Abstract
Background
This is an update of a review last published by Cochrane in June 2012 entitled "Cromolyn sodium for the prevention of chronic lung disease in preterm infants", which included two studies. This 2016 update identified no further studies.
Chronic lung disease (CLD) frequently occurs in preterm infants and has a multifactorial aetiology including inflammation. Cromolyn sodium is a mast cell stabiliser that inhibits neutrophil activation and neutrophil chemotaxis and therefore may have a role in the prevention of CLD.
Objectives
To determine the effect of prophylactic administration of cromolyn sodium on the incidence of CLD at 28 days or 36 weeks' postmenstrual age (PMA), mortality, or the combined outcome of mortality and CLD at 28 days or 36 weeks' PMA in preterm infants.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 4), MEDLINE via PubMed (1966 to 12 May 2016), Embase (1980 to 12 May 2016), and CINAHL (1982 to 12 May 2016). We searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
We included randomised or quasi‐randomised controlled clinical trials involving preterm infants. Initiation of cromolyn sodium administration was during the first two weeks of life. The intervention had to include administration of cromolyn sodium by nebuliser or metered dose inhaler with or without spacer device versus placebo or no intervention. Eligible studies had to include at least one of the following outcomes: overall mortality, CLD at 28 days, CLD at 36 weeks' PMA, or the combined outcome mortality and CLD at 28 days.
Data collection and analysis
We used the standard method for Cochrane as described in the Cochrane Handbook for Systematic Reviews of Interventions. We reported risk ratio (RR) and risk difference (RD) with 95% confidence intervals (CI) for dichotomous outcomes and mean difference (MD) with 95% CI for continuous data. The meta‐analysis used a fixed‐effect model. We examined heterogeneity using the I2 statistic. We assessed the quality of evidence for the main comparison at the outcome level using the GRADE approach.
Main results
We identified two eligible studies with small numbers of infants enrolled (64 infants). Prophylaxis with cromolyn sodium did not result in a statistically significant effect on the combined outcome of mortality and CLD at 28 days (typical RR 1.05, 95% CI 0.73 to 1.52; typical RD 0.03, 95% CI ‐0.20 to 0.27; 2 trials, 64 infants; I2 = 0% for both RR and RD); mortality at 28 days (typical RR 1.31, 95% CI 0.52 to 3.29; I2 = 73% typical RD 0.06, 95% CI ‐0.13 to 0.26; I2 = 87%; 2 trials, 64 infants) (very low quality evidence); CLD at 28 days (typical RR 0.93, 95% CI 0.53 to 1.64; I2 = 40%; typical RD ‐0.03, 95% CI ‐0.27 to 0.20; I2 = 38%; 2 trials, 64 infants) or at 36 weeks' PMA (RR 1.25, 95% CI 0.43 to 3.63; RD 0.08, 95% CI ‐0.29 to 0.44; 1 trial, 26 infants). There was no significant difference in CLD in survivors at 28 days (typical RR 0.97, 95% CI 0.58 to 1.63; typical RD ‐0.02, 95% CI ‐0.29 to 0.26; I2 = 0% for both RR and RD; 2 trials, 50 infants) or at 36 weeks' PMA (RR 1.04, 95% CI 0.38 to 2.87; RD 0.02, 95% CI ‐0.40 to 0.43; 1 trial, 22 infants). Prophylaxis with cromolyn sodium did not show a statistically significant difference in overall neonatal mortality, incidence of air leaks, necrotising enterocolitis, intraventricular haemorrhage, sepsis, and days of mechanical ventilation. There were no adverse effects noted. The quality of evidence according to GRADE was very low for one outcome (mortality to 28 days) and low for all other outcomes. The reasons for downgrading the evidence was due to design (risk of bias in one study), inconsistency between the two studies (high I2 values for mortality at 28 days for both RR and RD), and lack of precision of estimates (small sample sizes). Further research does not seem to be justified.
Authors' conclusions
There is currently no evidence from randomised trials that cromolyn sodium has a role in the prevention of CLD. Cromolyn sodium cannot be recommended for the prevention of CLD in preterm infants.
Plain language summary
Cromolyn sodium for the prevention of chronic lung disease in preterm infants
Review question: What is the effect of prophylactic administration of cromolyn sodium on the incidence of chronic lung disease at 28 days or 36 weeks' postmenstrual age (PMA), mortality, or the combined outcome of mortality or chronic lung disease at 28 days or 36 weeks' PMA in preterm infants.
Background
Cromolyn sodium administered in the first few days of life has not been shown to prevent chronic lung disease in preterm infants. Preterm babies (babies born before 37 weeks' PMA) often need to be given oxygen for lung problems for many weeks because of chronic lung disease. This is due, in part, to inflammation (swelling) within the lungs. Theoretically, cromolyn sodium is a drug that might help prevent this inflammation. It is relatively safe and side effects are rare. It can be given by nebuliser or aerosol inhaler in the first few days of life to try to prevent chronic lung disease.
Study characteristics
We found only two studies enrolling 64 infants. In one of the two studies, there was a low risk of bias whereas in the second study there were concerns about how the infants had been put into treatment groups, and whether parents and doctors were aware of which treatment was given (random sequence generation, allocation concealment and blinding of outcomes assessment).
Study funding sources
We found no studies that received funding from the industry.
Key results
Prophylaxis with cromolyn sodium did not result in an important effect on the combined outcome of mortality or chronic lung disease at 28 days of age, chronic lung disease at 28 days; chronic lung disease at 28 days or at 36 weeks' PMA; or chronic lung disease in survivors at 28 days or at 36 weeks' PMA. This review of trials found no strong evidence that cromolyn sodium can prevent or reduce chronic lung disease and further research does not seem to be justified.
Quality of evidence
The quality of evidence was low for most measures.
Summary of findings
Summary of findings for the main comparison. Cromolyn sodium for the prevention of chronic lung disease in preterm infants.
