Abstract
Background
Kawasaki disease (KD), or mucocutaneous syndrome, is the leading cause of childhood‐acquired heart disease in the developed world. There is much controversy on how best to treat children with KD and in particular who may benefit from additional treatment beyond the standard intravenous immunoglobulin (IVIG) and aspirin, such as the addition of corticosteroids.
Objectives
To assess the impact of corticosteroid use on the incidence of coronary artery abnormalities in KD as either first‐line or second‐line treatment. Corticosteroids may be given alone or in conjunction with other accepted KD treatments. Secondary objectives include the effect of steroids on mortality, the time taken for laboratory parameters to normalise, the duration of acute symptoms (such as fever), the long‐term impact of steroid use and evaluating their safety in KD and their efficacy in relevant population subgroups.
Search methods
The Cochrane Vascular Information Specialist searched Cochrane Vascular's Specialised Register (25 November 2016) and the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 10) in the Cochrane Library (searched 25 November 2016). Trial registries were also searched for details of ongoing or unpublished studies.
Selection criteria
We selected randomised trials involving children with all severities of KD who were treated with corticosteroids, including different types of corticosteroid and different durations of treatment.
Data collection and analysis
MJS and GMC independently selected studies, assessed evidence quality and extracted data. This process was overseen by AJW.
Main results
Seven trials consisting of 922 participants were included in this analysis. Trials ranged from 32 to 242 participants. On pooled analysis, corticosteroids reduced the subsequent occurrence of coronary artery abnormalities (odds ratio (OR) 0.29, 95% confidence interval (CI) 0.18 to 0.46; 907 participants; 7 studies; I² = 55%) without resultant serious adverse events (no events, 737 participants) and mortality (no events, 915 participants). In addition, corticosteroids reduced the duration of fever (mean difference (MD) −1.65 days, 95% CI −3.31 to 0.00; 210 participants; 2 studies; I² = 88%), time for laboratory parameters (erythrocyte sedimentation rate (ESR) and C‐reactive protein (CRP)) to normalise (MD −2.80 days, 95% CI −4.38 to −1.22; 178 participants; 1 study) and length of hospital stay (MD −1.41 days, 95% CI −2.36 to −0.46; 39 participants; 1 study). No studies detailed outcomes beyond 24 weeks. Subgroup analysis showed some potential groups that may benefit more than others; however, further randomised controlled trials are required before this can be the basis for clinical action.
Evidence quality was graded according to the GRADE system. Evidence was considered high quality for the incidence of serious adverse events, mortality and time for laboratory parameters to normalise. Evidence was considered moderate for the incidence of coronary artery abnormalities due to potential inconsistencies in data geography and patient benefits according to grouping. Evidence was moderate for duration of clinical symptoms (fever, rash) due to potential subjectivity in measurement. Evidence was moderate for length of hospital stay as only one study recorded this outcome. This means that we are reasonably confident that the true effect is close to that estimated in this work.
Authors' conclusions
Moderate‐quality evidence shows that use of steroids in the acute phase of KD can be associated with improved coronary artery abnormalities, shorter duration of hospital stay and a decreased duration of clinical symptoms. High‐quality evidence shows reduced inflammatory marker levels. There were insufficient data available regarding incidence of adverse effects attributable to steroids, mortality and long‐term (> 1 year) coronary morbidity. Certain groups, including those based in Asia, those with higher risk scores, and those receiving longer steroid treatment may have greater benefit from steroid use, especially with decreasing rates of heart problems, but more tests are needed to answer these questions. Evidence presented in this study suggests that treatment with a long course of steroids should be considered for all children diagnosed with KD until further studies are performed.
Plain language summary
Using steroids to treat Kawasaki disease
Review question
We reviewed the use of a set of drugs known as steroids in children affected by Kawasaki disease for the reduction in the chance of future heart problems as well as the effect on the duration of fever, signs of infection in the blood and the number of days spent in hospital.
Background
We currently have a limited understanding of Kawasaki disease and how best to manage it. This is important as one of the long‐term consequences can involve the heart, putting the child at higher risk of life‐shortening outcomes.
Study characteristics
Evidence is current to November 2016. Male and female children diagnosed with Kawasaki disease were included in this review. We selected only randomised clinical trials. Trials compared the use of steroids against not using steroids. This review involves seven trials and 922 participants.
Key results
Steroids appear to reduce the risk of heart problems after Kawasaki disease without causing any important side effects. They also reduce the length of symptoms (fever and rash), length of hospital stay, and blood markers associated with being unwell. Certain groups, including those based in Asia, those with higher risk scores, and those receiving longer steroid treatment, may have greater benefit from steroid use, especially with decreasing rates of heart problems, but more tests are needed to answer these questions. More tests are also needed to obtain a more accurate marker of the risk of serious side effects and to determine if there is a lower chance of death when using steroids. Evidence presented in this review suggests that treatment with a long course of steroids should be considered for all children diagnosed with Kawasaki disease until further studies are performed.
Quality of the evidence
Evidence quality was graded according to the GRADE system. Evidence was considered high quality for serious adverse events, mortality and time for laboratory parameters to normalise. Evidence was considered moderate quality for the risk of future heart problems, duration of clinical symptoms (fever, rash) and length of hospital stay. This means that we are reasonably confident that the true effect is close to that estimated in this work.
Summary of findings
Summary of findings for the main comparison. Summary of findings table: Corticosteroids versus no corticosteroid use for treatment if Kawasaki disease in children.
| Corticosteroids versus no steroid use for the treatment of Kawasaki disease in children | ||||||
| Patient or population: children diagnosed with Kawasaki disease Setting: hospital‐based Intervention: corticosteroids Comparison: no corticosteroid use | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no corticosteroid use | Risk with corticosteroids | |||||
| Incidence of coronary artery abnormalities Duration: 2 to 6 weeks |
Study population | OR 0.29 (0.18 to 0.46) | 907 (7 RCTs) | ⊕⊕⊕⊝ MODERATE1, 2 | ||
| 168 per 1000 | 56 per 1000 (35 to 85) | |||||
| Moderate | ||||||
| 114 per 1000 | 36 per 1000 (23 to 56) | |||||
| Incidence of serious adverse effects attributable to steroid use Duration: 2 to 6 weeks |
Study population | not estimable | 737 (6 RCTs) | ⊕⊕⊕⊕ HIGH | No cases of adverse events attributable to steroids use are recorded by the included studies | |
| see comments | ||||||
| Mortality (all‐cause) Duration: 2 to 6 weeks |
Study population | not estimable | 915 (7 RCTs) | ⊕⊕⊕⊕ HIGH | No deaths are recorded by the included studies | |
| see comments | ||||||
| Duration of clinical symptoms: fever, rash (days) Duration: N/A |
‐ | The mean duration of clinical symptoms (fever, rash) in the intervention group was 0.97 days fewer (1.2 fewer to 0.74 fewer) | ‐ | 210 (2 RCTs) | ⊕⊕⊕⊝ MODERATE3 | |
| Time for laboratory parameters to normalise: CRP, ESR (days) Duration: N/A |
‐ | The mean time for laboratory parameters (CRP, ESR) to normalise in the intervention group was 2.8 days fewer (4.38 fewer to 1.22 fewer) | ‐ | 178 (1 RCT) | ⊕⊕⊕⊕ HIGH | |
| Length of hospital stay (days) Duration: N/A |
‐ | The mean length of hospital stay in the intervention group was 1.41 days fewer (2.36 fewer to 0.46 fewer) | ‐ | 39 (1 RCT) | ⊕⊕⊕⊝ MODERATE4 | |
| Longer‐term (> 1 year post disease onset) coronary morbidity (non‐aneurysmal) Duration: N/A |
see comment | see comment | see comment | see comment | None of the included studies included data on outcomes (including coronary morbidity (non‐aneurysmal) > 1 year after study enrolment | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CRP: C‐reactive protein; ESR: Erythrocyte sedimentation rate; OR: Odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 Further investigation to reduce potential confounding required to explain inconsistencies in data (i.e. geographical variation), however overall effect unlikely to change (likely beneficial) 2 Downgraded by one level: large group and effect size, however subgroup analysis suggests that those with low‐risk scores or receiving single‐dose treatment may not benefit 3 Downgraded by one level: significant heterogeneity within the data, likely due to the subjective nature of the included outcome (i.e. rash) 4 Downgraded by one level: length of hospital stay measured in one small study only (n = 39)
Background
Description of the condition
Kawasaki disease (KD), or mucocutaneous syndrome, is the leading cause of childhood‐acquired heart disease in the developed world (Kato 1996). Originally described by Kawasaki 1967, it is a medium vessel vasculitis of unclear aetiology that has been linked with an abnormal host response to an infectious trigger. Generally affecting children less than five years old, peak onset is between 18 and 24 months. The incidence in those aged under 5 years varies widely throughout the world, including 8.4 per 100,000 in the UK, 17.5 per 100,000 to 20.8 per 100,000 in the USA, and 239.6 per 100,000 in Japan (Gardner‐Medwin 2002; Harnden 2002; Holman 2003; Nakamura 2012; Singh 2015). Rate of recurrence is approximately 3600 per 100,000, whilst acute mortality occurred in just one of the 23,730 cases Nakamura analysed in the 2009 to 2010 period (Nakamura 2012). Such varied epidemiology has strengthened theories linking KD with genetics and one or more infectious agents (Wood 2009).
KD is a multisystem vasculitis but its most important complication involves the predisposition for coronary artery vasculitis leading to aneurysms in up to 25% of untreated patients (Burns 1996). Such complications render the coronary vessels vulnerable to stenoses and thromboses, with subsequent risk of myocardial infarction and death (Daniels 2012). Furthermore, these thromboses act as focus for accelerated KD vasculopathy, increasing cardiovascular risk.
There is no diagnostic test for KD but laboratory findings typically show a raised white cell count, erythrocyte sedimentation rate (ESR) and C‐reactive protein (CRP). Diagnosis is generally based on clinical symptoms from one of two major sets of criteria. The Diagnostic Guidelines of the Japan KD Research Committee require any five from (1) fever longer than five days, (2) conjunctivitis, (3) lymphadenopathy, (4) polymorphous rash, (5) oral and perioral changes, and (6) changes in the extremities (Ayusawa 2005). The American Heart Association guidelines are similar, requiring a total of five of the aforementioned six but also stipulating that fever must feature (Newburger 2004). Diagnosis is complicated by these symptoms being prevalent in various common childhood viral exanthems. Symptoms may also occur sequentially rather than simultaneously. This variability leads to the idea of 'incomplete' KD, where KD is suspected but only three of five diagnostic criteria are satisfied.
