Abstract
Background
Restless legs syndrome (RLS) is defined as the spontaneous movement of the limbs (mainly legs) associated with unpleasant, sometimes painful sensation which is relieved by moving the affected limb. Prevalence of RLS among people on dialysis has been estimated between 6.6% and 80%. RLS symptoms contribute to impaired quality of life and people with RLS are shown to have increased cardiovascular morbidity and mortality.
Various pharmacological and non‐pharmacological interventions have been used to treat primary RLS. However, the evidence for use of these interventions in people with chronic kidney disease (CKD) is not well established. The agents used in the treatment of primary RLS may be limited by the side effects in people with CKD due to increased comorbidity and altered drug pharmacokinetics.
Objectives
The aim of this review was to critically look at the benefits, efficacy and safety of various treatment options used in the treatment of RLS in people with CKD and those undergoing renal replacement therapy (RRT). We aimed to define different group characteristics based on CKD stage to assess the applicability of a particular intervention to an individual patient.
Search methods
We searched the Cochrane Kidney and Transplant Specialised Register to 12 January 2016 through contact with the Information Specialist using search terms relevant to this review.
Selection criteria
Randomised controlled trials (RCT) and quasi‐RCTs that assessed the efficacy of an intervention for RLS in adults with CKD were eligible for inclusion. Studies investigating idiopathic RLS or RLS secondary to other causes were excluded.
Data collection and analysis
Two authors independently assessed studies for eligibility and conducted risk of bias evaluation. Results were expressed as risk ratios (RR) and their 95% confidence intervals (CI) for dichotomous outcomes, and mean difference (MD) and 95% CI for continuous outcomes.
Main results
We included nine studies enrolling 220 dialysis participants. Seven studies were deemed to have moderate to high risk of bias. All studies were small in size and had a short follow‐up period (two to six months). Studies evaluated the effects of six different interventions against placebo or standard treatment. The interventions studied included aerobic resistance exercise, gabapentin, ropinirole, levodopa, iron dextran, and vitamins C and E (individually and in combination).
Aerobic resistance exercise showed a significant reduction in severity of RLS compared to no exercise (2 studies, 48 participants: MD ‐7.56, 95% CI ‐14.20 to ‐0.93; I2 = 65%), and when compared to exercise with no resistance (1 study, 24 participants: MD ‐11.10, 95% CI ‐17.11 to ‐5.09), however there was no significant reduction when compared to ropinirole (1 study, 22 participants): MD ‐0.55, 95% CI ‐6.41 to 5.31). There were no significant differences between aerobic resistance exercise and either no exercise or ropinirole in the physical or mental component summary scores (using the SF‐36 form). Improvement in sleep quality varied. There was no significant difference in subjective sleep quality between exercise and no exercise; however one study reported a significant improvement with ropinirole compared to resistance exercise (MD 3.71, 95% CI 0.89 to 6.53). Using the Epworth Sleepiness Scale there were no significant differences between resistance exercise and no exercise, ropinirole, or exercise with no resistance. Two studies reported there were no adverse events and one study did not mention if there were any adverse events. In one study, one patient in each group dropped out but the reason for dropout was not reported. Two studies reported no adverse events and one study did not report adverse events.
Gabapentin was associated with reduced RLS severity when compared to placebo or levodopa, and there was a significant improvement in sleep quality, latency and disturbance reported in one study when compared to levodopa. Three patients dropped out due to lethargy (2 patients), and drowsiness, syncope and fatigue (1 patient).
Because of a short duration of action, rebound and augmentation were noted with levodopa treatment even though it conferred some benefit in reducing the symptoms of RLS. Reported adverse events were severe vomiting, agitation after caffeine intake, headaches, dry mouth, and gastrointestinal symptoms.
One study (25 participants) reported iron dextran reduced the severity of RLS at weeks one and two, but not at week four. Vitamins C, E and C plus E (1 study, 60 participants) helped the symptoms of RLS with minimal side effects (nausea and dyspepsia) but more evidence is needed before any conclusions can be drawn.
Authors' conclusions
Given the small size of the studies and short follow‐up, it can only be concluded that pharmacological interventions and intra‐dialytic exercise programs have uncertain effects on RLS in haemodialysis patients. There have been no studies performed in non‐dialysis CKD, peritoneal dialysis patients, or kidney transplant recipients. Further studies are warranted before any conclusions can be drawn. Aerobic resistance exercise and ropinirole may be suitable interventions for further evaluation.
Plain language summary
Interventions for treating restless legs syndrome in patients with chronic kidney disease
What is the issue?
People with restless legs syndrome (RLS) have an irresistible urge to move their limbs to relieve themselves of unpleasant sensations. RLS is common in chronic kidney disease (CKD) patients, however the cause is unknown. Patients with RLS often have reduced quality of life and increased risk of developing heart disease.
Medications such as dopamine agonists, benzodiazepines, anti‐epileptics, iron and non‐pharmacological agents such as exercise that were used to treat primary RLS were also used to treat RLS in CKD patients. However, these agents may be unsuitable to CKD patients due to associated co‐morbidity and altered pharmacokinetics in CKD patients.
What did we do?
We searched Cochrane Kidney and Transplant's Specialised Register to 12 January 2016. Nine studies reported enrolling 220 stable, adult haemodialysis patients of both sexes, were included in the review.
What did we find?
The quality of the studies was deemed to be moderate. Of the nine studies, one was sponsored by a pharmaceutical company and funding sources were reported for only two other studies. The included studies were heterogeneous, small in size and had short follow‐up periods.
The interventions studied included exercise, gabapentin, ropinirole, levodopa, iron dextran, and vitamins C and E (individually and in combination). All interventions reduced the severity of RLS compared to a control. Intradialytic aerobic exercise reduced the severity of RLS however the safety of this intervention is unclear. Resistance exercise did not improve sleep quality but improved the mental health component on a quality of life questionnaire. This improvement in mental health component was not significant when compared to no exercise or ropinirole. Ropinirole reduced the symptoms of RLS and improved quality of sleep without any reported side effects. Gabapentin and levodopa improved the symptoms of RLS; however there were several adverse events reported included lethargy, drowsiness and fatigue for gabapentin, and vomiting, agitation, headaches, dry mouth, and gastrointestinal symptoms for levodopa. Iron dextran infusion reduced the symptoms of RLS but was only significant up to two weeks after treatment. Vitamin C, E and their combination also reduced RLS symptoms with minimal side effects. Small size and short duration of follow‐up were the major drawbacks of these studies.
Conclusions
The small number of studies, small sample sizes and short duration of follow up make it difficult to draw any firm conclusions. The effects of aerobic exercise and other pharmacological agents on RLS are uncertain in haemodialysis patients. There is a need to perform high quality randomised studies to establish the best treatment for RLS in patients with CKD. Aerobic exercise and ropinirole may be suitable interventions for further evaluation.
Background
Description of the condition
The symptoms of restless legs syndrome (RLS) were first described in 1672 by Sir Thomas Willis. It is defined as the spontaneous movement of the limbs (mainly legs) associated with unpleasant, sometimes painful sensation and relieved by moving the affected limb. This definition has not been significantly modified since it was first defined in 1945 (Ekbom 1945).
Diagnostic criteria for RLS was published by the International Restless Legs Syndrome Study Group (IRLSSG) in 1995 (Walters 1995) and were subsequently revised by the National Institute of Health consensus conference in 2002 (Allen 2003) to include the following.
Urge to move the legs usually with unpleasant sensations in the legs; arms and other body parts are occasionally involved
Symptoms begin or worsen during periods of rest or inactivity
Symptoms partially or totally relieved by movement and as long as the activity continues
Symptoms are worse during the evening or night than during the day.
In addition, separate modified criteria were established for diagnosing RLS in special populations, for RLS augmentation, and for assessment of RLS in epidemiologic studies.
Prevalence of RLS in the general population is 5% to 15% whereas the prevalence in dialysis patients is 6.6% to 80% (Al‐Jahdali 2009). RLS contributes to impaired quality of life (QOL) (Mucsi 2005) and patients with RLS have increased cardiovascular morbidity and mortality (La Manna 2011).
Description of the intervention
There is emerging evidence with strong recommendations advocating the use of various agents to treat primary RLS. Dopaminergic agents have been established as first line treatment options for primary RLS. Anti‐epileptic drugs, benzodiazepines, opiates, alpha‐adrenergic blockers, iron supplementation, aerobic exercise, and Botox have been used in the treatment of people with primary RLS (Agarwal 2011; Giannaki 2010; Hornyak 2014).
