Abstract
Background
Elevated levels of total cholesterol and low‐density lipoprotein play an important role in the development of atheromas and, therefore, in cardiovascular diseases. Cholesterol biosynthesis follows a circadian rhythm and is principally produced at night (between 12:00 am and 6:00 am). The adjustment of hypolipaemic therapy to biologic rhythms is known as chronotherapy. Chronotherapy is based on the idea that medication can have different effects depending on the hour at which it is taken. Statins are one of the most widely used drugs for the prevention of cardiovascular events. In usual clinical practice, statins are administered once per day without specifying the time when they should be taken. It is unknown whether the timing of statin administration is important for clinical outcomes.
Objectives
To critically evaluate and analyse the evidence available from randomised controlled trials regarding the effects of chronotherapy on the effectiveness and safety of treating hyperlipidaemia with statins.
Search methods
We searched the CENTRAL, MEDLINE, Embase, LILACS, ProQuest Health & Medical Complete, OpenSIGLE, Web of Science Conference Proceedings, and various other resources including clinical trials registers up to November 2015. We also searched the reference lists of relevant reviews for eligible studies.
Selection criteria
We included randomised controlled trials (RCTs), enrolling people with primary or secondary hyperlipidaemia. To be included, trials must have compared any chronotherapeutic lipid‐lowering regimen with statins and any other statin lipid‐lowering regimen not based on chronotherapy. We considered any type and dosage of statin as eligible, as long as the control and experimental arms differed only in the timing of the administration of the same statin. Quasi‐randomised studies were excluded.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane. We extracted the key data from studies in relation to participants, interventions, and outcomes for safety and efficacy. We calculated odds ratios (OR) for dichotomous data and mean differences (MD) for continuous data with 95% confidence intervals (CI). Using the GRADE approach, we assessed the quality of the evidence and we used the GRADEpro Guideline Development Tool to import data from Review Manager to create 'Summary of findings' tables.
Main results
This review includes eight RCTs (767 participants analysed in morning and evening arms). The trials used different lipid‐lowering regimens with statins (lovastatin: two trials; simvastatin: three trials; fluvastatin: two trials; pravastatin: one trial). All trials compared the effects between morning and evening statin administration. Trial length ranged from four to 14 weeks. We found a high risk of bias in the domain of selective reporting in three trials and in the domain of incomplete outcome data in one trial of the eight trials included. None of the studies included were judged to be at low risk of bias.
None of the included RCTs reported data on cardiovascular mortality, cardiovascular morbidity, incidence of cardiovascular events, or deaths from any cause. Pooled results showed no evidence of a difference in total cholesterol (MD 4.33, 95% CI ‐1.36 to 10.01), 514 participants, five trials, mean follow‐up 9 weeks, low‐quality evidence), low‐density lipoprotein cholesterol (LDL‐C) levels (MD 4.85 mg/dL, 95% CI ‐0.87 to 10.57, 473 participants, five trials, mean follow‐up 9 weeks, low‐quality evidence), high‐density lipoprotein cholesterol (HDL‐C) (MD 0.54, 95% CI ‐1.08 to 2.17, 514 participants, five trials, mean follow‐up 9 weeks, low‐quality evidence) or triglycerides (MD ‐8.91, 95% CI ‐22 to 4.17, 510 participants, five trials, mean follow‐up 9 weeks, low‐quality evidence) between morning and evening statin administration.
With regard to safety outcomes, five trials (556 participants) reported adverse events. Pooled analysis found no differences in statins adverse events between morning and evening intake (OR 0.71, 95% CI 0.44 to 1.15, 556 participants, five trials, mean follow‐up 9 weeks, low‐quality evidence).
Authors' conclusions
Limited and low‐quality evidence suggested that there were no differences between chronomodulated treatment with statins in people with hyperlipidaemia as compared to conventional treatment with statins, in terms of clinically relevant outcomes. Studies were short term and therefore did not report on our primary outcomes, cardiovascular clinical events or death. The review did not find differences in adverse events associated with statins between both regimens. Taking statins in the evening does not have an effect on the improvement of lipid levels with respect to morning administration. Further high‐quality trials with longer‐term follow‐up are needed to confirm the results of this review.
Plain language summary
The effect of the timing of statin administration on hyperlipidaemia
Background
Cardiovascular disease (CVD), which comprises heart attacks (myocardial infarction), angina, and strokes, is the principal cause of death in the world and is a major cause of morbidity worldwide. High blood cholesterol is linked to CVD events and is an important risk factor. Therefore, decreasing high blood cholesterol is an important way to reduce the chances of suffering a CVD event. Blood cholesterol may come from foods that are high in fat, and is also produced by some of our body’s organs (most of this production is at night (between 12:00 am and 6:00 am).
Statins ‐ cholesterol‐lowering drugs ‐ (e.g. simvastatin, lovastatin, pravastatin, atorvastatin) are the first‐choice treatments for preventing CVD when high blood cholesterol exists. In usual clinical practice, statins are given once per day, without specifying the time when they should be taken. The aim of this review is to analyse whether the timing of taking the statin influences the reduction of CVD events, improves blood cholesterol levels, or affects treatment safety.
Study characteristics
We found eight randomised controlled trials that compared the effects between morning and evening statin administration in 767 people. Each trial evaluated different types and doses of statins. These trials were published between 1990 and 2013 and were conducted in the USA, Canada, Germany, Finland, Japan, South Korea and Thailand. This review includes evidence identified up to November 2015.
Key results
No trials assessed CVD clinical events or deaths. Evaluation of the available evidence indicated that there were no differences between evening or morning administration of statins in terms of lipid levels (total cholesterol, low‐density lipoprotein cholesterol (LDL‐C), high‐density lipoprotein cholesterol (HDL‐C), triglycerides). Additionally, there was no difference in the rate of adverse events associated with statins between both regimens.
Quality of the evidence
The evidence in this review is of low quality because of study limitations and imprecision. Larger studies are required to confirm these results.
Summary of findings
Summary of findings for the main comparison. Chronotherapy for the treatment of hyperlipidaemia.
| Chronotherapy for the treatment of hyperlipidaemia | ||||||
|
Participant or population: people with hyperlipidaemia
Settings: primary care
Intervention: evening statin dose Comparison: morning statin dose | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Morning statin dose | Evening statin dose | |||||
| Cardiovascular mortality | See comment | See comment | Not estimable | 0 (0) | See comment | Included RCTs did not report on this outcome |
| Cardiovacular morbidity | See comment | See comment | Not estimable | 0 (0) | See comment | Included RCTs did not report this outcome |
| At least 1 adverse event Follow‐up: mean 9 weeks | Study population | OR 0.71 (0.44 to 1.15) | 556 (5 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 188 per 1000 | 141 per 1000 (92 to 210) | |||||
| Total Cholesterol (mg/dL) Follow‐up: mean 9 weeks | The mean total cholesterol (mg/dL) in the intervention groups was 4.33 higher (1.36 lower to 10.01 higher) | 514 (5 studies) | ⊕⊕⊝⊝ low1,2 | |||
| LDL‐C (mg/dL) Follow‐up: mean 9 weeks | The mean LDL‐C (mg/dL) in the intervention groups was 4.85 higher (0.87 lower to 10.57 higher) | 473 (5 studies) | ⊕⊕⊝⊝ low1,2 | |||
| HDL‐C (mg/dL) Follow‐up: mean 9 weeks | The mean HDL‐C (mg/dL) in the intervention groups was 0.54 higher (1.08 lower to 2.17 higher) | 514 (5 studies) | ⊕⊕⊝⊝ low1,2 | |||
| Triglycerides (mg/dL) Follow‐up: mean 9 weeks | The mean triglycerides (mg/dL) in the intervention groups was 8.91 lower (22 lower to 4.17 higher) | 510 (5 studies) | ⊕⊕⊝⊝ low1,2 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded due to study limitations (unclear or high risk of bias) in the studies included. 2 Downgraded for imprecision due to very wide confidence interval.
Background
Description of the condition
Coronary cardiopathy (disease of the blood vessels that supply the cardiac muscle) and cerebrovascular disease (disease of the blood vessels that supply the brain) are the most frequent cardiovascular diseases (CVD) (WHO 2009). CVD represents the principal cause of death in the world as suggested by the following data obtained from different sources: 17.1 million people died due to CVD in 2004, which represents 29% of total deaths worldwide (WHO 2009). CVD affects women and men equally in all countries (WHO 2009), independently of their income level (Graham 2007; Lloyd‐Jones 2010; Petersen 2005; Redberg 2009; WHO 2009; WHOSIS 2009). In Europe, CVD causes more than 4.3 million deaths each year (almost half of total deaths) (European Heart Network 2008), and in the USA it causes one out of every three deaths, which means more deaths than those caused by cancer, chronic lower respiratory diseases and accidents combined (Lloyd‐Jones 2010). CVD will possibly continue to be the principal cause of death in the world in future (WHO 2009).
CVD is mainly caused by obstructions in the blood vessels that supply the heart or brain due to the formation of fat deposits (atheromas). The flow of blood to the heart or brain is thus made more difficult. One of the principal factors that is clearly associated with the formation of these fat deposits is the presence of high cholesterol levels in the blood. For this reason, hypercholesterolaemia is one of the principal risk factors for CVD (WHO 2009).
Description of the intervention
Statins are the first choice for lipid‐lowering agents according to the principal clinical practice guidelines (NICE 2008; San Vicente 2008; Stone 2014). Their mechanism of action is based on the inhibition of one of the initial steps of cholesterol biosynthesis (Smith 2009). Cholesterol synthesis is principally at night (between 12:00 am and 6:00 am) following a circadian rhythm repeated every 24 hours (Galman 2005; Jones 1990; Parker 1982; Santosa 2007). This periodic synthesis would allow for the adjustment of hypolipaemic therapy to biologic rhythms, which is known as chronotherapy. Chronotherapy is based on the idea that medication can have different effects depending on the hour at which it is taken (Sánchez 2005). However, in usual clinical practice statins are administered once per day, without specifying the time of day when they should be taken and, therefore, without taking into consideration the circadian rhythm of cholesterol (NZGG 2003; San Vicente 2008; SIGN 2007).
How the intervention might work
Elevated levels of total cholesterol and low‐density lipoprotein cholesterol (LDL‐C) play an important role in the development of atheromas and, therefore, in CVD (Kannel 1979; Kannel 1992; Keys 1980; LaRosa 2003; Tyroler 1990). A reduction in LDL‐C levels to those recommended by the clinical guidelines has shown a favourable effect on cardiovascular morbidity/mortality (Downs 1998; Pedersen 2004; Sacks 1996; Shepherd 1995). Thus, for example, a reduction of 1% can decrease the risk of coronary disease by 2% (Baigent 2010; LRC‐CPPT 1984), and a reduction of 1 mmol/L can reduce the risk of stroke by 10% (Law 2003).
Different types of cardiovascular events (myocardial infarction, sudden death and stroke) follow a circadian rhythm, with an increase in incidence between 6:00 am and 12:00 pm (Cohen 1997; Cooke 1994; Elliott 1998; ISIS‐2 1992; Muller 1994). It is plausible that this excess in cardiovascular risk at certain hours of the day is parallel to the circadian pattern of variables like blood pressure or cholesterol synthesis (Cooke‐Ariel 1998; Kozak 2003).
Statins have been shown to decrease the risk of CVD for secondary prevention (Baigent 2005; Institute for Clinical Systems Improvement 2009). In principle, statins with a shorter half‐life (one to five hours) would be more effective if taken in the evening, whereas those with a longer half‐life could be equally effective when taken at any hour of the day. This is because the period of greatest activity for short half‐life statins (i.e. lovastatin, simvastatin) would coincide with the cholesterol biosynthesis peak. A systematic review evaluated the effect of statins on blood cholesterol levels according to the time they were taken (morning versus evening) and concluded that there were sufficient data to support the evening administration of simvastatin to achieve an optimal lowering of LDL‐C levels (Plakogiannis 2007). The review also concluded that rigorous and robust trials were necessary to determine the best administration time to achieve optimal LDL‐C lowering for lovastatin, pravastatin, rosuvastatin, atorvastatin, and fluvastatin (Plakogiannis 2007). However, some studies have suggested that a morning administration of some statins is associated with a smaller reduction in LDL‐C levels, as compared to evening administration (specifically, 8.5 mg/dL smaller) (Haffner 1995; Hunninghake 1990; Saito 1991).
Why it is important to do this review
There are studies that have been completed or are currently being undertaken on applied chronotherapy for the treatment of cardiovascular risk factors in hypertension (Zhao 2003) or hyperlipidaemia (Plakogiannis 2007), or in other pathologies, including colorectal cancer (Liao 2010) and glaucoma (Luu 2010). However, Cochrane lacks systematic reviews on the effects of chronotherapy on the effectiveness and safety of hyperlipidaemia treatment for statins.
In people with high cardiovascular risk, statins are one of the most utilised drugs for the prevention of cardiovascular events (Graham 2007). Cronotherapy can be easily applied to any type of patient and it is economical. These advantages could improve the effectiveness, safety and efficiency of statin treatment.
Objectives
To critically evaluate and analyse the evidence available from randomised controlled trials (RCTs) regarding the effects of chronotherapy on the effectiveness and safety of treating hyperlipidaemia with statins.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs) as eligible regardless of their publication status or duration. We did not include quasi‐randomised controlled trials (see glossary in Appendix 1).
Types of participants
People of any age (at start of trial) with a confirmed diagnosis of hyperlipidaemia. We admitted any definition of hyperlipidaemia as long as it was reported by the study’s authors or deducible according to any current or past definition. We considered people with primary or secondary hyperlipidaemia and at any risk of cardiovascular disease (with or without history of cardiovascular disease). Primary hyperlipidemias are those caused by specific genetic abnormalities; secondary hyperlipidemias or acquired hyperlipidemias are those resulting from another underlying disorder, such as diabetes, hypothyroidism, nephrotic syndrome, chronic renal failure, obstructive liver disease or drugs (NCEP 2002). We did not consider those studies evaluating the chronobiologic effects of the administration of statins in normolipidaemic people as eligible.
Types of interventions
Intervention
Any chronotherapeutic lipid‐lowering regimen with statins. Chronotherapy or ‘chronomodulated therapy’, is defined as the practice of administering medical treatment at certain times of the day that are thought to be optimal for enhanced activity or lessened toxicity (Stedman 2010).
Comparison
Any other statin lipid‐lowering regimen not based on chronotherapy.
We included studies that assessed the effects of the timing of statin administration in its efficacy and safety (for example, comparing evening versus morning administration of lovastatin). We considered any type and dosage of statin as eligible, as long as the control and experimental arms differed only by the timing of the administration of the same statin.
Types of outcome measures
Primary outcomes
Efficacy outcomes
Cardiovascular mortality (reported as dichotomous data, when possible), defined as mortality secondary to myocardial infarction, unstable angina, heart failure, stroke, peripheral artery disease, complication of vascular procedures, or sudden death.
Cardiovascular morbidity (reported as dichotomous data, when possible), such as non‐fatal angina, myocardial infarction, peripheral vascular events, or stroke.
Global incidence of cardiovascular events (reported as dichotomous data, when possible), including cardiovascular deaths and non‐fatal cardiovascular events.
Deaths from any cause (reported as dichotomous data, when possible).
We planned, when possible, to group outcome data into those measured at six months, at one year, at two years, and at more than two years.
Safety outcomes
We considered an ‘adverse effect’ to be an unfavourable outcome that occurred during or after the use of a drug or other intervention for which the causal relation between the intervention and the event was at least a reasonable possibility (Loke 2011). When various types of adverse effects were reported, in order to address them in a more organised manner, we tried to narrow down this broad focus.
We considered the following safety outcomes associated with statins (reported as dichotomous data).
Participants with at least one adverse effect.
Participants with at least one serious adverse effect, as defined by the study authors.
Participants with myopathy or myotoxicity, as defined by the study authors.
Participants with liver dysfunction. We considered any definition supported by the study authors, such as elevated transaminases up to three times the normal levels.
Participants reporting symptoms possibly caused by the drug, such as muscle pain or gastrointestinal symptoms.
Participants with any other adverse effects considered as relevant by the study authors.
We did not consider participants who withdrew or dropouts as surrogate markers for safety or tolerability because of its potential for bias (Loke 2011).
Secondary outcomes
-
Change in lipid levels (mg/dL). The 'change' means the difference between the values at the baseline and at the end of follow‐up (reported as quantitative data, when possible).
Total cholesterol (TC)
Low‐density lipoprotein cholesterol (LDL‐C)
High‐density lipoprotein cholesterol (HDL‐C)
Triglycerides
Coronary revascularisation (angioplasty or bypass grafting), reported as dichotomous data, when possible.
Quality of life (measured with a validated scale).
Compliance with treatment (reported as dichotomous data, when possible).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to find reports of relevant RCTs.
The Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library Issue 10, 2015) (searched on 27 November 2015);
MEDLINE In process (Ovid; 1946 to 27 November 2015) (searched on 27 November 2015);
Embase (Ovid; 1980 to 2015 week 47) (searched on 30 November 2015);
LILACS (BIREME; 1982 to October 2012) (searched on 18 October 2012);
Science Citation Index Expanded (SCI‐EXPANDED) Web of Science (Thomson Reuters; 1970 to 28 November 2015) (searched on 30 November 2015);
Conference Proceedings Citation Index ‐ Science (CPCI‐S) Web of Science (Thomson Reuters; 1990 to 28 November 2015) (searched on 30 November 2015);
ProQuest Health & Medical Complete (searched on 5 November 2012).
We designed exhaustive searches for each database; combining them with appropriate methodological filters to retrieve RCTs. The sensitivity‐maximising version of the Cochrane RCT filter (Lefebvre 2011) was applied to MEDLINE and adaptations of it to the other databases where appropriate. Details of these strategies are available in Appendix 2. No restrictions regarding language or date of publication were used.
Searching other resources
We searched OpenGrey (www.opengrey.eu) and checked the following proceedings and abstracts presented at relevant conferences and meetings from 1987 (first statin authorized by the FDA) to April 2013:
World Organization of Family Doctors (WONCA);
European Society of Cardiology (ESC);
EuroPRevent;
American Heart Association (AHA);
American College of Cardiology (ACC);
American Society of Health‐System Pharmacists (ASHSP)
International Society of Chronobiology (ISC);
American Association of Medical Chronobiology and Chronotherapeutics;
World Congress of Chronobiology (WCC);
Sociedad Española de Cardiología;
Sociedad Española de Medicina de Familia y Comunitaria (SEMFyC).
We searched the following clinical trials registers for ongoing trials and trial results in July 2012:
Clinicaltrials.gov (www.clinicaltrials.gov);
International Standard Randomized Controlled Trial Number Register (www.controlled‐trials.com/isrctn/);
International Clinical Trials Registry Platform (www.who.int/trialsearch/);
Clinical Study results (www.clinicalstudyresults.org/).
We checked the reference lists of all relevant studies identified to find additional relevant citations (for example, systematic reviews and all included studies). We searched the Cochrane Database of Systematic Reviews to identify related reviews (19 October 2012). We also searched the Science Citation Index Expanded (SCI‐EXPANDED, Web of Science) to identify additional articles of interest that have cited the studies included in the review (10 March 2013).
We contacted experts in the field and the contact author of each included study to find out about further published or unpublished studies eligible for inclusion.
Data collection and analysis
Selection of studies
At least two review authors (JMIP, JMFT, AAA, PGA, IFE, LCS, PMC, OPL) independently checked the titles and abstracts resulting from the searches on electronic databases and classified them into three groups: ‘exclude’, ‘unsure’ or ‘potentially eligible’, using a form developed to document the process. We retrieved the full‐text versions of all those references classified as ‘unsure’ or ‘potentially eligible’ for definitive assessment of eligibility. At that stage, we only excluded those papers classified by both review authors as ‘exclude’.
We tried to obtain further information about any trial published only as an abstract. If a full report was not available, and we could not obtain the information from the study authors after 30 days, we excluded the abstract.
Using another form developed to document the process, we classified the full‐text copies into three groups (‘exclude’, ‘unsure’, or ‘include’), according to pre‐stated criteria (see Criteria for considering studies for this review). We resolved any disagreement through discussion. If finally there was no consensus, we consulted a third review author. If there was insufficient information to determine the eligibility of a study (full texts classified as ‘unsure’), we added the article to those 'awaiting assessment' and we asked the study authors for clarification. If finally we could not obtain the information, we excluded the study. We have detailed all relevant studies labelled as ‘excluded’ after the assessment of the full text, with the reasons for their exclusion, in the Characteristics of excluded studies.
We did not mask trial results or publication details during the selection of the studies.
Data extraction and management
At least two review authors (JMIP, JMFT, AAA, PGA, IFE, LCS, PMC, OPL) independently extracted the data from trial reports, using specially designed data extraction forms. We piloted this template in five trials to ensure its suitability. We extracted information about the methods used in the trial reports and details of:
participants (inclusion and exclusion criteria, number in each group, age, gender, setting, comparability at baseline regarding risk factors for cardiovascular disease, etc);
interventions (dosage, schedule, compliance, timing, comparison group etc);
duration of the follow‐up;
outcomes (primary and secondary outcomes);
results; and
risk of bias and other information.
The review authors resolved discrepancies on data extraction through discussion and the re‐examination of study reports. Where there was no consensus, a third review author settled the discrepancies.
When the lipid levels were expressed as mmol/L, they were transformed to mg/dL (total cholesterol, LDL‐C and HDL‐C: 1 mmol/l = 38.6697 mg/dL; triglycerides: 1 mmol/l = 88.5739 mg/dL). The studies included a mixture of change‐from‐baseline and final value scores. For each study, we used the difference in means (and SD) for the change of lipid levels between baseline and post‐treatment. We tried to extract or calculate this information from the report. When we could not obtain this information, we imputed it (see Dealing with missing data).
One review author (MNP) entered the data into Review Manager 5 (RevMan) (RevMan 2014) and another checked the data entered manually (JMIP). Studies reported in non‐English language journals were translated before assessment.
Dealing with duplicate publications
Where more than one publication relating to the same trial existed, we only included the study once, and used the most complete data from all the publications available.
Assessment of risk of bias in included studies
We assessed the risk of bias of each included study, according to the criteria of the Cochrane tool for assessing risk of bias (Higgins 2011a). We considered the following domains.
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data addressed (attrition bias)
Selective reporting (reporting bias)
Other bias
At least two review authors (JMIP, JMFT, AAA, PGA, IFE, LCS, PMC, OPL), not masked to the study details, had independently labelled each domain as a low, high, or unclear risk of bias. We resolved any disagreements by discussion and consensus and, if necessary, with the involvement of a third review author (MNP or JMIP). Overall, we summarised the risk of bias for each outcome in two different manners: (Higgins 2011a).
Within each study across domains: we defined each outcome (or class of outcomes) as having a ‘low risk of bias’ only if it met all the domains; as ‘high risk of bias’ if it demonstrated high risk of bias for one or more of them; or as ‘unclear risk of bias’ if it demonstrated unclear risk of bias for at least one domain without any of them described as ‘high risk of bias’.
Across studies: we defined each outcome (or class of outcomes) as having a ‘low risk of bias’ if most information was from studies at low risk of bias; as ‘high risk of bias’ if the proportion of information from studies at high risk of bias was sufficient enough to affect the interpretation of the results; or as ‘unclear risk of bias’ if most information was from studies at low or unclear risk of bias.
Measures of treatment effect
We performed the analyses using the RevMan 5 (RevMan 2014) statistical package provided by Cochrane and using Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011) as a guide. For dichotomous outcomes, we expressed results as odds ratios (OR) with 95% confidence intervals (CI). For continuous data we used the mean difference (MD) with standard deviations (SD).
Dealing with missing data
We described missing outcomes of the included studies by reporting proportions of randomised participants for whom no outcome data was obtained (with reasons) by outcome and by randomised group. We addressed the potential impact of the missing outcomes on the results of the included studies in the assessment of risk of bias and we described its impact on the findings of the review in the discussion section.
For all outcomes, we tried to carry out ‘analyses on an intention‐to‐treat’ principle (see glossary in Appendix 1). We planned to contact the primary study authors to request missing data and for the clarification of issues. Where we could not obtain this information, we performed an ‘available case analysis’ (see glossary in Appendix 1).
Regarding ‘change in lipid levels’, most studies did not report the SD of the change (Deeks 2011, section 9.4.5.2). Where unreported, we tried to obtain this information by looking carefully in the report for statistics that allow for its calculation (Higgins 2011b, section 7.7.3). If finally it was not possible to calculate, we imputed the missing SDs following the suggestions provided by section 16.1.3.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
Contacting trialists
For unpublished data, or where data were incomplete in published papers, we planned to obtain the information from the primary study authors.
Assessment of heterogeneity
We checked for heterogeneity considering the following factors:
The characteristics of the studies (clinical or methodological).
The forest plot of results of the studies. We checked the presence or absence of overlap in the confidence intervals of their results visually.
The results of the Chi² test for statistical heterogeneity (we considered trial results as heterogeneous if P < 0.10).
The results of the I² statistic for the quantification of the heterogeneity (Higgins 2003). We judged the importance of the observed value of the I² statistic depending on the magnitude and direction of effects and the strength of evidence for heterogeneity (moderate to high heterogeneity defined as I² at 50% or more) (Deeks 2011).
Assessment of reporting biases
We attempted to minimise reporting bias by including both published and unpublished studies, by extracting data on outcomes from the publication with the most mature data (in the case of studies with multiple publications), and by not excluding studies solely on the basis of the publication language. We planned to assess publication bias in two different ways: graphically, by visual assessment of funnel plots (see glossary in Appendix 1), and statistically, following guide provided by Sterne 2011 for statistical testing for funnel plot asymmetry.
Data synthesis
We combined the outcome measures from the individual trials in a meta‐analysis to provide a pooled effect estimate for each outcome only if the studies were clinically and methodologically comparable. We meta‐analysed data using a fixed‐effect model. Where significant heterogeneity existed (I² statistic was more than 50%), we used a random‐effects model. We carried out sensitivity analyses to assess the effect of the statistical model chosen for meta‐analysis (fixed‐effect model versus random‐effects model) (see Sensitivity analysis).
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses when we detected statistically significant differences between treatment groups and if there were enough studies.
Age (mean or median): under 30 years versus 30 to 65 years versus more than 65 years.
Gender: 50% or more versus fewer than 50% of participants were male.
Diabetes: 25% or more versus fewer than 25% of participants were diabetic.
Previous cardiovascular events: 25% or more versus fewer than 25% of participants with previous cardiovascular events.
Mean duration of treatment with statins: less than 12 months versus 12 months or more.
Mean LDL‐C baseline levels (mg/dL): less than 100 mg/dL versus 100 mg/dL to 129 mg/dL versus 130 mg/dL or more.
There were insufficient studies in each comparison to perform the subgroup analysis planned. We decided to perform a post‐hoc subgroup analysis based on the different follow‐up of the studies.
Sensitivity analysis
We performed the following sensitivity analyses.
Assessing the effect of the statistical model chosen for meta‐analysis (fixed‐effect model versus random‐effects model).
Exploring the influence of missing data: we made a sensitivity analysis that was not planned in protocol to explore the influence on effect size of studies with losses greater than 25%.
Repeating the meta‐analysis using relative risks (RR) for dichotomous outcomes.
For future updates, we plan to perform the following sensitivity analyses, when possible.
Risk of bias: excluding studies with any domain assessed as 'low' or 'unclear'.
Assumptions taken in the ‘available case analysis’:
for dichotomous outcomes, considering the ‘best‐case’ and ‘worst‐case’ scenarios (Gamble 2005). We defined the ‘best‐case’ scenario as all participants with missing outcomes in the experimental intervention group having good outcomes and all those with missing outcomes in the control intervention group having poor outcomes; the ‘worst‐case’ scenario will be the converse (Higgins 2011c); and
for continuous data, we plan to conduct a sensitivity analysis assuming a fixed difference between the actual mean for the missing data and the mean assumed by the analysis (Higgins 2011c).
Study size: repeating the meta‐analysis excluding very large studies (if present).
We plan to repeat the meta‐analysis excluding any unpublished studies.
Results
Description of studies
Results of the search
Our search identified 7482 potential studies. Of these, we retrieved 28 for further investigation by screening titles and abstracts. We have listed one study (Nakaya 1990) in the table of Characteristics of studies awaiting classification, pending a Japanese translation in future updates. We translated one trial from Japanese (Nakaya 1995). After full‐text assessment, we included eight studies. The study selection process is shown in Figure 1.
1.
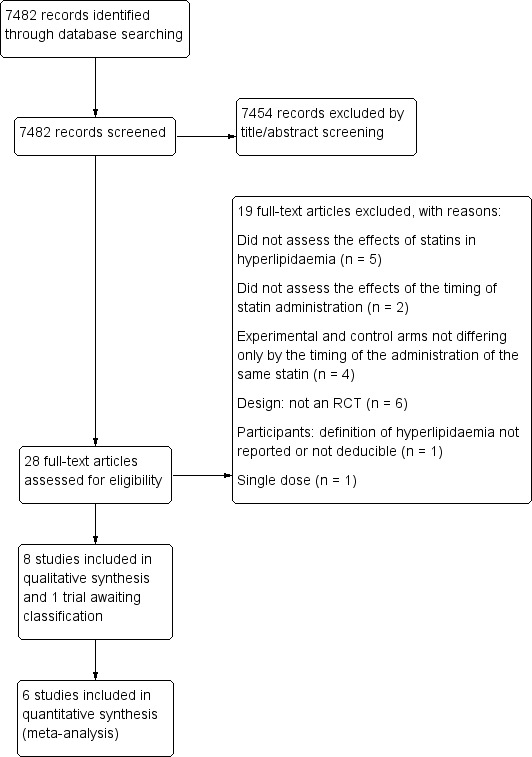
Study flow diagram
Included studies
Types of studies
All included studies were parallel RCTs. The trials were conducted worldwide (Germany (Kruse 1993; Scharnagl 2006), USA (Davignon 1990; Hunninghake 1990), Canada (Davignon 1990), Finland (Davignon 1990), Japan (Nakaya 1995), South Korea (Kim 2013), and Thailand (Tharavanij 2010).
Treatment duration varied from four weeks in two studies (Kruse 1993; Nakaya 1995), eight weeks in three studies (Hunninghake 1990; Kim 2013; Scharnagl 2006), 12 weeks in two (Saito 1991; Tharavanij 2010) and 14 weeks in one study (Davignon 1990). The mean follow‐up was nine weeks. No trials were stopped prematurely.
Types of participants
All included studies enrolled participants with hyperlipidaemia although not all using the same criteria.
Total analysed participants in morning and evening arms were 767, ranging from 22 to 234 among studies included. The mean age of participants was 56 years, and 57.6% were women. Nakaya 1995 only included men.
Baseline total cholesterol levels ranged from 237 mg/dL (Kim 2013) to 435 mg/dL (Kruse 1993), LDL‐C from 158 mg/dL (Kim 2013) to 360 mg/dL (Kruse 1993), HDL‐C from 38 mg/dL (Kruse 1993) to 58 mg/dL (Scharnagl 2006), and triglycerides from 127 mg/dL (Hunninghake 1990) to 191 mg/dL (Nakaya 1995).
Interventions
All trials compared the effect between morning and evening statin administration. However, when the statin was taken was not homogeneous across studies:
Kruse 1993 compared 6:00 am to 10:00 am versus 5:00 pm to 9:00 pm;
Tharavanij 2010 compared 6:00 am to 10:00 am versus 7:00 pm to 10:00 pm;
Davignon 1990 compared before morning meal versus before evening meal;
Saito 1991 compared after morning meal versus after evening meal;
Hunninghake 1990 compared before breakfast versus bedtime; and
three studies did not specify the time of day when the statin was taken (Kim 2013; Nakaya 1995; Scharnagl 2006).
The analysed statins were lovastatin (two studies, doses of 20 mg (Kruse 1993) and 40 mg (Davignon 1990)), simvastatin (three studies, doses of 2.5 mg and 5 mg (Saito 1991), 10 mg (Tharavanij 2010) and 20 mg (Kim 2013)), fluvastatin (two studies, doses of 10 mg (Nakaya 1995) and 80 mg (Scharnagl 2006)), and pravastatin (one study, dose of 40 mg (Hunninghake 1990)).
Four studies (Davignon 1990; Hunninghake 1990; Nakaya 1995; Saito 1991) were multi‐arm trials (see Characteristics of included studies). In Davignon 1990 we considered two arms for the analyses (40 mg daily dose of lovastatin in the morning versus evening administration). In Hunninghake 1990 we analysed two arms (40 mg daily dose of pravastatin in the morning versus evening administration). In Nakaya 1995 we analysed two arms (10 mg fluvastatin in the morning versus evening administration). In Saito 1991 we combined the results of two arms with different doses of statin (2.5 mg and 5 mg of simvastatin in a morning dose) and the results of two arms with the same doses of statin (2.5 mg and 5 mg of simvastatin) in an evening dose.
Outcomes
Primary Outcomes
None of the included studies provided data on deaths from any cause or cardiovascular mortality or morbidity.
Five of the studies (Kim 2013; Nakaya 1995; Saito 1991; Scharnagl 2006; Tharavanij 2010) reported all the safety outcomes we considered.
Participants with at least one adverse effect (Kim 2013; Nakaya 1995; Saito 1991; Scharnagl 2006; Tharavanij 2010).
Participants with at least one serious adverse effect, as defined by the study authors (Kim 2013; Scharnagl 2006; Tharavanij 2010).
Participants with myopathy or myotoxicity, as defined by the study authors (Kim 2013; Nakaya 1995; Tharavanij 2010).
Participants with liver dysfunction (Kim 2013; Nakaya 1995; Saito 1991; Scharnagl 2006; Tharavanij 2010).
Participants reporting symptoms possibly caused by the drug, such as gastrointestinal symptoms (Kim 2013; Nakaya 1995; Saito 1991; Scharnagl 2006).
Secondary outcomes
All the included studies reported the baseline lipid levels of total cholesterol, LDL‐C, HDL‐C, and triglycerides, and the levels at the end of the study, but only one (Kim 2013) presented the difference in means (with standard deviation) in changes of lipid levels from the baseline.
