Abstract
Background
Axillary surgery is an established part of the management of primary breast cancer. It provides staging information to guide adjuvant therapy and potentially local control of axillary disease. Several alternative approaches to axillary surgery are available, most of which aim to spare a proportion of women the morbidity of complete axillary dissection.
Objectives
To assess the benefits and harms of alternative approaches to axillary surgery (including omitting such surgery altogether) in terms of overall survival; local, regional and distant recurrences; and adverse events.
Search methods
We searched the Cochrane Breast Cancer Group Specialised Register, MEDLINE, Pre‐MEDLINE, Embase, CENTRAL, the World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov on 12 March 2015 without language restrictions. We also contacted study authors and checked reference lists.
Selection criteria
Randomised controlled trials (RCTs) including women with clinically defined operable primary breast cancer conducted to compare axillary lymph node dissection (ALND) with no axillary surgery, axillary sampling or sentinel lymph node biopsy (SLNB); RCTs comparing axillary sampling with SLNB or no axillary surgery; RCTs comparing SLNB with no axillary surgery; and RCTs comparing ALND with or without radiotherapy (RT) versus RT alone.
Data collection and analysis
Two review authors independently assessed each potentially relevant trial for inclusion. We independently extracted outcome data, risk of bias information and study characteristics from all included trials. We pooled data according to trial interventions, and we used hazard ratios (HRs) for time‐to‐event outcomes and odds ratios (OR) for binary outcomes.
Main results
We included 26 RCTs in this review. Studies were at low or unclear risk of selection bias. Blinding was not done, but this was only considered a source of bias for outcomes with potential for subjectivity in measurements. We found no RCTs of axillary sampling versus SLNB, axillary sampling versus no axillary surgery or SLNB versus no axillary surgery.
No axillary surgery versus ALND Ten trials involving 3849 participants compared no axillary surgery versus ALND. Moderate quality evidence showed no important differences between overall survival of women in the two groups (HR 1.06, 95% confidence interval (CI) 0.96 to 1.17; 3849 participants; 10 studies) although no axillary surgery increased the risk of locoregional recurrence (HR ranging from 1.10 to 3.06; 20,863 person‐years of follow‐up; four studies). It was uncertain whether no surgery increased the risk of distant metastasis compared with ALND (HR 1.06, 95% CI 0.87 to 1.30; 946 participants; two studies). Low‐quality evidence indicated no axillary surgery decreased the risk of lymphoedema compared with ALND (OR 0.31, 95% CI 0.23 to 0.43; 1714 participants; four studies).
Axillary sampling versus ALND Six trials involving 1559 participants compared axillary sampling versus ALND. Low‐quality evidence indicated similar effectiveness of axillary sampling compared with ALND in terms of overall survival (HR 0.94, 95% CI 0.73 to 1.21; 967 participants; three studies) but it was unclear whether axillary sampling led to increased risk of local recurrence compared with ALND (HR 1.41, 95% CI 0.94 to 2.12; 1404 participants; three studies). The relative effectiveness of axillary sampling and ALND for locoregional recurrence (HR 0.74, 95% CI 0.46 to 1.20; 406 participants; one study) and distant metastasis was uncertain (HR 1.05, 95% CI 0.74 to 1.49; 406 participants; one study). Lymphoedema was less likely after axillary sampling than after ALND (OR 0.32, 95% CI 0.13 to 0.81; 80 participants; one study).
SLNB versus ALND Seven trials involving 9426 participants compared SLNB with ALND. Moderate‐quality evidence showed similar overall survival following SLNB compared with ALND (HR 1.05, 95% CI 0.89 to 1.25; 6352 participants; three studies; moderate‐quality evidence). Differences in local recurrence (HR 0.94, 95% CI 0.24 to 3.77; 516 participants; one study), locoregional recurrence (HR 0.96, 95% CI 0.74 to 1.24; 5611 participants; one study) and distant metastasis (HR 0.80, 95% CI 0.42 to 1.53; 516 participants; one study) were uncertain. However, studies showed little absolute difference in the aforementioned outcomes. Lymphoedema was less likely after SLNB than ALND (OR ranged from 0.04 to 0.60; three studies; 1965 participants; low‐quality evidence). Three studies including 1755 participants reported quality of life: Investigators in two studies found quality of life better after SLNB than ALND, and in the other study observed no difference.
RT versus ALND Four trials involving 2585 participants compared RT alone with ALND (with or without RT). High‐quality evidence indicated that overall survival was reduced among women treated with radiotherapy alone compared with those treated with ALND (HR 1.10, 95% CI 1.00 to 1.21; 2469 participants; four studies), and local recurrence was less likely in women treated with radiotherapy than in those treated with ALND (HR 0.80, 95% CI 0.64 to 0.99; 22,256 person‐years of follow‐up; four studies). Risk of distant metastasis was similar for radiotherapy alone as for ALND (HR 1.07, 95% CI 0.93 to 1.25; 1313 participants; one study), and whether lymphoedema was less likely after RT alone than ALND remained uncertain (OR 0.47, 95% CI 0.16 to 1.44; 200 participants; one study).
Less surgery versus ALND When combining results from all trials, treatment involving less surgery was associated with reduced overall survival compared with ALND (HR 1.08, 95% CI 1.01 to 1.16; 12,864 participants; 19 studies). Whether local recurrence was reduced with less axillary surgery when compared with ALND was uncertain (HR 0.90, 95% CI 0.75 to 1.09; 24,176 participant‐years of follow up; eight studies). Locoregional recurrence was more likely with less surgery than with ALND (HR 1.53, 95% CI 1.31 to 1.78; 26,880 participant‐years of follow‐up; seven studies). Whether risk of distant metastasis was increased after less axillary surgery compared with ALND was uncertain (HR 1.07, 95% CI 0.95 to 1.20; 2665 participants; five studies). Lymphoedema was less likely after less axillary surgery than with ALND (OR 0.37, 95% CI 0.29 to 0.46; 3964 participants; nine studies).
No studies reported on disease control in the axilla.
Authors' conclusions
This review confirms the benefit of SLNB and axillary sampling as alternatives to ALND for axillary staging, supporting the view that ALND of the clinically and radiologically uninvolved axilla is no longer acceptable practice in people with breast cancer.
Plain language summary
Surgical removal of underarm lymph nodes in breast cancer
Review question
This review aimed to compare the benefits of surgical removal of underarm lymph nodes with the potential harms associated with this surgical procedure. The review also aimed to learn whether complete removal of all underarm nodes could be replaced by procedures that remove only a small number of lymph nodes.
Background
Surgical removal of underarm (axillary) lymph nodes is often part of the initial surgical treatment for patients with operable breast cancer. If cancer has spread to these lymph nodes, patients are advised to undergo additional treatments, such as chemotherapy or radiotherapy, to help treat their disease. If cancer has not spread to these lymph nodes, patients are spared extra treatments (with extra side effects). Surgical removal of lymph nodes can lead to short‐term surgical complications (such as infection and wound healing problems) and long‐term problems (such as shoulder stiffness, pain and arm swelling (lymphoedema)) when fluid accumulation causes restricted function and discomfort.
Modern strategies use a stepwise approach by first removing a small number of nodes and removing the others only if cancer is found at the first stage. This first stage can consist of ‘random’ axillary sampling, whereby the surgeon removes a small number of nodes (typically four) that can be felt. Alternatively, surgeons can use sentinel node techniques to identify those nodes most likely to contain cancer, leading to removal of as few nodes as possible. For patients with cancer in the sentinel nodes (or sample), complete removal of all underarm lymph nodes (axillary lymph node dissection) is usually recommended; however, radiotherapy to the axilla can also be given to obliterate any cancer cells in the lymph nodes. Some studies have explored alternative approaches such as no surgical treatment to the underarm nodes.
Study characteristics
The evidence is current to March 2015. The review identified 26 randomised controlled trials that compared axillary lymph node dissection (ALND) with alternative approaches involving less axillary surgery. Patients in these trials had operable primary breast cancer, and some trials included patients with palpably enlarged axillary lymph nodes. Ten trials including 3849 patients compared ALND with no axillary surgery. Six trials including 1559 patients compared ALND with axillary sampling. Seven trials including 9426 patients compared ALND with sentinel lymph node biopsy (SLNB). Four trials including 2585 patients compared ALND (with or without radiotherapy) with radiotherapy alone.
Key results
Moderate‐quality evidence suggests that patients treated with approaches involving lesser axillary surgery (such as axillary sampling or SLNB) do not have a reduced chance of survival compared with those treated with ALND. Moderate‐quality evidence indicates that overall survival is slightly reduced in patients who receive radiotherapy (but no axillary surgery) when compared with ALND. If survival is assumed to be 81% five years after surgery with ALND, then the evidence suggests it would be between 77% and 81% after treatment with radiotherapy alone.
Moderate‐quality evidence suggests that patients who have no axillary lymph nodes removed at all are at increased risk of locoregional recurrence (regrowth of cancer, in the breast, mastectomy scar area or underarm glands). If it is assumed that 86% of patients receiving ALND are free of locoregional recurrence five years after surgery, evidence suggests that the corresponding figure for patients who have no lymph nodes removed at all would be between 66% and 76%. For patients treated with axillary sampling, low‐quality evidence suggests that between 73% and 87% would be free of locoregional recurrence at five years.
Axillary recurrence rates were reported only in SLNB versus ALND trials, and researchers remain uncertain about the best treatment for this outcome because rates were very low (occurring in less than 1% of patients).
Low‐quality evidence suggests that patients treated with ALND are at increased risk of lymphoedema compared with those treated with SLNB or no axillary surgery. On the basis of this evidence, we would expect that out of every 1000 patients receiving ALND, 132 would experience lymphoedema at one year after surgery, compared with between 22 and 115 of those receiving SLNB. Other long‐term harms such as pain, impaired arm movement and numbness were also more likely with ALND than with SLNB.
Summary of findings
Background
Description of the condition
Invasive breast cancer occurs when uncontrolled, abnormal growth and division of cells in the lobules or ducts of the breast spreads to surrounding tissue. The Union Internationale Contre le Cancer staging system for breast cancer (UICC 1987) reflects how, when left untreated, cancer cells may spread locally to breast tissue and lymph glands in the axilla (stages I to III) and through the bloodstream and lymphatic system to other parts of the body (stage IV).
Description of the intervention
Removal of regional lymph nodes during attempts to achieve a curative excision for management of most cancers has a long history (Halsted 1895). Its aim consists of both local control of axillary disease and determination of stage to permit appropriate adjuvant therapy. Axillary surgery is a key component of breast cancer management, with UK clinical guidelines specifying that minimal surgery (preferably sentinel lymph node biopsy (SLNB)) should be performed to stage the axilla for patients with early invasive breast cancer and clinically negative axillary lymph nodes (NICE 2009).
Several alternative approaches to axillary surgery may be used.
Axillary clearance ‐ removal of all nodal tissue in the axilla by dissection up to the level of the axillary vein (Craig 1998) ‐ was previously the standard practice in many units. Full axillary clearance carries increased morbidity when compared with breast surgery alone, with 10% to 15% incidence of chronic arm lymphoedema (Kissin 1986), 9% incidence of late seroma, 2.2% infection rate, 12% breast oedema and 0.3% risk of damage to the long thoracic nerve (Senofski 1991). Other problems include shoulder stiffness ("frozen shoulder"), which can be severe (Kissin 1986). Immediate axillary node clearance is not considered appropriate in the absence of evidence of cancer spread determined by biopsy before surgery.
Axillary node sampling ‐ removal of four or five axillary nodes from the lower axilla (Craig 1998) ‐ involves removal of individual nodes, leaving axillary fat and most nodes and lymphatics intact. As a result, virtually none of the complications listed for axillary clearance are associated with this procedure. Women whose sampled axillary nodes contain cancer may need subsequent axillary clearance or radiotherapy. This previously popular approach was once considered appropriate.
Sentinel lymph node biopsy (Kelley 1998) ‐ a procedure in which the lymphatic pathway from the site of breast cancer is tracked with the use of a radio‐isotope or blue lymphatic dye ‐ allows biopsy of the first lymph node or nodes (sentinel node). Sentinel nodes are most likely to involve spread of cancer, and this approach allows accurate assessment of whether the cancer has spread along with removal of a small number of nodes (typically three or fewer).
In some patients who are not candidates for adjuvant therapies, surgeons may omit axillary surgery altogether to avoid additional morbidity (EBCTCG 1998, Walsh 1989). This has led some surgeons to spare some frail women with breast cancer from undergoing staging of the clinically uninvolved axilla by means of sentinel node biopsy or full clearance (Yancik 1989).
How the intervention might work
Removal of axillary nodes can improve local control of axillary disease while providing information on cancer stage that can be used to guide adjuvant therapy.
Why it is important to do this review
Arguments for and against each of these procedures are complicated and, as a result, practice is variable. Statistical synthesis of outcomes for these procedures will offer surgeons and patients a more reliable evidence base on which they can make difficult decisions concerning treatment.
Objectives
To assess the benefits and harms of alternative approaches to axillary surgery (including omitting such surgery altogether) in terms of overall survival; local, regional and distant recurrences; and adverse events.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials.
Types of participants
Women with clinically defined operable primary breast cancer, that is, primary tumour not fixed to underlying structures (includes tumour‐node‐metastasis (TNM) classifications T1‐3 and T4b with only minor skin involvement, N0‐1 and M0) nor to mobile lymph nodes (UICC 1987).
Types of interventions
-
Axillary lymph node dissection (ALND) versus no axillary surgery at the time of primary surgery
-
With the following subgroups for both arms:
Radiotherapy
No radiotherapy
-
-
ALND versus axillary sampling at the time of primary surgery
-
With the following subgroups for both arms:
Radiotherapy
No radiotherapy
-
And the following subgroups for the limited axillary staging arm:
Further treatment for histologically node‐positive cases
No further treatment for histologically node‐positive cases
-
-
ALND versus SLNB at the time of primary surgery
-
With the following subgroups for both arms:
Radiotherapy
No radiotherapy
-
And the following subgroups for the limited axillary staging arm:
Further treatment for histologically node‐positive cases
No further treatment for histologically node‐positive cases
-
-
Axillary sampling versus sentinel node biopsy at the time of primary surgery
-
With the following subgroups for both arms:
Radiotherapy
No radiotherapy
-
And the following subgroups for both arms:
Further treatment for histologically node‐positive cases
No further treatment for histologically node‐positive cases
-
-
Axillary sampling versus no axillary surgery at the time of primary surgery
-
With the following subgroups for both arms:
Radiotherapy
No radiotherapy
-
And the following subgroups for the limited axillary staging arm:
Further treatment for histologically node‐positive cases
No further treatment for histologically node‐positive cases
-
-
SLNB versus no axillary surgery at the time of primary surgery
-
With the following subgroups for both arms
Radiotherapy
No radiotherapy
-
And the following subgroups for the limited axillary staging arm:
Further treatment for histologically node‐positive cases
No further treatment for histologically node‐positive cases
-
-
ALND with no radiotherapy versus no axillary surgery with radiotherapy
With no subgroups
For all studies involving full axillary surgery or axillary sampling, the number of nodes removed and the method of node analysis used were recorded when available, to indicate whether an adequate sampling or clearance procedure was performed.
Types of outcome measures
Primary outcomes
Survival ‐ overall (interval between start of treatment or randomisation and death)
Disease control in the axilla (interval between start of treatment and the need for second‐line treatment or palliative treatment or regional recurrence in the axilla)
Breast cancer recurrence, either locally within the breast (local recurrence) or distantly as metastatic disease (distant recurrence), with time to recurrence and site of recurrence recorded
Adverse events (surgical complications) including acute local surgical complications, such as haematoma, infection, wound dehiscence or seroma, and acute systemic complications, such as chest infection, deep venous thrombosis, pulmonary embolism, cardiac failure, cardiac ischaemia and cerebrovascular accident
Long‐term complications including lymphoedema, shoulder stiffness, paraesthesia, pain, loss of functional capacity, winging of scapula and wound contracture or scarring
Secondary outcomes
Quality of life (measured on a validated scale)
Psychological and psychosocial variables (measured on validated scales)
Search methods for identification of studies
Electronic searches
The Trials Search Co‐ordinator for the Cochrane Breast Cancer Review Group searched the Specialised Register of the Group on 16 March 2015. Details of sources and search strategies used to populate this register are provided in the Group module in the Cochrane Library (http://onlinelibrary.wiley.com/o/cochrane/clabout/articles/BREASTCA/frame.html) . We have extracted for consideration studies coded as "AXILLARY NODE(S)", "EARLY BREAST CANCER", "LOCALLY ADVANCED BREAST CANCER", "PSYCHOSOCIAL" or "SURGERY" on the Specialised Register.
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; Issue 2) in the Cochrane Library on 16 March 2015. See Appendix 1 for the search strategy used.
In addition, an information specialist searched the following databases while using the search terms and strategy identified in Appendix 2: MEDLINE via OvidSP (2007 to 12 March 2015), PreMEDLINE via OvidSP (12 March 2015) and Embase via OvidSP (2002 to 12 March 2015). We used a validated filter to identify reports of RCTs in our initial search of MEDLINE (Lefebvre 2001), and for updated searches, we used the revised filter (Lefebvre 2011). We used the Scottish Intercollegiate Guidelines Network RCT filter in our search of Embase (http://www.sign.ac.uk/methodology/filters.html).
We also searched on 16 March 2015 the World Health Organization International Clinical Trials Registry Portal (WHO ICTRP) (Appendix 3) and ClinicalTrials.gov (Appendix 4), for prospectively registered and ongoing trials.
Searching other resources
We searched (on 12 March 2015) conference proceedings from the American Society of Clinical Oncology (ASCO) 41st to 50th Annual Meetings (2005 to 2014) via Journal of Clinical Oncology (http://jco.ascopubs.org/site/meetings). We also searched (on 12 March 2015) conference proceedings from the San Antonio Breast Cancer (SABCS) 29th to 37th Annual Symposium Meetings (2006 to 2014) via the Cancer Research website (http://cancerres.aacrjournals.org/).
We contacted the authors of included and ongoing trials by email and asked them if they knew of any relevant studies. This yielded no additional studies. We also checked the reference lists of included studies and published reviews to look for relevant studies.
Data collection and analysis
Selection of studies
Two review authors (NB, MSH or MA) screened the titles and abstracts of references identified by electronic searches to identify publications of potentially eligible trials. We obtained a copy of the full‐text article for each reference reporting a potentially eligible trial, and we applied the review selection criteria to each trial. We reported all exclusions of potentially eligible trials in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) diagram (Figure 1) and, in some cases, in the Characteristics of excluded studies table. We used trial publications to assess each trial's eligibility, and for unpublished trials, we obtained information from the trial protocol or the next best available resource. When necessary and possible, we sought additional information from the principal investigator. Two review authors (NB, MSH or MA) independently assessed each potentially eligible trial for inclusion in the review and resolved discrepancies in eligibility judgements by discussion.
1.
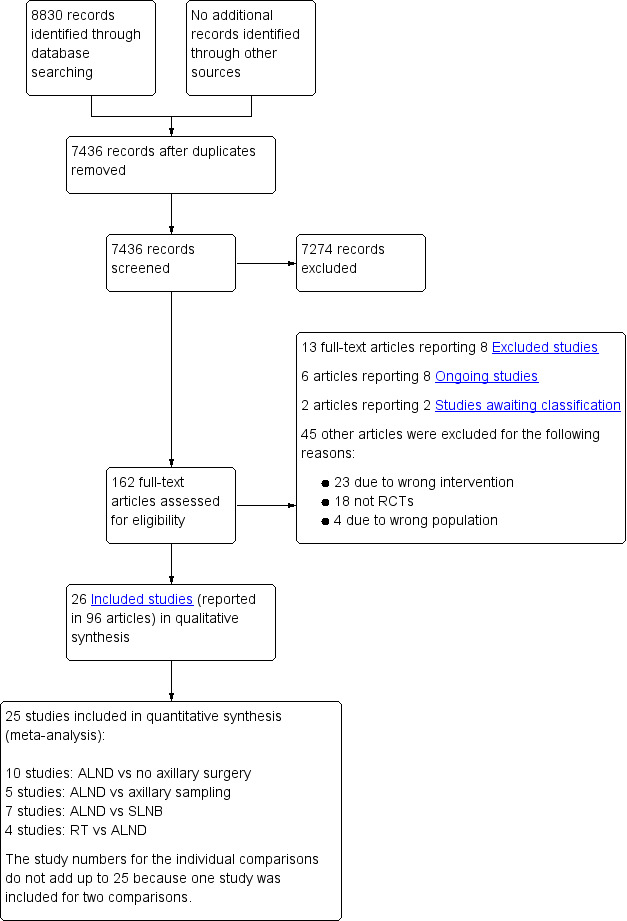
Study flow diagram.
Data extraction and management
We extracted data from published trial reports and entered them onto an electronic form (using Microsoft Word). Two review authors (NB, MSH or MA) independently extracted data from each trial and resolved disagreements regarding data extraction by discussion. The Early Breast Cancer Trialists' Collaborative Group (Clarke 2005) has published a meta‐analysis based on individual participant data for many of the included trials. We used this meta‐analysis as an additional source of outcome data for trials included in this review.
We contacted the authors of included and ongoing trials by email and asked them to share unpublished data from their trials and to clarify details about their trial that were unclear or missing from the published reports.
Assessment of risk of bias in included studies
We assessed the risk of bias of included studies by applying standard Cochrane methods for randomised trials as outlined in Higgins 2011. We assessed selection bias (random sequence generation, allocation concealment; two items) and reporting bias (selective reporting; one item) at study level, and detection bias (blinding of outcome assessment; one item) and attrition bias (incomplete outcome data; one item) at outcome level. We did not assess detection bias for the outcome of survival because this in an objective outcome, and we did not assess performance bias (one item) because blinding of healthcare personnel and participants is not possible for the interventions considered in this review.
Measures of treatment effect
For dichotomous data, we used odds ratio (OR) as the measure of treatment effect. For continuous data, we used the standardised mean difference (SMD). For time‐to‐event (survival) data, we used the hazard ratio (HR). For our meta‐analysis of time‐to‐event outcomes in Review Manager 5.3 (RevMan), we used 'O‐E' (observed minus expected) and 'V' (variance) statistics or hazard ratios for each trial. If these values were not reported for a given trial, we calculated them from available statistics, if possible, using the methods described in Tierney 2007.
Unit of analysis issues
Some trials performed serial measurements of arm volume and/or function over the first months and years after surgery. For our analysis, we used the measurement at one year post operation (or at the nearest time point after one year for trials not reporting data at the one‐year time point). One trial (NSABP B‐04) included three treatment comparison groups. This presented an issue only for analysis of less versus more axillary surgery (Analysis 5.1); to avoid double‐counting of the ALND group, we omitted the comparison of radiotherapy versus ALND in clinically node negative study participants.
5.1. Analysis.
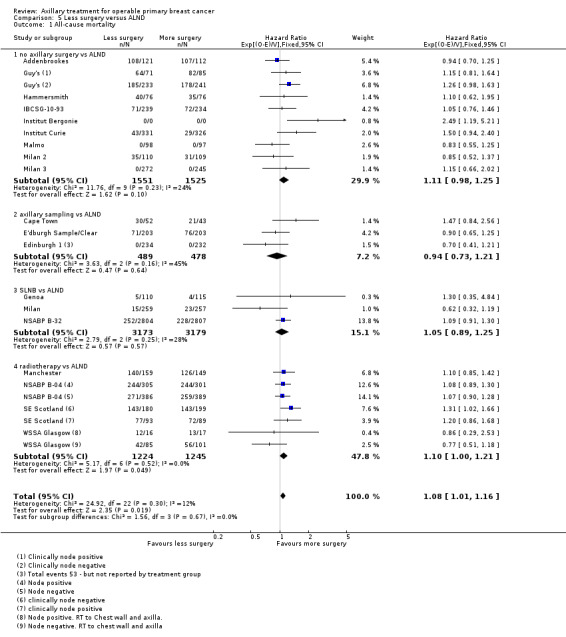
Comparison 5 Less surgery versus ALND, Outcome 1 All‐cause mortality.
Dealing with missing data
We analysed only data available in trial reports or obtained through contact with trial authors. We did not attempt data imputation.
Assessment of heterogeneity
We assessed statistical heterogeneity (variability in intervention effects) in meta‐analyses by using the I2 statistic, which we interpreted alongside magnitude and direction of effects. We regarded an I2 value of 30% to 60% as indicating potentially important heterogeneity and downgraded the overall quality of evidence for that outcome (owing to inconsistency) in the summary of findings tables. If heterogeneity was greater than 50%, we did not pool effect estimates but instead used the range of effects reported by individual studies.
Assessment of reporting biases
We checked reporting bias by using funnel plots and checked that outcomes measured in individual trials were reported in trial publications. If we suspected reporting bias for a given outcome, we downgraded the overall quality of the evidence in the summary of findings table owing to reporting/publication bias.
Data synthesis
We statistically synthesised time‐to‐event outcomes that were entered into RevMan as ‘O–E' and 'Variance’ outcomes by using a fixed‐effect model (the random‐effects model is not an option for this analysis in RevMan). We analysed dichotomous outcomes by using fixed‐effect (Mantel‐Haenszel method) and random‐effects (DerSimonian and Laird) models (Sensitivity analysis).
For summary of findings tables (Table 1; Table 2; Table 3; Table 4), we used the GRADE approach to assign an overall assessment of the quality of the evidence. In addition to the risk of bias assessment, the GRADE quality rating includes assessments of inconsistency, indirectness and imprecision of results, and of the likelihood of publication bias. We prioritised Primary outcomes for inclusion in summary of findings tables and organised them according to Types of interventions.
Summary of findings for the main comparison. No axillary surgery compared with full axillary surgery for operable primary breast cancer.
| No axillary surgery compared with full axillary surgery for operable primary breast cancer | |||||
| Patient or population: women with operable primary breast cancer Settings: hospital Intervention: no axillary surgery Comparison: full axillary surgery | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Full axillary surgery | No axillary surgery | ||||
| All‐cause mortality | 92% overall survival at 5 yearsa | 92% overall survival at 5 years (91% to 93%) | HR 1.06 (0.96 to 1.17) | 3849 (10 studies) | ⊕⊕⊕⊝ moderateb |
| Locoregional recurrence | 86% locoregional recurrence‐free survival at 5 yearsc | 71% locoregional recurrence‐free survival at 5 years (66% to 76%) | HR 2.35 (1.91 to 2.89) | 20,863d (5 studies) | ⊕⊕⊕⊝ moderatee |
| Lymphoedema Increase in arm circumference Follow‐up: 1 or more years | 236 per 1000 | 87 per 1000 (66 to 117) | OR 0.31 (0.23 to 0.43) | 1714 (4 studies) | ⊕⊕⊝⊝ lowe,f |
| Arm or shoulder movement impairment Follow‐up: 1 or more years | 91 per 1000 | 67 per 1000 (47 to 95) | OR 0.72 (0.49 to 1.05) | 1495 (5 studies) | ⊕⊝⊝⊝ very lowf,g |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; OR: odds ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aAssumed risk is taken from full axillary surgery arm of Institut Curie. bConfidence interval around the effect estimate includes both no effect and appreciable harm associated with no axillary surgery. cAssumed risk is taken from full axillary surgery arm of Institut Curie, local or axillary recurrence rates. dPerson‐years of follow‐up. eSubstantial heterogeneity (I2 > 50%). fUnclear blinding of outcome assessment. gConsiderable heterogeneity (I2 > 75%).
Summary of findings 2. Axillary sampling compared with full axillary surgery for operable primary breast cancer.
| Axillary sampling compared with full axillary surgery for operable primary breast cancer | ||||||
| Patient or population: women with operable primary breast cancer Settings: hospital Intervention: axillary sampling Comparison: full axillary surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Full axillary surgery | Axillary sampling | |||||
| All‐cause mortality | 82% overall survival at 5 yearsa | 83% overall survival at 5 years (79% to 87%) | HR 0.94 (0.73 to 1.21) | 967 (3 studies) | ⊕⊕⊝⊝ lowb,c | |
| Local recurrence | 85% local recurrence‐free survival at 5 yearsd | 80% local recurrence free survival at 5 years (71% to 86%) | HR 1.41 (0.94 to 2.12) | 1404 (3 studies) | ⊕⊕⊝⊝ lowe,f | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aAssumed risk is taken from full axillary surgery arm of E'dburgh Sample/Clear. bSubstantial heterogeneity. cConfidence interval for the effect includes both appreciable benefit and harm with axillary sampling. dAssumed risk taken from full axillary surgery arm of Cardiff. eNo blinding of outcome assessment or blinding not reported. fConfidence interval for effect includes both no difference and appreciable harm with axillary sampling. Low number of events.
Summary of findings 3. Sentinel node biopsy compared with full axillary surgery for operable primary breast cancer.
| Sentinel node biopsy compared with full axillary surgery for operable primary breast cancer | |||||
| Patient or population: women with operable primary breast cancer Settings: hospital Intervention: sentinel node biopsy Comparison: full axillary surgery | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Full axillary surgery | Sentinel node biopsy | ||||
| All‐cause mortality | 96% overall survival at 5 yearsa | 96% overall survival at 5 years (95% to 96%) | HR 1.05 (0.89 to 1.25) | 6352 (3 studies) | ⊕⊕⊕⊝ moderateb |
| Lymphoedema Patient‐reported lymphoedema of any severity Follow‐up: 12 months | 132 per 1000 | 48 per 1000 (22 to 115) | OR 0.33 (0.15 to 0.86) | 815 (3 studies) | ⊕⊕⊝⊝ lowb,c |
| Subjective arm movement impairment Follow‐up: 12 months | 100 per 1000 | 40 per 1000 (24 to 69) | OR 0.38 (0.22 to 0.67) | 877 (2 studies) | ⊕⊝⊝⊝ very lowb,d,e |
| Paraesthesia Follow‐up: 12 months | 776 per 1000 | 343 per 1000 (238 to 444) | OR 0.15 (0.09 to 0.23) | 495 (2 studies) | ⊕⊕⊝⊝ lowd,e |
| Pain Follow‐up: 12 months | 177 per 1000 | 86 per 1000 (61 to 126) | OR 0.44 (0.3 to 0.67) | 877 (2 studies) | ⊕⊕⊝⊝ lowd,e |
| Numbness Follow‐up: 12 months | 346 per 1000 | 185 per 1000 (152 to 222) | OR 0.43 (0.34 to 0.54) | 1799 (3 studies) | ⊕⊕⊕⊝ moderatef |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; OR: odds ratio. | |||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aAssumed risk taken from the full axillary surgery arm of Milan. bLow number of events. cIncomplete follow‐up for patient‐reported lymphoedema in ALMANAC. Event rates not reported in Addenbrookes 2. dModerate or substantial heterogeneity. eNo blinding or blinding not reported. fNo explanation provided.
Summary of findings 4. Radiotherapy alone compared with full axillary surgery for operable primary breast cancer.
| Radiotherapy alone compared with full axillary surgery for operable primary breast cancer | |||||
| Patient or population: women with operable primary breast cancer Settings: hospital Intervention: radiotherapy alone Comparison: full axillary surgery | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Full axillary surgery | Radiotherapy alone | ||||
| All‐cause mortality | 81% overall survival at 5 yearsa | 79% overall survival at 5 years (77% to 81%) | HR 1.1 (1 to 1.21) | 2469 (4 studies) | ⊕⊕⊕⊕ high |
| Local recurrence | 90% local recurrence‐free survival at 5 yearsb | 92% local recurrence‐free survival at 5 yearsa (90% to 93%) | HR 0.8 (0.64 to 0.99) | 22,256c (4 studies) | ⊕⊕⊕⊕ high |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio. | |||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aAssumed risk from full axillary surgery arm of NSABP B‐04 using mean 5‐year overall survival in combined N+ and N‐ groups. bAssumed risk from full axillary surgery arm of NSABP B‐04, using mean 5‐year risk for local or regional recurrence in combined lymph node‐positive and ‐negative groups. cPerson‐years of follow‐up.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses.
Radiotherapy versus no radiotherapy.
Further treatment versus no further treatment for histologically node‐positive participants.
Age groups (18 to 49 years; 50 to 69 years; 70 to 79 years; 80 years and older).
We were not able to analyse results by age group. When evidence suggested potentially important between‐study statistical heterogeneity (I2 value of 30% to 60%), we compared fixed‐effect and random‐effects estimates to check whether the intervention effect was sensitive to the type of model used, although it should be noted that such comparisons were not possible for analyses of time‐to‐event outcomes, as already outlined in the Data synthesis section.
Sensitivity analysis
To examine the robustness of our results, we performed sensitivity analyses that included only studies with low risk of bias for allocation concealment. Moreover, we planned to undertake sensitivity analyses to examine short‐term and long‐term morbidity outcomes only for studies with low risk of bias for blinded assessment of these outcomes. However, we considered none of the studies to be at low risk of bias for these items, so we could not perform these analyses.
Results
Description of studies
Results of the search
In total, we screened 7436 references for inclusion in this review (Figure 1). We retrieved full‐text articles for 163 references to potentially relevant publications to check inclusion eligibility. Of these,13 full‐text articles reported on eight trials that appeared relevant but did not meet all of the inclusion criteria (AATRM‐048‐13‐2000; ACOSOG Z0011; Buenos Aires; Copenhagen; Edinburgh SES; IBCSG‐23‐01; IPO‐P; OTOASOR). See Excluded studies section.
We identified six articles reporting on eight possibly eligible ongoing trials (AMAROS; GF‐GS 01; KiSS; NCT01717131; NCT02167490; NCT02271828; SNAC2; SOUND). Two studies (ISRCTN88463711; Semiglazov 2003) await classification. We excluded 45 other full‐text articles for the following reasons: 23 used ineligible Types of interventions, four included ineligible Types of participants and 18 were the wrong Types of studies.
The remaining 97 articles were reports of 26 eligible RCTs included in this review. We contacted the authors of included studies by email to ask about other relevant trials for inclusion in the review, but this yielded no additional studies.
Included studies
This review includes 26 studies that performed 27 treatment comparisons.
Full axillary surgery versus no axillary surgery
Ten studies compared axillary lymph node dissection (ALND) versus no axillary surgery (N = 3849; Addenbrookes; Guy's; Hammersmith; IBCSG‐10‐93; Institut Curie; Institut Bergonie; Malmo; Milan 2; Milan 3; NSABP B‐04).
The Malmo trial compared ALND plus radiotherapy (RT) versus no ALND and no RT. In one trial (IBCSG‐10‐93), only those treated with conservative breast surgery received RT. In Addenbrookes; Guy's; Hammersmith; Institut Curie; Institut Bergonie; Milan 2; and Milan 3, all study participants received RT. NSABP B‐04 reported a three‐group comparison of ALND, no ANLD plus RT and no ALND for patients with clinically negative axillary nodes. Patients in the ALND arm received limited RT to the chest wall. We included the ALND and no ALND arms of NSABP B‐04 for this comparison.
Five studies excluded patients with clinically involved lymph nodes (Institut Bergonie; Institut Curie; Malmo; Milan 2; Milan 3), whereas the remaining five studies included these patients only when clinically involved nodes were mobile and were not fixed to underlying structures (Addenbrookes; Guy's; Hammersmith; IBCSG‐10‐93; NSABP B‐04).
Seven studies (Addenbrookes; Guy's; Hammersmith; IBCSG‐10‐93; Malmo; Milan 2; NSABP B‐04) did not provide extra treatment for participants with histologically positive axillary lymph nodes. In Institut Curie, Institut Bergonie and Milan 3, such individuals could receive chemotherapy or hormone therapy.
Full axillary surgery versus axillary sampling
Six trials compared ALND versus axillary sampling (N = 1559; Cape Town; Cardiff; E'dburgh Sample/Clear; Edinburgh 1; Ostersund; Xu 2003). Of these trials, only Cape Town did not provide RT as part of the randomised treatment.
In Cardiff, E'dburgh Sample/Clear, Edinburgh 1 and Ostersund, participants with histologically positive sampled axillary lymph nodes received additional RT. In Xu 2003, RT was provided only for participants with more than three positive axillary lymph nodes and for those with a primary tumour in the central quadrant. In Cape Town, participants with histologically positive sampled nodes did not receive additional treatment.
Four trials (Cape Town; Cardiff; E'dburgh Sample/Clear; Edinburgh 1) included patients with clinically involved axillary nodes, provided such nodes were mobile. In the Ostersund and Xu 2003 trials, inclusion criteria were unclear.
Full axillary surgery versus sentinel node biopsy
Seven trials compared ALND versus sentinel lymph node biopsy (SLNB) (N = 9426; Addenbrookes 2; ALMANAC; Genoa; GIVOM Sentinella; Milan; NSABP B‐32; SNAC).
In three studies (Genoa; GIVOM Sentinella;Milan), only participants treated with breast‐conserving surgery received RT, which meant that some of the participants in Genoa and GIVOM Sentinella did not receive RT. In the remaining trials (Addenbrookes 2; ALMANAC; NSABP B‐32; SNAC), participants received RT according to local treatment protocols, which meant that in practice, most participants received RT.
In all of these trials, participants with histologically positive sentinel lymph nodes received further treatment. Treatment for histologically positive lymph nodes consisted of ALND (Addenbrookes; Genoa; GIVOM Sentinella; NSABP B‐32; Milan; SNAC) or the choice of ALND or RT to the axilla (ALMANAC).
Addenbrookes 2; ALMANAC; Genoa; GIVOM Sentinella; NSABP B‐32 and SNAC excluded patients with clinically involved axillary nodes, but it was unclear whether the Milan trial excluded such individuals.
Axillary sampling versus SLNB
We identified no studies for this comparison.
Axillary sampling versus no axillary surgery
We identified no studies for this comparison.
SLNB versus no axillary surgery
We identified no studies for this comparison.
Full axillary surgery with no RT versus no axillary surgery with RT
Four trials compared ALND without RT versus RT alone (N = 2585; Manchester; NSABP B‐04; SE Scotland; WSSA Glasgow). One of these trials (NSABP B‐04) performed a three‐group comparison of ALND, no ANLD plus RT and no ALND with clinically negative axillary nodes. Participants in the ALND arm of this trial did receive limited RT to the chest wall. We included in this review the ALND and no ALND plus RT arms of NSABP B‐04. This trial randomised participants with clinically positive nodes to ALND or no ANLD plus RT; we analysed these results separately. All of these trials included patients with clinically involved axillary nodes provided such nodes were mobile. None of these trials specified that they provided extra treatments for participants with histologically positive axillary nodes.
Excluded studies
We excluded eight trials from this review (see Excluded studies table for full details). We excluded two otherwise relevant trials because treatment allocation was not randomised; instead, investigators decided treatment group on the basis of month of birth (Buenos Aires) or order of entry into the trial (Copenhagen). We excluded the Edinburgh South East Scotland trial (Edinburgh SES) because it did not involve axillary surgery or lymph node biopsy.
We excluded five trials comparing ALND versus no further axillary surgery because trial entry or inclusion depended on the results of SLNB (AATRM‐048‐13‐2000; ACOSOG Z0011; IBCSG‐23‐01; IPO‐P; OTOASOR). All of these trials excluded patients with clinically involved axillary nodes before their primary surgery. The IPO‐P trial included only those with negative SLNB. Remaining trials included only patients with a positive SLNB (AATRM‐048‐13‐2000; ACOSOG Z0011; IBCSG‐23‐01; OTOASOR). AATRM‐048‐13‐2000 included only patients with sentinel lymph node micrometastases.
Risk of bias in included studies
We summarised in Figure 2 the risk of bias of included studies.
2.
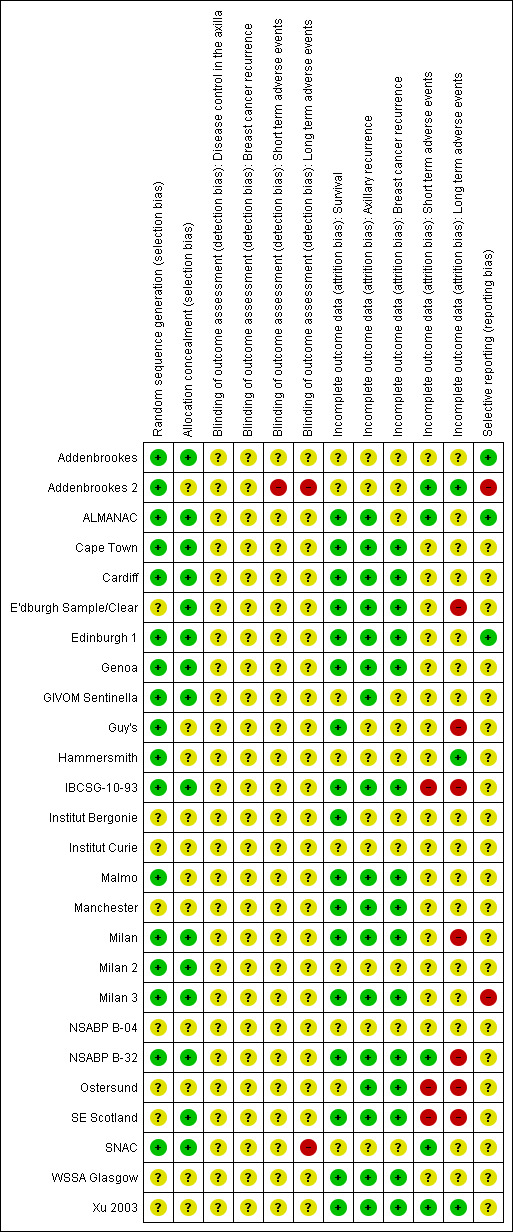
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In all, 17 trials clearly reported random sequence generation (Addenbrookes; Addenbrookes 2; ALMANAC; Cape Town; Cardiff; Edinburgh 1; Genoa; GIVOM Sentinella; Guy's; Hammersmith; IBCSG‐10‐93; Malmo; Milan; Milan 2; Milan 3; NSABP B‐32; SNAC), and the remaining nine trials provided unclear information on this (E'dburgh Sample/Clear; Institut Bergonie; Institut Curie; Manchester; NSABP B‐04; Ostersund;SE Scotland; WSSA Glasgow; Xu 2003).
Allocation concealment was adequate in 15 trials (Addenbrookes; ALMANAC; Cape Town; Cardiff; E'dburgh Sample/Clear; Edinburgh 1; Genoa; GIVOM Sentinella; IBCSG‐10‐93; Milan; Milan 2; Milan 3; NSABP B‐32; SE Scotland; SNAC) and unclear in the other 11 trials (Addenbrookes 2; Guy's; Hammersmith; Institut Bergonie; Institut Curie; Malmo; Manchester; NSABP B‐04; Ostersund; WSSA Glasgow; Xu 2003). In trials with unclear risk of selection bias, we did not observe obvious differences in the baseline characteristics of treatment groups, although Malmo, Ostersund and WSSA Glasgow poorly reported baseline characteristics.
Blinding
Two studies were at high risk of detection bias due to lack of blinding of outcome assessment or disease recurrence and adverse event outcomes (Addenbrookes 2; SNAC2). All other studies were at unclear risk of detection bias due to poor reporting.
Incomplete outcome data
Seventeen trials had low risk of incomplete overall survival data (ALMANAC; Cape Town; Cardiff; E'dburgh Sample/Clear; Edinburgh 1; Genoa; Guy's; IBCSG‐10‐93; Institut Bergonie; Malmo; Manchester; Milan; Milan 3; NSABP B‐32; SE Scotland; WSSA Glasgow; Xu 2003). The remaining trials were at unclear risk of bias due to incomplete outcome data because they did not report overall survival or the completeness of their reporting was uncertain. We observed a similar pattern for outcomes related to breast cancer recurrence and disease control in the axilla (Figure 2).
We judged five trials to be at low risk of bias because they provided incomplete data for short‐term adverse events (Addenbrookes 2; ALMANAC; NSABP B‐32; SNAC; Xu 2003); all of these trials involved SLNB. Three trials were at high risk (IBCSG‐10‐93; Ostersund; SE Scotland), and the remainder were at uncertain risk. We noted a similar pattern for long‐term adverse events, with three trials at low risk of bias (Addenbrookes 2; Hammersmith; Xu 2003), seven trials at high risk (E'dburgh Sample/Clear; Guy's; IBCSG‐10‐93; Milan; NSABP B‐32; Ostersund; SE Scotland) and the remainder at uncertain risk.
Selective reporting
Three trials were at low risk of bias due to selective reporting (Addenbrookes; ALMANAC; Edinburgh 1). Addenbrookes 2 and Milan 3 were at high risk of bias due to selective reporting of some outcomes on the basis of statistical significance. The remaining trials were at uncertain risk of bias due to selective reporting.
Other potential sources of bias
Trials typically reported intention‐to‐treat analyses, but in four trials it was unclear whether such analyses were performed (Cape Town; NSABP B‐04; Ostersund; WSSA Glasgow). We included two trials that performed per‐protocol analysis (Malmo; Milan) because study authors stated that per‐protocol results were similar to intention‐to‐treat results (Malmo), or because protocol violations were few (Milan).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
We recorded in Table 5 time‐to‐event statistics extracted for each trial. We listed in Table 6 the definitions of adverse event outcomes used in each study, and we summarised in Table 7 adverse events at various time points after treatment.
