Abstract
Background
Platelet‐rich therapies are being used increasingly in the treatment of musculoskeletal soft tissue injuries such as ligament, muscle and tendon tears and tendinopathies. These therapies can be used as the principal treatment or as an augmentation procedure (application after surgical repair or reconstruction). Platelet‐rich therapies are produced by centrifuging a quantity of the patient’s own blood and extracting the active, platelet‐rich, fraction. The platelet‐rich fraction is applied to the injured tissue; for example, by injection. Platelets have the ability to produce several growth factors, so these therapies should enhance tissue healing. There is a need to assess whether this translates into clinical benefit.
Objectives
To assess the effects (benefits and harms) of platelet‐rich therapies for treating musculoskeletal soft tissue injuries.
Search methods
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (25 March 2013), the Cochrane Central Register of Controlled Trials (CENTRAL 2013 Issue 2), MEDLINE (1946 to March 2013), EMBASE (1980 to 2013 Week 12) and LILACS (1982 to March 2012). We also searched trial registers (to Week 2 2013) and conference abstracts (2005 to March 2012). No language or publication restrictions were applied.
Selection criteria
We included randomised and quasi‐randomised controlled trials that compared platelet‐rich therapy with either placebo, autologous whole blood, dry needling or no platelet‐rich therapy for people with acute or chronic musculoskeletal soft tissue injuries. Primary outcomes were functional status, pain and adverse effects.
Data collection and analysis
Two review authors independently extracted data and assessed each study's risk of bias. Disagreement was resolved by discussion or by arbitration by a third author. We contacted trial authors for clarification of methods or missing data. Treatment effects were assessed using risk ratios for dichotomous data and mean differences (MD) or standardised mean differences (SMD) for continuous data, together with 95% confidence intervals. Where appropriate, data were pooled using the fixed‐effect model for RR and MD, and the random‐effects model for SMD. The quality of the evidence for each outcome was assessed using GRADE criteria.
Main results
We included data from 19 small single centre trials (17 randomised and two quasi‐randomised; 1088 participants) that compared platelet‐rich therapy with placebo, autologous whole blood, dry needling or no platelet‐rich therapy. These trials covered eight clinical conditions: rotator cuff tears (arthroscopic repair) (six trials); shoulder impingement syndrome surgery (one trial); elbow epicondylitis (three trials); anterior cruciate ligament (ACL) reconstruction (four trials), ACL reconstruction (donor graft site application) (two trials), patellar tendinopathy (one trial), Achilles tendinopathy (one trial) and acute Achilles rupture surgical repair (one trial). We also grouped trials into 'tendinopathies' where platelet‐rich therapy (PRT) injections were the main treatment (five trials), and surgical augmentation procedures where PRT was applied during surgery (14 trials). Trial participants were mainly male, except in trials including rotator cuff tears, and elbow and Achilles tendinopathies.
Three trials were judged as being at low risk of bias; the other 16 were at high or unclear risk of bias relating to selection, detection, attrition or selective reporting, or combinations of these. The methods of preparing platelet‐rich plasma (PRP) varied and lacked standardisation and quantification of the PRP applied to the patient.
We were able to pool data for our primary outcomes (function, pain, adverse events) for a maximum of 11 trials and 45% of participants. The evidence for all primary outcomes was judged as being of very low quality.
Data assessing function in the short term (up to three months) were pooled from four trials that assessed PRT in three clinical conditions and used four different measures. These showed no significant difference between PRT and control (SMD 0.26; 95% confidence interval (CI) ‐0.19 to 0.71; P value 0.26; I² = 51%; 162 participants; positive values favour PRT). Medium‐term function data (at six months) were pooled from five trials that assessed PRT in five clinical conditions and used five different measures. These also showed no difference between groups (SMD ‐0.09, 95% CI ‐0.56 to 0.39; P value 0.72; I² = 50%; 151 participants). Long‐term function data (at one year) were pooled from 10 trials that assessed PRT in five clinical conditions and used six different measures. These also showed no difference between groups (SMD 0.25, 95% CI ‐0.07 to 0.57; P value 0.12; I² = 66%; 484 participants). Although the 95% confidence intervals indicate the possibility of a poorer outcome in the PRT group up to a moderate difference in favour of PRT at short‐ and long‐term follow‐up, these do not translate into clinically relevant differences.
Data pooled from four trials that assessed PRT in three clinical conditions showed a small reduction in short‐term pain in favour of PRT on a 10‐point scale (MD ‐0.95, 95% CI ‐1.41 to ‐0.48; I² = 0%; 175 participants). The clinical significance of this result is marginal.
Four trials reported adverse events; another seven trials reported an absence of adverse events. There was no difference between treatment groups in the numbers of participants with adverse effects (7/241 versus 5/245; RR 1.31, 95% CI 0.48 to 3.59; I² = 0%; 486 participants).
In terms of individual conditions, we pooled heterogeneous data for long‐term function from six trials of PRT application during rotator cuff tear surgery. This showed no statistically or clinically significant differences between the two groups (324 participants).
The available evidence is insufficient to indicate whether the effects of PRT will differ importantly in individual clinical conditions.
Authors' conclusions
Overall, and for the individual clinical conditions, there is currently insufficient evidence to support the use of PRT for treating musculoskeletal soft tissue injuries. Researchers contemplating RCTs should consider the coverage of currently ongoing trials when assessing the need for future RCTs on specific conditions. There is need for standardisation of PRP preparation methods.
Keywords: Female; Humans; Male; Platelet‐Rich Plasma; Achilles Tendon; Achilles Tendon/injuries; Anterior Cruciate Ligament Reconstruction; Blood Transfusion, Autologous; Platelet Transfusion; Platelet Transfusion/methods; Randomized Controlled Trials as Topic; Rotator Cuff Injuries; Shoulder Impingement Syndrome; Shoulder Impingement Syndrome/therapy; Soft Tissue Injuries; Soft Tissue Injuries/therapy; Tendinopathy; Tendinopathy/therapy; Tennis Elbow; Tennis Elbow/therapy
Plain language summary
Platelet‐rich therapies for musculoskeletal soft tissue injuries
What is the medical problem?
Muscle, ligament and tendon injuries frequently occur during activities such as sports, and may be due to tissue degeneration. These injuries are more frequent in particular parts of the body, such as the tendons located in the shoulder, elbow, knee and ankle.
What treatments are available?
Several treatment options are available. These include conservative methods, such as physical therapy, and surgery, for example to repair torn tendons. Another, increasingly popular, therapy is platelet‐rich therapy.
What is platelet‐rich therapy?
Platelets form part of blood. They produce growth factors that assist in repair and regeneration of tissue. It is possible that if a high concentration of platelets is applied to an injury, healing may progress faster. Platelet‐rich therapy involves the production of a platelet‐rich (concentrated) fraction of the patient's own blood. This is then applied, such as by an injection, to the site of injury.
Does it work?
This review set out to examine the evidence to see if platelet‐rich therapy (PRT) works in practice.
We searched medical databases (until March 2013) and registers of new studies (until March 2012) and found 19 studies that compared PRT with a control condition (such as no PRT). These involved a total of 1088 participants. Most participants were men, except in trials involving shoulder (rotator cuff) injuries, and elbow and Achilles tendinopathies (sometimes called tendinitis), where similar numbers of women were included.
The 19 trials covered eight types of injury, some of which were being treated surgically: rotator cuff tears (surgical repair) (six trials); shoulder impingement syndrome (surgery to release trapped tissues in the shoulder) (one trial); tennis elbow (three trials); knee ligament reconstruction using a section of tendon from the patient (four trials); the donor site of the tendon used for knee ligament reconstruction (two trials); patellar tendinopathy (jumper's knee) (one trial); Achilles tendinopathy (tendinitis) (one trial); and acute rupture of the Achilles tendon (surgical repair) (one trial).
The quality of the evidence is very low, partly because most trials used flawed methods that mean their results may not be reliable. The trials also used different ways of preparing and applying the platelet‐rich plasma. We were only able to pool data for our primary outcomes (function, pain, adverse events) for a maximum of 11 studies and 45% of participants.
When we pooled the limited data that was available for all these conditions, we found very weak (very low quality) evidence for a slight benefit of PRT in pain in the short term (up to three months). However, pooled data do not show that PRT makes a difference in function in the short, medium or long term. There was weak evidence that suggested that adverse events (harms) occurred at comparable, low rates in people treated with PRT and people not treated with PRT.
In terms of individual conditions, we were able to pool results from six studies and found no differences in long‐term function between those who received PRT during rotator cuff surgery and those who did not.
In conclusion, the available evidence is insufficient to to support the use of PRT for treating musculoskeletal soft tissue injuries or show whether the effects of PRT vary according to the type of injury. Any future research in this area should bear in mind the several studies currently going on and should consider the need for standardisation of the PRP preparation.
Background
Description of the condition
Musculoskeletal soft tissues include tendons, ligaments, cartilage and muscles. Treatment of musculoskeletal soft‐tissue injuries ranges from 'wait‐and‐see' approaches through to surgery. Studies report diverse rates of effectiveness of therapy and sometimes poor outcomes (Schepull 2011).
Musculoskeletal soft tissue injuries are very common, particularly in sports–active adults (Clayton 2008; Hootman 2002). A survey conducted in 1986 of a cohort of physically active adults found that a quarter of these had sustained a musculoskeletal injury during the past year (Hootman 2002). Both Clayton 2008 and Hootman 2002 reported that the largest category was soft tissue injuries of the knee. However, many more people with more minor soft tissue injuries will remain undiagnosed and unreported because they do not seek medical attention.
Musculoskeletal soft tissue injuries can be either acute or chronic. Acute injuries mainly involve tearing of anatomical structure(s) and haematoma formation after a traumatic event. These trigger the recovery process ‐ cellular proliferation, regeneration, repair and remodeling processes (Lee 2011). Chronic injuries, which are sometimes referred to as overuse or cumulative trauma injuries, are common with increasing age and sports participation but there is still a lack of knowledge about their aetiology and pathogenesis (Maffulli 2003). The underlying processes of chronic injuries are degenerative, and these are mainly characterised by neovascularisation and absence of inflammation (Foster 2009; Khan 1999).
The clinical features of musculoskeletal soft‐tissue injuries include local pain and impaired performance. Physical examination may show swelling and bruising in the site of injury. Initial 'first aid' treatment of acute injuries is summed up by the acronym RICE, which stands for rest, ice, compression and elevation. Otherwise, standard treatments include pain killers, including oral non‐steroidal anti‐inflammatory drugs (NSAIDs), bracing and physiotherapy (Paoloni 2005). Overall, tendon, ligament and muscle injuries are more commonly treated by a combination of treatment methods such as bracing followed by physiotherapy (Paoloni 2005). When surgery is recommended, platelet‐rich therapies can act as adjuvant or complementary treatments. In these cases, surgical repair or reconstruction is the main intervention.
Description of the intervention
Platelet‐rich plasma is derived from centrifuging whole blood extracted from the patient, resulting in a platelet‐rich fraction in which the platelet concentration is higher than that of whole blood (Foster 2009; Lee 2011). Its anticipated role is to act as a biological enhancer for tissue healing (Dohan 2009; Foster 2009). Dohan 2009 proposed a comprehensive classification for platelet concentrates based on their biological properties and potential clinical uses of each concentrate: 1) pure platelet rich plasma (P‐PRP); 2) leucocyte and platelet rich plasma (L‐PRP); 3) pure platelet rich fibrin (P‐PRF); and 4) leucocyte and platelet rich fibrin (L‐PRF). Dohan 2009 also stated the indications for each platelet‐derived product, based on their biological properties (Dohan 2009).
Platelet‐rich therapies can be used as a sole or main treatment when the injury is being treated by conservative interventions, or as an additional therapy alongside other conservative interventions or when surgery is performed. Platelet‐rich plasma for conservatively treated injuries is applied after the identification of the area of injury based on physical examination and sometimes imaging (such as ultrasound), and area of maximum tenderness. Some clinicians use dynamic musculoskeletal ultrasound to identify the area for injection with platelet‐rich plasma (Foster 2009; Lee 2011). The site for applying platelet‐rich therapy can be indirectly visualised during arthroscopic surgery.
This review compared platelet‐rich therapy with no platelet‐rich therapy, or a placebo or 'whole blood' control. Injections, for example such as of saline, can be considered as possible placebo control interventions (De Vos 2010a). Dry needling could be considered a 'whole blood' control (Kiter 2006).
How the intervention might work
A high concentration of platelets and growth factors are produced from whole blood by the preparation process (Dohan 2009). Growth factors such as transforming growth factor–β (TGF‐β), platelet‐derived growth factor (PDGF), insulin‐like growth factor (IGF‐I, IGF‐II), fibroblast growth factor (FGF), epidermal growth factor, vascular endothelial growth factor (VEGF) and endothelial cell growth factor are responsible for enhancing tissue recovery (Foster 2009; Lee 2011). The intervention works by delivering these growth factors to the injury site, where they are assumed to enhance tissue regeneration and improve angiogenesis (formation of blood vessels) (Dohan 2009). Thus it is assumed that the autologous preparation (from the patient's own blood) could help to 'empower' the biocellular environment for promoting and accelerating the healing process.
The reported incidence of patients with side effects of platelet‐rich therapy is low, with an average of 2% to 5% (Filardo 2010). The vast majority of reported side effects relate to local tenderness and pain, which tend to last less than two days (Filardo 2010; Peerbooms 2010).
Why it is important to do this review
Platelet‐rich therapies are becoming widely used, mostly within sports medicine where early return to function is a major concern. The supposition that these therapies could enhance tissue recovery, allowing early return to activities and sports, has led to the promotion of their use for a myriad of conditions (Lee 2011), but without clear proof of their clinical effectiveness (Dohan 2009; Schepull 2011). These therapies also have some media‐induced appeal (Foster 2009), and commercial interest from manufacturers supplying the blood preparation kits.
This has led to a growing number of clinical studies testing the properties and effectiveness of platelet‐rich therapies for musculoskeletal injuries (Filardo 2010; Peerbooms 2010; Schepull 2011). However, published systematic reviews, e.g. De Vos 2010b, Lee 2011 and Taylor 2011, have included studies other than randomised trials, or studies testing other treatments in combination with platelet‐rich therapies; together these act to decrease both the internal and external validity of these reviews. None of these reviews has provided clear evidence regarding effectiveness and safety of platelet‐rich therapies. By restricting our focus to randomised or quasi‐randomised controlled trials comparing platelet‐rich therapy with either a no intervention, or placebo intervention, control, as well as updating the search for trials, this review aimed to provide a reliable summary of the best evidence to inform decisions on the use of platelet‐rich therapies for treating musculoskeletal soft‐tissue injuries.
Objectives
To assess the effects (benefits and harms) of platelet‐rich therapies for treating musculoskeletal soft tissue injuries.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials and quasi‐randomised controlled trials (where the allocation is not strictly random, for example, by date of birth, hospital record number, alternation) comparing platelet‐rich therapy with no platelet‐rich therapy or a placebo for musculoskeletal soft tissue injuries in adults.
Types of participants
People with musculoskeletal soft tissue injuries being treated either conservatively or surgically (for example, repair and reconstruction). Injuries include:
traumatic injuries, for example, Achilles tendon rupture, anterior cruciate ligament (ACL) injuries, rotator cuff tears, ankle sprains, hamstring muscle tears, meniscal and labral lesions;
tendinopathies (acute or chronic), for example, Achilles 'tendinitis', lateral epicondylitis (tennis elbow), rotator cuff 'tendinitis', patellar 'tendinitis' (jumper's knee).
We did not place any restrictions in terms of the diagnostic methods or criteria used by individual studies, or the duration of the injury. We excluded trials that focused on treating osteoarthritis.
Types of interventions
We considered studies in which platelet‐rich therapies were used as the only treatment, or as an additional or adjunctive treatment to conservative or surgical treatment that was provided to all trial participants. Such studies compared platelet‐rich therapy (intervention) with no platelet‐rich therapy or placebo. There was no restriction based on treatment dosage, usage and number of procedures or injections. We excluded studies that evaluated only other blood‐derived alternatives, such as whole blood injections. Subsequent to the protocol, we accepted trials that used whole blood injection or dry needling controls. However, we excluded trials with active agent controls such as steroid injections, as used in Peerbooms 2010.
Types of outcome measures
We categorised the outcome measurements as short term (up to 12 weeks follow‐up), medium term (between 12 weeks and one year follow‐up) and long term (more than one year follow‐up).
Primary outcomes
Functional evaluation (assessed by subjective assessment questionnaires such as Disabilities of the Arm, Shoulder and Hand questionnaire (Hudak 1996), Victorian Institute of Sports Assessment ‐ Achilles questionnaire (VISA‐A) (Robinson 2001), and American Orthopaedic Foot and Ankle Society (AOFAS) foot questionnaire (Kitaoka 1994)).
Pain (assessed by subjective scales such as visual analogue scales (VAS) (Revill 1976)).
Local and systemic adverse effects of platelet‐rich therapy (or placebo) administration (including infection and anaphylactic reaction).
Secondary outcomes
Recovery time: return to sports, and return to day‐to‐day or work activities.
Non‐return to previous activities: sports, work or decrease in the level of activity.
Quality of life (assessed by subjective assessment questionnaires such as Short Form (36) Health Survey (SF‐36) (Brazier 1992) and World Health Organization Quality of Life (WHOQoL) (Masthoff 2005).
Recurrence of the condition.
Need for a secondary treatment procedure (for example, surgery).
Participant satisfaction.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (25 March 2013), the Cochrane Central Register of Controlled Trials (2013 Issue 2), MEDLINE (1946 to March Week 2 2013), MEDLINE In‐Process & Other Non‐Indexed Citations (March 2013), EMBASE (1980 to 2013 Week 12), and LILACS (1982 to March 2013). No language restrictions were applied. We also searched Current Controlled Trials and the WHO International Clinical Trials Registry Platform for ongoing and recently completed trials (until March Week 2 2013).
In MEDLINE (Ovid Online), we combined the subject‐specific search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (sensitivity‐maximising version) (Lefebvre 2011). Search strategies for The Cochrane Library (CENTRAL), MEDLINE, EMBASE and LILACS are shown in Appendix 1.
Searching other resources
We searched reference lists of articles, reviews and non‐scholarly Internet sources for relevant studies. Additionally, we contacted other researchers or experts in the field for relevant data in terms of published, unpublished or ongoing studies. We searched the conference abstracts of the following conferences (2005 to March 2012): SICOT (Société Internationale de Chirurgie Orthopédique et de Traumatologie), AOSSM (American Orthopaedic Society for Sports Medicine) and AAOS (American Academy of Orthopaedic Surgeons).
Data collection and analysis
Selection of studies
Two review authors (VM and MT) independently screened titles and abstracts of the references identified by the searches. We retrieved full copies of all potentially relevant studies. The same two authors independently performed study selection. Any disagreements were resolved by discussion or, when necessary, by involving a third author (JB or ML).
Data extraction and management
Two review authors (VM and MT) independently extracted data using a pre‐piloted data extraction form. Any disagreements were resolved by discussion or, when necessary, by involving a third author (JB).
Assessment of risk of bias in included studies
The risk of bias of the included studies was assessed independently by two review authors (VM and ML) using The Cochrane Collaboration's 'Risk of bias' tool (Higgins 2011). Disagreements were resolved by discussion or by involving a third author (JB). The following domains were assessed: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other bias (for example, differences in follow‐up procedures between the intervention groups that might result in 'different diagnostic activity’). Each domain was judged in terms of whether it was at 'high', 'low' or 'unclear' risk of bias.
Measures of treatment effect
We presented risk ratios with 95% confidence intervals (CI) for dichotomous outcomes and mean differences (MD) with 95% CIs for continuous outcomes. We presented standardised mean differences (SMD) when pooling continuous data from outcomes that used different scales. Where appropriate in future, we intend to report the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH) both with 95% CIs.
Unit of analysis issues
The unit of randomisation in all studies included in this review was the individual participant. If we include cluster randomised trials in a future update, we will opt if possible to consider data at the level of the group, keeping the unit of analysis the same as the unit of randomisation. Where appropriate corrections have not been made, or cannot be obtained from trial authors, we will consider presenting the data for such trials where the disparity between the units of analysis and randomisation is small. We avoided unit of analysis issues relating to repeated observations, such as by performing separate analyses for different follow‐up times, and issues relating to the reporting of outcome by studies that tested multiple applications of platelet‐rich therapy over time.
Dealing with missing data
We contacted trial authors with requests to supply any missing data, such as number of participants, details of dropouts, means, measures of uncertainty (standard deviation or error) or number of events. If we were unsuccessful in acquiring missing data, we presented the available data and did not impute missing values.
Assessment of heterogeneity
We assessed heterogeneity by visual inspection of the forest plots. We used the I² test to provide an objective measurement of statistical heterogeneity. The heterogeneity was quantified using the I² statistic with a rough guide for interpretation as follows: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and 75% to 100% considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
In meta‐analyses where there were more than 10 studies on a single condition, we planned to assess the potential for publication bias (small study effects) by generating funnel plots.
Data synthesis
When appropriate, results of comparable groups of studies were pooled in meta‐analysis using the fixed‐effect model, except for standardised mean differences where a random‐effects model was used. We calculated pooled risk ratios with 95% CIs for dichotomous outcomes. When two or more studies presented continuous data derived from the same instrument of evaluation (with the same units of measurement), data were pooled as a mean difference with 95% CI. When the studies expressed the same variable using different instruments and different units of measurement, we used the standardised mean difference with 95% CI.
Subgroup analysis and investigation of heterogeneity
We were unable to perform all the planned subgroup analyses due to lack of data (seeDifferences between protocol and review). We performed two subgroup analyses in order to explore different estimated effects.
Grouping trials by condition (for example, rotator cuff tear, ACL reconstruction, chronic Achilles tendinopathy).
Grouping trials according to whether platelet rich therapy (PRT) was the main treatment for tendinopathies or a surgical augmentation procedure.
We investigated whether the results of subgroups were significantly different by inspecting the overlap of confidence intervals and performing the test for subgroup differences available in RevMan (RevMan 2012).
We investigated heterogeneity further by seeing the effects of removing single trial outliers.
Should sufficient data be available in future, we will consider conducting the following additional subgroup analyses.
Acute versus chronic injuries.
Different methods for PRTs (e.g. simple versus multiple doses; methods for PRT separation) (Dohan 2009).
Modalities for using in surgical procedures (as an augmentation procedure after repair or reconstruction; utilisation in the harvesting or donor site).
Groups at risk for non‐healing (e.g. smoking, diabetes).
Commercial versus laboratory‐prepared kits.
Sensitivity analysis
We performed sensitivity analyses to evaluate the impact of removing studies at high or unclear risk of selection bias (primarily in terms of inadequate allocation concealment) and detection bias (lack of assessor blinding) from the analysis. We also conducted sensitivity analyses to investigate the effects of missing data.
Quality assessment
We used the GRADE approach to assess the quality of evidence relating to the primary outcomes for overall result (section 12.2, Higgins 2011).
Results
Description of studies
Results of the search
The search was completed in March 2013. We screened records from the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (6 records); Cochrane Central Register of Controlled Trials (26), MEDLINE (98), EMBASE (99), LILACS (7), Current Controlled Trials (5) and the WHO International Clinical Trials Registry Platform (15). We also identified three potentially eligible studies from other sources (references search).
The search identified a total of 39 studies for potential inclusion, for which full reports were obtained. Upon study selection, 19 were included (Almeida 2012; Antuna 2013; Castricini 2011; Cervellin 2012; Creaney 2011; De Vos 2010; Everts 2008; Gumina 2012; Krogh 2013; NCT01029574; Orrego 2008; Randelli 2011; Rodeo 2012; Schepull 2010; Thanasas 2011; Vadalà 2013; Valenti Nín 2009; Vogrin 2010; Wasterlain 2013), four were excluded (Ferrero 2012; Figueroa 2010; Radice 2009; Silva 2009) and 16 are ongoing studies (ACTRN12612000982819; EUCTR201300047832ES; IRCT2013052313442N1; ISRCTN10464365; ISRCTN95369715; NCT01000935; NCT01170312; NCT01440725; NCT01509274; NCT01518335; NCT01600326; NCT01668953; NCT01765712; NCT01812564; NCT01833598; NCT01851044). No studies await classification. Further details of the process of screening and selecting studies for inclusion in the review are illustrated in Figure 1.
1.
Study flow diagram
Included studies
Individual characteristics of the 19 studies are described in the Characteristics of included studies section. One study was reported in three reports from the same population (De Vos 2010). All but two studies were published as full reports (NCT01029574; Wasterlain 2013); data for the two exceptions were collected by direct contact. Antuna 2013 also provided extra data. All studies were published in English.
Design
Seventeen studies were randomised and two were quasi‐randomised (Orrego 2008; Vogrin 2010). Most of the studies were conducted from 2005 to 2013. Ten studies did not have a pre‐published protocol/trial registration document (Cervellin 2012; Creaney 2011; Everts 2008; Orrego 2008; Randelli 2011; Schepull 2010; Thanasas 2011; Vadalà 2013; Valenti Nín 2009; Vogrin 2010). The randomisation methods were described in most of the trials; however, in some studies reporting was unclear (Cervellin 2012; Creaney 2011; Vadalà 2013). Most of the studies failed partially or entirely in the blinding procedure, as described in the Characteristics of included studies. Additionally, some studies did not report the platelet concentration (number of platelets per mm³), indicating that the authors did not quantify the platelet concentrations or its products.
Setting
Studies were conducted in different settings in Europe: Italy (Castricini 2011; Cervellin 2012; Gumina 2012; Randelli 2011; Vadalà 2013); Spain (Antuna 2013; Valenti Nín 2009); The Netherlands (De Vos 2010; Everts 2008); Denmark (Krogh 2013); Greece (Thanasas 2011); UK (Creaney 2011); Slovenia (Vogrin 2010); Sweden (Schepull 2010); and in the Americas: Brazil (Almeida 2012; NCT01029574); Chile (Orrego 2008); and the USA (Rodeo 2012; Wasterlain 2013). All were single‐centre trials.
Sample sizes
The studies included a total of 1088 participants, with 59 participants being lost during follow‐up. Trials population sizes ranged from 23 (Wasterlain 2013), to 150 (Creaney 2011). One trial had an substantial loss to follow‐up after three months (27 participants out of 40) and only reported full follow‐up data for this period (Krogh 2013).
Participants
Participant characteristics differed among study populations often reflecting the different clinical conditions covered by these trials. Populations in studies concerning mainly sports injuries (lateral epicondylitis, ACL reconstruction, patellar tendinopathy, Achilles tendinopathy and Achilles ruptures) included mainly young and active adults, whereas studies concerning degenerative conditions (chronic impingement syndrome and rotator cuff tears) mainly included an older population. Studies mainly included men. However, for rotator cuff ruptures, elbow epicondylitis and Achilles tendinopathies, there were similar proportions of male and female participants. Most of the studies did not specify whether the participants had a previous history of sports activity. This information was mostly available for ACL and Achilles tendon injuries, where sports activity status is a major concern. Three trials reported that their population included people with some level of sports activity (Cervellin 2012; De Vos 2010; Schepull 2010). Cervellin 2012 reported that all participants were at a 'high level' of sports activity. A study‐by‐study description of age, gender and sport activity can be found in the 'Participants' sections of the Characteristics of included studies.
Conditions and interventions
The conditions and main treatment of included trials were grouped into the following seven categories, one category being further subdivided into two. Notably, there were no included trials on sprains or muscle injuries. Further details of the various PRT interventions can be found in the Characteristics of included studies table.
Rotator cuff tears (surgical repair)
Six studies assessed the application of platelet‐rich plasma (PRP) to the repair site after arthroscopic rotator cuff repair (Antuna 2013; Castricini 2011; Gumina 2012; NCT01029574; Randelli 2011; Rodeo 2012). Three studies included participants with complete rotator cuff tears (Antuna 2013; Randelli 2011; Rodeo 2012). Two studies included participants with large rotator cuff tears (Gumina 2012; NCT01029574), and one study included small and moderate tears (Castricini 2011). Studies mostly assessed rotator cuff tears before surgery by means of physical examination and magnetic resonance imaging (MRI). In all studies, PRP was applied at the time of, or after, arthroscopic repair and with the aid of the arthroscopic device (PRP was applied mostly through one of the portals and its positioning checked by the arthroscope) and its preparation was carried out using a specific kit. In Castricini 2011, Gumina 2012 and Rodeo 2012, the PRP matrix was attached to the suture anchor, which was then passed down the arthroscopic cannula to the repair site. In the other three trials, PRP was applied directly to the repair site (with syringe and needles) (Antuna 2013; NCT01029574; Randelli 2011). In all six trials, PRP was applied in a single procedure to the suture site before closure of the surgical wounds.
