Abstract
Background
Nitric oxide (NO) is a major endogenous regulator of vascular tone. Inhaled nitric oxide (iNO) gas has been investigated as treatment for persistent pulmonary hypertension of the newborn.
Objectives
To determine whether treatment of hypoxaemic term and near‐term newborn infants with iNO improves oxygenation and reduces rate of death and use of extracorporeal membrane oxygenation (ECMO), or affects long‐term neurodevelopmental outcomes.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 1), MEDLINE via PubMed (1966 to January 2016), Embase (1980 to January 2016) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to January 2016). We searched clinical trials databases, conference proceedings and reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials. We contacted the principal investigators of studies published as abstracts to ascertain the necessary information.
Selection criteria
Randomised studies of iNO in term and near‐term infants with hypoxic respiratory failure, with clinically relevant outcomes, including death, use of ECMO and oxygenation.
Data collection and analysis
We analysed trial reports to assess methodological quality using the criteria of the Cochrane Neonatal Review Group. We tabulated mortality, oxygenation, short‐term clinical outcomes (particularly use of ECMO) and long‐term developmental outcomes.
Statistics: For categorical outcomes, we calculated typical estimates for risk ratios and risk differences. For continuous variables, we calculated typical estimates for weighted mean differences. We used 95% confidence intervals and assumed a fixed‐effect model for meta‐analysis.
Main results
We found 17 eligible randomised controlled studies that included term and near‐term infants with hypoxia.
Ten trials compared iNO versus control (placebo or standard care without iNO) in infants with moderate or severe severity of illness scores (Ninos 1996; Roberts 1996; Wessel 1996; Davidson 1997; Ninos 1997; Mercier 1998; Christou 2000; Clark 2000; INNOVO 2007; Liu 2008). Mercier 1998 compared iNO versus control but allowed back‐up treatment with iNO for infants who continued to satisfy the same criteria for severity of illness after two hours. This trial enrolled both preterm and term infants but reported most results separately for the two groups. Ninos 1997 studied only infants with congenital diaphragmatic hernia.
One trial compared iNO versus high‐frequency ventilation (Kinsella 1997).
Six trials enrolled infants with moderate severity of illness scores (oxygenation index (OI) or alveolar‐arterial oxygen difference (A‐aDO2)) and randomised them to immediate iNO treatment or iNO treatment only after deterioration to more severe criteria (Barefield 1996; Day 1996; Sadiq 1998; Cornfield 1999; Konduri 2004; Gonzalez 2010).
Inhaled nitric oxide appears to have improved outcomes in hypoxaemic term and near‐term infants by reducing the incidence of the combined endpoint of death or use of ECMO (high‐quality evidence). This reduction was due to a reduction in use of ECMO (with number needed to treat for an additional beneficial outcome (NNTB) of 5.3); mortality was not affected. Oxygenation was improved in approximately 50% of infants receiving iNO. The OI was decreased by a (weighted) mean of 15.1 within 30 to 60 minutes after the start of therapy, and partial pressure of arterial oxygen (PaO2) was increased by a mean of 53 mmHg. Whether infants had clear echocardiographic evidence of persistent pulmonary hypertension of the newborn (PPHN) did not appear to affect response to iNO. Outcomes of infants with diaphragmatic hernia were not improved; outcomes were slightly, but not significantly, worse with iNO (moderate‐quality evidence).
Infants who received iNO at less severe criteria did not have better clinical outcomes than those who were enrolled but received treatment only if their condition deteriorated. Fewer of the babies who received iNO early satisfied late treatment criteria, showing that earlier iNO reduced progression of the disease but did not further decrease mortality nor the need for ECMO (moderate‐quality evidence). Incidence of disability, incidence of deafness and infant development scores were all similar between tested survivors who received iNO and those who did not.
Authors' conclusions
Inhaled nitric oxide is effective at an initial concentration of 20 ppm for term and near‐term infants with hypoxic respiratory failure who do not have a diaphragmatic hernia.
Plain language summary
Nitric oxide for respiratory failure in infants born at or near term
Review question: Is inhaled nitric oxide gas, in addition to standard therapy, beneficial for babies born at full term who have lung disease leading to low levels of oxygen in the blood? Specifically, does it reduce the death rate or the number of babies who require highly invasive ECMO treatment?
Background: Nitric oxide is a naturally occurring molecule that relaxes blood vessels and is active in the lungs when mixed with the gases that a patient is breathing.
Study characteristics: In a search updated to February 2016, review authors identified a total of 17 studies for inclusion in the review. Most of the results reported in this review were obtained from 10 studies of moderate to high quality, which compared inhaled nitric oxide (iNO) versus standard therapy without iNO. Six studies compared iNO started when babies were less sick against waiting to see if they deteriorated, then treating them later. These studies were smaller, and only one was a high‐quality trial.
Key results: Inhaled nitric oxide is safe and can help some full‐term babies with respiratory failure who have not responded to other methods of support. Inhaled nitric oxide increases levels of oxygen in babies' blood, and babies are more likely to survive without needing ECMO, a highly invasive therapy with many complications. Unfortunately, benefits of iNO are not clear in babies whose respiratory failure is due to a diaphragmatic hernia. Inhaled nitric oxide has shown no short‐term or long‐term adverse effects. No signs suggest that iNO given earlier is more beneficial or results in more babies treated, and the number who die or who need ECMO is not significantly reduced.
Summary of findings
Background
Description of the condition
Persistent pulmonary hypertension of the newborn (PPHN) is an important cause of cardiorespiratory failure in the near‐term neonate (> 34 weeks), as a primary condition of neonatal maladaptation or secondary to other diseases such as hyaline membrane disease (HMD), meconium aspiration, infection and congenital diaphragmatic hernia (CDH). A review of the Oxford Database of Perinatal Trials performed in 1996, which included PPHN as an outcome, showed that at that time, no single randomised controlled trial (RCT) had demonstrated that PPHN could be prevented by any perinatal intervention. PPHN is a common underlying factor among infants who qualify for treatment with extracorporeal membrane oxygenation (ECMO).
Before inhaled nitric oxide (iNO) was available, conventional therapy involved paralysis, sedation and induction of alkalosis by hyperventilation and bicarbonate. None of these therapies were proven by prospective randomised trials to reduce mortality nor the need for ECMO. In addition, no clinically evaluated selective pulmonary vasodilator was free of systemic side effects.
Description of the intervention
Addition of low concentrations of nitric oxide gas to the inhaled gas mixture.
How the intervention might work
Regulation of vascular muscle tone at the cellular level occurs, in part, via nitric oxide (NO). Nitric oxide is generated enzymatically by one of three NO synthases from L‐arginine. NO activates guanyl cyclase by binding to its heme component, leading to the production of cyclic guanosine monophosphate (cGMP), which leads to relaxation of vascular smooth muscle.
Abman and coworkers (Abman 1990) showed, in the late gestation ovine fetus, that inhibition of NO production caused foetal pulmonary and systemic hypertension with attenuation of the rise in pulmonary blood flow at delivery. They also demonstrated that inhibition of NO formation impeded the pulmonary vasodilation produced by ventilation, increasing oxygen tension and shear stress (Cornfield 1992), and that inhalation of NO by the ovine foetus caused sustained and selective pulmonary vasodilation (Kinsella 1992a).
In newborn mammals, several models of pulmonary hypertension are reversed by iNO (Fratacci 1991; Frostell 1991; Roberts 1993; Etches 1994). None of these studies reported an effect on systemic vascular resistance.
In adults with pulmonary hypertension, the initial observation by Higenbottam and coworkers (Higenbottam 1988) that iNO decreases pulmonary vascular resistance was confirmed by Pepke‐Zaba and coworkers (Pepke‐Zaba 1991), who showed that iNO (40 ppm) reduced pulmonary hypertension with no change in systemic vascular resistance. In adults with acute respiratory distress syndrome, Rossaint and coworkers (Rossaint 1993) showed a reduction in pulmonary arterial pressure and a decrease in intrapulmonary shunting within 40 minutes of inhalation of NO. In these patients, improvement in oxygenation was due largely to improved ventilation/perfusion matching.
Why it is important to do this review
Two uncontrolled reports in neonates with PPHN from separate groups working in Denver (Kinsella 1992b) and Boston (Roberts 1992) indicated that iNO rapidly produced a significant improvement in preductal oxygen saturation, with no detectable toxicity. The Boston group (Roberts 1992) revealed that concentrations of 80 ppm of iNO were required to achieve a response, whereas the Denver group (Kinsella 1992b) observed beneficial effects with only 10 to 20 ppm. This latter group reported on nine additional infants treated with iNO, eight of whom showed a sustained beneficial response (Kinsella 1993). Finer and coworkers conducted a prospective evaluation of the dose response to iNO in infants referred for possible ECMO therapy (Finer 1994). Inhalation of NO was associated with significant improvements in partial pressure of arterial oxygen (PaO2) and decreases in alveolar‐arterial oxygen difference (A‐aDO2) and oxygenation index (OI) among 14 of 21 studied infants. Investigators noted no difference in response between 5 ppm and higher concentrations.
Preliminary evidence led to prospective randomised controlled clinical trials conducted to evaluate the role of iNO in infants with respiratory failure.
Objectives
To determine whether treatment of hypoxaemic term and near‐term newborn infants with iNO improves oxygenation and reduces rate of death and use of extracorporeal membrane oxygenation (ECMO), or affects long‐term neurodevelopmental outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised prospective clinical trials.
Types of participants
Participants were newborn infants (< 1 month of age) with hypoxaemia suspected to be due to lung disease, pulmonary hypertension with right‐to‐left shunting or both. We included only studies in term and near‐term infants (> 34 weeks' gestation). In all studies, investigators made an effort to exclude infants with intracardiac shunting due to structural congenital heart disease. As infants with congenital diaphragmatic hernia (CDH) may respond differently from other near‐term infants (from preliminary data), as far as possible results for these infants were evaluated separately.
Types of interventions
Studies compared administration of iNO gas versus control ‐ no gas or placebo gas.
Types of outcome measures
Primary outcomes
Death or use of ECMO
Death before hospital discharge
Use of ECMO before hospital discharge
Secondary outcomes
Improvement in oxygenation (as a dichotomous variable) within 30 to 60 minutes
Effects on oxygenation index after 30 to 60 minutes of therapy (both absolute values and change from baseline)
Effects on partial pressure of arterial oxygen (PaO2) after 30 to 60 minutes of therapy (both absolute values and change from baseline)
Neurodevelopmental disability at 18 to 24 months
Cerebral palsy
Cognitive impairment at 18 to 24 months
Deafness
Outcomes added post hoc
Change in oxygenation index after treatment
Change in PaO2 after treatment
Chronic lung disease
Search methods for identification of studies
We used Cochrane criteria and standard methods and those of the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register).
Electronic searches
For the 1998 review, we used standard methods of the Neonatal Collaborative Review Group. We performed the first MEDLINE search in March 1997, using the search engine 'Melvyl medline plus' and the following search terms: 'key word=nitric', and 'subject heading = infant, newborn'.
For the 2016 update, we conducted a comprehensive search that included the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 1) in the Cochrane Library; MEDLINE via PubMed (1966 to January 2016); Embase (1980 to January 2016); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to January 2016), using the following search terms: (Nitric OR Nitrix Oxide), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). We applied no language restrictions.
Searching other resources
For the 1998 review, we searched Society for Pediatric Research meeting abstracts on diskette for 1995, 1996 and 1997, using the terms 'control, controls or controlled' and 'nitric or NO'.
For the 2016 update, we searched clinical trials registries for ongoing and recently completed trials (clinicaltrials.gov; the World Health Organization International Trials Registry and Platform (www.whoint/ictrp/search/en/); and the ISRCTN Registry). We also searched conference abstracts from the Pediatric Academic Societies (PAS) and the European Society for Paediatric Research (ESPR). We carried out searches in Abstracts 2 View (2000 to 2014) and Pediatric Research.
Data collection and analysis
For each included study, we collected information regarding method of randomisation, blinding, drug intervention, stratification and whether the trial was a single‐centre or multi‐centre study. We noted information regarding trial participants, including gestational age criteria, birth weight criteria and other inclusion or exclusion criteria. We analysed information on clinical outcomes, including death or use of ECMO, death before hospital discharge, use of ECMO before hospital discharge, improvement in oxygenation (as a dichotomous variable) within 30 to 60 minutes, effects on OI after 30 to 60 minutes of therapy (both absolute values and changes from baseline), effects on PaO2 after 30 to 60 minutes of therapy (both absolute values and changes from baseline), neurodevelopmental disability at 18 to 24 months, cerebral palsy, cognitive impairment at 18 to 24 months and deafness.
Selection of studies
We included all randomised and quasi‐randomised controlled trials fulfilling the selection criteria described in the previous section. All review authors reviewed results of the search, separately selected studies for inclusion and resolved disagreements by discussion.
Data extraction and management
KB, TP and GA extracted, assessed and coded all data for each study, for studies newly added to this version of the review, using the same categories as were used in the previous version. We replaced any standard error of the mean reported with the corresponding standard deviation and resolved disagreements by discussion. For each study, one review author (KB) entered final data into Review Manager (RevMan 2014), and the other review authors (NF, TP, GA) checked the data. All review authors reviewed the protocol, analysis and draft manuscript.
Assessment of risk of bias in included studies
We employed standard methods of the Cochrane Neonatal Group. We assessed methodological quality of studies by using the following key criteria: allocation concealment (blinding of randomisation), blinding of intervention, completeness of follow‐up and blinding of outcome measurement/assessment. We assessed each criterion as introducing high, low or unclear risk. We included this information in the Characteristics of included studies table.
In addition, review authors independently assessed risk of bias for each study by using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We assessed methodological quality of studies by using the following criteria.
Sequence generation (evaluating possible selection bias). For each included study, we described the method used to generate the allocation sequence as adequate (any truly random process, e.g. random number table; computer random number generator), inadequate (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number; or an unverifiable process, e.g. coin toss) or unclear.
Allocation concealment (evaluating possible selection bias). For each included study, we described the method used to conceal the allocation sequence as adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes), inadequate (open random allocation; unsealed or non‐opaque envelopes; alternation; date of birth) or unclear.
Blinding (evaluating possible performance bias). For each included study, we described methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes, and we assessed methods as adequate, inadequate or unclear for participants; adequate, inadequate or unclear for study personnel; and adequate, inadequate or unclear for outcome assessors.
Incomplete outcome data (evaluating possible attrition bias through withdrawals, drop‐outs, protocol deviations). For each included study and for each outcome, we described the completeness of data including attrition and exclusions from analysis. We stated whether attrition and exclusions were reported, numbers included in the analysis at each stage (compared with total randomised participants) and reasons for attrition or exclusion when reported, and whether missing data were balanced across groups or were related to outcomes. We assessed methods as adequate (< 20% missing data), inadequate (≥ 20% missing data) or unclear.