However, the evidence for use of these agents in people with chronic kidney disease (CKD) is not well established. The usual agents that have been tried in the treatment of primary RLS may be limited by the side effects in people with CKD.
Kidney impairment can alter drug pharmacokinetics and pharmacodynamics, and consequently people with kidney impairment are at risk of developing adverse effects. People with CKD need to take many drugs and are at high risk for drug interactions and drug‐related problems (Manley 2003).
How the intervention might work
Advanced brain imaging techniques have implicated dysfunction of subcortical areas as causative in primary RLS.
Iron deficiency is considered to be common in primary RLS and there is an inverse relation between iron levels and the severity of RLS symptoms (Sun 1998). Abnormality of the body's ability to use and store iron may lead to the dopamine dysfunction. Iron probably works by both improving anaemia as well as being a cofactor for tyrosine hydroxylase, a rate limiting step in dopamine production pathway in the nigrostriatal area (Sloand 2004).
Evidence for the hypothesis implicating dopaminergic dysfunction as a cause for RLS came from the chance finding of symptom relief on small doses of levodopa (Akpinar 1982) and exacerbation of symptoms with dopamine antagonists (Winkelmann 2001). Dopamine agonists directly stimulate dopamine receptors and are generally superior to levodopa in treating RLS.
The pathophysiology of RLS in people with CKD is not well understood. Inadequate dialysis leading to toxin accumulation, iron deficiency, hyperparathyroidism and high calcium and phosphate levels have been implicated in the pathogenesis by various studies. Most drug interventions used for people with CKD have been extrapolated from experience in treating people with primary RLS. Some interventions specific to those with CKD have been tried based on anecdotal evidence.
Dopamine pathways have been implicated in the pathophysiology of RLS for a long time. It is known that iron is involved in a rate‐limiting step required to convert tyrosine to levodopa that is later decarboxylated to dopamine. Correcting functional iron deficiency using iron supplementation may help reduce the symptoms of RLS in haemodialysis patients.
Gabapentin (an anticonvulsant) has been shown to help RLS in non‐uraemic patients (Lee 2016). This drug is excreted by the kidney (77%) and has a long‐half lifetime. Gabapentin modulates various receptor sites and alters dopamine, serotonin and norepinephrine release.
Levodopa was shown to be beneficial in ameliorating the symptoms from RLS in non‐uraemic patients. However it has adverse effects of rebound, augmentation and tolerance to beneficial effects.
Ropinirole is a synthetic dopamine agonist with relatively large D3 and D4 affinity. Because of its short plasma half‐life and liver metabolism it may be safer to use in patients with kidney failure.
High oxidative stress has been implicated in pathogenesis of RLS and the use of anti‐oxidants vitamin C and E may relieve the symptoms of RLS.
Why it is important to do this review
Abnormal limb movement disorders that affect sleep, such as periodic limb movement disorder and RLS, are reported to be common among people with CKD and those undergoing dialysis (Kavanagh 2004). These disorders, which are often both under‐recognised and treated, are thought to cause significant sleep disruption and reduce QOL (Mucsi 2005). As well as experiencing poor QOL, mounting evidence suggests people with disturbed sleep patterns are also at increased risk of developing hypertension and cardiovascular diseases (Walters 2010).
In view of the coexistence of RLS with other sleep disturbances in people with kidney disease, it is difficult to identify people with RLS alone. It has only been in the last 15 years that diagnostic criteria were agreed internationally and are being used to identify participants for inclusion in therapeutic studies.
The available evidence concerning treatment of RLS in the context of kidney impairment is limited, and the best options to treat these patients remain unclear.
Objectives
The aim of this review was to critically look at the benefits and safety of various treatment options used in the treatment of RLS in people with CKD and those undergoing RRT. We aimed to define different group characteristics based on CKD stage to assess the applicability of particular interventions to individual patients.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods), looking at the specified therapeutic interventions for people with RLS associated with CKD stages 3 to 5, including different modes of RRT were included.
Types of participants
Inclusion criteria
All RCTs that recruited adults of both sexes over the age of 18 years with CKD stages 3 to 5 with secondary RLS as diagnosed using the criteria set out by the IRLSSG were eligible for inclusion. Patients undergoing peritoneal dialysis or haemodialysis, and those who had received a kidney transplant were included.
Exclusion criteria
Idiopathic RLS
RLS secondary to other causes (e.g. diabetes, amyloid)
RLS predating onset of kidney disease.
People with diagnoses of periodic limb movement disorders not specifically diagnosed as RLS were excluded from our analyses.
Types of interventions
Non‐pharmacological interventions not related to renal replacement therapy (RRT) including exercise, smoking cessation and alcohol intake reduction
-
Pharmacological interventions (of any dose, combination, duration) versus placebo, no treatment, or other pharmacological agent. The following agents were included.
Iron/erythropoietic stimulating agents to adequately treat anaemia
Benzodiazepines: clonazepam, diazepam
Dopamine agonists: ropinirole, pramipexole, pergolide, cabergoline
Antiepileptics: gabapentin, pregabalin
Other medications/treatments: vitamins, minerals, nutritional supplements
Any other intervention that was found to be relevant such as changes to dialysis regimens, kidney transplantation, enhancing dialysis adequacy and optimising electrolyte balance.
Types of outcome measures
Reduction in symptoms and signs, assessed by questionnaires (International RLS Scale, Johns Hopkins RLS Severity Scale) or by objective testing (Suggested Immobilisation Test (SIT) and actigraphy)
Reduction in sleep disturbances related to RLS, assessed by questionnaires (RLS‐6 scale, Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS)) or by objective testing (actigraphy)
Adverse events related to interventions.
Each of the interventions described may relate to subsets of participants, depending on the stage of CKD, underlying diagnoses, RRT mode and demographic variables. Applicability was assessed by analysing studies for particular patient group characteristics and adverse events relating to specific interventions in that group.
Primary outcomes
The effects of interventions in reducing symptom severity due to RLS in people with CKD and potential complications related to the use of such interventions.
Secondary outcomes
Improvement in QOL
Improvement in quality of sleep
Reduction in cardiovascular mortality
Applicability to individual patients.
Search methods for identification of studies
Electronic searches
We searched Cochrane Kidney and Transplant Specialised Register (to 12 January 2016) through contact with the Information Specialist using search terms relevant to this review. The Specialised Register contains studies identified from the following sources.
Quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register were identified through search strategies for CENTRAL, MEDLINE, and EMBASE, based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about the Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies, and clinical practice guidelines.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts that were relevant to the review. Titles and abstracts were screened independently by two authors, who discarded studies that were not applicable; however studies and reviews that included relevant data or information on studies were retained initially. Two authors independently assessed the retrieved abstracts, and where necessary assessed the full text of these studies to determine which satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, records were grouped together and the publication with the most complete data was included. Where relevant, outcome data that were only published in earlier versions was used. Disagreements were resolved by consultation with all authors.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Were reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
Where continuous scale of measurements was used to assess the effects of treatment (improvement in the sleeping score, improvement in the international restless leg syndrome scale score, improvement in Johns Hopkins RLS Severity Scale score), the mean difference (MD) was used, or the standardised mean difference (SMD) when different scales were used.
Dealing with missing data
We attempted to contact study authors to obtain missing data.
We calculated the standard deviations when not reported and data were available either in the paper or provided by study authors.
Assessment of heterogeneity
Duration of treatment, dose and form of drugs were assessed to ensure there was no heterogeneity. Non‐pharmaceutical interventions (e.g. the form and frequency of exercise) were also assessed to ensure there was no heterogeneity.
Where applicable, heterogeneity was analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test (Higgins 2003). I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Duration of treatment, dose and form of drugs, and follow‐up period were assessed to determine heterogeneity.
Non‐pharmaceutical interventions (e.g. the form and frequency of exercise) and follow‐up period were assessed to determine heterogeneity.
Assessment of reporting biases
We did not identify sufficient numbers of RCTs to examine for publication bias using funnel plots (Higgins 2011).
Data synthesis
Data were to be pooled using the random‐effects model but the fixed‐effect model was also to be used to ensure robustness of the model chosen and susceptibility to outliers. Data were not pooled due to significant heterogeneity identified in outcomes measured.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analysis to explore possible sources of heterogeneity (e.g. participants, interventions and study quality). Heterogeneity among participants could be related to age (e.g. less than 65 years versus more than 65 years old), renal pathology (for example diabetic kidney disease, glomerulonephritis, renovascular disease, polycystic kidney disease). Heterogeneity in treatments could be related to prior agent(s) used and the agent, dose and duration of therapy.
Subgroup analysis was not done due to limited number of studies investigating the effect of a particular intervention. Adverse events were tabulated and assessed with descriptive techniques, as they were different for the various agents used.