| Cromolyn sodium for the prevention of chronic lung disease in preterm infants | ||||||
| Patient or population: preterm infants Setting: neonatal intensive care units Intervention: cromolyn sodium Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with cromolyn sodium | |||||
| Mortality or CLD at 28/30 days | Study population | RR 1.05 (0.73 to 1.52) | 64 (2 RCTs) | ⊕⊕⊝⊝ Low 1, 2 | ||
| 625 per 1000 | 656 per 1000 (456 to 950) | |||||
| Mortality to 28 days | Study population | RR 1.31 (0.52 to 3.29) | 64 (2 RCTs) | ⊕⊝⊝⊝ Very low 1, 2, 3 | ||
| 188 per 1000 | 246 per 1000 (98 to 617) | |||||
| CLD at 28 days | Study population | RR 0.93 (0.53 to 1.64) | 64 (2 RCTs) | ⊕⊕⊝⊝ Low 1, 2 | ||
| 438 per 1000 | 407 per 1000 (232 to 717) | |||||
| CLD at 36 weeks' PMA | Study population | RR 1.25 (0.43 to 3.63) | 26 (1 RCT) | ⊕⊕⊝⊝ Low 1, 2 | ||
| 308 per 1000 | 385 per 1000 (132 to 1000) | |||||
| CLD among survivors to 28 days | Study population | RR 0.97 (0.58 to 1.63) | 50 (2 RCTs) | ⊕⊕⊝⊝ Low 1, 2 | ||
| 538 per 1000 | 522 per 1000 (312 to 878) | |||||
| CLD among survivors to 36 weeks' PMA | Study population | RR 1.04 (0.38 to 2.87) | 22 (1 RCT) | ⊕⊕⊝⊝ Low 1, 2 | ||
| 400 per 1000 | 416 per 1000 (152 to 1000) | |||||
| Days on mechanical ventilation | The mean days on mechanical ventilation was 0 | The mean days on mechanical ventilation in the intervention group was 1 undefined higher (6.41 lower to 8.41 higher) | ‐ | 28 (1 RCT) | ⊕⊕⊝⊝ Low 1, 2 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CLD: chronic lung disease; PMA: postmenstrual age; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Blinding of randomisation in one study could not be determined. Blinding of that outcome could not be determined.
2 Downgraded two levels due to risk of bias and imprecision of estimates.
3 Downgraded three levels due to risk of bias, imprecision of estimate and inconsistency across studies (moderate to high I2 statistic).
Background
Description of the condition
Chronic lung disease (CLD), defined as oxygen dependency at 28 days or at 36 weeks' postmenstrual age (PMA) is a pulmonary disorder that frequently occurs in preterm infants (Northway 1967; Shennan 1988). It is the consequence of unresolved or abnormally repaired lung damage and has a multifactorial aetiology that has been described extensively by previous authors. These factors include exposure to high oxygen concentrations, volume‐derived trauma, barotrauma, sepsis and inflammation (Avery 1987; Paita 1991; Rojas 1995). In the past decades, the survival rate of very low birth weight infants has increased and the prevalence of CLD remains high (Parker 1992). The incidence varies depending on the population studied, the diagnostic criteria used and variation between centres in clinical management (Avery 1987; Hack 1991; Lee 2000; O'Brodovich 1985; Shennan 1988). CLD may be associated with chronic respiratory difficulties, prolonged and recurrent hospitalisation, growth restriction and death (Lee 2000; O'Brodovich 1985). The administration of antenatal corticosteroids to mothers likely to give birth preterm reduces neonatal mortality and the incidence of respiratory distress syndrome (RDS) but not the incidence of CLD (Crowley 2000). Administration of prophylactic natural surfactant extract does not reduce the incidence of CLD but does reduce the combined outcome of death or CLD (Soll 1997).
Neonates who develop CLD have elevated levels of endothelin‐1, interleukin (IL)‐6, IL‐8, and high ratios of IL‐1B to IL‐1 receptor antagonists (IL‐1ra) in tracheobronchial lavage when compared to neonates with RDS or controls (Bagchi 1994; Niu 1998; Rindfleisch 1996; Tullus 1996). One study showed elevated levels of tumour necrosis factor (TNF) alpha in neonates who subsequently developed CLD (Tullus 1996), but another study did not (Bagchi 1994).
Description of the intervention
Cromolyn sodium was originally characterised as a mast cell stabiliser (Hoag 1991). However, it also inhibits neutrophil activation (Kay 1987), neutrophil chemotaxis (Bruijnzeel 1989), macrophage activation, tachykinin action, eicosanoid and cytokine release, and adhesion molecule expression (Yazid 2009). Cromolyn sodium was, therefore, thought to modulate the inflammatory process in the lung.
One in vitro study showed that cromolyn sodium stimulates the anti‐inflammatory intracellular protein annexin‐A1 trafficking and release (Yazid 2009). Cromolyn sodium inhibits eicosanoid release due to inhibition of a phosphatase PP2A (phosphoprotein phosphatase; EC 3.1.3.16), which probably forms part of a control loop to limit annexin‐A1 release (Yazid 2009).
In adults exposed to organic dust, cromolyn sodium attenuates the increase in neutrophils, IL‐6, TNF‐alpha, myeloperoxidase and soluble intracellular adhesion molecule (ICAM)‐1 in bronchoalveolar lavage (Larsson 2001).
In one small non‐randomised cohort comparison study of cromolyn sodium use in infants with CLD, Viscardi 1994 found that there was a decrease in peak inspiratory pressure, a reduction in intermittent mandatory ventilation requirements and an increase in dynamic compliance after two weeks of cromolyn sodium therapy. Total airway resistance did not change significantly. Compared to matched controls there were no differences in duration of need for supplemental oxygen, ventilation or hospitalisation. These results suggested that cromolyn therapy might improve pulmonary function in some infants with established CLD (Viscardi 1994). Initiating cromolyn sodium treatment in the first few days of life in infants at risk of CLD might inhibit the neutrophil influx and the release of inflammatory mediators in the lung and thereby decrease the lung injury leading to CLD.
How the intervention might work
Cromolyn sodium may be delivered by nebuliser or pressurised aerosol with or without a spacer device. With either method only 0.22% to 1.3% of the dose reaches the lungs (Fok 1996; Grigg 1992). Aerosolised products tend to be deposited in the central lung region rather than in the periphery (Fok 1996). Humidification of the gas reduces lower respiratory tract deposition of aerosolised products (Diot 1995). Addition of a spacer device between the nebuliser and the endotracheal tube (Harvey 1995), and synchronising nebulisation with inspiratory airflow (Diot 1995), increase deposition. There is considerable intersubject variability in lung deposition (Fok 1996). All these factors will modify therapeutic effects.
Cromolyn is thought to be a relatively safe drug. However, there are occasional reports in the literature of adverse effects. These include dermatitis (Settipane 1979; Sheffer 1975), urticaria (Menon 1977; Sheffer 1975), eosinophilic pulmonary infiltrates (Lobel 1972; Sheffer 1975), pulmonary allergic granulomatosis (Burgher 1974; Sheffer 1975), and anaphylaxis (Ahmad 1983; Brown 1981).
Why it is important to do this review
This review updates the existing review "Cromolyn sodium for the prevention of chronic lung disease in preterm infants" published in the Cochrane Database of Systematic Reviews which was last updated in 2012 (Ng 2001; Ng 2012).
Objectives
To determine the effect of prophylactic administration of cromolyn sodium on the incidence of CLD at 28 days or 36 weeks' postmenstrual age (PMA), mortality, or the combined outcome of mortality and CLD at 28 days or 36 weeks' PMA in preterm infants.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled clinical trials.
Types of participants
Preterm infants (less than 37 weeks' PMA).
Types of interventions
The intervention had to include administration of cromolyn sodium by nebuliser or metered dose inhaler with or without spacer device versus placebo or no intervention. Treatment with cromolyn sodium had to be initiated during the first two weeks of life.