Selective reporting bias. For each included study for which the protocol was available (through trials registers), we investigated the possibility of selective outcome reporting bias and reported what we found. We assessed methods as adequate (when it was clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review had been reported), inadequate (when not all of the study's prespecified outcomes had been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; or study failed to include results of a key outcome that would have been expected to have been reported) or unclear.
Other sources of bias. We noted other possible sources of bias (e.g. whether a potential source of bias was related to the specific study design, whether the trial was stopped early owing to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as yes, no or unclear.
Funding sources. For each study, we attempted to determine the nature and, if possible, the role of the funding source. Using published or registered protocols and the final publication of the main trial results, we determined whether funds for the trial were obtained from local agencies (e.g. research groups, hospital foundations, university funds), charitable sources (local, national or international foundations), government agencies or industry sources. If potential funding conflicts of interest were evident (particularly with industry funding), we evaluated the role of the funding source. We determined that when funding was received entirely from industry, studies were at high risk of bias. In cases of funding received in part from industry sources, we evaluated risk of bias according to the apparent role of the funding source.
Measures of treatment effect
We performed statistical analyses using Review Manager 5 software (RevMan 2014). We analysed categorical data using risk ratio (RR) and risk difference (RD). For statistically significant outcomes, we calculated the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH). We analysed continuous data by using weighted mean difference (WMD) and standardised mean difference (SMD). We reported 95% confidence intervals (CIs) for all estimates.
Assessment of heterogeneity
We estimated treatment effects reported by individual trials and examined heterogeneity among trials by inspecting forest plots and quantifying the impact of heterogeneity using the I² statistic. We graded the degree of heterogeneity as less than 25% (no heterogeneity), 25% to 49% (low heterogeneity), 50% to 75% (moderate heterogeneity) or greater than 75% (substantial heterogeneity). If we noted statistical heterogeneity (I² > 50%), we explored possible causes (e.g. differences in study quality, participants, intervention regimens or outcome assessments).
Data synthesis
If we identified multiple studies and thought they were sufficiently similar, we performed meta‐analysis by using RevMan version 5.3, as supplied by Cochrane. For categorical outcomes, we calculated typical estimates of RR and RD, each with its 95% CI, and for continuous outcomes, WMD or a summary estimate for SMD, each with its 95% CI. We used a fixed‐effect model for meta‐analysis. When we judged meta‐analysis to be inappropriate, we analysed and interpreted separately subgroups of individual trials that were very similar.
Quality of evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: survival without the need for ECMO (or its converse ‐ death or use of ECMO) and the two components of that outcome ‐ death (before hospital discharge) and use of ECMO. The long‐term outcome that we considered important was evaluation of neurodevelopmental disability at 18 to 24 months (components of which were cerebral palsy, cognitive impairment and deafness).
Two review authors (KB and TP) independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from RCTs as high quality but downgraded evidence one level for serious (or two levels for very serious) limitations on the basis of the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates and presence of publication bias. We used the GRADEpro Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence according to one of four grades.
High: We are very confident that the true effect lies close to the estimate of effect.
Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different.
Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect.
Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
The major subgroups that we analysed included trials that compared iNO versus no iNO and trials that compared immediate nitric oxide treatment upon enrolment in the trial versus selective treatment only when the infant's condition deteriorated, to satisfy rescue treatment criteria. We considered separately the unique study comparing high‐frequency ventilation versus iNO (Kinsella 1997) and did not include this study in the meta‐analysis.
Among studies that compared iNO versus control, we examined three subgroups: trials that did not allow iNO in the control group; the one study (Mercier 1998) that allowed iNO at the physician's discretion (with no specific criteria) after a relatively brief two‐hour period; and trials that enrolled participants with diaphragmatic hernia.
We used the I2 statistic for investigation of heterogeneity in each subgroup.
Sensitivity analysis
For major outcomes, we performed sensitivity analysis by running the meta‐analysis both while including and while excluding studies at higher risk of bias.
Results
Description of studies
Results of the search
The previous version of this review identified 14 randomised controlled trials. Updated searches revealed 652 records after duplicates were removed; after screening, review authors identified three new RCTs for inclusion in the review (Figure 1).
1.
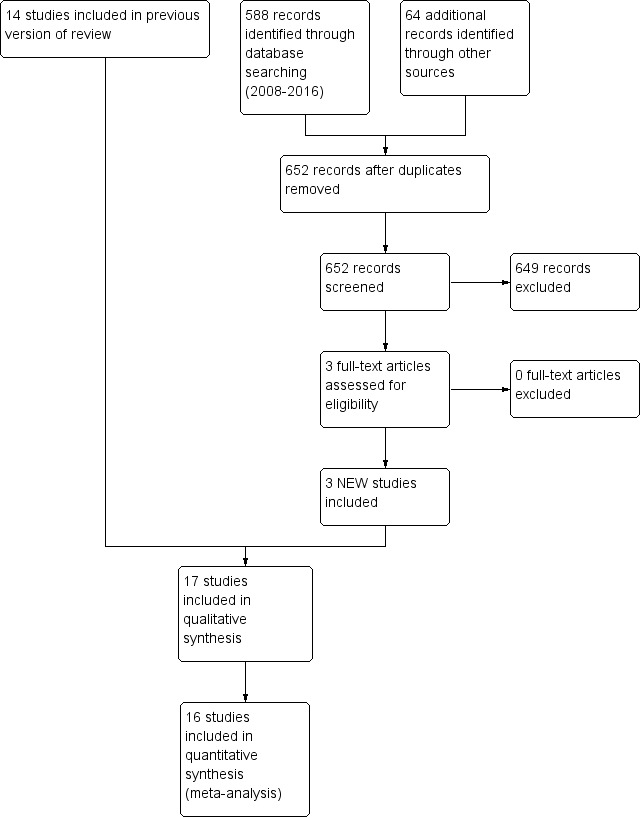
Study flow diagram: review update.
Included studies
In all, we identified 17 RCTs including term and near‐term infants with hypoxaemic respiratory failure (Barefield 1996; Day 1996; Ninos 1996; Roberts 1996; Wessel 1996; Davidson 1997; Kinsella 1997; Ninos 1997; Mercier 1998; Sadiq 1998; Cornfield 1999; Christou 2000; Clark 2000; Konduri 2004; INNOVO 2007; Liu 2008; Gonzalez 2010).
Ten of these trials (Ninos 1996; Roberts 1996; Wessel 1996; Davidson 1997; Ninos 1997; Mercier 1998; Christou 2000; Clark 2000; INNOVO 2007; Liu 2008) compared iNO versus control (placebo or standard care without iNO in infants with moderate or severe severity of illness scores), and 10 did not allow use of iNO in control groups. One trial (Mercier 1998) allowed treatment of both control and intervention participants after 2 hours of iNO at the physician's discretion, without clear criteria for treatment initiation in the control group. We therefore included this trial in group 1 but as a separate subgroup. Two trials applied other restrictions to eligibility criteria: One study enrolled only infants with diaphragmatic hernia (Ninos 1997) and compared iNO versus placebo, while not allowing open‐label iNO. The other study (Liu 2008) included only infants with meconium aspiration and compared iNO versus no iNO (for this trial, no back‐up ECMO was available).
One trial randomised infants to iNO or high‐frequency ventilation (Kinsella 1997), crossed participants over to the other treatment in case of failure, then combined treatments if the cross‐over was not successful.
Six of these trials (Barefield 1996; Day 1996; Sadiq 1998; Cornfield 1999; Konduri 2004; Gonzalez 2010) randomised infants with moderate severity of illness scores (defined by OI or A‐aDO2) to an intervention group that received immediate iNO and compared them with controls who were treated without iNO unless they deteriorated to more serious severity scores, in which case iNO was used.
These three groups of trials are investigating different questions: 'Is iNO preferable to no treatment?' 'Is iNO preferable to high‐frequency ventilation, or is the combination better?' 'Is early iNO preferable to later iNO?' Therefore, we divided the trials post hoc into the three groups.
One of the above studies enrolled infants of any gestational age but reported most descriptive and outcome data separately for preterm (< 33 weeks' gestation) and near‐term (≥ 33 weeks') babies (Mercier 1998). These studies varied in size from n = 17 (Barefield 1996) to n = 235 (Ninos 1996). Eligibility criteria have been reasonably homogeneous; some studies excluded infants with pulmonary hypoplasia, some excluded infants with pulmonary hypoplasia of any cause and others specifically excluded only those with congenital diaphragmatic hernia (Barefield 1996; Ninos 1996; Roberts 1996; Wessel 1996; Davidson 1997; Mercier 1998; Christou 2000; Konduri 2004), whereas the remaining studies included infants with these conditions (Day 1996; Kinsella 1997; Sadiq 1998; Cornfield 1999; Clark 2000). The Ninos group ran a parallel RCT of infants with congenital diaphragmatic hernia (Ninos 1997). Most studies limited participants to near‐term infants (≥ 34 weeks: Ninos 1996; Kinsella 1997; Wessel 1996; Cornfield 1999; Christou 2000; Clark 2000; ≥ 35 weeks: Barefield 1996; 'full term': Roberts 1996; Davidson 1997), but Day 1996 included preterm infants (five of 50 participants).
Hypoxaemic respiratory failure was required for entry into each of these studies, but exact criteria differed and are noted in the Characteristics of included studies table.
Many, but not all, of the studies (Day 1996; Roberts 1996; Wessel 1996; Davidson 1997; Kinsella 1997; Sadiq 1998; Cornfield 1999; Christou 2000) also required echocardiographic evidence of PPHN. Clark 2000 required clinical or echocardiographic evidence of PPHN, and Ninos 1996 required no evidence of PPHN.
Many studies reported data regarding short‐term effects on oxygenation as percentage or absolute change in PaO2 and OI. Data for all percentages, absolute changes and absolute values were available to us only for the Mercier 1998 study. Other studies provided results in one way or another.
Trials comparing iNO versus control (no iNO)
Ninos 1996 was a multi‐centre trial that included participants 14 days of age or younger without congenital structural heart disease. Although all infants in this trial received an echocardiogram before randomisation, an echocardiographic diagnosis of PPHN was not an inclusion criterion for this trial, as it was for many of the other trials herein reported. Infants were randomly assigned to receive 20 ppm of iNO or placebo; in this study, the placebo gas was 100% oxygen. The primary hypothesis was that administration of iNO to such infants would reduce the risk of death or the need for ECMO by 120 days from 50% in control infants to 30% in infants receiving iNO. This study encouraged full aggressive conventional therapy including high‐frequency ventilation provided by centres experienced in its use, use of a bovine surfactant as well as maintenance of arterial blood pressure above 45 mmHg, induction of alkalosis (target pH 7.45 to 7.60) with encouragement for use of sedation and/or paralysis and vasopressors and volume expansion as necessary to maintain blood pressure. The external data safety monitoring committee recommended termination of the trial after the second planned review of data revealed that the trial had reached the predetermined boundary of statistical significance, at which time 235 infants (121 controls and 114 infants in the iNO group) had been enrolled. These two groups were well matched for their clinical characteristics and blood gas values and were nearly identical in the use of support treatments at the time of randomisation. Approximately 55% of infants were receiving high‐frequency ventilation at the time of randomisation, and approximately 72% had received surfactant before randomisation. More than 90% of all infants had received volume and vasopressor support, neuromuscular blockade and sedation before randomisation.
The Ninos 2000 publication presents follow‐up of survivors of the two Ninos studies who underwent comprehensive neurodevelopmental assessment at 18 to 24 months of age. A secondary hypothesis of the original study was that administration of iNO would lead to no increase in neurodevelopmental disability at 18 to 24 months. Of 235 infants enrolled, 36 died, and 176 of the 199 survivors were assessed at follow‐up: 88 control infants and 85 iNO infants. In addition, this study included survivors of the parallel trial of infants with congenital diaphragmatic hernia (Ninos 1997). Among the original 53 enrolled infants, 29 were survivors and follow‐up was available for eight of 13 iNO survivors and for 14 of 16 control infants. Survivors were well matched for their neonatal characteristics. Investigators performed structured neurological examinations and hearing tests, and blinded assessors most often assigned Bayley Scales of Infant Development (BSID) scores.
Roberts 1996 conducted a multi‐centre trial including infants who had fraction of inspired oxygen (FiO2) of 1.0 and postductal PaO2 of 55 mmHg or less on two consecutive determinations 30 minutes apart. Researchers excluded infants who had polycythaemia (hematocrit ≥ 70%) or uncorrected hypotension (defined as mean aortic blood pressure < 40 mmHg) or an unevacuated pneumothorax. They also excluded infants who had received treatment with high‐frequency oscillatory ventilation or jet ventilation and included infants who had previously received surfactant without sustained improvement in oxygenation. All infants had FiO2 reduced to 0.9 and were enrolled if they maintained PaO2 greater than 85% of their previous baseline. An interim analysis after 50 participants were enrolled demonstrated that iNO increased systemic oxygenation significantly compared with the control gas. A total of 28 control group infants and 30 iNO infants appeared well matched in terms of diagnoses, blood gases, oxygen indices and ventilator settings.
Wessel 1996 was a single‐centre, open‐label trial in which treatment of participants included sedation and neuromuscular blockade. Infants in this trial were permitted to have previously received surfactant therapy or high‐frequency ventilation. Investigators initiated iNO therapy at 80 ppm after FiO2 was reduced to 0.97, with a protocol that lowered the iNO dose to 40 ppm after one hour and with continued weaning if tolerated. Researchers discontinued iNO when a participant was cannulated for ECMO, or when the clinician chose to initiate high‐frequency ventilation. They evaluated the effectiveness of iNO by assessing alterations in oxygenation, mortality and use of ECMO. Investigators enrolled 51 participants, of whom they excluded two. Researchers randomised 23 included infants to receive conventional treatment and 26 to receive iNO. Only four participants in this study actually received surfactant treatment, and, as in previous studies, the most common diagnosis was meconium aspiration (in 45% of enrolled participants). This group has published follow‐up data, including neurodevelopmental outcomes that were obtained by telephone interview from 60 of 83 survivors of the original trial. They conducted the interview when participants were between one and four years of age.
Davidson 1997 performed a multi‐centre study (funded by manufacturers) that compared three different doses of iNO (5 ppm, 20 ppm and 80 ppm) versus nitrogen placebo. Researchers hypothesised that iNO would reduce the incidence of a sequela included in the PPHN Major Sequelae Index (MSI), which they constructed. This index included death, ECMO, neurological sequelae and chronic lung disease. Investigators enrolled 155 infants with echocardiographic evidence of PPHN and PaO2 between 40 and 100 mmHg in 100% oxygen, with a wide range of illness severity at enrolment. They excluded infants who received surfactant therapy and did not allow concurrent high‐frequency ventilation. They defined failure as PaO2 less than 40 mmHg for longer than 30 minutes. This study was terminated early owing to poor enrolment. Researchers enrolled 155 infants; they entered 41 into the control group and 114 into one of the three iNO groups, with 41 receiving 5 ppm, 36 receiving 20 ppm and 37 receiving 80 ppm.
