Abstract
Background
Herpes zoster ophthalmicus affects the eye and vision, and is caused by the reactivation of the varicella zoster virus in the distribution of the first division of the trigeminal nerve. An aggressive management of acute herpes zoster ophthalmicus with systemic antiviral medication is generally recommended as the standard first‐line treatment for herpes zoster ophthalmicus infections. Both acyclovir and its prodrug valacyclovir are medications that are approved for the systemic treatment of herpes zoster. Although it is known that valacyclovir has an improved bioavailability and steadier plasma concentration, it is currently unclear as to whether this leads to better treatment results and less ocular complications.
Objectives
To assess the effects of valacyclovir versus acyclovir for the systemic antiviral treatment of herpes zoster ophthalmicus in immunocompetent patients.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register; 2016, Issue 5), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to June 2016), Embase (January 1980 to June 2016), Web of Science Conference Proceedings Citation Index‐Science (CPCI‐S; January 1990 to June 2016), BIOSIS Previews (January 1969 to June 2016), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 13 June 2016.
Selection criteria
We considered all randomised controlled trials (RCTs) in which systemic valacyclovir was compared to systemic acyclovir medication for treatment of herpes zoster ophthalmicus. There were no language restrictions.
Data collection and analysis
Two review authors independently selected trials, evaluated the risk of bias in included trials, and extracted and analysed data. We did not conduct a meta‐analysis, as only one study was included. We assessed the certainty of the evidence for the selected outcomes using the GRADE approach.
Main results
One study fulfilled the inclusion criteria. In this multicentre, randomised double‐masked study carried out in France, 110 immunocompetent people with herpes zoster ophthalmicus, diagnosed within 72 hours of skin eruption, were treated, with 56 participants allocated to the valacyclovir group and 54 to the acyclovir group. The study was poorly reported and we judged it to be unclear risk of bias for most domains.
Persistent ocular lesions after 6 months were observed in 2/56 people in the valacyclovir group compared with 1/54 people in the acyclovir group (risk ratio (RR) 1.93 (95% CI 0.18 to 20.65); very low certainty evidence. Dendritic ulcer appeared in 3/56 patients treated with valacyclovir, while 1/54 suffered in the acyclovir group (RR 2.89; 95% confidence interval (CI) 0.31 to 26.96); very low certainty evidence), uveitis in 7/56 people in the valacyclovir group compared with 9/54 in the acyclovir group (RR 0.96; 95% CI 0.36 to 2.57); very low certainty evidence). Similarly, there was uncertainty as to the comparative effects of these two treatments on post‐herpetic pain, and side effects (vomiting, eyelid or facial edema, disseminated zoster). Due to concerns about imprecision (small number of events and large confidence intervals) and study limitations, the certainty of evidence using the GRADE approach was rated as low to very low for the use of valacyclovir compared to acyclovir.
Authors' conclusions
This review included data from only one study, which had methodological limitations. As such, our results indicated uncertainty of the relative benefits and harms of valacyclovir over acyclovir in herpes zoster ophthalmicus, despite its widespread use for this condition. Further well‐designed and adequately powered trials are needed. These trials should include outcomes important to patients, including compliance.
Plain language summary
Valacyclovir compared with acyclovir for the treatment of herpes zoster ophthalmicus in people with an otherwise normal immune system
What is the aim of this review? The aim of this Cochrane Review was to find out if valacyclovir performs better than acyclovir in the treatment of a painful itchy rash caused by the chickenpox virus (herpes zoster ophthalmicus). Cochrane researchers collected and analysed all relevant studies to answer this question and found one study.
Key messages There is uncertainty as to the benefits and harms of valacyclovir compared with acyclovir for the treatment of herpes zoster ophthalmicus.
What was studied in the review? Herpes zoster ophthalmicus is a painful itchy rash that appears on one side of the forehead. If the rash reaches the eye it may lead to visual impairment. This is because the infection can damage the front of the eye. Herpes zoster is caused by the chickenpox virus, which can remain in the body for many years after the original chickenpox infection, and may get reactivated. Doctors can treat herpes zoster ophthalmicus with acyclovir. This is an antiviral medication that kills the chickenpox virus. Valacyclovir is a modified version of acyclovir that may need to be taken less frequently as it is better absorbed by the body.
What are the main results of the review? The review authors found one relevant study from France. This study compared valacyclovir 1000 mg taken three times a day for seven days with acyclovir 800 mg taken five times a day for seven days. The company that makes valacyclovir (Glaxo) funded the study.
The review authors are uncertain whether valacyclovir has any benefit over acyclovir in the treatment of herpes zoster ophthalmicus. They judged the certainty of the evidence to be very low because the study was small and there were some problems with the way it was reported.
How up‐to‐date is this review? The Cochrane researchers searched for studies that had been published up to June 2016.
Summary of findings
for the main comparison.
| Valacyclovir compared with acyclovir for the systemic treatment of herpes zoster ophthalmicus in immunocompetent patients | ||||||
|
Patient or population: Adult immunocompetent patients with herpes zoster ophthalmicus
Setting: No restriction on the type or location of the health service (primary, secondary and tertiary care) Intervention: Systemic valacyclovir Comparison: Systemic acyclovir | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with acyclovir | Risk with valacyclovir | |||||
| Occurrence of ocular involvement | 19 per 1000 | 36 per 1000 (3 to 382) | RR 1.93 (0.18 to 20.65) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1 2 | Data were only available on persistent ocular lesions after 6 months. |
| Ocular involvement ‐ Dendritic ulcer | 19 per 1000 | 54 per 1000 (6 to 49) | RR 2.89 (0.31 to 26.96) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1 2 | ‐ |
| Ocular involvement ‐ Uveitis | 167 per 1000 | 125 per 1000 (50 to 312) | RR 0.96 (0.36 to 2.57) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1 2 | ‐ |
| Pain at week 24 (self‐reported; yes) | 56 per 1000 | 53 per 1000 (11 to 254) | RR 0.96 (0.20 to 4.57) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1 2 | ‐ |
| Adverse effects ‐ Vomiting | 37 per 1000 | 54 per 1000 (9 to 308) | RR 1.45 (0.25 to 8.32) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1 2 | ‐ |
| Adverse effects ‐ Eyelid or facial oedema | 56 per 1000 | 18 per 1000 (2 to 167) | RR 0.32 (0.03 to 3.00) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ‐ |
| Adverse effects ‐ Disseminated zoster | 19 per 1000 | 6 per 1000 (0 to 143) | RR 0.32 (0.01 to 7.73) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ‐ |
| * The risk in the intervention group (valacyclovir) (and its 95% confidence interval) is based on the assumed risk in the comparison group (acyclovir treatment) and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1 We downgraded 1 level for risk of bias, as the authors did not report on random sequence generation, allocation concealment and masking of staff, and the extent to which bias had been avoided was largely unclear (‐1).
2 We downgraded 2 levels for imprecision as there were few events and very wide confidence intervals.
Background
Description of the condition
Herpes zoster is the result of reactivation of a prior varicella zoster virus infection. Herpes zoster ophthalmicus arises when a latent infection of the trigeminal ganglion is reactivated and involves the ophthalmic division of the trigeminal nerve (Arvin 1996). Approximately 10% to 20% of herpes zoster infections have an ophthalmic involvement, 20% to 70% of which end with ocular involvement (Ragozzino 1982; Womack 1983). Accordingly, effective treatment for the prevention of ocular involvement is necessary, as ocular involvement can lead to debilitating chronic pain and severe vision impairment.