Types of outcome measures
Primary outcomes
Mortality within the study period.
Combined outcome of CLD or mortality at 28 days.
CLD at 28 days and 36 weeks' PMA.
Secondary outcomes
Number of days on oxygen.
Number of days on ventilator.
Patent ductus arteriosus (PDA).
Pulmonary interstitial emphysema (PIE).
Pneumothorax.
Any grade of intraventricular haemorrhage (IVH).
Necrotising enterocolitis (NEC).
Sepsis.
Adverse effects.
Search methods for identification of studies
We performed the search strategy to identify studies according to the guidelines of the Cochrane Neonatal Review Group.
Electronic searches
We used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register).
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 4) in the Cochrane Library; MEDLINE via PubMed (1966 to 12 May 2016); Embase (1980 to 12 May 2016); and CINAHL (1982 to 12 May 2016) using the following search terms: (cromolyn sodium OR cromoglycate), plus database‐specific limiters for randomised controlled trials (RCTs) and neonates (see Appendix 1 for the full search strategies for each database). We applied no language restrictions.
Searching other resources
We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization's International Trials Registry and Platform www.whoint/ictrp/search/en/, and the ISRCTN Registry).
For this update, we searched abstracts from the Pediatric Academic Societies' Annual Meetings (2000 to 2016) on 12 May 2016 on their website PAS2View and Web of Science using the two previously identified trials as starting points (Viscardi 1997; Watterberg 1993).
Data collection and analysis
We used the standard methods of Cochrane (Higgins 2011) and the Cochrane Neonatal Review Group.
Selection of studies
We first reviewed identified reports to determine whether the trial had a concurrent control group. If not, we excluded the study. The method of assignment to control and intervention groups was then determined and if not random or quasi‐random, we discarded the trial.
Data extraction and management
Two review authors (GN, AO) independently selected studies and extracted and assessed data. We then compared the data and resolved any differences by discussion. Information on the trial participants included birth weight, gestational age (GA) at birth, need for mechanical ventilation, and sex. Information on clinical outcomes included CLD at 28 days, CLD at 36 weeks' PMA, combined outcome of CLD or mortality at 28 days, mortality at 28 days, IVH, NEC, air leaks, sepsis, and adverse effects due to cromolyn sodium. Information on hospital stay and days on oxygen or on mechanical ventilation was also sought.
Assessment of risk of bias in included studies
Two review authors (GN, AO) independently assessed the risk of bias (low, high or unclear) of all included trials using the Cochrane 'Risk of bias' tool for the following domains (Higgins 2011):
sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective reporting (reporting bias);
any other bias.
This information is included in the Characteristics of included studies table. See Appendix 2 for a more detailed description of risk of bias for each domain.
If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
Measures of treatment effect
We performed statistical analyses using Review Manager 5 (RevMan 2011). We analysed categorical data using risk ratio (RR), risk difference (RD) and the number needed to treat for an additional beneficial outcome (NNTB) or an additional harmful outcome (NNTH). We analysed continuous data using mean difference (MD). We reported the 95% confidence interval (CI) on all estimates.
Assessment of heterogeneity
We evaluated heterogeneity between trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I2 statistic (Higgins 2003). For this update of the review, we categorised heterogeneity as: none if less than 25%, low if 25% to 49%, moderate if 50% to 74% and high if 75% or more. We used a fixed‐effect model for meta‐analysis.
Data synthesis
We used the standard methods of the Cochrane Neonatal Review Group to synthesise data using RR, RD and NNTB if there was a statistically significant reduction in RD and NNTH if there was a statistically significant increase in the RD. We performed a meta‐analysis of the data from the included trials using a fixed‐effect model.
Quality of evidence
For the update in 2016, we used the GRADE approach to assess the quality of evidence (Schünemann 2013) for the following (clinically relevant) outcomes: CLD (defined as oxygen dependency at 28 days of life or at 36 weeks' postmenstrual age with compatible chest x‐ray signs), mortality within the study period and, the number of days on mechanical ventilation.
Two review authors independently assessed the quality of the evidence for each of the outcomes. We considered evidence from RCTs as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro Guideline Development Tool to create a 'Summary of findings' table to report the quality of the evidence (GRADEpro GDT).
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analysis based on GA or birth weight if data were available.
Sensitivity analysis
As the review included only two studies, sensitivity analysis was not possible.
Results
Description of studies
Results of the search
We identified two eligible studies, both single centre. We excluded no eligible studies from analysis. The results of previous and current searches are shown in the study flow diagram: review update (Figure 1).
1.

Study flow diagram: review update.
Included studies
In the trial by Watterberg 1993, 38 infants needing ventilatory support at birth were randomised to receive cromolyn sodium or saline. The preterm infants had a mean GA standard deviation (SD) of 25.9 ± 0.9 weeks and a mean (SD) birth weight of 845 ± 81 g in the treatment group and a mean GA (SD) of 26 ± 1.5 weeks and a mean (SD) birth weight of 851 ± 150 g in the control group. Infants were stratified into groups weighing below 1000 g and weighing 1000 to 2000 g. Watterberg 1993 stated that allocation to treatment groups was by sequential numbers, but it was unclear exactly what this involved and, therefore, whether blinding occurred was also unclear. The infants were comparable in severity of illness on entry into the study (defined as fractional inspired oxygen concentration (FiO2) and respiratory acuity score). Infants were enrolled into the study less than 12 hours after intubation for RDS. Cromolyn sodium 20 mg or normal saline 2 mL were given nebulised every six hours for the duration of intubation. The length of time that the infants were intubated and, therefore, received treatment, was lacking. The study was discontinued when 10 babies of birth weight under 1000 g and 28 babies of birth weight from 1000 to 2000 g had been enrolled. Although we had not specified subgroup analyses by birth weight, data from this study could be obtained only from babies over 1000 g for some outcomes.
In the trial by Viscardi 1997, 26 infants requiring ventilation on day one for RDS with a greater than 75% predicted probability of oxygen dependence at 28 days were randomised to receive either nebulised cromolyn sodium 20 mg or normal saline 2 mL every six hours. The 12‐hour CLD predictive score was similar in the two groups. There was no difference between groups in FiO2, alveolar‐arterial oxygen gradient or ventilatory settings on the day of study entry. The infants received the drug from day three to day 28 of life. One infant was withdrawn at day seven at parental request but was included in the analyses of outcomes. Seventy‐seven per cent of infants in the cromolyn sodium group and 100% of infants in the placebo group received exogenous surfactant. The primary outcome was the concentration of cytokines in lung lavage in these infants.
Neither trial collected data regarding the amount and site of drug deposition in the lungs. Neither trials documented the numbers of infants receiving postnatal dexamethasone.