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors on effect size.
Repeating the analysis excluding unpublished studies
Repeating the analysis taking account of risk of bias, as specified
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results
Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country.
We were unable to perform sensitivity analysis due to insufficient numbers of studies in each intervention category.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification
Results of the search
The searches returned 29 records. After grouping these records into studies (22 studies), nine studies (11 records) were excluded after full‐text review and nine studies (14 records) were included in the analyses. Four recently completed studies will be assessed in a future update of this review (NCT00996944; NCT01537042; NCT02011191; Razazian 2015) (Figure 1).
1.
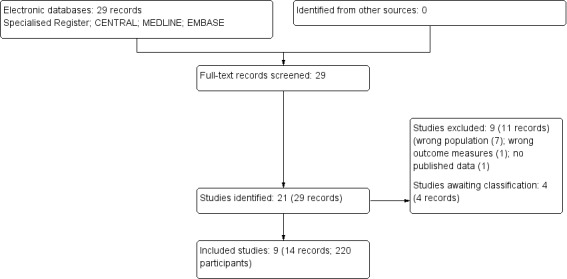
Study selection flow diagram
Included studies
We included nine studies that involved 220 participants undergoing RRT (Giannaki 2013; Giannaki 2013a; Micozkadioglu 2004; Mortazavi 2013; Pellecchia 2004; Sagheb 2012; Sloand 2004; Thorp 2001; Trenkwalder 1995). Eight studies only enrolled haemodialysis patients and one study enrolled haemodialysis and peritoneal dialysis patients (Sloand 2004) (Characteristics of included studies).
We contacted the authors for either missing data or clarification of the reported data; one author responded to our request.
Design
All nine studies were RCTs. Four studies were crossover studies (Micozkadioglu 2004; Pellecchia 2004; Thorp 2001; Trenkwalder 1995). Eight of the studies involved two comparisons whilst one (Sagheb 2012) included four groups for comparison. Three studies were open‐labelled studies (Micozkadioglu 2004; Mortazavi 2013; Pellecchia 2004), one was single blinded (Giannaki 2013a), and the other five were double‐blind (Giannaki 2013; Sagheb 2012; Sloand 2004; Thorp 2001; Trenkwalder 1995).
Sample sizes
Studies included within this review were relatively small in size, varying from 11 to 60 participants.
Setting
Studies were conducted in dialysis centres located in a number of countries and may reflect inherent variations in treatment and clinical practices. Study countries included Greece (Giannaki 2013; Giannaki 2013a); Iran (Mortazavi 2013; Sagheb 2012); USA (Sloand 2004; Thorp 2001); Germany (Trenkwalder 1995); Italy (Pellecchia 2004), and Turkey (Micozkadioglu 2004).
Participants
Participants were diagnosed with RLS based on criteria proposed by the IRLSSG. Updated IRLSSG standard criteria for RLS (Allen 2003) were used to identify participants for inclusion into the studies in all but two (Trenkwalder 1995, Thorp 2001). However criteria used by both studies were very similar to IRLSSG and were included in this review.
Interventions
Distinctly different interventions were assessed in the included studies.
-
Exercise interventions
Aerobic exercise versus no exercise (Mortazavi 2013)
Resistance exercise with either ropinirole or exercise with no resistance (Giannaki 2013a).
-
Pharmacological agents
Levodopa versus placebo (Trenkwalder 1995)
Gabapentin versus placebo (Thorp 2001)
Gabapentin versus levodopa (Micozkadioglu 2004)
Ropinirole versus levodopa (Pellecchia 2004)
Iron dextran versus placebo (Sloand 2004)
Vitamin C, vitamin E and placebo in four different combinations (Sagheb 2012).
Outcomes
The primary outcome in all studies was change in RLS severity from baseline to study end. Changes in QOL and sleep were included in the review when reported. None of the studies reported data on cardiovascular morbidity and mortality.
RLS severity was measured using different rating scales. Thorp 2001 and Trenkwalder 1995 pre‐dated publication of the validated IRLSSG RLS severity scoring tool which includes 10 questions each with five options scored from 0 to 4, giving a maximum score of 40. Four studies used this as the scoring tool (Giannaki 2013; Giannaki 2013a; Mortazavi 2013; Sagheb 2012). Micozkadioglu 2004 and Pellecchia 2004 applied a six question‐based IRLSSG scoring tool (introduced before the current IRLSSG tool) with a maximum score of 24. Three studies used their own non‐validated scoring tools (Thorp 2001; Trenkwalder 1995; Sloand 2004).
QOL was measured using validated SF‐36 questionnaire in three studies (Giannaki 2013; Micozkadioglu 2004; Mortazavi 2013). Trenkwalder 1995 used 13 visual analogue scales, each measuring different QOL components.
Sleep quality was assessed and reported in five studies using tools such as PSQI and ESS (Giannaki 2013; Giannaki 2013a; Micozkadioglu 2004; Pellecchia 2004; Trenkwalder 1995).
Adverse events were reported in all but one study (Mortazavi 2013) (Table 1).
1. Adverse events.
| Study | Intervention: number of events | Comments | Control: number of events | Comments |
| Giannaki 2013 | Resistance exercise: 0/16 | No adverse events | No exercise: 0/16 | No adverse events |
| Giannaki 2013a | Exercise with no resistance: 0/12 | No adverse events | Exercise with no resistance: 0/12 | No adverse events |
| Mortazavi 2013 | Exercise: not reported | Not reported | No exercise: not reported | Not reported |
| Thorp 2001 | Gabapentin: 2/15 | Withdrawal due to lethargy in gabapentin group. | Placebo: 1/16 | Myocardial infarction in placebo group prior to receiving gabapentin |
| Micozkadioglu 2004 | Gabapentin: 1/15 | Withdrawal due to fatigue, syncope in gabapentin group | Levodopa: not reported | Not reported |
| Trenkwalder 1995 | Levodopa: adverse effects were pooled and not reported separately for dialysis patients | Adverse effects were pooled and not reported separately for dialysis patients | Placebo: adverse effects were pooled and not reported separately for dialysis patients. | Adverse effects were pooled and not reported separately for dialysis patients. |
| Pellecchia 2004 | Ropinirole: 0/13 | No adverse events | Levodopa: 1/13 | Withdrawal due to vomiting in levodopa group |
| Sloand 2004 | Iron dextran: 2/11 had nausea/vomiting & 2/11 had headaches |
Nausea/vomiting Headache |
IV saline: 3/14 had nausea | Nausea |
| Sagheb 2012 | Vitamin C & E, vitamin C & placebo, vitamin E & placebo: 3/45 had nausea 3/45 had dyspepsia |
Nausea Dyspepsia |
Placebo: 1/15 had nausea | Nausea |
Outcomes were measured at different time points in the studies introducing significant heterogeneity. All studies had baseline severity scores. Two studies measured outcomes at six months (Giannaki 2013; Giannaki 2013a), one study measured the outcomes at 16 weeks (Mortazavi 2013), one study at eight weeks (Sagheb 2012), two studies at six weeks (Thorp 2001; Pellecchia 2004) and three studies at four weeks (Micozkadioglu 2004; Trenkwalder 1995; Sloand 2004). There were no long‐term follow‐up results assessing the effects of these interventions.
Excluded studies
Eleven studies were excluded (Characteristics of excluded studies).
Studies investigating uraemic neuropathy were excluded (Ausserwinkler 1989; Facchini 2004; FINESSE Study 2010; Kramer 1988; Okada 2000; Sprenger 1983; Walker 1996).
Included polysomnographic measurements of periodic limb movements (PLM) as primary outcomes in their analyses. We excluded this from our analysis as RLS is distinctly different from PLM disorder, which is a condition that occurs during sleep and is involuntary whilst RLS is voluntary movement of the limbs in order to relieve discomfort in the limbs (Pieta 1998).
One study registered in 2002 has never been published and the web link is no longer active (N0203077917).
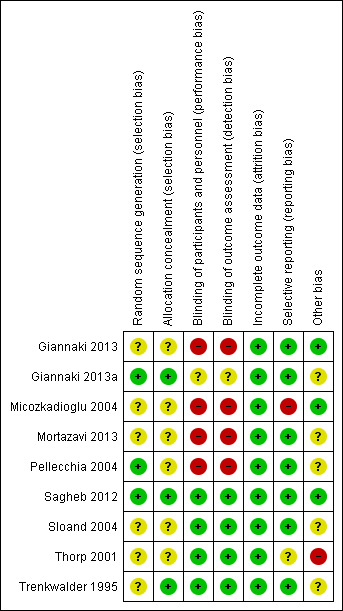
Risk of bias in included studies
Please see Figure 2; Figure 3. Overall the risk of bias was deemed to be moderate.
