerosis 2000;149(1):199‐205. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bays 2011 {published data only}
- Bays HE, Schwartz S, Littlejohn T III, Kerzner B, Krauss RM, Karpf DB, et al. MBX‐8025, a novel peroxisome proliferator receptor‐agonist: lipid and other metabolic effects in dyslipidemic overweight patients treated with and without atorvastatin. Journal of Clinical Endocrinology and Metabolism 2011;96(9):2889‐97. [EMBASE: 2011500391] [DOI] [PubMed] [Google Scholar]
Berthold 2004 {published data only}
- Berthold HK, Unverdorben S, Zittermann A, Degenhardt R, Baumeister B, Unverdorben M, et al. Age‐dependent effects of atorvastatin on biochemical bone turnover markers: a randomized controlled trial in postmenopausal women. Osteoporosis International 2004;15(6):459‐67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bertolami 2002 {published data only}
- Bertolami MC, Ramires JAF, Nicolau JC, Novazzi JP, Bodanese LC, Giannini SD. Open, randomized, comparative study of atorvastatin and simvastatin, after 12 weeks treatment, in patients with hypercholesterolemia alone or with combined hypertriglyceridemia [Comparacao da seguranca e eficacia da atorvastatina com a sinvastatina apos doze semanas, em portadores de hipercolesterolemia pura ou associada a hipertrigliceridemia]. Revista Brasileira de Medicina 2002;59(8):577‐84. [EMBASE: 2002368925] [Google Scholar]
Best 1996 {published data only}
- Best JD, Micholson GC, O'Neal DN, Kotowicz MA, Tebbutt NC, Chan K‐W, et al. Atorvastatin and simvastatin reduce elevated cholesterol in non‐insulin dependent diabetes. Diabetes, Nutrition and Metabolism 1996;9(2):74‐80. [EMBASE: 1996183161] [Google Scholar]
Bevilacqua 2004 {published data only}
- Bevilacqua M, Guazzini B, Righini V, Barrella M, Toscano R, Chebat E. Metabolic effects of fluvastatin extended release 80 mg and atorvastatin 20 mg in patients with type 2 diabetes mellitus and low serum high‐density lipoprotein cholesterol levels: a 4‐month, prospective, open‐label, randomized, blinded ‐ end point (probe) trial. Current Therapeutic Research Clinical and Experimental 2004;65(4):330‐44. [EMBASE: 2004439205] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blagden 2007 {published data only}
- Blagden MD, Chipperfield R, Blagden MD, Chipperfield R. Efficacy and safety of ezetimibe co‐administered with atorvastatin in untreated patients with primary hypercholesterolaemia and coronary heart disease. Current Medical Research & Opinion 2007;23(4):767‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bloomfield 2009 {published data only}
- Bloomfield D, Carlson GL, Sapre A, Tribble D, McKenney JM, Littlejohn TW III, et al. Efficacy and safety of the cholesteryl ester transfer protein inhibitor anacetrapib as monotherapy and coadministered with atorvastatin in dyslipidemic patients. American Heart Journal 2009;157(2):352‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bo 2001 {published data only}
- Bo M, Nicolello MT, Fiandra U, Mercadante G, Piliego T, Fabris F. Treatment of heterozygous familial hypercholesterolemia: atorvastatin versus simvastatin. Atherosclerosis Supplement 2001;2(2):97‐8. [CENTRAL: CN‐00444460] [PubMed] [Google Scholar]
- Bo M, Nicolello MT, Fiandra U, Mercadante G, Piliego T, Fabris F. Treatment of heterozygous familial hypercholesterolemia: atorvastatin vs simvastatin. Nutrition Metabolism and Cardiovascular Disease 2001;11(1):17‐24. [MEDLINE: ] [PubMed] [Google Scholar]
Bogsrud 2013 {published data only}
- Bogsrud MP, Langslet G, Ose L, Arnesen KE, Stuen MC, Malt UF, et al. No effect of combined coenzyme Q10 and selenium supplementation on atorvastatin‐induced myopathy. Scandinavian Cardiovascular Journal 2013;47(2):80‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Branchi 1999 {published data only}
- Branchi A, Fiorenza AM, Rovelli A, Torri A, Muzio F, Macor S, et al. Lowering effects of four different statins on serum triglyceride level. European Journal of Clinical Pharmacology 1999;55(7):499‐502. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Branchi 2001 {published data only}
- Branchi A, Fiorenza AM, Torri A, Muzio F, Berra C, Colombo E, et al. Effects of low doses of simvastatin and atorvastatin on high‐density lipoprotein cholesterol levels in patients with hypercholesterolemia. Clinical Therapeutics 2001;23(6):851‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Branchi A, Fiorenza AM, Torri A, Muzio F, Rovellini A, Berra C, et al. Effect of atorvastatin 10 mg and of simvastatin 20 mg on serum triglyceride level. Atherosclerosis 2000;151(1):47. [CENTRAL: CN‐00444522] [Google Scholar]
- Branchi A, Fiorenza AM, Torri A, Muzio F, Rovellini A, Berra C, et al. Effects of atorvastatin 10 mg and simvastatin 20 mg on serum triglyceride levels in patients with hypercholesterolemia. Current Therapeutic Research 2001;62(5):408‐15. [EMBASE: 2001204501] [Google Scholar]
Branchi 2002 {published data only}
- Branchi A, Fiorenza AM, Torri A, Muzio F, Colombo E, Valle ED, et al. Atorvastatin increases HDL cholesterol in hypercholesterolemic patients. Evidence of a relationship with baseline HDL cholesterol. Nutrition Metabolism & Cardiovascular Disease 2002;12(1):24‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Broncel 2005 {published data only}
- Broncel M, Marczyk I, Chojnowska‐Jezierska J, Michalska M, Sikora J, Kostka B. The comparison of simvastatin and atorvastatin effects on hemostatic parameters in patients with hyperlipidemia type II [Porownanie wplywu simwastatyny i atorwastatyny na wybrane parametry ukladu krzepniecia u chorych z hiperlipidemia typu II]. Polski Merkuriusz Lekarski 2005;18(106):380‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Brown 1998 {published data only}
- Brown AS, Bakker‐Arkema RG, Yellen L, Henley RW Jr, Guthrie R, Campbell CF, et al. Treating patients with documented atherosclerosis to National Cholesterol Education Program‐recommended low‐density‐lipoprotein cholesterol goals with atorvastatin, fluvastatin, lovastatin and simvastatin. Journal of the American College of Cardiology 1998;32(3):665‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bruni 2003 {published data only}
- Bruni F, Puccetti L, Pasqui AL, Pastorelli M, Bova G, Cercignani M, et al. Different effect induced by treatment with several statins on monocyte tissue factor expression in hypercholesterolemic subjects. Clinical & Experimental Medicine 2003;3(1):45‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bruni 2004 {published data only}
- Bruni F, Pasqui AL, Pastorelli M, Bova G, Renzo M, Cercignani M, et al. Effect of atorvastatin on different fibrinolysis mechanisms in hypercholesterolemic subjects. International Journal of Cardiology 2004;95(2‐3):269‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bruni 2005 {published data only}
- Bruni F, Pasqui AL, Pastorelli M, Bova G, Cercignani M, Palazzuoli A, et al. Different effect of statins on platelet oxidized‐LDL receptor (CD36 and LOX‐1) expression in hypercholesterolemic subjects. Clinical & Applied Thrombosis/Hemostasis 2005;11(4):417‐28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Budinski 2009 {published data only}
- Budinski D, Arneson V, Hounslow N, Gratsiansky N. Pitavastatin compared with atorvastatin in primary hypercholesterolemia or combined dyslipidemia. Clinical Lipidology 2009;4(3):291‐302. [IDS Number: 455FM] [Google Scholar]
Buldak 2012 {published data only}
- Buldak L, DulawaBuldak A, Labuzek K, Okopien B. Effects of 90‐day hypolipidemic treatment on insulin resistance, adipokines and proinflammatory cytokines in patients with mixed hyperlipidemia and impaired fasting glucose. International Journal of Clinical Pharmacology & Therapeutics 2012;50(11):805‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CAP 2008 {published data only}
- Bonnet J, McPherson R, Tedgui A, Simoneau D, Nozza A, Martineau P, et al. Comparative effects of 10‐mg versus 80‐mg atorvastatin on high‐sensitivity C‐reactive protein in patients with stable coronary artery disease: results of the CAP (Comparative Atorvastatin Pleiotropic effects) study. Clinical Therapeutics 2008;30(12):2298‐313. [EMBASE: 2009030462] [DOI] [PubMed] [Google Scholar]
- McPherson R, Bonnet J, Tedgui A, Martineau P, Simoneau D, Nozza A, et al. Atorvastatin's effect on inflammatory markers (hsCRP, IL‐6 and IL‐18) in the comparative atorvastatin pleiotropic effects (CAP) study. Canadian Journal of Cardiology 2007;23 Suppl C:222C‐3C. [PREV200800219825] [Google Scholar]
- Pfizer Inc. A Multicenter, Randomized Double‐Blind Study Comparing the Pleiotropic Effects of Atorvastatin 10 mg and 80 mg Over a 26‐Week Period in Subjects With Coronary Atherosclerosis (CAP: Comparative Atorvastatin Pleiotropic Effects). NCT00163202 Protocol No. A2581065 2005.
Castano 2003a {published data only}
- Castano G, Mas R, Fernandez L, Illnait J, Mesa M, Alvarez E, et al. Comparison of the efficacy and tolerability of policosanol with atorvastatin in elderly patients with type II hypercholesterolaemia. Drugs & Aging 2003;20(2):153‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Castano 2003b {published data only}
- Castano G, Fernandez L, Mas R, Illnait J, Mesa M, Fernandez JC. Comparison of the effects of policosanol and atorvastatin on lipid profile and platelet aggregation in patients with dyslipidaemia and type 2 diabetes mellitus. Clinical Drug Investigation 2003;23(10):639‐50. [EMBASE: 2003424476] [DOI] [PubMed] [Google Scholar]
Castro 2008 {published data only}
- Castro PF, Miranda R, Verdejo HE, Greig D, Gabrielli LA, Alcaino H, et al. Pleiotropic effects of atorvastatin in heart failure: role in oxidative stress, inflammation, endothelial function, and exercise capacity. Journal of Heart & Lung Transplantation 2008;27(4):435‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Catalano 2009 {published data only}
- Catalano G, Julia Z, Frisdal E, Vedie B, Fournier N, Goff W, et al. Torcetrapib differentially modulates the biological activities of HDL2 and HDL3 particles in the reverse cholesterol transport pathway. Arteriosclerosis, Thrombosis & Vascular Biology 2009;29(2):268‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cerda 2010 {published data only}
- Cerda A, Genvigir FDV, Arazi SS, Hirata MH, Dorea EL, Bernik MMS, et al. Influence of SCARB1 polymorphisms on serum lipids of hypercholesterolemic individuals treated with atorvastatin. Clinica Chimica Acta; International Journal of Clinical Chemistry 2010;411(9‐10):631‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cerda A, Genvigir FDV, Rodrigues AC, Willrich MAV, Dorea EL, Bernik MMS, et al. Influence of polymorphisms and cholesterol‐lowering treatment on SCARB1 mRNA expression. Journal of Atherosclerosis and Thrombosis 2011;18(8):640‐51. [EMBASE: 2011474438] [DOI] [PubMed] [Google Scholar]
CEZAR 2009 {published data only}
- Ostad Mir A, Eggeling S, Tschentscher P, Schwedhelm E, Boger R, Wenzel P, et al. Flow‐mediated dilation in patients with coronary artery disease is enhanced by high dose atorvastatin compared to combined low dose atorvastatin and ezetimibe: results of the CEZAR study. Atherosclerosis 2009;205(1):227‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CHALLENGE 2002 {published data only}
- Karalis DG, Ross AM, Vacari RM, Zarren H, Kafonek S, Tambone L. Comparing the efficacy of atorvastatin vs simvastatin in hypercholesterolemic patients with and without CHD. Atherosclerosis Supplement 2001;2(2):95. [CENTRAL: CN‐00446012] [Google Scholar]
- Karalis DG, Ross AM, Vacari RM, Zarren H, Scott R. Comparison of efficacy and safety of atorvastatin and simvastatin in patients with dyslipidemia with and without coronary heart disease. American Journal of Cardiology 2002;89(6):667‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Karalis DG, Ross AM, Vacari RM, Zarren H, Scott R. Efficacy and safety of atorvastatin and simvastatin. Cardiology Review 2003;20(2):30‐3. [EMBASE: 2003099804] [DOI] [PubMed] [Google Scholar]
Chan 2002 {published data only}
- Chan DC, Nguyen MN, Watts GF, Ooi EM, Barrett PHR. Effects of atorvastatin and n‐3 fatty acid supplementation on VLDL apolipoprotein C‐III kinetics in men with abdominal obesity. The American Journal of Clinical Nutrition. United States: Metabolic Research Centre School of MedicinePharmacology Royal Perth Hospital University of Western Australia Perth Western Australia., 2010; Vol. 91, issue 4:900‐6. [MEDLINE: ] [DOI] [PubMed]
- Chan DC, Watts GF, Barrett PH, Beilin LJ, Mori TA. Effect of atorvastatin and fish oil on plasma high‐sensitivity C‐reactive protein concentrations in individuals with visceral obesity. Clinical Chemistry 2002;48(6):877‐83. [MEDLINE: ] [PubMed] [Google Scholar]
- Chan DC, Watts GF, Barrett PH, Beilin LJ, Redgrave TG, Mori TA. Regulatory effects of HMG CoA reductase inhibitor and fish oils on apolipoprotein B‐100 kinetics in insulin‐resistant obese male subjects with dyslipidemia. Diabetes 2002;51(8):2377‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chan DC, Watts GF, Barrett PH, Mori TA, Beilin LJ, Redgrave TG. Mechanism of action of a 3‐hydroxy‐3‐methylglutaryl coenzyme a reductase inhibitor on apolipoprotein B‐100 kinetics in visceral obesity. Journal of Clinical Endocrinology & Metabolism 2002;87(5):2283‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chan DC, Watts GF, Barrett PHR, Martins IJ, James AP, Mamo JCL, et al. Effect of atorvastatin on chylomicron remnant metabolism in visceral obesity: a study employing a new stable isotope breath test. Journal of Lipid Research 2002;43(5):706‐12. [MEDLINE: ] [PubMed] [Google Scholar]
- Chan DC, Watts GF, Mori TA, Barrett PH, Beilin LJ, Redgrave TG. Factorial study of the effects of atorvastatin and fish oil on dyslipidaemia in visceral obesity. European Journal of Clinical Investigation 2002;32(6):429‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chan 2008 {published data only}
- Chan KC, Chou HH, Huang CN, Chou MC. Atorvastatin administration after percutaneous coronary intervention in patients with coronary artery disease and normal lipid profiles: impact on plasma adiponectin level. Clinical Cardiology 2008;31(6):253‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen F, Maccubbin D, Yan L, Sirah W, Chen E, Sisk CM, et al. Lipid‐altering efficacy and safety profile of co‐administered extended release niacin/laropiprant and simvastatin versus atorvastatin in patients with mixed hyperlipidemia. International Journal of Cardiology 2013;167(1):225‐31. [EMBASE: 2013353165] [DOI] [PubMed] [Google Scholar]
CHESS 2003 {published data only}
- Ballantyne CM, Blazing MA, Hunninghake DB, Davidson MH, Yuan Z, DeLucca P, et al. Effect on high‐density lipoprotein cholesterol of maximum dose simvastatin and atorvastatin in patients with hypercholesterolemia: results of the Comparative HDL Efficacy and Safety Study (CHESS). American Heart Journal 2003;146(5):862‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ballantyne CM, Hustad CM, Yuan Z, DeLucca P, Palmisano J. Efficacy and safety of simvastatin versus atorvastatin: results of the Comparative HDL‐C Efficacy and Safety Study (Chess). Atherosclerosis Supplement 2002;3(2):72. [CENTRAL: CN‐00444318] [Google Scholar]
CHEST 2003 {published data only}
- Ansell BJ, Watson KE, Weiss RE, Fonarow GC. hsCRP and HDL effects of statins trial (CHEST): rapid effect of statin therapy on C‐reactive protein and high‐density lipoprotein levels A clinical investigation. Heart Disease 2003;5(1):2‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CHIBA 2008 {published data only}
- Yokote K, Bujo H, Hanaoka H, Shinomiya M, Mikami K, Miyashita Y, et al. Multicenter collaborative randomized parallel group comparative study of pitavastatin and atorvastatin in Japanese hypercholesterolemic patients. Collaborative study on hypercholesterolemia drug intervention and their benefits for atherosclerosis prevention (CHIBA study). Atherosclerosis 2008;201(2):345‐52. [EMBASE: 2008556198] [DOI] [PubMed] [Google Scholar]
- Yokote K, Bujo H, Hanaoka H, Shinomiya M, Mikami K, Shirai K, et al. A randomized, open‐label trial comparing the efficacy and safety of pitavastatin with atorvastatin in Japanese patients with hypercholesterolemia. Diabetes 2007;56 Suppl 1:A233. [PREV200700477138] [Google Scholar]
- Yokote K, Saito Y, CHIBA. Influence of statins on glucose tolerance in patients with type 2 diabetes mellitus: subanalysis of the collaborative study on hypercholesterolemia drug intervention and their benefits for atherosclerosis prevention (CHIBA study). Journal of Atherosclerosis and Thrombosis. Japan, 2009; Vol. 16, issue 3:297‐8. [MEDLINE: ] [DOI] [PubMed]
Cho 2011 {published data only}
- Cho YK, Hur SH, Han CD, Park HS, Yoon HJ, Kim H, et al. Comparison of ezetimibe/simvastatin 10/20 mg versus atorvastatin 20 mg in achieving a target low density lipoprotein‐cholesterol goal for patients with very high risk. Korean Circulation Journal 2011;41(3):149‐53. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chu 2006a {published data only}
- Chu CS, Lee KT, Lee MY, Su HM, Voon WC, Sheu SH, et al. Effects of rosiglitazone alone and in combination with atorvastatin on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. American Journal of Cardiology 2006;97(5):646‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chu 2006b {published data only}
- Chu CS, Kou HS, Lee CJ, Lee KT, Chen SH, Voon WC, et al. Effect of atorvastatin withdrawal on circulating coenzyme Q10 concentration in patients with hypercholesterolemia. Biofactors 2006;28(3‐4):177‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chu 2006c {published data only}
- Chu CS, Lee KT, Lee MY, Su HM, Voon WC, Sheu SH, et al. Effects of atorvastatin and atorvastatin withdrawal on soluble CD40L and adipocytokines in patients with hypercholesterolaemia. Acta Cardiologica 2006;61(3):263‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chu 2007 {published data only}
- Chu CS, Lee KT, Lee ST, Lu YH, Lin TH, Voon WC, et al. Effects of atorvastatin on ventricular late potentials and repolarization dispersion in patients with hypercholesterolemia. Kaohsiung Journal of Medical Sciences 2007;23(5):217‐24. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Claeys 2004 {published data only}
- Claeys MJ, Cosyns B, Hoffer E, Carlier M, Missault L, Cools F, et al. Short‐term effect of atorvastatin on ischaemic threshold in hypercholesterolaemic patients with stable ischaemic heart disease. Acta Cardiologica 2004;59(3):269‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
COMETS 2005 {published data only}
- Schuster H. Does rosuvastatin improve lipid levels in patients with the metabolic syndrome more effectively than atorvastatin?. Nature Clinical Practice Cardiovascular Medicine 2006;3(2):74‐5. [EMBASE: 2006071305] [DOI] [PubMed] [Google Scholar]
- Stalenhoef AF, Ballantyne CM, Sarti C, Murin J, Tonstad S, Rose H, et al. A comparative study with rosuvastatin in subjects with metabolic syndrome: results of the COMETS study. European Heart Journal 2005;26(24):2664‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CORALL 2005 {published data only}
- Simsek S, Schalkwijk CG, Wolffenbuttel BH. Effects of rosuvastatin and atorvastatin on glycaemic control in Type 2 diabetes‐‐‐the CORALL study: associated included citation for the CORALL 2005 trial. Diabetic Medicine 2012;29(5):628‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wolffenbuttel BH, Franken AA, Vincent HH, Dutch Corall Study Group. Cholesterol‐lowering effects of rosuvastatin compared with atorvastatin in patients with type 2 diabetes ‐ CORALL study. Journal of Internal Medicine 2005;257(6):531‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Crouse III 1999 {published data only}
- Crouse III Jr, Frohlich J, Ose L, Mercuri M, Tobert JA. Effects of high doses of simvastatin and atorvastatin on high‐density lipoprotein cholesterol and apolipoprotein A‐1. American Journal of Cardiology 1999;83(10):1476‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wiklund O, Mattsson‐Hulten L, Hurt‐Camejo E, Oscarsson J. Effects of simvastatin and atorvastatin on inflammation markers in plasma. Journal of Internal Medicine 2002;251(4):338‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cubeddu 2006 {published data only}
- Cubeddu LX, Cubeddu RJ, Heimowitz T, Restrepo B, Lamas GA, Weinberg GB. Comparative lipid‐lowering effects of policosanol and atorvastatin: a randomized, parallel, double‐blind, placebo‐controlled trial. American Heart Journal 2006;152(5):982.e1‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CURVES 1998 {published data only}
- Jones P, Kafonek S, Laurora I, Hunninghake D. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin and fluvastatin in patients with hypercholesterolemia (the CURVES study). American Journal of Cardiology 1998;81(5):582‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kafonek S, CURVES Investigators. The CURVES study: a comparison of the dose efficacy of atorvastatin with simvastatin, pravastatin, lovastatin, and fluvastatin. XIII WORLD CONGRESS OF CARDIOLOGY. 1998:1003‐7. [IDS Number: BL74L]
DALI 2001 {published data only}
- Diabetes Atorvastin Lipid Intervention (DALI) Study Group. The effect of aggressive versus standard lipid lowering by atorvastatin on diabetic dyslipidemia: the DALI study: a double‐blind, randomized, placebo‐controlled trial in patients with type 2 diabetes and diabetic dyslipidemia. Diabetes Care 2001;24(8):1335‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Davidson 2002 {published data only}
- AstraZeneca. A 12 week, randomized, double‐blind, placebo‐controlled, multicenter trial to evaluate the efficacy and safety of ZD4522 5 mg and 10 mg and atorvastatin 10 mg in the treatment of subjects with hypercholesterolemia (4522IL/0024). Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research Office of Biostatistics 1999‐2000:12‐7. [NDA 21‐366]
- Blasetto JW, Stein EA, Brown WV, Chitra R, Raza A. Efficacy of rosuvastatin compared with other statins at selected starting doses in hypercholesterolemic patients and in special population groups. American Journal of Cardiology 2003;91 Suppl 5A:3C‐10C. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Davidson M, Ma P, Stein E, Hutchinson HG, Caplan R, Raza A, et al. Rosuvastatin is more effective than atorvastatin at improving the lipid profiles of patients with primary hypercholesterolaemia. Atherosclerosis Supplements 2001;2(2):38‐9. [CENTRAL: CN‐00445003] [Google Scholar]
- Davidson M, Ma P, Stein EA, Gotto AM Jr, Raza A, Chitra R, et al. Comparison of effects on low‐density lipoprotein cholesterol and high‐density lipoprotein cholesterol with rosuvastatin versus atorvastatin in patients with type IIa or IIb hypercholesterolemia. American Journal of Cardiology 2002;89(3):268‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Della‐Morte 2011 {published data only}
- Della‐Morte D, Moussa I, Elkind MS, Sacco RL, Rundek T. The short‐term effect of atorvastatin on carotid plaque morphology assessed by computer‐assisted gray‐scale densitometry: a pilot study. Neurological Research 2011;33(9):991‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratchford EV, Gutierrez J, Lorenzo D, McClendon, Rundek T. Short‐term effect of atorvastatin on carotid artery elasticity: a pilot study. Stroke 2011;42(12):3460‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Demir 2001 {published data only}
- Demir M, Acarturk E, Sasmaz I, Cayli M, Kilinc Y. Effects of atorvastatin on lipid profile and coagulation parameters. Current Therapeutic Research Clinical and Experimental 2001;62(10):691‐8. [493FT] [Google Scholar]
Despres 2002 {published data only}
- Delaval D, Despres JP, Salomon H. Micronized fenofibrate compared to atorvastatin in low HDL‐cholesterol patients (<40 mg/dl). Atherosclerosis Supplements 2001;2(2):48‐9. [CENTRAL: CN‐00445059] [Google Scholar]
- Despres JP, Lemieux I, Salmon H, Delaval D. Effects of micronized fenofibrate versus atorvastatin in the treatment of dyslipidaemic patients with low plasma HDL‐cholesterol levels: a 12 week randomized trial. Journal of Internal Medicine 2002;251(6):490‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Diepeveen 2005 {published data only}
- Diepeveen SH, Verhoeven GW, Palen J, Dikkeschei LD, Tits LJ, Kolsters G, et al. Effects of atorvastatin and vitamin E on lipoproteins and oxidative stress in dialysis patients: a randomised‐controlled trial. Journal of Internal Medicine 2005;257(5):438‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
DISCOVERY 2005 {unpublished data only}
- AstraZeneca. An open‐label, randomised, multi‐centre, phase IIIb/IV, parallel group study to compare the efficacy and safety of rosuvastatin and atorvastatin in subjects with type IIa and IIb hypercholesterolaemia (DISCOVERY). Protocol No. D3560L00009 2005.