Shoulder impingement syndrome (surgery)
One study assessed PRP application after open decompression for shoulder impingement syndrome (Everts 2008), with no intervention as a control. The surgeons inserted a needle into the subacromial space after open decompression and PRP was applied intracapsularly after deltoid and subcutaneous layers were closed. Before skin closure, the researcher also applied a small quantity (3 mL) into the subcutaneous layer.
Elbow epicondylitis
The three studies on lateral epicondylitis used ultrasound‐guided application of PRP to the origin of the elbow tendons as the intervention (Creaney 2011; Krogh 2013; Thanasas 2011). The studies differed with regard to the time span between symptoms and intervention. Creaney 2011 included participants with at least six months of symptoms who had been treated unsuccessfully with physiotherapy. Krogh 2013 and Thanasas 2011 included participants with at least three months of symptoms and permitted the inclusion of participants who had had previous injections as treatment. None of the studies reported on prior sports participation. One study reported that the procedure was aided by an experienced radiologist and two applications were performed over the time span of one month (Creaney 2011). Two studies considered autologous whole blood as a control (Creaney 2011; Thanasas 2011), and one considered dry needling using a peppering technique as a control (Krogh 2013). Creaney 2011 did not state whether they used a specific kit for PRP preparation.
Anterior cruciate ligament (ACL) reconstruction
Graft donor site
Almeida 2012 and Cervellin 2012 used a PRP preparation with the aim of reducing the morbidity of the ACL reconstruction donor site. Both studies used patellar tendons (bone‐tendon‐bone) as grafts and performed PRP applications after tendon harvesting, as a part of the operative procedure (before surgical wound closing). Both studies considered the standard procedure as the control intervention ‐ with no dedicated intervention in the tendon harvest site. Co‐interventions between the groups were similar in both studies.
Anterior cruciate ligament reconstruction (augmentation procedure)
Four studies used PRP in the arthroscopic reconstruction procedure (Orrego 2008; Vadalà 2013; Valenti Nín 2009; Vogrin 2010). All studies but Valenti Nín 2009 used hamstring tendons as grafts. Valenti Nín 2009 used patellar (bone‐tendon‐bone) graft. All studies considered the standard procedure as the control intervention, with no additional therapy in the course of reconstruction. A specific kit was used for PRP preparation. Orrego 2008, Vadalà 2013 and Valenti Nín 2009 applied PRP in the graft before insertion (as they awaited the formation of a clot). After insertion, it was also applied into the bone tunnels with the aid of the arthroscope, prior to closing wounds. The procedure in Vogrin 2010 differed, as they applied PRP after graft insertion.
Patellar tendinopathy
One study compared a single ultrasound‐guided application of PRP with dry needling control in patellar tendinopathy (Wasterlain 2013). They used a specific kit for PRP preparation. After the procedure, all participants were instructed to undergo an eccentric (muscular activation in which the muscle fibres lengthen to lower a load) five‐phase exercise programme.
Chronic Achilles tendinopathy
One study assessed the results of PRP application in participants with chronic Achilles tendinopathy and used saline injections as controls (De Vos 2010). A blinded physician performed the injections under ultrasound guidance, making five small deposits at various sites of the degenerated area of the tendon through each of three puncture locations. After the procedure, all participants were instructed to carry out the usual care, which consisted of a pre‐defined eccentric exercise programme.
Acute Achilles tendon rupture (surgical repair)
One study compared the intraoperative application of PRP in Achilles tendon ruptures after standard repair (Schepull 2010). Application consisted of the application of PRP to the repair site and, after closure, transdermally. Standard repair with no PRP was used as a control. The researchers added two tantalum beads proximally and distally to the ruptured tendon ends with the aim of measuring tendon properties, such as elasticity modulus, using roentgen stereophotogrammetric analysis.
Grouping by basic treatment categories
These studies could be grouped also into two categories: main treatment for 'tendinopathies' and surgical augmentation procedures. Studies were considered to belong in the tendinopathy group when the main treatment was injections and no surgery was performed (repair or reconstruction). This included the following selection from the above conditions: elbow epicondylitis (Creaney 2011; Krogh 2013; Thanasas 2011), patellar tendinopathies (Wasterlain 2013) and chronic Achilles tendinopathies (De Vos 2010). Studies were considered for the surgical augmentation group when surgery was the main treatment procedure. This included the following selection from the above conditions: rotator cuff tears repair (Antuna 2013; Castricini 2011; Gumina 2012; NCT01029574; Randelli 2011; Rodeo 2012), shoulder impingement syndrome surgery (Everts 2008), ACL reconstruction (Almeida 2012; Cervellin 2012; Orrego 2008; Vadalà 2013; Valenti Nín 2009; Vogrin 2010), and surgical repair of acute Achilles rupture (Schepull 2010).
Outcomes
The primary outcomes listed in our protocol were mostly reported in the studies. All the studies assessed function or pain, or both, using at least one validated instrument. Complications and adverse effects related to PRT were assessed in nine trials (Almeida 2012; Antuna 2013; Castricini 2011; Cervellin 2012; De Vos 2010; Everts 2008; Krogh 2013; Schepull 2010; Vadalà 2013).
Outcomes that are not reported by this review but that were part of the outcome assessment in the included trials are briefly summarised here for completeness. In particular, several trials prospectively collected imaging data and objective physical measures of function.
Almeida 2012 and Cervellin 2012 assessed patellar tendon harvest site healing by MRI (measurement of gap area from the harvest site, gap filling, assessment of new bone formation) and Almeida 2012 also assessed the patellar height by using the Insall‐Savati index derived from plain radiographs.
Three studies assessed the integrity of the rotator cuff repair using MRI (Antuna 2013; Castricini 2011; NCT01029574). One study used ultrasound assessment to evaluate tendon healing (Rodeo 2012). Krogh 2013 performed ultrasound assessment of elbow tendons and evaluated doppler changes and tendon thickness.
Orrego 2008 and Valenti Nín 2009 performed MRI assessments after ACL reconstructions to establish the maturation status of the graft (graft signal intensity, osteo‐ligamentous interface, tunnel widening), and Vadalà 2013 evaluated the femoral and tunnel enlargement (assessed by computed tomography (CT)) after reconstruction.
Schepull 2010 performed a roentgen stereophotogrammetric analysis (using tantalum beads) as a method to quantify Achilles tendon strain per load and also estimated of elasticity modulus (using callus dimensions from CT). De Vos 2010 performed a sonographic evaluation to assess Achilles tendon structure and neovascularisation.
Shoulder strength was measured by Randelli 2011 and Rodeo 2012. Range of shoulder motion was assessed by Everts 2008. Knee isokinetic testing was performed by Almeida 2012. Objective knee anterior laxity following ACL reconstruction was assessed by Valenti Nín 2009 and Vogrin 2010.
Excluded studies
We excluded four studies due to the lack of randomisation (Ferrero 2012; Figueroa 2010; Radice 2009; Silva 2009), as described in Characteristics of excluded studies.
Ongoing studies
PRP effectiveness for 'new' indications are currently being studied in six ongoing studies: greater trochanteric pain syndrome (ACTRN12612000982819); gluteus muscle tendinitis (NCT01600326); muscle strains or ruptures (NCT01440725; NCT01812564); plantar fascitis (NCT01509274); and ankle sprains (NCT01518335).
Evidence from other ongoing studies should enhance the available evidence for: elbow epicondylitis (EUCTR201300047832ES; NCT01668953; NCT01833598; NCT01851044); rotator cuff tears treatment (IRCT2013052313442N1), and surgery (ISRCTN10464365; NCT01000935; NCT01170312); Achilles tendinopathies (ISRCTN95369715), and ACL reconstruction (NCT01765712).
Details of the 16 ongoing studies are described in Characteristics of ongoing studies.
Risk of bias in included studies
The review authors' judgements of the risk of bias for each domain listed in Assessment of risk of bias in included studies are detailed below and summarised for each trial in Figure 2.
2.
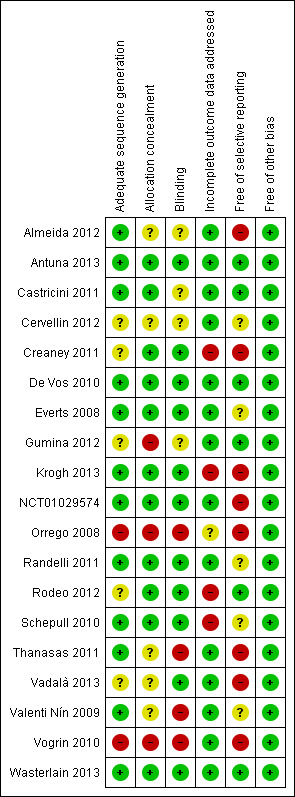
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Selection bias of individual trials was assessed mainly by judging the method of allocation concealment and verifying the similarity of the treatment groups by inspecting baseline characteristics. Most studies reported the use of sequentially‐numbered opaque envelopes. Three studies failed to report the process of allocation to groups (Thanasas 2011; Vadalà 2013; Valenti Nín 2009), and two studies were quasi‐randomised (Orrego 2008; Vogrin 2010). Orrego 2008 allocated participants following a "constant rotation" and Vogrin 2010 allocated participants by odd or even numbers. One study reported that the envelope was opened three days prior to surgery (Gumina 2012). Three studies demonstrated homogeneity of baseline characteristics between groups (Thanasas 2011; Valenti Nín 2009; Vogrin 2010), two studies did not perform a priori analysis (Orrego 2008; Vadalà 2013), and one study reported that groups were not the same with regard to age and baseline Constant scores (Gumina 2012). We judged that three trials were at high risk of selection bias (Gumina 2012; Orrego 2008; Vogrin 2010).
Blinding
Eleven trials reported that participants and follow‐up assessors were blinded or partially blinded to the procedure (Antuna 2013; Creaney 2011; De Vos 2010; Everts 2008; Krogh 2013; NCT01029574; Randelli 2011; Rodeo 2012; Schepull 2010; Vadalà 2013; Wasterlain 2013). Two studies reported that only the MRI assessor was blinded (Orrego 2008; Valenti Nín 2009). As this review concerns mostly patient‐reported outcomes and not imaging outcomes, both studies were judged as being at high risk of detection bias.
Thanasas 2011 reported that the participants were not blinded to the procedure, but the follow‐up assessor was. In Vogrin 2010, neither participants nor assessors were blinded. In four studies (Almeida 2012; Castricini 2011; Cervellin 2012; Gumina 2012), there was a lack of information regarding which of the assessors were blinded, or blinding, or the blinding procedure was not reported.
Our considerations of performance bias do not appear in the risk of bias tables. Most of the studies reported the same co‐interventions (mainly post‐intervention care and rehabilitation) in each treatment group and thus were at low risk. Additionally, interventions were mostly performed by surgeons (one or two in each trial) or radiologists with ultrasound guidance. The three studies that did not state who carried out the intervention were judged as being at unclear risk of performance bias (Cervellin 2012; Schepull 2010; Thanasas 2011).
Incomplete outcome data
Most of the studies reported no loss to follow‐up or small losses that were balanced between groups. Four studies (Creaney 2011; Krogh 2013; Rodeo 2012; Schepull 2010) were judged as being at high risk of attrition bias due either to data missing in an unbalanced manner between groups (Creaney 2011; Rodeo 2012; Schepull 2010), or to the study suffering great losses to follow‐up in the long term (Krogh 2013). One study failed to report the characteristics of the losses to follow‐up (Orrego 2008). As most of the studies did not suffer important losses (and thus were at low risk of bias), this is a minor concern in this review. We presented only the short‐term findings (no follow‐up losses) for Krogh 2013 in our analyses.
Selective reporting
Ten of the included studies did not provide any a priori protocol or trial registration details for the study (Cervellin 2012; Creaney 2011; Everts 2008; Orrego 2008; Randelli 2011; Schepull 2010; Thanasas 2011; Vadalà 2013; Valenti Nín 2009; Vogrin 2010); these were judged to be at unclear or high risk of selective reporting bias. Although studies reported the outcomes described in their methodology sections, some did not provide key endpoints during the follow‐up, such as pain (Almeida 2012), and functional scales (Vadalà 2013). As protocols and outcome assessments were not ideally reported or conducted, some important outcomes, such as adverse effects, may have been under‐recognised or under‐reported (high risk of bias).
Other potential sources of bias
As co‐interventions were mainly the same in the study groups and, for most studies, participants were unable to change interventions, there were no cross‐overs or differences between groups in the other care provided. As an exception, participants recruited for Creaney 2011 and Wasterlain 2013 changed treatments after failure had occurred. However, these studies remained at low risk of other bias as the authors conducted intention‐to‐treat analyses.
Additional quality assessment
We systematically assessed two other items (sample size calculations and validation of the platelet‐rich concentrate) that related more to trial quality than bias. The findings for individual trials are reported in the Characteristics of included studies. These assessments contributed to our consideration of the evidence in terms of imprecision and applicability.
Sample size calculations were reported in 11 trials (Castricini 2011; Cervellin 2012; Creaney 2011; De Vos 2010; Krogh 2013; NCT01029574; Randelli 2011; Rodeo 2012; Thanasas 2011; Vadalà 2013; Wasterlain 2013). However, only nine of these applied to the primary outcomes of our review (Castricini 2011; Cervellin 2012; Creaney 2011; De Vos 2010; Krogh 2013; NCT01029574; Randelli 2011; Thanasas 2011; Wasterlain 2013). This consideration is important because studies that are underpowered are more likely to incur a type II error (where sample sizes were not sufficient to detect differences between the arms of comparison).
Information about validation of the platelet‐rich concentrate through platelet quantification after preparation, was not available in eight studies (Antuna 2013; Castricini 2011; Cervellin 2012; De Vos 2010; Orrego 2008; Randelli 2011; Rodeo 2012; Vadalà 2013). In one study (Schepull 2010), the authors prepared PRP the night before the intervention; however, the viability of the sample was checked before surgery.
Effects of interventions
In keeping with the intentions stated in our protocol, we first present the overall findings for the 19 trials. Given the sparse nature of the data, this is currently restricted to primary outcomes only. The results in the first set of analyses are subgrouped by the eight different conditions listed in Included studies. We then present the overall findings subgrouped by whether PRT was the main treatment for a tendinopathy or an augmentation procedure for a condition requiring surgery. Finally, we present a complete summary of the evidence available for each of the eight individual conditions.
The data for function and pain are presented for three time periods: short term (up to three months); medium term (over three months, under one year; usually six months); and long term (one year or more).
Overall analysis
Function
Data, derived from four different functional scores, pooled from four trials assessing PRT for three clinical conditions, showed no statistically significant difference between PRT and control for short‐term function (SMD 0.26; 95% CI ‐0.19 to 0.71; P value 0.26; I² = 51%; 162 participants, four trials; positive values favour PRT; seeAnalysis 1.1). The significant results of the test for subgroup differences (P value 0.06, I² = 64.6%) reflected the inclusion of the results from De Vos 2010 (54 participants), which differed in direction of effect from the other two subgroups.
1.1. Analysis.
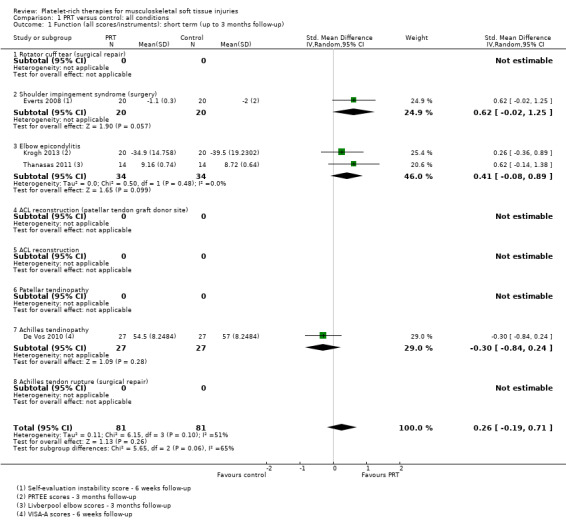
Comparison 1 PRT versus control: all conditions, Outcome 1 Function (all scores/instruments): short term (up to 3 months follow‐up).
Data, derived from five different functional scores, pooled from five trials assessing PRT for five clinical conditions, showed no difference (P value 0.72) between PRT and control for medium‐term function (SMD ‐0.09, 95% CI ‐0.56 to 0.39; I² = 50%; 151 participants, five trials; seeAnalysis 1.2). The significant result of the test for subgroup differences (P value 0.09, I² = 49.7%) reflects the spread of results for the five conditions evaluated here.
1.2. Analysis.
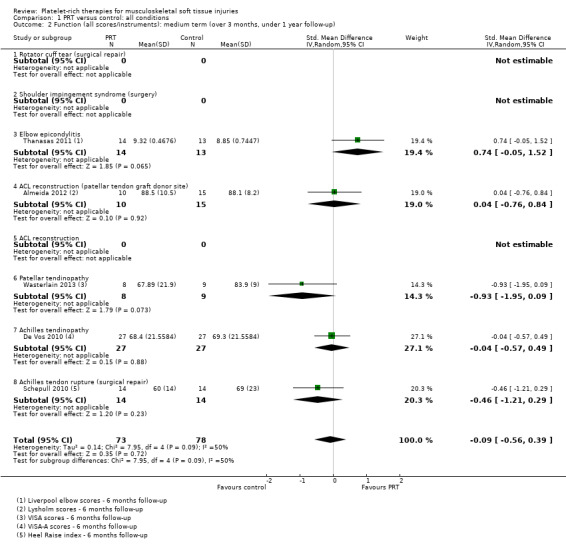
Comparison 1 PRT versus control: all conditions, Outcome 2 Function (all scores/instruments): medium term (over 3 months, under 1 year follow‐up).
Data on long‐term function derived from six different functional scores, pooled from 10 trials assessing PRT for five clinical conditions, showed no statistically significant difference between PRT and control (SMD 0.25, 95% CI ‐0.07 to 0.57; P value 0.12; I² = 66%; 484 participants, 10 trials; seeAnalysis 1.3). The significant results of the test for subgroup differences (P value 0.009, I² = 70.3%), reflect the results from Cervellin 2012, which were strongly in favour of PRT. Upon removal of Cervellin 2012 (40 participants), the pooled results showed less difference between the two groups (SMD 0.15, 95% CI ‐0.11 to 0.41) and the test for subgroup differences shows no difference (I² = 0%) (data not shown). The heterogeneity in the results for six trials (324 participants) of PRT for surgical repair of rotator cuff tears is also marked (P value 0.02, I² = 63%). As reported below, the pooled results of the five trials in this category reporting Constant scores showed a small but clinically non‐significant difference in favour of PRT. Thus the clinical significance of the upper 95% confidence limit of the pooled findings in Analysis 1.3 is doubtful.
1.3. Analysis.
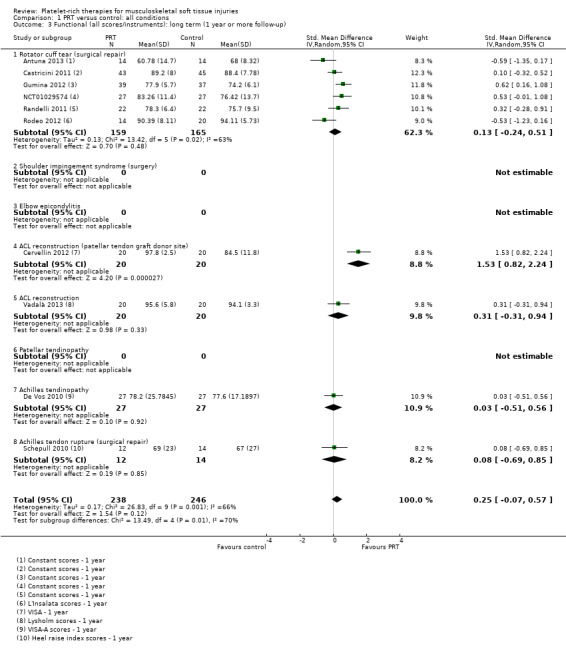
Comparison 1 PRT versus control: all conditions, Outcome 3 Functional (all scores/instruments): long term (1 year or more follow‐up).
Pain
Data pooled from four trials covering three conditions, showed a clinically small but statistically significant reduction in short‐term pain in favour of PRT (mean difference (MD) ‐0.95, 95% confidence interval (CI) ‐1.41 to ‐0.48; I² = 0%; 175 participants, four trials; see Analysis 1.4). Significantly heterogeneous data (P value 0.002; I² = 89.7%) for medium‐term pain were available from two trials (47 participants; two conditions), which reported in favour of PRT and control, respectively; we decided against pooling these data (seeAnalysis 1.5). Similarly, we decided not to pool data for long‐term pain based on a similar finding of significant heterogeneity of the results of two trials on one condition (P value 0.08; I² = 67%; 81 participants) (seeAnalysis 1.6).
1.4. Analysis.
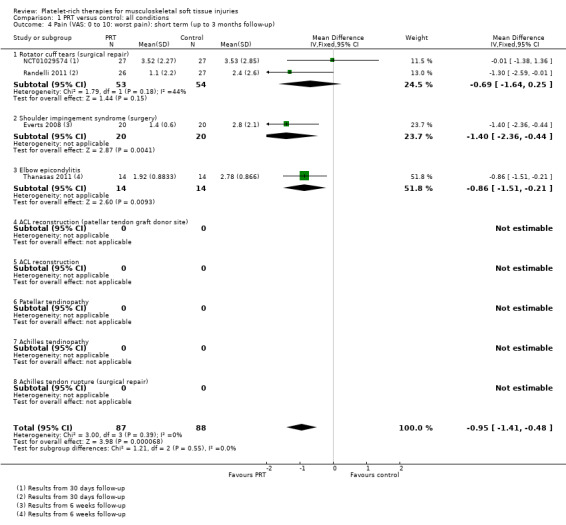
Comparison 1 PRT versus control: all conditions, Outcome 4 Pain (VAS: 0 to 10: worst pain): short term (up to 3 months follow‐up).
1.5. Analysis.
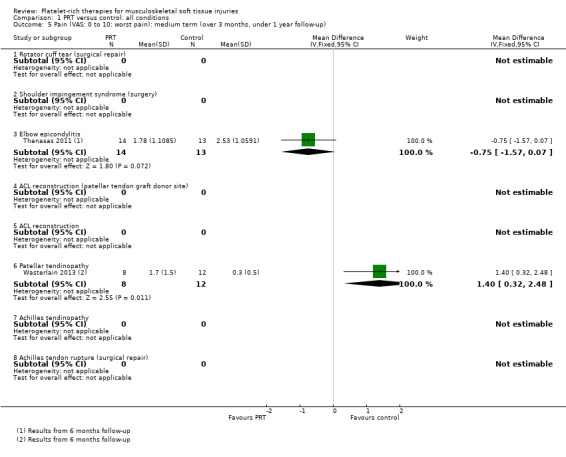
Comparison 1 PRT versus control: all conditions, Outcome 5 Pain (VAS: 0 to 10: worst pain): medium term (over 3 months, under 1 year follow‐up).
1.6. Analysis.
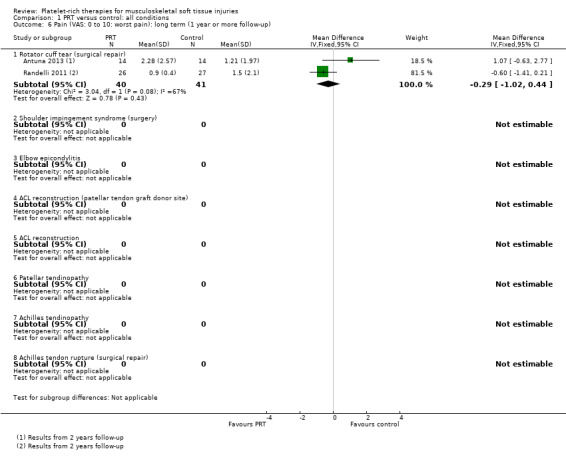
Comparison 1 PRT versus control: all conditions, Outcome 6 Pain (VAS: 0 to 10: worst pain): long term (1 year or more follow‐up).
Adverse effects
Four trials reported adverse effects and a further seven trials reported that there were no adverse effects. Pooled data showed no evidence of a significant difference between the two groups (7/241 versus 5/245; risk ratio (RR) 1.31, 95% CI 0.48 to 3.59; I² = 0%; 486 participants, 11 trials; see Analysis 1.7). The adverse effects were concerns about persisting pain prompting clinical contact in Krogh 2013 (seven participants), one shoulder adhesive capsulitis in each group in NCT01029574, one tendon repair rupture in the control group in Randelli 2011 and one re‐rupture and one deep infection in Schepull 2010.
1.7. Analysis.
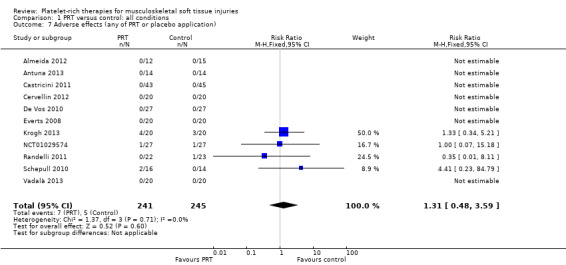
Comparison 1 PRT versus control: all conditions, Outcome 7 Adverse effects (any of PRT or placebo application).
Other outcomes
Our secondary outcomes were reported in only a few trials. The results are presented under the separate conditions.
Returns to sports: De Vos 2010 (chronic Achilles tendinopathy).
Quality of life (SF‐12): Wasterlain 2013 (patellar tendinopathy).
Recurrence (retear) of condition: Antuna 2013 and Randelli 2011 (rotator cuff tears) and Schepull 2010 (Achilles tendon rupture).
Patient satisfaction: Antuna 2013 (rotator cuff repair) and De Vos 2010 (chronic Achilles tendinopathy).
Subgroup analysis: PRT as a main treatment of tendinopathies versus as a surgical augmentation procedure for a musculoskeletal soft tissue injury
We subgrouped the trials into two categories: 'tendinopathies' in which PRT injections were the main treatment (Creaney 2011; De Vos 2010; Krogh 2013; Thanasas 2011; Wasterlain 2013), and 'surgical augmentation procedures' where PRT was applied during surgery (Almeida 2012; Antuna 2013; Castricini 2011; Cervellin 2012; Everts 2008; Gumina 2012; NCT01029574; Orrego 2008; Randelli 2011; Rodeo 2012; Schepull 2010; Vadalà 2013; Valenti Nín 2009; Vogrin 2010).
Function
Data on short‐term function were available from three tendinopathy trials and one augmentation trial.The test for subgroup differences was not significant (P value 0.26; I² = 20.7%) (see Analysis 2.1). Moderately heterogenous data pooled from the tendinopathy trials showed no difference between PRT and control in short‐term function (SMD 0.14, 95% CI ‐0.38 to 0.67; P value 0.13; I² = 50%; 182 participants, three trials).
2.1. Analysis.
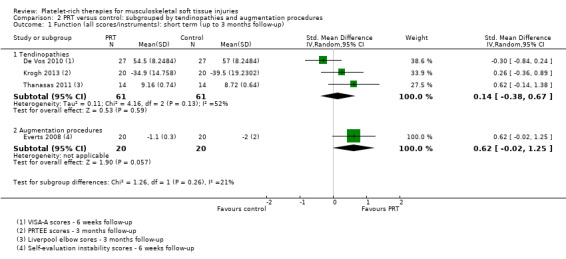
Comparison 2 PRT versus control: subgrouped by tendinopathies and augmentation procedures, Outcome 1 Function (all scores/instruments): short term (up to 3 months follow‐up).
Data on medium‐term function were available from three tendinopathy trials and two augmentation trials. The test for subgroup differences was not significant (P value 0.69; I² = 0%) (see Analysis 2.2). Significantly heterogeneous data pooled for the three tendinopathy trials showed little evidence (P value 0.95) of a difference between in PRT and control in medium‐term function (SMD ‐0.02, 95% CI ‐0.83 to 0.78; I² = 70%; 98 participants, three trials). A similar finding of little difference (P value 0.42) applied to pooled function data for the two augmentation trials (SMD ‐0.22, 95% CI ‐0.77 to 0.32; I² = 0%; 53 participants, two trials).
2.2. Analysis.
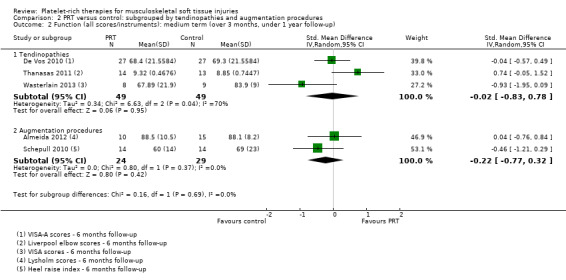
Comparison 2 PRT versus control: subgrouped by tendinopathies and augmentation procedures, Outcome 2 Function (all scores/instruments): medium term (over 3 months, under 1 year follow‐up).