Description of the intervention
It is thought that the prompt and effective treatment of KD can decrease the incidence of its cardiac sequelae. Accepted and proven initial pharmacological management involves intravenous immunoglobulin (IVIG) at a dose of 2 g/kg in a single 12‐hour infusion alongside 30 to 50 mg/kg of aspirin in four divided doses (Eleftheriou 2013). This has been shown to limit the duration of the acute phase of KD as well as reduce the long‐term coronary sequelae from 25% to 4.7% (Levin 1991). Both medications have already been subject to Cochrane Reviews (Baumer 2006; Oates‐Whitehead 2003). Plasma exchange is also used in certain institutions (Hokosaki 2012).
Patients do not always respond to the above regimen. A subset of KD patients, approximately 20%, have clinical symptoms that are resistant to the first dose of IVIG and aspirin after 48 hours. This group has been proven to be at higher risk for cardiac sequelae (Brogan 2002). Various systems for identifying this group have been formulated, including the Kobayashi score; however, while specific, these have shown poor sensitivity in Western populations compared with the Japanese groups in which they were devised (Kobayashi 2008; Sleeper 2011). Currently accepted identifiers for this high‐risk group include the following (Eleftheriou 2013).
Resistance to IVIG.
Very young age of onset (< 12 months).
Severe inflammatory markers.
Clinical features of shock.
Existing arterial aneurysms.
Kobayashi score greater than or equal to five.
These high‐risk patients have a higher prevalence of coronary aneurysms, especially giant aneurysms (> 8 mm), and have associated greater long‐term cardiovascular morbidity and mortality (Tatara 1987). Current consensus on management recommends a repeat dose of IVIG which facilitates disease defervescence in approximately half of the patients (Hashino 2001b). Measures to improve the success rate have been reviewed and the utility of intravenous steroids (IVS) with or without infliximab has shown mixed results. Current data on infliximab are insufficiently powered to draw conclusions (Davies 2013). IVS have long been used in vasculitides similar to KD; however, their use in KD has been subject to long‐standing controversy due to earlier works showing a deleterious effect (Levin 2013). That stated, it is now widely believed that these studies were subject to significant selection bias, with only the most severe cases, bearing the greatest probability of a poor outcome, given steroids (Kato 1979).
There have been recent gains in knowledge regarding the use of corticosteroids in KD. The early use of steroids has been advocated, but only in high‐risk patients (as defined above). This acknowledged, it remains unclear how best to use corticosteroids (Chen 2013). A recent editorial has highlighted some of the critical issues with current meta‐analyses in this area (Levin 2013). Problems include varying inclusion criteria and populations, differing methodologies of included works and an overall lack of power with respect to data on side effects. It is not currently known which demographic groups show the greatest benefit with respect to coronary sequelae, or if there is the potential for complications with IVS treatment. Furthermore, the most effective types, frequencies and doses of steroid have not yet been clarified, nor whether steroids should be administered alongside IVIG, aspirin or infliximab (Levin 2013).
How the intervention might work
Steroid treatment is already utilised in a broad range of vasculitides to great effect. Furthermore, steroids were a key part of KD treatment prior to the advent of IVIG. Research into the exact pathological mechanisms is ongoing and current theories of KD pathogenesis implicate immunological responses to infectious agents (Eleftheriou 2013). Such reactions are thought to be controllable via steroid administration due to a reduction in inflammatory mediator transcription. In the context of KD, this may mean a reduction in fever as well as lower levels of inflammation, leading to a reduction in the formation of coronary abnormalities and subsequent incidence of future cardiovascular sequelae (Levin 2013). It therefore remains important to ascertain the utility of IVS in KD.
Why it is important to do this review
There is much controversy regarding how best to identify the high‐risk patients who may benefit from additional treatment beyond the standard IVIG and aspirin. Furthermore, this situation is complicated by early reports of harmful effects of steroids in KD, although these conclusions are now considered to be the result of selection bias as only the sickest patients were investigated (Kato 1979). Current markers used to indicate resistance to IVIG include very young age of onset (< 12 months), high inflammatory markers, clinical features of shock, existing arterial aneurysms, or a Kobayashi score greater than or equal to five. The utility of corticosteroids in KD as a whole remains unclear despite several meta‐analyses and we seek to use this work to clarify the situation through a more targeted approach to analysis. In addition, it is unclear how best to define the patients that may benefit from corticosteroids the most, for example whether ethnic origin, severity of KD or pre‐steroid treatment status may help define the optimum population. It is hoped that the whole group and subgroup analyses of this work will cast greater light on this issue.
Objectives
To assess the impact of corticosteroid use on the incidence of coronary artery abnormalities in KD as either first‐line or second‐line treatment. Corticosteroids may be given alone or in conjunction with other accepted KD treatments. Secondary objectives assess the effect of steroids on mortality, the time taken for laboratory parameters to normalise, the duration of acute symptoms (such as fever), the long‐term impact of steroid use and evaluating their safety in KD and their efficacy in relevant population subgroups.
Methods
Criteria for considering studies for this review
Types of studies
We searched all randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation methods are not completely random, for example using alternation). Cross‐over trials were not included as the response to steroid intervention may depend on timing and previous treatment state. We excluded all studies not conforming to the RCT format.
Types of participants
We included all children (less than 19 years old) diagnosed with KD worldwide in the analysis. The diagnosis of KD had to fulfil the Diagnostic Guidelines of the Japan KD Research Committee. This requires any five of the following (Ayusawa 2005).
Fever for more than five days, non‐responsive to anti‐pyrexial agents.
Conjunctivitis: bilateral bulbar, non‐suppurative.
Lymphadenopathy: cervical, generally more than 1.5 cm.
Polymorphous exanthem, no crusts or vesicles.
Oral and perioral changes: strawberry tongue, cracked erythematous lips, diffuse oropharyngeal erythema.
Changes in the extremities, either acute (< 2 weeks): erythema and oedema of the palms and soles; or convalescent: desquamation at the fingertips.
An accepted exception to the above for a positive KD diagnosis exists if only four criteria are fulfilled but cardiac complications are found at echocardiography or angiography. We also accepted American Heart Association guideline definitions (Newburger 2004). These are similar, requiring a total of five of the above six, but stipulating that fever must feature. Therefore fulfilment of the American criteria automatically fulfils the Japanese criteria.
Corticosteroids could be part of the initial treatment for KD or form part of the second‐line treatment after failure of first‐line treatment that did not include steroids. The comparison group had to be in parallel. Cross–over trials were not eligible for inclusion.
Participants with positive blood cultures were excluded.
Types of interventions
All forms of corticosteroid therapy in conjunction with any combination of no treatment, placebo, immunoglobulin, aspirin or infliximab for the treatment of KD were considered the intervention of interest. That stated, the use of corticosteroids had to be the only difference in management between trial arms.
Comparator groups included any of:
placebo;
immunoglobulin only;
aspirin only;
immunoglobulin and aspirin;
infliximab only;
infliximab and immunoglobulin;
infliximab, immunoglobulin and aspirin.
Types of outcome measures
Primary outcomes
The incidence of coronary artery abnormalities (measured via diameter or z‐scores; Boston scores (de Zorzi 1998)) per study group found at either cardiac angiography or echocardiography within three months of KD diagnosis. Coronary abnormality was defined using either the de Zorzi criteria (a coronary dimension that is ≥ 2.5 standard deviations (SDs) above the mean for body surface area) (de Zorzi 1998); or the Japanese Ministry of Health criteria (Research Committee on Kawasaki Disease 1984), as follows.
Lumen > 3 mm in children < 5 years old;
Lumen > 4 mm in children > 5 years old;
Internal diameter of a segment measuring ≥ 1.5 times that of an adjacent segment.
The incidence of any serious adverse effects per study group that is attributable to the administration of steroids at any point after treatment initiation. Known side effects of steroids in other diseases include, for example, immunosuppression with resultant opportunistic infection and avascular necrosis of the femoral head.
Secondary outcomes
Mortality (all‐cause).
Duration of clinical symptoms: fever and rash.
Time for laboratory parameters to normalise: CRP and ESR.
Length of hospital stay.
Longer‐term (greater than one‐year post‐disease onset) coronary morbidity (non‐aneurysmal).
Search methods for identification of studies
We included studies reported as full‐text or published as abstract only.
Electronic searches
The Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials:
The Cochrane Vascular Specialised Register (searched 25 November 2016);
The Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 10) in the Cochrane Library (searched 25 November 2016).
See Appendix 1 for details of the search strategy used to search CENTRAL.
The Cochrane Vascular Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE Ovid, Embase Ovid, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used, are described in the Specialised Register section of the Cochrane Vascular module in the Cochrane Library (www.cochranelibrary.com).
The CIS searched the following trial registries for details of ongoing and unpublished studies;
ClinicalTrials.gov (www.ClinicalTrials.gov).
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch).
ISRCTN Register (www.isrctn.com/).
See Appendix 2 for details of the search strategies used.
Searching other resources
We contacted the authors of trials that met the eligibility criteria identified by the searches as ongoing or unpublished trials. We also searched reference lists of relevant trials for further publications.
Data collection and analysis
Selection of studies
Two review authors (MJS and GMC) independently applied the selection criteria to the studies identified by the search strategy. This included independently assessing whether the studies fulfilled the inclusion and exclusion criteria. If insufficient information was available to decide whether a study was truly eligible we contacted the study authors to request further information. A third review author (AJW) resolved disagreements.
Data extraction and management
Two review authors (MJS and GMC) independently extracted data using a modified version of the Cochrane Vascular standard data extraction form. These data were then brought together and monitored for discrepancies by a third review author (AJW), before being entered into Review Manager 5 software (RevMan 2014). We contacted study authors for any required information that was not included in the published works. The key information gathered in the data collection form included the following.
General study information: publication type.
Fulfilment of eligibility criteria: study type, interventions, outcomes measured and reasons for exclusion.
Study methods: allocations methods, study dates, duration, ethical approval and statistical methods.
Participants: methods of recruitment, consent, total number, treatment groups, age, sex, race, KD severity, subgroup analyses reported and eligibility criteria.
Intervention (steroids): number of participants, dosing, frequency, duration, delivery method, providers, compliance and concomitant treatment.
Outcomes: coronary diameters (acute), coronary diameter z‐scores (acute), coronary abnormality (long term), duration of clinical symptoms (e.g. fever), adverse effects, duration of laboratory parameter abnormality (e.g. CRP) and duration of hospital stay.
Assessment of risk of bias in included studies
Risk of bias was assessed using the recommended Cochrane tool as described in section 8.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Two review authors (MJS and GMC) performed this independently, and resolved any disagreements by discussion with a third review author (AJW). The domains assessed included:
sequence generation (selection bias);
allocation sequence concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective outcome reporting (reporting bias);
other bias.