DISCOVERY ALPHA 2006 {published data only}
- Binbrek AS, Elis A, Al‐Zaibag M, Eha J, Keber I, Cuevas AM, et al. Rosuvastatin versus atorvastatin in achieving lipid goals in patients at high risk for cardiovascular disease in clinical practice: a randomized, open‐label, parallel‐group, multicenter study (DISCOVERY alpha study). Current Therapeutic Research Clinical and Experimental 2006;67(1):21‐43. [IDS Number: 024LM] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dobreanu 2007 {published data only}
- Dobreanu M, Dobreanu D, Fodor A, Bacarea A, Dobreanu M, Dobreanu D, et al. Integrin expression on monocytes and lymphocytes in unstable angina short term effects of atorvastatin. Romanian Journal of Internal Medicine 2007;45(2):193‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Dogra 2007 {published data only}
- Dogra G, Irish A, Chan D, Watts G, Dogra G, Irish A, et al. A randomized trial of the effect of statin and fibrate therapy on arterial function in CKD. American Journal of Kidney Diseases 2007;49(6):776‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ECLIPSE 2008 {published data only}
- AstraZeneca. A 24‐Week, Randomised, Open‐Label, Parallel‐Group, Multicentre Study Which Compares the Efficacy and Safety of Rosuvastatin 10, 20 and 40 mg With Atorvastatin 10, 20, 40 and 80 mg When Force‐Titrated in the Treatment of Patients With Primary Hypercholesterolemia and Either a History of Coronary Heart Disease (CHD) or Clinical Evidence of Atherosclerosis or a CHD Risk Equivalent (10‐Year Risk Score >20%) ECLIPSE ‐ An Evaluation to Compare Lipid‐Lowering Effects of Rosuvastatin and Atorvastatin In Force‐Titrated Patients: a Prospective Study of Efficacy and Tolerability. Protocol No. D3569C00002 2005.
- Faergeman O, Hill L, Windler E, Wiklund O, Asmar R, Duffield E, et al. Efficacy and tolerability of rosuvastatin and atorvastatin when force‐titrated in patients with primary hypercholesterolemia: results from the ECLIPSE study. Cardiology 2008;111(4):219‐28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Economides 2004 {published data only}
- Economides PA, Caselli A, Tiani E, Khaodhiar L, Horton ES, Veves A. The effects of atorvastatin on endothelial function in diabetic patients and subjects at risk for type 2 diabetes. Journal of Clinical Endocrinology & Metabolism 2004;89(2):740‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Farnier 2000 {published data only}
- Farnier M, Portal JJ, Maigret P. Efficacy of atorvastatin compared with simvastatin in patients with hypercholesterolemia. Journal of Cardiovascular Pharmacology and Therapeutics 2000;5(1):27‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ferreira 2007 {published data only}
- Ferreira GA, Navarro TP, Telles RW, Andrade LE, Sato EI. Atorvastatin therapy improves endothelial‐dependent vasodilation in patients with systemic lupus erythematosus: an 8 week controlled trial. Rheumatology 2007;46(10):1560‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Franiak‐Pietryga 2009 {published data only}
- Franiak‐Pietryga I, Balcerak M, Sikora J, Duchnowicz P, Koter‐Michalak M, Stetkiewicz T, et al. The effect of atorvastatin on the erythrocyte plasma membrane and C‐reactive protein in menopausal women with metabolic syndrome. Przeglad Menopauzalny 2009;13(4):233‐8. [EMBASE: 2009617064] [Google Scholar]
Fu 2013 {published data only}
- Fu Q, Li YP, Gao Y, Yang SH, Lu PQ, Jia M, et al. Lack of association between SLCO1B1 polymorphism and the lipid‐lowering effects of atorvastatin and simvastatin in Chinese individuals. European Journal of Clinical Pharmacology 2013;69(6):1269‐74. [DOI] [PubMed] [Google Scholar]
- Li YP, Zhang LR, Jia M, Hu XJ. CYP3AP1 [superscript] star3 Allele Is Associated With Lipid‐Lowering Efficacy of Simvastatin and Atorvastatin in Chinese Women. Journal of Clinical Pharmacology 2011;51(2):181‐8. [International Pharmaceutical Abstracts Accession Number is 48‐04778] [DOI] [PubMed] [Google Scholar]
Geiss 2001 {published data only}
- Geiss HC, Otto C, Schwandte P, Parhofer KG. Effect of atorvastatin on low‐density lipoprotein subtypes in patients with different forms of hyperlipoproteinemia and control subjects. Metabolism Clinical & Experimental 2001;50(8):983‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gokkaya 2008 {published data only}
- Gokkaya SC, Ozden C, Levent OO, Hakan KH, Guzel O, Memis A, et al. Effect of correcting serum cholesterol levels on erectile function in patients with vasculogenic erectile dysfunction. Scandinavian Journal of Urology & Nephrology 2008;42(5):437‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goldberg 2009 {published data only}
- Goldberg AC, Bays HE, Ballantyne CM, Kelly MT, Buttler SM, Setze CM, et al. Efficacy and safety of ABT‐335 (fenofibric acid) in combination with atorvastatin in patients with mixed dyslipidemia. American Journal of Cardiology 2009;103(4):515‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mohiuddin SM, Thakker KM, Setze CM, Kelly MT. Evaluating optimal lipid levels in patients with mixed dyslipidemia following short‐ and long‐term treatment with fenofibric acid and statin combination therapy: a post hoc analysis. Current Medical Research & Opinion 2011;27(5):1067‐78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goudevenos 2000 {published data only}
- Goudevenos JA, Bairaktari ET, Chatzidimou KG, Milionis HJ, Mikhailidis DP, Elisaf MS. The effect of atorvastatin on serum lipids lipoprotein (a) and plasma fibrinogen levels in primary dyslipidaemia ‐ a pilot study involving serial sampling. Current Medical Research and Opinion 2000;16(4):269‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grossman 2000 {unpublished data only}
- Grossman SS. The effect of atorvastatin on blood glucose levels in type 2 diabetic patients. DAI‐B 61/07 2000:p. 3531. [ProQuest document ID: 727719711]
Guerin 2000 {published data only}
- Guerin M, Lassel TS, Goff W, Farnier M, Chapman MJ. Action of atorvastatin in combined hyperlipidemia: preferential reduction of cholesteryl ester transfer from HDL to VLDL1 particles. Arteriosclerosis, Thrombosis & Vascular Biology 2000;20(1):189‐97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guerin 2002 {published data only}
- Guerin M, Egger P, Goff W, Soudant C, Dupuis R, Chapman MJ. Atorvastatin reduces postprandial accumulation and cholesteryl ester transfer protein‐mediated remodeling of triglyceride‐rich lipoprotein subspecies in type IIB hyperlipidemia. Journal of Clinical Endocrinology and Metabolism 2002;87(11):4991‐5000. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Guerin M, Egger P, Soudant C, Goff W, Tol A, Dupuis R, et al. Dose‐dependent action of atorvastatin in type IIB hyperlipidemia: preferential and progressive reduction of atherogenic apo‐B‐containing lipoprotein subclasses (VLDL‐2, IDL, small dense LDL) and stimulation of cellular cholesterol efflux. Atherosclerosis 2002;163(2):287‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guerin 2008 {published data only}
- Guerin M, Goff W, Duchene E, Julia Z, Nguyen T, Thuren T. Inhibition of CETP by torcetrapib attenuates the atherogenicity of postprandial TG‐rich lipoproteins in type IIB hyperlipidemia. Arteriosclerosis, Thrombosis & Vascular Biology 2008;28(1):148‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gumprecht 2011 {published data only}
- Gumprecht J, Gosho M, Budinski D, Hounslow N. Comparative long‐term efficacy and tolerability of pitavastatin 4 mg and atorvastatin 20‐40 mg in patients with type 2 diabetes mellitus and combined (mixed) dyslipidaemia. Diabetes, Obesity and Metabolism 2011;13(11):1047‐55. [EMBASE: 2011546830] [DOI] [PubMed] [Google Scholar]
Guo 2013 {published data only}
- Guo YL, Liu J, Xu RX, Zhu CG, Wu NQ, Jiang LX, et al. Short‐term impact of low‐dose atorvastatin on serum proprotein convertase subtilisin/kexin type 9. Clinical Drug Investigation 2013;33(12):877‐83. [EMBASE: 2013791565] [DOI] [PubMed] [Google Scholar]
Han 2008 {published data only}
- Han H, Xue J, Zhang J‐Y, Xu F‐H, Wang Y‐H. Efficacy and safety of rosuvastatin and atorvastatin in aged patients with hypercholesterolemia. Chinese Journal of New Drugs and Clinical Remedies 2008;27(2):120‐3. [PREV200800281522] [Google Scholar]
Harangi 2009 {published data only}
- Harangi M, Mirdamadi HZ, Seres I, Sztanek F, Molnar M, Kassai A, et al. Atorvastatin effect on the distribution of high‐density lipoprotein subfractions and human paraoxonase activity. Translational Research: The Journal of Laboratory & Clinical Medicine 2009;153(4):190‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
HD‐ROWS 2012 {published data only}
- Backes JM, Gibson CA, Ruisinger JF, Moriarty PM. The high‐dose rosuvastatin once weekly study (the HD‐ROWS). Journal of Clinical Lipidology 2012;6(4):362‐7. [EMBASE: 2012436875] [DOI] [PubMed] [Google Scholar]
HeFH 2003 {published data only}
- Stein E, Strutt KL, Miller E, Southworth H. Rosuvastatin (20, 40 and 80 mg) reduces LDL‐C, raises HDL‐C and achieves treatment goals more effectively than atorvastatin (20, 40 and 80 mg) in patients with heterozygous familial hypercholesterolaemia [abstract]. Atherosclerosis Supplements 2001;2(2):90‐1. [CENTRAL: CN‐00447845] [Google Scholar]
- Stein EA, Strutt K, Southworth H, Diggle PJ, Miller E, HeFH Study Group. Comparison of rosuvastatin versus atorvastatin in patients with heterozygous familial hypercholesterolemia. American Journal of Cardiology 2003;92(11):1287‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Her 2010 {published data only}
- Her AY, Kim JY, Kang SM, Choi D, Jang Y, Chung N, et al. Effects of atorvastatin 20 mg, rosuvastatin 10 mg, and atorvastatin/ezetimibe 5 mg/5 mg on lipoproteins and glucose metabolism. Journal of Cardiovascular Pharmacology and Therapeutics 2010;15(2):167‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hernandez 2011 {published data only}
- Hernandez C, Francisco G, Ciudin A, Chacon P, Montoro B, Llaverias G, et al. Effect of atorvastatin on lipoprotein (a) and interleukin‐10: a randomized placebo‐controlled trial. Diabetes & Metabolism 2011;37(2):124‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Herregods 2008 {published data only}
- AstraZeneca. An open‐label, randomised, multi‐centre, phase IIIb, parallel group study to compare the efficacy and safety of rosuvastatin and atorvastatin in subjects with type IIa and IIb hypercholesterolaemia. Protocol No. D3560L00011 2004.
- Herregods MC, Daubresse JC, Michel G, Lamotte M, Vissers E, Vandenhoven G. Discovery Belux: comparison of rosuvastatin with atorvastatin in hypercholesterolaemia. Acta Cardiologica 2008;63(4):493‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hogue 2008a {published data only}
- Hogue JC, Lamarche B, Deshaies Y, Tremblay AJ, Bergeron J, Gagne C, et al. Differential effect of fenofibrate and atorvastatin on in vivo kinetics of apolipoproteins B‐100 and B‐48 in subjects with type 2 diabetes mellitus with marked hypertriglyceridemia. Metabolism Clinical & Experimental 2008;57(2):246‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hogue 2008b {published data only}
- Hogue JC, Lamarche B, Tremblay AJ, Bergeron J, Gagne C, Couture P, et al. Differential effect of atorvastatin and fenofibrate on plasma oxidized low‐density lipoprotein, inflammation markers, and cell adhesion molecules in patients with type 2 diabetes mellitus. Metabolism: Clinical & Experimental 2008;57(3):380‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hoogerbrugge 1998 {published data only}
- Hoogerbrugge N. Effects of atorvastatin on serum lipids of patients with familial hypercholesterolaemia. Journal of Internal Medicine 1998;244(2):143‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hoogerbrugge 1999 {published data only}
- Hoogerbrugge N, Jansen H. Atorvastatin increases low‐density lipoprotein size and enhances high‐density lipoprotein cholesterol concentration in male, but not in female patients with familial hypercholesterolemia. Atherosclerosis 1999;146(1):167‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Huang 2012 {published data only}
- Huang ZQ, Wu YT, Wang R, Huang W, Chen L. Effects of pitavastatin and atorvastatin on blood lipids and blood glucose in elderly type 2 diabetic patients. Zhongguo Xinyao yu Linchuang Zazhi 2012;31(10):614‐7. [BIOSIS:PREV201300107728] [Google Scholar]
Hufnagel 2000 {published data only}
- Hufnagel G, Michel C, Vrtovsnik F, Queffeulou G, Kossari N, Mignon F. Effects of atorvastatin on dyslipidaemia in uraemic patients on peritoneal dialysis. Nephrology Dialysis Transplantation 2000;15(5):684‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hunninghake 1998 {published data only}
- Hunninghake D, Bakker‐Arkema RG, Wigand JP, Drehobl M, Schrott H, Early JL, et al. Treating to meet NCEP‐recommended LDL cholesterol concentrations with atorvastatin, fluvastatin, lovastatin, or simvastatin in patients with risk factors for coronary heart disease. Journal of Family Practice 1998;47(5):349‐56. [MEDLINE: ] [PubMed] [Google Scholar]
Hunninghake 2001a {published data only}
- Hunninghake D, Insull W, Knopp R, Davidson M, Lohrbauer L, Jones P, et al. Comparison of the efficacy of atorvastatin versus cerivastatin in primary hypercholesterolemia. American Journal of Cardiology 2001;88(6):635‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hunninghake 2001b {published data only}
- Hunninghake D, Insull W Jr, Toth P, Davidson D, Donovan JM, Burke SK. Coadministration of colesevelam hydrochloride with atorvastatin lowers LDL cholesterol additively. Atherosclerosis 2001;158(2):407‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hunninghake 2003 {published data only}
- Hunninghake DB, Ballantyne CM, Maccubbin DL, Shah AK, Gumbiner B, Mitchel YB. Comparative effects of simvastatin and atorvastatin in hypercholesterolemic patients with characteristics of metabolic syndrome. Clinical Therapeutics 2003;25(6):1670‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hwang 2004 {published data only}
- Hwang YS, Tsai WC, Lu YH, Lin CC, Chen YF. Effect of atorvastatin on the expression of CD40 ligand and P‐selectin on platelets in patients with hypercholesterolemia. American Journal of Cardiology 2004;94(3):364‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ikewaki 2009 {published data only}
- Ikewaki K, Terao Y, Ozasa H, Nakada Y, Tohyama J, Inoue Y, et al. Effects of atorvastatin on nuclear magnetic resonance‐defined lipoprotein subclasses and inflammatory markers in patients with hypercholesterolemia. Journal of Atherosclerosis and Thrombosis 2009;16(1):51‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Illingworth 2001 {published data only}
- Illingworth DR, Crouse JR III, Hunninghake DB, Davidson MH, Escobar ID, Stalenhoef AFH, et al. A comparison of simvastatin and atorvastatin up to maximal recommended doses in a large multicenter randomized clinical trial. Current Medical Research and Opinion 2001;17(1):43‐50. [MEDLINE: ] [PubMed] [Google Scholar]
- Kastelein JJ, Isaacsohn JL, Ose L, Hunninghake DB, Frohlich J, Davidson MH, et al. Comparison of effects of simvastatin versus atorvastatin on high‐density lipoprotein cholesterol and apolipoprotein A‐I levels. American Journal of Cardiology 2000;86(2):221‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
IRIS 2007 {published data only}
- AstraZeneca. A 6‐Week, Randomized, Open‐Label, Comparative Study to Evaluate the Efficacy and Safety of Rosuvastatin and Atorvastatin in the Treatment of Hypercholesterolemia in South Asian Subjects (IRIS). Protocol No. D3560L00026/4522US0006 2005.