Data on long‐term function were available from one tendinopathy trial and nine augmentation trials. The test for subgroup differences was not significant (P value 0.44; I² = 0%) (see Analysis 2.3). Significantly heterogeneous data (I² = 69%) pooled for the nine augmentation trials showed no statistically significant difference between PRT and control in long‐term function (SMD 0.28, 95% CI ‐0.08 to 0.64; P value 0.13; 430 participants, nine trials).
2.3. Analysis.
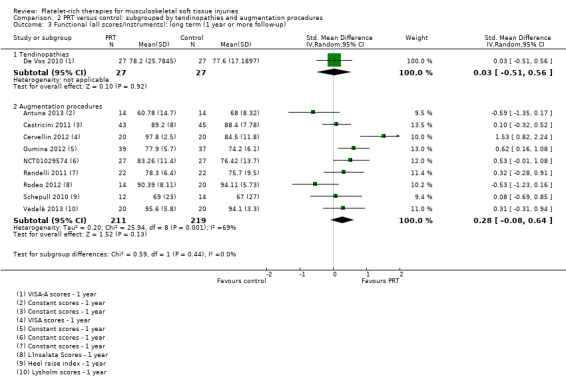
Comparison 2 PRT versus control: subgrouped by tendinopathies and augmentation procedures, Outcome 3 Functional (all scores/instruments): long term (1 year or more follow‐up).
Pain
Data on short‐term pain were available from one tendinopathy trial and three augmentation trials. The test for subgroup differences was not significant (P value 0.91; I² = 0%) (see Analysis 2.4). Slightly heterogeneous pooled data (I² = 30%) for augmentation procedures showed some clinically small benefit of PRT in short‐term pain (MD ‐1.04, 95% CI ‐1.71 to ‐0.37; 147 participants, three trials). Medium‐term pain data were only available from two tendinopathy trials; and long‐term pain data from two augmentation trials. As above, we did not pool these data given their clearly significant heterogeneity (see Analysis 2.5 and Analysis 2.6).
2.4. Analysis.
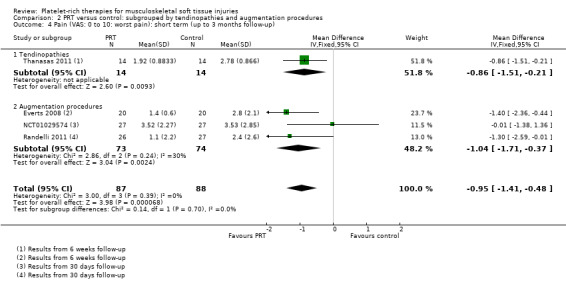
Comparison 2 PRT versus control: subgrouped by tendinopathies and augmentation procedures, Outcome 4 Pain (VAS: 0 to 10: worst pain): short term (up to 3 months follow‐up).
2.5. Analysis.
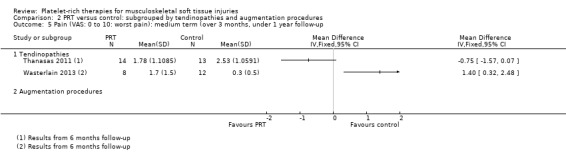
Comparison 2 PRT versus control: subgrouped by tendinopathies and augmentation procedures, Outcome 5 Pain (VAS: 0 to 10: worst pain): medium term (over 3 months, under 1 year follow‐up.
2.6. Analysis.
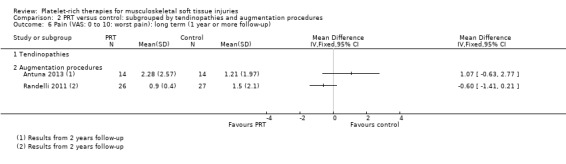
Comparison 2 PRT versus control: subgrouped by tendinopathies and augmentation procedures, Outcome 6 Pain (VAS: 0 to 10: worst pain): long term (1 year or more follow‐up).
Adverse effects
Adverse effects were reported in one tendinopathy trial and in three augmentation trials (see Analysis 2.7).
2.7. Analysis.
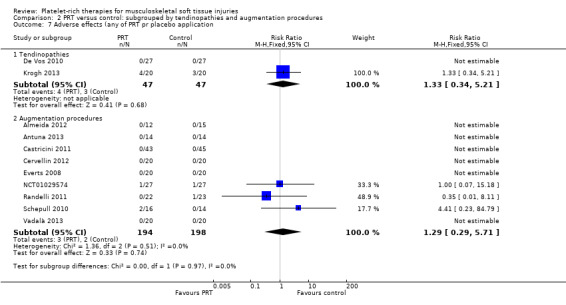
Comparison 2 PRT versus control: subgrouped by tendinopathies and augmentation procedures, Outcome 7 Adverse effects (any of PRT pr placebo application.
Analysis of individual conditions
Rotator cuff tears (surgical repair)
Six studies compared standard arthroscopic rotator cuff repair with or without PRP application at the repair site (Antuna 2013; Castricini 2011; Gumina 2012; NCT01029574; Randelli 2011; Rodeo 2012). Twelve participants were lost to follow‐up. Data were available for 291 participants.
Function
Functional status at one‐year follow‐up was documented in all six studies; the Constant score was reported in five studies (Antuna 2013; Castricini 2011; Gumina 2012; NCT01029574; Randelli 2011). A minimal clinically important difference of 10.4 for this commonly used score has been estimated in a study of rotator cuff surgery patients (Kukkonen 2013). Pooled Constant scores at one‐year follow‐up showed a clinically non‐significant difference in favour of PRT (MD 2.47, CI 95% 0.68 to 4.26; I² = 50%; 290 participants, five trials; see Analysis 3.1). The Constant scores at two years after the intervention from two trials were not pooled because these were significantly heterogeneous (I² = 85%; see Analysis 3.2) (Antuna 2013; Randelli 2011). Results based on other scores at one‐ or two‐year follow‐ups are shown in Analysis 3.3: UCLA (University of California Los Angeles) scores, two trials (NCT01029574; Randelli 2011); Analysis 3.4: SST (Simple Shoulder Test), two trials (Gumina 2012; Randelli 2011); Analysis 3.5 and Analysis 3.6: DASH (Disabilities of the Arm, Shoulder and Hand), one trial (Antuna 2013); Analysis 3.7 L'Insalata, one trial (Rodeo 2012); and Analysis 3.8: ASES (American Shoulder and Elbow Surgeons), one trial (Rodeo 2012). These show some variation in the direction and size of effect among different trials. When Constant data from five studies were pooled with the L'Insalata scores for Rodeo 2012 they showed little difference between the two groups (SMD 0.13, 95% CI ‐0.24 to 0.51; I² = 63%; 323 participants, six trials; see Analysis 3.9. However, the results of these six trials were significantly heterogenous.
3.1. Analysis.
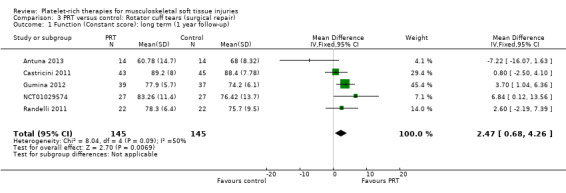
Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 1 Function (Constant score): long term (1 year follow‐up).
3.2. Analysis.
Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 2 Function (Constant score): long term (2 year follow‐up).
3.3. Analysis.
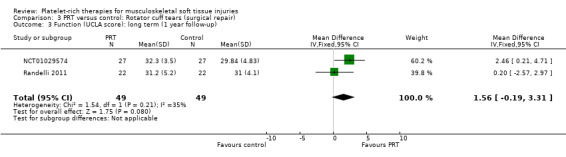
Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 3 Function (UCLA score): long term (1 year follow‐up).
3.4. Analysis.
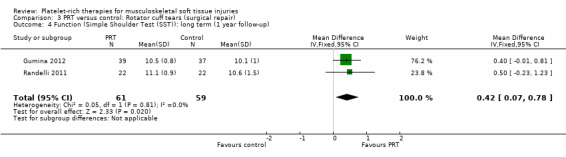
Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 4 Function (Simple Shoulder Test (SST)): long term (1 year follow‐up).
3.5. Analysis.
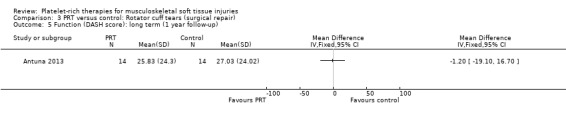
Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 5 Function (DASH score): long term (1 year follow‐up).
3.6. Analysis.
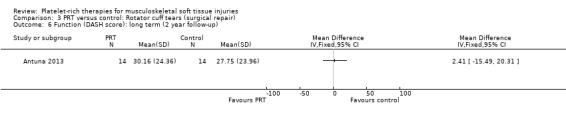
Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 6 Function (DASH score): long term (2 year follow‐up).
3.7. Analysis.
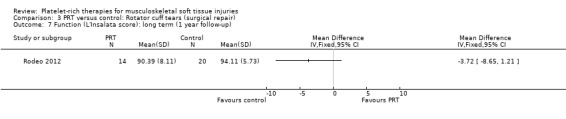
Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 7 Function (L'Insalata score): long term (1 year follow‐up).
3.8. Analysis.
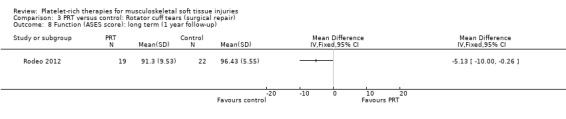
Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 8 Function (ASES score): long term (1 year follow‐up).
3.9. Analysis.
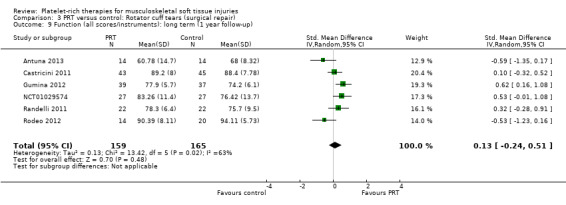
Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 9 Function (all scores/instruments): long term (1 year follow‐up).
Pain
Analysis 3.10 presents pain score data from two studies (105 participants) in the immediate post‐operative period (seven days). Pooled data showed some benefit of PRP (MD ‐1.40, 95% CI ‐2.44 to ‐0.36). The results at 30 days were heterogeneous, with those in Randelli 2011 still favouring PRT but those in NCT01029574 showing no difference between the two groups (MD ‐0.69, 95% CI ‐1.64 to 0.25; I² = 44%; 105 participants, two trials; see Analysis 3.13). Results at one‐year and two‐year follow‐ups were again from two trials with heterogenous results. Pooled results at one year showed little difference between the two groups (‐0.30, 95% CI ‐1.20 to 0.61; I² = 33%; 82 participants, two trials; see Analysis 3.12). The clearly heterogeneous results at two years (I² = 67%) meant that we did not pool these results (see Analysis 3.11).
3.10. Analysis.
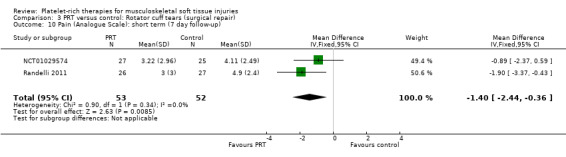
Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 10 Pain (Analogue Scale): short term (7 day follow‐up).
3.13. Analysis.
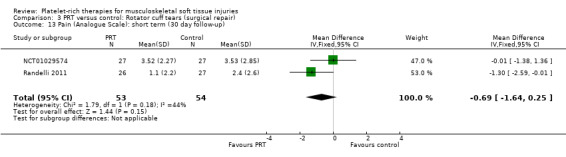
Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 13 Pain (Analogue Scale): short term (30 day follow‐up).
3.12. Analysis.
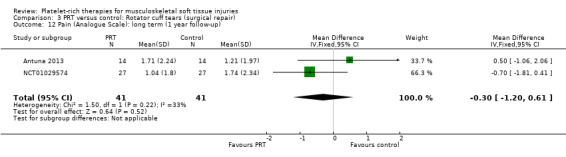
Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 12 Pain (Analogue Scale): long term (1 year follow‐up).
3.11. Analysis.
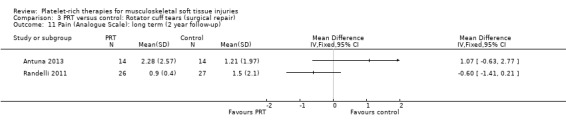
Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 11 Pain (Analogue Scale): long term (2 year follow‐up).
Retear rates
The three studies that assessed retear rates found fewer retears in the PRP group after one year (10/101 versus 19/98; RR 0.55, 95% CI 0.30 to 1.01; I² = 25%; see Analysis 3.14) (Castricini 2011; Gumina 2012; Randelli 2011). However, after two years, pooled results from two studies demonstrated more comparable rates of retear in the two groups (19/36 versus 22/37; RR 0.88, 95% CI 0.59 to 1.32; I² = 14%; see Analysis 3.15) (Antuna 2013; Randelli 2011).
3.14. Analysis.
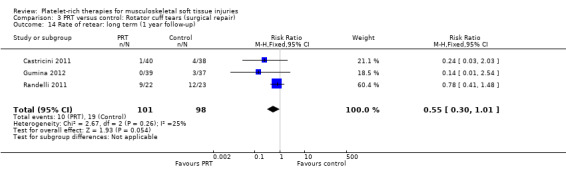
Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 14 Rate of retear: long term (1 year follow‐up).
3.15. Analysis.
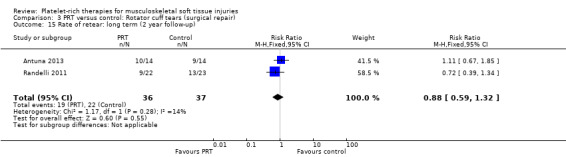
Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 15 Rate of retear: long term (2 year follow‐up).
Participant satisfaction
One study assessed participant satisfaction after two years and found that two participants, one from the PRP group and one from the control group, were dissatisfied with the procedure (satisfied participants: RR 1.00, 95% CI 0.81 to 1.23; see Analysis 3.16) (Antuna 2013).
3.16. Analysis.
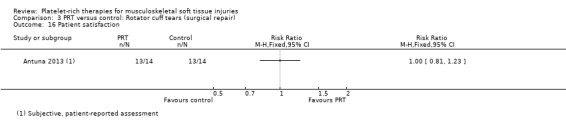
Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 16 Patient satisfaction.
Shoulder impingement syndrome (surgery)
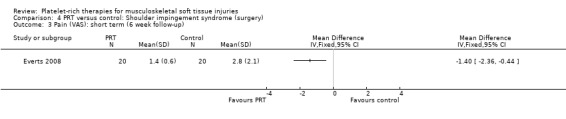
One study compared PRP versus no PRP application augmenting open subacromial decompression for shoulder impingement syndrome in 40 participants (Everts 2008); because of the limited availability of data at three‐month follow‐up, we present the data for six weeks here. (Data for pain were extracted from a graph.)
At six weeks, the PRP group had less pain (MD ‐1.40, 95% CI ‐2.36 to ‐0.44; see Analysis 4.3) and better function as assessed using the Shoulder Index Score (SIS), which measures pain and activities of daily living (MD ‐0.90, 95% CI ‐1.79 to ‐0.01; see Analysis 4.1). One participant in the PRP group and two participants in the control group had instability at six weeks (seeAnalysis 4.2). No adverse effects were reported.
4.3. Analysis.
Comparison 4 PRT versus control: Shoulder impingement syndrome (surgery), Outcome 3 Pain (VAS): short term (6 week follow‐up).
4.1. Analysis.
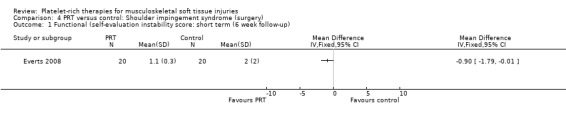
Comparison 4 PRT versus control: Shoulder impingement syndrome (surgery), Outcome 1 Functional (self‐evaluation instability score: short term (6 week follow‐up).
4.2. Analysis.
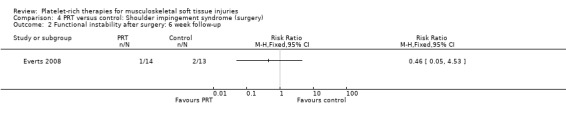
Comparison 4 PRT versus control: Shoulder impingement syndrome (surgery), Outcome 2 Functional instability after surgery: 6 week follow‐up.
Elbow epicondylitis
Three studies compared ultrasound‐guided PRP injections versus controls (Creaney 2011; Krogh 2013; Thanasas 2011). Two studies used autologous whole blood controls (Creaney 2011; Thanasas 2011), and Krogh 2013 used saline injections. (Krogh 2013 also assessed glucocorticoid injections in a third arm, but this intervention was not considered in this review.) Creaney 2011 applied two injections during a one‐month term, while the others applied a single injection (Krogh 2013; Thanasas 2011). Creaney 2011 included participants with 'resistant elbow tendinopathy' while Krogh 2013 and Thanasas 2011 included participants with lateral epicondylitis. Together, the three studies assessed 219 participants, with data available for pooling for 151 participants. Krogh 2013 reported a high rate of loss to follow‐up after three months, but no losses until three months. As the trial authors suggested in their report, we have only included the three‐month results here.
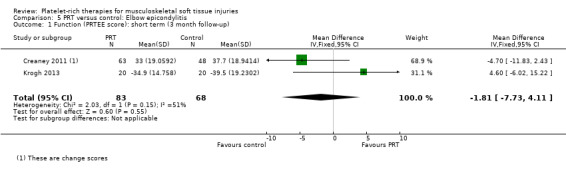
Function
Function was assessed using the PRTEE (Patient‐Rated Tennis Elbow Evaluation) in Creaney 2011 and Krogh 2013, while the Liverpool Elbow score was used in Thanasas 2011. The results of the individual scores at short‐ and medium‐term follow‐ups are shown in Analysis 5.1, Analysis 5.2, Analysis 5.3 and Analysis 5.4. The results were heterogenous with those in Krogh 2013 and Thanasas 2011 tending to favour PRT whilst the converse applied in Creaney 2011. Creaney 2011 noted that the favourable PRTEE change scores at six months in the control group (autologous blood) (MD ‐11.00, 95% CI ‐18.07 to ‐3.93) should be viewed cautiously because of the exclusion of some participants submitted for surgery because of treatment failure: "caution is advised against concluding that there is a true difference". Pooled results from two trials using different scores (final scores were not available for Creaney 2011) for function at short‐term follow‐up favoured PRT (SMD 0.40, 95% CI ‐0.08 to 0.89; 68 participants, two trials; seeAnalysis 5.5).
5.1. Analysis.
Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 1 Function (PRTEE score): short term (3 month follow‐up).
5.2. Analysis.
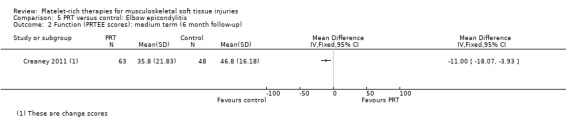
Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 2 Function (PRTEE scores): medium term (6 month follow‐up).
5.3. Analysis.
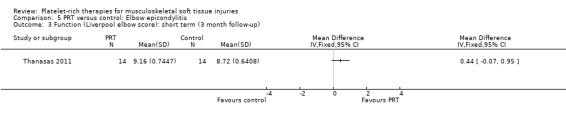
Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 3 Function (Liverpool elbow score): short term (3 month follow‐up).
5.4. Analysis.
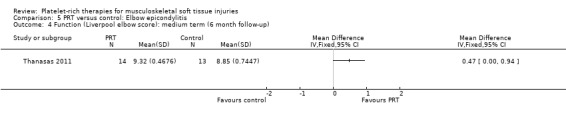
Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 4 Function (Liverpool elbow score): medium term (6 month follow‐up).
5.5. Analysis.
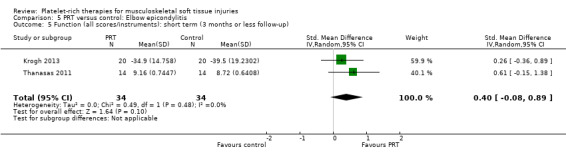
Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 5 Function (all scores/instruments): short term (3 months or less follow‐up).
Pain
Data from Thanasas 2011 showed lower pain scores in the PRP group at six weeks (MD ‐0.86, 95% CI ‐1.51 to ‐0.21; see Analysis 5.6) and six months (MD ‐0.75, 95% CI ‐1.57 to 0.07; see Analysis 5.7). Krogh 2013 also assessed pain related to the injection itself, querying by mail whether "injection therapy had caused any additional pain on a numeric rating scale from 0 to 10" and reported that PRP injections were more painful than saline injections.
5.6. Analysis.
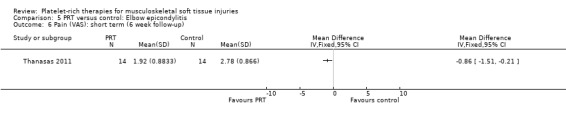
Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 6 Pain (VAS): short term (6 week follow‐up).
5.7. Analysis.
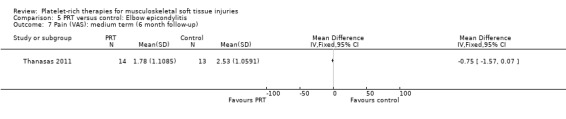
Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 7 Pain (VAS): medium term (6 month follow‐up).
Complications
Krogh 2013 reported that four participants in the PRP group and three in the saline group contacted the institution due to concerns about persisting pain.
Anterior cruciate ligament (ACL) reconstruction
Graft donor site
Two studies assessed the effects of PRP application at the patellar tendon donor site (Almeida 2012; Cervellin 2012). Both studies added PRP to the site of patellar tendon defect after harvesting. The studies were very similar in their design and outcomes. In all, there were 67 participants, of whom 65 were assessed at follow‐up. These studies analysed function and pain scores and also had a specific analysis of MRI parameters, such as patellar tendon graft area measurements. Almeida 2012 found no difference in function at six‐month follow‐up, when based on Tegner scores (MD 0.30, 95% CI ‐0.72 to 1.32; see Analysis 6.1) or Lysholm scores (MD 0.40, 95% CI ‐7.32 to 8.12; see Analysis 6.2). In contrast, Cervellin 2012 reported differences favouring the PRP group at one‐year follow‐up (MD 13.30, 95% CI 8.01 to 18.59; see Analysis 6.3). One study highlighted that the findings might demonstrate that PRP application may be of little relevance from the clinical perspective (Almeida 2012). Almeida 2012 found lower pain scores in the PRP group in the first day after surgery (MD ‐1.30, 95% CI ‐2.23 to ‐0.37; see Analysis 6.4). The studies reported that there were no adverse effects or complications.
6.1. Analysis.
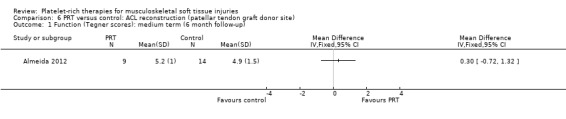
Comparison 6 PRT versus control: ACL reconstruction (patellar tendon graft donor site), Outcome 1 Function (Tegner scores): medium term (6 month follow‐up).
6.2. Analysis.
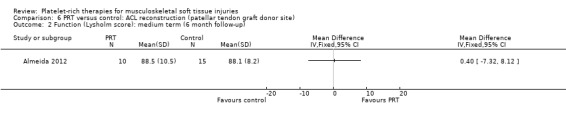
Comparison 6 PRT versus control: ACL reconstruction (patellar tendon graft donor site), Outcome 2 Function (Lysholm score): medium term (6 month follow‐up).
6.3. Analysis.
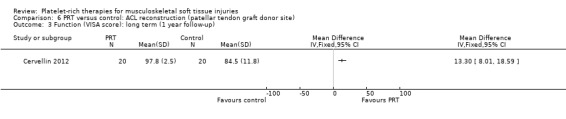
Comparison 6 PRT versus control: ACL reconstruction (patellar tendon graft donor site), Outcome 3 Function (VISA score): long term (1 year follow‐up).
6.4. Analysis.
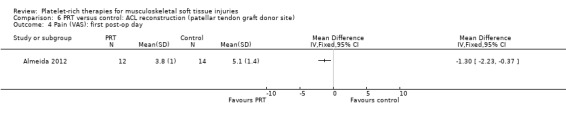
Comparison 6 PRT versus control: ACL reconstruction (patellar tendon graft donor site), Outcome 4 Pain (VAS): first post‐op day.
Anterior cruciate ligament (ACL) reconstruction ‐ augmentation procedure
Four studies analysed the effects of PRP application during the ACL reconstruction procedure (Orrego 2008; Vadalà 2013; Valenti Nín 2009; Vogrin 2010). There were 203 participants, but the data for five were lost. All studies reported the application of PRP to the knee bone tunnels or in the inner area of the graft, or both. No difference between groups was found for the IKDC (International Knee Documentation Committee) result at one year, either in the scores (MD ‐1.40, 95% CI ‐6.01 to 3.21; one trial; seeAnalysis 7.1) or in the numbers of people with good or better results (94/96 versus 94/97; RR 1.01, 95% CI 0.96 to 1.07; see Analysis 7.2). Vadalà 2013 also found no difference in the Lysholm scores (see Analysis 7.3). This trial specifically reported that there were no adverse effects associated with the procedure.
7.1. Analysis.
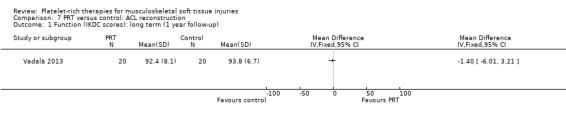
Comparison 7 PRT versus control: ACL reconstruction, Outcome 1 Function (IKDC scores): long term (1 year follow‐up).
7.2. Analysis.
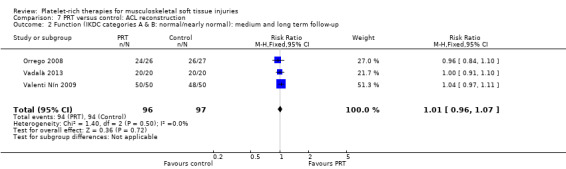
Comparison 7 PRT versus control: ACL reconstruction, Outcome 2 Function (IKDC categories A & B: normal/nearly normal): medium and long term follow‐up.
7.3. Analysis.
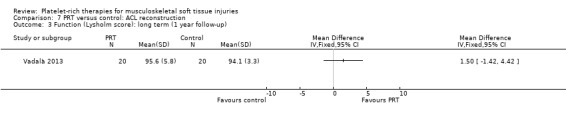
Comparison 7 PRT versus control: ACL reconstruction, Outcome 3 Function (Lysholm score): long term (1 year follow‐up).
Patellar tendinopathy
Wasterlain 2013 compared ultrasound‐guided application of PRP with dry needling control in 23 people with patellar tendinopathy. Three participants were lost to final follow‐up at six months. The protocol permitted participants to switch treatments if not satisfied, and analyses were performed on an intention‐to‐treat basis. Assessment at six months demonstrated that the dry needling participants tended towards higher VISA scores (MD ‐16.01, 95% CI ‐32.28 to 0.26; see Analysis 8.1), and Tegner scores (MD 0.60, 95% CI ‐2.44 to 1.24; seeAnalysis 8.2). In contrast, Lysholm scores favoured PRP (MD 15.50, 95% CI 0.55 to 30.45; see Analysis 8.3). PRP group participants had less pain at six‐month follow‐up (MD 1.40, 95% CI 0.32 to 2.48; seeAnalysis 8.4). No complications or adverse effects were found. No difference between groups was found for quality of life assessed using the SF‐12 (MD ‐1.60, 95% CI ‐5.66 to 2.46; see Analysis 8.5). As sample sizes were small, results from this study should be considered with caution.
8.1. Analysis.
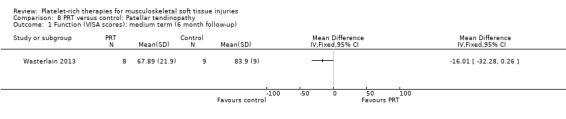
Comparison 8 PRT versus control: Patellar tendinopathy, Outcome 1 Function (VISA scores): medium term (6 month follow‐up).
8.2. Analysis.
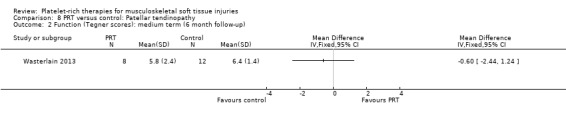
Comparison 8 PRT versus control: Patellar tendinopathy, Outcome 2 Function (Tegner scores): medium term (6 month follow‐up).