Herpes zoster itself typically presents as an itchy and painful rash of limited duration. In the case of acute herpes zoster ophthalmicus, the dermatome of the ophthalmic division of the trigeminal nerve is affected. However, some patients suffer further complications and have a long lasting and severe course. In particular, the ocular complications of herpes zoster ophthalmicus, such as scleritis, uveitis, vasculitis, and especially acute retinal necrosis, represent high risk for the patient to develop significant vision loss (Kanski 2008). In addition, patients with herpes zoster ophthalmicus are at greater risk of developing postherpetic neuralgia and of developing persistent neuropathic pain that lasts long after the initial rash has healed (Gross 2003).
Description of the intervention
An aggressive management of acute herpes zoster with antiviral medication can reduce the duration and severity of the acute zoster manifestation, and in particular, in the case of herpes zoster ophthalmicus, may prevent more serious complications (Gross 2003). Accordingly, an aggressive management of acute herpes zoster ophthalmicus with potent antiviral medication is integral to most current treatment guidelines (Dworkin 2007; Gross 2003).
As one of the most commonly used antiviral drugs, acyclovir represents the mainstay of antiviral herpes zoster treatment. However, its poor bioavailability and need for frequent daily dosing prompted the development of later generation antiviral agents with improved pharmacokinetics and lower dosing frequency. Valacyclovir is a modified product of acyclovir (l‐valyl ester of acyclovir). After oral administration, it is rapidly converted to acyclovir in the gastrointestinal tract and liver. Thus, the plasma levels of acyclovir are three to five times greater after the oral intake of valacyclovir compared to the direct oral intake of acyclovir. Simplified and shortened medication regime should improve compliance and therefore, might also improve efficacy. Accordingly, in recent years, there has been an increasing amount of literature on the use of valacyclovir as an antiviral agent for the treatment of herpes zoster, and it is now considered a promising alternative to conventional acyclovir regimes.
In common ophthalmic literature, acyclovir and other oral antiviral medication (valacyclovir, famciclovir, brivudin) are reported as similar in efficiency (Cohen 2013; Kanski 2008; Pavan‐Langston 2008). Nevertheless, one trial showed an improved effect in immunocompetent adults having herpes zoster (Beutner 1995), while another reported no difference in patients with herpes zoster ophthalmicus (Colin 2000). Both focused on the properties of valacyclovir in comparison to acyclovir.
How the intervention might work
Antiviral medication is integral to most current treatment guidelines and represents an important pillar in the treatment of herpes zoster ophthalmicus (Dworkin 2007; Gross 2003). Acyclovir (standard dose for herpes zoster ophthalmicus: 800 mg five times daily for seven to 10 days) and valacyclovir (standard dose for herpes zoster ophthalmicus: 1000 mg three times daily for seven days) have both been approved for the treatment of herpes zoster, and are widely used (Dworkin 2007). Both acyclovir and valacyclovir work by stopping the herpes zoster virus from reproducing and infecting more cells. Inside the cells of the body, acyclovir is phosphorylated specifically by the viral thymidine kinases, and thus selectively activated in those cells that are infected with herpes zoster. After being activated by the addition of phosphate groups, acyclovir is incorporated into the viral deoxyribonucleic acid (DNA) strand by the enzyme DNA polymerase. Once incorporated, acyclovir acts as a DNA chain terminator. By blocking the action of the viral DNA polymerase, acyclovir prevents the herpes zoster virus from multiplying. This controls the infection and helps the immune system to deal with it.
Valacyclovir is the esterified version of acyclovir and hence, an acyclovir prodrug. It is characterised by greater bioavailability than acyclovir. This higher oral bioavailability is mediated by a carrier‐mediated intestinal absorption (the human intestinal peptide transporter (Gou 1999)), followed by a rapid conversion into its active form, acyclovir, by ester hydrolysis in the small intestine (De Clercq 2006). During this rapid first‐pass metabolism, valacyclovir is split into acyclovir and the essential amino acid valine (Perry 1996). The steady‐state plasma concentration of acyclovir after oral doses of valacyclovir (1 gram three times daily) is described to be similar to those after intravenous acyclovir (three times daily) application (Ormrod 2000). Similarly, the vitreous penetration of orally administrated valacyclovir is comparable to that of intravenous acyclovir, at least in non‐inflamed eyes (Huynh 2008). Therefore, valacyclovir might be equal to intravenous acyclovir administration and possibly superior to oral acyclovir administration, resulting in less complications in patients with herpes zoster ophthalmicus.
Why it is important to do this review
Although it is known that valacyclovir has an improved bioavailability and steadier plasma concentrations, it is currently unclear whether this finally leads to comparable treatment results and less ocular complications. Therefore, an up‐to‐date systematic review is warranted to compare the effects of valacyclovir versus acyclovir for the systemic antiviral treatment of herpes zoster ophthalmicus.
Objectives
To assess the effects of valacyclovir versus acyclovir for the systemic antiviral treatment of herpes zoster ophthalmicus in immunocompetent patients.
Methods
Criteria for considering studies for this review
Types of studies
This review was conducted according to our published Cochrane protocol (Schuster 2015). We included randomised controlled trials (RCTs) only. We did not use sample size, language, or publication status to determine whether or not a study was included.
Types of participants
Immunocompetent adults of both sexes, with a clinical diagnosis of herpes zoster affecting the ophthalmic part of the trigeminal nerve. We excluded studies with participants who had a compromised immune system, such as patients with acquired immune deficiency syndrome (AIDS), or patients treated with immunosuppressive drugs. We included studies that included subsets of relevant participants if the data for the relevant subsets were reported separately (in such cases, we only included the data for the relevant subsets).
Types of interventions
We considered any trial where systemic valacyclovir was compared to systemic acyclovir medication, at any dose, for the treatment of herpes zoster ophthalmicus. There was no restriction to any type or location of the health service (primary, secondary and tertiary care). We included studies regardless of the time of onset of intervention after first symptoms.
Types of outcome measures
From each trial, we selected the measure considered to be most appropriate for each of the pre‐defined outcomes of this review. If an outcome had been reported in several different ways, preference was given to the outcome measure that was used and documented frequently in the field, as opposed to a novel or not validated measure. The time point of most outcome measures was defined as 12 months after primary infection, defined as a period of six to 18 months. Time points for occurrence are meant as any occurrence up to this time point and do not mean persistent occurrence up to this time point. In cases in which time points of outcome measures were only available at other points of time, data were not included in the primary analyses, but reported descriptively.
While the primary outcome summarizes any occurrence of ocular involvement during the disease, secondary outcomes did differentiate ocular manifestation into intraocular involvement (defined as severe manifestation due to risk of blindness) and superficial ocular affection (defined as simple ocular manifestation).
Primary outcomes
The primary outcome measure was
Occurence of ocular involvement (time point: 12 months after infection), defined as signs of any ocular manifestations (e.g. conjunctivitis, superficial keratitis, stromal keratitis, dendritic ulcer, scleritis, uveitis, vasculitis, optic neuritis, chorioretinitis, acute retinal necrosis)
Secondary outcomes
Secondary outcome measures included:
Occurrence of severe ocular manifestation (Intraocular involvement: e.g. scleritis, uveitis, vasculitis, optic neuritis, chorioretinitis, acute retinal necrosis; time point: 12 months after infection).
Occurrence of simple ocular manifestation (primary superficial ocular affection: e.g. conjunctivitis, superficial keratitis, episcleritis, stromal keratitis, dendritic ulcer; time point: 12 months after infection).
Occurrence of vision loss (best corrected visual acuity of 6/60 or less; time point: 12 months after infection).
Occurrence and severity (pain intensity) of postherpetic neuralgia (time point: 12 months after infection).
Second eye involvement in ocular manifestation of herpes zoster ophthalmicus (time point: more than 12 months after infection).