- Deedwania PC, Gupta M, Stein M, Ycas J, Gold A, IRIS Study Group. Comparison of rosuvastatin versus atorvastatin in South‐Asian patients at risk of coronary heart disease (from the IRIS Trial). American Journal of Cardiology 2007;99(11):1538‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Issa 2012 {published data only}
- Issa MH, Cerda A, Genvigir FD, Cavalli SA, Bertolami MC, Faludi AA, et al. Atorvastatin and hormone therapy effects on APOE mRNA expression in hypercholesterolemic postmenopausal women. Journal of Steroid Biochemistry & Molecular Biology 2012;128(3‐5):139‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
J‐CLAS 1997 {published data only}
- Nakamura H, Ohashi Y, Maruhama Y, Ninomiya K, Toyota T, Oikawa S, et al. Efficacy of atorvastatin in primary hypercholesterolemia. American Journal of Cardiology 1997;79(9):1248‐52. [MEDLINE: ] [PubMed] [Google Scholar]
Jin 2012 {published data only}
- Jin D, Wu Y, Zhao L, Guo J, Zhang K, Chen Z. Atorvastatin reduces serum HMGB1 levels in patients with hyperlipidemia. Experimental and Therapeutic Medicine 2012;4(6):1124‐6. [EMBASE: 2012594723] [DOI] [PMC free article] [PubMed] [Google Scholar]
Joukhadar 2001 {published data only}
- Joukhadar C, Klein N, Prinz M, Schrolnberger C, Vukovich T, Wolzt M, et al. Similar effects of atorvastatin, simvastatin and pravastatin on thrombogenic and inflammatory parameters in patients with hypercholesterolemia. Thrombosis & Haemostasis 2001;85(1):47‐51. [MEDLINE: ] [PubMed] [Google Scholar]
Kadikoylu 2003 {published data only}
- Kakikoylu G, Yukselen V, Yavasoglu I, Bolaman Z. Hemostatic effects of atorvastatin versus simvastatin. Annals of Pharmacotherapy 2003;37(4):478‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kadoglou 2011 {published data only}
- Kadoglou NPE, Vrabas IS, Kapelouzou A, Lampropoulos S, Sailer N, Kostakis A, et al. Impact of atorvastatin on serum vaspin levels in hypercholesterolemic patients with moderate cardiovascular risk. Regulatory Peptides 2011;170(1‐3):57‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kajinami 2003 {published data only}
- Kajinami K, Takekoshi N, Matsui S, Kanemitsu S, Okubo S, Kanayama S, et al. Effect of pretreatment vitamin D levels on in vivo effects of atorvastatin on bone metabolism in patients with heterozygous familial hypercholesterolemia. American Journal of Cardiology 2003;92(9):1113‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kassai 2007 {published data only}
- Kassai A, Illyes L, Mirdamadi HZ, Seres I, Kalmar T, Audikovszky M, et al. The effect of atorvastatin therapy on lecithin:cholesterol acyltransferase, cholesteryl ester transfer protein and the antioxidant paraoxonase. Clinical Biochemistry 2007;40(1‐2):1‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Keles 2008 {published data only}
- Keles T, Akar BN, Kayhan T, Canbay A, Sahin D, Durmaz T, et al. The comparison of the effects of standard 20 mg atorvastatin daily and 20 mg atorvastatin every other day on serum LDL‐cholesterol and high sensitive C‐reactive protein levels. Anadolu Kardiyoloji Dergisi 2008;8(6):407‐12. [MEDLINE: ] [PubMed] [Google Scholar]
Kim 2010 {published data only}
- Kim SH, Park K, Hong SJ, Cho YS, Sung JD, Moon GW, et al. Efficacy and tolerability of a generic and a branded formulation of atorvastatin 20 mg/d in hypercholesterolemic Korean adults at high risk for cardiovascular disease: a multicenter, prospective, randomized, double‐blind, double‐dummy clinical trial. Clinical Therapeutics 2010;32(11):1896‐905. [EMBASE: 2010647967] [DOI] [PubMed] [Google Scholar]
Kim 2013 {published data only}
- Kim SH, Seo MK, Yoon MH, Choi DH, Hong TJ, Kim HS. Assessment of the efficacy and tolerability of 2 formulations of atorvastatin in Korean adults with hypercholesterolemia: a multicenter, prospective, open‐label, randomized trial. Clinical Therapeutics 2013;35(1):77‐86. [EMBASE: 2013044605] [DOI] [PubMed] [Google Scholar]
Kocic 2002 {published data only}
- Kocic R, Pavlovic D, Kocic G, Lalic NM. Beneficial effect of atorvastatin therapy in diabetics. Atherosclerosis: Risk Factors, Diagnosis, and Treatment. Bologna: MEDIMOND S R L, 2002:321‐5. [IDS Number: BV31J ISBN: 88‐323‐2707‐4]
Koh 2010 {published data only}
- Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, Shin EK, et al. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. Journal of the American College of Cardiology 2010;55(12):1209‐16. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kom 2007 {published data only}
- Kom GD, Schwedhelm E, Maas R, Schneider L, Benndorf R, Boger RH. Impact of atorvastatin treatment on platelet‐activating factor acetylhydrolase and 15‐F(2trans)‐isoprostane in hypercholesterolaemic patients.. British Journal of Clinical Pharmacology 2007;63(6):672‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kosmidou 2008 {published data only}
- Kosmidou MS, Hatzitolios AI, Adamidou A, Giannopoulos S, Raikos N, Parharidis G, et al. Effects of atorvastatin on red‐blood cell Na(+)/Li(+) countertransport in hyperlipidemic patients with and without hypertension. American Journal of Hypertension 2008;21(3):303‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kotani 2012 {published data only}
- Kotani K, Yamada T, Miyamoto M, Ishibashi S, Taniguchi N, Gugliucci A. Influence of atorvastatin on serum amyloid A‐low density lipoprotein complex in hypercholesterolemic patients. Pharmacological Reports: PR 2012;64(1):212‐6. [EMBASE: 2012333843] [DOI] [PubMed] [Google Scholar]
Koter 2002 {published data only}
- Koter M, Broncel M, Chojnowska‐Jezierska J, Klikczynska K, Franiak I. The effect of atorvastatin on erythrocyte membranes and serum lipids in patients with type‐2 hypercholesterolemia. European Journal of Clinical Pharmacology 2002;58(8):501‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kowalski 2006 {published data only}
- Kowalski J, Barylski M, Banach M, Grycewicz J, Irzmanski R, Pawlicki L. Neutrophil superoxide anion generation during atorvastatin and fluvastatin therapy used in coronary heart disease primary prevention. Journal of Cardiovascular Pharmacology 2006;48(4):143‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kukharchuk 2007 {published data only}
- Kukharchuk VV, Kaminni AI, Kukharchuk VV, Kaminni AI. Assessment of hypolipidemic efficacy and safety of various doses of atorvastatin. Kardiologiia 2007;47(10):51‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Kural 2004 {published data only}
- Kural BV, Orem C, Uydu HA, Alver A, Orem A. The effects of lipid‐lowering therapy on paraoxonase activities and their relationships with the oxidant‐antioxidant system in patients with dyslipidemia. Coronary Artery Disease 2004;15(15):277‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Labios 2005 {published data only}
- Labios M, Martinez M, Gabriel F, Guiral V, Martinez E, Aznar J. Effect of atorvastatin upon platelet activation in hypercholesterolemia, evaluated by flow cytometry. Thrombosis Research 2005;115(4):263‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lavallee 2009 {published data only}
- Lavallee PC, Labreuche J, Gongora‐Rivera F, Jaramillo A, Brenner D, Klein IF, et al. Placebo‐controlled trial of high‐dose atorvastatin in patients with severe cerebral small vessel disease. Stroke 2009;40(5):1721‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lawrence 2004 {published data only}
- Lawrence JM. Reid J, Taylor GJ, Stirling C, Reckless JP. The effect of high dose atorvastatin therapy on lipids and lipoprotein subfractions in overweight patients with type 2 diabetes. Atherosclerosis 2004;174(1):141‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
LCP‐AtorFen 2009 {published data only}
- Davidson MH, Rooney MW, Drucker J, Eugene Griffin H, Oosman S, Beckert M. Efficacy and tolerability of atorvastatin/fenofibrate fixed‐dose combination tablet compared with atorvastatin and fenofibrate monotherapies in patients with dyslipidemia: a 12‐week, multicenter, double‐blind, randomized, parallel‐group study. Clinical Therapeutics 2009;31(12):2824‐38. [EMBASE: 2010047966] [DOI] [PubMed] [Google Scholar]
- Davidson, MH, Rooney M, Drucker J, Griffin HE, Beckert M. Atorvastatin/fenofibrate 40/100 mg fixed‐dose combination tablet (LCP‐AtorFen 40/100 mg) offers improved efficacy over 40 mg atorvastatin and higher dose 145 mg fenofibrate in patients with dyslipidemia. Circulation 2008;118 Suppl 2(18):S1139. [PREV200900200229] [Google Scholar]
Lee 2007 {published data only}
- Lee SH, Chung N, Kwan J, Kim DI, Kim WH, Kim CJ, et al. Comparison of the efficacy and tolerability of pitavastatin and atorvastatin: an 8‐week, multicenter, randomized, open‐label, dose‐titration study in Korean patients with hypercholesterolemia. Clinical Therapeutics 2007;29(11):2365‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2011 {published data only}
- Lee SH, Kang SM, Park S, Jang Y, Chung N, Choi D. The effects of statin monotherapy and low‐dose statin/ezetimibe on lipoprotein‐associated phospholipase A2. Clinical Cardiology 2011;34(2):108‐12. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2012 {published data only}
- Lee SH, Park S, Kang SM, Jang Y, Chung N, Choi D. Effect of atorvastatin monotherapy and low‐dose atorvastatin/ezetimibe combination on fasting and postprandial triglycerides in combined hyperlipidemia. Journal of Cardiovascular Pharmacology & Therapeutics 2012;17(1):65‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2013 {published data only}
- Lee JH, Kang HJ, Kim HS, Sohn DW, Oh BH, Park YB. Effects of ezetimibe/simvastatin 10/20 mg vs. atorvastatin 20 mg on apolipoprotein B/apolipoprotein A1 in Korean patients with type 2 diabetes mellitus: results of a randomized controlled trial. American Journal of Cardiovascular Drugs 2013;13(5):343‐51. [EMBASE: 2013633599] [DOI] [PubMed] [Google Scholar]
Lemieux 2003 {published data only}
- Lemieux I, Salomon H, Despres JP. Contribution of apo CIII reduction to the greater effect of 12‐week micronized fenofibrate than atorvastatin therapy on triglyceride levels and LDL size in dyslipidemic patients. Annals of Medicine 2003;35(6):442‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Leung 2002 {published data only}
- Leung AWS, Tam KM, Chiu CS. An open‐label, uncontrolled, prospective study of the effects of atorvastatin 10 mg on low‐density lipoprotein cholesterol levels in Chinese adults with coronary artery disease and hypercholesterolemia. Current Therapeutic Research 2002;63(6):399‐408. [EMBASE: 2002283535] [Google Scholar]
Li 2010 {published data only}
- Li J, Sun YM, Wang LF, Li ZQ, Pan W, Cao HY, et al. Comparison of effects of simvastatin versus atorvastatin on oxidative stress in patients with coronary heart disease. Clinical Cardiology 2010;33(4):222‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lins 2004 {published data only}
- Lins RL, Carpentier YA, Clinical Investigators Study Group. Plasma lipids and lipoproteins during atorvastatin (atvs) up‐titration in hemodialysis patients with hyperlipidemia: a placebo‐controlled study [abstract]. Nephrology Dialysis Transplantation 2002;17 Suppl 1:124. [CENTRAL: CN‐00509325] [Google Scholar]
- Lins RL, Matthys KE, Billiouw JM, Dratwa M, Dupont P, Lameire NH, et al. Lipid and apoprotein changes during atorvastatin up‐titration in hemodialysis patients with hypercholesterolemia: a placebo‐controlled study. Clinical Nephrology 2004;62(4):287‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Llaverias 2008 {published data only}
- Llaverias G, Pou J, Ros E, Zambon D, Cofan M, Sanchez A, et al. Monocyte gene‐expression profile in men with familial combined hyperlipidemia and its modification by atorvastatin treatment. Pharmacogenomics 2008;9(8):1035‐54. [EMBASE: 2008403034] [DOI] [PubMed] [Google Scholar]
Loughrey 2013 {published data only}
- Loughrey BV, McGinty A, Young IS, McCance DR, Powell LA. Increased circulating CC chemokine levels in the metabolic syndrome are reduced by low‐dose atorvastatin treatment: evidence from a randomized controlled trial. Clinical Endocrinology 2013;79(6):800‐6. [EMBASE: 2013713441] [DOI] [PubMed] [Google Scholar]
LUNAR 2012 {published data only}
- Ballantyne CM, Pitt B, Loscalzo J, Cain VA, Raichlen JS. Alteration of relation of atherogenic lipoprotein cholesterol to apolipoprotein B by intensive statin therapy in patients with acute coronary syndrome (from the Limiting UNdertreatment of lipids in ACS With Rosuvastatin [LUNAR] Trial). American Journal of Cardiology 2013;111(4):506‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pitt B, Loscalzo J, Monyak J, Miller E, Raichlen J. Comparison of lipid‐modifying efficacy of rosuvastatin versus atorvastatin in patients with acute coronary syndrome (from the LUNAR study). The American Journal of Cardiology 2012;109(9):1239‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lupattelli 2012 {published data only}
- Lupattelli G, Siepi D, Vuono S, Roscini AR, Crisanti F, Covelli D, et al. Cholesterol metabolism differs after statin therapy according to the type of hyperlipemia. Life Sciences 2012;90(21‐22):846‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ma 2000 {published data only}
- Ma P, Hegele R, Yale JF, Schwartz B. CAVEAT: a randomised, double‐blind, parallel group evaluation of cerivastatin 0.4 mg and 0.8 mg compared to atorvastatin 10 mg and 20 mg once daily in patients with combined (type IIb) dyslipidaemia. British Journal of Cardiology 2000;7(12):780‐6. [EMBASE: 2001009668] [Google Scholar]
- Ma P, Hegele, Yale JF, Schwartz B. CAVEAT: a comparison of cerivastatin 0.4 mg and 0.8 mg with atorvastatin 10 mg and 20 mg in patients with combined (type IIB) dyslipidaemia. Atherosclerosis Supplement 2001;2(2):48. [CENTRAL: CN‐00446513] [Google Scholar]
Mabuchi 2005 {published data only}
- Mabuchi H, Higashikata T, Kawashiri M, Katsuda S, Mizuno M, Nohara A, et al. Reduction of serum ubiquinol‐10 and ubiquinone‐10 levels by atorvastatin in hypercholesterolemic patients. Journal of Atherosclerosis & Thrombosis 2005;12(2):111‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mabuchi 2007 {published data only}
- Mabuchi H, Nohara A, Kobayashi J, Kawashiri MA, Katsuda S, Inazu A, et al. Effects of CoQ10 supplementation on plasma lipoprotein lipid, CoQ10 and liver and muscle enzyme levels in hypercholesterolemic patients treated with atorvastatin: a randomized double‐blind study. Atherosclerosis 2007;195(2):e182‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Macin 2005 {published data only}
- Macin SM, Perna ER, Farias EF, Franciosi V, Cialzeta JR, Brizuela M, et al. Atorvastatin has an important acute anti‐inflammatory effect in patients with acute coronary syndrome: results of a randomized, double‐blind, placebo‐controlled study. American Heart Journal 2005;149(3):451‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Magen 2004 {published data only}
- Magen E, Viskoper R, Mishal J, Priluk R, Berezovsky A, Laszt A, et al. Resistant arterial hypertension and hyperlipidemia: atorvastatin, not vitamin C, for blood pressure control. Israel Medical Association Journal 2004;6(12):742‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Majima 2007 {published data only}
- Majima T, Komatsu Y, Fukao A, Ninomiya K, Matsumura T, Nakao K. Short‐term effects of atorvastatin on bone turnover in male patients with hypercholesterolemia. Endocrine Journal 2007;54(1):145‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Maki 2011 {published data only}
- Maki KC, Bays HE, Dicklin MR, Johnson SL, Shabbout M. Effects of prescription omega‐3‐acid ethyl esters, coadministered with atorvastatin, on circulating levels of lipoprotein particles, apolipoprotein CIII, and lipoprotein‐associated phospholipase A2 mass in men and women with mixed dyslipidemia. Journal of Clinical Lipidology 2011;5(6):483‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mandosi 2010 {published data only}
- Mandosi E, Fallarino M, Gatti A, Carnovale A, Rossetti M, Lococo E, et al. Atorvastatin down regulates monocyte CD36 expression, nuclear NFB and TNFalpha levels in type 2 diabetes. Journal of Atherosclerosis and Thrombosis 2010;17(6):539‐45. [EMBASE: 2010396113] [DOI] [PubMed] [Google Scholar]
Manuel‐Y‐Keenoy 2004 {published data only}
- Manuel‐Y‐Keenoy B, Vinckx M, Vertommen J, Gaal L, Leeuw I. Impact of Vitamin E supplementation on lipoprotein peroxidation and composition in Type 1 diabetic patients treated with Atorvastatin. Atherosclerosis 2004;175(2):369‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marais 1997 {published data only}
- Marais AD, Firth JC, Bateman ME, Byrnes P, Martens C, Mountney J. Atorvastatin: an effective lipid‐modifying agent in familial hypercholesterolemia. Arteriosclerosis, Thrombosis and Vascular Biology 1997;17(8):1527‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marchesi 2000 {published data only}
- Marchesi S, Lupattelli G, Siepi D, Schillaci G, Vaudo G, Roscini AR, et al. Short‐term atorvastatin treatment improves endothelial function in hypercholesterolemic women. Journal of Cardiovascular Pharmacology 2000;36(5):617‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marketou 2006 {published data only}
- Marketou ME, Zacharis EA, Nikitovic D, Ganotakis ES, Parthenakis FI, Maliaraki N, et al. Early effects of simvastatin versus atorvastatin on oxidative stress and pro‐inflammatory cytokines in hyperlipidemic subjects. Angiology 2006;57(2):211‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McInnes 2014 {published data only}
- McInnes IB, Kim HY, Lee SH, Mandel D, Song YW, Connell CA, et al. Open‐label tofacitinib and double‐blind atorvastatin in rheumatoid arthritis patients: a randomised study. Annals of the Rheumatic Diseases 2014;73(1):124‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McKenney 1998 {published data only}
- McKenney J, McCormick L. The effect of atorvastatin and niacin on lipoprotein subclasses in patients with mixed hyperlipidemia. Atherosclerosis 1997;134:58. [CENTRAL: CN‐00446694] [Google Scholar]
- McKenney JM, McCormack LS, Weiss S, Koren M, Kafonek S, Black DM. A randomized trial of the effects of atorvastatin and niacin in patients with combined hyperlipidemia or isolated hypertriglyceridemia. American Journal of Medicine 1998;104(2):137‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MERCURY I 2004 {published data only}
- Cheung RC, Morrell JM, Kallend D, Watkins C, Schuster H. Effects of switching statins on lipid and apolipoprotein ratios in the MERCURY I study. International Journal of Cardiology 2005;100(2):309‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Insull W. Treatment of hypercholesterolemia in patients with the metabolic syndrome: how do various statins compare?. Nature Clinical Practice Cardiovascular Medicine 2006;3(3):134‐5. [EMBASE: 2006101984] [Google Scholar]
- Schuster H, Barter PJ, Stender S, Cheung RC, Bonnet J, Morrell JM, et al. Effects of switching statins on achievement of lipid goals: Measuring Effective Reductions in Cholesterol Using Rosuvastatin Therapy (MERCURY I) study. American Heart Journal 2004;147(4):705‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schuster H, Palmer MK, Ditmarsch M. The MERCURY I open‐label extension study ‐ subgroup analysis in patients with diabetes. British Journal of Diabetes and Vascular Disease 2008;8(3):142‐7. [EMBASE: 2008340601] [Google Scholar]
- Stender S, Schuster H, Barter P, Watkins C, Kallend D, MERCURY I Study Group. Comparison of rosuvastatin with atorvastatin, simvastatin and pravastatin in achieving cholesterol goals and improving plasma lipids in hypercholesterolemic patients with or without the metabolic syndrome in the MERCURY I trial. Diabetes, Obesity & Metabolism 2004;7(4):430‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MERCURY II 2006 {published data only}
- AstraZeneca. An Open‐label, Randomized, Multi‐center, Phase IIIb, Parallel Group Switching Study to Compare the Efficacy and Safety of Lipid Lowering Agents Atorvastatin and Simvastatin with Rosuvastatin in High Risk Subjects with Type IIa and IIb Hypercholesterolemia (MERCURY II). Protocol No. D3560C00068 (4522IL/0068) 2004.