8.3. Analysis.
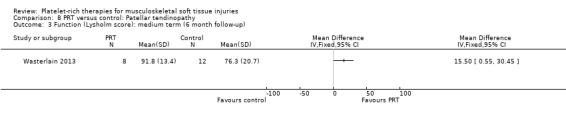
Comparison 8 PRT versus control: Patellar tendinopathy, Outcome 3 Function (Lysholm score): medium term (6 month follow‐up).
8.4. Analysis.
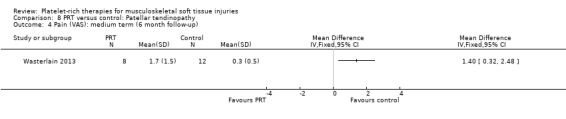
Comparison 8 PRT versus control: Patellar tendinopathy, Outcome 4 Pain (VAS): medium term (6 month follow‐up).
8.5. Analysis.
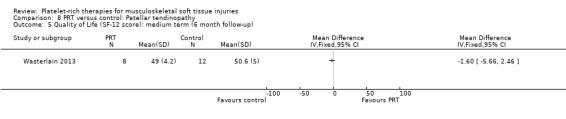
Comparison 8 PRT versus control: Patellar tendinopathy, Outcome 5 Quality of Life (SF‐12 score): medium term (6 month follow‐up).
Chronic Achilles tendinopathy
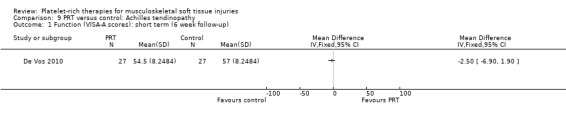
One study compared PRP versus placebo (saline) injection in 54 participants with chronic Achilles tendinopathy (De Vos 2010); both groups received eccentric exercises. No participants were lost during the follow‐up period. The authors stated a priori that a 12‐point difference in VISA‐A scores was the relevant difference to be detected.
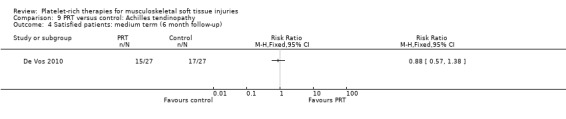
De Vos 2010 found no difference between the two groups in function assessed using VISA‐A score in the short term (six weeks: see Analysis 9.1), medium term (six months: see Analysis 9.2) and long term (one year: see Analysis 9.3). Similar numbers of participants in the two groups indicated they were satisfied with their outcome in the medium‐term (seeAnalysis 9.4) and long‐term (seeAnalysis 9.5). Return to sports was also similar in the two groups at medium‐term (see Analysis 9.6) and long‐term follow‐up (seeAnalysis 9.7).
9.1. Analysis.
Comparison 9 PRT versus control: Achilles tendinopathy, Outcome 1 Function (VISA‐A scores): short term (6 week follow‐up).
9.2. Analysis.
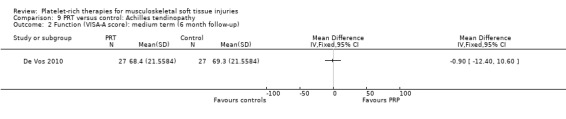
Comparison 9 PRT versus control: Achilles tendinopathy, Outcome 2 Function (VISA‐A score): medium term (6 month follow‐up).
9.3. Analysis.
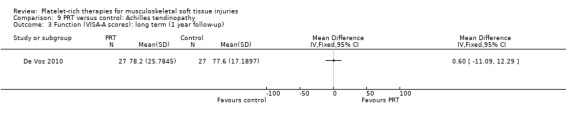
Comparison 9 PRT versus control: Achilles tendinopathy, Outcome 3 Function (VISA‐A scores): long term (1 year follow‐up).
9.4. Analysis.
Comparison 9 PRT versus control: Achilles tendinopathy, Outcome 4 Satisfied patients: medium term (6 month follow‐up).
9.5. Analysis.
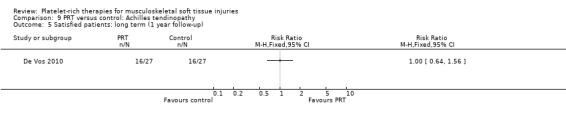
Comparison 9 PRT versus control: Achilles tendinopathy, Outcome 5 Satisfied patients: long term (1 year follow‐up).
9.6. Analysis.
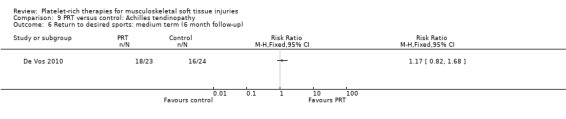
Comparison 9 PRT versus control: Achilles tendinopathy, Outcome 6 Return to desired sports: medium term (6 month follow‐up).
9.7. Analysis.
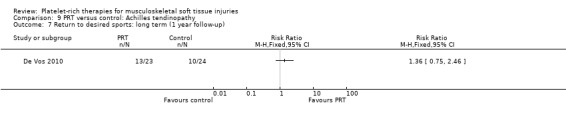
Comparison 9 PRT versus control: Achilles tendinopathy, Outcome 7 Return to desired sports: long term (1 year follow‐up).
Acute Achilles tendon rupture (surgical repair)
One study compared PRP application in acute surgical repair of acute Achilles tendon ruptures in 30 participants (Schepull 2010), four of whom were lost to follow‐up at one‐year follow‐up. Assessment of function was based on the heel‐raise index, a validated test used to evaluate calf muscle function. There was no difference between the PRP and no PRP groups in the heel‐raise index results at 19‐week follow‐up (MD ‐9.00, 95% CI ‐23.10 to 5.10; seeAnalysis 10.1), or at one year (MD 2.00, 95% CI ‐17.22 to 21.22; seeAnalysis 10.2). The authors reported two complications in the PRP group, one re‐rupture and one deep infection, and no complications with the controls (RR 4.41, 95% CI 0.23 to 84.79; see Analysis 10.3). This study also looked at the influence of PRP on the mechanical properties of the tendon using tantalum beads as landmarks for three‐dimensional radiographic studies; these results are not reported here.
10.1. Analysis.
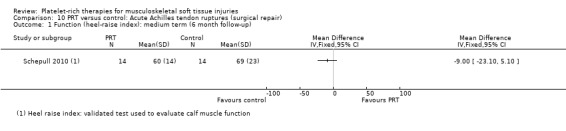
Comparison 10 PRT versus control: Acute Achilles tendon ruptures (surgical repair), Outcome 1 Function (heel‐raise index): medium term (6 month follow‐up).
10.2. Analysis.
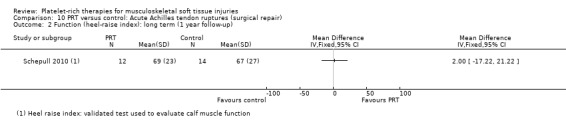
Comparison 10 PRT versus control: Acute Achilles tendon ruptures (surgical repair), Outcome 2 Function (heel‐raise index): long term (1 year follow‐up).
10.3. Analysis.
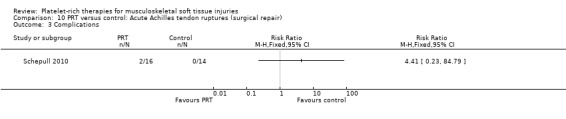
Comparison 10 PRT versus control: Acute Achilles tendon ruptures (surgical repair), Outcome 3 Complications.
Sensitivity analyses
Three trials were considered to be at high risk of selection bias from failure to conceal allocation. Two were quasi‐randomised (Orrego 2008; Vogrin 2010), and envelopes were opened three days before surgery in the third trial (Gumina 2012). Both quasi‐RCTs assessed ACL reconstruction, but data were available from Orrego 2008 only. Removing this trial from Analysis 7.2 did not affect the result: the RR changed from 1.01 (95% CI 0.96 to 1.07) to 1.03 (95% CI 0.97 to 1.09). Gumina 2012, one of the rotator cuff repair trials, was more influential. Removing this trial from Analysis 1.3 changed the pooled result from SMD 0.25 (95% CI ‐0.07 to 0.57) to SMD 0.20 (95% CI ‐0.15 to 0.56); removing it from the augmentation procedures in Analysis 2.3 changed the result from SMD 0.28 (95% CI ‐0.08 to 0.64) to SMD 0.23 (95% CI ‐0.18 to 0.63); and removing it from Analysis 3.9 changed the result from SMD 0.29 (95% CI ‐0.01 to 0.60) to SMD 0.21 (95% CI ‐0.13 to 0.55).
Discussion
This comprehensive systematic review aimed to assess the use of PRT as a treatment option for musculoskeletal soft tissue injuries. From the clinical perspective, there are questions regarding its clinical effectiveness and the possibility of adverse effects. Hence, this review and its focus on these key clinical endpoints.
Summary of main results
We included data on 1088 participants from 19 small single‐centre trials (17 randomised and two quasi‐randomised) that compared platelet‐rich therapy (PRT) with placebo, autologous whole blood, dry needling or no platelet‐rich therapy. These 19 trials covered eight clinical conditions: rotator cuff tears (arthroscopic repair) (six trials); shoulder impingement syndrome surgery (one trial); elbow epicondylitis (three trials); anterior cruciate ligament (ACL) reconstruction (four trials): hamstrings autologous graft (three trials), patellar tendon autologous graft (one trial)), ACL reconstruction (donor graft site application ‐ patellar tendon autologous graft) (two trials), patellar tendinopathy (one trial), Achilles tendinopathy (one trial) and acute Achilles rupture surgical repair (one trial). There were no trials available that evaluated PRT for sprains or muscle injuries; however, trial results will be available soon (see Ongoing studies). In our overall analyses, which compared PRT versus control (no PRT, autologous whole blood, dry needling or placebo), we presented the results subgrouped by these eight conditions. For function and pain, we presented separate results for short‐term (up to three months); medium‐term (usually six months) and long‐term (usually one year).
The results for function were available for a maximum of 45% of the participants included in the review and usually far less. These showed no statistically significant differences between the PRT and no PRT (control) in short‐term function (P value 0.26; 162 participants, four trials), in medium‐term function (P value 0.72; 151 participants, five trials) and in long‐term function (P value 0.12; 484 participants, 10 trials). In each case, the 95% confidence intervals indicated the possibility of a poorer outcome in the PRT group up to a moderate difference in favour of PRT at both short‐ and long‐term follow‐up. In all three analyses, the results of the individual trials were statistically heterogeneous, and significantly so at long‐term follow‐up. Sensitivity analyses where single outlier trials were removed showed the lack of robustness of these findings (with the effect moving closer to the null), as did the removal of the results for one trial at a high risk of selection bias. The interpretation of the SMD results is hampered by the variety of condition‐ or limb‐specific functional scores. However, based on the finding of clinically insignificant findings from the pooled results of five rotator cuff repair trials reporting Constant scores at long‐term follow‐up as a guide (MD 2.47, CI 95% 0.68 to 4.26; see Analysis 3.1), it is unlikely that the upper limit of the 95% confidence interval for long‐term function (0.57) and, by corollary, that of short‐term function (0.71), translates to a clinically important difference. This impression is reinforced when the SMD results (random‐effects) of these five trials are viewed using the same data as in Analysis 3.1 (SMD 0.25, 95% CI ‐0.10 to 0.61; not shown).
Homogenous data pooled from four trials, and three conditions, showed a small reduction in short‐term pain in favour of PRT; however, the clinical significance of a mean 0.95 difference in a 10‐point visual analogue scale (VAS) is marginal. Four of the 11 trials reporting on adverse outcomes reported a total of 12 adverse effects, with no significant difference between groups in the pooled result. Seven of these adverse effects were concerns about persisting pain that led to clinical contact in one trial.
Subgrouping by PRT therapy for tendinopathies (five trials) and surgical augmentation procedures (14 trials) was more revealing in the distribution of results in the analyses (with tendinopathies dominating short‐term and medium‐term function analyses, and augmentation dominating the long‐term analysis) and the substantial heterogeneity in the results of the trials within each subgroup. Where pooled, the results of trials in each category did not differ markedly from the overall findings.
Overall, the available evidence is insufficient to indicate whether the effects of PRT will differ importantly in individual clinical conditions. Primary outcome data could be pooled for just two individual conditions: rotator cuff tears (arthroscopic repair) (six trials) and elbow epicondylitis (three trials). The results for the former were heterogenous; the pooled results for long‐term function for all six trials showed no statistically or clinically significant differences between the two groups (324 participants). The results of the elbow epicondylitis trials were also heterogeneous. Pooled results from two trials showed no statistically or clinically significant differences between the two groups (151 participants) in short‐term function. (Change score data only were available for the largest trial; this precluded pooling with final score short‐term and medium‐term function data from the other trials on this condition.)
Overall completeness and applicability of evidence
The current evidence base on which to decide whether to use PRT for treating musculoskeletal injuries is weak, as it consists of 19 small single‐centre studies reporting a variety of outcome measures, several of which were not directly relevant to clinical outcomes. In particular, several trials reported on the effects of PRT on tissue healing by the use of imaging methods, such as magnetic resonance imaging (MRI) or ultrasound. However, as the relationship between these outcomes and symptoms or function is unclear, we have not reported these findings in this review. We focused on outcomes of direct relevance to patients required to assess whether PRT actually works in practice.
Overall, data from less than half of the 1088 trial participants were included in any analysis.
These trials covered a variety of conditions, which could be subgrouped according to whether PRT was used as the main treatment for tendinopathies or as a surgical augmentation procedure. These groups could be subgrouped also into eight clinical conditions. In general, the demographic characteristics, such as gender, age and sports activity level of the trial participants in these eight categories were representative. However, caution is still required as the available evidence for each category may not be applicable overall, given the clinical variation within them, for example complete versus partial rotator cuff tears and acute versus chronic tendinopathies.
As well as clinical heterogeneity in the review population, there was heterogeneity in the application of PRT. It is possible that the effectiveness of the intervention may vary depending on how the platelet‐rich plasma (PRP) is prepared, but currently there is no consensus regarding standardisation for research or clinical use. There are several preparation methods for platelet products, which are likely to be a source of heterogeneity for the assessment and comparison of the effectiveness of PRT. Additionally, some of the proposed protocols lacked documented a priori validation (Dohan 2009; De Long 2012). A specific explanation of these difficulties and differences can be found in the literature (De Long 2012; Dohan 2009; Europe 2007); however, this is based mostly on empirical evidence and basic science studies. While conceptually, PRT is supposed to enhance healing, it is possible that various platelet‐rich products, including those applied in the included trials, have different biological mechanisms. This again undermines a more general application.
While doing this review, we decided to extend our acceptable controls to include autologous whole blood and dry needling, as used in two elbow tendinopathy studies. Both these interventions are aimed at increasing the blood available at the injured site and thus do not meet the characteristics of a placebo fully. However, they can still be considered to be a control, given that PRT is an 'improved' fraction of whole blood in terms of the concentration of growth factors. We did not include pharmacological controls such as steroids (Peerbooms 2010), which would change the question to a comparison between two 'active' interventions. We have now made this exclusion explicit in Types of interventions.
The timing and duration of expected benefit of PRT should also be considered when considering the plausibility of the results. For instance: would any early improvement be expected to persist? At any rate, the potential for a small, but clinically marginal effect, in favour of PRT for short‐term pain is of questionable value in the context of the lack of evidence in favour of PRT for short‐term or long‐term function.
Quality of the evidence
This is discussed for the overall population only. The quality of the evidence available for all primary outcomes for which data were pooled (short‐term function, long‐term function, short‐term pain and adverse effects) was downgraded three levels in each case: one for limitations in design and implementation that related to potential risk of bias, often selective reporting bias (discussed below); one for inconsistency of results in terms of the variety in conditions under test but also the lack of standardisation in the PRT intervention (discussed further below); and one because most studies included in the review did not contribute to the outcome. Thus overall we judged the evidence to be of very low quality, which indicates that we are very uncertain about the estimates for all outcomes.
Randomisation methodologies and allocation concealment were adequate in most of the trials, but there were some key methodological concerns regarding this clinically heterogeneous group of trials that assessed a wide variety of conditions. Despite the fact we were assessing recent research (participants recruited after 2005), many studies had not made public an a priori research protocol or trial registration document. The latter are important for research transparency and, on particular, help identify and probably reduce selective reporting. It is possible, that selective reporting may result in researchers not reporting adverse effects, or reporting imaging analysis or other surrogate outcomes that tend not to have a clear relation to functional status.
A major concern particular to PRP research is the methodology for its preparation. There are a wide variety of PRP preparation protocols. Studies used different preparation methodologies with mostly minor, and occasionally major, modifications from preparation instructions derived from specific commercial kits. In addition, classification proposals of platelet‐rich products are available (De Long 2012; Dohan 2009; Europe 2007) and have demonstrated from the clinical science perspective that the effectiveness of these products may be strongly linked to three key items: 1) the absolute number of platelets, 2) the manner in which platelet activation occurs, and 3) the presence or absence of white cells. We have described the PRP preparation protocols in each of the studies in the Description of studies section. Other variations that were also recognised include the time span between the PRP preparation and delivery (studies varied from the night before the procedure to intraoperative preparation); the method of PRP delivery, such as image‐guided, arthroscope‐guided, direct vision‐guided or no guidance; the number of PRP applications; and post‐operative interventions (casting, anti‐inflammatory drugs). The variation in these methodologies among the trials reduces the quality of the evidence.
Potential biases in the review process
A comprehensive search strategy with no language restriction was conducted. Handsearching from retrieved studies and other available systematic reviews confirmed our trial findings. Nevertheless, we have some concerns that some studies may have been missed during the review process. Since PRP is a novel therapy with ongoing studies, it is possible that we might have missed some new relevant research. As a safeguard, we have contacted well known researchers in the area twice (authors of narrative reviews, researchers who conducted non randomised trials and contacts from trial registry database). We have received some feedback declaring that no new research is being conducted by them and also from authors declaring that they have been conducting clinical trials, however, no data are, as yet, available. We also made contact with authors who did not provide data after declaring that research assessments were finished. Some authors were contacted to provide some additional data (Almeida 2012; Krogh 2013; NCT01029574), but Krogh 2013 did not provide this, as the trialists considered that raw data should not be circulated. Almeida 2012 and NCT01029574 provided their doctoral theses as the source of data and the author of NCT01029574 is currently preparing his research for publication. Wasterlain 2013 has also provided data prior to publication elsewhere.
Where possible, we followed the methods in our protocol. All key changes are listed in Differences between protocol and review. As stated above, these changes included extending our acceptable controls to include autologous whole blood and dry needling, but also clarifying that other active agents (such as steroid injections) were not acceptable controls.
Agreements and disagreements with other studies or reviews
We found some narrative reviews and three systematic reviews that partially (Chahal 2012; Taylor 2011), or considerably (Sheth 2012), overlapped with our analysis. All of these reviews focused on functional outcomes, such as pain and functional scores, but included studies other than randomised trials.
Chahal 2012 conducted a systematic review that assessed PRP as an augmentation procedure after full‐thickness rotator cuff repair. The authors included randomised controlled trials and cohort studies with a minimum one‐year follow‐up assessment. The authors stated that their a priori hypothesis was that PRP has no effect on the rate of retears or improvement in functional status. The authors included five studies in their analysis, two of which were RCTs included in our review. Using the Detsky scale for quality assessment, the authors described the included studies as high quality. No differences between groups were found for functional assessments or overall rates of retear. Subgroup analysis suggested that there were lower rates of retear for small to medium tears in the PRP group. As in our assessment, the authors highlighted the clinical heterogeneity of the included studies, such as the tear patterns (size, number of involved tendons), surgical technique implemented (single versus double row fixation) and PRP preparation methodology.
Sheth 2012 conducted a systematic review that assessed PRP for 'orthopaedic indications' and therefore included a broader range of conditions than our review. The authors opted to include studies other than randomised trials, such as cohort studies, and also included trials with corticosteroid control groups. They searched for pain, function and healing endpoints and included 33 studies, 23 of which were randomised controlled studies. Quality assessment used the GRADE approach that resulted in assessments of 'very low' quality for all but one of the studies. Pooled analysis for pain scores demonstrated no benefit of PRP in all time frames or in dedicated analysis for RCTs and non RCTs. The authors have highlighted the same difficulties that we found in summarising the results due to the heterogeneity of conditions and autologous blood products. They also advised that trials of larger sample sizes would be required to detect minimally important differences in pain and function.
Taylor 2011 performed a systematic review that included tendon and ligament injuries. The authors included both randomised and non randomised studies. No quality assessment was performed. The authors highlighted the difference in PRP terminology as well as demonstrating concerns about the preparation methodology. They also assessed platelet quantification. The authors did not perform quantitative synthesis and stated in their results that PRP generally has no effect compared with other treatments. Despite this, the authors concluded that PRP has "several potential advantages" such as "faster recovery" and '"possibly, a reduction in recurrence".
Authors' conclusions
Implications for practice.
The available evidence base for assessing the effects of platelet‐rich therapies (PRT) for treating musculoskeletal soft tissue injuries comprises a diverse collection of small trials that applied PRT in various ways for treating tendinopathies or as an augmentation procedure for surgically treated soft tissue injuries. There is very low quality evidence from a subset of these trials for a marginal short‐term benefit in pain from PRT; however, other very low quality evidence indicates that the use of PRT does not appear to have a clinically relevant effect on short‐term or long‐term function. Very low quality evidence showed no difference in adverse effects between the PRT and the various control interventions. Overall, and for the individual conditions, there is currently insufficient evidence to support the use of PRT for treating these injuries.
Implications for research.
This is an active research field, as shown by the large number of ongoing studies that are likely to be included in future updates. The findings of this review and assessment of the coverage of current ongoing trials should be considered for assessing the need and viability of future RCTs on specific conditions. An important preliminary to further PRT clinical research would be the development of a standardised methodology for PRP preparation. This may need some additional input from basic scientific research. Consensus methodology for PRP preparation is a key way to increase confidence in the generalisability of study findings.
As well as condition‐specific RCTs, more general RCTs that include a wider range of participants, with flexible inclusion criteria, should be considered. For these, a priori subgroup analysis of different clinical populations should be established. Methodological safeguards, such as allocation concealment, independent, possibly blinded, assessment and efforts to avoid participant loss to follow‐up are key. Short‐term (less than three months) and long‐term assessment (one year of longer) of pain and functional outcome data should be collected. A dedicated evaluation of adverse effects is also required.
Feedback
Data error for short‐ and medium‐term function in lateral epicondylitis subgroup, 22 April 2014
Summary
We are conducting a systematic review related to autologous blood injection for the treatment of lateral epicondylitis. In the data collection process, we read a Cochrane systematic review entitled "Platelet‐rich therapies for musculoskeletal soft tissue injuries"1. This article enabled us to clearly understand the current evidence regarding the use of platelet‐rich therapies in treating musculoskeletal diseases. We also read a randomised controlled trial written by Creaney et al.2, which was selected for meta‐analysis within the Cochrane systematic review. We discovered that this trial demonstrated that autologous blood injection improved outcome measures more than platelet‐rich plasma injections did at all follow‐up times. However, in the Cochrane systematic review, we discovered that all the meta‐analyses included in this trial were calculated using the wrong effect direction (Analysis 1.1, 1.2, 2.1, 2.2, 5.1, 5.2, 5.5 and 5.6), which may have significantly influenced the results. Therefore, we suggest that this error be corrected.
In our review, we found two randomised controlled trials reporting on the comparison of autologous blood injection and platelet‐rich plasma injection in treating lateral epicondylitis2,3. The meta‐analysis of these two trials indicated that the efficacy of autologous blood injection is non significantly different from that of platelet‐rich plasma injection (standardised mean difference ‐0.03; 95% confidence interval ‐1.09 to 1.03) in treating lateral epicondylitis.
Footnotes
1. Moraes VY, Lenza M, Tamaoki MJ, Faloppa F, Belloti JC. Platelet‐rich therapies for musculoskeletal soft tissue injuries. Cochrane Database of Systematic Reviews 2013;12:CD010071.
2. Creaney L, Wallace A, Curtis M, Connell D. Growth factor‐based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: a prospective, single‐blind, randomised trial of autologous blood injections versus platelet‐rich plasma injections. British Journal of Sports Medicine 2011;45:966‐71.
3. Thanasas C, Papadimitriou G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet‐rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. American Journal of Sports Medicine 2011;39:2130‐4.
I certify that I have no affiliations with or involvement in any organisation or entity with a financial interest in the subject matter of my feedback.
Reply
We are very grateful for this important feedback. On returning to Creaney 2011, we realised that we had failed to note that they presented change scores rather than final scores. Given that final value and change scores should not be combined as standardised mean differences, we have removed the change score data of Creaney 2011 from Analyses 1.1, 1.2, 2.1, 2.2 and 5.5; and deleted Analysis 5.6 (now replaced). We have corrected Analyses 5.1 and 5.2 and rewritten all sections that were affected by this error. Importantly, this has resulted in very little change to our overall results and none to our conclusions. However, it has changed our conclusions for lateral epicondylitis and given that we could not pool the data for all three trials in this category, we have removed mention of this in the Abstract results and Plain language summary; and adjusted our Discussion accordingly.
Contributors
Feedback submitted by:
Hung‐Chou Chen, MD and Tsan‐Hon Liou, MD, PhD (email: peter_liou@s.tmu.edu.tw) Department of Physical Medicine and Rehabilitation, Shuang Ho Hospital, Taipei Medical University, Taipei, Taiwan
Reply prepared by:
Vinícius Y Moraes, Review Contact Author Helen Handoll, Co‐ordinating Editor, Cochrane Bone, Joint and Muscle Trauma Group Xavier Griffin, Feedback Editor, Cochrane Bone, Joint and Muscle Trauma Group
What's new
| Date | Event | Description |
|---|---|---|
| 25 April 2014 | Amended | We removed data for Creaney 2011 from several analyses of short‐term and medium‐term function results. |
| 25 April 2014 | New citation required but conclusions have not changed | The data error that we have corrected did not affect our overall results or conclusions. However, it has changed our findings for lateral epicondylitis. Given that we could not pool the data for all three trials in this category, we have removed mention of this in the Abstract results and Plain language summary; and adjusted our Discussion accordingly. |
| 25 April 2014 | Feedback has been incorporated | Feedback pointed out a data error in the results for lateral epicondylitis. Change scores for one trial (Creaney 2011) had been erroneously interpreted as final scores. This has now been corrected. |
Notes
The first version of the full review was published in December 2013. This citation was published in April 2014 after reader feedback was incorporated and minor changes made.
Acknowledgements
We would like to thank Lindsey Elstub, Joanne Elliott, Catherine Deering and Laura MacDonald for editorial assistance. We would like to thank Helen Handoll, Robert Jan De Vos and Janet Wale for feedback on the review. We would like to thank William Gillespie and Helen Handoll for editorial feedback on the protocol.