Excluded studies
We excluded no eligible studies from analysis. The 2016 search identified two abstracts. We excluded Fisher 1992 as it was an abstract of an RCT of nine infants that reported on biochemical mediators (elastase) in lung lavage fluids. The study reported no clinical outcomes except for median days of mechanical ventilation and supplementary oxygen, but gave no ranges or SDs. The second abstract (Watterberg 1991) was listed as a secondary study to Watterberg 1993.
Risk of bias in included studies
A 'Risk of bias' graph is shown in Figure 2 and a 'Risk of bias' summary in Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Both studies were of small size (26 and 38 infants enrolled). Watterberg 1993 included a sample size calculation but Viscardi 1997 did not.
Allocation
Viscardi 1997 stated that the randomisation process and the allocation to groups were blinded. In the study by Watterberg 1993, blinding of randomisation was unclear. Watterberg 1993 enrolled infants by sequential numbers but it was unclear what this actually meant. Viscardi 1997 accomplished randomisation by a computer‐generated table of random numbers.
Blinding
Both studies blinded the intervention although in the study by Viscardi 1997 the pharmacist was aware of treatment assignment. In the study by Viscardi 1997, the clinical outcome was CLD at 28 days of life. A single radiologist who was blinded to the treatment regimens and the outcomes reviewed chest x‐rays.
Incomplete outcome data
Both Viscardi 1997 and Watterberg 1993 analysed the data on an intention‐to‐treat basis and there was complete follow‐up of all infants.
Selective reporting
The protocols for the trials were not available to us, so we could not judge if there were any deviations from the protocols.
Other potential sources of bias
We did not identify any other concerns regarding potential bias in the two studies.
Effects of interventions
See: Table 1
Cromolyn sodium versus placebo
Mortality or chronic lung disease at 28/30 days (Outcome 1.1)
Both Viscardi 1997 and Watterberg 1993 assessed mortality or CLD at 28/30 days. Neither found a significant effect. The meta‐analysis of the results of both trials found no evidence of effect (typical RR 1.05, 95% CI 0.73 to 1.52; I2 = 0%; typical RD 0.03, 95% CI ‐0.20 to 0.27; I2 = 0%) (Analysis 1.1). These results were based on two trials, 64 infants and 41 events.
1.1. Analysis.

Comparison 1 Cromolyn sodium versus placebo, Outcome 1 Mortality or chronic lung disease (CLD) at 28/30 days.
Mortality to 28 days (Outcome 1.2)
Both Viscardi 1997 and Watterberg 1993 assessed mortality to 28 days. Neither found a significant effect. The meta‐analysis of the results of both trials found no evidence of effect (typical RR 1.31, 95% CI 0.52 to 3.29; I2 = 73%; typical RD 0.06, 95% CI ‐0.13 to 0.26; I2 = 87%) (Analysis 1.2). These results were based on two trials, 64 infants and 14 events.
1.2. Analysis.

Comparison 1 Cromolyn sodium versus placebo, Outcome 2 Mortality to 28 days.
Chronic lung disease at 28 days (Outcome 1.3)
Both Viscardi 1997 and Watterberg 1993 assessed CLD at 28 days. Neither found a significant effect. The meta‐analysis of the results of both trials found no evidence of effect (typical RR 0.93, 95% CI 0.53 to 1.64; I2 = 40%; typical RD ‐0.03, 95% CI ‐0.27 to 0.20; I2 = 38%) (Analysis 1.3). These results were based on two trials, 64 infants and 27 events.
1.3. Analysis.
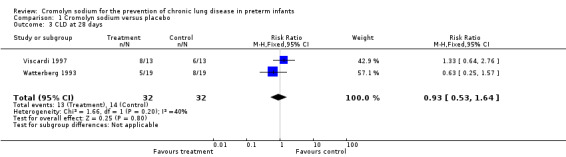
Comparison 1 Cromolyn sodium versus placebo, Outcome 3 CLD at 28 days.
Chronic lung disease at 36 weeks' postmenstrual age (Outcome 1.4)
The study by Viscardi 1997 found no evidence of effect (RR 1.25, 95% CI 0.43 to 3.63; RD 0.08, 95% CI ‐0.29 to 0.44) (Analysis 1.4). These results are based on one trial, 26 infants and nine events. The study by Watterberg 1993 did not report CLD at 36 weeks' PMA.
1.4. Analysis.
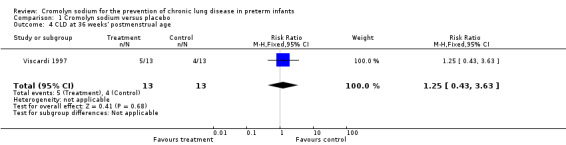
Comparison 1 Cromolyn sodium versus placebo, Outcome 4 CLD at 36 weeks' postmenstrual age.
Chronic lung disease among survivors at 28 days (Outcome 1.5)
Both Viscardi 1997 and Watterberg 1993 assessed CLD among survivors at 28 days. Neither found a significant effect. The meta‐analysis of the results of both trials found no evidence of effect (typical RR 0.97, 95% CI 0.58 to 1.63; I2 = 0%; typical RD ‐0.02, 95% CI ‐0.29 to 0.26; I2 = 0%) (Analysis 1.5). These results were based on two trials, 50 surviving infants and 27 events.
1.5. Analysis.
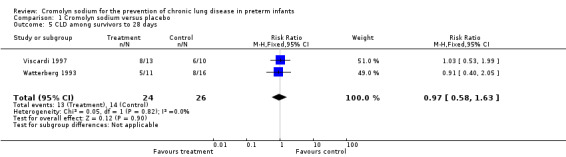
Comparison 1 Cromolyn sodium versus placebo, Outcome 5 CLD among survivors to 28 days.
Chronic lung disease among survivors at 36 weeks' postmenstrual age (Outcome 1.6)
The study by Viscardi 1997 found no evidence of effect (RR 1.04, 95% CI 0.38 to 2.87; RD 0.02, 95% CI ‐0.40 to 0.43) (Analysis 1.6). These results were based on one trial, 22 surviving infants and nine events. The study by Watterberg 1993 did not look at CLD among survivors at 36 weeks' PMA.
1.6. Analysis.

Comparison 1 Cromolyn sodium versus placebo, Outcome 6 CLD among survivors to 36 weeks' postmenstrual age.
Intraventricular haemorrhage (Outcome 1.7)
Both Viscardi 1997 and Watterberg 1993 assessed IVH. Neither found a significant effect. The meta‐analysis of the results of both trials found no evidence of effect (typical RR 0.89, 95% CI 0.63 to 1.27; I2 = 15%; typical RD ‐0.06, 95% CI ‐0.26 to 0.13; I2 = 0%) (Analysis 1.7). These results were based on two trials, 64 infants and 36 events.
1.7. Analysis.
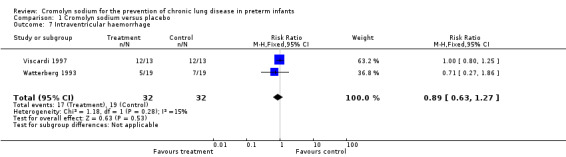
Comparison 1 Cromolyn sodium versus placebo, Outcome 7 Intraventricular haemorrhage.