- Ballantyne CM, Bertolami M, Hernandez Garcia HR, Nul D, Stein EA, Theroux P, et al. Achieving LDL cholesterol, non‐HDL cholesterol, and apolipoprotein B target levels in high‐risk patients: Measuring Effective Reductions in Cholesterol Using Rosuvastatin therapY (MERCURY) II. American Heart Journal 2006;151(5):975.e1‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ballantyne CM, Raichlen JS, Cain VA. Statin therapy alters the relationship between apolipoprotein B and low‐density lipoprotein cholesterol and non‐high‐density lipoprotein cholesterol targets in high‐risk patients ‐ The MERCURY II (Measuring Effective Reductions in Cholesterol Using Rosuvastatin therapY II) trial. Journal of the American College of Cardiology 2008;52(8):626‐32. [DOI: 10.1016/j.jacc.2008.04.052] [DOI] [PubMed] [Google Scholar]
Milionis 2003 {published data only}
- Milionis HJ, Kakafika AI, Tsouli SG, Athyros VG, Bairaktari ET, Seferiadis KI, et al. Effects of statin treatment on uric acid homeostasis in patients with primary hyperlipidemia. American Heart Journal 2004;148(4):635‐40. [CENTRAL: CN‐00504472] [DOI] [PubMed] [Google Scholar]
Milionis 2004 {published data only}
- Milionis H, Papkostas J, Kakafika A, Chasiotis G, Seferiadis K, Elisaf MS. Comparative effects of atorvastatin, simvastatin, and fenofibrate on serum homocysteine levels in patients with primary hyperlipidemia. Journal of Clinical Pharmacology 2003;43(8):825‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mirdamadi 2008 {published data only}
- Mirdamadi HZ, Sztanek F, Derdak Z, Seres I, Harangi M, Paragh G, et al. The human paraoxonase‐1 phenotype modifies the effect of statins on paraoxonase activity and lipid parameters. British Journal of Clinical Pharmacology 2008;66(3):366‐74. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
MODEST 2009 {published data only}
- Kanat M, Serin E, Tunckale A, Yildiz O, Sahin S, Bolayirli M, et al. A multi‐center, open label, crossover designed prospective study evaluating the effects of lipid lowering treatment on steroid synthesis in patients with Type 2 diabetes (MODEST Study). Journal of Endocrinological Investigation 2009;32(10):852‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kanat M, Yildiz O, Tunckale A, Ceyhan BO, Karagoz Y, Altuntafl Y, et al. Intensive lipid reduction and proinflammatory markers in the MODEST study. Turkish Journal of Endocrinology and Metabolism 2010;14(2):31‐4. [EMBASE: 2010630338] [Google Scholar]
Monteiro 2008 {published data only}
- Monteiro CMC, Oliveira L, Izar MCO, Santos AO, Povoa RMS, Fischer SM, et al. Early effects of lipid lowering treatment in subjects with metabolic syndrome and acute coronary syndromes. International Journal of Atherosclerosis 2008;3(2):93‐9. [EMBASE: 2010215110] [Google Scholar]
Mori 2013 {published data only}
- Mori H, Okada Y, Tanaka Y. Effects of pravastatin, atorvastatin, and rosuvastatin in patients with type 2 diabetes mellitus and hypercholesterolemia. Diabetology International 2013;4(2):117‐25. [EMBASE: 2013457978] [Google Scholar]
Morishita 2001 {published data only}
- Morishita E, Minami S, Ishino C, Kanno M, Uotani C, Asakura H, et al. Atorvastatin reduces plasma levels of factor VII activity and factor VII antigen in patients with hyperlipidemia. Journal of Atherosclerosis and Thrombosis 2001;9(1):72‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mullen 2000 {published data only}
- Mullen MJ, Wright D, Donald AE, Thorne S, Thomson H, Deanfield JE. Atorvastatin but not L‐arginine improves endothelial function in type I diabetes mellitus: a double‐blind study. Journal of the American College of Cardiology 2000;36(2):410‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Murrow 2012 {published data only}
- Murrow JR, Sher S, Au S, Uphoff I, Patel R, Porkert M, et al. The differential effect of statins on oxidative stress and endothelial function: atorvastatin versus pravastatin. Journal of Clinical Lipidology 2012;6(1):42‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Muscari 2001 {published data only}
- Muscari A, Bastagi L, Poggiopollini G, Tomassetti V, Massarelli G, Boni P, et al. Short term effect of atorvastatin and vitamin E on serum levels of C3, a sensitive marker of the risk of myocardial infarction in men. Cardiovascular Drugs & Therapy 2001;15(5):453‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nagila 2009 {published data only}
- Nagila A, Permpongpaiboon T, Tantrarongroj S, Porapakkham P, Chinwattana K, Deakin S, et al. Effect of atorvastatin on paraoxonase1 (PON1) and oxidative status. Pharmacological Reports: PR 2009;61(5):892‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Naoumova 1996 {published data only}
- Naoumova RP, Marais AD, Mountney J, Firth JC, Rendell NB, Taylor GW, et al. Plasma mevalonic acid, an index of cholesterol synthesis in vivo, and responsiveness to HMG‐CoA reductase inhibitors in familial hypercholesterolaemia. Atherosclerosis 1996;149(2):203‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Naoumova 1997 {published data only}
- Naoumova RP, Dunn S, Rallidis L, Abu‐Muhana O, Neuwirth C, Rendell NB, et al. Prolonged inhibition of cholesterol synthesis explains the efficacy of atorvastatin. Journal of Lipid Research 1997;38(7):1496‐500. [MEDLINE: ] [PubMed] [Google Scholar]
Naoumova 2003 {published data only}
- Naoumova RP, Patel DD, O'Neill FH, Thompson GR, Knight BL. Treatment with atorvastatin alters interleukin‐12 and ‐10 gene expression. European Journal of Clinical Investigation 2003;33(1):88‐91. [EMBASE: 2003018849] [DOI] [PubMed] [Google Scholar]
NASDAC 2005 {published data only}
- Jones PH, McKenney JM, Karalis DG, Downey J. Comparison of the efficacy and safety of atorvastatin initiated at different starting doses in patients with dyslipidemia. American Heart Journal 2005;149(1):e1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Karalis DG, Ishisaka DY, Luo D, Ntanios F, Wun CC. Effects of increasing doses of atorvastatin on the atherogenic lipid subclasses commonly associated with hypertriglyceridemia. American Journal of Cardiology 2007;100(3):445‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nawrocki 1995 {published data only}
- Nawrocki JW, Weiss SR, Davidson MH, Sprecher DL, Schwartz SL, Lupien PJ, et al. Reduction of LDL cholesterol by 25% to 60% in patients with primary hypercholesterolemia by atorvastatin, a new HMG‐CoA reductase inhibitor. Perfusion 1996;9(3):109‐14. [IDS Number: UM602] [DOI] [PubMed] [Google Scholar]
- Nawrocki JW, Weiss SR, Davidson MH, Sprecher DL, Schwartz SL, Lupien PJ, et al. Reduction of LDL‐cholesterol by 25% to 60% in patients with primary hypercholesterolemia by atorvastatin, a new HMG‐CoA reductase inhibitor. Arterosclerosis, Thrombosis & Vascular Biology 1995;15(5):678‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Neil 1999 {published data only}
- Neil H, Fowler G, Patel H, Eminton Z, Maton S. An assessment of the efficacy of atorvastatin in achieving LDL cholesterol target levels in patients with coronary heart disease: a general practice study. International Journal of Clinical Practice 1999;53(6):422‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Nordøy 2001 {published data only}
- Nordøy A, Hansen JB, Brox J, Svensson B. Effects of atorvastatin and omega‐3 fatty acids on LDL subfractions and postprandial hyperlipemia in patients with combined hyperlipemia. Nutrition Metabolism & Cardiovascular Diseases 2001;11(1):7‐16. [MEDLINE: ] [PubMed] [Google Scholar]
Nozue 2008 {published data only}
- Nozue T, Michishita I, Ito Y, Hirano T, Nozue T, Michishita I, et al. Effects of statin on small dense low‐density lipoprotein cholesterol and remnant‐like particle cholesterol in heterozygous familial hypercholesterolemia. Journal of Atherosclerosis & Thrombosis 2008;15(3):146‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Okopien 2004 {published data only}
- Okopien B, Krysiak R, Herman ZS. Effect of monthly atorvastatin treatment on hemostasis. International Journal of Clinical Pharmacology & Therapeutics 2004;42(11):589‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Okopien 2005 {published data only}
- Okopien B, Krysiak R, Haberka M, Herman ZS. Effect of monthly atorvastatin and fenofibrate treatment on monocyte chemoattractant protein‐1 release in patients with primary mixed dyslipidemia. Journal of Cardiovascular Pharmacology 2005;45(4):314‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Olsson 2001 {published data only}
- Astrazeneca. A Randomised, Parallel‐Group Dose‐Response Study with the HMG‐CoA Reductase Inhibitor ZD4522 and Atorvastatin in Subjects with Primary Hypercholesterolaemia. Protocol No. 4522IL/0008 2000.
- Olsson AG, Pears J, McKellar J, Mizan J, Raza A. Effect of rosuvastatin on low‐density lipoprotein cholesterol in patients with hypercholesterolemia. American Journal of Cardiology 2001;88(5):504‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pears JS, Olsson AG, Caplan RJ, McKellar J, Raza A. Dose‐ranging study of the HMG‐CoA reductase inhibitor ZD4522 in patients with primary hypercholesterolemia. Canadian Journal of Cardiology 2000;16(Supplement F):196F. [0828‐282X ER] [Google Scholar]
Olsson 2002 {published data only}
- AstraZeneca. A randomised, double‐blind, multinational, multicentre trial to compare the short‐term and long‐term efficacy and safety of ZD4522 and atorvastatin in the treatment of subjects with hypercholesterolaemia (4522IL/0026). Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research Office of Biostatistics 1999‐2000:25‐33. [NDA 21‐366]
- Blasetto JW, Stein EA, Brown WV, Chitra R, Raza A. Efficacy of rosuvastatin compared with other statins at selected starting doses in hypercholesterolemic patients and in special population groups. American Journal of Cardiology 2003;91 Suppl 5A:3C‐10C. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Olsson AG, Istad H, Luurila O, Ose L, Stender S, Tuomilehto J, et al. Effects of rosuvastatin and atorvastatin compared over 52 weeks of treatment in patients with hypercholesterolemia. American Heart Journal 2002;144(6):1044‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ong 2011 {published data only}
- Ong LM, Punithavathi N, Lena YLL, Mahanim O, Leekha S. Long term efficacy and safety of a generic atorvastatin in usual clinical care setting. Medical Journal of Malaysia 2011;66(3):214‐9. [PubMed] [Google Scholar]
Ooi 1997 {published data only}
- Ooi TC, Heinonen T, Alaupovic P, Davignon J, Leiter L, Lupien PJ, et al. Efficacy and safety of a new hydroxymethylglutaryl‐coenzyme A reductase inhibitor, atorvastatin, in patients with combined hyperlipidemia: comparison with fenofibrate. Arteriosclerosis Thrombosis and Vascular Biology 1997;17(9):1793‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oranje 2001 {published data only}
- Oranje WA, Sels JPJE, Rondas‐Colbers GJWM, Lemmens PJMR, Wolffenbuttel BHR. Effect of atorvastatin on LDL oxidation and antioxidants in normocholesterolemic type 2 diabetic patients. Clinica Chimica Acta 2001;311(2):91‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Orem 2002 {published data only}
- Orem C, Orem A, Calapoglu M, Baykan M, Uydu HA, Erdol C. Plasma fibronectin level and its relationships with lipids, lipoproteins and C‐reactive protein in patients with dyslipidaemia during lipid‐lowering therapy. Acta Cardiologica 2002;57(6):421‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Orr 2009 {published data only}
- Orr JS, Dengo AL, Rivero JM, Davy KP. Arterial destiffening with atorvastatin in overweight and obese middle‐aged and older adults. Hypertension 2009;54(4):763‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ozerkan 2006 {published data only}
- Ozerkan F, Ozdogan O, Zoghi M, Nalbantgil S, Yavuzgil O, Remzi Onder M. Effects of atorvastatin 10 mg/d on insulin resistance: a 12‐week, open‐label study in hyperlipidemic patients. Current Therapeutic Research Clinical and Experimental 2006;67(1):44‐54. [EMBASE: 2006272912] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozsoy 2003 {published data only}
- Ozsoy RC, Kastelein JJP, Arisz L, Koopman MG. Atorvastatin and the dyslipidemia of early renal failure. Atherosclerosis 2003;166(1):187‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pacanowski 2008 {published data only}
- Pacanowski MA, Frye RF, Enogieru O, Schofield RS, Zineh I. Plasma coenzyme Q10 predicts lipid‐lowering response to high‐dose atorvastatin. Journal of Clinical Lipidology 2008;2(4):289‐97. [EMBASE: 2008370721] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paiva 2005 {published data only}
- Paiva H, Laasko J, Lehtimaki T, Isomustajarvi M, Ruokonene I, Laaksonen R. Effect of high‐dose statin treatment on plasma concentrations of endogenous nitric oxide synthase inhibitors. Journal of Cardiovascular Pharmacology 2003;41(2):219‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Paiva H, Thelen KM, Coster R, Smet J, Paepe B, Mattila KM, et al. High‐dose statins and skeletal muscle metabolism in humans: a randomized, controlled trial. Clinical Pharmacology & Therapeutics 2005;78(1):60‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PAPAGO‐T 2013 {published data only}
- Liu PY, Lin LY, Lin HJ, Hsia CH, Hung YR, Yeh HI, et al. Pitavastatin and Atorvastatin double‐blind randomized comPArative study among hiGh‐risk patients, including thOse with Type 2 diabetes mellitus, in Taiwan (PAPAGO‐T Study). PLoS ONE [Electronic Resource] 2013;8(10):e76298. [EMBASE: 2013618552] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papathanasiou 2008 {published data only}
- Papathanasiou AI, Lourida ES, Tsironis LD, Goudevenos JA, Tselepis AD. Short‐ and long‐term elevation of autoantibody titers against oxidized LDL in patients with acute coronary syndromes. Role of the lipoprotein‐associated phospholipase A2 and the effect of atorvastatin treatment. Atherosclerosis 2008;196(1):289‐97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Parhofer 2000 {published data only}
- Parhofer KG, Hugh P, Barrett R, Schwandt P. Atorvastatin improves postprandial lipoprotein metabolism in normolipidemic subjects. Journal of Clinical Endocrinology & Metabolism 2000;85(11):4224‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Parhofer 2003 {published data only}
- Parhofer KG, Laubach E, Barrett PH. Effect of atorvastatin on postprandial lipoprotein metabolism in hypertriglyceridemic patients. Journal of Lipid Research 2003;44(6):1192‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Park 2010 {published data only}
- Park JS, Kim YJ, Choi JY, Kim YN, Hong TJ, Kim DS, et al. Comparative study of low doses of rosuvastatin and atorvastatin on lipid and glycemic control in patients with metabolic syndrome and hypercholesterolemia. The Korean Journal of Internal Medicine 2010;25(1):27‐35. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pfizer Inc 16 {unpublished data only}
- Pfizer Inc. Phase 2, multi‐center, double‐blind, placebo‐controlled, randomized, parallel group, dose response study of the safety and efficacy of CP‐529,414 combination with open‐label atorvastatin once daily (QD) for 12 weeks in subjects with hypercholesterolemia and without overt cardiovascular disease. Protocol No. A5091049 2005.
Pfizer Inc 19 {unpublished data only}
- Pfizer Inc. To compare the effects on lipoprotein fractions and safety of atorvastatin with that of fenofibrate. Protocol 981‐55 1998. [NDA: 20‐702S003]
Pirkova 2007 {published data only}
- Pirkova AA, Samoilova EV, Ameliushkina VA, Kaminnyi AI, Titov VN, Prokazova NV, et al. Effect of atorvastatin therapy on the level of secretory phospholipase A2 group IIA and on the modification of low density lipoproteins in patients with ischemic heart disease. Kardiologiia 2007;47(4):37‐40. [MEDLINE: ] [PubMed] [Google Scholar]
PITCH 2012 {published data only}
- Han KH, Rha SW, Kang HJ, Bae JW, Choi BJ, Choi SY, et al. Evaluation of short‐term safety and efficacy of HMG‐CoA reductase inhibitors in hypercholesterolemic patients with elevated serum alanine transaminase concentrations: PITCH study (pitavastatin versus atorvastatin to evaluate the effect on patients with hypercholesterolemia and mild to moderate hepatic damage). Journal of Clinical Lipidology 2012;6(4):340‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Plakogiannis 2002 {published data only}
- Plakogiannis R. The chronologic effects of atorvastatin: morning versus evening administration in hyperlipidemic adults, 2002. proquest.umi.com/pqdweb?did=726006791&sid=1&Fmt=2&clientId=6993&RQT=309&VName=PQD (accessed 5 November 2012). [ProQuest document ID: 726006791]
- Plakogiannis R, Cohen H, Taft D. Effects of morning versus evening administration of atorvastatin in patients with hyperlipidemia. American Journal of Health‐System Pharmacy 2005;62(23):2491‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pontrelli 2002 {published data only}
- Pontrelli L, Parris W, Adeli K, Cheung RC. Atorvastatin treatment beneficially alters the lipoprotein profile and increases low‐density lipoprotein particle diameter in patients with combined dyslipidemia and impaired fasting glucose/type 2 diabetes. Metabolism 2002;51(3):334‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PRAT 2013 {published data only}
- Sasaki J, Otonari T, Uchida Y, Ikeda Y, Biro S, Kono S, et al. Effects of pravastatin and atorvastatin on HDL cholesterol and glucose metabolism in patients with dyslipidemia and glucose intolerance: the PRAT study. Journal of Atherosclerosis & Thrombosis 2013;20(4):368‐79. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Puato 2010 {published data only}
- Puato M, Faggin E, Rattazzi M, Zambon A, Cipollone F, Grego F, et al. Atorvastatin reduces macrophage accumulation in atherosclerotic plaques: a comparison of a nonstatin‐based regimen in patients undergoing carotid endarterectomy 20195400. Stroke 2010;41(6):1163‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Puccetti 2002 {published data only}
- Puccetti L, Pasqui AL, Pastorelli M, Bova G, Cercignani M, Palazzuoli A, et al. Time‐dependent effect of statins on platelet function in hypercholesterolaemia. European Journal of Clinical Investigation 2002;32(12):901‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Puccetti 2005 {published data only}
- Puccetti L, Pasqui AL, Pastorelli M, Ciani F, Palazzuoli A, Gioffre W, et al. 3'UTR/T polymorphism of lectin‐like oxidized low‐density lipoprotein receptor‐1 (LOX‐1) is associated with modified anti‐platelet activity of atorvastatin in hypercholesterolemic subjects. Atherosclerosis 2005;183(2):322‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PULSAR 2006 {published data only}
- Anonymous. First open‐label study to compare rosuvastatin to atorvastatin in high‐risk patients shows benefits of effective statin starting dose. Cardiovascular Journal of Africa 2007;18(1):60. [EMBASE: 2008249348] [Google Scholar]
- AstraZeneca. A 6‐week Open‐label, Randomised, Multicentre, Phase IIIb, Parallel‐group Study to Compare the Efficacy and Safety of Rosuvastatin (10 mg) with Atorvastatin (20 mg) in Subjects with Hypercholesterolaemia and Either a History of Coronary Heart Disease (CHD) or Clinical Evidence of Atherosclerosis or a CHD Risk Equivalent (10‐year Risk Score of >20%). Protocol No. 4522IL/0102 (D3569C00001) 31 2005.