Appendices
Appendix 1. Search strategies
The Cochrane Library (Wiley Online Library)
#1 MeSH descriptor: [Platelet‐Rich Plasma] this term only (109) #2 MeSH descriptor: [Blood Transfusion, Autologous] this term only (585) #3 platelet rich near/3 (plasma or therap* or fibrin):ti,ab,kw (400) #4 PRP or PRF:ti,ab,kw (575) #5 (platelet near/3 (gel or concentrate)) or buffy layer:ti,ab,kw (256) #6 #1 or #2 or #3 or #4 or #5 (1533) #7 MeSH descriptor: [Soft Tissue Injuries] this term only (68) #8 MeSH descriptor: [Athletic Injuries] this term only (425) #9 MeSH descriptor: [Tendon Injuries] explode all trees (358) #10 MeSH descriptor: [Sprains and Strains] this term only (265) #11 MeSH descriptor: [Contusions] this term only (80) #12 (injur* or trauma* or lesion* or damage* or wound* or destruction* or oedema* or edema* or haematoma or hematoma or contusion* or bruis*or concus* or commotion* or pressur* or soreness or sprain* or strain* or tear*):ti,ab (101791) #13 #7 or #8 or #9 or #10 or #11 or #12 (102192) #14 MeSH descriptor: [Muscle, Skeletal] explode all trees (6303) #15 MeSH descriptor: [Tendons] explode all trees (773) #16 MeSH descriptor: [Ligaments, Articular] explode all trees (881) #17 MeSH descriptor: [Cartilage] this term only (71) #18 Soft tissue or muscl* or muscul* or ligament* or tendon* or tendin* or cartilage or sport* or athlet*:ti,ab,kw (32683) #19 #14 or #15 or #16 or #17 or #18 (32912) #20 #13 and #19 (8811) #21 #6 and #20 in Trials (26)
MEDLINE (Ovid Online)
1 Platelet‐Rich Plasma/ (1151) 2 Blood Transfusion, Autologous/ (6445) 3 (platelet rich adj3 (plasma or therap* or fibrin)).tw. (5638) 4 (PRP or PRF).tw. (11577) 5 ((platelet adj3 (gel or concentrate)) or buffy layer).tw. (963) 6 or/1‐5 (21986) 7 Soft Tissue Injuries/ (3070) 8 Athletic Injuries/ (19389) 9 exp Tendon Injuries/ (13273) 10 "Sprains and Strains"/ (3528) 11 Contusions/ (3830) 12 (injur* or trauma* or lesion* or damage* or wound* or destruction* or oedema* or edema* or haematoma or hematoma or contusion* or bruis*or concus* or commotion* or pressur* or soreness or sprain* or strain* or tear*).ti,ab. (2642999) 13 or/7‐12 (2657298) 14 exp Muscle, Skeletal/ or exp Tendons/ or exp Ligaments, Articular/ or Cartilage/ (248193) 15 (Soft tissue or muscl* or muscul* or ligament* or tendon* or tendin* or cartilage or fasci* or sport* or athlet*).tw. (843242) 16 or/14‐15 (916244) 17 and/13,16 (228703) 18 and/6,17 (448) 19 Randomized controlled trial.pt. (344171) 20 Controlled clinical trial.pt. (85489) 21 randomized.ab. (261956) 22 placebo.ab. (142192) 23 Drug therapy.fs. (1589895) 24 randomly.ab. (191042) 25 trial.ab. (270177) 26 groups.ab. (1233724) 27 or/19‐26 (3077008) 28 exp Animals/ not Humans/ (3782734) 29 27 not 28 (2630973) 30 and/18,29 (98)
EMBASE (Ovid Online)
1 Thrombocyte Rich Plasma/ (3901) 2 Blood Autotransfusion/ (7695) 3 (platelet rich adj3 (plasma or therap* or fibrin)).tw. (6506) 4 (PRP or PRF).tw. (13501) 5 ((platelet adj3 (gel or concentrate)) or buffy layer).tw. (1299) 6 or/1‐5 (26378) 7 Soft Tissue Injury/ (5234) 8 Sport Injury/ (22890) 9 exp Tendon Injury/ (15120) 10 Muscle Injury/ (6922) 11 injury/ (235475) 12 Contusion/ (4904) 13 (injur* or trauma* or lesion* or damage* or wound* or destruction* or oedema* or edema* or haematoma or hematoma or contusion* or bruis*or concus* or commotion* or pressur* or soreness or sprain* or strain* or tear*).ti,ab. (3106246) 14 or/7‐13 (3196492) 15 exp Skeletal Muscle/ or exp Tendon/ or exp Ligament/ or Cartilage/ (278183) 16 (soft tissue or muscl* or muscul* or ligament* or tendon* or tendin* or cartilage or fasci* or sport* or athlet*).tw. (970606) 17 or/15‐16 (1035966) 18 and/14,17 (276922) 19 and/6,18 (641) 20 Randomized Controlled Trial/ (339220) 21 Clinical Trial/ (876390) 22 Controlled Clinical Trial/ (395071) 23 Randomization/ (61054) 24 Single Blind Procedure/ (17145) 25 Double Blind Procedure/ (113723) 26 Crossover Procedure/ (36496) 27 Placebo/ (215018) 28 Prospective Study/ (228504) 29 ((clinical or controlled or comparative or placebo or prospective* or randomi#ed) adj3 (trial or study)).tw. (678344) 30 (random* adj7 (allocat* or allot* or assign* or basis* or divid* or order*)).tw. (165154) 31 ((singl* or doubl* or trebl* or tripl*) adj7 (blind* or mask*)).tw. (152220) 32 (cross?over* or (cross adj1 over*)).tw. (65061) 33 ((allocat* or allot* or assign* or divid*) adj3 (condition* or experiment* or intervention* or treatment* or therap* or control* or group*)).tw. (209062) 34 RCT.tw. (11133) 35 or/20‐34 (1777377) 36 Case Study/ or Abstract Report/ or Letter/ (881007) 37 35 not 36 (1740801) 38 and/19,37 (140) 39 limit 38 to human (99)
LILACS (BIREME IAHx interface)
(MH:"Platelet‐Rich Plasma" OR MH:"Blood Transfusion, Autologous" OR platelet‐rich or "platelet rich" OR PRP OR PRF OR (platelet AND (gel OR concentrate)) OR "buffy layer") AND ((MH:"Soft Tissue Injuries" OR MH:"Athletic Injuries" OR MH:C26.874$ OR MH:"Sprains and Strains" OR MH:"Contusions" OR injur$ OR trauma$ OR lesion$ OR damage$ OR wound$ OR destruction$ OR oedema$ OR edema$ OR haematoma OR hematoma OR contusion$ OR bruis$ OR concus$ OR commotion$ OR pressur$ OR soreness OR sprain$ OR strain$ OR tear$) AND (MH:A02.633.567$ OR MH:A02.880$ OR MH:A02.835.583.512$ OR MH:Cartilage OR "soft tissue" OR soft‐tissue OR muscl$ OR muscul$ OR ligament$ OR tendon$ OR fasci$ OR tendin$ OR cartil$ OR sport$ OR athlet$))
Limits: Humans = 7
Data and analyses
Comparison 1. PRT versus control: all conditions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Function (all scores/instruments): short term (up to 3 months follow‐up) | 4 | 162 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.19, 0.71] |
| 1.1 Rotator cuff tear (surgical repair) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Shoulder impingement syndrome (surgery) | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.62 [‐0.02, 1.25] |
| 1.3 Elbow epicondylitis | 2 | 68 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.08, 0.89] |
| 1.4 ACL reconstruction (patellar tendon graft donor site) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 ACL reconstruction | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 Patellar tendinopathy | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 Achilles tendinopathy | 1 | 54 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.84, 0.24] |
| 1.8 Achilles tendon rupture (surgical repair) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Function (all scores/instruments): medium term (over 3 months, under 1 year follow‐up) | 5 | 151 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.56, 0.39] |
| 2.1 Rotator cuff tear (surgical repair) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Shoulder impingement syndrome (surgery) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Elbow epicondylitis | 1 | 27 | Std. Mean Difference (IV, Random, 95% CI) | 0.74 [‐0.05, 1.52] |
| 2.4 ACL reconstruction (patellar tendon graft donor site) | 1 | 25 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.76, 0.84] |
| 2.5 ACL reconstruction | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Patellar tendinopathy | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.95, 0.09] |
| 2.7 Achilles tendinopathy | 1 | 54 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.57, 0.49] |
| 2.8 Achilles tendon rupture (surgical repair) | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐1.21, 0.29] |
| 3 Functional (all scores/instruments): long term (1 year or more follow‐up) | 10 | 484 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.07, 0.57] |
| 3.1 Rotator cuff tear (surgical repair) | 6 | 324 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.24, 0.51] |
| 3.2 Shoulder impingement syndrome (surgery) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Elbow epicondylitis | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 ACL reconstruction (patellar tendon graft donor site) | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 1.53 [0.82, 2.24] |
| 3.5 ACL reconstruction | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.31, 0.94] |
| 3.6 Patellar tendinopathy | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.7 Achilles tendinopathy | 1 | 54 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.51, 0.56] |
| 3.8 Achilles tendon rupture (surgical repair) | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.69, 0.85] |
| 4 Pain (VAS: 0 to 10: worst pain): short term (up to 3 months follow‐up) | 4 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐0.95 [‐1.41, ‐0.48] |
| 4.1 Rotator cuff tears (surgical repair) | 2 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐1.64, 0.25] |
| 4.2 Shoulder impingement syndrome (surgery) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐1.4 [‐2.36, ‐0.44] |
| 4.3 Elbow epicondylitis | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐0.86 [‐1.51, ‐0.21] |
| 4.4 ACL reconstruction (patellar tendon graft donor site) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 ACL reconstruction | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Patellar tendinopathy | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Achilles tendinopathy | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.8 Achilles tendon rupture (surgical repair) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Pain (VAS: 0 to 10: worst pain): medium term (over 3 months, under 1 year follow‐up) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Rotator cuff tear (surgical repair) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Shoulder impingement syndrome (surgery) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Elbow epicondylitis | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐1.57, 0.07] |
| 5.4 ACL reconstruction (patellar tendon graft donor site) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.5 ACL reconstruction | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.6 Patellar tendinopathy | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.4 [0.32, 2.48] |
| 5.7 Achilles tendinopathy | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.8 Achilles tendon rupture (surgical repair) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Pain (VAS: 0 to 10: worst pain): long term (1 year or more follow‐up) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Rotator cuff tear (surgical repair) | 2 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐1.02, 0.44] |
| 6.2 Shoulder impingement syndrome (surgery) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Elbow epicondylitis | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 ACL reconstruction (patellar tendon graft donor site) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.5 ACL reconstruction | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.6 Patellar tendinopathy | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.7 Achilles tendinopathy | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.8 Achilles tendon rupture (surgical repair) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Adverse effects (any of PRT or placebo application) | 11 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.48, 3.59] |
Comparison 2. PRT versus control: subgrouped by tendinopathies and augmentation procedures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Function (all scores/instruments): short term (up to 3 months follow‐up) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Tendinopathies | 3 | 122 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.38, 0.67] |
| 1.2 Augmentation procedures | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.62 [‐0.02, 1.25] |
| 2 Function (all scores/instruments): medium term (over 3 months, under 1 year follow‐up) | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Tendinopathies | 3 | 98 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.83, 0.78] |
| 2.2 Augmentation procedures | 2 | 53 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.77, 0.32] |
| 3 Functional (all scores/instruments): long term (1 year or more follow‐up) | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Tendinopathies | 1 | 54 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.51, 0.56] |
| 3.2 Augmentation procedures | 9 | 430 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.08, 0.64] |
| 4 Pain (VAS: 0 to 10: worst pain): short term (up to 3 months follow‐up) | 4 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐0.95 [‐1.41, ‐0.48] |
| 4.1 Tendinopathies | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐0.86 [‐1.51, ‐0.21] |
| 4.2 Augmentation procedures | 3 | 147 | Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐1.71, ‐0.37] |
| 5 Pain (VAS: 0 to 10: worst pain): medium term (over 3 months, under 1 year follow‐up | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Tendinopathies | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Augmentation procedures | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Pain (VAS: 0 to 10: worst pain): long term (1 year or more follow‐up) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Tendinopathies | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Augmentation procedures | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Adverse effects (any of PRT pr placebo application | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Tendinopathies | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.34, 5.21] |
| 7.2 Augmentation procedures | 9 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.29, 5.71] |
Comparison 3. PRT versus control: Rotator cuff tears (surgical repair).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Function (Constant score): long term (1 year follow‐up) | 5 | 290 | Mean Difference (IV, Fixed, 95% CI) | 2.47 [0.68, 4.26] |
| 2 Function (Constant score): long term (2 year follow‐up) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Function (UCLA score): long term (1 year follow‐up) | 2 | 98 | Mean Difference (IV, Fixed, 95% CI) | 1.56 [‐0.19, 3.31] |
| 4 Function (Simple Shoulder Test (SST)): long term (1 year follow‐up) | 2 | 120 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [0.07, 0.78] |
| 5 Function (DASH score): long term (1 year follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Function (DASH score): long term (2 year follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Function (L'Insalata score): long term (1 year follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Function (ASES score): long term (1 year follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Function (all scores/instruments): long term (1 year follow‐up) | 6 | 324 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.24, 0.51] |
| 10 Pain (Analogue Scale): short term (7 day follow‐up) | 2 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐2.44, ‐0.36] |
| 11 Pain (Analogue Scale): long term (2 year follow‐up) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12 Pain (Analogue Scale): long term (1 year follow‐up) | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.20, 0.61] |
| 13 Pain (Analogue Scale): short term (30 day follow‐up) | 2 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐1.64, 0.25] |
| 14 Rate of retear: long term (1 year follow‐up) | 3 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.30, 1.01] |
| 15 Rate of retear: long term (2 year follow‐up) | 2 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.59, 1.32] |
| 16 Patient satisfaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 4. PRT versus control: Shoulder impingement syndrome (surgery).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional (self‐evaluation instability score: short term (6 week follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Functional instability after surgery: 6 week follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Pain (VAS): short term (6 week follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 5. PRT versus control: Elbow epicondylitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Function (PRTEE score): short term (3 month follow‐up) | 2 | 151 | Mean Difference (IV, Fixed, 95% CI) | ‐1.81 [‐7.73, 4.11] |
| 2 Function (PRTEE scores): medium term (6 month follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Function (Liverpool elbow score): short term (3 month follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Function (Liverpool elbow score): medium term (6 month follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Function (all scores/instruments): short term (3 months or less follow‐up) | 2 | 68 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.08, 0.89] |
| 6 Pain (VAS): short term (6 week follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Pain (VAS): medium term (6 month follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 6. PRT versus control: ACL reconstruction (patellar tendon graft donor site).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Function (Tegner scores): medium term (6 month follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Function (Lysholm score): medium term (6 month follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Function (VISA score): long term (1 year follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Pain (VAS): first post‐op day | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 7. PRT versus control: ACL reconstruction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Function (IKDC scores): long term (1 year follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Function (IKDC categories A & B: normal/nearly normal): medium and long term follow‐up | 3 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.96, 1.07] |
| 3 Function (Lysholm score): long term (1 year follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 8. PRT versus control: Patellar tendinopathy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Function (VISA scores): medium term (6 month follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Function (Tegner scores): medium term (6 month follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Function (Lysholm score): medium term (6 month follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Pain (VAS): medium term (6 month follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Quality of Life (SF‐12 score): medium term (6 month follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 9. PRT versus control: Achilles tendinopathy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Function (VISA‐A scores): short term (6 week follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Function (VISA‐A score): medium term (6 month follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Function (VISA‐A scores): long term (1 year follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Satisfied patients: medium term (6 month follow‐up) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Satisfied patients: long term (1 year follow‐up) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Return to desired sports: medium term (6 month follow‐up) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Return to desired sports: long term (1 year follow‐up) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 10. PRT versus control: Acute Achilles tendon ruptures (surgical repair).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Function (heel‐raise index): medium term (6 month follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Function (heel‐raise index): long term (1 year follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Almeida 2012.
| Methods | Randomised controlled trial: allocation concealment by computer‐generated randomisation. Participants were followed for 6 months Trial conducted: Sao Paulo University Medical School, Brazil; recruitment November 2008‐February 2010 |
|
| Participants |
Participants: 27 undergoing ACL reconstruction Included participants: patients with ACL injuries, bone maturity and aged < 45 years Excluded participants: complex ligament lesions, osteoarthritis, previous surgeries at the same joint, post operative infection, arthrofibrosis, reoperation, inadequate follow‐up and thrombocytopenia Age: PRT group mean (range): 25.8 years (18‐44) No PRT mean (range): 23.1 years (15‐34) Gender: PRT group (number of participants men:women): 10:2 No PRT(number of participants men:women): 14:1 Sports activity: not available |
|
| Interventions | All participants underwent ACL reconstruction with bone‐patellar tendon bone graft 1. PRT (number of participants = 12). Single and intraoperative intervention: 450 mL blood, resulted in 30‐50 mL PRP. Remaining blood was returned to the participant. To generate PRP gel, CaCl2 and autologous thrombin was added. PRP gel applied in patellar tendon harvest site PRT preparation: kit: Haemonetics MCS+/ 995‐E Quantification of platelet concentrates after preparation: platelet concentration 1,185,166/mm3 (SD 404.472/mm3), which represented an average increase of 7.65 (range 3.82‐26.03) times the basal levels of platelets; white blood cells 0.91/mm3 (SD 0.81/mm3) 2. No PRT (number of participants = 15): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol |
|
| Outcomes | VAS MRI (to assess the patellar tendon harvest site healing: gap area of the patellar tendon harvest site, cross‐sectional area of the patellar tendon, patellar height by the Insall‐Salvati index) Lysholm Questionnaire IKDC Kujala Questionnaire Tegner Questionnaire Isokinetic strength measurements |
|
| Other quality issues |
Sample size: the authors did not calculate the sample size Validation of PRT: available |
|
| Notes | The authors provided extra information after request (academic thesis): measures of dispersion (standard deviation) for VAS, Lysholm, IKDC, Kujala and Tegner scores The authors provided the study protocol / trial registration details, ID: NCT01111747 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Computer‐generated sequence was used |
| Allocation concealment | Unclear risk | Not reported |
| Blinding All outcomes | Unclear risk | Probably not blinded |
| Incomplete outcome data addressed All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups |
| Free of selective reporting | High risk | The study protocol is available and one primary outcome (pain) was measured only within the first 24 hours after surgery, which was preplanned in the study's protocol. In addition, the clinical follow‐up period is short for participants who underwent ACL surgery |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Antuna 2013.
| Methods | Randomised controlled pilot trial: computer‐generated randomisation performed and kept in opaque envelopes Participants were followed for 2 years. Follow‐up assessors were blinded to the outcomes Trial conducted: Hospital Universitario La Paz, Madrid, Spain; recruitment: May 2007‐June 2009 |
|
| Participants |
Participants: 28 undergoing arthroscopic repair of rotator cuff tears Included participants: adults with massive rotator cuff tears (postero‐superior rotator cuff, 2 tendons, > 5 cm) that failed conservative treatment. Diagnosis performed by clinical examinations and MRI. Participant final eligibility occurred after intraoperative visual inspection Excluded participants: evidence of anterosuperior tears that affected the subscapularis; previous surgery on the affected shoulder; major joint trauma to the shoulder; radiographic osteoarthritis; major medical condition that affects quality of life; workers' compensation claims and unwillingness to be followed for the duration of the study. Participants with haematological abnormalities were also excluded Age: mean (range): 65 years (53‐77) PRT group mean (range): 64.5 years (55‐77) No PRT mean (range): 64.9 years (53‐75) Gender (number of men:women): 22:6 PRT group: not available No PRT: not available Sports activity: not available |
|
| Interventions | All participants underwent arthroscopic repair of rotator cuff tears with absorbable anchors PRT (number of participants = 14):Single, intraoperative intervention, as an augmentation therapy: 120 mL blood resulted in 6 mL PRF applied over the repair site, under endoscopic visualisation PRT preparation: kit: Vivostat PRF (Alleroed, Denmark) Quantification of platelet concentrates after preparation: not reported No PRT (number of participants = 14): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol |
|
| Outcomes | Constant score DASH VAS MRI (with regard to integrity of repair) |
|
| Other quality issues |
Sample size: the authors did not calculate the sample size. Authors report that their sample is underpowered Validation of PRT: PRT concentration/validation was not reported |
|
| Notes | Pilot trial. The authors provided extra information upon request: measures of dispersion (standard deviation) for VAS and Constant scores. In addition, there was insufficient information about whether baseline was balanced. The authors have provided the study protocol/trial registration details, ID: NCT01612845 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Used computer‐generated sequence |
| Allocation concealment | Low risk | Used sequentially‐numbered, opaque and sealed envelopes. The envelope was only opened following intraoperative inspection of the shoulder |
| Blinding All outcomes | Low risk | The surgeon was not blinded to the treatment allocation, but the research assistant performing follow‐up evaluations and the radiologist were blinded |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Low risk | The study protocol was available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Castricini 2011.
| Methods | Randomised controlled trial: participants followed for at least 16 months. It is not clear if clinical assessors and participants were not blinded to the procedure. MRI assessors were blinded to the procedure Trial conducted: Department of Orthopaedic and Trauma Surgery, Ospedale Civile, Jesi, Italy; recruitment: from January 2007‐April 2008 |
|
| Participants |
Participants: 88 undergoing arthroscopic repair of rotator cuff tears Included participants: participants with repairable small or medium rotator cuff tears (supraspinatus), as assessed in the operative procedure Excluded participants: presence of inflammatory joint disease; irreparable or partial lesions; acromioclavicular arthritis; rotator cuff arthropathy; subscapularis tendon abnormalities; workers' compensation claims; prior surgery on the affected shoulder Age: PRT group mean (range): 55.2 years (37‐69) No PRT mean (range): 55.5 years (41‐72) Gender: PRT group(number of men:women):23:22 No PRT (number of men:women): 17:26 Sports activity: not available |
|
| Interventions | All patients underwent arthroscopic repair with double row fixation. PRT was applied as an augmentation procedure PRT (number of participants = 43): single platelet‐rich fibrin matrix ‐ 9 mL blood centrifuged for 6 minutes PRP separated and CaCl2 was added for a 2‐phase centrifugation PRT preparation: kit: Cascade Autologous Platelet System Quantification of platelet concentrates after preparation: not assessed No PRT (number of participants = 45): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol |
|
| Outcomes | Constant Score MRI (integrity of the rotator cuff repair, retear) |
|
| Other quality issues |
Sample size: adequate power for Constant Validation of PRT: PRT concentration/validation was not reported |
|
| Notes | The authors have provided the study protocol/trial registration details, ID: ISRCTN49643328 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | The authors used a random numbers table to allocate study participants |
| Allocation concealment | Low risk | Used sequentially‐numbered, opaque and sealed envelopes |
| Blinding All outcomes | Unclear risk | Clinical assessors and participants were probably not blinded to the procedure, but MRI assessors were blinded to the procedure |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Low risk | The study protocol was available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Cervellin 2012.
| Methods | Randomised controlled trial: 2 blocks of 20 participants that were randomly selected by an external researcher. It is not clear how the allocation blocks were created. Participants followed for 12 months. Participants and radiologists were blinded to the intervention Trial conducted: Department of Sports Traumatology and Arthroscopic Surgery of the Galeazzi Orthopaedic Institute of Milan: recruitment: 2008‐2009 |
|
| Participants |
Participants: 40 undergoing arthroscopic ACL reconstruction Included participants: adults requiring ACL reconstruction Excluded participants: associated ligament damage; associated immune‐rheumatologic pathologies; chondropathies (Outerbrigde > III); pre‐existing anterior knee pain; femoropatellar pathologies and previous surgery on the same knee Age: PRT group mean (range): 22.9 years (18‐29) No PRT mean (range): 22.7 years (19‐27) Gender: PRT group: not available No PRT: not available Sports activity: included patients were in "high level" of sports activity |
|
| Interventions | All patients underwent ACL reconstruction with bone‐patellar tendon graft PRT (number of participants = 20): single, intra operative intervention, 54 mL blood plus 6 mL citrate anticoagulant, 15 minutes centrifugation. Buffy coat containing PRP was centrifuged with participant's thrombin (from another venous puncture) and applied after jellified. PRP gel was applied in the patellar and tendon bone plug harvest site and fixed with peritenon suture PRT preparation: kit: Gravitional Platelet Separation (GPS II). Addiction of CaCl2 and autologous thrombin Quantification of platelet concentrates after preparation: not assessed No PRT (number of participants = 20): no platelet‐rich therapy controls Co‐Interventions: same rehabilitation protocol |
|
| Outcomes | VISA VAS MRI (assessment of new bone formation in the graft site; gap filling > 70% considered as satisfactory) |
|
| Other quality issues |
Sample size: the authors did not calculate the sample size Validation of PRT: PRT concentration or its validation was not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Not reported |
| Allocation concealment | Unclear risk | Not reported |
| Blinding All outcomes | Unclear risk | Participants and radiologists were blinded to the intervention |
| Incomplete outcome data addressed All outcomes | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | The study protocol was not available. Relevant outcomes were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Creaney 2011.
| Methods | Randomised controlled trial: randomisation held in sealed envelopes. Not clear how the allocation sequence was generated. Participants followed for 6 months. Participants were blinded to the procedure. Assessors were independent Trial conducted: no details available; recruitment: no details available |
|
| Participants |
Participants: 150 with elbow tendinopathy Included participants: adults with elbow tendinopathy (< 6 months' duration) that had failed to respond to physical therapy exercises Exluded participants: previous injection therapies (e.g. Corticoid) Age: PRT group mean (range):53 years (not available) No PRT mean (range): 48 years (not available) Gender: PRT group (number of men:women): 46:34 No PRT (number of men:women): 45:25 Sports activity: not available |
|
| Interventions | All participants underwent 2 injections (at 0 and 1 month) with previous local anaesthesia (2 mL bupivacaine). Injections performed by ultrasound guidance by an musculoskeletal radiologist PRT (number of participants = 80): 8.5 mL blood sample, tube with citrate anticoagulant PRT preparation: no kit. Preparation: 15 minutes of centrifugation, 1.5 mL platelet‐rich plasma siphoned from buffy coat layer Quantification of platelet concentrates after preparation: 10 random samples of blood demonstrated a 2.8‐fold (CI 2.3‐3.5) elevation from baseline for the platelet concentration No PRT (number of participants = 70): autologous blood injections ‐ details not reported Co‐interventions: same rehabilitation protocol for both groups |
|
| Outcomes | PRTEE | |
| Other quality issues |
Sample size: powered for PRTEE Validation of PRT: quantification reported |
|
| Notes | Participants who did not improve with the proposed intervention (failure) had the option to undergo surgical treatment. This study was included using an inclusion criterion that differed from the published protocol: autologous whole blood was considered as a control intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Not reported |
| Allocation concealment | Low risk | Used sequentially‐numbered, opaque and sealed envelopes |
| Blinding All outcomes | Low risk | Participants and outcomes assessors were blinded |
| Incomplete outcome data addressed All outcomes | High risk | Missing outcome data were balanced in numbers across intervention groups. In addition, intention‐to‐treat analyses were performed. However, the data available for PTREE did not include 7 versus 12 participants who had subsequent surgery |
| Free of selective reporting | High risk | The study protocol is not available and the authors evaluated only 1 primary outcome (PRTEE). In addition, the clinical follow‐up period was short for participants who underwent elbow tendinopathy treatment |
| Free of other bias | Low risk | Participants were permitted to receive other treatments. However, authors performed analysis as intention‐to‐treat |
De Vos 2010.
| Methods | Randomised controlled trial: block randomisation (12 participants per block). Randomisation was made by sealed blank envelopes. Participants were pre‐stratified according to whether pre‐injury activity levels were high‐ or low‐level, based on a score that assesses ankle‐related activity. Participants were followed for 24 months (researcher was not blinded) and 52 months (researcher was blinded). Researchers divided the study protocol into 2 reports Trial conducted: The Hague Medical Center Antoniushove, Leidschendam, the Netherlands; recruitment: 28 August 2008‐29 January 2009 |
|
| Participants |
Participants: 54 with chronic Achilles tendinopathy, participants were contacted by email or telephone for the first consultation Included participants: presence of midportion achilles tendinopathy (2‐7 cm proximal to the insertion on the calcaneous), and aged between 18‐70 years. Diagnosis based on clinical findings (painful and thickened tendon in relation to activity and on palpation) Excluded participants: clinical suspicion of other musculoskeletal (insertional disorders and tendon rupture) injuries; inflammatory internal disorders or use of specific medications that can cause tendinopathy (fluoroquinolones); previous performance of a complete heavy load eccentric exercise program or inability to perform it or previous injection with PRP Age: PRT group mean (SD): 49 years (8.1) No PRT mean (SD): 50 years (9.4) Gender: PRT group (number of men:women): 13:14 No PRT (number of men:women): 13:14 Sports activity (active, PRT:no PRT): 22:24 |
|
| Interventions | All participants received a single injection. Previous local anaesthesia (2 mL bupivacaine (Marcaine)). All injections performed by ultrasonographic guidance by an experienced sports physician at 3 different locations proximal to the Achilles tendon insertion PRT (number of participants = 27): blood sample (54 mL) resulted in 4 mL PRP. Additional 6 mL citrate was added Preparation: 15 minutes centrifugation with the addition of 0.3 mL sodium bicarbonate (bicarbonate was added to match tissue pH. 4 mL was collected for infiltration) PRT preparation (number of participants = 27): kit: Recover Platelet separation kit (Gravitational Platelet Separation ‐ GPS III). No addition of CaCl2 or thrombin Quantification of platelet concentrates after preparation: no No PRT: saline injection Co‐interventions: same rehabilitation protocol both groups. Paracetamol (acetaminophen) was used as rescue medication in both groups |
|
| Outcomes | VISA‐A score Participant satisfaction (good or excellent reported satisfaction was considered as satisfied) Return to sports activity (cut‐off: return to desired sport on a pre‐injury level) Sonographic evaluation (tendon structure and neovascularisation) |
|
| Other quality issues |
Sample size: powered for VISA‐A Validation of PRT: PRT concentration/validation was not reported |
|
| Notes | JAMA 2010 ‐ Premilinary communication; AJSM 2011; BJSM 2011 ‐ Final reports The authors provided the study protocol/trial registration details, ID: NCT00761423 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | A block randomisation was performed with a block size of 12 participants |
| Allocation concealment | Low risk | Used sequentially‐numbered, opaque and sealed envelopes |
| Blinding All outcomes | Low risk | Personnel, participants and outcomes assessors were blinded |
| Incomplete outcome data addressed All outcomes | Low risk | No participants were lost to follow‐up |
| Free of selective reporting | Low risk | The study protocol is available and all expected outcomes were assessed |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Everts 2008.