Measures of treatment effect
The effect measure of choice for dichotomous data was the odds ratio (OR) with a 95% confidence interval (CI). This is a ratio between the corticosteroid intervention group and its parallel comparator group.
We managed continuous data, including time‐to‐event data, using mean differences (MD) and 95% CIs. If necessary, we planned to use a standardised mean difference (SMD) for studies that measured the same outcome but used different methods.
Unit of analysis issues
The unit of analysis was each participant recruited into a trial.
Dealing with missing data
We accounted for all missing data due to dropouts via an intention‐to‐treat (ITT) analysis. We reported if the individual trials carried this out. If they did not then we have endeavoured to apply an ITT analysis. In the event that we have been unable to do this then we utilised a per protocol analysis. We have explained all post‐allocation dropouts. We have followed up any data missing from the published document with the study’s original authors. If data still remain absent then we have considered this in the 'Risk of bias' assessments.
Assessment of heterogeneity
We took heterogeneity into account using the I² statistic for quantification of variability (< 40% = likely low heterogeneity; 40% to 60% = possible moderate heterogeneity; > 60% = possible significant heterogeneity). We also used the Chi² test (limit = degrees of freedom) and P values (10% significance threshold). Where heterogeneity exceeded generally accepted limits (> 60% heterogeneity) we subgrouped the analysis in a logical manner to explain these differences and reduce remaining heterogeneity. These methods were reinforced by visual recognition on forest plots to assess for overlapping CIs.
Assessment of reporting biases
Had there been more than eight trials, we planned to screen for publication and reporting bias using funnel plot asymmetry and measure using tests as outlined in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For smaller studies, we took into account 'the small‐study effect', where smaller studies can show larger treatment effects due to poor methodology, heterogeneity, selection bias, chance or artefact. We also tried to find eligible studies that have been registered and should have been completed, but are without available published data. It should be noted that the 'Risk of bias' assessment has taken into account selective outcome reporting.
Data synthesis
Statistical analysis took place using a fixed‐effect model if there was low heterogeneity, and a random‐effects model if there was significant heterogeneity (I² > 60%). We undertook outcome analyses using an ITT model. Two‐sided P values less than or equal to 0.05 were considered significant and all analyses were undertaken using Review Manager 5 (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
Planned subgroup analyses (data permitting) included:
type of steroid used;
steroid dosing;
steroid treatment frequency;
total steroid treatment duration;
steroid route of administration;
first‐line versus second‐line management;
geographical distribution of trial participants, ethnicity;
KD severity (non‐high risk versus high risk as detailed earlier);
recognised concomitant treatments for KD (as detailed earlier in the text).
We also looked at employing further subgroup analyses if heterogeneity remained significant (I² > 60%). These subgroups were tested using a Chi² P value threshold of 0.05.
Sensitivity analysis
We planned to perform a sensitivity analysis to explore causes of heterogeneity and the robustness of the results if there were sufficient data available. We planned to include the following factors in the sensitivity analysis.
Type of study design (RCT versus quasi‐RCT).
Low risk of bias trials versus high risk of bias trials.
Rates of dropouts for each treatment group.
Summary of findings
We presented the main findings of the review results concerning the quality of evidence, the magnitude of effect of the interventions examined and the sum of available data on the outcomes (Types of outcome measures) in a 'Summary of findings' table, according to the GRADE principles as described by Higgins 2011 and Atkins 2004. We used the GRADEpro (GRADEproGDT) software (www.guidelinedevelopment.org/) to assist in the preparation of the 'Summary of findings' table.
Results
Description of studies
Results of the search
See Figure 1.
1.
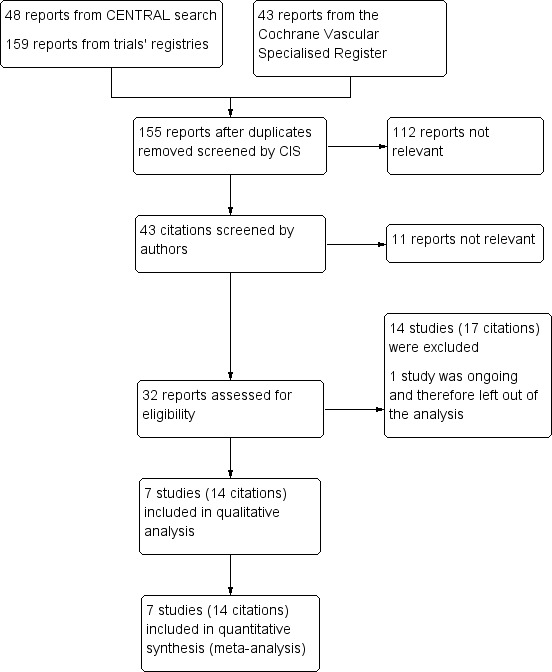
Study flow diagram.
Included studies
See Characteristics of included studies.
After applying the aforementioned inclusion and exclusion criteria, we identified seven trials (14 reports) suitable for inclusion within this review (Ikeda 2006; Inoue 2006; Kobayashi 2012; Newburger 2007; Ogata 2012; Okada 2003; Sundel 2003). This process is summarised in a flow diagram (Figure 1). Full‐text publications were obtained from the websites of the original source of publication. When studies had multiple publications, data were combined in order to give as complete an interpretation of available data as possible.
Two trials were conducted in North America (Newburger 2007; Sundel 2003); and five in Japan (Ikeda 2006; Inoue 2006; Kobayashi 2012; Ogata 2012; Okada 2003). Three trials were single centre (Ikeda 2006; Ogata 2012; Sundel 2003); and four trials were multicentre (Inoue 2006; Kobayashi 2012; Newburger 2007; Okada 2003).
The seven trials consisted of 922 participants and ranged from 32 to 242 participants. Three studies segmented high‐risk participants using criteria as set out in Table 2 (Ikeda 2006; Kobayashi 2012; Ogata 2012).
1. High‐risk subjects' criteria.
| Study | High risk criteria |
| Ikeda 2006 | Developed own risk score based upon IVIG unresponsiveness in a multiple logistic regression analysis. 42/178 randomly identified KD patients were deemed high risk. |
| Kobayashi 2012 | Kobayashi risk score of 5 or greater (≤ 4 days fever pre‐diagnosis, ≤ 12 years old, CRP ≥ 10 mg/dL, ≤ 300 platelets, ALT ≥ 100, sodium ≤ 133, neutrophils ≥ 80%) |
| Ogata 2012 | Egami score of 3 or greater (≤ 6 months old, ≤ 4 days fever pre diagnosis, ≤ 300 platelets, CRP ≥ 7 mg/dL, ALT ≥ 80) |
ALT: alanine transaminase CRP: C‐reactive protein IVIG: intravenous immunoglobulin KD: Kawasaki disease
Coronary artery abnormality was the primary outcome in four studies (Ikeda 2006; Inoue 2006; Kobayashi 2012; Newburger 2007). Duration of fever was the primary outcome in two studies (Ogata 2012; Sundel 2003). Cytokine levels was the primary outcome in one study (Okada 2003).
Further details including dosing and baseline characteristics can be found in Characteristics of included studies.
Excluded studies
See Characteristics of excluded studies.
Fourteen studies (17 reports) were excluded from the review (Asai 1985; Hashino 2001; ISRCTN74427627; Jibiki 2005; Kato 1979; Kusakawa 1983; Miura 2008; Nakamura 1985; Nonaka 1995; Ogata 2009; Sekine 2012; Seto 1983; Xu 2002; Yuan 2000).
Six studies did not assess the outcomes of this review (Jibiki 2005; Miura 2008; Nakamura 1985; Ogata 2009; Sekine 2012; Yuan 2000). Attempts were made to contact the study authors without success. For four studies, study outcome data were not available and again study authors were contacted without success (ISRCTN74427627; Kato 1979; Kusakawa 1983; Nonaka 1995). Hashino 2001 was excluded due to trial design affecting the comparability of results with the included works. Two studies combined steroids with an additional intervention meaning steroids were not the only differential factor between the trial arms (Asai 1985; Xu 2002).
One trial is ongoing and there are currently no suitable data available for review (JPRN‐UMIN000009524).
Risk of bias in included studies
All included studies were assessed for bias as illustrated in Figure 2 and Figure 3 and Characteristics of included studies.
2.
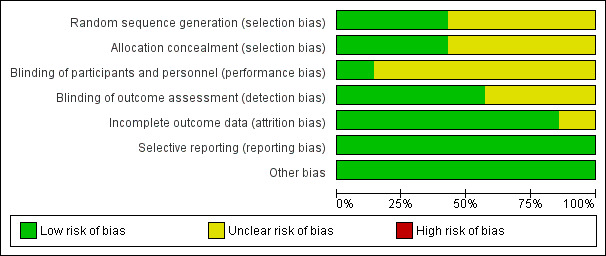
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
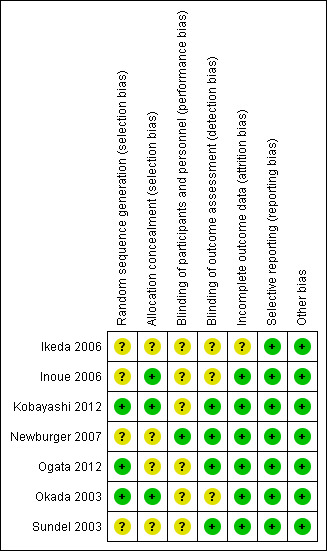
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All included studies are randomised controlled trials (Ikeda 2006; Inoue 2006; Kobayashi 2012; Newburger 2007; Ogata 2012; Okada 2003; Sundel 2003).
Random sequence generation
Three studies were deemed to be of low risk of bias from random sequence generation due to the use of recognised and accepted approaches to this step (e.g. use of computer generation) (Kobayashi 2012; Ogata 2012; Okada 2003). Four studies were deemed to be of unclear risk of bias because although the use of random allocation was stated, there was no detail regarding the exact approach (Ikeda 2006; Inoue 2006; Newburger 2007; Sundel 2003).
Allocation concealment
Four studies were deemed of unclear bias for allocation concealment due to inadequate detail being published (Ikeda 2006; Newburger 2007; Ogata 2012; Sundel 2003). Three were regarded as low risk of allocation disclosure due to the use of centrally held databases (Inoue 2006; Kobayashi 2012; Ogata 2012).
Blinding
Five trials were non‐blinded (Inoue 2006; Kobayashi 2012; Ogata 2012; Okada 2003; Sundel 2003); one trial was double blinded (Newburger 2007); and one trial did not state whether participants or investigators were blinded (Ikeda 2006). Therefore six studies were deemed at unclear risk of performance bias (Ikeda 2006; Inoue 2006; Kobayashi 2012; Ogata 2012; Okada 2003; Sundel 2003), and one study was deemed at low risk of performance bias (Newburger 2007).