- Clearfield MB, Amerena J, Bassand JP, García HRH, Miller SS, Sosef FFM, et al. Comparison of the efficacy and safety of rosuvastatin 10 mg and atorvastatin 20 mg in high‐risk patients with hypercholesterolemia – Prospective study to evaluate the Use of Low doses of the Statins Atorvastatin and Rosuvastatin (PULSAR). Trials 2006;7(35):not applicable. [EMBASE: 2007065270] [DOI] [PMC free article] [PubMed] [Google Scholar]
Puurunen 2013 {published data only}
- Puurunen J, Piltonen T, Puukka K, Ruokonen A, Savolainen MJ, Bloigu R, et al. Statin therapy worsens insulin sensitivity in women with polycystic ovary syndrome (PCOS): a prospective, randomized, double‐blind, placebo‐controlled study. Journal of Clinical Endocrinology & Metabolism 2013;98(12):4798‐807. [EMBASE: 2013778908] [DOI] [PubMed] [Google Scholar]
Qi 2013 {published data only}
- Qi Y, Liu J, Ma C, Wang W, Liu X, Wang M, et al. Association between cholesterol synthesis/absorption markers and effects of cholesterol lowering by atorvastatin among patients with high risk of coronary heart disease. Journal of Lipid Research 2013;54(11):3189‐97. [EMBASE: 2013670884] [DOI] [PMC free article] [PubMed] [Google Scholar]
RADAR 2005 {published data only}
- Bergheanu SC, Reijmers T, Zwinderman AH, Bobeldijk I, Ramaker R, Liem AH, et al. Lipidomic approach to evaluate rosuvastatin and atorvastatin at various dosages: investigating differential effects among statins. Current Medical Research & Opinion 2008;24(9):2477‐87. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bergheanu SC, Tol A, Dallinga‐Thie GM, Liem A, Dunselman PH, Bom JG, et al. Effect of rosuvastatin versus atorvastatin treatment on paraoxonase‐1 activity in men with established cardiovascular disease and a low HDL‐cholesterol. Current Medical Research & Opinion 2007;23(9):2235‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jukema JW, Liem AH, Dunselman PH, Sloot JA, Lok DJ, Zwinderman AH. LDL‐C/HDL‐C ratio in subjects with cardiovascular disease and a low HDL‐C: results of the RADAR (Rosuvastatin and Atorvastatin in different Dosages And Reverse cholesterol transport) study. Current Medical Research & Opinion 2005;21(11):1865‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Karalis IK, Bergheanu SC, Wolterbeek R, Dallinga‐Thie GM, Hattori H, Tol A, et al. Effect of increasing doses of rosuvastatin and atorvastatin on apolipoproteins, enzymes and lipid transfer proteins involved in lipoprotein metabolism and inflammatory parameters. Current Medical Research and Opinion 2010; Vol. 26, issue 10:2301‐13. [MEDLINE: ] [DOI] [PubMed]
Raison 2002 {published data only}
- Raison J, Rudnichi A, Safar ME. Effects of atorvastatin on aortic pulse wave velocity in patients with hypertension and hypercholesterolaemia: a preliminary study. Journal of Human Hypertension 2002;16(10):705‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Reinares 2002 {published data only}
- Reinares L, Figueredo A, Rueda A, Pontes JC, Rodriguez A, Ruiz‐Yague M, et al. Atorvastatin reduces expression of the CCR2 and MAC‐1 receptors on monocytes, and plasma levels of monocyte chemoattractant protein‐1 and C‐reactive protein, in patients with coronary heart disease. Clinical Drug Investigation 2002;22(1):1‐8. [EMBASE: 2002067878] [Google Scholar]
Reiter 2005 {published data only}
- Reiter M, Wirth S, Pourazim A, Baghestanian M, Minar E, Bucek RA. Statin therapy has no significant effect on skin tissue cholesterol: results from a prospective randomized trial. Clinical Chemistry 2005;51(1):252‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RESPOND 2007 {published data only}
- Pfizer Inc. A Multi‐National, Prospective Randomized Double‐Blind, Multi‐Center, Placebo‐Controlled Study to Evaluate Efficacy and Safety of a Fixed Combination Therapy of Amlodipine and Atorvastatin in the Treatment of Concurrent Hypertension and Hyperlipidemia ‐ the RESPOND Trial. Protocol No. A3841003 2003.
- Preston RA, Harvey P, Herfert O, Dykstra G, Jukema JW, Sun F, et al. A randomized, placebo‐controlled trial to evaluate the efficacy, safety, and pharmacodynamic interaction of co‐administered amlodipine and atorvastatin in 1660 patients with concomitant hypertension and dyslipidemia: the RESPOND trial. Journal of Clinical Pharmacology 2007;47(12):1555‐69. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rodrigues 2013 {published data only}
- Rodrigues AC, Sobrino B, Genvigir FD, Willrich MA, Arazi SS, Dorea EL, et al. Genetic variants in genes related to lipid metabolism and atherosclerosis, dyslipidemia and atorvastatin response. Clinica Chimica Acta 2013;417:8‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rodriguez‐Roa 2008 {published data only}
- Rodriguez‐Roa E, Tellez R, Rodriguez F, Gonzalez M. Comparative evaluation of clinical effectiveness of two atorvastatin formulations in patients with or without cardiovascular disease: a national multicentric study. Revista Latinoamericana de Hipertension 2008;3(4):129‐35. [EMBASE: 2008497379] [Google Scholar]
ROMEO 2011 {published data only}
- NCT00395486. A 6‐week, Randomised, Open‐label, Parallel Group, Multi‐centre Study to Compare the Efficacy of Rosuvastatin 10mg With Atorvastatin 10mg in the Treatment of Metabolic Syndrome Subjects With Raised LDL‐C. https://clinicaltrials.gov/ct2/show/NCT00395486 (accessed 2 March 2015).
Rosales 2012 {published data only}
- Rosales A, Alvear M, Cuevas A, Saavedra N, Zambrano T, Salazar LA. Identification of pharmacogenetic predictors of lipid‐lowering response to atorvastatin in Chilean subjects with hypercholesterolemia. Clinica Chimica Acta 2012;413(3‐4):495‐501. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rosenson 2009 {published data only}
- Rosenson RS, Otvos JD, Hsia J. Effects of rosuvastatin and atorvastatin on LDL and HDL particle concentrations in patients with metabolic syndrome: a randomized, double‐blind, controlled study. Diabetes Care 2009;32(6):1087‐91. [EMBASE: 2009272130] [DOI] [PMC free article] [PubMed] [Google Scholar]
SAGE 2007 {published data only}
- Deedwania P, Stone PH, Bairey Merz CN, Cosin‐Aguilar J, Koylan N, Luo D, et al. Effects of intensive versus moderate lipid‐lowering therapy on myocardial ischemia in older patients with coronary heart disease: results of the Study Assessing Goals in the Elderly (SAGE). Circulation 2007;115(6):700‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Deedwania PC. Study Assessing Goals in the Elderly Steering Committee and Investigators. Effect of aggressive versus moderate lipid‐lowering therapy on myocardial ischemia: the rationale, design, and baseline characteristics of the Study Assessing Goals in the Elderly (SAGE). American Heart Journal 2004;148(6):1053‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pfizer Inc. A Prospective, Randomized, Double‐blind, Multi‐center Study Comparing the Effects of Aggressive Lipid Lowering with Moderate Lipid Lowering on the Reduction of Total Duration of Myocardial Ischema in the Elderly as Measured by Holter Monitoring by Comparing the Maximal Doses of Two Statins: Study Assessing Goals in the Elderly (SAGE). Protocol No. 981‐400‐421‐429 2004.
Sakabe 2004 {published data only}
- Sakabe K, Fukuda N, Wakayama K, Nada T, Shinohara H, Tamura Y. Lipid‐altering changes and pleiotropic effects of atorvastatin in patients with hypercholesterolemia. American Journal of Cardiology 2004;94(4):497‐500. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sakabe 2008a {published data only}
- Sakabe K, Fukuda N, Fukuda Y, Wakayama K, Nada T, Morishita S, et al. Gender differences in short‐term effects of atorvastatin on lipid profile, fibrinolytic parameters, and endothelial function. Nutrition Metabolism & Cardiovascular Diseases 2008;18(3):182‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saklamaz 2005 {published data only}
- Saklamaz A, Comlekci A, Temiz A, Caliskan S, Ceylan C, Alacacioglu A, et al. The beneficial effects of lipid‐lowering drugs beyond lipid‐lowering effects: a comparative study with pravastatin, atorvastatin, and fenofibrate in patients with type IIa and type IIb hyperlipidemia. Metabolism: Clinical & Experimental 2005;54(5):677‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sansanayudh 2010 {published data only}
- Sansanayudh N, Wongwiwatthananukit S, Putwai P, Dhumma‐Upakorn R. Comparative efficacy and safety of low‐dose pitavastatin versus atorvastatin in patients with hypercholesterolemia. The Annals of Pharmacotherapy 2010;44(3):415‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sardo 2002 {published data only}
- Sardo MA, Castaldo M, Cinquegrani M, Bonaiuto M, Maesano A, Versace A, et al. Effects of atorvastatin treatment on sICAM‐1 and plasma nitric oxide levels in hypercholesterolemic subjects. Clinical and Applied Thrombosis/Hemostasis 2002;8(3):257‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sari 2007 {published data only}
- Sari R, Eray E. The effect of atorvastatin treatment on insulin resistance, leptin, and highly sensitive C‐reactive protein in hypercholesterolemic patients. Endocrinologist 2007;17(6):315‐7. [EMBASE: 2007624268] [Google Scholar]
Sasaki 2008 {published data only}
- Sasaki J, Ikeda Y, Kuribayashi T, Kajiwara K, Biro S, Yamamoto K, et al. A 52‐week, randomized, open‐label, parallel‐group comparison of the tolerability and effects of pitavastatin and atorvastatin on high‐density lipoprotein cholesterol levels and glucose metabolism in Japanese patients with elevated levels of low‐density lipoprotein cholesterol and glucose intolerance. Clinical Therapeutics 2008;30(6):1089‐101. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sathyapalan 2009 {published data only}
- Sathyapalan T, Kilpatrick ES, Coady AM, Atkin SL. The effect of atorvastatin in patients with polycystic ovary syndrome: a randomized double‐blind placebo‐controlled study. Journal of Clinical Endocrinology & Metabolism 2009;94(1):103‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Save 2006 {published data only}
- Save V, Patil N, Moulik N, Rajadhyaksha G. Effect of atorvastatin on type 2 diabetic dyslipidemia. Journal of Cardiovascular Pharmacology & Therapeutics 2006;11(4):262‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schneck 2003 {published data only}
- Schneck DW, Knopp RH, Ballantyne CM, McPherson R, Chitra RR, Simonson SG. Comparative effects of rosuvastatin and atorvastatin across their dose ranges in patients with hypercholesterolemia and without active arterial disease. American Journal of Cardiology 2003;91(1):33‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schneider 2004 {published data only}
- Schneider JG, Eynatten M, Parhofer KG, Volkmer JE, Schiekofer S, Hamann A, et al. Atorvastatin improves diabetic dyslipidemia and increases lipoprotein lipase activity in vivo. Atherosclerosis 2004;175(2):325‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Eynatten M, Liu D, Bluemm A, Schuster T, Baumann M, Lutz J, et al. Changes in adiponectin multimer distribution in response to atorvastatin treatment in patients with type 2 diabetes. Clinical Endocrinology 2009;71(1):27‐32. [EMBASE: 2009287090] [DOI] [PubMed] [Google Scholar]
Schrott 1998 {published data only}
- Pfizer Inc. Crystalline Form Atorvastatin Dose‐Ranging. Protocol No. 981‐96 1997.
- Schrott H, Fereshetian AG, Knopp RH, Bays H, Jones PH, Littlejohn III TW, et al. A multicenter, placebo‐controlled, dose‐ranging study of atorvastatin. Journal of Cardiovascular Pharmacology & Therapeutics 1998;3(2):119‐24. [EMBASE: 1999101186] [DOI] [PubMed] [Google Scholar]
Schwartz 2004 {published data only}
- AstraZeneca. A 24‐Week, Randomized, Double‐Blind, Multicenter Trial to Evaluate the Efficacy and Safety of Starting and Maximum Doses of ZD4522 and Atorvastatin in the Treatment of High Risk Hypercholesterolemic Subjects (4522IL/0025). Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research Office of Biostatistics 1999‐2000:17‐25. [NDA 21‐366]
- Schwartz GG, Bolognese MA, Tremblay BP, Caplan R, Hutchinson H, Raza A, et al. Efficacy and safety of rosuvastatin and atorvastatin in patients with hypercholesterolemia and a high risk of coronary heart disease: a randomized, controlled trial. American Heart Journal 2004;148(1):e4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shabana 2013 {published data only}
- Shabana MF, Mishriki AA, Issac MS, Bakhoum SW. Do MDR1 and SLCO1B1 polymorphisms influence the therapeutic response to atorvastatin? A study on a cohort of Egyptian patients with hypercholesterolemia. Molecular Diagnosis & Therapy 2013;17(5):299‐309. [EMBASE: 2013633604] [DOI] [PubMed] [Google Scholar]
Shimabukuro 2011 {published data only}
- Shimabukuro M, Higa M, Tanaka H, Shimabukuro T, Yamakawa K, Masuzaki H. Distinct effects of pitavastatin and atorvastatin on lipoprotein subclasses in patients with Type 2 diabetes mellitus. Diabetic Medicine: a Journal of the British Diabetic Association 2011;28(7):856‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shishehbor 2003 {published data only}
- Shishehbor MH, Aviles RJ, Brennan ML, Fu X, Goormastic M, Pearce GL, et al. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA 2003;289(13):1675‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SHUKRA 2008 {unpublished data only}
- AstraZeneca. A Phase IV, 6‐Week, Randomised, Double‐blind, Multicentre, Parallel Group, Comparative Study to Evaluate the Efficacy of Rosuvastatin 5 mg and Atorvastatin 10 mg in UK Asian Subjects with Primary Hypercholesterolaemia SHUKRA ‐ Study of Asian Patients with Hypercholesterolaemia in the UK Rosuvastatin 5 mg versus Atorvastatin 10 mg. Protocol No. D3560L00060 2008.
Simons 1998 {published data only}
- Simons LA. Comparison of atorvastatin alone versus simvastatin +/‐ cholestyramine in the management of severe primary hypercholesterolaemia (the six cities study). Australian & New Zealand Journal of Medicine 1998;28(3):327‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Singh 2008 {published data only}
- Singh U, Devaraj S, Jialal I, Siegel D. Comparison effect of atorvastatin (10 versus 80 mg) on biomarkers of inflammation and oxidative stress in subjects with metabolic syndrome. The American Journal of Cardiology 2008;102(3):321‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinski 2009 {published data only}
- Sinski M, Lewandowski J, Ciarka A, Bidiuk J, Abramczyk P, Dobosiewicz A, et al. Atorvastatin reduces sympathetic activity and increases baroreceptor reflex sensitivity in patients with hypercholesterolaemia and systemic arterial hypertension. Kardiologia Polska 2009;67(6):613‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Sirtori 2005 {published data only}
- Sirtori CR, Calabresi L, Pisciotta L, Cattin L, Pauciullo P, Montagnani M, et al. Effect of statins on LDL particle size in patients with familial combined hyperlipidemia: a comparison between atorvastatin and pravastatin. Nutrition Metabolism & Cardiovascular Diseases 2005;15(1):47‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SLIM 2009 {published data only}
- Knopp RH, Retzlaff BM, Fish B, Dowdy A, Twaddell B, Nguyen T, et al. The SLIM study: slo‐niacin and atorvastatin treatment of lipoproteins and inflammatory markers in combined hyperlipidemia. Journal of Clinical Lipidology 2009;3(3):167‐78. [EMBASE: 2009284377] [DOI] [PMC free article] [PubMed] [Google Scholar]
SOLAR 2007 {published data only}
- AstraZeneca. A 12‐Week, Randomized, Open‐label, 3‐Arm Parallel‐group, Multicenter, Phase IIIb Study Comparing the Efficacy and Safety of Rosuvastatin with Atorvastatin and Simvastatin Achieving NCEP ATP III LDL‐C Goals in High‐risk Subjects with Hypercholesterolemia in the Managed Care Setting (SOLAR study). Protocol No. D3560L00023 2005.
- Insull W Jr, Ghali JK, Hassman DRY, As JW, Gandhi SK, Miller E. Achieving low‐density lipoprotein cholesterol goals in high‐risk patients in managed care: comparison of rosuvastatin, atorvastatin, and simvastatin in the SOLAR trial. Mayo Clinic Proceedings 2007;82(5):543‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Insull W, Ycas J, Miller E. Effect of three statins at starting dose on achieving national LDL‐C goals in hypercholesterolemic patients with or without diabetes in a managed‐care setting. Diabetes 2005;54 Suppl 1:A568. [IDS Number: 931QZ] [Google Scholar]
Sposito 2003 {published data only}
- Sposito AC, Santos RD, Amancio RF, Ramires JA, Chapman MJ, Maranhao RC. Atorvastatin enhances the plasma clearance of chylomicron‐like emulsions in subjects with atherogenic dyslipidemia: relevance to the in vivo metabolism of triglyceride‐rich lipoproteins. Atherosclerosis 2003;166(2):311‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
STARSHIP 2006 {published data only}
- AstraZeneca. Report on the Randomized Treatment Phase. A 6‐week, Randomized, Open‐Label, Comparative Study to Evaluate the Efficacy and Safety of Rosuvastatin and Atorvastatin in the Treatment of Hypercholesterolemia in Hispanic Subjects (STARSHIP). Protocol No. D3560L00027 (4522US/0007) 2005.
- Lloret R, Ycas J, Stein M, Haffner S, STARSHIP Study Group. Comparison of rosuvastatin versus atorvastatin in Hispanic‐Americans with hypercholesterolemia (from the STARSHIP trial). American Journal of Cardiology 2006;98(6):768‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
STELLAR 2003 {published data only}
- Ai M, Otokozawa S, Asztalos BF, Nakajima K, Stein E, Jones PH, et al. Effects of maximal doses of atorvastatin versus rosuvastatin on small dense low‐density lipoprotein cholesterol levels. The American Journal of Cardiology 2008; Vol. 101, issue 3:315‐8. [MEDLINE: ] [DOI] [PubMed]
- AstraZeneca. A 6‐Week, Open‐Label, Dose‐Comparison Study to Evaluate the Safety and Efficacy of Rosuvastatin Versus Atorvastatin, Pravastatin, and Simvastatin in Subjects with Hypercholesterolemia (STELLAR). Protocol No. 4522IL/0065_LTE/D3560C00065 2005.