Quality of life (if assessed by standardised questionnaire; time points: under antiviral medication, three months after treatment and 12 months after treatment).
Adverse effects
Adverse effects (diarrhoea, vomiting, constipation, dizziness, headache, death, others) were also recorded within 12 months after infection.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register (2016, Issue 5)), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to June 2016), Embase (January 1980 to June 2016), Web of Science Conference Proceedings Citation Index‐Science (CPCI‐S) (January 1990 to June 2016), BIOSIS Previews (January 1969 to June 2016), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 13 June 2016.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), Embase (Appendix 3), CPCI‐S (Appendix 4), BIOSIS (Appendix 5), ISRCTN (Appendix 6), ClinicalTrials.gov (Appendix 7) and the ICTRP (Appendix 8).
Searching other resources
In addition, we searched the reference lists of all included studies and current reviews and guidelines for the management of herpes zoster ophthalmicus, and contacted corresponding authors of guidelines for management of herpes zoster. We also contacted the companies selling valacyclovir currently to the European and US market, and asked whether there were additional studies comparing valacyclovir to acyclovir for the treatment of herpes zoster ophthalmicus.
Data collection and analysis
Selection of studies
Two review authors (AKS, JT) independently assessed titles and abstracts from the search results and scanned them against the inclusion criteria. Initially, a list was made for included and excluded studies, as well as for studies assessed as unsure. The two review authors (AKS, JT) then discussed these lists. For potentially relevant studies, the two review authors independently read the full‐text articles to determine whether the articles met the pre‐specified selection criteria. They discussed disagreements in order to make a final decision; if agreement between the two authors was not achieved, a third review author (BCH) was contacted to reach final consent.
Data extraction and management
Two review authors (AKS, JT) independently extracted the data of the identified studies (study characteristics, study results and assessments of the risk of bias), using a pre‐specified data extraction form. The review authors (AKS, JT) met to double‐check all discrepancies; if agreement between them was not achieved, a third review author (BCH) was contacted to reach final consent.
If a publication required translation, the two review authors independently extracted relevant data from the translated article and sought further quality checks from the translator. If there were missing or unclear data, we contacted the corresponding authors twice, at least three months apart, for further information (Beutner 1995, Colin 2000).
We collected demographic data of participants, and if mentioned, previous ophthalmic diseases, interventions or operations, and follow‐up intervals for all primary outcomes and their corresponding findings.
In the description of study characteristics, we presented data as reported in the original publications.
All data were entered into Review Manager 5.3 (RevMan 2014) by one review author (AKS); another review author (JT) then compared the entered data against the data extraction forms.
Assessment of risk of bias in included studies
We used Cochrane's 'risk of bias' tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews and Interventions (Higgins 2011). For missing information about study design, we contacted the corresponding authors twice (at least three months apart) for further information. Two review authors (AKS, JT) independently performed 'risk of bias' assessment; disagreements were solved in a meeting. If necessary, a third review author (BCH) was contacted to reach final consent.
Measures of treatment effect
Due to the low number of studies, we abstained from combining studies in a meta‐analysis and reported the results descriptively instead, by calculating measures of treatment effect for the included study. If we would have found more studies, we had planned to calculate effect measures as mean differences (MD) for continuous data (time point of becoming acute pain free, time point of skin healing, severity of postherpetic neuropathy and quality of life. Data in publications only allowed us to report proportion of persistent ocular lesions and persistent pain six months after treatment. When assessment procedures varied in scale or principle of measuring method, we would have summarised continuous outcomes as standardized mean differences.
We calculated risk ratios (RR) for dichotomous data (occurrence of postherpetic neuropathy, occurrence of simple and severe ocular manifestations, occurrence of adverse effects). We calculated the 95% confidence intervals (CIs) for all outcomes. We reported categorical data descriptively.
Unit of analysis issues
As the chosen medication was administered systemically, we analysed participants based on their randomisation to treatment type, and not on individual eyes. This is in accordance with the outcomes defined for this review that relate to participants and not to eyes. Bilateral herpes zoster ophthalmicus is infrequent except in patients with acute retinal necrosis syndrome (which is a rare major ocular complication), so we anticipated that one eye per person would be reported.
Dealing with missing data
Due to the low number of studies, we abstained from combining studies in a meta‐analysis and reported the results descriptively instead. Nevertheless, if outcome data (loss to follow‐up data or non‐reporting outcome data) were missing or incomplete, we contacted the corresponding authors twice, at least three months apart, for further information.
Assessment of heterogeneity
Due to the low number of studies, we abstained from combining studies in a meta‐analysis and reported the results descriptively instead. We had prespecified that heterogeneity among enough trials would be assessed using the heterogeneity statistics Chi² and I², complemented by visual exploration of forest plots. We had planned to consider I² values above 50% as substantial heterogeneity (Higgins 2002). For testing the significance of heterogeneity, we would have considered the Chi² statistic; P = 0.1 or less would indicate significant heterogeneity.
Assessment of reporting biases
Due to the low number of studies, we abstained from combining studies in a meta‐analysis and reported the results descriptively instead. If we had included 10 or more studies in the review, we had planned to assess potential publication bias using a funnel plot. Funnel plots with fewer than 10 trials should be avoided, as the power of both visual inspection and regular testing is small when fewer than 10 trials are plotted (Sterne 2011). In addition, we had planed to assess selective outcome reporting bias by using an outcome reporting matrix (the ORBIT classification), as previously described by others (Kirkham 2010).
Data synthesis
Due to the low number of studies, we abstained from combining studies in a meta‐analysis and reported the results descriptively instead. We assessed the certainty of the level of evidence for the selected outcomes using the GRADE approach (GRADEpro 2014).
We had planned to compare and combine studies using a DerSimonian‐Laird random‐effects model to calculate pooled estimates with 95% CIs (DerSimonian 1986). A random‐effects model was chosen because we expected that studies would differ in the nature of patients (e.g. Caucasian, Asian etc), age, and gender (the ratio of women to men). We had planned to further analyse any results with I² values over 50% for sources of clinical heterogeneity and methodological differences. If no methodological or clinical reasons could be found to explain strong statistical heterogeneity,we would not have proceeded with the meta‐analysis. If only a very small number of trials had met the inclusion criteria, we had planned to report the results descriptively and not perform a meta‐analysis. All secondary outcomes were analysed in an explorative way.
Subgroup analysis and investigation of heterogeneity
Due to the low number of studies, we abstained from performing subgroup analyses or analyses of heterogeneity.
Had we included more studies, we had planned to perform subgroup analyses and investigate heterogeneity for:
Starting point of therapy: comparing participants with initial treatment (up to and including 72 hours) to participants with delayed start of treatment (longer than 72 hours).
-
Dosage regime: comparing standard dosage regime (valacyclovir: 1000 mg three times daily for at least seven days, oral acyclovir: 800 mg five times daily for at least seven days, intravenous acyclovir: concentration of 15 mg per kilogram bodyweight per day for seven days) to other dosage regimes.
Additional use of topical medication.
Time point of outcome observation after treatment.
Sensitivity analysis
Due to the low number of studies, we did not perform any sensitivity analyses. We had planned to conduct sensitivity analyses to determine the impact of excluding industry‐funded studies (industry‐funded studies versus industry‐independent studies), unpublished studies (full reports versus abstracts or unpublished), and studies with missing data.
Summary of findings table
In an amendment to our published protocol, we prepared a 'summary of findings' table and graded the certainty of the evidence using GRADE (GRADEpro 2014). GRADE considers the following criteria: study limitations, indirectness, imprecision, inconsistency, and publication bias.
We included the following outcomes in the summary of findings table.