| Methods | Randomised controlled trial: participants were allocated after randomisation derived from sealed envelopes. It is not clear how the randomisation sequence was generated. Participants and assessors were blinded to the intervention. Trial conducted: no details available; recruitment: no details available |
|
| Participants |
Participants: 40 undergoing open surgery for shoulder impingement syndrome Included participants: impingement syndrome (stage II), diagnosed at least 6‐months preoperatively. Participants with typical anterior shoulder pain during elevation, loss of active and passive shoulder motion and positive response to 3 subacromial infiltrations (local anaesthetics and corticoids) performed in a 6‐month period Excluded participants: presence of rotator cuff injury; frozen shoulder; acromioclavicular joint disorder; glenohumeral joint degenerative arthritis; shoulder instability; shoulder and elbow disorders; hand disorders; post‐traumatic disorder; participants with diseases that would affect post‐operative wound healing or who were treated for acute shoulder dysfunction Age: PRT group mean (SD): 52 years (11) No PRT mean (SD): 50 years (14) Gender: PRT group (number of men:women): 7:8 No PRT (number of men:women): 5:10 Sports activity: not available |
|
| Interventions | All participants underwent open subacromial decompression PRT (number of participants = 20): single intraoperative platelet‐leucocyte gel application. From 52 mL blood, 12 mL used to prepare intervention. Citrate dextrose and autologous thrombin were used for gel formation PRT preparation: kit: Magellan Autologous Platelet Separator System (MAPS) Quantification of platelet concentrates after preparation: 1183 SD 396/109/L, 5.7‐fold increase from baseline No PRT (number of participants = 20): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol both groups |
|
| Outcomes | ASES (American Shoulder and Elbow Surgeons scoring system) VAS ADL Shoulder range of motion Use of pain medication |
|
| Other quality issues |
Sample size: the authors did not calculate the sample size Validation of PRT: quantification reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Drew random numbers |
| Allocation concealment | Low risk | Used sequentially‐numbered, opaque and sealed envelopes |
| Blinding All outcomes | Low risk | Participants and assessors were blinded to the intervention |
| Incomplete outcome data addressed All outcomes | Low risk | No participants were lost to follow‐up |
| Free of selective reporting | Unclear risk | The study protocol was not available. It appears that the study’s prespecified primary and secondary outcomes that are of interest in the review have been reported in the prespecified way |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Gumina 2012.
| Methods | Randomised controlled trial: allocation concealment derived from randomisation (sealed envelopes). Participants were followed for a mean of 13 months Trial conducted: Orthopaedic Clinic, University of Rome 'Sapienza', Rome, Italy; recruitment: from June‐December 2009 |
|
| Participants |
Participants: 80 undergoing arthroscopic repair of rotator cuff tears Included participants: reparable large full‐thickness posterosuperior rotator cuff tears Excluded participants: partial‐thickness tear; small or massive full‐thickness tear; traumatic tear; biceps instability; labral pathology amenable to surgical treatment; os acromiale; degenerative arthritis of the glenohumeral joint; autoimmune or rheumatologic disease; previous surgery in the same shoulder and Workers' compensation claims Age: PRT group mean (SD): 60 years (4.4) No PRT mean (SD): 63 years (5.9) Gender: PRT group (number of men:women): 20:19 No PRT (number of men:women): 21:16 Sports activity: not available |
|
| Interventions | All participants underwent arthroscopic rotator cuff repair PRT (number of participants = 40): single, intraoperative intervention (platelet‐leukocyte membrane), 10 mL blood was centrifuged for 10 minutes at 120 x g. The product was added to gluconate and batroxobin, for 20‐30 minutes (product is a platelet‐leukocyte membrane) PRT preparation: kit: RegenKit, Regen Lab, Le Mont‐Sur‐Lausanne, Switzerland) Quantification of platelet concentrates after preparation: white blood cells (7 x 103/mm3), platelet (> 400 x 103/mm3), 1.7 times greater than the normal level in whole blood. No PRT (number of participants = 40): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol for both groups |
|
| Outcomes | Constant scores Simple Shoulder Test MRI (repair integrity): Sugaya classification |
|
| Other quality issues |
Sample size: a priori power calculations not available Validation of PRT: quantification reported |
|
| Notes | In the intervention group, 1 membrane was used for each repair anchor 4 follow‐up losses (1 in the PRT group), reasons not known The authors provided the study protocol/trial registration details, ID: ISRCTN93082180 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Randomisation reporting was unclear |
| Allocation concealment | High risk | The envelope was opened 3 days prior to surgery rather than during surgery |
| Blinding All outcomes | Unclear risk | The study was probably not blinded |
| Incomplete outcome data addressed All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups |
| Free of selective reporting | Low risk | The study protocol was available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Krogh 2013.
| Methods | Randomised controlled trial: endpoint assessors and participants were blinded to the procedure. Allocation sequence controlled by randomisation performed as blocks of 6 participants. Study's outcomes were measured at 3 months Trial conducted: Diagnostic Centre, Region Hospital Silkeborg, Silkeborg, Denmark; recruitment: from January 2009‐July 2010 |
|
| Participants |
Participants: 40 with elbow lateral epicondylitis Included participants: participants with symptoms for more than 3 months Excluded participants: participants < 18 years old; treated with glucocorticoid injection in previous 3 months; previous tennis elbow surgery; inflammatory diseases; neck pain on the ipsilateral side and chronic pain syndromes Lateral epicondylitis defined as pain on the lateral side of the elbow for at least 3 months, pain at the lateral epicondyle on direct palpation and during resisted dorsiflexion of the wrist. Ultrasonography was also performed at the origin of the extensor tendon; required a definite sign of tendinopathy with colour Doppler flow of at least grade 2 at baseline Age: PRT group mean (SD): 47.6 years (7.1) No PRT mean (SD): 44.7 years (7.9) Gender: PRT group (number of men:women): 9:11 No PRT (number of men:women): 9:11 Sports activity: not available |
|
| Interventions | All participants underwent platelet‐rich plasma or glucocorticoid or saline ultrasound‐guided single injection. A blood sample was collected from all participants, and all interventions were prepared out of the reach of the participant PRT (number of participants = 20): PRP: 3.0‐3.5 mL PRP derived from 27 mL blood. Blood was centrifuged at 3200 rpm for 15 minutes, before the addition of 3 mL citrate. Bicarbonate was added to the PRP to achieve physiological pH. PRT preparation: Recover GPS II system (Biomet Biologics Inc, Warsaw, Indiana) Quantification of platelet concentrates after preparation: 8‐fold (compared with whole blood) No PRT (number of participants = 20): saline (3 mL of 0.9%) Co‐interventions: same rehabilitation protocol for both groups |
|
| Outcomes | Pain section of the PRTEE questionnaire Functional disability of the PRTEE questionnaire Safety (adverse events) Injection‐related pain Ultrasound assessment: colour doppler changes and tendon thickness |
|
| Other quality issues |
Sample size: the authors calculated the sample size based on the PRTEE pain domain at 12 months (we expect that this based on another population) Validation of PRT: quantification reported |
|
| Notes | We excluded all the analyses relating to glucocorticoid intervention (not considered as placebo) The authors provided the study protocol/trial registration details, ID: NCT 01109446 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Used permuted blocks of 6 participants |
| Allocation concealment | Low risk | Used sequentially‐numbered, opaque and sealed envelopes |
| Blinding All outcomes | Low risk | The participant and outcome assessors were blinded to the treatment, but the treating physician was not |
| Incomplete outcome data addressed All outcomes | High risk | Only 13 out of 40 participants in the 2 groups completed 12 months' follow‐up |
| Free of selective reporting | High risk | The study protocol is not available and the clinical follow‐up period was short for participants who underwent elbow tendinopathy treatment |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
NCT01029574.
| Methods | Randomised controlled trial: randomisation performed by coin toss and concealment was kept in sealed, opaque envelopes. participants and outcome assessors (clinical and imaging) were blinded to the procedure. Participants were followed for 12 months Trial conducted: Sao Paulo University Medical School, Brazil; recruited: September 2008‐December 2013 |
|
| Participants |
Participants: 54 undergoing arthroscopic repair of rotator cuff tears Inclusion criteria: skeletally‐mature participants with no previous affected shoulder surgery. Complete supraspinatus tear, assessed by MRI, with small tendon retraction (< 3 cm). Pain and disability for > 3 months, not improving by standard non operative care. Absence of: other rotator cuff tears, anatomical abnormalities such as cyst that could potentially jeopardise the repair; rotator cuff fatty degeneration (Grades 2, 3 and 4), osteoarthritis (glenohumeral and acromioclavicular), or other conditions that could influence the results (mental and rheumatic disorders, pregnancy, infection) Exclusion criteria: unrepairable lesion; necessity to convert to open surgery; intraoperative identification of previously unrecognised injuries Age: PRT group mean (SD): 54.1 years (6.6) No PRT mean (SD): 55.3 years (8.3) Gender: PRT group (number of men:women): 8:19 No PRT (number of men:women): 9:18 Sports activity: not available |
|
| Interventions | All participants had arthroscopic supraspinatus repair with anchors PRP (number of participants = 27): single intraoperative application. 400 mL whole blood provided 30 mL PRP. After PRP separation, blood was returned by the apheresis device. Sodium citrate and autologous thrombin were added Quantification of platelet concentrates after preparation: 8‐fold (compared with whole blood) PRT preparation: kit: Haemonetics MCS+ 9000® and 994‐CFE (Haemonetics Corporation MA, USA) No PRP (number of participants = 27): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol both groups |
|
| Outcomes | Constant score UCLA VAS Frequency of rerupture (assessed by MRI) |
|
| Other quality issues |
Sample size: the authors set an a priori calculation of sample size for the primary endpoint Validation of PRT: the authors quantified the concentration of platelet concentrate |
|
| Notes | Sample size was calculated for Constant scores as primary endpoint. The authors provided extra information after request (academic thesis): measures of dispersion (standard deviation) for VAS, Constant and UCLA scores. The authors provided the study protocol/trial registration details, ID: NCT01029574 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Sequence generated by internet‐based coin toss |
| Allocation concealment | Low risk | Used sequentially‐numbered, opaque and sealed envelopes |
| Blinding All outcomes | Low risk | Assessors and participants were blinded to the procedure |
| Incomplete outcome data addressed All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups |
| Free of selective reporting | High risk | Most of the outcomes were reported, but with discrepancies among primary and secondary outcomes |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Orrego 2008.
| Methods | Quasi‐randomised controlled trial: participants were allocated to an intervention consecutively, following a predefined sequence. Outcomes were measured at 3 and 6 months Trial conducted: Departamento de Traumatología, Hospital Militar de Santiago,Chile; recruitment:from January 2005‐December 2006 |
|
| Participants |
Participants: 53 undergoing ACL reconstruction Inclusion criteria: mature skeleton, clinical instability, MRI showing total rupture of the ACL and voluntary acceptance of participation in the study Exclusion criteria: capsulo‐ligamentous injuries Age mean (range): 30 years (15‐57) PRT group mean (range): not available No PRT mean (range): not available Gender (number of men:women): 99:17 PRT group (number of men:women): not available No PRT (number of men:women:): not available Sports activity: not available |
|
| Interventions | 4‐arm intervention: 1. Standard semitendinosus‐gracilis graft ACL reconstruction 2. Standard semitendinosus‐gracilis graft ACL reconstruction augmentation with platelet concentrate 3. Standard semitendinosus‐gracilis graft ACL reconstruction with bone plug association 4. Standard semitendinosus‐gracilis graft ACL reconstruction and platelet concentrate and bone plug association PRT (number of participants = 26): single PRP application, 67 mL blood produced 10 mL PRP. Blood centrifuged for 10 minutes and clothing derived from participants' thrombin (obtained after a 10‐minute centrifugation). CaCl2 was added to the PRP product. A 2‐step application was performed: the graft was immersed in the PRP clot and PRP was injected in the bone femoral tunnel PRT preparation: kit: Biomet GPS II ( Warsaw, Indiana) Quantification of platelet concentrates after preparation: not reported No PRT (number of participants = 27): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol |
|
| Outcomes | MRI assessments: maturation of the graft (graft signal intensity, osteo ligamentous interface, tunnel widening) IKDC |
|
| Other quality issues |
Sample size: the authors calculated the sample size; however, it is not clear if for the main endpoint Validation of PRT: PRT concentration or its validation was not reported |
|
| Notes | For this review's purposes, data from interventions numbered as 3 and 4 were excluded (not considered as placebo) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | High risk | Quasi‐randomised clinical trial |
| Allocation concealment | High risk | Quasi‐randomised clinical trial |
| Blinding All outcomes | High risk | Only the MRI assessor was blinded |
| Incomplete outcome data addressed All outcomes | Unclear risk | Missing outcome data were probably balanced in numbers across intervention groups |
| Free of selective reporting | High risk | The study protocol is not available and the clinical follow‐up period was short for participants who underwent to ACL surgery |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Randelli 2011.
| Methods | Randomised controlled trial: participants were randomised utilising block procedure. Participants had final follow‐up at 24 months Trial conducted: Department of Scienze Medico Chirurgiche, University of Milano, IRCCS Policlinico San Donato, Milano, Italy; recruitment: from April 2007‐January 2008 |
|
| Participants |
Participants: 53 undergoing arthroscopic repair of rotator cuff tears Inclusion criteria: a complete rotator cuff tear confirmed intraoperatively; agreed to wear a dedicated brace for 4 weeks postoperatively; had a preoperative platelet count > 150,000; minimum preoperative haemoglobin of 11.0 g/dL; no infectious diseases or diseases that may have limited follow‐up; BMI < 33 Exclusion criteria: previous rotator cuff repair; active infection; osteomyelitis or sepsis, or distant infections; osteomalacia or other metabolic bone disorders; unco‐operative or had disorders that made them incapable of following directions, or who were unwilling to return for follow‐up examinations; vascular insufficiency, muscular atrophy, or neuromuscular diseases of the affected arm; cigarette smokers; had received steroid injection(s) in the affected shoulder Age: PRT group mean (range): 61.6 years (8.3) No PRT mean (range): 59.5 years (10.7) Gender: PRT group (number of men:women): 8:19 No PRT (number of men:women): 13:13 Sports activity: not available |
|
| Interventions | Participants were submitted to arthroscopic rotator cuff repair (single row repair, absorbable anchors) by a single surgeon. Acromioplasty was performed in all cases PRT (number of participants = 26): single, intraoperative injection. 54 mL blood mixed with 6 mL citrate as an anticoagulant. The product was centrifuged for 15 minutes at 3200 rpm. PRP was separated and centrifuged (2 minutes) to increase fibrinogen concentration and mixed with PRP. A final 6 mL PRP was applied through the arthroscopic portals PRT preparation: kit: GPS II, Biomet Biologics (Warsaw, IN) Quantification of platelet concentrates after preparation: not reported No PRT (number of participants = 27): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol |
|
| Outcomes | Constant score SST UCLA score VAS Strength in external rotation Rate of retear |
|
| Other quality issues |
Sample size: the authors calculated the sample size Validation of PRT: the exact composition of the PRP was unknown |
|
| Notes | Authors had 8 follow‐up losses (4 in each group). Pain was measured in short intervals in the early postoperative period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | A block randomised procedure was used to generate a randomisation list |
| Allocation concealment | Low risk | Used sequentially‐numbered, opaque and sealed envelopes |
| Blinding All outcomes | Low risk | The participants and outcome assessors were blinded to the treatment |
| Incomplete outcome data addressed All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups |
| Free of selective reporting | Unclear risk | The study protocol is not available but all expected outcomes were assessed |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Rodeo 2012.
| Methods | Randomised controlled trial Trial conducted: Sports Medicine and Shoulder Service, The Hospital for Special Surgery, New York, New York, USA; recruitment: no details available Participants were followed for 24 months |
|
| Participants |
Participants: 80 undergoing arthroscopic repair of rotator cuff tears Inclusion criteria: participants ≥ 40 years of age for whom non operative treatment had failed Exclusion criteria: people undergoing revision, mini‐open, or open procedures; people with concomitant labral tears. Age: PRT group mean (range): 59.5 years (10.7) No PRT mean (range): 59.5 years (10.7) Gender: PRT group (number of men:women): 23:17 No PRT (number of men:women): 21:18 Sports activity: not available |
|
| Interventions | All participants underwent arthroscopic rotator cuff repair with bone anchors PRT (number of participants = 40): single intraoperative application, PRFM, 9 mL blood produced a PRFM product. Fibrin matrix was produced after a second centrifugation step, by the addition of CaCl2 PRT preparation: kit: Cascade Autologous Platelet System, Musculoskeletal Transplant Foundation, Edision, New Jersey, USA) Quantification of platelet concentrates after preparation: not stated No PRT (number of participants = 40): no platelet‐rich therapy controls Quantification of platelet concentrates after preparation: not reported Co‐interventions: same rehabilitation protocol |
|
| Outcomes | Ultrasound assessment (tendon healing) ASES Score L' Insalata score Shoulder strength |
|
| Other quality issues |
Sample size: author stopped trial as it had detected no benefit (target: 65 participants per group) Validation of PRT: the exact composition of the PRP was unknown |
|
| Notes | Participants lost to follow‐up: n = 5 (PRT), n = 7 (no PRT) The authors provided the study protocol/trial registration details, ID: NCT01029574 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Not reported |
| Allocation concealment | Low risk | Used sequentially‐numbered, opaque and sealed envelopes |
| Blinding All outcomes | Low risk | The participants and outcome assessors were blinded to the treatment |
| Incomplete outcome data addressed All outcomes | High risk | Reasons for missing outcome data were not reported and there was imbalance in numbers across intervention groups |
| Free of selective reporting | Low risk | The study protocol was available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review were reported in the pre‐specified way |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
Schepull 2010.
| Methods | Randomised controlled trial: randomisation occurred as blocks of 6 participants, assignment kept in sealed envelopes. Allocation concealment was kept until the operative time. Participants and outcome assessors were blind to the intervention. Participants were followed for 1 year Trial conducted: Linköping University, Linköping, Sweden; recruitment: September 2007‐April 2008 |
|
| Participants |
Participants: 30 undergoing open repair of acute achilles tendon rupture Inclusion criteria: participants aged 18‐60 years, with an acute (< 3 days) rupture of Achilles tendon Exclusion criteria: diabetes mellitus; a history of cancer or lung or heart diseases; or diseases that could compromise the locomotor system Age: PRT group mean (range): 39.8 years (6.2) No PRT mean (range): 39.4 years (8.3) Gender: PRT group (number of men:women): 13:3 No PRT (number of men:women): 11:3 Sports activity: All participants were recreational athletes injured during sports or sports‐related activities |
|
| Interventions | All participants underwent open repair of acute Achilles tendon injuries, with implantation of tantalum beads to aid in image analyses PRT (number of participants = 16): 450 mL blood derived a mean volume of 21 mL PRP. PRP was prepared and stored, with constant rotation, up to 20 hours before use. Platelet viability was assessed, and found to have been maintained in all cases PRT preparation: no dedicated kit. Authors stated that they utilised a credited procedure (Europe 2007) Quantification of platelet concentrates after preparation: 3673 (SD 1051) x 109 platelets per mL No PRT (number of participants = 14): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol |
|
| Outcomes | Tendon strain per load: distance between the tantalum beads (roentgen stereophotogrammetric analysis (RSA)) while participants resisted different dorsal flexion moment over the ankle joint Estimate of elasticity modulus (using callus dimensions from computed tomography) Functional outcome: heel‐raise index and Achilles tendon Total Rupture Score |
|
| Other quality issues |
Sample size: the authors did not calculate the sample size Validation of PRT: the exact composition of the PRP is unknown |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Used permuted blocks of 6 participants |
| Allocation concealment | Low risk | Used sequentially‐numbered, opaque and sealed envelopes |
| Blinding All outcomes | Low risk | The participants and outcome assessors were blinded to the treatment |
| Incomplete outcome data addressed All outcomes | High risk | Missing outcome data were not balanced in numbers across intervention groups; more participants in the PRP group were lost to follow‐up (4/16 (25%) PRP versus 0/14 (0%) control) |
| Free of selective reporting | Unclear risk | The study protocol is not available, but all expected outcomes were assessed |
| Free of other bias | Low risk | The study appears to be free of other bias |
Thanasas 2011.
| Methods | Randomised controlled trial. Blocks were randomised from a sequence derived from an Internet‐based program Participants were followed for 6 months. Only the outcome assessors were blinded to the procedure Trial conducted: Department of Orthopaedic Surgery, Red Cross Hospital, Athens, Greece; recruitment: no details available |
|
| Participants |
Participants: 29 with elbow lateral epicondylitis Inclusion criteria: clinically diagnosed lateral epicondylitis (based on symptoms, site of tenderness, and pain elicited with resisted active extension of the wrist in pronation and elbow extension); no history of trauma; duration ≥ 3 months; no previous local injection treatment of any kind; no medical history of rheumatic disorder; and no signs of posterior interosseous nerve entrapment Exclusion criteria: recent onset of symptoms (< 3 months); history of trauma; medical comorbidities such as rheumatoid arthritis; previous local injections (e.g. cortisone); and suspicion of nerve involvement Age: PRT group mean (range): 35.9 years (34‐55) No PRT mean (range): 36.6 years (29‐52) Gender: PRT group (number of men:women): 5:10 No PRT (number of men:women): 3:11 Sports activity: not available |
|
| Interventions | All participants received 1 ultrasound‐guided injection for lateral epicondylitis at the origin of wrist extensors with a peppering technique (single skin insertion, deep peripheral multiple sites of injection) PRT (number of participants = 14): 55 mL blood produced 3‐6 mL PRP. Used 3 mL anticoagulant, but no activator, since authors stated that in vivo contact with collagen is responsible for activation Quantification of platelet concentrates after preparation: 235,000/mL to 1,292,500/mL (5.5 times, on average).An average ratio for white blood cells was reported as: 111/1 (platelets/leukocytes) PRT preparation: kit: GPS III, Biomet Biologics (Warsaw, IN) No PRT (number of participants = 15): 3 mL autologous peripheral whole blood, deep at the origin of wrist extensors with a peppering technique (single skin insertion, deep peripheral multiple sites of injection) under aseptic technique with the assistance of ultrasound guidance Co‐interventions: same rehabilitation protocol. Painkiller and ice therapy were prescribed in both groups |
|
| Outcomes | Pain (VAS) Liverpool elbow score |
|
| Other quality issues |
Sample size: the authors calculated the sample size. However they did not provide the estimate of the effect that they intended to identify in group comparisons Validation of PRT: the exact composition of the PRP is unknown |
|
| Notes | This study was included using an inclusion criterion that differed from the published protocol: autologous whole blood was considered as a control intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | The sequence generation was performed by a computer random number generator |
| Allocation concealment | Unclear risk | Not reported |
| Blinding All outcomes | High risk | Only outcomes assessors were blinded |
| Incomplete outcome data addressed All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups |
| Free of selective reporting | High risk | The study protocol is not available and the clinical follow‐up period was short for participants who underwent to elbow tendinopathy treatment |
| Free of other bias | Low risk | The study appears to be free of other bias |
Vadalà 2013.
| Methods | Randomised controlled trial. Sequence generation and allocation methodology were not reported. Participants were followed for a mean of 14.7 months Trial conducted: no details available; recruitment: no details available |
|
| Participants |
Participants: 40 undergoing ACL reconstruction Inclusion criteria: participants with chronic instability (> 30 days of trauma) Exclusion criteria: age > 50 years; concomitant medial or lateral collateral ligament injuries; degenerative joint disease or chondral damage (MRI or radiographic examinations) Age mean (range): 34.5 years (18‐48) PRT group mean (range): not available No PRT mean (range): not available Gender: all were men PRT group (number of men:women): 20:0 No PRT (number of men:women): 20:0 Sports activity: not available |
|
| Interventions | All patients underwent arthroscopic ACL reconstruction with hamstring graft PRT (number of participants = 20): single intraoperative application. PRP was applied in the femoral and tibial tunnel. 10 mL blood was centrifuged, thrombin and calcium gluconate added few minutes before its application in order to obtain a thick and adhesive gel PRT preparation: kit: PRP Fast Biotech kit (MyCells PPT‐Platelet Preparation Tube) Quantification of platelet concentrates after preparation: not reported No PRT (number of participants = 20): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol |
|
| Outcomes | Tunnel enlargement (assessed by CT) Tegner activity score Lysholm score IKDC score |
|
| Other quality issues |
Sample size: the authors did not calculate the sample size Validation of PRT: PRP preparation methodology was not clear and there are some inconsistencies between sections of the manuscript |
|
| Notes | The authors described different quantities for PRP preparation and application | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Not reported |
| Allocation concealment | Unclear risk | Not reported |
| Blinding All outcomes | Low risk | Outcome assessors were blinded |
| Incomplete outcome data addressed All outcomes | Low risk | No participants were lost to follow‐up |
| Free of selective reporting | High risk | The study protocol is not available and the authors did not report outcomes at each time point |
| Free of other bias | Low risk | The study appears to be free of other bias |
Valenti Nín 2009.
| Methods | Randomised controlled trial: participants were randomised by a computer‐generated sequence. MRI assessors were blinded to the intervention. Participants' last assessment performed at 12 months. Trial conducted: Clínica Universitaria of Navarra, Pamplona, Spain; recruitment: no details available | |
| Participants |
Participants: 100 undergoing ACL reconstruction Inclusion criteria: ACL disruption stabilised by an orthopaedic surgeon; positive Lachman e pivot‐shift test and MRI; no prior knee surgery and normal contra‐lateral knee Exclusion criteria: previous knee pathology or symptoms before ACL rupture Age: PRT group mean (range): 26.1 years (14‐57) No PRT mean (range): 26.6 years (15‐59) Gender: PRT group (number of men:women): 40:10 No PRT (number of men:women): 12:38 Sports activity: not available |
|
| Interventions | ACL reconstruction with patellar tendon allograft fixed by cross‐pin fixation (proximal) and interference screws (distal) PRT (number of participants = 50): 40 mL blood provided 4 mL platelet‐enriched gel Quantification of platelet concentrates after preparation: 3‐5 fold increase in platelet concentration over baseline PRT preparation: no dedicated kit. Authors stated that they used a modified reported method (Sonnleitner 2000). No PRT (number of participants = 50): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol |
|
| Outcomes | Pain (VAS) Anterior Laxity (KT‐1000) IKDC Protein‐C MRI (graft status, tunnel placement, graft position) Radiographs (graft healing) |
|
| Other quality issues |
Sample size: the authors did not calculate the sample size Validation of PRT: not available |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | The sequence generation was performed by a computer random number generator |
| Allocation concealment | Unclear risk | Not reported |
| Blinding All outcomes | High risk | Only the MRI assessors were blinded |
| Incomplete outcome data addressed All outcomes | Low risk | No participants were lost to follow‐up |
| Free of selective reporting | Unclear risk | The study protocol is not available but all expected outcomes were assessed. Complications were not assessed |
| Free of other bias | Low risk | The study appears to be free of other bias |
Vogrin 2010.
| Methods | Quasi‐randomised controlled trial: sequence generated by the presence of odd or even numbers. Participants followed for 6 months after the procedure Trial conducted: Department of Orthopedic Surgery, University Hospital Maribor, Maribor, Slovenia; recruitment: February‐June 2008 |
|
| Participants |
Participants: 55 undergoing ACL reconstruction Inclusion criteria: participants with unstable knee resulting from ACL rupture; aged 18‐50 years Exclusion criteria: inflammatory diseases; diabetes mellitus; developed knee osteoarthrosis; malignant diseases; allergy to contrast media, renal diseases and thrombocytopenia Age: PRT group (mean ± SD): 35.4 years ± 10.0 No PRT (mean ± SD): 33.0 years ± 12.5 Gender: PRT group (number of men:women): 13:9 No PRT (number of men:women): 17:6 Sports activity: not available |
|
| Interventions | Arthroscopic ACL reconstruction with semitendinosus and gracilis tendons (fixed with 2 cross pins in the femur and 1 interference screw in the tibia) PRT (number of participants = 28): single, intraoperative application in the bone tunnels after graft placement. 52 mL blood mixed with 8 mL calcium citrate as anticoagulant. The authors pre‐defined the PRP volume as 6 mL, and the process resulted in 6 mL of PRP. The product was activated with human thrombin and applied in the surgical site PRT preparation: kit: Magellan autologous platelet separator (Medtronic Biologic Therapeutics and Diagnostics, Minneapolis, MN, USA) Quantification of platelet concentrates after preparation: 962 (552‐1326) g/L; participants' average blood platelet concentration:192 g/L No PRT (number of participants = 27): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol |
|
| Outcomes | Knee stability (KT‐ 2000) Tegner activity score Lysholm score IKDC score |
|
| Other quality issues |
Sample size: the authors did not calculate the sample size Validation of PRT: quantification reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | High risk | Sequence generated by odd or even date ‐ quasi‐randomised |
| Allocation concealment | High risk | Quasi‐randomised clinical trial |
| Blinding All outcomes | High risk | The participants and outcome assessors were not blinded to the treatment |
| Incomplete outcome data addressed All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups |
| Free of selective reporting | High risk | The study protocol is not available and the clinical follow‐up period was short for participants who underwent to ACL surgery |
| Free of other bias | Low risk | The study appears to be free of other bias |
Wasterlain 2013.