- Asztalos BF, Maulf F, Dallal GE, Stein E, Jones PH, Horvath KV, et al. Comparison of the effects of high doses of rosuvastatin versus atorvastatin on the subpopulations of high‐density lipoproteins. American Journal of Cardiology 2007;99(5):681‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Barrios V, Lobos J M, Serrano A, Brosa M, Capel M, Alvarez Sanz C. Cost‐effectiveness analysis of rosuvastatin vs generic atorvastatin in Spain. Journal of Medical Economics 2012;15 Suppl 1:45‐54. [CENTRAL: CN‐00858418 NEW] [DOI] [PubMed] [Google Scholar]
- Chong PH, Varner D. Cost‐efficacy analysis of 3‐hydroxy‐3‐methylglutaryl coenzyme a reductase inhibitors based on results of the STELLAR trial: clinical implications for therapeutic selection. Pharmacotherapy 2005;25(2):270‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Costa‐Scharplatz M, Ramanathan K, Frial T, Beamer B, Gandhi S. Cost‐effectiveness analysis of rosuvastatin versus atorvastatin, simvastatin, and pravastatin from a Canadian health system perspective. Clinical Therapeutics 2008; Vol. 30, issue 7:1345‐57. [MEDLINE: ] [DOI] [PubMed]
- Costa‐Scharplatz M, Ramanathan K, Frial T, Beamer B, Gandhi S. Cost‐effectiveness analysis of rosuvastatin versus atorvastatin, simvastatin, and pravastatin from a Canadian health system perspective. Clinical Therapeutics 2008;30(7):1345‐57. [EMBASE: 2008371628] [DOI] [PubMed] [Google Scholar]
- Deedwania PC, Hunninghake DB, Bays HE, Jones PH, Cain VA, Blasetto JW. Effects of rosuvastatin, atorvastatin, simvastatin, and pravastatin on atherogenic dyslipidemia in patients with characteristics of the metabolic syndrome. American Journal of Cardiology 2005;95(3):360‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hirsch M, O'Donnell JC, Jones P. Rosuvastatin is cost‐effective in treating patients to low‐density lipoprotein‐cholesterol goals compared with atorvastatin, pravastatin and simvastatin: analysis of the STELLAR trial. European Journal of Cardiovascular Prevention & Rehabilitation. 2005;12(1):18‐28. [MEDLINE: ] [PubMed] [Google Scholar]
- Jones PH, Davidson MH, Stein EA, Bays HE, McKenney JM, Miller E, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). American Journal of Cardiology 2003;92(2):152‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jones PH, Hunninghake DB, Ferdinand KC, Stein EA, Gold A, Caplan RJ, et al. Statin therapies for elevated lipid levels compared across doses to rosuvastatin study group. Effects of rosuvastatin versus atorvastatin, simvastatin, and pravastatin on non‐high‐density lipoprotein cholesterol, apolipoproteins, and lipid ratios in patients with hypercholesterolemia: additional results from the STELLAR trial. Clinical Therapeutics 2004;26(9):1388‐99. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- McKenney JM, Jones PH, Adamczyk MA, Cain VA, Bryzinski BS, Blasetto JW. Comparison of the efficacy of rosuvastatin versus atorvastatin, simvastatin, and pravastatin in achieving lipid goals: results from the STELLAR trial. Current Medical Research & Opinion 2003;19(8):689‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Miller PS, Smith DG, Jones P. Cost effectiveness of rosuvastatin in treating patients to low‐density lipoprotein cholesterol goals compared with atorvastatin, pravastatin, and simvastatin (a US analysis of the STELLAR trial). American Journal of Cardiology 2005;95(11):1314‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Otokozawa S, Ai M, Himbergen T, Asztalos BF, Tanaka A, Stein EA, et al. Effects of intensive atorvastatin and rosuvastatin treatment on apolipoprotein B‐48 and remnant lipoprotein cholesterol levels. Atherosclerosis 2009; Vol. 205, issue 1:197‐201. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
- Thongtang N, Ai M, Otokozawa S, Himbergen TV, Asztalos BF, Nakajima K, et al. Effects of maximal atorvastatin and rosuvastatin treatment on markers of glucose homeostasis and inflammation: associated included citation for the STELLAR trial. American Journal of Cardiology 2011;107(3):387‐92. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himbergen TM, Matthan NR, Resteghini NA, Otokozawa S, Ai M, Stein EA, et al. Comparison of the effects of maximal dose atorvastatin and rosuvastatin therapy on cholesterol synthesis and absorption markers. Journal of Lipid Research 2009;50(4):730‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stojakovic 2007 {published data only}
- Stojakovic T, Putz‐Bankuti C, Fauler G, Scharnagl H, Wagner M, Stadlbauer V, et al. Atorvastatin in patients with primary biliary cirrhosis and incomplete biochemical response to ursodeoxycholic acid. Hepatology 2007;46(3):776‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SToP AF 2011 {published data only}
- Negi S, Shukrullah I, Veledar E, Bloom HL, Jones DP, Dudley SC. Statin therapy for the prevention of atrial fibrillation trial (SToP AF trial). Journal of Cardiovascular Electrophysiology 2011;22(4):414‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
STRENGTH 2008 {published data only}
- Voora D, Shah SH, Reed CR, Zhai J, Crosslin DR, Messer C, et al. Pharmacogenetic predictors of statin‐mediated low‐density lipoprotein cholesterol reduction and dose response. Circulation. Cardiovascular Genetics 2008;1(2):100‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voora D, Shah SH, Spasojevic I, Ali S, Reed CR, Salisbury BA, et al. The SLCO1B1*5 genetic variant is associated with statin‐induced side effects. Journal of the American College of Cardiology 2009; Vol. 54, issue 17:1609‐16. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
Stulc 2008 {published data only}
- Stulc T, Vrablik M, Kasalova Z, Marinov I, Svobodova H, Ceska R. Leukocyte and endothelial adhesion molecules in patients with hypercholesterolemia: the effect of atorvastatin treatment. Physiological Research 2008;57(2):184‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Suleiman 2012 {published data only}
- Suleiman M, Koestler C, Lerman A, Lopez‐Jimenez F, Herges R, Hodge D, et al. Atorvastatin for prevention of atrial fibrillation recurrence following pulmonary vein isolation: a double‐blind, placebo‐controlled, randomized trial. Heart Rhythm 2012;9(2):172‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SUPREME 2009 {published data only}
- Insull W Jr, Basile JN, Vo AN, Jiang P, Thakkar R, Padley RJ. Efficacy and safety of combination therapy with niacin extended‐release and simvastatin versus atorvastatin in patients with dyslipidemia: the SUPREME Study. Journal of Clinical Lipidology 2009;3(2):109‐18. [EMBASE: 2009130987] [DOI] [PubMed] [Google Scholar]
- Insull W Jr, Toth PP, Superko HR, Thakkar RB, Krause S, Jiang P, et al. Combination of niacin extended‐release and simvastatin results in a less atherogenic lipid profile than atorvastatin monotherapy. Vascular Health and Risk Management 2010; Vol. 6:1065‐75. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
Szapary 2004 {published data only}
- Szapary L, Horvath B, Marton Z, Alexy T, Kesmarky G, Habon T, et al. Short‐term effect of low‐dose atorvastatin on haemorheological parameters, platelet aggregation and endothelial function in patients with cerebrovascular disease and hyperlipidaemia. CNS Drugs 2004;18(3):165‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tagle 2000 {published data only}
- Tagle M, Salazar A, Gomez L, Alava Y, Bajana W. Clinical observation of the efficacy and safety of atorvastatin in the therapy of patients with hypercholesterolemia [Observacion clinica de la eficacia y seguridad de la administracion de atrovastatina en el tratamiento de pacientes con hipercolesterolemia]. Endocrinologia y Nutricion 2000;47(6):156‐60. [EMBASE: 2000318241] [Google Scholar]
Takebayashi 2005 {published data only}
- Takebayashi K, Matsumoto S, Wakabayashi S, Inukai Y, Matsutomo R, Aso Y, et al. The effect of low‐dose atorvastatin on circulating monocyte chemoattractant protein‐1 in patients with type 2 diabetes complicated by hyperlipidemia. Metabolism: Clinical & Experimental 2005;54(9):1225‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tan 2002 {published data only}
- Tan KC, Chow WS, Tam SC, Ai VH, Lam CH, Lam KS. Atorvastatin lowers C‐reactive protein and improves endothelium‐dependent vasodilation in type 2 diabetes mellitus. Journal of Clinical Endocrinology & Metabolism 2002;87(2):563‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tanaka 2001 {published data only}
- Tanaka A, Yamada N, Saito Y, Kawakami M, Ohashi Y, Akanuma Y. A double‐blind trial on the effects of atorvastatin on glycemic control in Japanese diabetic patients with hypercholesterolemia. Clinica Chimica Acta 2001;312(1‐2):41‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
TARGET TANGIBLE 1999 {published data only}
- Maerz W, Wollschlaeger H, Klein G, Neiss A, Wehling M. Safety of low‐density lipoprotein cholesterol reduction with atorvastatin versus simvastatin in a coronary heart disease population (the TARGET TANGIBLE trial). American Journal of Cardiology 1999;84(1):7‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Maerz W, Wollschlager H, Klein G, Neiss A, Wehling M. Comparison of the safety of atorvastatin and simvastatin in patients with coronary heart disease ‐ the target tangible trial. Atherosclerosis 1999;144(1):29. [CENTRAL: CN‐00446640] [DOI] [PubMed] [Google Scholar]
- Marz W, Wollschlager H, Klein G, Neiss A, Wehling M. Efficacy and safety of atorvastatin versus simvastatin in type II diabetes patients with coronary heart disease. Diabetologia 1999;42(Supplement 1):A19. [IDS Number: 225CC] [Google Scholar]
- Marz W, Wollschlager H, Klein G, Neiss A, Wehling M. Safety of low‐density lipoprotein cholesterol reduction with atorvastatin versus simvastatin in a coronary heart disease population (the TARGET TANGIBLE Trial). Perfusion 1999;12(10):427‐36. [IDS Number: 252QE] [DOI] [PubMed] [Google Scholar]
- Ruof J, Klein G, Marz W, Wollschlager H, Neiss A, Wehling M. Lipid‐lowering medication for secondary prevention of coronary heart disease in a German outpatient population: the gap between treatment guidelines and real life treatment patterns. Preventive Medicine 2002;35(1):48‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tateishi 2011 {published data only}
- Tateishi J. Efficacy of three potent statins in patients with hypercholesterolemia who were newly prescribed statins. Therapeutic Research 2011;32(12):1653‐61. [EMBASE: 2012051834] [Google Scholar]
Tekin 2004 {published data only}
- Tekin A, Tekin G, Guzelsoy D, Kaya A, Gurel CV, Yigit Z, et al. Effects of atorvastatin (10 mg) on hemostatic and inflammatory parameters in hyperlipidemic patients with angiographically proven coronary artery disease. American Journal of Cardiology 2004;94(2):206‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tekten 2004 {published data only}
- Tekten T, Ceyhan C, Ercan E, Onbasili AO, Turkoglu C. The effect of atorvastatin on platelet function in patients with coronary artery disease. Acta Cardiologica 2004;59(3):311‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tomas 2004 {published data only}
- Tomas JP, Moya JL, Campuzano R, Barrios V, Megias A, Ruiz S, et al. Noninvasive assessment of the effect of atorvastatin on coronary microvasculature and endothelial function in patients with dyslipidemia. Revista Espanola de Cardiologia 2004;57(10):909‐15. [MEDLINE: ] [PubMed] [Google Scholar]
Tousoulis 2005 {published data only}
- Tousoulis D, Antoniades C, Vassiliadou C, Toutouza M, Pitsavos C, Tentolouris C, et al. Effects of combined administration of low dose atorvastatin and vitamin E on inflammatory markers and endothelial function in patients with heart failure. European Journal of Heart Failure 2005;7(7):1126‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tousoulis 2006 {published data only}
- Tousoulis D, Bosinakou E, Kotsopoulou M, Antoniades C, Katsi V, Stefanadis C. Effects of early administration of atorvastatin treatment on thrombotic process in normocholesterolemic patients with unstable angina. International Journal of Cardiology 2006;106(3):333‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tousoulis 2011 {published data only}
- Tousoulis D, Koniari K, Antoniades C, Papageorgiou N, Miliou A, Noutsou M, et al. Combined effects of atorvastatin and metformin on glucose‐induced variations of inflammatory process in patients with diabetes mellitus. International Journal of Cardiology 2011;149(1):46‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tsunoda 2011 {published data only}
- Tsunoda S. Efficacy and safety of rosuvastatin 2.5 mg and atorvastatin 10 mg in patients with hypercholesterolemia. Therapeutic Research 2011;32(11):1507‐12. [EMBASE: 2011711049] [Google Scholar]
Undas 2006a {published data only}
- Undas A, Brummel‐Ziedins KE, Potaczek DP, Stobierska‐Dzierzek B, Bryniarski L, Szczeklik A, et al. Atorvastatin and quinapril inhibit blood coagulation in patients with coronary artery disease following 28 days of therapy. Journal of Thrombosis & Haemostasis 2006;4(11):2397‐404. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Uydu 2012 {published data only}
- Uydu H A, Yildirmis S, Orem C, Calapoglu M, Alver A, Kural B, et al. The effects of atorvastatin therapy on rheological characteristics of erythrocyte membrane, serum lipid profile and oxidative status in patients with dyslipidemia. Journal of Membrane Biology 2012;245(11):697‐705. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vansant 2001 {published data only}
- Vansant G, Mertens A, Muls E. The effect of atorvastatin on postprandial lipidaemia in overweight or obese women homozygous for apo E3. Acta Cardiologica 2001;56(3):149‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
VISION 2013 {published data only}
- Yoshida H, Shoda T, Yanai H, Ikewaki K, Kurata H, Ito K, et al. Effects of pitavastatin and atorvastatin on lipoprotein oxidation biomarkers in patients with dyslipidemia. Atherosclerosis 2013;226(1):161‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
VYTAL 2006 {published data only}
- Goldberg RB, Guyton JR, Mazzone T, Weinstock RS, Polis A, Edwards P, et al. Ezetimibe/simvastatin vs atorvastatin in patients with type 2 diabetes mellitus and hypercholesterolemia: the VYTAL study. Mayo Clinic Proceedings 2006;81(12):1579‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Goldberg RB, Guyton JR, Mazzone T, Weinstock RS, Polis AB, Tipping D, et al. Relationships between metabolic syndrome and other baseline factors and the efficacy of ezetimibe/simvastatin and atorvastatin in patients with type 2 diabetes and hypercholesterolemia. Diabetes Care 2010; Vol. 33, issue 5:1021‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
- Guyton JR, Goldberg RB, Mazzone T, Weinstock RS, Polis A, Rosenberg E, et al. Lipoprotein and apolipoprotein ratios in the VYTAL trial of ezetimibe/simvastatin compared with atorvastatin in type 2 diabetes. Journal of Clinical Lipidology 2008;2(1):19‐24. [EMBASE: 2008082220] [DOI] [PubMed] [Google Scholar]
- Merck, Co Inc. Ezetimibe/Simvastatin (Vytorin) versus Atorvastatin in Type 2 Diabetes Patients with Hypercholesterolemia: the VYTAL Study. Merck Protocol Number: 077 2005.
- Tomassini JE, Mazzone T, Goldberg RB, Guyton JR, Weinstock RS, Polis A, et al. Effect of ezetimibe/simvastatin compared with atorvastatin on lipoprotein subclasses in patients with type 2 diabetes and hypercholesterolaemia. Diabetes, Obesity & Metabolism 2009; Vol. 11, issue 9:855‐64. [MEDLINE: ] [DOI] [PubMed]
VYTELD 2010 {published data only}
- Foody JM, Brown WV, Zieve F, Adewale AJ, Flaim D, Lowe RS, et al. Safety and efficacy of ezetimibe/simvastatin combination versus atorvastatin alone in adults >=65 years of age with hypercholesterolemia and with or at moderately high/high risk for coronary heart disease (the VYTELD study). American Journal of Cardiology 2010;106(9):1255‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yip A, Hegele RA. Lipid modification in the elderly using the combination of a statin and a cholesterol absorption inhibitor: associated included citation for the VYTELD 2010 trial. Expert Opinion on Pharmacotherapy 2011;12(4):675‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
VYVA 2005 {published data only}
- Abate N, Catapano AL, Ballantyne CM, Davidson MH, Polis A, Smugar SS, et al. Effect of ezetimibe/simvastatin versus atorvastatin or rosuvastatin on modifying lipid profiles in patients with diabetes, metabolic syndrome, or neither: results of two subgroup analyses. Journal of Clinical Lipidology 2008;2(2):91‐105. [EMBASE: 2008124668] [DOI] [PubMed] [Google Scholar]
- Ballantyne CM, Abate N, King TR, Yuan Z, Palmisano J, Tershakovec A. Ezetimibe/Simvastatin versus atorvastatin for attainment of apolipoprotein B and C‐reactive protein goals: a VYVA substudy. Journal of the American College of Cardiology 2006;47 Suppl A(4):335A. [IDS Number: 015CV] [Google Scholar]
- Ballantyne CM, Abate N, Yuan Z. Dose‐comparison study of the combination of ezetimibe and simvastatin (Vytorin) versus atorvastatin in patients with hypercholesterolemia: the Vytorin Versus Atorvastatin (VYVA) study. American Heart Journal 2005;149(5):882. [IDS Number: 932NN] [DOI] [PubMed] [Google Scholar]
- Ballantyne CM, Abate N, Yuan Z, King TR, Palmisano J. Dose‐comparison study of the combination of ezetimibe and simvastatin (Vytorin) versus atorvastatin in patients with hypercholesterolemia: the Vytorin Versus Atorvastatin (VYVA) study. American Heart Journal 2005;149(3):464‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ballantyne CM, Abate N, Yuan Z, King TR, Palmisano J. Prevention and rehabilitation ‐ dose‐comparison study of the combination of ezetimibe and simvastatin (Vytorin) versus atorvastatin in patients with hypercholesterolemia: the Vytorin Versus Atorvastatin (VYVA) study. American Heart Journal 2005;149(3):464‐73. [DOI: 10.1016/j.ahj.2004.11.023; IDS Number: 917RV] [DOI] [PubMed] [Google Scholar]
- Davidson MH, Abate N, Ballantyne CM, Catapano AL, Xu X, Lin J, et al. Ezetimibe/simvastatin compared with atorvastatin or rosuvastatin in lowering to specified levels both LDL‐C and each of five other emerging risk factors for coronary heart disease: non‐HDL‐cholesterol, TC/HDL‐C, apolipoprotein B, apo‐B/apo‐A‐I, or C‐reactive protein. Journal of Clinical Lipidology 2008;2(6):436‐46. [EMBASE: 2008584795] [DOI] [PubMed] [Google Scholar]
- Merck, Co Inc. Dose‐comparison study of the combination of ezetimibe and simvastatin (Vytorin) versus atorvastatin in patients with hypercholesterolemia: the Vytorin Versus Atorvastatin (VYVA) study. Merck Protocol Number: 051‐0 2004.