Occurrence of ocular involvement
Ocular involvement ‐ dendritic ulcer
Ocular involvement ‐ uveitis
Pain at week 24 (self‐reported; yes)
Adverse effects ‐ vomiting
Adverse effects ‐ eyelid or facial oedema
Adverse effects ‐ disseminated herpes zoster
Time point for analysis was defined as 12 months, and if data were not available, the closest time point to 12 months in the time span of six to 18 months.
Results
Description of studies
Results of the search
The electronic searches yielded a total of 2072 references (Figure 1). The Cochrane Information Specialist (CIS) scanned the search results, removed 344 duplicates and then removed 1034 references which were not relevant to the scope of the review. We screened the remaining 694 reports and obtained the following 19 full‐text reports for further assessment: Anonymous 1996; Barsic 2004; Bell 1996; Beutner 1995; Carrington 1994; Chen 2006; Cochener 1997; Colin 1997; Colin 2000; Desmond 2002; Grant 1997; Grose 1997; Jubelt 2002; Li 1999; Lin 2001; Liu 2000; Wood 1998; Xu 2000; Yan 1999). This search resulted finally in three references meeting the prespecified inclusion criteria (Cochener 1997; Colin 1997; Colin 2000). These three references, however, related to the same study (publication: Colin 2000 and two meeting abstracts: Cochener 1997; Colin 1997).
1.
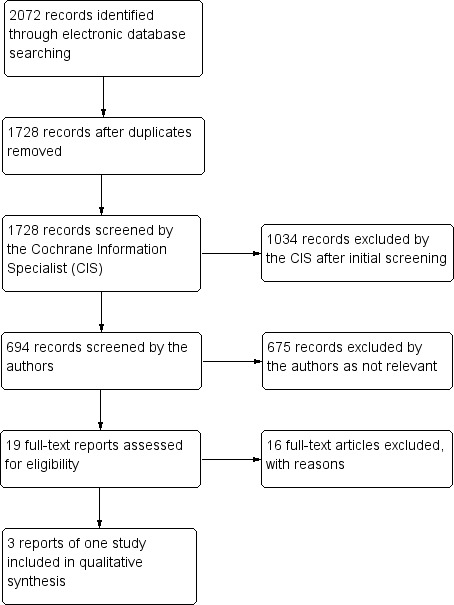
Study flow diagram.
Included studies
We included one study in this review, which reported on a comparison of valacyclovir to acyclovir in patients with herpes zoster ophthalmicus (Colin 2000).
Colin 2000 included 110 participants who had herpes zoster ophthalmicus in a multicentre, randomised, double‐masked study that compared valacyclovir 1000 mg three times daily for seven days to acyclovir 800 mg five times daily for seven days. This study was carried out in France. Main outcome measures were the frequency, severity, and duration of ocular complications, patient reports of zoster‐associated pain, and the healing progress of skin lesions. In addition, overall treatment tolerance was assessed, reporting incidence and types of adverse effects descriptively. This study only reported extractable data for time point 6 months.
Excluded studies
We excluded 16 studies after reviewing the full‐text copies. Reasons for excluding these studies can be found in the Characteristics of excluded studies table.
Risk of bias in included studies
As described in the following sections in more detail (and shown in Figure 2), the overall risk of bias, according to Higgins 2011 criteria, was unclear. Despite repeated inquiries, missing information were not provided by the study authors of the original study.
2.
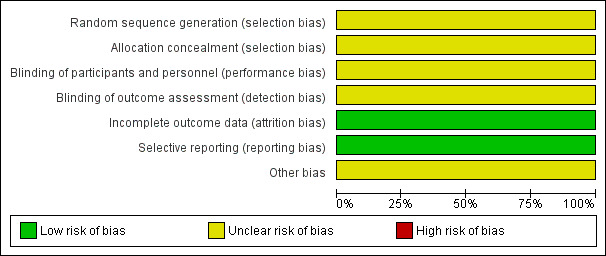
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Risk of selection bias was unclear as there was not sufficient information given by the authors.
Blinding
Masking of study medication was sufficiently described and carried out, but the masking of observers was unclear. Further, success and efficacy of masking was unclear.
Incomplete outcome data
Participants withdrawing informed consent was explained in detail. In addition, the authors used an intention‐to‐treat analysis. Thus there was low risk of attrition bias.
Selective reporting
Selective reporting bias could not be safely ruled out as an a priori published protocol of the study was not available. When comparing the publication Colin 2000 with the two published abstracts Colin 2000, we did not find differences with respect to reported outcomes.
Other potential sources of bias
Some of the study authors were sponsored by the pharmaceutical company marketing valacyclovir, and it was unclear whether a conflict of interest existed.
Effects of interventions
See: Table 1
As only one study was included in the review, we did not carry out a meta‐analysis but report the findings of this single study descriptively.
Colin 2000 reported similar incidence of ocular complications between the two study groups being treated with either valacyclovir or acyclovir. At enrolment, 13% (7/56) of the participants in the valacyclovir group and 7% (4/54) in the acyclovir group already had ocular involvement. Primary ocular complications in the observation time (up to six months) were: conjunctivitis in 54% (30/56) of the valacyclovir group and 52% (28/54) of the acyclovir group (relative risk (RR) 1.03; 95% confidence interval (CI 0.72 to 1.47)), punctate keratitis in 39% (22/56) and 48% (26/54), respectively (RR 0.82 (95% CI 0.53 to 1.25), and dendritic keratitis in 11% (6/56 and 6/54) of each group (RR 0.96 (95% CI 0.33 to 2.81); Analysis 1.1; Table 2). The total number of participants with dendritic ulcer (3/56 and 1/54, respectively) and episcleritis (4/56 and 1/54, respectively) were small and did not show a statistical difference between the two groups (Analysis 1.1). Participants with ocular complications of scleritis, vasculitis, optic neuritis, chorioretinitis, or acute retinal necrosis were not reported in the included publication (Colin 2000) and therefore these complications could not be analysed. Persistent ocular lesions after six months were reported in 2/56 in the valacyclovir group and 1/54 in the acyclovir group (RR 1.93 (95% CI 0.18 to 20.65)) (Analysis 1.1).
1.1. Analysis.
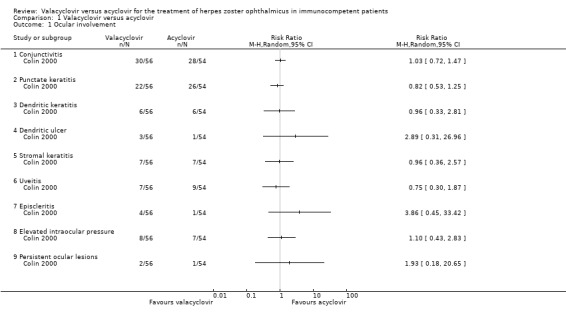
Comparison 1 Valacyclovir versus acyclovir, Outcome 1 Ocular involvement.