Four trials analysed compliance with treatment, in different ways: by counting the number of pills initially prescribed and those returned by the participant on the last visit day (Davignon 1990; Kim 2013); by pill count, percentage of prescribed doses taken, defined as the number of opening/closing events recorded divided by the number of prescribed doses in the period, multiplied by 100; and time compliance, defined as percentage of total dosing events recorded within the defined time intervals of 6:00 am to 10:00 am for the morning regime and 5:00 pm to 9:00 pm for the evening regime (Kruse 1993). One study did not report the method used for measuring drug compliance (Tharavanij 2010). Only one study reported enough information to know the SD (Kruse 1993).
None of the included studies provided data on coronary revascularisation or quality of life.
Excluded studies
See Characteristics of excluded studies.
We excluded 19 full‐text articles:
Six studies were not RCTs (Arca 2007; Erdogan 2010; Illingworth 1986; Nozaki 1996; Plakogiannis 2005; Schwartz 2009).
Five studies did not assess the effects of statins in hyperlipidaemia (Cilla 1996; Martin 2002; Triscari 1995; Wallace 2003; Yoon 2011).
Two studies did not assess the effects of the timing of statin administration (Kele, 2008; Matsuzawa 1991).
In four studies, experimental and control arms did not differ only by the timing of the administration of the same statin (Dujovne 1994; Hunninghake 1998; Insull 1994; Stein 1997).
One study did not consider hyperlipidaemia as an inclusion criteria (Lund 2002).
One study had an unacceptable lipid‐lowering regimen (single dose) (Fauler 2007).
Studies awaiting classification
There was one study awaiting classification (Nakaya 1990).
Risk of bias in included studies
We have described risk of bias in the Characteristics of included studies section and illustrated it in Figure 2 and Figure 3. In total, we deemed four trials to be at an unclear risk of bias (Kim 2013; Kruse 1993; Nakaya 1995; Scharnagl 2006), with the remainder considered to be at high risk (Davignon 1990; Hunninghake 1990; Saito 1991; Tharavanij 2010). None of the studies included was judged to be at low risk of bias.
2.
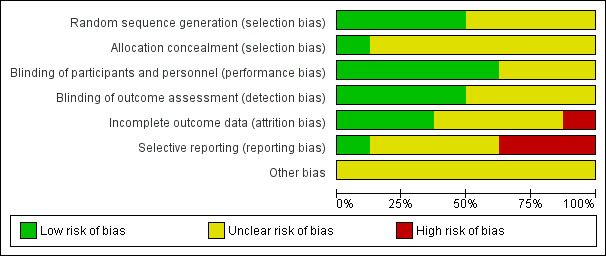
Risk of bias graph: authors' judgements about each risk of bias item presented as percentages across all included studies
3.
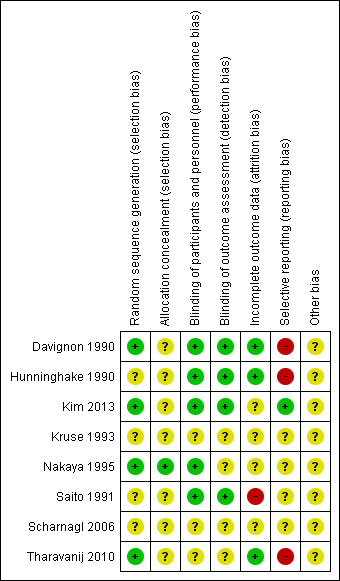
Risk of bias summary: authors' judgements about each risk of bias item for each included study
Allocation
All included trials reported that the allocation sequence was generated randomly, although only four contained enough information about the methodology used to allocate treatments: Davignon 1990; Tharavanij 2010 and Nakaya 1995 used a blocked randomisation method for random sequence generation and Kim 2013 used a random‐number table.
Two studies described allocation concealment: Nakaya 1995 used central allocation and Saito 1991 used sequentially numbered envelopes. However, we judged Saito 1991 to have an unclear risk of bias, as the report did not include whether or not the assignment envelopes were used with appropriate safeguards.
Blinding
In five trials, it was possible that participants and personnel were blinded (Davignon 1990; Hunninghake 1990; Kim 2013; Nakaya 1995; Saito 1991). Two trials that blinded participants and personnel did not appear to blind outcome assessment (Hunninghake 1990; Nakaya 1995). Blinding of outcome assessment was only explicitly stated in one study (Davignon 1990). We also judged the risk of bias to be low for blinding of outcome assessment in Hunninghake 1990 and Saito 1991 because the determination of serum lipid parameters was realised in a central laboratory. Three trials did not report any information about blinding (Kruse 1993; Scharnagl 2006; Tharavanij 2010).
Incomplete outcome data
The duration of follow‐up varied between four (Kruse 1993) and 14 weeks (Davignon 1990). Three studies reported post‐randomisation losses of less than 10% (Davignon 1990; Hunninghake 1990; Tharavanij 2010) during the study; two studies described losses between 10% and 20% (Kim 2013; Scharnagl 2006); one study reported losses greater than 25% (Saito 1991). One trial reported no losses (Nakaya 1995). One trial did not report number of withdrawals, but the denominator values suggested complete follow‐up (Kruse 1993). Two studies reported the number and reasons for losses separately for both arms (Hunninghake 1990; Kim 2013). The Kim 2013 trial described different attrition in both arms (9.4% versus 19.1%). The remaining three studies did not report reasons for losses to follow‐up (Saito 1991; Scharnagl 2006; Tharavanij 2010).
Selective reporting
The study protocol was not available for almost all studies, so it was difficult to make a judgment on the possibility of selective reporting bias. Four of the eight studies included did not provide enough information to assess the risk of bias and were judged as having an unclear risk of bias (Kruse 1993; Nakaya 1995; Saito 1991; Scharnagl 2006). A high risk of bias was considered in three trials (Davignon 1990; Hunninghake 1990; Tharavanij 2010 ), given that some relevant results were described incompletely. Davignon 1990 reported data only in a graph and Hunninghake 1990 did not report the number of participants analysed in each arm. Only Kim 2013 was considered as having a low risk of bias.
Other potential sources of bias
The overall assessment for other potential sources of bias was unclear. There was not enough information to assess whether an important risk of bias existed.
Effects of interventions
See: Table 1
The eight included trials (1129 participants randomised) evaluated chronotherapeutic lipid‐lowering regimens with statins in 767 people. All trials compared the effect between morning and evening statin administration. We present results for the primary and secondary outcomes of the review, if they were evaluated in the study, and where information was available.
Primary outcomes
Efficacy outcomes
None of the included RCTs reported data on efficacy outcomes: cardiovascular mortality, cardiovascular morbidity, incidence of cardiovascular events, or deaths from any cause.
Safety outcomes
Five trials (556 participants) reported the incidence of adverse events (Kim 2013; Nakaya 1995; Saito 1991; Scharnagl 2006; Tharavanij 2010). Ninety‐two adverse events were reported. None of these trials individually found a difference in the rate of adverse events between morning and evening statin regimens.
Meta‐analysis of the five trials (Kim 2013; Nakaya 1995; Saito 1991; Scharnagl 2006; Tharavanij 2010) showed no differences in the incidence of at least one adverse event between the two statin schedules (Analysis 2.1: OR 0.71, 95% CI 0.44 to 1.15, I2 = 0%, 556 participants). Only Scharnagl 2006 reported two serious adverse events (elevation in the ratio between the concentrations of the enzymes alanine transaminase (ALT) and aspartate transaminase (AST)to more than 3 upper limits of normal (ULN) in two consecutive visits of one participant) in the evening‐dose group. Kim 2013 and Tharavanij 2010 reported that no participant presented serious adverse events (Analysis 2.2: OR 0.21, 95% CI 0.01 to 4.43, 418 participants).
2.1. Analysis.
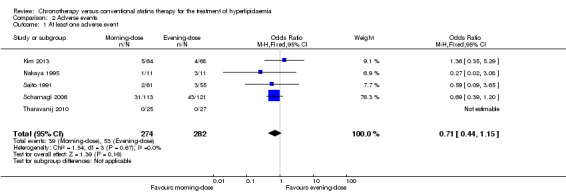
Comparison 2 Adverse events, Outcome 1 At least one adverse event.
2.2. Analysis.
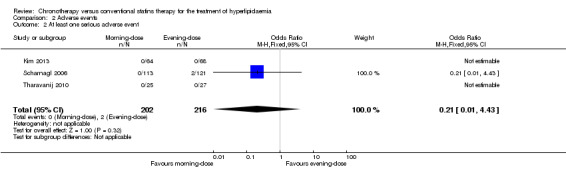
Comparison 2 Adverse events, Outcome 2 At least one serious adverse event.
No difference was found in the incidence of adverse events classified as:
myopathy or myotoxicity (three trials: Kim 2013; Nakaya 1995; Tharavanij 2010; Analysis 2.3): OR 0.33, 95% CI 0.03 to 3.28, 206 participants;
liver dysfunction (five trials: Kim 2013; Nakaya 1995; Saito 1991; Scharnagl 2006; Tharavanij 2010; Analysis 2.4): OR 1.42, 95% CI 0.27 to 7.44, 551 participants; or
gastrointestinal symptoms (four trials: Kim 2013; Nakaya 1995; Saito 1991; Scharnagl 2006; Analysis 2.5): OR 1.17, 95% CI 0.46 to 3.00, 504 participants.
2.3. Analysis.
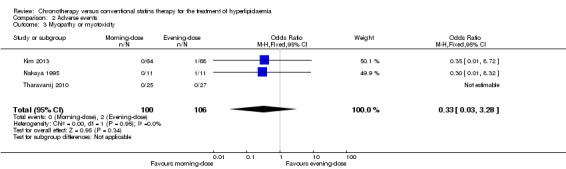
Comparison 2 Adverse events, Outcome 3 Myopathy or myotoxicity.
2.4. Analysis.
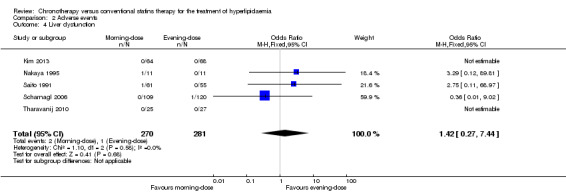
Comparison 2 Adverse events, Outcome 4 Liver dysfunction.
2.5. Analysis.

Comparison 2 Adverse events, Outcome 5 Gastrointestinal symptoms.
Secondary outcomes
Total cholesterol (mg/dL)
Five trials provided data on total cholesterol (Kim 2013; Kruse 1993; Nakaya 1995; Saito 1991; Scharnagl 2006). The chronotherapeutic lipid‐lowering regimen had no effect on total cholesterol (mg/dL) (Analysis 1.1: (MD 4.33, 95% CI ‐1.36 to 10.01), 514 participants, low‐quality evidence). Three studies (Kruse 1993; Nakaya 1995; Scharnagl 2006) reported cholesterol data with follow‐up at four weeks and two studies reported data with follow‐up at eight weeks (Kim 2013; Scharnagl 2006). Both pooled analyses showed no effect between morning and evening statin administration (Analysis 1.2: MD 3.88, 95% CI ‐3.66 to 11.43, 275 participants and MD 1.01, 95% CI ‐5.43 to 7.45, 352 participants, respectively). There was no significant heterogeneity in these analyses.
1.1. Analysis.
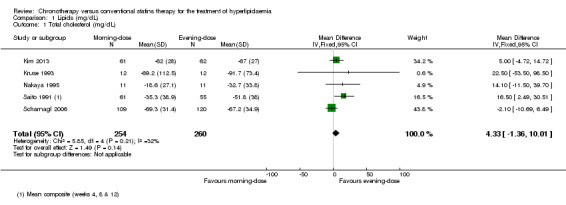
Comparison 1 Lipids (mg/dL), Outcome 1 Total cholesterol (mg/dL).
1.2. Analysis.
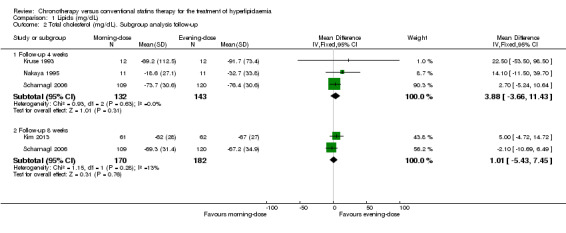
Comparison 1 Lipids (mg/dL), Outcome 2 Total cholesterol (mg/dL). Subgroup analysis follow‐up.
LDL‐C (mg/dL)
We found low‐quality evidence that chronotherapeutic lipid‐lowering regimen with statins had no effect on LDL‐C (mg/dL) levels (Analysis 1.5: MD 4.85 mg/dL, 95% CI ‐0.87 to 10.57, five trials, 473 participants, median follow‐up 4 weeks, I2 = 0%). These trials reported LDL‐C levels at different time points. Five studies (Kruse 1993; Nakaya 1995; Saito 1991; Scharnagl 2006; Tharavanij 2010) compared morning versus evening statin administration at four weeks. Pooled results showed lowered lipid levels (LDL‐C) with the evening regimen (Analysis 1.6: MD 12.30, 95% CI 2.40 to 22.20, 405 participants, I2 = 26%). Similarly the comparison between both regimens followed up at eight weeks, based on four studies (Kim 2013; Saito 1991; Scharnagl 2006; Tharavanij 2010), showed a small effect with the evening regimen (Analysis 1.6: MD 8.81, 95% CI 0.21 to 17.42, 480 participants, I2 = 52%). Only one study (Saito 1991) reported data based on 12 weeks' follow‐up, and there was no difference in LDL‐C levels between the two schedules (Analysis 1.6: MD 14, 95% CI ‐3.49 to 31.49, 107 participants).
1.5. Analysis.
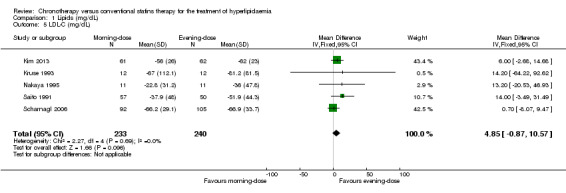
Comparison 1 Lipids (mg/dL), Outcome 5 LDL‐C (mg/dL).
1.6. Analysis.
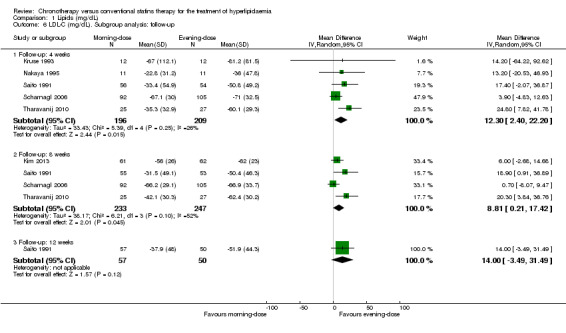
Comparison 1 Lipids (mg/dL), Outcome 6 LDL‐C (mg/dL). Subgroup analysis: follow‐up.
HDL‐C (mg/dL)
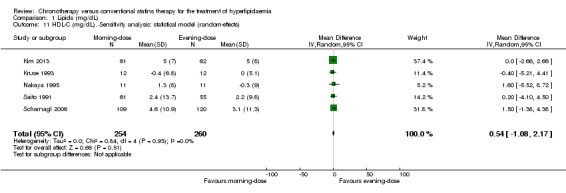
Five trials provided data on HDL‐C (mg/dL) (Kim 2013; Kruse 1993; Nakaya 1995; Saito 1991; Scharnagl 2006). We found no difference between morning and evening statin schedules on HDL‐C levels (Analysis 1.9: MD 0.54, 95% CI ‐1.08 to 2.17, 514 participants, low‐quality evidence).
1.9. Analysis.

Comparison 1 Lipids (mg/dL), Outcome 9 HDL‐C (mg/dL).
Statin regimens did not present an effect on HDL‐C levels in studies with follow‐up at 4 weeks (Kruse 1993; Nakaya 1995; Scharnagl 2006) (Analysis 1.10: MD 0.28, 95% CI ‐2.02 to 2.57, 275 participants, I2 = 0%). Studies with follow‐up at eight weeks (Kim 2013; Scharnagl 2006) also showed no differences between the two statin schedules (Analysis 1.10: MD 0.69, 95% CI ‐1.26 to 2.64, 352 participants, I2 = 0%).
1.10. Analysis.
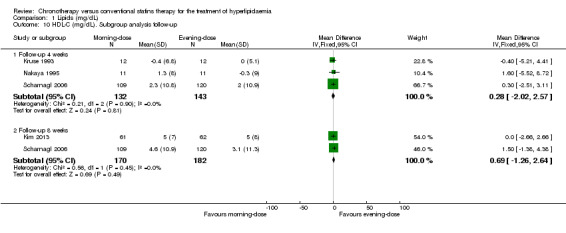
Comparison 1 Lipids (mg/dL), Outcome 10 HDL‐C (mg/dL). Subgroup analysis follow‐up.
