Abstract
Background
Nicotine receptor partial agonists may help people to stop smoking by a combination of maintaining moderate levels of dopamine to counteract withdrawal symptoms (acting as an agonist) and reducing smoking satisfaction (acting as an antagonist).
Objectives
To review the efficacy of nicotine receptor partial agonists, including varenicline and cytisine, for smoking cessation.
Search methods
We searched the Cochrane Tobacco Addiction Group's specialised register for trials, using the terms ('cytisine' or 'Tabex' or 'dianicline' or 'varenicline' or 'nicotine receptor partial agonist') in the title or abstract, or as keywords. The register is compiled from searches of MEDLINE, EMBASE, and PsycINFO using MeSH terms and free text to identify controlled trials of interventions for smoking cessation and prevention. We contacted authors of trial reports for additional information where necessary. The latest update of the specialised register was in May 2015, although we have included a few key trials published after this date. We also searched online clinical trials registers.
Selection criteria
We included randomised controlled trials which compared the treatment drug with placebo. We also included comparisons with bupropion and nicotine patches where available. We excluded trials which did not report a minimum follow‐up period of six months from start of treatment.
Data collection and analysis
We extracted data on the type of participants, the dose and duration of treatment, the outcome measures, the randomisation procedure, concealment of allocation, and completeness of follow‐up.
The main outcome measured was abstinence from smoking at longest follow‐up. We used the most rigorous definition of abstinence, and preferred biochemically validated rates where they were reported. Where appropriate we pooled risk ratios (RRs), using the Mantel‐Haenszel fixed‐effect model.
Main results
Two trials of cytisine (937 people) found that more participants taking cytisine stopped smoking compared with placebo at longest follow‐up, with a pooled risk ratio (RR) of 3.98 (95% confidence interval (CI) 2.01 to 7.87; low‐quality evidence). One recent trial comparing cytisine with NRT in 1310 people found a benefit for cytisine at six months (RR 1.43, 95% CI 1.13 to 1.80).
One trial of dianicline (602 people) failed to find evidence that it was effective (RR 1.20, 95% CI 0.82 to 1.75). This drug is no longer in development.
We identified 39 trials that tested varenicline, 27 of which contributed to the primary analysis (varenicline versus placebo). Five of these trials also included a bupropion treatment arm. Eight trials compared varenicline with nicotine replacement therapy (NRT). Nine studies tested variations in varenicline dosage, and 13 tested usage in disease‐specific subgroups of patients. The included studies covered 25,290 participants, 11,801 of whom used varenicline.
The pooled RR for continuous or sustained abstinence at six months or longer for varenicline at standard dosage versus placebo was 2.24 (95% CI 2.06 to 2.43; 27 trials, 12,625 people; high‐quality evidence). Varenicline at lower or variable doses was also shown to be effective, with an RR of 2.08 (95% CI 1.56 to 2.78; 4 trials, 1266 people). The pooled RR for varenicline versus bupropion at six months was 1.39 (95% CI 1.25 to 1.54; 5 trials, 5877 people; high‐quality evidence). The RR for varenicline versus NRT for abstinence at 24 weeks was 1.25 (95% CI 1.14 to 1.37; 8 trials, 6264 people; moderate‐quality evidence). Four trials which tested the use of varenicline beyond the 12‐week standard regimen found the drug to be well‐tolerated during long‐term use. The number needed to treat with varenicline for an additional beneficial outcome, based on the weighted mean control rate, is 11 (95% CI 9 to 13). The most commonly reported adverse effect of varenicline was nausea, which was mostly at mild to moderate levels and usually subsided over time. Our analysis of reported serious adverse events occurring during or after active treatment suggests there may be a 25% increase in the chance of SAEs among people using varenicline (RR 1.25; 95% CI 1.04 to 1.49; 29 trials, 15,370 people; high‐quality evidence). These events include comorbidities such as infections, cancers and injuries, and most were considered by the trialists to be unrelated to the treatments. There is also evidence of higher losses to follow‐up in the control groups compared with the intervention groups, leading to a likely underascertainment of the true rate of SAEs among the controls. Early concerns about a possible association between varenicline and depressed mood, agitation, and suicidal behaviour or ideation led to the addition of a boxed warning to the labelling in 2008. However, subsequent observational cohort studies and meta‐analyses have not confirmed these fears, and the findings of the EAGLES trial do not support a causal link between varenicline and neuropsychiatric disorders, including suicidal ideation and suicidal behaviour. The evidence is not conclusive, however, in people with past or current psychiatric disorders. Concerns have also been raised that varenicline may slightly increase cardiovascular events in people already at increased risk of those illnesses. Current evidence neither supports nor refutes such an association, but we await the findings of the CATS trial, which should establish whether or not this is a valid concern.
Authors' conclusions
Cytisine increases the chances of quitting, although absolute quit rates were modest in two recent trials. Varenicline at standard dose increased the chances of successful long‐term smoking cessation between two‐ and three‐fold compared with pharmacologically unassisted quit attempts. Lower dose regimens also conferred benefits for cessation, while reducing the incidence of adverse events. More participants quit successfully with varenicline than with bupropion or with NRT. Limited evidence suggests that varenicline may have a role to play in relapse prevention. The most frequently recorded adverse effect of varenicline is nausea, but mostly at mild to moderate levels and tending to subside over time. Early reports of possible links to suicidal ideation and behaviour have not been confirmed by current research.
Future trials of cytisine may test extended regimens and more intensive behavioural support.
Keywords: Humans; Alkaloids; Alkaloids/adverse effects; Alkaloids/therapeutic use; Azepines; Azepines/adverse effects; Azepines/therapeutic use; Azocines; Azocines/adverse effects; Azocines/therapeutic use; Benzazepines; Benzazepines/adverse effects; Benzazepines/therapeutic use; Bupropion; Bupropion/therapeutic use; Counseling; Counseling/methods; Heterocyclic Compounds, 4 or More Rings; Heterocyclic Compounds, 4 or More Rings/adverse effects; Heterocyclic Compounds, 4 or More Rings/therapeutic use; Nicotine; Nicotine/adverse effects; Nicotine/antagonists & inhibitors; Nicotinic Agonists; Nicotinic Agonists/adverse effects; Nicotinic Agonists/therapeutic use; Quinolizines; Quinolizines/adverse effects; Quinolizines/therapeutic use; Randomized Controlled Trials as Topic; Smoking; Smoking/drug therapy; Smoking Cessation; Smoking Cessation/methods; Substance Withdrawal Syndrome; Substance Withdrawal Syndrome/prevention & control; Varenicline; Varenicline/therapeutic use
Can nicotine receptor partial agonists, including cytisine and varenicline, help people to stop smoking?
Background
When people stop smoking they experience cravings to smoke and unpleasant mood changes. Nicotine receptor partial agonists aim to reduce these withdrawal symptoms and the pleasure people usually experience when they smoke. The most widely‐available treatment in this drug type is varenicline, which is available world‐wide as an aid for quitting smoking. Cytisine is a similar medication, but is only available in Central and Eastern European countries and through internet sales.
Study characteristics
We searched for randomised controlled trials testing varenicline, cytisine or dianicline. We found 39 studies of varenicline compared to placebo, bupropion or nicotine patches. We also found four trials of cytisine, one of which compared it to nicotine replacement therapy. We include one trial of dianicline, which is no longer in development, and so not available to use as a quitting aid. To be included, trials had to report quit rates at least six months from the start of treatment. We preferred the strictest available definition of quitting, and results which had been biochemically confirmed by testing blood or bodily fluids.We conducted full searches up to May 2015, although we have also included several key trials published after that date.
Key findings
From the information we found (27 trials, 12,625 people), varenicline at standard dose more than doubled the chances of quitting compared with placebo. Low‐dose varenicline (four trials, 1266 people) roughly doubled the chances of quitting, and reduced the number and severity of side effects. The number of people stopping smoking with varenicline was higher than with bupropion (five trials, 5877 people) or with NRT (eight trials, 6264 people). Based on the evidence so far, we can calculate that varenicline delivers one extra successful quitter for every 11 people treated, compared with smokers trying to quit without varenicline.
The most common side effect of varenicline is nausea, but this is mostly at mild or moderate levels and usually clears over time. People taking varenicline appear to have about a 25% increased chance of a serious adverse event, although these include many which are unrelated to the treatment. We also note that more people were lost from the control groups than from the varenicline groups by the end of the trials, which may mean that the count of events in the control groups is lower than it should be. After varenicline became available to use, there were concerns that it could be linked with an increase in depressed mood, agitation, or suicidal thinking and behaviour in some smokers. However, the latest evidence does not support a link between varenicline and these disorders, although people with past or current psychiatric illness may be at slightly higher risk. There have also been concerns that varenicline may slightly increase heart and circulatory problems in people already at increased risk of these illnesses. The evidence is currently unclear whether or not they are caused or made worse by varenicline, but we should have clearer answers to these questions when a further study is published later this year.
Quality of the evidence
The varenicline studies were generally of high quality, providing evidence that we consider to be reliable and robust. We rate the quality of the evidence comparing varenicline with NRT as moderate quality (we are reasonably confident of the stability of the evidence), since in some of them the participants knew which treatment they were receiving (i.e. non‐blinded open‐label trials). We judge the evidence from the cytsine trials to be of low quality (we have limited confidence in the evidence), as there are only two trials, with relatively low numbers included.
Summary of findings
Summary of findings for the main comparison.
Nicotine receptor partial agonists for smoking cessation
| Varenicline versus placebo or other first‐line treatments for smoking cessation | ||||||
| Patient or population: Individuals who smoke tobacco Setting: Varied Intervention: Varenicline Comparison: Varied controls | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control | Corresponding risk with varenicline | |||||
| Varenicline vs placebo: continuous/sustained abstinence at longest follow‐up (24+ weeks) | Study population (where risk refers to quitters) | RR 2.24 (2.06 to 2.43) | 12,625 (27 RCTs) | ⊕⊕⊕⊕ HIGH 1, 2 | ||
| 111 per 1000 | 250 per 1000 (230 to 271) | |||||
| Varenicline vs bupropion: continuous/sustained abstinence (24 weeks) | Study population (where risk refers to quitters) | RR 1.39 (1.25 to 1.54) | 5877 (5 RCTs) |
⊕⊕⊕⊕ HIGH | ||
| 171 per 1000 | 238 per 1000 (214 to 264) | |||||
| Varenicline vs NRT: point prevalence abstinence (24 weeks) | Study population (where risk refers to quitters) | RR 1.25 (1.14 to 1.37) | 6264 (8 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | ||
| 189 per 1000 | 237 per 1000 (216 to 259) |
|||||
| Varenicline vs placebo: number of participants reporting SAEs in duration of trials (trials reporting no events in either group excluded) | Study population (where risk refers to SAEs) | RR 1.25 (1.04 to 1.49) | 15,370 (29 RCTs) | ⊕⊕⊕⊕ HIGH | ||
| 30 per 1000 | 39 per 1000 (32 to 48) | |||||
| Varenicline vs placebo: number of participants reporting cardiac SAEs, including deaths, in duration of trials | Study population (where risk refers to SAEs) | RR 1.36 (0.91 to 2.04) |
8587 (21 studies) | ⊕⊕⊕⊝ MODERATE 4 | ||
| 9 per 1,000 | 12 per 1,000 (8 to 17) |
|||||
| Varenicline vs placebo: number of participants reporting nausea in duration of trials | Study population (where risk refers to SAEs) | RR 3.27 (3.00 to 3.55) |
14963 (32 studies) |
⊕⊕⊕⊕ HIGH | ||
| 85 per 1,000 | 277 per 1,000 (254 to 301) |
|||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).The assumed risk in the comparison group is calculated as the median risk in control groups. CI: Confidence interval; RR: Risk ratio; SAEs: Serious adverse events | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Moderate heterogeneity detected, however all but two studies showed positive effect of varenicline, so did not downgrade on this basis.
2Lack of smaller trials with negative findings suggests possible publication bias. However, earliest studies reported 2006. We are reasonably confident that licensing and subsequent trials have been registered online in clinical trials registries. Thus absence of negative studies may be marker of sustained efficacy rather than suppression or selective management of data.
3Downgraded once as three of the eight studies were rated at high risk of bias due to using an open‐label design.
4Downgraded once due to imprecision; CIs do not rule out an increase in risk
Summary of findings 2.
Nicotine receptor partial agonists for smoking cessation
| Cytisine versus placebo for smoking cessation | ||||||
| Patient or population: Individuals who smoke tobacco Setting: Varied Intervention: Cytisine Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Corresponding risk with Cytisine | |||||
| Cytisine vs placebo: continuous abstinence at longest follow‐up (24+ weeks) | Study population (where risk refers to quitters) | RR 3.98 (2.01 to 7.87) | 937 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 | ||
| 21 per 1000 | 85 per 1000 (43 to 169) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).The assumed risk in the comparison group is calculated as the median risk in control groups. CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Imprecision rated 'very serious' (downgraded two levels on this basis) as only two studies, and fewer than 300 events in each arm.
Background
Smoking is the main preventable cause worldwide of morbidity and premature death. Based on data from 2004, 12% of all deaths globally among adults aged 30 years and over were attributable to tobacco, with 5 million adults dying due directly to tobacco use (WHO 2012). The list of illnesses known to be linked to smoking includes cancers of the cervix, pancreas, kidneys and stomach, aortic aneurysms, acute myeloid leukaemia, cataracts, pneumonia, and gum disease. These are in addition to the long‐established links between tobacco use and such illnesses as lung cancer, cardiovascular diseases, and emphysema, and with prematurity, sudden infant death syndrome and low birth weight in the babies of maternal smokers (Surgeon General 2004). There is a growing understanding of the neurochemical basis of nicotine addiction (Fagerström 2006). There is strong evidence that dependence upon nicotine reflects the effects of the drug at neuronal nicotinic receptors in the brain (Benowitz 1999; Hogg 2007; Picciotto 1999). More recent studies have explored the potential of neuronal nicotinic acetylcholine receptors (nAChRs) as targets for a variety of therapeutic interventions (Hogg 2007). It is thought that the addictive properties of nicotine are mediated mainly through its action as an agonist at α4β2nAChRs, which stimulates the release of dopamine (Coe 2005). Pharmacotherapies to aid smoking cessation have been developed which exploit this mechanism, by acting as nicotine receptor partial agonists.
Cytisine
Cytisine was developed as a treatment for tobacco dependence in Bulgaria in the 1960s, and is available in some eastern and central European countries and through internet sales, under the trade name of Tabex (Foulds 2004; Tutka 2005; Tutka 2006). Its manufacturers, Sopharma Pharmaceuticals, developed their phytoproduct from the plant Cytisus Laburnum L. (Golden Rain). Although cytisine (Tabex) is not licensed for use as a smoking cessation aid across most countries outside Eastern Europe (Walker 2014), studies by Vinnikov 2008 and West 2011 have highlighted the potential of this drug, especially in countries with lower average incomes and where smoking cessation programmes are not supported by insurance plans or by a national health service. In many regions, it may be considerably cheaper to continue smoking than to embark upon a course of pharmacotherapy for smoking cessation. West 2011 reports that a pack of cigarettes in China costs between 15¢ and 73¢, compared with a course of nicotine replacement therapy (NRT) (USD 230), bupropion (USD 123), or varenicline (USD 327). Similarly, a pack of 20 cigarettes in India costs around USD 1.10, or 5¢ for a pack of bidis, compared with USD 150 for a course of NRT, USD 100 for bupropion and USD 200 for varenicline. Tabex is currently available in Poland for the equivalent of USD 15 for a course of treatment, and in Russia for the equivalent of USD 6 as an over‐the‐counter medication. There is also heightened interest and activity in cytisine in New Zealand, where it is found in the seeds of the native Kowhai tree, widely used in traditional Māori healing (Thompson‐Evans 2011). The current update adds a large single‐blind randomised non‐inferiority trial comparing cytisine with NRT, conducted in New Zealand between 2011 and 2013 (Walker 2014).
Dianicline
In 2006, Sanofi‐Aventis registered two trials of dianicline, their version of a nicotine receptor partial agonist (Tonstad 2011; Ameridian 2007). However, unfavourable results have led to the withdrawal of this drug from further development (Kirchhoff 2009). We have been unable to locate results for the AMERIDIAN trial, and present only the EURODIAN trial report in this review.
Varenicline
Varenicline was developed by Pfizer Inc to counteract the effects of nicotine on the nAChRs. The drug was based on the naturally‐occurring alkaloid compound cytisine described above, which had been shown to be an effective partial agonist for α4β2 receptors (Papke 1994; Slater 2003).
Varenicline was developed in 1997 (Coe 2005), and is described as a selective nicotinic receptor partial agonist. It was designed to selectively activate the α4β2nAChR, mimicking the action of nicotine and causing a moderate and sustained release of mesolimbic dopamine (Sands 2005). This, it was suggested, should counteract withdrawal symptoms consequent upon low dopamine release during smoking cessation attempts. However, because it is a partial agonist at these receptors, it elicits some dopamine overflow, but not the substantial increases evoked by nicotine. Perhaps more importantly, it blocks the effects of a subsequent nicotine challenge on dopamine release from the mesolimbic neurones thought pivotal to the development of nicotine dependence (Coe 2005). Although varenicline has been shown to be a partial agonist at heteromeric neuronal nicotine receptors, there is now evidence that it may also be a full agonist at the homomeric α7 receptor (Mihalak 2006).
Multicentre trials of varenicline have been conducted or are currently underway in the USA, Canada, Latin America, Europe, Australia, the Middle East and the Far East. There have also been studies in specific patient groups, including the following conditions: cardiovascular disease, chronic obstructive pulmonary disease, diabetes, drug or alcohol dependence, head and neck cancers, HIV infection, bipolar disorders, depression, schizophrenia or schizoaffective disorders.
Varenicline was approved as a prescription‐only aid to smoking cessation in 2006 by the American Food and Drug Administration under the trade name Chantix, and by the European Medicines Evaluation Agency under the trade name Champix. In July 2007 it was approved by the National Institute for Health and Clinical Excellence (NICE) for prescribing by the UK National Heath Service (ASH 2006; NICE 2007). Post‐marketing surveillance has raised subsequent concerns about possible links between varenicline and major health risks, including suicidal ideation and behaviour, depression, and serious adverse cardiovascular events (FDA 2008). We consider these findings in the Discussion section of this review, and in our meta‐analyses.
Objectives
To review the efficacy of nicotine receptor partial agonists, including varenicline and cytisine, for smoking cessation.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials.
Types of participants
Adult smokers. Trials which target users of smokeless tobacco are not included in this review, but are listed among the Excluded Studies. Interventions for smokeless tobacco use cessation are covered in a companion review (Ebbert 2011).
Types of interventions
Selective nicotine receptor partial agonists, including cytisine, dianicline and varenicline, or any other in this class of drug as they reach Phase 3 trial stage. The efficacy of lobeline is covered in an earlier Cochrane review (Stead 2003).
For this update, and in anticipation of current ongoing trials reaching publication, we have extended the range of analyses to cover the following intervention types and subgroups:
I. Varenicline versus other pharmacotherapies:
Varenicline versus placebo
Varenicline versus bupropion
Varenicline versus NRT
Varenicline versus mecamylamine
Combination treatments (e.g. varenicline + NRT) versus single‐therapy treatment, where the addition of varenicline is the intervention being tested
Varenicline tablets versus other formulations (e.g. patch, in solution)
II. Variations in usage:
Flexible quit dates
Variable dosages
Preloading (before TQD)
Reducing to quit
Maintenance therapy (relapse prevention)
Harm reduction
III. Specific patient groups:
Cardiovascular disease (CVD)
Chronic obstructive pulmonary disease (COPD)
Asthma
Schizophrenia/bipolar/psychiatric disorder
Depression
Substance use disorder/methadone‐maintained
Alcohol‐dependent smokers
HIV
Diabetes
Head and neck cancer
Varenicline in pregnancy
Long‐term use of NRT
IV. Settings/subgroups:
Hospital inpatients/perioperative patients
Smokers who have previously failed to quit on varenicline or NRT or bupropion
Light or heavy smokers
Varenicline by gender
Varenicline in ethnic groups
We have not considered for inclusion any trials of varenicline used for conditions other than smoking cessation, such as alcoholism, cocaine dependence, Parkinson's disease, spinocerebellar degeneration, etc.
Types of outcome measures
A minimum of six months abstinence is the primary outcome measure. We have used sustained cessation rates in preference to point prevalence, and we have preferred biochemically verified rates to rates based on self report of quitting. In analysis, we treat participants lost to follow‐up as continuing smokers. We have recorded any adverse effects of treatment.
Search methods for identification of studies
We searched the Tobacco Addiction Review Group specialised register for trials, using the terms ('cytisine' or 'Tabex' or 'dianicline' or 'varenicline' or 'nicotine receptor partial agonist') in the title or abstract, or as keywords. This register has been developed from electronic searching of MEDLINE, EMBASE, and PsycINFO, together with handsearching of specialist journals, conference proceedings and reference lists of previous trials and overviews. The most recent search of the Register was in May 2015, and included reports of trials indexed in the Cochrane Central Register of Controlled trials (CENTRAL), issue 5, 2015; MEDLINE (via OVID) to update 20150501; EMBASE (via OVID) to week 201519; PsycINFO (via OVID) to update 20150506. See the Tobacco Addiction Group Module for details of the search strategies for these databases.
We also searched UK and US online clinical trials registers for ongoing and recently completed trials. Trials which may be candidates for inclusion (i.e. RCTs of smoking cessation interventions using a nicotine receptor partial agonist with a minimum follow‐up of six months), and for which results are not yet available, are listed in the Characteristics of ongoing studies table. We contacted the authors of ongoing studies of varenicline and cytisine where necessary.
We made a strategic decision to delay publication of this update until we could access the findings of the Pfizer EAGLES 2016 trial (NCT014569360) in April 2016. Although we did not conduct full‐scale top‐up searches during this waiting period, we checked the status of all ongoing studies known to us, and identified published results for six of them: two were journal articles (Baker 2016; Eisenberg 2016), now included studies, and four had posted their results on the ClinicalTrials.gov database; we have added two of the trials (NCT00828113; NCT01347112) to the included studies, and the other two (NCT01308736; NCT01806779) to the excluded studies.
Data collection and analysis
We checked the abstracts of studies generated by the search strategy for relevance, and acquired full reports of any trials that might be suitable for the review. One author (KC) extracted the data, and a second author (NLH) checked them. We resolved any discrepancies by mutual consent, or by recourse to a third author (TL). Studies that did not meet the inclusion criteria are listed in the Characteristics of excluded studies table, with reasons for their exclusion.
Studies were evaluated on the basis of the quality of the randomisation procedure and allocation concealment, as described in the Cochrane Handbook (Higgins 2011). The following information about each trial, where it is available, is reported in the table Characteristics of included studies:
Country and setting (e.g. primary care, community, hospital outpatient/inpatient)
Method of selection of participants
Definition of smoker used
Methods of randomisation and allocation, and blinding of trialists, participants and assessors
Demographic characteristics of participants (e.g. average age, sex, average cigarettes/day)
Intervention and control description (dose, provider, duration, number of visits, etc.)
Outcomes including definition of abstinence used, and biochemical validation of cessation
Proportion of participants with follow‐up data
Any adverse events
Sources of funding
Studies in the Characteristics of included studies table are grouped by the type of treatment being tested (cytisine, dianicline, varenicline). Quit rates are calculated based on the numbers of people randomised to an intervention, and excluding any deaths or untraceable moves, in accordance with the Russell Standard (West 2005). We regard those who drop out or are lost to follow‐up as continuing smokers. We have noted any deaths and adverse events in the results section. Effects are expressed as risk ratios ((number of events in intervention condition/intervention denominator)/(number of events in control condition/control denominator)). For cessation a risk ratio greater than 1 indicates that more people are quitting in the intervention condition. For adverse events, a risk ratio greater than 1 indicates that more people experience adverse events in the intervention condition.
Where appropriate, we have conducted meta‐analyses of the included studies, using the Mantel‐Haenszel fixed‐effect model, provided that there was no significant heterogeneity. We assessed statistical heterogeneity between trials using the I² statistic which describes the percentage of total variation between studies that is due to heterogeneity rather than chance (Higgins 2003). Values over 50% suggest moderate heterogeneity, and values over 75% substantial heterogeneity.
For studies of disease‐specific patients (section III) and for patients in different settings (section IV), we have conducted and reported sensitivity analyses, treating them as subgroups of the main analyses and testing for subgroup differences.
For this update, we have produced 'Summary of findings' tables covering the main outcomes of smoking abstinence for varenicline versus placebo, varenicline versus bupropion, varenicline versus NRT (all in Table 1), and cytisine versus placebo (Table 2); and incidence of serious adverse events for the comparison of varenicline versus placebo. Our grading decisions are based on the five GRADE considerations: study limitations in design or execution (risks of bias), inconsistency of results, indirectness of evidence, imprecision of results, and publication bias. Evidence from studies is rated as high quality (i.e. we are very confident of the findings), through moderate, low, and very low quality (i.e. the true effect is likely to be substantially different from the estimate of effect).
We include in this review the Tobacco Addiction Group glossary of tobacco‐specific terms (Appendix 1).
Results
Description of studies
Included studies
Full details of the included studies are given in the Characteristics of included studies tables.
For this update, we now have 44 trials (previously 24) which met our inclusion criteria. Four trials (Scharfenberg 1971; Vinnikov 2008; Walker 2014; West 2011) evaluated cytisine (Tabex) for smoking cessation, covering 3461 participants, 2102 of whom took cytisine. One trial of 602 smokers, 300 of whom took the active treatment, tested the Sanofi‐Aventis drug dianicline for smoking cessation (Tonstad 2011). The remaining 39 trials tested varenicline in a variety of populations and settings, and against various comparators. Two trials, formerly classified as 'Ongoing studies' have now posted their findings on the www.ClinicalTrials.gov website, and we now treat them as included studies (NCT00828113; NCT01347112), albeit with limited information on design and findings. The trials cover more than 25,200 participants, 11,801 of whom took varenicline (see Appendix 2).
Nine studies which we originally treated as excluded are now classified as included studies, so that they can contribute data to the meta‐analyses for neuropsychiatric adverse events. These studies are flagged with an asterisk in the study ID, indicating that they do not contribute to the efficacy findings (Brandon 2011*; Ebbert 2011*; Faessel 2009*; Fagerström 2010*; Garza 2011*; Hughes 2011*; McClure 2013* NCT00944554; Meszaros 2013*; Mitchell 2012*). We have not completed Characteristics of included studies tables or 'Risk of bias' assessments for these nine studies, but have recorded our judgements on why they are not eligible to be included in the efficacy findings.
Cytisine
Cytisine versus placebo was tested as a cessation aid in Germany (Scharfenberg 1971), in Kyrgyzstan (Vinnikov 2008), and in Poland (West 2011). Scharfenberg 1971 was set in a smoking cessation clinic in what was then East Germany, Vinnikov 2008 was set in a Kyrgyz mining company, and West 2011 in a Warsaw smoking cessation clinic. A recent New Zealand non‐inferiority trial (Walker 2014) compared cytisine to NRT in a population of smokers contacting a national smoking quitline. The trials used 1.5 mg Tabex tablets over a 20‐day (Scharfenberg 1971) or 25‐day (Vinnikov 2008; Walker 2014; West 2011) treatment period, with behavioural support kept to a minimum in order to reduce programme costs. Vinnikov 2008 and Walker 2014 assessed their participants to six months, West 2011 to 12 months, and Scharfenberg 1971 to two years. Both Vinnikov 2008 and West 2011 verified claims of abstinence by testing expired carbon monoxide (CO) levels, while the remaining two trials relied upon self report without biochemical validation.
Dianicline
The dianicline trial (Tonstad 2011) was set in 22 sites across six European countries. Dianicline was administered as a 40 mg tablet twice a day for seven weeks, with brief counselling at each contact. Final follow‐up of the participants was at 26 weeks, with claims of abstinence verified by expired CO and by plasma cotinine samples.
Varenicline
Study design
Thirty‐four studies were double‐blinded randomised trials; the remaining five were open‐label. Three of the open‐label trials compared varenicline with NRT (Aubin 2008; Baker 2016; Tsukahara 2010), one compared varenicline with NRT and with placebo (Heydari 2012), and one compared varenicline plus counselling with counselling alone (Carson 2014 (formerly Smith 2012)).
Setting
Seventeen studies were set in the USA, two in Japan, two in Denmark, one each in Australia, Canada, Iran and the UK, one in both Taiwan and Korea, one in both China and Singapore, two in North America (USA and Canada), and ten in multiple countries. The trials were conducted in smoking cessation clinics, hospitals, universities and other research centres.
Participants
Participants in the majority of the trials were adult smokers, willing to make a quit attempt (Aubin 2008; Baker 2016; Bolliger 2011; Cinciripini 2013; EAGLES 2016; Eisenberg 2016; Gonzales 2006; Gonzales 2014; Heydari 2012; Jorenby 2006; Nakamura 2007; NCT01347112; Niaura 2008; Niaura 2008; Nides 2006; Oncken 2006; Rennard 2012; Tsai 2007; Tsukahara 2010; Wang 2009; Williams 2007). Several trials were conducted in clinical subgroups, including hospital inpatients (Carson 2014; Steinberg 2011; Wong 2012), and disease‐specific patient groups (CVD: Rigotti 2010; acute coronary syndrome Eisenberg 2016; COPD: Tashkin 2011; asthma: Westergaard 2015; substance use disorder: Nahvi 2014a; Stein 2013; alcohol abuse: NCT01347112; depression: Anthenelli 2013; bipolar/schizophrenia; schizoaffective disorder: Chengappa 2014; Evins 2014; Williams 2012). EAGLES 2016 enrolled two cohorts of adult smokers with and without histories of psychiatric disorders, including primary affective disorders (70%), anxiety disorders (19%), psychotic disorders (9.5%) and personality disorders (0.6%). Two trials targeted subgroups of smokers who were failing to respond to smoking cessation pharmacotherapies, either by increasing the dosage (Hajek 2015) or by switching to different medications (Rose 2013). Three studies focused on relapse prevention in successful quitters (NCT00828113; Tonstad 2006), or in people with schizophrenia who had successfully quit (Evins 2014). De Dios 2012 tested varenicline in Latino light smokers, and Ebbert 2015 in adult smokers unwilling to quit abruptly but prepared to reduce their smoking as a run‐up to quitting completely. Tønnesen 2013 tested varenicline as an aid to weaning ex‐smokers off extended use of NRT.
Interventions
Thirty‐three of the 39 trials used the standard 12‐week regimen of varenicline, routinely titrating the first week up to the recommended daily dose of 1 mg twice a day. Three trials (Nakamura 2007; Nides 2006; Oncken 2006) compared different dosage arms of varenicline against a placebo arm. One trial in non‐responders regulated dosage up to the target quit date (day 21) to a maximum of 5 mg a day (Hajek 2015), and another allowed participants to regulate their own dosage throughout the treatment phase (Niaura 2008). NCT00828113 is a randomised trial comparing extended (52‐week) and standard (12‐week) courses of varenicline.
Of the eight trials that used NRT as a comparator condition, five (Aubin 2008; Baker 2016; De Dios 2012; EAGLES 2016; Rose 2013) provided a 12‐week course, reducing the dosage as a weaning process, while two trials (Heydari 2012; Tsukahara 2010) provided an eight‐week course, with only the Tsukahara 2010 trial progressively reducing the dosage to the end of treatment. Stein 2013 gave a 24‐week course of NRT, tailored to the level of nicotine dependency, and matched to the duration of the placebo and varenicline arms of the trial.
The five trials which used bupropion all supplied the standard regimen of 150 mg twice a day, four of them for 12 weeks (Cinciripini 2013; EAGLES 2016; Gonzales 2006; Jorenby 2006) and Nides 2006 for seven weeks.
Comparisons
Twenty‐six RCTs compared varenicline to an identical placebo regimen (Anthenelli 2013; Bolliger 2011; Chengappa 2014; EAGLES 2016; Ebbert 2015; Eisenberg 2016; Evins 2014; Gonzales 2014; Hajek 2015; Nahvi 2014a; Nakamura 2007; NCT01347112; Niaura 2008; Oncken 2006; Rennard 2012; Rigotti 2010; Steinberg 2011; Tashkin 2011; Tonstad 2006; Tonstad 2011; Tsai 2007; Wang 2009; Westergaard 2015; Williams 2007; Williams 2012; Wong 2012); all these trials used the standard 12‐week course of treatment, apart from Ebbert 2015 (24 weeks, 'reduce to quit'), Evins 2014 (40 weeks, relapse prevention) and Williams 2007 (52 weeks, a safety trial). Four trials (Aubin 2008; Baker 2016; Rose 2013; Tsukahara 2010) used NRT as the comparator rather than a placebo, while three more trials (De Dios 2012; Heydari 2012; Stein 2013) used both NRT and placebo as comparator conditions, in a three‐arm study design. EAGLES 2016 was a four‐arm triple‐dummy trial, comparing varenicline, bupropion and NRT with a placebo. Four trials (Cinciripini 2013; Gonzales 2006; Jorenby 2006; Nides 2006) compared varenicline with bupropion and with placebo. One trial (Carson 2014) compared varenicline plus quitline counselling to quitline counselling alone.
Outcomes
As a condition of inclusion, all the trials reported cessation at least six months from the start of the intervention. Seventeen of 39 studies reported longest follow‐up at six months (point prevalence or continuous abstinence) (Bolliger 2011; Chengappa 2014; Cinciripini 2013; De Dios 2012; EAGLES 2016; Eisenberg 2016; Nahvi 2014a; NCT01347112; Rennard 2012; Rose 2013; Stein 2013; Steinberg 2011; Tsai 2007; Tsukahara 2010; Wang 2009; Westergaard 2015; Williams 2012), and 20 studies to 12 months. Hajek 2015, relevant for the exploration of dose variability, reported abstinence only to 12 weeks, and is not included in the main efficacy findings. Evins 2014 followed its participants until week 64, as part of a relapse prevention initiative.
All the trials except one (NCT01347112) used biochemical verification of abstinence by expired CO, at cut‐offs ranging from 5 to 10 ppm, at one or more time points. Baker 2016 validated outcomes at both 9 ppm and 5 ppm cut‐off levels. Heydari 2012 and Wong 2012 did not report their cut‐offs. Carson 2014 tested "a random sub‐set of subjects" (51/103 quitters). Five trials (Cinciripini 2013; De Dios 2012; Stein 2013; Tønnesen 2013; Wong 2012) also used salivary or urinary cotinine testing to confirm abstinence claims.
Excluded studies
Eight of the excluded studies tested cytisine (Granatowicz 1976; Kempe 1967; Maliszewski 1972; Metelitsa 1987; Monova 2004; Ostrovskaia 1994; Paun 1968; Schmidt 1974), and the remaining 48 tested varenicline, but did not meet our eligibility criteria to be treated as an included study.
The excluded studies are briefly described, with reasons for exclusion, in the Characteristics of excluded studies tables. Seven of the excluded studies (Ebbert 2014; Hajek 2013; Hoogsteder 2014; Koegelenberg 2014; NCT01806779; Ramon 2014; Rose 2014) administered varenicline to all participants, and tested the addition of another pharmacotherapy (nicotine replacement therapy, bupropion, or nicotine vaccine). Since varenicline was not primarily the intervention being tested, the findings of these trials are covered in the reviews which address the relevant adjunctive treatments. Swan 2010, which we had classified as an included study in the 2012 update, is now an excluded study, as all the participants received varenicline, and the intervention being tested was the addition and relative merits of internet‐ and telephone‐based counselling. Two trials (NCT01308736; NCT01806779), formerly treated as 'Ongoing studies', have now posted their findings on the www.ClinicalTrials.gov website, and we now report them as excluded studies.
Risk of bias in included studies
Among the cytisine trials, we rated Vinnikov 2008, Walker 2014 and West 2011 as being at low risk of bias in their randomisation and allocation procedures; Scharfenberg 1971 gave no details about these, and was therefore rated as unclear. We rated Walker 2014 at high risk of bias for a lack of blinding of participants and personnel. This study may also have been at risk of bias for providing cytisine free of charge but NRT at a cost of NZD 3 per item. Although Vinnikov 2008 invokes the Russell Standard criteria (West 2005) in support of the conduct of their trial, they excluded 26 participants who took no medication from the denominator; we have reinstated them for our meta‐analyses, in order to present an intention‐to‐treat estimate, i.e. all people randomised, excluding only those who died or who moved away. Of the 39 varenicline trials, 23 reported randomisation and allocation procedures in sufficient detail to be assessed as being at minimal risk in their attempts to control selection bias. Fifteen trials (Chengappa 2014; Cinciripini 2013; De Dios 2012; Heydari 2012; NCT00828113; NCT01347112; Oncken 2006; Rose 2013; Stein 2013; Tashkin 2011; Tsukahara 2010; Wang 2009; Westergaard 2015; Williams 2007; Williams 2012) gave insufficient information for this to be confirmed. A sensitivity analysis removing these trials made no difference to the findings. None of the trials reported any assessment of the integrity of the double‐blinding procedure. For the relapse prevention trials (Evins 2014; NCT00828113; Tonstad 2006), the integrity of the double‐blind phase may be questionable, since all randomised participants had successfully used varenicline during the open‐label phase.
All except eight of the included studies reported prolonged, sustained or continuous abstinence as their most rigorous estimate of efficacy; De Dios 2012; Heydari 2012; Nahvi 2014a; NCT00828113; Westergaard 2015; Williams 2007; Williams 2012; and Wong 2012 all reported only point prevalence abstinence. Steinberg 2011 used repeated point prevalence at 4, 12 and 24 weeks, which we have treated as sustained abstinence for the purposes of our meta‐analyses. 'Continuous abstinence' as defined in the remaining trials excluded the first eight weeks of treatment, and could more accurately be termed 'prolonged abstinence' (Hughes 2003).
Aubin 2008 was an unblinded open‐label trial, which may have led to the differential drop‐out rates after randomisation, with nine participants assigned to nicotine patch declining to take part compared with two in the varenicline group. We rated four open‐label trials of NRT versus varenicline (Aubin 2008; Baker 2016; Heydari 2012; Tsukahara 2010) at high risk of bias for being unblinded. Nakamura 2007 was assessed as being at high risk of selective reporting bias, since they reported continuous abstinence rates for all participants, but demographic information, craving and withdrawal measures for the highly nicotine‐dependent smokers only. Cinciripini 2013 reported changing interventions (from nortriptyline to varenicline) three months into their study, but found no differences between the varenicline and nortriptyline cohorts and therefore combined them for analysis. Heydari 2012 used an eight‐week course of varenicline (presumably to match the standard NRT regimen), which might be expected to have compromised its efficacy.
Two trials which posted their results on the www.ClinicalTrials.gov website are rated at high risk of bias for attrition and losses to follow‐up. NCT00828113, comparing long‐term and standard doses of varenicline, lost 60% from each of the groups by twelve‐month follow‐up, while NCT01347112, a small study of alcohol‐dependent smokers using varenicline to quit, lost 25% from the varenicline group and 71% from the placebo group at 24 weeks. This study also relied upon self report, rather than biochemical validation of abstinence.
Our judgements on the risks of bias of all the included studies are summarised in Figure 1.
Figure 1.
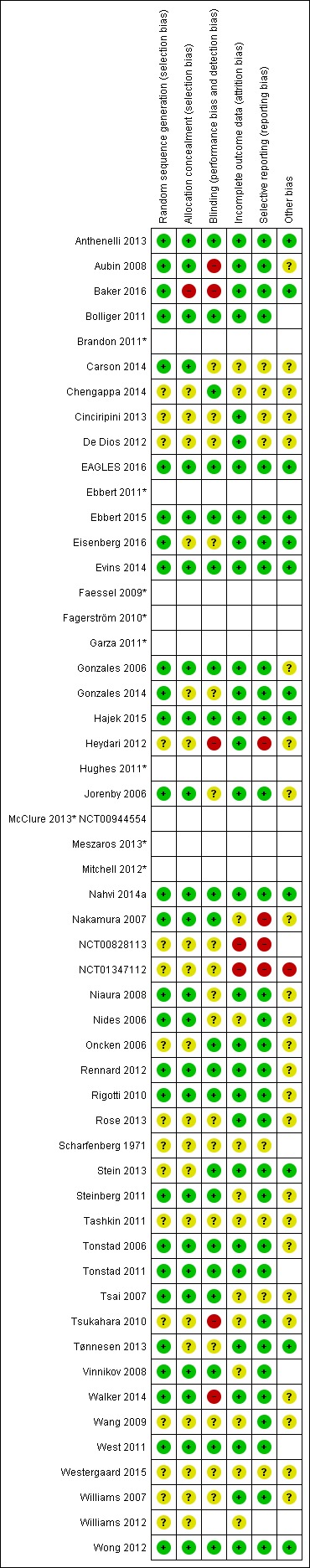
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
1. Cessation
Cytisine
We pooled the findings of two cytisine trials, covering 937 participants, 470 of whom took the active drug. Both trials reported continuous abstinence rates at longest follow‐up (24 weeks in Vinnikov 2008 and 52 weeks in West 2011), delivering an RR of 3.98 (95% CI 2.01 to 7.87; low‐quality evidence; Analysis 1.1). We have not combined these recent trials with Scharfenberg 1971, as the design and conduct of the latter is of indeterminate quality, using self‐reported point prevalence abstinence and without biochemical verification of its results. A sensitivity analysis combining the three trials increased the I² statistic from 0% to 68%, indicating substantial heterogeneity between the older study and the recent ones. The RR for Scharfenberg 1971 at two‐year follow‐up was 1.61 (95% CI 1.24 to 2.08; Analysis 1.2), and at six months 1.91 (95% CI 1.53 to 2.37; analysis not shown).
Analysis 1.1.
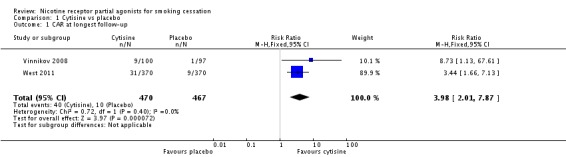
Comparison 1 Cytisine vs placebo, Outcome 1 CAR at longest follow‐up.
Analysis 1.2.
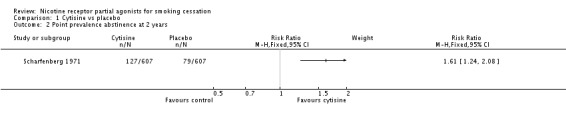
Comparison 1 Cytisine vs placebo, Outcome 2 Point prevalence abstinence at 2 years.
The largest cytisine trial (Walker 2014) compared it with NRT, and reported non‐verified continuous abstinence at six months. Although this study (in 1360 participants) was designed as a test of non‐inferiority, it demonstrated a significant benefit for cytisine over NRT, with a RR of 1.43 (95% CI 1.13 to 1.80; Analysis 2.1). The primary endpoint finding (at one month) also favoured cytisine: RR 1.30 (95% CI 1.12 to 1.51; analysis not shown).
Analysis 2.1.
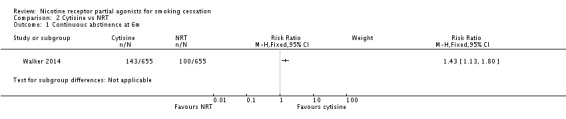
Comparison 2 Cytisine vs NRT, Outcome 1 Continuous abstinence at 6m.
The cytisine trials did not for the most part identify more adverse events in the intervention than the control arm; Scharfenberg 1971 reported similar rates of mild adverse events (nausea, restlessness, insomnia, irritability) in the cytisine and placebo groups at four weeks (23.4% and 20% respectively in abstinent participants), but did not report long‐term rates for the full study population. Vinnikov 2008 reported 10 events in eight participants (four from each group), including dyspepsia, nausea and headache. West 2011 reported gastrointestinal disorders at higher rates in the cytisine than in the placebo group (13.8% vs 8.1%, P = 0.02). Walker 2014 reported significantly more adverse events (nausea, vomiting, sleep disorders) in the cytisine group compared with the NRT group (4.6% versus 0.03%; P = 0.0002), but similar rates of serious adverse events in the cytisine (6.9%) and the NRT (6.0%) groups.
Dianicline
The one trial of dianicline that has published its findings (Tonstad 2011) reported continuous abstinence at 26 weeks. The quit rate among 300 dianicline users was 16.7%, compared with a placebo quit rate of 13.9% in 302 participants; this yields an RR of 1.20 (95% CI 0.82 to 1.75; Analysis 3.1). Results from the companion trial (Ameridian 2007) have not been made available to us by the manufacturers. Development of the drug has now been abandoned by Sanofi‐Aventis.
Analysis 3.1.
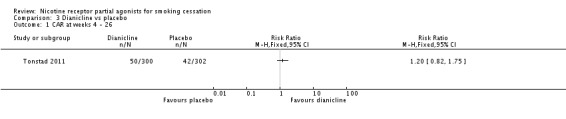
Comparison 3 Dianicline vs placebo, Outcome 1 CAR at weeks 4 ‐ 26.
Varenicline
The evidence base includes 39 methodologically sound clinical trials, involving more than 25,290 participants, 11,801 of whom received varenicline (see Appendix 2). Where point prevalence measures were the only ones reported, we have noted this in footnotes for each analysis.
The Nides 2006 and Nakamura 2007 comparisons chosen for our primary meta‐analysis were between the 1.0 mg twice a day group and the placebo group, since this matched the regimen now recommended for clinical practice. For the Oncken 2006 trial we combined the 1.0 mg twice a day titrated and non‐titrated groups for the meta‐analysis, since titration did not affect cessation rates.
I Varenicline versus other pharmacotherapies
1.1. Varenicline versus placebo
The pooled risk ratio (RR) for validated continuous abstinence six months or more from the start of the intervention (longest follow‐up) is 2.24 (95% CI 2.06 to 2.43; 27 trials, 12,625 participants, I² = 60%; high‐quality evidence; Analysis 4.1; Figure 2). This finding is consistent with that reported in the previous version of this review, which included 14 trials and 6166 participants. The current RR is based on 27 cessation trials of varenicline (26 versus placebo, and one (Carson 2014) versus counselling only). Although the control group did not receive placebo medication, we have included Carson 2014 in the main meta‐analysis; a sensitivity analysis excluding it made no appreciable difference to the estimate. All the trials in this analysis delivered varenicline at the standard dosage (1 mg twice a day) for 12 weeks, apart from Heydari 2012 and Nides 2006 (eight weeks). Limiting the analysis to the 15 studies with 12‐month follow‐up made little difference to the result (RR 2.29, 95% CI 2.02 to 2.60; 5904 participants). Six‐month abstinence rates for all 25 studies reporting this measure yielded a virtually identical RR of 2.25 (95% CI 2.08 to 2.44; 12,304 participants, I² = 66%; Analysis 4.2).
Analysis 4.1.
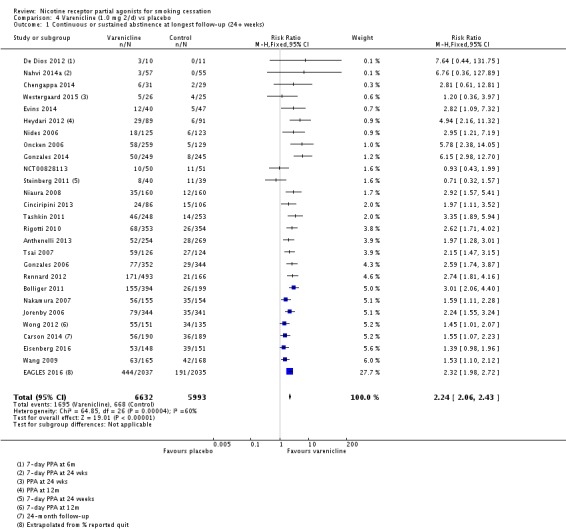
Comparison 4 Varenicline (1.0 mg 2/d) vs placebo, Outcome 1 Continuous or sustained abstinence at longest follow‐up (24+ weeks).
Figure 2.
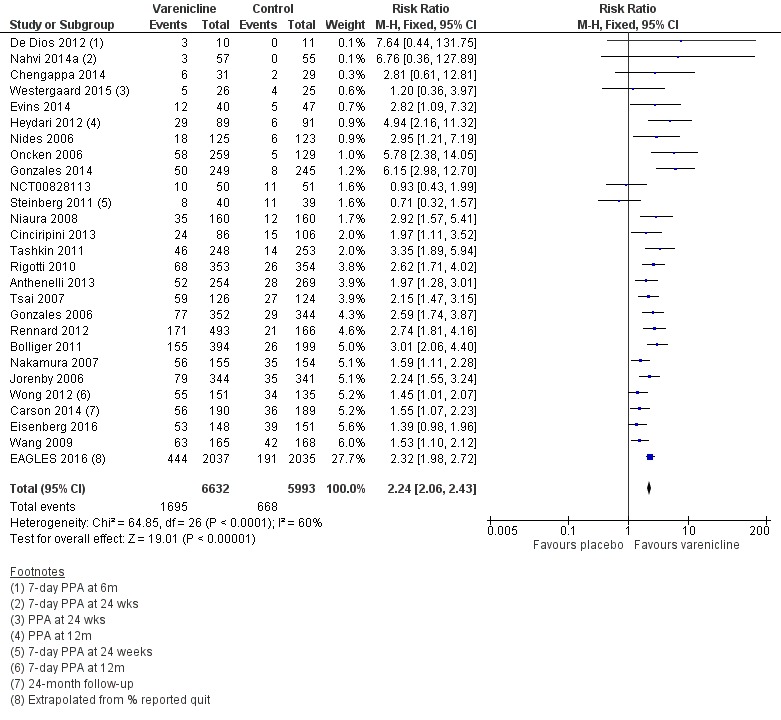
Varenicline (1.0 mg 2/d) vs placebo, outcome: 3.1 Continuous abstinence at longest follow‐up (24+ weeks)
Analysis 4.2.
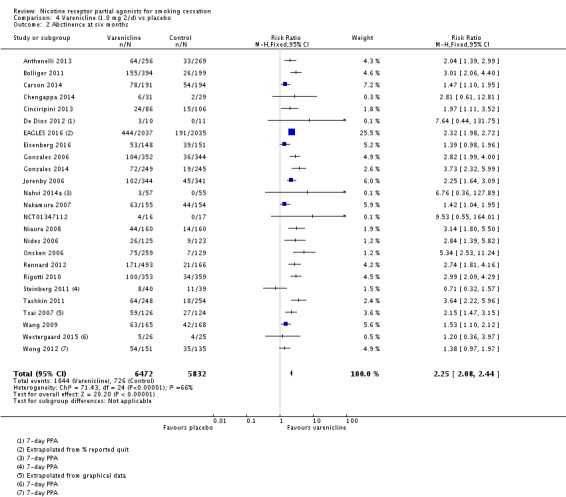
Comparison 4 Varenicline (1.0 mg 2/d) vs placebo, Outcome 2 Abstinence at six months.
The EAGLES 2016 trial presents results separately for the two constituent cohorts, with and without a history of psychiatric disorders. The groups without a psychiatric history in all cases and at both time points (12 and 24 weeks) achieved higher quit rates than the groups in the psychiatric cohort. The RR in the non‐psychiatric cohort for varenicline versus placebo was 2.42 (95% CI 1.97 to 2.99), with quit rates of 25.5% and 10.5% respectively; the corresponding measures in the psychiatric cohort were RR 2.20 (95% CI 1.73 to 2.80), and quit rates of 18.3% and 8.3% respectively. Treating the psychiatric cohort as a subgroup of the main analysis and testing for subgroup differences found no significant difference between the psychiatric cohort and the remaining trials (Chi² = 0.02, P = 0.88, I² = 0%; analysis not shown).
We have excluded from the main analysis four trials which tested extended varenicline treatment. Ebbert 2015 ('Reduce to quit') and Stein 2013 (substance‐abusing smokers on methadone maintenance) both tested 24 weeks of varenicline, and NCT00828113 and Williams 2007 (a safety trial) both prescribed 12 months of treatment. Pooling these data demonstrated a clear benefit for varenicline, with a RR of 3.64 (95% CI 2.81 to 4.72; 2170 participants, I² = 78%; Analysis 4.3). A sensitivity analysis removing NCT00828113, which is at high risk of attrition bias, increased the RR to 4.15 (95% CI 3.14 to 5.49) and dropped the I² to 0%.
Analysis 4.3.
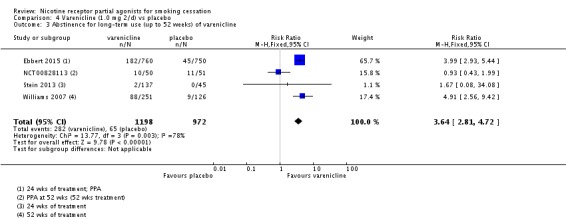
Comparison 4 Varenicline (1.0 mg 2/d) vs placebo, Outcome 3 Abstinence for long‐term use (up to 52 weeks) of varenicline.