Mercier 1998 was a randomised multi‐centre trial conducted at 33 French and Belgian neonatal units. Investigators enrolled 204 infants into the trial, 107 of whom were near‐term infants. Near‐term infants were entered at an OI between 15 and 40, which was confirmed on two blood gases taken one hour apart, and were treated with iNO at 10 ppm or with no iNO. Researchers excluded infants with pulmonary hypoplasia, including congenital diaphragmatic hernia. The primary outcome measure was OI at two hours. If an infant exceeded OI of 40 during the two‐hour period, iNO therapy was allowed. After the two‐hour assessment, further therapy was provided at the discretion of the physician, and it is not reported how many infants received iNO at this time. The randomisation procedure stratified infants according to gestation, mode of ventilation and pulmonary diagnosis. Two‐thirds of the infants had received surfactant, and just over half (57%) were given high‐frequency oscillatory ventilation. Thirty percent of the infants had meconium aspiration syndrome, 25% idiopathic PPHN and 45% RDS. Enrolment into the study was terminated because of slowing recruitment after 204 of the originally planned 360 infants had been entered. ECMO was not available as back‐up therapy; therefore, the number of infants dying was the same as the number of infants dying or requiring ECMO.
Christou 2000 randomised 42 infants of at least 34 weeks' gestation with respiratory failure and PaO2 less than 100 mmHg on 100% oxygen, one of whom proved to have congenital heart disease and was removed from the study, leaving 41 study infants. Researchers compared iNO at 40 ppm versus standard therapy, without placebo gas. After one hour, they decreased the dose to 20 ppm if tolerated and made daily attempts at weaning iNO. The study was terminated early after an ad hoc committee reviewed the data.
Clark 2000 randomised 248 near‐term infants who were four days of age or younger with OI greater than 25, to 20 ppm of iNO or nitrogen placebo. Infants were not eligible if their PaO2 was less than 30 mmHg and they were considered to be in urgent need of ECMO for refractory hypotension (mean blood pressure < 35 mmHg). Investigators did not exclude infants with congenital diaphragmatic hernia, and these data are available as a separate stratum, allowing comparison of results with those of Ninos 1997. Researchers stratified infants by one of five diagnostic categories and then randomised them within that stratum. These strata included meconium aspiration, pneumonia, respiratory distress syndrome, lung hypoplasia syndromes including congenital diaphragmatic hernia and idiopathic persistent pulmonary hypertension. Echocardiographic or clinical confirmation of pulmonary hypertension was required. The primary outcome variable was the need for ECMO. After 24 hours of treatment with 20 ppm, investigators reduced the dose to 5 ppm for up to another 96 hours. Researchers evaluated secondary outcome variables of oxygen dependence at 30 days and neurological abnormality defined clinically or on ultrasonography.
INNOVO 2007 randomised infants with more than 33 weeks' gestation if they had severe respiratory failure in the first 28 days of life, and 'if the responsible clinician was uncertain about whether an infant might benefit from iNO'. Researchers initiated iNO at 20 ppm or provided control intervention (without placebo gas). No cross‐over was allowed. Investigators enrolled 60 infants at European centres. Six of 31 controls received iNO; four iNO babies did not receive the gas, three improved and one died before the gas could be started. The primary outcome was death or severe disability at one year of age. Sample size was determined by a time limit for study duration.
Liu 2008, which was conducted in China, limited participants to infants with meconium aspiration syndrome. Investigators required that infants have gestational age greater than 36 weeks and birth weight of at least 2.5 kg, and that intubated infants have an OI greater than 15. Infants did not receive surfactant; all underwent echocardiography during the initial workup but were not required to have pulmonary hypertension to be eligible. The primary outcome variable is not clear; iNO at 15 ppm or no additional gas was given, and iNO could be increased to 20 ppm in cases of poor response.
Trials comparing iNO at moderate compared with severe criteria for illness severity
Barefield 1996 compared iNO versus 'conventional treatment'. Infants had been treated with induction of alkalosis, both metabolic and respiratory, to obtain a pH of 7.65 or greater with partial pressure of carbon dioxide (PCO2) of 25 to 35 mmHg, and all infants were paralysed with vecuronium and sedated. Infants were not allowed to receive other vasodilators during the trial and were initially randomised to receive no iNO, iNO at a dose of 20 ppm for PaO2 of 40 to 99 mmHg and iNO at 40 ppm for PaO2 less than 40 mmHg; increases were allowed up to 80 ppm. Treatment failure occurred if PaO2 was less than 80 mmHg for one hour "despite alkalosis", less than 40 mmHg for longer than one hour or less than 30 mmHg for 30 minutes. When control infants met treatment failure criteria, they were treated with iNO. The primary outcome of this trial was the need for ECMO or treatment failure.
Day 1996 was a single‐centre study in which investigators randomised infants with OI between 25 and 40 to receive conventional therapy or 20 ppm of iNO, then treated control infants whose OI increased to greater than 40 with open‐label iNO. As a result, 22 infants treated in the randomised portion of the trial presented with OI between 25 and 40; 11 received iNO, and 11 conventional therapy. Much of this report combines infants randomised to iNO and some non‐randomised infants who received iNO as well as control infants who crossed over to iNO. We have included data only when they were clearly derived from the initially randomised comparison.
Sadiq 1998 randomised infants with birth weight > 2 kg on assisted ventilation with 100% oxygen and an A‐aDO2 between 500 and 599 mmHg on two blood gases at least one hour apart to iNO (10 to 80 ppm) or standard medical management. Echocardiographic evidence of pulmonary hypertension was required, and infants must have received at least one dose of surfactant before enrolment. The primary outcome criterion was "severe pulmonary hypertension", which was defined as A‐aDO2 greater than 600 mmHg. If infants satisfied this definition, then iNO and other therapies including ECMO were allowed. Infants with congenital diaphragmatic hernia were enrolled, and only those with "lethal anomalies" were excluded.
Cornfield 1999 was a randomised study conducted at three centres that compared 2 ppm of iNO versus control. The hypothesis of this study was that low‐dose iNO would acutely improve oxygenation. The study was unblinded. Investigators enrolled 38 full‐term infants with OI over 25, nine of whom had a congenital diaphragmatic hernia. After the initial one‐hour period, if infants in either group had OI greater than 35, they were considered treatment failures and received 20 ppm of iNO. Thus the trial could be considered a trial of moderate (OI > 25) compared with severe (OI > 35) criteria for iNO, even though investigators used different concentrations for those criteria.
Konduri 2004 randomised infants at ≥ 34 weeks' gestation on assisted ventilation with OI between 15 and 25 and with FiO2 of 0.80 on any two arterial blood gases, at least 15 minutes and not more than 12 hours apart, to iNO at 5 to 20 ppm or placebo (nitrogen gas). Investigators started iNO at 5 ppm and increased the dose to a maximum of 20 ppm when they noted a partial response (adjustments were made by a single unmasked therapist, and the remainder of the team were masked). Researchers excluded infants with congenital diaphragmatic hernia or cardiac malformation and those older than 14 days. The primary outcome was death or the need for ECMO. The control group received iNO if OI exceeded 25.
Gonzalez 2010, a two‐centre study conducted in Chile, randomised near‐term and term infants with OI between 10 and 30 to receive iNO at 20 ppm or control; if within 48 hours, OI worsened to 40 or more, then controls received iNO. Infants who remained with OI greater than 40 were treated with high‐frequency ventilation. All infants had echocardiographic evidence of PPHN, but investigators did not state whether they received surfactant. The primary outcome variable was progression to OI over 40.
Trials comparing iNO versus high‐frequency ventilation
Kinsella 1997 compared iNO versus high‐frequency ventilation rather than against 'standard therapy'; if infants failed the first therapy, they were crossed over to the alternate therapy, then to combined treatment. Data from this study included in this review relate only to the first comparison between initially randomised groups. This multi‐centre study excluded infants with lethal abnormalities and proscribed the use of surfactant after enrolment. The definition of success in this trial was achievement of a PaO2 greater than 60 mmHg with assigned therapy. Initial randomisation was to iNO 20 to 40 ppm or to high‐frequency ventilation with the SensorMedics oscillator (SensorMedics Corporation, Yorba Linda, California, USA). The trial had enrolled 205 infants when it was terminated as the result of an interim analysis that demonstrated lack of efficacy. Thirty‐one percent of the infants included in this trial had received surfactant before enrolment. As this is the only trial included in this group, we did not perform a meta‐analysis, and all infants had the possibility of receiving iNO at some point in the trial. Therefore, we included data on short‐term oxygenation changes after iNO but did not analyse clinical outcome data.
All of the studies described above, with the exception of Kinsella 1997, Day 1996 and Clark 2000, excluded infants with congenital diaphragmatic hernia (CDH). This patient population is unique in presentation and pathophysiology. As a result, NINOS investigators performed a parallel study of infants with CDH using an identical study protocol to the main NINOS study (Ninos 1997). They enrolled 53 infants (28 control, 25 iNO treated), and their primary hypothesis was that iNO would reduce the occurrence of death or the need for ECMO at 120 days. Treated and control groups were well matched, with baseline OI of 45.8 ± 16.3 in controls and 44.5 ± 14.5 in iNO‐treated infants.
Excluded studies
See the Characteristics of excluded studies table.
Risk of bias in included studies
The overall quality of these studies is highly variable. The highest‐quality studies were fully blinded, adequately powered, multi‐centre RCTs with external data monitoring groups that examined clinically important outcomes (Ninos 1996;Ninos 1997;Clark 2000; Konduri 2004). Another group of studies of intermediate quality used variable degrees of blinding or examined primarily oxygenation outcomes (Roberts 1996; Davidson 1997; Mercier 1998;Sadiq 1998; INNOVO 2007). A third group of studies consisted of single (or few)‐centre studies that were unblinded and included very small sample sizes (Barefield 1996; Day 1996; Wessel 1996;Cornfield 1999; Christou 2000; Liu 2008;Gonzalez 2010).
Kinsella 1997 is a unique study of high quality that had a rather complex protocol and cannot be directly compared with the remaining studies, as investigators compared iNO versus high‐frequency ventilation. Several studies were terminated early. In the case of Ninos 1996, this occurred because predetermined stopping rules were satisfied. In other cases (Davidson 1997; Mercier 1998; Sadiq 1998; Konduri 2004), it occurred because of slowing enrolment; in Christou 2000, it followed examination of data.
Risk of bias summaries are shown in Figure 2 and Figure 3.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
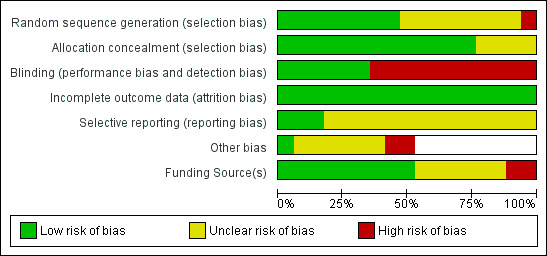
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Studies at low risk of bias
Ninos 1996: In this masked multi‐centre trial, investigators masked allocation, intervention and outcome assessment. Investigators performed preplanned interim analyses and used standard stopping rules. They noted the significance of the primary outcome variable after performing the second analysis.
Ninos 1997: This trial was designed in an unusual and pragmatic fashion to run simultaneously at the same centres as the main study (Ninos 1996), with a sample size that was planned by terminating enrolment when the main Ninos study was complete. Investigators performed allocation, intervention and outcome analysis in a masked fashion. Investigators reported long‐term neurodevelopmental follow‐up of infants in Ninos 1996, and in Ninos 1997, they reported survival of 87% for Ninos 1996 (n = 173) and 76% for Ninos 1997 (n = 53). All but seven assessments were blinded to original treatment. Non‐blinded BSID administrators assessed control infants and six iNO‐treated infants.
Clark 2000: This multi‐centre trial reported masked allocation and intervention and complete follow‐up. The published manuscript does not mention a prespecified sample size.
Konduri 2004: This multi‐centre study used central randomisation allocation and masking of the intervention. Researchers compared thresholds of OI 15 to 25 versus OI > 25. Unfortunately, slow enrolment led to early termination of the study and a reduction in power. This was done without review of results and should not affect the validity of study conclusions.
Studies at intermediate risk of bias
Roberts 1996: Sample size determination leaves this study open to the criticism of little protection against type 1 error, in that study authors reported that they planned to stop after enrolling 50 participants if results showed significant improvement in oxygenation. Review authors made no adjustments to the critical P value for multiple looks at the data. Researchers did not report the study hypothesis.
Davidson 1997: A three‐to‐one randomisation scheme enabled the provision of dose‐response oxygenation information but limited the number of controls. This study was terminated early and was therefore underpowered to detect clinical benefit.
Mercier 1998: This multi‐centre trial reported masked allocation and complete follow‐up but no masking of the intervention. Many protocol violations were noted (44 of the 54 assigned to control therapy received iNO, and only 55 of the 62 assigned to iNO received this treatment).
Sadiq 1998: This multi‐centre study included a relatively small sample and was terminated owing to slowing enrolment. The intervention was not masked. Review authors requested data on death and ECMO, and the principal investigator provided them.
INNOVO 2007: The term section of the INNOVO study enrolled only 60 participants from 27 participating hospitals over four years (15 hospitals actually contributed patients). Recruitment ended when the preterm portion of the trial ended. The entry criteria made it difficult to extrapolate study results to other populations, but infants were very sick with 25% mortality, and almost half had OI over 40 at enrolment.
Gonzalez 2010: This two‐centre trial randomised near‐term infants with OI between 10 and 30 to iNO at 20 ppm or to no iNO. All infants whose condition deteriorated to reach OI of 40 received iNO and could receive high‐frequency ventilation; if already receiving iNO, investigators added high‐frequency ventilation. ECMO in case of failure was not available. Randomisation was described only as sequenced sealed envelopes and may have been masked, but this is not certain. Investigators calculated the sample size on the basis of a very large predicted effect and achieved this goal. They did not mask the intervention.
Studies at higher risk of bias
Barefield 1996: This very small study included somewhat unbalanced groups at enrolment; the mean OI at entry was 26 in the control group and 38 in the treatment group. Infants receiving iNO also appeared to have a lower pH (7.46 ± 0.06 vs 7.61 ± 0.07), higher partial pressure of arterial carbon dioxide (PaCO2) (40 ± 7 vs 28 ± 4 mmHg) and lower PaO2 (49 ± 7 vs 63 ± 7 mmHg). Study authors did not clearly describe the study hypothesis. The sample size calculation required 24 participants per group, but the basis for this calculated sample size is not clear. Study authors did not explain why the study was discontinued before the calculated sample size was enrolled.