1. Ocular involvement.
| Valacyclovir versus acyclovir in the systemic treatment of herpes zoster ophthalmicus in immunocompetent patients to reduce ocular involvement | ||||||
| Patient or population: Adult immunocompetent patients with herpes zoster ophthalmicus Setting: No restriction on the type or location of the health service (primary, secondary and tertiary care) Intervention: Systemic valacyclovir Comparison: Systemic acyclovir | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with acyclovir | Risk with Valacyclovir | |||||
| Development of conjunctivitis | 519 per 1000 | 534 per 1000 (373 to 762) | RR 1.03 (0.72 to 1.47) | 110 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ‐ |
| Development of punctate keratitis | 481 per 1000 | 395 per 1000 (255 to 602) | RR 0.82 (0.53 to 1.25) | 110 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ‐ |
| Development of dendritic keratitis | 111 per 1000 | 107 per 1000 (37 to 312) | RR 0.96 (0.33 to 2.81) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 | ‐ |
| Development of dendritic ulcer | 19 per 1000 | 54 per 1000 (6 to 499) | RR 2.89 (0.31 to 26.96) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 | ‐ |
| Development of stromal keratitis | 130 per 1000 | 124 per 1000 (47 to 333) | RR 0.96 (0.36 to 2.57) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 | ‐ |
| Development of uveitis | 167 per 1000 | 125 per 1000 (50 to 312) | RR 0.75 (0.30 to 1.87) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 | ‐ |
| Development of episcleritis | 19 per 1000 | 71 per 1000 (8 to 619) | RR 3.86 (0.45 to 33.42) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 | ‐ |
| Development of elevated intraocular pressure | 130 per 1000 | 143 per 1000 (56 to 367) | RR 1.10 (0.43 to 2.83) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded 1 level for risk of bias, as the authors did not report on random sequence generation, allocation concealment and masking of staff, and the extent to which bias had been avoided was largely unclear (‐1).
2 We downgraded 1 level for imprecision as confidence intervals were wide and compatible with both benefit or harm.
3 We downgraded 2 levels for imprecision as there were few events and very wide confidence intervals.
Zoster‐associated pain was comparable between the two study groups at all examined time points (valacyclovir versus acyclovir; presentation: 51/56 versus 47/54, week four: 14/56 versus 17/54, week eight: 8/56 versus 10/54, week 16: 3/56 versus 6/54, week 24: 3/56 versus 3/54; Table 2; Analysis 1.2). Healing of skin lesions was comparable in both groups. The incidence of adverse effects was similar in both groups. The most frequent adverse effects were vomiting (5% versus 3%; RR 1.45 (95% CI 0.25 to 8.32; Analysis 1.3), and eyelid or facial oedema (2% versus 5%; RR 0.32 (95% CI 0.03 to 2.00; Analysis 1.3; Table 3). Three serious adverse events were observed, two in the valacyclovir group (rectorrhagia, kidney stone; Analysis 1.3; Table 3) and one in the acyclovir group (disseminated zoster; Analysis 1.3).
1.2. Analysis.
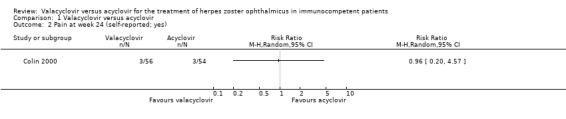
Comparison 1 Valacyclovir versus acyclovir, Outcome 2 Pain at week 24 (self‐reported; yes).
1.3. Analysis.
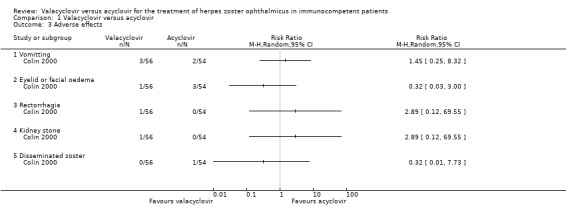
Comparison 1 Valacyclovir versus acyclovir, Outcome 3 Adverse effects.
2. Adverse effects.
| Valacyclovir versus acyclovir in the systemic treatment of herpes zoster ophthalmicus in immunocompetent patients ‐ an analysis of adverse effects | ||||||
| Patient or population: Adult immunocompetent patients with herpes zoster ophthalmicus Setting: No restriction on the type or location of the health service (primary, secondary and tertiary care) Intervention: Systemic valacyclovir Comparison: Systemic acyclovir | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with acyclovir | Risk with Valacyclovir | |||||
| Vomiting | 37 per 1000 | 54 per 1000 (9 to 308) | RR 1.45 (0.25 to 8.32) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ‐ |
| Eyelid or facial oedema | 56 per 1000 | 18 per 1000 (2 to 167) | RR 0.32 (0.03 to 3.00) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ‐ |
| Rectorrhagia | 0 per 1000 | 0 per 1000 (0 to 0) | RR 2.89 (0.12 to 69.55) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ‐ |
| Kidney stone | 0 per 1000 | 0 per 1000 (0 to 0) | RR 2.89 (0.12 to 69.55) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ‐ |
| Disseminated zoster | 19 per 1000 | 6 per 1000 (0 to 143) | RR 0.32 (0.01 to 7.73) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded 1 level for risk of bias, as the authors did not report on random sequence generation, allocation concealment and masking of staff, and the extent to which bias had been avoided was largely unclear (‐1).
2 We downgraded 2 levels for imprecision as there were few events and very wide confidence intervals
We downgraded the certainty of the evidence due to concerns about study limitations (downgraded one level) and precision of the effect estimates (downgraded one or two levels) (Table 1).
Individual data were not provided in a way which would have allowed stratification into any occurrence of ocular involvement, or occurrence of simple or severe ocular manifestations. Therefore we reported the different diseases descriptively and not the initially intended classification into simple and severe manifestation.
Discussion
The present study analysed whether the occurrence of complications for herpes zoster ophthalmicus differed when treated with valacyclovir compared to acyclovir. The most obvious finding to emerge from this review was that available data for the use of valacyclovir in the treatment of herpes zoster ophthalmicus in immunocompetent people were sparse. Our systematic search of the scientific literature revealed that, thus far, only one study was specifically tailored to examine whether the well‐established and widely applied treatment approach with valacyclovir was comparable to the standard treatment regimen using acyclovir. Thus, we concluded that there was currently only weak clinical trial evidence for the use of systemic valacyclovir in the treatment of herpes zoster ophthalmicus in immunocompetent people.
This was especially the case for severe complications, which however, were considered to be the most relevant reason for using aggressive antiviral medication in this condition. Thus far, there was only one single comparative study that included a total of 110 participants. Therefore, based on the generally low prevalence of severe complications in immunocompetent people, the total number of participants with dendritic ulcer and episcleritis in this study was too small to draw any conclusion about differences between acyclovir and valacyclovir in this condition. Furthermore, participants with scleritis, vasculitis, optic neuritis, chorioretinitis, or acute retinal necrosis did not present in this study at all. Nevertheless, treatment with valacyclovir might be efficacious in these conditions.
Summary of main results
One study fulfilled the inclusion criteria and compared valacyclovir to acyclovir in immunocompetent patients with herpes zoster ophthalmicus. In this multicentre, randomised double‐masked study, 110 participants with herpes zoster ophthalmicus, diagnosed within 72 hour of skin eruption, were treated. The most frequent ocular complications noted in each group were conjunctivitis (54% valacyclovir and 52% acyclovir), superficial keratitis (39% and 48%, respectively), and dendritic keratitis (11% in each group). Further, post‐herpetic pain, tolerability of medication, and side‐effect profiles were equivalent between the two groups, indicating that both medications were efficacious for the treatment of herpes zoster ophthalmicus.
Overall completeness and applicability of evidence
As there was only one randomised controlled trial included in this systematic review, there was only limited evidence for the use of valacyclovir compared to acyclovir in herpes zoster ophthalmicus. Treatment for other patient groups, such as patients with HIV infection, is currently under investigation and for this purpose, a Cochrane protocol has been published (Olusanya 2010). Arora et al. reported that the use of valacyclovir in two dosages were safe and efficacious therapies for reduction of zoster‐associated pain and zoster‐associated abnormal sensation in patients who were immunocompromised (Arora 2008).