1.2. Varenicline versus bupropion
Five trials (Cinciripini 2013; EAGLES 2016; Gonzales 2006; Jorenby 2006; Nides 2006) compared varenicline to bupropion. Although the Nides 2006 trial tested three dosing variants of varenicline, we have used the '1 mg twice a day' arm for our analysis, since this matches the regimen now recommended for clinical practice. The pooled RR for the five trials at six months was RR 1.39 (95% CI 1.25 to 1.54; 5877 participants, I² = 0%; moderate‐quality evidence; Analysis 5.1), in favour of varenicline. We conducted a sensitivity analysis to test the effect of excluding Nides 2006, which had included previous users of bupropion, but the RR remained steady, at 1.37 (95% CI 1.23 to 1.52). The three‐month and 12‐month results were in line with the main finding (Analysis 5.2; Analysis 5.3).
Analysis 5.1.
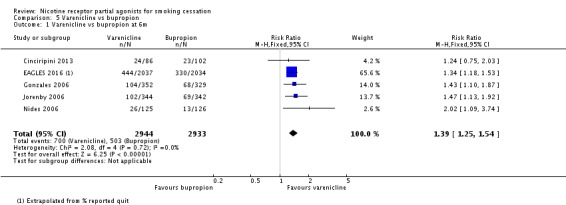
Comparison 5 Varenicline vs bupropion, Outcome 1 Varenicline vs bupropion at 6m.
Analysis 5.2.
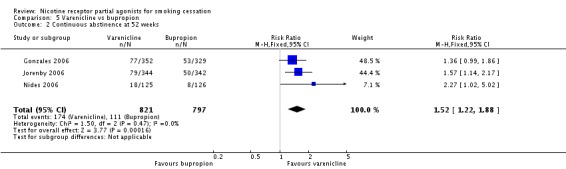
Comparison 5 Varenicline vs bupropion, Outcome 2 Continuous abstinence at 52 weeks.
Analysis 5.3.
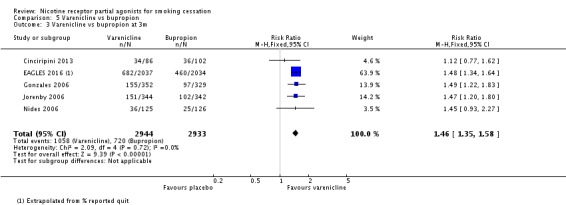
Comparison 5 Varenicline vs bupropion, Outcome 3 Varenicline vs bupropion at 3m.
The EAGLES 2016 trial demonstrated higher quit rates for this comparison in the non‐psychiatric than in the psychiatric cohort, with a RR of 1.36 (95% CI 1.15 to 1.60; non‐psychiatric), compared with RR 1.28 (95% CI 1.05 to 1.57; psychiatric). Quit rates were 25.5% for varenicline and 18.8% for bupropion in the non‐psychiatric cohort, and 18.3% for varenicline and 13.7% for bupropion in the psychiatric cohort.
1.3 Varenicline versus NRT
Eight trials tested varenicline against nicotine replacement therapy. Three trials were open‐label (Aubin 2008; Baker 2016; Tsukahara 2010), and one trial was an open‐label comparison of varenicline, NRT and no pharmacotherapy. Baker 2016 compared nicotine patch (the reference treatment) against varenicline and against combination NRT (patch plus lozenge). Three trials were placebo‐controlled three‐arm studies, with De Dios 2012 and Stein 2013 testing varenicline against a placebo tablet and against NRT, and Rose 2013 comparing varenicline, bupropion and NRT, with all participants receiving an active treatment plus two dummy treatments. EAGLES 2016 was a double‐blind four‐arm trial, comparing varenicline, bupropion and NRT against placebo. The pooled analysis indicates a benefit for varenicline over NRT. The RR at 24 weeks was 1.25 (95% CI 1.14 to 1.37; 6264 participants, I² = 39%; moderate‐quality evidence; Analysis 6.1). Removing the three open‐label trials (all at high risk of bias for blinding) from the analysis slightly strengthened the effect estimate (RR 1.34, 95% CI 1.19 to 1.50), and increased the I² value to 47%. Stein 2013 treated its participants for 24 weeks rather than the standard 12; removing it from the analysis made little difference to the result or to the I² value. For Baker 2016, Analysis 6.1 uses the varenicline/patch comparison; substituting the combination NRT arm for the nicotine patch arm made minimal difference to the study or meta‐analysis findings.
Analysis 6.1.
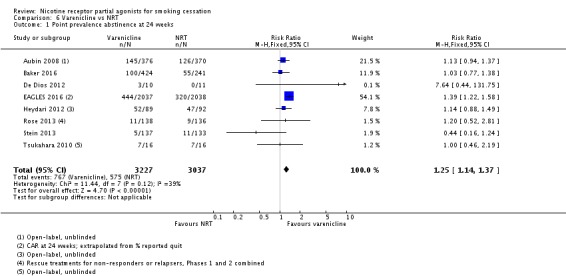
Comparison 6 Varenicline vs NRT, Outcome 1 Point prevalence abstinence at 24 weeks.
The EAGLES 2016 trial again demonstrated higher quit rates for this comparison in the non‐psychiatric than in the psychiatric cohort, with a RR of 1.38 (95% CI 1.17 to 1.63; non‐psychiatric), compared with RR 1.41 (95% CI 1.15 to 1.74; psychiatric). Quit rates were 25.5% for varenicline and 18.5% for NRT in the non‐psychiatric cohort, and 18.3% for varenicline and 13.0% for NRT in the psychiatric cohort.
1.4 Varenicline versus mecamylamine
No trials currently report on this comparison.
1.5 Combination varenicline treatment versus single‐therapy treatment
No trials currently report on this comparison.
1.6 Varenicline tablets versus other formulations
No trials currently report on this comparison.
II Variations in usage
2.1 Flexible quit date
One large multicentre study (Rennard 2012) allowed participants to select their own quit date anywhere between 8 and 35 days after joining the study. The trial found a clear benefit for varenicline over placebo, with an RR of 2.74 (95% CI 1.81 to 4.16; 659 participants; Analysis 7.1). By the end of the four‐week 'quit window' (day 35), 80.5% of the varenicline group had made a quit attempt, compared with 73.3% of the placebo group. Varenicline participants were also found to have made an earlier quit attempt (median day 17) than the placebo participants (median day 24) (P = 0.0074).
Analysis 7.1.
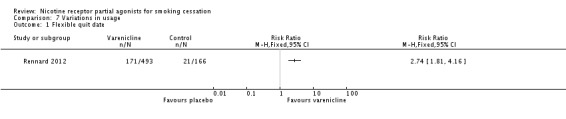
Comparison 7 Variations in usage, Outcome 1 Flexible quit date.
2.2 Variable dosages
Low‐dose varenicline versus placebo
Four trials investigated this comparison (Nakamura 2007; Niaura 2008; Nides 2006; Oncken 2006). For this review, we have combined the titrated and non‐titrated arms of the Oncken 2006 trial, as there were no detectable differences between the arms for any outcomes. Three of the trials prescribed half the recommended daily dosage, either as a single 1 mg tablet or as two 0.5 mg doses, while Niaura 2008 allowed participants to regulate their own dosage at anywhere between 0.5 mg and 2.0 mg a day. The regimen favoured varenicline over placebo, with a RR at 52 weeks of 2.08 (95% CI 1.56 to 2.78; 1266 participants; Analysis 7.2). The Niaura 2008 trial found that those on varenicline settled on a mean modal dose of 1.35 mg a day, compared with 1.63 mg a day for the placebo group.
Analysis 7.2.
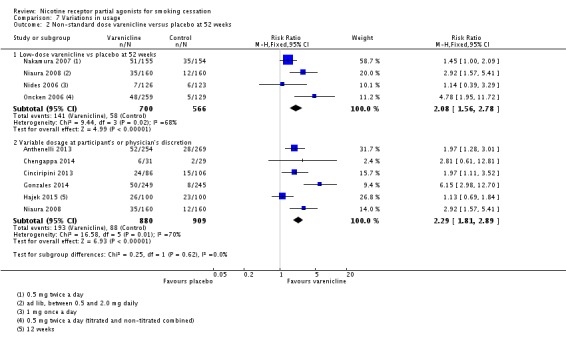
Comparison 7 Variations in usage, Outcome 2 Non‐standard dose varenicline versus placebo at 52 weeks.
Variable dosing at the participant's or physician's discretion
Six studies (Anthenelli 2013; Chengappa 2014; Cinciripini 2013; Gonzales 2014; Hajek 2015; Niaura 2008) explored the option of reducing the dosage to moderate side effects, either at the physician's behest or within the participant's own control. While this may have made the treatment more tolerable, it appeared not to have compromised efficacy, yielding a RR against placebo of 2.29 (95% CI 1.81 to 2.89; 1789 participants; I² = 70%; Analysis 7.2), which is very close to the point estimate for the main analysis, but with a wider confidence interval.
Standard‐dose versus low‐dose varenicline
Three trials (Nakamura 2007; Nides 2006; Oncken 2006) tested the standard regimen (1 mg twice a day) against half the recommended daily dose, either as a single 1 mg tablet or as two 0.5 mg doses, and found a modest advantage for the standard dosage: RR 1.25 (95% CI 1.00 to 1.55; 1079 participants; Analysis 7.3).
Analysis 7.3.
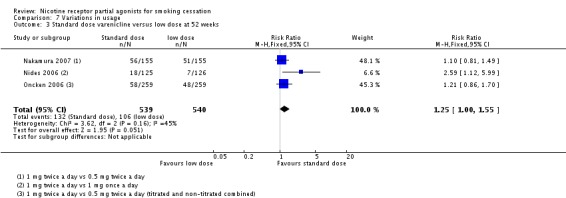
Comparison 7 Variations in usage, Outcome 3 Standard dose varenicline versus low dose at 52 weeks.
Standard dose versus high‐dose varenicline
In one recent trial (Hajek 2015; not included in the main analyses), 200 smokers who were judged not to be responding to the standard dose of varenicline (no strong nausea, no clear reduction in smoking enjoyment, and less than 50% smoking reduction after 10 days) were allocated to additional treatment (varenicline or placebo) up to the target quit date (day 21). Participants maintained that dosage for three weeks, but could reduce it if side effects became intolerable. Participants could take up to 3 mg a day in addition to the standard daily dose of 2 mg. The trial found a marginal but non‐significant benefit for quit rates with the higher dosing schedule, with an RR at 12 weeks of 0.88 (95% CI 0.54 to 1.44; Analysis 7.4), but noted a trend in the varenicline group for more fatigue and decreased appetite, and significantly higher levels of nausea and vomiting.
Analysis 7.4.
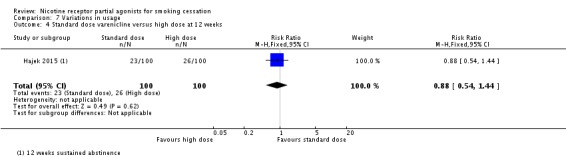
Comparison 7 Variations in usage, Outcome 4 Standard dose varenicline versus high dose at 12 weeks.
2.3 Preloading (before the TQD)
No trials currently report on this comparison.
2.4 Reducing to quit
One recent trial (Ebbert 2015) tested varenicline against placebo in 1510 smokers disinclined to quit abruptly, but willing to reduce their smoking gradually as a gateway to quitting. Treatment was given in this trial for 24 weeks rather than the standard regimen of 12 weeks, with participants asked to reduce their smoking rate by 50% by week 4, by at least 75% by week 8, and by 100% by week 12. After 12 months, the RR for quitting was 3.99 (95% CI 2.93 to 5.44; Analysis 7.5) in favour of varenicline.
Analysis 7.5.
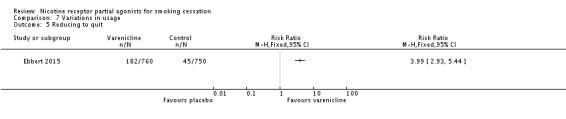
Comparison 7 Variations in usage, Outcome 5 Reducing to quit.
2.5 Maintenance therapy (relapse prevention)
Two trials have tested varenicline as an aid to relapse prevention in smokers who had successfully quit on varenicline. Tonstad 2006 randomised 1208 quitters to a further 12 weeks of either varenicline or placebo, while Evins 2014 randomised 87 quitters with schizophrenia, schizoaffective or bipolar disorder to a further 40 weeks of either varenicline or placebo treatment. We note that the integrity of the blinding in these trials may be questionable, as all the participants had already used open‐label varenicline to achieve abstinence. At 12 months, the RR in favour of varenicline was 1.24 (95% CI 1.08 to 1.42; Analysis 7.6). Heterogenity was high, at 82%, possibly reflecting the relatively extended treatment period in the smaller trial. A random‐effects analysis eliminated the significant difference (RR 1.75, 95% CI 0.71 to 4.33).
Analysis 7.6.
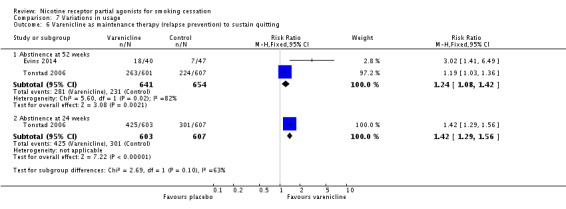
Comparison 7 Variations in usage, Outcome 6 Varenicline as maintenance therapy (relapse prevention) to sustain quitting.
2.6 Harm reduction
No trials currently report on this comparison.
III Specific patient groups
3.1 Cardiovascular disease (CVD)
Rigotti 2010 compared varenicline to placebo in a trial of 714 people with stable cardiovascular disease. Eisenberg 2016 randomised 302 smokers admitted for acute coronary syndrome to 12 weeks of treatment plus 12 weeks follow‐up. At longest follow‐up (52 weeks and 24 weeks respectively), the RR was 1.88 (95% CI 1.4 to 2.47; 1006 participants; I² = 81%; Analysis 8.1) in favour of varenicline. Treating the trials as a subgroup of the main analysis (Analysis 4.1) and testing for subgroup differences demonstrated no significant difference between them (Chi² = 1.70, P = 0.19, I² = 41.1%; analysis not shown).
Analysis 8.1.
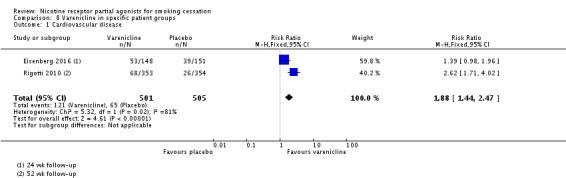
Comparison 8 Varenicline in specific patient groups, Outcome 1 Cardiovascular disease.
3.2 COPD
Tashkin 2011 compared varenicline to placebo in 504 adult smokers with mild to moderate COPD. At 52 weeks, the RR was 3.35 (95% CI 1.89 to 5.94; Analysis 8.2) in favour of varenicline.
Analysis 8.2.
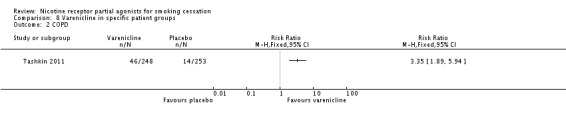
Comparison 8 Varenicline in specific patient groups, Outcome 2 COPD.
3.3 Asthma
Westergaard 2015 compared varenicline to placebo in 52 young adults (aged 19 to 40) with asthma. At six months, there was no difference in quit rates between the intervention and control arms (RR 1.25, 95% CI 0.38 to 4.14; Analysis 8.3).
Analysis 8.3.
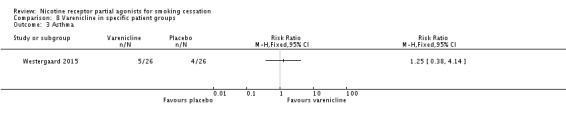
Comparison 8 Varenicline in specific patient groups, Outcome 3 Asthma.
3.4 Schizophrenia/bipolar/psychiatric disorder
Four trials tested varenicline against placebo in smokers diagnosed with bipolar disorder (Chengappa 2014), with a history of various psychiatric disorders (and at least one‐third of the cohort stably taking psychotropic medications (EAGLES 2016), with schizophrenia, schizoaffective or bipolar disorders (Evins 2014), and with schizophrenia or schizoaffective disorder (Williams 2012). The pooled analysis found a benefit for varenicline at six months, with a RR of 2.28 (95% CI 1.82 to 2.87; 2332 participants, I² = 0%; Analysis 8.4). Treating the trials as a subgroup of the main analysis (Analysis 4.1) and testing for subgroup differences demonstrated no significant difference between them (Chi² = 0.10, P = 0.76, I² = 0%; analysis not shown).
Analysis 8.4.
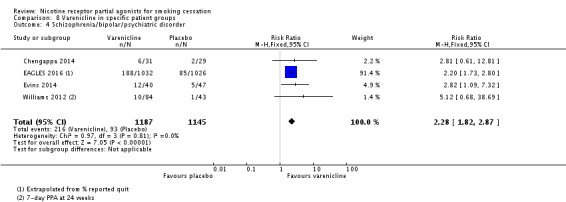
Comparison 8 Varenicline in specific patient groups, Outcome 4 Schizophrenia/bipolar/psychiatric disorder.
3.5 Depression
Anthenelli 2013 compared varenicline to placebo in 523 adult smokers with current or past depression. At 52 weeks, the RR was 1.97 (95% CI 1.28 to 3.01; Analysis 8.5) in favour of varenicline.
Analysis 8.5.
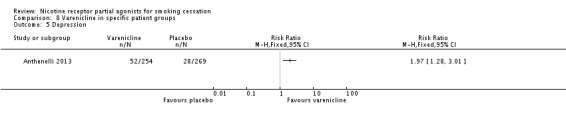
Comparison 8 Varenicline in specific patient groups, Outcome 5 Depression.
3.6 Substance use disorder/methadone‐maintained
Two trials tested varenicline against placebo in smokers on methadone treatment for substance use disorder. Nahvi 2014a covered 112 outpatients in New York, and Stein 2013 315 outpatients in New England. The latter study included a combination NRT arm (patch + ad lib nicotine gum), which is included in Analysis 6.1. The pooled analysis did not find an effect of varenicline: RR 3.72 (95% CI 0.50 to 27.59; I² = 0%: Analysis 8.6). Treating the trials as a subgroup of the main analysis (Analysis 4.1) and testing for subgroup differences demonstrated no significant difference between them (Chi² = 0.25, P = 0.62, I² = 0%; analysis not shown).
Analysis 8.6.
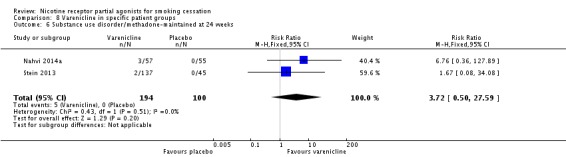
Comparison 8 Varenicline in specific patient groups, Outcome 6 Substance use disorder/methadone‐maintained at 24 weeks.
3.7 Alcohol‐dependent smokers
NCT01347112, which posted its results on the www.ClinicalTrials.gov website, reported cessation rates of 25% (4/16) for the varenicline group, and 0% (0/17) for the placebo group. These findings were not biochemically verified, and the study sustained high losses, putting it at high risk of bias.
3.8 HIV
No trials currently report on this comparison, although NCT00918307 includes a conference abstract giving preliminary findings. No results have been posted on the www.ClinicalTrials.gov trials registry database.
3.9 Diabetes
No trials currently report on this comparison.
3.10 Head and neck cancer
No trials currently report on this comparison.
3.11 Varenicline in pregnancy
No trials currently report on this comparison.
3.12 Varenicline for long‐term use of NRT
Tønnesen 2013 aimed to wean 139 ex‐smokers off long‐term use of NRT. All had been consuming an average of 16 NRT units a day for approximately six years. Participants were randomly allocated to varenicline or placebo for the standard 12‐week treatment phase, and were followed up to 52 weeks. The trial did not find a difference between the varenicline and placebo arms for participants, either for having smoked (10% in the varenicline group and 11.6% in the placebo group between weeks 36 and 52) or for not using NRT, with a RR of 1.31 (95% CI 0.83 to 2.08; Analysis 8.8).
Analysis 8.8.
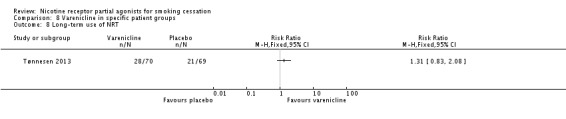
Comparison 8 Varenicline in specific patient groups, Outcome 8 Long‐term use of NRT.
IV Different settings and subgroups
4.1 Hospital inpatients/perioperative patients
Three trials currently address this population of smokers. Carson 2014 targeted adult smokers admitted to hospital for smoking‐related acute illnesses, Steinberg 2011 adult smokers admitted with any diagnosis, and Wong 2012 adult smokers admitted for non‐cardiac elective surgery. The pooled analysis at longest follow‐up favoured varenicline treatment, with a RR of 1.39 (95% CI 1.09 to 1.77; 744 participants, I² = 36%; Analysis 9.1). Treating the trials as a subgroup of the main analysis (Analysis 4.1) and testing for subgroup differences demonstrated a significant difference between the hospital group and the remaining trials (Chi² = 15.87, P < 0.0001, I² = 93.7%; analysis not shown). This may be linked to the negative findings of the Steinberg 2011 trial.
Analysis 9.1.
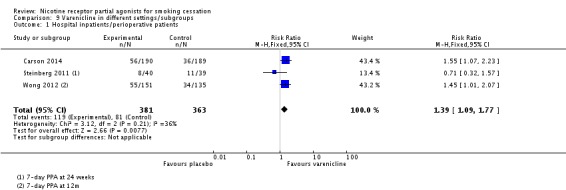
Comparison 9 Varenicline in different settings/subgroups, Outcome 1 Hospital inpatients/perioperative patients.
4.2 Smokers who have previously failed to quit on varenicline or NRT or bupropion
Gonzales 2014 tested varenicline versus placebo in a group of smokers who had previously used varenicline for two weeks or more, at least three months prior to admission to the study, and had failed to quit but were motivated to try again. The trial found a clear benefit for varenicline, with a RR at 52 weeks of 6.15 (95% CI 2.98 to 12.70; 494 participants; Analysis 9.2).
Analysis 9.2.
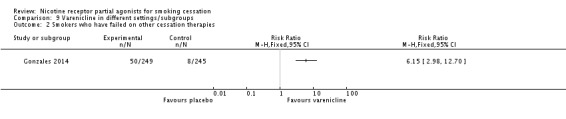
Comparison 9 Varenicline in different settings/subgroups, Outcome 2 Smokers who have failed on other cessation therapies.
4.3 Light or heavy smokers
De Dios 2012 is a small pilot study conducted in 32 Latino light smokers (smoking 10 or fewer cigarettes a day), randomising to varenicline, NRT or placebo tablets. The six‐month result, although favouring the varenicline arm, did not achieve statistical significance: RR 7.64 (95% CI 0.44 to 131.75; Analysis 9.3)
Analysis 9.3.
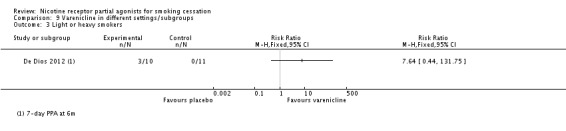
Comparison 9 Varenicline in different settings/subgroups, Outcome 3 Light or heavy smokers.
4.4 Varenicline by gender
No trials currently address this comparison, although a recent meta‐analysis (McKee 2015) presents abstinence data stratified by gender from 16 RCTs (supplied by Pfizer). Their meta‐analysis demonstrates, compared with other smoking cessation treatments, greater efficacy for short‐ and immediate‐term outcomes in women smokers versus men, and equal efficacy for abstinence at one year.
4.5 Varenicline in ethnic groups
No trials currently report on this comparison.
2. Craving and withdrawal
The results of the trials included in our review lend support to the theoretical basis for the development of varenicline. Its properties as a partial agonist, causing moderate activation of the α4β2nAChR, may be expected to mitigate craving and withdrawal symptoms, while its antagonist properties in blocking nicotine binding may lead to reduced smoking satisfaction and reduced psychological reward in those who continue to smoke while taking the drug. The varenicline trials which tested withdrawal and craving all reported its superiority over placebo in reducing withdrawal symptoms, as measured on the Minnesota Nicotine Withdrawal Scale or the Wisconsin Smoking Withdrawal Scale; craving, as measured on the Brief Questionnaire of Smoking urges; and enjoyment of concurrent smoking, as measured on the modified Cigarette Evaluation Questionnaire.Those trials (Nides 2006; Oncken 2006; Nakamura 2007; Niaura 2008) which measured the effects of varying dosage detected greater reductions in craving and withdrawal symptoms in the standard dose groups (1.0 mg twice a day) than in the reduced dose groups. Hajek 2015 noted similar disparities in enjoyment of smoking when participants moderated their own dosage up to the TQD. Full details of the comparative incidence of craving and withdrawal symptoms are shown in Appendix 3.
3. Adverse events (AEs)
The predominant adverse event for varenicline was mild to moderate nausea, subsiding over time, at rates between 6% (Stein 2013) and 51% (Nahvi 2014a), but with almost half the studies reporting levels between 24% and 29%. The trials testing non‐standard regimens found a dose‐response relationship for the incidence of nausea: rates ranged from 17.5% (0.3 mg daily) to 52% (1.0 mg twice daily) in Nides 2006, and from 7.2% (0.25 mg twice daily) to 24.4% (1.0 mg twice daily) in Nakamura 2007. Self regulation of treatment in Niaura 2008 appeared to reduce rates of nausea, with 13.4% of varenicline users reporting it compared with 5.2% of the placebo group. Both titration and dosage levels affected the incidence and severity of nausea in Oncken 2006, with the lower dose resulting in rates of 16.3% (titrated) and 22.6% (non‐titrated), compared with 34.9% (titrated) and 41.9% (non‐titrated) in the standard dosage groups. Hajek 2015 allowed participants to increase their dosage up to 5 mg a day by the TQD, and reported nausea rates of 80% in the varenicline group compared with 18% among the placebo participants. In Gonzales 2006 and Jorenby 2006, an average of 9.5% in the varenicline groups discontinued treatment but remained in the trial for follow‐up, compared with an average of 14% in the bupropion groups and 8% in the placebo groups. Discontinuation rates for any adverse event were highest in Williams 2007, where participants took the trial medication for a year, at 28.3% in the varenicline group and 10.3% in the control group. In the 12‐week open‐label phase of Evins 2014, 31.8% of participants taking varenicline discontinued the study because of adverse events, or for non‐adherence to the protocol, or because they no longer wished to stop smoking. In Phase 1 of Rose 2013, 62 of 112 (55%) non‐responders to NRT assigned to varenicline withdrew or were lost to follow‐up, but this was a comparable attrition rate to those lost from the NRT group (60%) and from the bupropion group (58%), and appeared not to be associated with adverse events. The study also noted that 25% of participants across all three conditions reduced their dosage at some point during treatment.
Adverse events were monitored weekly during treatment from weeks one to seven (Gonzales 2006; Jorenby 2006; Nides 2006; Oncken 2006), weekly throughout 12 weeks of treatment (Anthenelli 2013; Aubin 2008; Bolliger 2011; Carson 2014; Cinciripini 2013; EAGLES 2016; Ebbert 2015; Evins 2014; Gonzales 2014; Nakamura 2007; Niaura 2008; Rennard 2012; Rigotti 2010; Tashkin 2011; Tsai 2007; Wang 2009), or fortnightly throughout 12 weeks of treatment (Rose 2013; Tønnesen 2013). Stein 2013 monitored participants at weeks two and four, for adherence and adverse events. Tonstad 2006 monitored at week 13 (end of open‐label phase) and at week 25 (end of double‐blind phase), and Williams 2007 monitored weekly from weeks one to eight and then monthly to week 52. Baker 2016 monitored adverse events and delivered counselling at weeks 1, 4, 8 and 12. Steinberg 2011 collected adverse event data through self report at weeks 2, 4, 12 and 24, and Nahvi 2014a in four visits over 12 weeks of treatment. Hajek 2015 followed the UK's NHS Stop Smoking Service protocol and monitored weekly for the first month post‐TQD, and again at 12‐week end of treatment. The trials reported only those adverse events occurring in at least 5% of the varenicline groups, and at higher rates than in the placebo groups, with the exception of Bolliger 2011, Nahvi 2014a, Nakamura 2007, Stein 2013, Steinberg 2011, and Tønnesen 2013 (any occurrence), Anthenelli 2013 (occurring in 1% of either group), Ebbert 2015 (occurring in at least 2% of either group), Chengappa 2014, Cinciripini 2013, Gonzales 2014, and Hajek 2015 (at least 5% in either group), Evins 2014 (occurring in 10% of either group), and Rennard 2012 (any event occurring in at least 5% of either group, and psychiatric events in at least 1% of either group).
Meta‐analyses of the four main adverse events in the varenicline versus placebo groups yielded RRs of 3.27 (95% CI 3.00 to 3.55; 32 studies; 14,963 participants; I² = 22%) for nausea (Analysis 10.1); 1.49 (95% CI 1.35 to 1.65; 29 studies; 14,447 participants; I² = 0%) for insomnia (Analysis 10.2); 2.12 (95% CI 1.88 to 2.38; 26 studies; 13,682 participants; I² = 62%) for abnormal dreams (Analysis 10.3); and 1.17 (95% CI 1.07 to 1.29; 25 studies; 13,835 participants; I² = 27%) for headache (Analysis 10.4). All differences were statistically significant.
Analysis 10.1.
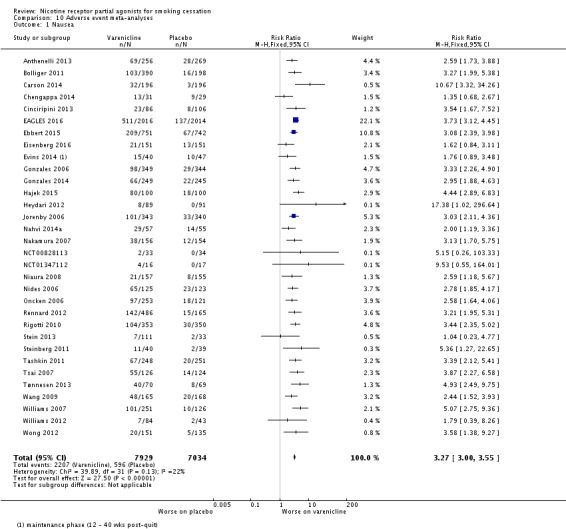
Comparison 10 Adverse event meta‐analyses, Outcome 1 Nausea.
Analysis 10.2.
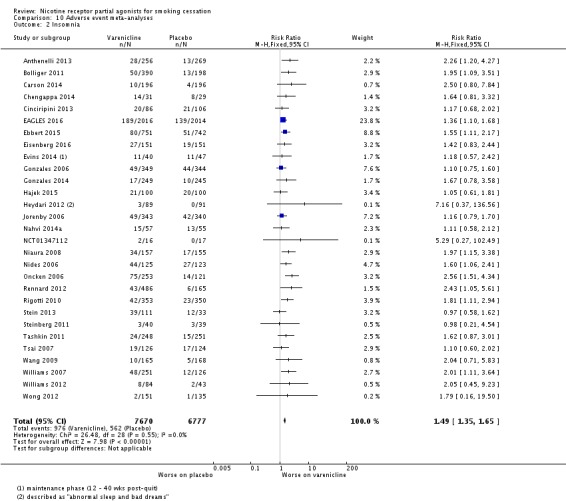
Comparison 10 Adverse event meta‐analyses, Outcome 2 Insomnia.
Analysis 10.3.
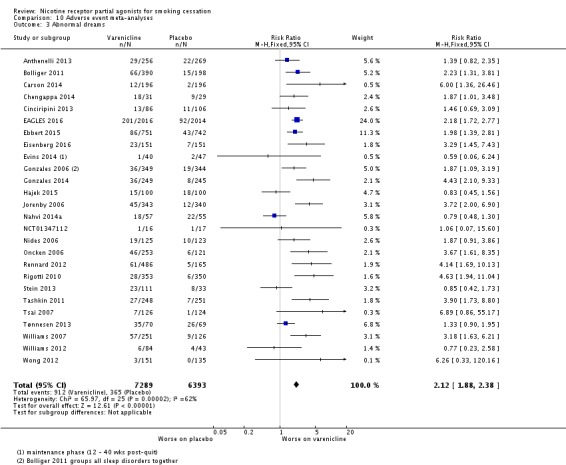
Comparison 10 Adverse event meta‐analyses, Outcome 3 Abnormal dreams.
Analysis 10.4.
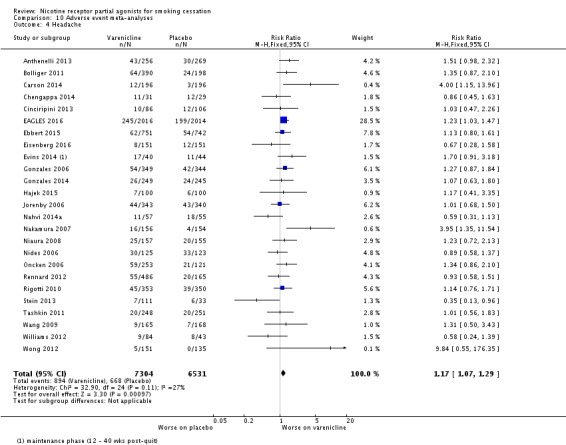
Comparison 10 Adverse event meta‐analyses, Outcome 4 Headache.
4. Serious Adverse Events (SAEs)
A serious adverse event (SAE) may be defined as any untoward medical occurrence that resulted in death; was life‐threatening; required inpatient hospitalisation or prolongation of existing hospitalisation; resulted in persistent or significant disability or incapacity; or resulted in a congenital anomaly or birth defect (Nakamura 2007). Vinnikov 2008 reported no SAEs in their cytisine trial, while West 2011 reported seven, none of which was deemed to be related to the medication, and Scharfenberg 1971 gave no information about the incidence of SAEs in either group. Walker 2014, comparing cytisine with NRT (no placebo group), reported 56 SAEs in 45 participants taking cytisine (eight of the SAEs occurring in one person), and 45 SAEs in 39 participants taking NRT. One person died in each group, but neither death (one alcohol‐related asphyxiation and one heart attack) was deemed to be treatment‐related.
Among the varenicline studies, there were no treatment‐related deaths in any of the intervention groups during treatment or follow‐up phases. However, Carson 2014 reported 13 fatalities during the first 12 months of the study period, in a population of inpatients admitted for acute episodes of smoking‐related illnesses. All the deaths (six in the varenicline + counselling group and seven in the counselling‐only group) were in people with known underlying comorbidities, including COPD, bradycardia, arrhythmia, lung cancer, stroke and non‐ST‐elevation myocardial infarction. The authors do not attribute any of the deaths to study medication (Carson 2011).
Non‐fatal SAEs occurred in 29 of the varenicline trials. We discounted from this analysis four trials which did not report any SAEs (De Dios 2012; Heydari 2012; Westergaard 2015; Wong 2012) and a further four which had no placebo group (Aubin 2008; Baker 2016; Rose 2013; Tsukahara 2010). Event counts for Analysis 11.1 and Analysis 11.2 are of individuals reporting one or more SAEs. Analysis 11.1 demonstrates an RR of 1.25 (95% CI 1.04 to 1.49; 15,370 participants, I² = 0%; high‐quality evidence), indicating an increased risk of SAEs in the varenicline groups compared with the placebo groups. A secondary analysis restricted to SAEs occurring within or immediately after the treatment phase demonstrated a similar effect (RR 1.25, 95% CI 1.02 to 1.52; 15,000 participants, I² = 0%). A sensitivity analysis using a Peto odds ratio (appropriate for the analysis of rare events) made no difference to the findings. Details of the SAEs among 32 of the included studies are given in Appendix 4.
Analysis 11.1.
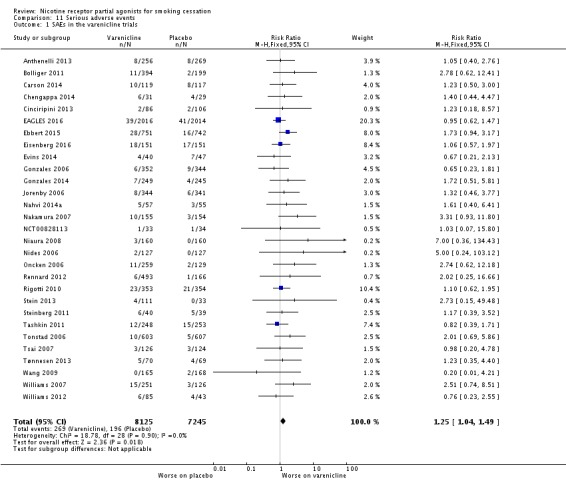
Comparison 11 Serious adverse events, Outcome 1 SAEs in the varenicline trials.
Analysis 11.2.
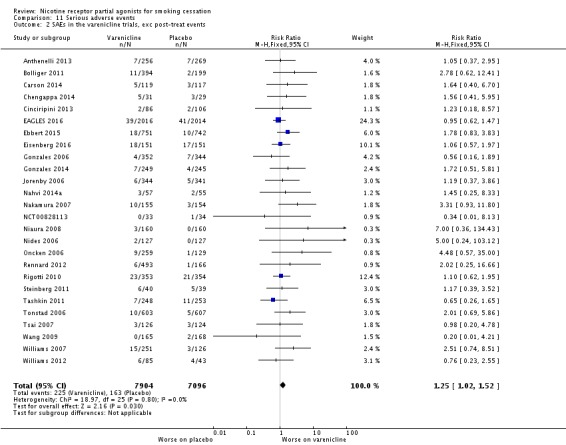
Comparison 11 Serious adverse events, Outcome 2 SAEs in the varenicline trials, exc post‐treat events.
We also include a meta‐analysis of cardiac SAEs including deaths (Analysis 11.4). There were 92 events in 8587 participants from 21 studies. There were more events in the varenicline groups and the confidence intervals are wide and do not rule out an increase (RR 1.36, 95% CI 0.91 to 2.04; I² = 0%; moderate‐quality evidence).
Analysis 11.4.
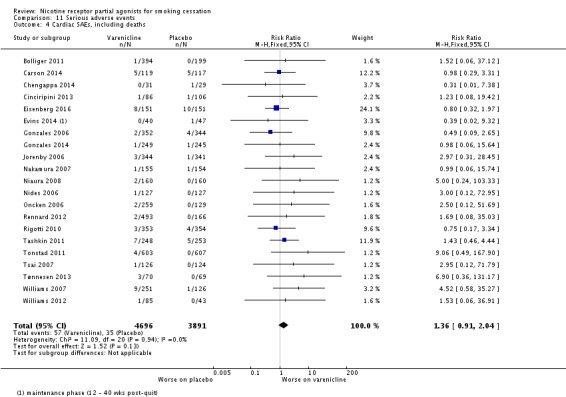
Comparison 11 Serious adverse events, Outcome 4 Cardiac SAEs, including deaths.
For this update. we have conducted new meta‐analyses of neuropsychiatric adverse events, using any study included in the efficacy findings which reported the incidence of these events, plus nine studies (flagged with an asterisk in the study ID) excluded from the efficacy analyses, but offering data on safety. The RR for depression is 0.94 (95% CI 0.77 to 1.14; 36 studies; 16,189 participants, I² = 0%; Analysis 10.5), with non‐significantly lower rates in the varenicline groups. The RR for suicidal ideation is 0.68 (95% CI 0.43 to 1.07; 24 studies; 11,193 participants, I² = 0; Analysis 10.6), with border‐line non‐significantly lower rates in the varenicline groups. It should be noted that all five events in the varenicline group for suicidal ideation occurred in the psychiatric cohort, with none reported in the non‐psychiatric group.
Analysis 10.5.
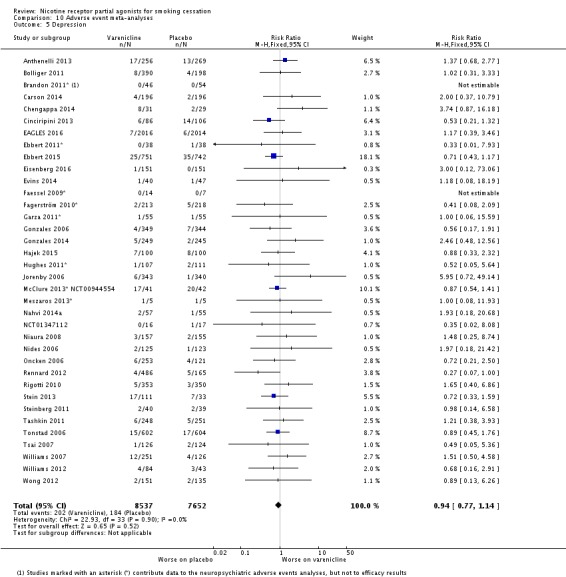
Comparison 10 Adverse event meta‐analyses, Outcome 5 Depression.
Analysis 10.6.
Comparison 10 Adverse event meta‐analyses, Outcome 6 Suicidal ideation.
The EAGLES Study
EAGLES 2016, a double‐blind triple‐dummy RCT, is the largest trial to have been conducted with varenicline, and was stratified by the presence (n = 4074) or absence (n = 3984) of a history of psychiatric disorders. The authors estimate that at least one‐third of the psychiatric cohort participants were stably taking psychotropic medications throughout the course of the study. The primary safety endpoint was a composite measure of 16 neuropsychiatric adverse events, including anxiety, depression, feeling abnormal, and hostility (all rated as severe), and agitation, aggression, delusions, hallucinations, homicidal ideation, mania, panic, paranoia, psychosis, suicidal ideation, suicidal behaviour, and completed suicide (all rated as moderate or severe). Outcomes were assessed using the Columbia Suicide Severity Rating Scale (C‐SSRS), the Suicide Behavior Questionnaire ‐ Revised (SBQ‐R), and the Hospital Anxiety and Depression Scale (HADS) at visits throughout the treatment and follow‐up phases of the study.
Rates of neuropsychiatric AEs were similar across all four treatment groups, with more AEs in the psychiatric than in the non‐psychiatric cohort. Event rates in the psychiatric cohort during treatment and up to 30 days after were varenicline 6.5%, bupropion 6.7%, NRT 5.2% and placebo 4.9%; the corresponding rates in the non‐psychiatric cohort were 1.3%, 2.2%, 2.5% and 2.4% respectively. The risk difference between groups was significantly lower for the varenicline group compared with placebo in the non‐psychiatric cohort (RD ‐1.28, 95% CI ‐2.40 to ‐0.15); all other differences in the remaining comparisons (varenicline, bupropion, NRT, all versus placebo) in both cohorts were statistically non‐significant. The study authors interpret this as indicating that none of the first‐line smoking cessation treatments compared with placebo significantly increases the risk of neuropsychiatric adverse events in smokers with or without psychiatric disorders.
An analysis by treatment group in the psychiatric cohort, assessing the incidence of AEs categorised by severity (severe or serious), discontinuation and corrective intervention (including medication, psychotherapy, counselling and hospitalisation), found few differences between the groups: counts of severe AEs were identical across the active treatment groups (14 in each), with the placebo group reporting 13. Serious AEs were similar: varenicline six, bupropion five, NRT and placebo three each. AEs leading to permanent treatment discontinuation were varenicline 16, bupropion and placebo 15 each, and NRT 12, while AEs requiring intervention were varenicline and NRT seven each, bupropion 12, and placebo 11. Based on the upper limits of the confidence intervals, the authors conclude that it is highly unlikely that varenicline and buporpion contribute to neuropsychiatric adverse events of moderate to severe intensity at a rate above 1.5% in smokers without a psychiatric disorder, and above 4% in smokers with such disorders. These estimates are also consistent with no increase in neuropsychiatric event rates in either population of smokers.
The authors report the limitations of their findings, confirming that the results may not be generalisable to smokers with untreated or unstable psychiatric disorders. For the psychiatric cohort, they confined recruitment to smokers with any of four major disease categories (mood, anxiety, psychosis and borderline personality disorder), and did not include smokers with current substance use disorders or imminent risk of suicide. They also point out that light smokers (fewer than 10 cigarettes a day) were excluded from the study population, and that the trial has low power to detect rare neuropsychiatric events.
The evidence currently presented demonstrates an inconsistent pattern between the two cohorts in AE event rates, with the psychiatric cohort reporting higher rates in the active treatment groups compared with placebo, and the reverse pattern in the non‐psychiatric cohort. This difference is in line with current SPC warnings that care should be taken in people with a history of psychiatric illness, and with the FDA 2015 Drug Safety Communication advising that they were unable to draw reliable conclusions on these issues. The reliance in this study on a composite safety endpoint, covering a mix of adverse and serious adverse events, also precludes firm conclusions about the risk levels for individual disorders, which may be elevated for some components and reduced for others. We await further details of the findings to explore the robustness of the risk profile.
Discussion
This update now covers four trials of cytisine, and 39 trials of varenicline. Full searches were conducted to May 2015, although we have included key trials which we obtained after this date.
Summary of main results
Cytisine
Two studies (Vinnikov 2008; West 2011) have demonstrated a benefit for cytisine over placebo. However, absolute quit rates were relatively low, with Vinnikov 2008 reporting 9% for cytisine and 1% for placebo at 24 weeks, and West 2011 8.4% and 2.4% respectively at 52 weeks. The authors note that their deliberately parsimonious intervention for the Polish trial, with a 25‐day regimen (in accordance with the manufacturer's guidelines; Tabex 2011) and minimal behavioural support, may have limited the achievable cessation rates. Further trials with modified regimens may need to be conducted to explore the balance between the positives of affordability and availability within lower‐ and middle‐income economies and the modest efficacy demonstrated to date. A recent non‐inferiority trial comparing cytisine with NRT (Walker 2014) demonstrated a benefit for cytisine at six months, with continuous abstinence rates of 21.8% and 15.3% respectively.
Varenicline
The evidence from 27 trials in 12,625 participants indicates that varenicline increases the chances of successful smoking cessation between two‐ and three‐fold compared with placebo. This estimate has remained stable, despite the growing inclusion of pragmatic trials in real‐world settings and in particular groups of smokers normally excluded from clinical trials, e.g. in lower‐ and middle‐income countries, and in disease‐specific populations. Long‐term use of varenicline (two trials of 24 weeks, two trials of 52 weeks) delivered an unequivocal advantage for varenicline over placebo, without a concomitant increase in adverse or serious adverse events.
In five trials (5877 participants), varenicline was shown to increase the probability of quitting more than bupropion. Eight trials (three of them open‐label) in 6264 people compared varenicline with nicotine patches, and found a modest but clear benefit for varenicline. One trial found no differences between any of the three tested treatments (varenicline, nicotine patch, and nicotine patch plus lozenge).
More smokers quit successfully with varenicline than with a placebo or an alternative intervention in all the populations and subgroups that we reviewed, including variations in usage (flexible versus fixed quit dates, different dosages, reducing to quit, relapse prevention therapy), in disease‐specific groups of patients (cardiovascular, COPD, schizophrenia and psychiatric disorders, depression), and in various subgroups or settings (hospital inpatients, smokers who failed to quit on other therapies). The exceptions to these findings were standard versus high‐dose varenicline, and varenicline versus placebo in young adults with asthma, in Latino light smokers, in methadone‐maintained substance abusers, and in long‐term users of NRT. In these instances the results favoured varenicline, but did not reach statistical significance.
The number needed to treat for an additional beneficial outcome (NNTB) can be derived from the pooled difference between placebo and treatment quit rates. However, absolute quit rates vary considerably between trials, according to the definition of cessation, length of follow‐up, the population treated and the extent of the counselling and follow‐up support given. The risk ratio should be independent of these factors and can be used to derive NNTBs for the assumed placebo rates that will apply in each local setting. We estimated a control quit rate with behavioural support at six months of 7.5%, derived from the weighted mean of the control event rates in the first few varenicline trials conducted in the USA. Based on this rate, the NNTB for varenicline is 11 (95% CI 9 to 13). For comparison we can estimate NNTBs from recent meta‐analyses of nicotine replacement therapy (NRT) (RR 1.60, 95% CI 1.53 to 1.68, Stead 2012) and bupropion (RR 1.62, 95% CI 1.49 to 1.76, Hughes 2014). Assuming the same 7.5% rate in the behavioural support‐only conditions, the NNTB for all types of NRT is 23 (95% CI 20 to 25), and the NNTB for bupropion is 22 (95% CI 18 to 28).
Adverse events
The main adverse effect of varenicline was nausea, which was generally mild to moderate, diminished over time, and was associated with low discontinuation rates. Those trials which tested levels of dosage and the presence or absence of titration found an increase in adverse events (apart from headache) with increasing dosage, and also found that titration appeared to reduce the incidence of nausea. The transitory nature of this adverse event may find further support in the relapse prevention study (Tonstad 2006), which reported nausea in 33.5% of varenicline users in the open‐label phase; once the successful quitters were randomised to varenicline or placebo, rates of nausea fell to 1.2% in the varenicline group and 0.7% in the placebo group. This virtual elimination of nausea as an adverse event may suggest that habituation over 12 weeks of treatment had resolved the condition. However, it is also plausible that those who suffered most with adverse events during the open‐label phase may not have successfully completed treatment or, having quit, would be less likely to accept the invitation to take part during the double‐blind phase. It would therefore be unwise to draw too strong an inference from the difference in rates between the two phases of the study.
New for this update are analyses of neuropsychiatric events (depression and suicidal ideation). In both cases, the event rates were higher in the placebo groups than in the varenicline groups, although neither point estimate reached statistical significance.
Serious adverse events
Our meta‐analysis of serious adverse event (SAE) data from 29 trials suggests there may be a 25% increased risk of such events among the varenicline groups compared with the controls. While this finding (RR 1.25, 95% CI 1.04 to 1.49; 15,370 participants; I² = 0%) reaches statistical significance, it must be noted that it is based on simple counts across the trials of participants reporting one or more such events, and does not distinguish between events attributed and those unrelated to treatment. A sensitivity analysis removing events known to have occurred after the treatment phase made a negligible difference to the point estimate (see Analysis 11.2). This finding should also be considered in the light of the higher losses to follow‐up in the control arm (mean of 28.4%) of most of the studies (25/29) compared with the varenicline arm (mean of 23.8%) (see Analysis 12.1), making it likely that the event rate in the intervention groups was consistently underestimated. A calculation of the number needed to treat for an additional harmful outcome (NNTH), based on a typical control rate of 2%, returned a figure of 143 (95% CI 74 to 556), i.e. one additional SAE for every 143 people treated with varenicline.
Analysis 12.1.
Comparison 12 Losses to follow‐up, Outcome 1 Participants remaining at end of varenicline trials.