2.
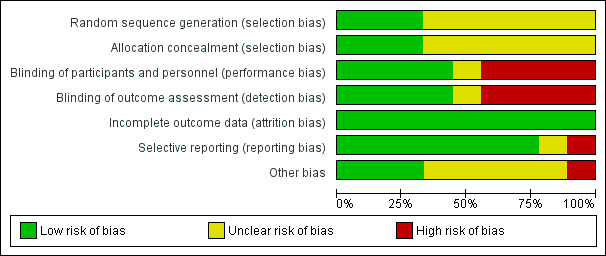
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation processes were clearly described in three studies (Giannaki 2013a; Pellecchia 2004; Sagheb 2012) whilst others were deemed to have unclear risk of bias.
Allocation concealment was adequate in only three studies (Giannaki 2013a; Pellecchia 2004; Sagheb 2012). The remaining six studies were assessed as unclear due to the lack of information regarding the allocation process.
Blinding
Four studies (Giannaki 2013; Micozkadioglu 2004; Mortazavi 2013; Pellecchia 2004) had high risk of bias due to inadequate blinding. The risk of bias was unclear in Giannaki 2013a as the participants were asked to change the dialysis shifts depending on their allocation, thereby introducing some performance bias. Other studies were deemed to have unclear risk due to lack of information even though they were described as double blinded.
Incomplete outcome data
All of the studies were thought to have low risk for attrition bias as the dropouts were low and all the studies provided details related to dropouts.
Selective reporting
Selective reporting bias in general has been deemed to be low except for one study (Micozkadioglu 2004), where the data has been presented selectively in relation to SF‐36 and sleep parameters.
Other potential sources of bias
One study was funded by a pharmaceutical company (Thorp 2001). Funding source was reported in only two other studies (Giannaki 2013; Sagheb 2012).
Effects of interventions
Exercise interventions
Three studies assessed the effect of exercise on the severity of RLS in haemodialysis patients: resistance exercise versus no exercise or ropinirole (Giannaki 2013), resistance exercise versus exercise with no resistance (Giannaki 2013a), and aerobic exercise to no exercise (Mortazavi 2013).
Severity of restless legs syndrome
Giannaki 2013 and Mortazavi 2013 reported a significant reduction in the scores of RLS severity between the exercise group and no exercise group (Analysis 1.1.1 (2 studies, 48 participants): MD ‐7.56, 95% CI ‐14.20 to ‐0.93; I2 = 65%).
1.1. Analysis.
Comparison 1 Exercise interventions, Outcome 1 Severity.
Giannaki 2013a study reported a significant reduction in the scores of severity of RLS in the resistance exercise group when compared to the exercise with no resistance group (Analysis 1.1.2 (1 study, 24 participants): MD ‐11.10, 95% CI ‐17.11 to ‐5.09).
Giannaki 2013 reported no evidence of reduction in the severity scores between the resistance exercise group and ropinirole group (Analysis 1.1.3 (1 study, 22 participants): MD ‐0.55, 95% CI ‐6.41 to 5.31).
Quality of Life
Two studies assessed the changes in QOL using SF‐36 (Giannaki 2013; Mortazavi 2013)
Giannaki 2013 did not report overall SF‐36 scores but presented physical component and mental component summary scores separately. They reported no significant difference in the physical component summary scores (Analysis 1.2.1 (1 study, 24 participants): (MD 5.40, 95% CI ‐4.82 to 15.62) or mental component summary scores (Analysis 1.3.1 (1 study, 24 participants); MD 6.20, 95% CI ‐8.54 to 20.94) between the aerobic exercise group and the no exercise group.
1.2. Analysis.
Comparison 1 Exercise interventions, Outcome 2 QOL: physical component score.
1.3. Analysis.
Comparison 1 Exercise interventions, Outcome 3 QOL: mental component summary score.
Similarly Giannaki 2013 reported no significant difference in the physical component summary scores (Analysis 1.2.2 (1 study, 22 participants): MD ‐8.60, 95% CI ‐18.92 to 1.72) or mental component summary scores (Analysis 1.3.2 (1 study 22 participants): MD ‐14.50, 95% CI ‐31.58 to 2.58) between the aerobic exercise group and the ropinirole group.
Mortazavi 2013 reported a non‐significant improvement in the QOL, however the data presented was not in the standard format and attempts to contact the authors have not been successful.
Quality of sleep
Two studies assessed the quality of sleep (Giannaki 2013; Giannaki 2013a). Giannaki 2013 reported no significant difference in subjective sleep quality at six months for resistance exercise versus no exercise (Analysis 1.4.1 (1 study, 22 participants): MD ‐1.13, 95% CI ‐3.71 to 1.45) and resistance exercise and exercise with not resistance (Analysis 1.4.2 (1 study, 24 participants): MD ‐0.50, 95% CI ‐2.93 to 1.93). Giannaki 2013 reported a significant improvement in subjective sleep quality in the ropinirole group when compared to resistance exercise group (Analysis 1.4.3 (1 study, 22 participants): MD 3.71, 95% CI 0.89 to 6.53).
1.4. Analysis.
Comparison 1 Exercise interventions, Outcome 4 Quality of sleep: subjective measure.
Giannaki 2013 reported no significant difference in the Epworth Sleepiness Scale scores when resistance exercise was compared with no exercise (Analysis 1.5.1 (1 study, 22 participants): (MD ‐0.07, 95% CI ‐3.47 to 3.33) or ropinirole (Analysis 1.5.3 (1 study, 22 participants): MD 2.07, 95% CI ‐2.15 to 6.29). Giannaki 2013a reported no significant difference in the Epworth Sleepiness Scale scores between resistance exercise and exercise with no resistance (Analysis 1.5.2 (1 study, 24 participants): MD 0.80, 95% CI ‐2.42 to 4.02). Mortazavi 2013 did not measure sleep parameters.
1.5. Analysis.
Comparison 1 Exercise interventions, Outcome 5 Quality of sleep: Epworth Sleepiness Scale.
Adverse events
Giannaki 2013 and Giannaki 2013a reported there were no adverse events in the study whilst Mortazavi 2013 did not mention if there were any adverse events. In Giannaki 2013, one patient in each group dropped out but the reason for dropout was not reported.
Pharmacological interventions
Severity of restless legs syndrome
Six studies investigated pharmacological intervention for treating RLS: gabapentin versus placebo (Thorp 2001); levodopa versus placebo (Trenkwalder 1995); iron dextran versus placebo (Sloand 2004); vitamin C, vitamin E and placebo in four different combinations (Sagheb 2012); gabapentin versus levodopa (Micozkadioglu 2004); and ropinirole versus levodopa (Pellecchia 2004);
Gabapentin versus placebo
Thorp 2001 assessed RLS severity at six weeks and at the end of a second treatment period. A non‐validated self‐administered RLS severity questionnaire based on the criteria developed by IRLSSG was used. The questionnaire consisted of four questions, each with a score ranging from 0 (symptoms never present) to 2 (symptoms constantly present). Eligible participants should have all four criteria with at least two present constantly giving a minimum score of six.
Thorp 2001 reported gabapentin significantly reduced the severity of RLS when compared to placebo (Analysis 2.1.1 (1 study, 26 participants): MD‐2.80, 95% CI ‐4.53 to ‐1.07).
2.1. Analysis.
Comparison 2 Pharmacological interventions, Outcome 1 Severity.
Levodopa versus placebo
Trenkwalder 1995 used their own RLS severity scoring tool. Participants scored severity of RLS from 0 (no symptoms) to 10 (very strong urge) depending on the urge to move the legs at the time of falling asleep.
Of the recruited 32 adult participants, 28 finished the study and 11 were haemodialysis patients. Results of the haemodialysis patients were reported separately. There was no significant difference in the severity of RLS between levodopa and placebo (Analysis 2.1.2 (1 study, 22 participants): MD ‐0.90, 95% CI ‐4.14 to 2.34).
Vitamins C and E versus placebo
Sagheb 2012 assessed RLS severity by IRLSSG RLS scoring tool at baseline and eight weeks. Means of RLS severity score decreased significantly in all vitamin groups compared to placebo: vitamin C (Analysis 2.1.3 (1 study, 30 participants): MD ‐6.90, 95% CI ‐9.23 to ‐4.57); vitamin E (Analysis 2.1.4 (1 study, 30 participants): MD ‐7.00, 95% CI ‐10.39 to ‐3.61); and vitamin C plus vitamin E (Analysis 2.1.5 (1 study, 30 participants): MD ‐7.20, 95% CI ‐10.28 to ‐4.12).