Day 1996: Study authors did not state how the sample size was calculated and reported no study hypothesis. The method of randomisation is unclear and was described as a 'blind draw'. Researchers reported the study objective as to "review the acute effects" of iNO.
Wessel 1996: This small single‐centre study provided unmasked intervention and did not adequately describe allocation; review authors could not ascertain whether it was masked.
Cornfield 1999: Study authors did not clearly describe the randomisation procedure, and masking of allocation is unclear. Study authors planned to enrol 60 participants. They planned an interim analysis at two‐thirds of full enrolment to be performed blind. The study was terminated after this interim analysis (n = 38) because a secondary analysis (i.e. response to 20 ppm after failure of initial therapy) differed between groups. Early termination of this trial was the result of an unexpected finding with limited clinical significance, and no difference in clinically important outcomes seriously limited the power of this study.
Christou 2000: This study included a limited sample size, as the study was terminated after Ninos 1996 and Roberts 1996 reported results. Study authors did not explicitly state the hypothesis, and they listed study objectives as determining whether iNO improves oxygenation in infants given high‐frequency ventilation; however, not all infants received this mode of ventilation.
Liu 2008: This single‐centre trial noted no prespecified sample size and is the only trial that restricted enrolment to infants with meconium aspiration syndrome; ECMO was not available as rescue therapy. The intervention was unmasked, and researchers provided little detail about the mechanics of randomisation.
Effects of interventions
Summary of findings for the main comparison. Inhaled NO compared with control for respiratory failure in infants born at or near term.
| Inhaled NO compared with control for respiratory failure in infants born at or near term | ||||||
| Patient or population: respiratory failure in infants born at or near term Setting: neonatal intensive care units Intervention: inhaled NO Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control | Risk with inhaled NO | |||||
| Death or use of ECMO; studies that did not allow back‐up use of iNO in controls | Study population | RR 0.66 (0.57 to 0.77) | 859 (8 RCTs) | ⊕⊕⊕⊕ High | ||
| 540 per 1000 | 356 per 1000 (308 to 416) | |||||
| Death or use of ECMO; infants with diaphragmatic hernia | Study population | RR 1.09 (0.95 to 1.26) | 84 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | ||
| 870 per 1000 | 948 per 1000 (826 to 1000) | |||||
| Death before hospital discharge; studies that did not allow back‐up use of iNO in controls | Study population | RR 0.89 (0.60 to 1.31) | 860 (8 RCTs) | ⊕⊕⊕⊕ High | ||
| 120 per 1000 | 106 per 1000 (72 to 157) | |||||
| Death before hospital discharge; infants with diaphragmatic hernia | Study population | RR 1.20 (0.74 to 1.96) | 84 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | ||
| 391 per 1000 | 470 per 1000 (290 to 767) | |||||
| Use of ECMO before hospital discharge; studies that did not allow back‐up use of iNO in controls | Study population | RR 0.60 (0.50 to 0.71) | 815 (7 RCTs) | ⊕⊕⊕⊕ High | ||
| 514 per 1000 | 308 per 1000 (257 to 365) | |||||
| Use of ECMO before hospital discharge; infants with diaphragmatic hernia | Study population | RR 1.27 (1.00 to 1.62) | 84 (2 RCTs) | ⊕⊕⊕⊝ Moderatea,b | ||
| 674 per 1000 | 856 per 1000 (674 to 1000) | |||||
| Neurodevelopmental disability at 18 to 24 months among survivors | Study population | RR 0.97 (0.66 to 1.44) | 301 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | ||
| 265 per 1000 | 257 per 1000 (175 to 382) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to the estimate of effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aSmall numbers of participants studied.
bSubgroup of participants from only 2 trials evaluated.
Summary of findings 2. Inhaled NO at moderate compared with severe criteria for illness severity in respiratory failure among infants born at or near term.
| Inhaled NO at moderate compared with severe criteria for illness severity in respiratory failure among infants born at or near term | ||||||
| Patient or population: infants born at or near term in respiratory failure Setting: neonatal intensive care units Intervention: inhaled NO at moderate criteria for illness severity (earlier iNO) Comparison: inhaled NO at severe criteria for illness severity (later iNO) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with inhaled NO at severe criteria for illness severity | Risk with Inhaled NO at moderate criteria for illness severity | |||||
| Death or requirement for ECMO | Study population | RR 0.88 (0.62 to 1.27) | 495 (5 RCTs) | ⊕⊕⊝⊝ Lowa,b | ||
| 192 per 1000 | 169 per 1000 (119 to 244) | |||||
| Death before hospital discharge | Study population | RR 0.69 (0.38 to 1.26) | 495 (5 RCTs) | ⊕⊕⊕⊝ Moderateb | ||
| 100 per 1000 | 69 per 1000 (38 to 126) | |||||
| Use of ECMO before hospital discharge | Study population | RR 1.01 (0.66 to 1.54) | 439 (4 RCTs) | ⊕⊕⊕⊝ Moderateb | ||
| 144 per 1000 | 146 per 1000 (95 to 222) | |||||
| Progression to severe criteria | Study population | RR 0.66 (0.55 to 0.79) | 512 (6 RCTs) | ⊕⊕⊕⊝ Moderateb | ||
| 595 per 1000 | 392 per 1000 (327 to 470) | |||||
| Neurodevelopmental disability at 18 to 24 months among survivors | Study population | RR 1.13 (0.74 to 1.74) | 234 (1 RCT) | ⊕⊕⊕⊝ Moderateb | ||
| 248 per 1000 | 280 per 1000 (183 to 431) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to the estimate of effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aHighly variable risk ratio.
bVery wide confidence intervals.
Comparison 1: iNO versus control (no iNO) in infants with hypoxic respiratory failure
Outcome 1.1: Death or use of ECMO
This outcome was reported by 10 trials (Analysis 1.1). Nine studies (Ninos 1996; Roberts 1996; Wessel 1996; Davidson 1997; Ninos 1997; Mercier 1998; Christou 2000; Clark 2000; INNOVO 2007; Liu 2008) did not allow use of iNO in controls who did not respond. One of these trials (Liu 2008) did not have ECMO available; therefore, the frequency of death reported is equal to death or requirement for ECMO. One study gave the randomised intervention for only two hours before allowing iNO among controls (Mercier 1998).
1.1. Analysis.
Comparison 1 Inhaled NO versus control, Outcome 1 Death or use of ECMO.
One study (Ninos 1997) included infants with CDH and reported the CDH stratum of Roberts 1996 as a separate subgroup. Investigators reported all outcomes of this subgroup separately (see below).
Four studies (Ninos 1996; Christou 2000; Clark 2000 and the non‐CDH stratum of Roberts 1996) found a statistically significant reduction in the combined outcome of death or requirement for ECMO. Meta‐analysis of the eight trials in the subgroup without back‐up iNO treatment and without participants with CDH revealed that iNO treatment resulted in a reduction in the incidence of death or requirement for ECMO (typical RR 0.66, 95% CI 0.57 to 0.77; eight studies, 859 infants; typical RD ‐0.18, 95% CI ‐0.25 to ‐0.12) (high‐quality evidence) and showed little heterogeneity (I2 = 21%). Sensitivity analysis revealed that exclusion of studies at higher risk of bias (Wessel 1996; Liu 2008) had no effect on the risk ratio of this outcome but reduced heterogeneity (I2) to zero.
The study that allowed back‐up iNO among controls (Mercier 1998) did not report a significant effect for this outcome.
Outcome 1.2: Death before hospital discharge
The same studies also reported death (Analysis 1.2). None of the individual studies found a significant effect. Likewise, meta‐analysis revealed no evidence of effect (typical RR 0.89, 95% CI 0.60 to 1.31; eight studies, 860 infants; typical RD ‐0.01, 95% CI ‐0.05 to 0.03) (high‐quality evidence). On sensitivity analysis, after trials at high risk of bias were excluded, the RR was 0.93 and confidence intervals were wide.
1.2. Analysis.
Comparison 1 Inhaled NO versus control, Outcome 2 Death before hospital discharge.
Outcome 1.3: Use of ECMO before hospital discharge
Seven studies reported this outcome (Ninos 1996; Roberts 1996; Wessel 1996; Davidson 1997; Christou 2000; Clark 2000; INNOVO 2007). Meta‐analysis of results of these studies in this subgroup supported a significant effect (typical RR 0.60, 95% CI 0.50 to 0.71; seven studies, 815 infants; typical RD ‐0.20, 95% CI ‐0.27 to ‐0.14) (Analysis 1.3). Thus, the number needed to treat with iNO for an additional beneficial outcome (NNTB) to prevent one infant from requiring ECMO was 5 (95% CI 3.7 to 7.1) (high‐quality evidence). On sensitivity analysis, exclusion of Wessel 1996 had no effect (Liu 2008 did not have ECMO available).
1.3. Analysis.
Comparison 1 Inhaled NO versus control, Outcome 3 Use of ECMO before hospital discharge.
Outcome 1.4: Failure to improve oxygenation (PaO2)
Two studies in this group reported this outcome (Ninos 1996; Roberts 1996). Both trials reported a statistically significant benefit of iNO, with fewer infants failing to have improved oxygenation (Analysis 1.4).
1.4. Analysis.
Comparison 1 Inhaled NO versus control, Outcome 4 Failure to improve oxygenation (PaO2).
Outcome 1.5: OI 30 to 60 minutes after the start of treatment
Five studies reported this outcome (Ninos 1996; Roberts 1996; Davidson 1997; Clark 2000; Liu 2008) (Analysis 1.5) and found a statistically significant benefit of iNO. The results are remarkably homogeneous across studies. Meta‐analysis shows that OI 30 to 60 minutes after therapy commenced was significantly lower in the iNO group (weighted mean difference ‐8.45, 95% CI ‐11.42 to ‐5.48; five studies, 709 infants). Christou 2000 reported median changes in OI after 60 minutes of ‐18 in the iNO group and 0 in the control group. Mercier 1998 reported OI results at two hours after the start of treatment as median and interquartile ranges. Therefore, we cannot currently add these data to our analysis. However, the direction and magnitude of the effect of iNO were very similar: Among controls, median OI at baseline was 21.7 with little change at two hours (median 19.4), whereas in those given iNO, median OI at baseline was 25.9 and fell to 15.8 at two hours. The two‐hour OI was significantly different between groups, as was the absolute change in OI (median ‐2.7 in the control group vs ‐7.8 in the iNO group).
1.5. Analysis.
Comparison 1 Inhaled NO versus control, Outcome 5 Oxygenation index 30 to 60 minutes after treatment.
Outcome 1.6: PaO2 30 to 60 minutes after treatment
Four studies reported this outcome (Ninos 1996; Roberts 1996; Davidson 1997; Clark 2000) (Analysis 1.6). All found statistically significant benefit of iNO. Study results are remarkably homogeneous. Meta‐analysis shows that PaO2 30 to 60 minutes after treatment was statistically significantly higher in the iNO group (weighted mean difference 32.62 mmHg, 95% CI 23.56 to 41.67; five studies, 707 infants). Christou 2000 reported median changes in PaO2 of 53 mmHg in the iNO group and 2 mmHg in the control group.
1.6. Analysis.
Comparison 1 Inhaled NO versus control, Outcome 6 PaO2 30 to 60 minutes after treatment.
Outcomes 1.7: Change in OI after treatment; and 1.8: Change in PaO2 after treatment
Only the Ninos trials reported these outcomes; both showed substantial improvement in PaO2 and decreased OI (Analysis 1.7; Analysis 1.8). For both outcomes, the change was statistically significant only among infants in the main trial who did not have congenital diaphragmatic hernia.
1.7. Analysis.
Comparison 1 Inhaled NO versus control, Outcome 7 Change in oxygenation index after treatment.
1.8. Analysis.
Comparison 1 Inhaled NO versus control, Outcome 8 Change in PaO2 after treatment.
Outcomes 1.9 to 1.13: Neurodevelopmental and other late outcomes
Four studies reported these outcomes (Ninos 1996; Wessel 1996; Davidson 1997; Ninos 1997) (Analysis 1.9; Analysis 1.10; Analysis 1.11; Analysis 1.12; Analysis 1.13). The Ninos 2000 publication reported neurodevelopmental outcomes from both Ninos 1996 and Ninos 1997, and Lipkin 2002 reported outcomes of Davidson 1997. Wessel 1996 reported neurodevelopmental outcomes obtained by telephone interview.
1.9. Analysis.
Comparison 1 Inhaled NO versus control, Outcome 9 Neurodevelopmental disability at 18 to 24 months among survivors.
1.10. Analysis.
Comparison 1 Inhaled NO versus control, Outcome 10 Hearing impairment in at least 1 ear among survivors.
1.11. Analysis.
Comparison 1 Inhaled NO versus control, Outcome 11 Cerebral palsy among survivors.
1.12. Analysis.
Comparison 1 Inhaled NO versus control, Outcome 12 BSID MDI > 2 SD below the mean.
1.13. Analysis.
Comparison 1 Inhaled NO versus control, Outcome 13 BSID PDI > 2 SD below the mean.
For survivors of Ninos 1996, investigators performed BSID II testing (n = 154), a neurological examination (n = 172) and hearing tests (n = 157). Results showed no significant differences in the occurrence of neurodevelopmental sequelae between iNO and control infants: 18/87 control infants and 19/85 iNO infants did not have normal neurological examination findings, and 9/87 controls compared with 10/85 iNO infants had cerebral palsy (CP). Investigators observed no differences in the occurrence of hearing impairment (defined as a threshold > 40 db; 23/82 evaluated controls vs 24/75 evaluated iNO infants) nor in scores on the BSID (Mental Development Index (MDI) 87 ± 18.7 for control infants vs 85 ± 21.7 for iNO infants; Physical Development Index (PDI) 93.6 ± 17.5 for control infants vs 85.7 ± 21.2 for iNO infants). One or more neurodevelopmental disabilities occurred in 26/87 control infants compared with 29/85 iNO infants. The occurrence of seizures was less among iNO infants (13/87 control infants vs 4/85 iNO infants; P = 0.046). In addition, investigators reported no differences in requirements for later hospital readmission, use of home medications, apnoea monitors, home oxygen, use of gastrostomy tubes or requirement for speech therapy. Researchers did not report outcomes of all infant testing conducted in Ninos 1997 and stated that they found no differences in the mean results of BSID examinations, and proportions with scores lower than 70 were similar.
Investigators from Davidson 1997 examined 35 control infants and 94 iNO‐treated infants at an average of 13 months of age by performing a neurological examination ‐ Bayley Scales of Infant Development (BSID) ‐ and audiological assessment. The prevalence of CP, developmental delay (BSID scores > 2 SD below the mean) and hearing impairment (defined as mild 25 to 50 db threshold, or severe > 50 db) was not different between groups. The general health of infants in terms of hospitalisation and growth was not different between groups.