Four studies had blinded outcome reporters and are coded as low risk of bias (Kobayashi 2012; Newburger 2007; Ogata 2012; Sundel 2003); one study had non‐blinded outcome reporters (Inoue 2006); and two studies did not mention if outcome reporters were blinded and was therefore judged as 'unclear' for detection bias (Ikeda 2006; Okada 2003).
Incomplete outcome data
Intention‐to‐treat analysis was mentioned in two studies (Kobayashi 2012; Newburger 2007). Inoue 2006 reported key outcome variables as per protocol analysis with an intention‐to‐treat analysis also performed. Three further studies were able to account for all or most of the data (Ogata 2012; Okada 2003; Sundel 2003). These were all judged to be of low risk of bias. One study did not state how data were handled and was therefore coded as unclear risk of bias (Ikeda 2006).
All trials had a relatively short follow‐up period, up to five weeks, so there was limited loss to follow‐up, which is consistent throughout the studies. No study had a dropout rate exceeding 20%.
Selective reporting
The protocol for one study was published before initiation of the trial (Newburger 2007). The published methodology including outcome measures reported by the remainder of the trials was consistent with that reported in the respective results section (Ikeda 2006; Inoue 2006; Kobayashi 2012; Ogata 2012; Okada 2003; Sundel 2003). The risk of selective reporting bias for these studies has therefore been coded as low.
Other potential sources of bias
None noted.
Effects of interventions
See: Table 1
Primary outcomes
Incidence of coronary artery abnormality
Seven trials on 907 participants assessed incidence of coronary artery abnormality (Ikeda 2006; Inoue 2006; Kobayashi 2012; Newburger 2007; Ogata 2012; Okada 2003; Sundel 2003). The pooled data of steroid use versus no steroid use demonstrate an OR of 0.29 (95% CI 0.18 to 0.46, P < 0.00001) (Analysis 1.1). There was possible moderate heterogeneity of 55% and this was reduced in subgroup analysis (see below).
1.1. Analysis.
Comparison 1 Corticosteroids vs no corticosteroid use, Outcome 1 Incidence of coronary artery abnormalities.
Incidence of serious adverse events attributable to steroid use
From six trials with 737 participants, no serious adverse events attributable to corticosteroid use were noted and the effects were deemed not estimable (Analysis 1.2) (Inoue 2006; Kobayashi 2012; Newburger 2007; Ogata 2012; Okada 2003; Sundel 2003). Ikeda 2006 did not disclose sufficient information for inclusion despite attempts to contact the authors.
1.2. Analysis.
Comparison 1 Corticosteroids vs no corticosteroid use, Outcome 2 Incidence of serious adverse effects attributable to steroid use.
Secondary outcomes
Mortality (all cause)
From the seven trials with 915 participants no deaths were recorded within the observed study period (Analysis 1.3) (Ikeda 2006; Inoue 2006; Kobayashi 2012; Newburger 2007; Ogata 2012; Okada 2003; Sundel 2003).
1.3. Analysis.
Comparison 1 Corticosteroids vs no corticosteroid use, Outcome 3 Mortality (all‐cause).
Duration of clinical symptoms: fever, rash
Two studies reported data on the duration of clinical symptoms (days), showing a significant reduction in time (MD −1.65 days, 95% CI −3.31 to 0.00; participants = 210; studies = 2; I² = 88%; P = 0.05) (Inoue 2006; Okada 2003). There was possible significant heterogeneity at 88% which was not explained by subgroup analysis for high‐ and low‐risk scores (Analysis 4.2). In both studies a child was considered afebrile if their temperature was below 37.5 °C for more than 24 hours; Inoue 2006 measured axillary temperature and Okada 2003 simply measured "body temperature", but does not state the site. This may account for the heterogeneity as body temperature can vary depending on measurement site.
4.2. Analysis.
Comparison 4 Subgroup: High‐risk scores vs lower‐risk scores or all participants if risk score not calculated in study, Outcome 2 Duration of clinical symptoms.
Time for laboratory parameters to normalise: CRP, ESR
One study involving 178 participants directly measured the time for laboratory parameters to normalise (Inoue 2006). Analysis showed a MD of −2.80 days (95% CI −4.38 to −1.22; P = 0.0005) in the steroid group versus the non‐steroid group (Analysis 1.5).
1.5. Analysis.
Comparison 1 Corticosteroids vs no corticosteroid use, Outcome 5 Time for laboratory parameters to normalise: CRP, ESR.
Length of hospital stay
One study reported on the length of hospital stay with steroid use versus non‐steroid use and demonstrated a reduction in days stayed in hospital (MD −1.41 days, 95% CI −2.36 to −0.46; participants = 39; P = 0.004) (Analysis 1.6) (Sundel 2003).
1.6. Analysis.
Comparison 1 Corticosteroids vs no corticosteroid use, Outcome 6 Length of hospital stay.
Longer‐term (> 1 year post disease onset) coronary morbidity
None of the included studies included data on outcomes (including coronary morbidity (non‐aneurysmal) more than 1 year after study enrolment.
Subgroup analysis
Planned subgroup analyses are listed below with comments on the ability for them to be performed.
Type of steroid used: this subgroup analysis was performed for the outcome coronary artery abnormalities in six of the included studies (Inoue 2006; Kobayashi 2012; Newburger 2007; Ogata 2012; Okada 2003; Sundel 2003), where the type and duration of steroid used was stated. When looking at the use of steroids as a single intravenous (IV) dose there was no evidence of effect with regards to coronary artery abnormalities (OR 0.56, 95% CI 0.29 to 1.08; participants = 277; studies = 3; I² = 36%) (Newburger 2007; Okada 2003; Sundel 2003). When this was followed with an oral course of steroids fewer coronary artery abnormalities were identified in the corticosteroid group (OR 0.13, 95% CI 0.05 to 0.32; participants = 452; studies = 3; I² = 0%) (Inoue 2006; Kobayashi 2012; Okada 2003). The P value for the test for subgroup differences is 0.009. Subgroupings explained the heterogeneity identified for the combined analysis (Analysis 2.1).
Steroid dosing: this subgroup analysis was not applicable as steroid dosing was broadly classed into one‐off IV methylprednisolone, or a longer tapering course of prednisolone, and the subgroup analysis for this was performed in the subgroup analysis 1 above.
Steroid treatment frequency: this subgroup analysis was not applicable as steroid dosing was broadly classed into one‐off IV methylprednisolone, or a longer tapering course of prednisolone, and the subgroup analysis for this was performed in the subgroup analysis 1 above.
Total steroid treatment duration: this subgroup analysis was not applicable as steroid dosing was broadly classed into one‐off IV methylprednisolone, or a longer tapering course of prednisolone, and the subgroup analysis for this was performed in the subgroup analysis 1 above.
Steroid route of administration: this subgroup analysis was not applicable as steroid dosing was broadly classed into one‐off IV methylprednisolone, or a longer tapering course of prednisolone. All steroids in the initial phase were given intravenously, and the subgroup analysis for this was performed in the subgroup analysis 1 above.
First‐line versus second‐line management: there were insufficient data to perform this subgroup analysis.
Geographical distribution of trial participants, ethnicity: this subgroup analysis found that the use of steroids appears to have an increased beneficial effect on coronary artery abnormalities in the studies conducted in Japan (Ikeda 2006; Inoue 2006; Kobayashi 2012; Ogata 2012; Okada 2003; OR 0.14, 95% CI 0.07 to 0.29, 678 participants) versus no evidence of benefit in North America (Newburger 2007; Sundel 2003; OR 0.77, 95% CI 0.37 to 1.59, 229 participants). The P value for test for subgroup differences is 0.001; however it is important to note that this analysis is vulnerable to confounding as most participants who received a one‐off dose of steroid were from the North American cohorts (Analysis 3.1).
KD severity (non‐high risk versus high risk as detailed earlier): on analysis of high‐risk participants only there is strong evidence of effect (OR 0.13, 95% CI 0.06 to 0.29; participants = 377; studies = 3; I² = 0%) (Ikeda 2006; Kobayashi 2012; Ogata 2012). When looking at low‐risk participants alone the benefit was weaker and potentially lost (OR 0.53, 95% CI 0.29 to 1.00; participants = 530; studies = 5; I² = 6%) (Ikeda 2006; Inoue 2006; Newburger 2007; Okada 2003; Sundel 2003). These groups proved to be significantly different (P = 0.007). In both subgroups heterogeneity was reduced to negligible levels (Analysis 4.1).
Recognised concomitant treatments for KD (as detailed earlier in the text): there were insufficient data to perform this subgroup analysis.
2.1. Analysis.
Comparison 2 Subgroup: One‐off steroid use vs longer course of steroids, Outcome 1 Coronary artery abnormalities.
3.1. Analysis.
Comparison 3 Subgroup: Geography, Outcome 1 Coronary artery abnormalities.
4.1. Analysis.
Comparison 4 Subgroup: High‐risk scores vs lower‐risk scores or all participants if risk score not calculated in study, Outcome 1 Coronary artery abnormalities.
Sensitivity analysis and reporting bias
A sensitivity analysis was not required for this review as all studies were randomised controlled trials, no studies were found to have a significant risk of bias and dropout rates were not significantly different between studies.
As there were only seven trials included in this Cochrane Review, we have not screened for publication and reporting bias by assessing funnel plot asymmetry using tests as outlined in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Discussion
Summary of main results
Pooled data show that the use of steroids in the acute phase of KD in children can lead to reduced incidence of coronary artery aneurysms, duration of clinical symptoms (fever, rash), time for laboratory parameters to normalise (CRP, ESR) and length of hospital stay. There were insufficient data available regarding incidence of adverse effects attributable to steroids, mortality and long‐term (> 1 year) coronary morbidity.
Subgroup analysis demonstrated that with respect to coronary abnormality:
the greatest benefit was in children in Japan versus those in North America, but the use of different regimens may have contributed to the different outcomes in Japanese and American studies;
the greatest benefit was in children with high‐risk scores versus those with low‐risk scores, although both display benefit;
there is a benefit of steroids if taken over a prolonged course versus the potential for no benefit if steroids are given as a one‐off dose.
However, potential confounding of the subgroup analyses must be noted here – those studies completed in North America are both considered part of the lower‐risk group and employed single‐dose regimens (Newburger 2007; Sundel 2003).
Subgroup analysis demonstrated that with respect to duration of clinical symptoms both groups with high‐ and low‐risk scores benefit from steroid treatment, with the greatest benefit in those with high‐risk scores although the numbers of studies and participants on which this is based are small.