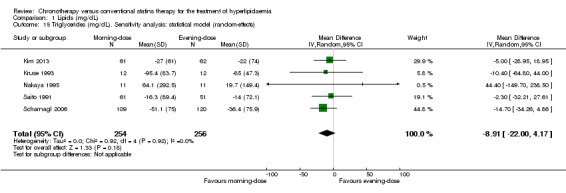
Triglycerides (mg/dL)
Five trials provided data on triglycerides levels (Kim 2013; Kruse 1993; Nakaya 1995; Saito 1991; Scharnagl 2006). Meta‐analysis of these trials did not introduce differences between the morning and evening group (Analysis 1.13: MD ‐8.91, 95% CI ‐22 to 4.17, 510 participants, I2 = 0%, low‐quality evidence). Three studies reported data with follow‐up at four weeks (Kruse 1993; Nakaya 1995; Scharnagl 2006) (Analysis 1.14: MD ‐8.24, 95% CI ‐26.58 to 10.09, 275 participants, I2 = 0%). Three studies reported data with follow‐up at eight weeks (Hunninghake 1990; Kim 2013; Scharnagl 2006) (Analysis 1.14: MD ‐10.82, 95% CI ‐25.97 to 4.33, 352 participants, I2 = 0%).
1.13. Analysis.
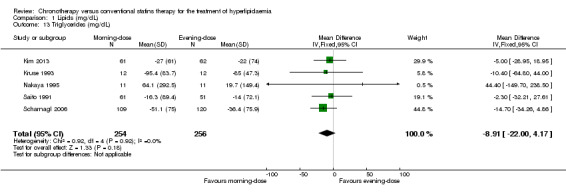
Comparison 1 Lipids (mg/dL), Outcome 13 Triglycerides (mg/dL).
1.14. Analysis.
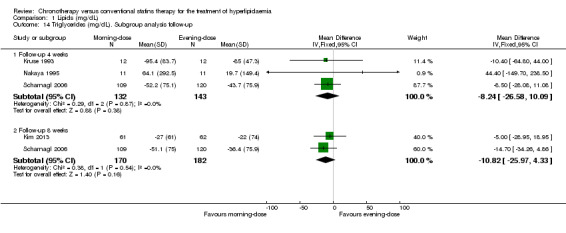
Comparison 1 Lipids (mg/dL), Outcome 14 Triglycerides (mg/dL). Subgroup analysis follow‐up.
Coronary revascularisation
No study reported coronary revascularisation associated with chronotherapeutic lipid‐lowering regimens.
Quality of life
No study reported health‐related quality of life measures.
Compliance with treatment
In Kruse 1993, there were no significant differences between the participant groups with regard to time compliance (Analysis 3.1: MD 4.60, 95% CI ‐16.05 to 25.25, 24 participants).
3.1. Analysis.
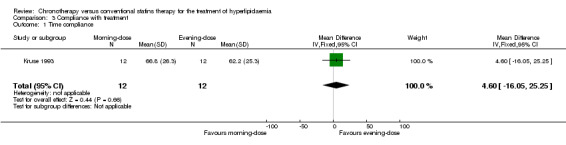
Comparison 3 Compliance with treatment, Outcome 1 Time compliance.
The other three studies that examined adherence were not included in meta‐analysis because they did not provide the SD and the estimation method was different. In Davignon 1990, the average dose actually taken (based on medication returned) was 96% of the specified dose for the morning and evening groups. In Kim 2013, compliance (by measuring the number of pills initially prescribed against those returned at the end of the study) was calculated to be 91.5% in the morning‐dose group and 92.3% in the evening‐dose group (P = 0.935). In Tharavanij 2010, compliance (the method used was not reported) did not differ between the two groups (96%).
Thus, compliance was good and similar in both groups in all trials.
Sensitivity analysis
Statistical model for meta‐analysis
We performed a sensitivity analysis using a random‐effects model. As expected, there were no differences between the results of any outcome with respect to the analysis under the fixed‐effect model because the heterogeneity was low (Analysis 1.3; Analysis 1.7; Analysis 1.11; Analysis 1.15).
1.3. Analysis.

Comparison 1 Lipids (mg/dL), Outcome 3 Total cholesterol (mg/dL). Sensitivity analysis: statistical model (random‐effects).
1.7. Analysis.
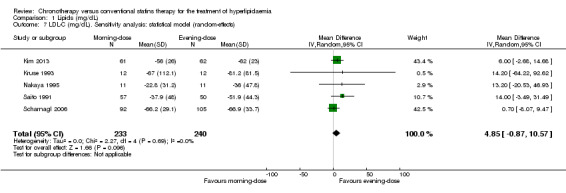
Comparison 1 Lipids (mg/dL), Outcome 7 LDL‐C (mg/dL). Sensitivity analysis: statistical model (random‐effects).
1.11. Analysis.
Comparison 1 Lipids (mg/dL), Outcome 11 HDL‐C (mg/dL). Sensitivity analysis: statistical model (random‐effects).
1.15. Analysis.
Comparison 1 Lipids (mg/dL), Outcome 15 Triglycerides (mg/dL). Sensitivity analysis: statistical model (random‐effects).
Levels of missing data
Most of the included trials had low levels of missing data (less than 20%). Only one trial reported losses greater than 25% (Saito 1991). We performed a sensitivity analysis to explore the impact of the levels of missing data on the chronotherapy statin regimen for the lipid levels. Excluding Saito 1991, similar findings were demonstrated: LDL‐C (Analysis 1.8: MD 3.76, 95% CI ‐2.29 to 9.81, 366 participants, I2 = 0%); total cholesterol (Analysis 1.4: MD 1.93, 95% CI ‐4.29 to 8.15, 398 participants, I2 = 0%); HDL‐C (Analysis 1.12: MD 0.60, 95% CI ‐1.15 to 2.35, 398 participants, I2 = 0%); and triglycerides (Analysis 1.16: MD ‐10.48, 95% CI ‐25.03 to 4.08, 398 participants, I2 = 0%).
1.8. Analysis.
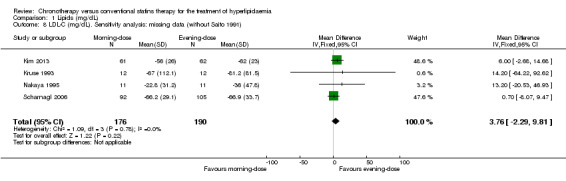
Comparison 1 Lipids (mg/dL), Outcome 8 LDL‐C (mg/dL). Sensitivity analysis: missing data (without Saito 1991).
1.4. Analysis.
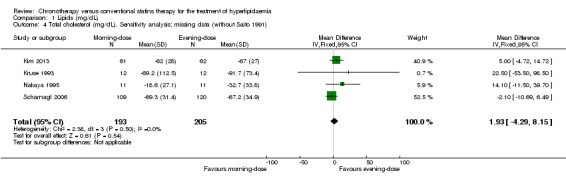
Comparison 1 Lipids (mg/dL), Outcome 4 Total cholesterol (mg/dL). Sensitivity analysis: missing data (without Saito 1991).
1.12. Analysis.
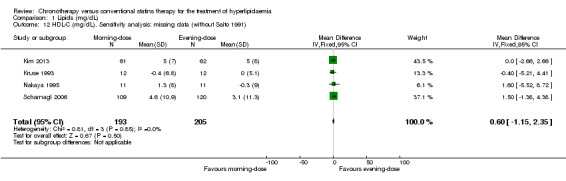
Comparison 1 Lipids (mg/dL), Outcome 12 HDL‐C (mg/dL). Sensitivity analysis: missing data (without Saito 1991).
1.16. Analysis.

Comparison 1 Lipids (mg/dL), Outcome 16 Triglycerides (mg/dL). Sensitivity analysis: missing data (without Saito 1991).
Measures of effects size chosen for meta‐analysis
We repeated the meta‐analysis using relative risks (RR) for dichotomous outcomes (adverse events). There were no significant differences in the results (see Analysis 2.6; Analysis 2.7; Analysis 2.8; Analysis 2.9; Analysis 2.10).
2.6. Analysis.
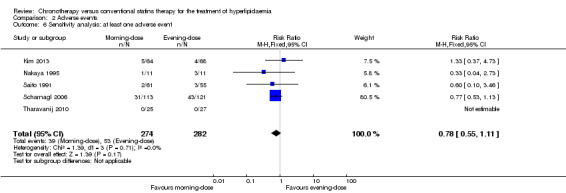
Comparison 2 Adverse events, Outcome 6 Sensitivity analysis: at least one adverse event.
2.7. Analysis.

Comparison 2 Adverse events, Outcome 7 Sensitivity analysis: at least one serious adverse event.
2.8. Analysis.
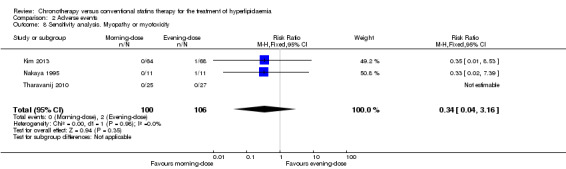
Comparison 2 Adverse events, Outcome 8 Sensitivity analysis. Myopathy or myotoxicity.
2.9. Analysis.

Comparison 2 Adverse events, Outcome 9 Sensitivity analysis. Liver disfunction.
2.10. Analysis.
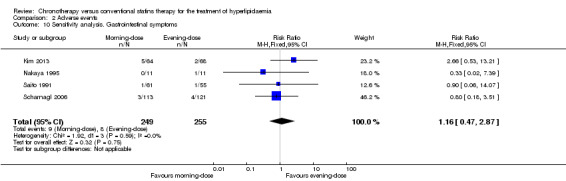
Comparison 2 Adverse events, Outcome 10 Sensitivity analysis. Gastrointestinal symptoms.
Discussion
Summary of main results
We did not find RCTs that analysed the influence of chronotherapy of statins in cardiovascular mortality, cardiovascular morbidity, incidence of cardiovascular events, or deaths from any cause.
Only five trials (556 participants) of low quality reported adverse events (92 adverse events). We found no difference in adverse events between morning and evening statin regimens. In any case, the data should be taken with caution because of the short‐term follow‐up (mean of 9 weeks) and the low number of events that occurred.
We found low‐quality evidence about the influence of chronotherapy on lipid levels. When statins were administered in the evening, we did not find any effect on the improvement of total cholesterol, LDL‐C, HDL‐C, or triglycerides levels with respect to morning administration.
Overall completeness and applicability of evidence
Only one trial (Kim 2013) provided data on the difference in means and standard deviation in changes of lipid levels from the baseline. For the rest of the studies, we imputed the missing SDs from Kim 2013, following the suggestions provided by section 16.1.3.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
Two studies were not included in meta‐analysis: Davignon 1990 (because the efficacy results were only shown in a graph and the report did not allow for the SD of the change in lipid levels at the end of the study to be imputed) and Hunninghake 1990 (because data were reported as a geometric mean and the number of participants analysed was not clear).
In one study (Saito 1991), we combined the results of the two arms with different doses of statin, but with the same administration timing, as indicated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
Four of the included trials (Davignon 1990; Hunninghake 1990; Nakaya 1995; Saito 1991) had more comparative arms than the interest of this review, and this could decrease the robustness of the trials' findings.
The confidence in the results of the review is low due to the studies' limitations and imprecision.
Quality of the evidence
We used the GRADE approach to assess the quality of the evidence (GRADE Working Group 2004) and the GRADEpro Guideline Development tool (GRADEpro GDT) to import data from RevMan 5 (RevMan 2014) to create 'Summary of findings' tables. None of the included RCTs reported data on our primary efficacy outcomes: cardiovascular mortality, cardiovascular morbidity, incidence of cardiovascular events, or deaths from any cause.
Only low‐quality evidence was available for the meta‐analysis for the safety outcomes and the change in lipid levels (Kim 2013; Kruse 1993; Nakaya 1995; Saito 1991; Scharnagl 2006; Tharavanij 2010). We downgraded it due to the risk of methodological bias and imprecision due to the confidence interval being too wide. Therefore, further research is very likely to have an important impact on our confidence in the estimate regarding effect, and is likely to change the estimate.
Potential biases in the review process
We used Cochrane methodology to conduct a comprehensive search to identify all related available trials.
Regarding the ‘change in lipid levels’, all but one study (Kim 2013) did not report the SD of the change. Where unreported, we imputed the missing SDs following the suggestions provided by section 16.1.3.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). Since we have not used the original data from the studies, differences in the results may arise.
All included studies enrolled participants with hyperlipidaemia although not all used the same criteria. In fact, the baseline total cholesterol levels ranged from 237 mg/dL (Kim 2013) to 435 mg/dL (Kruse 1993), LDL‐C from 158 mg/dL (Kim 2013) to 360 mg/dL (Kruse 1993), HDL‐C from 38 mg/dL (Kruse 1993) to 58 mg/dL (Scharnagl 2006) and triglycerides from 127 mg/dL (Hunninghake 1990) to 191 mg/dL (Nakaya 1995).
Agreements and disagreements with other studies or reviews
We have found only one review related to the chronotherapy efficacy of statins (Plakogiannis 2007) which had several differences with regard to our work. It is a narrative review without a comprehensive search of studies (until 2006). They included seven studies, but only two of them fulfil our requirements (Hunninghake 1990; Saito 1991). Two of them were not RCTs (Illingworth 1986; Plakogiannis 2005), the other two analysed a healthy population (Cilla 1996; Martin 2002) and Wallace 2003 people without hyperlipidaemia. Their conclusions support the evening administration of simvastatin based only on the study of Saito 1991. Our analysis, which includes more studies (only RCTs concerning people with hyperlipidaemia), suggest that there is no difference in efficacy when the statin is administered in the evening.
Authors' conclusions
Implications for practice.
The available evidence shows no difference in effect of different timings of statin intake. The statin administration in the evening instead of the morning does not have an effect on the improvement of total cholesterol, LDL‐C, HDL‐C, or triglycerides levels. There were no differences in adverse events between morning and evening intake.
Implications for research.
It is necessary to study the effects of chronotherapy in cardiovascular mortality, cardiovascular morbidity, incidence of cardiovascular events, or deaths from any cause. Future randomised trials should be designed and conducted rigorously, especially the randomisation procedure, the blinding of participants, and evaluators of outcomes, and should be reported correctly. It is not clear if the statin half‐life may play a role in the effect of the timing of the administration and there are not enough studies with each statin to reach a conclusion on this issue.
Acknowledgements
We acknowledge the contribution of Jesús López Alcalde in the protocol development and support in the first phase of the review.
We acknowledge the contribution of Abel González and Sergio Maeso‐Martínez in the selection of the studies.
We acknowledge the contribution of Marta García Solano in the co‐ordination of the protocol.
Iberoamerican Cochrane Centre for support in the translation of the background section.
Referees and Cochrane Heart Group editors.
Appendices
Appendix 1. Glossary
| Available case analysis | Analysis that includes data on only those whose results are known, using as a denominator the total number of people who had data recorded for the particular outcome in question (Higgins 2011c). |
| Funnel plot | Simple scatter plot of the intervention effect estimates from individual studies against some measure of each study’s size or precision (Sterne 2011). |
| Intention‐to‐treat analysis | Analysis that fulfils the next principles: 1) keeps participants in the intervention groups to which they were randomised, regardless of the intervention they actually received; 2) there is a measurement of outcome data on all participants; and 3) includes all randomised participants in the analysis (Higgins 2011c). |
| Quasi‐randomised controlled clinical trial (Q‐RCT) | Type of study where the participants (or groups of participants) are assigned prospectively to an intervention or to a control group (or more) using a process that attempts but does not achieve true randomisation (for example, alternation of allocation, birth dates or week days). |
| Small‐study effects | A tendency for estimates of the intervention effect to be more beneficial in smaller studies (Sterne 2011). |
Appendix 2. Search strategies
The reference for the Cochrane precision‐maximising RCT filter used for MEDLINE and terms as suggested to be used as RCT filter for Embase is: Lefebvre 2011
CENTRAL
#1 MeSH descriptor Hyperlipidemias explode all trees
#2 MeSH descriptor Dyslipidemias, this term only
#3 MeSH descriptor Cholesterol, this term only
#4 MeSH descriptor Cholesterol, HDL, this term only
#5 MeSH descriptor Cholesterol, LDL, this term only
#6 (hyperlipid?emia*)
#7 (cholesterol*)
#8 (cholesteryl)
#9 (lip?emia*)
#10 (hypercholesterol?emia*)
#11 (hypercholester?emia*)
#12 (hyperlip?emia*)
#13 (triglycerid*)
#14 (hypertriglycerid?emia*)
#15 (dyslipid?emia*)
#16 (lipoprotein*)
#17 (dyslipidprotein?emia*)
#18 hyperlipoprotein?emia*
#19 (LDL)
#20 (HDL)
#21 (lipid* near/2 low*)
#22 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21)
#23 MeSH descriptor Chronotherapy explode all trees
#24 MeSH descriptor Circadian Rhythm explode all trees
#25 MeSH descriptor Drug Administration Schedule, this term only
#26 (chronotherap*)
#27 (chronomod*)
#28 (chronopharm*)
#29 (circadian)
#30 (morning):ti or (morning):ab
#31 (afternoon):ti or (afternoon):ab
#32 (evening):ti or (evening):ab
#33 (night):ti or (night):ab
#34 (time related)
#35 ((drug )near/6 (time* or rhythm*))
#36 ((medicat*) near/6 (time* or rhythm*))
#37 (#23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36)
#38 (#22 AND #37)
MEDLINE Ovid
1 exp Hyperlipidemias/
2 Dyslipidemias/
3 Cholesterol/
4 Cholesterol, HDL/
5 Cholesterol, LDL/
6 hyperlipid?emia*.tw.