Participants remaining at end of varenicline trials
| Study | Placebo [%] | Varenicline [%] | Bupropion [%] | NRT [%] | Chi² and P value |
|---|---|---|---|---|---|
| Anthenelli 2013 | 179/269 (66.5%) | 175/256 (68.4) | 0.20, P = 0.66 | ||
| Aubin 2008 | 247/378 (65.3) | 230/379 (60.7) | 1.76, P = 0.18 | ||
| Baker 2016 | 389/424 (91.7) | Patch: 226/241 (93.8) C‐NRT: 408/421 (96.9) | 10.42, P = 0.005** | ||
| Bolliger 2011 | 156/199 (78.4) | 336/394 (85.3) | 4.44, P = 0.04* | ||
| Carson 2014 | 160/196 (81.6) | 165/196 (84.2) | 0.45, P = 0.50 | ||
| Chengappa 2014 | 20/29 (69.0) | 24/31 (77.4) | 0.55, P = 0.46 | ||
| Cinciripini 2013 | 76/106 (71.7) | 65/86 (75.6) | 73/102 (71.6) | 0.37, P = 0.54 | |
| De Dios 2012 | 7/11 (63.6) | 7/10 (70.0) | 9/11 (81.8) | 0.10, P = 0.76 | |
| EAGLES 2016 | 1552/2035 (76.3) | 1598/2037 (78.4) | 1586/2034 (80.0) | 1557/2038 (76.4) | 4.24, P = 0.24 |
| Ebbert 2015 | 516/750 (68.8) | 559/760 (73.6) | 4.16, P = 0.04* | ||
| Eisenberg 2016 | 112/151 (74.2) | 118/151 (78.1) | 0.66, P = 0.42 | ||
| Evins 2014 | 33/40 (82.5) | 26/47 (55.3) | 7.31, P = 0.007** | ||
| Gonzales 2006 | 187/344 (54.4) | 213/352 (60.5) | 184/329 (55.9) | 2.90, P = 0.23 | |
| Gonzales 2014 | 144/247 (58.8) | 169/251 (67.9) | 4.35, P = 0.04 | ||
| Hajek 2015 | 60/100 (60.0) | 66/100 (66.0) | 0.77, P = 0.38 | ||
| Heydari 2012 | 91/91 (100.0) | 89/89 (100.0) | 92/92 (100.0) | 0, P = 1 | |
| Jorenby 2006 | 204/341 (59.8) | 240/344 (69.8) | 221/342 (64.6) | 7.42, P = 0.02* | |
| Nahvi 2014a | 43/55 (78.2) | 49/57 (86.0) | 1.16, P = 0.28 | ||
| Nakamura 2007 | 132/154 (85.7) | 124/155 (79.5) | 1.78, P = 0.18 | ||
| NCT00828113 | 20/51 (39.2) | 20/50 (40.0) | 0.01, P = 0.94 | ||
| NCT01347112 | 12/16 (75.0) | 5/17 (29.4) | 10.24, P = 0.001** | ||
| Niaura 2008 | 89/160 (55.6) | 100/160 (62.5) | 1.56, P = 0.21 | ||
| Nides 2006 | 68/127 (53.5) | 77/127 (60.6) | 68/128 (53.1) | 1.83, P = 0.40 | |
| Oncken 2006 | 40/129 (31.0) | 146/253 (57.7) | 24.38, P < 0.001** | ||
| Rennard 2012 | 132/166 (79.5) | 432/493 (87.6) | 6.62, P = 0.01* | ||
| Rigotti 2010 | 289/359 (80.5) | 302/355 (85.1) | 2.61, P = 0.11 | ||
| Stein 2013 | 35/45 (77.8) | 115/137 (83.9) | 107/133 (80.5) | 1.05, P = 0.59 | |
| Steinberg 2011 | 21/39 (53.8) | 22/40 (55.0) | 0.01, P = 0.92 | ||
| Tashkin 2011 | 157/254 (61.8) | 176/250 (70.4) | 4.14, P = 0.04* | ||
| Tonstad 2006 | 463/607 (76.3) | 494/603 (81.9) | 5.83, P = 0.02* | ||
| Tsai 2007 | 117/124 (94.4) | 120/126 (95.2) | 0.10, P = 0.75 | ||
| Tsukahara 2010 | 14/16 (87.5) | 14/16 (87.5) | 0, P = 1 | ||
| Tønnesen 2013 | 54/69 (78.3) | 61/70 (87.1) | 1.92, P = 0.17 | ||
| Walker 2014 | 473/655 (72.2) [cytisine] | 482/655 (73.6) | 0.31, P = 0.58 | ||
| Wang 2009 | 161/168 (95.8) | 158/165 (95.8) | 0.001, P = 0.97 | ||
| Westergaard 2015 | 14/26 (53.8%) | 19/26 (73.1%) | 2.07, P = 0.15 | ||
| Williams 2007 | 59/126 (46.8) | 135/251 (53.8) | 1.63, P = 0.20 | ||
| Williams 2012 | 40/43 (93.0) | 75/85 (88.2) | 0.72, P = 0.40 | ||
| Wong 2012 | 119/135 (88.1) | 134/151 (88.7) | 0.02, P = 0.88 |
Neuropsychiatric SAEs
Post‐marketing surveillance has raised continuing safety issues concerning varenicline. In February 2008 the US Food and Drug Administration (FDA 2008) issued a public health advisory, reporting that an association between varenicline and an increased risk of behaviour change, agitation, depressed mood, suicidal ideation and behaviour "appears increasingly likely". Three months later, the FDA approved changes to the product labelling, including a boxed warning, and a Medication Guide produced by Pfizer Inc.
Tonstad 2010a points out the complexities of separating treatment‐related events during the cessation process from those associated with nicotine withdrawal, with normalisation of monoamine oxidase levels, and possibly with increased caffeine levels. Any causal relationship between varenicline and serious neuropsychiatric events must be convincingly disentangled from possible confounding factors. A review of the ten trials completed up to the end of 2008 (Tonstad 2010b) found no significant excess incidence of disorders in varenicline users compared with control groups (RR 1.02, 95% CI 0.86 to 1.22), apart from sleep disorders (RR 1.70, 95% CI 1.50 to 1.92). However, although the absolute risk of depressed mood disorders and disturbances appears to be low in these study populations (varenicline 2.8% vs placebo 1.9%), the RR of 1.42 (95% CI 0.96 to 2.08), while not statistically significant, suggests an increased likelihood of such disorders for varenicline users. It must also be noted that these trials excluded participants with current or recent depression, panic disorder, psychosis, bipolar disorder or alcohol/drug abuse or dependence, and represent an atypically 'healthy' population of smokers. The findings may not be readily generalisable to a mixed real‐world population of smokers.
Recent studies have explored possible links between varenicline use and suicidal ideation and behaviour. Any such evaluation is complicated by the fact that people who smoke have a two‐ to three‐fold increased risk of suicide (Hemmingsson 2003; Miller 2000). A UK cohort study (Gunnell 2009) evaluating rates of fatal and non‐fatal self harm, suicidal thoughts and depression in users of varenicline compared with NRT and bupropion found no clear evidence of an association. The hazard ratio for self harm among people using varenicline compared with NRT was 1.12 (95% CI 0.67 to 1.88), and compared with bupropion was 1.17 (95% CI 0.59 to 2.32). Similarly, current evidence did not detect an effect for an increase in risks of depression or suicidal thoughts associated with varenicline compared with the other two medications. Although the upper level of the confidence interval for the self‐harm estimate does not preclude the possibility of a two‐fold increase for varenicline users, the data broadly confirm that the absolute incidence was low. The Gunnell 2009 analysis has subsequently been updated by Thomas 2013, using validated outcomes, and deploying instrumental variable analysis to counter residual confounding. The updated analyses confirmed that people taking varenicline were no more likely than those taking NRT to suffer fatal or non‐fatal self harm (HR 0.88, 95% CI 0.52 to 1.49) or treated depression (HR 0.75, 95% CI 0.65 to 0.87). The interim report by the UK‐based Drug Safety Research Unit of their cohort study of prescription event monitoring (Kasliwal 2009) has found no evidence of an excess of suicidal thoughts or behaviours; both of the reported suicide attempts in the cohort of 2682 patients occurred in people with a previous history of psychiatric illness and with precipitating factors for the event. A similar study conducted in New Zealand by the Intensive Medicines Monitoring Programme identified one suicide (0.03%, 95% CI 0.007% to 0.16%) in a cohort of 3415 recipients of dispensed varenicline prescriptions (Harrison‐Woolrych 2011).
Thomas 2014 used MHRA yellow card data to review the incidence of depression and suicidal behaviour spontaneously reported for 110 different drugs, including varenicline. Varenicline was ranked first for the number of reports per million prescriptions for depression (248, 95% CI 233 to 264), second behind paroxetine for non‐fatal suicidal behaviour (172 per million, 95% CI 159 to 185), and sixth for completed suicide behind clozapine, citalopram, fluoxetine, paroxetine and venlafaxine (10 per million, 95% CI 8 to 14).
Three recent retrospective cohort studies have reported on the incidence of psychiatric events, criminal offending and traffic accidents and offences (Molero 2015), on cardiovascular and neuropsychiatric events in users of varenicline (Kotz 2015), and on neuropsychiatric events in varenicline compared with NRT users in the Military Health System (Meyer 2013). The Swedish study (Molero 2015) reviewed a cohort of 7,917,436 adults, of whom 69,757 had been treated with varenicline between 2006 and 2009. They found that varenicline use was not associated with suicidal behaviour, with criminal offending, with traffic accidents or offences, or with psychoses. There were, however, marginally increased risks of anxiety (hazard ratio (HR) 1.23, 95% CI 1.01 to 1.51) and of mood conditions (HR 1.31, 95% CI 1.06 to 1.63) in people with a pre‐existing psychiatric disorder. In those without an existing psychiatric disorder, the risks were elevated but not statistically significantly different. In the English study (Kotz 2015), data from 753 NHS general practices were reviewed to compare recipients of NRT (106,759; the reference group) with users of varenicline (51,450) and bupropion (6557), for the incidence of neuropsychiatric and cardiovascular events. Varenicline was not associated with an increased risk for any neuropsychiatric conditions, compared with NRT users. The hazard ratio for depression was 0.66 (95% CI 0.63 to 0.69), and for self harm 0.56 (95% CI 0.46 to 0.68), compared with rates in NRT users. However, note comments below on this study and the Thomas 2013 data. Meyer 2013 compared propensity‐matched cohorts of people prescribed varenicline or NRT (10,814 in each group) within the American military system for rates of hospitalisation for neuropsychiatric events within 30 days of prescription. The adjusted HR at 30 days was 1.14 (95% CI 0.56 to 2.34), and at 60 days 1.11 (0.59 to 2.10), indicating no evidence for an elevated risk of hospitalisation among varenicline users compared with those taking NRT (reference group).
In contrast with these broadly reassuring findings, Moore 2011 used the FDA's Adverse Events Reporting System (AERS) to assess the occurence of suicidal behaviour or depression in 9575 case reports of varenicline use, and in 1751 case reports of bupropion use for smoking cessation. They concluded that varenicline was linked to a steep increase in depression or self‐injurious behaviours (OR 8.4, 95% CI 6.8 to 10.4) compared with NRT. While this report highlights continuing concerns about safety, it must be noted that inferences drawn from spontaneous reporting systems should be treated with caution. Because of heightened media coverage and FDA warnings, suicidal ideation or behaviour during varenicline use may be more likely to be reported to the AERS than if the patient exhibited the same features while on NRT, for example. The FDA caution against ascribing a causal connection between a drug and the event, pointing out that there is often insufficient information on the report forms to evaluate the event, and that not all adverse events will be reported to them: "Therefore, AERS cannot be used to calculate the incidence of an adverse event in the U.S. population" (FDA 2012). The limitations of this class of data include potential confounding by indication (i.e. the patient's condition may predispose to higher rates of adverse event); under‐reporting; double‐counting from multiple sources; the use of concomitant medications; and lack of representativeness which limits generalisability (Gibbons 2011). The Institute for Safe Medication Practice (ISMP) has questioned whether a spike in routine reports of serious adverse events for varenicline submitted to the AERS in the third quarter of 2010 is associated with a delay in passing the information from Pfizer to the FDA, and with classifying such events as "expected" rather than fast‐tracking them (ISMP 2011). In light of the constraints of spontaneous reporting systems, Moore and colleagues compared reports submitted for NRT use (to estimate baseline risks for smokers) with those submitted for antibiotic use (as a proxy for population‐based risks), which indicated that smokers may already be at a fourfold‐increased risk of suicidal behaviour or depression, regardless of which cessation aids they use. The authors recommended that varenicline should not be offered as a first‐line treatment for smoking cessation, and noted that the Veterans Affairs Center for Medication Safety shared this precautionary approach (VA 2011). It is notable, however, that a matched observational study of more than 28,000 participants commissioned by the FDA and conducted by the VA Center found no difference in rates of hospitalisation for psychiatric events between users of NRT and users of varenicline; the hazard ratio for varenicline versus NRT was 0.76; 95% CI 0.40 to 1.46 (FDA 2011a). A recent FDA Drug Safety Communication (FDA 2011a, October 2011) concludes that "Based on FDA’s assessment of currently available data, the Agency continues to believe that the drug’s benefits outweigh the risks and the current warnings in the Chantix drug label are appropriate".
Cardiovascular SAEs
Following the publication of Rigotti 2010 (testing varenicline in people with stable cardiovascular disease (CVD)), the FDA issued a Drug Safety Communication (FDA 2011b) advising that varenicline may be associated with a small increased risk of certain cardiovascular adverse events in people with CVD. A systematic review of 14 trials (Singh 2011) claims that varenicline may increase the risk of serious cardiovascular events among tobacco users, with a meta‐analysis yielding a Peto odds ratio of 1.72 (95% CI 1.09 to 2.71). While the authors describe this as "a 72% increased risk", it should be noted that the incidence of such events was low, at 1.06% in varenicline users and 0.82% in the placebo group, returning an absolute difference of 0.24%, i.e. about 1 in 400. The review has some acknowledged limitations, including the validity of the classification of the cardiac events (unadjudicated, other than those derived from the Rigotti trial); higher losses to follow‐up in the placebo groups, which may underestimate the true rate of control events; and the choice of a Peto rather than a Mantel‐Haenszel odds ratio (the latter just missing statistical significance [M‐H OR 1.56, 95% CI 0.99 to 2.44]). The choice of a random‐effects rather than a fixed‐effect model would also lower the point estimate (OR 1.47, 95% CI 0.92 to 2.34). The assumption of a baseline risk rate of 5.57% for cardiac adverse events in their calculation of a number needed to treat for an additional harmful outcome (NNTH) may also be questionable, since it is based on the Rigotti study population of smokers with established cardiac disease, while the event counts used to derive the odds ratio come from trials which were mostly in unusually 'healthy' trial participants.
These concerns were echoed by an observational prospective cohort study of dispensed prescriptions for varenicline in New Zealand between April 2007 and November 2010 (Harrison‐Woolrych 2012). The study, conducted by the Intensive Medicines Monitoring Programme, covered all patients who received varenicline, and has so far identified 172 cardiovascular adverse events within that cohort. Forty‐eight of these were classified as myocardial ischaemia (including 12 reports of myocardial infarction and eight of angina), and 50 were classified as hypotensive events. Within each of these two subgroups, the investigators considered that two key cases may have been triggered by the use of varenicline. Twenty‐seven episodes of dysrythmia were also reported, two of which culminated in sudden death; one was attributed to pre‐existing heart disease, while the other displayed no definitive underlying cause. Although this cohort was subject to raised baseline risks because of their smoking and to multiple confounding factors, the authors speculate on possible mechanisms of dysregulation of blood pressure, which could have contributed to the events.
A Danish cohort study (Svanström 2012) compared propensity‐matched cohorts of people prescribed varenicline or bupropion (17,926 in each group) from 2007 to 2010 for rates of acute coronary syndrome, ischaemic stroke, and cardiovascular death six months from the start of treatment. The study found no excess of events in the varenicline group (6.9 cases per 1000 person‐years) compared with the bupropion group (7.1 cases per 1000 person‐years). The hazard ratio (varenicline versus bupropion) for acute coronary syndrome was 1.20 (95% CI 0.75 to 1.91), for ischaemic stroke 0.77 (95% CI 0.40 to 1.48), and for cardiovascular death 0.51 (95% CI 0.13 to 2.02). The presence or absence of a history of cardiovascular disease did not affect the overall findings.
The Kotz 2015 cohort study, briefly reported above, found significantly reduced risks of ischaemic heart disease (HR 0.80, 95% CI 0.72 to 0.87), of cerebral infarction (HR 0.62, 95% CI 0.52 to 0.73), of heart failure (HR 0.61, 95% CI 0.45 to 0.83) and of arrhythmia (HR 0.73, 95% CI 0.60 to 0.88) in varenicline users compared to NRT users.
A recent systematic review and meta‐analysis (Sterling 2016) of varenicline and cardiovascular serious adverse events included 38 RCTs (12,706 participants) published up to 2015, and found no evidence of an association, in people with (RR 1.04, 95% CI 0.57 to 1.89) or without (RR 1.03, 95% CI 0.64 to 1.64) cardiovascular illness. This study also analysed all‐cause mortality, and found no difference between the varenicline and placebo groups (RR 0.88, 95% CI 0.50 to 1.52).
We await results from the CATS study (NCT01574703), conducted among participants in the EAGLES 2016 study, and designed to monitor the incidence of major cardiovascular events (MACEs) for 28 weeks after the completion of the EAGLES 2016 trial. The CATS study was completed in July 2015, and is expected to report later this year.
Overall completeness and applicability of evidence
We have followed standard Cochrane methodology to perform this update. Figure 3 (a funnel plot of the main analysis) appears to identify a lack of smaller trials with negative findings. However, the earliest studies in this review were reported in 2006, and we are reasonably confident that the licensing and subsequent trials have been routinely registered online in clinical trials registries. The absence of negative studies may be more a marker of sustained efficacy than of the suppression or selective management of data.
Figure 3.
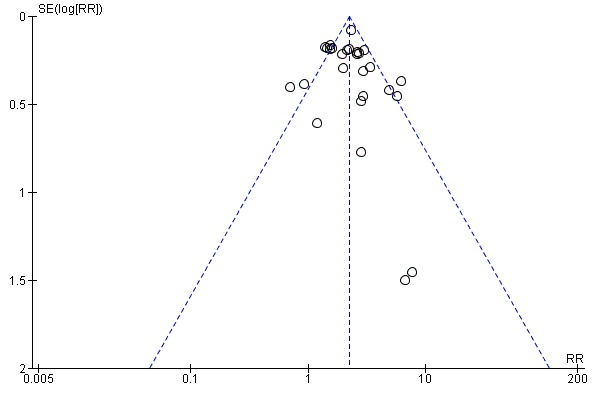
Funnel plot of comparison: 4 Varenicline (1.0 mg 2/d) vs placebo, outcome: 4.1 Continuous or sustained abstinence at longest follow‐up (24+ weeks).
Varenicline's efficacy for smoking cessation is now well established, with the point estimate remaining unchanged as more studies (including non‐Pfizer trials) accumulate. Trials are now being conducted and reported in patient groups originally excluded from the earlier studies, and more flexible regimens appear not to compromise levels of efficacy. However, concerns about possible adverse events in vulnerable individuals mean that varenicline is unlikely to be made available as an over‐the‐counter option, or outside the supervision of a health professional. The costs of treatment have hitherto restricted usage to high‐income countries, although trials are increasingly being conducted in low‐ and middle‐income countries. Cytisine, an unlicensed treatment in the European Union and the USA, is an affordable alternative available in parts of Eastern Europe and Russia, and for online purchase worldwide, and may have the potential to meet the needs of smokers wishing to quit in areas of economic constraint.
Quality of the evidence
We judge the current evidence from the cytsine trials to be of low quality, meaning that we have limited confidence in the evidence; only two trials contribute to the meta‐analysis, with relatively small numbers taking part. We rated the evidence from studies comparing varenicline with placebo and bupropion as comparators as being of high quality, i.e. reliable and robust. We rate the evidence from studies that compared varenicline with NRT as moderate quality (i.e. we are reasonably confident of the stability of the evidence), since three of them were non‐blinded open‐label trials.
Potential biases in the review process
We have delayed publication of this update in order to be able to report on the EAGLES 2016 trial. Our information on that trial has been drawn from the trial registry summary (NCT01456936) with results posted on May 3rd 2016, from Society for Research on Nicotine and Tobacco (SRNT) conference abstracts, from a presentation made at the SRNT conference in Chicago (March 3rd 2016), from correspondence with the Pfizer Medical Information Department, and from the in‐press release of the initial findings in The Lancet. Further results as they become available may moderate our findings in this update.
The comprehensive searches for this update are current to May 2015. Since May 2015 we have checked the status of all ongoing studies, and have generated monthly routine searches of PubMed (keywords 'varenicline' and 'cytisine') to identify any additional relevant research. Studies collected in this way include Baker 2016; EAGLES 2016; Ebbert 2015; Eisenberg 2016; Hajek 2015; Westergaard 2015. However, we cannot vouch for the completeness of the evidence base beyond the May 2015 search date, and may have missed some relevant reports or developments.
All the varenicline trials reported in this review apart from seven (Carson 2014; De Dios 2012; Heydari 2012; Nahvi 2014a; Rose 2013; Stein 2013; Tsukahara 2010) were funded and/or supported by Pfizer Inc, the manufacturers of varenicline. Evidence from systematic reviews suggests that industry‐funded trials, although conducted to a high standard, are more likely to have outcomes favourable to the product sponsor than studies with other sponsors (Etter 2007; Walsh 2011). However, a sensitivity analysis removing them from the main analysis made no difference to the result. Future updates of this review are increasingly likely to cover findings from community‐based independently‐conducted trials.
Although we have reported information from studies other than RCTs for the incidence and likelihood of adverse events and serious adverse events, it is important to acknowledge the risks of relying upon evidence from cohort studies, surveys, and prescription event‐monitoring data. There is, for example, evidence from observational studies (Kotz 2015; Thomas 2013) of residual confounding contributing to the observed reductions in the hazard ratios for depression and death. The lower rates in the varenicline users are attributable not to a protective effect conferred by the treatment but to baseline differences between the varenicline and NRT cohorts; the former were healthier, wealthier and younger (Davies 2015). The Thomas 2015 study (see below), based on 39 RCTs, found no significant reductions in depression, self harm or death rates, compared with placebo.
Agreements and disagreements with other studies or reviews
Reviews of controlled studies of cytisine (Etter 2006; Etter 2008; Tutka 2005; Tutka 2006; Tutka 2008) have focused upon its potential as an established and affordable aid to smoking cessation. Many of the early cytisine studies excluded from this review are discussed and evaluated in Etter 2006. A recent systematic review and network meta‐analysis (Leaviss 2014) has compared the efficacy and cost effectiveness of cytisine (two trials: Vinnikov 2008; West 2011) versus varenicline (21 trials). While the analysis found both treatments to be effective for smoking cessation, cytisine delivered more quality‐adjusted life‐years at a lower cost than varenicline. Cytisine was also associated with lower rates of headache and nausea than varenicline.
A Cochrane overview and network meta‐analysis of a number of pharmacological interventions for smoking cessation (Cahill 2013) assessed 12 Cochrane reviews published to November 2012, and therefore drew on the previous version of this review. Comparisons between varenicline, bupropion and single‐treatment NRT found varenicline to be superior to both treatments (OR 1.59; 95% credible interval 1.29 to 1.96, and OR 1.57, 95% credible interval 1.29 to 1.91 respectively). Varenicline demonstrated comparable efficacy to combination NRT (OR 1.06, 95% credible interval 0.75 to 1.48), but the number of NRT trials informing this comparison was low (nine trials). The direct comparisons between varenicline and placebo, varenicline and bupropion and varenicline and NRT reported in the EAGLES 2016 trial confirmed in all cases the network results of the same comparisons in Cahill 2013. A 2012 network meta‐analysis (Mills 2012), comparing high‐dose and combination NRT versus varenicline and versus bupropion across 146 RCTs, found varenicline (11 trials) to be superior to placebo and to bupropion at all time points, and similar in efficacy to standard and to high‐dose NRT.
Attention in recent years has tended to shift from efficacy (now clearly established) to adverse and serious adverse events. A meta‐analysis of gastrointestinal adverse events associated with varenicline use in 12 RCTs (Leung 2011) found that the drug produced higher rates of nausea (NNTH of 5), constipation (NNTH of 24), and flatulence (NNTH of 35). Another meta‐analysis in 12 RCTs of adverse effects during varenicline use (Drovandi 2015) found elevated rates of discontinuation attributable to adverse effects (OR 1.47, 95% CI 1.19 to 1.81) among the varenicline users compared to placebo, and higher rates of nausea, insomnia and headache. Since publication of the Singh 2011 systematic review, a number of other meta‐analyses and commentaries have addressed the risks of cardiovascular adverse events associated with varenicline usage. Two reviews which covered largely the same research as the Singh review did not demonstrate a statistically significantly raised event rate for cardiovascular disorders (Prochaska 2012, 22 studies; Ware 2013, 15 studies); the discrepancies were attributed by the Singh team to differences in interpretation of the outcomes and to modifications to the statistical computations. Mills 2013, a network meta‐analysis of 63 RCTs of NRT, bupropion and varenicline, found no elevated risk of serious cardiovascular events associated with any of the treatments, although trials of NRT demonstrated an increased risk for less serious events. The RR for major adverse cardiovascular events (MACEs) in varenicline compared with placebo was 1.34 (95% credible interval 0.66 to 2.66; 18 trials).
Thomas 2015 is a systematic review and meta‐analysis of 39 RCTs (10,761 participants), assessing the risk of neuropsychiatric adverse events among users of varenicline. The authors found no evidence of an increased risk of suicide or attempted suicide (Peto odds ratio (OR) 1.67, 95% CI 0.33 to 8.57), suicidal ideation (Peto OR 0.58, 95% CI 0.28 to 1.20), depression (Peto OR 0.96, 95% CI 0.75 to 1.22) or death (Peto OR 1.05, 95% CI 0.47 to 2.38) associated with varenicline. There was no evidence that the risk of depression and suicidal ideation differed by age, sex, ethnicity, smoking status, the presence or absence of psychiatric illness, or study sponsorship. This analysis included varenicline prescribed for any indication; our own analyses of depression (Analysis 10.5) and suicidal ideation (Analysis 10.6) use most of the same studies (including nine trials which did not contribute to our efficacy analyses), but we have dropped six trials which did not target smoking cessation, and have included four studies not then available to the Thomas team (Carson 2014; Ebbert 2015; Hajek 2015; Nahvi 2014a).
Because they are relatively rare, the incidence of serious neuropsychiatric events associated with varenicline has tended to be examined through retrospective cohort studies and prescription event monitoring studies, rather than through randomised trials, and has been considered in the Discussion section above. Gibbons 2013 is a re‐analysis of SAE neuropsychiatric data from 17 RCTs of varenicline, stratifying by the presence or absence of psychiatric disorders. The analysis found no excess of suicidal thoughts or behaviour in the varenicline group without psychiatric disorders (0.47 events per 1000, compared with 1.46 per 1000 in the placebo group), nor in the varenicline group with a history of psychiatric disorders (14.57 events per 1000, compared with 15.39 per 1000 in the placebo group). No suicides were reported in any of the groups. The same study also analysed a Department of Defence data set comparing events in a varenicline cohort (19,933 people) versus a cohort of NRT users (15,867 people) between August 2006 and August 2007 (i.e. before the FDA issued a black‐box warning). Rates of neuropsychiatric events in this cohort were 2.28% for varenicline and 3.1% for nicotine patch.
Authors' conclusions
Varenicline at standard dosage (1.0 mg twice a day) increased the chances of successful long‐term smoking cessation by more than two‐fold compared with pharmacologically unassisted quit attempts.
Varenicline at reduced dosage remained an effective aid to smoking cessation, delivering success rates similar to those achieved with nicotine replacement and bupropion, and appearing to reduce the impact of adverse events in the early weeks of treatment.
More people quit successfully with varenicline than with bupropion.
Eight trials of varenicline versus nicotine replacement therapy indicate a modest but unequivocal benefit for varenicline.
Limited evidence suggests that varenicline may have a role to play in relapse prevention.
The most commonly reported adverse effect of varenicline is nausea, but mostly at mild to moderate levels and tending to subside over time.
Users of varenicline may have an elevated risk of any serious adverse event, with rates about 25% higher than in those not using the drug.
Evidence from randomised controlled studies does not confirm a causal link between varenicline and psychiatric adverse events in people without a history of psychiatric disorders.
The evidence is less clearcut for the relationship between varenicline and neuropsychiatric events in people with past or current psychiatric disorders. The largest RCT suggests there may be up to a 4% increased risk of moderate‐to‐severe neuropsychiatric events in smokers with psychiatric disorders taking varenicline, compared with a 1.5% increased risk in smokers without these disorders. These estimates are also consistent with no increased risk in either cohort.
The imminent publication of data from a large recent trial should provide more definitive data on how varenicline may impact on major cardiovascular events.
Cytisine was shown to be effective and affordable, although absolute quit rates were modest.
Dianicline was no more effective than placebo in helping smokers to quit. Development of the drug has been suspended by the manufacturers.
Further varenicline trials may be useful in disorder‐specific groups of patients, excluded from the earlier trials.
Future trials should continue to investigate the long‐term success of extended treatment compared with standard 12‐week treatment.
The incidence of serious adverse events should continue to be monitored through controlled trials, and described with greater precision than is currently reported.
Further exploration of safety issues in people with past or current psychiatric disorders may still be warranted.
Additional trials of cytisine are needed to explore variations in the drug regimen and in the level of behavioural support needed to boost quit rates.
Acknowledgements
We thank Lindsay Stead of the Cochrane Tobacco Addiction Group, for extensive searching and support. We thank Paul Aveyard for advice and support on the content of each version of this review. We thank Karl Fagerström, John Stapleton, David Balfour and Nancy Rigotti for reading and commenting on earlier drafts. Our especial thanks go to Jean‐Francois Etter, who shared with us the bibliography and findings of his cytisine review, and made available to us original and translated versions of otherwise inaccessible publications. We also thank Serena Tonstad, Cheryl Oncken, Karen Reeves and Kristin Carson for supplying unpublished data for use in the review; David Gonzales, Douglas Jorenby, Stephen Rennard and Christian Westergaard for additional information; Piotr Tutka for cytisine reprints; and Rumen Nikolov of Sopharma (Bulgaria) for the Monova 2004 final trial report. We thank Denis Vinnikov for supplying his cytisine trial report, with additional unpublished data. We thank Cristina Russ of Pfizer Inc (New York) for information on trials in progress and details of SAEs. We thank Carlos Jiménez‐Ruiz, Mira Harrison‐Woolrych (Director of the Intensive Medicines Monitoring Programme) and Robert Gibbons for article reprints, Malgorzata Bala for providing non‐English language copies of cytisine trials, Irena U Beecher and Sonya Beecher for translation and help with Polish‐language papers, and Sebastian Straube for German‐language translations and data extraction.
Appendices
Appendix 1. Glossary of tobacco‐related terms
| Term | Definition |
| Abstinence | A period of being quit, i.e. stopping the use of cigarettes or other tobacco products. May be defined in various ways; see also: point prevalence abstinence; prolonged abstinence; continuous/sustained abstinence |
| Biochemical verification | Also called 'biochemical validation' or 'biochemical confirmation': A procedure for checking a tobacco user's report that he or she has not smoked or used tobacco. It can be measured by testing levels of nicotine or cotinine or other chemicals in blood, urine, or saliva, or by measuring levels of carbon monoxide in exhaled breath or in blood. |
| Bupropion | A pharmaceutical drug originally developed as an antidepressant, but now also licensed for smoking cessation; trade names Zyban, Wellbutrin (when prescribed as an antidepressant) |
| Carbon monoxide (CO) | A colourless, odourless highly poisonous gas found in tobacco smoke and in the lungs of people who have recently smoked, or (in smaller amounts) in people who have been exposed to tobacco smoke. May be used for biochemical verification of abstinence. |
| Cessation | Also called 'quitting' The goal of treatment to help people achieve abstinence from smoking or other tobacco use, also used to describe the process of changing the behaviour |
| Continuous abstinence | Also called 'sustained abstinence' A measure of cessation often used in clinical trials involving avoidance of all tobacco use since the quit day until the time the assessment is made. The definition occasionally allows for lapses. This is the most rigorous measure of abstinence |
| 'Cold Turkey' | Quitting abruptly, and/or quitting without behavioural or pharmaceutical support. |
| Craving | A very intense urge or desire [to smoke]. See: Shiffman et al 'Recommendations for the assessment of tobacco craving and withdrawal in smoking cessation trials' Nicotine & Tobacco Research 2004: 6(4): 599‐614 |
| Dopamine | A neurotransmitter in the brain which regulates mood, attention, pleasure, reward, motivation and movement |
| Efficacy | Also called 'treatment effect' or 'effect size': The difference in outcome between the experimental and control groups |
| Harm reduction | Strategies to reduce harm caused by continued tobacco/nicotine use, such as reducing the number of cigarettes smoked, or switching to different brands or products, e.g. potentially reduced exposure products (PREPs), smokeless tobacco. |
| Lapse/slip | Terms sometimes used for a return to tobacco use after a period of abstinence. A lapse or slip might be defined as a puff or two on a cigarette. This may proceed to relapse, or abstinence may be regained. Some definitions of continuous, sustained or prolonged abstinence require complete abstinence, but some allow for a limited number or duration of slips. People who lapse are very likely to relapse, but some treatments may have their effect by helping people recover from a lapse. |
| nAChR | [neural nicotinic acetylcholine receptors]: Areas in the brain which are thought to respond to nicotine, forming the basis of nicotine addiction by stimulating the overflow of dopamine |
| Nicotine | An alkaloid derived from tobacco, responsible for the psychoactive and addictive effects of smoking. |
| Nicotine Replacement Therapy (NRT) | A smoking cessation treatment in which nicotine from tobacco is replaced for a limited period by pharmaceutical nicotine. This reduces the craving and withdrawal experienced during the initial period of abstinence while users are learning to be tobacco‐free. The nicotine dose can be taken through the skin, using patches, by inhaling a spray, or by mouth using gum or lozenges. |
| Outcome | Often used to describe the result being measured in trials that is of relevance to the review. For example smoking cessation is the outcome used in reviews of ways to help smokers quit. The exact outcome in terms of the definition of abstinence and the length of time that has elapsed since the quit attempt was made may vary from trial to trial. |
| Pharmacotherapy | A treatment using pharmaceutical drugs, e.g. NRT, bupropion |
| Point prevalence abstinence (PPA) | A measure of cessation based on behaviour at a particular point in time, or during a relatively brief specified period, e.g. 24 hours, 7 days. It may include a mixture of recent and long‐term quitters. cf. prolonged abstinence, continuous abstinence |
| Prolonged abstinence | A measure of cessation which typically allows a 'grace period' following the quit date (usually of about two weeks), to allow for slips/lapses during the first few days when the effect of treatment may still be emerging. See: Hughes et al 'Measures of abstinence in clinical trials: issues and recommendations'; Nicotine & Tobacco Research, 2003: 5 (1); 13‐25 |
| Relapse | A return to regular smoking after a period of abstinence |
| Secondhand smoke | Also called passive smoking or environmental tobacco smoke [ETS] A mixture of smoke exhaled by smokers and smoke released from smouldering cigarettes, cigars, pipes, bidis, etc. The smoke mixture contains gases and particulates, including nicotine, carcinogens and toxins. |
| Self efficacy | The belief that one will be able to change one's behaviour, e.g. to quit smoking |
| SPC [Summary of Product Characteristics] | Advice from the manufacturers of a drug, agreed with the relevant licensing authority, to enable health professionals to prescribe and use the treatment safely and effectively. |
| Tapering | A gradual decrease in dose at the end of treatment, as an alternative to abruptly stopping treatment |
| Titration | A technique of dosing at low levels at the beginning of treatment, and gradually increasing to full dose over a few days, to allow the body to get used to the drug. It is designed to limit side effects. |
| Withdrawal | A variety of behavioural, affective, cognitive and physiological symptoms, usually transient, which occur after use of an addictive drug is reduced or stopped. See: Shiffman et al 'Recommendations for the assessment of tobacco craving and withdrawal in smoking cessation trials' Nicotine & Tobacco Research 2004: 6(4): 599‐614 |
Appendix 2. Participant numbers in varenicline trials
| Study | Varenicline | Placebo | Bupropion | NRT | TOTAL |
| Anthenelli 2013 | 256 | 269 | 525 | ||
| Aubin 2008 | 378 | 379 | 757 | ||
| Baker 2016 | 424 | 662 | |||
| Bolliger 2011 | 394 | 199 | 593 | ||
| Carson 2014 | 196 | 196 | 392 | ||
| Chengappa 2014 | 31 | 29 | 60 | ||
| Cinciripini 2013 | 86 | 106 | 102 | 294 | |
| De Dios 2012 | 10 | 11 | 11 | 32 | |
| EAGLES 2016 | Psych 1032 Non‐psych 1005 |
Psych 1026 Non‐psych 1009 |
Psych 1033 Non‐psych 1001 |
Psych 1025 Non‐psych 1013 |
4116 4028 |
| Ebbert 2015 | 760 | 750 | 1510 | ||
| Eisenberg 2016 | 151 | 151 | 302 | ||
| Evins 2014 | 40 | 47 | 87 | ||
| Gonzales 2006 | 352 | 344 | 329 | 1025 | |
| Gonzales 2014 | 249 | 245 | 494 | ||
| Hajek 2015 | 100 | 100 | 200 | ||
| Heydari 2012 | 89 | 91 | 92 | 272 | |
| Jorenby 2006 | 344 | 341 | 342 | 1027 | |
| Nahvi 2014a | 57 | 55 | 112 | ||
| Nides 2006 | 128 (0.3 x 1) 128 (1.0 x 1) 127 (1.0 x 2)* |
127 |
128 |
638 |
|
| NCT00828113 | 50 (Extended) 51 (Standard) |
101 | |||
| NCT01347112 | 16 | 17 | 33 | ||
| Oncken 2006 | 129 (0.5NT) 130 (0.5T) 129 (1.0NT)* 130 (1.0T)* |
129 |
647 |
||
| Williams 2012 | 85 | 43 | 128 | ||
| Rennard 2012 | 493 | 166 | 659 | ||
| Stein 2013 | 137 | 45 | 133 | 315 | |
| Tønnesen 2013 | 70 | 69 | 139 | ||
| Tonstad 2006 | 1927 Phase 1 [603] Phase 2* |
[607] Phase 2 |
1927 | ||
| Nakamura 2007 | 153 (0.25x2) 156 (0.5x2) 156 (1.0x2)* |
154 |
619 |
||
| Tsai 2007 | 126 | 124 | 250 | ||
| Williams 2007 | 251 | 126 | 377 | ||
| Niaura 2008 | 160 | 160 | 320 | ||
| Wang 2009 | 165 | 168 | 333 | ||
| Rigotti 2010 | 355 | 359 | 714 | ||
| Rose 2013 | 112 | 114 | 226 | ||
| Steinberg 2011 | 40 | 39 | 79 | ||
| Tsukahara 2010 | 16 | 16 | 32 | ||
| Tashkin 2011 | 250 | 254 | 504 | ||
| Westergaard 2015 | 26 | 25 | 52 | ||
| Wong 2012 | 151 | 135 | 286 | ||
| TOTALS | 11,801 * used in primary MA | 7109 | 2935 | 3445 | 25,290 |
Appendix 3. Measures of craving, withdrawal and reinforcement
| Study | MNWS or WSWS | QSU‐B Total Craving score | mCEQ (for smokers) |
|
Carson 2014 (wk 1) 1.0 mg bid vs placebo |
At week 1, Likert scale: Craving: V: 3.36; placebo: 4.45 Anxiety: V: 3.19; placebo: 4.25 Confidence: V: 7.95; placebo: 7.02 Motivation: V: 8.22; placebo: 7.50 |
||
| Gonzales 2006 (wks 1 ‐ 7) 1.0 mg bid vs placebo |
Urge to smoke: ‐0.54 (P < .001) ES: ‐0.57*N Negative affect: ‐0.19 (P < .001) ES: ‐0.30 Restlessness: ‐0.14 (P < .01) ES: ‐0.16 Increased appetite: +0.12 (P = .04) ES: 0.15 Insomnia: +0.05 (P = .36) ES: 0.06 | ‐0.45 (1.69 V, 2.13 P); P < 0.001; ES: ‐0.33 |
Baseline to wk 1: diff in changes between V&P: Smoking satisfaction: ‐0.60 (P < .001) ES: ‐0.47 Psych Reward: ‐0.50 (P < .001) ES: ‐0.37 Enjoy resp tract: ‐0.34 (P < .001) ES: ‐0.21 Craving reduction: ‐0.52 (P < .001) ES: ‐0.33 Aversion: ‐0.18 (P = .053) ES: ‐0.19 |
| Jorenby 2006 (wks 1 ‐ 7) 1.0 mg bid vs placebo |
Diff in mean change in: Urge to smoke: ‐0.48 (P < .001) Negative affect: ‐0.13 (P = 0.001) Restlessness:‐0.10(P = 0.05) Increased appetite: +0.07 (P = 0.22) Insomnia: +0.10 (P = 0.07) |
‐0.44 ; (P < .001) [Factor 1 (pleasure) ‐0.56; (P < .001) Factor 2 (negative affect relief) ‐0.27 (P < .001)] |
Baseline to wk 1: diff in changes between V&P: Smoking satisfaction: ‐0.44 (P < .001) Psych Reward: ‐0.32 (P < .001) Enjoy resp tract: ‐0.22 (P = 0.01) Craving reduction: ‐0.25 (P = 0.04) Aversion: 0 (P = 0.96) |
| Nides 2006 (wks 1 ‐ 7) 1.0 mg bid vs placebo |
Diff in mean change in: Urge to smoke: wk 1 ‐1.14; wk 2 ‐1.19; wk 3 ‐1.57; wk 4 ‐1.81; wk 5 ‐1.88; wk 6 ‐2.04; wk 7 ‐1.61(P < .001 for wks 1 ‐ 6, P < .01 wk 7) |
Total score: wk 1 ‐7.00; wk 2 ‐10.71; wk 3 ‐12.72; wk 4 ‐14.08; wk 5 ‐13.24; wk 6 ‐14.94; wk 7 ‐14.38 (wks 1, 3, 5 P < .001, wks 2, 4, 6, 7 P < .01) |
Baseline to wk 1: diff in changes between V&P: Smoking satisfaction: ‐1.62 Psych Reward: ‐0.35 Enjoy resp tract: ‐0.29 Craving reduction: ‐0.13 Aversion:‐0.79 |
| Oncken 2006 (MNWS: wks 1 ‐ 12; mCEQ wks 1 ‐ 7) 1.0 mg bid vs placebo |
Diff in mean change in Urge to Smoke score (extrapolated from graph): Wk 7: ‐0.2, Wk 12 ‐0.5; (P < .001 for both) |
Baseline to wk 7: diff in changes between V&P: (extrapolated from graph) Smoking satisfaction: ‐1.3 (P < 0.01) Psych reward: ‐2.2 (P < 0.001) Enjoy resp tract: ‐0.6 (P < 0.001) |
|
| Tonstad 2006 1.0 mg bid vs placebo |
Diff in mean change in Urge to Smoke score (extrapolated from graph): All participants: Wk 13: ‐0.35, Wk 25 ‐0.25; Abstainers only: Wk 13: ‐0.30, Wk 25 +0.02 |
|
|
| Tsai 2007 (wks 1 ‐ 6) 1.0 mg bid vs placebo |
Diff in mean change in Urge to Smoke: Wks 1 ‐ 6: ‐0.40 (P < 0.001) |
Mean total score, wks 1 ‐ 6: ‐0.39 (P < 0.001) |
Mean diff wks 1 ‐ 6: V vs P: Smoking satisfaction: ‐0.39 (P < 0.008) |
| Nakamura 2007 (wks 1 ‐ 7) 1.0 mg bid vs placebo (Nicotine‐dependent group only) |
Diff in mean change in: Urge to Smoke score: ‐0.51 (P < 0.001) Negative Affect score: ‐0.28 (P < 0.001) Restlessness score: ‐0.38 (P < 0.001) Appetite+ score: ‐0.09 (P = 0.481) Insomnia score: 0.56 (P = 0.380) |
Mean total score: ‐0.51 (P < 0.001) Factor 1 [pleasure] mean diff: ‐0.60 (P < 0.001) Factor 2 [negative affect] mean diff: ‐0.38 (P < 0.001) |
Mean diff wks 1 ‐ 7: V vs P: Smoking satisfaction: ‐0.74 (P < 0.001) Psych reward: ‐0.53 (P < 0.001) Enjoy resp tract: ‐1.00 (P < 0.001) Craving reduction: ‐0.45 (P < 0.001) Aversion:‐0.38 (P < 0.0007) |
|
Steinberg 2011 1.0 mg bid vs placebo |
At 4 wks, Varenicline group had score of ‐1.45, placebo +0.11 | ||
| Aubin 2008 (wks 1 ‐ 7) 1.0 mg bid vs NRT |
Diff in mean change in: Urge to Smoke score: ‐0.32 (P < 0.001); E.S. ‐0.37 Negative Affect score: ‐0.16 (P < 0.001); E.S. ‐0.21 Restlessness score: ‐0.20 (P < 0.001); E.S. ‐0.21 Appetite + score: 0.09 (P = 0.116); E.S. 0.12 Insomnia score: ‐0.07 (P = 0.207); E.S. ‐0.07 |
Mean diff wks 1 ‐ 7: V vs NRT: Smoking satisfaction: ‐0.54 (P < 0.001); E.S. ‐0.43 Psych reward: ‐0.32 (P = 0.001) E.S. ‐0.26 Enjoy resp tract: ‐0.39 (P < 0.001); E.S. ‐0.25 Craving reduction: ‐0.52 (P < 0.001); E.S. ‐0.32 Aversion: ‐0.07 (P = 0.436); E.S. 0.08 |
|
|
Niaura 2008 1 ‐ 4 x 0.5 mg ad lib |
Diff in Urge to smoke, all pts: Wk 1: ‐0.4; Wk 2: ‐0.4; Wk 3 ‐0.6; Wk 4 ‐0.5; Wk 5 ‐0.6; Wk 6 ‐0.5; Wk 7 ‐0.4; Wk 12 ‐0.6. Diff in withdrawal, all pts: Wk 1: ‐0.4; Wk 2: ‐0.7; Wk 3 ‐0.7; Wk 4 ‐1.1; Wk 5 ‐0.3; Wk 6 ‐0.4; Wk 7 ‐0.2; Wk 12 ‐0.9. |
Diff in changes between V&P: Smoking satisfaction: Enjoy resp tract: Wk 1 ‐0.1; Wk 2 ‐0.3; Wk 3 ‐0.4; Wk 4 ‐0.5; Wk 5 ‐0.5; Wk 6 ‐0.5; Wk 7 ‐0.4 |
|
|
Tsukahara 2010 1.0 mg bid vs NRT |
Diff in withdrawal score (all symptoms), V vs NRT: Wk 2 2.36; Wk 4 0.64; Wk 8 0.78; Wk 12 0.08 | ||
| Cinciripini 2013 | Significantly higher reward score (∼ 3.8) in placebo relapsers than in varenicline relapsers (∼ 2.7; P = 0.01) (extrapolated from graph) |
||
| Evins 2014 | WSWS measured over 12 wks open‐label varenicline: Total score: Baseline:59.9 ‐ wk 12: 50.77 Urge to smoke: Baseline: 11.85 ‐ wk12: 8.2 Irritability: Baseline: 5.62 ‐ wk 12: 4.46 Depression: Baseline: 6.61 ‐ wk 12: 5.8 Increased appetite: Baseline: 11.79 ‐ wk 12: 11.88 Difficulty concentrating: Baseline: 5.86 ‐ wk 12: 5.15 Insomnia: Baseline: 8.52 ‐ wk 12: 8.25 Anxiety: Baseline: 8.84 ‐ wk 12: 7.04 |
||
| Tønnesen 2013 | For V vs placebo at 12 wks: Craving: ‐0.26 for V (P < 0.0001) Appetite: ‐0.14 for V (P = 0.001) Total symptoms score: ‐0.16 (P = 0.002) |
||
| Hajek 2015 | Smoking enjoyment ratings up to TQD: Baseline/pre‐randomizarion: V: 2.5, placebo: 2.5 Day 15 (≤ 3 mg/day): V: 1.8, placebo: 2.1 Day 18 (≤ 4 mg/day): V: 1.7, placebo: 2.1 TQD (day 21; ≤ 5 mg/day): V: 1.6, placebo: 2.0 |
Appendix 4. Serious Adverse Events and deaths
| Study ID | Period | Placebo | Varenicline | Bupropion | NRT |
| VARENICLINE trials | |||||
| Anthenelli 2013 | During treatment or within 30 days of last dose | Intentional self injury; Depression with suicidal ideation; Agitation; Depression |
Psychotic disorder and depression; Suicidal ideation |
||
| Post‐treatment to week 52 | Intentional self injury 2 other SAEs |
2 deaths: overdose of clonazepam and morphine sulfate; Accidental fall 3 other SAEs |
|||
|
Aubin 2008 |
During treatment or within 30 days of last dose |
Depression*; Constipation |
Bile duct ca, sepsis; Gastrointestinal bleed; MI (2); Chest pain (2); Salivary gland tumour; Aggravation of old knee trauma |
||
| Acute ethanol intoxication; Suicidal ideation*; No deaths reported |
Abdominal cyst; No deaths reported |
||||
| Baker 2016 | Post‐treatment to week 52 | Hospitalised due to allergic reaction to varenicline | None reported | ||
| Bolliger 2011 |
During treatment or within 30 days of last dose |
Thyroid neoplasm; Appendicitis, peritonitis, diverticulitis; No deaths reported |
Abortion (possibly *); Hypersensitivity; Overdose; Bronchitis and asthma; Nasal septum deviation; Suicidal ideation + depressed mood; Suicidal ideation*; Tachycardia, bradychardia +dyspnoea; Panic attack; Injury; Appendicitis No deaths reported |
||
| Carson 2014 | During treatment |
(active control: counselling): Depressive episodes x 2; Agitation; 4 N‐STEMI (died); 2 lung ca (died) 1 stroke (died) |
Atrial fibrillation; Depressive episodes x 3; Aggression; 1 arrhythmia (died) 1 bradycardia (died) 2 respiratory/COPD (died) 2 N‐STEMI (died) |
||
| Post‐treatment to 52 weeks | Depressive episode | ||||
| Chengappa 2014 | During treatment | Alcohol intoxication; Exacerbation of asthma; Pregnancy; |
Exacerbation of anxiety; Rash; Agitation, hostility, alcohol abuse, drug abuse*; Hypoxia, asthma with COPD; Tremulousness, grogginess, left‐arm weakness; |
||
| Post‐treatment to 52 weeks | Chest pain, left‐hand numbness | Pneuomonia | |||
| Cincripini 2013 | Diabetes; Chest pain* |
Chest pain; Psychiatric hospitalisation |
Bilateral mammoplasty; Facial paralysis; Syncope* |
||
| EAGLES 2016 |
During treatment or within 30 days of last dose (Detail of SAES given in ClinicalTrials.gov posted results) |
16 (non‐psych) 25 (psych) 2 deaths (suicide, non‐psych; pulmonary embolism, psych) |
16 (non‐psych) 23 (psych) 2 deaths (heroin OD non‐psych; CV event, psych) |
19 (non‐psych) 29 (psych) |
21 (non‐psych) 24 (psych) |
| > 30 days after last dose | 2 deaths (RTA, non‐psych; MI, non‐psych) | 1 death (lung cancer, psych) | 3 deaths (prostate cancer, non‐psych; oesophageal adenoma, psych; sepsis, psych) | ||
| Ebbert 2015 | During treatment or within 30 days of last dose | 10 x suicidal ideation | 6 x suicidal ideation | ||
| Post‐treatment to week 52 | 18 other SAEs | 10 other SAEs | |||
| Eisenberg 2016 | Within 30 days of treatment | 3 MIs 5 unstable anginas Ischaemic cardiomyopathy Sick sinus syndrome Ruptured pseudoaneurysm Bowel obstruction COPD Rheumatoid arthritis Road traffic accident Melena Non‐cardiac chest pain Allergic reaction |
2 deaths (1 cardiac, 1 sudden death undefined) 3 MIs Unstable angina Depression Pulmonary embolism TIA Arrythmia and ICD implant 2 gastric bleeds 2 suspected unstable anginas (ruled out) Peroneal embolus with septic cellulitis Bowel surgery Dehydration Syncope Wound infection |
||
| Evins 2014 | Randomization phase | Sepsis (died); MI; Depressed mod and discontinuation of meds; Suicidal ideation; Worsened psychosis; Worsened psychosis and MJ intoxication; Manic symptoms |
Pancreatitis; Hyperglycaemia; Depressed mood; Worsened psychotic symptoms |
||
|
Gonzales 2006 |
During treatment or within 7 days of last dose |
Lung cancer; Acute MI; Acute exacerbation of schizophrenia; Chest pain (2); UTI Atrial fibrillation |
Abdominal pain; Atrial fibrillation*; Pneumonia; Possible stroke |
Cholecystitis, septic shock; Headache; Grand mal seizure* |
|
| Post‐treatment to week 52 | Mediastinal mass; Fall, fractured elbow, collapsed lung, death unexplained; I death (as above) |
Non‐cardiac chest pain; Acute appendicitis; No deaths reported |
Appendicitis; UTI; No deaths reported |
||
| Gonzales 2014 | During treatment or within 30 days of last dose | Acute coronary syndrome; Ligament rupture; Hyperventilation; Drug sensitivity |
Knee arthroplasty; pyelonephritis; Intervertebral disc protrusion; Ankle fracture; Chest pain*; Drug sensitivity to amoxicillin; Drug sensitivity to hair dye |
||
| Post‐treatment to week 52 | Acute on chronic alcoholism (died) | ||||
|
Jorenby 2006 |
During treatment or within 7 days of last dose |
Ruptured ovarian cyst; Ischaemic heart disease; Ruptured appendix; Pneumonia; Allergic reaction |
Lung or brain cancer; Acute coronary syndrome; Chest pain; Dehydration, periorbital cellulitis; Acute psychosis, emotional lability; Vertigo, raised BP, chest pain* |
Ectopic pregnancy; Angiodoema*; Gunshot wound; Post‐op bleeding; Lower Leg pain; Breast cancer (female) |
|
| Post‐treatment to week 52 | Appendicitis; No deaths reported |
Staphylococcal cellulitis; Acute psychosis; No deaths reported |
Occlusion coronary artery; Miscarriage; 1 death (RTA) |
||
| Nahvi 2014a | During treatment | Chest pain; Alcohol detoxification |
Hypoglycaemia; Alcohol and cocaine rehab; Knee replacement |
||
| Post‐treatment to 24 weeks | Total hip relacement | Alcohol rehab, acute cholecystitis; Asthma exacerbation |
|||
|
Nakamura 2007 |
During treatment | Subarachnoid haemorrhage; Contusion; Foot fracture |
0.25 mg bid: Gastroenteritis; Cholecystitis*; Gastric cancer; Cholecystitis, peritonitis; Herpes Zoster 0.5mg bid: Haemorrhoids, intestinal prolapse; Pituitary haemorrhage; 1 mg bid: Neurosensory deafness; Angina pectoris*, intervertebral disc protrusion, MS; 1 death (RTA) |
||
| NCT00828113 | During treatment | Road traffic accident | |||
| More than 30 days after treatment | Bladder surgery | ||||
|
Niaura 2008 |
Post‐treatment or within 30 days of last dose | None reported No deaths reported |
MI; Ventricular fibrillation; Spontaneous abortion; No deaths reported |
||
|
Nides 2006 |
During treatment | None reported No deaths reported |
Transient ischaemic attack*, transient loss of vision*; No deaths reported |
Syncope, possible seizure*; Grand mal convulsion*; Bloody diarrhoea*; No deaths reported |
|
|
Oncken 2006 |
During treatment | Syncope | Syncope; Duodenal ulcer; Cholesteatoma; Generalised tonic‐clonic seizure; Unstable angina; Paroxysmal supraventricular tachycardia; Cholelithiasis; Aseptic meningitis; Relapsing MS |
None reported | |
| Post‐treatment | Suicide attempt; No deaths reported |
Carcinoid colon cancer; Diabetes (> 30d post‐treat); No deaths reported |
None reported No deaths reported |
||
| Rennard 2011 | During treatment and within 30 days of last dose | Suicidal ideation | Intervertebral disc protrusion x 2; Carotid artery stenosis; Syncope; PAOD; Ureteric calculus |
||
|
Rigotti 2010 |
During treatment | Atrial fibrillation*; Congestive cardiac failure*; Chest pain*; Acute coronary syndrome*; + 17 others 1 death (unrelated) |
Chest pain*; MI*; Gingival bleeding*; CVA*; + 19 others |
||
| Post‐treatment to week 52 | 4 deaths (unrelated) | 2 deaths (unrelated) | |||
| Stein 2013 | During and post‐treatment | Rashes x 2 | Heart attack | ||
| Steinberg 2011 | During and post‐treatment to week 24 | 5 events (no detail) No deaths |
6 events (no detail) No deaths |
||
|
Tashkin 2010 |
During treatment or within 28 days of last dose | Bronchitis; CVA; Cholelithiasis; Lung cancer; Pneumonia; Musculoskeletal pain; Acute MI; Appendicitis; COPD; Chest pain; Anxiety |
Acute MI; Vocal cord polyp; Hyperkeratosis; Back pain; Angina pectoris; CVA; Cellulitis; |
||
| Post‐treatment | COPD; Pneumonia; Palpitations; Chest pain; 1 death (amyotrophic lateral sclerosis) |
L Ventricular dysfunction; Aortic valve stenosis; COPD; Chest pain; Laryngeal cancer; 2 deaths (1 cardiac, I RTA) |
|||
| Tønnesen 2013 | Post‐treatment | Rectal cancer with ileostomy, peritonitis; Gall stone; Malignant melanoma; Exacerbation of COPD |
Stroke; Severe constipation; Bradycardia; Cardiac arrest; Probable dengue fever |
||
|
Tonstad 2006 |
Open‐label phase (during treatment) | R Breast indeterminate path; Suicidal ideation; Menorrhagia; Glandular adenocarcinoma; Diminshed vision; Accidental injury; Headache; Abdominal pain*; Acute psychosis*; Acute pancreatitis; Neopharyngeal carcinoma; Raised AST, ALT, LDH, CPK; Grand mal convulsion; Atrial fibrillation; Ureteral stones; Persistent epistaxis; Worsening kidney stones; Loss of teeth, dislocated shoulder |
|||
| Open‐label phase (post‐treatment) | MI; Miscarriage |
||||
| Double‐blind phase (during treatment) | Increased dysmenorrhoea; Appendicitis; Spinal cord compression; Abdominal pain |
Injury to tibial artery; Uterine & bladder prolapse, suspected MI; Alcohol poisoning, costal fracture; Transient vision loss*; MI |
|||
| Double‐blind phase (post‐ treatment) | Acute cholecystitis; No deaths reported |
Colon cancer; Tumour; Ovarian tumour; Cerebral infarct, deep cerebral vein thrombosis; Supraventricular tachycardia; 3 deaths (unrelated) |
|||
| Tsai 2007 | During treatment or within 28 days of last dose | 3 RTAs; No deaths reported |
Unstable angina*; Acute pyelonephritis; Peritonitis, acute appendicitis; No deaths reported |
||
|
Tsukahara 2010 |
None reported | None reported | |||
|
Wang 2009 |
During treatment or within 7 days of last dose | Intestinal ulcer; 1 not described; No deaths reported |
None reported No deaths reported |
||
|
Williams 2007 |
Up to week 52 | Vertebral compression fracture; DVT, pulmonary embolism; Worsening coronary artery disease; No deaths reported |
Coronary artery disease; Herniated disc; Bilateral subcapsular cataracts* Chest pain, hypoglycaemia; Sinus bradycardia, hypotension, ventricular bigeminy, coronary angioplasty; Stroke; Cardiac catheterization; Tachycardia; Suspected GI bleed; Saphenous vein occlusion, ischaemia; MI, DVT; Ileus; Chest wall pain; Non‐cardiac chest pain, chronic bronchitis, pneumonia, chest pain; Spinal stenosis; No deaths reported |
||
| Williams 2012 | During treatment and within 30 days of last dose | Hyperglycaemia; Breast cancer; Aggression; Suicidal ideation; No deaths |
Chest pain; Convulsion; "Psychiatric symptom"; Suicidal ideation; Suicidal attempt; Asthma; 1 death |
||
| CYTISINE trials | |||||
| Scharfenberg 1971 | CYTISINE | Not reported | Not reported | ||
| Vinnikov 2008 | CYTISINE | None reported | None reported | ||
| Walker 2014 | 56 events in 45 people (see Table S5 in NEJM supplementary data) |
45 events in 39 people (see Table S5 in NEJM supplementary data) |
|||
| West 2011 | CYTISINE | COPD (died); Stroke (died); Lung cancer (died) |
Stroke; Tracheal cancer; Cardiac arrest (died); Lung cancer (died); |
||
| DIANICLINE trial | |||||
| Tonstad 2011 | DIANICLINE | Subileus; Thrombophlebitis; SVT; No deaths reported |
Appendicitis; Severe asthma; MI; No deaths reported |
||
|
* Possibly, probably or definitely attributable to study medication Abbreviations: ALT: Alanine transaminase; AST: Aspartate transaminase; BP: blood pressure; COPD: Chronic Obstructive Pulmonary Disease; CPK: Creatine phosphokinase; CVA: cerebrovascular accident; DVT: deep vein thrombosis; LDH: Lactate dehydrogenase; MI: myocardial infarction; MS: Multiple Sclerosis; N/STEMI: non‐ST‐elevation myocardial infarction; PAOD: peripheral arterial occlusive disease; RTA: road traffic accident; SVT: supraventricular tachycardia; UTI: urinary tract infection | |||||
Data and analyses
Comparison 1.