Quality of the evidence
Due to concerns about imprecision (due to small number of events and large confidence intervals) and relevant study limitations (random sequence generation, allocation concealment and masking of staff), the certainty of evidence was downgraded from high to low and very low. The included study reported a similar incidence of ocular complications, and comparable zoster‐associated pain and rate of healing of skin lesions between the two study groups being treated with either valacyclovir or acyclovir. For adverse events, similar frequencies were reported. When analysing reported adverse events, it remains unclear whether eyelid and facial oedema is the consequence of therapy or rather reflects insufficient therapy.
Potential biases in the review process
As we could only include one study in this review, there might be a risk of publication bias regarding other conducted but not registered or published studies. We systematically searched available clinical trial registrations, but registration of a clinical trial was not available in the 1990s.
Agreements and disagreements with other studies or reviews
Similar results as in the included study were reported by another study that provided a subgroup analysis of participants with herpes zoster ophthalmicus based on the primary inclusion criteria of herpes zoster (Beutner 1995). This study was conducted as a multicentre, randomised, three‐arm, double‐masked, double‐dummy study. In a subgroup analysis, Beutner and colleagues included 119 participants with herpes zoster ophthalmicus, which were randomised to either valacyclovir 1000 mg three times daily for seven days, to valacyclovir 1000 mg three times daily for 14 days, or to acyclovir 800 mg five times daily for seven days. They demonstrated comparable duration of pain, similar duration of abnormal sensations, comparable time to cessation of new lesion formation, and similar time to development of at least 50% crusting or healing of the rash between the three study groups. Ocular involvement was present in 34 (29%) participants with ophthalmic herpes zoster at presentation. During the observation period, an additional 17 participants presented with ocular involvement. Twenty‐three participants had serious ocular involvement (keratitis, uveitis, iritis, corneal, or scleral involvement), while 28 participants had minor ocular involvement (conjunctivitis, 'red eye', or excessive lacrimation). In more than 90% of these participants, the ocular involvement resolved within five weeks.
There are severe ocular involvements stated to be related to herpetic diseases. Two case reports described sufficient therapies using oral valacyclovir in patients with acute retinal necrosis (Emerson 2006; Taylor 2012). Taking into account the low number of events for severe complications overall, it must be assumed that the statistical power to detect differences was insufficient, and further research should be undertaken to investigate the effects of valacyclovir on the occurrence of rare but clinically relevant ocular complications.
However, the uncertainty of evidence in our review does not necessarily indicate that valacyclovir is less efficacious. A recent systematic review showed that oral treatment with valacyclovir compared to acyclovir resulted in a significant reduction in risk of herpes zoster‐associated pain up to 112 days in participants with herpes zoster, including ophthalmicus, but did not further evaluate ophthalmic complications (McDonald 2012).
Authors' conclusions
Implications for practice.
Based on the one included study, there is uncertainty in evidence that valacyclovir as systemic treatment option for herpes zoster ophthalmicus is different or comparable to acyclovir. Valacyclovir may offer theoretical advantages, especially in cases in which the patient's compliance is not consistent. In such cases, the greater bioavailability and simplified dosing regimen of valacyclovir may allow for improved compliance rates (De Clercq 2006).
Implications for research.
As there is only one randomised controlled trial comparing systemic valacyclovir to acyclovir in patients with herpes zoster ophthalmicus, further well‐designed RCTs are needed to investigate valacyclovir in the treatment of herpes zoster ophthalmicus. Sample size should be calculated to meet rare complications, such as severe intraocular complications; the follow‐up period should be at least six months to investigate permanent post‐herpetic neuralgia.
Acknowledgements
We acknowledge assistance from the Cochrane Eyes and Vision (CEV) for creating and executing the electronic search strategies. We thank Catey Bunce, Simon Taylor, and Stephanie Watson for their comments on the protocol and review and Jennifer Evans and Anupa Shah for their assistance throughout the review process.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Herpes Zoster Ophthalmicus] this term only #2 MeSH descriptor: [Herpes Zoster] this term only #3 MeSH descriptor: [Herpesvirus 3, Human] this term only #4 human herpesvirus 3 or human herpes virus 3 #5 (herpes or varicella) near/2 zoster* #6 MeSH descriptor: [Chickenpox] this term only #7 chicken pox or chickenpox #8 shingles #9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 #10 MeSH descriptor: [Acyclovir] this term only #11 acyclovir or aciclovir #12 #10 or #11 #13 valacyclovir or valaciclovir or valciclovir or valcyclor or valcyclovir #14 #9 and #12 and #13
Appendix 2. MEDLINE (Ovid) search strategy
1. randomised controlled trial.pt. 2. (randomised or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. Herpes Zoster Ophthalmicus/ 14. Herpes Zoster/ 15. Herpesvirus 3, Human/ 16. (human herpesvirus 3 or human herpes virus 3).tw. 17. ((herpes or varicella) adj2 zoster$).tw. 18. Chickenpox/ 19. (chicken pox or chickenpox).tw. 20. shingles.tw. 21. or/13‐20 22. Acyclovir/ 23. (acyclovir or aciclovir).tw. 24. or/22‐23 25. (valacyclovir or valaciclovir or valciclovir or valcyclor or valcyclovir).tw. 26. or/13‐21 27. 21 and 24 and 26 28. 12 and 27
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase (Ovid) search strategy
1. exp randomised controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. Herpes Zoster Ophthalmicus/ 34. Herpes Zoster/ 35. Varicella Zoster Virus/ 36. (human herpesvirus 3 or human herpes virus 3).tw. 37. ((herpes or varicella) adj2 zoster$).tw. 38. Chickenpox/ 39. (chicken pox or chickenpox).tw. 40. shingles.tw. 41. or/33‐40 42. Aciclovir/ 43. (acyclovir or aciclovir).tw. 44. or/42‐43 45. valaciclovir/ 46. (valacyclovir or valaciclovir or valciclovir or valcyclor or valcyclovir).tw. 47. or/45‐46 48. 41 and 44 and 47 49. 32 and 48
Appendix 4. Web of Science Conference Proceedings Citation Index ‐ Science (CPCI‐S) search strategy
#11 #8 AND #9 AND #10 #10 TS=(valacyclovir or valaciclovir or valciclovir or valcyclor or valcyclovir) #9 TS=(acyclovir or aciclovir) #8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 #7 TS=shingles #6 TS=(chicken pox OR chickenpox) #5 TS=varicella zoster #4 TS= (human herpesvirus 3 OR human herpes virus 3) #3 TS=Herpesvirus 3, Human #2 TS= Herpes Zoster #1 TS=Herpes Zoster Ophthalmicus
Appendix 5. BIOSIS search strategy
#11 #8 AND #9 AND #10 #10 TS=(valacyclovir or valaciclovir or valciclovir or valcyclor or valcyclovir) #9 TS=(acyclovir or aciclovir) #8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 #7 TS=shingles #6 TS=(chicken pox OR chickenpox) #5 TS=varicella zoster #4 TS= (human herpesvirus 3 OR human herpes virus 3) #3 TS=Herpesvirus 3, Human #2 TS= Herpes Zoster #1 TS=Herpes Zoster Ophthalmicus
Appendix 6. ISRCTN search strategy
(herpes zoster OR varicella zoster OR herpesvirus OR chicken pox OR chickenpox OR shingles) AND (acyclovir OR aciclovir) AND (valacyclovir OR valaciclovir OR valciclovir OR valcyclor OR valcyclovir) AND (eye OR ophthalmic OR ophthalmicus OR ocular)
Appendix 7. ClinicalTrials.gov search strategy
(herpes zoster OR varicella zoster OR herpesvirus OR chicken pox OR chickenpox OR shingles) AND (acyclovir OR aciclovir) AND (valacyclovir OR valaciclovir OR valciclovir OR valcyclor OR valcyclovir) AND (eye OR ophthalmic OR ophthalmicus OR ocular)
Appendix 8. ICTRP search strategy
herpes zoster OR varicella zoster OR herpesvirus OR chicken pox OR chickenpox OR shingles = Condition AND acyclovir OR aciclovir = Intervention
Data and analyses
Comparison 1. Valacyclovir versus acyclovir.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ocular involvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Conjunctivitis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Punctate keratitis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Dendritic keratitis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Dendritic ulcer | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Stromal keratitis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Uveitis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Episcleritis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Elevated intraocular pressure | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.9 Persistent ocular lesions | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Pain at week 24 (self‐reported; yes) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Vomitting | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Eyelid or facial oedema | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Rectorrhagia | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Kidney stone | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Disseminated zoster | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Colin 2000.