Overall completeness and applicability of evidence
All studies in this review collected data on the stated primary outcomes of this review: coronary artery abnormalities and serious adverse events attributable to steroids use. All relevant participants, interventions and outcomes have been investigated. Overall, the evidence collected is highly applicable to this review. The evidence demonstrates that steroids have some benefit in the acute treatment of KD in the populations studied in this review. That stated, further data looking at different ethnicity subgroups, disease risk scores, and duration of steroid use are required for a more complete guide. In particular, an investigation outside Japan employing risk stratification and IV steroid treatment followed by oral doses would be beneficial.
None of the studies demonstrated any serious adverse events during their follow‐up periods due to the use of steroids.
Difficulties remain with the application of the results of this review to Western populations, where there is no comparable severity risk score. The identification of groups who might gain the greatest benefit from steroids will remain problematic until a reliable risk stratification score is developed for this group.
Overall the results of this review are applicable to the majority of children worldwide diagnosed with KD, and should serve as a guide to the treating clinician.
Quality of the evidence
See Table 1
All studies included in this meta‐analysis were randomised trials, with variable incidence in the blinding of participants and outcome assessors. However, we do not believe the risks of bias identified in this work would affect the direction of the reported outcome variables. Trials reported data using a mixture of per protocol and intention‐to‐treat analyses. The overall quality of the current evidence can be considered moderate. This is represented by several inconsistencies in the different findings incorporated within this review.
We graded evidence quality according to the GRADE system. We considered evidence was high quality for the incidence of adverse events, mortality and time for laboratory parameters to normalise. We considered evidence was moderate for the incidence of coronary artery abnormalities, duration of clinical symptoms (fever, rash) and length of hospital stay. We downgraded coronary artery abnormality evidence as subgroup analysis suggests that those with low‐risk scores or receiving single‐dose treatment may not have benefit. We downgraded 'duration of clinical symptoms' due to the large heterogeneity. We downgraded 'length of hospital stay' as only one very small study recorded this outcome. Subgroup analysis suggests that those with low‐risk scores or receiving single‐dose treatment may not have benefit.
Overall, this means that we are reasonably confident that the true effect is close to that estimated in this work.
Potential biases in the review process
There are no known biases to disclose in the implementation of this review. There were no limitations within the search and this study followed the predefined protocol following Cochrane guidelines.
Agreements and disagreements with other studies or reviews
The results of this review reflect the shifting paradigm regarding the use of steroids in KD and show that steroids are beneficial in the treatment of KD, fitting with their use in other vasculitic diseases. The included studies suggest that steroids enhance the resolution of fever and inflammatory markers and are associated with improved coronary artery outcomes. This is thought to be due to suppression of the inflammatory reaction in KD that causes the coronary artery abnormalities (influx of neutrophils, large mononuclear cells and lymphocytes which destroy the internal elastic lamina, followed by myofibroblast proliferation causing a coronary aneurysm (Eleftheriou 2013)). In addition, other recent systematic reviews on this question have come to similar conclusions on the efficacy of steroids for KD (Chen 2013; Zhu 2012).
Authors' conclusions
Implications for practice.
Moderate‐quality evidence shows that use of steroids in the acute phase of KD can be associated with improved coronary artery abnormalities, shorter duration of hospital stay and a decreased duration of clinical symptoms. High‐quality evidence shows improved rates of reduced inflammatory markers with the use of steroids. There were insufficient data available regarding incidence of adverse effects attributable to steroids, mortality and long‐term (> 1 year) coronary morbidity during the study periods included in this analysis.
There appears to be an increased benefit to patients of Japanese origin, higher risk score and receiving a prolonged course of steroids. That stated, there is a risk of confounding in these conclusions. Therefore more tests are needed to answer these questions. Evidence presented in this study suggests that treatment with a long course of steroids should be considered for all children diagnosed with KD until further studies are performed.
Implications for research.
This meta‐analysis partially answers the question of the utility of steroids in the treatment of the acute phase of KD. However, the follow‐up periods were generally short and data are lacking to ascertain the long‐term benefit for patients.
Further trials and increased follow‐up of previous trials is required to evaluate the longer‐term implications of steroid use in KD. More trials outside Japan of appropriate steroid use and treatment duration are also required.
Notes
This review replaces the withdrawn protocol 'Steroid hormone treatment for Kawasaki disease in children' (Jones 2014).
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MESH DESCRIPTOR Mucocutaneous Lymph Node Syndrome EXPLODE ALL TREES | 74 |
| #2 | kawasaki*:TI,AB,KY | 182 |
| #3 | (mucocutaneous near5 syndrome):TI,AB,KY | 121 |
| #4 | #1 OR #2 OR #3 | 193 |
| #5 | MESH DESCRIPTOR Glucocorticoids EXPLODE ALL TREES | 13302 |
| #6 | steroid*:TI,AB,KY | 19295 |
| #7 | (corticosteroid* or corticoid* or glucocorticoid*):TI,AB,KY | 15794 |
| #8 | dexamethasone :TI,AB,KY | 5923 |
| #9 | methylprednis*:TI,AB,KY | 3345 |
| #10 | prednisone:TI,AB,KY | 6256 |
| #11 | prednisolone:TI,AB,KY | 4294 |
| #12 | hydroxycorticosteroid*:TI,AB,KY | 113 |
| #13 | corticosterone:TI,AB,KY | 84 |
| #14 | #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 | 44334 |
| #15 | #4 AND #14 | 48 |
Appendix 2. Trials' registries searches
WHO
69 records for 65 trials found Kawasaki clinical trials in children
Clinicaltrials.gov
86 studies found for Kawasaki/Child
ISRCTN
4 results found for Kawasaki
Appendix 3. Glossary of terms
| Term | Definition |
| Aetiology | Cause of a condition |
| Defervesence | The abatement of a fever |
| Deleterious | Negative effect |
| Desquamation | Loss of the outermost layer of a surface |
| Erythematous | Reddening ‐ a term generally reserved for the skin |
| Exanthem | A virus known to be associated with a rash |
| Mucocutanoeous | Type of bodily surface, such as that inside the mouth |
| Oropharyngeal | Area of the body encompassing the mouth and throat |
| Polymorphous rash | Rash of varying appearance |
| Sequelae | Consequence |
| Stenosis | Restriction in the diameter of a vessel |
| Thrombosis | The formation of a blood clot within a blood vessel |
Data and analyses
Comparison 1. Corticosteroids vs no corticosteroid use.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of coronary artery abnormalities | 7 | 907 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.18, 0.46] |
| 2 Incidence of serious adverse effects attributable to steroid use | 6 | 737 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Mortality (all‐cause) | 7 | 915 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Duration of clinical symptoms: fever, rash | 2 | 210 | Mean Difference (IV, Random, 95% CI) | ‐1.65 [‐3.31, 0.00] |
| 5 Time for laboratory parameters to normalise: CRP, ESR | 1 | 178 | Mean Difference (IV, Fixed, 95% CI) | ‐2.80 [‐4.38, ‐1.22] |
| 6 Length of hospital stay | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐1.41 [‐2.36, ‐0.46] |
1.4. Analysis.
Comparison 1 Corticosteroids vs no corticosteroid use, Outcome 4 Duration of clinical symptoms: fever, rash.
Comparison 2. Subgroup: One‐off steroid use vs longer course of steroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Coronary artery abnormalities | 6 | 729 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.18, 0.50] |
| 1.1 One‐off steroid use | 3 | 277 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.29, 1.08] |
| 1.2 Longer course of steroids | 3 | 452 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.05, 0.32] |
Comparison 3. Subgroup: Geography.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Coronary artery abnormalities | 7 | 907 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.18, 0.46] |
| 1.1 Centres in Japan | 5 | 678 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.07, 0.29] |
| 1.2 Centres in North America | 2 | 229 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.37, 1.59] |
Comparison 4. Subgroup: High‐risk scores vs lower‐risk scores or all participants if risk score not calculated in study.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Coronary artery abnormalities | 7 | 907 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.18, 0.46] |
| 1.1 High‐risk scores | 3 | 377 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.06, 0.29] |
| 1.2 Lower‐risk scores or all participants in study if risk score not calculated | 5 | 530 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.29, 1.00] |
| 2 Duration of clinical symptoms | 2 | 210 | Mean Difference (IV, Random, 95% CI) | ‐1.65 [‐3.31, 0.00] |
| 2.1 High‐risk scores | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐2.6 [‐3.74, ‐1.46] |
| 2.2 Lower‐risk scores or all participants in study if risk score not calculated | 1 | 178 | Mean Difference (IV, Random, 95% CI) | ‐0.9 [‐1.13, ‐0.67] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ikeda 2006.
| Methods | Participants "randomly assigned" and stratified into high‐ or low‐risk groups Unclear how many centres |
|
| Participants | 178 with a diagnosis of KD were randomly assigned No statement of baseline characteristics at enrolment between groups 90 assigned to steroid, 88 to control and they were divided as follows: Low risk:
High risk:
|
|
| Interventions | Participants given control (IVIG) or treatment (IVIG + prednisolone) No information on route, duration or dose of steroid used |
|
| Outcomes | Coronary artery abnormalities Unclear at what time point outcome measure assessed |
|
| Notes | No statement of blinding participants or researchers No clear dose, route or duration of steroid use Uncear source of funding No clear definition of "coronary artery abnormalities" No indication of when measurements were taken after treatment Undertaken at Gunma Children's Medical Centre, Japan |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned" Comment: unclear how this was done |
| Allocation concealment (selection bias) | Unclear risk | Not stated Comment: impossible to know if participants or physicians were blinded to the allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated Comment: impossible to know if participants or physicians were blinded to the study treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated Comment: impossible to know if there was any blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All data present |
| Selective reporting (reporting bias) | Low risk | Published methodology consistent with that reported in published results |
| Other bias | Low risk | None identified |
Inoue 2006.