1. Summary time‐to‐event statistics.
| Study | Outcome reported | Observed | Expected | Variance | HR | 95% CIs | P value | Follow‐up | Notes |
| Addenbrookes | Overall mortality |
ALND: 107/112 No ALND: 108/121 |
o‐e = ‐3.1 | 46.5 | 0.94 | (0.70 to 1.25) | NA | 15 years? | Taken from Clarke 2005 (Appendix web figure 9b), then inverted to reflect that more surgery is our control and less surgery is our research condition The number of patients reported by Clarke 2005 differs from that reported by Brinkley (1971). |
| Addenbrookes | Breast cancer mortality |
ALND: 74/112 No ALND: 78/121 |
o‐e = ‐2.2 | 32.8 | ‐ | ‐ | NA | 15 years? | Taken from Clarke 2005 (Appendix web figure 9b), then inverted to reflect that more surgery is our control and less surgery is our research condition. Not included in meta‐analysis |
| Addenbrookes | Isolated local recurrence |
ALND: 7 events/1148 women‐years No ALND: 15 events/1218 women‐years |
o‐e = 3.3 | 5.4 | 1.8 | (0.79 to 4.28) | NA | 5 years? | Taken from Clarke 2005 (Appendix web figure 9b), then inverted to reflect that more surgery is our control and less surgery is our research condition |
| ALMANAC | Overall mortality |
ALDN: 7/476 SLNB: 7/478 |
NA | NA | NA | NA | NA | 1 year | Cannot calculate o‐e. Not included in meta‐analysis |
| ALMANAC | Axillary recurrence |
ALDN: 4/476 SLNB: 1/478 |
NA | NA | NA | NA | NA | 1 year | Cannot calculate o‐e. Not included in meta‐analysis |
| Cape Town | Overall mortality |
ALND: 21/43 Simple: 30/52 |
o‐e = 4.74 | 12.35 | 1.47 | (0.84 to 2.56) | 0.1775 | 10 years | Tierney et al (2007) method 7 used log‐rank test results from figure 1. Cape Town |
| Cape Town | Overall mortality (node‐negative) |
ALND: 14/21 Simple: 26/30 |
o‐e = 1.8 | 7.6 | ‐ | ‐ | NA | Taken from Clarke 2005 (Appendix web figure 9a; Groote‐Schuur), then O‐E sign changed to reflect that more surgery is our control and less surgery is our research condition. Not included in meta‐analysis | |
| Cape Town | Overall mortality (node‐positive) |
ALND: 19/22 Simple: 22/25 |
o‐e = ‐1.9 | 7.7 | ‐ | ‐ | NA | Taken from Clarke 2005 (Appendix web figure 9b; Groote‐Schuur), then O‐E sign changed to reflect that more surgery is our control and less surgery is our research condition. Not included in meta‐analysis | |
| Cape Town | Isolated local recurrence (node‐negative) |
ALND: 3/206 women‐years Simple: 8/232 women‐years |
o‐e = 1.7 | 2.3 | 2.09 | (0.58 to 7.63) | NA | Taken from Clarke 2005 (Appendix web figure 9a; Groote‐Schuur), then inverted to reflect that more surgery is our control and less surgery is our research condition | |
| Cape Town | Isolated local recurrence (node‐positive) |
ALND: 5/134 women‐years Simple: 9/173 women‐years |
o‐e = 0.0 | 2.0 | 1.00 | (0.25 to 4.00) | NA | Taken from Clarke 2005 (Appendix web figure 9b; Groote‐Schuur), then inverted to reflect that more surgery is our control and less surgery is our research condition | |
| Cape Town | Axillary recurrence |
ALND: 2/43 Simple: 8/52 |
NA | NA | NA | NA | NA | 10 years | Cannot calculate o‐e. Not included in meta‐analysis |
| Cape Town | Any locoregional recurrence |
ALND: 11/43 Simple: 19/52 |
NA | NA | NA | NA | NA | 10 years | Cannot calculate o‐e. Not included in meta‐analysis |
| Cape Town | Distant metastases |
ALND: 11/43 Simple: 13/52 |
NA | NA | NA | NA | NA | 10 years | Cannot calculate o‐e. Not included in meta‐analysis |
| Cardiff | Overall survival |
ALND: N = 97 Sampling: N =103 Total events = 152 Fig 2 data: ALND: 23/97 Sampling: 13/103 |
o‐e: 7.4 | 38 | 1.21 | (0.29 to 0.99) | 0.23 | 20 years | HR calculated using log‐rank P value from Stewart et al (1994, page 42) by Tierney 2007 method 8, 9. Owing to non‐proportionality of hazard rates, HR cannot be included in meta‐analysis |
| Cardiff | Disease‐free survival |
ALND: 97 Sampling: 103 |
5.87 | 7.75 | 2.13 | (1.05 to 4.31) | 0.035 | 20 years | Log‐rank P value Tierney 2007 method 8, 9 (page 43 & Fig 5 Stewart et al, 1994) |
| Cardiff | Locoregional recurrence (chest wall, axilla, supraclavicular/internal mammary nodes) |
ALND: 19/94 Sampling: 31/99 Fig 4: ALND: 11/97 Sampling: 22/103 |
o‐e: 6.46 | 11.78 | 1.73 | (0.87 to 3.42) | NA | 20 years | Tierney et al (2007) method 4 used and data from Figure 4 & page 42 Stewart et al (1994) |
| Cardiff | Distant relapse |
ALND: 43/94 Sampling: 59/99 |
o‐e: 8.4 | 24.87 | 1.4 | (0.99 to 1.71) | 0.092 | 20 years | Data from Table 6, Stewart et al (1994): excludes patients with radiotherapy violations. Per‐protocol analysis ‐ not included in meta‐analysis |
| Cardiff | Breast cancer recurrence (total) (locoregional and distant relapse) |
ALND: 62/94 Sampling: 90/99 |
o‐e: 12.77 | 36.71 | 1.42 | (1.18 to 1.61) | 0.035 | 20 years | Calculated from Stewart et al (1994) (excludes RT violations) per‐protocol analysis Risk of overestimation not certain as these are first events or total events.‐ not included in meta‐analysis |
| Edinburgh 1 | Overall survival |
ALND: ?/232 Sampling: ?/234 Total events = 53 ALND: 207/232 Sampling: 190/234 |
o‐e: ‐4.66 | 13.25 | 0.7 | (0.41 to 1.21) | 0.20 | 5 years | HR calculated using log rank P ‐ figure 2, Chetty (2000) |
| Edinburgh 1 | Axillary recurrence |
ALND: /232 Sampling: /234 |
o‐e: ‐0.15 | 13.25 | 0.99 | (0.58 to 1.69) | 0.94 | Up to 8 years | Log‐rank P value Tierney 2007 method 7, 8, 9 used Fig 3 Chetty (2000) |
| Edinburgh 1 | Local recurrence in the breast |
ALND: 14/232 Sampling: 15/234 |
o‐e: ‐0.10 | 7.24 | 0.99 | (0.48 to 2.04) | 0.97 | Up to 8 years |
Tierney 2007 method 7, 8, 9 used Table 6 & page 87 Chetty (2000) |
| Edinburgh 1 | Distant recurrence |
ALND: 29/232 Sampling: 29/234 |
Not available | Not available | Not available | Not available | NA | Up to 8 years | Table 6, Chetty (2000). Unable to estimate HR ‐ not included in analysis |
| E'dburgh Sample/Clear | Overall survival |
ALND: 76/203 Sampling: 71/203 |
o‐e: ‐3.81 | 36.55 | 0.90 | (0.62 to 1.25) | NA | 13 years |
Tierney 2007 method 3 used (using 1995 data – Clarke 2005 paper reports more deaths) Fig 1 and page 82 HR (CI) in Forrest et al (1995) ‐ inverted the HR |
| E'dburgh Sample/Clear | Distant metastases |
ALND: 51/203 Sampling: 53/203 |
o‐e: 1.5 | 30.78 | 0.92 | (0.67 to 1.35) | NA | 13 years | Tierney 2007 method 3 used (using 1995 data), Fig 2 and HR (CI) page 82 in Forrest et al (1995), inverted the HR |
| E'dburgh Sample/Clear | Locoregional relapse (chest wall, axilla, supraclavicular) |
ALND: 38/203 Sampling: 29/203 |
o‐e: ‐4.9 | 16.32 | 0.74 | (0.46 to 1.20) | NA | 13 years |
Tierney 2007 method 3 used (using 1995 data) Method 3 Fig 3 from HR (CI), page 82 in Forrest et al (1995), inverted the HR |
| Genoa | Overall survival |
ALND: 4/115 SLNB: 5/110 |
o‐e: 0.58 | 2.22 | 1.32 | (0.35 to 4.92) | 0.679 | 5 years | Log‐rank P value (Canavese 2009 ‐ fig 3) Tierney 2007 method 7 used Fig 3 KM curve gives P = 0.679. I assumed that was correct as it appears on the graph. The text value (page 20) may be a typo 0.697. HR are similar; CI differ |
| Genoa | Axillary recurrence |
ALND: 1/115 SLNB: 0/110 |
NA | NA | NA | NA | NA | 5 years | Not included in meta‐analysis |
| Genoa | Breast cancer recurrence (local and contralateral recurrence, axillary and distant metastases) |
ALND: 10/115 SLNB: 8/110 |
NA | NA | NA | NA | NA | 5 years | Not included in meta‐analysis |
| Genoa | 5‐Year event‐free survival |
ALND: 12/115 SLNB: 10/110 |
o‐e: ‐0.85 | 5.45 | 0.86 | (0.37 to 1.98) | 0.715 | 5 years | Log‐rank P value from Fig 2, Canavese (2009) method 7 Tierney 2007 used |
| GIVOM Sentinella | Overall survival |
ALND: 14/352 SLNB: 21/345 |
NA | NA | NA | NA | NA | 5 years | Not included in meta‐analysis |
| GIVOM Sentinella | Disease‐free survival |
ALND: 28/352 SLNB: 39/345 |
o‐e = 1.18 | 16.3 | 1.08 | 0.769 | 5 years | Method 7 Tierney 2007 used | |
| GIVOM Sentinella | Axillary recurrence |
ALND: 0/352 SLNB: 1/345 |
NA | NA | NA | NA | NA | 5 years | Cannot calculate o‐e. Not included in meta‐analysis |
| GIVOM Sentinella | Locoregional recurrence |
ALND: 3/352 SLNB: 16/345 |
NA | NA | NA | NA | NA | 5 years | Cannot calculate o‐e. Not included in meta‐analysis |
| GIVOM Sentinella | Distant recurrence |
ALND: 16/352 SLNB: 11/345 |
NA | NA | NA | NA | NA | 5 years | Cannot calculate o‐e. Not included in meta‐analysis |
| Guy's | Overall mortality (clinically node negative) |
ALND: 178/241 No ALND (wide excision): 185/233 |
o‐e = 13.8 | 80.7 | 1.26 | (0.98 to 1.63) | 0.1 | 15 years? | Taken from Clarke 2005 (Appendix web figure 10a), then inverted to reflect that more surgery is our control and less surgery is our research intervention |
| Guy's | Overall mortality (clinically node positive) |
ALND: 82/85 No ALND (wide excision): 64/71 |
o‐e = 4.3 | 30.9 | 1.15 | (0.81 to 1.64) | 0.4 | 15 years? | Taken from Clarke 2005 (Appendix web figure 10b), then inverted to reflect that more surgery is our control and less surgery is our research intervention |
| Guy's | Breast cancer mortality (clinically node negative) |
ALND: 122/241 No ALND (wide excision): 142/233 |
o‐e = 13.8 | 58.8 | ‐ | ‐ | 0.07 | 15 years? | Taken from Clarke 2005 (Appendix web figure 10a), then inverted to reflect that more surgery is our control and less surgery is our research intervention Not included in meta‐analysis |
| Guy's | Breast cancer mortality (clinically node positive) |
ALND: 53/85 No ALND (wide excision): 54/71 |
o‐e = 6.2 | 23.6 | ‐ | ‐ | 0.2 | 15 years? | Taken from Clarke 2005 (Appendix web figure 10b), then inverted to reflect that more surgery is our control and less surgery is our research intervention. Not included in meta‐analysis |
| Guy's | Isolated local recurrence (clinically node negative) |
ALND: 35 events/3267 women‐years No ALND: 81 events/2383 women‐years |
o‐e = 29.5 | 26.4 | 3.06 | (2.09 to 4.48) | < .00001 | 5 years? | Taken from Clarke 2005 (Appendix web figure 10a), then inverted to reflect that more surgery is our control and less surgery is our research intervention |
| Guy's | Isolated local recurrence (clinically node positive) |
ALND: 17 events/873 women‐years No ALND: 31 events/519 women‐years |
o‐e = 10.5 | 10.8 | 2.64 | (1.46 to 4.80) | 0.001 | 5 years? | Taken from Clarke 2005 (Appendix web figure 10b), then inverted to reflect that more surgery is our control and less surgery is our research intervention |
| Hammersmith | Overall survival |
Radical: 35/76 Simple: 40/76 |
o‐e = 1.44 | 11.78 | 1.13 | (0.64 to 2.00) | NA | 8 years | Extracted from Fig 3, Burn et al (1968) Tierney 2007 method 10 on Simple is input as "research" and radical as "control". Min and max follow‐up input as 3‐96 months |
| Hammersmith | Local recurrence |
Radical: 10/76 Simple: 11/76 |
NA | NA | NA | NA | NA | 4‐9 years | Not included in meta‐analysis |
| Hammersmith | Mean time to recurrence |
Radical: 15.7 months Simple: 25.9 months |
NA | NA | NA | NA | NA | 4‐9 years | Not included in meta‐analysis |
| IBCSG‐10‐93 | Overall survival |
ALND: 72/234 Surgery only: 71/239 |
o‐e = 1.76 (survival curves cross) | 36.05 | 1.05 | (0.76 to 1.46) | 0.77 | 6‐7 years | HR reported on page 340 of IBCSG (2006), used Tierney 2007 method 3 |
| IBCSG‐10‐93 | Disease‐free survival |
ALND: 92/234 Surgery only: 89/239 |
o‐e = 2.6 | 44.69 | 1.06 | (0.79 to 1.42) | 0.69 | 6‐7 years | HR reported on page 340 of IBCSG (2006), used Tierney 2007 method 3 |
| IBCSG‐10‐93 | Axilla recurrence (as first event) |
ALND: 2/234 Surgery only: 6/239 |
NA | NA | NA | NA | NA | 6‐7 years | Not included in meta‐analysis |
| Institut Bergonie | Overall survival (whole follow‐up period) ITT |
no ALND: NR ALND: NR |
o‐e = 6.42 | 7.04 | 2.49 | 90% CI (1.34 to 4.63) | NA | Whole follow‐up period (unclear how long that is) | HR reported on page 566 of Avril (2011), used Tierney 2007 method 3 |
| Institut Bergonie | Event‐free survival (whole follow‐up period) ITT |
no ALND: 44/297 ALND: 31/297 |
o‐e = 8.75 | 18.37 | 1.61 | 90% CI (1.1 to 2.37) | NA | Whole follow‐up period (unclear how long that is) | HR reported on page 566 of Avril (2011), used Tierney 2007 method 3 |
| Institut Bergonie | Axillary event |
Within 5 years: no ALND: 4/297 ALND: 0/310 After 5 years: no ALND: 2/297 ALND: 0/310 |
NA | NA | NA | NA | NA | Not included in meta‐analysis | |
| Institut Bergonie | Lymph node (excl axillary) event |
Within 5 years: no ALND: 1/297 ALND: NA After 5 years: no ALND: 0/297 ALND: NA |
NA | NA | NA | NA | NA | Not included in meta‐analysis | |
| Institut Bergonie | Breast/chest wall event |
Within 5 years: no ALND: 5/297 ALND: 4/310 After 5 years: no ALND: 0/297 ALND: 8/310 |
NA | NA | NA | NA | NA | Not included in meta‐analysis | |
| Institut Bergonie | Metastatic event |
Within 5 years: no ALND: 4/297 ALND: 1/310 After 5 years: no ALND: 2/297 ALND: 2/310 |
NA | NA | NA | NA | NA | Not included in meta‐analysis | |
| Institut Bergonie | Contralateral breast cancer |
Within 5 years: no ALND: 2/297 ALND: 1/310 After 5 years: no ALND: 2/297 ALND: 1/310 |
NA | NA | NA | NA | NA | Not included in meta‐analysis | |
| Institut Bergonie | Other site cancer | Within 5 years: no ALND: 5/297 ALND: 5/310 After 5 years: no ALND: 5/297 ALND: 4/310 |
NA | NA | NA | NA | NA | Not included in meta‐analysis | |
| Institut Curie | Overall survival | RT: 43/331; ALND: 29/326 | o‐e = 7 | 17.3 | 1.50 | (0.94 to 2.40) | NA | Taken from Clarke 2005 (Appendix web figure 10a), then inverted to reflect that more surgery is our control and less surgery is our research condition | |
| Institut Curie | Isolated local recurrence | RT: 39/2045 women‐years; ALND: 34/2126 women‐years | o‐e = 1.6 | 17.5 | 1.10 | (0.69 to 1.75) | NA | Taken from Clarke 2005 (Appendix web figure 10a), then inverted to reflect that more surgery is our control and less surgery is our research condition | |
| Institut Curie | Axilla recurrence | RT: 12/332; ALND: 5/326 | o‐e = 3.86 | 3.53 | 3.93 | ‐ | 0.04 | Table 6 in Louis‐Sylvestre (2004), method 7 in Tierney 2007 | |
| Institut Curie | Disease‐free survival |
RT: 5 years: 82 (SD = 2.1)% 10 years: 72 (SD = 2.5)% 15 years: 65.5 (SD = 2.7)% |
ALND: 5 years: 83.3 (SD 2)% 10 years: 72.6 (SD 2.5)% 15 years: 64.3 (SD 2.9)%. |
NA | NA | NA | NA | o‐e cannot be extracted because P values not reported past NS in Table 6 in Louis‐Sylvestre (2004). Not included in meta‐analysis | |
| Institut Curie | Metastases |
RT: 5 years: 12.8 (SD 1.9)% 10 years: 21 (SD 2.3)% 15 years: 24.9 (SD 2.5)% |
ALND: 5 years: 10.8 (SD 1.7)% 10 years: 18.3 (SD 2.2)% 15 years: 25.8 (SD 2.6)% |
NA | NA | NA | NA | O‐e cannot be extracted because P values not reported past NS in Table 6 in Louis‐Sylvestre (2004). Not included in meta‐analysis | |
| Malmo | Overall survival | ALND + RT: ?/97 Mastectomy alone: ?/98 (total event rate = 91) | o‐e = ‐4.19 | 22.75 | 0.83 | (0.55 to 1.25) | 0.38 | 15‐20 years | Using P = 0.38 reported on page 558 of Borgstrom (1994) and Tierney 2007 method 8. The o‐e is calculated on the basis of a total event rate of N = 91, and total N = 97 in the ALND + RT group and N = 98 in mastectomy alone group (i.e. intent‐to‐treat numbers), and using the only P value reported, which was for per‐protocol analysis that study authors stated did not differ from intention‐to‐treat analyses |
| Malmo | Chest wall recurrence | ALND + RT: 2/97 Mastectomy alone: 11/98 | NA | NA | NA | NA | NA | 15‐20 years | Cannot calculate o‐e. Not included in meta‐analysis |
| Manchester | Overall survival |
Radical: 126/149 Simple + RT: 140/159 |
o‐e = 5.4 | 58.6 | 1.10 | (0.85 to 1.42) | NA | 15 years | Taken from Clarke 2005 (Appendix web figure 10b), then inverted to reflect that more surgery is our control and less surgery is our research condition |
| Manchester | Death from breast cancer |
Radical: 100/149 Simple + RT: 112/159 |
o‐e = 2.8 | 46 | 1.06 | (0.80 to 1.42) | NA | 15 years | Taken from Clarke 2005 (Appendix web figure 10b), then inverted to reflect that more surgery is our control and less surgery is our research condition |
| Manchester | Local recurrence |
Radical: 48 events/997 women‐years Simple + RT: 41 events/1113 women‐years |
o‐e = ‐5.7 | 19.9 | 0.75 | (0.48 to 1.17) | NA | 15 years | Taken from Clarke 2005 (Appendix web figure 10b), then inverted to reflect that more surgery is our control and less surgery is our research condition |
| Milan | Death from any cause (OS) |
ALND = 23/257 SLNB = 15/259 |
o‐e = ‐4.34 | 9.08 | 0.62 | (0.32 to 1.19) | 0.15 | 10 years | Log‐rank P (Tierney 2007 method 7); ALND is control |
| Milan | Breast cancer recurrence (local recurrence, regional lymph node metastases, distant metastases) |
ALND = 26/257 SLNB = 23/259 |
o‐e = ‐2.25 | 12.02 | 0.83 | (0.47 to 1.46) | 0.52 | 10 years | Log‐rank P (Tierney 2007 method 7); ALND is control |
| Milan | Distant metastasis |
ALND = 20/257 SLNB = 17/259 |
o‐e = ‐2.04 | 9.19 | 0.80 | (0.42 to 1.53) | 0.50 | 10 years | Log‐rank P from table 4 Veronesi (2010) (Tierney 2007 method 7); ALND is control |
| Milan | Axillary metastasis |
ALND = 0/257 SLNB = 2/259 |
o‐e = 0.97 | 0.50 | 6.96 | (0.44 to 111.3) | 0.17 | 10 years | Log‐rank P from table 4 Veronesi (2010) (Tierney 2007 method 8 and 9); ALND is control |
| Milan | Local recurrence |
ALND = 4/257 SLNB = 4/259 |
o‐e = ‐0.12 | 2.00 | 0.94 | (0.24 to 3.76) | 0.93 | 10 years | Log‐rank P from table 4 Veronesi (2010) (Tierney 2007 method 7); ALND is control |
| Milan | Supraclavicular metastasis |
ALND = 2/257 SLNB = 0/259 |
o‐e = ‐1.02 | 0.50 | 0.13 | (0.01 to 2.09) | 0.15 | 10 years | Log‐rank P from table 4 Veronesi (2010) (Tierney 2007 method 8, 9); ALND is control |
| Milan | Contralateral breast cancer |
ALND = 10/257 SLNB = 9/259 |
o‐e = ‐0.81 | 4.47 | 0.84 | (0.34 to 2.07) | 0.71 | 10 years | Log‐rank P from table 4 Veronesi (2010) (Tierney 2007 method 7); ALND is control |
| Milan 2 | Overall survival |
ALND = 31/109 No ALND = 35/110 |
o‐e = ‐2.72 | 16.43 | 0.85 | (0.52 to 1.37) | Median = 150 months | HR reported on page 922 of Martelli (2012). Using Tierney 2007 method 3 o Please note, the curves cross; also the HR used for extraction of o‐e and its variance is adjusted for tumour grade and oestrogen‐receptor status |
|
| Milan 2 | Breast cancer deaths |
ALND: 8/109 No ALND: 10/110 |
o‐e = 1.33 | 4.06 | 1.39 | ‐ | ‐ | Median = 150 months | HR reported in Table 7 of Martelli (2012). Tierney 2007 method 3 o Please note, the curves cross; also the HR used for extraction of o‐e and its variance is adjusted for tumour grade and oestrogen‐receptor status. Not included in meta‐analysis |
| Milan 2 | Axillary relapse |
ALND: 0/109 No ALND: 4/110 |
NA | NA | NA | NA | NA | Median = 150 months | Table 6 of Martelli (2012), cannot calculate o‐e |
| Milan 2 | Recurrence (ipsilateral breast tumour) |
ALND: 4/109 No ALND: 7/110 |
NA | NA | NA | NA | NA | Median = 150 months | Table 6 of Martelli (2012), cannot calculate o‐e |
| Milan 2 | Distant metastases |
ALND: 9/109 No ALND: 9/110 |
o‐e = ‐2.68 | 5.93 | 0.64 | (0.28 to 1.42) | NA | Median = 150 months | HR reported in Table 7 of Martelli (2012). Tierney 2007 method 3 Please note, the curves cross; also the HR used for extraction of o‐e and its variance is adjusted for tumour grade and oestrogen‐receptor status |
| Milan 3 | Overall survival |
10‐year ALND: 93.3% (95% CI 89.4‐95.8) no ALND: 91.5% (95% CI 87‐94.4) |
o‐e = 1.76 | 12.33 | 1.15 | (0.66 to 2.02) | P = .436 | Median = 127.5 months | Agresti (2014) Figure 3A and Tierney 2007 method 11 Please note, the curves cross at the very end, also HR used for extraction of o‐e |
| Milan 3 | Death from breast cancer |
ALND: 17/272 no ALND: 15/245 |
NA | NA | NA | NA | P = 1.00 | Median = 127.5 months | Not included in meta‐analysis |
| Milan 3 | Disease‐free survival |
10‐year ALND: 92.4% (95% CI 88.5‐95.1) no ALND: 91.3% (95% CI 86.7‐94.3) |
o‐e= ‐0.13 | 10.7 | 0.99 | (0.54 to 1.8) | P = .97 | Median = 127.5 months | Agresti (2014) Figure 3A andTierney 2007 method 11 Please note, the curves cross at the very end; also the HR used for extraction of o‐e |
| Milan 3 | Distant metastases |
ALND: 23/272 no ALND: 20/245 |
NA | NA | NA | NA | P = 1.00 | Median = 127.5 months | Not included in meta‐analysis |
| Milan 3 | Axillary recurrence |
ALND: 0/272; no ALND: 22/245 |
NA | NA | NA | NA | NA | Median = 127.5 months | Not included in meta‐analysis |
| Milan 3 | Local recurrence |
ALND: 14/272 no ALND: 11/245 |
NA | NA | NA | NA | P = .839 | Median = 127.5 months | Not included in meta‐analysis |
| Milan 3 | Contralateral breast cancer |
ALND: 13/272 no ALND: 14/245 |
`NA | NA | NA | NA | P = .695 | Median = 127.5 months | Not included in meta‐analysis |
| NSABP B‐04 | Overall survival: node negative: ALND vs no ALND |
ALND = 259/389 No ALND = 256/384 |
o‐e = ‐5 | 117.3 | 0.96 | (0.80 to 1.15) | NA | 15 years? | Taken from Clarke 2005 Lancet (Appendix web figure 9a), then inverted to reflect that more surgery is our control and less surgery is our research condition |
| NSABP B‐04 | Overall survival: node negative: ALND vs no ALND + RT |
ALND = 259/389 No ALND + RT = 271/386 |
o‐e = 8.6 | 122.2 | 1.07 | (0.90 to 1.28) | NA | 15 years? | Taken from Clarke 2005 (Appendix web figure 10a), then inverted to reflect that more surgery is our control and less surgery is our research condition |
| NSABP B‐04 | Overall survival: node positive: ALND vs no ALND + RT |
ALND = 244/301 No ALND + RT = 244/305 |
o‐e = 8.3 | 109.4 | 1.08 | (0.89 to 1.30) | NA | 15 years? | Taken from Clarke 2005 (Appendix web figure 10b), then inverted to reflect that more surgery is our control and less surgery is our research condition |
| NSABP B‐04 | Local isolated recurrence: node negative: ALND vs no ALND |
ALND = 35 events/3949 women‐years No ALND = 94 events/3335 women‐years |
o‐e = 31.5 | 29.2 | 2.94 | (2.05 to 4.23) | NA | 5 years? | Taken from Clarke 2005 (Appendix web figure 9a), then inverted to reflect that more surgery is our control and less surgery is our research condition |
| NSABP B‐04 | Local isolated recurrence: node negative: ALND vs no ALND + RT |
ALND = 35 events/3949 women‐years No ALND + RT = 18 events/3896 women‐years |
o‐e = ‐8.7 | 13 | 0.51 | (0.30 to 0.88) | NA | 5 years? | Taken from Clarke 2005 (Appendix web figure 10a), then inverted to reflect that more surgery is our control and less surgery is our research condition |
| NSABP B‐04 | Local isolated recurrence: node positive: ALND vs no ALND + RT |
ALND = 45 events/2268 women‐years No ALND + RT = 42 events/2025 women‐years |
o‐e = ‐0.5 | 20.8 | 0.98 | (0.64 to 1.50) | NA | 5 years? | Taken from Clarke 2005 (Appendix web figure 10b), then inverted to reflect that more surgery is our control and less surgery is our research condition |
| NSABP B‐04 | Disease‐free survival: node negative: ALND vs no ALND |
ALND = 281/362 No ALND + RT = 287/365 |
o‐e = 9.36 | 138.3 | 1.07 | (0.91 to 1.27) | 0.39 | 25 years | FIsher (2008) page 568 (radical vs total mastectomy) Tierney 2007 method 3, calculated from the date of mastectomy, events considered in determination of disease‐free survival were the first local, regional or distant recurrence of tumour; contralateral breast cancer or a second primary tumour other than a tumour in the breast; and death with no evidence of cancer |
| NSABP B‐04 | Disease‐free survival: node negative: ALND vs no ALND + RT |
ALND = 281/362 No ALND + RT = 292/352 |
o‐e = 8.3 | 142.39 | 1.06 | (0.90 to 1.25) | 0.49 | 25 years | FIsher (2008) page 568 (radical vs total mastectomy + RT) Tierney 2007 method 3, calculated from the date of mastectomy, events considered in determination of disease‐free survival were the first local, regional or distant recurrence of tumour; contralateral breast cancer or a second primary tumour other than a tumour in the breast; and death with no evidence of cancer |
| NSABP B‐04 | Disease‐free survival: node positive: ALND vs no ALND + RT |
ALND = 254/292 No ALND + RT = 258/294 |
o‐e = 14.46 | 127.57 | 1.12 | (0.94 to 1.33) | 0.20 | 25 years | FIsher (2008) page 568, Tierney 2007 method 3, calculated from the date of mastectomy, events considered in determination of disease‐free survival were the first local, regional or distant recurrence of tumour; contralateral breast cancer or a second primary tumour other than a tumour in the breast; and death with no evidence of cancer |
| NSABP B‐04 | Relapse‐free survival: node negative: ALND vs no ALND |
ALND = 154/362 No ALND + RT = 182/365 |
o‐e = 10.17 | 77.61 | 1.14 | (0.91 to 1.42) | 0.27 | 25 years | FIsher (2008) page 568 Tierney 2007 method 3; calculated from the date of mastectomy, events considered in determination of relapse‐free survival were the first local, regional or distant recurrence; or an event in the contralateral breast |
| NSABP B‐04 | Relapse‐free survival: node negative: ALND vs no ALND + RT |
ALND = 154/362 No ALND + RT = 163/352 |
o‐e = ‐2.9 | 71.05 | 0.96 | (0.76 to 1.21) | 0.74 | 25 years | FIsher (2008) page 568, Tierney 2007 method 3, calculated from the date of mastectomy, events considered in determination of relapse‐free survival were the first local, regional or distant recurrence; or an event in the contralateral breast |
| NSABP B‐04 | Relapse‐free survival: node positive: ALND vs no ALND + RT |
ALND = 178/292 No ALND + RT = 183/294 |
o‐e = 7.63 | 88.52 | 1.09 | (0.89 to 1.35) | 0.40 | 25 years | FIsher (2008) page 568, Tierney 2007 method 3, calculated from the date of mastectomy, events considered in determination of relapse‐free survival were the first local, regional or distant recurrence; or an event in the contralateral breast |
| NSABP B‐04 | Time to distant metastasis: node negative: ALND vs no ALND |
ALND = 101/362 No ALND + RT = 107/365 |
o‐e = 8.44 | 88.52 | 1.1 | (0.89 to 1.35) | 0.39 | 25 years | FIsher (2008) page 569, Tierney 2007 method 3 |
| NSABP B‐04 | Time to distant metastasis: node negative: ALND vs no ALND + RT |
ALND = 101/362 No ALND + RT = 111/352 |
o‐e = 6.69 | 86.9 | 1.08 | (0.88 to 1.34) | 0.44 | 25 years | FIsher (2008) page 569, Tierney 2007 method 3 |
| NSABP B‐04 | Time to distant metastasis: node positive: ALND vs no ALND + RT |
ALND = 120/292 No ALND + RT = 127/294 |
o‐e = 5.98 | 88.41 | 1.07 | (0.87 to 1.32) | 0.51 | 25 years | FIsher (2008) page 569, Tierney 2007 method 3 |
| NSABP B‐32 | Overall survival (all randomised participants, i.e. node+ and node‐) |
ALND = 228 (deaths)/2807 SLN = 252 (deaths)/2804 |
10.32 | 119.7 | 1.09 | (0.91 to 1.3) | 0.35 | 10 years | From Julian (2013) using Tierney 2007 method 4. Contacted author (Krag) to confirm direction of effect |
| NSABP B‐32 | Disease‐free survival (all randomised participants, i.e. node+ and node‐) |
ALND = 455/2807 SLN = 475/2804 |
4.6 | 232.39 | 1.02 | (0.9 to 1.16) | 0.72 | 10 years | From Julian (2013) using Tierney 2007 method 4. Contacted author (Krag) to confirm direction of effect |
| NSABP B‐32 | Local/regional recurrence (all randomised participants, i.e. node+ and node‐) |
ALND = 121/2807 SLN = 112/2804 |
‐2.37 | 58.16 | 0.96 | (0.74 to 1.24) | 0.77 | 10 years | From Julian (2013) using Tierney 2007 method 4. Contacted author (Krag) to confirm direction of effect |
| NSABP B‐32 | Axillary recurrence (all randomised participants, i.e. node+ and node‐) |
ALND = 6/2807 SLN = 14/2804 |
NA | NA | NA | NA | NA | 10 years | o‐e cannot be calculated. Not included in meta‐analysis |
| NSABP B‐32 | Overall survival (for SLN‐neg) |
ALND = 219 (dead)/1975 SLN = 245 (dead)/2011 |
o‐e = 12.07 | 115.64 | 1.11 | (0.93 to 1.33) | 0.27 | 10 years | From Julian (2013) using Tierney 2007 method 4 |
| NSABP B‐32 | Disease‐free survival (for SLN‐neg) | ALND = 456 (diseased)/1975 SLN = 465 (diseased)/2011 |
o‐e = 2.29 | 230.23 | 1.01 | (0.89 to 1.15) | 0.92 | 10 years | From Julian (2013) using Tierney 2007 method 4 |
| NSABP B‐32 | Local regional recurrence |
ALND = 85 (events)/1975 SLN = 80 (events)/2011 |
o‐e = ‐2.11 | 41.21 | 0.95 | (0.7 to 1.29) | 0.77 | 10 years | From Julian (2013) using Tierney 2007 method 4 |
| NSABP B‐32 | Local recurrence in SLN‐negative participants |
ALND = 54 (events)/1975 SLN = 49 (events)/2011 |
o‐e = ‐3.03 | 25.69 | 0.89 | (0.6 to 1.31) | 0.55 | Mean = 95.6 months | From Krag (2010) page 930 using logrank P = 0.55 Tierney 2007 method 7 |
| NSABP B‐32 | Regional recurrence in SLN‐negative participants |
ALND = 8 (events)/1975 SLN = 14 (events)/2011 |
o‐e = 2.77 | 5.09 | 1.72 | (0.72 to 4.11) | 0.22 | Mean = 95.6 months | From Krag (2010) page 930 using log rank P = 0.22 Tierney 2007 method 7 |
| NSABP B‐32 | Distant recurrence in SLN‐negative patients |
ALND = 55 (events)/1975 SLN = 64 (events)/2011 |
o‐e = 3.91 | 29.82 | 1.14 | (0.8 to 1.64) | Mean = 95.6 months | From Krag (2010) Figure 4 Tierney 2007 method 3 | |
| Ostersund | Recurrence in the axilla |
ALND: 0/57 Sampling: 1/54 |
NA | NA | NA | NA | NA | Median: 30 (range, 5‐76) months | From Borup‐Chistesen (1993) table IV. Recurrence is reported only out of N = 111 (57 + 54) participants who did not have metastases in axillary lymph nodes after dissection or biopsy. Cannot calculate o‐e on the basis of available data |
| Ostersund | Local recurrence |
ALND: 4/57 Sampling: 1/54 |
NA | NA | NA | NA | NA | Median: 30 (range, 5‐76) months | From Borup‐Chistesen (1993) table IV. Recurrence is reported only out of N = 111 (57 + 54) participants who did not have metastases in axillary lymph nodes after dissection or biopsy. Cannot calculate o‐e on the basis of available data |
| Ostersund | Distant recurrence |
ALND: 1/57 Sampling: 4/54 |
NA | NA | NA | NA | NA | Median: 30 (range, 5‐76) months | From Borup‐Chistesen (1993) table IV. Recurrence is reported only out of N = 111 (57 + 54) participants who did not have metastases in axillary lymph nodes after dissection or biopsy. Cannot calculate o‐e on the basis of available data |
| SE Scotland | Overall survival: node negative: ALND vs Simple + RT |
ALND = 143/199 Simple + RT = 143/180 |
o‐e = 17.5 | 65.7 | 1.31 | (1.02 to 1.66) | NA | 15 years? | Taken from Clarke 2005 (Appendix web figure 10a), then inverted to reflect that more surgery is our control and less surgery is our research condition |
| SE Scotland | Overall survival: node positive: ALND vs Simple + RT |
ALND = 72/89 Simple + RT = 77/93 |
o‐e = 6.3 | 34.1 | 1.20 | (0.86 to 1.68) | NA | 15 years? | Taken from Clarke 2005 (Appendix web figure 10b), then inverted to reflect that more surgery is our control and less surgery is our research condition |
| SE Scotland | Local isolated recurrence: node negative: ALND vs no ALND + RT |
ALND = 26 events/2880 women‐years Simple + RT = 21 events/2204 women‐years |
o‐e = ‐0.5 | 11.3 | 0.96 | (0.53 to 1.71) | NA | 5 years? | Taken from Clarke 2005 (Appendix web figure 10a), then inverted to reflect that more surgery is our control and less surgery is our research condition |
| SE Scotland | Local isolated recurrence: node positive: ALND vs no ALND + RT |
ALND = 24 events/943 women‐years Simple + RT = 17 events/878 women‐years |
o‐e = ‐2.9 | 9.8 | 0.74 | (0.40 to 1.39) | NA | 5 years? | Taken from Clarke 2005 (Appendix web figure 10b), then inverted to reflect that more surgery is our control and less surgery is our research condition |
| WSSA Glasgow | Overall survival ‐ node negative |
ALND: 56/101 Simple + RT to chest wall & axilla: 42/85 |
o‐e = ‐5.5 | 21.4 | 0.77 | (0.51 to 1.18) | NA | 15 years? | CAUTION: same control group used twice for these data Taken from Clarke 2005 (Appendix web figures 9a and 10a), then inverted to reflect that more surgery is our control and less surgery is our research condition |
| WSSA Glasgow | Overall survival ‐ node positive |
ALND: 13/17 Simple + RT to chest wall & axilla: 7/9 |
o‐e = ‐0.5 | 3.3 | 0.86 | (0.29 to 2.53) | NA | 15 years? | CAUTION: same control group used twice for these data Taken from Clarke 2005 (Appendix web figures 9b and 10b). then inverted to reflect that more surgery is our control and less surgery is our research condition |
| WSSA Glasgow | Isolated local recurrence ‐ node negative |
ALND: 15/510 py Simple + RT to chest wall & axilla: 13/483 py |
o‐e = 0.0 | 6.7 | 1.00 | (0.47 to 2.13) | NA | 5 years? | CAUTION: same control group used twice for these data Taken from Clarke 2005 (Appendix web figures 9a and 10a), then inverted to reflect that more surgery is our control and less surgery is our research condition |
| WSSA Glasgow | Isolated local recurrence ‐ node positive |
ALND: 3/69 py Simple + RT to chest wall & axilla: 1/41 py |
o‐e = ‐0.5 | 0.9 | 0.57 | (0.07 to 4.53) | NA | 5 years? | CAUTION: same control group used twice for these data Taken from Clarke 2005 (Appendix web figures 9b and 10b), then inverted to reflect that more surgery is our control and less surgery is our research condition |
| Xu 2003 | 10‐year overall survival |
Level I clearance: 75/93 ALND: 71/88 |
NA | NA | NA | NA | NA | 10 years | o‐e could not be calculated as no P values reported. Not included in meta‐analysis |
| Xu 2003 | 10‐year disease‐free survival |
Level I clearance: 72/93 ALND: 68/88 |
NA | NA | NA | NA | NA | 10 years | o‐e could not be calculated as no P values reported. Not included in meta‐analysis |
| Xu 2003 | Breast cancer recurrence |
Level I clearance: 19/93 ALND: 17/88 |
NA | NA | NA | NA | NA | 10 years? | o‐e could not be calculated as no P values reported. Not included in meta‐analysis |
| Xu 2003 | Local recurrence |
Level I clearance: 3.2% ALND: 2.3% |
NA | NA | NA | NA | NA | 10 years? | o‐e could not be calculated as no P values reported. Not included in meta‐analysis |
| Xu 2003 | Distant metastasis |
Level I clearance: 19/93 ALND: 15/88 |
NA | NA | NA | NA | NA | 10 years? | o‐e could not be calculated as no P values reported. Not included in meta‐analysis |
Figures in bold were reported in the original publication; others were derived (see Notes column).
2. Morbidity definitions.
| Study | Oedema | Shoulder function | Skin graft | Delayed healing | Activity | Attitude | Other | Notes |
| Guy's | Slight: 0‐2.5 cm Moderate: 2.5‐4.5 cm Severe > 4.5 cm Circumference of both arms measured 7.5 cm below the acromion, 18 cm above and 10 cm below the olecranon and at the wrist Presumably difference between arm circumference |
Arm function: Good: uses arm freely Fair: cannot do usual tasks Poor: very unsatisfactory use of arm Appears to be assessed by patient questionnaire |
Good: normal activity, back at work or resumed usual activities Fair: light work only because of operation; not resumed usual activities Poor: inactive. Assessed by patient questionnaire |
Good: no complaints Fair: some complaints Poor: very unhappy about experience Assessed by patient questionnaire |
||||
| ACOSOG Z0011 | Lympheoedema (subjective) – according to patient self‐report or physician diagnosis Lympheoedema (objective): 2 cm or greater postop increase in ipsilateral arm circumference |
Axillary paraesthesia – patient reported Brachial plexus injury – determined by physician on examining the patient |
||||||
| Addenbrookes | 1. Mild oedema 2. Gross oedema (estimated by measuring the circumference of each arm with the arm extended at points 11 inches and 22 inches from the tips of the middle finger. An increase of 1 inch in the circumference of the arm on the side of the operation at either or both points was taken to indicate some degree of oedema) |
Stiff shoulder | Need for skin graft | Sufficient to cause postponement of radiotherapy until at least 2 months after the operation. Although incidence of delayed healing varied between surgeons, each showed the same trend of higher incidence following a radical operation | ||||
| Addenbrookes 2 | Subjective lymphoedema: patient reported Objective lymphoedema: circumferential arm measurement at 4 cm intervals from the wrist (approximately 10 measurements) used to calculate arm volume. Volume corrected using measurements from contralateral arm |
Range of movement measured by recording degrees of flexion, abduction and internal and external rotation using goniometer Sensory function tested using pinprick, light touch |
Global Severity Index (GSI; low values better), Beck's Depression Inventory, Spielberger's State‐Trait anxiety, MAC, SF‐36 (measured psychological morbidity and quality of life) | |||||
| ALMANAC | Change in ipsilateral arm volume at each follow‐up visit was expressed as a % increase from pretreatment value. Ratios of presurgery to postsurgery arm volumes were compared on a log‐transformed scale. The contralateral arm was used as a control for evaluations of arm volume Also patient rated as mild, moderate or severe |
Assessed by goniometric measurement of arm movement (flexion, abduction, internal rotation and external rotation). Changes between visits calculated by subtraction The contralateral arm was used as a control for arm and shoulder function |
QoL: Fact‐B+4 Anxiety: Spielberger STAI |
|||||
| Cardiff ‐ Local | No morbidity data | |||||||
| Cardiff ‐ St Mary's | Oedema of arm 72 cm | Restricted elevation 720 degrees | Measured but not reported | Axillary pain; numbness or paraesthesia on operated sides; aesthetic appearance of axillary scar | ||||
| Edinburgh 1 | Arm swelling measured by water displacement, circumference 15 cm above and below the olecranon process | Shoulder mobility assessed by measuring elevation through flexion, abduction, medial and lateral rotation | Shoulder muscle power assessed using graduated spring to measure flexion, extension, abduction and adduction of the shoulder joint | |||||
| E'dburgh Sample/Clear | Arm welling (arm circumference 15 cm above and 10 cm below olecranon) | Objective assessment via adduction with internal rotation; abduction with external rotation, difference in height reached between treated and non‐treated arms by stretching above head, measurement of an abduction movement without shoulder rotation whilst lying on a flat, hard surface | Power (cm/kg) of pectoralis major by repeated lifting of a 3.5 kg weight as fast as possible over 45 seconds, comparing treated and untreated arm | Sample from study only, level B evidence | ||||
| GIVOM Sentinella | Lympheodema was assessed by comparing the circumference of the operated vs the non‐operated arm at 15 cm above the epicondyle Unclear what difference in circumference constituted lymphoedema |
Assessed by the surgeon by evaluating active and passive flexion, abduction, internal and external rotation, and classified on a scale 0 (normal mobility) to 3 (severe mobility) restriction Winged scapula reported as present/absent |
Axillary and arm pain reported by patients on a scale from 0 (absent) to 3 (continuous/severe) Numbness assessed by the surgeon by comparing skin sensitivity in operated and non‐operated arms. Rated 0 (absent) to 3 (severe) |
|||||
| Guy's | Reports lymphoedema; categorised as none, slight, moderate and severe | Reports arm function as good, fair or poor | Reports activity as good, fair or poor | Reports attitude as good, fair or poor | Pts in no axillary surgery + RT arm reported fibrosis of breast and sometimes "marbling" of the overlying skin. Both occurred in <5% of cases | |||
| Hammersmith | Impairted function of the shoulder joint and swollen arm: no definitions given, but it is stated that the methodology included volumetric measurement of the upper limb and that an attempt was made to ally objective measurements with the patient’s subjective expression of discomfort or disability | Impairted function of the shoulder joint and swollen arm: no definitions given, but it is stated that the methodology included volumetric measurement of the upper limb and that an attempt was made to ally objective measurements with the patient’s subjective expression of discomfort or disability | In evaluating morbidity, attempts made to ally objective measurements with patient's subjective expression of disability or discomfort. Expectation that after RM, slight increase in volume of ipsilateral arm, or after RT, some discomfort and stiffness to shoulder, but these do not amount to morbidity | |||||
| IBCSG‐10‐93 | ≥ 5% increase in arm circumference from baseline | QOL: A core questionnaire plus a surgical module specific to this trial. Four linear analogue scales on the core questionnaire were used: well‐being, mood, appetite and perceived adjustment/coping. After 1993, 6 additional scales were added: tiredness, hot flashes, nausea/vomiting, perceived social support, arm restriction and subjective health estimation. Surgical module measured swelling, numbness, weakness, pain, stiffness, performance of daily activities and global measure of arm/hand/shoulder/chest bother |
||||||
| IBCSG‐23‐01 | No definitions for functional outcomes reported | |||||||
| Institut Bergonie | No definitions for functional outcomes reported | |||||||
| IPO‐P | An increase in arm volume was defined as an increase > 2 cm, comparing the circumference of the operated upper limb (at 3 points: the wrist, the midpoint of the forearm and the midpoint of the upper arm) with its non‐operated counterpart | Patients were asked to lift their operated arm (maximum possible abduction): abduction ≥ 90° was considered adequate; abduction < 90° was considered abnormal | Patients were asked: Is your arm painful in a resting position (yes/no)? Does the inside of your arm feel more numb (yes/no)? |
|||||
| Manchester | ||||||||
| Milan | Arm swelling was assessed by comparing the circumference of treated and untreated arms 15 cm above the lateral epicondyle | Arm mobility was judged by asking the patient to rate restriction in movement on a scale 0 to 100 Numbness assessed by comparing skin sensitivity on inside and outside of the upper arm – classified as yes/no |
Aesthetic appearance of scar judged by patient (rated good or bad) | Postoperative pain was evaluated as continuous (> 50% of the day), sporadic or absent | ||||
| NSABP B‐04 | Ipsilateral and contralateral measurement of arm circumference at 15 cm below the acromion process and 15 cm below the olecranon: An increase in arm circumference ≥ 2 cm in ipsilateral arm (below or above the elbow) indicated arm oedema | |||||||
| NSABP B‐32 | Arm volume measured using volume of water displaced determined by the difference between treated and untreated arms (relative arm volume difference = [ipsilateral‐contralateral]/[contralateral] × 100%) | Arm mobility in degrees was determined by measuring the straight lateral abduction of both ipsilateral and contralateral arms using a standard orthopaedic goniometer to determine the angle between lateral chest wall and humerus (relative shoulder abduction deficit = [ipsilateral‐contralateral]/[contralateral] × 100%) | Numbness and tingling were assessed by self‐report by asking patients if they were currently experiencing any numbness or any tingling anywhere in ipsilateral and contralateral arms. OR of SLN compared with ALND Adverse events: no details reported |
|||||
| Ostersund | Arm volume measured using volume of water displaced. A cutoff of 10% increase in volume was used as the arbitrary cut point | Shoulder mobility (flexion, abduction and rotation) was determined with the help of a 360° scale placed on a wall with the centre at shoulder height | ||||||
| SNAC | Arm volume was estimated using 6 measures of arm circumference at 10 cm intervals starting 10 cm from the tip of the index finger. Upper limb swelling was expressed as percentage change in volume from baseline | Abduction and flexion measured using goniometer Arm morbidity measured using the 15‐item SSSS scale developed for the study, with each rated from 0 (no trouble at all) to 10 (worst I can imagine) and averaged to obtain overall score |
||||||
| SE Scotland | Increase in circumference of forearm by at least 3 cm | Failure to abduct the arm beyond a right angle | ||||||
| Xu 2003 | Postoperative swelling: middle grade (diameter is 3–6 cm enlargement on the involved upper arm or forearm compared with the contralateral part) |