Cytisine vs placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CAR at longest follow‐up | 2 | 937 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.98 [2.01, 7.87] |
| 2 Point prevalence abstinence at 2 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 2.
Cytisine vs NRT
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Continuous abstinence at 6m | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 3.
Dianicline vs placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CAR at weeks 4 ‐ 26 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 4.
Varenicline (1.0 mg 2/d) vs placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Continuous or sustained abstinence at longest follow‐up (24+ weeks) | 27 | 12625 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.24 [2.06, 2.43] |
| 2 Abstinence at six months | 25 | 12304 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [2.08, 2.44] |
| 3 Abstinence for long‐term use (up to 52 weeks) of varenicline | 4 | 2170 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.64 [2.81, 4.72] |
Comparison 5.
Varenicline vs bupropion
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Varenicline vs bupropion at 6m | 5 | 5877 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.25, 1.54] |
| 2 Continuous abstinence at 52 weeks | 3 | 1618 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.22, 1.88] |
| 3 Varenicline vs bupropion at 3m | 5 | 5877 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.35, 1.58] |
Comparison 6.
Varenicline vs NRT
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Point prevalence abstinence at 24 weeks | 8 | 6264 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.14, 1.37] |
Comparison 7.
Variations in usage
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Flexible quit date | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Non‐standard dose varenicline versus placebo at 52 weeks | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Low‐dose varenicline vs placebo at 52 weeks | 4 | 1266 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [1.56, 2.78] |
| 2.2 Variable dosage at participant's or physician's discretion | 6 | 1789 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.29 [1.81, 2.89] |
| 3 Standard dose varenicline versus low dose at 52 weeks | 3 | 1079 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.00, 1.55] |
| 4 Standard dose varenicline versus high dose at 12 weeks | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.54, 1.44] |
| 5 Reducing to quit | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Varenicline as maintenance therapy (relapse prevention) to sustain quitting | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Abstinence at 52 weeks | 2 | 1295 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.08, 1.42] |
| 6.2 Abstinence at 24 weeks | 1 | 1210 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.29, 1.56] |
Comparison 8.
Varenicline in specific patient groups
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiovascular disease | 2 | 1006 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.44, 2.47] |
| 2 COPD | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Asthma | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Schizophrenia/bipolar/psychiatric disorder | 4 | 2332 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.82, 2.87] |
| 5 Depression | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Substance use disorder/methadone‐maintained at 24 weeks | 2 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.72 [0.50, 27.59] |
| 7 Alcohol‐dependent smokers | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8 Long‐term use of NRT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Analysis 8.7.
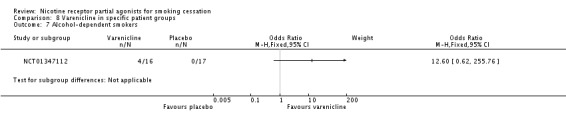
Comparison 8 Varenicline in specific patient groups, Outcome 7 Alcohol‐dependent smokers.
Comparison 9.
Varenicline in different settings/subgroups
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospital inpatients/perioperative patients | 3 | 744 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.09, 1.77] |
| 2 Smokers who have failed on other cessation therapies | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Light or heavy smokers | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 10.
Adverse event meta‐analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nausea | 32 | 14963 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [3.00, 3.55] |
| 2 Insomnia | 29 | 14447 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.35, 1.65] |
| 3 Abnormal dreams | 26 | 13682 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [1.88, 2.38] |
| 4 Headache | 25 | 13835 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.07, 1.29] |
| 5 Depression | 36 | 16189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.77, 1.14] |
| 6 Suicidal ideation | 24 | 11193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.43, 1.07] |
Comparison 11.
Serious adverse events
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SAEs in the varenicline trials | 29 | 15370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.04, 1.49] |
| 2 SAEs in the varenicline trials, exc post‐treat events | 26 | 15000 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.02, 1.52] |
| 3 Neuropsychiatric SAEs (not deaths) | 23 | 8955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.57, 1.19] |
| 4 Cardiac SAEs, including deaths | 21 | 8587 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.91, 2.04] |
Analysis 11.3.
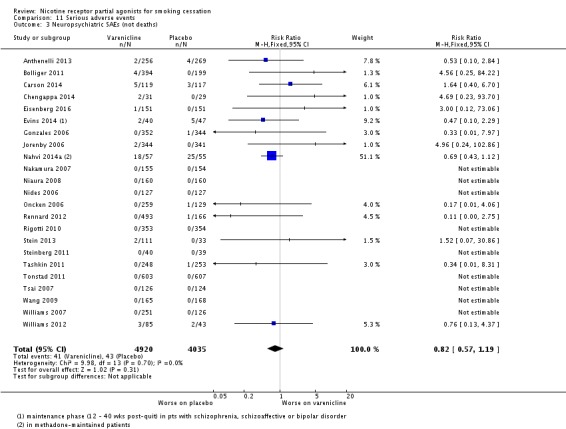
Comparison 11 Serious adverse events, Outcome 3 Neuropsychiatric SAEs (not deaths).
Comparison 12.
Losses to follow‐up
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants remaining at end of varenicline trials | Other data | No numeric data |
Comparison 13.
Sensitivity analysis
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ITT treatment vs per protocol control | 28 | 12422 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.65, 1.94] |
| 2 Continuous abstinence at 9 ‐ 12 weeks | 24 | 12339 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.49 [2.33, 2.65] |
| 3 Continuous abstinence at 24 weeks | 26 | 14016 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [2.26, 2.63] |
Analysis 13.1.
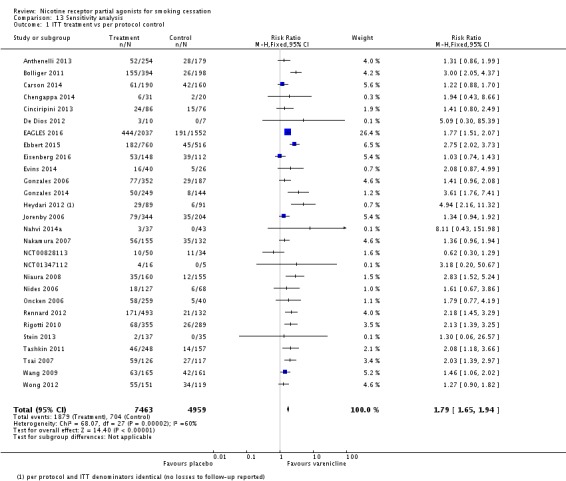
Comparison 13 Sensitivity analysis, Outcome 1 ITT treatment vs per protocol control.
Analysis 13.2.
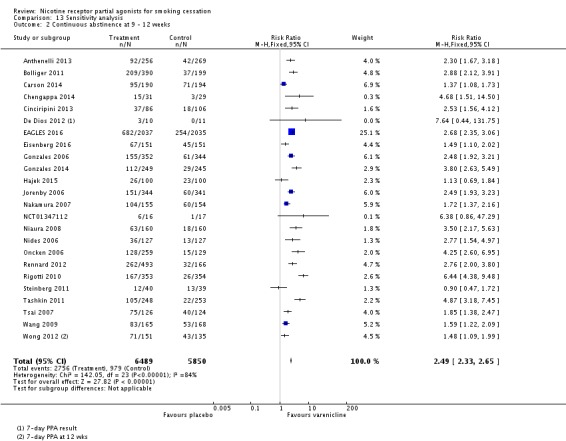
Comparison 13 Sensitivity analysis, Outcome 2 Continuous abstinence at 9 ‐ 12 weeks.
Analysis 13.3.
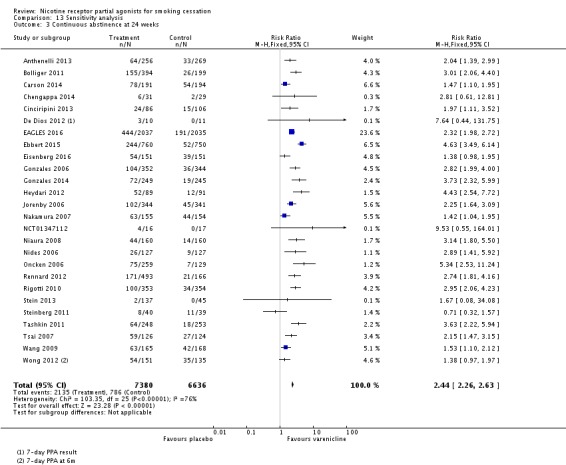
Comparison 13 Sensitivity analysis, Outcome 3 Continuous abstinence at 24 weeks.
What's new
Last assessed as up‐to‐date: 12 May 2015.
| Date | Event | Description |
|---|---|---|
| 31 January 2016 | New citation required and conclusions have changed | Additional comparisons. Analyses expanded and restructure. SAE information updated |
| 31 January 2016 | New search has been performed | 39 trials of varenicline now included |
History
Protocol first published: Issue 3, 2006 Review first published: Issue 1, 2007
| Date | Event | Description |
|---|---|---|
| 16 May 2013 | Amended | Minor change made to labelling on forest plot. |
| 14 March 2012 | New citation required and conclusions have changed | Safety profile modified, as new possible cardiovascular and psychiatric adverse events information incorporated. Efficacy findings unchanged but confirmed. |
| 14 March 2012 | New search has been performed | Seven new included studies (5 varenicline, 1 cytisine, 1 dianicline) and 14 new excluded studies added, plus safety data. |
| 13 January 2011 | Amended | Vinnikov trial of cytisine added to Studies awaiting Classification, for inclusion in next update. |
| 8 November 2010 | New search has been performed | Six new RCTs added; sources of funding added for all trials. Ongoing trials section expanded. |
| 8 November 2010 | New citation required and conclusions have changed | Surveillance data and secondary analyses do not support fears about safety. Efficacy conclusions strengthened but unchanged. |
| 17 July 2008 | Amended | Date of last search amended (2007 corrected to 2008); Source of support added. |
| 12 May 2008 | New citation required and conclusions have changed | Three new included trials, switch in the MA metric from OR to RR, updated background section and new safety information. |
| 15 March 2008 | New search has been performed | New search conducted. |
| 30 August 2007 | Amended | Converted to new review format. |
| 15 November 2006 | New citation required and conclusions have changed | Substantive amendment. |
Differences between protocol and review
For this 2016 update, we have restructured the analyses to accommodate increasing variation in the settings, populations, comparisons and regimens of trials that have been conducted.
For this 2016 update we include 'Summary of findings' tables for the main comparisons.
Characteristics of studies
Characteristics of included studies [author‐defined order]
Scharfenberg 1971
| Methods | Country: East Germany Aim: To test the efficacy of cytisine for smoking cessation Setting: smoking cessation clinic, Magdeburg, July‐December 1967 Study Design: double‐blind placebo‐controlled randomised trial Analysis: Chi squared test (P < 0.1) |
|
| Participants | 1214 smokers recruited from 1452 applicants through smoking clinics and via initial press releases. 88.2% M. 2.5% of participants smoked < 10 CPD, 42.4% 10 ‐ 20 CPD, 48.9% 21 ‐ 30 CPD, 5.2% > 30 CPD 40.4% had smoked > 20yrs. 40.6% had tried to quit at least once before. Randomised to cytisine (607) or placebo (607) Exclusion criteria not stated (214 volunteers excluded at initial screening) | |
| Interventions | 1. 20‐day course of cytisine. 1.5 mg tabs: Days 1 ‐ 3 6/day; days 4 ‐ 12 5/day; days 13 ‐ 16 4/day; days 17 ‐ 20 3/day. 2. Placebo tablets, same regimen Behavioural support: None | |
| Outcomes | Self‐reported abstinence at 4 wks, 6m and 2 yrs ITT analysis. Attrition rate 34% by longest follow‐up | |
| Treatment type | Medication: CYTISINE [TABEX] | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | "a numbered pouch" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | not stated |
| Selective reporting (reporting bias) | Unclear risk | not stated |
Vinnikov 2008
| Methods | Country: Kyrgyzstan Setting: Mining company (Kumtor Operating Company) Aim: To test the efficacy and safety of cytisine for smoking cessation in a workplace setting Study Design: Double‐blind placebo‐controlled parallel‐group RCT Analysis: Logistic regression used to assess influence of cytisine use, age, weight, CPD, smoking duration, previous quit attempts, FTND score and exhaled CO levels |
|
| Participants | 197 adult smokers, aged 20+, smoking at least 15 CPD, no prior use of cytisine, and motivated to quit Randomised to cytisine (100) or placebo (97). 26 (15 cytisine, 11 placebo) who took no medication were excluded from trial report 97% men, mean age 39, mean CPD 22, mean FTND 5.3, 86% had tried to quit previously; mean previous quit attempts 3.3 Exclusion criteria: Standard pharmacotherapy trial criteria |
|
| Interventions | Tabex tablets (1.5 mg cytisine): 1. First 3 days: 6 tabs per day; reduce smoking by half 2. Days 4 ‐ 12: 5 tabs per day; stop smoking completely 3. Days 13 ‐ 16: 4 tabs per day 4. Days 17 ‐ 20: 3 tabs per day 5. Days 21 ‐ 22: 2 tabs per day 6. Days 23 ‐ 25: 1 tab per day Placebo tablets, same regimen Treatment period was 25 days, with TQD Day 5. All participants received "behavior counselling" (no further detail) |
|
| Outcomes | Primary outcome: CO‐validated CAR from Day 5 to wk 8 Secondary outcome: CO‐validated CAR from Day 5 to wk 26 Validation was by expired CO ≤ 8ppm Other outcomes: Change in health‐related QoL measures, changes in body weight, adverse events, SAEs Attrition to 8 wks was 6 in cytisine group and 7 in placebo group; to 26 wks 10 in cytisine group and 16 in placebo group | |
| Treatment type | Medication: CYTISINE [TABEX] | |
| Notes | New for 2012 update Additional information supplied by the author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done by independent statistician in an Excel programme and the randomization key was kept by an independent person" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Nor patients neither investigators did not know where Tabex and where placebo were"; "follow‐up was blind". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 26 participants who did not take a single treatment dose were excluded from denominators by authors (restored to our MAs) |
| Selective reporting (reporting bias) | Low risk | Expected and predicted outcomes reported |
West 2011
| Methods | Country: Poland Setting: Smoking cessation clinic in Warsaw Aim: To test the efficacy and safety of cytisine for smoking cessation with minimal counselling and support. Dates conducted: December 2007 ‐ September 2010 Study Design: Single‐centre, double‐blind placebo‐controlled parallel‐group RCT Analysis: Power calculation (80%, alpha = 0.05, to detect a between‐group difference of 6 percentage points for primary outcome) |
|
| Participants | 740 healthy adults, smoking 10+ CPD, motivated to quit. Randomised to cytisine (370) or placebo (370) 46.5% men, mean age 48, mean CPD 23, prior quit attempts 82%, mean FTND 6.2 Exclusions were current psychiatric disorder or any medical condition contraindicated on cytisine label |
|
| Interventions | Tabex tablets (1.5 mg cytisine): 1. First 3 days: 6 tabs per day 2. Days 4 ‐ 12: 5 tabs per day 3. Days 13 ‐ 16: 4 tabs per day 4. Days 17 ‐ 20: 3 tabs per day 5. Days 21 ‐ 22: 2 tabs per day 6. Days 23 ‐ 25: 1 tab per day Placebo tablets, same regimen Treatment period was 25 days. Quitting advice, randomisation and drugs dispensed at baseline visit; phone calls at TQD + 1 wk later (+ optional clinic visit). Clinic visit 4 wks post‐TQD, then phone calls at 6m and 12m, with visit to confirm abstinence if claimed. Behavioural support was minimal, to simulate likelihood of real‐world conditions in countries where Tabex is available |
|
| Outcomes | Primary: CO‐validated abstinence 12m after end of treatment. Abstinence defined as smoking < 5 cigs during preceding 6m, and none in week before visit. Secondary outcomes: sustained CO‐validated abstinence at 6m follow‐up; 2‐wk PPA at 4 wks; 7‐day PPA at 12m Validation was expired CO < 10ppm Attrition: 79 (cytisine) and 89 (placebo) participants were lost to follow‐up over 12m. Drug discontinuation or reduction rates similar in both groups: 6.2% for cytisine and 4.6% for placebo Other outcomes: Adverse events, SAEs |
|
| Treatment type | Medication: CYTISINE [TABEX] | |
| Notes | New for 2012 update The trial was funded by the UK National Prevention Research Initiative, Cancer Research UK, and the National Institute for Health Research. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "performed by a statistician at Sopharma, who generated a list of study‐group assignments for 740 participants with nQuery Advisor software. Assignments were made in variable block sizes of either 20 (10 cytisine, 10 placebo) or 10 (5 and 5)" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Trial staff and participants were unaware of the group assignments and the randomization scheme" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts and attrition fully reported |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes reported |
Walker 2014
| Methods | Country: New Zealand Setting: National Quitline Aim: "a non‐inferiority trial to investigate whether cytisine was at least as effective as nicotine‐replacement‐therapy" Study Design: parallel‐group non‐inferiority RCT Dates conducted: March 2011 ‐ February 2013 Analysis: Power calculation (90%, 1‐tailed, alpha = 0.05) and assuming a 20% loss to follow‐up, to detect a 5% difference in 1‐month quit rates; cytisine 1‐month quit rate was assumed to be 55%, with a non‐inferiority margin of 5% |
|
| Participants | 1310 daily smokers, callers to the NZ National Quitline, aged 18+, motivated to quit. Allocated to cytisine (655) or to open‐label NRT (655). Mean age 38, 57% women, 33% NZ Maori, mean CPD 19, mean FTND 5.4 | |
| Interventions | All participants received standard Quitline support, i.e. average 3 x 10 ‐ 15‐minute calls over 8 wks 1. 25‐day course of cytisine (Tabex) tablets, + NRT vouchers in case they needed them AFTER completing the cytisine course 2. Usual care, i.e. 8‐week course of NRT (patch, gum or lozenge), tailored to dependence level, supplied by vouchers |
|
| Outcomes | Self‐reported CAR (5 cigarettes or fewer) at 1m CAR and 7‐day PPA (no smoking) at 1 wk, 1m, 2m and 6m. Adverse events Validation: None used |
|
| Treatment type | Medication: CYTISINE [TABEX] / NRT OPEN‐LABEL | |
| Notes | Funding by Health Research Council of New Zealand; cytisine supplied at no cost by Sopharma New for 2016 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated, by computer ... in a 1:1 ratio" |
| Allocation concealment (selection bias) | Low risk | "Randomization was stratified with the use of minimization according to sex, ethnicity (Maori, Pacific Islander, or non‐Maori and non–Pacific Islander), and cigarette dependence, which was determined by means of the Fagerström Test of Cigarette Dependence, in which smokers were assigned to one of two groups: those with scores of 5 or lower, indicating lower dependence, and those with scores greater than 5, indicating greater dependence" |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Participants and researchers collecting outcome data were aware of treatment allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses fully reported. By 6m, 182 cytisine participants (28%) lost to follow‐up, and 16 withdrawals; 173 NRT participants (26%) lost to follow‐up, and 14 withdrawals. 19 cytisine users crossed over to NRT, and 1 NRT user crossed over to cytisine. ITT analyses conducted |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Unclear risk | Cytisine was supplied free, while NRT users had to pay a nominal charge (NZD 3 for an 8‐wk course of each NRT item); Duration of treatment differed (25 days vs 8 wks), but 1º outcome set to 1m to counteract this |
Tonstad 2011
| Methods | Countries: France, Spain, Belgium, Sweden, Denmark, Norway Setting: 22 research centres Aim: To test the efficacy and safety of dianicline for smoking cessation Dates conducted: June 2006 ‐ June 2007 Study Design: Double‐blind placebo‐controlled parallel group RCT Study name: EURODIAN study Analysis: Power calculation (72% ‐ 99%, alpha = 0.05, for an OR of 2 ‐ 2.4, given a placebo quit rate of 7.5% ‐ 15%); ITT denominators used |
|
| Participants | 602 healthy adult volunteers, smoking 10+ CPD within previous 2m, aged 18+; allocated to dianicline (300), or placebo (302). 42% men, mean age 45, mean CPD 21, mean previous quit attempts 3.4, mean FTND score 5.75. Treatment groups were comparable at baseline Exclusion criteria: Standard pharmacotherapy trial criteria, plus any quit attempt in previous 3m, any use of bupropion, NRT, tobacco other than cigarettes 3+ times in previous 3m | |
| Interventions | 1. Dianicline 40 mg bid for 7 wks (not titrated). 2. Placebo inactive tablets, same regimen TQD was set for days 3 ‐ 7 following baseline visit All participants received standardised brief counselling (≤ 10 mins, based on Smoke‐Free and Living It) at each visit Weekly visits throughout wks 1 ‐ 7, then (for treatment completers) at wks 8, 10, 14, 18, 22 and 26 Smoking status and brief advice at each visit Participants completed smoking diaries |
|
| Outcomes | Primary outcome: CO‐confirmed CAR for wks 4 ‐ 7 Secondary outcomes: CO‐confirmed CAR at 26 wks. PPA wks 4 ‐ 7 Validation by expired CO < 10 ppm (all visits) and plasma cotinine ≤ 8μg/L (wks 4 and 7) Other outcomes: adverse events, SAEs; craving and withdrawal symptoms Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) 25.2% dianicline and 23% placebo participants did not complete the study. AE‐related dropouts were 4.3% dianicline and 7.6% placebo |
|
| Treatment type | Medication: DIANICLINE | |
| Notes | New for 2012 update The trial was funded by Sanofi‐Aventis. "The sponsor did not play a role in writing of the manuscript" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "a predefined, central, and computer‐generated randomization accessed through an Interactive Voice Response System assigned participants on a 1:1 ratio" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Participants and investigators were blinded to drug treatment assignments" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts fully reported |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes covered |
Anthenelli 2013
| Methods | Country: USA (9 centres) and international (24 centres, across Bosnia & Herzogovina, Croatia, Germany, Hungary, Romania, Russian Federation, Spain) Setting: Academic clinical trial centres and smoking cessation clinics Aim: To assess the efficacy and safety of 12 weeks of varenicline treatment or placebo for smoking cessation, with 40 weeks of non‐treatment follow‐up, in adults with current or past depression (MDD) Study Design: Double‐blind placebo‐controlled RCT Dates conducted: March 2010 ‐ June 2012 Analysis: Power calculation of 250 in each arm (80%, alpha = 0.05) to detect an OR of 2.35, assuming a placebo efficacy rate of 7% |
|
| Participants | 525 adult smokers, aged 18 ‐ 75, smoking at least 10 CPD, motivated to quit, diagnosed with unipolar MDD without psychotic features. 37% male, mean age 46, av CPD at baseline 22, mean FTND 5.9. Allocated to varenicline (256) or placebo (269) Exclusion criteria: Current or past diagnosis of dementia, schizophrenia, schizoaffective disorder, or other psychotic disorder, bipolar I disorder, bipolar II disorder. People with antisocial, schizotypal, or any other personality disorder severe enough to compromise their ability to comply with the study requirements Current use of either bupropion or nortryptiline |
|
| Interventions | 1. Varenicline 1 mg x 2/day, titrated for first wk 2. Placebo inactive tablets, same regimen All participants received manual‐guided SC support, telephone support and one‐to‐one 10‐minute counselling by the same person where possible. Participants in both groups could reduce the dosage if they wished TQD was set for wk 1 visit Treatment period was 12 wks. Visits at screening, baseline, weekly for wks 1 ‐ 12, and then at wks 13, 16, 24, 32, 40, 52 (or early termination); phone calls at wks 14, 20, 28, 36, 44 and 48. Weekly pill counts to assess adherence Safety data were reviewed regularly by an external independent data safety monitoring committee |
|
| Outcomes | Primary: CO‐confirmed CAR for wks 9 ‐ 12 Secondary: CO‐confirmed CAR for wks 9 ‐ 24, 9 ‐ 52; 7‐day PPA at wks 12, 24, 52; AEs and SAEs Verification: CO < 10 ppm |
|
| Treatment type | Medication: VARENICLINE | |
| Notes | New for 2016 update Funding by Pfizer; Dept of VA merit review award; NIAAA grant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible participants were randomly assigned to varenicline or placebo in a 1:1 ratio by using a computer generated, 4‐block randomization scheme at each site." |
| Allocation concealment (selection bias) | Low risk | "Randomization was stratified by antidepressant medication use at baseline (any vs. none) and baseline Montgomery–Åsberg Depression Rating Scale (MADRS) score (11 vs. 11) (32). Investigators obtained participant identification numbers and randomized study drug assignments by using a Web‐based or telephone call‐in computerized drug management system." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The study drug was supplied in blinded bottles by the sponsor to the study sites, where they were dispensed according to computerized instructions." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 68.4% of varenicline group completed study (lost 15.6% in treatment and 16% in follow‐up); 66.6% of placebo group completed study (lost 21.9% in treatment and 11.5% in follow‐up) |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None noted |
Aubin 2008
| Methods | Country: Belgium, France, Netherlands, UK, USA Setting: 24 research centres Aim: To compare the efficacy of varenicline with nicotine patch, both open‐label Dates conducted: January 2005 ‐ June 2006 Study design: Open‐label randomised trial Analysis: Power calculation (90%, alpha = 0.05) based on expected OR of 1.75 at wk 12; logistic regression model including terms for treatment, centre and country |
|
| Participants | Healthy adults, recruited from smoking cessation clinics or by local advertising, aged 18 ‐ 75, weight > 45.5 kg, BMI 15 ‐ 38, smoking ≥ 15 CPD. Varenicline arm 378, NRT arm 379. Mean age 42.9, 49.2% men, 93% white. Mean CPD 22.7. Previous use of nicotine patch 47.4%, previous use of bupropion 20%. Mean FTND 5.5. Exclusion criteria: Standard pharmacotherapy trial criteria, + participants must not have been in a varenicline trial in previous year, or used NRT in previous 6m | |
| Interventions | 1. Varenicline 1mg x 2/day for 12 wks, titrated 1st wk 2. Nicotine patch (21 mg wks 2 ‐ 6, 14 mg wks 7 ‐ 9, 7 mg wks 10 ‐ 11) No placebo control group All participants received Clearing the Air S‐H booklet at baseline, and brief counselling (≤ 10 mins) at each clinic visit or by phone. TQD was at wk 1 visit. Weekly visits throughout treatment phase, plus a phone call 3 days post‐TQD In follow‐up phase, clinic visits at wks 13, 16, 24, 32, 40, 48 and 52, plus brief phone calls at wks 14, 20, 28, 36 and 44 | |
| Outcomes | CO‐confirmed CAR for last 4 wks treatment (varenicline wks 9 ‐ 12, NRT wks 8 ‐ 11) CO‐confirmed CAR at wks 9 ‐ 24 and 9 ‐ 52 (varenicline) and 8 ‐ 24 and 8 ‐ 52 (NRT) 7‐day PPA at EoT and at wks 24 and 52 Other outcomes: Weight change, withdrawal symptoms (using MNWS and mCEQ), adverse events Validation was by expired CO ≤ 10 ppm Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) Attrition in treatment phase was 17.3% varenicline, 20.3% NRT. Losses to follow‐up 17% in each group 65.7% of varenicline and 62.2% of NRT groups completed study | |
| Treatment type | Medication: VARENICLINE / NRT OPEN‐LABEL | |
| Notes | The trial was funded by Pfizer Inc New for 2008 update Denominator used in trial report is all treated (V 376, Pl 370). We have used all randomised [378/379], which tips the RR into statistical significance Not included in main MA, as no placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a central computer‐generated sequence" |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Using an open‐label design" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Missing CO data were assumed to be < 10 ppm provided other conditions were met", i.e. no NRT other than prescribed patches. Missing = negative assumption reduced successes by 1 in each group |
| Selective reporting (reporting bias) | Low risk | All predicted outcomes fully reported, + analysis by country and treatment centre |
| Other bias | Unclear risk | Different duration of regimens, but effect sizes similar in last 4 wks of each course |
Baker 2016
| Methods | Country: USA Setting: 2 University sites in Wisconsin (Madison, Milwaukee) Aim: To compare the efficacy of varenicline with nicotine patch, and with combination NRT (C‐NRT) Dates conducted: May 2012 ‐ November 2015 Study design: Open‐label randomised trial (no placebo) Analysis: Logistic regression, comparing varenicline and C‐NRT arms against nicotine patch (reference) arm. Power calculations based on detecting a 10% difference, with > 80% power; numbers required: patch 227, varenicline and C‐NRT 387 |
|
| Participants | Healthy adults, recruited from participants in the ongoing Wisconsin Smokers Health Study or by media and community outreach, aged 17+, smoking ≥ 5 CPD, motivated to quit. Varenicline arm 424, nicotine patch arm 241, combination NRT arm 421 Mean age 48.1, 47.9% men, 67% white. Mean CPD 17. Mean FTND 4.8 Exclusion criteria: Standard pharmacotherapy trial criteria, CO < 4 ppm, no suicide attempts in previous 5 years, or current suicidal ideation,diagnosis or treatment of psychoses in previous 10 years |
|
| Interventions | 1. Varenicline 1mg x 2/day for 12 wks, titrated 1st wk 2. Nicotine patch: 11+ CPD on 21 mg wks 1 ‐ 8, 14 mg wks 9 ‐ 10, 7 mg wks 11 ‐ 12; 5 ‐ 10 CPD on 14 mg wks 1 ‐ 10, 7 mg wks 11 ‐ 12 3. Nicotine patch as for (2), plus nicotine lozenge (2 mg or 4 mg), at least 5 times a day for 12 wks No placebo control group. All participants received counselling (20 mins at visits 1, 2 and 3, and 10 mins by phone and at visits 4, 5) at 1 week pre‐TQD and at TQD, wks 1, 4, 12 post‐TQD, plus phone call at wk 8 In follow‐up phase, participants were contacted at wks 26 and 52 by phone | |
| Outcomes | All comparisons were based on varenicline and C‐NRT versus patch (reference arm), and on varenicline versus C‐NRT CO‐confirmed PPA at wk 26 CO‐confirmed PA from day 7 post‐TQD to day 181 CO‐confirmed PPA at wks 4, 12, 52 Other outcomes: Adherence, withdrawals, adverse events Validation was by expired CO ≤ 9 ppm and ≤ 5 ppm Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) Withdrawal rates were 8.3% varenicline, 6.2% nicotine patch, 3.1% C‐NRT |
|
| Treatment type | Medication: VARENICLINE / NRT OPEN‐LABEL | |
| Notes | The trial was funded by grant 5R01HL109031 from National Heart, Lung, and Blood Institute, and by grant K05CA139871 from the National Cancer Insitute New for 2016 update Not included in the main MA, as no placebo group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐based randomisation" |
| Allocation concealment (selection bias) | High risk | "Treatment assignment was unblinded" [open‐label] |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Treatment assignment was unblinded" [open‐label]. "The follow‐up telephone assessments were intended to be blinded, but a database search by interviewers could have revealed treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low rates of attrition, ITT analysis used |
| Selective reporting (reporting bias) | Low risk | All predicted outcomes reported, protocol available |
| Other bias | Low risk | None noted |
Bolliger 2011
| Methods | Countries: Brazil, Colombia, Costa Rica, Egypt, Jordan, Lebanon, Mexico, Saudi Arabia, South Africa, United Arab Emirates, Venezuela Setting: 42 research centres (51.2% Latin America, 30.6% Africa, 18.2% Middle East) Aim: To test the efficacy and tolerability of varenicline in regions not previously exposed to smoking cessation RCTs of varenicline Dates conducted: April 2008 ‐ August 2009 Study Design: Double‐blind placebo‐controlled RCT Analysis: Power calculation (90%, alpha = 0.05); ITT denominators and logistic regression analysis (step‐down procedure) |
|
| Participants | 593 adults, recruited from smoking cessation clinics, aged 18 ‐ 75, weight > 45.5 kg, BMI 15 ‐ 38, smoking ≥ 10 CPD, motivated to quit. Randomised to varenicline 394 (390 got medication), or placebo 199 (198 got medication). Mean age 43.5, 63.6% men, mean CPD 23.8, mean FTND 6.0. 55% had no prior quit attempt Exclusion criteria: Standard pharmacotherapy trial criteria, + participants must not have used NRT, bupropion, clonidine or nortriptyline in previous 6m | |
| Interventions | 1. Varenicline 1mg x 2/day, titrated during wk 1 2. Placebo inactive tablets, same regimen Treatment period was 12 wks. All participants received You can quit smoking self‐help booklet (available in English, Spanish, Portugese and Arabic) at baseline, and brief counselling (≤ 10 mins) at each clinic or telephone contact. TQD set for wk 1. Clinic visits at wks 2, 3, 4, 6, 8, 10 and 12 throughout treatment phase, plus a phone call 3 days post‐TQD In follow‐up phase, clinic visits at wks 13, 16, 20 and 24, plus brief phone calls at wks 14, 18 and 22 | |
| Outcomes | Primary outcome: CO‐validated CAR at 9 ‐12 wks Secondary outcomes: CO‐validated CAR at 9 ‐ 24 wks; 7‐day PPA at wks 12 and 24 Other outcomes: Adverse events, clinically significant changes in vital signs, SAEs. Abstinence was assessed using the Nicotine‐Use Inventory (NUI); validation was by expired CO ≤ 10 ppm Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis). [4 (V) 1 (P) who did not receive allocated intervention reincluded in denominators in this analysis.] Attrition in treatment phase was 11.2% (V) and 20.6% (P); in follow‐up phase 2.5% (V) and 0.5% (P) | |
| Treatment type | Medication: VARENICLINE | |
| Notes | New for 2012 update The study was funded and managed by Pfizer Inc |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a block randomization within each site, eligible participants were randomly assigned in a 2:1 ratio to receive varenicline or placebo" |
| Allocation concealment (selection bias) | Low risk | "a web‐based or telephone call‐in drug management system directed by the sponsor" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "All of the study personnel and participants were blinded to treatment assignment until the end of the nontreatment follow‐up phase" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts and attrition fully reported |
| Selective reporting (reporting bias) | Low risk | All predicted and expected outcomes reported |
Carson 2014
| Methods | Country: Australia Setting: Respiratory, cardiology, neurology, vascular and general medicine wards of 3 Adelaide (South Australia) hospitals Aim: To evaluate efficacy and safety of varenicline + quitline counselling vs quitline counselling alone in people admitted with smoking‐related acute illnesses Study Design: Phase II/III open‐label single‐blind RCT Dates conducted: August 2008 ‐ December 2011 Power calculation: 196 participants per arm, based on a 15% difference (45% vs 30%) at 52 wks, giving 80% power, 0.05 2‐sided significance Study name: Smoking Termination Opportunity for inPatients (STOP) |
|
| Participants | 392 adult smokers, aged 18 ‐ 75, smoking 10 CPD+, willing to quit, admitted with acute smoking‐related illnesses; randomised to varenicline + counselling (196) or counselling alone (196) Mean age 53, 32% women, 96% white, mean CPD 25, mean FTND 5.6, mean baseline LoS 6.5 days Exclusion criteria: Standard pharmacotherapy criteria, acute or pre‐existing psychiatric illness, history of psychosis or suicidal ideation, use of varenicline in past 12m |
|
| Interventions | 1. Varenicline 1.0 mg x 2/d for 12 wks, including wk 1 at titrated dose (described as standard MIMS dosing schedule), + counselling 2. Counselling only Both groups received Quit SA 5A behavioural counselling, i.e. maximum of 8 calls over 3m. Also booklet Quit because you can, + stickers and fridge magnets. Participants had to set a TQD within 1st 2 wks Contacts were attempted with all participants at days 3 and 5, wks 1, 2, 3, 4, 12 (EoT). Additional contacts at wks 26 and 52 | |
| Outcomes | Primary outcome: Self‐reported CAR (< 5 cigs in total) (2 wks ‐ 12m) Secondary outcomes: CAR at 4, 12 and 26 wks. 7‐day PPA each week for 1st 4 wks; craving; prevalence of I/P smoking; Reduced hospital bed utilisation; Reduction in healthcare costs CO validation ≤ 10 ppm used only in "a random sub‐set of subjects" |
|
| Treatment type | Medication: VARENICLINE | |
| Notes | Partially funded by the Department of Respiratory Medicine, Queen Elizabeth Hospital, Adelaide, SA; information based on unpublished data supplied by authors, + published 2014 study report New for 2012 update (study ID was Smith 2012; changed for 2015 update) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A pre‐defined, central, computer‐generated randomization sequence was used to assign subjects in a 1:1 ratio to either 12 weeks of treatment with varenicline plus Quitline‐counseling or 12 weeks of Quitline‐counseling alone." |
| Allocation concealment (selection bias) | Low risk | "using opaque, sealed envelopes with consecutive numbers" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Open‐label design. Attempt at single‐blinding (statistical investigator). "Participants and investigators were not blinded to treatment assignment". "Randomization and allocation concealment were performed by respiratory staff independent of the study" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Missing data from questionnaire (e.g., a question missed when administering follow‐up) were randomly imputed via a computer programme" 84% varenicline completed the study at 52 wks, vs 82% in the placebo group |
| Selective reporting (reporting bias) | Unclear risk | None noted |
| Other bias | Unclear risk | None noted |
Chengappa 2014
| Methods | Country: Pittsburgh, USA Setting: 2 outpatient clinics, Western Psychiatric Institute and Clinic; and Dubois Medical Regional Center, Pennsylvania Aim: To assess the efficacy and safety of varenicline to assist in smoking cessation among patients with bipolar disorder who were euthymic and motivated to quit smoking Study Design: Double‐blind placebo‐controlled RCT Dates conducted: February 2010 ‐ March 2013 Analysis: Power calculation of 60 in each arm Randomised placebo‐controlled quadruple‐blind trial |
|
| Participants | 60 outpatient smokers with DSMIV‐diagnosed bipolar disorder, aged 18 ‐ 65, stable state or on medication, willing to quit in the next 30 days, 10+ CPD; randomised to varenicline (31) or placebo (29) Mean age 46, 69% women, 66% white, mean CPD 18.1, mean FTND 6.2 Exclusions: Bupropion use (for SC); usual pharmacological criteria |
|
| Interventions | 1. Varenicline 1 mg x 2/day, titrated for first wk 2. Placebo inactive tablets, same regimen All participants received 15‐minute SC counselling at each visit. CO tested and pill counts at each visit. Participants in both groups could reduce the dosage if they wished. TQD was set for wk 2 onwards (i.e. full dosage reached) Treatment period was 12 wks. Weekly pill counts to assess adherence Safety data were reviewed blind monthly by an external independent data safety and monitoring board (DSMB) |
|
| Outcomes | Primary: 7‐day PPA, CO‐verified, at 12 wks Secondary outcomes: 7‐day PPA at 24 wks; CA at 12, 24 wks Validation: CO < 10 ppm |
|
| Treatment type | Medication: VARENICLINE | |
| Notes | New for 2016 update Funding from the National Insitute of Mental Health, and Pfizer |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated, other than "stratified by gender" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The treatment assignment was blinded to participating subjects, raters, investigators and statisticians" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 24 participants in each group completed treatment phase, and 24 (77%) and 20 (69%) completed full study in varenicline and placebo groups respectively Data were analysed using ITT with LOCF |
| Selective reporting (reporting bias) | Unclear risk | None noted |
| Other bias | Unclear risk | 8 participants (4 in each arm) were on bupropion for depression; 3/15 varenicline quitters and 1/3 placebo quitters were on long‐term bupropion |
Cinciripini 2013
| Methods | Country: Houston, TX, USA Setting: University of Texas MD Anderson Cancer Center Aim: To assess the relative efficacy of varenicline and bupropion SR plus intensive counselling on smoking cessation and emotional functioning Study Design: Double‐blind placebo‐controlled RCT Dates conducted: August 2006 ‐ October 2007 Analysis: "our sample size provided adequate power for assessing our primary outcome of prolonged abstinence at EOT (ie, ß= 0.99 for differences relative to placebo for varenicline and ß = 0.84 for differences relative to placebo for bupropion SR) but modest power for detecting drug group differences ( ß = 0.74)." |
|
| Participants | 294 volunteer smokers, aged 18 ‐ 65, 5+ CPD, fluent in English, no uncontrolled chronic illness, baseline CO > 6 ppm. Mean age 44, 39% women, 66% white, mean CPD 20, mean FTND 4.5, mean baseline CO 24.5 ppm. Allocated to varenicline (86), bupropion (102) or placebo (106) Exclusions: Usual pharma exclusions, current or history of psychotic disorder, moderate or high risk of suicidality, contra‐indications to varenicline or bupropion |
|
| Interventions | 1. Varenicline: 12‐week course (1 mg x 2/day) + non‐active bupropion course (placebo) 2. Bupropion: 12‐week course (150 mg x 2/day) + non‐active varenicline course (placebo) 3. Placebo: 12‐week course (placebo pill x 2/day) Blinded study physician could adjust dosages to reduce side effects if required throughout study All participants got intensive counselling, i.e. 6 x in‐person 30‐minute individual counselling sessions and 4 x 15‐minute phone calls during treatment phase, based on MI techniques. During follow‐up, each participant got a 15‐minute in‐person visit at 3m and 6m, and a 15‐minute phone call at 4m |
|
| Outcomes | Primary: PA at EoT Secondary: PA at 3m post‐quit, 6m post‐quit; CA at 3m post‐quit, 6m post‐quit; 7‐day PPA at EoT, 3m post‐quit, 6m post‐quit Validation: CO < 10 ppm. Self‐reported abstainers were asked to send a salivary cotinine sample (< 15 ng/mL) by post |
|
| Treatment type | Medication: VARENICLINE and BUPROPION | |
| Notes | New for 2016 update Funding: NIDA grant DA017073, NCI grant P50CA70907; varenicline supplied by Pfizer |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Adaptive randomization (minimization) was used to stratify the groups for sex, race/ethnicity, history of depression, and baseline smoking rate." |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study physician was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to treatment and follow‐up reported, and key variables with significant differences (FTND, years of education) identified between those who stayed in and those who left. ITT analysis conducted. By 6m, 21/86 for varenicline (24.4%), 29/102 for bupropion (28.4%) and 30/106 for placebo (28.3%) had been lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | None noted |
| Other bias | Unclear risk | Study began as nortriptyline vs bupropion; 3 months later, 19 people had been recruited to bupropion and 18 to placebo; nortriptyline was replaced as the target treatment by varenicline. The nortriptyline phase group (cohort 1) had 19 days of medication and 3 counselling sessions before TQD, whereas varenicline phase group (cohort 2) had 12 days of medication and 2 counselling sessions before TQD. No differences were found between the 2 cohorts, nor between overall findings and cohort 2 findings. Authors therefore combined both groups into a single study cohort for analysis. |
De Dios 2012
| Methods | Country: Rhode Island and Massachussetts, USA Setting: Butler Hospital, RI Aim: To assess the relative efficacy of varenicline and NRT on smoking cessation in Latino light smokers (< 10 CPD) Study Design: Feasibility double‐blind placebo‐controlled 3‐arm RCT Dates conducted: April 2010 ‐ July 2010 Analysis: No power calculation, as this was a pilot study with small sample size |
|
| Participants | 32 Latino volunteer light smokers (≤ 10 CPD), aged 18+, willing to set a quit date. Mean age 42, 53.1% women, mean CPD 7.6, mean FTND 2.9. Allocated to varenicline (10), NRT (11), placebo (11) Exclusions: Usual pharmacological conditions, on NRT or smokeless tobacco, history of suicide attempts, chronic or acute psychiatric disorder, employed as a pilot, driver or heavy machinery operator |
|
| Interventions | 1. Varenicline 12‐wk treatment course, titrated 1st wk 2. NRT 24‐hour patch: 12 wks: 4 wks @ 14 mg, 8 wks @ 7 mg 3. Varenicline‐placebo, i.e. identical tablet, same regimen All participants received a 30‐minute face‐to‐face "culturally informed" smoking cessation behavioural intervention, + a non‐tailored self‐help brochure, all available in both English and Spanish. All participants were compensated for attendance, and could receive travel vouchers if necessary |
|
| Outcomes | Primary: 7‐day PPA at 6m Secondary: 7‐day PPA at wks 1, 2, 1m, 2m, 3m, 4m; adherence Validation: CO < 5 ppm; salivary cotinine (not for the NRT group) > 10 mg/nL Adverse events not reported in detail, although study reports that "There was no pattern that suggested a higher side‐effect profile for those in the varenicline group" |
|
| Treatment type | Medication: VARENICLINE / NRT | |
| Notes | New for 2016 update Funding: NCI grant R01CA0129226‐S1 (De Dios); NIDA grant K24‐DA000512 (Stein); NIDA grant R01‐DA1234, NCI grant K07‐CA95623 (Stanton) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Study personnel and participants in the two‐pill groups (varenicline and varenicline‐placebo) were blinded to treatment condition. The research pharmacy maintained the study blind." NRT group could not be blinded to treatment; outcome assessment blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were fully reported; per protocol and ITT analyses conducted |
| Selective reporting (reporting bias) | Unclear risk | None noted |
| Other bias | Unclear risk | None noted |
EAGLES 2016
| Methods | Countries: Argentina, Australia, Brazil, Bulgaria, Canada, Chile, Denmark, Finland, Germany, Mexico, New Zealand, Russian Federation, Slovakiam South Africa, Spain, USA Setting: multiple research centres Aim: To evaluate the efficacy of varenicline, bupropion SR, nicotine patch and placebo for smoking cessation, and to assess how far this is moderated by the presence of psychiatric disorders Dates conducted: November 2011 ‐ January 2015 Study Design: Phase 4 triple‐dummy, double‐blind placebo‐controlled parallel‐group RCT Study name: EAGLES (Evaluating Global Events in a Global Smoking Cessation Study) |
|
| Participants | Treatment‐seeking adult smokers, aged 18 ‐ 75, smoking at least 10 CPD, with exhaled CO > 10 ppm at screening. Participants in the psychiatric disorder cohort had to have a current or lifetime stable psychiatric diagnosis, confirmed by Structured Clinical Interview for DSM IV disorders (SCID), i.e. no acute exacerbation in the previous 6 months, no changes to treatment for 3 months, not imminently likely to change treatment, and not at risk of self harm. Allocation for the pyshiatric cohort was balanced across four diagnostic group disorders, i.e. mood, anxiety, psychotic, personality 44% men, mean age 46, mean CPD 20.7, mean FTND 5.8 Exclusions: Past or current diagnosis of schizophreniform or delusional disorders, all delirium, dementia, and other cognitive disorders, and all substance‐induced disorders (other than nicotine) In the psychiatric disorders group, 70% had primary affective disorders, 19% anxiety disorders, 9.5% psychotic disorders, 0.6% personality disorders, and at least ⅓ were taking psychotropic medications Participants were grouped by the presence (4116) or absence (4028) of a history of psychiatric disorders Psychiatric disorders: varenicline 1032; bupropion 1033, NRT patch 1025, placebo 1026 No psychiatric disorders: varenicline 1005; bupropion 1001, NRT patch 1013, placebo 1009 Safety analyses were conducted in cohorts of 4074 (psychiatric) and 3984 (non‐psychiatric) |
|
| Interventions | 1. Varenicline, 1 mg x 2/day (1 wk titrated, then 11 weeks full dose) 2. Bupropion SR, 150 mg x 2/day (titrated for 3 days, then full dose for 11 weeks) 3. Nicotine patch, 21 mg x 7 weeks, 14 mg x 2 wks, 7 mg x 2 weeks (11 weeks) 4. Triple‐dummy placebo for each arm of the trial (12 weeks) All participants received counselling (up to 10 mins) at all contacts, and were encouraged to complete all visits even if treatment was discontinued Participants were monitored at weeks 1 ‐ 6, 8, 12, 13, 16, 20, 24; contacts were up to 15 face‐to‐face visits and 11 telephone visits |
|
| Outcomes | At least 1 SAE of anxiety depression, feeling abnormal, or hostility, and/or moderate or severe AE of agitation, aggression, delusions, hallucinations, homicidal ideation, mania, panic paranoia, psychosis, suicidal ideation/behaviour/completed 4‐week abstinence confirmed by CO < 10 ppm at wks 9 ‐ 12, and 15‐wk abstinence at weeks 9 ‐ 24 In the non‐psychiatric cohort, 78.9% completed treatment, and 78.4% completed the study In the psychiatric cohort, 74.2% completed treatment, and 77.8% completed the study |
|
| Treatment type | VARENICLINE / BUPROPION / NRT | |
| Notes | Trial was funded by Pfizer and GlaxoSmithKline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomisation schedule ... using a block size of 8 (1:1:1:1 ratio) for each of the 20 diagnosis by region combinations" |
| Allocation concealment (selection bias) | Low risk | "Investigators obtained participant identification numbers via a web‐based or telephone call‐in drug management system" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Study product kit codes did not allow deciphering of randomised treatment or block size. As such, participants, investigators, and research personnel were masked to treatment assignment" "The triple dummy design feature required participants to take study medication as masked tablets dispensed in separate varenicline and bupropion pill bottles each with matching placebo along with with either applying active or placebo patches on a daily basis" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All losses fully accounted for; ITT analysis conducted throughout |
| Selective reporting (reporting bias) | Low risk | All protocol‐reported outcomes were addressed |
| Other bias | Low risk | None noted |
Ebbert 2015
| Methods | Country: 65 centres in 10 countries: USA (14), Australia (4), Canada (6), Czech Republic (7), Egypt (3), Germany (7), Japan (6), Mexico (4), Taiwan (7), UK (7) Setting: Clinics, hospitals, academic research centres Aim: To determine the efficacy and safety of varenicline for increasing smoking abstinence rates through smoking reduction Study Design: Double‐blind placebo‐controlled multinational RCT Study name: Reduce to Quit Dates conducted: July 2011 ‐ July 2013 Analysis: "A sample size of 1404 randomized participants in a 1:1 ratio (702 in each group) was estimated to provide 90% or more power to detect a difference between varenicline and placebo of 10.3% in the primary end point of CAR during weeks 15 through 24, assuming a CAR of 17.2% for varenicline and 6.9% for placebo using a 2‐group, continuity–corrected, 2‐sided χ² test. A P value of .05 or less was considered significant" |
|
| Participants | 1510 adult smokers, unwilling to quit abruptly (within the next month), aged 18+, smoking mean 10+ CPD, interested in trying to quit within 3 months. Mean age 44.5, 43.7% women, mean CPD 20.7, mean FTND 5.5. Allocated to varenicline (760) or placebo (750) Exclusions: suicidal behaviour in previous 2 years or history of suicide attempts; major depression, anxiety; diagnosis of psychosis, panic disorder, PTSD, schizophrenia |
|
| Interventions | 1. Varenicline 24 wks, titrated 1st wk (12 wks to quit + 12 wks post‐quit) 2. Placebo 24 wks, titrated 1st wk (12 wks to quit + 12 wks post‐quit) All participants asked to reduce their smoking rate by 50% by wk 4, by 75%+ by wk 8, and 100% by wk 12. Individual 10‐minute counselling at each visit (18 face‐to‐face and 10 phone calls), + a copy of Clearing the air: quit smoking today. |
|
| Outcomes | Primary: CAR at wks 15 ‐ 24 Secondary: CAR at wks 21 ‐ 24, 15 ‐ 52, 21 ‐ 52; 7‐day PPA at wks 24, 52 Validation: CO < 10 ppm |
|
| Treatment type | Medication: VARENICLINE | |
| Notes | New for 2016 update Funding: Pfizer |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomized to receive varenicline or placebo for 24 weeks of treatment in a 1:1 ratio using a computer generated block randomization schedule within site" |
| Allocation concealment (selection bias) | Low risk | "Investigators obtained participant identification numbers and treatment group assignments through a web‐based or telephone call‐in drug management system" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Participants, investigators, and research personnel were blinded to randomization until after the database was locked" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses fully reported. ITT analyses conducted for efficacy (760 varenicline, 750 placebo), and treated denominators for safety outcomes (751 varenicline, 742 placebo) |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None noted |
Eisenberg 2016
| Methods | Country: 40 centres in USA and Canada Setting: Hospitals Aim: To determine the efficacy and safety of varenicline for increasing smoking abstinence rates through smoking reduction Study Design: Double‐blind placebo‐controlled multicentre RCT Study name: Evaluation of varenicline in smoking cessation for patients post‐acute coronary syndrome (EVITA) Dates conducted: not stated Analysis: "The sample size was estimated assuming a 7 day point prevalence abstinence rate of 24% at 24 weeks in patients receiving placebo.With this assumption, 150 patients per study arm would achieve a >80% power to identify a >15% absolute increase in abstinence rates (24% to 39%) using a two‐tailed α of 0.05". |
|
| Participants | 302 adult smokers, aged 18+, smoking 10+ CPD, interested in trying to quit, hospitalised in USA or Canada for acute coronary syndrome (MI or unstable angina). Mean age 55, 25% women, mean CPD 21.5 Allocated to varenicline (151) or placebo (151) Exclusions: Excessive alcohol, history of panic disorder, psychosis, bipolar disease, dementia, renal or hepatic impairment, current or recent drug use, history of suicidal ideation/attempt or family history of suicide |
|
| Interventions | 1. Varenicline 12 wks, titrated 1st wk 2. Placebo 12 wks, titrated 1st wk Medication was begun in hospital. All participants received low‐intensity counselling Follow‐up at wks 1, 2 and 8 by phone, and clinic visits at wks 4, 12 and 24 |
|
| Outcomes | Primary: 7‐day PPA at wk 24 Secondary: CAR at all follow‐up visits, 7‐day PPA at other follow‐up visits, ≥ 50% reduction in CPD Measures of side effects and SAEs Validation: CO ≤ 10 ppm |
|
| Treatment type | Medication: VARENICLINE | |
| Notes | New for 2016 update Funded by Pfizer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized to either varenicline or matching placebo... Randomization was performed by enrolling center personnel and stratified by center using a computer‐generated list of permuted blocks of 2 and 4" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as "double‐blind", but no further detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses fully reported; ITT analyses conducted |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None noted |
Evins 2014
| Methods | Country: USA Setting: Massachussetts General Hospital and 9 other community mental health centres in Massachussetts, Michigan, New Hampshire, Indiana, Alabama, Minnesota Aim: To determine whether smokers diagnosed with schizophrenia and bipolar disease have higher rates of prolonged tobacco abstinence with maintenance pharmacotherapy than with standard treatment Study Design: Double‐blind placebo‐controlled RCT Dates conducted: March 2008 ‐ April 2012 Analysis: "The study was powered to show differences between varenicline and placebo for point‐prevalence abstinence at week 52. Assuming a 35% to 40% relapse in the varenicline group and a 75% to 80% relapse in the placebo group, estimates based on trials of bupropion involving smokers with schizophrenia, we estimated that 48 participants per study group would provide 91% to 99% power and 40 patients per study group would provide 85% to 98% power to detect a treatment effect using a 2‐group Fisher exact test with a .05, 2‐sided significance level" |
|
| Participants | 247 outpatient smokers with schizophrenia, schizoaffective or bipolar disorder, aged 18 ‐ 70, CPD 10+, 87 of whom met the abstinence criteria after 12 wks of open‐label varenicline to enter this relapse prevention trial. Randomised to varenicline (40) or control (47). Mean age 48, 37% women, 74% white, mean FTND 5.9, mean CPD 23.2 | |