| Methods | Mulitcenter (12 centres in Gunma and Saitama, Japan) prospective non‐blinded randomised. September 2000 to March 2005. |
|
| Participants | 588 children referred to the medical institutions. 410 found to be ineligible (389 because parents decided against participation, 17 because of a previous diagnosis of KD, and 4 because a coronary artery abnormality was present before randomisation) 178 participants who were all diagnosed with KD (had at least 5 of the following: fever (> 38 °C), non‐exudative conjunctival injection, changes in the oropharynx (including mucosal erythema; dry, cracked lips; and "strawberry tongue"), changes in the extremities (including palmar and plantar erythema), oedema of the hands and feet or periungual desquamination, rash and cervical lymphadenopathy Groups well balanced in respect of baseline demographic and clinical characteristics, alongside risk score. All participants followed up for at least 2 months (range 2 to 50 months). Control: 88 Steroid: 90 |
|
| Interventions |
Control:
Treatment:
Excluding 1 participant whose steroid treatment was discontinued at the discretion of the treating physician, duration of steroid treatment 18 to 100 days (median 23 days). Total dose of prednisolone ranged from 23.5 to 90 mg/kg (median 32 mg/kg) |
|
| Outcomes |
Primary:
Secondary:
|
|
| Notes | Study terminated on 31 March 2005 by data monitoring committee at the time of the deadline despite a lower enrolment rate than expected Unclear source of funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "centre randomly assigned the patient" Comment: no clear statement of mechanism for random assignment |
| Allocation concealment (selection bias) | Low risk | Quote: "Centrally maintained table of random numbers" Comment: likely adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Non‐blinded Comment: unclear how this would have influenced results |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Echocardioraphy operators were non‐blinded Quote: "findings reviewed in a non blinded manner" Unclear if laboratory team processing blood samples were blinded Comment: unclear how this would have influenced results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Key outcome variables are reported as per protocol analysis with intention‐to‐treat analysis variable performed |
| Selective reporting (reporting bias) | Low risk | Published methodology consistent with that reported in published results |
| Other bias | Low risk | None identified |
Kobayashi 2012.
| Methods | Multicentre (74 hospitals in Japan), prospective, randomised, blinded end points 29 September 2008 to 2 December 2010 |
|
| Participants | Eligible participants had a diagnosis of KD (Japanese diagnostic guidelines) and a risk score of 5 points or higher, which emphasised the positive predictive value of no response to initial treatment with IVIG (2 points for each of the following: blood sodium < 133, < 4 days of fever at diagnosis, AST > 100, neutrophils > 80% of total WCC; 1 point for each of the following: platelet count < 30 x 104, CRP > 100, age < 12 months) Excluded were children with a history of KD, those diagnosed on or after day 9 of fever, those with coronary artery abnormalities prior to enrolment, those who were afebrile prior to enrolment, those who had received steroids in the previous 30 days before the study, those who had received IVIG in the previous 180 days before the study, those with concomitant severe medical disorders, or those with suspected infectious disease 2014 children assessed for eligibility. 1547 did not meet inclusion criteria (1436 had low risk scores, 44 had previous diagnosis of KD, 24 had defervescence, 16 had suspected infectious disease, 12 had presence of coronary artery abnormality at diagnosis, 10 had illness for > 9 days, 3 had pre‐existing severe disease, 1 had a history of IVIG use, 1 had history of steroid use, 219 declined to participate. 248 participants randomly assigned. Steroid group: 125 (4 excluded: 1 withdrew consent, 2 had presence of coronary artery abnormality, 1 had misdiagnosis of KD) Control group: 123 (2 excluded: 1 had presence of coronary artery abnormalities at enrolment, 1 did not receive IVIG) No significant differences between groups at baseline |
|
| Interventions |
Control:
Steroid group:
|
|
| Outcomes |
Primary end point:
Secondary end point:
|
|
| Notes | Funding source: Japanese Ministry of Health, Labour and Welfare – had no role in study design, data collection, data analysis, data interpretation or writing of the report RASIE study. Registered with University Hospital Medical Information Network clinical trials registry, number UMIN000000940 Pre‐planned interim analysis after enrolment of the 200th participant in June 2010 demonstrated significance difference in the incidence of coronary artery abnormalities between the 2 treatment groups (P < 0.0001), therefore independent data and safety monitoring committee recommended termination of the study. Study terminated on 2 December 2010 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated randomisation sequence" Comment: likely adequate and performed |
| Allocation concealment (selection bias) | Low risk | Quote: "Centrally maintained table of random numbers" Comment: likely adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Participant and treating physician non‐blinded" Comment: unclear how this would have influenced results |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Blinded end point" Echocardiography assessors blinded Comment: appears adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Intention to treat analysis used" Comment: appears adequate |
| Selective reporting (reporting bias) | Low risk | Published methodology consistent with that reported in published results |
| Other bias | Low risk | None identified |
Newburger 2007.
| Methods | Multicentre (8 centres in North America), randomised, double‐blind, placebo‐controlled trial. December 2002 to December 2004 |
|
| Participants | All participants between day 4 and day 10 of illness Inclusion criteria:
Exclusion criteria:
589 children identified. 276 were ineligible (185 met at least 1 exclusion criterion. 95 did not meet inclusion criteria) 313 considered eligible, of these 199 parents granted consent 199 children randomised Treatment: 101 participants Control: 98 participants No significant differences between groups at baseline randomisation |
|
| Interventions |
Control:
Treatment:
If children had recurrent fever > 36 h after the initial infusion (and no alternative source was found) then a further dose of IVIG 2 mg/kg, then if they remained pyrexial after a further 36 h another dose of IVIG 2 mg/kg was administered |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Notes | ClinicalTrials.gov number: NCT00132080 Funding: National Institutes of Health and Higgins Family Cardiology Research Fund No relevant potential conflict of interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned" with use of "dynamic balancing" at each centre Comment: no information of what "dynamic balancing" entailed |
| Allocation concealment (selection bias) | Unclear risk | Not stated Comment: impossible to know if participants or physicians were blinded to the allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind" Comment: appears adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind" Comment: appears adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Interim analysis reviewed by an independent data and safety monitoring board." Data excluded for "6 patients who were discovered to have met an exclusion criteria, for 2 patients who did not receive IV steroid despite randomisation into this group, and 8 patients who were enrolled because they had coronary abnormalities but did not meet the classic criteria for KD." |
| Selective reporting (reporting bias) | Low risk | Pre‐trial protocol published on ClinicalTrials.gov and consistent with methodology and results published |
| Other bias | Low risk | None identified |
Ogata 2012.
| Methods | Single centre in Japan (Kitasato University Hospital), unblinded with stratification of participants into high‐ and low‐risk groups. Participants were then randomly assigned to each treatment group April 2007 to November 2010 |
|
| Participants |
Incluson criteria:
Exclusion criteria:
122 participants enrolled into the study ‐ all Japanese 100% consent rate for parents High‐risk group (Egami score > 3): aspirin + IVIG = 26, aspirin + IVIG + steroid = 22 Low‐risk group (Egami score < 2; Table 2): aspirin alone = 6, aspirin + IVIG = 62 No significant differences at baseline between groups in the high‐risk group |
|
| Interventions |
Low risk group: Treatment:
Control:
High risk group: Treatment:
Control:
|
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Notes | Trial number: UMIN000005021 No conflict of interest reported Grant‐in‐aid for scientific research from Ministry of Education, Culture, Sports, Science and Technology; a Parents' Association Grant at Kitasato University School of Medicine, and a grant‐in‐aid from the Kawasaki Disease Research Centre in Japan |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Randomly assigned using a "random number list" Comment: appears adequate |
| Allocation concealment (selection bias) | Unclear risk | Not stated Comment: impossible to know if participants or physicians were blinded to the allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Non‐blinded Comment: unclear how this would have influenced results |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blinded" Comment: appears adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data included in analysis |
| Selective reporting (reporting bias) | Low risk | Published methodology consistent with that reported in published results |
| Other bias | Low risk | None identified |
Okada 2003.
| Methods | Multicentre (9 centres in Gunma Prefecture, Japan), prospective, randomised. Once participants identified, randomised into treatment groups June 2001 to May 2002 |
|
| Participants |
Inclusion criteria:
32 participants recruited:
Age, sex distribution, and severity score were similar in the 2 groups |
|
| Interventions |
Control:
Treatment:
|
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Notes | Unclear source of funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomised via a controller in the registration centre" Comment: appears adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "Controller in the registration centre" Comment: efforts made to separate randomisation from the study trial, reducing chance of information spread |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Non‐blinded Comment: unclear how this would have influenced results |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if blinded Comment: unclear how this would have influenced results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all participants appears in analysis |
| Selective reporting (reporting bias) | Low risk | Published methodology consistent with that reported in published results |
| Other bias | Low risk | None identified |
Sundel 2003.
| Methods | Single centre (Boston, North America). Prospective randomised trial. Once recruited participants were randomised to each treatment group February 1998 to November 2000 |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
71 met eligibility criteria. 10 declined consent. 20 not enrolled due to insufficient time prior to IVIG infusion. 2 excluded due to coronary artery abnormalities 39 participants recruited:
No significant differences in demographic data at enrolment |
|
| Interventions |
Control:
Treatment:
|
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Notes | Unclear funding source | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned" Comment: unclear how this was done |
| Allocation concealment (selection bias) | Unclear risk | Not stated Comment: impossible to know if participants or physicians were blinded to the allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Participants and investigators were not blinded to treatment assignment" Comment: unclear risk |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Outcome markers were generally measured objectively (e.g. temp, laboratory values), or were read by blinded observers unaware of patients' treatment status (echocardiograms)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 95% of children had ECHO at 2 weeks 93% of children had ECHO at 6 weeks (1 in treatment group, 2 in control group) |
| Selective reporting (reporting bias) | Low risk | Published methodology consistent with that reported in published results |
| Other bias | Low risk | None identified |
AST: aspartate aminotransferase CRP: C‐reactive protein ECHO: cardiac echocardiogram IV: intravenous IVIG: intravenous immunoglobulin KD: Kawasaki disease LAD: left anterior descending RCA: right coronary artery WCC: white cell count
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Asai 1985 | Intervention included dipyridamole as well as prednisolone as the difference between the groups. Effect of steroids therefore not ascertainable. Furthermore primary outcome— arterial changes — poorly defined |
| Hashino 2001 | Steroids only used if children were non‐responders to 2 treatments with IVIG. These IVIG non‐responsive children were then divided into groups allocated to receiving steroid (9 children) and those who received another dose of IVIG (8 children). This caused a significant delay in the administration of any steroids Due to the steroid treatment group being classed as non‐responders to IVIG, and the delay in receiving treatment, this study was not included as it is not comparable to any of the other studies used in this review |
| ISRCTN74427627 | Study outcome data not available despite attempts to contact author |
| Jibiki 2005 | Outcome not as per inclusion criteria of the review. Investigators assessed the laboratory mechanism of action of IVIG and steroid therapy in KD |
| Kato 1979 | Study outcome data not available despite attempts to contact author. Available outcome information does not provide objective data but categorises into aneurysm vs no aneurysm with inadequate detail on exact approach |
| Kusakawa 1983 | Study outcome data not available despite attempts to contact author. Available outcome information does not provide objective data but categorises into aneurysm versus no aneurysm with inadequate detail on exact approach |
| Miura 2008 | Outcome not as per inclusion criteria. Investigators assessed the laboratory mechanism of action of IVIG and steroid therapy in KD |
| Nakamura 1985 | Outcome not as per inclusion criteria of the review. The outcome of this study was regarding platelet laboratory processes, which was not an outcome measure of this review |
| Nonaka 1995 | Study outcome data not available despite attempts to contact author. Available outcome information does not provide objective data but categorises into aneurysm vs no aneurysm with inadequate detail on exact approach |
| Ogata 2009 | Outcome not as per inclusion criteria of the review. Investigators assessed the molecular mechanism of action of IVIG and steroid therapy in KD |
| Sekine 2012 | Outcome not as per inclusion criteria of the review. The outcome of this study was oxidative stress, which was not an outcome measure of this review |
| Seto 1983 | Inadequate information on methodology for validation, specifically no evidence of randomisation |
| Xu 2002 | Interventions included immunoglobulin versus steroids, meaning steroids were not the only differential factor between the groups and therefore their effect cannot be clearly defined. Inadequate information on methodology, specifically around randomisation, despite contacting authors |
| Yuan 2000 | Outcome not as per inclusion criteria of the review. Study does not clearly define coronary aneurysm or how this was measured |
IVIG: intravenous immunoglobulin
Characteristics of ongoing studies [ordered by study ID]
JPRN‐UMIN000009524.