3. Morbidity data at each time point.
| Study | Outcome | Measurement | Follow‐up period 1 | Follow‐up period 2 | Notes |
| ACOSOG Z0011 | Wound infection | Determined by treating physician | SLND: 11/371; SLND + ALND: 31/373 | ||
| ACOSOG Z0011 | Axillary seroma | Determined by treating physician | SLND: 21/371; SLND + ALND: 53/373 | ||
| ACOSOG Z0011 | Brachial plexus injury | Determined by treating physician | At 6 months: SLND: 3/415; SLND + ALND: 5/406 At 1 year: SLND: 0/415; SLND + ALND: 1/406 |
||
| ACOSOG Z0011 | Axillary paraesthesia | Patient reported | 30 days: SLND: 43/371; SLND + ALND: 174/373 | 6 months: SLND: 35/288; SLND + ALND: 146/335 | |
| ACOSOG Z0011 | Axillary paraesthesia | Patient reported | 12 months: SLND: 24/268; SLND + ALND: 113/287 | ||
| ACOSOG Z0011 | Lymphoedema (objective) | Arm measurement | 30 days: SLND: 17/272; SLND + ALND: 23/255 | 6 months: SLND: 21/271; SLND + ALND: 29/270 | |
| ACOSOG Z0011 | Lymphoedema (objective) | Arm measurement | 12 months: SLND: 14/226; SLND + ALND: 26/242 | ||
| ACOSOG Z0011 | Lymphoedema (subjective) | Patient reported/physician diagnosis | 6 months: SLND: 19/339; SLND + ALND: 27/327 | 12 months: SLND: 12/268; SLND + ALND: 37/288 | |
| ACOSOG Z0011 | Lymphoedema (subjective) | Patient reported/physician diagnosis | > 12 months: SLND: 14/253; SLND + ALND: 52/272 | ||
| Addenbrookes | Mild oedema | Follow‐up was at least 12 months in most cases. ALND = 7/91; Simple = 5/113 |
|||
| Addenbrookes | Stiff shoulder | ALND = 6/91; Simple = 8/113 | |||
| Addenbrookes | Skin graft | Need for skin graft | ALND = 4/91; Simple = 2/113 | ||
| Addenbrookes | Delayed healing | Need to delay postoperative RT | ALND = 18/91; Simple = 7/113 | ||
| Addenbrookes | Gross oedema | Arm measurement | ALND = 0/91; Simple = 0/113 | ALND = 12/45; Simple = 6/53 | |
| Addenbrookes 2 | Seroma | ALND: 33/155; SLNB: 20/143 | |||
| Addenbrookes 2 | Lymphoedema (objective) | Arm volume changes |
12 months: ALND: mean (SE) = 56.4 (10.9); SLNB: mean (SE) = 18.6 (13.8), difference mean (SE) = 37.8 (17.6) Mean (1, 3, 6, 12 months): ALND: mean (SE) = 53.1 (8.1); SLNB: mean (SE) = 17.7 (9.2), difference mean (SE) = 35.4 (12.2) |
Max: ALND: mean (SE) = 113.7 (9.7); SLNB: mean (SE) = 78.4 (12), difference mean (SE) = 35.3 (15.3) | |
| Addenbrookes 2 | Lymphoedema (subjective) |
Patient reported | 1 month: OR = 0.34 (95% CI 0.11 to 0.9); 3 months: OR = 0.4 (95% CI 0.16 to 0.94); 6 months: OR = 0.25 (95% CI 0.08 to 0.66) | 12 months: OR = 0.36 (95% CI 0.15 to 0.86); mean: OR = 0.3 (95% CI 0.18 to 0.68) | Odds ratios: SLNB/ALND; i.e. lower favours SLNB |
| Addenbrookes 2 | Paraesthesia | ALND: 130/155; SLNB: 92/140 | |||
| Addenbrookes 2 | Numbness | ALND: 115/155; SLNB: 68/143 | |||
| Addenbrookes 2 | Loss of pinprick | ALND: 118/155; SLNB: 77/140 | |||
| Addenbrookes 2 | Loss of light touch | ALND: 121/155; SLNB: 81/140 | |||
| Addenbrookes 2 | QOL (immediate postop) | Study authors note QOL scores were usually higher (better) in the SLND group and significantly so in the immediate postoperative period (P < 0.01). No significant effect of node positive/negative | |||
| Addenbrookes 2 | MAC scale (12 months) | Study authors no significant difference in MAC scores during 1 year follow‐up. No significant effect of node positive/negative | |||
| Addenbrookes 2 | BSI – somatisation (immediate postop) | SLND group scored lower (better) than ALND in the immediate postoperative period (P < 0.001) | |||
| Addenbrookes 2 | Quality of life | GSI level | 12 months: ALND: mean (SE, N) = 49.7 (1.1, 143); SLNB: mean (SE, N) = 48.4 (0.9, 134), difference mean (SE) = 1.3 (1.4) | OR for morbid GSI: study/control (95% CI) 0.55 (0.08 to 2.94) | |
| Addenbrookes 2 | Quality of life | SF‐36 (immediate postoperative) |
Physical combined: ALND: mean (SD, N) = 38.6 (8.2, 143); SLNB: mean (SD, N) = 42.3 (10.4, 134), difference mean (95% CI) = 3.7 (1.2 to 6.1) Physical functioning: ALND: mean (SD, N) = 41.3 (9, 143); SLNB: mean (SD, N) = 44.5 (8.1, 134), difference mean (95% CI) = 3.2 (1.1 to5.4) |
Vitality: ALND: mean (SD, N) = 48.2 (10.2, 143); SLNB: mean (SD, N) = 51.8 (9.8, 134), difference mean (95% CI) = 3.7 (1.1 to 6.2) | |
| Addenbrookes 2 | Shoulder movement (mean reduction) | Flexion, extension, abduction, internal rotation, external rotation | Flexion: ALND: mean (SD, N) = 13 (32.9, 141); SLNB: mean (SD, N) = 6.7 (15.6, 134), difference mean (95% CI) = 6.3 (0.1 to 12.6); Extension: ALND: mean (SD, N) = ‐1.5 (10.7, 139); SLNB: mean (SD, N) = ‐2.2 (8.1, 134), difference mean (95% CI) = 0.7 (‐1.5 to 3.3); Abduction: ALND: mean (SD, N) = 6.3 (11.5, 138); SLNB: mean (SD, N) = 3.1 (15.7, 132), difference mean (95% CI) = 3.2 (‐0.5 to 6.3) | Internal rotation: ALND: mean (SD, N) = 1.7 (12.7, 139); SLNB: mean (SD, N) = 0.3 (12, 134), difference mean (95% CI) = 1.4 (‐1.5 to 4.4); External rotation: ALND: mean (SD, N) = 2.9 (12.3, 139); SLNB: mean (SD, N) = 1.5 (11, 134), difference mean (95% CI) = 1.4 (‐1.5 to 4.4) | |
| ALMANAC | Axillary drain usage | ALND: 359/453; SLNB: 75/449 | |||
| ALMANAC | Infection rate of surgical wounds | ALND: 72/476; SLNB: 52/478 | |||
| ALMANAC | Lymphoedema | Patient‐assessed; moderate/severe | 1 month: ALND: 7/419; SLNB: 1/428 3 months: ALND: 12/395; SLNB: 4/417 |
6 months: ALND: 13/414; SLNB: 2/432 12 months: ALND: 10/403 SLNB: 4/412 |
|
| ALMANAC | Lymphoedema | Mean (95% CI) change in arm vol compared with pretreatment | 1 month: ALND = 1.022 (1.013‐1.032); SLNB = 1.003 (0.997‐1.01) 3 months: ALND = 1.044 (1.035‐1.053); SLNB = 1.019 (1.01‐1.028) |
6 months: ALND = 1.058 (1.048‐1.069); SLNB = 1.022 (1.011‐1.032) 12 months: ALND = 1.061 (1.048‐1.074); SLNB = 1.028 (1.016‐1.039) |
|
| ALMANAC | Sensory loss | Median area of sensory loss (cm2; range) | 1 month: ALND = 40 (1‐489); SLNB = 32 (2‐254) 3 months: ALND = 47 (0‐1139); SLNB = 48 (0‐327) |
6 months: ALND = 39 (0.4‐2827); SLNB = 32 (0‐201) 12 months: ALND = 35 (0.8‐1013); SLNB = 59 (0.2‐342) |
Event rates for self‐assessed sensory loss also reported in Mansel 2006 for these follow‐up periods, but not extracted |
| ALMANAC | Intercostobrachial nerve damage | Clinician assessment; severe | 1 month: ALND: 10/392; SLNB: 6/409 3 months: ALND: 10/373; SLNB: 4/397 |
6 months: ALND: 10/394; SLNB: 4/410 12 months: ALND: 5/384 SLNB: 5/400 |
|
| ALMANAC | Shoulder function | Mean change in shoulder function (degrees): flexion | 1 month: ALND = 9.8; SLNB = 5.8 3 months: ALND = 3.7; SLNB = 2 |
6 months: ALND = 1.6; SLNB = 2 12 months: ALND = 0.1; SLNB = 2.7 |
95% CI can also be extracted |
| ALMANAC | Shoulder function | Mean change in shoulder function (degrees): abduction | 1 month: ALND = 12.9; SLNB = 6.5 3 months: ALND = 4.2; SLNB = 1.9 |
6 months: ALND = 2.3; SLNB = 1.5 12 months: ALND = 1.9; SLNB = 2.5 |
95% CI can also be extracted |
| ALMANAC | Shoulder function | Mean change in shoulder function (degrees): external rotation | 1 month: ALND = 1.2; SLNB = 0.7 3 months: ALND = 1.2; SLNB = 0.2 |
6 months: ALND = 1; SLNB = 0.6 12 months: ALND = 0.7; SLNB = 0.6 |
95% CI can also be extracted |
| ALMANAC | Shoulder function | Mean change in shoulder function (degrees): internal rotation | 1 month: ALND = 0.9; SLNB = 0.4 3 months: ALND = 0.7; SLNB = 1 |
6 months: ALND = 0.8; SLNB = 0.2 12 months: ALND = 0.4; SLNB = 1.7 |
95% CI can also be extracted |
| ALMANAC | Quality of life | Measures: mean trial outcome index; trial outcome index reduced by ≥ 5 points from baseline (n/N); mean arm functioning subscale score; substantial arm swelling or tenderness (n/N); substantial numbness on ipsilateral side (n/N); mean FACT‐B+4 score | Means (95% CI) and event rates can be extracted for each time point (baseline, 1, 3, 6 and 12 months) | ||
| ALMANAC | State and trait anxiety | Mean and 95% CI can be extracted for each time point (baseline, 1, 3, 6 and 12 months) | |||
| Cardiff | Morbidity | Objective complaints: restricted elevation 720 degrees | Not stated: full axillary surgery, neg nodes = 25% (×2 = 7.47, P < 0.01); no axilary surgery, neg nodes = 0%; full axillary surgery + radical RT, positive nodes = 67%; no axillary surgery + local RT = 37% | Sample of 85 patients only from Cardiff site | |
| Cardiff | Morbidity | Objective complaints: oedema of arm, 72 cm | Not stated: full axillary surgery, neg nodes = 46% (×2 = 6.02, P < 0.03); no axillary surgery, neg nodes = 15%; full axillary surgery + radical RT, positive nodes = 58%; no axillary surgery + local RT = 37% | Sample of 85 patients only from Cardiff site | |
| Cardiff | Morbidity | Subjective complaints: limited arm movement | Not stated: full axillary surgery, neg nodes = 21%; no axillary surgery, neg nodes = 8%; full axillary surgery + radical RT, positive nodes = 8%; no axillary surgery + local RT = 21% | Sample of 85 patients only from Cardiff site | |
| Cardiff | Morbidity | Subjective complaints: swollen arm | Not stated: full axillary surgery, neg nodes = 43%; no axillary surgery, neg nodes = 23%; full axillary surgery + radical RT, positive nodes = 58%; no axillary surgery + local RT = 37% | Sample of 85 patients only from Cardiff site | |
| Edinburgh 1 | Morbidity | Lateral shoulder rotation (mean (SE) difference (cm) from preoperative value (N)) | 6 months: Sampling + RT: 1.91 (SE = 0.56) (N = 64), sampling ‐ RT: 0.34 (SE = 0.59) (N = 59); ALND: 0.13 (SE = 0.39) (N = 132) | 12 months: Sampling + RT: 1.75 (SE = 0.56) (N = 66), Sampling ‐ RT: 0.72 (SE = 0.62) (N = 55); ALND: 0.77 (0.4) (N = 128) | Figure 4, Chetty 2000 paper |
| Edinburgh 1 | Morbidity | Lateral shoulder rotation (mean (SE) difference (cm) from preoperative value (N)) | 24 months: Sampling + RT: 1.57 (SE = 0.6) (N = 60), Sampling ‐ RT: ‐0.48 (SE = 0.65) (N = 52); ALND: 0.38 (SE = 0.43) (N = 117) | 36 months: Sampling + RT: 2.19 (SE = 0.59) (N = 59), Sampling ‐ RT: 0.43 (SE = 0.64) (N = 50); ALND: 0.24 (SE = 0.43) (N = 110) | Figure 4, Chetty 2000 paper |
| Edinburgh 1 | Morbidity | Arm volume (mean (SE) percentage of preoperative arm volume (N)) | 6 months: Sampling + RT: 100.69 (SE = 0.779) (N = 56), Sampling ‐ RT: 102.04 (SE = 0.766) (N = 58); ALND: 103.57 (SE = 0.519) (N = 126) | 12 months: Sampling + RT: 100.95 (SE = 0.81) (N = 59), Sampling ‐ RT: 102.47 (SE = 0.85) (N = 54); ALND: 103.74 (SE = 0.57) (N = 119) | Figure 5, Chetty 2000 paper |
| Edinburgh 1 | Morbidity | Arm volume (mean (SE) percentage of preoperative arm volume (N)) | 24 months: Sampling + RT: 100.84 (SE = 1.03) (N = 54), Sampling ‐ RT: 100.81 (SE = 1.06) (N = 51); ALND: 104.37 (SE = 0.73) (N = 108) | 36 months: Sampling + RT: 100.01 (SE = 1.03) (N = 52), Sampling ‐ RT: 101.28 (SE = 1.07) (N = 48); ALND: 104.07 (SE = 0.73) (N = 103) | Figure 5, Chetty 2000 paper |
| E'dburgh Sample/Clear | Morbidity | Subjective arm | Not stated; full axillary surgery, positive node (Nil 8/12; intermittent 1/12; persistent 3/12); full axillary surgery, ‐negative node (nil 22/28; intermittent 1/28; persistent 5/28); Sample + RT, positive node (nil 17/28; intermittent 2/28; persistent 9/28); Sample, negative node (nil 23/26; intermittent 1/26; persistent 2/26) | Morbidity data to be included in discussion only; sample chosen from alphabetical pt list of patients free of local or systemic disease | |
| E'dburgh Sample/Clear | Morbidity | Subjective mobility | Not stated; full axillary surgery, positive node (normal 12/12; reduced 0/12); full axillary surgery, negative node (normal 22/28; reduced 6/28); Sample + RT, negative node (normal 12/28; reduced 16/28); Sample, negative node (normal 24/26; reduced 2/26) | See comments in Aitken paper | |
| E'dburgh Sample/Clear | Morbidity | Subjective interference with daily activities | Not stated; full axillary surgery, positive node (nil 12/12; occasional 0/12; severe 0/12); full axillary surgery, negative node (nil 24/28; occasional 4/28; severe 0/28); Sample + RT, positive node (nil 16/28; occasional 8/28; severe 4/28); Sample, negative node (nil 24/26; occasional 4/26; severe 0/26) | See comments in Aitken paper | |
| E'dburgh Sample/Clear | Morbidity | Objective assessment ‐ shoulder joint mobility | See comments in Aitken paper | ||
| WSSA Glasgow | Psychological morbidity | Use in discussion only | |||
| GIVOM Sentinella | Lymphoedema | Assessed by physician, reported as odds ratio (95% CI): SLNB/ALND | 6 months: 0.37 (0.2 to 0.7) 12 months: 0.48 (0.2 to 0.9) |
18 months: 0.59 (0.3 to 1.2) 24 months: 0.52 (0.2 to 1.1) |
|
| GIVOM Sentinella | Shoulder movement restriction | Assessed by physician, reported as odds ratio (95% CI): SLNB/ALND | 6 months: 0.47 (0.3 to 0.8) 12 months: 0.73 (0.4 to 1.4) 12 months: raw data extracted from graph (SLNB 17/336, ALND 23/341) |
18 months: 0.62 (0.3 to 1.3) 24 months: 0.44 (0.2 to 1.0) |
|
| GIVOM Sentinella | Axillary/arm pain | Assessed by physician, reported as odds ratio (95% CI): SLNB/ALND | 6 months: 0.52 (0.3 to 0.8) 12 months: 0.76 (0.5 to 1.3) 12 months: raw data extracted from graph (SLNB 30/336, ALND 39/341) |
18 months: 0.84 (0.5 to 1.5) 24 months: 0.90 (0.5 to 1.6) |
|
| GIVOM Sentinella | Numbness | Assessed by physician, reported as odds ratio (95% CI): SLNB/ALND | 6 months: 0.64 (0.4 to 0.9) 12 months: 0.53 (0.3 to 0.8) 12 months: raw data extracted from graph (SLNB 41/336, ALND 71/341) |
18 months: 0.37 (0.2 to 0.6) 24 months: 0.54 (0.3 to 0.9) |
|
| GIVOM Sentinella | Winged scapula | Assessed by physician | Study authors report rate too low to analyse | ||
| GIVOM Sentinella | Health‐related quality of life: SF‐36 – physical component | Assessed by patients using validated questionnaires | No significant differences found between group means of SF‐36 physical component (Del Bianco, 2008) | ||
| GIVOM Sentinella | Health‐related quality of life: SF‐36 – mental component | Assessed by patients using validated questionnaires | No significant differences found between group means of SF‐36 mental component (Del Bianco, 2008) | ||
| GIVOM Sentinella | Health‐related quality of life: SF‐36 HRQOL domains | Assessed by patients using validated questionnaires | No significant differences found between groups on all HRQOL domains of SF‐36 (Zavagno, 2008) | ||
| GIVOM Sentinella | Health‐related quality of life: psychological general well‐being index | Assessed by patients using validated questionnaires | 6, 12 months: significantly better PGWB general and anxiety domain scores in SLNB group than in ALND group (Del Bianco, 2008) | 24 months: no significant differences between PGWB general and anxiety domain scores of both groups.(Del Bianco, 2008) | |
| Guy's | Morbidity | Arm function | 3 months: ALND: Good: 44/90, Fair: 41/90, Poor: 5/90; No ALND: Good: 59/77, Fair: 18/77, Poor: 0/77 | 15 months: ALND: Good: 83/100, Fair: 14/100, Poor: 3/100; No ALND: Good: 70/88, Fair: 17/88, Poor: 1/88 | Sample only |
| Guy's | Morbidity | Lymphoedema | 3 months: ALND: None: 18/93, Slight: 66/93, Moderate: 6/93, Severe: 3/93; No ALND: None: 36/81, Slight: 43/81, Moderate: 0/81, Severe: 2/81 | 15 months: ALND: None: 27/104, Slight: 71/104 Moderate: 6/104, Severe: 0/104; No ALND: None: 39/91, Slight: 52/91, Moderate: 0/91, Severe: 0/91 | Sample only |
| Guy's | Morbidity | Activity | 3 months: ALND: Good: 45/92, Fair: 46/92, Poor: 1/92; No ALND: Good: 62/80, Fair: 16/80, Poor: 2/80 | 15 months: ALND: Good: 85/101, Fair: 14/101, Poor: 2/101; No ALND: Good: 78/92, Fair: 13/92, Poor: 1/92 | Sample only |
| Guy's | Morbidity | Attitude | 3 months: ALND: Good: 81/92, Fair: 9/92, Poor: 2/92; No ALND: Good: 71/80, Fair: 7/80, Poor: 2/80 | 15 months: ALND: Good: 91/101, Fair: 8/101, Poor: 2/101; No ALND: Good: 87/92, Fair: 5/92, Poor: 0/92 | Sample only |
| Hammersmith | Postoperative deaths | Radical: 0/95; Simple: 0/100 | |||
| Hammersmith | Morbidity | Shoulder function | At 4‐year minimum follow‐up in survivors: Radical: 6/95; Simple = 18/100 | Consequential morbidity, at time of publication Methodology not reported, all patients included | |
| Hammersmith | Morbidity | Arm swelling (including volumetric measurement of upper limb) | At 4‐year minimum follow‐up in survivors: Radical: 7/95; Simple = 3/100 | Consequential morbidity, at time of publication Methodology not reported, all patients included | |
| IBCSG‐10‐93 | Lymphoedema | Physician reported | Not significantly different between treatments | ||
| IBCSG‐10‐93 | Arm circumference | Physician reported | Not significantly different between treatments | ||
| IBCSG‐10‐93 | Performance of daily activities | Physician reported | Not significantly different between treatments | ||
| IBCSG‐10‐93 | Arm pain | Physician reported | Baseline: ALND 5/175, surgery 8/194; 1st postoperative: ALND 38/164, surgery 12/168; 3 months: ALND 16/161, surgery 9/171; 6 months: ALND 17/174, surgery 11/177 | 9 months: ALND 21/160, surgery 8/164; 12 months: ALND 13/189, surgery 8/190; 18 months: ALND 14/173, surgery 7/183; 24 months: ALND 12/165, surgery 8/164 |
|
| IBCSG‐10‐93 | Restricted arm movement | Physician reported | Baseline: ALND 9/174, surgery 6/194; 1st postoperative: ALND 64/163, surgery 25/168; 3 months: ALND 23/161, surgery 10/170; 6 months: ALND 21/176, surgery 9/176 | 9 months: ALND 21/160, surgery 7/163; 12 months: ALND 19/188, surgery 6/187; 18 months: ALND 10/171, surgery 7/182; 24 months: ALND 12/165, surgery 7/164 |
|
| IBCSG‐10‐93 | QOL ‐ bothered scores | Patient reported | No significant differences at any time point (baseline, 1st postoperative, 3, 6, 9, 12, 18 and 24 months) | ||
| IBCSG‐10‐93 | QOL ‐ arm movement scores | Patient reported | At 1st postoperative surgery alone, reported less restriction in use of their arm than ALND (P < .0001). Otherwise, no significant differences | ||
| IBCSG‐10‐93 | QOL ‐ numbness scores | Patient reported | At 1st postoperative surgery alone, reported less severe postsurgery numbness than ALND (P < .0001). Otherwise, no significant differences | ||
| IBCSG‐10‐93 | QOL ‐ coping scores | Patient reported | No significant differences at any time point (baseline, 1st postoperative, 3, 6, 9, 12, 18 and 24 months) | ||
| IBCSG‐23‐01 | Postoperative infection | Physician assessed | Surgery alone: 0/467 ALND: 1/464 |
||
| IBCSG‐23‐01 | Sensory neuropathy | Physician assessed |
Any: Surgery alone: 55/453 ALND: 82/447 Grade 3‐4: Surgery alone: 0/453 ALND: 1/447 |
||
| IBCSG‐23‐01 | Lymphoedema | Physician assessed |
Defined as long term: Any: Surgery alone: 15/453 ALND: 59/447 Grade 3‐4: Surgery alone: 0/453 ALND: 3/447 |
||
| IBCSG‐23‐01 | Motor neuropathy | Physician assessed |
Any: Surgery alone: 13/453 ALND: 37/447 Grade 3‐4: Surgery alone: 1/453 ALND: 3/447 |
||
| Institut Bergonie | Arm fatigue | Unclear | Moderate/severe: no ALND: N = 4/258; ALND: N = 24/273 | ||
| Institut Bergonie | Shoulder mobility | Unclear | Restricted somewhat or severely: no ALND: N = 5/257; ALND: N = 21/271 | ||
| Institut Bergonie | Parasthesia | Unclear | Moderate/severe: no ALND: N = 6/258; ALND: N = 41/274 | ||
| Institut Bergonie | Lymphoedema | Unclear | Minor/major difference: no ALND: N = 3/258; ALND: N = 29/275 | ||
| Institut Bergonie | Other functional impairments | Unclear | Minor/major: no ALND: N = 12/263; ALND: N = 16/276 | ||
| Institut Bergonie | Number of patients with functional impairments | Unclear | Minor: no ALND: N = 23/265; ALND: N = 78/278 | ||
| IPO‐P | Upper limb circumference > 2 cm | Measured as per definition | 6 months: Obs: 6/57; ALND: 10/49 12 months: Obs: 8/57; ALND: 15/49 |
24 months: Obs: 8/57; ALND: 14/49 48 months: Obs: 4/57; ALND: 19/49 |
|
| IPO‐P | Pain at rest | Patient reported | 6 months: Obs: 9/57; ALND: 9/49 12 months: Obs: 11/57; ALND: 14/49 |
24 months: Obs: 9/57; ALND: 10/49 48 months: Obs: 3/57; ALND: 7/49 |
|
| IPO‐P | Parasthesias | Patient reported? | 6 months: Obs: 10/57; ALND: 28/49 12 months: Obs: 6/57; ALND: 29/49 |
24 months: Obs: 5/57; ALND: 34/49 48 months: Obs: 6/57; ALND: 30/49 |
|
| IPO‐P | Shoulder dysfunction | Measured as per definition | 6 months: Obs: 5/57; ALND: 5/49 12 months: Obs: 4/57; ALND: 8/49 |
24 months: Obs: 0/57; ALND: 6/49 48 months: Obs: 2/57; ALND: 11/49 |
|
| Milan | Morbidity | Axillary pain (sporadic/continuous) | 6 months: ALND: 91/100; SNLB = 16/100 | 24 months: ALND: 39/100; SNLB = 8/100 | |
| Milan | Morbidity | Numbness/Parasthesia on operated side | 6 months: ALND: 85/100; SNLB = 2/100 | 24 months: ALND: 68/100; SNLB = 1/100 | |
| Milan | Morbidity | Arm mobility, 80%‐100% | 6 months: ALND: 73/100; SNLB = 100/100 | 24 months: ALND: 79/100; SNLB = 100/100 | |
| Milan | Morbidity | Arm mobility, 60%‐79% | 6 months: ALND: 22/100; SNLB = 0/100 | 24 months: ALND: 18/100; SNLB = 0/100 | |
| Milan | Morbidity | Arm mobility, 40%‐59% | 6 months: ALND: 5/100; SNLB = 0/100 | 24 months: ALND: 2/100; SNLB = 0/100 | |
| Milan | Morbidity | Arm mobility, 20%‐39% | 6 months: ALND: 0/100; SNLB = 0/100 | 24 months: ALND: 1/100; SNLB = 0/100 | |
| Milan | Morbidity | Arm mobility, < 20% | 6 months: ALND: 0/100; SNLB = 0/100 | 24 months: ALND: 0/100; SNLB = 0/100 | |
| Milan | Morbidity | Aesthetic appearance of axillary scar: bad | 6 months: ALND: 9/100; SNLB = 2/100 | 24 months: ALND: 15/100; SNLB = 0/100 | |
| Milan | Morbidity | Arm swelling < 1 cm difference in circumference | 6 months: ALND: 44/100; SNLB = 11/100 | 24 months: ALND: 38/100; SNLB = 6/100 | |
| Milan | Morbidity | Arm swelling 1‐2 cm difference in circumference | 6 months: ALND: 17/100; SNLB = 0/100 | 24 months: ALND: 25/100; SNLB = 1/100 | |
| Milan | Morbidity | Arm swelling >2 cm difference in circumference | 6 months: ALND: 8/100; SNLB = 0/100 | 24 months: ALND: 12/100; SNLB = 0/100 | |
| Milan | Morbidity | Arm swelling, any | 6 months: ALND: 69/100; SNLB = 11/100 | 24 months: ALND: 75/100; SNLB = 7/100 | |
| NSABP B‐04 | Arm oedema | Arm swelling ≥ 2 cm difference in circumference | No. of patients with data: ALND: N = 577; no ALND + RT: N = 568 no ALND: N = 312 both node + and node‐ patients. Final measurement was 2 to 5 years after surgery Arm oedema recorded at least once: ALND: 58.1%; no ALND + RT: 38.2%; no ALND: 39.1% (P < 0.001) Oedema always: ALND: 3.6%; no ALND + RT: 0.9%; no ALND: 1% No measurement after first oedema: ALND: 9.2%; no ALND + RT: 5.8%; no ALND: 3.2% Oedema always after first oedema: ALND: 6.1%; no ALND + RT: 3.2%; no ALND: 2.6% Intermittent, final measurement oedema: ALND: 11.8%; no ALND + RT: 4.9%; no ALND: 8.6%; Total with oedema on final measurement: ALND: 30.7%; no ALND + RT: 14.8%; no ALND: 15.4% (P < 0.001) |
Oedema once, then resolution: ALND: 15.9%; no ALND + RT: 15.3%; no ALND: 16.7% Intermittent, final measurement no oedema: ALND: 11.4%; no ALND + RT: 8.1%; no ALND: 7.1% Total with no oedema on final measurement (after at least 1 measurement of oedema): ALND: 27.3%; no ALND + RT: 23.4%; no ALND: 23.8 Arm oedema ≥ 4 cm difference in circumference recorded at least once: ALND: 21.5%; no ALND + RT: 11.4%; no ALND: 13.1% |
|
| NSABP B‐32 | Adverse events (grade 3 or greater surgery related) | No details reported | ALND: 14/2788 SLN: 12/2800 Must include most of SLN positive and negative patients |
Peri‐surgery | |
| NSABP B‐32 | Arm mobility/shoulder abduction deficit (objective) | Physician assessed | 6 months: < 5%: ALND: 1299/1667; SLN: 1468/1744 5%‐10%: ALND: 218/1667; SLN: 176/1744 ≥ 10%: ALND: 150/1667; SLN: 99/1744 |
||
| NSABP B‐32 | Arm volume difference (objective) | Physician assessed | 6 months: < 5%: ALND: 1187/1677; SLN: 1363/1759 5%‐10%: ALND: 277/1677; SLN: 236/1759 ≥ 10%: ALND: 211/1677; SLN: 158/1759 |
12 months: < 5%: ALND: 1170/1639; SLN: 1345/1705 5%‐10%: ALND: 252/1639; SLN: 215/1705 ≥ 10%: ALND: 216/1639; SLN: 147/1705 |
These data are also available for 18 and 30 months |
| NSABP B‐32 | Arm volume difference (objective) | Physician assessed | 24 months: < 5%: ALND: 1062/1517; SLN: 1184/1504 5%‐10%: ALND: 243/1517; SLN: 197/1504 ≥ 10%: ALND: 212/1517; SLN: 123/1504 |
36 months: < 5%: ALND: 990/1421; SLN: 1156/1459 5%‐10%: ALND: 227/1421; SLN: 194/1459 ≥ 10%: ALND: 203/1421; SLN: 109/1459 |
These data are also available for 18 and 30 months |
| NSABP B‐32 | Tingling (subjective) | Self‐reported | 6 months: ALND (N = 388/1693), SLN (N = 184/1766) 12 months: ALND (N = 305/1640), SLN (N = 158/1713) 18 months: ALND (N = 272/1566), SLN (N = 138/1638) |
24 months: ALND (N = 236/1521), SLN (N = 137/1588) 30 months: ALND (N = 219/1448), SLN (N = 116/1502) 36 months: ALND (N = 193/1431), SLN (N = 110/1463) |
|
| NSABP B‐32 | Numbness (subjective) | Self‐reported | 6 months: ALND (N = 821/1693), SLN (N = 257/1769) 12 months: ALND (N = 679/1641), SLN (N = 216/1713) 18 months: ALND (N = 592/1567), SLN (N = 174/1638) |
24 months: ALND (N = 554/1523), SLN (N = 157/1587) 30 months: ALND (N = 473/1450), SLN (N = 137/1504) 36 months: ALND (N = 445/1430), SLN (N = 119/1463) |
|
| NSABP B‐32 | Shoulder abduction deficit ≥ 5% (in those with < 5% at baseline) | Physician assessed | 6 months: ALND (N = 275/1449), SLN (N = 201/1519) | ||
| NSABP B‐32 | Shoulder abduction deficit ≥ 5% (in those with < 5% at baseline) | Physician assessed | 36 months: ALND (N = 314/1136), SLN (N = 192/1151) | ||
| NSABP B‐32 | Numbness (in those with none at baseline) | Self‐reported | 36 months: ALND (N = 407/1336), SLN (N = 103/1371) | ||
| NSABP B‐32 | Tingling (in those with none at baseline) | Self‐reported | 36 months: ALND (N = 175/1329), SLN (N = 90/1343) | ||
| Ostersund | Seroma | Patients with percutaneous aspiration in outpatient department | ALND: 17/50; sampling: 10/50 | Adverse events reported only for the 1987‐89 sample; i.e. for N = 100/200 | |
| Ostersund | Postoperative discharge (mL), median (range) | ALND: 250 (25‐1610); sampling: 130 (0‐1785) | Adverse events reported only for the 1987‐89 sample; i.e. for N = 100/200 | ||
| Ostersund | Duration of postop drainage (days) (median, range) | ALND: 4 (1‐11); sampling: 2.1 (1 ‐11) | Adverse events reported only for the 1987‐89 sample; i.e. for N = 100/200 | ||
| Ostersund | Arm volume increase | ≥ 10% | ALND: 14/47; sampling: 0/48 | Adverse events reported only for the 1987‐89 sample; i.e. for ca N = 100/200 | |
| Ostersund | Subjective sensation of swelling in women without objective increase in arm volume | Any | ALND: 12/33; sampling: 9/48 | Adverse events reported only for the 1987‐89 sample; i.e. for ca N = 100/200 | |
| Ostersund | Shoulder mobility (mean decrease compared with baseline) | 7.5° decrease for whole sample of 95 patients | Adverse events reported only for the 1987‐89 sample; i.e. for ca N = 100/200 | ||
| Ostersund | Axillary paraesthesia (impairment of sensibility in the axilla) | ALND: 17/48; sampling: 19/48 | Adverse events reported only for the 1987‐89 sample; i.e. for ca N = 100/200 | ||
| Ostersund | Inner upper arm paraesthesia (impairment of sensibility in the inner upper arm) | ALND: 24/48; sampling: 4/48 | Adverse events reported only for the 1987‐89 sample; i.e. for ca N = 100/200 | ||
| SE Scotland | Delayed healing | ALND: 27/100; Simple + RT: 8/100 | |||
| SE Scotland | Haematoma | ALND: 24/100; Simple + RT: 6/100 | |||
| SE Scotland | Infection | ALND: 9/100; Simple + RT: 6/100 | |||
| SE Scotland | DVT | ALND: 4/100; Simple + RT: 1/100 | |||
| SE Scotland | Pulmonary embolism | ALND: 1/100; Simple + RT: 1/100 | |||
| SE Scotland | Chest infection | ALND: 6/100; Simple + RT: 3/100 | |||
| SE Scotland | Severe skin reaction | ALND: 0/100; Simple + RT: 5/100 | |||
| SE Scotland | Nausea and vomiting | ALND: 0/100; Simple + RT: 2/100 | |||
| SE Scotland | Tracheitis | ALND: 0/100; Simple + RT: 2/100 | |||
| SE Scotland | Skin grafts | ALND: 10/100; Simple + RT: 0/100 | |||
| SE Scotland | Arm oedema | ALND: 10/100; Simple + RT: 5/100 | |||
| SE Scotland | Limitation of shoulder movement | ALND: 4/100; Simple + RT: 14/100 | |||
| SNAC | Haematoma | Any | ALND: 30/539; SLNB: 38/544 | ||
| SNAC | Seroma | Any | ALND: 195/539; SLNB: 93/544 | ||
| SNAC | Infection | Any | ALND: 73/539; SLNB: 48/544 | ||
| SNAC | Arm morbidity | Mean changes in arm morbidity (patient reported, overall summary average score of 15 items; unclear if it is SEM or SD reported) from baseline | Node+ and node‐ patients: average of measures taken at 6 and 12 months: ALND: 7 (N = 457); SLNB: 4.4 (N = 456) 1 month: ALND: 2.2 (0.2); SLNB: 1.4 (0.15) 6 months: ALND: 1.1 (0.2); SLNB: 0.8 (0.15) 12 months: ALND: 1.05 (0.2); SLNB: 0.8 (0.15) |
24 months: ALND: 1.05 (0.2); SLNB: 0.75 (0.15) 36 months: ALND: 1.05 (0.2); SLNB: 0.7 (0.15) |
|
| SNAC | Arm symptoms | Mean changes in arm symptoms (patient reported, average of 7 items; unclear if it is SEM or SD reported) from baseline | Node+ and node‐ patients: average of measures taken at 6 and 12 months: ALND: 9.7 (N = 457); SLNB: 5.5 (N = 456) 1 month: ALND: 2.1 (0.2); SLNB: 1.2 (0.1) 6 months: ALND: 1.3 (0.15); SLNB: 0.8 (0.1) 12 months: ALND: 1.25 (0.15); SLNB: 0.7 (0.1) |
24 months: ALND: 1.25 (0.15); SLNB: 0.7 (0.1) 36 months: ALND: 1.2 (0.2); SLNB: 0.65 (0.15) |
|
| SNAC | Arm swelling | Mean changes in arm swelling (patient reported, 1 item; unclear if it is SEM or SD reported) from baseline | Node+ and node‐ patients: average of measures taken at 6 and 12 months: ALND: 7.3 (N = 457); SLNB: 3.4 (N = 456) 1 month: ALND: 1.25 (0.2); SLNB: 0.75 (0.15) 6 months: ALND: 0.9 (0.15); SLNB: 0.55 (0.1) 12 months: ALND: 0.95 (0.15); SLNB: 0.45 (0.1) |
24 months: ALND: 1 (0.2); SLNB: 0.55 (0.15) 36 months: ALND: 1 (0.2); SLNB: 0.55 (0.15) |
|
| SNAC | Arm dysfunctions | Mean arm dysfunctions change (patient reported, average of 3 items; unclear if it is SEM or SD reported) from baseline | Node+ and node‐ patients: average of measures taken at 6 and 12 months: ALND: 5.5 (N = 457); SLNB: 3.6 (N = 456) 1 month: ALND: 1.9 (0.15); SLNB: 1.35 (0.15) 6 months: ALND: 0.8 (0.1); SLNB: 0.65 (0.1) 12 months: ALND: 0.75 (0.1); SLNB: 0.6 (0.1) |
24 months: ALND: 0.7 (0.1); SLNB: 0.55 (0.1) 36 months: ALND: 0.8 (0.1); SLNB: 0.5 (0.1) |
|
| SNAC | Arm disabilities | Mean arm disabilities (patient‐reported change, average of 4 items; unclear if it is SEM or SD reported) from baseline | Node+ and node‐ patients: average of measures taken at 6 and 12 months: ALND: 3.4 (N = 457); SLNB: 2.9 (N = 456) 1 month: ALND: 2.2 (0.2); SLNB: 1.4 (0.15) 6 months: ALND: 0.75 (0.1); SLNB: 0.55 (0.1) 12 months: ALND: 0.65 (0.1); SLNB: 0.45 (0.1) |
24 months: ALND: 0.6 (0.1); SLNB: 0.5 (0.1) 36 months: ALND: 0.7 (0.1); SLNB: 0.45 (0.1) |
|
| SNAC | Arm volume | Increase in arm volume (percentage change from clinician ratings from baseline; unclear if it is SEM or SD reported) | Average of measures taken at 6 and 12 months: ALND: 4.2% (N = 509); SLNB: 2.8% (N = 519) All patients: 1 month: ALND: 0.8% (0.4); SLNB: 0.9% (0.4), P = 0.67 6 months: ALND: 3.5% (0.8); SLNB: 2.4% (0.7), P = 0.02 12 months: ALND: 4.6% (0.8); SLNB: 3% (0.8), P = 0.001 Node‐negative patients: 1 month: ALND: 0.8% (0.4); SLNB: 0.3% (0.4), P = 0.16 6 months: ALND: 3.5% (0.8); SLNB: 1.9% (0.5), P = 0.004 12 months: ALND: 4.6% (0.8); SLNB: 2.2% (0.7), P = 0.001 |
All patients: 24 months: ALND: 5.8% (1); SLNB: 3.9% (0.7), P = 0.006 36 months: ALND: 5.8% (1); SLNB: 4.0% (1), P = 0.02 Node‐negative patients: 24 months: ALND: 5.8% (1); SLNB: 3% (0.7), P = 0.001 36 months: ALND: 5.8% (1); SLNB: 3.1% (1), P= 0.004 |
|
| SNAC | Arm volume | Number with an increase in arm volume ≥ 15% (percentage change from clinician ratings from baseline) | All patients: 1 month: ALND: 5/544; SLNB: 3/544 6 months: ALND: 29/544; SLNB:21/544 12 months: ALND: 47/544; SLNB: 29/544 (P = 0.02) Node‐negative patients only: 1 month: ALND: 4/363; SLNB: 1/356 6 months: ALND: 16/363; SLNB: 9/356 12 months: ALND: 28/363; SLNB: 13/356 (P = 0.02) |
All patients: 24 months: ALND: 81/544; SLNB: /544 (P = 0.001) 36 months: ALND: 82/544; SLNB: /544 (P = 0.01) Node‐negative patients only: 24 months: ALND: 47/363; SLNB: 25/356 (P = 0.01) 36 months: ALND: 49/363; SLNB: 25/356 (P = 0.006) |
|
| SNAC | Lateral abduction | Lateral abduction (change from clinician ratings from baseline; degrees; unclear if it is SEM or SD reported ‐ have assumed it is SEM for calculations) | Average of measures taken at 6 and 12 months (percentage change from baseline: ALND: 4.4% (N = 509); SLNB: 2.5% (N = 519) Node+ and node‐ patients (read off graph): Baseline: ALND: 158 (1); SLNB: 157 (1) 1 month: ALND: 131 (2); SLNB: 144 (2) 6 months: ALND: 150 (1); SLNB: 151 (1) 12 months: ALND: 150 (1); SLNB: 151 (1) |
Node+ and node‐ patients (read off graph): 24 months: ALND: 151 (1); SLNB: 152 (1) 36 months: ALND: 150 (1); SLNB: 151 (1) |
|
| SNAC | Forward flexion | Forward flexion (degrees; unclear if it is SEM or SD reported ‐ have assumed it is SEM for calculations) | Node+ and node‐ patients (read off graph): Baseline: ALND: 157 (1); SLNB: 158 (1) 1 month: ALND: 137 (2); SLNB: 148 (1.5) 6 months: ALND: 150 (1); SLNB: 152 (1) 12 months: ALND: 151 (1); SLNB: 151 (1) |
Node+ and node‐ patients (read off graph): 24 months: ALND: 152 (1); SLNB: 152 (1) 36 months: ALND: 152 (1); SLNB: 151 (1) |
|
| Xu 2003 | Postoperative swelling (oedema) | Measurement of arm diameter | Level I clearance: 3/93 ALND: 7/88 |
||
| Xu 2003 | Involved upper limb disorder | Unclear | Level I clearance: 0/93 ALND: 0/88 |
||
| Xu 2003 | Cerebrovascular accident | Unclear | Level I clearance: 0/93 ALND: 2/88 |
||
| Xu 2003 | Cardiovascular events | Unclear | Level I clearance: 2/93 ALND: 1/88 |
We reported relative effects of treatments on time‐to‐event outcomes and noted that HRs less than 1.0 favour the 'less axillary surgery' arm, and HRs greater than 1.0 favour the 'more axillary surgery' arm. Similarly, for adverse event rates, ORs less than 1.0 favour the 'less axillary surgery' arm, and ORs greater than 1.0 favour the 'more axillary surgery' arm.
No axillary surgery versus full axillary surgery
Overall survival
All 10 trials comparing ALND versus no axillary surgery reported overall survival. The HR for death from any cause was 1.06 (95% confidence interval (CI) 0.96 to 1.17; 3849 participants; 10 studies; Analysis 1.1) with no statistically significant heterogeneity (I2 = 26%; P = 0.20). We downgraded evidence for this outcome from high to moderate quality owing to imprecision: The confidence interval of the effect estimate includes both no difference between treatment groups and appreciable harm associated with no axillary surgery (Table 1). For the single trial that did not use RT (NSABP B‐04), the HR was 0.96 (95% CI 0.80 to 1.15; 773 participants; one study; Analysis 1.1). For trials that used RT, the HR was 1.11 (95% CI 0.98 to 1.25; 3076 participants; nine studies; Analysis 1.1) with no statistically significant heterogeneity (I2 = 24%; P = 0.23).
1.1. Analysis.
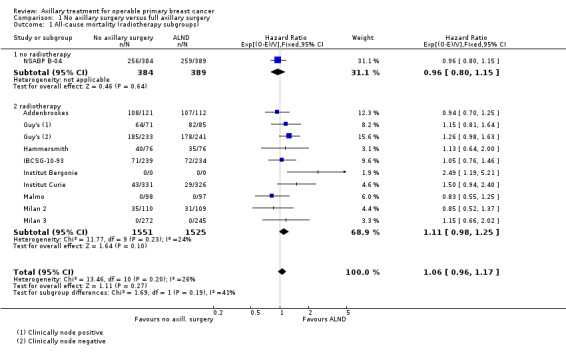
Comparison 1 No axillary surgery versus full axillary surgery, Outcome 1 All‐cause mortality (radiotherapy subgroups).
For the subgroup of studies that provided additional treatment to participants with histologically positive axillary nodes (Institut Bergonie; Institut Curie; Milan 3), no axillary surgery was associated with increased risk of overall mortality (HR 1.51, 95% CI 1.09 to 2.09; 1174 participants; three studies; Analysis 1.2.1) with no statistically significant heterogeneity (I2 = 25%; P = 0.27).
1.2. Analysis.
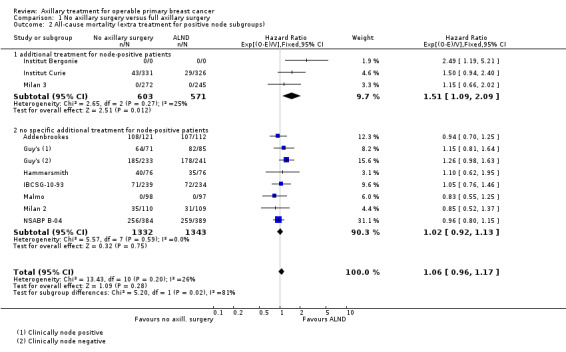
Comparison 1 No axillary surgery versus full axillary surgery, Outcome 2 All‐cause mortality (extra treatment for positive node subgroups).
For the subgroup of studies that did not provide additional treatment to participants with histologically positive axillary nodes (Addenbrookes; Guy's; Hammersmith; IBCSG‐10‐93; Malmo; Milan 2; NSABP B‐04), the HR for overall mortality was 1.02 (95% CI 0.92 to 1.13; 2675 participants; seven studies; Analysis 1.2.2) with no statistically significant heterogeneity (I2 = 0%; P = 0.59).
For the subgroup of studies with adequate allocation concealment (Addenbrookes; IBCSG‐10‐93; Milan 2; Milan 3), the HR for death from any cause was 0.98 (95% CI 0.81 to 1.18; 1442 participants; four studies; Analysis 1.13.1) with no statistically significant heterogeneity (I2 = 0%; P = 0.81).
1.13. Analysis.
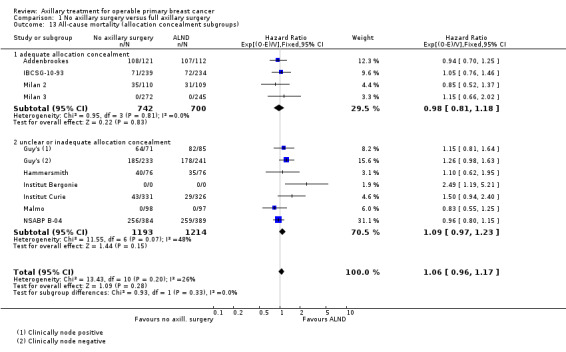
Comparison 1 No axillary surgery versus full axillary surgery, Outcome 13 All‐cause mortality (allocation concealment subgroups).
Disease control in the axilla
Trials comparing full axillary surgery with no axillary surgery did not report disease control in the axilla.
Breast cancer recurrence
Local recurrence
Included studies not separately report time to local recurrence.
Locoregional recurrence
We were able to extract locoregional recurrence time‐to‐event data for four of the nine included trials. No axillary surgery was associated with increased risk of locoregional recurrence (with HR ranging from 1.10 to 3.06; 20,863 person‐years of follow‐up; four studies; Analysis 1.3) but heterogeneity was substantial (I2 = 71%; P = 0.007); for this reason, we downgraded evidence for this outcome to moderate quality (Table 1).
1.3. Analysis.
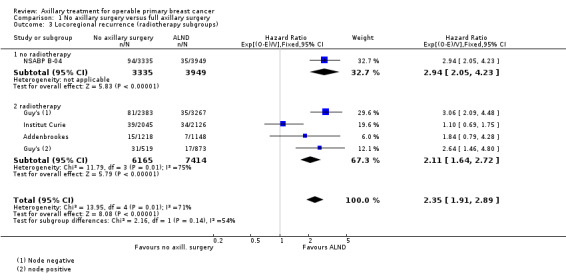
Comparison 1 No axillary surgery versus full axillary surgery, Outcome 3 Locoregional recurrence (radiotherapy subgroups).
For the single trial that provided additional treatment to participants with histologically positive axillary nodes (Institut Curie), the HR for locoregional recurrence was 1.10 (95% CI 0.69 to 1.75; 4171 person‐years of follow‐up; one study; Analysis 1.4.1). For the remaining trials (Addenbrookes; Guy's; NSABP B‐04), which provided no specific additional treatment to participants with histologically positive axillary nodes, no axillary surgery was associated with increased risk of locoregional recurrence (HR 2.83, 95% CI 2.25 to 3.57; 16,692 person‐years of follow‐up; three studies) with no statistically significant heterogeneity (I2 = 0%; P = 0.74).
1.4. Analysis.
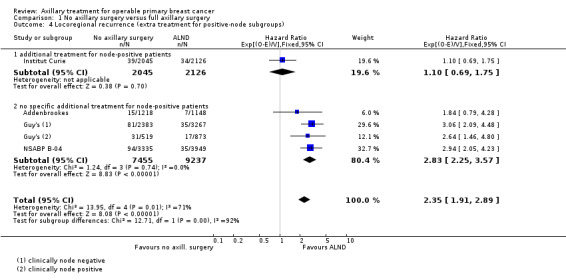
Comparison 1 No axillary surgery versus full axillary surgery, Outcome 4 Locoregional recurrence (extra treatment for positive‐node subgroups).