Necrotising enterocolitis (Outcome 1.8)
Both Viscardi 1997 and Watterberg 1993 assessed NEC. Neither found a significant effect. The meta‐analysis of the results of both trials found no evidence of effect (typical RR 1.25, 95% CI 0.37 to 4.24; I2 = 17%; typical RD 0.03, 95% CI ‐0.14 to 0.20; I2 = 6%) (Analysis 1.8). These results were based on two trials, 64 infants and nine events.
1.8. Analysis.

Comparison 1 Cromolyn sodium versus placebo, Outcome 8 Necrotising enterocolitis.
Patent ductus arteriosus (Outcome 1.9)
Both Viscardi 1997 and Watterberg 1993 assessed PDA. Neither found a significant effect. The meta‐analysis of the results of both trials found no evidence of effect (typical RR 0.88, 95% CI 0.65 to 1.18; I2 = 0%; typical RD ‐0.09, 95% CI ‐0.31 to 0.12; I2 = 0%). These results were based on two trials, 64 infants and 47 events.
Air leak (pulmonary interstitial emphysema, pneumothorax) (Outcome 1.10)
Both Viscardi 1997 and Watterberg 1993 assessed air leak (PIE, pneumothorax). Neither found a significant effect. The meta‐analysis of the results of both trials found no evidence of effect (typical RR 0.67, 95% CI 0.32 to 1.39; I2 = 0%; typical RD ‐0.13, 95% CI ‐0.34 to 0.09; I2 = 0%) (Analysis 1.10). These results are based on two trials, 64 infants and 20 events.
1.10. Analysis.
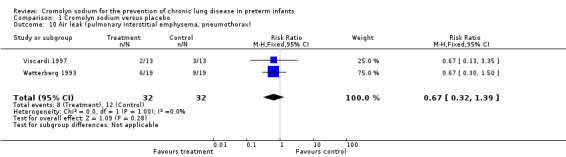
Comparison 1 Cromolyn sodium versus placebo, Outcome 10 Air leak (pulmonary interstitial emphysema, pneumothorax).
Days on mechanical ventilation (Outcome 1.11)
This is only commented on in the study by Watterberg 1993, who did not report days on mechanical ventilation for babies under 1000 g; therefore, this outcome was analysed only for babies over 1000 g. There was no evidence of effect (MD 1.00 day, 95% CI ‐6.41 to 8.41) (Analysis 1.11). This result was based on one trial and 28 infants.
1.11. Analysis.

Comparison 1 Cromolyn sodium versus placebo, Outcome 11 Days on mechanical ventilation.
Sepsis (Outcome 1.12)
Both Viscardi 1997 and Watterberg 1993 assessed sepsis. Neither found a significant effect. The meta‐analysis of the results of both trials found no evidence of effect (typical RR 0.82, 95% CI 0.41 to 1.63; I2 = 0%; typical RD ‐0.06, 95% CI ‐0.28 to 0.15; I2 = 0%) (Analysis 1.12). These results were based on two trials, 64 infants and 20 events.
1.12. Analysis.
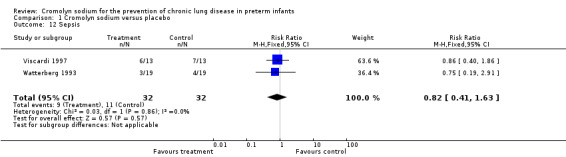
Comparison 1 Cromolyn sodium versus placebo, Outcome 12 Sepsis.
Other effects: cytokines in lung lavage
Viscardi 1997 showed that although the prestudy (day three) cytokine concentrations of cromolyn treated and control groups were similar, IL‐8 concentration in lavage from cromolyn sodium‐treated infants decreased 2.45‐fold from day three to day seven, whereas IL‐8 concentrations increased 1.7‐fold in lavage of control infants during the same interval. IL‐8 concentrations were 4.9‐fold lower on day seven in lavage from cromolyn sodium‐treated infants (median 2245 pg/mL) compared to levels in control infants (median 11,009 pg/mL) (P = 0.051). TNF‐alpha was 3.6‐fold lower on day seven in lavage from the cromolyn sodium‐treated infants (median 19.95 pg/mL) compared with lung lavage from control infants (median 70.9 pg/mL) (P = 0.04). There was a trend towards lower lavage levels of IL‐1B on day seven in cromolyn sodium‐treated infants (median 46.75 pg/mL) compared to lavage levels in control infants (median 208.2 pg/mL) (P = 0.13) but IL‐1ra concentrations were unaffected by cromolyn sodium treatment (day seven cromolyn sodium‐treated infants median 21.5 ng/mL, day seven control‐treated infants median 23 ng/mL). The IL‐1B:IL‐1ra ratio was lower in day seven lavage from cromolyn sodium‐treated infants compared to the ratio in control infant lavage (cromolyn sodium median 0.002, control median 0.005) (P = 0.09). It is difficult to interpret these results since there were no clinical benefits noted.
Adverse effects
Cromolyn sodium seemed to be a well‐tolerated drug without adverse effects and there were no reported adverse responses to nebulisation.
Discussion
Summary of main results
Both included studies involved very small numbers of infants (26 (Viscardi 1997) and 38 (Watterberg 1993)). The sample sizes were so small that no reliable estimate of treatment effect was provided. The wide CIs showed that clinically important effect sizes on major outcomes have not been excluded. Neither of these trials assessed drug deposition and it is possible that the drug was not received in effective doses at the correct sites.
Prophylaxis with cromolyn sodium did not result in a statistically significant effect on the combined outcome of mortality or CLD at 28 days, mortality at 28 days, CLD at 28 days or at 36 weeks' PMA. There was no significant difference in CLD in survivors at 28 days or at 36 weeks' PMA. Prophylaxis with cromolyn sodium did not show a statistically significant difference in overall neonatal mortality, incidence of air leaks, NEC, IVH, sepsis and days of mechanical ventilation. There were no adverse effects noted.
Overall completeness and applicability of evidence
To date only 64 infants have been enrolled in two studies that were published in 1993 and 1997.
Although the study by Viscardi 1997 showed a reduction in cytokines in lung lavage from infants treated with cromolyn sodium, this did not translate into clinical benefits. Neither of the two eligible studies found a reduction in CLD or mortality. There was moderate heterogeneity for the outcome of mortality to 28 days for RR (I2 = 73%) but high for RD (I2 = 87%). For all the other outcomes, there was no or low heterogeneity. Cromolyn sodium had no statistically significant effect on the outcomes of IVH, NEC, PDA, air leak, days on mechanical ventilation or sepsis. There were no adverse effects noted.
Cromolyn sodium was given for different duration in the two studies. In the Watterberg 1993 study, cromolyn sodium was given for the duration of intubation. In the Viscardi 1997 study, it was given for 25 days. The age of entry to the studies differed. In the Watterberg 1993 study, the age of entry was 12 hours after intubation and in the Viscardi 1997 study it was on day three of life.