Wessel 1996 used different scales and relied on a telephone interview to record outcome data. Researchers observed no differences in the developmental quotient calculated from the Motor and Social Development Scale of the 1981 Child Health Supplement to the National Health Interview Survey. The reliability, reproducibility and validity of this scale have received little attention. The number of infants with a quotient less than 70 was greater among the control group (4/25) than among the iNO group (0/35). Although cerebral palsy, visual disability and hearing and speech disabilities were reported, it is unclear how these terms were defined, and the incidence of overall neurological disability included seizures. Therefore, it is not possible for review authors to add any of these data to the meta‐analysis, but they do appear to show no evidence of neurodevelopmental impairment due to iNO therapy. The study included an assessment of general and pulmonary health; 31% of iNO participants (n = 35) and 20% of control participants (n = 20) had required hospital readmission, and 14% of iNO participants and 24% of control participants were found to have reactive airways disease; neither of these events was statistically significant.
Definitions used by the Ninos group and by Lipkin and coworkers for the overall incidence of neurodevelopmental disability were dissimilar. The Ninos group reported the incidence of having one or more disabilities (CP, BSID MDI or PDI < 2 SD, blindness or hearing impairment). Lipkin 2002 reported the incidence of mild (one or two mild impairments, including mild neurological abnormalities and mild reduction in scores on the BSID ‐ between 1 and 2 SDs below the mean) and severe impairments (CP, more than two mild impairments, or at least one severe impairment). If we proceed on the assumption that the definition of severe impairment in Lipkin 2002 is similar enough to the Ninos definition of one or more neurodevelopmental disabilities for a combined analysis, we find no effect of iNO (typical RR of adverse outcomes 0.97, 95% CI 0.66 to 1.44) (low‐quality evidence).
Comparison 1, subgroup analysis: iNO versus control (no iNO) in infants with hypoxic respiratory failure (subgroup of infants with CDH)
Infants with CDH do not appear to share the benefits of iNO; indeed it has been suggested that outcomes could be worse in infants with CDH who receive iNO compared with control infants. We have combined the results of Ninos 1997 with the diaphragmatic hernia stratum of Clark 2000 ‐ the only other study from which such information can be extrapolated.
Outcome 1.1.3: Death or requirement for ECMO in infants with CDH
The incidence of death or requirement for ECMO was 40/46 control infants and 36/38 infants with iNO (RR 1.09, 95% CI 0.95 to 1.26; two studies, 84 infants; RD 0.08, 95% CI ‐0.04 to 0.20) (Analysis 1.1.3).
Outcome 1.2.3: Death in infants with CDH
Mortality rate was not changed: 18/46 control infants compared with 18/38 infants with iNO (RR 1.20, 95% CI 0.74 to 1.96; two studies, 84 infants; RD 0.08, 95% CI ‐0.13 to 0.29) (Analysis 1.2.3).
Outcome 1.3.2: Requirement for ECMO in infants with CDH
Results showed a barely significant increase in the requirement for ECMO: 31/46 control infants compared with 32/38 infants with iNO (RR 1.27, 95% CI 1.00 to 1.62; two studies, 84 infants; RD 0.18, 95% CI 0.01 to 0.35) (Analysis 1.3.2). This occurred despite the fact that infants with CDH who received iNO were more likely to improve their oxygenation (data from Ninos 1997 only; all control infants failed to improve oxygenation compared with 20/24 iNO infants; RR of failure to improve oxygenation 0.83, 95% CI, 0.70 to 1.00). The mean difference in PaO2, however, was small and was not statistically significant. PaO2 30 minutes after treatment increased by 7.8 mmHg in iNO infants compared with 1.1 mmHg in control infants (MD 6.7, 95% CI 15.7 to ‐2.3), and OI decreased by 2.7 in iNO infants compared with an increase of 4.0 among control infants (MD ‐6.70, 95% CI ‐18.39 to +4.99).
Outcomes 1.10.2 and 1.11.2: Neurodevelopmental outcomes in infants with CDH
Survivors with CDH (from the Ninos 1997 study) had comparable neurodevelopmental outcomes at follow‐up. Mean BSID MDI among controls was 73.6 (SD 18) and PDI was 77.2 (SD 14.4); among iNO‐treated infants, mean MDI was 69.1 (SD 17) and PDI was 75.8 (SD 25.8). Both control infants and iNO infants had a high rate of sensorineural hearing loss (4/14 control infants vs 3/8 iNO infants) (Analysis 1.10.2).
Comparison 2. iNO started for moderate compared with severe disease criteria
All five trials included in this group all showed no incremental benefit for death or requirement for ECMO when iNO was started earlier.
Outcome 2.1: Death or use of ECMO
The RR of death or ECMO was 0.88 (95% CI 0.62 to 1.27; five studies, 495 infants; RD ‐0.02, 95% CI ‐0.09 to 0.04). On sensitivity analysis, when data from Barefield and Cornfield were excluded, leaving only the results of Konduri, Gonzalez and Sadiq, review authors found no effect on the conclusion of effect (RR 0.83, 95% CI 0.54 to 1.2) (Analysis 2.1).
2.1. Analysis.
Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 1 Death or use of ECMO.
Outcome 2.2: Death before hospital discharge
The RR of death with earlier treatment was 0.69 (95% CI 0.38 to 1.26; five studies, 495 infants; RD ‐0.03, 95% CI ‐0.08 to 0.02). On sensitivity analysis, we found no impact on the conclusion of no effect (RR 0.70, 95% CI 0.34 to 1.43) (Analysis 2.2).
2.2. Analysis.
Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 2 Death before hospital discharge.
Outcome 2.3: Use of ECMO before hospital discharge
Four studies reported the requirement for ECMO (ECMO was not available for infants during Gonzalez 2010). The RR for needing ECMO was 1.01 (95% CI 0.66 to 1.54; four studies, 439 infants; RD 0.00, 95% CI ‐0.06 to 0.06). On sensitivity analysis, we found that the RR for needing ECMO, after studies at higher risk of bias were excluded, was 0.90 (95% CI 0.52 to 1.58) (Analysis 2.3).
2.3. Analysis.
Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 3 Use of ECMO before hospital discharge.
Outcome 2.4: Progression to severe disease criteria
Treatment at lower disease severity appears to prevent progression to severe disease (i.e. fewer iNO participants progressed to satisfying rescue or severe disease criteria compared with control infants; RR 0.66, 95% CI 0.55 to 0.79; six studies, 512 infants; RD ‐0.20, 95% CI ‐0.28 to ‐0.12). On sensitivity analysis, we found that the RR was 0.59 (95% CI 0.48 to 0.74) (Analysis 2.4).
2.4. Analysis.
Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 4 Progression to severe disease criteria.
Outcome 2.5: Chronic lung disease
Gonzalez 2010, Konduri 2004 and Sadiq 1998 reported frequency of requiring oxygen at 28 days of age. Early iNO confers no advantage over late iNO for this outcome (RR 0.91, 95% CI 0.54 to 1.53; three studies, 437 infants; RD ‐0.01, 95% CI ‐0.07 to 0.05).
Outcomes 2.6 to 2.8: Neurodevelopmental outcomes
One study reported longer‐term neurodevelopmental outcomes (Konduri 2004). Analysis revealed no difference in frequency of neurological impairment or developmental delay at 18 to 24 months of age (Analysis 2.6), hearing loss requiring amplification (Analysis 2.7), nor grades of cerebral palsy (Analysis 2.8). Investigators showed no difference in severe CP but a lower BSID PDI score at 18 months among infants who received iNO early.
2.6. Analysis.
Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 6 Neurodevelopmental disability at 18 to 24 months among survivors.
2.7. Analysis.
Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 7 Hearing impairment among survivors.
2.8. Analysis.
Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 8 Cerebral palsy among survivors.
Other possibly important outcomes
Gonzalez 2010, Konduri 2004 and Sadiq 1998 reported duration of mechanical ventilation and duration of oxygen therapy in the two groups. Konduri and Sadiq also reported duration of hospitalisation. Gonzalez reported outcomes as median and range, Konduri as median and interquartile range and Sadiq as mean and standard deviation. Therefore, meta‐analysis cannot be performed, but we have tabulated the results (Table 3).
1. Additional important outcomes.
| Study | Ventilator days | Oxygen days | Hospitalisation days | |
| Gonzalez | Early iNO | Median 6, range 3‐28 | Median 11.5, range 5‐90 | |
| Late iNO | Median 8, range 4‐37 | Median 18, range 6‐142 | ||
| Konduri | Early iNO | Median 8, IQR 6‐12 | Median 13, IQR 9‐19 | Median 17, IQR 12‐22 |
| Late iNO | Median 8, IQR 6‐12 | Median 13, IQR 9‐19 | Median 18, IQR 12‐30 | |
| Sadiq | Early iNO | Mean 8,7, SD 4 | Mean 14, SD 8 | Mean 21, SD 14 |
| Late iNO | Mean 10, SD 6 | Mean 18, SD 17 | Mean 21, SD 11 |
IQR: interquartile range; SD: standard deviation.
Comparison 3. iNO versus high‐frequency ventilation in infants with hypoxic respiratory failure
Only one study compared iNO versus high‐frequency ventilation. Kinsella 1997 found that 23% of infants treated with high‐frequency ventilation compared with 28% treated with iNO had a successful response, defined as PaO2 greater than 60 mmHg. After cross‐over, 21% of 75 infants with high‐frequency ventilation failure responded to iNO, whereas 14% of 77 infants with iNO failure responded to high‐frequency ventilation (not significant). This study demonstrated that response to iNO was equivalent to response to high‐frequency ventilation among near‐term infants with hypoxic respiratory failure. Study authors evaluated infants who failed to respond to either therapy singly (n = 125) and found that 32% responded to the combination. Study authors concluded that the combination of high‐frequency ventilation and iNO was the most effective therapy for infants who failed to respond to either treatment alone. This study suggests that use of high‐frequency ventilation may be valuable in establishing adequate lung volumes in such a way that iNO therapy may then be efficacious.
As all infants were eligible to receive iNO during preparation of the protocol, this study is not informative for the outcome of death or ECMO. As this is the only study included in this group, we have not performed any meta‐analysis.
Discussion
Main results
First, use of inhaled nitric oxide (iNO) at doses of 10 to 80 ppm is supported by studies that enrolled near‐term, hypoxic, mechanically ventilated neonates with an oxygenation index (OI) of 25 or greater, or partial pressure of arterial oxygen (PaO2) less than 100 mmHg in 100% oxygen. Use of iNO in these circumstances decreases the need for extracorporeal membrane oxygenation (ECMO). All but one of the trials that compared iNO versus no iNO had ECMO available for participants who were failing, and no effect on mortality was evident. One very small trial from China (Liu 2008), which had no ECMO available, showed a small reduction in mortality, which may have been due to chance. ECMO is invasive and expensive and is associated with clinically important complications. Therefore, a reduction in ECMO requirement with less invasive and safer treatment provides benefit for patients. However, reviewed trials showed significant differences with respect to the use of other therapies, such as high‐frequency ventilation or surfactant. Ninos 1996,Ninos 1997,Day 1996 and Wessel 1996 allowed both treatments, Clark 2000 "encouraged" both treatments, whereas others proscribed such management or excluded infants who received such treatments (Barefield 1996 proscribed both; Roberts 1996 disallowed high‐frequency ventilation; Davidson 1997 excluded infants who received surfactant therapy and did not allow concurrent high‐frequency ventilation).
Second, studies that enrolled infants at moderate disease severity and randomised them to immediate iNO, or to iNO only if their condition deteriorated, showed no incremental benefit of giving iNO earlier, even if infants receiving earlier iNO were less likely to experience a deterioration in their oxygenation.
Third, the Ninos 2000 publication provides reassuring information regarding long‐term neurodevelopmental outcomes and demonstrates that infants who receive iNO are not at increased risk of neurodevelopmental sequelae, consistent with the observations of Rosenberg and coworkers (Rosenberg 1997), and did not experience increased postdischarge pulmonary complications, consistent with the report of Dobyns and coworkers (Dobyns 1999).
Fourth, clinical or echocardiographic evidence of pulmonary hypertension was not universally present in all reported trials; therefore, use of iNO should be considered for near‐term infants with hypoxic respiratory failure, with or without clear evidence of pulmonary hypertension. Inhaled NO can improve ventilation/perfusion mismatching and may improve oxygenation by this mechanism in infants without documented pulmonary hypertension.
Limits/toxicity issues
Nitric oxide reacts with oxygen to form nitrogen dioxide (NO2). Both NO and NO2 are toxic, causing death in dogs at concentrations between 0.1% and 2% due to methaemoglobinaemia, hypoxaemia and pulmonary edema (Greenbaum 1967). In humans, exposure to 2.3 ppm NO2 for five hours produced a 14% decrease in serum glutathione peroxidase activity and a 22% decrease in alveolar permeability 11 hours after the start of exposure, suggesting that even very low concentrations of NO2 may produce a delayed response (Rasmussen 1992). Occupational health guidelines set 25 ppm as the limit for eight hours/day NO exposure in the workplace, and 3 ppm (measured as the time‐weighted average) as the limit for NO2 (MMWR 1988). It has been demonstrated that using 80 ppm iNO in a neonatal ventilator with fraction of inspired oxygen (FiO2) of 0.9 could produce 5 ppm NO2 in less than 20 seconds (Bouchet 1993). Ongoing monitoring of inhaled gas for NO2 is therefore mandatory.
The condition of infants receiving iNO, even those who have not demonstrated a positive response, may deteriorate when iNO is discontinued. Davidson and coworkers (Davidson 1999) reviewed the results of their previous trial (Davidson 1997) and reported a 42 ± 101% increase in OI when iNO was withdrawn from participants who did not demonstrate a positive response to iNO. They reported that minimising the iNO dose to 1 ppm before discontinuation was associated with the least deterioration, so it appears that this practice should be followed for infants who are being weaned from iNO, regardless of their initial response.
Methaemoglobin levels must also be carefully monitored, and significant methaemoglobinaemia has been reported after accidental overdose of a neonate with > 135 ppm of iNO (Heal 1995). Long‐term treatment (up to 23 days for a newborn, 53 days for an adult) has not been shown to increase toxicity. When NO reacts with superoxide, peroxynitrite is rapidly formed, and this can result in membrane lipid peroxidation (Beckman 1990). No information is available to date with respect to the actual formation of such peroxynitrites in human patients receiving iNO, but the potential remains for resultant tissue injury, especially in the lung, with damage to surfactant and its related proteins (Haddad 1993). NO inhibits platelet aggregation and adhesion and in this way plays an important role in vascular homeostasis (Radomski 1993). Although it is believed that iNO does not exert systemic effects because of the previously mentioned interaction with haemoglobin, Hogman and coworkers have reported increased bleeding times in rabbits and humans exposed to 30 ppm of iNO (Hogman 1993; Hogman 1994).