In subgroup analyses of trials according to use of RT (Analysis 1.3), no axillary surgery was associated with increased risk of locoregional recurrence (HR ranging from 1.10 to 3.06; 13,579 person‐years of follow‐up; three studies; Analysis 1.3.2) but heterogeneity was substantial (I2 = 75%; P = 0.008). For the single trial that did not use RT (NSABP B‐04), no axillary surgery was associated with increased risk of locoregional recurrence (HR 2.94, 95% CI 2.05 to 4.23; 7284 person‐years of follow‐up; one study; Analysis 1.3.1).
We judged allocation concealment as adequate in only one of the trials reporting locoregional recurrence (Addenbrookes). We were uncertain about whether no axillary surgery was associated with increased risk of locoregional recurrence in this trial (HR 1.84, 95% CI 0.79 to 4.28).
Distant metastasis
We were able to extract distant metastasis time‐to‐event data for two trials (Milan 2; NSABP B‐04). The HR for distant metastasis was 1.06 (95% CI 0.87 to 1.30; 946 participants; two studies; Analysis 1.5) with moderate heterogeneity (I2 = 40%; P = 0.20).
1.5. Analysis.
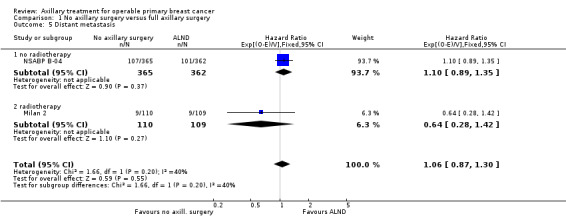
Comparison 1 No axillary surgery versus full axillary surgery, Outcome 5 Distant metastasis.
One of the trials (Milan 2) had adequate allocation concealment, but its results indicate uncertainty about the relative rates of distant metastasis with the two treatment options (HR 0.64, 95% CI 0.28 to 1.42; 219 participants; one study).
Institut Curie reported the rate of metastases but provided insufficient detail for extraction of time‐to‐event outcomes. In this trial, at 15 years of follow‐up, the rate of metastasis was 24.9% for no axillary surgery versus 25.8% for axillary lymph node dissection (P reported as not significant).
Long‐term adverse events
Lymphoedema
Four of the included trials reported the rate of lymphoedema, defined as an increase in arm circumference, at 12 or more months after surgery (Addenbrookes; Guy's; Institut Bergonie; NSABP B‐04). The Addenbrookes, Guy's and Institut Bergonie trials used RT. NSABP B‐04 was a three‐arm trial, but we included the two "no radiotherapy" arms for this comparison. No axillary surgery was associated with decreased risk of lymphoedema at 12 or more months post surgery (OR 0.31, 95% CI 0.23 to 0.43; fixed‐effect model; 1714 participants; four studies; Analysis 1.6). We downgraded evidence for this outcome to low quality owing to substantial heterogeneity (I2 = 69%; P = 0.02) and unclear blinding of the outcome assessment (Table 1). A random‐effects model yielded a similar result (OR 0.22, 95% CI 0.08 to 0.57; random‐effects model; 1714 participants; four studies; I2 = 69%; P = 0.02; Analysis 1.7).
1.6. Analysis.
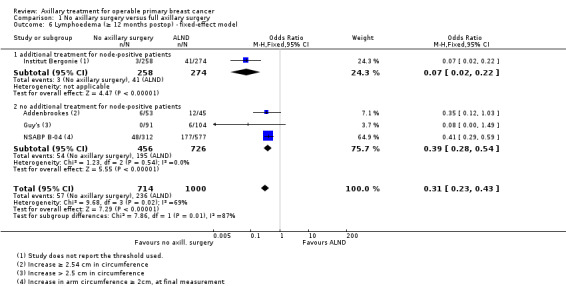
Comparison 1 No axillary surgery versus full axillary surgery, Outcome 6 Lymphoedema (≥ 12 months postop) ‐ fixed‐effect model.
1.7. Analysis.
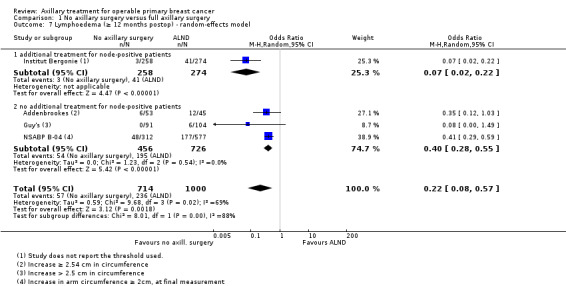
Comparison 1 No axillary surgery versus full axillary surgery, Outcome 7 Lymphoedema (≥ 12 months postop) ‐ random‐effects model.
Subgroup analysis of trials that did not provide additional treatment to participants with histologically positive axillary lymph nodes (Addenbrookes; Guy's, NSABP B‐04) revealed that no axillary surgery was associated with decreased risk of lymphoedema (OR 0.40, 95% CI 0.28 to 0.55; 1182 participants; three studies) and showed no important heterogeneity (I2 = 0%; P = 0.54).
We judged allocation concealment as adequate in only one of the trials reporting lymphoedema (Addenbrookes). Its results were consistent with results of the pooled analysis (HR 0.35; 95% CI 0.12 to 1.03; 98 participants).
Arm or shoulder movement impairment
Five trials (Addenbrookes; Guy's; Hammersmith; IBCSG‐10‐93; Institut Bergonie), involving 1495 participants, reported impairment of arm or shoulder function at 12 or more months after surgery (Analysis 1.8). Results show considerable heterogeneity (I2 = 78%; P = 0.001), with the OR for any impairment of function ranging from 0.24 to 3.26. We downgraded evidence for this outcome to very low quality owing to heterogeneity and unclear blinding of outcome assessment (Table 1).
1.8. Analysis.
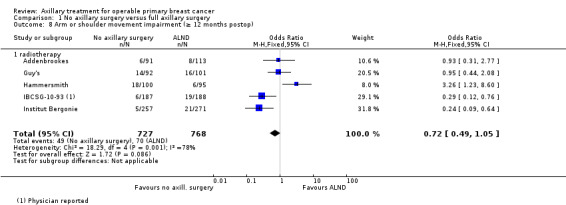
Comparison 1 No axillary surgery versus full axillary surgery, Outcome 8 Arm or shoulder movement impairment (≥ 12 months postop).
Differences between trials in the definitions of arm and shoulder impairment are a possible source of this heterogeneity. All trials provided RT, but in both Guy's and Hammersmith trials, the no axillary surgery group received more extensive RT than the ALND group.
Analysis restricted to trials with adequate allocation concealment (Addenbrookes; IBCSG‐10‐93) suggests fewer participants with arm or shoulder movement impairment in the no axillary surgery than in the ALND group (HR 0.46, 95% CI 0.23 to 0.93) but with potentially important heterogeneity (I2 = 59%; P = 0.12).
Arm pain
One study reported arm pain. In IBCSG‐10‐93, the OR for arm pain at 12 or more months was 0.60 (95% CI 0.24 to 1.47; 379 participants; Analysis 1.9).
1.9. Analysis.
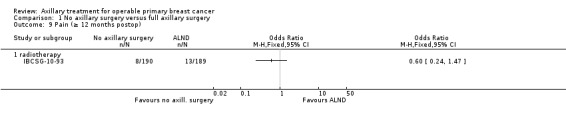
Comparison 1 No axillary surgery versus full axillary surgery, Outcome 9 Pain (≥ 12 months postop).
Paraesthesia
One study reported on paraesthesia. In Institut Bergonie, paraesthesia at 12 or more months after surgery was less likely in the no axillary surgery group (OR 0.14, 95% CI 0.06 to 0.32; 532 participants; Analysis 1.10).
1.10. Analysis.
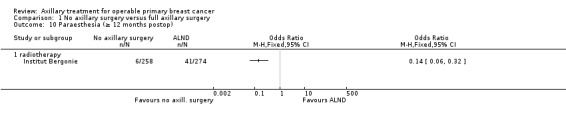
Comparison 1 No axillary surgery versus full axillary surgery, Outcome 10 Paraesthesia (≥ 12 months postop).
Short‐term adverse events
One trial (Addenbrookes) reported acute adverse events (surgical complications).
Delayed healing
Delayed healing was less likely in the no axillary surgery group (OR 0.27, 95% CI 0.11 to 0.67; 204 participants; one study; Analysis 1.11).
1.11. Analysis.
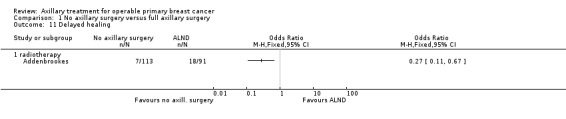
Comparison 1 No axillary surgery versus full axillary surgery, Outcome 11 Delayed healing.
Skin grafts
Skin grafts were less likely in the no axillary surgery group (OR 0.39, 95% CI 0.07 to 2.19; 204 participants; one study; Analysis 1.12).
1.12. Analysis.
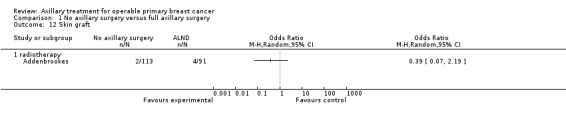
Comparison 1 No axillary surgery versus full axillary surgery, Outcome 12 Skin graft.
Quality of life
IBCSG‐10‐93 was the only trial that measured quality of life outcomes; investigators reported no statistically significant differences in quality of life, bother and coping scores between treatment groups during the two years of postoperative follow‐up.
Psychological and psychosocial outcomes
The included studies did not report on these outcomes.
Axillary sampling versus full axillary surgery
Overall survival
Five trials (Cape Town; Cardiff; E'dburgh Sample/Clear; Edinburgh 1; Xu 2003) reported time to death from any cause, but we excluded Cardiff data from the meta‐analysis owing to non‐proportionality of hazard rates (i.e. survival curves cross at 12 years' follow‐up) and the published report provided insufficient detail to include Xu 2003. In the remaining three trials (Cape Town; E'dburgh Sample/Clear; Edinburgh 1), heterogeneity in the HR for overall mortality was substantial (HR 0.94, 95% CI 0.73 to 1.21; 967 participants; three studies; I2 = 45%; P = 0.16; Analysis 2.1). We downgraded this evidence to low quality owing to substantial heterogeneity and serious imprecision (Table 2).
2.1. Analysis.
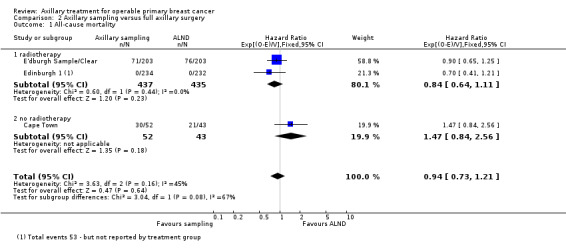
Comparison 2 Axillary sampling versus full axillary surgery, Outcome 1 All‐cause mortality.
Subgroup analysis of the two trials that provided RT (E'dburgh Sample/Clear; Edinburgh 1) yielded an HR of 0.84 (95% CI 0.64 to 1.11; 872 participants; two studies; Analysis 2.1) with no significant heterogeneity (I2 = 0%; P = 0.44), and for the trial that did not use RT (Cape Town), an HR of 1.47 (95% CI 0.84 to 2.56; 85 participants).
We conducted no sensitivity analysis for this outcome because all trials were at low risk of bias owing to allocation concealment.
Disease control in the axilla
Included studies did not report disease control in the axilla, but two trials reported axillary recurrence (see below).
Breast cancer recurrence
Local recurrence
Five trials that performed six treatment comparisons reported local recurrence (Cape Town (1) and (2); Cardiff; Edinburgh 1; Ostersund; Xu 2003), but we could not extract time‐to‐event data from Ostersund and Xu 2003. The HR for local recurrence was 1.41 (95% CI 0.94 to 2.12; 1404 participants; three studies; Analysis 2.2) with no heterogeneity (I2 = 0%; P = 0.91). In the Ostersund trial, one out of 54 participants in the axillary sampling arm experienced local recurrence compared with four of 57 participants in the ALND arm. In Xu 2003, local recurrence rates were 3.2% and 2.3% in the axillary sampling and ALND arms, respectively (181 participants; P value reported as greater than 0.05). We downgraded evidence for local recurrence to low quality on the basis of few events and serious imprecision (Table 2). We performed no sensitivity analysis for this outcome because all trials were at low risk of bias owing to allocation concealment.
2.2. Analysis.
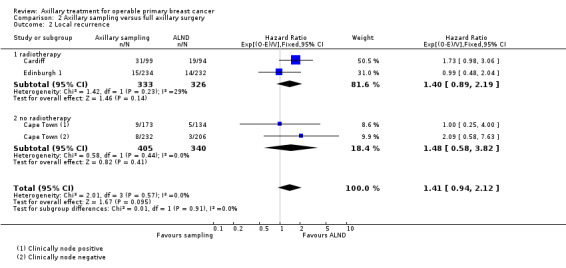
Comparison 2 Axillary sampling versus full axillary surgery, Outcome 2 Local recurrence.
Axillary recurrence
Two trials reported axillary recurrence rates (Cape Town; Edinburgh 1), but we were able to extract time‐to‐event data only from Edinburgh 1, yielding an HR for axillary recurrence of 0.99 (95% CI 0.58 to 1.69; 466 participants; Analysis 2.3) with axillary lymph node sampling versus dissection. In Cape Town, rates of axillary recurrence were 8/52 for axillary lymph node sampling and 2/43 for ALND.
2.3. Analysis.
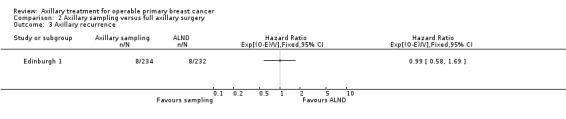
Comparison 2 Axillary sampling versus full axillary surgery, Outcome 3 Axillary recurrence.
Locoregional recurrence
Two trials (Cape Town; E'dburgh Sample/Clear) reported locoregional recurrence, but we could extract time‐to‐event data only from E'dburgh Sample/Clear, yielding an HR for locoregional recurrence of 0.74 (95% CI 0.46 to 1.20; 406 participants; one study; Analysis 2.4). In the Cape Town trial, 19 of 52 participants in the axillary sampling group experienced locoregional recurrence compared with 11 of 43 in the ALND group.
2.4. Analysis.
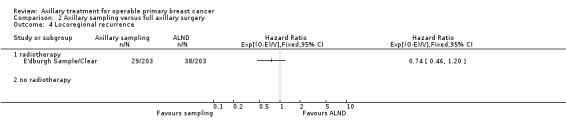
Comparison 2 Axillary sampling versus full axillary surgery, Outcome 4 Locoregional recurrence.
Distant metastasis
Four trials reported distant metastasis (Cape Town; Cardiff; E'dburgh Sample/Clear; Xu 2003). We were able to extract time‐to‐event data only extracted from the Cardiff and E'dburgh Sample/Clear trials, but we did not include data from Cardiff in the meta‐analysis owing to the non‐proportionality of HRs. In E'dburgh Sample/Clear, the HR for distant metastasis was 1.05 (95% CI 0.74 to 1.49; 406 participants; Analysis 2.5). In the Cape Town trial, distant metastasis occurred at a rate of 13 of 52 participants in the axillary sampling group compared with 11 of 43 participants in the ALND group. In Xu 2003, distant metastasis rates were 19/93 and 15/88 in the axillary sampling and ALND arms, respectively (181 participants; P value reported as greater than 0.05).
2.5. Analysis.
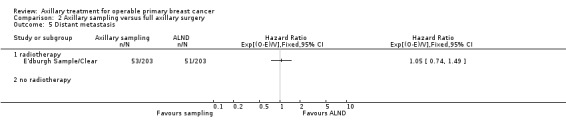
Comparison 2 Axillary sampling versus full axillary surgery, Outcome 5 Distant metastasis.
Long‐term adverse events
Lymphoedema
Two trials reported on lymphoedema. In the Cardiff trial, lymphoedema at 12 or more months after surgery (defined as an increase in arm circumference) was less likely in the axillary sampling group than in the ALND group (OR 0.32, 95% CI 0.13 to 0.81; 85 participants; one study; Analysis 2.6). In Xu 2003, postoperative lymphoedema occurred in 3/93 participants in the axillary sampling group compared with 7/88 in the ALND group, but it was unclear at what time this measurement was taken.
2.6. Analysis.
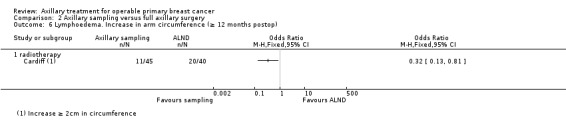
Comparison 2 Axillary sampling versus full axillary surgery, Outcome 6 Lymphoedema. Increase in arm circumference (≥ 12 months postop).
Arm or shoulder movement impairment
One trial (Edinburgh 1) reported shoulder lateral rotation at 12‐months follow‐up, noting a relatively small decrease in range of movement when compared with baseline in both the axillary sampling and ALND groups (mean difference (MD) ‐0.05 cm, 95% CI ‐1.50 to 1.40; 191 participants; one study; Analysis 2.7).
2.7. Analysis.
Comparison 2 Axillary sampling versus full axillary surgery, Outcome 7 Shoulder lateral rotation (12 months postop).
Short‐term adverse events
Seroma
One trial collected data on seroma formation. In the Ostersund trial, seroma occurred at a rate of 10 of 50 participants in the axillary sampling group compared with 17 of 50 participants in the ALND group (OR 0.49, 95% CI 0.20 to 1.20; 100 participants; one study; Analysis 2.8).
2.8. Analysis.
Comparison 2 Axillary sampling versus full axillary surgery, Outcome 8 Seroma.
Quality of life
The included studies did not report this outcome.
Psychological and psychosocial outcomes
The included studies did not report these outcomes.
Sentinel node biopsy versus full axillary surgery
Overall survival
Five trials reported overall mortality (ALMANAC; Genoa; GIVOM Sentinella; Milan; NSABP B‐32), but we were able to extract time‐to‐event data from only three studies (Genoa; Milan; NSABP B‐32). The HR for overall mortality was 1.05 (95% CI 0.89 to 1.25; 6352 participants; three studies; Analysis 3.1) with minimal heterogeneity (I2 = 28%; P = 0.25). We rated evidence for overall mortality as moderate quality owing to imprecision. The confidence interval of the effect estimate included both no differences between treatment groups and appreciable harm associated with SLNB (Table 3). In the ALMANAC trial, the overall mortality rate for the year after surgery was seven out of 478 women (1.5%) in the sentinel node group versus seven out of 476 women (1.5%) in the full axillary surgery group. In the GIVOM Sentinella trial, the overall mortality rate over the five years after surgery was 21 out of 345 women (6.1%) in the sentinel node group versus 14 out of 352 women (4.0%) in the full axillary surgery group.
3.1. Analysis.
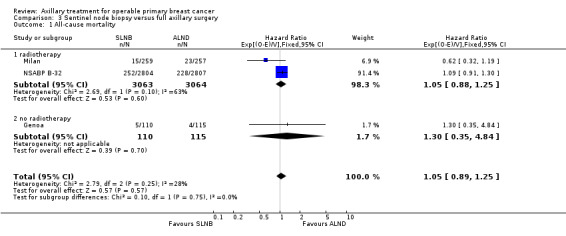
Comparison 3 Sentinel node biopsy versus full axillary surgery, Outcome 1 All‐cause mortality.
We conducted no sensitivity analysis for this outcome because all trials were at low risk of bias owing to allocation concealment.
Disease control in the axilla
The included studies did not report disease control in the axilla, although five trials reported axillary recurrence (see below).
Breast cancer recurrence
Local recurrence
Data reveal uncertainty about the relative effectiveness of SLNB and ALND in terms of local recurrence (HR 0.94, 95% CI 0.24 to 3.77; 516 participants; one study; Milan; Analysis 3.2).
3.2. Analysis.
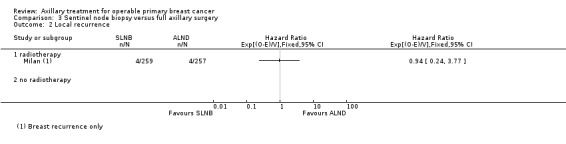
Comparison 3 Sentinel node biopsy versus full axillary surgery, Outcome 2 Local recurrence.
Axillary recurrence
Five trials, involving 7487 participants, reported axillary recurrence (ALMANAC; GIVOM Sentinella; Genoa; NSABP B‐32; Milan), but event rates were low, and we were able to extract time‐to‐event data only from Milan. Results derived from Milan suggest uncertainty about whether axillary recurrence is more likely with SLNB than with ALND (HR 6.96, 95% CI 0.44 to 111.25; 516 participants; one study; Analysis 3.3). In ALMANAC, the rate of axillary local recurrence during the first year after surgery was 1/478 (0.2%) in the SLNB group versus 4/476 (0.8%) in the ALND group. In GIVOM Sentinella, axillary recurrence rates over the five years after surgery were 1/345 (0.3%) in the SLNB group versus 0/352 (0%) in the ALND group. In Genoa, axillary recurrence rates were 0/110 (0%) in the SLNB group versus 1/115 (0.8%) in the ALND group. In NSABP B‐32, axillary recurrence rates were 14/2804 (0.5%) in the SLNB group versus 6/2807 (0.2%) in the ALND group.
3.3. Analysis.
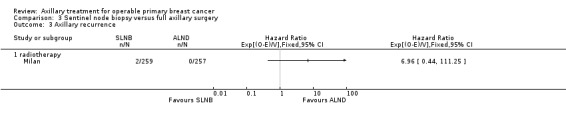
Comparison 3 Sentinel node biopsy versus full axillary surgery, Outcome 3 Axillary recurrence.
We conducted no sensitivity analysis for this outcome because all trials were at low risk of bias owing to allocation concealment.
Locoregional recurrence
Two trials reported locoregional recurrence (GIVOM Sentinella; NSABP B‐32), but we were able to extract time‐to‐event data only from NSABP B‐32. Data reveal uncertainty about whether SLNB or ALND was more effective in terms of locoregional recurrence (HR 0.96, 95% CI 0.74 to 1.24; 5611 participants; one study; Analysis 3.4). In GIVOM Sentinella, locoregional recurrence rates were 16/345 (4.6%) in the SLNB group versus 3/352 (0.9%) in the ALND group.
3.4. Analysis.
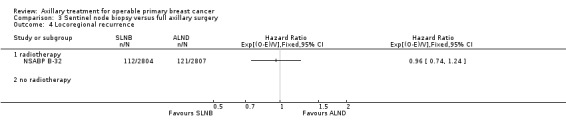
Comparison 3 Sentinel node biopsy versus full axillary surgery, Outcome 4 Locoregional recurrence.
Distant metastasis
Two studies reported distant metastases (GIVOM Sentinella; Milan), but we were able to extract time‐to‐event data only from Milan. The relative effectiveness of SLNB and ALND in terms of distant metastasis was uncertain (HR 0.80, 95% CI 0.42 to 1.53; 516 participants; one study; Analysis 3.5). In GIVOM Sentinella, distant metastasis rates were 11/3345 (3.2%) in the SLNB group versus 16/352 (4.5%) in the ALND group.
3.5. Analysis.
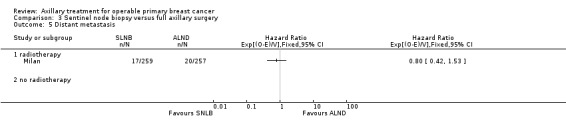
Comparison 3 Sentinel node biopsy versus full axillary surgery, Outcome 5 Distant metastasis.
Long‐term adverse events
Lymphoedema
Four studies reported objectively measured lymphoedema at 12 or more months after surgery (ALMANAC; GIVOM Sentinella; Milan; SNAC). Investigators measured lymphoedema by using arm circumference (GIVOM Sentinella; Milan) or arm volume (ALMANAC; SNAC). Increased arm circumference at 12 months after surgery was less likely with SLNB than with ALND (OR 0.48, 95% CI 0.26 to 0.92; 677 participants ‐ Analysis 3.6 OR 0.04, 95% CI 0.00 to 0.60; 200 participants ‐ Analysis 3.6 and OR 0.60, 95% CI 0.37 to 0.96, 1088 participants ‐ Analysis 3.6) for the GIVOM Sentinella, Milan and SNAC trials, respectively. We did not pool results owing to heterogeneity (I2 = 51%; P = 0.13), and we conducted no sensitivity analysis for this outcome because all trials were at low risk of bias owing to allocation concealment.
3.6. Analysis.
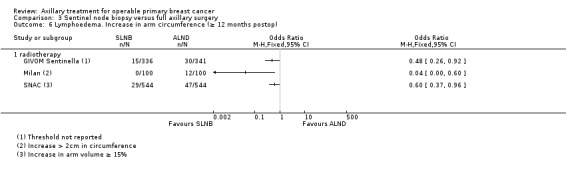
Comparison 3 Sentinel node biopsy versus full axillary surgery, Outcome 6 Lymphoedema. Increase in arm circumference (≥ 12 months postop).
The ALMANAC trial reported the mean ratio in arm volume at baseline compared with 12 months after surgery. In the sentinel lymph node group, this was 1.03 (95% CI 1.02 to 1.04) compared with 1.06 (95% CI 1.05 to 1.07) in the ALND group (P = 0.096; two sided t‐test).
In ALMANAC, Addenbrookes 2 and SNAC, patient‐reported lymphoedema (of any severity) was less likely in the SLNB group than in the ALND group (OR 0.33, 95% CI 0.23 to 0.47; fixed‐effect model; 1903 participants; three studies; Analysis 3.7) with no heterogeneity (I2 = 0%; P = 0.96). The random‐effects model produced the same result. We downgraded evidence on patient‐reported lymphoedema to moderate quality owing to incomplete follow‐up (Table 3). Restricting this analysis to trials with adequate allocation concealment (ALMANAC and SNAC) yielded a similar result (OR 0.33, 95% CI 0.22 to 0.48; fixed‐effect model).
3.7. Analysis.
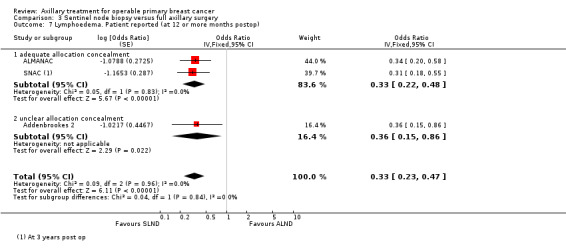
Comparison 3 Sentinel node biopsy versus full axillary surgery, Outcome 7 Lymphoedema. Patient reported (at 12 or more months postop).
Shoulder or arm movement impairment
The Addenbrookes 2, ALMANAC and SNAC trials measured change in the range of shoulder movement from baseline to 12 months after surgery. Results showed no statistically significant differences between SLNB and ALND groups when change in the range of movement was compared from baseline to 12 months post surgery, for flexion (MD 1.55°, 95% CI ‐0.19° to 3.29°; 2257 participants; three studies; Analysis 3.8), abduction (MD ‐1.02°, 95% CI ‐2.79° to 0.75°; 2252 participants; three studies; Analysis 3.9), internal rotation (MD 0.50°; 95% CI ‐1.10° to 2.09°; 1227 participants; two studies; Analysis 3.10) or external rotation (MD ‐0.56°; 95% CI ‐2.21° to 1.09°; 1227 participants; two studies; Analysis 3.11). Except for external rotation, heterogeneity was substantial or considerable for all shoulder movement comparisons.
3.8. Analysis.
Comparison 3 Sentinel node biopsy versus full axillary surgery, Outcome 8 Shoulder flexion (12 months postop).
3.9. Analysis.
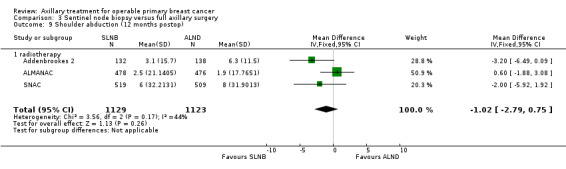
Comparison 3 Sentinel node biopsy versus full axillary surgery, Outcome 9 Shoulder abduction (12 months postop).
3.10. Analysis.
Comparison 3 Sentinel node biopsy versus full axillary surgery, Outcome 10 Shoulder internal rotation (12 months postop).
3.11. Analysis.
Comparison 3 Sentinel node biopsy versus full axillary surgery, Outcome 11 Shoulder external rotation (12 months postop).
In two trials (GIVOM Sentinella and Milan), subjective arm movement impairment was less likely with SLNB than with ALND. This difference was statistically significant in the Milan trial (OR 0.02, 95% CI < 0.00 to 0.31; 200 participants; Analysis 3.12) but not in the GIVOM Sentinella trial (OR 0.74, 95% CI 0.39 to 1.41; 677 participants; Analysis 3.12), and heterogeneity in the pooled estimate was considerable (I2 = 88%; P = 0.004). We downgraded evidence on subjective arm movement impairment to low quality owing to heterogeneity and lack of blinding (Table 3). We conducted no sensitivity analysis for this outcome because all trials were at low risk of bias owing to allocation concealment.
3.12. Analysis.
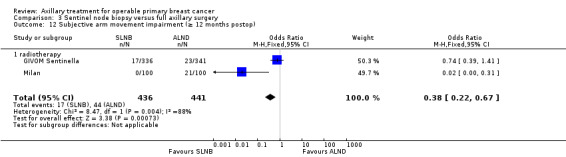
Comparison 3 Sentinel node biopsy versus full axillary surgery, Outcome 12 Subjective arm movement impairment (≥ 12 months postop).
The SNAC trial reported subjective arm disability rated on a scale from 0 (no trouble at al) to 10 (the worst I can imagine). At one year postoperatively, mean arm disability ratings were low in both groups: 0.65 (standard error (SE) 0.1) in the ALND group compared with 0.45 (SE 0.1) in the SLNB group.
Pain
Two trials reported pain at 12 or more months after surgery (GIVOM Sentinella; Milan). Pain was less likely to be reported in the sentinel lymph node group than in the axillary dissection group. This difference was statistically significant in the Milan trial (OR 0.14, 95% CI 0.06 to 0.31; 200 participants; Analysis 3.13) but not in the GIVOM Sentinella trial (OR 0.76, 95% CI 0.46 to 1.25; 677 participants; Analysis 3.13), and heterogeneity was considerable in the pooled estimate (I2 = 92%; P = 0.0005). We downgraded evidence on pain to low quality owing to heterogeneity and lack of blinding (Table 3).
3.13. Analysis.
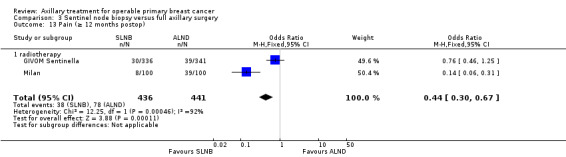
Comparison 3 Sentinel node biopsy versus full axillary surgery, Outcome 13 Pain (≥ 12 months postop).
Paraesthesia
Two trials reported paraesthesia at 12 or more months after surgery (Addenbrookes 2; Milan). Both trials found that paraesthesia was less likely in the sentinel lymph node group than in the axillary dissection group. For the Milan trial (OR < 0.00, 95% CI <0.00 to 0.04; 200 participants; Analysis 3.14) and the Addenbrookes 2 trial (OR 0.37, 95% CI 0.21 to 0.64; 295 participants; Analysis 3.14), heterogeneity was considerable in the pooled estimate (I2 = 95%; P < 0.00001). We downgraded evidence on paraesthesia to low quality owing to heterogeneity and lack of blinding (Table 3).
3.14. Analysis.
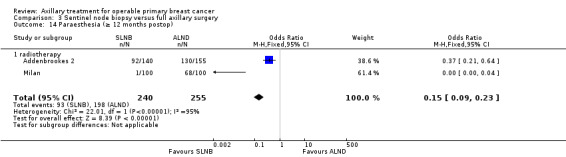
Comparison 3 Sentinel node biopsy versus full axillary surgery, Outcome 14 Paraesthesia (≥ 12 months postop).
Numbness
Three trials reported numbness or sensory deficit at 12 or more months after surgery (Addenbrookes 2;ALMANAC; GIVOM Sentinella). All found that numbness was less likely in the SLNB group than in the ALND group (OR 0.43, 95% CI 0.34 to 0.54; 1799 participants; Analysis 3.15) with limited heterogeneity (I2 = 20%; P = 0.29). Restricting this analysis to trials with adequate allocation concealment (ALMANAC; GIVOM Sentinella) yielded a similar result (OR 0.47, 95% CI 0.36 to 0.61).
3.15. Analysis.
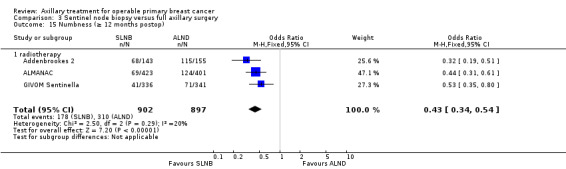
Comparison 3 Sentinel node biopsy versus full axillary surgery, Outcome 15 Numbness (≥ 12 months postop).
Short‐term adverse events
Seroma
The Addenbrookes 2 and SNAC trials reported that seroma was less likely with SLNB than with ALND (OR 0.60, 95% CI 0.33 to 1.11; 298 participants; Analysis 3.16; OR 0.36; 95% CI 0.27 to 0.48; 1083 participants; Analysis 3.16 respectively) but with considerable heterogeneity (I2 = 53%; P = 0.14).
3.16. Analysis.
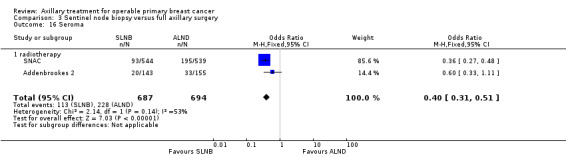
Comparison 3 Sentinel node biopsy versus full axillary surgery, Outcome 16 Seroma.
Wound infection
The ALMANAC and SNAC trials reported that wound infection was less likely with SLNB than with ALND (OR 0.65, 95% CI 0.50 to 0.85; 2074 participants; Analysis 3.17).
3.17. Analysis.
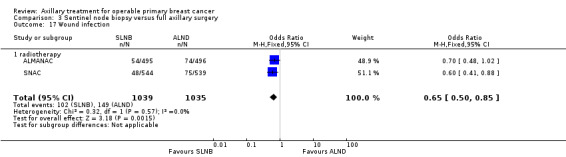
Comparison 3 Sentinel node biopsy versus full axillary surgery, Outcome 17 Wound infection.
Brachial plexus injury
The ALMANAC trial reported the rate of brachial plexus injury at six months postoperatively (OR 0.38, 95% CI 0.12 to 1.22; 804 participants).
Quality of life
We did not conduct statistical meta‐analysis because of differences in the scales used, but results from three trials (Addenbrookes 2; ALMANAC; GIVOM Sentinella) suggested that SLNB was associated with better quality of life, at least in the immediate postoperative period.
Addenbrookes 2 reported that quality of life scores were usually higher (better) in the SLND group than in the ALND group, and significantly so in the immediate postoperative period (P < 0.01).
ALMANAC measured a trial outcome index (TOI, derived from the sum of scores on physical and well‐being subscales and on breast cancer concerns subscales of the FACT‐B+4 (Functional Assessment of Cancer Therapy, Breast, for patients with lymphoedema questionnaire) before surgery and repeatedly in the following 18 months. Participants in the SLND group recovered more quickly to their baseline TOI value than those in the ALND group. This occurred at 12 months for the SLND group compared with 18 months for the ALND group (P < 0.01). Global quality of life (measured with the total FACT‐B+4 score) was significantly better in the SLND group than in the ALND group at most time points following surgery (at one month, P < 0.001; at three months, P = 0.04; at six months, P = 0.059; at 12 months, P = 0.024; at 18 months, P = 0.019). .
GIVOM Sentinella reported no significant differences between SLNB and ALND groups on the physical and health‐related quality of life components of the Short Form (SF)‐36 measure.
Psychological and psychosocial outcomes
Although three trials reported psychological outcomes, we did not pool their results owing to insufficient detail in reporting and differences in measurement scales used.
The Addenbrookes 2 trial reported no significant differences between SLND and ALND groups in Mental Adjustment to Cancer scores, depressive symptoms (measured on the Beck Depression Inventory) or state anxiety (measured by the Spielberger State/Trait Anxiety Inventory) during the first year after surgery.
ALMANAC reported that Spielberger State/Trait Anxiety Inventory scores were slightly lower (better) in the SLNB group than in the ALND group during the first year after surgery, but this difference was not statistically significant.
GIVOM Sentinella reported no significant differences between SLNB and ALND groups on the mental health‐related quality of life components of the SF‐36. Participants in the SLNB group scored significantly better than those in the ALND group in general and anxiety domains of the psychological well‐being measure within the first 12 months after surgery, but this difference was no longer statistically significant at two years after surgery.
Full axillary surgery with no radiotherapy versus no axillary surgery with radiotherapy
Overall survival
Four studies involving seven treatment comparisons reported that overall survival was reduced among participants treated with RT compared with those treated with ALND (HR 1.10, 95% CI 1.00 to 1.21; 2469 participants; Analysis 4.1) with no heterogeneity (I2 = 0%; P = 0.63). We graded this evidence as high quality (Table 4). Only one of the trials (SE Scotland) was at low risk of bias owing to allocation concealment; this trial was consistent with the pooled analysis showing reduced overall survival among patients treated with RT compared with those treated with ALND (HR 1.27, 95% CI 1.04 to 1.54).
4.1. Analysis.
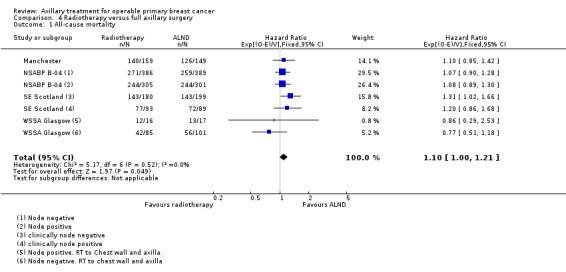
Comparison 4 Radiotherapy versus full axillary surgery, Outcome 1 All‐cause mortality.
Disease control in the axilla
Trials included in this comparison did not report disease control in the axilla.
Breast cancer recurrence
Local recurrence
Four studies involving seven treatment comparisons reported that local recurrence was less likely among participants treated with RT compared in those treated with ALND (HR 0.80, 95% CI 0.64 to 0.99; 22256 person‐years of follow‐up; four studies;Analysis 4.2) with no heterogeneity (I2 = 0%; P = 0.63). We graded this evidence as high quality (Table 4). Only one trial (SE Scotland) was at low risk of bias owing to allocation concealment; results showed uncertainty about whether local recurrence was less likely in patients treated with RT compared with those treated with ALND (HR 0.85, 95% CI 0.56 to 1.30).
4.2. Analysis.
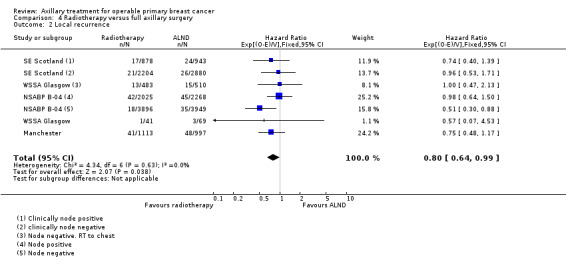
Comparison 4 Radiotherapy versus full axillary surgery, Outcome 2 Local recurrence.
Locoregional recurrence
The trials included for this comparison did not report locoregional recurrence.
Distant metastasis
One trial (NSABP B‐04) that performed two treatment comparisons reported that the HR for distant metastasis for RT alone versus ALND alone was 1.07 (95% CI 0.93 to 1.25; 1313 participants; Analysis 4.3).
4.3. Analysis.
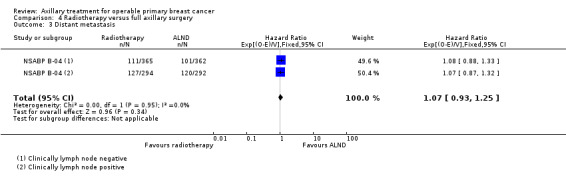
Comparison 4 Radiotherapy versus full axillary surgery, Outcome 3 Distant metastasis.
Long‐term adverse events
Lymphoedema
One trial (SE Scotland) reported lymphoedema at 12 or more months after treatment and used a definition of 2 cm or greater increase in arm circumference. In the RT group, 5 out of 100 participants had lymphoedema compared with 10 out of 100 in the axillary surgery group (OR 0.47, 95% CI 0.16 to 1.44; 200 participants; Analysis 4.4).
4.4. Analysis.
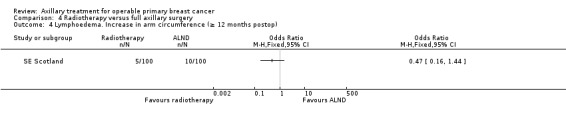
Comparison 4 Radiotherapy versus full axillary surgery, Outcome 4 Lymphoedema. Increase in arm circumference (≥ 12 months postop).
Short‐term adverse events
Delayed healing, wound infection and skin graft
One trial (SE Scotland) involving 200 participants reported that acute adverse events ‐ delayed healing (OR 0.24, 95% CI 0.10 to 0.55; Analysis 4.5), wound infection (OR 0.65, 95% CI 0.22 to 1.89; Analysis 4.6), skin graft (OR 0.04, 95% CI 0.00 to 0.74; Analysis 4.7) and haematoma (OR 0.20, 95% CI 0.08 to 0.52; Analysis 4.8) ‐ were less likely with radiotherapy than with axillary surgery.
4.5. Analysis.
Comparison 4 Radiotherapy versus full axillary surgery, Outcome 5 Delayed healing.
4.6. Analysis.
Comparison 4 Radiotherapy versus full axillary surgery, Outcome 6 Wound infection.
4.7. Analysis.
Comparison 4 Radiotherapy versus full axillary surgery, Outcome 7 Skin graft.
4.8. Analysis.
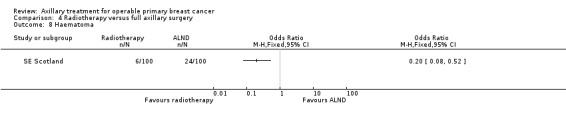
Comparison 4 Radiotherapy versus full axillary surgery, Outcome 8 Haematoma.
Quality of life
The trials included for this comparison did not report quality of life.
Psychological and psychosocial outcomes
The trials included for this comparison did not report psychological and psychosocial outcomes.
Less axillary surgery versus axillary lymph node dissection
Overall survival
When all trials were combined, the HR for overall mortality was 1.08 (95% CI 1.01 to 1.16, when HR > 1 favours ALND; 12,864 participants; 19 studies; Analysis 5.1) with no significant heterogeneity (I2 = 12%; P = 0.30).
Trials comparing no axillary surgery (with or without RT) versus ALND reported increased mortality with less axillary surgery (HR 1.10, 95% CI 1.02 to 1.19; 5545 participants; 13 studies; I2 = 6%; obtained by combining analyses 5.1.1 and 5.1.4 (not shown)), but trials comparing axillary sampling or SLNB versus ALND did not report increased mortality (HR 1.01, 95% CI 0.88 to 1.17; 7319 participants; six studies; obtained by combining analyses 5.1.2 and 5.1.3 (not shown)).
We performed subgroup analysis that was based on use of radiotherapy. Trials using RT in both treatment groups reported no difference in overall survival between less axillary surgery and more axillary surgery groups (HR 1.06, 95% CI 0.96 to 1.16; 10,075 participants; 13 studies; Analysis 5.2.1) with no important heterogeneity (I2 = 28%; P = 0.15). Similarly, results showed no differences between groups for trials that did not use RT in either group (HR 1.00, 95% CI 0.85 to 1.19; 1093 participants; three trials; Analysis 5.2.3) with no important heterogeneity (I2 = 8%; P = 0.34). Trials that used RT only in the less axillary surgery arm reported reduced overall survival for the less axillary surgery arm compared with the ALND arm (HR 1.10, 95% CI 1.00 to 1.21; 2469 participants; four trials; Analysis 5.2.2) with no heterogeneity (I2 = 0%; P = 0.52).
5.2. Analysis.
Comparison 5 Less surgery versus ALND, Outcome 2 All‐cause mortality (radiotherapy subgroups).
We conducted subgroup analysis according to whether additional treatment was given to participants with histologically positive nodes and excluded trials in which one of the treatment arms received no axillary staging. Trials that provided additional treatment to participants with histologically positive axillary nodes (E'dburgh Sample/Clear; Edinburgh 1; Genoa; Milan) reported uncertainty whether less axillary surgery was the more effective treatment in terms of overall survival (HR 0.82, 95% CI 0.64 to 1.05; 1613 participants; four trials; Analysis 5.3) with no heterogeneity (I2 = 0%; P=0.61). They also described uncertainty about relative effectiveness in the only trial (Cape Town) that did not provide additional treatment to those with histologically positive nodes (HR 1.47, 95% CI 0.84 to 2.56; 95 participants; Analysis 5.3).
5.3. Analysis.
Comparison 5 Less surgery versus ALND, Outcome 3 All‐cause mortality (additional treatment for histologically positive nodes).
Breast cancer recurrence
Local recurrence
Study results show uncertainty about whether local recurrence was reduced with less axillary surgery when compared with ALND (HR 0.90, 95% CI 0.75 to 1.09, when HR > 1 favours ALND; 24,176 participants; eight studies; Analysis 5.4).
5.4. Analysis.
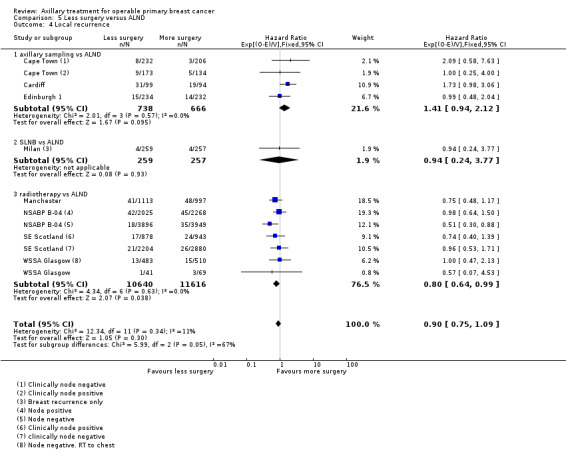
Comparison 5 Less surgery versus ALND, Outcome 4 Local recurrence.
Locoregional recurrence
Locoregional recurrence was more likely with less surgery than with ALND (HR 1.53, 95% CI 1.31 to 1.78, when HR > 1 favours ALND; 26,880 participant years of follow‐up; seven studies; Analysis 5.5).
5.5. Analysis.
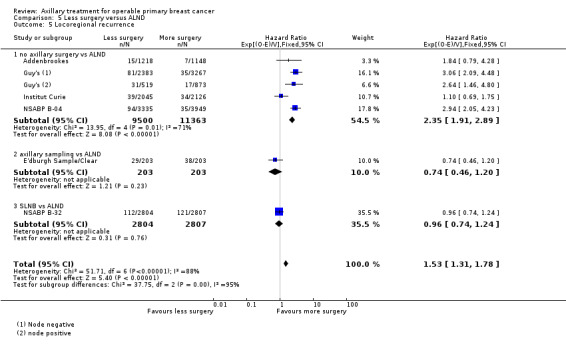
Comparison 5 Less surgery versus ALND, Outcome 5 Locoregional recurrence.
Distant metastasis
Results reveal uncertainty about whether distant metastasis was more likely in patients treated with less axillary surgery than in those receiving ALND (HR 1.07, 95% CI 0.95 to 1.20, when HR >1 favours ALND; 2665 participants; five studies; Analysis 5.6).
5.6. Analysis.
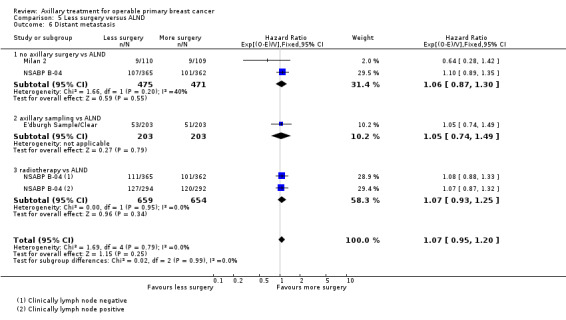
Comparison 5 Less surgery versus ALND, Outcome 6 Distant metastasis.
Long‐term adverse effects
Lymphoedema (defined as an increase in arm circumference at 12 or more months postoperatively) was less likely with less axillary surgery than with ALND (OR 0.37, 95% CI 0.29 to 0.46; fixed‐effect model; 3964 participants; nine studies; I2 = 52%; Analysis 5.7). The random‐effects model produced a similar result (OR 0.35, 95% CI 0.23 to 0.53; random‐effects model; 3964 participants; nine studies; I2 = 52%).