The available evidence does not justify further trials using the protocols for cromolyn sodium administration used to date.
Quality of the evidence
The quality of evidence according to GRADE was very low for one outcome (mortality to 28 days) and low for all the other reported outcomes (Table 1). The reasons for downgrading the evidence was due to design (risk of bias in one study), inconsistency between the findings of the two studies for one outcome (high I2 values for mortality at 28 days for both RR and RD) and lack of precision of estimates due to small sample sizes.
Potential biases in the review process
We are not aware of any biases as reviewers for this review update.
Agreements and disagreements with other studies or reviews
We are unaware of any other studies or reviews of this topic in newborn infants.
Authors' conclusions
Implications for practice.
Cromolyn sodium cannot be recommended for the prevention of chronic lung disease in preterm infants.
Implications for research.
Although results are available only from a small number of infants randomised, the available evidence does not appear to justify further trials using the protocols for drug administration reviewed here unless a more efficient type of delivery device than the jet nebuliser is employed.
What's new
| Date | Event | Description |
|---|---|---|
| 27 January 2020 | Amended | Arne Ohlsson deceased. |
History
Protocol first published: Issue 2, 2001 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 12 May 2016 | New search has been performed | No new trials identified |
| 12 May 2016 | New citation required but conclusions have not changed | No new trials identified. Summary of finding table added. |
| 19 April 2012 | New citation required but conclusions have not changed | No new trials were identified, but two additional studies were quoted in the background information. |
| 13 April 2012 | New search has been performed | This updates the review "Cromolyn sodium for the prevention of chronic lung disease in preterm infants|" (Ng 2001). |
| 4 August 2009 | New search has been performed | This review updates the existing review "Cromolyn sodium for the prevention of chronic lung disease in preterm infants" published in the Cochrane Database of Systematic Reviews (Ng 2001). Updated search found no new trials. No changes to conclusions. |
| 16 September 2008 | Amended | Converted to new review format. |
| 30 June 2006 | New search has been performed | This review updates the existing review "Cromolyn sodium for the prevention of chronic lung disease in preterm infants)" initially published in The Cochrane Library, Issue 2, 2001. In an updated search to April 2006 no new eligible studies were identified. The conclusion remains unchanged: there is no evidence from randomised trials that cromolyn sodium is effective in preventing chronic lung disease in preterm infants. |
| 7 November 2000 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to acknowledge the help of Elizabeth Uleryk, Director of the Hospital Library at the Hospital for Sick Children, Toronto, Ontario for help with search strategies in the original version of the review. We acknowledge the help of Ms Yolanda Brosseau (né Montagne) for doing the literature searches for this update of the review.
Appendices
Appendix 1. Standard search methodology
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomised controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomised [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomised controlled trial or controlled clinical trial or randomised or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomised controlled trial OR controlled clinical trial OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. 'Risk of bias' tool
The following issues were evaluated and entered into the risk of bias table.
Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk.
Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk.
Blinding (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorised the methods as:
low risk, high risk or unclear risk for participants;
low risk, high risk or unclear risk for personnel;
low risk, high risk or unclear risk for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data);
unclear risk.
Selective reporting bias. Were reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk (where it was clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review were reported);
high risk (where not all the study's prespecified outcomes were reported; one or more reported primary outcomes were not prespecified outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk.
Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk;
unclear risk.
If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Cromolyn sodium versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality or chronic lung disease (CLD) at 28/30 days | 2 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.73, 1.52] |
| 2 Mortality to 28 days | 2 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.52, 3.29] |
| 3 CLD at 28 days | 2 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.53, 1.64] |
| 4 CLD at 36 weeks' postmenstrual age | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.43, 3.63] |
| 5 CLD among survivors to 28 days | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.58, 1.63] |
| 6 CLD among survivors to 36 weeks' postmenstrual age | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.38, 2.87] |
| 7 Intraventricular haemorrhage | 2 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.63, 1.27] |
| 8 Necrotising enterocolitis | 2 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.37, 4.24] |
| 9 Patent ductus arteriosus | 2 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.65, 1.18] |
| 10 Air leak (pulmonary interstitial emphysema, pneumothorax) | 2 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.32, 1.39] |
| 11 Days on mechanical ventilation | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐6.41, 8.41] |
| 12 Sepsis | 2 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.41, 1.63] |
1.9. Analysis.

Comparison 1 Cromolyn sodium versus placebo, Outcome 9 Patent ductus arteriosus.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Viscardi 1997.
| Methods | Randomised, prospective, double‐blind, placebo‐controlled trial Blinding of randomisation: yes Blinding of intervention: yes Complete follow up: yes Blinding of outcome: yes, CXRs were reviewed by a single observer blinded to the treatment regimens |
|
| Participants | Number of infants entered into the study: 26 Mean BW (SD): 687 ± 46 g in the treatment group and 702 ± 35 g in the control group Mean GA (SD): 24.6 ± 0.4 weeks in the treatment group and 25.1 ± 0.4 weeks in the control group Age of enrolment into study: day 3 Other characteristics: high probability of oxygen dependence at 28 days predicted by a CLD score at 12 hours of age Exclusion criteria: documented sepsis, congenital cardiopulmonary anomalies Study location: University of Maryland Hospital Study period: not stated |
|
| Interventions | Nebulised cromolyn sodium 20 mg (n = 13) or 2 mL normal saline placebo (n = 13) every 6 hours from day 3 until day 28 | |
| Outcomes | Primary outcomes: CLD at 28 days of life and at 36 weeks' postmenstrual age. Changes in inflammatory cell populations and the cytokines in lung lavage in response to cromolyn therapy Secondary outcomes: number of ventilator days, duration of oxygen supplementation, PDA, air leak, sepsis, NEC and IVH |
|
| Notes | 100% of the control group and 77% of cromolyn sodium‐treated infants received exogenous surfactant. No adverse effects that could be attributed to the administration of cromolyn sodium were noted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was accomplished by a computer‐generated table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: yes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes (however, pharmacist aware of treatment assignment). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | CXRs were reviewed by a single observer blinded to the treatment regimens. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes. |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us so we could not judge if there were any deviations. |
| Other bias | Low risk | Appeared free of other bias. |
Watterberg 1993.