- Pearson T, Ballantyne C, Sisk C, Shah A, Veltri E, Maccubbin D. Comparison of effects of ezetimibe/simvastatin versus simvastatin versus atorvastatin in reducing C‐reactive protein and low‐density lipoprotein cholesterol levels. American Journal of Cardiology 2007;99(12):1706‐13. [EMBASE: 2007276098] [DOI] [PubMed] [Google Scholar]
- Polis AB, Abate N, Catapano AL, Ballantyne CM, Davidson MH, Smugar SS, et al. Low‐density lipoprotein cholesterol reduction and goal achievement with ezetimibe/simvastatin versus atorvastatin or rosuvastatin in patients with diabetes, metabolic syndrome, or neither disease, stratified by National Cholesterol Education Program risk category. Metabolic Syndrome and Related Disorders 2009; Vol. 7, issue 6:601‐10. [MEDLINE: ] [DOI] [PubMed]
Wang 2001 {published data only}
- Wang KY, Ting CT. A randomized, double‐blind, placebo‐controlled, 8‐week study to evaluate the efficacy and safety of once daily atorvastatin (10 mg) in patients with elevated LDL‐cholesterol. Japanese Heart Journal 2001;42(6):725‐38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang 2012 {published data only}
- Wang GH, Zhang X, Cao J, Li HT, Yin D, Zhou CJ. Lipid‐lowering efficacy and safety of rosuvastatin calcium in treatment of patients with chronic kidney disease. Guangdong Yixue 2012;33(1):125‐7. [CENTRAL: CN‐00858145 NEW] [Google Scholar]
WATCH 2001 {published data only}
- McPherson R, Angus C, Murray P, Genest J Jr. Efficacy of atorvastatin in achieving national cholesterol education program low‐density lipoprotein targets in women with severe dyslipidemia and cardiovascular disease or risk factors for cardiovascular disease: the Women's Atorvastatin Trial on Cholesterol (WATCH). American Heart Journal 2001;141(6):949‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wei 2001 {unpublished data only}
- Wei L. Clinical features and risk of coronary heart disease in familial hypercholesterolaemia and studies on hypolipidaemic drug treatment in Hong Kong Chinese. DAI‐B 62/01 2001:p. 151. [ProQuest document ID: 728458161]
Welder 2010 {published data only}
- Welder G, Zineh I, Pacanowski MA, Troutt JS, Cao G, Konrad RJ, et al. High‐dose atorvastatin causes a rapid sustained increase in human serum PCSK9 and disrupts its correlation with LDL cholesterol. Journal of Lipid Research 2010;51(9):2714‐21. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wierzbicki 1998 {published data only}
- Wierzbicki AS, Lumb PJ, Semra YK, Crook MA. High‐dose atorvastatin therapy in severe heterozygous familial hypercholesterolaemia. Quarterly Journal of Medicine 1998;91(4):291‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Willrich 2008 {published data only}
- Genvigir FD, Soares SA, Hirata MH, Willrich MA, Arazi SS, Rebecchi IM, et al. Effects of ABCA1 SNPs, including the C‐105T novel variant, on serum lipids of Brazilian individuals. Clinica Chimica Acta 2008;389(1‐2):79‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rebecchi IM, Rodrigues AC, Arazi SS, Genvigir FD, Willrich MA, Hirata MH, et al. ABCB1 and ABCC1 expression in peripheral mononuclear cells is influenced by gene polymorphisms and atorvastatin treatment. Biochemical Pharmacology 2009;77(1):66‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rodrigues AC, Perin PMS, Purim SG, Silbiger VN, Genvigir FDV, Willrich MAV, et al. Pharmacogenetics of OATP transporters reveals that SLCO1B1 c.388a>G variant is determinant of increased atorvastatin response. International Journal of Molecular Sciences 2011;12(9):5815‐27. [EMBASE: 2011537606] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willrich MA, Hirata MH, Genvigir FD, Arazi SS, Rebecchi IM, Rodrigues AC, et al. CYP3A53A allele is associated with reduced lowering‐lipid response to atorvastatin in individuals with hypercholesterolemia. Clinica Chimica Acta 2008;398(1‐2):15‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Willrich MA, Rodrigues AC, Cerda A, Genvigir FD, Arazi SS, Dorea EL, et al. Effects of atorvastatin on CYP3A4 and CYP3A5 mRNA expression in mononuclear cells and CYP3A activity in hypercholesterolemic patients: associated included citation for the Willrich 2008 trial. Clinica Chimica Acta 2013;421:157‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wu 2002 {published data only}
- Wu CC, Sy R, Tanphaichitr V, Hin ATT, Suyono S, Lee YT. Comparing the efficacy and safety of atorvastatin and simvastatin in Asians with elevated low‐density lipoprotein‐cholesterol ‐ a multinational, multicenter, double‐blind study. Journal of the Formosa Medical Association 2002;101(7):478‐87. [MEDLINE: ] [PubMed] [Google Scholar]
Wu 2005 {published data only}
- Wu SC, Shiang JC, Lin SL, Wu TL, Huang WC, Chiou KR, et al. Efficacy and safety of statins in hypercholesterolemia with emphasis on lipoproteins. Heart & Vessels 2005;20(5):217‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yoshitomi 2005 {published data only}
- Yoshitomi Y, Ishii T, Kaneki M, Tsujibayashi T, Sakurai S, Nagakura C, et al. Relationship between insulin resistance and effect of atorvastatin in non‐diabetic subjects. Journal of Atherosclerosis & Thrombosis 2005;12(1):9‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ZAPE 2003 {unpublished data only}
- Munoz MT, Alonso M, Oyarzabal M, Mainou C, Fabiani F, Sol JM, et al. Safety and efficacy of atorvastatin vs colestipol in children and adolescents with familial hypercholesterolemia. Circulation 2003;108 Suppl 4:IV‐689. [PREV200400052747] [Google Scholar]
- Pfizer Inc. A 1 Year, Open‐Label, Randomized Parallel Group Multicenter Study for the Comparison of Atorvastatin vs. Colestipol on the Treatment of Children and Adolescents with Familial Hypercholesterolemia (FH) and Hypercholesterolemia. Protocol 981‐336 2003.
Zhu 2000 {published data only}
- Zhu Q. Effects of atorvastatin treatment on the oxidatively modified low‐density lipoprotein in hyperlipidemic patients. MAI 38/01 2000:p116. [ProQuest document ID: 732074361] [DOI] [PubMed]
- Zhu Q, McMaster J, Mymin D, Dembinski T, Hatch G, Choy PC, et al. Effects of atorvastatin treatment on the oxidatively modified low density lipoprotein in hyperlipidemic patients. Molecular and Cellular Biochemistry 2000;207(1):9‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTFAST 2 2004 {published data only}
- Farsang C. Athyros V. Gaw A. ACTFAST‐2 Investigators and Steering Committee Members. A multicentre, open study to assess the effect of individualizing starting doses of atorvastatin according to baseline LDL‐C levels on achieving cholesterol targets: the Achieve Cholesterol Targets Fast with Atorvastatin Stratified Titration (ACTFAST‐2) study. Current Medical Research & Opinion 2007;23(8):1945‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pfizer Inc. Achieve Cholesterol Targets Fast with Atorvastatin Stratified Titration: a Multicenter, Twelve‐week Treatment, Single‐step Titration, Open‐label Study Assessing the Percentage of Dyslipidemic High‐risk Patients Achieving Low Density Lipoprotein Cholesterol (LDL‐C) Target with Atorvastatin Starting Doses of 10 mg, 20 mg, 40 mg, and 80 mg. Protocol No. A2581095 2004.
ADSL 2003 {unpublished data only}
- Pfizer. The atorvastatin efficacy and safety study in diabetic patients to determine starting dose for effectively reducing lipids (the ADSL study). Protocol No. A2581080 2003.
Ahmed 2008 {published data only}
- Ahmed S, Ullah E, Ahmed M, Abbas R, Khan MA, Iqbal J. Efficacy of combination of ezetimibe and simvastatin versus atorvastatin in reducing low density lipoprotein‐cholesterol in male patients of hypercholesterolemia, at Bahawalpur. Medical Forum Monthly. Medical Forum (Monthly) (Gujjar Singh, Lahore 5460, Pakistan), 2008; Vol. 19, issue 5:3‐9.
Alrasadi K 2008 {published data only}
- Alrasadi K, Awan Z, Alwaili K, Ruel I, Hafiane A, Krimbou L, et al. Comparison of treatment of severe high‐density lipoprotein cholesterol deficiency in men with daily atorvastatin (20 mg) versus fenofibrate (200 mg) versus extended‐release niacin (2 g). American Journal of Cardiology 2008;102(10):1341‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Alvarez 1999 {published data only}
- Alvarez ML, Errasti P, Gomez G, Lavilla FJ, Ballester B, Garcia I, et al. Effect of atorvastatin of the treatment of hypercholesterolemia after renal transplantation. Transplantation Proceedings 1999;31(6):2328‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arad 2005 {published data only}
- Arad Y, Newstein D, Roth M, Guerci AD. Rationale and design of the St. Francis Heart Study: a randomized clinical trial of atorvastatin plus antioxidants in asymptomatic persons with elevated coronary calcification. Controlled Clinical Trials 2001;22(5):553‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. Journal of the American College of Cardiology 2005;46(1):166‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Argent 2003 {published data only}
- Argent E, Kainer G, Aitken M, Rosenberg AR, Mackie FE. Atorvastatin treatment for hyperlipidemia in pediatric renal transplant recipients. Pediatric Transplantation 2003;7(1):38‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ASG 2008 {published data only}
- Atorvastatin Study Group. Flexible initial dosing of atorvastatin based upon initial low‐density lipoprotein cholesterol levels in type 2 diabetic patients. Korean Journal of Internal Medicine 2008;23(1):22‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
AstraZeneca 3 {unpublished data only}
- AstraZeneca. A Multicenter, Randomized, Double‐blind, Parallel‐group, Dose Titration (10 mg and 20 mg) Study to Compare the Efficacy and Safety of Rosuvastatin versus Atorvastatin in Patients with Primary Hypercholesterolemia. Protocol No. 4522SP/0001 2004.
AstraZeneca 4 {unpublished data only}
- AstraZeneca. A 6‐week, Randomised, Open‐label, Parallel Group, Multi‐centre Study to Compare the Efficacy of Rosuvastatin 10mg with Atorvastatin 10mg in the Treatment of Non‐diabetic Metabolic Syndrome Subjects with Raised LDL‐C. Protocol No. D3560L00053 2007.
ATGOAL 2005 {published data only}
- McKenney JM, Davidson MH, Saponaro J, Thompson PD, Bays HE. Use of a treatment algorithm to achieve NCEP ATP III goals with atorvastatin. Journal of Cardiovascular Pharmacology 2005;46(5):594‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pfizer Inc. A Multicenter, Eight‐Week Treatment, Single Step Titration, Open‐Label Study Assessing the Percentage of Dyslipidemic Patients Achieving Low Density Lipoprotein Cholesterol (LDL‐C) Target with Atorvastatin Starting Doses of 10 mg, 20 mg, 40 mg, and 80 mg (AT GOAL). Protocol No. A2581068 2002. [DOI] [PubMed]
- Pfizer Inc. A Multicenter, Eight‐Week Treatment, Single Step Titration, Open‐Label Study Assessing the Percentage of Dyslipidemic Subjects Achieving Low Density Lipoprotein Cholesterol Target With Atorvastatin Starting Doses of 10 mg, 20 mg, 40 mg, and 80 mg (Latin American Atorvastatin ATGOAL Study). Protocol No. A2581104 2005.
Athyros 2008 {published data only}
- Athyros VG, Tziomalos K, Kakafika AI, Koumaras H, Karagiannis A, Mikhailidis DP, et al. Effectiveness of ezetimibe alone or in combination with twice a week atorvastatin (10 mg) for statin intolerant high‐risk patients. American Journal of Cardiology 2008;101(4):483‐5. [DOI] [PubMed] [Google Scholar]
Atorvastatin 2008 {published data only}
- Atorvastatin Study Group. Flexible initial dosing of atorvastatin based upon initial low‐density lipoprotein cholesterol levels in type 2 diabetic patients. Korean Journal of Internal Medicine 2008;23(1):22‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Banyai 2001 {published data only}
- Banyai S, Banyai M, Falger J, Jansen M, Alt E, Derfler K, et al. Atorvastatin improves blood rheology in patients with familial hypercholesterolemia (FH) on long‐term LDL apheresis treatment. Atherosclerosis 2001;159(2):513‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boh 2011 {published data only}
- Boh M, Opolski G, Poredos P, Ceska R, Jezovnik M. Therapeutic equivalence of the generic and the reference atorvastatin in patients with increased coronary risk. International Angiology 2011;30(4):366‐74. [CENTRAL: CN‐00798821] [PubMed] [Google Scholar]
Bolewski 2008 {published data only}
- Bolewski A, Lipiecki J, Plewa R, Burchardt P, Siminiak T. The effect of atorvastatin treatment on lipid profile and adhesion molecule levels in hypercholesterolemic patients: relation to low‐density lipoprotein receptor gene polymorphism. Cardiology 2008;111(2):140‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bonet 2002 {published data only}
- Bonet LA, Martinez‐Dolz L, Vives A, Soriano JR, Saez AO, Gisbert FD, et al. Lipid‐lowering effect of atorvastatin in heart transplantation. Transplantation Proceedings 2002;34(1):179‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brown 2004 {published data only}
- Brown SL, Raal FJ, Panz VR, Stevens BA, Veller MG. High‐dose atorvastatin therapy is required for significant improvement of endothelial function in heterozygous familial hypercholesterolaemic patients. Cardiovascular Journal of Southern Africa 2004;15(2):70‐5. [MEDLINE: ] [PubMed] [Google Scholar]
CAPABLE {unpublished data only}
- Ferdinand KC, Flack JM, Saunders E, Victor R, Watson K, Kursun A, et al. Amlodipine/atorvastatin single‐pill therapy for blood pressure and lipid goals in African Americans: influence of the metabolic syndrome and type 2 diabetes mellitus. Journal of Clinical Hypertension 2009; Vol. 11, issue 10:585‐93. [DOI] [PMC free article] [PubMed]
- Flack JM, Victor R, Watson K, Ferdinand KC, Saunders E, Tarasenko L, et al. Improved attainment of blood pressure and cholesterol goals using single‐pill amlodipine/atorvastatin in African Americans: the CAPABLE trial. Mayo Clinic Proceedings 2008;83(1):35‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pfizer Inc. Clinical Utility of CADUET in Simultaneously Achieving Blood Pressure and Lipid Endpoints in a Specific Patient Population (CAPABLE). Protocol No. A3841025 2005.
Carnevale 2010 {published data only}
- Carnevale R, Pignatelli P, Santo S, Bartimoccia S, Sanguigni V, Napoleone L, et al. Atorvastatin inhibits oxidative stress via adiponectin‐mediated NADPH oxidase down‐regulation in hypercholesterolemic patients. Atherosclerosis 2010;213(1):225‐34. [DOI] [PubMed] [Google Scholar]
COMPELL 2007 {published data only}
- McKenney JM, Jones PH, Bays HE, Knopp RH, Kashyap ML, Ruoff GE, et al. Comparative effects on lipid levels of combination therapy with a statin and extended‐release niacin or ezetimibe versus a statin alone (the COMPELL study). Atherosclerosis 2007;192(2):432‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Conard 2008 {published data only}
- Conard SE, Bays HE, Leiter LA, Bird SR, Rubino J, Lowe RS, et al. Efficacy and safety of ezetimibe added on to atorvastatin (20 mg) versus up‐titration of atorvastatin (to 40 mg) in hypercholesterolemic patients at moderately high risk for coronary heart disease. The American Journal of Cardiology 2008; Vol. 102, issue 11:1489‐94. [DOI] [PubMed]
Costa 2003 {published data only}
- Costa A, Casamitjana R, Casals E, Alvarez L, Morales J, Masramon X, et al. Effects of atorvastatin on glucose homeostasis, postprandial triglyceride response and C‐reactive protein in subjects with impaired fasting glucose. Diabetic Medicine 2003;20(9):743‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Davis 2000 {published data only}
- Davis M, Atwal AS, Nair DR, Jagroop IA, Seifalian AM, Mikhailidis DP, et al. The effect of short‐term lipid lowering with atorvastatin on carotid artery media thickness in patients with peripheral vascular disease: a pilot study. Current Medical Research and Opinion 2000;16(3):198‐204. [MEDLINE: ] [PubMed] [Google Scholar]
Di Renzo 2008 {published data only}
- Renzo L, Noce A, Angelis S, Miani N, Daniele N, Tozzo C, et al. Anti‐inflammatory effects of combined treatment with acetyl salicylic acid and atorvastatin in haemodialysis patients affected by Normal Weight Obese syndrome. Pharmacological Research 2008;57(2):93‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
DISCOVERY PENTA 2005 {published data only}
- Fonseca FA, Ruiz A, Cardona‐Munoz EG, Silva JM, Fuenmayor N, Marotti M. The DISCOVERY PENTA study: a DIrect Statin COmparison of LDL‐C Value ‐ an Evaluation of Rosuvastatin therapY compared with atorvastatin. Current Medical Research & Opinion 2005;21(8):1307‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dogra 2002 {published data only}
- Dogra GK, Watts GF, Herrmann S, Thomas MA, Irish AB. Statin therapy improves brachial artery endothelial function in nephrotic syndrome. Kidney International 2002;62(2):550‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dujovne 2000 {published data only}
- Dujovne CA, Harris WS, Altman R, Overhiser RW, Black DM. Effect of atorvastatin on hemorheologic‐hemostatic parameters and serum fibrinogen levels in hyperlipidemic patients. American Journal of Cardiology 2000;85(3):350‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Faludi 2004 {published data only}
- Faludi AA, Aldrighi JM, Bertolami MC, Saleh MH, Silva RA, Nakamura Y, et al. Progesterone abolishes estrogen and/or atorvastatin endothelium dependent vasodilatory effects. Atherosclerosis 2004;177(1):89‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Farsang 2007 {published data only}
- Farsang C, Athyros V, Gaw A, Investigators and Steering Committee Members. A multicentre, open study to assess the effect of individualizing starting doses of atorvastatin according to baseline LDL‐C levels on achieving cholesterol targets: the Achieve Cholesterol Targets Fast with Atorvastatin Stratified Titration (ACTFAST‐2) study. Current Medical Research & Opinion 2007;23(8):1945‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ferrer‐Garcia 2008 {published data only}
- Ferrer‐Garcia JC, Sanchez‐Ballester E, Albalat‐Galera R, Berzosa‐Sanchez M, Herrera‐Ballester A. Efficacy of atorvastatin for achieving cholesterol targets after LDL‐cholesterol based dose selection in patients with type 2 diabetes. Journal of Cardiovascular Pharmacology & Therapeutics 2008;13(3):183‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gandelman 2011 {published data only}
- Gandelman K, Glue P, Laskey R, Jones J, LaBadie R, Ose L. An eight‐week trial investigating the efficacy and tolerability of atorvastatin for children and adolescents with heterozygous familial hypercholesterolemia. Pediatric Cardiology 2011;32(4):433‐41. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Geiss 1999 {published data only}
- Geiss HC, Parhofer KG, Schwandt P. Atorvastatin compared with simvastatin in patients with severe LDL hypercholesterolaemia treated by regular LDL apheresis. Journal of Internal Medicine 1999;245(1):47‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goldammer 2002 {published data only}
- Goldammer A, Wiltschnig S, Heinz G, Jansen M, Stulnig T, Horl WH, et al. Atovastatin in low‐density lipoprotein apheresis‐treated patients with homozygous and heterozygous familial hypercholesterolemia. Metabolism: Clinical & Experimental 2002;51(8):976‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gupta 2004 {published data only}
- Gupta A, Gupta V, Thapar S, Bhansali A. Lipid‐lowering drug atorvastatin as an adjunct in the management of diabetic macular edema. American Journal of Ophthalmology 2004;137(4):675‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ishigami 2003 {published data only}
- Ishigami M, Yamashita S, Sakai N, Hirano K‐I, Hiraoka H, Nakamura T, et al. Atorvastatin markedly improves type III hyperlipoproteinemia in association with reduction of both exogenous and endogenous apolipoprotein B‐containing lipoproteins. Atherosclerosis 2003;168(2):359‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jafari 2003 {published data only}
- Jafari M, Ebrahimi R, Ahmadi‐Kashani BS, Balian H, Bashir M. Efficacy of alternate‐day dosing versus daily dosing of atorvastatin. Journal of Cardiovascular Pharmacology & Therapeutics 2003;8(2):123‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jose 2012 {published data only}
- Jose MA, Anandkumar S, Narmadha MP, Sandeep M. A comparative effect of atorvastatin with other statins in patients of hyperlipidemia. Indian Journal of Pharmacology 2012;44(2):261‐3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jyoti 2008 {published data only}
- Jyoti N, Rai J, Singh S. Comparative evaluation of atorvastatin and rosuvastatin in patients of dyslipidemia. JK Practitioner. India: JK Practitioner (P.O. Box 884, Srinagar, Kashmir 190001, India), 2008; Vol. 15, issue 1‐4:27‐35.