| Methods | Multicentre, randomised, double‐masked study that was conducted in France between 1 July 1994 and 16 October 1995. | |
| Participants | Immunocompetent patients aged 18 years or older with herpes zoster ophthalmicus, diagnosed within 72 hours of skin eruption, were eligible for this study. People with other pre‐existing ocular disorders, renal failure, immune dysfunction, or diseases necessitating cytotoxic or immunosuppressive treatment, liver failure, or intolerance or hypersensitivity to acyclovir were ineligible. Women of childbearing age who were not using effective contraceptions or who were pregnant or breast‐feeding were excluded. |
|
| Interventions | Participants were randomly allocated to either valacyclovir (1 g three times daily for 7 days) or acyclovir (800 mg five times daily for 7 days), the other tablets of the five‐times daily regime were substituted with placebo tablets. | |
| Outcomes | The aim was to compare efficacy and safety of valacyclovir and acyclovir. Ocular symptoms, ocular movement, pupil diameter and reflexes, conjunctivae, sclera and episclera, cornea, anterior chamber, ocular tension, and retina were investigated. The ophthalmic examination focused on the frequency, severity and duration of ocular complications. Participants assessed ocular pain severity on a 4‐point scale (0 to 3) and on a visual analogue scale. Sensorineural disorders and healing of skin lesions were assessed at each visit. Tolerance was assessed on the basis of adverse effects and changes in laboratory parameters on days 1 (inclusion) and 7. | |
| Notes | The study was supported by Glaxo Wellcome, France. There was no declaration of interest among the primary researchers. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sufficient information is not given by the authors. |
| Allocation concealment (selection bias) | Unclear risk | Sufficient information is not given by the authors. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Sufficient information is not given by the authors. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Sufficient information is not given by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants withdrawing informed consent is explained in detail. |
| Selective reporting (reporting bias) | Low risk | Reported outcomes of the two abstracts and the main publication did not differ. |
| Other bias | Unclear risk | Some of the study authors were sponsored by the pharmaceutical company marketing valacyclovir and it is unclear whether a conflict of interest exists. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anonymous 1996 | Review article without primary data. |
| Barsic 2004 | Participants did not have herpes zoster ophthalmicus, but genital herpes or varicella zoster virus infections. |
| Bell 1996 | Review article without primary data. |
| Beutner 1995 | Randomisation was performed without regard to localisation of herpes zoster. Frequency of ocular complications was reported overall and not separately for valacyclovir or acyclovir. We were not able to get in contact with the authors. |
| Carrington 1994 | Review article without primary data. |
| Chen 2006 | No subgroup of participants with herpes zoster ophthalmicus was reported. |
| Desmond 2002 | Model building based on data of other primary studies. No primary participant data. |
| Grant 1997 | Cost‐consequence model based on data of other primary studies. No primary participant data. |
| Grose 1997 | Review article. No primary participant data. |
| Jubelt 2002 | Secondary report of a study comparing valacyclovir to famciclovir. No control group with acyclovir. |
| Li 1999 | No subgroup of participants with herpes zoster ophthalmicus was reported. |
| Lin 2001 | No subgroup of participants with herpes zoster ophthalmicus was reported. |
| Liu 2000 | No subgroup of participants with herpes zoster ophthalmicus was reported. |
| Wood 1998 | Secondary data analysis based on data of two primary studies. No primary participant data. |
| Xu 2000 | No treatment with valacyclovir in the treatment group. |
| Yan 1999 | We were unable to obtain a copy of this paper. |
Differences between protocol and review
According to the prespecified protocol, an estimation of distribution for non‐continuous data as suggested by Altman 1996 was originally planned, as well as a transformation of data if necessary (Schuster 2015). However, due to the low number of studies, we abstained from performing any meta‐analytic analyses, but reported data descriptively.
As the primary outcome "occurrence of ocular involvement" could not be determined based on the study reports, we included "persistent ocular lesions" as outcome. This outcome summarizes ocular involvements being persistent till the end of the study (six months).
Contributions of authors
The review was written by AK Schuster (AKS), J Tesarz (JT), MN Jarczok (MNJ), BC Harder (BCH), and FC Schlichtenbrede (FCS). AKS and JT developed the first draft of the protocol, MNJ made corrections and contributions to the statistical section, BCH and FCS gave clinical advice on its importance, the selection of outcomes, their measurement, and the subgroup analysis. They made corrections and contributions to these paragraphs. All authors approved the final review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEV Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
AKS: None known. BCH: None known. FCS: None known. MNJ: None known. JT: None known.
New
References
References to studies included in this review
Colin 2000 {published data only}
- Cochener B, Hoang‐Xuan T, Rolland B, Colin J. A double blind randomized trial to compare safety and efficacy of valaciclovir and aciclovir for treatment of herpes zoster ophthalmicus. Investigative Ophthalmology and Visual Science. 1997; Vol. 38:ARVO E‐Abstract 965.