7 cholesterol*.tw.
8 cholesteryl.tw.
9 lip?emia*.tw.
10 hypercholesterol?emia*.tw.
11 hypercholester?emia*.tw.
12 hyperlip?emia*.tw.
13 triglycerid*.tw.
14 hypertriglycerid?emia*.tw.
15 dyslipid?emia*.tw.
16 lipoprotein*.tw.
17 dyslipidprotein?emia*.tw.
18 hyperlipoprotein?emia*.tw.
19 LDL.tw.
20 HDL.tw.
21 (lipid* adj2 low*).tw.
22 or/1‐21
23 exp Chronotherapy/
24 exp Circadian Rhythm/
25 Drug Administration Schedule/
26 chronotherap*.tw.
27 chronomod*.tw.
28 chronopharm*.tw.
29 circadian.tw.
30 morning.ti,ab.
31 afternoon.ti,ab.
32 evening.ti,ab.
33 night.ti,ab.
34 time related.tw.
35 ((drug or medicat*) adj6 (time* or rhythm*)).tw.
36 or/23‐35
37 22 and 36
38 randomized controlled trial.pt.
39 controlled clinical trial.pt.
40 randomized.ab.
41 placebo.ab.
42 clinical trials as TS.sh.
43 randomly.ab.
44 trial.ti.
45 38 or 39 or 40 or 41 or 42 or 43 or 44
46 exp animals/ not humans.sh.
47 45 not 46
48 37 and 47
Embase Ovid
1 exp Hyperlipidemias/
2 Dyslipidemias/
3 Cholesterol/
4 Cholesterol, HDL/
5 Cholesterol, LDL/
6 hyperlipid?emia*.tw.
7 cholesterol*.tw.
8 cholesteryl.tw.
9 lip?emia*.tw.
10 hypercholesterol?emia*.tw.
11 hypercholester?emia*.tw.
12 hyperlip?emia*.tw.
13 triglycerid*.tw.
14 hypertriglycerid?emia*.tw.
15 dyslipid?emia*.tw.
16 lipoprotein*.tw.
17 dyslipidprotein?emia*.tw.
18 hyperlipoprotein?emia*.tw.
19 LDL.tw.
20 HDL.tw.
21 (lipid* adj2 low*).tw.
22 or/1‐21
23 exp Chronotherapy/
24 exp Circadian Rhythm/
25 Drug Administration Schedule/
26 chronotherap*.tw.
27 chronomod*.tw.
28 chronopharm*.tw.
29 circadian.tw.
30 morning.ti,ab.
31 afternoon.ti,ab.
32 evening.ti,ab.
33 night.ti,ab.
34 time related.tw.
35 ((drug or medicat*) adj6 (time* or rhythm*)).tw.
36 or/23‐35
37 22 and 36
38 random$.tw.
39 factorial$.tw.
40 crossover$.tw.
41 cross over$.tw.
42 cross‐over$.tw.
43 placebo$.tw.
44 (doubl$ adj blind$).tw.
45 (singl$ adj blind$).tw.
46 assign$.tw.
47 allocat$.tw.
48 volunteer$.tw.
49 crossover procedure/
50 double blind procedure/
51 randomized controlled trial/
52 single blind procedure/
53 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52
54 (animal/ or nonhuman/) not human/
55 53 not 54
56 37 and 55
LILACS
(MH:Hyperlipidemias OR MH:Dyslipidemias OR MH:Cholesterol OR MH:"Cholesterol, HDL" OR MH:"Cholesterol, LDL" OR TW:hyperlipid?emia* OR TW:cholesterol* OR TW:cholesteryl OR TW:lip?emia* OR TW:hyperocholesterol?emia* OR TW:hypercholester?emia* OR TW:hyperlip?emia* OR TW:triglycerid* OR TW:hypertriglycerid?emia* OR TW:dyslipid?emia* OR TW:lipoprotein* OR TW:dyslipidprotein?emia* OR TW:hyperlipoprotein?emia* OR TW:LDL OR TW:HDL OR TW:(LIPID* ADJ2 LOW*))AND (MH:Chronotherapy OR MH:"Circadian Rhythm" OR MH:"Drug Administration Schedule" OR TW:chronotherap* OR TW:chronomod* OR TW:chronopharm* OR TW:circadian OR TI:morning OR TI:afternoon OR AB:morning OR AB:afternoon OR TI:evening OR TI:night OR AB:evening OR AB:night OR TW:"time related" OR TW:((drug OR medicat*) ADJ6 (time* OR rhythm*)))
Science Citation Index Expanded (SCI‐EXP) and Conference Proceedings Citation Index – Science (CPCI‐S) on Web of Science (Thomson Reuters)
# 31 #30 AND #29
# 30 TS=((random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*))
# 29 #28 AND #17
# 28 #27 OR #26 OR #25 OR #24 OR #23 OR #22 OR #21 OR #20 OR #19 OR #18
# 27 TS=(((drug or medicat*) near/6 (time* or rhythm*)))
# 26 TS=("time related")
# 25 TS=(night)
# 24 TS=(evening)
# 23 TS=(afternoon)
# 22 TS=(morning)
# 21 TS=(circadian)
# 20 TS=(chronopharm*)
# 19 TS=(chronomod*)
# 18 TS=(chronotherap*)
# 17 #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
# 16 TS=(lipid* near/2 low*)
# 15 TS=(HDL)
# 14 TS=(LDL)
# 13 TS=(hyperlipoprotein?emia*)
# 12 TS=(dyslipidprotein?emia*)
# 11 TS=(lipoprotein*)
# 10 TS=(dyslipid?emia*)
# 9 TS=(hypertriglycerid?emia*)
# 8 TS=(triglycerid*)
# 7 TS=(hyperlip?emia*)
# 6 TS=(hypercholester?emia*)
# 5 TS=(hypercholesterol?emia*)
# 4 TS=(lip?emia*)
# 3 TS=(cholesteryl)
# 2 TS=(cholesterol*)
# 1 TS=(hyperlipid?emia*)
ProQuest Health & Medical Complete
(MESH.EXACT.EXPLODE("Hyperlipidemias") OR MESH.EXACT("Dyslipidemias") OR MESH.EXACT("Cholesterol") OR MESH.EXACT("Cholesterol, HDL") OR MESH.EXACT("Cholesterol, LDL") OR ft("hyperlipid?emia*" OR "cholesterol*" OR "cholesteryl" OR "lip?emia*" OR "hyperocholesterol?emia*" OR "hypercholester?emia*" OR "hyperlip?emia*" OR "triglycerid*" OR "hypertriglycerid?emia*" OR "dyslipid?emia*" OR "lipoprotein*" OR "dyslipidprotein?emia*" OR "hyperlipoprotein?emia*" OR "LDL" OR "HDL" OR ("LIPID*" ADJ2 "LOW*"))) AND (MESH.EXACT.EXPLODE("Chronotherapy") OR MESH.EXACT.EXPLODE("Circadian Rhythm") OR MESH.EXACT("Drug Administration Schedule") OR ft("chronotherap*" OR "chronomod*" OR "chronopharm*" OR "circadian") OR ti(("morning" OR "afternoon")) OR ab(("morning" OR "afternoon")) OR ti(("evening" OR "night")) OR ab(("evening" OR "night")) OR ft("time related") OR ft(("drug" OR "medicat*") ADJ6 ("time*" OR "rhythm*"))) AND (ft("randomized controlled trial") OR ft("controlled clinical trial") OR ab("randomized") OR ab("placebo") OR ("drug therapy") OR ab("randomly") OR ab("trial") OR ab("groups")) NOT (MESH.EXACT.EXPLODE("Animals") NOT mesh("humans"))
www.clinicalstudyresults.org
Generic Name : Atorvastatin, lovastatin, pravastatin sodium, fluvastatin, simvastatin, rosuvastatin, pitavastatin.
Study Name: Chronotherapy, chronotherapeutic, chronomodulated, chronopharmacology, circadian, afternoon, Chronobiology, Morning, Awakening, Evening, Night.
Clinicaltrials.gov
1)Conditions: Chronobiology disorders.
2) (Interventions: Hydroxymethylglutaryl‐CoA Reductase Inhibitors) AND (Free text search: Chronobiology or awakening or chronotherapy or chronotherapeutic or chronomodulated or chronopharmacology or afternoon or Morning or evening or night or circadian ).
3) (Conditions: Hyperlipidemias) AND (Free text search: Chronobiology or awakening or chronotherapy or chronotherapeutic or chronomodulated or chronopharmacology or afternoon or Morning or evening or night or circadian).
The ISRCTN registry (www.controlled‐trials.com/)
Free text search:
1) Chronotherapy
2) Chronotherapeutic, chronomodulated, chronobiology, chronopharmacology, Circadian
3) Statins and morning.
4) Statins and afternoon, statins and night, statins and awakening
5) Statins and evening
6) Statins and time
International Clinical Trials Registry Platform (ICTRP) www.who.int/trialsearch
Contained in the title: Chronobiology, chronopharmacology or chronomodulated or Chronotherap* or Circadian or Evening or Morning or Afternoon or Night or Awakening
Opengrey
(Chronotherapy OR Circadian OR Drug Administration Schedule OR chronomodulated OR chronopharmacology OR Circadian rhythm) AND (Hyperlipidemia OR Dyslipidemia OR Cholesterol OR+HDL OR LDL OR OR lipemia OR hypercholesteremia OR triglyceridenmia OR hypertriglyceridemia OR dyslipidemia OR lipoprotein OR dyslipidproteinemia OR hyperlipoproteinemia)
Data and analyses
Comparison 1. Lipids (mg/dL).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total cholesterol (mg/dL) | 5 | 514 | Mean Difference (IV, Fixed, 95% CI) | 4.33 [‐1.36, 10.01] |
| 2 Total cholesterol (mg/dL). Subgroup analysis follow‐up | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Follow‐up 4 weeks | 3 | 275 | Mean Difference (IV, Fixed, 95% CI) | 3.88 [‐3.66, 11.43] |
| 2.2 Follow‐up 8 weeks | 2 | 352 | Mean Difference (IV, Fixed, 95% CI) | 1.01 [‐5.43, 7.45] |
| 3 Total cholesterol (mg/dL). Sensitivity analysis: statistical model (random‐effects) | 5 | 514 | Mean Difference (IV, Random, 95% CI) | 5.73 [‐2.05, 13.52] |
| 4 Total cholesterol (mg/dL). Sensitivity analysis: missing data (without Saito 1991) | 4 | 398 | Mean Difference (IV, Fixed, 95% CI) | 1.93 [‐4.29, 8.15] |
| 5 LDL‐C (mg/dL) | 5 | 473 | Mean Difference (IV, Fixed, 95% CI) | 4.85 [‐0.87, 10.57] |
| 6 LDL‐C (mg/dL). Subgroup analysis: follow‐up | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Follow‐up: 4 weeks | 5 | 405 | Mean Difference (IV, Random, 95% CI) | 12.30 [2.40, 22.20] |
| 6.2 Follow‐up: 8 weeks | 4 | 480 | Mean Difference (IV, Random, 95% CI) | 8.81 [0.21, 17.42] |
| 6.3 Follow‐up: 12 weeks | 1 | 107 | Mean Difference (IV, Random, 95% CI) | 14.0 [‐3.49, 31.49] |
| 7 LDL‐C (mg/dL). Sensitivity analysis: statistical model (random‐effects) | 5 | 473 | Mean Difference (IV, Random, 95% CI) | 4.85 [‐0.87, 10.57] |
| 8 LDL‐C (mg/dL). Sensitivity analysis: missing data (without Saito 1991) | 4 | 366 | Mean Difference (IV, Fixed, 95% CI) | 3.76 [‐2.29, 9.81] |
| 9 HDL‐C (mg/dL) | 5 | 514 | Mean Difference (IV, Fixed, 95% CI) | 0.54 [‐1.08, 2.17] |
| 10 HDL‐C (mg/dL). Subgroup analysis follow‐up | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Follow‐up 4 weeks | 3 | 275 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐2.02, 2.57] |
| 10.2 Follow‐up 8 weeks | 2 | 352 | Mean Difference (IV, Fixed, 95% CI) | 0.69 [‐1.26, 2.64] |
| 11 HDL‐C (mg/dL). Sensitivity analysis: statistical model (random‐effects) | 5 | 514 | Mean Difference (IV, Random, 95% CI) | 0.54 [‐1.08, 2.17] |
| 12 HDL‐C (mg/dL). Sensitivity analysis: missing data (without Saito 1991) | 4 | 398 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐1.15, 2.35] |
| 13 Triglycerides (mg/dL) | 5 | 510 | Mean Difference (IV, Fixed, 95% CI) | ‐8.91 [‐20.00, 4.17] |
| 14 Triglycerides (mg/dL). Subgroup analysis follow‐up | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 Follow‐up 4 weeks | 3 | 275 | Mean Difference (IV, Fixed, 95% CI) | ‐8.24 [‐26.58, 10.09] |
| 14.2 Follow‐up 8 weeks | 2 | 352 | Mean Difference (IV, Fixed, 95% CI) | ‐10.82 [‐25.97, 4.33] |
| 15 Triglycerides (mg/dL). Sensitivity analysis: statistical model (random‐effects) | 5 | 510 | Mean Difference (IV, Random, 95% CI) | ‐8.91 [‐20.00, 4.17] |
| 16 Triglycerides (mg/dL). Sensitivity analysis: missing data (without Saito 1991) | 4 | 398 | Mean Difference (IV, Fixed, 95% CI) | ‐10.48 [‐25.03, 4.08] |
Comparison 2. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 At least one adverse event | 5 | 556 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.44, 1.15] |
| 2 At least one serious adverse event | 3 | 418 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.43] |
| 3 Myopathy or myotoxicity | 3 | 206 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.28] |
| 4 Liver dysfunction | 5 | 551 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.27, 7.44] |
| 5 Gastrointestinal symptoms | 4 | 504 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.46, 3.00] |
| 6 Sensitivity analysis: at least one adverse event | 5 | 556 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.55, 1.11] |
| 7 Sensitivity analysis: at least one serious adverse event | 3 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.41] |
| 8 Sensitivity analysis. Myopathy or myotoxicity | 3 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.16] |
| 9 Sensitivity analysis. Liver disfunction | 5 | 551 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.28, 7.15] |
| 10 Sensitivity analysis. Gastrointestinal symptoms | 4 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.47, 2.87] |
Comparison 3. Compliance with treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time compliance | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 4.60 [‐16.05, 25.25] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Davignon 1990.
| Methods | Study design: prospective, randomised, double‐blind, clinical trial Setting/location: 13 centres. Country: USA, Canada and Finland Period of study: not reported Unit of randomisation: participant Unit of analysis: participant | |
| Participants |
Inclusion criteria: primary hypercholesterolaemia, with elevated LDL‐C levels and normal triglycerides concentrations (type IIa phenotype) or with mild hypertriglyceridaemia (type IIb phenotype). The participants were at high risk for myocardial infarction Exclusion criteria: premenopausal status in women, unless highly unlikely to conceive, triglycerides level > 3.95 mmol/L, alcohol intake > 10 drinks per week, impaired hepatic function or abnormal results of liver function test; myocardial infarction or coronary bypass surgery within the previous 4 months; or diabetes mellitus or glucose intolerance, defined as a fasting glucose level > 7.8 mmol/L Enrolled: not reported Randomised: 290 M‐DG: n = 49; E‐DG: n = 47; lovastatin 80 mg evening: 49; and lovastatin 40 mg twice a day: 48; and probucol 500 mg: 97) Withdrawals: 7 M‐DG: n = not described, E‐DG: n = at least 1, other arms: n = at least 5). 1 was withdrawn because non co‐operation but was not reported the group Participants assessed (ITT analysis): 289 (not reported the group of the participant not analysed) Participants assessed (safety analysis): 289 (not reported the group of the participant not analysed) Definition of hyperlipidaemia: primary hypercholesterolaemia, with elevated LDL‐C levels and normal triglycerides concentrations (type IIa phenotype) or with mild hypertriglyceridaemia (type IIb phenotype) Baseline characteristics: Age (years) (mean (SD)): M‐DG: 48; E‐DG: 52.5; lovastatin 80 mg evening: 49.1; lovastatin 40 mg twice daily: 51; and probucol 500 mg: 49.4 Total cholesterol (TC) (mg/dL) (mean (SD)): M‐DG: 365.43 (SD not reported); E‐DG: 370.07 (SD not reported) LDL‐C (mg/dL) (mean (SD)): M‐DG: 289.64 (SD not reported); E‐DG: 296.60 (SD not reported) HDL‐C (mg/dL) (mean (SD)): M‐DG: 43.31 (SD not reported); E‐DG: 48.72 (SD not reported) Triglycerides (mg/dL) (mean (SD)): M‐DG: 143.49 (SD not reported); E‐DG: 117.80 (SD not reported) |
|
| Interventions |
Type of interventions: 40 mg morning vs 40 mg evening vs 80 mg evening vs 40 mg twice‐daily doses of lovastatin vs 500 mg twice‐daily doses of probucol M‐DG: 40 mg daily dose of lovastatin in the morning E‐DG: 40 mg daily dose of lovastatin in the evening Lovastatin 80 mg evening: 80 mg daily dose of lovastatin in the evening Lovastatin 40 mg twice daily: 40 mg twice‐daily dose of lovastatin (am + pm) Probucol 500 mg twice daily: 500 mg twice‐daily dose of probucol (am + pm) Duration of intervention: 14 weeks |
|
| Outcomes | Change in lipid levels: TC, LDL‐C, HDL‐C and triglycerides levels (mg/dL) Drug compliance (consumption) Safety outcomes |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were then randomised to 1 of 5 treatments after having been stratified by diagnosis" "The randomisation schedule was organized in blocks of 5, so as to ensure that within each centre the number of patients in each group was approximately equal" (page 23B trial report) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The double‐dummy technique was used to render the study double blind" "In the case of once‐each‐morning regimen and the once‐each‐evening regimen, a placebo capsule matching lovastatin was taken in the evening and morning respectively, to ensure that all patients took 1 capsule and 1 tablet twice daily." (page 23B trial report) Comment: Probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The laboratory maintained the results of the lipid and apolipoproteins analyses from 2 weeks onward and did not reveal them to the clinics or the coordinating center until the completion of the study" (page 23B trial report) Comment: biochemicals data analysed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “The study was completed by 283 of the 290 subjects enrolled” (7/290, 2.4%) "analyzable lipid data, using the all‐patients‐treated approach were available for 289 patients” (page 25B trial report) Comment: the percentage of withdrawals was very low; therefore we consider that there is a low risk of bias |
| Selective reporting (reporting bias) | High risk | The results are only shown in a graph without SD There is not protocol available |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias existed |
Hunninghake 1990.