| Methods | Randomised, prospective, double‐blind, placebo‐controlled trial Blinding of randomisation: cannot be determined. Infants were stratified into 2 groups, BW < 1000 g and BW 1000 g to 2000 g, and enrolled by 'sequential numbers' Blinding of intervention: yes Complete follow up: yes Blinding of outcome: could not be determined Study location: Newborn Intensive Care Unit of the University of New Mexico Hospital (Albuquerque) Study period: November 1987 to December 1989 |
|
| Participants | Number of infants entered into the study: 38 Mean BW (SD): < 1000 g arm: 845 ± 81 g in the treatment group and 851 ± 150 g in the placebo group; 1000 g to 2000 g arm: 1353 ± 184 grams in the treatment group and 1331 ± 227 g in the control group Mean GA (SD): < 1000 g arm: 25.9 ± 0.9 weeks in the treatment group and 26.2 ± 1.5 weeks in the control group; > 1000 g to 2000 g arm: 29.9 ± 1.8 weeks in the treatment group and 30.8 ± 2 weeks in the control group Age of enrolment into study: < 12 hours after intubation |
|
| Interventions | Aerosolised cromolyn sodium 20 mg (n = 19) or 2 mL normal saline placebo (n = 19) every 6 hours while intubated | |
| Outcomes | Primary outcome: survival without oxygen dependence (oxygen saturations > 90% in FiO2 < 0.25 at 30 days of life) Secondary outcomes: number of days dependent on mechanical ventilation during the first 30 days of life, the intensity of mechanical ventilation and pulmonary complications |
|
| Notes | No adverse effects that could be attributed to the administration of cromolyn sodium were noted. Days on mechanical ventilation were not reported for babies weighing < 1000 g. Therefore, this outcome was analysed in the review only for babies weighing > 1000 g | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Infants were stratified into 2 groups, BW < 1000 g and BW 1000 to 2000 g, and enrolled by 'sequential numbers'. |
| Allocation concealment (selection bias) | Unclear risk | Blinding of randomisation could not be determined. Communication with Dr Watterberg did not lead to further clarification. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of the intervention: yes. Infants received either cromolyn sodium 20 mg or 2 mL of normal saline placebo by aerosol every 6 hours while intubated. Individual drug ampoules were prepared and coded by Fisons Corp, and the code was not available to the investigators. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Could not be determined. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes. |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us so we could not judge if there were any deviations. |
| Other bias | Low risk | Appeared free of other bias. |
BW: birth weight; CLD: chronic lung disease; CXR: chest x‐ray; FiO2: fractional inspired oxygen concentration; GA: gestational age; IVH: intraventricular haemorrhage; n: number of infants; NEC: necrotising enterocolitis; PDA: patent ductus arteriosus; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Fisher 1992 | An abstract of a randomised controlled trial of 9 infants that reported on biochemical mediators (elastase) in lung lavage fluids. No clinical outcomes reported except for median days of mechanical ventilation and supplementary oxygen, but no ranges or standard deviations reported. |
Differences between protocol and review
For this update in 2016, we added the methodology and plan for 'Summary of findings' tables and GRADE recommendations, which were not included in the original protocol or the originally published review. We expanded on the categories in the 'Risk of bias' tables.
Contributions of authors
GN: literature search and identification of trials for inclusion; evaluation of methodological quality of included trials; abstraction of data; verifying and entering data into Review Manager 5; and writing text of review.
AO: literature search and identification of trials for inclusion; evaluation of methodological quality of included trials; abstraction of data; verifying and entering data into Review Manager 5; and revision of the final review.
GN and AO wrote the original review and updated the review in 2000 and 2006.
The July 2009 update was conducted centrally by the Cochrane Neonatal Review Group staff (Yolanda Montagne, Roger Soll, Diane Haughton) and reviewed and approved by GN and AO.
The April 2012 update was conducted by GN and AO. The literature searches were conducted by Yolanda Montagne.
The May 2016 update was conducted by GN and AO. The literature searches in 2016 were conducted by Yolanda Brosseau (né Montagne).
Sources of support
Internal sources
Chelsea and Westminster Hospital, UK.
Mount Sinai Hospital, Toronto, Ontario, Canada.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
Declarations of interest
GN has no declarations of interest.
AO has no declarations of interest.
Deceased
Edited (no change to conclusions)
References
References to studies included in this review
Viscardi 1997 {published data only}
- Viscardi RM, Hasday JD, Gumpper KF, Taciak V, Campbell AB, Palmer TW. Cromolyn sodium prophylaxis inhibits pulmonary proinflammatory cytokines in infants at high risk for bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine 1997;156(5):1523‐9. [DOI] [PubMed] [Google Scholar]
Watterberg 1993 {published data only}
- Watterberg KL, Murphy S. Can cromolyn sodium (Cr) alter the development of bronchopulmonary dysplasia?. Pediatric Research 1991;29(4 Part 2):238A. [Google Scholar]
- Watterberg KL, Murphy S. Failure of cromolyn sodium to reduce the incidence of bronchopulmonary dysplasia: a pilot study. Pediatrics 1993;91(4):803‐6. [PubMed] [Google Scholar]
References to studies excluded from this review
Fisher 1992 {published data only}
- Fisher J, Brodsky N, Wilson B, Porat R. Effect of cromolyn sodium on respiratory status of ventilated preterm infants. Pediatric Research 1992;31:307A. [Google Scholar]
Additional references
Ahmad 1983
- Ahmad S. Cromolyn sodium and anaphylaxis. Annals of Internal Medicine 1983;99(6):882‐3. [DOI] [PubMed] [Google Scholar]
Avery 1987
- Avery ME, Tooley WH, Keller JB, Hurd S, Bryan MH, Cotton RB, et al. Is chronic lung disease in low birth weight infants preventable? A survey of eight centers. Pediatrics 1987;79(1):26‐30. [PubMed] [Google Scholar]
Bagchi 1994
- Bagchi A, Viscardi RM, Taciak V, Ensor JE, McCrea KA, Hasday JD. Increased activity of interleukin‐6 but not tumor necrosis factor alpha in lung lavage of premature infants is associated with the development of bronchopulmonary dysplasia. Pediatric Research 1994;36(2):244‐51. [DOI] [PubMed] [Google Scholar]
Brown 1981
- Brown LA, Kaplan RA, Benjamin PA, Hoffman LS, Shearer WT. Immunoglobulin E‐mediated anaphylaxis with inhaled cromolyn sodium. Journal of Allergy and Clinical Immunology 1981;68(6):416‐20. [DOI] [PubMed] [Google Scholar]
Bruijnzeel 1989
- Bruijnzeel PL, Warringa RA, Kok PT. Inhibition of platelet‐activating factor and zymosan‐activated serum‐induced chemotaxis of human neutrophils by nedocromil sodium, BN 52021 and sodium cromoglycate. British Journal of Pharmacology 1989;97(4):1251‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burgher 1974
- Burgher LW, Kass I, Schenken JR. Pulmonary allergic granulomatosis: a possible drug reaction in a patient receiving cromolyn sodium. Chest 1974;66(1):84‐6. [DOI] [PubMed] [Google Scholar]
Crowley 2000
- Crowley P. Prophylactic corticosteroids for preterm birth. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD000065.pub2] [DOI] [PubMed] [Google Scholar]
Diot 1995
- Diot P, Morra L, Smaldone GC. Albuterol delivery in a model of mechanical ventilation. Comparison of metered‐dose inhaler and nebulizer efficiency. American Journal of Respiratory and Critical Care Medicine 1995;152(4 Pt 1):1391‐4. [DOI] [PubMed] [Google Scholar]
Fok 1996
- Fok TF, Monkman S, Dolovich M, Gray S, Coates G, Paes B, et al. Efficiency of aerosol medication delivery from a metered dose inhaler versus jet nebulizer in infants with bronchopulmonary dysplasia. Pediatric Pulmonology 1996;21(5):301‐9. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University. GRADEpro GDT. Hamilton (ON): McMaster University, 2014.