Authors' conclusions
Implications for practice.
On the basis of currently available information, iNO has been established as effective therapy for near‐term and term infants with hypoxic respiratory failure unresponsive to other therapy, with the possible exclusion of infants with congenital diaphragmatic hernia. Its use reduces the need for ECMO (with number needed to treat for an additional beneficial outcome (NNTB) of 5.3), and it appears to be the only selective pulmonary vasodilator for which proof of efficacy is available.
Lack of effect on systemic haemodynamics, coupled with relative safety of administration when appropriately monitored, and lack of significant longer‐term neurodevelopmental sequelae support the use of iNO in preference to other vasodilators.
It appears to be appropriate to institute therapy for those who are severely ill (with OI ≥ 25, or PaO2 < 100 mmHg in 100% oxygen); commencing therapy earlier does not appear to further reduce ECMO requirements or mortality (however, some uncertainty surrounds this, as confidence intervals of the RR are very wide (0.61 to 1.24)). Instituting therapy at lower severity of illness criteria may prevent progression to higher disease severity.
Implications for research.
Additional studies with untreated control groups in term and near‐term infants with hypoxic respiratory failure would not be ethically acceptable.
Comparative trials with other active agents, or with different doses of iNO, may be valuable. Indeed, the minimal effective dose remains unclear. Finer 2001 suggested that starting treatment at 2 ppm was as effective as starting treatment at higher concentrations, whereas Cornfield 1999 suggested that starting treatment at 2 ppm was not effective in preventing deterioration. This discrepancy warrants further investigation by an adequately powered study.
Moreover, treating at less severe criteria for poor oxygenation has not been shown to improve mortality or ECMO requirements, or other reported clinical outcomes, compared with waiting to see whether the patient's condition deteriorates. However, substantial uncertainty remains regarding effects on death or ECMO, with 95% confidence intervals including the possibility of a 40% reduction in death or ECMO, or a 25% increase. Also, earlier treatment may prevent disease progression, and long‐term outcomes could be affected by preventing more severe illness. This should be further investigated.
Finally, other unanswered questions include the following: Does pretreatment with surfactant improve response to iNO in humans (as it appears to in some animal models)? Is iNO safer and more effective than other vasodilators delivered by inhalation such as inhaled prostacyclin?
What's new
| Date | Event | Description |
|---|---|---|
| 19 July 2016 | New citation required but conclusions have not changed | Although new trials were added, conclusions remain unchanged. |
| 5 February 2016 | New search has been performed | We identified 3 new trials, added a flow chart and added GRADE Summary of findings tables. Review structure has changed; several trials referred to as 'allowing back‐up use of iNO in controls' are now referred to as 'comparing NO at moderate vs severe severity of illness criteria'. We entered an additional study into this group. |
History
Protocol first published: Issue 1, 1997 Review first published: Issue 1, 1998
| Date | Event | Description |
|---|---|---|
| 22 August 2008 | Amended | We converted this review to new review format. |
| 31 May 2006 | New search has been performed | This updates the review, "Nitric oxide for respiratory failure in infants born at or near term", published in the Cochrane Library (2001, Issue 2) (Finer 2001). This update includes 2 additional trials (Konduri and Sadiq). Both of these trials provided intervention at moderate disease severity rather than waiting until severe disease occurred. |
| 31 May 2006 | New citation required and conclusions have changed | We have made substantive amendments. |
Acknowledgements
We are very grateful for the translation from Chinese of the article by Liu 2008, provided by Dr David Corpman.
Appendices
Appendix 1. Search strategy 2016
(Nitric OR Nitrix Oxide) with database specific terms:
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh])) Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial) CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Clinicaltrials.gov: (infant)
Controlled‐trials.com: (infant)
WHO Trials database: (infant OR neonate)
Data and analyses
Comparison 1. Inhaled NO versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or use of ECMO | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Death or use of ECMO; studies that did not allow back‐up use of iNO in controls | 8 | 859 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.57, 0.77] |
| 1.2 Death or use of ECMO; studies that allowed back‐up use of iNO in controls | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.34, 4.16] |
| 1.3 Death or use of ECMO; infants with diaphragmatic hernia | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.95, 1.26] |
| 2 Death before hospital discharge | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Death; studies that did not allow back‐up use of iNO in controls | 8 | 860 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.60, 1.31] |
| 2.2 Death; studies that allowed back‐up use of iNO in controls | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.34, 4.16] |
| 2.3 Death; infants with diaphragmatic hernia | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.74, 1.96] |
| 3 Use of ECMO before hospital discharge | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Use of ECMO before hospital discharge; studies that did not allow back‐up use of iNO in controls | 7 | 815 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.50, 0.71] |
| 3.2 Use of ECMO before hospital discharge; infants with diaphragmatic hernia | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.00, 1.62] |
| 4 Failure to improve oxygenation (PaO2) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Failure to improve PaO2; studies that did not allow back‐up use of iNO in controls | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Failure to improve PaO2; infants with diaphragmatic hernia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Oxygenation index 30 to 60 minutes after treatment | 6 | 753 | Mean Difference (IV, Fixed, 95% CI) | ‐8.59 [‐11.53, ‐5.65] |
| 5.1 OI 30 to 60 minutes after treatment; studies that did not allow back‐up use of iNO in controls | 5 | 709 | Mean Difference (IV, Fixed, 95% CI) | ‐8.45 [‐11.42, ‐5.48] |
| 5.2 OI 30 to 60 minutes after treatment; infants with diaphragmatic hernia | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐16.1 [‐38.04, 5.84] |
| 6 PaO2 30 to 60 minutes after treatment | 5 | 707 | Mean Difference (IV, Fixed, 95% CI) | 32.62 [23.56, 41.67] |
| 6.1 PaO2 after 30 to 60 minutes; studies that did not allow back‐up use of iNO in controls | 4 | 663 | Mean Difference (IV, Fixed, 95% CI) | 43.91 [32.30, 55.51] |
| 6.2 PaO2 after 30 to 60 minutes; infants with diaphragmatic hernia | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 15.10 [0.64, 29.56] |
| 7 Change in oxygenation index after treatment | 2 | 277 | Mean Difference (IV, Fixed, 95% CI) | ‐13.61 [‐18.53, ‐8.70] |
| 7.1 Change in OI; studies that did not allow back‐up use of iNO in controls | 1 | 233 | Mean Difference (IV, Fixed, 95% CI) | ‐15.1 [‐20.52, ‐9.68] |
| 7.2 Change in OI; infants with diaphragmatic hernia | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐6.7 [‐18.39, 4.99] |
| 8 Change in PaO2 after treatment | 2 | 277 | Mean Difference (IV, Fixed, 95% CI) | 15.27 [7.18, 23.36] |
| 8.1 Change in PaO2; studies that did not allow back‐up use of iNO in controls | 1 | 233 | Mean Difference (IV, Fixed, 95% CI) | 50.4 [32.14, 68.66] |
| 8.2 Change in PaO2; infants with diaphragmatic hernia | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 6.70 [‐2.32, 15.72] |
| 9 Neurodevelopmental disability at 18 to 24 months among survivors | 2 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.66, 1.44] |
| 10 Hearing impairment in at least 1 ear among survivors | 2 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.72, 1.68] |
| 10.1 Hearing impairment among survivors; studies that did not allow back‐up use of iNO in controls | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.71, 1.84] |
| 10.2 Hearing impairment among survivors; infants with diaphragmatic hernia | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.39, 2.19] |
| 11 Cerebral palsy among survivors | 3 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.62, 2.45] |
| 11.1 Cerebral palsy among survivors; studies that did not allow back‐up use of iNO in controls | 2 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.49, 2.14] |
| 11.2 Cerebral palsy among survivors; infants with diaphragmatic hernia | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.33 [0.45, 154.78] |
| 12 BSID MDI > 2 SD below the mean | 2 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.38, 1.12] |
| 13 BSID PDI > 2 SD below the mean | 2 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.25, 0.94] |
Comparison 2. Inhaled NO at moderate compared with severe criteria for illness severity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or use of ECMO | 5 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.62, 1.27] |
| 2 Death before hospital discharge | 5 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.38, 1.26] |
| 3 Use of ECMO before hospital discharge | 4 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.66, 1.54] |
| 4 Progression to severe disease criteria | 6 | 512 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.55, 0.79] |
| 5 Chronic lung disease | 3 | 437 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.54, 1.53] |
| 6 Neurodevelopmental disability at 18 to 24 months among survivors | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.74, 1.74] |
| 7 Hearing impairment among survivors | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.03, 2.95] |
| 8 Cerebral palsy among survivors | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.53, 3.39] |
2.5. Analysis.
Comparison 2 Inhaled NO at moderate compared with severe criteria for illness severity, Outcome 5 Chronic lung disease.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barefield 1996.
| Methods | Single‐centre randomised study. Masking of allocation: yes. Masking of intervention: no. Completeness of follow‐up: yes. Masking of outcome measurement: no | |
| Participants | 17 near‐term infants ≥ 35 weeks with PaO2 < 100 mmHg on 100% oxygen on ventilator Patients with congenital diaphragmatic hernia were excluded. | |
| Interventions | iNO at 20 to 40 ppm increased to 80 if PaO2 stayed < 100 mmHg. Use of iNO allowed in case of failure of control treatment, if PaO2 was (1) < 80 mmHg (10.7 kPa) for longer than 1 hour, (2) < 40 mmHg (5.3 kPa) beyond 1 hour or (3) < 30 mmHg (4 kPa) beyond 30 minutes High‐frequency ventilation not allowed during study | |
| Outcomes | Primary outcome: 'treatment failure' or meeting ECMO criteria (defined as PaO2 < 80 mmHg for > 1 hour, < 40 mmHg after 1 hour or < 30 mmHg after 30 minutes) Secondary outcomes: oxygenation index, PaO2, alveolar arterial oxygen gradient, after 30 and 60 minutes; death and ultimate use of high‐frequency ventilation or ECMO | |
| Notes | Admission OI in control group averaged 26, in treatment group 38 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Funding Source(s) | Low risk | Charitable foundation (March of Dimes) |
Christou 2000.
| Methods | Single‐centre randomised study. Masking of allocation: yes. Masking of intervention: no. Completeness of follow‐up: yes. Masking of outcome: no | |
| Participants | 42 near‐term infants ≥ 34 weeks with PaO2 < 100 mmHg on 100%oxygen on ventilator. 41 infants after exclusion of 1 case of congenital heart disease. Patients with diaphragmatic hernia were excluded. Some evidence showing increased pulmonary artery pressure on echocardiography required | |
| Interventions | 40 ppm iNO reduced to 20 ppm after 1 hour. Combined therapy with high‐frequency ventilation and iNO allowed | |
| Outcomes | Death before discharge or requirement for ECMO. Secondary outcomes included changes in oxygenation and duration of ventilation and oxygen therapy. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Other bias | High risk | Study terminated early after ad hoc committee reviewed the data |
| Funding Source(s) | Low risk | Supported by local and government agencies |
Clark 2000.
| Methods | Multi‐centre randomised trial. Masking of allocation: yes. Masking of intervention: yes. Completeness of follow‐up: yes. Masking of outcome: yes | |
| Participants | 248 near‐term infants, ≥ 34 weeks, ≤ 4 days of age, with OI ≥ 25 | |
| Interventions | 20 ppm iNO or nitrogen placebo. Inhaled NO gas weaned to 5 ppm after 24 hours for a maximum of 96 hours | |
| Outcomes | Death before discharge, need for ECMO, chronic lung disease, neurological injury | |
| Notes | No calculation of sample size for the trial is described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cards on which treatment assignments were written were randomly ordered (shuffled by hand 3 times) and placed in sequentially numbered opaque envelopes in blocks of 8. |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Other bias | Unclear risk | Uncertain how sample size was determined |
| Funding Source(s) | High risk | Funded "in part" by INOtherapeutics; no other sources listed |
Cornfield 1999.
| Methods | Three‐centre randomised trial. Masking of allocation: cannot tell. Masking of intervention: no. Completeness of follow‐up: yes. Masking of outcome: no | |
| Participants | 38 near‐term infants with OI ≥ 25, < 1 week old, with echocardiographically proven pulmonary hypertension | |
| Interventions | Inhaled NO at 2 ppm or no therapy | |
| Outcomes | Primary outcome: failure, defined as OI > 35 after 1 hour of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Other bias | Low risk | |
| Funding Source(s) | Low risk | Local funds and charitable sources |
Davidson 1997.
| Methods | Multi‐centre randomised trial, with nitrogen used as placebo gas | |
| Participants | 155 term infants with echocardiographic evidence of pulmonary hypertension. PaO2 between 40 and 100 mmHg in 100% oxygen. Randomised equally to each of the 4 groups Excluded infants with congenital diaphragmatic hernia or other causes of pulmonary hypoplasia Did not allow surfactant therapy or concurrent high‐frequency ventilation | |
| Interventions | Inhaled nitric oxide at 5, 20 or 80 ppm or control, gas stopped upon 'failure', defined as PaO2 < 40 mmHg for longer than 30 minutes | |
| Outcomes | Major sequelae index: composite index of death, ECMO, neurological sequelae or bronchopulmonary dysplasia Oxygenation | |
| Notes | Terminated early because of poor enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised by a "scratch‐off card" |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo‐controlled masked trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Other bias | Unclear risk | Terminated early for poor enrolment |
| Funding Source(s) | High risk | Industry funded (Ohmeda) |
Day 1996.
| Methods | Single‐centre randomised parallel‐group study | |
| Participants | 22 term or premature infants with OI > 25 and < 40, plus right‐to‐left ductal shunting or estimated peak right ventricular pressure > 75% of systemic systolic pressure | |
| Interventions | 20 ppm iNO. High‐frequency jet ventilation allowed concurrently. Back‐up use of iNO allowed in case of failure of control treatment. Few details of other therapy given | |
| Outcomes | Primary outcomes: oxygenation index, PaO2, echocardiographic Doppler changes | |
| Notes | If the condition of infants in the controlled trial deteriorated to an OI > 40, iNO was given. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised by "blind draw" |
| Allocation concealment (selection bias) | Unclear risk | Not clearly described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Other bias | Unclear risk | No sample size calculation presented |
| Funding Source(s) | Low risk | Local sources |
Gonzalez 2010.
| Methods | Two‐centre randomised trial | |
| Participants | 56 infants > 34 weeks, < 48 hours old, with OI between 10 and 30 | |
| Interventions | Immediate iNO at 20 ppm, or no iNO unless OI increases to > 40 | |
| Outcomes | Primary outcome was the proportion with OI increasing to > 40. Secondary outcomes were death, days of ventilation and chronic lung disease. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Opaque sealed sequential envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Funding Source(s) | Unclear risk | Local (university) sources and industry (AGA, SA) |
INNOVO 2007.