| Methods | Study design: randomised, double‐blind, parallel, placebo‐controlled study Setting/location: six lipid treatment centres. Country: USA Period of study: not reported Unit of randomisation: participants Unit of analysis: participants | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Enrolled: 228 participants Randomised: 196 participants (not reported data by group) Withdrawals: 8 participants: 2 for personal reasons (1 morning and 1 placebo group), 2 for open heart surgery (1 evening and 1 twice‐daily group), 1 because of chronic lymphocytic leukaemia (evening group), 1 was lost to follow‐up (evening group), 1 was withdrawn because of erroneous randomisation and 1 due to gastrointestinal discomfort (placebo group) Participants assessed (efficacy): 184 participants (M‐DG: n = 48, E‐DG: n = 43, PG: n = 46 and twice‐daily group: n = 47). 12 participants were excluded from the efficacy analysis: 9 deviation from entrance criteria and 3 for change in concomitant medication potentially affecting lipid metabolism (page 222 trial report) Participants assessed (safety analysis): 196 participants Definition of hyperlipidaemia: fasting LDL‐C concentration ≥ 85th percentile for age and sex than North American populations and a Trygliceride concentration ≤ 250 mg/dL Baseline characteristics: Age (years) (mean): M‐DG: 53.3, E‐DG: 54, PG: 53.5 and twice‐daily group: 52.6 Total cholesterol (TC) (mg/dL) (mean (SD)): M‐DG: 320.2 (10.1), E‐DG: 320.6 (11.2), PG: 326 (9.3) and twice‐daily group: 322.9 (10.1) LDL‐C (mg/dL) (mean): M‐DG: 242.7, E‐DG: 244.6, PG: 249.8 and twice‐daily group: 244.7. HDL‐C (mg/dL) (mean (SD)): M‐DG: 44.5 (1.6), E‐DG: 44.5 (1.9), PG: 46.4 (1.6) and twice‐daily group: 44.1 (1.6) T riglycerides (mg/dL) (mean (SD)): M‐DG: 55.7 (3.1), E‐DG: 55.3 (4.6), PG: 55.3 (3.5) and twice‐daily group: 59.9 (3.5) |
|
| Interventions |
Type of interventions: morning vs evening vs twice‐daily doses of pravastatin
M‐DG: 40 mg daily dose of pravastatin in the morning
E‐DG: 40 mg daily dose of pravastatin in the evening
Twice‐daily group (TDG): 20 mg twice daily (am + pm) of pravastatin Placebo group (PG): placebo Duration of intervention: 14 weeks (figure 1 trial report) Pre‐randomisation period: 6 weeks Treatment phase: 8 weeks |
|
| Outcomes | Change in lipid levels: TC, LDL‐C, HDL‐C and triglycerides levels (mg/dL) Drug compliance (consumption) Safety outcomes |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Pravastatin and placebo were supplied as identical‐appearing tablets in blister cards. To maintain double‐blind conditions during the treatment phase, drug supplies were packaged so each patient received the same number of tablets each day." (page 221 trial report) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Blood chemistry analysis was performed centrally at the university of Cincinnati" (page 221 trial report) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "228 patients were enrolled, of which 196 qualified and were randomised to receive pravastatin or placebo. A total of 188 patients completed 8 weeks of double‐blind treatment." (8/196; 4.1%) "The included population (evaluated for efficacy) comprised 184 patients" (page 222 trial report) |
| Selective reporting (reporting bias) | High risk | Not reported the number of analysed patients in each arm There is no protocol available |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias existed |
Kim 2013.
| Methods |
Study design: prospective, randomised, double‐blind, phase III clinical trial
Setting/location: multicenter (hospitals: Seoul Metropolitan Boramae, Korea University Guro, Soonchunhyang University, Kangdong Sacred Heart, Ewha Womans University Mokdong, Bundang Jaesaeng General and Dongguk University Ilsan. Country: South Korea
Period of study: from 21 July 2008‐19 June 2009
Unit of randomisation: participants
Unit of analysis: participants Trial test: equivalence Analysis strategy: intention‐to‐treat |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Enrolled: 298 (130 participants finally did not follow the therapeutic lifestyle control and 36 participants did not meet the inclusion criteria after the therapeutic lifestyle control). Randomised: 132 (M‐DG: n = 64, E‐DG: n = 68) Excluded (post‐randomisation): 9 participants (3 participants in the M‐DG & 6 participants in the E‐DG). Reasons: in both arms the participants were excluded because they lacked blood tests. Participants assessed (ITT analysis): 123 (M‐DG: n = 61, E‐DG: n = 62). Participants assessed (safety analysis): 132 (M‐DG: n = 61, E‐DG: n = 62) Withdrawals: M‐DG: 3/61 (4.9%). Reasons: 1 participant discontinued due to constipation, 1 participant due to unspecified chest discomfort and 1 participant for refusal to follow‐up E‐DG: 7/62 (11.3%). Reasons: 2 participants were dropped due to concomitant medication (exclusion criteria), 1 participant for refusal to follow‐up, 4 participants for arthralgia, dyspepsia, dizziness, pyelonephritis and peripheral coldness Definition of hyperlipidaemia: LDL‐C between 100 mg/dL and 200 mg/dL Baseline characteristics: Female (%): M‐DG: 57.4, E‐DG: 53.2 Age (years) (mean (SD)): M‐DG: 58.7 (8.3), E‐DG: 58.5 (9.5) Total cholesterol (TC) (mg/dL) (mean (SD)): M‐DG: 236.1 (28.9), E‐DG: 238.4 (31.1) LDL‐C (mg/dL) (mean (SD)): M‐DG: 155.0 (22.3), E‐DG: 160.6 (25.0) HDL‐C (mg/dL) (mean (SD)): M‐DG: 48.6 (9.7), E‐DG: 50.3 (11.3) Triglycerides (mg/dL) (mean (SD)): M‐DG: 157.1 (65.2), E‐DG: 147.3 (63.1) Duration of hypercholesterolaemia (years) (mean): not reported |
|
| Interventions | Type of interventions: morning vs evening doses of simvastatin M‐DG: 20 mg daily dose of controlled‐release simvastatin (one 20 mg tablet) in the morning and a placebo tablet in the evening E‐DG: 20 mg daily dose of controlled‐release simvastatin (one 20 mg tablet) in the evening and a placebo tablet in the morning Duration of intervention: 8 weeks | |
| Outcomes |
Primary endpoint: change in LDL‐C levels (mg/dL) Secondary endpoints:
Safety outcomes: any adverse event including hepatic and renal functions, ECG and vital signs |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomly assigned using a randomisation table" (page 1351 trial report) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The processes were blinded during the entire study period" (page 1352 trial report) Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The processes were blinded during the entire study period" (page 1352 trial report) Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The percentage of randomised participants without known results can be significantly different in both arms (4.7 % vs 8.8%) (figure 1 trial report) (19/132; 14.4%) |
| Selective reporting (reporting bias) | Low risk | Clinical trial registered in ClinicalTrials.gov (Identifier: NCT00973115). All outcomes from protocol were reported in the results of the trial |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias existed |
Kruse 1993.
| Methods |
Study design: prospective, randomised trial
Setting/location: not reported.Country: Germany
Period of study: not reported (published in 1993)
Unit of randomisation: participant
Unit of analysis: participant Trial test: not reported Analysis strategy: intention‐to‐treat |
|
| Participants |
Inclusion criteria: outpatients with familiar hypercholesterolaemia Exclusion criteria: not reported Randomised: 24 (M‐DG: n = 12, E‐DG: n = 12) Participants assessed: 24 participants Withdrawals: no withdrawals Definition of hyperlipidaemia: not reported Baseline characteristics: Female (%): M‐DG: 25, E‐DG: 33 Age (years) (mean (SD)): M‐DG: 48.4 (11.4), E‐DG: 45 (9.7) Total cholesterol (TC) (mg/dL) (mean (SD)): M‐DG: 424.6 (129.9), E‐DG: 450.9 (87.0) LDL‐C (mg/dL) (mean (SD)): M‐DG: 338.8 (111), E‐DG: 379.7 (80.1) HDL‐C (mg/dL) (mean (SD)): M‐DG: 36.4 (10.8), E‐DG: 40.2 (8.1) Triglycerides (mg/dL) (mean (SD)): M‐DG: 178.9 (92.1), E‐DG: 130.2 (52.3) |
|
| Interventions | Type of interventions: morning vs evening doses of lovastatin M‐DG: 20 mg daily dose of lovastatin in the morning (regime 6:00 am‐10:00 am) E‐DG: 20 mg daily dose of lovastatin in the evening (regime 5:00 pm‐9:00 pm) Duration of intervention: 8 weeks. Quote: "The patients received placebo first in order to achieve stable baseline lipid values (washout period)... and all patients were randomly assigned to receive placebo...for 4 weeks. The washout period was followed by a second period of 4 weeks during which the patients were to take lovastatin 20 mg" (page 211 trial report) | |
| Outcomes | Drug compliance (consumption and time compliance) Change in lipid levels: TC, LDL‐C, HDL‐C and triglycerides levels (mg/dL) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "..and all patients were randomly assigned to receive placebo.." (page 211 trial report) Comment: insufficient information to make a judgement |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and study personnel not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Did not report number of withdrawals Comment: denominator values suggested complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias existed |
Nakaya 1995.
| Methods |
Study design: prospective, randomised trial
Setting/location: Tokai University Tokyo Hospital. Country: Japan
Period of study: August‐December 1990
Unit of randomisation: participant
Unit of analysis: participant Trial test: not reported Analysis strategy: intention‐to‐treat |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Randomised: 22 Participants assessed (ITT analysis): 22 Definition of hyperlipidemia: not described Baseline characteristics: Total cholesterol (TC) (mg/dL) (mean (SD)): M‐DG: 243.6 (9.4), E‐DG: 248.7 (10) & ME‐G: 239.3 (7.5) LDL‐C (mg/dL) (mean (SD)): M‐DG: 156.9 (8.3), E‐DG: 160.6 (12.5) & ME‐G: 156.9 (7.6) HDL‐C (mg/dL) (mean (SD)): M‐DG: 50.8 (2.9), E‐DG: 51.2 (3.8) & ME‐G: 50.4 (2.1) Triglycerides (mg/dL) (mean (SD)): M‐DG: 190.7 (43.4), E‐DG: 192.8 (37.6) & ME‐G: 160 (12.5) |
|
| Interventions |
Type of interventions: morning vs evening doses of fluvastatin
M‐DG: fluvastatin 10 mg
E‐DG: fluvastatin 10 mg Morning‐Evening group (ME‐G): fluvastatin 5 mg (twice a day) Duration of intervention: Washout period: 2 weeks (placebo) Treatment phase: 4 weeks Follow‐up observation period: 2 weeks (placebo) |
|
| Outcomes | Lipid levels: TC, LDL‐C, HDL‐C and triglycerides (mg/dL) | |
| Notes | Translated (original in Japanese) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study described a permuted‐block randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation controlled by the Pharmacy Service |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The authors were concerned to blind the treatment, all tablets were similar and indistinguishable |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not available the flow chart of the study |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias existed |
Saito 1991.
| Methods |
Study design: double‐blind placebo controlled study
Setting/location: not reported. Country: not reported
Period of study: not reported (published in 1991)
Unit of randomisation: participant
Unit of analysis: participant Trial test: not reported Analysis strategy: not reported |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Randomised: 172 participants (not reported by arm) Participants assessed at 0 weeks: 147 participants (M‐DG1: n = 29, E‐DG1: n = 27, M‐DG2: n = 32, E‐DG2: n = 28, PG: n=31) Withdrawals (subject decision): 46 participants (M‐DG1: n = 7, E‐DG1: n = 10, M‐DG2: n = 10, E‐DG2: n = 10, PG: n=9) Participants assessed at + 4 weeks: 101 participants (M‐DG1: n = 22, E‐DG1: n = 17, M‐DG2: n = 22, E‐DG2: n = 18, PG: n = 22) Definition of hyperlipidaemia: a serum cholesterol value of at least 220 mg/dL at each determination. The serum cholesterol was determined at least twice at intervals of 2‐4 weeks Baseline characteristics: Total cholesterol (TC) (mg/dL) (mean (SD)): (M‐DG1: 273.0 (39.6), E‐DG1: 274.9 (37.2), M‐DG2: 277.4 (49.8), E‐DG2: 288.8 (46.9), PG: 285.8 (44.6)) LDL‐C (mg/dL) (mean (SD)): (M‐DG1: 182.7 (46.8), E‐DG1: 195.9 (36.7), M‐DG2: 194.2 (48.1), E‐DG2: 204.3 (52.2), PG: 200.2 (54.3)) HDL‐C (mg/dL) (mean (SD)): (M‐DG1: 54.4 (24.3), E‐DG1: 47 (15), M‐DG2: 52.7 (17.9), E‐DG2: 53.2 (13), PG: 49.9 (14.3)) Triglycerides (mg/dL) (mean (SD)): (M‐DG1: 179.6 (105.3), E‐DG1: 160.3 (72.3), M‐DG2: 152.5 (77.4), E‐DG2: 156.3 (68.9), PG: 178.4 (124.5)) |
|
| Interventions |
Type of interventions: morning vs evening doses of simvastatin (figure 1 trial report)
M‐DG1: 2.5 mg daily dose of simvastatin with a placebo tablet of simvastatin 5 mg tablet in the morning and a placebo tablet of simvastatin 2.5 mg with a placebo tablet of simvastatin 5 mg in the evening
E‐DG1: a placebo tablet of simvastatin 2.5 mg with a placebo tablet of simvastatin 5 mg in the morning and 2.5 mg daily dose of simvastatin with a placebo tablet of simvastatin 5 mg in the evening
M‐DG2: 5 mg daily dose of simvastatin with a placebo tablet of simvastatin 2.5 mg in the morning and a placebo tablet of simvastatin 2.5 mg with a placebo tablet of simvastatin 5 mg in the evening
E‐DG2: a placebo tablet of simvastatin 5 mg with a placebo tablet of simvastatin 2.5 mg in the morning and 5 mg daily dose of simvastatin with a placebo tablet of simvastatin 2.5 mg tablet in the evening PG: a placebo tablet of simvastatin 2.5 mg with a placebo tablet of simvastatin 5 mg in the morning and in the evening Duration of intervention: 20 weeks including:
During the experimental period, the participants were instructed not to change their eating habits and alcohol consumption was prohibited on the day before test were performed. Concomitant administration of drugs known to affect serum lipid levels was prohibited |
|
| Outcomes | Lipid levels: TC, LDL‐C, HDL‐C, apolipoproteins and triglycerides levels (mg/dL) Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "was placed in envelopes for allocation to the subjects" (page 817 trial report) Comment: did not report if the assignment envelopes were used with appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "To preserve the double‐blind nature of this study, the double‐dummy method was applied" (page 817 trial report) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The determination of serum lipid parameters was realized in a central laboratory |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The authors did not report adequately the number and sources of withdrawals by arm (total: 46/172; 26.7%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias existed |
Scharnagl 2006.