Grigg 1992
- Grigg J, Arnon S, Jones T, Clarke A, Silverman M. Delivery of therapeutic aerosols to intubated babies. Archives of Disease in Childhood 1992;67(1 Spec No):25‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hack 1991
- Hack M, Horbar JD, Malloy MH, Tyson JE, Wright E, Wright L. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Network. Pediatrics 1991;87(5):587‐96. [PubMed] [Google Scholar]
Harvey 1995
- Harvey CJ, O'Doherty MJ, Page CJ, Thomas SHL, Nunan TO, Treacher DF. Effect of a spacer on pulmonary aerosol deposition from a jet nebuliser during mechanical ventilation. Thorax 1995;50(1):50‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoag 1991
- Hoag JE, McFadden ER. Long term effect of cromolyn sodium on non‐specific bronchial hyperresponsiveness: a review. Annals of Allergy 1991;66:53‐63. [PubMed] [Google Scholar]
Kay 1987
- Kay AB, Walsh GM, Moqbel R, MacDonald AJ, Nagakura T, Carroll MP, et al. Disodium cromoglycate inhibits activation of human inflammatory cells in vitro. Journal of Allergy and Clinical Immunology 1987;80(1):1‐8. [DOI] [PubMed] [Google Scholar]
Larsson 2001
- Larsson K, Larsson BM, Sandström T, Sundblad BM, Palmberg L. Sodium cromoglycate attenuates pulmonary inflammation without influencing bronchial responsiveness in healthy subjects exposed to organic dust. Clinical and Experimental Allergy 2001;31(9):1356‐68. [DOI] [PubMed] [Google Scholar]
Lee 2000
- Lee SK, McMillan DD, Ohlsson A, Pendray M, Synnes A, Whyte R, et al. Variations in practice and outcomes in the Canadian NICU Network 1996‐1997. Pediatrics 2000;106(5):1070‐9. [DOI] [PubMed] [Google Scholar]
Lobel 1972
- Lobel H, Machtey I, Eldror MY. Pulmonary infiltrates with eosinophilia in an asthmatic patient treated with disodium cromoglycate. Lancet 1972;2(7785):1032. [DOI] [PubMed] [Google Scholar]
Menon 1977
- Menon MP, Das AK. Asthma and urticaria during disodium cromoglycate treatment. Scandinavian Journal of Respiratory Diseases 1977;58(3):145‐50. [PubMed] [Google Scholar]
Niu 1998
- Niu JO, Munshi UK, Siddiq MM, Parton LA. Early increase in endothelin‐1 in tracheal aspirates of preterm infants: correlation with bronchopulmonary dysplasia. Journal of Pediatrics 1998;132(6):965‐9. [DOI] [PubMed] [Google Scholar]
Northway 1967
- Northway WH, Rosan RC, Porter DW. Pulmonary disease following respirator therapy of hyaline‐membrane disease. New England Journal of Medicine 1967;276(7):357‐68. [DOI] [PubMed] [Google Scholar]
O'Brodovich 1985
- O'Brodovich HM, Mellins RB. Bronchopulmonary dysplasia ‐ unresolved neonatal acute lung injury. American Review of Respiratory Disease 1985;132(3):694‐709. [DOI] [PubMed] [Google Scholar]
Paita 1991
- Paita M, Gabbert D, Weinstein MR, Peters ME. Multivariate assessment of traditional risk factors for chronic lung disease in very low birth weight neonates. Journal of Pediatrics 1991;119(2):285‐92. [DOI] [PubMed] [Google Scholar]
Parker 1992
- Parker RA, Lindstrom DP, Cotton RB. Improved survival accounts for most but not all of the increase in bronchopulmonary dysplasia. Pediatrics 1992;90(5):663‐8. [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rindfleisch 1996
- Rindfleisch MS, Hasday JD, Taciak V, Broderick K, Viscardi RM. Potential role of interleukin‐1 in the development of bronchopulmonary dysplasia. Journal of Interferon and Cytokine Research 1996;16(5):365‐73. [DOI] [PubMed] [Google Scholar]
Rojas 1995
- Rojas MA, Gonzales A, Bancalari E, Claure N, Poole C, Siva‐Nieto G. Changing trends in the epidemiology and pathogenesis of neonatal chronic lung disease. Journal of Pediatrics 1995;126(4):605‐10. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. gdt.guidelinedevelopment.org/app/handbook/handbook.html (accessed 2 January 2017).
Settipane 1979
- Settipane GA, Klein DE, Boyd GK, Sturam JH, Freye HB, Weltman JK. Adverse effects to cromolyn. JAMA 1979;241(8):811‐3. [PubMed] [Google Scholar]
Sheffer 1975
- Sheffer AL, Rocklin RE, Goetzi EJ. Immunologic components of hypersensitivity reactions to cromolyn sodium. New England Journal of Medicine 1975;295(24):1220‐4. [DOI] [PubMed] [Google Scholar]
Shennan 1988
- Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988;82(4):527‐32. [PubMed] [Google Scholar]
Soll 1997
- Soll R, Özek E. Prophylactic animal derived surfactant extract for preventing morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews 1997, Issue 4. [DOI: 10.1002/14651858.CD000511] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tullus 1996
- Tullus K, Noack GW, Burman LG, Nilsson R, Wretlind B, Brauner A. Elevated cytokine levels in tracheobronchial aspirate fluids from ventilator treated neonates with bronchopulmonary dysplasia. European Journal of Pediatrics 1996;155(2):112‐6. [DOI] [PubMed] [Google Scholar]
Viscardi 1994
- Viscardi RM, Adeniyi‐Jones SC. Retrospective study of the effectiveness of cromolyn sodium in bronchopulmonary dysplasia. Neonatal Intensive Care 1994;7:18‐20. [Google Scholar]
Yazid 2009
- Yazid S, Solito E, Christian H, McArthur S, Goulding N, Flower R. Cromoglycate drugs suppress eicosanoid generation in U937 cells by promoting the release of Anx‐A1. Biochemical Pharmacology 2009;77(12):1814‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Ng 2001
- Ng GY, Ohlsson A. Cromolyn sodium for the prevention of chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003059] [DOI] [PubMed] [Google Scholar]
Ng 2012
- Ng G, Ohlsson A. Cromolyn sodium for the prevention of chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD003059.pub2] [DOI] [PubMed] [Google Scholar]