Iron dextran versus normal saline
Sloand 2004 assessed RLS severity by a locally developed (University of Rochester) scoring tool based on three questions each with a score ranging from 0 to 10 at baseline, 1, 2 and 4 weeks post infusion. Baseline median RLS severity score was 9 in the placebo group and 7 in the iron group. The placebo group did not show any difference in the scores at the end of follow‐up period. In the iron dextran group, week 1 scores changed by ‐2 (IQR ‐6 to ‐1, P = 0.03), week 2 scores by ‐3 (IQR ‐5 to ‐2, P = 0.01). By week four the effects were no longer significant.
Ropinirole versus levodopa
In Pellecchia 2004 participants reported severity on a tool consisting of a 6‐item questionnaire developed by IRLSSG, at the end of six weeks of treatment with each agent. Ropinirole significantly reduced the severity of RLS in the ropinirole group compared to the levodopa group (Analysis 2.1.6 (1 study, 22 participants): MD ‐6.70, 95% CI ‐9.96 to ‐3.44).
Gabapentin versus levodopa
In Micozkadioglu 2004 RLS severity scoring tool consisted of six questions (each with a score ranging from 0 to 4). Both drugs were shown to be effective in reducing the symptoms. Severity score decreased to a median score of 10 in participants treated with Levodopa and to 3 in gabapentin group from a baseline median score of 17 (P < 0.001).
Gabapentin was effective in all treated participants whilst Levodopa was effective in 13/14 participants. Contrasting gabapentin, symptoms in levodopa group recurred in a few hours in most of the participants confirming its short duration of action.
Quality of Life
Gabapentin versus placebo
Thorp 2001 did not assess the effect of gabapentin on QOL.
Levodopa versus placebo
Trenkwalder 1995 used 13 visual analogue scales that measured various aspects of QOL. Levodopa was reported to significantly improve general well‐being and few other aspects. They reported non‐significant improvement in subjective QOL in relation to life satisfaction (Analysis 2.2.1, (1 study, 22 participants): MD 6.80, 95% CI ‐1.31 to 14.91) and reduction in scores of negative feelings and complaints (Analysis 2.3, (1 study, 22 participants): MD ‐2.70, 95% CI ‐11.06 to 5.66).
2.2. Analysis.
Comparison 2 Pharmacological interventions, Outcome 2 QOL: life satisfaction.
2.3. Analysis.
Comparison 2 Pharmacological interventions, Outcome 3 QOL: negative feelings & complaints.
Vitamins C and E versus placebo
Sagheb 2012 did not assess the effect of vitamins C and E on QOL.
Iron dextran versus normal saline
Sloand 2004 did not assess the effect of iron dextran infusion on QOL.
Ropinirole versus levodopa
Pellecchia 2004 did not assess the effect of either ropinirole or levodopa on QOL.
Gabapentin versus levodopa
Micozkadioglu 2004 reported gabapentin was shown to significantly improve the scores in general health, body pain and social function components of SF‐36. They did not report the composite scores.
Quality of sleep
Gabapentin versus placebo
Thorp 2001 did not assess the effect of gabapentin on quality of sleep.
Levodopa versus placebo
Trenkwalder 1995 reported subjective ratings of sleep quality (quality of sleep, nocturnal awakening). Subjective quality of sleep was reported to be significantly better with levodopa compared to placebo (Analysis 2.4.1, (1 study, 22 participants): MD 2.10, 95% CI 0.37 to 3.83).
2.4. Analysis.
Comparison 2 Pharmacological interventions, Outcome 4 Quality of sleep.
Vitamins C and E versus placebo
Sagheb 2012 did not assess the effect of intervention of quality of sleep.
Iron dextran versus normal saline
Sloand 2004 did not assess effect of iron dextran infusion on quality of sleep.
Ropinirole versus levodopa
Pellecchia 2004 reported sleep time significantly increased with ropinirole when compared to levodopa (Analysis 2.4.2 (1 study, 22 participants): MD 2.10, 95% CI 0.98 to 3.22).
Gabapentin versus levodopa
Micozkadioglu 2004 reported significant improvement in sleep quality, sleep latency and sleep disturbance with gabapentin when compared with levodopa, however data were not provided.
Adverse events
Gabapentin versus placebo
Two participants in the gabapentin group dropped out due to lethargy. One participant died due to myocardial infarction in the placebo group before gabapentin was administered.
Levodopa versus placebo
Two haemodialysis patients discontinued the study due to reasons not related to the drug use (Wegener's granulomatosis and deterioration in general health).
A total of 33 adverse events were reported in 15 participants but they are not separable between idiopathic RLS group and uraemic RLS group. Nine adverse events were possibly related to levodopa whilst one event was deemed severe (agitation after caffeine intake). Most frequent events were headaches, dry mouth and GI symptoms.
Vitamins C and E versus placebo
Two participants in the vitamin C and vitamin E, one in the vitamin E and placebo, and one in the double placebo group reported nausea during the first week of treatment. One patient in the vitamins C and E, one in the vitamin C and placebo, and one in the vitamin E and placebo group reported dyspepsia during the first two weeks of the treatment.
Iron dextran versus normal saline
Nausea and vomiting occurred in 2/11 participants in the iron group and 3/14 participants in the placebo group. Headache was reported in 2/11 in the iron group.
Ropinirole versus levodopa
In the levodopa group, one participant discontinued the study due to severe vomiting related to the treatment. No adverse events noted in ropinirole group whilst morning rebound was not reported in the other group.
Gabapentin versus levodopa
One participant dropped out from gabapentin group due to symptoms of drowsiness, syncope, and fatigue, thought to be related to the use of gabapentin.
Discussion
Summary of main results
This systematic review included only studies with stable haemodialysis patients as subjects as there were no studies identified in other CKD stages. Our systematic review demonstrated that in small clinical studies, aerobic exercise improved the symptoms of RLS significantly however the safety of this intervention is not clear. The effect of aerobic exercise on sleep quality was not significant.
It is worth noting that in Giannaki 2013a, patients in the exercise group had improved dialysis adequacy at the end of the study period (Kt/V improved from 1.10 ± 0.0 baseline versus 1.25 ± 0.1 post exercise, P = 0.041) when compared to the control group (1.2 ± 0.0 baseline versus 1.2 ± 0.4 post exercise, P > 0.05). As it is known that aerobic exercise improves the dialysis adequacy, and accumulation of uraemic toxins due to poor dialysis adequacy may be implicated in RLS, it is difficult to know if the benefit seen in the exercise group is related to exercise per se or better dialysis adequacy.
Ropinirole was shown to significantly improve the symptoms of RLS, sleep and mental component summary score of SF‐36 without any adverse events in two small studies. Gabapentin, even though it improved the symptoms of RLS, is not well tolerated, possibly due to its accumulation in patients with kidney failure. There were more dropouts due to adverse effects in the gabapentin groups. Levodopa also reduced the symptoms of RLS but due to its short acting nature was associated with rebound and augmentation. Iron dextran led to temporary improvement in the symptoms of RLS with minimal side effects. Vitamin C, vitamin E and their combination were shown to improve the symptoms of RLS, however this is a small study and further evidence is needed before recommending these agents.
Overall completeness and applicability of evidence
We planned to find the best intervention to treat RLS in patients with CKD across stages 3 to 5. However our searches did not yield any studies that included patients with stages of CKD other than those who are on haemodialysis. This possibly reflects the ease with which this group of patients could be approached and recruited as they attend in hospital haemodialysis three times a week. Hence, these results are applicable only to haemodialysis patients and cannot be generalised to patients with other stages of CKD.
We wanted to assess the effect of treatment intervention on cardiovascular morbidity and mortality. However none of the studies included these data. Included studies were relatively small in size with a short follow‐up duration, which may make it difficult to collect cardiovascular morbidity and mortality data in these studies.
Included studies were conducted in various parts of the world. It is well recognised that the clinical practices related to dialysis therapy differ significantly amongst different centres. Except for one study, there was no data available on parameters related to dialysis such as dialysis adequacy. This will be an important consideration when we try to generalise the results for haemodialysis patients.
Quality of the evidence
The quality of evidence in this review is hampered by the small size of the studies and shorter duration of follow‐up and as such it should be interpreted cautiously. Although all of the studies were randomised, the process of randomisation was only described in three studies which were of low risk. Four of the included studies were open‐label thereby introducing performance and detection bias.