| Methods | Multi‐centre parallel‐group randomised controlled trial | |
| Participants | 60 full‐term infants ≥ 34 weeks with severe hypoxic respiratory failure for whom attending physician was unsure whether iNO was indicated | |
| Interventions | iNO at 20 ppm or no iNO | |
| Outcomes | Survival without severe disability to 1 year of age | |
| Notes | Sample size determined by limit to trial duration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation by random number generator with minimisation |
| Allocation concealment (selection bias) | Low risk | Enrolled before allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes reported |
| Selective reporting (reporting bias) | Low risk | Protocol registered (ISRCTN 17821339). Primary outcome variables consistent with publication |
| Funding Source(s) | Low risk | Government agency |
Kinsella 1997.
| Methods | Randomised multi‐centre controlled parallel‐group trial of iNO compared with high‐frequency ventilation | |
| Participants | 205 near‐term infants, OI > 40. Stratified by disease process; infants with diaphragmatic hernia (n = 34) were included as a separate stratum | |
| Interventions | iNO at 40 ppm was compared with high‐frequency ventilation with the SensorMedics oscillator. Initial randomisation was followed by back‐up treatment with alternate therapy in cases of failure. This was followed by further cross‐over to combination treatment with high‐frequency ventilation and iNO if alternate therapy failed. iNO therapy was administered via a standard time‐cycled pressure‐limited ventilator. High‐frequency ventilation was aimed at a lung recruitment strategy. Surfactant treatment after enrolment was prohibited. iNO dose was increased to 40 ppm in case of failure to maintain PaO2 > 60 mmHg. | |
| Outcomes | Sustained PaO2 ≥ 60 mmHg. Failure defined as PaO2 < 60 mmHg after 2 hours of therapy or lack of improvement in PaO2 before 2 hours. Some data on infants with diaphragmatic hernia were presented separately. | |
| Notes | Complex study design; we abstracted only results from the initial randomisation. All infants who failed were exposed to iNO at some stage in the protocol. Study was stopped after interim analysis, suggesting no difference between initial treatment limbs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Funding Source(s) | Low risk | Local and government sources |
Konduri 2004.
| Methods | Multi‐centre randomised controlled trial | |
| Participants | All 302 enrolled (3 excluded, as they turned out to have congential heart disease) infants were ≥ 34 weeks' gestation, with hypoxic respiratory failure and OI between 15 and 25, while receiving ≥ 80% oxygen, on 2 blood gases between 15 minutes and 12 hours apart | |
| Interventions | iNO at 5 ppm; iNO could be increased to 20 ppm in the case of partial response; treated up to 14 days. Controls received nitrogen, or iNO if OI increased to > 25. | |
| Outcomes | Primary outcome: occurrence or death or requirement for ECMO Secondary hypotheses were that early iNO therapy would (1) reduce the probability of using standard iNO therapy; (2) decrease progression to severe respiratory failure, defined as OI > 40; and (3) would not increase neurodevelopmental impairment among surviving infants at 18 to 24 months of age. | |
| Notes | Study terminated early because of slowing enrolment; 75% of anticipated sample enrolled; study terminated without knowledge of results at that point | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computerised randomisation |
| Allocation concealment (selection bias) | Low risk | Randomised by telephone after enrolment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked gas administration |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | Study registered in 2000 (after start of trial but before completion); NCT00005773. Primary and secondary outcomes match registration documents. |
| Other bias | Unclear risk | Early termination without examination of data |
| Funding Source(s) | Unclear risk | Government agency, partial industry support |
Liu 2008.
| Methods | Single‐centre randomised trial | |
| Participants | 46 infants with meconium aspiration syndrome, over 36 weeks, > 2.5 kg, OI > 15 | |
| Interventions | iNO at 15 ppm or no additional gas | |
| Outcomes | Primary outcome variable unclear; outcomes reported include changes in OI and in echocardiography, survival, certain medical complications and duration of assisted ventilation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not clearly reported but may be acceptable; 'random number method' |
| Allocation concealment (selection bias) | Unclear risk | No relevant information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of all participants reported |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Funding Source(s) | Unclear risk | Uncertain |
Mercier 1998.
| Methods | Randomised multi‐centre trial of iNO | |
| Participants | 204 infants; 107 near term, ≥ 33 weeks' gestation. OI 15 to 40, on 2 blood gases 1 hour apart. Congenital diaphragmatic hernia excluded, congenital heart disease excluded, < 7 days of age only | |
| Interventions | iNO at 10 ppm for 2 hours, continued if response. Controls could be treated after 2 hours. | |
| Outcomes | Primary outcome: change in OI at 2 hours after initiation of treatment. Secondary outcomes: death, brain injury, long‐term oxygen therapy, duration of hospitalisation | |
| Notes | ECMO not available as back‐up therapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked study gas administration |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Other bias | Unclear risk | Terminated early for poor enrolment |
| Funding Source(s) | Unclear risk | Government agency and industry support |
Ninos 1996.
| Methods | Randomised multi‐centre study, with oxygen used as placebo gas | |
| Participants | 235 near‐term infants, ≥ 34 weeks' gestation, OI > 25 on 2 blood gases, 15 minutes apart. Congenital diaphragmatic hernia excluded, congenital heart disease excluded, < 14 days of age only | |
| Interventions | iNO at 20 ppm, trial at 80 ppm if no response to 20 ppm (in treatment group). Comparison with control. Both groups received 'maximal therapy' before study entry, including surfactant in the majority, high‐frequency ventilation at experienced centres, muscle relaxation and inotropes. Induction of alkalosis with target pH of 7.45‐7.60 was also used as a guideline. All of these treatment strategies were continued in controls. Investigators were not allowed to start high‐frequency ventilation or to administer surfactant after study entry. | |
| Outcomes | Survival to 120 days or discharge home, without requiring ECMO. Secondary outcomes were oxygenation (OI and PaO2) after 30 minutes, length of hospital stay, days of assisted ventilation and incidence of air leak or bronchopulmonary dysplasia. Neurodevelopment at 18‐24 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Prepared by study centre |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation system |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked gas administration |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | Study registered, NCT00005776, in 2000 (after completion). Outcomes in registration documents are reported in the main publication. |
| Funding Source(s) | Unclear risk | Started with government agency support, industry support after study commenced |
Ninos 1997.
| Methods | Randomised multi‐centre trial of iNO in infants with diaphragmatic hernia, with oxygen used as placebo gas | |
| Participants | 53 near‐term infants with diaphragmatic hernia, ≥ 34 weeks' gestation, < 14 days of age | |
| Interventions | iNO at 20 ppm, trial at 80 ppm if no response to 20 ppm (in treatment group). Comparison with control. Both groups received 'maximal therapy' before study entry, including surfactant in the majority, high‐frequency ventilation at experienced centres, muscle relaxation and inotropes. Induction of alkalosis with target pH of 7.45‐7.60 was also used as a guideline. All of these treatment strategies were continued in controls. Investigators were not allowed to start high‐frequency ventilation or to administer surfactant after study entry. | |
| Outcomes | Survival to 120 days or discharge home, without requiring ECMO. Secondary outcomes were oxygenation (OI and PaO2) after 30 minutes, length of hospital stay, days of assisted ventilation and incidence of air leak or bronchopulmonary dysplasia. Neurodevelopment at 18‐24 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised blocked randomisation |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation system |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked gas administration |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Unclear risk | Study registered, NCT00005776, in 2000 (after completion). Outcomes as registered are reported. |
| Funding Source(s) | Low risk | Planned and commenced with government agency support; industry support provided after study commenced |
Roberts 1996.
| Methods | Multi‐centre randomised study, with nitrogen used for placebo gas | |
| Participants | 58 'full‐term infants' on FiO2 1.0 with PaO2 < 55 mmHg. All had echocardiographic signs of pulmonary hypertension. Patients excluded if they had received high‐frequency ventilation Patients with diaphragmatic hernia excluded, or other causes of pulmonary hypoplasia | |
| Interventions | iNO at 80 ppm or control. Control patients received conventional ventilation. Surfactant was not allowed during the study. | |
| Outcomes | Primary outcome was 'success', defined as improved OI to < 40, without a fall in PaO2 or hypotension. Secondary outcomes were oxygenation, both OI and PaO2, after 30 minutes of therapy. | |
| Notes | Study was terminated after an interim analysis showed an effect at P < 0.05. Original sample size was not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in the publication |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masked gas administration |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome assessment |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Other bias | High risk | Early termination after examination of data |
| Funding Source(s) | Low risk | Government agency |
Sadiq 1998.
| Methods | Multi‐centre randomised trial | |
| Participants | 87 infants > 2 kg birth weight, with A‐aDO2 500‐599 after surfactant on 2 gases 1 hour apart, on 100% oxygen with echocardiographic evidence of PPHN | |
| Interventions | iNO at 10 ppm or control; iNO increased up to 80 ppm until no further increases in arterial PaO2 occurred | |
| Outcomes | Primary outcome variable was progression to severe PPHN, defined as an A‐aDO2 persistently > 600. Secondary outcome variables included death, ECMO rate, length of hospitalisation, amount and duration of mechanical ventilation, number of days of oxygen use and need for supplemental oxygen at 28 days of life. | |
| Notes | Study was terminated early after approval of iNO by the Federal Drug Administration, as this impaired recruitment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation order was determined a priori, in blocks of 10, by coin toss with folded group assignment cards. |
| Allocation concealment (selection bias) | Low risk | Cards were placed in sequentially numbered opaque envelopes for each centre. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Other bias | Unclear risk | Early termination, but not because of examination of data |
| Funding Source(s) | Unclear risk | Not described |
Wessel 1996.
| Methods | Single‐centre randomised trial. Masking of allocation: not clear. Masking of intervention: no. Completeness of follow‐up: yes. Masking of outcome: no | |
| Participants | 49 near‐term infants ≥ 34 weeks, PaO2 < 100 mmHg on 100% oxygen; all had evidence of PPHN on echocardiography | |
| Interventions | iNO at 80 ppm, reduced to 40 ppm after 1 hour. All received muscle relaxants and sedation and conventional ventilation. | |
| Outcomes | Primary outcomes were oxygenation as well as death and need for ECMO. Secondary outcomes were duration of mechanical ventilation, duration of hospitalisation and need for oxygen after hospital discharge. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unmasked trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol found |
| Funding Source(s) | Low risk | Supported by local and charitable sources |
A‐aDO2: alveolar‐arterial oxygen difference. ECMO: extracorporeal membrane oxygenation. FiO2: fraction of inspired oxygen. iNO: inhaled nitric oxide. NO: nitric oxide. OI: oxygenation index. PaO2: partial pressure of arterial oxygen. PPHN: persistent pulmonary hypertension of the newborn
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Hoffman 1997 | Non‐randomised retrospective study; infants were treated or were not treated according to availability of inhaled nitric oxide. The time period over which infants were studied was different between control and inhaled nitric oxide groups. |
| Pinheiro 1998 | Randomised comparison of inhaled nitric oxide with intravenous nitroprusside. Study was stopped after enrolment of 25 participants owing to decreasing enrolment. Inhaled nitric oxide produced much greater improvements in oxygenation than were produced by nitroprusside. |
Differences between protocol and review
The original protocol did not divide trials into the three groups now presented in the review. The original protocol and review did not include GRADE recommendations and Summary of findings tables.
Review authors added the following outcomes post hoc (reported in trials but not prespecified): change in oxygenation index after treatment, change in PaO2 after treatment, chronic lung disease.