- Colin J, Cochener B, Lescale O, Hannouche D, Hoang‐Xuan T. Treatment of herpes zoster ophthalmicus: a double‐blind trial to compare valaciclovir and aciclovir. American Academy of Opththalmology. 1997:170. [DOI] [PubMed]
- Colin J, Prisant O, Cochener B, Lescale O, Rolland B, Hoang‐Xuan T. Comparison of the efficacy and safety of valaciclovir and acyclovir for the treatment of herpes zoster ophthalmicus. Ophthalmology 2000;107(8):1507‐11. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anonymous 1996 {published data only}
- Anonymous. Valacyclovir. Medical Letter on Drugs and Therapeutics 1996;38:3‐4. [PubMed] [Google Scholar]
Barsic 2004 {published data only}
- Barsic B, Begovac J, Mahovlic V, Dostal M, Stojkovic A, Topolovic Z, et al. Randomized, open, comparative, multicentric, pilot study on the efficacy and tolerability of peroral valacyclovir and acyclovir in patients with genital herpes and varicella zoster virus infections. Infektoloski Glasnik 2004;24(4):189‐92. [Google Scholar]
Bell 1996 {published data only}
- Bell AR, Murray A, Shukla S, Crooks RJ. New zoster treatment. Can aciclovir be improved?. Chemotherapie Journal 1996;5(10):20‐3. [Google Scholar]
Beutner 1995 {published data only}
- Beutner KR, Friedman DJ, Forszpaniak C, Andersen PL, Wood MJ. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrobial Agents and Chemotherapy 1995;39(7):1546‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carrington 1994 {published data only}
- Carrington D. Prospects for improved efficacy with antiviral prodrugs: Will valaciclovir and famciclovir meet the clinical challenge?. International Antiviral News 1994;2(4):50‐3. [Google Scholar]
Chen 2006 {published data only}
- Chen A, Sun X. Comparative study of treatment of herpes zoster with valaciclovir and acyclovir. Wuhan Daxue Xuebao (Yixue Ban) 2006;27(4):537‐8. [Google Scholar]
Desmond 2002 {published data only}
- Desmond RA, Weiss HL, Arani RB, Soong SJ, Wood MJ, Fiddian PA, et al. Clinical applications for change‐point analysis of herpes zoster pain. Journal of Pain and Symptom Management 2002;23(6):510‐6. [DOI] [PubMed] [Google Scholar]
Grant 1997 {published data only}
- Grant DM, Mauskopf JA, Bell L, Austin R. Comparison of valaciclovir and acyclovir for the treatment of herpes zoster in immunocompetent patients over 50 years of age: a cost‐consequence model. Pharmacotherapy 1997;17(2):333‐41. [PubMed] [Google Scholar]
Grose 1997 {published data only}
- Grose C, Wiedeman J. Generic acyclovir vs. famciclovir and valacyclovir. Pediatric Infectious Disease Journal 1997;16(9):838‐41. [DOI] [PubMed] [Google Scholar]
Jubelt 2002 {published data only}
- Jubelt B. Valacyclovir and famciclovir therapy in herpes zoster. Current Neurology and Neuroscience Reports 2002;2(6):477‐8. [DOI] [PubMed] [Google Scholar]
Li 1999 {published data only}
- Li C, Liu Z. Effect of valociclovir hydrochloride on patients with herpes zoster. Bulletin of Hunan Medical University 1999;24(6):Inside back cover. [PubMed] [Google Scholar]
Lin 2001 {published data only}
- Lin WR, Lin HH, Lee SS, Tsai HC, Huang CK, Wann SR, et al. Comparative study of the efficacy and safety of valaciclovir versus acyclovir in the treatment of herpes zoster. Journal of Microbiology, Immunology, and Infection 2001;34(2):138‐42. [PubMed] [Google Scholar]
Liu 2000 {published data only}
- Liu C, Shi XM, Ji MK. A randomised controlled trial of valaciclovir versus acyclovir in the treatment of 100 patients with herpes zoster. Journal of Clinical Dermatology 2000;29(1):43‐4. [Google Scholar]
Wood 1998 {published data only}
- Wood MJ, Shukla S, Fiddian AP, Crooks RJ. Treatment of acute herpes zoster: effect of early (< 48 h) versus late (48‐72 h) therapy with acyclovir and valaciclovir on prolonged pain. Journal of Infectious Diseases 1998;178(Suppl 1):S81‐4. [DOI] [PubMed] [Google Scholar]
Xu 2000 {published data only}
- Xu HZ, Mao LE, Luo BG, Zhou SX, Bian ZP. A randomised controlled trial of valaciclovir versus acyclovir in the treatment of herpes zoster. Journal of Clinical Dermatology 2000;29(5):288‐9. [Google Scholar]
Yan 1999 {published data only}
- Yan ZF. Comparison between the efficacies of valaciclovir and acyclovir in the treatment of herpes zoster. Shanghai Medicine 1999;20(6):21‐2. [Google Scholar]
Additional references
Altman 1996
- Altman DB, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arora 2008
- Arora A, Mendoza N, Brantley J, Yates B, Dix L, Tyring S. Double‐blind study comparing 2 dosages of valacyclovir hydrochloride for the treatment of uncomplicated herpes zoster in immunocompromised patients 18 years of age and older. Journal of Infectious Diseases 2008;197(9):1289‐95. [DOI] [PubMed] [Google Scholar]
Arvin 1996
- Arvin AM. Varicella‐zoster virus. Clinical Microbiology Reviews 1996;9(3):361‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochener 1997
- Cochener B, Hoang‐Xuan T, Rolland B, Colin J. A double blind randomized trial to compare safety and efficacy of valaciclovir and aciclovir for treatment of herpes zoster ophthalmicus. Investigative Ophthalmology and Visual Science. 1997; Vol. 38:ARVO E‐Abstract 965.
Cohen 2013
- Cohen JI. Clinical practice: Herpes zoster. New England Journal of Medicine 2013;369(3):255‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colin 1997
De Clercq 2006
- Clercq E, Field HJ. Antiviral prodrugs – the development of successful prodrug strategies for antiviral chemotherapy. British Journal of Pharmacology 2006;147(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dworkin 2007
- Dworkin RH, Johnson RW, Breuer J, Gnann JW, Levin MJ, Backonja M, et al. Recommendations for the management of herpes zoster. Clinical Infectious Diseases 2007;44(Suppl 1):S1‐26. [DOI] [PubMed] [Google Scholar]
Emerson 2006
- Emerson GG, Smith JR, Wilson DJ, Rosenbaum JT, Flaxel CJ. Primary treatment of acute retinal necrosis with oral antiviral therapy. Ophthalmology 2006;113(12):2259‐61. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Gou 1999
- Guo A, Hu P, Balimane PV, Leibach FH, Sinko PJ. Interactions of a nonpeptidic drug, valacyclovir, with the human intestinal peptide transporter (hPEPT1) expressed in a mammalian cell line. Journal of Pharmacology and Experimental Therapeutics 1999;289(1):448‐54. [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 25 May 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Gross 2003
- Gross G, Schöfer H, Wassilew S, Friese K, Timm A, Guthoff R, et al. Herpes zoster guideline of the German Dermatology Society (DDG). Journal of Clinical Virology 2003;26(3):177‐89. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Huynh 2008
- Huynh TH, Johnson MW, Comer GM, Fish DN. Vitreous penetration of orally administered valacyclovir. American Journal of Ophthalmology 2008;145(4):682‐6. [DOI] [PubMed] [Google Scholar]
Kanski 2008
- Kanski JJ. Klinische Ophthalmologie. München: Elsevier, Urban and Fischer, 2008. [Google Scholar]
Kirkham 2010
- Krikham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI] [PubMed] [Google Scholar]
McDonald 2012
- McDonald EM, Kock J, Ram FS. Antivirals for management of herpes zoster including ophthalmicus: a systematic review of high‐quality randomized controlled trials. Antiviral Therapy 2012;17(2):255‐64. [DOI] [PubMed] [Google Scholar]
Olusanya 2010
- Olusanya BA, Oshun PO. Management of herpes zoster ophthalmicus in people with HIV infection. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD008770] [DOI] [Google Scholar]
Ormrod 2000
- Ormrod D, Goa K. Valaciclovir: a review of its use in the management of herpes zoster. Drugs 2000;59(6):1317‐40. [DOI] [PubMed] [Google Scholar]
Pavan‐Langston 2008
- Pavan‐Langston D. Herpes zoster antivirals and pain management. Ophthalmology 2008;115(2 Suppl):S13‐20. [DOI] [PubMed] [Google Scholar]
Perry 1996
- Perry CM, Faulds D. Valaciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in herpesvirus infections. Drugs 1996;52(5):754‐72. [DOI] [PubMed] [Google Scholar]
Ragozzino 1982
- Ragozzino MW, Melton LJ 3rd, Kurland LT, Chu CP, Perry HO. Population‐based study of herpes zoster and its sequelae. Medicine 1982;61(5):310‐6. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Taylor 2012
- Taylor SR, Hamilton R, Hooper CY, Joshi L, Morarji J, Gupta N, et al. Valacyclovir in the treatment of acute retinal necrosis. BMC Ophthalmology 2012;12:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Womack 1983
- Womack LW, Liesegang TJ. Complications of herpes zoster ophthalmicus. Archives of Ophthalmology 1983;101(1):42‐5. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Schuster 2015
- Schuster AK, Tesarz J, Harder BC, Schlichtenbrede FC, Jarczok MN, Jonas JB. Valaciclovir versus aciclovir for the treatment of herpes zoster ophthalmicus in immunocompetent patients. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD011503] [DOI] [PMC free article] [PubMed] [Google Scholar]