| Trial name or title | A prospective randomised controlled trial of immunoglobulin plus prednisolone for KD patients with high risk for coronary abnormalities |
| Methods | Parallel, assessor‐blinded study Control group: Intravenous immunoglobulin (2 g/kg) over 24 h with aspirin (30 mg/kg/day). The dosage of aspirin can be reduced to 5 mg/kg/day after resolution of fever Treatment group: Intravenous immunoglobulin (2 g/kg) over 24 h plus intravenous prednisolone (2 mg/kg/day) with aspirin (30 mg/kg/day). The dosage of aspirin can be reduced to 5 mg/kg/day after resolution of fever Prednisolone will be given intravenously for 3 days at least and then can be given orally after resolution of fever. When concentration of C‐reactive protein becomes 1.0 mg/dL or less, the dose of prednisolone will be tapered over 9 days |
| Participants | Target sample size 240 Inclusion criteria: 1. Severe KD patients of risk score 4 points or more 2. Written informed consent is obtained from patients or their parents 3. Streptococcus, Epstein‐Barr virus, adenovirus, or yersinia infection, or measles, or Stevens‐Johnson syndrome is ruled out Exclusion criteria: 1. Patients with past histories of KD 2. Patients diagnosed as KD on the ninth day of illness or later 3. KD patients with coronary artery lesions before treatment 4. KD patients with defervescence before treatment 5. Patients given steroids within 28 days before treatment 6. Patients given IVIG within 180 days before treatment 7. Patients with severe underlining diseases 8. Patients with a concurrent infection |
| Interventions |
Control group: Intravenous immunoglobulin (2 g/kg) over 24 h with aspirin (30 mg/kg/day). The dosage of aspirin can be reduced to 5 mg/kg/day after resolution of fever Treatment group: Intravenous immunoglobulin (2 g/kg) over 24 h plus intravenous prednisolone (2 mg/kg/day) with aspirin (30 mg/kg/day). The dosage of aspirin can be reduced to 5 mg/kg/day after resolution of fever Prednisolone will be given intravenously for 3 days at least and then can be given orally after resolution of fever. When concentration of C‐reactive protein becomes 1.0 mg/dL or less, the dose of prednisolone will be tapered over 9 days |
| Outcomes |
Primary outcome: Incidence of coronary artery lesions within 4 weeks after primary treatment Secondary outcome: Incidence of coronary artery lesions at 4 weeks after primary treatment, z‐score of coronary artery diameters, incidence of resistance to primary treatment or relapse, duration of fever after primary treatment, serum concentrations of C‐reactive protein at 1 week and 2 weeks after primary treatment, and incidence of adverse events |
| Starting date | 17 December 2012 |
| Contact information | Taichi Kato Nagoya University Graduate School of Medicine Department of Pediatrics 65 Tsuruma‐cho, Showa‐ku, Nagoya 466‐8550, Japan +81‐52‐744‐2298 ktaichi@med.nagoya‐u.ac.jp |
| Notes |
IVIG: Intravenous immunoglobulin
Differences between protocol and review
In conducting this study it was decided that we would refer to coronary 'aneurysms' in the context of coronary 'abnormalities'. This is due to a wide variation in the literature, including trials included in this review, as to what the definition of an aneurysm is.
Odds ratio was used instead of risk ratio to report dichotomous data, in line with standard statistical analysis.
We renamed the outcome 'incidence of any adverse effects...' to 'incidence of serious adverse events' to reflect more accurately the side effects we intended to study.
Contributions of authors
AJW was involved in the conception, creation and reviewing of the protocol. AJW is the guarantor of the review. AJW and MJS were involved in the drafting of the protocol and identification of studies. GMC and AJW performed the analysis. RMRT was involved in the final reviewing and re‐drafting of the review for submission.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
AJW: none known GMC: none known MJS: none known RMRT: none known
New
References
References to studies included in this review
Ikeda 2006 {published data only}
- Ikeda K, Kobayashi T, Inoue Y, Okada Y, Tomomasa T, Shinohara M, et al. Risk stratification and effectiveness of intravenous immunoglobulin plus prednisolone as the initial treatment of kawasaki disease. European Journal of Pediatrics 2006;165(Suppl 1):38‐9. [Google Scholar]
Inoue 2006 {published data only}
- Inoue Y, Okada Y, Shinohara M, Kobayashi T, Kobayashi T, Tomomasa T, et al. A multicentre prospective randomized trial of corticosteroids in primary therapy for kawasaki disease: clinical course and coronary artery outcome. Journal of Pediatrics 2006;149(3):336‐41. [DOI] [PubMed] [Google Scholar]
Kobayashi 2012 {published data only}
- Anon. Randomized controlled trial to Assess Immunoglobulin plus Steroid Efficacy for Kawasaki disease (protocol). apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000000940 2008.
- Ikeda K, Saji T, Kobayashi T, Yahata T, Okamoto A, Arakawa H, et al. Optimal timing of initial treatment in severe Kawasaki disease: A sub‐analysis of the RAISE study. Cardiology in the Young 2013;23:S144. [Google Scholar]
- Ishii M, Ogata S, Honda T et Ogihatra Y. The efficacy and safety of new strategy for refractory Kawasaki disease. Cardiology in the Young 2011;18(21):(Suppl 1). [Google Scholar]
- Kobayashi T, Saji T, Otani T, Takeuchi K, Nakamura T, Arakawa H, et al. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease. Pediatrics International 2012;54(Suppl 1):53. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Saji T, Otani T, Takeuchi K, Nakamura T, Arakawa H, et al. Significance of primary therapy with intravenous immunoglobulin plus prednisolone for severe Kawasaki disease: result from Japanese multicenter randomized clinical trial. Circulation 2011;124:A9271. [Google Scholar]
- Kobayashi T, Saji T, Otani T, Takeuchi K, Nakamura T, Arakawa H, et al. RAISE study group investigators. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open‐label, blinded‐endpoints trial. Lancet 2012;379(9826):1613‐20. [DOI] [PubMed] [Google Scholar]
Newburger 2007 {published data only}
- Newburger JW, Sleeper LA, McCrindle BW, Minich LL, Gersony W, Vetter VL, Paediatric Heart Network Investigators. Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. New England Journal of Medicine 2007;356(7):663‐75. [DOI] [PubMed] [Google Scholar]
Ogata 2012 {published data only}
- Ishii M, Ogata S, Ogihara Y, Sato K, Ebato T. Clinical utility of a new strategy for preventing coronary artery lesions in refractory Kawasaki disease patients: a randomized prospective study. Pediatrics International 2012;54(Suppl 1):104. [Google Scholar]
- Ogata S, Ogihara Y, Honda T, Kon S, Akiyama K, Ishii M. Cortocosteroid pulse combination therapy for refractory Kawasaki disease: a randomized trial. Pediatrics 2012;129(1):e17‐e23. [DOI] [PubMed] [Google Scholar]
Okada 2003 {published data only}
- Okada Y, Shinohara M, Kobayashi T, Inoue Y, Tomomasa T, Kobayashi T, et al. Gunma Kawasaki Disease Study Group. Effect of corticosteroids in addition to intravenous gamma globulin therapy on serum cytokine levels in the acute phase of Kawasaki disease in children. Journal of Pediatrics 2003;143(3):363‐7. [DOI] [PubMed] [Google Scholar]
Sundel 2003 {published data only}
- Sundel RP, Baker AL, Fulton DR, Newburger JW. Randomized trial of pulse steroids in the initial treatment of Kawasaki disease (KD). Pediatric Research 2003;53:164. [DOI] [PubMed] [Google Scholar]
- Sundel RP, Baker AL, Fulton DR, Newburger JW. Corticosteroids in the initial treatment of Kawasaki disease: report of a randomised trial. Journal of Pediatrics 2003;142(6):611‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Asai 1985 {published data only}
- Asai T. Follow up data of prospective controlled study for the treatment, clinical sign and laboratory data of Kawasaki disease. Shonika. Pediatrics of Japan 1985;26:995‐1004. [Google Scholar]
Hashino 2001 {published data only}
- Hashino K, Ishii M, Iemura M, Akagi T, Kato H. Re‐treatment for immune globulin‐resistant Kawasaki disease: a comparative study of additional immune globulin and steroid pulse therapy. Pediatrics International 2001;43(3):211‐7. [DOI] [PubMed] [Google Scholar]
ISRCTN74427627 {published data only}
- ISRCTN74427627. Multi‐centre randomised placebo‐controlled trial of corticosteroids in the prevention of coronary artery abnormalities in acute Kawasaki Disease (KD). ISRCTN Register 2005 (date accessed 29th May 2016). [URL: http://www.isrctn.com/ISRCTN74427627]
Jibiki 2005 {published data only}
- Jibiki T, Terai M, Kurosaki T, Nakajima H, Suzuki K, Inomata H, et al. Efficacy of intravenous immune globulin therapy combined with dexamethasone for the initial treatment of acute Kawasaki disease. European Journal of Pediatrics 2004;163(4‐5):229‐33. [DOI] [PubMed] [Google Scholar]
Kato 1979 {published data only}
- Kato H, Koike S, Yokoyama T. Kawasaki disease: effect of treatment on coronary artery involvement. Pediatrics 1979;63(2):175‐9. [PubMed] [Google Scholar]
Kusakawa 1983 {published data only}
- Kusakawa S. A prospective study for treatment of Kawasaki Disease with 3 regimes: aspirin, flubiprofen, predonisolone + dipyridamole. Nippon Shonika Gakkai Zasshi 1983;87:2465‐91. [Google Scholar]
- Kusakawa S. A randomised controlled study of three different therapies for patients having Kawasaki disease. Nippon Shonika Gakkai Zasshi 1986;90:1844‐9. [Google Scholar]