| Methods |
Study design: prospective, randomised, double‐blind, multiple dose phase III clinical trial
Setting/location: 43 centres. Country: Germany
Period of study: not reported (published in 2006)
Unit of randomisation: participants
Unit of analysis: participants
Trial test: non inferiority Analysis strategy: intention‐to‐treat and per‐protocol analysis |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Screened for eligibility: 358 participants Randomised: 236 (the trial did not report the number of participants by group) Excluded (post‐randomisation): 2 participants (quote: "two patients were subsequently excluded from analysis because they did not receive active study treatment") Participants assessed (ITT analysis): including all participants for whom there was at least one LDL‐C measurement: 229 (M‐DG: n = 109, E‐DG: n = 120) Participants assessed (per protocol analysis): including all participants who did not have any severe protocol deviations: 197 (M‐DG: n = 92, E‐DG: n = 105) Participants assessed (safety analysis): 234 (M‐DG: n = 113 , E‐DG: n = 121) Definition of hyperlipidaemia: hypercholesterolaemia type IIa/b (Frederickson), LDL‐C ≥ 160 mg/dL and triglycerides < 400 mg/dL in the absence of lipid‐lowering treatment |
|
| Interventions |
Type of interventions: morning vs evening doses of fluvastatin XL
M‐DG: 80 mg daily dose of fluvastatin XL in the morning and a placebo tablet in the evening
E‐DG: 80 mg daily dose of fluvastatin XL in the evening and a placebo tablet in the morning
Duration of intervention: 8 weeks Baseline characteristics: Female (%): M‐DG: 65.1, E‐DG: 59.2 Age (years) (mean): M‐DG: 60.1, E‐DG: 60.6 Total cholesterol (TC) (mg/dL) (mean (SD)): M‐DG: 282.3 (32.6), E‐DG: 282.5 (35.4) LDL‐C (mg/dL) (mean (SD)): M‐DG: 189.9 (27.6), E‐DG: 188.5 (32.9) HDL‐C (mg/dL) (mean (SD)): M‐DG: 58.0 (16.5), E‐DG: 59.4 (16.3) Triglycerides (mg/dL) (mean (SD)): M‐DG: 176.0 (80.7), E‐DG: 176.4 (74.4) Duration of hypercholesterolaemia (years) (mean): M‐DG: 5.1, E‐DG: 5 Concomitant diseases: M‐DG: 89%, E‐DG: 91.7% Concomitant medications: M‐DG: 77.1%, E‐DG: 77.5% |
|
| Outcomes |
Primary endpoint: change in LDL‐C levels (mg/dL) between week 0 and week 8 Secondary endpoints:
Safety outcomes: any adverse event |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "..were randomised to receive fluvastatin..." (page 242 trial report) Comment: insufficient information to make a judgement |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "This was a prospective, double‐blind.." (page 242 trial report) Comment: no information provided about how they did it |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "This was a prospective, double‐blind.." (page 242 trial report) Comment: no information provided about how they did it |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported the reason of the participant exclusion in the ITT and per‐protocol analyses (39/236; 16.5%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias existed |
Tharavanij 2010.
| Methods | Study design: randomised double‐blind trial Setting/location: Thammasat University Hospital. Country: Thailand Period of study: not reported (published in 2010) Unit of randomisation: participants Unit of analysis: participants | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Enrolled: 60 participants Randomised: 57 participants. Quote: "Three subjects failed to follow‐up during the preliminary 2‐week run in period" Excluded (post‐randomisation): 1 (inclusion failure: abnormal liver function) Participants assessed: 52 (M‐DG: n = 25, E‐DG: n = 27) Withdrawals: 4 participants. and 4 subjects dropped out during treatment" (page 110 trial report) Definition of hyperlipidaemia: NCEP ATP III Baseline characteristics: Female (%): M‐DG: 60, E‐DG: 66.6 Age (years) (mean (SD)): M‐DG: 56.1 (8.5), E‐DG: 53.3 (10.4) Total cholesterol (TC) (mg/dL) (mean (SD)): M‐DG: 242.2 (41.4), E‐DG: 243.1 (28.4) LDL‐C (mg/dL) (mean (SD)): M‐DG: 171.6 (30.1), E‐DG: 172.1 (29.1) HDL‐C (mg/dL) (mean (SD)): M‐DG: 44.8 (9.9), E‐DG: 45.6 (9.3) Triglycerides (mg/dL) (mean (SD)): M‐DG: 155.1 (66.8), E‐DG: 141.0 (50.1) Duration of hypercholesterolaemia (years) (mean): not reported Hypertension (n, %): M‐DG: 14 (56), E‐DG: 13 (48.1) Diabetes n (%): M‐DG: 4 (16), E‐DG: 5 (18.5) Current smoker n (%): M‐DG: 1 (4), E‐DG: 1 (3.7) |
|
| Interventions | Type of interventions: morning vs evening doses of simvastatin M‐DG: 10 mg daily dose of simvastatin in the morning (regime 6:00 am‐10:00 am) E‐DG: 10 mg daily dose of simvastatin in the evening (regime 7:00 pm‐10:00 pm) Duration of intervention: 12 weeks | |
| Outcomes | Change in lipid levels: LDL‐C, TC, HDL‐C and triglycerides levels (mg/dL) Compliance: method of measure not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "enrolled participants were randomised by permuted block to receive 10 mg simvastatin in the morning or evening and placebo" (page 110 trial report) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and study personnel not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 57 participants were randomised and 52 participants completed the study (5/57; 8.7%) |
| Selective reporting (reporting bias) | High risk | Lipid levels by week were described incompletely |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias existed |
ALT: alanine transaminase
E‐DG: evening‐dose group HDL‐C: high‐density lipoprotein cholesterol
hs‐CRP: high‐sensitivity C‐reactive Protein ITT: intention‐to‐treat (analysis) LDL‐C: low‐density lipoprotein cholesterol M‐DG: morning‐dose group
ME‐G: morning‐evening group
RCT: randomised controlled trial
TC: total cholesterol ULN: upper limits of normal
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arca 2007 | Design: not a RCT |
| Cilla 1996 | Did not assess the effects of statins in hyperlipidaemia |
| Dujovne 1994 | Experimental and control arms not differing only by the timing of the administration of the same statin |
| Erdogan 2010 | Design: not a RCT |
| Fauler 2007 | Not acceptable lipid‐lowering regimen: single dose |
| Hunninghake 1998 | Experimental and control arms not differing only by the timing of the administration of the same statin |
| Illingworth 1986 | Design: not a RCT |
| Insull 1994 | Experimental and control arms not differing only by the timing of the administration of the same statin |
| Kele, 2008 | Did not assess the effects of the timing of statin administration |
| Lund 2002 | Participants: the study did not consider hyperlipidaemia as an inclusion criteria |
| Martin 2002 | Did not assess the effects of statins in hyperlipidaemia |
| Matsuzawa 1991 | Did not assess the effects of the timing of statin administration |
| Nozaki 1996 | Design: not a RCT |
| Plakogiannis 2005 | Design: not a RCT |
| Schwartz 2009 | Design: not a RCT |
| Stein 1997 | Experimental and control arms not differing only by the timing of the administration of the same statin |
| Triscari 1995 | Did not assess the effects of statins in hyperlipidaemia |
| Wallace 2003 | Did not assess the effects of statins in hyperlipidaemia |
| Yoon 2011 | Did not assess the effects of statins in hyperlipidaemia |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Nakaya 1990.
| Methods | Study design: randomised double‐blind trial Period of study: not reported (published in 1990) Unit of randomisation: participants Unit of analysis: participants |
| Participants | Hyperlipidaemic participants n = 66 participants |
| Interventions | Type of interventions: morning vs evening doses of simvastatin M‐DG: 10 mg daily dose of pravastatin after breakfast E‐DG: 10 mg daily dose of pravastatin after dinner Duration of intervention: 12 weeks |
| Outcomes | Change in lipid levels: TC, HDL‐C and triglycerides levels (mg/dL) Treatment side effects |
| Notes |
E‐DG: evening‐dose group HDL‐C: high‐density lipoprotein cholesterol TC: total cholesterol
Differences between protocol and review
Types of outcomes measures
We stated in protocol that we would group outcome data into those measured at six months, at one year, at two years, and at more than two years. This was not possible because the trials we have included were no longer than 14 weeks.
Search methods
We stated in the protocol that we planned to search the ProQuest Dissertations & Theses Database. Instead, we focused our search in ProQuest in a subject area: ProQuest Health & Medical Complete. We did not contact pharmaceutical companies to identify further uncompleted or unpublished studies due to operational time restraints.
Data extraction and risk of bias assessment
We planned to obtain additional information when necessary by contacting the authors. Given that most of the trials had been published in the beginning of the 20th century we did not try to contact them.
Dealing with missing data
The protocol stated that if SDs in changes of lipid levels from the baseline were unknown for more than 50% of the studies, we would not impute them and we would not include these studies in the meta‐analysis. However, only one trial provided data on the SD and it was imputed for the rest of the studies.
Subgroup analysis
There were insufficient studies in each comparison to perform the subgroup analysis planned in the protocol. We decided during the process of the review to include a subgroup analysis based on the different follow‐up of the studies. We will undertake planned subgroup analysis in future updates when possible.
Sensitivity analysis
We were unable to explore the robustness of the results by performing a sensitivity analysis on the basis of risk of bias because none of the included studies were rated as ‘low risk of bias’. We did not include any unpublished data or very large studies in the analyses, so these sensitivity analyses were not possible, either. We also did not perform the planned sensitivity analysis based on assumptions taken in the ‘available case analysis’ due to the small number of studies included. In future updates, we will try to perform these sensitivity analyses as planned in protocol when possible.
Contributions of authors
JMIP: Conceived the review question, co‐ordinated the review development, controlled and wrote the content of the review; identified references for the background; assessed the relevance and quality of papers, and extracted data; made an intellectual contribution and provided a clinical perspective to the manuscript, and approved the final version of the review prior to submission.
JMFT: Co‐ordinated the protocol and review development; assessed the relevance and quality of papers, extracted data, identified references for the background, designed, drafted and wrote the protocol, organised retrieval of papers, made an intellectual contribution and provided a clinical perspective for the manuscript; and approved the final version of the review prior to submission.
MNP: Provided statistical analysis and interpretation of data, extracted data, supported in the remainder sections of the review in writing, made an intellectual contribution and provided a clinical perspective for the manuscript; and approved the final version of the review prior to submission.
All other authors: Assessed the relevance and quality of papers, extracted data, edited the review, identified references for the background, made an intellectual contribution and provided a clinical perspective for the manuscript; and approved the final version of the review prior to submission.
Sources of support
Internal sources
UETS, Health Technology Assessment Unit. UCICEC de Atención Primaria. Agency Laín Entralgo (Cochrane Collaborating Centre), Madrid, Spain.
Servicio Madrileño de Salud (SERMAS), Spain.
External sources
Grant PI11/02257: “Acción Estratégica de Salud” (2011), Subprograma de proyectos de investigacion en salud. Plan Nacional de Investigación Científica, Desarrollo e InnovaciónTecnológica 2008‐2011(ISCIII), Spain.
Grant RS_AP10/5: La cronoterapia frente la terapia convencional en el tratamiento de la hiperlipedemia. Convocatoria de ayudas para el año 2010 de la Agencia Pedro Laín Entralgo de Formación, Investigación y Estudios Sanitarios de la Comunidadde Madrid, para la realización de proyectos de investigación en el campo de resultados en salud en Atención Primaria, Spain.
Declarations of interest
Jose Manuel Izquierdo‐Palomares: is member of the board of directors of the Spanish Society of Primary Care Pharmacists (SEFAP), whose financing is through membership fees and also accepts donations from pharmaceutical laboratories. Jesus Maria Fernandez‐Tabera: Grant PI11/02257: “Acción Estratégica de Salud” (2011), Subprograma de proyectos de investigacion en salud. Plan Nacional de Investigación Científica, Desarrollo e InnovaciónTecnológica 2008‐2011(ISCIII), Spain. Grant RS_AP10/5: La cronoterapia frente la terapia convencional en el tratamiento de la hiperlipedemia. Convocatoria de ayudas para el año 2010 de la Agencia Pedro Laín Entralgo de Formación, Investigación y Estudios Sanitarios de la Comunidad de Madrid, para la realización de proyectos de investigación en el campo de resultados en salud en Atención Primaria. Maria N Plana: none known Almudena Añino Alba: none known Pablo Gómez Álvarez: none known Inmaculada Fernandez‐Esteban: from January 1996 to July 1997 Inmaculada Fernández Esteban was employed by Glaxo‐Wellcome as a documentalist. This activity was not related to her work with this Cochrane review. Luis Carlos Saiz: none known Pilar Martin‐Carrillo: none known Óscar Pinar López: none known
New
References
References to studies included in this review
Davignon 1990 {published data only}
- Davignon J, Xhignesse M, Frohlich J, Hayden ML, Vanetta H, Mishkel MA, et al. A multicenter comparison of lovastatin and probucol for treatment of severe primary hypercholesterolemia. American Journal of Cardiology 1990;66(8):22b‐30b. [DOI] [PubMed] [Google Scholar]
Hunninghake 1990 {published data only}
- Hunninghake DB, Mellies MJ, Goldberg AC, Kuo PT, Kostis JB, Schrott HG, et al. Efficacy and safety of pravastatin in patients with primary hypercholesterolemia. II. Once‐daily versus twice‐daily dosing. Atherosclerosis 1990;85(2‐3):219‐27. [DOI] [PubMed] [Google Scholar]
Kim 2013 {published data only}
- Kim SH, Kim MK, Seo HS, Hyun MS, Han KR, Cho SW, et al. Efficacy and safety of morning versus evening dose of controlled‐release simvastatin tablets in patients with hyperlipidemia: a randomized, double‐blind, multicenter phase III trial. Clinical Therapeutics 2013 Sep;35(9):1350‐60. [DOI] [PubMed] [Google Scholar]
Kruse 1993 {published data only}
- Kruse W, Nikolaus T, Rampmaier J, Weber E, Schlierf G. Actual versus prescribed timing of lovastatin doses assessed by electronic compliance monitoring. European Journal of Clinical Pharmacology 1993;45(3):211‐5. [DOI] [PubMed] [Google Scholar]
Nakaya 1995 {published data only}
- Nakaya N, Goto Y, Inoue T. Clinical effect of fluvastatin (XU62‐320) on hyperlipidemia: dose frequency study in a double‐blind comparative method. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1995;11(Suppl 1):131–51. [Google Scholar]
Saito 1991 {published data only}
- Saito Y, Yoshida S, Nakaya N, Hata Y, Goto Y. Comparison between morning and evening doses of simvastatin in hyperlipidemic subjects. A double‐blind comparative study. Arteriosclerosis and Thrombosis 1991;11(4):816‐26. [DOI] [PubMed] [Google Scholar]
Scharnagl 2006 {published data only}
- Scharnagl H, Vogel M, Abletshauser C, Freisinger F, Stojakovic T, Marz W. Efficacy and safety of fluvastatin‐extended release in hypercholesterolemic patients: morning administration is equivalent to evening administration. Cardiology 2006;106(4):241‐8. [DOI] [PubMed] [Google Scholar]
Tharavanij 2010 {published data only}
- Tharavanij T, Wongtanakarn S, Lerdvuthisopon N, Teeraaunkul S, Youngsriphithak P, Sritipsukho P. Lipid lowering efficacy between morning and evening simvastatin treatment: a randomized double‐blind study. Journal of the Medical Association of Thailand [Chotmaihet thangphaet] 2010;93(Suppl 7):S109‐113. [PubMed] [Google Scholar]
References to studies excluded from this review
Arca 2007 {published data only}
- Arca M, Gaspardone A. Atorvastatin efficacy in the primary and secondary prevention of cardiovascular events. Drugs 2007;67(Suppl 1):29‐42. [DOI] [PubMed] [Google Scholar]
Cilla 1996 {published data only}
- Cilla DD, Gibson DM, Whitfield LR, Sedman AJ. Pharmacodynamic effects and pharmacokinetics of atorvastatin after administration to normocholesterolemic subjects in the morning and evening. Journal of Clinical Pharmacology 1996;36(7):604‐9. [DOI] [PubMed] [Google Scholar]
Dujovne 1994 {published data only}
- Dujovne CA, Davidson MH. Fluvastatin administration at bedtime versus with the evening meal: a multicenter comparison of bioavailability, safety, and efficacy. The American Journal of Medicine 1994;96(6a):37s‐40s. [DOI] [PubMed] [Google Scholar]