Potential biases in the review process
We made stringent attempts to limit bias in the review process by ensuring a comprehensive search for potentially eligible studies. Searches were conducted by the Information Specialist based on a pre‐defined search strategy. Our independent assessments of eligibility of studies for inclusion in this review and extraction of data minimised the potential for additional bias. We attempted to contact the authors of the studies to obtain all the relevant missing data.
Agreements and disagreements with other studies or reviews
de Oliveira 2010 published a systematic review of the pharmacological treatment for uraemic RLS. The authors opined that only a few therapeutic studies on patients with uraemia with RLS have been published, and there is insufficient scientific evidence to favour any specific therapeutic regimen for uraemia‐associated RLS in keeping with our conclusions. The authors concluded that therapy using levodopa, dopaminergic agonists, anticonvulsants, and clonidine may be effective, but further studies were needed. In contrast to our review that included only studies with RLS, de Oliveira 2010 did not differentiate RLS from periodic limb movement disorder and included studies reporting periodic limb movement index.
Since the publication of de Oliveira 2010 review in 2010, four further studies were published, three studies evaluating aerobic exercise (Giannaki 2013; Giannaki 2013a; Mortazavi 2013) and one exploring vitamin C, E and their combination (Sagheb 2012). There was no further expansion of knowledge with regards to pharmacological therapy in this group of patients; however aerobic exercise has been shown to be a potential intervention to treat RLS.
Authors' conclusions
Implications for practice.
The findings of this review have limited clinical implications because of the small number of studies with significant heterogeneity. As only haemodialysis patients are included in the studies, these results are not applicable to other stages of CKD. Aerobic exercise appears to be effective in reducing the symptoms of RLS in haemodialysis patients. However this intervention will rely on the physical abilities and motivation of the patients along with conducive environment and resources, which may make it difficult to administer in a non‐trial setting. Ropinirole seems to be effective in helping the symptoms of RLS without any side effects in one small study, and this needs to be confirmed in larger studies in the future. As dopamine agonists are used widely in clinical practice to treat RLS, there is a huge need to conduct high quality studies using these agents to establish evidence.
Implications for research.
Given the high prevalence of RLS in patients with CKD and lack of high quality clinical studies, further and larger studies exploring pharmacological and non‐pharmacological interventions in CKD patients are needed. In the haemodialysis group, intra‐dialytic exercise program may help the symptoms of RLS without any major adverse effects and this needs to be verified by a prospective and large sized RCT. There is no evidence for using dopamine agonists to treat RLS in CKD patients, but are used routinely in clinical practice. Ropinirole may be suitable for further assessment.
Acknowledgements
We would like to acknowledge the referees for their assessments and suggestions. We would also like to thank the UK Cochrane Centre in Oxford for their support in training and provision of help over the phone or in person. We would like to thank Cochrane and Kidney Transplant's Information Specialists for their help and Sheena Deery for her invaluable advice and suggestions.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Exercise interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severity | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Exercise versus no exercise | 2 | 48 | Mean Difference (IV, Random, 95% CI) | ‐7.56 [‐14.20, ‐0.93] |
| 1.2 Resistance exercise versus exercise with no resistance | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐11.1 [‐17.11, ‐5.09] |
| 1.3 Resistance exercise versus ropinirole | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐6.41, 5.31] |
| 2 QOL: physical component score | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Resistance exercise versus no exercise | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Resistance exercise versus ropinirole | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 QOL: mental component summary score | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Resistance exercise versus no exercise | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Resistance exercise versus ropinirole | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Quality of sleep: subjective measure | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Resistance exercise versus no exercise | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Resistance exercise versus exercise with no resistance | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Resistance exercise versus ropinirole | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Quality of sleep: Epworth Sleepiness Scale | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Resistance exercise versus no exercise | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Resistance exercise versus exercise with no resistance | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Resistance exercise versus ropinirole | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Pharmacological interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severity | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Gabapentin versus placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Levodopa versus placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Vitamin C versus placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Vitamin E versus placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Vitamin C & E versus placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Ropinirole versus levodopa | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 QOL: life satisfaction | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Levodopa versus placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 QOL: negative feelings & complaints | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Levodopa versus placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Quality of sleep | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Levodopa versus placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Ropinirole versus levodopa | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Giannaki 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised 2:1:1 but no details of how the randomisation was done |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Physical intervention versus drug (double blinded in the placebo versus ropinirole arms) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients aware of the intervention in physical versus drug groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient withdrew from each group (transplantation (2); chronic infection (1) |
| Selective reporting (reporting bias) | Low risk | All results of parameters assessed were clearly detailed. However, the reasons for dropouts were not specified; stated only that it was for reasons unrelated to the study |
| Other bias | Low risk | Government funding |
Giannaki 2013a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using customised randomisation software |
| Allocation concealment (selection bias) | Low risk | Used randomisation software |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Even though patients belonging to different groups dialysed on different days, the nature of the intervention (resistance vs exercise with no resistance) would make it easy for the participants to know which arm they have been randomised to |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Patients may have been influenced by the knowledge of their intervention when reporting the outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals in the study |
| Selective reporting (reporting bias) | Low risk | A number of exercise sessions were reported as incomplete (patients were unable to complete the full 45 min cycling). Reasons for this were stated as personal reasons. Those instances were not systematically recorded and, hence, authors were unable to indicate the level of compliance clearly. The authors gauged compliance as very high |
| Other bias | Unclear risk | Funding source not reported |
Micozkadioglu 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients and study personnel were aware of the type of intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 patient excluded (due to medication side effect) |
| Selective reporting (reporting bias) | High risk | Selectively reported three domains of SF‐36; data not provided to support conclusions reached with regard to sleep parameters |
| Other bias | Low risk | Funding source: "There was no pharmaceutical industry support of this study" |
Mortazavi 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data available |
| Selective reporting (reporting bias) | Low risk | Outcome in question was reported (severity of RLS) |
| Other bias | Unclear risk | Funding source not mentioned |
Pellecchia 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Open label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adverse events were clearly stated |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Funding source not reported |
Sagheb 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation with a fixed block size of four by randomisation sequence generator |
| Allocation concealment (selection bias) | Low risk | 1:1:1:1 random assignment to the 4 groups; this was done by an investigator who had no clinical involvement in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Used random sequence generating software |
| Selective reporting (reporting bias) | Low risk | Clear reporting of side effects. Study protocol clear enough from the methods section |
| Other bias | Low risk | Funded by a Grant from the Shiraz University of Medical Sciences, Shiraz, Iran |
Sloand 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both placebo and drug were infused during dialysis by infusion pump with the medication (or placebo) and tubing covered with an opaque obscuring sleeve so that neither the patient, investigator, nor study nurse could detect which was being administered |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear mention of the randomisation process |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both placebo and drug were infused during dialysis by infusion pump with the medication (or placebo) and tubing covered with an opaque obscuring sleeve so that neither the patient, investigator, nor study nurse could detect which was being administered |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Patients were blinded to the treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data available |
| Selective reporting (reporting bias) | Low risk | Protocol not available but published report contained expected outcome (Improvement in RLS severity) Side effects were also reported |
| Other bias | Unclear risk | Funding source not reported |
Thorp 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Treatment administered under the direct supervision on nurse at the end of dialysis treatment. One week washout period between 2 treatments |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear mention how randomised |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients, nurses, and physicians were blinded to treatment assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments (questionnaires) were carried out while subjects and staff were still blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clearly mention drop outs and withdrawals with specified reasons |
| Selective reporting (reporting bias) | Unclear risk | No study protocol but expected outcome (improvement in severity of RLS) is clearly discussed |
| Other bias | High risk | "Gabapentin was supplied by Parke‐Davis Pharmaceuticals" |
Trenkwalder 1995.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on the randomisation procedure |
| Allocation concealment (selection bias) | Low risk | Double blind |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No clear statement of blinding of outcome assessment, but assessments of outcome (polysomnographic studies, Actigraphy, subjective rating and physician's rating) were carried out at different points of the study while participants and investigators were still blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data regarding different assessment methods were clearly shown and data regarding both groups (idiopathic versus uraemic RLS) were clearly shown |
| Selective reporting (reporting bias) | Low risk | No protocol but outcomes (improvement in RLS) assessment clearly outlined |
| Other bias | Unclear risk | Funding source not reported |
CGI ‐ clinical global impression; CRP ‐ C‐reactive protein; HD ‐ haemodialysis; IRLSSG ‐ International RLS Study group; M/F ‐ male/female; PD ‐ peritoneal dialysis; QOL ‐ quality of life; RCT ‐ randomised controlled trial; RLS ‐ restless legs syndrome; SD ‐ standard deviation; SF‐36 ‐ short form 36 question survey; TSAT ‐ transferrin saturation; URR ‐ urea reduction ratio; VAS ‐ visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ausserwinkler 1989 | Wrong population: participants with uraemic polyneuropathy which is a sensorimotor polyneuropathy caused by uraemic toxins |
| Facchini 2004 | Wrong population: participants with uraemic polyneuropathy which is a sensorimotor polyneuropathy caused by uraemic toxins |
| FINESSE Study 2010 | Wrong population: participants with uraemic polyneuropathy which is a sensorimotor polyneuropathy caused by uraemic toxins |
| Kramer 1988 | It was a study of uraemic neuropathy, which is a sensorimotor polyneuropathy caused by uraemic toxins and it can be a cause for secondary RLS which is one of our exclusion criteria |
| N0203077917 | No data has been published since the study was first registered in the UK in 2002 |
| Okada 2000 | It was a study of uraemic neuropathy, which is a sensorimotor polyneuropathy caused by uraemic toxins and it can be a cause for secondary RLS which is one of our exclusion criteria |
| Pieta 1998 | It was a study of periodic limb movement disorder and not RLS, so excluded |
| Sprenger 1983 | Wrong population: participants with uraemic polyneuropathy which is a sensorimotor polyneuropathy caused by uraemic toxins |
| Walker 1996 | The primary outcome was measured objectively using polysomnography and there were no data on subjective improvement |
RLS ‐ restless legs syndrome
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00996944.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
NCT01537042.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes | Ratio from baseline in PLMI Change from baseline
|
| Notes |
|
NCT02011191.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
Razazian 2015.