| Interventions | All participants had received 12 wks open‐label varenicline, and were confirmed abstinent at wks 11 and 12 1. Varenicline 1 mg x 2/day for a further 40 wks, + tapered CBT relapse prevention counselling 2. Placebo, same regimen, i.e. CBT alone |
|
| Outcomes | Primary: 7‐day PPA at wk 52 (12 wks cessation treatment + 40 weeks relapse prevention treatment); Secondary: PPA and CAR at wk 64 (52 wks after achieving abstinence); effect of varenicline on psychiatric symptoms (Calgary Depression Scale for Schizophrenia, Brief Psychiatric Rating Scale, Schedule for Assessment of Negative Symptoms), nicotine withdrawal symptoms (Wisconsin SmokingWithdrawal Scale), health‐related quality of life (SF‐12), body mass index, and adverse events Validation: CO < 9 ppm |
|
| Treatment type | Medication: VARENICLINE | |
| Notes | New for 2016 update Funding: NIDA grant R01 DA021245, DHHS grant 05B1MACMHS, Pfizer |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was conducted via centralized computer‐generated random sequence performed by Massachusetts General Hospital research pharmacy staff members, who were not otherwise involved in the trial, in double‐blind fashion, in blocks of 4, stratified by study site and by a single categorical predictor that was a combination of psychiatric disorder and type of antipsychotic medication (eMethods 1 in the Supplement), using a permuted block design with 1:1 ratio" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Low risk | See above. "participants were followed up for biochemically verified abstinence and safety outcomes under double‐blind conditions through week 64" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses fully reported; by wk 52, 33/40 varenicline participants completed study, and 28/42 placebo participants. ITT analyses conducted |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | "Telephone follow‐up at week 76 for self report of smoking behavior from those who had achieved continuous abstinence from weeks 12 through 64 was added to the protocol after trial commencement to better evaluate the duration of continuous abstinence after discontinuation of maintenance treatment." |
Gonzales 2006
| Methods | Country: USA Setting: 19 research centres Aim: To test the efficacy and safety of varenicline for smoking cessation Dates conducted: June 2003 ‐ April 2005 Study Design: Double‐blind placebo‐controlled parallel‐group RCT Analysis: Power calculation (90%, alpha = 0.05); ITT denominators and logistic regression analysis (step‐down procedure) |
|
| Participants | 1025 healthy adult volunteers, recruited through media advertising. Allocated to varenicline (352), bupropion (329) or placebo (344). 54% men, 79% white, mean age 42.4, mean CPD 21, mean FTND score 5.3. No significant differences between groups at baseline Exclusion criteria: Standard pharmacotherapy trial criteria, + use of tobacco products other than cigarettes; use of NRT, clonidine, nortriptyline within last month; BMI < 15 or > 38 or weight < 45.5 kg; any prior use of bupropion or varenicline | |
| Interventions | 1. Varenicline 1 mg x 2/day 2. Bupropion 150 mg x 2/day 3. Placebo inactive tablets, same regimen Treatment period was 12 wks. All participants received Clearing the Air self‐help booklet at baseline, and brief counselling (≤ 10 mins) at each clinic visit. Weekly visits throughout treatment phase, plus a phone call 3 days post‐TQD In follow‐up phase, clinic visits at wks 13, 24, 36, 44 and 52, plus brief phone calls at wks 16, 20, 28, 32, 40 and 48 | |
| Outcomes | Primary outcome: CO‐validated CAR at 9 ‐ 12 wks Secondary outcomes: CO‐validated CAR at 9 ‐ 24 wks and 9 ‐ 52 weeks; 7‐day PPA at wks 12, 24 and 52 Other outcomes: Weight change, withdrawal symptoms (using MNWS, QSU‐brief and mCEQ), adverse events Validation was by expired CO ≤ 10 ppm Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) Attrition in treatment phase was 31.5%, losses to follow‐up 16% of treatment completers | |
| Treatment type | Medication: VARENICLINE / BUPROPION | |
| Notes | This trial had the same aims and study design as Jorenby 2006 The trial was funded by Pfizer Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "predefined ... computer‐generated randomization sequence", 1:1:1, using block size of 6, stratified by centre |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Participants and investigators were blinded to drug treatment assignments[, and] ... were not encouraged to guess their treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Considered abstinent if, at next non‐missed visit, they reported no smoking... Missing CO but otherwise OK considered abstinent, except at end of study, where all criteria had to be present |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes covered |
| Other bias | Unclear risk | None noted |
Gonzales 2014
| Methods | Country: 37 centres in 8 countries: USA (8), Australia (4), Belgium (4), Canada (4), Czech Republic (4), France (3), Germany (5), UK (5) Setting: Clinics, hospitals, academic research centres Aim: To evaluate the efficacy and safety of retreatment with varenicline in smokers who had taken varenicline for ≥ 2 weeks in a previous smoking cessation attempt Study Design: Double‐blind placebo‐controlled multinational RCT Dates conducted: December 2010 ‐ November 2012 Analysis: "A sample size of 490 participants randomized to varenicline or placebo in a 1:1 ratio was estimated to provide ≥90% power for a comparison of varenicline vs. placebo using a two‐group continuity corrected two‐sided χ² test at the 0.05 significance level for the primary end point (CAR for weeks 9–12), assuming an OR of 3.36 with a placebo CAR of 12% and a varenicline CAR of 31%. It was also estimated to provide 80% power for the treatment comparison in the key secondary end point (CAR for weeks 9–52) for an OR of at least 2.55 with a 6% CAR in the placebo group and 14% in the varenicline group." |
|
| Participants | 498 adult smokers (varenicline 251, placebo 247) with previous use of 2+ wks of varenicline at least 3m prior to screening, aged 18+, CPD 10+, motivated to quit. Mean age 47.5, 50.4% women, 93% white, mean CPD 20.5, mean FTND 5.5 | |
| Interventions | 1. Varenicline 12 wks, titrated in 1st wk, 1 mg x 2/day 2. Placebo, identical regimen Brief (< 10 mins) counselling at each contact. TQD set for wk 1 visit. Clinic visits at wks 1, 2, 3, 4, 6, 8, 9, 10, 11, 12; 13, 16, 24, 32, 40, 48, 52. Brief phone calls at wks 5, 7, 14, 20, 36, 44. Dosage could be halved if intolerable |
|
| Outcomes | Primary: CAR at wks 9 ‐ 12, 9 ‐ 52 Secondary: CAR at wks 9 ‐ 24; 7‐day PPA at wks 12, 24, 52 Validation: CO < 10 ppm |
|
| Treatment type | Medication: VARENICLINE | |
| Notes | New for 2016 update Funding: Pfizer |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible participants were randomly assigned to receive either varenicline or placebo at a 1:1 ratio for 12 weeks of drug treatment using computer‐generated block randomization within each site" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up fully reported. ITT analyses conducted |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None noted |
Hajek 2015
| Methods | Country: UK Setting: Specialist stop‐smoking clinic in London Aim: To determine whether increasing varenicline dose in people who show no response to the drug improves treatment efficacy in terms of tobacco withdrawal relief and abstinence rates Study Design: Double‐blind placebo‐controlled RCT Dates conducted: July 2011 ‐ February 2013 Analysis: ANOVA for continuous end points and Χ² for categorical end points. Sample size of 200 for 80% power to detect a difference in 4‐wk abstinence between 60% placebo and 80% varenicline. 2‐tailed P < 0.05 |
|
| Participants | 200 non‐responders to varenicline at day 12, from an initial cohort of 503 given varenicline while still smoking, randomised to varenicline (100) or placebo (100) add‐on treatment. Treatment‐seeking smokers, aged 18+; 28% women, 65% white, mean age 45.8 yrs, 20.5 cigs in previous wk, mean FTND 5.5 | |
| Interventions | 503 eligible consented volunteers began taking varenicline at standard dosage; at day 12, 204 were rated as non‐responders (no strong nausea, no reduction in smoking enjoyment, < 50% reduction in baseline smoking), and 200 were then randomised to additional varenicline or placebo. All participants received phone calls on days 15 and 18, with TQD at day 21 + phone call 24 hours later, and 4 x weekly supportive visits (as per standard NHS stop‐smoking treatment protocol). participants were invited to a 12‐week final visit for assessment 1. Varenicline: standard dose + initial increase of 0.5 mg x 2/day which could be increased by 0.5 twice daily up to a total of 5 mg/day. Dosage used at TQD was maintained for 3 wks, with an option to reduce it if necessary. From 4 wks, only standard dose was used 2. Placebo: same regimen, but with identical placebo pills |
|
| Outcomes | Smoking enjoyment and withdrawal symptoms weekly for 1st 4 wks; CAR at wks 1, 4, 12 after TQD Validation: CO < 9 ppm |
|
| Treatment type | Medication: VARENICLINE | |
| Notes | Funding: Pfizer Although this study did not assess abstinence beyond 3m, we have included it for assessment of variation in dosing, and for safety data. We have not pooled the efficacy findings with the other included studies, apart from sensitivity analysis 13.2 (CAR at 9 ‐ 12 wks) New for 2016 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomized to treatment arms using sequentially numbered prepackaged medication containers boxed according to a computer‐generated randomization list prepared by an independent statistician" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The authors were unblinded only after the data analysis was completed" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All losses during treatment and follow‐up reported; ITT analyses conducted |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None noted |
Heydari 2012
| Methods | Country: Tehran, Iran Setting: Smoking cessation clinics in the Tobacco Prevention and Control Research Centre, Shahid Beheshti University of Medical Sciences Aim: To evaluate the effectiveness of varenicline in the Iranian community of tobacco quitters and compare it with other treatment methods Study Design: 3‐arm randomised parallel clinical study Dates conducted: 2009 ‐ 2010 Analysis: 91 participants per group were required |
|
| Participants | 272 treatment‐seeking participants: Brief advice (91), NRT (92), varenicline (89). 41.2% women, mean age 42.5 yrs, mean FTND 5.5 | |
| Interventions | All participants were managed by the same physician. All received brief (5 mins) education and counselling at 4 x weekly sessions. TQD was day 14 1. Control group: no pharmacotherapy 2. NRT: 8 wks of 15 mg NRT patches 3. 8 wks of 1 mg x 2/day varenicline (titrated 1st wk) |
|
| Outcomes | Abstinence at 6m and 12m, in person or by phone, verified by expired CO (cut‐off value not given) | |
| Treatment type | Medication: VARENICLINE / NRT | |
| Notes | Funding: Masih Daneshvari Hospital Research Institute, Tehran New for 2016 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Smokers who attended the clinic for help in quitting were divided randomly" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label; blinding of outcome assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition: "Participants entered the study of their own accord and none left the study" |
| Selective reporting (reporting bias) | High risk | No information on potential differences between phone‐ and in‐person reporting of abstinence at 6m and 12m, nor of whether all such claims of abstinence were biochemically verified No information on SAEs, if any |
| Other bias | Unclear risk | Participants were all previous quit‐attempters Varenicline was given for 8 wks, i.e. ⅔ of the normal regimen, presumably to align it with the NRT dosage pattern Abstinence‐by‐gender data (Table 2) appears to contain an error for women on NRT at 12m; we have ignored this finding in favour of the combined‐genders data |
Jorenby 2006
| Methods | Country: USA Setting: 14 research centres Aim: To test the efficacy and safety of varenicline for smoking cessation Dates conducted: June 2003 ‐ March 2005 Study Design: Double‐blind placebo‐controlled RCT. Analysis: Power calculation (90%, alpha = 0.05); ITT denominators and logistic regression analysis (step‐down procedure) |
|
| Participants | 1027 healthy adult volunteers. Allocated to varenicline (344), bupropion (342) or placebo (341). 58% men, 84% white, mean age 43.3, mean CPD 22, mean FTND score 5.3. No significant differences between groups at baseline Exclusion criteria: Standard pharmacotherapy trial criteria, + use of tobacco products other than cigarettes; use of NRT, clonidine, nortriptyline within last month; BMI < 15 or > 38 or weight < 45.5 kg; any prior use of bupropion or varenicline | |
| Interventions | 1. Varenicline 1 mg x2/day 2. Bupropion 150 mg x2/day 3. Placebo inactive tablets, same regimen Treatment period was 12 wks. All participants received brief counselling (≤ 10 mins) at each clinic visit Weekly visits throughout treatment phase, plus a phone call 3 days post‐TQD In follow‐up phase, clinic visits at wks 13, 24, 36, 44 and 52, plus brief phone calls at wks 16, 20, 28, 32, 40 and 48 | |
| Outcomes | Primary outcome: CO‐validated CAR at 9 ‐ 12 wks Secondary outcomes: CO‐validated CAR at 9 ‐ 24 wks and 9 ‐ 52 wks; 7‐day PPA at wks 12, 24 and 52 Other outcomes: Weight change, withdrawal symptoms (using MNWS, QSU‐brief and mCEQ), adverse events Validation was by expired CO ≤ 10 ppm Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) Attrition in treatment phase was 29.3%, losses to follow‐up 8% of treatment completers | |
| Treatment type | Medication: VARENICLINE / BUPROPION | |
| Notes | This trial had the same aims and study design as Gonzales 2006. The trial was funded by Pfizer Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated list" |
| Allocation concealment (selection bias) | Low risk | "completed centrally ... and sites used an electronic system to assign participants to treatment" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "in a double‐blind manner" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | CA for missed visits: if self‐reported abstinent at next visit, assumed abstinent, except at wk 52 visit when all criteria had to be met |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes covered |
| Other bias | Unclear risk | None noted |
Nahvi 2014a
| Methods | Country: USA Setting: 3 urban outpatient clinics for substance use disorder (SUD) in the Bronx, NY Aim: to test the efficacy and safety of varenicline for smoking cessation among opioid‐dependent people on a maintenance regimen Study Design: Randomised quadruple‐blind controlled trial Dates conducted: August 2009 ‐ September 2011 Analysis: 50 participants in each arm would give 80% power to detect a 22% abstinence rate in the varenicline users (½ the expected rate in non‐MM participants) |
|
| Participants | 112 smokers in methadone treatment for substance abuse, aged 18+, CPD 5+, motivated to quit within next 6m. Allocated 57 varenicline, 55 placebo. 52% women, 54% Hispanic, mean CPD 15, mean FTND 4 | |
| Interventions | All participants set a TQD 1 wk after treatment began. All were offered structured, brief (≤ 10 mins) individual in‐person counselling by a physician or tobacco specialist at baseline and at 2‐, 4‐, 8‐ and 12‐wk visits. All participants were also offered free quitline support 1. Varenicline: 12‐wk standard regimen, titrated for 1st wk 2. Control: Identical placebo tablets and regimen |
|
| Outcomes | 7‐day PPA at 12 and 24 wks Validation: Expired CO < 8 ppm |
|
| Treatment type | Medication: VARENICLINE | |
| Notes | Funding: National Center for Research Resources grant UL1 RR025750 to SN, and the National Institute on Drug Abuse grants K23 DA025736 to SN and R25 DA023021 to SN and JHA New for 2016 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatment group allocation was computer‐generated, and stratified by the three clinic sites in blocks of six within each stratum" |
| Allocation concealment (selection bias) | Low risk | "a central data manager concealed the allocation sequence using a password‐protected file, assigned subjects to treatment groups and faxed pre‐printed medication orders to the study pharmacist. The pharmacist prepared the research medication by compounding varenicline tablets or placebo lactose powder to create identical‐appearing capsules. The pharmacist marked medication bottles with subjects’ study identification numbers, and delivered medications for individual study subjects to clinical sites, where they were distributed to each subject by the research assistant" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The pharmacist prepared the research medication by compounding varenicline tablets or placebo lactose powder to create identical‐appearing capsules. The pharmacist marked medication bottles with subjects’ study identification numbers, and delivered medications for individual study subjects to clinical sites, where they were distributed to each subject by the research assistant" "All subjects, research assistants, counsellors and physicians were blinded to treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses during treatment (varenicline 6, placebo 9) and during follow‐up (varenicline 2, placebo 3) fully reported; ITT analyses conducted |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None noted |
Nakamura 2007
| Methods | Country: Japan Setting: 19 study sites Aim: To test efficacy, safety and tolerability of 3 doses of varenicline over 12 wks Dates conducted: not stated Study Design: Double‐blind, placebo‐controlled, parallel group RCT Analysis: Power calculation (90%, alpha = 0.05) for 0.5 or 1.0 mg vs placebo; ITT denominators; also logistic regression (step‐down) with dose and study centre as categorical variables |
|
| Participants | 619 healthy Japanese adult volunteers, aged 20 ‐ 75, smoking ≥ 10 CPD. Allocated to varenicline 0.25 mg x 2/day (153), 0.5 mg x 2/day (156), 1.0 mg x 2/day (156) or placebo x 2/day (154). 1 participant withdrew before treatment, and is excluded from ITT denominator. 1 RTA death removed from varenicline group at 52 wks Participants stratified by level of nicotine dependence, measured by Tobacco Dependence Screener scale (≥ 5) and by FTND. 515 (83.3%) classified as nicotine dependent Demographic data only supplied for nicotine‐dependent group (515/618): 75% men, mean age 39.8, mean CPD 24, mean FTND score 5.6 Exclusion criteria: Standard pharmacotherapy trial criteria, + use of NRT within last 30 days, use of pipe tobacco, snuff, chewing tobacco, cigars within last 30 days and throughout trial | |
| Interventions | 1. Varenicline 0.25 mg x 2/day 2. Varenicline 0.50 mg x 2/day 3. Varenicline 1.00 mg x 2/day 4. Placebo tablet x 2/day Treatment period 12 wks, 1st wk titrated dosage. All participants received a smoking cessation booklet Clearing the Air at baseline, + brief counselling (≤ 10 mins) at each clinic visit. Weekly visits throughout treatment phase, plus a 5‐min phone call at TQD and +3 days post‐TQD In follow‐up phase, clinic visits at wks 13, 16, 24, 36, 44 and 52, plus brief phone calls at wks 20, 28, 32, 40 and 48 | |
| Outcomes | Primary outcome: CO‐validated CAR at 9 ‐ 12 wks Secondary outcomes: CO‐validated CAR at 9 ‐ 24 wks and 9 ‐ 52 wks; 7‐day PPA at wks 2, 12, 24 and 52 Validation was by expired CO ≤ 10 ppm Other outcomes: Withdrawal symptoms (using MNWS, QSU‐brief and mCEQ), adverse events Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) Attrition in treatment phase was 6.4%, losses to follow‐up 11.4% of treatment completers (excluding 1 death) | |
| Treatment type | Medication: VARENICLINE | |
| Notes | Trial was funded by Pfizer Inc New for 2008 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated list of random numbers" |
| Allocation concealment (selection bias) | Low risk | "randomized to 1 of the 4 treatment groups in a 1:1:1:1 ratio using a central procedure" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐blinding of subjects and investigators was maintained throughout the study". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No comment on level or handling of missing data |
| Selective reporting (reporting bias) | High risk | CARs for all participants reported, but demographics, withdrawal and craving measures, and PPA for nicotine‐dependent group only |
| Other bias | Unclear risk | None noted |
| Methods | Randomised quadruple‐blind placebo‐controlled trial | |
| Participants | 101 adult smokers | |
| Interventions | All get 13 weeks varenicline, then half continue and half switch to placebo, until week 52 | |
| Outcomes | Biochemically confirmed abstinence (at 52 weeks) CO‐confirmed at ≤ 10 ppm | |
| Treatment type | VARENICLINE | |
| Notes | Study results posted on clinicaltrials.gov June 2012, updated October 2015 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Stated to be quadruple‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition rates: varenicline 30/50 (27 withdrawals, 3 lost), placebo 31/51 (26 withdrawals, 5 lost) |
| Selective reporting (reporting bias) | High risk | Results unpublished; available only on www.ClinicalTrials.gov |
| Methods | Phase II/III randomised quadruple‐blind placebo‐controlled trial | |
| Participants | 33 adult alcohol‐dependent smokers | |
| Interventions | Varenicline 1 mg bid for 12 weeks vs placebo | |
| Outcomes | Prolonged abstinence at 12 weeks (end of treatment), and at 6m Abstinence self‐reported, not biochemically confirmed | |
| Treatment type | VARENICLINE | |
| Notes | Study results posted on www.clinicaltrials.gov May 2014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition rates: 4/16 varenicline group (1 withdrawal, 3 lost), 12/17 placebo group (7 withdrawals, 5 lost) |
| Selective reporting (reporting bias) | High risk | Results unpublished; available only on www.ClinicalTrials.gov |
| Other bias | High risk | Trial planned to include 70 participants, but recruited only 33 |
Niaura 2008
| Methods | Country: USA Setting: 5 research centres Aim: To test the efficacy and safety of varenicline in smokers allowed to modify their own dosage regimen. Dates conducted: December 2001 ‐ June 2003 Study Design: Double‐blind placebo‐controlled RCT Analysis: Power calculation (90%, alpha = 0.05); ITT denominators and logistic regression analysis (step‐down procedure) |
|
| Participants | 320 healthy adult volunteers, aged 18 ‐ 65, smoking ≥ 10 CPD. Allocated to varenicline (160), or placebo (160) 52% men, 91% white, mean age 42, mean CPD 22, mean FTND score 5.4 Exclusion criteria: Standard pharmacotherapy trial criteria, + use of NRT within last 3m | |
| Interventions | 1. 0.5 mg varenicline ad lib, from 1 to 4 per day as wished 2. Placebo tablets ad lib, from 1 to 4 per day as wished Treatment period 12 wks, 1st wk titrated dosage up to 0.5 mg x 2/day. All participants received a smoking cessation booklet Clearing the Air at baseline, + brief counselling (≤ 10 mins) at each clinic visit. Weekly visits throughout treatment phase In follow‐up phase, clinic visits at wks 13, 24, and 52 wks, plus monthly phone calls between visits | |
| Outcomes | Primary outcome: CAR at 4 ‐ 7, 9 ‐ 12 and 9 ‐ 52 wks Validation was by expired CO ≤ 10 ppm Secondary outcomes: CO‐confirmed CAR at 9 ‐ 24 wks; CO‐confirmed 7‐day PPA Other outcomes: Mean modal dosage; withdrawal symptoms (using MNWS, QSU‐brief and mCEQ), adverse events Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) Attrition in treatment phase was 22% in varenicline group and 29% in placebo group; losses to follow‐up by wk 52 were 36% from varenicline group and 43% from placebo group | |
| Treatment type | Medication: VARENICLINE | |
| Notes | The trial was funded by Pfizer Inc New for 2010 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly permuted blocks and a pseudo‐random number generator" |
| Allocation concealment (selection bias) | Low risk | "participants were assigned in a 1:1 ratio to varenicline treatment or placebo in the numerical order that they were accepted to the study" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "double‐blind" but no further information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data imputed if prior and subsequent abstinence confirmed, otherwise assumed still smoking |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes covered |
| Other bias | Unclear risk | None noted |
Nides 2006
| Methods | Country: USA Setting: 7 research centres Aim: To test efficacy, tolerability and safety of 3 doses of varenicline over 6 wks Dates conducted: February 2000 ‐ January 2003 Study Design: Phase 2 double‐blind placebo‐controlled RCT Analysis: Power calculation (80%, 2‐tailed, alpha = 0.05); Dunnett adjustment for multiple comparisons used for primary endpoint (CQR within treatment phase). ORs and CIs least squares mean estimates. Not powered for varenicline/bupropion comparison |
|
| Participants | 638 healthy volunteer smokers, aged 18 ‐ 65, smoking at least 10 CPD on average. 48% men, 87% white, av age 42, av CPD 20, mean FTND 5.5. Allocated to varenicline group 1 (128), group 2 (128), group 3 (127), bupropion (128), placebo≤ (127) Exclusion criteria: Standard pharmacotherapy trial criteria, + use of bupropion within previous 12m, use of NRT within past 3m | |
| Interventions | 1. varenicline tartrate 0.3 mg x 1/day for 6wks, + 1 wk placebo 2. varenicline tartrate 1.0 mg x 1/day for 6 wks, + 1 wk placebo 3. varenicline tartrate 1.0 mg x 2/day for 6 wks, + 1 wk placebo 4. bupropion 150 mg x 2/day (titrated in wk 1) for 7 wks 5. placebo tablets x 2/day for 7 wks All groups received self‐help booklet Clearing the Air at baseline, + brief (≤ 10 mins) counselling at weekly clinic visits throughout treatment phase. At each visit smoking status reported and verified; lab samples taken at screening, baseline and wks 1, 2, 4, 6 and 7 Follow‐up phase (optional): Clinic visits at wks 12, 24, 52 for brief counselling, smoking status and vital signs. Phone calls every 4 wks from wk 16 | |
| Outcomes | Primary outcome: Continuous verified 4‐wk abstinence for any part of treatment period Secondary outcomes: CQR wks 4 ‐ 7; CQR from wk 4 to wks 12, 24, and 52 Other outcomes: Weight change; reduction of craving and withdrawal using MNWS, QSU‐brief and mCEQ; adverse events Validation was by expired CO ≤ 10 ppm Trial report ITT analysis based on numbers treated (N = 626); for consistency our MA used numbers randomised (N = 638). Attrition was 30% during treatment period, 25% of follow‐up consenters lost during follow‐up phase | |
| Treatment type | Medication: VARENICLINE / BUPROPION | |
| Notes | Previous users of bupropion > 12m before were not excluded, unlike Gonzalez and Jorenby trials; prior use ranged from 13% to 20.6% across groups Denominator in trial report is all treated; we have used all randomised in our MA The trial was funded by Pfizer Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated using a method of randomly permuted blocks and a pseudo‐random number generator" |
| Allocation concealment (selection bias) | Low risk | "assigned ... medication to subjects in numerical order of acceptance into the study" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "double‐blind", "to preserve treatment blinding" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes covered |
| Other bias | Unclear risk | None noted |
Oncken 2006
| Methods | Country: USA Setting: 10 research centres Aim: To evaluate efficacy and safety of 4 varenicline dose regimens Dates conducted: Not stated Study Design: Phase 2 double‐blind placebo‐controlled RCT Analysis: Power calculation (90%, 2‐tailed, alpha = 0.05); Logistic regression with treatment and centre as independent variables. Likelihood ratio Chi² statistic |
|
| Participants | 647 healthy volunteer smokers, aged 18 ‐ 65, smoking at least 10 CPD. 49.5% men, 80% white, av CPD 21, mean FTND 5.5. Allocated to group 1 (129), group 2 (130), group 3 (129), group 4 (130) or placebo (129) Exclusion criteria: Standard pharmacotherapy trial criteria, + use of NRT or bupropion within last 3m; use of marijuana or tobacco other than cigarettes with last month. | |
| Interventions | 1. 0.5 mg nontitrated (2/day for 12 wks) 2. 0.5 mg titrated (wk1 1/day, wks 2 ‐ 12 2/day) 3. 1.0 mg nontitrated (2/day for 12 wks) 4. 1.0 mg titrated (0.5 mg 1/day for 3 days, 0.5 mg 2/day for 4 days, 1.0 mg 2/day wks 2 ‐ 12) 5. placebo tablets 2/d 12 wks All groups received self‐help booklet at baseline, + brief (≤ 10 mins) counselling at weekly clinic visits throughout treatment phase, and phone call 3 days post‐TQD. At each visit smoking status reported and CO verified; vital signs, weight and adverse events. Urine, blood tests and ECGs at screening, baseline, wks 1, 2, 4, 7 and 12. Follow‐up phase: smoking status + CO measured at wks 13, 24, 52; self‐reported status by phone at wks 16, 20, 28, 32, 36, 40, 44 | |
| Outcomes | Primary outcome: Continuous verified 4‐wk abstinence at wks 4 ‐ 7 and 9 ‐ 12 Secondary outcomes: Continuous verified abstinence at wks 2 ‐ 12 and 9 ‐ 52; 7‐day PPA throughout treatment phase and at wks 12, 24 and 52 Other outcomes: weight change; craving and withdrawal changes using MNWS and mCEQ; adverse events Validation was by expired CO ≤ 10 ppm Cessation analyses were ITT (all participants randomised), while tolerability and safety analyses were based only on those known to have used the intervention drug (N = 627). Attrition was 27% during treatment phase, and 22% of follow‐up consenters lost in follow‐up phase | |
| Treatment type | Medication: VARENICLINE | |
| Notes | For cessation analyses, titrated and nontitrated results were reported separately and pooled. 24‐wk continuous cessation data supplied by authors The trial was funded by Pfizer Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | "Eligible subjects were randomly assigned to 1 of 5 groups" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Subjects and investigators were blinded to the study drug treatment [, and] were not encouraged to guess their treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing COs or visits OK if confirmed abstinent before and after missed measure |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes covered |
| Other bias | Unclear risk | None noted |
Rennard 2012
| Methods | Countries: Argentina, Brazil, Canada, China, Czech Republic, France, Germany, Hungary, Italy, Korea, Mexico, Taiwan, UK, USA Setting: 33 research centres Aim: To evaluate efficacy and safety of varenicline allowing a self‐selected quit date Dates conducted: September 2008 ‐ December 2009 Study Design: Double‐blind placebo‐controlled RCT Analysis: Power calculation (90%, alpha = 0.05) assuming a true abstinence rate at 9 ‐ 12 wks of 0.24 (placebo) and 0.46 (varenicline); Logistic regression with treatment and centre as independent variables |
|
| Participants | 659 healthy volunteer smokers, aged 18 ‐ 75, motivated to quit, smoking at least 10 CPD. 60% men, mean age 43, 68% white, mean CPD 21, mean FTND 5.5, 66% had tried to quit at least once before. Allocated to varenicline (493) or placebo (166) Exclusion criteria: Standard pharmacotherapy trial criteria, + use of NRT, bupropion, clonidine or nortriptyline within last 3m, ever use of varenicline; use of marijuana or tobacco other than cigarettes with last month | |
| Interventions | 1. Varenicline 1 mg x 2/day, titrated in 1st wk 2. Placebo inactive tablets, same regimen Participants could choose their own quit date between days 8 and 35 Treatment period was 12 wks. All participants received Clearing the Air: Quit smoking today booklet at baseline, + brief counselling (≤ 10 mins) at each clinic visit. Weekly visits throughout treatment phase, and in follow‐up phase clinic visits at wks 13, 16, 20 and 24. Phone calls at wks 14, 18 and 22 |
|
| Outcomes | Primary outcome: CO‐validated CAR at 9 ‐ 12 wks Secondary outcomes: CO‐validated CAR at 9 ‐ 24 wks; 7‐day PPA at wks 12 and 24 Other outcomes: Adverse events, SAEs; timing and number of quit attempts Validation was by expired CO ≤ 10 ppm Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) Attrition to end of study (24 wks) was 12.4% from varenicline, 20.5% from placebo | |
| Treatment type | Medication: VARENICLINE | |
| Notes | New for 2012 update. Additional information supplied by the authors The study was funded and managed by Pfizer Inc |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "a predefined, central, computer‐generated randomization sequence...assigned subjects in a 3:1 ratio". Block size: 4, stratified by centre |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Triple‐blind (participant, care‐giver, investigator) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts and attrition rates fully reported |
| Selective reporting (reporting bias) | Low risk | All predicted and expected outcomes reported |
| Other bias | Unclear risk | None noted |
Rigotti 2010
| Methods | Country: 15 countries in Europe, Asia, Americas Setting: 39 research centres Aim: To evaluate efficacy and safety of varenicline in patients with stable CVD Dates conducted: February 2006 ‐ August 2008 Study Design: Phase 3 double‐blind placebo‐controlled RCT Analysis: Logistic regression with treatment group and study site as independent variables |
|
| Participants | 714 adult smokers, aged 35 ‐ 75, smoking at least 10 CPD, with stable CVD and motivated to quit. 79% men, 80% white, mean CPD 22, mean FTND 5.6. Allocated to varenicline (355) or placebo (359), stratified by site Exclusion criteria: Standard pharmacotherapy trial criteria, + use of NRT or bupropion within previous month. All had been diagnosed for at least 2m with CVD, but not hypertension alone | |
| Interventions | 1. Varenicline 1.0 mg 2/day for 12 wks, including wk 1 at titrated dose 2. Placebo tablets as above Both groups received brief (≤ 10mins) counselling at weekly clinic visits throughout treatment phase, and phone call 3 days post‐TQD. At each visit smoking status reported and CO verified; vital signs, weight and adverse events. Urine, blood tests and ECGs at screening, baseline, wks 12 and 52 Follow‐up phase: smoking status + CO measured at wks 13, 16, 24, 32, 40 and 52; counselling and self‐reported status by phone at wks 14, 20, 28, 36 and 44 | |
| Outcomes | Primary outcome: CO‐validated CAR at wks 9 ‐ 12 Secondary outcomes: CO‐validated CAR at wks 9 ‐ 52 and 9 ‐ 24; 7‐day PPA at wks 12, 24 and 52 Other outcomes: Adverse events; serious adverse events; cardiovascular events; changes in blood pressure and heart rate Validation was by expired CO ≤ 10 ppm Cessation analyses were ITT (all participants randomised minus deaths), while tolerability and safety analyses were based only on those known to have used the intervention drug (N = 703). Attrition was 17.5% from the varenicline group and 20.3% from the placebo group during treatment phase, and 14.9% varenicline and 19.5% placebo who did not complete the study. This includes 2 deaths in the varenicline group and 5 in the placebo group by 52‐wk follow‐up | |
| Treatment type | Medication: VARENICLINE | |
| Notes | The study was funded by Pfizer Inc New for 2010 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study sponsor conducted the randomization centrally using a computer‐generated list that prespecified the order of treatment allocation" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as "double‐blind" (participants and study implementation). Cardiovascular outcomes "were reviewed separately and adjudicated under blinded conditions by an independent event committee made up of 3 board‐certified cardiologists" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analyses conducted; participants who missed a visit but had validated abstinence at next visit were considered continuously abstinent. But 52‐wk status had to be attended and confirmed |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes covered |
| Other bias | Unclear risk | None noted |
Rose 2013
| Methods | Country: USA Setting: Duke University Medical Center, Durham, NC Aim: "Given the safety and tolerability profile of nicotine replacement therapy, our rationale in this study was to use nicotine replacement therapy as an initial line of treatment, and then identify early on which smokers are unlikely to benefit from nicotine alone" Study Design: Randomised double‐blind parallel‐arm adaptive treatment trial in 2 phases Dates conducted: Not stated Analysis: Logistic regression |
|
| Participants | 606 adult smokers, motivated to quit, aged 18 ‐ 65, mean CPD 10+ for 3 yrs, expired CO level 10+ ppm. 46% women, 63% white, mean CPD 21.7, mean FTND 5.8. Participants could receive up to USD 320 for study participation | |
| Interventions | Two phase study: All participants seen weekly for 2 wks before TQD, and attended 4 ‐ 6 sessions after the TQD. At each session, participant received brief (< 15 mins) support, + clinical trial materials. Smoking diaries, expired CO, withdrawal symptoms and reports of adverse events were collected each time. Participants were recontacted at 6m, and those reporting abstinence were invited to return to give a CO sample All participants were given open‐label active NRT patch, either 42 mg/day (baseline CO > 30 ppm) or 21 mg/day (baseline CO < 30 ppm) for 2 wks; dose reductions allowed if side effects dictated. At 1 wk, participants were classified as 'responders' (reduced ad lib smoking by > 50%, CO‐verified) or 'non‐responders' (< 50%) Phase 1 (12 weeks): Non‐responders only (N = 371 ‐ 36 who withdrew, = 335) allocated to: 1. Double‐blind varenicline, stopping NRT (N = 112) 2. Double‐blind augmentation of NRT with bupropion (N = 109) 3. Continuation on open‐label NRT alone (N = 114) All participants received dummy (placebo) versions of the other 2 treatments as well as their own active treatment. Phase 2: 235 responders after wk 1 assessed at 1st wk after TQD (wk 3). Lapsers (N = 105) were assigned a 2nd TQD 1 wk later, and were allocated to the same 3 double‐blind treatment conditions as Phase 1 non‐responders. 1. Double‐blind varenicline, stopping NRT (N = 36) 2. Double‐blind augmentation of NRT with bupropion (N = 34) 3. Continuation on open‐label NRT alone (N = 35) Non‐lapsers (N = 130) remained on open‐label NRT throughout study duration All participants received dummy (placebo) versions of the other 2 treatments as well as their own active treatment. 47 participants were excluded from the analysis (27 Phase 1, 20 Phase 2) because of using contra‐indicated medications during the study or failing to meet other entry requirements. 1 individual died before end of treatment, and 1 was excluded for extreme CO change from the mean sample range |
|
| Outcomes | Primary: CAR at wks 8 ‐ 11 Secondary: CA from TQD for 11 wks (EoT); 7‐day PPA at 6m: CA from TQD to 6m Validation: CO ≤ 10 ppm AEs and SAEs (reported, but not by treatment group) |
|
| Treatment type | Medication: VARENICLINE, BUPROPION, NRT | |
| Notes | Funding by a grant from Philip Morris USA, with NRT supplied free by GSK Phase 1 and Phase 2 groups combined for varenicline vs NRT analysis New for 2016 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses fully reported; exclusions for protocol violations or contra‐indicated medicines. 1 death and 1 'rogue' CO reading excluded |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Unclear risk | Unexplained disparity between CONSORT (N = 103) and Results table (N = 108) denominators for rescue varenicline group |
Stein 2013
| Methods | Country: USA Setting: 9 methadone‐maintained treatment centres in New England Aim: "[to] test varenicline versus placebo, and include a comparison condition of combination nicotine replacement therapy" Study Design: Randomised 3‐armed double‐blind controlled trial Dates conducted: December 2008 ‐ January 2012 Analysis: Sample sizes of 132 (varenicline) and 44 (placebo) estimated to give 80% power to detect quit rates of 20% and 2.5% respectively; the study was not powered to detect differences between varenicline and combination NRT |
|
| Participants | 315 adult methadone‐maintained smokers, smoking 10+ CPD, willing to set a quit date within the 1st wk Allocated 3:1:3 to varenicline (137): placebo (45): combination NRT (133). Mean age 39.9, 47.6% women, 78.5% white, mean CPD 20, mean FTND 5.7 | |
| Interventions | All participants received a standardised 15‐min session of advice to quit (5As model), and were asked to set a TQD for 8 days time. All made monthly visits for support and top‐up medication 1. Varenicline: 24‐wk course of varenicline tablets, 1st wk titrated 2. Placebo: 24‐wk course of identical tablets and regimen 3. Combination NRT: 24‐wk course of NRT patch (42 mg for > 30 CPD, 21 mg if < 30 CPD), + ad lib nicotine gum (4 mg) as needed Participants were paid USD 30 for the baseline assessment and USD 40 for the 6m assessment |
|
| Outcomes | Primary: 7‐day PPA at 6m Secondary: CA from wk 2 to 6m; for non‐quitters: CPD reduction in the 28 days prior to 6m assessment Validation: CO < 8 ppm; urinary cotinine in varenicline and placebo participants claiming abstinence |
|
| Treatment type | Medication: VARENICLINE / NRT | |
| Notes | Funding: NCI grant RO1 CA129226; MDS supported by a NIDA mid‐career investigator award K24 DA000512 New for 2016 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were randomized to treatment after completing the baseline assessment". No further information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐blind"; research assistants were "blind to participant group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to treatment and follow‐up reported; ITT analyses conducted |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None noted |
Steinberg 2011
| Methods | Country: New Jersey, USA Setting: Robert Wood Johnson Hospital (584‐bed University‐based) Aim: To evaluate efficacy and safety of varenicline in hospital inpatients Dates conducted: August 2007 ‐ March 2009 Study Design: Phase III triple‐blind pilot RCT |
|
| Participants | 79 adult smokers, aged 18+, smoking 10+ CPD; randomised to varenicline (40) or placebo (39) 59% men, mean age: 51, 72% white, 57% > 20 cpd, 40% FTND > 6 Admission diagnoses 57% CVD, 14% orthopaedic, 13% pulmonary, 16% other Exclusion criteria: Standard pharmacotherapy criteria, + current use of any SC medications |
|
| Interventions | 1. Varenicline 1.0 mg x 2/day for 12 wks, including wk 1 at titrated dose 2. Placebo tablets as above Initial visit by Clinic Co‐ordinator of local Tobacco Dependence Program for 5 ‐ 10 min counselling Subsequent sessions of 15 mins post‐discharge After discharge, data collection sessions at 4, 12 and 26 wks, + 1 phone call at 2 wks with research nurse USD 25 gift card for attendance at each follow‐up visit |
|
| Outcomes | Primary outcome: 7‐day PPA at 26 wks Secondary outcomes: 7‐day PPA at 4, 12 wks. Repeated PPA at 4, 12 and 24 wks. AEs and SAEs; withdrawal and craving on MNWS; motivation; CPD; utilisation of OP services; composite medical outcome Validation: CO validation ≤ 8 ppm. Self report accepted if unable to attend |
|
| Treatment type | Medication: VARENICLINE | |
| Notes | Study was funded and support by Robert Wood Johnson Foundation and Pfizer Repeated PPA at 4, 12 and 24 wks used as strictest definition of abstinence and included in main MA New for 2012 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized in a 1:1 ratio through centralized telephone randomization process by the study statistician and hospital research pharmacist" |
| Allocation concealment (selection bias) | Low risk | see above |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The subject, research nurse, and treatment staff were blinded to treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis conducted; unvalidated smoking status included where ascertained for non‐attenders, but % of unvalidated status not reported |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes covered, except for detailed identification of SAEs |
| Other bias | Unclear risk | None noted |
Tashkin 2011
| Methods | Country: USA (17 centres), Spain (3 centres), France (4 centres), Italy (3 centres) Setting: 27 research centres. Aim: To test efficacy and safety of varenicline in smokers with COPD Dates conducted: May 2006 ‐ April 2009 Study Design: Double‐blind placebo‐controlled RCT Analysis: Power calculation (81% to detect a diff in CAR 9 ‐ 52 wks based on an OR of 2.21 and a placebo rate of 9%); ITT denominators. Logistic regression with treatment group and study site as independent variables |
|
| Participants | 504 adult smokers with mild‐to‐moderate COPD, aged 35+, smoking 10+ CPD, motivated to quit; allocated to varenicline (250), or placebo (254). 62% men, mean age 57, CPD 24 ‐ 25, FTND score 5.9 ‐ 6.2 Treatment groups were comparable at baseline Exclusion criteria: Standard pharmacotherapy trial criteria, + treatment with systemic corticosteroids or hospitalised for COPD in previous 4 wks | |
| Interventions | 1. Varenicline 1.0 mg x 2/day for 12 wks, preceded by 1 wk titrated dose 2. Placebo tablets as above Both groups received SC educational booklet, + brief (≤ 10mins) counselling at weekly clinic visits throughout treatment phase, and phone call 3 days post‐TQD. At each visit smoking status reported and CO verified; throughout treatment and at wk 52 lung function, respiratory symptoms, weight, BP, pulse, temperature, ECGs, haematology and serum chemistry assessed, + adverse events Follow‐up phase: smoking status + CO measured at wks 13, 16, 24, 32, 40, 48 and 52; counselling and self‐reported status by phone at wks 14, 20, 28, 36 and 44 | |
| Outcomes | Primary outcome: CO‐validated CAR at wks 9 ‐ 12 Secondary outcomes: CO‐validated CAR at wks 9 ‐ 52 and 9 ‐ 24; 7‐day PPA at wks 12, 24 and 52 Other outcomes: Adverse events; serious adverse events; weight change Validation was by expired CO ≤ 10 ppm Cessation analyses were ITT (all participants randomised), while tolerability and safety analyses were based only on those known to have used the intervention drug (N = 499). Attrition was 17% in the varenicline group and 24% in the placebo group during treatment phase, and 29% varenicline and 38% placebo who did not complete the study. This includes 2 deaths in the varenicline group and 1 in the placebo group | |
| Treatment type | Medication: VARENICLINE | |
| Notes | The study was funded by Pfizer Inc New for 2010 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "participants were randomized" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "double blind" but details not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | All expected and predicted outcomes covered |
| Other bias | Unclear risk | None noted |
Tønnesen 2013
| Methods | Country: Denmark Setting: 1 hospital‐based smoking cessation specialist clinic Aim: "to evaluate whether varenicline used for 12 weeks would be more effective than placebo to get long‐term NRT users to stop using NRT" Study Design: Randomised placebo‐controlled quadruple‐blind trial Dates conducted: Not given Analysis: Sample sizes of 66 in each group, estimated to give 80% power to detect quit rates of 50% and 25% respectively at 12 weeks in active and placebo groups |
|
| Participants | 139 adult ex‐smokers, aged 18+, reporting long‐term (> 11m) abstinence, using flexible‐dose NRT (i.e. > 4 pieces of nicotine gum/sublingual tablets or lozenges per day, or > 3 inhaler cartridges per day, or > 10 puffs of nasal spray per day), wishing and willing to try to stop using NRT; allocated to varenicline (70) or placebo (69) Participants used gums (2 mg 68.3%; 4 mg 11.5%), inhalers (5.8%), sublingual tablets (7.2%), lozenge (9.4%); mean daily NRT unit intake was 16 (SD 8.1), and mean NRT usage had lasted 6 years. Mean age 54.6, 54% women, mean CPD when smoking 23.5, mean FTND (recalled) 6.5 |
|
| Interventions | All participants attended clinic visits at wks 0, 2, 4, 6, 9, 12, 52, + 2 phone calls at wks 26 and 38. Each visit included assessments, < 5 mins counselling from SC nurses. All participants advised to gradually reduce NRT and to stop completely by TQD at 1 ‐ 2 wks 1. Varenicline: standard 12‐wk regimen, titrated 1st wk 2. Placebo: identical tablets, same regimen |
|
| Outcomes | 7‐day PPA at 12 weeks, not smoking or on NRT; also no NRT (7‐day PPA) + abstinence at 52 wks. CAR from wk 2 to wk 52, proven abstinent at all clinic visits Validation: expired CO < 7 ppm and plasma cotinine < 15 ng/ml |
|
| Treatment type | Medication: VARENICLINE | |
| Notes | Not included in the main analysis, as smoking cessation was not the aim Funding was from an Independent Investigator Grant from Pfizer A/S, Denmark and Pfizer, Europe New for 2016 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomized to active or placebo using a computer‐generated list with random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as a "double‐blind" trial. No additional information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | By 52 wks, 9 had dropped out of the varenicline group and 15 out of the placebo group (PRISMA flow diagram says 15, text says 14). ITT analyses conducted |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None noted |
Tonstad 2006
| Methods | Country: USA (6 centres) and 'international' (18 centres, across Canada, Czech Republic, Denmark, Norway, Sweden, UK*) Setting: 24 research centres Aim: To test the efficacy and safety of extended varenicline treatment for preventing relapse in adults who have quit smoking on open‐label varenicline Dates conducted: April 2003 ‐ February 2004 (initial recruitment phase) Study Design: Double‐blind placebo‐controlled RCT. Analysis: Power calculation (80%, alpha = 0.05); ITT denominators and logistic regression analysis for binary data, and Kaplan‐Meier curve for time to first lapse |
|
| Participants | 1210 successful quitters (62.8% of initial cohort) following a 12‐wk open‐label course of varenicline for smoking cessation, randomised to varenicline (603) or placebo (607) for a further 12 wks. 49% men, 97% white, mean age 45, BMI < 15 or > 38 or weight < 45.5 kg, mean CPD 21, mean FTND score 5.4 Exclusion criteria: Standard pharmacotherapy trial criteria, + use of marijuana or tobacco products other than cigarettes within last month; use of NRT, bupropion, clonidine, nortriptyline within last month | |
| Interventions | 1. Varenicline 1 mg x 2/day for 11 wks after 1 wk titrated dosage 2. Placebo tablets, same regimen All participants also received brief counselling (≤ 10 mins) at each clinic visit throughout treatment phase (wks 13 ‐ 24). Treatment phase clinic visits were at wks 13, 14, 16, 20 and 24 Follow‐up phase: 5 visits and 4 phone calls from wks 25 ‐ 52 | |
| Outcomes | Primary outcome: Relapse prevention: maintenance of CO‐validated CAR at 24 wks Secondary outcome: CO‐validated CAR at wk 52; 7‐day PPA at wks 24 and 52. 2 deaths removed from varenicline denominator at 52 wks Other outcomes: weight change, withdrawal symptoms (using MNWS), time to first lapse, adverse events Validation was by expired CO ≤ 10 ppm Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) Attrition was 12% during treatment phase, and 10% of treatment completers lost during follow‐up phase | |
| Treatment type | Medication: VARENICLINE | |
| Notes | * additional information supplied by author The trial was funded by Pfizer Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization sequence (stratified by center with a block size of 4)" |
| Allocation concealment (selection bias) | Low risk | "a single, centralised [system]" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐blind treatment phase"; "participant blinding was maintained during this [non‐treatment follow‐up] phase" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing COs were considered abstinent if other criteria OK; at wk 52 all criteria had to be met |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes covered |
| Other bias | Unclear risk | None noted |
Tsai 2007
| Methods | Country: Taiwan and Korea Setting: 5 sites in each country Aim: To test the efficacy and safety of varenicline for smoking cessation in Taiwanese and Korean smokers. Dates conducted: February 2005 ‐ March 2006 Study Design: Double‐blind placebo‐controlled RCT Analysis: Power calculation (I am happy to talk to the CEU team and Jo while you're away, to keep things moving forward. (≥ 90%, alpha = 0.05); ITT denominators and logistic regression model including treatment and centre |
|
| Participants | 250 healthy adult volunteers, motivated to quit, aged 18 ‐ 75; allocated to varenicline (126), or placebo (124). 89% men, mean age 40.3, BMI < 15 or > 38 or weight < 45.5 kg, mean CPD 24, mean FTND score 5.1. Treatment groups were comparable at baseline Exclusion criteria: Standard pharmacotherapy trial criteria | |
| Interventions | 1. Varenicline 1.0 mg x 2/day 2. Placebo tablet x 2/day Treatment period 12 wks, 1st wk titrated dosage. All participants received a smoking cessation booklet Clearing the Air at baseline, + brief counselling (≤ 10 mins) at each clinic visit. Clinic visits at baseline and at wks 1, 2, 3, 4, 6, 8, 10, 12, plus a 5‐min phone call at +3 days post‐TQD, and at wks 5, 7, 9, 11 In follow‐up phase, clinic visits at wks 13, 16, 20, 24 plus brief phone calls at wks 14, 18, 22 | |
| Outcomes | Primary outcome: CO‐validated CAR at 9 ‐ 12 wks Secondary outcomes: CO‐validated CAR at 9 ‐ 24 wks; 7‐day PPA at wks 12 and 24 Validation was by expired CO ≤ 10 ppm Other outcomes: Withdrawal symptoms (using MNWS, QSU‐brief and mCEQ), adverse events Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) Attrition in treatment phase was 2.8%, losses to follow‐up 2.5% of treatment completers | |
| Treatment type | Medication: VARENICLINE | |
| Notes | Trial was funded by Pfizer Inc New for 2008 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly permuted blocks" (block size=4) |
| Allocation concealment (selection bias) | Low risk | web‐ and telephone‐based assignment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Subjects, investigators, study staff and sponsor personnel |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information, but very high compliance rates |
| Selective reporting (reporting bias) | Unclear risk | All expected and predicted outcomes covered |
| Other bias | Unclear risk | None noted |
Tsukahara 2010
| Methods | Country: Japan Setting: Cessation clinic in Fukuoka University Hospital Aim: To test the efficacy and safety of varenicline for smoking cessation in Japanese smokers Dates conducted: Aug 2008 ‐ November 2009 Study Design: Randomised controlled open‐label trial Study name: The VN‐SEESAW Study |
|
| Participants | 32 adult smokers, motivated to quit, allocated to varenicline (16) or nicotine patch (16). 75% men, mean age 46, mean CPD 28 (varenicline), 25 (patch), mean TDS (addiction) score 7.6, mean Brinkman index score (CPD x yrs smoking) 702. 71% had tried to quit previously, and 7% had used nicotine patches before Standard pharmacotherapy trial exclusion criteria, plus attendance at any smoking cessation clinic during previous 12m | |
| Interventions | 1. Open‐label varenicline 1.0 mg x 2/day for 12 wks, following 1 wk titration 2. Open‐label nicotine patch for 8 wks (52.5 mg/day for 4 wks, 35 mg/day for 2 wks, 17.5 mg/day for 2 wks) No non‐treatment or placebo control group Varenicline group received 8 clinic visits and nicotine group 5 visits over 12 wks, with 5 brief counselling sessions (≤ 10 mins) | |