Erdogan 2010 {published data only}
- Erdogan D, Özaydın M, Türker Y, Karabacak M, Varol E, Doğan A. Influence of statin therapy on circadian variation of acute myocardial infarction. Anadolu Kardiyol Derg 2010;10(5):429‐33. [DOI] [PubMed] [Google Scholar]
Fauler 2007 {published data only}
- Fauler G, Abletshauser C, Erwa W, Loser R, Witschital K, Marz W. Time‐of‐intake (morning versus evening) of extended‐release fluvastatin in hyperlipemic patients is without influence on the pharmacodynamics (mevalonic acid excretion) and pharmacokinetics. International Journal of Clinical Pharmacology and Therapeutics 2007;45(6):328‐34. [DOI] [PubMed] [Google Scholar]
Hunninghake 1998 {published data only}
- Hunninghake DB. Clinical efficacy of cerivastatin: phase IIa dose‐ranging and dose‐scheduling studies. American Journal of Cardiology 1998;82(4b):26j‐31j. [DOI] [PubMed] [Google Scholar]
Illingworth 1986 {published data only}
- Illingworth DR. Comparative efficacy of once versus twice daily mevinolin in the therapy of familial hypercholesterolemia. Clinical Pharmacology and Therapeutics 1986;40(3):338‐43. [DOI] [PubMed] [Google Scholar]
Insull 1994 {published data only}
- Insull W, Black D, Dujovne C, Hosking J D, Hunninghake D, Keilson L, et al. Efficacy and safety of once‐daily vs twice‐daily dosing with fluvastatin, a synthetic reductase inhibitor, in primary hypercholesterolemia. Archives of Internal Medicine 1994;154(21):2449‐55. [PubMed] [Google Scholar]
Kele, 2008 {published data only}
- Kele T, Akar Bayram N, Kayhan T, Canbay A, Sahin D, Durmaz T, et al. The comparison of the effects of standard 20 mg atorvastatin daily and 20 mg atorvastatin every other day on serum LDL‐cholesterol and high sensitive C‐reactive protein levels. Anadolu Kardiyoloji Dergisi [Anatolian Journal of Cardiology] 2008;8(6):407‐12. [PubMed] [Google Scholar]
Lund 2002 {published data only}
- Lund TM, Torsvik H, Falch D, Christophersen B, Skardal R, Gullestad L. Effect of morning versus evening intake of simvastatin on the serum cholesterol level in patients with coronary artery disease. American Journal of Cardiology 2002;90(7):784–6. [DOI] [PubMed] [Google Scholar]
Martin 2002 {published data only}
- Martin PD, Mitchell PD, Schneck DW. Pharmacodynamic effects and pharmacokinetics of a new HMG‐CoA reductase inhibitor, rosuvastatin, after morning or evening administration in healthy volunteers. British Journal of Clinical Pharmacology 2002;54(5):472‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matsuzawa 1991 {published data only}
- Matsuzawa Y, Nakaya N, Kita T, Saito Y, Nakai T, Yamamoto A, et al. Clinical evaluation of pravastatin (CS‐514) in once daily therapy on hyperlipidemia: double‐blind comparative study between once daily and twice daily therapy. Rinsho Hyoka (Clinical Evaluation) 1991;19(1):47‐92. [Google Scholar]
Nozaki 1996 {published data only}
- Nozaki S, Nakagawa T, Nakata A, Yamashita S, Kameda‐Takemura K, Nakamura T, et al. Effects of pravastatin on plasma and urinary mevalonate concentrations in subjects with familial hypercholesterolaemia: a comparison of morning and evening administration. European Journal of Clinical Pharmacology 1996;49(5):361‐4. [DOI] [PubMed] [Google Scholar]
Plakogiannis 2005 {published data only}
- Plakogiannis R, Cohen H, Taft D. Effects of morning versus evening administration of atorvastatin in patients with hyperlipidemia. American Journal of Health‐System Pharmacy: AJHP: official journal of the American Society of Health‐System Pharmacists 2005;62(23):2491‐4. [DOI] [PubMed] [Google Scholar]
Schwartz 2009 {published data only}
- Schwartz JB, Verotta D. Population analyses of atorvastatin clearance in patients living in the community and in nursing homes. Clinical Pharmacology and Therapeutics 2009;86(5):497‐502. [DOI] [PubMed] [Google Scholar]
Stein 1997 {published data only}
- Stein E, Sprecher D, Allenby KS, Tosiello RL, Whalen E, Ripa SR. Cerivastatin, a new potent synthetic HMG Co‐A reductase inhibitor: effect of 0.2 mg daily in subjects with primary hypercholesterolemia. Journal of Cardiovascular Pharmacology and Therapeutics 1997;2(1):7‐16. [DOI] [PubMed] [Google Scholar]
Triscari 1995 {published data only}
- Triscari J, Rossi L, Pan HY. Chronokinetics of pravastatin administered in the PM compared with AM dosing. American Journal of Therapeutics 1995;2(4):265‐8. [DOI] [PubMed] [Google Scholar]
Wallace 2003 {published data only}
- Wallace A, Chinn D, Rubin G. Taking simvastatin in the morning compared with in the evening: randomised controlled trial. BMJ 2003;327(7418):788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoon 2011 {published data only}
- Yoon Hyung Sik, Kim Sung Ho, Kim Jeong Kyung, Ko Sang Hun, Ko Jae Ee, Park Soo Jin, et al. Comparison of effects of morning versus evening administration of ezetimibe/simvastatin on serum cholesterol in patients with primary hypercholesterolemia. [Erratum appears in Annals of Pharmacotherapy 2011 Sep;45(9):1172]. Annals of Pharmacotherapy 2011;45(7‐8):841‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Nakaya 1990 {published data only}
- Nakaya N, Kita T, Matsuzawa Y, Saito Y, Nakai T, Yamamoto A, et al. Clinical study of pravastatin (CS‐514) at once daily in hyperlipidemia: double‐blind comparative study between morning and evening therapy. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1990;6(9):1803–28. [Google Scholar]
Additional references
Baigent 2005
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366(9493):1267‐78. [DOI] [PubMed] [Google Scholar]
Baigent 2010
- Baigent C, Blackwell L, Emberson J, Holland LE, Reith C. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376(9753):1670‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1997
- Cohen MC, Rohtla KM, Lavery CE, Muller JE, Mittleman MA. Meta‐analysis of the morning excess of acute myocardial infarction and sudden cardiac death. The American Journal of Cardiology 1997;79(11):1512‐6. [DOI] [PubMed] [Google Scholar]
Cooke 1994
- Cooke HM, Lynch A. Biorhythms and chronotherapy in cardiovascular disease. American Journal of Hospital Pharmacy 1994;51(20):2569‐80. [PubMed] [Google Scholar]
Cooke‐Ariel 1998
- Cooke‐Ariel H. Circadian variations in cardiovascular function and their relation to the occurrence and timing of cardiac events. American Journal of Health‐System Pharmacy 1998;55(Suppl 3):S5‐11. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Downs 1998
- Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998;279(20):1615‐22. [DOI] [PubMed] [Google Scholar]
Elliott 1998
- Elliott WJ. Circadian variation in the timing of stroke onset: a meta‐analysis. Stroke 1998;29(5):992‐6. [DOI] [PubMed] [Google Scholar]
European Heart Network 2008
- European Heart Network (EHN). Cardiovascular disease statistics 2008. www.ehnheart.org/cdv‐statistics.html (accessed 30 December 2010).
Galman 2005
- Galman C, Angelin B, Rudling M. Bile acid synthesis in humans has a rapid diurnal variation that is asynchronous with cholesterol synthesis. Gastroenterology 2005;129(5):1445‐53. [DOI] [PubMed] [Google Scholar]
Gamble 2005
- Gamble C, Hollis S. Uncertainty method improved on best‐worst case analysis in a binary meta‐analysis. Journal of Clinical Epidemiology 2005;58(6):579‐88. [DOI] [PubMed] [Google Scholar]
GRADE Working Group 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime, Inc). Available from www.gradepro.org. GRADEpro Guideline Development Tool. McMaster University (developed by Evidence Prime, Inc). Available from www.gradepro.org, 2015.
Graham 2007
- Graham I, Atar D, Borch‐Johnsen K, Boysen G, Burell G, Cifkova R, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited experts). European Heart Journal 2007;28(19):2375‐414. [DOI] [PubMed] [Google Scholar]
Haffner 1995
- Haffner S, Orchard T, Stein E, Schmidt D, LaBelle P. Effect of simvastatin on Lp(a) concentrations. Clinical Cardiology 1995;18(5):261‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Institute for Clinical Systems Improvement 2009
- Institute for Clinical Systems Improvement (ICSI). Lipid management in adults. Health Care Guideline 2009. www.icsi.org/guidelines_and_more/gl_os_prot/cardiovascular/lipid_management_3/lipid_management_in_adults__4.html (accessed 30 December 2010).
ISIS‐2 1992
- ISIS‐2 (Second International Study of Infarct Survival) Collaborative Group. Morning peak in the incidence of myocardial infarction: experience in the ISIS‐2 trial. European Heart Journal 1992;13(5):594‐8. [PubMed] [Google Scholar]
Jones 1990
- Jones PJ, Schoeller DA. Evidence for diurnal periodicity in human cholesterol synthesis. Journal of Lipid Research 1990;31(4):667‐73. [PubMed] [Google Scholar]
Kannel 1979
- Kannel WB, Castelli WP, Gordon T. Cholesterol in the prediction of atherosclerotic disease. New perspectives based on the Framingham study. Annals of Internal Medicine 1979;90(1):85‐91. [DOI] [PubMed] [Google Scholar]
Kannel 1992
- Kannel WB, Wilson PW. Efficacy of lipid profiles in prediction of coronary disease. American Heart Journal 1992;124(3):768‐74. [DOI] [PubMed] [Google Scholar]
Keys 1980
- Keys A. Alpha lipoprotein (HDL) cholesterol in the serum and the risk of coronary heart disease and death. Lancet 1980;2(8195 pt 1):603‐6. [DOI] [PubMed] [Google Scholar]
Kozak 2003
- Kozak M. Circadian rhythms in cardiovascular diseases ‐ arrhythmias [Cirkadianni rytmy u kardiovaskularnich onemocneni ‐ arytmie]. Vnitrni Lekarstvi 2003;49(4):297‐301. [PubMed] [Google Scholar]
LaRosa 2003
- LaRosa JC. Reduction of serum LDL‐C levels: a relationship to clinical benefits. American Journal of Cardiovascular Drugs 2003;3(4):271‐81. [DOI] [PubMed] [Google Scholar]
Law 2003
- Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta‐analysis. BMJ (Clinical research ed) 2003;326(7404):1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liao 2010
- Liao C, Li J, Bin Q, Cao Y, Gao F. Chronomodulated chemotherapy versus conventional chemotherapy for advanced colorectal cancer: a meta‐analysis of five randomised controlled trials. International Journal of Colorectal Disease 2010;25(3):343‐50. [DOI] [PubMed] [Google Scholar]
Lloyd‐Jones 2010
- Lloyd‐Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, Simone G, et al. Heart disease and stroke statistics ‐ 2010 update: a report from the American Heart Association. Circulation 2010;121(7):e46‐215. [DOI] [PubMed] [Google Scholar]
Loke 2011
- Loke YK, Price D, Herxheimer A. Chapter 14: Adverse effects. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
LRC‐CPPT 1984
- LRC‐CPPT 1984. The Lipid Research Clinics Coronary Primary Prevention Trial results II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA 1984;251(3):365‐74. [PubMed] [Google Scholar]
Luu 2010
- Luu KT, Raber SR, Nickens DJ, Vicini P. A model‐based meta‐analysis of the effect of latanoprost chronotherapy on the circadian intraocular pressure of patients with glaucoma or ocular hypertension. Clinical Pharmacology and Therapeutics 2010;87(4):421‐5. [DOI] [PubMed] [Google Scholar]
Muller 1994
- Muller JE, Mangel B. Circadian variation and triggers of cardiovascular disease. Cardiology 1994;85 Suppl 2:3‐10. [DOI] [PubMed] [Google Scholar]
NCEP 2002
- National Cholesterol Education Program. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002;106(25):3143‐421. [PubMed] [Google Scholar]
NICE 2008
- National Institute for Health and Clinical Excellence. NICE clinical guideline 67: Lipid Modification. Cardiovascular risk assessment and the primary and secondary prevention of cardiovascular disease. www.nice.org.uk/nicemedia/pdf/CG67NICEguideline.pdf (accessed 30 December 2010).
NZGG 2003
- New Zealand Guidelines Group (NZGG). Assessment and management of cardiovascular risk. www.nzgg.org.nz/guidelines/dsp_guideline_popup.cfm?guidelineCatID=3&guidelineID=35 (accessed 30 December 2010).
Parker 1982
- Parker TS, McNamara DJ, Brown C, Garrigan O, Kolb R, Batwin H, et al. Mevalonic acid in human plasma: relationship of concentration and circadian rhythm to cholesterol synthesis rates in man. Proceedings of the National Academy of Sciences of the United States of America 1982;79(9):3037‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pedersen 2004
- Pedersen TR, Kjekshus J, Berg K, Haghfelt T, Faergeman O, Faergeman G, et al. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). 1994. Atherosclerosis. Supplements 2004;5(3):81‐7. [DOI] [PubMed] [Google Scholar]
Petersen 2005
- Petersen S, Peto V, Rayner M, Leal J, Luengo‐Fernandez R, Gray A. European Cardiovascular Disease Statistics. London: British Heart Foundation, 2005. [Google Scholar]
Plakogiannis 2007
- Plakogiannis R, Cohen H. Optimal low‐density lipoprotein cholesterol lowering ‐ morning versus evening statin administration. The Annals of Pharmacotherapy 2007;41(1):106‐10. [DOI] [PubMed] [Google Scholar]
Redberg 2009
- Redberg RF, Benjamin EJ, Bittner V, Braun LT, Goff DC Jr, Havas S, et al. AHA/ACCF [corrected] 2009 performance measures for primary prevention of cardiovascular disease in adults: a report of the American College of Cardiology Foundation/American Heart Association task force on performance measures (writing committee to develop performance measures for primary prevention of cardiovascular disease): developed in collaboration with the American Academy of Family Physicians; American Association of Cardiovascular and Pulmonary Rehabilitation; and Preventive Cardiovascular Nurses Association: endorsed by the American College of Preventive Medicine, American College of Sports Medicine, and Society for Women's Health Research. Circulation 2009;120(13):1296‐336. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sacks 1996
- Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. The New England Journal of Medicine 1996;335(14):1001‐9. [DOI] [PubMed] [Google Scholar]
San Vicente 2008
- San Vicente R, Pérez I, Ibarra J, Berraondo I, Uribe F, Garcia de Madinabeitia JU, et al. Clinical practice guide on lipid management as a cariovascular risk factor 2008 [Guía de práctica clínica sobre el manejo de los lípidos como factor de riesgo cardiovascular 2008]. www.guiasalud.es/GPC/GPC_433_Lipidos_compl_cast.pdf (accessed 30 December 2010).
Santosa 2007
- Santosa S, Varady KA, AbuMweis S, Jones PJ. Physiological and therapeutic factors affecting cholesterol metabolism: does a reciprocal relationship between cholesterol absorption and synthesis really exist?. Life Sciences 2007;80(6):505‐14. [DOI] [PubMed] [Google Scholar]
Shepherd 1995
- Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. The New England Journal of Medicine 1995;333(20):1301‐7. [DOI] [PubMed] [Google Scholar]
SIGN 2007
- Scottish Intercollegiate Guidelines Network (SIGN). SIGN 97: Risk estimation and the prevention of cardiovascular disease. A National Clinical Guideline 2007. www.sign.ac.uk/pdf/sign97.pdf (accessed 30 December 2010).
Smith 2009
- Smith MEB, Lee NJ, Haney E, Carson S. Drug class review: HMG‐CoA reductase inhibitors (statins). Update 5. www.ohsu.edu/drugeffectiveness/reports/final.cfm (accessed 30 December 2010).
Stedman 2010
- Stedman. Stedman's Medical Dictionary. www.drugs.com/medical_dictionary.html (accessed 30 December 2010).
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Stone 2014
- Stone NJ, Robinson J, Lichtenstein AH, Bairey Merz C, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 2014;63(25_PA):2889‐934. [DOI] [PubMed] [Google Scholar]
Sánchez 2005
- Sánchez Merino J, Gil Guillén VF. Chronobiology, chronotherapy and vascular risk [Cronobiología, cronoterapia y riesgo vascular]. Revista Clinica Española 2005;205(6):283‐6. [DOI] [PubMed] [Google Scholar]
Tyroler 1990
- Tyroler HA. Serum lipoproteins as risk factors: recent epidemiologic studies in individuals with and without prevalent cardiovascular disease. European Heart Journal 1990;11 Suppl H:21‐5. [DOI] [PubMed] [Google Scholar]
WHO 2009
- World Health Organization (WHO). Cardiovascular disease (CVDs) 2009. www.who.int/mediacentre/factsheets/fs317/en/index.html (accessed 30 December 2010).
WHOSIS 2009
- World Health Organization Statistical Information System (WHOSIS) 2009. Statistical Information System. www.who.int/whosis/en/ (accessed 30 December 2010).
Zhao 2003
- Zhao P, Xu P, Wan CM, Li BY, Wang ZR. Chronotherapy versus conventional drug therapy for hypertension. Cochrane Database of Systematic Reviews 2003, Issue 2. Art. No.: CD004184. [DOI: 10.1002/14651858.CD004184] [DOI] [Google Scholar]
References to other published versions of this review
Fernandes‐Tabera 2011
- Fernandes‐Tabera JM, López‐Alcalde J, Gómez Álvarez P, Izquierdo‐Palomares JM, Martin‐Carrillo P, Cauto‐Aragonés P, et al. Chronotherapy versus conventional statins therapy for the treatment of hyperlipidaemia. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD009462] [DOI] [PMC free article] [PubMed] [Google Scholar]