| Methods | Randomised controlled trial: randomisation sequence was generated by coin toss. Allocation concealment was kept in opaque envelopes that were opened on the day of the intervention. Orthopaedic surgeon and assessors were blinded to the procedure until 26‐weeks follow‐up, except for those for whom the procedure failed. Participants followed for 6 months Trial conducted: Stanford University School of Medicine, California USA; recruitment: October 2009‐June 2012 |
|
| Participants |
Participants: 23 with patellar tendinopathy Inclusion criteria: > 18 years old; diagnosed patellar tendinopathy; persistence of symptoms after 6 weeks of physical therapy with eccentric exercise Exclusion criteria: previous injection or surgery in the affected knee; inability to complete participant‐reported outcomes Age: PRT group mean (SD): 28 (8) No PRT mean (SD): 40 (14) Gender: PRT group (number of men:women): 8:1 No PRT (number of men:women): 12:0 Sports activity: not available |
|
| Interventions | Patellar tendon ultrasound‐guided treatment: single dry needling or PRP with the aid of a board‐certified radiologist For both groups, tendinopathy area was penetrated 10 times PRT (number of participants = 10): 55 mL blood resulted in 6 mL leukocyte‐rich PRP, injected into the patellar tendon during the dry needling procedure Quantification of platelet concentrates after preparation: not reported PRT preparation: kit: GPS III (Biomet Inc, Warsaw, IN, USA) No PRT (number of participants = 13): dry needling, as described above, and the 55 ml of blood that had been drawn was discarded Co‐interventions: same post procedure interventions, same rehabilitation protocol |
|
| Outcomes | VISA Tegner VAS Lysholm SF‐12 |
|
| Other quality issues |
Sample size: small sample size was powered for VISA, assuming an 13‐point effect size Validation of PRT: quantification not reported |
|
| Notes | Participants who were not satisfied with the procedure were allowed to receive other treatments. Analyses were performed on an intention‐to‐treat basis The authors provided the study protocol/trial registration details, ID: NCT01406821 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Sequence generated by coin toss |
| Allocation concealment | Low risk | Used sequentially‐numbered, opaque and sealed envelopes |
| Blinding All outcomes | Low risk | Participants and assessors were blinded |
| Incomplete outcome data addressed All outcomes | Low risk | Separate analysis were performed for participants who failed the allocated intervention, as a per protocol analysis and an intention to treat analysis |
| Free of selective reporting | Low risk | Data reported as depicted in the study protocol. Short follow‐up |
| Free of other bias | Low risk | Patients were permitted to receive other treatments. However, authors performed analysis as intention‐to‐treat |
Abbreviations
> = greater/more than < = less/fewer than ≥ = greater/more than or equal to ACL = anterior cruciate ligament ADL = activities of daily living AJSM = the American Journal of Sports Medicine ASES = American Shoulder and Elbow Surgeons' scoring system BMI = body:mass index BJSM = the British Journal of Sports Medicine CT = computed tomography DASH = Disabilities of the Arm Shoulder and Hand questionnaire IKDC = International Knee Documentation Committee JAMA = Journal of the American Medical Association MRI = magnetic resonance imaging PRF = platelet‐rich fibrin PRFM = platelet‐rich fibrin matrix PRP = platelet‐rich plasma PRT = platelet‐rich therapy PRTEE = Patient‐Related Tennis Elbow Evaluation SF‐12 = the Short Form health survey SST = Simple Shoulder Test UCLA = University of California, Los Angeles score VAS = visual analogue scale VISA = Victorian Institute Sports Assessment VISA‐A = Victorian Institute of Sports Assessment ‐ Achilles questionnaire
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ferrero 2012 | This was not a randomised study |
| Figueroa 2010 | This was not a randomised study |
| Radice 2009 | This was not a randomised study |
| Silva 2009 | This was not a randomised study |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12612000982819.
| Trial name or title | Northland, New Zealand Musculoskeletal Group Study on the effectiveness of platelet‐rich plasma for the treatment of greater trochanteric pain syndrome |
| Methods | Study design: randomised trial Random sequence generation: computer‐generated randomisation Allocation concealment: concealment by use of coded identifier for intervention Masking: single blinded |
| Participants | Location: not reported Target sample size (N): 48 Inclusion criteria: spontaneous pain in the lateral aspect of the hip Exclusion criteria: serious medical or psychologic disorders; history of operation in the same area; use of anticoagulants; pregnancy; high‐performance athletes; low haemoglobin or platelet count; inability to understand questionnaires |
| Interventions |
PRP: platelet‐rich plasma given with local anaesthetic as a single once‐off injection into the focal area of pain and tenderness over the outer hip (details not reported) Controls or placebo or no intervention (standard care): placebo (saline and local anaesthetic) |
| Outcomes |
Primary outcomes: pain according to NCS
Secondary outcomes: function (using Brief Pain Inventory); sleep (using Brief Pain Inventory); utilisation of health resources (consultations, medication use, other interventions) using participant recall Timing of outcomes measurement: 6 months |
| Starting date |
Main ID: ACTRN12612000982819
Date of registration: September 2012 Last refreshed on: not reported Date of first enrolment: January 2013 Status: completed |
| Contact information |
Name: Dr Grant Thompson
Address: PO Box 4274, Kamo, Whangarei 0141, New Zealand Telephone: +64 9 4594400 Email: grant@kensingtonmews.com Affiliation: not reported |
| Notes |
EUCTR201300047832ES.
| Trial name or title | Pilot randomised trial to assess the safety and potential efficacy of platelet rich plasma tenotomy for the treatment of chronic epicondylitis |
| Methods | Study design: randomised trial Random sequence generation: not reported Allocation concealment: not reported Masking: single blind (details not reported) |
| Participants | Location: not reported Target sample size (N): not reported Inclusion criteria: participants of both sexes aged 35‐75 years; pain in the arm scoring ≥ 3 points on VAS; values of BMI between 20‐35; possibility for observation during the follow‐up period; epicondylitis diagnosed Exclusion criteria: BMI > 35; systemic autoimmune rheumatic disease (connective tissue diseases and vasculitis systemic necrotising); poorly‐controlled diabetes mellitus (glycosylated haemoglobin above 9%); blood disorders (thrombopathy, thrombocytopenia, anaemia with Hb < 9); having immunosuppressive therapy and/or warfarin, or treatment with corticosteroids during the 3 months prior to inclusion in the study; treatment with NSAIDs, or oral corticosteroids within 15 days prior to inclusion in the study; severe heart disease |
| Interventions |
PRP: PRP injection (details were not reported) Controls or placebo or no intervention (standard care): lidocaine wet tenotomy |
| Outcomes |
Primary outcomes: DASH
Secondary outcomes: structural changes (by ultrasound) in the tendon secondary to treatment with PRP; assessment of whether the application of this technology is feasible; assessment of the feasibility of the protocol Timing of outcomes measurement: baseline, 6th week, and 3, 6 and 12 months |
| Starting date |
Main ID: EUCTR201300047832ES
Date of registration: April 2013 Last refreshed on: August 2013 Date of first enrolment: July 2013 Status: ongoing or finished |
| Contact information | Name: Isabel Andi Ortiz Address: Plaza de Cruces 48003 Barakaldo, Spain Telephone: 00349460060007005 Email: isabel.andiaortiz@osakidetza.net Affiliation: Basque Health Service |
| Notes |
IRCT2013052313442N1.
| Trial name or title | A randomised controlled trial: comparing the effectiveness of ultrasound guided injection of platelet rich plasma and shoulder physiotherapy on pain and function of patients with partial thickness rotator cuff tears |
| Methods | Study design: randomised trial Random sequence generation: not reported Allocation concealment: not reported Masking: not blinded |
| Participants | Location: not reported Target sample size (N): 40 Inclusion criteria: established rotator cuff tear (traumatic or degenerative) on MRI that should not be massive or full thickness in the radiologist's report; shoulder pain or dysfunction at a level of severity that makes the participant seek a medical intervention or surgery; all participants should have failed 2‐week treatments with NSAIDs and 6 weeks of physical therapy; provide written informed consent Exclusion criteria: pregnancy or active breastfeeding; presence of a tumour, metastatic disease, active infections; platelet count < 100,000 per μL or Hgb < 10 g/dL; gross instability of the glenohumeral joint; superior labral lesions requiring surgical repair; people with painful cervical spine pathology; previous surgery on the shoulder joint; national‐ or international‐level athletes |
| Interventions |
PRP: ultrasound guided, 3 mL PRP and 2 mL lidocaine injected directly into rotator cuff at the site of tear. Preparation must contain a platelet count of 100,000 per unit or be 5 times the basal level of the normal platelet count Controls or placebo or no intervention (standard care): 15 sessions of shoulder physiotherapy |
| Outcomes | Primary outcomes: Constant score; VAS Secondary outcomes: WORC Timing of outcomes measurement: 4, 8, 12 weeks |
| Starting date |
Main ID: IRCT201011205214N1
Date of registration: June 2011 Last refreshed on: July 2013 Date of first enrolment: December 2010 Status: complete |
| Contact information | Name: Dr Ramin Kordi Address: The Sports and Exercise Medicine Research Centre, Jalal Al Ahmad street, opposite the Shariati Hospital, Tehran, Tehran, Islamic Republic Of Iran Telephone: +98 2188 630227 Email: ramin_kordi@tums.ac.ir Affiliation: The Sports and Exercise Medicine Research Centre |
| Notes |
ISRCTN10464365.
| Trial name or title | A randomised controlled trial to assess the effectiveness of treating subacromial impingement and partial thickness rotator cuff tears with the administration of platelet rich plasma during arthroscopic decompression surgery |
| Methods | Study design: randomised trial Random sequence generation: computer generated randomisation system Allocation concealment: not reported Masking: not blinded |
| Participants |
Location: unknown
Target sample size (N): 34
Inclusion criteria: people with shoulder impingement syndrome or a partial thickness rotator cuff tear, with diagnosis confirmed using ultrasound scan by a trained member of the research team; failed conservative treatment; listed for arthroscopic subacromial decompression; male or female, aged 35‐75 years old
Exclusion criteria: full thickness rotator cuff tears; people with a history of significant trauma (fracture, dislocation/instability, etc.), surgery, osteoarthritis or other significant pathology of the affected shoulder not related to the rotator cuff; person is unable to consent for themselves; no conservative treatment; previous surgery on affected shoulder Contraindications to PRP: history of diabetes mellitus; platelet abnormality or platelet count < 100 x 109/L; haematological disorder; serum haemoglobin < 11 g/dL; use of systemic cortisone; use of any anticoagulant; evidence of gangrene/ulcers or peripheral vascular disease; history of hepatic or renal impairment or dialysis; person is known to have a psychological, developmental, physical, emotional or social disorder that may interfere with compliance with study requirements; history of alcohol or drug abuse; person has a religious or cultural conflict with the use of platelet gel treatment or blood products; has inadequate venous access for blood draw; is currently receiving or has received radiation or chemotherapy within the last 3 months prior to the study; pregnant women, or women who are lactating or planning pregnancy during the course of the study; any other significant disease or disorder that, in the opinion of the Investigator, may either put the participants at risk because of participation in the study, or may influence the result of the study, or the participant's ability to participate in the study |
| Interventions |
PRP: subacromial decompression plus an autologous PRP concentrate injection into the rotator cuff tendon (gel sprayed directly to the decompression area) Controls or placebo or no intervention (standard care): subacromial decompression (alone) |
| Outcomes | Primary outcomes: Oxford Shoulder Score Secondary outcomes: functional shoulder assessments; EQ5D; Oxford Satisfaction Index Timing of outcomes measurement: baseline and 3 weeks, 3, 6, 12 months posttreatment |
| Starting date |
Main ID: ISRCTN10464365
Date of registration: January 2011 Last refreshed on: June 2013 Date of first enrolment: unknown Status: completed |
| Contact information | Name: Andrew Carr Address: Windmill Road, Headington, Oxford Telephone: Email: andrew.carr@ndorms.ox.ac.uk Affiliation: University of Oxford |
| Notes |
ISRCTN95369715.
| Trial name or title | Achilles Tendinopathy Management: a randomised controlled trial comparing platelet rich plasma with an eccentric loading programme |
| Methods | Study design: randomised trial Random sequence generation: not reported Allocation concealment: not reported Masking: not reported |
| Participants | Location: Target sample size (N): 20 Inclusion criteria: midsubstance Achilles tendinopathy diagnosed clinically through pain on palpation at a level of 2‐6 cm above the tendon insertion and ultrasonography; tendinopathy must cause pain during loading activities and limit those activities; duration of at least 3 months; participants > 18 years old and of either sex Exclusion criteria: tendinopathies secondary to systemic conditions such as rheumatoid arthritis and diabetes; insertional Achilles tendinopathies; pregnancy; previous Achilles rupture or surgery; dislocation or fracture of the lower limb within the preceding 12 months |
| Interventions |
PRP: injected into the Achilles tendinopathy, PRP preparation protocol available Controls or placebo or no intervention (standard care): eccentric loading programme |
| Outcomes |
Primary outcomes: VISA‐ A
Secondary outcomes: EQ‐5D and complications Timing of outcomes measurement: at 6, 12, 24, 30, 36 and 52 weeks |
| Starting date |
Main ID: ISRCTN95369715
Date of registration: December 2009 Last refreshed on: February 2010 Date of first enrolment: February 2010 Status: completed |
| Contact information | Name: Matthew Costa Address: Clifford Bridge Road, Coventry, UK CV2 2DX Telephone: Email: N.K.Bains@warwick.ac.uk Affiliation: Warwick Medical School, Clinical Sciences Research Institute |
| Notes |
NCT01000935.
| Trial name or title | Impact of autologous platelet rich plasma on enhancing repair of rotator cuff tendons: a multicentre randomised controlled trial |
| Methods | Study design: randomised controlled trial Random sequence generation: not reported Allocation concealment: not reported Masking: double blind (subject, outcome assessor) |
| Participants | Location: Target sample size (N): Inclusion criteria: age >18 years; diagnosis of partial or full thickness rotator cuff tear of ≤ 3 cm; confirmed by MRI or US within a period of 6 months prior to booking surgery; the final inclusion will be based on arthroscopic assessment of the tear size and lack of significant concurrent pathology Exclusion criteria: unable to speak or read English; nonrepairable tear; acute tears (< 6 month); evidence of major joint trauma, infection, avascular necrosis, chronic dislocation, inflammatory arthropathy, frozen shoulder; concurrent pathology of SLAP lesions, Bankart lesions, or advanced osteoarthritis of the glenohumeral joint; previous surgery of the affected shoulder; bone marrow pathology; abnormal platelet count; serum haemoglobin concentration < 11 g/dL or hematocrit < 34%; use of systemic cortisone; current use of anticoagulants (i.e. aspirin); use of an investigational drug and/or blood donation within 3 months prior to surgery; substance or alcohol abuse; heavy smoking (> 20 cigarettes/day, based on definition of the World Health Organization (WHO)); psychiatric illness that precludes informed consent |
| Interventions |
PRP: PRP will be applied to the surgical site after completion of the repair (methods not reported) Standard‐of‐care: arthroscopic repair |
| Outcomes | Primary outcomes: visual analogue pain scale Secondary outcomes: adverse effects; MRI; patient‐focused outcomes: short WORC; ASES form; the CM score Timing of outcomes measurement: pain diary (1‐30 days), 6 weeks, 3 months, 6 months |
| Starting date |
Main ID: NCT01000935
Date of registration: September 2009 Last refreshed on: August 2013 Date of first enrolment: March 2011 Status: recruiting |
| Contact information | Name: Richard Holtby, MD Address: Sunnybrook Health Sciences Centre Telephone: not reported Email: helen.razmjou@sunnybrook.ca, gail.gunnis@sunnybrook.ca Affiliation: Sunnybrook Health Sciences Centre |
| Notes |
NCT01170312.
| Trial name or title | Arthroscopic surgery and platelet rich plasma In rotator cuff tear evaluation (ASPIRE): the use of platelet rich plasma following arthroscopic repair of rotator cuff tears, a pilot study |
| Methods | Study design: randomised trial Random sequence generation: not reported Allocation concealment: not reported Masking: double blind (subject, investigator, outcome assessor) |
| Participants | Location: not reported Target sample size (N): 25 Inclusion criteria: men or women; 18‐70 years of age; primary, traumatic or degenerative rotator cuff tears measuring 3 cm or less; rotator cuff tears requiring arthroscopic repair within 18 months of initial diagnosis; provision of informed consent Exclusion criteria: rotator cuff tears secondary to a fracture; an associated dislocation at the time of randomisation; rotator cuff tears that have had prior surgical repair or revision arthroscopy; nonsurgical rotator cuff‐associated treatment during month prior to randomisation, including corticosteroid injection and antiinflammatory treatment; prior PRP injection; pre‐existing conditions associated with upper extremity pain, including arthritis, ongoing infection, carpal tunnel syndrome, cervical neuropathy or other nerve pathology, local malignancy, and systemic disorders (e.g. uncontrolled diabetes, hypothyroidism); gross shoulder instability; people with an active infection; women who are pregnant, or plan to become pregnant in the next 12 months; a preoperative platelet count < 125,000 and a preoperative haemoglobin of 7.5 g/dL or less; likely problems with follow‐up (i.e. people with no fixed address, or reporting a plan to move out of town, or intellectually‐challenged people without adequate family support); inability to read and speak English; participating in another ongoing trial that would interfere with the assessment of the primary or secondary outcomes in this trial; any other reason (in the judgment of the surgeon) |
| Interventions |
PRP: ACP ‐ details not reported Controls or placebo or no intervention (standard care): saline ‐ details not reported |
| Outcomes | Primary outcomes: pain score Secondary outcomes: adverse events; use of healthcare resources; physical function; revision surgery Timing of outcomes measurement: 6 weeks |
| Starting date |
Main ID: NCT01170312
Date of registration: July 2010 Last refreshed on: November 2012 Date of first enrolment: September 2010 Status: completed |
| Contact information | Name: Mohit Bhandari Address: not reported Telephone: not reported Email: not reported Affiliation: McMaster University |
| Notes |
NCT01440725.
| Trial name or title | Multicenter double blind, with evaluator blinding, parallel, randomised clinical trial, to assess the efficacy of platelet rich plasma for treatment of muscle rupture with haematoma |
| Methods | Study design: randomised trial Random sequence generation: not reported Allocation concealment: not reported Masking: double‐blind (evaluator blinded) |
| Participants | Location: not reported Target sample size (N): Inclusion criteria: adults over 18 years; lesion with haematoma at the gastrocnemius muscle or the lower portion of the rectus femoral muscle; acceptance of participation in the clinical trial; surgical treatment of the muscle injury not indicated Exclusion criteria: history of bleeding disorders; inability to follow‐up the patient; use of corticosteroids, acetylsalicylic acid (aspirin) and NSAIDs during the study |
| Interventions |
PRP: autologous PRP injection (details not reported) Controls or placebo or no intervention (standard care): evacuation of haematoma |
| Outcomes | Primary outcomes: time to complete recovery from muscular lesions Secondary outcomes: adverse effects to treatments; pain; percentage of healing; percentage of muscular lesion recurrence; quality of the regenerated area Timing of outcomes measurement: weekly assessment for 8 weeks, then 12 months |
| Starting date |
Main ID: NCT01440725
Date of registration: September 2011 Last refreshed on: January 2013 Date of first enrolment: October 2009 Status: completed |
| Contact information | Name: Mª José Martínez Zapata, Address: not reported Telephone: not reported Email: not reported Affiliation: Centro Cochrane Iberoamericano, Servicio de Epidemiología Clínica y Salud Pública, Sant Pau, Barcelona, Spain |
| Notes |
NCT01509274.
| Trial name or title | Treatment of plantar fasciitis with injection of platelet rich plasma Into the origin of the plantar fascia: a prospective, randomised and double blinded study |
| Methods | Study design: randomised trial Random sequence generation: not reported Allocation concealment: not reported Masking: double blind (subject, investigator, outcome assessor) |
| Participants | Location: Target sample size (N): 90 participants Inclusion criteria: 18‐70 years of age; pain at the insertion of the plantar fascia on calcaneus; a VAS score of at least 4 at the insertion of the plantar fascia on calcaneus taking the first step in the morning; symptoms for 6‐12 months; ability to understand Danish and give informed consent Exclusion criteria: previously operated on in the same ankle or foot; pain in the foot anywhere other than the insertion of the plantar fascia on calcaneus on palpation; inflammatory disease; diabetes; previous rupture of the Achilles tendon; previous treatment with plasma injections; ongoing infection treated with antibiotics; treatment with steroids during the trial; treatment of the plantar fasciitis exceeding conservative treatment; use of crutches, walker or similar; pregnancy |
| Interventions |
PRP: plasma (3 mL plasma injected once into the plantar fascia) Controls or placebo or no intervention (standard care): 2 arms ‐ saline (3 mL saline injected once into the plantar fascia) and physiotherapy (3 times a day for 8 weeks) plus heel cap |
| Outcomes |
Primary outcomes: pain (VAS score)
Secondary outcomes: not provided Timing of outcomes measurement: at inclusion and after 1, 2, 3, 6 and 12 months |
| Starting date |
Main ID: NCT01509274
Date of registration: 10 January 2012 Last refreshed on: 16 January 2012 Date of first enrolment: August 2011 Status: recruiting participants |
| Contact information | Name: Bjørn Nedergaard Address: not provided Telephone: not provided Email: bspn77@gmail.com Affiliation: Kolding Sygehus |
| Notes |
NCT01518335.
| Trial name or title | A double blind, randomised, placebo controlled study evaluating the use of platelet rich plasma therapy for acute ankle sprains in the Emergency Department |
| Methods | Study design: randomised trial Random sequence generation: not reported Allocation concealment: not reported Masking: double blind (subject, caregiver) |
| Participants | Location: not reported Target sample size (N): 38 Inclusion criteria: severe ankle sprain, X‐ray completed Exclusion criteria: pregnancy/breastfeeding; police custody; active infection; metastatic disease/tumours; history of thrombocytopenia; allergy to ester or amine anaesthetics; taking anticoagulant medication; peripheral vascular disease; known coagulopathy |
| Interventions |
PRP: platelet rich plasma injection (details not reported) Controls or placebo or no intervention (standard care): standard care |
| Outcomes | Primary outcomes: LEFS Secondary outcomes: change in pain from baseline (details not reported) Timing of outcomes measurement: day 0; days 2‐3, days 8‐10, day 30 |
| Starting date |
Main ID: NCT01518335
Date of registration: December 2011 Last refreshed on: February 2013 Date of first enrolment: June 2009 Status: completed |
| Contact information | Name: Adam Rowden Address: not reported Telephone: not reported Email: not reported Affiliation: Einstein Healthcare Network |
| Notes |
NCT01600326.
| Trial name or title | A prospective comparison of ultrasound guided percutaneous platelet rich plasma injection versus tenotomy for treatment of gluteus minimus and medius tendinosis |
| Methods | Study design: randomised trial Random sequence generation: not reported Allocation concealment: not reported Masking: open label |
| Participants | Location: University of Michigan Hospital Target sample size (N): 30 Inclusion criteria: adult subjects with a diagnosis of tendinosis of the hip referred to Dr Jacobson for the treatment of tendinosis by tenotomy Exclusion criteria: not reported |
| Interventions |
PRP: ultrasound‐guided percutaneous PRP injection (methods not reported) Controls or placebo or no intervention (standard care): tenotomy (alone) |
| Outcomes |
Primary outcomes: pain
Secondary outcomes: effectiveness of PRP injection Timing of outcomes measurement: 15 days, and 30 days after intervention |
| Starting date |
Main ID: NCT01600326
Date of registration: 1 May 2012 Last refreshed on: 17 July 2013 Date of first enrolment: July 2010 Status: recruiting participants |
| Contact information | Name: Jon Jacobson, MD Address: University of Michigan Hospital, USA Telephone: +1 734 9364365 Email: jjacobsn@umich.edu Affiliation: University of Michigan Hospital |
| Notes |
NCT01668953.
| Trial name or title | Impact of platelet rich plasma over alternative therapies in patients with lateral epicondylitis |
| Methods | Study design: randomised trial Random sequence generation: not reported Allocation concealment: not reported Masking: single blinded |
| Participants | Location: multicentre Target sample size (N): 60 participants Inclusion criteria: adult men or women aged ≥ 20 years; clinical diagnosis of lateral epicondylitis based on site of pain, pain elicited with active extension of the wrist in pronation and elbow extension; documented sonographic diagnosis of common extensor tendinosis and possible tear based on abnormal echo texture (tendon thickening, anechoic areas, areas of hypoechogenicity, loss of fibrillar pattern); chronic symptoms (≥ 3 months); pain of at least 5/10 on a VAS; provision of informed consent Exclusion criteria: acute symptom onset (< 3 months); history of acute elbow trauma, rheumatoid arthritis,malignancy; pregnant or planning on becoming pregnant; requiring antiplatelet medication for the treatment of heart attack, stroke or other medical conditions; previous surgery for lateral epicondylitis; local injections, including steroids within the past 6 months; signs of other causes for lateral elbow pain (posterior interosseous nerve entrapment, osteochondral lesion); problems likely, in the judgment of the investigator, with maintenance of follow‐up; previous randomisation in this study or a competing study |
| Interventions |
PRP: Arthrex ACP system Controls or no intervention (standard care): whole blood injection, dry needle fenestration |
| Outcomes | Primary outcomes: rate of recruitment; ability to recruit 60 participants within a 12‐month period; adherence to study protocol Secondary outcomes: pain reduction (VAS); functional disability, Liverpool elbow score; psychological impairment (depression and anxiety), HADS; quality of life (SF‐12) Timing of outcomes measurement: 1, 2, 3, 6, 12 months |
| Starting date |
Main ID: NCT01668953
Date of registration: 16 August 2012 Last refreshed on: 26 July 2013 Date of first enrolment: Status: recruiting participants |
| Contact information | Name: Meg Chiavaras, PhD, MD Address: not reported Telephone: +1 905 5212100 ext 46521 Email: meg.chiavaras@gmail.com Affiliation: McMaster University |
| Notes |
NCT01765712.
| Trial name or title | Effect of intraoperative application of autologous PRP on post operative morbidity in ACL reconstruction using autologous bone patellar tendon bone graft harvest |
| Methods | Study design: randomised trial Random sequence generation: Allocation concealment: Masking: double blind (subject, investigator, outcome assessor) |
| Participants | Location: not reported Target sample size (N): Inclusion criteria: primary ACL reconstruction; outerbridge ≤ 2; minimum follow‐up of 2 years; no ligamentous secondary injury; willingness to participate in study Exclusion criteria: any previous knee injury prior history of anterior knee pain; outerbridge classification 3 or greater; revision ACL; diabetic or smoker; workers compensation patient; pregnant or nursing women; anybody with limited proficiency in English |
| Interventions |
PRP: ACL reconstruction bone patellar tendon bone autograft, PRP to be added to the participant's bone graft chips and placed into the donor site at the end of the case Controls or placebo or no intervention (standard care): ACL reconstruction bone patellar tendon bone autograft (standard care) |
| Outcomes | Primary outcomes: anterior knee pain Secondary outcomes: radiographic assessment of tunnel positioning; quantification of healing at the bony defect post operative strength (single leg hop test); post operative range of motion Timing of outcomes measurement: 2 weeks, 1, 3, 6, 12, 18, 24 months |
| Starting date |
Main ID: NCT01765712
Date of registration: 3 January 2013 Last refreshed on: 8 January 2013 Date of first enrolment: Status: recruiting participants |
| Contact information | Name: Brian Walters Address: not reported Telephone: not reported Email: not reported Affiliation: North Shore Long Island Jewish Health System |
| Notes |
NCT01812564.