Kaya 2009 {published data only}
- Kaya C, Cengiz SD, Berker B, Demirtas S, Cesur M, Erdogan G, et al. Comparative effects of atorvastatin and simvastatin on the plasma total homocysteine levels in women with polycystic ovary syndrome: a prospective randomized study. Fertility & Sterility 2009;92(2):635‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kearns 2008 {published data only}
- Kearns AK, Bilbie CL, Clarkson PM, White CM, Sewright KA, O'Fallon KS, et al. The creatine kinase response to eccentric exercise with atorvastatin 10 mg or 80 mg. Atherosclerosis 2008;200(1):121‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Krysiak 2010 {published data only}
- Krysiak R, Gdula‐Dymek A, Bachowski R, Okopien B, Krysiak R, Gdula‐Dymek A, et al. Pleiotropic effects of atorvastatin and fenofibrate in metabolic syndrome and different types of pre‐diabetes. Diabetes Care 2010;33(10):2266‐70. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamon‐Fava 2007 {published data only}
- Lamon‐Fava S, Diffenderfer MR, Barrett PH, Buchsbaum A, Matthan NR, Lichtenstein AH, et al. Effects of different doses of atorvastatin on human apolipoprotein B‐100, B‐48, and A‐I metabolism. Journal of Lipid Research 2007;48(8):1746‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2010 {published data only}
- Lee CW, Baek SH, Hong TJ, Choi YJ, Kim YJ, Ahn TH, et al. A multicenter, eight‐week treatment, single‐step titration, open‐label study assessing the percentage of Korean dyslipidemic patients achieving LDL cholesterol target with atorvastatin starting doses of 10 mg, 20 mg and 40 mg. Cardiovascular Drugs & Therapy 2010;24(2):181‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lundberg 2010 {published data only}
- Lundberg S, Lundahl J, Gunnarsson I, Jacobson SH. Atorvastatin‐induced modulation of monocyte respiratory burst in vivo in patients with IgA nephropathy: a chronic inflammatory kidney disease. Clinical Nephrology. Germany: Nephrology Unit, Division of Medicine, Karolinska University Hospital, Karolinska Institute, Stockholm, Sweden. sigrid.lundberg@karolinska.se, 2010; Vol. 73, issue 3:221‐8. [DOI] [PubMed]
McVey 1999 {published data only}
- McVey D, Patel H, Eminton Z, Maton S. An assessment of the efficacy of atorvastatin in treating patients with dyslipidaemia to target LDL‐cholesterol goals: the atorvastatin matrix study. International Journal of Clinical Practice 1999;53(7):509‐13. [MEDLINE: ] [PubMed] [Google Scholar]
Meas 2009 {published data only}
- Meas T, Laloi‐Michelin M, Virally M, Peynet J, Giraudeaux V, Kevorkian JP, et al. Switching fibrate to statin in type 2 diabetic patients: consequences on lipid profile. European Journal of Internal Medicine 2009;20(2):197‐200. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nawawi 2003 {published data only}
- Nawawi H, Osman NS, Yusoff K, Khalid BA. Reduction in serum levels of adhesion molecules, interleukin‐6 and C‐reactive protein following short‐term low‐dose atorvastatin treatment in patients with non‐familial hypercholesterolemia. Hormone & Metabolic Research 2003;35(8):479‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nicholls 2011 {published data only}
- Nicholls SJ, Brewer HB, Kastelein JJ, Krueger KA, Wang MD, Shao M, et al. Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol: a randomized controlled trial. JAMA 2011;306(19):2099‐109. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Paolisso 2000 {published data only}
- Paolisso G, Barbagallo M, Petrella G, Ragno E, Barbieri M, Giordano M, et al. Effects of simvastatin and atorvastatin administration on insulin resistance and respiratory quotient in aged dyslipidemic non‐insulin dependent diabetic patients. Atherosclerosis 2000;150(1):121‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Perez‐Castrillon 2007 {published data only}
- Perez‐Castrillon JL, Vega G, Abad L, Sanz A, Chaves J, Hernandez G, et al. Effects of atorvastatin on vitamin D levels in patients with acute ischemic heart disease. American Journal of Cardiology 2007;99(7):903‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pfizer Inc 10 {unpublished data only}
Pfizer Inc 11 {unpublished data only}
- Pfizer Inc. A Phase 3, Double‐Blind, Randomized, Multisite Trial of the Efficacy, Safety, and Tolerability of the Fixed Combination Torcetrapib/atorvastatin Administered Orally, Once Daily for 12 Months, Compared to Atorvastatin Alone, Titrated Based on NCEP ATP‐III LDL‐C Goals in Subjects with Fredrickson Types IIa and IIb Dyslipidemia. Protocol No. A5091019 2006.
Pfizer Inc 13 {unpublished data only}
- Pfizer Inc. Phase 3, Multi‐site, Double‐blind, Randomized, Forced Titration, Parallel Group Evaluation of the Efficacy, Safety and Tolerability of Fixed Combination Torcetrapib(CP‐529‐414)/atorvastatin Administered Orally, Once Daily (QD) for Eighteen Weeks, Compared with Atorvastatin Alone, in Subjects with Fredrickson Type IV Hypertriglyceridemia. Protocol No. A5091025 2006.
Pfizer Inc 14 {unpublished data only}
- Pfizer Inc. Phase 3, Multi‐center, Double‐blind, Randomized, Crossover Study of the Efficacy, Safety, and Tolerability of Fixed Combination Torcetrapib (CP‐529,414)/atorvastatin, Compared with Atorvastatin Therapy Alone, and Fenofibrate Alone, in Subjects with Fredrickson Type III Hyperlipoproteinemia (Familial Dysbetalipoproteinemia). Protocol No. A5091024 2006.
Pfizer Inc 5 {unpublished data only}
Puccetti 2001 {published data only}
- Puccetti L, Bruni F, Bova G, Cercignani M, Palazzuoli A, Console E, et al. Effect of diet and treatment with statins on platelet‐dependent thrombin generation in hypercholesterolemic subjects. Nutrition Metabolism & Cardiovascular Diseases 2001;11(6):378‐87. [MEDLINE: ] [PubMed] [Google Scholar]
Puccetti 2011 {published data only}
- Puccetti L, Santilli F, Pasqui AL, Lattanzio S, Liani R, Ciani F, et al. Effects of atorvastatin and rosuvastatin on thromboxane‐dependent platelet activation and oxidative stress in hypercholesterolemia. Atherosclerosis. Ireland: Center for Atherosclerosis Research, University of Siena, v.le Bracci, 53100 Siena, Italy. puccetti@unisi.it, 2011; Vol. 214, issue 1:122‐8. [DOI] [PubMed]
Riahi 2006 {published data only}
- Riahi S, Schmidt EB, Amanavicius N, Karmisholt J, Jensen HS, Christoffersen RP, et al. The effect of atorvastatin on heart rate variability and lipoproteins in patients treated with coronary bypass surgery. International Journal of Cardiology 2006;111(3):436‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Riesen 2002 {published data only}
- Riesen WF, Engler H, Risch M, Korte W, Noseda G. Short‐term effects of atorvastatin on C‐reactive protein. European Heart Journal 2002;23(10):794‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Roberto 2010 {published data only}
- Roberto C, Pasquale P, Serena DS, Simona B, Valerio S, Laura N, et al. Atorvastatin inhibits oxidative stress via adiponectin‐mediated NADPH oxidase down‐regulation in hypercholesterolemic patients. Atherosclerosis. Ireland: Elsevier Ireland Ltd (P.O. Box 85, Limerick, Ireland), 2010; Vol. 213, issue 1:225‐34. [DOI] [PubMed]
Schaefer 2002 {published data only}
- Schaefer EJ, McNamara JR, Tayler T, Daly JA, Gleason JA, Seman LJ, et al. Effects of atorvastatin on fasting and postprandial lipoprotein subclasses in coronary heart disease patients versus control subjects. American Journal of Cardiology 2002;90(7):689‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schaefer 2004 {published data only}
- Schaefer EJ, McNamara JR, Tayler T, Daly JA, Gleason JL, Seman LJ, et al. Comparisons of effects of statins (atorvastatin, fluvastatin, lovastatin, pravastatin, and simvastatin) on fasting and postprandial lipoproteins in patients with coronary heart disease versus control subjects. American Journal of Cardiology 2004;93(1):31‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schuster 1998 {published data only}
- Schuster H, Berger J, Luft FC. Randomized, double‐blind, parallel‐group trial of atorvastatin and fluvastatin on plasma lipid levels in patients with untreated hyperlipidaemia. British Journal of Cardiology 1998;5(11):597‐602. [EMBASE: 1998398852] [Google Scholar]
Soedamah‐Muthu 2003 {published data only}
- Soedamah‐Mathu SS, Colhoun HM, Thomason MJ, Betteridge DJ, Durrington PN, Hitman GA, et al. The effect of atorvastatin on serum lipids, lipoproteins and NMR spectroscopy defined lipoprotein subclasses in type 2 diabetic patients with ischaemic heart disease. Atherosclerosis 2003;167(2):243‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Son 2013 {published data only}
- Son JW, Kim DJ, Lee CB, Oh S, Song KH, Jung CH, et al. Effects of patient‐tailored atorvastatin therapy on ameliorating the levels of atherogenic lipids and inflammation beyond lowering low‐density lipoprotein cholesterol in patients with type 2 diabetes. Journal of Diabetes Investigation 2013;4(5):466‐74. [Biosis PREV201300771684] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sparks 2005 {published data only}
- Sparks DL, Sabbagh MN, Connor DJ, Lopez J, Launer LJ, Petanceska S, et al. Atorvastatin therapy lowers circulating cholesterol but not free radical activity in advance of identifiable clinical benefit in the treatment of mild‐to‐moderate AD. Current Alzheimer Research 2005;2(3):343‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tutunov 2008 {published data only}
- Tutunov VS, Popkova TV, Novikova DS, Nasonov EL, Kukhrchuk VV, Tutunov VS, et al. Comparative assessment of antiinflammatory action of atorvastatin in ischemic heart disease and rheumatoid arthritis. Kardiologiia 2008;48(9):4‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Undas 2006b {published data only}
- Undas A, Celinska‐Lowenhoff M, Lowenhoff T, Szczeklik A. Statins, fenofibrate, and quinapril increase clot permeability and enhance fibrinolysis in patients with coronary artery disease. Journal of Thrombosis & Haemostasis 2006;4(5):1029‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
VYMET 2009 {published data only}
- Robinson JG, Ballantyne CM, Grundy SM, Hsueh WA, Parving HH, Rosen JB, et al. Lipid‐altering efficacy and safety of ezetimibe/simvastatin versus atorvastatin in patients with hypercholesterolemia and the metabolic syndrome (from the VYMET study). American Journal of Cardiology 2009;103(12):1694‐703. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Robinson JG, Ballantyne CM, Hsueh W, Rosen J, Lin J, Shah A, et al. Achievement of specified low‐density lipoprotein cholesterol, non‐high‐density lipoprotein cholesterol apolipoprotein B, and high‐sensitivity C‐reactive protein levels with ezetimibe/simvastatin or atorvastatin in metabolic syndrome patients with and without atherosclerotic vascular disease (from the VYMET study). Journal of Clinical Lipidology 2011;5(6):474‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Robinson JG, Ballantyne CM, Hsueh WA, Rosen JB, Lin J, Shah AK, et al. Age, abdominal obesity, and baseline high‐sensitivity C‐reactive protein are associated with low‐density lipoprotein cholesterol, non‐high‐density lipoprotein cholesterol, and apolipoprotein B responses to ezetimibe/simvastatin and atorvastatin in patients with metabolic syndrome. Journal of Clinical Lipidology 2013;7(4):292‐303. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Villa J, Pratley RE. Ezetimibe/simvastatin or atorvastatin for the treatment of hypercholesterolemia in patients with the metabolic syndrome: the VYMET study. Current Diabetes Reports. Current Medicine Group LLC (5 Marine View Plaza, Suite 218, Hoboken NJ 07030, United States), 2010; Vol. 10, issue 3:173‐5. [DOI] [PubMed]
Wanner 2005 {published data only}
- Marz W, Genser B, Drechsler C, Krane V, Grammer TB, Ritz E, et al. Atorvastatin and low‐density lipoprotein cholesterol in type 2 diabetes mellitus patients on hemodialysis: associated excluded citation for the Wanner 2005 trial. Clinical Journal of the American Society of Nephrology (CJASN) 2011;6(6):1316‐25. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. New England Journal of Medicine 2005;353(3):238‐48. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wierzbicki 1999 {published data only}
- Wierzbicki AS, Christ ER, Chik G, Lumb PJ, Semra YK, Crook MA. A comparison of atorvastatin with previous therapy in treatment of patients with severe hyperlipidaemias. Atherosclerosis 1999;144 Suppl 1:34. [IDS Number: 203BM] [Google Scholar]
- Wierzbicki AS, Lumb PJ, Cheung J, Crook MA. Fenofibrate‐simvastatin therapy compared to simvastatin‐resin therapy and atorvastatin for familial hypercholesterolaemia. Atherosclerosis 1997;134(1‐2):63. [CENTRAL: CN‐00448364] [Google Scholar]
- Wierzbicki AS, Lumb PJ, Semra Y, Chik G, Christ ER, Crook MA. Atorvastatin compared with simvastatin‐based therapies in the management of severe familial hyperlipidaemias. Quarterly Journal of Medicine 1999;92(7):387‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Winkler 2004 {published data only}
- Weltzien P, Winkler K, Friedrich I, Schmitz H, Hauck P, Hoffmann M, et al. Fenofibrate and atorvastatin in patients with combined hyperlipidemia and dense low density lipoproteins. Diabetologia 2002;45 Suppl 2:A389. [IDS Number: 590HM] [Google Scholar]
- Winkler K, Weltzien P, Friedrich I, Schmitz H, Nickell HH, Hauck P, et al. Qualitative effect of fenofibrate and quantitative effect of atorvastatin on LDL profile in combined hyperlipidemia with dense LDL. Experimental & Clinical Endocrinology & Diabetes 2004;112(5):241‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yamamoto 2000 {published data only}
- Yamamoto A, Harada‐Shiba M, Kawaguchi A, Oi K, Kubo H, Sakai S, et al. The effect of atorvastatin on serum lipids and lipoproteins in patients with homozygous familial hypercholesterolemia undergoing LDL‐apheresis therapy. Atherosclerosis 2000;153(1):89‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yang 2007 {published data only}
- Yang B, Shi X‐M, Ma C‐Y, Feng X‐Y, Liu K, Chen D, et al. Relationship between serum uric acid and carotid intima‐media thickness in Tibetan: effect of atorvastatin therapy on hyperuricemia. Journal of Clinical Rehabilitative Tissue Engineering Research 2007;11(21):4248‐50. [EMBASE: 2007338185] [Google Scholar]
Yildiz 2007 {published data only}
- Yildiz A, Cakar MA, Baskurt M, Okcun B, Guzelsoy D, Coskun U. The effects of atorvastatin therapy on endothelial function in patients with coronary artery disease. Cardiovascular Ultrasound 2007;5:51. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yilmaz 2005 {published data only}
- Yilmaz AK, Kayardi M, Toktamis A, Tomul ZD, Nur N. The effects of clarithromycin added to atorvastatin treatment on serum lipid profiles: a randomised clinical trial. Pakistan Journal of Medical Sciences 2005;21(2):174‐7. [EMBASE: 2005315853] [Google Scholar]
Additional references
Abramson 2013
- Abramson JD, Rosenberg HG, Jewell N, Wright JM. Should people at low risk of cardiovascular disease take a statin?. BMJ 2013;347:f6123. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Adams 2014
- Adams SP, Sekhon SS, Wright JM. Rosuvastatin for lowering lipids. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD010254] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bandolier 2004
- Bandolier. Cholesterol lowering with statins. Bandolier 2004:121‐2.
CDC 2011
- Centers for Disease Control and Prevention (CDC). Million hearts: strategies to reduce the prevalence of leading cardiovascular disease risk factors ‐‐ United States, 2011. MMWR ‐ Morbidity & Mortality Weekly Report 2011;60(36):1248‐51. [MEDLINE: ] [PubMed] [Google Scholar]
CTT 2005
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R, Cholesterol Treatment Trialists' (CTT) Collaborators. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366(9493):1267‐78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Edwards 2003
- Edwards JE, Moore RA. Statins in hypercholesterolaemia: a dose‐specific meta‐analysis of lipid changes in randomised, double blind trials. BMC Family Practice 2003;4:18. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elmberger 1991
- Elmberger PG, Kalen A, Lund E, Reihner E, Eriksson M, Berglund L, Angelin B, Dallner G. Effects of pravastatin and cholestyramine on products of the mevalonate pathway in familial hypercholesterolemia. Journal of Lipid Research 1991;32(6):935‐40. [MEDLINE: ] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gaw 2000
- Gaw A, Packard CJ, Shepherd J. Statins: The HMG CoA Reductase Inhibitors in Perspective. London, England: Martin Dunitz Ltd, 2000. [ISBN 1853174688] [Google Scholar]
Goodman 2011
- Goodman LS, Brunton LL, Chabner B, Knollmann BC. Goodman & Gilman's Pharmacological Basis of Therapeutics. 12th Edition. New York: McGraw‐Hill, 2011. [ISBN: 9780071624428] [Google Scholar]
Grundy 2004
- Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr, Stone NJ, Coordinating Committee of the National Cholesterol Education Program. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Journal of the American College of Cardiology 2004;44(3):720‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Heran 2008
- Heran BS, Wong MMY, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003823.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org, 2011. [Google Scholar]
Holmqvist 2008
- Schmidt A. Non‐lipid lowering effects of statins. In: Gunnar N Holmqvist editor(s). Statins: Indications and Uses, Safety and Modes of Action. 1. New York: Nova Science, Inc, Dec 2008:73‐90. [ISBN‐10: 1606921037 ISBN‐13: 978‐1606921036] [Google Scholar]
IMS 2012
- Institute for Health Informatics. The use of medicines in the United States: review of 2011. Medicines in US Report 2012:32.
Kreatsoulas 2010
- Kreatsoulas C, Anand SS. The impact of social determinants on cardiovascular disease. Canadian Journal of Cardiology 2010;26(Suppl C):8C‐13C. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Law 2003
- Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta‐analysis. BMJ 2003;326(7404):1423. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liao 2005
- Liao JK, Laufs U. Pleiotropic effects of statins. Annual Review of Pharmacology and Toxicology 2005;45:89‐118. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lins 2003
- Lins RL, Matthys KE, Verpooten GA, Peeters PC, Dratwa M, Stolear JC, Lameire NH. Pharmacokinetics of atorvastatin and its metabolites after single and multiple dosing in hypercholesterolaemic haemodialysis patients. Nephrology Dialysis Transplantation 2003;18(5):967‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lipitor Prescribing Information 2012
- Highlights of Prescribing Information, 2012. labeling.pfizer.com/ShowLabeling.aspx?id=587 (accessed 8 November 2012).
Marcoff 2007
- Marcoff L, Thompson PD. The role of coenzyme Q10 in statin‐associated myopathy: a systematic review. Journal of the American College of Cardiology 2007;49(23):2231‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mills 2008
- Mills EJ, Rachlis B, Wu P, Devereaux PJ, Arora P, Perri D. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta‐analysis involving more than 65,000 patients. Journal of the American College of Cardiology 2008;52(22):1769‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moghadasian 1999
- Moghadasian MH. Clinical pharmacology of 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitors. Life Sciences 1999;65(13):1329‐37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Naci 2013
- Naci H, Brugts JJ, Fleurence R, Ades AE. Dose‐comparative effects of different statins on serum lipid levels: a network meta‐analysis of 256,827 individuals in 181 randomized controlled trials. European Journal of Preventive Cardiology 2013;20(4):658‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NCEP 2002
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106(25):3143‐421. [MEDLINE: ] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Roger 2011
- Roger VL, Go AS, Lloyd‐Jones DM, Adams RJ, Berry JD, Brown TM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics ‐‐ 2011 update: a report from the American Heart Association. Circulation 2011;123(4):e18‐e209. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schachter 2004
- Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundamental & Clinical Pharmacology 2004;19(1):117‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Smith 2009
- Smith MEB, Lee NJ, Haney E, Carson S. Drug Class Review. HMG‐CoA Reductase Inhibitors (Statins) and Fixed‐dose Combination Products Containing a Statin. Final Report Update 5, 2009. www.ncbi.nlm.nih.gov/books/NBK47273/pdf/TOC.pdf (last accessed 8 November 2012). [PubMed]
Taylor 2013
- Taylor F, Huffman MD, Macedo AF, Moore THM, Burke M, Davey Smith G, Ward K, Ebrahim S. Statins for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD004816.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Therapeutics Initiative 2010
- Therapeutics Initiative. Do statins have a role in primary prevention? An update. Therapeutics Letter 2010;77(Mar‐Apr):1‐2. [Google Scholar]
Thompson 2005
- Thompson JF, Man M, Johnson KJ, Wood LS, Lira ME, Lloyd DB, Banerjee P, Milos PM, Myrand SP, Paulauskis J, Milad MA, Sasiela WJ. An association study of 43 SNPs in 16 candidate genes with atorvastatin response. Pharmacogenomics Journal 2005;5(6):352‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tsang 2002
- Tsang MB, Adams SP, Jauca C, Wright JM. In Some Systematic Reviews Placebos May Not Be Necessary: An Example From a Statin Dose‐Response Study. 10th Cochrane Colloquium Abstracts; 2002 31 Jul‐3 Aug; Stavanger, Norway. 2002:Poster 29.
Uusitupa 1992
- Uusitupa MIJ, Miettinen TA, Happonen P, Ebeling T, Turtola H, Voutilainen E, Pyorala K. Lathosterol and other noncholesterol sterols during treatment of hypercholesterolemia with lovastatin alone and with cholestyramine or guar gum. Arteriosclerosis & Thrombosis 1992;12(7):807‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Adams 2010
- Wright JM, Adams SP, Tsang M. Lipid lowering efficacy of atorvastatin. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD008226] [DOI] [Google Scholar]
Adams 2012
- Adams SP, Tsang M, Wright JM. Lipid lowering efficacy of atorvastatin. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD008226.pub2] [DOI] [PubMed] [Google Scholar]