5.7. Analysis.
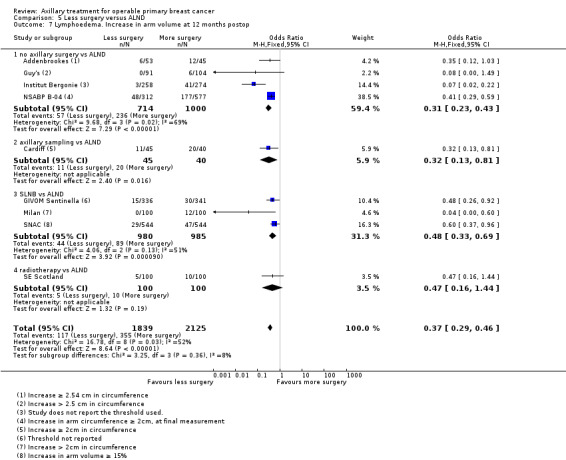
Comparison 5 Less surgery versus ALND, Outcome 7 Lymphoedema. Increase in arm volume at 12 months postop.
Paraesthesia
Three trials reported paraesthesia at 12 or more months after surgery (Institut Bergonie; Addenbrookes 2; Milan). All trials found paraesthesia less likely in the less axillary surgery group than in the more axillary surgery group. For Institut Bergonie (OR 0.14, 95% CI 0.06 to 0.32; 532 participants; Analysis 5.8), for Milan (OR < 0.00, 95% CI <0.00 to 0.04; 200 participants; Analysis 5.8) and for Addenbrookes 2 (OR 0.37, 95% CI 0.21 to 0.64; 295 participants; Analysis 5.8); heterogeneity was considerable in the pooled estimate (I2 = 91%; P < 0.0001).
5.8. Analysis.
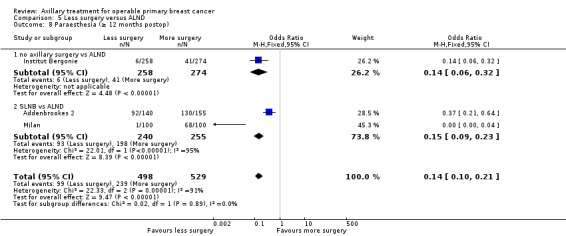
Comparison 5 Less surgery versus ALND, Outcome 8 Paraesthesia (≥ 12 months postop).
Pain
Three trials reported pain at 12 or more months after surgery (IBCSG‐10‐93; GIVOM Sentinella; Milan). Pain was less likely to be reported in the less surgery group than in the more surgery group. This difference was statistically significant in the Milan trial (OR 0.14, 95% CI 0.06 to 0.31; 200 participants; Analysis 5.9) but not in the GIVOM Sentinella trial (OR 0.76, 95% CI 0.46 to 1.25; 677 participants; Analysis 5.9) or the IBCSG‐10‐93 trial (OR 0.60 95% CI 0.24 to 1.47; 379 participants; Analysis 5.9), and heterogeneity was considerable in the pooled estimate (I2 = 84%; P < 0.0001).
5.9. Analysis.
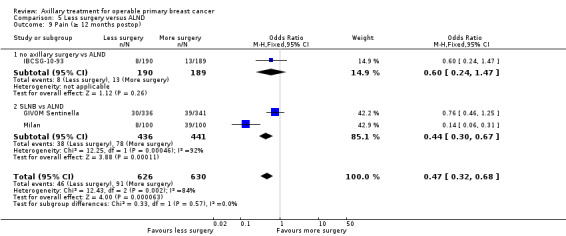
Comparison 5 Less surgery versus ALND, Outcome 9 Pain (≥ 12 months postop).
Short‐term side effects
Delayed healing
The Addenbrookes and SE Scotland trials reported delayed wound healing was less likely with less surgery than with more surgery (OR 0.25, 95% CI 0.13 to 0.46; 404 participants; fixed‐effect model; two studies; I2 = 0%; Analysis 5.10). The random‐effects model produced a similar result (OR 0.25, 95% CI 0.13 to 0.47; 404 participants; random‐effects model; two studies; I2 = 0%).
5.10. Analysis.
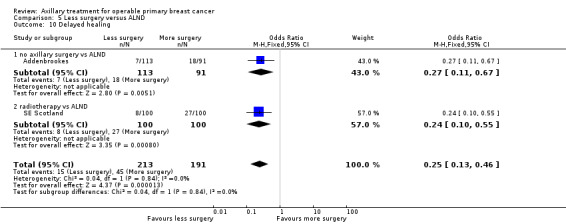
Comparison 5 Less surgery versus ALND, Outcome 10 Delayed healing.
Seroma
Seroma was less likely with less axillary surgery than with ALND (OR 0.40, 95% CI 0.32 to 0.52; 1481 participants; fixed‐effect model; three studies; I2 = 14%; Analysis 5.11). The random‐effects model produced a similar result (OR 0.42, 95% CI 0.31 to 0.56; 1481 participants; random‐effects model; three studies; I2 = 14%).
5.11. Analysis.
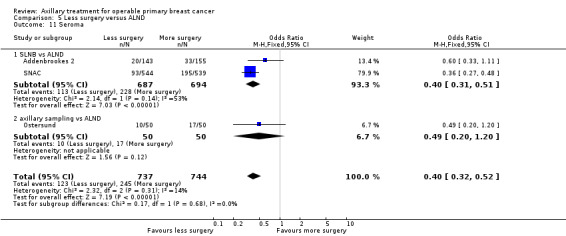
Comparison 5 Less surgery versus ALND, Outcome 11 Seroma.
Wound infection
Wound infection was less likely with less axillary surgery than with ALND (OR 0.65, 95% CI 0.50 to 0.84; fixed‐effect model; 2274 participants; three studies; I2 = 0%; Analysis 5.12). The random‐effects model yielded the same result.
5.12. Analysis.
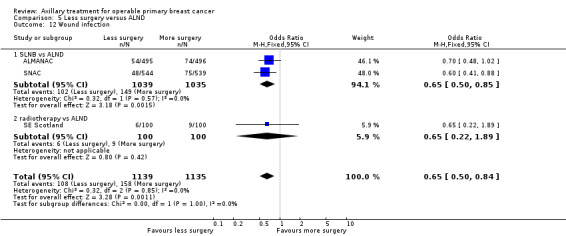
Comparison 5 Less surgery versus ALND, Outcome 12 Wound infection.
Skin graft
Data reveal uncertainty about whether skin graft was less likely with less axillary surgery than with ALND (OR 0.15, 95% CI 0.04 to 0.57; fixed‐effect model; 404 participants; two studies; I2 = 49%; Analysis 5.13). The random‐effects model suggested that skin graft was less likely with less axillary surgery than with ALND (OR 0.17, 95% CI 0.02 to 1.64; random‐effects model; 404 participants; two studies; I2 = 49%).
5.13. Analysis.
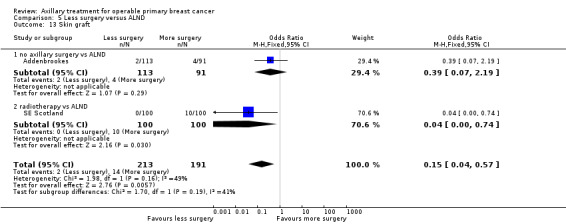
Comparison 5 Less surgery versus ALND, Outcome 13 Skin graft.
Haematoma
The SNAC and SE Scotland trials reported haematoma. In the SNAC trial there were similar rates of haematoma in the less surgery group than more surgery group (OR 1.27, 95% CI 0.78 to 2.09; 1083 participants; Analysis 5.14). In the SE Scotland trial haematoma was less likely in the less surgery group than the more surgery group (OR 0.20, 95% CI 0.08 to 0.52; 200 participants; Analysis 5.14. There was considerable heterogeneity in the pooled estimate (I2 = 91%; P = 0.0007).
5.14. Analysis.
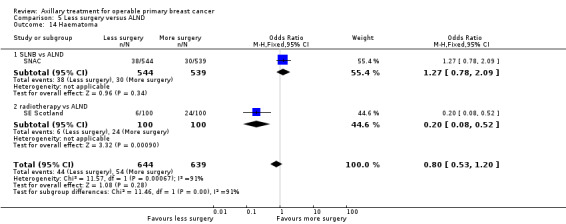
Comparison 5 Less surgery versus ALND, Outcome 14 Haematoma.
Quality of life, psychological and psychosocial outcomes
Only trials comparing SLND versus ALND reported these outcomes, so we could perform no additional analyses.
Discussion
Summary of main results
Risk of overall mortality was not increased when participants were treated with axillary sampling or sentinel lymph node biopsy (SLNB) versus axillary lymph node dissection (ALND). Treatment omitting all axillary surgery was associated with increased risk of overall mortality compared with ALND, but this was noted only in trials comparing radiotherapy (RT) alone versus ALND.
Axillary lymph node dissection was associated with increased risk of lymphoedema and surgical adverse events compared with less axillary surgery.
Overall completeness and applicability of evidence
We found no trials that performed the following comparisons: sentinel node biopsy versus axillary sampling, no axillary surgery versus axillary sampling and no axillary surgery versus sentinel node biopsy.
Adverse event data were limited, particularly for older trials comparing no surgery, RT or axillary sampling versus ALND. Quality of life data were limited to three trials. Sentinel lymph node trials provided limited data on long‐term overall survival and breast cancer recurrence; these trials were often designed to compare quality of life and adverse effects. Substantial heterogeneity in adverse event trial results was often due to differences among adverse event definitions between trials.
Some trials reported data in a way that precluded inclusion in the time‐to‐event meta‐analysis, and although we contacted study authors, we obtained no additional data.
Applicability of some of the comparisons in this review to current breast cancer practice is questionable, particularly for comparisons involving no axillary surgery. Use of adjuvant therapies differs between current practice and many of the included trials ‐ more effective adjuvant systemic therapies are available today. Similarly, RT regimens used in the older trials are most likely less effective and are associated with more side effects:
Patients with breast cancer today are likely to differ from those who participated in older trials, and breast cancer is more likely to be detected at an earlier stage.
Quality of the evidence
The included studies were at low or unclear risk of selection bias. Selection bias was typically unclear because trial publications did not fully report methods of random sequence generation or allocation concealment used and study authors did not reply when we contacted them to request additional information about conduct of the trial. We performed sensitivity analyses for trials with adequate allocation concealment and found that these results generally were consistent with findings of the main analyses.
Risk of attrition bias tended to be lower for survival and for breast cancer recurrence than for adverse events. This sometimes occurred because adverse event assessments were done for a subset of the trial population. This subgroup of participants assessed for adverse events could be systematically different from the trial population as a whole, especially in the case of assessment for long‐term adverse events when patients may have died or may have been too sick to participate.
The included trials did not include blinding (and it was probably infeasible), but this was considered a source of bias only for outcomes with potential subjectivity in measurement (i.e. breast cancer recurrence and adverse events). Detection bias could lead to overestimation of adverse events in patients with more extensive axillary surgery. Similarly, patients receiving less extensive axillary surgery could be checked more carefully for breast cancer recurrence.
For these reasons, we downgraded the quality of the evidence for adverse effects (Table 1; Table 2; Table 3; Table 4).
Potential biases in the review process
The meta‐analyses of time‐to‐event outcomes conducted for this review used the fixed‐effect model because only fixed‐effect meta‐analytical methods are available in RevMan for ‘O–E' and 'Variance’ outcomes. This could affect interpretation of results by yielding narrower confidence intervals for the pooled hazard ratio in the presence of heterogeneity than would be obtained with a random‐effects model. This is particularly the case for Analysis 5.1 (which compares overall survival with more surgery vs less surgery), in which the underlying assumption of the fixed‐effect model is unlikely to be true, given the different types of interventions and patient populations included.
Agreements and disagreements with other studies or reviews
Kell 2010 reported a meta‐analysis of seven trials of SLNB versus axillary clearance (Addenbrookes 2; ALMANAC; GIVOM Sentinella; Milan; SNAC; ACOSOG Z0011; NSABP B‐32). Compared with axillary clearance, SLNB was associated with reduced risk of postoperative wound infection (odds ratio (OR) 0.58, 95% confidence interval (CI) 0.42 to 0.80), of postoperative seroma (OR 0.40, 95% CI 0.31 to 0.51) and of arm swelling at six months postoperatively (OR 0.30, 95% CI 0.14 to 0.66). These results are consistent with findings of the current review.
Wang 2011 also analysed trials examining the sentinel lymph node versus axillary clearance (Addenbrookes 2; ALMANAC; GIVOM Sentinella; Genoa; Milan; SNAC; ACOSOG Z0011; NSABP B‐32). Comparison of SLNB with ALND revealed no statistically significant difference in overall survival (hazard ratio (HR) 1.07, 95% CI 0.90 to 1.27) or regional lymph node recurrence (OR 1.65, 95% CI 0.77 to 3.56). Postoperative complications were less likely with SLNB than with ALND, including lymphoedema (OR 0.24, 95% CI 0.11 to 0.53), numbness (OR 0.19, 95% CI 0.11 to 0.33), infection (OR 0.50, 95% CI 0.36 to 0.70) and seroma (OR 0.39, 95% CI 0.31 to 0.49). These results are consistent with findings of the current review.
In the Early Breast Cancer Trialists Group, meta‐analysis of individual participant data (Clarke 2005) revealed that axillary clearance versus effective axillary RT involved little absolute difference (< 10%) in five‐year risk of local recurrence, as well as little difference in breast cancer mortality (when combined with other local treatment comparisons). The current review observed an increase in overall mortality with RT with no axillary surgery compared with axillary clearance, but the absolute difference at five years was on the order of a few percent (Table 4), and had a random‐effects model been possible, greater uncertainty would surround this estimate.
Authors' conclusions
Implications for practice.
This review confirms the evidence base for the current widespread approach to staging of disease and treatment of the axilla in patients with operable early breast cancer. Evidence showing a small but significant survival benefit with ALND (when compared with no axillary surgery) and the impact that this procedure has on systemic therapy planning and provision of prognostic information is balanced against increased incidence of harmful side effects, particularly lymphoedema. Full axillary clearance of the clinically and radiologically uninvolved axilla is no longer considered acceptable practice. In the absence of any direct comparisons, both sentinel node biopsy and axillary node sampling are considered appropriate choices for axillary staging followed by treatment with surgery or RT.
Implications for research.
Emerging evidence (ACOSOG Z0011) suggests that overall survival is not improved by further surgical lymph node clearance of the axilla in a subset of patients undergoing breast conservation with surgery and RT to the breast, and systemic therapy has resulted in revised American Society of Clinical Oncology (ASCO) guidelines pertaining to treatment when one or two sentinel nodes contain metastases (Lyman 2014). These guidelines state that women without sentinel lymph node metastases should not undergo ALND, and that most women with one to two metastatic sentinel lymph nodes planning to receive breast‐conserving surgery with whole breast RT should not undergo ALND. However, evidence from ACOSOG Z0011 has not yet resulted in a widespread change in practice outside the USA. Further evidence is required to confirm this finding – trials are under way (e.g. Goyal 2014) to address some of the issues raised by ACOSOG Z0011 (such as inclusion of patients with micrometastases and exclusion of patients undergoing mastectomy) and will be included in future reviews.
What's new
| Date | Event | Description |
|---|---|---|
| 29 May 2019 | Review declared as stable | This review entails a historical overview of the management of axillary nodes. In the last decade, practice has changed and questions now exist on the management of positive sentinel lymph node, omission of sentinel lymph node biopsy when preoperative nodal imaging is negative, or omission of sentinel lymph node biopsy in neoadjuvant therapy. Therefore new Cochrane review topics will reflect these current questions |
History
Protocol first published: Issue 2, 2004 Review first published: Issue 1, 2017
| Date | Event | Description |
|---|---|---|
| 28 September 2017 | Amended | Data from the NSABP B‐04 study (specifically on women who were node‐negative) were added to Analysis 5.1 (subgroup 5.1.4). This addition did not change the results of this review |
| 24 February 2009 | Amended | Changed from protocol to full review |
| 15 October 2008 | Amended | Converted to new review format |
Notes
We have added a new review author, Eifiona Wood, to the protocol (10/05/2004). We have added a new comparison to the protocol along with the following text added to the section titled "Criteria for considering studies for this review" (10/05/2004). 7) Full axillary surgery with no radiotherapy versus no axillary surgery with radiotherapy. No subgroups. We added comparison '7' to the original protocol in response to retrieval of large numbers of trial reports pertaining to this question. The review authors recognise that, unlike comparisons 1 through 6, comparison 7 does not address the effectiveness of axillary surgery. A regimen in comparison 1 ‐ full axillary surgery plus radiotherapy ‐ was standard practice but has been largely discontinued because of the illogic of irradiating the axilla subsequent to removal of the lymph nodes. The regimen in comparison 7 ‐ no axillary surgery with radiotherapy ‐ reflects more current practice; although it is considered irrelevant to a younger, fitter population, some clinicians still consider it a viable treatment option for older women.
Acknowledgements
Professor RE Coleman, University of Sheffield, UK, and the North Trent Cancer Research Network provided advice and support. We thank Andrew Cleves for his input and contributions. We also thank Lixin Ma (School of Public Health, Hebei University, China) for assistance provided in translating a Chinese paper, and Dr Liliya‐Eugenevna Ziganshina (Department of Basic and Clinical Pharmacology, Kazan Federal University, Russian Federation, and The Nordic Cochrane Centre) for assistance provided in translating two Russian papers.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Breast Neoplasms] explode all trees #2 breast near cancer* #3 breast near neoplasm* #4 breast near carcinoma* #5 breast near tumour* #6 breast near tumor* #7 #1 or #2 or #3 or #4 or #5 or #6 #8 MeSH descriptor: [Sentinel Lymph Node Biopsy] explode all trees #9 sentinel lymph node biopsy or SLNB or SNB or SLN or (sentinel near node) #10 MeSH descriptor: [Axilla] explode all trees #11 axilla* near (surg* or sampl* or stag*) #12 MeSH descriptor: [Neoplasm Staging] explode all trees #13 MeSH descriptor: [Lymph Node Excision] explode all trees #14 lymphadenectomy #15 (block or lymph node or axillary) near dissection #16 (block or lymph node or axillary) near clearance #17 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 #18 #7 and #17
Appendix 2. MEDLINE search strategy
MEDLINE via OVIDSp
1 exp Breast Neoplasms/
2 exp "Neoplasms, Ductal, Lobular, and Medullary"/
3 exp Fibrocystic Breast Disease/
4 or/1‐3
5 exp Breast/
6 breast.tw.
7 5 or 6
8 (breast adj milk).ti,ab,sh.
9 (breast adj tender$).ti,ab,sh.
10 8 or 9
11 7 not 10
12 exp Neoplasms/
13 11 and 12
14 exp Lymphedema/
15 14 and 11
16 (breast adj25 neoplasm$).ti,ab,sh.
17 (breast adj25 cancer$).ti,ab,sh.
18 (breast adj25 tumour$).ti,ab,sh.
19 (breast adj25 tumor$).ti,ab,sh.
20 (breast adj25 carcinoma$).ti,ab,sh.
21 (breast adj25 adenocarcinoma$).ti,ab,sh.
22 (breast adj25 sarcoma$).ti,ab,sh.
23 (breast adj50 dcis).ti,ab,sh.
24 (breast adj25 ductal).ti,ab,sh.
25 (breast adj25 infiltrating).ti,ab,sh.
26 (breast adj25 intraductal).ti,ab,sh.
27 (breast adj25 lobular).ti,ab,sh.
28 (breast adj25 medullary).ti,ab,sh.
29 or/16‐28
30 4 or 13 or 15 or 29
31 exp Mastectomy/
32 30 or 31
33 (mammary adj25 neoplasm$).ti,ab,sh.
34 (mammary adj25 cancer$).ti,ab,sh.
35 (mammary adj25 tumour$).ti,ab,sh.
36 (mammary adj25 tumor$).ti,ab,sh.
37 (mammary adj25 carcinoma$).ti,ab,sh.
38 (mammary adj25 adenocarcinoma$).ti,ab,sh.
39 (mammary adj25 sarcoma$).ti,ab,sh.
40 (mammary adj50 dcis).ti,ab,sh.
41 (mammary adj25 ductal).ti,ab,sh.
42 (mammary adj25 infiltrating).ti,ab,sh.
43 (mammary adj25 intraductal).ti,ab,sh.
44 (mammary adj25 lobular).ti,ab,sh.
45 (mammary adj25 medullary).ti,ab,sh.
46 or/33‐45
47 32 or 46
48 exp Breast Self‐Examination/
49 (breast adj25 self$).ti,ab,sh.
50 (breast adj25 screen$).ti,ab,sh.
51 exp Mammography/
52 or/47‐51
53 mammograph$.tw.
54 53 and 11
55 52 or 54
56 randomized controlled trial.pt.
57 controlled clinical trial.pt.
58 randomized controlled trials.sh.
59 random allocation.sh.
60 double‐blind method.sh.
61 single‐blind method.sh.
62 or/56‐61
63 clinical trial.pt.
64 exp Clinical Trials/
65 (clin$ adj25 trial$).ti,ab.
66 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab.
67 placebos.sh.
68 placebo$.ti,ab.
69 random$.ti,ab.
70 research design.sh.
71 or/63‐70
72 62 or 71
73 55 and 72
74 (animals not humans).sh.
75 73 not 74
76 exp Sentinel Lymph Node Biopsy/
77 (sentinel adj2 node).mp.
78 (SN or SNB or SLN or SLNB).mp.
79 exp Axilla/
80 exp Neoplasm Staging/
81 exp Lymph Node Excision/
82 lymphadenectomy.mp.
83 (axill$ adj3 (surg$ or sampl$ or stag$)).mp.
84 ((block or lymph node or axillary) adj dissection).mp.
85 ((block or lymph node or axillary) adj clearance).mp.
86 or/76‐85
87 75 and 86
Appendix 3. WHO ICTRP search strategy
Basic search
1. Axillary staging for operable primary breast cancer 2. Breast cancer AND (axillary sampling OR axillary staging OR axillary surgery OR sentinel node biopsy OR sentinel lymph node biopsy)
Advanced search
1. Title: Axillary staging for operable primary breast cancer Recruitment status: ALL
2. Condition: Breast cancer Intervention: axillary sampling OR axillary staging OR axillary surgery OR sentinel node biopsy OR sentinel lymph node biopsy Recruitment status: ALL
Appendix 4. ClinicalTrials.gov search strategy
Basic search
1. Axillary staging for operable primary breast cancer 2. Breast cancer AND (axillary sampling OR axillary staging OR axillary surgery OR sentinel node biopsy OR sentinel lymph node biopsy)
Advanced search
1. Search terms: Axillary staging for operable primary breast cancer Recruitment: all studies Study results: all studies Study type: all studies Gender: all studies
2. Conditions: breast cancer Interventions: axillary sampling OR axillary staging OR axillary surgery OR sentinel node biopsy OR sentinel lymph node biopsy Recruitment: all studies Study results: all studies Study type: all studies Gender: all studies
Data and analyses
Comparison 1. No axillary surgery versus full axillary surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality (radiotherapy subgroups) | 10 | 3849 | Hazard Ratio (95% CI) | 1.06 [0.96, 1.17] |
| 1.1 no radiotherapy | 1 | 773 | Hazard Ratio (95% CI) | 0.96 [0.80, 1.15] |
| 1.2 radiotherapy | 9 | 3076 | Hazard Ratio (95% CI) | 1.11 [0.98, 1.25] |
| 2 All‐cause mortality (extra treatment for positive node subgroups) | 10 | 3849 | Hazard Ratio (95% CI) | 1.06 [0.96, 1.17] |
| 2.1 additional treatment for node‐positive patients | 3 | 1174 | Hazard Ratio (95% CI) | 1.51 [1.09, 2.09] |
| 2.2 no specific additional treatment for node‐positive patients | 7 | 2675 | Hazard Ratio (95% CI) | 1.02 [0.92, 1.13] |
| 3 Locoregional recurrence (radiotherapy subgroups) | 4 | 20863 | Hazard Ratio (95% CI) | 2.35 [1.91, 2.89] |
| 3.1 no radiotherapy | 1 | 7284 | Hazard Ratio (95% CI) | 2.94 [2.05, 4.23] |
| 3.2 radiotherapy | 3 | 13579 | Hazard Ratio (95% CI) | 2.11 [1.64, 2.72] |
| 4 Locoregional recurrence (extra treatment for positive‐node subgroups) | 4 | 20863 | Hazard Ratio (95% CI) | 2.35 [1.91, 2.89] |
| 4.1 additional treatment for node‐positive patients | 1 | 4171 | Hazard Ratio (95% CI) | 1.10 [0.69, 1.75] |
| 4.2 no specific additional treatment for node‐positive patients | 3 | 16692 | Hazard Ratio (95% CI) | 2.83 [2.25, 3.57] |
| 5 Distant metastasis | 2 | 946 | Hazard Ratio (95% CI) | 1.06 [0.87, 1.30] |
| 5.1 no radiotherapy | 1 | 727 | Hazard Ratio (95% CI) | 1.10 [0.89, 1.35] |
| 5.2 radiotherapy | 1 | 219 | Hazard Ratio (95% CI) | 0.64 [0.28, 1.42] |
| 6 Lymphoedema (≥ 12 months postop) ‐ fixed‐effect model | 4 | 1714 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.23, 0.43] |
| 6.1 additional treatment for node‐positive patients | 1 | 532 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.22] |
| 6.2 no additional treatment for node‐positive patients | 3 | 1182 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.28, 0.54] |
| 7 Lymphoedema (≥ 12 months postop) ‐ random‐effects model | 4 | 1714 | Odds Ratio (M‐H, Random, 95% CI) | 0.22 [0.08, 0.57] |
| 7.1 additional treatment for node‐positive patients | 1 | 532 | Odds Ratio (M‐H, Random, 95% CI) | 0.07 [0.02, 0.22] |
| 7.2 no additional treatment for node‐positive patients | 3 | 1182 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.28, 0.55] |
| 8 Arm or shoulder movement impairment (≥ 12 months postop) | 5 | 1495 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.49, 1.05] |
| 8.1 radiotherapy | 5 | 1495 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.49, 1.05] |
| 9 Pain (≥ 12 months postop) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 radiotherapy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Paraesthesia (≥ 12 months postop) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1 radiotherapy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Delayed healing | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.1 radiotherapy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Skin graft | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 12.1 radiotherapy | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 All‐cause mortality (allocation concealment subgroups) | 10 | 3849 | Hazard Ratio (95% CI) | 1.06 [0.96, 1.17] |
| 13.1 adequate allocation concealment | 4 | 1442 | Hazard Ratio (95% CI) | 0.98 [0.81, 1.18] |
| 13.2 unclear or inadequate allocation concealment | 6 | 2407 | Hazard Ratio (95% CI) | 1.09 [0.97, 1.23] |
Comparison 2. Axillary sampling versus full axillary surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 3 | 967 | Hazard Ratio (95% CI) | 0.94 [0.73, 1.21] |
| 1.1 radiotherapy | 2 | 872 | Hazard Ratio (95% CI) | 0.84 [0.64, 1.11] |
| 1.2 no radiotherapy | 1 | 95 | Hazard Ratio (95% CI) | 1.47 [0.84, 2.56] |
| 2 Local recurrence | 3 | 1404 | Hazard Ratio (95% CI) | 1.41 [0.94, 2.12] |
| 2.1 radiotherapy | 2 | 659 | Hazard Ratio (95% CI) | 1.40 [0.89, 2.19] |
| 2.2 no radiotherapy | 1 | 745 | Hazard Ratio (95% CI) | 1.48 [0.58, 3.82] |
| 3 Axillary recurrence | 1 | Hazard Ratio (95% CI) | Totals not selected | |
| 4 Locoregional recurrence | 1 | Hazard Ratio (95% CI) | Totals not selected | |
| 4.1 radiotherapy | 1 | Hazard Ratio (95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 no radiotherapy | 0 | Hazard Ratio (95% CI) | 0.0 [0.0, 0.0] | |
| 5 Distant metastasis | 1 | Hazard Ratio (95% CI) | Totals not selected | |
| 5.1 radiotherapy | 1 | Hazard Ratio (95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 no radiotherapy | 0 | Hazard Ratio (95% CI) | 0.0 [0.0, 0.0] | |
| 6 Lymphoedema. Increase in arm circumference (≥ 12 months postop) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 radiotherapy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Shoulder lateral rotation (12 months postop) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 radiotherapy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Seroma | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 radiotherapy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Sentinel node biopsy versus full axillary surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 3 | 6352 | Hazard Ratio (95% CI) | 1.05 [0.89, 1.25] |
| 1.1 radiotherapy | 2 | 6127 | Hazard Ratio (95% CI) | 1.05 [0.88, 1.25] |
| 1.2 no radiotherapy | 1 | 225 | Hazard Ratio (95% CI) | 1.30 [0.35, 4.84] |
| 2 Local recurrence | 1 | Hazard Ratio (95% CI) | Totals not selected | |
| 2.1 radiotherapy | 1 | Hazard Ratio (95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 no radiotherapy | 0 | Hazard Ratio (95% CI) | 0.0 [0.0, 0.0] | |
| 3 Axillary recurrence | 1 | Hazard Ratio (95% CI) | Totals not selected | |
| 3.1 radiotherapy | 1 | Hazard Ratio (95% CI) | 0.0 [0.0, 0.0] | |
| 4 Locoregional recurrence | 1 | Hazard Ratio (95% CI) | Totals not selected | |
| 4.1 radiotherapy | 1 | Hazard Ratio (95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 no radiotherapy | 0 | Hazard Ratio (95% CI) | 0.0 [0.0, 0.0] | |
| 5 Distant metastasis | 1 | Hazard Ratio (95% CI) | Totals not selected | |
| 5.1 radiotherapy | 1 | Hazard Ratio (95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 no radiotherapy | 0 | Hazard Ratio (95% CI) | 0.0 [0.0, 0.0] | |
| 6 Lymphoedema. Increase in arm circumference (≥ 12 months postop) | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 radiotherapy | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Lymphoedema. Patient reported (at 12 or more months postop) | 3 | Odds Ratio (Fixed, 95% CI) | 0.33 [0.23, 0.47] | |
| 7.1 adequate allocation concealment | 2 | Odds Ratio (Fixed, 95% CI) | 0.33 [0.22, 0.48] | |
| 7.2 unclear allocation concealment | 1 | Odds Ratio (Fixed, 95% CI) | 0.36 [0.15, 0.86] | |
| 8 Shoulder flexion (12 months postop) | 3 | 2257 | Mean Difference (IV, Fixed, 95% CI) | 1.55 [‐0.19, 3.29] |
| 8.1 radiotherapy | 3 | 2257 | Mean Difference (IV, Fixed, 95% CI) | 1.55 [‐0.19, 3.29] |
| 9 Shoulder abduction (12 months postop) | 3 | 2252 | Mean Difference (IV, Fixed, 95% CI) | ‐1.02 [‐2.79, 0.75] |
| 9.1 radiotherapy | 3 | 2252 | Mean Difference (IV, Fixed, 95% CI) | ‐1.02 [‐2.79, 0.75] |
| 10 Shoulder internal rotation (12 months postop) | 2 | 1227 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐1.10, 2.09] |
| 10.1 radiotherapy | 2 | 1227 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐1.10, 2.09] |
| 11 Shoulder external rotation (12 months postop) | 2 | 1227 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐2.21, 1.09] |
| 11.1 radiotherapy | 2 | 1227 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐2.21, 1.09] |
| 12 Subjective arm movement impairment (≥ 12 months postop) | 2 | 877 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.22, 0.67] |
| 12.1 radiotherapy | 2 | 877 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.22, 0.67] |
| 13 Pain (≥ 12 months postop) | 2 | 877 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.30, 0.67] |
| 13.1 radiotherapy | 2 | 877 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.30, 0.67] |
| 14 Paraesthesia (≥ 12 months postop) | 2 | 495 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.09, 0.23] |
| 14.1 radiotherapy | 2 | 495 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.09, 0.23] |
| 15 Numbness (≥ 12 months postop) | 3 | 1799 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.34, 0.54] |
| 15.1 radiotherapy | 3 | 1799 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.34, 0.54] |
| 16 Seroma | 2 | 1381 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.31, 0.51] |
| 16.1 radiotherapy | 2 | 1381 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.31, 0.51] |
| 17 Wound infection | 2 | 2074 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.50, 0.85] |
| 17.1 radiotherapy | 2 | 2074 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.50, 0.85] |
| 18 Brachial plexus injury at 6 months postop | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 18.1 radiotherapy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.18. Analysis.
Comparison 3 Sentinel node biopsy versus full axillary surgery, Outcome 18 Brachial plexus injury at 6 months postop.
Comparison 4. Radiotherapy versus full axillary surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 4 | 2469 | Hazard Ratio (95% CI) | 1.10 [1.00, 1.21] |
| 2 Local recurrence | 4 | 22256 | Hazard Ratio (95% CI) | 0.80 [0.64, 0.99] |
| 3 Distant metastasis | 1 | 1313 | Hazard Ratio (95% CI) | 1.07 [0.93, 1.25] |
| 4 Lymphoedema. Increase in arm circumference (≥ 12 months postop) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Delayed healing | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Wound infection | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Skin graft | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Haematoma | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. Less surgery versus ALND.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 19 | 12864 | Hazard Ratio (95% CI) | 1.08 [1.01, 1.16] |
| 1.1 no axillary surgery vs ALND | 9 | 3076 | Hazard Ratio (95% CI) | 1.11 [0.98, 1.25] |
| 1.2 axillary sampling vs ALND | 3 | 967 | Hazard Ratio (95% CI) | 0.94 [0.73, 1.21] |
| 1.3 SLNB vs ALND | 3 | 6352 | Hazard Ratio (95% CI) | 1.05 [0.89, 1.25] |
| 1.4 radiotherapy vs ALND | 4 | 2469 | Hazard Ratio (95% CI) | 1.10 [1.00, 1.21] |
| 2 All‐cause mortality (radiotherapy subgroups) | 19 | 13637 | Hazard Ratio (95% CI) | 1.07 [1.00, 1.14] |
| 2.1 radiotherapy (same in both groups) | 13 | 10075 | Hazard Ratio (95% CI) | 1.06 [0.96, 1.16] |
| 2.2 radiotherapy (in less surgery group only) | 4 | 2469 | Hazard Ratio (95% CI) | 1.10 [1.00, 1.21] |
| 2.3 no radiotherapy | 3 | 1093 | Hazard Ratio (95% CI) | 1.00 [0.85, 1.19] |
| 3 All‐cause mortality (additional treatment for histologically positive nodes) | 5 | 1708 | Hazard Ratio (95% CI) | 0.90 [0.72, 1.14] |
| 3.1 additional treatment for histologically positive nodes | 4 | 1613 | Hazard Ratio (95% CI) | 0.82 [0.64, 1.05] |
| 3.2 no additional treatment for histologically positive nodes | 1 | 95 | Hazard Ratio (95% CI) | 1.47 [0.84, 2.56] |
| 4 Local recurrence | 8 | 24176 | Hazard Ratio (95% CI) | 0.90 [0.75, 1.09] |
| 4.1 axillary sampling vs ALND | 3 | 1404 | Hazard Ratio (95% CI) | 1.41 [0.94, 2.12] |
| 4.2 SLNB vs ALND | 1 | 516 | Hazard Ratio (95% CI) | 0.94 [0.24, 3.77] |
| 4.3 radiotherapy vs ALND | 4 | 22256 | Hazard Ratio (95% CI) | 0.80 [0.64, 0.99] |
| 5 Locoregional recurrence | 6 | 26880 | Hazard Ratio (95% CI) | 1.53 [1.31, 1.78] |
| 5.1 no axillary surgery vs ALND | 4 | 20863 | Hazard Ratio (95% CI) | 2.35 [1.91, 2.89] |
| 5.2 axillary sampling vs ALND | 1 | 406 | Hazard Ratio (95% CI) | 0.74 [0.46, 1.20] |
| 5.3 SLNB vs ALND | 1 | 5611 | Hazard Ratio (95% CI) | 0.96 [0.74, 1.24] |
| 6 Distant metastasis | 3 | 2665 | Hazard Ratio (95% CI) | 1.07 [0.95, 1.20] |
| 6.1 no axillary surgery vs ALND | 2 | 946 | Hazard Ratio (95% CI) | 1.06 [0.87, 1.30] |
| 6.2 axillary sampling vs ALND | 1 | 406 | Hazard Ratio (95% CI) | 1.05 [0.74, 1.49] |
| 6.3 radiotherapy vs ALND | 1 | 1313 | Hazard Ratio (95% CI) | 1.07 [0.93, 1.25] |
| 7 Lymphoedema. Increase in arm volume at 12 months postop | 9 | 3964 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.29, 0.46] |
| 7.1 no axillary surgery vs ALND | 4 | 1714 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.23, 0.43] |
| 7.2 axillary sampling vs ALND | 1 | 85 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.13, 0.81] |
| 7.3 SLNB vs ALND | 3 | 1965 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.33, 0.69] |
| 7.4 radiotherapy vs ALND | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.16, 1.44] |
| 8 Paraesthesia (≥ 12 months postop) | 3 | 1027 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.10, 0.21] |
| 8.1 no axillary surgery vs ALND | 1 | 532 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.06, 0.32] |
| 8.2 SLNB vs ALND | 2 | 495 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.09, 0.23] |
| 9 Pain (≥ 12 months postop) | 3 | 1256 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.32, 0.68] |
| 9.1 no axillary surgery vs ALND | 1 | 379 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.24, 1.47] |
| 9.2 SLNB vs ALND | 2 | 877 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.30, 0.67] |
| 10 Delayed healing | 2 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.13, 0.46] |
| 10.1 no axillary surgery vs ALND | 1 | 204 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.11, 0.67] |
| 10.2 radiotherapy vs ALND | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.10, 0.55] |
| 11 Seroma | 3 | 1481 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.32, 0.52] |
| 11.1 SLNB vs ALND | 2 | 1381 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.31, 0.51] |
| 11.2 axillary sampling vs ALND | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.20, 1.20] |
| 12 Wound infection | 3 | 2274 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.50, 0.84] |
| 12.1 SLNB vs ALND | 2 | 2074 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.50, 0.85] |
| 12.2 radiotherapy vs ALND | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.22, 1.89] |
| 13 Skin graft | 2 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.04, 0.57] |
| 13.1 no axillary surgery vs ALND | 1 | 204 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.07, 2.19] |
| 13.2 radiotherapy vs ALND | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.74] |
| 14 Haematoma | 2 | 1283 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.53, 1.20] |
| 14.1 SLNB vs ALND | 1 | 1083 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.78, 2.09] |
| 14.2 radiotherapy vs ALND | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.08, 0.52] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Addenbrookes.
| Methods |
Study design: RCT Country: UK Study period: 1958‐1965 Inclusion criteria: clinical stage II breast cancer (a tumour of any size but confined to the breast tissue with mobile axillary nodes present on the same side, no skin infiltration or muscle involvement) and judged by the surgeon to be suitable for treatment allocation including postoperative radiotherapy Exclusion criteria: none listed, but some patients were excluded owing to age, poor general condition or the surgeon’s opinion that their tumour was unsuitable for treatments provided in the trial Length of follow up: 5‐12 years |
|
| Participants |
No. in trial arms: simple: N = 113; ALND: N = 91 Age: simple: mean = 54 years; ALND: mean = 54 years Stage distribution: stage II (entry requirement) Proportion node positive: simple: 47/113 (42/113 were negative and 24/113 were nil – no node histopathology – possibly because no nodes were removed); ALND: 51/91 (39/91 were negative and 1/91 was nil – no node histopathology) Pathological type of breast cancer: not reported |
|
| Interventions | Modified simple mastectomy (removal of breast tissue without removal of the pectoral muscle. This might include removal of accessible axillary glands with no block dissection of the axilla) + x‐ray therapy vs radical mastectomy (removal of breast tissue and sternal head of the pectoralis‐major muscle and the pectoralis‐minor muscle, together with block dissection of the axilla. The surgeon might remove the internal mammary nodes if he wished) + x‐ray therapy | |
| Outcomes | Survival, recurrence‐free survival, oedema of the arm, shoulder stiffness, skin graft, delayed healing | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: no minimum for the simple mastectomy arm – accessible nodes were optionally removed, and some participants had no nodes removed for histopathology Nodes removed radical mastectomy arm: not reported Nodes removed simple mastectomy arm: not reported Method of node pathological analysis: not reported Further treatment for node‐positive cases: no |
|
| Radiotherapy |
Both arms: X‐ray therapy was administered as soon after surgery as possible, typically within 3‐4 weeks. Two 30 × 10 cm longitudinal fields were used to treat the whole pectoral area, axilla and supraclavicular and internal‐mammary‐node regions in a single block. Bolus was used and a minimum tumour dose of 3250r was given, during an overall time of 18 days, by means of 250 kV rays of h.v.l. 2.7 mm Cu. If wide separation of the fields was necessary, an extra direct field was used to build up the dose centrally and over the supraclavicular area RT same in all trial arms? yes |
|
| Hormone and chemotherapy | Both arms: no details reported | |
| Notes | N = ≥ 3 ALND patients had tumours > 5 cm in diameter (i.e. stage III by the 1961 international scheme of clinical staging). Baseline differences? ALND group included a larger proportion with inner quadrant tumours. Intention to treat analyses? No details were provided, and for long‐term adverse events, data are missing from N = 106 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Once entered into the trial, the drawing of an odd or even number from a random number table decided the type of treatment. This procedure was performed by personnel who were not in any way concerned with clinical examination or treatment of participants. |
| Allocation concealment (selection bias) | Low risk | See cell above. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | No details were provided. Outcome might have been affected by blinding. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | No details were provided. Outcome might have been affected by blinding. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | No details were provided. Outcome might have been affected by blinding. |
| Incomplete outcome data (attrition bias) Survival | Unclear risk | Patients entered into the trial were not reported in Brinkley et al (1966) – only those who received treatment were reported. |
| Incomplete outcome data (attrition bias) Axillary recurrence | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Unclear risk | Patients entered into the trial were not reported in Brinkley et al (1966) – only those who received treatment were reported. |
| Incomplete outcome data (attrition bias) Short term adverse events | Unclear risk | Patients entered into the trial were not reported in Brinkley et al (1966) – only those who received treatment were reported. |
| Incomplete outcome data (attrition bias) Long term adverse events | Unclear risk | Patients entered into the trial were not reported in Brinkley et al (1966) – only those who received treatment were reported. In the 1971 paper, results were reported for 98/114 participants who were still alive. |
| Selective reporting (reporting bias) | Low risk | Major outcomes were reported. |
Addenbrookes 2.
| Methods |
Study design: RCT Country: UK Study period: 1999‐2003 Inclusion criteria: Tumour diameter < 3 cm, histological diagnosis of invasive breast cancer Exclusion criteria: prior treatment for breast cancer, pregnancy, clinically involved axillary nodes, multi‐focal breast cancer or previous diagnostic excision biopsy Length of follow‐up (median and range): All participants were reviewed at 3‐monthly intervals for the first year after surgery. The study planned to observe participants yearly until 5 years. |
|
| Participants |
No. in trial arm: ALND: N =155; SLNB: N = 143 Age: ALND: mean (SD) = 58 (10.6) years; SLNB: mean (SD) = 57 (9.5) years Stage distribution: not reported Proportion node positive: ALND: 26%; SLNB: 34% Pathological type of breast cancer: not reported |
|
| Interventions | Wide local excision/mastectomy + ALND (level 2 axillary node dissection) vs SLNB (sentinel lymph node biopsy was done via a combined method of blue dye and radioisotope – then, mastectomy/wide local excision was done as planned. ALND was done as a second procedure if the sentinel node was positive) | |
| Outcomes | Arm volume change, subjective lymphoedema, seroma, sensory findings (numbness, loss of pinprick sensation, loss of light touch sensation, paraesthesia), range of shoulder movement, psychological morbidity | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: not reported Nodes removed ALND arm: not reported Nodes removed SLNB arm: not reported Method of node pathological analysis: combined method of blue dye and isotope with intraoperative detection by gamma probe. All SLNs < 5 mm diameter were bisected, and both halves were histologically examined. Nodes > 5 mm were sliced into 3 or more sections and examined histologically. Blocks were sectioned at 3 levels of 100 μm and stained with hematoxylin and eosin. If no metastases were found in H&E‐stained sections, serial sections from all levels of all blocks were stained with low‐molecular‐weight cytokeratin antibody CAM5.2 to identify micrometastases. Nodes > 5 mm were cut into 3 mm sections; those < 5 mm were embedded as a whole. Further treatment for node‐positive cases: yes (ALND) |
|
| Radiotherapy |
RT ALND only arm: Participants received radiotherapy according to local protocols. N = 137/88% received radiotherapy. RT SLND arm: Participants received radiotherapy according to local protocols. N = 132/92% received radiotherapy. RT same in all trial arms? unclear |
|
| Hormone and chemotherapy | Participants received chemotherapy and endocrine therapy according to local protocols. ALND: 23% received chemotherapy and 74% endocrine therapy; SLNB: 30% received chemotherapy and 80% endocrine therapy. | |
| Notes |
Baseline differences?Table 5 shows comparable baseline characteristics. Text reports no significant differences between groups. Intention to treat analyses? Short‐term and long‐term adverse events: Main analysis was done on an intention‐to‐treat basis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator was used. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes. Study does not mention whether they were opaque. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Short term adverse events | High risk | No blinding was reported – and it is unlikely that treating clinicians would have been blinded to the degree of surgery. |
| Blinding of outcome assessment (detection bias) Long term adverse events | High risk | No blinding was reported – and it is unlikely that treating clinicians would have been blinded to the degree of surgery. |
| Incomplete outcome data (attrition bias) Survival | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Axillary recurrence | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Short term adverse events | Low risk | Most participants were analysed for primary endpoints (134/143 in SLNB and 143/155 in ALND groups). |
| Incomplete outcome data (attrition bias) Long term adverse events | Low risk | Most participants were analysed for primary endpoints (134/143 in SLNB and 143/155 in ALND groups). |
| Selective reporting (reporting bias) | High risk | Some quality of life outcomes were reported only if statistically significant (QOL, BIS and MAC scale). |
ALMANAC.
| Methods |
Study design: RCT Country: UK Study period: 1999‐2003 Inclusion criteria: patients of either sex who were younger than 80 years and were scheduled to have a wide local excision or mastectomy for clinically node‐negative invasive breast cancer regardless of tumour size Exclusion criteria: multi‐centric cancer, previous ipsilateral breast or axillary surgery other than benign excision biopsy, previous irradiation of the ipsilateral axilla or breast, preexisting limb disease causing swelling, known allergy to human albumin or Patent Blue V, pregnancy or breast feeding, inability to complete quality of life questionnaires in English Length of follow‐up: 12 months |
|
| Participants |
No. in trial arms: SLNB: N = 495 (4 male); ALND: N = 496 (1 male) Age: SLNB: mean (SD) = 57.4 (9.9) years; ALND: mean (SD) 57.9 (9.8) years Stage distribution: not reported, but tumour size was as follows: SLNB: ≤ 20 mm, N = 354; 20.1‐50 mm, N = 125; > 50 mm, N = 10. ALND: ≤ 20 mm, N = 378; 20.1‐50 mm, N = 99; > 50 mm, N = 9. Proportion node positive: SLNB: N = 127/495; ALND: N = 116/496 Pathological type of breast cancer: SLNB: invasive ductal, N = 360; invasive lobular, N = 40; other, N = 95. ALND: invasive ductal, N = 356; invasive lobular, N = 43; other, N = 97 |
|
| Interventions | Sentinel lymph node biopsy (SLNB; using a pharmaceutical compound and a blue dye with preoperative lymphoscintigraphy) + breast‐conserving procedure/mastectomy vs standard axillary lymph node dissection (ALND; level I‐III or 4‐node axillary sampling) + breast‐conserving procedure/mastectomy Participants with metastatic disease in SNL were offered delayed ALND or axillary radiotherapy. When no SLN could be identified, ALND was performed. |
|
| Outcomes | Arm morbidity, quality of life, state and trait anxiety, axillary recurrence rate, survival | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: not reported Nodes removed clearance arm: participants (N = 123) who received 4‐node sampling: median (range) = 5 (2‐25) nodes per participant; participants (N = 373) who received ALND: median (range) = 15 (1‐42) nodes per participant Nodes removed SNLB: median (range) = 2 (1‐11) per participant Method of node pathological analysis: All lymph nodes were examined by standard hematoxylin‐eosin staining. Nodes smaller than 5 mm were bisected and stained; larger nodes were sectioned at 3 mm intervals, and single sections H&E stained. No intraoperative histopathology or immunohistochemistry was used. Further treatment for node positive cases: yes (ALND or radiotherapy) |
|
| Radiotherapy |
Both arms: Participants were treated with adjuvant radiotherapy according to standard institutional protocols. RT same in all trial arms? not reported |
|
| Hormone and chemotherapy | Both arms: Participants were treated with adjuvant systemic therapy according to standard institutional protocols. | |
| Notes | N = 37 were excluded because of substantial protocol deviation, or because they dropped out of the study (i.e. no data were available for analysis), leaving 954 participants available for intention‐to‐treat analyses of efficacy outcomes. Baseline differences? The paper states that the 2 groups of participants were similar with respect to participant and tumour characteristics. Intention‐to‐treat analyses? Paper states that intention‐to‐treat analysis was employed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list was used. |
| Allocation concealment (selection bias) | Low risk | Central allocation was performed by fax. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | No details were provided. |
| Incomplete outcome data (attrition bias) Survival | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Axillary recurrence | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Short term adverse events | Low risk | Data appear to be available for the vast majority/all participants. |
| Incomplete outcome data (attrition bias) Long term adverse events | Unclear risk | Follow‐up was incomplete (e.g. for lymphoedema self‐assessment at 3 months in ALND arm, only 395/476 participants were included; see Table 6, Mansell 2006). |
| Selective reporting (reporting bias) | Low risk | All major outcomes within the stated follow‐up period appear to be reported. |
Cape Town.