| Outcomes | CO‐confirmed CAR at 9 ‐ 12 wks, and self‐reported at 9 ‐ 24 wks by phone interview Validation by expired CO < 8 ppm at 12 wks, but not at 24 wks Other outcomes: Safety and tolerability by wk 12, using MNWS at wks 2, 4, 8 and 12. Also used Stress Check List and Strait‐trait Anxiety Inventory Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) Attrition in treatment phase was 12.5% from each group | |
| Treatment type | Medication: VARENICLINE / NRT OPEN‐LABEL | |
| Notes | The study was supported by the Japanese Ministry of Education, Science and Culture, Fukuoka University and FU‐Global program Not included in main MA, as no placebo group New for 2010 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "by computer" allocating men: women 3:1 to reflect Japanese smoking prevalence (M: 40%, F: 12%). |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants and personnel were not blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes covered |
| Other bias | Unclear risk | None noted |
Wang 2009
| Methods | Country: China (10 sites), Singapore (3 sites), Thailand (2 sites) Aim: To test the efficacy and safety of varenicline for smoking cessation in Chinese, Singaporean and Thai smokers Dates conducted: Not stated Study Design: Double‐blind placebo‐controlled RCT. Analysis: Power calculation (≥ 90%, alpha = 0.05); ITT denominators and logistic regression model including treatment with site, country, FTND score, CPD and time to first cigarette. No interactions found |
|
| Participants | 333 healthy adult volunteers, aged 18 ‐ 75; allocated to varenicline (165), or placebo (168). 97% men, mean age 39, BMI > 15 and < 38 or weight > 45.5 kg, mean CPD 20, mean FTND score 5.4. Treatment groups were comparable at baseline. 58% had never tried to quit before Exclusion criteria: Standard pharmacotherapy trial criteria, plus any use of NRT or bupropion in previous 6m | |
| Interventions | 1. Varenicline 1.0 mg x 2/day 2. Placebo tablet x 2/day Treatment period 12 wks, 1st wk titrated dosage. All participants received a smoking cessation booklet at baseline, + brief counselling (≤ 10 mins) at each clinic visit, except for wks 5 and 7, when counselling was conducted by phone In follow‐up phase, clinic visits at wks 13, 16, 20, 24 plus brief phone calls at wks 14, 18, 22. Dosing and CO checked at each visit, and lab samples taken at wks 12 and 24 | |
| Outcomes | Primary outcome: CO‐confirmed CAR for wks 9 ‐ 12 Secondary outcomes: CO‐confirmed CAR for wks 9 ‐ 24; 7‐day PPA at 24 wks Validation by expired CO < 10 ppm Other outcomes: adverse events; long‐term quit rates Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) Attrition in treatment phase was 3.0% in varenicline group, and 3.6% in placebo group. By wk 24, 4.2% of had dropped out of each group | |
| Treatment type | Medication: VARENICLINE | |
| Notes | The trial was funded by Pfizer Inc New for 2010 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "eligible subjects were randomized in a 1:1 ratio" |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "double‐blind", but no further information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information, but very high compliance rates |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes covered |
| Other bias | Unclear risk | None noted |
Westergaard 2015
| Methods | Country: Denmark Aim: To evaluate the effect of varenicline on tobacco cessation in young smokers suffering from asthma Dates conducted: Not stated Study Design: Double‐blind placebo‐controlled RCT |
|
| Participants | 52 young (aged 19 ‐ 40) smokers with asthma, randomised to varenicline (26) or placebo (26). CPD ≥ 10; FTND 5.6 | |
| Interventions | 1. Varenicline: presumed standard regimen: Varenicline 1.0 mg x 2/day 2. Placebo tablet x 2/day No further details |
|
| Outcomes | Primary: presumed PPA at 12 wks Secondary: presumed PPA at 0, 6, 24 wks Validation by expired CO < 10 ppm Also assessed asthma symptom score, general health quality score (15D) and methacholine challenge |
|
| Treatment type | Medication: VARENICLINE | |
| Notes | Author supplied further details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. ""randomized, placebo‐controlled, double‐blinded trial" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated. "double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated; ITT analysis conducted |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Other bias | Unclear risk | Not stated |
Williams 2007
| Methods | Country: USA and Australia Setting: 9 research centres (8 USA, 1 Aus) Aim: To test the safety of long‐term (12m) use of varenicline in smokers trying to quit Study Design: Double‐blind placebo‐controlled RCT Dates conducted: October 2003 ‐ March 2005 |
|
| Participants | 377 adult smokers, aged 18 ‐ 75, smoking at least 10 CPD. 49.9% men, 88.6% white, av CPD at baseline 23, mean FTND 5.5 in treatment group, 6.05 in control group. Allocated to varenicline (251) or placebo (126) Exclusion criteria: Standard pharmacotherapy trial criteria, + no use of NRT, antidepressants, antipsychotics, naltrexone during study period. | |
| Interventions | 1. Varenicline 1mg x 2/day, titrated for first wk 2. Placebo inactive tablets, same regimen All participants received S‐H booklet Clearing the Air. Brief counselling (≤ 10 mins) at each visit TQD was 1st day of wk 1 visit (7 ‐ 10 days post‐randomisation) Treatment period was 52 wks. Weekly visits throughout wks 1 ‐ 8, then every 4 wks to wk 52, + wk 53 assessment Blood and urine samples taken at screening, baseline, wks 2, 12, 24, 36, 52 (or early termination) Complete physical exam at baseline, wks 24 and 52; BP, pulse and weight measured at all visits, ECG at screening, baseline, wks 2, 24 and 52 (or early termination) | |
| Outcomes | Primary outcome: Safety of smokers treated continuously with varenicline over 52 wks, measured at wk 53 by level and tolerability of adverse events and incidence of SAEs Secondary outcome: 7‐day CO‐verified PPA at all clinic visits (expired CO ≤ 10 ppm) Other outcomes: Weight change; changes in vital signs Attrition was 46.2% in varenicline group, 53.2% in control group by end of study | |
| Treatment type | Medication: VARENICLINE | |
| Notes | This was a safety study, with cessation rates collected as a secondary outcome The trial was funded and conducted by Pfizer Inc In the first version of this review, this trial appeared as Reeves 2006 (unpublished data) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation 2:1 varenicline to placebo. No detailed information reported |
| Allocation concealment (selection bias) | Unclear risk | No information reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing CO and/or visit taken as smokers |
| Selective reporting (reporting bias) | Low risk | Primary outcome was safety, so minimal cessation data |
| Other bias | Unclear risk | None noted |
Williams 2012
| Methods | Countries: Canada, USA Setting: 12 sites Aim: To evaluate primarily safety, but also efficacy of varenicline in smokers with schizophrenia or schizoaffective disorders Dates conducted: May 2008 ‐ April 2010 Study Design: Double‐blind placebo‐controlled RCT. Sample size [120] was considered sufficient to detect a between‐group difference in 7‐day PPA "for a medium effect size" |
|
| Participants | 128 adults, diagnosed with stable schizophrenia or schizoaffective disorders, smoking at least 15 CPD and motivated to quit. Randomised to varenicline (85) or placebo (43). 77% men aged 18 ‐ 75 | |
| Interventions | 1. Varenicline 1.0 mg x 2/d for 12 wks, including wk 1 at titrated dose 2. Placebo tablets as above. Weekly clinic visits, for safety and efficacy, ≤ 30‐min counselling sessions; after treatment phase, clinic visits at wks 13, 16, 20, 24, with brief phone calls at wks 14, 18 and 22. Follow‐up sessions included brief (≤ 10 mins) counselling. AEs collected to 30 days after treatment, and neuropsychiatric AEs to wk 24 |
|
| Outcomes | Primary outcome: N of participants with adverse and serious adverse events from baseline to 30 days after end of treatment (12 wks). N of participants with psychiatric adverse events, including suicidal ideation or behaviour Secondary outcomes: CO‐confirmed PPA at wks 12 and 24; 50%+ reduction in CPD; change in CPD from baseline. Assessments on mood and psychiatric scales Validation was by exhaled CO ≤ 10 ppm Dropouts in treatment phase: 14 (varenicline), 3 (placebo); follow‐up phase: 10 (varenicline), 3 (placebo) 1 varenicline participant died during follow‐up phase |
|
| Treatment type | Medication: VARENICLINE | |
| Notes | The study was funded by Pfizer New for 2012 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomized (2:1) to varenicline or placebo ... and were stratified according to antipsychotic medication type (typical vs atypical)." |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not yet reported |
Wong 2012
| Methods | Country: Canada Setting: 2 Toronto hospitals Aim: "to determine the effectiveness and safety of a perioperative smoking cessation intervention including varenicline and counseling versus placebo and counseling to increase short‐ and long‐term abstinence in surgical patients" Study Design: Randomised placebo‐controlled double‐blind trial Dates conducted: June 2008 ‐ November 2010 Analysis: Sample sizes of 145 in each group, estimated to give 80% power to detect a risk difference of 15% at 12 months between active and placebo groups |
|
| Participants | 286 non‐cardiac elective surgery patients, smoking 10+ CPD, no abstinence > 3m in last year, scheduled for surgery in the next 8 ‐ 30 days. Allocated to varenicline (151) or placebo (135). Mean age 52.6, 47% women, mean CPD 17.4, mean FTND 4.8 | |
| Interventions | All participants received 2 standardised 15‐min counselling sessions by researchers, 1 in pre‐op clinic and 1 24 hours after surgery, supplemented by written materials. All participants retained the same counsellor throughout the process Weekly counselling phone calls for 4 weeks, and at the end of 8 weeks. From 3 ‐ 12 months, phone calls every 4 weeks for smoking status, nicotine dependence, stage of change, CPD, brief (< 5 mins) counselling. TQD was set for 24 hours before surgery, and medication begun 7 days before TQD 1. Varenicline: 12 wks standard regimen, 1st wk titrated 2. Placebo: Identical‐looking tablets and regimen Participants were invited to visit the hospital at 3, 6, and 12m, for assessment and testing. Participants unable to visit the hospital were sent a self‐test urinary kit |
|
| Outcomes | 7‐day PPA at 12m; abstinence on TQD; 7‐day PPA at 3m and 6m. Self‐reported changes in CPD and stage of change at 3, 6 and 12m Validation: Expired CO and urinary cotinine (cut‐offs not given) |
|
| Treatment type | Medication: VARENICLINE | |
| Notes | Supported by Canadian academic institutes and Pfizer Canada New for 2016 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Smokers were randomly assigned to receive varenicline (Pfizer Inc., Kirkland, Quebec, Canada) or matching placebo using a computer‐generated randomization list at each center. A stratified randomization with blocks of 40, based on the smoker’s stage of change, was employed because the stage of change may predict successful abstinence from smoking." |
| Allocation concealment (selection bias) | Low risk | "The patient assignments were placed into sequentially numbered, opaque sealed envelopes, and were kept by an independent research pharmacist at each center who was not involved with patient care or outcome assessments. For each patient, the research pharmacist opened the envelope and provided the research coordinator with the medication or placebo (lactose, identical in appearance) according to the randomization schedule." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The patients, healthcare personnel, and research staff were blinded to the randomization throughout the study period." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses fully reported: Varenicline: 6 discontinued treatment, 11 discontinued follow‐up. Placebo: 6 discontinued treatment, 10 discontinued follow‐up. ITT analyses conducted |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None noted |
Brandon 2011*
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Treatment type | ||
| Notes | Treated as an included study in order to contribute to adverse event meta‐analyses; Excluded for efficacy: Short‐term (15 days) RCT, to test craving and psychological reward; cessation was not an outcome | |
Ebbert 2011*
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Treatment type | ||
| Notes | Treated as an included study in order to contribute to adverse event meta‐analyses; Excluded for efficacy: Pilot study of varenicline for smokeless tobacco users. 12‐wk outcome (EoT) reported, not long‐term post‐treatment. | |
Faessel 2009*
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Treatment type | ||
| Notes | Treated as an included study in order to contribute to adverse event meta‐analyses; Excluded for efficacy: Outcomes were safety, tolerability and pharmacokinetics, not smoking cessation. | |
Fagerström 2010*
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Treatment type | ||
| Notes | Treated as an included study in order to contribute to adverse event meta‐analyses; Excluded for efficacy: 431 smokeless tobacco users in Norway and Sweden, randomised to varenicline or placebo; CAR assessed at 12 and 26 weeks. | |
Garza 2011*
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Treatment type | ||
| Notes | Treated as an included study in order to contribute to adverse event meta‐analyses; Excluded for efficacy: 110 abstinent smokers treated with varenicline or placebo, to assess incidence and severity of neuropsychiatric symptoms; not a cessation trial. | |
Hughes 2011*
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Treatment type | ||
| Notes | Treated as an included study in order to contribute to adverse event meta‐analyses; Excluded for efficacy: 218 smokers not ready to quit assigned to varenicline or placebo for 2 ‐ 8 weeks for cigarette reduction; abstinence was not the outcome of interest, although measured in those who made a quit attempt. Primary outcome was incidence of quit attempts over 6m. | |
McClure 2013* NCT00944554
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Treatment type | ||
| Notes | Treated as an included study in order to contribute to adverse event meta‐analyses; Excluded for efficacy: New for 2016 update. Laboratory study following an RCT of varenicline in a programmed lapse; abstinence only to 4 weeks. | |
Meszaros 2013*
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Treatment type | ||
| Notes | Treated as an included study in order to contribute to adverse event meta‐analyses; Excluded for efficacy: New for 2016 update. Pilot study (10 participants, only 4 completers), only followed to 3m; objective was reduction, not cessation. | |
Mitchell 2012*
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Treatment type | ||
| Notes | Treated as an included study in order to contribute to adverse event meta‐analyses; Excluded for efficacy: New for 2016 update. Varenicline was for drinking reduction, not smoking cessation; only followed for 12 weeks. | |
BMI: Body Mass Index (kg/m2) CAR: Continuous Abstinence Rate CO: carbon monoxide COPD: chronic obstructive pulmonary disease CPD: cigarettes per day CQR: continuous quit rate CVD: cardiovascular disease EoT: end of treatment FTND: Fagerström Test for Nicotine Dependence ITT: intention‐to‐treat LOCF: last observation carried forward MA: meta‐analysis MDD: major depressive disorder MI: motivational interviewing mCEQ: Modified Cigarette Evaluation Questionnaire MNWS: Minnesota Nicotine Withdrawal Scale PA prolonged abstinence PPA: point‐prevalence abstinence QoL: quality of life QSU‐brief: Brief Questionnaire of Smoking Urges RCT: randomised controlled trial SAE: serious adverse event SC: smoking cessation TQD: target quit date
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Burstein 2006 | RCT of tolerability and safety of varenicline in 24 elderly (≥ 65) smokers for 1 week. Not a cessation trial. |
| Chantix 2006 | 39 smokers randomised to NRT alone (17) or varenicline + NRT (22) for 12 days to test safety and side effects of co‐administration. 36% of combined group discontinued, compared with 6% of NRT alone group. |
| Cui 2012 | Open‐label non‐randomised pre/post study of 36 HIV+ participants; all got varenicline, assessed at wks 12 and 24. |
| Dezee 2013 | New for 2016 update. RCT in which all participants were given varenicline; intervention tested was in‐person vs internet counselling. |
| Dutra 2012 | 53 participants with schizophrenia given varenicline + CBT. Abstinence assessed at 12 weeks (end of treatment). |
| Ebbert 2009a | Open‐label, single‐arm Phase II study, for safety and efficacy of varenicline plus bupropion. |
| Ebbert 2009b | Cohort analysis of 104 participants on varenicline + NRT and 135 participants treated prior to release of varenicline (93% used NRT). |
| Ebbert 2014 | New for 2016 update. RCT in which all participants were given varenicline; the intervention being tested was bupropion vs placebo. See also Hong 2011 NCT00492349 |
| Falk 2014 | Varenicline was used for alcohol reduction, not for smoking |
| Fatemi 2013 NCT01111149 | New for 2016 update. 3‐arm RCT of varenicline, bupropion and placebo; only assessed to end of treatment (12 weeks). |
| Ferketich 2012 | New for 2016 update. Pilot study of varenicline vs NRT; participants could choose their treatment; intervention being tested was the addition of a lung cancer screening programme. |
| Ferketich 2013 | New for 2016 update. Safety of varenicline among smokers enrolled in the Lung HIV study. Participants could choose varenicline or NRT, and were only followed for 3 months. |
| Frye 2013 | New for 2016 update. Small (9 participants) feasibility study in bipolar participants, open‐label, followed only until end of treatment (12 weeks). |
| Fucito 2011 | RCT of 30 heavy‐drinking smokers, assigned to pre‐treatment varenicline or placebo, prior to 4 wks varenicline; primary outcome was effects on drinking behaviour, but smoking status at end of study (8 wks) was also measured. |
| Granatowicz 1976 | Polish uncontrolled study of 1968 smokers, 71% taking cytisine, followed for 6m. |
| Gray 2012 | New for 2016 update. Pilot study of varenicline vs bupropion in older adolescents; outcome was reduction rather than cessation, and participants were only followed for 3 months. |
| Hajek 2011 | 101 smokers randomised to preloaded varenicline or placebo; abstinence not measured beyond 12 weeks. |
| Hajek 2013 | New for 2016 update. All were given varenicline, with the intervention tested being the addition of a NRT patch. Only followed to 3 months. |
| Hartwell 2014 | Varenicline for drinking and smoking; smoking topography and pharmacogenetics rather than smoking cessation |
| Hawk 2012 NCT00835900 | New for 2016 update. RCT of extended pre‐TQD varenicline vs standard regimen; all participants got varenicline, and were followed only until end of treatment (12 weeks). |
| Hong 2011 NCT00492349 | New for 2016 update. Secondary analysis to Ebbert 2014, looking at depression in recipients of varenicline + bupropion vs varenicline alone |
| Hoogsteder 2014 | New for 2016 update. All participants were given open‐label varenicline; the intervention being tested was the addition of NicVAX. |
| Hsueh 2014 | New for 2016 update. Open‐label cohort study of smokers taking varenicline or NRT. |
| Jain 2014 | New for 2016 update. RCT of smokeless tobacco users in India (to be covered in our review of interventions for smokeless tobacco). |
| Jennings 2014 | New for 2016 update. The EUROACTION PLUS study; a complex nurse‐led intervention for smokers at high risk of CVD. Varenicline was a treatment option. Only followed to 16 weeks. |
| Jiménez‐Ruiz 2013 | New for 2016 update. Cohort study of smokers not responding to standard varenicline dosage by 8 weeks treated with varenicline 3 mg/day in 2 Spanish smoking cessation clinics. |
| Kempe 1967 | Bulgarian 1965 observational uncontrolled study of 30 male smokers given cytisine (Tabex) for 25 days and followed up for 6m. |
| Koegelenberg 2014 | New for 2016 update. All participants took varenicline; the intervention being tested was the addition of NRT. |
| Maliszewski 1972 | Polish uncontrolled study of 14 smokers on a 25‐day course of cytisine (Tabex); followed up for 2 wks. |
| Marakulin 1984 | Russian trial of 620 smokers; no placebo, but autogenic training for control group. Follow‐up 6 wks |
| McColl 2008 | RCT of varenicline's potential as an abuse drug in smokers and non‐smokers; not a smoking cessation trial. |
| McNaughton 2013 | New for 2016 update. All participants received varenicline; the intervention being tested, as a relapse prevention aid, was interactive voice response phone calls |
| Metelitsa 1987 | Russian uncontrolled study of 281 smokers, comparing anabasine hydrochloride, cytisine or a combination of both drugs, taken as biosoluble film on a paper or fabric patches. Followed for 6 ‐ 14m. |
| Mocking 2013 | New for 2016 update. 7‐day administration of varenicline for emotional and cognitive processing in non‐smokers. |
| Mocking 2014 | New for 2016 update. 7‐day administration of varenicline for cortisol levels, not for smoking cessation. |
| Monova 2004 | Bulgarian RCT of 150 moderate+ smokers; investigators did not instruct participants to stop smoking, but monitored their smoking behaviour during and after a 25‐day course of cytisine (Tabex). Follow‐up was 60 days. |
| NCT00502216 | New for 2016 update. Study of varenicline and naltrexone for tolerability and weight gain in smokers, not cessation. |
| NCT01308736 | Outcome was 50% reduction in smoking, not abstinence (none succeeded in quitting completely). |
| NCT01806779 | All participants got varenicline; the addition of bupropion was the intervention being tested. |
| Nollen 2011 | RCT of 72 black smokers; all received varenicline, but half got extended counselling and half a single session. Cessation only measured to 3m endpoint. |
| Ostrovskaia 1994 | Russian uncontrolled study of 74 smokers, comparing anabasin, cytisine or combination therapy, in film patches. (Relates to Metelitsa 4‐stage study). Followed for 6 ‐ 14m. |
| Park 2011 | RCT of 49 smokers with lung cancer randomised to varenicline or placebo; Follow‐up only for 12 weeks to end of treatment. |
| Patterson 2010 | New for 2016 update. Short‐term (3‐week) study of propensity to relapse with working memory deficits after 10 days of varenicline. |
| Paun 1968 | Bulgarian controlled trial of cytisine (Tabex) (366 smokers) vs placebo (239 smokers) but followed only for 8 wks. Observational study of 230 cytisine‐users followed for 26 wks, but no comparator group. |
| Pfizer 2006 | Phase II flexible‐dosing trial of varenicline in 312 participants. Treatment lasted 12 weeks, and cessation outcomes reported for continuous abstinence through weeks 9 ‐ 12. |
| Poling 2010 | RCT of varenicline in 31 methadone‐maintained smokers; trial lasted 3m, and reduction was an outcome of interest (though 3m abstinence was reported). |
| Ramon 2014 | New for 2016 update. RCT in which all participants received varenicline; intervention being tested was the addition of NRT. |
| Rose 2014 | New for 2016 update. RCT of varenicline versus varenicline + bupropion, in smokers who had failed to quit on NRT. All got varenicline. |
| Schlienz 2014 | New for 2016 update. 4 weeks treatment with varenicline; outcome was impact on behavioural economic indices, not smoking cessation. |
| Schmidt 1974 | Non‐randomised trial of 16 smoking cessation preparations, including cytisine (Tabex) (200 smokers); participants allocated to treatment 'by chance', and followed up over 3m. Placebo group not directly matched to cytisine (Tabex) group. |
| Schnoll 2011 | New for 2016 update. RCT of open‐label varenicline + counselling; intervention being tested was recruitment strategies, not smoking cessation. |
| Shim 2011 | 60 smokers with schizophrenia randomised to varenicline or placebo for 8 weeks; assessment at end of treatment, reduction but not abstinence rates reported. |
| Sicras‐Mainar 2010 | Multicentre observational non‐randomised non‐controlled study. |
| Stapleton 2008 | Non‐randomised trial of 412 attenders at a London smoking cessation clinic, choosing either NRT (single product or combination) or varenicline. NRT arm were historical controls. Effectiveness and safety were assessed separately in a subset of 111 participants receiving treatment for mental illness. |
| Stoyanov 1972 | 87 smokers (17 of them psychiatric patients); observational study with no comparator group and short but unstated length of follow‐up. |
| Swan 2010 | All participants were given varenicline (treated as an included study for 2012 update). |
| Weiner 2011 | 9 smokers with schizophrenia randomised to varenicline or placebo; final assessment was at 12 weeks (end of treatment). |
| Zatonski 2006 | Polish uncontrolled observational study of 342 smokers; at 12 months 13.8% abstinent. |
Characteristics of studies awaiting assessment [ordered by study ID]
Wiratmoko 2013
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Abstract only; further details awaited |
Yujie 2014
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Abstract only; further details awaited |
Zincir 2013
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not in English; may be too short‐term to include |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12613000854730
| Trial name or title | TALANOA Samoa: A randomised controlled trial to evaluate the efficacy of a cessation support programme for smokers delivered by radio |
| Methods | Open‐label parallel‐group efficacy RCT |
| Participants | Up to 130 adults (16+), tobacco smoker, speaking Samoan, resident in Auckland NZ |
| Interventions | 10 weekly ½‐hour radio programmes of behavioural advice |
| Outcomes | Prevalence of quitting at 3m, CO‐verified (< 10 ppm). N of quit attempts, successful or not |
| Starting date | September 2013 |
| Contact information | PI: v.nosa@auckland.ac.nz; Scientific enquiries: d.gentles@auckland.ac.nz |
| Notes |
ACTRN12614000329662
| Trial name or title | Examination of mechanism of action of pre‐quit use of nicotine patch and varenicline for smoking cessation [PQT] |
| Methods | Open‐label parallel‐group efficacy RCT |
| Participants | Up to 216 adults (18+), smoking 15+ CPD, high motivation to quit, willing and able to take either medication. |
| Interventions | (i) Varenicline, 2 wks before TQD and 4 wks after; or (ii) NRT 21 mg patch, starting 2 wks before TQD |
| Outcomes | Primary: 1. CPD reduction in 1st 2 wks 2. Measures of craving 3. Smoking satisfaction Secondary: 1. CO‐validated abstinence at 28 days post‐TQD |
| Starting date | March 2014 |
| Contact information | PI and enquiries: stuart.ferguson@utas.edu.au |
| Notes |
ACTRN12614000876695
| Trial name or title | Improving radiotherapy outcomes with smoking cessation: feasibility trial in head and neck cancer patients [Health Steps] |
| Methods | Parallel blinded safety/efficacy RCT |
| Participants | Up to 40 head‐and‐neck cancer patients, smoking at least 5 CPD, scheduled for radiotherapy |
| Interventions | 3m varenicline + 10 sessions manual‐based MI; control get TAU, no varenicline |
| Outcomes | Feasibility and acceptability, i.e. compliance, tolerability Continuous abstinence up to 6m post‐radiotherapy, CO‐validated |
| Starting date | August 2014 |
| Contact information | PI and enquiries: ben.britton@hnehealth.nsw.gov.au |
| Notes |
Ameridian 2007
| Trial name or title | Efficacy and safety of dianicline treatment as an aid to smoking cessation in cigarette smokers (AMERIDIAN) |
| Methods | Double‐blind placebo‐controlled RCT; companion study to EURODIAN trial (see Tonstad 2011). |
| Participants | 600 adult smokers in USA, Canada |
| Interventions | Dianicline 40 mg bid for 7 wks, vs placebo (same regimen) |
| Outcomes | CAR at wks 4 ‐ 7. Craving and withdrawal symptoms |
| Starting date | September 2006 |
| Contact information | Sanofi‐Aventis |
| Notes | Information taken from ClinicalTrials.gov; results not yet reported. |
EUCTR2009‐017599‐26‐IT
| Trial name or title | Efficacy and safety of smoking cessation with varenicline tartrate in diabetic smokers: a double‐blind, placebo‐controlled randomised trial |
| Methods | RCT |
| Participants | Elderly adults, aged 75+, with type 2 diabetes |
| Interventions | 1 mg x 2/day |
| Outcomes | CQR at wk 24 |
| Starting date | 22nd January 2010 |
| Contact information | |
| Notes |
ISRCTN25441641
| Trial name or title | Evaluation of the impact of systematic delivery of cessation interventions on delivery of smoking cessation in secondary care [Exploring ways to help hospital patients stop smoking] |
| Methods | RCT |
| Participants | Adult smokers |
| Interventions | NRT + counselling, with varenicline or bupropion offered to those who do not wish to take NRT |
| Outcomes | 1m abstinence after discharge from hospital |
| Starting date | October 2010 |
| Contact information | Kapka Nilan, University of Nottingham, UK |
| Notes |
Nahvi 2014b
| Trial name or title | Varenicline smoking cessation treatment for methadone maintenance patients |
| Methods | Open‐label parallel‐group efficacy RCT |
| Participants | 100 adult methadone‐maintained smokers, at least 5 CPD, interested in quitting |
| Interventions | Directly‐observed varenicline treatment versus TAU (self‐administered varenicline) |
| Outcomes | CO‐verified abstinence at 12 wks Varenicline adherence; tobacco use measures; reduction in CPD |
| Starting date | July 2011 |
| Contact information | Shadi Nahvi |
| Notes |
| Trial name or title | Comparison of varenicline and placebo for smoking cessation in schizophrenia |
| Methods | Randomised double‐blind placebo‐controlled trial |
| Participants | 44 smokers with schizophrenia |
| Interventions | 12 weeks varenicline 1.0 mg x 2/day vs placebo |
| Outcomes | PPA at 12 weeks, neuropsychiatric symptoms |
| Starting date | November 2007 |
| Contact information | E Weiner |
| Notes |
| Trial name or title | The effect of varenicline (Chantix) and bupropion (Zyban) on smoking lapse behavior |
| Methods | Randomised triple‐blind factorial trial |
| Participants | 60 adult smokers |
| Interventions | 8‐day course of varenicline, bupropion or placebo |
| Outcomes | Latency to initiate ad‐lib smoking |
| Starting date | April 2007 |
| Contact information | SA McKee |
| Notes |
| Trial name or title | Contingency management and pharmacotherapy for smoking cessation (Donaghue) |
| Methods | Open‐label parallel‐group efficacy RCT |
| Participants | 59 adults, smoking 10+ CPD, motivated to quit (intended to recruit 70) |
| Interventions | All on 12 wks varenicline + brief counselling; tested intervention is the addition of contingent prizes for quitting |
| Outcomes | CO‐ and cotinine‐verified abstinence at wks 5, 12 and 24 |
| Starting date | May 2008 |
| Contact information | Sheila M Alessi |
| Notes |
| Trial name or title | Improving varenicline adherence and outcomes in homeless smokers |
| Methods | Open‐label parallel‐group efficacy RCT |
| Participants | 428 adult homeless smokers, at least 5 CPD |
| Interventions | Varenicline + MI sessions vs varenicline + brief advice (testing MI) |
| Outcomes | 7‐day PPA at 6m Adherence at 12 wks and 6m; moderating effects of psychiatric comorbidities |
| Starting date | September 2007 |
| Contact information | Kolawole S Okuyemi |
| Notes |
| Trial name or title | Smoking study with behavioural therapy for hypertensive patients (VANQUISH) |
| Methods | Open‐label parallel‐group efficacy RCT |
| Participants | 260 hypertensive adult smokers |
| Interventions | Varenicline alone vs varenicline + behavioural therapy |
| Outcomes | Abstinence at 5, 6, 8, 12, 24, 36, 52 wks |
| Starting date | April 2009 |
| Contact information | William B White |
| Notes |
| Trial name or title | Methadone maintenance treatment and smoking cessation (MMTASC) |
| Methods | Randomised double‐blind placebo‐controlled trial |
| Participants | 112 smokers on methadone maintenance for opioid dependence |
| Interventions | Varenicline 1.0 mg x 2/day vs placebo for 12 wks |
| Outcomes | 7‐day PPA at 26 weeks |
| Starting date | May 2009 |
| Contact information | Milan Khara |
| Notes |
| Trial name or title | Comparison of the efficacy and safety of varenicline versus placebo for smoking cessation among HIV‐infected patients (Inter‐ACTIV) |
| Methods | Randomised quadruple‐blind placebo‐controlled trial |
| Participants | 254 smokers diagnosed with HIV infection |
| Interventions | Varenicline 1.0 mg x 2/day vs placebo for 12 weeks |
| Outcomes | CAR for wks 9 ‐ 48 |
| Starting date | October 2009 |
| Contact information | Patrick Mercie |
| Notes |
| Trial name or title | Exercise or relaxation for smoking cessation |
| Methods | Open‐label parallel‐group efficacy RCT |
| Participants | 364 postmenopausal (aged 45+) women smokers, 10+ CPD, motivated to quit and to exercise |
| Interventions | Varenicline + counselling + exercise programme vs varenicline + counselling + relaxation programme |
| Outcomes | Abstinence at wks 12 and 64 |
| Starting date | March 2009 |
| Contact information | Cheryl Oncken |
| Notes |
| Trial name or title | Smoking cessation treatment for head and neck cancer patients |
| Methods | Randomised open‐label trial |
| Participants | 30 smokers diagnosed with head and neck cancer |
| Interventions | Varenicline 1.0 mg x 2/day vs 21 mg nicotine patch for 8 weeks |
| Outcomes | CAR at wks 5 ‐ 8 |
| Starting date | July 2009 |
| Contact information | Benjamin Toll |
| Notes |
| Trial name or title | Treatment of smoking among individuals with PTSD |
| Methods | Single‐blind parallel‐group RCT |
| Participants | 166 treatment‐seeking smokers, aged 18 ‐ 75, ≥ 10 CPD, diagnosed with chronic PTSD |
| Interventions | Varenicline + SC counselling + CBT vs varenicline + SC counselling |
| Outcomes | 7‐day PPA at end of treatment and at 6m |
| Starting date | January 2009 |
| Contact information | Edna B Foa |
| Notes |
| Trial name or title | Effectiveness of varenicline vs. varenicline plus bupropion or placebo for smoking cessation |
| Methods | Randomised triple‐blind placebo‐controlled trial |
| Participants | 350 adult smokers |
| Interventions | (Varenicline + bupropion) vs (varenicline + placebo) vs double placebo, for 12 weeks |
| Outcomes | Quit rate at 12 weeks |
| Starting date | May 2010 |
| Contact information | Paul Cinciripini |
| Notes |
NCT01067612
| Trial name or title | Extended treatment for smoking cessation |
| Methods | Randomised open‐label trial |
| Participants | 400 adult smokers |
| Interventions | 10‐wk open‐label phase of CBT + bupropion and NRT; those still smoking at 10 wks will be switched to 16 wks of varenicline. All will get CBT to 26 wks |
| Outcomes | Smoking abstinence at 52 and 104 wks |
| Starting date | March 2010 |
| Contact information | Joel D Killen |
| Notes |
| Trial name or title | Varenicline for smoking cessation/reduction in patients with bipolar disorder |
| Methods | Randomised placebo‐controlled quadruple‐blind trial |
| Participants | 30 adult smokers with bipolar disorder |
| Interventions | Varenicline flexible dosing (0.5 to 2.0 mg/day) vs placebo for 10 weeks. All get group CBT. |
| Outcomes | Smoking cessation and safety at 10 weeks |
| Starting date | November 2009 |
| Contact information | Tony George |
| Notes |
| Trial name or title | Maintaining nonsmoking |
| Methods | Open‐label 4‐arm randomised trial |
| Participants | 271 adult smokers (5+ CPD), who have all completed 12‐wk course of varenicline + counselling |
| Interventions | After treatment, participants are randomised to: 1. Extended brief contact, or 2. Extended health education, or 3. Extended relapse prevention + varenicline, or 4. Extended relapse prevention |
| Outcomes | Smoking status at 12, 24, 52, 64 and 104 wks |
| Starting date | May 2010 |
| Contact information | Not named (U of California, San Francisco) |
| Notes |
| Trial name or title | Safety and efficacy of varenicline in patients with acute coronary syndrome |
| Methods | Randomised placebo‐controlled double‐blind trial |
| Participants | 100 adult smokers with acute coronary syndrome |
| Interventions | Varenicline 100 [sic] mg bid |
| Outcomes | Nicotine levels at 1 month; recurrent myocardial ischaemia |
| Starting date | January 2008 |
| Contact information | Marc Cohen |
| Notes |
| Trial name or title | Smoking cessation program in the preadmission clinic: the use of a teachable moment |
| Methods | Double‐blind parallel‐group RCT |
| Participants | 300 smokers scheduled for elective surgery, aged 18+, smoking 10+ CPD |
| Interventions | Varenicline vs placebo |
| Outcomes | Abstinence at 24 and 52 wks |
| Starting date | November 2007 |
| Contact information | Francis Chung |
| Notes |
| Trial name or title | Varenicline in residential treatment (ViRT) |
| Methods | Phase IV randomised triple‐blind controlled trial |
| Participants | 50 smokers undergoing inpatient treatment for alcohol dependence |
| Interventions | Varenicline 1 mg x 2/day for 12 weeks vs placebo |
| Outcomes | Abstinence at end of treatment and 30‐day CAR at 6m |
| Starting date | June 2011 |
| Contact information | Laurie Zawertailo |
| Notes |
| Trial name or title | Smoking cessation study in healthy adolescent smokers |
| Methods | Phase IV randomised triple‐blind placebo‐controlled trial |
| Participants | 300 healthy adolescents (12 ‐ 19 yrs) smoking at least 5 CPD, with at least 1 failed quit attempt |
| Interventions | Varenicline 1 mg x 2/day vs varenicline 0.5 mg x 2/day vs placebo |
| Outcomes | CAR at weeks 9 ‐ 12, 9 ‐ 24, 9 ‐ 52; 7‐day PPA at wks 12, 24, 52; CPD reduction |
| Starting date | April 2011 |
| Contact information | Pfizer Inc |
| Notes |
| Trial name or title | Pharmacogenetics of nicotine addiction treatment |
| Methods | Phase III randomised triple‐blind placebo‐controlled trial |
| Participants | 1350 adult smokers, stratified by nicotine metabolite ratio (NMR) |
| Interventions | Varenicline 1 mg x 2/day + placebo patch vs NRT patch + placebo pills vs placebo pills + placebo patch |
| Outcomes | 7‐day PPA at 11 weeks, CAR at 11 weeks, cost effectiveness, time to relapse |
| Starting date | January 2011 |
| Contact information | Caryn Lerman |
| Notes |
| Trial name or title | Smoking cessation program in the preadmission clinic: the combination of counseling, pharmacotherapy and quit line |
| Methods | Randomised open‐label controlled trial |
| Participants | 296 adult smokers scheduled for elective surgery |
| Interventions | Counselling + 12 weeks varenicline + proactive telephone support, vs standard care (brief information + smokers help line) |
| Outcomes | 4‐wk CAR at 4, 12, 24 and 52 weeks; 24 hr PPA |
| Starting date | December 2010 |
| Contact information | Francis Chung |
| Notes |
| Trial name or title | Efficacy and safety of smoking cessation with varenicline tartrate in diabetic smokers (DIASMOKE) |
| Methods | Randomised double‐blind placebo‐controlled trial |
| Participants | 300 adult smokers with type 2 diabetes |
| Interventions | Varenicline 1 mg x 2/day for 12 weeks vs placebo |
| Outcomes | CAR at week 24; safety; CAR at week 52; adverse events |
| Starting date | June 2011 |
| Contact information | Riccardo Polosa |
| Notes |
| Trial name or title | Varenicline inpatient study [VIP] |
| Methods | Double‐blind parallel‐group RCT |
| Participants | 80 (40m, 40f) smokers hospitalised, 10+ CPD, with admission of at least 3 days |
| Interventions | Varenicline + counselling vs placebo + counselling |
| Outcomes | 7‐day PPA after 4 wks of treatment |
| Starting date | August 2011 |
| Contact information | Judith J Prochaska |
| Notes |
| Trial name or title | Varenicline for adolescent smoking cessation |
| Methods | Double‐blind parallel‐group RCT |
| Participants | 166 adolescents aged 14 ‐ 21, daily smoker for 6+ months, motivated to quit and failed at least 1 quit attempt |
| Interventions | Varenicline vs placebo for 12 wks |
| Outcomes | Self‐reported CPD; CO‐validated smoking status at 26 wks; adverse events |
| Starting date | August 2012 |
| Contact information | Kevin M Gray |
| Notes |
| Trial name or title | Smoking habits and smoking cessation in young adults |
| Methods | Single‐blind 4‐arm parallel‐group RCT |
| Participants | 300 young adults (18 ‐ 26), smoking at least 1 CPD |
| Interventions | Varenicline, 10 mg nicotine patch, 15 mg nicotine patch, placebo |
| Outcomes | CAR at 12m |
| Starting date | May 2012 |
| Contact information | Tuula Toljamo |
| Notes |
| Trial name or title | Tobacco dependence in beast cancer patients trial of varenicline (Chantix) |
| Methods | Double‐blind parallel‐group RCT |
| Participants | 30 women smokers diagnosed with breast cancer, scheduled for mastectomy and breast reconstruction |
| Interventions | Varenicline + counselling vs placebo + counselling |
| Outcomes | PPA and CA at 2 yrs |
| Starting date | February 2012 |
| Contact information | Jamie Ostroff |
| Notes |
| Trial name or title | Clinical trial to evaluate the efficacy of smoking cessation (COMBIVAR) |
| Methods | Double‐blind parallel‐group RCT |
| Participants | Adult smokers (18 ‐ 65), smoking 20+ CPD |
| Interventions | Varenicline + nicotine patches versus varenicline + placebo patches |
| Outcomes | CAR at wk 12, 24, 36. 52; safety |
| Starting date | February 2012 |
| Contact information | Josep Maria Ramon Torrell |
| Notes | Will be excluded, as intervention being tested is nicotine patches |
| Trial name or title | Varenicline treatment of alcohol dependence in smokers |
| Methods | Double‐blind parallel‐group RCT |
| Participants | Smokers seeking treatment for alcohol dependence |
| Interventions | Varenicline versus placebo, 16 weeks |
| Outcomes | Primarily N of drinking days, but also self‐reported abstinence in the last month of treatment |
| Starting date | February 2012 |
| Contact information | SS O'Malley |
| Notes | May be excluded, as primarily about alcohol abuse |
| Trial name or title | Study to evaluate cardiac assessments following different treatments of smoking cessation medications in subjects with and without psychiatric disorders [CATS] |
| Methods | Double‐blind 4‐arm parallel‐group RCT |
| Participants | 6800 adult smokers, 10+ CPD, motivated to quit; Neuropsychiatric subgroup must have 'proper diagnosis as outlined in protocol' |
| Interventions | Placebo, varenicline, NRT patch, bupropion |
| Outcomes | 1. Time to major adverse cardiac event (MACE) up to 52 wks 2. Abstinence at wk 12, 24 |
| Starting date | May 2012 |
| Contact information | Pfizer,GSK |
| Notes |
| Trial name or title | Tailored tobacco quitline for rural veterans |
| Methods | Double‐blind parallel‐group RCT |
| Participants | 50 adult smokers, rural‐dwelling veterans |
| Interventions | Tailored behavioural and pharmacotherapy group vs Enhanced standard of care + pharmacotherapy group |
| Outcomes | Treatment satisfaction; 7‐day PPA and PA at 6m |
| Starting date | June 2012 |
| Contact information | Mark VanderWeg |
| Notes |
| Trial name or title | Reducing cardiovascular disease by combining smoking cessation pharmacotherapy and behavioural counseling (RW) |
| Methods | Open‐label parallel‐assignment RCT |
| Participants | Adult smokers, 10+ CPD |
| Interventions | Nicotine patch versus nicotine patch + gum or inhaler versus varenicline |
| Outcomes | CO‐confirmed CAR at 10, 22 and 52 weeks |
| Starting date | July 2011 |
| Contact information | Heather Tulloch |
| Notes | May be related to Tulloch 2014 |
| Trial name or title | Varenicline for light smokers (ChanLight) |
| Methods | Double‐blind parallel‐group RCT |
| Participants | Adult smokers, smoking 5 ‐ 10 CPD for last 6m |
| Interventions | Varenicline versus placebo |
| Outcomes | Abstinence at 12 weeks (end of treatment) |
| Starting date | July 2012 |
| Contact information | Jon Ebbert |
| Notes |
| Trial name or title | Efficacy of varenicline on smoking cessation at the acute phase of an exacerbation of chronic obstructive pulmonary disease (SAVE) |
| Methods | Double‐blind parallel‐group RCT |
| Participants | 276 adult patients hospitalised with acute COPD, smoking 10+ CPD, motivated to quit |
| Interventions | Varenicline + counselling vs placebo + counselling |
| Outcomes | Abstinence at 1 yr; side effects and tolerance at 3m |
| Starting date | August 2012 |
| Contact information | Francis Couturaud |
| Notes |
| Trial name or title | Varenicline for nicotine dependence among those with HIV/AIDS |
| Methods | Double‐blind parallel‐group RCT |
| Participants | 350 adult smokers diagnosed with HIV, 5+ CPD |
| Interventions | Varenicline + counselling vs placebo + counselling |
| Outcomes | 7‐day PPA and CA cotinine‐confirmed at 24 wks |
| Starting date | October 2012 |
| Contact information | Robert A Schnoll |
| Notes |
| Trial name or title | Extended varenicline treatment for smoking among cancer patients |
| Methods | Double‐blind parallel‐group RCT |
| Participants | 400 adult smokers with a current or last 5 years cancer diagnosis, 5+ CPD |
| Interventions | 24 wks varenicline + counselling vs 12 wks varenicline + 12 wks placebo + counselling |
| Outcomes | 7‐day PPA, CA, PA CO‐verified at wk 24, wk 52 |
| Starting date | January 2013 |
| Contact information | |
| Notes |
| Trial name or title | Varenicline or nicotine patch in promoting smoking cessation among current smokers |
| Methods | Open‐lable parallel‐group RCT |
| Participants | 300 adult smokers calling quitline |
| Interventions | Varenicline + counselling vs nicotine patch + counselling |
| Outcomes | 4m quit rate |
| Starting date | October 2012 |
| Contact information | Martin Mahoney |
| Notes |
| Trial name or title | A smoking intervention study using scheduled gradual reduction with varenicline to help with cessation |
| Methods | Double‐blind factorial RCT |
| Participants | 192 adult smokers, 10+ CPD |
| Interventions | 4‐wk scheduled gradual reduction programme + varenicline vs 4‐wk scheduled gradual reduction programme + placebo |
| Outcomes | PA at 4, 12 wks |
| Starting date | December 2012 |
| Contact information | Joel Erblich |
| Notes |
| Trial name or title | The Canadian HIV Quit Smoking Trial: tackling the co‐morbidities of depression and cardiovascular disease in HIV+ smokers (CANQUIT) |
| Methods | Open‐label 4‐arm factorial RCT |
| Participants | 256 adults HIV+ smokers, 5+ CPD, willing to set a quit date |
| Interventions | NRT, NRT + HIV‐tailored quit smoking counselling, varenicline, varenicline + HIV‐tailored quit smoking counselling |
| Outcomes | 7‐day PPA and 4‐wk CA at wk 48, CO‐verified |
| Starting date | January 2014 |
| Contact information | |
| Notes |
| Trial name or title | Varenicline lapse study |
| Methods | Double‐blind cross‐over RCT |
| Participants | 50 adult smokers, 10+ CPD, not trying to quit, with schizophrenia or schizoaffective disorder; controls not on psychotropic meds or diagnosed with any Axis 1 disorder |
| Interventions | Varenicline vs placebo |
| Outcomes | Time to lapse |
| Starting date | June 2013 |
| Contact information | Tony George |
| Notes |
| Trial name or title | Dissemination of a tailored tobacco quitline for rural veteran smokers |
| Methods | Double‐blind parallel‐group RCT |
| Participants | 500 adult veteran daily smokers, willing to try to quit |
| Interventions | Tailored intervention (behavioural and meds) vs Enhanced standard of care |
| Outcomes | 7‐day and 30‐day PPA at 6m |
| Starting date | July 2013 |
| Contact information | Mark W Vander Weg |
| Notes |
| Trial name or title | Improving adherence to smoking cessation medication among PLWHA (HIV) |
| Methods | Open‐label RCT |
| Participants | 220 adult HIV+ smokers, 5+ CPD, willing to quit |
| Interventions | Varenicline (standard care), vs varenicline: text messages + adherence behavioural therapy |
| Outcomes | Adherence to treatment; abstinence at wks 1, 4, 8, EoT, 3m |
| Starting date | March 2013 |
| Contact information | Donna Shelley |
| Notes |
| Trial name or title | Smoking cessation strategies in community cancer programs for lung and head and neck cancer patients |
| Methods | Open‐label 12‐arm RCT |
| Participants | 180 adult smoking patients with current lung, head and neck cancer diagnosis, smoked at least 1 cigarette within 4 wks of enrolment |
| Interventions | High vs low intensity counselling, long‐acting vs PRN NRT, bupropion, varenicline in various combinations |
| Outcomes | 7‐day CO‐confirmed PPA at 8 wks |
| Starting date | July 2014 |
| Contact information | Joseph Valentino |
| Notes | May be too short |
| Trial name or title | Early in‐hospital initiation of pharmacotherapy for smoking cessation, Patients after ACS |
| Methods | Double‐blind parallel‐group RCT |
| Participants | 300 adult smokers with ACS |
| Interventions | Varenicline vs placebo |
| Outcomes | CAR at 1m, 6m, 1 yr after hospitalisation; SAE rate |
| Starting date | June 2014 |
| Contact information | Haim Lotan |
| Notes |
| Trial name or title | Internet‐based medication adherence program for nicotine dependence treatment |
| Methods | Double‐blind parallel‐group RCT |
| Participants | 70 adult members of Group Health insurance, smoking 10+ CPD, motivated to quit, smart phone access |
| Interventions | Online self help + varenicline vs augmented online self help + varenicline |
| Outcomes | 7‐day PPA at 5m |
| Starting date | October 2014 |
| Contact information | Sherryl Catz, Larry An |
| Notes |
| Trial name or title | The MATCH (medication aids for tobacco cessation) Study |
| Methods | Open‐label RCT |
| Participants | 1500 adult smokers, 10+ CPD, motivated to quit |
| Interventions | Bupropion + weekly motivational emails vs varenicline + weekly motivational emails |
| Outcomes | CA at 12, 26, 52 wks |
| Starting date | May 2014 |
| Contact information | Laurie Zawertailo |
| Notes |
| Trial name or title | Pilot study of nicotine nasal spray and varenicline on smoking in methadone‐maintained patients |
| Methods | Double‐blind 4‐arm cross‐over RCT |
| Participants | 20 adult smokers on methadone maintenance, 10+ CPD |
| Interventions | Nasal spray (active and placebo), varenicline (active and placebo), taken in different orders |
| Outcomes | Proportion of CPD taken within 4 hours of receiving methadone dose; abstinence, CO‐verified |
| Starting date | December 2014 |
| Contact information | Theresa Winhusen |
| Notes |
| Trial name or title | Reward sensitivity and pharmacotherapy for smoking cessation |
| Methods | Double‐blind parallel‐group RCT |
| Participants | 90 adults smokers, 5+ CPD, |
| Interventions | Varenicline + placebo patch vs nicotine patch + placebo tablet; all get behavioural counselling |
| Outcomes | CAR at EoT, 3m, 6m |
| Starting date | April 2015 |
| Contact information | Paul Cinciripini |
| Notes |
| Trial name or title | Varenicline and combined nicotine replacement therapy (NRT) for smoking cessation |
| Methods | Double‐blind 5‐arm cross‐over RCT |
| Participants | 310 adult smokers, 5+ CPD |
| Interventions | Varenicline vs nicotine patch + lozenge vs extra tablets or patches vs switch to different therapy vs extra tablet or patch |
| Outcomes | 7‐day PPA at 12 wks |
| Starting date | May 2015 |
| Contact information | Paul Cinciripini |
| Notes |
| Trial name or title | Randomised clinical trial to reduce harm from tobacco |
| Methods | Single‐blind parallel‐group RCT |
| Participants | 6000 adult smokers, Vitality beneficiaries |
| Interventions | Standardised Vitality program vs Vitality + choice of e‐cigarette/varenicline/bupropion/NRT vs Vitality + choice of meds + deposit‐refund programme |
| Outcomes | CO‐verified abstinence at 6m, 12m |
| Starting date | January 2015 |
| Contact information | Scott Halpern |
| Notes |
| Trial name or title | Genetically informed smoking cessation trial |
| Methods | Double‐blind 3‐arm parallel‐group RCT |
| Participants | 720 adults, 5+ CPD, motivated to quit smoking |
| Interventions | NRT + counselling vs varenicline + counselling vs combination NRT + counselling |
| Outcomes | 7‐day PPA at wk 12, wk 24 |
| Starting date | May 2015 |
| Contact information | Li‐Shiun Chen |
| Notes |
| Trial name or title | Advancing tobacco use treatment for African‐American smokers (KIS‐IV) |
| Methods | Double‐blind parallel‐group RCT |
| Participants | 500 adult A‐A smokers, 5+ CPD, motivated to quit |
| Interventions | Varenicline vs placebo |
| Outcomes | 7‐day PPA at 6m |
| Starting date | April 2015 |
| Contact information | Lisa Sanderson Cox |
| Notes |
| Trial name or title | Penn State TXT2Quit study |
| Methods | Single‐blind parallel‐group RCT |
| Participants | 150 adult smokers, 4+ CPD, motivated to quit |
| Interventions | Varenicline + motivation text messages vs varenicline alone |
| Outcomes | 7‐day PPA CO‐verified at wk 12 |
| Starting date | January 2015 |
| Contact information | Jonathan Foulds |
| Notes |
Reid 2010
| Trial name or title | Varenicline versus transdermal nicotine patch for smoking cessation in patients with coronary heart disease |
| Methods | Randomised open‐label trial |
| Participants | 60 adult smokers |
| Interventions | Varenicline or NRT patch for 12 weeks |
| Outcomes | CO‐confirmed CAR for wks 12 ‐ 26 |
| Starting date | April 2009 |
| Contact information | Robert Reid |
| Notes |
Rohsenow 2015
| Trial name or title | Varenicline helps smokers with SUD stop smoking without harming recovery |
| Methods | Randomised quadruple‐blind placebo‐controlled trial |
| Participants | 274 adult smokers with substance use disorders |
| Interventions | Varenicline vs NRT patches for 12 weeks, plus motivational advice |
| Outcomes | 7‐day PPA at 3, 6 and 12m |
| Starting date | |
| Contact information | Damaris_Rohsenow@brown.edu |
| Notes | New for 2016 update; extraction based on Powerpoint slides in 137 participants |
Smith 2013b
| Trial name or title | Varenicline for cognitive deficits and cigarette smoking in schizophrenia |
| Methods | Randomised double‐blind placebo‐controlled trial |
| Participants | 60 adult smokers with schizophrenia |
| Interventions | Varenicline 1 ‐ 2 mg/day vs placebo |
| Outcomes | Cotinine‐verified cessation, + Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS), Positive and Negative Syndrome Scale (PANNS), Hamilton Depression Scale |
| Starting date | September 2008 |
| Contact information | RC Smith |
| Notes |
Tulloch 2014
| Trial name or title | Flexible and extended dosing of nicotine replacement therapy or varenicline in comparison to fixed dose nicotine replacement therapy for smoking cessation: the FLEX trial |
| Methods | Randomised controlled trial |
| Participants | 737 adult smokers |
| Interventions | NRT vs combination NRT vs varenicline |
| Outcomes | CAR wks 5 ‐ 52, + neuropsychiatric and withdrawal symptoms |
| Starting date | |
| Contact information | hetulloch@ottawaheart.ca |
| Notes | New for 2016 update |
Van Rossem 2015
| Trial name or title | Helping more smokers to quit by combining varenicline with counselling for smoking cessation. The COVACO randomized controlled trial |
| Methods | Open‐label RCT |
| Participants | 295 primary‐care smoking patients, no minimum CPD |
| Interventions | Varenicline + brief GP advice vs varenicline + PN or GP extended counselling |
| Outcomes | PA at wk 52 |
| Starting date | |
| Contact information | C van Rossem |
| Notes |
This list does not include all registered studies of varenicline, dianicline and cytisine. It covers only those studies expected to be eventual candidates for inclusion within future updates of this review, i.e. RCTs of smoking cessation interventions with a minimum follow‐up of six months, or for shorter duration if safety issues are the main outcome.