| Methods |
|
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
|
| Outcomes |
|
| Notes |
|
CGI ‐ Clinical Global Impressions; CKD ‐ chronic kidney disease; CVD ‐ cardiovascular disease; DBP ‐ diastolic blood pressure; ESKD ‐ end‐stage kidney disease; HD ‐ haemodialysis; IRLSSG ‐ International Restless Legs Syndrome Study Group Rating Scale; PD ‐ peritoneal dialysis; PLMI ‐ periodic limb movements index; PLMSAI ‐ Periodic Limb Movement During Sleep Arousal Index; PSQI ‐ Pittsburgh Sleep Quality Index; QOL ‐ quality of life; RCT ‐ randomised controlled trial; RLS ‐ restless legs syndrome; RLS‐6 ‐ Restless Legs‐6 Rating Scale; RLS‐QOL ‐ Restless Legs‐Quality of Life SBP ‐ systolic blood pressure
Contributions of authors
Draft the protocol: MS, SG
Study selection: MS, SG
Extract data from studies: MS, SG
Enter data into RevMan: MS, SG
Carry out the analysis: MS, SG
Interpret the analysis: MS, NA, SG
Draft the final review: MS, NA, SG
Disagreement resolution: MS, NA, SG
Update the review: MS, NA, SG
Declarations of interest
Seerapani Gopaluni: none known
Mohamed Sheriff: none known
Naim A Ahmadouk: none known
New
References
References to studies included in this review
Giannaki 2013 {published data only}
- Giannaki CD, Sakkas GK, Karatzaferi C, Hadjigeorgiou GM, Lavdas E, Kyriakides T, et al. Effect of exercise training and dopamine agonists in patients with uremic restless legs syndrome: a six‐month randomized, partially double‐blind, placebo‐controlled comparative study. BMC Nephrology 2013;14:194. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giannaki 2013a {published data only}
- Giannaki CD, Hadjigeorgiou GM, Karatzaferi C, Maridaki MD, Koutedakis Y, Founta P, et al. A single‐blind randomized controlled trial to evaluate the effect of 6 months of progressive aerobic exercise training in patients with uraemic restless legs syndrome. Nephrology Dialysis Transplantation 2013;28(11):2834‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Micozkadioglu 2004 {published data only}
- Micozkadioglu H, Ozdemir FN, Kut A, Sezer S, Saatci U, Haberal M. Gabapentin versus levodopa for the treatment of restless legs syndrome in hemodialysis patients: an open‐label study. Renal Failure 2004;26(4):393‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mortazavi 2013 {published data only}
- Mortazavi M, Vahdatpour B, Ghasempour A, Taheri D, Shahidi S, Moeinzadeh F, et al. Aerobic exercise improves signs of restless leg syndrome in end stage renal disease patients suffering chronic hemodialysis. Thescientificworldjournal 2013;2013:628142. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi M, Vahdatpour B, Shahidi S, Ghasempour A, Taheri D, Dolatkhah S, et al. Aerobic exercise improves signs of restless leg syndrome in end stage renal disease patients suffering chronic hemodialysis [abstract]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii281. [EMBASE: 70766123] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi M, Vahdatpour B, Shahidi S, Ghasempour A, Taheri S, Ghasami M. The effect of aerobic exercise on the symptoms of restless leg syndrome and quality of life in hemodialysis patients [abstract no: P407]. Iranian Journal of Kidney Diseases 2011;5(Suppl 2):63‐4. [EMBASE: 70673915] [Google Scholar]
Pellecchia 2004 {published data only}
- Pellecchia MT, Vitale C, Sabatini M, Longo K, Amboni M, Bonavita V, et al. Ropinirole as a treatment of restless legs syndrome in patients on chronic hemodialysis: an open randomized crossover trial versus levodopa sustained release. Clinical Neuropharmacology 2004;27(4):178‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sagheb 2012 {published data only}
- Fallahzadeh MK, Akbari H, Sohrabi Nazari S, Sagheb MM. Efficacy of vitamins C, E and their combination for treatment of restless legs syndrome in hemodialysis patients; a randomized, double‐blind, placebo‐controlled trial [abstract no: 404]. Iranian Journal of Kidney Diseases 2011;5(Suppl 2):22‐3. [EMBASE: 70673845] [DOI] [PubMed] [Google Scholar]
- Sagheb MM, Dormanesh B, Fallahzadeh MK, Akbari H, Sohrabi NS, Heydari ST, et al. Efficacy of vitamins C, E, and their combination for treatment of restless legs syndrome in hemodialysis patients: a randomized, double‐blind, placebo‐controlled trial. Sleep Medicine 2012;13(5):542‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sloand 2004 {published data only}
- Halterman MW, Sloand JA. An update on the use of intravenous iron for the treatment of restless legs syndrome in end stage renal disease [Actualizacion sobre el uso de hierro intravenoso para el tratamiento del sindrome de piernas inquietas asociado a insuficiencia renal terminal]. Salud(i)Ciencia 2007;15(5):860‐5. [EMBASE: 2007548364] [Google Scholar]
- Sloand JA, Shelly MA, Feigin A, Bernstein P, Monk RD. A double‐blind, placebo‐controlled trial of intravenous iron dextran therapy in patients with ESRD and restless legs syndrome. American Journal of Kidney Diseases 2004;43(4):663‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thorp 2001 {published data only}
- Thorp ML, Morris CD, Bagby SP. A crossover study of gabapentin in treatment of restless legs syndrome among hemodialysis patients. American Journal of Kidney Diseases 2001;38(1):104‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Trenkwalder 1995 {published data only}
- Trenkwalder C, Stiasny K, Pollmacher T, Wetter T, Schwarz J, Kohnen R, et al. L‐dopa therapy of uremic and idiopathic restless legs syndrome: a double‐blind, crossover trial. Sleep 1995;18(8):681‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wetter TC, Trenkwalder C, Pollmacher T, Stiasny K, Kohnen R, Kazenwadel J, et al. Effect of levodopa therapy of idiopathic and uremic restless legs syndrome: a double blind crossover trial [abstract]. Journal of Neurology 1995;242(6 Suppl 2):S81. [CENTRAL: CN‐00507525] [Google Scholar]
- Wetter TC, Trenkwalder C, Stiasny K, Pollmacher T, Kazenwadel J, Kohnen R, et al. Treatment of idiopathic and uremic restless legs syndrome with L‐dopa‐‐a double‐blind cross‐over study [Behandlung des idiopathischen und uramischen restless‐legs‐syndrom mit L‐Ddpa‐‐eine doppelblinde cross‐over‐studie]. Wiener Medizinische Wochenschrift 1995;145(17‐18):525‐7. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies excluded from this review
Ausserwinkler 1989 {published data only}
- Ausserwinkler M, Schmidt P. Clonidine is effective in the treatment of 'restless leg' syndrome in chronic uraemic patients [abstract]. Nephrology Dialysis Transplantation 1988;3(4):530. [CENTRAL: CN‐00260393] [Google Scholar]
- Ausserwinkler M, Schmidt P. Successful clonidine treatment of restless leg syndrome in chronic kidney insufficiency [Erfolgreiche behandlung des "restless legs"‐syndroms bei chronischer Niereninsuffizienz mit clonidin]. Schweizerische Medizinische Wochenschrift [Journal Suisse de Medicine] 1989;119(6):184‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Facchini 2004 {published data only}
- Facchini MG, Mambelli E, Checchia G, Balboni M, Bainotti S, Santoro A. Lorenz therapyTM a new treatment of uremic neuropathy [abstract no: SU‐PO379]. Journal of the American Society of Nephrology 2004;15(Oct):616A. [CENTRAL: CN‐00583895] [Google Scholar]
- Facchini MG, Mambelli E, Checchia G, Gaggi R, Santoro A. The Lorenz™ therapy: a new tool in the treatment of uremic neuropathy [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:375. [CENTRAL: CN‐00509181]
FINESSE Study 2010 {published data only}
- Jardine M, Krishnan A, Gallagher M, Snelling P, Hawley C, Pussell B, et al. Uraemic neuropathy remains ubiquitious: rationale and feasibility of the FINESSE (Filtration in the Neuropathy of End Stage kidney disease Symptom Evolution) trial [abstract no: 235]. Nephrology 2010;15(Suppl 4):88. [EMBASE: 70467239] [Google Scholar]
Kramer 1988 {published data only}
- Kramer G, Suss W, Kohler H, Schneider B, Hopf HC. Ganglioside therapy in uremic polyneuropathy [Gangliosid‐therapie der uramischen polyneuropathie]. Medizinische Klinik 1988;83(1):7‐11. [MEDLINE: ] [PubMed] [Google Scholar]
N0203077917 {published data only}
- Bingham C. Pergolide for restless legs in uraemia. www.nihr.ac.uk/Profile/Pages/NRRResults.aspx?publication_id=N0203077917 (link no longer active) 2002.