| Methods |
Study design: RCT Country: South Africa Study period: 1968‐1971 Inclusion criteria: female patients aged < 76 years with clinical T1‐2, N0‐1 and M0 breast cancer and fit for surgery Exclusion criteria: patients with breast cancer with any of the following features: (1) lump > 5 cm, (2) palpable/fixed/atypical nodes, (3) deep fixation, (4) skin infiltration or ulceration, (5) any form of oedema of the skin of the breast, (6) metastases Length of follow‐up: 40 months‐10 years |
|
| Participants |
No. in trial arms: simple: N = 51 or 52; ALND: N = 43 or 44 (see notes below) Age: simple: median (range) = 54 (23‐75) years; ALND: median (range) = 53 (31‐69) years Stage distribution: simple: T1: N = 8; T2: N = 39; T3: N = 4. ALND: T1: N =5; T2: N = 37; T3: N = 1 Proportion node positive: simple: 16/51 or 52; ALND: 22/43 or 44 Pathological type of breast cancer: not reported |
|
| Interventions | Simple mastectomy alone if nodes were not clinically palpable or with local excision of enlarged nodes vs radical mastectomy (ALND; mastectomy, axillary clearance and excision of pectoral muscles) | |
| Outcomes | Locoregional recurrence, distant metastases, survival | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: not reported Nodes removed clearance arm: not reported Nodes removed simple arm: not reported Method of node pathological analysis: not reported Further treatment for node‐positive cases: no |
|
| Radiotherapy |
Both arms: none initially, but a combination of RT and dromostanolone was given on relapse RT same in all trial arms: yes, none |
|
| Hormone and chemotherapy | Both arms: See cell above. | |
| Notes | Helman (1992) states that 51 participants received simple mastectomy and 44 received ALND; however, Dent (1996) states that 52 participants received simple mastectomy and 43 received ALND. Trial was terminated early owing to relatively high local recurrence rate after simple mastectomy. Baseline differences? very limited number of participant characteristics reported. Dent (1996): Table 5 shows stage and pathological N1, possible excess of T3 and N0 in simple group? Intention to treat analyses? Helman (1992) states that 51 participants received simple mastectomy and 44 received ALND; however, Dent (1996) states that 52 participants received simple mastectomy and 43 received ALND. No additional details were provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by drawing lots (Dent, 1996, page 870). |
| Allocation concealment (selection bias) | Low risk | Selection of lots was blinded (Dent, 1996, page 870). |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | No details were reported. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | No details were reported. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Survival | Low risk | Outcomes appear to be reported for all participants (although the Clarke 2005 paper describes 3 additional participants in the simple mastectomy arm). |
| Incomplete outcome data (attrition bias) Axillary recurrence | Low risk | Outcomes appear to be reported for all participants (although the Clarke 2005 paper describes 3 additional participants in the simple mastectomy arm). |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Low risk | Outcomes appear to be reported for all participants (although the Clarke 2005 paper describes 3 additional participants in the simple mastectomy arm) |
| Incomplete outcome data (attrition bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Long term adverse events | Unclear risk | Outcome was not reported. |
| Selective reporting (reporting bias) | Unclear risk | Long‐term and short‐term adverse events were not reported. |
Cardiff.
| Methods |
Study design: RCT Country: UK Study period: 1967‐1973 Inclusion criteria: patients with primary breast cancer with tumours of TNM (1958) stages I and II (T1, T2, N0, N1, M0) Exclusion criteria: locally advanced or metastatic. No further criteria were reported, but see also ‘Notes’. Length of follow up: median (range) = 20.6 (17‐24) years |
|
| Participants |
No. in trial arms: sampling: N = 103; ALND: N = 97 Age: sampling: median (range) = 55 (31‐85) years; ALND: median (range) = 55 (28‐81) years Stage distribution (clinical): sampling: T1: N = 10; T2: N = 93. ALND: T1: N = 11; T2: N = 86 Proportion node positive: sampling: N = 37/74, N = 29 ‘not known’; ALND: N = 34/94, N = 3 ‘not known’ Pathological type of breast cancer: not reported, but Site of tumour was as follows: sampling: medial: N = 54; other: N = 49. ALND: medial: N = 54; other: N = 43 |
|
| Interventions | Total mastectomy (preserving both pectoral muscles) + dissection of the axillary tail of the breast to the level of the axillary fat, at which point those lower axillary nodes lying close to the upper border of the axillary tail were removed for biopsy. In the protocol, it was stated that the surgeon was responsible for defining lymph nodes for histological examination, if necessary extending the dissection by removal of a portion of fat from the lower axilla. If sampled nodes were free of tumour, or if the surgeon had failed to identify any nodes for histological examination, no further treatment was given vs radical mastectomy with total removal of the breast and in continuity dissection of axillary nodes at levels I, II and III (which could include removal of the pectoralis major and minor muscles (Halsted operation) or preservation of the pectoralis major (Patey operation)). | |
| Outcomes | Local recurrence‐free rates, distant disease‐free rates, event‐free survival, overall survival | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: not reported Nodes removed clearance arm: not reported Nodes removed sampling arm: not reported Method of node pathological analysis: not reported Further treatment for node‐positive cases: yes (radiotherapy) |
|
| Radiotherapy |
Sampling: For those with histopathological involvement of these lower axillary nodes, the axilla was irradiated to ‘eradicate residual disease’. Treatment consisted of 40 Gy delivered from a 60Co source in 10 fractions over 4 weeks. ALND: Radical postoperative radiotherapy was given if axillary node involvement was histologically confirmed. The dose of radiation was 40 Gy to the chest wall (from a 60Co source), 35 Gy to supraclavicular and internal mammary regions and 40 Gy to the axilla by 300 Kv photons, each delivered in 10 fractions over 4 weeks. RT same in all trial arms? no |
|
| Hormone and chemotherapy | None reported | |
| Notes | N = 1 participant who emigrated in 1982 was lost to follow‐up. Sampling: N = 5/103 patients were ineligible (N = 3 were over 75 years of age, N = 1 had previous cancer of the cervix and N = 1 had non‐invasive DCIS [?]). ALND: N = 8/97 patients were ineligible (N = 3 were over 75 years of age, N = 3 had previous cancer of the breast and N = 2 had non‐invasive DCIS [?]). Baseline differences? The 2 groups of participants appear to be similar with respect to reported participant and tumour characteristics. Intention to treat analyses? Paper states that intention‐to‐treat analysis was employed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was completed with the use of sealed cards supplied by the Medical Computing Unit in Cardiff. |
| Allocation concealment (selection bias) | Low risk | See cell above. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Survival | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Axillary recurrence | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Long term adverse events | Unclear risk | Outcome was not reported. |
| Selective reporting (reporting bias) | Unclear risk | Short‐term and long‐term adverse events were not reported. |
E'dburgh Sample/Clear.
| Methods |
Study design: RCT Country: Scotland Study period: 1980‐1983 Inclusion criteria: patients with clinically operable invasive breast cancer (T1, T2, operable T3; N0, N1; M0). Fit enough for surgery and radiotherapy Exclusion criteria: those not available for continuous follow‐up, with in situ cancer, Paget’s disease of the nipple, multiple ipsilateral or contralateral breast cancer Length of follow‐up (median and range): 11.0 (2‐13) years |
|
| Participants |
No. in trial arms: sampling: N = 203; ALND: N = 203 Age: sampling: median (range) = 58.7 (25.7‐77.1) years; ALND: median (range) = 57 (29.6‐76) years Stage distribution: not reported (most had T1 or T2 tumour and N0 or N1 nodes, some with operable T3 tumours were also enrolled) Proportion node positive: sampling: N = 88/203; ALND: N = 80/203 Pathological type of breast cancer: not reported |
|
| Interventions | Radical mastectomy with axillary node clearance (ALND; via the Patey technique, fat and nodal tissue were dissected to the level of the first rib) vs mastectomy with axillary node sample (sampling; the breast was dissected from the underlying chest wall from medial to lateral and the axillary tail mobilised). Nodes were identified by inspection and palpation of the axillary tail and connected fat, and 4 were removed for histological examination. | |
| Outcomes | Overall survival, distant recurrence, locoregional recurrence, reduced arm mobility, severe interference with daily activities, persistent arm swelling | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: 4 nodes for axillary sample Nodes removed sampling arm: mean 6, median 4 (range, 0‐19) Nodes removed clearance arm: mean 20, median 20 (range, 5‐46) Method of node pathological analysis: sampling: Samples of the axillary tail of breast and related fat were palpated, and additional nodes dissected out, then fixed. ALDN: Specimens were assessed radiologically for determination of node distribution. Specimens then were placed on a cork board, and the nodes dissected out; these were then labelled separately or in groups and were fixed. Sections of all nodes were examined by histology. Further treatment for node positive cases: yes (radiotherapy) |
|
| Radiotherapy |
RT node sampling arm: Postoperative radiotherapy (6‐MeV) was given to 82/86 participants with positive nodes, and to 2 with no identified nodes. Dose ranged from 4000 cGy to 4250 cGy; number of fractions ranged from 10 to 20 in 4 weeks (the radiotherapy protocol was modified over the course of the trial). RT node clearance arm: none RT same in all trial arms? no |
|
| Hormone and chemotherapy |
Sampling: endocrine therapy (tamoxifen or oophorectomy) 84/203, chemotherapy (CMF) 10/203, no endocrine or chemotherapy 109/203 ALND: endocrine therapy (tamoxifen or oophorectomy) 96/203, chemotherapy (CMF) 8/203, no endocrine or chemotherapy 99/203 |
|
| Notes | Protocol violations: sampling: N = 16, ALND: N =7 Baseline differences? Groups appear to be comparable at baseline. Intention‐to‐treat analyses? Survival, disease control in the axilla, breast cancer recurrence: Paper states that data were analysed according to the intention‐to‐treat principle. Long‐term adverse events: Arm morbidity was reported for only 33.2% of included participants chosen alphabetically from those known to be free of local and systemic disease; therefore, we have not included them. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details were provided. |
| Allocation concealment (selection bias) | Low risk | Central randomisation was performed by telephone from Scottish Cancer Trials Office (except for first 8 weeks, when participants were randomised in theatre with sequentially numbered cards). |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | No details were reported. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | No details were reported. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | No details were reported. |
| Incomplete outcome data (attrition bias) Survival | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Axillary recurrence | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Long term adverse events | High risk | Arm morbidity was reported for only 33.2% of included patients chosen alphabetically from those known to be free of local and systemic disease; therefore, we have not included these data. |
| Selective reporting (reporting bias) | Unclear risk | Data on short‐term and long‐term adverse event outcomes are missing. |
Edinburgh 1.
| Methods |
Study design: RCT Country: Scotland Study period: 1987‐1995 Inclusion criteria: < 70 years old, unilateral invasive breast cancer of clinical size ≤ 4 cm, no evidence of metastatic disease, considered suitable for either study intervention Exclusion criteria: clinically multi‐centric tumour or considered locally inoperable (T4), fixed axillary nodes (N2), history of previous invasive carcinoma at any site (except skin basal cell carcinoma) Length of follow up: median = 4.1 years |
|
| Participants |
No. in trial arms: axillary clearance: N = 232; axillary sampling: N = 234 Age: axillary clearance: median = 54 years; axillary sampling: median = 54 years Stage distribution: not reported Proportion node positive: axillary clearance: N = 78/232; axillary sampling: N = 66/234 Pathological type of breast cancer: axillary clearance: no special type, N = 177; lobular, N = 11; tubular, N = 16; non‐invasive, N = 5; other, N = 23. Axillary sampling: no special type, N = 176; lobular, N = 11; tubular, N = 13; non‐invasive, N = 3; other, N = 31 |
|
| Interventions | Axillary node clearance (level III) vs axillary node sampling (obtain ≥ 4 palpable lymph nodes from the axilla, starting at the axillary tail and working upwards) | |
| Outcomes | Survival, recurrence, range of shoulder movement (6, 12, 24 and 36 months), shoulder muscle power (6, 12, 24 and 36 months), arm swelling (6, 12, 24 and 36 months) | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: axillary clearance: level III; axillary sampling: ≥ 4 palpable lymph nodes Nodes removed clearance arm: median (range) = 15 (4‐36) Nodes removed sampling arm: median (range) = 5 (2‐12) Method of node pathological analysis: not reported Further treatment for node‐positive cases: yes (radiotherapy) |
|
| Radiotherapy |
RT node clearance arm: RT to the breast (45 Gy/20 fractions/4 wk or 45 Gy/25 fractions/5 wk for larger breasts + a boost to tumour bed by interstitial implant (20 Gy to 85% reference isodose) or electrons (15 Gy at 100% isodose/5 daily fractions/1 wk, but not to the axilla (all adjuvant)) RT node sampling arm: RT to the breast (as above) and regional lymphatics (45 Gy/20 fractions/4 wk) and to the axilla when sampling revealed involved nodes (apart from in N = 5, who were also included in another trial and did not receive RT). N = 39 with node‐negative axilla receiving RT to the axilla (all adjuvant) RT SNB arm: NA RT same in all trial arms? no |
|
| Hormone and chemotherapy |
Axillary clearance: tamoxifen N = 163, chemotherapy N = 26, ovarian suppression N = 11, chemotherapy + tamoxifen N = 10, none N = 22 (all adjuvant) Axilla sampling: tamoxifen N = 174, chemotherapy N = 28, ovarian suppression N = 6, chemotherapy + tamoxifen N = 9, none N = 17 (all adjuvant) |
|
| Notes | Participants in both groups received postoperative adjuvant hormone or chemotherapy, depending on the results of pathology, including axillary node histology and oestrogen receptor status. Baseline differences? probably, but no statistical analyses compared groups at baseline Intention‐to‐treat analyses? survival, disease control in the axilla and breast cancer recurrence: stated in paper that intention‐to‐treat analyses were employed. Long‐term adverse events: stated in paper that analysis was performed per actual treatment received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | List was derived via randomised permuted blocks of 8. |
| Allocation concealment (selection bias) | Low risk | Central allocation was conducted by the Scottish Cancer Trials Office. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | No details were provided. |
| Incomplete outcome data (attrition bias) Survival | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Axillary recurrence | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Long term adverse events | Unclear risk | Data were reported for N = 126‐132 in the axillary clearance group, and for N = 114‐123 in the axilla sampling group. |
| Selective reporting (reporting bias) | Low risk | All major outcomes appear to have been reported apart from short‐term adverse events. |
Genoa.
| Methods |
Study design: RCT, non‐inferiority Country: Italy Study period: 1998‐2001 Inclusion criteria: 18‐75 years, primary invasive breast cancer as revealed by mammography and cytohistology, clinically negative axillary lymph nodes, unifocal tumour ≤ 3 cm as estimated by echography Exclusion criteria: previous surgery on the same breast or on the ipsilateral axilla, chronic life‐threatening disease possibly preventing adjuvant therapy Length of follow‐up: event‐free survival: median = 5.5 ± 1.4 years. Overall survival: median = 5.6 ± 1.3 years |
|
| Participants |
No. in trial arms: SLNB: N = 110; ALND: N = 115 Age: SLNB: median (range) = 60 (35‐75) years; ALND: median (range) = 59 (28‐75) years Stage distribution: SLNB: pTis N = 1, pT1mic N = 2, pT1a N = 11, pT1b N = 24, pT1c N = 59, pT2 N = 13; pN0 N = 77, pN1mic N = 5, pN1a N = 21, pN2a N = 6, pN3a N = 1. ALND: pTis N = 1, pT1mic N = 0, pT1a N = 10, pT1b N = 18, pT1c N = 57, pT2 N = 29; pN0 N = 79, pN1mic N = 11, pN1a N = 17, pN2a N = 5, pN3a N = 3 Proportion node positive: SLNB: N = 33/110; ALND: N = 36/115 Pathological type of breast cancer: SLNB: ductal NOS, N = 107; lobular, N = 1; in situ, N = 1; other, N = 1. ALND: ductal NOS, N = 110; lobular, N = 2; in situ, N = 1; other, N = 2 |
|
| Interventions | Breast surgery (mastectomy or conservative quadrantectomy carried out according to standard criteria) + sentinel lymph node biopsy (SLNB; identified by breast lymphoscintigraphy and lymphatic dye mapping) + axillary lymph node dissection (ALND) vs breast surgery + SLNB + ALND only if SLN was found to be positive at the intraoperative evaluation. Any participant whose SLNs could not be identified received ALND independently of the treatment assigned. | |
| Outcomes | 5‐Year event‐free survival and 5‐year overall survival, axillary recurrence in those who did not undergo axillary lymph node dissection, sensitivity and predictive value of SLNB in ALND arm | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: not reported Nodes removed clearance arm: N = 211, mean = 1.83 per participant Nodes removed SNB + clearance: N = 194, mean 1.76 per participant Method of node pathological analysis: SLN bisected on major axis, and 5 pairs of frozen sections, each 4 μm thick, were cut every 10 μm in each half of the node. The first, third and fifth sections were stained with hematoxylin‐eosin. If negative, then second and fourth sections were tested with immunohistochemistry for cytokeratins, via cytokeratin mAb and horseradish peroxidase. Remaining tissue was embedded in paraffin for postoperative evaluation. Further treatment for node‐positive cases: yes (ALND and/or adjuvant therapy) |
|
| Radiotherapy |
ALND or SLNB: Only participants who received conservative surgery were given radiotherapy (50 Gy/8 wk) to the ipsilateral breast. No RT was given to the axilla. RT same in all trial arms? yes |
|
| Hormone and chemotherapy | Both arms: The choice of adjuvant chemotherapy and/or hormone therapy regimen, when given, was based on the main prognostic factors of the primary tumour (nodal status, tumour size, tumour grading, hormonal receptor status). | |
| Notes | No SLN was found in 3 patients who had ALND (1 control/2 research). Study was powered for 2570 participants; only 248 were recruited, and the trial was interrupted when participants became aware of promising SLNB procedure and refused randomisation to ALND. Baseline differences? No statistically significant differences between groups were noted at baseline. Intention‐to‐treat analyses? Paper stated that intention‐to‐treat analyses were employed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list was used. |
| Allocation concealment (selection bias) | Low risk | Central allocation was conducted by the Epidemiology and Clinical Trials Unit of the Institute. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Survival | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Axillary recurrence | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Long term adverse events | Unclear risk | Outcome was not reported. |
| Selective reporting (reporting bias) | Unclear risk | Short‐term and long‐term adverse events were not reported. |
GIVOM Sentinella.
| Methods |
Study design: RCT (non‐inferiority) Country: Italy Study period: 1999‐2004 Inclusion criteria: patients with invasive breast cancer ≤ 3 cm and clinically negative axilla Exclusion criteria: non‐palpable tumours, multiple tumours, ductal carcinoma in situ, tumours > 3 cm, clinically positive axilla, distant metastases, previous neoadjuvant therapy, pregnancy, > 80 years of age Length of follow‐up: median (IQR) = 55.6 (42.4‐63.1) months |
|
| Participants |
No. in trial arms: ALND: N = 352; SLNB: N = 345 Age: ALND: mean (SD) = 58.2 (10.6) years; SLNB: mean (SD) = 57.6 (10.4) years Stage distribution: not reported, but size of tumour was as follows: ALND: T1a, N = 7; T1b, N = 72; T1c, N = 208; T2 (≤ 3 cm), N = 63; T4, N = 0; not available, N = 2. SLNB: T1a N, 12; T1b N, 67; T1c N, 198, T2 (≤ 3 cm), N = 63; T4 N = 3, not available, N = 2 Proportion node positive: ALND: N = 108/334 (with identified SLN); SLNB: N = 99/328 (with identified SLN) Pathological type of breast cancer: not reported |
|
| Interventions | SLNB + ALND (at least nodes located at the I‐II Berg levels were removed) vs SLNB with frozen section and histological examination followed by ALND if SLNB was positive. All participants had surgical treatment of the primary tumour before SLNB. | |
| Outcomes | Disease‐free survival, overall survival, physical morbidity, quality of life | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: axillary clearance: see Interventions Nodes removed ALND arm: not reported Nodes removed SNLB + ALND: not reported Method of node pathological analysis: For frozen section analysis, sentinel lymph nodes of diameter 5 mm or less were bisected, larger nodes were sectioned every 2 to 3 mm. For each sample, 2 frozen sections made at 40 μm were analysed. For the definitive analysis, 2 consecutive 5 μm sections were cut from a paraffin block, 40 μm apart from each other. These sections were hematoxylin‐eosin stained and immunostained with a monoclonal antibody to cytokeratin. Further treatment for node positive cases: yes (ALND and/or adjuvant therapy) |
|
| Radiotherapy | All participants who underwent conservative breast surgery (ALND: N = 297; SLNB: N = 293) received radiation to the ipsilateral breast with 50 Gy of high‐energy photons. RT same in all trial arms? yes |
|
| Hormone and chemotherapy | Participants with unfavourable prognostic features were given chemotherapy or hormone therapy according to the practice of the treating centre. | |
| Notes |
ALND: N = 323/334 (with identified SLN) underwent ALDN (level I‐II‐III dissection: N = 268; level I‐II dissection: N = 55). In 11 cases, scheduled completion of ALDN was not performed owing to protocol violation. SLNB: N = 94/99 (with positive SLN) received ALND (level I‐II‐III dissection: N = 78; level I‐II dissection: N = 16). Five participants refused ALND completion. Designed as a non‐inferiority study that aimed to recruit 1498 participants. Trial was stopped early owing to participant and clinician preference for SLNB. Baseline differences? Groups appear to be comparable, but no statistical analyses are reported to compare groups at baseline. Intention‐to‐treat analyses? All statistical analyses were based on the intent‐to‐treat principle. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participant randomisation was carried out by telephone through the Clinical Trials and Biostatistics Unit of Padova, via computer‐generated random numbers to select random permuted blocks stratified by participating centre. Block lengths of 4 and 6 were randomly varied. |
| Allocation concealment (selection bias) | Low risk | See cell above. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | No details were provided. |
| Incomplete outcome data (attrition bias) Survival | Unclear risk | Study authors report that all participants randomised were analysed for primary endpoint (5‐year DFS; Zavagno, 2008), but survival curves show incomplete follow‐up to 60 months. |
| Incomplete outcome data (attrition bias) Axillary recurrence | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Unclear risk | Study authors report that all participants randomised were analysed for primary endpoint (5‐year DFS; Zavagno, 2008), but survival curves show incomplete follow‐up to 60 months. |
| Incomplete outcome data (attrition bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Long term adverse events | Unclear risk | > 90% and 75% of participants, respectively, completed morbidity assessments by surgeons up until 18 months and at 24 months. |
| Selective reporting (reporting bias) | Unclear risk | All major outcomes appear to have been reported apart from short‐term adverse events. |
Guy's.
| Methods |
Study design: 2 RCTs Country: UK Study period: 1961‐1975 (RCT 1, 1961‐1970; RCT 2, 1971‐1975) Inclusion criteria: women with Manchester stage I or 2 (T1‐2, N0‐1 [RCT1], M0) breast cancer judged suitable for radical mastectomy or extended tylectomy (wide excision). RCT 1 included only women aged ≥ 50, whereas RCT 2 included women of any age but restricted disease classifications to T1‐2, N0‐1a, M0. Exclusion criteria: none listed Length of follow‐up: median follow up = 24.7 years |
|
| Participants |
No. in trial arms: wide excision: N = 305; ALND: N = 324 Age: wide excision: mean (range) = 58 (27‐80) years; ALND: mean (range) = 56 (25‐90) years (P = 0.03) Stage distribution: not reported, but tumour size was ≤ 2 cm: N = 83 in wide excision and N = 77 in ALND group; > 2 and ≤ 5 cm: N = 190 in wide excision and N = 209 in ALND group; > 5 cm: N = 29 in wide excision and N = 28 in ALND group (P = 0.63) Proportion node positive: 46% of participants treated via radical mastectomy had pathologically involved axillary nodes. Wide excision: clinically node positive 71/304 (from Clarke 2005 meta‐analysis web figures 10A/B); ALND: clinically node positive 85/326 (from Clarke 2005 meta‐analysis web figures 10A/B) Pathological type of breast cancer: histology: grade I: N = 63 in wide excision and N = 72 in ALND; grade II: N = 169 in wide excision and N = 176 in ALND; grade III: N = 60 in wide excision and N = 64 in ALND; lobular: N = 4 in wide excision and N = 2 in ALND; other: N = 9 in wide excision and N = 10 in ALND; contralateral tumour: N = 28 in wide excision and N = 41 in ALND (P = 0.9) |
|
| Interventions | Extended tylectomy, or wide excision, of the lump, together with surrounding breast tissue within 3 cm of palpable or visible growth + thiotepa + radiotherapy vs radical mastectomy (standard Halsted operation, except that the clavicular head of the pectoralis major muscle was conserved) + synoperative thiotepa + radiotherapy | |
| Outcomes | Overall survival, breast cancer survival, distant recurrence, local recurrence, arm function, lymphoedema, activity, attitude | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: not reported Nodes removed clearance arm: not reported Nodes removed wide excision arm: not reported Method of node pathological analysis: All nodes were sectioned in specimens removed at radical mastectomy. No further details were reported. Further treatment for node positive cases: no |
|
| Radiotherapy |
Wide excision: same as ALND with the exception that overall treatment time to supraclavicular triangle and axilla was 12 days (i.e. 25‐27 Gy) and breast was treated with parallel opposing fields on a 6 MeV linear accelerator via “Lincolnshire bolus” to bring the peak dose to the surface. Tumour dose = 3500‐3800 rads in 3 weeks (an additional 35‐38 Gy) ALND: RT to the axilla, supraclavicular triangle and internal mammary chain via a 300 kV machine with 10 × 8 cm field sizes for the axilla and supraclavicular triangle and 15 × 7.5 cm field sizes for the internal mammary chain. Supraclavicular and axillary fields directed to cross at the apex of the axilla giving a tumour dose at this point of 2500‐2700 rads. Treatment was given 5 days a week for 18 days (25‐27 Gy). RT same in all trial arms? no |
|
| Hormone and chemotherapy | Both arms: synoperative thiotepa at doses of 2 mg per 6.4 kg body weight with premedication, 1.5 mg per 6.4 kg body weight on second postoperative day and 1 mg per 6.4 kg body weight on fourth postoperative day. However, no patient entering the trial after 1968 received thiotepa. | |
| Notes | No. in trial arms differs slightly from that reported in the Clarke 2005 meta‐analysis (web figures 10A and B): ALND: N = 326, wide excision: N = 304 Baseline differences? With Bonferroni adjustment for multiple comparisons, the age difference is no longer statistically significant. Intention‐to‐treat analyses? Survival, disease control in the axilla and breast cancer recurrence: no details reported. Long‐term adverse events: outcomes reported only for N = 77‐92 for wide excision arm, and for N = 90‐104 for ALND arm |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out by drawing a ticket from a box. |
| Allocation concealment (selection bias) | Unclear risk | It is unclear whether allocation could be seen on the ticket. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | No details were provided. |
| Incomplete outcome data (attrition bias) Survival | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Axillary recurrence | Unclear risk | No details were reported. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Unclear risk | No details were reported. |
| Incomplete outcome data (attrition bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Long term adverse events | High risk | Outcomes were reported only for RCT 1 and only for N = 77‐92 from the wide excision arm, and for N = 90‐104 from the ALND arm. |
| Selective reporting (reporting bias) | Unclear risk | Short‐term adverse events were not reported, and long‐term adverse events were reported for < 1/3 of participants. |
Hammersmith.
| Methods |
Study design: RCT Country: UK Study period: 1965‐1970 Inclusion criteria: patients with clinical stage T1N0, T2N0, T1N1 and T2N1 primary lesions and no evidence of distant metastatic disease; patients with T3 lesions for which the T3 category was decided solely on the size of the tumour; and patients with clinically involved axillary nodes were included, irrespective of the size and position of nodes, but only if they remained mobile. Exclusion criteria: patients with lesions that had excessive skin tethering or any attachment to pectoral muscles, patients with fixed axillary nodes (N2) or involved supraclavicular nodes (N3) Length of follow up: 4‐9 years (median not reported. If recruitment was at a constant rate, median follow‐up would be 6.5 years by 1974) |
|
| Participants |
No. in trial arms: radical: N = 95; simple: N = 100 Age: not reported Stage distribution: not reported Proportion node positive: not reported by trial arm (overall 79/195 – 41% had clinically involved nodes at time of trial entry) Pathological type of breast cancer: not reported |
|
| Interventions | Simple total mastectomy + postoperative radiotherapy vs radical mastectomy (Halsted) + postoperative radiotherapy | |
| Outcomes | Overall survival, short‐term postoperative mortality, local recurrence, morbidity (stiff shoulder, swollen arm) | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: not reported Nodes removed clearance arm: not reported Nodes removed SNLB: not reported Method of node pathological analysis: not reported Further treatment for node‐positive cases: no |
|
| Radiotherapy |
Radical: postoperative radiotherapy to the apex of the axilla and to supraclavicular, infraclavicular and internal mammary lymph nodes Simple: postoperative radiotherapy to the chest wall, axilla and supraclavicular, infraclavicular and internal mammary lymph nodes RT same in all trial arms? no |
|
| Hormone and chemotherapy | All but 1 participant who were premenopausal or within 10 years of stopping menstruation also received ‘prophylactic’ oophorectomy, which usually was carried out at the time of mastectomy. | |
| Notes | 100% follow up (1974), although some follow‐up was conducted by post. Need to locate final trial report if it was ever published Baseline differences? For allocation of participants, paired stratification was employed with the following stratification factors: age, menopausal status, child‐bearing history and exact clinical stage (TNM). No further details were reported. Intention‐to‐treat analyses? Data were reported only for 76 matched participant pairs. 22% of participants were excluded from analysis because they were unmatched. Were these unmatched participants different in a systematic way? |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated to 1 or another of the 2 treatment groups after matching, via random number tables. |
| Allocation concealment (selection bias) | Unclear risk | For allocation of participants, paired stratification was employed with the following stratification factors: age, menopausal status, child‐bearing history and exact clinical stage (TNM). No further details were reported. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | No details were provided. |
| Incomplete outcome data (attrition bias) Survival | Unclear risk | Data were reported only for the 76 matched participant pairs. 22% of participants were excluded from analysis because they were unmatched. Were these unmatched participants different in a systematic way? |
| Incomplete outcome data (attrition bias) Axillary recurrence | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Unclear risk | Data were reported only for the 76 matched participant pairs. 22% of participants were excluded from analysis because they were unmatched. Were these unmatched participants different in a systematic way? |
| Incomplete outcome data (attrition bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Long term adverse events | Low risk | All 195 participants were measured for stiff shoulder/swollen arm. Follow‐up was reported as 100%. |
| Selective reporting (reporting bias) | Unclear risk | Short‐term adverse events were not reported. |
IBCSG‐10‐93.
| Methods |
Study design: RCT (originally conceived as a non‐inferiority trial – see notes Country: international Study period: 1993‐2002 Inclusion criteria: postmenopausal patients aged ≥ 60 years with clinically node‐negative operable breast cancer. All patients had a histologically proven unilateral breast cancer of stage T1a‐b, T2a‐b, T3, N0 or M0 with ER‐positive or ER‐negative primary tumours. Exclusion criteria: treatment started before randomisation, prior or concurrent malignancy Length of follow up: median = 6.6 years |
|
| Participants |
No. in trial arms: surgery alone: N = 239; ALND: N = 234 Age: surgery alone: median (range) = 74 (60‐91) years; ALND: median (range) = 74 (60‐91) years Stage distribution: not reported, but tumour size was as follows: surgery alone: ≤ 20 mm, N = 137; > 20 mm, N = 100; unknown, N = 2. ALND: ≤ 20 mm, N = 126; > 20 mm, N = 100; unknown, N = 8 Proportion node positive: surgery alone: not examined (axilla not dissected in N = 232/239); ALND: N = 64/230 (axilla not dissected in N = 4) Pathological type of breast cancer: not reported, but ER status was as follows: surgery alone: positive, N = 201; negative, N = 31; unknown, N = 7. ALND: positive, N = 179; negative, N = 46; unknown, N = 9 |
|
| Interventions | Surgery alone (total mastectomy, N = 106; breast‐conserving surgery with (N = 77) or without (N = 56) radiotherapy) vs surgery (total mastectomy, N = 105; breast‐conserving surgery with (N = 78) or without (N = 51) radiotherapy) + axillary clearance | |
| Outcomes | Quality of life (including adverse events), disease‐free survival, overall survival | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: not reported Nodes removed clearance arm: not reported Nodes removed no axillary surgery: not reported Method of node pathological analysis: not reported Further treatment for node‐positive cases: no |
|
| Radiotherapy |
Both arms: Radiotherapy using 2 tangential fields was recommended after breast‐conserving surgery. No further details were reported. RT same in all trial arms? not reported |
|
| Hormone and chemotherapy |
HRT: surgery alone: no, N = 184; yes, N = 52; unknown, N = 3. ALND: no, N = 184; yes, N = 50 Both arms: Participants were treated with adjuvant tamoxifen (20 mg) for 5 years. In August 2002, IBCSG Scientific Commmittee made a recommendation to discontinue tamoxifen for participants with endocrine non‐responsive tumours. |
|
| Notes | N = 19 did not meet protocol eligibility criteria, but these patients were included in intention‐to‐treat analyses. Originally designed as a non‐inferiority trial with estimated sample size of 1020 – poor accrual meant a change in design to assess whether avoiding ALND improved quality of life Baseline differences? Paper states that baseline characteristics were balanced according to randomly assigned treatment arms Intention‐to‐treat analyses? Survival, disease control in the axilla, breast cancer recurrence: Paper states that intention‐to‐treat analysis was employed. Short‐term and long‐term adverse events: data not available for all participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted blocks randomisation schedule was produced by use of pseudo‐random numbers generated by a congruence method. |
| Allocation concealment (selection bias) | Low risk | Random assignment was performed centrally. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | No details were provided. |
| Incomplete outcome data (attrition bias) Survival | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Axillary recurrence | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Short term adverse events | High risk | Data were available only for subgroups of surgery alone participants and ALND participants. |
| Incomplete outcome data (attrition bias) Long term adverse events | High risk | Data were available only for subgroups of surgery alone participants and ALND participants. |
| Selective reporting (reporting bias) | Unclear risk | Some adverse events were not reported. |
Institut Bergonie.
| Methods |
Study design: RCT (equivalence trial) Country: France Study period: 1995‐2005 Inclusion criteria: postmenopausal female patients aged ≥ 50 years with early invasive breast cancer (tumour size ≤ 10 cm) Exclusion criteria: patients with inflammation, palpable axillary nodes (clinical N+), metastasis, prior contralateral invasive cancer or other carcinoma or limited survival prognosis (< 10 years) Length of follow‐up: 5 years |
|
| Participants |
No. in trial arms (these are reported per protocol): no ALND: N = 297 (ITT, N = 312); ALND: N = 310 (ITT, N = 313) Age: no ALND: median (range) = 62.6 (50‐81) years; ALND: mean (range) = 61.6 (50‐87) years Stage distribution (histological tumour size): no ALND: mean = 7.1 mm; 1‐5 mm, N = 86; 6‐10 mm, N = 196; > 10 mm, N = 9; missing, N = 6. ALND: mean = 7.25 mm; 1‐5 mm, N = 82; 6‐10 mm, N = 208; > 10 mm, N = 19; missing, N = 1 Proportion node positive: 42 ALND participants Pathological type of breast cancer: no ALND: invasive ductal, N = 232; invasive lobular, N = 23; other, N = 42. ALND: invasive ductal, N = 236; invasive lobular, N = 28; other: N = 45 |
|
| Interventions | Standard surgery was performed according to the same technique for all eligible patients: radical modified mastectomy or lumpectomy involving an excision ≥ 10 mm surrounding the tumour with section slices for histological analysis to ensure free margins. For the ALND group, axillary lymph node clearance was standard and was limited to nodes inferior to the axillary vein (Berg levels I and II): no ALND (standard surgery + adjuvant treatment if indicated) vs ALND (surgery + standard axillary lymph node clearance + adjuvant treatment if indicated) | |
| Outcomes | 5‐year overall survival, event‐free survival, functional outcomes | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: 10 or more Nodes removed clearance arm: see "Interventions" Nodes removed no ALND arm: none Method of node pathological analysis: not reported Further treatment for node‐positive cases: yes (adjuvant chemotherapy if histologically or biologically indicated) |
|
| Radiotherapy |
All lumpectomy participants and most mastectomy participants as indicated (i.e. with involved nodes): 50 Gy over the whole breast or chest wall with no axillary irradiation RT same in all trial arms? yes |
|
| Hormone and chemotherapy | Both arms: Participants with oestrogen‐ or progesterone‐positive receptors or unknown status received 20 mg tamoxifen daily from surgery for 3 (participants randomised before 23/9/02) or 5 (participants randomised after 23/9/02) years. For negative receptor participants, no endocrine therapy was prescribed, but adjuvant chemotherapy was prescribed as indicated. If histologically or biologically indicated, adjuvant chemotherapy was prescribed after surgery according to the practices of each centre. | |
| Notes | At the first interim analysis, enrolment was stopped early (600 enrolled instead of the 1600 expected) owing to lack of equivalence in OS, better than predicted survival in the no ALND arm and changes in clinical practice (e.g. sentinel lymph node dissection, changes in adjuvant endocrine therapy). Baseline differences? Groups appear to be comparable at baseline, except in terms of receipt of adjuvant therapy with 270 and 6 of the 297 no ALND participants receiving endocrine and chemotherapy, respectively, compared with 203 and 26 of 310 ALND participants, respectively. Intention‐to‐treat analyses? Data available only on an intention‐to‐treat basis for overall survival. Remaining outcomes are reported per protocol. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization was performed by block, stratified by centre and by operation time: either histological diagnosis was known and randomisation was performed after histological analysis; or, randomisation was performed intra‐operatively and was based on extemporaneously‐assessed size." No further information was provided. |
| Allocation concealment (selection bias) | Unclear risk | See cell above. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | No details were provided. |
| Incomplete outcome data (attrition bias) Survival | Low risk | All data appear to have been included as intention‐to‐treat. |
| Incomplete outcome data (attrition bias) Axillary recurrence | Unclear risk | Data were reported only per protocol with data missing from 15 no ALND and 3 ALND participants. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Unclear risk | Data were reported only per protocol with data missing from 15 no ALND and 3 ALND participants. |
| Incomplete outcome data (attrition bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Long term adverse events | Unclear risk | Data were reported only per protocol for 543/625 participants. |
| Selective reporting (reporting bias) | Unclear risk | All major outcomes appear to have been reported apart from short‐term adverse events. |
Institut Curie.
| Methods |
Study design: RCT Country: France Study period: 1982‐1987 Inclusion criteria: female patients aged < 70 years with no history of previous cancer, no previous treatment, presenting with a unilateral invasive carcinoma < (Louis‐Sylvestre 2004) or ≤ (Cabanes 1992) 3 cm, no clinically involved axillary lymph node (N0, Louis‐Sylvestre 2004; or N0‐N1a, Cabanes 1992) and non‐metastatic (M0) disease Exclusion criteria: patients age > 70 years with cancer at another site (apart from basal cell carcinoma and intraepithelial carcinoma of the cervix), patients who could not be regularly followed up at the Institut Curie Length of follow up: median (range) = 180 (12‐221) months |
|
| Participants |
No. in trial arms: RT: N = 332; ALND: N = 326 Age: RT: mean = 50.6 years; ALND: mean = 52 years Stage distribution: RT: T1, N = 233; T2, N = 99; clinical N0, N = 256; clinical N1a, N = 76. ALND: T1, N = 207; T2, N = 119; clinical N0, N = 270; clinical N1a, N = 56 Proportion node positive: 68/322 who received ALND (i.e. 2 RT participants and 320 ALND participants (see also notes)) Pathological type of breast cancer: RT: invasive intraductal, N = 286; other, N = 46. ALND: invasive intraductal, N = 268; other, N = 58 |
|
| Interventions | Lumpectomy (wide local excision of the tumour with macroscopically healthy margins) + RT to the breast and axillary and internal mammary lymph nodes vs lumpectomy (wide local excision (with macroscopically healthy margins) + axillary dissection (limited to nodes inferior to the axillary vein; level I and lower level II nodes) + RT to supraclavicular and internal mammary lymph nodes in participants with histologically confirmed metastatic lymph nodes. If medial or central tumour was diagnosed in this group, internal mammary lymph nodes were also irradiated. | |
| Outcomes | Overall survival, local and lymph node recurrence, metastases, disease‐free survival | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: not reported Nodes removed clearance arm: see "Interventions" Nodes removed RT arm: none Method of node pathological analysis: not reported Further treatment for node‐positive cases: yes (hormone or chemotherapy) |
|
| Radiotherapy |
Both arms: 55 Gy fractionated over 6 weeks to the breast. 10‐15 Gy boost to the tumour bed Axillary nodes: 50 Gy Internal mammary nodes and supraclavicular nodes: 45 Gy RT same in all trial arms? no |
|
| Hormone and chemotherapy |
Both arms: Adjuvant medical treatment was available depending on the number of lymph nodes invaded and menopausal status. Chemotherapy: RT: N = 9; ALND: N = 19 Hormone therapy: RT: N = 8; ALND: N = 14 |
|
| Notes | The treatment protocol was not followed in 15 participants (RT: N = 2, N1 patients underwent dissection; N = 4, underwent mastectomy; ALND: N = 6, did not have dissection (and consequently received no treatment of the axilla); N = 3, underwent mastectomy). In addition, 7 N1 participants (RT: N = 6; ALND: N = 1) were enrolled, although they should not have been included in the protocol. N = 11 were lost to follow‐up at 5 years, and N = 58 were lost to follow‐up at 10 years, but unclear to which group they belonged Baseline differences? Groups appear to be comparable at baseline. Intention‐to‐treat analyses? Cabanes (1992) and Louis‐Sylvestre (2004; from which data were extracted): Both state that participants with protocol violations were maintained in the group to which they had initially been assigned for purposes of statistical analysis, which was conducted in an intention‐to‐treat fashion. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Paper states that randomisation was done by sealed envelopes (equilibrated every 6 participants) in the operating theatre after verification that participants satisfied the inclusion criteria. No further details were provided. |
| Allocation concealment (selection bias) | Unclear risk | See cell above. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | No details were reported. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | No details were reported. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Survival | Unclear risk | N = 11 were lost to follow‐up at 5 years; N = 58 were lost to follow‐up at 10 years, but it is unclear to which group they belonged. |
| Incomplete outcome data (attrition bias) Axillary recurrence | Unclear risk | See cell above. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Unclear risk | See cell above. |
| Incomplete outcome data (attrition bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Long term adverse events | Unclear risk | Outcome was not reported. |
| Selective reporting (reporting bias) | Unclear risk | Long‐term and short‐term adverse events were not reported. |
Malmo.
| Methods |
Study design: RCT Country: Sweden Study period: 1969‐1974 Inclusion criteria: patients with microscopically verified breast cancer ≤ 5 cm and clinically node negative Exclusion criteria: none reported Length of follow‐up: range = 15‐20 years |
|
| Participants |
No. in trial arms: ALND + RT: N = 97; mastectomy only: N = 98 Age: ALND + RT: mean (SD) = 54.6 (10.2) years; mastectomy only: mean (SD) = 57.7 (10) years Stage distribution: not reported, but Size of tumour was as follows: ALND + RT: mean (SD) = 2 (1) cm; mastectomy only: mean (SD) = 1.9 (1) cm Proportion node positive: ALND: 28/97; mastectomy only, N = 3 at surgery and N = 11 during first postoperative year Pathological type of breast cancer: not reported |
|
| Interventions | ALND + RT vs mastectomy alone | |
| Outcomes | Survival, chest wall recurrence | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: not reported Nodes removed ALND + RT arm: not reported Nodes removed mastectomy arm: not reported, but presumably none? Method of node pathological analysis: not reported Further treatment for node‐positive cases: no |
|
| Radiotherapy |
ALDN + RT: Postoperative radiotherapy was delivered with conventional x‐rays to the axilla (140 kV, HVL 6.6 mm Cu) and chest wall (100 kV, HVL 2.7 mm Cu) with surface doses to the chest wall of 31.5 Gy in 3.5 Gy fractions, and to the axilla of 28 Gy in 4 Gy fractions, 5 times a week. Supraclavicular and parasternal nodes were treated with cobalt‐60 or electrons, with peak absorbed doses of 48 Gy in fractions of 3 Gy, 4 times per week. Mastectomy only: If axillary metastases were diagnosed later on during follow‐up, axillary dissection with postoperative radiotherapy was performed. RT same in all trial arms? no |
|
| Hormone and chemotherapy | Not reported | |
| Notes | N = 8 ALDN + RT and N = 6 mastectomy only participants were not strictly treated according to protocol. Baseline differences? very few baseline characteristics reported Intention‐to‐treat analyses? Survival: Per‐protocol results are presented, but study authors state in the text that results of intention‐to‐treat analyses were similar without presenting data for these analyses. Disease control in the axilla and breast cancer recurrence: Some participants were not treated according to protocol; it is unclear if they are included in the analyses, and, if yes, it is unclear how they are included. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables were used (page 557, Borgstrom 1994). |
| Allocation concealment (selection bias) | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Survival | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Axillary recurrence | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Long term adverse events | Unclear risk | Outcome was not reported. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes are incompletely reported, and adverse events are not reported at all. |
Manchester.
| Methods |
Study design: RCT Country: UK Study period: 1970‐1975 Inclusion criteria: new cases of clinical stage II (T1‐2, N1, M0) breast carcinoma Exclusion criteria: males, women aged > 70 years, history of cancer of the opposite breast, intercurrent disease, unavailable for follow‐up, pregnancy and lactation Length of follow up: 5‐10 years |
|
| Participants |
No. in trial arms: simple mastectomy + postoperative radiotherapy (PORT): N = 159; ALND: N = 149 Age: simple mastectomy + PORT: mean (SD) = 55.2 (9.6) years; ALND: mean (SD) = 55.1 (9.9) years (latter value includes only N = 148) Stage distribution: T2 = 83% in both groups Proportion node positive: not reported Pathological type of breast cancer: not reported |
|
| Interventions | Simple mastectomy (removal of the whole breast including pectoral fascia but without intentional removal of any axillary node; thin skin flaps were to be avoided and transverse incisions preferred) + PORT vs radical mastectomy (removal of the whole breast with dissection of axillary nodes; removal of pectoral muscles up to the individual surgeon) | |
| Outcomes | Local recurrence rate, breast cancer death, overall survival | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: not reported Nodes removed clearance arm: not reported Nodes removed sampling arm: NA Nodes removed SNLB: NA Method of node pathological analysis: not reported Further treatment for node‐positive cases: no |
|
| Radiotherapy |
Simple mastectomy arm: Participants were treated with adjuvant radiotherapy according to 1 of the following 2 techniques. 1. Quadrate technique (3 fields (300 kV) at a tangent to the chest wall, irradiating the chest wall, the parasternal region and the axilla; also a field to the supraclavicular fossa and a posterior field to the apex of the of the axilla (est dose 3700 rads in 3 weeks); or 2. Peripheral and tangent pair technique as follows. a. Single megavoltage (4 MV) field consisting of irradiation of the parasternal, supraclavicular and axillary regions from the front (given dose 4000 rads in 3 weeks); or b. Parallel pair of fields to the chest wall, 300 kV (mid‐dose 3000 rads in 3 weeks; max dose to the skin 3800‐4500 rads). RT same in all trial arms? no. RT given only in simple mastectomy arm |
|
| Hormone and chemotherapy | Both arms: Participants who were premenopausal or < 3 years postmenopausal were offered artificial menopause by x‐ray or surgical castration. | |
| Notes | Treatment of N = 20 and 16, respectively, deviated from protocol in the simple mastectomy + PORT and radical mastectomy arms. However, all participants were analysed according to randomised treatment allocation (i.e. intention to treat‐analyses were performed). Baseline differences? Paper states that the 2 groups of participants were similar with respect to age, menopausal status and tumour site within the breast. Intention‐to‐treat analyses? Paper states that intention‐to‐treat analysis was employed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly allocated, with stratification by surgeon, to one or other of the treatment groups under comparison." (Lythgoe 1978, page 744). No additional details were provided. |
| Allocation concealment (selection bias) | Unclear risk | "Patients were randomly allocated, with stratification by surgeon, to one or other of the treatment groups under comparison." (Lythgoe 1978, page 744). No additional details were provided. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Survival | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Axillary recurrence | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Long term adverse events | Unclear risk | Outcome was not reported. |
| Selective reporting (reporting bias) | Unclear risk | Short‐term and long‐term adverse events were not reported. |
Milan.