ACS: acute coronary syndrome CAR: continuous abstinence rate COPD: chronic obstructive pulmonary disease CPD: cigarettes per day CQR: continuous quit rate MI: motivational interviewing PLWHA: people living with HIV/AIDS PN: psychiatric nurse PPA: point prevalence abstinence PTSD: post‐traumatic stress disorder TAU: treatment as usual
Contributions of authors
KC: Performed clinical trials register searching, extracted data, conducted the analyses and wrote the review. NL‐H: Extracted data, contributed to the writing and updating process. TF: Conducted the neuropsychiatric adverse event meta‐analyses and advised on statistical interpretation. KT: Contributed data and advice on neuropsychiatric adverse event data. TL: Gave editorial and conceptual support.
All authors contributed to text and findings, and approved the final version.
Sources of support
Internal sources
Department of Primary Care Health Sciences, University of Oxford, UK.
National School for Health Research School for Primary Care Research, UK.
External sources
NHS Research and Development Fund, UK.
Declarations of interest
Kate Cahill : None known Nicola Lindson‐Hawley: NLH is a co‐applicant on the Preloading Trial, which is funded by the NIHR HTA. The study treatment is nicotine patches which are provided free of charge by GlaxoSmithKline. Tom Fanshawe: None known Kyla Thomas: None known Tim Lancaster: None known Robert West, who is an editor for the Tobacco Addiction Group, has ruled himself out of participating in the editorial process for this review, as he is a member of the varenicline advisory board for Pfizer Inc.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
- Anthenelli RM, Morris C, Ramey TS, Dubrava SJ, Tsilkos K, Russ C, et al. Effects of varenicline on smoking cessation in adults with stably treated current or past major depression: a randomized trial.[Summary for patients in Ann Intern Med. 2013 Sep 17;159(6):I‐36; PMID: 24042380]. Annals of Internal Medicine 2013;159(6):390‐400. [CENTRAL: 921009; CRS: 9400126000000199; EMBASE: 2013581859; PUBMED: 24042367] [DOI] [PubMed] [Google Scholar]; Pfizer Inc. Safety and efficacy of 12 weeks of varenicline for smoking cessation in smokers with depression. clinicaltrials.gov/ct2/ (accessed 2/11/2010)2010. [ClinicalTrials.gov ID NCT01078298]
- Aubin H‐J, Bobak A, Britton JR, Oncken C, Billing CB, Gong J, et al. Authors' reply [to JE Rose]. Thorax 2008;63(8):752. [DOI] [PMC free article] [PubMed] [Google Scholar]; Aubin H‐J, Bobak A, Britton JR, Oncken C, Billing CB, Gong J, et al. Authors' reply [to T Hillman]. Thorax 2008;63(8):752‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Aubin H‐J, Bobak A, Britton JR, Oncken C, Billing CB, Gong J, et al. Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised, open‐label trial. Thorax 2008;1:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Aveyard P. The place of varenicline in smoking cessation treatment. Thorax 2008;63(8):666‐8. [DOI] [PubMed] [Google Scholar]; Hillman T, Rajakulasingam K, Bhowmik A. Clinically significant outcomes in smoking cessation. Thorax 2008;63(8):752. [PubMed] [Google Scholar]; Rose JE. Pre‐cessation varenicline treatment vs post‐cessation NRT: an uneven playing field. Thorax 2008;63(8):751. [PubMed] [Google Scholar]
- Baker TB, Piper ME, Stein JH, Smith SS, Bolt DM, Fraser DL, et al. Effects of nicotine patch vs varenicline vs combination nicotine replacement therapy on smoking cessation at 26 weeks. JAMA 2016;315(4):371‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolliger CT, Issa JS, Posadas‐Valay R, Safwat T, Abreu P, Correia EA, et al. Effects of varenicline in adult smokers: a multinational, 24‐week, randomized, double‐blind, placebo‐controlled study. Clinical Therapeutics 2011;33(4):465‐77. [CENTRAL: 799588; CRS: 9400123000011547; PUBMED: 21635992] [DOI] [PubMed] [Google Scholar]
- Brandon TH, Drobes DJ, Unrod M, Heckman BW, Oliver JA, Roetzheim RC, et al. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology (Berl) 2011;218(2):391‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinn M, Daziel K, Carson K, Labiszewski N, Esterman A, Smith B. Cost effectiveness of an inpatient smoking cessation intervention for patients with tobacco related illnesses (Stop Trial): a multi‐centre RCT [Abstract]. Respirology (Carlton, Vic.) 2013;18(Suppl 2):16 [O027]. [CENTRAL: 849080; CRS: 9400123000018279; EMBASE: 71010964] [Google Scholar]; Carson K, Smith BJ, Peters MJ, Veale AJ, Esterman AJ. Superiority of a course of varenicline tartrate plus counselling over counselling alone for smoking cessation: a 24‐month randomized controlled trial for inpatients. Respirology 2015;20:115. [DOI] [PubMed] [Google Scholar]; Carson KV, Brinn MP, Smith BJ, Garside CG, Goldsworthy SJ, Fitridge RA. Respirology 2010, 15 (Suppl 1):A30. Varenicline tartrate and counselling versus counselling alone in a randomised controlled trial for inpatient smoking cessation: 3 month interim results [Abstract]. Respirology 2010;15(Suppl 1):A30. [Google Scholar]; Carson KV, Smith BJ, Brinn MP, Peters MJ, Fitridge R, Koblar SA, et al. Safety of varenicline tartrate and counseling versus counseling alone for smoking cessation: a randomized controlled trial for inpatients (STOP study). Nicotine & Tobacco Research 2014;16(11):1495‐502. [CENTRAL: 1036725; CRS: 9400050000000132; EMBASE: 2014920189; PUBMED: 25031315] [DOI] [PubMed] [Google Scholar]; Hnin K, Carson K, Brinn M, Jannes J, Ameer F, Esterman A, et al. Triggers resulting in relapse: cohort analysis from the smoking termination opportunity for inpatients (STOP) trial [Abstract]. Respirology (Carlton, Vic.) 2014;19(Suppl 2):40 [TO 076]. [CENTRAL: 996495; CRS: 9400050000000097; EMBASE: 71605471] [Google Scholar]; Smith BJ, Carson KV, Brinn MP, Labiszewski NA, Peters MJ, Fitridge R, et al. Smoking termination opportunity for in patients (STOP): Superiority of a course of varenicline tartrate plus counselling over counselling alone for smoking cessation: A 12‐month randomised controlled trial for inpatients. Thorax 2013;68(5):485‐6. [CENTRAL: 849081; CRS: 9400107000000003; EMBASE: 2013242202; NCT01141855/ClinicalTrials.gov; PUBMED: 22993168] [DOI] [PubMed] [Google Scholar]; Smith BJ, Peters MJ, Fitridge RA, Esterman AJ, Litt JC, Horowitz JD, et al. Varenicline tartrate and counselling versus counselling alone in a randomised controlled trial for inpatient smoking cessation: 6 month interim results. Not stated. 2011. [Google Scholar]
- Chengappa KNR, Perkins KA, Brar JS, Schlicht PJ, Turkin SR, Hetrick ML, et al. Varenicline for smoking cessation in bipolar disorder: A randomized, double‐blind, placebo‐controlled study. Journal of Clinical Psychiatry 2014;75(7):765‐72. [CENTRAL: 1014641; CRS: 9400129000003150] [DOI] [PubMed] [Google Scholar]; NCT01010204. Varenicline treatment for smoking cessation in patients with bipolar disorder (BEST). ClinicalTrials.gov/ct2/ 2009 (accessed 2nd November 2010). [ClinicalTrials.gov ID NCT01010204]
- Cinciripini PM, Karam‐Hage M. Randomised controlled trial: Study suggests varenicline safe and effective among adults with stable depression. Evidence‐based Medicine 2014;19(3):92. [CRS: 9400129000002225; EMBASE: 2014382469; PUBMED: 24482150] [DOI] [PMC free article] [PubMed] [Google Scholar]; Cinciripini PM, Robinson JD, Karam‐Hage M, Minnix JA, Lam C, Versace F, et al. Effects of varenicline and bupropion sustained‐release use plus intensive smoking cessation counseling on prolonged abstinence from smoking and on depression, negative affect, and other symptoms of nicotine withdrawal. JAMA Psychiatry (Chicago, Ill.) 2013;70(5):522‐33. [CENTRAL: 863881; CRS: 9400107000000437; EMBASE: 2013278203; PUBMED: 23536105] [DOI] [PMC free article] [PubMed] [Google Scholar]; Hays JT. Varenicline may reduce negative effect while aiding smoking cessation. Evidence‐based Medicine 2014;19(1):23. [CRS: 9400129000003304; PUBMED: 23990527] [DOI] [PubMed] [Google Scholar]; NCT00507728. Pharmacogenetics, emotional reactivity and smoking. ClinicalTrials.gov/ct2/ (accessed 13th October 2010). [ClinicalTrials.gov ID NCT00507728]
- Dios MA, Anderson BJ, Stanton C, Audet DA, Stein M. Project Impact: a pharmacotherapy pilot trial investigating the abstinence and treatment adherence of Latino light smokers. Journal of Substance Abuse Treatment 2012;43(3):322‐30. [CENTRAL: 836798; CRS: 9400123000013063; EMBASE: 2012562711; PUBMED: 22377389] [DOI] [PMC free article] [PubMed] [Google Scholar]
- A phase 4, randomized, double blind, active and placebo controlled multicenter study evaluating the neuropsychiatric safety and efficacy of 12 weeks varenicline tartrate 1mg BID and bupropion hydrochloride 150 mg BID for smoking cessation in subjects with and without a history of psychiatric disorders [EAGLES]. clinicaltrials.gov/ct2/show/NCT01456936 (accessed 1st March 2016). ; Anthenelli R, Benowitz N, West R, Aubin L, McRae T, Lawrence D, et al. Reports of suicidal ideation and behavior in the EAGLES trial. Proceedings of the Society for Research on Nicotine and Tobacco, USA 3rd ‐ 5th March. 2016; Vol. SYM5D:8. [Google Scholar]; Anthenelli RM, Benowitz NL, West R, Aubin L, McRae T, Lawrence D, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double‐blind, randomised, placebo‐controlled clinical trial. Lancet 2016 April 22nd [Epub ahead of print]. [DOI: 10.1016/S0140-6736(16)30272-0] [DOI] [PubMed]; Benowitz N, Evins AE, West R, Aubin L, McRae T, Lawrence D, et al. EAGLES trial: study design and neuropsychiatric safety results. Proceedings of the Society for Research on Nicotine and Tobacco, USA 3rd ‐ 5th March. 2016; Vol. SYM5B:7. [Google Scholar]; Prochaska J. Neuropsychiatric risk concerns in the context of smoking, quitting, and cessation pharmacotherapy use. Proceedings of the Society for Research on Nicotine and Tobacco, USA 3rd ‐ 5th March. 2016; Vol. SYM5A:7. [Google Scholar]; Prochaska J, Benowitz N, West R, Anthenelli R. Evaluating adverse events in a global smoking cessation study (EAGLES): a randomized controlled tiral comparing the safety and efficacy of the first‐line smoking cessation aids in smokers with and without pychiatric disorders. Proceedings of the Society for Research on Nicotine and Tobacco, USA 3rd ‐ 5th March. 2016; Vol. SYM5:7. [Google Scholar]; West R, Benowitz N, Evins AE, Aubin L, McRae T, Lawrence D, et al. Relative efficacy of varenicline, bupropion SR, and nicotine transdermal patch in aiding smoking cessation in the EAGLES trial. Proceedings of the Society for Research on Nicotine and Tobacco, USA 3rd ‐ 5th March. 2016; Vol. SYM5C:8. [Google Scholar]
- Ebbert JO, Croghan IT, North F, Schroeder DR. A pilot study to assess smokeless tobacco use reduction with varenicline. Nicotine & Tobacco Research 2011;13(9):820‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian H. Comment on Ebbert 2015 'Reduce to Quit' trial. www.ncbi.nlm.nih.gov/pubmed/?term=25688780 Feb 18 2015 (accessed 11th June 2015). ; Ebbert JO, Hughes JR, West RJ, Rennard SI, Russ C, McRae TD, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA 2015;313(7):687‐94. [CENTRAL: 1050651; CRS: 9400131000000054; PUBMED: 25688780] [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT01370356. A study to evaluate the efficacy and safety of varenicline compared to placebo for smoking cessation through reduction. clinicaltrials.gov/ct2/ 2011 (accessed 18th October 2011). [ClinicalTrials.gov ID NCT01370356]
- Eisenberg MJ, Windle SB, Roy N, Old W, Grondin F, Bata I, et al. Varenicline for smoking cessation in hospitalized patients with acute coronary syndrome. Circulation 2016;133(1):21‐30. [DOI] [PubMed] [Google Scholar]; Windle SB, Bata I, Madan M, Abramson BL, Eisenberg MJ. A randomized controlled trial of the efficacy and safety of varenicline for smoking cessation after acute coronary syndrome: Design and methods of the Evaluation of Varenicline in Smoking Cessation for Patients Post‐Acute Coronary Syndrome trial. American Heart Journal 2015;170(4):635‐40. [DOI] [PubMed] [Google Scholar]
- Evins AE. Extended duration pharmacotherapy with varenicline prevents relapse to smoking in adult smokers with schizophrenia. Neuropsychopharmacology 2013;38:S63‐4. [CENTRAL: 993941; CRS: 9400130000000397; EMBASE: 71278012] [Google Scholar]; Evins AE, Cather C, Pratt SA, Pachas GN, Hoeppner SS, Goff DC, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: A randomized clinical trial. JAMA 2014;311(2):145‐54. [CENTRAL: 921056; CRS: 9400130000000074; EMBASE: 2014041619; PUBMED: 24399553] [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT00621777. A study of varenicline for prevention of relapse to smoking in patients with schizophrenia. clinicaltrials.gov/ct2/show/NCT00621777 2008 (accessed 14th April 2008). ; Pachas GN, Cather C, Pratt SI, Hoeppner B, Nino J, Carlini SV, et al. Varenicline for smoking cessation in schizophrenia: safety and effectiveness in a 12‐week open‐label trial. Jounrla of Dual Diagnosis 2012;8(2):117‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faessel H, Ravva P, Williams K. Pharmacokinetics, safety, and tolerability of varenicline in healthy adolescent smokers: a multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study. Clinical Therapeutics 2009;31:177‐89. [clinicaltrials.gov ID: NCT00463918] [DOI] [PubMed] [Google Scholar]
- Fagerström K, Gilljam H, Metcalfe M, Tonstad S, Messig M. Stopping smokeless tobacco with varenicline: randomised double blind placebo controlled trial. BMJ 2010;341:c6549. [DOI: 10.1136/bmjc6549] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza D, Murphy M, Tseng L‐J, Riordan HJ, Chatterjee A. A double‐blind randomized placebo‐controlled pilot study of neuropsychiatric adverse events in abstinent smokers treated with varenicline or placebo. Biological Psychiatry 2011;69:1075‐82. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Jorenby DE, Brandon T, Arteaga C, Lee TC. Delayed quitting, lapse recovery and long‐term outcomes for quitters taking varenicline, bupropion and placebo. Society for Research on Nicotine and Tobacco Annual Meeting, Portland OR, Feb 27‐March 1st. 2008. [Google Scholar]; Gonzales D, Jorenby DE, Brandon TH, Arteaga C, Lee TC. Emergent adverse psychiatric symptoms by therapy during 12 weeks of treatment with varenicline, bupropion SR, or placebo for smoking cessation. Society for Research on Nicotine and Tobacco Europe: Rome, September 23‐262008. ; Gonzales D, Jorenby DE, Brandon TH, Arteaga C, Lee TC. Immediate versus delayed quitting and rates of relapse among smokers treated successfully with varenicline, bupropion SR or placebo. Addiction 2010;105(11):2002‐13. [DOI: 10.1111/j.1360-0443.2010.03058.x] [DOI] [PMC free article] [PubMed] [Google Scholar]; Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an α4ß2 nicotinic acetylcholine receptor partial agonist, vs sustained‐release bupropion and placebo for smoking cessation. JAMA 2006;296(1):47‐55. [DOI] [PubMed] [Google Scholar]; Hays JT, Leischow SJ, Lawrence D, Lee TC. Adherence to treatment for tobacco dependence: association with smoking abstinence and predictors of adherence. Nicotine & Tobacco Research 2010;12(6):574‐81. [DOI] [PubMed] [Google Scholar]; Heffner JL, Lee TC, Arteaga C, Anthenelli RM. Predictors of post‐treatment relapse to smoking in successful quitters: pooled data from two phase III varenicline trials. Drug and Alcohol Dependence 2010;109:120‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jackson KC, Nahoopii R, Said Q, Dirani R, Brixner D. An employer‐based cost‐benefit analysis of a novel pharmacotherapy agent for smoking cessation. Journal of Occupational and Environmental Medicine 2007;49(4):453‐60. [DOI] [PubMed] [Google Scholar]; Nides M, Glover ED, Reus VI, Christen AG, Make BJ, Billing CB, et al. Varenicline versus bupropion SR or placebo for smoking cessation: a pooled analysis. American Journal of Health Behavior 2008;32(6):664‐75. [DOI] [PubMed] [Google Scholar]; O'Brien CP. A new medication for the worst addiction. Current Psychiatry Reports 2007;9(5):347‐8. [PubMed] [Google Scholar]; Ravva P, Gastonguay MR, French JL, Tensfeldt TG, Faessel HM. Quantitative assessment of exposure‐response relationships for the efficacy and tolerability of varenicline for smoking cessation. Clinical Pharmacology and Therapeutics 2010;87(3):336‐44. [DOI] [PubMed] [Google Scholar]; Tonstad S. Practical implementation of varenicline as an aid to smoking cessation in clinical practice. Pneumologia 2009;58(3):167‐74. [PubMed] [Google Scholar]; West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. UK National Smoking Cessation Conference Proceedings, London, June 2007. 2007. [Google Scholar]; Xenakis JG, Kinter ET, Ishak KJ, Ward AJ, Marton JP, Willke RJ, et al. A discrete‐event simulation of smoking‐cessation strategies based on varenicline pivotal trial data. PharmacoEconomics 2011;29:497‐510. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Hajek P, Pliamm L, Nackaerts K, Tseng L‐J, McRae TD, et al. Retreatment with varenicline for smoking cessation in smokers who have previously taken varenicline: A randomized, placebo‐controlled trial. Clinical Pharmacology and Therapeutics 2014;96(3):390‐6. [CENTRAL: 1014162; CRS: 9400129000003147; EMBASE: 2014565570; PUBMED: 24911368] [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT01244061. A multi‐national study to assess how effective and safe the smoking cessation medicine varenicline is in smokers who have already tried varenicline in the past as a prescription medicine from their usual healthcare provider. ClinicalTrials.gov/ct2/ 2010 (accessed 18th October 2011). [ClinicalTrials.gov ID NCT01244061]
- Hajek P, McRobbie H, Myers Smith K, Phillips A, Cornwall D, Dhanji AR. Increasing varenicline dose in smokers who do not respond to the standard dosage: a randomized clinical trial. JAMA Internal Medicine 2015;175(2):266‐71. [CENTRAL: 1047635; CRS: 9400050000000143; EMBASE: 2015718793; PUBMED: 25545858] [DOI] [PubMed] [Google Scholar]
- Heydari G, Talischi F, Tafti SF, Masjedi MR. Quitting smoking with varenicline: parallel, randomised efficacy trial in Iran. International Journal of Tuberculosis and Lung Disease 2012;16(2):268‐72. [CENTRAL: 814663; CRS: 9400123000012712; EMBASE: 2012026893; PUBMED: 22236931] [DOI] [PubMed] [Google Scholar]
- Hughes JR, Rennard SI, Fingar JR, Talbot SK, Callas PW, Fagerström KO. Efficacy of varenicline to prompt quit attempts in smokers not currently trying to quit: a randomized placebo‐controlled trial. Nicotine & Tobacco Research 2011;13(10):955‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT00595868. Efficacy of varenicline in ambivalent smokers. ClinicalTrials.gov/ct2/show/NCT00595868 2007 (accessed 2nd January 2016).
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an α4ß2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained‐release bupropion for smoking cessation. JAMA 2006;296(1):56‐63. [DOI] [PubMed] [Google Scholar]; O'Brien CP. A second varenicline trial. Current Psychiatry Reports 2007;9(5):348‐8. [PubMed] [Google Scholar]
- McClure EA, Vandrey RG, Johnson MW, Stitzer ML. Effects of varenicline on abstinence and smoking reward following a programmed lapse. Nicotine & Tobacco Research 2013;15(1):139‐48. [CENTRAL: 873078; CRS: 9400123000018200; EMBASE: 2012756138; PUBMED: 22573730] [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT00944554. Varenicline for relapse prevention. ClinicalTrials.gov/ct2/ 2009 (accessed 2nd November 2010). [CRS: 9400123000011434]
- Meszaros ZS, Abdul‐Malak Y, Dimmock JA, Wang D, Ajagbe TO, Batki SL. Varenicline treatment of concurrent alcohol and nicotine dependence in schizophrenia: a randomized, placebo‐controlled pilot trial. Journal of Clinical Psychopharmacology 2013;33(2):243‐7. [CENTRAL: 908808; CRS: 9400123000017959; EMBASE: 2013155799; PUBMED: 23422399] [DOI] [PubMed] [Google Scholar]; Meszaros ZS, Abdul‐Malak Y, Dimmock JA, Wang D, Batki SL. Varenicline treatment of alcohol and nicotine dependence in schizophrenia: Problems encountered in a pilot trial. American Journal on Addictions / American Academy of Psychiatrists in Alcoholism and Addictions 2012;21(4):393‐4. [CENTRAL: 834600; CRS: 9400123000015660; EMBASE: 70815316] [Google Scholar]; NCT00727103. Varenicline treatment in alcohol and nicotine dependent patients with schizophrenia. ClinicalTrials.gov/ct2/ (accessed 13th October 2010).
- Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Varenicline decreases alcohol consumption in heavy‐drinking smokers. Psychopharmacology 2012;223(3):299‐306. [CENTRAL: 845347; CRS: 9400050000000038; PUBMED: 22547331] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01027754. Smoking cessation treatment for methadone maintenance patients. ClinicalTrials.gov/ct2/ 2009 (accessed 2nd November 2010). [ClinicalTrials.gov ID NCT01027754] ; Nahvi S, Ning Y, Segal KS, Richter KP, Arnsten JH. Varenicline efficacy and safety among methadone maintained smokers: A randomized placebo‐controlled trial. Addiction (Abingdon, England) 2014;109(9):1554‐63. [CENTRAL: 997902; CRS: 9400129000002315; PUBMED: 24862167] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerström K, Nakamura M, Cho H, Tsa S, Wang C, Davies S, et al. Varenicline treatment for smoking cessation in Asian populations: a pooled analysis of placebo‐controlled trials conducted in six Asian countries. Current medical Research & Opinion 2010;26(9):2165‐73. [DOI] [PubMed] [Google Scholar]; Igarashi A, Takuma H, Fukada T, Tsutani K. Cost‐utility analysis of varenicline, an oral smoking‐cessation drug, in Japan. Pharmacoeconomics 2009;27(3):247‐61. [DOI] [PubMed] [Google Scholar]; Nakamura M, Oshima A, Fujimoto Y, Maruyama N, Ishibashi T, Reeves KR. Efficacy and tolerability of nicotinic varenicline, an alpha4beta2 acetylcholine receptor partial agonist, in a 12‐week, randomized, placebo‐controlled, dose‐response study with 40‐week follow‐up for smoking cessation in Japanese smokers. Clinical Therapeutics 2007;29(6):1040‐56. [CENTRAL: 611262; CRS: 9400123000004511; EMBASE: 2007380498; PUBMED: 17692720] [DOI] [PubMed] [Google Scholar]; Nakamura M, Oshima A, Fujimoto Y, Maruyama N, Ishibashi T, Reeves KR. Efficacy and tolerability of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, in a 12‐week, randomized, placebo‐controlled, dose‐response study with 40‐week follow‐up for smoking cessation in Japanese smokers. Clinical Therapeutics 2007;29(6):1040‐56. [DOI] [PubMed] [Google Scholar]; Nakamura M, Oshima A, Ohkura M, Arteaga C, Suwa K. Predictors of lapse and relapse to smoking in successful quitters in a varenicline post hoc analysis in japanese smokers. Clinical Therapeutics 2014;36(6):918‐27. [CENTRAL: 995377; CRS: 9400129000003045; EMBASE: 2014542011] [DOI] [PubMed] [Google Scholar]
- NCT00828113. Varenicline for long‐term smoking cessation. ClinicalTrials.gov/ct2/ 2009 (accessed 2nd November 2010).
- NCT01347112. Varenicline treatment for active alcoholic smokers. ClinicalTrials.gov/ct2/ 2011 (accessed 18th October 2011).
- Niaura R, Taylor Hays J, Jorenby DE, Leone FT, Pappas JE, Reeves KR, et al. The efficacy and safety of varenicline for smoking cessation using a flexible dosing strategy in adult smokers: a randomized controlled trial. Current Medical Research and Opinion 2008;24(7):1931‐41. [clinicaltrials.gov ID: NCT00150228] [DOI] [PubMed] [Google Scholar]
- Nides M, Glover ED, Reus VI, Christen AG, Make BJ, Billing CB, et al. Varenicline versus bupropion SR or placebo for smoking cessation: a pooled analysis. American Journal of Health Behavior 2008;32(6):664‐75. [DOI] [PubMed] [Google Scholar]; Nides M, Oncken C, Gonzalez D, Rennard S, Watsky EJ, Anziano R, et al. Smoking cessation with varenicline, a selective α4β2 nicotinic receptor partial agonist. Archives of Internal Medicine 2006;166:1561‐8. [DOI] [PubMed] [Google Scholar]; Oncken C, Watsky E, Reeves K, Anziano R. Varenicline is efficacious and well tolerated in promoting smoking cessation: Results from a 7‐week, randomized, placebo‐ and bupropion‐controlled trial [POS1‐047]. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20‐23 March 2005; Prague, Czech Republic. 2005. [Google Scholar]
- Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Archives of Internal Medicine 2006;166(15):1571‐7. [CENTRAL: 567305; CRS: 9400123000004048; EMBASE: 2006391945; PUBMED: 16908789] [DOI] [PubMed] [Google Scholar]; Oncken C, Watsky E, Reeves K, Anziano R. Smoking cessation with varenicline, a selective nicotinic receptor partial agonist: results from a phase 2 study [POS1‐046]. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20‐23 March 2005; Prague, Czech Republic. 2005. [Google Scholar]
- Hughes JR, Russ C, Messig MA. Association of deferring a quit attempt with smoking cessation success: a secondary analysis. Journal of Substance Abuse Treatment 2014;46(2):264‐7. [CENTRAL: 911546; CRS: 9400129000000234; EMBASE: 2013742404; PUBMED: 24074849] [DOI] [PubMed] [Google Scholar]; Hughes JR, Russ CI, Arteaga CE, Rennard SI. Efficacy of a flexible quit date versus an a priori quit date approach to smoking cessation: a cross‐study analysis. Addictive Behaviors 2011;36(12):1288‐91. [CENTRAL: 830553; CRS: 9400123000011672; PUBMED: 21872998] [DOI] [PubMed] [Google Scholar]; Rennard S, Hughes J, Cinciripini PM, Kralikova E, Raupach T, Arteaga C, et al. A randomized placebo‐controlled trial of varenicline for smoking cessation allowing flexible quit dates. Nicotine & Tobacco Research 2012;14(3):343‐50. [CENTRAL: 831332; CRS: 9400123000014196; PUBMED: 22080588] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockene I, Salmoirago‐Blotcher E. Varenicline for smoking cessation in patients with coronary heart disease [editorial]. Circulation 2010;121(2):188‐90. [DOI] [PubMed] [Google Scholar]; Rigotti N, Pipe A, Benowitz N, Arteaga C, Garza D, Tonstad S, et al. A randomized trial of varenicline for smoking cessation in patients with cardiovascular disease: analysis of efficacy by baseline characteristics. Circulation 2010;Conference:2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease. Circulation 2010;121(2):221‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S. Response to letter regarding article, efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation 2010;122(9):e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00894166. Evaluation of a tailored smoking cessation treatment algorithm based on initial treatment response and genotype (ConNIC3). ClinicalTrials.gov/ct2/ 2009 (accessed 2nd November 2010). ; Rose JE, Behm FM. Adapting smoking cessation treatment according to initial response to precessation nicotine patch. American Journal of Psychiatry 2013;170(8):860‐7. [CENTRAL: 873084; CRS: 9400107000001313; EMBASE: 2013550171; PUBMED: 23640009] [DOI] [PMC free article] [PubMed] [Google Scholar]; Uhl GR, Walther D, Musci R, Fisher C, Anthony JC, Storr CL, et al. Smoking quit success genotype score predicts quit success and distinct patterns of developmental involvement with common addictive substances. Molecular Psychiatry 2014;19(1):50‐4. [CRS: 9400129000002878; PUBMED: 23128154] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benndorf S, Scharfenberg G, Kempe G, Wendekamm R, Winkelvoss E. Smoking cessation treatment with cytisin (Tabex®): half‐yearly outcomes for former smokers abstinent at four weeks from beginning of treatment [Medikamentöse raucherentwöhnung mit cytisin (Tabex®): ergebnisse der halbjahresbefragung bei den vier wochen nach kurbeginn abstinenten ehemaligen rauchern]. Das Deutsche Gesundheitwesen 1970;24:774‐6. [PubMed] [Google Scholar]; Benndorf S, Scharfenberg G, Kempe G, Winkelvoss E, Wendekamm R. Further reports on a double blind trial of the Bulgarian cytisine compound Tabex® on 1214 smokers wishing to quit and practical experience in conducting clinics for such smokers [Weitere mitteilungen über einen doppelten blindversuch mit dem cytisinhaltigen bulgarischen präparat Tabex an 1214 entwöhnungswilligen rauchern und praktische erfahrungen bei der durchfürung einer sprechstunde für entwöhnungswillige raucher]. Das Deutsche Gesundheitswesen 1969;24:1135‐40. [Google Scholar]; Benndorf S, . Kempe G, Scharfenberg G, Wendekamm R, Winkelvoss E. Results of smoking cessation treatment with cytisin (Tabex®) [Ergebnisse der medikamentösen raucherentwöhnung mit cytisin (Tabex®)]. Das Deutsche Gesundheitwesen 1968;23(44):2092‐6. [PubMed] [Google Scholar]; Scharfenberg G, Benndorf S, Kempe G. Cytisine (Tabex®) as a treatment for smoking cessation [Cytisin (Tabex®) als medikamentöse raucherentwöhnungshilfe]. Das Deutsche Gesundheitwesen 1971;26(10):463‐5. [PubMed] [Google Scholar]
- Dios MA, Anderson BJ, Caviness CM, Stein MD. Early quit days among methadone‐maintained smokers in a smoking cessation trial. Nicotine & Tobacco Research 2014;16(11):1463‐9. [CENTRAL: 1042497; CRS: 9400129000003420] [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT00790569. Varenicline versus nicotine replacement for methadone‐maintained smokers. ClinicalTrials.gov/ct2/ 2008 (accessed 2nd November 2010). [CRS: 9400123000011408] ; Stein MD, Caviness CM, Kurth ME, Audet D, Olson J, Anderson BJ. Varenicline for smoking cessation among methadone‐maintained smokers: A randomized clinical trial. Drug and Alcohol Dependence 2013;133(2):486‐93. [CENTRAL: 870955; CRS: 9400107000001457; EMBASE: 2013694276; PUBMED: 23953658] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MB, Randall J, Greenhaus S, Schmelzer AC, Richardson DL, Carson JL. Tobacco dependence treatment for hospitalized smokers: a randomized, controlled, pilot trial using varenicline. Addictive Behaviors 2011;36(12):1127‐32. [DOI] [PubMed] [Google Scholar]
- Antoniu SA, Trofor AC. Varenicline for smoking cessation intervention in chronic obstructive pulmonary disease. Expert Opinion on Pharmacotherapy 2011;12(16):2595‐7. [DOI] [PubMed] [Google Scholar]; Kotz D, Schayck OCP. What justifies a placebo‐controlled trial of varenicline for smoking cessation in patients with COPD? [comment]. Chest 2011;139:968‐9. [DOI] [PubMed] [Google Scholar]; Tashkin DP, Rennard S, Hays JT, Ma W, Lawrence D, Lee TC. Effects of varenicline on smoking cessation in mild‐to‐moderate COPD: a randomized controlled trial. Chest 2011;139:591‐9. [DOI] [PubMed] [Google Scholar]; Tashkin DP, Rennard S, Taylor Hays J, Lawrence D, Marton JP, Lee TC. Lung function and respiratory symptoms in a 1‐year randomized smoking cessation trial of varenicline in COPD patients. Respiratory Medicine 2011;105(11):1682‐90. [CENTRAL: 811557; CRS: 9400123000011713; 6378; PUBMED: 21621992] [DOI] [PubMed] [Google Scholar]
- Bolin K, Mörk A‐C, Wilson K. Smoking‐cessation therapy using varenicline: the cost‐utility of an additional 12‐week course of varenicline for the maintenance of smoking abstinence. Journal of Evaluation in Clinical Practice 2009;15:478‐85. [DOI] [PubMed] [Google Scholar]; Hajek P, Tønneson P, Arteaga C, Russ C, Tonstad S. Varenicline in prevention of relapse to smoking: effect of quit pattern on response to extended treatment. Addiction 2009;104:1597‐1602. [DOI] [PubMed] [Google Scholar]; Knight C, Howard P, Baker CL, Marton JP. The cost‐effectiveness of an extended course (12 + 12 weeks) of varenicline compared with other available smoking cessation strategies in the United States: an extension and update to the BENESCO model. Value in Health 2010;13(2):209‐14. [DOI] [PubMed] [Google Scholar]; Knight CJ, Howard PA, Baker CL. An evaluation of the cost‐effectiveness of an extended course of varenicline in preventing smokers who have quit from relapsing [PSM3]. Value in Health2007; Vol. 10, issue 6:A472. ; Lee JH, Jones PG, Bybee K, O'Keefe JH. A longer course of varenicline therapy improves smoking cessation rates. Preventive Cardiology 2008;11(4):210‐4. [DOI] [PubMed] [Google Scholar]; O'Brien CP. Varenicline as maintenance therapy. Current Psychiatry Reports 2007;9(5):348‐8. [DOI] [PubMed] [Google Scholar]; Tonstad S, Tønnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA 2006;296(1):64‐71. [clinicaltrials.gov ID: NCT00143286] [DOI] [PubMed] [Google Scholar]
- Tonstad S, Holme I, Tønnesen P. Dianicline, a novel α4β2 nicotinic acetylcholine receptor partial agonist, for smoking cessation: a randomized placebo‐controlled clinical trial. Nicotine & Tobacco Research 2011;13(1):1‐6. [DOI] [PubMed] [Google Scholar]
- Fagerström K, Nakamura M, Cho H, Tsa S, Wang C, Davies S, et al. Varenicline treatment for smoking cessation in Asian populations: a pooled analysis of placebo‐controlled trials conducted in six Asian countries. Current medical Research & Opinion 2010;26(9):2165‐73. [DOI] [PubMed] [Google Scholar]; Tsai S‐T, Cho H‐J, Cheng H‐S, Kim C‐H, Hsueh K‐C, Billing CB, Williams KE. A randomized, placebo‐controlled trial of varenicline, a selective α4β2 nicotinic acetylcholine receptor partial agonist, as a new therapy for smoking cessation in Asian smokers. Clinical Therapeutics 2007;29(6):1027‐39. [DOI] [PubMed] [Google Scholar]
- Fujiwara H. Smoking is a disease and smokers are patients. Circulation Journal 2010;74(4):628‐9. [DOI] [PubMed] [Google Scholar]; Tsukahara H, Noda K, Keijiro S. A randomized controlled open comparative trial of varenicline vs nicotine patch in adult smokers. Circulation Journal 2010;74(4):771‐8. [DOI] [PubMed] [Google Scholar]
- NCT00977249. Varenicline for long‐term NRT users. ClinicalTrials.gov/ct2/ 2009 (accessed 2nd November 2010). [CRS: 9400123000011440] ; Tønnesen P, Mikkelsen K. Varenicline to stop long‐term nicotine replacement use: a double‐blind, randomized, placebo‐controlled trial. Nicotine & Tobacco Research 2013;15(2):419‐27. [CENTRAL: 861602; CRS: 9400123000018068; EMBASE: 2013064611; PUBMED: 23024246] [DOI] [PubMed] [Google Scholar]
- Vinnikov D, Brimkulov N, Burjubaeva A. A double‐blind, randomised, placebo‐controlled trial of cytisine for smoking cessation in medium‐dependent workers. Journal of Smoking Cessation 2008;3(1):57‐62. [Google Scholar]
- Walker N, Howe C, Bullen C, McRobbie H, Glover M, Parag V, et al. Study protocol for a non‐inferiority trial of cytisine versus nicotine replacement therapy in people motivated to stop smoking. BMC Public Health 2011;11(1):880. [CENTRAL: 814611; CRS: 9400123000011474; EMBASE: 22104038; 6430; PUBMED: 22104038] [DOI] [PMC free article] [PubMed] [Google Scholar]; Walker N, Howe C, Glover M, McRobbie H, Barnes J, Nosa V, et al. Cytisine versus nicotine for smoking cessation. New England Journal of Medicine 2014;371(25):2353‐62. [CENTRAL: 1037839; CRS: 9400129000003864; EMBASE: 2014610901] [DOI] [PubMed] [Google Scholar]; Zatonski W, Zatonski M. Cytisine versus nicotine for smoking cessation. New England Journal of Medicine 2015;372(11):1072. [CRS: 9400131000001342; PUBMED: 25760363] [DOI] [PubMed] [Google Scholar]
- Fagerström K, Nakamura M, Cho H, Tsa S, Wang C, Davies S, et al. Varenicline treatment for smoking cessation in Asian populations: a pooled analysis of placebo‐controlled trials conducted in six Asian countries. Current medical Research & Opinion 2010;26(9):2165‐73. [DOI] [PubMed] [Google Scholar]; Wang C, Xiao D, Chan KPW, Pothirat C, Garza D, Davies S. Varenicline for smoking cessation: a placebo‐controlled, randomized study. Respirology 2009;14(3):384‐92. [clinicaltrials.gov ID: NCT00371813] [DOI] [PubMed] [Google Scholar]
- West R, Zatonski W. Cytisine increased smoking cessation in adults. Annals of Internal Medicine 2012;156(2):JC1‐6. [CENTRAL: 897954; CRS: 9400123000012701; EMBASE: 2012033460] [DOI] [PubMed] [Google Scholar]; West R, Zatonski W, Cedzynska M, Lewandowska D, Pazik J, Aveyard PA, et al. Placebo‐controlled trial of cytisine for smoking cessation. New England Journal of Medicine 2011;365:1193‐200. [Current Controlled Trials ID: ISRCTN37568749] [DOI] [PubMed] [Google Scholar]
- Westergaard CG, Porsbjerg C, Backer V. The effect of smoking cessation on airway inflammation in young asthma patients. Clinical & Experimental Allergy 2013;44:353‐61. [DOI] [PubMed] [Google Scholar]; Westergaard CG, Porsbjerg C, Backer V. The effect of varenicline on smoking cessation in a group of young asthma patients. American Journal of Respiratory and Critical Care Medicine 2014;189:A1091. [DOI] [PubMed] [Google Scholar]; Westergaard CG, Porsbjerg C, Backer V. The effect of varenicline on smoking cessation in a group of young asthma patients. Respiratory Medicine 2015;109(11):1416‐22. [DOI: 10.1016/j.rmed.2015.07.017] [DOI] [PubMed] [Google Scholar]
- Reeves K, Watsky E, Williams K, Azoulay S, Billing B, Gong J. The safety of varenicline taken for 52 weeks for smoking cessation [RPOS3‐54]. Society for Research on Nicotine and Tobacco 12th Annual Conference Orlando Fla, USA2006. ; Spangler JG, Williams KE, Reeves KR, Billing CB, Pennington AM, Gong J. Comment and reply: A double‐blind study evaluating the long‐term safety of varenicline for smoking cessation. Current Medical Research and Opinion 2008;24(2):577‐9. [DOI] [PubMed] [Google Scholar]; Williams KE, Reeves KR, Billing CB, Pennington AM, Gong J. A double‐blind study evaluating the long‐term safety of varenicline for smoking cessation. Current Medical Research and Opinion 2007;23(4):793‐801. [DOI] [PubMed] [Google Scholar]
- NCT00644969. Smoking cessation study for patients with schizophrenia or schizoaffective disorder. ClinicalTrials.gov 2011 (accessed 2nd November 2011). ; Williams JM, Anthenelli RM, Morris CD, Treadow J, Thompson JR, Yunis C, et al. A randomized, double‐blind, placebo‐controlled study evaluating the safety and efficacy of varenicline for smoking cessation in patients with schizophrenia or schizoaffective disorder. Journal of Clinical Psychiatry 2012;73(5):654‐60. [CENTRAL: 836870; CRS: 9400123000013848; EMBASE: 2012321245; PUBMED: 22697191] [DOI] [PubMed] [Google Scholar]
- NCT00937508. Smoking cessation program in the pre‐admission clinic. ClinicalTrials.gov/ct2/ 2009 (accessed 2nd November 2010). ; Wong J, Abrishami A, Yang Y, Zaki A, Friedman Z, Selby P, et al. A perioperative smoking cessation intervention with varenicline: a double‐blind, randomized, placebo‐controlled trial. Anesthesiology 2012;117(4):755‐64. [CENTRAL: 835869; CRS: 9400123000016221; EMBASE: 2012567287; PUBMED: 22890119] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Burstein AH, Fullerton T, Clark DJ, Faessel HM. Pharmacokinetics, safety, and tolerability after single and multiple oral doses of varenicline in elderly smokers. Journal of Clinical Pharmacology 2006;46:1234‐40. [DOI] [PubMed] [Google Scholar]
- Pfizer Inc. Chantix prescribing information. http://www.chantix.com/content/Prescribing_Information.jsp (accessed 10th February 2007)2006.