| Trial name or title | Use of platelet rich plasma in the management of acute hamstring muscle strain injury |
| Methods | Study design: randomised trial Random sequence generation: not reported Allocation concealment: not reported Masking: double blind (subject, investigator, outcome assessor) |
| Participants | Location: Target sample size (N): Inclusion criteria: acute onset posterior thigh pain; MRI confirmed Grade I, II hamstring lesions; < 5 days from injury; able to perform physiotherapy at ASPETAR (5 sessions/week); available for follow‐up; male; age > 18 years Exclusion criteria: diabetes; immunocompromised state; overlying skin infection; re‐injury or chronic ongoing hamstring injury; unwilling to comply with follow‐up; contraindication to MRI; needle phobia; bleeding disorder or other medical contraindication to injection; medication increasing bleeding risk (e.g. Plavix); concurrent other injury inhibiting rehabilitation |
| Interventions |
PRP: complex growth factor preparations (PRP) in combination with exercise therapy Controls or placebo or no intervention (standard care): 2 groups: 1) PPP injections in combination with exercise therapy (control injection and usual care) and 2) exercise therapy (usual care) |
| Outcomes | Primary outcomes: time to return to play Secondary outcomes: recurrent hamstring lesions; pain during walking, jogging, running, sprinting, acceleration and during training; pain with isometric contraction against resistance assessed with the VAS; length and width of pain area during palpation and location of pain on palpation; passive straight leg raising test; full knee extension test at rest; 90 degrees hip flexion test; (painful) resisted knee flexion test at 90 degrees; pain with resisted hip extension test at 30 degrees; slump test; MRI scoring; hamstring strength; adverse effects Timing of outcomes measurement: every 7 days, 3 weeks after injury (MRI), 2 months, 1 year |
| Starting date |
Main ID: NCT01812564
Date of registration: 6 February 2013 Last refreshed on: 13 March 2013 Date of first enrolment: November 2009 Status: recruiting participants |
| Contact information | Name: Johannes Tol, MD PhD Address: not reported Telephone: +97444132142 Email: johannes.tol@aspetar.com Affiliation: ASPETAR |
| Notes |
NCT01833598.
| Trial name or title | Percutaneous needle tenotomy (PNT) versus platelet rich plasma (PRP) with PNT in the treatment of chronic tendinosis |
| Methods | Study design: randomised trial Random sequence generation: not reported Allocation concealment: not reported Masking: single blind (subject) |
| Participants | Location: Icahn School of Medicine at Mount Sinai Target sample size (N): 86 Inclusion criteria: aged 18‐100 years; with pain (≥ 5/10 pain on the VAS) that is a direct result of tendinopathy as determined by history of injury and study team member physician's best judgment; ≥3 months of pain after injury that has failed conservative treatments or after corticosteroid treatment (must be 3 months after corticosteroid injection to avoid theoretical tendon rupture) Exclusion criteria: taking coumadin; known coagulopathy or bleeding dyscrasia listed by patient report (patients will be asked if they have a bleeding disorder) and/or past medical history; taking fluoroquinolones; prior PNT or PRP for the affected tendon(s); known systemic illness such as vasculitis; an autoimmune or an inflammatory disease; uncontrolled diabetes; presence of other musculoskeletal injury or tendon rupture; pregnant or planning to become pregnant during the study. Those taking aspirin or NSAIDs are not excluded |
| Interventions |
PRP: percutaneous needle tenotomy with peritendinous platelet rich plasma injection Controls or placebo or no intervention (standard care): percutaneous needle tenotomy (alone) |
| Outcomes |
Primary outcomes: pain
Secondary outcomes: activity level, complications Timing of outcomes measurement: 2, 4, 6, 8, 12 weeks |
| Starting date |
Main ID: NCT01833598
Date of registration: April 2013 Last refreshed on: July 2013 Date of first enrolment: September 2012 Status: enrolling participants |
| Contact information | Name: Alexandra Voigt Address: Icahn School of Medicine at Mount Sinai Telephone: +972 2126 599379 Email: Alexandra.voigt@mountsinai.org Affiliation: Mount Sinai School of Medicine |
| Notes |
NCT01851044.
| Trial name or title | The effect of platelet rich plasma on lateral epicondylitis the treatment of lateral epicondylitis: the effect of platelet rich plasma on healing ‐ a randomised controlled double blinded trial |
| Methods | Study design: randomised controlled trial Random sequence generation: not reported Allocation concealment: not reported Masking: double blind (subject, investigator, outcome assessor) |
| Participants | Location: University of Tampere Target sample size (N): 120 Inclusion criteria: symptoms for > 3 months; primary conservative treatment (physiotherapy, NSAIDs etc.) has been tried Exclusion criteria: significant systemic diseases; any surgical operation on the affected elbow |
| Interventions |
PRP: 9 mL autologous venous blood centrifuged using the Arthrex ACP® Double Syringe System and 2 mL PRP injected to the proximal insertion of the extensor carpi radialis brevis muscle Controls or placebo or no intervention (standard care): saline injections (1 arm) and whole blood injections (1 arm) |
| Outcomes | Primary outcomes: pain (VAS scale) and DASH score Secondary outcomes: grip strength (Jamar); need for NSAIDs; duration of the potential sick leave due to lateral epicondylitis Timing of outcomes measurement: 52 weeks |
| Starting date |
Main ID: NCT01851044
Date of registration: May 2013 Last refreshed on: May 2013 Date of first enrolment: not reported Status: |
| Contact information | Name: Olli Leppänen Address: Hatanpää City Hospital, Tampere, Finland Telephone: +358 405 866581 Email: olli.v.leppanen@uta.fi Affiliation: University of Tampere |
| Notes |
Abbreviations
> = greater/more than < = less/fewer than ≥ = greater/more than or equal to ≤ = less/fewer than or equal to ACL = anterior cruciate ligament ACP = autologous conditioned plasma ASES = American Shoulder and Elbow Surgeons' scoring system ASPETAR = Qatar's Orthopaedic and Sports Medicine Hospital BMI = body:mass index CM = Constant‐Murley score DASH = Disabilities of the Arm Shoulder and Hand questionnaire EQ‐5D = Euroqol 5D a standardised instrument for measuring quality of life HADS = Hospital Anxiety and Depression Scale Hgb = haemoglobin LEFS = Lower extremity Function Scale MRI = magnetic resonance imaging NCS = numeric rating scale NSAIDs = non‐steroidal anti‐inflammatories PPP = platelet poor plasma PRP = platelet‐rich plasma SF‐12 = the Short Form health survey SLAP = Superior Labral Anterior and Posterior lesions US = ultrasound VAS = visual analogue scale VISA‐A = Victorian Institute of Sports Assessment ‐ Achilles questionnaire WORC = Western Ontario Rotator Cuff outcome measure
Differences between protocol and review
When trials included more than one measure of function, we chose the Constant score rather than UCLA, ASES and L’Insalata scores as it is the most commonly used tool in the literature for assessment of shoulder function.
We opted to include autologous whole blood and dry needling as control interventions.
We opted not to present 'Summary of findings' tables given the heterogeneity of the underlying conditions in the included trials.
Most of our planned subgroup analyses were not performed because of a lack of data. We introduced a modified subgroup analysis (main treatment for tendinopathies versus surgical augmentation procedure), as this seemed appropriate in the context of the available data.
Sensitivity analyses were restricted to testing the effects of including quasi‐randomised studies in the meta‐analysis and to exploring the effects of obvious outliers on heterogeneity and test for subgroup differences.
Contributions of authors
All authors contributed to the review. VM, ML MT, JB and FF drafted the review and all authors provided comments and approved the final version. The guarantor of this review is Vinícius Ynoe de Moraes.
Sources of support
Internal sources
Escola Paulista de Medicina ‐ Universidade Federal de São Paulo, Brazil.
External sources
No sources of support supplied
Declarations of interest
Vinícius Y Moraes ‐ none known Mário Lenza ‐ none known Marcel Jun Tamaoki ‐ none known Flávio Faloppa ‐ none known João Carlos Belloti ‐ none known
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Almeida 2012 {published data only}
- Almeida AM. Platelet‐rich plasma in the regeneration of the patellar ligament after harvesting its central third: a prospective randomised study [Efeito do plasma rico em plaquetas na regeneração do terço central do ligamento da patela: estudo prospectivo randomizado] (Thesis). São Paulo: Universidade de São Paulo, Faculdade de Medicina, 2013. [Google Scholar]
- Almeida AM, Demange MK, Sobrado MF, Rodrigues MB, Pedrinelli A, Hernandez AJ. Patellar tendon healing with platelet‐rich plasma. American Journal of Sports Medicine 2012;40(6):1283‐8. [DOI] [PubMed] [Google Scholar]
Antuna 2013 {published data only}
- Antuna S, Barco R, Martinez JM, Sanchez JM. Effect of platelet‐rich fibrin on rotator cuff repair (PRP‐Fibrin). http://clinicaltrials.gov/show/NCT01612845 (accessed 04 December 2013).
- Antuna SA, Barco Laakso R, Martinez Diez JM, Sanchez Marquez JMM. PRP‐fibrin for arthroscopically repaired massive rotator cuff tears: a prospective randomized clinical trial. In: American Academy of Orthopaedic Surgeons Annual Meeting; 2012 Mar 7‐11; San Francisco (Ca) 2012.
- Antuña S, Barco J, Martínez Díes JM, Sánchez Márquez JM. Platelet‐rich fibrin in arthroscopic repair of massive rotator cuff tears: A prospective randomized pilot clinical trial. Acta Orthopaedica Belgica 2013;79:25‐30. [PubMed] [Google Scholar]
- Moraes V. Personal communication. Email to: S Antuna 18 April 2013.
Castricini 2011 {published data only}
- Castricini R, Longo UG, Benedetto M, Panfoli N, Pirani N, Zini R, et al. Platelet‐rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. American Journal of Sports Medicine 2011;39(2):258‐65. [DOI: 10.1177/0363546510390780] [DOI] [PubMed] [Google Scholar]
Cervellin 2012 {published data only}
- Cervellin M, Girolamo L, Bait C, Denti M, Volti P. Autologous platelet‐rich plasma gel to reduce donor‐site morbidity after patellar tendon graft harvesting for anterior cruciate ligamente reconstruction: a randomized, controlled clinical study. Knee Surgery, Sports Traumatology, Arthroscopy 2012;20(1):114‐20. [DOI: 10.1007/s00167-011-1570-5] [DOI] [PubMed] [Google Scholar]
Creaney 2011 {published data only}
- Creaney L, Wallace A, Curtis M, Connell D. Growth factor‐based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: a prospective, single‐blind, randomised trial of autologous blood injections versus platelet‐rich plasma injections. British Journal of Sports Medicine 2011;45(12):966‐71. [DOI: 10.1136/bjsm.2010.082503] [DOI] [PubMed] [Google Scholar]
De Vos 2010 {published data only}
- Jonge S, Vos RJ, Weir A, Schie HT, Bierma‐Zeinstra SM, Verhaar JA, et al. One‐year follow‐up of platelet‐rich plasma treatment in chronic Achilles tendinopathy. American Journal of Sports Medicine 2011;39(8):1623‐9. [DOI] [PubMed] [Google Scholar]
- Vos RJ, Weir A, Tol JL, Verhaar JA, Weinans H, vanSchie HT. No effects of PRP on ultrasonographic tendon structure and neovascularization in chronic midportion Achilles tendinopathy. British Journal of Sports Medicine 2011;45(5):387‐92. [DOI: 10.1136/bjsm.2010.076398] [DOI] [PubMed] [Google Scholar]
- Vos RJ, Weir A, Schie HT, Bierma‐Zeinstra SM, Verhaar JA, Weinans H, et al. Platelet‐rich plasma injection for chronic Achilles tendinopathy. JAMA 2010;303(2):144‐9. [DOI] [PubMed] [Google Scholar]
Everts 2008 {published data only}
- Everts PA, Devilee RJ, Mahoney CB, Erp A, Oosterbos CJ, Stellenboom M, et al. Exogenous application of platelet‐leukocyte gel during open subacromial decompression contributes to improved patient outcome. European Surgical Research 2008;40(2):203‐10. [DOI: 10.1159/000110862] [DOI] [PubMed] [Google Scholar]
Gumina 2012 {published data only}
- Gumina S, Campagna V, Ferrazza G, Giannicola G, Fratalocchi F, Milani A, et al. Use of platelet‐leukocyte membrane in arthroscopic repair of large rotator cuff tears. Journal of Bone and Joint Surgery ‐ American Volume 2012;94(15):1345‐52. [DOI: 10.2106/JBJS.K.00394] [DOI] [PubMed] [Google Scholar]
Krogh 2013 {published data only}
- Krogh TP, Fredberg U, Stengaard‐Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet‐rich plasma, glucocorticoid, or saline: a randomized, double‐blind, placebo‐controlled trial. American Journal of Sports Medicine 2013;41(3):625‐35. [DOI: 10.1177/0363546512472975] [DOI] [PubMed] [Google Scholar]
NCT01029574 {published data only}
- Malavolta E, Ferreira Neto AA. Platelet rich plasma on arthroscopic repair of the complete rotator cuff lesions: a prospective and randomized study. http://clinicaltrials.gov/ct2/show/NCT01029574 (accessed 26 August 2013).
- Moraes V. Personal communication. Email to: E Malavolta.
Orrego 2008 {published data only}
- Orrego M, Larrain C, Rosales J, Valenzuela L, Matas J, Durruty J, et al. Effects of platelet concentrate and a bone plug on the healing of hamstring tendons in a bone tunnel. Arthroscopy 2008;24(12):1373‐80. [DOI: 10.1016/j.arthro.2008.07.016] [DOI] [PubMed] [Google Scholar]
Randelli 2011 {published data only}
- Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2‐year follow‐up. Journal of Shoulder and Elbow Surgery 2011;20(4):518‐28. [DOI: 10.1016/j.jse.2011.02.008] [DOI] [PubMed] [Google Scholar]
Rodeo 2012 {published data only}
- Rodeo SA, Delos D, Williams RJ, Adler RS, Pearle A, Warren RF. The effect of platelet‐rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. American Journal of Sports Medicine 2012;40(6):1234‐41. [DOI: 10.1177/0363546512442924] [DOI] [PubMed] [Google Scholar]
Schepull 2010 {published data only}
- Schepull T, Kvist J, Norrman H, Trinks M, Berlin G, Aspenberg P. Autologous platelet have no effect on the healing of human achilles tendon ruptures: a randomized single‐blind study. American Journal of Sports Medicine 2011;39(1):38‐47. [DOI: 10.1177/0363546510383515] [DOI] [PubMed] [Google Scholar]
Thanasas 2011 {published data only}
- Thanasas C, Papadimitriou G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet‐rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. American Journal of Sports Medicine 2011;39(10):2130‐4. [DOI: 10.1177/0363546511417113] [DOI] [PubMed] [Google Scholar]
Vadalà 2013 {published data only}
- Vadalà A, Iorio R, Carli A, Ferretti M, Paravani D, Caperna L, et al. Platelet‐rich plasma: does it help reduce tunnel widening after ACL reconstruction?. Knee Surgery Sports Traumatology Arthroscopy 2013;21(4):824‐9. [DOI: 10.1007/s00167-012-1980-z] [DOI] [PubMed] [Google Scholar]
Valenti Nín 2009 {published data only}
- Valentí Nín JR, Gasque GM, Azcárate AV, Beola JD, Gonzalez MH. Has platelet‐rich plasma any role in anterior cruciate ligament allograft healing?. Arthroscopy 2009;25(11):1206‐13. [DOI: 10.1016/j.arthro.2009.06.002] [DOI] [PubMed] [Google Scholar]
Vogrin 2010 {published data only}
- Vogrin M, Rupreht M, Crnjac A, Dinevski D, Krajnc Z, Recnik G. The effect of platelet‐derived growth factors on knee stability after anterior cruciate ligament reconstruction: a prospective randomized clinical study. Wiener Klinische Wochenschrift 2010;122(Suppl 2):91‐5. [DOI: 10.1007/s00508-010-1340-2] [DOI] [PubMed] [Google Scholar]
Wasterlain 2013 {unpublished data only}
References to studies excluded from this review
Ferrero 2012 {published data only}
- Ferrero G, Fabbro E, Orlandi D, Martini C, Lacelli F, Serafini G, et al. Ultrasound‐guided injection of platelet‐rich plasma in chronic Achilles and patellar tendinopathy. Journal of Ultrasound 2012;15(4):260‐6. [DOI: 10.1016/j.jus.2012.09.006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Figueroa 2010 {published data only}
- Figueroa D, Melean P, Calvo R, Vasiman A, Zilleruelo N, Figueroa F, et al. Magnetic resonance imaging evaluation of the integration and maturation of semitendinosus‐gracilis graft in anterior cruciate ligament reconstruction using autologous platelet concentrate. Arthroscopy 2010;26(10):1318‐25. [DOI: 10.1016/j.arthro.2010.02.010] [DOI] [PubMed] [Google Scholar]
Radice 2009 {published data only}
- Radice F, Yánez R, Gutiérrez V, Rosales J, Pinedo M, Coda S. Comparison of magnetic resonance findings in anterior cruciate ligament grafts with or without autologous platelet‐derived growth factors. Arthroscopy 2010;26(1):50‐7. [DOI: 10.1016/j.arthro.2009.06.030] [DOI] [PubMed] [Google Scholar]
Silva 2009 {published data only}
- Silva A, Sampaio R. Anatomic ACL reconstruction: does the platelet‐rich plasma accelerate tendon healing?. Knee Surgery, Sports Traumatology, Arthroscopy 2009;17(6):676‐82. [DOI: 10.1007/s00167-009-0762-8] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12612000982819 {published data only}
- Thompson G. Northland lateral hip pain platelet‐rich plasma treatment study. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612000982819 (accessed 26 August 2013).
EUCTR201300047832ES {published data only}
- Gomes JI. Pilot randomised trial to assess the safety and potential efficacy of platelet rich plasma tenotomy for the treatment of chronic epicondylitis. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2013‐000478‐32 (accessed 26 August 2013). [EudraCT: 2013‐000478‐32 ]
IRCT2013052313442N1 {published data only}
- Raeissadat SA. Study of the effects of local platelet‐rich plasma (PRP) injection versus autologous blood injection in patients with lateral epicondylitis in PM & R clinic of Modarres hospital from 2011 to 2013. http://www.irct.ir/searchresult.php?id=13442&number=1 (accessed 26 August 2013).
ISRCTN10464365 {published data only}
- Carr A. Platelet rich plasma and rotator cuff tendons. http://www.controlled‐trials.com/ISRCTN10464365/ (accessed 26 August 2013).
ISRCTN95369715 {published data only}
- Costa M. Achilles tendinopathy management: platelet rich plasma versus eccentric loading programme. http://www.controlled‐trials.com/ISRCTN95369715/ (accessed 26 August 2013).
NCT01000935 {published data only}
- Razmjou H. Impact of autologous platelet rich plasma on healing of rotator cuff repairs. http://clinicaltrials.gov/ct2/show/record/NCT01000935 (accessed 26 August 2013).
NCT01170312 {published data only}
- Bhandari M. Arthroscopic surgery and platelet rich plasma in rotator cuff tear evaluation (A.S.P.I.R.E.): the use of platelet rich plasma following arthroscopic repair of rotator cuff tears, a pilot study. http://clinicaltrials.gov/show/NCT01170312 (accessed 26 August 2013).
NCT01440725 {published data only}
- Martínez‐Zapata MJ. Multicenter double blind, with evaluator blinding, parallel, randomized clinical trial, to assess the efficacy of platelet rich plasma for treatment of muscle rupture with haematoma. http://clinicaltrials.gov/show/NCT01440725 (accessed 26 August 2013).
NCT01509274 {published data only}
- Nedergaard B. Treatment of plantar fasciitis with injection af platelet rich plasma into the origin of the plantar fascia. http://clinicaltrials.gov/ct2/show/NCT01509274 (accessed 26 August 2013).
NCT01518335 {published data only}
- Rowden A. A doubleblind, randomized, placebo controlled study evaluating the use of platelet rich plasma therapy for acute ankle sprains in the emergency department. http://clinicaltrials.gov/show/NCT01518335 (accessed 26 August 2013). [DOI] [PubMed]
NCT01600326 {published data only}
- Jacobson J. A prospective comparison of ultrasound‐guided percutaneous platelet‐rich plasma injection versus tenotomy for treatment of gluteus minimus and medius tendinosis. http://clinicaltrials.gov/ct2/show/NCT01600326.
NCT01668953 {published data only}
- Chiavaras M. Comparison of platelet rich plasma and alternative therapies for the treatment of tennis elbow (lateral epicondylitis) (IMPROVE). http://clinicaltrials.gov/ct2/show/NCT01668953 (accessed 26 August 2013).
NCT01765712 {published data only}
- Walters B. Effect of intraoperative application of autologous PRP on post operative morbidity in ACL reconstruction using autologous bone patellar tendon bone graft harvest. http://clinicaltrials.gov/show/NCT01765712 (accessed 26 August 2013).
NCT01812564 {published data only}
- Tol J. Use of platelet rich plasma in the management of acute hamstring muscle strain injury. http://clinicaltrials.gov/show/NCT01812564 (accessed 26 August 2013).
NCT01833598 {published data only}
- Voigt A. Percutaneous needle tenotomy (PNT) versus platelet rich plasma (PRP) with pnt in the treatment of chronic tendinosis. http://clinicaltrials.gov/show/NCT01833598 (accessed 26 August 2013).
NCT01851044 {published data only}
- Leppänen OV. The effect of platelet rich plasma on lateral epicondylitis. http://clinicaltrials.gov/show/NCT01851044 (accessed 26 August 2013).
Additional references
Brazier 1992
- Brazier JE, Harper R, Jones NM, O'Cathain A, Thomas KJ, Usherwood T, et al. Validating the SF‐36 health survey questionnaire: new outcome measure for primary care. BMJ 1992;305(6846):160‐4. [Google Scholar]
Chahal 2012
- Chahal J, Thiel GS, Mall N, Heard W, Bach BR, Cole BJ, et al. The role of platelet‐rich plasma in arthroscopic rotator cuff repair: a systematic review with quantitative synthesis. Arthroscopy 2012;28(11):1718‐27. [DOI] [PubMed] [Google Scholar]
Clayton 2008
- Clayton RA, Court‐Brown CM. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury 2008;39(12):1338‐44. [DOI] [PubMed] [Google Scholar]
De Long 2012
- Long JM, Russel RP, Mazzocca AD. Platelet‐rich plasma: the PAW classification system. Arthroscopy 2012;28(7):998‐1009. [DOI] [PubMed] [Google Scholar]
De Vos 2010a
- Vos RJ, Weir A, Schie HT, Bierma‐Zeinstra SM, Verhaar JA, Weinans H, et al. Platelet‐rich plasma injection for chronic Achilles tendinopathy. JAMA 2010;303(2):144‐9. [DOI] [PubMed] [Google Scholar]
De Vos 2010b
- Vos RJ, Veldhoven PL, Moen MH, Weir A, Tol JL, Maffulli N. Autologous growth factor injections in chronic tendinopathy: a systematic review. British Medial Bulletin 2010;95(1):63‐77. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Dohan 2009
- Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet‐rich plasma (P‐PRP) to leucocyte‐ and platelet‐rich fibrin (L‐PRF). Trends in Biotechnology 2009;27(3):158‐67. [DOI] [PubMed] [Google Scholar]
Europe 2007
- Council of Europe. Guide to the preparation, use and quality assurance of blood components. 13th Edition. Strasbourg, France: Council of Europe, 2007. [Google Scholar]
Filardo 2010
- Filardo G, Kon E, Della Villa S, Vincentelli F, Fornasari PM, Marcacci M. Use of platelet‐rich plasma for the treatment of refractory jumper's knee. International Orthopaedics 2010;34(6):909‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foster 2009
- Foster TE, Puskas BL, Mandelbaum BL, Gerhardt MB, Rodeo SA. Platelet‐rich plasma: from basic science to clinical applications. American Journal of Sports Medicine 2009;37(11):2259‐72. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hootman 2002
- Hootman JM, Macera CA, Ainsworth BE, Addy CL, Martin M, Blair SN. Epidemiology of musculoskeletal injuries among sedentary and physically active adults. Medicine and Science in Sports and Exercise 2002;34(5):838‐44. [DOI] [PubMed] [Google Scholar]
Hudak 1996
- Hudak PL, Amadio PC, Bombardier C, Boland A, Fischer T, Flatow EL, et al. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). American Journal of Industrial Medicine 1996;29(6):602‐8. [DOI] [PubMed] [Google Scholar]
Khan 1999
- Khan KM, Cook JL, Bonar F, Harcourt P, Astrom M. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Medicine 1999;27(6):393‐408. [DOI] [PubMed] [Google Scholar]
Kitaoka 1994
- Kitaoka H, Alexander I, Adelaar R, Nunley J, Myerson M, Sanders M. Clinical rating systems for the ankle‐hindfoot, midfoot, hallux, and lesser toes. Foot & Ankle International 1994;15(7):349‐53. [DOI] [PubMed] [Google Scholar]
Kiter 2006
- Kiter E, Celikbas E, Akkaya S, Demirkan F, Kiliç BA. Comparison of injection modalities in the treatment of plantar heel pain: a randomized controlled trial. Journal of the American Podiatric Medical Association 2006;96(4):293‐6. [DOI] [PubMed] [Google Scholar]
Kukkonen 2013
- Kukkonen J, Kauko T, Vahlberg T, Joukainen A, Aärimaa V. Investigating minimal clinically important difference for Constant score in patients undergoing rotator cuff surgery. Journal of Shoulder and Elbow Surgery 2013;22(12):1650‐5. [DOI] [PubMed] [Google Scholar]
Lee 2011
- Lee K, Wilson JJ, Rabago DP, Baer GS, Jacobson JA, Borrero CG. Musculoskeletal applications of platelet‐rich plasma: fad or future?. American Journal of Roentgenology 2011;196(3):628‐36. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Maffulli 2003
- Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clinical Journal of Sports Medicine 2003;22(4):675‐92. [DOI] [PubMed] [Google Scholar]
Masthoff 2005
- Masthoff E, Trompenaars F, Heck G, Hodiamont P, Vries J. Validation of the WHO Quality of Life assessment instrument (WHOQOL‐100) in a population of Dutch adult psychiatric outpatients. European Psychiatry 2005;20(7):465‐73. [DOI] [PubMed] [Google Scholar]
Paoloni 2005
- Paoloni JA, Orchard JW. The use of therapeutic medications for soft‐tissue injuries in sports medicine. Medical Journal of Australia 2005;183(7):384‐8. [DOI] [PubMed] [Google Scholar]
Peerbooms 2010
- Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double‐blind randomized controlled trial: platelet‐rich plasma versus corticosteroid injection with a 1‐year follow‐up. American Journal of Sports Medicine 2010;38(2):255‐62. [DOI] [PubMed] [Google Scholar]
Revill 1976
- Revill SI, Robinson JO, Rosen M, Hogg MI. The reliability of a linear analogue for evaluating pain. Anaesthesia 1976;31(9):1191‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Robinson 2001
- Robinson JM, Cook JL, Purdam C, Visentini PJ, Ross J, Maffulli N, et al. The VISA‐A questionnaire: a valid and reliable index of the clinical severity of Achilles tendinopathy. British Journal of Sports Medicine 2001;35(5):335‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schepull 2011
- Schepull T, Kvist J, Norrman H, Trinks M, Berlin G, Aspenberg P. Autologous platelets have no effect on the healing of human achilles tendon ruptures. American Journal of Sports Medicine 2011;39(1):38‐47. [DOI] [PubMed] [Google Scholar]
Sheth 2012
- Sheth U, Simunovic N, Klein G, Fu F, Einhorn TA, Schemitsch E, et al. Efficacy of autologous platelet‐rich plasma use for orthopaedic indications: a meta‐analysis. Journal of Bone and Joint Surgery ‐American Volume 2012;94(4):298‐07. [DOI] [PubMed] [Google Scholar]
Sonnleitner 2000
- Sonnleitner D, Huemer P, Sullivan D. A simplified technique for producing platelet‐rich plasma and platelet concentrate for intraoral bone grafting techniques: A technical note. International Journal of Oral & Maxillofacial Implants 2000;15(6):879‐82. [PubMed] [Google Scholar]
Taylor 2011
- Taylor DW, Petrera M, Hendry M, Theodoropoulos JS. A systematic review of the use of platelet‐rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clinical Journal of Sport Medicine 2011;21(4):344‐52. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Moraes 2012
- Moraes VY, Lenza M, Tamaoki MJ, Faloppa F, Belloti JC. Platelet rich therapies for musculoskeletal soft‐tissue injuries. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD010071] [DOI] [Google Scholar]
Moraes 2013
- Moraes VY, Lenza M, Tamaoki MJ, Faloppa F, Belloti JC. Platelet‐rich therapies for musculoskeletal soft tissue injuries. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD010071.pub2] [DOI] [PubMed] [Google Scholar]