| Methods |
Study design: randomised controlled trial Country: Italy Study period: 1998 to 1999 Inclusion criteria: women aged 40‐75 years with invasive primary breast cancer ≤ 2 cm, treated with breast‐conserving surgery Exclusion criteria: history of other cancer (except non‐melanoma skin cancer), multi‐centric breast cancer and previous excisional biopsy Length of follow‐up ( median and range): 102 months (1‐120 months) |
|
| Participants |
No. in trial arm: ALND: N = 257; SLNB: N = 259 Age: ALND: median (range) = 56 (40‐75) years; SLNB: median (range) = 55 (40‐75) years Stage distribution: not reported Proportion node positive: ALND: 83/259; SLNB: 92/259 Histological type of breast cancer: ALND: ductal infiltrating, N = 212; lobular infiltrating, N = 20; other, N = 25. SLNB: ductal infiltrating, N = 209; lobular infiltrating, N = 18; other, N = 32 |
|
| Interventions | Sentinel lymph node biopsy (SLNB) plus axillary lymph node dissection (ALND) vs SLNB followed by ALND only if metastases were found in the SLN. Both groups also received breast‐conserving surgery. | |
| Outcomes | Overall survival, breast cancer‐related events (axillary metastases, supraclavicular metastases, intrabreast tumour reappearance, distant metastases), contralateral breast cancer, axillary pain, numbness or paraesthesia on operated side, arm mobility, aesthetic appearance of axillary scar, arm swelling (difference between circumference of treated and untreated arms) | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: not reported, but at least 1 sentinel node should have been removed Nodes removed ALND arm: 429 SLN from 257 participants (mean = 1.66 SLN/participant; mean non‐sentinel lymph nodes/participant = 24) Nodes removed SLNB arm: 424 SLN from 259 participants (mean = 1.63 SLN/participant; mean non‐sentinel lymph nodes/participant = 24) Method of node pathological analysis: Each sentinel node was bisected along major axis, embedded in optimal‐cutting‐temperature compound, then frozen in isopentane cooled with liquid nitrogen (SLNs < 5 mm diameter were embedded and frozen whole). 15 pairs of 4 µm thick sections were cut at 50 µm intervals, from each half node (60 sections/node). Any remaining tissue was sectioned at 100 µm intervals. If more than 1 sentinel node was found, all were analysed in this way. One section of each pair was hematoxylin and eosin stained; if this was ambiguous, the other section of the pair was stained for cytokeratins. Further treatment for node‐positive cases: yes (ALND) |
|
| Radiotherapy |
RT ALND arm: 50 Gy to ipsilateral breast over 8 weeks, with 10 Gy boost to skin surrounding the surgical scar RT SLND arm: 50 Gy to ipsilateral breast over 8 weeks, with 10 Gy boost to skin surrounding the surgical scar RT same in all trial arms?yes |
|
| Hormone and chemotherapy |
ALND: hormonal therapy: N = 133; chemotherapy: N = 21; both hormonal and chemotherapy: N = 99; neither: N = 4 ALND: Hormonal therapy: N = 126; chemotherapy: N = 16; both hormonal and chemotherapy: N = 106; neither: N = 11 Significantly more women in ALND arm had chemotherapy than in SLNB arm, but rates of hormone therapy ‐ both hormone and chemotherapy and no hormone or chemotherapy ‐ did not differ between groups. |
|
| Notes |
Baseline differences? Groups appear comparable. Intention‐to‐treat analyses? Survival, disease control in axilla and breast cancer recurrence: per‐protocol analysis employed, but few protocol violations (7/264 ALDN participants and 9/268 SLNB participants were excluded from analyses). Long‐term adverse events: no intention‐to‐treat analyses undertaken. Only women with negative sentinel nodes (who did not go on to have ALND) were included in the SLND group for long‐term adverse events analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated permuted blocks |
| Allocation concealment (selection bias) | Low risk | Randomised after resection of tumour. Data centre telephoned surgeon with treatment group information. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | No information was provided. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | No information was provided. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | No information was provided. |
| Incomplete outcome data (attrition bias) Survival | Low risk | Participants are accounted for at 10‐year follow‐up (Veronesi 2010). |
| Incomplete outcome data (attrition bias) Axillary recurrence | Low risk | Participants are accounted for at 10‐year follow‐up (Veronesi 2010). |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Low risk | Participants are accounted for at 10‐year follow‐up (Veronesi 2010). |
| Incomplete outcome data (attrition bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Long term adverse events | High risk | Only a sample of 100 women from each group was included in this analysis. The SLND group sample was biased – see below. |
| Selective reporting (reporting bias) | Unclear risk | Short‐term adverse events were not reported. |
Milan 2.
| Methods |
Study design: randomised clinical trial Country: Italy Study period: 1996 to 2000 (trial entry period) Inclusion criteria: women with primary operable breast cancer ≤ 2 cm in mammographic diameter, clinically negative axillary nodes, aged 65 to 80 years Exclusion criteria: synchronous bilateral breast cancer, distant metastases at diagnosis, history of other malignancy (except basal cell carcinoma or intraepithelial cervical cancer) Length of follow‐up: ALND: median (range) = 150 (125‐175) months. No ALND: median (range) = 149 (124‐174) months |
|
| Participants |
No. in trial arm: ALND: N = 109; no ALDN: N = 110 Age: ALND: median (range) = 70 (65‐80 ) years; no ALND: median (range) = 70 (65‐80 ) years Stage distribution: ALDN: T1a, N = 2; T1b, N = 30; T1c, N = 69; T2, N = 8. No ALDN: T1a, N = 6; T1b, N = 44; T1c, N = 52; T2, N = 8 Proportion node positive: ALDN: 25/109. No ALDN: not reported, but 2/110 (1.8%) required delayed axillary dissection for overt axillary disease during follow‐up Pathological type of breast cancer: ALDN: Infiltrating ductal carcinoma, N = 60; infiltrating lobular carcinoma, N = 20; other infiltrating carcinoma, N = 29. No ALDN: infiltrating ductal carcinoma, N = 61; infiltrating lobular carcinoma, N = 19; other infiltrating carcinoma, N = 30 |
|
| Interventions | Quadrantectomy plus axillary dissection (all 3 Berg levels removed) vs quadrantectomy alone | |
| Outcomes | Overall mortality, breast cancer mortality, breast events (ipsilateral tumour recurrence, contralateral breast cancer, distant metastases) | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: not reported Nodes removed axillary dissection arm: not reported Nodes removed no axillary dissection arm: not reported Method of node pathological analysis: not reported Further treatment for node‐positive cases: no |
|
| Radiotherapy |
RT ALND arm: postoperative RT to residual breast within 4 weeks of surgery. Axillary, supraclavicular and internal nodes were NOT irradiated, but RT fields used typically included the lower part of level I of the axilla. 50 Gy over 5 weeks, with a supplemental boost of 10 Gy to the tumour bed RT no ALND arm: postoperative RT to residual breast within 4 weeks of surgery. Axillary, supraclavicular and internal nodes were NOT irradiated, but RT fields used typically included the lower part of level I of the axilla. 50 Gy over 5 weeks, with a supplemental boost of 10 Gy to the tumour bed RT same in all trial arms? yes |
|
| Hormone and chemotherapy | All women were prescribed 10 mg tamoxifen twice daily after surgery for 5 years. 15% discontinued tamoxifen owing to side effects. | |
| Notes |
Baseline differences? possible excess of stage T1c in axillary dissection arm ‐ Table 5 (page 3, Martelli et al 2005). No P values were reported. Intention‐to‐treat analyses? yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list was reported (page 242, Martelli et al 2005), but it was not reported how this list was derived. |
| Allocation concealment (selection bias) | Low risk | Allocation was performed by calling data centre manager at study centre (page 2, Martelli et al 2005). |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | This was not reported. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | This was not reported. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Survival | Unclear risk | 14 participants were excluded from analysis for protocol violation. It is unclear to which group they were randomised. |
| Incomplete outcome data (attrition bias) Axillary recurrence | Unclear risk | 14 participants were excluded from analysis for protocol violation. It is unclear to which group they were randomised. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Unclear risk | 14 participants were excluded from analysis for protocol violation. It is unclear to which group they were randomised. |
| Incomplete outcome data (attrition bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Long term adverse events | Unclear risk | Outcome was not reported. |
| Selective reporting (reporting bias) | Unclear risk | Short‐term and long‐term adverse events were not reported. |
Milan 3.
| Methods |
Study design: single‐centre RCT (non‐inferiority) Country: Italy Study period: 1998 to 2003 Inclusion criteria: patients with mammographically detected T1 N0 breast cancer, aged 65 to 80 years Exclusion criteria: bilateral or pluricentric breast cancer, distant metastases, history of previous malignancy or histological evidence of non‐infiltrating carcinoma only. Patients with unexpected pathological findings of bifocal breast cancer (smaller lesion close to the reference cancer); patients with T1 disease with tumour size > 2 cm at final histology were not excluded Length of follow‐up: median (IQR) = 127.5 (112.5‐141.1) months |
|
| Participants |
No. in trial arm: ALND: N = 272; no ALND: N = 245 Age: ALND: mean (SD) = 52.7 (7.5) years; no ALND: mean (SD) = 52.5 (7.9) years Stage distribution: ALDN: T1A/B, N = 92; T1C, N = 174; T2, N = 6. No ALDN: T1A/B, N = 88; T1C, N = 154; T2, N = 3 Proportion node positive (histopathologically confirmed): ALDN: 78/272 participants; no ALDN: not reported Pathological type of breast cancer: ALDN: invasive ductal carcinoma, N = 179; invasive ductal carcinoma + invasive lobular carcinoma, N = 29; invasive lobular carcinoma, N = 40; other, N = 24. No ALDN: invasive ductal carcinoma, N = 154; invasive ductal carcinoma + invasive lobular carcinoma, N = 36; invasive lobular carcinoma, N = 32; other, N = 23 |
|
| Interventions | Quadrantectomy + complete ALND (3 Berg levels) vs quadrantectomy without ALND | |
| Outcomes | Disease‐free survival, overall survival, local recurrence, distant metastases, axillary relapse | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: not reported, beyond 3 Berg levels Nodes removed axillary dissection arm: median (range) = 20 (11‐43) Nodes removed no axillary dissection arm: not reported Method of node pathological analysis: Formalin‐fixed paraffin‐embedded surgical specimens were sectioned and stained with hematoxylin and eosin. Tumours considered to be positive for oestrogen receptor/progesterone receptors if > 10% of tumour cell nuclei were immunostained. Further treatment for node‐positive cases: yes, see the 2 cells below |
|
| Radiotherapy |
RT ALND arm: postoperative RT to the operated breast, with no attempt to include the axilla or supraclavicular or internal mammary lymph nodes in the irradiation fields. Participants (N = 132) with node‐negative, oestrogen receptor‐positive and grade I‐II received RT and no adjuvant treatment (outlined in cell below); patients (N = 140) with node‐positive and/or oestrogen receptor‐negative and/or grade III received adjuvant treatment followed by radiotherapy. RT no ALND arm: postoperative RT to the operated breast, with no attempt to include the axilla or supraclavicular or internal mammary lymph nodes in the irradiation fields. Participants (N = 158) with oestrogen receptor‐positive and up to 1 of the following features: grade III, HER2‐positive or laminin receptor‐positive received RT and no adjuvant treatment (outlined in cell below); patients (N = 87) with oestrogen receptor‐negative with or without more than 1 of the following features: grade III, HER2‐positive or laminin receptor‐positive received adjuvant treatment followed by radiotherapy. RT same in all trial arms? yes, it seems so |
|
| Hormone and chemotherapy | Anthracycline‐based adjuvant chemotherapy consisted of epirubicin 120 mg/m2 every 3 weeks for 4 cycles followed by cyclophosphamide 600 mg/m2 on days 1 and 8, methotrexate 40 mg/m2 on days 1 and 8 and 5‐fluorouracil 600 mg/m2 on days 1 and 8 every 4 weeks for 4 cycles. Hormonal treatment for all participants after chemotherapy consisted of tamoxifen 20 mg/d for 5 years. 140/ 272 (51%) participants in the ALND arm received chemotherapy, and 87/245 (36%) in the no ALND arm received chemotherapy (difference was significant at P < 0.001). |
|
| Notes |
Baseline differences? possible difference in proportion of participants with a favourable prognostic profile: ALND = 48.5%; no ALND = 64.5% Intention‐to‐treat analyses? no, the only analyses presented were conducted on an as‐treated basis. Among randomised participants, 14 ALND participants and 34 no ALND participants did not receive assigned treatment and were excluded from analyses. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Email contact with the corresponding author confirmed that "The women for trial INT09/98 were randomised by calling the data manager at the study coordination centre. After the inclusion and exclusion criteria had been checked, eligible women were assigned to axillary dissection vs no axillary surgery using a randomisation list." |
| Allocation concealment (selection bias) | Low risk | See cell above. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | No information was reported. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | No information was reported. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | No information was reported. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | No information was reported. |
| Incomplete outcome data (attrition bias) Survival | Low risk | 14/286 ALND participants and 34/279 no ALND participants did not receive assigned treatment and were excluded from analyses. |
| Incomplete outcome data (attrition bias) Axillary recurrence | Low risk | 14/286 ALND participants and 34/279 no ALND participants did not receive assigned treatment and were excluded from analyses. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Low risk | 14/286 ALND participants and 34/279 no ALND participants did not receive assigned treatment and were excluded from analyses. |
| Incomplete outcome data (attrition bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Long term adverse events | Unclear risk | Outcome was not reported. |
| Selective reporting (reporting bias) | High risk | No morbidity outcomes were reported. |
NSABP B‐04.
| Methods |
Study design: RCT Country: USA and Canada Study period: 1971‐1974 Inclusion criteria: women with primary operable potentially curable breast cancer, with tumours confined to breast or breast and axilla, with tumours moveable in relation to underlying muscle and chest wall, with axillary nodes moveable in relation to chest wall and neuromuscular bundle, with no arm oedema Exclusion criteria: pregnancy, lactation previous treatment for current neoplasm, prior or concomitant cancer other than an effectively managed basal or squamous cell skin tumour, bilateral breast cancer, tumour other than a carcinoma, inflammatory tumour, skin ulceration > 2 cm, peau d’orange involving more than one‐third of the skin of the breast, satellite or parasternal nodules, fixation of axillary lymph nodes (> 2 cm), lymph nodes elsewhere suspected of containing tumour unproved by biopsy to be negative, poor surgical risks precluding any treatment options, presence of non‐malignant systemic disease making prolonged follow‐up unlikely Length of follow‐up: 25 years |
|
| Participants |
No. in trial arms: clinically node negative: ALND: N = 389; total mastectomy + RT: N = 386; total mastectomy alone: N = 384. Clinically node positive: ALND: N = 301; total mastectomy + RT: N = 305 Age: clinically node negative: ALND: 56.5 years; total mastectomy + RT: 55.6 years; total mastectomy alone: 56.4 years. Clinically node positive: ALND: 55.3 years; total mastectomy + RT: 55.3 years Stage distribution: not reported, but Pathologic size of tumour was (for 1599/1665 participants): clinically node negative: ALND: 3.2 (SD 1.99) cm; total mastectomy + RT: 3.4 (SD 2.25) cm; total mastectomy alone: 3.1 (SD 1.73) cm. Clinically node positive: ALND: 3.7 (SD 2.02) cm; total mastectomy + RT: 3.7 (SD 1.95) cm Proportion node positive: See No. in trial arms entry above. Pathological type of breast cancer (for 1578/1665 participants): clinically node negative: ALND: infiltrating duct not otherwise stated (NOS) pure 46.3%, infiltrating duct NOS combinations 35.1%, medullary 3.5%, lobular 5.6%, mucoid 2.9%, tubular 0.9%, other 5.6%. Total mastectomy + RT: infiltrating duct NOS pure 48.5%, infiltrating duct NOS combinations 31%, medullary 3.3%, lobular 5.4%, mucoid 3.3%, tubular 1.5%, other 6.9%. Total mastectomy alone: infiltrating duct NOS pure 41.2%, infiltrating duct NOS combinations 37.2%, medullary 6%, lobular 7.1%, mucoid 2%, tubular 1.1%, other 5.4%. Clinically node positive: ALND: infiltrating duct NOS pure 57.1%, infiltrating duct NOS combinations 25.6%, medullary 8.4%, lobular 4.4%, mucoid 1.5%, tubular 0.4%, other 2.6%. Total mastectomy + RT: infiltrating duct NOS pure 62.1%, infiltrating duct NOS combinations 23.4%, medullary 3.9%, lobular 4.3%, mucoid 1.1%, tubular 0.7%, other 4.6% |
|
| Interventions | Participants were clinically assessed to be axillary node positive or axillary node negative before randomisation, then were randomly assigned to the following treatments: If node negative: radical mastectomy (see below) vs total mastectomy (see below) + regional radiation vs total mastectomy alone. Participants designated as having clinically negative axillary nodes who had a total mastectomy and subsequently developed clinical evidence of axillary node involvement in the absence of other manifestations of disease were managed as follows. biopsy of involved nodes was performed to determine their status. If such nodes were reported as tumour positive, an axillary dissection was performed. If node positive: radical mastectomy vs total mastectomy + regional radiation. Radical mastectomy: removal of breast, pectoral muscles and axillary content en bloc. Total (simple) mastectomy: total removal of breast tissue in that area bounded by the midline of the sternum extending superiorly to the supraclavicular space, posteriorly along the lateral edge of the latissimus dorsi and inferiorly to the costal margin. Removal of the nipple was included. The pectoral fascia but not the pectoral muscles, together with an adequate excision of skin affected by tumour, was removed. No operative intervention was permissible in the axilla beyond the border of the pectoral muscle per protocol. |
|
| Outcomes | Disease‐free survival, overall survival, arm oedema | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: axillary clearance: see Interventions Nodes removed ALND arm: median = 15.5 nodes, mean = 17.7 nodes (range, 3‐63) Nodes removed total mastectomy: two‐thirds of participants having a total mastectomy had no nodes in the specimen; in 90%, ≤ 5, in 97%, ≤ 10. Median = 0 nodes, mean = 2 nodes (range, 0‐31) Method of node pathological analysis: not reported Further treatment for histological node‐positive cases: no (but in the clinical node negative arm ‐ ALND was done if nodes became clinically involved and histological evidence showed node metastasis on biopsy) |
|
| Radiotherapy |
Participants in the total mastectomy + RT arm Clinically negative axillary node: Both internal mammary and supraclavicular nodes received a tumour dose of 45 Gy in 25 fractions. Both chest wall and mid‐axilla received a tumour dose of 50 Gy in 25 fractions. Clinically positive axillary node: as for clinically node‐negative participants + an additional 10‐20 Gy boost to the mid‐axilla RT same in all trial arms? no |
|
| Hormone and chemotherapy | None received adjuvant systemic therapy. | |
| Notes | 68/365 node‐negative women who received total mastectomy alone subsequently had pathological confirmation of positive ipsilateral nodes. Positive nodes were identified within 2 years of surgery in 51/68, > 2‐5 years after surgery in 10/68, > 5‐10 years after surgery in 6/68 and > 10 years after surgery in 1/68. Median (range) time from mastectomy to identification of positive axillary nodes = 14.8 (3‐134.5) months. Baseline differences? Groups appear to be comparable at baseline. Intention‐to‐treat analyses? not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details were reported. |
| Allocation concealment (selection bias) | Unclear risk | No details were reported. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Survival | Unclear risk | Data were reported for clinically node negative: ALND: N = 362/389; total mastectomy + RT: N = 352/386; total mastectomy alone: N = 365/384. Clinically node positive: ALND: N = 292/301; total mastectomy + RT: N = 294/305 |
| Incomplete outcome data (attrition bias) Axillary recurrence | Unclear risk | Data were reported for clinically node negative: ALND: N = 362/389; total mastectomy + RT: N = 352/386; total mastectomy alone: N = 365/384. Clinically node positive: ALND: N = 292/301; total mastectomy + RT: N = 294/305 |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Unclear risk | Data were reported for clinically node negative: ALND: N = 362/389; total mastectomy + RT: N = 352/386; total mastectomy alone: N = 365/384. Clinically node positive: ALND: N = 292/301; total mastectomy + RT: N = 294/305 |
| Incomplete outcome data (attrition bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Long term adverse events | Unclear risk | Outcome was not reported. |
| Selective reporting (reporting bias) | Unclear risk | Short‐term and long‐term adverse events were not reported. |
NSABP B‐32.
| Methods |
Study design: RCT (multi‐centre) Country: USA and Canada Study period: 2001‐2004 Inclusion criteria: patients with operable invasive primary breast cancer and clinically node negative Exclusion criteria: none listed Length of follow up: median (for all participants) = 131.1 months; median (for SLN‐negative participants) = 9.4 years |
|
| Participants |
Total N = 5611, but data reported only in full publications for pathologically SLN‐negative participants: No. in trial arms: ALND: N = 1975; SLN: N = 2011 Age: ALND: ≤ 49 years: N = 488; ≥ 50 years: N = 1490; SLN: ≤ 49 years: N = 491; ≥ 50 years: N = 1520 Stage distribution: not reported Proportion node positive: Pathologically node‐positive participants were not included in the present analyses. Pathological type of breast cancer: not reported, but clinical tumour size was reported: ALND: ≤ 2 cm: N = 1655; 2.1‐4 cm: N = 291; ≥ 4.1 cm: N = 32; SLN: ≤ 2 cm: N = 1689; 2.1‐4 cm: N = 294; ≥ 4.1 cm: N = 28 |
|
| Interventions | SLN resection + ALND vs SLN resection without ALND if SLN were negative, and with ALND if SLN were positive or if no SLN were identified during SLN resection | |
| Outcomes | Survival, regional control, morbidity, quality of life | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: ALND: not reported Nodes removed ALND arm: not reported Nodes removed SLN resection: not reported Method of node pathological analysis: All SLNs were fixed and paraffin‐embedded, and serial sections were obtained in 2‐3 mm slices. Staining with hematoxylin and eosin was done, and immunohistochemistry was reserved for confirmation of suspected metastases. |
|
| Radiotherapy |
Patients in the ALND arm: not reported Patients in the SLN arm: not reported RT same in all trial arms? unclear, but 1618/1975 ALND participants and 1650/2011 SLN participants received RT |
|
| Hormone and chemotherapy | 1680/1975 ALND participants and 1694/2011 SLN participants received systemic adjuvant therapy (not further specified). | |
| Notes |
A majority of data were reported only for pathologically SLN‐negative participants: In addition to these participants, N = 829 were pathologically SLN‐positive/SLN‐not assessed in the ALND group, and N = 793 SLN‐positive/SLN‐not assessed in the SLN group. A substudy was conducted within the whole study, which studied quality of life: "By design, the sub study included all SN‐negative patients randomly assigned at participating institutions designated as members of the Community Clinical Oncology Program, a National Cancer Institute program that encourages clinical trial participation by community‐based physicians." This substudy included data from 356 and 391 ALND and SNL participants, respectively; these data are not included here, as it is unclear how participating institutions designated as members of the Community Clinical Oncology Program differ from participating institutions not designated as members of the Community Clinical Oncology Program. Email contact with study authors allowed us to include results for all randomised participants (i.e. both node‐positive and node‐negative participants for the following outcomes: overall survival, disease‐free survival, local/regional recurrence and axillary recurrence. Baseline differences? Groups appear to be comparable at baseline. Intention‐to‐treat analyses? yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation was performed with use of a biased coin minimisation method. |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned in a 1:1 ratio at the NSABP Biostatistical Centre. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | No information was reported. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | No information was reported. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | No information was reported. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | No information was reported. |
| Incomplete outcome data (attrition bias) Survival | Low risk | All data from those participants were included. |
| Incomplete outcome data (attrition bias) Axillary recurrence | Low risk | All data from those participants were included. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Low risk | All data from those participant were included. |
| Incomplete outcome data (attrition bias) Short term adverse events | Low risk | Most participants appear to have been included. |
| Incomplete outcome data (attrition bias) Long term adverse events | High risk | Data were reasonably complete at baseline, but progressively larger proportions of data were missing at week 1, weeks 2‐3 and months 6, 12, 18, 24, 30 and 36. |
| Selective reporting (reporting bias) | Unclear risk | Data from SLN‐positive participants were not reported in detail, and poor reporting of short‐term adverse events precluded treatment group comparisons. |
Ostersund.
| Methods |
Study design: RCT Country: Sweden Study period: 1985‐1987 and 1989‐1991 Inclusion criteria: none listed directly, but it seems that included patients had to be residents of the hospital’s catchment area with operable breast cancer Exclusion criteria: none listed, but N = 62 patients who were residents of the catchment area who had breast cancer diagnosed during study periods were not included in the study for the following reasons: N = 31 elderly or disabled patients treated with tamoxifen only, N = 23 elderly patients who had simple mastectomy or lumpectomy without axillary staging, N = 4 patients at stage IV on admission, N = 4 for other reasons Length of follow‐up: median (range) = 30 (5‐76) months (for participants without histologically confirmed lymph node involvement in the axilla) |
|
| Participants |
No. in trial arms: axillary clearance: N = 100 (N = 50 from each time period); axillary sampling: N = 100 (N = 50 from each time period) Age (1987‐89 and 1989‐91 samples): axillary clearance: median (range) = 60 (31‐85) years; axillary sampling: median (range) = 60 (37‐84) years Age (1987‐89 sample only): axillary clearance: mean (SD) = 59 (12) years; axillary sampling: mean (SD) = 61 (13) years Stage distribution: not reported Proportion node positive: axillary clearance: N = 43/100; axillary sampling: N = 46/100 Pathological type of breast cancer: not reported, but tumour diameter was reported Tumour diameter (1987‐89 and 1989‐91 samples): axillary clearance: median (range) = 21 (7‐70) mm; axillary sampling: median (range) = 21 (9‐80) mm Tumour diameter (1987‐89 sample only): axillary clearance: mean (SD) = 24 (11) mm (?); axillary sampling: mean (SD) = 23 (9) mm (?) Primary surgery (1987‐89 and 1989‐91 samples): axillary clearance: total mastectomies N = 67, partial mastectomies N = 33; axillary sampling: total mastectomies N = 63, partial mastectomies N = 37 Primary surgery (1987‐89 sample only): axillary clearance: total mastectomies N = 33, partial mastectomies N = 17; axillary sampling: total mastectomies N = 33, partial mastectomies N = 17 |
|
| Interventions | Axillary dissection (aimed to remove all fat tissue in axilla up to the axilla vein. No muscles were divided. The vein and the nerves to the anterior serratus and latissimus dorsi muscles were identified and carefully exposed. No attempt was made to save the intercostobrachial nerves; procedure corresponds to level II clearance) vs axillary node sampling (aimed to excise axillary fat containing lymph nodes. If no nodes were palpable, the lower half of the axillary fat was excised. Any suspected pathological nodes were also removed. No special efforts were made to identify the vein or the nerves) All: In general, women < 70 years or with T1 tumours (largest diameter on mammograms < 2 cm) received partial mastectomy, and women with T2 tumours or > 70 years with T1 tumours received mastectomy. |
|
| Outcomes | Recurrence (1987‐89 & 1989‐91 samples), operating time (1987‐89 sample only), postoperative discharge (1987‐89 sample only), duration of postoperative drainage (1987‐89 sample only), hospital stay (1987‐89 sample only), seroma (1987‐89 sample only), shoulder mobility (12 months; 1987‐89 sample only), arm volume (3, 6, 12 months; 1987‐89 sample only), sensibility (6 months; 1987‐89 sample only) | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: axillary clearance: not reported beyond details in ‘Interventions’. Axillary sampling: not reported beyond details in ‘Interventions’ Nodes removed clearance arm (1987‐89 and 1989‐91 samples): median (range) = 8.5 (0‐16); median (range) positive nodes: 2 (1‐14) Nodes removed sampling arm (1987‐89 and 1989‐91 samples): median (range) = 6 (0‐14); median (range) positive nodes: 2 (1‐9) Nodes removed clearance arm (1987‐89 sample only): mean (range) = 7.2 (3‐16) Nodes removed sampling arm (1987‐89 sample only): mean (range) = 4.5 (0‐10) Nodes removed SNB + clearance: NA Method of node pathological analysis: histopathological examination (axillary fat was cut into slices 55 mm thick, and each slice was crushed manually and searched for lymph nodes, including microscopy) Further treatment for node‐positive cases: yes (radiotherapy) |
|
| Radiotherapy |
All: postoperative RT given to women < 70 years (1) after partial mastectomy, (2) with T2 tumour irrespective of N status, (3) with lymph node metastases. RT included the axilla (except in 3 participants with partial mastectomy; clearance N = 2, sampling N = 1, who received RT to the breast only). The type of axillary operation did not influence indications for or extent of RT. RT generally began 1 month after surgery and was given over 4‐5 weeks. Radiation to the axilla was delivered with mega‐voltage photons, averaging 43 (38‐46) Gy to the anterior port. Radiation after mastectomy was given with electrons to the thoracic wall in doses averaging 38 Gy. After partial mastectomy, 58 Gy was given to the breast with photons. RT same in all trial arms? yes |
|
| Hormone and chemotherapy | Chemotherapy was not used, but tamoxifen was given to N = 24 postmenopausal women with nodal metastases (clearance N = 11, sampling N = 13). | |
| Notes | For the 1987‐89 sample, follow‐up of 95 participants was complete follow‐up. Of the remaining 5 participants, 2 moved out of the area and 2 died of disseminated disease (1 of each from each treatment group and 1 dissection participant could not participate in final follow‐up). Baseline differences? Only a few baseline characteristics were reported. Intention‐to‐treat analyses? not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was reported beyond that participants were randomised. |
| Allocation concealment (selection bias) | Unclear risk | No information was reported. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | No details were provided. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | No details were provided. |
| Incomplete outcome data (attrition bias) Survival | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Axillary recurrence | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Low risk | Data appear to be available for all participants. |
| Incomplete outcome data (attrition bias) Short term adverse events | High risk | Outcome was reported only for the 1987‐1989 sample, that is, for 50/100 participants. |
| Incomplete outcome data (attrition bias) Long term adverse events | High risk | Outcome was reported only for the 1987‐1989 sample, that is, for 50/100 participants. |
| Selective reporting (reporting bias) | Unclear risk | Survival was not reported (but this may be reasonable given the low rates of recurrence). However, adverse events were reported only for the 1987‐89 sample. |
SE Scotland.
| Methods |
Study design: RCT Country: Scotland Study period: 1964‐1971 Inclusion criteria: operable breast cancer (stage I, II and some III), age 35 to 60 years Exclusion criteria: skin involvement wider than the tumour, ulceration > 3 cm, peau d'orange wide of the tumour, tumour fixed to the chest wall, homolateral axillary nodes fixed to each other or to adjacent structures, homolateral supraclavicular or infraclavicular nodes moveable or fixed, oedema of the arm, distant metastases detected by clinical examination or X‐rays of chest and pelvis Length of follow‐up (median and range): 5‐12 years |
|
| Participants |
No. in trial arms: axillary clearance: N = 256 (N = 288 in Clarke 2005 meta‐analysis); simple mastectomy: N = 242 (N = 273 in Clarke 2005 meta‐analysis) Age: axillary clearance: mean (SD) = 54.7 (9.2) years; simple mastectomy: mean (SD) = 55.4 (8.8) years Stage distribution: axillary clearance: stage I: N = 144, stage II: N = 60, stage III: N = 52. Simple mastectomy: stage I: N = 131, stage II: N = 64, stage 3: N = 47 Proportion node positive: axillary clearance: N = 89/288; simple mastectomy: N = 93/273 Pathological type of breast cancer: not reported |
|
| Interventions | Radical mastectomy (breast, pectoral muscles and axillary contents were removed en bloc) vs simple mastectomy (breast removed) plus (postoperative) radiotherapy | |
| Outcomes | Overall survival, breast cancer recurrence, long‐term and short‐term complications | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol Nodes removed axillary dissection arm: not reported, but see "Interventions" Nodes removed no axillary dissection arm: none Method of node pathological analysis: not reported Further treatment for node‐positive cases: no |
|
| Radiotherapy |
RT ALND arm: none RT simple mastectomy: 45 Gy to the breast/chest wall/internal mammary nodes in 10 fractions over 4 weeks. 42.5 Gy to the axilla and supraclavicular regions in 10 fractions over 4 weeks RT same in all trial arms? no |
|
| Hormone and chemotherapy | All participants aged 35‐60 years were given prophylactic bilateral oophorectomy. Participants who refused oophorectomy were given ovarian irradiation (aged 41‐59 years) or were withdrawn from the trial (aged 35‐40 years and aged 41‐59 years who refused ovarian irradiation). | |
| Notes | 1099 participants were randomised, and 512/1099 were withdrawn owing to benign breast tumour; an additional 89 participants were excluded from study publications owing to protocol violations, leaving 498 treated within the trial protocol (Hamilton 1977); however, data do not match Clarke 2005 numbers. All participants in the per‐protocol analysis had bilateral surgical oophorectomy or ovarian ablation by radiotherapy, some included in the Clarke 2005 analysis may not have received this. We have assumed that the reason participant numbers are higher in the Clarke 2005 analysis is that investigators included some of the 89 patients excluded owing to protocol violations. Baseline differences? Groups appear to be comparable at baseline. Intention‐to‐treat analyses? No. N = 89 were excluded owing to protocol violations. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | Random allocation was conducted by central office. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | No details were reported. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | No details were reported. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | No details were reported. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | No details were reported. |
| Incomplete outcome data (attrition bias) Survival | Low risk | The Clarke 2005 analysis includes 561 of the eligible 587 participants (i.e. included participants + those excluded for protocol violations). |
| Incomplete outcome data (attrition bias) Axillary recurrence | Low risk | Although no participants have been lost to follow‐up, data are reported only for per‐protocol treated participants. These numbers seem to be balanced between groups. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Low risk | The Clarke 2005 analysis contains 561 of the 587 eligible patients (i.e. included participants + those excluded for protocol violations). |
| Incomplete outcome data (attrition bias) Short term adverse events | High risk | Data were reported only for the first 100 participants included in each group. |
| Incomplete outcome data (attrition bias) Long term adverse events | High risk | Data were reported only for the first 100 participants included in each group. |
| Selective reporting (reporting bias) | Unclear risk | Thsi trial was conducted in 1964‐1971; still, no updated results have been published for short‐term and long‐term adverse events. |
SNAC.
| Methods |
Study design: RCT Country: Australia Study period: 2001‐2005 Inclusion criteria: patients with primary unifocal breast cancer, ≤ (Gill 2009) or < (Gill 2004; Smith 2009; Ung 2004) 3 cm in diameter, node negative on clinical evaluation, WHO PS 0‐1 and able to maintain regular follow‐up Exclusion criteria: surgery for prior ipsilateral breast cancer or prior ipsilateral axillary surgery, < 18 years old, pregnant, allergic to blue dye or radioisotope, multi‐centric cancer, ductal carcinoma in situ, evidence of metastatic disease Length of follow‐up: 12 months |
|
| Participants |
No. in trial arms: SLNB: N = 544; ALND: N = 544 Age: SLNB: ≤ 30 years, N = 2; 30‐49 years, N = 118; 50‐69 years, N = 354; ≥ 70 years, N = 71. ALND: age ≤ 30 years, N = 2; 30‐49 years, N = 117; 50‐69 years, N = 358; ≥ 70 years, N = 66 Stage distribution: not reported, but Primary tumour size was as follows: SLND: ≤ 1 cm, N = 149; > 1‐2 cm, N =243; > 2‐3 cm, N = 101; ≥ 3 cm, N = 48. ALND: ≤ 1 cm, N = 146; > 1‐2 cm, N =244; > 2‐3 cm, N = 103; ≥ 3 cm N = 42 Proportion node positive: SLNB: 159/544 (sentinel node); ALND: 137/544 (sentinel node positive) Pathological type of breast cancer: not reported |
|
| Interventions | Sentinel lymph node biopsy (SLNB; performed with blue dye together with preoperative radioisotope lymphoscintigraphy (N = 954) or blue dye alone (N = 119) + axillary clearance if any node from the SLND was positive (regardless of its location. If a sentinel node was not identified, axillary clearance was performed during the initial procedure) vs standard level I and II axillary lymph node dissection (ALND; removal of all anatomical level I and II nodes). All participants also had wide local excision or mastectomy | |
| Outcomes | Arm morbidity, surgery‐related morbidity | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: SLND (all nodes judged to be hot, blue or both) followed by level I and level II axillary node dissection Nodes removed clearance arm: mean = 16 (lower and upper quartiles = 12 and 20, respectively) nodes per participant Nodes removed SNLB: mean = 16 (lower and upper quartiles = 10 and 20, respectively) nodes per participant Across both groups, the mean number of sentinel nodes removed was 1.8 (SD = 1). Method of node pathological analysis: SLNs sliced grossly into 2 mm slices embedded in paraffin blocks, sectioned in 4 steps at 200‐micron intervals and H&E stained. Sections were also prepared on coated slides with anti‐keratin antibody CAM 5.2 to facilitate visualisation of smaller metastases. 33 women in the SLND arm had intraoperative pathology. Nodes from axillary clearance were examined with 1 H&E section. Further treatment for node‐positive cases: yes (ALND) |
|
| Radiotherapy |
Both arms: Postoperative adjuvant therapies were prescribed at the discretion of local clinicians according to national guidelines based on standard criteria. RT same in all trial arms? not reported |
|
| Hormone and chemotherapy | Both arms: No participants had received neoadjuvant chemotherapy. See also cell above. | |
| Notes | Data for several outcomes were missing. Baseline differences? The 2 groups of participants appear to be balanced with respect to participant characteristics. Intention‐to‐treat analyses? Paper states that all analyses were performed on an intention‐to‐treat basis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central random assignment was performed by staff at the National Health and Medical Research Council Clinical Trials Centre on the basis of a computerised minimisation algorithm for balancing randomisation for each institution and the following characteristics: age < 50 years, palpable primary tumour, planned lymphatic mapping with blue dye alone. |
| Allocation concealment (selection bias) | Low risk | See cell above. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | No details were reported. |
| Blinding of outcome assessment (detection bias) Long term adverse events | High risk | Arm volume, shoulder movement and sensation were measured by a clinician who was not blinded to participants' treatment groups. Participants assessed arm morbidity subjectively by using study‐specific scales; they were not blinded. |
| Incomplete outcome data (attrition bias) Survival | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Axillary recurrence | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Short term adverse events | Low risk | Data appear to be available for 539/544 ALND participants and for 544/544 SLNB participants. |
| Incomplete outcome data (attrition bias) Long term adverse events | Unclear risk | Data appear to be available for 456‐519/544 SLND participants and for 457‐509/544 ALND participants. |
| Selective reporting (reporting bias) | Unclear risk | Survival, disease‐free survival and recurrence were not reported. Arm swelling and symptoms were assessed but were not reported at 1 month. |
WSSA Glasgow.
| Methods |
Study design: 3‐arm RCT Country: Scotland Study period: 1972‐1977 Inclusion criteria: aged ≤ 76 years, operable breast cancer, no deep fixation or skin involvement, no fixation of axillary lymph nodes Exclusion criteria: none reported Length of follow‐up: 5 years in EBCTCG 1990 |
|
| Participants | Simple mastectomy with radiotherapy to the chest wall but not to nodal areas (Arm A) vs simple mastectomy with radiotherapy to both chest wall and nodal areas (Arm B) vs simple mastectomy with axillary clearance and radiotherapy to the chest wall but not to nodal areas (Arm C) No. in trial arm: Arm A: N = 123; Arm B: N = 94; Arm C: N = 118 Age median and range: not reported Stage distribution: not reported Proportion node positive: Arm A: N = 16/123; Arm B: N = 9/94; Arm C: N = 17/118 Pathological type of breast cancer: not reported |
|
| Interventions | Simple mastectomy with radiotherapy to the chest wall but not to nodal areas (Arm A) vs simple mastectomy with radiotherapy to both chest wall and nodal areas (Arm B) vs simple mastectomy with axillary clearance and radiotherapy to the chest wall but not to nodal areas (Arm C) | |
| Outcomes | Overall survival, local recurrence | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: see the next 3 lines Arm A: Protocol specifies no disturbance of nodes. Arm B: Protocol specifies no disturbance of nodes. Arm C: Axillary contents were removed. Method of node pathological analysis: not reported Further treatment for node‐positive cases: no |
|
| Radiotherapy |
Arm A: Radiotherapy to chest wall (42 Gy in 2.1 Gy fractions) Arm B: Radiotherapy to chest wall (42 Gy in 2.1 Gy fractions) and nodal areas, including axilla and supraclavicular fossa (42 Gy in 2.1 Gy fractions) Arm C: Radiotherapy to chest wall (42 Gy in 2.1 Gy fractions) RT same in all trial arms? no |
|
| Hormone and chemotherapy | Not reported | |
| Notes | Study included 3 arms: 1. Simple mastectomy with RT to chest wall but not to nodal areas; 2: Simple mastectomy with RT to both chest wall and nodal areas, including axilla and supraclavicular fossa; and 3: Simple mastectomy with axillary clearance plus RT to chest wall but not to nodal areas: results derived from arms 1 and 3 only. Data from meta‐analysis forest plot only Central randomisation Sealed cards Baseline differences? not reported Intention‐to‐treat analyses? not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Numbered envelopes: It is unclear how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes: It is unclear whether envelopes were opaque. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | This was not reported. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | This was not reported. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Survival | Low risk | Data appear to be available for all included participants. |
| Incomplete outcome data (attrition bias) Axillary recurrence | Low risk | Data appear to be available for all included participants. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Low risk | Data appear to be available for all included participants. |
| Incomplete outcome data (attrition bias) Short term adverse events | Unclear risk | Outcome was not reported. |
| Incomplete outcome data (attrition bias) Long term adverse events | Unclear risk | Outcome was not reported. |
| Selective reporting (reporting bias) | Unclear risk | Short‐term and long‐term adverse events were not reported. |
Xu 2003.
| Methods |
Study design: RCT Country: China Study period: 1992‐2003 Inclusion criteria: "Females with invasive breast cancer of stage Ⅰor Ⅱ, who were hospitalised from Jun 1992 to October 1995, agreed and signed the informed consent form" Exclusion criteria: none reported Length of follow‐up (median and range): 99.5 months (12‐136 months) |
|
| Participants |
No. in trial arm: Axillary dissection level 1 ± ovariectomy: N = 96; ALND ± ovariectomy: N = 96 Age median and range: Axillary dissection level 1 ± ovariectomy: 50.4 (31‐69) years; ALND ± ovariectomy: 48.3 (29‐69) years Stage distribution: Axillary dissection level 1 ± ovariectomy: clinical stage I/II: N = 17/79; TMN stage T1/T2/T3: N = 20/74/2; TMN stage N0/1/4/10: N = 62/23/8/3; ALND ± ovariectomy: clinical stage I/II: N = 12/84; TNM stage T1/T2/T3: N = 15/78/3; TNM stage N0/1/4/10: N = 56/26/11/3 Proportion node positive: unclear, but possibly as reported in the lines above Pathological type of breast cancer: not reported, but ER status was as follows: Axillary dissection level 1 ± ovariectomy: ER +/‐: N = 64/32; ALND ± ovariectomy: ER+/‐: N = 64/32 |
|
| Interventions | Mastectomy and axillary dissection (level I axillary lymph nodes were cleared) ± ovariectomy (16 participants received ovariectomy) vs radical mastectomy ± ovariectomy (20 participants received ovariectomy; 35 underwent Halsted radical mastectomy; and 61 had a modified radical mastectomy operation (retaining pectoralis major muscle and medialis and lateralis branches of the thoracic nerve, cutting off the pectoralis minor muscle. The clearing scope of the axillary lymph node is the same as that for a Halsted radical mastectomy)). | |
| Outcomes | 10‐Year overall survival, 10‐year disease‐free survival, local recurrence, upper limb oedema, distant metastasis, involved upper limb disorder, cardiovascular events, cerebrovascular accident | |
| Axillary node surgery |
Minimum no. nodes to be removed according to protocol: see the next 3 lines Axillary dissection level 1 ±ovariectomy: Level Ⅰ lymph node clearance (only the lower axillary lymph nodes were cleared) ALND ±ovariectomy: Halsted radical mastectomy (all upper, middle and lower axillary lymph nodes were cleared) was performed for 35 participants, and 61 were treated with modified radical mastectomy (type Ⅱ). Method of node pathological analysis: "Confirmed by pathological examination" Further treatment for node‐positive cases: yes |
|
| Radiotherapy | "Postoperative radiotherapy was delivered to the internal mammary and clavicle area, to the metastasis in patients with axillary lymph node number ≥ 4, or to patients whose primary tumour were located inside to the nipple." Radiotherapy was given to 30 participants in the axillary dissection level 1 ± ovariectomy arm and to 42 in the ALND ± ovariectomy arm. RT same in all trial arms? yes |
|
| Hormone and chemotherapy | Postoperative adjuvant CMF chemotherapy was administered to participants with breast cancer stage Ⅰ‐Ⅱ, tumour size > 1 cm. The chemotherapy regimen was composed of CTX 500 mg/m2, 5‐FU 500 mg/m2, MTX 30 mg/m2. Axillary dissection level 1 ±ovariectomy: 34 participants completed 6 cycles of chemotherapy. ALND ±ovariectomy: 35 participants completed 6 cycles of chemotherapy Oral tamoxifen was given to participants after chemotherapy, to participants intolerant to chemotherapy and to ER‐positive participants (10 mg daily, 2 times a day). |
|
| Notes | The study was published in Chinese and was kindly translated and data extracted by Lixin Ma (School of Public Health, Hebei University, China). Risk of bias was discussed by 2 review authors. One review author entered this information into Review Manager. Baseline differences? Groups appear to be comparable at baseline. Intention‐to‐treat analyses? no. Analyses were per‐protocol. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "192 cases invasive breast cancer patients diagnosed as phase Ⅰ‐Ⅱ during the period from Jun 1992 to October 1995 signed informed consent, and participated in this study. They were randomly divided into two groups. 96 cases were in MAD ± ovariectomized group and 96 cases in RM± ovariectomized group." No further information was reported. |
| Allocation concealment (selection bias) | Unclear risk | "The selected patients were then acknowledged and allocated to two groups through sealed envelope." No further information was reported. |
| Blinding of outcome assessment (detection bias) Disease control in the axilla | Unclear risk | Information was collected from clinical records and clinical examination. No further information was reported. |
| Blinding of outcome assessment (detection bias) Breast cancer recurrence | Unclear risk | Information was collected from clinical records and clinical examination. No further information was reported. |
| Blinding of outcome assessment (detection bias) Short term adverse events | Unclear risk | Information was collected from clinical records and clinical examination. No further information was reported. |
| Blinding of outcome assessment (detection bias) Long term adverse events | Unclear risk | Information was collected from clinical records and clinical examination. No further information was reported. |
| Incomplete outcome data (attrition bias) Survival | Low risk | 10‐Year follow‐up: loss to follow‐up: 3 participants in the level I clearance group; 8 in the ALND group. Participant flow chart was unavailable. |
| Incomplete outcome data (attrition bias) Axillary recurrence | Low risk | 10‐Year follow‐up: Loss to follow‐up: 3 participants in the level I clearance group; 8 in the ALND group. Participant flow chart was unavailable. |
| Incomplete outcome data (attrition bias) Breast cancer recurrence | Low risk | 10‐Year follow‐up: Loss to follow‐up: 3 participants in the level I clearance group; 8 in the ALND group. Participant flow chart was unavailable. |
| Incomplete outcome data (attrition bias) Short term adverse events | Low risk | 10‐Year follow‐up: Loss to follow‐up: 3 participants in the level I clearance group; 8 in the ALND group. Participant flow chart was unavailable. |
| Incomplete outcome data (attrition bias) Long term adverse events | Low risk | 10‐Year follow‐up: Loss to follow‐up: 3 participants in the level I clearance group; 8 in the ALND group. Participant flow chart was unavailable. |
| Selective reporting (reporting bias) | Unclear risk | Not enough information is available, and reporting of morbidity outcomes is limited. |
5‐FU: 5‐fluorouracil. ALND: axillary lymph node dissection. BIS: bispectral index scale. CMF: cyclophosphamide, methotrexate, 5‐fluorouracil. CTX: cyclophosphamide. DFS: disease‐free survival. ER: oestrogen receptor. H&E: hematoxylin and eosin. IQR: interquartile ratio. ITT: intention‐to‐treat. NA: not applicable. MAC: minimal alveolar concentration. MTX: methotrexate. QOL: quality of life. RCT: randomised controlled trial. RT: radiotherapy. SD: standard deviation. SLN: sentinel lymph node. SLNB: sentinel lymph node biopsy. WHO PS: World Health Organization Perfomance Scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| AATRM‐048‐13‐2000 | Inclusion criteria included positive sentinel lymph node: Participants were randomised before sentinel lymph node biopsy but were included only if the biopsy indicated micrometastasis. |
| ACOSOG Z0011 | Participants were eligible only if they had positive sentinel lymph node biopsy: Randomisation took place after sentinel lymph node biopsy results were known. |
| Buenos Aires | Participants were not randomised: Participants born on even months received axillary lymph node dissection (ALND), and those born on odd months were given wide tumour excision. |
| Copenhagen | Participants were not randomised: On arrival, participants were given consecutive numbering of their records. Participants with even numbers were allocated to the axillary lymph node dissection (ALND) group, and those with odd numbers were allocated to the simple mastectomy + radiotherapy (RT) group. |
| Edinburgh SES | Study compared radiotherapy vs no radiotherapy after simple mastectomy in clinically node‐negative women. |
| IBCSG‐23‐01 | Participants were eligible only if they had positive sentinel lymph node biopsy: Randomisation took place after sentinel lymph node biopsy results were known. |
| IPO‐P | Participants were eligible only if they had negative sentinel lymph node biopsy: Randomisation took place after sentinel lymph node biopsy results were known. |
| OTOASOR | Study compared completion axillary lymph node dissection vs axillary nodal irradiation in participants with sentinel lymph node‐positive primary invasive breast cancer. |
Characteristics of studies awaiting assessment [ordered by study ID]
ISRCTN88463711.