- Kusakawa S. Studies on the treatment of Kawasaki disease during acute stage. Nippon Shonika Gakkai Zasshi 1985;89:814‐18. [Google Scholar]
Miura 2008 {published data only}
- Miura M, Kohno K, Ohki H, Yoshiba S, Sugaya A, Satoh M. Effects of methylprednisolone pulse on cytokine levels in Kawasaki disease patients unresponsive to intravenous immunoglobulin. European Journal of Pediatrics 2008;167(10):1119‐23. [DOI] [PubMed] [Google Scholar]
Nakamura 1985 {published data only}
- Nakamura T. Studies on activated platelets in Kawasaki disease. Nippon Shonika Gakkai Zasshi 1985;89:1845‐60. [Google Scholar]
Nonaka 1995 {published data only}
- Nonaka Z, Maekawa K, Okabe T, Eto Y, Kubo M. Randomized controlled study of intravenous prednisolone and gammaglobulin treatment in 100 cases with Kawasaki disease. In: Kato H editor(s). Kawasaki Disease. Amsterdam: Elsevier Science, 1995:328‐31. [Google Scholar]
- Nonaka Z, Usui N, Mackawa K. The combination therapy with gamma‐globulin and prednisolone is an supportive or alternative for Kawasaki disease in high risk. Pediatric Research 2000;47(4):565. [Google Scholar]
Ogata 2009 {published data only}
- Ogata S, Ogihara Y, Nomoto K, Akiyama K, Nakahata Y, Sato K, et al. Clinical score and transcript abundance patterns identify kawasaki disease patients who may benefit from addition of Methylprednisolone. Pediatric Research 2009;66(5):577‐84. [DOI] [PubMed] [Google Scholar]
Sekine 2012 {published data only}
- Sekine K, Mochizuki H, Inoue Y, Kobayashi T, Suganuma E, Matsuda S, et al. Regulation of oxidative stress in patients with Kawasaki disease. Inflammation 2012;35(3):952‐8. [DOI] [PubMed] [Google Scholar]
Seto 1983 {published data only}
- Seto S. A controlled trial of steroid pulse therapy for Kawasaki disease. Nippon Shonika Gakkai Zasshi 1983;87:399‐406. [Google Scholar]
Xu 2002 {published data only}
- Xu JH, Luo XL. Observation of coronary artery diseases in kawasaki diseases with methylprednisolone. Journal of applied Clinical Pediatrics 2002;17(6):680‐1. [Google Scholar]
Yuan 2000 {published data only}
- Yuan Y. Analysis of recent effects for corticosteroid in treating Kawasaki disease. Chinese Journal of Practical Paediatrics 2000;15:49. [Google Scholar]
References to ongoing studies
JPRN‐UMIN000009524 {published data only}
- JPRN‐UMIN000009524. A prospective randomized controlled trial of immunoglobulin plus prednisolone for Kawasaki disease patients with high risk for coronary abnormalities. WHO clinical trials 2012 (date accessed 29th May 2016). [URL: http://apps.who.int/trialsearch/Trial.aspx?TrialID=JPRN‐UMIN000009524]
Additional references
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ayusawa 2005
- Ayusawa M, Sonobe T, Uemura S, Ogawa S, Nakamura Y, Kiyosawa N, et al. Kawasaki Disease Research Committee. Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition). Pediatrics International 2005;47(2):232‐4. [DOI] [PubMed] [Google Scholar]
Baumer 2006
- Baumer JH, Love SJ, Gupta A, Haines LC, Maconochie I, Dua JS. Salicylate for the treatment of Kawasaki disease in children. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD004175.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brogan 2002
- Brogan P, Bose A, Burgner D, Shingadia D, Tulloh R, Michie C, et al. Kawasaki disease: an evidence based approach to diagnosis, treatment, and proposals for future research. Archives of Disease in Childhood 2002;86(4):286‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burns 1996
- Burns JC, Shike H, Gordon JB, Malhotra A, Schoenwetter M, Kawasaki T. Sequelae of Kawasaki disease in adolescents and young adults. Journal of the American College of Cardiology 1996;28(1):253‐7. [DOI] [PubMed] [Google Scholar]
Chen 2013
- Chen S, Dong Y, Yin Y, Krucoff MW. Intravenous immunoglobulin plus corticosteroid to prevent coronary artery abnormalities in Kawasaki disease: a meta‐analysis. Heart 2013;99(2):76‐82. [DOI] [PubMed] [Google Scholar]
Daniels 2012
- Daniels LB, Gordon JB, Burns JC. Kawasaki disease: late cardiovascular sequelae. Current Opinion in Cardiology 2012;27(6):572‐7. [DOI] [PubMed] [Google Scholar]
Davies 2013
- Davies S, Gold‐von Simson G. Should infliximab be used as an adjuvant to IVIG in the treatment of children with Kawasaki disease who are at high risk for resistance to conventional therapy?. Pediatric Cardiology 2013;34(7):1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Zorzi 1998
- Zorzi A, Colan SD, Gauvreau K, Baker AL, Sundel RP, Newburger JW. Coronary artery dimensions may be misclassified as normal in Kawasaki disease. Journal of Pediatrics 1998;133(2):254–8. [DOI] [PubMed] [Google Scholar]
Eleftheriou 2013
- Eleftheriou D, Levin M, Shingadia D, Tulloh R, Klein NJ, Brogan PA. Management of Kawasaki disease. Archives of Disease in Childhood 2013;99(1):74‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gardner‐Medwin 2002
- Gardner‐Medwin JM, Dolezalova P, Cummins C, Southwood TR. Incidence of Henoch‐Schönlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet 2002;360(9341):1197‐202. [DOI] [PubMed] [Google Scholar]
Harnden 2002
- Harnden A, Alves B, Sheikh A. Rising incidence of Kawasaki disease in England: analysis of hospital admission data. BMJ 2002;324(7351):1424‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hashino 2001b
- Hashino K, Ishii M, Iemura M, Akagi T, Kato H. Re‐treatment for immune globulin‐resistant Kawasaki disease: a comparative study of additional immune globulin and steroid pulse therapy. Pediatrics International 2001;43(3):211‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hokosaki 2012
- Hokosaki T, Mori M, Nishizawa T, Nakamura T, Imagawa T, Iwamoto M, et al. Long‐term efficacy of plasma exchange treatment for refractory Kawasaki disease. Pediatrics International 2012;54(1):99‐103. [DOI] [PubMed] [Google Scholar]
Holman 2003
- Holman RC, Curns AT, Belay ED, Steiner CA, Schonberger LB. Kawasaki syndrome hospitalizations in the United States; 1997 and 2000. Pediatrics 2003;112(3 Pt 1):495‐501. [DOI] [PubMed] [Google Scholar]
Jones 2014
- Jones KS, Baumer JH, Gupta A, Dua JS. Steroid hormone treatment for Kawasaki disease in children. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD003284.pub3] [DOI] [Google Scholar]
Kato 1996
- Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, et al. Long‐term consequences of Kawasaki disease. A 10‐ to 21‐year follow‐up study of 594 patients. Circulation 1996;94(6):1379‐85. [DOI] [PubMed] [Google Scholar]
Kawasaki 1967
- Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi 1967;16(3):178‐222. [PubMed] [Google Scholar]
Kobayashi 2008
- Kobayashi T, Inoue Y, Morikawa A. Risk stratification and prediction of resistance to intravenous immunoglobulin in Kawasaki disease. Japanese Journal of Clinical Medicine 2008;66(2):332‐7. [PubMed] [Google Scholar]
Levin 1991
- Levin M, Tizard EJ, Dillon MJ. Kawasaki disease: recent advances. Archives of Disease in Childhood 1991;66(12):1369‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levin 2013
- Levin M. Steroids for Kawasaki disease: The devil is in the detail. Heart 2013;99(2):69‐70. [DOI] [PubMed] [Google Scholar]
Nakamura 2012
- Nakamura Y, Yashiro M, Uehara R, Sadakane A, Tsuboi S, Aoyama Y, et al. Epidemiologic features of Kawasaki disease in Japan: results of the 2009‐2010 nationwide survey. Journal of Epidemiology 2012;22(3):216‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newburger 2004
- Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long‐term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 2004;110(17):2747‐71. [DOI] [PubMed] [Google Scholar]
Oates‐Whitehead 2003
- Oates‐Whitehead RM, Baumer JH, Haines L, Love S, Maconochie IK, Gupta A, et al. Intravenous immunoglobulin for the treatment of Kawasaki disease in children. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD004000] [DOI] [PMC free article] [PubMed] [Google Scholar]
Research Committee on Kawasaki Disease 1984
- Ministry of Health and Welfare Japan. Report of Subcommittee on standardisation of diagnostic criteria and reporting of coronary artery lesions in Kawasaki Disease. Research Committee on Kawasaki Disease 1984.
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Singh 2015
- Singh S, Vignesh P, Burgner D. The epidemiology of Kawasaki disease: a global update. Archives of Disease in Childhood 2015;100(11):1084‐8. [DOI] [PubMed] [Google Scholar]
Sleeper 2011
- Sleeper LA, Minich LL, McCrindle BM, Li JS, Mason W, Colan SD, et al. Pediatric Heart Network Investigators. Evaluation of Kawasaki disease risk‐scoring systems for intravenous immunoglobulin resistance. Journal of Pediatrics 2011;158(5):831‐5, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tatara 1987
- Tatara K, Kusakawa S. Long‐term prognosis of giant coronary aneurysm in Kawasaki disease: an angiographic study. Journal of Pediatrics 1987;111(5):705‐10. [DOI] [PubMed] [Google Scholar]
Wood 2009
- Wood LE, Tulloh RM. Kawasaki disease in children. Heart 2009;95(10):787‐92. [DOI] [PubMed] [Google Scholar]
Zhu 2012
- Zhu BH, LV HT, Sun L, Zhang JM, Cao L, Jia HL, et al. A meta‐analysis on the effect of corticosteroid therapy in Kawasaki disease. European Journal of Pediatrics 2012;171(3):571‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Wardle 2014
- Wardle AJ, Kiddy HC, Seager MJ, Tulloh RMR, Levin M. Corticosteroids for the treatment of Kawasaki disease in children. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD011188] [DOI] [PMC free article] [PubMed] [Google Scholar]