Okada 2000 {published data only}
- Okada H, Moriwaki K, Kanno Y, Sugahara S, Nakamoto H, Yoshizawa M, et al. Vitamin B6 supplementation can improve peripheral polyneuropathy in patients with chronic renal failure on high‐flux haemodialysis and human recombinant erythropoietin. Nephrology Dialysis Transplantation 2000;15(9):1410‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pieta 1998 {published data only}
- Pieta J, Millar T, Zacharias J, Fine A, Kryger M. Effect of pergolide on restless legs and leg movements in sleep in uremic patients. Sleep 1998;21(6):617‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sprenger 1983 {published data only}
- Sprenger KB, Bundschu D, Lewis K, Spohn B, Schmitz J, Franz HE. Improvement of uremic neuropathy and hypogeusia by dialysate zinc supplementation: a double‐blind study. Kidney International ‐ Supplement 1983;16:S315‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Walker 1996 {published data only}
- Walker SL, Fine A, Kryger MH. L‐DOPA/carbidopa for nocturnal movement disorders in uremia. Sleep 1996;19(3):214‐8. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00996944 {published data only}
- NCT00996944. Clinical evaluation of ropinirole IR (immediate release) tablets in patients who are diagnosed with symptomatic restless legs syndrome (RLS) associated with chronic kidney disease (CKD) managed with haemodialysis (including haemofiltration and haemodiafiltration). www.clinicaltrials.gov/ct2/show/study/NCT00996944 (accessed 21 July 2016).
NCT01537042 {published data only}
- NCT01537042. A sleep laboratory study to investigate the safety and efficacy of the rotigotine skin patch in subjects with restless legs syndrome and end‐stage renal disease requiring hemodialysis. www.clinicaltrials.gov/ct2/show/NCT01537042 (accessed 21 July 2016).
NCT02011191 {published data only}
- Moretti H. Biotin deficiency and restless legs syndrome: evidence for a causal relationship from randomized, double‐blind, placebo‐controlled trial. www.clinicaltrials.gov/ct2/show/NCT02011191 (accessed 21 July 2016).
Razazian 2015 {published data only}
- Razazian N, Azimi H, Heidarnejadian J, Afshari D, Ghadami MR. Gabapentin versus levodopa‐c for the treatment of restless legs syndrome in hemodialysis patients: a randomized clinical trial. Saudi Journal of Kidney Diseases & Transplantation 2015;26(2):271‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Agarwal 2011
- Agarwal P, Sia C, Vaish N, Roy‐Faderman I. Pilot trial of onabotulinumtoxina (Botox) in moderate to severe restless legs syndrome. International Journal of Neuroscience 2011;121(11):622‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Akpinar 1982
- Akpinar S. Treatment of restless legs syndrome with levodopa plus benserazide. Archives of Neurology 1982;39(11):739. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Al‐Jahdali 2009
- Al‐Jahdali HH, Al‐Qadhi WA, Khogeer HA, Al‐Hejaili FF, Al‐Ghamdi SM, Al Sayyari AA. Restless legs syndrome in patients on dialysis. Saudi Journal of Kidney Diseases & Transplantation 2009;20(3):378‐85. [MEDLINE: ] [PubMed] [Google Scholar]
Allen 2003
- Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Medicine 2003;4(2):101‐19. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Oliveira 2010
- Oliveira MM, Conti CF, Valbuza JS, Carvalho LB, do Prado GF. The pharmacological treatment for uremic restless legs syndrome: evidence‐based review. Movement Disorders 2010;25(10):1335‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ekbom 1945
- Ekbom K. Restless legs. Acta Medica Scandinavica 1945;121(S158):7‐123. [DOI] [PubMed] [Google Scholar]
Giannaki 2010
- Giannaki CD, Sakkas GK, Hadjigeorgiou GM, Karatzaferi C, Patramani G, Lavdas E, et al. Non‐pharmacological management of periodic limb movements during hemodialysis session in patients with uremic restless legs syndrome. ASAIO Journal 2010;56(6):538‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hornyak 2014
- Hornyak M, Scholz H, Kohnen R, Bengel J, Kassubek J, Trenkwalder C. What treatment works best for restless legs syndrome? Meta‐analyses of dopaminergic and non‐dopaminergic medications.[Erratum appears in Sleep Med Rev. 2014 Aug;18(4):367‐8]. Sleep Medicine Reviews 2014;18(2):153‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kavanagh 2004
- Kavanagh D, Siddiqui S, Geddes CC. Restless legs syndrome in patients on dialysis. American Journal of Kidney Diseases 2004;43(5):763‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
La Manna 2011
- Manna G, Pizza F, Persici E, Baraldi O, Comai G, Cappuccilli ML, et al. Restless legs syndrome enhances cardiovascular risk and mortality in patients with end‐stage kidney disease undergoing long‐term haemodialysis treatment. Nephrology Dialysis Transplantation 2011;26(6):1976‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2016
- Lee DO, Buchfuhrer MJ, Garcia‐Borreguero D, Avidan AY, Ahmed M, Hays R, et al. Efficacy of gabapentin enacarbil in adult patients with severe primary restless legs syndrome. Sleep Medicine 2016;19:50‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Manley 2003
- Manley HJ, McClaran ML, Overbay DK, Wright MA, Reid GM, Bender WL, et al. Factors associated with medication‐related problems in ambulatory hemodialysis patients. American Journal of Kidney Diseases 2003;41(2):386‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mucsi 2005
- Mucsi I, Molnar MZ, Ambrus C, Szeifert L, Kovacs AZ, Zoller R, et al. Restless legs syndrome, insomnia and quality of life in patients on maintenance dialysis. Nephrology Dialysis Transplantation 2005;20(3):571‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sun 1998
- Sun ER, Chen CA, Ho G, Earley CJ, Allen RP. Iron and the restless legs syndrome. Sleep 1998;21(4):371‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Walters 1995
- Walters AS. Toward a better definition of the restless legs syndrome. The International Restless Legs Syndrome Study Group. Movement Disorders 1995;10(5):634‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Walters 2010
- Walters A, Rye D. Evidence continues to mount on the relationship of restless legs syndrome/periodic limb movements in sleep to hypertension, cardiovascular disease, and stroke. Sleep 2010;33(3):287. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Winkelmann 2001
- Winkelmann J, Schadrack J, Wetter TC, Zieglgansberger W, Trenkwalder C. Opioid and dopamine antagonist drug challenges in untreated restless legs syndrome patients. Sleep Medicine 2001;2(1):57‐61. [EMBASE: 2001086037] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gopaluni 2013
- Gopaluni S, Sherif M, Ahmadouk NA. Interventions for chronic kidney disease‐associated restless legs syndrome. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD010690] [DOI] [PMC free article] [PubMed] [Google Scholar]