- Cui Q, Robinson L, Elston D, Smaill F, Cohen J, Quan C, et al. Safety and tolerability of varenicline tartrate (Champix®/Chantix®) for smoking cessation in HIV‐infected subjects: a pilot open‐label study. AIDS patient Care and STDs 2012;26(1):12‐19. [DOI: 10.1089/apc.2011.0199] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CM, Wink JS, DeZee KJ. Use of the Patient Health Questionnaire‐2 to predict suicidal ideations in patients taking varenicline. American Journal on Addictions / American Academy of Psychiatrists in Alcoholism and Addictions 2012;21(4):356‐62. [CRS: 9400123000016466; PUBMED: 22691015] [DOI] [PubMed] [Google Scholar]; Dezee KJ, Wink JS, Cowan CM. Internet versus in‐person counseling for patients taking varenicline for smoking cessation. Military Medicine 2013;178(4):401‐5. [CENTRAL: 959429; CRS: 9400130000000072; PUBMED: 23707824] [DOI] [PubMed] [Google Scholar]
- Dutra SJ, Stoeckel LE, Carlini SV, Pizzagalli DA, Evins AE. Varenicline as a smoking cessation aid in schizophrenia: effects on smoking behavior and reward sensitivity. Psychopharmacology (Berl) 2012;219(1):25‐34. [DOI: 10.1007/s00213-011-2373-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbert JO, Croghan IT, Sood A, Schroeder DR, Hays JT, Hurt RD. Varenicline and bupropion sustained‐release combination therapy for smoking cessation. Nicotine & Tobacco Research 2009;11(3):234‐9. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Burke MV, Hays JT, Hurt RD. Combination treatment with varenicline and nicotine replacement therapy. Nicotine & Tobacco Research 2009;11(5):572‐6. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Hatsukami DK, Croghan IT, Schroeder DR, Allen SS, Hays JT, et al. Combination varenicline and bupropion SR for tobacco‐dependence treatment in cigarette smokers: A Randomized Trial. JAMA 2014;311(2):155‐63. [CENTRAL: 921055; CRS: 9400130000000073; EMBASE: 2014041620; PUBMED: 24399554] [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT00935818. Varenicline and bupropion for smoking cessation (CHANBAN). ClinicalTrials.gov/ct2/ 2009 (accessed 2nd November 2010).
- Falk DE, Litten RZ, Ryan ML, Fertig JB. A double‐blind, placebo‐controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. Alcoholism, Clinical and Experimental Research 2014;38(Supplement s1):139A. [CENTRAL: 993967; CRS: 9400129000002290; EMBASE: 71503638] [Google Scholar]; Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, et al. Journal of Addiction Medicine 2013;7(4):277‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Yousefi MK, Kneeland RE, Liesch SB, Folsom TD, Thuras PD. Antismoking and potential antipsychotic effects of varenicline in subjects with schizophrenia or schizoaffective disorder: a double‐blind placebo and bupropion‐controlled study. Schizophrenia Research 2013;146(1‐3):376‐8. [CENTRAL: 877011; CRS: 9400107000001596; PUBMED: 23507358] [DOI] [PubMed] [Google Scholar]; NCT01111149. Varenicline and smoking cessation in schizophrenia (VSCS). ClinicalTrials.gov/ct2/ 2010 (accessed 2nd November 2010).
- Ferketich AK, Otterson GA, King M, Hall N, Browning KK, Wewers ME. A pilot test of a combined tobacco dependence treatment and lung cancer screening program. Lung Cancer (Amsterdam, Netherlands) 2012;76(2):211‐5. [CENTRAL: 814368; CRS: 9400123000012566; EMBASE: 2012198269; PUBMED: 22088938] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferketich AK, Diaz P, Browning KK, Lu B, Koletar SL, Reynolds NR, et al. Safety of varenicline among smokers enrolled in the lung HIV study. Nicotine & Tobacco Research 2013;15(1):247‐54. [CRS: 9400123000018197; EMBASE: 2012756151; PUBMED: 22589421] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye MA, Ebbert JO, Prince CA, Lineberry TW, Geske JR, Patten CA. A feasibility study of varenicline for smoking cessation in bipolar patients with subsyndromal depression. Journal of Clinical Psychopharmacology 2013;33(6):821‐3. [CRS: 9400129000001031; PUBMED: 23963060] [DOI] [PubMed] [Google Scholar]
- Fucito LM, Toll BA, Wu R, Romano DM, Tek C, O'Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology 2011;215(4):655‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granatowicz J. Smoking cessation through the use of cytisine and other therapy. World Smoking Health 1976;1:8‐11. [Google Scholar]
- Gray KM, Carpenter MJ, Lewis AL, Klintworth EM, Upadhyaya HP. Varenicline versus bupropion XL for smoking cessation in older adolescents: A randomized, double‐blind pilot trial. Nicotine & Tobacco Research 2012;14(2):235‐9. [CENTRAL: 830824; CRS: 9400123000012679; EMBASE: 2012065214; PUBMED: 21778151] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhelaria RK, Rothberg M. Is varenicline effectiveness declining in randomized trials?. Archives of Internal Medicine 2011;171(19):1770‐1. [DOI] [PubMed] [Google Scholar]; Hajek P, McRobbie H, Myers K, Stapleton J, Dhanji AR. Is varenicline effectiveness declining in randomized trials? ‐ Reply. Archives of Internal Medicine 2011;171(19):1771‐2. [DOI] [PubMed] [Google Scholar]; Hajek P, McRobbie HJ, Myers KE, Stapleton J, Dhanji A‐R. Use of varenicline for 4 weeks before quitting smoking. Archives of Internal Medicine 2011;171(8):770‐7. [DOI] [PubMed] [Google Scholar]; Simon JA. Smoking cessation interventions: a primer for physicians. Archives of Internal Medicine 2011;171(8):777‐8. [DOI] [PubMed] [Google Scholar]
- Hajek P, Smith KM, Dhanji A‐R, McRobbie H. Is a combination of varenicline and nicotine patch more effective in helping smokers quit than varenicline alone? A randomised controlled trial. BMC Medicine 2013;11(1):140. [CENTRAL: 963647; CRS: 9400130000000078; EMBASE: 2013375051; PUBMED: 23718718] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell EE, Roche DJ, Ray LA. Pharmacogenetics of naltrexone and varenicline in heavy drinking smokers. Alcoholism, Clinical and Experimental Research 2014;38(Supplement s1):223A. [CENTRAL: 993964; CRS: 9400129000002287; EMBASE: 71503974] [Google Scholar]; Roche DJ, Bujarski S, Hartwell E, Green R, Ray LA. Combined varenicline and naltrexone treatment reduces smoking topography intensity in heavy‐drinking smokers. Pharmacology, Biochemistry, and Behavior 2015;134:92‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk LWJ, Ashare RL, Lohnes SF, Schlienz NJ, Rhodes JD, Tiffany ST, et al. The effects of extended pre‐quit varenicline treatment on smoking behavior and short‐term abstinence: a randomized clinical trial. Clinical Pharmacology and Therapeutics 2012;91(2):172‐80. [CENTRAL: 814229; CRS: 9400123000012021; PUBMED: 22130118] [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT00835900. An alternative dosing schedule for varenicline for smoking cessation. ClinicalTrials.gov/ct2/ 2009 (accessed 2nd November 2010).
- Hong LE, Thaker GK, McMahon RP, Summerfelt A, Rachbeisel J, Fuller RL, et al. Effects of moderate‐dose treatment with varenicline on neurobiological and cognitive biomarkers in smokers and nonsmokers with schizophrenia or schizoaffective disorder. Archives of General Psychiatry 2011;68(12):1195‐206. [CENTRAL: 810278; CRS: 9400123000016481; 6348; PUBMED: 21810630] [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT00492349. Varenicline adjunctive treatment in schizophrenia. ClinicalTrials.gov/ct2/ (accessed 13th October 2010).
- Hoogsteder PH, Kotz D, Spiegel PI, Viechtbauer W, Schayck OC. Efficacy of the nicotine vaccine 3'‐AmNic‐rEPA (NicVAX) co‐administered with varenicline and counselling for smoking cessation: a randomized placebo‐controlled trial. Addiction (Abingdon, England) 2014;109(8):1252‐9. [CENTRAL: 1000793; CRS: 9400129000002390; PUBMED: 24894625] [DOI] [PubMed] [Google Scholar]
- Hsueh K‐C, Hsueh S‐C, Chou M‐Y, Pan L‐F, Tu M‐S, McEwen A, et al. Varenicline versus transdermal nicotine patch: a 3‐year follow‐up in a smoking cessation clinic in Taiwan. Psychopharmacology 2014;231(14):2819‐23. [CENTRAL: 1050988; CRS: 9400129000003096; EMBASE: 2014439532; PUBMED: 24522334] [DOI] [PubMed] [Google Scholar]
- Jain R, Jhanjee S, Jain V, Gupta T, Mittal S, Goelz P, et al. A double‐blind placebo‐controlled randomized trial of varenicline for smokeless tobacco dependence in India. Nicotine & Tobacco Research 2014;16(1):50‐7. [CENTRAL: 1000326; CRS: 9400129000002785; PUBMED: 23946326] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings C, Kotseva K, Bacquer D, Hoes A, Velasco J, Brusaferro S, et al. Effectiveness of a preventive cardiology programme for high CVD risk persistent smokers: the EUROACTION PLUS varenicline trial. European Heart Journal 2014;35(21):1411‐20. [CENTRAL: 996426; CRS: 9400129000002224; EMBASE: 2014386940; PUBMED: 24616337] [DOI] [PubMed] [Google Scholar]
- Jiménez‐Ruiz CA, Barrios M, Peña S, Cicero A, Mayayo M, Cristóbal M, et al. Increasing the dose of varenicline in patients who do not respond to the standard dose. Mayo Clinic Proceedings 2013;88(12):1443‐5. [DOI] [PubMed] [Google Scholar]
- Bacvarov VI. Smoking cessation through medication: remarks on G. Scharfenberg, E Winkelvoss and S. Benndorf, Munch Med Wschr. 109 (1967) 1687‐1689. Munchener Medizinische Wochenschrift 1967;109(50):2663‐5. [PubMed] [Google Scholar]; Kempe G. Observation about the Bulgarian medicine for smoking withdrawal Tabex, produced by Pharmachim‐Sofia. Savr Med 1967;18(4):355‐6. [Google Scholar]
- Koegelenberg CF. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: A randomized clinical trial. [German]. Zeitschrift fur Gefassmedizin 2014;11(3):26. [CENTRAL: 994156; CRS: 9400129000002802; EMBASE: 2014813108; PUBMED: 25005652] [DOI] [PubMed] [Google Scholar]
- Maliszewski L, Straczynski A. Therapeutic use of Tabex. Wiadomoscie Lekarskie 1972;25(24):2207‐10. [PubMed] [Google Scholar]
- Marakulin VS, Komarov VM, Chuprin VV. Treatment of nicotinism [in Russian]. Voenno‐Meditsinskii Zhurnal 1984;1:55‐8. [PubMed] [Google Scholar]
- McColl SL, Burstein AH, Reeves KR, Billing CB, Stolar M, Sellers EM. Human abuse liability of the smoking cessation drug varenicline in smokers and nonsmokers. Clinical Therapeutics and Pharmacology 2008;83(4):607‐14. [DOI] [PubMed] [Google Scholar]
- McNaughton B, Frohlich J, Graham A, Young QR. Extended interactive voice response telephony (IVR) for relapse prevention after smoking cessation using varenicline and IVR: a pilot study. BMC Public Health 2013;13:824. [CENTRAL: 1015643; CRS: 9400129000003157] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metelitsa VI. Pharmacological agents in controlling smoking. Biulleten Vsesoiuznogo Kardiologicheskogo Nuachnogo Tsentra AMN SSSR 1987;10(1):109‐12. [PubMed] [Google Scholar]
- Mocking RJ, Patrick Pflanz C, Pringle A, Parsons E, McTavish SF, Cowen PJ, et al. Effects of short‐term varenicline administration on emotional and cognitive processing in healthy, non‐smoking adults: a randomized, double‐blind, study. Neuropsychopharmacology 2013;38(3):476‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMPTY
- Monova A, Monova D, Petrov V. Peneva E, Todorova M, Petrova M. Final report on double blind, placebo controlled, randomized clinical study for evaluation of efficacy, safety and tolerability of Tabex in patients with chronic nicotinism (tabacism): TAB ‐ SPH ‐ 04014. Sopharma plc unpublished report2004.
- NCT00502216. Naltrexone and varenicline: weight gain and tolerability in smokers. ClinicalTrials.gov/show/NCT00502216 2007 (accessed 2nd January 2016). [CRS: 9400123000016609] ; NCTNCT00502216. Naltrexone and varenicline: weight gain and tolerability on cigarette smokers. ClinicalTrials.gov/ct2/show/NCTNCT00502216 2008 (accessed 14th April 2008).
- NCT01308736. Varenicline‐aided cigarette reduction in smokers not ready to quit. ClinicalTrials.gov/show/NCT013087362011. [CRS: 9400131000001788]
- NCT01806779. Combination bupropion/varenicline for smoking cessation in male smokers. ClinicalTrials.gov/show/NCT01806779 2013 (accessed 2nd January 2016). [CRS: 9400107000001317]
- Buchanan TS, Berg CJ, Cox LS, Nazir N, Benowitz NL, Yu L, et al. Adherence to varenicline among African American smokers: an exploratory analysis comparing plasma concentration, pill count, and self‐report. Nicotine & Tobacco Research2012; Vol. 14, issue 9:1083‐91. [CENTRAL: 834603; CRS: 9400123000015692; EMBASE: 2012554324; PUBMED: 22367976] [DOI] [PMC free article] [PubMed]; Nollen NL, Cox LS, Nazir N, Ellerbeck EF, Owen A, Pankey S, et al. A pilot clinical trial of varenicline for smoking cessation in Black smokers. Nicotine & Tobacco Research 2011;13(9):868‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrovskaia TP. Results of clinical investigation of anti‐nicotine drug patches. Meditsinskaia Tekhnika 1994;3:42‐3. [PubMed] [Google Scholar]
- Park ER, Japuntich S, Temel J, Lanuti M, Pandiscio J, Hilgenberg J, et al. A smoking cessation intervention for thoracic surgery and oncology clinics. Journal of Thoracic Oncology 2011;6(6):1059‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C. Effects of Chantix on relapse prevention for smoking cessation. ClinicalTrials.gov/ct2/ 2009 (accessed 2nd November 2010). ; Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, et al. Working memory deficits predict short‐term smoking resumption following brief abstinence. Drug and Alcohol Dependence 2010;106(1):61‐4. [CENTRAL: 731184; CRS: 9400123000005478; PUBMED: 19733449] [DOI] [PMC free article] [PubMed] [Google Scholar]; Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, et al. Varenicline improves mood and cognition during smoking abstinence. Biological Psychiatry 2009;65(2):144‐9. [CENTRAL: 687104; CRS: 9400123000005157; PUBMED: 18842256] [DOI] [PMC free article] [PubMed] [Google Scholar]; Rudnick ND, Strasser AA, Phillips JM, Jepson C, Patterson F, Frey JM, et al. Mouse model predicts effects of smoking and varenicline on event‐related potentials in humans. Nicotine & Tobacco Research 2010;12(6):589‐97. [CENTRAL: 751949; CRS: 9400123000005659; PUBMED: 20395358] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paun D, Franze J. [Smoking cessation with cytisine 'Tabex' tablets] [German] [Raucherentwöhnung mit cytisinhaltigen "Tabex" tabletten]. Sonderduck aus das deutsche Gesundheitwesen 1968;23(44):2088‐91. [PubMed] [Google Scholar]; Paun D, Franze Y. Tabex: registering and treatment of smokers with chronic bronchitis in the consultation against tobacco smoking ‐ Berlin. Medico‐Biologic Information 1970;3:15‐9. [Google Scholar]
- Pfizer Inc. Flexible Dosing Trial. NDA 21‐928 [reported in CDER 2006]2006.
- Poling J, Rounsaville B, Gonsai K, Severino K, Sofuoglu M. The safety and efficacy of varenicline in cocaine using smokers maintained on methadone: a pilot study. The American Journal on Addictions 2010;19:401‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon JM, Morchon S, Baena A, Masuet‐Aumatell C. Combining varenicline and nicotine patches: a randomized controlled trial study in smoking cessation. BMC Medicine 2014;12(1):172. [CENTRAL: 1066789; CRS: 9400129000003408] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01303861. Concurrent bupropion/varenicline for smoking cessation (ConNic4). ClinicalTrials.gov/ct2/ 2011 (accessed 18th October 2011). ; Rose JE, Behm FM. Combination treatment with varenicline and bupropion in an adaptive smoking cessation paradigm. American Journal of Psychiatry 2014;171(11):1199‐205. [CENTRAL: 1021652; CRS: 9400129000003903; EMBASE: 2014905509; PUBMED: 24934962] [DOI] [PMC free article] [PubMed] [Google Scholar]; Rose JE, Behm FM. Combination varenicline/bupropion treatment benefits male NRT‐Nonresponders. Society for Research on Nicotine and Tobacco 19th Annual Meeting March 13‐16 Boston MA. 2013:261. [CRS: 9400107000001311] [Google Scholar]
- Schlienz NJ, Hawk LWJ, Tiffany ST, O'Connor RJ, Mahoney MC. The impact of pre‐cessation varenicline on behavioral economic indices of smoking reinforcement. Addictive Behaviors 2014;39(10):1484‐90. [CENTRAL: 993961; CRS: 9400129000002210; EMBASE: 2014413435; PUBMED: 24949949] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt F. [Medical support of nicotine withdrawal. Report on a double blind trial in over 5000 smokers (author's transl)] [German] [Medikamentose unterstutzung der raucherentwohnung: bericht uber versuche an uber 5000 rauchern im doppelblindversuch]. Munch Med Wachr 1974;116(11):557‐64. [PubMed] [Google Scholar]
- Schnoll RA, Cappella J, Lerman C, Pinto A, Patterson F, Wileyto EP, et al. A novel recruitment message to increase enrollment into a smoking cessation treatment program: preliminary results from a randomized trial. Health Communication 2011;26(8):735‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JC, Jung D, Oh M, Kong B, Ha T, Cho D, et al. Varenicline treatment for smoking cessation in people with schizophrenia: a randomized double‐blind placebo‐controlled trial. Schizophrenia Bulletin 2011;37:320‐1. [Google Scholar]; Shim JC, Jung DU, Jung SS, Seo YS, Cho DM, Lee JH, et al. Adjunctive varenicline treatment with antipsychotic medications for cognitive impairments in people with schizophrenia: a randomized double‐blind placebo‐controlled trial. Neuropsychopharmacology 2012;37(2):660‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicras‐Mainar A, Navarro‐Artieda R, Diaz‐Cerezo S, Sanz de Burgoa V. Effectiveness of varenicline compared with bupropion and nicotine replacement therapy (NRT) for smoking cessation in two smoking specialized units in the primary care setting. Society for Research on Nicotine and Tobacco Europe Conference: Bath, UK, 6‐9 October2010.
- Stapleton JA, Watson L, Spirling LI, Smith R, Milbrandt A, Ratcliffe M, et al. Varenicline in the routine treatment of tobacco dependence: a pre‐post comparison with nicotine replacement therapy and an evaluation in those with mental illness. Addiction 2008;103(1):146‐54. [DOI] [PubMed] [Google Scholar]
- Stoyanov S, Yanachkova M. [On the therapeutic effectiveness and tolerability of Tabex] [Bulgarian]. Savremenna Medicina 1972;23(6):30‐3. [Google Scholar]
- Catz SL, Jack LM, McClure JB, Javitz HS, Deprey M, Zbikowski SM, et al. Adherence to varenicline in the COMPASS smoking cessation intervention trial. Nicotine & Tobacco Research 2011;13(5):361‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Halperin AC, McAfee TA, Jack LM, Catz SL, McClure JB, Deprey TM, et al. Impact of symptoms experienced by varenicline users on tobacco treatment in a real world setting. Journal of Substance Abuse Treatment 2009;36:428‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]; McClure JB, Swan GE, Catz SL, Jack L, Javitz H, McAfee T, et al. Smoking outcome by psychiatric history after behavioral and varenicline treatment. Journal of Substance Abuse and Treatment 2010;38:394‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]; McClure JB, Swan GE, Jack L, Catz SL, Zbikowski SM, McAfee TA, et al. Mood, side‐effects and smoking outcomes among persons with and without probably lifetime depression taking varenicline. Journal of General Internal Medicine 2009;24:563‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Swan GE, McClure JB, Jack LM, Zbikowski SM, Javitz HS, Catz SL, et al. Behavioral counseling and varenicline treatment for smoking cessation. American Journal of Preventive Medicine 2010;38(5):482‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zbikowski SM, Jack LM, McClure JB, Deprey M, Javitz HS, McAfee TA, et al. Utilization of services in a randomized trial testing phone‐ and web‐based interventions for smoking cessation. Nicotine & Tobacco Research 2011;13(5):319‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner E, Buchholz A, Coffay A, Liu F, McMahon RP, Buchanan RW, et al. Varenicline for smoking cessation in people with schizophrenia: a double blind randomized pilot study. Schizophrenia Research 2011;129:94‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatonski W, Cedzynska M, Przewozniak K, Karpinska E, Lewandowska D, Pstrucha E, et al. An open label observational study of herbal cytisine (Tabex) as an aid to smoking cessation [POS1‐058]. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20‐23 March 2005; Prague, Czech Republic. 2005. [Google Scholar]; Zatonski W, Cedzynska M, Tutka P, West R. An uncontrolled trial of cytisine (Tabex) for smoking cessation. Tobacco Control 2006;15(6):481‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Wiratmoko MR, Yunus F, DSusanto A, Ginting TT, Kekalih A. Efficacy of varenicline, an nicotinic acetylcholine receptor partial agonist, vs placebo for smoking cessation. A randomized controlled trial. Respirology (Carlton, Vic.) 2013;18(Suppl 4):66 [OS205]. [CENTRAL: 989836; CRS: 9400129000000811; EMBASE: 71371580] [Google Scholar]
- Yujie W, Huliang L. Efficacy and safety of varenicline for smoking cessation in patients with CAD undergoing PCI. Journal of the American College of Cardiology 2014;64(16 SUPPL. 1):C207. [CENTRAL: 1054389; CRS: 9400129000003754; EMBASE: 71665049] [Google Scholar]
- Zincir SB, Zincir S, Kaymak E, Sunbul EA. Comparison of the effectiveness of varenicline, extended‐release bupropion and nicotine replacement therapy on the success and the maintenance of a smoking cessation program. Bulletin of Clinical Psychopharmacology 2013;23(3):224‐30. [CENTRAL: 914104; CRS: 9400126000000177; EMBASE: 2013628205] [Google Scholar]
References to ongoing studies
- Nosa V, Glover M. TALANOA Samoa: A randomised controlled trial to evaluate the efficacy of a cessation support programme for smokers delivered via radio. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364690 2013 (accessed 2nd January 2016).
- ACTRN12614000329662. Examination of mechanism of action of pre‐quit use of nicotine patch and varenicline for smoking cessation. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=365947 2014 (accessed 2nd January 2016). ; Ferguson SG, Walters JA, Lu W, Wells GP, Schuz N. Examination of the mechanism of action of two pre‐quit pharmacotherapies for smoking cessation. BMC Public Health 2015;15(1):1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACTRN12614000876695. Improving radiotherapy outcomes with smoking cessation: Feasibility trial in head and neck cancer patients. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12614000876695 2014 (accessed 2nd January 2016).
- NCT00387946. Efficacy and safety of dianicline treatment as an aid to smoking cessation in cigarette smokers (AMERIDIAN). ClinicalTrials.gov NCT00387946 2007 (accessed 20th November 2011). [CRS: 9400123000011388]
- EUCTR2009‐017599‐26‐IT. Efficacy and safety of smoking cessation with varenicline tartrate in diabetic smokers: a double‐blind, placebo‐controlled, randomized trial. www.clinicaltrialsregister.eu/ctr‐search/search?query=2009‐017599‐26 2009 (accessed 30th July 2015).
- ISRCTN25441641. Evaluation of the impact of systematic delivery of cessation interventions on delivery of smoking cessation in secondary care. www.isrctn.com/ISRCTN25441641 2010 (accessed 30th July 2015).
- Nahvi S, Segal KS, Litwin AH, Arnsten JH. Rationale and design of a randomized controlled trial of varenicline directly observed therapy delivered in methadone clinics. Addiction Science & Clinical Practice 2014;9:9. [CENTRAL: 1037892; CRS: 9400129000003243] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00554840. Comparison of varenicline and placebo for smoking cessation in schizophrenia. ClinicalTrials.gov/ct2/show/NCT00554840 2007 (accessed 2nd January 2016).
- NCT00580853. The effect of varenicline (Chantix) and bupropion (Zyban) on smoking lapse behavior. ClinicalTrials.gov/ct2/ (accessed 13th October 2010).
- NCT00683280. Contingency management and pharmacotherapy for smoking cessation. ClinicalTrials.gov/show/NCT00683280 2008 (accessed 2nd January 2016). [CRS: 9400131000001774]
- NCT00786149. Improving varenicline adherence and outcomes in homeless smokers. ClinicalTrials.gov/show/NCT00786149 2007 (accessed 2nd January 2016). [CRS: 9400131000001776]
- NCT00879177. Contingency management for initiating smoking abstinence in patients with hypertension. ClinicalTrials.gov/show/NCT00879177 2009 (accessed 2nd January 2016). [CRS: 9400131000001778]
- NCT00906386. Methadone maintenance treatment and smoking cessation (MMTASC). ClinicalTrials.gov/ct2/ 2009 (accessed 2nd November 2010).
- NCT00918307. Comparison of the efficacy and safety of varenicline versus placebo for smoking cessation among HIV‐infected patients. A randomized double blind controlled trial. ClinicalTrials.gov/show/NCT00918307 2009 (accessed 2nd January 2016). [CRS: 9400129000001223] ; NCT00918307. Efficacy and safety of varenicline amongst HIV‐infected patients (Inter‐ACTIV). ClinicalTrials.gov/ct2/ 2009 (accessed 2nd November 2010).
- NCT00921388. Exercise for smoking cessation in postmenopausal women. ClinicalTrials.gov/show/NCT00921388 2009 (accessed 2nd January 2016). [CRS: 9400129000001195]
- NCT00931021. Smoking cessation treatment for head and neck cancer patients. ClinicalTrials.gov/ct2/ 2009 (accessed 2nd November 2010). [CRS: 9400123000011426]
- NCT00937235. Treatment of smoking among individuals with PTSD: a phase II, randomized study of varenicline and cognitive behavioral therapy. ClinicalTrials.gov/show/NCT00937235 2009 (accessed 2nd January 2016). [CRS: 9400131000001780]
- NCT00943618. Combining varenicline and bupropion for smoking cessation. ClinicalTrials.gov/ct2/ 2009 (accessed 2nd November 2010).
- NCT01067612. Extended treatment for smoking cessation [NRT, bupropion and varenicline]. ClinicalTrials.gov/ct2/ 2010 (accessed 2nd November 2010).
- NCT01093937. Study of varenicline for smoking cessation/reduction in patients with bipolar disorder. ClinicalTrials.gov/ct2/ 2010 (accessed 2nd November 2010).
- NCT01162239. Maintaining nonsmoking. ClinicalTrials.gov/show/NCT01162239 2010 (accessed 2nd January 2016). [CRS: 9400131000001784]
- NCT01170338. Safety and efficacy of varenicline in patients with acute coronary syndrome. ClinicalTrials.gov/ct2/ 2010 (accessed 2nd November 2010).
- NCT01243203. Smoking cessation program in the preadmission clinic: the use of a teachable moment. ClinicalTrials.gov/show/NCT01243203 2007 (accessed 2nd January 2016). [CRS: 9400131000001786]
- NCT01286584. Varenicline in residential treatment (ViRT). ClinicalTrials.gov/ct2/ 2011 (accessed 18th October 2011).
- NCT01312909. A twelve‐week, randomized, double‐blind, placebo‐controlled, parallel‐group, dose‐ranging study with follow‐up evaluating the safety and efficacy of varenicline for smoking cessation in healthy adolescent smokers. ClinicalTrials.gov/show/NCT01312909 2011 (accessed 2nd January 2016). [CRS: 9400123000015499] ; NCT01312909. Smoking cessation study in healthy adolescent smokers. ClinicalTrials.gov/ct2/ 2011 (accessed 18th October 2011); Vol. ClinicalTrials.gov ID. [CRS: 9400123000011462]
- Chenoweth MJ, Novalen M, Hawk LWJ, Schnoll RA, George TP, Cinciripini PM, et al. Known and novel sources of variability in the nicotine metabolite ratio in a large sample of treatment‐seeking smokers. Cancer Epidemiology, Biomarkers & Prevention 2014;23(9):1773‐82. [CRS: 9400129000003416] [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT01314001. Pharmacogenetics of nicotine addiction treatment. ClinicalTrials.gov/ct2/ 2011 (accessed 18th October 2011).
- NCT01320462. Smoking cessation program in the preadmission clinic. ClinicalTrials.gov/ct2/ 2010 (accessed 18th October 2011).
- NCT01387425. Efficacy and safety of smoking cessation with varenicline tartrate in diabetic smokers (DIASMOKE). ClinicalTrials.gov/ct2/ 2011 (accessed 18th October 2011). [CRS: 9400123000011472]
- NCT01413516. A Two‐Part Pilot Study of Dosing, Safety and Efficacy of Varenicline Initiated During an Acute Smoke‐free Hospitalization and Continued Post‐Hospitalization. Http://clinicaltrials.gov/show/NCT014135162011. [CRS: 9400131000001790]
- NCT01509547. A randomized controlled trial of varenicline for adolescent smoking cessation. ClinicalTrials.gov/show/NCT01509547 2012 (accessed 2nd January 2016). [CRS: 9400131000001792]
- NCT01531049. Smoking cessation in young adults in Northern Finland. ClinicalTrials.gov/show/NCT01531049 2012 (accessed 2nd January 2016). [CRS: 9400131000001772]
- NCT01532232. Treatment of tobacco dependence in breast cancer patients: a randomized trial of varenicline (Chantix). ClinicalTrials.gov/show/NCT01532232 2012 (accessed 2nd January 2016). [CRS: 9400131000001794]
- NCT01538394. Clinical trial to evaluate the efficacy of smoking cessation. ClinicalTrials.gov/ct2/results?term=NCT01538394 2012 (accessed 30th July 2015).
- NCT01553136. 1/2‐multi‐site study: varenicline treatment of alcohol dependent smokers. ClinicalTrials.gov/show/NCT01553136 2012 (accessed 2nd January 2016). [CRS: 9400131000001798]
- NCT01574703. Study to evaluate cardiac assessments following different treatments of smoking cessation medications in subjects with and without psychiatric disorders [CATS]. ClinicalTrials.gov/show/NCT01574703 (accessed 2nd January 2016).
- NCT01592695. Tailored tobacco cessation program for rural veterans with comorbid depression, alcoholism or obesity. ClinicalTrials.gov/show/NCT01592695 2012 (accessed 2nd January 2016). [CRS: 9400131000001800]
- NCT01623505. Reducing cardiovascular disease by combining smoking cessation pharmacotherapy and behavioural counselling. ClinicalTrials.gov/ct2/results?term=NCT01623505 2011 (accessed 30th July 2015).
- NCT01639560. Varenicline for light smokers (ChanLight). ClinicalTrials.gov/ct2/show/NCT01639560?term=NCT01639560&rank=1 2012 (accessed 30th July 2015).
- NCT01694732. Efficacy of varenicline associated with intensive counselling versus placebo of varenicline associated with intensive counselling on smoking cessation at the acute phase of an exacerbation of chronic obstructive pulmonary disease (COPD). A multicenter randomized double‐blind trial. ClinicalTrials.gov/show/NCT01694732 2012 (accessed 2nd January 2016). [CRS: 9400131000001802]
- NCT01710137/S. A placebo controlled trial of varenicline for smoking among those with HIV/AIDS. ClinicalTrials.gov/show/NCT01710137 2012 (accessed 2nd January 2016). [CRS: 9400129000001228]
- NCT01756885. Extended duration varenicline for smoking among cancer patients: a clinical trial. ClinicalTrials.gov/show/NCT01756885 2013 (accessed 2nd January 2016). [CRS: 9400131000001804]
- NCT01771627. Pilot study of varenicline vs. nicotine patch delivered by a telephone quitline to promote smoking cessation. ClinicalTrials.gov/show/NCT01771627 2012 (accessed 2nd January 2016). [CRS: 9400131000001806]
- NCT01772641. A combination of scheduled reduced smoking with varenicline to enhance cessation. ClinicalTrials.gov/show/NCT01772641 2012 (accessed 2nd January 2016). [CRS: 9400131000001808]
- NCT01800019. The Canadian HIV quit smoking trial: tackling the co‐morbidities of depression and cardiovascular disease in HIV+ smokers. ClinicalTrials.gov/show/NCT01800019 2014 (accessed 2nd January 2016). [CRS: 9400129000001211]
- NCT01850953. Evaluating the effects of varenicline on smoking lapse in smokers with and without schizophrenia: implications for treatment. ClinicalTrials.gov/show/NCT01850953 2013 (accessed 2nd January 2016). [CRS: 9400131000001810]
- NCT01892813. Tailored tobacco intervention. ClinicalTrials.gov/show/NCT01892813 2013 (accessed 2nd January 2016). [CRS: 9400131000001812]
- NCT01898195. Improving adherence to smoking cessation medication among PLWHA. ClinicalTrials.gov/show/NCT01898195 2013 (accessed 2nd January 2016). [CRS: 9400129000001221]
- NCT02048917. Optimization of smoking cessation strategies in community cancer programs for newly diagnosed lung and head and neck cancer patients. Clinicaltrials.gov/show/NCT02048917 2014 (accessed 2nd January 2016). [CENTRAL: 1013511; CRS: 9400050000000094]
- NCT02106637. Early in‐hospital initiation of pharmacotherapy for smoking cessation, concomitant with nurse‐led support, in patients after an acute coronary syndrome (ACS). ClinicalTrials.gov/show/NCT02106637 2014 (accessed 2nd January 2016). [CRS: 9400131000001817]
- NCT02136498. Internet‐based medication adherence program for nicotine dependence treatment. ClinicalTrials.gov/show/NCT02136498 2014 (accessed 2nd January 2016). [CRS: 9400131000001052]
- NCT02146911. Evaluating the real‐world effectiveness of varenicline and bupropion for long‐term smoking cessation. ClinicalTrials.gov/show/NCT02146911 2014 (accessed 2nd January 2016). [CRS: 9400131000001819]
- NCT02147132. A pilot randomized, placebo‐controlled, crossover study of the effect of the nicotine nasal spray and varenicline on cigarette smoking following methadone dosing in methadone‐maintained patients. ClinicalTrials.gov/show/NCT02147132 2014 (accessed 2nd January 2016). [CRS: 9400131000001821]
- NCT02162849. The effects of behavioral counseling plus nicotine replacement therapy (NRT) or varenicline on smoking cessation among smokers high and low in intrinsic reward sensitivity. ClinicalTrials.gov/show/NCT02162849 2015 (accessed 2nd January 2016). [CRS: 9400131000001823]
- NCT02271919. Varenicline and combined nicotine replacement therapy (NRT) for initial smoking cessation and rescue Treatment in smokers: a randomized pilot trial. ClinicalTrials.gov/show/NCT02271919 2015 (accessed 2nd January 2016). [CRS: 9400131000001825]
- NCT02328794. Randomized clinical trial to reduce harm from tobacco. ClinicalTrials.gov/show/NCT02328794 2015 (accessed 2nd January 2016). [CRS: 9400131000001081]
- NCT02351167. Genetically informed smoking cessation trial. ClinicalTrials.gov/show/NCT02351167 2015 (accessed 2nd January 2016). [CRS: 9400131000001827]
- NCT02360631. Advancing tobacco use treatment for African American smokers. ClinicalTrials.gov/show/NCT02360631 2015 (accessed 2nd January 2016). [CRS: 9400131000001829]
- NCT02367391. Pilot randomized trial of an automated smoking cessation intervention via mobile phone text messages as an adjunct to varenicline in primary care. ClinicalTrials.gov/show/NCT02367391 2015 (accessed 2nd January 2016). [CRS: 9400131000001054] [DOI] [PMC free article] [PubMed]
- NCT00959972. Varenicline versus transdermal nicotine patch for smoking cessation in patients with coronary heart disease. http://clinicaltrials.gov/ct2/ 2009 (accessed 2nd November 2010). ; Reid RD, Armstrong A, McDonnell L, Aitken DA, Robert L, LaRue A, et al. Varenicline versus transdermal nicotine patch for smoking cessation in patients with coronary heart disease: A pilot randomized trial. Canadian Journal of Cardiology 2010;26(Supplement D):53D. [CRS: 9400123000014547; EMBASE: 70674067] [Google Scholar]
- NCT00756275. Varenicline and motivational advice for smokers with substance use disorders. ClinicalTrials.gov/ct2/show/NCT00756275 2008 (accessed 13th October 2010). [ClinicalTrials.gov ID NCT00756275] ; Rohsenow DJ, Tidey JW, Martin RA, Colby SM, Monti PM. Varenicline helps smokers with SUD stop smoking without harming recovery (POS5‐63). Society for Research on Nicotine and Tobacco 21st Annual Meeting February 25‐28 Philadelphia. 2015. [CRS: 9400131000000039] [Google Scholar]
- NCT00802919. Varenicline for cognitive deficits and cigarette smoking in schizophrenia. ClinicalTrials.gov/ct2/show/NCT00802919 2008 (accessed 2nd November 2010); Vol. ClinicalTrials.gov ID. ; Smith RC, Amiaz R, Mei S, Maayan L, Jin H, Boules S, et al. Varenicline effects on smoking, cognition, and psychiatric symptoms in schizophrenia. Neuropsychopharmacology 2013;38:S364‐5. [CENTRAL: 990503; CRS: 9400050000000054; EMBASE: 71278474] [Google Scholar]
- Tulloch H, Pipe A, Els C, Aitken D, Clyde M, Corran B, et al. Flexible and extended dosing of nicotine replacement therapy or varenicline in comparison to fixed dose nicotine replacement therapy for smoking cessation: rationale, methods and participant characteristics of the FLEX trial. Contemporary Clinical Trials 2014;38(2):304‐13. [CENTRAL: 999078; CRS: 9400129000003060; EMBASE: 2014493992] [DOI] [PubMed] [Google Scholar]
- EUCTR2009‐016446‐50/NL. Helping more smokers to quit by combining varenicline with counselling for smoking cessation. The COVACO randomized controlled trial. ClinicalTrialsregister.eu/ctr‐search/trial/2009‐016446‐50/NL 2009 (accessed 2nd January 2016). ; Rossem C, Spigt M, Smit ES, Viechtbauer W, Mijnheer KK, Schayck CP, et al. Combining intensive practice nurse counselling or brief general practitioner advice with varenicline for smoking cessation in primary care: Study protocol of a pragmatic randomized controlled trial. Contemporary Clinical Trials 2015;41:298‐312. [CENTRAL: 1066790; CRS: 9400131000001477; EMBASE: 2015828707] [DOI] [PubMed] [Google Scholar]
Additional references
- Raw M, McNeill A, Arnott D. Varenicline: guidance for health professionals on a new prescription‐only stop smoking medication. www.ash.org.uk/html/cessation/ASHVareniclineguidance.pdf (accessed November 2006)2006.
- Benowitz NL. Nicotine addiction. Primary Care 1999;26:611‐31. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta‐analysis. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD009329.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carston K. Personal communication December 2011.
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. Varenicline: an alpha4beta2 nAChR nicotinic receptor partial agonist for smoking cessation. Journal of Medicinal Chemistry 2005;48:3474‐7. [DOI] [PubMed] [Google Scholar]
- Davies NM, Taylor G, Taylor AE, Martin RM, Munafò MR, Thomas KH. Cardiovascular and neuropsychiatric risks of varenicline: too good to be true?. Lancet Respiratory Medicine 2015;3(12):e39. [DOI] [PubMed] [Google Scholar]
- Drovandi AD, Chen CC, Glass BD. Adverse effects cause varenicline discontinuation: a meta‐analysis. Current Drug Safety Sep 27th 2015 [Epup ahead of print]. [DOI] [PubMed]
- Ebbert JO, Montori VM, Erwin PJ, Stead LF. Interventions for smokeless tobacco use cessation. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD004306.pub4] [DOI] [PubMed] [Google Scholar]
- Etter J‐F. Cytisine for smoking cessation: a literature review and a meta‐analysis. Archives of Internal Medicine 2006;166:1‐7. [DOI] [PubMed] [Google Scholar]
- Etter JF, Burri M, Stapleton J. The impact of pharmaceutical company funding on results of randomized trials of nicotine replacement therapy for smoking cessation: a meta‐analysis. Addiction 2007;102(5):815‐22. [DOI] [PubMed] [Google Scholar]
- Etter JF, Lukas RJ, Benowitz NL, West R, Dresler CM. Cytisine for smoking cessation: a research agenda. Drug and Alcohol Dependence 2008;92(1‐3):3‐8. [DOI] [PubMed] [Google Scholar]
- Fagerström K, Balfour DJK. Neuropharmacology and potential efficacy of new treatments for tobacco dependence. Expert Opinion on Investigational Drugs 2006;15(2):107‐16. [DOI] [PubMed] [Google Scholar]
- US Food, Drug Administration. FDA issues Public Health Advisory on Chantix. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116849.htm 2008 (accessed 3rd September 2015).
- U. S. Food, Drug Administration. FDA drug safety communication: safety review update of Chantix (varenicline) and risk of neuropsychiatric adverse events. http://www.fda.gov/Drugs/DrugSafety/ucm276737.htm (accessed 19/1/2012) October 2011.
- U.S. Food, Drug Administration. FDA drug safety communication: Chantix (varenicline) may increase the risk of certain cardiovascular adverse events in patients with cardiovascular disease. http://www.fda.gov/Drugs/DrugSafety/ucm259161.htm (accessed 19/1/2012) June 2011.
- U.S. Food, Drug Administration. Adverse Events Reporting System (AERS). http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/default.htm (accessed 5/1/2012)2012.
- U. S. Food, Drug Administration. FDA updates label for stop smoking deug Chantix (varenicline) to include potential alcohol interaction, rare risk of seizures and studies of side effects on mood, behavior, or thinking. www.fda.gov/Drugs/DrugSafety/ucm436494.htm (accessed 18th March 2016).
- Foulds J, Burke M, Steinberg M, Williams JM, Ziedonis DM. Advances in pharmacotherapy for tobacco dependence. Expert Opinion on Emerging Drugs 2004;9(1):39‐53. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Mann JJ. Strategies for quantifying the relationship between medications and suicidal behaviour. Drug Safety 2011;34(5):375‐95. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Mann JJ. Varenicline, smoking cessation and neuropsychiatric adverse events. American Journal of Psychiatry 2013;170(12):1460‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnell D, Irvine D, Wise L, Davies C, Martin RM. Varenicline and suicidal behaviour: a cohort study based on data from the General Practice Research Database. BMJ 2009;339:b3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison‐Woolrych M, Ashton J. Psychiatric adverse events associated with varenicline: an intensive postmarketing prospective cohort study in New Zealand. Drug Safety 2011;34(9):763‐72. [DOI] [PubMed] [Google Scholar]
- Harrison‐Woolrych M, Maggo S, Tan M, Savage R, Ashton J. Cardiovascular events in patients taking varenicline: a case series from intensive postmarketing surveillance in New Zealand. Drug Safety 2012;35(1):33‐43. [DOI] [PubMed] [Google Scholar]
- Hemmingsson T, Kriebel D. Smoking at age 18‐20 and suicide during 26 years of follow‐up ‐ how can the association be explained?. international Journal of Epidemiology 2003;32:1000‐4. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;7414:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. http://www.cochrane‐handbook.org2011.
- Hogg RC, Bertrand D. Partial agonists as therapeutic agents at neuronal nicotinic acetylcholine receptors. Biochemical Pharmacology 2007;73:459‐68. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip‐Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine & Tobacco Research 2003;5(1):13‐25. [PubMed] [Google Scholar]
- Hughes JR, Stead LF, Hartmann‐Boyce J, Cahill K, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD000031.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute for Safe Medication Practice. Quartewatch: 2010 Quarter 3. http://www.ismp.org/quarterwatch/pdfs/2010Q3.pdf (accessed 19/1/2012)2011.
- Kasliwal R, Wilton LV, Shakir SA. Safety and drug utilization profile of varenicline as used in general practice in England: interim results from a prescription‐event monitoring study. Drug Safety 2009;32(6):499‐507. [DOI] [PubMed] [Google Scholar]
- Kirchhoff VD, Nguyen HT, Soczynska JK, Woldeyohannes H, McIntyre RS. Discontinued psychiatric drugs in 2008. Expert Opinion on Investigational Drugs 2009;18(10):1431‐43. [DOI] [PubMed] [Google Scholar]
- Kotz D, Viechtbauer W, Simpson C, Schayck OCP, West R, Sheikh A. Cardiovascular and neuropsychiatric risks of varenicline: a retrospective cohort study. Lancet 2015 [Epub ahead of print];S2213‐2600(15):320‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaviss J, Sullivan W, Ren S, Everson‐Hock E, Stevenson M, Stevens JW, et al. What is the clinical effectiveness and cost‐effectiveness of cytisine compared with varenicline for smoking cessation? a systematic review and economic evaluation. Health Technology Assessment 2014;18(33):1‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LK, Patafio FM, Rosser WW. Gastrointestinal adverse effects of varenicline at maintenance dose: a meta‐analysis. BMC Clinical Pharmacology 2011;11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Smith PH, Kaufman M, Mazure CM, Weinberger AH. Sex differences in varenicline efficacy for smoking cessation: a meta‐analysis. Nicotine & Tobacco Research 2015 Oct 6th [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Meyer TE, Taylor LG, Xie S, Graham DJ, Mosholder AD, Williams JR, et al. Neuropsychiatric events in varenicline and nicotine replacement patch users in the Military Health System. Addiction (Abingdon, England) 2013;108(1):203‐10. [CRS: 9400123000018430; PUBMED: 22812921] [DOI] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at a4ß2 and a full agonist at a7 neuronal nicotinic receptors. Molecular Pharmacology 2006;70(3):801‐5. [DOI] [PubMed] [Google Scholar]
- Miller M, Hemenway D, Rimm E. Cigarettes and suicide: a prospective study of 50000 men. American Journal of Public Health 2000;90(5):768‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills EJ, Wu P, Lockhart I, Thorlund K, Puhan M, Ebbert JO. Comparisons of high‐dose and combination nicotine replacement therapy, varenicline and bupropion for smoking cessation: a systematic review and multiple‐treatment meta‐analysis. Annals of Medicine 2012;44(6):588‐97. [DOI] [PubMed] [Google Scholar]
- Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta‐analysis. Circulation 2014;129(1):28‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molero Y, Lichtenstein P, Zetterqvist J, Hellner Gumpert C, Fazel S. Varenicline and risk of psychiatric conditions, suicidal behaviour, criminal offending, and transport accidents and offences: population based cohort study. BMJ 2015;351:h2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TJ, Furberg CD, Glenmullen J, Maltsberger JT, Singh S. Suicidal behaviour and depression in smoking cessation treatments. PLoS ONE 2011;6(11):e27016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence. Varenicline for smoking cessation. http://www.nice.org.uk/nicemedia/pdf/TA123Guidance.pdf (accessed 14th April 2008). London: National Institute for Health and Clinical Excellence, 2007.
- Papke RL, Heinemann SF. Partial agonist properties of cytisine on neuronal nicotinic receptors containing the beta2 subunit. Molecular Pharmacology 1994;45:142‐9. [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Changeux JP. Use of knockout mice to determine the molecular basis for the actions of nicotine. Nicotine & Tobacco Research 1999;1(Suppl 2):S121‐5. [DOI] [PubMed] [Google Scholar]
- Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta‐analysis. BMJ 2012;344:e2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands SB, Brooks PR, Chambers LK, Coe JW, Liu Y, Rollema H, et al. A new therapy for smoking cessation: varenicline, a selective nicotinic receptor partial agonist [SYM10C]. Society for Research on Nicotine and Tobacco 11th Annual Meeting. 20‐23 March 2005; Prague, Czech Republic. 2005:14. [Google Scholar]
- Singh S, Loke YK, Spangler JG, Furberg CD. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta‐analysis. Canadian Medical Association Journal 2011;183(12):1359‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater YE, Houlihan LM, Maskell PD, Exley R, Bermudez I, Lukas RJ, et al. Halogenated cytisine derivatives as agonists at human neuronal nicotinic acetylcholine receptor subtypes. Neuropharmacology 2003;44:503‐15. [DOI] [PubMed] [Google Scholar]
- Stead LF, Hughes JR. Lobeline for smoking cessation. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD000124] [DOI] [PubMed] [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Hartmann‐Boyce J, Cahill K, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD000146.pub4] [DOI] [PubMed] [Google Scholar]
- Sterling LH, Windle SB, Filion KB, Touma L, Eisenberg MJ. Varenicline and adverse cardiovascular events: a systematic review and meta‐analysis of randomized controlled trials. Journal of the American Heart Association 2016;5:e002849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Surgeon General. The health consequences of smoking: a report of the Surgeon General. www.cdc.gov/tobacco/sgr/sgr_2004/index.htm (accessed 25/1/2006)2004. [PubMed]
- Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ 2012;345:e7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopharma AD. http://www.tabex.net/41814_packageinsert.phtml (accessed 2/11/2011). Biogenic Stimulants Inc, 2011.
- Thomas KH, Martin RM, Davies NM, Metcalfe C, Windmeijer F, Gunnell D. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ 2013;347:f5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KH, Martin RM, Potokar J, Pirmohamed M, Gunnell D. Reporting of drug induce depression and non‐fatal suicidal behaviour in the UK from 1998 to 2011. BMC Pharmacology & Toxicology 2014;15:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KH, Martin RM, Knipe DW, Higgins JPT, Gunnell D. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta‐analysis. BMJ 2015;350:h1109. [PROSPERO 2014:CRD42014009224] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson‐Evans TP, Glover MP, Walker N. Cytisine's potential to be used as a traditional healing method to help indigenous people stop smoking: a qualitative study with Māori. Nicotine & Tobacco Research 2011;13(5):353‐60. [DOI] [PubMed] [Google Scholar]
- Tonstad S, Els C. Varenicline: smoking cessation in patients with medical and psychiatric comorbidity. Clinical Medicine Insights: Therapeutics 2010;2:681‐95. [Google Scholar]
- Tonstad S, Davies S, Flammer M, Russ C, Hughes J. Psychiatric adverse events in randomized, double‐blind, placebo‐controlled clinical trials of varenicline: a pooled analysis. Drug Safety 2010;33(4):289‐301. [DOI] [PubMed] [Google Scholar]
- Tutka P, Zatoński W. Cytisine for the treatment of nicotine addiction: from a molecule to therapeutic efficacy. Pharmacological Reports 2005;58:777‐98. [PubMed] [Google Scholar]
- Tutka P, Mróz T, Zatoński W. Cytisine ‐ renaissance of well known alkaloid. Pharmacological aspects of efficacy in the treatment of tobacco dependence [Polish]. Farmakoterapia w Psychiatrii i Neurologii 2006;1:33‐9. [Google Scholar]
- Tutka P. Nicotinic receptor partial agonists as novel compounds for the treatment of smoking cessation. Expert Opinion on Investigational Drugs 2008;17(10):1473‐85. [DOI] [PubMed] [Google Scholar]
- VA Center for Medication Safety. Varenicline criteria for prescribing. http://www.pbm.va.gov/Clinical%20Guidance/Criteria%20For%20Use/Varenicline%20Criteria%20for%20Prescribing.doc (accessed 19/1/2012)2011.
- Walsh RA. Australia's experience with varenicline: usage, costs and adverse reactions [letter]. Addiction 2011;106:449‐52. [DOI] [PubMed] [Google Scholar]
- Ware JH, Vetrovec GW, Miller AB, Tosh A, Gaffney M, Yunis C, et al. Cardiovascular safety of varenicline: patient‐level meta‐analysis of randomized, blinded, placebo‐controlled trials. American Journal of Therapeutics 2013;20(3):235‐46. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100:299‐303. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Global Report: Mortality Attributable to Tobacco. apps.who.int/iris/bitstream/10665/44815/1/9789241564434_eng.pdf. Geneva: World Health Organization, 2012 (accessed 8th September 2015).
References to other published versions of this review
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD006103.pub2] [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD006103.pub3] [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD006103.pub5] [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD006103.pub6] [DOI] [PubMed] [Google Scholar]
- Hey K, Lancaster T, Bala M. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD006103] [DOI] [PubMed] [Google Scholar]