Contributions of authors
The original protocol was written by Drs Finer and Barrington, and both review authors equally completed each revision of the review. All four review authors have edited the latest revision of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201600005C
Declarations of interest
Dr Barrington was chair of the iNO Therapeutics Canadian Medical Advisory Committee for a single meeting, for which he received expenses and an honorarium. Dr Finer previously served as a member of the iNO Therapeutics Advisory Committee.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Barefield 1996 {published data only}
- Barefield ES, Karke VA, Phillips JB, Carlo WA. Inhaled nitric oxide in term infants with hypoxemic respiratory failure. Journal of Pediatrics 1996;129:279‐86. [DOI] [PubMed] [Google Scholar]
- Barefield ES, Karke VA, Phillips JB, Carlo WA. Randomized, controlled trial of inhaled NO in ECMO candidates. Pediatric Research 1995;37:195A. [Google Scholar]
Christou 2000 {published data only}
- Christou H, Marter LJ, Wessel DL, Allred EN, Kane JW, Thompson JE, et al. Inhaled nitric oxide reduces the need for extracorporeal membrane oxygenation in infants with persistent pulmonary hypertension of the newborn. Critical Care Medicine 2000;28:3722‐7. [DOI] [PubMed] [Google Scholar]
Clark 2000 {published data only}
- Clark RH, Keuser TJ, Walker MW, Southgate MS, Huckaby JL, Perez JA, et al. for the Clinical Inhaled Nitric Oxide Research Group. Low‐dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. New England Journal of Medicine 2000;342:469‐74. [DOI] [PubMed] [Google Scholar]
Cornfield 1999 {published data only}
- Cornfield DN, Maynard RC, deRegnier RA, Guiang SF 3rd, Barbato JE, Milla CE. Randomized, controlled trial of low‐dose inhaled nitric oxide in the treatment of term and near‐term infants with respiratory failure and pulmonary hypertension. Pediatrics 1999;104:1089‐94. [DOI] [PubMed] [Google Scholar]
Davidson 1997 {published data only}
- Davidson D, Barefield ES, Kattwinkel J, Dudell G, Damask M, Straube R, et al. Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: a randomized, double‐masked, placebo‐controlled, dose‐response, multicenter study. Pediatrics 1998;101:325‐34. [DOI] [PubMed] [Google Scholar]
- Davidson D, Barefield ES, Kattwinkel J, Dudell G, Damask M, Straube R, et al. I‐NO/PPHN Study Group. A double masked, randomized, placebo controlled, dose response study of inhaled nitric oxide for the treatment of persistent pulmonary hypertension of the newborn. Pediatric Research 1997;41:144A (abstract). [DOI] [PubMed] [Google Scholar]
Day 1996 {published data only}
- Day RW, Lynch JM. Acute hemodynamic and blood gas effects of inhaled nitric oxide in newborns with lung disease and pulmonary hypertension. Pediatric Research 1996;39:330A. [Google Scholar]
- Day RW, Lynch JM, White KS, Ward RM. Acute response to inhaled nitric oxide in newborns with respiratory failure and pulmonary hypertension. Pediatrics 1996;98:698‐705. [PubMed] [Google Scholar]
Gonzalez 2010 {published data only}
- Gonzalez A, Fabres J, D'Apremont I, Urcelay G, Avaca M, Gandolfi C, et al. Randomized controlled trial of early compared with delayed use of inhaled nitric oxide in newborns with a moderate respiratory failure and pulmonary hypertension. Journal of Perinatology 2010;30(6):420‐4. [DOI] [PubMed] [Google Scholar]
INNOVO 2007 {published data only}
- Field D, Elbourne D, Hardy P, Fenton AC, Ahluwalia J, Halliday HL, et al. INNOVO Trial Collaborating Group. Neonatal ventilation with inhaled nitric oxide vs. ventilatory support without inhaled nitric oxide for infants with severe respiratory failure born at or near term: the INNOVO multicentre randomised controlled trial. Neonatology 2007;91(2):73‐82. [DOI] [PubMed] [Google Scholar]
Kinsella 1997 {published data only}
- Kinsella JP, Truog WE, Walsh WF, Goldberg RN, Bancalari E, Clark RH, et al. Randomized, multicenter trial of inhaled nitric oxide and high frequency ventilation in severe persistent pulmonary hypertension of the newborn. Pediatric Research 1996;39:222A. [DOI] [PubMed] [Google Scholar]
- Kinsella JP, Truog WE, Walsh WF, Goldberg RN, Bancalari EE, Mayock DE, et al. Randomized, multicenter trial of inhaled nitric oxide and high‐frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. Journal of Pediatrics 1997;131:55‐62. [DOI] [PubMed] [Google Scholar]
Konduri 2004 {published data only}
- Konduri GG, Solimano A, Sokol GM, Singer J, Ehrenkranz RA, Singhal N, et al. Neonatal Inhaled Nitric Oxide Study Group. A randomized trial of early versus standard inhaled nitric oxide therapy in term and near‐term newborn infants with hypoxic respiratory failure. Pediatrics 2004;113(3 Pt 1):559‐64. [DOI] [PubMed] [Google Scholar]
- Konduri GG, Vohr B, Robertson C, Sokol GM, Solimano A, Singer J, et al. Early inhaled nitric oxide therapy for term and near‐term newborn infants with hypoxic respiratory failure: neurodevelopmental follow‐up. Journal of Pediatrics 2007;150(3):235‐40.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2008 {published data only}
- Liu CQ, Ma L, Tang LM, He XJ, Wei SF, Wang SX, et al. A randomized controlled study on the efficacy of inhaled nitric oxide in treatment of neonates with meconium aspiration syndrome. Zhonghua Er Ke za Zhi [Chinese Journal of Pediatrics] 2008;46(3):224‐8. [PubMed] [Google Scholar]
Mercier 1998 {published data only}
- Franco‐Belgium Collaborative NO Trial Group. Early compared with delayed inhaled nitric oxide in moderately hypoxaemic neonates with respiratory failure: a randomised controlled trial. Lancet 1999;354:1066‐71. [PubMed] [Google Scholar]
- Mercier JC, Dehan M, Breart G, Clement S, O'Nody P. Inhaled nitric oxide in neonatal respiratory failure. Pediatric Research 1998;43:290A. [Google Scholar]
Ninos 1996 {published and unpublished data}
- The Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full‐term and nearly full term infants with hypoxic respiratory failure. New England Journal of Medicine 1997;336:597‐604. [DOI] [PubMed] [Google Scholar]
- The Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in term and near‐term infants: neurodevelopmental follow‐up of the The Neonatal Inhaled Nitric Oxide Study Group (NINOS). Journal of Pediatrics 2000;136:611‐7. [DOI] [PubMed] [Google Scholar]
Ninos 1997 {unpublished data only}
- The Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. Pediatrics 1997;99:838‐45. [DOI] [PubMed] [Google Scholar]
- The Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in term and near‐term infants: neurodevelopmental follow‐up of the The Neonatal Inhaled Nitric Oxide Study Group (NINOS). Journal of Pediatrics 2000;136:611‐7. [DOI] [PubMed] [Google Scholar]
Roberts 1996 {published data only}
- Roberts JD Jr, Fineman J, Morin FC III, Shaul PW, Rimar S, Schreiber MD, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. New England Journal of Medicine 1997;336:605‐10. [DOI] [PubMed] [Google Scholar]
- Roberts JD Jr, Fineman J, Morin FC III, Shaul PW, Rimar S, Schreiber MD, et al. Inhaled nitric oxide gas improves oxygenation in PPHN. Pediatric Research 1996;39:241A. [Google Scholar]
Sadiq 1998 {published and unpublished data}
- Sadiq FH, Illinois Multicenter Trial. Treatment of persistent pulmonary hypertension of the newborn with nitric oxide: a randomized trial. Pediatric Research 1998;43:192A. [Google Scholar]
- Sadiq HF, Mantych G, Benawra RS, Devaskar UP, Hocker JR. Inhaled nitric oxide in the treatment of moderate persistent pulmonary hypertension of the newborn: a randomized controlled, multicenter trial. Journal of Perinatology 2003;23:98‐103. [DOI] [PubMed] [Google Scholar]
Wessel 1996 {published data only}
- Ellington M, O'Reilly D, Allred EN, McCormick MC, Wessel DL, Kourembanas S. Child health status, neurodevelopmental outcome, and parental satisfaction in a randomized controlled trial of nitric oxide for persistent pulmonary hypertension of the newborn. Pediatrics 2001;107:1351‐6. [DOI] [PubMed] [Google Scholar]
- Wessel D, Adatia I, Thompson J, Kane J, Marter L, Stark A, et al. Improved oxygenation in a randomized trial of inhaled nitric oxide for PPHN. Pediatric Research 1996;39:252A. [DOI] [PubMed] [Google Scholar]
- Wessel DL, Adatia I, Marter LJ, Thompson JE, Kane JW, Stark AR, et al. Improved oxygenation in a randomized trial of inhaled nitric oxide for persistent pulmonary hypertension of the newborn. Pediatrics 1997;100(5):e7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Hoffman 1997 {published data only}
- Hoffman GM, Ross GA, Day SE, Rice TB, Nelin LD. Inhaled nitric oxide reduces the utilization of extracorporeal membrane oxygenation in persistent pulmonary hypertension of the newborn. Critical Care Medicine 1997;25:352‐9. [DOI] [PubMed] [Google Scholar]
Pinheiro 1998 {published data only}
- Pinheiro JMB, Carey T. Inhaled NO vs. intravenous nitroprusside in neonatal pulmonary hypertension: randomized trial at a center without ECMO. Pediatric Research 1998;43:294A. [Google Scholar]
Additional references
Abman 1990
- Abman SH, Chatfield BA, Hall SL, McMurtry IF. Role of endothelium‐derived relaxing factor during transition of pulmonary circulation at birth. American Journal of Physiology 1990;259:H1921‐7. [DOI] [PubMed] [Google Scholar]
Beckman 1990
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxides. Proceedings of the National Academy of Sciences of the United States of America 1990;87:1620‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bouchet 1993
- Bouchet M, Renaudin MH, Raveau C, Mercier JC Dehan M, Zupan V. Safety requirement for use of inhaled nitric oxide in neonates. Lancet 1993;341:968‐9. [DOI] [PubMed] [Google Scholar]
Cornfield 1992
- Cornfield DN, Chatfield BA, McQueston JA, McMurtry IF, Abman SH. Effects of birth‐related stimuli on L‐arginine‐dependent pulmonary vasodilation in ovine fetus. American Journal of Physiology 1992;262:H1474‐81. [DOI] [PubMed] [Google Scholar]
Davidson 1999
- Davidson D, Barefield ES, Kattwinkel J, Dudell G, Damask M, Straube R, et al. Safety of withdrawing inhaled nitric oxide therapy in persistent pulmonary hypertension of the newborn. Pediatrics 1999;104:231‐6. [DOI] [PubMed] [Google Scholar]
Dobyns 1999
- Dobyns EL, Griebel J, Kinsella JP, Abman SH, Accurso FJ. Infant lung function after inhaled nitric oxide therapy for persistent pulmonary hypertension of the newborn. Pediatric Pulmonology 1999;28:24‐30. [DOI] [PubMed] [Google Scholar]
Etches 1994
- Etches PC, Finer NN, Barrington KJ, Graham AJ, Chan WKY. Nitric oxide reverses pulmonary hypertension in the newborn piglet. Pediatric Research 1994;35:15‐9. [DOI] [PubMed] [Google Scholar]
Finer 1994
- Finer NN, Etches PC, Kamstra B, Tierney AJ, Peliowski A, Ryan CA. Inhaled nitric oxide in infants referred for extracorporeal membrane oxygenation: dose response. Journal of Pediatrics 1994;124:302‐8. [DOI] [PubMed] [Google Scholar]
Fratacci 1991
- Fratacci MD, Frostell CG, Chen TY, Wain JC, Robinson DR, Zapol WM. Inhaled nitric oxide ‐ A selective pulmonary vasodilator of heparin‐protamine vasoconstriction in sheep. Anesthesiology 1991;75:990‐9. [DOI] [PubMed] [Google Scholar]
Frostell 1991
- Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM. Inhaled nitric oxide ‐ A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation 1991;83:2038‐47. [DOI] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- McMaster University. GRADEpro [www.gradepro.org]. Ontario: McMaster University, 2014.
Greenbaum 1967
- Greenbaum R, Bay J, Hargreaves MD, Kain ML, Kelman GR, Nunn JF, et al. Effects of higher oxides of nitrogen on the anaesthetized dog. British Journal of Anaesthesia 1967;39:393‐404. [DOI] [PubMed] [Google Scholar]
Haddad 1993
- Haddad IY, Ischiropoulos H, Holm BA, Beckman JS, Baker JR, Matalon S. Mechanisms of peroxynitrite‐induced injury to pulmonary surfactants. American Journal of Physiology 1993;265:L555‐64. [DOI] [PubMed] [Google Scholar]
Heal 1995
- Heal CA, Spencer SA. Methaemoglobinaemia with high‐dose nitric oxide administration. Acta Paediatrica 1995;84:1318‐9. [DOI] [PubMed] [Google Scholar]
Higenbottam 1988
- Higenbottam T, Pepke‐Zaba J, Scott J, Wollman P, Coutts C, Wallwork J. Inhaled "endothelium‐derived relaxing factor" (EDRF) in primary hypertension (PPH). American Review of Respiratory Disease 1988;137:A107. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hogman 1993
- Hogman M, Frostell C, Arnberg H, Hedenstierma G. Bleeding time prolongation and NO inhalation. Lancet 1993;341:1664‐5. [DOI] [PubMed] [Google Scholar]
Hogman 1994
- Hogman M, Frostell C, Arnberg H, Sandhagen B, Hedenstierna G. Prolonged bleeding time during nitric oxide inhalation in the rabbit. Acta Physiologica Scandinavica 1994;151:125. [DOI] [PubMed] [Google Scholar]
Kinsella 1992a
- Kinsella JP, McQueston JA, Rosenberg AA, Abman SH. Hemodynamic effects of exogenous nitric oxide in ovine transitional pulmonary circulation. American Journal of Physiology 1992;263:H875‐80. [DOI] [PubMed] [Google Scholar]
Kinsella 1992b
- Kinsella JP, Neish SR, Shaffer E, Abman SH. Low‐dose inhalational nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 1992;340:819‐20. [DOI] [PubMed] [Google Scholar]
Kinsella 1993
- Kinsella JP, Neish SR, Ivy DD, Shaffer E, Abman SH. Clinical responses to prolonged treatment of persistent pulmonary hypertension of the newborn with low doses of inhaled nitric oxide. Journal of Pediatrics 1993;123:103‐8. [DOI] [PubMed] [Google Scholar]
Lipkin 2002
- Lipkin PH, Davidson D, Spivak L, Straube R, Rhines J, Chang CT. Neurodevelopmental and medical outcomes of persistent pulmonary hypertension in term newborns treated with nitric oxide. Journal of Pediatrics 2002;140:306‐10. [DOI] [PubMed] [Google Scholar]
MMWR 1988
- National Institute for Occupational Safety and Health. NIOSH recommendations for occupational safety and health standards. MMWR 1988;37:21. [PubMed] [Google Scholar]
Ninos 2000
- Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in term and near‐term infants: neurodevelopmental follow‐up of the neonatal inhaled nitric oxide study group (NINOS). Journal of Pediatrics 2000;136:611‐7. [DOI] [PubMed] [Google Scholar]
Pepke‐Zaba 1991
- Pepke‐Zaba J, Higenbottam TW, Dinh‐Xuan AT, Stone D, Wallwork J. Inhaled nitric oxide as a cause of selective pulmonary vasodilation in pulmonary hypertension. Lancet 1991;338:1173‐4. [DOI] [PubMed] [Google Scholar]
Radomski 1993
- Radomski MW, Moncada S. Regulation of vascular homeostasis by nitric oxide. Thrombosis and Haemostasis 1993;70:36‐41. [PubMed] [Google Scholar]
Rasmussen 1992
- Rasmussen TR, Kjaergaard SK, Tarp U, Pedersen OF. Delayed effects of NO2 exposure on alveolar permeability and glutathione peroxidase in healthy humans. American Review of Respiratory Disease 1992;146:654‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 1992
- Roberts JD, Polaner DM, Lang P, Zapol WM. Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 1992;340:818‐9. [DOI] [PubMed] [Google Scholar]
Roberts 1993
- Roberts JD Jr, Chen T‐Y. Inhaled nitric oxide reverses pulmonary vasoconstriction in the hypoxic and acidotic newborn lamb. Circulation Research 1993;72:246. [DOI] [PubMed] [Google Scholar]
Rosenberg 1997
- Rosenberg AA, Kennaugh JM, Moreland SG, Fashaw LM, Hale KA, Torielli FM, et al. Longitudinal follow‐up of a cohort of newborn infants treated with inhaled nitric oxide for persistent pulmonary hypertension. Journal of Pediatrics 1997;131:70‐5. [DOI] [PubMed] [Google Scholar]
Rossaint 1993
- Rossaint R, Falke KJ, Lopez F, Slama K, Pison U, Zapol WM. Inhaled nitric oxide for the adult respiratory distress syndrome. New England Journal of Medicine 1993;328:399‐405. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GWG. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. www.guidelinedevelopment.org/handbook Updated October 2013.
References to other published versions of this review
Finer 1997
- Finer NN, Barrington KJ. Nitric oxide in respiratory failure in full‐term and nearly full‐term newborn infants. Cochrane Database of Systematic Reviews 1997, Issue 4. [DOI: 10.1002/14651858.CD000399] [DOI] [Google Scholar]
Finer 1999
- Finer NN, Barrington KJ. Nitric oxide in respiratory failure in full‐term and nearly full‐term newborn infants. Cochrane Database of Systematic Reviews 1999, Issue 1. [DOI: 10.1002/14651858.CD000399] [DOI] [Google Scholar]
Finer 2001
- Finer NN, Barrington KJ. Nitric oxide in respiratory failure in full‐term and nearly full‐term newborn infants (Cochrane Review). Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD000399.pub2] [DOI] [Google Scholar]