| Methods |
Study design: randomised controlled trial (RCT) Country: United Kingdom |
| Participants |
Inclusion criteria: histologically proven breast cancer, tumour size no greater than 4 cm, no skin involvement, aged < 70 years, no medical contraindications to treatment protocols Exclusion criteria: none listed |
| Interventions | Surgery (wide local excision) and axillary node sampling, followed by radiotherapy to the breast and, if the sample is positive, radiotherapy to the axillary lymph nodes vs surgery (wide local excision) and axillary lymph node dissection (ALND) + radiotherapy to the breast |
| Outcomes | Not reported |
| Notes |
Semiglazov 2003.
| Methods | Study design: described as randomised; no further information reported |
| Participants | 212 patients with T1‐2N0M0 breast cancer (superficial tumours no larger than 2.5 cm in diameter) |
| Interventions | Modified mastectomy by Patey‐Dyson (1985‐90, 207 participants) vs organ‐sparing treatment (segmental resection of a breast + axillary dissection + radiotherapy – 1985‐97, 211 participants): sectorial or segmental resection performed 1 cm away from the tumour margin with axillary resection at the I‐II level. Radiotherapy done on gamma‐therapeutic apparatus “Rocus” with the use of classic fractionation (2 Gy daily 5 times a week) at a summative local dosage (SLD) applied to the breast of 50‐60 Gy. To the bed of the tumour, 10 Gy was applied additionally in 5 fractions. Zones of lymphatic collectors (axillary‐subclavian and parasternal) in cases when metastases were found were radiotreated with the analogous regimen (SLD = 40 Gr). All participants with receptor‐positive tumours received hormonal therapy with tamoxifen 20 mg daily for 5 years. Those with receptor‐negative tumours received adjuvant chemotherapy CMF (cyclophosphamide + methotrexate + 5‐fluorouracil) or FAC (5‐flurouracil + doxorubicin + cyclophosphamide) up to 6 courses. |
| Outcomes | Survival, local recurrence, distant metastasis |
| Notes | Paper was published in Russian and, after initial translation of sections related to treatment group allocation and axillary treatment by Dr Liliya‐Eugenevna Ziganshina (Department of Basic and Clinical Pharmacology, Kazan Federal University, Russian Federation), which showed that these sections did not provide sufficient detail, we emailed study author on 16/6/15 to ask for additional study details, specifically answers to the following two questions: 1. How were participants allocated to receive EITHER modified mastectomy OR organ‐sparing treatment (segmental resection of a breast + axillary dissection + radiotherapy)? Were they randomised to either of these treatment groups, and, if yes, how were they randomised? We would appreciate it if you would give us as much detail as possible about the recruitment and treatment allocation process. 2. Exactly what interventions did the 2 treatment groups receive to the axilla? Again, we are interested in learning as much detail as possible, including the level of node clearance (level I, I, or III). On 9/7/15, we received the following response: "Thank you for your attention to our studies performed in 1985 and 1990, “Sparing and organ‐saving operations in breast cancer,” and “The modern organ‐ and function‐sparing surgical treatment in oncology. "The first trial included patients with clinically early breast cancer (c)T1‐2N0M0. The second one included only patients with (c)T1N0M0. Patients were randomly assigned in a 1:1 ratio to receive Patey‐Dyson modified mastectomy versus segmental resection of the breast + axillary lymph node dissection up to level I or level II (in case of detection of axillary metastases in level I nodes as a result of intraoperative biopsy – in 20% of conservative surgery arm and 23% in modified mastectomy group). Randomization was done centrally at the department of Epidemiology and Statistics at the N.N. Petrov Research Institute of Oncology operation office with a computer program and a minimization technique, taking into account age, histologic type and grade (G) and hormone‐receptor status. The same principles were used in the second trial in which patients with (c)T1N0M0 were undergoing breast conservative surgery ± radiotherapy. Sentinel lymph node biopsy with the use of radio‐tracer has been routinely performed in (c)N0 patients in our institute for ten years by now. In 2014 we initiated a study to evaluate the role of the sentinel node biopsy in patients who had undergone neoadjuvant systemic therapy." Study author emailed again on 13/7/15, as no clear response had been received to the second question in our original email, i.e. exactly which interventions did patients receive to the axilla (e.g. what is a Patey‐Dyson modified mastectomy). Our second email was re‐sent on 17/8/15, as no response had been received. To date, we have received no response. |
Characteristics of ongoing studies [ordered by study ID]
AMAROS.
| Trial name or title | AMAROS |
| Methods |
Study design: RCT (multi‐centre, non‐inferiority) Country: Europe |
| Participants |
Inclusion criteria: patients with operable unifocal invasive breast cancer (5‐30 mm) and clinically node negative Exclusion criteria: metastatic disease, previous treatment of the axilla by surgery or radiotherapy, previous treatment of cancer (except basal cell carcinoma of the skin and in situ carcinoma of the cervix), pregnancy |
| Interventions | Women were randomised before surgery and SLNB to the treatment they would receive if their SLNB proved positive. Women with negative SLNB received no additional treatment. Those with a positive lymph node received axillary lymph node dissection (level I and II) or axillary radiation therapy. Patients could also receive adjuvant systemic chemo/endocrine therapy according to local guidelines. |
| Outcomes | Regional control, survival, long‐term morbidity |
| Starting date | 2001 |
| Contact information | Emiel Rutgers, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, Netherlands. Email: e.rutgers@nki.nl |
| Notes | Target number of participants is 4766; up until December 2008, more than 4000 participants had been enrolled. |
GF‐GS 01.
| Trial name or title | GF‐GS 01/NCT00144898 |
| Methods |
Study design: RCT Country: France |
| Participants |
Inclusion criteria: women aged 18‐90 years with clinically node‐negative operable unifocal N0 breast cancer (clinical tumour size < 30 mm) Exclusion criteria: none listed |
| Interventions | ALND vs SLN resection |
| Outcomes | Recurrence‐free survival |
| Starting date | 2003 |
| Contact information | Alain LEIZOROVICZ, Université Claude Bernard Lyon I (responsible party), Gilles Houvenaeghel, Institut Paoli Calmette (principal investigator) |
| Notes |
KiSS.
| Trial name or title | KiSS (Klinisch‐Interdisziplinäre‐SentinelNode‐Studie) |
| Methods |
Study design: RCT Country: Germany |
| Participants |
Inclusion criteria: histologically proven unifocal breast cancer < 25 mm diameter, clinically and sonographically unsuspicious ipsilateral axillary lymph nodes Exclusion criteria: none listed |
| Interventions | SLNB + ALND vs SLNB + ALND only if the SLN was positive. Women received adjuvant therapy according to St. Gallen and AGO eV guidelines. |
| Outcomes | Axillary recurrence, shoulder and arm morbidity |
| Starting date | Unclear, but the trial was definitely running from November 20000 until September 2002 |
| Contact information | Contacted study author on Helms (2009): R Kreienberg, +49 731 500 58501, rolf.kreienberg@uniklinik‐ulm.de |
| Notes | Although some trial data are published in the Schem (2011) abstract, this trial is not published in full in any of the identified publications (Helms 2009 published only data from a subgroup of about 10% of participants), and we cannot extract relevant data for full inclusion of this study. |
NCT01717131.
| Trial name or title | NCT01717131/Institut Paoli‐Calmettes |
| Methods |
Study design: RCT Country: France |
| Participants |
Inclusion criteria: patients aged ≥ 18 years with (histologically or cytologically (by fine‐needle biopsy)) proven, invasive (unifocal tumour, TI‐T2 (up to 5 cm, clinical or imagery)) breast cancer, clinically N0 and M0, who have received no previous therapy (neoadjuvant or hormone therapy), for whom conservative surgery with SLN technique is feasible from the start in terms of carcinoembryology, and who are affiliated with a social security system of benefiting from such a system. The clinicaltrials.gov record further states, "All patients with lymph node involvement (GS+), whatever the size of the metastasis (macro‐metastasis, cellular cluster or isolated tumour cells)". Exclusion criteria: tumour > 5 cm, indication of neoadjuvant therapy by chemotherapy or hormone therapy, history of breast cancer (ipsilateral, i.e. recurrence, or contralateral breast, history of any invasive cancer other than a past cutaneous cancer correctly treated, initial metastatic disease known, presence of clinical axillary adenopathy, contraindication to surgical excision, contraindication to the SLN technique, pregnant women, women of child‐bearing potential, lactating women, patients deprived of liberty or under supervision of a guardian, impossibility to undergo medical examination of the study for geographical, social or psychological reasons |
| Interventions | ALND vs no ALND |
| Outcomes | Disease‐free survival, axillary recurrence rate, overall survival |
| Starting date | 2012 |
| Contact information | Dominique Genre and Sandra Cournier, +33 0491223778, bec@ipc.unicancer.fr |
| Notes |
NCT02167490.
| Trial name or title | Sentinel Node Vs Observation After Axillary Ultra‐souND |
| Methods |
Study design: RCT Country: Italy |
| Participants |
Inclusion criteria: breast cancer < 2 cm, clinically negative axilla, any age, candidates to receive breast‐conserving surgery + radiotherapy, negative preoperative assessment of the axilla (ultrasound with or without FNAC in case 1, doubtful node is found), written informed consent must be signed and dated by both participant and investigator before inclusion, participants must be accessible for follow‐up Exclusion criteria: synchronous distant metastases, previous malignancy, bilateral breast cancer, multi‐centric or multi‐focal breast cancer, previous primary systemic therapy, pregnancy or breastfeeding, preoperative diagnosis (cytology or histology) of axillary lymph node metastases, preoperative radiological evidence of multiple involved or suspicious nodes, psychiatric, addictive or any disorder that may compromise ability to give informed consent for participation in this study |
| Interventions | SLNB ± axillary dissection vs no axillary surgical staging (no axillary dissection will be performed in case of negative SLN or in the presence of isolated tumour cells or micrometastases. SLNB will be completed by axillary dissection in the presence of macrometastases diagnosed in the SLN) |
| Outcomes | Distant disease‐free survival, distant recurrence, disease‐free survival, overall survival, axillary recurrence |
| Starting date | 2014 |
| Contact information | Nicole Rotmensz, MS; Tel: +39 02 57489810; email: nicole.rotmensz@ieo.it Claudia Sangalli, MS; Tel: +39 02 57489840; email: claudia.sangalli@ieo.it |
| Notes | Other study ID number: IEO S637/311 |
NCT02271828.
| Trial name or title | Omitting sentinel node procedure in breast cancer patients undergoing breast conserving therapy |
| Methods |
Study design: RCT Country: The Netherlands |
| Participants |
Inclusion criteria: female, aged 18 years or older, pathologically confirmed invasive breast carcinoma, clinical T1‐2 tumour, will be treated with lumpectomy and whole breast radiotherapy, clinically node‐negative status: no signs of axillary lymph node metastases at physical examination and preoperative axillary ultrasound (or negative cyto/histopathology), written informed consent Exclusion criteria: clinically node‐positive preoperative, bilateral breast cancer, evidence of metastatic disease, history of invasive breast cancer, previous treatment of the axilla with surgery or radiotherapy (except surgery for hidradenitis suppurativa or for other superficially located skin lesions, such as nevi), pregnant or nursing, other prior malignancies within the past 5 years (except successfully treated basal cell and squamous cell skin cancer, carcinoma in situ of the cervix or carcinoma in situ of the ipsilateral or contralateral breast) or unsuccessfully treated malignancies > 5 years before randomisation, unable or unwilling to give informed consent |
| Interventions | SLNB vs no SLNB (or other SLN procedure) |
| Outcomes | Regional recurrence rate |
| Starting date | 2015 |
| Contact information | Marjolein L Smidt, MD, PhD, Maastricht University Medical Centre, Maastricht, the Netherlands Hans JW de Wilt, MD, PhD, Radboud University Medical Centre, Nijmegen, the Netherlands |
| Notes | Other study ID numbers: BOOG 2013‐08, BOOG 2013‐08, KWF UM 2014‐6679 |
SNAC2.
| Trial name or title | SNAC2/ACTRN12605000409673 |
| Methods |
Study design: RCT (multi‐centre) Country: New Zealand, Australia (?) |
| Participants |
Inclusion criteria: histologically or cytologically confirmed invasive breast cancer, single or multiple ipsilateral primary breast cancer, primary breast cancer may be less than or greater than 3 cm Exclusion criteria: in situ carcinoma only, clinically involved nodes for which the investigator deems axillary clearance is essential, evidence of metastatic disease, previous breast cancer or in situ carcinoma in the same breast |
| Interventions | SLNB (+ ALND if SLNB positive) vs SLNB + ALND |
| Outcomes | Locoregional recurrence, overall survival, distant disease‐free survival |
| Starting date | 2006 |
| Contact information | Dr Ian Campbell (Study Chair), Department of Surgery, Waikato Hospital, Private Bag 3200, Hamilton, New Zealand, Tel: +64 7 8398899 (Ext. 8279), email: CAMPBELI@waikatodhb.govt.nz Xanthi Coskinas (Trial Co‐ordinator), National Health and Medical Research Council (NHMRC) Clinical Trials Centre, Locked Bag 77, Camperdown NSW 1450, Australia. Tel: +61 2 95625049, email: xanthi.coskinas@ctc.usyd.edu.au. Trial web site: http://www.ctc.usyd.edu.au/trials/cancer/breast.htm |
| Notes |
SOUND.
| Trial name or title | SOUND (Sentinel node vs Observation After Axillary UltraSouND) |
| Methods |
Study design: RCT Country: Italy |
| Participants |
Inclusion criteria: breast cancer ≤ 2 cm and clinically negative axilla, any age, candidates to receive breast‐conserving surgery + radiotherapy, negative preoperative assessment of the axilla (ultrasound with or without FNAC in case 1 doubtful node is found), written informed consent must be signed and dated by the participant and the investigator before inclusion, patients must be accessible for follow‐up Exclusion criteria: synchronous distant metastases, previous malignancy, bilateral breast cancer, multi‐centric or multi‐focal breast cancer, previous primary systemic therapy, pregnancy or breastfeeding, preoperative diagnosis (cytology or histology) of axillary lymph node metastases, preoperative radiological evidence of multiple involved or suspicious nodes, patients with psychiatric/addictive/any disorder that compromises the ability to give informed consent for participation in the study |
| Interventions | SLND with axillary dissection in the presence of macrometastases diagnosed in the sentinel lymph node and SLND without axillary dissection in the case of negative sentinel lymph node or in the presence of isolated tumour cells or micrometastases vs no axillary surgical staging |
| Outcomes | Distant disease‐free survival, cumulative incidence of distant recurrences, cumulative incidence of axillary recurrences, disease‐free survival, overall survival, quality of life, evaluation of type of adjuvant treatment administered |
| Starting date | 2012 |
| Contact information | Oreste Gentilini, oreste.gentilini@ieo.it |
| Notes |
ALND: axillary lymph node dissection. FNAC: fine‐needle aspiration cytology. RCT: randomised controlled trial. SLN: sentinel lymph node. SLNB: sentinel lymph node biopsy.
Differences between protocol and review
We searched trial registries to comply with new Cochrane methodological standards
We analysed breast cancer recurrence separately for local recurrence, locoregional recurrence and distant metastasis
The protocol states that when the eligibility of a trial is judged, the results section of the publication would be masked, but results were not masked when review authors judged eligibility
The protocol predates the current Cochrane risk of bias tool, which we used for the review
With the exception of Prof Malcolm W Reed, the review authors are different from those listed in the protocol
We have updated the background section of the review
We used the GRADE approach to interpret review findings
We included an additional comparison of less surgery versus ALND, which combines comparisons 1, 2, 3 and 7 (see Types of interventions section)
Contributions of authors
NB, MSH and MA screened literature searches and extracted and analysed data. MWR interpreted results and prepared the discussion and implications for practice. EH designed and carried out literature searches. MWR, LW and DH conceived of the protocol. LW, DH, EW and CB drafted the protocol. MWR and Professor RE Coleman commented on the content of the protocol.
Sources of support
Internal sources
North Trent Cancer Research Network, UK.
External sources
No sources of support supplied
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Addenbrookes {published data only}
- Brinkley D, Haybittle JL. Treatment of stage‐II carcinoma of the female breast. Lancet 1971;2(733):1086‐7. [DOI] [PubMed] [Google Scholar]
- Brinkley D, Haybittle SL. Treatment of stage‐II carcinoma of the female breast. Lancet 1966;2(7458):291‐5. [DOI] [PubMed] [Google Scholar]
Addenbrookes 2 {published data only}
- Purushotham AD, Britton TMB, Klevesath MB, Chou P, Agbaje OF, Duffy SW. Lymph node status and breast cancer‐related lymphedema. Annals of Surgery 2007;246(1):42‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushotham AD, Upponi S, Klevesath MB, Bobrow L, Millar K, Myles JP, et al. Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. Journal of Clinical Oncology 2005;23(19):4312‐21. [DOI] [PubMed] [Google Scholar]
ALMANAC {published data only}
- Clarke D, Mansel RE. The learning curve in sentinel node biopsy (SNB) in breast cancer: results from the ALMANAC trial. Breast Cancer Research and Treatment. 69 2001; Vol. 69, issue 3:212.
- Fleissig A, Fallowfield LJ, Langridge CI, Johnson L, Newcombe RG, Dixon JM, et al. Post‐operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Research and Treatment 2006;95:279‐93. [DOI] [PubMed] [Google Scholar]
- Mansel RE. The ALMANAC trial: initial experience. Breast Cancer Research and Treatment. 64 2000; Vol. 64, issue 1:36.
- Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC trial. Journal of the National Cancer Institute 2006;98(9):599‐609. [DOI] [PubMed] [Google Scholar]
- Mansel RE, Goyal A, Fallowfield L, Newcombe RG. Sentinel node biopsy in breast cancer: the first results of the randomized multicenter ALMANAC trial. Journal of Clinical Oncology 2004;22(14):4S. [Google Scholar]
Cape Town {published data only}
- Dent DM, Gudgeon CA, Murray EM. Mastectomy with axillary clearance versus mastectomy without it. Late results of a trial in which patients had no adjuvant chemo‐, radio‐ or endocrine therapy. South African Medical Journal 1996;86(6):670‐1. [PubMed] [Google Scholar]
- Helman P, Bennett MB, Louw JH, Wilkie W, Madden P, Silber W, et al. Interim report on trial of treatment for operable breast cancer. South African Medical Journal 1972;46(38):1374‐5. [PubMed] [Google Scholar]
Cardiff {published data only}
- Forrest AP, Roberts MM, Cant EL, Henk M, Hughes LE, Hulbert M, et al. Simple mastectomy and pectoral node biopsy: the Cardiff St. Mary's trial. World Journal of Surgery 1977;1(3):320‐3. [DOI] [PubMed] [Google Scholar]
- Forrest AP, Roberts MM, Preece P, Henk JM, Campbell H, Hughes LE, et al. The Cardiff‐St Mary's trial. British Journal of Surgery 1974;61(10):766‐9. [DOI] [PubMed] [Google Scholar]
- Forrest APM, Stewart HJ, Roberts MM, Steele RJC. Simple mastectomy and axillary node sampling (pectoral node biopsy) in the management of primary breast cancer. Annals of Surgery 1982;196(3):371‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MM, Blumgart LH, Davies M, Henk JM, Forrest AP, Campbell H, et al. Simple versus radical mastectomy. Preliminary report of the Cardiff breast trial. Lancet 1973;1(812):1073‐6. [DOI] [PubMed] [Google Scholar]
- Roberts MM, Davies M, Henk M, Forrest AP, Blumgart LH, Gleave EN, et al. A clinical trial of treatment in early breast cancer. British Journal of Surgery 1973;60(4):305‐6. [PubMed] [Google Scholar]
- Roberts MM, Furnival IG, Forrest AP. The morbidity of mastectomy. British Journal of Surgery 1972;59(4):301‐2. [PubMed] [Google Scholar]
- Stewart HJ, Everington D, Forrest APM. The Cardiff local therapy trial ‐ Results at 20 years. Breast 1994;3:40‐5. [Google Scholar]
E'dburgh Sample/Clear {published data only}
- Aitken RJ, Gaze MN, Rodger A, Chetty U, Forrest AP. Arm morbidity within a trial of mastectomy and either nodal sample with selective radiotherapy or axillary clearance. British Journal of Surgery 1989;76(6):568‐71. [DOI] [PubMed] [Google Scholar]
- Forrest AP, Everington D, McDonald CC, Steele RJ, Chetty U, Stewart HJ. The Edinburgh randomized trial of axillary sampling or clearance after mastectomy. British Journal of Surgery 1995;82(11):1504‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Forrest APM, Stewart HJ, Roberts MM, Steele RJC. Simple mastectomy and axillary node sampling (pectoral node biopsy) in the management of primary breast cancer. Annals of Surgery 1982;196(3):371‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambah PA, Dixon JM, Prescott RJ, Forrest JW. Long term results of randomized studies of axillary clearance vs non‐targeted axillary sampling. Breast Cancer Research & Treatment. 64 2000; Vol. 64, issue 1:38. [MEDLINE: ]
- Steele R, Forrest A, Gibson T, Stewart H, Chetty U. The efficacy of lower axillary sampling in obtaining lymph node status in breast cancer: a controlled randomized trial. British Journal of Surgery 1985;72:368‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Edinburgh 1 {published data only}
- Chetty U, Jack W, Prescott RJ, Tyler C, Rodger A. Management of the axilla in operable breast cancer treated by breast conservation: a randomized clinical trial. Edinburgh Breast Unit. British Journal of Surgery 2000;87(2):163‐9. [DOI] [PubMed] [Google Scholar]
- Duncan W, Forrest APM, Gray N, Hamilton T, Langlands AO, Prescott RJ, et al. New Edinburgh primary breast cancer trials. British Journal of Cancer 1975;32:628‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Genoa {published data only}
- Canavese G, Catturich A, Vecchio C, Tomei D, Gipponi M, Villa G, et al. Sentinel node biopsy compared with complete axillary dissection for staging early breast cancer with clinically negative lymph nodes: results of randomized trial. Annals of Oncology 2009;20(6):1001‐7. [DOI] [PubMed] [Google Scholar]
GIVOM Sentinella {published data only}
- Bianco P, Zavagno G, Burelli P, Scalco G, Barutta L, Carraro P, et al. Morbidity comparison of sentinel lymph node biopsy versus conventional axillary lymph node dissection for breast cancer patients: results of the sentinella‐GIVOM Italian randomised clinical trial. European Journal of Surgical Oncology 2008;34(5):508‐13. [DOI] [PubMed] [Google Scholar]
- Zavagno G. A randomized clinical trial on sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer: results of the sentinella/GIVOM trial. Annals of Surgery 2008;247(2):207‐13. [DOI] [PubMed] [Google Scholar]
- Zavagno G. Clinical impact of false‐negative sentinel lymph nodes in breast cancer. European Journal of Surgical Oncology 2008;34(6):620‐5. [DOI] [PubMed] [Google Scholar]
Guy's {published data only}
- Atkins H, Hayward JL, Klugman DJ, Wayte AB. Treatment of early breast cancer: a report after ten years of a clinical trial. British Medical Journal 1972;2(811):423‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentiman IS. Long‐term follow‐up of the first breast conservation trial: Guy's wide excision study. Breast 2000;9:1‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hayward J. Conservative surgery in the treatment of early breast cancer. British Journal of Surgery 1974;61(10):770‐1. [DOI] [PubMed] [Google Scholar]
- Hayward JL. The Guy's trial of treatments of 'early' breast cancer. World Journal of Surgery 1977;1:314‐6. [DOI] [PubMed] [Google Scholar]
Hammersmith {published data only}
- Burn JI. 'Early' breast cancer: the Hammersmith trial. An interim report. British Journal of Surgery 1974;61(10):762‐5. [DOI] [PubMed] [Google Scholar]
- Burn JI, Davey J, Sellwood RA, Deeley TJ, Azzopardi J, Li J. A prospective comparative clinical trial in early carcinoma of the female breast. British Journal of Surgery 1968;55(11):869. [PubMed] [Google Scholar]
IBCSG‐10‐93 {published data only}
- Goldhirsch A, Gelber RD, Castiglione M, Price KN, Rudenstam CM, Lindtner J, et al. The best available adjuvant treatments are within the framework of clinical trials. The International Breast Cancer Study Group. Israel Journal of Medical Sciences 1995;31(2‐3):144‐54. [PubMed] [Google Scholar]
- International Breast Cancer Study Group, Rudenstam CM, Zahrieh D, Forbes JF, Crivellari D, Holmberg SB, et al. Randomized trial comparing axillary clearance versus no axillary clearance in older patients with breast cancer: first results of International Breast Cancer Study Group Trial 10‐93 [see comment]. Journal of Clinical Oncology 2006;24(3):337‐44. [DOI] [PubMed] [Google Scholar]
Institut Bergonie {published data only}
- Avril A, Bouedec G, Lorimier G, Classe JM, Tunon‐de‐Lara C, Giard S, et al. Phase III randomized equivalence trial of early breast cancer treatments with or without axillary clearance in post‐menopausal patients: results after 5 years of follow‐up. European Journal of Surgical Oncology 2011;37:563‐70. [DOI] [PubMed] [Google Scholar]
Institut Curie {published data only}
- Cabanes PA, Salmon RJ, Vilcoq JR, Durand JC, Fourquet A, Gautier C, et al. Value of axillary dissection in addition to lumpectomy and radiotherapy in early breast cancer. The Breast Carcinoma Collaborative Group of the Institut Curie [see comments]. Lancet 1992;339(8804):1245‐8. [DOI] [PubMed] [Google Scholar]
- Louis‐Sylvestre C, Clough K, Asselain B, Vilcoq JR, Salmon RM, Campana F, et al. Axillary treatment in conservative management operable breast cancer: dissection or radiotherapy? Results of a randomised study with 15 years of follow‐up. Journal of Clinical Oncology 2004;22(1):97‐101. [DOI] [PubMed] [Google Scholar]
- Louis‐Sylvestre C, Clough KB, Falcou M‐C, Salmon RJ, Fourquet A, Vilcoq JR. A randomized trial comparing axillary dissection and axillary radiotherapy for early breast cancer: 15 year results. Breast Cancer Research and Treatment. 69 2001; Vol. 69, issue 3:212.
- Salmon RJ, Vilcoq JR, Durand JC, Cabanes PA, Asselain B. Lumpectomy with or without axillary dissection for early breast cancer: results of a prospective randomised trial. Breast Cancer Research and Treatment. 19 1991; Vol. 19, issue 2:212.
Malmo {published data only}
- Borgstrom S, Linell F, Tennvall‐Nittby L, Ranstam J, Tennvall Nittby L. Mastectomy only versus radical mastectomy and postoperative radiotherapy in node negative, resectable breast cancer. A randomized trial. Acta Oncologica 1994;33(5):557‐60. [DOI] [PubMed] [Google Scholar]
Manchester {published data only}
- Lythgoe JP, Leck I, Swindell R. Manchester regional breast study: preliminary results. Lancet 1978;8067:744‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lythgoe JP, Palmer MK. Manchester regional breast study: 5 and 10 year results. British Journal of Surgery 1982;69:693‐6. [DOI] [PubMed] [Google Scholar]
Milan {published data only}
- Veronesi U. Sentinel lymph node biopsy in breast cancer: ten‐year results of a randomized controlled study. Annals of Surgery 2010;251(4):595‐600. [DOI] [PubMed] [Google Scholar]
- Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, et al. A randomized comparison of sentinel‐node biopsy with routine axillary dissection in breast cancer [comment]. New England Journal of Medicine 2003;349(6):546‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Milan 2 {published data only}
- Martelli G, Boracchi P, Ardoino I, Lozza L, Bohm S, Vetrella G, et al. Axillary dissection versus no axillary dissection in older patients with T0N0 breast cancer: 15‐year results of a randomized controlled trial. Annals of Surgery 2012;256:920‐4. [DOI] [PubMed] [Google Scholar]
- Martelli G, Boracchi P, Palo M, Pilotti S, Oriana S, Zucali R, et al. A randomized trial comparing axillary dissection to no axillary dissection in older patients with T0N0 breast cancer: results after 5 years of follow‐up. Annals of Surgery 2005;242:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Milan 3 {published data only}
- Agresti R. Axillary dissection vs no axillary surgery in T1N0 breast cancer patients: a randomized clinical trial (INT 09/98). European Journal of Cancer. 2013; Vol. Conference:S457.
- Agresti R, Martelli G, Menard S, Ferraris C, Maugen I, Pelliteri C, et al. Conservative surgery with or without axillary clearance in T1N0 breast cancer: ten‐year results of INT 09/98 randomised trial. European Journal of Cancer. 2012; Vol. Conference:S45.
- Agresti R, Martelli G, Sandri M, Tagliabue E, Carcangiu ML, Maugeri I, et al. Axillary lymph node dissection versus no dissection in patients with T1N0 breast cancer: a randomized clinical trial (INT09/98). Cancer 2014;120:885‐93. [DOI] [PubMed] [Google Scholar]
NSABP B‐04 {published data only}
- Deutsch M, Land S, Begovic M, Sharif S. The incidence of arm edema in women with breast cancer randomized on the National Surgical Adjuvant Breast and Bowel Project study B‐04 to radical mastectomy versus total mastectomy and radiotherapy versus total mastectomy alone. International Journal of Radiation Oncology, Biology, Physics 2008;70:1020‐4. [DOI] [PubMed] [Google Scholar]
- Fisher B. The operative management of primary breast cancer. International Journal of Radiation Oncology, Biology, Physics 1977;2(9‐10):989‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty‐five‐year follow‐up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation [see comment]. New England Journal of Medicine 2002;347(8):567‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fisher B, Montague E, Redmond C, Barton B, Borland D, Fisher ER, et al. Comparison of radical mastectomy with alternative treatments for primary breast cancer. A first report of results from a prospective randomized clinical trial. Cancer 1977;39:2827‐39. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fisher B, Montague E, Redmond C, Deutsch M, Brown GR, Zauber A, et al. Findings from NSABP protocol No. B‐04: comparison of radical mastectomy with alternative treatments for primary breast cancer. Cancer 1980;46(1):1‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fisher B, Redmond C, Fisher ER, Bauer M, Wolmark N, Wickerham DL, et al. Ten‐year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with or without radiation. New England Journal of Medicine 1985;312(11):674‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NSABP B‐32 {published data only}
- Julian TB, Anderson SJ, Krag DN, Harlow SP, Costantino JP, Ashikaga T, et al. 10‐yr follow‐up results of NSABP‐32, a randomized phase III clinical trial to compare sentinel node resection (SNR) to conventional axillary dissection (AD) in clinically node‐negative breast cancer patients. Journal of Clinical Oncology 2013;31(15 Suppl):1000. [Google Scholar]
- Julian TB, Anderson SJ, Krag DN, Weaver DL, Costantino JP, Ashikaga T, et al. Abstract S2‐05: 10‐yr follow‐up results of occult detected sentinel node disease: NSABP B‐32, a randomized phase III clinical trial to compare sentinel node resection (SNR) to conventional axillary dissection (AD) in clinically node‐negative breast cancer patients. Cancer Research 2013;73:S2‐05. [Google Scholar]
- Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, et al. Technical outcomes of sentinel‐lymph‐node resection and conventional axillary‐lymph‐node dissection in patients with clinically node‐negative breast cancer: results from the NSABP B‐32 randomised phase III trial.[see comment]. Lancet Oncology 2007;8(10):881‐8. [DOI] [PubMed] [Google Scholar]
- Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel‐lymph node resection compared with conventional axillary‐lymph node dissection in clinically node‐negative patients with breast cancer: overall survival findings from the NSABPB‐32 randomised phase 3 trial. Lancet Oncology 2010;11:927‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krag DN, Ashikaga T, Harlow SP, Skelly JM, Julian TB, Brown AM, et al. Surgeon training, protocol compliance, and technical outcomes from breast cancer sentinel lymph node randomized trial. Journal of the National Cancer Institute 2009;101(19):1356‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land SR, Kopec JA, Julian TB, Brown AM, Anderson SJ, Krag DN, et al. Patient‐reported outcomes in sentinel node‐negative adjuvant breast cancer patients receiving sentinel‐node biopsy or axillary dissection: National Surgical Adjuvant Breast and Bowel Project phase III protocol B‐32. Journal of Clinical Oncology 2010;28(25):3929‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land SR, Kopec JA, Lee M, Haile SR, Ritter MW, Krag D, et al. Quality of life in breast cancer patients receiving sentinel‐node (SN) biopsy alone or with axillary dissection (AD): results from NSABP Protocol B‐32. ASCO Meeting Abstracts 2008;26(15 Suppl):9533. [Google Scholar]
Ostersund {published data only}
- Borup CS, Jansson C. Axillary biopsy compared with dissection in the staging of lymph nodes in operable breast cancer. A randomised trial. European Journal of Surgery 1993;159(4):159‐62. [PubMed] [Google Scholar]
- Borup Christensen S, Lundgren E. Sequelae of axillary dissection vs. axillary sampling with or without irradiation for breast cancer. A randomized trial. Acta Chirurgica Scandinavica 1989;155(10):515‐9. [PubMed] [Google Scholar]
SE Scotland {published data only}
- Bruce J. Operable cancer of the breast. A controlled clinical trial. Cancer 1971;28:1443‐52. [DOI] [PubMed] [Google Scholar]
- Hamilton T, Fraser J, Bruce J. Carcinoma of the breast ‐ a clinical trial. British Journal of Surgery 1969;56(8):615. [PubMed] [Google Scholar]
- Hamilton T, Langlands AO. A clinical trial in the management of operable cancer of the breast. Journal of the Royal College of Surgeons of Edinburgh 1977;22(1):52‐5. [PubMed] [Google Scholar]
- Hamilton T, Langlands AO, Prescott RJ. The treatment of operable cancer of the breast: a clinical trial in the South‐East region of Scotland. British Journal of Surgery 1974;61(10):758‐61. [DOI] [PubMed] [Google Scholar]
- Langlands AO, Prescott RJ, Hamilton T. A clinical trial in the management of operable cancer of the breast. British Journal of Surgery 1980;67(3):170‐4. [DOI] [PubMed] [Google Scholar]
SNAC {published data only}
- Gill G, SNAC Trial Group of the Royal Australasian College of Surgeons (RACS) and NHMRC Clinical Trials Centre. Sentinel‐lymph‐node‐based management or routine axillary clearance? One‐year outcomes of sentinel node biopsy versus axillary clearance (SNAC): a randomized controlled surgical trial. Annals of Surgical Oncology 2009;16(2):266‐75. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Gill PG, Wetzig N, Sourjina T, Gebski V, Ung O, et al. Comparing patients' and clinicians' assessment of outcomes in a randomised trial of sentinel node biopsy for breast cancer (the RACS SNAC trial). Breast Cancer Research & Treatment 2009;117(1):99‐109. [DOI] [PubMed] [Google Scholar]
- Wetzig N, Gill PG, Zannino D, Stockler MR, Gebski V, Ung O, et al. Sentinel lymph node based management or routine axillary clearance? Three‐year outcomes of the RACS Sentinel Node Biopsy Versus Axillary Clearance (SNAC) 1 Trial. Annals of Surgical Oncology 2014;22:17‐23. [DOI] [PubMed] [Google Scholar]
- Wetzig NR, Gill PG, Ung O, Collins J, Kollias J, Gillett D, et al. Participation in the RACS sentinel node biopsy versus axillary clearance trial. ANZ Journal of Surgery 2005; Vol. 75, issue 3:98‐100. [DOI] [PubMed]
WSSA Glasgow {published data only}
- Early Breast Cancer Trialists Collaborative Group. Effects of radiotherapy and surgery in early breast cancer. New England Journal of Medicine 1995;333(292):1444‐55. [DOI] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). West of Scotland study of treatment of mammary carcinoma. Treatment of Early Breast Cancer 1990;1:117. [Google Scholar]
Xu 2003 {published data only}
- Xu H, Zhang B, Zhang Q. The comparison between mastectomy plus axillary dissection and radical mastectomy in patients with I and II stage breast cancer: follow‐up of a randomized controlled study of 192 cases. Chinese Journal of Practical Surgery 2003;23:614‐6. [Google Scholar]
References to studies excluded from this review
AATRM‐048‐13‐2000 {published data only}
- Sola MS, Alberro JA, Fraile M, Santesteban P, Ramos M, Fabregas R, et al. Complete axillary lymph node dissection versus clinical follow‐up in breast cancer patients with sentinel node micrometastasis: final results from the multicenter clinical trial AATRM 048/13/2000. Annals of Surgical Oncology 2013;20:120‐7. [DOI] [PubMed] [Google Scholar]
ACOSOG Z0011 {published data only}
- Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011;305:569‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Annals of Surgery 2010;252:426‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencranz PW, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. Journal of Clinical Oncology 2007;25(24):3657‐63. [DOI] [PubMed] [Google Scholar]
- Olson JA Jr, McCall LM. Impact of immediate versus delayed axillary node dissection on surgical outcomes in breast cancer patients with positive sentinel nodes: results from American College of Surgeons Oncology Group trials Z0010 and Z0011. Journal of Clinical Oncology 2008;26(21):3530‐5. [DOI] [PubMed] [Google Scholar]
Buenos Aires {published data only}
- Gori J, Castano R, Engel H, Toziano M, Fischer C, Maletti G. Conservative treatment vs. mastectomy without radiotherapy in aged women with breast cancer ‐ a prospective and randomized trial. Zentralblatt fur Gynakologie 2000;122(6):311‐7. [PubMed] [Google Scholar]
Copenhagen {published data only}
- Johansen H, Kaae S, Schiodt T. Simple mastectomy with postoperative irradiation versus extended radical mastectomy in breast cancer. A twenty‐five‐year follow‐up of a randomized trial. Acta Oncologica 1990;29(6):709‐15. [DOI] [PubMed] [Google Scholar]
Edinburgh SES {published data only}
- Stewart HJ, Jack WJ, Everington D, Forrest AP, Rodger A, McDonald CC, et al. South‐east Scottish trial of local therapy in node negative breast cancer. The Breast 1994;3(1):31‐9. [Google Scholar]
IBCSG‐23‐01 {published data only}
- Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, et al. Axillary dissection versus no axillary dissection in patients with sentinel‐node micrometastases (IBCSG 23‐01): a phase 3 randomised controlled trial. Lancet Oncology 2013;14(4):297‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, et al. S3‐1. Update of International Breast Cancer Study Group Trial 23‐01 to compare axillary dissection versus no axillary dissection in patients with clinically node negative breast cancer and micrometastases in the sentinel node. Cancer Research 2012;71(24 Suppl):S3‐1. [Google Scholar]
IPO‐P {published data only}
- Fougo JL, Dinis‐Ribeiro M, Araujo C, Dias T, Reis P, Giesteira L, et al. Impact of lymphadenectomy on axillary recurrence and morbidity of the upper limb in breast cancer patients with negative sentinel node. A prospective randomised study. Cirugia Espanola 2011;89(5):307‐16. [DOI] [PubMed] [Google Scholar]
OTOASOR {published data only}
- Savolt A, Musonda P, Matrai Z, Polgar C, Renyi‐Vamos F, Rubovszky G, et al. Optimal treatment of the axilla after positive sentinel lymph node biopsy in early invasive breast cancer. Early results of the OTOASOR trial. Orvosi Hetilap 2013;154:1934‐42. [DOI] [PubMed] [Google Scholar]
- Savolt A, Polgar C, Musonda P, Matrai Z, Renyi‐Vamos F, Toth L, et al. Does the result of completion axillary lymph node dissection influence the recommendation for adjuvant treatment in sentinel lymph node‐positive patients?. Clinical Breast Cancer 2013;13:364‐70. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ISRCTN88463711 {published data only}
Semiglazov 2003 {published data only}
- Semiglazov VF, Chagunava OL. Sparing and organ‐saving operations in breast cancer. Voprosy Onkologii 1990;36:535‐9. [PubMed] [Google Scholar]
- Semiglazov VF, Maksimov SI, Semiglazov VV, Kosnikov AG. The modern organ‐ and function‐sparing surgical treatment in oncology. Vestnik Rossisko Akademii Meditsinskikh Nauk/Rossiskaia Akademiia Meditsinskikh Nauk 2003;10:34‐8. [PubMed] [Google Scholar]
References to ongoing studies
AMAROS {published data only}
- Donker M, Tienhoven G, Straver ME, Meijnen P, Velde CJH, Mansel RE, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981‐22023 AMAROS): a randomised, multicentre, open‐label, phase 3 non‐inferiority trial. Lancet Oncology 2014;15:1303‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straver ME, Meijnen P, Tienhoven G, Velde CJ, Mansel RE, Bogaerts J, et al. Role of axillary clearance after a tumor‐positive sentinel node in the administration of adjuvant therapy in early breast cancer. Journal of Clinical Oncology 2010;28(5):731‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straver ME, Meijnen P, Tienhoven G, Velde CJ, Mansel RE, Bogaerts J, et al. Sentinel node identification rate and nodal involvement in the EORTC 10981‐22023 AMAROS trial. Annals of Surgical Oncology 2010;17:1854‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
GF‐GS 01 {published data only}
- GF‐GS 01/NCT00144898. Ongoing study 2003.
KiSS {published data only}
- Helms G, Kuhn T, Moser L, Remmel E, Kreienberg R. Shoulder‐arm morbidity in patients with sentinel node biopsy and complete axillary dissection ‐ data from a prospective randomised trial. European Journal of Surgical Oncology 2009;35(7):696‐701. [DOI] [PubMed] [Google Scholar]
- Schem C, Jonat W, Ostertag H. Observation or standard axillary dissection after sentinel‐node biopsy in breast cancer: final results from the German KISS study. Journal of Clinical Oncology 2011;29(15 Suppl):1012. [Google Scholar]
NCT01717131 {published data only}
- NCT01717131/Institut Paoli‐Calmettes. Ongoing study 2012.
NCT02167490 {published data only}
- Sentinel Node Vs Observation After Axillary Ultra‐souND. Ongoing study 2014.
NCT02271828 {published data only}
- Omitting sentinel node procedure in breast cancer patients undergoing breast conserving therapy. Ongoing study 2015.
SNAC2 {published data only}
- SNAC2/ACTRN12605000409673. Ongoing study 2006.
SOUND {published data only}
- Gentilini O, Veronesi U. Abandoning sentinel lymph node biopsy in early breast cancer? A new trial in progress at the European Institute of Oncology of Milan (SOUND: Sentinel node vs Observation after axillary UltraSouND). Breast 2012;21(5):678‐81. [DOI] [PubMed] [Google Scholar]
Additional references
Clarke 2005
- Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15‐year survival: an overview of the randomised trials. Lancet 2005;366(9503):2087‐106. [DOI] [PubMed] [Google Scholar]
Craig 1998
- Craig PH, Silverstein MJ. Small invasive carcinomas: the role of axillary lymph node dissection. In: Bland KI, Copeland EN editor(s). The Breast, Comprehensive Management of Benign and Malignant Disease. 2nd Edition. Philadelphia: Saunders, 1998:1072‐93. [Google Scholar]
EBCTCG 1998
- Early Breast Cancer Trialists Collaborative Group. Polychemotherapy for early breast cancer: an overview of the randomised trials. The Lancet 1998;352:930‐42. [PubMed] [Google Scholar]
Goyal 2014
- Goyal A. POSNOC ‐ A randomised trial of armpit (axilla) treatment for women with early stage breast cancer (ISRCTN54765244), 2014. www.isrctn.com/ISRCTN54765244 accessed 12 December 2016.
Halsted 1895
- Halsted W. The results of operations for the cure of cancer of the breast performed at the Johns Hopkins Hospitals from June 1889 to January 1894. Johns Hopkins Hospital Bulletin 1895;4:297‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed.) 2011;343:d5928. [PUBMED: 22008217] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kell 2010
- Kell ML, Burke JP, Barry M, Morrow M. Outcome of axillary staging in early breast cancer: a meta‐analysis. Breast Cancer Research and Treatment 2010;120:441‐7. [DOI] [PubMed] [Google Scholar]
Kelley 1998
- Kelley MC, Giuliano AE. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. In: Bland KI, Copeland EN editor(s). The Breast, Comprehensive Management of Benign and Malignant Disease. 2nd Edition. Philadelphia: Saunders, 1998:1100‐11. [Google Scholar]
Kissin 1986
- Kissin MW, Querci della Rovere G, Easton D, Westbury G. Risk of lymphoedema following the treatment of breast cancer. British Journal of Surgery 1986;73:580‐4. [DOI] [PubMed] [Google Scholar]
Lefebvre 2001
- Lefebvre C, Clarke M. Identifying randomised trials. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd Edition. London: BMJ Publishing Group, 2001. [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London: The Cochrane Collaboration, 2011. [Google Scholar]
Lyman 2014
- Lyman GH, Temin S, Edge SB, Newman LA, Turner RR, Weaver DL, et al. Sentinel lymph node biopsy for patients with early‐stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. Journal of Clinical Oncology 2014;32(13):1365‐83. [PUBMED: 24663048] [DOI] [PubMed] [Google Scholar]
NICE 2009
- National Institute for Health and Care Excellence. Early and locally advanced breast cancer: diagnosis and treatment. Early and Locally Advanced Breast Cancer: Diagnosis and Treatment (CG80). London: NICE, 2009. [PubMed] [Google Scholar]
RevMan [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Senofski 1991
- Senofski GM, Moffat FL, Davis K, Masri MM, Clark KC, Robinson DS. Total axillary lymphadenectomy in the management of breast cancer. Archives of Surgery 1991;126:1336‐42. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8(16):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
UICC 1987
- Union Internationale Contre le Cancer. TNM classification of malignant tumours.. In: Hermanek P, Sobin LH editor(s). TNM Classification of Malignant Tumours. 4th Edition. Berlin: Springer‐Verlag, 1987:80‐89.. [Google Scholar]
Walsh 1989
- Walsh SJ, Begg CB, Carbone PP. Cancer chemotherapy in the elderly. Seminars in Oncology 1989;16:66‐75. [PubMed] [Google Scholar]
Wang 2011
- Wang Z, Wu LC, Chen JQ. Sentinel lymph node biopsy compared with axillary lymph node dissection in early breast cancer: a meta‐analysis. Breast Cancer Research and Treatment 2011;129:675‐89. [DOI] [PubMed] [Google Scholar]
Yancik 1989
- Yancik R, Ries LG, Yates JW. Breast cancer in ageing women. A population based study of contrasts in stage, surgery and survival. Cancer 1989;63:976‐81. [DOI] [PubMed] [Google Scholar]


